Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/127/157. The contractual start date was in December 2013. The draft report began editorial review in April 2021 and was accepted for publication in March 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Abdel-Fattah et al. This work was produced by Abdel-Fattah et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Abdel-Fattah et al.
Chapter 1 Introduction
Parts of this chapter are reproduced from Abdel-Fattah et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/. The text below includes minor additions and formatting changes to the original text.
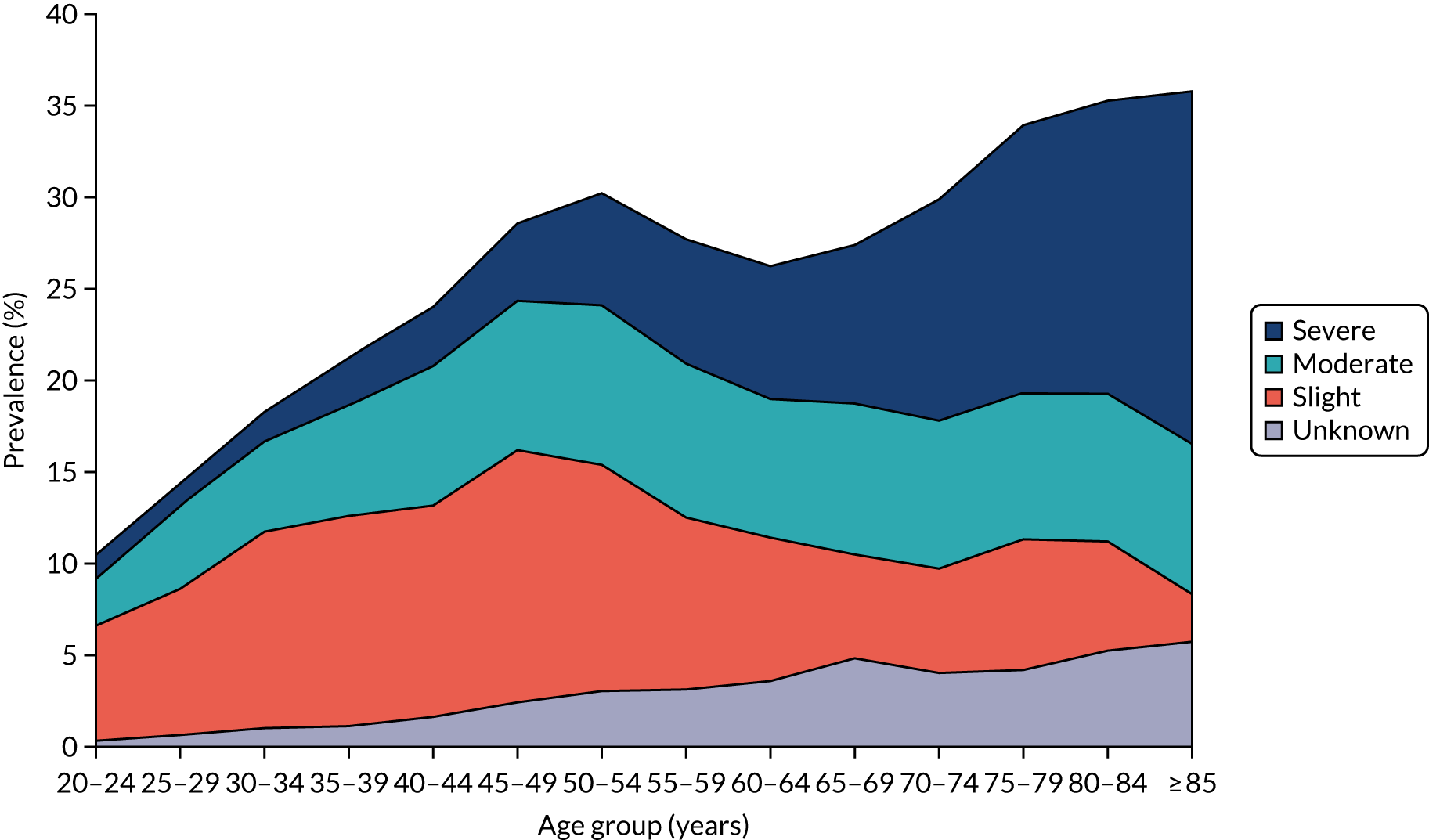
Urinary incontinence (UI) is involuntary leakage of urine. 2 It can affect both men and women, but it generally affects twice as many women as men. 3 The prevalence varies according to the population and the tool used. In a UK study published in 2009, 23% of the population reported UI. 4 Among women, the incidence of at least monthly UI is highest among those of white ethnicity (7.3/100 person-years), followed by those of Asian ethnicity (5.7/100 person-years). 5 The Leicestershire Medical Research Council Incontinence Study reported that over one-third of community-dwelling women aged ≥ 40 years had significant urinary symptoms, with 12% experiencing UI weekly. 6 The Epidemiology of Incontinence in the County of Nord-Trøndelag (EPINCONT) study among women aged > 20 years in Norway reported the prevalence of UI according to its severity within different age groups. 7 The authors7 reported that the prevalence of severe UI was 29% (range 11–72%) and affects more elderly women (Figure 1).
There are several types of UI according to the aetiology. The International Continence Society first published the definitions of the types of UI in 2002 and revised them in 2009. 8–10
The following are the most common types of UI:
-
Stress urinary incontinence (SUI) is involuntary leakage of urine on effort or on sneezing or coughing.
-
Urgency urinary incontinence (UUI) is involuntary leakage of urine associated with urgency. Urgency itself is a sudden compelling desire to pass urine that is difficult to defer.
-
Mixed urinary incontinence (MUI) is involuntary loss of urine associated with urgency and also with effort, sneezing or coughing.
-
Overactive bladder (OAB) is urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection (UTI) or other obvious pathology. It is referred to as ‘OAB wet’ or ‘OAB dry’, depending on whether or not the urgency is associated with UUI. OAB is a symptom syndrome (clinical diagnosis) and can be diagnosed by cystometry with urodynamic findings of detrusor overactivity (DO).
Urinary incontinence can be progressive. The Nurses’ Health Study of almost 24,000 women aged 54–79 years showed that 9.2% of women leaked at least monthly. 11 After 2 years, 32% of these women progressed to report UI on a weekly basis. Wennberg et al. 12 compared two cross-sectional studies for the same cohort of Swedish women over 20 years (n = 2911) in 1991 and 2007 and found no significant differences in the prevalence of UI and/or the proportion of women seeking medical treatment for UI.
Burden of urinary incontinence
Although UI is not life-threatening, its effect on the physical and psychological well-being of women has been well demonstrated. 13 UI is a condition that causes personal and hygiene problems, with a detrimental impact on women’s quality of life (QoL). 12,14 UI in women is associated with low self-esteem and can lead to social disabilities and isolation. 6 Norton et al. 15 showed that 25% of women waited > 5 years before seeking help because of embarrassment or fear of surgery, 60% avoided leaving their home, 50% felt different from others and 45% avoided public transport because of fear of UI.
Urinary incontinence can affect health directly through skin irritation and ulceration, infection and the need for catheterisation (e.g. among the elderly) and its associated complications, or indirectly through the development of avoidance behaviour such as reduction of physical activity, social interaction and sexual activity and/or limitation in employment and productivity at work. UI also has a great impact on the psychological well-being of those experiencing it. 16 It is therefore not surprising to find UI associated with many comorbidities. In the national audit for continence care, those aged ≥ 65 years had comorbidities spanning the major organ systems. 17 Impaired mobility dominated the profile outside mental health and care home settings; within these settings, dementia, depression and recurrent falls were common. For those aged < 65 years, depression, neurological disease and hypertension predominated as associated conditions across the settings, and dementia and impaired mobility were common associated conditions within the mental health and care home settings. 17 Elderly people experiencing UI are twice as likely to be depressed. 18 Less than one-third of women experiencing ‘moderate’ or ‘severe’ UI were found to be receiving health or social services for their condition. 19
A number of studies showed a direct relationship between UI and women’s sexual function. In one study,15 50% reported avoiding sexual activity because of fear of UI, and 25–50% reported dyspareunia, lack of orgasm and/or negative impact on their marital status. The ability of surgery for SUI to improve sexual function has been debatable, especially given the poor level of evidence available. In a number of studies, women reported improvement in sexual function following continence surgery. 20–22 In other studies, it was associated with a risk of developing dyspareunia (up to 15% in some studies). 23–25
The financial burden of UI is immense, either directly to the individual through the need to buy incontinence products or medical care, or indirectly through limiting employment opportunities. The cost to the health-care system is even greater. In 2000, the annual cost to the NHS for the management of UI in women aged ≥ 40 years was £301M, equivalent to 0.3% of the total NHS budget. 26 In the same year, the annual costs borne by women were estimated at £230M, or £290 per woman per year. 14,27 In the Prospective Urinary Incontinence Research (PURE) study, the annual costs of treatment for female UI were estimated at €359 (£248) in the UK/Ireland. 28 Suboptimal continence management among the elderly often results in catheterisation and bedsores, with the associated health-care costs. In the UK, the harm resulting from the use of indwelling catheters costs the NHS between £1.0B and £2.5B and accounts for ≈ 2100 deaths per year. 29
Surgical treatment of SUI is costly. The lifetime risk for women having surgery for SUI is 3.6% in the UK and 13% in the USA. 30,31 Hospital Episode Statistics for England show that, between 2008 and 2017, > 100,000 continence procedures were performed in England. 32 Similarly, 165,000 surgical continence procedures were performed in the USA in 1995, which accounts for almost 2% of the US health-care budget. 33 In 2003, a systematic review of the clinical effectiveness and cost-effectiveness of different surgical treatments for the management of SUI in the UK showed a decrease in the cost of surgical treatment for every woman with UI owing to the development of mid-urethral sling (MUS) procedures, which necessitate a shorter hospital stay and are associated with more rapid recovery and return to normal activities than previous procedures, such as colposuspension and traditional slings. 34 More recently (2019), an updated systematic review and network meta-analysis reported that ‘over a lifetime, retropubic MUS is, on average, the least costly and most effective surgery. However, the high level of uncertainty makes robust estimates difficult to ascertain’. 35
Treatment of stress urinary incontinence
Stress urinary incontinence is the most common type of UI among women. 8 Treatment pathways for SUI generally start with lifestyle changes and pelvic floor muscle training (PFMT). A Cochrane systematic review of randomised trials found that, compared with no treatment or placebo, women treated with PFMT were more likely to report improvement or cure of UI. 36 In 2020, the Optimal PFMT for Adherence Long term (OPAL) study found no added value for biofeedback to augment PFMT. 37 Pharmacological treatment for SUI is generally not effective; there is one medication (duloxetine) that is licensed for the treatment of SUI, but its effectiveness is limited and its tolerability is poor. A Cochrane review of 10 randomised controlled trials (RCTs) comparing duloxetine with placebo or PFMT showed that, when assessed subjectively, the cure effect size of duloxetine was only 3%; when assessed objectively, it resulted in no added benefit. 38
Other conservative treatment options include mechanical devices/pessaries (e.g. urethral plugs, vaginal devices) to support the bladder neck. 39,40 Among women with SUI or MUI, ≈ 50% treated with continence pessaries are satisfied at 1 year of follow-up. 41 Women who do not respond to conservative measures have the option of progressing to surgery.
Surgical treatment
Historically, there has been > 200 surgical operations described for treating SUI; the majority have come and gone with time. 42 They are generally classified as procedures that augment urethral closure by increasing outflow resistance (e.g. slings and urethral bulking) or that support the bladder neck/proximal urethra by elevating the bladder neck and proximal urethra to be intra-abdominal (e.g. Marshall–Marchetti–Krantz procedure, colposuspension). 43
Burch colposuspension
Up to the mid-1990s, Burch colposuspension (BC) was the most commonly performed continence procedure worldwide. BC is performed via a transverse lower-abdominal incision and had a reasonable success rate of ≈ 80% at 5 years’ follow-up. 44 BC is associated with an up to 30% risk of development of posterior wall prolapse, a 25% risk of postoperative voiding difficulties and an 18% risk of de novo OAB symptoms (urgency and UUI). 45
The first laparoscopic colposuspension (LC) was described in 1991. Several studies have reported patient-reported and objective success rates of 70–98%. 46–48 Kitchener showed that LC has a similar effectiveness to the open colposuspension, with shorter operating time. 49 LC has the advantage of less postoperative pain, a shorter hospital stay and shorter recovery time. 50 Interestingly, laparoscopic skills were not widely available in urology or gynaecology at the time LC was introduced, hence LC was offered only in certain centres. At the same time (1996), synthetic MUSs, namely retropubic tension-free vaginal tapes (RP-TVTs), were introduced.
Traditional slings
Traditional slings were first described by Aldridge51 in 1942; they require a combined abdominal and vaginal approach. Several studies comparing traditional MUSs with BC showed that the patient-reported cure rate was lower with the traditional slings at 1 year of follow-up [risk ratio (RR) 0.75, 95% confidence interval [CI], 0.62 to 0.90). BC was associated with fewer perioperative complications, shorter duration of use of indwelling catheter and less long-term voiding dysfunction (VD). 52–54
Synthetic mid-urethral slings, mesh, tapes
In 1996, Ulmsten et al. 55 presented the first MUS: the RP-TVT (Figure 2), which was revolutionary to continence surgery worldwide. MUS was developed with the aim of transforming continence surgery into a day case procedure. MUS primarily depended on the integral theory for continence which was first described by Petros and Ulmsten and later upheld by DeLancey’s hammock hypothesis. 56,57 In both, the pubourethral ligaments and the vaginal hammock structure constitute the main continence mechanism, disrupted in women with SUI, and require re-enforcement during surgical treatment.
FIGURE 2.
The RP-TVT needle and the position of its hammock.
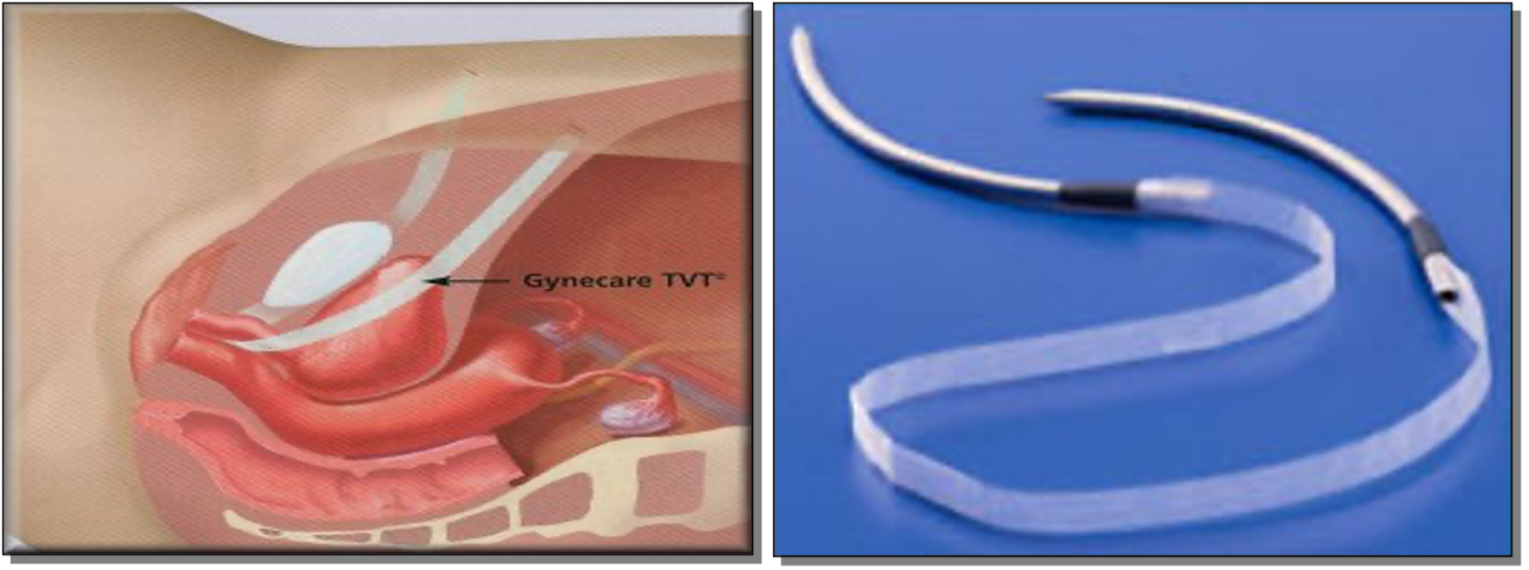
The RP-TVT is considered the first generation of standard-length MUSs. It utilises a type-1 polypropylene mesh strip to create a suburethral hammock at the mid-urethral level. One main advantage of this procedure is that it is placed in a tension-free fashion (i.e. supporting the urethra). The RP-TVT procedure was easier to learn than LC, and soon evidence accumulated to show that it had a similar success rate and comparable pattern of postoperative complications to BC, and showing that subsequent prolapse development was much higher with BC. 58 RP-TVT was introduced as a day procedure under local anaesthetic (LA). However, the British Society of Urogynaecology (BSUG) surgical database in 2010 showed that 97% of MUS procedures in the UK were performed under general anaesthetic (GA). 59 The main concern with RP-TVT is bladder injury, with a reported rate of ≈ 6.3%, and is mainly attributed to the blind retropubic trajectory of the insertion needles/trocars. 58
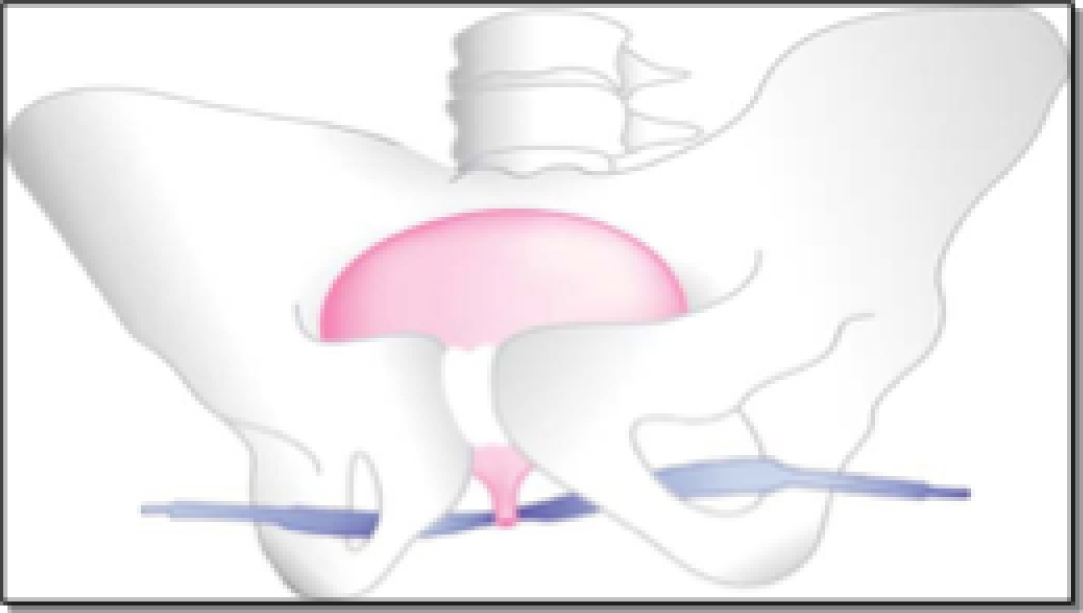
The second generation of standard-length MUSs, the transobturator tension-free vaginal tape (TO-TVT), (Figure 3) was developed as an outside–in TO-TVT by Delorme60 in 2001, followed by the introduction of the inside–out tension-free vaginal tape – obturator (TVT-O) TO-TVT by de Leval and Waltregny in 2003. 61 The main aim was to keep the concept and benefits of the RP-TVT (i.e. tension-free strip of polypropylene mesh supporting the mid-urethra), but to avoid the blind retropubic trajectory to reduce the risk of bladder injury and the more serious, but rare, bowel and vascular injury. In TO-TVT procedures, the insertion trocars pass in a more horizontal fashion (than they do with RP-TVT procedures) through the bilateral obturator complexes, with the skin incisions in the upper medial thighs. These theoretical benefits of TO-TVT materialised, with very low bladder injury rates and lower voiding difficulty rates than with RP-TVT. 62 However, more patients experienced groin/thigh pain with TO-TVTs, especially with the inside–out technique (i.e. TVT-O). 63 This was attributed to the passage of the mesh sling through the adductor muscles and the obturator complexes in the upper thigh (see Figure 3).
FIGURE 3.
The TO-TVT.
At the time of the single-incision mini-sling (SIMS) trial design, a number of systematic reviews and the Cochrane review reported no evidence of significant differences, at 12 months’ follow-up, in the patient-reported and objective cure rates between TO-TVT (RR 1.01, 95% CI 0.96 to 1.05) and RP-TVT (RR 0.96, 95% CI 0.93 to 0.99). 63–69 Similarly, there were no significant differences in hospital stay (median 0.01 days, 95% CI –0.09 to 0.11 days) or recovery time (median 0.00 weeks, 95% CI –0.14 to 0.13 weeks). The TO-TVT procedure was significantly shorter in operative time (17 minutes, compared with 27 minutes for RP-TVT). Groin pain was more common (12%) with TO-TVT than with RP-TVT, but postoperative VD was significantly less with TO-TVT than with RP-TVT (4% vs. 7%, respectively, RR 0.63, 95% CI 0.44 to 0.89). No bladder injury occurred with TO-TVT; with RP-TVT, the rate of bladder injury was 7%. 70
Mid-urethral slings (both RP-TVT and TO-TVT) rapidly became the most common continence procedures worldwide. 30 Between April 2008 and March 2017, 100,516 MUS procedures were performed in England, compared with 1195 for all other procedures. 32 The Scottish independent review on transvaginal mesh implants71 analysed routinely collected data in Scotland and showed immediate postoperative adverse events (AEs) of 3.7% for RP-TVT, 2.5% for TO-TVT and 7.8% for colposuspension, with similar rates for repeat surgery or for later complications, when comparing standard mid-urethral sling (SMUS) procedures with open colposuspension. 71 The report recommended RP-TVT as the preferred mesh-based procedure for surgical treatment of SUI among women.
Single-incision mini-slings
Single-incision mini-slings were introduced in 2006 with the aim of keeping the advantages of SMUSs, but avoiding both the retropubic trajectory and the perforation of the adductor muscles to reduce the risks of bladder injury and upper thigh pain. 72 It also involves less surgical dissection and shorter mesh length (8–14 cm, compared with 17 ± 2.87 cm for TO-TVT and 20.4 ± 0.8 cm for RP-TVT). A number of small studies showed that the SIMS procedure was more likely to be performed under LA, and had lower incidence of immediate postoperative pain, shorter hospital stay and quicker recovery. 73–76
In 2008, the National Institute for Health and Care Excellence (NICE) produced interventional procedures guidance (IPG) on the SIMS procedure. It found no RCTs evaluating its clinical effectiveness and cost-effectiveness, compared with those of other continence procedures. The NICE IPG recommended that SIMS procedures be confined to research and/or performed under special governance conditions (NICE IPG 262). 77 A Cochrane systematic review in 2011 reported lower patient-reported and objective success rates for SIMSs than for SMUSs, with 6–12 months’ follow-up, and higher incidences of repeat continence surgery and de novo UUI. However, SIMSs were associated with less operative time, less immediate postoperative groin pain and shorter recovery. 78
The introduction of SIMSs was associated with great enthusiasm as a truly ambulatory procedure. A number of SIMS devices (Figure 4) were introduced into clinical practice rather quickly, without any robust assessment of their effectiveness or safety. At the time of design of the SIMS trial, a number of SIMS procedures were used in clinical practice, such as Minitape® (Mpathy Medical Devices Ltd, Glasgow, UK), MiniArc® (American Medical Systems, Inc., Minnetonka, MN, USA), Ophira Mini Sling System (Promedon, Córdoba, Argentina), Zippere™ (ProSurg, Inc., San Jose, CA, USA), Contasure-Needleless® (NeoMedic Ltd, Watford, UK), Solyx™(Boston Scientific, Marlborough, MA, USA), Ajust™ (C.R. Bard, Inc., New Providence, NJ, USA), Altis® (Coloplast A/S, Humlebæk, Denmark) and Tissue Fixation System® (TFS) (Adelaide, SA, Australia) (see Figure 4). TVT Secur™ (Ethicon, Inc., Bridgewater, NJ, and Cincinnati, OH, USA) was withdrawn from clinical practice in 2013 by its manufacturer for ‘commercial reasons’.
FIGURE 4.
Types of SIMSs: TVT Secur (Johnson & Johnson, New Brunswick, NJ, USA), Minitape, Mini sling, Zippere and MiniArc, Solyx, Ajust, Epilog, Ophira, Needleless and Altis.
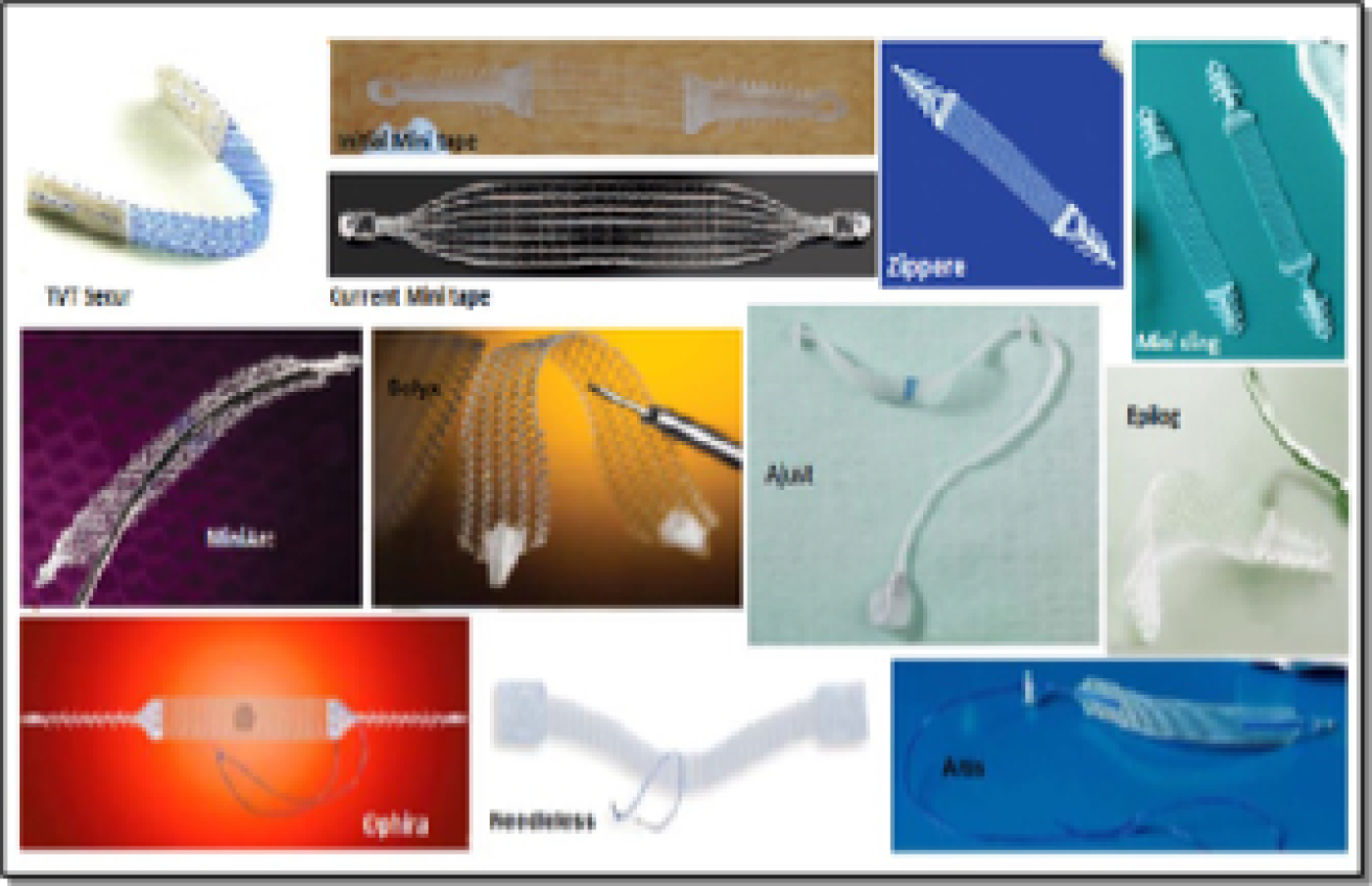
Single-incision mini-slings fundamentally differ from SMUSs because they have a shorter trajectory of insertion, and therefore need a robust anchoring mechanism to the obturator complex with a strong post-insertion pull-out force. 79,80 All clinically used SIMSs share the same mesh material (type-1 polypropylene) and the same insertion technique, through a single vaginal incision; however, they differ in the type/robustness of the anchorage mechanism used. 81 A number of more recently developed SIMSs, such as Ajust and Altis, have an added advantage in that they allow post-anchorage adjustment of the sling tension and have been shown in independent animal studies, assessing their immediate and delayed extraction forces, to be associated with the strongest and most robust anchoring mechanism to the obturator complex. 79,80
At the time of SIMS trial design, several observational studies have shown promising results for SIMSs. The objective and patient-reported success rates were 82–91% and 80–85%, respectively, at 12 months’ follow-up. SIMSs were associated with rapid recovery, low levels of postoperative pain and a short hospital stay. 73,74,82,83 There were, however, reports of potentially higher rates of postoperative voiding difficulty, vaginal exposure, de novo urgency and reoperation rate. 83–85
To our knowledge, our group was the first in the UK to evaluate the adjustable anchored SIMS (Ajust) in a series of interlinked projects. A multicentre prospective cohort study of the adjustable anchored SIMS Ajust among 100 women has shown its acceptability (75%) and feasibility (97%) under LA. 74 A multicentre prospective RCT comparing the SIMS Ajust with the SMUS TO-TVT, with a minimum of 12 months of follow-up, showed no significant differences in the patient-reported success rates [odds ratio (OR) 0.895, 95% CI 0.344 to 2.330; p = 1.000], the objective success rates (OR 0.929, 95% CI 0.382 to 2.258; p = 1.00) or the reoperation rates (OR 0.591, 95% CI 0.136 to 2.576; p = 0.721) between the two groups. 86 Comparable numbers of women in both groups reported significant improvement in QoL (p = 0.190) and sexual function (p = 0.699). Similar results were reached by a Dutch group in a similar small RCT. 87 In addition, a number of observational studies assessing adjustable anchored SIMSs, across multiple countries (UK, France, Italy, USA and Israel) and with varying cohort sizes and lengths of follow-up (6–12 months), have shown similar patient-reported and objective success rates of 85–91%. 73,88,89
Evidence on the longer-term outcomes of adjustable anchored SIMSs emerged. In July 2012, one RCT reported its 5-year follow-up comparing an adjustable anchored SIMS (TFS) with a SMUS. 90 The objective and patient-reported success rates were 83% and 89%, respectively, in the SIMS (TFS) group, compared with 75% and 78%, respectively, in the SMUS group (p = 0.16). Naumann et al. 91 reported their prospective observational study of 51 women who underwent a SIMS procedure (Ajust) with 20–29 months’ follow-up; the patient-reported success rate was 86%.
We conducted the first health economic analysis of the adjustable anchored SIMS Ajust, compared with the SMUS TO-TVT. 92 Results have shown an incremental total cost saving to the health service of £142 per procedure with the adjustable anchored SIMS, not counting the further potential economic gain of earlier return to work among these women. There were no significant differences in quality-adjusted life-years (QALYs) generated, compared with the SMUS.
An updated systematic review and meta-analysis comparing the effectiveness and complications of SIMSs with those of SMUSs for the surgical management of female SUI included a total of 26 RCTs (n = 3308 women). 81 The results showed that, after excluding RCTs evaluating TVT Secur, which was clinically irrelevant having been excluded from clinical practice, there was no evidence of significant differences between SIMSs and SMUSs in patient-reported success rates (RR 0.94, 95% CI 0.88 to 1.00) and objective success rates (RR 0.98, 95% CI 0.94 to 1.01) at a mean follow-up of 18.6 months. These results were sustained on comparing the SIMS with TO-TVT and RP-TVT separately.
In the same review, meta-analyses showed that SIMSs lead to significantly earlier return to normal activities and to work [weighted mean differences (WMDs) of –5.08 (95% CI –9.59 to –0.56) and –7.20 (95% CI –12.43 to –1.98), respectively], and to lower immediate postoperative pain scores (WMD –2.94, 95% CI –4.16 to –1.73).
Single-incision mini-slings had numerically higher rates of repeat continence surgery (RR 2.00, 95% CI 0.93 to 4.31), but this difference was not statistically significant. We urged caution in interpretation of results because of the heterogeneity of the small trials, including lack of blinding of the assessors, which can be a source of bias; the level of incomplete data, leading to attrition bias; and the relatively short term of follow-up.
The Cochrane review in 2014 included data from 3290 patients from 31 studies and showed that SIMSs were less effective than other tapes, but once data from the withdrawn TVT Secur were excluded, the difference was no longer statistically significant. 93 The authors concluded that there was not enough evidence to show difference between the SIMS and the SMUS, and recommended an adequately powered RCT with long-term follow-up to evaluate the clinical effectiveness and cost-effectiveness of SIMSs, compared with those of SMUSs.
The SIMS trial compares the patient-reported success rate and cost-effectiveness of SIMSs with those of SMUSs.
Chapter 2 Methods
Parts of this chapter are reproduced from Abdel-Fattah et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are also reproduced from Beard et al. 94 Contains public sector information licensed under the Open Government Licence v3.0.
Trial design
The trial was a pragmatic, multicentre non-inferiority RCT comparing adjustable anchored SIMSs with tension-free SMUSs in the surgical management of SUI among women. The trial protocol has been published in an open-access journal. 1
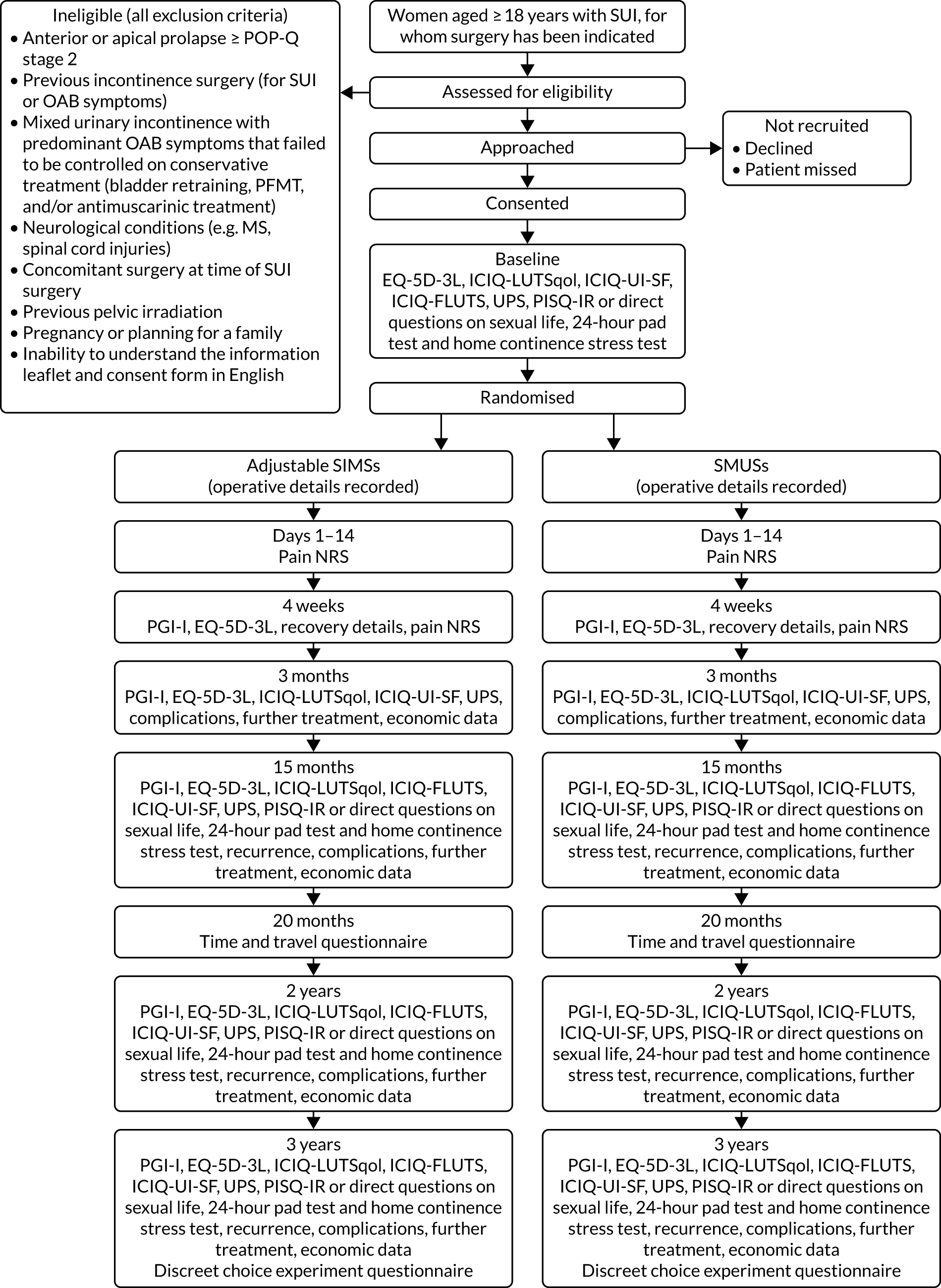
The trial design is presented in Figure 5.
FIGURE 5.
Trial design. EQ-5D-3L, EuroQol-5 Dimensions, three-level version; ICIQ-FLUTS, International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms; ICIQ-LUTSqol, International Consultation on Incontinence Questionnaire-Lower Urinary Tract Symptoms-Quality of Life; ICIQ-UI-SF, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form; MS, multiple sclerosis; NRS, numerical rating scale; PGI-I, Patient Global Impression of Improvement; PISQ-IR, Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire, International Urogynecological Association-Revised; POP-Q, Pelvic Organ Prolapse Quantification System; UPS, Urgency Perception Scale.
Interventions
The interventions compared were tension-free SMUSs, including RP-TVT and TO-TVT, and adjustable anchored SIMSs, which fulfilled the following criteria of robust anchorage and post-insertion adjustability:
-
made of type-1 polypropylene mesh – monofilament and macroporous (pore size ≥ 75 µm).
-
robustly anchored to obturator complex (robust insertion is defined as immediate pull-out force of 12 N and/or 4-week pull-out force of 30 N).
-
fully adjustable sling post insertion/anchorage.
-
proven feasibility to be done under LA.
-
minimum of level-2 evidence showing their safety and short-term (minimum 3 months) patient-reported outcomes.
Two types of SIMSs used in the UK fulfilled these criteria at the time of the study: Ajust and Altis.
Standard MUS procedures were performed under GA or deep intravenous sedation, whereas SIMS procedures were offered under LA as standard, but all participants were informed that they could opt for GA. A participant’s request for a GA was respected at all stages of the trial/procedure. A standard LA protocol (previously published and successfully used in two previous studies74,86) was used as a LA guide (see Appendix 1).
All participants received a preoperative analgesia (30–60 minutes prior to the operation): paracetamol and a non-steroidal anti-inflammatory drug (diclofenac sodium or ibuprofen), a vaginal application of EMLA™ cream (AstraZeneca plc, Cambridge, UK) (a 5% emulsion preparation, containing 2.5% each of lidocaine and prilocaine) and an optional 10 ml of intraurethral Instillagel® (Almed GmbH, Berlin, Germany) (anaesthetic, antiseptic lubricant). All participants also received preoperative/intraoperative prophylactic broad-spectrum antibiotics. A cystoscopy (rigid or flexible) was performed in all women following insertion of the sling. Postoperatively, all participants underwent a voiding assessment, including assessment for post-voiding residual urine volume using a bedside bladder scanner, when available at the collaborating centre (see Appendix 1).
Adjustable anchored single-incision mini-slings
The choice of adjustable anchored SIMS (Ajust or Altis) was dependant on the device used as standard in the collaborating centre and/or surgeon preference and experience.
A standard combination of fast- and delayed-action LA (dose was dependant on participant’s body weight) was infiltrated vaginally into either side of the mid-urethra, the vaginal angles (sulci) and behind the inferior pubic ramus into the obturator complex (e.g. using a curved black spinal needle and/or pudendal block needle). When possible, women were accompanied throughout the procedure by a health-care professional for support. The women’s bladders were emptied with a catheter. An adjustable anchored SIMS (meeting the prespecified criteria described previously) was used. The standard insertion steps for the adjustable anchored SIMS (Ajust and Altis) were as follows: women were positioned in lithotomy position with hips flexed at 90–100 °; LA infiltration was conducted as above; a suburethral vertical vaginal incision (≈ 1.5 cm) was made; and bilateral paraurethral tunnels were created reaching to the posterior margin of the inferior pubic ramus, but without piercing the obturator membrane. Further infiltration of LA into the obturator complex was carried out; the SIMS, with the ‘fixed anchor’ end mounted on the applicator, was introduced through the pre-dissected paraurethral tunnel until reaching behind the inferior pubic ramus. The applicator then pivoted slowly behind the ramus, allowing the fixed anchor to maintain its position in the obturator complex (membrane and obturator internus muscle) at points equivalent to 10 and 2 o’clock in relation to the urethral orifice. The insertion steps were repeated on the other side, allowing the ‘adjustable anchor’ to be fixed in the contralateral side. With the SIMS now robustly anchored, the tension was then adjusted as required to achieve continence while avoiding voiding difficulty. The cough stress test (CST) was conducted when possible. For Ajust, the adjustable anchor was then locked (this was not required with Altis). A cystoscopy was then performed to exclude lower urinary tract (LUT) injury and the vaginal incision was closed.
Standard tension-free mid-urethral slings
The choice of RP-TVT or TO-TVT was dependant on the standard procedure and device used in the collaborating centre and/or surgeon preference and experience.
Retropubic tension-free vaginal tape
The RP-TVTs were type-1 polypropylene mesh (monofilament and macroporous, with a pore size of ≥ 75 µm). The procedure (developed by Ulmsten and Petros55,56) was done under GA or intravenous sedation as per the standard practice of each surgeon. Women were positioned in lithotomy position. The women’s bladders were emptied with a Foley catheter. Close to the superior rim of the pubic bone, two 1-cm long transverse incisions 3 cm either side of the mid-line were made after injection of LA into the abdominal skin just above the symphysis pubis, down along the back of the pubic bone to the retropubic space and vaginally into the periurethral area. An incision of ≈ 1.5 cm was made in the mid-line of the suburethral vaginal wall, followed by dissection of the periurethral tunnels to allow introduction of the RP-TVT needle. A stent was then inserted into the Foley catheter to deviate the uretherovesical junction away from the path of the needle. The RP-TVT needle perforated the urogenital diaphragm and was brought up to the abdominal incision as close as possible to the back of the pubic bone. The procedure was then repeated on the other side, and a cystoscopy was performed to exclude LUT injury. The CST was then performed, according to the surgeon’s standard technique; the sling adjusted in a tension-free fashion; and the incisions closed.
Transobturator tension-free vaginal tape
The TO-TVTs were type-1 polypropylene mesh (monofilament and macroporous, with a pore size of ≥ 75 µm). All procedures were performed under GA (as originally described by Delorme60 and de Leval and Waltregny61 for the outside–in and inside–out routes, respectively). The lithotomy position was used with hips hyperflexed at 100–110 °. LA was infiltrated into the vaginal angles in a similar regime to the one used in the adjustable SIMS insertion (see previous section). The women’s bladders were emptied with a Foley catheter. A suburethral longitudinal vaginal incision of ≈ 1.5 cm was made, and bilateral paraurethral tunnels were created, reaching to the posterior margin of the inferior pubic ramus. Bilateral groin incisions were made 1–2 cm lateral to the labio-femoral fold and 2 cm above level of the urethra. The TO-TVT trocar was inserted from groin incisions at 90 ° to pierce the groin muscles, obturator muscles and membranes, and then guided by the surgeon’s finger to the vaginal incision. The TO-TVT was then mounted on the trocar and the trocar was withdrawn in reverse order. The previous two steps were repeated on the contralateral side, achieving a horizontal suburethral placement, and the TO-TVT was then adjusted until tension free. For the inside–out technique of insertion, the TO-TVT was introduced in the reverse route, from the vaginal incision towards the groin, using the winged guide to protect the LUT. A cystoscopy was performed to exclude LUT injury. Vaginal and skin incisions were then closed.
Setting
Clinical centres
The trial was conducted in 21 secondary and tertiary care acute hospital settings across the UK. NHS Grampian was the clinical co-ordinating centre, housing the chief investigator.
Each collaborating centre had at least one participating surgeon who was competent in performing SIMS procedures under LA prior to enrolling in the RCT. This experience was demonstrated by the surgeons having performed an average of 12 adjustable anchored SIMS procedures (with six or more procedures performed under LA) in the preceding year. Clinical experts in the trial team watched the surgeons performing two SIMS procedures under LA in their local hospitals and deemed them eligible for the trial. All collaborating centres also had at least one participating surgeon who was experienced in at least one type of SMUS (RP-TVT or TO-TVT) and had performed an adequate workload (an average of 20 procedures) in the preceding 2 years. In 20 out of 21 centres, the same surgeon was experienced in both procedures (SIMS and SMUS procedures). In five centres, at least one additional surgeon participated in the trial and was experienced in either procedure.
Population
The population comprised women aged ≥ 18 years with SUI, who had been referred to one of the collaborating centres from across the UK, and for whom MUS surgery had been indicated. Women had completed their families and failed or declined conservative treatment: PFMT. All women had either urodynamic stress incontinence or urodynamic mixed incontinence with predominant SUI bothering symptoms. Women with pure symptoms and signs of SUI, and no symptoms of OAB or voiding difficulties, were included without urodynamic investigations, as per NICE clinical guidelines 171,19 at the time. Patients were discussed in the local multidisciplinary team meetings as per standard local practice.
Preoperative urodynamic investigations included free uroflowmetry, post-voiding residual urine volume assessment and subtracted filling cystometry. Other tests, such as urethral pressure profile and leak point pressures, were not mandatory.
We excluded women if they had one or more of the following:
-
anterior or apical prolapse ≥ stage 2 on the Pelvic Organ Prolapse Quantification system
-
previous incontinence surgery (for SUI or OAB)
-
MUI with predominant OAB symptoms (defined as OAB failed to be controlled on conservative treatment, such as bladder retraining, PFMT and/or antimuscarinic treatment)
-
neurological conditions (e.g. multiple sclerosis, spinal cord injuries)
-
concomitant surgery at time of SUI surgery
-
previous pelvic irradiation
-
pregnancy or planning for a family
-
inability to understand the information leaflet and consent form in English.
There were 14 minor breaches to the exclusion criteria of concomitant surgery at the time of SUI surgery. These are detailed in Appendix 5, Table 35.
Identifying participants
Local procedures to identify participants at the participating centres were different, and the timing and mode of approach to patients and the consent process varied to accommodate both the variability at the centres and the needs of the patients. When possible, the patient information leaflet (PIL) was sent to patients together with their clinic appointments, ensuring that they had ample time (> 24 hours) to consider participating before being approached by the research team at the clinic.
Patients likely to require MUS surgery for SUI and who met the eligibility criteria were identified at the pre-assessment clinics, urodynamic clinics and outpatient urology/gynaecology clinics by their consultant, clinical team or a research nurse (RN).
A baseline invitation letter was available to centres to send to potential participants before they were approached at the centre. This contained local details and was personalised for each centre with the hospital trust logo. Patient address labels were then added, and the letters sent out with a copy of a PIL. Between February 2014 and June 2016, patients were also given a detailed surgical information patients’ leaflet, produced by the Scottish Pelvic Floor Network, on various MUSs. After June 2016, the surgical information was updated according to national guidelines and incorporated into the trial PIL.
Alternatively, these documents were given to patients attending clinics to read before the trial was discussed with them. The consultant or RN introduced the trial to the patient, provided them with the PIL and answered any queries. Patients whose first approach was at the clinic were given as much time as they required to consider participation. Patients could decide to participate during their hospital visit or take the recruitment pack home and decide later.
A log was taken of all potentially eligible patients assessed to document the reasons for non-inclusion in the trial (e.g. the reason why they were ineligible or declined to participate) to inform the Consolidated Standards of Reporting Trials (CONSORT) diagram. Brief details of potentially eligible patients were recorded in the screening logs at each centre (as an aid to monitoring potential participant inclusion). As the screening logs held personal data of potential participants, who had not given consent to participate, these screening logs were not shared with the trial office; they were seen only by the centre’s research staff.
If a patient decided to participate during the hospital visit, they signed the consent form and completed the baseline questionnaire at this visit. If required, the baseline questionnaire could also be completed at home and returned in the stamped addressed envelope (addressed to the centre) provided. At the hospital visit, they also received the 24-hour pad test and the home continence stress test (see Appendix 1) to complete at home 48 hours prior to admission for surgery, and returned them to the RN at the surgery appointment. 1
Some patients decided to participate during the hospital visit, whereas others agreed to be contacted at home by the local RN, taking home the recruitment pack containing the PIL, consent form, baseline questionnaire, stamped addressed envelope, the 24-hour pad test and the home continence stress test. In the latter case, typically, the patient was telephoned by the local RN to discuss any questions about participating in the trial. If the patient agreed to participate, they completed and signed the consent form, then completed the baseline questionnaire, and returned both to the centre in the stamped addressed envelope. The 24-hour pad test and the home continence stress test were completed at home (48 hours prior to admission for surgery) and returned to the RN at the surgery appointment.
The pads returned at the surgery visit for the 24-hour pad test were weighed, the weight and number of pads was recorded, and the pads were disposed of by the local team.
Informed consent
The PIL explained that the trial was investigating the use of adjustable anchored SIMSs and tension-free SMUSs for the surgical management of SUI among women. Signed informed consent forms were obtained from all participants. Patients who could not give informed consent (e.g. due to incapacity) were not eligible to participate. The participant’s permission was sought to contact them about any potential long-term follow-up for the SIMS trial and to inform their general practitioner (GP) that they were taking part in this trial.
Randomisation
Participants were allocated using a remote web-based randomisation service at the Centre for Healthcare Randomised Trials (CHaRT) in the University of Aberdeen. Participants were allocated 1 : 1 to either the SIMS or the SMUS (RP-TVT or TO-TVT) using a minimisation algorithm based on centre and previously supervised PFMT within the previous 2 years (yes/no). Participants were given a unique trial number on randomisation. An e-mail was automatically sent to the RN at the trial centre and to the trial office detailing the randomisation allocation and trial number.
Participants had to complete (and, if completed remotely, return) the baseline questionnaire before being informed of their allocated treatment.
Trial outcome measures and schedule of assessment
The SIMS trial outcomes and schedule of measurement are detailed in Table 1
| Measure | Baseline | Surgery details | Days 1–14 | 4 weeks | 3 months | 15 monthsa | 20 months | 2 years | 3 years |
|---|---|---|---|---|---|---|---|---|---|
| Clinical/surgery details | ○ | ○ | |||||||
| Pain NRS/daily text messaging | ● | ● | |||||||
| Recovery | ● | ● | |||||||
| PGI-I scale | ● | ● | ● | ● | ● | ||||
| EQ-5D-3L | ○ | ● | ● | ● | ● | ● | |||
| ICIQ-LUTSqol | ○ | ● | ● | ● | ● | ||||
| ICIQ-FLUTS | ○ | ● | ● | ● | |||||
| ICIQ-UI-SF and UPS | ○ | ● | ● | ● | ● | ||||
| PISQ-IR or direct questions on dyspareunia and coital incontinence | ○ | ● | ● | ● | |||||
| 24-hour pad test | ○ | ● | ● | ● | |||||
| Home continence stress test | ○ | ● | ● | ● | |||||
| Health-care resource use/complications/recurrence/further treatment | ● | ● | ● | ● | |||||
| Time and travel questionnaire | ● | ||||||||
| DCE | ● |
We collected data using participant questionnaires at baseline, at 4 weeks and 3 months postoperatively, and at 15 months and 2 and 3 years post randomisation. We chose 15 months post randomisation to reflect the average waiting time for surgery, which was up to 3 months.
Baseline assessment comprised the following:
-
demographic data – baseline use of anticholinergics/prophylactic antibiotics/clean intermittent self-catheterisation (CISC); the urodynamics diagnosis; any previous relevant surgery; 24-hour pad test and home continence stress test.
-
symptom severity questionnaires – International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF), Urgency Perception Scale (UPS) and International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms (ICIQ-FLUTS). 95–97
-
quality-of-life questionnaires – EuroQol-5 Dimensions, three-level version (EQ-5D-3L), and International Consultation on Incontinence Questionnaire-Lower Urinary Tract Symptoms-Quality of Life (ICIQ-LUTSqol). 98,99
-
sexual function – as part of a substudy comparing the two approaches to assessing sexual function:
-
half the cohort (50% selected at random; see the following paragraph for discussion) were given the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire, International Urogynecological Association-Revised (PISQ-IR)100
-
the other 50% received direct questions on dyspareunia and coital incontinence, derived and modified from the International Consultation on Incontinence Questionnaire-Female Sexual Matters Associated with Lower Urinary Tract Symptoms (ICIQ-FLUTSsex). 101
-
At the time of the RCT design, the majority of the Project Management Group (PMG) members felt that the PISQ-IR was long and intrusive. In the interest of reducing participant burden, especially with potentially intrusive questionnaires, we decided to undertake a substudy to assess two approaches of assessing the impact of the procedure on participants’ sexual function: the PISQ-IR versus simple direct questions on sexual function. Hence, only 50% of participants (randomly selected) received the PISQ-IR; the rest received direct questions on dyspareunia and coital incontinence (derived and modified from the ICIQ-FLUTSsex). 100,101
Operative data were collected, comprising operative time, blood loss, intraoperative complications, postoperative voiding assessment and duration of hospital stay. We also collected pain scores and analgesia use in recovery, at hospital discharge and daily up to 14 days postoperatively.
Follow-up data comprised the following.
-
At 4 weeks post operation, participants completed the Patient Global Impression of Improvement (PGI-I) scale and the EQ-5D-3L, and provided information on pain (i.e. a pain score) and return to normal activities. At 3 months post operation, participants also completed the ICIQ-UI-SF and the ICIQ-LUTSqol, and provided information on AEs and additional treatments. At 15 months and 2 and 3 years post randomisation, they also completed the ICIQ-FLUTS and sexual function assessment as explained above.
-
On completion of the questionnaires at 15 months, 2 years and 3 years post randomisation, participants were sent the 24-hour pad test and home continence stress test to complete.
-
At 20 months, participants were asked to complete an additional health economic data questionnaire, which included the patient time and travel costs questionnaire. Sending this questionnaire at 20 months aimed to minimise participant burden when completing the primary outcome questionnaire at 15 months.
-
The discrete choice experiment (DCE) was sent to all participants on completion of their questionnaire at 3 years.
See Table 1 for the source and timing of measures.
Outcomes
The primary outcome was patient-reported success as measured by the PGI-I scale at 15 months post randomisation. We dichotomised the PGI-I scale responses: ‘success’ was defined as ‘very much improved’ or ‘much improved’, and the rest of the responses were defined as failures. This definition of ‘success’ is widely used within the research field of surgical treatment of SUI; therefore, it facilitates comparison of our results with those of other trials in the literature.
We chose patient-reported success rate as the primary outcome as it reflects patient experience, compared with the objective measures, which can overestimate the success of SUI surgery. The PGI-I scale is a simple, direct and easy-to-use scale that is intuitively understandable to clinicians and patients. It is widely used for assessment of patient-reported outcomes following surgical and conservative interventions for treatment of UI. It has excellent construct validity. 102
Secondary outcomes comprised the following: AEs, such as bladder/urethral injuries; blood loss of ≥ 200 ml; postoperative voiding difficulties; pain; mesh exposure; dyspareunia; long-term self-catheterisation; worsening urgency; postoperative pain using a pain numerical rating scale, assessed on days 1–14; objective success rates, assessed by the 24-hour pad test; LUT symptoms, as measured using the ICIQ-FLUTS and ICIQ-UI-SF; health-related QoL profile derived from the EQ-5D-3L, pain scores and ICIQ-LUTSqol; impact on sexual function, derived from the PISQ-IR; and reoperation rates for SUI. Operative AEs were collected from operative data collection sheets. Other AEs were collected at each time point as they were reported by participants, reviewed and confirmed by the relevant centre, and onward reported as appropriate. Data on participants receiving extra treatments as outpatients or inpatients were also collected as supplementary hospital visits’ reports.
Compliance with allocated treatment
The SIMS trial was designed as a pragmatic trial and compliance with trial intervention was monitored using a question on the surgery case report form (CRF).
Safety reporting
We defined AEs as any untoward medical event affecting a participant. AEs were recorded from the time of joining the trial until follow-up was complete. Each initial AE was considered for severity, causality and expectedness, and reclassified as a serious event when appropriate.
Adverse events did not include the following:
-
continuous and persistent disease or symptom, present before the trial, which failed to improve, such as urgency, urgency incontinence, VD, pain or dyspareunia
-
treatment failure – persistence or recurrence of UI.
Worsening pain or the site of pain changing were AEs.
We identified the following as potentially expected AEs linked to surgery.
-
Intraoperative complications: bleeding, bladder/urethral injury, bowel injury, nerve injury (obturator/dorsal nerve of clitoris), injury to blood vessels, hypersensitivity to the LA or GA and/or any of the medications or materials used, pain, shaking/dizziness, change of procedure or device and/or type of anaesthesia.
-
Immediate postoperative complications: pain in the hip/thigh or the vagina, infection (chest, urinary tract), bleeding, fever, haematuria, syncope, dizziness, voiding difficulties/urinary retention and thromboembolism.
-
Later postoperative complications: pain in the hip/thigh or the vagina, vaginal mesh exposure, mesh erosion to the LUT, haematoma, abscess formation and nerve injury. In addition, new onset or worsening of any of the following: dyspareunia, vaginal discharge, voiding difficulties/urinary retention, long-term self-catheterisation (CISC) and urgency/urgency incontinence.
We adhered to the standard definition of serious adverse events (SAEs) as those leading to death or life-threatening, unplanned hospitalisation or prolongation of existing hospitalisation (except for social/geographical reasons),leading to persistent or significant disability or incapacity, or otherwise considered medically significant.
Hospitalisations for treatment planned prior to randomisation and hospitalisation for elective treatment of a pre-existing condition, or complication arising from either, were not considered to be AEs or SAEs.
Adverse events were assessed in respect of seriousness to determine if they were a SAE by the local principal investigator (PI), the chief investigator or their deputies.
A total of 27 SAEs were reported during the trial, (see Appendix 5, Table 34). All SAEs were reviewed by the sponsor from 11 September 2014. The two SAEs reported prior to this date were sent to sponsor on 19 September 2014 for their review. The Data Monitoring Committee (DMC) reviewed all SAEs annually at first, but from September 2017 to the end of the trial, it reviewed all SAEs as they occurred.
Blinding
Baseline data were collected prior to randomisation using self-completed questionnaires. It was not possible to blind the participants, given the nature of the procedures (SIMS procedure under LA and SMUS procedure under GA). Surgeons could not be blinded for obvious surgical reasons. Outcome assessment was primarily through participant-completed postal questionnaires.
Sample size
The aim of the trial was to determine the clinical effectiveness and cost-effectiveness of adjustable anchored SIMSs, compared with those of tension-free SMUSs, and so the trial was designed to show non-inferiority. If SIMSs were superior in having shorter hospital stays, less postoperative pain, earlier recovery and greater cost-effectiveness, then 10% was the maximum inferiority margin acceptable, as determined by expert clinicians.
Published literature at the time of the trial design suggested that the 15-month success rate was approximately 85% for the SMUS arm. Several smaller studies indicated a similar success rate for SIMSs. Power estimates were obtained by simulating trials of a fixed sample size and using the proportion of simulated trials where the lower bound of a two-sided 95% CI for the difference in success rates (SIMS – SMUS) was > –10%. These simulations showed that 275 women randomised to each arm would give 90% power. Adjusting the total of 550 participants to allow for 15% dropout gave a required sample size of 650 participants. In November 2016, this was reduced to 600 owing to difficulties completing recruitment within time and budget. Under the same assumptions, this reduced the power to 88%. There was no interim analysis of the primary outcome to inform the re-estimation of statistical power.
Statistical analysis of outcomes
Predefined statistical analyses were included in the published protocol. 1 All statistical analyses were based on all randomised women, regardless of whether they complied with their randomised surgery. The comparisons were between those who were randomised to receive a SIMS and those who were randomised to receive a SMUS.
The primary outcome was the PGI-I dichotomised to success and failure. Success was defined as a response of ‘very much improved’ or ‘much improved’; all other responses were classed as failure. The primary outcome was analysed using a generalised linear model (GLM) with binomial family and log-link function. Fixed effects were included for the treatment (receiving a SIMS procedure) and having received supervised PFMT within the previous 2 years. Robust variances were used to adjust for clustering by centre. Statistical significance was at one-sided 2.5%, as standard for a non-inferiority design, with CIs calculated at the usual 95% width. An intention-to-treat (ITT) analysis was performed with all participants remaining in their randomised group. A prespecified per-protocol analysis was also carried out of participants who received their allocated randomised surgery. The adjusted difference is obtained from the difference between the predictive margin of the SIMS and SMUS groups.
The primary outcome was tested in a non-inferiority framework with a margin of 10%. The null hypothesis was that SIMSs were inferior to SMUSs by at least 10%; a p-value of < 0.025 would indicate that the null hypothesis could be rejected, thereby suggesting that SIMSs were non-inferior to SMUSs.
The prespecified statistical analysis plan did not provide precise details on dealing with missing data; post hoc, we have chosen to use multiple imputation using chained equations to account for missing data on the primary and secondary outcomes. The imputation model used the treatment variable; the baseline characteristics of age, body mass index (BMI) and receipt of PFMT; number of previous deliveries; and the responses to the questions at baseline on how often a participant leaks, the amount leaked and how much urinary leakage interferes with daily life. Collected outcomes for other participants and the baseline measures of the outcomes were also used in the imputation model. If baseline data were missing for a participant, they were imputed with the centre mean or median, as appropriate.
Subgroup analyses of the primary outcome between the following groups were carried out at the stricter one-sided 0.5% level, and are therefore summarised with 99% CIs:
-
urodynamic stress incontinence versus urodynamic mixed incontinence
-
types of adjustable anchored SIMS (Ajust and Altis) versus each type of SMUS (i.e. RP-TVT and TO-TVT, separately)
-
age – above and below the observed median age of the recruited women
-
a post hoc analysis for age – < 65 years, compared with those aged ≥ 65 years
-
a post hoc comparison between devices withdrawn from clinical use and those still available
-
a post hoc comparison between those who had received supervised PFMT in the previous 2 years and those who had not.
Secondary outcomes with multiple categories (such as satisfaction categories) were analysed using ordered logistic regression clustered by centre and with adjustment for PFMT. Secondary outcomes that are continuous outcomes and measured repeatedly (such as the EQ-5D-3L; the ICIQ-UI-SF; the ICIQ-FLUTS filling, voiding and incontinence scores; the ICIQ-LUTSqol and the PISQ-IR) were analysed using a mixed-effects repeated time model, with random effects for centre and participant and fixed effects for the treatment, the respective outcome at baseline and PFMT.
Secondary outcomes that measure QoL, such as EQ-5D-3L, or incontinence secondary outcomes, such as the ICIQ-UI-SF, the three ICIQ-FLUTS outcomes, the specific QoL measure ICIQ-LUTSqol and the PISQ-IR, were tested under a superiority framework.
Non-responder analysis
Descriptive data comparing the baseline characteristics of participants who did respond with those of participants who did not respond at 15 months are displayed in Appendix 2, Table 29; the t-test (continuous outcomes) and chi-squared test (categorical outcomes) were used to estimate the statistical significance of the differences between responders and non-responders.
Sensitivity analyses
The secondary outcomes (i.e. ICIQ-UI-SF score; ICIQ-FLUTS filling, voiding and incontinence scores; ICIQ-LUTSqol score; and PISQ-IR score) were all measured at baseline and the repeated measures mixed-effects model included a fixed effect for the baseline measure. The GLM for objective success using the 24-hour pad test has a fixed effect for the pad test weight at baseline. For participants for whom follow-up measures were recorded but the baseline measure was missing, the baseline measure was imputed by the centre mean.
When the primary outcome was missing, it was imputed by pattern-mixture modelling; this is presented with the effect size from the multiple imputation using chained equations and a complete-case analysis. The results from the imputations are shown along with the observed effect size on a forest plot (see Figure 8).
Economic evaluation
A cost–utility analysis was conducted alongside the RCT and a cost–benefit analysis was also conducted using the results from the DCE. Our primary health economic evaluation is from a health service provider’s (NHS) perspective; however, we also present data from a wider societal perspective. These data include costs to patients of time and travel, costs to carers and family members and costs to society as a whole, estimated from lost productivity as a result of time off work/away from normal activities. Full details are given in Chapters 7 and 8.
Research ethics and regulatory approvals
The SIMS trial received a favourable ethics opinion from the North of Scotland Research Ethics Committee (REC) on 12 December 2013 (REC reference number: 13/NS/0143).
Protocol amendments
There were seven protocol amendments; these are summarised in Appendix 2. All amendments were reviewed by the sponsor. Substantial amendments were then submitted for approval to the REC. Amendments involving changes to the protocol were reviewed by the funder and the Trial Steering Committee (TSC) before being submitted to the REC for approval. Non-substantial amendments were submitted to the REC when the next substantial amendment was submitted for review or included in the annual REC report.
Management of the trial
The trial management team, based in the CHaRT at the University of Aberdeen, provided day-to-day support for the recruiting centres led by a local PI. The PIs, in most cases supported by RNs, trial co-ordinators or dedicated staff, were responsible for all aspects of local organisation, including recruitment of participants, delivery of the interventions and notification of any problems or unexpected developments during the trial period.
