Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/82/04. The contractual start date was in May 2016. The draft report began editorial review in January 2020 and was accepted for publication in September 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Daniels et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this report have been reproduced from Daniels et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Group B Streptococcus disease
Group B Streptococcus (GBS) (Streptococcus agalactiae) is a ubiquitous bacterium that forms part of the normal bacterial flora of the gut and genital tract. In adults, GBS is an occasional cause of serious systemic infections in immunocompromised people, but is more commonly seen as an opportunistic pathogen of the female urogenital tract. 2 However, if a neonate, whose immune system is immature, is exposed to GBS then it can lead to sepsis and death. Most systemic GBS infections usually present within 24 hours of delivery as rapidly progressing septicaemia. However, early-onset GBS disease of the newborn is defined by the National Institute for Health and Care Excellence (NICE) as occurring within the first 72 hours of life,3 and by the Royal College of Obstetricians and Gynaecologists (RCOG) as occurring before the first 7 days of age. 4,5 Exposure to GBS present in the genital tract of the mother during birth is thought to be the most common route for early-onset colonisation in the neonate. The incidence of early-onset GBS sepsis can be decreased if women colonised with GBS or who have risk factors associated with early-onset GBS infection are given intrapartum antibiotic prophylaxis (IAP) during labour. Any infection with GBS in babies between 7 days and 3 months of age is deemed late onset and is more often associated with localised infections (especially meningitis and pneumonia). Colonisation from environmental sources is thought to be the most common cause of late-onset GBS infection and is beyond the remit of the Group B Streptococcus 2 (GBS2) trial.
Early-onset group B Streptococcus infection
Maternal colonisation
The gastrointestinal tract is the natural reservoir of GBS in humans and is the likely source of vaginal colonisation. Asymptomatic maternal colonisation with GBS has been reported at a global level of 15%,6 although this figure can vary with race, region and the method of laboratory culture used for its detection. 6,7 A previous review of UK studies indicated an overall colonisation rate of 18% from vaginal/rectal swabs [95% confidence interval (CI) 16% to 21%], higher than from vaginal swabs alone. 8
Transmission
Neonates with early-onset GBS infection show initial colonisation mainly in the mucous membranes of their respiratory tract, and the major route of vertical transmission at the time of birth is thought to be through aspiration of vaginal, rectal and amniotic aerosols during birth. Vertical transmission in utero is thought to occur as a consequence of prolonged rupture of membranes (although GBS can cross intact membranes9) and is regarded as one of the causes of stillbirth. 10 Colonisation of the mother is less predictive for late-onset GBS infection, with prematurity being the major risk factor. 11
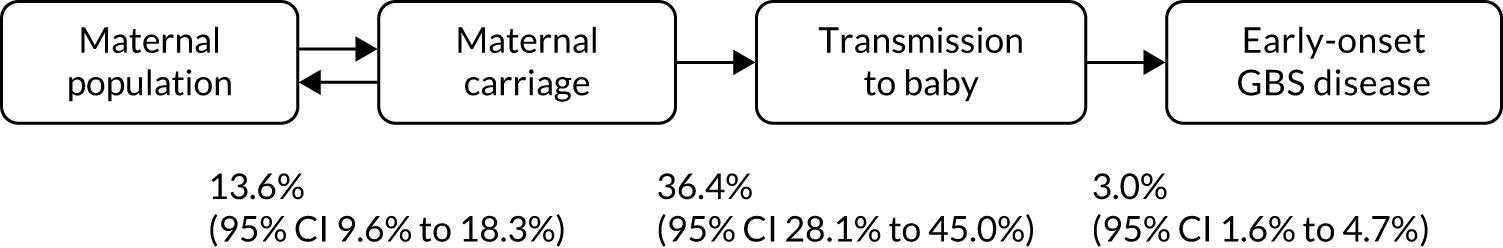
The association between the rates of maternal colonisation, transmission and infection has been established (Figure 1). A meta-analysis of six studies of the maternal and baby colonisation rates in an untreated general population showed a transmission rate between the colonised mother and her baby of 36.4% (95% CI 28.1% to 45.0%). A further analysis in the same report gave an average incidence of 3.0% (95% CI 1.6% to 4.7%) of babies born to colonised mothers who went on to develop early-onset GBS disease. 8 There is some evidence that maternal colonisation with GBS is associated with preterm birth, particularly ascending infection due to GBS bacteriuria. 12
FIGURE 1.
Model of colonisation, transmission and early-onset GBS disease.
Burden of early-onset group B Streptococcus infection
Group B Streptococcus remains one of the most important causes of severe early-onset infection in newborn infants. A systematic review estimated that the global incidence of early-onset GBS infection was 0.41 (95% CI 0.36 to 0.47) per 1000 live births, with the highest burden found in the Caribbean and South Africa. 13 Enhanced surveillance in the UK and Ireland from 2014 to 2015 showed an incidence of culture-proven GBS infection in babies aged < 90 days of 0.94 (95% CI 0.88 to 1.00) per 1000 live births. 14 In this study, 60% of cases were early-onset infection, a rate of 0.57 (95% CI 0.52 to 0.62) per 1000 live births, an increase from 0.48 per 1000 live births in 2000. In the USA, the incidence of early-onset GBS infection fell from 1.7 per 1000 live births in the early 1990s to 0.22 per 1000 live births in 2014, without any concurrent rise in Gram-negative sepsis. 15
The global case–fatality ratio is estimated at 8.4%, with a fourfold variation between Africa and high-income countries,13 whereas in the UK it was 6.2% in 2014, an improvement from 9.6% in 2001, possibly because of the improvements in neonatal care. Mortality is much higher in preterm babies. Oddie and Embleton16 found that preterm infants accounted for 38% of all cases and 83% of the deaths from early-onset GBS infection. Information on morbidity among survivors is less clear, but significant long-term morbidity, including impaired psychomotor development, has been reported in up to 30% of survivors. 17
It is highly likely that some cases of serious early-onset sepsis caused by GBS are unrecognised because cultures of blood and cerebrospinal fluid are negative. By taking into account superficial swab culture results from all neonates who underwent a septic screen in the first 72 hours of life, Luck et al. 18 concluded that the true incidence of early-onset GBS disease in the UK may be as high as 3.6 per 1000 live births (i.e. over seven times higher than previously estimated). Epidemiological studies have suggested that various factors present at the time of birth are associated with the neonate having an increased risk of developing GBS disease, presenting as either an early- or late-onset infection. UK surveillance data suggest that only 35% of infants with early-onset GBS infection were born to mothers with one or more risk factors,14 as defined by the RCOG guidelines implemented at that time. 19
Maternal risk factor for neonatal disease
Maternal risk factors for vertical GBS transmission to the newborn disease have been suggested to include the following.
Prematurity
Colonised premature babies are at a high risk of developing early-onset GBS infection, as their immune system is immature and they are less likely to have received passive immunity transplacentally. The pooled odds of maternal colonisation in preterm births, compared with births at > 37 weeks’ gestation, was 1.53 (95% CI 1.14 to 2.05). Birth weight is highly correlated with prematurity and inversely related to developing early-onset GBS infection. The UK surveillance study indicated an incidence of 2.24 (95% CI 1.31 to 3.59) early-onset GBS infection cases per 1000 live births in babies weighing < 1500 g at birth, compared with 0.43 (95% CI 0.38 to 0.49) per 1000 live births in those weighing ≥ 2500 g at birth. 14
Prelabour rupture of membranes
Prelabour rupture of membranes (PROM) with a delay in progress to established labour would be expected to lead to an increased likelihood of ascending infection and baby colonisation in utero, although there is debate as to what, if any, role the presence of GBS plays in the induction of PROM. Rupture of the membranes > 18 hours before birth is significantly associated with early-onset GBS infection (odds ratio 25.8, 95% CI 10.2 to 64.8) compared with non-infected infants. 17 Therefore, babies born to mothers who experience preterm labour with PROM of any duration, or preterm labour if there is suspected or confirmed intrapartum rupture of membranes lasting > 18 hours, are especially thought to be at risk of developing early-onset GBS infection. UK NICE guidelines recommend that induction of labour at term is offered at 24 hours after PROM. 3
Maternal fever
Intrapartum fever is also highly associated with the development of early-onset GBS infection (odds ratio 10.0, 95% CI 2.4 to 40.8). 16 In a UK surveillance study, 19% of babies with early-onset GBS infection were born to mothers with intrapartum pyrexia and 31% to mothers with suspected chorioamnionitis. 14
Previous baby with group B Streptococcus disease
The proportion of women giving birth at term who have had a previous baby with early-onset GBS infection was estimated at 0.08%. These women are considered to be at higher risk of another infected baby, than multiparous women whose previous babies were not affected, although the data are insufficient to describe the size of this increased risk. 20
Group B Streptococcus detected in current pregnancy
Group B Streptococcus bacteriuria is associated with higher risk of chorioamnionitis and neonatal disease, although the data are insufficient to quantify the increased risk. 21,22 In the 2014 UK surveillance study, 9% of mothers of GBS-infected babies were known to be colonised with GBS, although a figure of 5% was used in a model of the effectiveness of screening. 23
Testing for risk of group B Streptococcus disease
The aim of maternal GBS testing is to prevent early-onset GBS infection. However, no tests discriminate between colonised mothers who will or will not transmit GBS to their babies, or between babies who will or will not develop early-onset GBS infection. Instead, there are several methods for identifying GBS maternal colonisation in late pregnancy or during labour.
Tests for maternal group B Streptococcus colonisation
Bacteriological culture
The sensitivity of testing methods based on the culture identification of maternal colonisation of GBS depends on the timing of specimen collection, the source of the specimen and the culture technique used by the microbiology laboratory. A systematic review of prospective studies showed that a single vaginal/rectal swab has a positive predictive value (i.e. the proportion positive at 35–37 weeks’ gestation who remain positive at term labour) of 70.2% and a negative predictive value of 95.2%, but there were variations in testing methods. 24 Overall, using retrospective and prospective studies and different types of swabs and culture methods, sensitivities of the antenatal tests ranged from 51% to 71% and specificities were consistently around 85%. 24
Molecular tests for group B Streptococcus colonisation
Development of molecular methods that allow rapid bedside detection of microorganisms, such as polymerase chain reaction (PCR) methods, offers the potential to target use of antibiotics more specifically than was previously possible. Previous experience shows that implementation of complex point-of-care (POC) tests for GBS colonisation are technically feasible. 25 However, the practical value of any POC test depends on accurate results being reliably available within the clinically required time frame. To this end, careful consideration is required of a number of factors, including the expected frequency of testing, achievable results turnaround times, the amount of hands-on test time, strategies to deal with test failure and assurance of the ongoing availability of sufficient trained staff able to undertake testing when required.
The majority of the commercially available test systems required multiple preparation and incubation steps. What is required, is a system that can use swabs directly and be operated by midwives on a maternity unit. In the light of these limitations, the technology used in the GBS2 trial was the Cepheid GeneXpert® system (Cepheid, Maurens-Scopont France), which is feasible as a POC test. The Xpert® GBS test (Cepheid) for this platform allows detection of GBS within 35 minutes of placing a swab into a cartridge and loading one machine, with a hands-on preparation time of < 2 minutes.
Accuracy of GeneXpert rapid test to detect group B Streptococcus colonisation
The Cepheid GeneXpert GBS system has been available since 2008 and several groups have assessed its accuracy in studies, although with different swabbing strategies and reference standard comparators. The manufacturer cites a sensitivity (1 – false-positive rate) of 91.9% (95% CI 84.7% to 96.5%) and a specificity of 95.6% (95% CI 95.0% to 98.9%) for intrapartum vaginal/rectal samples, compared with selective enrichment culture of a second intrapartum swab. 26 A meta-analysis, undertaken in 2014, of nine studies, which used an enrichment culture method for the reference standard, obtained estimates of the average accuracy of the test of sensitivity 96.4% (95% CI 90.8% to 98.6%) and specificity 98.9% (95% CI 97.5% to 99.5%) (Jane Daniels, University of Nottingham, 2014, personal communication). Overall, the quality of the studies was rated as being adequate, but the potential for biases remained, from the lack of blinding, the use of only vaginal swabs and the inappropriate use of non-enrichment culture as the reference standard, which could underestimate colonisation rates. A subsequent meta-analysis of 15 studies using various test PCR platforms produced a pooled sensitivity of 93.7% (95% CI 92.1% to 95.3%) and specificity of 97.6% (95% CI 97.0 % to 98.1%). 27 These accuracy parameters, together with the turnaround time, suggest that the GeneXpert system meets the proposed criteria set by the US Centers for Disease Control and Prevention for a clinically useful GBS test, namely that it should consist of a simple bedside kit that can be used by delivery suite staff, have a turnaround time of < 30 minutes, and have a sensitivity and specificity of ≥ 90%. 26
Current policy for prevention of early-onset group B Streptococcus infection
The strategy recommended by the UK’s RCOG is to consider and offer IAP to women who have risk factors identified during the pregnancy or in labour for having a baby with early-onset GBS infection. 4 Reviews by the UK National Screening Committee (NSC)24,28 and NICE3 have endorsed this approach.
The risk factors to consider in this approach, and the management options available in the original,29 the 201219 and the 20174 revised guidance are summarised below.
Women with a previous baby with group B Streptococcus disease
-
2003, 2012, 2017 guidelines: offer IAP.
Women with group B Streptococcus bacteriuria in the current pregnancy
-
2003 guidelines: consider IAP.
-
2012 and 2017 guidelines: offer IAP.
Women with an incidental finding of vaginal or rectal group B Streptococcus colonisation, or from an intentional test, in the current pregnancy
-
2003 guidelines: consider IAP.
-
2012 and 2017 guidelines: offer IAP.
Prematurity of < 37 weeks’ gestation without known group B Streptococcus colonisation
-
2003 guidelines: discuss IAP.
-
2012 guidelines: do not offer IAP in women presenting in established preterm labour with intact membranes and with no other risk factors for GBS, unless they are known to be colonised with GBS.
-
2017 guidelines: recommend IAP to all women in confirmed preterm labour.
Prolonged rupture of membranes > 18 hours
-
2003 guidelines: consider IAP.
-
2012 guidelines: states that the evidence for IAP is unclear for women at term with PROM.
-
2017 guidelines: if a woman is known to be colonised then she should be offered IAP and the induction of labour. If colonisation status is negative or unknown, offer the induction of labour immediately or by 24 hours.
Fever in labour > 38 °C
-
2003 guidelines: discuss IAP.
-
2012 and 2017 guidelines: offer IAP.
The 2017 revision recommends that (1) antenatal antibiotics (before labour starts or membranes rupture) are not offered to women, even if proven to be colonised with GBS by a vaginal or rectal swab, and (2) women undergoing planned caesarean in absence of labour and with intact membranes, whether at term or preterm, do not require GBS-specific antibiotics. A further recommendation is that women who were diagnosed as GBS carriers in a previous pregnancy, the opportunity for culture-based testing in the late third trimester or IAP without testing should be offered in subsequent pregnancies.
NICE issued guidance in 2012 on antibiotics for the prevention and treatment of early-onset neonatal infection. 3 The guidance recommends that IAP should be offered to women who have had:
-
a previous baby with an invasive GBS infection
-
GBS colonisation, bacteriuria or infection in the current pregnancy.
The guidance suggests that IAP is considered for women in preterm labour if there is:
-
PROM of any duration
-
suspected or confirmed intrapartum rupture of membranes lasting > 18 hours.
For women with PROM at term, including prolonged (> 24 hours) rupture, the use of prophylactic antibiotics is not recommended. 30
Evidence for group B Streptococcus testing strategies
Introducing universal testing for maternal GBS colonisation into the UK health-care system has been considered alongside other testing and vaccination strategies from the perspectives of both cost-effectiveness and overtreatment. The UK NSC reviewed the evidence for universal and risk factor-based screening in 2012 and again in 2017, and at both times concluded that there was insufficient evidence against its standardised criteria to justify a change from the current risk factor-based screening approach to guide administration of IAP. 24,28 The NSC estimated that the number of women needing to receive IAP on the basis of culture-based testing at 35–37 weeks to prevent one case of early-onset GBS infection missed by the risk factor approach was 1000–1500 in the 2012 analysis and 1675–1854 in the 2017 analysis. In the 2019 hypothetical cohort, an additional 96,260 women would receive IAP under a testing policy; however, with the test having a positive predictive power of 0.2%, the overwhelming majority of infants born to women receiving IAP would never have been at risk. 23 However, the model’s input parameters have been called into question, as the risk factor strategy emerged with an early-onset GBS infection rate of 0.49 per 1000 live births, which is significantly lower than UK surveillance data suggest. 14,23
A French study31 assessed whether or not rapid intrapartum testing for GBS reduced the proportion of women inappropriately given IAP, compared with a hypothetical situation in which the national screening programme standard of antenatal testing prevailed. The French investigators assessed the diagnostic performance of both the GeneXpert test and microbiological screening at 34–38 weeks’ gestation compared with a reference standard of microbiological culture of intrapartum swabs. IAP was directed during the study by the rapid test, and the adequacy of this strategy compared with what hypothetically would have been followed by use of the 35–37 weeks’ gestation culture result. The authors31 concluded that the universal rapid intrapartum test would correspond to an absolute risk reduction of 0.925%, equivalent to 108 more women needing to be tested in labour and provided IAP to prevent a single case of early-onset GBS infection that would be missed by the culture-based testing. 31
Acceptability and implementation of testing for maternal group B Streptococcus colonisation
Even if the trial shows the superiority of one test over the other, the likelihood of successful implementation will depend on the acceptability of the test for childbearing women and for maternity care professionals. The previous UK GBS rapid testing study24 did examine the acceptability of the swabbing process and the information provided, but in a hypothetical situation in which the results were not being used to direct care. Although study participation was less than half of those approached, among the women who did agree to participate, there was a high level of satisfaction with the process, with 80.5% of women satisfied or very satisfied with the information provided, 94.3% of women happy or very happy with the way the swabs were taken and 94.1% of women confident in its use in routine care. The NSC consider evidence that the criterion, namely that any test should be acceptable to the target population, has been met as uncertain. 24
Cost-effectiveness of group B Streptococcus testing for maternal group B Streptococcus colonisation
At the time of the initiation of the GBS2 trial, the most relevant cost-effectiveness evidence was a 2010 decision model, which suggested that a policy of routine culture testing at 35–37 weeks was considered the most cost-effective once routine untargeted IAP was eliminated as a potential strategy. 32 Intrapartum testing using first-generation PCR tests was not cost-effective unless the average cost of testing was reduced from £29.95 to £7.00. The 2017 NSC review did not identify any new cost-effectiveness estimates relevant to testing in a UK setting. 4 Economic models do not yet have the data to incorporate aspects such as the effect of widespread use of antibiotics on the development of antibiotic resistance and the impact this will have, the impact of maternal and neonatal microbiomes, and the effect of very rare but potentially catastrophic anaphylaxis in labour.
Antibiotic resistance
Antibiotic resistance is considered an imminent threat to human health and the impact of antibiotic resistance spans all ages, including infants. 33–35 Children may be colonised with antibiotic-resistant bacteria early in life. 36 Antibiotic (treatment)-resistant bacteria have been increasingly shown to cause early- and late-onset neonatal sepsis and neonatal intensive care unit sepsis outbreaks. 37,38 Studies of transmission of bacteria from mothers to their infants have predominantly focused on the causative agents of early neonatal sepsis: Streptococcus agalactiae (GBS) and Escherichia coli. 39,40 Risk factors for neonatal colonisation with multiantibiotic-resistant strains of E. coli, for example extended-spectrum β-lactamase (ESBL)-producing strains, have been described, but the relative contribution of maternal carriage at the time of birth is uncertain. 41 As part of an Olympics surveillance project and in collaboration with Public Health England in 2014, ESBL-producing Enterobacteriaceae were isolated from 20% of women of childbearing age (i.e. 15–45 years) who submitted a faecal sample for laboratory examinations in the north-east of London. 42 At the same time, colonisation with ESBL-producing Enterobacteriaceae has been demonstrated in 7% of neonatal intensive care unit infants of < 31 weeks’ gestational age recruited into a multicentre double-blind placebo-controlled randomised probiotic feeding study in the south-east of England. 43
Strategies for control of antibiotic resistance have focused on antibiotic stewardship programmes designed to reduce the selection pressure for resistance and early detection of carriers. Carriers of antibiotic-resistant microbes can be identified by screening and actions can be taken to prevent the spread from carriers to others, including the implementation of contact precautions and decontamination of colonised individuals, when this is feasible. By contrast with methicillin-resistant Staphylococcus aureus (MRSA), there is currently no reliable method of decontamination of individuals colonised with antibiotic-resistant Enterobacteriaceae, including those with ESβLs. ESβLs have recently emerged in community-acquired E. coli and Klebsiella pneumoniae, and their identification as causal agents of infections in neonatal units and the lack of effective therapeutic options is a worrying development. 36
Prevalence of antibiotic resistant group B Streptococcus
Penicillin remains the first choice of antibiotic for GBS prophylaxis and treatment. 44–47 However, since 2008, strains with reduced susceptibility have emerged. Studies in Japan have detected alarming rates of resistance, with 14.7% of GBS isolates testing resistant to penicillin, a marked increase from 2.3% in 2005–6, albeit from a range of samples and populations and not vaginal/rectal swabs from pregnant women. 48 Although resistance to penicillin appears to be low outside Japan, resistance to the secondary antibiotics of choice, clindamycin and erythromycin, is high, and so it is possible that these will not forever be available as an alternative for penicillin-allergic women. Clindamycin was removed as a recommended alternative to benzylpenicillin in the 2017 RCOG guidelines, as the resistance rate in the UK is reported as 16%. 14 Other research highlights substantial risks of adverse consequences arising from exposure of the fetus and newborn infant to unnecessary antibiotics, including necrotising enterocolitis, inflammatory bowel disease, fungal infection49 and cerebral palsy. 50
Chapter 2 Aims of the GBS2 trial and design
The aim of the GBS2 trial was to establish whether or not, a strategy of targeted IAP based on the results of a rapid intrapartum test for maternal GBS colonisation can reduce unnecessary maternal and neonatal antibiotic exposure in women with risk factors for their babies developing early-onset GBS disease, and if the rapid test can accurately diagnose GBS colonisation in clinical practice.
Primary objectives
-
To determine if the use of the rapid intrapartum test for maternal GBS colonisation reduces maternal and neonatal antibiotic exposure, compared with usual care in which IAP is based on maternal risk factors, in a cluster randomised trial.
-
To determine the real-time accuracy of the rapid intrapartum test for GBS colonisation among women in labour with risk factors for GBS transmission, compared with the reference standard of selective enrichment culture, in a cross-sectional study nested within the randomised cohort.
Secondary objectives
-
To evaluate if the rapid intrapartum test reduces IAP in the mother for any indication compared with usual care.
-
To evaluate the effect of the rapid test, compared with the usual-care strategy, on neonatal exposure to antibiotics, neonatal morbidity and neonatal mortality.
-
To evaluate if timely IAP administration can be achieved with a rapid intrapartum test to ensure adequate antibiotic exposure by establishing a standard operating procedure for use of the test.
-
To evaluate the cost and cost-effectiveness of using the rapid intrapartum test compared with usual care.
-
To evaluate the antibiotic resistance profile of GBS and the colonisation by other antibiotic-resistant bacteria of the mother from the intrapartum vaginal/rectal swab, and the risk of such colonisation in the baby at 6 weeks of age.
-
To evaluate the colonisation rate of antibiotic-resistant bacteria, particularly E. coli, MRSA and vancomycin-resistant enterococci (VRE), in vaginal/rectal samples from women.
-
To evaluate the extent to which colonisation of specific resistant bacteria or resistance elements of the mother at the time of birth increase the risk of carriage of those specific bacteria or elements to the infant at 6 weeks of postnatal age.
-
To gather some information on peripartum risk factors for transmission (e.g. mode of birth, gestational age and antibiotic exposure).
Rationale for trial design
A randomised comparison provides the most reliable data to compare test strategies for IAP to prevent early-onset GBS infection. It was decided that individual randomisation would not be feasible for the GBS2 trial because of two factors. First, if a GeneXpert system is available on a maternity ward, given its presumed high accuracy, it would be difficult and potentially unethical not to offer this test to all women in labour. Second, women would have to provide consent at the point of diagnosis of labour. The first GBS study51 showed that obtaining consent for research delays the start of the testing process and inevitably means that many women are not approached by midwives for participation. This undermines the principle that it is the strategy of targeted testing being compared and, hence, is not generalisable at the population level.
The cluster trial randomised different maternity units to follow either the rapid testing or the usual-care strategy of offering IAP to all women with risk factors, and required that all women with risk factors followed the same strategy within each unit. In cluster trials, it is important that either all eligible participants are identified prior to the unit being randomised, which is possible with a prevalent population, or outcome data from all eligible participants are included in the analysis. As intrapartum risk factors can be identified only at the time when the screening strategy needs to be applied, it was necessary to include all women with risk factors in the analysis. If consent was sought, there would be selection by midwife (overtly or unintentionally, because of time pressures) and resulting in women declining to provide swabs or data for research. The selection bias caused by the need to approach and individually consent participants within a cluster leads to unreliable estimates of screening effectiveness. 52 However, if the testing strategy is adopted as standard practice by the maternity unit and anonymous routinely collected data are retrieved, consent for research is unnecessary (although clinical consent for vaginal/rectal and neonatal swabs would prevail).
For the test to be proven useful, it will need to detect a higher proportion of GBS maternal carriage than other tests, but not at the cost of low specificity and overuse of IAP. The appropriate evaluation of the test’s performance was to follow a classic test accuracy design. The rapid test performed on intrapartum rectovaginal swabs was the index test, which was compared with microbiological culture of duplicate swabs serving as the reference standard. The a priori sample size was computed to determine the sensitivity of the rapid test within a 10% margin, but with the ability to assess specificity with greater precision.
If a rapid POC test improves the detection of maternal GBS colonisation in the intrapartum period, then it is likely that important cost implications will be seen for the health-care sector. For example, IAP will be avoided for many women who test negative and appropriate treatment for those who test positive should lead to a reduction in admission to neonatal intensive care. The accuracy of the test must be carefully examined and established for its impact on both false-positive and false-negative results, and the costs and outcomes that follow based on decisions made as a result of the test must be evaluated. For example, the rapid test may detect additional cases of maternal GBS colonisation compared with the usual-care risk factor-based strategy, which will increase the use of antibiotics prescribed in the intrapartum period; however, it could ultimately reduce the risk of early-onset GBS infection and avoid costly neonatal admissions. Alternatively, replacement of the risk factor-based strategy with the rapid test may lead to an increased number of false positives, resulting in the administration of unnecessary IAP, or it could lead to an increased number of false-negative test results and consequential adverse outcomes.
This trial provided an opportunity to correlate maternal carriage of resistant bacteria with early colonisation of the corresponding infant. Maternal vaginal/rectal samples collected for the trial and used for the enrichment culture reference standard, and neonatal swabs and routinely collected specimens, were reprocessed to determine the presence of antibiotic-resistant Gram-negative bacteria, focusing particularly on ESBL-producing strains using selective media. Strains isolated from mothers and their infants were typed to determine the degree of relatedness. The outcomes from this part of the trial include the carriage rates of antibiotic-resistant Gram-negatives in the trial population of pregnant women, the risk of colonisation in their infant(s) and the peripartum risk factors for transmission (e.g. mode of birth, maternal comorbidities and colonising species).
