Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/23/02. The contractual start date was in July 2018. The draft report began editorial review in February 2021 and was accepted for publication in June 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Perry et al. This work was produced by Perry et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Perry et al.
Chapter 1 Introduction
Parts of this report are reproduced or adapted from Perry et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
In the UK, there are 250,000 fractures in children each year, with one-third of individuals sustaining a fracture during their childhood. 2 The rate of childhood fractures is so high that, among adults, it is surpassed by the rate among women aged ≥ 85 years only; in no age group is the rate of fractures in men higher than that in children. 2 Torus (buckle) fractures of the distal radius are the most common fractures in children. 3
Children’s bones are very flexible compared with adult bones. In adults, a fracture leads to a complete disruption of the cortex of the bone, such that the broken bone is usually unstable and needs support from a cast or even surgical fixation. However, in children, the bones can crush or ‘buckle’, such that there is deformation but no break in the cortex. These fractures are at a low risk of complications or deformity in those who are skeletally immature and the fractures almost universally heal well. 4 Torus fractures of the distal radius (i.e. when the radius bone ‘buckles’) are the most frequently seen torus fractures.
There is considerable variation in the management of torus fractures. Treatment can include plaster cast immobilisation or the use of a removable rigid splint or more flexible splints. The differences in practice arise from a long-standing doctrine saying that fractures should be treated by rigid immobilisation5 and a simpler treatment method has not yet been widely implemented, despite evidence suggesting that they are frequently as effective, or perhaps even more effective. 6–10 The proponents of rigid forms of immobilisation (i.e. cast/splint) argue that these maximise pain relief and minimise the occurrence of complications (i.e. refracture). However, there is growing evidence showing an absence of complications even with less rigid constructs and growing acceptance that rigid immobilisation may not improve pain control, may inhibit the return to normal function, and that patients may safely be discharged at diagnosis. 9,11,12
The most comprehensive review of the evidence is a 2018 Cochrane review, which identified the quality of the evidence on treatments for treating wrist fractures in children as low or very low. 13 Ten randomised controlled trials (RCTs) have investigated different types of immobilisation, including an aggregate of 695 children with buckle fractures or similar minimally displaced stable fractures. Six trials compared a removable splint with a cast,6–8,10,14 and four [one unpublished: Jones S, Smith I, Jones MW. Treatment of distal radius buckle fractures, British Orthopaedic Congress, 2001, poster abstract no. 41.] compared a bandage with a cast. 15–17 No trials have compared a bandage with a removable splint, or considered ‘no treatment’. The recovery appeared broadly similar regardless of treatment, but little attention had been given to patient-reported outcomes. Insufficient evidence was available to assess the time taken to resume usual physical activities, pain or satisfaction. Two RCTs,9,11 involving 404 children, investigated the effect on recovery of the location where casts were removed – either in hospital or, in the case of a ‘soft cast’ or ‘half-cast’, at home. There were no refractures or complications, but there was low-quality evidence for greater parent satisfaction in the home removal group. To our knowledge, no further RCTs have been published since this Cochrane review.
The 2016, the National Institute for Health and Care Excellence (NICE) guideline for non-complex fractures made recommendations on the management of these injuries. 18 The NICE review concluded that torus fractures of the distal radius should not be immobilised in a non-removable rigid cast, and advocated discharge from the emergency department (ED) without the need for outpatient follow-up. NICE recommended that bandaging or soft casts should be the mainstay of treatment for torus fractures, but questioned whether or not any treatment was necessary at all. NICE recommended a trial to determine the optimal treatment for torus fractures as one of the five research priorities within the non-complex fracture review, particularly addressing whether or not no immobilisation was as efficacious as bandages or splinting.
Despite the available evidence and guidelines supporting a move away from cast immobilisation and outpatient follow-up, a recent survey of practice in 100 UK EDs demonstrated that 40% of EDs were using casts in the treatment of this fracture, and 60% were arranging outpatient clinic follow-up. 10 Similarly, a survey in Ireland found that 70% of EDs were using traditional casts and clinic follow-up. 15 Internationally, recent studies from the USA and Australia have demonstrated very high rates of cast immobilisation and follow-up, with associated high rates of radiographical follow-up. 7,8,14
Given the very large number of these injuries, identifying the optimal treatment strategy could have profound effects on childhood pain, the number of days of school absence and the cost to the NHS. Even apparently minor modifications in the care pathway of a very common fracture, such as discontinuing the use of manufactured wrist splints or reducing follow-up, could have very large financial implications across the NHS. A multicentre trial is likely to have wider financial benefits by promoting best practice across the NHS, such as reducing the reliance on follow-up outpatient visits and follow-up radiography.
We initially planned a trial to address the NICE research recommendation, comparing ‘no immobilisation’ with ‘bandages or splinting’. However, early participation from families revealed that this trial was unlikely to recruit successfully, as families felt that ‘no intervention’ was unacceptable, favouring the offer of a bandage even if it were not used. The trial interventions were, therefore, adapted based on the recommendation of parents and young people to replace ‘no intervention’ with the offer of a bandage.
Objectives
The aim of this project was to establish whether or not treating children with a torus fracture of the distal radius with the offer of a soft bandage and immediate discharge (i.e. offer of a bandage) provides the same recovery, in terms of pain and function, as treating them with rigid immobilisation and follow-up as per the protocol of the treating centre (i.e. rigid immobilisation).
The primary objective was to quantify and draw inferences on observed differences in the Wong–Baker FACES Pain Rating Scale (‘Wong–Baker Scale’) scores between the offer of a bandage and rigid immobilisation at 3 days post randomisation.
The secondary objectives were to:
-
assess differences in the Wong–Baker Scale scores between trial treatment groups at 1 day, 7 days, 3 weeks and 6 weeks post randomisation
-
determine differences in the use of regular analgesia between trial treatment groups at 1 day, 3 days and 7 days post randomisation.
-
quantify and draw inferences on functional recovery using the patient-report outcomes measurement system (PROMIS) upper extremity limb score for Children Computer Adaptive Test between the trial treatment groups at 3 days, 7 days, 3 weeks and 6 weeks post randomisation
-
quantify and draw inferences on observed differences in health-related quality of life (HRQoL) using EuroQol-5 Dimensions, youth version (EQ-5D-Y), between trial treatment groups at 3 days, 7 days, 3 weeks and 6 weeks post randomisation
-
determine differences in the number of days of school absence between trial treatment groups up to 6 weeks post randomisation
-
determine differences in the complication rate between trial treatment groups, including the need for further hospital attendance up to 6 weeks post randomisation
-
investigate, using appropriate statistical and economic analysis methods, the resource use and comparative cost-effectiveness between trial treatment groups during the first 6 weeks post randomisation.
Chapter 2 Methods
The final protocol (reproduced with permission of The British Editorial Society of Bone & Joint Surgery19) and the statistical and health economic analysis plan (reproduced with permission of The British Editorial Society of Bone & Joint Surgery20) have been published and some of the content has been reproduced in this monograph. These are Open Access publications distributed under the terms of the Creative Commons Attribution CC BY-NC-ND 4.0 licence, which permits others to copy and redistribute the material in any medium or format, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc-nd/4.0/. All protocol versions can be found on the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) Journals Library website. 21 See Appendix 2, Table 32, for the summary of changes implemented with each protocol version.
Throughout this publication, the term ‘parent’ means parent or guardian, as appropriate.
Trial design
All children aged 4–15 years with a radiologically confirmed torus fracture of the distal radius were potentially eligible for inclusion. Randomisation, stratified by recruitment centre and age, was facilitated through a computer randomisation service provided by the Oxford Clinical Trials Research Unit (OCTRU). Patients were randomly allocated 1 : 1 to either the offer of a bandage group or the rigid immobilisation group.
The primary outcome was the Wong–Baker Scale score, which was assessed at days 1, 3 and 7, as well as weeks 3 and 6 post randomisation. Functional and quality-of-life outcome data were collected using the PROMIS and EQ-5D-Y questionnaires at days 3 and 7 and at weeks 3 and 6 post randomisation. Data on the number of complications, the number of days of school absence and a resource use questionnaire were collected over the initial 6 weeks period post randomisation. Case report forms were completed electronically with data received centrally by the University of Oxford.
Participants
Patients were screened in the ED from the participating trial recruitment centres. It was anticipated that there would be a large seasonal variation in the number of screened and recruited participants as, in contrast to adults, children suffer more fractures in mid-summer than in mid-winter (approximately three times more), with weather being significantly related to the number of fractures as it is correlated with the time spent playing outside. 22 Recruitment centres were directed to screen all patients meeting the inclusion criteria for the study, with a poster (see Appendix 3) detailing the eligibility criteria to clinicians. The number of eligible and recruited patients, as well as the number of patients who declined consent, were recorded.
Inclusion criteria
Patients were eligible for inclusion in the FORCE (FOrearm fracture Recovery in Children Evaluation) trial if:
-
There was radiographical evidence of a torus fracture of the distal radius whereby there was a cortical deformation within the distal third of the radius but no break in the cortex. These could be associated with an ipsilateral fracture to the ulna (the ulna fracture could be buckle, greenstick or otherwise).
-
They were aged 4–15 years.
-
Randomisation could occur at a recruitment centre that was able to definitively treat the injury (e.g. an ED).
Exclusion criteria
Patients were excluded from this trial if:
-
The injury had occurred > 36 hours previously.
-
The treating clinician judged that there was a cortical disruption of the radius on radiographs (i.e. a greenstick fracture).
-
The patient had sustained an additional fracture at the time of the index fracture (with the exception of ipsilateral ulna fractures). Any child with bilateral torus fractures was therefore excluded.
-
There was evidence that the patient and/or parent would be unable to adhere to trial procedures or complete follow-up, such as insufficient English-language comprehension, developmental delay or a developmental abnormality, or no parental access to a mobile phone with internet access.
Consent
Recruitment took place in 23 recruitment centres in England from 21 NHS trusts that treated children with torus fractures of the distal radius. Eligible patients were identified by the clinical team. After introduction of the study concept by the clinical team, a member of the local research team presented the patient and parents with age-appropriate participant information sheets or online study information and verbal explanation of the trial procedures. The patient/parent were then given the opportunity to discuss any issues related to the trial with the local research team and their family and friends. The parent was then asked to sign an electronic informed consent form, and children from the age of 8 years were invited to sign an electronic assent form. Assent was taken where appropriate; however, the absence of assent did not exclude the patient from the study if consent had been obtained from the parent. If a child indicated that they did not want to take part, the child was not included in the study.
The FORCE trial was part of an ongoing NIHR-funded Study Within A Trial (SWAT) [TRials Engagement in Children and Adolescents (TRECA) NIHR Health Services and Delivery Research 14/21/2123] investigating the effects of the mode of information delivery to children and parents. The FORCE trial was one of a number of host trials embedding the TRECA intervention. Recruitment centres were randomised as clusters. All patients and parents received the same content, with information presented differently – either in paper format or through electronic multimedia information. Full details of the SWAT will be published elsewhere. 24
Decline consent and withdrawals
Participants (or their parents) were able to decline consent initially or withdraw consent for the trial at any time without prejudice. A decision to decline consent or withdraw did not affect the standard of care the patient received. Participants (or their parents) could withdraw by contacting the central research team by telephone or e-mail. If a patient withdrew, any data collected up until the time of withdrawal were retained by the research team and included in the final analysis. Withdrawn patients or patients deemed ineligible after randomisation were not replaced.
Randomisation
Those patients who consented to take part in the trial had their treatment allocated using a secure, centralised, online-encrypted randomisation service provided by OCTRU. All hospital treatment areas had access to the internet so accessed the randomisation service in real time (i.e. there were no delays in patient treatment).
Consented participants were randomised to one of two treatment groups (1 : 1). Randomisation was implemented using stratification by centre and age (4–7 years and 8–15 years), with randomisation schedules prepared by the trial statistician using variable block sizes of 2, 4 and 6, and embedded in the online system.
Stratification by centre helped to ensure that any clustering effect related to the centre was equally distributed in the trial groups. The catchment area (i.e. the local population served by the hospital) was similar for all of the hospitals; each hospital was a children’s injury unit dealing with these fractures on a daily basis. All of the recruitment centres, and indeed all hospitals throughout the NHS, use these techniques as part of their normal practice (i.e. staff were already equally familiar with both forms of treatment). This could not eliminate the clinician-specific effect of an individual at any one recruitment centre. 25 However, as the procedures were commonplace across the NHS, many clinicians were involved in the management of this group of patients (probably between 20 and 50 clinicians at each recruitment centre, including consultants, trainees and specialist nurses). Therefore, it was anticipated that each individual clinician would treat a handful of those enrolled in the trial only, reducing the risk of a clinician-specific effect on the outcome in any one recruitment centre.
Stratification on the basis of age ensured that the treatments were balanced across the age groups. This took into account differences in the properties of the primary outcome by age, with the score tending to linearity in those ≥ 8 years old, but behaving non-linearly for those aged < 8 years. 26 Furthermore, there was a discontinuity within the secondary outcome instruments (i.e. self-reports for those in the older group and proxy reports for those in the younger group). The trial therefore considered children aged 4–7 years separately from those aged 8–15 years to ensure the maximum validity of the result generated and to maximise the generalisability of the trial results.
Blinding
Participants and their parents could not be blind to their treatment. The treating clinician was, of course, not blind to the treatment they were providing. However, the treating clinical team did not take part in the follow-up assessment of the participants. The outcome data were collected directly from the participant and/or their parent.
Trial treatments
All of the hospitals involved in this trial were familiar with both treatment techniques. All of the participants received analgesia at the discretion of the treating clinician, as per local guidelines. In the absence of local guidelines, clinicians were advised to adhere to the Royal College of Emergency Medicine best practice guidelines for the management of acute pain in children. 27
This trial compared two approaches to treating torus fractures of the distal radius in children: the offer of a soft bandage and immediate discharge, or rigid immobilisation and follow-up as per the protocol of the treating centre.
The offer of a soft bandage and immediate discharge
A simple bandage, such as a gauze bandage or similar, was offered to participants. Whether or not to use, and when to discontinue the use of, the bandage was at the discretion of the child and their parents. For those choosing to use the bandage at the outset, this was applied in the ED. The bandage technique involved application to the wrist from the middle of the forearm to the level of the metacarpophalangeal joints. For those choosing not to use the bandage at the outset, a bandage was offered should they wish to apply this at home. Participants were discharged from the ED with no further planned outpatient follow-up (as per NICE guidance18). It was advised that the child could return to activities as pain allowed, a point of contact for any ongoing concern was provided and no specific restrictions on movement were in place. It was advised that the bandage should not be worn for more than 3 weeks. Details were sought from the patient and/or parent related to the duration that the bandage was worn.
Rigid immobilisation and follow-up as per the protocol of the treating centre
A rigid splint was applied that was either manufactured to conform to the wrist (e.g. futura splints) or was moulded to conform the wrist (e.g. backslab, plaster cast). The study was pragmatic and the exact type of splint was not prescribed to treating clinicians. A record was made of the type of splint used. Treatment advice and follow-up was as per the usual practice of the treating centre. Details were sought from the patient and/or parent related to the duration that the rigid immobilisation was worn.
Rehabilitation
Physiotherapy did not typically form a part in the management of these injuries, and no specific guidelines were offered to clinicians or patients.
Outcome measures
Outcomes from participants were collected at regular intervals during the 6-week follow-up period (Table 1 shows the collection times for all of the trial outcomes).
| Outcome | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Day 1 | Day 3 | Day 7 | Week 3 | Week 6 | |
| Wong–Baker Scale score | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Functional recovery – PROMIS | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Analgesia use | ✗ | ✗ | ✗ | |||
| EQ-5D-Y score | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Days absent from school | ✗ | ✗ | ||||
| Complications | ✗ | |||||
| Health-care use | ✗ | ✗ | ✗ | |||
Primary outcome
The primary outcome measure for this study was the Wong–Baker Scale,28 which is a validated self-reported tool. It is an ordinal assessment of pain using a series of six facial expressions to illustrate the degree of pain intensity. A numerical rating is assigned to each face (from 0, ‘no hurt’, to 10, ‘hurts worst’). It has been validated for use among children aged > 3 years, including in the paediatric ED,29 with its use being most established in those aged > 5 years. 6,30 It has been identified as an excellent measure of pain when estimating the effect of treatments in the ED, and is highly correlated with the visual analogue scale (VAS) (r = 0.90; p < 0.001). 29 The test–retest reliability is excellent (r = 0.90; p < 0.001). 31 The Wong–Baker Scale is widely used in clinical practice, forming part of the Royal College of Emergency Medicine ‘composite tool for the assessment of pain in children’ produced in 2013 as part of a best practice guideline,18 and was recently specifically highlighted for use by the NICE major trauma guidelines. 32
Secondary outcomes
The secondary outcome measures in this trial were as follows.
Functional recovery: patient-reported outcomes measurement system (PROMIS Bank v2.0) upper extremity limb score for Children Computer Adaptive Test
Patient-reported outcomes measurement system is a collection of patient-reported health status tools available for children and adults that were developed in collaboration with the US National Institute for Health to be disease non-specific. 33 These tools can be administered to healthy children as well as to children with a variety of chronic health conditions. These tools are self-reported by those aged ≥ 8 years and proxy reported in those aged < 8 years. PROMIS is available in full (30 questions), in short form (eight questions) or as a Computer Adaptive Test (average of eight questions). A Computer Adaptive Test enables the answer from one question to inform the choice of the next question, so each child completing a Computer Adaptive Test answers a distinct set of questions to arrive at their score.
Analgesia use
In patients with torus fractures, pain is usually controlled with simple analgesics such as paracetamol or ibuprofen. Patients are typically asked to purchase these over the counter, but outside pharmacy hours they may be given a short supply in hospital. Information concerning the use (i.e. yes/no) and type of analgesia (i.e. paracetamol, ibuprofen, other) in the last 24 hours was collected on days 1, 3 and 7 post randomisation. This information was self-reported by participants aged 8–15 years and proxy reported for those aged 4–7 years.
Quality of life: EQ-5D-Y
This is the child-friendly version of the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), which has been especially adapted in terms of language for children aged 8–11 years and for adolescents aged 12–18 years. 34,35 A proxy version is available for younger children. The age appropriateness in terms of feasibility, reliability and validity in children and adolescents has been established. 36 This was self-reported by participants aged 8–15 years and proxy reported for those aged 4–7 years.
Days of absence from school/child care
School absence due to the index injury was recorded, as well as the day of purchased child care and working days lost because of the child’s injury. This was proxy reported for all participants.
Complications
All complications were recorded. Particular note was made of hospital reattendance related to the index injury, including for reasons of inadequate analgesia, refracture or worsening of the fracture.
Satisfaction
Parent-reported satisfaction with clinical treatment received was collected on day 1 and at week 6 after randomisation using a seven-point scale from 1 (extremely satisfied) to 7 (extremely unsatisfied).
Health-care use
Resource use data for the economic evaluation were collected during the trial period from online questionnaires sent to participants at 3 and 6 weeks post randomisation. These health resource questionnaires were proxy reported.
The questionnaires covered two survey periods: baseline to 3 weeks post randomisation, and 3–6 weeks post randomisation. Questionnaires captured NHS and Personal Social Services (PSS) resource use associated with the index injury, including the frequency of use of outpatient care, community care and social care services. Questionnaires also recorded private care (e.g. physiotherapy), direct medical costs (e.g. medications), direct non-medical costs (e.g. help with child care) and indirect costs (e.g. carer absenteeism) associated with the injury. Free-text responses (applicable to all of the ‘other’ options) were reclassified to the appropriate cost category, were excluded if deemed unrelated/irrelevant to the trial by clinical experts or were analysed collectively as ‘other’ in the descriptive analysis.
Audit of the radiographical diagnosis of the torus fractures
The Trial Steering Committee (TSC) recommended performing an audit on the radiographical diagnosis of torus fractures to ascertain whether or not the enrolment of participants complied with the inclusion criteria.
The audit was conducted from a sample taken of the first 250 participants enrolled into the trial, which included participants from 17 recruitment centres. The local research team reviewed the clinical records of those recruited to determine the findings of the ‘formal’ report that is produced after review of the radiographs by a radiologist; this review was part of the routine clinical care.
Data management: questionnaire completion
The parent was prompted to complete the questionnaires with or on behalf of the child at days 1, 3 and 7, and at weeks 3 and 6. In addition, children aged > 12 years (with parents’ agreement) were prompted directly to complete the questionnaires. A direct link to the online questionnaire was sent by text message and/or e-mail. If there was no response to the initial and reminder messages within a specified time frame (the time allowed varied for each of the time points), an attempt was made to contact the parent by telephone to obtain the outcome data for the time point. Exact timelines and frequency of telephone calls were specified in the data management plan.
Once the final questionnaire was completed, a £10 gift voucher was offered as compensation for any costs (i.e. mobile phone data) incurred while completing the outcome measure assessments.
Adverse event management
Serious adverse events (SAEs) were entered onto the SAE reporting form and reported to the central study team. Once notified, causality and expectedness were assessed by the chief investigator or trial’s nominated clinician. Some adverse events that are foreseeable as part of the proposed treatment were not reported on a SAE reporting form; they were instead recorded on a complications reporting form. These foreseeable SAEs included recall to hospital outpatient/ED with a diagnosis of an alternative fracture pattern, or a worsening fracture deformity (with or without the need for differing inpatient or outpatient treatment).
All participants experiencing SAEs were followed up as per protocol (PP) until the end of the trial. All unexpected SAEs or suspected unexpected serious adverse reactions that occurred between date of consent and the 6-week follow-up point had to be reported to the sponsor and ethics committee.
Statistical analysis
Sample size
The primary outcome was the six-category Wong–Baker Scale score at 3 days. 30 The Wong–Baker Scale has demonstrated a very high correlation with a standard 0–100 mm VAS. 29 Each face equates to 2 points on the six-category Wong–Baker Scale. The minimally clinical important difference on the Wong–Baker Scale is one face (i.e. 2 points), which was determined in the setting of the paediatric ED. 29 This trial was designed to investigate equivalence of the offer of a bandage to the use of rigid immobilisation, assessing the difference in means on the Wong–Baker Scale at 3 days post randomisation. Assuming an equivalence margin of 1 point (half of the minimally clinical important difference), 90% power, conducting two one-sided tests at 2.5% significance and a standard deviation (SD) of 2.3 (based on results from a feasibility study37), 278 patients (139 per group) with primary outcome data were required to show equivalence.
The Wong–Baker Scale is a categorical outcome that may behave non-linearly in some instances (i.e. the magnitude of pain within the intervals is not uniform), with non-linearity most likely in younger age groups, tending to linearity in those aged > 8 years. 38 Therefore, the trial was powered for equivalence separately in the two subpopulations (i.e. 4–7 years and 8–15 years), which is also important for the secondary outcomes.
Allowing for 20% loss to follow-up inflated the sample size to 348 in each of the subpopulations (174 per group). Given that the primary outcome was measured at 3 days post randomisation, the loss to follow-up inflation could be readily adjusted to ensure that the study recruited effectively and efficiently. Sample size calculations were performed in PASS [PASS 13 Power Analysis and Sample Size Software (2014); NCSS, LLC, Kaysville, UT, USA; www.ncss.com/software/pass].
We planned on collecting primary outcome data for a minimum of 556 patients, a minimum of 278 in the 4–7 years age group and a minimum of 278 in the 8–15 years age group. Allowing for 20% loss to follow-up, we anticipated recruiting 696 patients in total (348 patients in each group).
Analysis plan
The statistical and health economic analysis plan for this trial has been published previously. 20 The methods used for the statistical analysis are summarised here.
General analysis principles
Two analysis populations were considered, the intention-to-treat (ITT) population and the PP population. The ITT population included all participants who were randomised, with participants analysed according to the group to which they were randomised, regardless of the treatment that they actually received. Participants were excluded from the PP population if (1) they did not receive the treatment to which they were randomised or changed from their allocated treatment prior to the primary outcome time point (day 3), (2) they did not provide sufficient follow-up data for analysis (i.e. did not provide a primary outcome) or (3) following randomisation, they were found not to satisfy the eligibility criteria for the study.
As this is an equivalence trial, a maximum clinical difference was prespecified for the primary outcome. This specified the level within which the two treatments can be considered not to differ in any clinically meaningful way. The null hypothesis tested in this trial was that a difference greater than the maximum clinical difference existed between the treatments in either direction. The trial was designed to disprove this in favour of the alternative that no clinically important difference exists. Analyses of the primary outcome were performed for the ITT population and repeated for the PP population. Equivalence was required in both populations for equivalence to be claimed. 39,40 Analyses of all secondary outcomes were performed for the ITT population and repeated for the PP population.
All the main analyses in this trial were performed using the available case data set. A sensitivity analysis of the primary outcome exploring a variety of missing not at random scenarios was planned in the case that > 10% of the data were missing. Owing to the small number of missing primary outcome data, no imputation was performed for this data set. In addition, no imputation was performed for missing data on any of the secondary outcomes.
A significance level of 0.05 was used throughout and 95% confidence intervals (CIs) reported. All secondary analyses were considered as supporting the primary outcome analyses. All analyses were conducted using Stata® 15.1 (StataCorp LP, College Station, TX, USA). Analyses of the primary (i.e. Wong–Baker Scale) and key secondary (i.e. PROMIS) outcomes were independently repeated by a statistician not involved in the trial to validate the results.
Descriptive analyses
The flow of participants through each stage of the trial, including the number of individuals screened, eligible, randomised, receiving allocated treatment and included in the primary analysis, was summarised using a Consolidated Standards of Reporting Trials (CONSORT) flow chart. Reasons for ineligibility, loss to follow-up and exclusion from the primary analysis were also summarised. Baseline comparability of the two groups in terms of stratification factors, baseline characteristics and primary and secondary outcomes at baseline were summarised using numbers for binary and categorical variables, and either means with SDs or medians with interquartile ranges (IQRs) for continuous variables. These descriptive analyses were performed for the overall trial population and separately for the two age groups (i.e. 4–7 years and 8–15 years).
The number of losses to follow-up and withdrawals, along with reasons for these, were summarised by treatment group at each time point. The number and percentage of participants with missing data for each outcome at each time point were also summarised, along with reasons for missingness, when known. The patterns of missingness were explored and suitability of missing data assumptions considered.
Compliance with treatment
The numbers of participants who fell into the following categories were summarised by treatment group, along with reasons for not receiving or changing from allocated treatment: (1) they received their allocated treatment until removal of treatment, (2) they received another treatment at baseline, (3) they changed from their allocated treatment prior to the primary outcome time point (day 3) or (4) they changed from their allocated treatment after the primary outcome time point. For those receiving rigid immobilisation, the number and percentage receiving each type of immobilisation (i.e. splint or cast) were summarised, as was the average length of time the rigid immobilisation was worn. For those offered a bandage, the number and percentage who had this applied prior to discharge from the ED and the number who applied it at home were summarised. The average length of time that the bandage was worn was also summarised.
Analysis of primary outcome
The Wong–Baker Scale scores at 3 days post randomisation were summarised by treatment group using means and SDs. A multivariable linear regression model adjusting for stratification factors (age and recruitment centre) and participant sex was used to compare the two groups with the adjusted difference, 95% CI and a p-value. The assumption of approximate normality of the residuals was assessed graphically and confirmed to be appropriate. An unadjusted t-test was also performed. These analyses were repeated separately for the two age groups (i.e. 4–7 years and 8–15 years), with the results reported in a similar manner.
Analyses utilising all time points (baseline to 6 weeks) were also performed using multilevel linear regression models with repeated measures (level 1) nested within participants (level 2) and adjusted for recruitment centre (level 3) and participant sex and age (fixed effects). The model included a treatment by time interaction, and the Wong–Baker Scale scores at each time point were summarised by treatment group using means and SDs, and reported alongside the adjusted difference (with 95% CI). As a supplementary analysis, the parameter estimates from this model were used to calculate summary statistics area under the curve (AUC) estimates for each treatment group41 to investigate total pain. The differences between the two groups were calculated and compared using a t-test. This approach was also used to investigate total pain from baseline to 3 days post randomisation.
Analysis of secondary outcomes
Continuous secondary outcomes (PROMIS, and EQ-5D-Y utility and VAS) were analysed using repeated-measures mixed-effects multilevel linear regression, similar to the one used for Wong–Baker Scale scores. Scores at each time point were summarised by treatment group using means and SDs, and adjusted differences and associated 95% CIs were also reported.
