Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its proceeding programmes as project number 08/1815/234. The contractual start date was in October 2008. The final report began editorial review in October 2012 and was accepted for publication in February 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Ridsdale et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Introduction to epilepsy in the context of the NHS
The stated policy of the NHS is to empower and support people with long-term conditions to understand their own needs and self-manage them. 1 In UK surveys of people with epilepsy (PWE), there has been a consistent demand for better provision of information. 2–5 One survey of patients with poorly controlled epilepsy found that one-third reported not being told what epilepsy was, > 90% wanted more information about the disease and ∼ 75% felt that they had not been given enough information about the side effects of antiepileptic drugs (AEDs). Over 60% wanted to talk to someone other than a consultant about epilepsy. 4 PWE (and their carers) frequently lack the confidence, however, to seek out such information. 6 To date, the NHS has not implemented a routine programme of education or rehabilitation for PWE.
The National Service Framework for Long-Term Conditions7 includes epilepsy as a long-term condition. Most adults with epilepsy experience paroxysmal loss of consciousness, and between attacks they do not have obvious signs such as weakness, rigidity or incoordination that might benefit from physiotherapy. Disability between attacks is likely to be cognitive, psychological and social and so is largely hidden. Perhaps this is why rehabilitation and advice on self-care have not generally been given a high profile in research or in practice.
The National Institute for Health and Care Excellence (NICE) advocates self-management education for adults with epilepsy, aiming to achieve improvement in seizure frequency, increase individuals' understanding of epilepsy and adherence to medication, decrease fear of seizures and reduce hazardous self-management strategies. 8 A Cochrane review9 concluded that so far there is some evidence of benefit from self-management approaches, but there is insufficient evidence of improvement in health. Further research in this area has been recommended. 10,11
Poor epilepsy control is associated with unnecessary hospital admissions,12 which NHS policy aims to prevent. 13 Self-management education is designed, amongst other things, to reduce this, and providing information should receive high priority. 8 Improving health-related quality of life (HRQoL) for people with long-term conditions and ensuring that people feel supported to manage their conditions are NHS outcome targets. 13 In the UK, self-management programmes have been tested and adopted for other chronic conditions [e.g. diabetes: Dose Adjustment for Normal Eating (DAFNE),14 Diabetes Education and Self Management for Ongoing and Newly Diagnosed (DESMOND),15 X-PERT;16 arthritis17,18].
What is the epidemiology of epilepsy?
The World Health Organization and the International League Against Epilepsy (ILAE) reported that epilepsy is the most common serious neurological condition, affecting approximately 50 million people worldwide. 19 With 0.6–1.0% of adults affected at any point in time,20–22 there are approximately 315,000 adults with epilepsy in England. The lifetime prevalence of seizures is 2–5%. 23 Epilepsy attacks are intermittent and their frequency and severity are variable. After diagnosis most people achieve control of attacks – studies show that 50–70% of PWE have had no attacks in the previous year. 24,25 Although these people have a long-term condition, most are able to participate in their social setting and adjust to the initial distress of a potentially stigmatising condition. 26–28
The impact of poor epilepsy control
On an individual level
The same studies found that 30–50% of PWE have had an epilepsy attack in the previous year and 40% have two or more seizures per year. 24,25 People with poorly controlled epilepsy face restrictions in activity, such as driving, which in turn affects work and social participation. Indeed, epilepsy alone accounts for 1% of all global disability, as measured by productive life-years lost. 29 Poor epilepsy control is also associated with higher rates of psychological distress, including anxiety and depression, and perceived stigma. 30,31
On acute hospital service use
One study24 found that 18% of PWE had attended a hospital emergency department (ED) and 9% had been admitted to hospital for epilepsy in the previous year. A different study32 found that 13% of PWE attended an ED for epilepsy, with a mean number of visits of 0.3. Shohet et al. 33 explored this issue using data from the 2004 Quality and Outcomes Framework (QOF). 34 The QOF operates as a means of linking the income of English primary care general medical practices to care quality. As part of this, practices annually report the percentage of PWE (aged ≥ 16 years) registered on AEDs who were seizure free in the last 12 months. Shohet et al. found that poor epilepsy control measured according to the QOF34 was strongly associated with higher levels of emergency epilepsy-related hospitalisations. For those PWE-related emergency admissions, the mean number of admissions was 1.3. There is a gap in the evidence with regard to the frequency of ED use by PWE. However, use of health-care services is often not evenly distributed. 35 Some people attend infrequently or not at all, whereas others use services frequently.
Moore et al. 36 examined reattendance within a 12-month period to an inner London hospital ED amongst the general ED population. Reattendance was unusual – only 24% reattended, most doing so on one occasion only. In contrast, those with chronic conditions with episodic relapse reattend more frequently. International evidence shows that up to 67% of patients with chronic obstructive pulmonary disease (COPD)37 and 42% with asthma37 or diabetes38 reattend within a 12-month period.
The distribution of ED use is unknown in epilepsy. However, there is evidence that it is the most frequent neurological reason for emergency readmission into hospital. 39 If some people do attend EDs more frequently, there is a gap in the evidence about what their characteristics are, whether this service use is appropriate or preventable and, if so, by what means.
What is the association between poor epilepsy control, deprivation and acute hospital service use?
There is a strong correlation between the prevalence of epilepsy and social deprivation. 40 Using evidence derived from the QOF, Ashworth et al. 41 found that patients with epilepsy living in socially deprived areas were less likely to have had seizure control in the previous year. Gladman et al. 42 found examples of some neurological conditions that were better managed in major cites, particularly those with specialist rehabilitation services. The implication is that, for some conditions, specialist rehabilitation can and has achieved better outcomes. However, despite the presence of specialist services in English cities, we know of no evidence showing that better epilepsy control has been achieved. Neurologists see patients referred for diagnosis. After diagnosis there has not been a strategic approach to systematically identify people in the catchment area with recurrent seizures, for example by their attendance at EDs. Less than one-quarter of epilepsy-related ED attendees are referred for follow-up,43 and patients with poorly controlled epilepsy may not expect much or ask for follow-up. Majeed et al. 44 found that there are a greater number of hospital emergency admissions amongst those living in deprived areas in London. Reactive acute services are not linked with rehabilitation services.
What is the consequence of poor epilepsy control in terms of NHS cost?
Epilepsy is costly. In the EU, the total cost of epilepsy was £15.5B in 2004,45 with the total cost per case being £2000–11,500. The largest element of health-care cost has often been found to be hospitalisations. 32,46 Six out of seven admissions for epilepsy are on an emergency basis. 47 Of all neurological conditions, epilepsy is associated with the highest rate of emergency readmissions within the same year. 39 In 2008–9, there were 37,140 NHS hospital admissions for which epilepsy was the primary diagnosis. 12 The average episode cost was £1514,48 indicating a total annual inpatient cost of £56.2M. There may also be significant indirect societal costs through lost/absent employment. 49
The costs are likely to be distributed unevenly because of differences in deprivation. 44 These costs are all estimated from research and national data sets with no in-depth research in specific areas of low or high service use. The health economic gain from any intervention may be greater when there are low epilepsy QOF scores, with high non-planned epilepsy admissions. On the other hand, when indicators suggest that epilepsy control is very poor, step-up care might need to be highly intensive. If the ratio of cost to benefit is low, new services may be deemed too expensive in the current economic climate.
There is a gap in the evidence with regard to the actual costs of ED use by PWE, particularly in deprived areas where use is likely to be high. As NHS commissioning becomes increasingly decentralised, this cost information will be important for commissioners. This will be weighed against the potential cost-effectiveness of any proactive intervention that is designed to improve the self-management of PWE.
What evidence is there with regard to usual care provided for people with epilepsy who attend the emergency department?
In England all PWE are expected to have a structured medical review of their epilepsy at least yearly by either a generalist or a specialist. 50 Evidence nationally is that 95% of adults on drug treatment have had their epilepsy reviewed in primary care in the last 15 months. 51 In contrast, there is no currently accepted care for those with established epilepsy who have visited an ED. NICE guidelines for epilepsy,50 however, indicate that, when seizures are not controlled or treatment fails, it is expected that a patient will be referred to tertiary services for assessment. The 2012 UK-wide National Audit of Seizure Management in Hospitals (NASH)52 showed that UK EDs initiated this for only one-third of PWE attending EDs.
The NASH also found that only a minority of PWE who had attended an ED had a basic neurological examination and that advice was not typically given to patients or their carers on seizure management. These findings suggest that there has been little change in care since a survey of usual ED care for PWE was carried out in the late 1990s by Reuber et al. 43 The NASH also found that there was great variability between hospitals, and some consensus is emerging that ED attendance is a lost opportunity to identify and help PWE who have poor control and self-management (Association of British Neurologists Conference, 2012, personal communication).
The need to know
All people with long-term conditions may benefit from some self-help education, especially when first diagnosed; however, this does not always occur. In a previous qualitative study of PWE53 one person said:
They didn't give me hardly anything (information), just sort of said, please take these tablets.
Female, 56 years
Another summarised the lack of information and support:
I was left high and dry.
Male, 59 years
In epilepsy the need is particularly great when the person's knowledge of epilepsy is low54 or when he or she experiences a negative psychological response to the diagnostic label or subsequent life events. The reaction to loss, whether it be to a disease with negative consequences or any other loss, has been characterised as including a range of responses such as fear, denial, anger, bargaining and depression as well as acceptance. 55 Negative psychological reactions to the diagnostic label of epilepsy, such as denial, may considerably limit an individual's ability to take on new information needed to manage his or her condition. Initial and subsequent experiences of seizures, with unconsciousness and possible injury, may trigger fear. This was described by people following other conditions involving a sudden loss of consciousness, such as subarachnoid haemorrhage, who also fear recurrence. 56,57 In this condition fear and catastrophic thinking has been conceptualised as a post-traumatic stress syndrome. 58 PWE, particularly those from minority ethnic groups, experience more than an internal struggle. Cultural beliefs in evil spirits may be associated with epilepsy, and this may have negative consequences for PWE and their quality of, and opportunities in, life. 59,60 When ethnic groups represent multiple small diverse minorities as they do in the UK, their special needs may not be recognised, as has been described for other conditions. 61
Our research group54 found that, after diagnosis by a neurologist, the median score for PWE using Jarvie et al.'s62 epilepsy knowledge questionnaire was 43 out of a maximum of 55, with a wide range of 12–51. Not having general education qualifications [General Certificate of Secondary Education (GCSE)], normally undertaken at 16 years in the UK, was associated with a lower knowledge of epilepsy (one-third of the general population have no qualifications). Compared with those in the highest knowledge of epilepsy quartile, those in the lowest quartile had a median score that was 12 points lower on the knowledge of epilepsy scale (26 vs. 48). 54 It is arguable that, after diagnosis, a course of learning should be tailored to the educational level of the PWE, with duration inversely related to educational attainment.
In another study we found that people with long-term epilepsy (average 23 years) did not have a higher median knowledge score (42.5) than those with newly diagnosed epilepsy. 63 As with those with new epilepsy, educational attainment was predictive of epilepsy knowledge. Specifically, those with no GCSEs had lower epilepsy knowledge scores (median score 39) than those with GSCEs or a higher qualification (median score 43). Lower epilepsy knowledge scores were also found in older people (37 vs. 43 in younger people), in those who left school earlier rather than later (40 vs. 43) and in those not belonging to an epilepsy self-help group (42 vs. 45). Multiple regression analysis showed that these predictors had independent effects and so the additive consequences of social disadvantage for knowledge of epilepsy are considerable.
Some people are affected more by their epilepsy than others. Like people with so-called ‘brittle’ diabetes, these people can have difficulties controlling their epilepsy. They may benefit from step-up care and advice, which might be called rehabilitation. The specific characteristics of those whose condition impacts on them more, in terms of distress to themselves and their families, reduced social participation and possibly inappropriate service use, need to be identified. The study investigators responded to a NHS call for research on rehabilitation. The research was funded by the National Institute for Health Research (NIHR) and allocated to a self-help research group. We have used the terminology of self-help generally here, but the issue of specific needs that may require specific services will become clear in the results and will be taken up in the discussion.
What is self-management?
There is no universally agreed definition of self-management. The US Institute of Medicine proposed that self-management is ‘the tasks that individuals must undertake to live with one or more chronic conditions. These tasks include having the confidence to deal with medical management, role management and emotional management of their conditions’ (p. 57). 64
Self-management can be enhanced by ‘self-management programmes’. ‘Self-management programmes’, as defined by the Department of Health, ‘are not simply about educating or instructing patients about their condition. They are based on developing the confidence and motivation of patients to use their own skills and knowledge to take effective control over life with a chronic illness’. 65 Self-management therefore aims to enhance patients' self-efficacy by helping to solve identified problems, with benefits for patients' clinical outcomes and quality of life (QoL) and reduced hospital utilisation. 66
Epilepsy self-management can be conceptualised as a range of actions and skills that help PWE feel more confident about making decisions about their epilepsy, acting to improve seizure control, their use of medication and living with epilepsy. 49 Good self-management therefore involves PWE working in partnership with health-care professionals to decide the best treatment and care plan for their epilepsy and to assist them in developing confidence and problem-solving skills and strategies to manage the emotional and physical challenges of epilepsy. 49
What is rehabilitation?
A critical review has been undertaken of evidence from the UK to support the concept of developing a rehabilitation strategy. 6 A rehabilitation service is not simply physical therapy. Rather, it can comprise a multidisciplinary team of people who work together towards common goals for each patient, involve and educate the patient and his or her family, have relevant knowledge and skills and can resolve most of the common problems faced by their patients. Here the rehabilitation process aims to maximise the participation of the patient in his or her social setting and minimise distress experienced by the patient and distress and stress experienced by the patient's family and carers. 67 As epilepsy is common, it is important to identify those who will benefit more specifically from rehabilitation services.
How should needs be matched with services for people with epilepsy?
Is it self-care or rehabilitation?
Individuals have described their experiences of epilepsy movingly. 68–71 Once diagnosed with epilepsy, people vary in their ability to manage their condition and participate fully in daily life. The needs of people and the skills of care providers need matching,72 with the addition of an overarching strategy. 73 Health services can be conceptualised as promoting and providing for self-help and/or rehabilitation. This includes prevention of death and disability by the comprehensive and systematic care of established disease. This is sometimes more a vision than a reality; nevertheless, care models lie on a continuum, which attempts to provide a theory about how services might match people's needs.
What is the current evidence on epilepsy self-management?
Two Cochrane reviews9,10 found only three self-management studies targeting adults with epilepsy. 74–76 A fourth study77 was published later. Psychological interventions have also been reviewed. 11 None of the interventions was trialled in the UK. Helgeson et al. 75 reported the 2-day Sepulveda Epilepsy Education (SEE) programme for adults in the USA. The Modular Service Package Epilepsy (MOSES)74 was evaluated in Europe and offered over 2 days. Olley et al.'s76 psychoeducational therapy programme was run in Nigeria. Pramuka et al. 77 trialled six weekly sessions of a psychosocial self-management programme in the USA.
Differences in study methodology prevented a direct comparison of findings in a meta-analysis. 18 Of the four self-management approaches for adults, MOSES74 has been evaluated in the greatest number of participants, across 22 epilepsy centres (mainly specialist epilepsy hospital units). Its evaluation was the most robust, with benefits in terms of improved knowledge about epilepsy, better seizure control and coping, and greater tolerance of, and fewer reported, side effects of AEDs. MOSES was delivered on an inpatient basis to groups of PWE by a pair of educational facilitators drawn from a medical/nursing and psychosocial background. An inpatient hospital course might facilitate access for PWE who cannot predict when they will have seizures, but would be costly to provide. In the current economic climate it is likely that NHS interventions need to be developed on an outpatient basis. Courses or sessions might also be targeted only at those PWE who have the greatest needs, in terms of poor epilepsy control and high service use.
What is the current evidence of the impact of epilepsy nurse specialist-led advice on self-management for people with epilepsy in ambulatory care?
Bradley and Lindsay9 reviewed specialist education and advice for neurological conditions and identified three previous trials of the impact of epilepsy nurses, two undertaken by our own group. 54,63,78 These trials were undertaken in areas that were not deprived. As most people achieved good seizure control, the trials focused on outcomes such as satisfaction with information provided, psychological distress and knowledge of epilepsy. In the trial of an epilepsy nurse specialist (ENS)-led self-management intervention for people with chronic epilepsy, there was improved patient satisfaction with the information provided and reduced depression scores in the group who had experienced no recent seizure. 53,63,79 Again, it was PWE with lower educational levels who were found to have the least knowledge of epilepsy. 63 In the trial of patients with newly diagnosed epilepsy, those who were in the lowest knowledge quartile at baseline improved their knowledge of epilepsy following an ENS-led self-management intervention. 63
A small US study with ‘hard’ outcomes
There is evidence from a US study80 that a nurse-led intervention can help patients manage their epilepsy and reduce hospital admissions. Nurses led on helping patients who had been hospitalised for epilepsy to manage their condition, and this was associated with a reduction in seizure-related readmission at 90 days (0/23 patients in the intervention group and 3/19 in the control group). This was a small but interesting trial. Results from some case series also suggest that nurse interventions may reduce ED visits and admissions. 81,82
An epilepsy nurse specialist-led self-management intervention in an area of poor epilepsy control
From the evidence, the potential for demonstrating change in outcomes and cost-effectiveness from ENS-led rehabilitation might be greater in the context of high levels of deprivation, poor epilepsy control and less social participation. We planned to achieve this by carrying out a study with patients in three London boroughs, namely Lambeth, Southwark and Lewisham. These boroughs are in the top 10% of English authorities for deprivation. 83 The mean level of practice-reported seizures in 2007 was lower (50%) than the national average (60%) [Mark Ashworth, general practitioner (GP) and Clinical Senior Lecturer, Department of Primary Care and Public Health Sciences, King's College London, 22 December 2011, personal communication]. There are three hospitals in this area: one lies in the north of Lambeth and Southwark, one in the south and one in Lewisham. One hospital had two ENSs who previously only saw patients referred by neurologists and neurosurgeons. They co-ordinate a multidisciplinary team approach to managing problems experienced by patients. The other hospitals had no ENSs. A sample of patients with poor epilepsy control could be identified by recruiting ED attendees for epilepsy at each hospital. In a previous audit we found that one out of 60 attendances were for epilepsy, with 40% of patients being admitted. The plan was for the nurses to offer clinic appointments following discharge for those PWE attending the ED for epilepsy, and lead on providing advice and support. The other hospitals would continue to provide usual medical care.
Aims
The aims of the study were to provide:
-
a description of people attending the ED for epilepsy, their use of the ED and their psychological state, knowledge of epilepsy, perception of stigma, QoL and needs
-
an economic evaluation of people attending the ED for epilepsy to determine the cost both for PWE and for society
-
quantitative evidence from a comparison of two groups, one receiving treatment as usual (TAU) and the other receiving TAU and an ENS-led self-management intervention
-
qualitative evidence of PWE's experiences of emergency services, the way in which services meet/do not meet their needs and their explanations of the process of, and rationale for, attendance
-
qualitative evidence from the group receiving the ENS-led self-management intervention
-
an economic evaluation of the cost-effectiveness of services both for an ENS-led self-management intervention and comparison groups before and after the ENS-led intervention.
Justification for use of mixed methods, staging and reporting in three streams
We will describe the work in three major streams: the first used quantitative methods, the second used qualitative methods and the third used health economic methodology. We gave priority to identifying those PWE with poor control and evaluating step-up care. There is some evidence that use of emergency medical services may be a proxy for poor control. Currently, there is comparatively little evidence on the characteristics of PWE who attend EDs, and no evidence from the PWE themselves about what the process is. In the current climate of recession the Department of Health and hospital medical services have strong drivers to identify the reasons why six out of seven epilepsy admissions are unplanned, to reduce these admissions, and to provide evidence on what the costs of reactive and proactive services might be.
We therefore used mixed methods to describe not only the demographic, psychological and social characteristics of PWE who use EDs but also their views of why and how they use EDs. We planned to describe not only whether an ENS-led self-management intervention reduces the use of EDs but also whether PWE regard the intervention as useful or as not useful and why.
To avoid the interviews contaminating the responses to the questionnaires, the qualitative component was scheduled to be carried out after the return of the final questionnaires, 1 year after recruitment of the participants. This sequence has a methodological advantage in terms of the prevention of contamination. We realise that the results of neither of the two enquiries about reasons for calling the ED and participants' views of the intervention can inform the intervention retrospectively; however, the results can be used to inform future interventions, which might as a consequence be designed differently.
Chapter 2 Quantitative component
We recruited people with established epilepsy from the EDs of three inner London hospitals and conducted a non-randomised trial (ISRCTN06469947). Those recruited from two of the hospitals formed a TAU cohort, whereas those attending the ED of the remaining hospital were offered the outpatient ENS-led self-management intervention plus TAU. Participants in both cohorts were assessed on recruitment (baseline) and then at 6 and 12 months following recruitment.
This chapter is split into two sections. In the first section we present the results from the baseline assessments, which occurred before any differences had occurred in the care given to the two cohorts. Results from this assessment are used to describe the characteristics, needs and previous service use of PWE attending EDs for epilepsy.
In the second section we describe the effects of the ENS-led self-management intervention by comparing the psychosocial outcomes and subsequent ED use of the two cohorts, adjusting for differences between them at baseline.
STUDY 1: THE CHARACTERISTICS OF PEOPLE WHO ATTEND HOSPITALS FOR EPILEPSY
The information collected from the baseline assessments was used to answer four specific questions concerning the characteristics, needs and previous service use of PWE attending EDs for epilepsy:
-
What is the pattern of use of EDs by PWE?
-
What are the characteristics of this population?
-
What standard of outpatient epilepsy care have they been receiving?
-
What factors are most associated with frequent ED visits (cross-sectional analysis)?
Methods
Recruitment
From May 2009 to March 2011 we prospectively recruited PWE attending the EDs of three London hospitals because of their condition. The hospitals were King's College Hospital (KCH), St. Thomas' Hospital (STH) and University Hospital Lewisham (UHL). These are inner London facilities with comparable EDs. Each is consultant led and offers a 24-hour service with full resuscitation facilities. Together they serve 1 million residents in the surrounding London boroughs of Southwark, Lambeth and Lewisham. 84 The prevalence of epilepsy in adults in this population is 0.51%. 85
Each of the boroughs has high levels of social deprivation and ethnic diversity, similar rates of emergency epilepsy admissions and a level of epilepsy control that is worse than the national average. 85,86 Epilepsy control is defined here as the percentage of PWE prescribed AEDs in the local population who were seizure free in the previous 12 months as recorded by primary care doctors in the boroughs as part of England's 2009–10 QOF. 85 According to this measure, 68% of PWE in the three boroughs were seizure free during the period of recruitment, whereas the national average was 74%.
Inclusion criteria
People with epilepsy may visit a hospital ED for a variety of reasons. These include for acute seizures, status epilepticus and seizure-related accidents (e.g. head injuries, burns) and causes (e.g. rash from anticonvulsants). To identify PWE visiting the ED with a wide range of presentations, we convened an expert panel of two emergency medicine and two neurology consultants to identify those symptoms and diagnoses by which the three EDs classified attendances that they considered potentially indicative of an epilepsy-related attendance. During the recruitment phase a research worker (AN) and a neurology consultant (LR) reviewed for eligibility ED records of patients falling into these categories.
People with established epilepsy were invited to participate in our study as long as their attendance was caused by their epilepsy and they were aged ≥ 18 years, had had epilepsy diagnosed for ≥ 1 year, could independently complete questionnaires and had no life-threatening or serious comorbidity (e.g. psychosis). For the purposes of the subsequent trial phase of the project, we also excluded those who had seen an ENS in the previous year, those referred to neurology for an outpatient appointment by the ED and, to maximise the comparability of patients in the two cohorts, those visiting the ED who did not reside within the local boroughs of Southwark, Lambeth or Lewisham.
Invitation letters were sent to eligible PWE shortly following their discharge from the ED. The Joint South London and Maudsley and the Institute of Psychiatry NHS Research Ethics Committee approved the study (08/H0807/86). Written informed consent was obtained from all participants.
Assessment
On recruitment, during a face-to-face appointment with a researcher (AN), participants completed validated generic and epilepsy-specific self-report questionnaires. 62,87–92 These included measures of their epilepsy-related ED visits and care over the previous 12 months, epilepsy-related QoL, seizure frequency, medication management skills, psychological distress, felt stigma, confidence in managing epilepsy (i.e. mastery) and epilepsy knowledge. Table 1 details the specific measures used.
Information collected on participants' epilepsy was restricted to that which was recorded in their primary and secondary care medical records. Deprivation levels were estimated by linking their postcodes to the Index of Multiple Deprivation. 93 As noted by previous studies,43,103,104 information on the seizures that led to the participants' ED attendances was not consistently reported in their ED records and so we do not present this information here.
| Purpose | Assessments used ina | Measure | Information | Range/interpretation |
|---|---|---|---|---|
| Epilepsy characteristics | 1 | Medical records | Information collected on participants' epilepsy was restricted to that recorded in their medical records, which were coded using the ILAE's 1989 classification system94 | – |
| Social deprivation | 1 | Index of Multiple Deprivation93 | Deprivation was estimated by linking participants' home postcodes to the Index of Multiple Deprivation | – |
| ED and health service use | 1, 2, 3 | Client Services Receipt Inventory95 | Examines contact (proportion, appointments number, duration) with different services for epilepsy, including the ED, neurology, ENSs, other hospital outpatient services and primary care doctors and nurses, and medications prescribed in the previous 12 months for assessment 1 and the previous 6 months for assessments 2 and 3 | – |
| Seizure frequency | 1, 2, 3 | Frequency scale96,97 | How many attacks have you had in the last 12 months (assessment 1)/6 months (assessments 2 and 3)? | 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, ≥ 10 |
| Seizure severity | 1, 2 | Liverpool Seizure Severity Scale 2.087 | Patient rates ‘most severe seizure/s’ in the previous 4 weeks against 12 items concerning loss of consciousness, confusion, postictal sleepiness, time to recovery and injury. Linear transformation of sum of responses produces a total score | Range 0–100; higher = increasing severity |
| Psychological distress | 1, 2, 3 | Hospital Anxiety and Depression Scale98 | Patient rates experience of seven anxiety and seven depression symptoms in the previous week. Symptoms of anxiety or depression relating also to physical disorders, such as headaches, insomnia, anergia and fatigue, are excluded | Range 0–21 for each scale; higher score = more disturbance. For each scale, 8–10 = borderline, ≥ 11 = valid case88 |
| QoL | 1, 2, 3 | Quality of Life in Epilepsy Inventory-1092 | 10-item measure of QoL in the previous 4 weeks. Covers epilepsy effects (memory, physical and mental effects of AEDs), mental health (energy, depression) and role functioning (seizure worry, work, driving, social limits) | Range 10–50; higher = lower QoL |
| Felt stigma | 1, 2 | Stigma of Epilepsy Scale99 | Patients asked to what extent, because of their epilepsy, they feel that some people (1) are uncomfortable with them, (2) treat them as inferior, (3) would prefer to avoid them. Each item is responded to using Taylor et al.'s90 scale: 0 = ‘not at all’; 1 = ‘yes, maybe’; 2 = ‘yes, probably’; 3 = ‘yes, definitely’ | Range 0–9; higher = more stigma; 0 = no felt stigma, ≥ 1 = stigmatised |
| Medication management | 1, 2, 3 | Epilepsy Self-Management Scale – medication subscale89b | Examines the frequency, over the previous 12 (assessment 1)/6 (assessments 2 and 3) months, with which patient performed behaviours associated with optimum adherence. Covers intentional and non-intentional non-adherence. Items rated on a scale ranging from ‘never’ to ‘always’ | Range 10–50; higher = better management |
| Information satisfaction | 1, 2 | Satisfaction with Information about Medicines Scale100 | 17-item scale asks patients to rate the amount of, and satisfaction with, medication information received. Items 1–9 address action and usage of their AEDs and 10–17 concern the potential problems of their medications. Items rated on a scale ranging from 1 = ‘none needed’ to 5 = ‘too much’ | Range, post recording, 0–17; higher = more satisfied |
| Epilepsy knowledge | 1, 2 | Epilepsy Knowledge Profile – General62 | 55-item true/false questionnaire (34 medical knowledge items, 21 social knowledge items). Social knowledge scale contains items on first aid for epilepsy | Range: medical knowledge scale 0–35, social knowledge scale 0–21; higher = more knowledge |
| Mastery | 1, 2, 3 | Epilepsy Mastery Scale101 | Epilepsy-specific six-item adaptation of Pearlin and Schooner's102 internal vs. external locus of control measure. Patients rate extent to which they perceive their epilepsy as being under their control. Example of item: ‘Sometimes I feel helpless in dealing with my seizures’. Items rated on a scale ranging from 1 = ‘strongly agree’ to 4 = ‘strongly disagree’ | Range 6–24; higher = greater perceived mastery |
The standard of epilepsy care reported to have been received by each of the participants in the 12 months preceding recruitment was compared with the following three NICE criteria for good epilepsy care. 8 The first concerns access to specialist services: ‘[i]f seizures are not controlled and/or there is diagnostic uncertainty or treatment failure, individuals should be referred to tertiary services . . . for further assessment’ (p. 44). The second relates to medical review: ‘[a]ll individuals with epilepsy should have a regular structured review . . . this . . . should be carried out at least yearly by either a generalist or specialist, depending on how well the epilepsy is controlled’ (p. 44). The third concerns prescribed AEDs: ‘individuals should be treated with a single antiepileptic drug . . . wherever possible’ (p. 56). The Clinical Standards Advisory Group104 further recommends that ‘Monotherapy should be the rule . . . in at least 50% of those with established or severe epilepsy’ (p. 44).
Statistical analysis
Representativeness
To examine how representative our sample was of the population from which it was recruited, the characteristics of the sample were compared with those of the group with established epilepsy who attended the EDs for epilepsy during the recruitment period but who were not recruited. The recruited sample was also then compared solely with those people who were eligible but who declined participation.