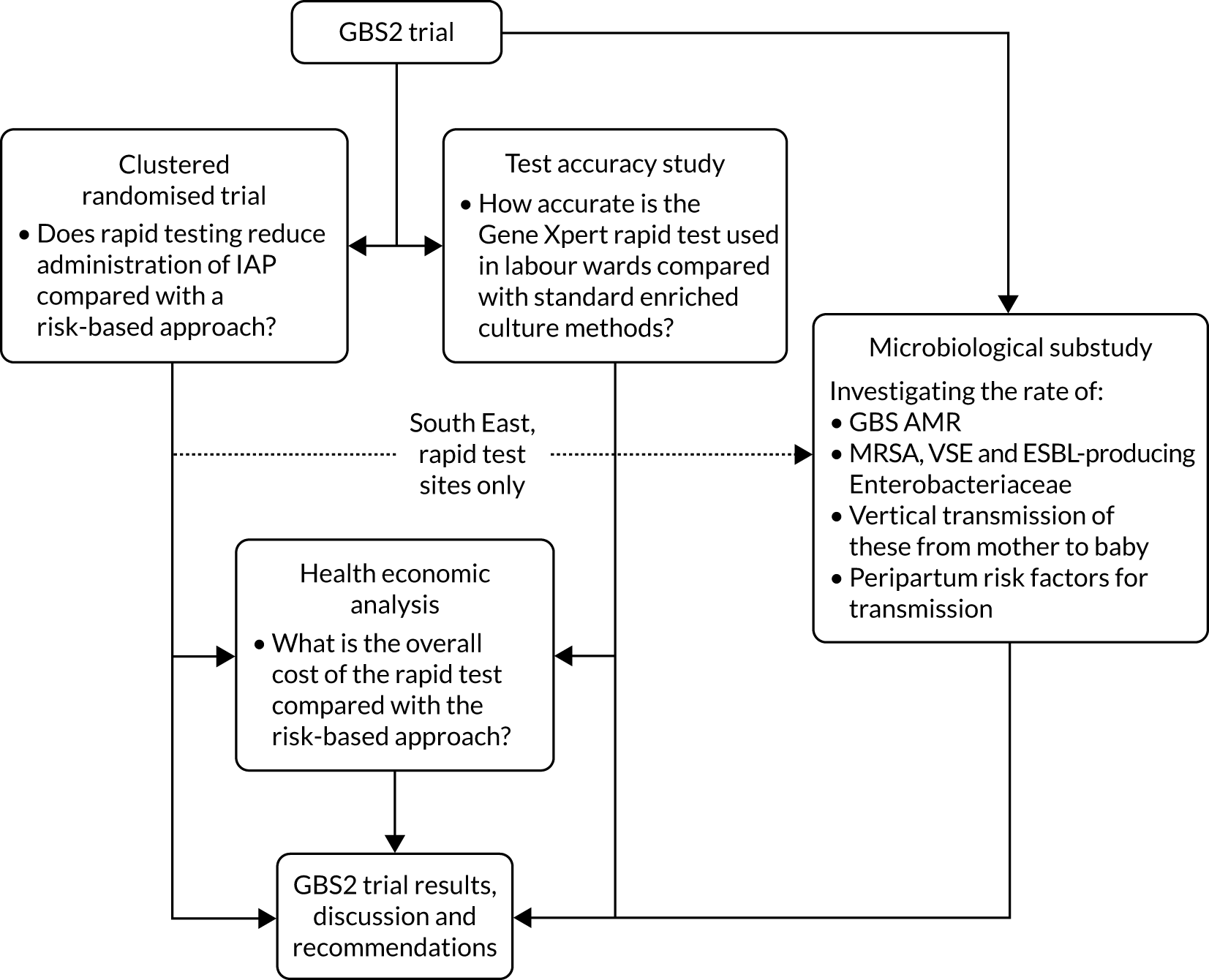
An overall trial schema for the GBS2 trial is shown in Figure 2.
FIGURE 2.
Overall trial schema for the GBS2 trial. AMR, antimicrobial resistance.
Chapter 3 Methods
Parts of this chapter have been reproduced from Daniels et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Trial design
The GBS2 trial was a multicentre, prospective, unblinded, parallel-cluster, randomised controlled trial (RCT), with a nested test accuracy study and a nested microbiological substudy, with economic evaluation.
The GBS2 trial had a favourable ethics opinion from the West Midlands – Edgbaston Research Ethics Committee (reference number 16/WM/0036), including waiver of individual research consent.
There were three substantial amendments to the protocol. The first amendment was approved on 18 April 2017, before the first unit started identifying women, and aligned eligibility criteria to those anticipated to be in the revised 2017 RCOG guidelines,4 which redefined the maternal risk factors for early-onset GBS infection. The second amendment was approved on 7 March 2018 and updated the recommended antibiotics, following the publication of the RCOG’s guidelines. The third amendment was approved on 3 December 2018 and formally capped each unit’s recruitment target at 86 women.
Cluster randomised controlled trial
The GBS2 trial was designed as a cluster RCT that was randomised at the level of the maternity unit. Sites were randomised to the rapid test strategy (i.e. IAP administration based on the rapid test results) or to usual care (i.e. the standard risk-based screening strategy and IAP offered to all women with risk factors).
Nested test accuracy study
To establish the real-time accuracy of the Cepheid GeneXpert system rapid POC test for GBS colonisation in women presenting to a labour ward with risk factors for early-onset GBS infection, the results of the rapid test were compared with the reference standard of selective enrichment culture in a prospective cohort study. The results of the rapid test preceded those of the culture test and interpretation of the reference standard was performed blind to the rapid test.
Process evaluation in rapid test sites
In the units that were allocated to rapid testing in the cluster randomised trial, we undertook an observational study to determine in real time the timings between rapid test results and commencement of IAP, and to identify if there were any failures in producing results once the tests were initiated on the machine.
Economic evaluation
The economic analysis is based on primary clinical data collected in the cluster RCT, adopting the UK NHS perspective. Full methods of the health economics analysis are described in Chapter 7.
Microbiological substudy
A substudy embedded in the GBS2 trial estimated the relatedness of certain bacterial species that are of public health concern that may colonise the mother during the birth of her baby and her child when they reach 6 weeks of age. The substudy also aimed to detect the antibiotic susceptibility of these strains. This substudy took place in participating units in London and the south-east of England (LSE) that were randomised to receive a rapid test system. The methods are described in Chapter 8.
Centres and participants
Eligibility of centres
Maternity units were eligible to participate in the GBS2 trial if they were prepared to accept a policy of rapid test-directed IAP administration to all women with risk factors for the duration of the trial period when allocated to the rapid test strategy. Maternity units also had to be prepared to include all preterm labours considered as high risk, irrespective of the implementation date of the RCOG’s guidelines and its current local policy. The trusts hosting the maternity units were also required to have access to microbiology facilities that were able to perform a selective enrichment bacteriological culture to detect GBS.
Randomisation
Randomisation of clusters was performed at the Birmingham Clinical Trials Unit using a minimisation algorithm programed in a Microsoft Excel® spreadsheet (Microsoft Corporation, Redmond, WA, USA) incorporating the following factors:
-
region [the Midlands (MID) or LSE]
-
pre-trial IAP rate (above or below the median)
-
the number of vaginal or emergency caesarean births (above or below the median).
The pre-trial IAP rates and the birth rates over a period of 12 months were determined from all potential recruiting units prior to randomisation. This estimate provided the trial with the size of the population eligible to be tested, and served as a minimisation variable and as the denominator for the IAP use calculation. Antibiotic data were derived from interrogation of hospital records and pharmacy prescribing databases at the level of the maternity unit, not at the individual level. Using the standard dosing regimen of antibiotics, the size of the eligible population and the assumptions regarding the average duration of antibiotic administration, we estimated the number of women who received IAP prior to the trial. The median value of the eligible population sizes and pre-trial IAP rates were calculated for the first 20 maternity units that were intending to participate in the GBS2 trial, and these were used as thresholds for dichotomising the minimisation variable data.
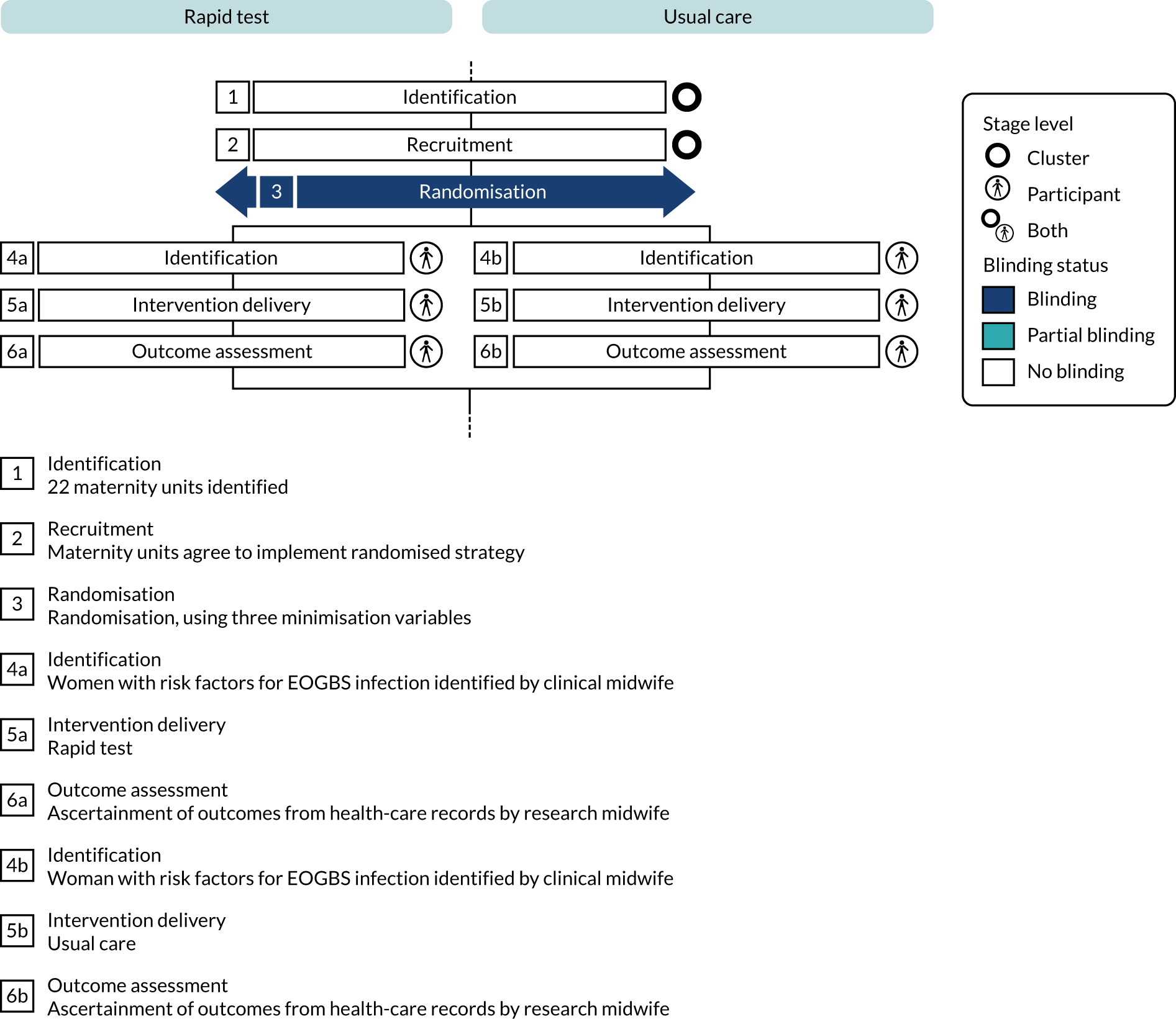
Blinding
Owing to the different study procedures under the two testing strategies, it was not possible to blind maternity unit staff to their allocation. A summary of the blinding status along the trial pathway is shown in Figure 3.
FIGURE 3.
Cluster randomised trial blinding timeline for the GBS2 trial. EOGBS, early-onset group B Streptococcus (disease/infection). This figure was produced using the Timeline Cluster Tool. 53
Setting
The cluster was defined as the maternity unit. Women were identified and screened for eligibility by clinical midwives and doctors in various locations, including the delivery suite, the maternity triage unit and the induction ward. No research-specific consent was obtained, although women in the rapid test units provided verbal clinical assent to have the vaginal/rectal swab.
Participant eligibility criteria
The eligibility pathway for the GBS2 trial is shown in Figure 4.
FIGURE 4.
The GBS2 trial eligibility criteria flow chart.
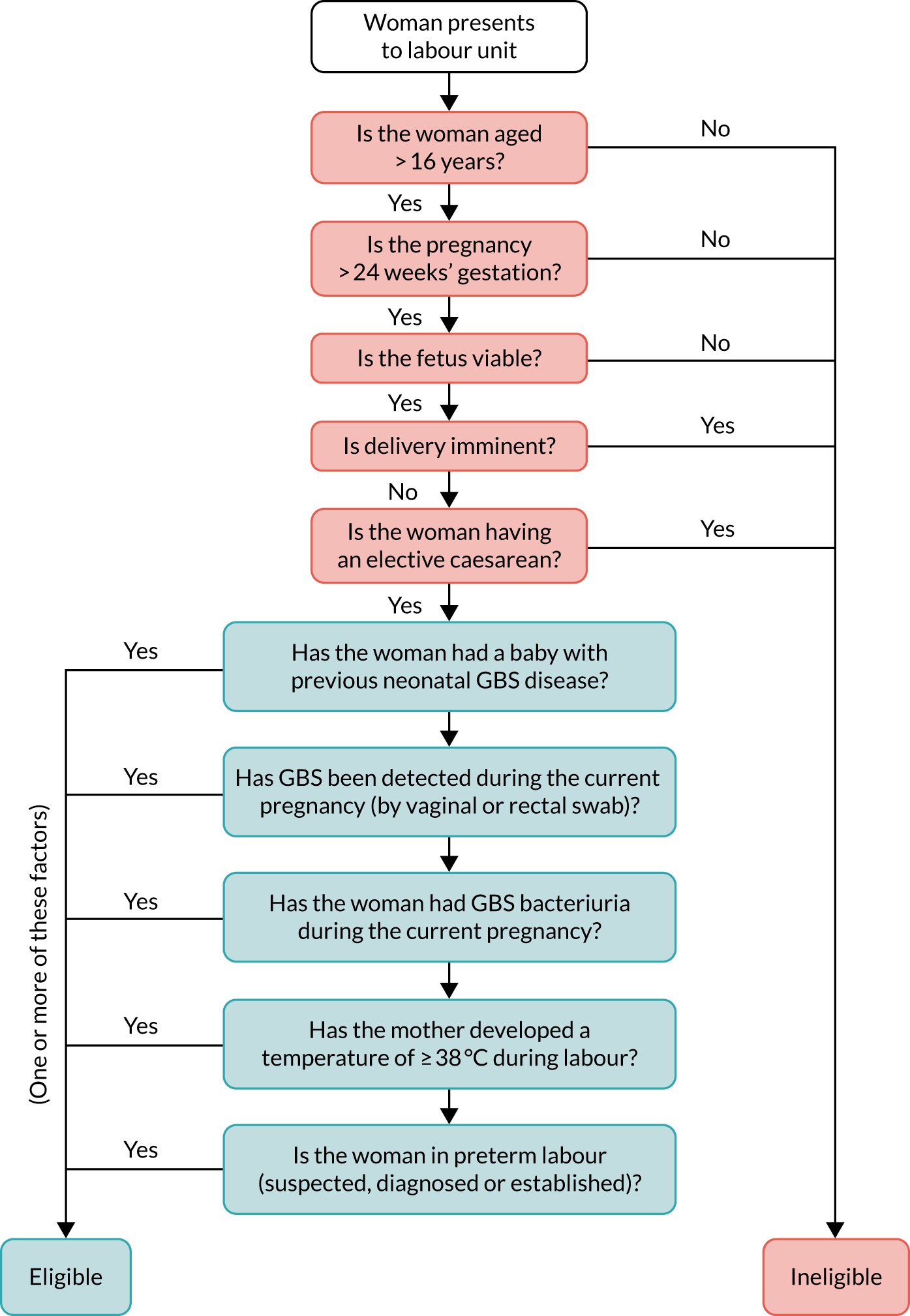
Women were eligible for inclusion in the GBS2 trial if they met one or more of the following criteria:
-
a previous baby with early- or late-onset neonatal GBS disease, as reported by the mother and documented in the maternal notes
-
GBS bacteriuria during the current pregnancy, as documented in the maternal notes, irrespective of whether or not the GBS bacteriuria was treated at the time of diagnosis with antibiotics
-
GBS colonisation of the vagina and/or rectum (determined from a vaginal/rectal swab) in current pregnancy, as documented in the maternal notes
-
preterm labour (< 37 weeks’ gestation) whether suspected, diagnosed or established, regardless of whether membranes were intact or there was PROM of any duration
-
maternal pyrexia (≥ 38 °C) observed at any point in labour, including clinically suspected/confirmed chorioamnionitis.
Women were screened for eligibility to participate in the GBS2 trial if they presented to the maternity unit in preterm labour (suspected, diagnosed or established), regardless of rupture of membranes, in term labour (latent or established) or if they were about to be induced.
Participant exclusion criteria
Women were ineligible for inclusion in the GBS2 trial if they were aged < 16 years; their pregnancy was at < 24 weeks’ gestation; they were in the second stage of labour at admission or considered likely to give birth to their baby imminently; they had planned an elective caesarean birth; or their baby was known to have died in utero or had a congenital anomaly incompatible with survival at birth.
Study procedures
The study procedures at each site varied according to the testing strategy randomly allocated to the participating maternity unit. The recommended antibiotic regimen for preventing early-onset GBS infection in both maternity unit groups of the trial were identical: intravenous administration of 3 g of benzylpenicillin as soon as possible after the onset of labour, and half of that dose at 4-hourly intervals until birth. If the woman was known to be allergic to penicillin she was offered a cephalosporin, and if she had a history of serious reactions to beta-lactams, vancomycin was indicated.
The protocol stipulated that all women in the usual-care group should have received IAP, whereas only those who tested positive or for whom a test result was not available should be offered IAP in the rapid test group (unless there was a clinical reason for prescribing IAP or the women had already had a previous baby with GBS infection and requested IAP).
Usual-care units (risk factor-based provision of intrapartum antibiotic prophylaxis)
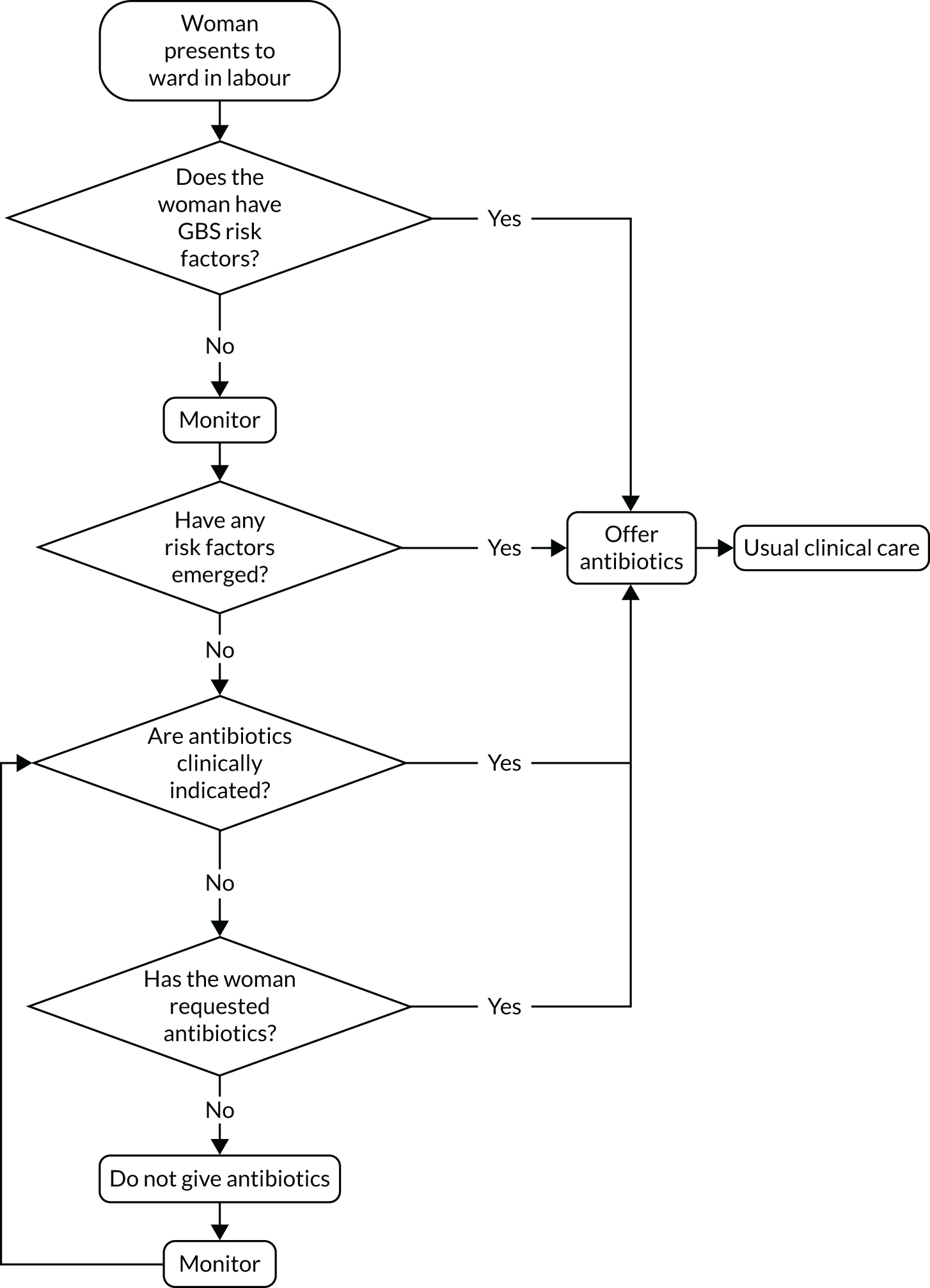
The study procedure for usual-care sites, in which IAP is offered to women with clinical risk factors, is outlined in Figure 5. All women with risk factors for early-onset GBS infection, whether initially apparent or emerging, were considered eligible for the study.
FIGURE 5.
Study procedures for usual practice maternity units.
Rapid test strategy
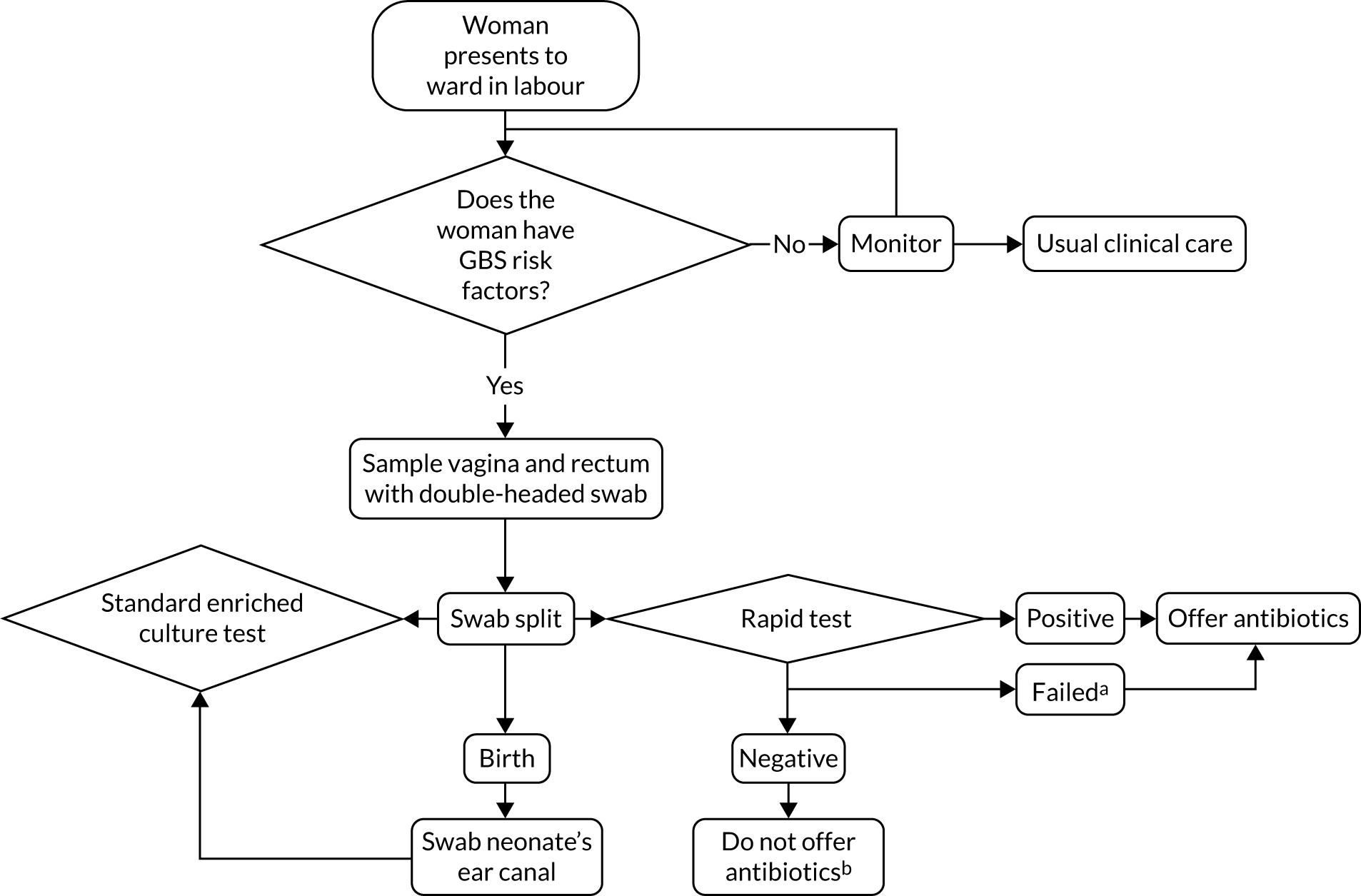
The units that were randomised to the rapid test received a GeneXpert® Dx IV GBS rapid testing system (Cepheid), which was installed and commissioned by a field specialist from the manufacturers. Each unit allocated to the rapid test screening policy was required to house the GeneXpert machine and computer centrally in the unit and have it operational at all times. Units were provided with a sufficient supply of Xpert GBS test cartridges (Cepheid) and double-headed swabs in transport tubes containing Stuart transport medium. In units participating in the substudy, additional single-headed swabs were also supplied. The use of the rapid test for screening was restricted to women who presented to the labour ward and who were eligible according to the participant eligibility criteria. A summary of the pathway for rapid test units is shown in Figure 6.
FIGURE 6.
Study procedures for rapid test maternity units. a, Failed tests include, but are not limited to, machine failure and failure to process swab in required time frame; and b, unless otherwise clinically indicated. This was at the discretion of local clinical care.
Obtaining a vaginal/rectal swab
Swabs were taken using a double-headed swab. Depending on the stage of labour, the swabs were obtained by either the woman herself or a suitably trained member of the woman’s care team. This could be on admission to the labour or induction ward before a vaginal examination was performed, or after a risk factor was detected (e.g. when maternal fever was observed). Swabs were first taken by gently rotating the swabs across the mucosa of the lower vagina. The same swab was then used to take a sample from the rectum, inserting the swab through the anal sphincter then gently rotating. The shafts of the double-headed swabs were then separated carefully. One was returned to the transport tube and sent to the local microbiology laboratory for selective enrichment culture to detect GBS (see Enrichment culture method) and the other was immediately used for the rapid test. For sites taking part in the microbiological substudy, and for participants who had consented to take part, an additional single-headed swab was taken and sent to the substudy laboratory (see Chapter 8).