Satisfaction scores at days 1 and 42 were summarised by treatment group using medians and IQRs and the two groups were compared using a Mann–Whitney U-test.
The number and percentage of participants using pain medication in the first 7 days post randomisation were summarised by treatment group. A mixed-effects logistic regression model adjusted for recruitment centre, and participant age and sex was used to compare the two treatment groups, and the adjusted odds ratio (OR) and associated 95% CI were reported. The risk difference and associated 95% CI were also reported. Details of non-standard ‘over-the-counter’ analgesics were summarised.
Logistic regression models were also used to compare the number of participants who reported missing school during the first 3 weeks post randomisation. The number of days of school missed was summarised using medians and IQRs and compared using a Mann–Whitney U-test.
The PROMIS scores, EQ-5D-Y scores and use of pain medication were proxy reported for the younger age group (i.e. 4–7 years) and self-reported for the older age group (i.e. 8–15 years); therefore, analyses of these outcomes were repeated including an interaction between treatment and age group, with results reported as described previously.
Analysis of complications
The number and percentage of participants experiencing a foreseeable complication (recall to hospital with a diagnosis of an alternative fracture pattern or worsening fracture deformity) were summarised by treatment group, along with details of these complications. A comparative analysis of complications by treatment group was planned; however, the overall number of complications was very small and so no formal comparative analysis was performed.
Exploratory analysis
The impact of the receipt of pain medication in the preceding 24 hours was explored by including an interaction between receipt of pain medication and treatment. This analysis was performed for the overall population and for the two age groups separately.
Within the rigid immobilisation group, there are two types of immobilisation that could be used: splint or cast. An exploratory analysis investigating the effect of type of immobilisation used on Wong–Baker Scale scores at 3 days post randomisation was planned; however, this analysis could not be performed as there was not a sufficient number of participants randomised to each type of immobilisation (at least 10% to each type).
Implications of the COVID-19 pandemic
The majority of participants in this trial had been recruited and completed follow-up prior to the lockdown restrictions first imposed at the outset of the COVID-19 pandemic (i.e. March 2020). As all follow-up was performed remotely and collected directly from the participants or their parents, it was anticipated that the most substantial impact of the pandemic and resulting restriction of activities imposed under the ‘UK lockdown’ (from 23 March 2020) on this trial was likely to be the reduction in recruitment rate. For completeness, some areas of potential impact, including the type of participants recruited (see Appendix 5, Tables 38 and 39) and the reported rates of school absence (see Appendix 5, Table 40), were explored.
Health economic analysis plan
The within-trial economic analysis was performed using individual patient-level data. The analytical approach took the form of a cost–utility analysis. Based on trial evidence, incremental and cost–utility ratios were calculated by taking a ratio of the difference in the mean costs and mean utility measure.
The trial was conducted in the UK, which has a national health service that provides publicly funded health care, mostly free of charge at the point of use. The primary economic analysis was from the NHS and PSS perspective. A secondary analysis included the perspective of patients and carers.
The economic analysis compared the costs and consequences of each group over the first 6 weeks after randomisation, with no extrapolation beyond the study period of 6 weeks, as prespecified in the health economics analysis plan,20 because cost and outcome had converged for treatment groups by 6 weeks.
Costing of the treatments
Some of the assumptions made when cleaning, analysing or costing the data included the following:
-
If a patient answered ‘no’ to a prompt question about resource use, the frequency of service use for this category of resources was equal to zero.
-
If the drug use box was checked at all time points, that is days 1, 3 and 7, drug use was considered continuous up to the last checked period.
-
Treatments were delivered by either accident and emergency junior doctors or emergency nurse practitioners.
Rigid immobilisation
Participants randomised to receive rigid immobilisation were given a futura splint, a backslab, a soft cast or a hard cast. The type of splint used was at the discretion of the treating centre. Staff at the recruitment centres were asked to indicate the exact splint materials used, and how long it took to apply the cast, splint or bandage. We based the cost of delivering rigid immobilisation on the median time required to deliver the treatment (i.e. cast/splint application) and the average cost for each clinician delivering the treatment. The unit cost of the rigid immobilisation was calculated as the median of the types of splint used (see Appendix 6, Table 42). The total cost per participant of the rigid immobilisation was calculated by adding the mean administration cost and the device cost.
Offer of a soft bandage
If the parent/child accepted the offer of a soft bandage, the bandage could be applied in the ED or at home by the parent. Staff at participants recruitment centres were asked to state the exact bandage materials used, and how long it took to apply the soft bandage. The unit cost of the soft bandage was taken as the median cost of the various types of soft bandage used (see Appendix 6, Table 42). The total cost of soft bandage treatment of each participant was calculated by summing the mean administration and soft bandage costs.
Valuation of resource use
Unit costs for each resource item associated with the trial were sourced from the latest national sources, such as the NHS Supply Chain Catalogue 2018/1942 and NHS Reference Costs 2015–16. 43
The unit costs of the different forms of immobilisation applied (i.e. futura-type splint, backslab, soft cast or hard cast) and the soft bandage were sourced from the latest NHS Supply Chain Catalogue 2018/19. 42
As the injury was primarily managed within the ED, any potential cost of hospitalisation, as defined by the Healthcare Resource Group code, was expected to be the same between the treatment groups.
The unit costs of direct medical costs that were not part of the trial treatments, such as outpatient care and community care, were sourced from the latest available NHS Reference Costs43 and Unit Costs of Health and Social Care. 44
The unit cost of medication related to wrist injury was sourced from the latest available British National Formulary (BNF)45 based on the assumed daily dose using BNF recommendations.
Measurement of broader resource use
Collection of unit costs for direct non-medical resource items, such as help with child care incurred by the participant’s carer, was not required because patient costs were obtained directly from the questionnaire. In addition, lost productivity was also obtained from the trial questionnaires. These costs were excluded from the base-case analysis as they were beyond the NHS/PSS perspective of the economic evaluation.
Cost per patient
The cost of health resource use per patient was computed by multiplying the frequency of health resource use rate by the unit cost of each resource item. Direct non-medical costs were obtained directly from the questionnaire.
All costs related to the most recent year for which unit cost data were available and were expressed in Great British pounds (GBPs).
Calculation of utilities and quality-adjusted life-years
The HRQoL of participants was estimated using the EQ-5D-Y, a child-friendly version of the EuroQol-5 Dimensions (EQ-5D), at baseline, 3 days, 7 days, 3 weeks and 6 weeks post randomisation. The EQ-5D-Y instrument estimates a respondent’s HRQoL (sometimes referred to informally as a ‘utility’) – in this context a preference-based valuation placed on an individual’s particular health outcome. The EQ-5D consists of five health state dimensions (i.e. mobility, self-care, usual activity, pain/discomfort and anxiety/depression). There are three levels of health status to choose from: no problems, some problems and a lot of problems. Each participant or their proxy reported the participant’s present health at the date of questionnaire completion. In addition, they self-rated their health at the time of survey completion using a VAS, a non-preference-based measure.
As there is no validated tariff for estimating EQ-5D utility based on the EQ-5D-Y, we used the UK time trade-off tariff for the adult version of the EQ-5D questionnaire. 46 A recent review of patterns and trends of measurement and valuation of childhood health utilities47 found that 78.7% of the studies employing the EQ-5D-Y used general adult-derived tariffs, with the rest providing no information about the tariff used.
Quality-adjusted life-years (QALYs) were calculated as the area under the utility curve of the EQ-5D utility scores using baseline data and data obtained 3 days, 7 days, 3 weeks and 6 weeks post randomisation using the trapezoidal rule. 48
Missing data
Imputation and estimation was conducted according to good practice guidance using the multiple imputation framework within Stata. 49 Multiple imputation provides unbiased estimates of treatment effects if data are missing at random (i.e. causes of missingness are explained by observed variables). This assumption was explored in the data using logistic regression of the missingness of costs and QALYs against baseline variables. 50 Imputation models used fully conditional multiple imputation by chained equations methods, which are appropriate when correlation occurs between variables. Each multiple imputation ‘draw’ provided a complete data set, which probabilistically reflected the distributions and correlations between variables. The imputation process was partitioned to run independently for the two treatment groups. Predictive mean matching drawn from the five nearest neighbours (knn = 5) was used to enhance the plausibility and robustness of imputed values, as normality may not be assumed. An analysis of multiple draws was conducted with Stata’s multiple imputation framework providing estimation adjusted for Rubin’s rule. 51 Within the imputation, missing costs and EQ-5D-Y scores were imputed for each period of follow-up and aggregated to overall patient costs and QALYs for each draw. All imputed variables acted as predictive variables, supplemented by trial baseline variables if significant and plausible predictors of missingness. Multiple imputation estimation models were bootstrapped to provide non-parametric estimates. Initially, the imputation model employed 10 draws, reflecting the proportion of missing data. To minimise the information loss of finite imputation sampling, the fraction of missing information was assessed, ensuring that the number of draws exceeded the fraction of missing information percentage.
Cost-effectiveness analysis
Using ITT principles, the incremental cost-effectiveness ratio (ICER) was estimated comparing (1) the offer of a soft bandage and immediate discharge (as the ‘new’ treatment) with (2) treatment with rigid immobilisation (‘current’ practice) with follow-up as per the protocol of the treating centre. The ICER and CI were estimated using bivariate analysis (complete-case analysis) or multiple imputed bivariate analysis (base-case analysis and other sensitivity analyses). Bootstrapped models were used to report summary estimates (median and percentiles) of group costs and QALYs and to graphically visualise the ICER plane, net monetary benefit (NMB), cost-effectiveness acceptability curve and expected value of perfect information. Value for money was determined by comparing the ICER with several willingness-to-pay thresholds, using an upper threshold for NICE ‘regular’ approvals of £30,000 per QALY,52 a central value of £20,000 per QALY and a lower value of £15,000 per QALY, which reflects the uncertainty about the true value appropriate to the NHS. 53
To assess the robustness of findings, base-case assumptions were explored using a range of supportive sensitivity analyses. A planned subgroup analysis explored the interaction of age group (i.e. 4–7 years or 8–15 years) with the findings. Further planned sensitivity analyses included a complete-case analysis (without imputation and assuming ‘missing completely at random’) and a broader societal perspective (including productivity losses and loss of earnings). Some participants were recorded in recruitment centre-reported data as having attended hospital but did not self-report these visits. To investigate the effect on the cost evaluation, a post hoc sensitivity analysis assumed that participants attended hospital at least as often as reported in the recruitment centre-reported data (i.e. if a participant reported no additional hospital visits, yet the recruitment centre had recorded one additional hospital visit for that participant, then ‘1’ was assumed to be the correct assignment). It was unclear if such visits occurred in outpatient departments or EDs; therefore, an average cost of hospital services was applied to any additional visits. The sensitivity analysis recalculated total NHS costs and explored the impact on cost-effectiveness.
Analyses and modelling were undertaken using Stata 16 and reporting follows the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 54
Ethics approval and monitoring
Ethics committee approval
The National Research Ethics Committee approved this study on 16 November 2018 (18/WM/0324).
Trial Management Group
The day-to-day management of the trial was the responsibility of the clinical trial manager, based at Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, and supported by the OCTRU staff. This was overseen by the Trial Management Group (TMG), which met monthly to assess overall trial progress. It was the responsibility of the trial manager to train the research teams at each of the recruitment centres. The trial statistician and health economist were closely involved in setting up data capture systems, design of databases and design of clinical reporting forms.
Trial Steering Committee
The TSC, which included independent members, provided overall supervision of the trial on behalf of the funder. Its terms of reference were agreed with the HTA programme and were drawn up in a TSC charter that outlined its roles and responsibilities. Meetings of the TSC took place at least once per year during the recruitment period. The responsibilities of the TSC included monitoring and supervising the progress of the trial towards its interim and overall objectives, reviewing relevant information at regular intervals from other sources (i.e. similar studies/trials newly reported in the literature), considering the recommendations of the Data Safety and Monitoring Committee (DSMC) and informing the funding body on the progress of the trial.
Data Safety and Monitoring Committee
The DSMC adopted a DAMOCLES charter, which defined its terms of reference and operation in relation to oversight of the trial. It did not perform any formal interim analyses of effectiveness. However, it reviewed summaries of data accrued to date by treatment group and assessed the screening algorithm against the eligibility criteria. It also considered emerging evidence from other related trials or research and reviewed related SAEs that had been reported.
Patient and public involvement
The trial was co-produced with families from the outset, including in the development of the funding application.
We initially planned a trial to address the NICE research recommendation, comparing ‘no immobilisation’ with ‘bandages or splinting’. However, this proposal was discussed with the Generation R Young Persons Advisory Group and at the Parents and Carers Forum at Alder Hey Hospital. Both groups were clear that ‘no treatment’ was not an acceptable treatment to families, favouring a ‘bandage’, even if young people or their parents chose not to use the bandage. ‘No immobilisation’ was therefore adapted, with guidance from young people and parents, to ‘the offer of a bandage’.
Throughout the study design, families helped determine the primary outcome tool and the primary outcome time point. Early pain, rather than function, was a key concern for families. How well that pain was resolved should be measured on several occasions during the first few days, and it was agreed that the primary outcome measure would be pain recorded on the third day following the injury. To determine the outcome tool, parents and children were shown a number of different pain tools with similar scientific properties (i.e. the Wong–Baker Scale and the FACES Pain Scale Revised)55 to ascertain which they preferred. The Wong–Baker Scale was the tool preferred by parents and children.
Parents also helped determine the method of collection of outcome data. Parents preferred to respond to periodic text messages rather than telephone or ‘diary’ responses. The timing of the data collection was chosen to coincide with school closure (i.e. 16.00), with reminders closer to the child’s bedtime (19.00).
Once funded, a competition was held using an online design agency to design the study logo. The process yielded around 10 excellent designs. To select the winning design, over 100 children in schools and hospitals voted for the winner. Alongside this, the trial team was engaged in undertaking school assemblies and broader ‘research’ education to engage the public in what was being undertaken.
To ensure ongoing patient and public involvement, a parent representative (PG) was actively involved in the day-to-day management of the trial. This parent representative offered regular insights into the trial from a parent perspective, and regularly discussed the trial progress at the Parents and Carers Forum at Alder Hey Hospital. In addition, a further independent parent representative was a member of the TSC.
One of the largest pieces of engagement at the beginning of the trial was the development of trial recruitment materials. This involved an iterative process of developing an ‘explainer video’. A script was co-produced by the trial team, and this was shared widely to ensure that the text was accessible to families, with the treatments presented in a ‘balanced’ way. The parent representative (PG) shared these with a Parents and Carers Forum, as well as families with children, to seek to ensure that the objectives of the team were met. Iterative changes were made to the text, and then an explainer was produced by a professional design company. Families again reviewed the animation produced. In the original animation, the rigid immobilisation was multicoloured, and children commented that they ‘prefer the rigid immobilisation’ because of the coloured splint. Based on this, the video was revised to ensure that children identified rigid immobilisation to be as equally appealing as the bandage.
A dissemination video that is closely aligned to the study explainer animation (i.e. using the same cartoon characters) has been produced. The text and animation in the video underwent a similar development process to the explainer animation to ensure that the trial result is broadly accessible to the public. The video will be shared through social media, and will be made available to hospitals to ensure that it is available to be played in radiology department and ED waiting rooms (i.e. the places it will be most relevant and impactful to affected families).
Chapter 3 Results
Screening and randomisation
Patient screening for potential study participants was open from 16 January 2019 to 13 July 2020. A total of 1907 patients were screened, of whom 394 were not eligible (Table 2 shows the reasons for ineligibility). The most common reason for ineligibility was that injury occurred > 36 hours previously. Appendix 4, Table 33, summarises the baseline characteristics of those patients who were randomised and those who were eligible but not randomised.
| Reason for ineligibility | Number of participants |
|---|---|
| Injury > 36 hours | 258 |
| Other fractures present (excluding ipsilateral ulna) | 77 |
| Unable to adhere to the trial procedures | |
| Insufficient English language | 41 |
| Developmental delay | 3 |
| Developmental abnormality | 9 |
| Othera | 6 |
| Total | 394 |
Of the 1513 eligible patients, 89 (5%) were not able to enrol in the study because they experienced internet problems, because their legal representative was absent or because there was a lack of clinician equipoise. The absence of clinician equipoise was rare and accounted for < 1% of the eligible patients who were not enrolled into the study. Of the 1424 eligible participants who were available to be recruited, 459 (32%) declined to participate; Table 3 shows the breakdown of the reasons for declining to consent. More than half of the patients/parents who declined to participate did so because they preferred treatment with rigid immobilisation.
| Reason for non-participation | Number of participants |
|---|---|
| Reasons for declining to consent | |
| Child did not want to take part | 31 |
| Parent did not want child to take part | 117 |
| Child did not want to complete questionnaires | 2 |
| Parent did not want to complete questionnaires | 20 |
| No reason given | 23 |
| Treatment preference (for rigid immobilisation) | 252 |
| Treatment preference (for offer of a bandage) | 4 |
| Other | 10 |
| Total | 459 |
| Reason for being unable to enrol | |
| Clinician had a treatment preference (no equipoise) | 14 |
| Internet problems | 29 |
| No legal parental representative present | 40 |
| Othera | 6 |
| Total | 89 |
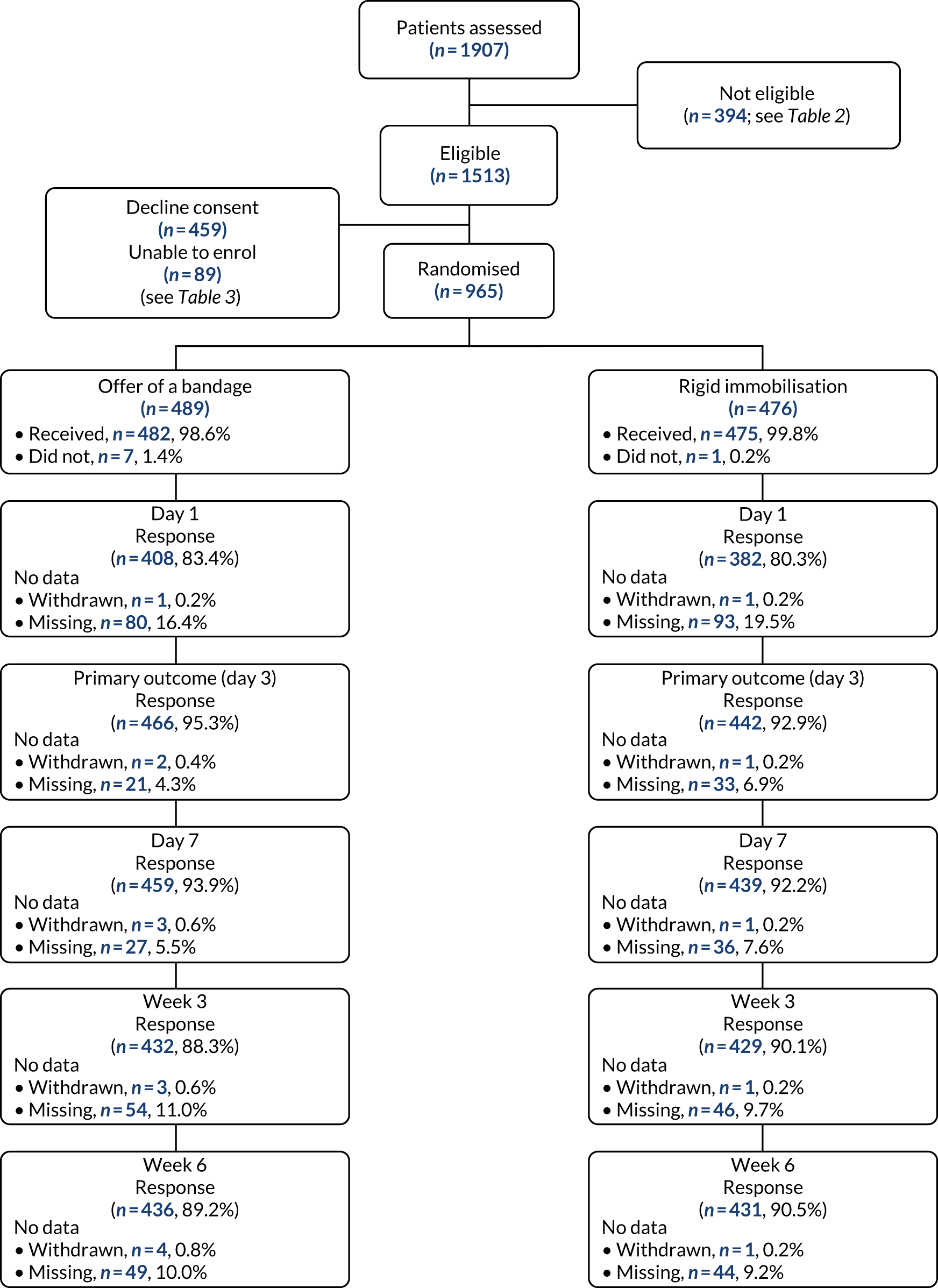
A total of 965 patients were recruited and randomised to the trial treatments. Peak recruitment times were between 15.00 and 19.00 (Figure 1). A total of 489 participants were allocated to the offer of a bandage group and 476 were allocated to the rigid immobilisation group. Figure 2 shows the CONSORT flow chart for screened patients. A total of 965 participants were included in the ITT population (offer of a bandage group, n = 489; rigid immobilisation group, n = 476) and 870 participants were included in the PP population (offer of a bandage group, n = 428; rigid immobilisation group, n = 442).
FIGURE 1.
Recruitment by time of day.
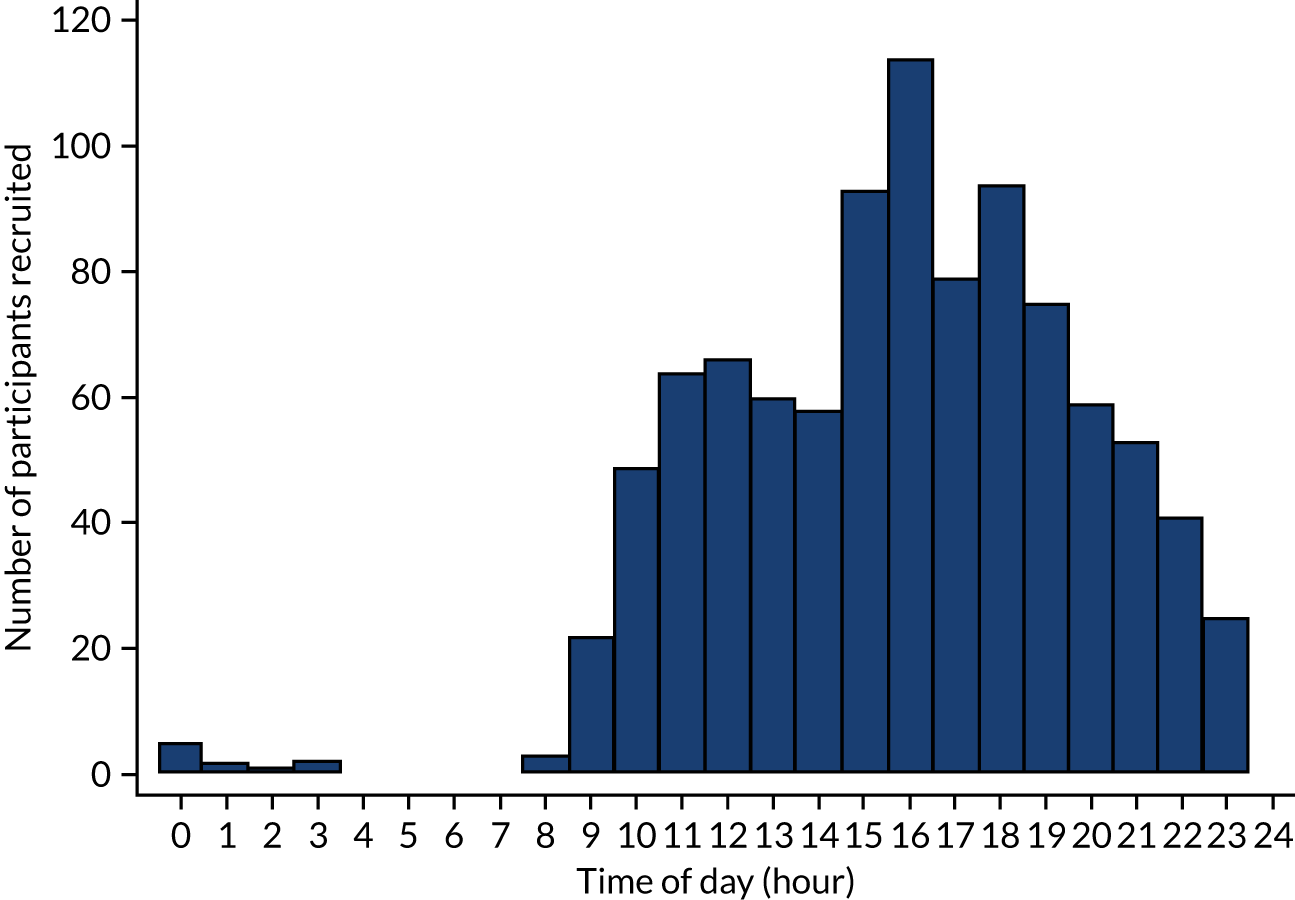
FIGURE 2.
