Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/115/82. The contractual start date was in August 2015. The draft report began editorial review in February 2021 and was accepted for publication in August 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Maheshwari et al. This work was produced by Maheshwari et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Maheshwari et al.
Chapter 1 Introduction
The study operated to a strict pre-agreed protocol, which has been published. 1
Parts of this chapter have been reproduced with permission from the published protocol, Maheshwari et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Infertility is common, affecting one in seven couples in the UK. 2 The National Institute for Health and Care Excellence (NICE) recommends in vitro fertilisation (IVF) as the definitive treatment for prolonged unresolved infertility. 3 The number of IVF treatments in the UK has continued to rise each year, from 6609 in 1999 to > 64,000 in 2013, resulting in > 20,000 pregnancies. 4
In vitro fertilisation
In vitro fertilisation treatment involves a number of consecutive steps. Initially, each woman is given external hormone injections to develop multiple ovarian follicles. The growth of these follicles is monitored by serial transvaginal ultrasound scans and, when these follicles reach maturity, the eggs within them are harvested surgically. Retrieved eggs are mixed with sperm by one of two methods: IVF, where motile sperm are placed surrounding the eggs, or intracytoplasmic sperm injection (ICSI), where a single sperm is selected and injected into the egg. Eggs mixed with sperm are then incubated to create embryos. Conventionally, these embryos are allowed to develop in the laboratory for a few days before one or two of them are selected for transfer into the uterus (i.e. fresh-embryo transfer) on day 3 (the cleavage stage) or day 5 (the blastocyst stage). Additional embryos are frozen and stored for replacement at a later date without the need for ovarian stimulation (i.e. frozen-embryo transfer).
Concerns with in vitro fertilisation
Despite being a widely used treatment in the UK and around the world, there are a number of concerns about conventional IVF.
Static success rates
In vitro fertilisation success rates remain modest, with a mean live birth rate of 25% per treatment involving a fresh-embryo transfer. Data for three consecutive years (2010 to 2012) from the American4 and European registries5 suggest that there was no improvement in IVF live birth rates over the 3-year period.
Ovarian hyperstimulation syndrome
Exogenous hormones used for ovarian stimulation are associated with a risk of ovarian hyperstimulation syndrome (OHSS), which is exacerbated if a woman becomes pregnant following fresh-embryo transfer. Moderate to severe OHSS is a complication unique to IVF treatment, occurring in around 1–5% of treatments,6 and often requiring in-patient care, resulting in significant NHS costs. Severe OHSS is associated with significant morbidity (including ascites, pleural and pericardial effusion, respiratory failure and intensive care admission) and, rarely, death.
Poor obstetric and perinatal outcomes
Pregnancies resulting from IVF are associated with a higher rate of maternal and perinatal complications than pregnancies resulting from spontaneous conception. A systematic review7 has shown that babies, even singletons, conceived following IVF are more likely than babies conceived without IVF treatment to die during the perinatal period [risk ratio (RR) 1.87, 95% confidence interval (CI) 1.48 to 2.37], to be delivered preterm (RR 1.54, 95% CI 1.47 to 1.62), to have a low birthweight (RR 1.65, 95% CI 1.56 to 1.75) and to have congenital anomalies (RR 1.67, 95% CI 1.33 to 2.09). Women who become pregnant as a result of IVF are more likely than those who become pregnant as a result of spontaneous conception to develop pre-eclampsia (RR 1.49, 95% CI 1.39 to 1.59), bleeding in pregnancy (RR 2.49, 95% CI 2.30 to 2.69) and diabetes (RR 1.48, 95% CI 1.33 to 1.66) and to require a caesarean section (RR 1.56, 95% CI 1.51 to 1.60).
Although the absolute number of women with OHSS and pregnancy-related complications associated with IVF is relatively small, the increasing number of women receiving IVF4 has meant that the NHS burden of dealing with its short- and long-term complications is a serious and growing problem.
A possible cause of suboptimal live birth rates, as well as adverse maternal and perinatal outcomes, following IVF is the impact of the exogenous hormones used for ovarian stimulation on the lining of the uterine cavity. High levels of oestrogen produced by the ovary in response to this treatment affect uterine receptivity, reducing the chances of successful implantation and placentation. Suboptimal placentation may lead to obstetric and perinatal complications. It has been suggested that avoiding embryo transfer when the uterus is less receptive could improve success rates and reduce complications in pregnancy and delivery. Such a strategy also reduces the risk of OHSS by ensuring that a pregnancy does not occur in the presence of hyperstimulated ovaries.
Evidence supporting frozen-embryo transfer
It is already known that the risk of severe OHSS is greatly reduced by a policy of freezing all embryos, followed by frozen-embryo transfer, compared with fresh-embryo transfer. 8 A systematic review of observational data9 showed that babies (singletons) conceived from frozen embryos have a reduced risk of perinatal morbidity (RR 0.68, 95% CI 0.48 to 0.96) and preterm delivery (RR 0.84, 95% CI 0.78 to 0.90), making IVF safer and more effective for women and babies.
Preliminary data from small randomised trials from the Islamic Republic of Iran10 and the USA11,12 in 2015 suggested that a strategy of not replacing embryos when they are created, but freezing them, followed by transferring thawed embryos into the uterus at a later date, improves pregnancy rates. A meta-analysis of data from these three randomised controlled trials (RCTs)8 has shown higher pregnancy rates following frozen-embryo transfer (odds ratio 1.32, 95% CI 1.10 to 1.59).
However, these existing trials have a number of significant limitations:
-
They reported implausibly high pregnancy rates (e.g. 84% per embryo transfer), which are far in excess of those reported by national and international registries. 4,5
-
Key outcomes, including healthy baby, live birth, costs, safety and acceptability, were not measured by any of the trials.
-
They were limited in terms of design, with highly selected populations, inadequate sample sizes and per-protocol analysis rather than intention to treat (ITT) and conduct, as all of the trials involved co-interventions that were not accounted for in the analysis.
One of the publications10 has been retracted on the grounds of serious methodological flaws. Hence, the evidence base, comprising two small trials, was not sufficiently robust to support a radical change in clinical practice. In addition, the results of these trials could not be directly applied to a UK setting because of very different regulatory and funding arrangements. There was, therefore, an urgent need to perform a definitive RCT in the UK evaluating elective freezing of embryos, followed by subsequent thawed frozen-embryo transfer, in terms of clinical effectiveness and cost-effectiveness.
Objective
Primary objective
The primary objective of the trial was to determine if a policy of freezing embryos, followed by thawed frozen-embryo transfer, results in a higher healthy baby rate than the current policy of transferring fresh embryos.
Secondary objectives
The secondary objectives of the trial were to assess if a policy of freezing embryos, followed by thawed frozen-embryo transfer, compared with the current policy of transferring fresh embryos, results in:
-
fewer complications associated with IVF treatment and pregnancy
-
greater cost-effectiveness from a health service and broader societal perspective.
Chapter 2 Methods
The study operated to a strict pre-agreed protocol1 and statistical analysis plan (SAP),13 both of which have been published.
Parts of this chapter have been reproduced with permission from the published protocol, Maheshwari et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter have also been reproduced with permission from the SAP, Bell et al. 13 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Design
The elective freeze (E-Freeze) trial was a pragmatic, multicentre, two-arm, parallel-group, non-blinded RCT conducted in the UK, comparing the freezing of all suitable embryos, followed by frozen-embryo transfer, with the current policy of fresh-embryo transfer. We undertook both clinical effectiveness and economic analysis. Details of the economic analysis are reported in Chapter 4.
Ethics approval and research governance
The E-Freeze trial protocol was approved by the North of Scotland Research Ethics Service (NoSRES) Committee (study reference 15/NS/0114). Local approval and site-specific assessments were obtained from each participating site. The trial was registered with the International Standard Randomised Controlled Trial Number (ISRCTN) Registry as ISRCTN61225414.
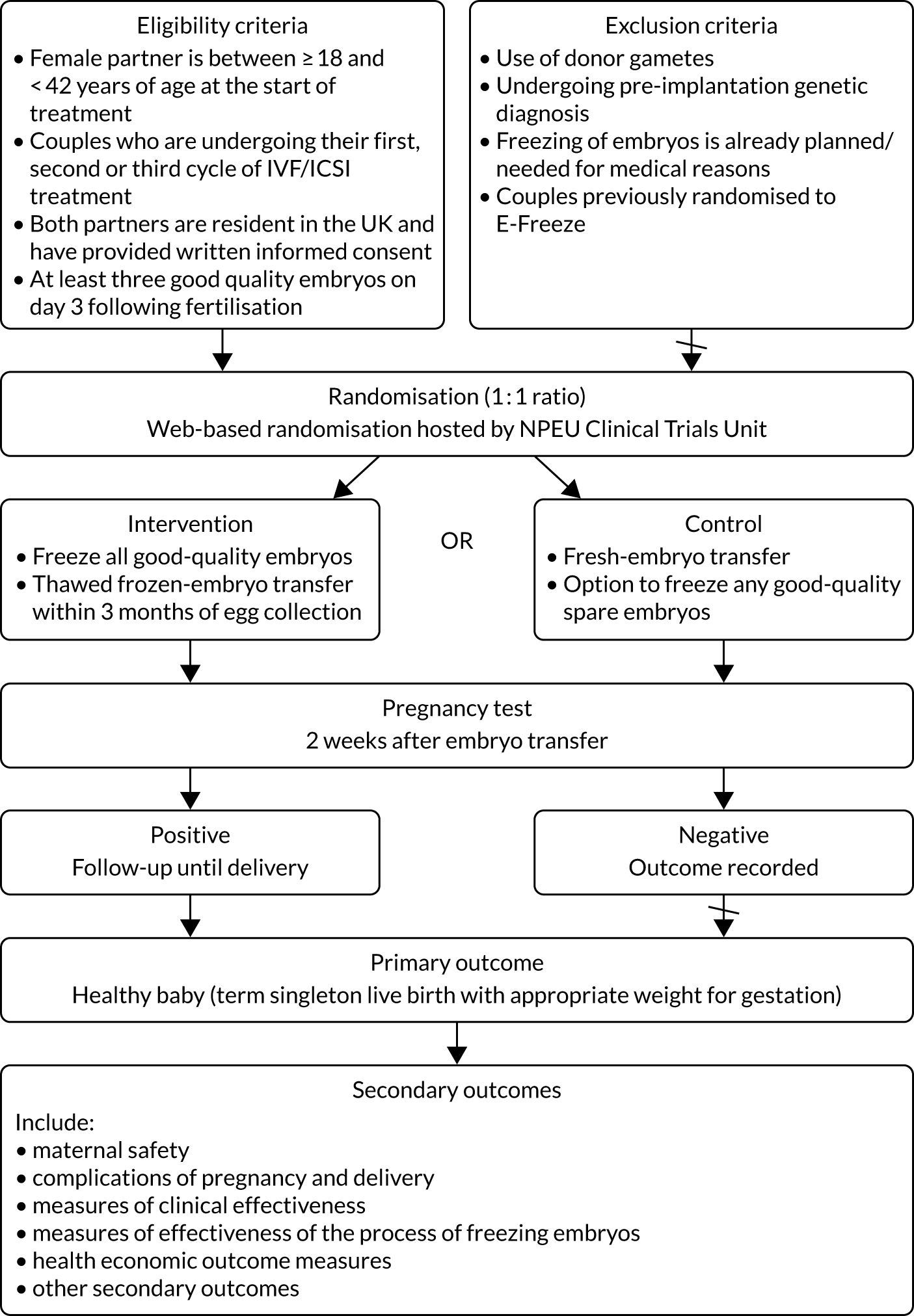
Participants
Participants were couples undergoing their first, second or third cycle of IVF/ICSI treatment in the participating clinics in the UK.
Inclusion criteria
-
The female partner was aged between ≥ 18 and < 42 years at the start of treatment (i.e. start of ovarian stimulation).
-
Couples were undergoing their first, second or third cycle of IVF/ICSI treatment, where a cycle is defined as egg collection following ovarian stimulation.
-
Both partners were resident in the UK.
-
Both partners were able to provide written informed consent.
-
They had at least three good-quality embryos [as defined by the Association of Clinical Embryologists (ACE)14] on day 3 after egg collection (note that the day of egg collection is counted as day 0). Good-quality embryos on day 3 were defined as those with 6–8 cells of grade 3/3 or above using the agreed national grading scheme. 14
At the start of the trial, only the first cycle was included. However, after discussion with the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) Monitoring Board, it was agreed that couples having their second or third cycle could also be included. This change to the eligibility criteria took effect from 12 April 2018.
A list of all amendments are described in Appendix 1.
Exclusion criteria
-
Couples were using donor gametes.
-
Pre-implantation genetic testing was planned.
-
Elective freezing of all embryos was planned for medical reasons (e.g. severe risk of OHSS).
-
Couples had been previously randomised to E-Freeze.
Setting
The trial was conducted in 18 IVF units across the UK. A list of all participating sites is presented in Appendix 2.
Participant selection and enrolment
Identifying participants
Potentially eligible couples were identified from clinic case notes. An invitation letter and participant information leaflet (PIL) were mailed to eligible couples prior to their clinic appointment. A PIL was also provided at patient information/open evenings attended by couples preparing for their IVF/ICSI treatment. This was usually at least 24 hours prior to their clinic appointment. Eligible couples were approached by a clinician involved in their care and were invited to participate in the trial. Those interested in participating were able to discuss the study with a research nurse on the same day or at a later date.
Consenting participants
Informed consent from both partners was obtained by an appropriately delegated member of the study team. Contact details and baseline characteristics that were necessary for randomisation were recorded by the research nurse immediately after consent was obtained. Consent forms were signed by both partners; however, this could be undertaken at two different time points, as not all appointments were attended by both partners. This could be undertaken at their clinic appointment or at a subsequent visit until the procedure of egg collection; all consent forms had to be signed before the procedure of egg collection took place (Figure 1).
FIGURE 1.
Process and timescale for consent and randomisation.
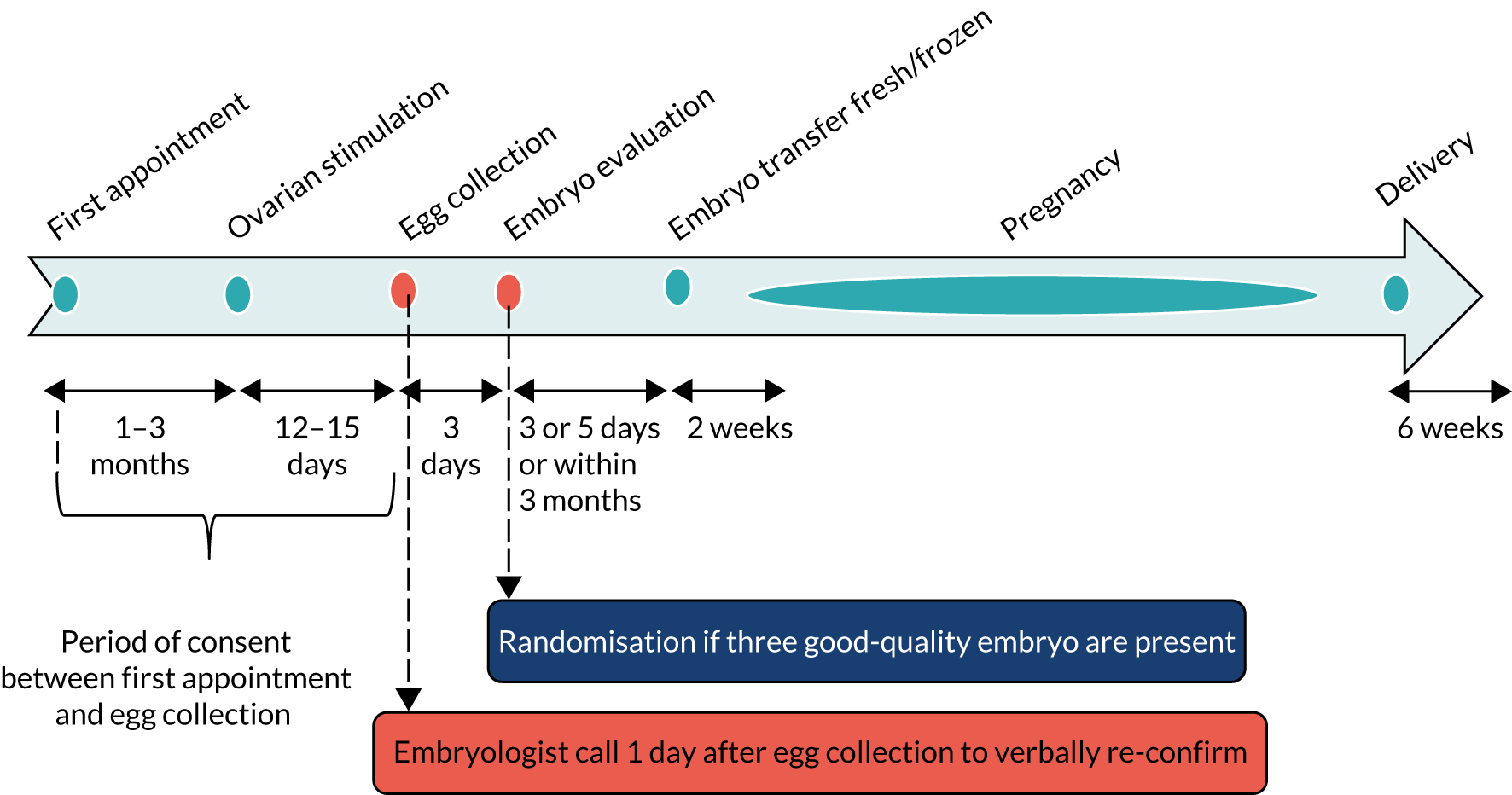
Couples who may have previously consented to take part in E-Freeze during their first or second cycle of IVF were still eligible to participate in E-Freeze if they had not been previously randomised into the trial. For couples who had previously consented but had not been randomised onto the trial, informed consent was reobtained for any participation during future cycles and a new study number was generated.
After consent, each partner completed a short questionnaire on how they were feeling emotionally (see Report Supplementary Material 1). Each participant sealed their questionnaire in an envelope after completion and questionnaires were destroyed (unopened) if the couple did not proceed to randomisation.
The data needed for randomisation were recorded in the bespoke consent and randomisation program developed by the National Perinatal Epidemiology Unit (NPEU) Clinical Trials Unit (CTU) at the University of Oxford (Oxford, UK).
Confirmation of consent
A routine telephone call was made to couples 1 day after egg collection to inform them of the outcome of fertilisation (see Figure 1). Consent was confirmed during this routine telephone call from the embryologist or research delegate.
Screening for final eligibility
A final eligibility check was carried out on day 3 post egg retrieval. Couples with a minimum of three good-quality embryos were eligible for randomisation to receive either fresh-embryo transfer (i.e. the fresh-embryo transfer arm) or freezing of all good-quality embryos, followed by subsequent transfer of thawed embryos within 3 months (i.e. the freeze-all arm) (Figure 2).
FIGURE 2.
Final eligibility criteria.
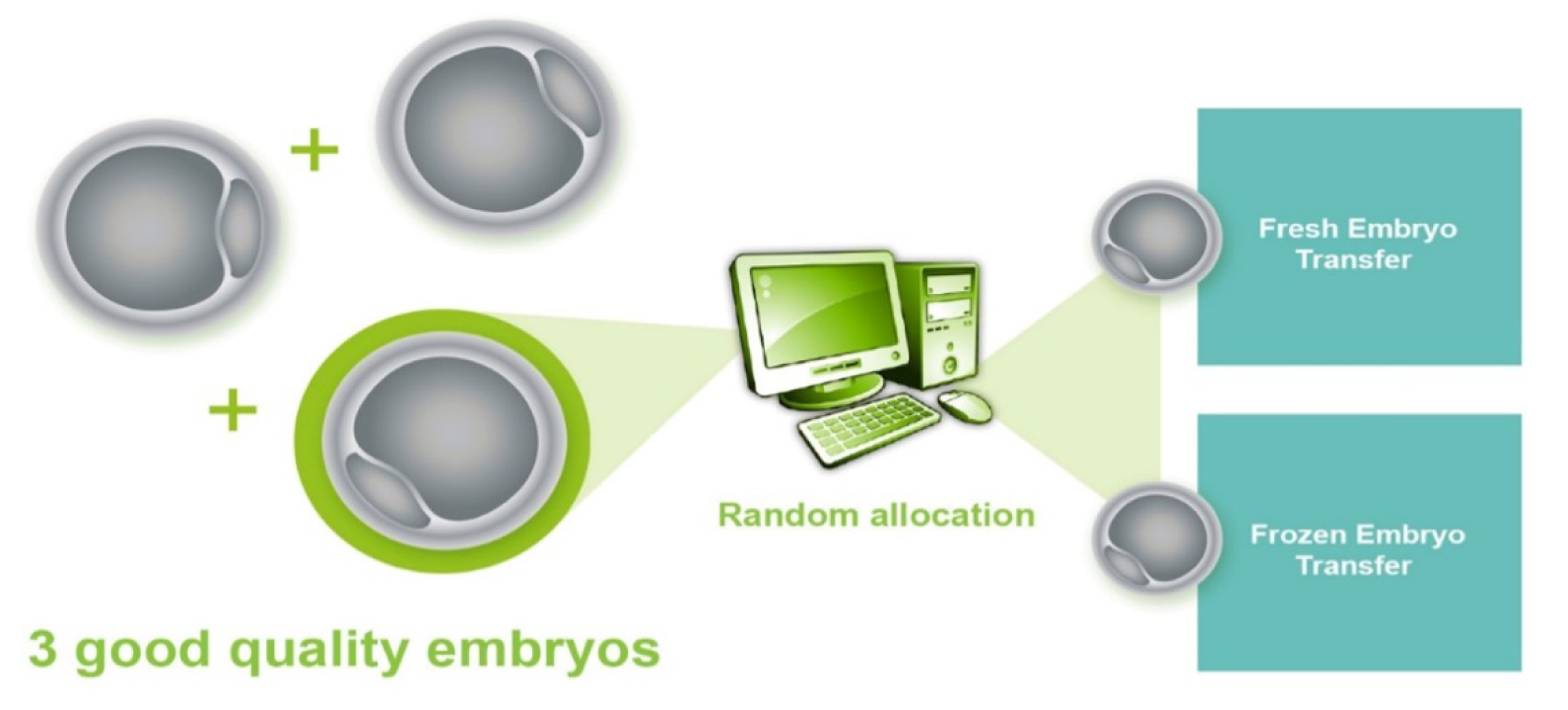
Good-quality embryos on day 3 were defined as those with 6–8 cells grade 3/3 or above using the agreed national grading scheme based on guidance from ACE in the UK. 14
Randomisation
Randomisation was performed after the creation of embryos, 3 days post egg collection, once all eligibility criteria were established, including ensuring that three or more good-quality embryos were available. This minimised the randomisation-to-intervention time interval as embryos were transferred at either the cleavage or the blastocyst stage (i.e. day 3 or 5 after egg collection, respectively). Couples were randomised (in an allocation ratio of 1 : 1) to a strategy of either fresh-embryo transfer or freezing of embryos, followed by thawing and replacement at a later date (typically 4–6 weeks later and almost always within 3 months of egg collection). Randomisation was undertaken by the research nurse or a delegated member of the research team using a secure web-based centralised system [with 24 hours per day, 7 days per week (24/7) telephone back-up, 365 days per year] hosted by the NPEU CTU (University of Oxford), ensuring allocation concealment. The randomisation employed a minimisation algorithm to balance across the following factors: fertility clinic, woman’s age (at the time of start of treatment, i.e. ovarian stimulation), primary/secondary infertility, self-reported duration of infertility, method of insemination (IVF, ICSI or a combination of both) and number of previous egg collections (i.e. cycles).
Communication of randomisation to couples
As part of routine practice, the embryologist contacted the couple by telephone to let them know the quality of their embryos (on day 3 after egg collection). The embryologist or research delegate confirmed to couples whether or not they fulfilled the final inclusion criteria (three or more good-quality embryos on day 3) and which arm they had been randomised to at the time of their routine telephone call on day 3. The research nurse then contacted the couple if they had not fulfilled the inclusion criteria to answer any queries and offer follow-up in the clinic.
Treatment plan
This study was a pragmatic, multicentre, two-arm, parallel-group, non-blinded RCT to evaluate the clinical effectiveness of the proposed intervention using the most rigorous gold-standard experimental methodology in real-life conditions. All clinical elements of IVF/ICSI treatment, apart from the randomised interventions, were carried out in accordance with local protocols. Blinding of the allocated intervention was not possible in this trial because of the nature of the treatments and statutory requirements of the regulatory body the Human Fertilisation and Embryology Authority (HFEA). 15 The process is detailed in the subsequent sections.
Standard-care arm
Women underwent fresh-embryo transfer at the cleavage or blastocyst stage in accordance with local protocols.
Intervention arm
All good-quality embryos were frozen in accordance with local protocols. Couples who were randomised to the freeze-all arm were contacted by the research nurse or research delegate within 3 working days post randomisation and arrangements were made for frozen-embryo transfer within 3 months of the egg retrieval process. This could involve a few visits to hospital for blood tests and ultrasounds to prepare the endometrium prior to embryo transfer.
At embryo transfer (in both arms), couples were asked to complete a short questionnaire to assess the additional costs related to the treatment (see Report Supplementary Material 2) and to repeat the emotions questionnaire (see Report Supplementary Material 1) that they filled in when they provided consent.
Ineligible and non-recruited participants
Details of all consenting couples were entered in a dedicated secure online database. It was anticipated that a proportion of those consented may not proceed to randomisation; the reasons for this were recorded (if available) and included the non-availability of three good-quality embryos on day 3. Couples not proceeding to randomisation were offered the most appropriate standard treatment. All clinics have access to supportive counselling as a requirement of the regulatory authority.
Follow-up
All randomised women carried out a pregnancy test 2 weeks (± 3 days) after embryo transfer. All women who had a positive pregnancy test at 2 weeks (± 3 days) underwent a transvaginal ultrasound scan afterwards (i.e. at 6–8 weeks of gestation) to identify the presence of a gestational sac with a fetal heartbeat, signifying an ongoing pregnancy.
Women who had an ongoing pregnancy were contacted by their research nurse (by telephone) to record pregnancy events and outcomes at 12 and 28 weeks of gestation and, again, at approximately 6 weeks after delivery. Outcomes presenting at ≥ 6 weeks post delivery were not recorded. All women who conceive by IVF/ICSI are followed up by their IVF centres routinely, as there is a mandatory requirement to report early-pregnancy outcomes, as well as delivery outcomes, including stillbirth, congenital anomalies and perinatal mortality, to the regulatory body (HFEA). Usually, this information is provided to each IVF clinic by the couples themselves. Alternatively, clinic staff contact couples by telephone to collect this information and report it to HFEA. In addition to data collected for reporting to HFEA, data were collected over the telephone at 12 and 28 weeks, and collected using questionnaires at embryo transfer for this trial.
Those who had a negative pregnancy test were not followed up any further as part of this trial.
Figure 3 presents a flow chart that explains the flow of participants through the trial.
FIGURE 3.
Flow chart for the study.
Outcomes
Primary outcome
The primary outcome was a healthy baby. A healthy baby was defined as a live, singleton baby born at term (between 37 and 42 completed weeks of gestation), with an appropriate weight for gestation (i.e. weight between the 10th and the 90th centile for that gestation, based on standardised charts).
Secondary outcomes
The secondary outcomes were separated into maternal safety, complications of pregnancy and delivery, measures of clinical effectiveness, measures of effectiveness of the process of freezing embryos, and health economic outcome measures.
Maternal safety outcome
-
Ovarian hyperstimulation syndrome, defined and classified as per the Royal College of Obstetricians and Gynaecologists (RCOG)’s Green-Top Guidelines. 6
Complications of pregnancy and delivery outcomes
-
Vanishing twin or triplet (defined as more fetal heartbeats than babies born, more gestational sacs than babies born or more gestational sacs than fetal heartbeats).
-
Miscarriage rate (defined as pregnancy loss prior to age of viability, i.e. 24 weeks of gestation).
-
Ectopic pregnancy.
-
Termination.
-
Gestational diabetes mellitus (GDM).
-
Multiple pregnancy (defined as more than one fetal heartbeat or more than one gestational sac).
-
Multiple births (including live and stillbirths).
-
Hypertensive disorders of pregnancy (e.g. chronic hypertension, pregnancy-induced hypertension, pre-eclampsia and eclampsia).
-
Most severe hypertensive disorder experienced (from least to worst severe: chronic hypertension, pregnancy-induced hypertension, pre-eclampsia and eclampsia).
-
Antepartum haemorrhage (i.e. any bleeding per vaginam after 28 weeks of pregnancy, including placenta praevia and placental abruption).
-
Onset of labour (i.e. spontaneous, induced or planned caesarean section).
-
Mode of delivery for each baby (i.e. normal vaginal delivery, instrumental vaginal delivery or caesarean section).
-
Preterm delivery (defined as delivery at < 37 completed weeks of gestation).
-
Very preterm delivery (defined as delivery at < 32 completed weeks of gestation).
-
Low birthweight (defined as weight of < 2500 g at birth).
-
Very low birthweight (defined as weight of < 1500 g at birth).
-
High birthweight (defined as weight of > 4000 g at birth).
-
High birthweight for gestational age (defined as birthweight > 90th centile for gestational age at delivery, based on standardised charts).
-
Low birthweight for gestational age (defined as birthweight < 10th centile for gestational age at delivery, based on standardised charts).
-
Congenital anomaly/birth defect (all congenital anomalies/birth defects identified to be included).
-
Perinatal mortality (stillbirth or late, as well as early, neonatal deaths, up to 28 days after birth).
Measures of clinical effectiveness outcomes
-
Live birth rate (this is a live birth episode, i.e. twins were counted as one birth).
-
Singleton live birth rate.
-
Singleton live birth rate at term.
-
Singleton baby with appropriate weight for gestation.
-
Pregnancy rate (defined as positive pregnancy test at 2 weeks ± 3 days after embryo transfer).
-
Clinical pregnancy rate (defined as the presence of at least one fetal heartbeat at ultrasound between 6 and 8 weeks’ gestation; ectopic pregnancy counts as a clinical pregnancy and multiple gestational sacs count as one clinical pregnancy).
Measures of the effectiveness of the process of freezing embryos outcomes
-
Total number of embryos frozen, thawed and transferred for all randomised couples.
-
Proportion of thawed embryos that were then transferred for all randomised couples.
-
No embryos survived thawing, leading to no embryo transfer.
Health economic outcome measures
-
Cost to the health service of treatment, pregnancy and delivery care.
-
Modelled long-term costs of health and social care, and broader societal costs.
Other secondary outcomes
-
Evaluation of emotional state (for both the female and the male partners).
Data collection
Data were collected at various time points, as shown in Figure 4. Data for both clinical and economic outcomes were collected using bespoke electronic case report forms (eCRFs) and entered directly into the study’s OpenClinica, version 3.0 (Waltham, MA, USA), electronic database by the centre’s research staff and trial team. Data were single entered only and, at the point of entry, the data underwent a number of checks to verify the validity and missingness of the data captured.
FIGURE 4.
Stages of data collection. a, Part of routine care.
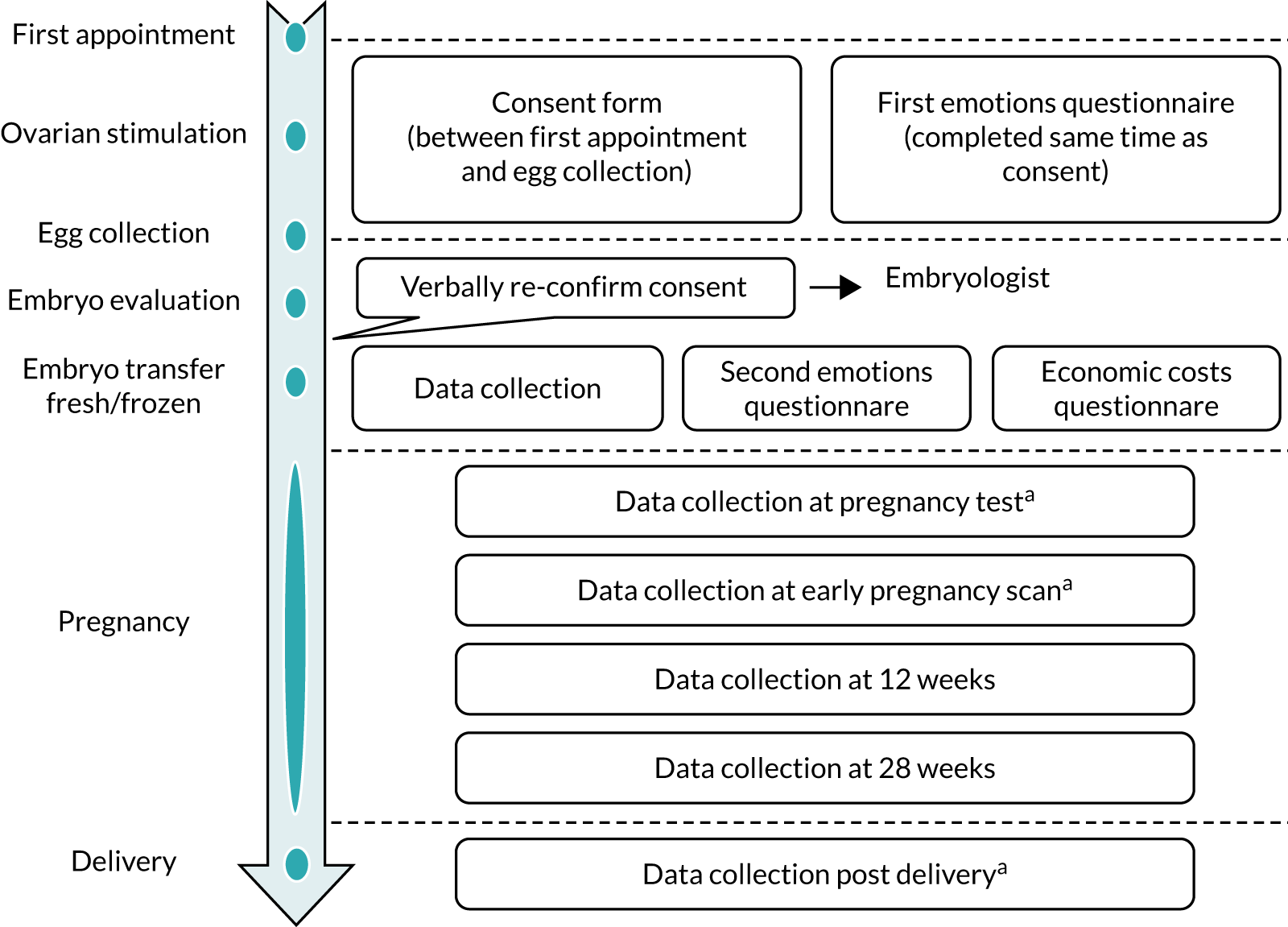
After consent and at embryo transfer, each partner completed a short paper-based questionnaire (see Report Supplementary Material 1) asking them how they were feeling. This was based on the State–Trait Anxiety Inventory (STAI). 16
A short questionnaire was provided to each partner for them to record the details of time and travel expenses accrued during their treatment as part of the economic evaluation (see Report Supplementary Material 2). This was completed at the time of embryo transfer.
Sample size
Sample size calculation
The proposed primary outcome for this trial was novel and is not currently reported by IVF clinics or national regulatory bodies. This meant that a number of assumptions were made to determine the expected event rate in the fresh-embryo transfer arm (receiving current standard treatment, i.e. fresh-embryo transfer).
Prior to commencing the trial, the most recent data from the HFEA,4 which collects data on all IVF cycles from all clinics in the UK, showed that 25% of all women undergoing one episode of IVF treatment involving a fresh-embryo transfer have a live birth and 20% have singleton live births. These values were for women of all age groups, not necessarily for women fulfilling the inclusion criteria for this trial in terms of the number of good-quality embryos in their IVF cycle. The live birth rate for first, second and third cycles was similar. 4 No data were available regarding the primary outcome for this study: the healthy baby rate (i.e. live singletons born between 37 and 42 weeks, with appropriate weight for gestation). For our trial population, we anticipated that the fresh-embryo transfer arm event rate was likely to be < 25% and possibly as low as 17%.
To provide relevant information regarding the event rate expected in the fresh-embryo transfer arm, we surveyed 10 IVF centres that expressed an interest in the study, collecting data on the number of live births in women aged < 42 years who were undergoing their first IVF treatment in 2012. The average live birth episode rate from this survey was 31% (95% CI 25% to 37%). Accurate data on the healthy baby rate in those with at least three good-quality embryos were not available. Although the live birth rate is expected to be higher in women with at least three good-quality embryos (who are likely to have a better prognosis), we anticipated that the healthy baby rate in our trial population would be towards the lower end of the CI, that is around 25%, taking into account the higher risk of preterm delivery and babies who are small for their gestational age following IVF. 9
The following assumptions were made for the sample size calculation.
We assumed a healthy baby rate of between 17% and 25% in women who were eligible for the trial (i.e. aged < 42 years, with three good-quality embryos) undergoing standard care (i.e. fresh-embryo transfer). Taking into account the extra time, effort and potential expense involved in freezing embryos, and the delay in embryo transfer of up to 3 months, a difference of at least 8% in absolute terms was considered to be clinically important by an expert panel of clinicians to recommend a change in clinical practice. With 90% power and using a two-sided, 5% level of statistical significance, a total of 1086 couples (i.e. 543 couples in each arm) would be required to be able to detect an absolute difference of 8% (from 17% to 25%) and 9% (from 25% to 34%) in the healthy baby rate in the fresh-embryo transfer arm and the freeze-all arm, respectively.
It is a regulatory requirement for clinics in the UK to report live birth outcomes (including number, weight and gestation) after all embryo transfers; hence, loss to follow-up was not anticipated. Therefore, we did not take into account loss to follow-up for these sample size calculations.
It was anticipated that a proportion of those who consented may not reach randomisation (e.g. those who did not have three good-quality, day 3 embryos or who required all embryos to be frozen for medical reasons); therefore, a larger number of participants would need to be consented. It was anticipated that the number of participants who did not have three good-quality embryos would be 50% out of those consented.
Statistical analysis
A detailed SAP was agreed and published13 prior to data lock. The analysis and presentation of results followed the most up-to-date recommendations of the Consolidated Standards of Reporting Trials (CONSORT) group. 17
Descriptive analysis
The flow of participants through each stage of the trial was summarised by trial arm. Demographic factors and clinical characteristics were summarised for all couples at trial entry, and separately for couples who delivered. Counts and percentages were reported for categorical variables, means [with standard deviations (SDs)] were reported for normally distributed continuous variables, and medians [with interquartile ranges (IQRs)] were reported for other continuous variables. No tests of statistical significance were performed and CIs were not calculated for differences between randomised arms on any baseline variable.
Primary analysis
All participants were analysed in the arms to which they were assigned, regardless of deviation from the protocol or treatment received under the ITT analysis principle. To perform the analyses for all outcomes on the ITT analysis population, the couple was included in the denominator once for all outcomes regardless of whether a pregnancy or a live birth occurred. Where this was a perinatal outcome, the woman was included once in the denominator. For neonatal secondary outcomes, the unit of analysis in the ITT analysis was the mother, and in cases of multiple pregnancy for which the infants’ outcomes differ, the worst outcome was reported.
Binary outcomes were analysed using a log-binomial regression model or a Poisson regression model with a robust variance estimator if the binomial model failed to converge. Linear regression was used for normally distributed continuous outcomes and quantile regression was used for skewed continuous outcomes. All comparative analyses were adjusted for the minimisation factors where possible. Fertility clinic was treated as a random effect in the models, where possible, and all other factors were treated as fixed effects. Both unadjusted and adjusted estimates are presented, but the primary inference is based on the adjusted analyses.
Comparative analyses entailed calculating the adjusted RR and 95% CI for the primary outcome, adjusted RRs and 99% CIs for all binary secondary outcomes, adjusted mean differences (MDs) and 99% CIs for normally distributed continuous secondary outcomes, or median differences and 99% CIs for skewed continuous secondary outcome variables. To account for the number of hypothesis tests performed, 99% CIs were used for all analyses of the secondary outcomes.
Customised birthweight centiles to calculate low weight for gestational age and high weight for gestational age were based on an existing, published, British model. 18
The following secondary outcomes were described only, and no formal statistical analysis comparing the arms was conducted:
-
chronic hypertension, pregnancy-induced hypertension, pre-eclampsia and eclampsia
-
most severe hypertensive disorder experienced (from least to worst severe: chronic hypertension, pregnancy-induced hypertension, pre-eclampsia and eclampsia).
Secondary analysis
The primary analysis for all primary and secondary outcomes was by ITT. Secondary analyses were performed to include clinically relevant denominators, such as the total number of women with a positive pregnancy test at 2 weeks ± 3 days after embryo transfer (for miscarriage), the total number of pregnant women with an ongoing pregnancy resulting in delivery (for pregnancy complications) and the total number of babies born (for birthweight and congenital anomalies). The adjusted analyses per total number of babies also accounted for the anticipated correlation in outcomes between multiple births. The rate of embryos not surviving after thawing (per embryo thawed) was reported for the intervention arm only.
Subgroup analysis
We performed subgroup analyses of the primary outcome on the following, as prespecified in the SAP:13
-
fertility clinic
-
woman’s age (at the time of start of treatment, i.e. ovarian stimulation): < 35, 35 to < 40 and ≥ 40 years
-
blastocyst-stage compared with cleavage-stage embryo transfer
-
single embryo transfer compared with multiple embryo transfer
-
number of previous embryo transfers: 0 compared with ≥ 1 (the groups 0, 1–3 and ≥ 4 were prespecified, but were reduced to two groups in the analysis because of low frequencies).
The consistency of the effect of type of embryo transfer across specific subgroups of couples was assessed for the primary outcome using the statistical test of interaction, in addition to the adjusted model. The results are presented in forest plots, with RRs, 95% CIs and the results of the interaction test.
In addition, for those receiving frozen-embryo transfer, the primary outcome was summarised for the following subgroups using numbers and percentages only:
-
natural cycles compared with hormone replacement cycles
-
vitrification compared with slow freezing.
Additional analysis
The following prespecified analyses were carried out for the primary outcome only:
-
per protocol – restricted to those who complied with the allocated intervention
-
as treated – grouping couples according to the intervention that they actually received (i.e. those randomised to frozen-embryo transfer but who received fresh-embryo transfer were in the fresh-embryo transfer arm in this analysis)
-
complier-average causal effect (CACE) analysis.
To assess the impact of non-compliance with the randomised allocation, that is women randomised to the frozen arm receiving fresh-embryo transfer (non-compliers), a CACE analysis was conducted. This analytic technique provides a robust estimate of the treatment effect among compliant participants. 19,20
The baseline characteristics of women randomised to the freeze-all arm were reported by compliance status, and the unadjusted event rate for the primary outcome was calculated for the observed compliers and non-compliers in the freeze-all arm. The CACE analysis assumed that the proportion of non-compliers in the fresh-embryo transfer arm (i.e. couples in the fresh-embryo transfer arm who would not have complied had they been randomised to the freeze-all arm) was the same as the proportion of non-compliers in the freeze-all arm. It also assumed that the event rate among the non-compliers in the freeze-all arm was the same as the event rate among the non-compliers in the fresh-embryo transfer arm. Applying these two assumptions, the unadjusted event rate for the primary outcome was calculated for the would-be compliers and would-be non-compliers in the fresh-embryo transfer arm. The unadjusted CACE RR with 95% CIs for the primary outcome was calculated using the event rates for compliant groups only (i.e. the observed compliers in the freeze-all arm and the would-be compliers in the fresh-embryo transfer arm). The CIs for the CACE-estimated RRs were calculated using bootstrapping methods. 21
Analysis of emotions questionnaire
The emotions questionnaires at randomisation and post embryo transfer captured responses to the STAI. 16 The response at randomisation was used as a covariate in an analysis of covariance (ANCOVA) model. Hypothesis testing investigated if there was a post-embryo transfer difference in the means of the two treatment arms after adjustment for responses at randomisation. To avoid bias, maximise the power of the study and obey the ITT principle, the missing-indicator method22 was used to replace missing baseline scores. This method replaces all missing baseline observations with the same value and an extra indicator variable is added to the model to indicate whether or not the value for that variable is missing. For any partially completed questionnaires, if one or two items were omitted, then the prorated score was obtained by calculating the mean weighted score for the completed items, multiplying by 20 and rounding to the next whole number. If three or more items were omitted, then the whole questionnaire was analysed as missing. Models were fitted separately for the female and male partners.
Economic evaluation
A formal economic evaluation was undertaken to assess the cost-effectiveness of the alternative approaches to treatment used in the trial.