In practice, a number of substances containing antimicrobial compounds administered vaginally may interfere with the results of the rapid test. The use of lubricant that contains antimicrobial substances, such as K-Y Jelly (Reckitt Benckiser, Slough, UK), for vaginal examination or for taking swabs was discouraged. It was suggested that sterile non-bacteriostatic fluid (e.g. sterile water or saline) was used when lubrication was necessary. Other procedures that may have involved antimicrobial substances include placing a pessary to induce labour, or chlorhexidine to cleanse the perineum or as a vaginal douche (although this is not currently indicated in NICE guidelines). 54 Nevertheless, as the GBS2 trial aimed to establish the use of a rapid test in a real-world setting and, indeed, vaginal examinations are usually undertaken to establish labour, women for whom antimicrobial substances were used were not excluded from the trial. Instead, the use of any antimicrobial substances after admission to the maternity and prior to the swab being taken was recorded.
Delayed labour
If more than 48 hours had elapsed since the test result became available and the woman had yet to give birth, the test result was regarded as invalid. In this situation, it was advised that the woman should be re-swabbed and the presence of GBS tested for again using both the rapid test and the microbiological techniques.
Conducting the rapid intrapartum test
After sampling, one swab was used to inoculate a test cartridge. Should the inoculated cartridge not have been loaded into the rapid test system and the test commenced within 15 minutes of the swab being introduced into the cartridge, the test was be deemed to have failed. The NHS or hospital number was entered into the GeneXpert software alongside the woman’s unique trial number. This trial number was identical on each item of study material associated with one woman and her child (or children). The test was conducted as per the operator manual. 55 It takes, on average, 35 minutes to get a result if GBS is present, 55 minutes to confirm if there is no GBS present and an error message is presented if the test has failed. Used test cartridges and swabs were disposed of in accordance with local policies for clinical waste. Instructions provided to sites for conducting the rapid test are available on request from the manufacturer.
Enrichment culture method
The second vaginal/rectal swab was sent to the local microbiology department where it was used to inoculate a selective enrichment medium prior to plating to detect the presence of any GBS, as per the current Public Health England recommendations. 56 Results from the microbiological cultures were returned to the care team using the usual reporting pathways and were recorded in the woman’s notes. Swabs, sent to the microbiology laboratory for the determination of the GBS colonisation status, were inoculated into Todd–Hewitt broth for overnight enrichment at 37 °C. This enriched broth was subcultured on chromogenic GBS agar and incubated aerobically overnight at 37 °C. GBS was identified by the presence of pink/red colonies. Microbiological data were transcribed to the GBS2 trial database by either a member of the microbiology department or a local research nurse. When manual data entry was required, the data entry screen did not allow review of the rapid test results by those outside the study office, therefore reducing the risk of review bias.
Neonatal ear canal swab
For eligible women in rapid test units, a single swab was taken from the baby’s ear canal as soon as convenient after birth. This swab was put into a transport tube, labelled with a numbered sticker and sent to microbiology for culture using the hospital’s usual request system to detect the presence of GBS, as per the mother’s swab (see Enrichment culture method). The neonatal swab was not used in the rapid test machine.
Outcome measures
Owing to the difference in the strategies for testing women and for directing IAP, it was not possible to blind women or their care team to the randomised allocation. Data were extracted from maternity and neonatal notes by research midwives within each unit who were involved in the implementation of the study, and therefore it was not possible to blind them to the randomised allocation.
Research midwives regularly collected data from consecutive eligible women regarding the use of antibiotics from the women’s health-care records, including information on whether or not any antibiotic was given, along with the date and time of IAP initiation.
Primary outcomes
-
The proportion of women who received only IAP for GBS prophylaxis, of all women identified with one or more risk factors for neonatal GBS infection.
-
Measures of test accuracy (i.e. the sensitivity and specificity of the GeneXpert GBS rapid test).
The indication for any intrapartum antibiotic administration was ascertained by making available the following options: GBS prophylaxis (in usual-care units only), a positive rapid test and a failed rapid test (both in rapid test units), pyrexia during labour, a caesarean birth, maternal request and other reason (to be specified). Multiple reasons could be recorded.
Secondary outcomes
Cluster randomised trial
Maternal
Intrapartum maternal antibiotic use for any indication
-
The proportion of women receiving any intrapartum antibiotic that has been indicated as being for GBS prophylaxis for a maternal clinical indication, such as pyrexia, on maternal request, prior to a caesarean or for any other reason, as a proportion of those women identified as having one or more risk factors for early-onset GBS infection.
Intrapartum maternal antibiotic use for any indication other than for a caesarean
-
The proportion of women receiving any intrapartum antibiotic that has been indicated as being for GBS prophylaxis for a maternal clinical indication, such as pyrexia, on maternal request or for any other reason other than for a caesarean, as a proportion of those women identified as having one or more risk factors for early-onset GBS infection.
Postpartum maternal antibiotic use for any indication
-
This was defined as those women receiving any postpartum antibiotic that had been indicated as being a maternal clinical indication, such as pyrexia, on maternal request or for any other reason, as a proportion of those women identified by the delivery suite midwives as having one or more risk factors for early-onset GBS infection. The period in which these data were collected was from birth until the mother’s discharge from either the hospital she gave birth or any hospital to which she was immediately transferred. Data on antibiotic use following any readmittance or prescribed by her general practitioner were not collected.
Time of intrapartum antibiotic prophylaxis exposure
-
This was defined as the duration between the start time of the first dose of IAP and the birth of the baby. Sufficient exposure was considered as an interval of either > 2 hours or > 4 hours before birth.
Neonatal
Neonatal antibiotic use for prophylaxis or treatment
-
The proportion of babies receiving antibiotic prophylaxis because of maternal GBS status or antibiotic treatment for suspected or confirmed neonatal infection, as a proportion of babies born to women identified as having risk factors for early-onset GBS infection.
Suspected neonatal infection
-
The number of babies prescribed antibiotics for presumed neonatal infection, as a proportion of all live born babies.
Neonatal group B Streptococcus colonisation rates
-
The rate of GBS-positive selective enrichment cultures from the neonatal ear swabs, as a proportion of all neonatal ear swabs cultured.
Neonatal mortality
-
Includes stillbirth rate and early neonatal death (before 7 days), and these are combined as the perinatal mortality rate.
Test accuracy secondary outcomes
Maternal and neonatal colonisation rates, and the mother-to-baby transmission rate.
Process outcomes
A number of process outcomes were also evaluated, for the rapid test units only, including the:
-
duration between a positive test becoming available on the GeneXpert machine and the time the result is collected by a midwife, and the duration between that point and the start of IAP
-
proportion of the cartridges on which the tests were not commenced within 15 minutes of inoculation, which is defined as an invalid test
-
proportion of tests initiated on the Cepheid GeneXpert machine that failed to produce a result within 55 minutes, which is defined as a failed test, or were reported as failed by the system.
Serious adverse event outcomes
Serious adverse events are expected to be extremely rare. There may be significant consequences of the failure to offer IAP to a woman with GBS risk factors and to women identified by rapid test as being colonised with GBS in terms of the increased risk of their baby developing early-onset GBS infection. Conversely, there is a risk of overtreatment if IAP is administered to women without risk factors or with a negative rapid test. However, these instances were considered outcomes of interest within the study, and not adverse events. The risk-based approach involves the noting of historical risk factors and the monitoring of women for emerging risk factors, such as chorioamnionitis. This presents no risk to the women other than a failure to identify and act on these risk factors. The rapid test requires a vaginal/rectal swab to be collected during labour, which is benign and presents no foreseeable risk of harm. As with almost any diagnostic test, there was a risk that testers may suffer an inoculation injury (most likely mucous membrane exposure) with clinical material. A proportion of women will receive antibiotics, particularly benzylpenicillin for IAP, which carry a very small risk of anaphylaxis. 58
The outcomes of economic evaluation and for microbiological substudy are provided in Chapters 7 and 8.
Statistical methods
For baseline characteristics, categorical data are summarised by frequencies and percentages. Continuous data are summarised by the number of responses, mean and standard deviation (SD) if deemed to be normally distributed, and number of responses, median and interquartile range (IQR) if data appear skewed. Tests of statistical significance were not undertaken. In accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline, both absolute and relative measures of treatment effects are reported, and so for the primary and secondary outcomes in the cluster randomised trial component both risk differences (RDs) and relative risks (RDs) are presented where possible. Estimates of differences between groups are presented with 95% two-sided CIs. p-values are reported from two-sided tests at the 5% significance level and no corrections are made for multiple testing.
In the primary analysis for the cluster randomised trial, a mixed-effects binomial regression with a log-link was used to estimate the RR, and a binomial model with identity link was used to estimate the RD. In the case of non-convergence of the binomial model with a log-link, a Poisson model with robust standard errors was fitted. If the binomial model with the identity link did not converge, then only a RR was reported. Both models allowed for clustering by maternity unit as a random effect. To correct the potential inflation of the type I error rate due to small number of clusters, the Kenward and Roger method59 was used.
Comparative estimates of differences between groups were adjusted for the hospital and the minimisation variables of region (i.e. LSE or MID), baseline IAP rate (i.e. equal to or greater than the median or less than the median) and the number of vaginal or emergency caesarean births (i.e. equal to or greater than the median or less than the median). These effects were all be assumed to be fixed in the regression models used. A secondary analysis for the primary outcome additionally adjusted for maternal temperature of ≥ 38 °C observed while in labour, any previous baby with GBS disease, GBS bacterium detected in current pregnancy, and woman in suspected/diagnosed/established preterm labour. When covariate adjustment was not practical, unadjusted estimates were produced and it was made clear in the output why this occurred (e.g. not possible because of low event rate, lack of model convergence or poor recording accuracy of covariates). Multiple imputation analysis, accounting for clustering, was planned if the proportion of observations with missing complete information on these covariates was > 5%.
Prespecified subgroup analyses were limited to the primary outcome. The following subgroup effects were investigated:
-
maternal temperature of ≥ 38 °C observed while in labour
-
previous baby with GBS disease
-
GBS bacterium detected in current pregnancy
-
preterm labour (< 37 weeks’ gestation) with intact membranes or rupture of membranes of any duration, whether suspected, diagnosed or established.
Treatment effects were summarised within each subgroup separately, and an interaction test was performed between each subgroup variable and the received allocation.
The diagnostic accuracy of the rapid test was estimated through the standard calculations of sensitivity and specificity. Estimates are presented with 95% CIs calculated using binomial exact methods. 60 We also undertook a binomial proportions test to compare the observed sensitivity with the hypothesised minimal performance value of 90%.
Sample size for the cluster randomised trial
The focus of sample size calculation was on the effectiveness of the rapid test. We aimed to recruit a minimum of eight clusters per randomisation group (i.e. a total of 16 maternity units). This was a realistic recruitment goal and was deemed sufficient to allow estimation of model parameters. We estimated that the sample size per cluster would be approximately 83 (this is informed by routinely collected data on number of eligible women giving birth). This equated to a total sample size of approximately 664 per strategy group to allow recruitment of the required number of individuals for the test accuracy study. As there were two strategy groups (i.e. usual care and rapid test), each with at least 664 participants, it was expected to provide a sample size of 1328 women. This was rounded up to a target sample size of 1340 women. To allow for dropouts at the level of the cluster, anticipating implementation issues in maternity units, the number of clusters was increased from 16 to 20.
The proportion of women receiving antibiotics to prevent vertical GBS transmission (i.e. the primary outcome in the cluster RCT) was expected to be in the region of 50–75% (based on the previous study51 and assuming better compliance with RCOG guidelines since that study). This primary outcome was a process outcome and so the within-cluster correlation of this outcome [the intracluster coefficient (ICC)] was expected to be higher than it would be for a clinical outcome. We therefore considered sensitivity of our calculations to a range of proportions in the usual-care units and a range of ICC values, which we believe to be quite conservative. All of our calculations allowed for 90% power and 5% significance. We did not acknowledge varying cluster sizes in our calculations, as we considered the coefficient of variation of unit birth sizes to be small (approximately 0.21 based on annual birth rates for 2013) and could be altered by varying the study duration at maternity units. However, given our conservative assumptions, and that the impact of the testing strategies on the primary outcome was expected to be large (i.e. a priori we expect the effect size to be large), we expected the impact of any varying cluster sizes to be minimal. For a range of values for the important parameters, as described above, we worked out the detectable differences and equivalent relative RR (Table 1).
| IAP rate in risk factor strategy | ICC, % (RR) | |||
|---|---|---|---|---|
| 0.2 | 0.1 | 0.05 | 0.01 | |
| 75 | 38 (0.51) | 48 (0.64) | 55 (0.73) | 63 (0.84) |
| 60 | 22 (0.37) | 32 (0.53) | 39 (0.65) | 47 (0.78) |
This means that this trial would have around 90% power to detect a reduction in proportion of women prescribed antibiotics to prevent GBS transmission from 75% to 63% (i.e. a RR of about 20%) for a low value of the ICC to a reduction from 75% to 38% for a very conservative value of the ICC (0.2), equating to a RR reduction of 50%.
Sample size for the test accuracy study
The sample size of the test accuracy study was dependent on the sensitivity of the rapid test. For the test to be proven useful, we needed to show that it would detect a higher proportion of GBS colonisation than other tests, but not at the cost of low specificity and/or unnecessary administration of IAP. Results from the Group B Streptococcus 1 (GBS1) study51 suggested that the most cost-effective test (if untargeted universal IAP was excluded) was antenatal culture for GBS at 35–37 weeks’ gestation. In the GBS1 study51 the sensitivity of this test was 75.8% (95% CI 47.2% to 91.5%). Therefore, if we could prove that the sensitivity of rapid test was > 90% then the results of the GBS2 study51 would be convincing. This was a stringent test and a lower threshold might also be adequate. We did not compare the rapid test with antenatal GBS culture testing within the study, but compared with this result from external literature. Therefore, we undertook a ‘one sample, sample size’ computation comparing against a fixed value.
We had data from a systematic review on the performance of the GeneXpert GBS test (J Daniels, personal communication). From the meta-analysis of nine studies, the pooled accuracy of the test was estimated, giving a sensitivity of 96.4% (95% CI 90.8% to 98.6%) and specificity of 98.9% (95% CI 97.5% to 99.5%). Sample size calculations are therefore based on showing that a test with sensitivity of 96.4% is greater than a fixed value of 90%. With a power of 90% to demonstrate this sensitivity, 167 cases of maternal GBS colonisation are required (or 136 at 80% power).
A sample size of 676 women would provide 90% chance of us accruing enough GBS-colonised women to have 90% power to show the sensitivity of the rapid intrapartum test to be statistically significantly (with p < 0.05) > 90% should the meta-analytical estimate of its performance (96.4%) be correct, while allowing for 10% loss from failed tests, based on the GBS prevalence observed in the GBS1 study,51 which was 29.8% (89/299, 95% CI 24.6% to 35.2%). Of the 606 participants with data, we would expect 167 to be GBS carriers and 439 to be negative for GBS colonisation. If the prevalence of GBS colonisation was actually at the lower end of the 95% CI from GBS1 study,51 namely 24.6%, then a total of 673 women would give a 90% chance of observing 136 cases of GBS colonisation, including 10% lost tests. The 95% CIs we would observe on sensitivities and specificities of 85%, 90%, 95% and 98% with a sample size of 676 women (606 women with data) are shown in Table 2, and these have adequate precision (sensitivity within 10% and specificity within 6%) for modelling.
| Point estimate | 95% CI for sensitivity (n = 167) | 95% CI for specificity (n = 439) |
|---|---|---|
| 85 | 78.7 to 90.1 | 81.3 to 88.2 |
| 90 | 84.2 to 94.0 | 86.8 to 92.7 |
| 95 | 90.9 to 97.9 | 92.5 to 96.8 |
| 98 | 94.8 to 99.6 | 96.2 to 99.1 |
Chapter 4 Characteristics of sites and participants
Parts of this chapter have been reproduced from Daniels et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
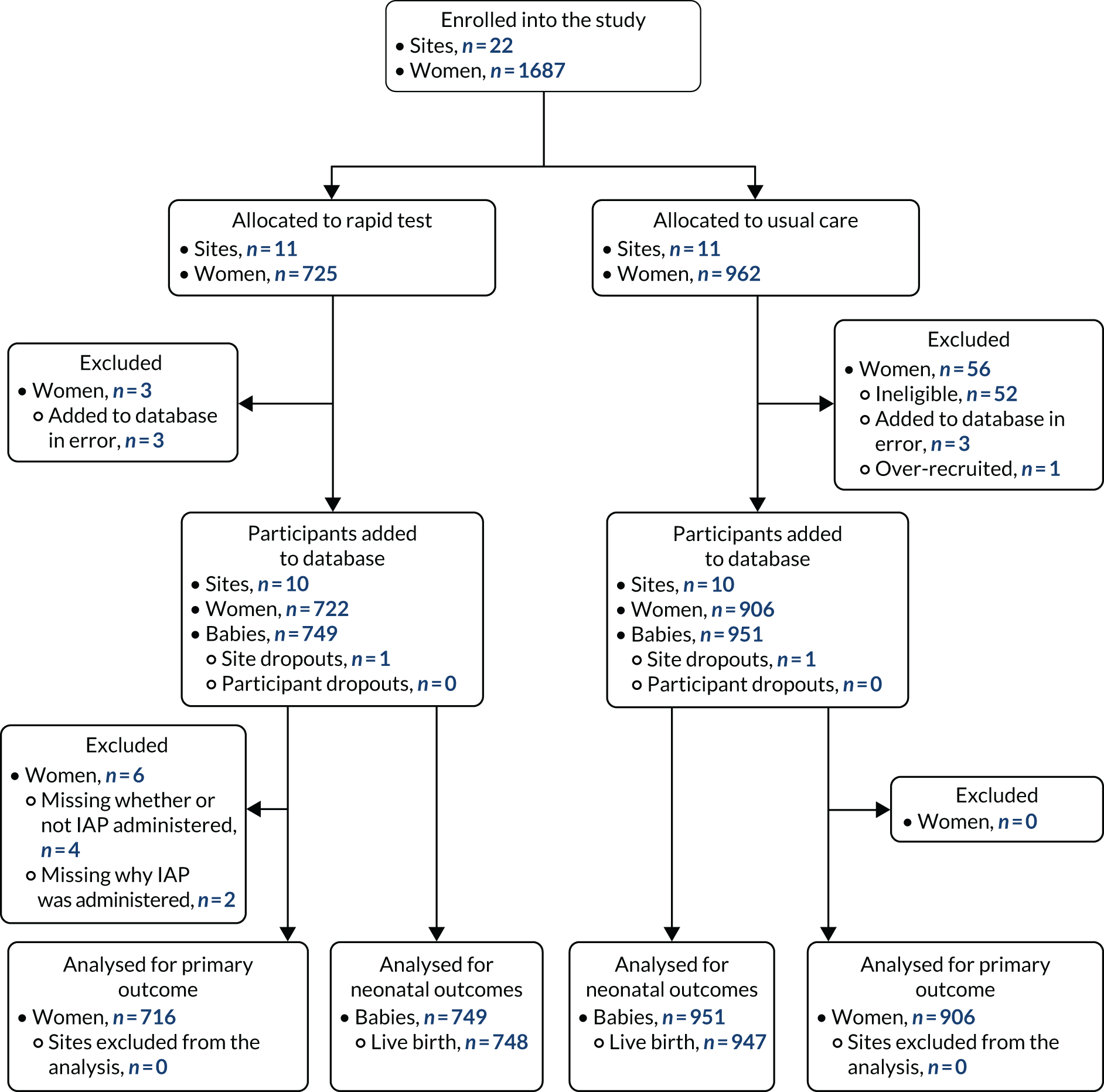
Accrual period
The first site opened to recruitment on 26 July 2017, and the trial closed to recruitment on 30 April 2019. Ten usual-care sites and eight rapid test sites met or exceeded their recruitment target of 86 women. As the sample size requirements were met (i.e. at least eight sites in each arm recruited 86 women per site), the Trial Steering Committee requested that recruitment ended.
Site-level randomisation
Overall, 22 maternity units agreed to participate and were randomised to usual-care or rapid test pathways. Initially, we randomised 20 sites (see Appendix 1). The allocations were revealed to the units at the same time, after all units had commited to participation. Following randomisation, two sites, one allocated to each strategy, requested withdrawal from the study. One usual-care site withdrew as it lost a key staff member and one rapid test site was not able to implement the rapid test using the no-consent model. We replaced these with two other sites that were randomised to usual-care and rapid test strategies, respectively. The anonymised maternity unit characteristics that were used within the minimisation algorithm are shown in Table 3. The units had around 2000–6000 births per year that were not planned elective caesareans. There was variation in the reported rates of IAP to prevent early-onset GBS infection.
| Site | Region | Annual number of vaginal or emergency caesarean deliveries | Estimated IAP rate (proportion of all deliveries receiving IAP) |
|---|---|---|---|
| Rapid test | |||
| R1 | MID | 4144 | 44.48 |
| R2 | LSE | 2385 | 24.12 |
| R3 | LSE | 2138 | 14.78 |
| R4 | LSE | 6021 | 22.77 |
| R5 | MID | 5583 | 32.64 |
| R6 | MID | 3952 | 24.71 |
| R7 | MID | 5627 | 7.24 |
| R8 | LSE | 4934 | 29.89 |
| R9 | LSE | 5105 | 25.84 |
| R10 | MID | 2148 | 10.01 |
| R11 | MID | 3567 | 62.7 |
| Usual care | |||
| U1 | MID | 2930 | 9.13 |
| U2 | LSE | 3701 | 29.22 |
| U3 | LSE | 2403 | 11.24 |
| U4 | LSE | 5050 | 31.73 |
| U5 | LSE | 4803 | 8.81 |
| U6 | MID | 5373 | 26.98 |
| U7 | MID | 1929 | 9.90 |
| U8 | MID | 2679 | 73.64 |
| U9 | LSE | 5231 | 87.28 |
| U10 | MID | 4291 | 12.06 |
| U11 | LSE | 2954 | 27.93 |
Individual-level accrual
The cumulative rate of accrual of women into the GBS2 trial, by screening pathway, is shown in Appendix 2. The date on which the woman was identified as having GBS risk factors was used as the date of study entry. The weekly total number of intended vaginal deliveries was requested from each unit to enable monitoring of the accrual rate. In the early phase of the trial, usual-care units were allowed to exceed their target of 86 women and continue to 100 women per unit. This was curtailed after the second unit reached a total of 100 women. At the end of the recruitment period, when sample size requirements were met, recruitment at two rapid test units was stopped: in one when 83 women had been recruited and in one after three women had been recruited. An estimate of the proportion of women recruited into the study of the total from the population giving birth vaginally or by emergency caesarean (using data from Table 3, pro rata for the duration of participation) is shown in Table 4.
| Site | Estimated number of vaginal or emergency caesarean deliveries during accrual period | Proportion of women accrued (% of all vaginal or emergency caesarean deliveries) |
|---|---|---|
| Rapid test | ||
| R1 | 1856 | 4.7 |
| R2 | 1684 | 5.1 |
| R3 | 1474 | 5.9 |
| R4 | 3209 | 1.0 |
| R5 | 1273 | 6.8 |
| R6 | 1791 | 4.8 |
| R7 | 1175 | 7.3 |
| R8 | 2562 | 3.1 |
| R9 | 1809 | 5.0 |
| R11 | 774 | 0.3 |
| Usual care | ||
| U1 | 1240 | 7.3 |
| U2 | 641 | 13.4 |
| U4 | 1013 | 8.5 |
| U5 | 739 | 13.5 |
| U6 | 2687 | 3.2 |
| U7 | 890 | 11.2 |
| U8 | 228 | 37.7 |
| U9 | 805 | 12.4 |
| U10 | 613 | 13.9 |
| U11 | 771 | 11.2 |
Characteristics of the included sites and participants
Maternity sites
Following randomisation, the maternity units were balanced according to the minimisation characteristics (Table 5).
| Minimisation factor | Rapid test (N = 10) | Usual care (N = 10) | Overall (N = 20) |
|---|---|---|---|
| Number of vaginal deliveries or emergency caesareans | |||
| Median (IQR) | 4539 (3567–5583) | 3996 (2930–5050) | 4218 (2942–5168) |
| Below median, n (%) | 5 (50) | 5 (50) | 10 (50) |
| Above median, n (%) | 5 (50) | 5 (50) | 10 (50) |
| Region, n (%) | |||
| LSE | 5 (50) | 5 (50) | 10 (50) |
| MID | 5 (50) | 5 (50) | 10 (50) |
| Estimated IAP rate as a proportion of all vaginal deliveries | |||
| Median (IQR) | 25.3 (22.8–32.6) | 27.5 (9.9–31.7) | 26.4 (13.4–32.2) |
| Below median, n (%) | 6 (60) | 4 (40) | 10 (50) |
| Above median, n (%) | 4 (40) | 6 (60) | 10 (50) |
Participants
Overall, 1687 women were identified and added to the trial database during the recruitment window for the 20 randomised sites. There were 52 women from two usual-care maternity units who were added to the database but who were ineligible for the study, as the database eligibility criteria initially did not reflect the protocol. A further three women in each group who, on closer examination, were added to the database in error, as they were not eligible and one woman’s record was duplicated. These women’s data were excluded from the analysis, leaving 1628 women in the data set.
The overall numbers of women recruited were 722 in rapid test sites and 906 in usual-care sites. There were 67 pairs of twins and three trios of triplets and, therefore, the number of babies available for assessment was 749 in the rapid test group and 951 in the usual-care units (Figure 7). Similar proportions of women in the two study groups were recruited from the two regions, and the women were similar in their parity and type of birth. A higher proportion of women in the rapid test sites entered labour after being induced than in the usual-care units (Table 6).
FIGURE 7.