The CONSORT flow chart. The numbers of responses are based on providing a Wong–Baker Scale score at each time point (ITT population)
Recruitment
Stratification factors by treatment groups
The stratification factors (i.e. recruitment centre and age group) are summarised by treatment group and overall using numbers and percentages in Table 4. There were around twice as many recruits in the older age group (i.e. 8–15 years) as in the younger age group (i.e. 4–7 years).
| Stratification factor | Number (%) of participants randomised | ||
|---|---|---|---|
| Treatment group | Total (N = 965) | ||
| Offer of a bandage group (N = 489) | Rigid immobilisation (N = 476) | ||
| Recruitment centre | |||
| Alder Hey Children’s Hospital (Liverpool) | 79 (16.2) | 77 (16.2) | 156 (16.2) |
| Birmingham Children’s Hospital (Birmingham) | 35 (7.2) | 35 (7.4) | 70 (7.3) |
| Bristol Royal Hospital for Children (Bristol) | 57 (11.7) | 54 (11.3) | 111 (11.5) |
| Darlington Memorial Hospital (Darlington) | 3 (0.6) | 2 (0.4) | 5 (0.5) |
| Evelina London Children’s Hospital (London) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Horton General Hospital (Oxford) | 10 (2.0) | 10 (2.1) | 20 (2.1) |
| Ipswich Hospital (Ipswich) | 33 (6.7) | 31 (6.5) | 64 (6.6) |
| John Radcliffe Hospital (Oxford) | 20 (4.1) | 17 (3.6) | 37 (3.8) |
| King George Hospital (London) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Leicester Royal Infirmary (Leicester) | 32 (6.5) | 34 (7.1) | 66 (6.8) |
| New Cross Hospital (Wolverhampton) | 25 (5.1) | 20 (4.2) | 45 (4.7) |
| Nottingham Children’s Hospital (Nottingham) | 21 (4.3) | 22 (4.6) | 43 (4.5) |
| Queen’s Hospital (Romford) | 8 (1.6) | 6 (1.3) | 14 (1.5) |
| Royal Berkshire Hospital (Reading) | 6 (1.2) | 5 (1.1) | 11 (1.1) |
| Royal Derby Hospital (Derby) | 53 (10.8) | 54 (11.3) | 107 (11.1) |
| Royal Devon and Exeter Hospital (Exeter) | 13 (2.7) | 11 (2.3) | 24 (2.5) |
| Royal London Hospital (London) | 20 (4.1) | 20 (4.2) | 40 (4.1) |
| Sheffield Children’s Hospital (Sheffield) | 30 (6.1) | 30 (6.3) | 60 (6.2) |
| St George’s Hospital (London) | 11 (2.2) | 11 (2.3) | 22 (2.3) |
| Sunderland Royal Hospital (Sunderland) | 16 (3.3) | 18 (3.8) | 34 (3.5) |
| University Hospital Southampton (Southampton) | 2 (0.4) | 3 (0.6) | 5 (0.5) |
| University Hospitals Coventry & Warwickshire (Coventry) | 12 (2.5) | 10 (2.1) | 22 (2.3) |
| Wexham Park Hospital (Slough) | 3 (0.6) | 4 (0.8) | 7 (0.7) |
| Age group (years) | |||
| 4–7 | 153 (31.3) | 147 (30.9) | 300 (31.1) |
| 8–15 | 336 (68.7) | 329 (69.1) | 665 (68.9) |
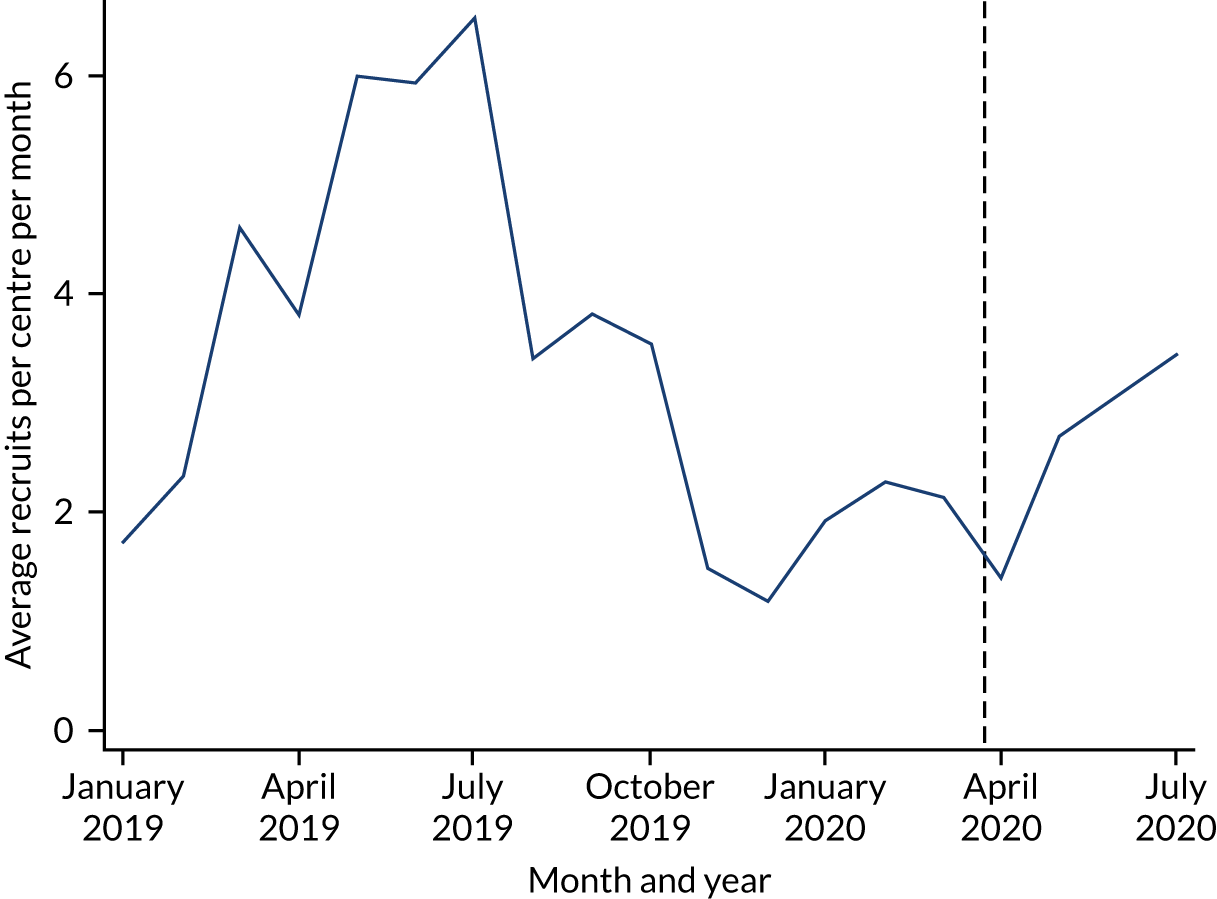
Recruitment by month
A seasonal pattern to recruitment was anticipated in this trial, with substantially more recruits in the summer months than in the winter months. The trend in terms of monthly recruitment rates is summarised in Figure 3. The rate of recruitment is calculated as the total number of recruits divided by the number of recruitment centres open.
FIGURE 3.
Recruits per recruitment centre per month. Note that the dashed line indicates 23 March 2020, when restrictions on activity were imposed in the UK owing to the lockdown associated with the COVID-19 pandemic.
Participants and treatments
Treatment allocation
Participants who did and participants who did not receive their allocated treatments at baseline are summarised in Table 5, along with details of the type of immobilisation used and whether a bandage was applied or given. The average duration of treatment, as well the number of patients still being treated, at 3 weeks post randomisation and the number who changed from their allocated treatment prior to 3 weeks post randomisation, is also summarised in Table 5. In addition, details of all hospital-initiated immobilisation changes are summarised by time of change and overall. This includes instances where a participant changed to another treatment within the same arm (e.g. from a splint to a cast).
| Treatment details | Offer of a bandage group (N = 489) | Rigid immobilisation group (N = 476) |
|---|---|---|
| Received allocated treatment at baseline, n (%) | 482 (98.6) | 475 (99.8) |
| Did not receive allocated treatment at baseline, n (%) | 7 (1.4) | 1 (0.2) |
| Reason did not receive allocated treatment, n (%) | ||
| Clinical decision | 1 (0.2) | 0 (0.0) |
| Child/parent decision | 6 (1.2) | 1 (0.2) |
| Immobilisation type used, n (%) | ||
| Futura splint (or similar) | 7 (1.4) | 451 (94.7) |
| Backslab | 0 (0.0) | 8 (1.7) |
| Soft cast full | 0 (0.0) | 11 (2.3) |
| Soft cast split | 0 (0.0) | 3 (0.6) |
| Hard cast split | 0 (0.0) | 1 (0.2) |
| Other | 0 (0.0) | 1 (0.2) |
| Bandage applied, n (%) | 458 (93.7) | 0 (0.0) |
| Bandage given, n (%) | 23 (4.7) | 0 (0.0) |
| Removed splint/cast/bandage completely by day 3, n (%) | 69/482 (14.3) | 4/475 (0.8) |
| Days splint/cast worn, median (IQR), (minimum, maximum), n | 13 (8–18), (8, 18), 2 | 18 (14–21), (1, 27), 241 |
| Still wearing splint/cast at week 3, n (%) | 3 (0.6) | 177 (37.2) |
| Days bandage worn, median (IQR), (minimum, maximum), n | 7 (4–16), (0, 32), 338 | 0 (0–0), (0, 0), 1 |
| Still wearing bandage at day week 3, n (%) | 50 (10.2) | 0 (0.0) |
| Changed to splint/cast before week 3, n (%) | 50 (10.2) | 0 (0.0) |
| Number of patients undergoing hospital-initiated immobilisation changes (e.g. splint to bandage, splint to splint, bandage to bandage or bandage to splint) | ||
| Day 1, n (%) | 10 (2.0) | 5 (1.1) |
| Day 3, n (%) | 22 (4.5) | 8 (1.7) |
| Day 7, n (%) | 20 (4.1) | 3 (0.6) |
| Day 21, n (%) | 9 (1.8) | 6 (1.3) |
| Total number of immobilisation changes | 61 | 22 |
| Total number of participants with at least one immobilisation change, n (%) | 53 (10.8) | 22 (4.6) |
Participants were considered to have crossed over if they changed from their allocated treatment on or before the 3-day follow-up time point. In total, there were 36 crossovers in the offer of a bandage group (7.4%) and one crossover in the rigid immobilisation group (0.2%). Crossovers are summarised by treatment group in Table 6. Participants who changed treatment after day 3 are also summarised. The reasons for changes are summarised, and the crossovers and later treatment changes are also summarised separately by age group.
| Treatment change | Offer of a bandage group (N = 489) | Rigid immobilisation group (N = 476) |
|---|---|---|
| Changed from allocated treatment by day 3 (crossover), n (%) | 36 (7.4) | 1 (0.2) |
| 4–7 years, n/N (%) | 15/153 (9.8) | 0/147 (0.0) |
| 8–15 years, n/N (%) | 21/336 (6.2) | 1/329 (0.3) |
| Reason for crossover, n (%) | ||
| Child/parent decision | 6 (1.2) | 1 (0.2) |
| Clinical decision | 1 (0.2) | 0 (0.0) |
| Pain | 18 (3.7)a | 0 (0.0) |
| Change of diagnosis (alternative fracture identified) | 1 (0.2) | 0 (0.0) |
| Other | 10 (2.0) | 0 (0.0) |
| Changed from allocated treatment after day 3, n (%) | 21 (4.3) | 0 (0.0) |
| 4–7 years, n/N (%) | 6/153 (3.9) | N/A |
| 8–15 years, n/N (%) | 15/336 (4.5) | N/A |
| Reason for change after day 3, n (%) | ||
| Pain | 11 (2.2) | 0 (0.0) |
| Change of diagnosis (alternative fracture identified) | 1 (0.2) | 0 (0.0) |
| Other | 9 (1.8)b | 0 (0.0) |
Available data
Follow-up was completed between 17 January 2019 and 27 August 2020. Availability of each of the primary and secondary outcomes at each follow-up time point from day 1 to week 6 post randomisation by allocated treatment and by age group is summarised in Appendix 4, Table 34. Overall follow-up rates were high: approximately 94% at the 3-day follow-up time point.
Withdrawals and protocol deviations
Only five participants withdrew during the trial. Withdrawals and reasons for these are reported by treatment group in Table 7. The time in days from randomisation to withdrawal is also summarised in Table 7.
| Withdrawal details | Offer of a bandage group (N = 489) | Rigid immobilisation group (N = 476) |
|---|---|---|
| Participant withdrawals, n (%) | 4 (0.8) | 1 (0.2) |
| Reasons for withdrawal, n (%) | ||
| Parent did not want to complete questionnaires | 2 (0.4) | 0 (0.0) |
| No reason | 1 (0.2) | 1 (0.2) |
| Other reason | 1 (0.2) | 0 (0.0) |
| Time to withdrawal (days), median (IQR), (minimum, maximum), n | 3 (1.5–19.5), (1, 35), 4 | 1 (1–1), (1, 1), 1 |
Twenty protocol deviations were recorded during the trial. These are summarised by treatment group in Table 8, along with the reasons for the deviation. Four protocol deviations relating to the diagnosis were recorded by recruitment centres. Owing to the pragmatic approach to diagnosis of torus fractures taken in this trial (i.e. the diagnosis was made by the treating clinician, thus emulating routine care), these were not considered eligibility errors. These deviations were recorded as ‘query about diagnostic criteria; participant remains eligible’ and participants were not excluded from the PP population for this reason.
| Protocol deviation details | Offer of a bandage group (N = 489) | Rigid immobilisation group (N = 476) |
|---|---|---|
| Participants with protocol deviations, n (%) | 16 (3.3) | 4 (0.8) |
| Type of deviation, n (%) | ||
| Consent | 1 (0.2) | 1 (0.2) |
| Eligibility error | 2 (0.4) | 0 (0.0) |
| Followed up | 1 (0.2) | 0 (0.0) |
| Not received the allocated treatment | 7 (1.4) | 1 (0.2) |
| Query about diagnostic criteria; participant remains eligible | 3 (0.6) | 1 (0.2) |
| Double randomisation | 1 (0.2) | 0 (0.0) |
| Randomised under wrong recruitment centre | 1 (0.2) | 1 (0.2) |
Baseline characteristics
Baseline participant characteristics
The baseline characteristics are summarised by treatment group, both overall and for each of the age groups separately, in Table 9. The treatment groups appear to be well balanced in terms of these characteristics at baseline. The proportion of female participants was larger in the younger age group (50.3%) than in the older age group (34.3%), but, otherwise, the two age groups were similar.
| Characteristic | Offer of a bandage group (N = 489) | Rigid immobilisation group (N = 476) | Total (N = 965) |
|---|---|---|---|
| Age (years), mean (SD), n | |||
| Overall | 9.61 (2.99), 489 | 9.69 (2.85), 476 | 9.65 (2.92), 965 |
| 4–7 | 6.10 (1.18), 153 | 6.33 (1.06), 147 | 6.21 (1.13), 300 |
| 8–15 | 11.21 (2.05), 336 | 11.20 (1.98), 329 | 11.20 (2.01), 665 |
| Female, n (%)a | |||
| Overall | 179 (36.6) | 200 (42.0) | 379 (39.3) |
| 4–7 years age group | 72 (47.1) | 79 (53.7) | 151 (50.3) |
| 8–15 years age group | 107 (31.8) | 121 (36.8) | 228 (34.3) |
| Right-side injury, n (%)b | |||
| Overall | 215 (44.0) | 197 (41.4) | 412 (42.7) |
| 4–7 years age group | 72 (47.1) | 61 (41.5) | 133 (44.3) |
| 8–15 years age group | 143 (42.6) | 136 (41.3) | 279 (42.0) |
| Mechanism of injury, n (%) | |||
| Low energy | |||
| Overall | 371 (75.9) | 352 (73.9) | 723 (74.9) |
| 4–7 years age group | 119 (77.8) | 106 (72.1) | 225 (75.0) |
| 8–15 years age group | 252 (75.0) | 246 (74.8) | 498 (74.9) |
| High energy | |||
| Overall | 100 (20.4) | 106 (22.3) | 206 (21.3) |
| 4–7 years age group | 31 (20.3) | 39 (26.5) | 70 (23.3) |
| 8–15 years age group | 69 (20.5) | 67 (20.4) | 136 (20.5) |
| Otherc | |||
| Overall | 18 (3.7) | 18 (3.8) | 36 (3.7) |
| 4–7 years age group | 3 (2.0) | 2 (1.4) | 5 (1.7) |
| 8–15 years age group | 15 (4.5) | 16 (4.9) | 31 (4.7) |
| Dominant hand, n (%) | |||
| Right | |||
| Overall | 420 (85.9) | 410 (86.1) | 830 (86.0) |
| 4–7 years age group | 135 (88.2) | 119 (81.0) | 254 (84.7) |
| 8–15 years age group | 285 (84.8) | 291 (88.4) | 576 (86.6) |
| Left | |||
| Overall | 62 (12.7) | 58 (12.2) | 120 (12.4) |
| 4–7 years age group | 13 (8.5) | 23 (15.6) | 36 (12.0) |
| 8–15 years age group | 49 (14.6) | 35 (10.6) | 84 (12.6) |
| Unsure/ambidextrous | |||
| Overall | 7 (1.4) | 8 (1.7) | 15 (1.6) |
| 4–7 years age group | 5 (3.3) | 5 (3.4) | 10 (3.3) |
| 8–15 years age group | 2 (0.6) | 3 (0.9) | 5 (0.8) |
| Injury side, n (%) | |||
| Dominant hand | |||
| Overall | 219 (44.8) | 207 (43.5) | 426 (44.1) |
| 4–7 years age group | 69 (45.1) | 63 (43.5) | 133 (44.3) |
| 8–15 years age group | 150 (44.6) | 143 (43.5) | 293 (44.1) |
| Non-dominant hand | |||
| Overall | 263 (53.8) | 261 (54.8) | 524 (54.3) |
| 4–7 years age group | 79 (51.6) | 78 (53.1) | 157 (52.3) |
| 8–15 years age group | 184 (54.8) | 183 (55.6) | 367 (55.2) |
| Not applicabled | |||
| Overall | 7 (1.4) | 8 (1.7) | 15 (1.6) |
| 4–7 years age group | 5 (3.3) | 5 (3.4) | 10 (3.3) |
| 8–15 years age group | 2 (0.6) | 3 (0.9) | 5 (0.8) |
Patient-reported outcome measures at baseline
Baseline data were collected from the participant or parent after consent was obtained and before randomisation occurred. The patient-reported outcome measures (PROMs) (i.e. the Wong–Baker Scale, PROMIS and EQ-5D-Y) are summarised by treatment group overall and for each age group separately in Table 10. The treatment groups appear to be well balanced at baseline.
| Scale | Offer of a bandage group (N = 489) | Rigid immobilisation group (N = 476) | Total (N = 965) |
|---|---|---|---|
| Wong–Baker, mean (SD), na | |||
| Overall | 5.21 (2.32), 489 | 4.91 (2.10), 476 | 5.07 (2.22), 965 |
| 4–7 years | 4.72 (2.85), 153 | 4.76 (2.46), 147 | 4.74 (2.66), 300 |
| 8–15 years | 5.44 (2.00), 336 | 4.98 (1.93), 329 | 5.21 (1.97), 665 |
| PROMIS upper extremity limb, mean (SD), nb | |||
| Overall | 25.0 (6.3), 489 | 25.6 (7.7), 476 | 25.3 (7.1), 965 |
| 4–7 years | 23.0 (6.5), 153 | 24.0 (7.6), 147 | 23.5 (7.0), 300 |
| 8–15 years | 25.8 (6.0), 336 | 26.3 (7.7), 329 | 26.1 (6.9), 665 |
| EQ-5D-Y utility, mean (SD), nc | |||
| Overall | 0.53 (0.34), 489 | 0.56 (0.34), 476 | 0.54 (0.34), 965 |
| 4–7 years | 0.51 (0.34), 153 | 0.59 (0.32), 147 | 0.55 (0.33), 300 |
| 8–15 years | 0.54 (0.35), 336 | 0.54 (0.35), 329 | 0.54 (0.35), 665 |
| EQ-5D-Y VAS, mean (SD), nd | |||
| Overall | 72.7 (22.6), 489 | 73.1 (22.7), 476 | 72.9 (22.6), 965 |
| 4–7 years | 80.5 (20.2), 153 | 79.3 (21.4), 147 | 79.9 (20.8), 300 |
| 8–15 years | 69.2 (22.9), 336 | 70.4 (22.7), 329 | 69.8 (22.8), 665 |
Primary outcome
Wong–Baker Scale scores at day 3
The primary outcome in the FORCE trial was the Wong–Baker Scale score at 3 days post randomisation. The Wong–Baker Scale scores at day 3 were similar in the offer of a bandage group (mean = 3.21, SD = 2.08) and the rigid immobilisation group (mean = 3.14, SD = 2.11). The trial was designed to test the equivalence of the two treatments in terms of the Wong–Baker Scale score, with a prespecified equivalence margin of 1 point. Comparisons between the two groups in both the ITT population (adjusted difference = –0.10, 95% CI –0.37 to 0.17) and the PP population (adjusted difference = –0.06, 95% CI –0.34 to 0.21) indicated that any difference between the two treatments was less than the prespecified 1 point on the Wong–Baker Scale and, therefore, equivalence was concluded (Table 11). The trial was also powered to separately assess equivalence in the two age groups. The results of these comparisons are also presented in Table 11 and indicate equivalence of the two treatments in both of these subgroups. Similarly, the prespecified PP analyses also demonstrate effect estimates that are well within the equivalence margins. These results are presented graphically in Figure 4, where they are compared with the equivalence margin.
| Age group | Mean (SD) scorea, N | Unadjusted difference (95% CI) | Adjusted difference (95% CI) | |
|---|---|---|---|---|
| Offer of a bandage group | Rigid immobilisation group | |||
| Overall | ||||
| ITT | 3.21 (2.08), 466 | 3.14 (2.11), 442 | –0.07 (–0.34 to 0.21) | –0.10 (–0.37 to 0.17) |
| PP | 3.17 (2.04), 428 | 3.14 (2.11), 442 | –0.03 (–0.31 to 0.24) | –0.06 (–0.34 to 0.21) |
| 4–7 years | ||||
| ITT | 2.78 (2.10), 147 | 2.85 (2.07), 139 | 0.07 (–0.41 to 0.56) | 0.05 (–0.44 to 0.53) |
| PP | 2.70 (2.02), 132 | 2.85 (2.07), 139 | 0.15 (–0.34 to 0.64) | 0.13 (–0.36 to 0.62) |
| 8–15 years | ||||
| ITT | 3.40 (2.04), 319 | 3.27 (2.12), 303 | –0.13 (–0.46 to 0.20) | –0.16 (–0.48 to 0.16) |
| PP | 3.39 (2.01), 296 | 3.27 (2.12), 303 | –0.11 (–0.44 to 0.22) | –0.14 (–0.46 to 0.19) |
FIGURE 4.
Day 3 Wong–Baker Scale score treatment effects compared with equivalence margin (dashed lines).
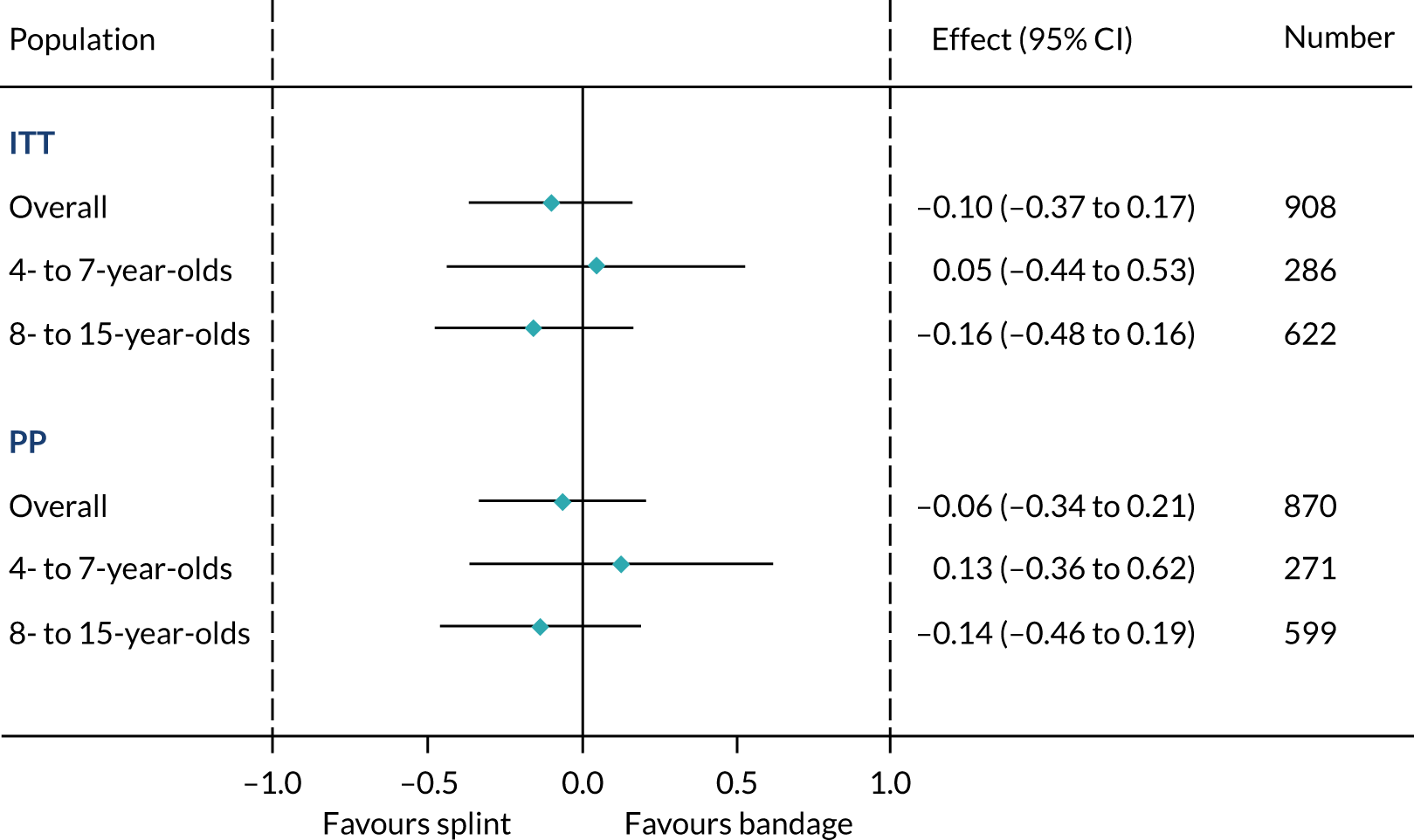
Wong–Baker Scale score from day 1 to week 6
In addition to the scores at 3 days post randomisation, the Wong–Baker Scale score was also collected at baseline and at days 1 and 7 and weeks 3 and 6. Scores are summarised by treatment group in Table 12. The Wong–Baker Scale scores over time are summarised separately for the two age groups in Figure 5, which demonstrates that, in the first few weeks after the injury, pain scores are slightly higher in the older age group (regardless of treatment received); however, this difference has disappeared by week 6. Adjusted differences between the two groups at each time point are also provided in Table 12, along with associated 95% CIs. All of the effect estimates and 95% CIs lie within the prespecified equivalence margin (1 point). AUCs up to day 3 post randomisation and up to week 6 post randomisation were also calculated for each group and compared. A statistically significant difference between the two groups in terms of AUC up to 3 days post randomisation is identified; however, it is clear that this value is not clinically significant.
| Time point | Wong–Baker Scale score, mean (SD), N | Adjusted difference (95% CI) | |
|---|---|---|---|
| Offer of a bandage group | Rigid immobilisation group | ||
| ITT population | |||
| Day 0a | 5.21 (2.32), 489 | 4.91 (2.10), 476 | – |
| Day 1a | 4.29 (2.25), 408 | 3.94 (2.13), 382 | –0.36 (–0.61 to -0.12) |
| Day 3a | 3.21 (2.08), 466 | 3.14 (2.11), 442 | –0.09 (–0.32 to 0.14) |
| Day 7a | 2.32 (1.81), 459 | 2.12 (1.68), 439 | –0.21 (–0.44 to 0.02) |
| Week 3a | 0.81 (1.32), 432 | 0.87 (1.39), 429 | 0.04 (–0.20 to 0.27) |
| Week 6a | 0.27 (0.81), 436 | 0.24 (0.77), 431 | –0.05 (–0.28 to 0.19) |
| AUC (to day 3)b | 11.74 (11.13 to 12.36) | 10.91 (10.27 to 11.56) | 0.83 (0.16 to 1.50), 0.02 |
| AUC (to week 6)b | 50.80 (44.35 to 57.25) | 48.09 (41.42 to 54.76) | 2.71 (–4.31 to 9.74), 0.45 |
| PP population | |||
| Day 0a | 5.15 (2.33), 428 | 4.86 (2.11), 442 | – |
| Day 1a | 4.15 (2.16), 371 | 3.94 (2.13), 378 | –0.22 (–0.47 to 0.03) |
| Day 3a | 3.17 (2.04), 428 | 3.14 (2.11), 442 | –0.05 (–0.29 to 0.18) |
| Day 7a | 2.29 (1.81), 415 | 2.13 (1.70), 428 | –0.17 (–0.41 to 0.07) |
| Week 3a | 0.81 (1.35), 398 | 0.87 (1.40), 422 | 0.06 (–0.19 to 0.30) |
| Week 6a | 0.27 (0.79), 398 | 0.24 (0.77), 422 | –0.04 (–0.28 to 0.20) |
FIGURE 5.
Wong–Baker Scale score by treatment group and age group from baseline to 6 weeks post randomisation (ITT population).