The resource use and costs were primarily estimated from a health and personal social services perspective. However, personal time and travel costs associated with any additional treatment-related visits that were not part of standard, routine data collection were also estimated using a short questionnaire administered at the time of embryo transfer (see Report Supplementary Material 2). This was completed by both partners.
Trial data collected using eCRFs were used to capture participant-level resource use associated with treatment up to the trial end points of delivery or failure to become pregnant following the initial transfer. The appropriate unit costs were used to value resource use events recorded in the case report forms (CRFs). The detailed methods of economic evaluation are described in Chapter 4.
Adverse event reporting
A Data Monitoring Committee (DMC) was established to ensure the independent monitoring of the data and the well-being of study participants. The DMC periodically reviewed study progress and outcomes, as well as reports of unexpected serious adverse events (SAEs). The DMC made recommendations regarding the continuance of the study or modification of the study protocol.
Adverse events
As per the protocols of the trial unit, an adverse event (AE) was defined as any untoward medical occurrence in a participant, which did not, necessarily, have to have a causal relationship with the intervention. Owing to the high incidence of AEs routinely expected in this patient population (e.g. abnormal laboratory findings, new symptoms), only those AEs identified as serious were recorded for the trial.
Serious adverse events
A SAE was any untoward medical occurrence that:
-
resulted in death
-
was life-threatening
-
required participant hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability/incapacity
-
was a congenital anomaly/birth defect
-
was an important medical event.
The term ‘severe’ was often used to describe the intensity (i.e. severity) of a specific event; however, the event itself may have been of relatively minor medical significance. This was not the same as ‘serious’, which was based on participant/event outcome or action criteria, usually associated with events that posed a threat to a participant’s life or functioning.
The term ‘life-threatening’ in the definition of serious refers to an event in which the participant was at risk of death at the time of the event; it does not refer to an event that hypothetically might have caused death had it been more severe. Medical and scientific judgement was exercised in deciding whether or not an AE was serious in other situations.
Foreseeable serious adverse events
Foreseeable SAEs were events that were expected in the patient population or as a result of the routine care/treatment of a patient. The events were foreseeable in women or couples undergoing IVF treatment and, therefore, did not need to be reported as SAEs. Data on foreseeable SAEs were collected on the eCRF as part of routine data collection.
The foreseeable events relating to the female partner or couple were:
-
OHSS
-
miscarriage
-
hypertensive disorders of pregnancy
-
antepartum haemorrhage
-
GDM
-
multiple pregnancy
-
no embryos surviving thawing.
The foreseeable events relating to the baby when born were:
-
low birthweight
-
very low birthweight
-
low weight for gestational age
-
high weight for gestational age
-
preterm delivery
-
very preterm delivery.
Unforeseeable serious adverse events
An unforeseeable SAE was any event that met the definition of a SAE and was not detailed as foreseeable. The following unforeseeable SAEs were reported:
-
maternal death
-
stillbirth
-
congenital anomaly detected antenatally or postnatally
-
neonatal death.
Unforeseeable SAEs were reported up to 6 weeks post delivery. They were reported to the NPEU CTU as soon as possible after staff at the site became aware of the event. The SAEs were reported in one of the following ways:
-
Using the clinical database OpenClinica – only staff with access to OpenClinica could report SAEs in this way; site staff were required to print the OpenClinica SAE form and obtain the information and signature of the study clinician carrying out the causality assessment. The completed and signed SAE form was e-mailed or faxed to the NPEU CTU. NPEU CTU staff were automatically informed by e-mail of any SAEs reported electronically.
-
By completing a SAE form that was e-mailed or faxed to the NPEU CTU. Paper copies were available with the trial documentation to enable anyone to report a SAE. Guidance for the research site was provided on the paper SAE reporting form.
-
If it was not possible to report a SAE using the methods detailed in points 1 and 2, the unforeseeable SAE could be reported by telephone and the SAE form was completed by staff at the NPEU CTU.
If any additional information regarding the SAEs became available, this was detailed on a new SAE form and e-mailed or faxed to the NPEU CTU or reported electronically using OpenClinica. The SAE forms were sent to the sponsor by the NPEU CTU as soon as possible after they were received. The chief investigator assessed whether or not a SAE was ‘related’ (i.e. resulting from administration of any of the research procedures) and ‘unforeseeable’ in relation to those procedures. Any reports of related and unforeseeable SAEs were submitted to the following places, in line with the protocols of the trial unit: the North of Scotland Research Ethics Committee (which gave a favourable opinion of the study), the sponsor and the centre at which the SAE occurred within 15 working days of the chief investigator becoming aware of the event. All recorded SAEs were reviewed by the DMC at regular intervals. The chief investigator informed all principal investigators (PIs) concerned of relevant information that adversely affected the safety of the participants.
Governance and monitoring
To ensure oversight and governance of the trial, a DMC and a Trial Steering Committee (TSC) were established, and a Project Management Group (PMG) was responsible for the day-to-day running of the trial.
Trial Steering Committee
The role of the TSC was to provide overall supervision of the study. The TSC monitored the progress of the study and conduct, and advised on its scientific credibility. The TSC considered and acted, as appropriate, on the recommendations of the DMC and, ultimately, carried the responsibility for deciding whether or not the trial needed to be stopped on grounds of safety or efficacy.
The TSC consisted of an independent chairperson and two other independent members. The committee members were deemed to be independent if they were not involved in study recruitment and were not employed by any organisation directly involved in the study’s conduct. Representatives from Fertility Network (London, UK) (patient/public involvement groups), the chief investigator and other investigators/co-applicants were joined by observers from the NPEU CTU. A TSC charter was prepared in advance of and agreed on at the first TSC meeting to document how the committee operated.
Data Monitoring Committee
A DMC that was independent of the applicants and the TSC reviewed the progress of the trial at frequent intervals and provided advice on the conduct of the trial to the TSC, which reported to the HTA programme manager. The committee periodically reviewed study progress and outcomes. The timing and content of the DMC reviews were detailed in a DMC charter, which was agreed on at its first meeting.
Project Management Group
The study was supervised on a day-to-day basis by the PMG. This group reported to the TSC, which had overall responsibility for the conduct of the study. The core PMG met regularly (i.e. at least monthly). The Co-Investigators Group (CIG) met at regular intervals during the trial, and comprised all co-applicants and the members of the core PMG. The full membership of the committees is listed in Appendix 5.
Trial management
The trial co-ordinating centre was the NPEU CTU, University of Oxford, where the trial manager was based. The NPEU CTU was responsible for trial oversight; information technology (IT) system/functions, such as randomisation, clinical and administrative databases; all programming and statistical analyses; servicing both the DMC and the TSC; and, in collaboration with the chief investigator and the local research nurse, the general day-to-day running of the study, including the recruitment of sites and training of staff. A 24/7 (365 days per year) emergency helpline was available for out-of-hours queries related to the trial. The economic analysis was conducted at the University of Aberdeen (Aberdeen, UK).
Risk assessment and monitoring
A study risk assessment and monitoring plan was completed as part of the development of this study by the NPEU CTU. This risk assessment and monitoring plan was reviewed at regular intervals during the study to ensure that appropriate and proportionate monitoring activity was performed.
Patient and public involvement
Patients and the public were involved at every step of the trial. At the conception of the trial, the Chief Executive of Fertility Network UK, Mrs Claire Lewis Jones, was consulted. She was involved in every meeting from the submission of the outline application to the NIHR HTA programme and the full application. Once funding was awarded, patient involvement continued during the design of the protocol and all patient facing information, including leaflets. Multiple members of Fertility Network UK were involved in publicising the trial, especially when recruitment was suboptimal and non-compliance was higher than expected. Patient representatives advised on recruitment strategies and the conduct of the trial at each step. They were part of both the DMC and the TSC. They were consulted when the inclusion criteria were amended from first cycle of IVF/ICSI to first, second and third cycle. We also took their advice when it was recommended to stop the trial, as communication to participants was crucial at that time. Patient representatives have been fully involved in the interpretation of the results, writing of this report and dissemination strategies.
Chapter 3 Results
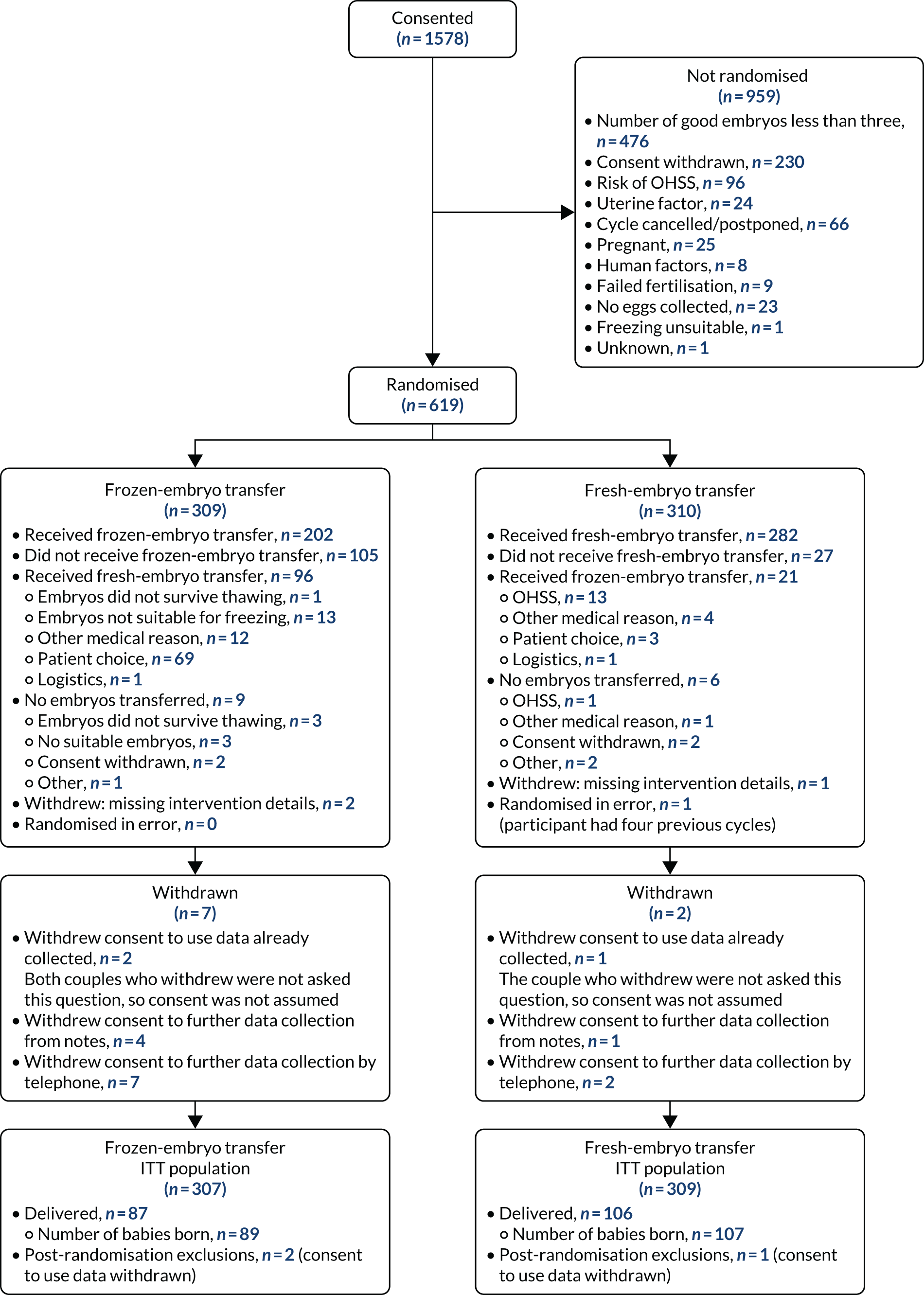
Between February 2016 and April 2019, 1578 participants consented and 619 couples were randomised: 310 to the fresh-embryo transfer arm and 309 to the freeze-all arm (Figure 5). One couple in the fresh-embryo transfer arm and two in the intervention arm withdrew consent to use their data; hence, the ITT analysis included 309 participants in the fresh-embryo transfer arm and 307 participants in the freeze-all arm.
FIGURE 5.
Flow of participants. Reproduced from Maheshwari et al. ,23 Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-Freeze), Human Reproduction, 2022, deab279, by permission of European Society of Human Reproduction and Embryology.
Recruitment and retention
When the trial initially started, in 2016, only the first cycle of IVF/ICSI was included. Owing to suboptimal recruitment (and after discussion with the funders), it was agreed that we could include the second and third cycles as well (decided on 12 April 2018).
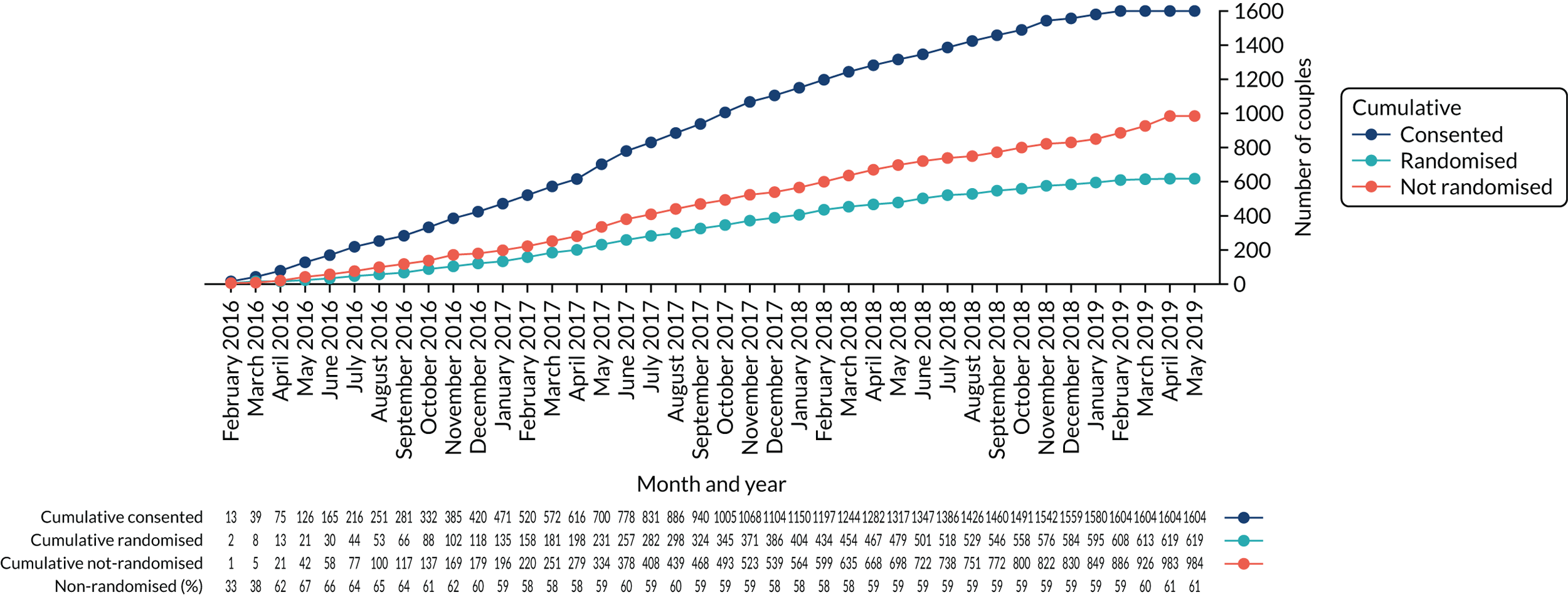
A total of 1578 couples provided consent to be enrolled in the trial. The time between consent and randomisation varied from 10 to 80 days, with a mean of 55.5 days. A total of 959 (60.8%) couples who provided consent were not randomised; the main reason for this (49.6%) was not meeting the final eligibility criterion of at least three good-quality embryos on day 3. Other reasons are described in the flow chart in Figure 5. Some couples (n = 25) became pregnant spontaneously during the time between consent and randomisation.
The proportion of couples not randomised after providing consent remained constant throughout the trial (Figure 6), even after changing the inclusion criteria to incorporate second- and third-cycle treatments.
FIGURE 6.
Proportion of consented and randomised participants.
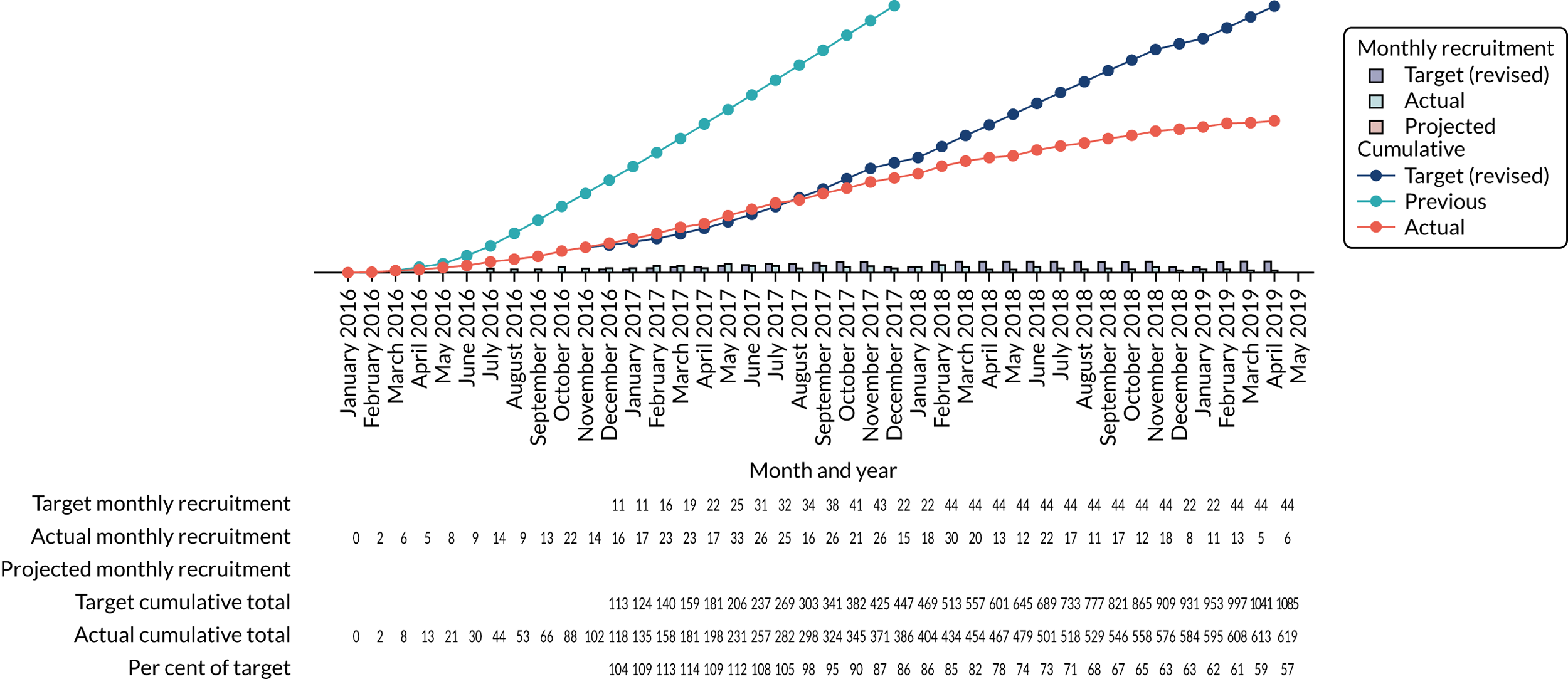
The monthly recruitment figures lagged behind target and plateaued after October 2018 (Figure 7). On 9 November 2018, the DMC recommended to the TSC that the trial should be halted, owing to the shortfall in recruitment and the high level of non-adherence. Following this recommendation, a joint meeting of the TSC and DMC was convened on 17 January 2019, with an independent chairperson because of disagreement between the TSC and the DMC, to agree scenarios for a monitoring meeting. After a monitoring meeting with the NIHR HTA programme on 29 January 2019, it was agreed that the trial would stop recruitment on 30 April 2019. It was felt that continuing the trial further would yield no benefit, as an adequate sample size was unlikely given the slow recruitment, compounded by non-adherence, which was particularly evident in the intervention arm (see Table 1).
FIGURE 7.
Final recruitment figures.
One-fifth of couples included in the analysis (117/616, 19%) did not adhere to their allocated intervention: 21 out of 309 (6.8%) in the fresh-embryo transfer arm and 96 out of 307 (31.3%) in the freeze-all arm. Non-adherence varied across clinics, with the rate in the intervention arm ranging from 0% to 86%. One clinic had a for-cause monitoring visit and was closed early in its recruitment, with non-adherence reaching almost 100%.
Table 1 shows the reasons for non-adherence in the trial arms. The most common reason for non-adherence in the freeze-all arm was patient choice (72% of those who did not receive their allocated intervention).
| Clinical characteristics | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|
| Received frozen-embryo transfer, n (%) | 202 (65.8) | 21 (6.8) |
| Time from egg collection to frozen-embryo transfer (days), median (IQR) | 63 (33–97) | 105 (84–138) |
| Received frozen-embryo transfer within 3 months of egg collection,a n (%) | 145 (71.8) | 7 (33.3) |
| Received fresh-embryo transfer, n (%) | 96 (31.3) | 282 (91.3) |
| Reason embryo transfer type is different from allocation, n (%) | 96 | 21 |
| Embryos did not survive after thawing | 1 (1.0) | 0 (0.0) |
| OHSS | 0 (0.0) | 13 (61.9) |
| Not suitable to freeze | 13 (13.5) | 0 (0.0) |
| Other medical reason | 12 (12.5) | 4 (19.0) |
| Patient choice | 69 (71.9) | 3 (14.3) |
| Logistics | 1 (1.0) | 1 (4.8) |
| Received no embryo transfer, n (%) | 9 (2.9) | 6 (1.9) |
| Reason no embryos were transferred, n (%) | 9 | 6 |
| Embryos did not survive after thawing | 3 (33.3) | 0 (0.0) |
| OHSS | 0 (0.0) | 1 (16.7) |
| No suitable embryos | 3 (33.3) | 0 (0.0) |
| Other medical reason | 0 (0.0) | 1 (16.7) |
| Consent withdrawn | 2 (22.2) | 2 (33.3) |
| Other | 1 (11.1) | 2 (33.3) |
Recruitment by sites
Eighteen clinics across the UK signed up for the trial. Only 13 clinics randomised any participants, of which four randomised > 50 participants. The number of recruited participants from each clinic is presented in Table 2.
| Fertility clinica | Number of participants (%) | |
|---|---|---|
| Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | |
| 1 | 11 (3.6) | 11 (3.6) |
| 2 | 14 (4.6) | 11 (3.6) |
| 3 | 90 (29.3) | 92 (29.8) |
| 4 | 49 (16.0) | 48 (15.5) |
| 5 | 31 (10.1) | 30 (9.7) |
| 6 | 21 (6.8) | 29 (9.4) |
| 7 | 11 (3.6) | 11 (3.6) |
| 8 | 24 (7.8) | 23 (7.4) |
| 9 | 7 (2.3) | 7 (2.3) |
| 10 | 10 (3.3) | 8 (2.6) |
| 11 | 29 (9.4) | 30 (9.7) |
| 12 | 1 (0.3) | 1 (0.3) |
| 13 | 9 (2.9) | 8 (2.6) |
Data missingness
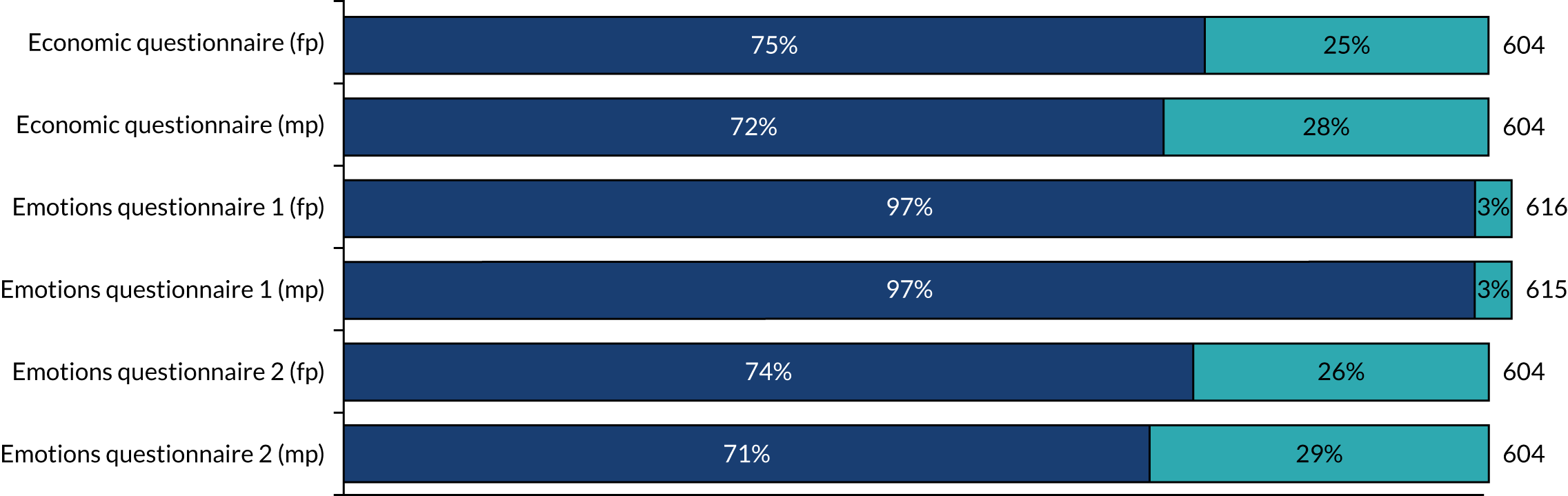
As is clear from Figures 8 and 9, there were very few missing data for all clinical outcomes. The emotions questionnaires were completed at consent and, again, at embryo transfer. The return rate was 97% for the first emotions questionnaire and > 70% for the second emotions questionnaire. Patients also completed an economics questionnaire, which had a return rate of > 70%.
FIGURE 8.
Data missingness on CRFs.
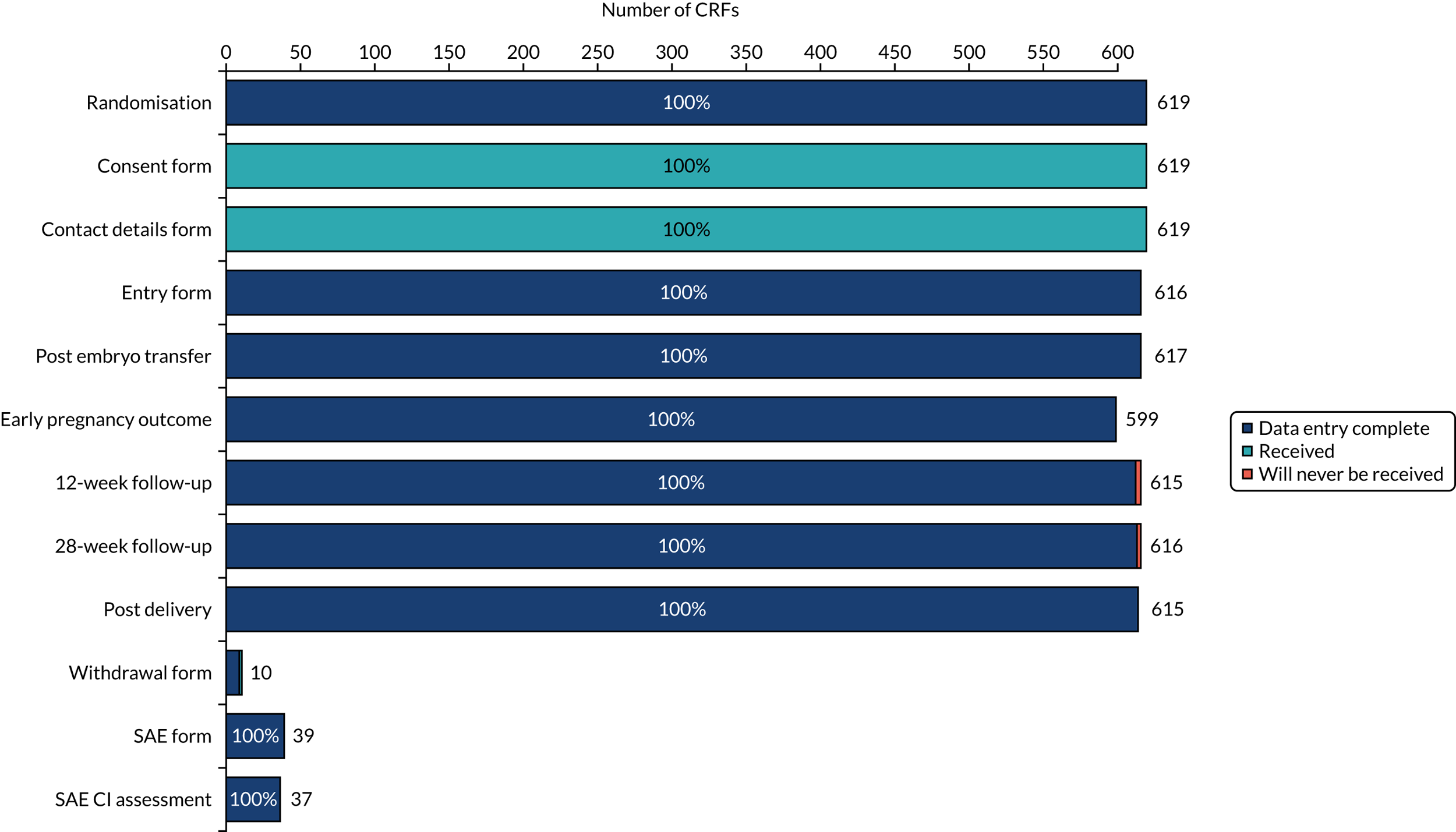
FIGURE 9.
Data missingness for patient-completed questionnaires. fp, female participant; mp, male participant.
Statistical analyses
Baseline comparability of randomised arms
The demographic and clinical characteristics at trial entry are described for all couples in the ITT population by trial arm.
Demographic characteristics at consent
Demographic characteristics at consent are described in Table 3.
| Characteristic | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|
| Woman’s age at ovarian stimulation (years) | ||
| < 35, n (%) | 153 (49.8) | 157 (50.8) |
| 35 to < 40, n (%) | 137 (44.6) | 139 (45.0) |
| ≥ 40, n (%) | 17 (5.5) | 13 (4.2) |
| Mean (SD) | 34.7 (3.8) | 34.6 (3.6) |
| Woman’s ethnicity, n (%) | ||
| White | 237 (82.0) | 221 (78.4) |
| Black | 8 (2.8) | 13 (4.6) |
| Asian | 28 (9.7) | 40 (14.2) |
| Mixed | 6 (2.1) | 5 (1.8) |
| Other | 10 (3.5) | 3 (1.1) |
| Not known | 12 | 16 |
| Missing | 6 | 11 |
| Woman’s smoking status, n (%) | ||
| Never smoked | 276 (89.9) | 282 (91.3) |
| Past smoker | 30 (9.8) | 26 (8.4) |
| Current smoker | 1 (0.3) | 1 (0.3) |
| Woman’s BMI (kg/m2) | ||
| Underweight (< 18.5), n (%) | 5 (1.6) | 5 (1.6) |
| Healthy weight (18.5–24.9), n (%) | 195 (63.7) | 187 (60.7) |
| Overweight (25–29.9), n (%) | 91 (29.7) | 102 (33.1) |
| Obese (30–34.9), n (%) | 12 (3.9) | 14 (4.5) |
| Very obese (> 35), n (%) | 3 (1.0) | 0 (0.0) |
| Mean (SD) | 24.1 (3.4) | 24.1 (3.2) |
| Missing, n | 1 | 1 |
| Type of infertility, n (%) | ||
| Primary | 237 (77.2) | 241 (78.0) |
| Secondary | 70 (22.8) | 68 (22.0) |
| Woman’s previous pregnancies, n (%) | ||
| 0 | 214 (69.7) | 220 (71.2) |
| 1 | 65 (21.2) | 63 (20.4) |
| 2 | 18 (5.9) | 16 (5.2) |
| > 2 | 10 (3.3) | 10 (3.2) |
| Woman’s previous live births, n (%) | ||
| 0 | 292 (95.1) | 295 (95.5) |
| 1 | 15 (4.9) | 12 (3.9) |
| 2 | 0 | 2 (0.6) |
| Main cause of infertility, n (%) | ||
| Ovulatory | 40 (13.0) | 32 (10.4) |
| Tubal | 29 (9.4) | 27 (8.7) |
| Endometriosis | 13 (4.2) | 11 (3.6) |
| Unexplained | 119 (38.8) | 131 (42.4) |
| Male factor, n (%) | 102 (33.2) | 102 (33.0) |
| Uterine | 1 (0.3) | 0 (0.0) |
| Low ovarian reserve | 2 (0.7) | 4 (1.3) |
| Other | 1 (0.3) | 2 (0.6) |
| Duration of infertility (months) | ||
| < 12, n (%) | 3 (1.0) | 3 (1.0) |
| 12 to < 24, n (%) | 31 (10.1) | 37 (12.0) |
| 24 to < 36, n (%) | 106 (34.5) | 99 (32.0) |
| 36 to < 48, n (%) | 80 (26.1) | 81 (26.2) |
| 48 to < 60, n (%) | 37 (12.1) | 38 (12.3) |
| ≥ 60, n (%) | 50 (16.3) | 51 (16.5) |
| Median (IQR) | 36 (24–48) | 36 (24–48) |
Age
The mean (SD) age of the female partner was 34.7 (3.8) years in the freeze-all arm and 34.6 (3.6) years in the fresh-embryo transfer arm. Most women (95.1%) were aged < 40 years and half (50.3%) were aged < 35 years. Age was a minimisation criterion.
Ethnicity
Women’s ethnicity was included in the eCRFs only part-way through the trial, on 12 April 2017. All attempts were made to collect these data retrospectively, but data were missing for some couples who were recruited prior to this date (freeze-all arm, n = 6; fresh-embryo transfer arm, n = 11). Most participants were of white ethnic background (80.2%) and 11.9% were Asian. A small proportion in both arms were of black, mixed or other ethnic backgrounds.
Woman’s smoking status
Regarding smoking status, 89.9% of women in the freeze-all arm and 91.3% in the fresh-embryo transfer arm had never smoked. A small proportion were previous smokers (9.8% in the freeze-all arm and 8.4% in the fresh-embryo transfer arm).
Woman’s body mass index
The mean body mass index (BMI) of the female partner was 24.1 kg/m2 (SD 3.4 kg/m2) in the freeze-all arm and 24.1 kg/m2 (SD 3.2 kg/m2) in the fresh-embryo transfer arm. Just over 60% were in the healthy weight category (as per internationally agreed criteria). 24 Almost one-third of female participants were overweight and 4.2% were obese. Overall, 95.3% had a BMI of < 30 kg/m2.
Type of infertility
Most women (77.2% in the freeze-all arm and 78% in the fresh-embryo transfer arm) had primary infertility. One-fifth had secondary infertility in both arms (22.8% vs. 22% in the freeze-all and fresh-embryo transfer arms, respectively). The type of infertility was a minimisation criterion.
Previous pregnancies
Over two-thirds of the participants (69.7% in the freeze-all arm vs. 71.2% in the fresh-embryo transfer arm) had had no previous pregnancy. In both arms, most participants (> 95%) had not had a previous live birth.
Main cause of infertility
The male factor and unexplained infertility constituted > 70% of the causes of infertility (72.0% in the freeze-all arm and 75.4% in the fresh-embryo transfer arm), followed by ovulatory factor (13.0% in the freeze-all arm and 10.4% in the fresh-embryo transfer arm). In both arms, a large proportion of participants had unexplained infertility (38.8% in the freeze-all arm and 42.4% in the fresh-embryo transfer arm).
Duration of infertility (months)
The median (IQR) duration of infertility was 36 months (24–48 months) in both arms. Overall, 28.6% of patients had a duration of infertility of > 48 months.
The demographic characteristics of participants randomised to the freeze-all arm were similar whether or not they complied with the allocated intervention.
Characteristics of treatment pre randomisation
The characteristics of the IVF treatment pre randomisation are described in Table 4. All proportions were similar in both arms unless specified.
| Characteristic | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|
| Endometrial scratch performed, n (%) | 4 (1.3) | 3 (1.0) |
| Stimulation regimen used, n (%) | 299 (97.4) | 301 (97.4) |
| Long | 73 (23.8) | 64 (20.7) |
| Short | 42 (13.7) | 48 (15.5) |
| Ultrashort | 7 (2.3) | 4 (1.3) |
| Antagonist | 177 (57.7) | 185 (59.9) |
| Total stimulation dose of FSH (IUs), mean (SD) | 2540 (1257) | 2543 (1259) |
| Adjuvants used (non-exclusive), n (%) | 9 (2.9) | 7 (2.3) |
| Aspirin | 0 (0.0) | 1 (0.3) |
| Heparin | 3 (1.0) | 0 (0.0) |
| Steroids | 2 (0.7) | 1 (0.3) |
| Growth hormone | 7 (2.3) | 6 (1.9) |
| DHEA | 0 (0.0) | 1 (0.3) |
| Blood test performed on day of trigger injection, n (%) | 135 (44.0) | 142 (46.0) |
| Trigger injection used, n (%) | ||
| Agonist | 19 (6.2) | 28 (9.1) |
| Dual trigger | 31 (10.1) | 32 (10.4) |
| HCG | 257 (83.7) | 249 (80.6) |
| Total number of eggs collected | ||
| 3–5, n (%) | 14 (4.6) | 16 (5.2) |
| 6–9, n (%) | 73 (23.8) | 77 (24.9) |
| 10–15, n (%) | 141 (45.9) | 121 (39.2) |
| > 15, n (%) | 79 (25.7) | 95 (30.7) |
| Median (IQR) | 12 (9–16) | 12 (9–17) |
| Method of insemination,a n (%) | ||
| IVF | 158 (51.5) | 159 (51.5) |
| ICSI | 139 (45.3) | 138 (44.7) |
| Split (IVF and ICSI) | 10 (3.3) | 12 (3.9) |
| Number of eggs fertilised normally (two pronuclei) | ||
| 3–5, n (%) | 69 (22.5) | 69 (22.3) |
| 6–9, n (%) | 139 (45.3) | 137 (44.3) |
| 10–15, n (%) | 76 (24.8) | 81 (26.2) |
| > 15, n (%) | 23 (7.5) | 22 (7.1) |
| Median (IQR) | 8 (6–11) | 8 (6–11) |
| Time lapse used, n (%) | 124 (40.4) | 126 (40.8) |
| Good-quality embryos created on day 3 | ||
| 3 or 4, n (%) | 131 (42.7) | 112 (36.2) |
| 5 or 6, n (%) | 70 (22.8) | 84 (27.2) |
| 7–10, n (%) | 80 (26.1) | 88 (28.5) |
| > 10, n (%) | 26 (8.5) | 25 (8.1) |
| Median (IQR) | 5 (3–7) | 5 (4–8) |
| Number of previous egg collections,a n (%) | ||
| 0 | 284 (92.5) | 286 (92.6) |
| 1 | 19 (6.2) | 17 (5.5) |
| 2 | 4 (1.3) | 5 (1.6) |
| ≥ 3 | 0 (0.0) | 1 (0.3) |
| Number of previous embryo transfers, n (%) | ||
| 0 | 284 (92.5) | 288 (93.2) |
| 1–3 | 22 (7.2) | 20 (6.5) |
| ≥ 4 | 1 (0.3) | 1 (0.3) |
Stimulation regimen and dose
The most common protocol used was an antagonist protocol (used in 58.8%), followed by a long protocol (used in 22.2%). The total stimulation dose was similar, with a mean dose of 2542 (SD 1257) international units (IUs). Most participants (> 80%) had human chorionic gonadotrophin (HCG) as a final booster injection, followed by dual trigger (10.2%) and, in a small proportion of participants, agonist trigger.
Adjuvants
Most treatments did not have add-ons or adjuvants, including endometrial scratch. A total of ≈ 40% used time lapse as the incubator, but this was similar in both arms.
Number of eggs collected
The median (IQR) number of eggs collected was 12 (range 9–16 eggs), with > 10 eggs collected from 70.8% of participants and > 15 eggs collected from 28.2%; in the case of 30 participants, fewer than six eggs were collected.
Method of insemination
The eggs and sperm were mixed by IVF or ICSI, with an almost equal split between the two methods (IVF, 51.5%; ICSI, 45%). The method of insemination was a minimisation criterion.
Number of embryos
The median number of embryos created was 8 (IQR 6–11), with 45 couples having more than 15 embryos. The median number of good-quality embryos created on day 3 was 5 (IQR 3–7) in the freeze-all arm and 5 (IQR 4–8) in the fresh-embryo transfer arm. The number of good-quality embryos created on day 3 was a minimisation criterion.
Number of previous treatments
Despite the inclusion of second and third cycles, most couples recruited had not previously undergone egg collection (92.5%) or embryo transfer (92.9%).
The clinical pre-randomisation characteristics of those randomised to the freeze-all arm were similar whether or not they complied with the allocated intervention.
Clinical characteristics post randomisation
The clinical characteristics of the embryo and the endometrium, which were collected post randomisation, are described for the ITT population by trial arm in Table 5.
| Characteristic | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|
| Had embryo transfer, effective, n | 298 | 303 |
| Stage of embryo at transfer, n (%) | ||
| Cleavage (day 3) | 11 (3.7) | 14 (4.6) |
| Cleavage (day 4) | 5 (1.7) | 6 (2.0) |
| Blastocyst (day 5) | 270 (90.6) | 281 (92.7) |
| Blastocyst (day 6) | 12 (4.0) | 1 (0.3) |
| < 3 days | 0 (0.0) | 1 (0.3) |
| Number of embryos transferred | ||
| 1, n (%) | 249 (83.6) | 247 (81.5) |
| 2, n (%) | – | 55 (18.2) |
| 3, n (%) | 49 (16.4) | 1 (0.3) |
| Median (IQR) | 1 (1–1) | 1 (1–1) |
| Number of remaining frozen embryos after transfera | ||
| 0, n (%) | 68 (22.8) | 61 (20.1) |
| 1, n (%) | 46 (15.4) | 52 (17.2) |
| 2, n (%) | 55 (18.5) | 55 (18.2) |
| ≥ 3, n (%) | 129 (43.3) | 135 (44.6) |
| Median (IQR) | 2 (1–4) | 2 (1–4) |
| Endometrial appearance, n (%) (percentage is of effective N: frozen, effective N = 167; fresh-embryo transfer, effective N = 169) | ||
| Triple layer | 152 (96.2) | 157 (96.3) |
| No triple layer | 6 (3.8) | 6 (3.7) |
| Unknown | 140 | 140 |
| Endometrial thickness (mm), mean (SD) (mean is of effective N: frozen, effective N = 188; fresh-embryo transfer, effective N = 189) | 9.3 (1.9) | 10.2 (2.3) |
| Not recorded, n | 119 | 120 |
Of those randomised, 298 out of 307 participants underwent embryo transfer in the freeze-all arm and 303 out of 309 participants underwent embryo transfer in the fresh-embryo transfer arm. Most transfers (93.8%) were at the blastocyst stage. Overall, > 80% of participants in both arms underwent single embryo transfer; the remaining participants had two embryos, except for one participant, who had three embryos.
The endometrial appearance was recorded in only half of the cases in both arms; in 96.3% of these cases, it was triple layer, with a mean thickness of > 9.3 mm (SD 1.9 mm) in the freeze-all arm and 10.2 mm (SD 2.3 mm) in the fresh-embryo transfer arm.
The number of embryos remaining frozen (i.e. spare embryos) after the first embryo transfer was similar in both arms (median 2, IQR 1–4); 78.5% of couples had at least one remaining embryo frozen after embryo transfer.
The clinical characteristics post randomisation of those randomised to the freeze-all arm were similar whether or not they complied with the allocated intervention.