The CONSORT flow diagram of sites and participants in the GBS2 study.
| Maternal characteristic | Rapid test (N = 722) | Usual care (N = 906) | Overall (N = 1628) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 29.3 (5.8) | 30.1 (5.8) | 29.7 (5.8) |
| Missing | 1 | 0 | 1 |
| Region, n (%) | |||
| LSE | 375 (52) | 458 (51) | 833 (51) |
| MID | 347 (48) | 448 (49) | 795 (49) |
| Onset of labour, n (%) | |||
| Spontaneous | 343 (48) | 527 (58) | 870 (53) |
| Induced | 354 (49) | 364 (40) | 718 (44) |
| Missing | 8 (1) | 0 (0) | 8 (< 1) |
| Type of delivery, n (%) | |||
| Spontaneous vaginal | 439 (61) | 542 (60) | 981 (60) |
| Instrumental | 102 (14) | 131 (14) | 233 (14) |
| Emergency caesarean | 173 (24) | 233 (26) | 406 (25) |
| Missing | 8 (1) | 0 (0) | 8 (< 1) |
| Multiparity, n (%) | |||
| Yes | 465 (64) | 585 (65) | 1050 (65) |
| No | 255 (35) | 321 (35) | 576 (35) |
| Missing | 2 (< 1) | 0 (0) | 2 (< 1) |
| If yes, number of previous pregnancies | |||
| Median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Missing | 0 | 0 | 0 |
| If yes, number of previous vaginal deliveries | |||
| Median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Missing | 0 | 0 | 0 |
In 93% of women there was one risk factor for neonatal GBS disease, and two risk factors in 6–7% of women. The most common risk factor was preterm labour. The distribution of risk factors among the study population is shown in Tables 7 and 8.
| Risk factor | Rapid test (N = 722), n (%) | Usual care (N = 906), n (%) | Overall (N = 1628), n (%) |
|---|---|---|---|
| Maternal temperature ≥ 38 °C | 66 (9) | 156 (17) | 222 (14) |
| Previous baby with GBS | 51 (7) | 53 (6) | 104 (6) |
| GBS detected in this pregnancy | 330 (46) | 332 (37) | 662 (41) |
| Preterm labour | 324 (45) | 432 (48) | 756 (47) |
| Number of risk factors | Combinations of risk factors | Rapid test (N = 722), n (%) | Usual care (N = 906), n (%) | Overall (N = 1628), n (%) |
|---|---|---|---|---|
| One | Maternal temperature ≥ 38 °C | 55 (8) | 139 (15) | 194 (12) |
| Previous baby with GBS | 35 (5) | 40 (4) | 75 (5) | |
| GBS detected in this pregnancy | 293 (41) | 278 (31) | 571 (35) | |
| Preterm labour | 291 (40) | 384 (42) | 675 (41) | |
| Total | 674 (93) | 841 (93) | 1515 (93) | |
| Two | Maternal temperature ≥ 38 °C + GBS detected in this pregnancy | 5 (< 1) | 8 (< 1) | 13 (< 1) |
| Maternal temperature ≥ 38 °C + preterm labour | 5 (< 1) | 7 (< 1) | 12 (< 1) | |
| Previous baby with GBS + GBS detected in this pregnancy | 8 (1) | 8 (< 1) | 16 (< 1) | |
| Previous baby with GBS + preterm labour | 6 (< 1) | 4 (< 1) | 10 (< 1) | |
| GBS detected in this pregnancy + preterm labour | 22 (3) | 36 (4) | 58 (6) | |
| Total | 46 (6) | 63 (7) | 109 (7) | |
| Three | Maternal temperature ≥ 38 °C + previous baby with GBS + GBS detected in this pregnancy | 1 (< 1) | 1 (< 1) | 2 (< 1) |
| Maternal temperature ≥ 38 °C + GBS detected in this pregnancy + preterm labour | 0 (0) | 1 (< 1) | 1 (< 1) | |
| Previous baby with GBS + GBS detected in this pregnancy + preterm labour | 1 (< 1) | 0 (0) | 1 (< 1) | |
| Total | 2 (< 1) | 2 (< 1) | 4 (< 1) |
Among those women with only one risk factor, more women in rapid test units had GBS detected in the current pregnancy (41%, 293/722) than those in the usual-care units (31%, 278/906). In addition, 15% (139/906) of women in usual-care units had a raised maternal temperature compared with 8% (55/722) of women in rapid test units. GBS was detected in the vaginal or rectal swab commonly in both groups, followed by diagnosis in the midstream sample (Table 9).
| GBS sampling method for culture | Rapid test (N = 722), n (%) | Usual care (N = 906), n (%) | Overall (N = 1628), n (%) |
|---|---|---|---|
| Mid-stream urine sample | 57 (17) | 81 (25) | 138 (21) |
| Vaginal or rectal swab | 251 (76) | 206 (64) | 457 (70) |
| Both | 7 (2) | 26 (8) | 33 (5) |
| Not stated | 15 (5) | 10 (3) | 25 (4) |
| Total | 330 | 323a | 653a |
Compliance to group allocation and test strategy
At a site level, complete compliance was achieved because the rapid test machines were supplied only to those sites allocated to the rapid test. In 53 women (7.35%), a swab was taken but the test failed to yield a result because of equipment problems; half of these problems arose in two maternity units where the GeneXpert machine developed an intermittent fault during the recruitment period. A further 23 tests were considered invalid, as > 15 minutes had passed between swabbing and initiating the test, with other reasons or no documented reason for the test failing in 24 attempts. There were 56 women who should have had a second test because their labours failed to progress within 48 hours, but who were missed. Among individual participants, for three women in the rapid test strategy group it is unknown whether or not a swab was taken and no test results are available. We were unable to calculate the duration between the test becoming available, the midwife collecting the result and the IAP being administered.
Of the 241 women in rapid test units who tested positive, 79% (190/241) received IAP to prevent GBS vertical transmission and a further 8% received antibiotics for other reasons. Of the 316 women who tested negative for GBS, 17% received IAP to prevent GBS and a further 39% did so for other indications. Table 10 shows the adherence to the test result.
| Rapid test result | Administration of antibiotics, n (%) | Missing IAP data | Total, n (%) | ||
|---|---|---|---|---|---|
| IAP for GBS (± other reasons) | Antibiotic for other reasons | No antibiotics | |||
| Positive | 190 (79) | 20 (8) | 31 (13)c | 0 (0) | 241 (33) |
| Negative | 52 (16)c | 124 (39) | 138 (44) | 2 (1) | 316 (44) |
| Faileda | 47 (47) | 26 (26) | 27 (27) | 0 (0) | 100 (14) |
| Not performedb | 7 (12) | 15 (27) | 33 (59) | 1 (2) | 56 (8) |
| Missing test data | 1 (12) | 2 (25) | 5 (63) | 0 (0) | 8 (1) |
| Total | 297 | 187 | 234 | 3 | 721 |
Table 11 provides the various indications for IAP for positive and negative rapid tests and women with failed or missed test.
| Reasons for IAPa | Usual care (N = 906), n (%) | Rapid test (N = 721), n (%)b | All (N = 1627), n (%) | ||||
|---|---|---|---|---|---|---|---|
| Positive (N = 241) | Negative (N = 316) | Test failed or invalid (N = 100) | Not performed (N = 56) | Missing test data (N = 8) | |||
| GBS colonisation | 328 (52) | 190 (30) | 52 (8) | 47 (7) | 7 (1) | 1 (0) | 625 (38) |
| Pyrexia | 122 (62) | 16 (8) | 48 (24) | 8 (4) | 3 (2) | 0 (0) | 197 (12) |
| Caesarean | 76 (52) | 18 (12) | 37 (25) | 6 (4) | 7 (5) | 2 (1) | 146 (9) |
| Maternal request | 0 (0) | 6 (22) | 19 (70) | 1 (4) | 1 (4) | 0 (0) | 27 (2) |
| Other reason | 120 (54) | 8 (4) | 57 (26) | 29 (13) | 6 (3) | 2 (1) | 222 (14) |
| No IAP | 304 (57) | 31 (6) | 138 (26) | 27 (5) | 33 (6) | 5 (1) | 538 (33) |
Chapter 5 Cluster randomised trial on effects of rapid intrapartum test for group B Streptococcus
Parts of this chapter have been reproduced from Daniels et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
This chapter reports the effects of rapid intrapartum GBS testing in reducing maternal and neonatal antibiotic usage primarily, and on other neonatal and process outcomes, compared with usual care, in which IAP is directed based on maternal risk factors alone.
Intrapartum antibiotics prophylaxis to prevent early-onset group B Streptococcus infection
There were no differences in the proportion of women who were administered IAP for GBS prophylaxis (RR 1.16, 95% CI 0.83 to 1.64). Overall, 41% (297/716) of women in the rapid test group were given IAP for GBS prophylaxis, compared with 36% (328/906) of women in the usual-care group. The estimates are provided in Table 12 after adjusting for cluster size, unit birth rate and IAP rates.
| IAP for GBS | Rapid test, n (%) | Usual care, n (%) | RDa (95% CI); p-value | RRb (95% CI); p-value |
|---|---|---|---|---|
| Yes | 297 (41) | 328 (36) | 0.05 (–0.07 to 0.18); 0.37 | 1.16 (0.83 to 1.64); 0.35 |
| No | 419 (59) | 578 (64) | ||
| Total | 716 | 906 | ||
| Missing | 6 | 0 |
A post hoc sensitivity analysis that adjusted for maternal risk factors did not show a difference between the two strategies (RR 0.96, 95% CI 0.80 to 1.16).
Maternal antibiotic administration for any indication
There were no differences between women in the rapid test units (n = 484, 67%) and women in the usual-care units (n = 602, 66%) in terms of receiving antibiotics for any indication, indications other than caesarean delivery and postpartum antibiotics to prevent or treat infection (Table 13).
| Intrapartum maternal antibiotic | Rapid test, n (%) | Usual care, n (%) | RDa (95% CI); p-value | RRb (95% CI); p-value |
|---|---|---|---|---|
| Intrapartum maternal antibiotic use for any indication | ||||
| Yes | 484 (67) | 602 (66) | –0.007 (–0.14 to 0.12); 0.91 | 0.99 (0.81 to 1.21); 0.92 |
| No | 234 (33) | 304 (34) | ||
| Total | 718 | 906 | ||
| Missing | 4 | 0 | ||
| Intrapartum maternal antibiotic use for any indication other than a caesarean | ||||
| Yes | 454 (63) | 559 (62) | 0.007 (–0.11 to 0.13); 0.90 | 1.01 (0.83 to 1.23); 0.89 |
| No | 263 (37) | 347 (38) | ||
| Total | 717 | 906 | ||
| Missing | 5 | 0 | ||
| Postpartum maternal antibiotic use for any indication | ||||
| Yes | 146 (20) | 203 (22) | –0.02 (–0.12 to 0.08); 0.71 | 0.92 (0.60 to 1.44); 0.71 |
| No | 567 (80) | 702 (78) | ||
| Total | 713 | 905 | ||
| Missing | 9 | 1 | ||
There was a significant increase in the proportion of women who received sufficient antibiotic exposure (i.e. IAP exposure > 4 hours before birth) in the rapid test group compared with those in the usual-care group (RD 0.16, 95% CI 0.06 to 0.27). There were no differences in the rates of women with IAP exposure > 2 hours between the two strategies (Table 14).
| IAP exposure | Rapid test, n (%) | Usual care, n (%) | RDa (95% CI); p-value | RRb (95% CI); p-value |
|---|---|---|---|---|
| > 2 hours before birth | ||||
| Yes | 376 (79) | 380 (72) | 0.11 (–0.24 to 0.45); 0.16 | 1.10 (0.98 to 1.24); 0.09c |
| No | 100 (21) | 149 (28) | ||
| Total | 476 | 529 | ||
| > 4 hours before birth | ||||
| Yes | 322 (68) | 288 (54) | 0.16 (0.06 to 0.27); 0.005 | 1.32 (1.12 to 1.55); 0.004 |
| No | 154 (32) | 241 (46) | ||
| Total | 476 | 529 | ||
| Missing exposure duration data | 8 | 73 | ||
| Did not receive IAP | 234 | 304 | ||
Neonatal antibiotics exposure
The rates of neonatal exposure to antibiotics were lowered by 29% (RR 0.71, 95% CI 0.54 to 0.95) in the rapid test group compared with the usual-care group (Table 15). This included all babies who started antibiotics within the first 7 days of life, regardless of maternal antibiotic exposure or indication for neonatal antibiotic prescription. The number of babies for whom suspected early-onset sepsis was the indication for antibiotics was lower in the rapid test units (25%) than in the usual-care units (39%) (RR 0.63, 95% CI 0.43 to 0.92; p = 0.02).
| Neonatal antibiotic use for prophylaxis or treatment | Rapid test, n (%) | Usual care, n (%) | RDa (95% CI); p-value | RRb (95% CI); p-value |
|---|---|---|---|---|
| Yes | 244 (33) | 412 (44) | –0.13 (–0.23 to –0.02); 0.02 | 0.71 (0.54 to 0.95); 0.02c |
| No | 493 (67) | 534 (56) | ||
| Missing information | 11 | 1 | ||
| Stillbirths | 1 | 4 | ||
| Total | 749 | 951 |
The predominant reason stated for administration of neonatal antibiotics was suspected early-onset infection, which was the stated reason in one-third of all babies (Table 16).
| Reason for i.v. antibiotic administration | Rapid test (N = 749), n (%) of all babiesa | Usual care (N = 951), n (%) of all babiesb | Overall (N = 1700), n (%) of all babies |
|---|---|---|---|
| Prophylaxis in newborn despite the mother receiving IAP at least 2 hours before birth | 61 (8) | 48 (5) | 109 (6) |
| Prophylaxis in newborn because the mother received IAP < 2 hours before birth | 21 (3) | 29 (3) | 50 (3) |
| Prophylaxis in newborn because the mother did not receive any IAP | 17 (2) | 15 (2) | 32 (2) |
| The baby had suspected early-onset neonatal sepsis | 187 (25) | 374 (39) | 561 (33) |
| Other reason | 43 (6)c | 75 (8)d | 118 (7) |
In most of the babies with suspected early-onset infection who were commenced on antibiotics, following further tests, such as microbiological culture of blood or cerebrospinal fluid, the decision was made not to administer the full course of antibiotics (Tables 17–19). There were 11 reports of GBS infection among 561 babies who received antibiotics, equivalent to a rate of 6.5 per 1000 live births. Of these 11 babies, all of their mothers (n = 11) had received IAP. Two babies died before discharge, both in the usual-care group, and one death was judged to be because of GBS (the other death was due to E. coli meningitis). Three of these 11 babies were in the rapid test group and the mothers of all three returned a positive rapid test result. All three of these babies returned an ear canal swab, but only one baby was positive for GBS. There were three perinatal deaths in the rapid test units and eight in the usual-care units, which included one and four stillbirths, respectively.
| Management route | Rapid test (N = 187), n (%) | Usual care (N = 374), n (%) | Overall (N = 561), n (%) |
|---|---|---|---|
| Infection subsequently ruled out, clinically well and antibiotic treatment discontinued | 138 (72) | 260 (73) | 398 (72) |
| Infection not microbiologically confirmed, but a full course of antibiotics given | 46 (24) | 80 (22) | 126 (23) |
| Infection caused by GBS | 3 (2) | 8 (2) | 11 (2) |
| Infection caused by another bacterium | 6 (3) | 10 (3) | 16 (3) |
| Risk factor | Rapid test group, n/N (%) | Usual-care group, n/N (%) | RDa (95% CI) | RRb (95% CI) | Interaction p-value |
|---|---|---|---|---|---|
| Maternal pyrexia (> 38 °C) in labour | |||||
| Yes | 24/65 (37) | 48/156 (31) | 0.02 (–0.16 to 0.20) | 1.09 (0.65 to 1.83) | 0.79 |
| No | 273/651 (42) | 280/750 (37) | 0.05 (–0.07 to 0.18) | 1.16 (0.82 to 1.64) | |
| Total | 716 | 906 | |||
| Previous baby with GBS disease | |||||
| Yes | 17/51 (34) | 23/53 (43) | –0.04 (–0.26 to 0.17) | 0.87 (0.48 to 1.59) | 0.26 |
| No | 280/665 (42) | 305/853 (36) | 0.06 (–0.07 to 0.19) | 1.19 (0.84 to 1.67) | |
| Total | 716 | 906 | |||
| GBS bacterium detected in current pregnancy | |||||
| Yes | 156/330 (47) | 130/331 (39) | 0.09 (–0.04 to 0.22) | 1.27 (0.89 to 1.81) | 0.18 |
| No | 141/386 (37) | 198/575 (34) | 0.03 (–0.10 to 0.15) | 1.06 (0.75 to 1.50) | |
| Total | 716 | 906 | |||
| Preterm labour | |||||
| Yes | 121/320 (38) | 152/432 (35) | 0.03 (–0.10 to 0.16) | 1.08 (0.76 to 1.55) | 0.33 |
| No | 176/396 (44) | 176/474 (37) | 0.08 (–0.05 to 0.21) | 1.23 (0.87 to 1.76) | |
| Total | 716 | 906 | |||
| IAP for GBS | Rapid test group, n (%) | Usual-care group, n (%) | RDa (95% CI) | RRb (95% CI) |
|---|---|---|---|---|
| Received | 294 (44) | 325 (42) | 0.02 (–0.13 to 0.17) | 1.07 (0.74 to 1.54) |
| Did not receive | 367 (56) | 442 (58) | ||
| Total | 661 | 767 | ||
| Missing data on IAP use | 4 | |||
| Missing data on reason for IAP | 2 |
Subgroup and sensitivity analyses
Performing subgroup analyses for each of the four risk factors indicated that there was no evidence of any interaction between the subgroup category and the comparison of the primary outcome, nor any significant difference between the strategy groups for any subgroup (see Table 17).
Serious adverse outcomes
There were no reports of maternal anaphylaxis because of antibiotic administration or any reports of inoculation injury.
Chapter 6 Results of the test accuracy study
Parts of this chapter have been reproduced from Daniels et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
This chapter reports the test accuracy parameters of the Xpert GBS test on the GeneXpert testing platform compared with selective enrichment culture as reference standard.
The secondary objectives were to (1) establish a standard operating procedure for the rapid test, with turnaround times compatible with provision of a suitable duration of antibiotic exposure to test positive mothers, and (2) determine the time to availability of test results in practice and the time remaining before birth to give an adequate antibiotic exposure for effective prevention of mother-to-baby transmission.
Development of a standard operating procedure and training
With the assistance of the field application specialist from Cepheid, a simple protocol for using the GeneXpert testing platform was developed. The field application specialist and trial manager trained research midwives and clinical midwives who were designated as GBS2 trial champions at each maternity unit at a face-to-face training session. The trained midwives were then responsible for cascading the training to all clinical midwives until there were sufficient numbers of midwives trained to be able to offer the test at all times and have someone capable of using the GeneXpert machine. The average time between the initial training of midwives on the GeneXpert machine and the first women accrued to the study was 12 weeks (range 3.5–20.8 weeks).
Test data completeness
Overall, 557 of the 722 (77%) women in the rapid test group contributed to positive or negative results with the rapid intrapartum test (whether first or subsequent tests), 619 of the 722 (86%) women contributed to selective enrichment culture results and 534 of the 722 (74%) women contributed information on both tests. A flow chart to illustrate the availability of data and diagnosis by selective enrichment culture is shown in Figure 8.
FIGURE 8.
Flow chart of test data from rapid test and selective enrichment culture.
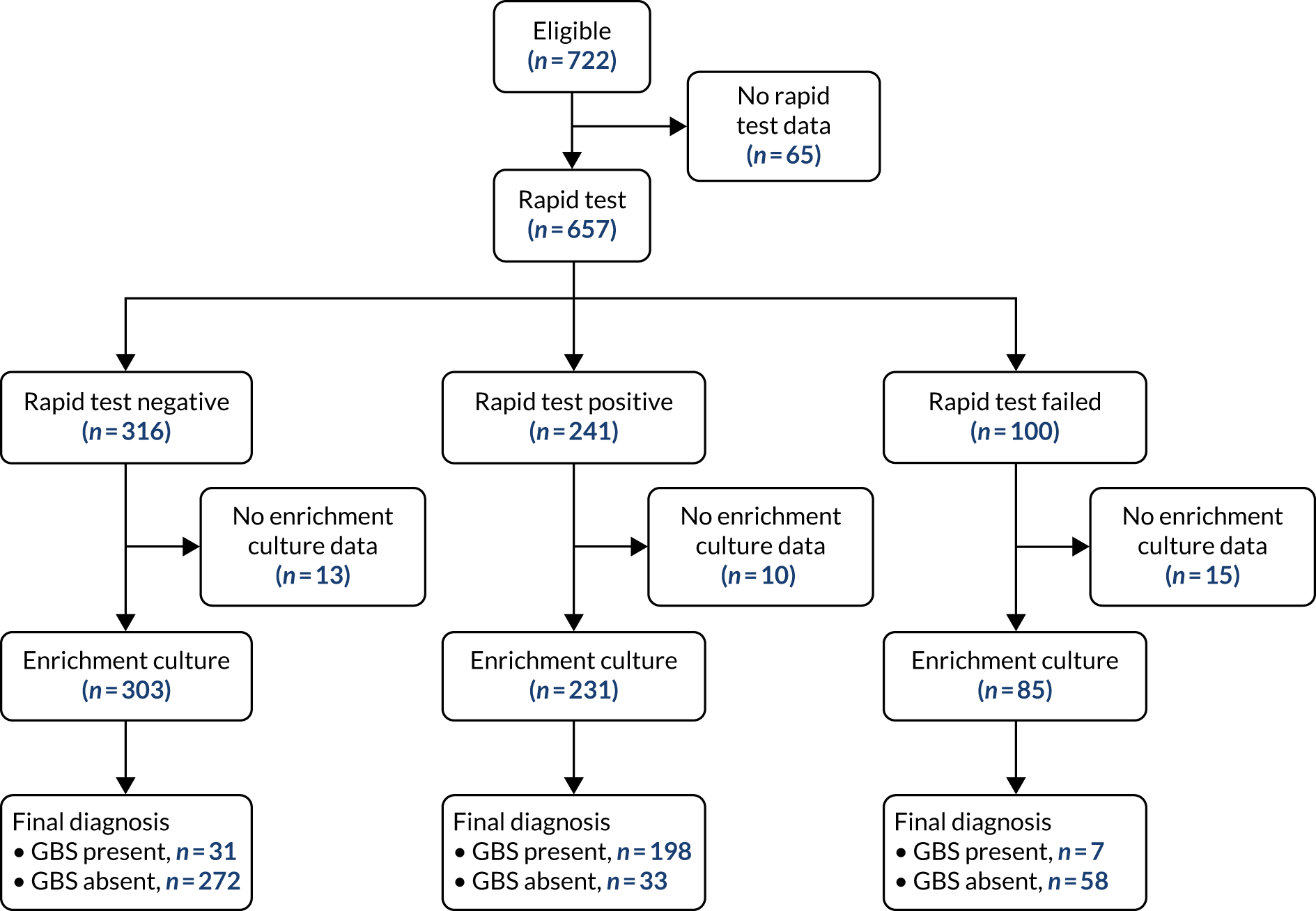
Although a vaginal/rectal swab was required by the protocol, this was not obtained in all cases, as women declined the rectal swab. In 90 women (i.e. 14% of all 667 women swabbed), only a vaginal swab was taken. There were also 253 (45%, 253/557) women with a test result who had vaginal cleansing with an antimicrobial solution that could have interfered with the viability of the swab sample and the test result. The reference standard data were not available for 4% (23/557) of women with test results and 15% (15/100) of those with failed rapid test. An ear canal swab was taken from 445 babies and a test result obtained by selective enrichment culture. A corresponding maternal colonisation status was known for 443 of these babies.
Prevalence of maternal and neonatal group B Streptococcus colonisation
The prevalence of GBS colonisation was similar for each test method (Table 20) and removing data from those women who provided a vaginal swab only did not have an impact on the prevalence.
| Swab results contributing to data | Number of women for whom tests provided the results (N = 722a), n (%) | Prevalence of GBS maternal colonisation, % (95% CI) |
|---|---|---|
| All women with test data | ||
| Rapid test | 557 (77) | 43 (39 to 48) |
| Selective enrichment culture | 619 (86) | 41 (37 to 45) |
| Excluding women contributing only vaginal swabs | ||
| Rapid test | 468 (65) | 45 (41 to 50) |
| Selective enrichment culture | 521 (72) | 44 (39 to 48) |
The neonatal colonisation rate was 11% (95% CI 8% to 14%) among 445 babies who had a result from selective enrichment culture of a neonatal ear swab in the rapid test units. The association between maternal and neonatal GBS colonisation is shown in Table 21. The concordance between maternal and neonatal colonisation status is 20%, irrespective of the method of determination of maternal status.
| Maternal GBS colonisation | Neonatal colonisation, n (%)a | |
|---|---|---|
| Present | Absent | |
| Selective enrichment cultureb | ||
| Positive | 34 (20) | 137 (80) |
| Negative | 10 (5) | 199 (95) |
| Rapid testc | ||
| Positive | 38 (21) | 146 (79) |
| Negative | 7 (3) | 196 (97) |
Diagnostic test accuracy of the rapid intrapartum test for maternal group B Streptococcus colonisation
The test result data for the 534 women providing results for both tests using vaginal/rectal or vaginal swab data are shown in Table 22. The sensitivity of the rapid test was 86% (95% CI 81% to 91%) and the specificity was 89% (95% CI 85% to 92%). The sensitivity of the rapid test was not statistically different from the expected sensitivity (p = 0.052) and specificity of 90% (p = 0.34).
| Test findings and accuracy | Selective enrichment culture | |
|---|---|---|
| Positive | Negative | |
| Rapid test, n (%) | ||
| Positive | 198 (86) | 22 (11) |
| Negative | 31 (14) | 272 (89) |
| Accuracy parameters, % (95% CI); p-value | ||
| Sensitivity | 86 (81 to 91); 0.052 | |
| Specificity | 89 (85 to 92); 0.34 | |
The results of the sensitivity analyses excluding the 87 women who provided a vaginal swab only, and the 245 women who had vaginal cleansing or lubrication with an antimicrobial solution, are provided in Tables 23 and 24, respectively. The sensitivity of the test increased slightly to 88% and 89%, respectively.
| Test findings and accuracy | Selective enrichment culture | |
|---|---|---|
| Positive | Negative | |
| Rapid test, n (%) | ||
| Positive | 177 (88) | 25 (10) |
| Negative | 24 (12) | 221 (90) |
| Accuracy parameters, % (95% CI); p-value | ||
| Sensitivity | 88 (84 to 93); 0.21 | |
| Specificity | 90 (86 to 94); 0.50 | |
| Test findings and accuracy | Selective enrichment culture | |
|---|---|---|
| Positive | Negative | |
| Rapid test, n (%) | ||
| Positive | 137 (89) | 13 (10) |
| Negative | 17 (11) | 123 (90) |
| Accuracy parameters, % (95% CI); p-value | ||
| Sensitivity | 89 (84 to 94); 0.37 | |
| Specificity | 89 (86 to 93); 0.34 | |
Chapter 7 Economic evaluation
This chapter reports the economic evaluation conducted alongside the GBS2 clinical trial. The aim of the economic evaluation was to explore whether or not a rapid intrapartum test for GBS was cost-effective in reducing maternal and neonatal antibiotic usage, compared with usual care, where the administration of IAP was directed based on maternal risk factors alone.
Methods
The economic analysis conducted alongside the GBS2 trial was based on primary clinical data collected in the cluster RCT, which was reported in earlier chapters. The principal clinical data of relevance to the economic evaluation included the use of IAP, maternal length of stay and neonatal length of stay. In the RCT, clusters were allocated to testing strategies of either usual care, based on the assessment of risk factors for GBS, or a rapid intrapartum test, which involved the use of the GeneXpert test and the results of the strategy informing IAP use. All resource use by the testing strategy group was recorded and unit costs applied.
The outcome of interest was the incidence of the use of maternal IAP that was avoided by a more targeted approach using the rapid test; hereafter this is referred to as the case of IAP avoided. The base-case cost-effectiveness analysis (CEA) results and the results of the sensitivity analyses were expressed in terms of the cost per case of IAP avoided. In the base-case analyses, costs and outcomes were based on antibiotics given specifically to reduce the risk of GBS transmission to the baby. As set out in the protocol, this included IAP for preventing vertical transmission of GBS from mother to baby only and IAP in combination with antibiotics for any indication. The length of hospital stay in the base case related only to the mother and the cost was derived from NHS reference costs for an indicative length of stay according to the mode of birth, and was not based on the trial data.
Sensitivity analyses were carried out:
-
Sensitivity analysis 1 – the length of stay for only the mother was included and was based on trial data, and the NHS reference costs for each mode of birth were removed from the analyses for both trial strategy groups.
-
Sensitivity analysis 2 – as sensitivity analysis 1, but with the costs of inpatient stay for infants added to the costs for both testing strategy groups of the trial based on the trial data.
The focus of the trial was on the period of labour and birth and, therefore, within a 12-month time frame, and so the discounting of costs and outcomes was not applicable. The results of the economic evaluation are reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 61
Resource use and costs
The CEA adopted the UK NHS perspective. In alignment with recommended practice, only direct costs to the health service were included in this analysis. 62 The data from the trial were at an individual woman level, as resource use data were collected via electronic health records. The health-care resource utilisation data were obtained on respondents’ usage from hospital admission until the discharge date. The main resource use monitored included the use of intrapartum antibiotics, and resource use associated with the midwife monitoring the woman, admission to labour and delivery units and the length of labour. In terms of undertaking the rapid test during labour, the resource use was based on the listed resource use from a previous time-and-motion study51 and was varied in sensitivity analysis.