Information on the non-recruited patients' characteristics was limited to that available in their ED records, as wider access to their medical records was not ethically permissible. We were able to compare the age, gender profile, deprivation status and ethnicity of participants in the groups, as well as the clinical urgency of the ED presentation as measured by the triage category that each patient was assigned to on arrival at the ED. 105 To permit an examination of the recruited and non-recruited groups' epilepsy and care generally, we also extracted information recorded by their primary care practices for the 2009–10 QOF. 106
As noted in the previous chapter, the QOF operates as a means of linking the income of primary care practices to care quality. For epilepsy, participating practices annually report (1) the percentage of PWE (aged ≥ 16 years) registered on AEDs who were seizure free in the last 12 months (indicator 8); (2) the percentage of PWE on AEDs who had a medication review in the last 15 months (indicator 7); and (3) the percentage of PWE on AEDs with a record of seizures in the last 15 months (indicator 6). There is variability between practices on these criteria. 41
Mann–Whitney, Kruskal–Wallis and chi-square tests were used to compare groups on the variables noted. Because information in ED records is often incomplete, each analysis was restricted to those without missing data. When missing data exceeded 5%, those with and without missing data were compared.
Pattern of emergency department use, attendees' characteristics and standard of epilepsy care
Descriptive statistics described the level of ED use, the standard of epilepsy care and the characteristics of the recruited patients. When data were not normally distributed, the median and interquartile range (IQR) were used to describe central tendency.
Association between level of previous emergency department use and baseline factors
Regression analyses were used to estimate relationships between the frequency of previous ED use reported at baseline and the other baseline variables. Coding of the variables is described in Table 2.
Unadjusted regression models were first run to determine the relationship between each of the baseline measures and the level of ED use in the previous 12 months. Variables significantly associated with ED use were then simultaneously entered into multiple regression analysis to identify parsimonious predictors.
Overdispersion and exclusion of zero values in baseline ED visit data meant that zero-truncated negative binomial regression (NBR) was the most appropriate regression technique. 35 Relative ED use is described in incidence rate ratios (IRRs), with corresponding 95% confidence intervals (CIs). The likelihood ratio test examined overdispersion and the Wald statistic provided the statistical significance of variables.
All p-values are two-sided and alpha set at 5%. Analyses were performed using Stata 12 (StataCorp LP, College Station, TX, USA), SPSS 17.0 (IBM, Armonk, NY, USA) and StatsDirect 2.7.8 (StatsDirect, Cheshire, UK).
Results
Participants
During the recruitment period, 943 people attended the EDs because of established epilepsy. Of these, 315 were eligible and 85 (27%) were recruited. We found no significant difference in the acceptance rates between ED sites.
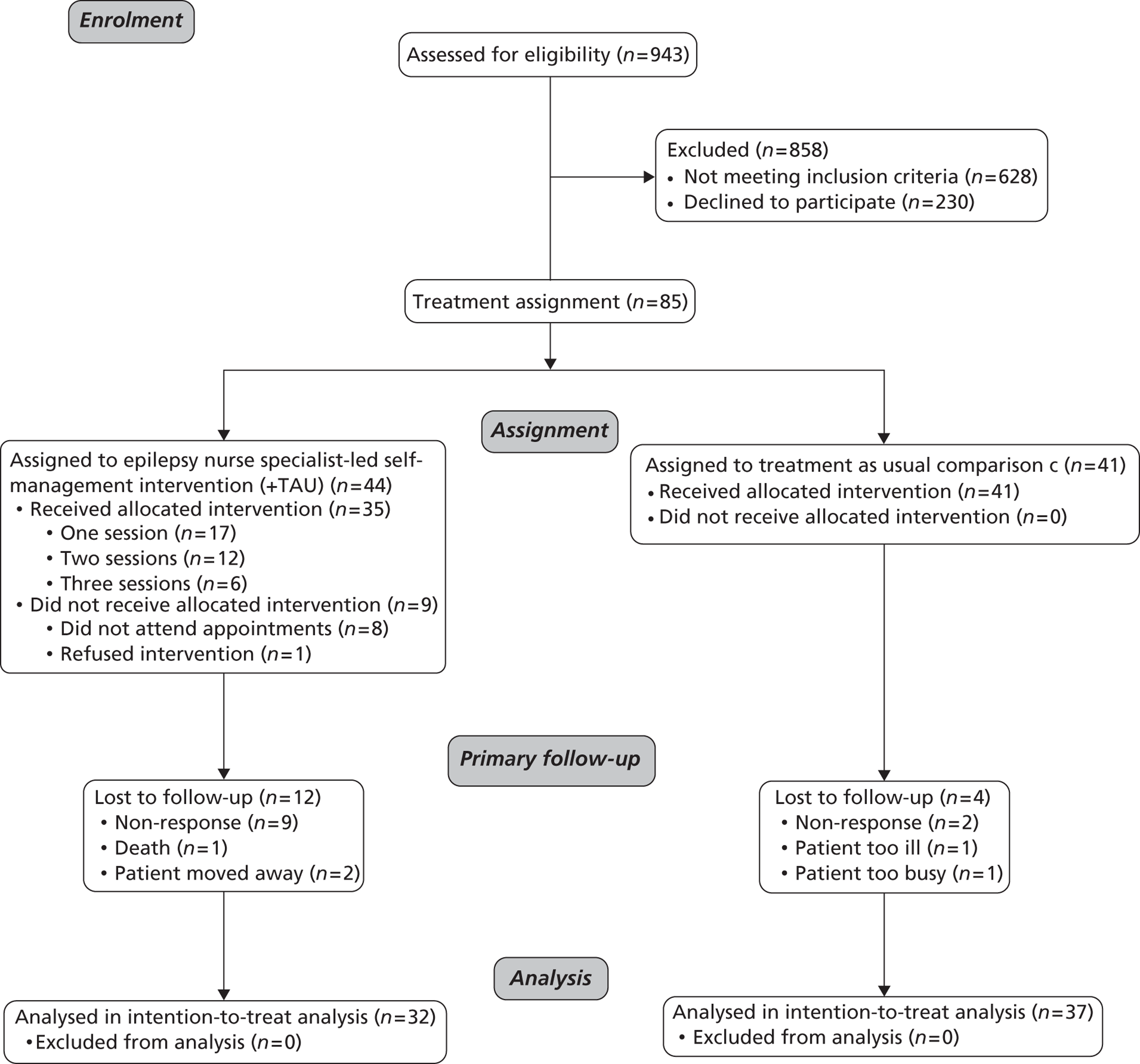
Reasons for exclusion were not living within one of the local boroughs served by the hospital (n = 352, 56.1%), unable to independently complete questionnaires (n = 115, 18.3%), having a serious comorbidity (n = 83, 13.2%), having consulted an ENS < 1 year previously (n = 43, 6.8%) or having been referred to neurology by the ED (n = 35, 5.6%) (Figure 1).
For those recruited, the median age at which epilepsy was diagnosed was 19 years (IQR 13.0–32.5 years) and the median time since diagnosis was 11 years (IQR 6–28 years). In total, 34% lived in areas with a deprivation score in the most deprived quintile for England. 84
The longstanding nature of the participants' diagnoses meant that their epilepsy tended to be described in their wider medical records according to the ILAE's older 1989 system of classification. 94 Specifically, 37 patients (43.5%) were recorded as having experienced both focal and generalised seizures, 37 (43.5%) only generalised seizures and six (7.1%) only partial seizures; for five patients (5.9%) no seizure type was recorded.
The recruited sample was representative of the population from which it was drawn (Table 3), with no significant differences being found between those who were recruited and those who were not recruited. However, when compared solely with those who were eligible but who declined participation, a significant difference was that more white than non-white ED attendees agreed to participate (p < 0.03).
FIGURE 1.
Flow diagram of participant recruitment, treatment allocation and retention.
| Factor | PWE attending the ED who consented to participate (n = 85) (group A) | PWE attending the ED who were not eligible to participate (n = 628) (group B) | PWE attending the ED who were eligible but who declined to participate (n = 230) (group C) | Difference (95% CI) group A vs. group B | Difference (95% CI) group A vs. group C |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 41.1 (16) | 40.9 (17) | 38.8 (16) | −0.27 (−4.11 to 3.57) | −2.36 (−6.37 to 1.65) |
| Males, n (%) | 45 (52.9) | 349 (55.6) | 140 (60.9) | −0.03 (−0.14 to 0.08) | −0.08 (−0.20 to 0.04) |
| Median triage priority (IQR)a (1 = see immediately, 5 = non-urgent) | 3 (3–3) | 3 (3–3.75) | 3 (3–3) | 0 (0 to 0) | 0 (0 to 0) |
| White ethnicity, n (%)b | 51 (60) | 270 (55.1) | 78 (45.6) | −0.05 (−0.16 to 0.07) | 0.14 (0.01 to 0.27)c |
| Median deprivation score (IQR)a (score closer to 1 = more deprivation) | 32.1 (24.3–37.7) | 29.5 (21.1–37.0) | 32.7 (28.7–38.3) | 2.3 (0.01 to 4.67) | −1.8 (−3.92 to 0.31) |
| Median score QOF indicator 8 (IQR)d (higher = more seizure free) | 70.4 (61.1–78.2) | 71.4 (62.2–78.8) | 67.9 (56.4–77.8) | −0.9 (−4.1 to 2.1) | 2.5 (−1.1 to 6) |
| Median score QOF indicator 7 (IQR)d (higher = better epilepsy care) | 96.2 (93.3–100) | 95.7 (93.5–100) | 96.8 (94.6–100) | 0 (0.0 to 0.7) | 0 (−1 to 0) |
| Median score QOF indicator 6 score (IQR)d (higher = better epilepsy care) | 97.2 (94.1–100) | 96.5 (93.3–100) | 97.1 (94.1–100) | 0 (0.0 to 1.1) | 0 (−0.3 to 0.4) |
Pattern of use of emergency departments by people with epilepsy
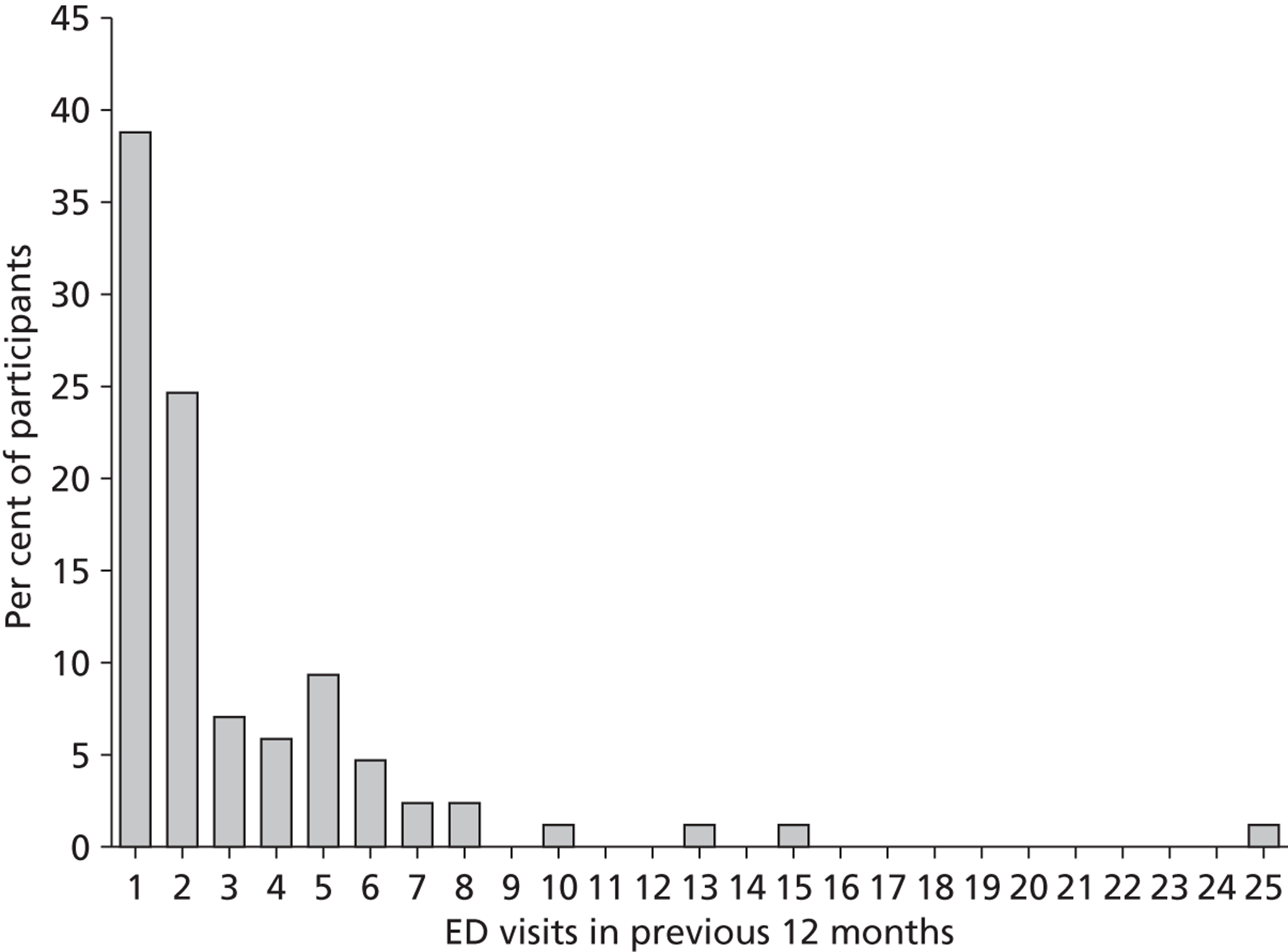
In the 12 months before the baseline assessment, the 85 PWE recruited from the EDs had together made 270 ED visits for epilepsy. Frequency of ED use amongst these PWE showed a strong positive skew (Figure 2). The median number of visits in the previous year was two (IQR 1–4, range 1–25), with 61% of participants reporting reattendance within 12 months. Specifically, 33 (39%) participants attended only once, 21 (25%) attended on two occasions and the remaining 31 (36%) attended on three or more occasions. This last group accounted for 72% of all ED visits. The median number of visits made by this subgroup was five (IQR 4–7).
Characteristics of people with epilepsy attending emergency departments
Seizures
All of the participants had experienced an epileptic seizure in the previous year. In total, 39 (46%) of the participants had experienced from two to nine seizures and 36 (42%) participants had experienced ≥ 10 seizures in the previous 12 months (Table 2). The median seizure severity score for the 54 (64%) participants who had a seizure in the 4 weeks preceding the baseline assessment was 57.5 (IQR 43.1–72.5).
Quality of life
Epilepsy-specific QoL, as represented by the mean total 10-item Quality of Life in Epilepsy (QOLIE-10) score, was 26.30 (SD 7.95). Higher scores on this measure indicate poorer QoL, and participants who reported having visited an ED the most in the previous 12 months reported the worst QoL. The mean QoL score was 28.30 (SD 8.7) for those PWE who had visited an ED on three or more occasions in the previous 12 months, 26.04 (SD 7.9) for those who had attended on two occasions and 24.57 (SD 6.9) for those who had visited once only.
Psychological distress
In total, 29 participants (34%) had a ‘case’ level of anxiety and/or depression, 28 (33%) had a ‘case’ level of anxiety and eight (9.4%) had a ‘case’ level of depression. Days since last seizure were not significantly associated with anxiety or depression score.
FIGURE 2.
Histogram of the number of participant-reported ED attendances in the previous 12 months. The distribution shows a positive skew (+3.40). In total, 39% of participants attended an ED once only.
Felt stigma
In total, 58 participants (68.2%) reported feeling stigmatised because of their epilepsy.
Epilepsy knowledge
Expressed as the mean per cent of correct answers, which is standard for this measure, the sample's scores on the different epilepsy knowledge scales were 70.7 (SD 10.8) for total score, 68.2 (SD 9.9) for social knowledge and 73.4 (SD 11.6) for medical knowledge. As an example, 24 (28.2%) of the participants stated incorrectly on the social knowledge scale that it was always necessary to call a doctor or ambulance if a person with epilepsy has a seizure, even if it occurred without complications.
| Factor | All participants (n = 85), n (%) | ED use, median (IQR) | Association with ED use, IRR (95% CI)a |
|---|---|---|---|
| Age (years), mean (SD) | 41.12 (16) | ||
| Youngest quartile (18–26 years) | 23 (27.1) | 2 (1–5) | 1.00 (reference) |
| Second quartile (27–42 years) | 21 (24.7) | 2 (1–4) | 1.29 (0.44 to 3.81) |
| Third quartile (43–51 years) | 20 (23.5) | 2 (2–5) | 1.07 (0.43 to 2.66) |
| Oldest quartile (52–89 years) | 21 (24.7) | 1 (1–2.5) | 0.42 (0.16 to 1.12) |
| Gender | |||
| Male | 45 (52.9) | 2 (1–4.5) | 1.00 (reference) |
| Female | 40 (47.10) | 2 (1–4) | 0.86 (0.39 to 1.89) |
| Ethnicity | |||
| White British | 51 (60) | 2 (1–4) | 1.00 (reference) |
| Other | 34 (40) | 2 (1–5) | 1.36 (0.59 to 3.10) |
| Social deprivation, median (IQR) | 32.07 (24.31–37.66) | ||
| Least deprived quartile (13.97–24.38) | 22 (25.9) | 1 (1–2.25) | 1.00 (reference) |
| Second quartile (24.39–32.07) | 21 (24.7) | 3 (1–6) | 2.37 (0.54 to 10.36) |
| Third quartile (32.08–37.62) | 21 (24.7) | 2 (1–4) | 1.12 (0.24 to 5.33) |
| Most deprived quartile (37.63–47.46) | 21 (24.7) | 2 (2–4.5) | 1.26 (0.28 to 5.69) |
| Epilepsy type | |||
| Focal | 49 (57.6) | 2 (1–3.5) | 1.00 (reference) |
| Generalised | 17 (20.0) | 2 (1–5) | 1.59 (0.69 to 3.70) |
| Undefined | 19 (22.4) | 2 (1–5) | 2.21 (0.89 to 5.49) |
| Seizure type | |||
| Partial and generalised | 37 (43.5) | 2 (1–4) | 1.00 (reference) |
| Generalised only | 37 (43.5) | 2 (1–4.5) | 0.64 (0.27 to 1.53) |
| Partial only | 6 (7.1) | 2 (1–3.75) | 0.54 (0.16 to 1.87) |
| Unknown | 5 (5.9) | 5 (1.5–7) | 2.16 (0.84 to 5.54) |
| Seizures in last year, median (IQR) | 6 (3–10) | – | 1.22 (1.08 to 1.37) |
| Medication management, median (IQR) | 36.0 (32.5–38.0) | ||
| Highest management quartile (39–40) | 24 (28.2) | 2 (1–3) | 1.00 (reference) |
| Second quartile (37–38) | 24 (28.2) | 1 (1–4.5) | 1.97 (0.49 to 7.97) |
| Third quartile (34–36) | 17 (20.0) | 2 (1–2.75) | 1.01 (0.41 to 2.49) |
| Lowest management quartile (21–33) | 20 (23.5) | 3 (1–6.75) | 2.58 (1.06 to 6.27) |
| Anxiety, median (IQR) | 8 (5.5–12) | ||
| Not anxious | 36 (42.4) | 2 (1–2) | 1.00 (reference) |
| Borderline | 21 (24.7) | 2 (1–5.5) | 2.29 (1.16 to 4.51) |
| Caseness | 28 (32.9) | 3 (1–5.75) | 3.67 (1.67 to 8.09) |
| Depression, median (IQR) | 5 (2–7) | ||
| Not depressed | 66 (77.6) | 2 (1–3) | 1.00 (reference) |
| Borderline | 11 (12.9) | 4 (1–6) | 2.45 (1.19 to 5.03) |
| Caseness | 8 (9.4) | 4 (2–13.25) | 5.07 (2.03 to 12.63) |
| Felt stigma, median (IQR) | 2 (0–3) | ||
| Least stigmatised quartile (0) | 27 (31.8) | 1 (1–2) | 1.00 (reference) |
| Second quartile (1–2) | 20 (23.5) | 2 (1–2.75) | 1.64 (0.67 to 4.02) |
| Third quartile (3) | 18 (21.2) | 2 (1–4) | 1.82 (0.84 to 3.93) |
| Most stigmatised quartile (4–8) | 20 (23.5) | 5 (2–7.75) | 5.88 (2.62 to 13.19) |
| Social knowledge, median (IQR) | 15 (13–16) | ||
| Most knowledgeable quartile (17–20) | 26 (30.6) | 1 (1–3) | 1.00 (reference) |
| Second quartile (16) | 37 (43.5) | 1 (1–2.5) | 1.41 (0.26 to 7.63) |
| Third quartile (14–15) | 13 (15.3) | 2 (1–3) | 1.07 (0.33 to 3.44) |
| Least knowledgeable quartile (8–13) | 9 (10.6) | 4 (1.75–6.25) | 3.55 (1.04 to 12.17) |
| Medical knowledge, median (IQR) | 26 (22–28) | ||
| Most knowledgeable quartile (29–32) | 26 (30.6) | 1 (1–2.5) | 1.00 (reference) |
| Second quartile (27–28) | 24 (28.2) | 2.5 (1–5) | 2.80 (0.98 to 8.03) |
| Third quartile (23–26) | 18 (21.2) | 2 (1–2.75) | 2.46 (0.59 to 10.12) |
| Least knowledgeable quartile (15–22) | 17 (20.0) | 2 (1–5) | 3.46 (1.21 to 9.88) |
Characteristics of emergency department attendees compared with those of the wider epilepsy population
Table 4 presents a comparison of the ED attendees and the wider epilepsy population on some of the key variables. In descending order of effect, this provides some evidence that ED attendees have experienced more seizures, perceive more epilepsy-related stigma, have recently experienced more anxiety and have a lower knowledge of epilepsy and its management.
Standard of outpatient epilepsy care that attendees had been receiving
Access to tertiary epilepsy services
Most participants (n = 68, 80%) considered their main epilepsy carer to be a hospital doctor rather than a primary care doctor. Forty-three (51%) were being seen in general neurology clinics, 23 (27%) in specifically named ‘epilepsy clinics’ and two (2%) by neuropsychiatry or neurosurgery services.
Frequency of medical review
Nearly all participants (n = 82, 96%) had received a medical review of their epilepsy in the previous 12 months, with 60 (71%) reporting attendance at a hospital clinic and 72 (85%) reporting attendance in primary care. The median number of outpatient appointments for epilepsy was four (IQR 2–9).
Number of antiepileptic drugs prescribed
In total, 44 participants (52%) were taking monotherapy and 38 (45%) were taking polytherapy (median 2, IQR 2–2.25); three were not taking AEDs at all.
Factors associated with frequency of emergency department visits
Because the dependent variable ED use was overdispersed (mean 3.17 < variance 12.89), we adopted a negative binomial model. With use of unadjusted regression analysis, we found that in descending order of importance increased felt stigma, increased depression, increased anxiety, lower social and medical epilepsy knowledge, reduced medication self-management skills and increased seizure frequency were each significantly associated with increased use of EDs by PWE (see Table 2).
| Factor | ED participants (n = 85) | Wider epilepsy population | Reference study details |
|---|---|---|---|
| Seizures in last year, n (%) | |||
| (n = 1630) | Moran et al.25 Postal survey of adults with active epilepsy identified through 80 primary care practices, geographically distributed across the UK | ||
| No seizures | 0 (0.0) | 843 (51.7) | |
| One seizure | 10 (11.8) | 129 (7.9) | |
| Two to nine seizures | 39 (45.9) | 280 (17.2) | |
| ≥ 10 seizures | 36 (42.4) | 378 (23.2) | |
| Epilepsy type, n (%) | |||
| (n = four studies) | Forsgren et al.107 Review of epidemiology of epilepsy type in European studies | ||
| Focal | 49 (57.6) | (33–65) | |
| Generalised | 17 (20.0) | (17–60) | |
| Undefined | 19 (22.4) | (2–8) | |
| Seizure type, n (%) | |||
| (n = four studies) | Forsgren et al.107 Review of epidemiology of epilepsy type in European studies | ||
| Partial and generalised | 37 (43.5) | (55–83) | |
| Generalised only | 37 (43.5) | (6–32) | |
| Partial only | 6 (7.1) | – | |
| Unknown | 5 (5.9) | (8–20) | |
| Anxiety, n (%) | |||
| (n = 1176) | Thapar.108 Survey of 82 adults (77 face-to-face, five postal) with active epilepsy from a epilepsy clinic in Glasgow, UK | ||
| Not anxious (0–7) | 36 (42.4) | 429 (36.5) | |
| Borderline (8–10) | 21 (24.7) | 487 (41.4) | |
| Caseness (≥ 11) | 28 (32.9) | 260 (22.1) | |
| Depression, n (%) | |||
| (n = 1185) | Thapar et al.109 Postal survey of adults with active epilepsy from a random selection of 82 primary care practices in Greater Manchester, UK | ||
| Not anxious (0–7) | 66 (77.6) | 878 (74.1) | |
| Borderline (8–10) | 11 (12.9) | 170 (14.3) | |
| Caseness (≥ 11) | 8 (9.4) | 137 (11.6) | |
| Felt stigma, n (%) | |||
| (n = 1571) | Taylor et al.90 Postal survey of adults with newly diagnosed epilepsy from UK hospital outpatient clinics recruited for SANAD trial comparing standard and new AEDs | ||
| None (0) | 27 (31.8) | 729 (46.4) | |
| Mild to moderate (1–6) | 50 (58.8) | 746 (47.5) | |
| High (7–9) | 8 (9.4) | 96 (6.1) | |
| Epilepsy knowledge, mean % correct | |||
| (n = five studies) | No single population reference is available. However, Elliot and Shneker110 identified and reviewed five previous European studies using the measure in the wider epilepsy population and reported the arithmetic mean scores. Three studies had recruited from hospital clinics, two from primary care and one from user support groups | ||
| Social knowledge scale | 68.2 | 71.8 | |
| Medical knowledge scale | 73.4 | 75.0 | |
| Total knowledge score | 70.7 | 74.3 | |
Multiple regression was performed for ED visits using those baseline variables that proved significant in the unadjusted analyses. The likelihood ratio test for alpha confirmed that the data were significantly overdispersed [χ2(1) = 21.68, p < 0.001]. The model predicting ED visits using the reduced list of variables remained statistically significant [χ2(9) = 81.03, p < 0.001]. Having a level of social epilepsy knowledge in the lowest quartile (p < 0.005), a sense of stigma in the highest quartile (p < 0.005), increased seizure frequency (p < 0.005) and less than optimal medication self-management (lowest quartile) (p < 0.05) remained significant in the adjusted model and predicted more frequent ED use.
Based on their respective IRRs, it was lack of social epilepsy-related knowledge (2.10, 95% CI 1.31 to 3.35) and greater perceived stigma (2.08, 95% CI 1.32 to 3.25) that were found to be most highly associated with ED use. On average, those with a social knowledge score in the lowest quartile had visited an ED in the previous year on two occasions more than those with more knowledge. Those with a felt stigma score in the highest quartile had visited an ED on three occasions more than those with lower felt stigma scores. Holding other variables constant, compared with those with better self-reported medication management, ED use was increased by 65% in those in the lowest quartile (IRR 1.65, 95% CI 1.02 to 2.67), and ED use increased by 11% for each category on the ordinal seizure frequency scale compared with the one below (IRR 1.11, 95% CI 1.04 to 1.19).
Summary
In total, 85 patients were recruited. The mean age of participants was 41 years and 53% were male. A total of 61% of participants reported reattending an ED within 12 months. PWE reported a mean of 3.2 and a median of two ED attendances in the last year. The rate of ED reattendance by PWE exceeds that of ED users in general and those with most chronic conditions. However, ED use was not homogeneous amongst participants, with some attending more frequently.
Compared with the wider epilepsy population and in descending order of effect, our results indicate that PWE attending the ED have experienced more seizures, perceive more epilepsy-related stigma, have recently experienced more anxiety and have lower knowledge of epilepsy and its management.
In the previous 12 months, the epilepsy outpatient care of most patients was consistent with standard criteria for quality.
Our cross-sectional analysis showed that, in descending order, lower epilepsy knowledge, higher perceived stigma, poorer self-medication management and higher seizure frequency were associated with the patient having made more ED visits.
STUDY 2: A COMPARISON OF THE GROUPS OF PEOPLE RECEIVING USUAL CARE AND AN EPILEPSY NURSE SPECIALIST-LED SELF-MANAGEMENT INTERVENTION, AND A COHORT STUDY OF PREDICTORS OF SERVICE USE
In this section we test the hypothesis that, compared with TAU alone, an ENS-led self-management intervention can reduce reattendance at the ED and improve well-being (ISRCTN06469947).
In the previous section we described how PWE were recruited from three similar inner London hospital EDs and completed self-report questionnaires on their service use and psychosocial well-being. To evaluate the effect of the ENS-led self-management intervention on well-being and subsequent ED use, participants who had attended the ED of one of the hospitals, KCH, were offered the intervention plus TAU whereas those attending the EDs of the two remaining hospitals, STH and UHL, were offered TAU alone. Participants in both groups were then reassessed 6 and 12 months later and the responses of the groups and their care were compared to determine the effect of the intervention. The similarity of the EDs made comparison of the patients' outcomes reasonable.
Methods
Treatment arms
The epilepsy nurse specialist-led self-management intervention (plus treatment as usual) group
Those from KCH were offered two one-to-one intervention sessions delivered on an outpatient basis at the hospital. The initial session was scheduled to last for 45–60 minutes and the second session for 30 minutes. The intervention was planned to be responsive to a patient's individual needs and so the number of sessions completed was permitted to vary slightly.
The intervention was informed by the premise that PWE, as opposed to medical care providers, are responsible for their day-to-day epilepsy management. As such, PWE need the knowledge, support and skills to mitigate disability and improve outcome. 111 Sessions were delivered by either one of the two ENSs based at KCH. Carers accompanied patients when PWE requested this.
To guide the intervention's delivery and record the information given and actions taken by the ENS during sessions, a comprehensive checklist was developed (see Appendix 2). The intervention started with the ENS reviewing the patient's epilepsy and checking that the AED(s) and dose that the patient reported taking was aligned with his or her prescription. The ENS identified any self-management problems that the patient was having, and factors relevant to their resolution. The ENS developed personalised care plans with the patient, helped the patient set goals (e.g. to socialise more, be comfortable talking about epilepsy, be less fearful about seizures), evaluated progress, provided the patient with the opportunity to ask questions and provided information.