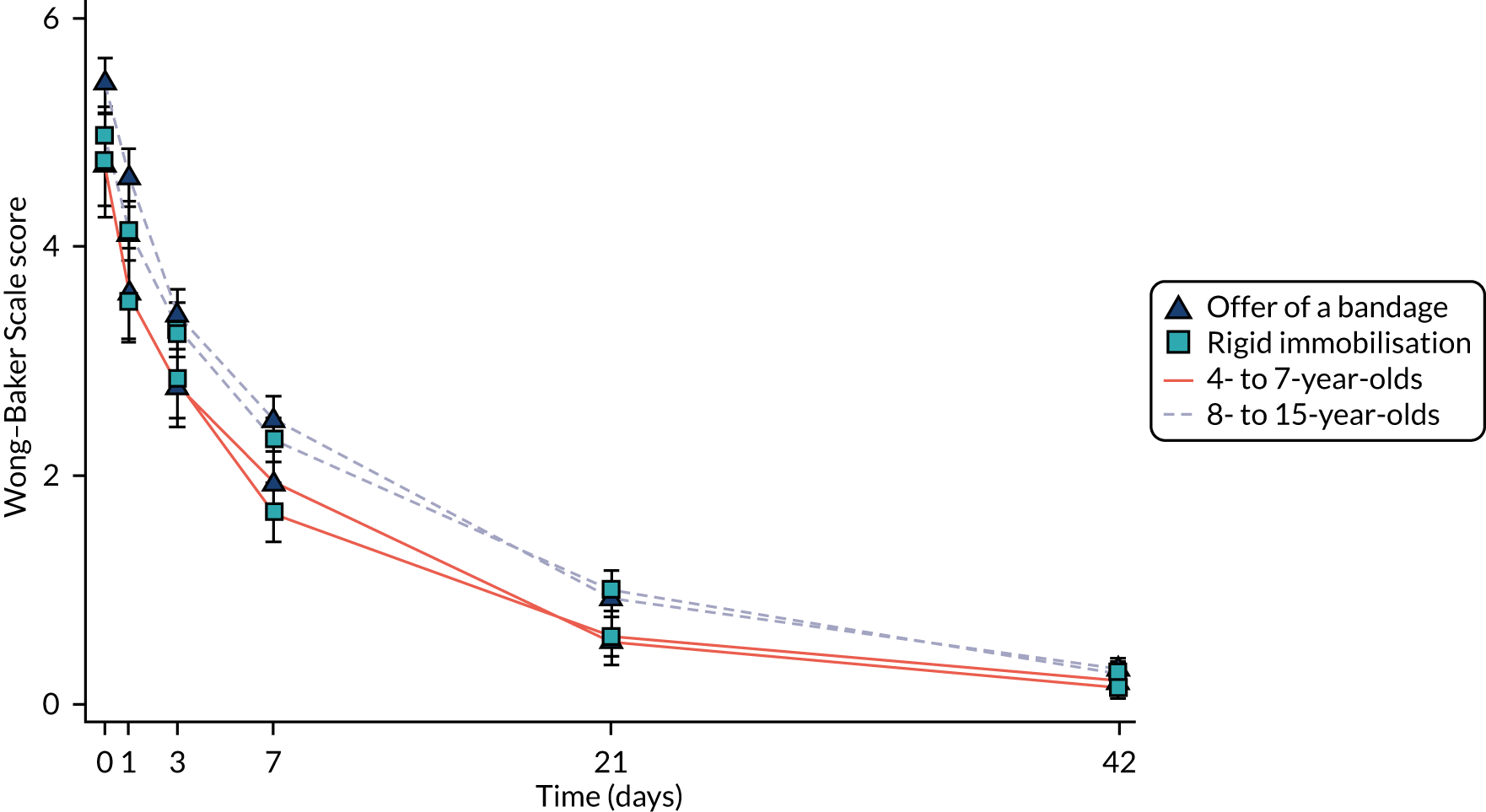
Secondary outcomes
Patient-reported outcome measures
Patient-reported outcomes measurement system scores from baseline to week 6 for the ITT population are summarised by treatment group overall and for each age group in Table 13. Trends over time are summarised graphically for each treatment group in each age group in Figure 6. In all groups, the scores increase over time, with a particularly marked increase between day 7 and week 3. Scores were, in general, slightly higher in the older age group than in the younger one. Comparisons between the two treatment groups at each time point overall and for each age group are presented in Table 13. No significant differences between the two groups were identified. This analysis was also repeated for the PP population (see Appendix 4, Table 35).
| Time point | Offer of a bandage group | Rigid immobilisation group | Adjusted difference (95% CI) | p-value |
|---|---|---|---|---|
| PROMIS score, mean (SD), na | ||||
| Overall | ||||
| Baseline | 25.0 (6.3), 489 | 25.6 (7.7), 476 | – | – |
| Day 3 | 28.4 (7.8), 462 | 27.8 (7.9), 441 | –0.50 (–1.58 to 0.57) | 0.36 |
| Day 7 | 34.7 (9.9), 456 | 34.5 (9.2), 437 | –0.12 (–1.20 to 0.96) | 0.82 |
| Week 3 | 46.6 (10.1), 431 | 46.3 (10.1), 426 | –0.26 (–1.36 to 0.83) | 0.64 |
| Week 6 | 52.8 (7.3), 434 | 52.6 (7.5), 428 | –0.20 (–1.29 to 0.90) | 0.72 |
| 4–7 years group | ||||
| Baseline | 23.0 (6.5), 153 | 24.0 (7.6), 147 | – | – |
| Day 3 | 27.1 (7.1), 145 | 27.0 (7.9), 138 | –0.31 (–2.23 to 1.60) | 0.75 |
| Day 7 | 33.6 (8.9), 144 | 34.2 (9.1), 137 | 0.69 (–1.23 to 2.62) | 0.48 |
| Week 3 | 44.6 (9.8), 138 | 44.9 (9.6), 134 | 0.32 (–1.63 to 2.27) | 0.75 |
| Week 6 | 49.8 (8.3), 142 | 50.9 (7.4), 136 | 0.97 (–0.96 to 2.90) | 0.33 |
| 8–15 years group | ||||
| Baseline | 25.8 (6.0), 336 | 26.3 (7.7), 329 | – | – |
| Day 3 | 28.9 (8.1), 317 | 28.2 (7.8), 303 | –0.57 (–1.86 to 0.72) | 0.39 |
| Day 7 | 35.2 (10.3), 312 | 34.7 (9.2), 300 | –0.48 (–1.78 to 0.82) | 0.47 |
| Week 3 | 47.5 (10.1), 293 | 46.9 (10.3), 292 | –0.52 (–1.84 to 0.80) | 0.44 |
| Week 6 | 54.2 (6.2), 292 | 53.4 (7.4), 292 | –0.75 (–2.08 to 0.57) | 0.27 |
| EQ-5D-Y utility score, mean (SD), nb | ||||
| Overall | ||||
| Baseline | 0.53 (0.34), 489 | 0.56 (0.34), 476 | – | – |
| Day 3 | 0.56 (0.27), 459 | 0.55 (0.27), 441 | –0.01 (–0.04 to 0.02) | 0.43 |
| Day 7 | 0.71 (0.23), 456 | 0.69 (0.24), 435 | –0.01 (–0.04 to 0.02) | 0.53 |
| Week 3 | 0.89 (0.16), 430 | 0.89 (0.16), 426 | –0.01 (–0.04 to 0.02) | 0.65 |
| Week 6 | 0.97 (0.10), 434 | 0.96 (0.10), 428 | –0.00 (–0.04 to 0.03) | 0.82 |
| 4–7 years age group | ||||
| Baseline | 0.51 (0.34), 153 | 0.59 (0.32), 147 | – | – |
| Day 3 | 0.60 (0.26), 145 | 0.57 (0.26), 139 | –0.04 (–0.09 to 0.02) | 0.20 |
| Day 7 | 0.73 (0.23), 144 | 0.74 (0.23), 137 | 0.01 (–0.05 to 0.06) | 0.77 |
| Week 3 | 0.91 (0.18), 138 | 0.91 (0.14), 134 | 0.00 (–0.05 to 0.06) | 0.90 |
| Week 6 | 0.97 (0.11), 142 | 0.97 (0.09), 136 | 0.00 (–0.05 to 0.06) | 0.88 |
| 8–15 years age group | ||||
| Baseline | 0.54 (0.35), 336 | 0.54 (0.35), 329 | – | – |
| Day 3 | 0.55 (0.28), 314 | 0.54 (0.27), 302 | –0.00 (–0.04 to 0.03) | 0.89 |
| Day 7 | 0.70 (0.23), 312 | 0.67 (0.24), 298 | –0.02 (–0.06 to 0.02) | 0.32 |
| Week 3 | 0.89 (0.15), 292 | 0.87 (0.17), 292 | –0.01 (–0.05 to 0.03) | 0.50 |
| Week 6 | 0.97 (0.09), 292 | 0.96 (0.11), 292 | –0.01 (–0.05 to 0.03) | 0.66 |
| EQ-5D-Y VAS score, mean (SD), nc | ||||
| Overall | ||||
| Baseline | 72.7 (22.6), 489 | 73.1 (22.7), 476 | – | – |
| Day 3 | 76.8 (19.5), 458 | 75.5 (19.8), 437 | –1.00 (–3.40 to 1.41) | 0.42 |
| Day 7 | 82.9 (19.5), 451 | 83.2 (17.7), 435 | 0.27 (–2.15 to 2.69) | 0.83 |
| Week 3 | 91.3 (16.2), 430 | 90.3 (16.3), 425 | –1.06 (–3.52 to 1.39) | 0.40 |
| Week 6 | 94.1 (15.2), 433 | 94.3 (15.9), 426 | 0.28 (–2.16 to 2.73) | 0.82 |
| 4–7 years age group | ||||
| Baseline | 80.5 (20.2), 153 | 79.3 (21.4), 147 | – | – |
| Day 3 | 79.5 (20.0), 145 | 80.9 (18.5), 138 | 1.28 (–2.98 to 5.54) | 0.56 |
| Day 7 | 87.3 (16.7), 142 | 88.6 (16.1), 137 | 1.14 (–3.14 to 5.42) | 0.60 |
| Week 3 | 95.5 (8.5), 138 | 94.2 (10.9), 133 | –1.51 (–5.85 to 2.82) | 0.49 |
| Week 6 | 96.4 (9.1), 142 | 96.2 (12.9), 136 | –0.43 (–4.72 to 3.86) | 0.84 |
| 8–15 years age group | ||||
| Baseline | 69.2 (22.9), 336 | 70.4 (22.7), 329 | – | – |
| Day 3 | 75.6 (19.1), 313 | 73.0 (19.9), 299 | –2.19 (–5.08 to 0.70) | 0.14 |
| Day 7 | 80.8 (20.4), 309 | 80.7 (17.9), 298 | –0.26 (–3.16 to 2.64) | 0.86 |
| Week 3 | 89.3 (18.5), 292 | 88.5 (17.9), 292 | –0.96 (–3.91 to 1.98) | 0.52 |
| Week 6 | 93.0 (17.3), 291 | 93.4 (17.1), 290 | 0.46 (–2.50 to 3.41) | 0.76 |
| Satisfaction, median (IQR), nd | ||||
| Overall | ||||
| Day 1 | 2 (1–2), 406 | 1 (1–2), 380 | – | < 0.001 |
| Week 6 | 1 (1–2), 433 | 1 (1–2), 425 | – | 0.12 |
FIGURE 6.
The PROMIS scores by treatment group and age group from baseline to 6 weeks post randomisation (ITT population).
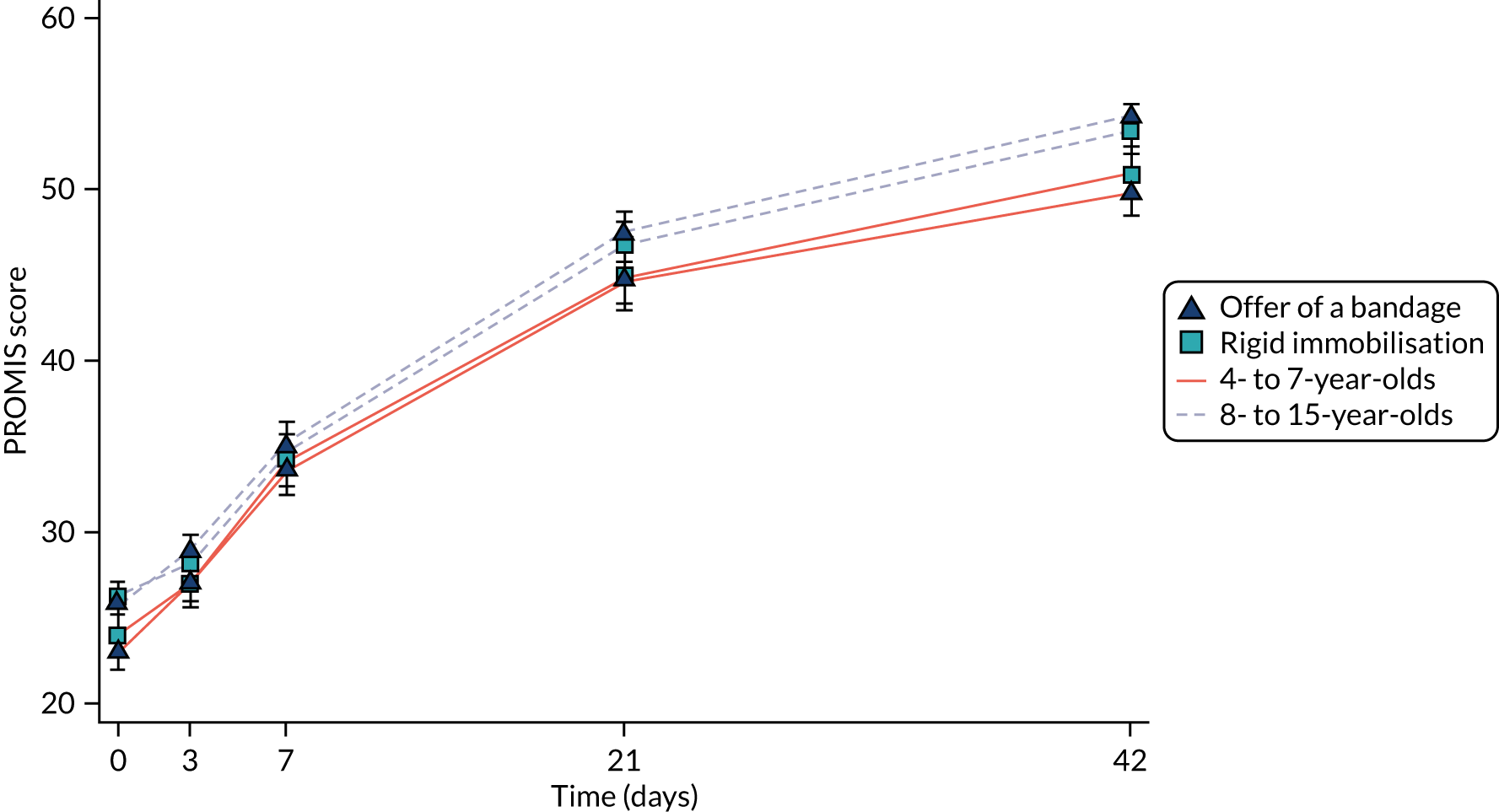
The EQ-5D-Y utility scores from baseline to week 6 for the ITT population are summarised by treatment group overall and for each age group in Table 13. Trends over time are summarised graphically for each treatment group in each age group in Figure 7. In all groups, the scores increased over time. There is little difference between the two age subgroups. Comparisons between the two treatment groups at each time point overall and for each age group are presented in Table 13. No significant differences between the two groups were identified. This analysis was also repeated for the PP population (see Appendix 4, Table 35).
FIGURE 7.
The EQ-5D-Y utility scores by treatment group and age group from baseline to 6 weeks post randomisation (ITT population).
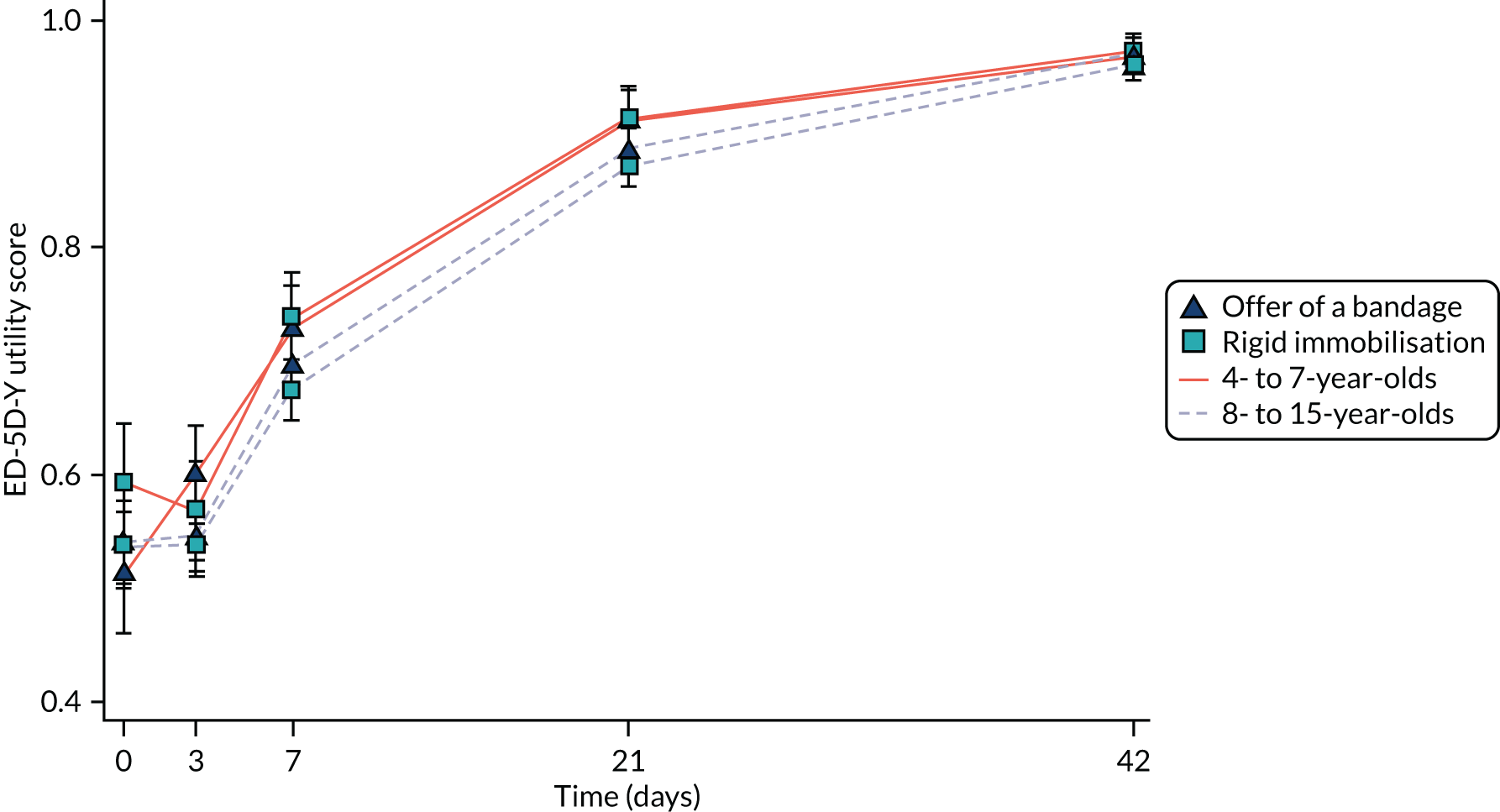
The EQ-5D-Y VAS scores from baseline to week 6 for the ITT population are summarised by treatment group overall and for each age group in Table 13. Trends over time are summarised graphically for each treatment group in each age group in Figure 8. In all groups, the scores increased over time. Scores were consistently higher in the younger age group than in the older age group. Comparisons between the two treatment groups at each time point overall and for each age group are presented in Table 13. No significant differences between the two groups were identified. This analysis was also repeated for the PP population (see Appendix 4, Table 35).
FIGURE 8.
The EQ-5D-Y VAS scores by treatment group and age group from baseline to 6 weeks post randomisation (ITT population).
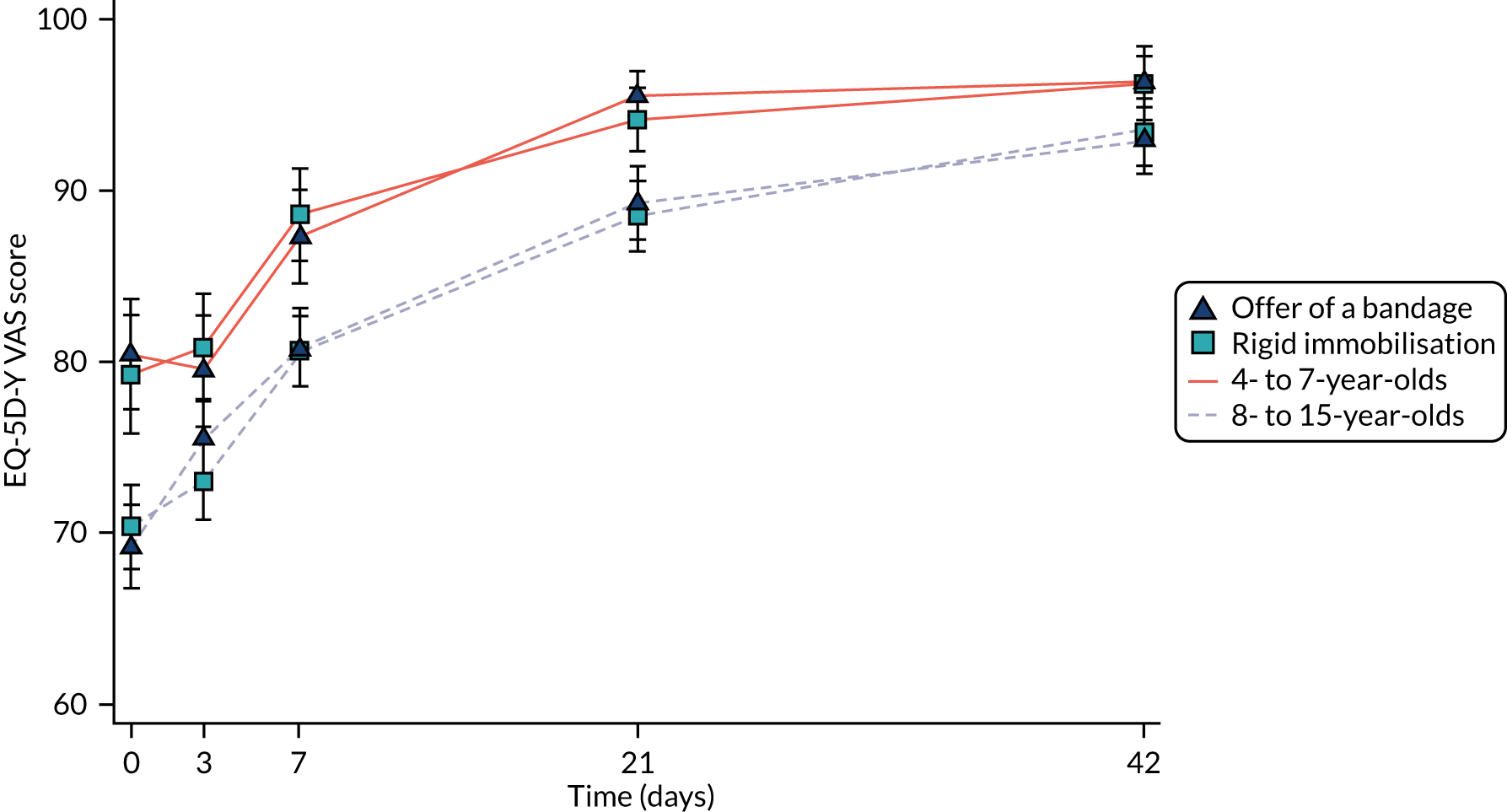
Satisfaction scores at day 1 and at week 6 are summarised separately by treatment group for the ITT population in Table 13. Overall satisfaction with the treatment received was very high, with a score of 1 representing ‘extremely satisfied’ and a score of 2 representing ‘very satisfied’. Mann–Whitney U-tests were used to compare the two groups at each time point. At day 1, satisfaction was significantly higher in the rigid immobilisation group than in the offer of a bandage group; however, by week 6 there was no significant difference between the groups (see Table 13). These analyses were repeated for the PP population and the results were similar (see Appendix 4, Table 35).
Receipt of pain medication
The numbers and percentages of participants who, at days 1, 3 and 7 post randomisation, had received pain medication in the previous 24 hours are summarised by treatment group in Table 14. The proportion of participants receiving pain medication decreased from 80% in the first 24 hours to only 25% by day 7. The proportion of participants receiving pain medication was larger in the offer of a bandage group than in the rigid immobilisation group at each time point. Comparisons between the two groups are reported at each time point (see Table 14). The difference between the two groups of the ITT population was significant at day 1 (OR 0.53, 95% CI 0.28 to 0.98; p = 0.04). No significant differences between the two groups were identified in the PP population (see Appendix 4, Table 36).
| Time point | Number in receipt of pain medication, n/N (%) | OR (95% CI); p-value | RD (%) (95% CI) | |
|---|---|---|---|---|
| Offer of a bandage group | Rigid immobilisation group | |||
| Overall | ||||
| Day 1 | 337/408 (82.6) | 297/382 (77.7) | 0.53 (0.28 to 0.98); 0.04 | –5.8 (–11.8 to 0.3) |
| Day 3 | 264/465 (56.8) | 227/442 (51.4) | 0.60 (0.36 to 0.99); 0.05 | –6.1 (–11.8 to –0.4) |
| Day 7 | 116/459 (25.3) | 100/439 (22.8) | 0.70 (0.40 to 1.22); 0.21 | –3.6 (–9.3 to 2.1) |
| 4–7 years age group | ||||
| Day 1 | 98/129 (76.0) | 95/123 (77.2) | 1.16 (0.41 to 3.33); 0.78 | 0.1 (–10.6 to 10.8) |
| Day 3 | 66/147 (44.9) | 70/139 (50.4) | 1.38 (0.56 to 3.40); 0.49 | 4.7 (–5.4 to 14.8) |
| Day 7 | 22/144 (15.3) | 22/137 (16.1) | 0.83 (0.27 to 2.52); 0.74 | –0.3 (–10.5 to 9.8) |
| 8–15 years age group | ||||
| Day 1 | 239/279 (85.7) | 202/259 (78.0) | 0.36 (0.17 to 0.79); 0.01 | –8.4 (–15.7 to –1.1) |
| Day 3 | 198/318 (62.3) | 157/303 (51.8) | 0.41 (0.22 to 0.76); 0.005 | –10.9 (–17.8 to –4.1) |
| Day 7 | 94/315 (29.8) | 78/302 (25.8) | 0.64 (0.33 to 1.24); 0.19 | –4.9 (–11.8 to 2.0) |
The proportion of participants receiving pain medication is also summarised separately for the two age groups in Table 14. More participants in the older age group than in the younger age group were receiving pain medication at each time point. Comparisons between the two treatment groups are also reported for each age group (see Table 14). No significant differences between the two treatment groups were identified for the younger age group; however, in the older age group, more participants in the offer of a bandage group than in the rigid immobilisation group were receiving pain medication at days 1 and 3.