Valuing resource use
Antibiotic prices were obtained from the British National Formulary63 and secondary care costs from the National Schedule of Reference Costs (2017–2018). 64 All resource use was multiplied by the unit cost to estimate the overall costs for each treatment allocation. All items, their relevant unit costs and the sources for the cost data are presented in Tables 25–27. All unit costs were valued at 2017/18 prices in UK pounds sterling. When necessary, costs from prior years were inflated to the current price year using the Hospital and Community Health Service index. 64
| Antibiotic name and dosage | Vial size | Unit price (£) | Source and additional information |
|---|---|---|---|
| Main antibiotic issued | |||
| Benzylpenicillin sodium | |||
| 600 mg | 2 | 6.01 | Injection vials (AAH Pharmaceuticals Ltd, Coventry, UK) |
| 25 | 75.12 | ||
| 1.2 g | 25 | 109.49 | |
| Cephalosporins | |||
| Cefotaxime | |||
| 500 mg | 10 | 21.00 | Solution for injection vials (Bowmed Ibisqus Ltd, Wrexham, UK) |
| 1 g | 10 | 35.00 | Solution for injection vials (Wockhardt UK Ltd, Wrexham, UK) |
| 2 g | 10 | 37.50 | Solution for injection vials (Wockhardt UK Ltd) |
| Cefuroxime | |||
| 750 mg (powder) | 1 | 2.52 | Powder for injection vials (Flynn Pharma Ltd, Stevenage, UK) |
| 750 mg (powder) | 5 | 11.72 | Powder for injection vials (Flynn Pharma Ltd) |
| 750 mg (powder) | 10 | 25.20 | Powder for injection vials (Flynn Pharma Ltd) |
| Ceftriaxone | |||
| 1 g | 1 | 9.58 | Solution for injection vials (Stravencon Ltd, London, UK) |
| 1 g | 10 | 95.80 | Solution for injection vials (Wockhardt UK Ltd) |
| Clindamycin | |||
| 150 mg per 1 ml | 5 | 29.50 | 300 mg/2 ml solution for injection ampoules (Bowmed Ibisqus Ltd) |
| 5 | 59.00 | 600 mg/4 ml solution for injection ampoules (Bowmed Ibisqus Ltd) | |
| Metronidazole | |||
| 5 mg per 1 ml | 20 | 63.86 | 500 mg/100 ml infusion bag (AAH Pharmaceuticals Ltd) |
| Other antibiotics issued | |||
| Amikacin | |||
| 50 mg per 1 ml | 5 | 10.33 | 100 mg/2 ml solution for injection vials (Bristol-Myers Squibb Pharmaceuticals Ltd, Middlesex, UK) |
| 250 mg per 1 ml | 5 | 60.00 | 500 mg/2 ml solution for injection vials (Pfizer Inc., New York, NY, USA) |
| Amoxicillin | |||
| 250 mg (powder) | 10 | 4.50 | Solution for injection vials (Bowmed Ibisqus Ltd) |
| 500 mg (powder) | 10 | 9.60 | Solution for injection vials (Wockhardt UK Ltd) |
| 1 g (powder) | 10 | 16.50 | Solution for injection vials (Bowmed Ibisqus Ltd) |
| Ampicillin | |||
| 500 mg (powder) | 10 | 78.30 | Solution for injection vials (AAH Pharmaceuticals Ltd) |
| Erythromycin | |||
| 1 g (powder) | 1 | 22.95 | Solution for injection vials (Advanz Pharma, Jersey, UK) |
| Gentamicin | |||
| 1 mg per 1 ml | 20 | 40.17 | 80 mg/80 ml infusion bags (AAH Pharmaceuticals Ltd) |
| Resource use item: test | Unit cost (£) | Source and additional information |
|---|---|---|
| Expert GBS cartridge | 28 | Cepheid information |
| Swab (vaginal or rectal) | 0.84 | Cepheid information |
| Cartridge GeneXpert system machine | 14,400 | Cepheid information. For 12 months out of a 3-year lease |
| Cost of midwife administering rapid test | 30 | Midwife time was costed as band 6 from PSSRU 201865 at £45/hour and approximately 40 minutes to take test, including time to take swab, insert cartridge and retrieve results |
| Resource use item | Unit cost (£) | Source and additional information |
|---|---|---|
| Vaginal births | ||
| Cost per admission into delivery suite visit | 1084.00 | National Schedule of Reference Costs (2017–2018);64 item NZ30C |
| Normal labour | 3009.27 | National Schedule of Reference Costs (2017–2018);64 item NZ30A,B,C |
| Normal birth with induced labour | 3722.93 | National Schedule of Reference Costs (2017–2018);64 item NZ31A,B,C |
| Assisted birth | 3333.33 | National Schedule of Reference Costs (2017–2018);64 item NZ40A,B,C |
| Assisted birth and induced labour | 4357.24 | National Schedule of Reference Costs (2017–2018);64 item NZ42A,B,C |
| Emergency caesarean | 5723.45 | National Schedule of Reference Costs (2017–2018);64 item NZ51A,B,C |
| Postnatal care | ||
| Day in a maternity ward | 284.13 | Petrou et al.66 |
| Day in an intensive care unit | 1294.05 | Coomarasamy et al.67 |
| Day in a high-dependency unit | 673.09 | Coomarasamy et al.67 |
| Neonatal care | ||
| Day in an intensive care unit | 1445.00 | National Schedule of Reference Costs (2017–2018);64 item XA02Z |
| Day in a high-dependency unit | 925.00 | National Schedule of Reference Costs (2017–2018);64 item XA03Z |
| Day in special care, with or without external carer | 520.00 | National Schedule of Reference Costs (2017–2018);64 item XA04Z |
| Day in normal care | 441.00 | National Schedule of Reference Costs (2017–2018);64 item XA05Z |
Economic analysis
All statistical analyses were conducted using Stata® version 15 (StataCorp LP, College Station, TX, USA). Initial analyses included conducting a cost–consequence analysis, detailing all costs and outcomes incurred in each trial strategy in a disaggregated form. This analysis was based on an intention-to-treat basis, meaning that within the analysis all women were analysed according to their maternity unit’s allocated testing strategy, regardless of whether the individual adhered to the site’s testing strategy or IAP was administered appropriately. 68
The total costs incurred by each woman during labour were calculated by multiplying the resource use volume by the unit costs. Moreover, mean costs and outcomes were generated for each trial testing strategy. The principal outcome on which the economic evaluation was based is the number of instances of IAP avoided. The timeliness of the test, in terms of the rapid test producing a result in time to administer intrapartum antibiotics before labour begins, was not factored in, as the cost of the rapid test is incurred regardless of whether or not the result was presented in time to be acted on. An incremental analysis was conducted if appropriately indicated by the cost–consequence analysis. An incremental analysis is estimated by comparing the differences in mean costs and outcomes between the two strategies using seemingly unrelated regression estimates. By dividing the cost differences by the outcome differences, the incremental cost-effectiveness ratio (ICER) is calculated. To account for the inherent skewness of the cost data, 95% CIs around the mean differences were calculated using the bias-corrected and accelerated bootstrap method. 69
Stochastic cost-effectiveness analysis
A stochastic CEA was conducted to examine the sensitivity of the results. The approach taken in the probabilistic sensitivity analysis was that all important variables relating to costs and clinical outcomes were given a distribution that describes the uncertainty surrounding the mean. The distributions were simulated 10,000 times. Each time, random numbers were drawn from the appropriate distributions. After each simulation, the incremental costs and effects were plotted in a cost-effectiveness plane that comprised four quadrants: (1) north-east, (2) north-west, (3) south-east and (4) south-west. The scatterplot that was produced represented the simulations. If dots from the scatterplot were in the north-east quadrant, then this indicated that the addition of the rapid test was more costly and more effective than usual care, based on risk factors. Dots in the north-west quadrant indicated that the rapid test was more costly and less effective than the usual care. Based on these simulations, the probability that the rapid test strategy would be cost-effective was presented. This was the standard approach for health economics following accepted guidelines (i.e. CHEERS)70 and was a presentation of results of cost-effectiveness studies that would be required by decision-makers, such as NICE.
Results were also presented using cost-effectiveness acceptability curves (CEACs) to reflect sampling variation and uncertainties in the cost-effectiveness value, when appropriate. The CEACs show the probability of the rapid test being cost-effective, compared with usual care, based on risk factors and observed data for a range of maximum monetary values (thresholds) that decision-makers might be willing to pay for a particular unit change in outcome. 71 We examine cost-effectiveness across a range of monetary willingness-to-pay (WTP) thresholds.
Additional sensitivity analyses were carried out:
-
Sensitivity analysis 3 – the proportion of midwife time to conduct the rapid test was reduced from 40 minutes in the base case to 10 minutes.
-
Sensitivity analysis 4 – only the women for whom IAP was given specifically for GBS and no other reason were analysed.
Results
Rapid intrapartum test
A total of 721 women gave birth in units randomised to the rapid test (one woman delivered elsewhere) and 906 women gave birth in usual-care units. Almost all women in the rapid test arm had at least one swab taken and reported (for three women it is unknown whether or not the swab was taken and no test results are available). However, more tests were issued within the rapid test strategy, depending on the length of labour and delivery stage. The data identified 26 women who had a repeat test performed because either the test was not started within 15 minutes of taking the swab or labour had not progressed within 48 hours of initial admission.
Risk factors
Of the total population, the most common risk factor was preterm labour, identified in 757 (46%) women. This was followed by a history of temperatures of ≥ 38 °C during labour, identified in 662 (41%) women (see Table 8). It should be noted that, for this analysis, any costs associated in assessing risk factors were assumed to be included within the ‘usual’ birth costs.
Vaginal deliveries
Vaginal deliveries were grouped into four categories: (1) spontaneous vaginal delivery following spontaneous onset, (2) vaginal delivery following induced labour, (3) instrumental delivery following spontaneous onset of labour and (4) instrumental delivery following induction of labour. These categories are based on the trial data set and the available cost data for these types of birth in the NHS reference costs. 64 Across both strategy groups, 584 (48.1%) women had spontaneous deliveries, 397 (32.7%) had a vaginal birth with induced labour, 124 (10.2%) had an assisted vaginal birth and 109 (9.0%) had an assisted and induced birth.
Caesarean deliveries
Elective caesareans were not included in this analysis, as they were part of the exclusion criteria. Women who gave birth by emergency caesarean section were included. In total, 406 (25.0%) women underwent an emergency caesarean section.
Resource use
The following resource use and costs were reported by category. The estimation of resource use and associated cost was carried out on complete-case data.
Antibiotics provided
The average doses of antibiotics administered per woman during labour are presented by testing strategy in Appendix 3, Table 33. The antibiotic usage was categorised in terms of loading doses and maintenance doses. Loading doses were high initial doses issued at the beginning of the treatment given prior to reduced maintenance dosages. 72 Overall, on average, women in the rapid test units received a slightly higher level of antibiotic in terms of loading doses. There was little variation in antibiotic for maintenance dose overall.
Average costs
To calculate the mean costs for each testing strategy, mean resource use was multiplied by the relevant unit cost. The mean cost varied between strategies for individual antibiotics (see Appendix 3,Table 34), but the overall the mean of the total antibiotic cost was higher in the rapid test arm.
Loading dose costs
There was variation in the costs regarding the different types of antibiotics issued. In terms of the costs for loading dosages, sizeable differences in costs were noted for benzylpenicillin, cephalosporins and metronidazole, as noted by the bootstrapped difference 95% CIs. With the exception of cephalosporins, the rapid test units showed a higher mean loading dose cost for both benzylpenicillin and metronidazole.
Maintenance dose costs
There was less variation in the mean costs for the maintenance dose of IAPs. Notable differences were observed for cephalosporins and metronidazole. A difference was noted for the benzylpenicillin maintenance dosage; here, the rapid test has a mean cost of £11.43, whereas the usual-care strategy has a mean cost of £9.06. Table 28 presents the combined costs for both the loading and the maintenance doses. There was a variation in the types of antibiotic issued. Clear differences between the two strategies existed for almost all antibiotics, with the data showing that typically the rapid test incurs higher mean total costs than usual care. The only exception being other antibiotics, namely amikacin, amoxicillin, ampicillin, erythromycin and gentamicin, outside the main group issued to women during labour. The other antibiotic average cost in the rapid test strategy was lower than the usual-care strategy (£12.65 vs. £19.63), with an adjusted mean difference of £6.98 less than the usual-care arm.
| Cost category | Rapid test (N = 722) | IAP based on risk factors (N = 906) | Bootstrapped differences | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (£) | SD (£) | n | Mean (£) | SD (£) | Adjusted mean difference (£) | 95% CI (£) | |
| Antibiotic | ||||||||
| Benzylpenicillin | 258 | 26.94 | 18.91 | 294 | 23.94 | 11.97 | 3.00 | 0.64 to 5.74 |
| Cephalosporins | 30 | 7.86 | 7.80 | 10 | 5.25 | 0.87 | 2.61 | 0.35 to 5.90 |
| Clindamycin | 22 | 30.31 | 17.68 | 29 | 29.91 | 19.63 | 0.40 | –9.39 to 11.18 |
| Metronidazole | 17 | 9.38 | 15.61 | 8 | 3.19 | 0 | 6.19 | 1.31 to 16.14 |
| Other | 108 | 12.65 | 18.67 | 125 | 19.63 | 31.59 | –6.98 | –14.43 to 0.83 |
| Cost of delivery | 722 | 4008.10 | 1047.27 | 906 | 3979.56 | 1095.95 | 28.55 | –76.57 to 130.63 |
Cost–consequences analysis
Table 29 presents the costs and outcomes assessed within the GBS2 trial. In the rapid test strategy, the costs included the following: the mean cost of IAP, the mean costs of birth/deliveries and the mean cost of issuing the rapid test. The mean cost of issuing the rapid test included the additional midwifery time per woman, the costs of vaginal/rectal swabs, the costs of a cartridge for the GeneXpert machine and the machine running time. By contrast, the costs of the usual-care strategy comprised the mean cost of IAP and the mean costs of the baby being born. The assumption was made that assessing for risk factors is an integral component of the overall cost of the baby being born. The mean cost per woman in the rapid testing strategy was approximately £4128, whereas under usual care the mean cost was approximately £4003 per woman.
| Costs and outcomes | Rapid test (SD) (n = 722) | Usual care (SD) (n = 906) |
|---|---|---|
| Costs (£) | ||
| Mean cost of IAP | 24.93 (22.35) | 23.81 (20.96) |
| Mean cost of deliveries | 4008.10 (1047.27) | 3979.56 (1095.56) |
| Mean cost of issuing rapid test (includes swab, cartridge, machine running time and midwifery time) | 94.686 (6.57) | |
| Mean total costs | £4127.71 | £4003.36 |
| Total costs | £2,939,262.00 | £3,615,951.10 |
| Outcomes | ||
| Number of women receiving IAP for GBS prophylaxis | 297 | 328 |
| Proportion of women receiving IAP for GBS prophylaxis, of all women in testing group | 0.41 | 0.36 |
Outcomes
The primary outcome of the trial was the proportion of women for whom IAP was used as prophylaxis against GBS infection in her baby. In the rapid test sites, IAP for GBS prophylaxis was administered to 297 women [proportion 0.41 (297/722)], compared with 328 women in the usual-care units [proportion 0.36 (328/906)]. A higher proportion of women in the rapid test units received IAP to prevent neonatal GBS disease, but there was no statistically significant difference from those in the usual-care units (see Table 12).
Base-case analysis
The results in Table 29 show that the mean cost per woman in the rapid test units was approximately £4128 and the mean proportion of women treated with antibiotics was 0.41. This compared with the mean cost per woman in usual-care units being approximately £4003 and a mean proportion of women treated with antibiotics of 0.36. Therefore, to achieve the outcome of averting antibiotic use, the rapid test was more costly and less effective than usual care, as it cost more and antibiotics use to prevent GBS infection was greater. The use of the rapid test in this analysis was said to be dominated by usual care. Therefore, producing an ICER is not applicable for the base case, as the cost–consequence analyses revealed the situation of dominance.
The cost-effectiveness plane for the base-case analysis is presented in Figure 9. The majority of the scatterplot dots (depicting paired incremental costs and outcomes) are in the north-west and south-west quadrants. Scatterplot dots falling in the north-west quadrant represent higher costs and worse outcome than the comparator, whereas scatterplot dots in the south-west quadrant represent worse outcome and lower costs. Therefore, Figure 9 suggests that the rapid test is less effective at reducing the use of IAP. However, it is uncertain whether the rapid test is likely to be more costly (north-west quadrant) or less costly (south-west quadrant) compared with usual care (represented by the origin). The CEAC (Figure 10) shows the probability of the rapid test being cost-effective at various values of decision-makers’ WTP per case of antibiotic avoided. The CEAC (see Figure 10) suggests that the probability of the rapid test being cost-effective is > 40% for all WTP values, tending to 0% as the WTP increases. Therefore, the probability of cost-effectiveness decreases as the WTP increases.
FIGURE 9.
Cost-effectiveness plane: the base case.
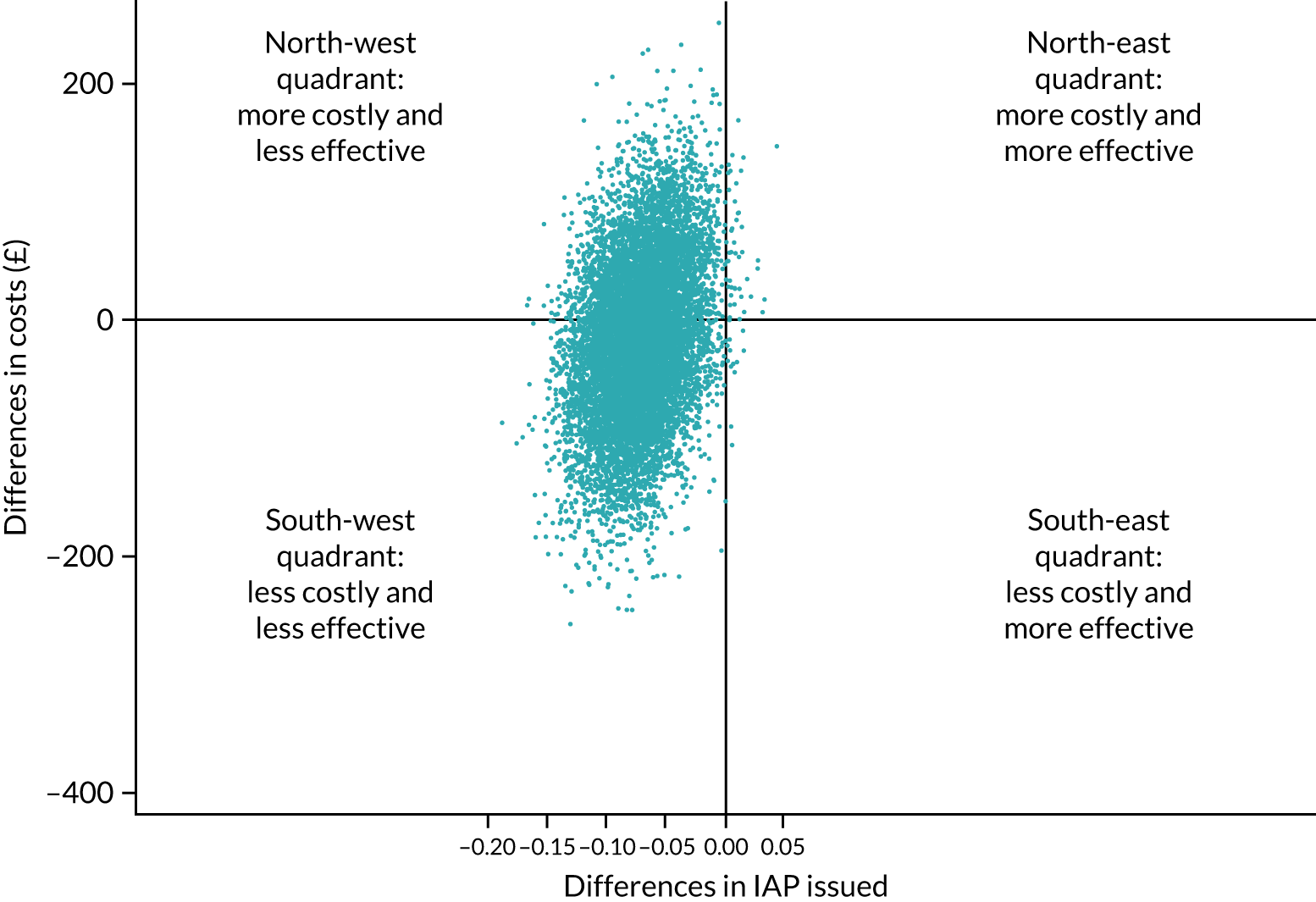
FIGURE 10.
Cost-effectiveness acceptability curve: the base case.
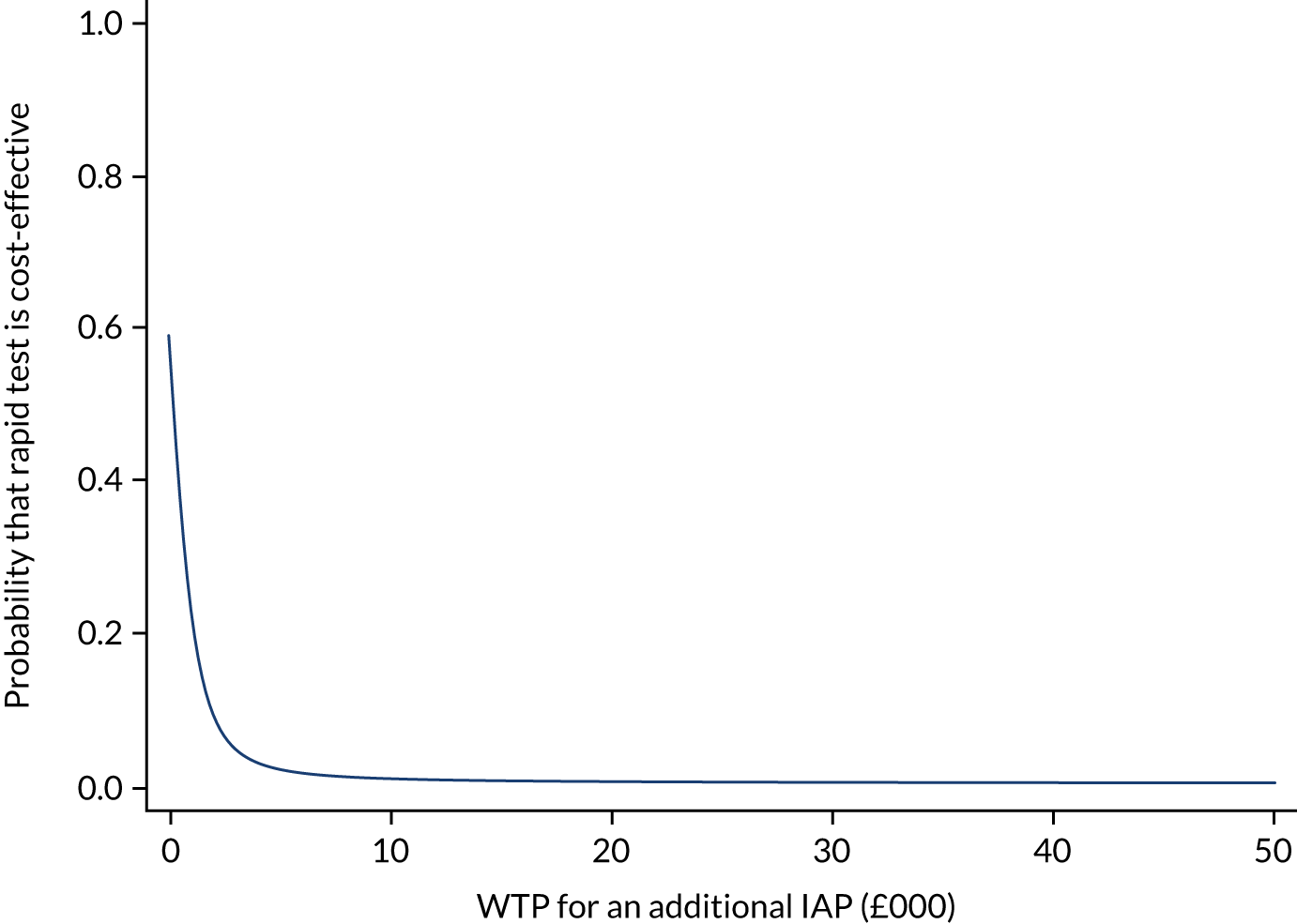
Sensitivity analyses were carried out:
-
Sensitivity analysis 1 – the length of stay for the mother was based on trial data, and the mode of birth (which includes costs of an indicative length of stay that was used in the base case) was removed from the analyses for both trial testing strategy groups.
-
Sensitivity analysis 2 – as for sensitivity analysis 1, but the costs of inpatient stay for newborns were added to the costs for both trial testing strategy groups based on the trial data.
The descriptive statistics for inpatient days for the mother and the babies on which these two sensitivity analyses are based are presented in the Appendix 3, Tables 35 and 36, respectively.
Sensitivity analysis 1
The mean length of stay and mean costs per strategy are presented in Appendix 3, Table 35. The cost-effectiveness plane for sensitivity analysis 1 is presented in Figure 11. The majority of the scatterplot dots now resided in the north-west quadrant and there were fewer in the south-west quadrant. This suggested that the true length of stay, based on the length of stay recorded in the trial, was actually more costly than the reference cost data implied. The test remained ineffective, but some uncertainty around the cost data was reduced, as the rapid test strategy was shown to be more costly than usual care. Figure 11 broadly supported the base-case analysis and showed that the rapid test strategy was more costly and less effective than usual care. The CEAC (Figure 12) was similar to that of the base case.
FIGURE 11.
Cost-effectiveness plane: mothers’ length of stay (sensitivity analysis 1).
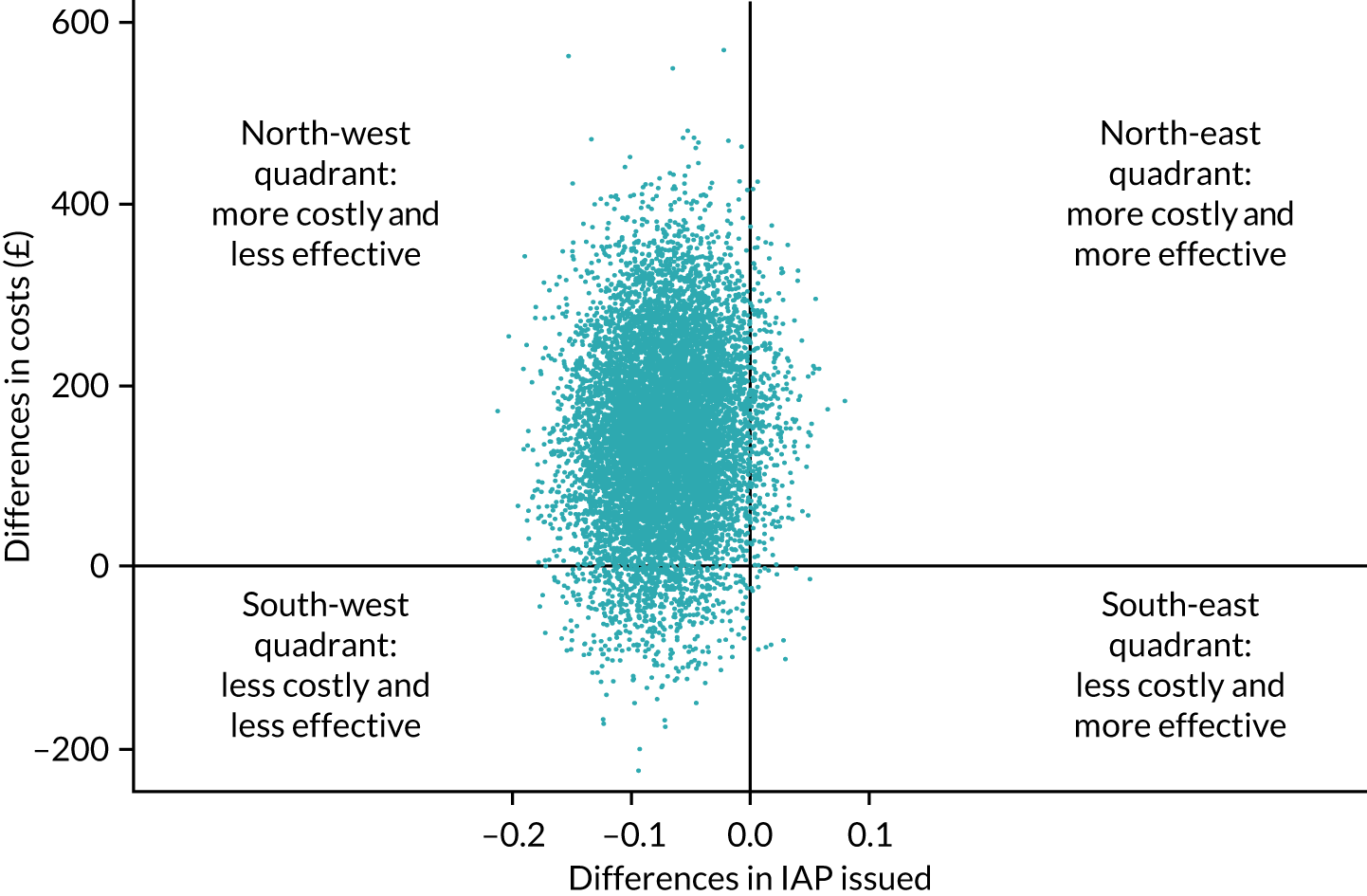
FIGURE 12.