Information provision formed a large component of the intervention. The information that could be provided included the causes of epilepsy; seizure first aid; the role and mechanism of action of AEDs; the importance of adherence to their medication and of taking the same brand; prescription charges; what to do if a dose is missed; potential seizure triggers; safety in the home; legal rights of, and benefits available for, PWE; and the contact details of support organisations. The ENS also informed patients about the name of their seizures and syndrome and, having reviewed their existing medical records, the probable cause of their epilepsy.
With regards to advice concerning seizure first aid, the ENS informed the patient what should and should not be done when a seizure occurs and, as a permanent record, provided the patient with an information pamphlet developed by the Epilepsy Society on first aid management of seizures. 112 As per these guidelines, participants were informed that, usually, when a person has an epileptic seizure, there is no need to call an ambulance. Emergency medical care is recommended only when any of the following apply: (1) it is the person's first seizure; (2) the person has injured him- or herself badly; (3) the person has trouble breathing after the seizure has stopped; (4) one seizure immediately follows another with no recovery in between; (5) the seizure lasts for 2 minutes longer than is usual or the seizure lasts for > 5 minutes and you do not know how long the person's seizures usually last for.
The ENS could make referrals through normal pathways to other services, tailored to the patient's requirements (e.g. counselling, social services, emergency rescue medication clinic). Any advice given and actions taken were communicated to the patient's primary care doctor. At appointments, participants had direct access to either of two ‘expert patients’ in the waiting room who were trained by the UK's Epilepsy Society, and were invited to join a service users group.
Before the trial, for reasons of limited service capacity, the ENSs accepted only direct referrals from neurologists and neurosurgeons. They ran clinics but, as for this study, did not independently prescribe AEDs. At the time of the intervention, one nurse had 8 years' experience working as an ENS and the other had 10 years' experience.
Treatment-as-usual comparison group
Following recruitment, no restrictions were placed on the services that TAU participants could access. At the time of the study no ENS services were part of TAU at STH or UHL.
Baseline and outcome assessments
Following their baseline assessment (assessment 1), the results of which were described in the previous section, participants were assessed again at 6 months (assessment 2) and 12 months (assessment 3) post recruitment. As at baseline, measures included those of their epilepsy-related ED visits and care, health-related QoL, seizure frequency, medication management skills, psychological distress, felt stigma, confidence in managing epilepsy (i.e. mastery) and epilepsy knowledge. Table 1 details the measures used at each assessment.
As for assessment 1, the questionnaires at assessment 2 were completed during a face-to-face follow-up appointment with AN, who was not blind to treatment allocation. However, assessment 3 was completed by post. Questionnaires on care received, service use and seizure frequency referred to the previous 12 months at assessment 1 and the previous 6 months at assessments 2 and 3.
Sample size
Jacoby et al. 32 found that 27% of PWE in the UK with uncontrolled epilepsy (more than one seizure a month) make at least one ED visit per year. We considered that the intervention might reduce this to 7% (rate ratio 0.26), partly by more effective self-management and partly by more frequent and appropriate use of non-emergency services. Following Parmar and Machin's113 formulae, full data on 60 participants in each treatment group would give 80% power to detect such a difference. We planned to recruit 160 subjects to allow for 25% loss to follow-up.
Statistical analysis
Treatment group equivalence and care received
Descriptive statistics describe the characteristics of those recruited into each of the treatment arms, those retained at each follow-up assessment and the epilepsy care that patients received following recruitment. Logistic regression tested for the significance of differences between the groups. Odds ratios (ORs) and 95% CIs are presented.
Effect of intervention on emergency department use
Analyses were performed using an intention-to-treat approach. The primary outcome was the number of ED visits that participants reported making over the previous 6 months at assessment 3. Secondary measures were ED visits reported to have been made over the 6 months preceding assessment 2 and psychosocial outcomes at assessments 2 and 3. A p-value of < 0.05 was considered significant for outcome analyses, with no adjustments made for multiple comparisons.
To examine the effect of the ENS-led self-management intervention on ED visits compared with TAU alone, NBR was used to determine whether treatment allocation predicted ED visits made over follow-up. Overdispersion of ED visits meant that NBR, with robust standard errors, was appropriate. 35
Unadjusted NBR analyses were first completed. However, to account for imbalances in baseline characteristics between the treatment groups, which may have confounded the estimated association between condition and subsequent ED use, we also completed adjusted NBR analyses. This involved first undertaking a process of model building, in which we examined the association between scores on each baseline measure and ED visits at assessments 2 and 3. Those covariates with a marginal statistical association (p < 0.10) were then included in the applicable adjusted NBR analyses. The adjustments made for each model are indicated in the table notes.
Estimates of treatment effect are presented in the form of IRRs with 95% CIs. IRRs < 1 represent a lower ED visit rate in the intervention group relative to TAU, whereas IRRs > 1 indicate a higher ED visit rate in the intervention group relative to TAU.
Effect of intervention on secondary outcome measures
For secondary outcomes, scores were treated as continuous, and linear regression, with robust standard errors, tested for treatment effects. Results from unadjusted and adjusted analyses are presented, with treatment effect estimates given in the form of unstandardised coefficients. Positive coefficients indicate an increase in the score on the outcome variable associated with receiving the ENS-led self-management intervention, whereas negative coefficients indicate the opposite.
All analyses were performed using Stata 11.
Results
Participants
Recruitment and baseline condition equivalence
Of the 85 recruited PWE, 44 were recruited from KCH and formed the intervention group and 41 came from STH and UHL and formed the comparison group. Figure 1 depicts their recruitment and retention.
At assessment 1 (baseline), participants in the two groups were broadly comparable (Table 5). The comparison group did, however, report having experienced significantly more seizures in the previous year [median seizure number 10 (IQR 1.2–4.5) vs. 5.5 (IQR 1.0–3.0) in the intervention group]. The groups also differed on the related seizure frequency indicator of the 2009–10 QOF measure. 85
Retention at follow-up
In total, 69 participants (81%) were retained at assessments 2 and 3. Loss to follow-up was not equal between the treatment arms and those lost differed from those retained in terms of baseline characteristics. A total of 37 participants (90%) from the comparison group were retained compared with 32 intervention participants (73%). This further imbalanced the baseline characteristics of the two groups (Table 6). As well as the intervention group still having had fewer seizures, it also now had fewer participants who felt highly stigmatised by epilepsy at baseline. At the same time, however, there were more in the intervention group who had a comorbid condition.
Reasons for loss are presented in Figure 1. Of note, one participant died of sudden unexplained death in epilepsy. This patient was allocated to the intervention study arm but failed to attend any appointments with the ENS.
Epilepsy care received by participants following recruitment
Assessment 2
No significant differences existed between the two groups in the proportion of participants who reported having consulted with a neurologist or a primary doctor, or who had accessed other hospital outpatient services for epilepsy over the 6 months preceding assessment 2 (all p > 0.05). However, significantly more participants in the intervention group (n = 27, 84%; median number of appointments 1, IQR 1–2) than in the comparison group (n = 2, 5%; OR 94.5, 95% CI 16.80 to 531.72) had seen an ENS in this time. The median appointment duration was 45 minutes (IQR 30–60 minutes).
Significantly more participants in the comparison group (n = 19, 51%; median number of appointments 1, IQR 1–2) than in the intervention group (n = 7, 22%; OR 0.27, 95% CI 0.10 to 0.77) had had an appointment with a nurse within their primary care medical practice. Participants frequently cited the reason for these appointments as being for AED level testing. The median duration of these appointments was 10 minutes (IQR 5–15 minutes).
| Baseline measure | Treatment groups at baseline, n (%) | OR (95% CI) | |
|---|---|---|---|
| Comparison (n = 41) | Intervention (n = 44) | ||
| Age at baseline (years) | |||
| 18–24 | 6 (14.6) | 8 (18.2) | 1.00 (reference) |
| 25–34 | 8 (19.5) | 12 (27.3) | 1.55 (0.56 to 4.31) |
| 35–45 | 7 (17.1) | 7 (15.9) | 0.92 (0.29 to 2.91) |
| 46–53 | 12 (29.3) | 8 (18.2) | 0.54 (0.19 to 1.50) |
| 54–89 | 8 (19.5) | 9 (20.5) | 1.06 (0.36 to 3.09) |
| Gender | |||
| Male | 22 (53.7) | 24 (54.5) | 1.00 (reference) |
| Female | 19 (46.3) | 20 (45.5) | 0.97 (0.41 to 2.28) |
| Ethnicity | |||
| Other | 17 (41.5) | 17 (38.6) | 1.00 (reference) |
| White British | 24 (58.5) | 27 (61.4) | 0.89 (0.37 to 2.13) |
| Years of formal education | |||
| 10 (least educated) | 2 (4.9) | 1 (2.3) | 1.00 (reference) |
| 11 | 24 (58.5) | 19 (43.2) | 0.54 (0.23 to 1.28) |
| 12 | 2 (4.9) | 2 (4.5) | 0.93 (0.12 to 7.00) |
| 13–15.5 | 6 (14.6) | 10 (22.7) | 1.72 (0.56 to 5.28) |
| 16–24 (most educated) | 7 (17.1) | 12 (27.3) | 1.82 (0.63 to 5.24) |
| Deprivation score | |||
| 13.97–22.70 (least deprived) | 5 (12.2) | 12 (27.3) | 1.00 (reference) |
| 23.36–28.98 | 9 (22.0) | 8 (18.2) | 0.79 (0.27 to 2.31) |
| 29.75–33.46 | 7 (17.1) | 10 (22.7) | 1.43 (0.48 to 4.22) |
| 33.56–38.31 | 11 (26.8) | 7 (15.9) | 0.52 (0.18 to 1.50) |
| 38.76–47.46 (most deprived) | 9 (22.0) | 7 (15.9) | 0.67 (0.22 to 2.02) |
| Comorbidity | |||
| None | 23 (56.1) | 20 (45.5) | 1.00 (reference) |
| Psychiatric and/or medical | 18 (43.9) | 24 (54.5) | 1.53 (0.65 to 3.63) |
| Years epilepsy diagnosed | |||
| 2–4 | 5 (12.2) | 10 (22.7) | 1.00 (reference) |
| 5–8 | 9 (22.0) | 7 (15.9) | 0.67 (0.22 to 2.02) |
| 9–15 | 7 (17.1) | 13 (29.5) | 2.04 (0.72 to 5.80) |
| 16–34 | 9 (22.0) | 8 (18.2) | 0.79 (0.27 to 2.31) |
| 35–67 | 11 (26.8) | 6 (13.6) | 0.43 (0.14 to 1.31) |
| ED visits last 12 months | |||
| 1 | 15 (36.6) | 18 (40.9) | 1.00 (reference) |
| 2 | 12 (29.3) | 10 (22.7) | 0.71 (0.27 to 1.90) |
| 3–4 | 3 (7.3) | 8 (18.2) | 2.82 (0.69 to 11.55) |
| 5–25 | 11 (26.8) | 8 (18.2) | 0.61 (0.22 to 1.71) |
| Seizures last 12 months | |||
| 1–2 | 7 (17.1) | 10 (22.7) | 1.00 (reference) |
| 3–5 | 6 (14.6) | 12 (27.3) | 2.19 (0.73 to 6.56) |
| 6–9 | 6 (14.6) | 8 (18.2) | 1.30 (0.41 to 4.15) |
| ≥ 10 | 22 (53.7) | 14 (31.8) | 0.40 (0.17 to 0.98) |
| Primary care QOF 8 score | |||
| 0.00–56.4 (fewer seizure free) | 6 (14.6) | 11 (25.0) | 1.00 (reference) |
| 58.3–65.4 | 10 (24.4) | 7 (15.9) | 0.59 (0.20 to 1.73) |
| 65.5–73.6 | 10 (24.4) | 6 (13.6) | 0.49 (0.16 to 1.51) |
| 75.9–78.9 | 10 (24.4) | 8 (18.2) | 0.69 (0.24 to 1.97) |
| 80.0–91.7 (more seizure free) | 5 (12.2) | 12 (27.3) | 2.70 (0.85 to 8.56) |
| Seizure severity score | |||
| 0–5 (least severe) | 13 (32.5) | 20 (45.5) | 1.00 (reference) |
| 7.5–50 | 10 (25) | 6 (13.6) | 0.47 (0.15 to 1.46) |
| 52.5–67.5 | 9 (22.5) | 8 (18.2) | 0.77 (0.26 to 2.24) |
| 70–90 (most severe) | 8 (20) | 10 (22.7) | 1.18 (0.41 to 3.38) |
| Seizure onset | |||
| Generalised or unknown | 19 (46.3) | 17 (38.6) | 1.00 (reference) |
| Focal | 22 (53.7) | 27 (61.4) | 1.37 (0.58 to 3.27) |
| AEDS prescribed | |||
| 0 | 1 (2.4) | 2 (4.5) | 1.00 (reference) |
| 1 | 18 (43.9) | 26 (59.1) | 1.85 (0.78 to 4.39) |
| 2 | 16 (39.0) | 13 (29.5) | 0.66 (0.27 to 1.62) |
| 3–5 | 6 (14.6) | 3 (6.8) | 0.43 (0.10 to 1.85) |
| Depression score | |||
| 0–1 (least symptoms) | 11 (26.8) | 2 (4.5) | 1.00 (reference) |
| 2–3 | 11 (26.8) | 26 (59.1) | 0.70 (0.26 to 1.93) |
| 4–5 | 4 (9.8) | 0 (0.0) | 2.72 (0.77 to 9.56) |
| 6–7 | 7 (17.1) | 13 (29.5) | 1.43 (0.48 to 4.22) |
| 8–19 (most symptoms) | 8 (19.5) | 3 (16.8) | 1.38 (0.49 to 3.88) |
| Anxiety score | |||
| 0–4 (least symptoms) | 7 (17.1) | 7 (15.9) | 1.00 (reference) |
| 5–7 | 10 (24.4) | 12 (27.3) | 1.16 (0.44 to 3.10) |
| 8–9 | 8 (19.5) | 6 (13.6) | 0.65 (0.20 to 2.09) |
| 10–12 | 9 (22.0) | 10 (22.7) | 1.05 (0.37 to 2.92) |
| 13–19 (most symptoms) | 7 (17.1) | 9 (20.5) | 1.25 (0.42 to 3.76) |
| QoL score | |||
| 13–18 (highest QoL) | 9 (22.0) | 7 (15.9) | 1.00 (reference) |
| 19–23 | 7 (17.1) | 11 (25.0) | 1.62 (0.56 to 4.71) |
| 24–26 | 6 (14.6) | 8 (18.2) | 1.30 (0.41 to 4.14) |
| 27–33 | 11 (26.8) | 8 (18.2) | 0.61 (0.22 to 1.71) |
| 34–36 (lowest QoL) | 8 (19.5) | 10 (22.7) | 1.21 (0.42 to 3.47) |
| Felt stigma score | |||
| 0 (least stigma) | 12 (29.3) | 15 (34.1) | 1.00 (reference) |
| 1–2 | 9 (22.0) | 10 (22.7) | 1.05 (0.37 to 2.92) |
| 3–4 | 8 (19.5) | 13 (29.5) | 1.73 (0.63 to 4.77) |
| 5–9 (most stigma) | 12 (29.3) | 6 (13.6) | 0.38 (0.13 to 1.15) |
| Medication management | |||
| 13–39 (lowest skills) | 5 (12.5) | 11 (25.0) | 1.00 (reference) |
| 40–44 | 6 (15.0) | 10 (22.7) | 1.90 (0.65 to 5.54) |
| 45–46 | 9 (22.5) | 10 (22.7) | 1.18 (0.44 to 3.16) |
| 47–48 | 8 (20.0) | 7 (15.9) | 0.93 (0.32 to 2.72) |
| 49–50 (highest skills) | 12 (30.0) | 6 (13.6) | 0.48 (0.16 to 1.41) |
| Satisfaction with medication information | |||
| 1–4 (least satisfied) | 7 (17.5) | 7 (16.3) | 1.00 (reference) |
| 5–7 | 8 (20.0) | 10 (23.3) | 1.21 (0.42 to 3.48) |
| 8–9 | 6 (15.0) | 9 (20.9) | 1.50 (0.48 to 4.71) |
| 10–11 | 8 (20.0) | 10 (23.3) | 1.21 (0.42 to 3.48) |
| 12–17 (most satisfied) | 11 (27.5) | 7 (16.3) | 0.51 (0.18 to 1.50) |
| Social knowledge | |||
| 8–12 (lowest knowledge) | 10 (24.4) | 5 (11.4) | 1.00 (reference) |
| 13–14 | 13 (31.7) | 13 (29.5) | 0.90 (0.36 to 2.29) |
| 15 | 9 (22.0) | 13 (29.5) | 1.49 (0.56 to 4.01) |
| 16–20 (highest knowledge) | 9 (22.0) | 13 (29.5) | 1.49 (0.56 to 4.01) |
| Medical knowledge | |||
| 15–21 (lowest knowledge) | 11 (26.8) | 7 (15.9) | 1.00 (reference) |
| 22–24 | 9 (22.0) | 8 (18.2) | 0.79 (0.27 to 2.31) |
| 25–26 | 7 (17.1) | 8 (18.2) | 1.08 (0.35 to 3.32) |
| 27–28 | 7 (17.1) | 11 (25.0) | 1.62 (0.56 to 4.71) |
| 29–32 (highest knowledge) | 7 (17.1) | 10 (22.7) | 1.43 (0.48 to 4.22) |
| Mastery | |||
| 6–12 (lowest confidence) | 10 (24.4) | 8 (18.2) | 1.00 (reference) |
| 13–14 | 8 (19.5) | 11 (25.0) | 1.38 (0.49 to 3.88) |
| 15 | 5 (12.2) | 8 (18.2) | 1.60 (0.47 to 5.40) |
| 16–17 | 8 (19.5) | 10 (22.7) | 1.21 (0.42 to 3.47) |
| 18–21 (highest confidence) | 10 (24.4) | 7 (15.9) | 0.59 (0.20 to 1.73) |
| Baseline measure | Treatments groups at assessment 2, n (%) | OR (95% CI) | Treatments groups at assessment 3, n (%) | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| Comparison (n = 37) | Intervention (n = 32) | Comparison (n = 37) | Intervention (n = 32) | |||
| Age at baseline (years) | ||||||
| 18–24 | 5 (13.5) | 5 (15.6) | 1.00 (reference) | 5 (13.5) | 2 (6.3) | 1.00 (reference) |
| 25–34 | 8 (21.6) | 8 (25.0) | 1.21 (0.39 to 3.73) | 7 (18.9) | 9 (28.1) | 1.68 (0.54 to 5.22) |
| 35–45 | 7 (18.9) | 5 (15.6) | 0.79 (0.22 to 2.82) | 7 (18.9) | 6 (18.8) | 0.99 (0.29 to 3.35) |
| 46–53 | 9 (24.3) | 6 (18.8) | 0.72 (0.22 to 2.32) | 12 (32.4) | 6 (18.8) | 0.48 (0.16 to 1.49) |
| 54–89 | 8 (21.6) | 8 (25.0) | 1.21 (0.39 to 3.73) | 6 (16.2) | 9 (28.1) | 2.02 (0.63 to 6.54) |
| Gender | ||||||
| Male | 20 (54.1) | 14 (43.8) | 1.00 (reference) | 20 (54.1) | 13 (40.6) | 1.00 (reference) |
| Female | 17 (45.9) | 18 (56.3) | 1.51 (0.58 to 3.95) | 17 (45.9) | 19 (59.4) | 1.72 (0.66 to 4.51) |
| Ethnicity | ||||||
| Other | 17 (45.9) | 10 (31.3) | 1.00 (reference) | 23 (62.2) | 21 (65.6) | 1.00 (reference) |
| White British | 20 (54.1) | 22 (68.8) | 0.54 (0.20 to 1.45) | 14 (37.8) | 11 (34.4) | 0.86 (0.32 to 2.33) |
| Years of formal education | ||||||
| 10 (least educated) | 1 (2.7) | 1 (3.1) | 1.00 (reference) | 2 (5.4) | 1 (3.1) | 1.00 (reference) |
| 11 | 21 (56.8) | 13 (40.6) | 0.52 (0.20 to 1.37) | 21 (56.8) | 12 (37.5) | 0.46 (0.17 to 1.21) |
| 12 | 2 (5.4) | 2 (6.3) | 1.17 (0.15 to 8.92) | 1 (2.7) | 2 (6.3) | 2.40 (0.20 to 28.28) |
| 13–15.5 | 6 (16.2) | 6 (18.8) | 1.19 (0.34 to 4.18) | 6 (16.2) | 7 (21.9) | 1.45 (0.43 to 4.90) |
| 16–24 (most educated) | 7 (18.9) | 10 (31.3) | 1.95 (0.64 to 5.97) | 7 (18.9) | 10 (31.3) | 1.95 (0.64 to 5.97) |
| Deprivation score | ||||||
| 13.97–22.70 (least deprived) | 5 (13.5) | 11 (34.4) | 1.00 (reference) | 4 (10.8) | 10 (31.3) | 1.00 (reference) |
| 23.36–28.98 | 7 (18.9) | 3 (9.4) | 0.44 (0.10 to 1.90) | 8 (21.6) | 3 (9.4) | 0.38 (0.09 to 1.57) |
| 29.75–33.46 | 7 (18.9) | 5 (15.6) | 0.79 (0.22 to 2.82) | 6 (16.2) | 7 (21.9) | 1.45 (0.43 to 4.90) |
| 33.56–38.31 | 11 (297) | 7 (21.9) | 0.66 (0.22 to 1.99) | 11 (29.7) | 6 (18.8) | 0.55 (0.17 to 1.71) |
| 38.76–47.46 (most deprived) | 7 (18.9) | 6 (18.8) | 0.99 (0.29 to 3.35) | 8 (21.6) | 6 (18.8) | 0.84 (0.25 to 2.76) |
| Comorbidity | ||||||
| None | 20 (54.1) | 12 (37.5) | 1.00 (reference) | 22 (59.5) | 11 (34.4) | 1.00 (reference) |
| Psychiatric and/or medical | 17 (45.9) | 20 (62.5) | 1.96 (0.74 to 5.18) | 15 (40.5) | 21 (65.6) | 2.80 (1.04 to 7.52) |
| Years epilepsy diagnosed | ||||||
| 2–4 | 4 (10.8) | 6 (18.8) | 1.00 (reference) | 5 (13.5) | 7 (21.9) | 1.00 (reference) |
| 5–8 | 9 (24.3) | 6 (18.8) | 0.72 (0.22 to 2.32) | 7 (18.9) | 4 (12.5) | 0.61 (0.16 to 2.34) |
| 9–15 | 7 (18.9) | 9 (28.1) | 1.68 (0.54 to 5.22) | 7 (18.9) | 9 (28.1) | 1.68 (0.54 to 5.22) |
| 16–34 | 7 (18.9) | 6 (18.8) | 0.99 (0.29 to 3.35) | 9 (24.3) | 7 (21.9) | 0.87 (0.28 to 2.71) |
| 35–67 | 10 (27.0) | 5 (15.6) | 0.50 (0.15 to 1.67) | 9 (24.3) | 5 (15.6) | 0.58 (0.17 to 1.96) |
| ED visits last 12 months | ||||||
| 1 | 14 (37.8) | 15 (46.9) | 1.00 (reference) | 14 (37.8) | 14 (43.8) | 1.00 (reference) |
| 2 | 12 (32.4) | 8 (25.0) | 0.69 (0.24 to 2.01) | 11 (29.7) | 8 (25.0) | 0.79 (0.27 to 2.31) |
| 3–4 | 3 (8.1) | 6 (18.8) | 2.62 (0.59 to 11.58) | 2 (5.4) | 6 (18.8) | 4.04 (0.74 to 21.91) |
| 5–25 | 8 (21.6) | 3 (9.4) | 0.38 (0.09 to 1.57) | 10 (27.0) | 4 (12.5) | 0.39 (0.11 to 1.39) |
| Seizures last 12 months | ||||||
| 1–2 | 7 (18.9) | 7 (21.9) | 1.00 (reference) | 5 (13.5) | 7 (21.9) | 1.00 (reference) |
| 3–5 | 5 (13.5) | 8 (25.0) | 2.13 (0.61 to 7.41) | 6 (16.2) | 8 (25.0) | 1.72 (0.52 to 5.68) |
| 6–9 | 5 (13.5) | 6 (18.8) | 1.48 (0.40 to 5.44) | 5 (13.5) | 7 (21.9) | 1.79 (0.50 to 6.38) |
| ≥ 10 | 20 (54.1) | 11 (34.4) | 0.45 (0.17 to 1.19) | 21 (56.8) | 10 (31.3) | 0.35 (0.13 to 0.94) |
| Primary care QOF 8 score | ||||||
| 0.00–56.4 (fewer seizure free) | 6 (16.2) | 9 (28.1) | 1.00 (reference) | 5 (13.5) | 9 (28.1) | 1.00 (reference) |
| 58.3–65.4 | 9 (24.3) | 6 (18.8) | 0.72 (0.22 to 2.32) | 10 (27.0) | 6 (18.8) | 0.62 (0.20 to 1.98) |
| 65.5–73.6 | 9 (24.3) | 4 (12.5) | 0.44 (0.12 to 1.63) | 10 (27.0) | 5 (15.6) | 0.50 (0.15 to 1.67) |
| 75.9–78.9 | 8 (21.6) | 7 (21.9) | 1.02 (0.32 to 3.22) | 9 (24.3) | 6 (18.8) | 0.72 (0.22 to 2.32) |
| 80.0–91.7 (more seizure free) | 5 (13.5) | 6 (18.8) | 1.48 (0.40 to 5.44) | 3 (8.1) | 6 (18.8) | 2.62 (0.59 to 11.58) |
| Seizure severity score | ||||||
| 0–5 (least severe) | 11 (30.6) | 14 (43.8) | 1.00 (reference) | 12 (32.4) | 15 (46.9) | 1.00 (reference) |
| 7.5–50 | 10 (27.8) | 5 (15.6) | 0.48 (0.14 to 1.61) | 10 (27.0) | 5 (15.6) | 0.50 (0.15 to 1.67) |
| 52.5–67.5 | 7 (19.4) | 6 (18.8) | 0.96 (0.28 to 3.24) | 9 (24.3) | 5 (15.6) | 0.58 (0.17 to 1.96) |
| 70–90 (most severe) | 8 (22.2) | 7 (21.9) | 0.98 (0.31 to 3.12) | 6 (16.2) | 7 (21.9) | 1.45 (0.43 to 4.90) |
| Seizure onset | ||||||
| Generalised or unknown | 17 (45.9) | 13 (40.6) | 1.00 (reference) | 19 (51.4) | 12 (37.5) | 1.00 (reference) |
| Focal | 20 (54.1) | 19 (59.4) | 1.24 (0.47 to 3.26) | 18 (48.6) | 20 (62.5) | 1.76 (0.67 to 4.64) |
| AEDS prescribed | ||||||
| 0 | 1 (2.7) | 0 (0.0) | 1.00 (reference) | 1 (2.7) | 1 (3.1) | 1.00 (reference) |
| 1 | 16 (43.2) | 19 (59.4) | 1.92 (0.73 to 5.04) | 16 (43.2) | 19 (59.4) | 1.92 (0.73 to 5.04) |
| 2 | 14 (37.8) | 10 (31.3) | 0.75 (0.27 to 2.05) | 15 (40.5) | 10 (31.3) | 0.67 (0.25 to 1.816) |
| 3–5 | 6 (16.2) | 3 (9.4) | 0.54 (0.12 to 2.36) | 5 (13.5) | 2 (6.3) | 0.43 (0.08 to 2.40) |
| Depression score | ||||||
| 0–1 (least symptoms) | 9 (24.3) | 3 (9.4) | 1.00 (reference) | 11 (29.7) | 1 (3.1) | 1.00 (reference) |
| 2–3 | 11 (29.7) | 8 (25.0) | 0.79 (0.27 to 2.31) | 8 (21.6) | 19 (59.4) | 1.02 (0.32 to 3.22) |
| 4–5 | 4 (10.8) | 6 (18.8) | 1.90 (0.48 to 7.53) | 4 (10.8) | 0 (0.0) | 1.90 (0.48 to 7.53) |
| 6–7 | 5 (13.5) | 6 (18.8) | 1.48 (0.40 to 5.44) | 7 (18.9) | 10 (31.3) | 1.20 (0.37 to 3.92) |
| 8–19 (most symptoms) | 8 (21.6) | 9 (28.1) | 1.42 (0.47 to 4.29) | 7 (18.9) | 2 (6.3) | 1.68 (0.54 to 5.22) |
| Anxiety score | ||||||
| 0–4 (least symptoms) | 7 (18.9) | 5 (15.6) | 1.00 (reference) | 7 (18.9) | 5 (15.6) | 1.00 (reference) |
| 5–7 | 9 (24.3) | 10 (31.3) | 1.41 (0.49 to 4.11) | 8 (21.6) | 10 (31.3) | 1.65 (0.55 to 4.90) |
| 8–9 | 8 (21.6) | 3 (9.4) | 0.38 (0.09 to 1.57) | 8 (21.6) | 3 (9.4) | 0.38 (0.09 to 1.57) |
| 10–12 | 7 (18.9) | 8 (25.0) | 1.43 (0.45 to 4.54) | 7 (18.9) | 8 (25.0) | 1.43 (0.45 to 4.54) |
| 13–19 (most symptoms) | 6 (16.2) | 6 (18.8) | 1.19 (0.34 to 4.18) | 7 (18.9) | 6 (18.8) | 0.99 (0.29 to 3.35) |
| QoL score | ||||||
| 13–18 (highest QoL) | 8 (21.6) | 4 (12.5) | 1.00 (reference) | 8 (21.6) | 4 (12.5) | 1.00 (reference) |
| 19–23 | 7 (18.9) | 9 (28.1) | 1.68 (0.54 to 5.22) | 7 (18.9) | 8 (25.0) | 1.43 (0.45 to 4.54) |
| 24–26 | 4 (10.8) | 5 (15.6) | 1.53 (0.37 to 6.32) | 5 (13.5) | 6 (18.8) | 1.48 (0.40 to 5.44) |
| 27–33 | 10 (27.0) | 6 (18.8) | 0.62 (0.19 to 1.98) | 10 (27.0) | 6 (18.8) | 0.62 (0.20 to 1.98) |
| 34–36 (lowest QoL) | 8 (21.6) | 8 (25.0) | 1.21 (0.39 to 3.73) | 7 (18.9) | 8 (25.0) | 1.43 (0.45 to 4.54) |
| Felt stigma score | ||||||
| 0 (least stigma) | 11 (29.7) | 13 (40.6) | 1.00 (reference) | 10 (27.0) | 13 (40.6) | 1.00 (reference) |
| 1–2 | 8 (21.6) | 7 (21.9) | 1.02 (0.32 to 3.22) | 9 (24.3) | 7 (21.9) | 0.87 (0.28 to 2.71) |
| 3–4 | 6 (16.2) | 7 (21.9) | 0.63 (0.24 to 1.67) | 7 (18.9) | 9 (28.1) | 1.68 (0.54 to 5.22) |
| 5–9 (most stigma) | 12 (32.4) | 5 (15.6) | 0.39 (0.12 to 1.26) | 11 (29.7) | 3 (9.4) | 0.25 (0.06 to 0.98) |
| Medication management | ||||||
| 13–39 (lowest skills) | 4 (11.1) | 7 (21.9) | 1.00 (reference) | 5 (13.9) | 6 (18.8) | 1.00 (reference) |
| 40–44 | 6 (16.7) | 8 (25.0) | 1.89 (0.62 to 5.79) | 4 (11.1) | 8 (25.0) | 2.96 (0.85 to 10.32) |
| 45–46 | 8 (22.2) | 7 (21.9) | 1.17 (0.40 to 3.42) | 7 (19.4) | 8 (25.0) | 1.59 (0.54 to 4.67) |
| 47–48 | 6 (16.7) | 5 (15.6) | 1.15 (0.35 to 3.80) | 8 (22.2) | 6 (18.8) | 0.99 (0.33 to 2.99) |
| 49–50 (highest skills) | 12 (33.3) | 5 (15.6) | 0.49 (0.16 to 1.53) | 12 (33.3) | 4 (12.5) | 0.40 (0.12 to 1.33) |
| Satisfaction with medication information | ||||||
| 1–4 (least satisfied) | 6 (16.7) | 5 (16.1) | 1.00 (reference) | 6 (16.7) | 5 (16.1) | 1.00 (reference) |
| 5–7 | 8 (22.2) | 6 (19.4) | 0.84 (0.25 to 2.78) | 8 (22.2) | 6 (19.4) | 0.84 (0.25 to 2.78) |
| 8–9 | 5 (13.9) | 7 (22.6) | 1.81 (0.51 to 6.47) | 4 (11.1) | 8 (25.8) | 2.78 (0.74 to 10.46) |
| 10–11 | 8 (22.2) | 6 (19.4) | 0.84 (0.25 to 2.78) | 7 (19.4) | 6 (19.4) | 0.99 (0.29 to 3.38) |
| 12–17 (most satisfied) | 9 (25.0) | 7 (22.6) | 0.88 (0.28 to 2.73) | 11 (30.6) | 6 (19.4) | 0.55 (0.17 to 1.72) |
| Social knowledge | ||||||
| 8–12 (lowest knowledge) | 9 (24.3) | 3 (9.4) | 1.00 (reference) | 9 (24.3) | 4 (12.5) | 1.00 (reference) |
| 13–14 | 10 (27.0) | 9 (28.1) | 1.06 (0.36 to 3.07) | 11 (29.7) | 8 (25.0) | 0.79 (0.27 to 2.31) |
| 15 | 9 (24.3) | 9 (28.1) | 1.22 (0.41 to 3.60) | 9 (24.3) | 8 (25.0) | 1.04 (0.34 to 3.13) |
| 16–20 (highest knowledge) | 9 (24.3) | 11 (34.4) | 1.63 (0.57 to 4.68) | 8 (21.6) | 12 (37.5) | 2.18 (0.75 to 6.33) |
| Medical knowledge | ||||||
| 15–21 (lowest knowledge) | 9 (24.3) | 4 (12.5) | 1.00 (reference) | 9 (24.3) | 5 (15.6) | 1.00 (reference) |
| 22–24 | 9 (24.3) | 7 (21.9) | 0.87 (0.28 to 2.71) | 9 (24.3) | 7 (21.9) | 0.87 (0.28 to 2.71) |
| 25–26 | 6 (16.2) | 4 (12.5) | 0.74 (0.19 to 2.92) | 7 (18.9) | 4 (12.5) | 0.61 (0.16 to 2.34) |
| 27–28 | 6 (16.2) | 10 (31.3) | 2.35 (0.74 to 7.48) | 5 (13.5) | 9 (28.1) | 2.50 (0.74 to 8.54) |
| 29–32 (highest knowledge) | 7 (18.9) | 7 (21.9) | 1.20 (0.37 to 3.92) | 7 (18.9) | 7 (21.9) | 1.20 (0.37 to 3.92) |
| Mastery | ||||||
| 6–12 (lowest confidence | 10 (27.0) | 7 (21.9) | 1.00 (reference) | 10 (27.0) | 7 (21.9) | 1.00 (reference) |
| 13–14 | 7 (18.9) | 7 (21.9) | 1.20 (0.37 to 3.92) | 6 (16.2) | 8 (25.0) | 1.72 (0.52 to 5.68) |
| 15 | 5 (13.5) | 7 (21.9) | 1.79 (0.50 to 6.38) | 4 (10.8) | 7 (21.9) | 2.31 (0.60 to 8.85) |
| 16–17 | 6 (16.2) | 5 (15.6) | 0.96 (0.26 to 3.52) | 8 (21.6) | 5 (15.6) | 0.67 (0.19 to 2.33) |
| 18–21 (highest confidence) | 9 (24.3) | 6 (18.8) | 0.72 (0.22 to 2.32) | 9 (24.3) | 5 (15.6) | 0.58 (0.17 to 1.96) |
Assessment 3
The only significant difference in the care reported to have been received over the 6 months preceding assessment 3 was that more participants in the intervention group (n = 14, 44%) had accessed other hospital outpatient services for their epilepsy than participants in the comparison group (n = 5, 14%; OR 4.98, 95% CI 1.53 to 16.23). These were typically noted by participants as being appointments for brain imaging and electroencephalography, which typically arose as a consequence of having seen the ENS (median number of appointments 1, IQR 1–2).