Post-randomisation characteristics of those who received frozen-embryo transfer
As shown in Table 6, 202 couples in the freeze-all arm and 21 couples in the fresh-embryo transfer arm received frozen-embryo transfer. Most couples had embryos frozen by vitrification (88.1% in the freeze-all arm and 95.2% in the fresh-embryo transfer arm). Most embryos were frozen at the blastocyst stage. The median number of embryos frozen among those who underwent frozen-embryo transfer was 4 (IQR 3–6). The median number of embryos thawed was one. One-fifth (19.8%) of those randomised to the freeze-all arm and 14.3% of those randomised to the fresh-embryo transfer arm who underwent frozen transfer had more than one embryo thawed. Very few embryos were thawed and discarded (i.e. this occurred in a total of 19 couples). The most common method used for endometrial preparation was artificial cycle with oestrogen and progesterone, or downregulation with gonadotropin-releasing hormone (GnRH) agonist.
| Characteristic | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|
| Received frozen transfer, n | 202 | 21 |
| Method of embryo freezing, n (%) | ||
| Vitrification | 178 (88.1) | 20 (95.2) |
| Slow freezing | 24 (11.9) | 1 (4.8) |
| Number of embryos frozen | ||
| 1, n (%) | 16 (7.9) | 1 (4.8) |
| 2, n (%) | 34 (16.8) | 2 (9.5) |
| ≥ 3, n (%) | 152 (75.2) | 18 (85.7) |
| Median (IQR) | 4 (3 to 6) | 4 (4 to 6) |
| Number of embryos thawed | ||
| 1, n (%) | 162 (80.2) | 18 (85.7) |
| 2, n (%) | 34 (16.8) | 2 (9.5) |
| ≥ 3, n (%) | 6 (3.0) | 1 (4.8) |
| Median (IQR) | 1 (1 to 1) | 1 (1 to 1) |
| Number of embryos thawed and discarded | ||
| 1, n (%) | 15 (7.4) | 0 |
| 2, n (%) | 2 (1.0) | 1 (4.8) |
| ≥ 3, n (%) | 1 (0.5) | 0 |
| Median (IQR) | 0 (0 to 0) | 0 (0 to 0) |
| Number of embryos thawed and refrozen, n (%) | ||
| ≥ 3 | 1 (0.5) | 0 |
| Number of embryos remaining frozen | ||
| 0, n (%) | 28 (13.9) | 1 (4.8) |
| 1, n (%) | 33 (16.3) | 4 (19.0) |
| 2, n (%) | 40 (19.8) | 1 (4.8) |
| ≥ 3, n (%) | 101 (50.0) | 15 (71.4) |
| Median (IQR) | 3 (1 to 4) | 3 (2 to 5) |
| Method of endometrial preparation for transfer, n (%) | ||
| Natural cycle | 6 (3.0) | 6 (28.6) |
| Natural cycle with HCG | 4 (2.0) | 0 |
| Artificial cycle with oestrogen and progesterone | 130 (64.4) | 7 (33.3) |
| Artificial cycle with oestrogen and progesterone and downregulation with GnRH agonist | 47 (23.3) | 6 (28.6) |
| Artificial cycle with oestrogen, progesterone and antagonist | 14 (6.9) | 2 (9.5) |
| Other | 1 (0.5) | 0 |
| Time from egg collection to embryo freezing, n (%) | ||
| Cleavage (day 3) | 14 (6.9) | 0 |
| Cleavage (day 4) | 3 (1.5) | 0 |
| Blastocyst (day 5) | 175 (86.6) | 19 (90.5) |
| Blastocyst (day 6) | 10 (5.0) | 1 (4.8) |
| < 3 days | 0 | 1 (4.8) |
Primary outcome
Intention-to-treat analysis
The ITT analysis (Table 7) showed that the healthy baby rate (i.e. term singleton live birth with appropriate weight for gestation) was 20.3% in the freeze-all arm and 24.4% in the fresh-embryo transfer arm (p = 0.28). There was no statistical difference with/without adjustment of confounding factors (adjusted RR 0.84, 95% CI 0.62 to 1.15). The proportion of singletons born was 27.7% in the freeze-all arm and 34.0% in the fresh-embryo transfer arm. The proportion of babies born at term was 25.4% in the freeze-all arm and 30.2% in fresh-embryo transfer arm. Similarly, the proportion with an appropriate weight for gestation was 22.5% in the freeze-all arm and 26.9% in the fresh-embryo transfer arm.
| Outcome | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | RR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Singleton baby born at term with an appropriate weight for gestation, n (%) (percentage is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 308) | 62 (20.3) | 75 (24.4) | 0.83 (0.62 to 1.12) | 0.84 (0.62 to 1.15) | 0.275 |
| Missing, n | 1 | 1 | |||
| Singleton, n (%) | 85 (27.7) | 105 (34.0) | |||
| Born at term, n (%) (percentage is of effective N: frozen, effective N = 307; fresh-embryo transfer, effective N = 308) | 78 (25.4) | 93 (30.2) | |||
| Missing, n | 0 | 1 | |||
| Appropriate weight for gestation, n (%) (percentage is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 308) | 69 (22.5) | 83 (26.9) | |||
| Missing, n | 1 | 1 | |||
Sensitivity analysis by compliance status of the freeze-all arm
When the analysis for the primary outcome was undertaken by compliance status of those randomised to the freeze-all arm, there was no difference in the outcome healthy baby rate between those who complied and those who did not comply with the allocated intervention (21.3% vs. 20.0%, respectively) (Table 8).
| Outcome | Treatment received, n (%) | |
|---|---|---|
| Frozen-embryo transfer (N = 202) | Fresh-embryo transfer (N = 96) | |
| Singleton baby born at term with an appropriate weight for gestation | 43 (21.3) | 19 (20.0) |
| Missing | 0 | 1 |
| Singleton | 56 (27.7) | 29 (30.2) |
| Born at term | 52 (25.7) | 26 (27.1) |
| Appropriate weight for gestation | 46 (22.8) | 23 (24.2) |
| Missing | 0 | 1 |
Sensitivity analysis: complier-average causal effect
The CACE RR was 0.77 (0.204/0.264). This was calculated for participants with no missing primary outcome data.
The values in Table 9 are calculated for the would-be non-compliers and would-be compliers of the fresh-embryo transfer arm, assuming the same non-compliance rate and event rate as the non-compliers of the freeze-all arm. 20 The CACE RR suggests that there is no difference in the healthy baby rate between the two arms.
| As per compliance status | Freeze-all arm (N = 306) | Fresh-embryo transfer arm (N = 308) | CACE RR (95% CI) | |||
|---|---|---|---|---|---|---|
| Compliance, n (%) | Primary outcome (n/N) | Event rate (%) | Primary outcome (n/N) | Event rate (%) | ||
| Compliers | 211 (69.0) | 43/211 | 20.4 | 56/212 | 26.4 | 0.77 (0.44 to 1.10) |
| Non-compliers | 95 (31.0) | 19/95 | 20.0 | 19/96 | 20.0 | |
| Total | 62/306 | 20.3 | 75/308 | 24.4 | ||
Exploratory analysis on primary outcome
In both arms, the healthy baby rate did not differ depending on whether the analysis was restricted to those who had received the allocated intervention (p = 0.45) or the analysis was undertaken as treated (p = 0.59) (Table 10).
| Analysis | Freeze-all arm | Fresh-embryo transfer arm | RR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Restricted per-protocol | |||||
| Total couples, excluding those who did not receive their allocated intervention, n | 202 | 282 | |||
| Singleton baby born at term with appropriate weight for gestation, n (%) (percentage is of effective N: frozen, effective N = 202; fresh-embryo transfer, effective N = 281) | 43 (21.3) | 70 (24.9) | 0.85 (0.61 to 1.19) | 0.87 (0.59 to 1.26) | 0.453 |
| Missing, n | 0 | 1 | |||
| As treated | |||||
| Total couples receiving each allocation, N | 223 | 378 | |||
| Singleton baby born at term with appropriate weight for gestation, n (%) (percentage is of effective N: frozen, effective N = 202; fresh-embryo transfer, effective N = 376) | 48 (21.5) | 89 (23.7) | 0.91 (0.67 to 1.24) | 0.91 (0.64 to 1.29) | 0.593 |
| Missing, n | 0 | 2 | |||
Subgroup analysis
The prespecified subgroup analysis for the primary outcome of healthy baby rate was undertaken based on the age of the female partner, number of previous embryo transfers, stage and number of embryos, and fertility clinic. As shown in Figure 10, there was no statistical difference in the healthy baby rate in different age groups (< 35, 35 to < 40 and > 40 years), number of previous embryo transfers (0 or ≥ 1), whether the transfer was undertaken at the cleavage or blastocyst stage, or whether one or two embryos were transferred (Table 11). There was a difference in the healthy baby rate between clinics; however, this is unlikely to be meaningful because of the very small numbers recruited by most clinics. Exploratory analyses were undertaken for method of endometrial preparation and type of freezing. Most frozen-embryo transfers were hormonally mediated and most embryos were frozen by vitrification.
FIGURE 10.
Subgroup analysis of the primary outcome. Adjusted for minimisation factors at randomisation. p-values from test of heterogeneity. Reproduced from Maheshwari et al. ,23 Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-Freeze), Human Reproduction, 2022, deab279, by permission of European Society of Human Reproduction and Embryology.
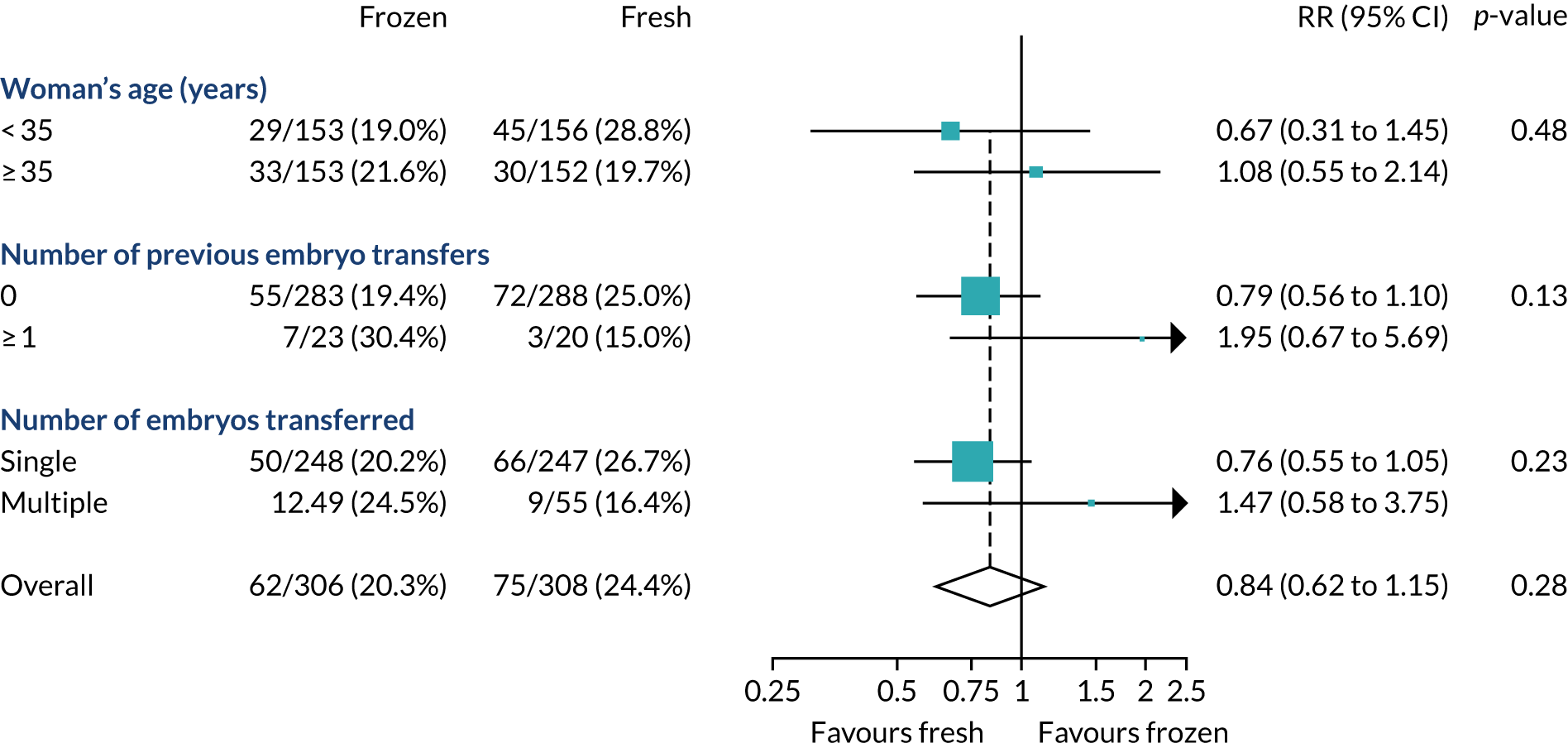
| Subgroup analysis | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | RRa (95% CI) | Interaction p-value |
|---|---|---|---|---|
| Woman’s age (years), n/N (%) | 0.100 | |||
| < 35 | 29/153 (19.0) | 45/156 (28.8) | 0.67 (0.31 to 1.45) | |
| 35 to < 40 | 32/136 (23.5) | 28/139 (20.1) | 1.15 (0.60 to 2.18) | |
| ≥ 40 | 1/17 (5.9) | 2/13 (15.4) | 0.41 (0.04 to 4.10) | |
| Fertility clinic, n/N (%) | < 0.001 | |||
| 1 | 2/11 (18.2) | 4/11 (36.4) | 0.45 (0.11 to 1.78) | |
| 2 | 2/13 (15.4) | 3/11 (27.3) | 0.60 (0.12 to 3.00) | |
| 3 | 15/90 (16.7) | 22/92 (23.9) | 0.67 (0.37 to 1.20) | |
| 4 | 9/49 (18.4) | 14/48 (29.2) | 0.68 (0.33 to 1.44) | |
| 5 | 5/31 (16.1) | 9/30 (30.0) | 0.54 (0.20 to 1.42) | |
| 6 | 7/21 (33.3) | 6/29 (20.7) | 1.62 (0.65 to 4.02) | |
| 7 | 4/11 (36.4) | 1/11 (9.1) | 4.46 (0.55 to 36.01) | |
| 8 | 3/24 (12.5) | 4/23 (17.4) | 0.73 (0.18 to 2.93) | |
| 9 | 2/7 (28.6) | 2/7 (28.6) | 0.87 (0.18 to 4.20) | |
| 10 | 0/10 (0.0) | 2/8 (25.0) | – | |
| 11 | 11/29 (37.9) | 7/30 (23.3) | 1.77 (0.79 to 3.97) | |
| 12 | 1/1 (100.0) | 0/1 (0.0) | – | |
| 13 | 1/9 (11.1) | 1/7 (14.3) | 0.88 (0.07 to 10.75) | |
| Previous embryo transfers, n/N (%) | 0.132 | |||
| 0 | 55/283 (19.4) | 72/288 (25.0) | 0.79 (0.56 to 1.10) | |
| ≥ 1 | 7/23 (30.4) | 3/20 (15.0) | 1.95 (0.67 to 5.69) | |
| Stage of embryo at transfer, n/N (%) | 0.821 | |||
| Cleavage | 1/16 (6.3) | 1/20 (5.0) | 1.16 (0.08 to 16.52) | |
| Blastocyst | 61/281 (21.7) | 73/281 (26.0) | 0.84 (0.61 to 1.16) | |
| Missing, n | 9 | 7 | ||
| Embryos transferred | 0.227 | |||
| Single, n/N (%) | 50/248 (20.2) | 66/247 (26.7) | 0.76 (0.55 to 1.05) | |
| Multiple, n/N (%) | 12/49 (24.5) | 9/55 (16.4) | 1.47 (0.58 to 3.75) | |
| Missing, n | 9 | 6 | ||
| Received frozen-embryo transfer, n | 202 | 21 | ||
| Method of endometrial preparation for transfer | ||||
| Natural cycle, n/N (%) | 0/10 (0.0) | 0/6 (0.0) | ||
| Hormone replacement cycle, n/N (%) | 43/191 (22.5) | 5/15 (33.3) | ||
| Missing, n | 1 | 0 | ||
| Method of embryo freezing, n/N (%) | ||||
| Vitrification | 37/178 (20.8) | 5/20 (25.0) | ||
| Slow freezing | 6/24 (25.0) | 0/1 (0.0) | ||
| Overall, n/N (%) | 62/306 (20.3) | 75/308 (24.4) | 0.84 (0.62 to 1.15) | 0.275 |
| Missing, n | 1 | 1 | ||
Secondary outcomes
Maternal safety: ovarian hyperstimulation syndrome
The risk of OHSS was lower in the freeze-all arm (3.6%) than in the fresh-embryo transfer arm (8.1%); however, this difference did not reach statistical significance (RR 0.44, 99% CI 0.15 to 1.30). The severity of OHSS was mild to moderate in the freeze-all arm, whereas six (1.9%) women had severe OHSS in the fresh-embryo transfer arm (Table 12).
| OHSS | Freeze-all arm (N = 307), n (%) | Fresh-embryo transfer arm (N = 309), n (%) | RR (99% CI) | |
|---|---|---|---|---|
| Unadjusted | Adjusteda | |||
| Women with OHSS | 11 (3.6) | 25 (8.1) | 0.44 (0.18 to 1.10) | 0.44 (0.15 to 1.30) |
| Severity | ||||
| Mild | 6 (2.0) | 7 (2.3) | ||
| Moderate | 5 (1.6) | 12 (3.9) | ||
| Severe | 0 (0.0) | 6 (1.9) | ||
Measures of clinical effectiveness
Table 13 presents the measures of clinical effectiveness.
| Measure | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | RR (99% CI) | |
|---|---|---|---|---|
| Unadjusted | Adjusteda | |||
| Live birth episode, n (%) | 87 (28.3) | 106 (34.3) | 0.83 (0.61 to 1.13) | 0.83 (0.65 to 1.06) |
| Singleton baby, n (%) | 85 (27.7) | 105 (34.0) | 0.81 (0.60 to 1.11) | 0.82 (0.64 to 1.06) |
| Singleton baby born at term, n (%) (percentage is of effective N: frozen, effective N = 307; fresh-embryo transfer, effective N = 308) | 78 (25.4) | 93 (30.2) | 0.84 (0.60 to 1.18) | 0.85 (0.67 to 1.08) |
| Missing, n | 0 | 1 | ||
| Singleton baby with appropriate weight for gestation, n (%) (percentage is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 308) | 68 (22.2) | 83 (26.9) | 0.82 (0.57 to 1.19) | 0.83 (0.55 to 1.26) |
| Missing, n | 1 | 1 | ||
| Pregnancy test 2 weeks after embryo transfer, n (%) | ||||
| Positive | 139 (45.3) | 154 (49.8) | 0.91 (0.73 to 1.13) | 0.91 (0.77 to 1.08) |
| Negative | 159 (51.8) | 149 (48.2) | ||
| Clinical pregnancy, n (%) | 104 (33.9) | 124 (40.1) | 0.84 (0.64 to 1.11) | 0.85 (0.65 to 1.11) |
| EPS performed, n (%) | 129 (42.0) | 139 (45.0) | ||
| Ongoing pregnancy, n/N (%) | 104/129 (80.6) | 123/139 (88.5) | ||
| Ectopic pregnancy, n/N (%) | 3/129 (2.3) | 5/139 (3.6) | ||
| Pregnancy of unknown location, n/N (%) | 3/129 (2.3) | 0/139 | ||
| Miscarriage, n/N (%) | 19/129 (14.7) | 11/139 (7.9) | ||
| EPS not performed, n (%) | 178 (58.0) | 170 (55.0) | ||
| Pregnancy lost before date of scan, n/N (%) | 10/178 (5.6) | 15/139 (8.8) | ||
| No embryo transfer or negative pregnancy test, n/N (%) | 165/178 (92.7) | 155/139 (91.2) | ||
| Other, n/N (%) | 3/178 (1.7) | 0/139 (0.0) | ||
There was no significant difference in the live birth rate between the freeze-all arm and the fresh-embryo transfer arm (28.3% vs. 34.3%; adjusted RR 0.83, 99% CI 0.65 to 1.06).
The singleton baby rate was 27.7% in the freeze-all arm and 34.0% in the fresh-embryo transfer arm, but the difference was not statistically significant (adjusted RR 0.82, 99% CI 0.64 to 1.06).
The rate of singleton babies born at term was 25.4% in the freeze-all arm and 30.2% in the fresh-embryo transfer arm. There was no statistically significant difference (adjusted RR 0.85, 99% CI 0.67 to 1.08). The details of gestational age were missing for one baby in the fresh-embryo transfer arm.
The rate of singleton babies with an appropriate weight for their gestational age was 22.2% in the freeze-all arm and 26.9% in the fresh-embryo transfer arm. There was no statistically significant difference (adjusted RR 0.83, 99% CI 0.55 to 1.26). Details were missing for one baby in each arm.
The rate of positive pregnancy tests was 45.3% in the freeze-all arm and 49.8% in the fresh-embryo transfer arm. There was no statistically significant difference (adjusted RR 0.91, 99% CI 0.77 to 1.08).
The clinical pregnancy rate was 33.9% in the freeze-all arm and 40.1% in the fresh-embryo transfer arm. There was no statistically significant difference (adjusted RR 0.85, 99% CI 0.65 to 1.11).
Complications of pregnancy and delivery
Tables 14 (ITT analysis) and 15 (clinically relevant denominators) present the complications in pregnancy and delivery. All complications in pregnancy and delivery are described in text with the clinically relevant denominator.
| Complication | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | RR (99% CI) | |
|---|---|---|---|---|
| Unadjusted | Adjusteda | |||
| Vanishing twin/triplet, n (%) | 4 (1.3) | 4 (1.3) | 1.01 (0.16 to 6.15) | – |
| Pregnancy loss, n (%) | ||||
| Miscarriage | 44 (14.3) | 40 (12.9) | 1.11 (0.66 to 1.87) | 1.09 (0.72 to 1.66) |
| Early (< 12 weeks’ gestation) | 34 (79.1) | 34 (85.0) | ||
| Late (12 to < 24 weeks’ gestation) | 9 (20.9) | 6 (15.0) | ||
| Gestational age unknown | 1 | 0 | ||
| Ectopic | 3 (1.0) | 6 (1.9) | 0.50 (0.08 to 3.07) | – |
| Termination | 2 (0.7) | 2 (0.6) | 1.01 (0.08 to 13.12) | – |
| Pregnancy of unknown location | 3 (1.0) | 0 (0.0) | ||
| GDM, n (%) (percentage is of effective N: frozen, effective N = 305; fresh-embryo transfer, effective N = 306) | 4 (1.3) | 4 (1.3) | 1.00 (0.16 to 6.13) | – |
| Missing | 2 | 3 | ||
| Multiple pregnancy, n (%) | 8 (2.6) | 5 (1.6) | 1.61 (0.38 to 6.89) | – |
| Multiple births, n (%) | 2 (0.7) | 1 (0.3) | 2.01 (0.09 to 46.88) | – |
| Hypertensive disorder, n (%) (percentage is of effective N: frozen, effective N = 305; fresh-embryo transfer, effective N = 306) | 8 (2.6) | 7 (2.3) | 1.15 (0.31 to 4.28) | – |
| Chronic hypertension | 0 (0.0) | 1 (0.3) | ||
| Pregnancy-induced hypertension | 4 (1.3) | 5 (1.6) | ||
| Pre-eclampsia | 5 (1.6) | 1 (0.3) | ||
| Eclampsia | 0 (0.0) | 0 (0.0) | ||
| Missing | 2 | 3 | ||
| Most severe hypertensive disorder experienced, n (%) (percentage is of effective N: frozen, effective N = 305; fresh-embryo transfer, effective N = 306) | ||||
| Chronic hypertension | 0 (0.0) | 1 (0.3) | ||
| Pregnancy-induced hypertension | 3 (1.0) | 5 (1.6) | ||
| Pre-eclampsia | 5 (1.6) | 1 (0.3) | ||
| Eclampsia | 0 (0.0) | 0 (0.0) | ||
| Missing | 2 | 3 | ||
| Antepartum haemorrhage (non-exclusive), n (%) (percentage is of effective N: frozen, effective N = 304; fresh-embryo transfer, effective N = 306) | 12 (3.9) | 13 (4.2) | 0.93 (0.34 to 2.55) | – |
| Placenta praevia | 1 (0.3) | 4 (1.3) | ||
| Placental abruption | 2 (0.7) | 1 (0.3) | ||
| Other | 4 (1.3) | 6 (2.0) | ||
| Unexplained | 6 (2.0) | 4 (1.3) | ||
| Missing | 3 | 3 | ||
| Onset of labour, n (%) (percentage is of effective N: frozen, effective N = 303; fresh-embryo transfer, effective N = 307) | ||||
| Spontaneous | 28 (9.2) | 50 (16.3) | 0.57 (0.32 to 1.00) | 0.57 (0.33 to 1.01) |
| Induced | 37 (12.2) | 39 (12.7) | ||
| Planned caesarean section | 18 (5.9) | 15 (4.9) | ||
| Missing | 4 | 2 | ||
| Mode of delivery, n (%) (percentage is of effective N: frozen, effective N = 303; fresh-embryo transfer, effective N = 307) | ||||
| Normal vaginal delivery | 28 (9.2) | 38 (12.4) | 0.75 (0.41 to 1.37) | 0.75 (0.54 to 1.05) |
| Instrumental vaginal delivery | 20 (6.6) | 30 (9.8) | 0.68 (0.33 to 1.38) | 0.69 (0.39 to 1.21) |
| Caesarean section | 35 (11.6) | 36 (11.7) | 0.99 (0.55 to 1.75) | 0.99 (0.67 to 1.47) |
| Missing | 4 | 2 | ||
| Preterm delivery (< 37 completed weeks of gestation), n (%) (percentage is of effective N: frozen, effective N = 307; fresh-embryo transfer, effective N = 308) | 9 (2.9) | 12 (3.9) | 0.75 (0.25 to 2.30) | – |
| Missing | 0 | 1 | ||
| Very preterm delivery (< 32 completed weeks of gestation), n (%) (percentage is of effective N: frozen, effective N = 307; fresh-embryo transfer, effective N = 308) | 2 (0.7) | 5 (1.6) | 0.40 (0.05 to 3.43) | – |
| Missing | 0 | 1 | ||
| Low birthweight (< 2500 g at birth), n (%) (percentage is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 309) | 7 (2.3) | 13 (4.2) | 0.54 (0.17 to 1.79) | – |
| Missing | 1 | 0 | ||
| Very low birthweight (< 1500 g at birth), n (%) (percentage is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 309) | 1 (0.3) | 8 (2.6) | 0.13 (0.01 to 1.92) | – |
| Missing | 1 | 0 | ||
| High birthweight (> 4000 g at birth), n (%) (percentage is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 309) | 10 (3.3) | 10 (3.2) | 1.01 (0.33 to 3.14) | – |
| Missing | 1 | 0 | ||
| Customised birthweight centile (in live births), mean (SD) (mean is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 308) | 53.1 (27.9) | 45.3 (27.9) | – | – |
| Missing, n | 1 | 1 | ||
| High weight for gestational age (> 90th centile), n (%) (percentage is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 308) | 9 (2.9) | 10 (3.2) | 0.91 (0.28 to 2.90) | – |
| Missing | 1 | 1 | ||
| Low weight for gestational age (< 10th centile), n (%) (percentage is of effective N: frozen, effective N = 306; fresh-embryo transfer, effective N = 308) | 8 (2.6) | 12 (3.9) | 0.67 (0.21 to 2.13) | – |
| Missing | 1 | 1 | ||
| Congenital anomaly/birth defect, n (%) (percentage is of effective N: frozen, effective N = 305; fresh-embryo transfer, effective N = 308) | 6 (2.0) | 7 (2.3) | 0.87 (0.21 to 3.57) | – |
| Tongue tie | 1 (0.3) | 2 (0.6) | ||
| Cleft palate | 1 (0.3) | 2 (0.6) | ||
| Other | 3 (1.0) | 1 (0.3) | ||
| Missing | 2 | 1 | ||
| Perinatal mortality up to 28 days after birth, n (%) | 1 (0.3) | 0 (0.0) | – | – |
| Stillbirth | 0 (0.0) | 0 (0.0) | ||
| Neonatal death up to 28 days after birth | 1 (0.3) | 0 (0.0) | ||
| Complication | Freeze-all arm, n (%) | Fresh-embryo transfer arm, n (%) | RR (99% CI) | |
|---|---|---|---|---|
| Unadjusted | Adjusteda | |||
| Women with a positive pregnancy test at 2 weeks ± 3 days after embryo transfer | ||||
| Sample size | 139 | 154 | ||
| Miscarriage | 44 (31.7) | 40 (26.0) | 1.22 (0.76 to 1.96) | 1.18 (0.76 to 1.84) |
| Early (< 12 weeks’ gestation) | 34/43 (79.1) | 34/40 (85.0) | ||
| Late (12 to < 24 weeks’ gestation) | 9/43 (20.9) | 6/40 (15.0) | ||
| Gestation unknown | 1 | 0 | ||
| Multiple pregnancy | 8 (5.8) | 5 (3.2) | 1.77 (0.42 to 7.46) | – |
| Pregnant women with an ongoing pregnancy resulting in delivery | ||||
| Sample size | 87 | 106 | ||
| GDM (percentage is of effective N: frozen, effective N = 85; fresh-embryo transfer, effective N = 103) | 4 (4.7) | 4 (3.9) | 1.21 (0.20 to 7.20) | – |
| Missing | 2 | 3 | ||
| Multiple pregnancy | 4 (4.6) | 4 (3.8) | 1.22 (0.20 to 7.25) | – |
| Hypertensive disorder (percentage is of effective N: frozen, effective N = 85; fresh-embryo transfer, effective N = 103) | 8 (9.4) | 7 (6.8) | 1.38 (0.39 to 4.97) | – |
| Chronic hypertension | 0 | 1 (1.0) | ||
| Pregnancy-induced hypertension | 4 (4.7) | 5 (4.9) | ||
| Pre-eclampsia | 5 (5.9) | 1 (1.0) | ||
| Eclampsia | 0 | 0 | ||
| Missing | 2 | 3 | ||
| Most severe hypertensive disorder experienced (percentage is of effective N: frozen, effective N = 85; fresh-embryo transfer, effective N = 103) | ||||
| Chronic hypertension | 0 | 1 (1.0) | ||
| Pregnancy induced hypertension | 3 (3.5) | 5 (4.9) | ||
| Pre-eclampsia | 5 (5.9) | 1 (1.0) | ||
| Eclampsia | 0 | 0 | ||
| Missing | 2 | 3 | ||
| Antepartum haemorrhage (percentage is of effective N: frozen, effective N = 84; fresh-embryo transfer, effective N = 103) | 11 (13.1) | 12 (11.7) | 1.12 (0.41 to 3.07) | – |
| Placenta praevia | 1 (1.2) | 4 (3.9) | ||
| Placental abruption | 2 (2.4) | 1 (1.0) | ||
| Other | 4 (4.8) | 6 (5.8) | ||
| Unexplained | 5 (6.0) | 3 (2.9) | ||
| Missing | 3 | 3 | ||
| Preterm delivery (< 37 completed weeks of gestation) (percentage is of effective N: frozen, effective N = 87; fresh-embryo transfer, effective N = 105) | 9 (10.3) | 12 (11.4) | 0.91 (0.31 to 2.65) | – |
| Missing | 0 | 1 | ||
| Very preterm delivery (< 32 completed weeks of gestation) (percentage is of effective N: frozen, effective N = 87; fresh-embryo transfer, effective N = 105) | 2 (2.3) | 5 (4.8) | 0.48 (0.06 to 4.03) | – |
| Missing | 0 | 1 | ||
| Onset of labour (percentage is of effective N: frozen, effective N = 83; fresh-embryo transfer, effective N = 104) | ||||
| Spontaneous | 28 (33.7) | 50 (48.1) | 0.70 (0.44 to 1.13) | 0.69 (0.43 to 1.11) |
| Induced | 37 (44.6) | 39 (37.5) | ||
| Planned caesarean section | 18 (21.7) | 15 (14.4) | ||
| Missing | 4 | 2 | ||
| Babies born | ||||
| Sample size | 89 | 107 | ||
| Mode of delivery for each baby (percentage is of effective N: frozen, effective N = 85; fresh-embryo transfer, effective N = 105) | ||||
| Normal vaginal delivery | 28 (32.9) | 38 (36.2) | 0.91 (0.54 to 1.53) | 0.92 (0.63 to 1.33) |
| Instrumental vaginal delivery | 20 (23.5) | 30 (28.6) | 0.82 (0.43 to 1.56) | 0.84 (0.56 to 1.27) |
| Caesarean section | 37 (43.5) | 37 (35.2) | 1.24 (0.77 to 1.97) | 1.21 (0.98 to 1.51) |
| Missing | 4 | 2 | ||
| Low birthweight (< 2500 g at birth) (percentage is of effective N: frozen, effective N = 88; fresh-embryo transfer, effective N = 105) | 8 (9.1) | 14 (13.1) | 0.69 (0.24 to 2.05) | – |
| Missing | 1 | 0 | ||
| Very low birthweight (< 1500 g at birth) (percentage is of effective N: frozen, effective N = 88; fresh-embryo transfer, effective N = 85) | 1 (1.1) | 8 (7.5) | 0.15 (0.01 to 2.28) | – |
| Missing | 1 | 0 | ||
| High birthweight (> 4000 g at birth) (percentage is of effective N: frozen, effective N = 88; fresh-embryo transfer, effective N = 85) | 10 (11.4) | 10 (9.3) | 1.22 (0.41 to 3.62) | – |
| Missing | 1 | 0 | ||
| High weight for gestational age (> 90th centile) (percentage is of effective N: frozen, effective N = 88; fresh-embryo transfer, effective N = 106) | 9 (10.2) | 10 (9.4) | 1.08 (0.35 to 3.33) | – |
| Missing | 1 | 1 | ||
| Low weight for gestational age (< 10th centile) (percentage is of effective N: frozen, effective N = 88; fresh-embryo transfer, effective N = 106) | 9 (10.2) | 12 (11.3) | 0.90 (0.31 to 2.64) | – |
| Missing | 1 | 1 | ||
| Congenital anomaly/birth defect (percentage is of effective N: frozen, effective N = 87; fresh-embryo transfer, effective N = 106) | 5 (5.7) | 5 (4.7) | 1.22 (0.25 to 5.95) | – |
| Tongue tie | 1 (1.1) | 2 (1.9) | ||
| Cleft palate | 1 (1.1) | 2 (1.9) | ||
| Other | 3 (3.4) | 1 (0.9) | ||
| Missing | 2 | 1 | ||
| Perinatal mortality up to 28 days after birth | 1 (1.1) | 0 | ||
| Stillbirth | 0 | 0 | ||
| Neonatal death up to 28 days after birth | 1 (1.1) | 0 | ||
Early-pregnancy loss
There were three ectopic pregnancies in the freeze-all arm and six in the fresh-embryo transfer arm (RR 0.50, 99% CI 0.08 to 3.07) (see Table 14). Two participants underwent a termination in each arm.
There was no statistically significant difference in the risk of miscarriage in pregnancies as a result of frozen-embryo transfer compared with that of fresh-embryo transfer (31.7% vs. 26.0%, respectively) (adjusted RR 1.18, 99% CI 0.76 to 1.84) (see Table 15).
Multiple pregnancies
There were four cases of vanishing twins in each arm. There were eight cases of multiple pregnancy in the freeze-all arm and five in the fresh-embryo transfer arm (see Table 14). For births, only two couples had multiple births in the freeze-all arm and one couple had a multiple birth in the fresh-embryo transfer arm.
Obstetric complications:
There was no difference in the risk of GDM in pregnancies as a result of frozen-embryo transfer and pregnancies as a result of fresh-embryo transfer (4.7% vs. 3.9%, respectively; RR 1.21, 99% CI 0.20 to 7.20).
There was no difference in the risk of hypertensive disorder in pregnancies as a result of frozen-embryo transfer and pregnancies as a result of fresh-embryo transfer (9.4% vs. 6.8%, respectively) (RR 1.38, 99% CI 0.39 to 4.97). There were no cases of eclampsia in the trial. There were five cases of pre-eclampsia (5.9%) in women who were pregnant as a result of frozen-embryo transfer and one (1%) in a woman pregnant as a result of fresh-embryo transfer.
The risk of antepartum haemorrhage was 13.1% in the freeze-all arm and 11.7% in the fresh-embryo transfer arm (RR 1.12, 99% CI 0.41 to 3.07) (see Table 15).
The risk of preterm delivery was 10.3% in deliveries in the freeze-all arm and 11.4% in those in the fresh-embryo transfer arm (RR 0.91, 99% CI 0.31 to 2.65). There was no difference in the risk of very preterm delivery between the freeze-all arm (2.3%) and the fresh-embryo transfer arm (4.8%) (RR 0.48, 99% CI 0.06 to 4.03).
There were no statistical differences between groups (adjusted RR 0.69, 99% CI 0.43 to 1.11) in the proportion of women undergoing spontaneous labour (frozen-embryo transfer arm, 33.7%, vs. fresh-embryo transfer arm, 48.1%), induced labour (44.6% vs. 37.5%, respectively) or planned caesarean section (21.7% vs. 14.4%, respectively). Data on onset of labour were missing for six couples (four in the frozen-embryo arm and two in the fresh-embryo arm).
A total of 196 babies (89 in the freeze-all arm and 107 in the fresh-embryo transfer arm) were born. In both arms, one-third of babies (freeze-all arm, 32.9%, vs. fresh-embryo transfer arm, 36.2%) were born by normal vaginal delivery (adjusted RR 0.92, 99% CI 0.63 to 1.33). The corresponding figures for instrumental vaginal delivery were 23.5% and 28.6%, respectively (adjusted RR 0.84, 99% CI 0.56 to 1.27), and for caesarean section were 43.5% and 35.2%, respectively (adjusted RR 1.21, 99% CI 0.98 to 1.51). Six couples (four in the freeze-all arm and two in the fresh-embryo transfer arm) had missing outcome data for mode of delivery.
The details of obstetric complications, with clinically relevant denominators, are reported in Table 15.
Neonatal outcomes
The risk of having a baby with a low birthweight was not statistically significantly different in the freeze-all arm and the fresh-embryo transfer arm (9.1% vs. 13.1%, respectively; RR 0.69, 99% CI 0.24 to 2.05). Similarly, the risk of delivering a low-birthweight baby was not significantly different between arms (1.1% vs. 7.5%, respectively; RR 0.15, 99% CI 0.01 to 2.28).
There was no statistically significant difference between arms in the proportion of babies born with a high birthweight (freeze-all arm, 11.4%, vs. fresh-embryo transfer arm, 9.3%; RR 1.22, 99% CI 0.41 to 3.62).
There was no statistically significant difference between arms in the risk of having a baby with a high birthweight for gestational age (freeze-all arm, 10.2%, vs. fresh-embryo transfer arm, 9.4%; RR 1.08, 99% CI 0.35 to 3.33).
There was no statistically significant difference between arms in the risk of having a baby with a low birthweight for gestational age (freeze-all arm, 10.2%, vs. fresh-embryo transfer arm, 11.3%; RR 0.90, 99% CI 0.31 to 2.64).
There was no difference between arms in the rate of congenital anomalies (freeze-all arm, 5.7%, vs. fresh-embryo transfer arm, 4.7%; RR 1.22, 99% CI 0.25 to 5.95). There was one neonatal death in the freeze-all arm and no neonatal deaths in the fresh-embryo transfer arm.
The details of neonatal outcomes, with clinically relevant denominator, are reported in Table 15.
Measures of effectiveness of the process of freezing embryos
The following measures were used to determine the effectiveness of the freezing process:
-
the total number of embryos frozen, thawed and transferred for all randomised couples
-
the proportion of thawed embryos that were then transferred for all randomised couples
-
no embryos surviving after thawing, leading to no embryo transfer.
To transfer 248 embryos, 280 embryos had to be thawed (i.e. 88.6% of embryos were suitable for transfer after being thawed) (Table 16).
| Measure | Couples (N = 616) |
|---|---|
| Total number of embryos | |
| Frozen | 967 |
| Thawed | 280 |
| Transferred | 248 |
| Percentage of thawed embryos that were then transferred | 88.6 |
Three couples in the freeze-all arm did not have any embryos to transfer because their embryos did not survive the freezing–thawing process (Table 17).
| Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | |
|---|---|---|
| No embryos survived thawing, leading to no embryo transfer, n (%) | 3 (1.0) | 0 (0.0) |
Evaluation of emotional status
The couples’ emotional state was assessed using the STAI questionnaire. 16 Both partners completed the questionnaire at two time points (at consent and at embryo transfer).
Table 18 reports the data from the STAI questionnaire.
| Parameters | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | MD (99% CI) | |
|---|---|---|---|---|
| Unadjusteda | Adjustedb | |||
| Consent | ||||
| Emotional state score | ||||
| Female partner complete responses, n | 295 | 295 | ||
| STAI score, mean (SD) | 35.8 (10.2) | 35.0 (10.5) | ||
| Male partner complete responses, n | 294 | 295 | ||
| STAI score, mean (SD) | 30.6 (8.8) | 29.9 (8.1) | ||
| Overall satisfaction with the IVF treatment process at consent, n/N (%) | ||||
| Female partner | ||||
| Very satisfied | 179/296 (60.5) | 174/294 (59.2) | ||
| Satisfied | 96/296 (32.4) | 102/294 (34.7) | ||
| Neither satisfied nor dissatisfied | 19/296 (6.4) | 17/294 (5.8) | ||
| Dissatisfied | 1/296 (0.3) | 1/294 (0.3) | ||
| Very dissatisfied | 1/296 (0.3) | 0/294 | ||
| Missing | 11 | 15 | ||
| Male partner | ||||
| Very satisfied | 190/295 (64.4) | 181/294 (61.6) | ||
| Satisfied | 87/295 (29.5) | 102/294 (34.7) | ||
| Neither satisfied nor dissatisfied | 17/295 (5.8) | 11/294 (3.7) | ||
| Dissatisfied | 1/295 (0.3) | 0/294 | ||
| Very dissatisfied | 0/295 | 0/294 | ||
| Missing | 12 | 15 | ||
| At embryo transfer | ||||
| Emotional state scores | ||||
| Female partner complete responses, nc | 218 | 227 | ||
| STAI score, mean (SD) | 38.2 (11.4) | 37.4 (10.8) | 0.0 (–2.4 to 2.3) | 0.0 (–2.2 to 2.2) |
| Male partner complete responses, nc | 203 | 218 | ||
| STAI score, mean (SD) | 33.5 (11.0) | 32.4 (9.1) | 0.1 (–1.9 to 2.2) | 0.1 (–2.4 to 2.6) |
| Overall satisfaction with the IVF treatment process at embryo transfer, n/N (%) | ||||
| Female partner | ||||
| Very satisfied | 119/217 (54.8) | 151/231 (65.4) | ||
| Satisfied | 78/217 (35.9) | 68/231 (29.4) | ||
| Neither satisfied nor dissatisfied | 8/217 (3.7) | 9/231 (3.9) | ||
| Dissatisfied | 3/217 (1.4) | 2/231 (0.9) | ||
| Very dissatisfied | 9/217 (4.1) | 1/231 (0.4) | ||
| Missing | 90 | 78 | ||
| Male partner | ||||
| Very satisfied | 123/203 (60.6) | 141/219 (64.4) | ||
| Satisfied | 68/203 (33.5) | 71/219 (32.4) | ||
| Neither satisfied nor dissatisfied | 9/203 (4.4) | 3/219 (1.4) | ||
| Dissatisfied | 2/203 (1.0) | 3/219 (1.4) | ||
| Very dissatisfied | 1/203 (0.5) | 1/219 (0.5) | ||
| Missing | 104 | 90 | ||
Emotional state score
A total of 295 women and 294 men in the freeze-all arm and 295 women and 295 men in the fresh-embryo transfer arm completed the questionnaire at consent. A total of 218 women and 203 men in the freeze-all arm and 227 women and 218 men in the fresh-embryo transfer arm completed the questionnaire at embryo transfer.
At the time of consent, the mean STAI score for female partners was 35.8 (SD 10.2) in the freeze-all arm and 35.0 (SD 10.5) in the fresh-embryo transfer arm. At embryo transfer, the mean STAI score for female partners was 38.2 (SD 11.4) in the freeze-all arm and 37.4 (SD 10.8) in the fresh-embryo transfer arm. There was no statistical difference between the two arms (adjusted MD 0.0, 99% CI –2.2 to 2.2).
At the time of consent, the mean STAI score for male partners was 30.6 (SD 8.8) in the freeze-all arm and 29.9 (SD 8.1) in fresh-embryo transfer arm. At embryo transfer, the mean STAI score for male partners was 33.5 (SD 11.0) in the freeze-all arm and 32.4 (SD 9.1) in the fresh-embryo transfer arm. There was no statistical difference between the two arms (adjusted MD 0.1, 99% CI –2.4 to 2.6).
Satisfaction with process at consent and embryo transfer
Most female partners who responded (92.9% in the freeze-all arm and 93.9% in the fresh-embryo transfer arm) were either satisfied or very satisfied at the time of consent.