Cost-effectiveness acceptability curve: mothers’ length of stay (sensitivity analysis 1).
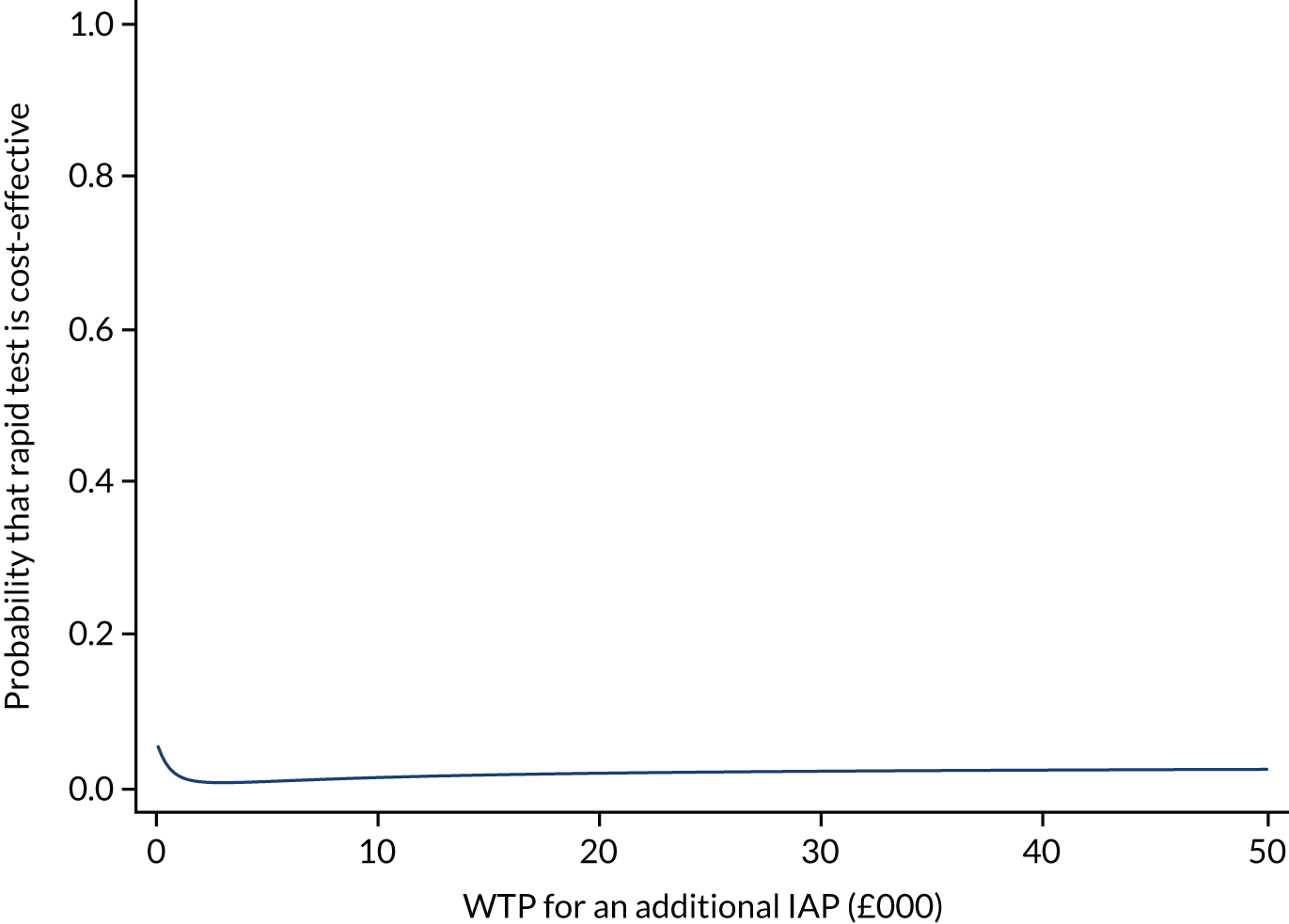
Sensitivity analysis 2
This was similar to sensitivity analysis 1, but the costs of inpatient stay for newborns by their level of care were added to the costs in both strategy groups of the trial, based on the trial data. When needed, assumptions were required as indicated in the Appendix 3, Table 36.
The cost-effectiveness plane for sensitivity analysis 2 is presented in Figure 13. The majority of the scatterplot dots have now shifted to the south-west quadrant, showing that although, overall, more antibiotics were prescribed in the rapid test units than in the usual-care units, the overall costs were now less for the rapid test strategy. This meant that the length of stay of babies and their corresponding costs were higher in the usual-care units. The implication was that these babies required more care than the babies in the rapid tests units, whose mothers had received IAP. Therefore, Figure 13 differed from the base-case analysis and suggested that the addition of the babies’ length of stay changes the costs of the rapid test strategy to be less costly than usual care overall. The CEAC (Figure 14) showed that the probability of being cost-effective declined as the WTP increased, primarily because more antibiotics were prescribed in the rapid test group and so the rapid test had failed to meet the objective of reducing the prescribing of antibiotics, despite being slightly cheaper overall.
FIGURE 13.
Cost-effectiveness plane: mothers’ and babies’ length of stay (sensitivity analysis 2).
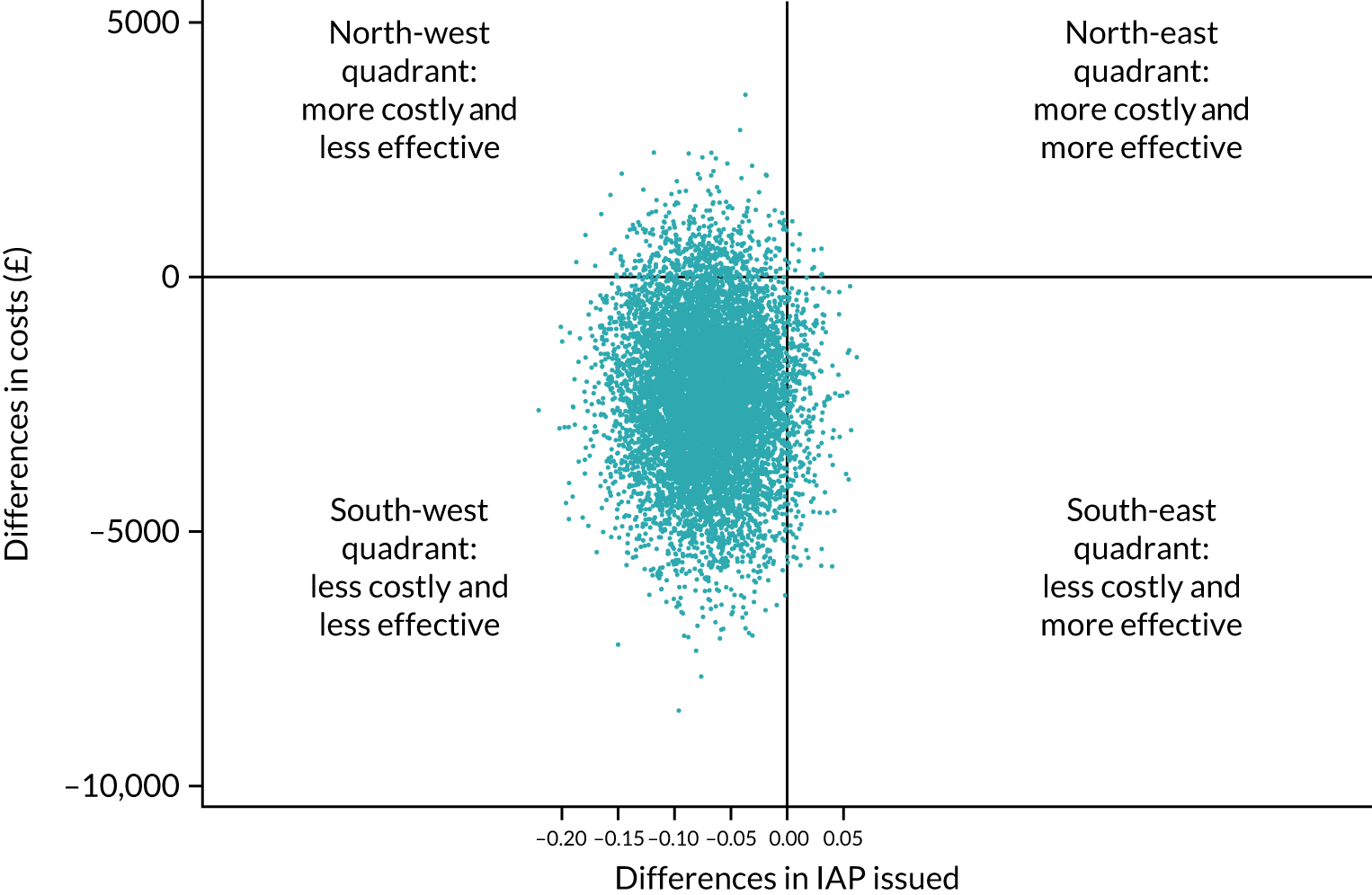
FIGURE 14.
Cost-effectiveness acceptability curve: mothers’ and babies’ length of stay (sensitivity analysis 2).
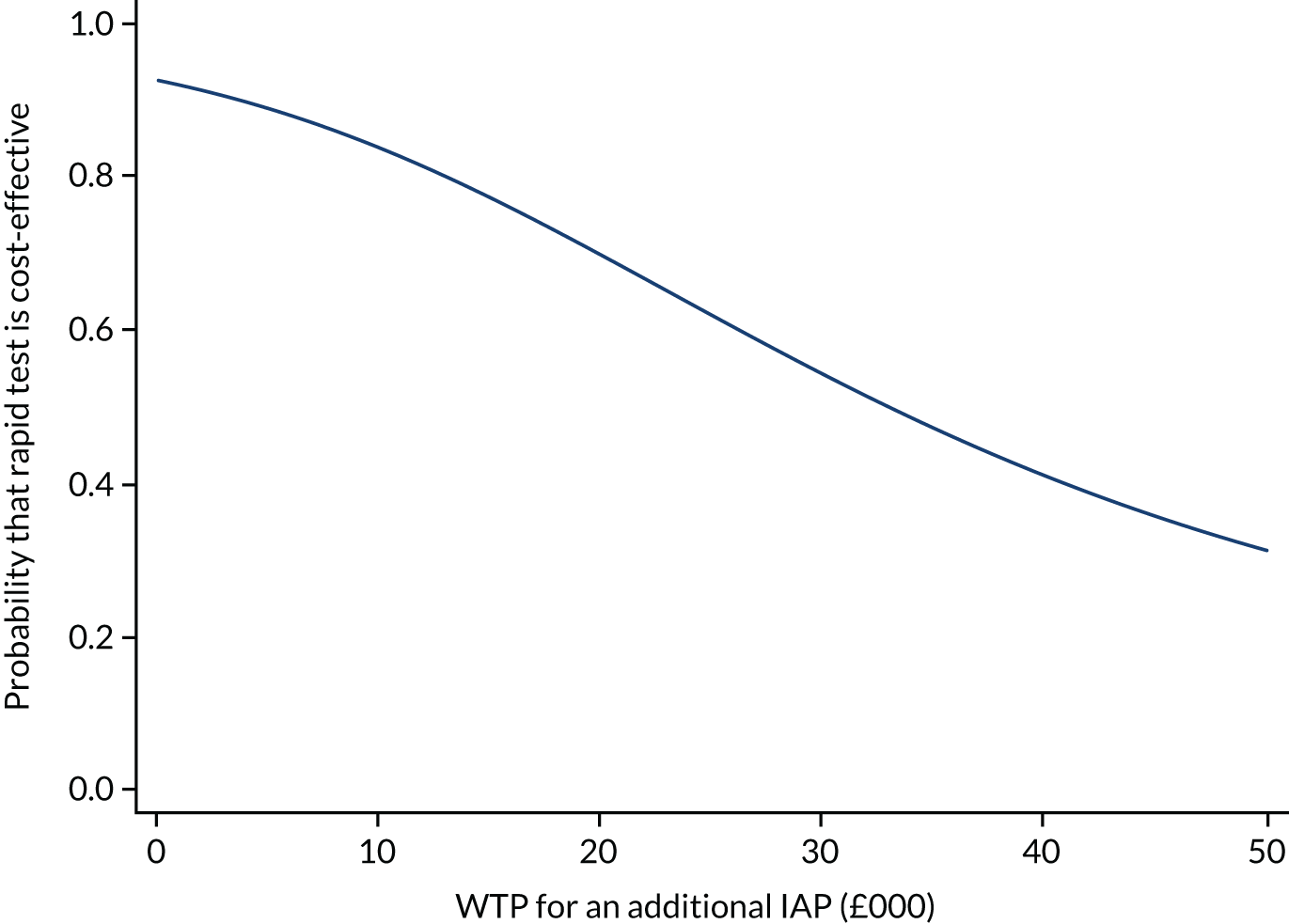
Additional probability sensitivity analyses were also conducted. Sensitivity analysis 3 reduced the length of midwife time in the rapid test strategy from 40 minutes to 10 minutes and showed no substantial change on the base case. Sensitivity analysis 4 was the same as the base case, but woman who were prescribed GBS prophylaxis only and not antibiotics for any other reason were included. No substantial change in the base case was observed, with the cost-effectiveness plane focusing on the origin.
Chapter 8 Microbiology substudy
Parts of this chapter have been reproduced from Daniels et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Substudy objectives
-
To determine the antibiotic resistance profile of any GBS isolated from the rectal vaginal swab taken from the mother at the time of labour, and to compare this with the antibiotic resistance profile from any faecal sample taken from the woman’s baby at 6 weeks of age.
-
To estimate the carriage rate of antibiotic-resistant bacteria, particularly E. coli, in rectal samples from women recruited to the GBS2 trial from centres in LSE who are assigned a rapid test system. In addition to antibiotic-resistant E. coli, we have also included MRSA and VRE.
-
To estimate the extent to which carriage of specific resistant bacteria or resistance elements by the mother at the time of birth increases the risk of carriage of those specific bacteria or elements by the infant at 6 weeks of postnatal age.
-
To gather some information on peripartum risk factors for transmission (e.g. mode of birth, maternal comorbidities, gestational age and antibiotic exposure).
Methods
The substudy recruited only in the participating centres in LSE that were randomised to receive a rapid test system. Written consent was sought for the 6-week follow-up of the children born to study participants in the participating centres. Vaginal/rectal swabs were taken from women who had risk factors associated with early-onset GBS infection around the time of birth using a third swab. This swab was taken alongside or directly after the first and second swabs, as in other centres assigned a rapid test (see Chapter 7).
Additional microbiological assessment of the third vaginal/rectal swab
The third swab was sent to the microbiology laboratory at Barts Health NHS Trust and stored in a Brain Heart Infusion Broth (Oxoid Ltd, Basingstoke, UK) with 10% glycerol cryopreservative broth at –80 °C. This swab was processed further only if notification of written consent for substudy participation had been obtained from the mother. The third swab was held for a period of 96 hours in the microbiology department of Barts Health NHS Trust to allow the woman to receive an invitation to participate in research and give informed consent. Should a woman have declined to provide consent, or if 96 hours had passed since the receipt of the third swab, then this third swab was not processed but instead was disposed of in a suitable manner compliant with local policies.
Obtaining a faecal sample from the infant
Once a period of 5 weeks had elapsed and following notification from the trial office that consent had been given, the substudy research assistant supplied the maternity unit with a faecal sample pot labelled with the baby’s unique study number. The research assistant then prompted the dispatch of a follow-up sample collection pack to the consenting mother. The research assistant recorded the number on the pot alongside the mother’s trial number. The maternity unit staff or research midwife/practitioner then checked with the community midwifery team that nothing untoward had happened to the child, then the research midwife/practitioner sent out a follow-up sample collection pack. This pack consisted of a faecal sample pot, a covering letter, instructions, a protective transport container and a prepaid addressed envelope to the woman’s home address. The letter requested that a sample of the child’s faecal material be collected from the child’s nappy. The instructions provided to the mother are shown in Report Supplementary Material 1.
When a sample of the baby’s faecal material was not forthcoming after the first request, then another request was sent to the mother at around 9 weeks after her baby was born. No further attempts to obtain a faecal sample were made.
Microbiological testing of infant faecal samples
Methods for the isolation of GBS are described elsewhere in this monograph. Following receipt of the infant faecal sample, the microbiology laboratory at Barts Health NHS trust informed the research assistant, who then recorded this against the woman’s unique trial number. The faecal sample was diluted 1 in 10 into a cryopreservation broth and then mixed by vortexing, before being stored at –80 °C.
Antibiotic resistance testing
The frozen sample was allowed to defrost at room temperature and then plated on to a range of culture media, including chromogenic MRSA agar (Oxoid Ltd), Slanetz–Bartley agar with vancomycin (Oxoid Ltd) and MacConkey agar (Oxoid Ltd). Presumptive isolates were identified using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Antibiotic resistance was tested using European Committee on Antimicrobial Susceptibility Testing methods and break points. 73 Enterobacterales were tested for sensitivity to ampicillin, piperacillin plus tazobactam, amoxicillin plus clavulanate, cefpodoxime, gentamicin, cefuroxime, amikacin, co-trimoxazole, temocillin, ceftazidime, ertapenem and ciprofloxacin on Muller–Hinton agar.
Molecular testing for Gram-negative antibiotic resistance genes
The EasyScreen™ Sample Processing Kit (SP006, Genetic Signatures Ltd, Newtown, NSW, Australia) is designed to rapidly isolate nucleic acids [deoxyribonucleic acid (DNA) and ribonucleic acid (RNA)] from clinical samples via an automated purification system. The system was used to look for antibiotic-resistant genes prevalent in Gram-negative bacteria. Reagents are supplied to convert all nucleic acids into 3base™ sequences (Genetic Signatures Ltd) and to perform a subsequent purification on the GS-mini platform (Genetic Signatures Ltd). The original maternal vaginal swab was removed from the –80 °C freezer and vortexed briefly in the Amies transport medium in which it was stored to release the bacteria. A 1-ml aliquot was removed and added to the GSL-combined reagents 1 and 2. Infant samples were removed from the –80 °C freezer and, after defrosting, 100 µl/ml was added to combined GSL reagents 1 and 2 for processing with the EasyScreen™ Sample Processing Kit (SP006).
The EasyScreen™ Sample Processing Kit lyses any microorganisms present and converts all cytosine bases to uracil (detected as thymine after PCR amplification) to create 3base™ DNA and RNA. The 3base™ nucleic acids have an increased homology compared with the genome that comprised the native four bases. This complexity reduction results in genomes that are more similar to each other, enabling the design of primers and probes that contain fewer mismatches, are more homologous, produce better amplification and, importantly, have less cross-reactivity when multiple strains of the same organism are present.
Extracted DNA was analysed using the EasyScreen™ ESBL/CPO Detection Kit (BL001, Genetic Signatures Ltd), which uses panels of multiplexed real-time PCR assays to provide rapid and accurate detection of 16 β-lactam and carbapenem-resistant pathogen targets. These were TEM, GES, OXA-48(like), MCR-1, VIM, CMY, CTX-M, KPC, OXA-23 (like), DHA, IMI, IMP, NDM, SHV, SME and OXA-51 (like). The kit includes an extraction control in the reaction mix to determine the reliability of the extracted nucleic acids and indicate the presence of any inhibitors after extraction from primary samples. PCR was performed on a Bio-Rad CFX96/384 Touch™ real-time PCR instrument. Reaction mixtures were set up in accordance with the manufacturer’s instructions and Ct values read in accordance with its recommendations.
Statistical methods
A binomial regression model with a log-link was used to estimate RRs. All estimates are reported along with 95% CIs. All analyses were conducted in SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA). Fisher’s exact test was used to compare proportions and a p-value of < 0.05 was considered statistically significant.
Results
Overall, 117 women provided samples for the substudy. There were 64 paired samples from 63 mothers and 64 infants (one set of twins). There were 60 paired samples available for molecular testing (59 mothers and 60 infants).
Group B streptococci were isolated from 39 (33%) of the 117 maternal samples. Of these, 32 out of 39 (82%, 95% CI 66% to 92%) isolates were tetracycline resistant, 9 out of 39 (23.1%, 95% CI 11% to 39%) were erythromycin resistant and 7 out of 39 (18%, 95% CI 8% to 34%) were clindamycin resistant, but there were no penicillin-resistant GBS isolates (0%, 95% CI 0% to 9%). GBS were isolated from only 2 of the 64 infant 6-week samples (3%, 95% CI 0% to 11%). There were no MRSA isolated from mother or infant samples. VRE were isolated from one infant and one unrelated maternal sample.
E. coli were isolated from 85 (73%) of the 117 maternal samples. Of these, resistance to ampicillin was found in 46 (54%) of the 85 samples, resistance to amoxycillin/clavulanate was found in 37 (44%) samples, resistance to trimethoprim/sulfamethozaxole was found in 21 (25%) samples, resistance to ciprofloxacin was found in five (6%) samples and resistance to gentamicin was found in four (5%) samples. ESBL-producing bacteria were cultured from three (4%) samples, and 18 (21%) samples were resistant to three or more antibiotic classes (multiresistant).
Gram-negative antibiotic resistance genes were identified in the 128 samples from mother–infant pairs. Genes identified in samples included TEM, CTX-M, SHV, OXA-23, 48, 51 (like), CMY, IMI, VIM, MCR-1, DHA, GES, KPC and NDM. Only TEM and CTX-M were present in ≥ 10 maternal samples. SHV (which is carried by Klebsiella pneumoniae predominantly) was found in 14 out of 60 (23%) infant samples compared with only 5 out of 59 (8%) maternal samples, possibly reflecting differences in mother and infant Klebsiella colonisation (Fisher’s exact test p = 0.04).
The RR of carriage of resistant E. coli or resistance genes in infants born to mothers with or without carriage of strains with specific characteristics is shown in Table 30. Although the point estimates for association between infants carrying any resistant E. coli or specific resistance genes were consistently higher when carriage was detected in the mother, the findings were significant only for co-trimoxazole-resistant and multidrug-resistant genes.
| Colonisation | Mother, n (%) | RR (95% CI)a | |
|---|---|---|---|
| Yes | No | ||
| Presence of any E. coli | |||
| Yes | 31 (61) | 6 (46) | 1.32 (0.70 to 2.47) |
| No | 20 (39) | 7 (54) | |
| Ampicillin resistant | |||
| Yes | 10 (43) | 9 (22) | 1.98 (0.94 to 4.16) |
| No | 13 (57) | 32 (78) | |
| Co-amoxiclav resistant | |||
| Yes | 7 (35) | 8 (18) | 1.93 (0.81 to 4.58) |
| No | 13 (65) | 36 (72) | |
| Co-trimoxazole resistant | |||
| Yes | 3 (27) | 3 (6) | 4.82 (1.12 to 20.8) |
| No | 8 (73) | 50 (94) | |
| Ciprofloxacin resistant | |||
| Yes | 1 (20) | 0 (0) | – |
| No | 4 (80) | 59 (100) | |
| Gentamicin resistant | |||
| Yes | 0 (0) | 0 (0) | – |
| No | 2 (100) | 62 (100) | |
| Multiple resistance | |||
| Yes | 3 (25) | 2 (4) | 6.50 (1.22 to 34.7) |
| No | 9 (75) | 50 (96) | |
| TEM resistance gene | |||
| Yes | 12 (57) | 13 (33) | 1.71 (0.96 to 3.06) |
| No | 9 (43) | 26 (67) | |
| CTX-M resistance gene | |||
| Yes | 8 (50) | 11 (25) | 2.00 (0.98 to 4.06) |
| No | 8 (50) | 33 (75) | |
In the adjusted analysis that included mode of birth, gestational age, neonatal and maternal peripartum antibiotic exposure, and neonatal intensive care admission, small numbers precluded the convergence of a ‘full’ model (i.e. one adjusted for all five variables) and, therefore, there are no estimates of risk ratios or CIs taking account of all variables. Instead, all adjusted analyses included a single adjustment variable. The results of the analyses are shown in Table 31, in which the analyses in each column are adjusted by only the single adjustment variable indicated in the column heading.
| Colonisation of baby | Adjustment variable, RR (95% CI)a | ||||
|---|---|---|---|---|---|
| Mode of birth | Gestational age | Neonatal antibiotic use | Maternal antibiotic use in peripartum period | Neonatal ICU admission | |
| E. coli detected | 1.29 (0.68 to 2.46) | 1.35 (0.72 to 2.52) | 1.31 (0.70 to 2.46) | 1.32 (0.70 to 2.49) | – |
| Ampicillin resistant | 2.29 (1.13 to 4.64) | 2.13 (1.04 to 4.39) | 1.91 (0.92 to 3.97) | 2.00 (0.95 to 4.23) | – |
| Co-amoxyclav resistant | 2.14 (0.88 to 5.19) | 2.24 (0.97 to 5.19) | 1.95 (0.84 to 4.53) | 1.84 (0.77 to 4.40) | – |
| Co-trimoxazole resistant | 4.44 (0.97 to 20.3) | 5.16 (1.21 to 22.0) | 4.71 (1.04 to 21.4) | 4.51 (1.03 to 19.7) | 4.55 (1.05 to 19.6) |
| Ciprofloxacin resistant | – | – | – | – | – |
| Gentamicin resistant | – | – | – | – | – |
| Multiple resistance | 5.14 (0.99 to 26.6) | 6.70 (1.29 to 34.8) | 9.52 (1.93 to 46.9) | 6.39 (1.17 to 34.8) | 6.13 (1.15 to 32.7) |
| TEM resistance gene | 1.83 (1.01 to 3.33) | 1.92 (1.07 to 3.46) | 1.89 (1.07 to 3.34) | 1.67 (0.91 to 3.06) | 1.87 (1.01 to 3.46) |
| CTX-M resistance gene | 1.87 (0.88 to 3.99) | 1.88 (0.93 to 3.79) | 1.88 (0.94 to 3.74) | 1.92 (0.95 to 3.89) | 1.86 (0.92 to 3.77) |
Chapter 9 Discussion
Main findings
The GBS trial did not find any evidence that use of a rapid POC intrapartum test for maternal GBS colonisation in women whose babies are considered to be at higher risk reduced the rates of intrapartum antibiotics administered to prevent early-onset GBS infection, compared with usual care where IAP is offered to women based on only risk factors. There were no differences between the two strategies in maternal antibiotics use for any indication. However, use of the rapid test reduced the neonatal exposure to antibiotics and fewer babies were administered with antibiotics for suspicion of early-onset infection than in usual care. The rapid intrapartum test, when administered in a real-life scenario, showed good sensitivity and specificity. Overall, 4 in 10 mothers with risk factors, and 1 in 10 of their babies, were colonised with GBS. For the outcomes of cost per case of maternal antibiotics avoided, rapid test was not more cost-effective than usual care.