Uptake of the epilepsy nurse specialist-led self-management intervention
Over the entire 12-month follow-up period, 35 (80%) of the 44 participants offered the intervention attended one or more sessions. Seventeen (39%) attended one ENS session, 12 (27%) attended two sessions and six (14%) attended three sessions. The first session took place on average 5 weeks following recruitment, the second 24 weeks following recruitment and the third 38 weeks following recruitment.
Using logistic regression, the baseline information on intervention participants did not significantly predict whether or not a patient received at least one intervention session (all p < 0.05).
At the time of the 6-month follow-up assessment, 29 (90.6%) of the 32 retained intervention participants had received at least one ENS-led self-management session. At the 12-month follow-up assessment, 30 (93.8%) of the 32 retained intervention participants had received at least one ENS-led self-management session.
Outcome analyses
Unadjusted analyses of effect of intervention on emergency department visits
To reiterate, the primary outcome assessment occurred 12 months post recruitment (assessment 3). Unadjusted analyses indicated that the rate of ED visits reported by the intervention group at assessment 3 was 55% lower than that of the comparison group (Table 7). This difference was not statistically significant (p = 0.113), with groups not significantly predicting ED use [Wald χ2 (1) = 2.52, p = 0.1127]. In addition, no significant difference was found in the rate of ED visits reported by the groups at the 6-month follow-up (assessment 2). Table 8 presents the pattern of ED use reported by participants in the treatment groups at baseline and follow-up.
No significant effect of the intervention on subsequent ED visits was found when analyses were restricted to include in the intervention group only those participants who had received at least one intervention session (Table 9).
Covariates for emergency department visits and adjusted analyses of effect of intervention
The baseline variables identified as most predictive of a greater number of ED visits following recruitment were, in descending order of importance, lower confidence in managing epilepsy (less mastery), higher number of prescribed AEDs, more felt stigma, higher number of baseline ED visits, greater seizure frequency and higher levels of depression and anxiety (Table 10). In multivariate analyses, greater felt stigma and less confidence in managing epilepsy remained significantly predictive of ED visits at assessment 3.
Including the covariates in the regression models for ED visits resulted in the models now significantly predicting the level of ED visits that participants reported having made 6 months [Wald χ2 (6) = 103.30, p < 0.0001] and 12 months [Wald χ2 (11) = 140.90, p < 0.0001] following recruitment. Treatment allocation, however, remained a non-significant predictor both when the data were analysed on an intention-to-treat basis (see Table 7) and when the data were analysed on an efficacy basis (see Table 9).
| Outcome measure | Assessment 2 (n = 69) | Assessment 3 (n = 69) | ||
|---|---|---|---|---|
| Unadjusted IRR/coefficient (95% CI) | Adjusted IRR/coefficient (95% CI) | Unadjusted IRR/coefficient (95% CI) | Adjusted IRR/coefficient (95% CI) | |
| Primary outcome measure | ||||
| ED visits | 1.07 (0.45 to 2.54) | 1.75 (0.93 to 3.28) | 0.45 (0.17 to 1.20) | 1.92 (0.68 to 5.41) |
| Secondary outcome measures | ||||
| QoL (higher = poorer QoL) | 1.29 (−2.35 to 4.94) | 0.98 (−1.40 to 3.36) | 2.65 (−1.06 to 6.37) | 3.20 (−0.59 to 6.98) |
| Seizure frequency (higher = more seizures) | −0.27 (−2.30 to 1.74) | 0.51 (−1.10 to 2.12) | −0.27 (−2.19 to 1.65) | 0.58 (−0.97 to 2.13) |
| Anxiety (higher = more symptoms) | −0.41 (−2.64 to 1.83) | −1.01 (−2.56 to 0.55) | −1.04 (−3.29 to 1.20) | −1.72 (−3.70 to 0.25) |
| Depression (higher = more symptoms) | 0.25 (−1.68 to 2.17) | −0.67 (−1.94 to 0.59) | 0.18 (−1.72 to 2.08) | −0.03 (−1.88 to 1.82) |
| Medication management skills (higher = better skills) | −2.70 (−4.63 to 0.77) | −1.28 (−2.94 to 0.38) | −1.26 (−5.50 to 2.97) | 1.85 (−1.47 to 44.99) |
| Mastery (higher = greater confidence) | −0.46 (−2.14 to 1.21) | −0.80 (−2.23 to 0.62) | 0.32 (−1.33 to 1.98) | −0.49 (−2.10 to 1.12) |
| Epilepsy social knowledge (higher = more knowledgeable) | 0.04 (−1.18 to 1.25) | −0.86 (−1.82 to 0.11) | – | – |
| Epilepsy medical knowledge (higher = more knowledgeable) | 0.32 (−1.54 to 2.17) | −0.94 (−2.22 to 0.34) | – | – |
| Felt stigma (higher = more stigmatisation) | −0.69 (−2.03 to 0.64) | 0.01 (−0.85 to 0.85) | – | – |
| Satisfaction with medication information (higher = more satisfied) | 0.31 (−1.43 to 0.82) | −0.16 (−2.40 to 2.08) | – | – |
| Assessment point | ED visits in the last 12 (baseline) and 6 (assessments 2 and 3) months, n (%) | |||
|---|---|---|---|---|
| 0 | 1 | 2–3 | ≥ 4 | |
| Baseline | ||||
| Comparison (n = 41) | 0 | 15 (36.6) | 13 (31.7) | 13 (31.7) |
| Intervention (n = 44) | 0 | 18 (40.9) | 15 (34.1) | 11 (25.0) |
| Assessment 2 | ||||
| Comparison (n = 37) | 23 (62.2) | 6 (16.2) | 4 (10.8) | 4 (10.8) |
| Intervention (n = 32) | 13 (40.6) | 10 (31.3) | 7 (21.9) | 2 (6.3) |
| Assessment 3 | ||||
| Comparison (n = 37) | 23 (62.2) | 4 (10.8) | 6 (16.2) | 4 (10.8) |
| Intervention (n = 32) | 22 (68.8) | 3 (9.4) | 6 (18.8) | 1 (3.1) |
| Outcome measure | Assessment 2 (n = 65) | Assessment 3 (n = 67) | ||
|---|---|---|---|---|
| Unadjusted IRR/coefficient (95% CI) | Adjusted IRR/coefficient (95% CI) | Unadjusted IRR/coefficient (95% CI) | Adjusted IRR/coefficient (95% CI) | |
| Primary outcome measure | ||||
| ED visits | 0.89 (0.37 to 2.16) | 1.72 (0.86 to 3.43) | 0.46 (0.17 to 1.25) | 2.24 (0.73 to 6.85) |
| Secondary outcome measures | ||||
| QoL (higher = poorer QoL) | 1.57 (−2.25 to 5.40) | 1.05 (−1.40 to 3.50) | 2.48 (−1.35 to 6.31) | 3.08 (−0.73 to 6.89) |
| Seizure frequency (higher = more seizures) | −0.29 (−2.43 to 1.83) | −0.18 (−1.82 to 1.47) | −0.33 (−2.30 to 1.64) | 0.49 (−1.06 to 2.04) |
| Anxiety (higher = more symptoms) | −0.28 (−2.63 to 2.07) | −0.97 (−2.61 to 0.67) | −1.12 (−3.42 to 1.17) | −1.57 (−3.54 to 0.40) |
| Depression (higher = more symptoms) | 0.30 (−1.71 to 2.32) | −0.55 (−1.88 to 0.78) | 0.09 (−1.86 to 2.03) | −1.79 (−3.83 to 0.25) |
| Medication management skills (higher = better skills) | 2.70 (−5.74 to 11.13) | 2.68 (−5.11 to 10.46) | −0.03 (−3.24 to 3.18) | 1.88 (−2.70 to 6.47) |
| Mastery (higher = greater confidence) | −0.70 (−2.40 to 0.99) | −0.97 (−2.34 to 0.40) | 0.30 (−1.40 to 2.00) | 1.32 (−2.75 to 5.38) |
| Epilepsy social knowledge (higher = more knowledgeable) | −0.24 (−1.58 to 1.11) | −0.77 (−1.87 to 0.34) | – | – |
| Epilepsy medical knowledge (higher = more knowledgeable) | 0.68 (−1.37 to 2.74) | −0.73 (−2.17 to 0.71) | – | – |
| Felt stigma (higher = more stigmatisation) | −1.01 (−2.61 to 0.59) | −0.24 (−1.26 to 0.78) | – | – |
| Satisfaction with medication information (higher = more satisfied) | 0.22 (−2.05 to 2.50) | 0.09 (−1.70 to 1.88) | – | – |
| Baseline measure | Assessment 2 | Assessment 3 | ||
|---|---|---|---|---|
| Unadjusted IRR (95% CI) | Adjusted IRR (95% CI) | Unadjusted IRR (95% CI) | Adjusted IRR (95% CI) | |
| Gender (0 = female; 1 = male) | 0.69 (0.31 to 1.55) | – | 0.97 (0.30 to 3.12) | – |
| Age (years) | 0.99 (0.98 to 1.02) | – | 1.01 (0.98 to 1.02) | – |
| Ethnicity (0 = white British; 1 = other) | 1.30 (0.52 to 3.25) | – | 2.40 (0.84 to 6.87) | – |
| Education (years) | 0.92 (0.80 to 1.06) | – | 0.94 (0.82 to 1.09) | – |
| Deprivation (higher = more deprivation) | 0.97 (0.93 to 1.01) | – | 0.99 (0.93 to 1.06) | – |
| Comorbidity (0 = none; 1 = present) | 0.94 (0.40 to 2.22) | – | 1.32 (0.45 to 3.83) | – |
| Duration of epilepsy (years) | 0.99 (0.97 to 1.02) | – | 0.99 (0.97 to 1.03) | – |
| Emergency visits last 12 months | 1.14 (1.10 to 1.19) | 1.14 (1.03 to 1.26) | 1.19 (1.10 to 1.29) | 1.05 (0.92 to 1.20) |
| QoL (higher = poor QoL) | 1.04 (0.99 to 1.10) | – | 1.09 (1.02 to 1.16) | 0.93 (0.86 to 1.01) |
| Seizure frequency | 1.09 (0.97 to 1.23) | – | 1.19 (1.03 to 1.38) | 0.91 (0.80 to 1.02) |
| Primary care QOF 8 score (higher = more seizure free) | 1.01 (0.98 to 1.03) | – | 0.98 (0.96 to 1.01) | – |
| Seizure severity (higher = more severe) | 1.01 (0.99 to 1.02) | – | 1.03 (1.01 to 1.04) | 1.02 (0.99 to 1.03) |
| Seizure localisation (0 = generalised or unknown; 1 = focal) | 0.55 (0.24 to 1.24) | – | 0.66 (0.23 to 1.96) | – |
| Number of AEDs prescribed | 1.56 (1.13 to 2.15) | 0.98 (0.68 to 1.41) | 1.69 (1.18 to 2.44) | 1.43 (0.83 to 2.47) |
| Depression (higher = more symptoms) | 1.12 (1.04 to 1.20) | 0.99 (0.88 to 1.12) | 1.16 (1.07 to 1.25) | 0.99 (0.87 to 1.14) |
| Anxiety (higher = more symptoms) | 1.13 (1.04 to 1.23) | 1.02 (0.94 to 1.11) | 1.10 (0.99 to 1.22) | – |
| Felt stigma (higher = more felt stigma) | 1.14 (1.01 to 1.30) | 0.97 (0.82 to 1.10) | 1.42 (1.19 to 1.69) | 1.32 (1.11 to 1.56) |
| Medication management skills (higher = better skills) | 0.97 (0.92 to 1.03) | – | 1.01 (0.96 to 1.06) | – |
| Satisfaction with medication information (higher = increased satisfaction) | 0.93 (0.83 to 1.04) | – | 0.89 (0.77 to 1.04) | – |
| Medical knowledge (higher = more knowledge) | 0.95 (0.87 to 1.03) | – | 0.92 (0.83 to 1.02) | – |
| Social knowledge (higher = more knowledge) | 0.88 (0.71 to 1.09) | – | 0.80 (0.60 to 1.06) | – |
| Mastery (higher = more confidence in managing epilepsy) | 0.85 (0.78 to 0.93) | 0.95 (0.87 to 1.04) | 0.77 (0.70 to 0.84) | 0.86 (0.77 to 0.96) |
| Model summary | χ2 (6) = 43.69, p < 0.0001 | χ2 (8) = 120.91, p < 0.0001 | ||
Effect of intervention on secondary outcomes
The effect of the intervention on the secondary outcome variables at assessments 2 and 3 is presented in Table 7. No significant effect of the intervention was found on any of the measures at the 12-month outcome assessment, with and without adjustment for covariates.
No significant effect of the intervention on the secondary outcomes was also found when analyses were restricted to include in the intervention group only those participants who had received at least one intervention session (see Table 9).
Summary
We tested a self-management intervention delivered by ENSs that aimed to reduce subsequent ED visits by PWE. We compared its effect with the effect of TAU alone.
In total, 80% of the participants offered the ENS-led intervention in our study attended at least one intervention session. This uptake rate is favourable when compared with that reported to have occurred in previous randomised (76–95%)54,63,78,114,115 and non-randomised (48%)116 trials of nurse interventions in the wider epilepsy population.
Recruitment for our study was slower than anticipated and the trial stopped with 69 participants instead of the planned-for 120. This is reflected in wider CIs for the key estimates and consequent ambiguity in some conclusions. The results from our adjusted analyses are, nevertheless, evidence against the possibility of the intervention leading to a large (50%) reduction in number of ED visits. No effects on the secondary psychosocial outcome measures were found.
Results from our analyses of baseline predictors of subsequent ED use suggest that, to improve patient outcomes and reduce visits, interventions may need to focus more on patients' perceptions of stigmatisation because of epilepsy, confidence in managing epilepsy, psychological distress and epilepsy knowledge.
Chapter 3 Qualitative component
A qualitative study was nested within the non-randomised trial. Qualitative methods allow issues of importance for patients to be identified and examined in depth117 and were used here to explore two main topics with participants. The first concerned patients' reasons for attending an ED for epilepsy, and the second addressed participants' views of the ENS-led self-management intervention, including how it compared with usual care and satisfied their perceived support needs.
For the qualitative study, semistructured interviews were completed with a subsample of participants from the intervention group and the TAU group. Participants from the intervention group were interviewed both about their reasons for attending an ED for epilepsy and about the intervention, whereas those from the TAU group were interviewed only about their reasons for attending an ED for epilepsy.
We have split this chapter into two sections. The first section presents explanations given by PWE for using emergency medical services, and the second describes their views of the intervention. We describe the methods used in the first section only.
STUDY 3: QUALITATIVE STUDY OF PATIENTS' VIEWS, EXPERIENCES OF THE SERVICE AND REASONS FOR ATTENDING THE EMERGENCY DEPARTMENT STUDY
We aimed to explore the perspectives of adults with epilepsy who had attended the ED about who had made the call for emergency medical services and their rationale.
Methods
Recruitment for nested qualitative study
To avoid biasing the questionnaire responses, participants for this nested study were interviewed after they had returned the 12-month follow-up questionnaire for the trial. Interviews were conducted until responses indicated that the saturation point had been reached and no further interviews were required. The first 24 trial participants who sequentially completed the final follow-up were invited for interview.
Procedure
Interviews were conducted by a researcher independent of the trial and intervention (CV; please see acknowledgements section). Depending on each participant's preference, interviews were undertaken at their home or in our university office. The majority of interviews lasted for 1 hour (IQR 44–69 minutes). If the participant asked for a family member or ‘significant other’ to be present at the interview, this was agreed by us.
The topic guide was informed by a literature review and four open interviews with PWE not involved in the trial but who had attended an ED because of their epilepsy and seen the ENS more generally.
To explore patients' reasons for attendance, the topic guide first required the interviewer to ask participants to recall their most recent epilepsy-related attendance at the ED, the circumstances leading to the attendance, who made the decision to call the emergency medical services and whether attendance was the users' preference or not. Those participants who had received the intervention were then asked about their experiences of the intervention; whether they found it helpful and, if so, how and why; and whether they felt that any improvements could be made to the intervention. Throughout the interview, participants were encouraged to talk freely, with the interviewer probing and prompting responses as required.
Data analysis
Interviews were audio recorded and transcribed verbatim. Data were analysed thematically117 using the software package NVivo 9 (qSR International, Southport, UK). The researchers AN and CV read each transcript line by line and generated codes through open coding and then categorised these thematically. Relationships between themes were then identified through constant comparison of the transcripts, codes and categories. LR and MM reviewed the codes and their application and suggested alternative interpretations, and further interrogation of the data was undertaken until consensus was reached about explanations, relationships and influences on behaviours.
Results
Of the 24 participants identified, 19 (80%) agreed to be interviewed. Two were not contactable, one had died, one was too busy to meet and one refused to be interviewed. The sample interviewed included patients of varying ages, ethnicities, epilepsy duration, seizure frequency and reported ED use in the last year (Table 11). Sixteen of the participants interviewed were from the trial intervention group and three were from the TAU comparison group.
Analysis of patients' reasons for attending the ED identified three major themes: (1) context and presence/absence of a ‘significant other’; (2) patients' perspective on whether calling the emergency medical services was the right choice; and (3) how patients defined ‘an emergency’. Quotations are presented to illustrate the themes and data extracts are anonymised.
Theme 1: context and presence/absence of a ‘significant other’
Participants distinguished between seizures that occurred within the home and seizures that occurred elsewhere.
Seizures occurring within the home
Approximately half of the respondents (9/19) reported that their most recent seizure occurred within the home. For most of these participants, the family members/significant others were key contributors towards the decision to attend the ED. Three participants described their significant others as familiar with their epilepsy and having witnessed seizures before. Patients described the way that their significant others' confidence/knowledge meant that they were in control in the event of a seizure:
He [patients' son] has seen that type before 'cos he had to call them [emergency services] regularly. About a year and a half ago I was having them every day, but I did say to him, I said time it before you call them. I said if it's under five minutes, leave it, just go find a neighbour, but if it's more than five minutes, then yes call them.
Participant number 15 (female, 34 years)
| ID | Treatment arm | Gender | Age (years) | Ethnicity | No. of ED visits in year before trial | Years diagnosed | No. of seizures in year before trial | Main epilepsy care | Epilespy onset | Seizure type/s | Of three areas,a number in which improvement perceived | Time since intervention completion (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TAU only | Male | 33 | White British | 1 | 16 | ≥ 10 | Hospital specialist | Focal | Combination partial and generalised | NA | NA |
| 2 | TAU only | Male | 47 | Other | 1 | 20 | 5 | None | Undefined | Undefined | NA | NA |
| 3 | TAU only | Female | 45 | White British | 2 | 39 | 3 | Hospital specialist | Focal | Combination partial and generalised | NA | NA |
| 4 | Intervention | Female | 91 | White British | 1 | 13 | 1 | Primary care | Focal | Combination partial and generalised | 0 | 12 |
| 5 | Intervention | Male | 56 | White British | 1 | 4 | 1 | Hospital specialist | Focal | Generalised seizures only | 1 | 10 |
| 6 | Intervention | Female | 68 | Other | 1 | 4 | 2 | Hospital specialist | Focal | Generalised seizures only | 1 | 20 |
| 7 | Intervention | Female | 34 | Other | 1 | 9 | 3 | Hospital specialist | Generalised | Generalised seizures only | 1 | 9 |
| 8 | Intervention | Male | 21 | White British | 1 | 6 | 5 | Hospital specialist | Focal | Partial seizures only | 1 | 10 |
| 9 | Intervention | Male | 59 | White British | 1 | 42 | 2 | Primary care | Generalised | Generalised seizures only | 2 | 15 |
| 10 | Intervention | Male | 26 | Other | 2 | 14 | 4 | Hospital specialist | Generalised | Generalised seizures only | 0 | 10 |
| 11 | Intervention | Male | 57 | White British | 2 | 56 | 2 | Hospital Specialist | Focal | Combination partial and generalised | 1 | 13 |
| 12 | Intervention | Female | 34 | White British | 2 | 10 | 5 | Hospital specialist | Focal | Combination partial and generalised | 1 | 10 |
| 13 | Intervention | Female | 47 | Other | 2 | 4 | 6 | Hospital specialist | Undefined | Generalised seizures only | 1 | 12 |
| 14 | Intervention | Female | 39 | White British | 2 | 25 | ≥ 10 | Hospital Specialist | Focal | Generalised seizures only | 1 | 17 |
| 15 | Intervention | Female | 34 | Other | 3 | 13 | ≥ 10 | Hospital Specialist | Generalised | Generalised seizures only | 2 | 14 |
| 16 | Intervention | Female | 60 | White British | 3 | 5 | ≥ 10 | Hospital specialist | Undefined | Generalised seizures only | 3 | 2 |
| 17 | Intervention | Male | 37 | Other | 6 | 3 | 6 | Hospital specialist | Focal | Generalised seizures only | 2 | 4 |
| 18 | Intervention | Female | 31 | Other | 8 | 18 | 8 | Hospital Specialist | Undefined | Undefined | 2 | 10 |
| 19 | Intervention | Male | 23 | White British | 13 | 7 | ≥ 10 | Hospital specialist | Generalised | Generalised seizures only | 2 | 18 |
These patients stated that, because their significant others were aware of what actions to carry out in the event of a seizure, their decision to contact medical help was based on whether they were regarded as requiring emergency care. One said:
Because it had been quite a while and because I hadn't presented with vomiting before. So it was obviously the new dimension of it that led me to call the ambulance.
Participant number 8 (male, 21 years)
Four patients and one accompanying friend described how the significant others had witnessed a seizure before but described them on that occasion as being uncertain of what was required. As a result they regarded emergency medical services as a safer option. For example, an accompanying friend said:
[I] just worried because I don't know anything about epilepsy and I don't . . . I mean I only know the bad things, I know it can be quite serious and things like that, and I know you can die from it so I decided . . . I was so worried I decided just to ring an ambulance . . . better safe than sorry.
Female friend of participant number 16 (female, 60 years)
There were two participants who had had a seizure at home and who lived alone. One said that living alone made her feel more vulnerable and fearful and so she sought medical attention on a routine basis:
I was afraid I might die, because it could kill. [I] want to talk to someone; I want to see people around me, just other people who can care for me.
Participant number 6 (female, 68 years)
The other described how she had support from her neighbours, with decisions to seek medical help arising from their uncertainty about the situation:
They were worried, they called the ambulance.
Participant number 4 (female, 91 years)
Seizures occurring outside the home
Nine respondents (9/19) reported that their most recent seizure that led to an emergency call and ED attendance occurred outside the home. In these circumstances many respondents described decisions to seek medical care as being made by someone outside their usual home-based network of family/friends, such as bystanders, work colleagues or the police.
Three respondents had had their recent seizure in the workplace. These respondents described having confidence in their work colleagues to make the right decision in the event of a seizure. This was based on them telling their work colleagues about their condition beforehand, to increase the possibility that they could provide first aid and decide whether calling the emergency medical services was necessary:
Usual routine is to lie patient on floor, time how long, if more than 5 minutes they phone the ambulance . . . because it is just round the corner.
Participant number 3 (female, 45 years)
Two participants had experienced their most recent seizure whilst on public transport. They reported relief when the decision was made by the public to contact the emergency medical services based on the unpredictability of their condition, their physical appearance/presentation of the seizure and their belief that the public have a social responsibility to call for medical help:
I'd have thought they were absolutely shocked and terrified . . . I gather it was quite unpleasant to watch. I don't feel anything, but you know, you kind of make the noise and you go rigid and shake and fall on the floor. Sometimes you bite your mouth so it probably looks, kind of, there is blood there. So I'd imagine someone just next [saying] ‘stop, stop the train’, and call someone. That's quite obvious.