Recruitment pauses
Recruitment to the trial paused at participating Scottish centres in June 2014 for 3 weeks when the Scottish Health Secretary requested the suspension of mesh implant surgery in Scotland. It was also paused briefly at all participating centres in December 2014 when the chief investigator was temporarily changed to John Norrie and James N’Dow, jointly, for a period of 8 months to allow sponsor investigation into a media report. The investigation did not find evidence of any inappropriate behaviour. The findings were accepted by the National Institute for Health and Care Research and Mohamed Abdel-Fattah resumed as chief investigator.
Trial oversight committees
Study Management Group
The Study Management Group was responsible for the day-to-day management of the trial. This group was chaired by the chief investigator and consisted of the trial manager, senior trial manager, data co-ordinator, health economist and statistician.
Project Management Group
The PMG was responsible for overseeing the management of the trial. This group consisted of the Study Management Group plus grant applicants, a patient and public involvement (PPI) representative and a senior programmer. Membership of the PMG is listed in the Acknowledgements.
Trial Steering Committee
The TSC was responsible for monitoring and supervising the progress of the SIMS trial. The committee met seven times between April 2014 and September 2020, at intervals agreed by the TSC. The TSC consisted of independent experts, a PPI representative, the chief investigator and key members of the PMG. Membership of the TSC is given in the Acknowledgements.
Data Monitoring Committee
The DMC was independent of the trial and responsible for monitoring safety and data integrity. The committee met nine times between April 2014 and September 2020, at intervals agreed by the committee. The trial statistician provided the data and analyses requested by the DMC prior to each meeting. The committee consisted of three independent experts. Membership of the DMC is given in the Acknowledgements.
Patient and public involvement
One of the trial PPI representatives was a grant holder and an active member of the PMG. As part of this role, she contributed extensively to the development and review of trial materials, including the protocol, PIL, questionnaires and participant newsletters, as well as to the trial processes, the 6-monthly funder reports and the final report. There was also an active PPI member of the TSC.
Chapter 3 Baseline data and operative details
This chapter describes how the trial population was formed, the clinical characteristics of the participants and the baseline measures used. We also describe the baseline operative details.
Trial recruitment
Between 4 February 2014 and 7 September 2017, we recruited 600 participants from 21 centres (see Appendix 3, Table 31). A total of 300 participants were allocated to receive an adjustable anchored SIMS and 300 were allocated to receive a tension-free SMUS. All centres recruited to both arms of the trial. The trial database was locked on 15 October 2020.
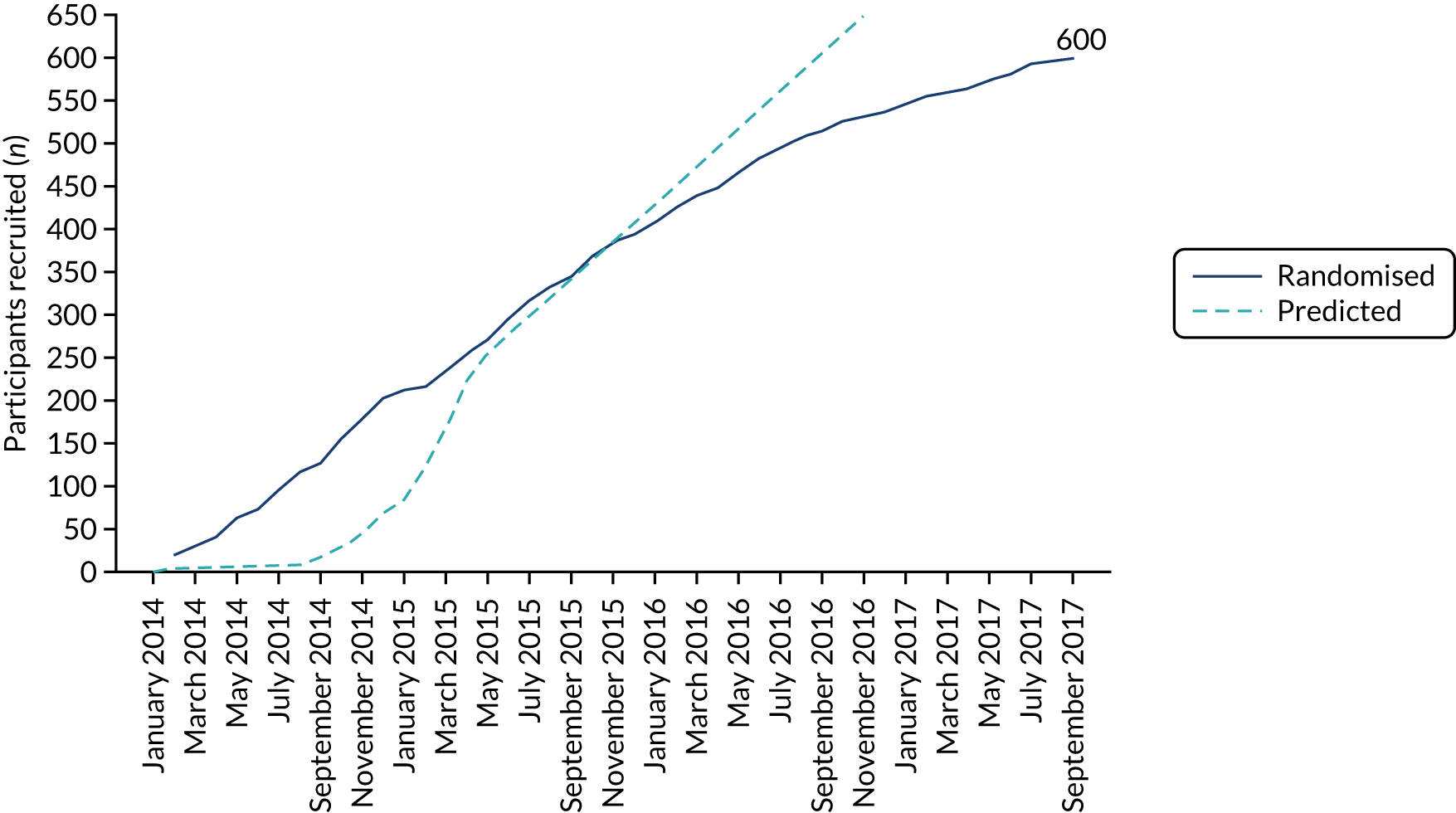
Appendix 3, Table 31, shows the number of participants randomised at each site to receive a SIMS and those randomised to receive a SMUS. The data show that there was no dominant site in the trial and that all sites allocated participants to both interventions. Figure 6 shows the monthly recruitment to the trial, compared with what was predicted. Although the trial required an extension to the recruitment period, Figure 6 shows that recruitment was at a consistent rate.
FIGURE 6.
Recruitment graph.
Participant flow
The progress of participants through the stages of the trial to the follow-up stages is shown in the CONSORT diagram (Figure 7). In total, 1040 participants were considered for entry to the trial. Of these participants, 163 (15.7%) failed to meet one or more of the eligibility criteria. Of the 877 who were eligible, 277 were excluded; the majority of these were excluded because the patient wanted a particular surgery or anaesthetic.
FIGURE 7.
The CONSORT diagram.
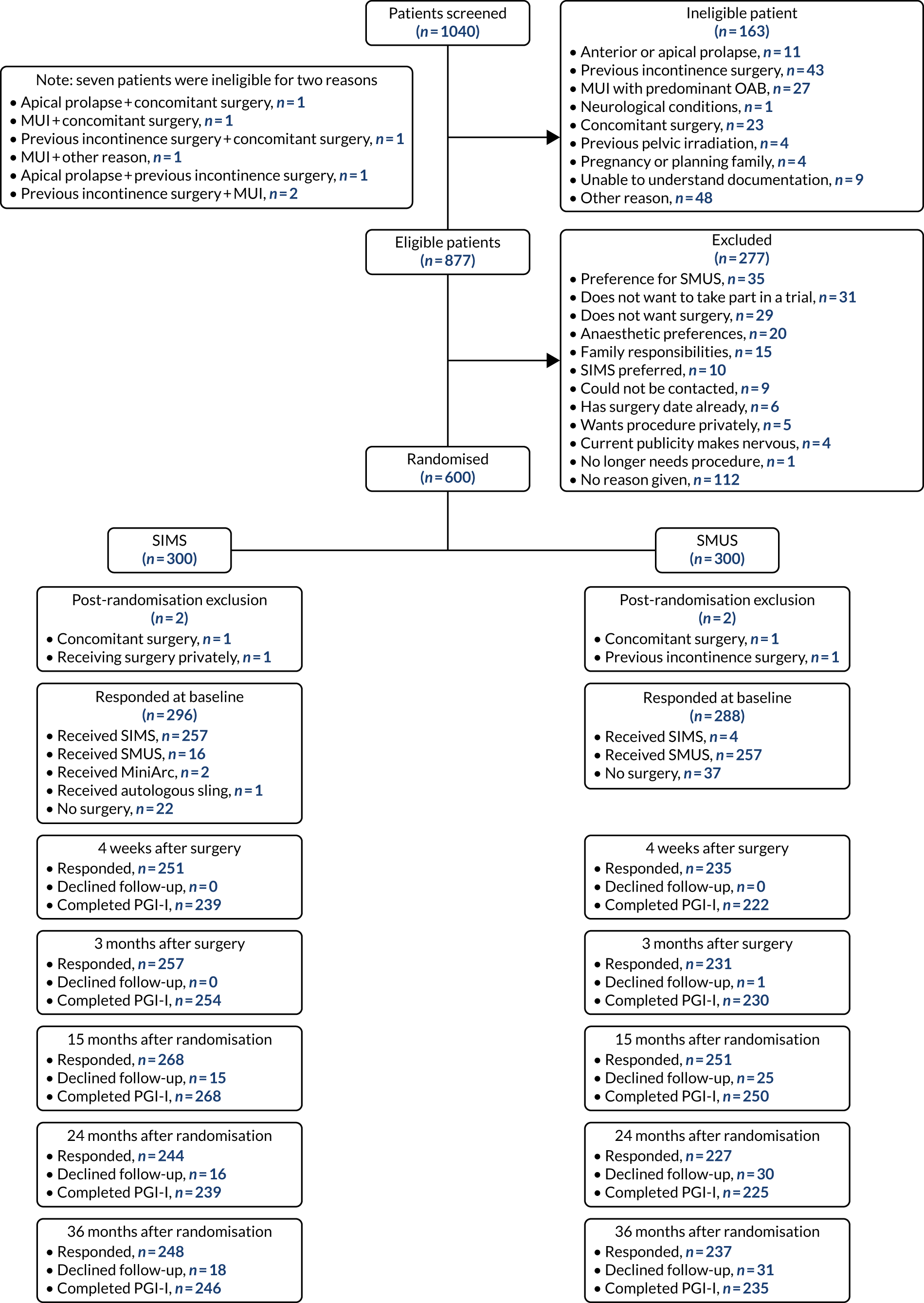
Four participants were excluded from the trial after entering. Two of these were in the SIMS group and were excluded for the following reasons: one was receiving concomitant surgery and one wanted to receive their surgery privately. In the SMUS group, one participant was excluded because of previous incontinence surgery and another because she was receiving prolapse surgery concomitantly.
The time from randomisation to intervention and the time from randomisation to each of the follow-up points were similar between the two randomised groups. These are shown in Appendix 3, Table 32.
Participant and sociodemographic factors
The mean age of participants was between 50 and 51 years. The mean BMI was similar in both groups, at very slightly < 29 kg/m2. Approximately 85% of participants in both groups had received PFMT within the previous 2 years. A slightly higher percentage of participants in the SIMS group than in the SMUS group were smokers [17.2% (n = 52) and 14.4% (n = 43), respectively]. There was a difference between the two groups in the percentage of participants on anticholinergic drugs at baseline: 20.1% (n = 60) in the SIMS group, compared with 11.7% (n = 35) in the SMUS group. Previous history of use of anticholinergic drugs was similar between the two groups. Previous gynaecology surgeries were similar between the two groups, although previous abdominal hysterectomy and anterior repairs had slightly higher percentages in the SIMS group. Table 2 shows the baseline characteristics of participants in both groups.
| Characteristic | Trial group | |
|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | |
| Age (years), mean (SD) | 50.4 (11.0) [n = 298] | 50.7 (10.9) [n = 298] |
| BMI (kg/m2), mean (SD) | 28.9 (5.5) [n = 297] | 28.7 (5.6) [n = 292] |
| Received PFMT in previous 2 years, n (%) | 254 (85) | 254 (85) |
| Obstetric history, n (%) | ||
| Parity | ||
| 0 | 10 (3.4) | 9 (3.0) |
| 1 | 41 (14) | 35 (12) |
| 2 | 130 (44) | 130 (44) |
| 3 | 81 (27) | 81 (27) |
| ≥ 4 | 34 (11) | 39 (13) |
| Missing | 2 (0.67) | 4 (1.3) |
| At least one forceps delivery | 38 (13) | 37 (12) |
| At least one vacuum delivery | 20 (6.7) | 21 (7.0) |
| All deliveries were caesareans | 9 (3.0) | 10 (3.4) |
| Manual job (heavy lifting), n (%) | 84 (28) | 84 (28) |
| Smoker, n (%) | 52 (17) | 43 (14) |
| Current or previous hormone replacement therapy, n (%) | 29 (9.7) | 26 (8.7) |
| On anticholinergic drugs at baseline, n (%) | 60 (20) | 35 (12) |
| Previous use of any anticholinergic drugs, n (%) | 49 (16) | 50 (17) |
| Experience recurrent UTIs, n (%) | 10 (3.4) | 11 (3.7) |
| Performing CISC, n (%) | 4 (1.3) | 1 (0.34) |
| On prophylactic low-dose antibiotics, n (%) | 2 (0.67) | 2 (0.67) |
| Previous gynaecology surgery, n (%) | 98 (33) | 87 (29) |
| Abdominal hysterectomy | 42 (14) | 30 (10) |
| Vaginal hysterectomy | 17 (5.7) | 20 (6.7) |
| Sacrospinous fixation | 1 (0.34) | 1 (0.34) |
| Anterior repair | 14 (4.7) | 6 (2.0) |
| Anterior mesh repair | 2 (0.67) | 5 (1.7) |
| Posterior repair | 6 (2.0) | 6 (2.0) |
| Sacrohysteropexy | 1 (0.34) | |
| Posterior mesh repair | 1 (0.34) | |
| Manchester repair | 1 (0.34) | |
| Other previous gynaecology surgery | 35 (12) | 31 (10) |
Clinical assessment and health status
Most women underwent preoperative urodynamics (95%, n = 571). The preoperative urodynamic diagnosis was urodynamic stress incontinence for 79% (n = 235) and 78% (n = 231) of the SIMS and SMUS groups, respectively, and mixed urodynamic UI was diagnosed for 12% (n = 36) and 11% (n = 33) of the SIMS and SMUS groups, respectively. A clinical diagnosis of pure SUI was used (without urodynamics) for only 4.7% (n = 14) and 3.7% (n = 11) of the SIMS and SMUS groups, respectively. For 77% (n = 459) of participants, the uroflowmetry diagnosis was normal. Although the ICIQ-FLUTS filling and voiding scores were low on average, some women had scores at the top of the scale, indicating the worst possible score. The ICIQ-UI-SF score, ICIQ-FLUTS incontinence score and ICIQ-LUTSqol score all suggested that women’s lives were negatively affected by their UI. Both coital incontinence and dyspareunia had higher frequencies at baseline among women in the SIMS group.
The EQ-5D-3L results showed a wide range of values, including the maximum score of 1.0; both the mean and median were > 0.8, suggesting very good health on average. There were also some very low scores, indicating that some women rated their health as very poor. The patient-reported baseline scores were similar between the two groups; this is also the case for the ICIQ-LUTSqol.
Table 3 shows the baseline questionnaire scores and Table 4 shows the urodynamics diagnoses for both groups. The urodynamic diagnosis by device is shown in Appendix 6, Table 38.
| Questionnaire | Trial group | |
|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | |
| ICIQ-UI-SF score, mean (SD) | 14.4 (3.3) [n = 284] | 14.4 (3.6) [n = 285] |
| Median (IQR) | 15.0 (12.0–17.0) | 14.0 (12.0–17.0) |
| ICIQ-FLUTS filling score, mean (SD) | 4.5 (2.7) [n = 291] | 4.9 (2.8) [n = 284] |
| Median (IQR) | 4.0 (3.0–6.0) | 5.0 (3.0–7.0) |
| ICIQ-FLUTS voiding score, mean (SD) | 1.9 (2.0) [n = 293] | 1.7 (2.0) [n = 286] |
| Median (IQR) | 2.0 (0.0–3.0) | 1.0 (0.0–3.0) |
| ICIQ-FLUTS incontinence score, mean (SD) | 11.0 (3.0) [n = 284] | 11.4 (3.1) [n = 286] |
| Median (IQR) | 11.0 (9.0–13.0) | 11.0 (9.0–14.0) |
| ICIQ-LUTSqol score, mean (SD) | 46.9 (11.7) [n = 286] | 46.6 (10.7) [n = 276] |
| Median (IQR) | 46.0 (38.0–55.0) | 45.0 (38.5–54.0) |
| EQ-5D-3L | 0.860 (0.200) [n = 286] | 0.834 (0.249) [n = 284] |
| PISQ-IR, mean (SD) | 3.3 (0.6) [n = 87] | 3.3 (0.6) [n = 91] |
| Median (IQR) | 3.3 (2.9–3.9) | 3.3 (2.9–3.8) |
| Coital incontinence, n/N (%) | 60/145 (41) | 52/145 (36) |
| Dyspareunia, n/N (%) | 25/145 (17) | 21/145 (14) |
| Urodynamics diagnosis | Trial group, n (%) | |
|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | |
| Cystometry diagnosis | ||
| Urodynamic stress incontinence | 235 (79) | 231 (78) |
| Urodynamic mixed incontinence | 36 (12) | 33 (11) |
| Equivocal | 3 (1.0) | 3 (1.0) |
| Not interpretable | 1 (0.34) | 1 (0.34) |
| Other | 2 (0.67) | 2 (0.67) |
| Clinical diagnosis SUI (no urodynamics performed) | 14 (4.7) | 11 (3.7) |
| Missing | 7 (2.3) | 17 (5.7) |
| Uroflowmetry diagnosis | ||
| Normal | 233 (78) | 226 (76) |
| Obstruction | 6 (2.0) | 4 (1.3) |
| Suboptimal | 12 (4.0) | 17 (5.7) |
| Equivocal | 4 (1.3) | 4 (1.3) |
| Not interpretable | 5 (1.7) | |
| Not recorded | 19 (6.4) | 10 (3.4) |
| Other | 18 (6.0) | 21 (7.0) |
| Missing | 6 (2.0) | 11 (3.7) |
Symptom severity
There were a wide range of pad test results, but the median in both groups was similar at 39 g and 40 g in the SIMS and SMUS groups, respectively.
The total ICIQ-UI-SF score was comparable between both groups, indicating similar symptom severity and impact on women’s QoL. There were also similar percentages of women in both groups describing severe symptoms (i.e. a score of ≥ 13). The participants’ responses to individual questions of ICIQ-UI-SF are shown in Table 5.
| Symptom severity | Trial group | |
|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | |
| Pad test weight (g), mean (SD) | 51.0 (58.1) [N = 234] | 56.4 (58.5) [N = 204] |
| Median (IQR) | 39.0 (24.0–60.0) | 40.0 (24.0–67.0) |
| ICIQ-UI-SF score, mean (SD) | 14.4 (3.3) [N = 284] | 14.4 (3.6) [N = 285] |
| Median (IQR) | 15.0 (12.0–17.0) | 14.0 (12.0–17.0) |
| How often do you leak urine?, n (%) | ||
| Once or less per week | 8 (2.7) | 7 (2.3) |
| Two or three times per week | 31 (10) | 37 (12) |
| Once per day | 29 (9.7) | 24 (8.1) |
| Several times per day | 192 (64) | 183 (61) |
| All the time | 33 (11) | 37 (12) |
| Missing | 5 (1.7) | 10 (3.4) |
| How much urine do you leak?, n (%) | ||
| Small amounts | 122 (41) | 106 (36) |
| Moderate amounts | 125 (42) | 131 (44) |
| Large amounts | 41 (14) | 50 (17) |
| Missing | 10 (3.4) | 11 (3.7) |
| How much does urinary leakage interfere with day-to-day activities?, mean (SD) | 7.3 (2.1) [N = 289] | 7.1 (2.2) [N = 286] |
| ICIQ-UI-SF severity, n (%) | N = 284 | N = 285 |
| Mild/moderate (< 13) | 79 (28) | 80 (28) |
| Severe (≥ 13) | 205 (72) | 205 (72) |
| Urgency perception: baseline, n (%) | ||
| No urgency | 49 (16) | 40 (13) |
| Mild urgency | 80 (27) | 85 (29) |
| Moderate urgency | 115 (39) | 117 (39) |
| Severe urgency | 49 (16) | 45 (15) |
| Not answered | 5 (1.7) | 11 (3.7) |
Operative details
Of the women allocated to receive an adjustable anchored SIMS, 92.6% (n = 276) received surgery, whereas 87.6% (n = 261) of those randomised to the tension-free SMUS group received surgery. Compliance with the allocated intervention/surgery was high: 86.2% (n = 257) in both groups.
The comparison between both groups for operative data collected is provided in Table 6 (for operative outcomes, see Table 12). In terms of the actual device received, 70.7% (n = 195) in the SIMS group received Altis and 22.5% (n = 62) received Ajust. Slightly more SMUS devices were TO-TVT [52.9% (n = 138)] than RP-TVT [45.6% (n = 119)]. Owing to the nature of the procedure, there were some differences between the two groups, for example in the type of anaesthesia and adjustment of the sling. More women in the SMUS group had their procedure by a senior trainee who was deemed competent by their consultant/local PI. In all such cases, the procedures were performed under supervision of the consultant.
| Operative data | Trial group, n (%) | |
|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | |
| Received any surgery | 276 (93) | 261 (88) |
| Type of procedure | N = 276 | N = 261 |
| Ajust | 62 (22) | – |
| Altis | 195 (71) | 4 (1.5) |
| RP-TVT | 7 (2.5) | 119 (46) |
| TO-TVT | 9 (3.3) | 138 (53) |
| Autologous fascial sling | 1 (0.36) | – |
| MiniArc | 2 (0.72) | – |
| Grade of surgeon | ||
| Subspecialist urogynaecologist | 65 (24) | 46 (18) |
| Consultant gynaecologist | 183 (66) | 160 (61) |
| Consultant urologist | 23 (8.3) | 9 (3.4) |
| Associate specialist/staff grade | 1 (0.36) | 3 (1.1) |
| Senior trainee | 4 (1.4) | 43 (16) |
| Type of anaesthesia | ||
| GA | 70 (25) | 238 (91) |
| Spinal | 5 (1.8) | 7 (2.7) |
| LA with IV sedation | 47 (17) | 14 (5.4) |
| LA with oral sedation | 26 (9.4) | 1 (0.38) |
| Local anaesthesia only | 128 (46) | 1 (0.38) |
| Local anaesthesia received during the procedure | 270 (98) | 235 (90) |
| Sling adjusted under CST guidance | 180 (65) | 15 (5.7) |
The majority of participants [91% (n = 238)] in the SMUS group had the procedure under GA, whereas the majority in the SIMS group [73% (n = 201)] had the procedure under LA, with or without sedation. Most participants received LA infiltration during the procedure. More women in the SIMS group had their sling adjusted under guidance of a CST.
Chapter 4 Patient-reported clinical outcomes
This chapter compares the clinical outcomes of the adjustable anchored SIMS with those of the tension-free SMUS at 4 weeks and at 3 months after surgery, and at 15, 24 and 36 months after randomisation.
Analysis populations
A total of 600 participants were randomised to receive either a SIMS or a SMUS device. There were two post-randomisation exclusions in each group; this chapter reports on the results from 596 participants.
Primary outcome
The primary outcome was the proportion of women who reported success at 15 months post randomisation (≈ 12 months post operation). Success was defined as a participant response of either ‘very much improved’ or ‘much improved’ to the PGI-I scale question ‘thinking about how you have been on average over the past four weeks, please describe how your incontinence is now, compared with how it was before your operation’. All other responses (improved, same, worse, much worse and very much worse) were classed as failure. A total of 212 participants out of 268 (79.1%) in the SIMS group and 189 out of 250 (75.6%) in the SMUS group reported success at 15 months. The adjusted absolute risk difference (RD) was 4.6 (95% CI –2.7 to 11.8; pNI < 0.001, where pNI is the non-inferiority hypothesis p-value). The SIMS was non-inferior to the SMUS at 15 months post randomisation: the lower bound of the 95% CI excluded the predefined non-inferiority margin of –10%. Similarly, at 3 years follow-up, patient-reported success rates in the SIMS group were non-inferior to those of the SMUS group at the 10% margin: 177 out of 246 (72%) participants in the SIMS group and 157 out of 235 (66.8%) in the SMUS group reported success (RD 5.7, 95% CI –1.3 to 12.8; pNI < 0.001). Table 7 reports this outcome at each time point. At each follow-up time point, the CI for the adjusted RDs excludes –10%; therefore, patient-reported success with SIMSs was non-inferior to success with SMUSs at all time points and up to 36 months’ follow-up. The per-protocol estimates at 4 weeks and at 3, 15, 24 and 36 months were similar to the ITT analysis.
| Analysis and primary outcome | Trial group, n/N (%) | Difference (95% CI); pNI-value | |
|---|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | ||
| ITT | |||
| Patient-reported success on the PGI-I scale at | |||
| 4 weeks | 183/239 (77) | 168/222 (76) | 1.3 (–5.0 to 7.6); < 0.001 |
| 3 months | 211/254 (83) | 187/230 (81) | 3.2 (–4.2 to 10.6); < 0.001 |
| 15 months | 212/268 (79) | 189/250 (76) | 4.6 (–2.7 to 11.8); < 0.001 |
| 24 months | 185/239 (77) | 167/225 (74) | 3.9 (–4.2 to 11.9); < 0.001 |
| 36 months | 177/246 (72) | 157/235 (67) | 5.7 (–1.3 to 12.8); < 0.001 |
| Per protocol | |||
| Patient-reported success on the PGI-I scale at | |||
| 4 weeks | 174/226 (77) | 167/218 (77) | 1.0 (–5.4 to 7.4); < 0.001 |
| 3 months | 200/241 (83) | 184/226 (81) | 3.0 (–3.8 to 9.9); < 0.001 |
| 15 months | 198/248 (80) | 185/241 (77) | 3.6 (–2.7 to 9.9); < 0.001 |
| 24 months | 175/223 (78) | 163/217 (75) | 3.2 (–5.1 to 11.5); < 0.001 |
| 36 months | 168/230 (73) | 153/226 (68) | 6.1 (–0.7 to 12.8); < 0.001 |
Primary outcome sensitivity analyses
Figure 8 shows the range of sensitivity analyses performed. Non-inferiority was shown in every case apart from when unlikely scenarios were used, that is when it was assumed that all SIMS participants missing at 15 months had a failure on the primary outcome and that all SMUS participants missing at 15 months had a success. Therefore, the primary outcome data are robust to missing data. The effect size from multiple imputation is close to the effect size in the complete-case analysis. The pNI-values in Figure 8 are non-inferiority p-values.
FIGURE 8.
Sensitivity analyses of the primary outcome.
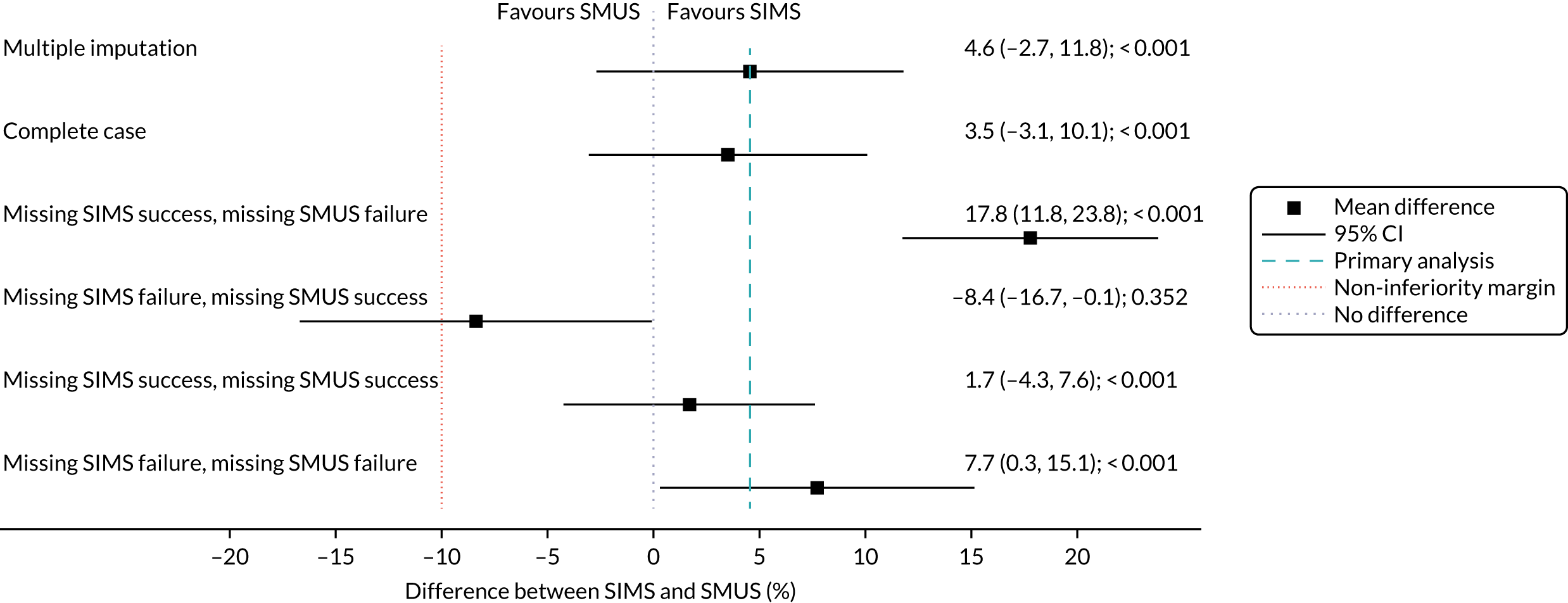
Subgroup analyses of the primary outcome
Subgroup analyses were performed to compare the following:
-
urodynamic diagnoses – SUI versus MUI
-
age – < median (i.e. 48 years) versus ≥ median
-
age – < 65 years versus ≥ 65 years (post hoc)
-
PFMT versus no PFMT (post hoc)
-
device availability – still available versus withdrawn from the market (post hoc).
The forest plot for the subgroups is shown in Figure 9, which shows the effect sizes in all of the subgroups. Table 8 provides a summary of the subgroups and includes the size of the interaction terms. The interaction is not statistically significant for any of the subgroups.
FIGURE 9.
Differences between treatments, by subgroup.
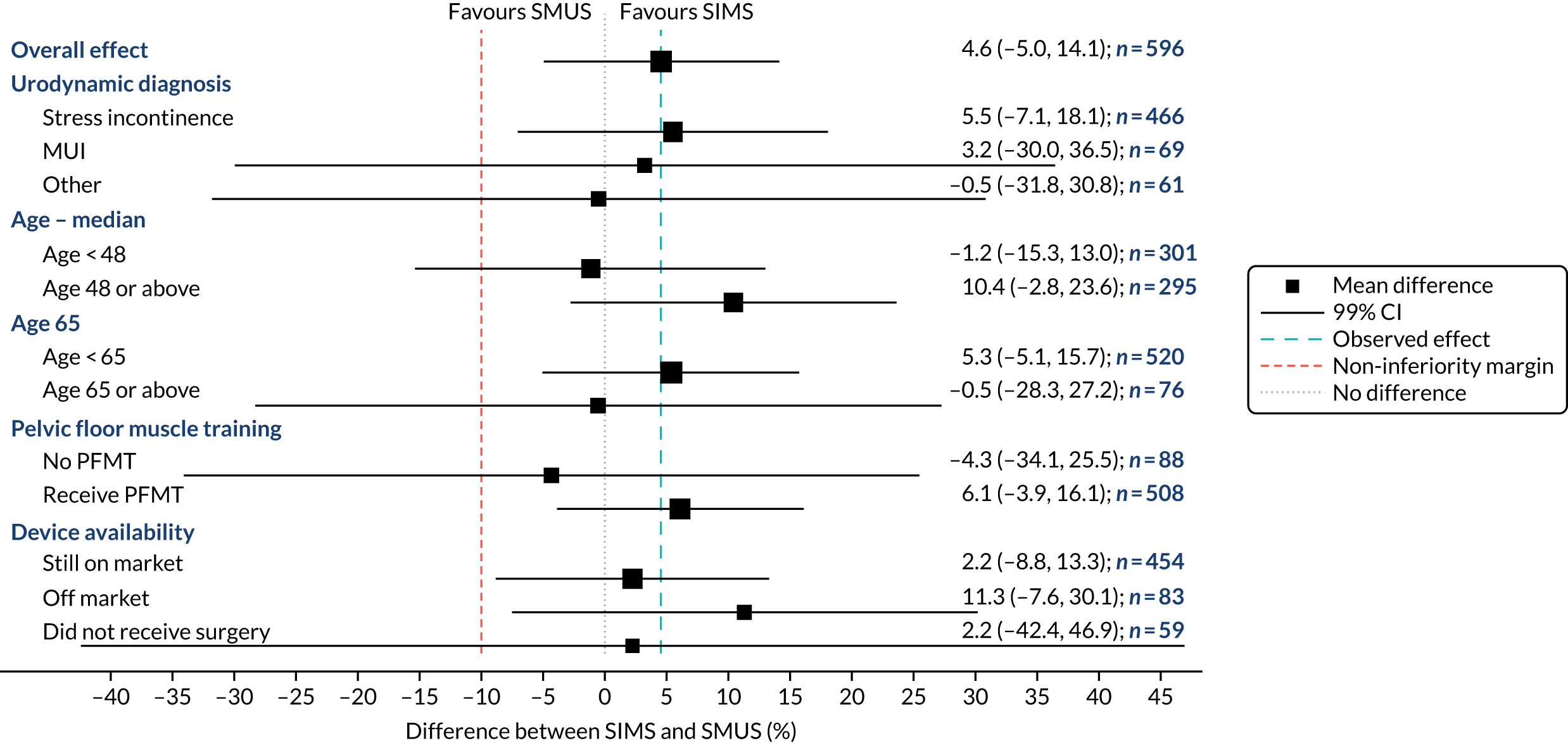
| Subgroup | Trial group, n/N (%) | Interaction term (99% CI); p-value | |
|---|---|---|---|
| SIMS | SMUS | ||
| Urodynamic diagnosis | |||
|
172/217 (79) | 151/203 (74) | –2.3 (–40.7 to 36.1); 0.56 |
|
24/31 (77) | 21/28 (75) | |
|
16/20 (80) | 17/19 (89) | |
| Age (years) | |||
|
99/131 (76) | 102/129 (79) | 11.6 (–8.4 to 31.5); 0.068 |
|
113/137 (82) | 87/121 (72) | |
|
188/235 (80) | 166/218 (76) | –5.9 (–36.2 to 24.5); 0.69 |
|
24/33 (73) | 23/32 (72) | |
| PFMT | |||
|
28/39 (72) | 28/36 (78) | 10.4 (–20.3 to 41.1); 0.19 |
|
184/229 (80) | 161/214 (75) | |
| Device availability | |||
|
157/200 (79) | 176/229 (77) | 9.1 (–13.9 to 32.1); 0.15 |
|
54/64 (84) | 12/16 (75) | |
|
1/4 (25) | 1/5 (20) | |
Most participants had urodynamic stress incontinence [SIMS group, 79% (n = 235); SMUS group, 78% (n = 231)], rather than urodynamic mixed incontinence [SIMS group, 12% (n = 36); SMUS group, 11% (n = 33)]. In both subgroups, the success rates were higher among those receiving a SIMS, but the difference is smaller in the urodynamic mixed incontinence subgroup.
The median age of participants was 48 years. Of those aged > 48 years, there was a higher success rate among those who received SIMSs, whereas, of those aged < 48 years, the success rate among those who received a SMUS was slightly higher. We presented another subgroup analysis according to age, which is clinically relevant: among those aged < 65 years, it appears that SIMSs have a higher success rate than SMUSs, whereas, among those aged ≥ 65 years, it appears that there is very little difference between the devices.
Only a small number of participants had not received PFMT. Of the women who did receive PFMT, there is a higher success rate in the SIMS group. Table 8 shows a large amount of uncertainty around the size of this interaction, so the difference in effects is due to the small sample size.
The post hoc subgroup analysis compared devices still available for clinical practice with those that are no longer available (see Table 8). In both subgroups, non-inferiority of the SIMS was confirmed.
Secondary outcomes
Patient-reported cure and objective success are reported in Table 9. We used a post hoc strict definition for patient-reported cure by utilising the first two questions in the ICIQ-UI-SF: if a participant responded that they never leak and the amount they leak was none, this was classed as cured. At all time points, the percentage cured was higher in the SIMS group, and the effect sizes and CIs show that SIMSs appear to be non-inferior to SMUSs.
| Cure and success | Trial group, n/N (%) | Effect size (95% CI); pNI-value | |
|---|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | ||
| Patient-reported cure | |||
| 3 months | 114/252 (45) | 83/224 (37) | 6.5 (–0.6 to 13.7); < 0.001 |
| 15 months | 93/241 (39) | 72/217 (33) | 6.4 (–1.2 to 13.9); < 0.001 |
| 24 months | 91/222 (41) | 76/205 (37) | 3.3 (–7.1 to 13.7); 0.006 |
| 36 months | 68/210 (32) | 62/202 (31) | 4.1 (–4.0 to 12.2); < 0.001 |
| Objective success (24-hour pad test) | |||
| 15 months | 102/119 (86) | 83/110 (75) | 5.2 (–5.9 to 16.2); 0.004 |
| 24 months | 99/114 (87) | 78/91 (86) | 6.3 (–2.4 to 15.1); < 0.001 |
| 36 months | 75/87 (86) | 64/79 (81) | 3.7 (–5.0 to 12.4); 0.001 |
Objective success was a participant with a 24-hour pad test weight gain of < 8 g. Participants were asked to complete a pad test only when they returned a completed participant questionnaire at the relevant time point. Owing to the COVID-19 pandemic, 22 participants were not sent a pad test to complete at 36 months. At all time points, the success rate was higher for the SIMS group, and the effect sizes indicate SIMSs are non-inferior to SMUS devices.
The full PGI-I scale responses at 4 weeks and at 3, 15, 24 and 36 months are shown in Appendix 4, Table 33. According to the responses to the seven-point PGI-I scale, there is a beneficial effect from receiving a SIMS, although the CI shows that the difference is not significant.
Quality of life and sexual function
The EQ-5D-3L scores increased from baseline to peak at 3 months; at 36 months, the EQ-5D-3L scores in both groups were lower than at baseline. Across all the ICIQ-LUTSqol outcomes, the pattern was similar: small differences favouring the SIMS, but with considerable uncertainty and no clear signal that one treatment was better than the other.
The PISQ-IR sexual function scores showed a small improvement from baseline to 15 months in both groups, although this improvement then diminished at 24 and 36 months. The effect size favours the SMUS group, although the difference is small with a CI that excludes a significant difference. The patient-reported secondary outcomes are shown in Table 10.
| Outcome and time point | Trial group | Difference (95% CI); p-value (superiority) | |
|---|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | ||
| QoL | |||
| EQ-5D-3L score | |||
| Baseline | 0.860 (0.200) [n = 286] | 0.834 (0.249) [n = 284] | |
| 4 weeks | 0.866 (0.166) [n = 239] | 0.838 (0.212) [n = 226] | 0.026 (–0.006 to 0.058); 0.11 |
| 3 months | 0.878 (0.194) [n = 255] | 0.855 (0.254) [n = 226] | 0.019 (–0.022 to 0.059); 0.36 |
| 15 months | 0.848 (0.243) [n = 249] | 0.825 (0.300) [n = 219] | 0.022 (–0.018 to 0.062); 0.28 |
| 24 months | 0.865 (0.244) [n = 232] | 0.816 (0.324) [n = 212] | 0.035 (–0.006 to 0.077); 0.097 |
| 36 months | 0.836 (0.261) [n = 217] | 0.821 (0.294) [n = 205] | 0.013 (–0.030 to 0.056); 0.55 |
| ICIQ-LUTSqol score | |||
| Baseline | 46.9 (11.7) [n = 286] | 46.6 (10.7) [n = 276] | |
| 3 months | 26.5 (10.2) [n = 237] | 27.9 (11.5) [n = 210] | –1.5 (–3.4 to 0.3); 0.10 |
| 15 months | 26.6 (10.2) [n = 230] | 27.6 (10.5) [n = 202] | –0.7 (–2.5 to 1.1); 0.43 |
| 24 months | 26.6 (11.0) [n = 211] | 28.4 (12.3) [n = 187] | –1.7 (–3.5 to 0.0); 0.057 |
| 36 months | 27.4 (10.7) [n = 203] | 28.3 (11.4) [n = 181] | –1.1 (–3.1 to 0.8); 0.24 |
| Sexual function | |||
| PISQ-IR score | |||
| Baseline | 3.3 (0.6) [n = 87] | 3.3 (0.6) [n = 91] | |
| 15 months | 3.7 (0.5) [n = 75] | 3.7 (0.5) [n = 55] | 0 (–0.2 to 0.1); 0.55 |
| 24 months | 3.7 (0.5) [n = 64] | 3.6 (0.6) [n = 54] | 0 (–0.1 to 0.1); 0.90 |
| 36 months | 3.6 (0.6) [n = 62] | 3.5 (0.6) [n = 54] | 0 (–0.1 to 0.1); 0.92 |
| Other urinary symptoms | |||
| ICIQ-UI-SF score | |||
| Baseline | 14.4 (3.3) [n = 284] | 14.4 (3.6) [n = 285] | |
| 3 months | 2.8 (4.7) [n = 234] | 3.3 (5.0) [n = 212] | –0.7 (–1.5 to 0.2); 0.14 |
| 15 months | 4.4 (5.0) [n = 219] | 4.7 (5.0) [n = 200] | –0.4 (–1.2 to 0.5); 0.40 |
| 24 months | 4.1 (4.8) [n = 197] | 4.9 (5.2) [n = 190] | –0.7 (–1.6 to 0.1); 0.088 |
| 36 months | 4.9 (4.8) [n = 195] | 5.3 (5.2) [n = 187] | –0.5 (–1.4 to 0.4); 0.29 |
| ICIQ-FLUTS filling score | |||
| Baseline | 4.5 (2.7) [n = 291] | 4.9 (2.8) [n = 284] | |
| 15 months | 3.4 (2.4) [n = 247] | 3.5 (2.5) [n = 220] | 0.1 (–0.3 to 0.5); 0.52 |
| 24 months | 3.2 (2.5) [n = 221] | 3.7 (2.6) [n = 206] | –0.3 (–0.7 to 0.1); 0.16 |
| 36 months | 3.6 (2.4) [n = 214] | 3.6 (2.4) [n = 199] | –0.0 (–0.4 to 0.4); 0.93 |
| ICIQ-FLUTS voiding score | |||
| Baseline | 1.9 (2.0) [n = 293] | 1.7 (2.0) [n = 286] | |
| 15 months | 2.1 (2.3) [n = 248] | 2.1 (2.1) [n = 217] | 0.0 (–0.4 to 0.3); 0.84 |
| 24 months | 1.9 (2.1) [n = 224] | 2.0 (2.0) [n = 210] | –0.1 (–0.5 to 0.3); 0.61 |
| 36 months | 1.9 (2.1) [n = 215] | 2.0 (2.1) [n = 199] | –0.1 (–0.5 to 0.2); 0.49 |
| ICIQ-FLUTS incontinence score | |||
| Baseline | 11.0 (3.0) [n = 284] | 11.4 (3.1) [n = 286] | |
| 15 months | 3.9 (4.1) [n = 241] | 4.4 (4.3) [n = 215] | –0.2 (–0.9 to 0.4); 0.49 |
| 24 months | 3.8 (3.9) [n = 221] | 4.1 (4.3) [n = 202] | –0.3 (–1.0 to 0.4); 0.40 |
| 36 months | 4.4 (4.2) [n = 211] | 4.5 (4.3) [n = 197] | –0.2 (–1.0 to 0.5); 0.52 |
Other urinary questionnaires’ scores
For all ICIQ-FLUTS domains, the between-group differences were small, and CIs were incompatible with a worthwhile difference favouring either treatment. For both the filling and incontinence domains, there were sizeable improvements at 36 months, compared with baseline, in both groups. However, the voiding domain did not show this trend, and there was a slight deterioration from baseline in the SMUS group.
Urgency perception as assessed by the UPS at 15, 24 and 36 months is shown in Table 11. At all time points, participants in the SIMS group reported less urgency. For urgency perception at 15 months, the OR was 1.3 (95% CI 0.8 to 2.0; p = 0.26); at 36 months, the effect size was (OR) 1.1 (95% CI 0.7 to 1.6; p = 0.81). These effect sizes favour the SIMS group, suggesting less urgency, but the CI excludes a significant effect.
| Urgency | Trial group, n (%) or n/N (%) | Effect sizea (95% CI) | |
|---|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | ||
| Urgency perception | |||
| 3 months | N = 253 | N = 228 | |
| No urgency | 75 (30) | 66 (29) | 1.1 (0.7 to 1.7) |
| Mild urgency | 111 (44) | 94 (41) | |
| Moderate urgency | 48 (19) | 48 (21) | |
| Severe urgency | 19 (7.5) | 20 (8.8) | |
| Not answered | 45/298 (15) | 70/298 (23) | |
| 15 months | N = 250 | N = 220 | |
| No urgency | 72 (29) | 59 (27) | 1.3 (0.8 to 2.0) |
| Mild urgency | 118 (47) | 92 (42) | |
| Moderate urgency | 42 (17) | 45 (20) | |
| Severe urgency | 18 (7) | 24 (11) | |
| Not answered | 48/298 (16) | 78/298 (26) | |
| 24 months | N = 232 | N = 213 | |
| No urgency | 84 (36) | 60 (28) | 1.4 (1.1 to 1.9) |
| Mild urgency | 92 (40) | 86 (40) | |
| Moderate urgency | 41 (18) | 43 (20) | |
| Severe urgency | 15 (6.5) | 24 (11) | |
| Not answered | 66/298 (22) | 85/298 (29) | |
| 36 months | N = 211 | N = 204 | |
| No urgency | 57 (27) | 58 (28) | 1.1 (0.7 to 1.6) |
| Mild urgency | 93 (44) | 77 (38) | |
| Moderate urgency | 44 (21) | 47 (23) | |
| Severe urgency | 17 (8.1) | 22 (11) | |
| Not answered | 87/298 (29) | 94/298 (32) | |
| Impact on urgency | |||
| 3 months | |||
| Cureb | 53/208 (25) | 48/191 (25) | 1.0 (0.7 to 1.5) |
| Improvedc | 73/208 (35) | 66/191 (35) | 1.0 (0.6 to 1.6) |
| No change | 66/208 (32) | 56/191 (29) | 1.1 (0.8 to 1.5) |
| Worsenedd | 16/208 (7.7) | 21/191 (11) | 0.7 (0.2 to 2.0) |
| New onsete | 22/49 (45) | 18/40 (45) | 1.0 (0.3 to 2.7) |
| Missing | 36/244 (15) | 56/247 (23) | |
| 15 months | |||
| Cureb | 51/206 (25) | 41/187 (22) | 1.2 (0.7 to 1.9) |
| Improvedc | 75/206 (36) | 58/187 (31) | 1.3 (0.7 to 2.3) |
| No change | 65/206 (32) | 65/187 (35) | 0.9 (0.5 to 1.6) |
| Worsenedd | 15/206 (7.3) | 23/187 (12) | 0.6 (0.3 to 1.3) |
| New onsete | 20/49 (41) | 14/40 (35) | 1.3 (0.5 to 3.5) |
| Missing | 38/244 (16) | 60/247 (24) | |
| 24 months | |||
| Cureb | 61/189 (32) | 42/181 (23) | 1.6 (1.0 to 2.3) |
| Improvedc | 59/189 (31) | 52/181 (29) | 1.1 (0.7 to 1.7) |
| No change | 55/189 (29) | 63/181 (35) | 0.8 (0.5 to 1.3) |
| Worsenedd | 14/189 (7.4) | 24/181 (13) | 0.5 (0.2 to 1.2) |
| New onsete | 18/49 (37) | 13/40 (33) | 1.2 (0.5 to 2.7) |
| Missing | 55/244 (23) | 66/247 (27) | |
| 36 months | |||
| Cureb | 38/171 (22) | 40/174 (23) | 1.0 (0.5 to 1.7) |
| Improvedc | 58/171 (34) | 53/174 (30) | 1.2 (0.8 to 1.8) |
| No change | 59/171 (35) | 54/174 (31) | 1.2 (0.7 to 2.0) |
| Worsenedd | 16/171 (9.4) | 27/174 (16) | 0.6 (0.3 to 1.1) |
| New onsete | 20/49 (41) | 12/40 (30) | 1.5 (0.7 to 3.5) |
| Missing | 73/244 (30) | 73/247 (30) | |
Of the women with preoperative urgency at baseline, the SIMS group participants appear more likely to report postoperative cure/improvement in urgency at almost all follow-up time points. However, the effect sizes rule out a significant difference between groups (see Table 11).
New-onset urgency was observed for only 89 participants. The SIMS participants appear more likely to develop new-onset urgency; however, the effect sizes rule out a significant difference between groups (see Table 11).
Operative outcomes
Table 12 shows operative outcomes. The procedure time for those receiving a SIMS was slightly shorter than for those receiving a SMUS. The postoperative stay was shorter for the SIMS group and the analysis of pain scores over the 14 days post operation also shows significantly lower pain scores in the SIMS group. Although the difference is not statistically significant, the blood loss for those receiving a SIMS does appear to be lower.
| Operative outcome | Trial group | Effect size (95% CI); p-value (superiority) | |
|---|---|---|---|
| SIMS (N = 276) | SMUS (N = 261) | ||
| Blood loss, n (%) | |||
|
134 (49) | 107 (41) | 0.72 (0.48 to 1.08); 0.11 |
|
126 (46) | 129 (49) | |
|
15 (5.4) | 23 (8.8) | |
|
1 (0.36) | 2 (0.77) | |
| Procedure time (minutes), mean (SD) | 39.2 (16.8) [n = 273] | 41.3 (11.6) [n = 258] | –2.2 (–5.9 to 1.6); 0.25 |
| Postoperative hospital stay (hours), mean (SD) | 7.2 (8.7) [n = 276] | 9.7 (10.7) [n = 261] | –2.5 (–4.7 to –0.3); 0.029 |
| Pain score up to 14 days post operation, mean (SD) | 19.8 (19.6) [n = 238] | 28.1 (22.2) [n = 213] | –8.3 (–12.8 to –3.8); 0.001 |
| Pain score up to 14 days post operation (text messaging), mean (SD) | 15.8 (14.0) [n = 114] | 26.0 (19.4) [n = 121] | –10.2 (–14.2 to –6.1); < 0.001 |
| Use of analgesia up to 14 days post operation (days), mean (SD) | 2.7 (3.6) [n = 238] | 3.5 (3.8) [n = 213] | 0.79 (0.64 to 0.98); 0.029 |
| Satisfactory voiding without any intervention, n/N (%) | 230/276 (83) | 206/261 (79) | 1.31 (0.68 to 2.54); 0.42 |
| Return to normal activities within 28 days, n/N (%) | 185/246 (75) | 160/226 (71) | 1.24 (0.86 to 1.80); 0.25 |
Table 12 also shows that women in the SIMS group were slightly more likely to have satisfactory voiding and to have returned to normal activities within 28 days, but there is a level of uncertainty around the estimates of the effect sizes.
Chapter 5 Safety data
This chapter reports the AEs recorded in the trial up to the 36-month follow-up point. The AEs that occurred during surgery and those that were self-reported by participants are included. Additional hospital visits and surgeries are also reported.
Adverse events
Adverse events reported by all collaborating teams/hospitals to have occurred during surgery and AEs reported by the participants at their follow-up are shown in Table 13.
| AEs | Trial group, n (%) | |
|---|---|---|
| SIMS (N = 276) | SMUS (N = 261) | |
| Operative | ||
| Any operative AE | 25 (9.0) | 20 (7.6) |
| Bladder injurya | – | 9 (3.4) |
| Urethral injury | – | 1 (0.38) |
| Blood loss of > 200 ml | 5 (1.8) | 5 (1.9) |
| GA complications | 1 (0.36) | – |
| Vaginal buttonhole | 6 (2.2) | 3 (1.1) |
| Need to use more than one kit | 7 (2.5) | |
| Anaesthesia changed | 7 (2.5) | 1 (0.38) |
| Need to insert trocar more than once | 1 (0.36) | – |
| Anaphylactic reaction to antibiotics | 1 (0.36) | – |
| Skin reaction in the area of surgery | – | 1 (0.38) |
| Intraoperative tonic–clonic seizure | 1 (0.36) | – |
| Self-catheterisation (CISC) | ||
| 3 months | 3 (1.1) | 7 (2.7) |
| 15 months | 2 (0.77) | |
| 24 months | 3 (1.1) | 3 (1.1) |
| 36 months | 3 (1.1) | 4 (1.5) |
| Groin or thigh pain | ||
| 3 months | 70 (25) | 56 (21) |
| 15 months | 41 (15) | 31 (12) |
| 24 months | 35 (13) | 29 (11) |
| 36 months | 39 (14) | 39 (15) |
| Use of analgesia | ||
| 3 months | 40 (14) | 22 (8.4) |
| 15 months | 24 (8.7) | 13 (5.0) |
| 24 months | 19 (6.9) | 12 (4.6) |
| 36 months | 21 (7.6) | 12 (4.6) |
| Tape/mesh exposure | ||
| 3 months | 5 (1.8) | 3 (1.1) |
| 15 months | 2 (0.72) | 2 (0.77) |
| 24 months | 1 (0.36) | – |
| 36 months | 1 (0.36) | – |
| Recurrent UTI | ||
| 3 months | 17 (6.2) | 15 (5.7) |
| 15 months | 30 (11) | 20 (7.7) |
| 24 months | 22 (8.0) | 19 (7.3) |
| 36 months | 21 (7.6) | 23 (8.8) |
| Indwelling catheter | ||
| 15 months | 1 (0.36) | 2 (0.77) |
| 36 months | 1 (0.36) | |
| Medications | N = 298 | N = 298 |
| Using anticholinergic drugs | ||
| 15 months | 33 (11) | 26 (8.7) |
| 24 months | 20 (6.7) | 22 (7.4) |
| 36 months | 19 (6.4) | 20 (6.7) |
| Using prophylactic low-dose antibiotics | ||
| 15 months | 6 (2.0) | 5 (1.7) |
| 24 months | 10 (3.4) | 7 (2.3) |
| 36 months | 6 (2.0) | 7 (2.3) |
| Using over-the-counter medicine for UI | ||
| 15 months | 3 (1.0) | 3 (1.0) |
| 24 months | 1 (0.34) | 1 (0.34) |
| 36 months | 2 (0.67) | 2 (0.67) |
| Other AEs | N = 298 | N = 298 |
| Sudden death at home as a result of drug overdose | – | 1 (0.34) |
| Admitted with right-sided weakness, facial droop and slurred speech. Consistent with a TIA | – | 1 (0.34) |
| Overdose of paracetamol | – | 1 (0.34) |
| Chemotherapy for secondary lung cancer | – | 1 (0.34) |
| Sexual functioning | N = 145 | N = 145 |
| Dyspareunia | ||
| 15 months | 25 (17) | 8 (5.5) |
| 24 months | 16 (11) | 8 (5.5) |
| 36 months | 17 (11) | 7 (4.8) |
| Coital incontinence | ||
| 15 months | 16 (11) | 7 (4.8) |
| 24 months | 12 (8.3) | 14 (9.7) |
| 36 months | 16 (11) | 7 (4.8) |
Operative AEs were reported in both groups. A total of 26 SIMS and 22 SMUS participants had operative AEs. Two participants in the SIMS group had two operative AEs; both required insertion of more than one device and had a change of anaesthesia to GA.
Nine out of 261 (3.4%) SMUS group participants experienced a bladder injury; no participants in the SIMS group experienced a bladder injury. The incidence of blood loss of > 200 ml was similar in both groups [SMUS, n = 5 (1.8%); SIMS, n = 5 (1.9%)]. Although the rates were low, vaginal buttonhole and the need to use more than one kit were more common in the SIMS group. In addition, the need to change anaesthetic was more common in the SIMS group [n = 7 (2.5%) vs. n = 1 (0.4%) in the SIMS and SMUS groups, respectively], possibly because SIMS participants were more likely to have had their procedure under LA.
The need for self-catheterisation was greater in the SMUS group at the earlier follow-up points, but by 24 and 36 months the rates were similar in both groups.
Groin or thigh pain was greater in the SIMS group initially; however, by 24 and 36 months any difference was smaller. Indeed, by 36 months, there was a slightly higher rate of groin or thigh pain among the SMUS participants. The analysis shows the differences in groin or thigh pain between the SIMS and SMUS groups: at 15 months, pain was 14.9% and 11.9% (RD 3.0, 95% CI –1.1 to 7.1; p = 0.144) in the SIMS and SMUS groups, respectively, and, at 36 months, it was 14.1% and 14.9% (RD –0.8, 95% CI –4.1 to 2.5; p = 0.613) in the SIMS and SMUS groups, respectively. The rate of use of analgesics at 15 months was 8.7% in the SIMS group and 5% in the SMUS group (RD 3.7, 95% CI 0.0 to 7.4; p = 0.047); at 36 months, it was 7.6% in the SIMS group and 4.6% in the SMUS group (RD 3.0, 95% CI –0.4 to 6.4; p = 0.081).
Tape/mesh exposure rates were higher for SIMS participants [9/276 (3.3%)] than for SMUS participants [5/261 (1.9%)] over the 36 months of follow-up (RD 1.3, 95% CI –1.7 to 4.4; p = 0.373). The difference in rates of exposure was initially higher in the SIMS group than in the SMUS group at 3 months [5/276 (1.8%) vs. 3/261 (1.1%) for the SIMS and SMUS groups, respectively]; it was similar at 15 months [2/276 (0.7%) vs. 2/261 (0.8%) for the SIMS and SMUS groups, respectively] and fell in both arms at 24 months [1/276 (0.4%) vs. 0/261 (0%) for the SIMS and SMUS groups, respectively] and 36 months [1/276 (0.4%) vs. 0/261 (0%) for the SIMS and SMUS groups, respectively]. One participant had persistent tape/mesh exposure following a procedure to cover the exposed mesh with vaginal walls; she was then treated with local excision of the exposed portion.
The rate of anticholinergic drug use was higher among SIMS participants at 15 months, but, by 24 and 36 months, the rate had decreased in both arms and was slightly higher among SMUS participants.
Rates of dyspareunia and coital incontinence were higher in the SIMS group at almost all time points, including at baseline. The rate of dyspareunia was 17.2% and 5.5% in the SIMS and SMUS groups, respectively (RD 11.8, 95% CI 3.5 to 20.1; p = 0.008), at 15 months; at 36 months, it was 11.7% in the SIMS group and 4.8% in the SMUS group (RD 7.0, 95% CI 1.9 to 12.1; p = 0.010). There was a similar trend for the rate of coital incontinence: at both 15 and 36 months, it was 11% in the SIMS group and 4.8% in the SMUS group (RD 6.0, 95% CI –0.9 to 12.9; p = 0.084). Nine SIMS and two SMUS participants reported dyspareunia at both time points, and 10 SIMS and three SMUS participants reported coital incontinence at both time points.
Similar rates were reported between groups at 15 and 36 months for recurrent UTI [RD 3.2 (95% CI –2.7 to 9.1; p = 0.268) and RD –1.2 (95% CI –5.5 to 3.0; p = 0.553), respectively] and for use of anticholinergic drugs [RD 2.3 (95% CI –2.2 to 6.9; p = 0.297) and RD –0.3 (95% CI –4.1 to 3.4; p = 0.854), respectively].
Additional consultations and surgeries
Additional hospital visits and consultations are reported in Table 14. Forty-one SIMS participants and 36 SMUS participants reported making additional relevant visits/consultations to either primary or secondary care. SMUS participants were more likely to attend the ward/outpatient clinic because of complete or incomplete urinary retention, mainly up to 15 months of follow-up. Only two additional participants had hospital visits for urinary retention between 15 and 36 months. Four of the participants had multiple (3–19) hospital visits.
| Additional hospital consultations | Trial group, n (%) | |
|---|---|---|
| SIMS (N = 276) | SMUS (N = 261) | |
| Hospital visit because of urinary retention | 3 (1.1) | 8 (3.1) |
| Overnight hospital stay because of urinary retention | 3 (1.2) | |
| Hospital visit with thigh pain | 7 (2.5) | 6 (2.3) |
| Overnight hospital stay because of thigh pain | 2 (0.72) | |
| Hospital visit with vagina pain | 11 (4.0) | 5 (1.9) |
| Overnight hospital stay because of vagina pain | 2 (0.77) | |
| Hospital visit with hip pain | 6 (2.2) | 2 (0.77) |
| Overnight hospital stay because of hip pain | 1 (0.38) | |
| Hospital visit with other pain | 6 (2.2) | 3 (1.1) |
| Overnight hospital stay because of other pain | 2 (0.72) | |
| Further investigations and assessments | N = 298 | N = 298 |
| Voiding assessment | 19 (6.4) | 23 (7.7) |
| Cystoscopy | 21 (7.0) | 14 (4.7) |
| Urodynamics | 13 (4.4) | 9 (3.0) |
| Additional surgical treatments | ||
| Laparoscopy in the postoperative period as a result of operative haematoma | – | 1/261 (0.38) |
| Additional treatments for UI | N = 276 | N = 261 |
| Botox® (Allergan plc, Dublin, Ireland) | 4 (1.4) | 3 (1.1) |
| Tibial nerve stimulation | 1 (0.36) | 1 (0.38) |
| Urethral bulking | 4 (1.4) | 1 (0.38) |
| Colposuspension | 1 (0.36) | 2 (0.77) |
| Autologous sling | 1 (0.36) | – |
| TO-TVT | 1 (0.36) | – |
| Additional surgical treatments for VD | ||
| Division/release of tape for VD | 1 (0.36) | 2 (0.77) |
| Additional surgical treatments for pain | ||
| Complete removal for pain | 3 (1.1) | – |
| Partial removal for pain | 1 (0.36) | 2 (0.77) |
| Sacroiliac joint/bilateral ischial tuberosity injection | 3 (1.1) | – |
| Additional surgical treatments for tape/mesh exposure | ||
| Partial removal/excision of eroded part of the tape/mesh | 4 (1.4) | 3 (1.1) |
| Recovering of the tape/mesh | 3 (1.1) | – |
The number of consultations because of pain was slightly higher in the SIMS group [24/276 (8.7%)] than in the SMUS group [16/261 (6.1%)], as shown in Table 14. A small number of participants (n = 10) reported four or more hospital visits over the 36 months, either for retention (n = 3) or pain (n = 7). These included two participants who reported 12 and 20 separate hospital visits for pain.
The rate of voiding assessment consultations was higher in the SMUS group, whereas cystoscopies and urodynamics were performed more often on SIMS participants. Although the rates of specific additional surgeries and treatments are relatively low, it can be seen that more SIMS participants required additional treatments for UI, pain and mesh exposure.
Additional surgical treatment
Additional surgical treatments are reported in Table 14. Twenty-four SIMS participants and 12 SMUS participants received surgical treatment over the 36-month period. These included further surgery for SUI [SIMS group, n = 7 (2.5%) vs. SMUS group, n = 3 (1.1%)] and complete or partial removal of tape/mesh because of pain [SIMS group, n = 4 (1.5%) vs. SMUS group, n = 2 (0.8%)] or because of mesh exposure [SIMS group, n = 4 (1.4%) vs. SMUS group, n = 3 (1.1%)].
Of the participants who received further treatment for SUI, four participants underwent surgery in the first year (TO-TVT, n = 1; autologous sling, n = 1; urethral bulking, n = 2), four underwent surgery between 1 and 2 years (colposuspension, n = 2; urethral bulking, n = 2) and two underwent surgery between 2 and 3 years (colposuspension, n = 1, urethral bulking, n = 1). All four participants who received colposuspension/autologous sling and one of the participants who received urethral bulking had previously received either a complete or partial tape removal. One participant in the SMUS group received tibial nerve stimulation and proceeded to receive Botox treatment.
Chapter 6 Patient-reported success and safety by procedure received
This chapter reports descriptive data on the primary and secondary outcomes, and safety data on the type of procedure received: Ajust, Altis, RP-TVT or TO-TVT. A total of 537 participants received surgery. Of the participants allocated to receive a SIMS procedure, 62 received Ajust. No SMUS participants received Ajust. In the SIMS group, 199 participants received an Altis sling, and four SMUS participants crossed over to receive Altis. In the SMUS group, 119 participants received a RP-TVT and 138 received a TO-TVT. Seven and five SIMS participants crossed over to receive a RP-TVT and a TO-TVT, respectively. The one SIMS participant who received an autologous fascial sling and the two SIMS participants who received MiniArc slings are not included in these summaries. We present descriptive data with no analysis performed.
Baseline characteristics
In Appendix 6, Tables 36 and 37, we present the baseline characteristics and data across all four groups. As we stated in the methods section, all surgeons were asked to identify their usual SMUS procedure (RP-TVT or TO-TVT) and their standard SIMS procedure (Altis, Ajust or other) to be used in the trial in advance of starting recruitment. Surgeons did not interchange between Ajust and Altis for the SIMS procedure, nor did they interchange between RP-TVT and TO-TVT for the SMUS procedure.