There is some evidence to suggest an association between multidrug-resistant Gram-negative bacterial colonisation in the mother and similar colonisation in the newborn at 6 weeks of age.
Strengths and weaknesses of the cluster randomised trial
The cluster randomised trial with the embedded test accuracy study successfully implemented the rapid test strategy to enable robust comparison with usual practice. Although two units withdrew soon after randomisation, we ensured that these were replaced with two other units. We recruited more than the required number of clusters to take into account potential dropout of sites after recruitment started, although that was not so in our case and the required sample size was reached. The usual-care units all started and completed their accruals ahead of the rapid test units, although this lack of synchronicity did not appear to influence the outcomes. However, it is possible that eligible women may have been missed in the rapid test arm as, unlike the usual-care arm where the data were collected from recorded notes, lack of trained staff availability, both during the day and night, may have influenced recruitment to the intervention arm.
This primary outcome was a process outcome and so the within-cluster correlation of this outcome, the ICC (i.e. the proportion of the total variance in the IAP rate that can be explained by variation between maternity units), was expected to be higher than it would be for a clinical outcome. We, therefore, considered the sensitivity of our calculations to a range of proportions in the usual-care group and a range of ICC values that we believed to be quite conservative. The ICC was calculated at the end of the trial at 0.06 (95% CI 0.03 to 0.12). This was in the range anticipated at the start of the trial and used in the sample size calculation. Analysis of the primary outcome allowed for clustering at a maternity unit level as a random effect and corrected for the small number of clusters.
Owing to the obvious differences in the two strategies, blinding of the clinical staff and women was not possible. The primary outcome was an objective measure that was recorded in the women’s health-care record, and so adjudication was not required. It is, however, entirely possible that awareness of the maternal GBS colonisation modified the threshold of clinicians for suspecting early-onset infection and prescribing antibiotics to babies. We did not define clinically suspected infection in the protocol. Knowledge of their unit’s allocation could influence the research midwives’ selection of reasons for antibiotic administration from the woman’s health-care records, but under the data protection restrictions of the ethics approval, source data verification by trial staff was not possible.
To compare strategies, a randomised comparison provides the most reliable data. We considered and rejected individual randomisation because of the risk of contamination (i.e. once the GeneXpert system was made available on a maternity unit, it would be difficult for midwifery staff not to offer this testing strategy to all consenting women, given the presumed high accuracy, thereby removing the benefit of randomisation). 74
In cluster trials, if participants cannot be identified in advance, it is important that of all those who are eligible follow their cluster’s allocated strategy, without overt or unintentional selection. The trial showed some evidence of differential ascertainment of participants across rapid test and usual-care units, both with respect to the number of participants and some of the characteristics of the participants.
There were imbalances in the number of women in whom GBS was detected earlier in pregnancy, with more cases detected in the rapid test units. These women might have been more aware of the test procedure and actively requested the intrapartum test. Conversely, there were more women with a diagnosis of a raised maternal temperature in the usual-care units, although closer inspection shows a wide range of rates in the rapid test units (1–36%), excluding the two units that did not reach their targets. This is further evidence of midwives approaching women for the rapid test for selective inclusion in the study. Adjusting the primary comparison for the risk factor rates did not substantially alter the result.
The proportion of women receiving IAP in the usual-care group was considerably lower than expected at 36%, compared with a projected 50–75%, given that all women should be considered for IAP by virtue of maternal risk factors being observed. There were very few missing study data on the outcome of antibiotics prescribed, but there are fewer sources of routinely collected data on IAP rates against which to compare our observation. In the previous UK study51 of rapid test accuracy, only 50% of women with a risk factor received IAP. The RCOG GBS audits found that most maternity units had local policies aligned with the RCOG guidelines, but these audits were undertaken before the 2017 revision of the guidelines. 75 The audits highlighted the occasional misinterpretation of the definition of IAP, antibiotics for other maternal reasons and antibiotics used as prophylaxis against wound infection in caesarean deliveries. It is possible that was some miscoding of the need for IAP in the usual-care arm. Notwithstanding this, two-thirds of women received intrapartum antibiotics of any kind. The survey of units prior to randomisation illustrated the difficultly in collecting IAP administration rate routinely, without resorting to audit of individual health-care records. Instead, a crude estimated rate was obtained from the number of vials of benzylpenicillin and clindamycin dispensed on each delivery suite, although this gave rates of IAP use ranging from 7% to 87%.
Strengths and weaknesses of the test accuracy study
The robust design and execution of our test accuracy study allows us to be confident that the estimates of accuracy are valid and identifies specific aspects of the testing process that maximise the accuracy. We minimised methodological bias by ensuring that the rapid tests and selective enrichment culture were performed independently and interpreted blind to each other. In addition, the double-headed swab used in this study was designed to ensure that the index and reference tests were undertaken on contemporaneous samples. Maternal colonisation was significantly higher than in the previous GBS test accuracy study (at 41% vs. 29% in the presence of risk factors). 51
There was also a good proportion of participants providing rapid test data that were verified by selective enrichment culture at 74%. The missing test data were equally distributed between missing rapid test and missing selective enrichment culture test results, with little overlap, and appeared to be due to systemic problems in conducting the tests, in different hospitals and for limited periods of time, rather than evidence of systematic partial verification based on rapid test results. The number of paired test data, at 534, were smaller than the 606 required by the sample size calculation, which was due in part to the number of failed rapid tests and the need to curtail accrual of data at the end of the study recruitment period. However, this was counterbalanced by the higher than anticipated maternal GBS colonisation rate, and so, although it was anticipated that there would be 167 women with GBS colonisation in a cohort of 606 women, we observed 229 positive selective enrichment cultures. This number of true-positive cases provided 96% post hoc power to determine whether or not the observed accuracy was less than the minimally acceptable threshold of 90% sensitivity; therefore, we can be confident that our observed results can be interpreted as clinically acceptable. The design of the trial had inherent spectrum bias, as only those women presenting with risk factors for early-onset GBS infection were included; however, previous work has shown that post-test probability of maternal colonisation did not vary substantially between women with risk factors present and women with risk factors absent. 51
Strengths and weaknesses of the economic evaluation
To our knowledge, ours is the first economic evaluation to be conducted alongside a RCT comparing a strategy of exploring rapid test with screening based on risk factors alone for higher risk of early-onset GBS infection. The resource use and outcome data were prospectively collected at different points in the trial. Unit costs were obtained from standard and recognised sources. The cost-effectiveness results also benefited from the robustness of the main analyses and sensitivity analyses. One of the key principles of health economic analysis is to maximise the health benefits from, and ensure the most efficient allocation of, scarce resources. It is plausible to incur analyses that suggest that a potentially small decrement in an outcome is acceptable on cost-effectiveness grounds if the potential cost saving is great enough to more than offset the loss in health outcome, and if the saved resources can be used to better effect elsewhere. However, this interpretation does not apply in the current analysis because of uncertainty in a number of areas. The principal issue in the current analysis is that the rapid test has not managed to achieve the hypothesised reduction in the use of prescribed antibiotics to reduce GBS transmission. Another limitation relates to the intermediate outcome on which the economic analysis is based. The outcome of ‘cost per additional antibiotic prescription avoided’ is severely limiting. It is an intermediate outcome, as it provides no indication of the pathways that would be followed for the infant based on any decision to prescribe antibiotics to the mother for preventing GBS. The key issue is the pathway and wellness of the baby in this study, for which there are no data. Such an intermediate outcome is credible only when the hypothesis is upheld by the results. What we do not know with any certainty is whether or not the antibiotics prescribed in the rapid test units helped to avoid cases of GBS that might otherwise have occurred. There was very limited swabbing of neonates to establish whether or not they incurred colonisation with GBS. This was recorded for only 40% of infants in the rapid tests units and, therefore, the results are potentially biased by the large number of missing data. Furthermore, these data were available only for the rapid test units as no swabbing of infants for colonisation was undertaken in the usual-care units.
It has been neither possible nor appropriate to conduct a model-based economic evaluation in this study. When analyses are based on an intermediate outcome, it can be appropriate for the health economic analysis to model beyond the outcome recorded in the trial to ascertain the likely impact on longer-term health outcomes. However, it is inappropriate and infeasible to attempt to model beyond the intermediate outcome in this case, as any assumptions required cannot be supported by any data. If it is assumed that women appropriately received more antibiotics based on the results of the rapid test, compared with usual care, then the assumptions in the model would be in the direction of preventing more cases of GBS colonisation. In the absence of data on GBS colonisation in the usual-care units, such an assumption is not supportable. Furthermore, such an assumption directly opposes the hypothesis that was predetermined in the trial design (i.e. that the rapid test will reduce the number of women who are prescribed antibiotics). Therefore, modelling beyond the intermediate outcome collected in the trial is not appropriate in these circumstances and would produce misleading results.
Public and parent involvement
We have been supported throughout the project by the charity Group B Strep Support (GBSS) (Haywards Heath, UK) and, in particular, its chief executive. Public and parent involvement was crucial in improving the acceptability of the GBS2 trial and promoting engagement of maternity units. We engaged with GBSS chief executive throughout, improving our understanding of the opinions and uncertainty surrounding testing for GBS among pregnant women, doctors, microbiologists and midwives, and the barriers to accessing testing that women encounter. This prompted us to provide a study-specific testing information leaflet to all eligible women. Members of the GBSS charity and a small group of parents on the GBSS mailing list commented on public-facing materials and posters to ensure that they were clear and comprehensive. The GBSS helpline took many telephone calls from women booked to give birth in maternity units allocated the rapid test strategy, supporting their decisions whether or not to consent to the swab and the implications of the test.
The substudy public-facing materials and approach for consent pathway were discussed and reviewed by Katie’s Team (London, UK), an East London-based patient and public involvement group that includes members of the public with lived experiences of pregnancy.
We will engage with GBSS regarding the dissemination of our findings, providing a plain English summary of the findings and the uncertainties around the evidence we have discussed here. This will be distributed via GBSS’s website, e-newsletter and social media channels. Any future research groups taking forward the research recommendations from this project would benefit from engaging with GBSS.
Interpretation of findings
Use of the rapid test did not appear to lower the use of IAP administered to the mother to prevent vertical transmission of GBS to the offspring. This could be for the following reasons. First, the rate of IAP for GBS in the usual-care arm, in which every participant should have been offered the IAP, was less than what was expected (at < 40%) and this may have influenced the findings. However, our work reflects current clinical practice and we would expect similar findings if rapid test was implemented. Second, when the outcome of maternal antibiotics for any indication was considered, although the overall rates of antibiotic use increased, it still does not show a reduction with rapid test. Third, clinicians still decided to administer IAP to prevent GBS in 17% of test-negative women, indicating reluctance in some instances to follow the test result, which may have affected the primary outcome. Conversely, 22% of test-positive women did not receive IAP. Furthermore, the test results were available in only four-fifths of all women and IAP was administered to fewer than half of these women.
However, we found a significant reduction in neonatal exposure to antibiotics in the rapid test units. Antibiotics are often started prophylactically in newborns when the clinicians suspected a high risk of neonatal sepsis based on symptoms in the newborn, maternal history and any treatment given to the mother. In our trial, fewer babies in the rapid test arm than in the usual care were suspected to have sepsis, a major indication for starting antibiotics. The high rates of women administered antibiotics 4 hours prior to birth in the rapid test (compared with the usual-care arm) and because neonatologists were less likely to commence antibiotics in rapid test-negative women could have influenced these findings.
The accuracy of the GeneXpert rapid test was marginally better than that of the previous generation of GBS test from Cepheid, the Smart GBS test, which had a reported sensitivity of 84% (95% CI 79% to 84%) and specificity of 87% (95% CI 85% to 89%). The first-generation test was performed in batches by a laboratory assistant, whereas in the GBS2 trial the test was performed in real time by clinical midwives, as a true POC test. Removing data from 244 women who had used an antimicrobial lubricant or cleanser prior to the swab being taken marginally increased the accuracy of the rapid test, compared with the complete population. Conversely, considering only those who were exposed to an antimicrobial solution gave a reduced sensitivity of the rapid test [61 rapid test positive/75 microbiological culture positive, 81% (95% CI 71% to 89%)]. This confirms that the accuracy of the rapid test can be influenced by the environment from which the swabs are taken, and that lubricant or cleanser containing antimicrobial substances, such as chlorhexidine, should be avoided. The NICE guidelines recommend using just water for hygienic cleaning,76 as there is no strong evidence for efficacy of antimicrobial solutions in reducing neonatal infection. 30
The results of the economic evaluation show dominance: the strategy of adding the rapid test to usual care is dominated by usual care alone in achieving the predetermined outcome. As dominance exists, an ICER is not appropriate. The results of the stochastic CEA and the presentation in the cost-effectiveness planes support the interpretation that the strategy of adding the rapid test to usual care is less effective than usual care alone in achieving the objective of reducing antibiotic use. Almost all of the scatterplot dots are to the west (less-effective quadrant) of the vertical axis (see Figures 9, 12 and 14); this is because more women in the rapid test arm are prescribed antibiotics than women in the usual-care arm. Whether this is ultimately more or less costly in the short term is more uncertain, as the scatterplot spanned the north- and south-west quadrants of the cost-effectiveness plane in the base-case analysis.
The sensitivity analyses broadly supports these base-case results, although there are some slight impacts on costs. In sensitivity analysis 1, where the length of stay for mothers is based on trial data, the impact is to increase the overall costs of the rapid test strategy compared with usual care. In sensitivity analysis 2, where the inpatient days for the baby, by level of care, are added to the calculation of costs, the impact is to reduce the overall cost of the rapid test strategy compared with usual care. This latter sensitivity analysis result may imply that some benefit was inferred onto the infants from the mothers in the rapid test units who received more antibiotics than those in the usual-care units. This may have led to fewer inpatient days for infants in the rapid test units and a reduction in overall costs. However, such an implication must be interpreted with caution.
Interpretation of the substudy
Seale and Millar77 reviewed the literature on perinatal transmission of antibiotic resistance from mothers to infants and found a paucity of relevant literature. A more recent systematic review78 reports evidence of carriage of indistinguishable antibiotic-resistant Gram-negative bacteria by mothers and infants, and also concludes that there is a paucity of relevant research and that ‘none of the included studies reported on risk factors for multidrug resistant Gram-negative bacteria transmission from colonised mothers to infants’. There remains little information on the contribution that maternal carriage of resistant bacteria or associated genes makes to carriage by their infants. Recent reports have suggested a weak (if any) association between mode of birth, maternal and infant antibiotic use or feeding practices on the carriage of antibiotic-resistant Gram-negative bacteria by infants. 79,80 Despite collecting data on parental carriage of antibiotic resistance, Hetzer et al. 81 did not include information in the analysis of risk factors for acquisition of antibiotic-resistant intestinal E. coli during the first year of life.
The identification of risk factors for perinatal transmission has the potential to allow the development of preventative strategies. A study published in 2010 reported that, among 114,999 women who gave birth between 1992 and 2007, approximately one-third had received an antibiotic during pregnancy. 82 The extent to which antibiotic prescribing in pregnancy is inappropriate is unknown. Antibiotic stewardship is a potential remediable route to reducing the selection and transmission of antibiotic-resistant bacteria during this critical period of life; however, the extent to which antibiotic usage in pregnancy can be constrained without prejudicing maternal or neonatal outcomes must be determined.
Implications for practice and future research
The GBS2 trial was unable to demonstrate if rapid test-based IAP in women with risk factors for early-onset GBS infection in newborn babies reduces the antibiotic usage for GBS prophylaxis in labour. CIs were wide and included both increases and decreases in the use of antibiotics, which might be clinically important (from a 7% decrease to an 18% increase). The GBS2 trial also highlights the low adherence to the current UK guidelines for GBS prophylaxis and also the widespread use of antibiotics in labour for other maternal indications.
There is work to be carried out to improve the current rate of IAP provision among women with risk factors for early-onset GBS infection. The rapid test did appear to reduce neonatal antibiotic administration, suggesting that neonatologists are willing to take the results of the test into account when considering whether or not to start antibiotic treatment. Intrapartum GBS colonisation status is a variable in the neonatal sepsis risk calculator83,84 that is gaining traction in the UK and has been shown to substantially reduce neonatal antibiotic use. 85 The accuracy of the rapid test shows that it has potential to inform both maternal and neonatal antibiotic use.
Use of the rapid test is feasible in clinical practice, with turnaround times compatible with clinical care. The requirements for installation of the rapid test and training of all clinical midwives to allow a unit to provide round-the-clock testing should not be underestimated. Previous research has suggested that midwives want to take ownership of the testing process51 and, anecdotally, once trained, midwives could incorporate the rapid test into their practice. Clinicians will need to be reassured regarding the performance of test, if IAP administration is to be based on rapid test results.
The rapid test is accurate in the diagnosis of maternal GBS colonisation, but there needs to be improvements in reducing the numbers of failed tests. Clinicians should be advised to administer IAP in the event of the failed test. Routine use of the rapid test is not a cost-effective strategy for avoiding maternal antibiotic administration. Any future studies should endeavour to collect data on neonatal clinical outcomes, such as babies colonised in all trial groups and complications of infection to enable economic modelling.
The GBS2 trial is limited to women with risk factors for early-onset GBS infection in their baby. Risk factors are associated only with about one-third of UK early-onset GBS infection. As the GBS2 trial demonstrates, the proportion of women approached for intrapartum testing was lower than previously reported risk factors rates, suggesting imperfect implementation of the testing strategy. The National Institute for Health Research has commissioned a further trial called GBS3 (HTA 17/86/06). This is a cluster randomised trial in 80 maternity units, comparing routine testing of all women intending vaginal birth with the same usual-care strategy. The routine testing units will be further randomised between the same intrapartum rapid test system and antenatal testing by selective enrichment culture. The primary outcome will be all-cause early-onset all-cause neonatal sepsis, both culture proven and clinically suspected, whereas the main outcome for the subrandomisation will be the proportion of women for whom a test result is available in time for adequate IAP to be administered. Approximately 320,000 women and their babies will be included in the study, which will use data from routinely collected sources. A full economic evaluation of the three strategies will be conducted with a long-term perspective. Interviews with parents and all cadres of maternity unit staff will describe qualitatively the barriers to implementation of the testing strategies.
Acknowledgements
Other members of the GBS2 Trial Collaborative Group
Clinical collaborators
-
Dr Shad Hussain (Consultant Neonatologist).
-
Professor Khaled Ismail (Consultant in Obstetrics and Gynaecology).
-
Ms Sal Higgins (Lead Research Midwife).
-
Dr Stephen Kempley (Consultant Neonatologist).
Trial delivery
-
Mr Kostas Tryposkiadis (Trial Statistician).
-
Ms Alicia Jakeman (Trial Co-ordinator).
-
Ms Gemma Slinn (Trials Management Team leader).
-
Mr Adrian Wilcockson (Database Developer).
Trial Steering Committee
-
Professor Paul Heath (chairperson).
-
Professor Kerry Hood.
-
Professor Stavros Petrou.
-
Professor Ben Stenson.
-
Dr Sarah McMullen.
-
Professor Julia Sanders.
-
Ms Alison Stanley.
Data Monitoring Committee
-
Professor Stephen Walters (chairperson).
-
Professor Patrick Bossuyt.
-
Professor Ruth Gilbert.
-
Dr Rhona Hughes.
Principal investigators at participating maternity units
See Appendix 1, Table 32.
Contributions of authors
Professor Jane Daniels (https://orcid.org/0000-0003-3324-6771) (Professor of Clinical Trials and Non-Clinical Lead Investigator) contributed to the design, delivery, analysis and interpretation of all components of the GBS2 trial, the first draft and overall editing of the final report.
Ms Emily F Dixon (https://orcid.org/0000-0003-3660-5354) (Trial Co-ordinator) was responsible for the day-to-day management and delivery of the cluster trial and test accuracy study, contributed to drafting the report and edited the final report.
Ms Alicia Gill (https://orcid.org/0000-0001-5019-6054) (Trial Statistician) performed the analyses for the cluster trial, accuracy study and microbiology study.
Dr Jon Bishop (https://orcid.org/0000-0003-1789-5886) (Senior Statistician) contributed to the analysis, the interpretation of the cluster trial, accuracy study and microbiology study, and the drafting of the report.
Ms Maria D’Amico (https://orcid.org/0000-0002-1457-6399) (Research Assistant) contributed to the design and delivery of all components of the study.
Mr Khaled Ahmed (https://orcid.org/0000-0001-5699-5834) (Senior Trial Co-ordinator) was responsible for the day-to-day management and delivery of the cluster trial and test accuracy study.
Dr Julie Dodds (https://orcid.org/0000-0002-6041-1456) (Senior Research Manager) contributed to the design and delivery of all components of the study.
Mr Kostas Tryposkiadis (https://orcid.org/0000-0002-2516-1180) (Trial Statistician) contributed to the analysis of the cluster trial and performed the randomisation.
Dr Mark Wilks (https://orcid.org/0000-0002-2170-1944) (Consultant Microbiologist) contributed to the design, analysis, the interpretation of the accuracy study and microbiology study.
Dr Michael Millar (https://orcid.org/0000-0002-5675-9679) (Consultant Microbiologist) contributed to the design, analysis, the interpretation of the accuracy study and microbiology study, and the drafting of the report.
Dr Shahid Husain (https://orcid.org/0000-0001-8910-9472) (Consultant Neonatologist) contributed to the design and interpretation of the cluster trial.
Dr Jim Gray (https://orcid.org/0000-0003-4169-3141) (Consultant Microbiologist) contributed to the design, analysis and the interpretation of the cluster trial accuracy study.
Ms Angela Whiley (https://orcid.org/0000-0001-8672-463X) (Research Scientist) contributed to the design and delivery of the microbiology study.
Dr Patrick V Moore (https://orcid.org/0000-0002-5205-8832) (Lecturer in Health Economics) contributed to the design and interpretation of the economic evaluation, performed the analysis and drafted the economic study chapter.
Ms Ruvimbo L Munetsi (https://orcid.org/0000-0002-3472-8548) (Research Associate) contributed to the design and interpretation of the economic evaluation, and performed the analysis.
Professor Karla Hemming (https://orcid.org/0000-0002-2226-6550) (Professor of Biostatistics) contributed to the design, analysis and interpretation of the cluster trial.
Professor Tracy Roberts (https://orcid.org/0000-0002-0624-0537) (Professor of Health Economics) contributed to the design, analysis and interpretation of the economic evaluation.
Mrs Jane Plumb (https://orcid.org/0000-0002-0738-3695) (Chief Executive of Group B Strep Support) contributed to the design and interpretation of the cluster trial and accuracy study, and led on parent and public involvement contributions.
Professor Jonathan Deeks (https://orcid.org/0000-0002-8850-1971) (Professor of Medical Statistics) contributed to the design, analysis and interpretation of the accuracy study.
Professor Khalid S Khan (https://orcid.org/0000-0001-5084-7312) (Professor of Women’s Health and Clinical Epidemiology and Initial Chief Clinical Investigator) contributed to the design, delivery and interpretation of the cluster trial and accuracy study.
Professor Shakila Thangaratinam (https://orcid.org/0000-0002-4254-460X) (Professor of Maternal and Perinatal Health and Chief Investigator) contributed to the design, delivery, analysis and interpretation of all components of the GBS2 trial, the first draft and overall editing of the final report.
Publication
Daniels JP, Dixon E, Gill A, Bishop J, Wilks M, Millar M, et al. Rapid intrapartum test for maternal group B streptococcal colonisation and its effect on antibiotic use in labouring women with risk factors for early-onset neonatal infection (GBS2): cluster randomised trial with nested test accuracy study. BMC Med 2022;20:9
Data-sharing statement
We shall make data available to the scientific community with as few restrictions as feasible. All data requests should be submitted to the corresponding author for consideration.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Daniels JP, Dixon E, Gill A, Bishop J, Wilks M, Millar M, et al. Rapid intrapartum test for maternal group B streptococcal colonisation and its effect on antibiotic use in labouring women with risk factors for early-onset neonatal infection (GBS2): cluster randomised trial with nested test accuracy study. BMC Med 2022;20. https://doi.org/10.1186/s12916-021-02202-2.
- Shabayek S, Spellerberg B. Group B streptococcal colonization, molecular characteristics, and epidemiology. Front Microbiol 2018;9. https://doi.org/10.3389/fmicb.2018.00437.
- National Institute for Health and Care Excellence . Antibiotics for the Prevention and Treatment of Early-Onset Neonatal Infection 2012.
- Royal College of Obstetricians and Gynaecologists . Prevention of Early-Onset Neonatal Group B Streptococcal Disease 2017.
- Royal College of Obstetricians and Gynaecologists . Group B Streptococcal Disease, Early-Onset 2017.
- Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, et al. Maternal colonization with group B streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S100-S111. https://doi.org/10.1093/cid/cix658.
- Gopal Rao G, Hiles S, Bassett P, Lamagni T. Differential rates of group B streptococcus (GBS) colonisation in pregnant women in a racially diverse area of London, UK: a cross-sectional study. BJOG 2019;126:1347-53. https://doi.org/10.1111/1471-0528.15648.
- Colbourn T, Gilbert R. An overview of the natural history of early onset group B streptococcal disease in the UK. Early Hum Dev 2007;83:149-56. https://doi.org/10.1016/j.earlhumdev.2007.01.004.
- Katz V, Bowes WA. Perinatal group B streptococcal infections across intact amniotic membranes. J Reprod Med 1988;33:445-9.
- Baker CJ, Barrett FF. Transmission of group B streptococci among parturient women and their neonates. J Pediatr 1973;83:919-25. https://doi.org/10.1016/S0022-3476(73)80524-4.
- Lin FY, Weisman LE, Troendle J, Adams K. Prematurity is the major risk factor for late-onset group B streptococcus disease. J Infect Dis 2003;188:267-71. https://doi.org/10.1086/376457.
- Bianchi-Jassir F, Seale AC, Kohli-Lynch M, Lawn JE, Baker CJ, Bartlett L, et al. Preterm birth associated with group B streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S133-S142. https://doi.org/10.1093/cid/cix661.
- Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S160-S172. https://doi.org/10.1093/cid/cix656.
- O’Sullivan CP, Lamagni T, Patel D, Efstratiou A, Cunney R, Meehan M, et al. Group B streptococcal disease in UK and Irish infants younger than 90 days, 2014–15: a prospective surveillance study. Lancet Infect Dis 2019;19:83-90. https://doi.org/10.1016/S1473-3099(18)30555-3.
- Schrag SJ, Farley MM, Petit S, Reingold A, Weston EJ, Pondo T, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics 2016;138. https://doi.org/10.1542/peds.2016-2013.
- Oddie S, Embleton ND. Risk factors for early onset neonatal group B streptococcal sepsis: case-control study. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7359.308.
- Adriaanse AH, Lagendijk I, Muytjens HL, Nijhuis JG, Kollée LA. Neonatal early onset group B streptococcal infection. A nine-year retrospective study in a tertiary care hospital. J Perinat Med 1996;24:531-8. https://doi.org/10.1515/jpme.1996.24.5.531.
- Luck S, Torny M, d’Agapeyeff K, Pitt A, Heath P, Breathnach A, et al. Estimated early-onset group B streptococcal neonatal disease. Lancet 2003;361:1953-4. https://doi.org/10.1016/S0140-6736(03)13553-2.
- Royal College of Obstetricians and Gynaecologists . The Prevention of Early-Onset Group B Streptococcal Disease 2012.
- Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al. Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11290.
- Anderson BL, Simhan HN, Simons KM, Wiesenfeld HC. Untreated asymptomatic group B streptococcal bacteriuria early in pregnancy and chorioamnionitis at delivery. Am J Obstet Gynecol 2007;196:524-5. https://doi.org/10.1016/j.ajog.2007.01.006.