Most female partners (90.8% in the freeze-all arm and 94.8% in the fresh-embryo transfer arm) were either satisfied or very satisfied at the time of embryo transfer.
Most male partners who responded (93.9% in the freeze-all arm and 96.3% in the fresh-embryo transfer arm) were either satisfied or very satisfied at the time of consent.
Most male partners (94.1% in the freeze-all arm and 96.8% in the fresh-embryo transfer arm) were either satisfied or very satisfied at the time of embryo transfer.
Serious adverse events
There were 30 AEs reported, as per the SAE reporting described in Chapter 2. None was directly related to the intervention. Two cases of ectopic pregnancies were assessed by the PI as related; however, the chief investigator and the sponsor’s assessment found that they were unrelated. Details of all SAEs are given in Appendix 4.
Chapter 4 Economic analysis
This chapter reports the economic evaluation of freezing all embryos, followed by frozen-embryo transfer, compared with fresh-embryo transfer, including a within-trial analysis of post-randomisation costs and outcomes up to and including delivery, and a model-based extrapolation of costs and outcomes over a complete cycle (including the subsequent transfer of the remaining frozen embryos in both arms). In addition, a within-trial cost–consequences summary is reported.
Objectives
The primary objective of the economic evaluation was to assess the incremental cost per additional healthy baby born of freezing all embryos, followed by frozen-embryo transfer, compared with fresh-embryo transfer in IVF. Two secondary economic objectives were to compare the cost and consequences between these embryo transfer strategies and to model the longer-term cost-effectiveness of frozen-embryo transfer compared with fresh-embryo transfer.
Methods
Study design and participants
Details of the trial design are provided in the published protocol1 and in Chapter 2. The economic analysis was based on all women randomised, except for three post-randomisation exclusions, and follows the same ITT principle as the statistical analysis in Chapter 3.
Cost and outcome assessment
Costs and outcomes were assessed from post randomisation up to and including delivery, using the trial eCRFs completed by the research staff post embryo transfer, during early pregnancy (i.e. 6–8 weeks’ gestation), at the 12- and 28-week follow-ups and 6 weeks post delivery, and the paper-based economic questionnaire completed by couples at embryo transfer. Health-care utilisation data collected post embryo transfer, during early pregnancy, at 28 weeks’ gestation and at 6 weeks post delivery were used in the economic analysis. Questions related to participant time and travel costs incurred during treatment were included in the embryo transfer economic questionnaire (see Report Supplementary Material 2). All costs are reported in 2018/19 Great British pounds. Adjustments for inflation were applied to unit costs, where necessary, using the NHS Cost Inflation Index (NHSCII). 25
Assessment of health service costs
Given that the economic evaluation seeks to inform the efficient allocation of scarce health-care resources, the base-case analysis adopted the NHS and Personal Social Services perspective. The effect on patient time and travel costs was considered separately as a secondary analysis.
Cost of the primary intervention
The cost of the embryo transfer procedure was estimated based on the health service resource use observed for each arm. The additional cost of freezing all embryos, followed by frozen-embryo transfer, was estimated from resource use data recorded in the eCRF post embryo transfer for each participant who received frozen-embryo transfer. The eCRF captured the number of monitoring visits, blood tests and ultrasound scans prior to embryo transfer, and the method of endometrial preparation. The cost of preparing frozen embryos was also included for the participants who received frozen-embryo transfer. The cost of embryo freezing was applied to all participants who received frozen-embryo transfer, as well as to the participants who had their remaining embryos frozen following a fresh-embryo transfer. The unit costs that were used to value the resource use associated with the intervention are reported in Appendix 5.
The costing approach assigned costs to each component of resource use to capture patient-level variation in costs. Each resource use item was mapped to an appropriate Healthcare Resource Group (HRG), where available, and costed using the relevant NHS reference cost. 26 The monitoring visit prior to transfer was assumed to be a 30-minute session led by a nurse. 25 The reported methods of endometrial preparation were valued based on routine regimens obtained from clinical advice and unit costs from the British National Formulary (BNF). 27 For embryo freezing and the preparation of frozen embryos, the time spent by an embryologist for each procedure was assumed to be 1 hour based on clinical advice. 25
Costs of ovarian hyperstimulation syndrome
The clinical management costs associated with OHSS and pregnancy were valued using the NHS Reference Costs 2018/19. 26 The resource use for managing OHSS was obtained from the eCRFs post embryo transfer and during early pregnancy. The non-elective inpatient stay cost for a 1-day stay was valued using the inpatient short stay cost, whereas inpatient stays of > 1 day were valued using the inpatient long stay cost, adjusted for length of stay using the excess bed-day cost if the stay was longer than the average length of stay. As some of the relevant data needed for the adjustment of inpatient long-stay costs were not available in the NHS Reference Costs 2018/19, the average length of stay for non-elective long stays and the non-elective excess bed-day cost were obtained from the NHS Reference Costs 2017/1828 and inflated. This approach was undertaken for all costs associated with inpatient stays in the analysis.
Costs of pregnancy outcomes
Data on pregnancy outcomes (e.g. miscarriage, biochemical pregnancy, ectopic pregnancy, pregnancy of unknown location and termination) were obtained from the eCRF during early pregnancy, at the 12- and 28-week follow-ups. Miscarriage and ectopic pregnancy were costed by applying the average reference cost per case, whereas termination cost varied by gestation. It was assumed that, on average, a minimum of one ultrasound scan and three beta-human chorionic gonadotropin (βHCG) blood tests would be required for biochemical pregnancy and pregnancy of unknown location. 29
Costs of antenatal care
The antenatal care (ANC) costs were based on the NHS Reference Costs 2018/1926 and varied according to the maternal complications reported. Primary and secondary care contacts during pregnancy and any complications were captured in the 28-week follow-up CRF and post-delivery eCRF. It was assumed that participants had a community midwife visit following an early pregnancy scan (EPS). The complications were grouped using the Code to Group: HRG4+ 2019/20 Local Payment Grouper30 workbook to determine the corresponding HRG. Episodes of care were costed by applying either the day-case reference cost for an inpatient day care visit or the non-elective inpatient cost (stay of ≥ 1 day), adjusted for length of stay using the excess bed-day cost.
For antenatal ultrasound scans, the resource use was obtained from the eCRFs during early pregnancy, at the 28-week follow-up and post delivery. The costs of ultrasound were determined using the NHS reference cost26 and were varied in accordance with the standard recommendation for antenatal ultrasound scanning of one scan throughout the pregnancy. Any additional ultrasound scans were costed as non-routine.
Costs of delivery
The delivery method was obtained from the post-delivery eCRF and valued based on the NHS reference cost. 26 The costs varied according to the delivery mode, onset method (with or without induction) and length of inpatient stay for delivery.
Participant travel and time costs
Participant costs associated with travelling to and from appointments and the time spent for a clinic visit during treatment were estimated from post randomisation to embryo transfer. Travel costs were estimated based on the number of visits to the clinic and travel expenses per visit or distance travelled by car, obtained from the economic questionnaire. Travel costs were estimated based on the expenses reported for participants who travelled using public transport and taxis, whereas the costs for travel by car were calculated using the mileage reported and the private car rate per mile of 45p per mile published by Her Majesty’s Revenue and Customs (HMRC). 31 Time costs, which account for time lost from productive activities, were estimated from the economic questionnaire based on the time taken to visit the clinic. Time taken away from normal productive activities was estimated in hours, and appropriate unit costs were used to estimate the opportunity cost of time. Gross age- and sex-specific wage rates obtained from the Annual Survey of Hours and Earnings (ASHE),32 published by the Office for National Statistics, were used to cost the time lost from paid employment. To estimate the cost associated with time lost for the accompanying male partner, the partner’s age was assumed to be the same as that of the participant. The cost of time lost from unpaid work was estimated using the value of unpaid work published by the Office for National Statistics. 33 Forgone leisure time was valued using the current value of non-working time, available from the Department for Transport. 34 The unit costs that were used to value the time lost are reported in Appendix 5.
Outcome measures
Effectiveness for the economic evaluation was measured in terms of the number of healthy babies born and the secondary clinical outcomes of the trial. Secondary outcomes included live births, maternal safety outcomes (i.e. OHSS), pregnancy outcomes, complications of pregnancy and delivery and adverse birth outcomes.
Statistical analysis of trial economic data
Aggregating costs and effects
Resource use, costs and health outcome data were summarised by trial arm, based on the participants who had the event of interest and by ITT. The IVF costs were broken down into the following categories: freezing of embryo, endometrial preparation, embryo transfer, monitoring visits prior to frozen-embryo transfer, blood tests prior to frozen-embryo transfer, transvaginal ultrasound scans prior to frozen-embryo transfer and preparation of frozen embryos. All cost elements were summed over the follow-up period (up to and including the cost of delivery) to estimate a total NHS cost per patient.
Cost data were fully present with respect to resource use associated with embryo transfers and OHSS; however, given that no further resource use data were collected for 15 participants who did not undergo embryo transfer, these participants were conservatively assigned no further treatment costs other than embryo freezing and thawing, where applicable. This assumption favours the freeze-all approach because three patients in the freeze-all arm may have incurred further work-up costs prior to cancellation of their embryo transfer owing to the embryos failing to survive the thawing process. Therefore, we also conducted a sensitivity analysis in which multiple imputation was used to impute the pre-embryo-transfer monitoring visit, blood test and scan costs for these three participants.
Elements of resource use data were also missing for a small number (n = 13) of resultant pregnancies. Two of these participants who had uncomplicated pregnancies were missing entries for the number of antenatal midwifery, outpatient and inpatient attendances between 12 and 28 weeks’ gestation only. These two participants were included in the complete-case analysis by assigning a zero cost to these elements; based on a comparison with similar participants with no missing data, it was considered plausible that these elements were missing because the costs were zero and they were not missing at random. Thus, for the complete-case analysis, we retained 616 participants for the cost-effectiveness analysis using treatment plus OHSS costs, and 605 participants for the analysis of total NHS costs (inclusive of ANC and delivery care).
Recognising the uncertainty in the above approach, we also conducted a sensitivity analysis using multiple imputation to impute plausible values for all 13 participants with missing ANC and delivery care cost elements, and the work-up costs for the three cancelled frozen-embryo transfer cycles where missingness could not be ascertained. We did not, however, impute missing values for the primary clinical effectiveness outcome, allowing 614 participants to be included in the cost-per-healthy-live birth multiple-imputation analysis and 616 participants to be included in the cost-per-live birth multiple-imputation analysis.
Within-trial cost-effectiveness and cost-consequence analysis
The within-trial economic analysis was performed on an ITT basis using individual participant-level data from the trial. The cost differences were summarised by ITT against the primary and secondary clinical outcomes using a cost–consequence balance sheet. All analyses were performed using Stata®, version 15 (StataCorp LLC, College Station, TX, USA).
For the within-trial cost-effectiveness analysis, two cost categories were used: treatment costs (including post-randomisation preparation and embryo transfer costs); and OHSS costs and full NHS costs, which, in addition to treatment and OHSS costs, included pregnancy and delivery costs. Cost-effectiveness was expressed in terms of the incremental cost per healthy baby and per live birth of freezing all embryos, followed by frozen-embryo transfer, compared with fresh-embryo transfer. The incremental treatment cost (inclusive of OHSS costs) per additional healthy baby born was estimated as the primary measure of cost-effectiveness, as the management and incidence rate of complications per pregnancy were similar between arms, with no statistically significant differences. Generalised linear models (GLMs), with adjustment for minimisation factors, were used to estimate MDs in costs and effects between the trial arms. For cost outcomes, a gamma family with log-link was selected using the modified Park test, Pearson’s correlation, Pregibon link and modified Hosmer–Lemeshow tests. 35 For the effectiveness outcomes (i.e. healthy babies and live births), a Poisson family with log-link was used, as per the statistical analysis. Recycled predictions were used to recover adjusted mean values by trial arm, as well as the incremental differences between trial arms. 35 The incremental cost-effectiveness ratio (ICER) for frozen-embryo transfer compared with fresh-embryo transfer was calculated as the difference in mean cost divided by the difference in mean effect. The variance surrounding the joint incremental costs and effects was characterised using non-parametric bootstrapping (i.e. 1000 iterations), with simulated output summarised graphically using cost-effectiveness plane and cost-effectiveness acceptability curves. For the sensitivity analysis using multiple imputation, the imputation model used chained equations with predictive mean matching (k = 5) to generate five imputed data sets (m = 5) nested within each bootstrapped resample (n = 1000). The imputation model included all of the missing cost elements, treatment allocation, women’s age and indicators for pregnancy and live birth as auxiliary variables.
Sensitivity analysis
The sensitivity analysis focused on the costing methodology for the initial interventions and missing data. A sensitivity analysis using multiple imputation chained equation was performed to assess the impact of missing data (including participants with partial missing data) on the robustness of the cost-effectiveness findings of the incremental NHS cost per baby born. The trial-based cost-effectiveness analysis was also conducted using pre-defined subgroups for women’s age at ovarian stimulation (< 35 years vs. ≥ 35 years).
Modelling of subsequent frozen-embryo transfers
Although the within-trial analysis is useful for informing cost-effectiveness in the short term, a longer time horizon is required to determine the relative cost-effectiveness of the alternative embryo transfer strategies in the context of routine IVF practice, whereby the subsequent transfer of the remaining frozen embryos can take place if the initial fresh-embryo transfer or frozen-embryo transfer fails to achieve a live birth. Therefore, a Markov model was developed to simulate progression through the subsequent transfer of the remaining frozen embryos for those failing to achieve live birth in both trial arms. This compares the policy of offering a full cycle of IVF using the freeze-all approach and a full cycle of IVF using the routine approach, in which a fresh embryo is used for the index transfer and the remaining frozen embryos are replaced in subsequent associated transfers. Although NICE3,36 recommends up to three full cycles of IVF treatment, with remaining good-quality embryos frozen and, subsequently, transferred as part of the same cycle, implementation of this guidance is low in England. 37 Furthermore, the most efficient approach to the first full IVF cycle is also likely to offer the best value for money if repeated full IVF cycles are permitted. Therefore, the economic model considered one full IVF cycle. The index transfer in the model was parametrised using event rates and costs derived from the ITT analysis of the E-Freeze trial data, including post-randomisation costs for the initial embryo transfer, OHSS costs and ongoing costs associated with pregnancies. The costs and outcomes associated with subsequent frozen-embryo transfers were extrapolated using several assumptions (see Figure 11 for details). The state transition diagram for the model is provided in Figure 11. The details of the derived model parameter inputs are provided in Results.
FIGURE 11.
State transition diagram for the Markov model. a, Tunnel states consisting of nine 4-week temporary states to capture progression through pregnancy. b, States were multiplied by 6 to allow for up to six subsequent frozen-embryo transfers.
The model structure is replicated for the fresh-embryo transfer and freeze-all arms of the E-Freeze trial and runs on a fixed 4-week Markov cycle. Couples start the model in the ‘index fresh embryo transfer’ or the ‘index frozen-embryo transfer’ state based on the observed distribution in the respective arms of the E-Freeze trial. Following this, couples proceed to embryo transfer and either achieve a positive pregnancy test result or fail to become pregnant. A small proportion also receive no transfer, as observed in both arms of the trial. Those who fail to become pregnant move to either the ‘not pregnant – frozen embryos remaining’ or the ‘cycle complete – no child’ state, depending on the availability of remaining frozen embryos. Those who become pregnant move to the ‘pregnant’ state, which is a tunnel state consisting of nine temporary states that can be occupied for one 4-week cycle only. This captures progression and outcomes through the stages of pregnancy. During pregnancy, women incur ANC costs relevant to their stage of pregnancy, as observed by trial arm in the E-Freeze trial. Women can also lose their pregnancy; in these situations, they incur the cost of this event and transition to the ‘not pregnant – frozen embryos remaining’ or ‘cycle complete – no child’ state.
For those who carry their pregnancy to ≥ 24 weeks’ gestation, birth outcomes are modelled as observed by trial arm in the E-Freeze trial, and the relevant costs of delivery care are applied. To fit with the model structure and cycle length, preterm deliveries were categorised into three mutually exclusive categories as follows: < 28 weeks’, 28–32 weeks’ and 33–36 weeks’ gestation. For those transitioning to the ‘not pregnant – frozen embryos remaining’ state, the observed numbers of remaining embryos by treatment allocation arm in E-Freeze were used, in conjunction with several assumptions informed by external data,38 to inform transitions through subsequent frozen transfers of remaining embryos (see Figure 11). The model states representing subsequent embryo transfers, pregnancy following subsequent transfer, and failure to achieve pregnancy following subsequent transfer (see Figure 11), are multiplied by six to allow for up to six frozen transfers for those failing to achieve a live birth. Once a live birth has been achieved, no further transfers are modelled.
For the key outcomes that drive differences in the live birth rate and healthy baby rate between the trial arms, the model utilises relative risks (and 95% CIs) for frozen-embryo transfer and fresh-embryo transfer (ITT) applied to the baseline probabilities of events observed in the fresh-embryo transfer arm of E-Freeze [positive pregnancy following embryo transfer, RR 0.91 (95% CI 0.77 to 1.08); any pregnancy loss following a positive pregnancy test, RR 1.18 (95% CI 0.76 to 1.84); delivery prior to 33 weeks’ gestation for ongoing pregnancies at 24 weeks’ gestation, RR 0.60 (95% CI 0.15 to 2.34); and delivery between 33 and < 37 weeks’ gestation for ongoing pregnancies at end of week 32 of gestation, RR 1.17 (95% CI 0.39 to 3.52)]. The relative risk for OHSS is also applied (0.44 95% CI, 0.15 to 1.3). For other parameters, trial arm-specific event counts and costs are derived from the observed E-Freeze outcome and cost data. The details of these derived model parameter inputs are provided in Results.
Following internal validation of the model output for the index embryo transfer against the trial-based cost-effectiveness findings, the model was used to extrapolate the costs and consequences of transferring the remaining frozen embryos. For this, the distribution of remaining embryos by trial arm in the E-Freeze trial for those not achieving live birth was used to estimate the proportion of women expected to have remaining embryos available for a second, third, fourth, fifth, sixth and seventh embryo transfer attempt. This required two key assumptions:
-
The remaining frozen embryos are thawed and replaced one at a time.
-
The transfer of each remaining frozen embryo has an equal chance of resulting in pregnancy and live birth, regardless of the approach to the first transfer and the total number of embryos remaining frozen.
For all subsequent frozen-embryo transfers, the event rates and costs were based on those observed for the index transfer in the frozen arm of the E-Freeze trial. The following adjustments were also made:
-
It was assumed that not all couples with remaining frozen embryos available after each failed transfer would return to use them (i.e. a discontinuation rate was applied after each failed transfer).
-
The survival rate for thawed embryos was set to 88%, in line with data from the E-Freeze trial.
-
The chance of pregnancy with subsequent frozen-embryo transfers was assumed to be lower, relative to the chance of pregnancy in the index transfer in the freeze-all arm of the E-Freeze trial.
-
A duration of three model cycles (i.e. 12 weeks) was assumed between each embryo transfer attempt.
The adjustments described in 1 and 3 above were based on an Australian population-based study reporting on the cumulative live birth rate (CLBR) following a freeze-all strategy compared with a fresh-embryo transfer strategy,38 including a good-prognosis group with a similar number of embryos available and a similar live birth rate as those of the E-Freeze cohort. Following each failed embryo transfer and using the reported data, a discontinuation rate was calculated as the proportion of women with remaining frozen embryos who did not return for a further transfer. This was taken as the average across the fresh-embryo transfer and freeze-all groups in the best prognosis subgroup of the Australian study. The relative adjustment to the pregnancy rate in subsequent frozen-embryo transfers compared with index frozen-embryo transfer cycles was derived using data reported for the freeze-all group of the best-prognosis subgroup of the Australian study. No more than six subsequent frozen-embryo transfers were incorporated in the model, as < 5% of the E-Freeze cohort had more than six embryos remaining frozen following failure of the index transfer, and the increase in the expected live birth rate from allowing a sixth transfer was < 0.1% compared with that from allowing up to five subsequent frozen-embryo transfers.
The model also included final birth outcome states, as it was originally intended to extrapolate longer-term costs and health outcomes per child born following treatment. This was in anticipation of a potential trade-off between maximising the live birth rate and maximising the health baby rate. However, the E-Freeze trial data indicated that, as a proportion of live births, the healthy baby rate was the same (71%) in both arms. Therefore, a decision was made not to include these in the current model. Although there were differences in the percentages of live births falling into the categories of preterm delivery and abnormal birthweight, these differences were based on very small numbers and, therefore, are highly uncertain. This would consequently translate into a high degree of uncertainty around any modelled difference in expected child health service costs or health outcomes.
Model-based analysis
The model was run probabilistically over a time horizon of 5 years, using 1000 random draws from distributions assigned to each clinical and cost input parameter. The results of the model were assessed in terms of the incremental cost per healthy baby and the incremental cost per live birth. Costs incurred beyond year 1 in the model were discounted using a rate of 3.5%, in line with the NICE reference case,39 but birth outcomes were not discounted because there is no guidance on the discount rate to apply to these outcomes in the context of fertility treatment. The probabilistic model output was summarised using cost-effectiveness scatterplots and cost-effectiveness acceptability curves, indicating the probability of each strategy being preferred on grounds of cost-effectiveness by increasing levels of societal willingness to pay per additional healthy baby or additional live birth. A further deterministic sensitivity analysis was undertaken to assess the robustness of the model findings to key structural uncertainties and costing assumptions.
Results
Health service resource use and costs
Table 19 summarises NHS resource use by trial arm and Table 20 summarises costs by trial arm. A total of 601 participants received an embryo transfer, with 117 participants not receiving their allocated treatment. As a result, 223 participants received frozen-embryo transfer, including 21 who were allocated to fresh-embryo transfer. Prior to frozen-embryo transfer, participants had additional monitoring visits, blood tests and transvaginal ultrasound scans. The crossover participants had slightly fewer monitoring visits and transvaginal scans, but more blood tests. The resource use associated with IVF translated to an average post-randomisation treatment cost of £1538 and £1216 in the freeze-all and fresh-embryo transfer arms, respectively, giving an unadjusted cost difference of £322. Compared with frozen-embryo transfer, a larger number of participants developed OHSS in the fresh-embryo transfer arm (8% vs. 4%, respectively), and participants in the fresh-embryo transfer arm had more outpatient visits and inpatient day case visits and a longer inpatient length of stay than participants in the freeze-all arm. The mean OHSS costs by treatment allocation were £17 for frozen-embryo transfer and £201 for fresh-embryo transfer.
| Variable | Number of observations | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|---|
| Fresh-embryo transfer, n (%) | 378 | 96 (31) | 282 (91) |
| Frozen-embryo transfer, n (%) | 223 | 202 (66) | 21 (7) |
| No transfer, n (%) | 15 | 9 (3) | 6 (2) |
| Care associated with frozen-embryo transfer | |||
| Sample size (n) | 223 | 202 | 21 |
| Monitoring visit prior to frozen-embryo transfer, mean (SD) | 223 | 2.19 (2.08) | 1.86 (1.39) |
| Blood test prior to frozen-embryo transfer, mean (SD) | 223 | 0.35 (0.81) | 0.62 (1.07) |
| Transvaginal ultrasound prior to frozen-embryo transfer, mean (SD) | 223 | 1.80 (1.51) | 1.38 (0.97) |
| Endometrial preparation for frozen-embryo transfer (n)a | 223 | 202 | 21 |
| Natural cycle, n (%) | 12 | 6 (3) | 6 (29) |
| Natural cycle with HCG, n (%) | 4 | 4 (2) | 0 (0) |
| Artificial cycle with oestrogen and progesterone, n (%) | 137 | 130 (64) | 7 (33) |
| Artificial cycle with oestrogen, progesterone and GnRH agonist, n (%) | 53 | 47 (23) | 6 (29) |
| Artificial cycle with oestrogen, progesterone and antagonist, n (%) | 16 | 14 (7) | 2 (10) |
| Other, n (%) | 1 | 1 (0) | 0 (0) |
| OHSS | |||
| Participants with OHSS, n (%) | 36 | 11 (4) | 25 (8) |
| Outpatient hospital visits, mean (SD) | 36 | 0.27 (0.90) | 2.32 (3.02) |
| Inpatient day case visits, mean (SD) | 36 | 1.27 (2.00) | 1.64 (2.06) |
| Inpatient length of stay, mean (SD) | 36 | 0.09 (0.30) | 0.68 (1.75) |
| Pregnancy outcomeb | |||
| 2 weeks post embryo transfer, n (%) | 601 | 298 | 303 |
| Positive | 293 | 139 (47) | 154 (51) |
| Negative | 308 | 159 (53) | 149 (49) |
| 6–8 weeks’ gestation, n (%) | 293 | 139 | 154 |
| Ongoing pregnancy | 227 | 104 (75) | 123 (80) |
| Biochemical pregnancy | 25 | 10 (7) | 15 (10) |
| Miscarriage | 30 | 19 (14) | 11 (7) |
| Ectopic pregnancy | 8 | 3 (2) | 5 (3) |
| Pregnancy of unknown location | 3 | 3 (2) | 0 (0) |
| 12 weeks’ gestation, n (%) | 227 | 104 | 123 |
| Ongoing pregnancy | 201 | 93 (89) | 108 (88) |
| Miscarriagec | 23 | 10 (10) | 13 (11) |
| Ectopic pregnancy | 1 | 0 (0) | 1 (1) |
| Termination | 2 | 1 (1) | 1 (1) |
| 28 weeks’ gestation, n (%) | 201 | 93 | 108 |
| Ongoing pregnancy | 189 | 87 (94) | 102 (94) |
| Miscarriage | 6 | 5 (5) | 1 (1) |
| Termination | 2 | 1 (1) | 1 (1) |
| Live birth | 4 | 0 (0) | 4 (4) |
| ANC | |||
| 6 to < 12 weeks’ gestation (n) | 293 | 139 | 154 |
| Antenatal ultrasound, mean (SD) | 293 | 0.93 (0.26) | 0.90 (0.30) |
| 12 to < 28 weeks’ gestation (n) | 201 | 93 | 108 |
| Community midwife visit, mean (SD) | 199 | 2.60 (1.64) | 2.61 (1.87) |
| Outpatient hospital visits, mean (SD) | 199 | 2.13 (1.88) | 2.25 (2.12) |
| Inpatient day case visits, mean (SD) | 199 | 0.18 (0.69) | 0.15 (0.53) |
| Inpatient length of stay, mean (SD) | 199 | 0.44 (3.16) | 0.33 (1.20) |
| Antenatal ultrasound, mean (SD) | 199 | 2.09 (1.68) | 2.08 (1.45) |
| Missing, n (%) | 2 | 0 (0) | 2 (2) |
| 28 weeks’ gestation to delivery (n) | 189 | 87 | 102 |
| Community midwife visit, mean (SD) | 181 | 3.13 (2.29) | 3.22 (2.42) |
| Outpatient hospital visits, mean (SD) | 181 | 2.80 (2.22) | 2.80 (2.19) |
| Inpatient day case visits, mean (SD) | 181 | 0.32 (0.84) | 0.41 (1.00) |
| Inpatient length of stay, mean (SD) | 181 | 0.60 (1.63) | 0.43 (2.30) |
| Antenatal ultrasound, mean (SD) | 181 | 2.30 (1.88) | 2.24 (1.71) |
| Missing, n (%) | 8 | 3 (3) | 5 (5) |
| Maternal complication | |||
| 12 to < 28 weeks’ gestation, n (%) | 201 | 93 | 108 |
| No maternal complications | 172 | 81 (87) | 91 (84) |
| Hypertensive disorder (non-exclusive) | 6 | 3 (3) | 3 (3) |
| GDM (non-exclusive) | 7 | 3 (3) | 4 (4) |
| Antepartum haemorrhage | 15 | 7 (8) | 8 (7) |
| Missing | 2 | 0 (0) | 2 (2) |
| 28 weeks’ gestation to delivery, n (%) | 189 | 87 | 102 |
| No maternal complications | 149 | 68 (78) | 81 (79) |
| Hypertensive disorder (non-exclusive) | 13 | 7 (8) | 6 (6) |
| GDM (non-exclusive) | 7 | 3 (3) | 4 (4) |
| Antepartum haemorrhage | 15 | 6 (7) | 9 (9) |
| Missing | 6 | 3 (3) | 3 (3) |
| Delivery, | |||
| Sample size (n) | 193 | 87 | 106 |
| Inpatient length of stay, mean (SD) | 185 | 3.15 (2.11) | 3.12 (1.97) |
| Missing, n (%) | 8 | 3 (3) | 5 (5) |
| Delivery mode, n (%) | |||
| Normal vaginal delivery | 66 | 28 (33) | 38 (36) |
| Instrumental vaginal delivery | 50 | 20 (23) | 30 (28) |
| C-section | 71 | 35 (41) | 36 (34) |
| Missing | 6 | 4 (5) | 2 (2) |
| Newborns delivered (live births and stillbirths), n (%) | |||
| Sample size | 193 | 87 | 106 |
| Still births | 0 | 0 (0) | 0 (0) |
| Neonatal deaths | 1 | 1 (1) | 0 (0) |
| Preterm newborns | 21 | 9 (10) | 12 (11) |
| Term newborns | 170 | 77 (89) | 93 (88) |
| Missing | 1 | 0 (0) | 1 (1) |
| Variable | Number of observations | Mean cost (£) (SD) | |
|---|---|---|---|
| Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | ||
| IVF costs | 616 | 1538.45 (473.67) | 1215.51 (221.17) |
| Freezing of embryo | 616 | 41.16 (15.96) | 38.14 (18.75) |
| Endometrial preparation | 616 | 131.88 (104.18) | 78.05 (50.45) |
| Embryo transfer | 616 | 1063.07 (185.05) | 1073.91 (151.37) |
| Monitoring visit prior to frozen-embryo transfer | 616 | 80.81 (111.32) | 7.08 (32.94) |
| Blood test prior to frozen-embryo transfer | 616 | 0.25 (0.75) | 0.05 (0.35) |
| Transvaginal ultrasound prior to frozen-embryo transfer | 616 | 189.66 (239.42) | 15.05 (68.56) |
| Preparation of frozen embryo | 616 | 31.60 (22.33) | 3.22 (11.93) |
| OHSS management costs | 616 | 16.73 (163.06) | 201.04 (1066.54) |
| Pregnancy loss costs | 616 | 89.03 (227.96) | 74.65 (205.10) |
| ANC costs | 607 | 743.70 (1713.17) | 803.21 (1787.25) |
| Delivery inpatient costs | 606 | 1051.62 (1989.32) | 1279.38 (2196.31) |
| Total NHS costa | 605 | 3431.15 (3507.87) | 3573.99 (3807.37) |
Of the 293 participants with a positive pregnancy test result at 2 weeks post embryo transfer, 100 suffered pregnancy loss. The number with pregnancy loss was slightly larger in the freeze-all arm, at 52 (37%), than in the fresh-embryo transfer arm, at 48 (31%). The mean cost of pregnancy loss was £89 and £75 for frozen-embryo transfer and fresh-embryo transfer, respectively. As a result, 193 participants had a live birth delivery, with more babies delivered in the fresh-embryo transfer arm than in the freeze-all arm. No obvious, notable differences were observed in the resource use associated with ANC in participants with ongoing pregnancy or in the delivery costs for those achieving live birth. The mean cost of ANC and delivery was higher in the fresh-embryo transfer arm (see Table 22) owing to the higher pregnancy rate and the smaller proportion of participants experiencing pregnancy loss.
The resource use from randomisation to delivery translated to a total, average, unadjusted NHS cost of £3431 in the freeze-all arm and £3574 in the fresh-embryo transfer arm, resulting in an unadjusted difference of £143. A breakdown of direct medical costs per participant experiencing each type of resource use event is presented in Appendix 6.
Participant travel and time costs
Table 21 presents the travel and time costs for attending clinic appointments between the time of treatment allocation and embryo transfer. A larger number of participants in the freeze-all arm reported at least one clinic visit (135 vs. 41 in the fresh-embryo transfer arm), and participants in the freeze-all arm also reported a larger average number of clinic visits (1.6 vs. 0.6 in the fresh-embryo transfer arm). This translated to an average travel cost of £30 for the thawed freeze-all arm and £25 for the fresh-embryo transfer arm.
| Variable | Number of observations | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|---|
| Clinic visit(s) between treatment allocation and embryo transfer, n (%) | |||
| Yes | 176 | 135 (44) | 41 (13) |
| No | 279 | 86 (28) | 193 (62) |
| Missing | 161 | 86 (28) | 75 (24) |
| Number of clinic visits, mean (SD) | 443 | 1.56 (2.08) | 0.55 (1.58) |
| Missing, n (%) | 186 | 104 (34) | 82 (27) |
| Total travel costs,a mean (SD) | 437 | 29.83 (73.32) | 25.38 (138.96) |
| Total time costs,b mean (SD) | 432 | 50.26 (82.86) | 26.54 (101.14) |
| Total patient costs,c mean (SD) | 429 | 80.09 (144.06) | 52.05 (236.13) |
Among the 164 participants who reported at least one clinic visit, women in the freeze-all arm reported spending less time in the clinic per visit than women in the fresh-embryo transfer arm. In total, 145 participants reported taking time off from paid work (see Appendix 7). The mean productivity cost associated with time away from usual activities was £50 for the freeze-all arm and £27 for the fresh-embryo transfer arm. When travel and time costs were summed, the average patient’s time and travel cost came to £80.09 and £52.05 for the freeze-all arm and the fresh-embryo transfer arm, respectively.
Cost-effectiveness analysis results
The cost-effectiveness analysis was conducted using the complete-cases data set, and the incremental cost per baby born is presented in Table 22. The adjusted mean treatment cost (including OHSS) per participant was £1402 for the fresh-embryo transfer arm and £1573 for the freeze-all arm, resulting in an adjusted MD of £171. The mean treatment cost was significantly higher in the freeze-all arm than in the fresh-embryo transfer arm. The mean adjusted healthy baby rate was 0.242 in the fresh-embryo transfer arm and 0.204 in the freeze-all arm, producing an adjusted MD of –0.039 (95% CI –0.104 to 0.023) in favour of the fresh-embryo transfer arm. The cost-effectiveness scatterplot, using 1000 bootstrapped iterations, in Figure 12 shows that frozen-embryo transfer was more costly than fresh-embryo transfer in the majority of iterations (≈ 99%). In addition, the healthy baby rate was lower for frozen-embryo transfer than fresh-embryo transfer in 89% of iterations, in line with the MD, in effect favouring fresh-embryo transfer over frozen-embryo transfer. Thus, frozen-embryo transfer was dominated by fresh-embryo transfer. Based on the cost-effectiveness acceptability curves in Figure 13, frozen-embryo transfer had a low chance of being cost-effective at all willingness-to-pay thresholds. Similar findings were noted for the cost-effectiveness analysis using live birth as the measure of effect. (see Table 24, and Figures 12 and 13).
| Cost | Cost (£), mean (95% CI) | Effect, mean (95% CI) | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| Treatment: healthy baby (n = 614) | |||||
| Fresh-embryo transfer | 1402.02 (1297.21 to 1516.44) | 0.242 (0.197 to 0.294) | |||
| Freeze all | 1572.88 (1518.45 to 1641.31) | 170.86 (60.77 to 284.18) | 0.204 (0.160 to 0.246) | –0.039 (–0.104 to 0.023) | Dominated |
| Treatment: live birth (n = 616) | |||||
| Fresh-embryo transfer | 1401.41 (1297.14 to 1516.62) | 0.341 (0.289 to 0.397) | |||
| Freeze all | 1571.55 (1516.11 to 1642.32) | 170.15 (66.79 to 288.57) | 0.285 (0.235 to 0.331) | –0.057 (–0.138 to 0.013) | Dominated |
| NHS: healthy baby (n = 605) | |||||
| Fresh-embryo transfer | 3551.41 (3137.70 to 3960.49) | 0.233 (0.189 to 0.281) | |||
| Freeze all | 3454.15 (3101.50 to 3869.45) | –97.25 (–622.81 to 460.94) | 0.193 (0.151 to 0.237) | –0.040 (–0.101 to 0.027) | 2425 |
| NHS: live birth (n = 605) | |||||
| Fresh-embryo transfer | 3551.41 (3137.70 to 3960.49) | 0.329 (0.278 to 0.377) | |||
| Freeze all | 3454.15 (3101.50 to 3869.45) | –97.25 (–622.81 to 460.94) | 0.273 (0.225 to 0.324) | –0.056 (–0.127 to 0.020) | 1742 |
FIGURE 12.
Trial-based incremental cost-effectiveness scatterplots for frozen-embryo transfer vs. fresh-embryo transfer. (a) Treatment costs: healthy baby; (b) treatment costs: live birth; (c) NHS costs: healthy baby; and (d) NHS costs: live birth.
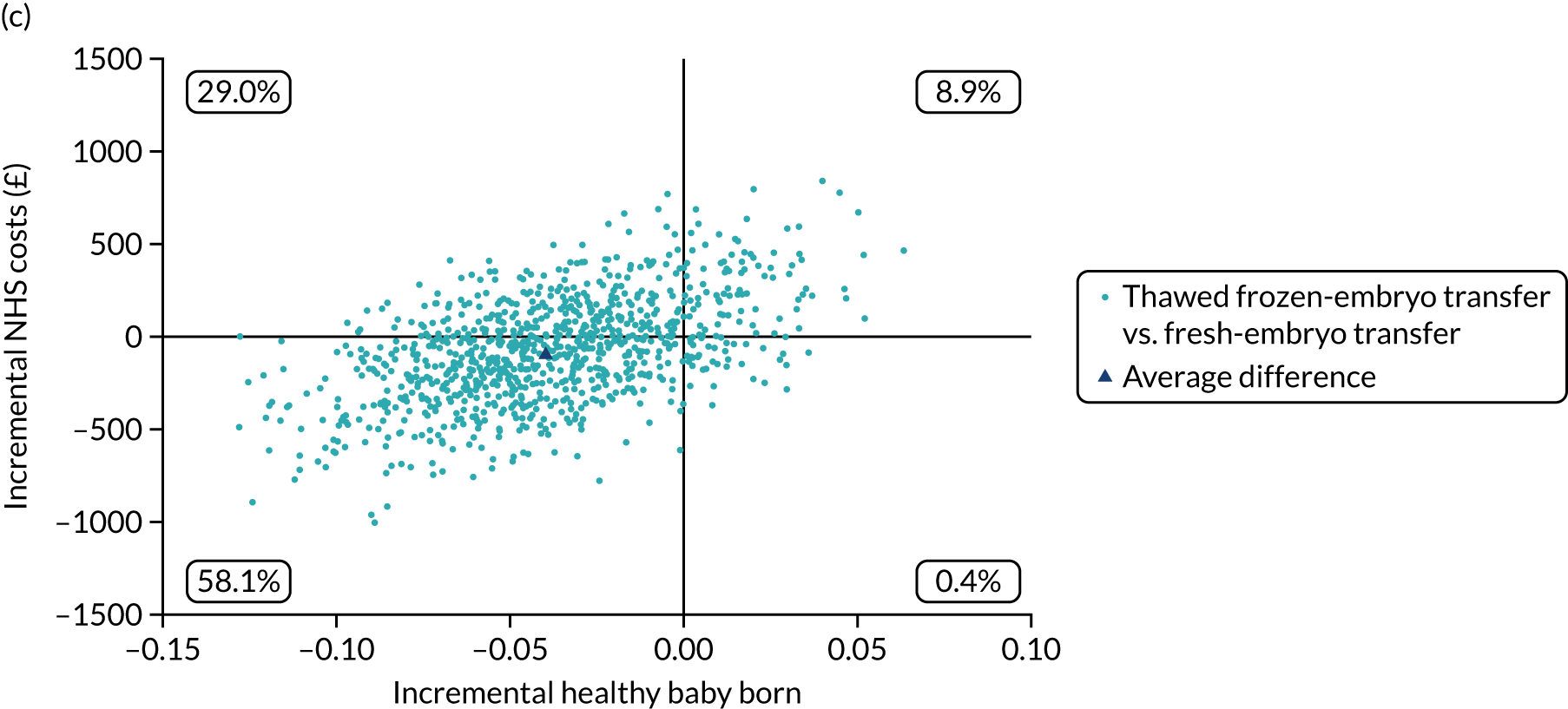
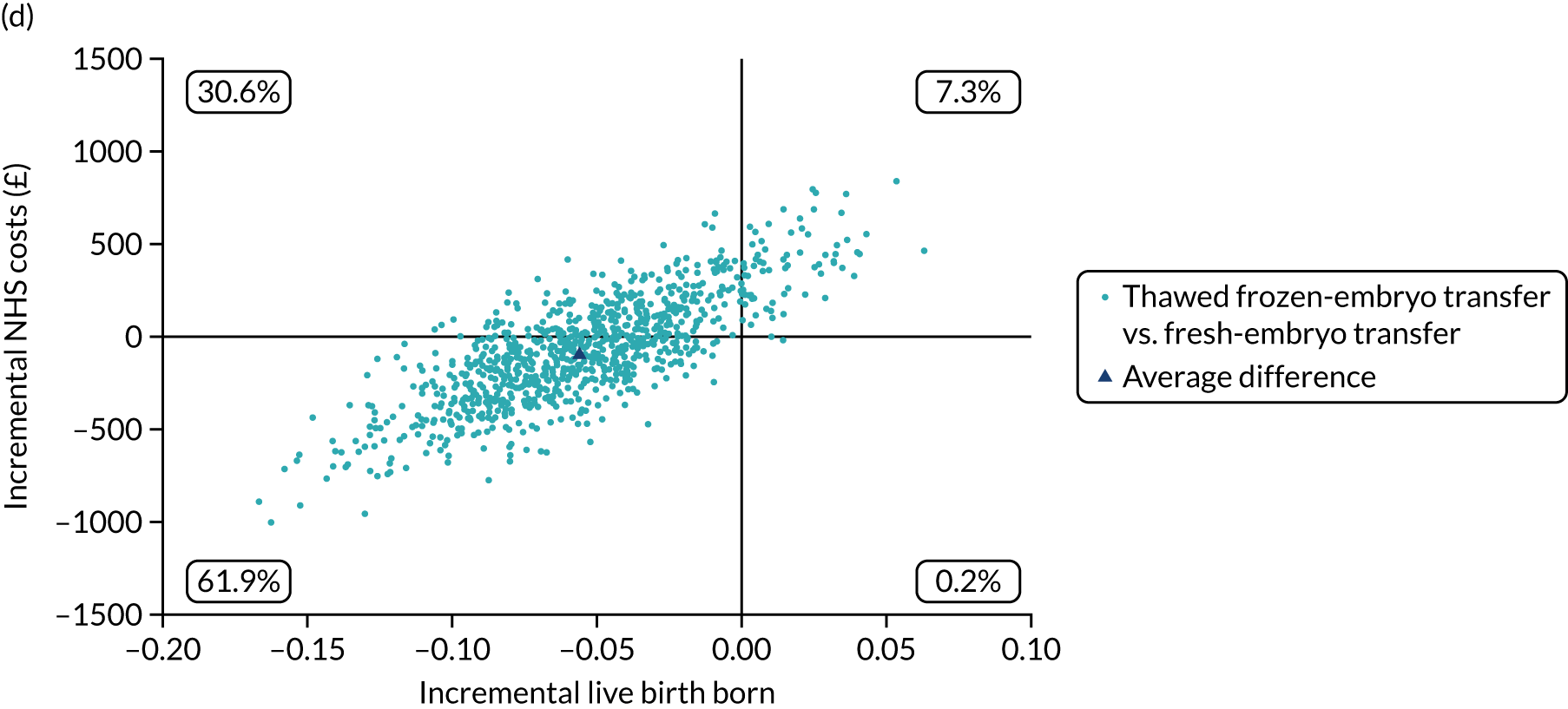
FIGURE 13.
Trial-based cost-effectiveness acceptability curves for frozen-embryo transfer vs. fresh-embryo transfer. (a) Treatment costs: healthy baby; (b) treatment costs: live birth; (c) NHS costs: healthy baby; and (d) NHS costs: live birth.