Primary outcome
The primary outcome is reported in Table 15. At 15 months (≈ 1 year post operation), the patient-reported success rate (defined as responses of very much or much improved on the PGI-I scale) was 83.6% (n = 51) for Ajust, 80% (n = 111) for TO-TVT, 78.5% (n = 150) for Altis and 73% (n = 84) for RP-TVT. By 36 months, the patient-reported success rate fell for all four procedures to 74% (n = 132) for Altis, 71% (n = 39) for Ajust, 69% (n = 74) for RP-TVT and 66% (n = 86) for TO-TVT.
| Outcome and time point | Procedure, n/N (%) | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| PGI-I scale: success | ||||
| 4 weeks | 45/56 (80) | 130/174 (75) | 71/97 (73) | 102/131 (78) |
| 3 months | 47/60 (78) | 156/185 (84) | 88/109 (81) | 104/127 (82) |
| 15 months | 51/61 (84) | 150/191 (79) | 84/115 (73) | 111/139 (80) |
| 24 months | 42/55 (76) | 136/172 (79) | 76/105 (72) | 95/123 (77) |
| 36 months | 39/55 (71) | 132/179 (74) | 74/107 (69) | 86/131 (66) |
| Patient-reported cure | ||||
| 3 months | 28/60 (47) | 82/183 (45) | 35/106 (33) | 50/124 (40) |
| 15 months | 25/54 (46) | 63/173 (36) | 34/101 (34) | 41/122 (34) |
| 24 months | 24/54 (44) | 64/158 (41) | 38/99 (38) | 40/110 (36) |
| 36 months | 21/49 (43) | 44/152 (29) | 34/95 (36) | 30/111 (27) |
| Objective success (24-hour pad test) | ||||
| 15 months | 19/21 (90) | 76/92 (83) | 36/46 (78) | 50/66 (76) |
| 24 months | 16/19 (84) | 79/90 (88) | 34/37 (92) | 45/56 (80) |
| 36 months | 11/13 (85) | 59/70 (84) | 26/31 (84) | 40/49 (82) |
Patient-reported cure, using the strict definition of no leakage reported on the ICIQ-UI-SF, is also shown in Table 15. The success rate for the Ajust sling does fall slightly from 3 months to 36 months, but is still 42.9% (n = 21) at 36 months. The drop in the cure rates in the same period is greater for both Altis and TO-TVT: at 3 months, the cure rates were 44.8% (n = 82) and 40.3% (n = 50), respectively, but, by 36 months, the cure rates were 28.9% (n = 44) and 27.0% (n = 30), respectively.
The objective success rates are based on the 24-hour pad test. Completion of the 24-hour pad test was low at all time points. Success rates are high for those who completed the test. Although the 15-month rates are high for the two SIMS procedures, by 36 months, the success rates are similar for all groups.
Secondary outcomes
The full breakdown of the seven-point PGI-I scale is shown in Appendix 6, Table 39. It is important to show the proportions both of the ‘improved’ subgroup and of the subgroups of those who reported no change or that the procedure made UI worse.
The summary statistics are mean, SD and number of participants.
Table 16 shows the EQ-5D-3L scores at baseline and at all possible follow-up points. There was a difference at baseline between the groups. In terms of the follow-up outcomes, all four groups had their highest EQ-5D-3L score at either 4 weeks or 3 months. There are fluctuations across time and between groups; the highest EQ-5D-3L score at 36 months is for Altis participants.
| Outcome and time point | Procedure, mean (SD) [n] | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| EQ-5D-3L score | ||||
| Baseline | 0.811 (0.246) [60] | 0.883 (0.173) [193] | 0.828 (0.251) [123] | 0.828 (0.256) [147] |
| 4 weeks | 0.864 (0.210) [55] | 0.870 (0.148) [174] | 0.843 (0.186) [102] | 0.831 (0.231) [131] |
| 3 months | 0.859 (0.227) [60] | 0.889 (0.183) [185] | 0.852 (0.253) [108] | 0.851 (0.252) [125] |
| 15 months | 0.817 (0.289) [56] | 0.868 (0.211) [179] | 0.832 (0.297) [101] | 0.807 (0.314) [124] |
| 24 months | 0.831 (0.308) [53] | 0.874 (0.224) [169] | 0.846 (0.282) [98] | 0.794 (0.351) [117] |
| 36 months | 0.782 (0.320) [51] | 0.856 (0.233) [158] | 0.842 (0.255) [96] | 0.791 (0.329) [112] |
| ICIQ-UI-SF score | ||||
| Baseline | 15.1 (3.1) [62] | 14.2 (3.3) [189] | 14.8 (3.5) [124] | 14.3 (3.7) [145] |
| 3 months | 4.3 (5.5) [59] | 3.7 (4.5) [167] | 4.7 (5.1) [97] | 3.8 (4.6) [120] |
| 15 months | 4.1 (5.0) [48] | 4.4 (4.8) [157] | 4.7 (5.2) [97] | 4.5 (4.7) [110] |
| 24 months | 4.3 (5.3) [49] | 3.9 (4.7) [139] | 5.3 (5.7) [90] | 4.4 (4.6) [104] |
| 36 months | 4.8 (5.2) [45] | 5.0 (4.8) [140] | 4.9 (5.0) [91] | 5.3 (5.0) [101] |
| ICIQ-FLUTS filling score | ||||
| Baseline | 5.1 (2.8) [62] | 4.3 (2.6) [194] | 5.0 (3.2) [124] | 4.7 (2.6) [145] |
| 15 months | 3.7 (2.7) [57] | 3.4 (2.5) [178] | 3.5 (2.5) [100] | 3.4 (2.3) [125] |
| 24 months | 3.4 (2.5) [50] | 3.3 (2.5) [161] | 3.8 (2.5) [97] | 3.6 (2.6) [113] |
| 36 months | 3.8 (2.5) [51] | 3.5 (2.5) [155] | 3.5 (2.4) [94] | 3.6 (2.3) [108] |
| ICIQ-FLUTS voiding score | ||||
| Baseline | 1.9 (2.0) [61] | 1.7 (1.9) [197] | 1.8 (2.1) [125] | 1.7 (2.0) [146] |
| 15 months | 2.3 (2.7) [56] | 2.0 (2.2) [180] | 2.2 (2.2) [99] | 2.2 (2.2) [123] |
| 24 months | 2.1 (2.3) [50] | 1.8 (2.0) [164] | 1.9 (1.9) [99] | 2.1 (2.1) [115] |
| 36 months | 2.3 (2.3) [51] | 1.7 (2.0) [156] | 1.9 (2.1) [94] | 2.2 (2.1) [108] |
| ICIQ-FLUTS incontinence score | ||||
| Baseline | 11.7 (3.3) [58] | 10.9 (2.9) [193] | 11.5 (3.2) [125] | 11.3 (3.1) [145] |
| 15 months | 3.6 (4.2) [56] | 3.9 (4.0) [173] | 4.6 (4.9) [98] | 4.1 (3.7) [122] |
| 24 months | 3.6 (4.2) [51] | 3.8 (3.9) [160] | 4.3 (4.7) [95] | 3.8 (3.9) [111] |
| 36 months | 4.1 (4.7) [51] | 4.6 (4.1) [152] | 4.2 (4.5) [93] | 4.7 (4.0) [107] |
| ICIQ-LUTSqol score | ||||
| Baseline | 49.9 (11.7) [59] | 46.0 (11.4) [193] | 48.2 (11.7) [123] | 46.1 (10.1) [139] |
| 3 months | 28.5 (14.0) [55] | 26.0 (8.9) [175] | 29.0 (12.1) [98] | 26.7 (10.5) [117] |
| 15 months | 26.2 (9.1) [53] | 26.6 (10.6) [166] | 28.6 (12.0) [89] | 26.7 (9.0) [118] |
| 24 months | 27.5 (12.3) [47] | 26.3 (10.7) [154] | 29.6 (13.4) [86] | 27.2 (11.0) [106] |
| 36 months | 27.7 (11.9) [48] | 27.5 (10.7) [148] | 28.8 (12.2) [87] | 27.6 (10.1) [97] |
| PISQ-IR sexual function score | ||||
| Baseline | 3.2 (0.7) [18] | 3.4 (0.6) [56] | 3.3 (0.6) [38] | 3.2 (0.6) [45] |
| 15 months | 3.5 (0.6) [19] | 3.8 (0.4) [51] | 3.8 (0.4) [30] | 3.6 (0.6) [28] |
| 24 months | 3.7 (0.6) [15] | 3.7 (0.5) [45] | 3.7 (0.6) [28] | 3.7 (0.6) [28] |
| 36 months | 3.4 (0.7) [14] | 3.6 (0.6) [45] | 3.6 (0.6) [28] | 3.5 (0.6) [27] |
The ICIQ-UI-SF is a measure of symptoms severity and impact on QoL. In all groups, there is a large improvement between baseline and 3 months in the ICIQ-UI-SF score. Scores seem stable in all four groups (see Appendix 6, Table 40) over the 36 months, indicating stability in improvement of SUI. Although there are again fluctuations between groups and across time, the observed differences between the groups at 15 months and 36 months seem to be small.
For the ICIQ-FLUTS domains, there is a small improvement for all four groups on the filling domain across the follow-up time points. There is a slight worsening in the voiding score between baseline and 36 months for the Ajust and TO-TVT participants. All four groups show sizeable improvements from baseline over the 36-month follow-up period on the ICIQ-FLUTS incontinence score.
There was an improvement in the ICIQ-LUTSqol scores from baseline across all groups. Similarly, there were changes across time on the PISQ-IR sexual functioning score. In all four groups, there was a small improvement from baseline to 36 months.
Appendix 6, Table 41, also presents all participants’ responses to the UPS at baseline and all follow-up points across all four groups. It was notable to see the relatively small percentage of participants in all groups (11–19%) who reported ‘no urgency’ at baseline, as assessed by the UPS. The percentages of participants reporting a degree of urgency at baseline were 85% (n = 53) in the Ajust group, 80% (n = 160) in the Altis group, 82% (n = 104) in the RP-TVT group and 89% (n = 131) in the TO-TVT group.
The impact of procedure on ‘urgency’ in all four groups at all follow-up points, as assessed by the UPS, is presented in Appendix 6, Table 41. The impact of a procedure on urinary urgency seems to start quite early. At 3 months, the percentages of those who reported cure of/improvement in preoperative urgency were relatively high in all groups: 53.8% (n = 28) for Ajust, 61.7% (n = 90) for Altis, 62.4% (n = 53) for RP-TVT and 58.4% (n = 66) for TO-TVT. At 36 months, the rates were stable for the Ajust and RP-TVT groups and reduced in the Altis and TO-TVT groups [59% (n = 23) for Ajust, 55% (n = 68) for Altis, 61% (n = 47) for RP-TVT and 48% (n = 48) for TO-TVT].
Safety
This section provides information on AEs occurring during surgery and those reported by participants in follow-up questionnaires. Further surgeries and consultations are also reported.
Adverse events
Adverse events during surgery and self-reported at follow-up are shown in Table 17. The numbers of RP-TVT and TO-TVT procedures are similar, but the table shows a higher frequency of bladder injuries for those receiving a RP-TVT. Instances of blood loss of > 200 ml and vaginal buttonholes are comparable across all four groups. Needing to use more than one kit is more common for SIMS devices.
| AEs | Procedure, n (%) | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| Operative | ||||
| Bladder injury | – | – | 8 (6.3) | 1 (0.68) |
| Urethral injury | – | – | 1 (0.79) | – |
| Blood loss of > 200 ml | 1 (1.6) | 4 (2.0) | 2 (1.6) | 3 (2.0) |
| Vaginal buttonhole | 1 (1.6) | 4 (2.0) | 2 (1.6) | 2 (1.4) |
| Need to use more than one kit | 1 (1.6) | 5 (2.5) | – | 1 (0.68) |
| Anaesthesia changed | – | 2 (1.0) | – | 6 (4.1) |
| Need to insert trocar more than once | – | 1 (0.50) | – | – |
| Anaphylactic reaction to antibiotics | – | 1 (0.50) | – | – |
| Skin reaction in the area of surgery | – | – | – | 1 (0.68) |
| Intraoperative tonic–clonic seizure | 1 (1.6) | – | – | – |
| Self-catheterisation (CISC) | ||||
| 3 months | – | 3 (1.5) | 5 (4.0) | 2 (1.4) |
| 15 months | – | – | 1 (0.79) | 1 (0.68) |
| 24 months | – | 3 (1.5) | 2 (1.6) | 1 (0.68) |
| 36 months | – | 3 (1.5) | 1 (0.79) | 3 (2.0) |
| Groin or thigh pain | ||||
| 3 months | 12 (19) | 53 (27) | 32 (25) | 28 (19) |
| 15 months | 4 (6.5) | 35 (18) | 13 (10) | 19 (13) |
| 24 months | 4 (6.5) | 32 (16) | 14 (11) | 14 (10) |
| 36 months | 7 (11) | 29 (15) | 21 (17) | 21 (14) |
| Use of analgesia | ||||
| 3 months | 4 (6.5) | 32 (16) | 13 (10) | 12 (8.2) |
| 15 months | 3 (4.8) | 18 (9.0) | 4 (3.2) | 11 (7.5) |
| 24 months | 3 (4.8) | 16 (8.0) | 5 (4.0) | 7 (4.8) |
| 36 months | 3 (4.8) | 17 (8.5) | 8 (6.3) | 5 (3.4) |
| Tape/mesh exposure | ||||
| 3 months | 2 (3.2) | 3 (1.5) | – | 3 (2.0) |
| 15 months | – | 2 (1.0) | – | 2 (1.4) |
| 24 months | – | 1 (0.50) | – | – |
| 36 months | – | 1 (0.50) | – | – |
| Recurrent UTI | ||||
| 3 months | 6 (9.7) | 11 (5.5) | 9 (7.1) | 6 (4.1) |
| 15 months | 8 (13) | 20 (10) | 14 (11) | 8 (5.4) |
| 24 months | 4 (6.5) | 16 (8.0) | 11 (8.7) | 10 (6.8) |
| 36 months | 2 (3.2) | 19 (9.5) | 10 (7.9) | 13 (8.8) |
| Using anticholinergic drugs | ||||
| 15 months | 12 (19) | 21 (11) | 8 (6.3) | 16 (11) |
| 24 months | 7 (11) | 14 (7.0) | 4 (3.2) | 17 (12) |
| 36 months | 10 (16) | 10 (5.0) | 8 (6.3) | 10 (6.8) |
| Using prophylactic low-dose antibiotics | ||||
| 15 months | 1 (1.6) | 4 (2.0) | 2 (1.6) | 3 (2.0) |
| 24 months | 1 (1.6) | 8 (4.0) | 4 (3.2) | 3 (2.0) |
| 36 months | 1 (1.6) | 5 (2.5) | 4 (3.2) | 3 (2.0) |
| Using over-the-counter medicine for UI | ||||
| 15 months | – | 3 (1.5) | 2 (1.6) | 1 (0.68) |
| 24 months | – | 1 (0.50) | 1 (0.79) | – |
| 36 months | – | 2 (1.0) | – | 2 (1.4) |
| Dyspareunia | N = 30 | N = 99 | N = 68 | N = 71 |
| 15 months | 3 (10) | 20 (20) | 5 (7.4) | 4 (5.6) |
| 24 months | 3 (10) | 12 (12) | 5 (7.4) | 4 (5.6) |
| 36 months | 4 (13) | 11 (11) | 5 (7.4) | 4 (5.6) |
| Coital incontinence | ||||
| 15 months | 2 (6.7) | 14 (14) | 3 (4.4) | 4 (5.6) |
| 24 months | 2 (6.7) | 11 (11) | 5 (7.4) | 8 (11) |
| 36 months | 3 (10) | 13 (13) | 4 (5.9) | 3 (4.2) |
A higher rate of CISC at earlier time points is seen in the RP-TVT group. By 36 months, CISC frequency is low in all groups (≤ 2%).
Groin and thigh pain have the highest frequency at both 15 and 24 months among the Altis participants; however, by 36 months, the rates are comparable in all groups [RP-TVT, 16.7% (n = 21); Altis, 14.6% (n = 29); TO-TVT, 14.3% (n = 21); and Ajust, 11.3% (n = 7)]. Analgesia use is observed to be highest in the Altis and RP-TVT groups, whereas the TO-TVT was least associated with use of analgesia [Altis, 8.5% (n = 17); RP-TVT, 6.3% (n = 8); Ajust, 4.8% (n = 3); and TO-TVT, 3.4% (n = 5)].
The rates of dyspareunia and coital incontinence were low in the TO-TVT and RP-TVT groups at 15 and 36 months’ follow-up. In the Altis group, there was almost a 50% reduction in dyspareunia rates at 36 months, compared with 15 months: 11.1% (n = 11) versus 20.2% (n = 20), respectively.
Further hospital consultations and procedures are reported in Table 18.
| Consultations and procedures | Procedure, n (%) | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| Further hospital consultations | ||||
| Hospital visit because of urinary retention | – | 2 (1.0) | 5 (4.0) | 4 (2.7) |
| Overnight hospital stay because of urinary retention | – | – | 2 (1.6) | 1 (0.68) |
| Hospital visit with thigh pain | 1 (1.6) | 6 (3.0) | 3 (2.4) | 3 (2.0) |
| Overnight hospital stay because of thigh pain | 1 (0.50) | 1 (0.68) | ||
| Hospital visit with vagina pain | 5 (8.1) | 6 (3.0) | 2 (1.6) | 3 (2.0) |
| Overnight hospital stay because of vagina pain | – | – | – | 2 (1.4) |
| Hospital visit with hip pain | – | 6 (3.0) | 1 (0.79) | 1 (0.68) |
| Overnight hospital stay because of hip pain | – | – | – | 1 (0.68) |
| Hospital visit with other pain | – | 5 (2.5) | – | 4 (2.7) |
| Overnight hospital stay because of other pain | 1 (1.6) | – | – | 1 (0.68) |
| Further investigations and assessments | ||||
| Voiding assessment | 5 (8.1) | 14 (7.0) | 9 (7.1) | 14 (9.5) |
| Cystoscopy | 5 (8.1) | 17 (8.5) | 4 (3.2) | 9 (6.1) |
| Urodynamics | 3 (4.8) | 10 (5.0) | 5 (4.0) | 4 (2.7) |
| Additional surgical treatments | ||||
| Laparoscopy as a result of operative haematoma | – | – | 1 (0.79) | – |
| Additional treatments for UI | ||||
| Botox | 1 (1.6) | 3 (1.5) | 3 (2.4) | – |
| Tibial nerve stimulation | – | 1 (0.50) | 1 (0.79) | – |
| Urethral bulking | 1 (1.6) | 3 (1.5) | 1 (0.79) | – |
| Colposuspension | 1 (1.6) | – | – | 2 (1.4) |
| Autologous sling | – | 1 (0.50) | – | – |
| TO-TVT | 1 (1.6) | – | – | – |
| Additional surgical treatments for VD | ||||
| Division/release of tape/mesh for VD | – | 1 (0.50) | 1 (0.79) | 1 (0.68) |
| Additional surgical treatments for pain | ||||
| Complete removal for pain | 1 (1.6) | 2 (1.0) | – | – |
| Partial removal for pain | – | 1 (0.50) | – | 2 (1.4) |
| Sacroiliac joint/bilateral ischial tuberosity injection | – | 2 (1.0) | – | 1 (0.68) |
| Additional surgical treatments for tape/mesh exposure | ||||
| Partial removal/excision of eroded part of the tape/mesh | 1 (1.6) | 3 (1.5) | – | 3 (2.0) |
| Recovering of the tape/mesh | 1 (1.6) | 2 (1.0) | – | – |
No RP-TVT participants required additional treatment for either pain or mesh exposure; the other three groups had comparable frequencies for this treatment.
Chapter 7 Discrete choice experiment
Background/introduction
Globally, policy-makers increasingly consider patient preferences as an integral part of their decision-making processes to ensure the delivery of patient-centred health-care services that are clinically effective and cost-effective. 103 Given that the SIMS trial has shown that SIMSs are clinically non-inferior to SMUSs, and given that neither strategy is clearly superior in terms of cost-effectiveness, patient preference will play a crucial role in deciding which is the most appropriate treatment to offer patients. The findings of the cost-effectiveness analyses were based on an NHS perspective, with the aim of the evaluation to maximise health outcomes, measured in terms of QALYs gained. Although useful for informing policy-makers, QALYs may not adequately capture all the outcomes that are of value to patients. They typically ignore concepts such as process of intervention delivery (e.g. type of anaesthetic), other non-health outcomes (e.g. time to return to work and usual activities after surgery) and patient preferences.
A DCE is a survey-based method that provides a more holistic measure of value that captures aspects of health and health care that may not typically be embedded within standard QALY measures. DCEs also have the advantage of evaluating the trade-offs that services users, in this case women with SUI who require surgical care, may be willing to make between different aspects of their care. For example, patients may prefer to receive a LA at their surgery if it allows them to return to work sooner, or they may be willing to sacrifice some improvement in SUI symptoms to lower the risk of procedure-related AEs.
The DCE method is used widely in research to elicit such benefit/risk trade-offs to better understand and account for patient preference when delivering health-care services. 104,105 This chapter describes the design, analysis and results of a DCE completed by a sample of the SIMS trial participants to elicit their preferences for different types of surgical care and outcomes (benefits and risks).
Methods
Discrete choice experiments have a strong grounding in the economic theory of preferences. Specifically, DCEs assume that the utility (value) of any good or service can be described by the value attributed to that service’s specific characteristics. 106 DCE surveys ask respondents to make several choices between two or more hypothetical service configurations that vary in terms of the service components (i.e. attributes). For example, the value attached to surgical procedures for SUI may comprise the value attached to the recovery time, impact on SUI symptoms, impact on daily activities and risk of AEs. In each choice task, respondents are asked to choose their preferred service configuration, the one that maximises their utility (level of satisfaction) that is dependent on the attribute’s levels. By observing the choices that respondents make over a series of choice tasks, we are provided with information on the trade-offs respondents are willing to make between attributes, which can help design patient-centred care that is informed and designed based on the integration of patient preference. By including a cost attribute within the DCE, it is then possible to calculate a monetary valuation of benefits, in terms of willingness to pay (WTP), which allows a direct comparison of the value associated with multiple different types of service configuration that vary depending on the set of attributes and levels included in the DCE. 107
Selection of attributes and levels for the discrete choice experiment
Discrete choice experiment attributes and levels were selected following a review of the literature to identify processes and outcomes of care that were of greatest value to service users and that were also collected in the SIMS trial. The attribute and level selection process involved engagement with clinical and patient representatives on the trial team to ensure that the attributes and levels selected for the DCE were realistic and captured the most important patient outcomes. This iterative approach was used to draft and revise the DCE questionnaire to ensure that the choices presented included attributes and levels that were realistic, tradeable, meaningful to patients and clinically relevant. The DCE included a cost attribute framed as a one-off out-of-pocket expense and the cost attribute to enable calculation of WTP. Appendix 7, Table 42, describes the attributes and levels included in the DCE.
Experimental design
Based on a DCE with a total of six attributes, (1 × 2 levels, 4 × 4 levels, 1 × 5 levels), there are a total of (21 × 44 × 51 =) 2560 different potential combinations of attributes and levels in an unrestricted full factorial design. This would lead to > 3 million unique choice sets. Therefore, we created a main-effects D-optimal experimental design using Ngene experimental design software (ChoiceMetrics, Sydney, NSW, Australia), reducing the number of potential choice tasks to 40. 108 The optimal experimental design of 40 choice tasks was further split into four blocks of 10 choice tasks to minimise respondent burden. Two further choice tasks were added to each block. Choice one was a warm-up exercise to familiarise respondents with the structure of the questions and a further repeated choice task was added as a consistency check. Each respondent therefore completed one block of 12 choice tasks.
The design was informed using priors for complications and level of symptom improvement obtained from a pilot version of the survey sent to 136 trial respondents. Ninety participants responded to the pilot: a response rate of 66%. Two further restrictions were placed on the combinations of attributes and levels that were presented to respondents within any one choice task alternative after inspection of the pilot data. The restrictions prevented respondents from being presented with ‘symptoms: very much improved’ in combination with ‘complications: intermittent self-catheterisation’ or ‘avoid activities: frequently’. These restrictions were imposed following further clinical and patient expert feedback to improve the plausibility of the choice tasks. The full experimental design code for the final survey can be found in Appendix 7.
Each choice task asked respondents to choose between two different surgical procedures, labelled ‘treatment A’ and ‘treatment B’, and an opt-out alternative, labelled ‘no treatment’, which varied in terms of the attributes and levels described in Appendix 7, Table 42. The opt-out ‘no treatment’ alternative was fixed across all choice tasks and included no surgery, but with no improvement in symptoms and no surgical-related complications, and it was assumed that women would occasionally avoid activities because of a fear of leaking. As no treatment was offered, the cost attribute for the opt-out alternative was £0. An example choice task is provided in Figure 10.
FIGURE 10.
Example choice task.
Questionnaire design
There were three sections in the questionnaire. Section 1 provided guidance for completing the survey, including a detailed description of all the attributes and levels. Respondents were first asked to tell us if they had experienced any of the complications included in the DCE and how long it took them to recover from the surgical procedure. They were then asked to imagine a baseline reference scenario to consider when completing the choice tasks, described as follows:
You leak a moderate amount of urine several times a day. You leak when you cough, sneeze or are physically active. Your urinary problem causes you to occasionally avoid activities due to fear of leaking. You always use pads to keep dry from your stress urinary incontinence.
Respondents were then asked to rate how acceptable improvements from this scenario would be to them (improved, much improved, very much improved). They were also asked to consider how often they would avoid activities because of a fear of urinary leakage in this scenario. The questions in section 1 were designed to encourage respondents to think about the attributes they would make choices about in the DCE stage of the survey.
Section 2 described the process of making choices and provided respondents with an example choice task. Respondents then proceeded to complete 12 choice tasks, indicating which of the three alternatives they would choose (treatment A, treatment B or no treatment) in each choice task. Immediately following the choice tasks, respondents were asked to agree or disagree (on a five-point scale ranging from ‘strongly disagree’ to ‘strongly agree’) with six statements describing their experience of the DCE survey in terms of understanding, level of information provided, relevance, plausibility, complexity and clarity.
The survey concluded in section 3 with demographic questions about education and income. These questions were included because evidence shows that ability to pay can have an impact on WTP estimates in DCEs. The income question was therefore used to adjust preferences and WTP for ability to pay. Respondents were also provided with an opportunity to give any further additional comments about the questionnaire.
The survey was sent as a postal questionnaire to SIMS trial participants who completed the follow-up questionnaire at 3 years. One reminder was sent to participants to encourage survey completion.
Discrete choice experiment data analysis
Responses to the DCE data were collated, merged with the experimental design and analysed using best-practice methods, according to random utility theory. 109 Under the random utility theory framework, each survey respondent (n) chooses their preferred treatment package (j) in each of the 10 different choice tasks (t). Data were analysed using conditional and mixed logistical regression models, allowing for multiple choices per respondent, to estimate the relative importance of the included attributes and levels. 110 The observable component of the utility function (Vnjt) is a linear additive function of the attributes and levels, such that:
The alternative specific constant (ASC) is represented by α, included as a normally distributed random parameter. The ASC describes the latent utility associated with choosing any surgical treatment package, compared with none (i.e. opting in). Type of anaesthetic (reference category: local), complication (reference category: none), symptoms (reference category: no improvement) and avoidance of activities (reference category: never) are included as categorical variables in the model, whereby the set of β parameters represents the marginal utility of each dummy-coded attribute level, compared with the reference category. Time to return to pre-surgery usual activities and cost are included in the model as continuous variables, where β6 and β13 describe the impact on utility of a 1-day reduction in time to return to usual activities and a £1 increase in cost, respectively. Mixed logit models were estimated with the ‘mixlogit’ command in Stata® version 14 (StataCorp LP, College Station, TX, USA), using maximum simulated likelihood, fitted with 50 Halton draws. 111
In total, we estimated six different DCE models. M1, M3 and M5 are conditional logit, errors component logit (random ASC) and random parameters logit (all random except cost, with a normal distribution), respectively. M2, M4 and M6 are the same as M1, M3 and M5, respectively, but with the inclusion of interaction terms to account for the impact of type of anaesthesia received on preferences for anaesthesia and the impact of income on preferences for the cost attribute.
The estimated utility parameters from M1 to M6 describe the impact on utility, but, to compare changes in all attributes in a single unit, it is necessary to calculate marginal rates of substitution between each attribute level (βk) and the cost attribute (β13) to estimate marginal WTP, calculated as:
The delta method, using the ‘NLCOM’ command in Stata, was used to compute CIs surrounding calculated marginal WTP.
Analysis of data quality and perceptions of the discrete choice experiment survey
As described in the questionnaire design, respondents were asked to agree or disagree on a five-point scale with six statements regarding the process of making choices within the survey. These data are tabulated and plotted graphically for illustration of the results.
Results
The final version of the DCE was sent to 325 out of 596 (55%) trial participants after the 3-year follow-up time point. Of these, 227 (70%) returned a questionnaire, leading to a final estimation sample of 227 respondents. Among a potential 6810 observations (227 respondents × 10 choice tasks × 3 alternatives), the final data set had 6390 observations, indicating a 94% choice task completion rate among respondents.
Sample characteristics
Appendix 7, Table 43, describes the sample characteristics of the women who returned the DCE questionnaire, compared, for reference, with the corresponding baseline data for all trial participants. Those who returned completed DCE questionnaires closely matched the demographics of the participants who took part in the trial. This provides reassurance that the DCE sample was, in general, a good representation of the preferences of those in the larger trial sample who received an index surgery.
Preferences for treatments and willingness to pay
The results of the respective DCE analysis models are provided in Table 19 and corresponding WTP values are provided in Table 20. M5 generates the best model fit and so these results will be considered the base-case WTP values for carrying forward to a cost–benefit analysis in Chapter 8. Across all six models analysed, women indicated a preference to have a surgical treatment as opposed to none, with the consequence of remaining in their current health state. GA was preferred to LA, with those who had GA in the trial indicating a stronger preference for procedures conducted under GA than those who had LA. Women prefer shorter times to return to normal activities and are willing to pay between £70 and £100 per day of reduction in recovery time following surgery. As would be expected, women attach the greatest value to achieving improvement in their UI symptoms and the avoidance of complications. In general, the complications with the greatest negative impact on utility were the need for intermittent self-catheterisation, and the experience of mesh exposure, although avoidance of dyspareunia and new-onset urgency incontinence were also valued. Women placed an approximately equal value to achieving an outcome of ‘very much improved’ and avoiding the need for intermittent self-catheterisation or experiencing mesh exposure. Women did not have a statistically significant preference against treatments for which they would only rarely have to avoid their usual activities, compared with never having to avoid activities. However, they were willing to pay between £1700 and £2200 for treatments that improved their frequency of avoiding daily activities because of a fear of leaking from occasionally to never. They were willing to pay more (between £4500 and £5700) to improve from frequently avoiding usual activities to never avoiding usual activities.
| Attribute | M1 | M2 | M3 | M4 | M5 | M6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Main effectsa | ||||||||||||
| ASC | 0.6365*** | 0.16 | 0.6413*** | 0.16 | 1.3131*** | 0.25 | 1.3273*** | 0.25 | 1.3071*** | 0.27 | 1.4533*** | 0.28 |
| Anaesthetic: general | 0.3091*** | 0.06 | 0.0881 | 0.09 | 0.3219*** | 0.07 | 0.0651 | 0.1 | 0.3728*** | 0.11 | 0.0063 | 0.18 |
| Complications | ||||||||||||
| Urge incontinence | –1.0111*** | 0.11 | –1.0140*** | 0.11 | –1.4026*** | 0.13 | –1.4059*** | 0.13 | –1.8327*** | 0.18 | –1.8507*** | 0.18 |
| Self-catheterisation | –1.4819*** | 0.11 | –1.4826*** | 0.12 | –1.6853*** | 0.13 | –1.6943*** | 0.13 | –2.4289*** | 0.21 | –2.6102*** | 0.24 |
| Dyspareunia | –1.0440*** | 0.11 | –1.0547*** | 0.11 | –1.4457*** | 0.13 | –1.4621*** | 0.13 | –1.8568*** | 0.19 | –1.9966*** | 0.2 |
| Extrusion or exposure | –1.3482*** | 0.11 | –1.3503*** | 0.11 | –1.5821*** | 0.12 | –1.5895*** | 0.12 | –2.3648*** | 0.22 | –2.5449*** | 0.21 |
| Return to normal activities (days) | –0.0121*** | 0 | –0.0121*** | 0 | –0.0146*** | 0 | –0.0148*** | 0 | –0.0173*** | 0 | –0.0191*** | 0 |
| Symptoms | ||||||||||||
| Very much improved | 1.6018*** | 0.12 | 1.6125*** | 0.12 | 1.9800*** | 0.14 | 1.9922*** | 0.14 | 2.6742*** | 0.19 | 2.6583*** | 0.2 |
| Much improved | 1.3439*** | 0.11 | 1.3446*** | 0.11 | 1.6729*** | 0.14 | 1.6763*** | 0.14 | 2.2584*** | 0.18 | 2.1672*** | 0.19 |
| Improved | 1.0789*** | 0.12 | 1.0804*** | 0.12 | 1.4246*** | 0.15 | 1.4349*** | 0.15 | 1.8672*** | 0.18 | 1.7238*** | 0.19 |
| Avoid activities | ||||||||||||
| Rarely | –0.0953 | 0.1 | –0.0901 | 0.1 | –0.1717 | 0.1 | –0.1672 | 0.1 | –0.2208 | 0.14 | –0.0998 | 0.15 |
| Occasionally | –0.2920** | 0.1 | –0.2826** | 0.1 | –0.3257** | 0.1 | –0.3087** | 0.1 | –0.4526** | 0.15 | –0.4173** | 0.15 |
| Frequently | –0.7770*** | 0.11 | –0.7705*** | 0.11 | –0.8559*** | 0.12 | –0.8495*** | 0.12 | –1.2194*** | 0.17 | –1.0903*** | 0.17 |
| Cost | –0.0002*** | 0 | –0.0002*** | 0 | –0.0002*** | 0 | –0.0002*** | 0 | –0.0002*** | 0 | –0.0002*** | 0 |
| Interaction effects | ||||||||||||
| Anaesthetic GA × received GA | – | – | 0.3696*** | 0.1 | – | – | 0.4298*** | 0.13 | – | – | 0.6170** | 0.23 |
| Cost × high incomeb | – | – | –0.0001 | 0 | – | – | –0.0002** | 0 | – | – | –0.0003*** | 0 |
| SDc | ||||||||||||
| ASC | – | – | – | – | 2.3043*** | 0.19 | 2.3216*** | 0.19 | 2.4013*** | 0.2 | 2.1989*** | 0.18 |
| Anaesthetic: general | – | – | – | – | – | – | – | – | 1.0257*** | 0.13 | 1.0436*** | 0.13 |
| Complications | ||||||||||||
| Urge incontinence | – | – | – | – | – | – | – | – | 0.9815*** | 0.23 | –0.9553*** | 0.2 |
| Self-catheterisation | – | – | – | – | – | – | – | – | 1.4458*** | 0.24 | 1.8017*** | 0.27 |
| Dyspareunia | – | – | – | – | – | – | – | – | 1.1548*** | 0.27 | 1.1302*** | 0.29 |
| Extrusion or exposure | – | – | – | – | – | – | – | – | 1.6333*** | 0.23 | 1.6913*** | 0.21 |
| Return to normal activities (days) | – | – | – | – | – | – | – | – | 0.0067 | 0.01 | –0.0094 | 0.01 |
| Symptoms | ||||||||||||
| Very much improved | – | – | – | – | – | – | – | – | –0.9451*** | 0.19 | 1.3020*** | 0.23 |
| Much improved | – | – | – | – | – | – | – | – | 0.2192 | 0.32 | 0.5010 | 0.22 |
| Improved | – | – | – | – | – | – | – | – | 0.2446 | 0.24 | 0.6137** | 0.21 |
| Avoid activities | ||||||||||||
| Rarely | – | – | – | – | – | – | – | – | –0.1474 | 0.17 | 0.0224 | 0.18 |
| Occasionally | – | – | – | – | – | – | – | – | 0.208 | 0.14 | 0.2708 | 0.16 |
| Frequently | – | – | – | – | – | – | – | – | 0.7906** | 0.26 | 0.5818** | 0.21 |
| Cost | – | – | – | – | – | – | – | – | – | – | –0.2282 | 0.22 |
| Model fit | ||||||||||||
| Log likelihood | –1917.53 | –1909.88 | –1665.58 | –1655.67 | –1582.17 | –1570.18 | ||||||
| Akaike information criterion | 3863.1 | 3851.8 | 3361.2 | 3345.3 | 3218.3 | 3202.4 | ||||||
| Bayesian information criterion | 3957.7 | 3960.0 | 3462.6 | 3460.3 | 3400.9 | 3412.0 | ||||||
| Observations (n) | 6390 | 6390 | 6390 | 6390 | 6390 | 6390 | ||||||
| WTP fora | WTP (£), mean (95% CI)b | |||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | |
| ASC | 3703 (2008 to 5397) | 3990 (2139 to 5842) | 7143 (4356 to 9931) | 8680 (4887 to 12,472) | 5721 (3309 to 8134) | 7581 (4161 to 11,002) |
| Anaesthetic: general | 1798 (958 to 2637) | 548 (–537 to 1633) | 1751 (932 to 2570) | 426 (–851 to 1702) | 1632 (610 to 2653) | 33 (–1778 to 1844) |
| Complications | ||||||
| Urge incontinence | –5881 (–7909 to –3853) | –6309 (–8624 to –3994) | –7630 (–10,046 to –5215) | –9194 (–12,740 to –5648) | –8022 (–10,661 to –5383) | –9654 (–13,469 to –5840) |
| Self-catheterisation | –8620 (–11,315 to –5925) | –9225 (–12,340 to –6109) | –9168 (–12,004 to –6333) | –11,080 (–15,318 to –6842) | –10,632 (–14,077 to –7187) | –13,616 (–19,173 to –8059) |
| Dyspareunia | –6073 (–8156 to –3990) | –6562 (–8953 to –4171) | –7865 (–10,345 to –5384) | –9562 (–13,241 to –5883) | –8128 (–10,931 to –5324) | –10,415 (–14,712 to –6118) |
| Extrusion or exposure | –7842 (–10,178 to –5506) | –8402 (–11,114 to –5689) | –8607 (–11,108 to –6106) | –10,395 (–14,179 to –6611) | –10,351 (–13,599 to –7104) | –13,276 (–18,290 to –8261) |
| Return to normal activities (days) | –70 (–110 to –30) | –75 (–119 to –31) | –80 (–121 to –38) | –97 (–152 to –42) | –76 (–119 to –33) | –100 (–159 to –41) |
| Symptoms | ||||||
| Very much improved | 9317 (6597 to 12,038) | 10,033 (6846 to 13,220) | 10,771 (7651 to 13,891) | 13,029 (8288 to 17,770) | 11,706 (8267 to 15,144) | 13,867 (8617 to 19,117) |
| Much improved | 7817 (5430 to 10,204) | 8366 (5610 to 11,122) | 9101 (6299 to 11,902) | 10,962 (6809 to 15,116) | 9885 (6885 to 12,886) | 11,305 (6912 to 15,698) |
| Improved | 6276 (4098 to 8454) | 6722 (4235 to 9209) | 7750 (5128 to 10,373) | 9384 (5576 to 13,191) | 8173 (5459 to 10,887) | 8992 (5188 to 12,796) |
| Avoid activities | ||||||
| Rarely | –554 (–1645 to 536) | –560 (–1731 to 610) | –934 (–2056 to 188) | –1094 (–2469 to 282) | –967 (–2199 to 266) | –520 (–2033 to 992) |
| Occasionally | –1698 (–2862 to –535) | –1758 (–3016 to –500) | –1772 (–2951 to –593) | –2019 (–3491 to –546) | –1981 (–3319 to –643) | –2177 (–3808 to –546) |
| Frequently | –4520 (–6182 to –2858) | –4794 (–6654 to –2934) | –4656 (–6334 to –2978) | –5555 (–7884 to –3226) | –5338 (–7258 to –3417) | –5688 (–8183 to –3193) |
Assessment of data quality and views on the survey
Figure 11 describes the participants’ responses to six debriefing questions about the survey content, asking about perceptions of survey complexity, learning through the survey, plausibility, relevance to policy, level of information provided and clarity of the choice tasks. Almost all respondents understood the concept of choosing between different treatment options (i.e. DCE choice tasks), which suggests that respondents were willing to make hypothetical trade-offs between benefits and risks. Of the respondents, 26% reported that they found the choice tasks to be confusing, but most felt that the process became easier the more choice tasks they answered. The majority of the respondents felt that the treatment options made sense and that their responses would have an impact on the future availability of treatments, suggesting a high degree of perceived survey consequentiality. Regarding the level of information provided in the choice tasks, about half of the respondents felt that they needed more information to inform their decision-making. Although this proportion would appear low, it must be considered that the volume of information provided is a trade-off between complete information and survey complexity.
FIGURE 11.
Participants’ perceptions of the DCE survey.
Chapter 8 Health economics: cost-effectiveness analysis
Parts of this chapter are reproduced from Abdel-Fattah et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are also reproduced from Glazener et al. 112 Contains public sector information licensed under the Open Government Licence v3.0.
Cost–utility analysis
Economic evaluation overview
The aim of this chapter is to present the cost-effectiveness of adjustable anchored SIMSs, compared with that of tension-free SMUSs, in the surgical management of female SUI. The primary economic objective was to conduct an ITT analysis alongside a RCT to determine the cost-effectiveness of SIMSs, compared with SMUSs, from a UK NHS perspective. Cost-effectiveness was measured using incremental cost-effectiveness ratios (ICERs) based on QALYs derived from responses to the generic EQ-5D-3L and the UI-specific ICIQ-LUTSqol measures over various time points during the 36-month follow-up period. The secondary economic objectives were to estimate costs from a societal perspective, to determine patient preferences for the processes and outcomes of alternative surgical procedures using a DCE (see Chapter 7) and to use the DCE results to conduct a cost–benefit analysis. Costs and QALYs accrued in the 24 and 36 months were discounted by 3.5% per annum, in line with NICE recommendations. 113 The base-case analysis was conducted as an imputed analysis owing to the impact of missing data. 114 The choice of method for handling missing data (multiple imputation) was grounded in the assumed missing data mechanism, missing at random, which was supported by the data. The methods and results were reported following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) recommendations and the analyses followed the health economics analysis plan. 115
Methods
Resource use and cost collection
Measuring resource use
The resource use data and costs for the within-trial analysis were broken into the following categories: intervention, consultations with primary health-care provider, consultation with secondary care professionals and procedures for subsequent treatment related to treating UI symptoms. Intervention resource use at the index surgery was derived based on several elements. The number and type of intervention kits, the staff involved in the index surgery, the time spent in surgery, the type of anaesthesia used and medications. Intervention resource use was captured through the operation CRF. This form specified the surgery duration, the grade of the surgeon performing the procedure and whether or not they were supervised, the type of procedure performed (RP-TVT/TO-TVT/SIMS), the grade of the anaesthetist, the type of anaesthesia used (general/spinal/local with intravenous sedation/LA with oral sedation/LA only), type of analgesics/anxiolytic/sedative (several specified) received, length of stay until discharge, and details of catheterisation during and/or after the procedure. It was assumed that there were three nurses at the surgery (two band 5 and one band 4). Postoperative resource use included information on duration of hospital stay, type of catheter and details of any return to theatre before hospital discharge.
Following discharge, primary and secondary care resource use data were collected at 3, 15, 24 and 36 months using patient questionnaires. The resource use data collected included the use of primary care GP services and referral to other NHS services for subsequent additional specialist management, such as physiotherapy or a district nurse. Secondary care resource use included inpatient re-admissions related to UI (including length of stay), repeat continence procedures carried out, hospitalisation for AEs (such as partial or complete tape removal) and outpatient attendances. If women answered ‘no’ to seeing a health professional, resource use was assumed to be zero. Secondary resource use was also recorded using CRFs completed retrospectively by RNs at the point of contact with the hospital. The trial office cross-checked any details of hospitalisations reported by participants against CRFs. When details were available from CRFs and participant questionnaires, the analysis was based on the CRF data. However, if matching CRFs were not available, sites were contacted directly to verify patient-reported resource use details. Any additional hospital contacts reported on questionnaires that were deemed related to UI and validated by the sites were included in the analysis.
Valuing NHS resource use (NHS unit costs)
Operation resource use
The base-case analysis used a component costing approach. Several surgical devices from different manufacturers were used in the trial as long as they met the prespecified criteria in the trial protocol. 1 Data on the type of device used for each woman was collected on the CRF. The unit cost of the device was based on the list price that the sites purchased them for. The unit costs were derived from personal communication with four sites. The intervention device costs were applied at the individual participant level. The grade of the operating surgeon and whether or not they were supervised, and the grade of the anaesthetist, were also collected on the CRF. An assumption was made about grade and the number of supplementary staff present during a typical surgical procedure (nurses and theatre assistants) based on the clinical opinion of experts working on the trial team. The unit costs of staff salaries were derived from a published source. 116 The resource use data for the different anaesthesia were based on a published study117 and the unit costs of different anaesthesia were based on the costs of the drugs administered. 118 Although most interventions were day cases, some women spent some days in hospital. The unit cost of overnight stay was based on the cost of elective inpatient excess bed-days for vaginal tape/MUS operations.
Follow-up costs
The follow-up primary care visit costs were derived from a published source116,118,119 and the secondary diagnostic and procedure costs were based on Healthcare Resource Group tariffs. The unit costs were measured in Great British pounds (£). The costs were based on 2018/19 prices. Unit costs/prices were obtained from the following published sources: the British National Formulary,118 reference costs119 and Unit Costs of Health and Social Care 2019,116 as indicated in Table 21.
| Resource | Unit cost (£) | Notes/source |
|---|---|---|
| Operation resource use | ||
| SIMS | Several | Price paid by sites for SIMS devices (prices ranged from £350 to £550) |
| SMUS | Several | Price paid by sites for SMUS devices (prices ranged from £327 to £584) |
| Consultant | 108 | Cost per hour based on a 48-hour week116 |
| Associate registrar | 105 | Cost per hour based on a 48-hour week116 |
| Specialist registrar | 43 | Cost per hour based on a 48-hour week116 |
| Nurse | 37 | Cost per hour for band 5116 |
| 28 | Cost per hour for band 4116 | |
| GA | 22 | Based on calculation of drugs and consumables (see Appendix 8, Table 44)117,118 |
| Spinal anaesthesia | 3 | Based on calculation of drugs (see Appendix 8, Table 44)118 |
| LA with sedation | 5 | Based on calculation of drugs (see Appendix 8, Table 44)118 |
| LA | 1 | Based on calculation of drugs (see Appendix 8, Table 44)118 |
| Analgesics and anxiolytics | Various prices | Costs based on CRF data for each participant118 |
| Theatre overheads | 420 | Hourly cost excluding supplies120 |
| Inpatient stay | 483 | Weighted average cost of elective inpatient excess bed-days (LB 51A and B) vaginal tape operations for UI119 |
| Indwelling catheter | 6 | Calculated cost per week of permanent catheter (see Appendix 8, Table 45)121 |
| In-and-out catheter | 6 | Unit cost per day of catheter Folysil® X-Tra (Coloplast A/S, Humlebæk, Denmark) (size 14), pack size 1. Assume no additional procedure time required if catheterised during surgery121 |
| Consultations with primary health-care professionals | ||
| Doctor surgery consultation | 39 | Per surgery consultation lasting 9.22 minutes116 |
| Doctor telephone call | 24 | Average cost per consultation116 |
| Nurse surgery consultation | 9 | Average cost per consultation116 |
| Nurse telephone call | 15 | Average cost per consultation116 |
| Physiotherapist | 9 | Average cost per consultation116 |
| District nurse | 15 | Average cost per consultation116 |
| Consultation with secondary health-care professionals/procedures for subsequent treatment | ||
| Outpatient urology | 108 | Average cost per outpatient attendance: consultant and non-consultant led. Service code 101 urology department119 |
| Outpatient gynaecology | 141 | Average cost per outpatient attendance: service code 502 gynaecology department119 |
| Physiotherapy/nurse | 58 | Average cost per outpatient attendance: service code 650 physiotherapy department119 |
| Pain management | 157 | Average cost per outpatient attendance: service code 191 pain management department119 |
| Neurology | 177 | Average cost per outpatient attendance: Service code 400 Neurology department119 |
| A&E | 168 | Average cost per outpatient attendance: service code 180 A&E department119 |
| Indwelling catheter | 6 | Calculated cost per week of permanent catheter (see Appendix 8, Table 45)121 |
| Disposable catheter | 30 | Calculated average cost per week of disposable catheter (see Appendix 8, Table 46)121 |
| Cystoscopy | 1546 Elective | LB09D intermediate endoscopic ureter procedures, ≥ 19 years119 |
| 1043 Day case | ||
| Urodynamics | 698 Elective | LB42A dynamic studies of urinary tract, ≥ 19 years119 |
| 368 Day case | ||
| Radiography | 31 | DAPF direct access plain film119 |
| MRI | 143 | RD01A MRI scan of one area, without contrast, ≥ 19 years119 |
| CT | 85 | RD20A CT scan of one area, without contrast, ≥ 19 years119 |
| SMUS | 2904 Elective | Weighted average of LB51 vaginal tape operations for UI, with CC score of 0 to 2+119 |
| 1549 Day case | ||
| SIMS | 2904 Elective | Weighted average of LB51 Vaginal Tape Operations for UI, with CC Score 0 to 2+119 |
| 1549 Day case | ||
| Botox injections | 1546 Elective | LB14Z intermediate endoscopic bladder procedures119 |
| 1043 Day case | ||
| Tibial nerve stimulation | 2795 Elective | LB80Z insertion of neurostimulator electrodes for treatment of UI119 |
| 1803 Day case | ||
| Pubovaginal slings | 4415 Elective | LB59Z major, open or laparoscopic, bladder neck procedures (female)119 |
| Colposuspension | 4415 Elective | LB59Z major, open or laparoscopic, bladder neck procedures (female)119 |
| Duloxetine | 0.99 | Cost per tablet118 |
| Antimuscarinic treatment | 0.52 | Average cost per tablet of reported antimuscarinics118 |
| Antibiotics | 0.19 | Average cost per tablet of reported antibiotics118 |
| Urethral dilation | 1546 Elective | LB14Z intermediate endoscopic bladder procedures119 |
| 1043 Day case | ||
| Insertion of suprapubic catheter | 1136 Elective | LB18Z attention to suprapubic bladder catheter119 |
| 400 Day case | ||
| Urethrolysis | 1546 Elective | LB14Z intermediate endoscopic bladder procedures119 |
| 1043 Day case | ||
| Complete removal of tape | 3239 Elective | Weighted average of MA03 major open lower genital tract procedures with CC score of 0–3+119 |
| 1811 Day case | ||
| Partial removal of tape | 2539 Elective | Weighted average of MA04 intermediate open lower genital tract procedures with CC score of 0–3+119 |
| 1452 Day case | ||
Participant-incurred unit costs
Participant resource use comprised three main elements: travel costs for making return visit(s) to NHS health care, time costs of travelling and attending NHS health care, and self-purchased health care. The time resource and unit cost of making a return journey to each type of health-care provider were obtained from the Participant Time and Travel Cost Questionnaire.
The participants reported how long they spent travelling to and attending their last visit to each type of health-care provider. Participants were asked what activity they would have been undertaking (e.g. paid work, leisure, housework) had they not attended the health-care provider. These data were presented in their natural units (e.g. hours) and costed using standard economic conventions (e.g. the Department of Transport estimates for the value of leisure time). Participants were asked if they were accompanied by a friend or a relative and, if so, to provide details of their time and travel. These unit time costs were multiplied by the number of health-care contacts derived from the health-care resource use questions to generate the opportunity cost of time and travel from the patient perspective. Details of unit costs applied to the various activities are included in Table 22.
| Activity | Unit cost (£) | Source and notes |
|---|---|---|
| Unit costs applied to participant and companion travel | ||
| Cost per mile travelled by car | 0.45 | HMRC (approved mileage rates for the first 10,000 miles)122 |
| Car parking charges | Various | As reported by participants |
| Cost of public transport (bus, train, taxi) | Various | As reported by participants |
| Unit costs applied to participant and companion time | ||
| Paid work | 16.52 | Hourly pay for female full-time employee jobs123 |
| Housework | 8.58 | Hourly rate124 |
| Child care | 15.28 | Hourly rate124 |
| Caring for a friend/family member | 9.75 | Hourly rate124 |
| Voluntary work | 14.43 | ONS123 |
| Retired | 5.03 | TAG data book125 |
| Leisure | 5.03 | TAG data book125 |
| Unemployed | 5.03 | TAG data book125 |
Data collected through the patient questionnaire were used to estimate the costs of self-purchased health-care items, including pads bought by participants, prescription costs and over-the-counter medications. All self-purchased health care related to items purchased for the management or treatment of UI.
Estimates of resource use were combined with unit costs to derive total costs for each item of resource use and each patient. The costs for each item of resource use for each patient were summed to produce a total cost for each patient and an average total cost per patient in each intervention arm.
Derivation of quality-of-life measures
Health-related QoL was measured using a standard generic tool and a disease-specific tool: the EQ-5D-3L and the ICIQ-LUTSqol, respectively. For both these tools, higher utility scores indicate better health as perceived by the participants.
Trial participants completed the EQ-5D-3L at baseline; at 4 weeks and at 3 months after their intervention; and at 15, 24 and 36 months after randomisation. The responses to the EQ-5D-3L questionnaire were valued using UK general population tariffs, based on the time trade-off technique to generate a utility score for every participant at each follow-up time point in the trial. 126 QALYs were calculated as the area under the curve defined by the utility values at baseline and at each follow-up. 127 Most women had to wait for some time for their surgery. Therefore, the base-case analysis QALY calculation assumed that utility remained constant between randomisation and surgery (i.e. women could not generate QALY gains before the point of surgery). The waiting time between randomisation and surgery was calculated using the dates recorded on the CRF. For the remaining time points, a linear interpolation between utility measurement time points was assumed.
The 4-week and 3-month questionnaires were triggered by the operation date. Women who did not get surgery did not receive the 4-week or 3-month questionnaires; therefore, QALY calculation area under the curve was estimated using data from baseline and 15, 24 and 36 months. Overall self-rated health status was also collected using the EuroQol Visual Analogue Scale (EQ-VAS) score (0 equals worst health and 100 equals best health). QoL data were also collected using items from the condition-specific tool ICIQ-LUTSqol for sensitivity analysis. These data were collected at baseline and at 3, 15, 24 and 36 months.
The ICIQ-LUTSqol is a psychometrically robust patient-completed questionnaire evaluating QoL in urinary incontinent patients for use in research and clinical practice across the world. The ICIQ-LUTSqol is the King’s Health Questionnaire (KHQ) adapted for use within the ICIQ structure and provides a measure to assess the impact of UI on QoL with particular reference to social effects. ICIQ-LUTSqol responses were converted into a utility index using a published algorithm. 128 There was no need to impute utility data as no deaths were recorded during the trial follow-up period.
Data analysis
All components of costs were described with the appropriate descriptive statistics when relevant: mean and SD for continuous and count outcomes, and numbers and percentages for dichotomous and categorical outcomes (e.g. numbers reporting problems on the EQ-5D-3L). All analyses were conducted using Stata version 14.1. Investigations were carried out for skewed cost data (i.e. a small proportion of participants incurring very high costs), using GLMs to test alternative model specifications for appropriate fit to the data. The GLM models allow for heteroscedasticity by selecting and specifying an appropriate distributional family for the data. This family offers alternative specifications to reflect the relationship between the mean and variance of the estimates under consideration (Glick 2015). 129,130 Two methods were used to identify the most appropriate distributional family: (1) a modified Park test and (2) the Akaike information criterion. For costs, the post-prediction test statistics indicated that the most appropriate family was inverse Gaussian. The goodness-of-fit statistics based on multiple tests (Pregibon link, modified Hosmer–Lemeshow and Pearson’s correlation) indicated that the log was the appropriate link. The QALY data were analysed using a Gauss family and an identity link. Analysis models were run to estimate the incremental effect of treatment group on costs and QALYs. Models were adjusted using a fixed effect for the minimisation covariate: previous supervised PFMT within the preceding 2 years (PFMT: yes/no), age and baseline QoL data (EQ-5D-3L or ICIQ-LUTSqol as appropriate); furthermore, the analyses used robust standard errors, clustered by centre.
Incremental cost per quality-adjusted life-year
Results of the cost–utility analysis are reported as incremental cost per QALY for each treatment group. ICERs were computed to determine the cost-effectiveness of SIMSs, compared with that of SMUSs. The point estimate of the ICER is calculated as:
where Ci and Cj are the mean NHS costs for the SIMS and the SMUS arms, respectively. Similarly, Ei and Ej are the mean QALYs in the SIMS and SMUS arms, respectively. The ICER is assessed against the NICE-recommended cost-effectiveness WTP threshold of £20,000 per QALY gained; additional information on the probability of cost-effectiveness for £0 and £30,000 WTP thresholds is also provided. 113
The joint density of incremental costs and incremental QALYs was derived using non-parametric bootstrapping. From the results of the bootstrapping, cost-effectiveness acceptability curves (CEACs) were created. CEACs are used to display the inherent uncertainty surrounding cost-effectiveness at various WTP threshold values for society’s WTP for a QALY. CEACs present results when the analysis follows a net benefit approach. This approach utilises a straightforward rearrangement of the cost-effectiveness decision rule used when calculating ICERs to create the net monetary benefit (NMB) for each bootstrapped iteration at increasing values of WTP per QALY:
where λ represents a decision-maker’s WTP for incontinence avoided or a QALY gained. If the above expression holds true for a given iteration and WTP threshold value (λ), then the intervention is considered cost-effective for that iteration. As society’s WTP is unknown, the NMB was calculated for several possible λ values, including the usual £20,000–30,000 range often adopted by policy-makers in the NHS. 113
Missing data
Missing data are a frequent problem in economic evaluations undertaken within a RCT setting, driven by the requirement to use multiple resource use items to calculate costs, and the repeated measures nature of questionnaires required to derive total costs and the QALY area under the curve. There are several possible methods that can be employed to account for missing data, such as mean or multiple imputation. For the analysis of costs, the mechanism of data missingness was investigated using logistical regression analysis, whereby the dependent binary variable (missing or not) was regressed on age, minimisation variables, baseline EQ-5D-3L utility score and baseline measures of type of UI. Imputation analysis was conducted as > 5% of both cost and QALY data needed for primary analysis were missing. The handling of missing data was based on the premise that the data were missing at random, and multiple imputation was employed. All imputation was completed using Stata’s multiple imputation procedure. Components of cost (primary and secondary care costs were imputed for each year) and utility data were imputed for each measurement time point, based on GLMs that were adjusted for minimisation variables, age and baseline utility. To preserve the allocation of participants’, the imputation was done on each randomised group. Imputations were generated using predictive mean matching drawn from the five k-nearest neighbours (k-NN = 5); predictive mean matching preserves distribution of the data and is more robust to violations of the normality assumption. The multiple imputation was run 20 times, generating 20 complete data sets, to ensure that stable results were combined using Rubin’s rules to generate estimates of costs. 131
Sensitivity analyses and subgroup analysis
Sensitivity analyses
The estimation of the QALYs used in the base-case analysis considered the time participants waited for surgery and incorporated a bottom-up approach in costing the intervention. Sensitivity analyses were performed to gauge the impact of varying these assumptions and/or parameter values on the base-case analysis.
-
The base-case analysis used multiple imputation of missing data. A complete-case analysis was performed as a sensitivity analysis to highlight the importance of this base-case assumption.
-
Sensitivity analyses on the QoL measure (i.e. the EQ-5D-3L) were conducted without adjusting for the time the women waited for the initial surgery after randomisation.
-
A sensitivity analysis was conducted using the utility values from the condition-specific QoL measure, ICIQ-LUTSqol.
-
Analyses were conducted to explore the impact of changing the discount rate used for 24- and 36-month costs and QALYs in accordance with NICE best-practice recommendations; the discount rate was varied from 0% to 6% per annum.
-
An analysis was conducted to explore the relaxation of the assumption that women who did not have surgery and did not have any CRF completed for follow-up visits had zero costs (see Appendix 8, Table 47).
Subgroup analysis
Depending on the availability of data, the following subgroup analyses were conducted:
-
urodynamic mixed incontinence versus urodynamic stress incontinence
-
age – above and below the observed median age of the recruited women
-
a post hoc analysis for age – < 65 years compared with those aged ≥ 65 years
-
a post hoc comparison between those who had received supervised PFMT in the previous 2 years and those who had not.
Cost–benefit analysis
The cost–benefit analysis compared SIMSs with SMUSs using a definition of net benefit whereby both costs and outcomes are measured in monetary values. This allows a direct calculation of net benefits (benefit, measured as WTP – cost) for each participant in the trial. To obtain monetary valuation of benefits, marginal WTP tariffs, calculated from the DCE base-case model (random parameters logit model; see M5 in Table 19), were mapped to clinically important and patient-relevant characteristics of the surgical procedure (type of anaesthesia) and trial outcomes (number of recovery days after surgery, complications, PGI-I scale and avoidance of activities). The mapping process and associated assumptions made to match DCE attributes with trial outcomes is described in Table 23.
| Attribute | DCE levels | WTP tariff (£) | Mapping description |
|---|---|---|---|
| ASC | Any surgery | 5721 | Mapped from the ASC in the DCE. Assumed that respondents who do not have a surgical treatment receive a WTP of £0 |
| No surgery | 0 | ||
| Type of anaesthesia | General | 1632 | Direct map to GA and LA. Assumes that all types of LA incur the same WTP tariff. Participants who received spinal anaesthesia only were assumed to receive the LA WTP tariff, as spinal anaesthesia was rare in the trial and not included as an attribute in the DCE |
| Local | 0 | ||
| Complications | New-onset UUI | –8022 | Obtained by calculating the presence of any urgency (mild, moderate or severe) reported at any of the annual follow-up time points for women who did not report urgency in their baseline questionnaire |
| Intermittent self-catheterisation | –10,632 | Mapped to patient-reported outcomes for any woman who reported using a disposable catheter at any of the annual questionnaire follow up time points | |
| Dyspareunia | –8128 | Mapped to sexual function questionnaire responses identifying pain during sexual intercourse according to the version of the annual questionnaire received by participants. Any report of any pain during sexual intercourse, regardless of severity, in any of the annual questionnaires is counted as experience of dyspareunia for the mapping process | |
| Mesh extrusion/erosion | –10,351 | Defined as any mesh exposure that required medical treatment and was experienced at any point over the 3-year follow-up of the trial. Treatment for mesh extrusion/erosion was verified by the relevant trial centre | |
| None | 0 | Absence of the above complications | |
| Number of recovery days | 3 days | –76 per day | A daily WTP tariff applied to each additional day required to return to normal activities following surgery. Mapped directly to data collected in the questionnaire at 4 weeks. Assumption that women who had not yet recovered from the surgical procedure at the time of the questionnaire incurred a tariff for 28 days (in the absence of data collected beyond this time point) |
| 13 days | |||
| 23 days | |||
| 33 days | |||
| Level of improvement in incontinence symptoms after surgery | Very much improved | 11,706 | Mapped to women’s report of PGI-I scale improvement level, on average, across the three annual questionnaires. If fewer than three annual questionnaires were available, an average score across the available questionnaires was obtained and used for the mapping. Data mapped directly for ‘very much improved’, ‘much improved’ and ‘improved’. Those who remained the same, or got worse, incurred a WTP tariff of £0 |
| Much improved | 9885 | ||
| Improved | 8173 | ||
| None | 0 | ||
| Avoid activities | Frequently | –5338 | Mapped to a composite score across nine questions from the ICIQ-LUTSqol, averaged over the 3-year follow-up, asking women about how UI affects their daily lives (specifically household tasks, job, physical activities, travel, social life, ability to see friends, relationships, sexual life and family life). Response options (‘not at all’ mapped to ‘never’, ‘slightly’ mapped to ‘rarely’, ‘moderately’ mapped to ‘occasionally’, and ‘a lot’ mapped to ‘frequently’) |
| Occasionally | –1981 | ||
| Rarely | –967 | ||
| Never | 0 | ||
| Cost of treatment | £1000 | Mapping not required; cost attribute used to derive WTP | |
| £2000 | |||
| £3500 | |||
| £5000 |
Benefits, measured as total WTP, were calculated for each woman in the trial by summing the marginal WTP tariffs from the DCE for each attribute level mapped to the corresponding patient outcomes from the trial. An average total WTP for women in each treatment arm (SIMS and SMUS) was then calculated for use in the cost–benefit analysis.
Total WTP was compared with total costs measured from both the NHS perspective and the DCE to generate estimates of net benefit and calculate benefit-to-cost ratios. All surgical treatments with a benefit-to-cost ratio of > 1 should be implemented on the grounds that they offer value for money from a societal welfare maximisation perspective. Similarly, all surgical treatments with a benefit-to-cost ratio of < 1 should not be implemented, or should be phased out if already in routine use, as the benefits would be insufficient to offset the costs of treatment and would lead to inefficiency.
We also report incremental net benefit for SIMSs versus SMUSs. Positive incremental net benefit values indicate an improvement in efficiency by adopting SIMSs over SMUSs, whereas negative incremental net benefit values indicate that SIMSs would reduce overall efficiency. As with the cost–utility analyses, results are reported based on multiple imputation of missing data, with imputations for WTP using multiple imputation with chained equations and using predictive mean matching, with 20 imputed data sets. Total WTP data were analysed using standard ordinary least squares regression models, with robust standard errors, clustered by centre. Bootstrapping was used to generate recycled predictions of cost and benefit (total WTP) pairs, and results are reported using scatterplots of the cost–benefit plane and cost–benefit acceptability curves to illustrate uncertainty around net benefit estimates.
Results
Details of missing cost and QoL data are presented in Appendix 8, Table 48. As can be seen from the table, it was not possible to calculate total costs across all time points for 60–70% of respondents, with the majority of missing data accruing from the participant-reported questionnaires (i.e. questionnaires, or responses within questionnaires, were missing). For QALYs, a complete set of utility time points was unavailable for approximately 40–50% of respondents. Therefore, it was deemed essential to conduct multiple imputation of missing data for the base-case analysis.