- Wood EG, Dillon HC. A prospective study of group B streptococcal bacteriuria in pregnancy. Am J Obstet Gynecol 1981;140:515-20. https://doi.org/10.1016/0002-9378(81)90226-X.
- Bevan D, White A, Marshall J, Peckham C. Modelling the effect of the introduction of antenatal screening for group B Streptococcus (GBS) carriage in the UK. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-024324.
- Bazian Ltd . Screening for Group B Streptococcal Infection in Pregnancy. External Review Against Programme Appraisal Criteria for the UK National Screening Committee (UK NSC 2012.
- Gray JW, Milner PJ, Edwards EH, Daniels JP, Khan KS. Feasibility of using microbiology diagnostic tests of moderate or high complexity at the point-of-care in a delivery suite. J Obstet Gynaecol 2012;32:458-60. https://doi.org/10.3109/01443615.2012.673034.
- Verani JR, McGee L, Schrag SJ. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease – revised guidelines from CDC, 2010. MMWR Recomm Rep 2010;59:1-36.
- Feuerschuette OHM, Silveira SK, Cancelier ACL, da Silva RM, Trevisol DJ, Pereira JR. Diagnostic yield of real-time polymerase chain reaction in the diagnosis of intrapartum maternal rectovaginal colonization by group B streptococcus: a systematic review with meta-analysis. Diagn Microbiol Infect Dis 2018;91:99-104. https://doi.org/10.1016/j.diagmicrobio.2018.01.013.
- UK National Screening Committee . The UK NSC Recommendation on Group B Streptococcus Screening in Pregnancy 2017.
- Royal College of Obstetricians and Gynaecologists . The Prevention of Early-Onset Group B Streptococcal Disease 2003.
- Ohlsson A, Shah VS, Stade BC. Vaginal chlorhexidine during labour to prevent early-onset neonatal group B streptococcal infection. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD003520.pub3.
- El Helali N, Nguyen JC, Ly A, Giovangrandi Y, Trinquart L. Diagnostic accuracy of a rapid real-time polymerase chain reaction assay for universal intrapartum group B streptococcus screening. Clin Infect Dis 2009;49:417-23. https://doi.org/10.1086/600303.
- Kaambwa B, Bryan S, Gray J, Milner P, Daniels J, Khan KS, et al. Cost-effectiveness of rapid tests and other existing strategies for screening and management of early-onset group B streptococcus during labour. BJOG 2010;117:1616-27. https://doi.org/10.1111/j.1471-0528.2010.02752.x.
- World Health Organization (WHO) . The Evolving Threat of Antimicrobial Resistance – Options for Action 2012.
- Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States 2019.
- Department of Health and Social Care (DHSC) . Infections and the Rise of Antimicrobial Resistance 2013.
- Medernach RL, Logan LK. The growing threat of antibiotic resistance in children. Infect Dis Clin North Am 2018;32:1-17. https://doi.org/10.1016/j.idc.2017.11.001.
- Cantey JB, Sreeramoju P, Jaleel M, Treviño S, Gander R, Hynan LS, et al. Prompt control of an outbreak caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit. J Pediatr 2013;163:672-9. https://doi.org/10.1016/j.jpeds.2013.03.001.
- Oteo J, Cercenado E, Vindel A, Bautista V, Fernandez-Romero S, Saez D, et al. Outbreak of multidrug-resistant CTX-M-15-producing Enterobacter cloacae in a neonatal intensive care unit. J Med Microbiol 2013;62:571-5. https://doi.org/10.1099/jmm.0.053017-0.
- Gibbs RS, Schrag S, Schuchat A. Perinatal infections due to group B streptococci. Obstet 2004;104:1062-76. https://doi.org/10.1097/01.AOG.0000144128.03913.c2.
- Friedman S, Shah V, Ohlsson A, Matlow AG. Neonatal Escherichia coli infections: concerns regarding resistance to current therapy. Acta Paediatr 2000;89:686-9. https://doi.org/10.1080/080352500750044007.
- Parm U, Metsvaht T, Sepp E, Ilmoja ML, Pisarev H, Pauskar M, et al. Risk factors associated with gut and nasopharyngeal colonization by common Gram-negative species and yeasts in neonatal intensive care units patients. Early Hum Dev 2011;87:391-9. https://doi.org/10.1016/j.earlhumdev.2011.02.007.
- Dave J, Millar M, Haigh j, Mead R, McEwan R, Phee LM, et al. Enhanced Surveillance for Multi-Drug Resistant Bacteria During the Olympic Period n.d.
- Millar MR, Seale J, Turton J, Wilks M, Costeloe K, Woodford N, et al. ESBL-producing Enterobacteriaceae in 24 neonatal units and associated networks in the south of England: no clustering of ESBL-producing Escherichia coli in units or networks. J Antimicrob Chemother 2016;71:1174-7. https://doi.org/10.1093/jac/dkv459.
- Kasahara K, Baltus AJ, Lee SH, Edelstein MA, Edelstein PH. Prevalence of non-penicillin-susceptible group B streptococcus in Philadelphia and specificity of penicillin resistance screening methods. J Clin Microbiol 2010;48:1468-9. https://doi.org/10.1128/JCM.02296-09.
- Capanna F, Emonet SP, Cherkaoui A, Irion O, Schrenzel J, Martinez de Tejada B. Antibiotic resistance patterns among group B streptococcus isolates: implications for antibiotic prophylaxis for early-onset neonatal sepsis. Swiss Med Wkly 2013;143. https://doi.org/10.4414/smw.2013.13778.
- Teatero S, Ferrieri P, Martin I, Demczuk W, McGeer A, Fittipaldi N. Serotype distribution, population structure, and antimicrobial resistance of group B streptococcus strains recovered from colonized pregnant women. J Clin Microbiol 2017;55:412-22. https://doi.org/10.1128/jcm.01615-16.
- Yan Y, Hu H, Lu T, Fan H, Hu Y, Li G, et al. Investigation of serotype distribution and resistance genes profile in group B streptococcus isolated from pregnant women: a Chinese multicenter cohort study. APMIS 2016;124:794-9. https://doi.org/10.1111/apm.12570.
- Seki T, Kimura K, Reid ME, Miyazaki A, Banno H, Jin W, et al. High isolation rate of MDR group B streptococci with reduced penicillin susceptibility in Japan. J Antimicrob Chemother 2015;70:2725-8. https://doi.org/10.1093/jac/dkv203.
- Dinsmoor MJ, Viloria R, Lief L, Elder S. Use of intrapartum antibiotics and the incidence of postnatal maternal and neonatal yeast infections. Obstet Gynecol 2005;106:19-22. https://doi.org/10.1097/01.AOG.0000164049.12159.bd.
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 2008;372:1319-27. https://doi.org/10.1016/S0140-6736(08)61203-9.
- Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al. Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13420.
- Eldridge S, Kerry S, Torgerson DJ. Bias in identifying and recruiting participants in cluster randomised trials: what can be done?. BMJ 2009;339. https://doi.org/10.1136/bmj.b4006.
- Caille A, Kerry S, Tavernier E, Leyrat C, Eldridge S, Giraudeau B. Timeline cluster: a graphical tool to identify risk of bias in cluster randomised trials. BMJ 2016;354. https://doi.org/10.1136/bmj.i4291.
- National Institute for Health and Care Excellence . Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth 2017.
- Cepheid . GeneXpert Dx System Operator Manual (Software Version 4) 2012.
- Public Health England . UK Standards for Microbiology Investigations – Detection of Carriage of Group B Streptococci (Streptococcus agalactiae). 2015.
- National Institute for Health and Care Excellence . Neonatal Infection (Early Onset): Antibiotics for Prevention and Treatment 2012.
- McCall SJ, Bunch KJ, Brocklehurst P, D’Arcy R, Hinshaw K, Kurinczuk JJ, et al. The incidence, characteristics, management and outcomes of anaphylaxis in pregnancy: a population-based descriptive study. BJOG 2018;125:965-71. https://doi.org/10.1111/1471-0528.15041.
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997;53:983-97. https://doi.org/10.2307/2533558.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404-13. https://doi.org/10.1093/biomet/26.4.404.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2019.
- Department of Health and Social Care (DHSC) . National Schedule of Reference Costs (2017–2018) 2019.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury; 2018.
- Petrou S, Cooper P, Murray L, Davidson LL. Economic costs of post-natal depression in a high-risk British cohort. Br J Psychiatry 2002;181:505-12. https://doi.org/10.1192/bjp.181.6.505.
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. PROMISE: first-trimester progesterone therapy in women with a history of unexplained recurrent miscarriages - a randomised, double-blind, placebo-controlled, international multicentre trial and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20410.
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670-4. https://doi.org/10.1136/bmj.319.7211.670.
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. https://doi.org/10.1192/bjp.187.2.106.
- Lamont T, Cousins D, Bischler A, Gerrett DJB. Safer loading doses of medicines: summary of a safety report from the National Patient Safety Agency. BMJ 2011;342. https://doi.org/10.1136/bmj.d33.
- Matuschek E, Brown DF, Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect 2014;20:O255-66. https://doi.org/10.1111/1469-0691.12373.
- Modi N, Doré CJ, Saraswatula A, Richards M, Bamford KB, Coello R, et al. A case definition for national and international neonatal bloodstream infection surveillance. Arch Dis Child Fetal Neonatal Ed 2009;94:F8-12. https://doi.org/10.1136/adc.2007.126458.
- Royal College of Obstetricians and Gynaecologists . Audit of Current Practice in Preventing Early-Onset Neonatal Group B Streptococcal Disease in the UK 2016.
- National Institute for Health and Care Excellence . Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth 2014.
- Seale J, Millar M. Perinatal vertical transmission of antibiotic-resistant bacteria: a systematic review and proposed research strategy. BJOG 2014;121:923-8. https://doi.org/10.1111/1471-0528.12746.
- Bulabula ANH, Dramowski A, Mehtar S. Transmission of multidrug-resistant Gram-negative bacteria from colonized mothers to their infants: a systematic review and meta-analysis. J Hosp 2019. https://doi.org/10.1016/j.jhin.2019.10.001.
- Islam MA, Amin MB, Roy S, Asaduzzaman M, Islam MR, Navab-Daneshmand T, et al. Fecal colonization with multidrug-resistant E. coli among healthy infants in rural Bangladesh. Front Microbiol 2019;10. https://doi.org/10.3389/fmicb.2019.00640.
- Zhang L, Kinkelaar D, Huang Y, Li Y, Li X, Wang HH. Acquired antibiotic resistance: are we born with it?. Appl Environ Microbiol 2011;77:7134-41. https://doi.org/10.1128/aem.05087-11.
- Hetzer B, Orth-Höller D, Würzner R, Kreidl P, Lackner M, Müller T, et al. Enhanced acquisition of antibiotic-resistant intestinal E. coli during the first year of life assessed in a prospective cohort study. Antimicrob Resist Infect Control 2019;8. https://doi.org/10.1186/s13756-019-0522-6.
- Petersen I, Gilbert R, Evans S, Ridolfi A, Nazareth I. Oral antibiotic prescribing during pregnancy in primary care: UK population-based study. J Antimicrob Chemother 2010;65:2238-46. https://doi.org/10.1093/jac/dkq307.
- Puopolo KM, Benitz WE, Zaoutis TE. Committee on Fetus and Newborn. Management of neonates born at ≥ 35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 2018;142. https://doi.org/10.1542/peds.2018-2894.
- Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics 2011;128:e1155-63. https://doi.org/10.1542/peds.2010-3464.
- Goel N, Shrestha S, Smith R, Mehta A, Ketty M, Muxworthy H, et al. Screening for early onset neonatal sepsis: NICE guidance-based practice versus projected application of the Kaiser Permanente sepsis risk calculator in the UK population. Arch Dis Child Fetal Neonatal Ed 2020;105:118-22. https://doi.org/10.1136/archdischild-2018-316777.
Appendix 1 Site details
| Site | Trust | Region | Principal investigator |
|---|---|---|---|
| Rapid test sites | |||
| Birmingham Heartlands Hospital | Heart of England NHS Foundation Trust | MID | Pallavi Karkhanis |
| Frimley Park Hospitala | Frimley Health NHS Foundation Trust | LSE | Anne Deans |
| Hinchingbrooke Hospital | North West Anglia NHS Foundation Trust | MID | NA |
| Newham General Hospitala | Barts Health NHS Trust | LSE | Sanjula Sharma |
| Queen’s Medical Centre | Nottingham University Hospitals NHS Trust | MID | Gemma Wright |
| Russell’s Hall Hospital | The Dudley Group NHS Foundation Trust | MID | Manjula Subramanian |
| St Richard’s Hospitala | Western Sussex Hospital NHS Trust | LSE | Irene Ray |
| Tunbridge Wells Hospitala | Maidstone and Tunbridge Wells NHS Trust | LSE | Dibyenda Datta |
| University Hospital Coventry | University Hospitals Coventry and Warwickshire NHS Trust | MID | Lauren Lacey |
| Walsall Manor Hospital | Walsall Healthcare NHS Trust | MID | Johnathon Pepper |
| Worthing Hospitala | Western Sussex Hospitals NHS Foundation Trust | LSE | Ruth Mason |
| Usual-care sites | |||
| Birmingham City Hospital | Sandwell and West Birmingham Hospitals NHS Trust | MID | Neil Shah |
| Burton Hospital | Burton Hospitals NHS Foundation Trust | MID | Katharina Anwar |
| George Eliot Hospital | George Eliot Hospital NHS Trust | MID | Neena Navaneetham |
| Homerton University Hospital | Homerton University Hospital NHS Foundation Trust | LSE | Shad Husain |
| Queen Charlotte’s and Chelsea Hospital | Imperial College Healthcare NHS Trust | LSE | Phillip Bennett |
| Royal Stoke University Hospital | University Hospitals of North Midlands NHS Trust | MID | Geraldine Masson |
| Royal Sussex County Hospital | Brighton and Sussex University Hospitals NHS Trust | LSE | NA |
| St Peter’s Hospital | Ashford and St Peter’s Hospitals NHS Foundation Trust | LSE | Hristina Raykova |
| The Royal London Hospital | Barts Health NHS Trust | LSE | Matt Hogg |
| Whipps Cross University Hospital | Barts Health NHS Trust | LSE | Bashir Dawalatly |
| Worcestershire Royal Hospital | Worcestershire Acute Hospitals NHS Trust | MID | Lakshmi Thirumalaikumar Kate Townsend |
Appendix 2 Recruitment
FIGURE 15.
Cumulative recruitment across both maternity unit groups for the GBS2 trial.
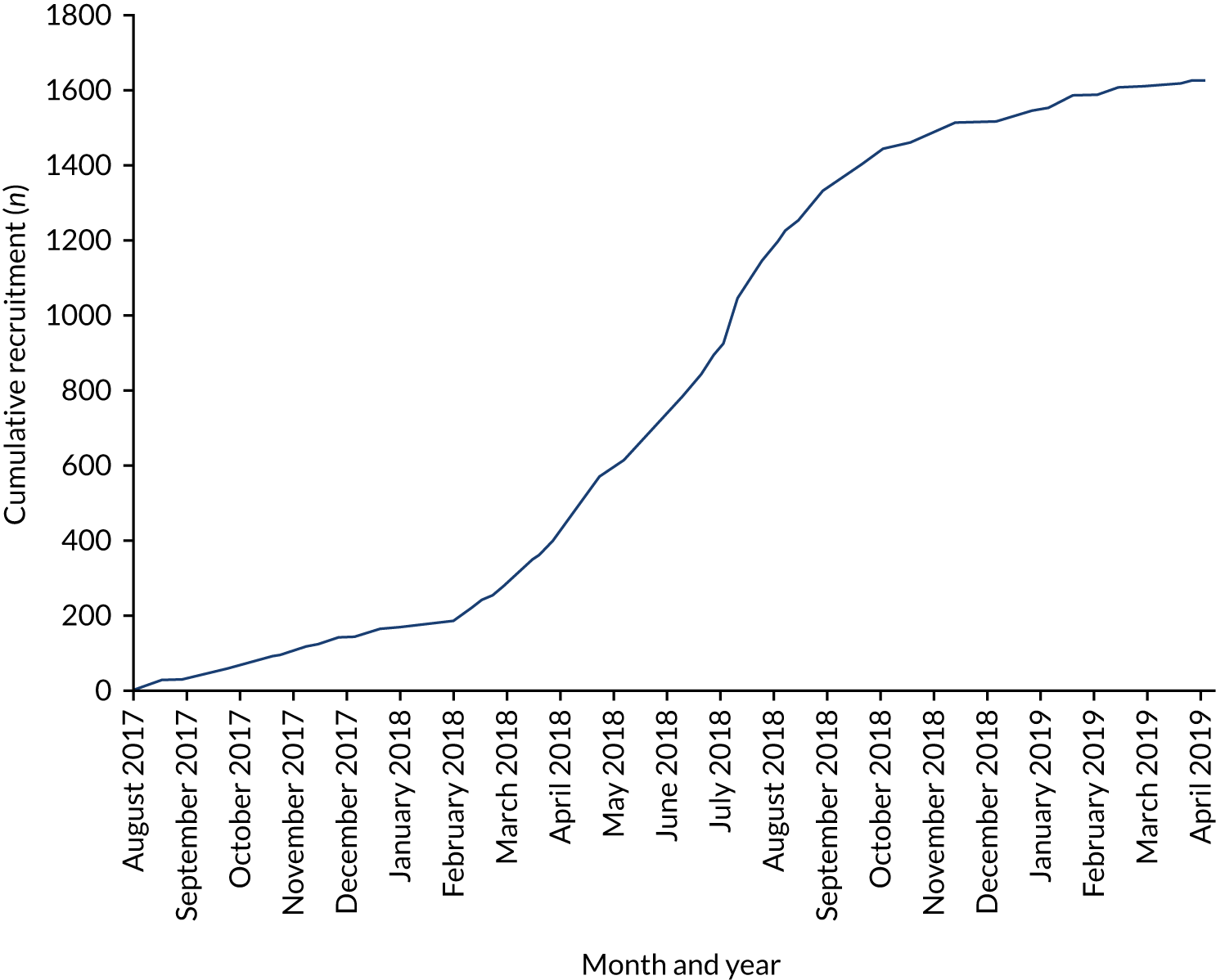
FIGURE 16.
Cumulative recruitment by arm for the GBS2 trial.
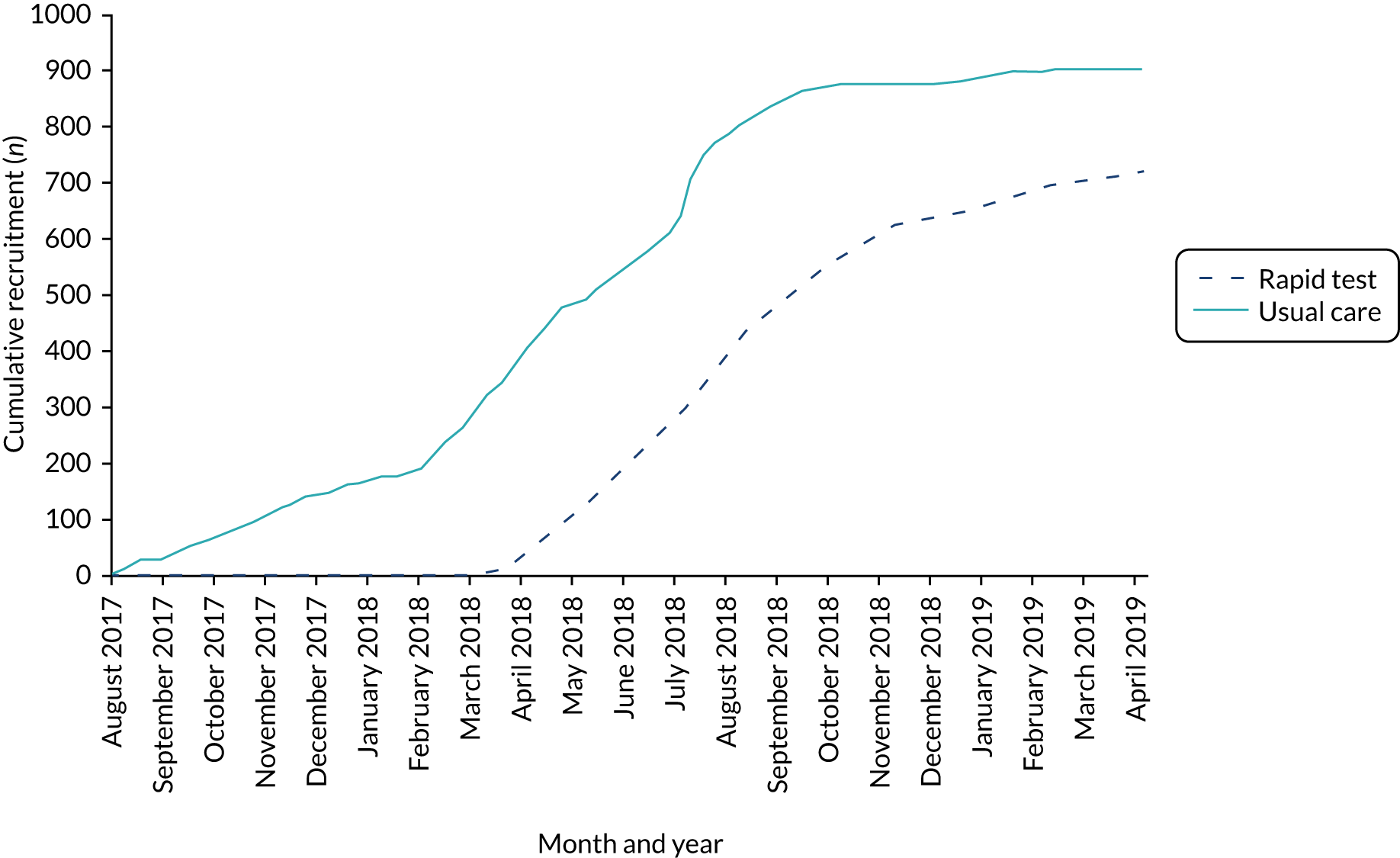
Appendix 3 Health economics tables
| Resource item | Rapid test | Usual care | Bootstrapped difference | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Adjusted mean difference | 95% CI | |
| Benzylpenicillin | ||||||
| Loading dose | 1.022 | 0.167 | 1.005 | 0.074 | 0.016 | –0.002 to 0.039 |
| Maintenance dose | 2.158 | 1.621 | 2.139 | 1.592 | 0.018 | –0.260 to 0.340 |
| Cephalosporin | ||||||
| Loading dose | 1.014 | 0.117 | 1 | 0 | 0.014 | 0 to 0.055a |
| Maintenance dose | 2.370 | 1.864 | 1.900 | 1.392 | 0.470 | –0.302 to 1.382 |
| Clindamycin | ||||||
| Loading dose | 1.067 | 0.253 | 1 | 0 | 0.067 | 0 to 0.190a |
| Maintenance dose | 1.615 | 1.121 | 1.7 | 1.081 | –0.085 | –0.857 to 0.682 |
| Metronidazole | ||||||
| Loading dose | 1.060 | 0.314 | 1 | 0 | 0.060 | 0 to 0.184a |
| Maintenance dose | 2.857 | 1.878 | 1.673 | 1.133 | 1.184 | 0.419 to 2.064a |
| Other | ||||||
| Loading dose | 1.172 | 0.729 | 1.010 | 0.101 | 0.162 | 0.046 to 0.326a |
| Maintenance dose | 2.600 | 2.380 | 4 | 5.048 | –1.400 | –3.088 to 0.129 |
| Cost | Rapid testing | Risk factors | Bootstrapped difference | |||
|---|---|---|---|---|---|---|
| Mean (£) | SD (£) | Mean (£) | SD (£) | Adjusted mean difference (£) | 95% CI (£) | |
| Benzylpenicillin | ||||||
| Loading dose | 15.18 | 2.13 | 14.80 | 2.40 | 0.38 | 0.02 to 0.80a |
| Maintenance dose | 16.73 | 20.35 | 15.53 | 11.08 | 1.20 | –1.96 to 4.84 |
| Cephalosporin | ||||||
| Loading dose | 4.57 | 1.58 | 5.41 | 0.74 | –0.85 | –1.60 to – 0.12a |
| Maintenance dose | 9.40 | 8.67 | 3.78 | 0 | 5.62 | 0 to 14.57a |
| Clindamycin | ||||||
| Loading dose | 17.70 | 4.82 | 17.70 | 1.78 | 1.98 | –1.69 to 2.60 |
| Maintenance dose | 23.11 | 16.00 | 30.68 | 20.58 | –7.57 | –21.63 to 4.35 |
| Metronidazole | ||||||
| Loading dose | 4.13 | 3.87 | 3.19 | 0 | 0.94 | 0 to 3.54a |
| Maintenance dose | 14.89 | 16.96 | 3.19 | 0 | 11.70 | 0 to 38.81a |
| Other | ||||||
| Loading dose | 7.86 | 11.32 | 11.61 | 21.21 | –3.76 | –9.67 to 0.61 |
| Maintenance dose | 14.49 | 22.99 | 19.65 | 31.41 | –5.15 | –16.84 to 6.41 |
| Level of care | Rapid testing (SD) (n = 698)a | Issuing IAP based on risk factors (SD) (n = 891)a |
|---|---|---|
| Mean costs (£) | ||
| Maternity ward days | 1074.68 (954.40) | 1209.30 (902.96) |
| Intensive care unit daysb | 673.09 | 0 |
| High-dependency unit days | 2588.10 (2588.10) | 2381.05 (2496.99) |
| Normal care days | 2616.45 (3206.86) | 1221.01 (996.34) |
| Total hospital days | 1126.14 (1090.53) | 1278.70 (1085.23) |
| Mean number of days | ||
| In a maternity ward | 2.80 (2.48) | 3.15 (2.35) |
| In a intensive care unitb | 1 | 0 |
| In a high-dependency unit | 2 (2) | 1.84 (1.93) |
| In inpatient normal care | 7.5 (9.19) | 3.51 (2.85) |
| Total hospital days | 2.85 (2.56) | 3.24 (2.46) |
| Level of care | Rapid testing (SD) (neonates n = 726;a mothers n = 697) | Issuing IAP based on risk factors (SD) (neonates n = 880;a mothers n = 835) |
|---|---|---|
| Mean costs (£) | ||
| Intensive care | 21,675 (23,376.24) | 14,906.32 (20,865.93) |
| High-dependency unit | 7400 (14,280.05) | 11,710.68 (21,850.20) |
| Special care unit | 4001.52 (4553.259) | 4260.86 (4996.15) |
| Normal careb | 957.43 (1313.91) | 963.14 (1510.63) |
| Total hospital days | 3718.46 (10,347.36) | 5130.96 (15,835.67) |
| Mean number of days | ||
| In intensive care | 15 (16.18) | 10.32 (14.44) |
| In a high-dependency unit | 8 (15.44) | 12.66 (23.62) |
| In a special care unit | 7.70 (8.76) | 8.19 (9.61) |
| Normal care daysb | 1.99 (3.22) | 2.18 (3.84) |
| Total hospital days | 5.4 (11.03) | 7.14 (16.46) |
List of abbreviations
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- DNA
- deoxyribonucleic acid
- ESBL
- extended-spectrum β-lactamase
- GBS
- group B Streptococcus
- GBS1
- Group B Streptococcus 1
- GBS2
- Group B Streptococcus 2
- GBSS
- Group B Strep Support
- IAP
- intrapartum antibiotic prophylaxis
- ICC
- intracluster coefficient
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- LSE
- London and the south-east of England
- MID
- Midlands
- MRSA
- meticillin-resistant Staphylococcus aureus
- NICE
- National Institute for Health and Care Excellence
- NSC
- National Screening Committee
- PCR
- polymerase chain reaction
- POC
- point of care
- PROM
- prelabour rupture of membranes
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- RD
- risk difference
- RNA
- ribonucleic acid
- RR
- relative risk
- SD
- standard deviation
- VRE
- vancomycin-resistant enterococci
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/BICF1187).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