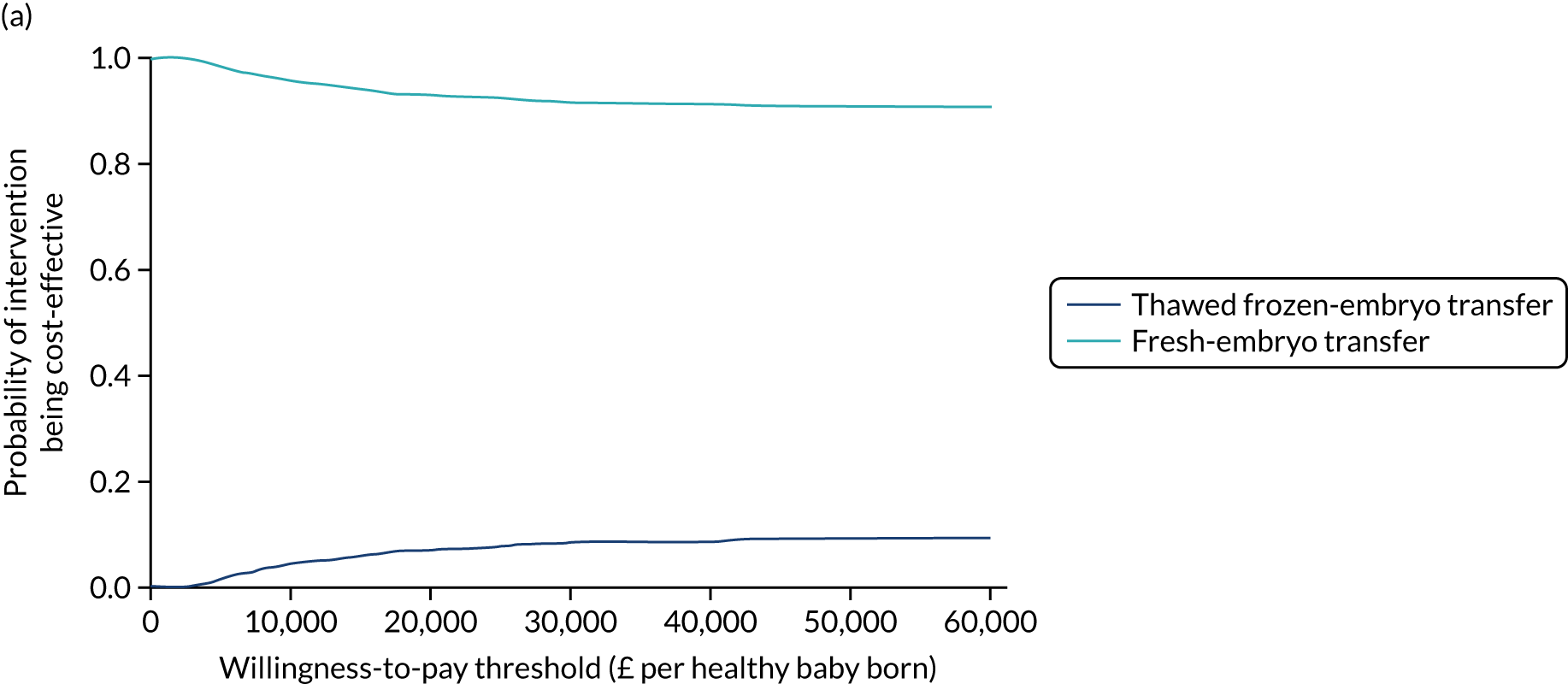
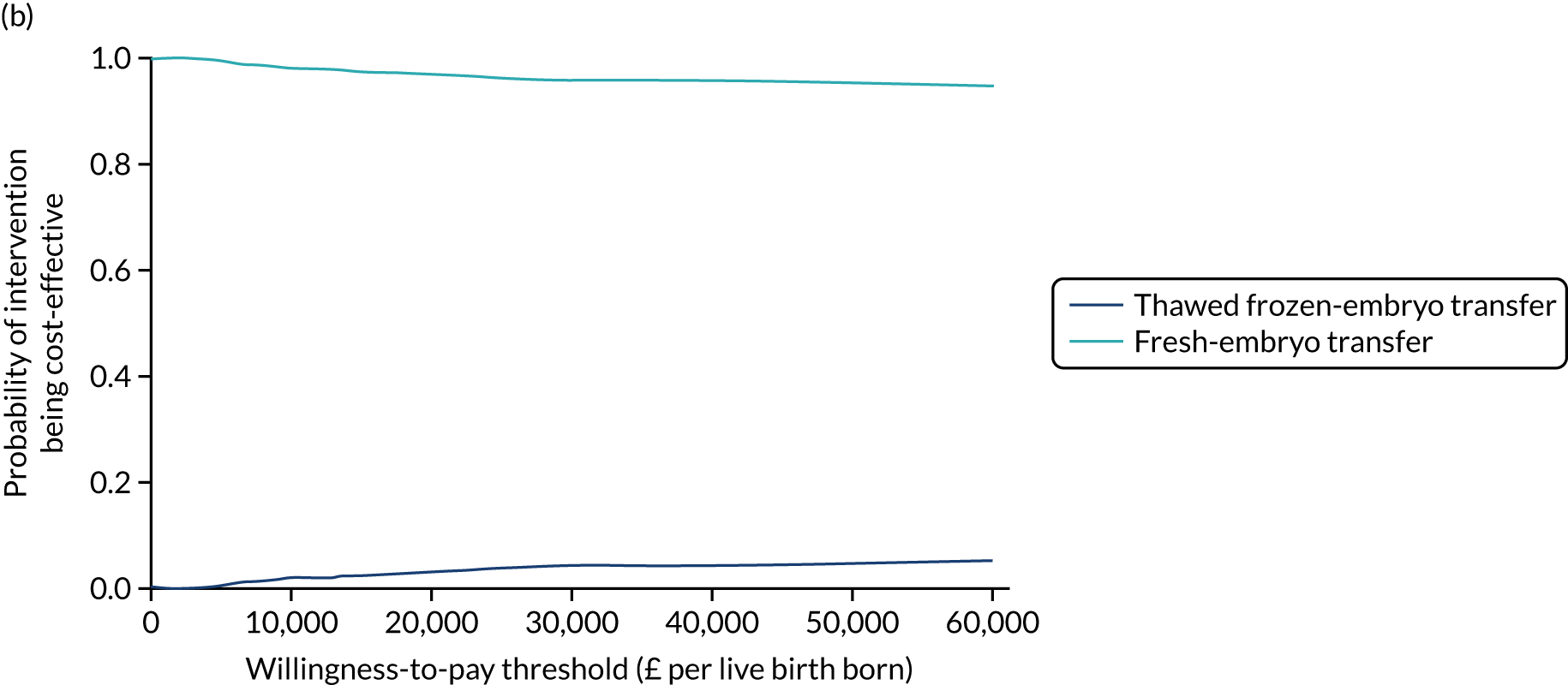
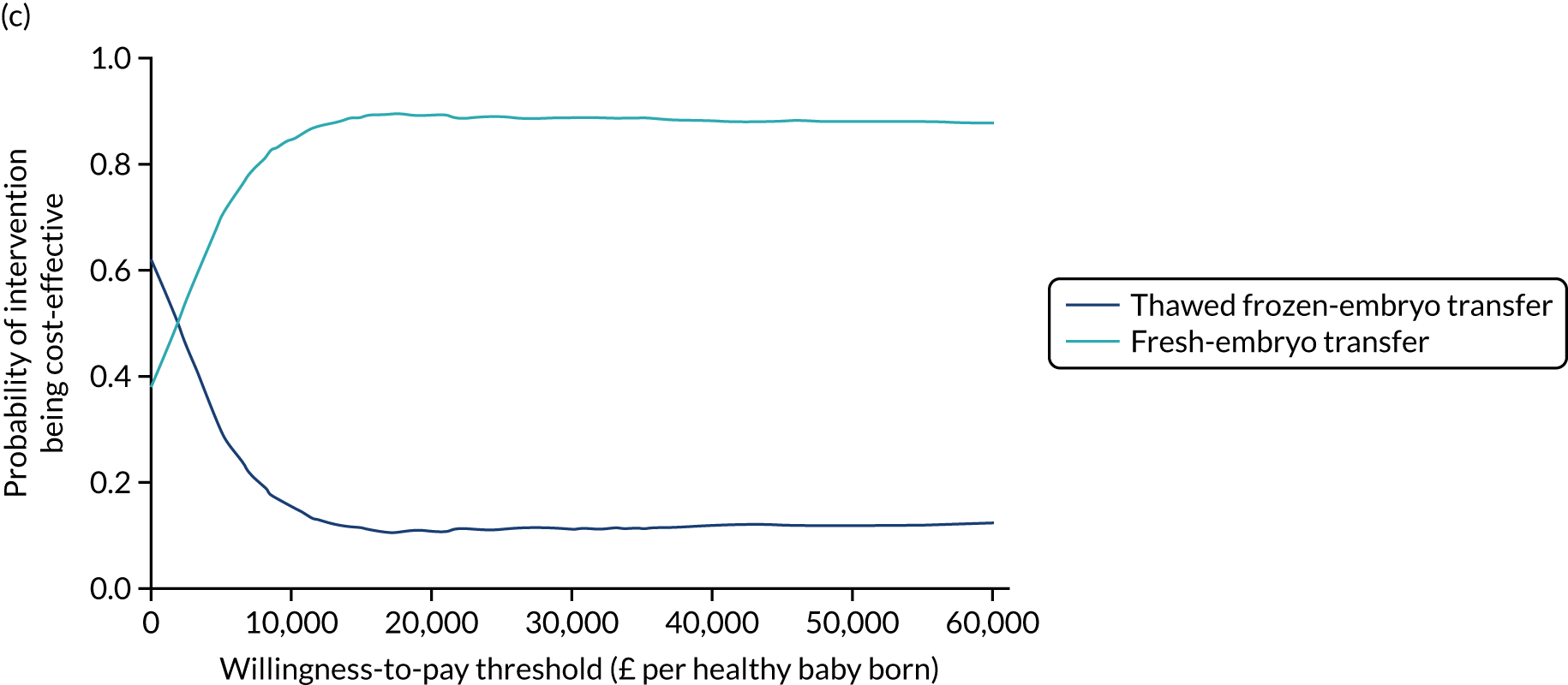
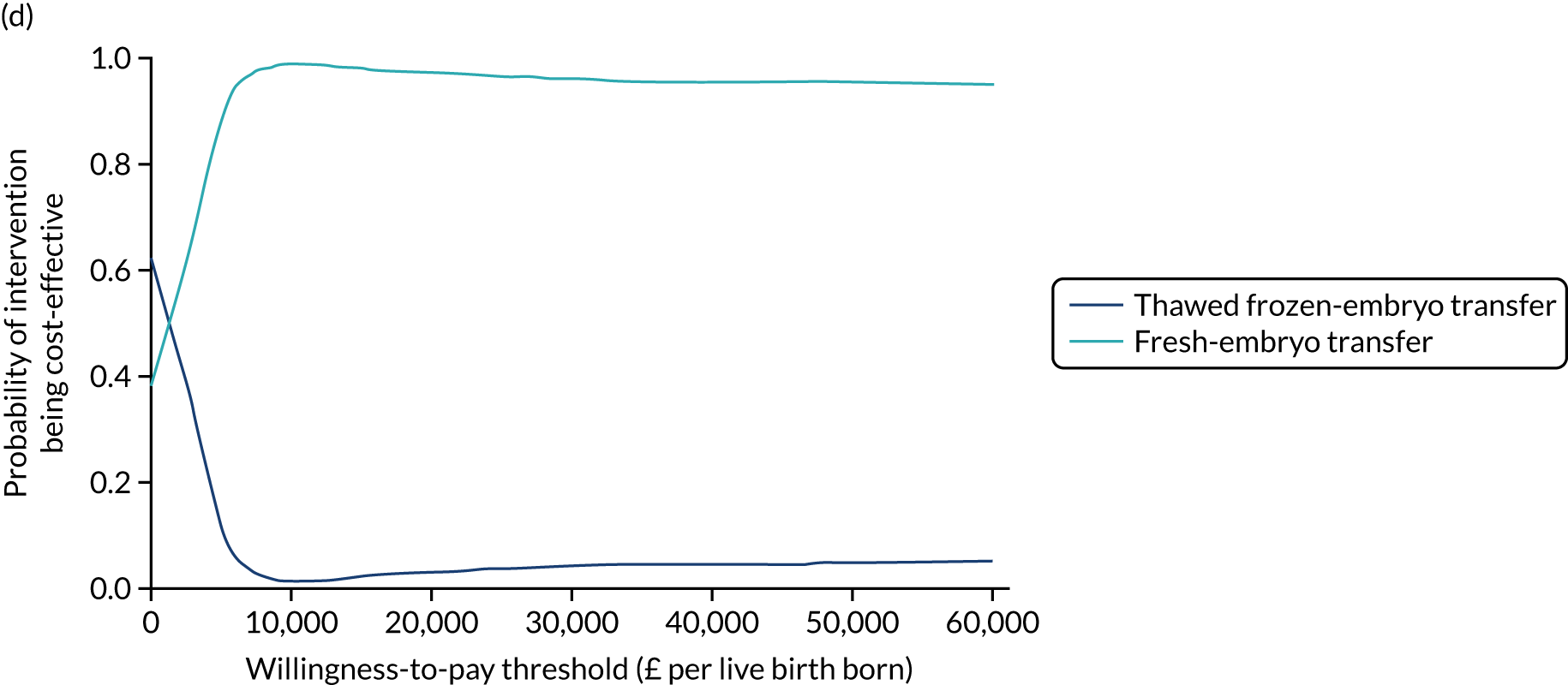
When the total NHS costs were used in the analysis, the adjusted mean NHS cost per participant was £3551 for the fresh-embryo transfer arm and £3454 for the freeze-all arm, resulting in an adjusted MD of £97 (fresh-embryo transfer vs. freeze all). The mean treatment costs for fresh-embryo transfer were slightly higher than those for frozen-embryo transfer because of higher ANC and delivery costs, driven by the higher pregnancy and delivery rates. The ICER for frozen-embryo transfer compared with fresh-embryo transfer, representing cost savings per unit reduction in effect, came to £2425 and £1742 for one less healthy baby and one less live birth, respectively.
Sensitivity and subgroup analyses results
As the cost of transvaginal scans was the main driver of the increased treatment costs for the freeze-all arm compared with the fresh-embryo transfer arm, several sensitivity analyses were conducted using alternative costing methodologies for the scan (Table 23). In addition, multiple imputation was conducted to assess the impact of the base-case assumptions around missing cost data (Table 24). Prespecified subgroup analyses based on age and the number of previous embryo transfers were also conducted (Table 25). The cost-effectiveness scatterplots and cost-effectiveness acceptability curves are shown in Appendix 8.
| Sensitivity analysis | Cost (£), mean (95% CI) | Effect, mean (95% CI) | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| Base-case analysis (NHS reference cost for transvaginal ultrasound scan: £160) | |||||
| Fresh-embryo transfer | 1402.02 (1297.21 to 1516.44) | 0.242 (0.197 to 0.294) | |||
| Freeze all | 1572.88 (1518.45 to 1641.31) | 170.86 (60.77 to 284.18) | 0.204 (0.160 to 0.246) | –0.039 (–0.104 to 0.023) | Dominated |
| Assuming the transvaginal scan cost was inclusive of a monitoring visit cost | |||||
| Fresh-embryo transfer | 1397.09 (1292.44 to 1509.98) | 0.242 (0.197 to 0.294) | |||
| Freeze all | 1508.76 (1461.13 to 1571.11) | 111.67 (5.19 to 221.82) | 0.204 (0.160 to 0.246) | –0.039 (–0.104 to 0.023) | Dominated |
| Using an abdominal scan cost (£53) to cost transvaginal scans | |||||
| Fresh-embryo transfer | 1392.54 (1288.81 to 1503.92) | 0.242 (0.197 to 0.294) | |||
| Freeze all | 1442.97 (1400.86 to 1498.28) | 50.42 (–55.54 to 157.41) | 0.204 (0.160 to 0.246) | –0.039 (–0.104 to 0.023) | Dominated |
| Trial-based sensitivity analysis of incremental costs per baby born (using multiple imputation assumptions) | Cost (£), mean (95% CI) | Effect, mean (95% CI) | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| Treatment costs: healthy baby (n = 614)a | |||||
| Fresh-embryo transfer | 1398.56 (1298.96 to 1514.05) | 0.242 (0.195 to 0.294) | |||
| Freeze all | 1580.80 (1520.49 to 1642.94) | 182.24 (63.49 to 290.63) | 0.204 (0.159 to 0.251) | –0.039 (–0.108 to 0.025) | Dominated |
| Treatment costs: live birth (n = 616)a | |||||
| Fresh-embryo transfer | 1398.10 (1299.28 to 1511.60) | 0.342 (0.292 to 0.394) | |||
| Freeze all | 1580.07 (1519.40 to 1647.80) | 181.96 (61.91 to 295.07) | 0.284 (0.236 to 0.334) | –0.057 (–0.128 to 0.013) | Dominated |
| NHS costs: healthy baby (n = 614)a,b | |||||
| Fresh-embryo transfer | 3600.62 (3207.12 to 4024.05) | 0.242 (0.195 to 0.294) | |||
| Freeze all | 3519.20 (3124.21 to 3946.27) | –81.41 (–652.38 to 492.33) | 0.204 (0.159 to 0.251) | –0.039 (–0.108 to 0.025) | 2109.12 |
| NHS costs: live birth (n = 616)a,b | |||||
| Fresh-embryo transfer | 3614.84 (3215.32 to 4029.97) | 0.342 (0.292 to 0.394) | |||
| Freeze all | 3534.61 (3162.81 to 3952.30) | –80.23 (–644.73 to 486.58) | 0.284 (0.236 to 0.334) | –0.057 (–0.128 to 0.013) | 1398.85 |
| Subgroup | Cost (£), mean (95% CI) | Effect, mean (95% CI) | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| Base-case analysis | |||||
| Fresh-embryo transfer | 1402.02 (1297.21 to 1516.44) | 0.242 (0.197 to 0.294) | |||
| Freeze all | 1572.88 (1518.45 to 1641.31) | 170.86 (60.77 to 284.18) | 0.204 (0.160 to 0.246) | –0.039 (–0.104 to 0.023) | Dominated |
| Maternal age: < 35 years | |||||
| Fresh-embryo transfer | 1456.12 (1283.09 to 1630.29) | 0.289 (0.215 to 0.364) | |||
| Freeze all | 1586.77 (1498.57 to 1709.66) | 130.65 (–16.23 to 311.72) | 0.190 (0.129 to 0.250) | –0.100 (–0.192 to –0.002) | Dominated |
| Maternal age: ≥ 35 years | |||||
| Fresh-embryo transfer | 1328.28 (1251.48 to 1427.94) | 0.202 (0.142 to 0.269) | |||
| Freeze all | 1577.28 (1500.75 to 1659.19) | 249.00 (123.09 to 362.21) | 0.212 (0.147 to 0.276) | 0.010 (–0.079 to 0.096) | 24,308 |
Alternative costing methodology
Table 23 shows the results of the sensitivity analyses. Using alternative, more conservative, assumptions for costing transvaginal ultrasound scans and pre-embryo transfer monitoring, the incremental treatment cost of frozen-embryo transfer was reduced and was no longer significant under the lowest scan-cost scenario. However, both alternative analyses led to similarly low probabilities of frozen-embryo transfer being cost-effective compared with fresh-embryo transfer (see Appendix 8).
Multiple imputation
Table 24 presents the results of sensitivity analyses using the multiple imputation approach. The incremental treatment costs were slightly increased (frozen-embryo transfer vs. fresh-embryo transfer) following this approach, and the total NHS cost savings (inclusive of pregnancy delivery costs) were slightly lower (frozen-embryo transfer vs. fresh-embryo transfer). The incremental NHS cost per healthy baby (£2109) and per live birth (£1399) was, consequently, slightly lower for fresh-embryo transfer than for frozen-embryo transfer.
Subgroup analyses
Table 25 reports the results of subgroup analyses by age. In women aged ≥ 35 years, the healthy baby rate was slightly higher in the freeze-all arm, although the difference was not statistically significant, leading to a positive ICER of £24,308 per additional healthy baby. Based on the results of the non-parametric bootstrap, frozen-embryo transfer was found to have a 46.5% chance of being the preferred intervention at the willingness-to-pay threshold of £20,000 per healthy baby (see Appendix 8). The cost-effectiveness findings for other subgroups remained less favourable to frozen-embryo transfer than to fresh-embryo transfer.
Costs and consequences summary
The summary of costs and consequences was consistent with the cost-effectiveness findings. Frozen-embryo transfer incurred higher treatment and patient costs than fresh-embryo transfer. There were no significant differences in all primary and secondary outcome measures between the trial arms (Table 26), although, directionally, the majority of the outcome measures favoured fresh-embryo transfer over frozen-embryo transfer. The exceptions to this were preterm delivery and low birthweight, which were proportionally higher in the fresh-embryo transfer arm than in the freeze-all arm, although this was based on small numbers of events, with no statistically significant differences found.
| Outcome | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) | Difference,a mean (95% CI) |
|---|---|---|---|
| Costs (£), mean (95% CI) | |||
| Treatment (IVF and OHSS) | 1572.88 (1518.45 to 1641.31) | 1402.02 (1297.21 to 1516.44) | 170.86 (60.77 to 284.18) |
| NHS | 3454.15 (3101.50 to 3869.45) | 3551.41 (3137.70 to 3960.49) | –97.25 (–622.81 to 460.94) |
| Patient | 124.75 (101.76 to 190.65) | 70.83 (37.45 to 106.44) | 53.92 (12.45 to 137.34) |
| Total | 3569.72 (3209.94 to 3986.87) | 3626.90 (3217.83 to 4044.10) | –57.18 (–578.94 to 500.62) |
| Consequences, mean (95% CI) | |||
| Healthy baby born | 0.204 (0.160 to 0.246) | 0.242 (0.1967 to 0.294) | –0.039 (–0.104 to 0.023) |
| Live birth | 0.285 (0.235 to 0.331) | 0.341 (0.289 to 0.397) | –0.057 (–0.138 to 0.013) |
| Maternal safety outcome, n (%) | |||
| OHSS | 11 (3.6) | 25 (8.1) | 0.44 (0.15 to 1.30)b |
| Complications of pregnancy and delivery, n (%) | |||
| Miscarriage | 44 (14.3) | 40 (12.9) | 1.09 (0.72 to 1.66)b |
| Ectopic pregnancy | 3 (1.0) | 6 (1.9) | 0.50 (0.08 to 3.07) |
| Termination | 2 (0.7) | 2 (0.6) | 1.01 (0.08 to 13.12) |
| GDM | 4 (1.3) | 4 (1.3) | 1.00 (0.16 to 6.13) |
| Hypertensive disorders of pregnancy | 8 (2.6) | 7 (2.3) | 1.15 (0.31 to 4.28) |
| Antepartum haemorrhage | 12 (3.9) | 13 (4.2) | 0.93 (0.34 to 2.55) |
| Preterm delivery | 9 (2.9) | 12 (3.9) | 0.75 (0.25 to 2.30) |
| Very preterm delivery | 2 (0.7) | 5 (1.6) | 0.40 (0.05 to 3.43) |
| Low birthweight | 7 (2.3) | 13 (4.2) | 0.54 (0.17 to 1.79) |
| Very low birthweight | 1 (0.3) | 8 (2.6) | 0.13 (0.01 to 1.92) |
| High birthweight | 10 (3.3) | 10 (3.2) | 1.01 (0.33 to 3.14) |
| High weight for gestational age | 9 (2.9) | 10 (3.2) | 0.91 (0.28 to 2.90) |
| Low weight for gestational age | 8 (2.6) | 12 (3.9) | 0.67 (0.21 to 2.13) |
| Congenital anomaly | 6 (2.0) | 7 (2.3) | 0.87 (0.21 to 3.57) |
| Perinatal mortality | 1 (0.3) | 0 (0.0) | – |
| Measure of clinical effectiveness outcomes, n (%) | |||
| Live birth episode | 87 (28.3) | 106 (34.3) | 0.83 (0.65 to 1.06)b |
| Singleton live birth | 85 (27.7) | 105 (34.0) | 0.82 (0.64 to 1.06)b |
| Singleton live birth at term | 78 (25.4) | 93 (30.2) | 0.85 (0.67 to 1.08)b |
| Singleton baby with appropriate weight for gestation | 68 (22.2) | 83 (26.9) | 0.83 (0.55 to 1.26)b |
| Pregnancy | 139 (45.3) | 154 (49.8) | 0.91 (0.77 to 1.08)b |
| Clinical pregnancy | 104 (33.9) | 124 (40.1) | 0.85 (0.65 to 1.11)b |
Modelling of subsequent frozen-embryo transfers
All of the derived parameter inputs for the cost-effectiveness model are provided in Appendix 9. The results of the modelling exercise, allowing for up to six subsequent frozen-embryo transfers, are provided in Table 27, and Figures 14 and 15. The results show that, allowing for the transfer of the remaining embryos, the incremental cost associated with frozen-embryo transfer can be expected to increase further, whereas the difference in effect can be expected to narrow slightly. This is driven by the higher initial failure rate in the freeze-all arm than that in the fresh-embryo transfer arm, resulting in a larger proportion of the cohort retuning for further frozen-embryo transfers than fresh-embryo transfers. Allowing for this, fresh-embryo transfer continues to dominate frozen-embryo transfer in terms of treatment costs per healthy baby and live birth, and in terms of NHS costs per health baby and live birth. Considering the uncertainty around the joint incremental costs and effect (see Figure 14), fresh-embryo transfer retains the higher chance of being preferred on grounds of cost-effectiveness across all values of willingness to pay per health baby or live birth, compared with frozen-embryo transfer (see Figure 15).
| Cost | Cost (£), mean | Effect, mean | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| Treatment: healthy baby | |||||
| Fresh-embryo transfer | 2870 | 0.381 | |||
| Freeze all | 3195 | 325 | 0.349 | –0.031 | Dominated |
| Treatment: live birth | |||||
| Fresh-embryo transfer | 2870 | 0.538 | |||
| Freeze all | 3195 | 325 | 0.503 | –0.035 | Dominated |
| NHS: healthy baby | |||||
| Fresh-embryo transfer | 6391 | 0.381 | |||
| Freeze all | 6560 | 169 | 0.349 | –0.031 | Dominated |
| NHS: live birth | |||||
| Fresh-embryo transfer | 6391 | 0.538 | |||
| Freeze all | 6560 | 169 | 0.503 | –0.035 | Dominated |
FIGURE 14.
Model-based cost-effectiveness scatterplots for frozen-embryo transfer vs. fresh-embryo transfer. (a) Treatment costs (including OHSS): healthy baby; (b) treatment costs (including OHSS): live birth; (c) NHS costs: healthy baby; and (d) NHS costs: live birth.
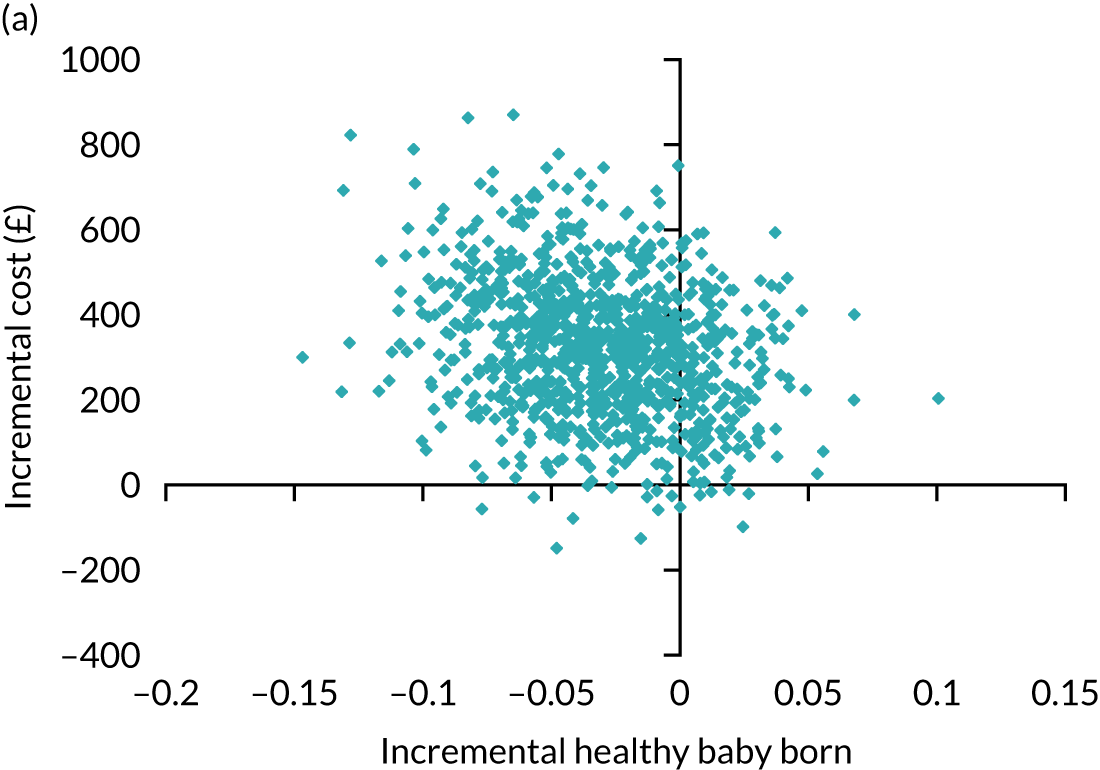
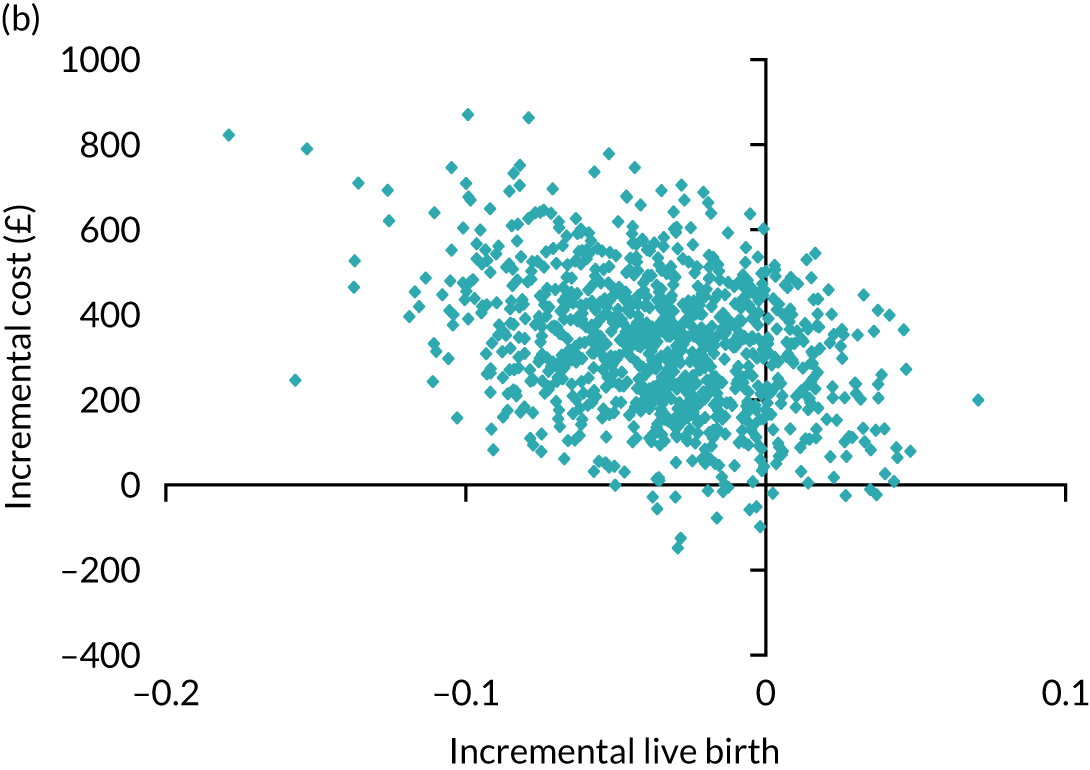
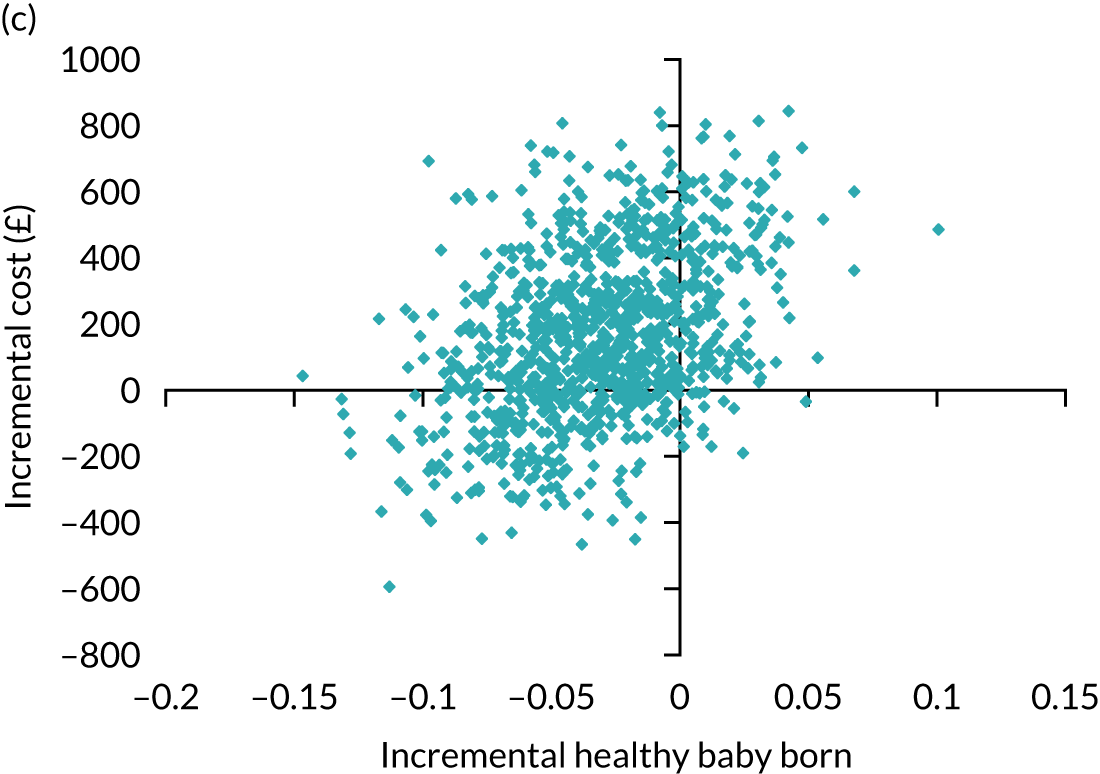
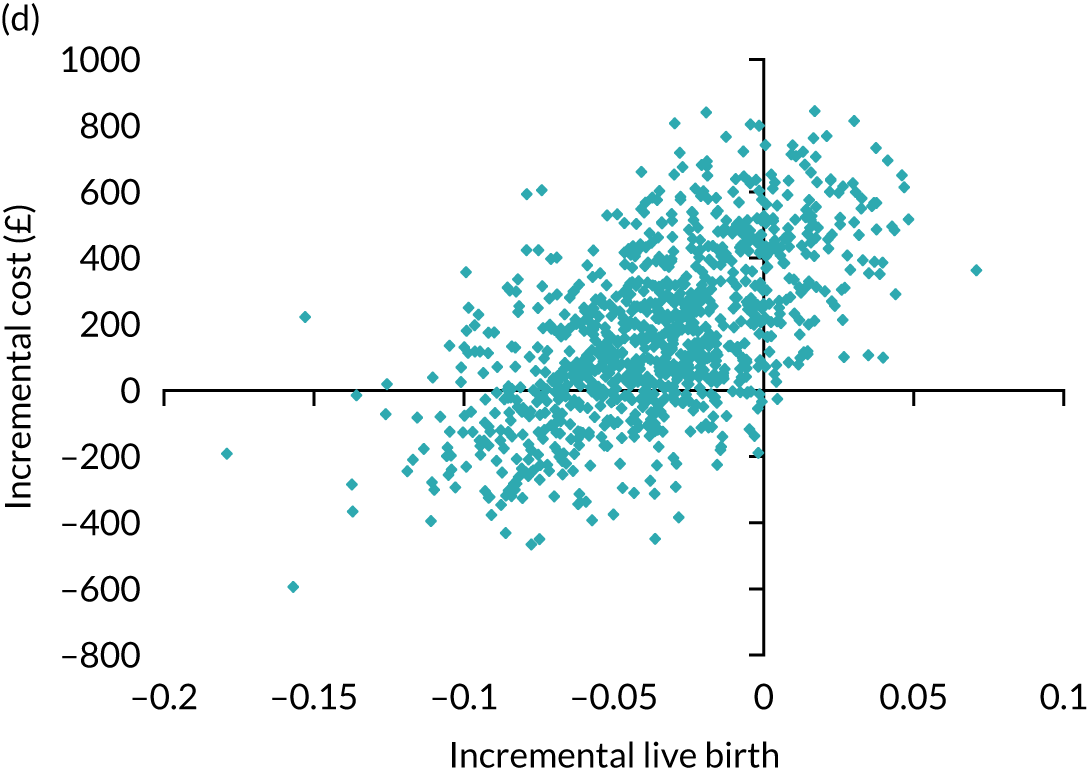
FIGURE 15.
Model-based cost-effectiveness scatterplots for frozen-embryo transfer vs. fresh-embryo transfer. (a) Treatment costs (including OHSS): healthy baby; (b) treatment costs (including OHSS): live birth; (c) NHS costs: healthy baby; and (d) NHS costs: live birth.
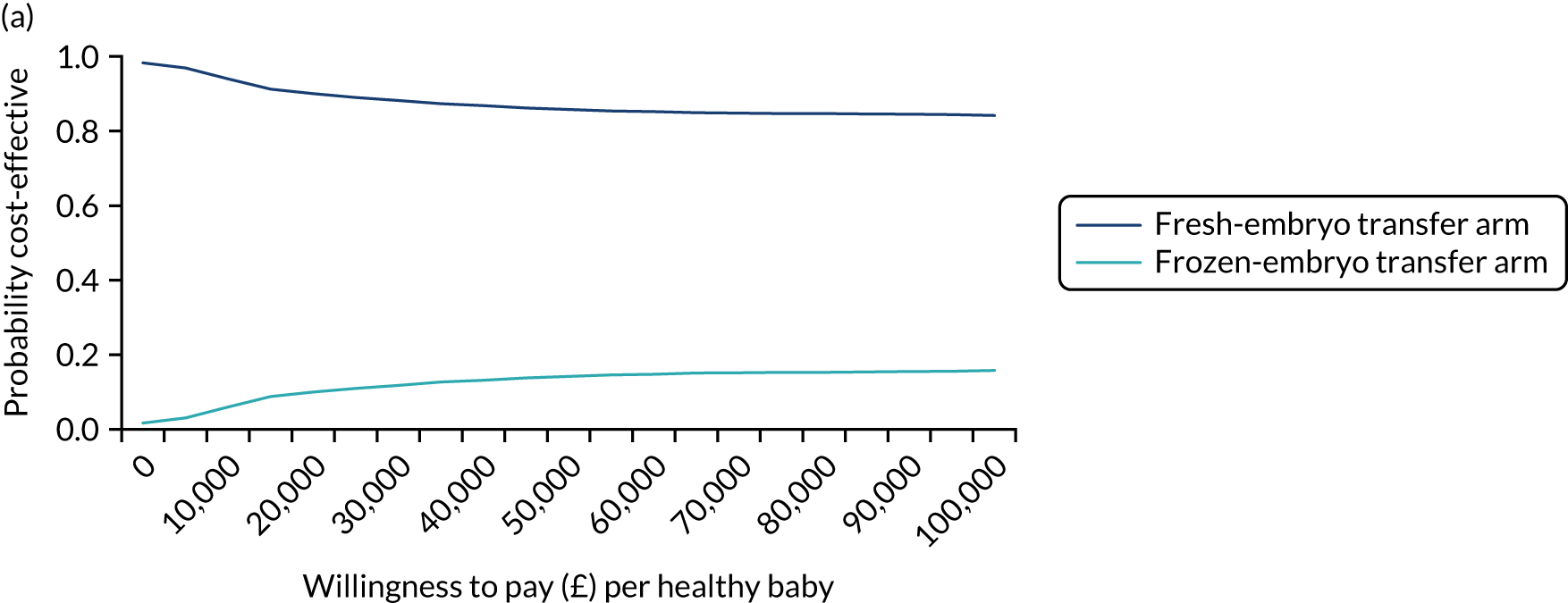
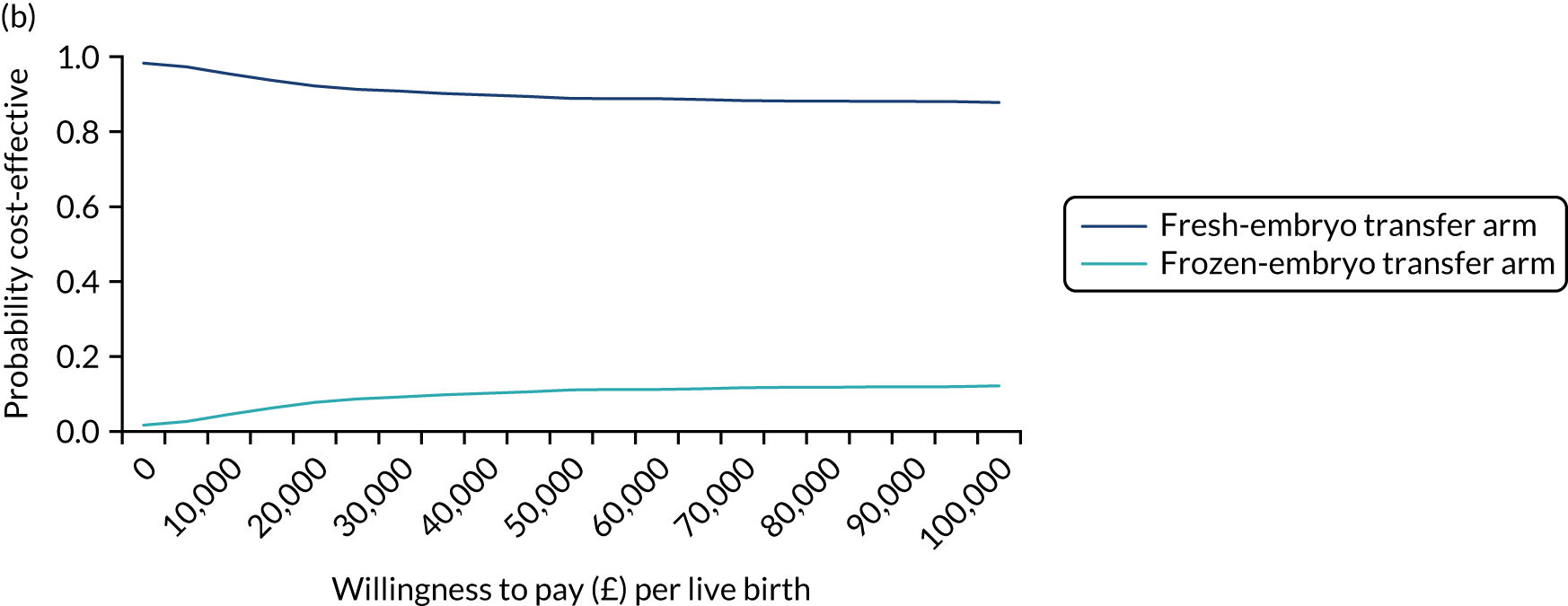
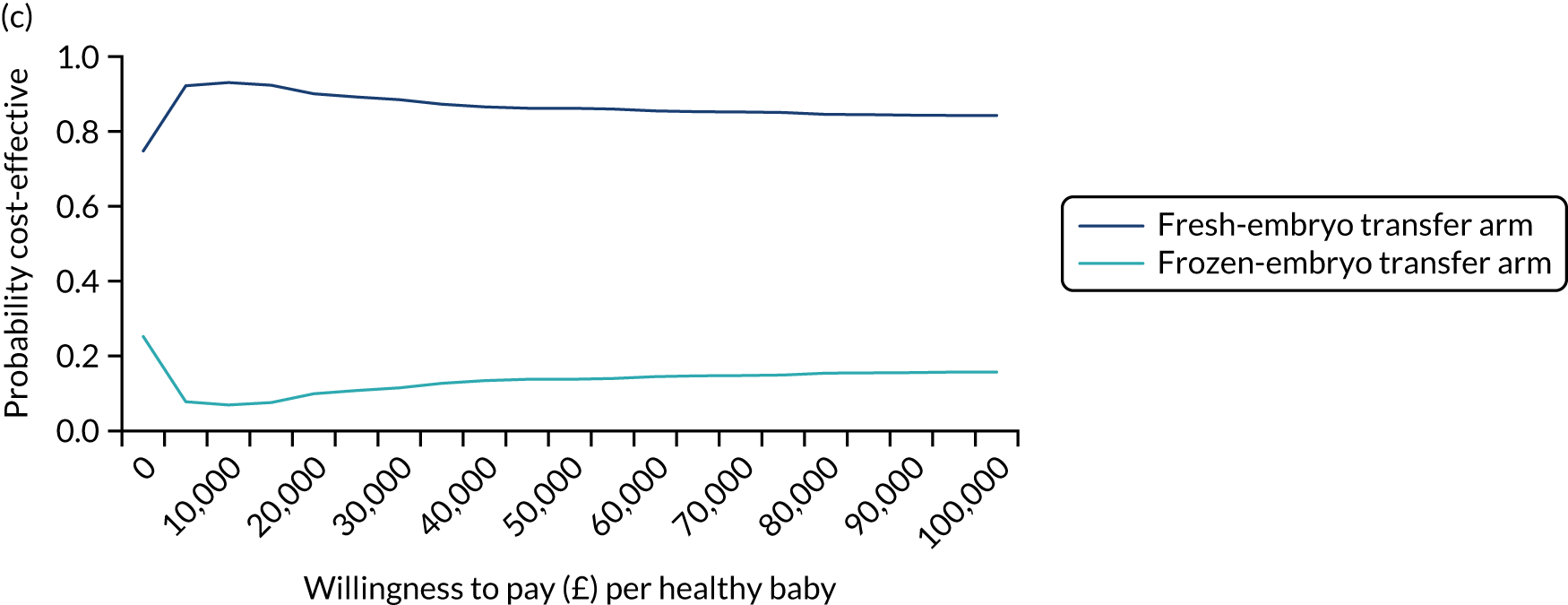
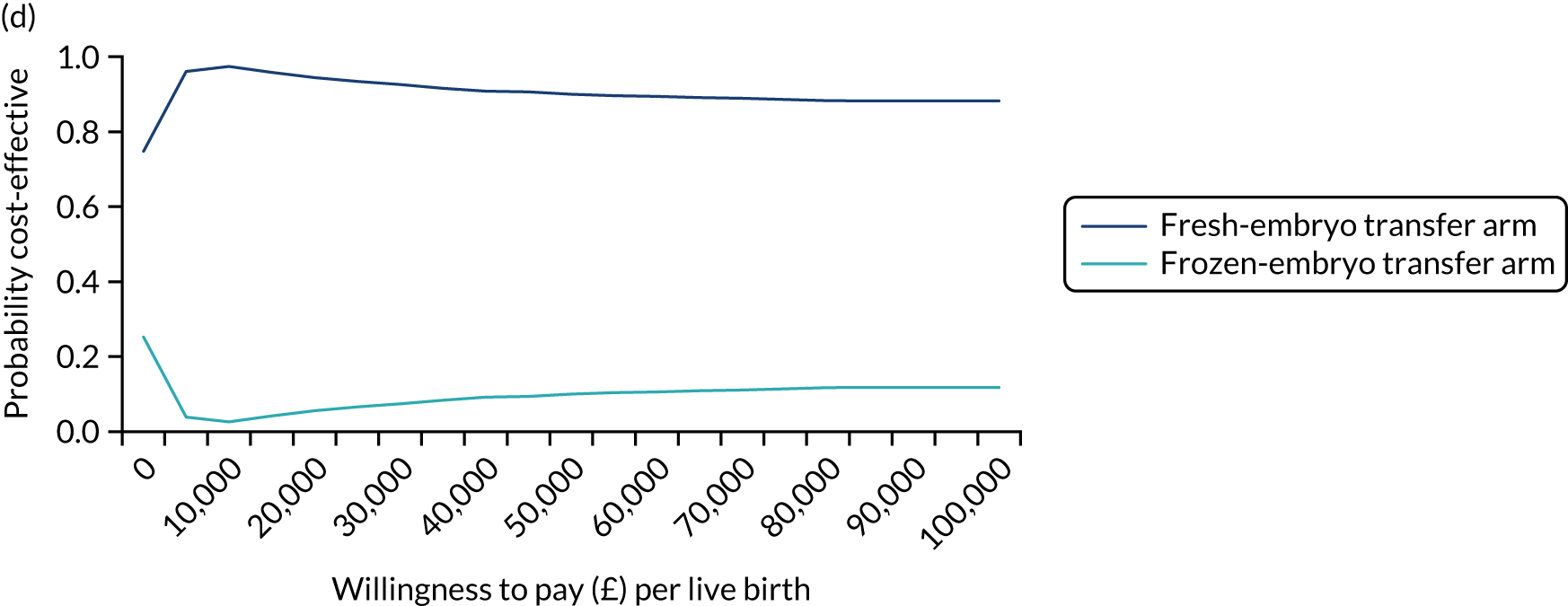
Model-based sensitivity analysis
Table 28 presents the results of several key sensitivity analyses around the model-based estimates of cost-effectiveness, using incremental treatment cost (including OHSS) per additional healthy baby as the measure of cost-effectiveness. Assuming no discontinuation among those eligible for subsequent frozen-embryo transfers and applying more conservative costs for transvaginal scans and monitoring prior to frozen-embryo transfer, fresh-embryo transfer remains, on average, more effective and less costly than thawed frozen-embryo transfer. Cost-effectiveness acceptability curves for the model-based sensitivity analysis are provided in Appendix 10.
| Sensitivity analysis | Cost (£), mean | Effect, mean | ICER | ||
|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | ||
| Base case | |||||
| Fresh-embryo transfer | 2870 | 0.381 | |||
| Freeze all | 3195 | 325 | 0.349 | –0.031 | Dominated |
| Assuming no discontinuation among those with embryos remaining for subsequent frozen-embryo transfer cycles | |||||
| Fresh-embryo transfer | 3124 | 0.404 | |||
| Freeze all | 3484 | 361 | 0.376 | –0.028 | Dominated |
| Using the lower ultrasound scan cost (£53) to cost transvaginal scans | |||||
| Fresh-embryo transfer | 2693 | 0.381 | |||
| Freeze all | 2880 | 187 | 0.349 | –0.031 | –6001 |
Chapter 5 Discussion and conclusions
Summary of main findings
A total of 1578 couples consented, 619 of whom were randomised (310 to the fresh-embryo transfer arm and 309 to the freeze-all arm). Most non-randomisations (n = 959) were owing to the non-availability of three good-quality embryos (n = 476). Of those randomised, 117 (19%) couples did not adhere to their allocated intervention: 96 (31.3%) in the freeze-all arm and 21 (6.8%) in the fresh-embryo transfer arm. Non-adherence was higher in the freeze-all arm than in the fresh-embryo transfer arm, with the most common reason being patient choice.