Intervention and follow-up costs (NHS perspective costs)
The following sections report descriptive statistics and cost difference results based on complete-case data. These costs include intervention costs up to the point of discharge and primary care and secondary care follow-up costs. The total NHS costs were calculated by multiplying the resource use (see Appendix 8, Table 49) by the appropriate unit costs outlined in Table 21.
Intervention costs
Details of costs are reported in Table 24. The cost of the intervention was, on average, lower for the SIMS group. On average, there were significant cost savings in the SIMS group in several of the resources that contributed to the operation costs: the type of anaesthesia administered, the availability of an anaesthetist in the index intervention and recovery time. The women in the SMUS group spent more time in theatre and recovery and more had GA for their surgery. The average cost of the intervention device was similar in both groups. Overall, the total cost of the initial intervention was significantly lower in the SIMS group, at –£180 (95% CI –£287 to –£73), than in the SMUS group.
| Resource | Trial group costs (£), mean (SD) [n] | SIMS vs. SMUS, mean differencea (95% CI) (£) | |
|---|---|---|---|
| SIMS | SMUS | ||
| Surgeon | 65 (38) [298] | 70 (41) [298] | –7 (–16 to 1) |
| Nurse | 72 (35) [298] | 74 (35) [298] | 4 (–11 to 3) |
| Supervised | 3 (15) [298] | 16 (37) [298] | –13 (–22 to –5) |
| Anaesthetist | 33 (49) [298] | 75 (39) [298] | –45 (–60 to –30) |
| Anaesthesia | 7 (9) [298] | 18 (8) [298] | –12 (–15 to –9) |
| Intervention device | 376 (147) [298] | 405 (175) [298] | –43 (112 to 26) |
| Analgesia | 13 (10) [298] | 9 (10) [298] | 3 (–1 to 8) |
| Theatre overheads | 297 (142) [298] | 303 (144) [298] | –18 (–47 to 11) |
| Recovery time | 113 (97) [298] | 131 (108) [298] | –21 (–35 to –8) |
| Overnight stay | 88 (206) [298] | 101 (244) [298] | –19 (–54 to 17) |
| In-and-out catheter | 1 (2) [298] | 0 (2) [298] | 0.1 (–0.4 to 0.6) |
| Indwelling catheter | 2 (5) [298] | 2 (6) [298] | 0.6 (–2 to 1) |
| Total intervention costs | 1069 (410) [298] | 1204 (530) [298] | –180 (–287 to –73) |
| Total 3-month costs | 21 (45) [231] | 28 (80) [204] | –8 (–19 to2) |
| 15 months: primary care costs | 71 (135) [202] | 62 (124) [165] | 6 (–22 to 35) |
| 15 months: secondary care costs | 184 (618) [275] | 174 (684) 265 | 21 (–80 to 121) |
| Total 15-month costs for primary and secondary care | 242 (637) [198] | 224 (777) [165] | 45 (–117 to 206) |
| Total 15-month costs (intervention, primary care and secondary care) | 1367 (593) [173] | 1587 (856) [146] | –218 (–369 to –68) |
| 24 months: primary care | 68 (179) [186] | 57 (156) [160] | 19 (–53 to 90) |
| 24 months: secondary care | 147 (774) [257] | 93 (883) [249] | –51 (–167 to 64) |
| Total 24-month costs for primary and secondary care | 227 (885) [184] | 92 (262) [157] | 150 (–37 to 338) |
| 36 months: primary care | 76 (280) [169] | 51 (131) [150] | 26 (–24 to 76) |
| 36 months: secondary care | 71 (328) [244] | 78 (408) [245] | 47 (–75 to 170) |
| Total 36-month costs for primary and secondary care | 124 (378) [169] | 100 (298) [149] | 48 (–73 to 169) |
| Total 36-month costs (intervention, primary care and secondary care)b | 1583 (1023) [101] | 1830 (1210) [83] | –238 (–508 to 32) |
Health service costs over trial follow-up period
The follow-up costs at 3, 15, 24 and 36 months were not significantly different between the groups. Details of secondary care resource use and costs are given in Appendix 8, Table 50. Overall, over the 36 months of follow-up, the mean cost saving for the SIMS group was –£254 per person (95% CI –£587 to £79). The results of the complete-case cost analysis should be interpreted cautiously, and in the light of the substantial amount of missing information to generate cost profiles.
Participant perspective costs
This analysis includes participant-incurred costs for attending their SIMS trial surgery and any follow-up visits that related to UI. Table 25 reports the costs of attending the index surgery, return to usual activities, appointments at their GP surgery and hospital outpatient and inpatient departments, over-the-counter purchases, payments to private health-care providers and purchases of pads. Women reported their return to usual activities in the questionnaire at 4 weeks after surgery; therefore, the maximum number of days (28) was used for those who reported that they had not returned to usual activities to estimate the opportunity cost of days away from usual activities. These costs were then summed across all the available cost data for participants and companion time and travel cost data to estimate the total cost over the 3-year follow-up period.
| Resource | Trial group costs (£), mean (SD) [n] | SIMS vs. SMUS, mean differencea (95% CI) (£) | |
|---|---|---|---|
| SIMS | SMUS | ||
| Intervention visit time off work and travel costs | 217 (103) [298] | 213 (123) [298] | –4 (–23 to 14) |
| Return to usual activities after index surgery | 1064 (606) [238] | 1192 (583) [223] | –145 (–275 to –14) |
| 3 months: patient time off work and travel to GP | 4 (13) [246] | 5 (15) [225] | |
| 15 months: patient time and travel | 7 (23) [231] | 5 (20) [201] | |
| 24 months: patient time and travel | 6 (26) [215] | 4 (25) [200] | |
| 36 months: patient time and travel | 4 (24) [204] | 2 (10) [185] | |
| Total: 36 months – GP visit patient time off and travel | 19 (79) [161] | 13 (33) [138] | 7 (–13 to 27) |
| 15 months: outpatient visit time and travel | 24 (75) [275] | 22 (64) [265] | |
| 24 months: outpatient visit time and travel | 14 (55) [257] | 8 (41) [249] | |
| 36 months: outpatient visit time and travel | 13 (71) [235] | 6 (400) [231] | |
| Total: 36 months – outpatient visit time and travel | 52 (185) [228] | 34 (118) [223] | 24 (–72 to 120) |
| 15 months: inpatient visit time and travel | 1 (11) [275] | 4 (33) [265] | |
| 24 months: inpatient visit time and travel | 5 (70) [257] | 1 (17) [249] | |
| 36 months: inpatient visit time and travel | 0 (0) [244] | 1 (12) [245] | |
| Total: 36 months – inpatient visit time and travel | 6 (74) [228] | 6 (38) [223] | 2 (–27 to 31) |
| Total: over-the-counter medicines | 1 (7) [183] | 2 (21) [157] | –2 (–6 to 2) |
| Total: pads purchase | 128 (244) [212] | 154 (258) [184] | –26 (–95 to 43) |
| Total: private health-care professional visit | 13 (100) [189] | 9 (60) [161] | 9 (–8 to 26) |
| Total paid | 137 (237) [175] | 154 (238) [144] | 20 (–52 to 92) |
| Total patient time and travel costsb | 1462 (739) [129] | 1560 (718) [106] | –89 (–280 to 102) |
| Total patient time and travel and NHS costs | 2935 (1363) [84] | 3305 (1709) [71] | –330 (–805 to 145) |
On average, women in the SMUS group took longer to return to usual activities than those in the SIMS group. Of the women who returned the questionnaire at 4 weeks, 61 out of 246 (27%) in the SIMS group and 66 out of 226 (29%) in the SMUS group had not returned to usual activities. For those who returned the questionnaire, the average time to return to usual activities was 12 (SD 7) days in the SIMS group and 15 (SD 8) days in the SMUS group. The cost difference in opportunity costs for return to usual activities was lower for the SIMS group (–£145, 95% CI –£275 to –£14) than for the SMUS group. The opportunity costs of time and travel, purchase of over-the-counter medicines and activities related to their urinary symptoms for the intervention, general practice visits, outpatient visits and inpatient stay were similar across the groups. The mean cost for the women who had complete cost data in the SIMS group [129/298 (43%)] was £1462 (SD £739); for the SMUS group [106/298 (36%)], it was £1560 (SD £718). The total cost difference was –£89 (95% CI –£280 to £102). The high SDs in both groups indicate that some women had high out-of-pocket costs.
Combining the women’s and the total NHS costs increased the cost difference to –£328 (95% CI –£804 to £147); however, it was not statistically significant. The wide CIs suggest that there was great variation in the costs incurred by the women. Some women had no costs and a few women had very high costs.
Table 26 provides descriptive data of complete-case utility scores and QALYs generated by combining utilities with duration of follow-up. The differences are based on linear regression models (GLMs), with adjustment for minimisation covariate, age and baseline utility score. The utility scores derived using the EQ-5D-3L indicate that the QoL at all time points was higher for the SIMS group, including at baseline. However, when calculating incremental QALYs adjusting for baseline imbalances in EQ-5D-3L utilities, SIMSs were associated with fewer QALYs gained over the 3 years (mean difference –0.089, 95% CI –0.156 to –0.023). There was little difference in ICIQ-LUTSqol utilities at any of the measurement time points, and there was no evidence of a difference in the derived QALYs between the groups. The EQ-VAS scores were higher in the SIMS group, but the differences in the scores were not statistically significant. These results need to be interpreted in the context that they are based on complete-case data across all utility measurement time points, of which between 43% and 47% are missing. It is important therefore to consider multiple imputation for the base-case cost-effectiveness analysis.
| Time point | Trial group, mean (SD) [n] | SIMS vs. SMUS, mean difference (95% CI)a | |
|---|---|---|---|
| SIMS | SMUS | ||
| Baseline | 0.860 (0.20) [286] | 0.834 (0.25) [284] | – |
| 4 weeks post surgery | 0.866 (0.17) [239] | 0.838 (0.21) [226] | 0.016 (–0.013 to 0.046) |
| 3 months post surgery | 0.878 (0.19) [255] | 0.855 (0.25) [226] | 0.002 (–0.031 to 0.034) |
| 15 months post randomisation | 0.848 (0.24) [249] | 0.825 (0.30) [219] | –0.010 (–0.053 to 0.030) |
| 24 months post randomisation | 0.865 (0.24) [232] | 0.816 (0.32) [212] | 0.015 (–0.025 to 0.055) |
| 36 months post randomisation | 0.836 (0.26) [217] | 0.821 (0.29) [205] | –0.004 (–0.041 to 0.033) |
| QALYsb,c | 2.376 (0.61) [171] | 2.387 (0.67) [159] | –0.089 (–0.156 to –0.023) |
| EQ-VAS | |||
| Baseline | 289 77 (22) | 287 73 (24) | – |
| 4 weeks | 244 81 (19) | 229 78 (19) | 2.137 (–0.366 to 4.641) |
| 3 months | 248 83 (19) | 225 79 (20) | 1.321 (–0.768 to 3.409) |
| 15 months | 252 79 (19) | 222 76 (20) | 0.385 (–1.464 to 2.234) |
| 24 months | 232 80 (20) | 214 77 (19) | 0.117 (–2.697 to 2.930) |
| 36 months | 220 78 (20) | 207 77 (20) | 0.197 (–3.683 to 4.078) |
| ICIQ-LUTSqol | |||
| Baseline | 0.94 (0.02) [291] | 0.94 (0.02) [284] | – |
| 3 months | 0.98 (0.02) [248] | 0.97 (0.02) [225] | 0.002 (–0.001 to 0.006) |
| 15 months | 0.98 (0.02) [247] | 0.98 (0.02) [218] | 0.001 (–0.003 to 0.005) |
| 24 months | 0.98 (0.02) [225] | 0.97 (0.02) [208] | 0.003 (–0.002 to 0.008) |
| 36 months | 0.98 (0.02) [217] | 0.97 (0.02) [201] | 0.003 (–0.002 to 0.008) |
| ICIQ-LUTSqol QALYb | 2.71 (0.15) [179] | 2.72 (0.13) [164] | 0.002 (–0.008 to 0.011) |
Numbers reporting problems on the EuroQol-5 Dimensions, three-level version, questionnaire
The proportion of women reporting problems was based on the number of women who reported that they had some or severe problems in the domains in the EQ-5D-3L questionnaire. The details of the number and percentage of women reporting problems are in Figures 12–17. The domain in which the lowest proportion of women reported problems, over all the time points that data were collected, was self-care (see Figures 12–17 and Appendix 8, Figure 24). At baseline, the proportion of women reporting problems was evenly distributed among the domains, apart from anxiety or depression, for which the proportion of women in SIMS group was lower (19%) than that in the SMUS group (33%) (see Figure 12). More women reported having problems with usual activities (SIMS group, 31%; SMUS group, 40%) at 4 weeks post surgery (see Figure 13) than at all the other time points, and more women in SIMS group (33%) than in the SMUS group (16%) reported having problems with pain or discomfort at 4 weeks post surgery. The proportion of women reporting problems with pain or discomfort and anxiety or depression at 15, 24 and 36 months ranged between 24% and 31% (see Figures 15–17). At 24 and 36 months, the number of women reporting problems in all domains apart from usual activities was higher than at baseline.
FIGURE 12.
Number and percentage of women reporting problems at baseline.
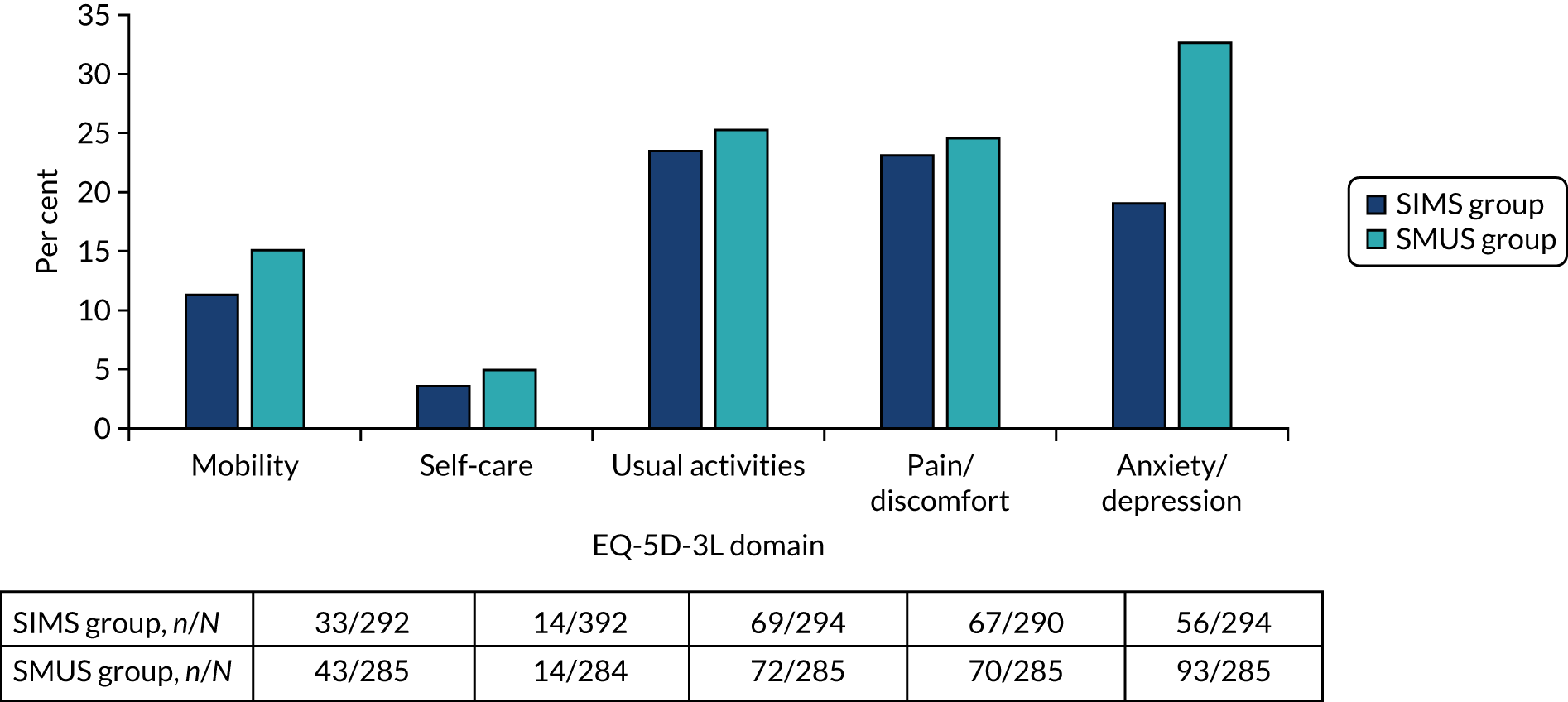
FIGURE 13.
Number and percentage of women reporting problems at 4 weeks.
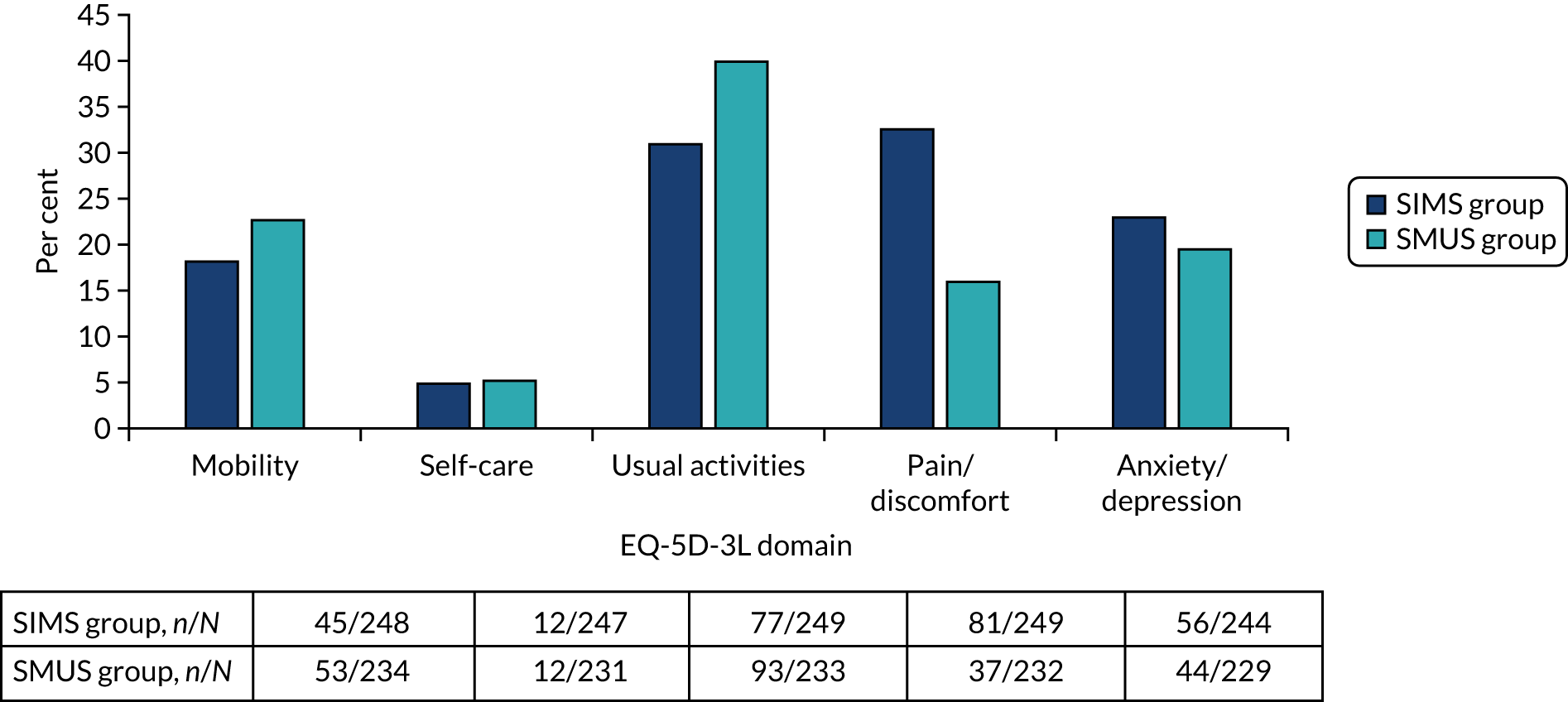
FIGURE 14.
Number and percentage of women reporting problems at 3 months.
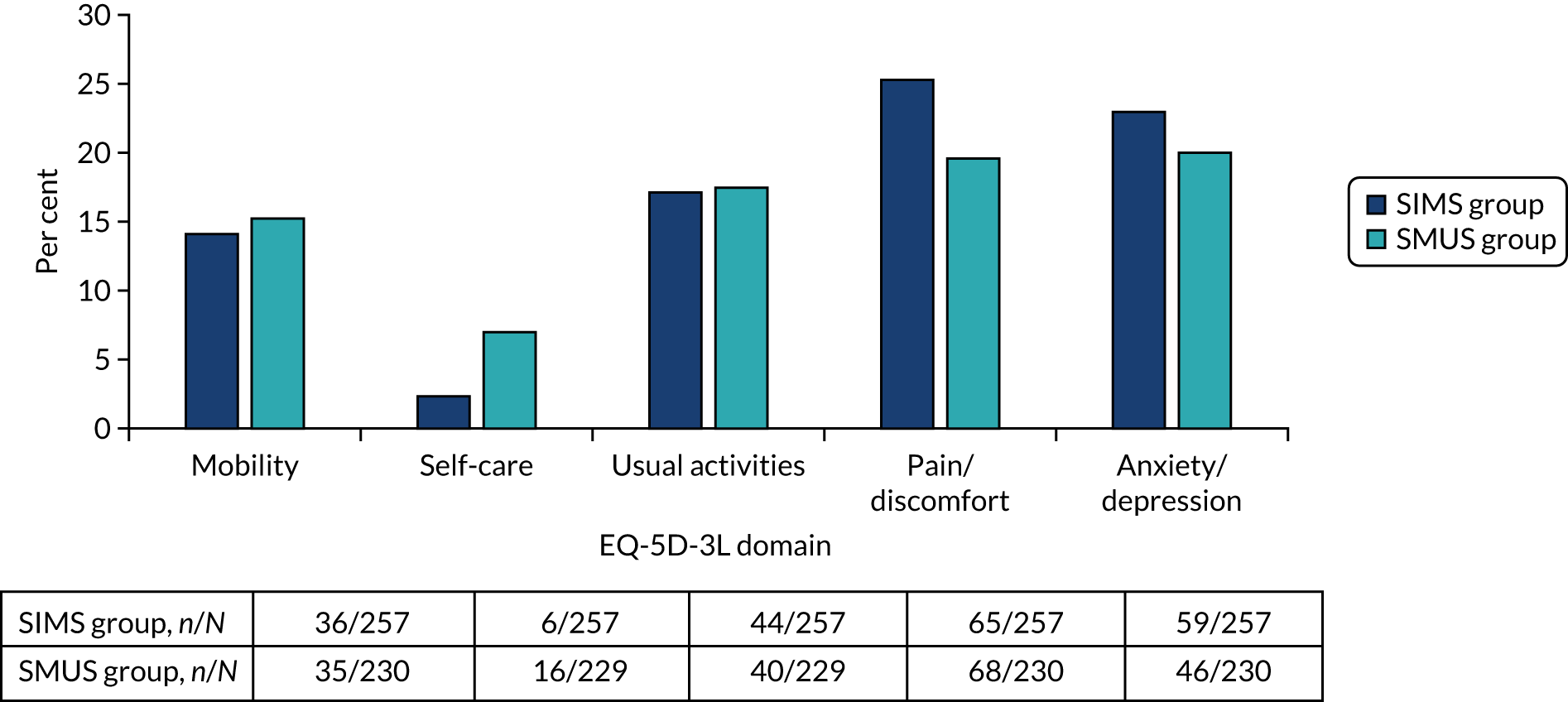
FIGURE 15.
Number and percentage of women reporting problems at 15 months.
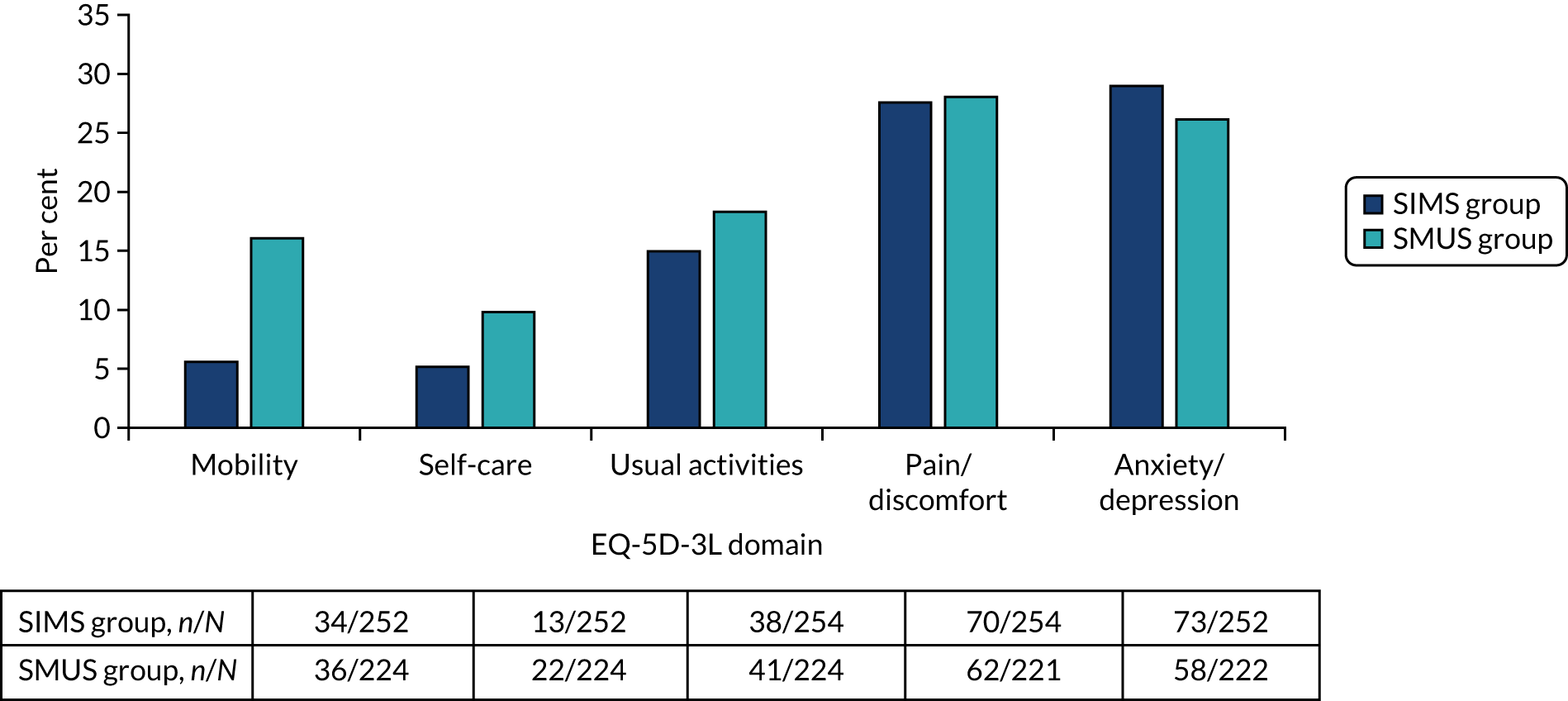
FIGURE 16.
Number and percentage of women reporting problems at 24 months.
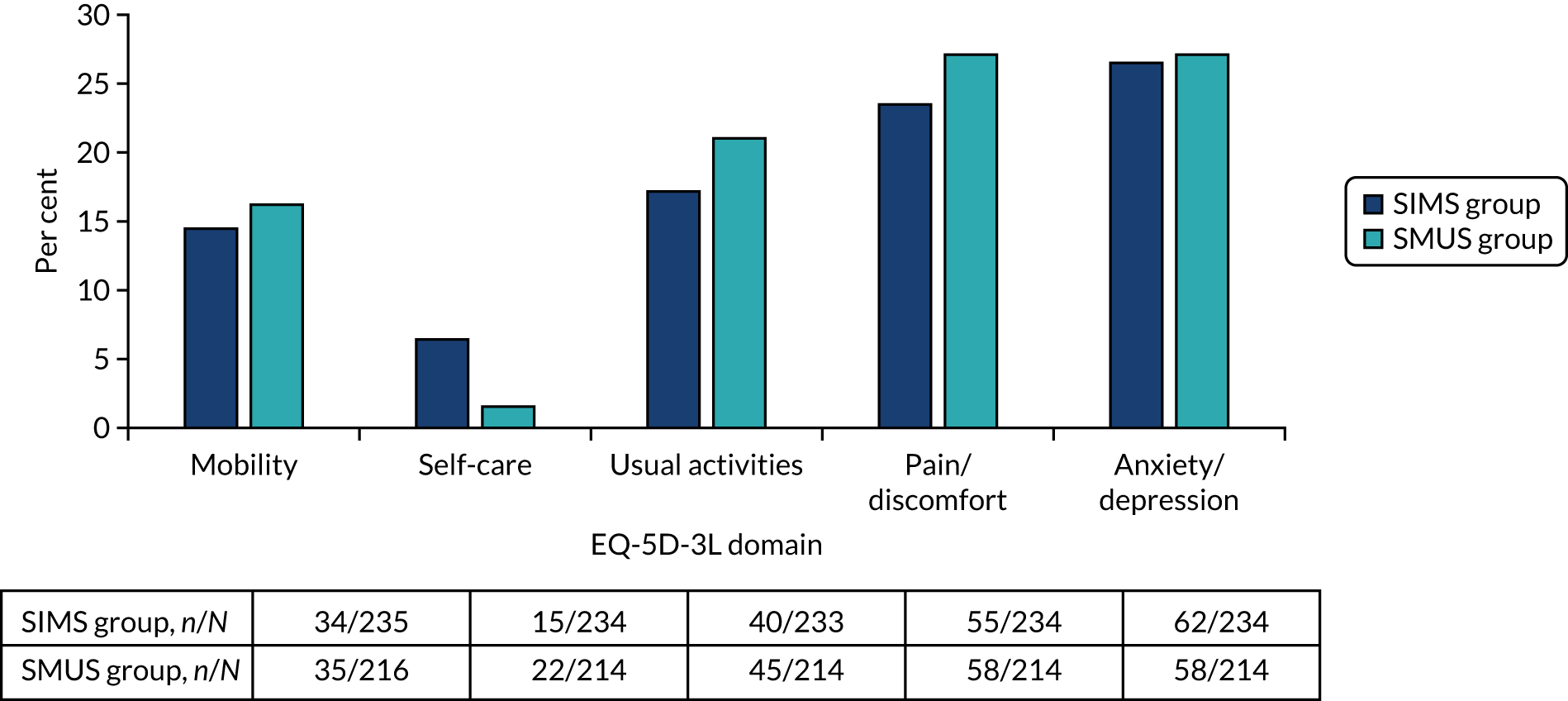
FIGURE 17.
Number and percentage of women reporting problems at 36 months.
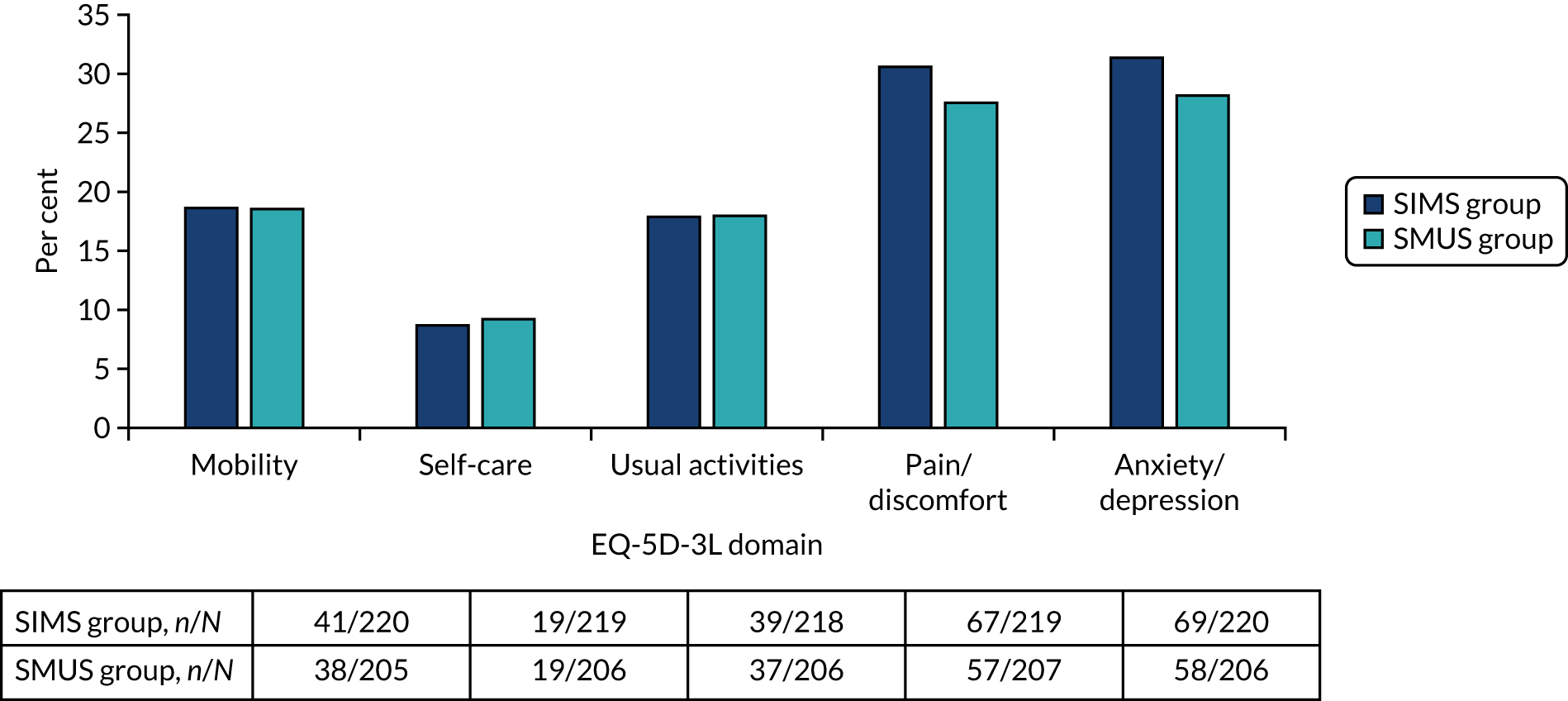
Cost-effectiveness analyses
Incremental cost-effectiveness analysis
Results of the within-trial analysis are reported as ICERs, calculated as the difference in costs divided by the differences in QALYs (SIMS vs. SMUS) over the 36-month follow-up period. To account for the substantial proportion of missing cost and QALY pair data (72%), the base-case and all sensitivity analyses, apart from the complete-case analysis, were conducted using multiple imputation of missing data. The base-case results in Table 27 show no differences in costs (–£6, 95% CI –£228 to £208) or QALYs (0.005, 95% CI –0.068 to 0.073) between the groups. The uncertainty in differences in costs and QALYs is illustrated by the width of the CIs and in Figure 18, which shows cost and QALY differences scattered in all the quadrants of the cost-effectiveness plane. The cost and QALY differences are in Figure 18 are well distributed in all quadrants of the cost-effectiveness plane. The CEAC (Figure 19) illustrates that there is a 56% probability that SIMSs will be considered cost-effective at the £20,000 WTP threshold value for a QALY.
| Intervention | Cost (£) | Cost difference (SIMS vs. SMUS)a (95% CI) (£) | QALY | QALY difference (SIMS vs. SMUS)a (95% CI) | ICER (SIMS vs. SMUS) (£) | Probability of being cost-effective at society’s threshold WTP values for a QALY (%) | ||
|---|---|---|---|---|---|---|---|---|
| £0 | £20,000 | £30,000 | ||||||
| Multiple imputation base-case analysis | ||||||||
| SIMS | 1696 | 2.347 | 51 | 56 | 56 | |||
| SMUS | 1702 | –6 (–228 to 208) | 2.342 | 0.005 (–0.068 to 0.073) | Dominatedb | 49 | 44 | 44 |
| Complete-case analysis (those with complete cost and QALY data) (SIMS group, n = 87; SMUS group, n = 77) | ||||||||
| SIMS | 1559 | 2.384 | 93 | 10 | 9 | |||
| SMUS | 1769 | –209 (–493 to 76) | 2.480 | –0.096 (–0.227 to –0.027) | 2187c | 7 | 90 | 91 |
| ICIQ-LUTSqol index imputation sensitivity analysis | ||||||||
| SIMS | 1696 | 2.706 | 51 | 48 | 49 | |||
| SMUS | 1702 | –6 (–228 to 208) | 2.708 | –0.001 (–0.029 to 0.023) | 4120 | 49 | 52 | 51 |
| Zero discount rate | ||||||||
| SIMS | 1714 | 2.520 | 50 | 62 | 63 | |||
| SMUS | 1715 | –2 (–229 to 217) | 2.508 | 0.011 (–0.061 to 0.081) | Dominated | 50 | 38 | 37 |
| 6% discount rate | ||||||||
| SIMS | 1685 | 2.321 | 53 | 56 | 56 | |||
| SMUS | 1693 | –9 (–227 to 202) | 2.317 | 0.005 (–0.068 to 0.071) | Dominated | 47 | 44 | 44 |
| Unadjusted QALYs | ||||||||
| SIMS | 1696 | 2.347 | 51 | 62 | 62 | |||
| SMUS | 1702 | –6 (–228 to 208) | 2.342 | 0.011 (–0.151 to 0.013) | Dominated | 49 | 38 | 38 |
| No assumption of CRF costs for those who did not have surgery | ||||||||
| SIMS | 1668 | 2.380 | 96 | 61 | 58 | |||
| SMUS | 1757 | –89 (–192 to 9) | 2.347 | 0.005 (–0.068 to 0.073) | Dominated | 4 | 39 | 42 |
| Societal perspective | ||||||||
| SIMS | 1887 | 2.347 | 53 | 56 | 56 | |||
| SMUS | 1.898 | –11 (–267 to 241) | 2.342 | 0.005 (–0.068 to 0.073) | Dominated | 47 | 44 | 44 |
| Subgroup analysis: aged < 65 years | ||||||||
| SIMS | 1646 | 2.368 | 89 | 62 | 61 | |||
| SMUS | 1715 | –69 (–179 to 39) | 2.362 | 0.006 (–0.055 to 0.064) | Dominated | 11 | 38 | 39 |
| Subgroup analysis: SUI | ||||||||
| SIMS | 1603 | 2.390 | 99 | 85 | 84 | |||
| SMUS | 1720 | –117 (–226 to –19) | 2.366 | 0.024 (–0.030 to 0.077) | Dominated | 1 | 15 | 16 |
| Subgroup analysis: had PFMT | ||||||||
| SIMS | 1661 | 2.360 | 79 | 54 | 53 | |||
| SMUS | 1703 | –42 (–141 to 53) | 2.360 | 0 (–0.066 to 0.061) | Dominated | 21 | 46 | 47 |
| Subgroup analysis: aged ≤ 48 years | ||||||||
| SIMS | 1637 | 2.400 | 81 | 97 | 97 | |||
| SMUS | 1686 | –49 (–169 to 70) | 2.340 | 0.060 (–0.004 to 0.132) | Dominated | 19 | 3 | 3 |
| Subgroup analysis: aged > 48 years | ||||||||
| SIMS | 1688 | 2.334 | 85 | 10 | 9 | |||
| SMUS | 1746 | –77 (–229 to 83) | 3.396 | –0.062 (–0.147 to 0.018) | 1255 | 15 | 90 | 91 |
FIGURE 18.
Incremental costs and QALYs for the SIMS group, compared with the SMUS group, using imputed costs and EQ-5D-3L QoL scores.
FIGURE 19.
The CEACs for the SIMS group vs. the SMUS group using imputed costs and EQ-5D-3L QoL scores.
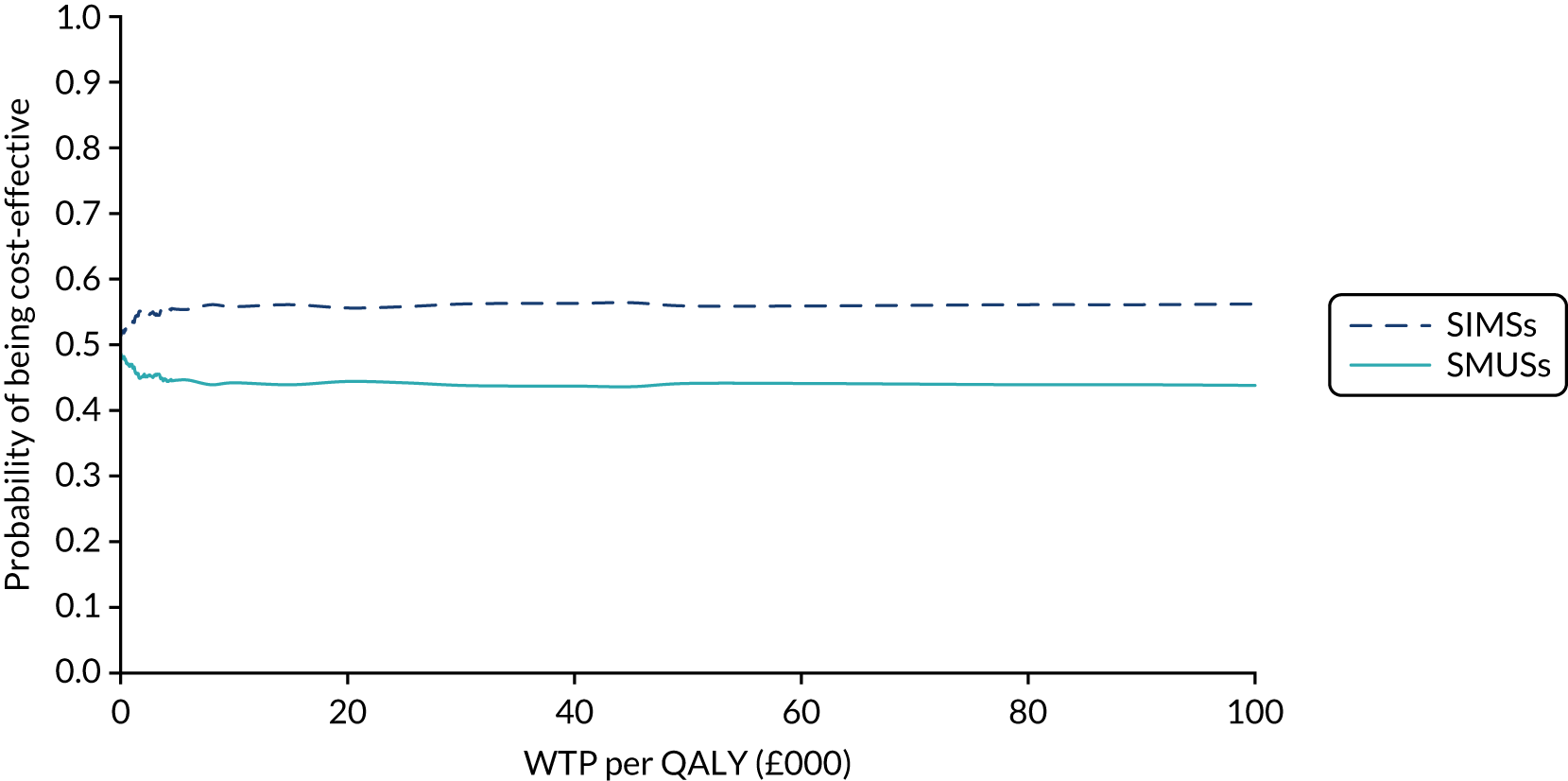
Deterministic sensitivity analysis
The results of the sensitivity analysis are presented in Table 27, and the scatterplots and CEACs are shown in Appendix 8, Figures 25–38. Some of the estimates of incremental cost and QALYs were sensitive to the approach taken.
The results of the complete-case sensitivity analysis suggest that, on average, SIMSs cost less than SMUSs (–£209, 95% CI –£493 to £76), but had fewer QALYs (–0.096, 95% CI –0.227 to 0.027). The estimates of cost and QALY differences fall mainly in the south-west quadrant of the cost-effectiveness plane (see Figures 25 and 26). SIMSs had an ICER of £2187 cost saving per QALY loss and a 10% chance that they would be considered cost-effective if society would require £20,000 cost savings to justify a QALY loss.
When the assumption was relaxed that the secondary care costs were zero for those who did not have surgery, the cost difference was higher for the SIMS group (–£89, 95% CI –£192 to £9) than for the SMUS group, and the QALY difference was 0.005 (base case) to 0.035 (95% CI –0.018 to 0.082). The probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold was higher than for the base-case analysis (61% vs. 56%) (see Figures 35 and 36).
The results that used ICIQ-LUTSqol data indicate that SIMSs cost less (–£6, 95% CI –£228 to £208) and were less effective (–0.001, 95% CI –0.029 to 0.023) than SMUSs. The ICER was £4120 cost saving per QALY loss and the probability that SIMSs are cost-effective at the £20,000 WTP threshold was 48% (see Figures 27 and 28).
The assumptions made in the calculation of the QALY, and discount rates applied to the costs, did not seem to have an impact on the overall results, with SIMSs always costing less than SMUSs and having more QALYs than SMUSs. The probability that SIMSs would be cost-effective at the £20,000 WTP threshold ranged between 56% and 62% for these analyses (see Figures 29–34). The societal perspective analysis that combined participant costs and NHS data did not have a substantial effect on overall findings for the cost–utility analysis (see Figures 37 and 38). SIMSs cost –£11 (95% CI –£267 to £241) less than SMUSs and had 0.005 (95% CI –0.068 to 0.073) more QALYs than SMUSs.
Subgroup analyses
Subgroup analyses were conducted; the results are presented in Table 27 for the analyses for which data were available.
Most of the women (78%) had a diagnosis of urodynamic stress incontinence. Results for this subgroup analysis indicate that SIMSs cost –£117 (95% CI –£226 to –£19) less and had 0.024 (95% CI –0.030 to 0.077) more QALYs than SMUSs. The probability that SIMSs would be cost-effective at the £20,000 WTP threshold was 85%.
Most of the women (87%) were aged < 65 years. Results for this subgroup analysis indicate that SIMSs cost –£69 (95% CI –£179 to £39] less and had 0.006 (95% CI –0.055 to 0.064) more QALYs than SMUSs. The probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold was 62%.
The number of women who had PFMT was equal in both group [254/298 (85%)]. Results for this subgroup analysis indicate that SIMSs cost –£42 (95% CI –£141 to £53) less and had 0.0003 (95% CI –0.066 to 0.061) more QALYs than SMUSs. The probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold was 54%.
For women who were aged ≤ 48 years, SIMSs cost –£49 (95% CI –£169 to £70) less and had 0.0003 (95% CI –0.066 to 0.061) more QALYs than SMUSs. The probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold was 97%. For women aged > 48 years, SIMSs cost –£77 (95% CI –£229 to £83) less and had –0.062 (95% CI –0.147 to 0.018) fewer QALYs than SMUSs. The probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold was 10%.
Cost–benefit analysis
The incremental cost–benefit analysis results are reported in Table 28 and in Figures 20 and 21. The cost difference is the same as for the base-case analysis and the incremental net benefit for SIMSs, compared with SMUSs, is –£941. The probability that SIMS would be considered cost-effective at the £20,000 WTP threshold is 9%.
| Intervention | Cost (£) | Cost difference (£) | Benefit (WTP) (£) | Benefit difference (£) | Incremental net benefit (SIMS vs. SMUS) (£) | Probability of being cost-beneficial at different thresholds of the benefit-to-cost ratio (%) | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | ||||||
| SIMS | 1696 | 7922 | 9 | 8 | 7 | |||
| SMUS | 1702 | –6 | 8869 | –947 | –941 | 91 | 92 | 93 |
| SIMS | 1887 | 7922 | 9 | 8 | 7 | |||
| SMUS | 1898 | –11 | 8869 | –947 | –936 | 91 | 92 | 93 |
FIGURE 20.
Scatterplot of incremental costs and incremental benefits (WTP) for SIMSs, compared with SMUSs.
FIGURE 21.
Cost–benefit acceptability curves: SIMS vs. SMUS using imputed costs and net benefit.

Chapter 9 Discussion
Parts of this chapter are reproduced from Abdel-Fattah et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/. The text below includes minor additions and formatting changes to the original text.
To our knowledge, the SIMS trial (conducted in 21 hospitals in the UK) is the largest trial worldwide to compare the clinical effectiveness and cost-effectiveness of SIMSs with those of SMUSs, and is possibly the largest trial to assess MUSs in women. Participants were randomised to receive either a SMUS (i.e. RP-TVT or TO-TVT) or the relatively new adjustable anchored SIMS, with initial follow-up of 3 years.
Principal findings
The results show that patient-reported success rates in the SIMS group were non-inferior to those of the SMUS group at all time points of assessment (4 weeks, 3 and 15 months, and 2 and 3 years). The primary outcome was clinical effectiveness, defined as patient-reported success at 15 months post randomisation (≈ 1year post surgery); the results show that adjustable anchored SIMSs were non-inferior to tension-free SMUSs at the 10% margin [SIMS group, 79.1% (212/268) vs. SMUS group, 75.6% (189/250), RD 4.6, 95% C I–2.7 to 11.8; pNI < 0.001]. Similarly, at 3 years’ follow-up, patient-reported success rates in the SIMS group were non-inferior to those of the SMUS group at the 10% margin: 72% and 66.8% for the SIMS and SMUS groups, respectively (RD 5.7, 95% CI –1.3 to 12.8; pNI < 0.001). The results were confirmed in the per-protocol analysis and across prespecified subgroups. More women in the SIMS group than in the SMUS group had their procedure under LA (73% vs. 6%, respectively). Those in the SIMS group had less postoperative pain 2 weeks post surgery, and a shorter hospital stay. The analysis showed that the initial rates of groin/thigh pain for the SIMS and SMUS groups at 15 months were 14.9% and 11.9%, respectively [RD 3.0, 95% CI –1.1 to 7.1; (superiority) p = 0.144], but by 36 months the rates were similar: 14.1% and 14.9% for the SIMS and SMUS groups, respectively (RD –0.8, 95% CI –4.1 to 2.5; p = 0.613). Tape/mesh exposure rates were higher for SIMS than for SMUS participants [9/276 (3.3%) vs. 5/261 (1.9%), respectively] (RD 1.3, 95% CI –1.7 to 4.4; p = 0.373) over the 36-month follow-up. More women in the SIMS group than in the SMUS group received further surgical treatment (n = 24 vs. n = 12, respectively) for UI or treatment of AEs over the 36 months. The total rates for mesh removal (partial/complete) for any indication were comparable [SIMS group, n = 8 (2.9%); SMUS group, n = 5 (1.9%)]. There were no significant differences in the scores of the QoL and the sexual function questionnaires, but more women in the SIMS group (17.2%) than in the SMUS group (5.5%) reported dyspareunia (RD 11.9%, 95% CI 3.5% to 20.1%). There is no evidence that SIMSs or SMUSs are superior on the grounds of cost-effectiveness.
Trial team and setting
The SIMS trial team included experienced trialists, statisticians, health economists, patient representatives and urogynaecologists with demonstrated experience in MUS and SIMS procedures under LA (clinical experts). The trial team were supported by a clinical trials unit (CHaRT) with a strong track record in designing and delivering complex surgical trials. The depth of experience in the team and the CHaRT enabled the trial to overcome the significant obstacles it faced in recruitment, as explained below.
The SIMS trial was pragmatic by design to ensure that findings would be generalisable to the wider NHS setting. The 21 collaborating hospitals are a mixture of high- and medium-volume centres, in using MUSs and in performing continence surgery in general, from England (n = 16), Wales (n = 2) and Scotland (n = 3). The surgeons included in the trial were a mixture of gynaecologists and urologists with varying degrees of seniority, but all had demonstrable experience in performing SMUS and/or SIMS procedures prior to enrolling in the trial. Clinical experts in the trial team visited the majority of collaborating hospitals prior to starting local recruitment to observe the collaborating surgeons’ performing SIMS procedures under LA, confirm surgeons’ competence as per protocol and discuss standardisation of surgical techniques and protocols. The choice of devices used was down to the surgeon’s experience and the local standard practice in the unit. Hence, the results are generalisable to all hospitals and surgeons in the UK.
Procedures
This was a pragmatic clinical trial, hence there were variations in the way the procedures were performed according to surgeons’ experiences (e.g. to tension the MUS using scissors versus babcock and/or using the CST versus not) and the local protocols. However, the trial team has taken a number of steps to help achieve a reasonable standardisation of the procedures and the relevant protocols.
-
The trial protocol described the SIMS and the SMUS procedure steps as originally described in the literature and provided guidance for a standardised LA protocol that was used successfully in a previous small RCT. 74 Guidance for the postoperative voiding assessment was also introduced to help standardise this process.
-
In the SMUS group (standard surgery arm), surgeons used their standard techniques; however, infiltration of LA at the start of the procedure was recommended even if the procedure was performed under GA or spinal anaesthetic. Intraoperative cystoscopy (rigid or flexible) was performed as a standard to ensure the detection of any LUT injury.
At the time of the SIMS trial design, MUSs were the most common surgical treatment for SUI worldwide. Between April 2008 and March 2017, 100,516 MUS procedures were performed in England, compared with 1195 for all other procedures. 32 In 2009, Smith et al. 67 showed that TO-TVT, compared with RP-TVT, is the most common primary continence procedure worldwide, with a minor margin. Despite MUSs originally being described as performed under LA and sedation, the surgical database of the BSUG in 2010 showed that RP-TVT and TO-TVT procedures are predominately performed under GA. 59
-
In the SIMS group (trial arm), the procedure was offered under LA with a CST and intraoperative cystoscopy (rigid or flexible) as standard.
The criteria laid out for adjustable anchored SIMSs were to ensure a robust anchoring mechanism and post-insertion adjustability. These were identified in previous systematic reviews and various basic science studies at the time of the trial design as key criteria for the success of mini-slings and represent the fundamental differences of SIMS, compared with the earlier version of mini-slings (TVT Secur), which were consistently shown to have inferior medium- and long-term success rates, whereas the Velcro® (Velcro IP Holdings LLC, Knutsford, UK) mechanism of anchorage (such as in the TVT Secur) was the weakest. We fully described this in the published protocol:1
All currently available SIMS share the same tape material (type 1 polypropylene) and the insertion technique through a single vaginal incision; however, they differ in the type/robustness of the anchorage mechanism used.[79,80] A number of recently developed SIMS, such as Ajust, Altis, and TFS, have an added advantage that allow post-anchorage adjustment of the sling tension and have been shown in independent animal studies, assessing their immediate and delayed extraction forces, to be associated with the strongest and most robust anchoring mechanism to the obturator complex. The Velcro® (Velcro IP Holdings LLC, Knutsford, UK) mechanism of anchorage (such as in the TVT Secur) was the weakest.[79,80]
The above have been highlighted in the European Association of Urology guidelines132 on the management of UI, in which MUSs were classified into two: (1) ‘tension free mid-urethral slings’, including all standard tension-free MUSs (SMUS: RP-TVT and TO-TVT) and TVT Secur; and (2) the ‘anchored mid-urethral slings’, which included the new adjustable anchored SIMS.
Compliance with the allocated intervention was high, at ≥ 95% in both arms. Cross-over between groups was 5.4% in the SIMS group (n = 16) and 1.5% in the SMUS group (n = 4). In addition, three SIMS participants underwent other procedures (MiniArc or autologous fascia sling). Cross-over was a result of patient preference, or unavailability of a specific device or an experienced surgeon on the day of surgery. These are all recognised events that can occur in standard day-to-day NHS practice, reflecting the pragmatic nature of the trial.
It was not possible to blind the surgeons or participants, given the nature of the procedures: the SIMS procedure is performed under LA, whereas the SMUS procedure is performed under GA. Primary outcome collection was predominantly patient-reported through postal questionnaires, essentially removing the clinical assessors’ bias.
Recruitment
The trial started recruitment in February 2014; however, since early 2013, there had been a highly publicised debate on the use of mesh in women with SUI and prolapse worldwide. MUS procedures were suspended in Scotland in June 2014, and in the whole of the UK in 2018; these suspensions are still in force today. Hence, the entire recruitment and follow-up in the SIMS trial occurred during the heightened public and medical debate on the safety and effectiveness of mesh-based procedures. This led to a significant slowdown in recruitment from June 2014 onwards and, consequently, an extension of the recruitment period to > 3 years. The recruitment target was also reduced to 600 participants without reviewing any outcome data, with a reduction in trial power from 90% to 88%. On the other hand, a significant advantage gained from these circumstances was the fact that participants were very much aware of the public debate over mesh procedures; this means the results are most unlikely to underestimate the AE rates as these were predominantly reported by participants on annual questionnaires.
A total of 600 women were recruited. Three of the four women excluded post randomisation were ineligible for the trial because of either previous continence surgery or receiving a concomitant procedure. One woman had the surgery privately and we were unaware of any operative details or operative date. Previous continence surgery is an exclusion criterion in most surgical trials assessing MUSs. Women who have previously had unsuccessful continence surgery require a different approach for investigation and management than those undergoing primary continence surgery. Management plans are often individualised depending on the type of previous continence surgery and time from index surgery to failure.
Undergoing concomitant prolapse surgery was an exclusion criterion. At the time of the trial design, there were three main reasons for this, all of which remain valid to date.
-
The SIMS procedure was offered under LA as standard. LA is not compatible with the vast majority of concomitant prolapse procedures.
-
The main trial hypothesis was that ‘patient-reported success rate following surgical treatment with adjustable anchored SIMS procedures is non-inferior to tension-free SMUS, while the former is associated with less postoperative pain, shorter hospital stay, earlier recovery and consequently earlier return to usual activities/work, and is more cost-effective than SMUS’. 1 If concomitant prolapse procedures are performed, they will be the main drivers of postoperative pain, length of hospital stay and recovery time. They can have very different patterns; for example, concomitant anterior repair is a moderate procedure with 1 or 2 days’ hospital stay, minimal postoperative pain and ≈ 4 weeks’ recovery, whereas concomitant anterior repair, vaginal hysterectomy and sacrospinous fixation is a major procedure with 4 days’ hospital stay, significant postoperative pain and a prolonged recovery period of 10–12 weeks. Hence, it would not be possible to determine with any degree of certainty the secondary outcomes of interest and to capture true differences between groups in postoperative pain, hospital stay and recovery.
-
Current literature shows that over two-thirds of SUI continence procedures are performed as stand-alone surgeries. In addition, if concomitant prolapse is present, there is currently uncertainty if it is best to treat both prolapse and SUI in the same setting or as interval surgery, that is correcting the prolapse first then conducting surgery for continence if needed later.
Randomisation
Randomisation was predominately done on the day the participant was added to the waiting list for surgery, rather than in the operating theatre. The trial team believed that it was necessary to randomise participants well in advance of the day of surgery for several reasons: the SIMS procedure was offered under LA as standard, compared with the SMUS procedure, which was offered under GA as a standard, with obvious different theatre requirements. In some recruiting centres, advance notice was required to ensure the availability of the device to be used on the day of surgery. Furthermore, in the context of the public debate on mesh, it was important that participants were given as much time as possible to further consider their options. At least one participant changed her mind after randomisation and opted for non-mesh procedure (autologous fascia sling).
At the time of the trial design, it was estimated that the waiting time for surgery (from randomisation) would be approximately 3 months (90 days), hence the decision to obtain the primary outcome at 15 months, that is ≈ 1 year postoperatively. Our results showed that the time from randomisation to surgery was similar between the two groups, and within our assumptions: SIMS group versus SMUS group – mean 65 (SD 69) days versus 64 (SD 59) days, respectively; median 48 [interquartile range (IQR) 16–93] days versus 47 (IQR 19–93) days, respectively. These were also confirmed on measuring the time between surgery and completing the primary outcome to be an average of 13.5 months: SIMS group versus SMUS group – mean 412 (SD 69) days versus 416 (SD 65) days, respectively; median 421 (IQR 379–453) days versus 431 (IQR 380–452) days, respectively.
Missing data
It is not possible to know whether or not data are missing at random (informative missingness) by inspecting the data. However, we felt that if those who were missing data on the primary outcome at 15 months shared roughly the same baseline characteristics with those who did have the primary outcome at 15 months, then that could be consistent with a missing-at-random assumption. In addition, the number of missing data was felt to be insufficient to meaningfully attempt sensitivity-type analyses (e.g. using the sigma-adjustment approach detailed in van Buuren’s133 Flexible Imputation of Missing Data under a missing-not-at-random assumption).
Operative outcomes
More women in the SIMS group than in the SMUS group had their procedure under LA (73% vs. 6%, respectively) and had their sling adjusted using the CST (65.2% vs. 5.7%, respectively). The results are in line with our first study in this series of interlinked projects showing the acceptability (71%) and feasibility (97%) of adjustable anchored SIMSs under LA. 74 The authors believe that the role of an accompanying nurse and continuous communication with the surgeon cannot be overemphasized for reducing patient anxiety during LA procedures. There is no robust evidence to prove or refute the impact of (1) the patient being awake and (2) undertaking an intraoperative CST on the results for SIMSs or SMUSs. In a study evaluating the CST among 90 women receiving SIMSs (Ajust), the authors concluded that:
The performance of a cough test during the placement of an adjustable single-incision sling for the treatment of SUI does not affect the functional outcome, and is therefore not necessary.
Engberts et al. 134
Similarly, there is low-quality evidence in the literature that the CST did not affect the outcome of the SMUSs. Interestingly, one study suggested that the CST may lead to overtreatment and complications. 135
More procedures in the SMUS group (n = 43, 16.5%) were performed by senior trainees who completed their training in performing MUS procedures. The SIMS procedure is not part of the standard training programme for clinicians; hence, only four SIMS procedures (1.4%) were performed by a senior trainee who was deemed competent by their supervisor.
The results show that SIMS participants had more favourable pain scores up to 14 days (difference –8.3, 95% CI –12.8 to –3.8; p = 0.001) and shorter postoperative hospital stays (difference –2.5, 95% CI –4.7 to –0.3; p = 0.029). The impact of an average of 2 hours less in postoperative stay is debatable and, in the authors’ opinion, is unlikely to be of real-life clinical significance. Most participants received LA infiltration regardless of the procedure (SIMS group, 98%; SMUS group, 90%). This is important to reduce the possible confounding effect of LA infiltration on the immediate postoperative pain assessment and hospital stay. Our results agree with those of previous small RCTs. In our previous small RCT136 comparing the SIMS Ajust with TO-TVT (n = 137), women in the SIMS Ajust group had a significantly lower postoperative pain profile (p < 0.001) up to 4 weeks postoperatively and shorter hospital stays [median 3.65 (IQR 2.49–4.96) days] than the TO-TVT group [median 4.42 (IQR 3.16–5.56) days] (95% CI –0.026 to 1.326). Our systematic review/meta-analysis81 in 2014 identified 26 RCTs (n = 3308) and showed that people receiving SIMSs had significantly lower postoperative pain scores than people receiving SMUSs: WMD –3.13, 95% CI –4.89 to –1.36.
In the SIMS trial, there were no significant differences between groups in participants returning to normal activities within 28 days. This is different to earlier smaller studies in the literature. In our previous small RCT comparing the SIMS Ajust with TO-TVT (n = 137),136 women in the SIMS Ajust group had significantly earlier return to normal activities (p = 0.025) than women in the TO-TVT group. In one RCT of SIMSs versus SMUSs, Schellart et al. 137 randomised 97 participants to the SIMS MiniArc and 96 participants to the SMUS Monarc (American Medical Systems, Inc.), and, in another, Xin et al. 138 compared the SIMS Ajust with the SMUS TVT-O (n = 368) using similar assessment tools. In both RCTs, the SIMS group had less immediate postoperative pain and shorter recovery time. Our systematic review/meta-analysis81 in 2014 (n = 3308) showed that SIMSs were associated with significantly earlier return to normal activities and to work (SIMS group, WMD –5.08, 95% CI –9.59 to –0.56; SMUS group, WMD –7.20, 95% CI –12.43 to –1.98).
Response rates
At 1 and 3 years post randomisation, the participant response rates were excellent, at 87% and 81%, respectively. Our results compared favourably with the Evaluation of Transobturator Tapes (E-TOT) study139 (n = 314) comparing the inside–out and outside–in TO-TVT in a single tertiary centre in the UK and using similar assessment tools for patient-reported outcomes. In the E-TOT study, the participants’ response rates were 88%, 70% and 68% at 1, 3 and 9 years of follow-up, respectively. 65,139,140 In 2019, Alexandridis et al. 141 reported a 73% (205/279) response rate at the 3-year follow-up in a multicentre (n = 7) RCT comparing the SIMS Ajust with the SMUS TO-TVT, and Nikpoor et al. 142 reported an 84% (207/246) response rate at the 3-year follow-up in a RCT of the SIMS MiniArc versus the SMUS tension-free vaginal tape (TVT) Abbrevo® (Ethicon, Inc.). In 2018, Lee and Cho143 reported a lower response rate of 68% (125/185) at 36 months of follow-up in their RCT. In 2016, Schellart et al. 137 reported a 77.5% (150/193) response rate at 36 months in their RCT comparing the SIMS MiniArc with the SMUS TO-TVT.
We have applied a number of successful strategies that led to excellent participant engagement and minimal participant drop-out rates.
-
Regular participant newsletters. These are instrumental to:
-
keep the participants well informed of the trial progress
-
provide balanced interpretation of significant media publications or events within the mesh debate and their impact, if any, on the SIMS trial (such as the Scottish Government and, later, the UK government decisions to halt the mesh procedures; the Cumberlege report144)
-
encourage participants to respond to yearly questionnaires, explain the importance of responding, provide updates on response rates and express gratitude to responders.
-
-
We provided alternative ways to respond to questionnaires, including full-length postal questionnaires, shortened questionnaires for non-responders and telephone follow-up for persistent non-responders. We also used text messages to capture daily postoperative pain in the immediate postoperative period.
-
Sending participants vouchers on returning completed questionnaires as a token of appreciation and recognition of their time spent on the trial.