The types of pain medication received at each time point are reported for the overall population in Table 15. Almost all the medication received was either paracetamol (approximately 80% of participants receiving pain medication at each time point) or ibuprofen (approximately 50% of participants receiving pain medication at each time point). Participants could report more than one type of medication at each time point.
| Type of pain medication | Offer of a bandage group, n/N (%) | Rigid immobilisation group, n/N (%) |
|---|---|---|
| Day 1 | ||
| Paracetamol | 275/337 (81.6) | 238/297 (80.1) |
| Ibuprofen | 183/337 (54.3) | 147/297 (49.5) |
| Other | 0/337 (0.0) | 1/297 (0.3) |
| Day 3 | ||
| Paracetamol | 205/264 (77.7) | 180/227 (79.3) |
| Ibuprofen | 139/264 (52.7) | 107/227 (47.1) |
| Other | 0/264 (0.0) | 0/227 (0.0) |
| Day 7 | ||
| Paracetamol | 91/116 (78.4) | 75/100 (75.0) |
| Ibuprofen | 53/116 (45.7) | 46/100 (46.0) |
| Other | 1/116 (0.9) | 0/100 (0.0) |
Wong–Baker Scale scores by pain medication status
Wong–Baker Scale scores at day 3 are summarised separately by treatment group for those who did and did not report taking pain medication in the preceding 24 hours in Table 16. Pain scores were higher among those who reported taking pain medication in the past 24 hours than among those who did not. The treatment effect estimates and associated 95% CIs all lie within the prespecified equivalence margin of 1 point. Summaries by treatment group and effect estimates and 95% CIs are also presented separately for the two age groups in Table 16. All effect estimates and CIs lie within the equivalence margin.
| Medication status | Offer of a bandage group,a mean (SD), n | Rigid immobilisation group,a mean (SD), n | Adjusted difference (95% CI) |
|---|---|---|---|
| Overall | |||
| Pain medication in last 24 hours | 3.96 (2.15), 264 | 3.87 (2.19), 227 | –0.11 (–0.45 to 0.23) |
| No pain medication in last 24 hours | 2.22 (1.48), 201 | 2.37 (1.72), 215 | 0.12 (–0.25 to 0.49) |
| 4–7 years age group | |||
| Pain medication in last 24 hours | 3.76 (2.34), 66 | 3.54 (2.31), 70 | –0.26 (–0.91 to 0.38) |
| No pain medication in last 24 hours | 1.98 (1.47), 81 | 2.14 (1.51), 69 | 0.17 (–0.45 to 0.78) |
| 8–15 years age group | |||
| Pain medication in last 24 hours | 4.03 (2.09), 198 | 4.01 (2.12), 157 | –0.04 (–0.45 to 0.36) |
| No pain medication in last 24 hours | 2.38 (1.47), 120 | 2.48 (1.81), 146 | 0.09 (–0.37 to 0.55) |
Complications
Overall, only eight participants experienced a complication during the trial, and these were relatively evenly split between the two treatment groups (Table 17). Seven of the complications were due to an alternative fracture being identified, with one caused by another reason. Details of the types of alternative fractures are also provided in Table 17.
| Complications and reasons | Offer of a bandage group (N = 489), n (%)a | Rigid immobilisation group (N = 476), n (%) |
|---|---|---|
| Any complications | 5 (1.0) | 3 (0.6) |
| Reason | ||
| Change of diagnosis (alternative fracture) | 4 (0.8) | 3 (0.6) |
| Otherb | 1 (0.2) | 0 (0.0) |
| Details of alternative fracture | ||
| Greenstick | 1 (0.2) | 1 (0.2) |
| Complete fracture – remains undisplaced | 3 (0.6) | 2 (0.4) |
School absence
The number and proportion of participants reporting school absence up to week 3 are summarised by treatment group in Table 18 and Appendix 4, Table 37. Around 24% of participants missed some school in the first 3 weeks post randomisation. Comparisons between the two treatment groups identified no significant differences in the proportions missing school. For participants who missed school, the average number of days missed was similar in both treatment groups at 1.5 days. These analyses were performed for both the ITT (see Table 18) and PP (see Appendix 4, Table 37) populations with similar results.
| Details of school absence | Offer of a bandage group (N = 430) | Rigid immobilisation group (N = 425) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Number of participants missing any days of school, n (%) | 112 (26.0) | 93 (21.9) | 0.79 (0.57 to 1.08) | 0.14 |
| Number of days of school missed,a median (IQR), (minimum, maximum), n | 1.5 (1–2), (0.5, 5), 112 | 1.5 (1–2), (0.5, 8), 93 | – | 0.37 |
Audit results
Fracture diagnoses performed during the screening of randomised patients were compared with the formal medical report. Twelve recruitment centres completed the audit, including 218 randomised participants. In 84% (95% CI 80% to 89%) of participants, the clinician confirming eligibility and the reporting radiologist agreed that there was a torus fracture. There was disagreement in 16% of cases: 7% (95% CI 4% to 10%) were reported to the radiologist to have ‘no fracture’, 7% (95% CI 4% to 10%) were reported as a greenstick fracture, 1% (95% CI 0% to 3%) were reported as a Salter–Harris II fracture, and 0.5% (95% CI 0% to 1%) were reported to have an unspecified ‘fracture’.
Health economic results
Use of health resource and data completeness
Health resource by treatment group is shown for the period from treatment to 3 weeks post randomisation in Table 19 and for the period from 3 to 6 weeks post randomisation in Table 20. Table 21 shows the response rate for resource use and EQ-5D-Y by follow-up points and treatment group.
| Type of care | Offer of a bandage group (N = 456) | Rigid immobilisation group (N= 443) | ||||
|---|---|---|---|---|---|---|
| Number reporting use of resource | Number reporting no use of resource | Missing, n (%) | Number reporting use of resource | Number reporting no use of resource | Missing, n (%) | |
| Outpatient care | ||||||
| Orthopaedic clinic (due to wrist injury) | 15 | 423 | 18 (4.0) | 31 | 395 | 17 (3.8) |
| Radiology (X-rays) | 5 | 434 | 17 (3.7) | 3 | 427 | 13 (2.9) |
| Physiotherapy | 0 | 440 | 16 (3.5) | 0 | 430 | 13 (2.9) |
| Emergency department | 19 | 420 | 17 (3.7) | 11 | 420 | 12 (2.7) |
| Community care (NHS) | ||||||
| General practitioner (surgery) | 7 | 430 | 19 (4.2) | 4 | 427 | 12 (2.7) |
| General practitioner (telephone/e-mail contact) | 1 | 438 | 17 (3.7) | 2 | 429 | 12 (2.7) |
| Practice nurse | 1 | 439 | 16 (3.5) | 0 | 431 | 12 (2.7) |
| District nurse | 0 | 440 | 16 (3.5) | 0 | 431 | 12 (2.7) |
| Physiotherapist | 1 | 439 | 16 (3.5) | 0 | 431 | 12 (2.7) |
| 111 advice | 1 | 439 | 16 (3.5) | 2 | 429 | 12 (2.7) |
| Community care (private) | ||||||
| Physiotherapy | 0 | 440 | 16 (3.5) | 0 | 431 | 12 (2.7) |
| Direct non-medical cost | 35 | 391 | 30 (6.6) | 37 | 387 | 19 (4.3) |
| Help with child care | 3 | 437 | 16 (3.5) | 2 | 429 | 12 (2.7) |
| Lost productivity | 32 | 408 | 16 (3.5) | 35 | 396 | 12 (2.7) |
| Type of care | Offer of a bandage group (N = 456) | Rigid immobilisation group (N = 443) | ||||
|---|---|---|---|---|---|---|
| Number reporting use of resource | Number reporting no use of resource | Missing, n (%) | Number reporting use of resource | Number reporting no use of resource | Missing, n (%) | |
| Outpatient care | ||||||
| Orthopaedic clinic (due to wrist injury) | 7 | 426 | 23 (5.0) | 4 | 424 | 15 (3.4) |
| Radiology (X-rays) | 1 | 436 | 19 (4.2) | 1 | 430 | 12 (2.7) |
| Physiotherapy | 1 | 435 | 20 (4.4) | 0 | 431 | 12 (2.7) |
| Emergency department | 3 | 434 | 19 (4.2) | 1 | 430 | 12 (2.7) |
| Community care (NHS) | ||||||
| General practitioner (surgery) | 0 | 436 | 20 (4.4) | 4 | 427 | 12 (2.7) |
| General practitioner (telephone/e-mail contact) | 1 | 436 | 19 (4.2) | 0 | 431 | 12 (2.7) |
| Practice nurse | 0 | 437 | 19 (4.2) | 0 | 431 | 12 (2.7) |
| District nurse | 0 | 437 | 19 (4.2) | 0 | 431 | 12 (2.7) |
| Physiotherapist | 1 | 436 | 19 (4.2) | 2 | 429 | 12 (2.7) |
| 111 advice | 1 | 436 | 19 (4.2) | 0 | 431 | 12 (2.7) |
| Community care (private) | ||||||
| Physiotherapy | 0 | 437 | 19 (4.2) | 0 | 431 | 12 (2.7) |
| Direct non-medical cost | 13 | 420 | 23 (5.0) | 14 | 409 | 20 (4.5) |
| Help with child care | 1 | 436 | 19 (4.2) | 2 | 429 | 12 (2.7) |
| Lost productivity | 12 | 425 | 19 (4.2) | 12 | 419 | 12 (2.7) |
| Time point | Percentage of missing responses | |
|---|---|---|
| Offer of a bandage group (N = 489) | Rigid immobilisation group (N = 476) | |
| Baseline | 0.00 | 0.00 |
| Day 3 | 6.13 | 7.35 |
| Day 7 | 6.75 | 8.61 |
| Week 3 | 12.07 | 10.50 |
| Week 6 | 11.25 | 10.08 |
| QALY (AUC) | 16.36 | 15.76 |
Health-care resource use and costs
Information on the type of interventions and the time to apply them was collected by a survey sent to recruitment centres.
Rigid immobilisation
The splints used within the study were Actimove® Manus Wrist Brace (BSN medical GmbH, Hamburg, Germany), Promedics Neoprene Wrist Thumb Splint (Promedics Orthopaedics Ltd, Glasgow, UK), Beagle Orthopaedic Paediatric D-ring Wrist Brace (Beagle Orthopaedic, Blackburn, UK), Promedics Wrist Brace, Promedics Standard Neobrace, Provectus Medical Ltd one-size wrist brace (Nelson, UK), Deltaform Futuro splint (Promedics Orthopaedics Ltd, Glasgow, UK), 3M soft cast (3M United Kingdom PLC, Bracknell, UK), BeneCare Universal Wrist Splint (BeneCare Direct, Manchester, UK) or Neoprene wrist brace. The application time for the splint varied from 24 seconds to 10 minutes (median 2 minutes) and time to explain the treatment to the child/parent varied from 30 seconds to 10 minutes. The application time for the backslab varied from 5 to 10 minutes, for the soft cast this varied from 4 to 10 minutes, for the hard cast this took 10 minutes and for the split hard cast this was 15 minutes, whereas the time to explain the treatment to the child/parent varied from 2 to 30 minutes. In the case of backslab, we assumed that patients had two plaster of Paris bandages BP (either a 7.5 cm × 2.7 m roll or a 5 cm × 2.7 m roll) and a synthetic undercast padding (either 5 cm × 2.7 m or 7.5 cm × 2.7 m). For hard cast, we assumed that patients had two fibreglass casting tapes (either 7.5 cm × 3.6 m or 5 cm × 3.6 m) and a synthetic undercast padding (either 5 cm × 2.7 m or 7.5 cm × 2.7 m). For the soft cast, we assumed that patients had a flexible casting tape (either 5 cm × 3.6 m or 7.5 cm × 3.6 m) and a synthetic undercast padding (either 5 cm × 2.7 m or 7.5 cm × 2.7 m). The unit costs of the different methods of rigid immobilisation are presented in Table 22.
| Resource item | Rigid immobilisation group | Offer of a bandage group | Unit type | Unit cost (£) |
|---|---|---|---|---|
| Futura splint | ✓ | ✗ | Item | 6.05 |
| Backslab | ✓ | ✗ | Item | 1.50 |
| Soft cast | ✓ | ✗ | Item | 4.97 |
| Hard cast | ✓ | ✗ | Item | 5.82 |
| Soft gauze bandage | ✗ | ✓ | Item | 0.78 |
Offer of a bandage
Participants randomised to receive the offer of a soft bandage were given a K-Band Urgo Type 1 Conforming Bandage (Urgo Medical UK, Loughborough, UK) in small, large or Hospiform® Elastic Conforming Bandage (Hartmann Limited, Heywood, UK), Ce-Fix Conforming Bandage (Richardson Healthcare Ltd, Hertfordshire, UK), Urgo K-Lite, Mölnlycke Tubigrip (Mölnlycke Health Care AB, Gothenburg, Sweden) or Hospicrepe® 233 type 2 cotton crepe bandage (Hartmann Limited, Heywood, UK). The application time for the soft bandage varied from 30 seconds to 10 minutes (median 2 minutes) and the time to explain the treatment to the child/parent also varied from 30 seconds to 10 minutes. We based the cost of delivering the soft bandage treatment on the median delivery time of application (as the median is more robust against outliers) and the average cost per hour for each clinician delivering the treatment. The unit cost of the soft bandage was calculated as the median of the aforementioned types of soft bandage cost (see Appendix 6, Table 42). The total cost of soft bandage treatment to each participant was calculated by summing the mean administration and soft bandage costs (see Table 22 for the calculated cost of the soft bandage).
Information about the use of other relevant NHS services was obtained by participant self-reported data at week 3 and 6. Estimates of health-care use have been presented based on the two reporting time periods for resource use: post treatment to 3 weeks (Table 23) and 3 to 6 weeks post randomisation (Table 24). These resource quantities were multiplied by the relevant unit cost (see Appendix 6, Table 41) to provide the estimated mean costs per patient from treatment to 3 weeks post randomisation (Table 25) and from 3 to 6 weeks post randomisation (Table 26).
| Type of care | Offer of a bandage group, mean (SE), n | Rigid immobilisation group, mean (SE), n | Difference | Bootstrap (95% CI) | p-valuea |
|---|---|---|---|---|---|
| Outpatient care | |||||
| Orthopaedic clinic (due to wrist injury) | 0.037 (0.009), 438 | 0.085 (0.015), 426 | –0.048 | –0.084 to -0.012 | 0.009 |
| Radiology (X-rays) | 0.011 (0.005), 439 | 0.009 (0.006), 430 | 0.002 | –0.013 to 0.016 | 0.783 |
| Physiotherapy | 0 (0), 440 | 0 (0), 430 | 0 | – | – |
| Emergency department | 0.048 (0.011), 439 | 0.032 (0.010), 431 | 0.015 | –0.014 to 0.044 | 0.306 |
| Community care (NHS) | |||||
| General practitioner (surgery) | 0.016 (0.006), 437 | 0.009 (0.005), 431 | 0.007 | –0.001 to 0.021 | 0.397 |
| General practitioner (telephone/e-mail contact) | 0.002 (0.002), 439 | 0.007 (0.005), 431 | –0.005 | –0.015 to 0.007 | 0.416 |
| Practice nurse | 0.002 (0.002), 440 | 0 (0), 431 | 0.002 | –0.002 to 0.007 | 0.338 |
| District nurse | 0 (0), 440 | 0 (0), 431 | 0 | – | – |
| Physiotherapist | 0 (0), 440 | 0 (0), 431 | – | – | |
| 111 advice | 0.002 (0.002), 440 | 0.005 (0.003), 431 | –0.002 | –0.010 to 0.005 | 0.548 |
| Type of care | Offer of a bandage group, mean (SE), n | Rigid immobilisation group, mean (SE), n | Difference | Bootstrap (95% CI) | p-valuea |
|---|---|---|---|---|---|
| Outpatient care | |||||
| Orthopaedic clinic (due to wrist injury) | 0.021 (0.008), 433 | 0.014 (0.007), 428 | 0.007 | –0.015 to 0.028 | 0.544 |
| Radiology (X-rays) | 0.002 (0.002), 437 | 0.004 (0.004), 431 | –0.002 | –0.012 to 0.007 | 0.646 |
| Physiotherapy | 0.002 (0.002), 436 | 0 (0), 431 | 0.002 | –0.002 to 0.006 | 0.325 |
| Emergency department | 0.006 (0.003), 437 | 0.002 (0.002), 431 | 0.004 | –0.004 to 0.003 | 0.332 |
| Community care (NHS) | |||||
| General practitioner (surgery) | 0 (0), 436 | 0.011 (0.006), 431 | –0.011 | –0.023 to 0.000 | 0.057 |
| General practitioner (telephone/e-mail contact) | 0.002 (0.002), 437 | 0 (0), 431 | 0.002 | –0.002 to 0.007 | 0.326 |
| Practice nurse | 0 (0), 437 | 0 (0), 431 | 0 | – | – |
| District nurse | 0 (0), 437 | 0 (0), 431 | 0 | – | – |
| Physiotherapist | 0.002 (0.0022), 437 | 0.004 (0.003), 431 | –0.002 | –0.009 to 0.005 | 0.544 |
| 111 advice | 0.002 (0.002), 437 | 0 (0), 431 | 0.002 | –0.002 to 0.006 | 0.297 |
| Type of care | Offer of a bandage group, mean (SE), n | Rigid immobilisation group, mean (SE), n | Difference | Bootstrap (95% CI) | p-valuea |
|---|---|---|---|---|---|
| NHS and PSS perspective | |||||
| Outpatient care | |||||
| Orthopaedic clinic (due to wrist injury) | 4.384 (1.144), 438 | 10.141 (1.848), 426 | –5.757 | –9.985 to -1.529 | 0.008 |
| Radiology (X-rays) | 0.353 (0.157), 439 | 0.288 (0.176), 430 | 0.065 | –0.402 to 0.531 | 0.786 |
| Physiotherapy | 0 (0), 440 | 0 (0), 430 | 0 | – | – |
| Emergency department | 5.549 (1.184), 439 | 3.767 (1.089), 431 | 1.781 | –1.778 to 5.341 | 0.327 |
| Community care (NHS) | |||||
| General practitioner (surgery) | 0.442 (0.167), 437 | 0.256 (0.128), 431 | 0.186 | –0.205 to 0.577 | 0.352 |
| General practitioner (telephone/e-mail contact) | 0.063 (0.063), 439 | 0.192 (0.143), 431 | –0.129 | –0.432 to 0.174 | 0.403 |
| Practice nurse | 0.095 (0.095), 440 | 0 (0), 431 | 0.095 | –0.090 to 0.281 | 0.314 |
| District nurse | 0 (0), 440 | 0 (0), 430 | 0 | – | – |
| 111 advice | 0.032 (0.032), 440 | 0.066 (0.046), 431 | –0.033 | –0.148 to 0.080 | 0.560 |
| Physiotherapist | 0 (0), 440 | 0 (0), 430 | 0 | – | – |
| Medication | 0.065 (0.335), 440 | 0.071 (0.323), 430 | –0.005 | –0.036 to 0.047 | 0.800 |
| Total (NHS and PSS) | 11.294 (2.061), 433 | 14.748 (2.294), 424 | –3.464 | –9.271 to 2.363 | 0.245 |
| Societal perspective | |||||
| Direct non-medical cost | |||||
| Help with child care | 0.014 (0.008), 440 | 0.034 (0.032), 431 | –0.020 | –0.088 to 0.048 | 0.565 |
| Lost productivity | 0.109 (0.023), 439 | 0.155 (0.030), 430 | –0.046 | –0.120 to 0.027 | 0.217 |
| Total (non-NHS) | 0.124 (0.024), 439 | 0.191 (0.047), 430 | –0.066 | –0.169 to 0.036 | 0.207 |
| Type of care | Mean (SE) cost (GBP), n | Difference | Bootstrap (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| Offer of a bandage group | Rigid immobilisation group | ||||
| NHS and PSS perspective | |||||
| Outpatient care | |||||
| Orthopaedic clinic (due to wrist injury) | 2.494 (0.993), 433 | 1.682 (0.883), 428 | 0.812 | –0.787 to 3.412 | 0.540 |
| Radiology (X-rays) | 0.071 (0.071), 437 | 0.143 (0.143), 431 | –0.073 | –0.400 to 0.255 | 0.662 |
| Physiotherapy | 0.089 (0.089), 436 | 0 (0), 431 | 0.089 | –0.084 to 0.262 | 0.312 |
| Emergency department | 0.796 (0.458), 437 | 0.269 (0.269), 431 | 0.527 | –0.482 to 1.531 | 0.306 |
| Community care (NHS) | |||||
| General practitioner (surgery) | 0 (0), 436 | 0.455 (0.119), 431 | –0.456 | –0.922 to 0.012 | 0.056 |
| General practitioner (telephone/e-mail contact) | 0.063 (0.063), 437 | 0 (0), 431 | 0.063 | –0.056 to 0.183 | 0.302 |
| Practice nurse | 0 (0), 437 | 0 (0), 431 | 0 | – | – |
| District nurse | 0 (0), 437 | 0 (0), 431 | 0 | – | – |
| Physiotherapist | 0.171 (0.171), 437 | 0.348 (0.245), 431 | –0.176 | –0.076 to 0.413 | 0.558 |
| 111 advice | 0.032 (0.032), 437 | 0 (0), 431 | 0.032 | –0.099 to 0.033 | 0.333 |
| Medication cost | 0.027 (0.225), 437 | 0.011 (0.102), 431 | 0.016 | –0.039 to 0.006 | 0.168 |
| Total (NHS and PSS) | 3.484 (1.285), 432 | 2.840 (0.988), 428 | 0.643 | –2.486 to 3.773 | 0.687 |
| Societal perspective | |||||
| Direct non-medical cost | |||||
| Help with child care | 0.003 (0.003), 437 | 0.034 (0.034), 4300 | –0.031 | –0.102 to 0.039 | 0.383 |
| Lost productivity | 0.067 (0.027), 437 | 0.045 (0.015), 431 | 0.022 | –0.036 to 0.081 | 0.461 |
| Total (non-NHS) | 0.071 (0.027), 437 | 0.080 (0.038), 430 | –0.009 | –0.102 to 0.084 | 0.845 |
Medication use was recorded at days 1, 3 and 7 and weeks 3 and 6 (see Table 14). The estimated cost of medications for each individual over these five time intervals can be seen in Table 27. The mean cost per participant of medication over the 6-week trial period was very low for both treatment groups. A summary of all included costs from the NHS and PSS perspective (treatment cost, NHS service utilisation cost and medication cost) over the trial period is given in Table 28. Based on complete data, using a bandage instead of rigid immobilisation resulted in a small but statistically significant saving of £12.55 (95% CI £5.30 to £19.51).
| Time point | Mean (SE) cost (GBP), n | Difference | Bootstrap (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| Offer of a bandage group | Rigid immobilisation group | ||||
| Day 1 | 0.903 (0.809), 409 | 0.898 (0.800), 389 | 0.004 | –0.108 to 0.100 | 0.937 |
| Day 3 | 1.249 (1.545), 451 | 1.303 (1.524), 435 | –0.053 | –0.146 to 0.252 | 0.602 |
| Day 7 | 0.741 (1.709), 446 | 0.969 (1.895), 429 | –0.227 | –0.012 to 0.467 | 0.063 |
| Week 3 | 0.065 (0.335), 428 | 0.071 (0.323), 425 | –0.005 | –0.036 to 0.047 | 0.800 |
| Week 6 | 0.027 (0.225), 433 | 0.011 (0.102), 424 | 0.016 | –0.039 to 0.006 | 0.168 |
| Cost category | Mean (SE) cost (GBP), n | Difference | Bootstrap (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| Offer of a bandage group | Rigid immobilisation group | ||||
| Treatment | 3.378 (0.168), 456 | 12.461 (0.03), 443 | –9.082 | –9.213 to -8.982 | 0.000 |
| Medication cost (day 1 to day 7) | 2.985 (0.159), 435 | 3.165 (0.165, 417 | –0.179 | –0.627 to 0.267 | 0.431 |
| NHS services (including medication cost, week 3 to week 6) | 14.716 (2.914), 410 | 17.085 (2.741), 409 | –2.369 | –10.101 to 5.363 | 0.548 |
| Total cost, NHS and PSS | 21.131 (2.923), 410 | 32.653 (2.722), 409 | –11.522 | –19.558 to –3.486 | 0.005 |
Table 29 presents the mean cost and standard error of non-NHS cost and any additional expenses that were borne by the participants. The mean cost of work loss by treatment allocation for the 6-week period is also shown in Table 29.
| Cost category | Mean (SE) cost (GBP), n | Difference | Bootstrap (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| Offer of a bandage group | Rigid immobilisation group | ||||
| Time off work | 0.169 (0.041), 456 | 0.195 (0.035), 443 | –0.025 | –0.084 to 0.134 | 0.649 |
| Child care | 0.017 (0.011), 456 | 0.067 (0.065), 443 | –0.051 | –0.083 to 0.183 | 0.461 |
| Total cost, non-NHS | 0.187 (0.042), 456 | 0.262 (0.078), 443 | –0.075 | –0.099 to 0.251 | 0.399 |
| Total societal cost, NHS and PSS and non-NHS | 20.171 (2.787), 409 | 33.619 (2.742), 408 | –13.448 | –21.158 to –5.737 | 0.001 |
Utility and quality-adjusted life-years
Utility scores were estimated using validated EQ-5D-Y questionnaires completed by participants at baseline, 3 days, 7 days, 3 weeks and 6 weeks. The summary statistics of the unadjusted EQ-5D-Y utility scores for all observed cases across all time points by treatment are presented in Table 30. EQ-5D-Y scores at 6 weeks were higher than the baseline post-injury scores in both treatment groups. The baseline EQ-5D-Y score was slightly higher in the rigid immobilisation group than in the offer of a bandage group. Consequently, unadjusted EQ-5D-Y utilities and QALYs should be interpreted with caution. Nonetheless, based on complete data, using the offer of a soft bandage instead of a rigid immobilisation resulted in a small and non-statistically significant increase in quality of life of 0.0012 QALYs (95% CI –0.001 to 0.002 QALYs). We adjusted for baseline imbalance in the economic evaluation within analyses.
| Time point | Mean (SE) score, n | Difference | Bootstrap (95% CI) | p-valueb | |
|---|---|---|---|---|---|
| Offer of a bandage group | Rigid immobilisation group | ||||
| EQ-5D-Y | |||||
| Baseline | 0.537 (0.016), 456 | 0.557 (0.629), 443 | –0.019 | –0.064 to 0.025 | 0.399 |
| 3 days | 0.563 (0.012), 444 | 0.548 (0.013), 432 | 0.015 | –0.021 to 0.052 | 0.408 |
| 7 days | 0.706 (0.011), 444 | 0.695 (0.011), 428 | 0.011 | –0.018 to 0.044 | 0.410 |
| 3 weeks | 0.895 (0.007), 430 | 0.885 (0.007), 428 | 0.009 | –0.012 to 0.032 | 0.388 |
| 6 weeks | 0.975 (0.004), 434 | 0.972 (0.004), 428 | 0.003 | –0.008 to 0.014 | 0.618 |
| QALYc (AUC) | 0.095 (0.001), 409 | 0.094 (0.001), 401 | 0.001 | –0.001 to 0.002 | 0.485 |
| EQ-5D-Y VAS | |||||
| Baseline | 72.728 (1.024), 456 | 73.288 (1.029), 443 | –0.560 | –2.226 to 3.347 | 0.693 |
| 3 days | 76.969 (0.895), 444 | 75.862 (0.916), 432 | 1.107 | –3.559 to 1.345 | 0.379 |
| 7 days | 83.064 (0.901), 444 | 83.540 (0.807), 428 | –0.476 | –1.922 to 2.874 | 0.697 |
| 3 weeks | 92.577 (0.589), 430 | 90.699 (0.732), 428 | 1.878 | –3.694 to –0.061 | 0.043 |
| 6 weeks | 94.744 (0.629), 434 | 96.129 (0.448), 428 | –1.385 | –0.119 to 2.889 | 0.071 |
Cost-effectiveness results
The ICER plane in Figure 9 shows the joint distribution of incremental cost and QALYs for the base-case analysis. Patients allocated to the offer of a bandage treatment experienced a marginally larger average quality of life gain (0.0013 QALYs, 95% CI –0.004 to 0.003 QALYs) and incurred lower average health costs (–£12.55, 95% CI –£19.51 to –£5.30) than those in the rigid immobilisation group (Table 31). The probability of the offer of a bandage being cost-effective was > 95% at each of the cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY (Figure 10 and see Table 31). The NMB associated with the offer of a bandage was found to be positive and increased with willingness to pay (Figure 11). Therefore, the base-case analysis indicates that the offer of a bandage rather than rigid immobilisation is likely to be cost-effective in the studied population.
FIGURE 9.
Incremental cost-effectiveness plane with 95% credible region, showing the base-case analysis of bandage compared with rigid immobilisation.