The ITT analysis showed that the healthy baby rate was 20.3% in the freeze-all arm and 24.4% in the fresh-embryo transfer arm (RR 0.84, 95% CI 0.62 to 1.15). Similar results were obtained from CACE analysis (RR 0.77, 95% CI 0.44 to 1.10), per-protocol analysis (RR 0.87, 95% CI 0.59 to 1.26) and as-treated analysis (RR 0.91, 95% CI 0.64 to 1.29). There was no statistical difference in the healthy baby rate across age groups (< 35, 35 to < 40 and > 40 years), the number of previous embryo transfers (0 or ≥ 1), whether it was cleavage or blastocyst transfer, or whether one or two embryos were transferred.
There was no evidence of a difference in live birth rate (28.3% vs. 34.3%; RR 0.83, 99% CI 0.65 to 1.06) or clinical pregnancy rate (33.9% vs. 40.1%; RR 0.85, 99% CI 0.65 to 1.11) between the freeze-all arm and the fresh-embryo transfer arm.
There were no statistical differences between the two arms in any of the obstetrics and perinatal outcomes (i.e. hypertensive disorders of pregnancy, antepartum haemorrhage, preterm delivery, very preterm delivery, onset of labour, mode of delivery, low birthweight, high birthweight, low birthweight for gestational age, high birthweight for gestational age and congenital anomalies).
The risk of ovarian hyperstimulation was 3.6% in the freeze-all arm and 8.1% in the fresh-embryo transfer arm (RR 0.44, 99% CI 0.15 to 1.30). There were 30 reported AEs, but these were not related to the intervention.
A total of 88.6% embryos survived the freezing–thawing process.
There was no statistical difference in STAI scores for male participants (MD 0.1, 99% CI –2.4 to 2.6) and female participants (MD 0.0, 99% CI –2.2 to 2.2) between the arms.
Following adjustment for minimisation criteria, the mean post-randomisation treatment cost (inclusive of OHSS) per woman randomised was £1395 (95% CI £1294 to £1505) in the fresh-embryo transfer arm and £1576 (95% CI 1514 to £1642) in the freeze-all arm. The mean between-group difference was £181 (95% CI £60 to £292). Based on the estimated difference in the healthy live birth rate (–0.039, 95% CI –0.101 to 0.027), fresh-embryo transfer was found to dominate frozen-embryo transfer, being, on average, less costly and more effective. Considering the joint uncertainty surrounding the estimated differences in costs and effects, the probability of fresh-embryo transfer being preferred on the grounds of cost-effectiveness was > 89% across all willingness-to-pay thresholds per additional healthy live birth.
When ANC and delivery costs were included in the cost-effectiveness analysis, the freeze-all strategy was, on average, less costly, owing to a smaller number of pregnancies and live births (MD –£75, 95% CI –£623 to £461). However, fresh-embryo transfer retained a higher probability of being cost-effective compared with frozen-embryo transfer above a willingness-to-pay threshold of £1921 per additional healthy live birth. Furthermore, when cumulative costs and outcomes associated with the transfer of the remaining frozen embryos were simulated using a Markov model, fresh-embryo transfer was found to be less costly and more effective than frozen-embryo transfer, even when including the ANC and delivery costs. The same pattern of results was observed when using live births as the measure of effectiveness.
The difference in treatment costs was found to be sensitive to the application of more conservative costs for monitoring ultrasound scans prior to frozen-embryo transfer, but the overall cost-effectiveness findings remained stable, with fresh-embryo transfer retaining a substantially higher probability of being cost-effective than frozen-embryo transfer.
Update and comparison with existing literature
The E-Freeze trial was planned in 2014, awarded funding in 2015 and started recruitment in 2016. Several trials40–46 across the world were conducted and published during this time (i.e. 2016–20) comparing freezing all embryos, followed by frozen-embryo transfer, with fresh-embryo transfer. Two trials report on hyper-responders (i.e. those who are at high risk of OHSS),40,41 and five reported on those who were predicted as normal responders (i.e. not at high risk for OHSS). 42–46
Table 29 summarises the trials reporting on normal responders that were published after the E-Freeze trial began. We present the aggregated meta-analysis on these trials on key clinical outcomes with and without incorporating data from E-Freeze trial. For the comparison of the results and data from the E-Freeze trial with the existing literature, we have included only those trials reporting on normal responders, the populations for which were similar to the population of the E-Freeze trial.
| Trial, country and centre type | Population | Randomisation detail | FET regimes | Conclusions (freeze-all vs. fresh-embryo transfer) |
|---|---|---|---|---|
| Vuong et al.,42 2018, Viet Nam, single centre | 782 women without PCOS undergoing their first or second cycle of IVF; mean age 32 years; day 3 embryo transfer | Had to have at least one grade 1 embryo on day 3 | Most FET by HRT cycle | Similar live birth (31.8% vs. 33.8%) and ongoing pregnancy (34.5% vs. 36.3%) rates |
| Shi et al.,43 2018, China, multiple centres | 2157 women (non-PCOS);first cycle of IVF; aged 20–35 years with good ovarian reserve; day 3 embryo transfer | Had to have five or more oocytes to be randomised | Natural cycles for most, artificial cycles for some | Similar live birth rate (48.7% vs. 50.2%) |
| Wei et al.,44 2019, China, multiple centres | 1650 women; first cycle of IVF; aged 20–35 years with regular menstrual cycles; blastocyst transfer only | Randomisation on day 3 after egg collection with four or more high-grade embryos | Natural (62%) or programmed cycles (35%) | Higher singleton live birth (50% vs. 40%) and live birth (53.2% vs. 41.3%) rates |
| Stromlund et al.,45 2020, Denmark, multiple centres | 453 couples; aged 18–39 years with regular menstrual cycle; AMH > 6.28 pmol/l; normal and high responders | Randomisation at start of stimulation | Modified natural cycle | Similar ongoing pregnancy (27.8% vs. 29.6%) and live birth (27.4% vs. 28.7%) rates |
| Wong et al.,46 2021, the Netherlands, single centre | 202 couples; aged 18–43 years; any indication of IVF; 205 couples; blastocyst transfer | Randomisation at start of downregulation | Artificial cycle | Similar CLBR (19% vs. 31%) |
The two trials on hyper-responders40,41 reported that the freeze-all approach improves the live birth rate and reduces the risk of OHSS in those who are hyper-responders.
The five trials on normal responders reported from Viet Nam,42 China, 43,44 Denmark45 and the Netherlands. 46 Our results are consistent with three of these trials,42,43,45 but are in contrast with the others. 44,46 Wei et al. 44 suggest that the singleton live birth rate is higher with freeze-all, followed by frozen-embryo transfer, and Wong et al. 46 showed that the live birth rate was significantly lower when freezing all embryos rather than using fresh embryos. There are differences in population, outcome measures and the timing of randomisation in each of the trials (Table 30), which may account for these differences.
| Complication | Risk of complications (%) | ||
|---|---|---|---|
| General population | Freeze-all arm | Fresh-embryo transfer arm | |
| Gestational diabetes | 1.5 | 4.7 | 3.9 |
| Hypertensive disorder (all) | 10–15 | 9.4 | 6.8 |
| Pre-eclampsia | 4–6 | 5.9 | 1.0 |
| Antepartum haemorrhage (all) | 6 | 13.1 | 11.7 |
| Preterm delivery | 5.0 | 10.3 | 11.4 |
| Very preterm delivery | 0.7 | 2.3 | 4.8 |
| Caesarean section | 16.2 | 43.5 | 35.2 |
| Low birthweight | 7.0 | 9.1 | 13.1 |
| Very low birthweight | 0.5 | 1.1 | 7.5 |
| High birthweight | 8–10 | 11.4 | 9.3 |
| High weight for gestational age | 15.9 (using intergrowth chart) | 10.2 | 9.4 |
| Low weight for gestational age | 7.6 | 10.2 | 11.3 |
| Congenital anomalies | 0.2 | 5.7 | 4.7 |
| Perinatal mortality | 2.6 | 1.1 | 0 |
The outcome healthy baby rate was not reported by any other trial. The closest comparison was singleton live birth, reported by Wei et al. 44 Hence, it is not possible to compare the primary outcome measure reported by the E-Freeze trial with any other studies in the literature.
Live birth rate
The live birth rate in our trial was 28.3% in the frozen-embryo transfer arm and 34.3% in the fresh-embryo transfer arm. Although there were no statistically significant differences between the two arms, these figures were similar to the live birth rates reported by the two trials from Europe45,46 and the trial from Viet Nam. 42 However, the rates are much lower than those of both of the trials reported from China. 43,44 This could be because the trials reporting from China had an upper age limit of 35 years and, therefore, included patients with a better prognosis.
The combined data from these five trials showed no difference in the outcome of live birth between the two arms, which is similar to the results of the E-Freeze trial (Figure 16).
Based on nearly 5246 women randomised to fresh-embryo transfer compared with frozen-embryo transfer, an aggregate-data meta-analysis did not seem to favour either fresh-embryo transfer or a strategy of freezeing all embryos, followed by frozen-embryo transfer, with a combined RR of 1.01 (99% CI 0.78 to 1.31). An updated meta-analysis, including randomised data from an additional 616 women from the E-Freeze trial, does not appear to result in a convincing change in the direction, size or precision of the overall effect, with a combined RR of 0.97 (95% CI 0.77 to 1.24).
Ovarian hyperstimulation syndrome
The risk of OHSS reported in the E-Freeze trial was 3.6% in the freeze-all arm and 8.1% in the fresh-embryo transfer arm. Most cases of OHSS were mild, with moderate to severe OHSS in only 1.6% in the freeze-all arm and 5.8% in the fresh-embryo transfer arm. This is higher than the rate quoted for moderate to severe OHSS in national statistics. 6 This difference is because of better ascertainment in the trial setting; it is well known that cases of OHSS are not reported in clinical practice. 49
The total OHSS figures were higher than those reported by other trials. This could be because we also reported mild OHSS, whereas other trials reported only moderate and severe OHSS. Combined data from the five trials reporting on OHSS42–46 showed a statistically significant reduction in OHSS when all embryos were frozen compared with fresh-embryo transfer. The addition of data from the E-Freeze trial did not change the direction or magnitude of these figures, but increased the precision (Figure 17).
Miscarriage
The risk of miscarriage per couple randomised was 14.3% in the frozen-embryo transfer arm and 12.9% in the fresh-embryo transfer arm. The corresponding figures for risk of miscarriage per pregnancy were 31.7% and 26.0%, respectively. Miscarriage rates were slightly higher in the E-Freeze trial than in other studies (ranging from 9.9% to just over 25% per pregnancy42–45). We were unable to explain this higher rate of miscarriage. To explore the higher risk of miscarriage, we undertook a post hoc analysis of the miscarriage rate per centre; there were no significant differences between the five clinics that contributed most data. The combined data from other trials showed no difference in miscarriage rates between trial arms, which is similar to the results reported in the E-Freeze trial. 42–45 Wong et al. 46 did not report on miscarriage per pregnancy; hence, their data are not included in the meta-analysis graph. The addition of E-Freeze to existing data does not change the direction, magnitude or precision of effect (Figure 18).
Obstetric and perinatal complications
There was no statistical difference in obstetric and perinatal complication between arms in the E-Freeze trial. Observational studies and meta-analysis of published data50–52 suggested that the risk of pre-eclampsia and large for gestational age (LGA) babies is increased in pregnancies that are a result of frozen-embryo transfer. The numbers of each individual complication were too small to draw any definite conclusions from this trial alone, or from any of the other existing trials individually. 42–46 In comparison with the risk in the general population (see Table 30), not all risks were higher in IVF pregnancies, irrespective of whether they were the result of fresh-embryo transfer or frozen-embryo transfer. Although this is reassuring, it is in contrast to the previous findings from systematic review of observational data7 and could be owing to small numbers of each complication in our trial.
Natural compared with hormone replacement therapy frozen-embryo transfer
Most of the frozen-embryo transfers in the E-Freeze trial were undertaken in hormonally mediated cycles. By contrast, worldwide,53 45% of cycles are natural cycles. This can be explained by the fact that participants in the E-Freeze trial were eager to receive treatment and wanted a planned date for their frozen-embryo transfer, after having their treatment delayed by participation in the trial. By contrast, the worldwide data are based on observational, non-randomised data and are more likely to be from patients undergoing transfer of surplus frozen embryos after fresh-embryo transfer has failed, rather than frozen-embryo transfer as their first treatment.
The two Chinese trials43,44 had a large proportion of participants who underwent endometrial preparation for frozen-embryo transfer using natural cycles. The two European trials45,46 and the Vietnamese trial42 had the largest number of patients undergoing hormonally mediated hormone replacement therapy (HRT) cycles.
Hormonally mediated frozen-embryo transfer is more convenient for the clinic and patients, as the date of thaw and transfer can be planned in accordance with the workload of the clinic and at the convenience of the staff and patients. Recently, some concerns have been raised that pregnancies following HRT-mediated frozen-embryo transfer may be at a higher risk of complications than those following a natural cycle. 54
Non-adherence
Non-adherence in the freeze-all arm was very high in this trial. Other trials have also shown non-adherence to the allocated intervention, but at much lower levels than those seen in the E-Freeze trial. The highest level was reported by Shi et al. 43 (freeze-all, 18%; fresh-embryo transfer, 15%). However, non-adherence was reported in both arms, whereas in the E-Freeze trial non-adherence was predominantly seen in the freeze-all arm (31.3%). This level of non-adherence occurred despite the fact that the trial was specifically designed to reduce non-adherence, with consent being reconfirmed just before randomisation and information being provided throughout the process. There could be two reasons for this non-adherence. The funding of IVF treatment is limited across the UK, especially in England, where most of the participating centres were based, and > 60% of treatments in England are funded by the patients themselves. Where funding is available, only one cycle of treatment is funded by most CCGs. Hence, there will be apprehension about freezing all embryos among clinicians, scientists and patients, especially when there are only one or two embryos. There is always a fear that the embryos may not survive the freezing–thawing process, leading to loss of funding for the only funded treatment. Our data showed that, on average, 86% of embryos survived the freezing–thawing process across the participating clinics; hence, the survival rate is far below 100%.
Although there was an intention and acceptance from patients to be randomised to either arm at the time of consent, this did not translate to real practice, with a noticeable preference seen for fresh-embryo transfer. Studies from two different parts of the world55,56 have shown that patients would accept the intervention of freeze-all, followed by frozen-embryo transfer, if freeze-all reduces the side effects and has at least equal, if not better, success rates. Both conditions must be fulfilled to accept the delay.
Economic analysis
The few published economic evaluations of freeze-all compared with fresh-embryo transfer have produced mixed findings. Roque et al. 57 used observational data from a private centre in Brazil to compare the cost per ongoing pregnancy among patients receiving treatment with each strategy. Although the total cost of treatment was higher per patient undergoing the freeze-all approach, the average cost per pregnancy was lower, given the substantially higher pregnancy rate in the non-randomised cohort (39.7% vs. 31.1%). Thus, Roque et al. 57 concluded that a freeze-all strategy was a cost-effective option compared with fresh-embryo transfer.
In an economic evaluation carried out using data from a RCT conducted in Viet Nam, Le et al. 58 reported higher mean costs in the freeze-all arm than in the fresh-embryo transfer arm over a full cycle, including the transfer of all embryos obtained from a single, controlled, ovarian hyperstimulation cycle. The live birth rate was also slightly higher in the freeze-all arm than in the fresh-embryo transfer arm (48.6% vs. 47.3%), but the average cost per live birth was higher. In an incremental analysis, the additional cost per additional live birth was estimated to be €30,997 per additional live birth (freeze-all vs. fresh-embryo transfer). The probability of cost-effectiveness for the freeze-all approach remained < 60% irrespective of the willingness to pay per additional live birth. Based on these results, Le et al. 58 concluded that the freeze-all approach did not constitute a cost-effective use of resources.
Our findings of increased treatment costs with the freeze-all approach, compared with the fresh-embryo transfer approach, are consistent with those in the published studies. 58 However, directionally, both the health baby rate and the live birth rate favoured fresh-embryo transfer in the E-Freeze trial, leading to a lower probability of the freeze-all approach being considered cost-effective. This pattern remained when the subsequent transfer of the remaining embryos was simulated, both with and without the inclusion of pregnancy and delivery costs.
A further economic analysis was carried out using data from a multinational RCT comparing a personalised embryo transfer strategy, guided by endometrial receptivity, with frozen-embryo transfer and fresh-embryo transfer. 59 The personalised embryo transfer strategy involved the freezing of all viable blastocysts and the use of an array test to predict each individual’s optimum window for implantation in a subsequent frozen-embryo transfer with hormonal replacement. Similarly, this study found the cost per embryo transfer to be higher in the freeze-all arm than in the fresh-embryo transfer arm, and reported a slightly lower live birth rate per first frozen-embryo transfer than for single fresh-embryo transfer. The personalised embryo transfer strategy generated the highest live birth rate, but incurred higher treatment costs than both the fresh-embryo transfer and the freeze-all arm. No such treatment arm was included in E-Freeze for comparison.
Strengths
To the best of our knowledge, this is the first and only trial in the UK comparing fresh-embryo transfer with a policy of electively freezing all embryos, followed by subsequent frozen-embryo transfer. In total, 18 clinics participated, of which 13 recruited participants.
The recruited trial population is consistent with what would be expected given the NHS-funded treatment criteria (i.e. age < 40 years, BMI < 30 kg/m2, non-smoker, no previous children). 3,60 The occurrence rate of aetiological causes of infertility in this trial were similar to those that have been reported in previous population-based studies for other aetiologies of infertility,61,62 except unexplained infertility, the proportion of which was higher in this trial. The duration of infertility was > 2 years for most participants, which is the minimum duration criterion for unexplained infertility used for accessing NHS-funded IVF, as per national guidance. 3,60
The E-Freeze trial was a pragmatic trial (i.e. except for randomisation, all other process pre and post randomisation were as per local protocols) involving multiple clinics across the UK. The participants were recruited from both the NHS and private clinics, given that > 60% of IVF in the UK is privately funded by couples themselves. The pragmatic nature of the trial provides a true reflection of what happens in clinical practice.
A healthy baby is a unique outcome chosen for this trial because it encompasses efficacy and safety together. All other trials on this topic have reported on live birth rate or ongoing pregnancy rates. 40–46 As all complications in pregnancy and delivery have an impact on the short- and long-term health of an individual, the E-Freeze trial was unique in taking a holistic view of the infant, rather than just focusing on a live birth.
A detailed economic analysis is presented, based on all costs incurred to the NHS as well as participants, from post randomisation to the delivery of the baby. Cost analysis also included modelling to incorporate the longer-term costs in each of the trial arms of a heathy baby if all remaining embryos were used. Real-time data from clinical record forms and participant questionnaires were used for cost analysis. The response rate for the questionnaires was > 70% from both partners. Of the seven existing trials across the world on this topic,40–46 only one Vietnamese trial has reported on an economic analysis conducted alongside the trial,58 stating similar conclusions to ours; however, the Vietnamese trial did not have a societal perspective and was not performed in an NHS setting.
The E-Freeze trial is the only trial on this topic exploring the emotions of both the male and the female partners. It is becoming clear that, along with the clinical outcome, patient perceptions are equally important for any process or procedure to be put in place. This is especially important when one is recommending radical changes to the treatment, such as how IVF treatment is delivered, with delay and uncertainty associated with it. As with the economic questionnaire, the emotions questionnaire had a good return rate of > 70% at both of the time points at which they were administered (i.e. at consent and the actual intervention). Most literature on emotions relates to women only; this trial was unique in exploring the emotions of both the male and the female partner’s STAI scores separately. None of the other trials on this topic has evaluated this aspect.
Although a lot of assumptions were made in the sample size calculations because of the unique nature of the primary outcome, the results suggested that most were correct. We anticipated that the healthy baby rate in our trial population would be towards the lower end of the CI, around 25%, taking into account the higher risk of preterm delivery and babies with a low birthweight for their gestational age following IVF. The healthy baby rate was 24.4% in the trial, which was in line with this assumption. We also anticipated that, of those couples who consented, 50% would not obtain three good-quality embryos. In the trial, 49.6% of those consented did not have at least three good-quality embryos and, hence, were not randomised. Therefore, the trial design and sample size calculation were robust, based on the assumptions, which were very close to what was seen in reality.
Limitations
The trial did not reach its predetermined sample size of 1086 and, therefore, lacked the power to provide a definitive answer to the research questions. This was compounded by the bias introduced from non-adherence to the allocated intervention, especially in the freeze-all arm, of up to 31%. The additional analysis by per protocol, as treated and CACE did not change any results. There was no difference in the baseline characteristics (i.e. demographic and clinical, pre and post randomisation) or primary outcome rate between those who adhered to the allocated intervention and those who did not adhere to the allocated intervention. The consistent result across all analysis types indicates that it is likely that non-adherence did not alter the result. However, this cannot be definitively concluded.
There was a significant drop in the number of participants from consent to randomisation, with most participants not reaching three good-quality embryos. This was a result of the broad inclusion criteria, which allowed patients to take part in the study regardless of ovarian reserve, rather than including only those with good ovarian reserve. This was agreed by the co-investigator group at the outset, after much debate, acknowledging that ovarian reserve tests predict only the number of eggs, not their quality. A total of 30 participants had fewer than six eggs; they would not have been included if we had strict criteria based on ovarian reserve tests.
There was a change in practice during the trial, as clinics moved from embryo transfer on day 3 to embryo transfer on day 5. The reason for randomisation at day 3 in this trial was that embryo transfer would be as close to randomisation as possible. When the trial protocol was written, most embryo transfers occurred at day 3. However, as practice changed to transfer on day 5, there was a gap between randomisation and the actual intervention, which provided an opportunity for clinicians and participants to change their mind, contributing to non-adherence.
It has been suggested that freeze-all will benefit those with a large number of eggs, but we did not plan a subgroup analysis based on the number of eggs obtained a priori, as a large number of eggs are at a higher risk of OHSS, which was an exclusion criterion. There are now data available from other trials, published after the E-Freeze trial started recruiting, reporting that the strategy of freeze-all benefits those with a large number of eggs. 40,41 This was revisited by the co-investigator group; however, it was agreed not to undertake a post hoc analysis because the number of patients with more than 15 eggs was small (45/616) and, therefore, it was unlikely to provide a clinically meaningful answer.
Some adverse birth outcomes (e.g. preterm delivery and low birthweight) can have a far-reaching impact on costs and child health outcomes. It was initially planned1 that modelling would be used to inform cost-effectiveness over an extended time horizon to capture the long-term costs and health outcomes for any infants born as a result of treatment. However, the number of infants with adverse birth outcomes in this trial was too small to inform a robust analysis of any expected differences in long-term outcomes. It was felt that this analysis would be best undertaken as part of an individual patient data (IPD) meta-analysis (IPD-MA), as explained in Implications for research.
Meaning of the study
For the strategy of freeze-all to be used as routine policy, it must be better in terms of safety, efficacy and cost, as it involves a delay for couples in getting to pregnancy. There is also extra work involved for clinics. Although there is a biological plausibility that freezing all embryos, followed by frozen-embryo transfer, will be better in terms of success rates, safety and, hence, costs for all couples undergoing IVF/ICSI, this has not been proven when tested in this trial or when the data from other trials were assembled. This is not dissimilar to other interventions in this field (e.g. personalised embryo transfer,59 endometrial scratch63 and preimplantation genetic testing64 for aneuploidy) that had a biological plausibility to improve success rates, but, when put to the stringent test of RCTs, were not proven to be more effective.
The health economic findings from the E-Freeze trial of a low probability of cost-effectiveness for the freeze-all approach compared with fresh-embryo transfer, in terms of NHS treatment costs per healthy baby and per live birth and higher costs incurred by participants, do not currently support the widespread use of the freeze-all approach in the NHS. However, further research to ascertain any differences in child health outcomes and costs between the alternative approaches is required (see Implications for research). Although the data did not reach statistical significance, there is a trend towards a reduction in the healthy baby, live birth and clinical pregnancy rates with the freeze-all approach compared with fresh-embryo transfer.
The absolute number of cases with OHSS was small in our trial. Although there is no statistically significant difference in any arm in the E-Freeze trial, the data collated from all trials together show a reduction in OHSS when freezing all embryos compared with fresh-embryo transfer. Hence, from a safety viewpoint, to reduce OHSS there could be a subgroup of participants who may still benefit from freezing all embryos, especially those at high risk of OHSS.
The level of non-adherence in the freeze-all arm suggests that patients still prefer fresh-embryo transfer to the freeze-all approach.
Implications for practice and policy
Based on the fact that the strategy of freezing all embryos, followed by frozen-embryo transfer, did not lead to a higher healthy baby rate or a higher live birth rate, and given it incurred slightly higher treatment costs, its widespread adoption as part of routine NHS practice is not supported by data from the E-Freeze trial. It should be advocated only when there is an indication that the freeze-all approach would be beneficial for the individual patient. There is no indication to recommend a change to current practice in the UK, which is fresh-embryo transfer and the freezing of spare embryos.
The NICE guidance does not specifically mention freeze-all, possibly because the last guidance was published in 2004, when freeze-all was not on the horizon. An update of the guidance was conducted in 2013, but this question was not looked at. 3 It is possible that the next update of the NICE guidance will specifically look at this question, as practice of freeze-all has been increasing. 65 Our results clearly show that freeze all is not a cost-effective strategy from a health-care perspective, despite accounting for the extra costs associated with OHSS in fresh-embryo transfer. In fact, the freeze-all approach is more costly, with no added benefit for normal responders.
The most common reason for non-adherence was patient choice. This indicates that the strategy is not ready to be accepted by patients. This is an equally important finding, as patients’ choices must be taken into consideration, with an equal weighting as that of other clinical evidence, when recommending a policy.
Implications for regulators
The Health Fertilisation and Embryology Authority regulates and licenses all clinics providing IVF treatment in the UK. HFEA has a traffic light system for treatment add-ons. 66 Treatment add-ons are optional, additional treatments that patients may be offered on top of their routine fertility treatment, usually at an additional cost. The treatment add-ons claim to improve the chances of a live birth. The traffic light rating system consists of three colours: red, green and amber. Green indicates that there is evidence, in the form of high-quality RCTs, showing that a treatment add-on can safely improve the live birth rate for someone undergoing fertility treatment. The freeze-all approach is currently amber. 67 An amber symbol is given for an add-on where there is ‘conflicting evidence’ to show that an add-on can improve live birth rates or that the add-on is safe for patients to use. The amber symbol ‘means that the evidence is not conclusive and further research is required, and the add-on should not be recommended for routine use’ (© HFEA. Reproduced with permission from HFEA66). Although there is evidence that the freeze-all approach is suitable for hyper-responders, for whom the risk of OHSS is high, there is no evidence that the chance of a live birth improves for normal responders. The lack of improvement in live birth rate in the freeze-all arm compared with the fresh-embryo transfer arm is clear from this trial, but adding the data from other trials confirms it. 42–46
Although the E-Freeze trial did not reach an adequate sample size on its own, the sample size is adequate when you combine the data from the other five trials42–46 that were published during the conduct of this trial. The combined sample size for these trials is much larger than the sample size that was proposed for the E-Freeze trial. The fact that adding the E-Freeze trial data to the data from other trials does not alter the precision, magnitude or direction of the effect means that the evidence is now stable for the live birth rate. There is no need for further RCTs in this area, as they would be unlikely to change the outcome for women undergoing IVF/ICSI. Any trial that has a larger sample size than this combined sample size is unlikely to be conducted in the UK given the recruitment difficulties in the current trial and the fact that it will cost a huge amount of taxpayers’ money. With the combined data from the other trials,42–46 as shown in Figure 15, the freeze-all approach could be assigned the red symbol to stop the practice of freezing all embryos (a practice which has been advocated by some clinics68) for all patients, including those who are not at a high risk of OHSS.
Implications for funders for NHS in vitro fertilisation/intracytoplasmic sperm injection treatments
Difficulties in recruiting to in vitro fertilisation studies in the UK
Most IVF/ICSI treatment across the UK is funded by patients. Where it is funded by the NHS, the full entitlement reported in the NICE guidance3 (i.e. three full cycles of IVF/ICSI) is not available for most patients. To randomise patients to a clinical IVF trial in such a set-up is always going to be difficult, especially when the intervention causes a delay and extra costs, and is the only funded treatment that patients may receive. This is, possibly, one of the reasons for the large proportion of patients who were non-compliant. RCTs are the gold standard; for successful recruitment in any future clinical trials in the UK in the field of IVF/ICSI, NHS funding must improve to the level recommended by NICE. 3
Implications for research
Further analyses of the E-Freeze trial data
Cumulative live birth rates
This trial evaluated the results from the first embryo transfer after randomisation only. However, most participants had any spare embryos frozen. The field of IVF is changing, and providers’ and patients’ perceptions of the procedure’s success are drifting from focusing on the results of the first embryo transfer to the cumulative results of a single ovarian stimulation cycle. We would like to look at the CLBR after all spare embryos are used. This is especially important as it is acknowledged that the CLBR is a more relevant and important outcome for both policy and the individual patient. This has been recognised by regulatory authorities for data reporting, and the most up-to-date data reporting includes the CLBR as the headline. 5,69 There have been concerns raised that the CLBR may be lower if all embryos are frozen. 46,70 Participants were asked to consent to long-term follow-up, to which the majority of participants agreed.
Follow-up of children born
The primary outcome of this study was the healthy baby rate; however, there are long-term implications. We would like to follow up children born from both kinds of embryo transfer to see if there is any impact on long-term outcomes.
Further analysis of State–Trait Anxiety Inventory scores
We plan to perform in-depth analysis of the STAI self-evaluation questionnaire to:
-
investigate if there was an association between baseline demographic and clinical characteristics and anxiety at the start and end of the IVF process
-
examine whether or not anxiety prior to IVF treatment is related to non-adherence to the allocated embryo transfer method.
We will also perform qualitative analysis on the free-text responses for both those participants who complied to their allocated intervention and those participants who did not to elicit the reasons behind non-compliance.
Further research questions raised
Individual patient data meta-analysis
The question of whether or not freezing all embryos, followed by thawed frozen-embryo transfer, is better than fresh-embryo transfer has received a lot of attention. When the E-Freeze trial was planned, several other trials across the world were also planned and conducted. Despite eight RCTs in total (including the E-Freeze trial)11,12,42–46 and multiple aggregated meta-analyses on this topic, there is still a lack of clarity around the effectiveness and cost-effectiveness of a strategy of elective embryo freezing or freeze-all in IVF. What is becoming clear from the published trial data and the data from the E-Freeze trial is that freeze-all is not an effective approach in all women undergoing IVF, can reduce the chances of pregnancy in some women and carries potential risks. The current meta-analyses of aggregated data50,51 have indicated that more data are needed to resolve the current uncertainty as to the groups of patients for whom the strategy of freeze-all would be beneficial.
As the randomised trials42–46 have a degree of clinical heterogeneity that could mask the potential benefits of frozen-embryo transfer in specific groups of women, rather than investing additional time and resources into further randomised trials, we believe that a patient IPD-MA offers a more efficient and cost-effective way of identifying these subgroups and providing a definitive answer to this important clinical question. The number of published IPD-MAs has increased considerably over the past decade in other areas of medicine. Evidence synthesis involving the collection and analysis of individual patient data (IPD) are considered the best method for assessing participants’ characteristics and provide more detailed and robust meta-analysis results. 71 Such an approach could also provide more power to detect any differences in the categories of adverse birth outcomes to better inform any expected differences in child health outcomes and costs.
An IPD-MA approach has both statistical and clinical advantages. Data from existing trials42–46 suggest that the freeze-all strategy is not effective for all patients but could improve the efficacy and safety of IVF in some women. Therefore, it is very important to identify subgroups of participants from the existing trials in whom a freeze-all strategy could be adopted. For example, the effectiveness of a freeze-all policy may vary by maternal age, number of available eggs and embryos, stage of development of an embryo prior to transfer and the laboratory method used to freeze embryos (i.e. slow vs. fast freezing). No individual trial is large enough to answer this question and conventional meta-analysis of aggregated data does not lend itself easily to the extraction of sufficient compatible data for meaningful subgroup analyses. By contrast, IPD will allow the effective categorisation of participants for subgroup analyses defined by single or multiple factors and, therefore, offers valuable clinical insights that are particularly relevant to our clinical question.
An IPD-MA will allow us to estimate the treatment effects adjusted for baseline factors where, previously, only unadjusted estimates were available. This has the advantage of increasing the statistical power and allowing an adjustment for potential confounding factors. Consistent inclusion and exclusion criteria could be applied across studies in a way that cannot be undertaken using published aggregated data. It is possible to verify the results of the original trials and request additional data from the triallists that are not available from the published reports.
The statistical analysis can be standardised across studies [e.g. the analysis method, how continuous variables (e.g. maternal age) are analysed] and we can combine data that have been recorded in different formats. Moreover, model assumptions can be assessed and more advanced methods can be applied when necessary.
One can estimate the incidence rate of clinically important, but less common, pregnancy and neonatal complications with greater precision in a randomised cohort of children born following either fresh-embryo transfer or frozen-embryo transfer. This will enable precise projections of any expected differences in child health outcomes and costs. An IPD approach will also help to inform future studies that develop prediction models to predict a couple’s success rate with fresh-embryo transfer compared with frozen-embryo transfer, which would not be possible with standard published data.
Conclusions
The results of this pragmatic, multicentre, two-arm, parallel-group, non-blinded RCT show no evidence of a difference in the healthy baby rate from freezing all embryos, followed by thawed frozen-embryo transfer, compared with the rate from fresh-embryo transfer. There was no statistical difference in OHSS, obstetrics or perinatal complications, or in stress and anxiety scores, between the groups.
The health economic analysis shows that freezing all embryos is not a cost-effective strategy; in fact, it is more costly in both the short and the longer terms and is, therefore, unsuitable for use in routine practice currently.
The decision to offer the freeze-all strategy should be balanced against potential benefit and harm for the mother and child. Rather than investing resources in further trials, we need to join our efforts to undertake an IPD-MA to reach definite answers and identify the subgroups who will benefit most from the freeze-all approach.
Acknowledgements
The authors would like to acknowledge all of the couples who participated in the trial and the site staff, without whom this research would not have been possible. We thank the members of the independent DMC and TSC, and the administrative and support colleagues at the NPEU CTU.
Funding and sponsorship
The E-Freeze trial was sponsored by the University of Aberdeen and NHS Grampian. The sponsor had no role in the study design or data collection, analysis and interpretation.
Independent Trial Steering Committee
Professor Richard Anderson (chairperson), Ms Kate Brian, Ms Gwenda Burns, Ms Helen Kendrew, Dr Umesh Acharya, Mr Lee Middleton, Ms Sue Avery, Ms Susan Seenan and Ms Aileen Feeney.
Independent Data Monitoring Committee
Mr Anthony Rutherford (chairperson), Dr Paul Knaggs, Dr Gillian Lockwood and Dr Elizabeth Allen.
Contributions of authors
Abha Maheshwari (https://orcid.org/0000-0002-3652-2447) (Chief Investigator) was responsible for data collection and management, the study design and writing the report; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Vasha Bari (https://orcid.org/0000-0001-8183-2455) (E-Freeze Acting Trial Manager) was responsible for data collection and management; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Jennifer L Bell (https://orcid.org/0000-0001-9571-0715) (Medical Statistician) was responsible for the data analysis and writing the report; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Siladitya Bhattacharya (https://orcid.org/0000-0002-4588-356X) (Co-investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Priya Bhide (https://orcid.org/0000-0003-0871-6508) (Principal Investigator) provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Ursula Bowler (https://orcid.org/0000-0002-0100-0155) (Senior Trials Manager) was responsible for data collection and management, and the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Daniel Brison (https://orcid.org/0000-0002-4307-1293) (Co-investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Tim Child (https://orcid.org/0000-0001-6668-0529) (Co-investigator and Principal Investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Huey Yi Chong (https://orcid.org/0000-0002-0768-1844) (Research Fellow and Health Economist) was responsible for the health economic analysis and writing the report; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Ying Cheong (https://orcid.org/0000-0001-7687-4597) (Principal Investigator) provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Christina Cole (https://orcid.org/0000-0002-8798-2136) (Core Trials Support and E-Freeze Trial Manager) was responsible for data collection and management, the study design and writing the report; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Arri Coomarasamy (https://orcid.org/0000-0002-3261-9807) (Co-investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Rachel Cutting (https://orcid.org/0000-0002-6786-1097) (Director of Compliance at Human Fertilisation and Embryology Authority, Co-investigator and Principal Investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Fiona Goodgame (https://orcid.org/0000-0002-4809-9746) (Acting E-Freeze Trial Manager, Administrator and Data Co-ordinator) was responsible for data collection and management, and writing the report; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Pollyanna Hardy (https://orcid.org/0000-0003-2937-8368) (Co-investigator and Senior Medical Statistician) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Haitham Hamoda (https://orcid.org/0000-0002-2330-1768) (Principal Investigator) provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Edmund Juszczak (https://orcid.org/0000-0001-5500-2247) (Co-investigator and former NPEU CTU Director, current Professor of Clinical Trials and Statistics in Medicine in Nottingham CTU) was responsible for data collection and management, the study design and the data analysis; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Yacoub Khalaf (https://orcid.org/0000-0002-5642-7367) (Co-investigator and Principal Investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Andrew King (https://orcid.org/0000-0001-7175-2718) (Head of Trials Programming) was responsible for data collection and management, and the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Jennifer J Kurinczuk (https://orcid.org/0000-0001-9554-6337) (Co-investigator and Director, NPEU) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Stuart Lavery (https://orcid.org/0000-0002-7380-3491) (Co-investigator and Principal Investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Clare Lewis-Jones (Co-Investigator and patient and public involvement representative) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Louise Linsell (https://orcid.org/0000-0003-3205-6511) (Lead Medical Statistician) was responsible for the data analysis and writing the report; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Nick Macklon (https://orcid.org/0000-0003-2436-2316) (Co-investigator and Principal Investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Raj Mathur (https://orcid.org/0000-0002-7550-1817) (Principal Investigator) provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
David Murray (https://orcid.org/0000-0001-9010-2905) (Senior Trials Programmer) was responsible for data collection and management, and the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Jyotsna Pundir (https://orcid.org/0000-0003-4183-8048) (Principal Investigator) provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Nick Raine-Fenning (https://orcid.org/0000-0001-5521-9059) (Co-investigator and Principal Investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Madhurima Rajkohwa (Co-investigator and Principal Investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Lynne Robinson (https://orcid.org/0000-0002-3309-554X) (Principal Investigator) provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Graham Scotland (https://orcid.org/0000-0001-5539-8819) (Co-investigator and Senior Health Economist) was responsible for the study design, the health economic analysis and writing the report; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Kayleigh Stanbury (https://orcid.org/0000-0002-8726-2411) (Senior Trials Manager) was responsible for data collection and management; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Stephen Troup (https://orcid.org/0000-0002-3342-4825) (Co-investigator and Principal Investigator) was responsible for the study design; provided intellectual input during the conduct of the study, modification of the protocol, delivery of the study, interpretation of the findings, recommendations and implications for policy and practice, and dissemination; and approved the final draft of the manuscript.