-
On occasions when communication between participants and the collaborating hospital was difficult, the trial office made appropriate inquiries at the request of some participants and helped them receive responses to their queries. These gestures were appreciated by both our participants and collaborating hospitals.
Primary outcome
The primary outcome was patient-reported success rate, defined as outcomes of ‘very much improved’ or ‘much improved’ on the PGI-I scale.
The patient-reported success rates reflect patients’ experiences, compared with the objective measures, which can overestimate the success of SUI surgery. Tincello and Alfirevic145 reported that objective assessment of cure rates gives an overoptimistic picture of the success of surgery for SUI. In a questionnaire-based survey including patients, incontinence nurses and surgeons, all groups rated patient-reported cure and improvement in patients’ QoL as the most important outcomes for surgical treatment of SUI and the authors concluded that the idea of objective assessment following surgical treatment for SUI should be re-examined.
The Patient Global Impression of Improvement scale
The Patient Global Impression scale is a global index that is widely used to rate the response of a condition to a therapy (transition scale). The PGI-I scale is a simple, direct and easy-to-use scale that is intuitively understandable to clinicians and patients. 146 The PGI-I scale has excellent construct validity, compared with various assessment variables: incontinence episode frequency, the Incontinence Quality of Life Questionnaire and the fixed-volume (400 ml) stress pad test. 147 In a benchmark study, Yalcin and Bump102 reported a secondary analysis of data from two double-blind, placebo-controlled clinical trials (n = 1133) that evaluated the treatment of women with predominant SUI. The authors showed that significant correlations (p < 0.0001) were observed between the PGI-I scale response categories and three independent measures of improvement in SUI (0.49, 0.33 and –0.43 for incontinence episode frequency, stress test and Incontinence Quality of Life Questionnaire results, respectively). This important study established the construct validity of the PGI-I scale for the evaluation of the baseline severity of, and treatment response among women with, SUI.
Since that study,102 the PGI-I scale has been widely used in clinical trials assessing surgical and conservative interventions for UI among women.
-
The botulinum toxin A versus placebo for refractory detrusor overactivity in women (RELAX) trial148 evaluated the PGI-I scale as an outcome assessment tool among women with refractory OAB symptoms requiring Botox treatment. The authors commented that their results showed that ‘the PGI-I scales are robust and valid instruments to assess disease severity, bother and improvement after treatment in women with detrusor overactivity’. 148
-
Brubaker et al. 149 used the PGI-I scale to measure the primary outcome in their RCT evaluating Botox treatment among women with refractory OAB. The authors showed that the PGI-I scale was able to detect differences in responses between groups.
-
The PGI-I scale was used to measure the primary outcome in our previous small RCT (n = 137)86 comparing the SIMS Ajust with the SMUS TO-TVT for the surgical treatment of SUI in women. 86 The results showed an excellent response rate at 1 and 4 years of follow-up.
-
The PGI-I scale was used to measure the primary outcome in the E-TOT study comparing inside–out and outside–in TO-TVTs, which reported outcomes at 1, 3 and 9 years. 65,139,140
-
The 10-year outcomes were recently (in 2016 and 2017) reported for two clinical trials of surgical treatment of SUI among women. 150,151 They both used the PGI-I scale to measure their primary patient-reported outcomes.
-
The PGI-I scale is currently used as the primary outcome comparing surgical interventions for the treatment of UUI in the Health Technology Assessment (HTA)-funded Female Urgency, Trial of Urodynamics as Routine Evaluation (FUTURE) study. 152
Patient-reported success rates
The patient-reported success rates in the SIMS group were non-inferior to those of the SMUS group at all time points of assessment (i.e. 4 weeks, 3 and 15 months, and 2 and 3 years). The primary outcome was clinical effectiveness, defined as patient-reported success at 15 months post randomisation; the results showed that adjustable anchored SIMSs were non-inferior to tension-free SMUSs at the 10% margin [SIMS group, 79.1% (212/268) vs. SMUS group, 75.6% (189/250), RD 4.6, 95% CI –2.7 to 11.8; pNI < 0.001]. At 2 and 3 years’ follow-up, patient-reported success rates in the SIMS group continued to be non-inferior to those of the SMUS group at the 10% margin [at 2 years: SIMS group, 77.4% (185/239) vs. SMUS group, 74.2% (167/225), RD 3.9, 95% CI –4.2 to 11.9; pNI < 0.001; at 3 years: SIMS group, 72% (177/246) vs. SMUS group, 66.8% (157/235), RD 5.7, 95% CI –1.3 to 12.8; pNI < 0.001]. We used ITT analysis, which is the standard analysis method for non-inferiority clinical trials, but we also undertook a per-protocol analysis, which showed similar results all time points. Similarly, non-inferiority was confirmed in a range of sensitivity analyses for the primary outcome. These all give assurances on the robustness of the results and provide certainty in concluding non-inferiority of patient-reported success in the adjustable anchored SIMS group, compared with the tension-free SMUS group, at up to 3 years of follow-up.
To our knowledge, the SIMS trial is the largest trial to assess SIMSs versus SMUSs. Previous studies were small, heterogeneous and at high risk of bias. 153–159
-
Schellart et al. 137 randomised 97 participants to the SIMS MiniArc and 96 participants to the SMUS Monarc (TO-TVT). At 1 year, the patient-reported (PGI-I scale) success rates were 83% and 86% (p = 0.46) for the MiniArc and Monarc groups, respectively, and the objective (CST) success rates were 89% and 91% (p = 0.65) for the MiniArc and Monarc groups, respectively. 137 At 3 years, the subjective cure rates were 86% in the MiniArc group and 87% in the Monarc group (RD –0.6%, 95% CI –12% to 11%). The objective cure rates were 89% in the MiniArc group and 88% in the Monarc group (RD 1.3%, 95% CI –9% to 11%). Lee and Cho143 reported 3 years’ follow-up for a RCT (n = 185) comparing the SIMS MiniArc with the SMUS RP-TVT. They showed that subjective cure rates were 85% and 87% for the MiniArc and RP-TVT groups, respectively, and objective cure rates were 84% and 89% for the MiniArc and RP-TVT groups, respectively, with no statistically significant difference between groups (p > 0.05). Xin et al. 138 compared the SIMS Ajust with the SMUS TVT-O in a RCT (n = 368) using similar assessment tools. At 1 year, no statistically significant differences in subjective and objective success rates were seen between groups (p = 0.171 and p = 0.195, respectively).
-
In 2019, Alexandridis et al. 141 reported a multicentre RCT (n = 279) comparing the SIMS Ajust with the SMUS TO-TVT, which used block randomisation. The main outcome evaluated was the subjective cure rate as reported through the ICIQ-UI-SF. At 3 years of follow-up, no significant difference was observed in success rates between the groups (50.9% vs. 51.5% for the Ajust and TO-TVT groups, respectively; p = 0.909). Nikpoor et al. 142 reported a RCT of the SIMS MiniArc versus the SMUS TVT Abbrevo (n = 246). At 3 years of follow-up, there was no statistically significant difference between the groups in subjective (59% vs. 69.5% for the MiniArc and TVT Abbrevo groups, respectively; p = 0.14) or objective (92.6% vs. 98.7% for the MiniArc and TVT Abbrevo groups, respectively; p = 0.12) cure rates. They also undertook a sensitivity analysis taking into account all missing data, which confirmed their results. Enzelsberger et al. 160 compared 90 women randomised to either the SIMS MiniArc or the SMUS TO-TVT in a single centre. At 24 months, the patient-reported success rates were not significantly different, at 82% and 86% for the MiniArc and TO-TVT groups, respectively. The authors concluded that the MiniArc reduces perioperative morbidity, while also associated with similar continence rates. Masata et al. 161 compared the outcomes of 100 women randomised to either the SIMS Ajust or a SMUS TO-TVT in a single centre. Their results showed that women in the Ajust group had significantly lower intensity, and shorter duration, of postoperative pain. At 2 years’ follow-up, there was no evidence of a significant difference in patient-reported success rates (Ajust, 83.3%; TVT-O, 82%).
-
These RCTs137,138,141–143,160,161 were all underpowered to detect non-inferiority. In addition, they compared specific SIMSs (Ajust, MiniArc) with TO-TVTs (Monarc, TVT Abbrevo) or RP-TVT, and hence were limited in generalisability. It was interesting to see a wide range of patient-reported success rates at 3 years (50–87%). This is most likely due to using different definitions for success and various assessment tools for the primary outcome. Nevertheless, all these RCTs showed no statistically significant differences in patient-reported and objective success rates between the SIMS and the SMUS groups. In contrast, a number of earlier reported RCTs showed different results. In 2013, Basu and Duckett162 compared the SIMS MiniArc with the SMUS RP-TVT in a small RCT (n = 71). Treatment was considered to have failed if patients documented SUI on the symptom domain of the KHQ or underwent repeat surgery for SUI. The overall 3-year failure rates were 52.6% and 9.0% (OR 10.0, 95% CI 2.6 to 38.4) in the MiniArc and RP-TVT groups, respectively. One explanation for the poor early outcomes could be the surgeons’ lack of experience in the new technique, compared with their standard technique. However, lack of surgeon experience was not to blame in the case of TVT Secur, the earliest type of SIMS. TVT Secur was introduced into clinical practice in 2006 and was withdrawn in 2013 because of inferior objective and patient-reported outcomes when compared with SMUSs. The manufacturer, however, announced that the withdrawal was based primarily on commercial reasons. 163 Unlike adjustable anchored SIMSs, the TVT Secur depended on the Velcro technique for stabilisation of the sling (no anchoring mechanism) and had no mechanism for adjusting the sling after insertion. In 2015, Masata et al. 164 reported a RCT (n = 197) comparing TVT-O with TVT Secur systems, H and U approaches. They concluded that:
at a minimum 5-year follow-up we observed a further decrease in the subjective cure rate and an increase in the number of failures in the TVT SECUR group compared to the TVT-O group, and this situation is different to that at 2-year check-up.
Masata et al. 164
Subgroup analyses
We conducted a number of pre-planned subgroup analyses of the primary outcome for clinically relevant subgroups.
Women with urodynamic mixed urinary incontinence
The International Continence Society definition of MUI emphasises the presence of SUI and components of OAB, that is urgency with or without frequency, nocturia and urgency incontinence. 8 MUI is considered more difficult to treat because of the need to mutually manage SUI and OAB symptoms, with the latter often being unpredictable, with evidence of flaring up and remission of symptoms over time. 165 There is a paucity of primary research in this area and the management of women with urodynamic mixed incontinence remains a subject of much debate. Duckett and Tamilselvi166 have shown that 63% of women with urodynamic mixed incontinence experience complete resolution of urgency symptoms after a RP-TVT procedure, with objective cure rates of 47% and 92% for DO and urodynamic stress incontinence, respectively. Similarly, Lee et al. 167 have shown a 79.4% cure rate among women with MUI, compared with 89.8% among women with SUI, with up to 6 years of follow-up; the difference was not statistically significant. It has been previously shown that, among women with SUI-predominant urodynamic mixed incontinence, TO-TVTs are associated with good patient-reported success rates of 75%, 73.8% and 65% at 1, 3 and 9 years, respectively. 168–170
In the SIMS trial, most participants had urodynamic stress incontinence (SIMS group, 79%; SMUS group, 78%), rather than urodynamic mixed incontinence (SIMS group, 12%; SMUS group, 11%). In both subgroups, the success rates were higher for those receiving SIMSs than for those receiving SMUSs; however, the difference was smaller in the urodynamic mixed incontinence subgroup.
Age
Age is another clinically relevant subgroup, so we conducted a subgroup analysis of the primary outcome according to age. The median age was 48 years; patient success rates were better for participants aged > 48 years in the SIMS group (see Table 8). We also conducted another post hoc subgroup analysis based on age: < 65 years versus ≥ 65 years. We chose 65 years as it is the retirement age in the UK; hence, day-to-day activities can significantly change at this age. There seems to be no significant differences between SIMSs and SMUSs in this subgroup.
In 2020, Ahn et al. 171 reported their study assessing the impact of age on outcomes of SMUS procedures among 262 women who underwent the procedure in 2010–2015. They divided women into three age groups (≤ 50 years, 51–59 years and ≥ 60 years) and found no significant differences in patient-reported success rates between RP-TVT and TO-TVT. In 2018, Engen et al. 172 presented the outcomes of 21,832 women with SUI or MUI who underwent MUS procedures between 1998 and 2016. Data were obtained from the Norwegian Female Incontinence Registry. The ‘very satisfied’ rates at 6–12 months post procedure (other options were ‘moderately satisfied’, ‘neither satisfied nor dissatisfied’, ‘moderately dissatisfied’ and ‘very dissatisfied’), per age decade, were as follows: 18–29 years – 79.4%, 30–39 years – 86.3%, 40–49 years – 87.1%, 50–59 years – 86.2%, 60–69 years – 81.1%, 70–79 years – 71.7% and 80–99 years – 61.8%. This is consistent with earlier reports from Malek et al. 173 in their retrospective study of nearly 700 women undergoing MUS procedures. The mean age of patients aged ≥ 70 years (n = 160) was 75.4 ± 4.5 years; the mean age for those aged < 70 years (n = 536) was 56.2 ± 9.4 years. A multivariable analysis revealed no difference in SUI failure rates among older cohorts, compared with younger cohorts (adjusted OR 1.7, 95% CI 0.9 to 3.1). Women aged < 70 years reported a greater impression of improvement than women aged ≥ 70 years (67.7% vs. 56.6%, respectively; p = 0.01).
Different definitions of patient-reported success
The results of a post hoc analysis for patient-reported ‘cure’ using the strict definition of a response of ‘no leakage’ on the ICIQ-UI-SF were as follows: SIMS group, 38.6%; SMUS group, 33.2%. Ward et al. 174 used a similar 2-year strict definition, and found 25% and 20% cure rates for those undergoing RP-TVT and colposuspension, respectively. Interestingly, in one observational study for the mid-term safety and efficacy of the SIMS Altis, using the same strict definition, the patient-reported cure rate was 88.2% among 110 women. 175,176
Some studies use different definitions of patient-reported success. Hence, we presented the full analysis of itemised PGI-I scale responses (very much improved, much improved, improved, same, worse, much worse and very much worse) between both groups at all time points of assessment (i.e. 3, 15, 24 and 36 months). It gives confidence in the results to see that, at 36 months, comparable percentages of women in the SIMS and SMUS groups reported their continence status to be the ‘same’ (9.3% vs. 7.7%, respectively), compared with before their procedure, whereas 7.3% and 10.2% in the SIMS and SMUS groups, respectively, reported their continence status to be ‘worse’, ‘much worse’ or ‘very much worse’. By 36 months, 4.3% and 2.3% in the SIMS and SMUS groups, respectively, had received further invasive intervention for UI (including both SUI and UUI).
Excluding devices that were withdrawn from clinical practice
We included a post hoc subgroup analysis for the primary outcome excluding the devices that were withdrawn from clinical practice. The findings did not change.
Unlike other studies in the literature, the SIMS RCT is, to our knowledge, the first to compare two MUS ‘technologies’, and not specific types of slings/devices, that is it compared standard-length tension-free MUSs (both retropubic and transobturator approaches) with adjustable anchored mini slings (SIMSs). Within each trial arm (SMUS and SIMS), the choice of device used in any specific patient/hospital was down to the surgeon experience/preference and availability at the specific hospital. Hence, the trial results are generalisable to any type of free SMUS and any type of adjustable anchored SIMS that were available at the time of the trial, or similar devices that may be available in the future, or those that might be reintroduced.
Objective success rates
We assessed the objective success using the validated 24-hour pad-test and found no statistically significant difference at 15 months (RD 5.2, 95% CI –5.9 to 16.2). We acknowledge the limitation that only 36% completed the 24-hour pad test.
Our systematic review/meta-analysis81 in 2013 identified 26 RCTs (n = 3308) and showed no significant differences between SIMSs (excluding TVT Secur) and SMUSs in patient-reported and objective success rates: RR 0.94 (95% CI 0.88 to 1.00) and RR 0.98 (95% CI 0.94 to 1.01) for SIMSs and SMUSs, respectively, at 18 months. In 2018, Kim et al. 177 included 29 RCTs (n = 3000) in a meta-analysis of long-term results (> 36 months) and found that SMUSs had significantly better objective success rates (OR 0.68, 95% CI 0.47 to 0.99; p = 0.04). However, objective cure was assessed using a range of methods among the included RCTs, with inevitable significant heterogeneity.
Pascom et al. 155 compared SIMSs with TO-TVT among 130 women. They defined objective cure as negative CST and pad tests, and subjective cure as patient-reported satisfaction and no desire for additional treatment. At 3 years’ follow-up, the objective cure rate was lower in the SIMS group than in the TO-TVT group (68.3% vs. 90.2%, respectively; p = 0.027); however, the subjective cure rates were similar for both groups. Schellart et al. 178 reported a multicentre international RCT comparing a SIMS (MiniArc, n = 97) with a SMUS [TO-TVT (Monarc), n = 96]. At 36 months’ follow-up, the subjective cure rates were 86% in the MiniArc group and 87% in the Monarc group (RD –0.6%, 95% CI –12% to 11%). The objective cure rates were 89% and 88% for the MiniArc and Monarc groups, respectively (RD 1.3%, 95% CI –9% to 11%). The authors concluded that the MiniArc (SIMS) is non-inferior to the Monarc TO-TVT (SMUS) with up to 3 years of follow-up in regard to subjective and objective cure rates.
We used the 24-hour pad test as our objective outcome assessment test. It is a standardised robust validated assessment tool for UI. Urodynamics is the only objective test that can reliably differentiate urodynamic stress incontinence and DO. There is agreement in the clinical community and the public that there is no justification for undertaking postoperative urodynamics, whether in a clinical or a research capacity. The CST is another test that can be used; however, contrary to the 24-hour pad test, it has no globally agreed standardisation method. This makes it difficult to compare results between trials. We considered the use of the CST in the standing position with bladder volume of 300 ml (as has been used in some clinical trials). However, it meant an extra hospital visit. In contrast, the 24-hour pad test is done in a patient’s own home, which is more patient friendly. In one study on the use of different outcome measures for SUI studies, the authors concluded: ‘We suggest that the minimum data set should include structured questions, diaries and the 24-hour pad test.’. 179
Safety
At the time of the SIMS trial design, SMUSs were the most common surgical procedures for treatment for SUI worldwide, with > 3.5 million mesh devices sold worldwide between 2005 and 2013. 180 However, the safety of mesh devices has faced significant scrutiny over the last decade, with patients reporting SAEs such as tape/mesh exposure, groin/thigh pain, dyspareunia and others. The mesh scrutiny is primarily regarding its safety profile, with several lawsuits against mesh manufacturers in various countries. 181 Some manufacturers have withdrawn their products from clinical practice. 93,182 In the UK, safety concerns have led to cross-party parliamentary groups; parliamentary debates; patient-/media-led campaigns; and, in Scotland, an independent inquiry. 183 NHS England instituted a pause in mesh procedures for SUI, which was further extended in 2019 to date. Similarly, in Scotland, mesh surgery was suspended in almost all circumstances in 2014, with a halt ordered in 2018 to date.
In July 2020, Baroness Cumberlege published her much anticipated report, First Do No Harm: Independent Medicines and Medical Devices Safety Review,144 looking into the response of England’s health-care system to patients’ reports of harm from drugs and medical devices, including transvaginal mesh for surgical treatment of SUI and prolapse. One of the main features was the testimony from hundreds of patients reporting lack of informed consent for their initial treatment, followed by years of dismissal by clinicians and regulators. Haskell184 reported that the review panel found reluctance in all parts of the system to collect evidence on potential harms.
In the light of these developments, it is clear that mesh devices, including the newer, less established, SIMSs, require thorough evaluation to assess their safety. The SIMS trial, including all the follow-up, was performed during heightened public mesh debate; hence, participants and clinicians are unlikely to have under-reported AEs. We have presented the AEs in Chapter 5. In the next section, we discuss some clinically relevant AEs in comparison with three main components of the reported literature.
-
Randomised controlled trials: two medical students conducted a systematic review and meta-analyses of the up-to-date literature (up to mid-January 2021) for RCTs comparing SIMSs with SMUSs as part of their intercalating Bachelor of Science thesis under supervision of the chief investigator.
-
Surgical databases in the UK: AEs can be better evaluated through large registers such as the BSUG/British Association of Urological Surgeons surgical database. BSUG has published its first national report, which included the first full 10 years of data collection (2008–17). 185 This included data on > 26,000 procedures: TO-TVT, n = 9411, and RP-TVT, n = 17,488. However, one main limitation for these databases was the voluntary, and possibly selective, nature of recording the AEs.
-
Large retrospective studies: a large study published in The Lancet in 2017 assessed the immediate and long-term outcomes following surgical mesh insertions for SUI using the Hospital Episode Statistics database used in England. 183,186 This was a retrospective cohort study of > 92,000 primary MUS procedures between April 2007 and March 2015, including RP-TVT (n = 56,648), TO-TVT (n = 34,704) and SIMSs (n = 834). Such a large study would be valuable in identifying AEs with relatively low risk rates, especially as it had 100% coverage of NHS patients (including private patients treated in an NHS setting) in England over an 8-year period. However, an inherited drawback will be dependence on accurate clinical coding and the lack of details on the AEs reported.
By including these three resources, we are able to appraise the AEs reported in the SIMS trial, compared with the most up-to-date literature in this field.
Intraoperative injuries
In the SIMS trial, there were no cases of major visceral injuries. Data from the BSUG surgical database showed ureteric and bowel injuries of 0.02% (i.e. one in 5000) and 0.006% (i.e. one in 17,000) for RP-TVT, that is rare and extremely rare, respectively. 185 In our study, only one case of retropubic haematoma following RP-TVT insertion occurred; it was diagnosed in the immediate postoperative period and confirmed by radiological imaging. Diagnostic laparoscopy was performed on day 1 post operation, and the haematoma was managed conservatively. It is debatable if the laparoscopy was absolutely necessary as the haematoma is retroperitoneal and imaging such as magnetic resonance imaging and computerised tomography could have sufficed to confirm stability in the haematoma size and exclude active bleeding. However, one advantage of the laparoscopy was to exclude any other organ injuries.
In the SIMS trial, intraoperative LUT injuries occurred exclusively in the SMUS group (3.8% of participants; RP-TVT 7.1% vs. TO-TVT 0.7%). Similarly, the Trial Of Mid-Urethral Slings (TOMUS) reported LUT injury rates in its RP-TVT and TO-TVT groups of 5.3% and 0%, respectively. 187 A large retrospective study has shown a 1% risk of LUT injuries with TO-TVT procedures. 188 Data from the BSUG database showed LUT injury rates of 3.8% and 0.6% for RP-TVT and TO-TVT procedures, respectively. 185 In the literature, 20 RCTs (n = 2613 participants) compared SIMSs with SMUSs with regard to LUT injuries. 86,90,138,141,154,155,157,158,160,162,164,189–197 Pooled data favoured neither SIMSs nor SMUSs (RR 0.69, 95% CI 0.35 to 1.36).
Mesh exposure
Our results showed higher tape/mesh exposure rates among the SIMS participants than the SMUS participants, with nine out of 276 (3.3%) in the SIMS group and five out of 261 (1.9%) in the SMUS group (mean difference 1.3, 95% CI –1.7 to 4.4; p = 0.373) reporting tape exposure over the 36 months of follow-up. Management of tape exposure included partial tape removal/limited excision of exposed mesh (SIMS group, 1.4%; SMUS group, 1.1%) and recovering of the exposed mesh using vaginal walls (SIMS group, 1.1%; SMUS group, 0%). In a large retrospective study of 316 women, the vaginal mesh exposure rate for TO-TVT was 5%. 198 Data from the BSUG database showed overall mesh complication rates of 2.9% for RP-TVT and 2.3% for TO-TVT. 185 In the current literature, 12 RCTs reported on vaginal mesh exposure (n = 1528 participants). 86,90,138,155,156,160,162,191–193,196,197 Pooled data did not favour either SIMSs (excluding TVT Secur) or SMUSs (RR 0.98, 95% CI 0.44 to 2.20).
Groin/thigh pain
We asked participants to report groin/thigh procedure-related pain, analgesia use and any other pain treatments received, but did not measure the impact of pain on day-to-day activities. The absence of preoperative pain measurement is a limitation, as we are unable to measure baseline pelvic pain from other pathology. The rates of groin or thigh pain and subsequent use of analgesics were higher in the SIMS group at 15 months: SIMS group, 14.9%; SMUS group, 11.9% (mean difference 3.0%, 95% CI –2.8% to 8.8%). However, by 3 years, there was a slightly higher rate of pain in the SMUS group: SIMS group, 14.1%; SMUS group, 14.9% (mean difference –0.8%, 95% CI –4.1% to 2.5%). Despite no statistically significant difference in pain rates at 15 months, all four participants receiving tape removal for pain up to 15 months were in the SIMS group. By 3 years, two women in the SMUS group underwent partial removal for pain, whereas there were no further removals for pain in the SIMS group. This might be attributed to the patients’/surgeons’ lower threshold for earlier removal of SIMSs, as the newer non-standard procedure, or the reluctance of the surgeons to remove the SMUS as the standard procedure. Furthermore, the shorter mesh in the SIMS gives the perception that most of the mesh could be removed vaginally without groin or retropubic dissection. However, the authors are aware of one report in the trial of difficulties in removing the SIMS anchors with vaginal dissection only.
In the TOMUS (n = 597),187 the neurological (pain) symptoms rate was higher in the TO-TVT group than in the RP-TVT group (9.4% vs. 4%, respectively; p = 0.01). Sabadell et al. 158 compared 60 women randomly assigned to either Ajust or TO-TVT. At 1 year of follow-up, three women in the Ajust group reported persistent thigh pain 1 year after surgery; none in the TO-TVT group reported pain. The authors concluded that, at 1 year, the Ajust SIMS showed non-inferior effectiveness, compared with TO-TVT, and added ‘Although not statistically significant, unexpectedly, more women reported persistent thigh pain in the Ajust group’. 158 In the literature, three RCTs (n = 585 participants) have compared immediate postoperative pain between those receiving SIMSs (excluding TVT Secur) and those receiving SMUSs at postoperative day 7. 138,155,161 Pooled data of postoperative pain favoured SIMSs over SMUSs (mean difference –0.58, 95% CI –0.73 to –0.39).
Dyspareunia
More women in the SIMS group than in the SMUS group reported dyspareunia at baseline (17.2% vs. 14.5%, respectively). The rate of dyspareunia continued to be higher in the SIMS groups at all follow-up time points. By 3 years, more women in the SIMS group than in the SMUS group reported dyspareunia (11.7% vs. 4.8%, respectively; RD 7.0%, 95% CI 1.9% to 12.1%). The reason for this higher rate in the SIMS group is unclear, but it is possible that the anchoring mechanism of the SIMS (compared with that of the tension free SMUS) potentially contributes to higher rates of dyspareunia.
Shah and Badlani199 explained that dyspareunia may occur because of mesh-related infection, exposure or abnormal healing leading to scarring. Alexandridis et al. 141 showed no significant difference in rates of dyspareunia between SIMS and SMUS groups at 3 years. Masata et al. 161 compared the outcomes of 100 women randomised to either Ajust or TO-TVT. 161 At 2 years of follow-up, two participants in the Ajust group mentioned de novo dyspareunia. The authors explained that clinical examination revealed palpable painful anchor in the obturator membrane in both women, and one patient required surgical removal of the anchor.
In the current literature, seven RCTs (n = 726 participants) have reported on dyspareunia at 1–3 years of follow-up. 154,157,158,161,178,192,200 Pooled data regarding the occurrence of dyspareunia did not favour SIMSs nor SMUSs (RR 1.26, 95% CI 0.57 to 2.82).
Repeat continence surgery
In the SIMS trial, over 3 years of follow-up, 4.3% and 2.3% of SIMS and SMUS participants, respectively, received further invasive intervention for UI (including both SUI and UUI). In the SMUS group, 1.2% (n = 3) received further SUI surgery, compared with 2.6% (n = 7) in the SIMS group. Five of the women receiving further SUI surgery underwent urethral bulking. Urethral bulking was a popular choice presumably because of the suspension of mesh procedures in the UK, whereas major surgery (colposuspension/autologous slings) could be less favoured by women and/or surgeons. It may be less favoured by surgeons because of lack of experience.
In the current literature, 13 RCTs (n = 1703 participants) comparing SIMSs (excluding TVT Secur) with SMUSs have reported on participants receiving repeat continence surgery at 1–3 years of follow-up. 86,90,142,155,157,161,162,178,192,196,200–202 Pooled data did not favour SIMSs nor SMUSs (RR 1.92, 95% CI 1.18 to 3.12). In a population-based retrospective study,183 the authors studied the records of 95,057 women in England, with a median follow-up of 5.5 years after a primary SMUS procedure (RP-TVT, n = 60,194; TO-TVT, n = 34,863). The results showed that the risk of reoperation for SUI was 1.3% (95% CI 1.3% to 1.4%) at 1 year, 3.5% (95% CI 3.4% to 3.6%) at 5 years and 4.5% (95% CI 4.3% to 4.7%) at 9 years after the index procedure.
Mesh removal
In a population-based retrospective study, the authors studied the records of 95,057 women in England, with a median follow-up of 5.5 years after a primary SMUS procedure (RP-TVT, n = 60,194; TO-TVT, n = 34,863). The results showed that the rate of SMUS removal was 1.4% (95% CI 1.3% to 1.4%) at 1 year, 2.7% (95% CI 2.6% to 2.8%) at 5 years and 3.3% (95% CI 3.2% to 3.4%) at 9 years. In the SIMS trial, the total rates for mesh removal (partial/complete) for any indication were comparable to those of the large study [SIMS group, 3% (n = 8); SMUS group, 2% (n = 5)]. However, we reported on mesh removals within the relevant clinical categories for better informing the clinical decision-making of both women and clinicians. Complete/partial mesh removal for pain was 1.5% in the SIMS group, compared with 0.8% in the SMUS group, as described in Chapter 5, Additional surgical treatment. In addition, partial removal/limited excision of mesh exposure was reported by 1.4% of participants in the SIMS group, compared with 1.1% of the SMUS group. None underwent complete tape/mesh removal for the sole indication of mesh exposure. The total rates for mesh removal (partial/complete) for any indication were comparable (SIMS group, 2.9%; SMUS group, 1.9%).
Quality of life
According to the World Health Organization, QoL is defined as an individual’s perception of their position in life, in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards and concerns. 203
Quality of life can be quite complicated to assess at long-term follow-up, as many other confounding factors may have developed in participants’ lives. The use of disease-specific questionnaires can help to overcome this issue to an extent. A previous RCT (n = 341)140 assessed the long-term outcomes (9 years) of SMUSs (TO-TVT) using the KHQ, which is the predecessor of the ICIQ-LUTSqol used in the SIMS trial;204 clinically significant improvement in QoL was seen in 76.8% of participants. Women reported improvement in all KHQ domains except the general health domain. Similarly, other studies by Ulrich et al. 151 and Serati et al. 150 reported a significant improvement in most QoL domains at 10 years of follow-up following a SMUS (TO-TVT) procedure. 150,151 The evidence, therefore, tends to confirm that the successful outcome of SMUSs can have a long-lasting positive effect on women’s QoL.
However, the impact of SIMSs on QoL is less clear. In a RCT (n = 137)136 comparing an adjustable anchored SIMS (Ajust) with a SMUS (TO-TVT), 82% of women showed clinically significant improvement in total KHQ score, compared with baseline, with no significant differences between the groups (Ajust group, 76.9%; TVT-O group, 87.7%, OR 2.143, 95% CI 0.805 to 5.704; p = 0.19). 136 All KHQ domains except general health showed significant improvement after both operations, compared with baseline, with no significant differences between groups. Pascom et al. 155 compared SIMSs with SMUSs among 130 women. Secondary outcomes included assessment of QoL using the Incontinence Quality of Life questionnaire and the Urogenital Distress Inventory-6 items (UDI-6). 205,206 Women in the SMUS group had better outcomes regarding the avoidance and limiting behaviour domain of the Incontinence Quality of Life questionnaire (p = 0.021) and UDI-6 scores (p = 0.026); otherwise, there was no evidence of significant differences between the groups in any of the other domains. The authors suggested that the better QoL improvement in the SMUS group could be explained by the significantly higher objective success rate in this group.
In the SIMS trial, scores of the QoL questionnaires generally improved compared with baseline, with no statistically significant differences between the SIMS and SMUS groups. We used the ICIQ-LUTSqol, a condition-specific validated multidimensional tool that is widely used for assessing QoL in clinical trials evaluating interventions in UI and pelvic organ prolapse, allowing comparison between our results and those of other RCTs in the literature. We also used the EQ-5D-3L, a general QoL assessment tool. EQ-5D-3L scores increased from baseline to peak at 3 months, but, at 3 years, the EQ-5D-3L score in both groups was lower than at baseline and there were no significant differences between groups (difference 0.013, 95% CI –0.030 to 0.056; p = 0.55). The agreement of the results between the general and disease-specific QoL tools provides reassurance in the results that there is no strong signal favouring SIMSs or SMUSs with regard to impact on women’s QoL at up to 3 years of follow-up.
Our results agree with the current evidence. In 2019, Alexandridis et al. ,141 in a multicentre RCT (n = 279) comparing the SIMS Ajust with the SMUS TO-TVT, assessed the impact on QoL using the same tool used in the SIMS trial, the ICIQ-LUTSqol. At 3 years’ follow-up, a similar improvement in scores, compared with baseline, was observed between the groups (2.8 ± 3.6 vs. 3.0 ± 3.9 in the Ajust and TO-TVT groups, respectively). Similar results were reported by Masata et al. 161 and Xin et al. 138 in RCTs comparing the SIMS Ajust with the SMUS TO-TVT. In the current literature, five RCTs (n = 713 participants) have reported on the impact of SIMSs on participants’ QoL at 2 and 3 years’ follow-up. 141,155,161,178,194 Pooled data did not favour either SIMSs or SMUSs (standardised mean difference 0.19, 95% CI –0.06 to 0.43). A significant limitation is the substantial heterogeneity: studies used a mixture of questionnaires (ICIQ-UI-SF, Incontinence Impact Questionnaire-7 items, UDI-6 and KHQ) to assess QoL.
Sexual function
Female sexual function is complex and multifactorial. It is estimated that 60% of women with UI experience female sexual dysfunction. 207 Several studies found that women with UI report lower intercourse frequency, coital incontinence, decreased libido, vulval and vaginal irritation from persistent urine leakage, dyspareunia, and avoidance or even abstinence from sexual activity. 208 It is therefore reasonable to assume that the resolution of UI and coital incontinence will positively influence a patient’s sexual life. However, the available data on the sexual activity of people who underwent SMUS procedures are inconsistent. 208 Some studies report improvement, whereas others show equivocal results or even deterioration. 209–214 In 2020, a study assessed the impact of SMUSs (RP-TVT) on sexual function using the PISQ-IR among 171 women at 1 year of follow-up. 208 They found that coital incontinence was reported by 56% of women before the surgery, and by 8.6% of women at the 1-year follow-up. The authors concluded that successful treatment of SUI with a SMUS significantly improves a patient’s sexual life. On the other hand, persistent incontinence appears to be the most probable cause of lack of improvement in sexual life.
One RCT140 assessed the long-term outcomes (9 years) of a SMUS (TO-TVT) using the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire-12 items (PISQ-12),215 which is the predecessor of the PISQ-IR used in the SIMS trial. The sexual function score showed improvement in 61% of participants and deterioration in 34.5% of participants. The authors commented that several confounding factors may have occurred in that decade of follow-up that would inevitably affect women’s sexual function, such as advancing age, development of prolapse and menopausal vaginal dryness.
In their systematic review, Kim et al. 177 reported that four RCTs showed better QoL postoperatively with a SMUS procedure than with a SIMS procedure (OR 2.02, 95% CI 1.18 to 2.85). However, Kim et al. 177 also found no significant difference between groups in sexual function after SIMS or SMUS surgery in four RCTs (OR –0.39, 95% CI –1.87 to 1.08). The PISQ-12 and the Female Sexual Function Index were used to assess sexual function among pooled studies.
In the SIMS trial, the scores of the sexual function questionnaire (i.e. the PISQ-IR) generally improved from baseline, with no statistically significant differences between the SIMS and SMUS groups. The International Urogynecological Association revised the PISQ-IR as a condition-specific validated multidimensional tool that assesses arousal, orgasm, partner-related and condition-specific global quality, condition impact and desire. The PISQ-IR is widely used for assessing sexual function in clinical trials evaluating interventions in UI and pelvic organ prolapse; hence our use of it allows comparison of our results with those of other RCTs in the literature. 216
However, at time of the SIMS trial design, some members of the trial team felt that the PISQ-IR was quite long and intrusive. Hence, participants were randomly assigned to either complete the PISQ-IR or answer direct questions on sexual function (dyspareunia and coital incontinence). Interestingly, a relatively high percentage of participants described dyspareunia and coital incontinence at baseline. However, participants undergoing a SIMS procedure were significantly more likely to report dyspareunia and coital incontinence than SMUS participants at all follow-up time points up to 3 years [dyspareunia: 11.7% vs. 4.8% (RD 7.0%, 95% CI 1.9% to 12.1%; p = 0.010) for the SIMS and SMUS groups, respectively; coital incontinence: 11% vs. 4.8% (RD 6.0%, 95% CI –0.9% to 12.9%; p = 0.084) for the SIMS and SMUS groups, respectively]. The discrepancy between these findings and the insignificant differences in the PISQ-IR scores, between groups, casts doubt on the ability of the PISQ-IR to reliably capture these specific outcomes.
Other urinary symptoms
We used the validated ICIQ-FLUTS, which assess three domains: voiding, filling and incontinence. The results showed that the between-group differences were small: CIs were incompatible with a worthwhile difference favouring either SIMSs or SMUSs. It was reassuring to see that the filling and incontinence symptoms have shown sizeable improvements across both groups at 3 years, compared with baseline. However, the voiding domain did not show this trend: there was a slight deterioration from baseline in the SMUS group. These are not surprising findings. Voiding symptoms are not typically impaired in women with UI, and hence are not expected to vastly improve following continence surgery. In addition, continence surgery including a MUS can be potentially obstructive, leading to new voiding symptoms such as slow stream, prolonged voiding and incomplete bladder-emptying. In a relatively smaller RCT (n = 137)86 comparing an adjustable anchored SIMS (Ajust) with a SMUS (TO-TVT), at 1 year of follow-up, analysis of all domains of the ICIQ-FLUTS showed no evidence of significant differences between the groups in any of the storage, voiding or continence domains.
Our results showed that participants in the SIMS group were more likely to report improvement or cure in preoperative urgency than those in the SMUS group. We used the UPS, which is a validated simple tool for assessment of urgency. Eighteen RCTs (n = 1876 participants) in the literature compared the occurrence of de novo, or worsening, urgency symptoms between those receiving a SIMS and those receiving a SMUS, and favoured neither treatment (RR 1.03, 95% CI 0.76 to 1.38). 86,90,154,155,157–162,189,191,192,194,196,197,217,218 Pooled data of these 18 RCTs should be interpreted with caution, as the results are confounded by significant heterogeneity because of the different assessment tools used.
Observational data
We presented descriptive data on the primary outcome and secondary outcomes, and safety data, by the type of procedure received by the 537 participants who underwent surgery (Ajust, n = 62; Altis, n = 199; RP-TVT, n = 126; and TO-TVT, n = 147) (the participant who received an autologous fascial sling and the two participants who received MiniArc slings are not included). These are observational data with purely descriptive analyses and no adjustment of underlying risk factors; therefore, they should be interpreted with caution. As per trial protocol, all surgeons were asked to identify their standard procedure (SMUS: RP-TVT or TO-TVT; SIMS: Altis, Ajust or other) in advance of starting recruitment.
The patient-reported success rates observed in all groups fell over the 3-year follow-up, which is not unexpected. At 15 and 36 months, the observed success rates were 84% and 71%, respectively, for Ajust; 80% and 66%, respectively, for TO-TVT; 79% and 74%, respectively, for Altis; and 73% and 69%, respectively, for RP-TVT. The decline in success rates overtime is not unexpected. The E-TOT RCT (n = 341)65 showed a 73% patient-reported success rate for the TO-TVT at the 3-year follow-up. This was a sizeable deterioration from the 81% success rate reported for this same cohort at 1 year.
In our observational data, the impact of the procedures on urinary urgency seemed to start quite early. At 3 months, the percentages of those who reported cure of/improvement in preoperative urgency were relatively high in all groups: Ajust, 54%; Altis, 62%; RP-TVT, 62%; and TO-TVT, 58%. At 36 months, the urgency cure/improvement rates were stable for the Ajust and RP-TVT groups, and relatively reduced for the Altis and TO-TVT groups (Ajust, 59%; Altis, 55%; RP-TVT, 61%; and TO-TVT, 48%).
In the SMUS group, the observational data showed that a higher percentage of women in the RP-TVT group than in the TO-TVT group reported groin/thigh pain and use of analgesia at 3 years (groin/thigh pain: 16.7% vs. 14.3% for the RP-TVT and TO-TVT groups, respectively; use of analgesia: 6.3% vs. 3.4% for the RP-TVT and TO-TVT groups, respectively). A slightly higher percentage of women in the TO-TVT group than in RP-TVT group reported using CISC (2% vs. 0.8%, respectively), but similar percentages in both groups required surgery for voiding difficulties. This is different from the current literature, in which groin/thigh pain is believed to be predominately associated with TO-TVT. Latthe et al. 219 showed that there was a significantly higher incidence of groin/hip pain among women receiving a TO-TVT (12%) than among those receiving a RP-TVT (1%). In the TOMUS (n = 597 women),187 there was no significant difference in patient-reported success rates between the RP-TVT (62.2%) and the TO-TVT (55.8%) groups at 12 months; the incidence of voiding difficulties necessitating surgery was higher in the RP-TVT group than in the TO-TVT group (2.7% vs. 0%, respectively; p = 0.004), whereas the rate of neurological (pain) symptoms was higher in the TO-TVT group than in the RP-TVT group (9.4% vs. 4% respectively; p = 0.01). 187 The Cochrane review on SMUSs showed few RCTs reporting medium-term (1–5 years, n = 683) and longer-term (> 5years, n = 714) follow-up. 220 The patient-reported success rate at medium-term follow-up was similar between RP-TVT and TO-TVT groups (RR 0.97, 95% CI 0.87 to 1.09, and RR 0.95, 95% CI 0.80 to 1.12, respectively). In the long term, patient-reported success rates were as follows: TO-TVT, 43–92%; RP-TVT, 51–88%. Postoperative VD was less frequent following a TO-TVT procedure (RR 0.53, 95% CI 0.43 to 0.65; moderate-quality evidence). Overall rates of groin pain were higher in the TO-TVT group than in the RP-TVT group (6.4% vs. 1.3%, respectively; RR 4.12, 95% CI 2.71 to 6.27), whereas the rate of suprapubic pain was lower in the TO-TVT group than in the RP-TVT group (0.8% vs. 2.9%, respectively; RR 0.29, 95% CI 0.11 to 0.78); both were of short duration. Differences between our results and the aforementioned literature are most likely due to the observational nature of our data on RP-TVTs versus TO-TVTs and the inherent selection bias that can arise. Another potential reason is that, in the SIMS trial, surgeons used their SMUS procedure of preference (RP-TVT vs. TO-TVT), which may reflect higher level of expertise in performing that procedure.
Our observational data also showed that Altis was associated with relatively higher rates of groin/thigh pain (18%) and dyspareunia (20%) at 15 months. However, by 36 months, the groin/thigh pain rates were comparable in all groups (RP-TVT, 16.7%; Altis, 14.6%; TO-TVT, 14.3%; and Ajust, 11.3%). There were differences in analgesia use across groups at 36 months, but TO-TVT was least associated with use of analgesia (Altis, 8.5%; RP-TVT, 6.3%; Ajust, 4.8%; and TO-TVT, 3.4%). In the Altis group, there was almost a 50% reduction in dyspareunia rates at 36 months, compared with 15 months (11% vs. 20%, respectively).
In 2020, a number of small observational-size studies reported on the short-term effectiveness of various types of SIMS. 175,176,221–225 Most of the studies had small cohorts and some were not independent from the industry. One prospective cohort study, of 116 women receiving Altis, reported groin/hip/thigh pain (8%), dyspareunia (1%) and tape exposure (3.5%) at 12 months’ follow-up, but, interestingly, no further new AEs at 24 months’ follow-up. 176,225 In contrast, in a 2014 observational study175 of the safety and efficacy of Altis, 22% reported AEs at 24 months: none had mesh exposures and 6.3% had self-limiting pain. 175
Health economics
Discrete choice experiment
The purpose of the DCE is to estimate trial participant preferences for different characteristics of surgical treatment for SUI (type of anaesthesia, surgery recovery time, impact on SUI symptoms, impact on daily activities, complications). Inclusion of a cost attribute within the DCE allows for an assessment of the value to women of any service configuration that varies in terms of the attributes and levels included in the DCE. This allows for an assessment of how women value the different benefit/risk trade-offs of treatment and may be useful for shared decision-making. For example, using the WTP tariffs calculated from M5 (base-case model) in Table 20, consider two different possible surgical options being evaluated (treatment A and treatment B).
Treatment A (WTP = £5721) is provided under GA (WTP = £1632), leads to no complications (WTP = £0), takes 7 days to recover from surgery (WTP = –£76 × 7 = –£532), leads to an improvement in incontinence symptoms (WTP = £8173) and means that the patient occasionally has to avoid daily activities because of a fear of leaking (WTP = –£1981). The total WTP for treatment A is £13,013.
Consider an alternative, treatment B, in which surgery is also provided (WTP = £5721), under LA (WTP = £0), with a shorter recovery time of 2 days (WTP = –£76 × 2 = –£152). Symptoms of UI are much improved (WTP = £9855), meaning that the patient rarely avoids usual activities because of a fear of leaking (WTP = –£967), but, in this case, the woman experiences mesh extrusion/erosion that requires additional treatment to resolve (WTP = –£10,351). The total WTP for treatment B is £4106.
This hypothetical example illustrates the potential value trade-offs that the DCE can inform. In this case, treatment A is preferred to treatment B because there are no complications, despite treatment B having better outcomes overall. This example illustrates that it is not always the most effective procedure in terms of preventing UI that would be preferred by women, and therefore the benefit/risk trade-offs of different procedures require careful collaborative consideration between women and their health-care providers.
To the best of our knowledge, ours is the only DCE study worldwide that allows estimation of WTP for surgical treatment of SUI. We are aware of one other study (Brazzelli et al. 35) that uses a DCE to explore UK women’s preferences for the processes and outcomes of treatment for SUI, but it does not include a cost attribute to enable WTP estimation. Brazzelli et al. 35 report the findings of an online DCE with UK patients (45% of sample) and non-patients (55% of sample), exploring women’s preferences for different surgical treatments for SUI. Although some of the attributes included in the DCE were comparable to ours (AEs and time to return to normal activities), others were not (chronic pain, risk of recurrence). For those attributes that can be compared between the studies, similar conclusions were drawn, in that patients prefer to have surgery as opposed to none. However, women in our study attached greater value to avoiding AEs than appears to be the case in Brazzelli et al. 35 Caution is required when making direct comparisons. Our study did not include ‘chronic pain’ as a specific attribute. Chronic pain is likely to be caused by AEs, for example mesh extrusion. This may explain the high WTP values associated with the avoidance of adverse events in our DCE. However, our study may not capture women’s preferences for the avoidance of all possible sources of chronic pain. Future research exploring whether or not women’s preferences for the avoidance of chronic pain are determined by the source cause of that pain would help inform future DCEs in this area. As Brazzelli et al. 35 did not include a cost attribute in their DCE, it is not possible to directly compare the valuations (WTP) elicited in our study with those elicited in theirs.
Strengths and limitations of the discrete choice experiment study
Our DCE study was based on rigorous iterative methodology, and piloting with a sample of the trial population, which led to some refinement and improvement of the final experimental design. Our study has a unique strength, compared with other DCEs or valuation exercises in the literature. The inclusion of a cost attribute in the DCE allows for the calculation of marginal rates of substitution, enabling a comparison of value attached to different characteristics of care and outcomes using a single metric (money). This enables an assessment of the benefit/risk trade-offs that women are willing to make, and is useful for shared decision-making, involving women directly in the resource allocation process. The DCE can be used to describe the total value of any treatment package that varies according to the characteristics included in the valuation exercise. Furthermore, including cost as an attribute allows for the results of the DCE to be integrated with the trial findings to enable a cost–benefit analysis to be conducted, which allows women’s preferences to be considered directly in the economic evaluation process and ensures that women’s views can be closely integrated into developing patient-centred and cost-effective policy recommendations.
A potential limitation of the DCE study is that preferences for the cost attribute are lower than has been observed in many other DCEs. This suggests that the values of the cost attribute may not have been sufficiently high enough to induce trade-offs against cost. The result is that WTP estimates for several attribute levels are beyond the maximum level value included in the DCE. This may be a cause for concern that outcomes are overvalued, and it is important to consider the validity of the estimates against other studies in the literature. As described in the literature review section, there are no other DCEs with which to compare our results. However, we are aware of a contingent valuation study that estimates WTP for resolution of incontinence problems. 226 Our DCE shows that women are willing to pay a one-off payment of £11,706 for the maximum improvement score on the PGI-I scale (very much improved). By comparison, a US contingent valuation study found that women were willing to pay US$70 (2005 values) per month for complete resolution of incontinence symptoms. Assuming an average age of 60 years and an average life expectancy of 80 years, this would suggest that women were willing to pay 70 × 12 × 20 = US$16,800. Using an online tool to inflate and convert to UK values, this would suggest that women in that study would be willing to pay £14,612 over their remaining lifetime to obtain full resolution of incontinence symptoms, a value somewhat higher that what we have estimated in our DCE. 227 Although there are many differences in study design, framing of cost and heterogeneity in payment for health care, the results nonetheless provide some reassurance that our WTP estimates are not unreasonable.
Cost-effectiveness
The base-case analysis results indicate that, over the 36-month follow-up period, on average, SIMSs cost less (–£6, 95% CI –£228 to £208) than SMUSs and SIMSs had, on average, 0.005 (95% CI –0.068 to 0.073) more QALYs than SMUSs. The QALY difference equates to just under an additional 2 days in perfect QoL accrued over the 36 months of follow-up. Neither cost nor QALY differences are statistically significant. There is a high level of uncertainty attached to these results because of the small differences in cost and, more importantly, small differences in QALYs between the groups. The cost and QALY differences are distributed in all the quadrants of the cost-effectiveness plane and there is no clear trend in these results. When the decision-maker is not willing to pay anything for an additional QALY, there is 51% chance that SIMSs are cost-effective. This increases to a 56% chance at a £20,000 WTP threshold. These results should be interpreted taking into consideration all the factors. There is no evidence that SIMSs or SMUSs are superior on the grounds of cost-effectiveness.
The intervention costs were lower for the SIMS group for most of the resources that were used in the index surgery, and the differences were statistically significant in the cost of the surgeons who were supervised, the time spent in surgery, the type of anaesthesia used (more women in the SMUS group had GA) and the time spent in recovery. The difference in costs over the 36-month follow-up period was mainly driven by the index surgery cost, as SIMSs, on average, cost more than SMUSs at the 24- and 36-month follow-ups. However, these differences in costs were not statistically significant.
The results were robust to several deterministic sensitivity analyses around the discount rate, method used to account for time from randomisation to surgery for QALY calculation and broadening the costing perspective to a societal one. In all cases, on average, SIMSs cost less, had more QALYs and thus were dominant over SMUSs. The probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold value for a QALY gained ranged between 56% and 62% across the analyses conducted.
The results of the complete-case data, the estimation of QoL utilities using the condition-specific instrument ICIQ-LUTSqol and the relaxation of the assumption that women who did not have surgery had zero secondary resource use costs (i.e. these values were considered missing) indicate that they were sensitive to the assumptions made in the base-case analysis. The complete-case analysis results reported that SIMSs cost less but were less effective than SMUSs. The ICER was £2187 for the QALY loss and the probability that SIMSs would be cost-effective at the £20,000 WTP for a QALY threshold was 10%. However, these results were based on < 30% of the women in each group: 87 out of 298 in the SIMS group and 77 out of 298 in the SMUS group. SIMSs had fewer QALYs than SMUSs in the analysis that used ICIQ-LUTSqol estimates for QoL. However, the probability that SIMSs would be cost-effective remained close to that reported in the baseline analysis. The QALY values were higher than those reported in the generic instrument (EQ-5D-3L); this could be explained by the fact that the range of values that can be derived from these instruments is different. The preference-based index produced from this instrument has a substantially smaller range of values than the established generic preference-based measures of health. 128 The lowest value is 0.77, whereas that of EQ-5D-3L is negative.
The results of most of the subgroup analyses suggest that SIMSs cost less and have more QALYs than SMUSs, and the probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold ranged from 10% to 97%. The probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold was 54% among women who had received PFMT and 62% among women aged ≤ 65 years. For women with a urodynamic stress incontinence diagnosis, the probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold was 85%; for women aged ≤ 48 years, this probability was 97%. However, for women aged > 48 years, the probability that SIMSs would be considered cost-effective at the £20,000 WTP threshold was 10%.
The cost–benefit analysis incorporates a valuation of outcomes that goes beyond health outcomes only, such as those captured in QoL measures such as the EQ-5D-3L, instead providing a more holistic measure of value that encapsulates women’s valuation of having surgery, weighing the procedural outcomes against recovery time, improvements in symptoms, risk of complications and impact on daily activities. When comparing these benefits with the NHS perspective costs over the 3 years of follow-up, the results show that both procedures generate positive net benefits, with benefit-to-cost ratios of 4.67 for SIMSs and 5.21 for SMUSs. Assuming that all interventions that generate a benefit-to-cost ratio of > 1 improve the efficiency of the health-care system, either surgery could be considered to offer value for money. However, in terms of identifying the optimal surgical procedure, we can consider that the incremental net benefits for SIMSs, compared with SMUSs (–£941) indicate that replacing SMUSs with SIMSs would not be the most efficient use of scarce NHS resources. The results are further illustrated using the scatterplots of the cost–benefit plane and the cost–benefit acceptability curves, indicating that SIMSs would be unlikely to be considered the optimal treatment strategy over the 3-year time horizon of the analysis.
Strengths and limitations of the health economic study
A key strength is that, to our knowledge, this is the first economic analysis performed with women who were followed up over 36 months, which allowed us to capture the costs and benefits related to the SIMS and SMUS interventions over a longer period. The economic analysis was also undertaken alongside the multicentre design that included women from all over the UK, thus increasing the generalisability and the validity of the results in the UK. A comprehensive microcosting that included detailed costing of the intervention device was undertaken, which adds to the generalisability of the results. Incorporation of a wider economic perspective on costs as a secondary analysis added value in terms of understanding the impact of non-health-care costs on women, their families and the economy, as a result of urodynamic stress incontinence symptoms and problems. The economic analysis was conducted using two perspectives of benefits (QALYs and WTP values). The DCE provided useful information in the valuation of UI treatment outcomes.
One of the challenges of the study was the number of missing cost and QALY data. Exploratory analysis conducted to predict missingness indicated that data were missing at random and the base-case analysis was conducted using multiple imputation data based on best practice. Another limitation of the study was that several of the women reported that they had not returned to usual activities at 4 weeks. An assumption was made to cap the days to 28 days (4 weeks after surgery), when they completed the questionnaire. This may be a conservative estimate that may have underestimated the cost of return to normal activities.
A previous cost-effectiveness analysis92 reported 12-month results that were similar to the results of this study: low and non-significant QALY differences and cost-savings for SIMSs. The study reported that SIMSs had an ICER of £48,419 cost saving per QALY loss, with an 80% probability that SIMSs would be cost-effective at the £20,000 WTP threshold. The findings of this study suggest that the SIMS follow-up costs at both 24 and 36 months were higher than those of the SMUS, and, at 36 months, the probability that SIMSs would be cost-effective reduced to 56%.
Another study35 that used a decision-analytic model to evaluate the cost-effectiveness of nine different surgical interventions for the treatment of women with SUI or stress-predominant MUI concluded that the RP-TVT was less costly and more effective than all other surgical interventions over a lifetime time horizon; therefore, it was a dominant strategy. The probabilistic results showed that RP-TVT and traditional sling have the highest probabilities of being cost-effective across all WTP thresholds over a lifetime time horizon. RP-TVT remains dominant over a 10-year time horizon in the cure model. The only major deviation from these findings was when the time horizon was reduced to 1 year. The most cost-effective surgical intervention was single-incision sling, which was similar to the results that were reported in Boyers et al. 92 There is need for long-term follow-up of these women to evaluate the long-term effectiveness of SIMSs.
The results of this study suggest that the probability that SIMSs would be cost-effective at the £20,000 WTP threshold is 56%. However, there is still some uncertainty over the longer time complication and failure rates for the devices used in the treatment of UI; therefore, long-term follow-up would be needed to establish the cost and QoL implications for these events.
Limitations in the SIMS trial
The main limitations are the lack of follow-up beyond 3 years, and the inadequate power to detect important differences in safety/AEs between SIMSs and SMUSs. Emerging evidence over the previous decade shows strong signals for late-onset AEs and/or decline in effectiveness in MUSs. 65,139,140,183 The first SIMS device (TVT Secur) was withdrawn from clinical practice in 2013 for commercial reasons following disappointingly low 2- to 3-year success rates. 163 In 2017, Karmakar et al. 140 reported a 9-year follow-up for a surgical RCT on transobturator tapes (TO-TVT) for surgical treatment of SUI among women (n = 341). They showed late-onset (> 3 years post operation) mesh extrusion (2%) and groin/thigh pain (4.3%), and 1.4% underwent surgical removal of the mesh and/or received regular analgesia. These results were different from the 3-year follow-up of the same cohort, at which point no procedure-related pain was reported by participants. 65 Compared with the 1-year outcomes of the same cohort, there was a significant reduction in the patient-reported success rate at 9 years (80% vs. 71.6%, respectively; p = 0.004). 139 In addition, significantly more women reported deterioration in sexual function at 9 years than at 1 year (34.5% vs. 4.3%, respectively). In 2018, a population-based retrospective study (95,000 women)183 reported a linear rise in the rate of reoperation following MUS insertion, including mesh removal rates, which were 2.6% (95% CI 2.5% to 2.7%) at 1 year, 5.5% (95% CI 5.4% to 5.7%) at 5 years and 6.9% (95% CI 6.7% to 7.1%) at 9 years.
The lack of an objective primary outcome may be a limitation. However, patient-reported outcomes better reflect patient experience than objective measures, which can overestimate SUI surgery success. 145 Urodynamics is the only objective test that can reliably differentiate urodynamic stress incontinence and DO. There is agreement within the clinical community and among the public that there is no justification for undertaking postoperative urodynamics, whether in a clinical or a research capacity. The CST is another test that can be used; however, contrary to the 24-hour pad test, it has no globally agreed standardisation method. This makes it difficult to compare results between trials. We considered the use of the CST in a standing position with a bladder volume of 300 ml (as has been used in some clinical trials). However, it meant an extra hospital visit. In contrast, the 24-hour pad test is done at a patient’s own home, which is more patient friendly.
In one study on the use of different outcome measures for SUI studies, the authors concluded ‘that the minimum data set should include structured questions, diaries and the 24-hour pad test’. 179 We used the PGI-I scale, which is ‘a robust validated and global review of the treatment outcome encompassing of the range of benefits and potential harms’. 179
Nevertheless, in the SIMS trial, the relatively low participant response rate for the pad test is certainly a limitation.
Another potential limitation is that several mesh devices have been withdrawn from clinical practice/market during the heightened public mesh debate (e.g. devices from American Medical Systems and Bard Pharmaceuticals). Our study compares two MUS technologies (tension-free SMUS and adjustable anchored SIMS), not specific devices. Nevertheless, most participants received devices still on the market (SMUS, 93%; and SIMS, 76%); hence, the study results are generalisable to similar types of SMUSs and SIMSs still available.
Excluding women with concomitant prolapse procedures can be a potential limitation. However, there are several reasons for excluding concomitant prolapse surgery, as explained previously, but mainly because SIMSs were offered under LA as standard in this RCT, which is not compatible with most concomitant prolapse procedures.
It was not possible to blind the surgeons or participants, given the procedures (SIMS procedure under LA and SMUS procedure under GA). Follow-up was primarily patient-reported through postal questionnaires, essentially removing any clinical assessor bias.
Conclusion
Adjustable anchored SIMSs are non-inferior to tension-free SMUSs for patient-reported and objective success rates up to 3 years of follow-up. There were no significant differences in QoL or sexual function scores, but more women in the SIMS group reported dyspareunia and/or underwent further surgery. There is no evidence that SIMSs or SMUSs are superior on the grounds of cost-effectiveness.
Further research
It is important to establish the long-term clinical effectiveness and cost-effectiveness of SIMSs. There are well-confirmed signals in the literature of late-onset AEs and decline in effectiveness with mesh-based procedures. Ten years’ follow-up of the SIMS trial has been funded by the HTA programme and is under way.
Acknowledgements
The authors thank the members of the TSC and the DMC who oversaw this trial.
Trial Steering Committee
-
Independent chairperson (from 2017): Christopher Mayne, Consultant Gynaecologist (retired 2017), Leicester General Hospital, Leicester, UK.
-
Independent chairperson (2014–2017): Khaled Ismail, Professor of Obstetrics, University of Birmingham, Birmingham, UK.
-
Independent triallist: the late Lynda Harper, Clinical Projects Manager, Medical Research Council Clinical Trials Unit at University College London, London, UK (2014–2020).
-
Independent clinician with relevant expertise (20142017): Christopher Mayne, Consultant Gynaecologist (retired 2017), Leicester General Hospital, Leicester, UK.
-
Independent PPI representative: Isobel Montgomery, Aberdeen, UK.
-
Independent clinician with relevant expertise (from 2017): Dudley Robinson, Consultant Urogynaecologist/Honorary Lecturer, King’s College Hospital, London, UK.
-
Independent triallist (from 2020): Eleanor Mitchell, Assistant Professor of Clinical Trials, University of Nottingham, Nottingham, UK.
Data Monitoring Committee
-
Independent chairperson (from 2015): Professor Peter Brocklehurst, Director of Birmingham Clinical Trials Unit, University of Birmingham, Birmingham, UK.
-
Independent chairperson (2014–2015): Doug Tincello, Professor of Urogynaecology, University of Leicester, Leicester, UK.
-
Independent statistician: Lee Middleton, University of Birmingham, Birmingham, UK.
-
Independent clinician with relevant expertise: Christian Phillips, Consultant Gynaecologist and Obstetrician, Basingstoke and North Hampshire Hospital, Basingstoke, UK.
Study Management Group
-
Chief investigator: Mohamed Abdel-Fattah.
-
Trial manager: Tracey Davidson.
-
Statistician: David Cooper.
-
Data co-ordinators: Zoe Batham, Patryca Bromm, Becky Bruce; Margery Heath, Zareen Thorlu-Bangura and Jessica Wood.
-
Senior trial manager: Alison McDonald.
-
Research governance: Gail Holland (manager, 2014–2016), Patricia Burns (manager, 2016–2019), Louise King (manager from 2019) and Stacey Dawson (officer, 2014 to present).
Project Management Group
The members of the Study Management Group were also members of the PMG, along with the following:
-
Grant-holders: Philip Assassa, James N’Dow, John Norrie, Graeme MacLennan and Kirsty McCormack.
-
Patient and public involvement: Judith Wardle.
-
CHaRT trial office: Gladys McPherson (2014–2016) and Mark Forrest (from 2016).
-
Health economist: Mary Kilonzo.
-
Quality assurance: Samantha Wileman.
Additional thanks
The authors would like to acknowledge the support of the following people and thank them for their contribution:
-
Kerry Duffus (primary budget manager) and Anne Buckle (budget manager).
-
Research and innovation department, University of Aberdeen.
-
Medical students: Kate Gillespie and Ahmed Mansor.
The authors would also like to extend their thanks to Lesley Dodd, Research Manager, HTA.
The authors would like to thank Phil Assassa for his substantive role in the trial, including obtaining funding, protocol design and participant recruitment; Dr Aethele Khunda for his contribution to the recruitment of participants and assistance with the writing and editing of the introduction chapter of this report (see Chapter 1); and Dr Anower Hossain for his work on the primary outcome results. The authors also thank Ahmed Mansor and Katie Gillespie (Bachelor of Science medical students) for their contributions to the systematic review.