Participant number 12 (female, 34 years)
Four participants explained that their most recent seizure occurred whilst they were on the street and/or in view of the public. They felt that, as they were in public, the public's responsibility was discharged by contacting the emergency medical services:
Well I suppose if someone just fell over on a balcony and didn't get up, you might think that you should probably call A&E [ED]. Kind of just something you do in the neighbourhood. I mean it was a classic kind of thing to do something, although not want to get involved, 'cos when the ambulance people turned up, he just walked off. So you know, I probably imagine that lots of people, perhaps me too, would do the same. You know, arrange for some emergency service to take up and take over, and then you just think, right, well I've done my bit [laughs].
Participant number 1 (male, 33 years)
Theme 2: perspectives on whether use of the emergency department was the ‘right choice’
Preference to attend the emergency department
Most participants reported that it was the right choice for either their significant other or those outside their social network (i.e. the public) to have contacted the emergency services. Some patients felt personally that it would not have been the choice that they would have made, but they regarded it as the right choice in the given situation that justified other peoples' decisions as the right course of action:
I was with my friend, so she was scared and she doesn't know what to do, you know, she was lost. So she needed to call the ambulance. And when they came around, I was still in it. [I] don't always like going into A&E, but she called them, so there was absolutely nothing I could do to stop them from doing their job.
Participant number 18 (female, 31 years)
Some participants believed that it was the right choice to attend the ED, based on the seizure or its consequences needing emergency medical care, particularly when treatment was required for injuries or new symptoms:
I mean I think in most circumstances whether there are new symptoms, you know . . . as soon as I can, and you know . . . A&E is the most appropriate thing, that's fine.
Participant number 8 (male, 21 years)
Another person felt that it was the right choice based on the fear of sudden death and the need for reassurance:
I don't want to die. [A]nything could happen, you see this epilepsy can happen to you, anywhere you know. So, happy to call 999 for me.
Participant number 17 (male, 37 years)
Preference to avoid the emergency department
Although most patients justified the reasons for calling the emergency medical services, three participants highlighted that it was either a situation in which they had no choice, or a decision that was not required as they had learnt to accept their condition and felt that the ED had little to offer. They thought that they would benefit more from recovering within the comfort of their own home:
There's not a lot they can actually do. Most times on arrival, you're aware, and they cannot offer you any more; wasting space for somebody else who can go ahead and use that.
Participant number 2 (male, 47 years)
Another participant described a level of medical care that was too much for her:
'Cos I feel like it's a fuss 'cos I'm there. It's only a fit I've had, 'cos I've got used to it now and I'm thinking they don't need to fuss round me and putting all these things on me when I know I'm OK . . . don't need it.
Participant number 13 (female, 47 years)
Theme 3: describing what ‘an emergency’ was generally
Most participants (n = 13) stated that only in particular medical circumstances would they feel that emergency medical services were required. Ten respondents mentioned specific criteria to use to determine when it was appropriate to call an ambulance. One said:
injury is the only cause I see for medical attention really to be checked over. If it's something minor, it doesn't need medical attention, it wastes hospital time.
Participant number 10 (male, 26 years)
However, many respondents proposed a broader social definition of an emergency state, which was influenced by the presence or absence of their significant others. They identified that, if their network of family/friends were present and able to cope, then calling the emergency medical services would not be necessary:
If I was at home then me mum can turn around and say to herself ‘right I can cope with that . . . I'll just stick her to bed’.
Participant number 3 (female, 45 years)
They described that their ED attendance was necessary when their significant other was absent, sometimes because the significant other was responsible for delivering their medication, as this respondent explained:
But if I'm not at my mum's and have one here, I always call the ambulance. [T]hat's why, Dr [Name] gave me diazepam to give to my mum. So if I go in one, she shoots it up to me, and then I come out of it.
Participant number 19 (male, aged 23 years)
Three respondents regarded calling the emergency medical services as routine. They expressed fear of the risk of sudden, unexpected death, possibly exacerbated by living alone, with no support network and poor knowledge of the condition. One said of her seizures:
Felt this thing was catching me, afraid I might die, and no one knows, that is why.
Participant number 6 (female, aged 68 years)
Summary
From the patients' perspective, use of emergency service pathways is appropriate when they are away from home or do not have someone who knows about seizure management nearby.
Hospitals providing regular sessions on seizure management might increase knowledge and confidence among patients and their supporters, as well as fostering participation by PWE. Future research should be designed to develop and evaluate this.
STUDY 4: PATIENTS' VIEWS AFTER 1 YEAR OF FOLLOW-UP OF THE EXTENT TO WHICH THE EPILEPSY NURSE SPECIALIST-LED SELF-MANAGEMENT INTERVENTION MET THEIR NEEDS
In Chapter 2 we presented the findings from our trial of the effect of an ENS-led self-management intervention on subsequent ED use and patient well-being. Full evaluation of complex health interventions, however, requires consideration of issues in addition to effectiveness. 118 This includes the acceptability of the intervention to patients, barriers to and facilitators of its uptake, and the benefits and costs perceived by patients. Such information can be used to inform treatment refinement. Data from the interviews with the intervention participants were used to address the following specific questions, which are important for a patient-based evaluation of the ENS-led self-management intervention:
-
Was the intervention valued by patients and, if so, why? Some have suggested that PWE who attend the ED might be reluctant to accept and engage in additional treatment41,119
-
Were any aspects of the intervention perceived by patients as particularly helpful or unhelpful and, if so, why? The intervention was ‘complex’, and identifying what were the ‘active ingredients’ of the intervention might help with future service development120
-
Did all patients who saw the ENS perceive similar benefits and limitations of the intervention or did it depend on specific participant characteristics?
Methods
The methods used have been described in the previous section. To answer the questions in this study, only the data from the 16 intervention participants (participants 4–19) (see Table 11) were analysed.
Results
Analysis of the transcripts provided insights into the effects of the intervention and how these occurred. Four key themes were identified. These were limitations of usual care; what the ENS-led self-management intervention added; specific ways that the intervention had helped; and some reasons why, for some participants, the intervention had a more limited benefit. Quotations are presented to illustrate themes. There has been minor editing of some to preserve anonymity and ensure meaning of extracts.
Theme 1: limitations of usual care
Participants consistently highlighted that their usual epilepsy care had not equipped them with sufficient information about epilepsy despite having typically been diagnosed with epilepsy for ≥ 5 years. Many described this as contributing to them lacking confidence in self-management. Participants described how they had often left usual care consultations with unresolved questions and uncertainties:
They told me it was epilepsy, but they didn't explain what it really meant . . . I didn't know whether it was affecting me personally, or my brain, or what. I didn't know.
Participant number 4 (female, 91 years)
Primary care doctors were felt to be available for patients to see but to be often lacking in expertise in epilepsy to enable them to satisfactorily answer their questions:
When you go to your family doctor and you ask them a question, they say, ‘Oh I don't know, why don't you hold that question for when you next see your consultant’, which is twice a year!
Participant number 15 (female, 34 years)
Hospital specialists, on the other hand, were frequently perceived to be poor listeners and not interested in the patient's perspective, or the wider psychosocial difficulties that patients were experiencing whilst living with epilepsy:
When I go and see my consultant, there's very much a sort of, ‘Right, this is your condition, this is what we're going to do. Any questions? No. All right.’
Participant number 8 (male, 21 years)
Theme 2: what the epilepsy nurse specialist-led self-management intervention offered and how it was different
All but one participant reported having valued or even enjoyed the experience of the intervention to some degree:
A generally very useful conversation. Ten out of ten.
Participant number 9 (male, 59 years)
First, it helped satisfy some participants' need for information about epilepsy:
She knew her subject very well. Whenever I asked a question, she was able to respond straightaway . . . I think that's a lot better service.
Participant number 12 (female, 34 years)
Sessions were also perceived to be more relaxed. This helped participants to feel able to ask questions and to be involved in the content of their care:
It was less formal than with the doctor. You felt that you could actually ask questions . . . With [nurse name] I felt it was far more about me . . .‘What do you want to know about?’
Participant number 8 (male, 21 years)
Participants appreciated the empathic approach adopted by the ENSs and valued their attention to the often broad challenges of living with epilepsy:
[Nurse name] is more helpful than my doctor. We talked about everything, about epilepsy and fertility, about sex . . . She introduced me to a support group for epilepsy. Suggested I go for counseling and so many things like that. But Dr [Name] will always talk to me only about epilepsy. Never the wider aspects.
Participant number 18 (female, 31 years)
The longer length of the nurse-run sessions compared with usual care was also valued as it permitted information to be thoroughly explained, and also meant that complex challenges in patients' lives could be explored and needs identified:
I have had chronic pelvic pain that goes back to when I gave birth to my son fourteen years ago . . . She [the nurse] asked physio if they could see me as a priority because the pain affects my sleep which affects my epilepsy, and then we have this constant circle. They did see me. They've discharged me now, but I'm maintaining it. If I can handle the pelvis, I can handle the epilepsy.
Participant number 15 (female, 34 years)
The nurses offered participants their office telephone number. Some commented on the usefulness of this as it meant that they had access to rapid support in managing challenges from their epilepsy as they arose.
Theme 3: specific benefits that participants received from the intervention
Reflecting the intended responsive nature of the intervention, a variety of benefits were identified that involved emotional well-being, ability to take antiepileptic medication and confidence in managing seizures.
The number of domains in which a participant perceived benefit to have occurred and the magnitude of this benefit was broadly linked with the number of ED visits that he or she reported having made in the year before trial recruitment, with those who had used the ED the most describing more benefit. In contrast, participants who had used the ED once in the last year typically reported fewer benefits and they tended to be restricted to emotional well-being. There was no suggestion that duration of epilepsy exerted a similar influence.
Emotional well-being
Eleven participants described having experienced a variety of emotional difficulties as a result of epilepsy. Eight considered that the intervention had had a positive impact on these. They described the intervention as being the first time that they had been asked about how they felt about their epilepsy, and it provided an opportunity for them to ‘get these things out and off one's shoulders’ (participant number 9). Some felt more comfortable with their diagnosis as a result:
I'm confident now . . . she made me into someone with the confidence to talk about it. It's really helped me talking about it. [T]he shame of myself as an epileptic patient has also drastically reduced.
Participant number 18 (female, 31 years)
One aspect that these participants identified as being particularly helpful in such adjustment was being provided with information about how common epilepsy is.
Ability to take antiepileptic medication
Half of the participants volunteered that they had previously experienced difficulties in taking their AEDs. Of these, six felt that the intervention had helped them, although how it helped varied. For some this included being taught strategies to help them to remember to take their tablets, whereas for others it was being educated about the importance of regular dosing and adhering to the prescribed regime:
She pointed out, you know, it's not just daily, but it's got to be at a certain time every day . . . I wasn't as good at timings . . . I'm not perfect with it now, but I do try to take it at the same time every day, in the morning and in the evening. That was kind of drummed home to me.
Participant number 12 (female, 34 years)
Participants also highlighted the importance of the nurse reviewing their medication(s) and their responses to them. For one patient this resulted in troublesome adverse effects being identified and, with the involvement of her primary care doctor, the individual switched to another AED.
Confidence in managing seizures
Seven participants reported having felt fearful about their seizures and the potential consequences:
Cancer, you're awake. I know you can die, but you're awake. I'd prefer something like that . . . Having epilepsy, you're going into a fit. You don't know if you're going to wake up or die.
Participant 19 (male, 23 years)
As well as restricting their social activities, participants described how this often led them to call for an ambulance when they believed that they were about to have, or had had, a seizure, regardless of whether the seizure involved complications. Four participants felt that, through the provision of information about seizures, triggers, risk management and appropriate first aid, the intervention helped them feel more confident in managing seizures, and knowing when it was necessary to seek emergency care:
Now I don't feel as if I need to go to hospital . . . I'm not so frightened of it . . . I was really frightened of it.
Participant number 16 (female, 60 years)
Participants reported that the nurse highlighted the possibility of wearing an epilepsy identification bracelet and/or carrying a card. Some described these items as giving them more confidence. As a result their epilepsy did not restrict them as much as before, because they did not feel the need to be accompanied by a carer who could explain their diagnosis to others should a seizure occur.
Theme 4: why for some the benefits were more limited
People with epilepsy who reported at baseline having used the ED on only one occasion reported the fewest benefits. The main reason given for this was that, although they felt that usual epilepsy care had not addressed all of their support needs, they believed that they had the capability, disposition and/or confidence to overcome these limitations by themselves. One said:
This is going to sound terrible . . . I think it [the intervention] is absolutely vital for somebody who is less intelligent, less self-aware; less inquisitive . . . I'm not trying to be pejorative or anything . . . I'm just saying that, you know, if I don't understand something, I go out and I find out about it . . . I'm a ‘why’ person.
Participant number 5 (male, 56 years)
These people felt that their epilepsy was not as ‘severe’ as that of others and that for them the experience of seizures and use of the ED was atypical. The seizures that led to these participants' ED visits were mostly described as being precipitated by an unusual event in their life, such as a virus or stress:
I'm totally independent. Don't need any looking after whatsoever . . . I am not the normal ‘run-of-the-mill’ in terms of people with epilepsy that you'll see at the emergency department . . . I had been at home. Been working really hard. I'd been out to a party the night before. Was stressed out with all sort of things . . . I was in the bathroom in the morning . . . fell down . . . hit my head and [partner's name] came and said, ‘Oh, you know, that's a hospital job’ . . . first time in a long, long time.
Participant number 9 (male, 59 years)
Summary
We have reported that the trial results ruled out the possibility that a brief ENS-led self-management intervention delivered on an outpatient basis could lead to a large reduction in subsequent ED use by PWE. To more fully understand the utility of ENS-led self-management interventions and how PWE who attend ED should be supported, we have described here the intervention from the perspective of patients. We found that the intervention satisfied most patients and identified what they valued about it. Our participants valued the intervention as it redressed limitations in their usual epilepsy care. Those who had previously used the ED more perceived the greatest benefits.
Not only is a self-management intervention delivered by ENSs seemingly acceptable to PWE who attend the ED, but there was also evidence suggesting that, with optimisation, such an intervention might reduce the number of ED visits, as some patients perceived improvements in domains that may be linked to ED use by PWE. If this succeeded in liberating anything up to six-seventh more resources than are currently spent on emergency hospitalisation for other epilepsy services, this could justify more research in terms of an efficiency saving, as well as enhancing patient-related outcomes. The question of health economic outcomes is taken up in Chapter 4.
Chapter 4 Health economics component
STUDY 5: AN ECONOMIC EVALUATION OF THE PREVIOUS SERVICE USE REPORTED BY PEOPLE ATTENDING THE EMERGENCY DEPARTMENT FOR EPILEPSY AND OF THE COST-EFFECTIVENESS OF THE EPILEPSY NURSE SPECIALIST-LED SELF-MANAGEMENT INTERVENTION
The economic evaluation aimed to describe the current costs of care for this patient group who attend the ED, and compare the cost-effectiveness of the ENS-led self-management intervention with that of TAU alone, primarily from a health-care perspective but with the inclusion of lost employment costs in further analyses.
Methods
This study entailed combining data on health service costs with appropriate outcomes. Given the need to provide suitable information for commissioning and policy-making, we followed NICE recommendations and used quality-adjusted life-years (QALYs) as the main outcome measure in the economic evaluation. As a secondary aim, we analysed baseline data to identify patient characteristics that were associated with service costs.
Costing
Contacts with the intervention nurses were collected centrally and other service use was measured with the Client Service Receipt Inventory (CSRI) for the 12 months before the baseline assessment and the 6 months before the 6-month and 12-month follow-ups. 95 The CSRI was interviewer administered with service use information self-reported by participants. Services included secondary care, primary care and social care. Data were collected on whether or not a service was used, the number of contacts and (when relevant) the typical contact duration. For inpatient care the number of days spent in hospital was recorded. Medication taken as a result of epilepsy was recorded at each time point. The CSRI also asked for information on lost work days (for those in employment) because of health problems.
Service costs were calculated by combining the service use data with appropriate national unit cost information. For most services, unit costs were obtained from the annual compendium from the University of Kent. 121 Medication costs were taken from routine Prescription Cost Analysis data. 122 Lost production was valued by combining lost work days with the national average wage rate. 123
Benefit measurement and valuation
The National Institute for Health and Clinical Excellence recommends that, when possible, economic evaluations use QALYs as the outcome measure. QALYs combine information on quantity of life and health-related QoL, with the latter measured on a scale anchored by 1 (full health) and 0 (death). The European Quality of Life-5 Dimensions (EQ-5D)124 combined with UK weights125 was used to generate the health-related QoL scores at baseline and each follow-up point. The total QALYs accrued for each were calculated using area under the curve methods, and QALYs were compared between the two groups using a linear regression model adjusting for baseline health-related QoL.
Baseline analyses
Baseline costs represent the costs of caring for patients with epilepsy prior to the intervention being provided. Use and costs of different services were described and variables associated with cost variations were identified using a generalised linear model with (1) service costs and (2) service costs plus lost employment costs used as the dependent variables. Independent variables included in the model were age, gender, ethnicity, marital status, duration of illness, type of seizure at onset, current type of seizures, presence of psychiatric/neurological comorbidities and presence of learning disabilities. The cost data were positively skewed and we specified a gamma distribution in the model. The relationship between independent variables and cost was assumed to be additive and we therefore used an identity link function. Patients were recruited from three centres and, to account for the potential dependence between patients within sites, we used the cluster option in the (Stata) model.
Follow-up analyses
Total service costs for the 12-month follow-up period were compared between the two groups using a linear regression model with adjustment for baseline service costs. As before, bootstrapped 95% CIs were generated around the regression coefficient representing the cost difference.
Health-care costs were combined with the QALY data in the form of an incremental cost-effectiveness ratio (ICER), calculated by dividing the incremental costs for the intervention group compared with the comparison group by the incremental QALY gain. The ICER is thus based on point estimates of cost and outcome differences. To address uncertainty round the ICER we generated 1000 resamples using bootstrapping with replacement and calculated cost and outcome differences for each resample. These 1000 cost–outcome pairs were plotted on a cost-effectiveness plane.
To aid interpretation of the results we generated cost-effectiveness acceptability curves (CEACs) using the net benefit approach. The latter is defined for each participant as the monetary value of the outcome (i.e. QALYs accrued) minus the cost of achieving this. A range of monetary values for a QALY gain were used, from £0 to £80,000, in increments of £5000. For each value, a regression model was used to estimate the difference in net benefit between the groups. Bootstrapping was used to generate 1000 regression coefficients for each model and the proportion of these that exceeded 1 represented the probability that the intervention was the most cost-effective option at that particular monetary value of 1 QALY. This information was plotted on a chart to produce the CEACs.
Data related to, at most, a 1-year period and so discounting of costs or outcomes was not applied.
All analyses were performed using Stata 11.
Results
Baseline analyses
During the 12 months before baseline all participants had ED contacts (Table 12). Around one-quarter of participants spent time as an inpatient and for these the average time in hospital was around 5 days. Relatively high numbers of participants also spent time in the clinical decision unit (which is a short-stay hospital unit attached to the ED), had outpatient contacts and saw a GP. In total, 47 (55.3%) of the 85 patients had been admitted to an inpatient hospital ward and/or an ED clinical decision unit for epilepsy in the previous 12 months. Nearly all took some form of epilepsy-related medication. Inpatient stays and time in the clinical decision unit accounted for 43% of service costs. ED visits accounted for 7% of total service costs, and medication accounted for 19%. The cost of lost employment was relatively low.
From Figure 3 it is clear that the costs were heavily skewed. One patient was an extreme outlier because of an extended period of time in hospital and this individual's data were excluded from the analysis of baseline costs. Table 13 shows the mean costs for the remaining 84 patients. Costs did not differ substantially by demographic characteristics; however, costs were lower for younger patients and for those who were divorced/separated. Costs appeared to follow a U-shaped distribution in relation to illness duration.
The regression of service costs on patient characteristics revealed that black and ethnic minority patients had costs that were on average £733 (95% CI £401 to £1065) higher than the average for white British patients. Total costs were also higher (by £714 on average) for black and ethnic minority patients (95% CI £384 to £1045), for women (difference £709; 95% CI £44 to £1374), for those whose initial seizure had an undefined onset compared with a focal onset (difference £733; 95% CI £11 to £1348) and for those whose current seizures were partial rather than undefined (difference £2398; 95% CI £605 to £4192). Other differences were non-significant and much of the cost variation was unexplained by the characteristics that we measured.
| Service | n (%) | Mean (SD) no. of contacts | Mean (SD) cost (£) |
|---|---|---|---|
| ED | 85 (100) | 3.2 (7.7) | 155 (176) |
| Inpatient stays | 20 (24) | 4.8 (7.7) | 452 (1690) |
| Clinical decision unit | 41 (48) | 1.8 (1.5) | 502 (795) |
| Neurology outpatient | 57 (67) | 2.4 (1.8) | 237 (276) |
| Other outpatient | 34 (40) | 3.1 (2.9) | 185 (354) |
| Day care | 3 (4) | 3.0 (1.7) | 16 (91) |
| GP contacts | 73 (86) | 6.7 (8.3) | 210 (283) |
| Epilepsy nurse | 0 (0) | – | 0 (0) |
| Practice nurse | 29 (34) | 2.1 (2.1) | 8 (17) |
| Physiotherapist | 5 (6) | 6.0 (10.1) | 6 (38) |
| Social worker | 6 (7) | 6.0 (9.9) | 14 (68) |
| Medication | 81 (95) | – | 425 (393) |
| Total health- and social-care cost | 2210 (2328) | ||
| Lost work days | 19 (22) | 8.1 (8.7) | 145 (421) |
| Total cost | 2355 (2455) |
FIGURE 3.
Distribution of baseline costs.
| Characteristic | Service cost (£) | Total cost (£) |
|---|---|---|
| Age (years) | ||
| < 26 | 1674 | 1810 |
| 26–50 | 2182 | 2376 |
| > 50 | 2114 | 2114 |
| Gender | ||
| Male | 2061 | 2119 |
| Female | 2021 | 2238 |
| Ethnicity | ||
| White British | 2068 | 2232 |
| Other | 2005 | 2081 |
| Marital status | ||
| Single | 2069 | 2216 |
| Cohabiting/married | 2055 | 2181 |
| Divorced/widowed | 1776 | 1776 |
| Illness duration (years) | ||
| < 6 | 2146 | 2297 |
| 6–10 | 1787 | 1993 |
| 11–20 | 1548 | 1718 |
| 21–30 | 2776 | 2829 |
| > 30 | 2326 | 2351 |
| Seizure onset | ||
| Undefined | 1883 | 1954 |
| Focal | 2044 | 2178 |
| Generalised | 2209 | 2388 |
| Seizure type | ||
| Partial | 2422 | 2555 |
| Generalised | 1974 | 2069 |
| Combination | 2102 | 2273 |
| Undefined | 1574 | 1634 |
| ED site | ||
| STH | 2046 | 2137 |
| KCH | 1935 | 2077 |
| UHL | 2320 | 2471 |
| Comorbidity | ||
| No | 1909 | 2043 |
| Yes | 2177 | 2303 |
| Learning disabilities | ||
| No | 1982 | 2113 |
| Yes | 2712 | 2826 |
Follow-up analyses
During the first 6 months of the follow-up period, more participants in the intervention group had ED contacts than participants in the comparison group, but for those who did, the number of contacts was fewer and this resulted in lower ED costs for the intervention group (Table 14). A similar number in each group had inpatient stays but these were longer for the comparison group and hence the costs of inpatient care were 527% higher for the comparison group than for the intervention group. Costs of other services were similar, and total service costs for the intervention group were 27% lower than for the comparison group.
In the 6 months before the 12-month follow-up there was little difference in ED use and costs, but there remained a difference in inpatient costs, which were lower for the intervention group (Table 15). Total service costs for the intervention group were 16% lower than for the comparison group.
The mean total service cost over the entire follow-up period was £2948 for the comparison group and £2202 for the intervention group. The difference in mean costs, adjusted for baseline costs, was £558, and this was not statistically significant (bootstrapped 95% CI −£2409 to £648).
Over the follow-up period the QALY gain for the intervention group was 0.786 and that for the comparison group was 0.807. The mean difference, adjusting for baseline utility, was 0.0211, which also was not statistically significant (bootstrapped 95% CI −0.09 to 0.04). Based on these average costs and QALY differences it can be seen that the intervention resulted in lower costs but fewer QALYs. The ICER was £26,445 (−£558/−0.0211). This means that it costs an extra £26,445 to achieve 1 extra QALY if the intervention is not used.
| Service | Comparison group (n = 37) | Intervention group (n = 32) | ||||
|---|---|---|---|---|---|---|
| n (%) | Mean (SD) no. of contacts | Mean (SD) cost (£) | n (%) | Mean (SD) no. of contacts | Mean (SD) cost (£) | |
| ED | 14 (38) | 2.9 (3.4) | 53 (122) | 17 (53) | 1.7 (1.3) | 44 (61) |
| Inpatient stays | 5 (14) | 11.6 (21.5) | 633 (3320) | 4 (13) | 2.7 (2.1) | 101 (384) |
| Clinical decision unit | 5 (14) | 1.8 (1.3) | 144 (451) | 11 (34) | 1.1 (0.3) | 222 (328) |
| Neurology outpatient | 23 (62) | 1.3 (0.6) | 119 (114) | 21 (66) | 1.2 (0.4) | 119 (102) |
| Other outpatient | 17 (46) | 2.2 (1.5) | 147 (216) | 15 (47) | 1.5 (0.9) | 106 (146) |
| Day care | 2 (5) | 2.5 (2.1) | 20 (99) | 3 (9) | 1.0 (0.0) | 14 (44) |
| GP contacts | 27 (73) | 3.6 (2.1) | 71 (79) | 25 (78) | 3.6 (2.3) | 105 (111) |
| Epilepsy nurse | 2 (5) | 1.0 (0.0) | 2 (7) | 27 (84) | 1.6 (0.7) | 51 (34) |
| Practice nurse | 20 (54) | 2.0 (2.1) | 8 (14) | 7 (22) | 1.4 (0.5) | 4 (8) |
| Physiotherapist | 2 (5) | 3.0 (1.4) | 3 (16) | 1 (3) | 2.0 (–) | 1 (6) |
| Social worker | 0 (0) | – | 0 (0) | 6 (19) | 3.3 (4.3) | 68 (238) |
| Medication | 35 (95) | – | 260 (245) | 31 (97) | – | 230 (204) |
| Total health- and social-care cost | 1461 (3643) | 1065 (781) | ||||
| Lost work days | 3 (8) | 4.7 (2.1) | 30 (111) | 6 (19) | 4.8 (3.9) | 73 (197) |
| Total cost | 1492 (3648) | 1138 (840) | ||||
| Service | Comparison group (n = 37) | Intervention group (n = 32) | ||||
|---|---|---|---|---|---|---|
| n (%) | Mean (SD) no. of contacts | Mean (SD) cost (£) | n (%) | Mean (SD) no. of contacts | Mean (SD) cost (£) | |
| ED | 14 (38) | 4.0 (5.0) | 74 (175) | 10 (31) | 2.2 (1.2) | 34 (60) |
| Inpatient stays | 8 (22) | 3.5 (4.8) | 306 (1036) | 2 (6) | 4.5 (2.1) | 114 (473) |
| Clinical decision unit | 9 (24) | 2.3 (1.1) | 337 (678) | 6 (19) | 2.0 (1.5) | 222 (598) |
| Neurology outpatient | 22 (59) | 1.4 (0.7) | 119 (124) | 19 (59) | 1.5 (0.6) | 133 (131) |
| Other outpatient | 5 (14) | 2.0 (1.2) | 40 (118) | 14 (44) | 1.4 (0.9) | 92 (138) |
| Day care | 1 (3) | 1.0 (–) | 4 (24) | 6 (19) | 2.0 (1.7) | 55 (153) |
| GP contacts | 22 (59) | 3.6 (2.2) | 92 (141) | 23 (72) | 4.1 (3.0) | 140 (240) |
| Epilepsy nurse | 3 (8) | 1.3 (0.6) | 2 (8) | 8 (25) | 1.5 (1.1) | 11 (31) |
| Practice nurse | 9 (24) | 1.9 (1.6) | 6 (25) | 6 (19) | 1.2 (0.4) | 2 (5) |
| Physiotherapist | 1 (3) | 2.0 (–) | 2 (9) | 3 (9) | 4.7 (1.5) | 14 (51) |
| Social worker | 1 (3) | 1.0 (–) | 3 (17) | 3 (9) | 2.0 (1.7) | 36 (154) |
| Medication | 35 (95) | – | 309 (299) | 30 (94) | – | 234 (248) |
| Total health- and social-care cost | 1293 (1764) | 1088 (944) | ||||
| Lost work days | 8 (22) | 8.0 (9.7) | 138 (434) | 4 (13) | 3.3 (2.2) | 33 (103) |
| Total cost | 1431 (1784) | 1121 (927) | ||||
The cost-effectiveness plane (Figure 4) indicates that the most likely outcome is that the intervention results in lower costs and a poorer outcome as measured by QALYs. There is a similar likelihood that the intervention results in the best outcome (lower costs and more QALYs) and the worst outcome (higher costs and fewer QALYs).
The CEACs (Figure 5) show that if a QALY is given a 0 value then the intervention is the most cost-effective option. However, as a QALY receives a higher value the probability that the intervention is cost-effective falls. NICE uses a threshold of £30,000 to determine the cost-effectiveness of services. At this point the comparison condition is marginally more likely to be the most cost-effective option.
FIGURE 4.
Cost-effectiveness plane showing uncertainty around the cost-effectiveness estimates.