Publication
Maheshwari A, Bell JL, Bhide P, Brison D, Child T, Chong HY, et al. Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-Freeze). Human Reprod 2022:deab279.
Data-sharing statement
Data will be shared in accordance with the NPEU Data Sharing policy. Requests for access to the data will be considered by the NPEU Data Sharing committee. Access to anonymised data can be requested from general@npeu.ox.ac.uk.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Maheshwari A, Bhattacharya S, Bowler U, Brison D, Child T, Cole C, et al. Study protocol: E-Freeze – freezing of embryos in assisted conception: a randomised controlled trial evaluating the clinical and cost effectiveness of a policy of freezing embryos followed by thawed frozen-embryo transfer compared with a policy of fresh-embryo transfer, in women undergoing in vitro fertilisation. Reprod Health 2019;16. https://doi.org/10.1186/s12978-019-0737-2.
- Oakley L, Doyle P, Maconochie N. Lifetime prevalence of infertility and infertility treatment in the UK: results from a population-based survey of reproduction. Hum Reprod 2008;23:447-50. https://doi.org/10.1093/humrep/dem369.
- National Institute for Health and Care Excellence . Fertility: Assessment and Treatment for People With Fertility Problems [CG156] n.d. www.nice.org.uk/guidance/cg156 (accessed 24 August 2015).
- Human Fertilisation and Embryology Authority . Fertility Treatment in 2012: Trends and Figures n.d. www.hfea.gov.uk/docs/FertilityTreatment2012TrendsFigures.PDF (accessed 24 August 2015).
- Society For Assisted Reproductive Technology . National Summary Report: Live Births Per Intended Egg Retrieval (All Embryo Transfers) 2018. www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2018 (accessed September 2020).
- Royal College of Obstetricians and Gynaecologists . Ovarian Hyperstimulation Syndrome, Management (Green-Top Guideline No. 5) n.d. www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg5/ (accessed 24 August 2015).
- Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 2012;18:485-503. https://doi.org/10.1093/humupd/dms018.
- Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, et al. Fresh-embryo transfer versus frozen-embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril 2013;99:156-62. https://doi.org/10.1016/j.fertnstert.2012.09.003.
- Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril 2012;98:368-77.e9. https://doi.org/10.1016/j.fertnstert.2012.05.019.
- Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh-embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet 2010;27:357-63. https://doi.org/10.1007/s10815-010-9412-9.
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril 2011;96:344-8. https://doi.org/10.1016/j.fertnstert.2011.05.050.
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertil Steril 2011;96:516-18. https://doi.org/10.1016/j.fertnstert.2011.02.059.
- Bell JL, Hardy P, Greenland M, Juszczak E, Cole C, Maheshwari A, et al. E-Freeze – a randomised controlled trial evaluating the clinical and cost effectiveness of a policy of freezing embryos followed by thawed frozen-embryo transfer compared with a policy of fresh-embryo transfer, in women undergoing in vitro fertilisation: a statistical analysis plan. Trials 2020;21. https://doi.org/10.1186/s13063-020-04441-9.
- Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A. BFS and ACE . Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb) 2008;11:131-46. https://doi.org/10.1080/14647270802302629.
- Human Fertilisation and Embryology Authority . Human Fertilisation and Embryology Authority: The New Version of the Code of Practice Is Now Available n.d. www.hfea.gov.uk/about-us/news-and-press-releases/2019-news-and-press-releases/new-version-of-the-code-of-practice-has-been-launched/ (accessed 15 December 2021).
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
- Consolidated Standards of Reporting Trials group . The CONSORT Statement n.d. www.consort-statement.org (accessed 15 December 2021).
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407-29. https://doi.org/10.1002/(SICI)1097-0258(19980228)17:4<407::AID-SIM742>3.0.CO;2-L.
- Dunn G, Maracy M, Dowrick C, Ayuso-Mateos JL, Dalgard OS, Page H, et al. Estimating psychological treatment effects from a randomised controlled trial with both non-compliance and loss to follow-up. Br J Psychiatry 2003;183:323-31. https://doi.org/10.1192/bjp.183.4.323.
- Hewitt CE, Torgerson DJ, Miles JNV. Is there another way to take account of noncompliance in randomized controlled trials?. Can Med Assoc J 2006;175:347-8. https://doi.org/10.1503/cmaj.051625.
- Opondo C, Halliday K, Witek-McManus S, Allen E. Estimating Intervention Effect in Cluster Randomised Controlled Trials With Non-Compliance n.d.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Maheshwari A, Bell JL, Bhide P, Brison D, Child T, Chong HY, et al. Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-Freeze). Human Reprod 2022. https://doi.org/10.1093/humrep/deab279.
- Centres for Disease Control and Prevention . Defining Adult Overweight &Amp; n.d. www.cdc.gov/obesity/adult/defining.html (accessed 15 December 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2018 19 2020. www.england.nhs.uk/national-cost-collection/#ncc1819 (accessed May 2020).
- Joint Formulary Committee . British National Formulary (online) n.d. https://bnf.nice.org.uk/ (accessed May 2020).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017 18 2019. https://improvement.nhs.uk/resources/reference-costs/ (accessed May 2020).
- Sagili H, Mohamed K. Review: Pregnancy of Unknown Location: An Evidence-Based Approach to Management n.d. https://elearning.rcog.org.uk/sites/default/files/Early%20pregnancy%20loss%20-%20management/sagili_tog_2008.pdf (accessed September 2020).
- National Casemix Office . Code to Group: HRG4+ 2019 20 Local Payment Grouper 2019. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/local-payment-grouper-2019-20 (accessed May 2020).
- HM Revenue and Customs . Expenses and Benefits: Business Travel Mileage for Employees’ Own Vehicles n.d. www.gov.uk/expenses-and-benefits-business-travel-mileage/rules-for-tax (accessed 6 May 2020).
- Office for National Statistics . Annual Survey of Hours and Earnings. 2019 Provisional n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/agegroupashetable6 (accessed May 2020).
- Office for National Statistics . Annual Survey of Hours and Earnings. 2016. Unpaid Work Calculator n.d. www.ons.gov.uk/visualisations/dvc376/index.html (accessed May 2020).
- Department for Transport . Transport Analysis Guidance (TAG) Data Book n.d. www.gov.uk/government/publications/tag-data-book (accessed May 2020).
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Handbooks in Health Economic Evaluation. Oxford: Oxford University Press; 2007.
- Howard S. The hidden costs of infertility treatment. BMJ 2018;361. https://pubmed.ncbi.nlm.nih.gov/29789345/ (accessed December 2021).
- Human Fertilisation and Embryology Authority . Fertility Treatement 2019: Trends and Figures n.d. https://hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2019-trends-and-figures/ (accessed June 2021).
- Li Z, Wang AY, Bowman M, Hammarberg K, Farquhar C, Johnson L, et al. Cumulative live birth rates following a ‘freeze-all’ strategy: a population-based study. Hum Reprod Open 2019;2019. https://doi.org/10.1093/hropen/hoz004.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/chapter/foreword (accessed December 2021).
- Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523-33. https://doi.org/10.1056/NEJMoa1513873.pmid:27509101.
- Aflatoonian A, Mansoori-Torshizi M, Farid Mojtahedi M, Aflatoonian B, Khalili MA, Amir-Arjmand MH, et al. Fresh versus frozen-embryo transfer after gonadotropin-releasing hormone agonist trigger in gonadotropin-releasing hormone antagonist cycles among high responder women: a randomized, multi-center study. Int J Reprod Biomed 2018;16:9-18. https://doi.org/10.29252/ijrm.16.1.9.
- Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, et al. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med 2018;378:137-47. https://doi.org/10.1056/NEJMoa1703768.
- Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018;378:126-36. https://doi.org/10.1056/NEJMoa1705334.
- Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet 2019;393:1310-18. https://doi.org/10.1016/S0140-6736(18)32843-5.
- Stormlund S, Sopa N, Zedeler A, Bogstad J, Prætorius L, Nielsen HS, et al. Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilisation in women with regular menstrual cycles: multicentre randomised controlled trial. BMJ 2020;370. https://doi.org/10.1136/bmj.m2519.
- Wong KM, van Wely M, Verhoeve HR, Kaaijk EM, Mol F, van der Veen F, et al. Transfer of fresh or frozen embryos: a randomised controlled trial. Hum Reprod 2021;36:998-1006. https://doi.org/10.1093/humrep/deaa305.
- Corps D. Office for National Statistics . Birth Characteristics in England and Wales: 2019 2020. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2019 (accessed September 2020).
- Hirst JE, Knight HE, Ohuma EO, Dwyer T, Hennig BD, Papageorghiou AT, et al. Social gradient of birthweight in England assessed using the INTERGROWTH-21st gestational age-specific standard. Arch Dis Child Fetal Neonatal Ed 2019;104:F486-92. https://doi.org/10.1136/archdischild-2018-315295.
- Human Fertilisation and Embryology Authority . Ovarian Hyperstimulation Syndrome n.d. www.hfea.gov.uk/media/2463/january-2018-ovarian-hyperstimulation-syndrome.pdf (accessed December 2021).
- Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is frozen-embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer?. Hum Reprod Update 2018;24:35-58. https://doi.org/10.1093/humupd/dmx031.
- Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen-embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update 2019;25:2-14. https://doi.org/10.1093/humupd/dmy033.
- Bosdou JK, Venetis CA, Tarlatzis BC, Grimbizis GF, Kolibianakis EM. Higher probability of live birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: a meta-analysis. Hum Reprod 2019;34:491-505. https://doi.org/10.1093/humrep/dey388.
- Weissman A. IVF Worldwide . Results: Frozen-Thawed Embryo Transfer 2008. https://ivf-worldwide.com/survey/frozen-thawed-embryo-transfer/results-frozen-thawed-embryo-transfer.html (accessed 21 January 2021).
- von Versen-Höynck F, Häckl S, Selamet Tierney ES, Conrad KP, Baker VL, Winn VD. Maternal vascular health in pregnancy and postpartum after assisted reproduction. Hypertension 2020;75:549-60. https://doi.org/10.1161/HYPERTENSIONAHA.119.13779.
- Stormlund S, Schmidt L, Bogstad J, Løssl K, Prætorius L, Zedeler A, et al. Patients’ attitudes and preferences towards a freeze-all strategy in ART treatment. Hum Reprod 2019;34:679-88. https://doi.org/10.1093/humrep/dez006.
- Abdulrahim B, Scotland G, Bhattacharya S, Maheshwari A. Assessing couples’ preferences for fresh or elective frozen-embryo transfer in in vitro fertilisation: a discrete choice experiment. Hum Reprod 2021;36:2891-903. https://doi.org/10.1093/humrep/deab207.
- Roque M, Valle M, Guimarães F, Sampaio M, Geber S. Cost-effectiveness of the freeze-all policy. JBRA Assist Reprod 2015;19:125-30. https://doi.org/10.5935/1518-0557.20150028.
- Le KD, Vuong LN, Ho TM, Dang VQ, Pham TD, Pham CT, et al. A cost-effectiveness analysis of freeze-only or fresh-embryo transfer in IVF of non-PCOS women. Hum Reprod 2018;33:1907-14. https://doi.org/10.1093/humrep/dey253.
- Simón C, Gómez C, Cabanillas S, Vladimirov I, Castillón G, Giles J, et al. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod Biomed Online 2020;41:402-15. https://doi.org/10.1016/j.rbmo.2020.06.002.
- The Scottish Government . National Infertility Group Report 2016 2016. www.gov.scot/Publications/2016/06/9960 (accessed May 2020).
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J 1985;291:1693-7. https://doi.org/10.1136/bmj.291.6510.1693.
- Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Hum Reprod 2008;23:538-42. https://doi.org/10.1093/humrep/dem431.
- Lensen S, Osavlyuk D, Armstrong S, Stadelmann C, Hennes A, Napier E, et al. A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med 2019;380:325-34. https://doi.org/10.1056/NEJMoa1808737.
- Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril 2019;112:1071-9.e7.
- Human Fertilisation and Embryology Authority . Fertility Treatment 2018: Trends and Figures n.d. www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2018-trends-and-figures/ (accessed 21 January 2021).
- Human Fertilisation and Embryology Authority . Treatment Add-Ons With Limited Evidence n.d. www.hfea.gov.uk/treatments/treatment-add-ons/ (accessed 21 January 2021).
- Human Fertilisation and Embryology Authority . Elective Freeze All Cycles n.d. www.hfea.gov.uk/treatments/treatment-add-ons/elective-freeze-all-cycles/ (accessed 21 January 2021).
- Lumby T. Frozen embryo pregnancy boost. Express 2018. www.express.co.uk/life-style/health/1010619/Frozen-embryo-pregnancy-ivf-baby-science-health-fertility (accessed 17 December 2021).
- Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus?. Hum Reprod 2016;31:572-81. https://doi.org/10.1093/humrep/dev336.
- Smith ADAC, Tilling K, Lawlor DA, Nelson SM. Live birth rates and perinatal outcomes when all embryos are frozen compared with conventional fresh and frozen-embryo transfer: a cohort study of 337,148 in vitro fertilisation cycles. BMC Med 2019;17. https://doi.org/10.1186/s12916-019-1429-z.
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340. https://doi.org/10.1136/bmj.c221.
- Office for National Statistics . UK Harmonised European Time Use Survey (HETUS), 2015 n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/articles/womenshouldertheresponsibilityofunpaidwork/2016-11-10 (accessed May 2020).
Appendix 1 Amendments
Appendix 2 Participating sites, principal investigators and research staff
| Recruiting site for E-Freeze | PI(s) | Research staff |
|---|---|---|
| Aberdeen Fertility Centre, Aberdeen | Professor Abha Maheshwari | Avril Kidd and Val Peddie |
| Birmingham Women’s Hospital, Birmingham | Dr Lynne Robinson and Dr Madhurima Rajkhowa | Faye Andrews, Nikkita Carden and Shanteela McCooty |
| Countess of Chester/IVI Cheshire | Mr Simon Wood | Nichola Kearsley |
| Glasgow Centre For Reproductive Medicine Clinic, Glasgow | Dr Marco Gaudoin | Laura McLuskey and Claire Wentworth |
| Guy’s Hospital, London | Professor Yacoub Khalaf | Oluyemisi Adegbile, Jean Bvumbe and Charlotte Yearwood-Martin |
| Hammersmith Hospital, London | Dr Stuart Lavery | Sara Barnett, Anna Bosanquet and Floria Cheng |
| Homerton Hospital, London | Dr Priya Bhide | Zameen Brar, Merve Digil, Monica James and Elizabeth Timlick |
| IVI Midland, Midlands | Dr Rhada Venkatakrishnan | Sue Lowbridge and Karen Mayne |
| Jessop Wing, Sheffield | Dr Rachel Cutting and Dr Helen Clarke | Elizabeth Taylor |
| King’s College Hospital, London | Dr Haitham Hamoda | Yusuf Beebeejaun, Nick Dalton-Brewer and Sarah Lensen |
| Liverpool Women’s Hospital, London | Miss Rebecca Lunt and Dr Stephen Troup | Sarah Hockenhull, Deborah Stephenson and Julie Wray |
| Nurture Fertility, Nottingham | Associate Professor Nick Raine-Fenning | Kathryn Cocking, Lynne Fogg, Michelle Parris-Larkin and Katie Smith |
| Oxford Fertility, Oxford | Professor Tim Child | Ginny Mounce |
| Princess Anne Hospital, Southampton | Professor Nick Macklon and Dr Ying Cheong | Jane Forbes, Teresa Gubbins and Susan Wellstead |
| Queen’s Hospital, Romford | Dr Sesh Sunkara | Annemarie McGregor |
| St Bartholomew’s Hospital, London | Dr Jyotsna Pundir | Alice Rossi, Amy Thomas and Zoi Vardavaki |
| St Mary’s Hospital, Manchester | Dr Raj Mathur | Katie Swindells, Clare Waters and Claudette Wright |
| University College Hospital, London | Dr Ephia Yasmin | Sarah Ekladios |
Appendix 3 Oversight committees
Data Monitoring Committee
-
Mr Anthony Rutherford, chairperson, independent member, consultant in reproductive medicine, The Leeds Centre for Reproductive Medicine, Leeds, UK.
-
Dr Paul Knaggs, independent member, consultant embryologist, Wales Fertility Institute, University Hospital of Wales, Cardiff, UK.
-
Dr Gillian Lockwood, independent member, director, Midland Fertility, Tamworth, UK.
-
Dr Elizabeth Allen, independent member, statistician, senior lecturer, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK.
Trial Steering Committee
-
Professor Richard Anderson, chairperson, independent member, professor of reproductive medicine, Medical Research Council University of Edinburgh Centre for Reproductive Health, the Queen’s Medical Research Institute, Edinburgh, UK.
-
Ms Kate Brian, independent member, London representative, Fertility Network, London, UK.
-
Ms Gwenda Burns, independent member, chief executive, Fertility Network, London, UK.
-
Ms Susan Seenan, independent member, chief executive, Infertility Network, Irvine, UK.
-
Ms Aileen Feeney, independent member, head of charity operations, Fertility Network, London, UK.
-
Ms Helen Kendrew, independent member, matron, Bath Fertility Centre, Bath, UK.
-
Dr Umesh Acharya, independent member, consultant in reproductive medicine, South West Centre for Reproductive Medicine, Plymouth, UK.
-
Mr Lee Middleton, independent member, senior statistician, Birmingham CTU, University of Birmingham, Birmingham, UK.
-
Professor Abha Maheshwari, non-independent member, consultant and honorary senior lecturer, University of Aberdeen, Aberdeen Maternity Hospital, Aberdeen, UK.
-
Associate Professor Edmund Juszczak, non-independent member, director, NPEU CTU, University of Oxford, Oxford, UK.
-
Professor Siladitya Bhattacharya, observer, professor of reproductive medicine and fertility, Aberdeen Maternity Hospital, Aberdeen, UK.
-
Ms Sue Avery, observer, director, Birmingham Women’s Fertility Centre, Assisted Conception Unit, Birmingham Women’s Hospital, Birmingham, UK.
-
Ms Kayleigh Stanbury, observer, senior trials manager, NPEU CTU, University of Oxford, Oxford, UK.
-
Ms Christina Cole, observer, E-Freeze trial manager/core trials support, NPEU CTU, University of Oxford, Oxford, UK.
Project Management Group
-
Professor Jennifer Kurinczuk, professor of perinatal epidemiology, director, NPEU CTU, University of Oxford, Oxford, UK.
-
Professor Abha Maheshwari, consultant and honorary senior lecturer, chief investigator, Aberdeen Maternity Hospital, Aberdeen, UK.
-
Associate Professor Ed Juszczak, director, NPEU CTU, University of Oxford, Oxford, UK.
-
Ms Kayleigh Stanbury, senior trials manager, NPEU CTU, University of Oxford, Oxford, UK.
-
Mr Andy King, head of trials programming, NPEU CTU, University of Oxford, Oxford, UK.
-
Mr David Murray, senior trials programmer, NPEU CTU, University of Oxford, Oxford, UK.
-
Ms Louise Linsell, senior trial statistician, NPEU CTU, University of Oxford, Oxford, UK.
-
Ms Jennifer Bell, trial statistician, NPEU CTU, University of Oxford, Oxford, UK.
-
Ms Christina Cole, Observer, E-Freeze Trial Manager/Core Trials Support, NPEU CTU, University of Oxford, Oxford, UK.
-
Ms Melanie Greenland, Trial Statistician, NPEU CTU, University of Oxford, Oxford, UK.
Appendix 4 Serious adverse events by trial arm
| SAE number | Trial arm | Centre ID | Description | Severity | Related | Action taken | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Fresh-embryo transfer | 1 | Patient unwell with hyperemesis, GP admitted the patient to hospital for IV fluids | Mild | Not related | None | Resolved |
| 2 | Fresh-embryo transfer | 1 | Patient attended clinic feeling unwell. Observations were recorded and bloods taken, and the results showed signs of OHSS. The patient was admitted for IV therapy, analgesia and management of symptoms | Moderate | Not related | None | Resolving |
| 3 | Fresh-embryo transfer | 3 | During the follow-up telephone call 3 months after birth, the mother reported that the baby had tongue tie | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Unknown |
| 4 | Fresh-embryo transfer | 4 | Participant diagnosed with late onset of OHSS. Admitted to local hospital for further tests and treatment | Moderate | Not related | None | Resolved |
| 5 | Fresh-embryo transfer | 4 | Woman reported that her daughter was born with a cleft palate. She said there is no other complication but that she will see a specialist to have it corrected later this year | Mild | Not related | None | Resolving |
| 6 | Fresh-embryo transfer | 4 | Infection post delivery. Patient complained of redness, pain and pus at site of C-section (infection in the uterus). Patient reported being given antibiotics for 10 days | Mild | Not related | None | Resolved |
| 7 | Fresh-embryo transfer | 4 | Patient had 20/40 [20 week] scan at her local hospital. Possible cardiac (fetal) anomaly seen. Referred to [name] hospital for confirmation. Scanned at 30 + 5/40 [weeks of gestation]. Confirmed fetal diagnosis of coarctation of aorta and ventricular septal defect. No other anomalies detected. No intervention for now. IOL at 38/40 [weeks of gestation]. Follow-up report: baby had surgery to correct heart defect as reported earlier, mother and baby home and well. This was reported during post-delivery follow-up call by site and transposed to the SAE follow-up form by the co-ordinating centre | Moderate | Not related | Discontinued | Resolved |
| 8 | Fresh-embryo transfer | 4 | Patient reported that her baby boy was tongue tied at birth and struggled to breastfeed. This was rectified immediately at 2 weeks post delivery. In addition, she reported that her son has one testicle that has failed to descend. He is booked to see the specialist | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Unknown |
| 9 | Fresh-embryo transfer | 5 | Fetal abnormality detected on antenatal ultrasound. Patient decided to terminate pregnancy | Severe | Not related | None | Resolved |
| 10 | Fresh-embryo transfer | 6 | Cleft soft palate noted on routine examination of the newborn. No other abnormalities. Reviewed by [location] cleft team [hospital name] while an inpatient. To be followed up as an outpatient. Safe to continue oral feeding | Moderate | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 11 | Fresh-embryo transfer | 8 | Patient had a termination, reported at the 12-week follow-up call. A congenital anomaly was noted but site detailed that the patient was unable to provide more information | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 12 | Fresh-embryo transfer | 10 | Patient was 4 weeks post partum when she had complained that she couldn't sleep or eat, had extreme emotions, paranoia, itchy body rash and flashing light before her eyes. Admitted to A&E | Moderate | Not related | None | Resolved |
| 13 | Fresh-embryo transfer | 11 | Ectopic pregnancy – confirmed [date] – admitted to hospital – left sided – home 4 days later | Moderate | Definitely | N/A [intervention(s) stopped prior to the event starting] | Resolved with sequelae |
| 14 | Fresh-embryo transfer | 11 | Postnatal period, approximately 2 weeks after birth, woman had seizure. Then another 2 weeks later. Now diagnosed as epileptic | Moderate | Not related | None | Resolved with sequelae |
| 15 | Frozen | 1 | Baby found to have a thickened nuchal area and abnormal location of heart outside the thorax | Severe | Not related | None | Resolved |
| 16 | Frozen | 2 | Patient reported being admitted to hospital when 11/40 [weeks of gestation] with severe pain from cyst on left ovary and overstimulated ovaries causing fluid in pelvis. Stayed in hospital for one week on painkillers and IV drip to help with nausea. Discharged, no follow-up and settled on its own with no intervention required | Moderate | Possibly | Resolved | |
| 17 | Frozen | 2 | Participant reports being admitted to hospital around 29 weeks’ gestation with a urinary tract infection. She was admitted for two nights on oral antibiotics and pain relief. Note: participant not treated within this trust so limited info available. Participant not able to recall exact medication | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 18 | Frozen | 2 | Participant reports being admitted into hospital around 32 weeks’ gestation with diarrohea. She was admitted for one night on IV fluids. Note: Participant not treated within this trust so limited info available. Participant not able to recall exact medication | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 19 | Frozen | 2 | Participant had prolonged hospitalisation due to ?Sepsis. Had raised CRP and creatinine and tachycardic. Was admitted to HDU post emergency C-section under GA. Was on IVAbx and IV fluids – was then observed and later discharged. Note: neonatal death also prolonged hospital stay as patient did not feel ready to go home following events | Moderate | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 20 | Frozen | 2 | Neonatal death. Post-mortem results now available. The pathologist’s opinion as to the cause of death: 1, hypoxic–ischaemic brain damage, 2, intrauterine infection manifesting in chorioamnionitis and intrauterine pneumonia | Severe | Not related | N/A [intervention(s) stopped prior to the event starting] | Fatal |
| 21 | Frozen | 3 | The baby was born on [date]. The site collected the data by telephone 1 month and 6 days later, when it was reported that the baby had a cleft palate | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Unknown |
| 22 | Frozen | 4 | The participant reported that her daughter was born at 41+5 weeks [of gestation]. Her baby had meconium aspiration during the delivery and had to stay in hospital for 10 days post delivery. The mother left hospital 4 days after delivery. Baby has been discharged on oxygen. She been on oxygen 4 weeks now since delivery and they are suggesting she may have another 6–8 weeks of oxygen therapy to come. Spoke to mother again on the [date] and she is happy to report that her baby is feeding well, her oxygen dose has been lowered to 0.05 l and her baby is sleeping well, growing as normal and thriving. She stated that the her baby is now down to a very low level of oxygen and that she is scheduled to see the doctor next week and expects that the oxygen therapy will be stopped | Moderate | Not related | None | Resolving |
| 23 | Frozen | 4 | Woman diagnosed with cholestasis in later stages of pregnancy | Moderate | Not related | None | Resolved |
| 24 | Frozen | 4 | Baby birthweight was 4400 g. During delivery he experienced shoulder dystocia. He also had tongue-tie, which was addressed through surgery to improve his feeding | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 25 | Frozen | 5 | Baby born on [date] with good Apgar score 9/1 + 10/5 min. Stopped breathing 18 minutes after birth. Ventilated for 2 days. Diagnosed with oesophageal atresia, tracheo-oesphageal fistula, small ventricular septal defect, patent foramen ovale and ductus arteriosus. Had repair surgery and closure of fistula on [date]. Tolerating feed well. Routine surgical and cardiology follow-up for heart murmur | Severe | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 26 | Frozen | 6 | After embryo transfer the patient has reported symptoms of OHSS. She has a positive BHCG and went to [place] with her symptoms of OHSS. We have been told she has had a chest draine as a result of OHSS. She has an appointment with us on [date]. We will receive and provide more information when we see her | Severe | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 27 | Frozen | 8 | Hirschprung: the baby has a temporary stoma | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Unknown |
| 28 | Frozen | 11 | Suspected ectopic pregnancy (Pain, per vagina bleeding, positive pregnancy test) at 6/40 pregnancy prior to pregnancy scan. Ultrasound revealed right sided ectopic. Admitted via ambulance to local hospital. Stable observation (+ surgery salpingectomy to remove) | Moderate | Definitely | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 29 | Frozen | 11 | Baby born on [date]. Site collected post-delivery data 3 months later. During the phone call, the mother reported that the baby had tongue-tie | Mild | Not related | N/A [intervention(s) stopped prior to the event starting] | Resolved |
| 30 | Frozen | 13 | Participant had pre-term and prolonged rapture of membranes from 29 weeks and was on erythromycin for that. According to electronic notes, participant had placenta abruption at 30+4 weeks [of gestation] and had emergency caesarean section. Baby is currently admitted at neonatal unit with parenteral nutrition. To this date, is diagnosed with prematurity. intrauterine growth restriction, jaundice, newborn feeding problem due to prematurity, respiratory disease syndrome, suspected sepsis and necrotising enterocolitis | Moderate | Not related | Resolving |
Appendix 5 Unit costs used in economic analysis (£)
| Resource | How it is measured | Source of measurement | Unit cost (£) | Source of valuation |
|---|---|---|---|---|
| IVF | See Cost of the primary intervention | Post-embryo transfer eCRF | ||
| Embryo transfer | Number of embryo transfers | 1095 per case | NHS Reference Costs 2018/1926 (MC11Z Implantation of Embryo) | |
| Embryo freezing | 1 hour spent by an embryologist | 47 per freezing | Unit Costs of Health and Social Care 201925 (Section IV, p. 143: Hospital-based scientific and professional staff) | |
| Monitoring visit | Number of monitoring visits | 56 per visit | Unit Costs of Health and Social Care 201925 (Section IV, p. 147: Hospital-based nurses, Band 6) | |
| Blood test | Number of blood tests | 1 per test | NHS Reference Costs 2018/1926 (DAPS04 Clinical Biochemistry) | |
| Transvaginal ultrasound scan | Number of transvaginal ultrasound scans | 160 per case | NHS Reference Costs 2018/1926 (MA36Z Transvaginal Ultrasound) | |
| Endometrial preparation | Treatment regimen used |
14 per natural cycle 52 per natural cycle with HCG 48 to 64 per artificial cycle with oestrogen and progesterone 114 per artificial cycle with oestrogen and progesterone, GnRH agonist 335 per artificial cycle with oestrogen and progesterone, antagonist 159 per luteal support for positive pregnancy |
British National Formulary (online) 27 | |
| Preparation of frozen embryo prior to transfer | 1 hour by an embryologist | 47 per case | Unit Costs of Health and Social Care 201925 (Section IV, p. 143: Hospital-based scientific and professional staff, band 6) | |
| OHSS | See Cost of OHSS | eCRFs post embryo transfer, early pregnancy | ||
| Outpatient attendance | Number of outpatient hospital visits | 126 per visit | NHS Reference Costs 2018/1926 (WF01 A 501 Non-Admitted Face-to-Face Attendance, Follow-up, Obstetrics) | |
| Day case | Number of inpatient day care visits | 792 per case | NHS Reference Costs 2018/1926 (MB09 Non-Malignant Gynaecological Disorders with Interventions) | |
| Inpatient stay | Number of inpatient nights |
1340 per short stay (one night) 861 per night of a long stay 454 per excess bed-day |
NHS Reference Costs 2018/1926 (MB09 Non-Malignant Gynaecological Disorders with Interventions) | |
| Pregnancy outcomes | See Cost of pregnancy outcomes | eCRFs at early pregnancy, 12 weeks’ follow-up and 28 weeks’ follow-up | ||
| Miscarriage | 619 per case | NHS Reference Costs 2018/1926 (MB08 Threatened or Spontaneous Miscarriage) | ||
| Ectopic pregnancy | 537 per case | NHS Reference Costs 2018/1926 (MA48Z Medical Treatment of Ectopic Pregnancy) | ||
| Pregnancy of unknown location | 170 per case | RCOG review,29 NHS Reference Costs 2018/1926 (MA36Z Transvaginal Ultrasound, DAPS04 Clinical Biochemistry) | ||
| Termination |
1016 per case from 9 to 14 weeks’ gestation 1450 per case from 14 to 20 weeks’ gestation |
NHS Reference Costs 2018/1926 (MA51Z Surgical, Abortion or Miscarriage Care from 14 to 20 weeks’ gestation; MA52 Surgical, Abortion or Miscarriage Care < 14 week’ gestation; MA54Z Medical, Abortion or Miscarriage Care from 14 to 20 weeks’ gestation; MA55 Medical, Abortion or Miscarriage Care from 9 to < 14 weeks’ gestation) | ||
| Biochemical pregnancy | 128 per case | Clinical advice, NHS Reference Costs 2018/1926 (NZ22Z Ante-Natal Specialised Non-Routine Ultrasound Scan, DAPS04 Clinical Biochemistry) | ||
| ANC | See Cost of ANC | eCRFs at early pregnancy, 28 weeks’ follow-up and post delivery | ||
| Community midwife visit | Number of community midwife visits | 58 per visit | NHS Reference Costs 2018/1926 (HVM N01 A Community Midwife, Ante Natal Visit) | |
| Outpatient attendance | Number of outpatient hospital visits | 126 per visit | NHS Reference Costs 2018/1926 (WF01 A 501 Non-Admitted Face-to-Face Attendance, Follow-up, Obstetrics) | |
| Day case | Number of inpatient day care visits |
NZ16: 331 per visit NZ18: 390 per visit NZ19: 641 per visit |
NHS Reference Costs 2018/1926 (NZ16 Ante-Natal Routine Observation, NZ18 Ante-Natal Complex Disorders, NZ19 Ante-Natal Major Disorders) | |
| Inpatient stay | Number of inpatient nights |
NZ16: 386 per short stay, 1220 per night of a long stay, 501 per excess bed-day NZ18: 602 per short stay, 853 per night of a long stay, 506 per excess bed-day NZ19: 417 per short stay, 801 per night of a long stay, 519 per excess bed-day |
NHS Reference Costs 2018/1926 (NZ16 Ante-Natal Routine Observation, NZ18 Ante-Natal Complex Disorders, NZ19 Ante-Natal Major Disorders) | |
| Antenatal ultrasound scan | Number of ultrasound scans |
NZ21Z: 118 per scan NZ22Z: 124 per scan |
NHS Reference Costs 2018/1926 (NZ21Z Ante-Natal Standard Routine Ultrasound Scan, NZ22Z Ante-Natal Specialised Non-Routine Ultrasound Scan) | |
| Delivery | See Cost of delivery | Post-delivery eCRF | ||
| Normal delivery | Number of inpatient nights |
NZ30 (Spontaneous): 1640 per short stay, 1224 per night of a long stay, 577 per excess bed-day NZ32-NZ34 (Induced): 1930 per short stay, 1202 per night of a long stay, 557 per excess bed-day (weighted average) |
NHS Reference Costs 2018/1926 (NZ30 Normal Delivery, NZ31 Normal Delivery, with Epidural or Induction, NZ32 Normal Delivery, with Epiducal and Induction, or with Post-Partum Surgical Intervention, NZ33 Normal Delivery, with Epidural or Induction, and with Post-Partum Surgical Intervention, NZ34 Normal Delivery, with Epidural, Induction and Post-Partum Surgical Intervention) | |
| Instrumental delivery | Number of inpatient nights |
NZ40 (Spontaneous): 1727 per short stay, 1077 per night of a long stay, 511 per excess day NZ42-NZ44 (Induced): 2255 per short stay, 1229 per night of a long stay, 577 per excess day (weighted average) |
NHS Reference Costs 2018/1926 (NZ40 Assisted Delivery, NZ41 Assisted Delivery, with Epidural or Induction, NZ42 Assisted Delivery, with Epidural and Induction, or with Post-Partum Surgical Intervention, NZ43 Assisted Delivery, with Epidural or Induction, and with Post-Partum Surgical Intervention, NZ44 Assisted Delivery, with Epidural, Induction and Post-Partum Surgical Intervention) | |
| Caesarean | Number of inpatient nights |
NZ50: 2913 per short stay, 1681 per night of a long stay, 1519 per excess bed-day, NZ51: 2862 per short stay, 1337 per night of a long stay, 563 per excess day |
NHS Reference Costs 2018/1926 (NZ50 Planned Caesarean Section, NZ51 Emergency Caesarean Section) | |
| Paid work | Amount of time spent per visit | Economic questionnaire | ASHE32 | |
| Age (years): female | ||||
| 22–29 | 11.43 per hour | |||
| 30–39 | 13.65 per hour | |||
| 40–49 | 13.54 per hour | |||
| Age (years): male | ||||
| 22–29 | 12.27 per hour | |||
| 30–39 | 15.67 per hour | |||
| 40–49 | 17.54 per hour | |||
| Unpaid work | Amount of time spent per visit | Economic questionnaire | ASHE32 | |
| Voluntary work | 14.43 per hour | |||
| At home looking after family or dependants | 14.92 per hour | Weighted average of childcare and adult care | ||
| In education | 10.37 per hour | Weighted average of unpaid work done by a full-time student72 | ||
| Other | 10.17 per hour | Weighted average across seveb unpaid work activities from ASHE32 | ||
| Leisure | 5.03 per hour | Transport Analysis Guidance (TAG) Data Book 34 | ||
Appendix 6 Direct medical costs by treatment allocation
| Variable | Number of observations | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|---|
| IVF costs (£), mean (SD) | 616 | 1538.45 (473.67) | 1215.51 (221.17) |
| Freezing of embryo | 616 | 41.16 (15.96) | 38.14 (18.75) |
| Endometrial preparation costs | 616 | 131.88 (104.18) | 78.05 (50.45) |
| Embryo transfer costs | 616 | 1063.07 (185.05) | 1073.91 (151.37) |
| Monitoring visit costs prior to frozen-embryo transfer | 223 | 122.82 (116.97) | 104.24 (77.95) |
| Blood test costs prior to embryo transfer | 223 | 0.39 (0.89) | 0.68 (1.18) |
| Transvaginal ultrasound costs prior to embryo transfer | 223 | 288.24 (242.30) | 221.50 (156.14) |
| Preparation of frozen embryo | 223 | 47.33 (0) | 47.33 (0) |
| OHSS management costs (£), mean (SD) | 36 | 467.02 (763.05) | 2484.84 (2947.32) |
| Pregnancy loss costs (£), mean (SD) | 100 | 525.61 (278.99) | 480.57 (276.47) |
| Miscarriage costs | 59 | 618.68 (0) | 618.68 (0) |
| Ectopic pregnancy costs | 9 | 536.95 (0) | 536.95 (0) |
| Pregnancy of unknown location costs | 3 | 169.93 (0) | 0 (0) |
| Termination costs | 4 | 1450.37 (0) | 1232.99 (307.42) |
| Biochemical pregnancy costs | 25 | 127.50 (0) | 127.50 (0) |
| ANC costs | |||
| 6 to < 12 weeks’ gestation (n) | 293 | 139 | 154 |
| ANC costs (£), mean (SD) | 293 | 169.10 (47.25) | 164.46 (54.20) |
| 12 to < 28 weeks’ gestation (n) | 201 | 93 | 108 |
| ANC costs (£), mean (SD) | 199 | 999.46 (1927.34) | 963.46 (1029.86) |
| No maternal complications, mean (SD) | 172 | 801.05 (744.31) | 786.17 (665.34) |
| Hypertensive disorder, mean (SD) | 5 | 1379.14 (1373.08) | 1155.42 (513.30) |
| GDM, mean (SD) | 6 | 976.98 (614.77) | 808.86 (313.08) |
| Antepartum haemorrhage, mean (SD) | 14 | 3598.54 (7065.07) | 2985.43 (2233.78) |
| > 1 complication, mean (SD) | 1 | 1761.13 (0) | 0 (0) |
| Missing, n (%) | 2 | 0 (0) | 2 (2) |
| 28 weeks’ gestation to delivery, n | 189 | 87 | 102 |
| ANC costs (£), mean (SD) | 181 | 1343.35 (1324.50) | 1254.90 (1752.03) |
| No maternal complications, mean (SD) | 148 | 1110.82 (1017.13) | 955.87 (676.20) |
| Hypertensive disorder, mean (SD) | 11 | 2361.85 (2201.93) | 1293.45 (326.47) |
| GDM, mean (SD) | 6 | 1300.58 (596.87) | 2536.77 (1478.13) |
| Antepartum haemorrhage, mean (SD) | 15 | 2811.74 (2126.38) | 3390.75 (5002.93) |
| > 1 complication, mean (SD) | 1 | 0 (0) | 1954.46 (0) |
| Missing, n (%) | 8 | 3 (3) | 5 (5) |
| Delivery inpatient costs (n) | 193 | 87 | 106 |
| Delivery costs (£), mean (SD) | 183 | 3873.02 (1908.82) | 3850.80 (2148.27) |
| Normal vaginal delivery costs, mean (SD) | 66 | 3180.55 (1336.91) | 2648.41 (1158.45) |
| Instrumental vaginal delivery costs, mean (SD) | 48 | 3105.21 (1610.56) | 3575.44 (1489.73) |
| C-section costs, mean (SD) | 69 | 4894.95 (2036.53) | 5376.53 (2505.25) |
| Missing, n (%) | 10 | 5 (6) | 5 (5) |
| Total NHS cost, mean (SD) | 605 | 3431.15 (3507.87) | 3573.99 (3807.37) |
Appendix 7 Resource use and costs related to travelling and time, by treatment allocation
| Variable | Number of observations | Freeze-all arm (N = 307) | Fresh-embryo transfer arm (N = 309) |
|---|---|---|---|
| Participant | |||
| Visited clinic (n) | 176 | 135 | 41 |
| Transport mode, n (%) | |||
| Train | 45 | 37 (27) | 8 (20) |
| Bus/tram | 5 | 5 (4) | 0 (0) |
| Car | 105 | 77 (57) | 28 (68) |
| Taxi | 5 | 4 (3) | 1 (2) |
| Hospital transport/ambulance | 0 | 0 (0) | 0 (0) |
| Walk/cycle | 5 | 5 (4) | 0 (0) |
| Other | 10 | 7 (5) | 3 (7) |
| Missing | 1 | 0 (0) | 1 (2) |
| Transport costs per visit (£), mean (SD) | 168 | 17.63 (23.33) | 29.16 (30.52) |
| Train costs | 41 | 11.25 (8.11) | 42.38 (41.72) |
| Bus/tram costs | 5 | 8.76 (5.19) | 0 (0) |
| Car costs | 103 | 20.50 (27.08) | 25.06 (28.15) |
| Taxi costs | 5 | 19.78 (10.71) | 30 (0) |
| Hospital transport/ambulance costs (£), mean (SD) | 0 | 0 (0) | 0 (0) |
| Walk/cycle costs, mean (SD) | 5 | 0 (0) | 0 (0) |
| Other transport costs, mean (SD) | 9 | 36.56 (34.27) | 29.13 (17.96) |
| Missing, n (%) | 8 | 5 (4) | 3 (7) |
| Other costs per visit (£), mean (SD) | 168 | 0.38 (4.39) | 2.84 (12.26) |
| Missing, n (%) | 8 | 5 (4) | 3 (7) |
| Travel costs per visit (£), mean (SD) | 168 | 18.02 (25.04) | 32.00 (38.92) |
| Missing, n (%) | 8 | 5 (4) | 3 (7) |
| Time spent to visit the clinic per visit (hours), mean (SD) | 164 | 2.80 (2.98) | 4.20 (3.92) |
| Time off paid work, mean (SD) | 145 | 2.84 (3.11) | 4.45 (4.29) |
| Time off unpaid work, mean (SD) | 9 | 2.50 (1.05) | 3.88 (1.18) |
| Time off leisure/social activities, mean (SD) | 10 | 2.36 (0.94) | 2.17 (1.61) |
| Missing, n (%) | 12 | 7 (5) | 5 (12) |
| Time cost per visit (£), mean (SD) | 164 | 33.07 (22.68) | 47.25 (27.00) |
| Cost of time lost from paid work, mean (SD) | 145 | 34.36 (23.11) | 50.38 (26.28) |
| Cost of time lost from unpaid work, mean (SD) | 9 | 32.79 (9.99) | 51.58 (24.09) |
| Cost of time lost from leisure activities, mean (SD) | 10 | 11.86 (4.75) | 10.90 (8.08) |
| Missing, n (%) | 12 | 7 (5) | 5 (12) |
| Accompanying partner | |||
| Clinic visit(s) between the time from treatment allocation and the time to embryo transfer, n (%) | |||
| Yes | 132 | 95 (31) | 37 (12) |
| No | 298 | 115 (37) | 183 (59) |
| Missing | 186 | 97 (32) | 89 (29) |
| Number of visits to clinic, mean (SD) | 419 | 1.03 (1.68) | 0.45 (1.35) |
| Missing, n (%) | 197 | 104 (34) | 93 (30) |
| Transport mode, n (%) | |||
| Train | 29 | 23 (24) | 6 (16) |
| Bus/tram | 4 | 4 (4) | 0 (0) |
| Car | 85 | 59 (62) | 26 (70) |
| Taxi | 2 | 1 (1) | 1 (3) |
| Hospital transport/ambulance | 0 | 0 (0) | 0 (0) |
| Walk/cycle | 4 | 4 (4) | 0 (0) |
| Other | 7 | 4 (4) | 3 (8) |
| Missing | 1 | 0 (0) | 1 (3) |
| Transport cost per visit (£), mean (SD) | 127 | 18.73 (24.63) | 29.23 (39.99) |
| Train, mean (SD) | 27 | 14.85 (17.65) | 22.75 (37.92) |
| Bus/tram, mean (SD) | 4 | 10.20 (4.69) | 0 (0) |
| Car, mean (SD) | 83 | 21.78 (28.25) | 27.35 (32.12) |
| Taxi, mean (SD) | 2 | 18.00 (0) | 30.00 (0) |
| Hospital transport/ambulance, mean (SD) | 0 | 0 (0) | 0 (0) |
| Walk/cycle, mean (SD) | 4 | 0 (0) | 0 (0) |
| Other transport, mean (SD) | 7 | 21.91 (17.44) | 21.60 (3.42) |
| Missing, n (%) | 5 | 3 (3) | 2 (5) |
| Other costs per visit (£), mean (SD) | 127 | 0 (0) | 3.09 (12.75) |
| Missing, n (%) | 5 | 3 (3) | 2 (5) |
| Travel cost per visit (£), mean (SD) | 127 | 18.73 (24.63) | 29.23 (39.99) |
| Missing, n (%) | 5 | 3 (3) | 2 (5) |
| Time spent to visit the clinic (hours), mean (SD) | 123 | 2.61 (1.83) | 4.00 (4.08) |
| Time off paid work, mean (SD) | 116 | 2.64 (1.86) | 4.18 (4.22) |
| Time off unpaid work, mean (SD) | 5 | 1.92 (0.63) | 2.75 (1.77) |
| Time off leisure/social activities, mean (SD) | 2 | 2.50 (0) | 1.00 (0) |
| Missing, n (%) | 9 | 5 (5) | 4 (11) |
| Indirect cost per visit (£), mean (SD) | 123 | 39.63 (25.63) | 51.14 (33.85) |
| Cost of time lost from paid work, mean (SD) | 116 | 40.32 (25.93) | 55.21 (32.59) |
| Cost of time lost from unpaid work, mean (SD) | 5 | 28.80 (9.44) | 13.16 (18.60) |
| Cost of time lost from leisure activities, mean (SD) | 2 | 12.58 (0) | 5.03 (0) |
| Missing, n (%) | 9 | 5 (5) | 4 (11) |
| Total travel costs,a mean (SD) | 415 | 20.20 (60.19) | 19.10 (101.72) |
| Total time costs,b mean (SD) | 411 | 37.66 (77.67) | 23.98 (92.86) |
| Total patient costs,c mean (SD) | 409 | 57.89 (120.38) | 42.40 (190.04) |
Appendix 8 Trial-based cost-effectiveness scatterplots and cost-effectiveness acceptability curves
FIGURE 19.