The Health Services Research Unit at the University of Aberdeen is core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
All staff at NHS trusts responsible for recruitment in the clinical centres were as follows.
| Barnsley Hospital NHS Foundation Trust | Mishell Cunningham, Allison Daniels, Meena Dass (PI) Carol Dennis, Khaled Farag (PI), Hazel Marsh, Bethany Oates, Michelle Reid, Amy Sanderson, Andrea Tyas |
| Borders Health Board | Mohamed Abdel-All (PI), Joy Dawson, Thiyagaraj Narrainen-Poulle (PI) |
| Brighton & Sussex University Hospitals NHS Trust | John Bell, Ayman Fouad (PI), Jane Gaylard, Sharif Ismail, Julie Newman, Emma Pitcher, Vuivun Wong (PI) |
| Cardiff and Vale University Health Board | Kiron Bhal (PI) and Joanna Davies |
| Countess of Chester Hospital NHS Foundation Trust | Kerry Barker-Williams, Lorraine Dinardo, Jenny Grounds, Mofid Ibraheim (PI), Nichola Kearsley, Sandra Leason and Charlotte Perkins |
| NHS Fife | Julie Aitken, Keith Boath, Linsey Burd, Chu Chin Lim (PI) and Isla Smith |
| NHS Grampian | Mohamed Abdel-Fattah (PI), Debjyoti Karmakar and Alyaa Mostafa |
| Great Western Hospitals NHS Trust | Tamer Abdelrazik (PI), Hilary Acourt, Natasha Beck, Angela Clarke, Rebecca Elliott-Jones, Hany Gamal-Eldeen (PI), Sarah Grayland, David Griffiths, Anita Leslie, Catherine Lewis-Clarke, Lisa Matter, Suzannah Pegler and Steven Windebank |
| Hywel Dda University Health Board | Islam Abdelrahman, Manal Elbadrawy, Debra Evans, Baba Gana (PI), Lucy Hill, Kathryn Malinovszky, Emma Perkins and Bryan Phillips |
| James Paget University Hospitals NHS Foundation Trust | Nadine Baldwin, Naomi Bruce, Ben Burton (PI), Mukta Chattopadhyay, Teresa Ferreira, Helen Nutt, Mumtaz Rasid (PI) and Helen Sutherland |
| Lancashire Teaching Hospitals NHS Foundation Trust | Christian Amoah (PI), Susan Flintoff, Anne Gardner, Abdo Khalil, Pauline MacDonald, Sanjeev Prashar (PI) and Brice Rodriguez |
| Milton Keynes University Hospital NHS Foundation Trust | Kerry Cayley, Edel Clare, Veronica Edgell, Rebecca Hinch, Salma Ibrahim (PI), Cheryl Padilla-Harris, Maryam Pezeshki (PI) and Carolyn Rooth |
| Torbay and South Devon NHS Foundation Trust | Pauline Mercer, Subramanian Narayanan (PI), Gabrielle de Selincourt and Lorraine Thornton |
| South Tees Hospitals NHS Foundation Trust | Gulsanga Afridi, Hazel Alexander, Callum Allison, Colette Anderson, Paul Ballard, Sarah Croft, Helen Harwood, Mary Hodgers, Aethele Khunda (PI), Gillian McIlhinney, Chou Pay Lim, Julie Potts and Shantha Samarage |
| The Mid Yorkshire Hospitals NHS Trust | Philip Assassa (PI), Fattah Azza (PI), Julie Ball, Sarah Buckley, Thelma Darian and Tracey Lowry |
| The Queen Elizabeth Hospital, King’s Lynn, NHS Foundation Trust | Shaker Elghannam, Eleanor Gilham, Mohamed Rabeih Elghorori (PI), Keyleigh Vine and Jane Wolfe |
| The Royal Wolverhampton NHS Trust | Khaled Afifi (PI), Efstathios Altanis, Lyndsay Bibb, Charles Cox, Amina Douglas, Ayman Elnaqa (PI), Laura Gardiner, Charles Icke, Sharon Kempson, Marian McCormick, Priscilla Mhembere, Olgah Ncube, Maisie Orgill, Jayne Rankin and Emma Virgilio |
| United Lincolnshire Hospitals NHS Trust | Asem Ali, Helen Ayre, Susie Butler, Caroline Collins, Amy Cunningham, Val Elliott, Claire Hewitt, Maisa Salman (PI) and Wendy Thompson |
| Western Sussex Hospitals NHS Foundation Trust | Waleed Al-Singary (PI), Yolanda Baird, Irvin Balagosa, Dawn Crowe, Marian Flynn-Batham, Linda Folkes, Sarah Funnell, Raquel Gomez Marcos, Sarah House, Dawn Hughes, Sally Moore, Carolyn Noren, Farzin Pakarian, Gillian Peters, Richard Pyper (PI), Matthew Smith and Tan Tsawayo |
| Wirral University Teaching Hospital NHS Foundation Trust | Mark Doyle (PI), Julie Grindey and Jeremy Weetch |
| York and Scarborough Teaching Hospitals NHS Foundation Trust | Olugbenga Adekanmi, Sophie Bennett, Isobel Birkinshaw, Zoe Coleman, Nicola Dean, Catherine Farrar, Andy Gibson, Latife Hardaker, Mustafa Hilmy (PI), Laura Howe, Debbie Hukins, Zoe Scott, Tom Smith, Rebecca Tait, John Whitwell and Monica Wright |
Contributions of authors
Mohamed Abdel-Fattah (https://orcid.org/0000-0002-8290-0613) (Professor, Clinical Chair in Gynaecology, Consultant Gynaecologist and Subspecialist Urogynaecologist, and Chief Investigator) contributed to the conception and the design of the trial, the recruitment of participants, the conduct of the study, the interpretation of the results and the writing/editing of the report.
David Cooper (https://orcid.org/0000-0002-9361-4399) (Statistician) conducted the statistical analyses and confirmed the accuracy and completeness of the data. David Cooper wrote the first draft of the four results chapters, which were reviewed/edited by all other authors, and reviewed the final report.
Tracey Davidson (https://orcid.org/0000-0002-4263-3389) (Trial Manager, Triallist) was responsible for the day-to-day management of the trial. Tracey Davidson contributed to the first draft of the methods chapter (see Chapter 2), which was reviewed/edited by other authors, and to the writing/editing of the final report.
Mary Kilonzo (https://orcid.org/0000-0002-3450-4536) (Health Economist) designed and conducted the within-trial economic evaluation and analysis and wrote the health economics chapter (see Chapter 8). She conducted the design of the DCE and commented on the DCE chapter (see Chapter 7) and on the final report. Mary Kilonzo confirms the accuracy and completeness of the health economics data.
Dwayne Boyers (https://orcid.org/0000-0002-9786-8118) (Research Fellow, Health Economist) contributed to the design and conduct of the within-trial economic evaluation and commented on the health economics chapter(see Chapter 8). He conducted the DCE analysis, wrote the DCE chapter (see Chapter 7) and commented on the final report. Dwayne Boyers confirms the accuracy and completeness of the health economics data.
Kiron Bhal (https://orcid.org/0000-0002-3559-0034) (Consultant Urogynaecologist) contributed to the recruitment of participants, interpretation of the data and conduct of the trial, and provided commentary on the final report.
Alison McDonald (https://orcid.org/0000-0002-0256-2889) (Senior Trial Manager) contributed to the conception, design and the conduct of the trial and provided commentary on the final report.
Judith Wardle (https://orcid.org/0000-0002-7318-6665) (Patient and Public Lead) contributed to the conception, design and the conduct of the trial and interpretation of the data, and provided commentary on the final report.
James N’Dow (https://orcid.org/0000-0001-5340-0081) (Professor of Urological Surgery, Co-chief Investigator from January to July 2015) contributed to the conception, design and the conduct of the trial.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Professor, CHaRT Director, Statistician and Triallist) contributed to the conception, design and conduct of the trial and the interpretation of results, and made significant contributions to the final report. Graeme MacLennon confirms the accuracy and completeness of the data.
John Norrie (https://orcid.org/0000-0001-9823-9252) [Professor of Statistics and Director of CHaRT (to 2016), Co-chief Investigator from 2015 to 2017] contributed to the conception, design and conduct of the trial, and provided significant contributions to the final report. John Norrie confirms the accuracy and completeness of the data.
Publications
Abdel-Fattah M, MacLennan G, Kilonzo M, Assassa RP, McCormick K, Davidson T, et al. The SIMS trial: adjustable anchored single-incision mini-slings versus standard tension-free midurethral slings in the surgical management of female stress urinary incontinence. A study protocol for a pragmatic, multicentre, non-inferiority randomised controlled trial. BMJ Open 2017;7:e015111.
Abdel-Fattah M, Cooper D, Davidson T, Kilonzo M, Hossain M, Boyers D, et al. Single-incision mini-slings for stress urinary incontinence in woman. N Engl J Med 2022;386:1230–43.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Abdel-Fattah M, MacLennan G, Kilonzo M, Assassa RP, McCormick K, Davidson T, et al. The SIMS trial: adjustable anchored single-incision mini-slings versus standard tension-free midurethral slings in the surgical management of female stress urinary incontinence. A study protocol for a pragmatic, multicentre, non-inferiority randomised controlled trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015111.
- D’Ancona C, Haylen B, Oelke M, Abranches-Monteiro L, Arnold E, Goldman H, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn 2019;38:433-77. https://doi.org/10.1002/nau.23897.
- Tennstedt SL, Link CL, Steers WD, McKinlay JB. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults: the Boston Area Community Health (BACH) Survey. Am J Epidemiol 2008;167:390-9. https://doi.org/10.1093/aje/kwm356.
- Buckley BS, Lapitan MC. Prevalence of urinary and faecal incontinence and nocturnal enuresis and attitudes to treatment and help-seeking among a community-based representative sample of adults in the United Kingdom. Int J Clin Pract 2009;63:568-73. https://doi.org/10.1111/j.1742-1241.2008.01974.x.
- Townsend MK, Curhan GC, Resnick NM, Grodstein F. The incidence of urinary incontinence across Asian, black, and white women in the United States. Am J Obstet Gynecol 2010;202:378.e1-7. https://doi.org/10.1016/j.ajog.2009.11.021.
- Perry S, Shaw C, Assassa P, Dallosso H, Williams K, Brittain KR, et al. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. Leicestershire MRC Incontinence Study Team. J Public Health Med 2000;22:427-34. https://doi.org/10.1093/pubmed/22.3.427.
- Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. Norwegian EPINCONT study . Epidemiology of Incontinence in the County of Nord-Trøndelag. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol 2000;53:1150-7. https://doi.org/10.1016/S0895-4356(00)00232-8.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:167-78. https://doi.org/10.1002/nau.10052.
- Abrams P, Artibani W, Cardozo L, Dmochowski R, van Kerrebroeck P, Sand P. International Continence Society . Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn 2009;28. https://doi.org/10.1002/nau.20737.
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 2010;21:5-26. https://doi.org/10.1007/s00192-009-0976-9.
- Lifford KL, Townsend MK, Curhan GC, Resnick NM, Grodstein F. The epidemiology of urinary incontinence in older women: incidence, progression, and remission. J Am Geriatr Soc 2008;56:1191-8. https://doi.org/10.1111/j.1532-5415.2008.01747.x.
- Wennberg AL, Molander U, Fall M, Edlund C, Peeker R, Milsom I. A longitudinal population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in women. Eur Urol 2009;55:783-91. https://doi.org/10.1016/j.eururo.2009.01.007.
- Herzog AR, Diokno AC, Brown MB, Fultz NH, Goldstein NE. Urinary incontinence as a risk factor for mortality. J Am Geriatr Soc 1994;42:264-8. https://doi.org/10.1111/j.1532-5415.1994.tb01749.x.
- Fultz NH, Burgio K, Diokno AC, Kinchen KS, Obenchain R, Bump RC. Burden of stress urinary incontinence for community-dwelling women. Am J Obstet Gynecol 2003;189:1275-82. https://doi.org/10.1067/S0002-9378(03)00598-2.
- Norton PA, MacDonald LD, Sedgwick PM, Stanton SL. Distress and delay associated with urinary incontinence, frequency, and urgency in women. BMJ 1988;297:1187-9. https://doi.org/10.1136/bmj.297.6657.1187.
- Wagg A, Harari D, Husk J, Lowe D, Lourtie J. National Audit of Continence Care: Combined Organisational and Clinical Report 2010. www.rcplondon.ac.uk/file/911/download (accessed 1 April 2021).
- Royal College of Physicians . National Audit of Continence Care (NACC) n.d. www.rcplondon.ac.uk/projects/outputs/national-audit-continence-care-nacc (accessed 4 January 2021).
- Ko Y, Lin SJ, Salmon JW, Bron MS. The impact of urinary incontinence on quality of life of the elderly. Am J Manag Care 2005;11:103-11.
- National Institute for Health and Care Excellence . Urinary Incontinence in Women: Management n.d. www.nice.org.uk/guidance/cg171 (accessed 26 January 2021).
- Glavind K, Tetsche MS. Sexual function in women before and after suburethral sling operation for stress urinary incontinence: a retrospective questionnaire study. Acta Obstet Gynecol Scand 2004;83:965-8. https://doi.org/10.1111/j.0001-6349.2004.00555.x.
- Elzevier HW, Venema PL, Lycklama á Nijeholt AA. Sexual function after tension-free vaginal tape (TVT) for stress incontinence: results of a mailed questionnaire. Int Urogynecol J Pelvic Floor Dysfunct 2004;15:313-18. https://doi.org/10.1007/s00192-004-1149-5.
- Roumeguére T, Quackels T, Bollens R, de Groote A, Zlotta A, Bossche MV, et al. Trans-obturator vaginal tape (TOT) for female stress incontinence: 1 year follow-up in 120 patients. Eur Urol 2005;48:805-9. https://doi.org/10.1016/j.eururo.2005.08.003.
- Brubaker L, Chiang S, Zyczynski H, Norton P, Kalinoski DL, Stoddard A, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol 2009;200:562.e1-7. https://doi.org/10.1016/j.ajog.2008.11.017.
- Serati M, Salvatore S, Uccella S, Artibani W, Novara G, Cardozo L, et al. Surgical treatment for female stress urinary incontinence: what is the gold-standard procedure?. Int Urogynecol J Pelvic Floor Dysfunct 2009;20:619-21. https://doi.org/10.1007/s00192-009-0850-9.
- Sentilhes L, Berthier A, Loisel C, Descamps P, Marpeau L, Grise P. Female sexual function following surgery for stress urinary incontinence: tension-free vaginal versus transobturator tape procedure. Int Urogynecol J Pelvic Floor Dysfunct 2009;20:393-9. https://doi.org/10.1007/s00192-008-0778-5.
- Turner DA, Shaw C, McGrother CW, Dallosso HM, Cooper NJ. MRC Incontinence Team . The cost of clinically significant urinary storage symptoms for community dwelling adults in the UK. BJU Int 2004;93:1246-52. https://doi.org/10.1111/j.1464-410x.2004.04806.x.
- Papanicolaou S, Hunskaar S, Lose G, Sykes D. Assessment of bothersomeness and impact on quality of life of urinary incontinence in women in France, Germany, Spain and the UK. BJU Int 2005;96:831-8. https://doi.org/10.1111/j.1464-410X.2005.05722.x.
- Monz B, Chartier-Kastler E, Hampel C, Samsioe G, Hunskaar S, Espuna-Pons M, et al. Patient characteristics associated with quality of life in European women seeking treatment for urinary incontinence: results from PURE. Eur Urol 2007;51:1073-81. https://doi.org/10.1016/j.eururo.2006.09.022.
- Feneley RC, Hopley IB, Wells PN. Urinary catheters: history, current status, adverse events and research agenda. J Med Eng Technol 2015;39:459-70. https://doi.org/10.3109/03091902.2015.1085600.
- Abdel-Fattah M, Familusi A, Fielding S, Ford J, Bhattacharya S. Primary and repeat surgical treatment for female pelvic organ prolapse and incontinence in parous women in the UK: a register linkage study. BMJ Open 2011;1. https://doi.org/10.1136/bmjopen-2011-000206.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 2014;123:1201-6. https://doi.org/10.1097/AOG.0000000000000286.
- NHS Digital . NHS Digital Publishes Statistics on Vaginal Mesh Procedures n.d. https://digital.nhs.uk/news-and-events/latest-news/nhs-digital-publishes-statistics-on-vaginal-mesh-procedures (accessed January 2021).
- Wagner TH, Hu TW. Economic costs of urinary incontinence in 1995. Urology 1998;51:355-61. https://doi.org/10.1016/S0090-4295(97)00623-7.
- Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al. Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7210.
- Brazzelli M, Javanbakht M, Imamura M, Hudson J, Moloney E, Becker F, et al. Surgical treatments for women with stress urinary incontinence: the ESTER systematic review and economic evaluation. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23140.
- Hay-Smith EJ, Bø Berghmans LC, Hendriks HJ, de Bie RA, van Waalwijk van Doorn ES. Pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD001407.pub3.
- Hagen S, Elders A, Stratton S, Sergenson N, Bugge C, Dean S, et al. Effectiveness of pelvic floor muscle training with and without electromyographic biofeedback for urinary incontinence in women: multicentre randomised controlled trial. BMJ 2020;371. https://doi.org/10.1136/bmj.m3719.
- Mariappan P, Ballantyne Z, N’Dow JM, Alhasso AA. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database Syst Rev 2005;3. https://doi.org/10.1002/14651858.CD004742.pub2.
- Staskin D, Bavendam T, Miller J, Davila GW, Diokno A, Knapp P, et al. Effectiveness of a urinary control insert in the management of stress urinary incontinence: early results of a multicenter study. Urology 1996;47:629-36. https://doi.org/10.1016/S0090-4295(96)00003-9.
- Peschers U, Zen Ruffinen F, Schaer GN, Schüssler B. The VIVA urethral plug: a sensible expansion of the spectrum for conservative therapy of urinary stress incontinence?. Geburtshilfe Frauenheilkd 1996;56:118-23. https://doi.org/10.1055/s-2007-1022276.
- Farrell SA, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Can 2004;26:113-17. https://doi.org/10.1016/S1701-2163(16)30486-8.
- Hinoul P, Roovers JP, Ombelet W, Vanspauwen R. Surgical management of urinary stress incontinence in women: a historical and clinical overview. Eur J Obstet Gynecol Reprod Biol 2009;145:219-25. https://doi.org/10.1016/j.ejogrb.2009.04.020.
- Abrams P, Hilton P, Lucas M, Smith T. A proposal for a new classification for operative procedures for stress urinary incontinence. BJU Int 2005;96:232-3. https://doi.org/10.1111/j.1464-410X.2005.05607.x.
- Feyereisl J, Dreher E, Haenggi W, Zikmund J, Schneider H. Long-term results after Burch colposuspension. Am J Obstet Gynecol 1994;171:647-52. https://doi.org/10.1016/0002-9378(94)90077-9.
- Chaliha C, Stanton SL. Complications of surgery for genuine stress incontinence. Br J Obstet Gynaecol 1999;106:1238-45. https://doi.org/10.1111/j.1471-0528.1999.tb08176.x.
- Tong JL, Zhu L, Lang JH. Effects of laparoscopic Burch colposuspension and tension-free vaginal tape in treatment of female stress urinary incontinence: a comparative study. Zhonghua Yi Xue Za Zhi 2008;88:3192-4.
- Andrada Hamer M, Persson J. Preoperative urethral parameters at rest and objective cure following laparoscopic colposuspension. Int Urogynecol J 2010;21:331-6. https://doi.org/10.1007/s00192-009-1034-3.
- Yoon WS, Lee HN, Lee YS, Jeung IC, Park EK. Laparoscopic colposuspension to the Cooper’s ligament after hysterectomy for uterovaginal prolapse. J Obstet Gynaecol Res 2013;39:714-9. https://doi.org/10.1111/j.1447-0756.2012.02038.x.
- Kitchener HC, Dunn G, Lawton V, Reid F, Nelson L, Smith AR. COLPO Study Group . Laparoscopic versus open colposuspension – results of a prospective randomised controlled trial. BJOG 2006;113:1007-13. https://doi.org/10.1111/j.1471-0528.2006.01035.x.
- Liu CY. Laparoscopic treatment for genuine urinary stress incontinence. Baillieres Clin Obstet Gynaecol 1994;8:789-98. https://doi.org/10.1016/s0950-3552(05)80056-2.
- Aldridge A. Transplantation of fascia for the relief of urinary stress incontinence. Am J Obstet Gynecol 1942;44:398-414. https://doi.org/10.1016/S0002-9378(42)90477-0.
- Enzelsberger H, Helmer H, Schatten C. Comparison of Burch and lyodura sling procedures for repair of unsuccessful incontinence surgery. Obstet Gynecol 1996;88:251-6. https://doi.org/10.1016/0029-7844(96)00193-7.
- Bai SW, Sohn WH, Chung DJ, Park JH, Kim SK. Comparison of the efficacy of Burch colposuspension, pubovaginal sling, and tension-free vaginal tape for stress urinary incontinence. Int J Gynaecol Obstet 2005;91:246-51. https://doi.org/10.1016/j.ijgo.2005.08.023.
- Albo M, Wruck L, Baker J, Brubaker L, Chai T, Dandreo KJ, et al. The relationships among measures of incontinence severity in women undergoing surgery for stress urinary incontinence. J Urol 2007;177:1810-14. https://doi.org/10.1016/j.juro.2007.01.032.
- Ulmsten U, Henriksson L, Johnson P, Varhos G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1996;7:81-5. https://doi.org/10.1007/BF01902378.
- Petros PP, Ulmsten U. An anatomical classification – a new paradigm for management of female lower urinary tract dysfunction. Eur J Obstet Gynecol Reprod Biol 1998;80:87-94. https://doi.org/10.1016/S0301-2115(98)00092-X.
- DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 1994;170:1713-20. https://doi.org/10.1016/S0002-9378(12)91840-2.
- Lapitan MC, Cody JD. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev 2012;6. https://doi.org/10.1002/14651858.CD002912.pub5.
- Assassa P, Moran P, Duckett J, Bsug B. Stress incontinence surgery in the UK (1). Pre-operative work up and intra-operative complications. Analysis of the British Society of Urogynaecology database. Neurourol Urodyn 2010;29:809-10.
- Delorme E. Transobturator urethral suspension: mini-invasive procedure in the treatment of stress urinary incontinence in women. Prog Urol 2001;11:1306-13.
- de Leval J, Waltregny D. New surgical technique for treatment of stress urinary incontinence TVT-Obturator: new developments and results. Surg Technol Int 2005;14:212-21.
- Ogah J, Cody JD, Rogerson L. Minimally Invasive Synthetic Suburethral Sling Operations for Stress Urinary Incontinence in Women (Review). Chichester: John Wiley & Sons Ltd; 2010.
- Latthe PM, Singh P, Foon R, Toozs-Hobson P. Two routes of transobturator tape procedures in stress urinary incontinence: a meta-analysis with direct and indirect comparison of randomized trials. BJU Int 2010;106:68-76. https://doi.org/10.1111/j.1464-410X.2009.09051.x.
- Madhuvrata P, Riad M, Ammembal MK, Agur W, Abdel-Fattah M. Systematic review and meta-analysis of ‘inside–out’ versus ‘outside–in’ transobturator tapes in management of stress urinary incontinence in women. Eur J Obstet Gynecol Reprod Biol 2012;162:1-10. https://doi.org/10.1016/j.ejogrb.2012.01.004.
- Abdel-Fattah M, Mostafa A, Familusi A, Ramsay I, N’dow J. Prospective randomised controlled trial of transobturator tapes in management of urodynamic stress incontinence in women: 3-year outcomes from the Evaluation of Transobturator Tapes study. Eur Urol 2012;62:843-51. https://doi.org/10.1016/j.eururo.2012.04.021.
- Abdel-Fattah M, Ramsay I. Transobturator tension free vaginal tapes: are they the way forward in the surgical treatment of urodynamic stress incontinence?. Int J Surg 2007;5:3-10. https://doi.org/10.1016/j.ijsu.2005.12.002.
- Smith AR, Dmochowski R, Hilton P, Nilsson CG, Reid F, Rovner E, et al. Incontinence. Plymouth: Health Publication Ltd; 2009.
- Novara G, Artibani W, Barber MD, Chapple CR, Costantini E, Ficarra V, et al. Updated systematic review and meta-analysis of the comparative data on colposuspensions, pubovaginal slings, and midurethral tapes in the surgical treatment of female stress urinary incontinence. Eur Urol 2010;58:218-38. https://doi.org/10.1016/j.eururo.2010.04.022.
- Ogah J, Cody DJ, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women: a short version Cochrane review. Neurourol Urodyn 2011;30:284-91. https://doi.org/10.1002/nau.20980.
- Liapis A, Bakas P, Giner M, Creatsas G. Tension-free vaginal tape versus tension-free vaginal tape obturator in women with stress urinary incontinence. Gynecol Obstet Invest 2006;62:160-4. https://doi.org/10.1159/000093320.
- Gillies T. Scottish Independent Review of the Use, Safety and Efficacy of Transvaginal Mesh Implants in the Treatment of Stress Urinary Incontinence and Pelvic Organ Prolapse in Women: Final Report March 2017 2017. www.gov.scot/publications/scottish-independent-review-use-safety-efficacy-transvaginal-mesh-implants-treatment-9781786528711/ (accessed 1 April 2021).
- Debodinance P, Lagrange E, Amblard J, Lenoble C, Lucot JP, Villet R, et al. TVT Secur: more and more minimally invasive. Preliminary prospective study of 110 cases. J Gynecol Obstet Biol Reprod 2008;37:229-36. https://doi.org/10.1016/j.jgyn.2008.01.008.
- Meschia M, Barbacini P, Baccichet R, Buonaguidi A, Maffiolini M, Ricci L, et al. Short-term outcomes with the Ajust™ system: a new single incision sling for the treatment of stress urinary incontinence. Int Urogynecol J 2011;22:177-82. https://doi.org/10.1007/s00192-010-1254-6.
- Abdel-Fattah M, Agur W, Abdel-All M, Guerrero K, Allam M, Mackintosh A, et al. Prospective multi-centre study of adjustable single-incision mini-sling (Ajust®) in the management of stress urinary incontinence in women: 1-year follow-up study. BJU Int 2012;109:880-6. https://doi.org/10.1111/j.1464-410X.2011.10471.x.
- Berkers J, Van Der Aa F, Hamid D, De Ridder D. The minimal invasive MiniArc sling versus Monarc trans-obturator sling system in the treatment of female stress urinary incontinence. Int Urogynecol J 2009;20:S253-S4.
- De Ridder D, Berkers J, Deprest J, Verguts J, Ost D, Hamid D, et al. Single incision mini-sling versus a transobutaror sling: a comparative study on MiniArc and Monarc slings. Int Urogynecol J 2010;21:773-8. https://doi.org/10.1007/s00192-010-1127-z.
- National Institute for Health and Care Excellence . Single-Incision Sub-Urethral Short Tape Insertion for Stress Urinary Incontinence in Women n.d. www.nice.org.uk/guidance/ipg262/documents/ipg262-singleincision-suburethral-short-tape-insertion-for-stress-urinary-incontinence-in-women-understanding-nice-guidance-ms-word-format2 (accessed 1 April 2021).
- Abdel-Fattah M, Ford JA, Lim CP, Madhuvrata P. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: a meta-analysis of effectiveness and complications. Eur Urol 2011;60:468-80. https://doi.org/10.1016/j.eururo.2011.05.003.
- Gadjiev N, Tabaza R, Kirschner-Hermanns R. Mini-sling: what is known about anchorage systems?. Neurourol Urodyn 2012;31:709-1102.
- Kocjancic E, Sedlar A. A strength comparison of immediate and delayed extraction forces of 5 different single incision slings anchor types: an animal model. Int Urogynecol J 2012;23.
- Mostafa A, Lim CP, Hopper L, Madhuvrata P, Abdel-Fattah M. Single-incision mini-slings versus standard midurethral slings in surgical management of female stress urinary incontinence: an updated systematic review and meta-analysis of effectiveness and complications. Eur Urol 2014;65:402-27. https://doi.org/10.1016/j.eururo.2013.08.032.
- Tommaselli GA, Di Carlo C, Gargano V, Formisano C, Scala M, Nappi C. Efficacy and safety of TVT-O and TVT-Secur in the treatment of female stress urinary incontinence: 1-year follow-up. Int Urogynecol J 2010;21:1211-17. https://doi.org/10.1007/s00192-010-1181-6.
- Hinoul P, Vervest HA, den Boon J, Venema PL, Lakeman MM, Milani AL, et al. A randomized, controlled trial comparing an innovative single incision sling with an established transobturator sling to treat female stress urinary incontinence. J Urol 2011;185:1356-62. https://doi.org/10.1016/j.juro.2010.11.083.
- Barber MD, Weidner AC, Sokol AI, Amundsen CL, Jelovsek JE, Karram MM, et al. Single-incision mini-sling compared with tension-free vaginal tape for the treatment of stress urinary incontinence: a randomized controlled trial. Obstet Gynecol 2012;119:328-37. https://doi.org/10.1097/AOG.0b013e318242a849.
- Dover C, Leverson G, McAchran S. Complication rates of single incision slings – meta-analysis of the world literature. Neurourol Urodyn 2012;31. https://doi.org/10.1016/j.juro.2012.02.595.
- Mostafa A, Agur W, Abdel-All M, Guerrero K, Lim C, Allam M, et al. Multicenter prospective randomized study of single-incision mini-sling vs. tension-free vaginal tape-obturator in management of female stress urinary incontinence: a minimum of 1-year follow-up. Urology 2013;82:552-9. https://doi.org/10.1016/j.urology.2013.02.080.
- Schweitzer K, Cromheecke A, Milani H, Van Eijndhoven D, Gietelink E, Hallenleben C, et al. A randomized controlled trial comparing the TVT-O® with the Ajust® as primary surgical treatment of female stress urinary incontinence. Int Urogynecol J 2012;23:S77-S8.
- Cornu J, Ciofu C, Sebe P, Peyrat L, Haab F. Cure of women stress urinary incontinence with the Ajust single incision sling: 1 year results. Neurourol Urodyn 2011;30.
- Lucente V, Cornu J, Sebe P, Peyrat L, Ciofu C, Haab F. Ajust single incision transobturator sling procedure for stress urinary incontinenvce in women. Int Urogynecol J 2011;22.
- Sivaslioglu AA, Unlubilgin E, Aydogmus S, Keskin L, Dolen I. A prospective randomized controlled trial of the transobturator tape and tissue fixation mini-sling in patients with stress urinary incontinence: 5-year results. J Urol 2012;188:194-9. https://doi.org/10.1016/j.juro.2012.02.2564.
- Naumann G, Hagemeier T, Zachmann S, Ali-Ani A, Skala C, Albrich S, et al. Ajust™ fully adjustable single incision sling for the treatment of stress urinary incontinence: 1 year follow-up on a new minimal-invasive treatment for female SUI. Int Urogynecol J 2011;22. https://doi.org/10.1007/s00192-012-1843-7.
- Boyers D, Kilonzo M, Mostafa A, Abdel-Fattah M. Single incision mini-slings versus standard mid-urethral slings in surgical management of female stress urinary incontinence: a cost-effectiveness analysis alongside a randomised controlled trial. Neurourol Urodyn 2012;31:726-7.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev 2014;6. https://doi.org/10.1002/14651858.CD008709.pub2.
- Beard DJ, Davies LJ, Cook JA, MacLennan G, Price A, Kent S, et al. Total versus partial knee replacement in patients with medial compartment knee osteoarthritis: the TOPKAT RCT. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24200.
- International Consultation on Incontinence Questionnaire Development Group . International Consultation on Incontinence Questionnaire – Urinary Incontinence Short Form (ICIQ-UI SF) n.d. https://iciq.net/wp-content/uploads/2019/08/Sample-ICIQ-UI-Short-Form.pdf (accessed 9 March 2020).
- Cardozo L, Coyne KS, Versi E. Validation of the urgency perception scale. BJU Int 2005;95:591-6. https://doi.org/10.1111/j.1464-410X.2005.05345.x.
- International Consultation on Incontinence Questionnaire Development Group . International Consultation on Incontinence Questionnaire – Female Lower Urinary Tract Symptoms Long Form Module (ICIQ-FLUTS LF) n.d. https://iciq.net/wp-content/uploads/2019/08/Sample-ICIQ-FLUTS-LF.pdf (accessed 9 March 2020).
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- International Consultation on Incontinence Questionnaire Development Group . International Consultation on Incontinence Questionnaire – Lower Urinary Tract Symptoms Quality of Life Module (ICIQ-LUTSqol) n.d. https://iciq.net/wp-content/uploads/2019/08/Sample-ICIQ-LUTSqol.pdf (accessed 9 March 2020).
- International Urogynecological Association . Appendix A: PISQ-IR Sexual Function for Women With POP, Urinary Incontinence and Or Fecal Incontinence n.d. www.iuga.org/images/content/PISQ-IR_Instrument_English.pdf (accessed 15 March 2020).
- International Consultation on Incontinence Questionnaire Development Group . International Consultation on Incontinence Questionnaire Female Sexual Matters Associated With Lower Urinary Tract Symptoms Module (ICIQ-FLUTSsex) n.d. https://iciq.net/iciq-flutssex (accessed 9 March 2021).
- Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol 2003;189:98-101. https://doi.org/10.1067/mob.2003.379.
- Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093-103. https://doi.org/10.1001/jama.2016.12195.
- Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics 2014;32:883-902. https://doi.org/10.1007/s40273-014-0170-x.
- Soekhai V, Whichello C, Levitan B, Veldwijk J, Pinto CA, Donkers B, et al. Methods for exploring and eliciting patient preferences in the medical product lifecycle: a literature review. Drug Discov Today 2019;24:1324-31. https://doi.org/10.1016/j.drudis.2019.05.001.
- Lancaster K. A new approach to consumer theory. J Politic Econ 1966;74:132-57. https://doi.org/10.1086/259131.
- Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Health Care. Dordrecht: Springer; 2008.
- ChoiceMetrics . NGENE User Guide and Reference Manual 2014.
- McFadden D, Zarembka P. Frontiers in Econometrics. New York, NY: Academic Press; 1973.
- McFadden D, Train K. Mixed MNL models for discrete response. J Appl Econom 2000;15:447-70. https://doi.org/10.1002/1099-1255(200009/10)15:5<447::AID-JAE570>3.0.CO;2-1.
- Hole AR. Fitting mixed logit models by using maximum simulated likelihood. Stata J 2007;7:388-401. https://doi.org/10.1177/1536867X0700700306.
- Glazener C, Boachie C, Buckley B, Cochran C, Dorey G. Conservative treatment for urinary incontinence in Men After Prostate Surgery (MAPS): two parallel randomised controlled trials. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15240.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal n.d. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 1 April 2021).
- Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Appl Health Econ Health Policy 2005;4:65-7. https://doi.org/10.2165/00148365-200504020-00001.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Hemming C, Constable L, Goulao B, Kilonzo M, Boyers D, Elders A, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess 2020;24. https://doi.org/10.3310/hta24130.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 4 March 2020).
- NHS England . 2018/19/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 23 May 2022).
- Information Services Division Scotland . R140X Theatre Services n.d. www.isdscotland.org/Health-topics/Finance/Costs/Detailed-Tables/Theatres.asp (accessed 20 September 2019).
- NHS Business Services Authority . NHS Electronic Drug Tariff n.d. www.drugtariff.nhsbsa.nhs.uk/#/00730181-DC/DC00730158/Home (accessed 20 June 2020).
- Her Majesty’s Revenue and Customs . Expenses and Benefits: Business Travel Mileage for Employees’ Own Vehicles n.d. www.gov.uk/expenses-and-benefits-business-travel-mileage/rules-for-tax (accessed 30 November 2019).
- Office for National Statistics . Annual Survey of Hours and Earnings: 2019 Revised Results n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/allemployeesashetable1 (accessed 15 December 2020).
- Office for National Statistics . Women Shoulder the Responsibility of ‘Unpaid Work’ n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/articles/womenshouldertheresponsibilityofunpaidwork/2016-11-10 (accessed 15 December 2020).
- Department for Transport . TAG Data Book 2020 n.d. www.gov.uk/government/publications/webtag-tag-data-book (accessed 15 December 2020).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. https://doi.org/10.1002/hec.901.
- Brazier J, Czoski-Murray C, Roberts J, Brown M, Symonds T, Kelleher C. Estimation of a preference-based index from a condition-specific measure: the King’s Health Questionnaire. Med Decis Making 2008;28:113-26. https://doi.org/10.1177/0272989X07301820.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2015.
- Drummond MF, Sculpher M, Torrance GW, O’Brien B, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004.
- Thüroff JW, Abrams P, Andersson KE, Artibani W, Chapple CR, Drake MJ, et al. EAU guidelines on urinary incontinence. Eur Urol 2011;59:387-400. https://doi.org/10.1016/j.eururo.2010.11.021.
- van Buuren S. Flexible Imputation of Missing Data. New York, NY: Chapman and Hall/CRC; 2018.
- Engberts MK, Schweitzer KJ, van Eijndhoven HWF, Cromheecke GJ, Naber HR, Huub van der Vaart C. A prospective observational cohort study of the Ajust® single incision sling performed under conscious sedation with local infiltration. Neurourol Urodyn 2019;38:1632-9. https://doi.org/10.1002/nau.24027.
- Simsek A, Kirecci SL, Bayar G, Horasanli K, Ozgor F, Gurbuz ZG. Evaluation of per-operative cough stress test during transobturator mid-urethral sling surgery. Arch Ital Urol Androl 2017;89:222-5. https://doi.org/10.4081/aiua.2017.3.222.
- Mostafa A, Agur W, Abdel-All M, Guerrero K, Lim C, Allam M, et al. A multicentre prospective randomised study of single-incision mini-sling (Ajust®) versus tension-free vaginal tape-obturator (TVT-O™) in the management of female stress urinary incontinence: pain profile and short-term outcomes. Eur J Obstet Gynecol Reprod Biol 2012;165:115-21. https://doi.org/10.1016/j.ejogrb.2012.06.022.
- Schellart RP, Oude Rengerink K, Van der Aa F, Lucot JP, Kimpe B, Dijkgraaf MG, et al. A randomised comparison of single-incision versus traditional transobturator midurethral sling in women with stress urinary incontinence: results of a 24-month follow-up. Int Urogynecol J 2016;27:871-7. https://doi.org/10.1007/s00192-015-2898-z.
- Xin X, Song Y, Xia Z. A comparison between adjustable single-incision sling and tension-free vaginal tape-obturator in treating stress urinary incontinence. Arch Gynecol Obstet 2016;293:457-63. https://doi.org/10.1007/s00404-015-3949-x.
- Abdel-Fattah M, Ramsay I, Pringle S, Hardwick C, Ali H, Young D, et al. Randomised prospective single-blinded study comparing ‘inside-out’ versus ‘outside-in’ transobturator tapes in the management of urodynamic stress incontinence: 1-year outcomes from the E-TOT study. BJOG 2010;117:870-8. https://doi.org/10.1111/j.1471-0528.2010.02544.x.
- Karmakar D, Mostafa A, Abdel-Fattah M. Long-term outcomes of transobturator tapes in women with stress urinary incontinence: E-TOT randomised controlled trial. BJOG 2017;124:973-81. https://doi.org/10.1111/1471-0528.14561.
- Alexandridis V, Rudnicki M, Jakobsson U, Teleman P. Adjustable mini-sling compared with conventional mid-urethral slings in women with urinary incontinence: a 3-year follow-up of a randomized controlled trial. Int Urogynecol J 2019;30:1465-73. https://doi.org/10.1007/s00192-019-04004-w.
- Nikpoor P, Karjalainen PK, Ow LL, Melendez-Munoz J, Leitch A, Ryan G, et al. Modified transobturator (TVT Abbrevo) and single incision (MiniArc) suburethral sling in women with stress urinary incontinence – a randomised controlled trial: 3 year follow up. Female Pelvic Med Reconstr Surg 2019;25:S92-S3.
- Lee S, Cho S. Single incision mid-urethral sling and tension-free vaginal tape procedure for the treatment of stress urinary incontinence: a 36-month follow-up randomized study. Int Urogynecol J 2018;29.
- Cumberlege J. First Do No Harm: The Report of the Independent Medicines and Medical Devices Safety Review 2020. www.immdsreview.org.uk/downloads/IMMDSReview_Web.pdf (accessed 1 April 2021).
- Tincello DG, Alfirevic Z. Important clinical outcomes in urogynecology: views of patients, nurses and medical staff. Int Urogynecol J Pelvic Floor Dysfunct 2002;13:96-8. https://doi.org/10.1007/s001920200022.
- Corcos J, Beaulieu S, Donovan J, Naughton M, Gotoh M. Symptom Quality of Life Assesment Committee of the First International Consultation on Incontinence . Quality of life assessment in men and women with urinary incontinence. J Urol 2002;168:896-905. https://doi.org/10.1016/S0022-5347(05)64540-5.
- Karantanis E, Fynes M, Moore KH, Stanton SL. Comparison of the ICIQ-SF and 24-hour pad test with other measures for evaluating the severity of urodynamic stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2004;15:111-16. https://doi.org/10.1007/s00192-004-1123-2.
- Tincello DG, Kenyon S, Abrams KR, Mayne C, Toozs-Hobson P, Taylor D, et al. Botulinum toxin A versus placebo for refractory detrusor overactivity in women: a randomised blinded placebo-controlled trial of 240 women (the RELAX study). Eur Urol 2012;62:507-14. https://doi.org/10.1016/j.eururo.2011.12.056.
- Brubaker L, Richter HE, Visco A, Mahajan S, Nygaard I, Braun TM, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol 2008;180:217-22. https://doi.org/10.1016/j.juro.2008.03.028.
- Serati M, Braga A, Athanasiou S, Tommaselli GA, Caccia G, Torella M, et al. Tension-free vaginal tape-obturator for treatment of pure urodynamic stress urinary incontinence: efficacy and adverse effects at 10-year follow-up. Eur Urol 2017;71:674-9. https://doi.org/10.1016/j.eururo.2016.08.054.
- Ulrich D, Tammaa A, Hölbfer S, Trutnovsky G, Bjelic-Radisic V, Tamussino K, et al. Ten-year follow-up after tension-free vaginal tape-obturator procedure for stress urinary incontinence. J Urol 2016;196:1201-6. https://doi.org/10.1016/j.juro.2016.05.036.
- Abdel-Fattah M, Mostafa A, Brown K, Guerrero K, Ward K, Cotterill N, et al. Female Urgency, Trial of Urodynamics As Routine Evaluation (FUTURE study); A Superiority Randomised Clinical Trial to Evaluate the Effectiveness and Cost Effectiveness of Invasive Urodynamic Investigations in Management of Women With Refractory Overactive Bladder Symptoms n.d. https://doi.org/10.1186/s13063-021-05661-3 (accessed 4 March 2021).
- Masata J, Svabik K, Zvara K, Hubka P, Toman A, Martan A. Comparison of the efficacy of tension-free vaginal tape obturator (TVT-O) and single-incision tension-free vaginal tape (Ajust™) in the treatment of female stress urinary incontinence: a 1-year follow-up randomized trial. Int Urogynecol J 2016;27:1497-505. https://doi.org/10.1007/s00192-016-3012-x.
- Maturana AP, Palos CC, Ghersel FR, Fernandes CE, Oliveira E. Randomized controlled trial comparing mini-sling with transobturator sling for the treatment of stress urinary incontinence. Int Urogynecol J 2020;31:1925-31. https://doi.org/10.1007/s00192-019-04145-y.
- Pascom ALG, Djehdian LM, Bortolini MAT, Jarmy-Di Bella ZIK, Delroy CA, Tamanini JTN, et al. Randomized controlled trial comparing single-incision mini-sling and transobturator midurethral sling for the treatment of stress urinary incontinence: 3-year follow-up results. Neurourol Urodyn 2018;37:2184-90. https://doi.org/10.1002/nau.23546.
- Jurakova M, Huser M, Belkov I, Janku P, Hudecek R, Stourac P, et al. Prospective randomized comparison of the transobturator mid-urethral sling with the single-incision sling among women with stress urinary incontinence: 1-year follow-up study. Int Urogynecol J 2016;27:791-6. https://doi.org/10.1007/s00192-015-2895-2.
- Tieu AL, Hegde A, Castillo PA, Davila GW, Aguilar VC. Transobturator versus single incision slings: 1-year results of a randomized controlled trial. Int Urogynecol J 2017;28:461-7. https://doi.org/10.1007/s00192-016-3128-z.
- Sabadell J, Palau-Gené M, Huguet E, Montero-Armengol A, Salicrú S, Poza JL. Multicentre randomized trial of the Ajust™ single-incision sling compared to the Align™ transobturator tape sling. Int Urogynecol J 2017;28:1041-7. https://doi.org/10.1007/s00192-016-3221-3.
- Gaber ME, Borg T, Samour H, Nawara M, Reda A. Two new mini-slings compared with transobturator tension-free vaginal tape for treatment of stress urinary incontinence: A 1-year follow-up randomized controlled trial. J Obstet Gynaecol Res 2016;42:1773-81. https://doi.org/10.1111/jog.13143.
- Enzelsberger H, Cemer I, Enzelsberger S, Schalupny J. MiniArc® versus Monarc® – Eine prospektiv randomisierte Vergleichsstudie zur operativen Therapie der weiblichen Stressharninkontinenz. Geburtshilfe Frauenheilkd 2010;70:499-502. https://doi.org/10.1055/s-0030-1249952.
- Masata J, Svabik K, Hubka P, Martan A. Randomized trial to compare the efficacy of TVT-O and single incision tape ajust in the treatment of stress urinary incontinent women – two year follow-up. Neurourol Urodyn 2015;34:S418-S20.
- Basu M, Duckett J. Three-year results from a randomised trial of a retropubic mid-urethral sling versus the MiniArc single incision sling for stress urinary incontinence. Int Urogynecol J 2013;24:2059-64. https://doi.org/10.1007/s00192-013-2125-8.
- Angleitner-Flotzinger J, Aigmueller T. Mid-term follow-up of the TVT-Secur midurethral sling for primary stress incontinence. Eur J Obstet Gynecol Reprod Biol 2014;180:24-7. https://doi.org/10.1016/j.ejogrb.2014.06.015.
- Masata J, Svabik K, Hubka P, Martan A. Randomized prospective trial of a comparison of the efficacy of TVT-O and TVT-Secur system in the treatment of stress urinary incontinent women – long-term results with a minimum of 5 years follow-up. Int Urogynecol J Pelvic Floor Dysfunct 2015;26:S137-S8.
- Irwin DE, Milsom I, Chancellor MB, Kopp Z, Guan Z. Dynamic progression of overactive bladder and urinary incontinence symptoms: a systematic review. Eur Urol 2010;58:532-43. https://doi.org/10.1016/j.eururo.2010.06.007.
- Duckett JR, Tamilselvi A. Effect of tension-free vaginal tape in women with a urodynamic diagnosis of idiopathic detrusor overactivity and stress incontinence. BJOG 2006;113:30-3. https://doi.org/10.1111/j.1471-0528.2005.00810.x.
- Lee JH, Cho MC, Oh SJ, Kim SW, Paick JS. Long-term outcome of the tension-free vaginal tape procedure in female urinary incontinence: a 6-year follow-up. Korean J Urol 2010;51:409-15. https://doi.org/10.4111/kju.2010.51.6.409.
- Abdel-Fattah M, Mostafa A, Young D, Ramsay I. Evaluation of transobturator tension-free vaginal tapes in the management of women with mixed urinary incontinence: one-year outcomes. Am J Obstet Gynecol 2011;205:150.e1-6. https://doi.org/10.1016/j.ajog.2011.03.018.
- Abdel-Fattah M, Hopper LR, Mostafa A. Evaluation of transobturator tension-free vaginal tapes in the surgical management of mixed urinary incontinence: 3-year outcomes of a randomized controlled trial. J Urol 2014;191:114-19. https://doi.org/10.1016/j.juro.2013.07.035.
- Abdel-Fattah M, Cao G, Mostafa A. Long-term outcomes for transobturator tension-free vaginal tapes in women with urodynamic mixed urinary incontinence. Neurourol Urodyn 2017;36:902-8. https://doi.org/10.1002/nau.23192.
- Ahn SH, Park YJ, Kong MK, Bai SW. Impact of age on outcomes of midurethral sling procedures in women. Int Urogynecol J 2020;31:785-9. https://doi.org/10.1007/s00192-019-04112-7.
- Engen M, Svenningsen R, Schiøtz HA, Kulseng-Hanssen S. Mid-urethral slings in young, middle-aged, and older women. Neurourol Urodyn 2018;37:2578-85. https://doi.org/10.1002/nau.23583.
- Malek JM, Ellington DR, Jauk V, Szychowski JM, Parden AM, Richter HE. The effect of age on stress and urgency urinary incontinence outcomes in women undergoing primary midurethral sling. Int Urogynecol J 2015;26:831-5. https://doi.org/10.1007/s00192-014-2594-4.
- Ward K, Hilton P. United Kingdom and Ireland Tension-free Vaginal Tape Trial Group . Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7355.67.
- Dias J, Xambre L, Costa L, Costa P, Ferraz L. Short-term outcomes of Altis single-incision sling procedure for stress urinary incontinence: a prospective single-center study. Int Urogynecol J 2014;25:1089-95. https://doi.org/10.1007/s00192-014-2355-4.
- Kocjancic E, Erickson T, Tu LM, Gheiler E, Van Drie D. Two-year outcomes for the Altis® adjustable single incision sling system for treatment of stress urinary incontinence. Neurourol Urodyn 2017;36:1582-7. https://doi.org/10.1002/nau.23156.
- Kim A, Kim MS, Park YJ, Choi WS, Park HK, Paick SH, et al. Clinical outcome of single-incision slings, excluding TVT-Secur, vs. standard slings in the surgical management of stress incontinence: an updated systematic review and meta-analysis. BJU Int 2019;123:566-84. https://doi.org/10.1111/bju.14447.
- Schellart RP, Zwolsman SE, Lucot JP, de Ridder DJMK, Dijkgraaf MGW, Roovers JWR. A randomized, nonblinded extension study of single-incision versus transobturator midurethral sling in women with stress urinary incontinence. Int Urogynecol J 2018;29:37-44. https://doi.org/10.1007/s00192-017-3362-z.
- Matharu GS, Assassa RP, Williams KS, Donaldson M, Matthews R, Tincello DG, et al. Objective assessment of urinary incontinence in women: comparison of the one-hour and 24-hour pad tests. Eur Urol 2004;45:208-12. https://doi.org/10.1016/j.eururo.2003.09.006.
- Medicines and Healthcare products Regulatory Agency . A Summary of the Evidence on the Benefits and Risks of Vaginal Mesh Implants n.d. www.gov.uk/government/publications/vaginal-mesh-implants-summary-of-benefits-and-risks (accessed 1 April 2021).
- Dyer O. Johnson and Johnson faces lawsuit over vaginal mesh devices. BMJ 2016;353. https://doi.org/10.1136/bmj.i3045.
- Moldovan CP, Marinone ME, Staack A. Transvaginal retropubic sling systems: efficacy and patient acceptability. Int J Womens Health 2015;7:227-37. https://doi.org/10.2147/IJWH.S59265.
- Morling JR, McAllister DA, Agur W, Fischbacher CM, Glazener CMA, Guerrero K, et al. Adverse events after first, single, mesh and non-mesh surgical procedures for stress urinary incontinence and pelvic organ prolapse in Scotland, 1997–2016: a population-based cohort study. Lancet 2016;389:629-40. https://doi.org/10.1016/S0140-6736(16)32572-7.
- Haskell H. Cumberlege review exposes stubborn and dangerous flaws in healthcare. BMJ 2020;370. https://doi.org/10.1136/bmj.m3099.
- British Society of Urogynaecology . Stress Urinary Incontinence Surgery in the UK 2008–2017: 1st National Report – Audit and Database Committee 2018 2018. https://bsug.org.uk/budcms/includes/kcfinder/upload/files/reports/BSUG-Stress-Incontinence-Surgery---1st-National-Report.pdf (accessed 1 April 2021).
- NHS Digital . Hospital Episode Statistics 2020 n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 31 March 2021).
- Richter HE, Albo ME, Zyczynski HM, Kenton K, Norton PA, Sirls LT, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med 2010;362:2066-76. https://doi.org/10.1056/NEJMoa0912658.
- Abdel-Fattah M, Ramsay I, Pringle S. Lower urinary tract injuries after transobturator tape insertion by different routes: a large retrospective study. BJOG 2006;113:1377-81. https://doi.org/10.1111/j.1471-0528.2006.01097.x.
- Resende A, Oliveira R, Botelho F, Silva C, Dinis P, Cruz F. 770 Mid-term follow-up of a randomized trial comparing TVT-O, TVT-Secur and MiniArc. European Urol Supp 2011;10. https://doi.org/10.1016/S1569-9056(11)60757-4.
- Enzelsberger H, Cemer I, Kostersitz E. Ophira (Minisling) versus Monarc (TOT) – a prospective randomized study for the treatment of female stress urinary incontinence at a follow-up of 20 months. Int Urogynecol J Pelvic Floor Dysfunct 2011;22:S1835-S6.
- Dati S, Rombolá P, Cappello S, Piccione E. M432 Single-incision minisling (Ajust) vs. obturator tension-free vaginal shortened tape (TVT-AbbrevO) in surgical management of female stress urinary incontinence. Int J Gynaecol Obstet 2012;119. https://doi.org/10.1016/S0020-7292(12)61622-1.
- Schweitzer KJ, Milani AL, van Eijndhoven HWF, Gietelink DA, Hallensleben E, Cromheecke GJ, et al. Postoperative pain after adjustable single-incision or transobturator sling for incontinence: a randomized controlled trial. Obstet Gynecol 2015;125:27-34. https://doi.org/10.1097/AOG.0000000000000604.
- Pastore AL, Palleschi G, Al Salhi Y, Riganelli L, Fuschi A, Autieri D, et al. Evaluation of sexual function and quality of life in women treated for stress urinary incontinence: tension-free transobturator suburethral tape versus single-incision sling. J Womens Health 2016;25:355-9. https://doi.org/10.1089/jwh.2015.5416.
- Fernandez-Gonzalez S, Martinez Franco E, Lin Miao X, Amat Tardiu L. Contasure-Needleless® compared with Monarc® for the treatment of stress urinary incontinence. Int Urogynecol J 2017;28:1077-84. https://doi.org/10.1007/s00192-016-3231-1.
- Gul M. A randomized prospective comparison of single incision mini-sling with transobrutator tape in women with stress urinary incontinence: 12 months follow-up results. J Endourol 2018;32:A28-A9.
- Gopinath D, Smith AR, Holland C, Reid FM. Why don’t women participate? A qualitative study on non-participation in a surgical randomised controlled trial. Int Urogynecol J 2013;24:969-75. https://doi.org/10.1007/s00192-012-1967-9.
- Fernandez S, Martinez-Franco E, Lin X. Contasure-Needleless compared with Monarc for the treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2015;26:S84-S5. https://doi.org/10.1007/s00192-014-2475-x.
- Abdel-Fattah M, Sivanesan K, Ramsay I, Pringle S, Bjornsson S. How common are tape erosions? A comparison of two versions of the transobturator tension-free vaginal tape procedure. BJU Int 2006;98:594-8. https://doi.org/10.1111/j.1464-410X.2006.06348.x.
- Shah HN, Badlani GH. Mesh complications in female pelvic floor reconstructive surgery and their management: a systematic review. Indian J Urol 2012;28:129-53. https://doi.org/10.4103/0970-1591.98453.
- Dogan O, Kaya AE, Pulatoglu C, Basbug A, Yassa M. A randomized comparison of a single-incision needleless (Contasure-Needleless®) mini-sling versus an inside-out transobturator (Contasure-KIM®) mid-urethral sling in women with stress urinary incontinence: 24-month follow-up results. Int Urogynecol J 2018;29:1387-95. https://doi.org/10.1007/s00192-018-3624-4.
- Van Rensburg JA, Jeffery ST, Sand Enbergh HA, Juul L, Steyn DW. Single incision-needleless and inside out TVT-O: a multicentre clinical equivalent randomised trial with preliminary 6 months and 1 year outcome for stress urine continence. Int Urogynecol J Pelvic Floor Dysfunct 2015;26:S136-S7.
- Lee J, Rosamilia A, Lim Y, Thomas E, Murray C, Leitch A, et al. MiniArc Monarc suburethral sling in women with stress urinary incontinence – an RCT – 60M follow up. Int Urogynecol J 2017;28:S80-S1.
- World Health Organization . The World Health Organization Quality of Life (WHOQOL) n.d. www.who.int/publications-detail-redirect/WHO-HIS-HSI-Rev.2012.03 (accessed 1 April 2021).
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol 1997;104:1374-9. https://doi.org/10.1111/j.1471-0528.1997.tb11006.x.
- Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996;47:67-71. https://doi.org/10.1016/S0090-4295(99)80384-7.
- Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) Research Group. Qual Life Res 1994;3:291-306. https://doi.org/10.1007/BF00451721.
- Duralde ER, Rowen TS. Urinary incontinence and associated female sexual dysfunction. Sex Med Rev 2017;5:470-85. https://doi.org/10.1016/j.sxmr.2017.07.001.
- Horosz E, Zwierzchowska A, Pomian A, Majkusiak W, Tomasik P, Barcz E. Impact of midurethral sling implantation on sexual function in women with stress urinary incontinence. J Clin Med 2020;9. https://doi.org/10.3390/jcm9051538.
- Zyczynski HM, Rickey L, Dyer KY, Wilson T, Stoddard AM, Gormley EA, et al. Sexual activity and function in women more than 2 years after midurethral sling placement. Am J Obstet Gynecol 2012;207:421.e1-6. https://doi.org/10.1016/j.ajog.2012.06.053.
- Naumann G, Steetskamp J, Meyer M, Laterza R, Skala C, Albrich S, et al. Sexual function and quality of life following retropubic TVT and single-incision sling in women with stress urinary incontinence: results of a prospective study. Arch Gynecol Obstet 2013;287:959-66. https://doi.org/10.1007/s00404-012-2669-8.
- Glavind K, Larsen T, Lindquist AS. Sexual function in women before and after tension-free vaginal tape operation for stress urinary incontinence. Acta Obstet Gynecol Scand 2014;93:986-90. https://doi.org/10.1111/aogs.12475.
- Marszalek M, Roehlich M, Racz U, Metzenbauer M, Ponholzer A, Rauchenwald M, et al. Sexual function after tension-free vaginal tape procedure. Urol Int 2007;78:126-9. https://doi.org/10.1159/000098069.
- Mazouni C, Karsenty G, Bretelle F, Bladou F, Gamerre M, Serment G. Urinary complications and sexual function after the tension-free vaginal tape procedure. Acta Obstet Gynecol Scand 2004;83:955-61. https://doi.org/10.1111/j.0001-6349.2004.00524.x.
- Kenton K, Stoddard AM, Zyczynski H, Albo M, Rickey L, Norton P, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol 2015;193:203-10. https://doi.org/10.1016/j.juro.2014.08.089.
- Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C. A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Int Urogynecol J Pelvic Floor Dysfunct 2003;14:164-8. https://doi.org/10.1007/s00192-003-1063-2.
- Rogers RG, Rockwood TH, Constantine ML, Thakar R, Kammerer-Doak DN, Pauls RN, et al. A new measure of sexual function in women with pelvic floor disorders (PFD): the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire, IUGA-Revised (PISQ-IR). Int Urogynecol J 2013;24:1091-103. https://doi.org/10.1007/s00192-012-2020-8.
- Fu Q, Lv J, Fang W, Jiang C, Gu Y, Leng J, et al. The clinical efficacy of needleless sling technique and TOT in the treatment of female stress urinary incontinence: a prospective randomized controlled trial. Int J Clin Exp Med 2017;10:7084-90.
- Merali S, Dolhaniuk C, Unger T. Stress incontinence in women; a pilot study comparing the MiniArc single incision sling system to the Monarc transobturator sling system. J Minim Invasive Gynecol 2012;19:S33-S4. https://doi.org/10.1016/j.jmig.2012.08.104.
- Latthe PM, Foon R, Toozs-Hobson P. Transobturator and retropubic tape procedures in stress urinary incontinence: a systematic review and meta-analysis of effectiveness and complications. BJOG 2007;114:522-31. https://doi.org/10.1111/j.1471-0528.2007.01268.x.
- Ford AA, Rogerson L, Cody JD, Aluko P, Ogah JA. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev 2017;7. https://doi.org/10.1002/14651858.CD006375.pub4.
- Keršič M, Keršič M, Kunič T, Garzon S, Laganá AS, Barbič M, et al. Single-incision mini-sling for the treatment of female stress urinary incontinence: is it actually inferior to transobturator vaginal tape and tension-free vaginal tape?. Gynecol Minim Invasive Ther 2020;9:123-30. https://doi.org/10.4103/GMIT.GMIT_78_19.
- Karakeçi A, Eftal TC, Keleş A, Gölbaşı C, Onur R. Single-incision midurethral sling shows less pain and similar success rate in a short-term follow-up compared to the transobturator tape method in the treatment of stress urinary incontinence. Turk J Urol 2020;46:63-8. https://doi.org/10.5152/tud.2019.19105.
- Erickson T, Roovers JP, Gheiler E, Parekh M, Parva M, Hanson C, et al. A multicenter prospective study evaluating efficacy and safety of a single-incision sling procedure for stress urinary incontinence. J Minim Invasive Gynecol 2021;28:93-9. https://doi.org/10.1016/j.jmig.2020.04.014.
- White AB, Kahn BS, Gonzalez RR, Rosamilia A, Anger JT, Eilber KS, et al. Prospective study of a single-incision sling versus a transobturator sling in women with stress urinary incontinence: 3-year results. Am J Obstet Gynecol 2020;223:545.e1-545.e11. https://doi.org/10.1016/j.ajog.2020.03.008.
- Kocjancic E, Tu LM, Erickson T, Gheiler E, Van Drie D. The safety and efficacy of a new adjustable single incision sling for female stress urinary incontinence. J Urol 2014;192:1477-82. https://doi.org/10.1016/j.juro.2014.05.101.
- Subak LL, Brown JS, Kraus SR, Brubaker L, Lin F, Richter HE, et al. The ‘costs’ of urinary incontinence for women. Obstet Gynecol 2006;107:908-16. https://doi.org/10.1097/01.AOG.0000206213.48334.09.
- Campbell and Cochrane Economics Methods Group, Evidence for Policy and Practice Information and Coordinating Centre . CCEMG – EPPI-Centre Cost Converter n.d. https://eppi.ioe.ac.uk/costconversion/default.aspx (accessed 15 March 2021).
Appendix 1 Guidance protocol and flow chart for postoperative voiding assessment
FIGURE 22.
Local anesthesia guidance. Chirocaine®, AbbVie Inc., Chicago, IL, USA. Carbostesin®, AstraZeneca plc, Cambridge, UK.

FIGURE 23.
Pathway for postoperative voiding assessment and management of VD for women following MUS procedure.
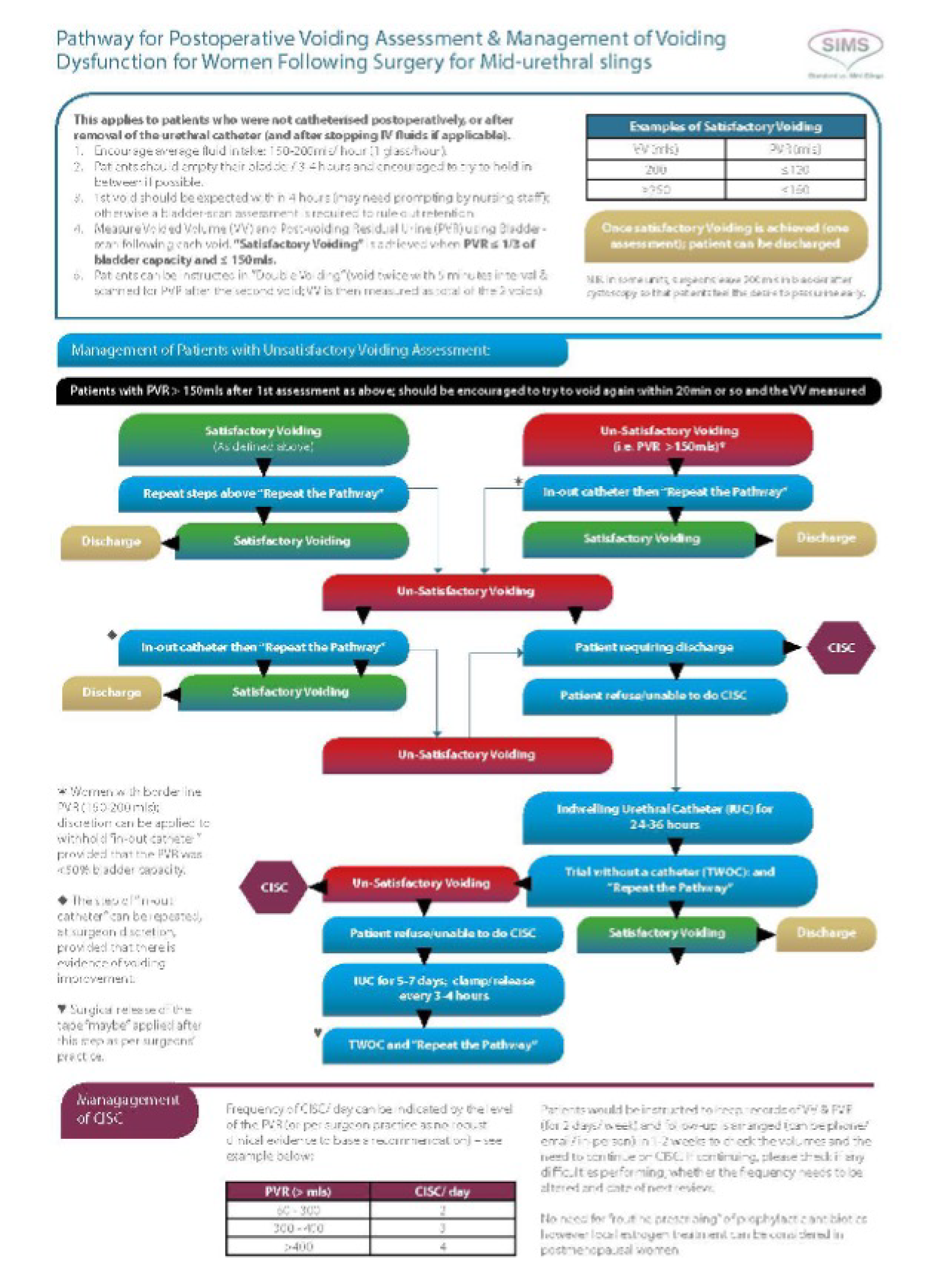
Objective assessment of urinary incontinence in the single-incision mini-sling trial: protocol
-
Participants will receive four or more pre-weighed pads in two transparent self-sealing plastic bags (for the 24-hours pad test), two tissue continence sheets (for the home continence stress tests), instructions on how to perform the tests and a test evaluation questionnaire.
-
Each participant will be asked to:
-
perform a standardised home continence stress test
-
perform the 24-hours pad test (as described by the International Continence Society) using the provided pre-weighed pads
-
repeat the home continence stress test at the end of the 24-hour pad test
-
report all their observations on the provided test questionnaire.
-
-
At the end of the tests, women will be asked to complete an open question regarding their experience of the tests. Women’s satisfaction/convenience with each test will also be assessed using 10-point Likert scales.
-
Preoperatively, participants will be asked to perform this test 24 hours prior to their operation and return any used pads and the test questionnaire to the local RN/team on the day of their surgery. The returned pads will be weighed using a gram-sensitive scale and the pad gain, if any, will be calculated and recorded.
-
At 1, 2 and 3 years postoperatively, participants will return the completed test questionnaire and any used pads in the self-addressed prepaid envelope provided within 24 hours of completion.
-
The returned pads will be weighed by the researcher using a gram-sensitive scale and the pad gain, if any, will be calculated and recorded.