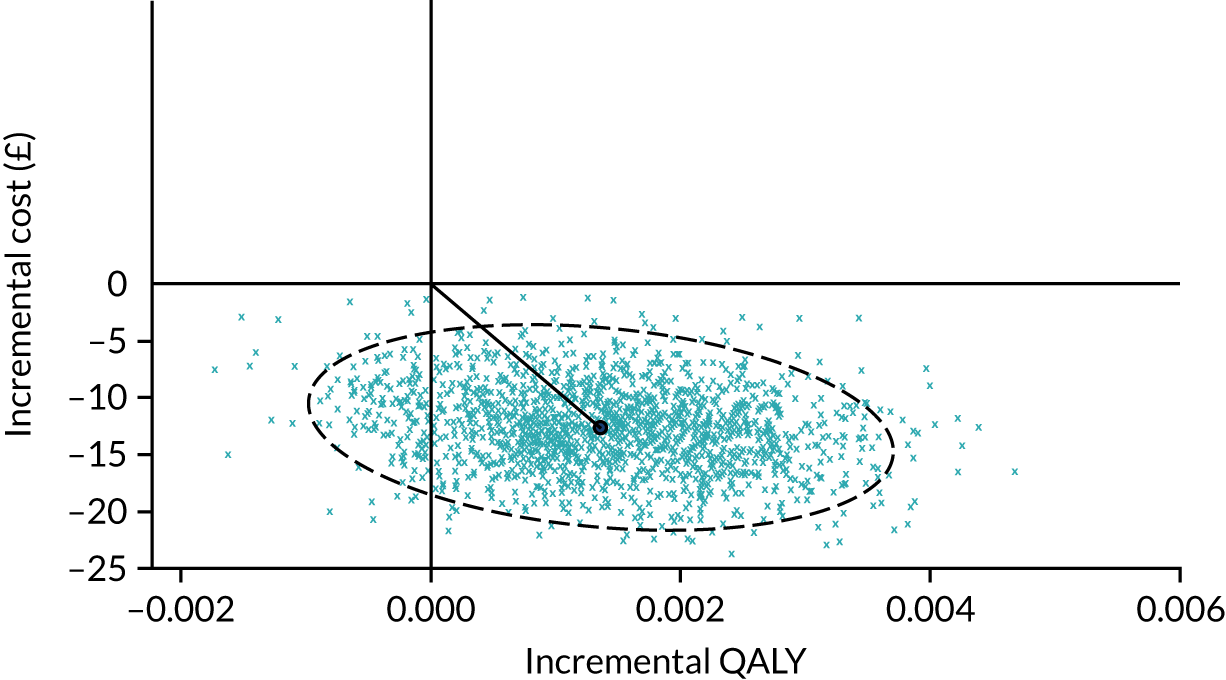
| Analysis | Incremental cost (£) (95% CI) | Incremental QALYs (95% CI) | ICER, £ (QALY) | Probability of cost-effectiveness at specified cost-effectiveness threshold | ||
|---|---|---|---|---|---|---|
| £15,000 | £20,000 | £30,000 | ||||
| Base case | ||||||
| NHS and PSS perspective: imputed costs and QALYs, covariate adjusted | –12.552 (–19.801 to –5.302) | 0.0013 (0.000 to 0.003) | –9311 (SE quadrant) | 0.9845 | 0.9760 | 0.9655 |
| Sensitivity | ||||||
|
–12.003 (–20.07; –3.94) | 0.0012 (0.003 to –0.001) | –10,680 (SE quadrant) | 0.9625 | 0.9515 | 0.9320 |
|
–12.302 (–19.483 to –5.121) | 0.0012 (0.000 to 0.003) | –9890 (NE quadrant) | 0.9800 | 0.9680 | 0.9555 |
|
–17.00 (–34 to –3) | 0.0013 (–0.002 to 0.007) | –35,241 (SE quadrant) | 0.7695 | 0.7350 | 0.6985 |
|
–11.00 (–18 to –3) | 0.0021 (0.004 to 0.000) | –6442 (NE quadrant) | 0.9805 | 0.9730 | 0.9670 |
|
–13.002 (–20.245 to –5.452) | 0.0013 (–0.004 to 0.003) | –9432 (SE quadrant) | 0.9825 | 0.9770 | 0.9653 |
FIGURE 10.
Base-case analysis of the offer of a bandage compared with rigid immobilisation: cost-effectiveness acceptability curve.
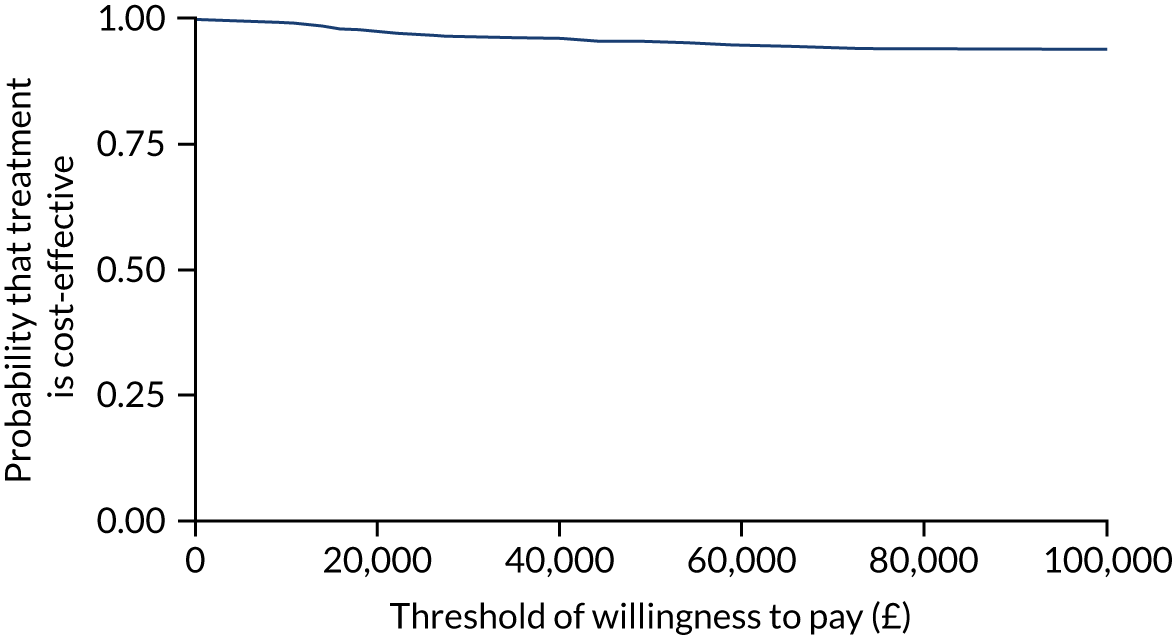
FIGURE 11.
Base-case analysis of the offer of a bandage compared with rigid immobilisation: NMB.
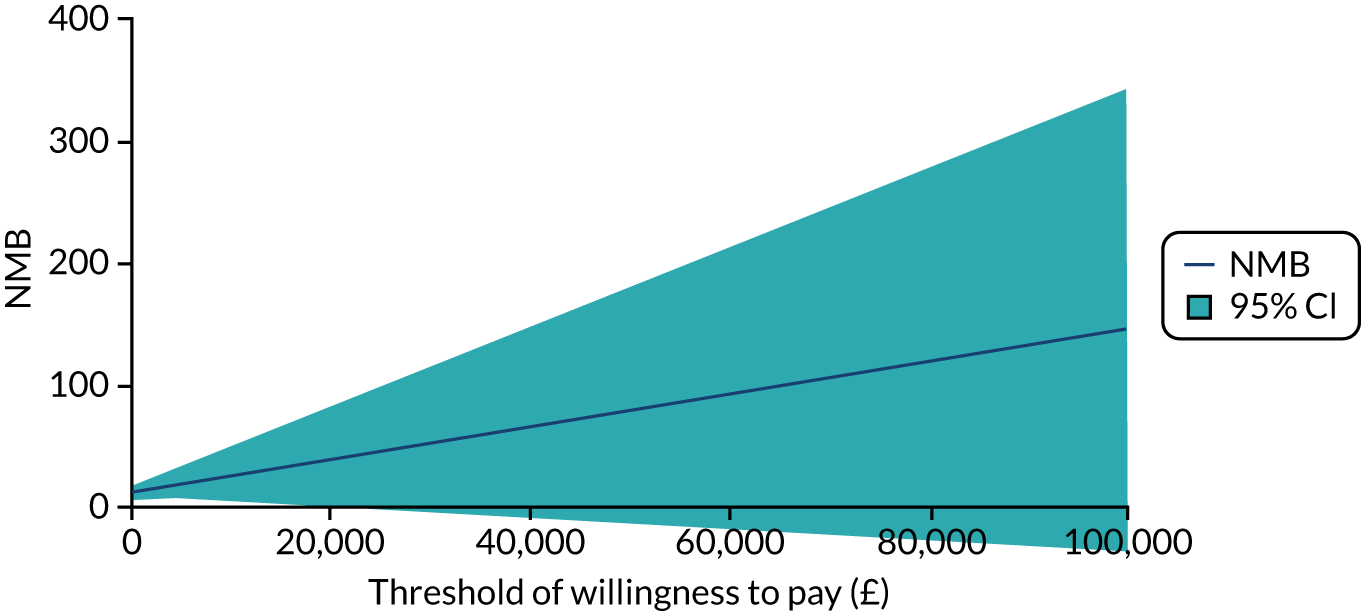
Sensitivity analyses showed similar results that supported the base-case finding: in each case, the offer of a bandage proved the dominant strategy. The ICER, when the complete-case analysis was implemented, was –£10,680 per QALY and for the societal perspective the ICER was –£9890 per QALY. The probability that use of a bandage was cost-effective when compared with rigid immobilisation within the studied population was generally > 95% in both sensitivity analyses.
The planned subgroup analysis of age found that, in the offer of a bandage group, costs were higher and quality-of-life gains smaller in the 4–7 years age group than in the 8–15 years age group, as shown in Table 31.
When the assumption was made that participants attended hospital at least as often as was identified by the recruitment centre-reported data, the results again supported the base-case analysis. The ICER was –£9432 per QALY. The probability that use of a bandage was cost-effective when compared with rigid immobilisation within the studied population was > 95% in this sensitivity analysis.
The expected value of perfect information per patient in the base-case analysis was about £1 at a willingness-to-pay threshold of £30,000 per QALY (Figure 12). Given that there are about 60,000 emergency attendances for torus fractures of the distal radius in children per year in Great Britain,2,3 realistic technology time horizons of 10–20 years suggest that further research to reduce uncertainty is unlikely to be appropriate.
FIGURE 12.
Base-case analysis of the offer of a bandage compared with rigid immobilisation: EVPI. EVPI, expected value of perfect information.
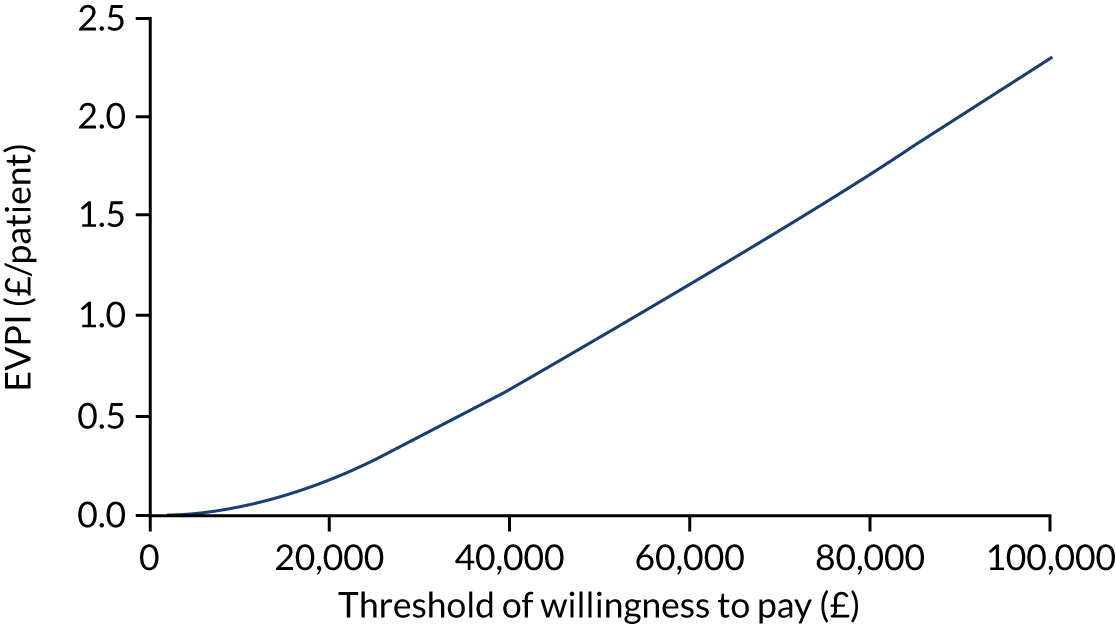
Chapter 4 Discussion
Recruitment
Paediatric Emergency Research United Kingdom and Ireland (PERUKI), an increasingly well-established network of clinicians, which aims to effectively deliver high-quality trials in paediatric trauma and emergency care, facilitated the delivery of this trial. At the outset of the trial, we were mindful of the seasonality of injuries likely to be encountered, and the overall number of patients screened in the trial was in keeping with the estimated rate of recruitment at the recruitment centres, including this marked seasonal effect. As expected, the rate of recruitment was substantially higher during the summer months, with approximately three times more patients both screened and recruited than during the winter months. The rate of recruitment among the younger group of patients was slower than anticipated, leading to a 3-month extension of the recruitment window. The key reason for this delay was an unanticipated imbalance between the age groups within the study, with the older group (i.e. 8–15 years) screened and recruited at twice the rate of those in the younger group (i.e. 4–7 years). As a result, we recruited considerably more children than planned in the older group.
Among the children who met the inclusion criteria, 20% of those screened were excluded because they did not meet the predetermined eligibility criteria. The majority of these (65%) were made up of children who presented to the recruitment centre > 36 hours after their injury. This exclusion criterion was decided on based on the primary outcome of pain measured at 3 days post randomisation, with the anticipation that children with fractures typically present immediately. It was therefore felt that those presenting > 36 hours after injury would already be approaching the point for which pain was already significantly resolving.
The number of potentially eligible patients presenting at each centre differed considerably, largely because of the size of their catchment populations. The key determinant driving the conversion of ‘screened’ to ‘recruited’ was patient preference. Among families, there was a strong preference for rigid immobilisation, with 252 patients/parents declining to participate for this reason. Conversely, only four families declined to participate because they had a preference for a bandage. Although we were unable to formally explore this preference through qualitative interviews, it was apparent that there was a pre-existing belief among parents and young people that ‘broken bones require immobilisation’. However, clinician preference was uncommon, with only 14 patients excluded based on clinician preference, presumably with their actions informed through evidence and their own experience of the infrequent nature of complications and reattendance with this injury.
The study was delivered almost wholly online; consent forms, case report forms, treatment details, details of complications and details of patient-reported outcomes were collected electronically and added directly to the database. There were relatively few difficulties encountered with consent, with only 29 patients unable to be enrolled because of technical difficulties. The online approach contributed to the study being widely accessible to clinical teams, with more than half of patients recruited outside normal working hours. The online approach also minimised errors within recruitment (i.e. there were few errors of consent or incomplete data fields throughout follow-up) and meant that the study material was easily accessible to all (i.e. through the internet). However, there were challenges in terms of an initial reluctance of some clinical/research teams to move online, a reliance on the availability of a ‘device’ in busy EDs, and inevitable ‘glitches’ that occured throughout the process (i.e. study enrolment was unable to be performed between 00.00 and 01.00, as this was the time that the database server updated, which contributed to some of the technical difficulties encountered).
Overall, 965 of the 1513 (64%) eligible patients were included within the trial. We can be confident that the patients who took part in the trial are broadly representative of those children with an acute torus fracture of the distal radius.
Participants and treatments
In total, 965 patients consented to take part in the trial. The mean age of the participants was slightly higher than previously anticipated, at 9.65 years. This accounted for the imbalance in the recruitment between the two age groups, with recruitment being extended until sufficient primary outcomes were acquired in both groups to reach the sample size (n = 278). Consequently, 300 patients were recruited in the 4–7 years age group, and 665 patients were recruited in the 8–15 years age group. As expected, most injuries were the result of low-energy trauma (75%). The injury was equally common in both sexes in the 4–7 years age group, but in the 8–15 years age group two-thirds of those screened and recruited were male, which reflects previous epidemiological data concerning sex disparities in childhood injuries. 56 This difference is believed to be a consequence of behavioural differences in boys and girls that emerge at an early age. 57
A total of 489 participants were randomised to the offer of a bandage group and 476 were randomised to the rigid immobilisation group. We anticipated that some patients would cross over following randomisation and, indeed, in the ED, seven patients received rigid immobilisation despite being randomised to the offer of a bandage group, and one received the offer of a bandage despite being randomised to the rigid immobilisation group. The crossovers were mostly driven by family preference, with families changing their mind after randomisation. By the point of the primary outcome (i.e. day 3), 36 patients had received rigid immobilisation despite being randomised to the offer of a bandage group, and one patient had received the offer of a bandage despite being randomised to the rigid immobilisation group. The additional crossovers occurring before day 3 were almost all related to pain. Crossovers after the initial treatment were all unidirectional (i.e. the offer of a bandage changing to rigid immobilisation) because families generally returned for reassurance or an escalation in care. After day 3, an additional 14 children crossed over from the offer of a bandage group to the rigid immobilisation group, such that 50 (10.2%) children randomised to the offer of a bandage group ultimately changed treatment. This imbalance could potentially pose a threat to the integrity of the trial, but, because the number of such crossovers was small in the context of a trial of 965 participants, this is very unlikely to have affected the results. Furthermore, the analysis undertaken considered the result according to both treatment received (PP) and by treatment randomised (ITT).
Although crossovers were generally unidirectional, changes in the immobilisation device occurred in both groups. Twenty-two participants in the rigid immobilisation group had further immobilisation changes after the initial treatment, which included splint changes or escalation in care from a removable splint to plaster cast immobilisation. Furthermore, care was frequently de-escalated at home: by day 3, 69 (14.3%) bandages and four (0.8%) casts had been removed.
Most participants in the rigid immobilisation group (451/476, 95%) were treated with a futura-type splint or similar. A prior study of UK practice found that 40% of hospitals were primarily using casts,58 so the widespread use of futura-type splints in the study suggests either that use of this practice has rapidly increased throughout the UK or that the centres involved in the trial are more innovative than centres not involved in the trial. Most patients in the offer of a bandage group (458/489, 94%) chose to have the bandage applied in the ED. Compliance with the treatments was good. The average duration of treatment use was 7 days in the offer of a bandage group and 18 days in the rigid immobilisation group. At 3 weeks, 37% of the rigid immobilisation group continued to wear the treatment, but only 10% of the offer of a bandage group did.
In terms of the primary outcome measure of pain at 3 days, there were 94.1% complete scores, with follow-up rates broadly similar across age groups and treatment groups. The early primary outcome time point allowed the trial to be efficiently concluded once the number of primary outcomes required to achieve 90% power in each age group of children had been collected.
At 6 weeks, the rate of completion of secondary outcome measures was 90% overall, with a slight discrepancy between age groups, the rate being 92.7% for 4- to 7-year-olds and 88.3% for 8- to 15-year-olds. The high rates of follow-up reflect the success of automated electronic participant follow-up in this patient population.
Of those participants who did not complete the 6 weeks’ follow-up for the trial, five withdrew, with the remaining participants failing to respond to prompts.
Results
Primary outcome
This trial showed equivalence in the Wong–Baker Scale scores at 3 days post randomisation between the offer of a bandage group and the rigid immobilisation group in the management of torus fractures of the distal radius in children aged 4–15 years. Both the ITT analysis (analysis by treatment randomised) and the PP analysis (analysis of participants who received their allocated treatment) confirmed equivalence. Furthermore, the trial was powered to separately assess equivalence in each of the age subgroups, and equivalence was confirmed for both groups.
The number of missing data at the primary end point was very small (approximately 5%); therefore, as per the statistical analysis plan,20 no attempt was made to account for missing data.
Secondary outcomes
In keeping with the primary analysis of pain at 3 days, this trial found no evidence of a difference between the two treatment groups at any of the time points up to the final 6-week follow-up, with the exception of day 1. For the day 1 follow-up, the ITT analysis demonstrated a small but statistically significant difference in pain scores (difference –0.36, 95% CI –0.61 to –0.12) favouring the rigid immobilisation group, but this difference was notably smaller than both the prespecified equivalence margin of 1 point and the minimal clinically important difference of 2 points. This finding was not significant in the PP analysis (difference –0.22, 95% –0.47 to 0.03). Interestingly, the number of participants receiving analgesia was similarly slightly larger in the offer of a bandage group than in the rigid immobilisation group, with approximately 5% more children receiving analgesia at each time point during the first 7 days (day 1, 83% vs. 78%; day 3, 57% vs. 51%; day 7, 25% vs. 23%). The analgesia used was, almost universally, simple ‘over-the-counter’ analgesia.
In keeping with the outcome of pain, the secondary outcomes of upper extremity function or quality of life identified no evidence of a difference between the two treatment groups at any of the time points during follow-up. Parental satisfaction was slightly better (extremely satisfied vs. very satisfied) at day 1 among those treated with a rigid immobilisation than among those receiving the offer of a bandage, but there was no difference at the completion of follow-up.
Although differences did not exist between the treatment groups, there were small differences between the age groups. Children in the older age group generally reported slightly higher pain and poorer quality of life at each time point than those in the younger group, but, conversely, this group also reported more rapid functional recovery. Although this difference is small, it may reflect a difference between self-reporting and proxy reporting, which alters the interpretation of the experience.
School attendance was similar, with participants in both groups, missing an average of 1.5 days of school.
The size of this study allowed particular consideration of complications, of which refracture and worsening deformity requiring intervention are the key concerns of families and clinicians alike. Of the 965 children, none was found to have a worsened deformity. One fracture was identified to have a refracture; the patient was initially randomised to the offer of a bandage group but crossed over to the rigid immobilisation group in week 1 because of pain and was treated with a splint. This patient then experienced refracture at around 3 weeks, following a fall. In total, only eight complications were reported, seven of which related to an alternative type than that originally diagnosed by the treating clinician – all of which were treated with cast immobilisation. Owing to the pragmatic nature of the study, these were not considered protocol deviations, as the treating clinician acted in accordance with their usual practice, and there is subjectivity in making the diagnosis. However, as these alternative fracture patterns inevitably were present from the outset, they amount to errors of radiographical interpretation and, therefore, of study eligibility.
Diagnosis audit
Given the unexpected age distribution of participants (i.e. imbalance between the younger and older patient groups) the FORCE trial TSC recommended that the trial team audit the diagnostic agreement between treating clinicians and reporting radiologists to ensure the validity of the diagnoses. The audit took place when the first 250 patients had been recruited to the trial. The decision was made to use the ‘reporting radiologist’ as a baseline, but it should be noted that the radiologist’s interpretation of the radiograph is also prone to misinterpretation, as there is no clear reference standard to follow.
In total, 12 recruitment centres participated in the audit, contributing 212 fractures. There was agreement between the treating clinician and the reporting radiologist in 85% of cases. Seven per cent of cases were reported by the radiologist to be less severe (i.e. no fracture) and 8% of cases were reported to be more severe (i.e. greenstick, growth plate injury or a complete fracture) than the report by the treating clinician. There was, therefore, broad consistency in the diagnosis of torus fractures, but there was some diagnostic uncertainty. Although, in a few cases, this resulted in crossover between treatment groups, the majority, after review by the treating emergency clinicians, continued to be treated as torus fractures. The absence of worsened deformity, irrespective of the fracture pattern, indicates that the treatment approach is likely to be appropriate even in the presence of diagnostic debate among expert clinicians.
The addition of the posters detailing the inclusion parameters for the trial (see Appendix 3, Figure 13) may have improved the diagnostic accuracy beyond that seen in usual care. However, 281 clinicians from 23 recruitment centres recruited patients to the trial, demonstrating the generalisability of the study findings.
Health economic evaluation
The unit cost of treatment was £12.55 higher in the rigid immobilisation group than in the offer of a bandage group, and quality of life was also marginally higher in the rigid immobilisation group (mean 0.0013 QALYs, 95% CI 0.000 to 0.003 QALYs). Based on the base-case analysis, the cost per patient of offer of a bandage was lower than the cost of rigid immobilisation from the NHS and PSS perspective.
At a £30,000 per QALY ceiling ratio, the offer of a bandage was the most cost-effective treatment for treating children with a torus fracture of the distal radius. A significant decrease in cost and small non-significant increase in quality of life combine to provide a positive NMB for the offer of a bandage and better than 95% probability of cost-effectiveness. The findings appeared to be robust when considering sensitivity analyses, although the evidence is less compelling for the older age group (i.e. 8–15 years).
Although missing data are usually an issue in an economic analysis and may introduce bias into the health economics results, the combined level of missingness of cost and outcome data (12.1%) in the FORCE trial was low, enhancing the robustness of findings and similarity between the imputed and complete-case model estimates.
Limitations
Recruiting patients to clinical trials in the context of emergencies is difficult, which is magnified when the patient group involves children. A concern before this trial started was that families and/or clinicians would not be willing to take part. This concern was unfounded regarding the clinicians, who were broadly in equipoise, with only 14 patients not enrolled owing to a clinician preference. However, families had a strong pre-existing preference for rigid immobilisation, with 252 declining to participate from the outset for this reason. This preference continued after randomisation, with seven patients declining to accept the allocated treatment as randomised and immediately changing treatment groups. Given the preference and the inability to blind families to the treatment allocation, it is likely that there was some bias in the reporting of patient-reported outcomes. This bias is likely to amplify the magnitude of the treatment effect, that is to overstate outcomes in the rigid immobilisation group. This is perhaps most evident in reports of patient satisfaction: satisfaction on follow-up day 1 was lower among participants randomised to the offer of a bandage group, despite only a small reduction in reported pain that was well below the minimal clinically important difference. Despite this bias, there was equivalence in the primary outcome and all other clinical outcomes at every time point in the trial.
Although a selection bias could emerge through the initial patient preference in the trial, these numbers are small compared with the size of the trial, and the demographics of those declining to participate in the trial were broadly similar to those included within the trial. Any potential selection bias therefore appears unlikely to affect the external validity of the results.
The exclusion criteria excluded participants in whom the injury had occurred > 36 hours previously. Although this was intended to ensure that the treated participants were recruited at a similar point on the recovery pathway, this does affect the generalisability of the findings to this group of patients. Nevertheless, clinically, it seems unlikely that the results would not equally apply to this patient group.
Research recommendations
Given the findings of this study, a clinical decision tool to determine which children require radiography for wrist injuries would be an important next step. Only fractures that require intervention need undergo radiography; therefore, differentiating these from sprains and torus fractures could be important in preventing overinvestigation and overtreatment of sprains and torus fractures. There is also a need to rationalise interventions for other common injuries in children (e.g. rigid immobilisation and follow-up for ‘toddler’s fractures’ of the tibia).
A future trial may similarly investigate whether or not bandage immobilisation and immediate discharge would be as good as rigid immobilisation and follow-up.
Chapter 5 Conclusions
In children with a torus fracture of the distal radius, there was clear evidence of equivalence in reported pain at 3 days post randomisation and throughout the entire 6-week follow-up period between those treated with the offer of a bandage and those treated with rigid immobilisation. There were no safety concerns in either group, which supports the strategy of immediate discharge of children with this injury from EDs. The offer of a bandage was the most cost-effective treatment for treating children, with a saving of –£12.55 per participant, which is significant because of the 60,000 children in the UK presenting with this injury each year.
Acknowledgements
Recruitment centres
| Hospital | NHS Trust |
|---|---|
| Alder Hey Children’s Hospital | Alder Hey Children’s NHS Foundation Trust |
| Birmingham Children’s Hospital | Birmingham Women’s and Children’s NHS Foundation Trust |
| Bristol Royal Hospital for Children | University Hospitals Bristol NHS Foundation Trust |
| Coventry University Hospital | University Hospitals Coventry & Warwickshire |
| Darlington Memorial Hospital | County Durham and Darlington NHS Foundation Trust |
| Evelina London Children’s Hospital | Guy’s and St Thomas’ NHS Foundation Trust |
| Horton General Hospital | Oxford University Hospitals NHS Foundation Trust |
| Ipswich Hospital | East Suffolk and North Essex NHS Foundation Trust |
| John Radcliffe Hospital | Oxford University Hospitals NHS Foundation Trust |
| King George Hospital | Barking, Havering and Redbridge University Hospitals NHS Trust |
| Leicester Royal Infirmary | University Hospitals of Leicester NHS Trust |
| New Cross Hospital | The Royal Wolverhampton NHS Trust |
| Nottingham Children’s Hospital | Nottingham University Hospitals NHS Trust |
| Queen’s Hospital | Barking, Havering and Redbridge University Hospitals NHS Trust |
| Royal Berkshire Hospital | Royal Berkshire NHS Foundation Trust |
| Royal Derby Hospital | Derby Teaching Hospitals NHS Foundation Trust |
| Royal Devon and Exeter Hospital | Royal Devon and Exeter NHS Foundation Trust |
| Royal London Hospital | Barts Health NHS Trust |
| Sheffield Children’s Hospital | Sheffield Children’s NHS Foundation Trust |
| St Georges Hospital | St George’s University Hospitals NHS Foundation Trust |
| Sunderland Royal Hospital | City Hospitals Sunderland NHS Foundation Trust |
| University Hospital Southampton | University Hospital Southampton NHS Foundation Trust |
| Wexham Park Hospital | Frimley Health NHS Foundation Trust |
Trial team
Trial management team
Mr Daniel Perry, chief investigator; Dr Ruth Knight, trial statistician; Associate Professor Susan Dutton, senior trial statistician; Dr Juul Achten, research manager; Professor Matthew Costa, mentor to chief investigator; Dr Damian Roland, PERUKI executive member; Dr Shrouk Messahel, PERUKI executive member; Mr James Widnall, lead for trainee and engagement; Ms Jennifer Preston, patient and public involvement manager; Dr Duncan Appelbe, senior information specialist; Mrs Louise Spoors, trial manager; Ms Amender Juss, trial co-ordinator; Mrs Amrita Athwal, senior trial manager; Dr Melina Dritsaki, trial health economist; Professor James Mason, senior trial health economist; Mrs Phoebe Gibson, public member; Miss Gurneet Sur, Miss Kinzah Abbasi and Mr Hugo Strachwitz, trial administrative co-ordinators; and Mrs Moe Byrne and Dr Peter Knapp, TRECA SWAT team.