Trial-based cost-effectiveness scatterplots and cost-effectiveness acceptability curves generated from sensitivity and subgroup analyses. (a) Sensitivity analysis: assuming that the transvaginal scan cost was inclusive of a monitoring visit; (b) sensitivity analysis: using standard ultrasound scan cost (£53) to cost transvaginal scans; (c) sensitivity analysis: multiple imputation (treatment costs, health baby); (d) sensitivity analysis: multiple imputation (treatment costs, live birth); (e) sensitivity analysis: multiple imputation (NHS costs, healthy baby); (f) sensitivity analysis: multiple imputation (NHS costs, live birth); (g) subgroup analysis: maternal age < 35 years; and (h) subgroup analysis: maternal age ≥ 35 years. Reproduced from Maheshwari et al. ,23 Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-Freeze), Human Reproduction, 2022, deab279, by permission of European Society of Human Reproduction and Embryology.
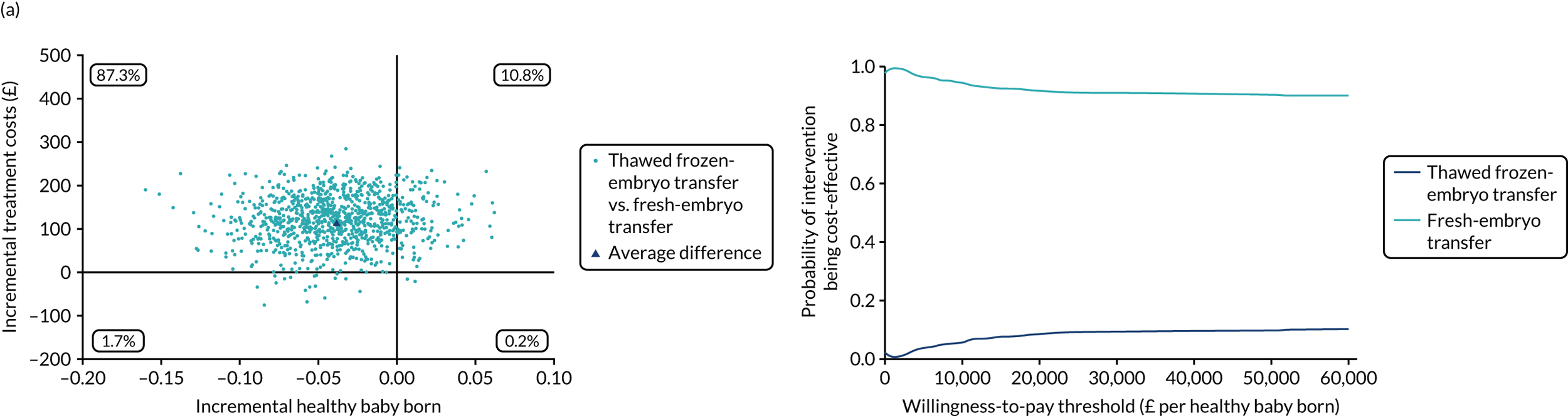
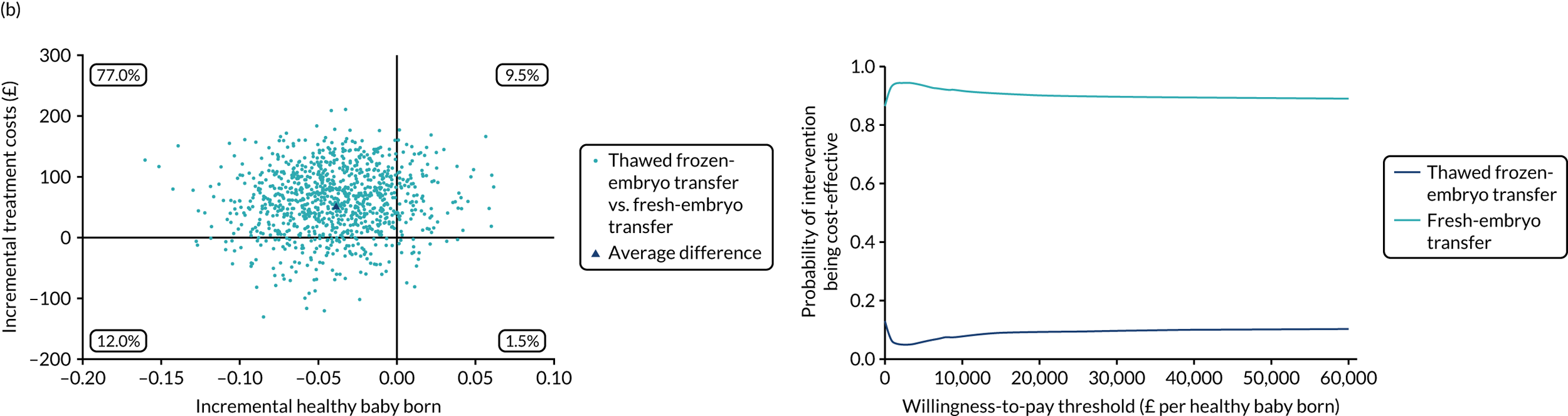
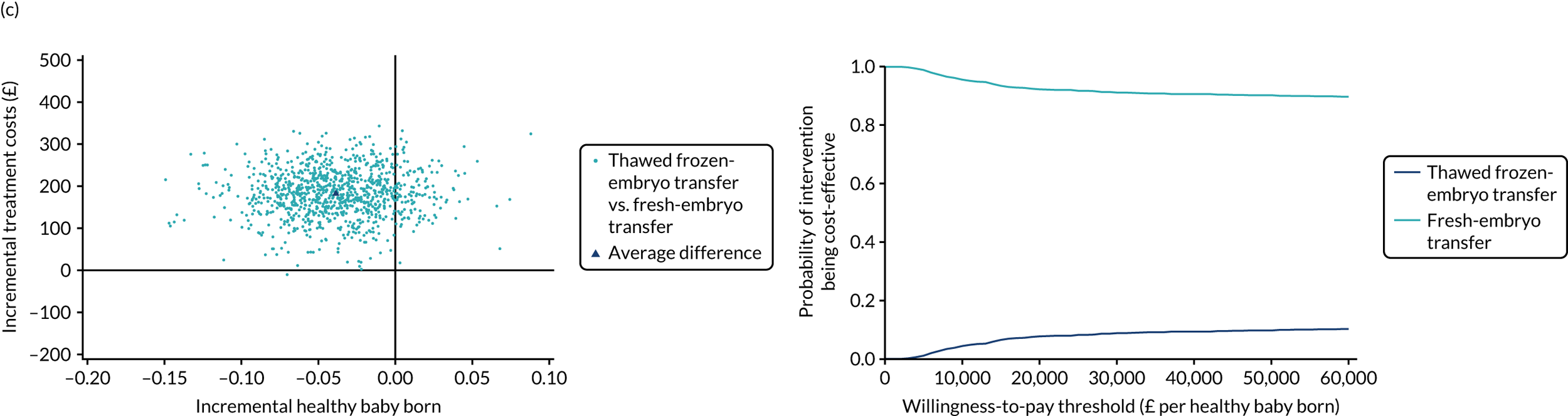
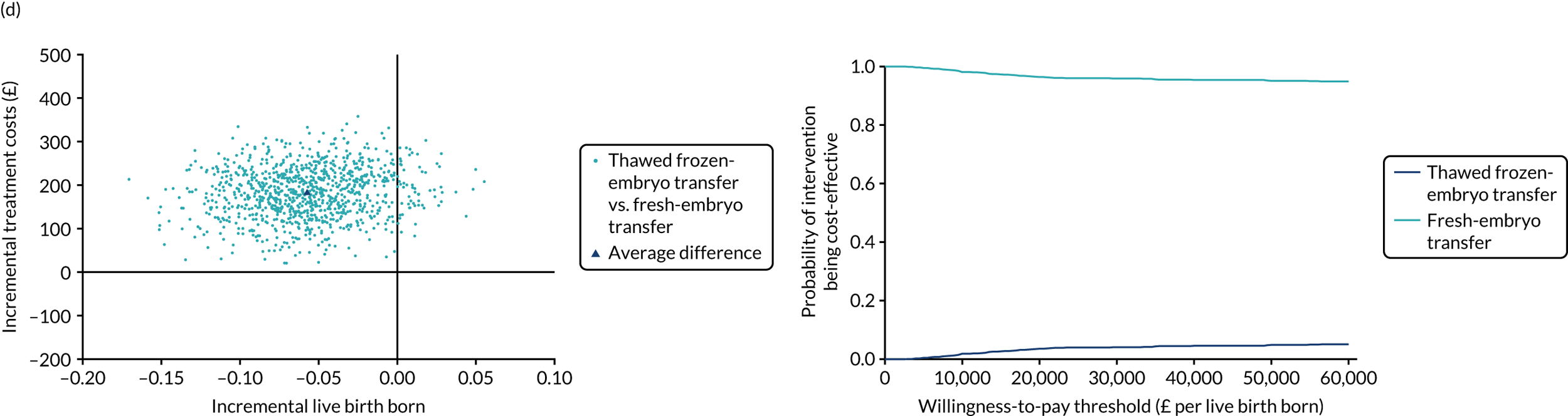
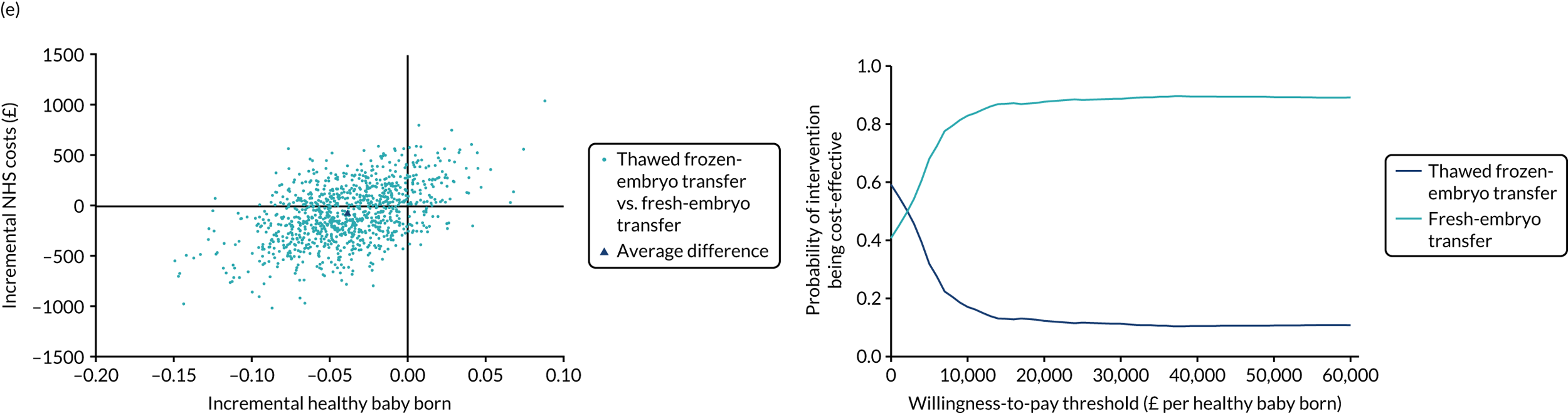
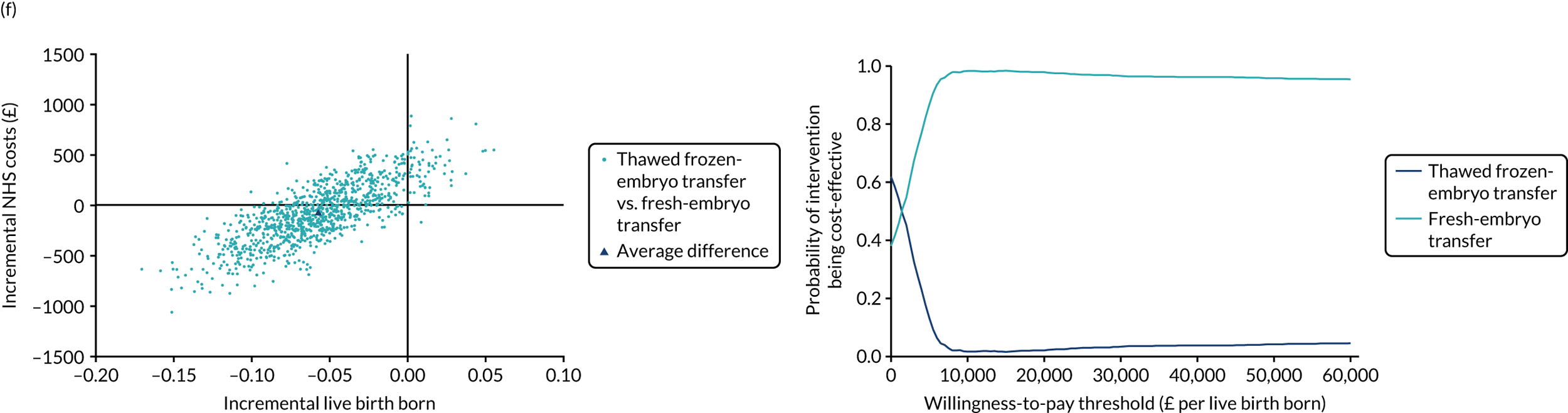
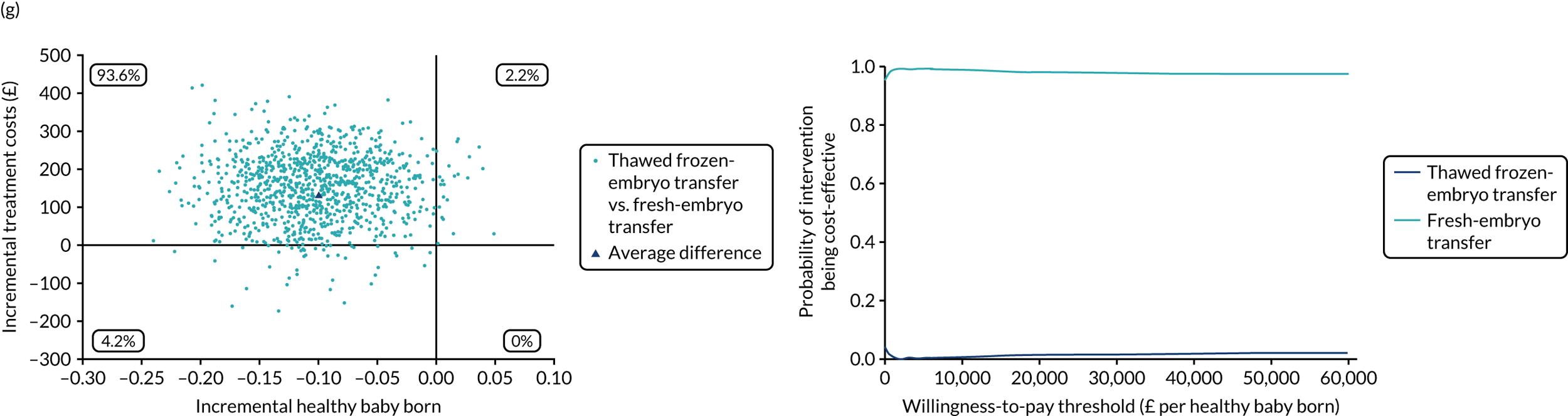
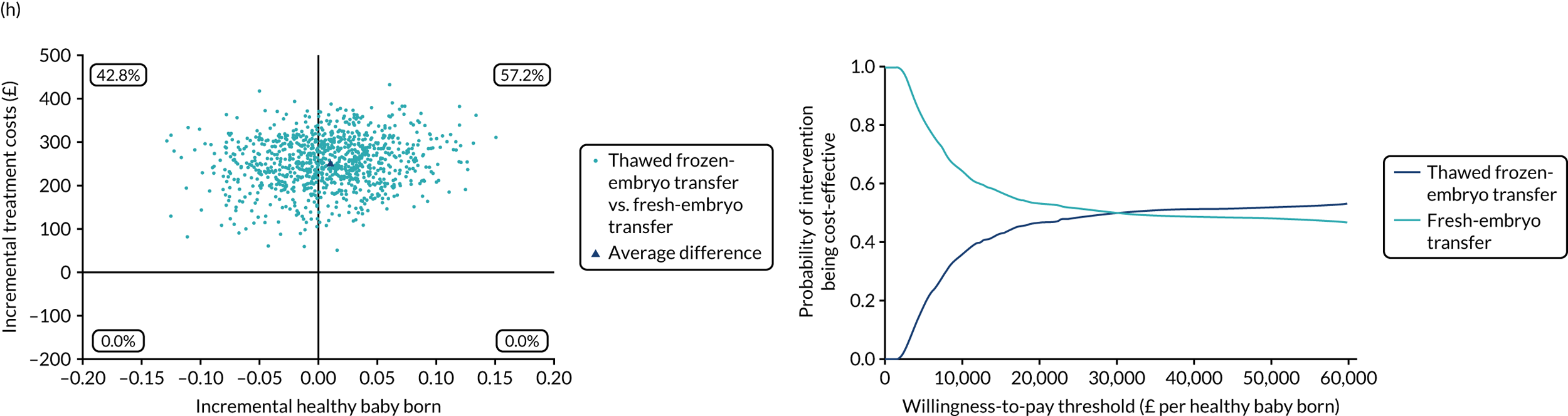
Appendix 9 Markov model parameter inputs (derived from the analysis of the E-Freeze trial cost and outcome data, unless otherwise noted)
| Parameter name | Description | Fresh-embryo transfer arm | Freeze-all arm | ||||
|---|---|---|---|---|---|---|---|
| Expected value | Distribution type | Distribution parameters | Expected value | Distribution type | Distribution parameters | ||
| Probabilities (index transfer) | |||||||
| prop_eFz | Proportion of embryo transfers that are frozen | 0.069 | Beta |
Alpha: 21 Beta: 282 |
0.678 | Beta |
Alpha: 202 Beta: 96 |
| prop_Fresh | Proportion of embryo transfers that are fresh | 0.931 | 1 – prop_eFz | 0.322 | 1-prop_eFz | ||
| p_transfer | Probability of planned transfer going ahead | 0.981 | Beta |
Alpha: 303 Beta: 6 |
0.971 | Beta |
Alpha: 298 Beta: 9 |
| p_OHSS | Probability of OHSS | 0.081 | Beta |
Alpha: 25 Beta: 284 |
= p_OHSS × RR_OHSS (see relative risk definition below) | ||
| p_test_positive | Probability of a positive pregnancy test following embryo transfer | 0.508 | Beta |
Alpha: 154 Beta: 149 |
= p_test_postive × RR_pos_test (see relative risk definition below) | ||
| p_early_loss | Probability of pregnancy loss by 8 weeks following a positive pregnancy test | 0.201 | Beta |
Alpha: 31 Beta: 123 |
= p_early_loss × RR_early_loss (see relative risk definition below) | ||
| p_miscarriage | Probability of pregnancy loss by 12 weeks in those ongoing at 8 weeks | 0.122 | Beta |
Alpha: 15 Beta: 108 |
= p_miscarriage × RR_miscarriage (see relative risk definition below) | ||
| p_late_miscarriage | Probability of pregnancy loss by 24 weeks in those ongoing at 12 weeks | 0.019 | Beta |
Alpha: 2 Beta: 105 |
= p_late_miscarriage × RR_late_miscarriage (see relative risk definition below) | ||
| p_delivery_25_28 | Probability of delivery by 28 weeks for ongoing pregnancies at 24 weeks | 0.038 | Beta |
Alpha: 4 Beta: 102 |
= p_delivery_25_28 × RRePT (see relative risk definition below) | ||
| p_delivery_29_32 | Probability of delivery by 32 weeks for ongoing pregnancies at 28 weeks | 0.020 | Beta |
Alpha: 2 Beta: 99 |
= p_delivery_29_32 × RR_vPreterm (see relative risk definition below) | ||
| p_delivery_33_36 | Probability of delivery by 36 weeks for ongoing pregnancies at 32 weeks | 0.061 | Beta |
Alpha: 6 Beta: 93 |
= p_delivery_33_36 × RR_Preterm (see relative risk definition below) | ||
| RR (freeze all vs. fresh-embryo transfer) | |||||||
| RR_OHSS | Relative risk of OHSS by ITT (freeze all vs. fresh-embryo transfer) | Reference group | 0.44 | Log-normal |
umeanoflogs: –0.821 sigmastddevoflogs: 0.551 |
||
| RR_pos_test | Relative risk for a positive test following embryo transfer (freeze all vs. fresh-embryo transfer) | Reference group | 0.913 | Log-normal |
umeanoflogs: –0.094 sigmastddevoflogs: 0.086 |
||
| RR_early_loss | Relative risk of any miscarriage conditional on a positive pregnancy test (freeze all vs. fresh-embryo transfer) | Reference group | 1.18 | Log-normal |
umeanoflogs: 0.166 sigmastddevoflogs: 0.226 |
||
| RR_ePT | Relative risk of delivery prior to 33 weeks for those ongoing at 24 weeks (freeze all vs. fresh-embryo transfer) | Reference group | 0.767 | Log-normal |
umeanoflogs: –0.505 sigmastddevoflogs: 0.692 |
||
| RR_late_miscarriage | Relative risk of any miscarriage conditional on a positive pregnancy test (freeze all vs. fresh-embryo transfer) | Reference group | 1.18 | Log-normal |
umeanoflogs: 0.166 sigmastddevoflogs: 0.226 |
||
| RR_miscarriage | Relative risk of any miscarriage conditional on a positive pregnancy test (freeze all vs. fresh-embryo transfer) | Reference group | 1.18 | Log-normal |
umeanoflogs: 0.166 sigmastddevoflogs: 0.226 |
||
| RR_Preterm | Relative risk of delivery prior to 37 weeks for those ongoing at 32 weeks (freeze all vs. fresh-embryo transfer) | Reference group | 1.377 | Log-normal |
umeanoflogs: 0.164 sigmastddevoflogs: 0.558 |
||
| RR_vPreterm | Relative risk of delivery prior to 33 weeks for those ongoing at 24 weeks (freeze all vs. fresh-embryo transfer) | Reference group | 0.767 | Log-normal |
umeanoflogs: –0.505 sigmastddevoflogs: 0.692 |
||
| Probabilities for subsequent frozen ETs | |||||||
| p_embryos_remaining | Proportion with frozen embryos remaining after the index ET cycle | 0.777 | Beta |
Alpha: 153 Beta: 44 |
0.787 | Beta |
Alpha: 166 Beta: 45 |
| p_embryos_remaining_ET1 | Proportion with frozen embryos remaining after first subsequent frozen ET | 0.745 | Beta |
Alpha: 114 Beta: 39 |
0.789 | Beta |
Alpha: 131 Beta: 35 |
| p_embryos_remaining_ET2 | Proportion with frozen embryos remaining after second subsequent frozen ET | 0.719 | Beta |
Alpha: 82 Beta: 32 |
0.679 | Beta |
Alpha: 89 Beta: 42 |
| p_embryos_remaining_ET3 | Proportion with frozen embryos remaining after third subsequent frozen ET | 0.622 | Beta |
Alpha: 51 Beta: 31 |
0.697 | Beta |
Alpha: 62 Beta: 27 |
| p_embryos_remaining_ET4 | Proportion with frozen embryos remaining after fourth subsequent frozen ET | 0.686 | Beta |
Alpha: 35 Beta: 16 |
0.629 | Beta |
Alpha: 39 Beta: 23 |
| p_embryos_remaining_ET5 | Proportion with frozen embryos remaining after fifth subsequent frozen ET | 0.629 | Beta |
Alpha: 22 Beta: 13 |
0.538 | Beta |
Alpha: 21 Beta: 18 |
| p_subsequent_fz1a | Probability of proceeding to first subsequent frozen cycle conditional on frozen embryos being available | 0.941 | Beta |
Alpha: 1720 Beta: 108 |
Same as the fresh-embryo transfer arm | ||
| p_subsequent_fz2a | Probability of proceeding to second subsequent frozen cycle conditional on frozen embryos being available | 0.902 | Beta |
Alpha: 1591 Beta: 173 |
Same as the fresh-embryo transfer arm | ||
| p_subsequent_fz3a | Probability of proceeding to third subsequent frozen cycle conditional on frozen embryos being available | 0.902 | Beta |
Alpha: 1591 Beta: 173 |
Same as the fresh-embryo transfer arm | ||
| p_subsequent_fz4a | Probability of proceeding to fourth subsequent frozen cycle conditional on frozen embryos being available | 0.902 | Beta |
Alpha: 1591 Beta: 173 |
Same as the fresh-embryo transfer arm | ||
| p_subsequent_fz5a | Probability of proceeding to fifth subsequent frozen cycle conditional on frozen embryos being available | 0.902 | Beta |
Alpha: 1591 Beta: 173 |
Same as the fresh-embryo transfer arm | ||
| p_subsequent_fz6a | Probability of proceeding to fifth subsequent frozen cycle conditional on frozen embryos being available | 0.902 | Beta |
Alpha: 1591 Beta: 173 |
Same as the fresh-embryo transfer arm | ||
| RR (subsequent frozen-embryo transfer versus index) | |||||||
| RR_preg_subs_vs_indexa | Relative risk of pregnancy in subsequent frozen cycles versus index frozen cycle | – | 0.785 | Log-normal |
umeanoflogs: –0.245 sigmastddevoflogs: 0.076 |
||
| Cost parameters | |||||||
| cOHSS | Cost of managing OHSS in those who get it | 2484.84 | Gamma | Alpha: [(2484.84)2]/[(589.46)2], lambda: (2484.84)/[(589.46)2] | 467.02 | Gamma |
Alpha: [(467.02)2]/[(230.68)2] Lambda: (467.02)/[(230.68)2] |
| cFreezing | Cost of freezing embryos by ITT | 37.22 | Gamma |
Alpha: [(37.22)2]/[(1.11)2] Lambda: (37.22)/[(1.11)2] |
39.78 | Gamma |
Alpha: [(39.78)2]/[(0.99)2] Lambda: (39.78)/[(0.99)2] |
| cEndo_prep_luteul_support | Cost of endometrial preparation and luteal support by ITT | 78.05 | Gamma |
Alpha: [(78.05)2]/[(2.87)2] Lambda: (78.05)/[(2.87)2] |
131.88 | Gamma |
Alpha: [(131.88)2]/[(5.95)2] Lambda: (131.88)/[(5.95)2] |
| cMonitoring_fz | Expected cost of monitoring prior to ET for those undergoing frozen ET | 104.24 | Gamma |
Alpha: [(104.24)2]/[(16.62)2] Lambda: (104.24)/[(16.62)2] |
122.82 | Gamma |
Alpha: [(122.82)2]/[(8.23)2] Lambda: (122.82)/[(8.23)2] |
| c_ultrasound_fz | Expected cost of scans prior to ET for those undergoing frozen ET | 221.50 | Gamma |
Alpha: [(221.50)2]/[(33.29)2] Lambda: (221.50)/[(33.29)2] |
288.24 | Gamma |
Alpha: [(288.24)2]/[(17.48)2] Lambda: (288.24)/[(17.48)2] |
| cBlood_tests_fz | Expected cost of blood tests prior to ET for those undergoing frozen ET | 0.68 | Deterministic | 0.39 | Deterministic | ||
| c_prep_thawed_embryos | Cost preparing frozen embryos for transfer | 47.33 | Deterministic | (Same as the fresh-embryo transfer arm) | Deterministic | ||
| cET | Cost of the ET procedure itself | 1095.18 | Deterministic | (Same as the fresh-embryo transfer arm) | Deterministic | ||
| cEarly_preg | Cost of early pregnancy, including confirmatory scan at 3–8 weeks, up to 12 weeks | 164.46 | Gamma |
Alpha: [(164.46)2]/[(4.37)2] Lambda: (164.46)/[(4.37)2] |
169.10 | Gamma |
Alpha: [(169.1)2]/[(4.01)2] Lambda: (169.1)/[(4.01)2] |
| cEarly_loss | Cost per pregnancy loss occurring by 8 weeks | 367.83 | Gamma |
Alpha: [(367.83)2]/[(42.78)2] Lambda: (367.83)/[(42.78)2] |
432.87 | Gamma |
Alpha: [(432.87)2]/[(39.20)2] Lambda: (432.87)/[(39.20)2] |
| c_Miscarriage | Cost per pregnancy loss occurring between 8 and 12 weeks | 639.69 | Gamma |
Alpha: [(639.69)2]/[(27.40)2] Lambda: (639.69)/[(27.40)2] |
694.29 | Gamma |
Alpha: [(694.29)2]/[(75.61)2] Lambda: (694.29)/[(75.61)2] |
| c_Late_Miscarriage | Cost per pregnancy loss occurring between 12 and 24 weeks | 1034.53 | Gamma |
Alpha: [(1034.53)2]/[(415.84)2] Lambda: (1034.53)/[(415.84)2] |
757.30 | Gamma |
Alpha: [(757.3)2]/[(138.62)2] Lambda: (757.3)/[(138.62)2] |
| cANC_12_28w | Cost of ANC between 12 and 28 weeks (in those ongoing at 12 weeks) | 963.46 | Gamma |
Alpha: [(963.46)2]/[(100.03)2] Lambda: (963.46)/[(100.03)2] |
999.46 | Gamma |
Alpha: [(999.46)2]/[(199.86)2] Lambda: (999.46)/[(199.86)2] |
| cANC_post_28w | Cost of ANC after 28 days up to but excluding delivery (in those ongoing at 28 weeks) | 1254.90 | Gamma |
Alpha: [(1254.90)2]/[(177.89)2] Lambda: (1254.90)/[(177.89)2] |
1343.35 | Gamma |
Alpha: [(1343.35)2]/[(142.00)2] Lambda: (1343.35)/[(142.00)2] |
| cDelivery | Cost of delivery in those who deliver post 24 weeks | 3850.80 | Gamma |
Alpha: [(3850.80)2]/[(208.66)2] Lambda: (3850.80)/[(208.66)2] |
3873.02 | Gamma |
Alpha: [(3873.02)2]/[(204.65)2] Lambda: (3873.02)/[(204.65)2] |
| c_Endo_prep_subs_fz | Cost of endometrial preparation and luteal support in subsequent frozen cycles | – | 131.88 | Gamma |
Alpha: [(131.88)2]/[(5.95)2] Lambda: (131.88)/[(5.95)2] |
||
| Birth outcomes (term infants) | |||||||
| prop_Term_lbw | Proportion of term births that are below 10th percentile for gestation | 0.097 | Dirichlet | Alpha: 9 | 0.115 | Dirichlet | Alpha: 9 |
| prop_Term_nbw | Proportion of term deliveries of normal birthweight (healthy term babies) | 0.806 | Dirichlet | Alpha: 75 | 0.795 | Dirichlet | Alpha: 62 |
| prop_Term_hbw | Proportion of term deliveries of high birthweight (macrosomia) | 0.097 | Dirichlet | Alpha: 9 | 0.090 | Dirichlet | Alpha: 7 |
| prop_term_lga_below1500g | Proportion of term low birthweight for gestational age infants < 1500 g | 0.111 | Dirichlet | Alpha: 1 | 0.000 | Dirichlet | Alpha: 0 |
| prop_term_lga_below2500g | Proportion of term low weight for gestational age infants 1500 g to < 2500 g | 0.111 | Dirichlet | Alpha: 1 | 0.143 | Dirichlet | Alpha: 1 |
| prop_term_lga_2500g_plus | proportion of term low birthweight for gestational age infants ≥ 2500 g | 0.778 | Dirichlet | Alpha: 7 | 0.795 | Dirichlet | Alpha: 6 |
Appendix 10 Cost-effectiveness acceptability curves for model-based sensitivity analysis
FIGURE 20.
Cost-effectiveness acceptability curves for model-based sensitivity analysis. (a) Base case; (b) no discontinuation among those eligible for subsequent embryo transfer; and (c) using the lower ultrasound scan cost (£53) to cost transvaginal scans. Reproduced from Maheshwari et al. ,23 Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-Freeze), Human Reproduction, 2022, deab279, by permission of European Society of Human Reproduction and Embryology.
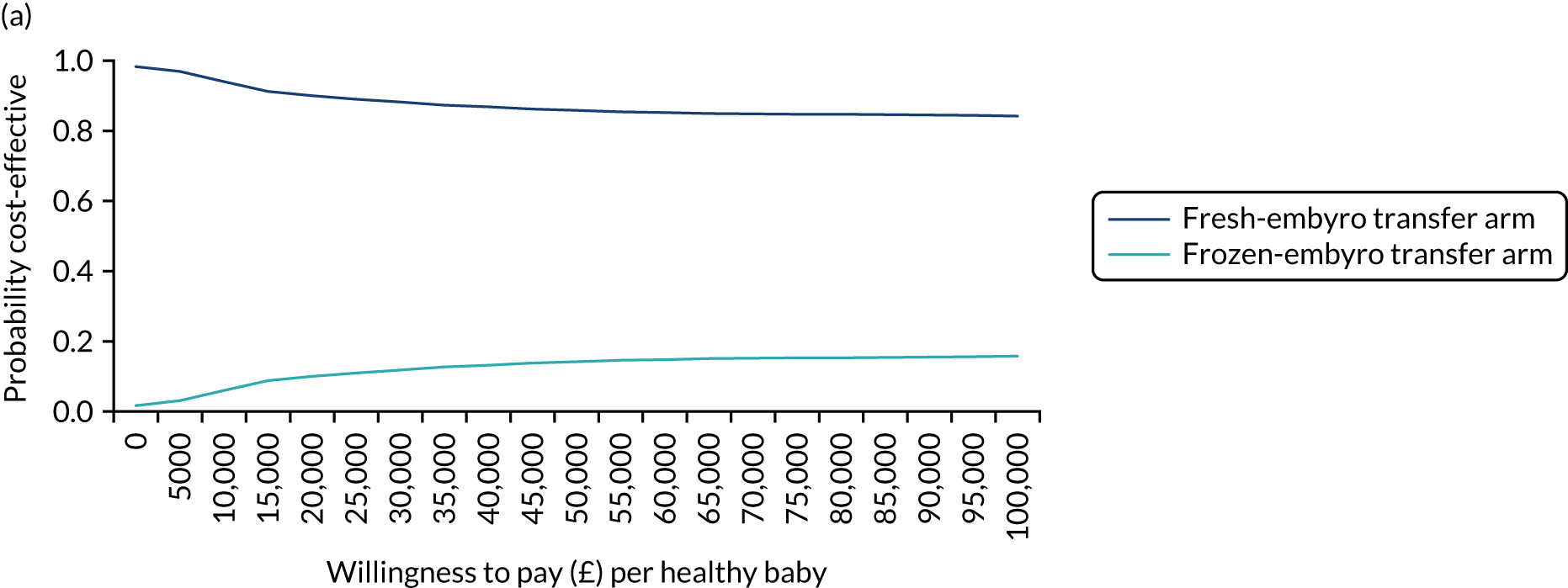
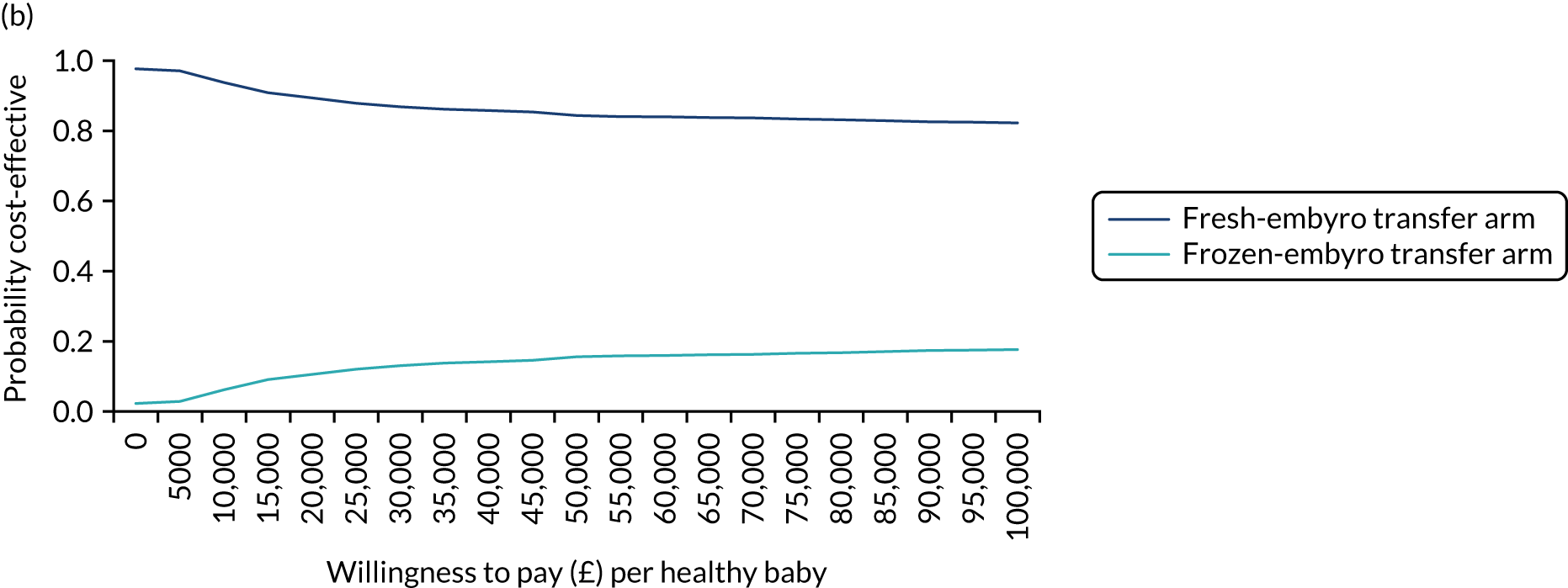
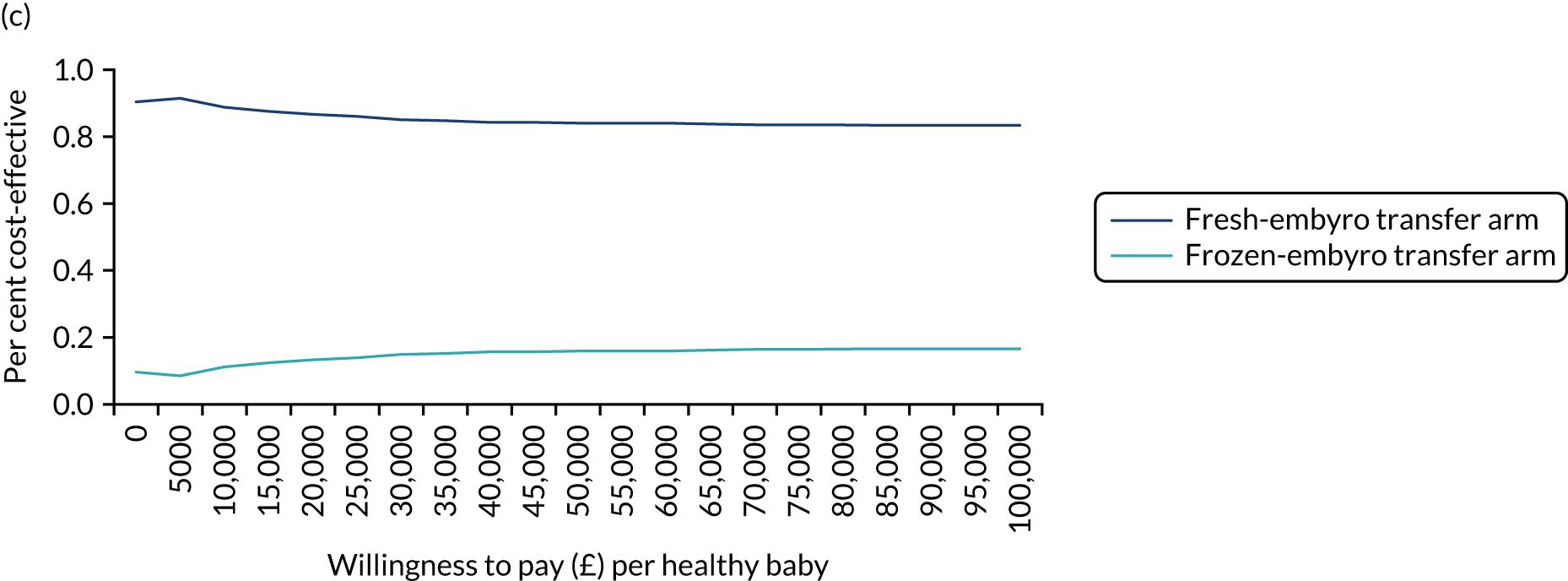
List of abbreviations
- 24/7
- 24 hours per day, 7 days per week
- ACE
- The Association of Clinical Embryologists
- AE
- adverse event
- ANC
- antenatal care
- ASHE
- Annual Survey of Hours and Earnings
- BMI
- body mass index
- CACE
- complier-average causal effect
- CI
- confidence interval
- CLBR
- cumulative live birth rate
- CRF
- case report form
- CTU
- Clinical Trials Unit
- DMC
- Data Monitoring Committee
- eCRF
- electronic case report form
- E-Freeze
- elective freeze
- GDM
- gestational diabetes mellitus
- GnRH
- gonadotropin-releasing hormone
- HCG
- human chorionic gonadotropin
- HFEA
- Human Fertilisation and Embryology Authority
- HRG
- Heathcare Resource Group
- HRT
- hormone replacement therapy
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICSI
- intracytoplasmic sperm injection
- IPD
- individual patient data
- IPD-MA
- individual patient data meta-analysis
- IQR
- interquartile range
- ITT
- intention to treat
- IVF
- in vitro fertilisation
- MD
- mean difference
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NPEU
- National Perinatal Epidemiology Unit
- OHSS
- ovarian hyperstimulation syndrome
- PI
- principal investigator
- PIL
- participant information leaflet
- PMG
- Project Management Group
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- RR
- risk ratio
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- STAI
- State–Trait Anxiety Inventory
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/AEFU1104).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.