Appendix 2 Missing data comparison and protocol amendments
| Data | 15 months | 36 months | ||||
|---|---|---|---|---|---|---|
| Available (N = 519) | Missing (N = 77) | Difference (95% CI)/p-value | Available (N = 485) | Missing (N = 111) | Difference (95% CI)/p-value | |
| Age (years), mean (SD) | 50.8 (10.9) [n = 519] | 49.1 (11.4) [n = 77] | –1.6 (–4.2 to 1.0) 0.224 | 50.8 (10.9) [n = 485] | 49.5 (11.3) [n = 111] | –1.3 (–3.6 to 1.0) 0.257 |
| BMI (kg/m2), mean (SD) | 28.6 (5.5) [n = 519] | 30.5 (5.7) [n = 70] | 1.9 (0.5 to 3.3) 0.007 | 28.7 (5.4) [n = 485] | 29.4 (6.0) [n = 104] | 0.8 (–0.4 to 1.9) 0.207 |
| Pad test weight (g), mean (SD) | 53.9 (59.1) [n = 420] | 45.0 (31.6) [n = 18] | –8.9 (–36.5 to 18.7) 0.527 | 53.3 (60.2) [n = 398] | 55.1 (33.7) [n = 40] | 1.8 (–17.3 to 20.8) 0.855 |
| How much does urinary leakage interfere with day-to-day activities? mean (SD) | 7.2 (2.1) [n = 509] | 6.9 (2.2) [n = 66] | –0.3 (–0.9 to 0.2) 0.263 | 7.2 (2.2) [n = 477] | 7.3 (2.0) [n = 98] | 0.1 (–0.4 to 0.6) 0.708 |
| ICIQ-UI-SF score, mean (SD) | 14.5 (3.4) [n = 503] | 14.1 (3.4) [n = 66] | –0.3 (–1.2 to 0.5) 0.438 | 14.4 (3.5) [n = 471] | 14.6 (3.3) [n = 98] | 0.2 (–0.6 to 0.9) 0.657 |
| EQ-5D-3L score, mean (SD) | 0.845 (0.231) [n = 506] | 0.860 (0.181) [n = 64] | 0.014 (–0.044 to 0.073) 0.629 | 0.848 (0.227) [n = 474] | 0.841 (0.223) [n = 96] | –0.007 (–0.0571 to 0.043) 0.779 |
| Received PFMT in previous 2 years, n (%) | 444 (86) | 64 (83) | p = 0.575 | 415 (86) | 93 (84) | p = 0.633 |
| Number of babies, n (%) | ||||||
| 0 | 17 (3.3) | 2 (2.6) | p < 0.001 | 17 (3.5) | 2 (1.8) | p < 0.001 |
| 1 | 67 (13) | 9 (12) | 61 (13) | 15 (14) | ||
| 2 | 234 (45) | 26 (34) | 223 (46) | 37 (33) | ||
| 3 | 138 (27) | 24 (31) | 130 (27) | 32 (29) | ||
| ≥ 4 | 62 (12) | 11 (14) | 53 (11) | 20 (18) | ||
| Missing | 1 (0.19) | 5 (6.5) | 1 (0.21) | 5 (4.5) | ||
| Cystometry diagnosis, n (%) | ||||||
| Urodynamic stress incontinence | 421 (81) | 45 (58) | p < 0.001 | 394 (81) | 72 (65) | p < 0.001 |
| Urodynamic mixed incontinence | 59 (11) | 10 (13) | 54 (11) | 15 (13) | ||
| Equivocal | 4 (0.77) | 2 (2.6) | 5 (1.0) | 1 (0.90) | ||
| Not interpretable | 2 (0.39) | 2 (0.41) | ||||
| Other | 4 (0.77) | 3 (0.62) | 1 (0.90) | |||
| Clinical diagnosis of SUI (no urodynamics performed) | 22 (4.2) | 3 (3.9) | 20 (4.1) | 5 (4.5) | ||
| Urodynamics not done and no clinical diagnosis | 7 (1.3) | 17 (22) | 7 (1.4) | 17 (15) | ||
| Uroflowmetry diagnosis, n (%) | ||||||
| Normal | 409 (79) | 50 (65) | p < 0.001 | 386 (80) | 73 (66) | p < 0.001 |
| Obstruction | 10 (1.9) | 7 (1.4) | 3 (2.7) | |||
| Suboptimal | 24 (4.6) | 5 (6.5) | 22 (4.5) | 7 (6.3) | ||
| Equivocal | 8 (1.5) | 6 (1.2) | 2 (1.8) | |||
| Not interpretable | 4 (0.77) | 1 (1.3) | 5 (1.0) | |||
| Not recorded | 26 (5.0) | 3 (3.9) | 23 (4.7) | 6 (5.4) | ||
| Other | 36 (6.9) | 3 (3.9) | 34 (7.0) | 5 (4.5) | ||
| Test not performed | 2 (0.39) | 15 (19) | 2 (0.41) | 15 (14) | ||
| How often do you leak urine?, n (%) | ||||||
| Once or less per week | 15 (2.9) | p < 0.001 | 14 (2.9) | 1 (0.90) | p < 0.001 | |
| Two or three times per week | 60 (12) | 8 (10) | 59 (12) | 9 (8.1) | ||
| Once per day | 43 (8.3) | 10 (13) | 42 (8.7) | 11 (9.9) | ||
| Several times per day | 336 (65) | 39 (51) | 313 (65) | 62 (56) | ||
| All the time | 61 (12) | 9 (12) | 54 (11) | 16 (14) | ||
| No response to question | 2 (0.39) | 1 (1.3) | 2 (0.41) | 1 (0.90) | ||
| Questionnaire missing | 2 (0.39) | 10 (13) | 1 (0.21) | 11 (9.9) | ||
| How much urine do you leak?, n (%) | ||||||
| Small amounts | 203 (39) | 25 (32) | p < 0.001 | 192 (40) | 36 (32) | p < 0.001 |
| Moderate amounts | 222 (43) | 34 (44) | 204 (42) | 52 (47) | ||
| Large amounts | 84 (16) | 7 (9.1) | 80 (16) | 11 (9.9) | ||
| No response to question | 8 (1.5) | 1 (1.3) | 8 (1.6) | 1 (0.90) | ||
| Questionnaire missing | 2 (0.39) | 10 (13) | 1 (0.21) | 11 (9.9) | ||
| Manual job (heavy lifting), n (%) | 151 (29) | 17 (22) | p = 0.202 | 142 (29) | 26 (23) | p = 0.216 |
| Smoker, n (%) | 82 (16) | 13 (17) | p = 0.808 | 68 (14) | 27 (24) | p = 0.007 |
| Amendment number | Protocol version | Description of changes [including author(s) of changes] | Date effective |
|---|---|---|---|
| 1 | New document | 21 October 2013 | |
| 1.1 | Amended document | 27 November 2013 | |
| 5 | 2 | Amended document (not used at sites) | 29 October 2014 |
| 5 | 2.1 | Amended document | 9 January 2015 |
| 6 | 2.2 | Amended document | 25 August 2015 |
| 11 | 3 | Amended document, amendment 11, REC amendment 12: 12-month extension to recruitment to November 2016 and update of annual questionnaire. Annual questionnaire reminder letter, postoperative test questionnaire and annual pad test letter. New shortened annual reminder questionnaires and labels for front of years 2 and 3 questionnaires regarding vouchers | 18 March 2016 |
| 12 | 3.1 | Amended document – amended flow chart and Gantt chart to reflect extension to recruitment from amendment 11 (REC amendment 12) | 24 October 2016 |
| 13 | 4 | Amended document | 5 December 2016 |
| 13 | 4.1 | Amended document, amendment 13. No cost extension to recruitment from November 2016 to end of July 2017. Change of status of John Norrie from co-chief investigator to grant holder – senior methodologist. Change of TSC chairperson and independent member | 23 January 2017 |
| 17 | 5 | Change to follow-up of non-respondents to 2- and 3-year questionnaires. Inclusion of telephone call/text follow-up | 16 March 2018 |
| 17 | 5.1 | Removal of text follow-up option | 23 April 2018 |
| 25 and NSA27 | 6 | Inclusion of long-term follow-up, appendix 6a | 13 July 2020 |
| Inclusion of appendix 7: COVID-19 data collection changes | |||
| NSA28 | 7 | Removal of appendix regarding long-term follow-up as funding not secured at this time | 31 August 2020 |
| NSA29 | 8 | Change of independent TSC member: removal of Lynda Harper and addition of Eleanor Mitchell | 23 October 2020 |
Appendix 3 Baseline tables
| Recruitment centre | Trial group, n (%) | Total, n (%) | |
|---|---|---|---|
| SIMS | SMUS | ||
| Aberdeen Royal Infirmary | 39 (13) | 38 (13) | 77 (13) |
| Milton Keynes University Hospital | 32 (11) | 32 (11) | 64 (11) |
| Pinderfields Hospital, Mid Yorkshire | 31 (10) | 31 (10) | 62 (10) |
| Countess of Chester Hospital | 30 (10) | 28 (9.3) | 58 (9.7) |
| James Cook University Hospital | 27 (9.0) | 27 (9.0) | 54 (9.0) |
| Borders General Hospital | 18 (6.0) | 18 (6.0) | 36 (6.0) |
| Great Western Hospital, Swindon | 16 (5.3) | 17 (5.7) | 33 (5.5) |
| United Lincolnshire Hospitals NHS Trust | 14 (4.7) | 14 (4.7) | 28 (4.7) |
| York Hospital | 13 (4.3) | 14 (4.7) | 27 (4.5) |
| Torbay Hospital, South Devon | 13 (4.3) | 11 (3.7) | 24 (4.0) |
| Royal Preston Hospital | 11 (3.7) | 11 (3.7) | 22 (3.7) |
| Wirral University Teaching Hospital NHS Foundation Trust | 9 (3.0) | 11 (3.7) | 20 (3.3) |
| University Hospital of Wales | 9 (3.0) | 9 (3.0) | 18 (3.0) |
| Barnsley Hospital NHS Foundation Trust | 9 (3.0) | 8 (2.7) | 17 (2.8) |
| Hywel Dda University Health Board, Carmarthen | 8 (2.7) | 8 (2.7) | 16 (2.7) |
| Princess Royal Hospital | 5 (1.7) | 5 (1.7) | 10 (1.7) |
| Wolverhampton Hospital | 3 (1.0) | 7 (2.3) | 10 (1.7) |
| James Paget University Hospital, Great Yarmouth | 5 (1.7) | 4 (1.3) | 9 (1.5) |
| Worthing Hospital | 4 (1.3) | 4 (1.3) | 8 (1.3) |
| Queen Elizabeth Hospital, King’s Lynn | 3 (1.0) | 2 (0.67) | 5 (0.83) |
| Queen Margaret Hospital, Dunfermline | 1 (0.33) | 1 (0.33) | 2 (0.33) |
| Total (N) | 300 | 300 | 600 |
| Time | Trial group | |
|---|---|---|
| SIMS | SMUS | |
| Time from randomisation to surgery (in days), mean (SD) | 65 (69) [n = 276] | 64 (59) [n = 261] |
| Median (IQR) | 48 [16–93] | 47 [19–93] |
| Time from randomisation to | ||
| 4-week follow-up, mean (SD) | 100 (89) [n = 204] | 99 (62) [n = 186] |
| Median (IQR) | 80 (47–126) | 85 (51–134) |
| 3-month follow-up, mean (SD) | 168 (75) [n = 216] | 165 (61) [n = 206] |
| Median (IQR) | 150 (119–196) | 151 (119–197) |
| 15-month follow-up, mean (SD) | 476 (30) [n = 230] | 479 (32) [n = 210] |
| Median (IQR) | 465 (459–489) | 464 (460–486) |
| 24-month follow-up, mean (SD) | 745 (36) [n = 187] | 747 (25) [n = 175] |
| Median (IQR) | 740 (734–753) | 739 (734–752) |
| 36-month follow-up, mean (SD) | 1143 (97) [n = 207] | 1143 (110) [n = 202] |
| Median (IQR) | 1111 (1101–1133) | 1109 (1101–1131) |
| Time from surgery to | ||
| 4-week follow-up, mean (SD) | 34 (56) [n = 204] | 29 (11) [n = 186] |
| Median (IQR) | 28 (28–29) | 28 (28–29) |
| 3-month follow-up, mean (SD) | 103 (16) [n = 216] | 101 (17) [n = 206] |
| Median (IQR) | 97 (92–109) | 95 (92–104) |
| 15-month follow-up, mean (SD) | 412 (69) [n = 226] | 416 (65) [n = 205] |
| Median (IQR) | 421 (379–453) | 431 (380–452) |
| 24-month follow-up, mean (SD) | 682 (81) [n = 184] | 686 (60) [n = 172] |
| Median (IQR) | 699 (662–729) | 705 (657–726) |
| 36-month follow-up, mean (SD) | 1083 (118) [n = 206] | 1079 (122) [n = 196] |
| Median (IQR) | 1074 (1032–1098) | 1069 (1026–1095) |
Appendix 4 Patient-reported clinical outcomes
| PGI-I scale | Trial group, n (%) or n/N (%) | Effect size (95% CI) | |
|---|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | ||
| 4 weeks | N = 239 | N = 222 | |
| Very much improved | 123 (51) | 108 (49) | 1.09 (0.81 to 1.47) |
| Much improved | 60 (25) | 60 (27) | |
| Improved | 33 (14) | 37 (17) | |
| Same | 13 (5.4) | 10 (4.5) | |
| Worse | 5 (2.1) | 5 (2.3) | |
| Much worse | 4 (1.7) | 1 (0.45) | |
| Very much worse | 1 (0.42) | 1 (0.45) | |
| Missing | 59/298 (20) | 76/298 (26) | |
| 3 months | N = 254 | N = 230 | |
| Very much improved | 165 (65) | 139 (60) | 1.26 (0.89 to 1.78) |
| Much improved | 46 (18) | 48 (21) | |
| Improved | 24 (9.4) | 21 (9.1) | |
| Same | 10 (3.9) | 10 (4.3) | |
| Worse | 5 (2.0) | 8 (3.5) | |
| Much worse | 4 (1.6) | 2 (0.87) | |
| Very much worse | 2 (0.87) | ||
| Missing | 44/298 (15) | 68/298 (23) | |
| 15 months | N = 268 | N = 250 | |
| Very much improved | 172 (64) | 156 (62) | 1.17 (0.79 to 1.73) |
| Much improved | 40 (15) | 33 (13) | |
| Improved | 27 (10) | 35 (14) | |
| Same | 14 (5.2) | 15 (6.0) | |
| Worse | 9 (3.4) | 8 (3.2) | |
| Much worse | 1 (0.37) | ||
| Very much worse | 5 (1.9) | 3 (1.2) | |
| Missing | 30/298 (10) | 48/298 (16) | |
| 24 months | N = 239 | N = 225 | |
| Very much improved | 152 (64) | 135 (60) | 1.19 (0.83 to 1.70) |
| Much improved | 33 (14) | 32 (14) | |
| Improved | 25 (10) | 28 (12) | |
| Same | 15 (6.3) | 16 (7.1) | |
| Worse | 8 (3.3) | 7 (3.1) | |
| Much worse | 4 (1.7) | 3 (1.3) | |
| Very much worse | 2 (0.84) | 4 (1.8) | |
| Missing | 59/298 (20) | 73/298 (24) | |
| 36 months | N = 246 | N = 235 | |
| Very much improved | 136 (55) | 124 (53) | 1.20 (0.87 to 1.67) |
| Much improved | 41 (17) | 33 (14) | |
| Improved | 28 (11) | 36 (15) | |
| Same | 23 (9.3) | 18 (7.7) | |
| Worse | 12 (4.9) | 19 (8.1) | |
| Much worse | 3 (1.2) | ||
| Very much worse | 3 (1.2) | 5 (2.1) | |
| Missing | 52/298 (17) | 63/298 (21) | |
Appendix 5 Safety data
| Event number | Patient ID | Date | Description of event | Summary of event | Action |
|---|---|---|---|---|---|
| 1 | 11011 | 6 March 2014 | Patient lost 807 ml of blood during the operation, which was managed by manual compression to her left obturator area |
|
Sponsor notified on 19 September 2014, after meeting on 11 September 2014 agreeing that all SAEs will be reviewed by sponsor. Sponsor in agreement with assessment (e-mail 19 September 2014) |
| Assessed by Mohamed Abdel-Fattah (chief investigator) | |||||
| 2 | 20007 | 13 August 2014 | The patient was unable to pass urine post operatively and complained of left leg pain. The patient did pass urine the next day and no abnormalities were detected regarding the leg pain. Patient discharged 14 August 2014 |
|
Sponsor notified on 19 September 2014, after meeting on 11 September 2014 agreeing that all SAEs will be reviewed by sponsor. Sponsor noted that the SAE is serious, expected and related (e-mail 19 September 2014) |
| Assessed by Aethele Khunda (PI) | |||||
| 3 | 20014 | 22 October 2014 | Patient was randomised to the SIMS group on 17 October 2014, but had an anaphylactic reaction in theatre from the antibiotics prior to surgery commencing. The patient was given steroids and admitted for 24-hour monitoring |
|
Sponsor notified on 27 October 2014. Sponsor noted that the SAE is serious, expected and related (e-mail 29 October 2014). Chief investigator content with sponsor assessment (29 October 2014) |
| Assessed by Mohamed Abdel-Fattah | |||||
| 3 (follow-on report) | 20014 | 9 November 2015 | Patient developed an anaphylactic reaction to co-amoxiclav prior to surgery. Operation was abandoned and the patient was given steroids and admitted overnight for observation. Following overnight observation, the patient went home well the next day. She was relisted and underwent her planned surgery on 17 November 2014. Her recovery was uneventful and she was discharged later the same day |
|
Sponsor notified on 3 December 2015 |
| Assessed by Aethele Khunda | |||||
| 4 | 20015 | 22 October 2014 | At the end of the operation it was noted that skin reactions had developed, initially in the area of surgery, then spread to legs and arms. This occurred after the Opsite (Smith & Nephew plc, Watford, UK) dressing was applied. Treated with antihistamine and steroids. Deteriorated in recovery, tryptase sent, arterial gases sent and monitored. Indwelling catheter inserted, IV fluids prescribed. Blood and urine cultures sent. Arterial line inserted and blood gases sent off. Refer to RVI for allergy screening. Admitted to gynaecology ward for observation overnight |
|
Sponsor notified on 27 October 2014. Sponsor noted that the SAE is serious, expected and related (e-mail 29 October 2014). Chief investigator content with sponsor assessment (29 October 2014) |
| Assessed by Mohamed Abdel-Fattah | |||||
| 4 (follow-on report) | 20015 | 9 November 2015 | The patient had a skin reaction in the area of surgery. SAE was notified on 22 October 2014. The reaction was suspected to be due to the dressing or iodine. RAST was performed and the participant had had a reaction to the povidone-iodine. This is likely to be the source of the reaction. It has been recommended that, in future, the participant uses chlorhexidine as a safe alternative for skin preparation |
|
Sponsor notified on 3 December 2015 |
| Assessed by Aethele Khunda | |||||
| 5 | 11077 | 31 October 2014 | Needed to insert trocar/needle more than once because of lack of robust anchoring on left side |
|
Sponsor notified on 10 November 2014. Sponsor noted that the SAE is serious, expected and related (e-mail 18 November 2014) |
| Assessed by Mohamed Abdel-Fattah | |||||
| 6 | 19002 | 11 November 2014 | Urinary retention post TVT/cystoscopy. Patient was not happy to go home with indwelling catheter and/or doing intermittent self-catheterisation |
|
Sponsor notified on 13 November 2014. Sponsor noted that the SAE is serious, expected and related (e-mail 18 November 2014) |
| Assessed by Maryam Pezeshki | |||||
| 7 | 11045 | 2 December 2014 | Developed pain in right thigh after operation. MRI showed spine problem. Neurophysiology studies showed possible nerve stretch (obdurate or lateral cutaneous nerve injury). On gabapentin |
|
Sponsor notified on 9 December 2014. E-mail update sent to sponsor on 29 April 15. Closed by sponsor on 16 June 2015 after follow-on report |
| Assessed by Mohamed Abdel-Fattah | |||||
| 7 (follow-on report) | 11045 | 8 May 2015 | Following mini sling procedure for stress incontinence under LA, patient developed pain in right buttock, thigh and groin. Report updated by PI on 15 June 2015 with the following information. Thigh pain could not be expected from a Minitape, only for an obturator tape (TVT-ob). The event is presumed to have occurred at the operation, but the symptoms did not start immediately after the operation. It is therefore difficult to attribute the symptoms the patient developed to the administration of the LA, or the procedure. It could have been the position at operation, but she was awake and therefore had muscle support and awareness, so unlikely |
|
Sponsor notified on 14 May 2015. Sponsor notified of updated report on 15 June 2015. Sponsor noted that the SAE is serious, expected and related, but will not over-ride the PI’s decision (e-mail 16 June 2015) |
| Assessed by Kevin Cooper | |||||
| 7 (follow-on report) | 11045 | 17 November 2015 | Further MDT convened by PI and recommended imaging + pain clinic management + physiotherapy. Imaging – MR neurography – not available in ARI (will not affect management, but for diagnosis) |
|
Updated report notified to sponsor 03/12/2015 |
| Assessed by Mohamed Abdel-Fattah | |||||
| 8 | 23016 | 11 May 2015 | Patient attended A&E for increasing right groin pain. Pain described as deep, severe ache with intermittent sharp pain. Redness and swelling to right inner thigh |
|
Sponsor notified on 12 May 2015. Sponsor noted that the SAE is serious, expected and related (e-mail 15 May 2015) |
| Assessed by Mofid Ibraheim | |||||
| 8 (follow-on report) | 23016 | 10 November 2015 | 18 May 2015: patient attended a follow-up appointment post TVT-O procedure. Pain persisting in right hip joint but no pain reported in the inguinal region. No voiding problems. Analgesia prescribed and follow-up appointment arranged for 3 months’ time 2 September 2015: patient attended follow-up appointment; pain resolved and patient discharged |
|
Sponsor notified. DMC notified |
| Assessed by Mofid Ibraheim | |||||
| 9 | 31008 | 28 October 2015 | Patient experienced a tonic–clonic seizure; seen straight away by anaesthetist. Small dose of propofol. Adjacent tape put in opposite side. Cystoscopy done. Patient recovered and mentally alert, coughed and allowed for tape adjustment. Vital signs maintained |
|
Sponsor notified on 30 October 2015. Sponsor noted that the SAE is serious, and not related (e-mail 6 November 2015) |
| Assessed by Meena Dass | |||||
| 9 (follow-on report) | 31008 | 2 November 2015 | Follow-on report. As previous SAE form. Patient admitted on 28 October 2015. Discharged on 29 October 2015 |
|
Sponsor notified on 3 November 2015. Sponsor noted that the SAE is serious and not related (e-mail 6 November 2015) |
| Assessed by Meena Dass | |||||
| 10 | 25007 | 27 May 2016 | Ongoing postoperative groin pain necessitating ongoing analgesia; inability to return to normal activities of daily living. Intervention required: antibiotics post operatively from GP plus amitriptyline (25 mg) prescribed by Waleed Al-Singary. Two clinic visits for MRI. Patient discussed at local urogynaecology MDT meeting: to be referred for second opinion to Andrew Simons, consultant urogynaecologist at St Richard’s. Possible intervention may include local infiltration for pain control |
|
Sponsor notified on 17 June 2016. After follow-on report, sponsor closed SAE on 10 October 2016 |
| Assessed by Waleed Al-Singary | |||||
| 10 (follow-on report) | 25007 | 27 May 2016 | Ongoing postoperative groin pain necessitating ongoing analgesia; inability to return to normal activities of daily living. Intervention required: referral to Andrew Simons at Chichester for review and possible further treatment |
|
Sponsor notified on 28 June 2016. Sponsor confirmed that no further information required; SAE closed (e-mail 10 October 2016) |
| Assessed by Waleed Al-Singary | |||||
| 10 (follow-on updated report) | 25007 | 27 June 2016 | Ongoing postoperative groin pain necessitating ongoing analgesia; inability to return to normal activities of daily living. Reassessment of expectedness and relatedness by PI |
|
Sponsor notified on 28 June 2016. Sponsor confirmed that no further information required; SAE closed (e-mail 10 October 2016) |
| Assessed by Waleed Al-Singary | |||||
| 11 | 23023 | 6 October 2016 |
Post operative pain. Pain detected postoperatively at home; patient has had follow-up in gynaecology outpatient department. Patient under the pain team and musculoskeletal team Postoperatively, patient experienced:Patient under care of pain and musculoskeletal teams for bilateral pain as this is the main complaint |
|
Sponsor notified on 14 October 2016. Sponsor confirmed that no further information required; SAE closed (e-mail 1 December 2016) |
| Assessed by Mofid Ibraheim | |||||
| 12 | 11027 | 28 November 2016 | Postoperative pain. Patient described hip pain on sitting down and persistence of UI in late 2014. She had a bad fall resulting in severe back pain prior to her continence referral in 2013 and described buttock pain after her prolapse surgery in 2013. The pain was investigated with hip radiography, which showed degenerative hip joint changes. This was followed by MRI for the pelvis and lumbar spine and later to the course of the tape, all of which were negative. The patient was discussed in the pelvic floor MDT meeting and appropriate referrals/follow-ups were arranged |
|
Sponsor notified on 28 November 2016. Sponsor confirmed that no further information required; SAE closed (e-mail 1 December 2016) |
| Assessed by Mohamed Abdel-Fattah | |||||
| 13 | 15022 | 15 March 2017 | Patient took paracetamol (1000 mg) every 4 hours for 10 days. Felt unwell; presented at GP. GP prescribed codeine in place of paracetamol. Patient presented at A&E the following day. Codeine overdose also confirmed |
|
Sponsor notified on 17 March 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 10 April 2017) |
| Assessed by Mustafa Hilmy (PI) | |||||
| 13 (follow-on report) | 15022 | 30 March 2017 | Participant took 1 g of paracetamol every 4 hours for 10 days, exceeding the maximum does recommended every 24 hours for 10 days. Attended A&E in Scarborough Hospital on advice from her GP. Was admitted for investigation and treatment of paracetamol overdose. She was found to have deranged liver function tests with an ALT level of 414 IU/l and an alkaline phosphatase level of 305 IU/l. She was treated with acetylcysteine and discharged the following day. On the EDN from this admission it also states she had a ‘confirmed codeine overdoes’. However, the participant denies this and there is no further evidence of this on her electronic medical records, such as blood tests or treatment records. The patient reports that her GP changed her from paracetamol to codeine the day before she attended A&E but she had only taken two doses in > 12 hours and had no symptoms consistent with codeine overdose. Suspected to be a clerical error |
|
Sponsor notified on 31 March 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 10 April 2017) |
| Assessed by Mustafa Hilmy (PI) | |||||
| 14 | 19034 | 7 June 2017 | Participant reported feeling some sharpness inside the vagina, especially during sexual intercourse. On examination by the PI, a small area of tape exposure was seen. Participant is awaiting an appointment to have resuturing of the vaginal skin over the tape exposure |
|
Sponsor notified on 8 June 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 9 June 2017) |
| Assessed by Maryam Pezeshki (PI) | |||||
| 14 (follow-on report) | 19034 | 30 October 2017 | Patient reported feeling sharpness inside vagina. On examination by Maryam Pezeshki, a small area of tape exposure was seen. Patient had resuturing of anterior vaginal wall over tape exposure on 25 October 2017 |
|
Sponsor notified on 30 October 2017 |
| Assessed by Maryam Pezeshki (PI) | |||||
| 15 | 24018 | 16 August 2017 | Patient experienced vaginal and left-sided groin pain 2 weeks following surgery, which necessitated admission to a neighbouring hospital (no immediate details available). Commenced on tramadol. Seen back in our hospital clinic on 15 August 2016. Persisting symptoms. Imaging requested; awaiting further urogynaecology MDT meeting |
|
Sponsor notified on 16 August 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 21 August 2017) |
| Assessed by Kiron Bhal (PI) | |||||
| 15 (follow-on report) | 24018 | 27 October 2017 | Patient experienced vaginal and left-sided groin pain 2 weeks following surgery, which necessitated admission to a neighbouring hospital (no immediate details available). Commenced on tramadol. Seen back in our hospital clinic on 15 August 2016. MRI was arranged, which did not show any abscess, etc. Post-operation follow-up at 4 weeks: persisting pain and patient debriefed. Referred to chronic pain team and to physiotherapy |
|
Notified sponsor 30 October 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 21 August 2017) |
| Assessed by Kiron Bhal (PI) | |||||
| 16 | 20047 | 14 September 2017 | Patient was experiencing pain with intercourse, necessitating surgical intervention. They returned to theatre on 25 July 2017 for excision of vaginal portion of TOT. Pain completely resolved following TOT excision |
|
Notified sponsor 15 September 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 21 September 2017) |
| Assessed by Aethele Khunda (PI) | |||||
| 17 | 29029 | 15 September 2017 | Patient acquired a massive retropubic haematoma during surgery. CT confirmed diagnosis |
|
Sponsor notified on 15 September 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 21 September 2017) |
| Assessed by Tamer Abdelrazik (PI) | |||||
| 17 (follow-on report) | 29029 | 18 September 2017 | Patient developed retro haematoma from TVT surgery. Haemoglobin low. Received 4 units of blood (transfused) and laparoscopy (day 3) with washout that showed disposal of haematoma retroperitoneal. No injury to bowel or bladder. Discharged on 10 September 2017 (day 4). Follow-up arranged as outpatient on 15 September 2017 |
|
Sponsor notified on 18 September 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 21 September 2017) |
| Assessed by Tamer Abdelrazik (PI) | |||||
| 18 | 19056 | 19 October 2017 | Since having TVT procedure, patient has been having pain in her groin and unable to completely empty bladder. Symptoms of UTI but no abnormality seen in mid-stream urine (MSU). Patient will have removal of tape |
|
Sponsor notified on 24 October 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 25 October 2017) |
| Assessed by Maryam Pezeshki (PI) | |||||
| 18 (follow-on report) | 19056 | 8 November 2017 | Since having TVT procedure, patient has been having pain in her groin and unable to completely empty bladder. Symptoms of UTI four times, but no abnormality seen in mid-stream urine. Patient had removal of TVT under GA on 27 October 2017 |
|
Sponsor notified on 13 November 2017. Sponsor confirmed that no further information required; SAE closed (e-mail 15 January 2018) |
| Assessed by Maryam Pezeshki (PI) | |||||
| 19 | 23047 | 20 April 2018 | Vaginal pain since SIMS procedure on 3 February 2017. Post operation, patient was troubled with cholecystitis and gallstones. Patient had MRI (negative), transvaginal scan (right small haemorrhagic cyst) and physiotherapy; pain settled and patient discharged |
|
Sponsor notified on 24 April 2018. Sponsor confirmed that no further information required; SAE closed (e-mail 26 April 2018) |
| Assessed by Mofid Ibraheim (PI) | |||||
| 20 | 18012 | 7 February 2019 | Seen by Derriford Hospital urogynaecology consultant. Admitted for removal of vaginal portion of TOT on 11 January 2018 owing to vaginal pain. Removal of vaginal portion of TOT. Reviewed at Derriford after 5 months. Pain had improved significantly. Still reported stress incontinence |
|
Sponsor notified on 8 February 2019. Sponsor confirmed that no further information required; SAE closed (e-mail 11 February 2019) |
| Assessed by Subramanian Narayanan (PI) | |||||
| 21 | 16039 | 23 May 2019 | Planned admission for GA cystoscopy and partial removal of transvaginal tape: operation findings – tape tight and close to bladder, neck contributing to patient’s reported symptoms |
|
Sponsor notified on 24 May 2019. Sponsor confirmed that no further information required; SAE closed (e-mail 29 May 2019) |
| Assessed by Mohamed Abdel-Fattah (chief investigator) | |||||
| 22 | 26007 | 4 June 2019 | Chest and lung MDT: patient was found to have lung metastasis but gynaecology primary cancer was not confirmed. Undergoing chemotherapy – 23 January 2018 |
|
Sponsor notified on 5 June 2019. Sponsor confirmed that no further information required; SAE closed (e-mail 12 June 2019) |
| Assessed by Mohamed Abdel-Fattah (chief investigator) | |||||
| 23 (follow-on report) | 26007 | 4 June 2019 | 28 February 2019: outpatient appointment – received chemotherapy and palliative care for ovarian cancer, peritoneum cancer treatment. Therapeutic diagnostic thoracentesis. Chemotherapy stopped because of intolerable side effects; discharged under the care of palliative care team and hospice. Patient died on 11 May 2019 |
|
Sponsor notified on 1 July 2019. Sponsor confirmed that no further information required; SAE closed (e-mail 2 July 2019) |
| Assessed by Mohamed Abdel-Fattah (chief investigator) | |||||
| 24 | 16039 | 9 July 2019 | Follow-on report: reviewed in clinic postoperatively; flow rate confirmed good flow, voiding and emptying bladder almost completely. Patient discharged from clinic |
|
Sponsor notified on 9 July 2019. Sponsor confirmed that no further information required; SAE closed (e-mail 12 July 2019) |
| Assessed by Mohamed Abdel-Fattah (chief investigator) | |||||
| 25 | 12016 | 9 March 2020 | Sudden death at home. Overdose of a drug: 1a mixed-drug intoxication – post-mortem report result |
|
Sponsor notified on 9 March 2020. Sponsor confirmed that no further information required; SAE closed (e-mail 10 March 2020) |
| Assessed by Mohamed Abdel-Fattah (chief investigator) | |||||
| 26 | 30003 | 25 September 2020 | Patient had LC with a spontaneous splenic capsular rupture post operation resulting in a 9-day stay and a blood transfusion |
|
Sponsor notified on 25 September 2020. Sponsor confirmed that no further information required; SAE closed (e-mail 28 September 2020) |
| Assessed by Mark Doyle (PI) | |||||
| 27 | 12025 | 6 October 2020 | Patient was admitted to hospital with right-sided weakness, facial droop and slurred speech. Consistent with a TIA. Diagnosis:
|
|
Sponsor notified on 6 October 2020. Sponsor confirmed that no further information required; SAE closed (e-mail 9 October 2020) |
| Assessed by Mohamed Abdel-Fattah (chief investigator) | |||||
| 27 (follow-on report) | 12025 | 8 October 2020 |
|
|
Sponsor notified on 9 October 2020. Sponsor confirmed that no further information required; SAE closed (e-mail 9 October 2020) |
| Assessed by Mohamed Abdel-Fattah (chief investigator) | |||||
| 28 | 20032 | 11 December 2020 | Admitted overnight on 9 May 2016 with abdominal pain. All investigation and imaging normal. Due to see consultant on 12 July 2017 but did not attend |
|
Sponsor notified on 18 December 2020. Sponsor confirmed that no further information required; SAE closed (e-mail 18 December 2020) |
| Assessed by Aethele Khunda |
| Breach number | Patient ID | Date of the breach report | Date of incident | Description of breach | Summary of breach, assessment and assessed by | Action |
|---|---|---|---|---|---|---|
| 01 | 16021 | 19 June 2015 | 11 February 2015 | Hysteroscopy for heaving periods. Patient had this procedure as concomitant surgery despite concomitant surgery being an exclusion criterion |
|
E-mail to all sites reminding sites that concomitant surgery is not allowed |
| 02 | 19007 | 19 June 2015 | 24 February 2015 | Patient had concomitant surgical procedure (removal of skin tag), despite concomitant surgery being an exclusion criterion |
|
E-mail to all sites reminding sites that concomitant surgery is not allowed |
| 03 | 20001 | 19 June 2015 | 21 July 2014 | Patient received concomitant surgery (cervical smear and copper coil inserted), despite concomitant surgery being an exclusion criterion |
|
E-mail to all sites reminding sites that concomitant surgery is not allowed |
| 04 | 20018 | 19 June 2015 | 6 March 2015 | Patient received concomitant surgery (bladder biopsy), despite concomitant surgery being an exclusion criterion |
|
E-mail to all sites reminding sites that concomitant surgery is not allowed |
| 05 | 20026 | 19 June 2015 | 7 April 2015 | Patient received concomitant surgery (bladder biopsy × 2 and a laparotomy), despite concomitant surgery being an exclusion criterion |
|
E-mail to all sites reminding sites that concomitant surgery is not allowed |
| 06 | 26011 | 15 March 2016 | 8 February 2016 | Patient received concomitant surgery (posterior pelvic floor repair), despite concomitant surgery being an exclusion criterion |
|
Clinical co-chief investigator spoke to PI at site reminding them that concomitant surgery is not allowed |
| 07 | 19038 | 15 March 2016 | 9 March 2016 | Patient received concomitant surgery [replacement Mirena® coil (Bayer AG, Leverkusen, Germany)], despite concomitant surgery being an exclusion criterion |
|
E-mail to site reminding them that concomitant surgery is not allowed |
| 08 | 18013 | 1 April 2016 | 14 March 2016 | Patient received concomitant procedure (planned hysteroscopy for missing coil), despite concomitant procedures being an exclusion criterion |
|
E-mail to site reminding them that concomitant surgery is not allowed |
| 09 | 16056 | 30 May 2016 | 18 May 2016 | Patient received concomitant surgery: bladder biopsy of bladder lesion |
|
No action, as this is a routine procedure if indicated by intraoperative finding during cystoscopy |
| 10 | 36001 | 5 July 2016 | 23 June 2016 | Patient did not receive randomised procedure because of non-supply of randomised sling in theatre |
|
Agreed corrective and preventative actions with site |
| 11 | 23039 | 9 August 2016 | 13 July 2016 | Patient received concomitant surgery: insertion of Mirena coil and removal of skin tag |
|
E-mail to site reminding them that concomitant surgery is not allowed |
| 12 | 30009 | 26 August 2015 | 6 August 2015 | Patient received concomitant surgery: anal skin tag removed |
|
E-mail to site reminding them that concomitant surgery is not allowed |
| 13 | 19044 | 26 August 2016 | 27 July 2016 | Patient received concomitant surgery: hysteroscopy and polypectomy |
|
E-mail to site reminding them that concomitant surgery is not allowed |
| 14 | 24008 | 29 August 2016 | 27 November 2015 | Patient received concomitant surgery: urethral dilation |
|
E-mail to site reminding them that concomitant surgery is not allowed |
| 15 | 19053 | 1 May 2017 | 26 April 2017 | Patient received concomitant surgery: vulval biopsy |
|
E-mail to site reminding them that concomitant surgery is not allowed |
| 16 | N/A | 6 March 2019 | 22 February 2019 | Two questionnaires and letters sent to incorrect participants |
|
Process for cross-checking of letter and envelope address checked when documents sent to participants. A 100% check of 100 SIMS trial documents mailed out will be double-checked under the new process |
Appendix 6 Patient-reported success and safety, by procedure
| Characteristic | Procedure | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| Age (years), mean (SD) | 49.7 (9.8) [n = 62] | 50.5 (11.2) [n = 199] | 51.1 (11.5) [n = 126] | 50.1 (10.1) [n = 147] |
| BMI (kg/m2), mean (SD) | 29.1 (4.9) [n = 62] | 28.8 (5.5) [n = 199] | 28.5 (5.8) [n = 126] | 29.3 (5.7) [n = 147] |
| Received PFMT in previous 2 years, n (%) | 60 (97) | 165 (83) | 107 (85) | 123 (84) |
| Obstetric history, n (%) | ||||
| Number of babies | ||||
| 1 | 12 (19) | 26 (13) | 18 (14) | 15 (10) |
| 2 | 27 (44) | 87 (44) | 52 (41) | 68 (46) |
| 3 | 13 (21) | 56 (28) | 32 (25) | 40 (27) |
| ≥ 4 | 9 (15) | 21 (11) | 21 (17) | 19 (13) |
| Missing | 1 (0.50) | |||
| At least one forceps delivery | 8 (13) | 26 (13) | 20 (16) | 16 (11) |
| At least one vacuum delivery | 5 (8.1) | 13 (6.5) | 12 (9.5) | 4 (2.7) |
| All deliveries were caesareans | 3 (4.8) | 6 (3.0) | 3 (2.4) | 4 (2.7) |
| Manual job (heavy lifting), n (%) | 16 (26) | 57 (29) | 36 (29) | 48 (33) |
| Smoker, n (%) | 15 (24) | 27 (14) | 19 (15) | 23 (16) |
| Current or previous hormone replacement therapy, n (%) | 5 (8.1) | 19 (9.5) | 10 (7.9) | 15 (10) |
| On anticholinergic drugs, n (%) | 15 (24) | 41 (21) | 7 (5.6) | 26 (18) |
| Previous use of anticholinergic drugs, n (%) | 6 (9.7) | 40 (20) | 21 (17) | 22 (15) |
| Performing CISC, n (%) | 3 (1.5) | |||
| On prophylactic low-dose antibiotics, n (%) | 1 (0.50) | 2 (1.4) | ||
| Previous gynaecology surgery, n (%) | 22 (35) | 62 (31) | 38 (30) | 45 (31) |
| Abdominal hysterectomy | 8 (13) | 24 (12) | 14 (11) | 15 (10) |
| Pelvic floor repair with mesh | 2 (3.2) | 1 (0.68) | ||
| Vaginal hysterectomy | 7 (11) | 10 (5.0) | 7 (5.6) | 13 (8.8) |
| Sacrospinous fixation | 1 (0.50) | 1 (0.68) | ||
| Anterior repair | 2 (3.2) | 12 (6.0) | 3 (2.4) | 3 (2.0) |
| Anterior mesh repair | 1 (1.6) | 1 (0.50) | 3 (2.4) | 2 (1.4) |
| Posterior repair | 1 (1.6) | 5 (2.5) | 3 (2.4) | 1 (0.68) |
| Sacrohysteropexy | 1 (1.6) | |||
| Posterior mesh repair | 1 (0.79) | |||
| Manchester repair | 1 (0.79) | |||
| Other previous gynaecology surgery | 8 (13) | 22 (11) | 15 (12) | 14 (9.5) |
| Questionnaire | Procedure | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| ICIQ-UI-SF score, mean (SD) | 15.1 (3.1) [n = 62] | 14.2 (3.3) [n = 189] | 14.8 (3.5) [n = 124] | 14.3 (3.7) [n = 145] |
| Median (IQR) | 15.0 (13.0–17.0) | 14.0 (12.0–17.0) | 15.0 (13.0–17.5) | 14.0 (12.0–16.0) |
| ICIQ-FLUTS filling score, mean (SD) | 5.1 (2.8) [n = 62] | 4.3 (2.6) [n = 194] | 5.0 (3.2) [n = 124] | 4.7 (2.6) [n = 145] |
| Median (IQR) | 5.0 (3.0–6.0) | 4.0 (2.0–6.0) | 4.0 (3.0–7.0) | 5.0 (3.0–7.0) |
| ICIQ-FLUTS voiding score, mean (SD) | 1.9 (2.0) [n = 61] | 1.7 (1.9) [n = 197] | 1.8 (2.1) [n = 125] | 1.7 (2.0) [n = 146] |
| Median (IQR) | 2.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) |
| ICIQ-FLUTS incontinence score, mean (SD) | 11.7 (3.3) [n = 58] | 10.9 (2.9) [n = 193] | 11.5 (3.2) [n = 125] | 11.3 (3.1) [n = 145] |
| Median (IQR) | 12.0 (9.0–14.0) | 11.0 (9.0–13.0) | 12.0 (10.0–14.0) | 11.0 (10.0–14.0) |
| ICIQ-LUTSqol score, mean (SD) | 49.9 (11.7) [n = 59] | 46.0 (11.4) [n = 193] | 48.2 (11.7) [n = 123] | 46.1 (10.1) [n = 139] |
| Median (IQR) | 51.0 (42.0–59.0) | 45.0 (37.0–54.0) | 47.0 (39.0–57.0) | 46.0 (40.0–53.0) |
| EQ-5D-3L score, mean (SD) | 0.811 (0.246) [n = 60] | 0.883 (0.173) [n = 193] | 0.828 (0.251) [n = 123] | 0.828 (0.256) [n = 147] |
| PISQ-IR sexual functioning, mean (SD) | 3.2 (0.7) [n = 18] | 3.4 (0.6) [n = 56] | 3.3 (0.6) [n = 38] | 3.2 (0.6) [n = 45] |
| Median (IQR) | 3.0 (2.7–3.9) | 3.4 (2.9–4.0) | 3.3 (3.0–3.7) | 3.3 (2.7–3.6) |
| Coital incontinence, n/N (%) | 13/30 (43) | 42/99 (42) | 29/68 (43) | 23/71 (32) |
| Dyspareunia, n/N (%) | 5/30 (17) | 16/99 (16) | 10/68 (15) | 12/71 (17) |
| Urodynamics diagnosis | Device, n (%) | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| Cystometry diagnosis | ||||
| Urodynamic stress incontinence | 47 (76) | 160 (80) | 107 (85) | 115 (78) |
| Urodynamic mixed incontinence | 10 (16) | 23 (12) | 13 (10) | 18 (12) |
| Equivocal | 3 (1.5) | 2 (1.4) | ||
| Not interpretable | 1 (1.6) | 1 (0.79) | ||
| Other | 1 (1.6) | 1 (0.50) | 2 (1.6) | |
| Clinical diagnosis of SUI (no urodynamics performed) | 2 (3.2) | 11 (5.5) | 10 (6.8) | |
| Missing | 1 (1.6) | 1 (0.50) | 3 (2.4) | 2 (1.4) |
| Uroflowmetry diagnosis | ||||
| Normal | 45 (73) | 162 (81) | 108 (86) | 106 (72) |
| Obstruction | 5 (2.5) | 3 (2.4) | 2 (1.4) | |
| Suboptimal | 12 (6.0) | 5 (4.0) | 8 (5.4) | |
| Equivocal | 2 (1.0) | 1 (0.79) | 3 (2.0) | |
| Not interpretable | 2 (1.6) | 3 (2.0) | ||
| Not recorded | 12 (19) | 6 (3.0) | 2 (1.6) | 7 (4.8) |
| Other | 5 (8.1) | 11 (5.5) | 4 (3.2) | 18 (12) |
| Missing | 1 (0.50) | 1 (0.79) | ||
| PGI-I scale | Procedure | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| 4 weeks | n = 56 | n = 174 | n = 97 | n = 131 |
| Very much improved, n (%) | 26 (46) | 92 (53) | 40 (41) | 72 (55) |
| Much improved, n (%) | 19 (34) | 38 (22) | 31 (32) | 30 (23) |
| Improved, n (%) | 6 (11) | 27 (16) | 17 (18) | 20 (15) |
| Same, n (%) | 2 (3.6) | 9 (5.2) | 4 (4.1) | 8 (6.1) |
| Worse, n (%) | 2 (3.6) | 4 (2.3) | 3 (3.1) | 1 (0.76) |
| Much worse, n (%) | 1 (1.8) | 3 (1.7) | 1 (1.0) | |
| Very much worse, n (%) | 1 (0.57) | 1 (1.0) | ||
| Missing, n/N (%) | 6/62 (9.7) | 25/199 (13) | 29/126 (23) | 16/147 (11) |
| 3 months | n = 60 | n = 185 | n = 109 | n = 127 |
| Very much improved, n (%) | 37 (62) | 124 (67) | 69 (63) | 72 (57) |
| Much improved, n (%) | 10 (17) | 32 (17) | 19 (17) | 32 (25) |
| Improved, n (%) | 6 (10) | 17 (9.2) | 11 (10) | 11 (8.7) |
| Same, n (%) | 3 (5.0) | 6 (3.2) | 4 (3.7) | 7 (5.5) |
| Worse, n (%) | 2 (3.3) | 4 (2.2) | 2 (1.8) | 5 (3.9) |
| Much worse, n (%) | 2 (3.3) | 2 (1.1) | 2 (1.8) | |
| Very much worse, n (%) | 2 (1.8) | |||
| Missing, n/N (%) | 2/62 (3.2) | 14/199 (7.0) | 17/126 (13) | 20/147 (14) |
| 15 months | n = 61 | n = 191 | n = 115 | n = 139 |
| Very much improved, n (%) | 42 (69) | 123 (64) | 76 (66) | 82 (59) |
| Much improved, n (%) | 9 (15) | 27 (14) | 8 (7.0) | 29 (21) |
| Improved, n (%) | 5 (8.2) | 22 (12) | 21 (18) | 13 (9.4) |
| Same, n (%) | 3 (4.9) | 8 (4.2) | 4 (3.5) | 12 (8.6) |
| Worse, n (%) | 2 (3.3) | 6 (3.1) | 5 (4.3) | 1 (0.72) |
| Much worse, n (%) | 1 (0.52) | |||
| Very much worse, n (%) | 4 (2.1) | 1 (0.87) | 2 (1.4) | |
| Missing, n/N (%) | 1/62 (1.6) | 8/199 (4.0) | 11/126 (8.7) | 8/147 (5.4) |
| 24 months | n = 55 | n = 172 | n = 105 | n = 123 |
| Very much improved, n (%) | 35 (64) | 114 (66) | 64 (61) | 72 (59) |
| Much improved, n (%) | 7 (13) | 22 (13) | 12 (11) | 23 (19) |
| Improved, n (%) | 7 (13) | 18 (10) | 12 (11) | 15 (12) |
| Same, n (%) | 1 (1.8) | 10 (5.8) | 7 (6.7) | 8 (6.5) |
| Worse, n (%) | 3 (5.5) | 4 (2.3) | 7 (6.7) | 1 (0.81) |
| Much worse, n (%) | 2 (3.6) | 2 (1.2) | 2 (1.9) | 1 (0.81) |
| Very much worse, n (%) | 2 (1.2) | 1 (1.0) | 3 (2.4) | |
| Missing, n/N (%) | 7/62 (11) | 27/199 (14) | 21/126 (17) | 24/147 (16) |
| 36 months | n = 55 | n = 179 | n = 107 | n = 131 |
| Very much improved, n (%) | 31 (56) | 99 (55) | 63 (59) | 66 (50) |
| Much improved, n (%) | 8 (15) | 33 (18) | 11 (10) | 20 (15) |
| Improved, n (%) | 8 (15) | 19 (11) | 15 (14) | 21 (16) |
| Same, n (%) | 5 (9.1) | 14 (7.8) | 8 (7.5) | 10 (7.6) |
| Worse, n (%) | 2 (3.6) | 9 (5.0) | 9 (8.4) | 11 (8.4) |
| Much worse, n (%) | 1 (1.8) | 2 (1.1) | ||
| Very much worse, n (%) | 3 (1.7) | 1 (0.93) | 3 (2.3) | |
| Missing, n/N (%) | 7/62 (11) | 20/199 (10) | 19/126 (15) | 16/147 (11) |
| Symptom | Procedure | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| Pad test weight (g), mean (SD) | 43.7 (29.3) [n = 54] | 55.0 (66.2) [n = 167] | 63.7 (70.7) [n = 99] | 47.3 (41.8) [n = 115] |
| Median (IQR) | 39.5 (21.0–55.6) | 41.0 (25.0–62.0) | 41.0 (27.0–73.0) | 37.0 (23.0–60.0) |
| ICIQ-UI-SF score, mean (SD) | 15.1 (3.1) [n = 62] | 14.2 (3.3) [n = 189] | 14.8 (3.5) [n = 124] | 14.3 (3.7) [n = 145] |
| Median (IQR) | 15.0 (13.0–17.0) | 14.0 (12.0–17.0) | 15.0 (13.0–17.5) | 14.0 (12.0–16.0) |
| How often do you leak urine?, n (%) | ||||
| Once or less per week | 2 (3.2) | 6 (3.0) | 3 (2.4) | 4 (2.7) |
| Two or three times per week | 6 (9.7) | 20 (10) | 16 (13) | 20 (14) |
| Once per day | 3 (4.8) | 25 (13) | 8 (6.3) | 7 (4.8) |
| Several times per day | 37 (60) | 129 (65) | 84 (67) | 96 (65) |
| All the time | 14 (23) | 17 (9) | 15 (12) | 20 (14) |
| Missing | 2 (1.0) | |||
| How much urine do you leak?, n (%) | ||||
| Small amounts | 26 (42) | 83 (42) | 41 (33) | 61 (41) |
| Moderate amounts | 27 (44) | 82 (41) | 59 (47) | 60 (41) |
| Large amounts | 9 (15) | 27 (14) | 26 (21) | 25 (17) |
| Missing | 7 (3.5) | 1 (0.68) | ||
| How much does urinary leakage interfere with day-to-day activities?, mean (SD) | 7.8 (1.7) [n = 62] | 7.1 (2.2) [n = 194] | 7.3 (2.3) [n = 124] | 7.1 (2.1) [n = 146] |
| ICIQ-UI-SF severity | n = 62 | n = 189 | n = 124 | n = 145 |
| Mild/moderate (< 13), n (%) | 13 (21) | 56 (30) | 30 (24) | 42 (29) |
| Severe (≥ 13%), n (%) | 49 (79) | 133 (70) | 94 (76) | 103 (71) |
| Urgency perception: baseline, n (%) | ||||
| No urgency | 8 (13) | 37 (19) | 21 (17) | 16 (11) |
| Mild urgency | 18 (29) | 53 (27) | 29 (23) | 51 (35) |
| Moderate urgency | 25 (40) | 78 (39) | 51 (40) | 60 (41) |
| Severe urgency | 10 (16) | 29 (15) | 24 (19) | 20 (14) |
| Not answered | 1 (1.6) | 2 (1.0) | 1 (0.79) | |
| Urgency perception and impact | Procedure | |||
|---|---|---|---|---|
| Ajust (N = 62) | Altis (N = 199) | RP-TVT (N = 126) | TO-TVT (N = 147) | |
| Urgency perception | ||||
| Baseline | n = 61 | n = 197 | n = 125 | n = 147 |
| No urgency, n (%) | 8 (13) | 37 (19) | 21 (17) | 16 (11) |
| Mild urgency, n (%) | 18 (30) | 53 (27) | 29 (23) | 51 (35) |
| Moderate urgency, n (%) | 25 (41) | 78 (40) | 51 (41) | 60 (41) |
| Severe urgency, n (%) | 10 (16) | 29 (15) | 24 (19) | 20 (14) |
| Not answered, n/N (%) | 1/62 (1.6) | 2/199 (1.0) | 1/126 (0.79) | |
| 3 months | n = 61 | n = 183 | n = 107 | n = 127 |
| No urgency, n (%) | 14 (23) | 59 (32) | 32 (30) | 35 (28) |
| Mild urgency, n (%) | 28 (46) | 75 (41) | 41 (38) | 59 (46) |
| Moderate urgency, n (%) | 14 (23) | 35 (19) | 24 (22) | 23 (18) |
| Severe urgency, n (%) | 5 (8.2) | 14 (7.7) | 10 (9.3) | 10 (7.9) |
| Not answered, n/N (%) | 1/62 (1.6) | 16/199 (8.0) | 19/126 (15) | 20/147 (14) |
| 15 months | n = 56 | n = 180 | n = 101 | n = 126 |
| No urgency, n (%) | 15 (27) | 52 (29) | 27 (27) | 34 (27) |
| Mild urgency, n (%) | 31 (55) | 82 (46) | 42 (42) | 53 (42) |
| Moderate urgency, n (%) | 7 (13) | 31 (17) | 20 (20) | 29 (23) |
| Severe urgency, n (%) | 3 (5.4) | 15 (8.3) | 12 (12) | 10 (7.9) |
| Not answered, n/N (%) | 6/62 (9.7) | 19/199 (9.5) | 25/126 (20) | 21/147 (14) |
| 24 months | n = 55 | n = 167 | n = 100 | n = 117 |
| No urgency, n (%) | 22 (40) | 60 (36) | 34 (34) | 26 (22) |
| Mild urgency, n (%) | 21 (38) | 64 (38) | 34 (34) | 56 (48) |
| Moderate urgency, n (%) | 7 (13) | 32 (19) | 21 (21) | 24 (21) |
| Severe urgency, n (%) | 5 (9.1) | 11 (6.6) | 11 (11) | 11 (9.4) |
| Not answered, n/N (%) | 7/62 (11) | 32/199 (16) | 26/126 (21) | 30/147 (20) |
| 36 months | n = 46 | n = 157 | n = 97 | n = 110 |
| No urgency, n (%) | 11 (24) | 46 (29) | 30 (31) | 26 (24) |
| Mild urgency, n (%) | 24 (52) | 61 (39) | 38 (39) | 45 (41) |
| Moderate urgency, n (%) | 9 (20) | 34 (22) | 18 (19) | 30 (27) |
| Severe urgency, n (%) | 2 (4.3) | 16 (10) | 11 (11) | 9 (8.2) |
| Not answered, n/N (%) | 16/62 (26) | 42/199 (21) | 29/126 (23) | 37/147 (25) |
| Impact on urgency, n/N (%) | ||||
| 3 months | ||||
| Cure | 10/52 (19) | 41/146 (28) | 22/85 (26) | 27/113 (24) |
| Improved | 18/52 (35) | 49/146 (34) | 31/85 (36) | 39/113 (35) |
| No change | 4/52 (7.7) | 12/146 (8.2) | 4/85 (4.7) | 17/113 (15) |
| Worsened | 20/52 (38) | 44/146 (30) | 28/85 (33) | 30/113 (27) |
| New onset | 4/8 (50) | 19/37 (51) | 11/21 (52) | 6/16 (38) |
| Missing | 1/53 (1.9) | 14/160 (8.8) | 19/104 (18) | 18/131 (14) |
| 15 months | ||||
| Cure | 12/48 (25) | 33/145 (23) | 18/81 (22) | 26/113 (23) |
| Improved | 24/48 (50) | 49/145 (34) | 25/81 (31) | 34/113 (30) |
| No change | 9/48 (19) | 50/145 (34) | 34/81 (42) | 36/113 (32) |
| Worsened | 3/48 (6.3) | 13/145 (9.0) | 4/81 (4.9) | 17/113 (15) |
| New onset | 4/8 (50) | 15/37 (41) | 10/21 (48) | 5/16 (31) |
| Missing | 5/53 (9.4) | 15/160 (9.4) | 23/104 (22) | 18/131 (14) |
| 24 months | ||||
| Cure | 18/46 (39) | 41/133 (31) | 20/79 (25) | 22/106 (21) |
| Improved | 14/46 (30) | 41/133 (31) | 25/79 (32) | 29/106 (27) |
| No change | 9/46 (20) | 41/133 (31) | 28/79 (35) | 39/106 (37) |
| Worsened | 5/46 (11) | 10/133 (7.5) | 6/79 (7.6) | 16/106 (15) |
| New onset | 4/8 (50) | 14/37 (38) | 6/21 (29) | 7/16 (44) |
| Missing | 7/53 (13) | 27/160 (17) | 25/104 (24) | 25/131 (19) |
| 36 months | ||||
| Cure | 10/39 (26) | 28/124 (23) | 18/77 (23) | 20/100 (20) |
| Improved | 13/39 (33) | 40/124 (32) | 29/77 (38) | 28/100 (28) |
| No change | 11/39 (28) | 44/124 (35) | 25/77 (32) | 32/100 (32) |
| Worsened | 5/39 (13) | 12/124 (9.7) | 5/77 (6.5) | 20/100 (20) |
| New onset | 6/8 (75) | 14/37 (38) | 8/21 (38) | 4/16 (25) |
| Missing | 14/53 (26) | 36/160 (23) | 27/104 (26) | 31/131 (24) |
Appendix 7 Discrete choice experiment: experimental design and tables
Design
;alts = A, B, NO
;rows = 40
;eff = (mnl,d)
;block = 4
;cond:
if(A.AVIOD = 4, A.IMPRO > 1),
if(B.AVIOD = 4, B.IMPRO > 1),
if(A.COMPL = 2, A.IMPRO > 1),
if(B.COMPL = 2, B.IMPRO > 1)
;model:
U(A) = b0[1] + b1[0]*TYPE[1,2] + b2.dummy[-0.635|-0.967|-0.665|-0.807]*COMPL[1,2,3,4,5] + b3.dummy[0|0|0]*DAYS[3,13,23,33] + b4.dummy[0.757|1.056|1.339]*IMPRO[1,2,3,4] + b5.dummy[0|0|0]*AVIOD[1,2,3,4] + b6.dummy[0|0|0]*COST[1000,2000,3500,5000]/
U(B) = b0[1] + [0]*TYPE[1,2] + b2.dummy[-0.635|-0.967|-0.665|-0.807]*COMPL[1,2,3,4,5] + b3.dummy[0|0|0]*DAYS[3,13,23,33] + b4.dummy[0.757|1.056|1.339]*IMPRO[1,2,3,4] + b5.dummy[0|0|0]*AVIOD[1,2,3,4] + b6.dummy[0|0|0]*COST[1000,2000,3500,5000] $
**Note that the expected design selected from the experimental design software was evaluation number 20,291 from the algorithm with a multinomial D-error of 0.294888.