Trial applicants
Daniel Perry, chief investigator.
Co-applicants: Juul Achten, Matthew Costa, Susan Dutton, Melina Dritsaki, James Mason, Damian Roland, Shrouk Messahel, Phoebe Gibson, James Widnall and Jennifer Preston.
Trial Steering Committee
Dr Catriona McDaid, Methodologist (chairperson and independent member, and a member of the HTA and Efficacy and Mechanism Evaluation editorial board); Professor Deborah Eastwood, Consultant Paediatric Orthopaedic Surgeon (independent member); Mr Fergal Monsell, Consultant Paediatric Orthopaedic Surgeon (non-independent member); Dr Emma Morely and Mrs Amy Moscrop (lay independent members); and Mr Daniel Perry, chief investigator.
Data Monitoring Committee
Professor Richard Body, Professor of Emergency Medicine (chairperson and independent member); Professor Steve Turner, Professor of Paediatrics (independent member); and Mr Charlie Welch, Statistician (independent member).
Statisticians
Dr Ruth Knight and Associate Professor Susan Dutton.
Health economists
Dr Melina Dritsaki and Professor James Mason.
Programming team
Duncan Appelbe, Patrick Julier, Lucy Eldridge and Malcolm Hart.
Research associates
Liz Lee, Louise Rogers, Sarah Sheedy, Tracey Bingham, Joanna Teuke, Kerri Mcgowan, Deborah Beeby, Rebecca Francis, Sam King, Helen Smith, Jin Li, Megan Meredith, Sally Beer, Hannah Thraves, Raine Astin-Chamberlain, Amina Rawat, Jayne Evans, Julie Morcombe, Vanessa Unsworth, Desislava Baramova, Heather Jarman, Louise Fairlie, Charlotte Busby, Rebecca Denyer, Catherine Yearsung, Heather Prowse, Joana Rocha, Lauren Whitty, Genevieve Roberts, Kevin Thorpe, Amanda Cowton, Mohammad Ahmad and Daniella Hydes.
Other acknowledgements
The FORCE trial was supported by the NIHR Oxford Biomedical Research Centre.
The FORCE trial was facilitated by a multidisciplinary team, including nurses, radiographers, physiotherapists, emergency clinicians and orthopaedic surgeons from different hospitals in the UK who were part of the PERUKI Collaborative.
Contributions of authors
Daniel C Perry (https://orcid.org/0000-0001-8420-8252) (Professor of Paediatric Orthopaedic Surgery, Paediatric Orthopaedics) was involved in the study conception and design, developed the protocol, had clinical responsibility, was a member of the TMG, and was involved in the writing and reviewing of the report.
Juul Achten (https://orcid.org/0000-0002-8857-5743) (Research Manager, Trial Management and Methodology) was involved in the study conception and design, developed the protocol, was a member of the TMG, was involved in the overall management of the trial and writing and reviewing of the report.
Ruth Knight (https://orcid.org/0000-0001-6810-2845) (Senior Statistician, Medical Statistics) was a member of the TMG, was responsible for the statistical analysis of the trial, and was involved in the writing and reviewing of report.
Susan J Dutton (https://orcid.org/0000-0003-4573-5257) (Associate Professor of Medical Statistics, Medical Statistics) was involved in the study conception and design, developed the protocol, was a member of the TMG, was responsible for overseeing the statistical analysis of the trial, and was involved in the writing and reviewing of the report.
Melina Dritsaki (https://orcid.org/0000-0002-1673-3036) (Senior Health Economist, Health Economics) was a member of the TMG, was responsible for the health economics analysis, and was involved in the writing and reviewing of the report.
James M Mason (https://orcid.org/0000-0001-9210-4082) (Professor of Health Economics, Health Economics) was involved in the study conception and design, developed the protocol, was a member of the TMG, was responsible for overseeing the health economic analysis of the trial, and was involved in the writing and reviewing of the report.
Duncan E Appelbe (https://orcid.org/0000-0003-1493-0391) (Senior Information Specialist, Medical Data Management and Analysis) was a member of the TMG, was responsible for the building and maintenance of the databases and the follow-up system of the study, and was involved in the reviewing of the report.
Damian T Roland (https://orcid.org/0000-0001-9334-5144) (Consultant in Paediatric Emergency Medicine) developed the protocol, was a member of the TMG and was involved in the writing and reviewing of the report.
Shrouk Messahel (https://orcid.org/0000-0003-0645-3070) (Consultant in Paediatric Emergency Medicine) developed the protocol, was a member of the TMG and was involved in the reviewing of the report.
James Widnall (https://orcid.org/0000-0003-4547-1466) (Consultant in Paediatric Trauma and Orthopaedics) developed the protocol, was a member of the TMG and was involved in the reviewing of the report.
Phoebe Gibson (https://orcid.org/0000-0003-2764-4888) (Patient Representative) developed the protocol, was a member of the TMG and was involved in the reviewing of the report.
Jennifer Preston (https://orcid.org/0000-0003-4800-234X) (Senior Patient and Public Involvement Manager) was a member of the TMG and was involved in the reviewing of the report.
Louise M Spoors (https://orcid.org/0000-0003-0488-0087) (Clinical Trials Manager, Trauma and Orthopaedics) developed the protocol, was a member of the TMG and was involved in the reviewing of the report.
Marta Campolier (https://orcid.org/0000-0002-7168-2534) (Clinical Trials Manager, Trauma and Orthopaedics) was involved in the writing and reviewing of the report.
Matthew L Costa (https://orcid.org/0000-0003-3644-1388) (Professor of Orthopaedic Trauma Surgery, Trauma and Orthopaedics) developed the protocol, was a member of the TMG and was involved in the reviewing of the report.
Publications
Achten J, Knight R, Dutton Sj, Costa ML, Mason J, Dritsaki M, et al. A multicentre prospective randomized equivalence trial of a soft bandage and immediate discharge versus current treatment with rigid immobilization for torus fractures of the distal radius in children. Bone Joint Open 2020;1:214–21.
Knight R, Dritsaki M, Mason J, Perry DC, Dutton SJ. The Forearm Fracture Recovery in Children Evaluation (FORCE) trial: statistical and health economic analysis plan for an equivalence randomized controlled trial of treatment for torus fractures of the distal radius in children. Bone Joint Open 2020;1:205–13.
Perry DC, Gibson P, Roland D, Messahel S. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ 2021;372:m4862.
Perry DC, Achten J, Knight R, Appelbe D, Dutton SJ, Dritsaki M, et al. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet 2022;400:39–47.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Perry DC, Achten J, Knight R, Appelbe D, Dutton SJ, Dritsaki M, et al. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet 2022;400:39-47. https://doi.org/10.1016/s0140-6736(22)01015-7.
- Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res 2004;19:1976-81. https://doi.org/10.1359/JBMR.040902.
- Baig MN. A review of epidemiological distribution of different types of fractures in paediatric age. Cureus 2017;9. https://doi.org/10.7759/cureus.1624.
- Solan MC, Rees R, Daly K. Current management of torus fractures of the distal radius. Injury 2002;33:503-5. https://doi.org/10.1016/S0020-1383(01)00198-X.
- Charnley J. The Closed Treatment of Common Fractures. Cambridge: Cambridge University Press; 2004.
- Davidson JS, Brown DJ, Barnes SN, Bruce CE. Simple treatment for torus fractures of the distal radius. J Bone Joint Surg Br 2001;83:1173-5. https://doi.org/10.1302/0301-620x.83b8.11451.
- Oakley EA, Ooi KS, Barnett PL. A randomized controlled trial of 2 methods of immobilizing torus fractures of the distal forearm. Pediatr Emerg Care 2008;24:65-70. https://doi.org/10.1097/PEC.0b013e318163db13.
- Plint AC, Perry JJ, Correll R, Gaboury I, Lawton L. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics 2006;117:691-7. https://doi.org/10.1542/peds.2005-0801.
- Symons S, Rowsell M, Bhowal B, Dias JJ. Hospital versus home management of children with buckle fractures of the distal radius. A prospective, randomised trial. J Bone Joint Surg Br 2001;83:556-60. https://doi.org/10.1302/0301-620x.83b4.11211.
- Williams KG, Smith G, Luhmann SJ, Mao J, Gunn JD, Luhmann JD. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care 2013;29:555-9. https://doi.org/10.1097/PEC.0b013e31828e56fb.
- Hamilton TW, Hutchings L, Alsousou J, Tutton E, Hodson E, Smith CH, et al. The treatment of stable paediatric forearm fractures using a cast that may be removed at home: comparison with traditional management in a randomised controlled trial. Bone Joint J 2013;95-B:1714-20. https://doi.org/10.1302/0301-620X.95B12.31299.
- Hill CE, Masters JP, Perry DC. A systematic review of alternative splinting versus complete plaster casts for the management of childhood buckle fractures of the wrist. J Pediatr Orthop B 2016;25:183-90. https://doi.org/10.1097/BPB.0000000000000240.
- Handoll HH, Elliott J, Iheozor-Ejiofor Z, Hunter J, Karantana A. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev 2018;12. https://doi.org/10.1002/14651858.CD012470.pub2.
- Karimi Mobarakeh M, Nemati A, Noktesanj R, Fallahi A, Safari S. Application of removable wrist splint in the management of distal forearm torus fractures. Trauma Mon 2013;17:370-2. https://doi.org/10.5812/traumamon.5094.
- Pountos I, Clegg J, Siddiqui A. Diagnosis and treatment of greenstick and torus fractures of the distal radius in children: a prospective randomised single blind study. J Child Orthop 2010;4:321-6. https://doi.org/10.1007/s11832-010-0269-3.
- West S, Andrews J, Bebbington A, Ennis O, Alderman P. Buckle fractures of the distal radius are safely treated in a soft bandage: a randomized prospective trial of bandage versus plaster cast. J Pediatr Orthop 2005;25:322-5. https://doi.org/10.1097/01.bpo.0000152909.16045.38.
- Kropman RH, Bemelman M, Segers MJ, Hammacher ER. Treatment of impacted greenstick forearm fractures in children using bandage or cast therapy: a prospective randomized trial. J Trauma 2010;68:425-8. https://doi.org/10.1097/TA.0b013e3181a0e70e.
- National Institute for Health and Care Excellence (NICE) . Fractures (Non-Complex): Assessment And Management 2016.
- Achten J, Knight R, Dutton SJ, Costa ML, Mason J, Dritsaki M, et al. A multicentre prospective randomized equivalence trial of a soft bandage and immediate discharge versus current treatment with rigid immobilization for torus fractures of the distal radius in children: protocol for the Forearm Fracture Recovery in Children Evaluation (FORCE) trial. Bone Jt Open 2020;1:214-21. https://doi.org/10.1302/2633-1462.16.BJO-2020-0014.R1.
- Knight R, Dritsaki M, Mason J, Perry DC, Dutton SJ. The Forearm Fracture Recovery in Children Evaluation (FORCE) trial: statistical and health economic analysis plan for an equivalence randomized controlled trial of treatment for torus fractures of the distal radius in children. Bone Jt Open 2020;1:205-13. https://doi.org/10.1302/2633-1462.16.BJO-2020-0015.R1.
- FORCE, The FOrearm fracture Recovery in Children Evaluation . A multi-centre prospective randomized equivalence trial of a soft bandage and immediate discharge versus current treatment with rigid immobilisation for torus fractures of the distal radius in children. Health Technol Assess n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/172302/#/ (accessed September 2021).
- Parsons N, Odumenya M, Edwards A, Lecky F, Pattison G. Modelling the effects of the weather on admissions to UK trauma units: a cross-sectional study. Emerg Med J 2011;28:851-5. https://doi.org/10.1136/emj.2010.091058.
- Knapp P, Stones C, Graffy J, Baines P, Preston J, Young B, et al. The TRECA study: TRials Engagement in Children and Adolescents. Health Serv Deliv Res 2021 n.d.
- Martin-Kerry J, Bower P, Young B, Graffy J, Sheridan R, Watt I, et al. Developing and evaluating multimedia information resources to improve engagement of children, adolescents, and their parents with trials (TRECA study): study protocol for a series of linked randomised controlled trials. Trials 2017;18. https://doi.org/10.1186/s13063-017-1962-z.
- Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT. Statistical assessment of the learning curves of health technologies. Health Technol Assess 2001;5. https://doi.org/10.3310/hta5120.
- Ayres-de-Campos D, Silva-Carvalho JL, Oliveira C, Martins-da-Silva I, Silva-Carvalho J, Pereira-Leite L. Inter-observer agreement in analysis of basal body temperature graphs from infertile women. Hum Reprod 1995;10:2010-16. https://doi.org/10.1093/oxfordjournals.humrep.a136227.
- Royal College of Emergency Medicine . Management of Pain in Children. Best Practice Guideline 2017. www.rcem.ac.uk/docs/RCEM%20Guidance/RCEM%20Pain%20in%20Children%20-%20Best%20Practice%20Guidance%20(REV%20Jul%202017).pdf (accessed 5 August 2021).
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs 1988;14:9-17.
- Garra G, Singer AJ, Taira BR, Chohan J, Cardoz H, Chisena E, et al. Validation of the Wong–Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med 2010;17:50-4. https://doi.org/10.1111/j.1553-2712.2009.00620.x.
- Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of faces scales for the self-report of pain intensity in children 2010;126:e1168-98. https://doi.org/10.1542/peds.2010-1609.
- Keck JF, Gerkensmeyer JE, Joyce BA, Schade JG. Reliability and validity of the Faces and Word Descriptor Scales to measure procedural pain. J Pediatr Nurs 1996;11:368-74. https://doi.org/10.1016/S0882-5963(96)80081-9.
- National Institute for Health and Care Excellence (NICE) . Major Trauma: Assessment and Initial Management 2016.
- HealthMeasures . PROMIS (Patient-Reported Outcomes Measurement Information System) n.d. www.healthmeasures.net/explore-measurement-systems/promis (accessed 15 June 2020).
- Eidt-Koch D, Mittendorf T, Greiner W. Cross-sectional validity of the EQ-5D-Y as a generic health outcome instrument in children and adolescents with cystic fibrosis in Germany. BMC Pediatr 2009;9. https://doi.org/10.1186/1471-2431-9-55.
- Wille N, Badia X, Bonsel G, Burström K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res 2010;19:875-86. https://doi.org/10.1007/s11136-010-9648-y.
- Ravens-Sieberer U, Erhart M, Rajmil L, Herdman M, Auquier P, Bruil J, et al. Reliability, construct and criterion validity of the KIDSCREEN-10 score: a short measure for children and adolescents’ well-being and health-related quality of life. Qual Life Res 2010;19:1487-500. https://doi.org/10.1007/s11136-010-9706-5.
- Widnall J, Capstick T, Wijesekera M, Messahel S, Perry DC. Pain scores in torus fractures. Bone Jt Open 2020;1:3-7. https://doi.org/10.1302/2633-1462.12.BJO-2019-0002.
- Oliveira A, Batalha L, Fernandes A, Gonçalves J, Viegas R. A functional analysis of the Wong–Baker Faces Pain Rating Scale: linearity, discriminability and amplitude. Rev Enferm Ref 2014;IV:121-30. https://doi.org/10.12707/riv14018.
- Chow SC, Wang H. On sample size calculation in bioequivalence trials. J Pharmacokinet Pharmacodyn 2001;28:155-69. https://doi.org/10.1023/a:1011503032353.
- Christensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol 2007;46:947-54. https://doi.org/10.1016/j.jhep.2007.02.015.
- Bell ML, King MT, Fairclough DL. Bias in area under the curve for longitudinal clinical trials with missing patient reported outcome data. SAGE 2014;4. https://doi.org/10.1177/2158244014534858.
- NHS Business Services Authority . NHS Supply Chain Catalogue 2018 19 2018. https://my.supplychain.nhs.uk/catalogue (accessed 3 March 2021).
- Department of Health . NHS Reference Costs 2015–16 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 5 March 2020).
- Personal Social Services Research Unit . Unit Costs of Health and Social Care n.d. www.pssru.ac.uk/project-pages/unit-costs/ (accessed 12 December 2018).
- Joint Formulary Committee . British National Formulary (Online) n.d. www.medicinescomplete.com (accessed 12 December 2018).
- Dolan P, Gudex C, Kind P, Williams A. Valuing health states: a comparison of methods. J Health Econ 1996;15:209-31. https://doi.org/10.1016/0167-6296(95)00038-0.
- Kwon J, Kim SW, Ungar WJ, Tsiplova K, Madan J, Petrou S. Patterns, trends and methodological associations in the measurement and valuation of childhood health utilities. Qual Life Res 2019;28:1705-24. https://doi.org/10.1007/s11136-019-02121-z.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Claxton K, Sculpher M, Palmer S, Culyer AJ. Causes for concern: is NICE failing to uphold its responsibilities to all NHS patients?. Heal Econ 2015;24:1-7. https://doi.org/10.1002/hec.3130.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- Morgan EM, Mara CA, Huang B, Barnett K, Carle AC, Farrell JE, et al. Establishing clinical meaning and defining important differences for Patient-Reported Outcomes Measurement Information System (PROMIS®) measures in juvenile idiopathic arthritis using standard setting with patients, parents, and providers. Qual Life Res 2017;26:565-86. https://doi.org/10.1007/s11136-016-1468-2.
- Jones S, Tyson S, Young M, Gittins M, Davis N. Patterns of moderate and severe injury in children after the introduction of major trauma networks. Arch Dis Child 2019;104:366-71. https://doi.org/10.1136/archdischild-2018-315636.
- Morrongiello BA, McArthur BA, Spence JR. Understanding gender differences in childhood injuries: examining longitudinal relations between parental reactions and boys’ versus girls’ injury-risk behaviors. Health Psychol 2016;35:523-30. https://doi.org/10.1037/hea0000275.
- Widnall J, Capstick T, Wijesekera M, Messahel S, Perry DC. Pain scores in torus fractures. Bone Jt Open 2020;1:3-7. https://doi.org/10.1302/2633-1462.12.bjo-2019-0002.
- NHS . NHS Electronic Drug Tariff 2020. www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff (accessed 3 March 2021).
- Department of Health and Social Care . Social Care – Charging for Care and Support 2017.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019 n.d. www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/ (accessed September 2021).
- Department of Health and Social Care . Social Care – Charging for Care and Support 2019.
- Curtis L, Burns A. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2015.
- Murgia M. NHS to trial artificial intelligence app in place of 111 helpline. Financial Times 2017.
- The Physiotherapy Centre . Prices: How Much 2020. www.thephysiocentre.co.uk/how_much (accessed 5 March 2020).
Appendix 1 FORCE Trial Collaborators
Shrouk Messahel, Alder Hey Children’s Hospital.
Phoebe Gibson, Alder Hey Parents and Carers Forum.
Stuart Hartshorn, Birmingham Children’s Hospital.
Fergal Monsell, Bristol Royal Hospital for Children.
Mark Lyttle, Bristol Royal Hospital for Children.
Temem (Tim) Hussan, Darlington Memorial Hospital.
Sylvester Gomes, Evelina London Children’s Hospital.
Anastasia Alcock, Evelina London Children’s Hospital.
Jennifer Preston, Experimental Arthritis Treatment Centre for Children, University of Liverpool.
Alex Novak, Horton General Hospital & John Radcliffe Hospital.
Nadine Darlow, Ipswich Hospital.
David Hartin, Ipswich Hospital.
Damian Roland, Leicester Royal Infirmary.
Emma Jenkinson, New Cross Hospital.
Christopher Gough, Nottingham Children’s Hospital.
Colin Gilhooley, Nottingham Children’s Hospital.
Darryl Wood, Queen’s Hospital & King George Hospital.
David Metcalfe, Royal Berkshire Hospital.
Manish Thakker, Royal Berkshire Hospital.
Graham Johnson, Royal Derby Hospital.
Andy Appelboam, Royal Devon and Exeter Hospital.
Tessa Davis, Royal London Hospital.
Louise Connor, Sheffield Children's Hospital.
Nicolas Nicolaou, Sheffield Children's Hospital.
Shammi Ramlakhan, Sheffield Children’s Hospital.
Rhys Beynon, St George’s Hospital.
Rahail Ahmad, St George’s Hospital.
Niall Mullen, Sunderland Royal Hospital.
Christopher McKie, Sunderland Royal Hospital.
Jane Bayreuther, University Hospital Southampton.
Dan Westacott, University Hospitals Coventry & Warwickshire.
Sarah Wilson, Wexham Park Hospital.
Yok Weng Tan, Birmingham Children’s Hospital.
Louise Morgans, Bristol Royal Hospital for Children.
Neha Jain, Bristol Royal Hospital for Children.
Aman Paul, Horton General Hospital and John Radcliffe Hospital (Oxford).
Charlotte Brown, Horton General Hospital and John Radcliffe Hospital (Oxford).
Catherine Nunn, Leicester Royal Infirmary.
Lisa Kehler, New Cross Hospital.
Krishna Vemulapalli, Queen’s Hospital and King George Hospital.
Kath O’Hagan, Royal Berkshire Hospital.
Joanna Weekes, Royal Derby Hospital.
Matt Long, Royal Devon and Exeter Hospital.
Aarani Somaskanthan, Royal London Hospital.
Emily Cadman, Royal London Hospital.
Lisa Armour, University Hospitals Coventry & Warwickshire.
Shahab Manouchehri, Wexham Park Hospital.
Appendix 2 Changes to the protocol
| Version and date | Summary of changes |
|---|---|
| 2.0: 9 January 2019 |
4.3 Outcomes: school absence – added ‘school absence, due to injury, will be recorded’ 4.5.1 Inclusion criteria (and throughout): changed to ‘4–15 years’ 4.5.1 Exclusion criteria: changed to ‘mobile telephone with internet access’ 4.5.2 Recruitment and consenting: a slight amendment and addition for clarification was required regarding child assent 4.5.5 Withdrawals: amended as no longer patient facing 4.6.2 Rigid splint immobilisation: the treatment advice has been clarified in line with the patient facing document 4.7 Adverse events: changed to ‘complications and serious adverse event management’. Adverse events were removed and these will be reported as complications in the case report forms 5.2 Economic evaluation: minor modification as required by the health economist Protocol appendix TRECA SWAT: added |
| 3.0: 13 November 2019 |
1. Contact details: update of trial management group members 4.5.2 Recruitment and consenting: changed further recruitment period from 6–8 months to 12–14 months 7. Project timetable and milestones: dates amended – end recruitment changed from December 2019 to June 2020, complete follow-up changed from February 2020 to August 2020, statistical and health economic analysis changed from May 2020 to October 2020, data review changed from June 2020 to December 2020 and final HTA report changed from July 2020 to January 2021 |
Appendix 3 Recruitment poster
FIGURE 13.
Recruitment poster for staff members of the recruitment centres.