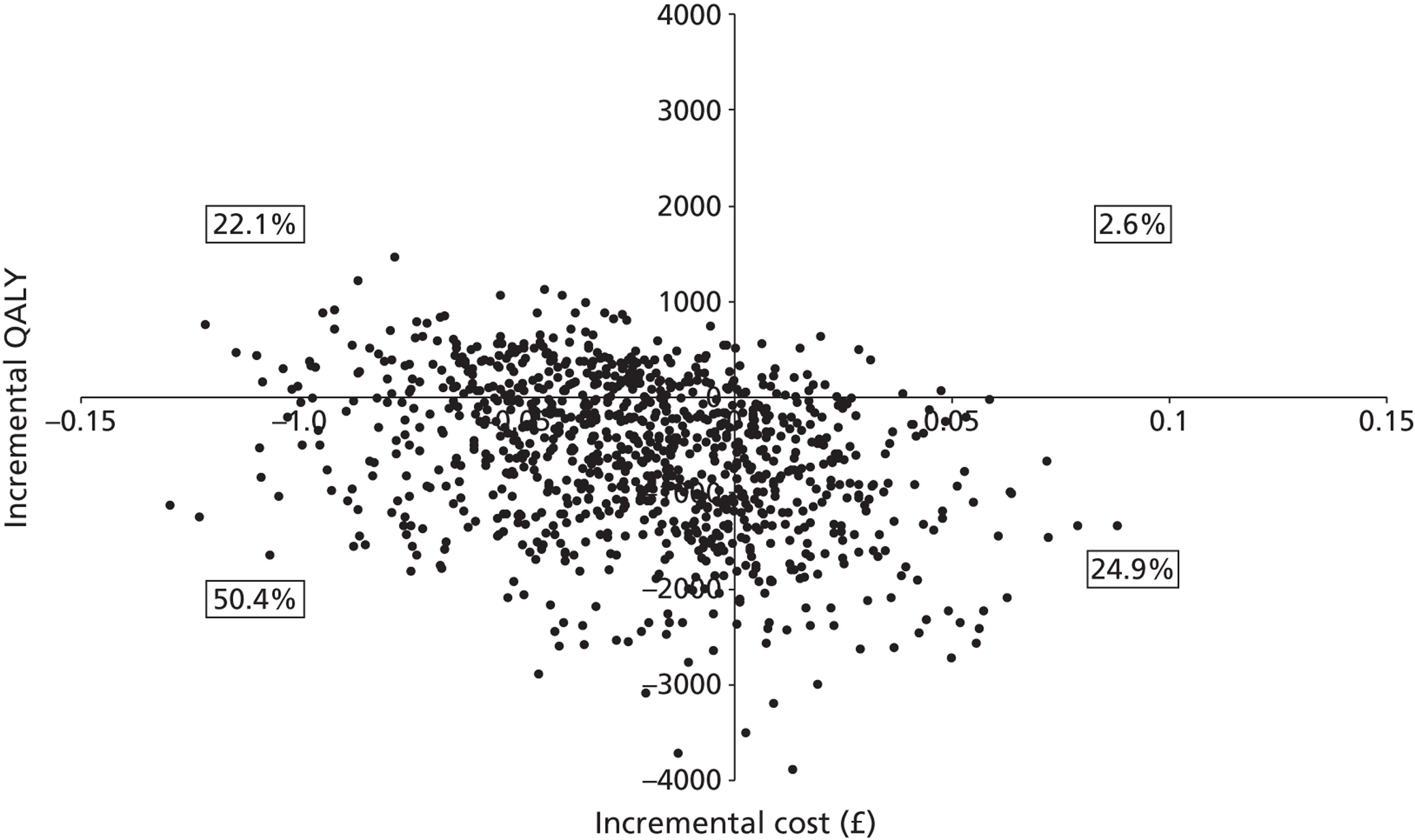
FIGURE 5.
Cost-effectiveness acceptability curves.
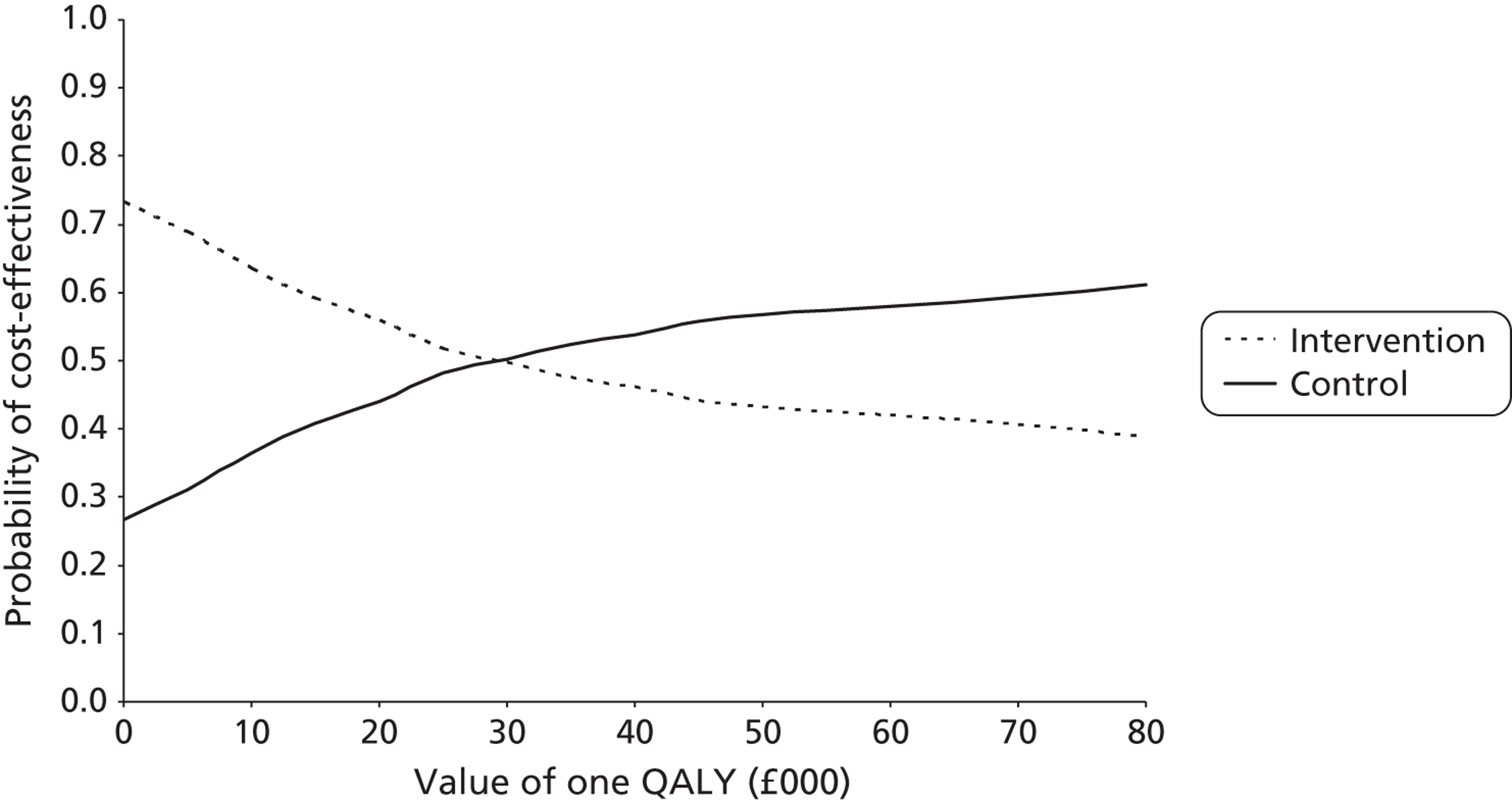
Summary
The 1-year costs before treatment allocation were on average £2355 per patient. Costs are significantly higher for black and minority ethic patients than for white British patients. The intervention reduced costs but did not improve outcomes according to the EQ-5D, a generic measure of health status. The ICER for standard care compared with the intervention was below the NICE threshold of £30,000 per QALY. However, there is substantial variation around the cost and QALY estimates, and the overall conclusion is that there is no evidence to suggest whether either the ENS intervention or TAU should be preferred.
Chapter 5 Discussion
Principal findings
Our project makes an important contribution to a small body of research. Previous studies from the UK and abroad describe attendance at EDs by PWE. 32,52,104,126–128 These studies mainly focused only on the appropriateness of ED attendance and lack of benefit from EDs for PWE who attend in the context of continuing care. No study had trialled an intervention for PWE that specifically aimed to reduce ED use, or explored patients' own reasons for attending the ED. Our mixed-methods project provides evidence on these issues and others. Our results provide new information on the frequency of ED use by PWE who attended the ED, and their characteristics. It describes patients' views of their routine epilepsy care, what ENS care added and who benefited most, as well as quantitative outcomes. Finally we are able to postulate potential targets that, if addressed, might reduce unnecessary attendance, provide efficiency savings and improve patient self-management in the future.
Pattern of emergency department use by people with epilepsy
The findings from our baseline assessment showed that the pattern of ED use by people with established epilepsy is different from that of general ED users, and that it is not homogeneous. In total, 39% of PWE attended the ED only once within the previous year, and > 60% reattended. Specifically, 25% had visited the ED on two occasions and the remaining 36% had attended on three or more occasions. This led to about one-third (36%) of ED attendees accounting for almost three-quarters (72%) of all visits made by the group.
Moore et al. 36 examined reattendance at the ED by general ED users within a 12-month period and found reattendance to be unusual – only 24% reattended, with most doing so on only one occasion. The pattern of ED use reported by PWE is most similar to that of people with COPD, another chronic relapsing condition. 37 It does, however, exceed that reported for people with diabetes or asthma. 37,38
In a US study, Bautista et al. 129 reported that the health-related QoL of PWE who had attended the ED was worse (weighted mean 27.8 on an epilepsy-related QoL instrument) than that of PWE who did not attend (weighted mean 23.4). We used the same epilepsy-related QoL instrument and found the QoL reported by our sample to be similarly low.
The characteristics of people with epilepsy who attend the emergency department
The results from the baseline assessment also showed that PWE recruited from south London were different from samples drawn from the wider epilepsy population. We found that all PWE attending the ED reported having a seizure in the previous year whereas, in the general population, Moran et al. 25 found that 48% of PWE are seizure free. In total, 46% attending the ED reported from two to nine seizures and 42% reported ≥ 10 seizures in the previous year compared with 16% reporting from two to nine seizures and 24% reporting ≥ 10 seizures in the previous year amongst PWE generally.
Anxiety was also more frequent amongst ED attendees. In total, 33% of ED attendees had ‘case’ levels of anxiety, compared with 22% in the general population of PWE. 108 However, ‘case’ levels of depression were not raised compared with other epilepsy population samples. 109
The ED attendees were also different in terms of the high proportion (68%) that felt stigmatised by their epilepsy. It has been reported that those with newly diagnosed epilepsy are at particular risk of perceived stigma. 130 Using the same scale, Taylor et al. 90 measured felt stigma amongst UK adults with newly diagnosed epilepsy but nevertheless found that fewer patients (53%) reported stigma.
Predictors of frequent emergency department use by people with epilepsy
Surprisingly, perhaps, we found that, in the previous 12 months, most of the PWE who we recruited from the ED had received epilepsy outpatient care that was in line with national guidelines. 50 This implies that current guidelines for quality excellence do not meet the needs of some people with poorly controlled epilepsy in terms of prevention of ED use. This is consistent with process evidence from a national audit suggesting that ED use offers little added value to care. 52 Using regression analysis we found that, in descending order of importance, less knowledge, more felt stigma, poorer medication management and greater seizure frequency predicted emergency visits before entering the study.
Twelve months after recruitment we found that the baseline variables identified as most predictive of increased numbers of ED visits were, in descending order of importance, lower confidence in managing epilepsy (less mastery), higher number of prescribed AEDs, more felt stigma, higher number of previous ED visits recorded at baseline, greater seizure frequency and higher levels of depression and anxiety. In multivariate analyses, greater felt stigma and lower perceived confidence in epilepsy management remained significantly predictive of ED visits at final assessment.
The results from our cross-sectional analyses of factors associated with previous use at baseline, and of the baseline factors that predicted ED use over follow-up, imply that these features require addressing through the development of interventions to prevent unnecessary ED visits. A case can be made for how these factors lead to ED use.
In terms of epilepsy knowledge, for example, it is possible that this mediates the relationship between seizures and whether or not a patient attends the ED as a result. One means by which this might occur is through the knowledge that a person has of first aid for seizures. Some patients may attend because of a lack of knowledge about what action to take. Most ED attendees do not require emergency treatment and present after an uncomplicated seizure. 24,131 Comparing knowledge of epilepsy scores among ED users and the wider epilepsy population shows lower knowledge among ED users. 110 For example, although one-third of our sample (incorrectly) stated that it was always necessary to call a doctor or ambulance if a person with epilepsy has a seizure, even if it occurred without complications, only 11% of a sample of the wider epilepsy population gave this answer. 132
Greater perceived stigma was also highly associated with ED use. This may be because patients who perceive epilepsy to be a stigmatising condition find it more difficult to engage with treatment planning. Buck et al. 133 found that those who reported stigma were more likely to miss taking their AEDs. Baker et al. 130 found that patients who felt stigmatised were also more likely to have experienced a seizure injury.
To a lesser extent, poorer medication self-management and increased seizure frequency also had independent roles in predicting ED use. This concurs with the results of previous studies. Poor adherence to AEDs is known to be associated with an increased number of ED visits, and the risk of seizure-related injuries is 21% higher during non-adherent periods. 134,135 The modest role of seizure frequency indicates that ED use is not simply a marker of seizure control.
Patients' explanations for attending an emergency department for epilepsy
This was a mixed-methods study including quantitative, qualitative and health economic analyses. From the perspective of PWE, the results of the study will be best represented by the stream of the study that collected and analysed the views of users. We found that users viewed their need to use the ED as contextual. It depended partly on whether their seizure occurred at home or in a public space, and partly on the knowledge, experience and confidence of those nearby of what to do. Other triggers were fear of death among PWE and others.
People with epilepsy frequently cannot make decisions themselves when they have a seizure, as they are unconscious or confused. From the patients' perspective, use of emergency medical services was regarded as appropriate, particularly when they are away from home and when they do not have someone who knows about seizure management nearby.
Our results concord with those of previous studies. An internet survey of public attitudes to witnessing a seizure found that two-thirds of respondents would promptly contact the emergency medical services. 136 Our participants said that they would expect this action to be taken by members of the public.
Fear of death or ‘death anxiety’ has also been described in a quantitative study of patients attending outpatient neurology clinics. 137 It was found to be associated with generalised epilepsy, higher anxiety scores and lower levels of education. We did not measure ‘death anxiety’ using quantitative methods but the cohort group in which this qualitative study was nested did have higher anxiety scores and a lower knowledge of epilepsy than PWE in other studies of the general epilepsy population.
Health economics of emergency department use
Emergency department visits at baseline accounted for 43% of total service costs, with medication accounting for 19% of total service costs. The regression of service costs on patient characteristics revealed that black and ethnic minority patients had costs that were higher on average by £733 (95% CI £401 to £1065). Total costs were also higher (by £714 on average) for black and ethnic minority patients (95% CI £384 to £1045). As part of the capacity development associated with this project (see Appendix 5), one of our MSc students (Fazia Sheikh, 2012) has carried out a literature review of the sociocultural perspectives of black and ethnic minority populations living with epilepsy. She found some evidence from the UK that South Asians hold alternative hypotheses about the cause of epilepsy and potential treatments, which may be important for clinicians to be aware of and address. 60 There is no evidence to date on the views of PWE from black ethnic minority communities, which compose 40–50% of the population of south London. However, there is evidence from research in West Africa, from where many of the black Africans originate, of PWE suffering discrimination, stigmatisation and social deprivation, and of women in particular suffering physical and sexual abuse because of their epilepsy. 59,138 This might also be experienced to a lesser degree by black ethnic minority PWE living in the UK, as a consequence of past experience, or through the influence of family or friends. This could be an important barrier to black ethnic minority PWE acquiring the knowledge and skills necessary for self-management. If this were so, these PWE might require a different and stepped-up intervention to address stigma for them to engage more fully with learning self-management skills.
Effect of the epilepsy nurse specialist-led self-management intervention on subsequent emergency department visits
Health service planners need interventions to reduce unnecessary emergency hospital visits by people with established epilepsy. In this study we have completed the first trial of an intervention to achieve this. We compared the effectiveness of a self-management intervention delivered by an ENS with the effectiveness of TAU alone for reducing subsequent ED visits. No statistically significant benefit was found in terms of reducing subsequent visits to the ED, nor was there any improvement in patient well-being.
Recruitment for our study was slower than anticipated and the trial stopped with 69 participants instead of the planned-for 120. This is reflected in wider CIs for the key estimates and consequent ambiguity in some conclusions. The results from our adjusted analyses are, nevertheless, evidence against the possibility of a large (50%) reduction in number of ED visits.
Why was the intervention not effective? First, although previous evidence had suggested that such interventions could reduce the frequency of ED visits,80–82 this came from studies using weak methodologies. This included studies comparing ED visits in patients before and after receiving the intervention, and which did not have a TAU comparison group. All reductions in the frequency of ED visits were therefore attributed to the effect of the intervention. However, the results from our baseline assessment show that, even without the specific support of a nurse, 40% of epilepsy attendees do not revisit an ED in the subsequent 12 months. We included a TAU comparison group to allow for this. The increased methodological rigour of our study may explain why our results are different.
Second, it is also perhaps not surprising in the context of a trial in a deprived area with a group of participants who had more seizures, greater levels of anxiety, a lower knowledge of epilepsy and its management and greater perceived epilepsy-related stigma than the average PWE, that two visits with an ENS lasting in total about 90 minutes failed to change the frequency of ED attendance or impact on secondary outcomes. More intensive interventions are used to improve self-management skills in those with other chronic, relapsing conditions. In type 1 diabetes, for example, a 5-day course is required to improve functioning (glycaemic control) and QoL. 139
Finally, the intervention was not designed to redress all of the factors found to be predictive subsequently, as they were not known. Even if they had been known, the size of the intervention was limited as NIHR terms and conditions required it to be self-funded by the hospital trust as an additional treatment cost. It was probably too dilute.
Cost-effectiveness of the epilepsy nurse specialist-led self-management intervention
The health economic evaluation provided a different perspective on the utility of the intervention. Specifically, during the first 6 months of the follow-up period, more participants in the intervention group had ED contacts than participants in the comparison group, but for those in the intervention group who did have contacts, the number of contacts was fewer and this resulted in lower ED costs for the intervention group. A similar number in each trial group had inpatient episodes, but the duration of the stays were longer for the comparison group and hence the costs of inpatient care were 527% higher than for the intervention group. Costs of other services were similar, and total service costs for the intervention group were 27% lower than those for the comparison group.
In the 6 months before the 12-month follow-up there was little difference in ED use and costs but there remained a difference in inpatient costs, which were lower for the intervention group. Total service costs for the intervention group were 16% lower than for the comparison group. The mean total service costs over the entire follow-up period were £2202 for the intervention group and £2948 for the comparison group. The difference in mean costs, adjusted for baseline costs, was £558. This was not statistically significant (bootstrapped 95% CI −£2409 to £648). We did not measure seizure severity at the 12-month follow-up and it is possible that the intervention group had a lower seizure severity.
In the economic evaluation the QALY gain over the follow-up period was 0.786 for the intervention group compared with 0.807 for the comparison group. This result was unexpected and runs counter to the finding that the more detailed and specific epilepsy QoL measure did not show a difference between the intervention and comparison groups. We would tend to give more credibility to the specific epilepsy QoL measure, which covers a longer period of a month rather than a day.
Patients' views of the epilepsy nurse specialist-led self-management intervention and how it compared with usual care
Qualitative evaluation of the ENS intervention, undertaken 1 year after the trial started, added to an understanding of why a lack of effect occurred in terms of a decline in frequency of ED visits after the intervention. About 40% of PWE used the ED only once in the year and felt that they did not need additional input to their self-management. The number of areas in which our participants perceived a benefit to have occurred from the intervention, and the extent of the benefit, were, however, associated with the degree to which the participants had previously visited the ED. Those who had used the ED the most tended to report the most benefit from the intervention. Our results suggest that offering some form of additonal advice and support only to those who attend the ED on more than one occassion in the previous year is perceived as helpful by PWE.
The intervention was almost universally valued by our participants. This finding counters speculation that PWE who have attended the ED may not accept or engage with additional treatment. 41,119 The main reason given for valuing the intervention was that it redressed limitations perceived in their usual epilepsy care. In particular, they felt that the content of the intervention sessions was tailored to their inividual needs, sessions were less time pressured and the ENSs were more considerate of, and attentive to, the broader aspects of living with epilespsy.
The areas in which participants reported benefits to have occurred – in particular, improved emotional well-being (including reduced perceived stigmatisation), better medication management skills and greater confidence in managing seizures – have been suggested as being causally related to ED visits by PWE. 134,135,140–144
As part of a mission for capacity building, we supervised four King's College London MSc Neuroscience students to undertake successful dissertations on epilepsy during the study (see Appendix 5). This contributed to our findings and complemented them. A qualitative study undertaken by one MSc student who was an ambulance clinician focused on the views of ambulance clinicians about their decision-making when called to PWE. 145 Participants reported that their previous experience was important, more so than training, in determining their confidence to manage PWE. But they added that training and guidelines were insufficient and that sometimes decisions were made to avoid a perceived threat of litigation. Ambulance clinicians reported that lack of someone to contact, and of information on patients, were important and could lead to PWE being transported to the ED unnecessarily. 145
Strengths and weaknesses of the project
As noted, our project makes an important contribution to a small body of research. However, our results should be interpreted in light of some limitations. In the following sections we describe the respective strengths and weaknesses of each of the main components of our project.
Quantitative component
Study 1: an evaluation of a group of people attending the emergency department for epilepsy, their use of emergency services and their psychological state, knowledge of epilepsy, perception of stigma, quality of life and needs
To evaluate the characteristics and needs of PWE attending the ED and their pattern of ED use, participants' responses to the questionnaires administered at the trial's baseline assessment – which occurred before any differences in care were implemented – were described and compared with findings from the literature in those from the wider epilepsy population.
The first potential limitation of this component of the project is that the participant acceptance rate into the study was low. Of those we invited, 27% agreed to participate. Low acceptance raises the possibility that those who agreed to participate in our study may not be representative of PWE who attend the ED, limiting the generalisability of the results. Information on the non-recruited patients' epilepsy was limited to their ED records, as wider access to their medical records was not ethically permissible. This meant that our comparison of participants and non-participants was restricted to age, gender, deprivation status, ethnicity and the clinical urgency of their ED presentation. 105 We also extracted information recorded by their primary care practices for the 2009–10 QOF85 to compare their epilepsy care generally. According to these measures we found that those who agreed to participate were representative of PWE attending the ED from which they were drawn, with one significant difference being that fewer eligible people of non-white ethnicity were recruited. It is well known that recruiting people from minority groups, as well as women and those of certain ages, is difficult and under-representation is common. 146
To help identify the determinants of ED use, in this study we examined the association between patients' characteristics and the number of ED visits that they reported having made over the previous 12 months. A limitation of this part of our study is that the cross-sectional design of the analysis means that conclusions cannot be made on the basis of the results about the direction of the relationship between the factors in our regression model. The directionality may be the other way around.
A third potential limitation is that, as the baseline assessment was part of our trial to compare the effect of the ENS-led self-management intervention with the effect of TAU, those who had recently seen an ENS and those referred to neurology by the ED were excluded from the study. This could have led to the exclusion of certain categories of patients, which limits the generalisability of our results to all PWE who attend the ED. However, the number excluded on the basis of these criteria was small (amounting to 12% of all exclusions).
Fourth, we used self-report data. This is common and accords well with concepts such as QoL, which emphasise the experience of the individual. However, the reliance on patient reports of seizure frequency introduces the possibility of bias. Although there is limited consensus on how else to measure seizures over a sustained period in community studies, many patients are not aware of, or are amnesic for, a proportion of seizures. 147 We also relied on patients to self-report their ED use as there is no national record of an individual's ED attendances, but PWE have been found to be reasonably accurate in recalling use of other health-care services, particularly hospital-based services, over the previous year. 24,32
Although the UK NHS differs from national health services in other countries in some specifics, it is publicly funded like those in the majority of Western countries. 148 A strength of our study is therefore that our results may be readily generalisable to many other countries. We also recruited from an urban, ethnically diverse population with a high degree of deprivation. Although findings may be less generalisable to rural and less deprived areas,149 the potential similarity of our multiethnic population to populations in metropolitan areas in other countries may mean that our evidence is generalisable internationally.
Study 2: quantitative evidence from a comparison of two groups, one receiving usual care and the other receiving an epilepsy nurse specialist-led intervention
The first limitation of our comparison of the two treatment groups was that treatment allocation was not randomised but rather was based on the site from which participants were recruited. Randomised studies produce more accurate estimates of treatment effect. 150 The advantage of randomisation is that, when undertaken properly, it reduces the potential for bias in the allocation of patients to different treatments and, on average, the groups are balanced on known and unknown covariates. 151 It is possible that unknown baseline differences existed between our treatment groups and these may have confounded the results and reduced the accuracy of our treatment effect estimate.
In considering how accurate our treatment effect estimate is we sought to minimise the likelihood of group differences by restricting recruitment to PWE from similar hospitals and similar areas. We also endeavoured to capture group differences by using a wide selection of baseline measures, with adjustment for differences detected. We also used prospective recruitment methods, which can make a non-randomised trial's estimate of effect similar to that of a randomised trial. 150
A second potential limitation to the study is that the researcher administering the assessments was not blind to the treatment allocation of the participants. This may have influenced the assessments in some way, even though the same scoring procedures were followed for each participant, and the outcome negative.
Finally, as already noted, recruitment was slower than anticipated and the trial stopped with 69 participants instead of the planned-for 120. This is reflected in wider CIs for the key estimates, and consequent loss of power, with ambiguity in some conclusions. The reason why the intended sample size was not achieved was because the 27% participant acceptance rate into the trial was lower than the rate of 50% that we had anticipated. It is now apparent from studies in the wider literature that low acceptance rates are common in studies on ED attendees with long-term conditions and in trials in which serial assessment is required. 139,152 When planning our project this information was not available and no previous studies had exclusively recruited PWE from EDs. In previous epilepsy nurse studies our group had obtained 80–84% recruitment rates. 54,63 Because studies had not been conducted in socially deprived areas or specifically with those with poor epilepsy control, we had considered an adjusted estimate of 50% to be reasonable. It became apparent subsequently that even this conservative estimate was too optimistic.
Future studies that intend to recruit PWE from EDs should factor in low uptake rates and implement evidence-based strategies to maximise recruitment. For example, we offered all participants a £20 shopping voucher as evidence suggests that financial incentives are generally found to facilitate recruitment. 153 Participant acceptance may be further maximised in future ED studies if a research worker is available to recruit participants directly from the ED. We did not have the resources to do this. We were funded for one research worker whereas patients attend the ED 24 hours a day, 7 days a week. AN needed to assess participants in the community at baseline and follow-up and was responsible for all of the project's administrative tasks. We therefore posted an invitation letter to those patients who were eligible, asking them to express their interest using a response slip and Freepost envelope. A potential limitation of such an approach is that, as a research team, we did not have an established relationship with the patients. This relationship and trust is what many potential participants depend on when considering participation in a trial. 154 Research worker(s) recruiting directly from the ED could more readily align themselves with the patients' treatment teams. The feasibility of recruiting PWE directly from the ED would require clarification. For example, patients may often be in a postictal state and, also, a stay in the ED is typically far shorter than a stay on a hospital ward.
It is also important to note that our study design obliged us to exclude a high number of patients on the basis of non-residence within one of the London boroughs served by the hospitals from which we recruited. Specifically, of the 628 patients we excluded, in 56% of cases this was because they did not live in one of the three boroughs directly served by the EDs. There were two reasons for excluding such patients. The first was to promote the similarity of the participants who compose the treatment arms in this non-randomised trial by ensuring that they came from areas with comparable levels of epilepsy control, social deprivation and ethnic diversity. The second reason was more pragmatic as many people attending inner London hospitals, such as STH, which is located next to a major transport hub (i.e. London Waterloo railway station), do not live in London. Recruiting such people would have potentially resulted in a lower rate of attendance at the intervention sessions and potentially a higher loss of participants to follow-up. A lower rate of exclusion would likely occur if future studies recruit from a less metropolitan area.
At follow-up we retained 81% of the participants who were recruited into our trial. This rate of retention is favourable when compared with that achieved by many previous epilepsy trials. 9,10,155 It is important to note, however, that the dropout rate was higher in the intervention group and there was some evidence that it was those participants who felt most stigmatised by their epilepsy that were more likely to be lost.
As loss to follow-up can introduce bias and mean reduced statistical power,156 future trials with PWE recruited from EDs should consider implementing strategies to maximise the retention of intervention participants. If a future trial were designed to test an intervention that is delivered in a location outside of the patient's home, one strategy to increase retention might be to offer participants free taxi travel to the location of the intervention. We suggest this because a difference between being in the intervention group and being in the comparison group in our study was that intervention group participants were requested to visit a hospital clinic on several occasions to receive the ENS-led self-management intervention. According to UK regulations a person who has suffered an epileptic seizure cannot legally drive for at least 1 year. This means that people with poorly controlled epilepsy often depend on public transport. Recent evidence shows, however, that those PWE who feel most stigmatised by their condition are more likely to be worried about the possibility of having a seizure, but at the same time have a smaller network of friends and family to support them, such as to accompany them on trips outside of the house in case a seizure occurs. 157,158 This might explain why participants in our study's intervention group who felt most stigmatised were less willing or able to stay in the study. Providing such people with the possibility of free travel by taxi to the intervention might lead to more of them staying in the study.
Qualitative component
Study 3: qualitative study of patients' views, experiences of the service and reasons for attending the emergency department
For this component of the project we interviewed 16 PWE from the intervention group and three from the TAU group after they had completed their final assessment as part of our trial. Participants for the trial were identified and recruited because they had experienced a seizure and had attended an ED. In the wider epilepsy population, not all people who have seizures attend an ED. Epidemiological data, for example, show that approximately half of PWE in the UK experience seizures each year,25 but only 13–18% attend an ED annually. 24,32 Therefore, a potential limitation to this qualitative study, which aimed to explore the reasons for ED attendance, is that we did not interview PWE who had experienced seizures but who did not attend an ED. Also, patients were interviewed just over a year after the ED visit that precipitated their initial recruitment. Nevertheless, most patients had experienced more seizures than ED attendances and they were able to describe episodes that led to the emergency services being called, as well as episodes when ED attendance was avoided.