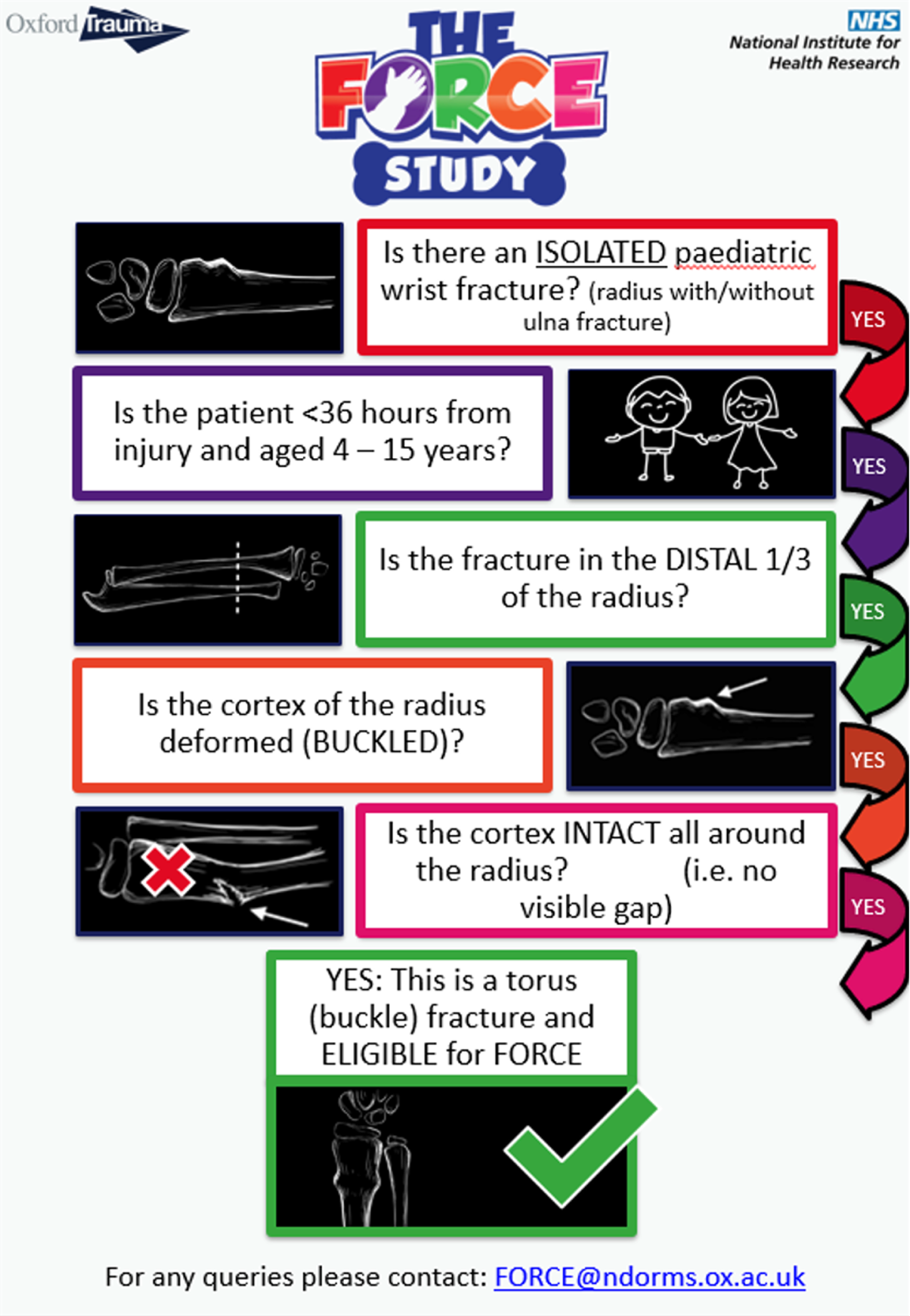
Appendix 4 Supplementary results
| Characteristic | Eligible but not randomised (N = 548) | Randomised (N = 965) |
|---|---|---|
| Centre, n (%) | ||
| Alder Hey Children’s Hospital | 45 (8.2) | 156 (16.2) |
| Birmingham Children’s Hospital | 44 (8.0) | 69 (7.2) |
| Bristol Royal Hospital for Children | 53 (9.7) | 112 (11.6) |
| Coventry University Hospital | 9 (1.6) | 22 (2.3) |
| Darlington Memorial Hospital | 2 (0.4) | 5 (0.5) |
| Evelina London Children’s Hospital | 27 (4.9) | 64 (6.6) |
| Horton General Hospital | 17 (3.1) | 37 (3.8) |
| Ipswich Hospital | 17 (3.1) | 66 (6.8) |
| John Radcliffe Hospital | 51 (9.3) | 45 (4.7) |
| King George Hospital | 39 (7.1) | 43 (4.5) |
| Leicester Royal Infirmary | 4 (0.7) | 13 (1.3) |
| New Cross Hospital | 53 (9.7) | 107 (11.1) |
| Nottingham Children’s Hospital | 40 (7.3) | 40 (4.1) |
| Queen’s Hospital | 69 (12.6) | 60 (6.2) |
| Royal Berkshire Hospital | 16 (2.9) | 22 (2.3) |
| Royal Derby Hospital | 35 (6.4) | 34 (3.5) |
| Royal Devon and Exeter Hospital | 6 (1.1) | 20 (2.1) |
| Royal London Hospital | 0 (0.0) | 2 (0.2) |
| Sheffield Children’s Hospital | 14 (2.6) | 11 (1.1) |
| St George’s Hospital | 1 (0.2) | 5 (0.5) |
| Sunderland Royal Hospital | 0 (0.0) | 1 (0.1) |
| University Hospital Southampton | 6 (1.1) | 7 (0.7) |
| Wexham Park Hospital | 0 (0.0) | 24 (2.5) |
| Sex, n (%) | ||
| Female | 232 (42.3) | 379 (39.3) |
| Male | 316 (57.7) | 586 (60.7) |
| Age (years), median (IQR), na | 10 (8–12), 548 | 9 (7–11), 965 |
| Ethnicity, n (%) | ||
| White | 388 (70.8) | 772 (80.0) |
| Black/African/Caribbean/black British | 15 (2.7) | 49 (5.1) |
| Asian/Asian British | 75 (13.7) | 98 (10.2) |
| Other ethnic group | 14 (2.6) | 21 (2.2) |
| Mixed/multiple ethnic groups | 17 (3.1) | 20 (2.1) |
| Not stated | 39 (7.1) | 5 (0.5) |
| Index of Multiple Deprivation, median (IQR), n | 4 (2–7), 548 | 4 (2–7), 965 |
| Outcome measure | Offer of a bandage group, n (%) | Rigid immobilisation group, n (%) | ||
|---|---|---|---|---|
| 4–7 years (N = 153) | 8–15 years (N = 336) | 4–7 years (N = 147) | 8–15 years (N = 329) | |
| Day 1 | ||||
| Wong–Baker Scale | 129 (84.3) | 279 (83.0) | 123 (83.7) | 259 (78.7) |
| Pain medication | 129 (84.3) | 279 (83.0) | 123 (83.7) | 259 (78.7) |
| Satisfaction | 129 (84.3) | 277 (82.4) | 123 (83.7) | 257 (78.1) |
| Day 3 | ||||
| Wong–Baker Scale | 147 (96.1) | 319 (94.9) | 139 (94.6) | 303 (92.1) |
| Pain medication | 147 (96.1) | 318 (94.6) | 139 (94.6) | 303 (92.1) |
| PROMIS | 145 (94.8) | 317 (94.3) | 138 (93.9) | 303 (92.1) |
| EQ-5D-Y | 145 (94.8) | 314 (93.5) | 139 (94.6) | 302 (91.8) |
| Day 7 | ||||
| Wong–Baker Scale | 144 (94.1) | 315 (93.8) | 137 (93.2) | 302 (91.8) |
| Pain medication | 144 (94.1) | 315 (93.8) | 137 (93.2) | 302 (91.8) |
| PROMIS | 144 (94.1) | 312 (92.9) | 137 (93.2) | 300 (91.2) |
| EQ-5D-Y | 144 (94.1) | 312 (92.9) | 137 (93.2) | 298 (90.6) |
| Day 21 | ||||
| Wong–Baker Scale | 138 (90.2) | 294 (87.5) | 135 (91.8) | 294 (89.4) |
| PROMIS | 138 (90.2) | 293 (87.2) | 134 (91.2) | 292 (88.8) |
| EQ-5D-Y | 138 (90.2) | 292 (86.9) | 134 (91.2) | 292 (88.8) |
| School attendance | 138 (90.2) | 292 (86.9) | 133 (90.5) | 292 (88.8) |
| Week 6 | ||||
| Wong–Baker Scale | 142 (92.8) | 294 (87.5) | 136 (92.5) | 295 (89.7) |
| PROMIS | 142 (92.8) | 292 (86.9) | 136 (92.5) | 292 (88.8) |
| EQ-5D-Y | 142 (92.8) | 292 (86.9) | 136 (92.5) | 292 (88.8) |
| School attendance | 142 (92.8) | 291 (86.6) | 136 (92.5) | 289 (87.8) |
| Satisfaction | 142 (92.8) | 291 (86.6) | 136 (92.5) | 289 (87.8) |
| Time point | Offer of a bandage group | Rigid immobilisation group | Adjusted difference (95% CI) | p-value |
|---|---|---|---|---|
| PROMIS score, mean (SD), na | ||||
| Overall | ||||
| Baseline | 25.0 (6.0), 428 | 25.5 (7.6), 442 | – | – |
| Day 3 | 28.7 (7.7), 424 | 27.8 (7.9), 441 | –0.79 (–1.89 to 0.30) | 0.16 |
| Day 7 | 35.0 (9.8), 412 | 34.4 (9.2), 427 | –0.49 (–1.60 to 0.62) | 0.39 |
| Week 3 | 46.8 (10.1), 397 | 46.2 (10.1), 419 | –0.63 (–1.75 to 0.49) | 0.27 |
| Week 6 | 53.0 (7.1), 396 | 52.6 (7.5), 420 | –0.38 (–1.51 to 0.74) | 0.50 |
| 4–7 years age group | ||||
| Baseline | 22.9 (6.4), 132 | 24.0 (7.6), 139 | – | – |
| Day 3 | 27.7 (7.0), 130 | 27.0 (7.9), 138 | –0.59 (–2.56 to 1.38) | 0.55 |
| Day 7 | 34.1 (8.5), 127 | 34.3 (9.1), 135 | 0.46 (–1.52 to 2.45) | 0.65 |
| Week 3 | 45.2 (10.0), 124 | 45.1 (9.5), 133 | 0.01 (–2.00 to 2.01) | 1.00 |
| Week 6 | 50.1 (8.2), 126 | 51.0 (7.4), 133 | 0.88 (–1.11 to 2.88) | 0.39 |
| 8–15 years age group | ||||
| Baseline | 25.9 (5.6), 296 | 26.2 (7.6), 303 | – | – |
| Day 3 | 29.1 (7.9), 294 | 28.2 (7.8), 303 | –0.87 (–2.18 to 0.45) | 0.20 |
| Day 7 | 35.4 (10.3), 285 | 34.5 (9.2), 292 | –0.91 (–2.24 to 0.43) | 0.18 |
| Week 3 | 47.6 (10.1), 273 | 46.8 (10.3), 286 | –0.90 (–2.25 to 0.46) | 0.19 |
| Week 6 | 54.3 (6.1), 270 | 53.4 (7.4), 287 | –0.94 (–2.30 to 0.41) | 0.17 |
| EQ-5D-Y utility score, mean (SD), nb | ||||
| Overall | ||||
| Baseline | 0.54 (0.34), 428 | 0.56 (0.34), 442 | – | – |
| Day 3 | 0.57 (0.27), 421 | 0.55 (0.27), 441 | –0.02 (–0.05 to 0.01) | 0.23 |
| Day 7 | 0.71 (0.23), 412 | 0.69 (0.24), 427 | –0.02 (–0.05 to 0.01) | 0.29 |
| Week 3 | 0.90 (0.16), 396 | 0.88 (0.16), 419 | –0.01 (–0.04 to 0.02) | 0.43 |
| Week 6 | 0.97 (0.10), 396 | 0.96 (0.11), 420 | –0.00 (–0.04 to 0.03) | 0.76 |
| 4–7 years age group | ||||
| Baseline | 0.52 (0.33), 132 | 0.60 (0.32), 139 | – | – |
| Day 3 | 0.62 (0.25), 130 | 0.57 (0.26), 139 | –0.05 (–0.11 to 0.01) | 0.08 |
| Day 7 | 0.75 (0.22), 127 | 0.74 (0.23), 135 | –0.01 (–0.06 to 0.05) | 0.83 |
| Week 3 | 0.92 (0.17), 124 | 0.92 (0.14), 133 | –0.00 (–0.06 to 0.05) | 0.89 |
| Week 6 | 0.97 (0.12), 126 | 0.97 (0.10), 133 | 0.01 (–0.05 to 0.06) | 0.82 |
| 8–15 years age group | ||||
| Baseline | 0.54 (0.34), 296 | 0.54 (0.35), 303 | – | – |
| Day 3 | 0.55 (0.27), 291 | 0.54 (0.27), 302 | –0.01 (–0.04 to 0.03) | 0.73 |
| Day 7 | 0.70 (0.23), 285 | 0.67 (0.24), 292 | –0.02 (–0.06 to 0.01) | 0.22 |
| Week 3 | 0.89 (0.16), 272 | 0.87 (0.17), 286 | –0.02 (–0.06 to 0.02) | 0.36 |
| Week 6 | 0.97 (0.08), 270 | 0.96 (0.11), 287 | –0.01 (–0.05 to 0.03) | 0.55 |
| EQ-5D-Y VAS score, mean (SD), nc | ||||
| Overall | ||||
| Baseline | 73.3 (22.0), 428 | 73.3 (22.7), 442 | – | – |
| Day 3 | 76.8 (19.4), 421 | 75.5 (19.8), 437 | –1.14 (–3.58 to 1.29) | 0.36 |
| Day 7 | 83.2 (19.3), 409 | 83.1 (17.8), 427 | –0.19 (–2.65 to 2.27) | 0.88 |
| Week 3 | 91.2 (16.1), 396 | 90.2 (16.4), 418 | –1.24 (–3.73 to 1.25) | 0.33 |
| Week 6 | 94.4 (14.3), 395 | 94.3 (16.0), 418 | –0.15 (–2.64 to 2.34) | 0.91 |
| 4–7 years age group | ||||
| Baseline | 82.2 (18.7), 132 | 79.2 (21.6), 139 | – | – |
| Day 3 | 80.1 (19.6), 130 | 80.9 (18.5), 138 | 0.66 (–3.66 to 4.99) | 0.76 |
| Day 7 | 87.8 (16.8), 125 | 88.8 (16.0), 135 | 0.91 (–3.47 to 5.29) | 0.68 |
| Week 3 | 95.7 (8.5), 124 | 94.3 (10.8), 132 | –1.64 (–6.05 to 2.77) | 0.47 |
| Week 6 | 96.2 (9.5), 126 | 96.2 (13.0), 133 | –0.26 (–4.64 to 4.13) | 0.91 |
| 8–15 years age group | ||||
| Baseline | 69.4 (22.2), 296 | 70.6 (22.8), 303 | – | – |
| Day 3 | 75.3 (19.1), 291 | 73.0 (19.9), 299 | –2.13 (–5.04 to 0.78) | 0.15 |
| Day 7 | 81.1 (20.0), 284 | 80.4 (17.9), 292 | –0.87 (–3.81 to 2.08) | 0.56 |
| Week 3 | 89.2 (18.3), 272 | 88.3 (18.1), 286 | –1.20 (–4.18 to 1.78) | 0.43 |
| Week 6 | 93.5 (16.0), 269 | 93.4 (17.2), 285 | –0.27 (–3.26 to 2.72) | 0.86 |
| Satisfaction score, median (IQR), nd | ||||
| Overall | ||||
| Day 1 | 2 (1–2), 369 | 1 (1– 2), 377 | – | < 0.001 |
| Week 6 | 1 (1–2), 395 | 1 (1–2), 417 | – | 0.20 |
| Time point | Offer of a bandage group, n/N (%) | Rigid immobilisation group, n/N (%) | OR (95% CI); p-value | RD (%) (95% CI) |
|---|---|---|---|---|
| Overall | ||||
| Day 1 | 304/371 (81.9) | 294/378 (77.8) | 0.57 (0.30 to 1.08); 0.08 | –5.1 (–11.4 to 1.1) |
| Day 3 | 239/427 (56.0) | 227/442 (51.4) | 0.65 (0.39 to 1.09); 0.10 | –5.2 (–11.0 to 0.7) |
| Day 7 | 105/415 (25.3) | 99/428 (23.1) | 0.73 (0.41 to 1.30); 0.28 | –3.1 (–9.1 to 2.8) |
| 4–7 years age group | ||||
| Day 1 | 86/116 (74.1) | 95/123 (77.2) | 1.39 (0.48 to 4.06); 0.54 | 1.9 (–9.1 to 13.0) |
| Day 3 | 57/132 (43.2) | 70/139 (50.4) | 1.60 (0.63 to 4.06); 0.32 | 6.5 (–3.9 to 17.0) |
| Day 7 | 19/127 (15.0) | 22/135 (16.3) | 0.89 (0.28 to 2.82); 0.85 | 0.2 (–10.4 to 10.8) |
| 8–15 years age group | ||||
| Day 1 | 218/255 (85.5) | 199/255 (78.0) | 0.37 (0.17 to 0.83); 0.01 | –8.3 (–15.8 to –0.8) |
| Day 3 | 182/295 (61.7) | 157/303 (51.8) | 0.43 (0.23 to 0.82); 0.01 | –10.3 (–17.3 to –3.3) |
| Day 7 | 86/288 (29.9) | 77/293 (26.3) | 0.67 (0.34 to 1.31); 0.24 | –4.4 (–11.6 to 2.7) |
| Details of school absence | Offer of a bandage group | Rigid immobilisation group | OR (95% CI) | p-value |
|---|---|---|---|---|
| Number of participants missing any days of school, n/N (%) | 97/396 (24.5) | 91/418 (21.8) | 0.85 (0.61 to 1.19) | 0.36 |
| Number of days of school missed,a median (IQR), (minimum, maximum), n | 1.5 (1–2), (0.5, 5), 97 | 1.5 (1–2), (0.5, 8), 91 | – | 0.38 |
Appendix 5 The COVID-19 implications
Owing to the restrictions imposed on normal life activities during the COVID-19 pandemic outbreak, the following areas of potential impact on the study management and results were explored:
-
Whether or not participants’ hospital presentations were more delayed during this period. The proportion of participants excluded because > 36 hours had passed since their injury was compared for those recruited prior to 23 March 2020 and those recruited after this date.
-
Whether or not the participants recruited during the pandemic/lockdown differed from those recruited prior to this (i.e. in terms of age, sex, mechanism of injury). Baseline summaries were performed separately for those recruited prior to 23 March 2020 and for those recruited after this date.
-
Whether or not school closures had an impact on the reported days of school absence. The analysis of school absence data was repeated separately for the subgroup of participants who completed follow-up prior to COVID-19-related school closures (i.e. 20 March 2020). Participants were included in this analysis only if they were randomised at least 6 weeks prior to 20 March 2020 (i.e. on or before 7 February 2020). As the final follow-up in this trial was performed on 27 August 2020, no post-COVID-related school closures group was considered.
The results from the exploratory analyses were the following:
-
The COVID-19 pandemic and resulting UK lockdown occurred while recruitment and follow-up for the FORCE trial were ongoing. Several sensitivity analyses were performed to assess the impact of this on the data.
-
The number and proportion of participants who were ineligible because their injury was > 36 hours old were summarised separately for those randomised before 23 March 2020 (235/579, 40.6%) and for those randomised on or after 23 March 2020 (23/47, 48.9%). The rate was found to be higher among the latter group; however, as only a small proportion of the total sample were screened in this phase, it is difficult to draw conclusions based on this result.
-
Stratification factors for those recruited before 23 March 2020 are compared with those recruited on or after that date in Table 38. Most of the key recruitment centres were the same in both periods, but there was some variation among the other recruitment centres. The proportion of recruits who were in the younger age group was larger after 23 March 2020, probably because of more targeted recruitment. Baseline characteristics were also compared for participants recruited before and after 23 March 2020 (Table 39) and the distributions were similar.
| Stratification factor | Before 23 March 2020 (N = 833), n (%) | On/after 23 March 2020 (N = 132), n (%) |
|---|---|---|
| Centre | ||
| Alder Hey Children’s Hospital (Liverpool) | 126 (15.1) | 30 (22.7) |
| Birmingham Children’s Hospital (Birmingham) | 58 (7.0) | 12 (9.1) |
| Bristol Royal Hospital for Children (Bristol) | 90 (10.8) | 21 (15.9) |
| Darlington Memorial Hospital (Darlington) | 5 (0.6) | 0 (0.0) |
| Evelina London Children’s Hospital (London) | 1 (0.1) | 0 (0.0) |
| Horton General Hospital (Oxford) | 20 (2.4) | 0 (0.0) |
| Ipswich Hospital (Ipswich) | 64 (7.7) | 0 (0.0) |
| John Radcliffe Hospital (Oxford) | 37 (4.4) | 0 (0.0) |
| King George Hospital (London) | 1 (0.1) | 0 (0.0) |
| Leicester Royal Infirmary (Leicester) | 55 (6.6) | 11 (8.3) |
| New Cross Hospital (Wolverhampton) | 44 (5.3) | 1 (0.8) |
| Nottingham Children’s Hospital (Nottingham) | 42 (5.0) | 1 (0.8) |
| Queen’s Hospital (Romford) | 14 (1.7) | 0 (0.0) |
| Royal Berkshire Hospital (Reading) | 0 (0.0) | 11 (8.3) |
| Royal Derby Hospital (Derby) | 98 (11.8) | 9 (6.8) |
| Royal Devon and Exeter Hospital (Exeter) | 8 (1.0) | 16 (12.1) |
| Royal London Hospital (London) | 35 (4.2) | 5 (3.8) |
| Sheffield Children’s Hospital (Sheffield) | 57 (6.8) | 3 (2.3) |
| St George’s Hospital (London) | 22 (2.6) | 0 (0.0) |
| Sunderland Royal Hospital (Sunderland) | 29 (3.5) | 5 (3.8) |
| University Hospital Southampton (Southampton) | 3 (0.4) | 2 (1.5) |
| University Hospitals Coventry & Warwickshire (Coventry) | 22 (2.6) | 0 (0.0) |
| Wexham Park Hospital (Slough) | 2 (0.2) | 5 (3.8) |
| Age group (years) | ||
| 4–7 | 242 (29.1) | 58 (43.9) |
| 8–15 | 591 (70.9) | 74 (56.1) |
| Characteristic | Before 23 March 2020 (N = 833) | On/after 23 March 2020 (N = 132) |
|---|---|---|
| Age (years), mean (SD), n | ||
| Overall | 9.78 (2.90), 833 | 8.86 (2.96), 132 |
| 4–7 | 6.23 (1.13), 242 | 6.14 (1.12), 58 |
| 8–15 | 11.23 (2.01), 591 | 11.00 (2.05), 74 |
| Female, n (%)a | ||
| Overall | 324 (38.9) | 55 (41.7) |
| 4–7 years age group | 125 (51.7) | 26 (44.8) |
| 8–15 years age group | 199 (33.7) | 29 (39.2) |
| Right-side injury, n (%)b | ||
| Overall | 366 (43.9) | 46 (34.8) |
| 4–7 years age group | 111 (45.9) | 22 (37.9) |
| 8–15 years age group | 255 (43.1) | 24 (32.4) |
| Mechanism of injury, n (%) | ||
| Low energy | ||
| Overall | 626 (75.2) | 97 (73.5) |
| 4–7 years age group | 182 (75.2) | 43 (74.1) |
| 8–15 years age group | 444 (75.1) | 54 (73.0) |
| High energy | ||
| Overall | 175 (21.0) | 31 (23.5) |
| 4–7 years age group | 57 (23.6) | 13 (22.4) |
| 8–15 years age group | 118 (20.0) | 18 (24.3) |
| Otherc | ||
| Overall | 32 (3.8) | 4 (3.0) |
| 4–7 years age group | 3 (1.2) | 2 (3.4) |
| 8–15 years age group | 29 (4.9) | 2 (2.7) |
| Dominant hand, n (%) | ||
| Right | ||
| Overall | 722 (86.7) | 108 (81.8) |
| 4–7 years age group | 206 (85.1) | 48 (82.8) |
| 8–15 years age group | 516 (87.3) | 60 (81.1) |
| Left | ||
| Overall | 100 (12.0) | 20 (15.2) |
| 4–7 years age group | 29 (12.0) | 7 (12.1) |
| 8–15 years age group | 71 (12.0) | 13 (17.6) |
| Unsure/ambidextrous | ||
| Overall | 11 (1.3) | 4 (3.0) |
| 4–7 years age group | 7 (2.9) | 3 (5.2) |
| 8–15 years age group | 4 (0.7) | 1 (1.4) |
Schools in the UK closed to most pupils on Friday 18 March 2020 and did not reopen fully until after recruitment had ended. The analysis of school absence was repeated, including only those participants who were randomised at least 6 weeks (i.e. the length of the follow-up period) prior to school closure (i.e. on or before 7 February 2020). Rates of reported school absence were slightly higher in the period associated with the lockdown than in the whole population; however, no significant differences between the two groups were identified (Table 40).
| Population | Offer of a bandage group | Rigid immobilisation group | OR (95% CI) | p-value |
|---|---|---|---|---|
| ITT | ||||
| Number of participants missing any days of school (by day 21), n/N (%) | 98/342 (28.7) | 77/338 (22.8) | 0.72 (0.51 to 1.03) | 0.07 |
| Number of days missed (to day 21), median (IQR), (minimum, maximum), n | 1.5 (1–2), (0.5, 5), 98 | 1.5 (1–2), (0.5, 8), 77 | – | 0.36 |
| Number of participants missing any days of school (days 21–42), n/N (%) | 76/341 (22.3) | 66/334 (19.8) | 0.85 (0.58 to 1.23) | 0.38 |
| Number of days missed (days 21–42), median (IQR), (minimum, maximum), n | 1.5 (1–2.25), (0.5, 7), 76 | 1.5 (1–2), (0.5, 7), 66 | – | 0.83 |
| PP | ||||
| Number of participants missing any days of school (by day 21), n/N (%) | 83/311 (26.7) | 75/333 (22.5) | 0.79 (0.55 to 1.15) | 0.22 |
| Number of days missed (to day 21), median (IQR), (minimum, maximum), n | 1.5 (1–2), (0.5, 5), 83 | 1.5 (1–2), (0.5, 8), 75 | – | 0.35 |
| Number of participants missing any days of school (days 21–42), n/N (%) | 61/307 (19.9) | 63/328 (19.2) | 0.94 (0.63 to 1.41) | 0.78 |
| Number of days of school missed (days 21–42),a median (IQR), (minimum, maximum), n | 1 (1–2), (0.5, 7), 61 | 1.5 (1–2), (0.5, 7), 63 | – | 0.82 |
Appendix 6 Health economics complementary tables
| Medication | Unit | Unit cost/day (£) | Source |
|---|---|---|---|
| Paracetamol | |||
| Child 4–5 years | 200 ml | 0.756 | NHS Electronic Drug Tariff 59 |
| Paracetamol Six Plus | |||
| Child 6–7 years | 100 ml | 0.528 | NHS Electronic Drug Tariff 59 |
| Child 8–9 years | 100 ml | 0.792 | NHS Electronic Drug Tariff 59 |
| Child 10–11 years | 100 ml | 1.056 | NHS Electronic Drug Tariff 59 |
| Child 12–15 years | 100 ml | 1.584 | NHS Electronic Drug Tariff 59 |
| Ibuprofen | |||
| Child 4–5 years | 500 ml | 0.335 | NHS Electronic Drug Tariff 59 |
| Child 6–7 years | 500 ml | 0.335 | NHS Electronic Drug Tariff 59 |
| Child 8–9 years | 500 ml | 0.447 | NHS Electronic Drug Tariff 59 |
| Ibuprofen Seven Plus | |||
| Child 10–11 years | 100 ml | 0.945 | NHS Electronic Drug Tariff 59 |
| Ibuprofen Twelve Plus | |||
| Child 12–15 years | 100 ml | 1.260 | NHS Electronic Drug Tariff 59 |
| Resource item | Unit | Unit cost (GBP) | Source |
|---|---|---|---|
| Interventions | |||
| Actimove Manus Wrist Brace | Each | 3.59 | NHS Supply Chain Catalogue 2018/19 42 |
| Promedics Neoprene Wrist Thumb Splint | Each | 7.94 | NHS Supply Chain Catalogue 2018/19 42 |
| Beagle Orthopaedic Paediatric D-ring Wrist Brace | Each | 5.15 | Trial |
| Promedics Wrist Brace | Pack of 6 | 19.44 | NHS Supply Chain Catalogue 2018/19 42 |
| Promedics Standard Neobrace | Pack of 5 | 24.44 | NHS Supply Chain Catalogue 2018/19 42 |
| Provectus Medical Ltd one-size wrist brace | Each | 4.83 | NHS Supply Chain Catalogue 2018/19 42 |
| Deltaform Futuro splint | Each | 14.40 | NHS Supply Chain Catalogue 2018/19 42 |
| 3M soft cast | Pack of 10 | 75.64 | NHS Supply Chain Catalogue 2018/19 42 |
| BeneCare Universal Wrist Splint | Each | 4.74 | NHS Supply Chain Catalogue 2018/19 42 |
| Neoprene wrist brace | Each | 4.20 | NHS Supply Chain Catalogue 2018/19 42 |
| K-Band Urgo Type 1 Conforming Bandage in small or large | Pack of 20 | 2.40 | Trial |
| Hospiform Elastic Conforming Bandage | Each | 0.26 | NHS Supply Chain Catalogue 2018/19 42 |
| Ce-Fix Conforming Bandage | Pack of 20 | 1.00 | Trial |
| Urgo K-Lite | Each | 0.39 | NHS Supply Chain Catalogue 2018/19 42 |
| Mölnylycke Tubigrip | Each | 2.32 | NHS Supply Chain Catalogue 2018/19 42 |
| Hospicrepe 233 type 2 cotton crepe bandage | Each | 0.60 | NHS Supply Chain Catalogue 2018/19 42 |
| Bandage plaster of Paris BP (7.5 cm × 2.7 m roll) | Pack of 24 | 16.13 | NHS Supply Chain Catalogue 2018/19 42 |
| Bandage of plaster of Paris BP (5 cm × 2.7 m roll) | Pack of 24 | 14.40 | NHS Supply Chain Catalogue 2018/19 42 |
| Undercast padding synthetic (5 cm × 2.7 m) | Pack of 6 | 1.18 | NHS Supply Chain Catalogue 2018/19 42 |
| Undercast padding synthetic (7.5 cm × 2.7 m) | Pack of 6 | 1.53 | NHS Supply Chain Catalogue 2018/19 42 |
| Fibreglass casing tape (7.5 cm × 3.6 m) | Pack of 10 | 30.77 | NHS Supply Chain Catalogue 2018/19 42 |
| Fibreglass casing tape (5 cm × 3.6 m) | Pack of 10 | 25.20 | NHS Supply Chain Catalogue 2018/19 42 |
| Flexible casting tape (5 cm × 3.6 m) | Pack of 10 | 53.79 | NHS Supply Chain Catalogue 2018/19 42 |
| Flexible casting tape (7.5 cm × 3.6 m) | Pack of 10 | 41.28 | NHS Supply Chain Catalogue 2018/19 42 |
| Outpatient care | |||
| Paediatric trauma and orthopaedics | Visit | 132.00 | NHS Reference Costs 2019: 21460 |
| Physiotherapy | Visit | 38.88 | PSSRU 2019,61 p. 68 |
| Radiology (X-rays) | Test | 31.00 | NHS Reference Costs 2017:a DAPF62 |
| Emergency department | Visit | 116.00 | NHS Reference Costs 2019: VB09Z60 |
| Community care (NHS) | |||
| General practitioner (surgery) | 9.22-minute visit | 39.23 | PSSRU 2019,61 p. 120 |
| General practitioner (telephone contact) | 7.1 minutes | 27.62 | aPSSRU 2015,63 p. 177 |
| Practice nurse | Hour visit | 42.00 | PSSRU 2019,61 p. 118 |
| 111 advice | Per call | 14.32 | Financial Times64 2017a |
| Physiotherapist | Session | 36.83 | PSSRU 2019,61 p. 82 |
| Community care (private) | |||
| Physiotherapy | Visit | 75.00 | The Physio Centre 202065 |
| Direct non-medical cost | |||
| Help with child care | – | Trial | |
| Lost productivity | – | Trial | |
List of abbreviations
- AUC
- area under the curve
- BNF
- British National Formulary
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DSMC
- Data Safety and Monitoring Committee
- ED
- emergency department
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-Y
- EuroQol-5 Dimensions, youth version
- FORCE
- FOrearm fracture Recovery in Children Evaluation
- GBP
- Great British pounds
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMB
- net monetary benefit
- OCTRU
- Oxford Clinical Trials Research Unit
- OR
- odds ratio
- PERUKI
- Paediatric Emergency Research United Kingdom and Ireland
- PP
- per protocol
- PROM
- patient-reported outcome measure
- PROMIS
- patient-reported outcomes measurement system
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SWAT
- Study Within A Trial
- TMG
- Trial Management Group
- TRECA
- TRials Engagement in Children and Adolescents
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