A second potential limitation is that only the first 24 participants to complete the trial were invited to be interviewed. These PWE do not necessarily represent the whole sample, the population of south London or, indeed, the UK population. It is possible that a different rationale for calling the emergency services might be used in rural areas, for example, where distance to the hospital requires more travel.
Study 4: patients' views after 1 year of follow-up of the extent to which the epilepsy nurse specialist-led self-management intervention met their needs
As noted for the previous qualitative study, we invited to interview the first 24 participants to complete the final trial assessment. Sixteen of these participants were from the intervention arm and they were asked about their views of the intervention. Because the ENSs delivering the intervention had not previously delivered self-management support to those who had attended the ED, it is possible that their ability to deliver the intervention was greater for later participants who were not interviewed by us. If this was the case, a limitation of our study is that its results may present a less optimistic view of the potential benefits of the intervention.
A second potential limitation is that the benefits that participants described could have been influenced by the skills of the particular nurses who delivered the intervention. It could be that different findings would emerge if different people delivered the intervention. This is not a feature unique to our intervention but is common to most psychosocial interventions, including those that are already part of mainstream health care, such as cognitive–behaviour therapy. 159 To promote replication of the intervention and its benefits, we have fully described it and the characteristics of those delivering it (see Chapter 2). Also, two ENSs delivered the intervention to limit the influence of an individual therapist.
Finally, in considering the generalisability of our results, our trial participants were recruited from a deprived urban UK population. Some of the difficulties that our participants reported with their usual epilepsy care and the benefits derived from the intervention may result from the views of people from such areas. However, similar limitations to usual care have been reported by PWE from other countries and by PWE from different areas in the UK. 160–162
Health economics component
Study 5: an economic evaluation of the previous service use reported by people attending the emergency department for epilepsy and of the cost-effectiveness of the epilepsy nurse specialist-led self-management intervention
The reliance on patient self-report of service use is a potential limitation of the economic evaluation, although a number of previous studies have shown this to be a reasonable method to use. 163,164 The strengths of the study are the use of QALYs as an outcome measure and the breadth of service use included.
Future research: implications
-
Testing workshops on seizure management for PWE and their significant others.
-
Testing self-management education programmes for PWE.
-
Exploring the perception of stigma and self-management.
-
Exploring the perception of stigma and self-management among ethnic minorities.
-
Evaluation of the needs of PWE who attend EDs in different areas of the UK.
-
Development of interventions to manage anxiety and death anxiety for PWE and their carers.
-
Risk factors for ED attendance and risk of death in epilepsy: mortality prevention.
Priority 1: testing workshops on seizure management for people with epilepsy and their significant others
As a consequence of our exploratory interviews, we believe that all district hospitals might in the future develop and provide regular workshops, for example monthly, on seizure management for PWE, who would be invited to come together with their significant others. These workshops would need to be provided repeatedly to enable PWE to bring different members of their family, a partner or new partner, friends as they become closer and work colleagues whenever they change their employment.
Practical training could be provided on triggers to seizures and how to manage them, as well as on prevention of injury, together with time provided for PWE and their significant others to discuss fears of death. This initiative might be led by ENSs in collaboration with clinicians in emergency medical services. This might be evaluated most rigorously by means of a randomised controlled trial. Mixed methods including a qualitative approach to evaluate users' perceptions of how to present the intervention to other potential users, how users and NHS providers should invite participation from significant others, and the timing, duration and content of the intervention should be considered to optimise participation as well as any benefits of the intervention. We have demonstrated the high cost of ED attendance with few benefits. The trial should be powered to measure potential efficiency savings by means of robust health economic evaluation.
Priority 2: testing self-management education programmes for people with epilepsy
In the UK, self-management programmes have been tested and adopted for other chronic conditions (e.g. diabetes: DAFNE,14 DESMOND,15 X-PERT;16 arthritis17,18). Given the perceived lack of information for PWE, their perception of stigma, their anxiety and their lack of confidence in managing their condition, we believe that there is scope to evaluate longer structured interventions designed to meet these needs. One option is a self-management learning programme for PWE, rather like the 1-week programme that has already been trialled for diabetes and rolled out in the NHS. 139
We have been funded by the NIHR Health Technology Assessment programme (ref. 09/165/01) to modify and test a 2-day course developed in Germany for PWE for the NHS context. 74 This is targeted specifically for people with chronic poorly controlled epilepsy.
From our research evidence and that of others, it is likely that, as in other conditions, for example diabetes, all PWE would benefit from a self-education programme from the time of first diagnosis. We have demonstrated a high level of variation in people's knowledge of epilepsy, which correlates with their general education. For some people step-up learning and support strategies will be required. This may be particularly important in areas of high social deprivation and for ethnic minority groups to tailor learning specifically. We have highlighted the higher cost of health services for our participants of black ethnicity. If more investment, linked to their specific needs, is effective in improving self-management, there is scope for greater efficiency savings as well as patient benefits.
Priority 3: exploring the perception of stigma and self-management
Our evidence suggests that the perception of stigma may be a particularly important mediator between seizures and ED use. Lack of acceptance of their condition by PWE (or their significant others) may prevent PWE from fully accepting their condition and engaging with health professionals to acquire the skills to manage it in the long term. It is currently unknown what support should be offered to PWE who experience felt stigma. When there is stigma and denial, as occurs in other long-term conditions such as addiction, motivational interviewing has been recommended to increase clients' sense of ownership and self-efficacy. It might be feasible to help PWE who also feel stigmatised to engage more fully with self-management by developing a purpose-designed motivational interviewing approach and testing it by means of a randomised control trial.
Priority 4: exploring the perception of stigma and self-management among ethnic minorities
We believe that we have identified a need for more research on the views of black ethnic minority people about epilepsy and self-management. It seems likely from the literature that they are more at risk of felt stigma and this may be a consequence of the beliefs and behaviours held by significant others. We are currently exploring the views of people from this population.
We have highlighted the significantly higher costs of health services incurred by those of black ethnicity. If more investment, linked to specific concerns, is effective in improving self-management, there is scope for greater efficiency savings as well as patient benefits. In a time of unprecedented international migration, we propose that more qualitative research might be carried out on the specific attitudes, beliefs and needs of PWE from ethnic minorities, particularly when addressing them has the potential to reduce health inequalities.
Priority 5: evaluation of the needs of people with epilepsy who attend emergency departments in different areas of the UK
We planned our study in an area of social deprivation in the inner city and completed it before the NASH was published. 165 The NASH has highlighted wide variations in standards of care, with, in particular, poor follow-up, communication and services for PWE attending EDs across the UK. NASH calls for more in-depth research and there is some momentum for hospitals to address the problems identified across the NHS.
Our findings will generalise best to similar metropolitan populations in the UK and abroad. We do not know how people behave in rural areas, where, for example, a trip to the ED may take much longer. Because of this we plan to replicate the first stage of the study, describing quantitatively the characteristics of PWE who attend an ED in a rural area. This is in collaboration with local physician scientists and will be funded by Epilepsy Bereaved. We believe that, although there has been some quantitative research carried out on PWE attending EDs, more can be learned by using mixed methods, including in-depth qualitative and economic research. This requires more funding but may result in efficiency saving in the longer term, as well as improvement in patient outcomes.
Priority 6: development of interventions to manage anxiety and death anxiety for people with epilepsy and their carers
There remains scope for developing and testing other interventions. Specifically, interventions are needed to identify and manage anxiety and death anxiety, for example, using cognitive–behavioural therapy. The way in which risk is communicated to patients has been researched in depth in dealing with, for example, genetic conditions. 166 PWE are at higher risk of death. In addition, sudden loss of consciousness can evoke fear of imminent death. 68–71 In this context more research is needed to develop and test ways to explain risk of death and manage death anxiety among PWE and their significant others.
Priority 7: risk factors for emergency department attendance and risk of death in epilepsy: mortality prevention
At a time of recession and public spending restraint, research can be targeted towards efficiency savings, including prevention of recurrent and clinically unnecessary ED use. We have ourselves focused to some extent on this. However, during the study we identified complex factors that predict ED use. At the same time, deaths that are amenable to medical intervention by identifying and treating risk factors such as hypertension, or preventable by individual behaviour change or public health measures such as smoking cessation/prevention have also been targeted in the UK's NHS policies. 167 Mortality from all causes in the general population of England and Wales declined by 16% between 1993 and 2005; in contrast, mortality with epilepsy recorded as an underlying cause increased by 31% in males and 39% in females during this period. 168
Epilepsy is ranked as the fifth highest amenable cause of years of life lost before the age of 75 years for males and the eighth highest for females. 169 A cohort study of PWE dying over an 8-year period reported that 30% of them died of accidents (mostly drowning and burns), 23% died suddenly, 16% died in status epilepticus and 14% committed suicide. 170 An audit of sudden epilepsy-related deaths in the UK found a lack of communication between professionals and with families and estimated that 40% of adult and 60% of child deaths were potentially avoidable through improved care. 171 The 2012 UK NASH52 found that advice was not typically given to patients who had attended an ED or their carers on seizure management, nor was there referral at the time for assessment by the neurology team or for follow-up by a relevant specialist.
Large-scale epidemiological work might show that frequent use of EDs is a proxy for, or marker of, greater need and risk of death in epilepsy. Our study was small but one out of 85 recruited participants died in the follow-up year. At the same time our research group undertook a case–control study to quantify risks of death in epilepsy, based on the UK General Practice Research Database, which included 1.5 million registered medical patients. 22 The risk factors for mortality identified were recorded alcohol problems, a ‘missed’ prescription for anticonvulsant drugs, a history of injuries, treatment for depression and one or more seizures in the past year. Some of the risk factors identified for death in epilepsy are also predictors of frequent ED use. In the current study people with primarily alcohol problems were excluded as a different nurse specialist service is responsible for their management at hospital. However, the NASH study52 found that epilepsy with alcohol overuse was a frequent correlate of ED attendance. In the current study predictors of frequent attendance were lower confidence in managing epilepsy, a higher number of prescribed AEDs, more felt stigma, a higher number of baseline ED visits, greater seizure frequency and higher levels of depression and anxiety.
People with long-term neurological conditions have received continuing and linked-up care. GPs have acknowledged a lack of competence and confidence in epilepsy management. 172 Until recently there was no funding for epilepsy care in general practice to support systematic identification and monitoring. 173 We believe that there is a need for large-scale programme research to identify risk factors and service use across primary and secondary care, which may link up processes to the goal of reducing avoidable death. This will include large-scale epidemiological work linking data sets, the development and testing of interventions, and mixed quantitative, qualitative and economic evaluation.
Chapter 6 Conclusions
Six out of seven hospital admissions for PWE are unplanned and are the result of them attending an ED. 47 One in five PWE attend an ED each year. 24 We have presented here the first in-depth study of the characteristics and needs of PWE attending an ED and our study was exploratory in this context. We found that > 60% of PWE who do come to an ED reattend in the same year and one-third attend three or more times. The average total health- and social-care cost of ED attendees is £2210 per year, and 50% of this is consumed by ED attendance and the hospital use that ensues. Evidence from the 2012 UK-wide NASH52 indicates that two-thirds of hospital emergency attendances are not clinically necessary and result in no benefit for the ongoing care and self-management of PWE.
Nonetheless, we found that PWE who do attend the ED report lower confidence in managing epilepsy (less mastery), a higher number of prescribed AEDs, more felt stigma, a higher number of baseline ED visits, greater seizure frequency and higher levels of depression and anxiety. The perception of feeling stigmatised and having less mastery were consistently predictive of frequent attendance at the ED, suggesting that these people do have unmet needs. On interview, people with recurrent seizures were able to describe why a particular seizure led to use of hospital services. They reported that they did not always have someone nearby with sufficient knowledge, experience or confidence who could help them, be it a family member, friend or work colleague. Some participants reported that they or a significant other were influenced by fear of death, and indeed one participant did die during the trial's 12-month follow-up period.
The study was designed as a ‘natural’ comparison between a hospital that had two ENSs who could offer two appointments to people who attended the ED and two hospitals that did not have any ENSs. The main outcome measure was frequency of use of the ED and this did not change after the intervention. However, duration of hospital stay following ED attendance was reduced for the group who received the ENS-led self-management intervention. After adjusting for differences at baseline, the mean total service cost over the entire follow-up period was £2948 for the comparison group and £2202 for the intervention group. This was not statistically significantly different; however, the study was small and underpowered to show this.
Participants who at baseline reported having used the ED the most, perceived the most, benefit from the intervention on interview. They described the intervention as improving on their usual care by providing information about managing their epilepsy and an opportunity to talk about their feelings. Benefits that participants reported included improved emotional well-being, improved confidence in managing seizures and improved medication adherence.
We did not know patients' perceptions of the reasons for hospital attendance at the outset. If we had known we might have targeted the intervention towards increasing the knowledge, confidence and skills of family, friends and colleagues, in addition to those of PWE. Equally, the issues of stigma and death anxiety, highlighted by the study of ED attendees, were not known and might require a step-up intervention of longer duration.
Acknowledgements
The Study Management Group comprised all of the Principal Investigators, Professor Leone Ridsdale (Chief Investigator), Professor Laura Goldstein, Professor Myfanwy Morgan, Professor Paul McCrone and Mr Paul Seed, and the study research associate, Dr Adam Noble.
We would like to thank the Study Advisory Group, which comprised Professor Ann Jacoby (Independent Chair, Professor of Medical Sociology, Division of Public Health at the University of Liverpool), Mr Bernard Gage (Epilepsy Carer, London Epilepsy Project), Dr Greg Rogers (GP with Specialist Interest in Epilepsy, Bethesda Medical Centre in Margate) and Mr Tunji Lasoye (Head of Emergency Department, King's College Hospital NHS Foundation Trust), and Ms Sara Lailey (Epilepsy Nurse Specialist, Department of Neurology, King's College Hospital NHS Foundation Trust), Ms Cathy Queally (Epilepsy Nurse Specialist, Department of Neurology, King's College Hospital NHS Foundation Trust) and Mrs Mary-Jane Atkins (Expert User, National Society for Epilepsy), who were also involved in the delivery of the intervention.
We also thank Dr Ed Glucksman (Director of Trauma, Emergency & Acute Medicine, King's College Hospital NHS Foundation Trust), Mr Andrew Parfitt (Consultant, Emergency Department, Guy's and St Thomas' Hospital NHS Foundation Trust), Ms Nicola Leete (Emergency Department, King's College Hospital NHS Foundation Trust), Mr Nadeem Nayeem (Clinical Director, Emergency Department, University Hospital Lewisham) and Dr Giles Walker (Associate Specialist in Emergency Medicine and Gastroenterology, Emergency Department, University Hospital Lewisham) for their assistance in identifying participants; Ms Cheryl Virdi (MSc, Department of Clinical Neuroscience, Institute of Psychiatry, King's College London) for completing the qualitative interviews; Dr Robert Delamont for helping with validation of recruitment criteria; and Dr Anne Laybourne (Postdoctoral Research Associate) for her support in compiling the final report.
Finally, we are very grateful to the people who participated in the study for contributing their time, sharing their experiences with us and allowing us to advance knowledge of the treatment of epilepsy.
Contribution of authors
Leone Ridsdale (Professor of Neurology and General Practice) was the Chief Investigator and led in the conception and design of the study, the supervision and co-ordination of the study, the interpretation of the data and, with Adam Noble, the drafting of the final report. Laura Goldstein (Professor of Clinical Neuropsychology) was a Principal Investigator and contributed to the original protocol, co-supervised the study research associate and contributed to the final report. Myfanwy Morgan (Professor of Sociology of Health) was a Principal Investigator and contributed to the original protocol, supervised the qualitative study with patients, including data collection and analysis, and advised on the relevant sections of the report. Paul McCrone (Professor of Health Economics) was a Principal Investigator and contributed to the original protocol, supervised the collection of health economic data, analysed these data and drafted the relevant sections of the report. Paul Seed (Senior lecturer in Medical Statistics) was a Principal Investigator and contributed to the original protocol, provided statistical expertise and contributed to the draft final report. Adam Noble (Postdoctoral Research Associate) was responsible for setting up the study in the three centres, for participant recruitment and assessment, for data analysis and interpretation and, with Leone Ridsdale, led the writing of the draft final report.
This project was funded by the NIHR Service Delivery and Organisation programme (project number 08/1808/247). Service support costs and excess treatment costs were provided by NHS R&D. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of Health.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health.
Publications
Ridsdale L. The social causes of inequality in epilepsy and developing a rehabilitation strategy: a UK-based analysis. Epilepsia 2009;50:2175–9.
Burrell L, Noble AJ, Ridsdale L. Decision making by ambulance clinicians in London when managing patients with epilepsy: a qualitative study. Emerg Med J 2013;30:236–40. doi:10.1136/emermed-2011-200388.
Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. Characteristics of people with epilepsy who attend emergency departments: prospective study of metropolitan hospital attendees. Epilepsia 2012;53:1820–8.
Noble AJ, Goldstein LH, Seed P, Ridsdale L. The characteristics of emergency attendees for epilepsy in London hospitals. J Neurol Neurosurg Psychiatry 2012;83:e1. doi:10.1136/jnnp-2011-301993.95.
Noble AJ, Virdi C, Morgan M, Ridsdale L. A nurse-led self-management intervention for people who attend emergency departments with epilepsy: the patients' view. J Neurol 2013;260:1022–30. doi:10.1007/s00415-012-6749-2.
Ridsdale L, Virdi C, Noble AJ, Morgan M. Explanations given by people with epilepsy for using emergency medical services: a qualitative study. Epilepsy Behav 2012;25:529–33.
Parts of the above papers have been reproduced in this report with permissions.
Presentations relating to this research project
Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. The characteristics of emergency attendees for epilepsy in London hospitals. Poster presentation at the Association of British Neurologists, Gateshead, 4–7 October 2011.
Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. The characteristics of emergency attendees for epilepsy in London hospitals. Poster presentation at the World Congress of Neurology, Morocco, 12–17 November 2011.
Ridsdale L, Goldstein LH, Seed P, McCrone P, Morgan M, Noble AJ. What are characteristics of people attending emergency departments with epilepsy? Oral presentation at the Society of Academic Primary Care, Glasgow, February 2012.
Ridsdale L, Goldstein LH, Seed P, McCrone P, Morgan M, Noble AJ. What are characteristics of people attending emergency departments with epilepsy? Oral presentation at the European Neurological Society, Neurology Medical Congress, Prague, Czech Republic, 9–12 June 2012.
Noble AJ, Morgan M, Virdi C, Ridsdale L. Can a nurse intervention promote the self-management of patients who attend emergency departments with established epilepsy? A nested qualitative study. Poster presentation at the 10th European Congress on Epileptology, London, 30 September–4 October 2012.
Noble AJ, Goldstein LH, Seed P, Ridsdale L. The characteristics of emergency attendees for epilepsy in London hospitals. J Neurol Neurosurg Psychiatry 2012;83:e1. doi:10.1136/jnnp-2011–301993.95.
References
- Help and advice for living life well with a long term condition. London: Department of Health; 2009.
- Services for patients with epilepsy. London: Clinical Standards Advisory Group; 1999.
- Goldstein LH, Minchin L, Stubbs P, Fenwick PB. Are what people know about their epilepsy and what they want from an epilepsy service related?. Seizure 1997;6:435-42. http://dx.doi.org/10.1016/S1059-1311(97)80017-9.
- Jain P, Patterson VH, Morrow JI. What people with epilepsy want from a hospital clinic. Seizure 1993;2:75-8. http://dx.doi.org/10.1016/S1059-1311(05)80106-2.
- Moran N, Poole K, Bell G, Solomon J, Kendall S, McCarthy M, et al. NHS services for epilepsy from the patient's perspective: a survey of primary, secondary and tertiary care access throughout the UK. Seizure 2000;9:559-65. http://dx.doi.org/10.1053/seiz.2000.0451.
- Ridsdale L. The social causes of inequality in epilepsy and developing a rehabilitation strategy: a UK-based analysis. Epilepsia 2009;10:2175-9.
- The national service framework for long-term conditions. London: Department of Health; 2005.
- Stokes T, Shaw EJ, Juarez-Garcia A, Camosso-Stefinovic J, Baker R. Clinical guidelines and evidence review for the epilepsies: diagnosis and management in adults and children in primary and secondary care. London: Royal College of General Practitioners; 2004.
- Bradley PM, Lindsay B. Care delivery and self-management strategies for adults with epilepsy. Cochrane Database Syst Rev 2009;4. http://dx.doi.org/10.1002/14651858.CD006244.pub2.
- Ramaratnam S, Baker GA, Goldstein LH. Psychological treatments for epilepsy. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.CD002029.pub2.
- Mittan RJ. Psychosocial treatment programs in epilepsy: a review. Epilepsy Behav 2009;16:371-80. http://dx.doi.org/10.1016/j.yebeh.2009.08.031.
- Health and Social Care Information Centre . Hospital Episode Statistics Online 2009. Hospital Episode Statistics 2008–2009 n.d. www.hscic.gov.uk/pubs/hes0809 (accessed 3 October 2012).
- The NHS outcomes framework 2011/12. London: Department of Health; 2010.
- Amiel S, Beveridge S, Bradley C, Gianfrancesco C, Heller S, James P, et al. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746-9.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4093.
- Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the diabetes X-PERT programme makes a difference. Diabet Med 2006;23:944-54. http://dx.doi.org/10.1111/j.1464-5491.2006.01906.x.
- Barlow JH, Turner AP, Wright CC. A randomized controlled study of the Arthritis Self-Management Programme in the UK. Health Educ Res 2000;15:665-80. http://dx.doi.org/10.1093/her/15.6.665.
- Buszewicz M, Rait G, Griffin M, Nazareth I, Patel A, Atkinson A, et al. Self management of arthritis in primary care: randomised controlled trial. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38965.375718.80.
- ILAE/IBE/WHO Global Campaign Against Epilepsy . Epilepsy in the WHO European Region: Fostering Epilepsy Care in Europe n.d. www.ibe-epilepsy.org/downloads/EURO%20Report%20160510.pdf (accessed 3 October 2012).
- Ridsdale L, Robins D, Fitzgerald A, Jeffery S, McGee L. Epilepsy monitoring and advice recorded: general practitioners' views, current practice and patients' preferences. Br J Gen Pract 1996;46:11-4.
- Shohet C, Yelloly J, Bingham P, Lyratzopoulos G. The association between the quality of epilepsy management in primary care, general practice population deprivation status and epilepsy-related emergency hospitalisations. Seizure 2007;16:351-5. http://dx.doi.org/10.1016/j.seizure.2007.02.005.
- Ridsdale L, Charlton J, Ashworth M, Richardson M, Gulliford M. Epilepsy mortality and risk factors for death in epilepsy: a population-based study. Br J Gen Pract 2011;61:e271-8. http://dx.doi.org/10.3399/bjgp11X572463.
- Hauser WA, Kurland LT. Epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia 1975;16:1-66. http://dx.doi.org/10.1111/j.1528-1157.1975.tb04721.x.
- Hart YM, Shorvon SD. The nature of epilepsy in the general population. II. Medical care. Epilepsy Res 1995;21:51-8. http://dx.doi.org/10.1016/0920-1211(95)00008-X.
- Moran NF, Poole K, Bell G, Solomon J, Kendall S, McCarthy M, et al. Epilepsy in the United Kingdom: seizure frequency and severity, anti-epileptic drug utilization and impact on life in 1652 people with epilepsy. Seizure 2004;13:425-33. http://dx.doi.org/10.1016/j.seizure.2003.10.002.
- Jacoby A. Epilepsy and the quality of everyday life. Findings from a study of people with well-controlled epilepsy. Soc Sci Med 1992;34:657-66.
- Leidy NK, Elixhauser A, Vickrey B, Means E, Willian MK. Seizure frequency and the health-related quality of life of adults with epilepsy. Neurology 1999;53:162-6. http://dx.doi.org/10.1212/WNL.53.1.162.
- Stavem K, Loge JH, Kaasa S. Health status of people with epilepsy compared with a general reference population. Epilepsia 2000;41:85-90. http://dx.doi.org/10.1111/j.1528-1157.2000.tb01510.x.
- The global burden of disease: 2004 update. Geneva: World Health Organization; 2008.
- Kimiskidis VK, Triantafyllou NI, Kararizou E, Gatzonis S, Fountoulakis KN, Siatouni A, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry 2007;6. http://dx.doi.org/10.1186/1744-859X-6-28.
- Ridsdale L, Robins D, Fitzgerald A, Jeffery S, McGee L. Epilepsy in general practice: patients' psychological symptoms and their perception of stigma. Br J Gen Pract 1996;46:365-6.
- Jacoby A, Buck D, Baker G, McNamee P, Graham-Jones S, Chadwick D. Uptake and costs of care for epilepsy: findings from a UK regional study. Epilepsia 1998;39:776-86.
- Shohet C, Yelloly J, Bingham P, Lyratzopolous G. The association between the quality of epilepsy management in primary care, general practice population deprivation status and epilepsy-related emergency hospitalisations. Seizure 2007;16:351-5. http://dx.doi.org/10.1016/j.seizure.2007.02.005.
- The Information Centre . The Quality and Outcomes Framework 2003 4 n.d. www.ic.nhs.uk/services/qof (accessed 30 June 2013).
- Elhai JD, Calhoun PS, Ford JD. Statistical procedures for analysing mental health services data. Psychiatry Res 2008;160:129-36. http://dx.doi.org/10.1016/j.psychres.2007.07.003.
- Moore L, Deehan A, Seed P, Jones R. Characteristics of frequent attenders in an emergency department: analysis of 1-year attendance data. Emerg Med J 2009;26:263-7. http://dx.doi.org/10.1136/emj.2008.059428.
- Emtner M, Hedin A, Andersson M, Janson C. Impact of patient characteristics, education and knowledge on emergency room visits in patients with asthma and COPD: a descriptive and correlative study. BMC Pulm Med 2009;9. http://dx.doi.org/10.1186/1471-2466-9-43.
- Egede LE. Patterns and correlates of emergency department use by individuals with diabetes. Diabetes Care 2004;27:1748-50. http://dx.doi.org/10.2337/diacare.27.7.1748.
- Whiston S, Coyle B, Chappel D. Health needs assessment for long term neurological conditions in North East England. Stockton on Tees: North East Public Health Observatory; 2009.
- Morgan CLI, Ahmed A, Kerr MP. Social deprivation and prevalence of epilepsy and associated health usage. J Neurol Neurosurg Psychiatry 2000;69:13-7. http://dx.doi.org/10.1136/jnnp.69.1.13.
- Ashworth M, Seed P, Armstrong D, Durbaba S, Jones R. The relationship between social deprivation and the quality of primary care: a national survey using indicators from the UK Quality and Outcomes Framework. Br J Gen Pract 2007;57:441-8.
- Gladman JRF, Jones RG, Radford K, Walker E, Rothera I. Person-centred dementia services are feasible, but can they be sustained?. Age Ageing 2007;36:171-6. http://dx.doi.org/10.1093/ageing/afl161.
- Reuber M, Hattingh L, Goulding PJ. Epileptological emergencies in accident and emergency: a survey at St James's University Hospital, Leeds. Seizure 2000;9:216-20. http://dx.doi.org/10.1053/seiz.2000.0386.
- Majeed A, Bardsley M, Morgan D, O’sullivan C, Bindman AB. Cross sectional study of primary care groups in London: association of measures of socioeconomic and health status with hospital admission rates. BMJ 2000;321:1057-60. http://dx.doi.org/10.1136/bmj.321.7268.1057.
- Pugliatti M, Beghi E, Forsgren L, Ekman M, Sobocki P. Estimating the cost of epilepsy in Europe: a review with economic modeling. Epilepsia 2007;48:2224-33. http://dx.doi.org/10.1111/j.1528-1167.2007.01251.x.
- Jennum P, Gyllenborg J, Kjellberg J. The social and economic consequences of epilepsy: a controlled national study. Epilepsia 2011;52:949-56. http://dx.doi.org/10.1111/j.1528-1167.2010.02946.x.
- Bruce M, Griffiths C, Brock A. Trends in mortality and hospital admissions associated with epilepsy in England and Wales during the 1990s. Health Stat Q 2004;21:23-9.
- NHS reference costs 2008–09. London: Department of Health; 2010.
- The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. London: National Clinical Guideline Centre; 2011.
- The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. London: NICE; 2012.
- The Information Centre . The Quality and Outcomes Framework 2010 11 n.d. www.ic.nhs.uk/services/qof (accessed 30 June 2013).
- Pearson M, Marson T, Dixon P, Billington K. National audit of seizure management in hospitals report. St Elsewhere's: Clinical Report 2012. www.nashstudy.org.uk/Newsletters/St%20Elsewhere's%20NASH%20Report.pdf (accessed 3 October 2012).
- Ridsdale L, Morgan M, O’Connor C. Promoting self-care in epilepsy: the views of patients on the advice they had received from specialists, family doctors and an epilepsy nurse. Patient Educ Counsel 1999;37:43-7. http://dx.doi.org/10.1016/S0738-399(98)00094-9.
- Ridsdale L, Kwan I, Cryer C. Newly diagnosed epilepsy: can nurse specialists help? A randomized controlled trial. Epilepsia 2000;41:1014-19. http://dx.doi.org/10.1111/j.1528-1157.2000.tb00287.
- Kubler-Ross E, Kessler D. On grief and grieving. Finding the meaning of grief though the five stages of loss. New York, NY: Scribner; 2005.
- Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. The characteristics of emergency attendees for epilepsy in London hospitals n.d.
- Noble AJ, Baisch S, Mendelow AD, Allen L, Kane P, Schenk T. Post-traumatic stress disorder explains reduced quiality of life in subarachnoid hemorrhage patients in both the short- and long-term. Neurosurgery 2008;63:1095-105.
- Noble AJ, Baisch S, Covey J, Mukerji N, Nath F, Schenk T. Subarachnoid haemorrhage patients' fears of recurrence are related to the presence of post-traumatic stress disorder. Neurosurgery 2011;69:323-32.
- Komolafe MA, Sunmonu TA, Afolabi OT, Komolafe EO, Fabusiwa FO, Groce N, et al. The social and economic impacts of epilepsy on women in Nigeria. Epilepsy Behav 2012;24:97-101. http://dx.doi.org/10.1016/j.yebeh.2011.11.019.
- Ismail H, Wright J, Rhodes P, Small N. Religious beliefs about causes and treatment of epilepsy. Br J Gen Pract 2005;55:26-31.
- Morgan M, Gulliford M, Detels R, Beaglehole R, Lansang M, Gulliford M. Oxford textbook of public health. Oxford: Oxford University Press; 2009.
- Jarvie S, Espie CA, Brodie MJ. The development of a questionnaire to assess knowledge of epilepsy. 1. General knowledge of epilepsy. Seizure 1993;2:179-85. http://dx.doi.org/10.1016/S1059-1311(05)80125-6.
- Ridsdale L, Kwan I, Cryer C. The effect of a special nurse on patients' knowledge of epilepsy and their emotional state. Epilepsy Evaluation Care Group. Br J Gen Pract 1999;49:285-9.
- Adams K, Grenier AC, Corrigan JM. 1st Annual Crossing the Quality Chasm Summit: a focus on communities. Washington, DC: National Academic Press; 2004.
- The expert patient: a new approach to chronic disease management for the 21st century. London: Department of Health; 2001.
- Bodenheimer T, Wagner E, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA 2002;288:1909-14. http://dx.doi.org/10.1001/jama.288.15.1909.
- Wade D, de Jong B. Recent advances in rehabilitation. BMJ 2000;320:1385-8. http://dx.doi.org/10.1136/bmj.320.7246.1385.
- Hawthorne S. Bird. And other writings on epilepsy. Melbourne: Spinifex Press; 1999.
- Robinson R. Electricity. London: Picador; 2006.
- Wagner AT, Myers KR. Illness in the academy: a collection of pathographies by academics. West Lafayette, IN: Purdue University Press; 2007.
- Hustvedt S. The shaking woman or a history of my nerves. London: Sceptre; 2011.
- Ridsdale L. Matching the needs with skills in epilepsy care. BMJ 1995;310:1219-20. http://dx.doi.org/10.1136/bmj.310.6989.1219.
- Muir Gray JA. How to get better value healthcare. Oxford: Offox Press; 2011.
- May TW, Pfafflin M. The efficacy of an educational treatment program for patients with epilepsy (MOSES): results of a controlled, randomized study. Epilepsia 2002;43:539-49. http://dx.doi.org/10.1046/j.1528-1157.2002.23801.x.
- Helgeson DC, Mittan R, Tan SY, Chayasirisobhon. Sepulveda epilepsy education: the efficacy of a psychoeducational treatment program in treating medical and psychosocial aspects of epilepsy. Epilepsia 1990;31:75-82. http://dx.doi.org/10.1111/j.1528-1157.1990.tb05363.x.
- Olley BO, Osinowo HO, Brieger WR. Psycho-educational therapy among Nigerian adult patients with epilepsy: a controlled outcome study. Patient Educ Counsel 2001;42:25-33. http://dx.doi.org/10.1016/S0738-3991(00)00087-2.
- Pramuka M, Hendrickson R, Zinski A, Van Cott AC. A psychosocial self-management program for epilepsy: a randomized pilot study in adults. Epilepsy Behav 2007;11:533-45. http://dx.doi.org/10.1016/j.yebeh.2007.06.013.
- Ridsdale L, Robins D, Cryer C, Williams H. Feasibility and effects of nurse run clinics for patients with epilepsy in general practice: randomised controlled trial. Epilepsy Care Evaluation Group. BMJ 1997;314:120-2.
- Scambler A, Scambler G, Ridsdale L, Robins D. Towards an evaluation of the effectiveness of an epilepsy nurse in primary care. Seizure 1996;5:255-8. http://dx.doi.org/10.1016/S1059-1311(96)80017-3.
- Schull DE, Tosch P, Wood M. Clinical nurse specialists as collaborative care managers. Nurs Manag 1992;37:30-3. http://dx.doi.org/10.1097/00006247-199203000-00011.
- Tatum WO, Al-Saadi S, Orth TL. Outpatient case management in low-income epilepsy patients. Epilepsy Res 2008;82:156-61. http://dx.doi.org/10.1016/j.eplepsyres.2008.07.017.
- Department of Health . Case-Study 2: Emergency Admissions for Epilepsy in Children 2012. www.rightcare.nhs.uk/index.php/nhs-atlas/case-study-2-emergency-admissions-for-epilepsy-in-children/ (accessed 3 October 2012).
- Department for Communities and Local Government . English Indices of Deprivation 2010 n.d. www.gov.uk/government/publications/english-indices-of-deprivation-2010 (accessed 3 October 2012).
- Public Health England . Health Profiles 2010 Regional Profiles n.d. www.apho.org.uk/resource/view.aspx?RID=95272 (accessed 3 October 2012).
- Health and Social Care Information Centre . Disease Prevalence, Quality and Outcomes Framework (QOF) for April 2009 – March 2010, England 2010. www.hscic.gov.uk/catalogue/PUB05534 (accessed 3 October 2012).
- NHS Right Care . The NHS Atlas of Variation in Healthcare 2010. www.rightcare.nhs.uk/atlas/qipp_nhsAtlas-LOW_261110c.pdf (accessed 3 October 2012).
- Scott-Lennox J, Bryant-Comstock L, Lennox R, Baker GA. Reliability, validity and responsiveness of a revised scoring system for the Liverpool Seizure Severity Scale. Epilepsy Res 2001;44:53-6. http://dx.doi.org/10.1016/S0920-1211(01)00186-3.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77.
- Dilorio C, Escoffery C, McCarty F, Yeager K, Henry T, Koganti A, et al. Evaluation of WebEase: an epilepsy self-management web site. Health Educ Res 2009;24:185-97.
- Taylor J, Baker GA, Jacoby A. Levels of epilepsy stigma in an incident population and associated factors. Epilepsy Behav 2011;21:255-60. http://dx.doi.org/10.1016/j.yebeh.2011.04.002.
- Cramer JA, Arrigo C, Van Hammee G, Bromfield EB. Comparison between the QOLIE-31 and derived QOLIE-10 in a clinical trial of levetiracetam. Epilepsy Res 2000;41:29-38. http://dx.doi.org/10.1016/S0920-1211(00)00127-3.
- Cramer JA, Perrine K, Devinsky O, Meador K. A brief questionnaire to screen for quality of life in epilepsy: the QOLIE-10. Epilepsia 1996;37:577-82. h\ttp://dx.doi.org/10.1111/j.1528-1157.1996.tb00612.x.
- Department for Communities and Local Government . English Indices of Deprivation 2010 2011. www.communities.gov.uk/publications/corporate/statistics/indices2010 (accessed 3 October 2012).
- Commission on Classification and Terminology of the International League Against Epilepsy . Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389-99.
- Beecham J, Knapp M, Thornicroft G. Measuring mental health needs. London: Gaskell; 2001.
- Thapar A, Kerr M, Harold G. Stress, anxiety, depression, and epilepsy: investigating the relationship between psychological factors and seizures. Epilepsy Behav 2009;14:134-40. http://dx.doi.org/10.1016/j.yebeh.2008.09.004.
- Thapar AJ. Examining the Association Between Depression and Seizures Amongest Adults With Epilepsy: A Longitudinal Analysis 2009.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Jacoby A. Felt versus enacted stigma: a concept revisited: evidence from a study of people with epilepsy in remission. Soc Sci Med 1994;38:269-74.
- Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research. Qual Health Care 2001;10:135-40. http://dx.doi.org/10.1136/qhc.0100135.
- Wagner AK, Keller SD, Kosinski M, Baker GA, Jacoby A, Hsu MA, et al. Advances in methods for assessing the impact of epilepsy and antiepileptic drug therapy on patients' health-related quality of life. Qual Life Res 1995;4:115-34. http://dx.doi.org/10.1007/BF01833606.
- Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav 1978;19:2-21. http://dx.doi.org/10.2307/2136319.
- Ryan J, Nash S, Lyndon J. Epilepsy in the accident and emergency department – developing a code of safe practice for adult patients. South East and South West Thames Accident and Emergency Specialty Sub-committees. J Accid Emerg Med 1998;15:237-43. http://dx.doi.org/10.1136/emj.15.4.237.
- Kitson A, Shorvon S. Services for patients with epilepsy: a report of a CSAG Committee. London: Department of Health; 2000.
- Mackway-Jones K. Emergency triage: Manchester Triage Group. London: BMJ Publishing Group; 1997.
- NHS Information Centre for Health and Social Care . Quality and Outcomes Framework – 2009–10, Practice Level n.d. http://data.gov.uk/dataset/quality-and-outcomes-framework-2009-10-practice-level (accessed 3 October 2012).
- Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe – a systematic review. Eur J Neurol 2005;12:245-53. http://dx.doi.org/10.1111/j.1468-1331.2004.00992.x.
- Thapar AK. The Quality of Care and the Quality of Life of People With Epilepsy 2001.
- Thapar A, Rolanda M, Harold G. Do depression symptoms predict seizure frequency or vice versa?. J Psychosom Res 2005;59:269-74. http://dx.doi.org/10.1016/j.jpsychores.2005.04.001.
- Elliott J, Shneker B. Patient, caregiver, and health care practitioner knowledge of, beliefs about, and attitudes toward epilepsy. Epilepsy Behav 2008;12:547-56. http://dx.doi.org/10.1016/j.yebeh.2007.11.008.
- Fraser RT, Johnson EK, Miller JW, Temkin N, Barber J, Caylord L, et al. Managing epilepsy well: self-management needs assessment. Epilepsy Behav 2011;20:291-8. http://dx.doi.org/10.1016/j.yebeh.2010.10.010.
- National Society for Epilepsy . When to Dial 999 2012. www.epilepsysociety.org.uk/AboutEpilepsy/Firstaid/Whentodial999 (accessed 3 October 2012).
- Parmar MK, Machin D. Survival analysis – a practical approach. New York, NY: Wiley; 1995.
- Warren E, Baker GA, Campion P, Chadwick DR, Jacoby A, Luker K. An evaluation of a nurse specialist/case manager intervention in the management of epilepsy. Report for the North West Regional Health Authority, R&Amp;D Directorate 1998.
- Helde G, Bovim G, Bråthen G, Brodtkorb E. A structured, nurse-led intervention program improves quality of life in patients with epilepsy: a randomized, controlled trial. Epilepsy Behav 2005;7:451-7. http://dx.doi.org/10.1016/j.yebeh.2005.06.008.
- Mills N, Bachmann MO, Harvey I, McGowan M. Effect of a primary care-based epilepsy specialist nurse service on quality of care from the patient's perspective: quasi-experimental evaluation. Seizure 1999;8:1-7.
- Morgan M, Ridsdale L, Ridsdale L. Evidence-based Practice for Primary Care. Edinburgh: Churchill Livingstone; 1998.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Farhidvash F, Singh P, Abou-Khalil B, Arain A. Patients visiting the emergency room for seizures: insurance status and clinic follow-up. Seizure 2009;18:644-7. http://dx.doi.org/10.1016/j.seizure.2009.08.001.
- A framework for development and evaluation of RCTs for complex interventions to improve health. London: Medical Research Council; 2000.
- Curtis L. Unit Costs of Health and Social Care 2010 2010.
- Health and Social Care Information Centre . Prescription cost analysis England 2011. Health and Social Care Information Centre, Prescribing and Primary Care Services 2011. www.hscic.gov.uk/pubs/prescostanalysis2011 (accessed 3 October 2012).
- Office for National Statistics . Annual Survey of Hours and Earnings (ASHE) – 2011 Provisional Results 2012. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/2012-provisional-results/stb-ashe-statistical-bulletin-2012.htmlhttp://data.gov.uk/dataset/annual_survey_of_hours_and_earnings (accessed 3 October 2012).
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Dolan P, Gudex C, Kind P, Williams A. A social tariff for EuroQol: results from a UK general population survey. York: Centre for Health Economics, University of York; 1995.
- Casado V. Neurological patient care in emergency departments. A review of the current situation in Spain. Neurologia 2011;26:233-8. http://dx.doi.org/10.1016/S2173-5808(11)70047-5.
- de Falco FA, Sterzi R, Toso V, Consoli D, Guidetti D, Provinciali L, et al. The neurologist in the emergency department. An Italian nationwide epidemiological survey. Neurol Sci 2008;29:67-75. http://dx.doi.org/10.1007/s10072-008-0864-y.
- Royl G, Ploner CJ, Möckel M, Leithner C. Neurologische Leitsymptome in einer Notaufnahme. Nervenarzt 2010;81:1226-30. http://dx.doi.org/10.1007/s00115-010-3020-x.
- Bautista RE, Glen ET, Wludyka PS, Shetty NK. Factors associated with utilization of healthcare resources among epilepsy patients. Epilepsy Res 2008;79:120-9. http://dx.doi.org/10.1016/j.eplepsyres.2008.01.003.
- Baker GA, Brooks J, Buck D, Jacoby A. The stigma of epilepsy: a European perspective. Epilepsia 1999;41:98-104. http://dx.doi.org/10.1111/j.1528-1157.2000.tb01512.x.
- Hunt N, Touquet VL. Known epileptic patients brought to the accident and emergency department. J R Coll Gen Pract 1986;36:224-5.
- Jarvie S. Self Perception and Psychosocial Functioning in People With Intractable Epilepsy 1993.
- Buck D, Jacoby A, Baker GA, Chadwick DW. Factors influencing compliance with antiepileptic drug regimes. Seizure 1997;6:87-93. http://dx.doi.org/10.1016/S1059-1311(97)80060-X.
- Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia 2008;49:446-54. http://dx.doi.org/10.1111/j.1528-1167.2007.01414.x.
- Faught E, Duh MS, Weiner JR, Guerin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology 2008;71:1572-8. http://dx.doi.org/10.1212/01.wnl.0000319693.10338.b9.
- Baxendale S, O’Toole A. Epilepsy myths: alive and foaming in the 21st century. Epilepsy Behav 2007;11:192-6. http://dx.doi.org/10.1016/j.yebeh.2007.04.019.
- Otoom S, Al-Jishi A, Montgomery A, Ghwanmeh M, Atoum A. Death anxiety in patients with epilepsy. Seizure 2007;16:142-6. http://dx.doi.org/10.1016/j.seizure.2006.10.014.
- Akinsulore A, Adewuya A. Psychosocial aspects of epilepsy in Nigeria: a review. Afr J Psychiatry 2010;13:351-6. http://dx.doi.org/10.4314/ajpsy.v13i5.63100.
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325.
- Lacey CJ, Salzberg MR, Roberts H, Trauer T, D’souza WJ. Psychiatric comorbidity and impact on health service utilization in a community sample of patients with epilepsy. Epilepsia 2009;50:1991-4. http://dx.doi.org/10.1111/j.1528-1167.2009.02165.x.
- Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. Characteristics of people with epilepsy who attend emergency departments: prospective study of metropolitan hospital attendees. Epilepsia 2012;53:1820-8. doi:10.1111/j.1528-1167.2012.03586.x.
- Pugh MJV, Copeland LA, Zeber J, Cramer JA, Amuan ME, Cavazos JE, et al. The impact of epilepsy on health status among younger and older adults 2005;46:1820-7. http://dx.doi.org/10.1111/j.1528-1167.2005.00291.x.
- Ridsdale L, Virdi C, Noble A, Morgan M. Explanations given by people with epilepsy for using emergency medical services: a qualitative study. Epilepsy Behav 2012;25:529-33. http://dx.doi.org/10.1016/j.yebeh.2012.09.034.
- Snow DG, Jackson SH, Skinner D, Burton RM, Williams AH. Do patients presenting to accident and emergency departments have low serum anticonvulsant concentrations?. Arch Emerg Med 1991;8:41-4. http://dx.doi.org/10.1136/emj.8.1.41.
- Burrell L, Noble A, Ridsdale L. Decision-making by ambulance clinicians in London when managing patients with epilepsy: a qualitative study. Emerg Med J 2013;30:236-40. doi:10.1136/emermed-2011-200388.
- Mosenifar Z. Population issues in clinical trials. Proc Am Thorac Soc 2007;4:185-8. http://dx.doi.org/10.1513/pats.200701-009GC.
- Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol 2007;64:1595-9. http://dx.doi.org/10.1001/archneur.64.11.1595.
- Colombo F, Tapay N. Paris: OECD; 2004.
- Tomlinson B. Report of the inquiry into London's health serice, medical education, and research. London: HM Stationary Office; 1992.
- Ioannidis JPA, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tekonidou MG, et al. Comparison of evidence of treatment effects in randomized and non-randomized studies. JAMA 2001;286:821-30.
- D’Agostino RBS, Kwan H. Measuring effectiveness: what to expect without a randomised control group. Med Care 1995;33:AS95-105.
- Tapp S, Lasserson TJ, Rowe B. Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database Syst Rev 2007;3. http://dx.doi.org/10.1002/14651858.CD003000.pub2.
- Treweek S, Pitkethly M, Cook J, Kjeldstrom M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010;4. http://dx.doi.org/10.1002/14651858.MR000013.pub4.
- Canvin K, Jacoby A. Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials 2006;7.
- Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess 2005;9.
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies?. Arch Dis Child 2008;93:458-61. http://dx.doi.org/10.1136/adc.2007.127316.
- Smith G, Ferguson PL, Saunders LL, Wagner JL, Wannamaker BB, Selassie AW. Psychosocial factors associated with stigma in adults with epilepsy. Epilepsy Behav 2009;16:484-90. http://dx.doi.org/10.1016/j.yebeh.2009.08.028.
- Austin JK, Dunn DW, Perkins SM, Shen J. Youth with epilepsy: development of a model of children's attitudes toward their condition. Child Health Care 2006;35:123-40. http://dx.doi.org/10.1207/s15326888chc3502_2.
- Huppert JD, Bufka LF, Barlow DH, Gorman JM, Shear MK, Woods SW. Therapists, therapist variables, and cognitive-behavioral therapy outcome in a multicenter trial for panic disorder. J Consult Clin Psychol 2001;69:747-55. http://dx.doi.org/10.1037//0022-006X.69.5.747.
- Elwyn G, Todd S, Hibbs R, Thapar A, Edwards P, Webb A, et al. A ‘real puzzle’: the views of patients with epilepsy about the organisation of care. BMC Fam Pract 2003;4.
- Ross J, Stefan H, Schauble B, Day R, Sander JW. European survey of the level of satisfaction of patients and physicians in the management of epilepsy in general practice. Epilepsy Behav 2010;19:36-42. http://dx.doi.org/10.1016/j.yebeh.2010.06.002.
- Wall M, Buchanan N, Baird-Lambert JA. The management of epilepsy: patients' perceptions and expectations. Med J Aust 1987;146:473-6.
- Calsyn RJ, Allen G, Morse GA, Smith R, Tempelhoff B. Can you trust self-report data provided by homeless mentally ill individuals?. Eval Rev 1993;17:353-66. http://dx.doi.org/10.1177/0193841X9301700306.
- Goldberg RW, Seybolt DC, Lehman A. Reliable self-report of health service use by individuals with serious mental illness. Psychiatr Serv 2002;53:879-81. http://dx.doi.org/10.1176/appi.ps.53.7.879.
- Pearson M, Marson T, Dixon P, Billington K. National audit of seizure management in hospitals report. St Elsewhere'S: Clinical Report 2012.
- Marteau TM. Communicating genetic risk information. Br Med Bull 1999;55:414-28. http://dx.doi.org/10.1258/0007142991902466.
- Wheller L, Baker A, Griffiths C, Rooney C. Trends in avoidable mortality in England and Wales, 1993–2005. Health Stat Q 2007;34:6-25.
- Office for National Statistics . Mortality Statistics: General Review of the Register General on Deaths in England and Wales 2005. www.statistics.gov.uk/downloads/theme_health/Dh1_38_2005/DH1_No_38.pdf (accessed 4 April 2011).
- Page A, Tobias M, Glover J. Australian and New Zealand atlas of avoidable mortality. Adelaide: University of Adelaide; 2006.
- Sander JW, Bell GS. Reducing mortality: an important aim of epilepsy management. J Neurol Neurosurg Psychiatry 2004;75:349-51. http://dx.doi.org/10.1136/jnnp.2003.029223.
- Hanna JN, Black M, Sander JW, Smithson WH, Appleton R, Fish DR. National sentinel clinical audit of epilepsy-related death. London: The Stationery Office; 2002.
- Thapar AK, Stott NCH, Richens A, Kerr M. Attitudes of GPs to the care of people with epilepsy. Fam Pract 1998;15:437-42. http://dx.doi.org/10.1093/fampra/15.5.437.
- Ridsdale L. No more neurophobia: welcome neurology in general practice. Br J Gen Pract 2009;59:567-9. http://dx.doi.org/10.3399/bjgp09X453756.
Appendix 1 Protocol
Version 4
Can a nurse-led self-management intervention reduce attendance at A&E and promote wellbeing for people with severe epilepsy?
The objective of this study is to test whether nurse led rehabilitation is more cost effective in meeting the needs of people with poorly controlled epilepsy as compared to usual care and is an evaluation of clinical effectiveness and cost effectiveness of services at two hospitals.
85 patients with acute epilepsy attacks will be recruited from (A) King's College Hospital and (B) Guy's and St Thomas' Foundation Trust and University Hospital Lewisham (approximately 50% from site A and 50% from sites B).
Inclusion criteria
-
Aged 18 and over.
-
Have epilepsy that has been diagnosed and treated with medication.
-
Be able to communicate in English sufficiently to complete questionnaires.
-
Be resident and registered with a GP in one of the three surrounding PCTs: Southwark, Lambeth and Lewisham.
Exclusion criteria
-
Having no diagnosis; new epilepsy leads to a referral to a neurologist.
-
Having already seen a specialist nurse for epilepsy in the prior year.
-
Alcohol or other substance misuse.
-
Other severe medical illness, such as psychosis or terminal cancer.
Patients attending the A&E sites due to epilepsy will be identified by weekly computerised searches of the hospital admission data-bases and with the assistance of the consultants Drs. Ed Glucksman, Tunji Lasoye and Nadeem Nayeem from King's College Hospital, Guys and St. Thomas' Hospital and University Hospital Lewisham A&E departments. The experienced research associate working on this project will send an invitation letter to those patients who have been identified as suitable. Attached to this letter will be the study information letter and an ‘expression of interest’ reply slip and pre-paid return envelope. The research associate will then explain the study in depth with any patients who express an interest. Written consent to participate will be taken by the research associate.
The Research Associate will coordinate the baseline and the follow-up data collection to achieve participation and collaboration throughout. Two competent and qualified nurses with a special interest in epilepsy will work with patients from the intervention group in order that they may be referred to specialist rehabilitation workers from a multidisciplinary team.
Baseline variables and questionnaires will be administered to all patients:
-
Age
-
Gender
-
Racial/ethnic group
-
Economic (Index of Multiple Deprivation for postcode) [1]
-
Quality of routine medical care (epilepsy components of Quality Outcomes Framework - QOF) in particular for general practices [2]
-
Liverpool Quality of life questionnaire [3]
-
A knowledge of epilepsy questionnaire [4]
-
Psychological distress measured using the Hospital Anxiety and Depression Scale (HADS) [5], and Stigma Scale [6].
-
Satisfaction with information on medications (SIMS) [7].
-
Medication management skills [8].
-
Mastery/confidence in managing epilepsy [9]
-
Quality of life in epilepsy measured by the 10-item Quality of Life in Epilepsy questionnaire QUOLIE-10 [10].
-
Quality-adjusted life years (QALYs) will be measured with the EQ-5D [11].
-
General and epilepsy-related service use in the past 6 or 12 months measured using a version of the Client Services Receipt Inventory (CSRI), modified for use in epilepsy [12].
Intervention Group
An appointment with a nurse with special interest in epilepsy will be offered after the patent has been discharged following an A&E attendance, including access to other members of the multidisciplinary team, and an expert-user group, in addition to usual medical care. The nurse will assess the patient identifying the nature and extent of their problems and the factors relevant to their resolution. Family and or carers will be included, provided the patient agrees. The nurse will help the patient set goals, provide information and support. They will also be offered specific treatments for example to social services, psychology, neurology, occupational therapy, an employment officer, the learning disability team. Further input will be as judged by nurse and client, but will include at least one follow-up appointment three months later. The duration of the intervention will be 3–6 months.
Comparison Group
The comparison group will be offered the usual medical care where no special epilepsy nurse is available and will receive usual medical care.
The baseline questionnaires will be repeated at 6 and 12 month follow up and additional measures taken:
-
Primary outcome will be re-attendance at A&E.
-
Secondary outcome with be unplanned readmissions to hospital for epilepsy.
-
A cost analysis of the above.
-
Seizure frequency and severity, the impact of epilepsy, perceived mastery, and medication management skills.
Nested Qualitative interview
A purposeful sample of those respondents' with a range of scores on the Seizure Severity Scale will be interviewed at either their home or a mutually convenient public place. Questions will cover:
-
Reasons for attendance at A&E and effects of the circumstance in which the seizure occurred and carers concerns and decision making.
-
Patients' views and satisfaction with the ways in which these interventions met or failed to meet their needs.
-
Specific benefits and limitations of the intervention examined in detail with particular reference to patients' perceptions of epilepsy.
-
Views of and adherence of patients' epilepsy medications and the extent to which the intervention met its objectives of enhancing respondents' feelings of empowerment, support and control in managing epilepsy.
Service Costs
-
Data on consultations with nurses and other staff.
-
Record the number of days off work due to health problems and specifically epilepsy.
The evidence produced from this study will be useful to inform practice improvement/development in deprived areas and has the potential to promote well being for people with epilepsy.
References
- Department for Communities and Local Government (2011) . English Indices of Deprivation 2010 n.d. URL: https://www.gov.uk/government/publications/english-indices-of-deprivation-2010 (accessed 3/10/2012).
- NHS Information Centre for Health and Social Care (2010) . Quality and Outcomes Framework – 2009-10, Practice Level n.d. URL: http://data.gov.uk/dataset/quality-and-outcomes-framework-2009-10-practice-level (accessed 3/10/2012).
- Baker GA, Smith DF, Dewey M, Jacob A, Chadwick DW. The initial development of a health-related quality of life model as an outcome measure in epilepsy. Epilepsy Research 1993;16:65-81.
- Jarvie S, Espie CA, Brodie MJ. The development of a questionnaire to assess knowledge of epilepsy: 1 – General knowledge of epilepsy. Seizure 1993;2:179-85.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70.
- Jacoby A. Felt versus enacted stigma: a concept revisited: evidence from a study of people with epilepsy in remission. Social Science &Amp; Medicine 1994;38:269-74.
- Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research. Quality in Health Care 2001;10:135-40.
- DiIorio C, Hennessy M, Manteuffel B. Epilepsy self-management: a test of a theoretical model. Nurs Res 1996;45:211-7.
- Wagner AK, Keller SD, Kosinski M, Baker GA, Jacoby A, . Advances in methods for assessing the impact of epilepsy and antiepileptic drug therapy on patients' health-related quality of life. Qual Life Res 1995;4:115-34.
- Cramer JA, Perrine K, Devinsky O, Meador K. A brief questionnaire to screen for quality of life in epilepsy: the QOLIE-10. Epilepsia 1996;37:577-82.
- Williams A. The role of the EUROQOL instrument in QALY calculations. York: Centre for Health Economics, University of York; 1995.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell; 1992.
Appendix 2 Epilepsy nurse specialist-led self-management intervention checklist
Epilepsy nurse specialist-led self-management intervention checklist (PDF download)
Appendix 3 Composite participant questionnaire pack
Appendix 4 Outputs
References
- Burrell L, Noble A, Ridsdale L. Decision-making by ambulance clinicians in London when managing patients with epilepsy: a qualitative study. Emerg Med J 2013;30:236-40. URL: doi:10.1136/emermed-2011-200388.
- Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. The characteristics of emergency attendees for epilepsy in London hospitals n.d.
- Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. The characteristics of emergency attendees for epilepsy in London hospitals n.d.
- Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. Characteristics of people with epilepsy who attend emergency departments: prospective study of metropolitan hospital attendees. Epilepsia 2012;53:1820-8. URL: doi:10.1111/j.1528-167.2012.03586.x.
- Noble AJ, Virdi C, Morgan M, Ridsdale L. Can a nurse intervention promote the self-management of patients who attend emergency departments with established epilepsy? A nested qualitative study n.d.
- Noble AJ, Virdi C, Morgan M, Ridsdale L. Can a nurse intervention promote the self-management of patients who attend emergency departments with established epilepsy? A nested qualitative study n.d.
- Ridsdale L. The social causes of inequality in epilepsy and developing a rehabilitation strategy: a UK-based analysis. Epilepsia 2009;50:2175-9.
- Ridsdale L, Alarcón G, Valentín A. Introduction to epilepsy. Cambridge: Cambridge University Press; 2012.
- Ridsdale L. Characteristics of people with epilepsy who attend emergency departments: prospective study of metropolitan hospital attendees n.d.
Appendix 5 Capacity development
References
- Burrell L, Noble A, Ridsdale L. Decision-making by ambulance clinicians in London when managing patients with epilepsy: a qualitative study. Emerg Med J 2013;30:236-40.
- Sheikh Faiza. A Study on Self-Management of Epilepsy Among Western and Non-Western African Patients in South London 2012.
- Virdi Cheryl. Can Nurse-Led Rehabilitation Reduce A&Amp;E Attendance and Promote Well-Being Amongst People With Severe Epilepsy? 2011.
- Nadeem Farah. Migraine Study 2011.
- AlNaffakh Mahmoud. Epilepsy and A&Amp;E Attendance 2009.
List of abbreviations
- AED
- antiepileptic drug
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CSRI
- Client Services Receipt Inventory
- ED
- emergency department
- ENS
- epilepsy nurse specialist
- EQ-5D
- European Quality of Life-5 Dimensions
- GCSE
- General Certificate of Secondary Education
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- ILAE
- International League Against Epilepsy
- IQR
- interquartile range
- IRR
- incidence rate ratio
- KCH
- King's College Hospital
- MOSES
- Modular Service Package Epilepsy
- NASH
- National Audit of Seizure Management in Hospitals
- NBR
- negative binomial regression
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PWE
- people with epilepsy
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- QoL
- quality of life
- SD
- standard deviation
- STH
- St. Thomas' Hospital
- TAU
- treatment as usual
- UHL
- University Hospital Lewisham