| Attribute | Levels | Description |
|---|---|---|
| Type of anaesthesia | General | |
| Local | ||
| Complications | New-onset UUI | An urgent desire to pass urine and sometimes urine leaks before you have time to get to the toilet |
| Intermittent self-catheterisation | Owing to temporary problems emptying the bladder fully, short-term self-catheterisation is required for a few days or weeks | |
| Dyspareunia | Pain in the pelvis during or after sexual intercourse | |
| Mesh extrusion/exposure | Exposure of mesh through the vaginal wall or nearby organ. This can happen soon, or years after surgery. Sometimes, further surgery might be needed to help relieve pain, or to remove the mesh | |
| None | You would not experience any of these complications | |
| Number of recovery days | 3 days | This means your usual activities, such as work or leisure, before you had your surgery, not usual activities before you had incontinence |
| 13 days | ||
| 23 days | ||
| 33 days | ||
| Level of improvement in incontinence symptoms after surgerya | Very much improved | You leak none or only a small amount of urine once a week or less. You never use pads to keep dry |
| Much improved | You leak a small amount of urine two or three times per week. You mainly leak when you are physically active. You occasionally use pads to keep dry | |
| Improved | You leak a moderate amount of urine once a day. You mainly leak when you are physically active. You often use pads to keep dry | |
| None | You leak a moderate amount of urine several times a day. You mainly leak when you cough, sneeze or are physically active. You always use pads to keep dry | |
| Avoid activities | Frequently | This means how often you avoid activities because of a fear of leaking urine. Activities might include socialising, physical activity, sex, travel or shopping |
| Occasionally | ||
| Rarely | ||
| Never | ||
| Cost of treatment | £1000 | This means all the costs involved with receiving treatment, such as the cost of the treatment itself, time off work and travel costs such as bus fares or petrol costs or car park charges. We know that you do not have to pay for NHS treatment, but please imagine a scenario in which you do. Think about how much each treatment would be worth to you, and whether or not you would be able and willing to pay for it |
| £2000 | ||
| £3500 | ||
| £5000 |
| Characteristics | DCE estimation sample (N = 227) | Trial population (N = 596) |
|---|---|---|
| Randomised group, n (%) | ||
| SIMS | 113 (50) | 298 (50) |
| SMUS | 114 (50) | 298 (50) |
| PFMT in the previous 2 years, n (%) | ||
| Yes | 190 (84) | 508 (85) |
| No | 37 (16) | 88 (15) |
| Type of anaesthesia, n (%) | ||
| General | 133 (59) | 308 (52) |
| Spinal | 9 (4.0) | 12 (2.0) |
| Local (with IV sedation) | 32 (14) | 61 (10) |
| Local (with oral sedation) | 14 (6.2) | 27 (4.5) |
| Local only | 37 (16) | 129 (22) |
| Nonea | 0 (0) | 59 (10) |
| Income category, n (%) | ||
| Low | 58 (26) | N/Ab |
| Moderate | 72 (32) | N/Ab |
| High | 40 (18) | N/Ab |
| Prefer not to say/missing | 57 (25) | N/Ab |
| Demographic/clinical characteristics, mean (SD) | ||
| Age (years) | 51 (10) | 51 (11) |
| Baseline EQ-5D-3L utility score | 0.838 (0.237) | 0.847 (0.226) |
Appendix 8 Health economics tables
| Drug | Unit price (£) | Price per | Resource use | Cost per average case (£) | Comments | Sources |
|---|---|---|---|---|---|---|
| GA | ||||||
| Propofol (Propofol-Lipuro, Baxter Healthcare Ltd, Newbury, UK), 1% injection | 19.36 | 20-ml ampoule | 1 ampoule | 3.87 | One ampoule is sufficient for a standard case | VUE117 BNF118 |
| Fentanyl (AAH Pharmaceuticals, Coventry, UK), 100 µg | 14.32 | 2-ml ampoule (50 µg/ml) | 1 ampoule | 1.43 | One ampoule is sufficient for a standard case | BNF118 VUE117 |
| Morphine | 15.00 | 1-ml vial | 1 vial | 1.50 | For pain relief | BNF118 VUE117 |
| Sevoflurane (volatile agent) (Sevoflurane Volatile Liquid, Baxter Healthcare Ltd) | 123.00 | 250-ml bottle | 25 ml | 12.30 | Baxter, Newbury, UK; 2013 | www.baxterhealthcare.co.uk/downloads/prescribing_information/hospital_products/anaesthesia_critical_care/sevoflurane_pi.pdf (accessed 8 August 2022) |
| Laryngeal mask | 29.50 | Box of 10 | 1 mask | 2.95 | PRO-Breathe Laryngeal Airway, disposable (PROACT Medical Ltd, Corby, UK) | www.proactmedical.co.uk/our-products/airway-management/pro-breathe-laryngeal-airways (accessed 14 June 2022) |
| Total cost of GA | 22.05 | Anaesthesia consumables cost per average case | ||||
| Spinal anaesthesia | ||||||
| Bupivacaine hydrochloride anhydrous injection (1%) | 18.3 | 10-ml ampoule | 1 ampoule | 1.83 | Resource use requirements | VUE117 BNF118 |
| Lidocaine Hydrochloride (Alliance Healthcare Ltd, Chessington, UK) | 11.00 | 10-ml ampoule | 1 ampoule | 1.10 | Resource use requirements | VUE117 BNF118 |
| Total cost of spinal anaesthesia | 2.93 | Anaesthesia consumables cost per average case | ||||
| Local with sedation anaesthesia | ||||||
| Propofol, 1% injection | 19.36 | 20-ml ampoule | 1 ampoule | 3.87 | 1 ampoule will be sufficient for a standard case, Dr Karen Cranfield (NHS Grampian, 2019) | VUE117 BNF118 |
| Lidocaine | 11.00 | 10-ml ampoule | 1 ampoule | 1.10 | Resource use requirements provided by Dr Karen Cranfield | VUE117 BNF118 |
| Total cost of local with sedation anaesthesia | 4.97 | Anaesthesia consumables cost per average case | ||||
| Local anaesthesia only | ||||||
| Lidocaine | 11.00 | 10-ml ampoule | 1 ampoule | 1.10 | Resource use requirements | VUE117 BNF118 |
| Resource | Product | Manufacturer | Pack size | Packs for 1 year | Unit cost (2019) (£) | Total cost (£) | Reference/notes |
|---|---|---|---|---|---|---|---|
| Sterile catheterisation insertion pack | Cath-it (1 pack) | Richardson Healthcare, Inc., Elstree, UK | 1 | 4 | 1.98 | 7.92 | NHS EDT121 2019 |
| Sterile lubricant for instillation | OptiLube sterile lubricating jelly (1 × 11-ml syringe) | Optimum Medical Ltd, Leeds, UK | 1 | 4 | 0.98 | 3.84 | NHS EDT121 2019 |
| Indwelling catheter | Folysil X-Tra (size 14), pack size 1 | Coloplast A/S | 1 | 6 (4 + 2 spares) | 6.37 | 38.22 | NHS EDT121 2019 |
| Leg bags (assumes patients have continuous drainage) | Simpla® Profile, 500 ml, 25-cm tube | Coloplast A/S | 10 | 3.09 | 25.66 | 153.96 | NHS EDT121 2019 |
| Catheter stabilisation device | Leg bag holder: AquaSleeve, size standard | Coloplast A/S | 4 | 2 | 8.64 | 17.00 | NHS EDT121 2019 |
| Night drainage bags | Single use, Prosys® (2 l) | Clinisupplies Ltd, Watford, UK | 10 | 37 | 3.06 | 113.22 | NHS EDT121 2019 |
| Total | Average annual cost | 334.16 | |||||
| Cost per week | 6.43 |
| Product | Manufacturer | Pack size (n) | Number of packs required for 1 year | Unit cost (2019) (£) | Total cost (£) | Reference/notes |
|---|---|---|---|---|---|---|
| Hi-slip® Plus | Bullen Healthcare, Liverpool, UK | 30 | 37 | 33.11 | 1225.07 | NHS EDT121 2019 |
| Advance™ | Hollister Ltd, Wokingham, UK | 25 | 44 | 34.37 | 1512.28 | NHS EDT121 2019 |
| SpeediCath® Compact | Coloplast A/S | 30 | 37 | 47.54 | 1758.98 | NHS EDT121 2019 |
| SpeediCath® | Coloplast A/S | 30 | 37 | 45.91 | 1698.67 | NHS EDT121 2019 |
| HydroSil® | Bard Ltd, Crawley, UK | 30 | 37 | 45.85 | 1696.45 | NHS EDT121 2019 |
| LoFric® Sense™ | Wellspect HealthCare Ltd, Stonehouse, UK | 30 | 37 | 43.65 | 1615.05 | NHS EDT121 2019 |
| Average cost for 1 full year | 1584.42 | |||||
| Cost for 1 week | 30.47 |
| CRF available | Base-case assumptions | Sensitivity analysis | Reason | |
|---|---|---|---|---|
| Had index surgery | ||||
| PQ returned and hospitalisation reported | No | CRF £0 | Checked with site | |
| PQ returned and hospitalisation not reported | No | CRF £0 | Not checked with site, but hospitalisation unlikely as PQ returned and no event(s) reported | |
| PQ not returned | Yes | CRF cost | CRF data will be accurate | |
| PQ not returned | No | CRF £0 | Treat as missing data | Assume that most sites would have returned a CRF when the patient attended, but accept that this is unknown with certainty |
| PQ returned | Yes | CRF cost | CRF cost takes precedence | |
| Did not have index surgery | ||||
| PQ returned and hospitalisation reported | No | CRF £0 | Checked with site | |
| PQ returned, but no hospitalisation reported | No | CRF £0 | Not checked with site, but hospitalisation unlikely as PQ returned and no event(s) reported (treat as missing for sensitivity analysis) | |
| PQ not returned | Yes | CRF cost | CRF cost takes precedence | |
| PQ not returned | No | CRF £0 | Treat as missing data | Assume that most sites would have returned a CRF when the patient attended, even if they did not have an index trial surgery, but accept that this is unknown with certainty (treat as missing data in sensitivity analysis) |
| PQ returned | Yes | CRF cost | CRF cost takes precedence | |
| Cost and QoL data | Trial group, n (%) | Total (N = 596), n (%) | |
|---|---|---|---|
| SIMS (N = 298) | SMUS (N = 298) | ||
| Cost data | |||
| 3-month PQ | 67 (22) | 94 (32) | 161 (27) |
| 15 months | |||
| PQ | 96 (32) | 133 (45) | 229 (38) |
| CRF | 43 (14) | 67 (22) | 110 (18) |
| Total (PQ and CRF) | 100 (34) | 133 (45) | 233 (39) |
| 24 months | |||
| PQ | 112 (38) | 138 (46) | 250 (42) |
| CRF | 62 (21) | 83 (28) | 145 (24) |
| Total (PQ and CRF) | 114 (38) | 141 (47) | 255 (43) |
| 36 months | |||
| PQ | 129 (43) | 148 (50) | 277 (46) |
| CRF | 75 (25) | 88 (30) | 163 (27) |
| Total (PQ and CRF) | 129 (43) | 149 (50) | 278 (47) |
| Total follow-up costs | 197 (66) | 215 (72) | 412 (69) |
| QoL data | |||
| EQ-5D-3L | |||
| Baseline | 12 (4) | 14 (5) | 26 (4) |
| 4 weeks | 59 (20) | 72 (24) | 131 (22) |
| 3 months | 43 (14) | 72 (24) | 115 (19) |
| 15 months | 49 (16) | 79 (27) | 128 (21) |
| 24 months | 66 (22) | 86 (29) | 152 (26) |
| 36 months | 81 (27) | 93 (31) | 174 (29) |
| QALY | 127 (43) | 141 (47) | 266 (45) |
| ICIQ-LUTSqol | |||
| Baseline | 7 (2) | 14 (5) | 21 (2) |
| 3 months | 50 (17) | 73 (24) | 123 (13) |
| 15 months | 51 (17) | 80 (27) | 131 (14) |
| 24 months | 73 (24) | 90 (30) | 163 (19) |
| 36 months | 81 (27) | 97 (33) | 178 (21) |
| Type | Trial group, mean (SD) [n] | |||
|---|---|---|---|---|
| SIMS | SMUS | |||
| Resource use | Cost (£) | Resource use | Cost (£) | |
| 3-month follow-up | ||||
| Doctor at surgery | 0.20 (0.62) [250] | 8 (25) [250] | 0.21 (0.66) [229] | 9 (26) [229] |
| Doctor at home | 0.01 (0.14) [252] | 0 (5) [252] | 0.00 (0.07) [230] | 0 (3) [230] |
| Doctor: telephone | 0.04 (0.29) [252] | 1 (7) [252] | 0.05 (0.27) [230] | 1 (6) [230] |
| Nurse at surgery | 0.05 (0.42) [252] | 0 (4) [252] | 0.05 (0.28) [229] | 0 (3) [229] |
| Nurse at home | 0.00 (0.00) [252] | 0 (0) [252] | 0.00 (0.00) [229] | 0 (0) [229] |
| Nurse: telephone | 0.03 (0.23) [252] | 0 (1) [252] | 0.06 (0.40) [229] | 0 (1) [229] |
| District nurse | 0.00 (0.06) [253] | 0 (1) [253] | 0.00 (0.07) [228] | 0 (1) [228] |
| Physiotherapist | 0.00 (0.06) [252] | 0 (1) [252] | 0.03 (0.20) [226] | 0 (2) [226] |
| Permanent catheter | 0.00 (0.00) [256] | 0 (0) [256] | 0.00 (0.00) [226] | 0 (0) [226] |
| Disposable catheter | 0.01 (0.11) [254] | 5 (43) [254] | 0.03 (0.17) [227] | 12 (69) [227] |
| Urinary medicine | 0.50 (1.49) [253] | 9 (25) [253] | 0.50 (1.47) [225] | 10 (27) [225] |
| Cystitis medicine | 0.11 (0.57) [254] | 0 (1) [254] | 0.13 (0.64) [226] | 0 (1) [226] |
| Long-term antibiotics | 0.03 (0.16) [255] | 1 (5) [255] | 0.03 (0.16) [223] | 1 (5) [223] |
| Pain medicine | 0.16 (0.37) [251] | 2 (4) [251] | 0.10 (0.30) [228] | 1 (3) [228] |
| Total cost | 21 (45) [231] | 28 (80) [204] | ||
| Year 1 follow-up | ||||
| Doctor at surgery | 0.23 (0.79) [246] | 9 (32) [246] | 0.21 (0.86) [213] | 8 (34) [213] |
| Doctor at home | 0.00 (0.00) [249] | 0 (0) [249] | 0.00 (0.00) [215] | 0 (0) [215] |
| Doctor: telephone | 0.12 (0.96) [249] | 3 (23) [249] | 0.07 (0.56) [215] | 2 (13) [215] |
| Nurse at surgery | 0.04 (0.34) [245] | 0 (3) [245] | 0.05 (0.33) [212] | 0 (3) [212] |
| Nurse at home | 0.00 (0.00) [245] | 0 (0) [245] | 0.00 (0.00) [212] | 0 (0) [212] |
| Nurse: telephone | 0.02 (0.29) [245] | 0 (1) [245] | 0.03 (0.25) [212] | 0 (1) [212] |
| District nurse | 0.01 (0.09) [244] | 0 (1) [244] | 0.00 (0.00) [212] | 0 (0) [212] |
| Physiotherapist | 0.10 (0.65) [244] | 1 (6) [244] | 0.05 (0.42) [210] | 0 (4) [210] |
| Permanent catheter | 0.00 (0.06) [245] | 1 (11) [245] | 0.01 (0.10) [215] | 2 (16) [215] |
| Disposable catheter | 0.00 (0.06) [251] | 3 (50) [251] | 0.01 (0.09) [221] | 7 (75) [221] |
| Urinary medicine | 0.27 (1.17) [252] | 14 (50) [252] | 0.38 (1.42) [220] | 17 (51) [220] |
| Cystitis medicine | 0.32 (1.08) [243] | 1 (2) [243] | 0.24 (1.00) [212] | 1 (2) [212] |
| Long-term antibiotics | 0.02 (0.15) [253] | 1 (8) [253] | 0.02 (0.15) [217] | 1 (9) [217] |
| Pain medicine | 0.10 (0.31) [229] | 1 (3) [229] | 0.07 (0.25) [199] | 1 (2) [199] |
| Total cost | 71 (135) [202] | 62 (124) [165] | ||
| Year 2 follow-up | ||||
| Doctor at surgery | 0.17 (0.61) [223] | 7 (24) [223] | 0.28 (1.59) [207] | 11 (64) [207] |
| Doctor at home | 0.00 (0.00) [227] | 0 (0) [227] | 0.00 (0.00) [210] | 0 (0) [210] |
| Doctor: telephone | 0.06 (0.48) [227] | 1 (12) [227] | 0.11 (0.91) [210] | 3 (22) [210] |
| Nurse at surgery | 0.02 (0.16) [226] | 0 (1) [226] | 0.02 (0.17) [206] | 0 (2) [206] |
| Nurse at home | 0.00 (0.00) [226] | 0 (0) [226] | 0.00 (0.00) [206] | 0 (0) [206] |
| Nurse: telephone | 0.01 (0.15) [226] | 0 (1) [226] | 0.00 (0.00) [206] | 0 (0) [206] |
| District nurse | 0.00 (0.07) [223] | 0 (1) [223] | 0.00 (0.07) [204] | 0 (1) [204] |
| Physiotherapist | 0.14 (0.72) [222] | 1 (6) [222] | 0.01 (0.14) [205] | 0 (1) [205] |
| Permanent catheter | 0.00 (0.00) [225] | 0 (0) [225] | 0.00 (0.00) [207] | 0 (0) [207] |
| Disposable catheter | 0.01 (0.11) [234] | 20 (179) [234] | 0.01 (0.12) [216] | 22 (186) [216] |
| Urinary medicine | 0.20 (0.96) [234] | 25 (94) [234] | 0.38 (1.45) [215] | 30 (97) [215] |
| Cystitis medicine | 0.22 (0.91) [223] | 1 (2) [223] | 0.18 (0.85) [201] | 0 (2) [201] |
| Long-term antibiotics | 0.04 (0.20) [231] | 4 (21) [231] | 0.03 (0.18) [209] | 4 (21) [209] |
| Pain medicine | 0.09 (0.28) [216] | 1 (3) [216] | 0.06 (0.24) [193] | 1 (2) [193] |
| Total cost | 68 (179) [186] | 57 (156) [160] | ||
| Year 3 follow-up | ||||
| Doctor at surgery | 0.14 (0.68) [210] | 6 (27) [210] | 0.11 (0.57) [195] | 5 (23) [195] |
| Doctor at home | 0.00 (0.00) [215] | 0 (0) [215] | 0.01 (0.14) [201] | 0 (6) [201] |
| Doctor: telephone | 0.03 (0.25) [215] | 1 (6) [215] | 0.03 (0.30) [201] | 1 (7) [201] |
| Nurse at surgery | 0.04 (0.30) [213] | 0 (3) [213] | 0.00 (0.00) [195] | 0 (0) [195] |
| Nurse at home | 0.01 (0.14) [213] | 0 (2) [213] | 0.00 (0.00) [195] | 0 (0) [195] |
| Nurse: telephone | 0.02 (0.25) [213] | 0 (1) [213] | 0.00 (0.00) [195] | 0 (0) [195] |
| District nurse | 0.01 (0.12) [213] | 0 (2) [213] | 0.02 (0.22) [194] | 0 (3) [194] |
| Physiotherapist | 0.02 (0.23) [212] | 0 (2) [212] | 0.06 (0.47) [195] | 1 (4) [195] |
| Permanent catheter | 0.00 (0.07) [213] | 2 (23) [213] | 0.00 (0.00) [200] | 0 (0) [200] |
| Disposable catheter | 0.01 (0.12) [213] | 22 (188) [213] | 0.02 (0.14) [206] | 31 (220) [206] |
| Urinary medicine | 0.24 (1.10) [213] | 26 (96) [213] | 0.45 (1.53) [205] | 33 (101) [205] |
| Cystitis medicine | 0.22 (0.87) [205] | 1 (2) [205] | 0.27 (1.22) [195] | 1 (3) [195] |
| Long-term antibiotics | 0.03 (0.17) [210] | 3 (19) [210] | 0.03 (0.18) [204] | 4 (21) [204] |
| Pain medicine | 0.11 (0.31) [196] | 1 (3) [196] | 0.07 (0.26) [179] | 1 (3) [179] |
| Total cost | 76 (280) [195] | 51 (131) [150] | ||
| Type | Trial group, mean (SD) [n] | |||
|---|---|---|---|---|
| SIMS | SMUS | |||
| Resource use | Cost (£) | Resource use | Cost (£) | |
| Year 1 | ||||
| Outpatient department | 0.35 (1.12) [275] | 44.93 (148.68) [275] | 0.33 (0.92) [265] | 40.19 (110.56) [265] |
| Ward review | 0.03 (0.18) [275] | 4.51 (24.61) [275] | 0.03 (0.22) [265] | 4.68 (29.97) [265] |
| Cystoscopy | 0.03 (0.19) [275] | 28.15 (174.51) [275] | 0.03 (0.17) [265] | 29.22 (177.70) [265] |
| Urine dipstick | 0.04 (0.22) [275] | 0.16 (0.97) [275] | 0.06 (0.33) [265] | 0.25 (1.41) [265] |
| Mid-stream specimen of urine | 0.01 (0.13) [275] | 0.05 (0.58) [275] | 0.03 (0.23) [265] | 0.13 (0.99) [265] |
| Urodynamics | 0.03 (0.19) [275] | 10.71 (69.48) [275] | 0.04 (0.26) [265] | 15.13 (101.67) [265] |
| Ultrasonography | 0.03 (0.18) [275] | 1.43 (10.05) [275] | 0.02 (0.12) [265] | 0.85 (6.84) [265] |
| Radiography | 0.01 (0.09) [275] | 0.23 (2.64) [275] | 0.00 (0.06) [265] | 0.12 (1.90) [265] |
| MRI | 0.04 (0.31) [275] | 6.44 (50.13) [275] | 0.01 (0.11) [265] | 1.82 (17.07) [265] |
| CT | 0.00 (0.06) [275] | 0.36 (6.03) [275] | 0.00 (0.06) [265] | 0.38 (6.14) [265] |
| SMUS | 0.00 (0.06) [275] | 5.63 (93.41) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| SIMS | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Botox injections | 0.01 (0.09) [275] | 7.59 (88.78) [275] | 0.01 (0.12) [265] | 7.87 (128.14) [265] |
| Tibial nerve stimulation | 0.00 (0.06) [275] | 6.56 (108.72) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Sacral nerve stimulation | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Pubovaginal/autologous slings | 0.00 (0.06) [275] | 4.95 (82.13) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Colposuspension | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.06) [265] | 16.66 (271.21) [265] |
| Duloxetine | 0.00 (0.06) [275] | 0.67 (11.10) [275] | 0.02 (0.15) [265] | 2.78 (27.60) [265] |
| Antibiotics | 0.01 (0.24) [275] | 0.78 (12.91) [275] | 0.01 (0.09) [265] | 0.40 (4.64) [265] |
| Antimuscarinics | 0.04 (0.22) [275] | 7.96 (40.47) [275] | 0.03 (0.18) [265] | 6.20 (33.12) [265] |
| Covering of tape extrusion | 0.01 (0.12) [275] | 10.56 (175.12) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Urethral dilation | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Catheter | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Intermittent catheterisation | 0.01 (0.19) [275] | 5.24 (68.57) [275] | 0.03 (0.23) [265] | 10.87 (82.18) [265] |
| Dilation | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.06) [265] | 7.23 (117.70) [265] |
| Partial tape removal | 0.00 (0.06) [275] | 5.28 (87.56) [275] | 0.00 (0.06) [265] | 9.58 (155.97) [265] |
| Complete tape removal | 0.01 (0.10) [275] | 19.76 (188.46) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Exposure from vagina | 0.00 (0.06) [275] | 6.59 (109.21) [275] | 0.00 (0.06) [265] | 6.83 (111.25) [265] |
| Excision to bladder | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Insertion of indwelling catheter | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.06) [265] | 3.36 (54.73) [265] |
| Tape cut | 0.00 (0.06) [275] | 5.28 (87.56) [275] | 0.00 (0.06) [265] | 5.48 (89.20) [265] |
| Urethrolysis | 0.00 (0.00) [275] | 0.00 (0.00) [275] | 0.00 (0.00) [265] | 0.00 (0.00) [265] |
| Inpatient stay | 0.00 (0.06) [275] | 0.00 (0.00) [275] | 0.02 (0.17) [265] | 0.00 (0.00) [265] |
| Total | 185.55 (622.75) [275] | 179.13 (711.09) [265] | ||
| Year 2 | ||||
| Outpatient department | 0.22 (0.80) [257] | 27.11 (96.43) [257] | 0.13 (0.68) [249] | 16.33 (88.17) [249] |
| Ward review | 0.00 (0.06) [257] | 0.55 (8.80) [257] | 0.01 (0.14) [249] | 1.47 (17.51) [249] |
| Cystoscopy | 0.04 (0.21) [257] | 38.73 (225.95) [257] | 0.02 (0.13) [249] | 17.77 (139.33) [249] |
| Urine dipstick | 0.02 (0.16) [257] | 0.08 (0.71) [257] | 0.01 (0.19) [249] | 0.05 (0.82) [249] |
| Mid-stream specimen of urine | 0.02 (0.15) [257] | 0.07 (0.66) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Urodynamics | 0.02 (0.14) [257] | 7.16 (50.93) [257] | 0.01 (0.09) [249] | 2.96 (32.91) [249] |
| Ultrasonography | 0.02 (0.15) [257] | 1.31 (8.47) [257] | 0.02 (0.17) [249] | 1.12 (9.34) [249] |
| Radiography | 0.00 (0.06) [257] | 0.12 (1.93) [257] | 0.00 (0.06) [249] | 0.12 (1.96) [249] |
| MRI | 0.00 (0.06) [257] | 0.63 (10.04) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| CT | 0.00 (0.06) [257] | 0.39 (6.24) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| SMUS | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| SIMS | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Botox injections | 0.01 (0.09) [257] | 8.12 (91.83) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Tibial nerve stimulation | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.03 (0.44) [249] | 50.69 (799.82) [249] |
| Sacral nerve stimulation | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Pubovaginal/autologous slings | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Colposuspension | 0.00 (0.06) [257] | 17.18 (275.40) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Duloxetine | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Antibiotics | 0.02 (0.26) [257] | 1.04 (13.75) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Antimuscarinics | 0.02 (0.12) [257] | 2.84 (22.63) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Covering of tape extrusion | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Urethral dilation | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Catheter | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Intermittent catheterisation | 0.02 (0.15) [257] | 5.60 (54.83) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Dilation | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Partial tape removal | 0.00 (0.06) [257] | 9.88 (158.38) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Complete tape removal | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Exposure from vagina | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Excision to bladder | 0.00 (0.06) [257] | 7.05 (112.97) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Insertion of indwelling catheter | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Tape cut | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Urethrolysis | 0.00 (0.00) [257] | 0.00 (0.00) [257] | 0.00 (0.00) [249] | 0.00 (0.00) [249] |
| Inpatient stay | 0.03 (0.38) [257] | 13.16 (183.15) [257] | 0.01 (0.09) [249] | 0.00 (0.00) [249] |
| Total follow-up costs | 149.34 (779.68) [257] | 94.39 (884.90) [249] | ||
| Year 3 | ||||
| Outpatient department | 0.13 (0.57) [244] | 17.41 (76.46) [244] | 0.08 (0.47) [245] | 9.65 (59.91) [245] |
| Ward review | 0.00 (0.06) [244] | 0.58 (9.03) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Cystoscopy | 0.02 (0.13) [244] | 18.13 (140.73) [244] | 0.02 (0.15) [245] | 18.06 (140.45) [245] |
| Urine dipstick | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.06) [245] | 0.02 (0.28) [245] |
| Mid-stream specimen of urine | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.06) [245] | 0.02 (0.28) [245] |
| Urodynamics | 0.01 (0.09) [244] | 3.02 (33.25) [244] | 0.01 (0.19) [245] | 4.51 (70.53) [245] |
| Ultrasonography | 0.01 (0.09) [244] | 0.46 (5.06) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Radiography | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| MRI | 0.00 (0.06) [244] | 0.66 (10.31) [244] | 0.00 (0.06) [245] | 0.66 (10.29) [245] |
| CT | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| SMUS | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| SIMS | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Botox injections | 0.00 (0.06) [244] | 4.27 (66.77) [244] | 0.01 (0.11) [245] | 12.77 (114.94) [245] |
| Tibial nerve stimulation | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Sacral nerve stimulation | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Pubovaginal/autologous slings | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Colposuspension | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.06) [245] | 18.02 (282.06) [245] |
| Duloxetine | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Antibiotics | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.06) [245] | 0.22 (3.42) [245] |
| Antimuscarinics | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Covering of tape extrusion | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Urethral dilation | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Catheter | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Intermittent catheterisation | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Dilation | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Partial tape removal | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.06) [245] | 10.36 (162.21) [245] |
| Complete tape removal | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Exposure from vagina | 0.00 (0.06) [244] | 7.42 (115.94) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Excision to bladder | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Insertion of indwelling catheter | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Tape cut | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Urethrolysis | 0.00 (0.00) [244] | 0.00 (0.00) [244] | 0.00 (0.00) [245] | 0.00 (0.00) [245] |
| Inpatient stay | 0.00 (0.00) [244] | 19.84 (188.93) [244] | 0.00 (0.06) [245] | 2.61 (32.41) [245] |
| Total follow-up costs | 71.78 (332.01) [244] | 78.87 (410.88) [245] | ||
FIGURE 24.
Number of women experiencing problems over the 36 months (EQ-5D-3L).
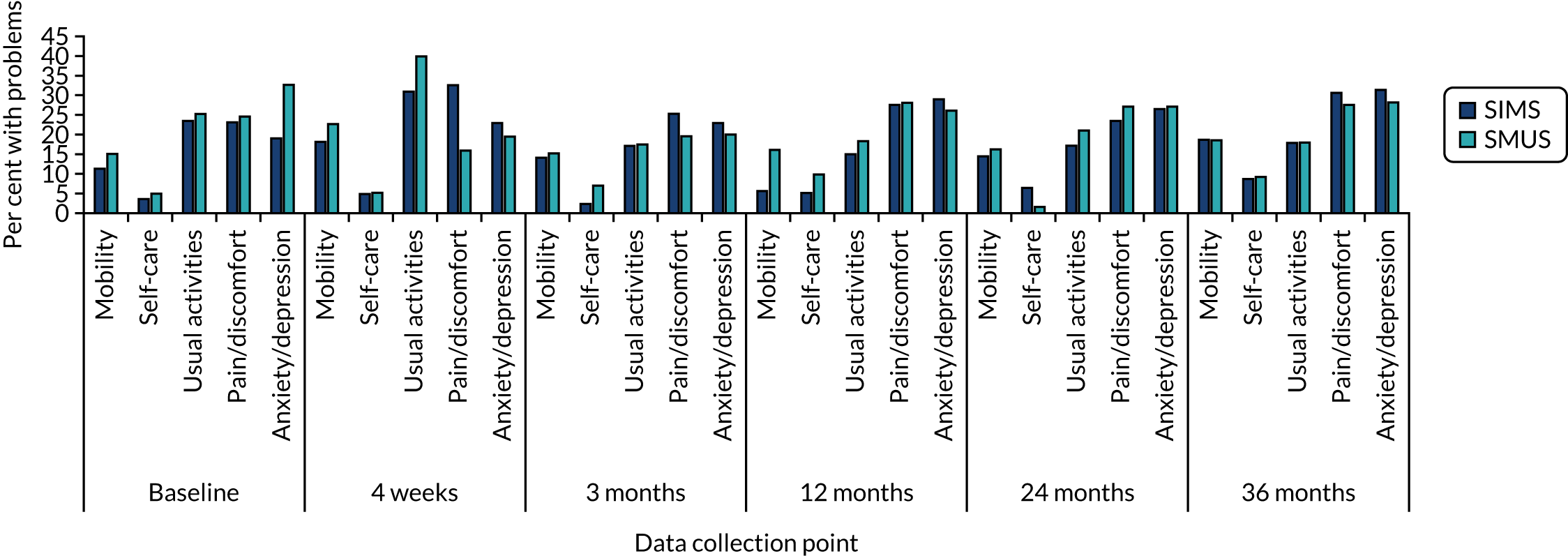
Cost-effectiveness acceptability curves
Complete-case data analysis
FIGURE 25.
Scatterplot of incremental costs and QALYs for SIMSs, compared with SMUSs, using complete-case data analysis.
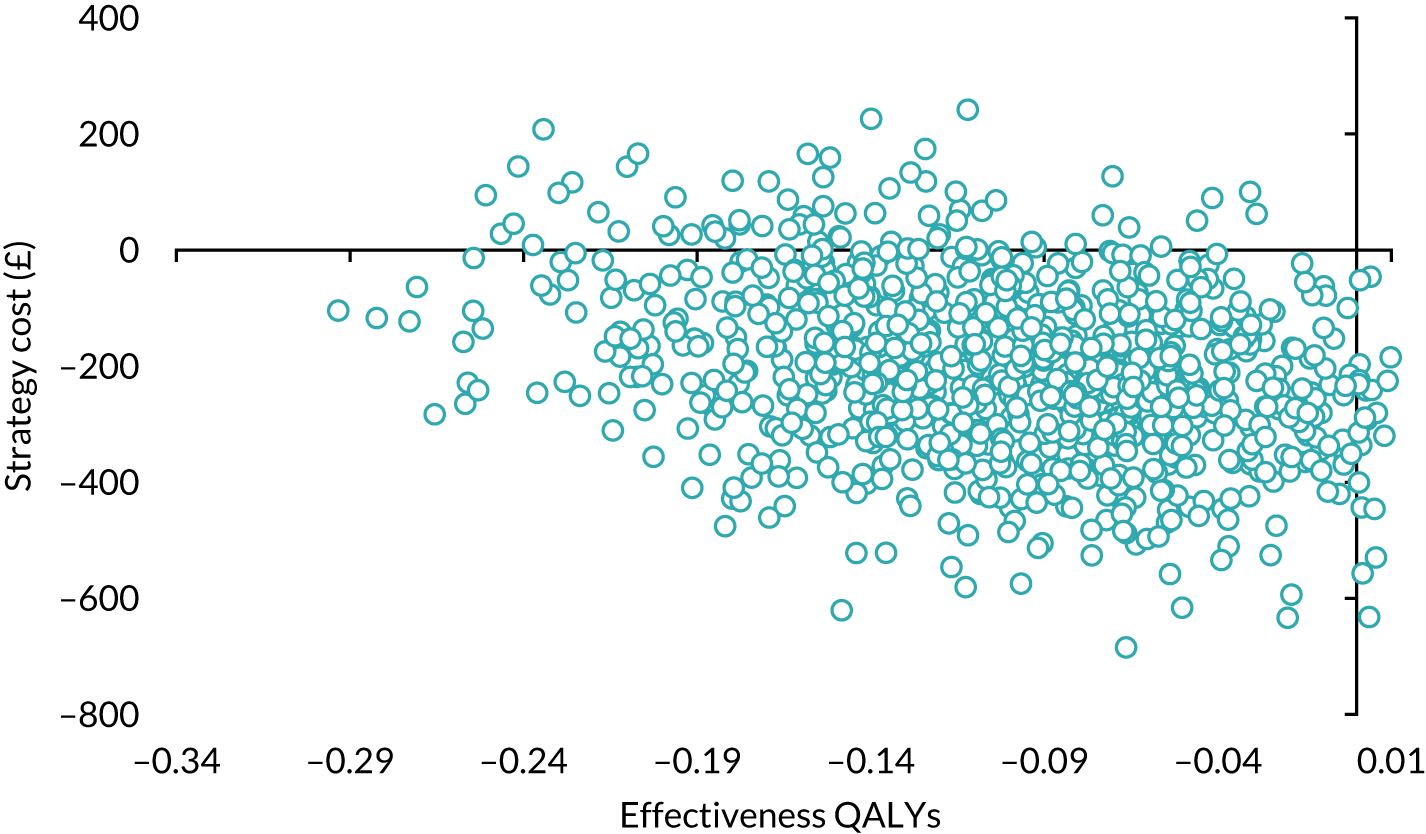
FIGURE 26.
Cost-effectiveness acceptability curve: SIMSs vs. SMUSs using complete-case data analysis.
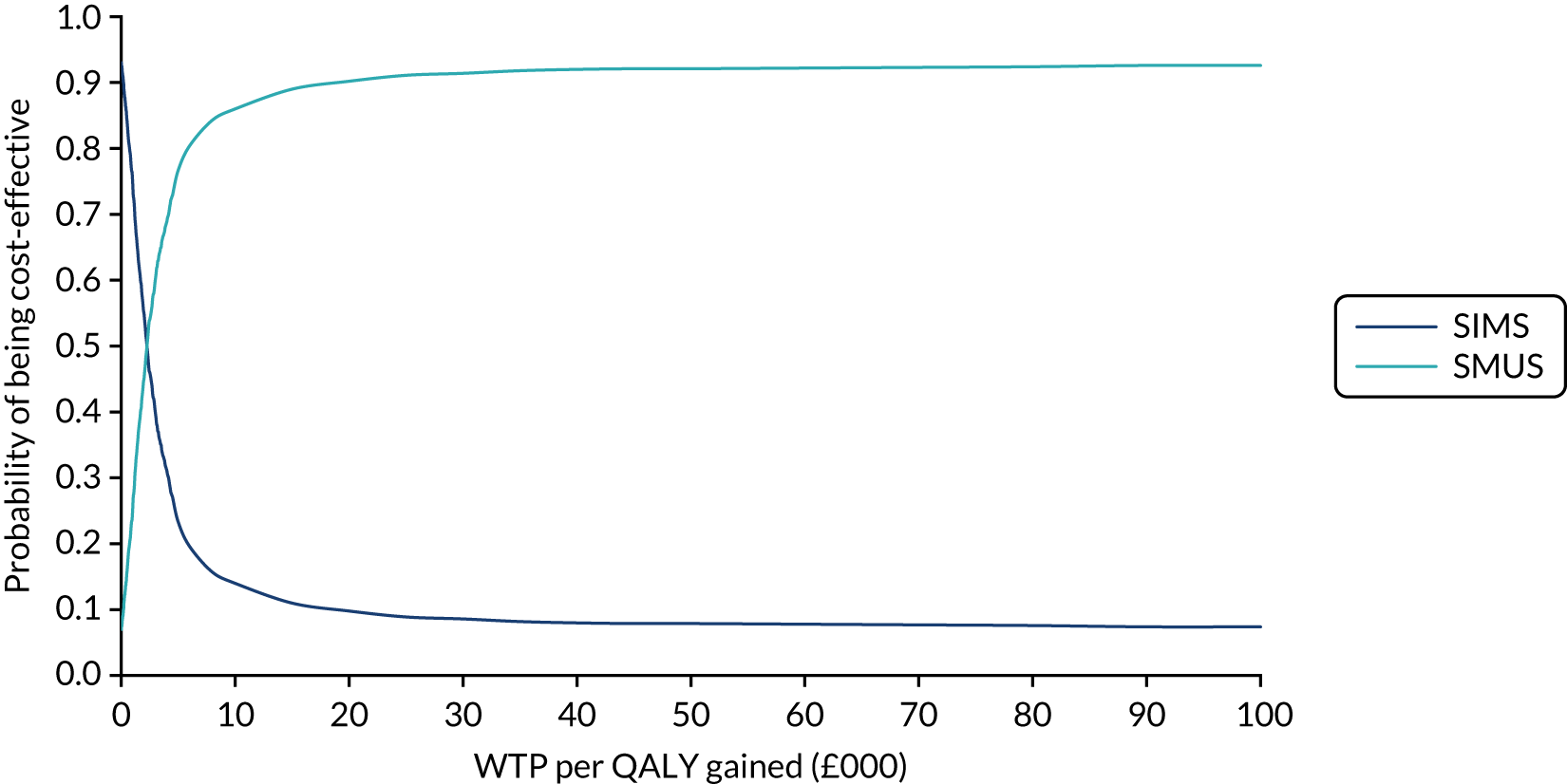
FIGURE 27.
Scatterplot of incremental costs and QALYs for SIMSs, compared with SMUSs, using ICIQ-LUTSqol score.
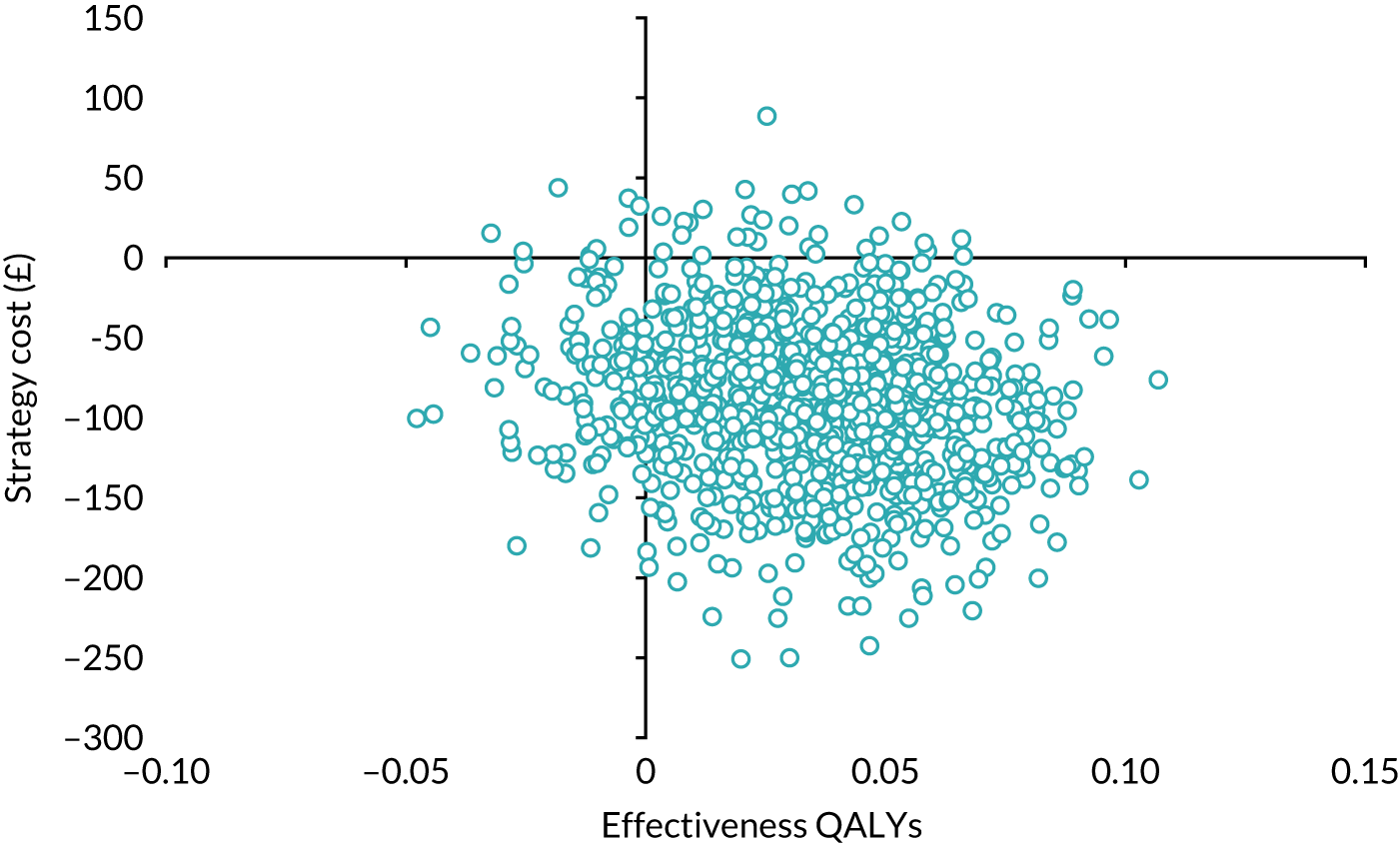
FIGURE 28.
Cost-effectiveness acceptability curve: SIMSs vs. SMUSs using ICIQ-LUTSqol scores. Undiscounted costs and QALYs.

FIGURE 29.
Scatterplot of incremental costs and QALYs for SIMSs, compared with SMUSs, using undiscounted costs and EQ-5D-3L QoL score.
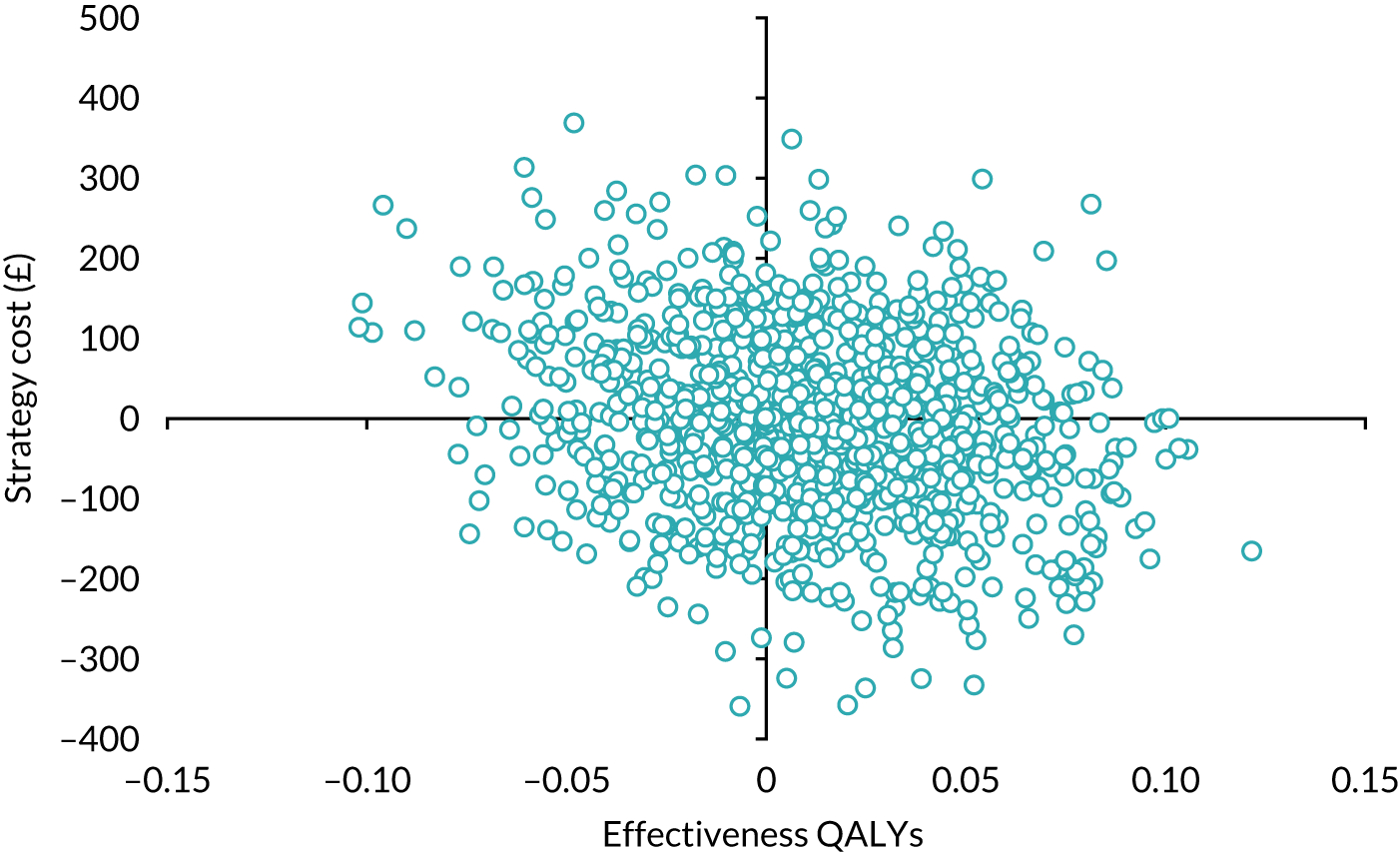
FIGURE 30.
Cost-effectiveness acceptability curve: SIMSs vs. SMUSs using undiscounted costs and EQ-5D-3L QoL scores. Undiscounted costs and QALYs.
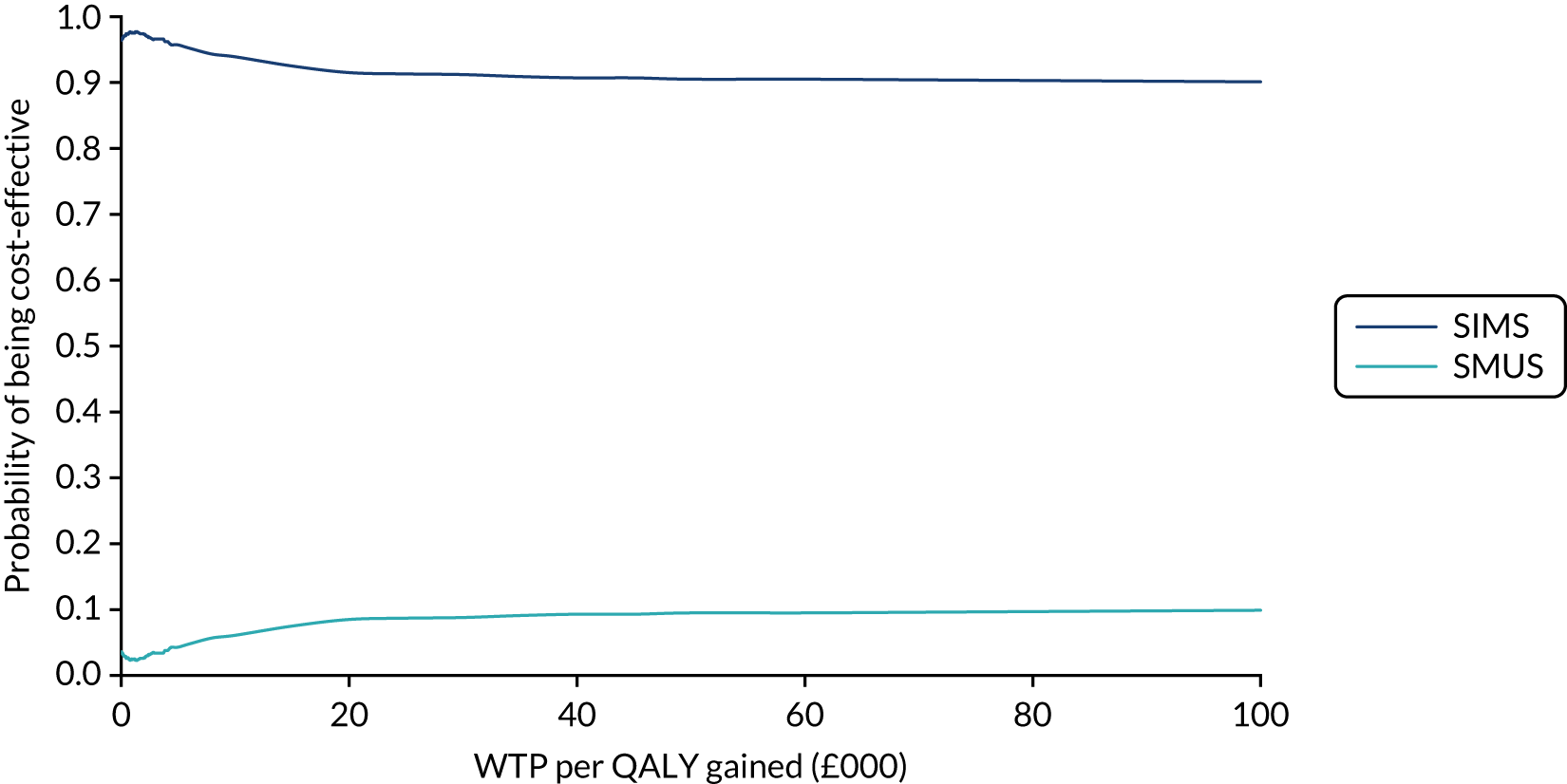
FIGURE 31.
Scatterplot of incremental costs and QALYs for SIMSs, compared with SMUSs, using a 6% discount rate for costs and EQ-5D-3L QoL scores.
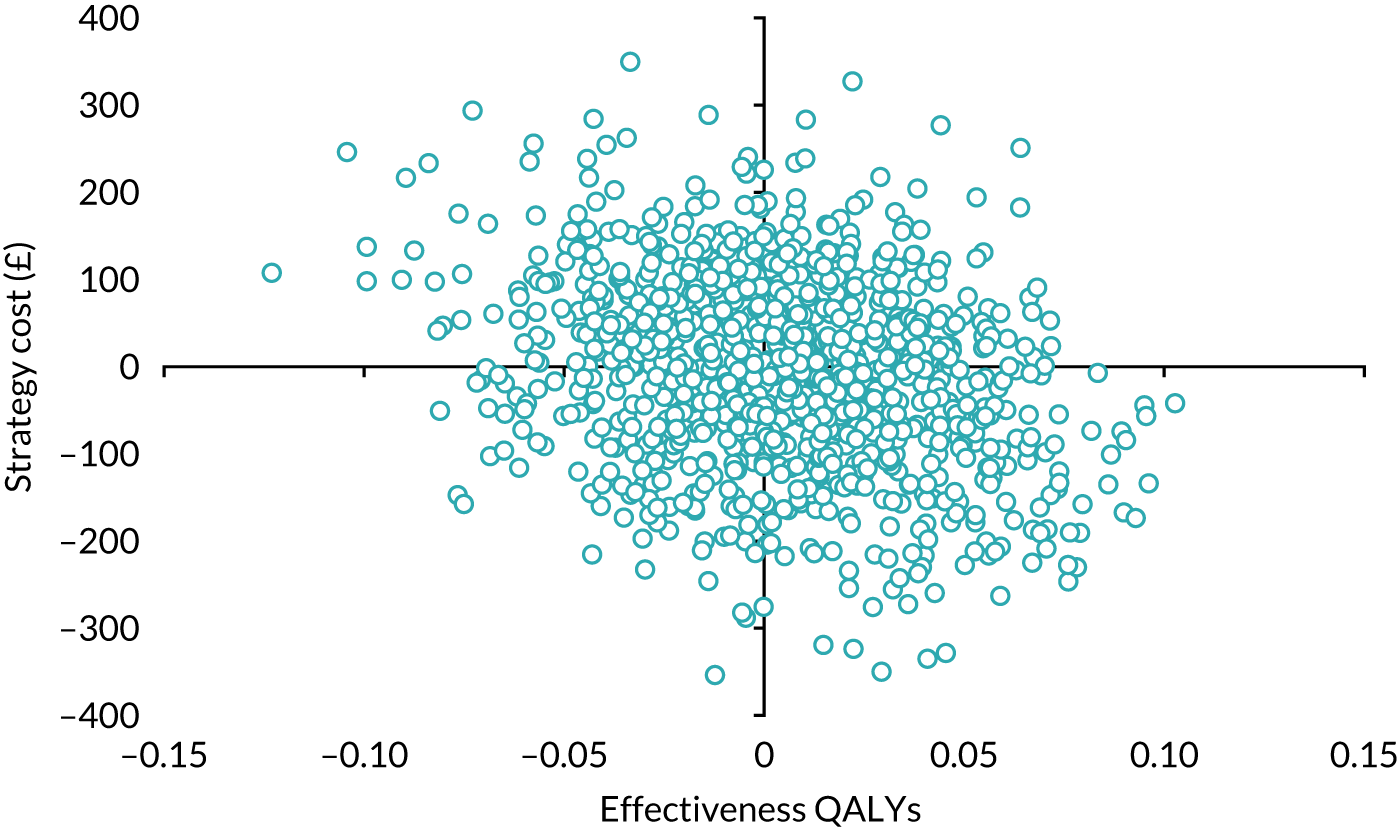
FIGURE 32.
Cost-effectiveness acceptability curve: SIMSs vs. SMUSs using a 6% discount rate for costs and EQ-5D-3L QoL scores.
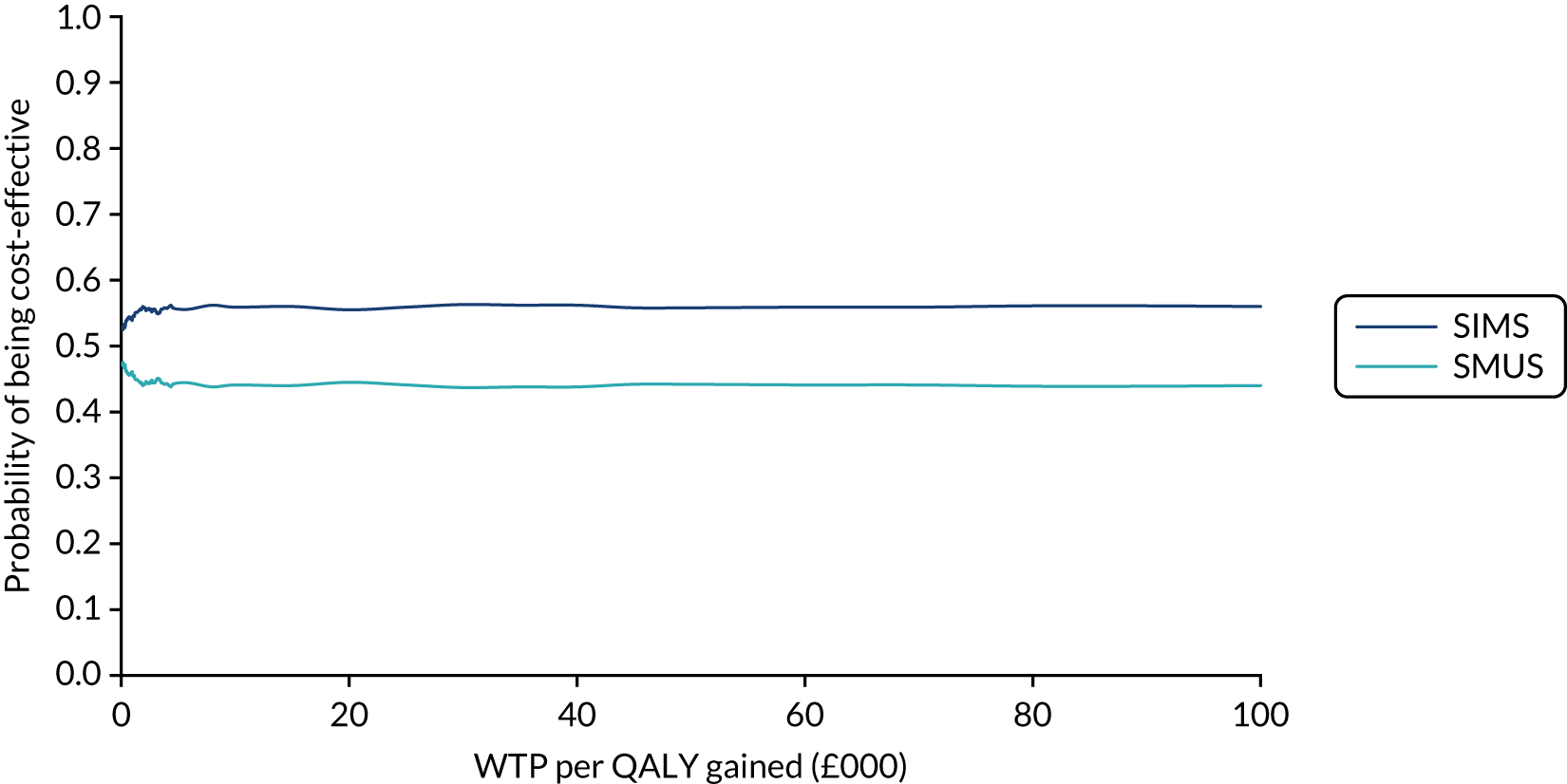
FIGURE 33.
Scatterplot of incremental costs and QALYs for SIMSs, compared with SMUSs, using unadjusted time waiting for surgery and EQ-5D-3L QoL scores.
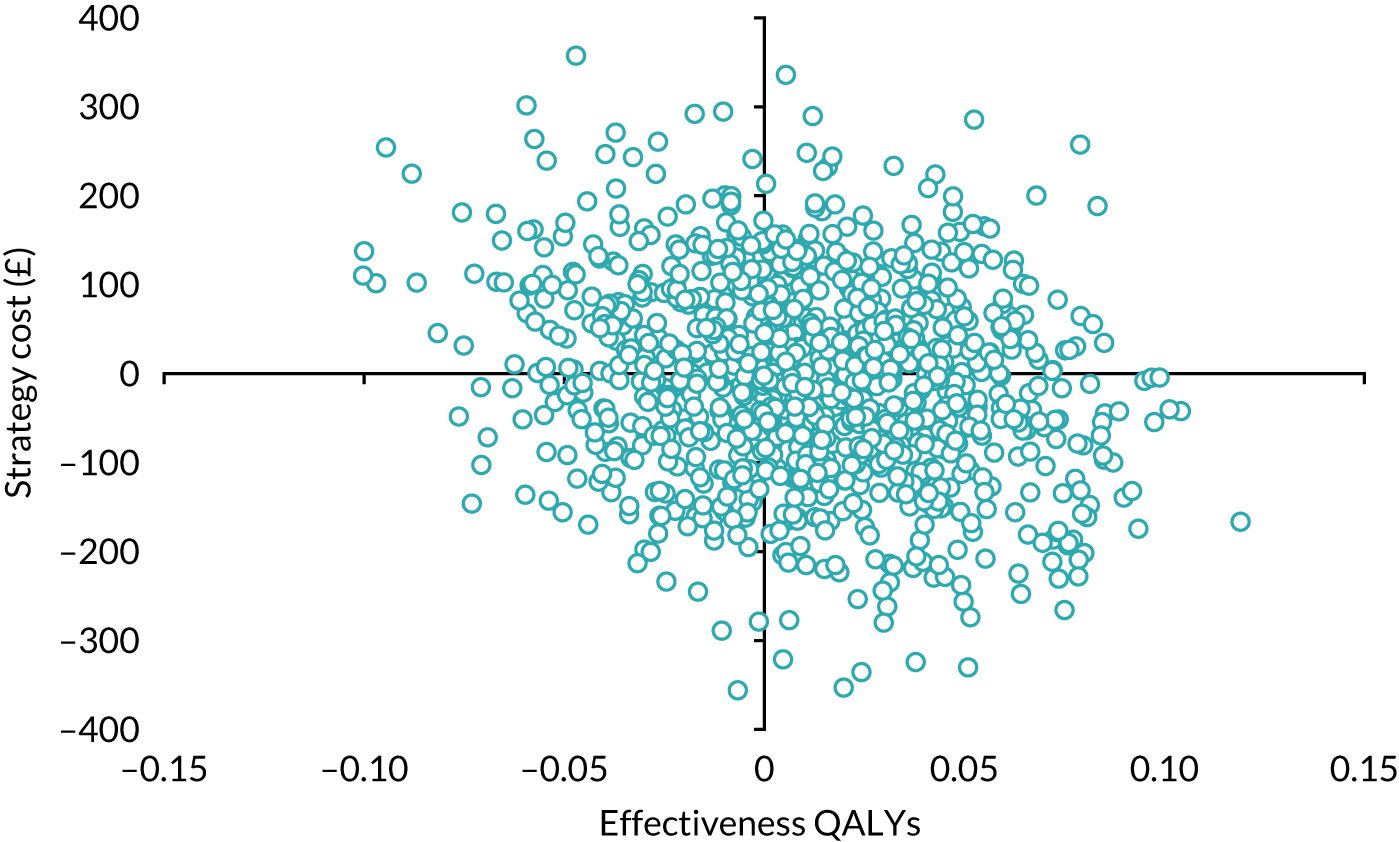
FIGURE 34.
Cost-effectiveness acceptability curve: SIMSs vs. SMUSs using unadjusted time waiting for surgery and EQ-5D-3L QoL scores.
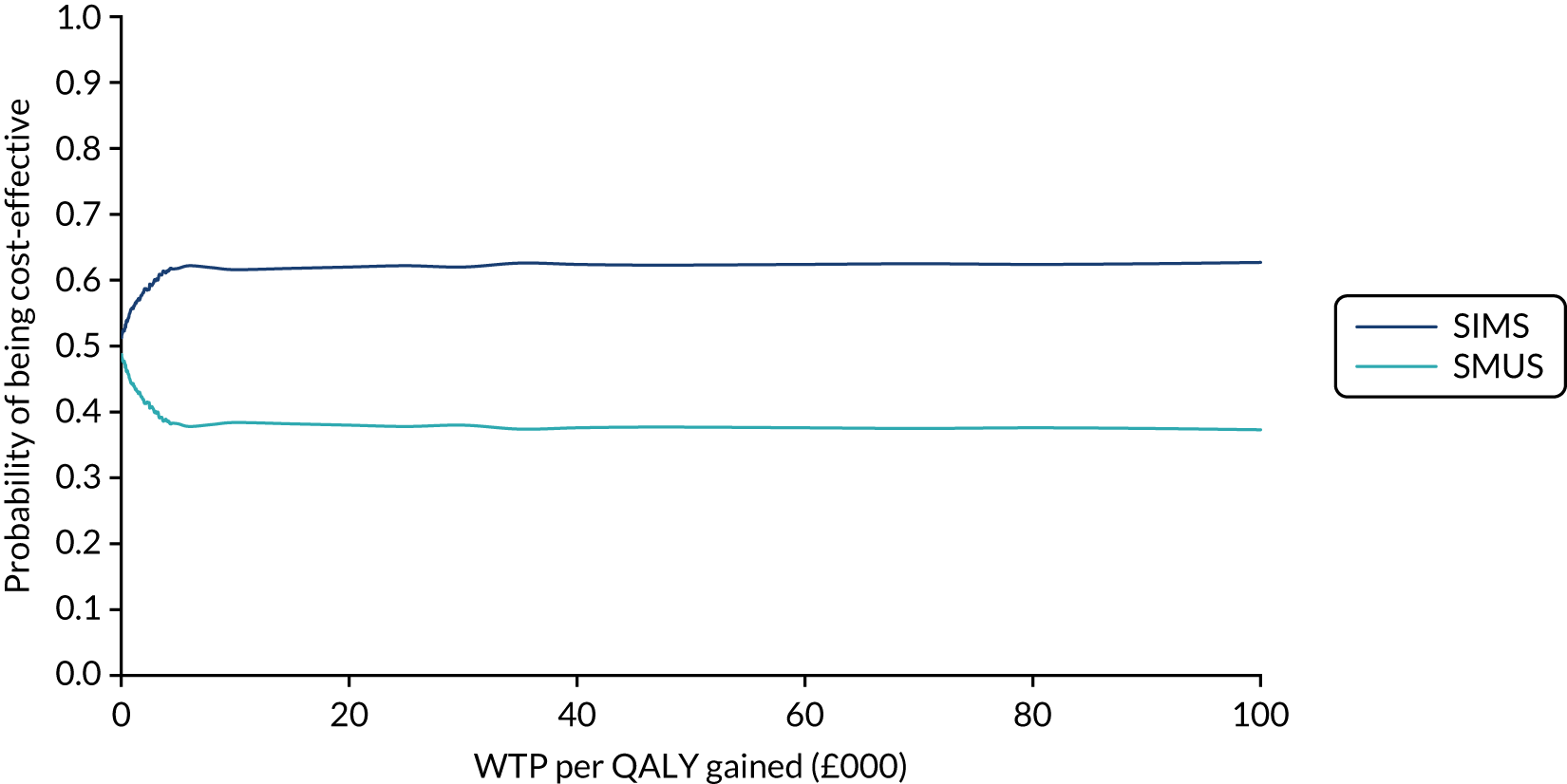
FIGURE 35.
Scatterplot of incremental costs and QALYs for SIMSs, compared with SMUSs, assuming that follow-up CRF costs for those who had no surgery were missing.
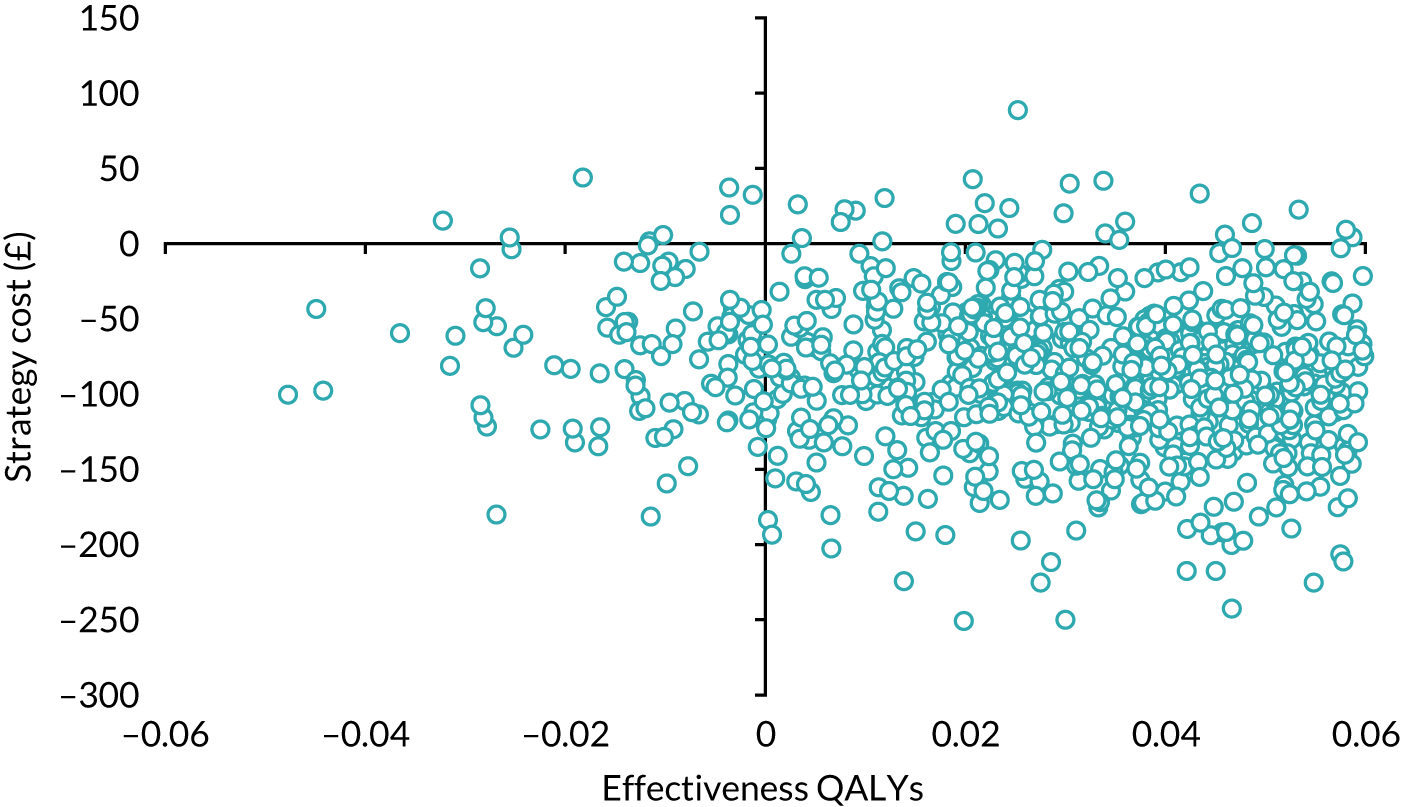
FIGURE 36.
Cost-effectiveness acceptability curve: SIMSs vs. SMUSs, assuming that follow-up CRF costs for those who had no surgery were missing.
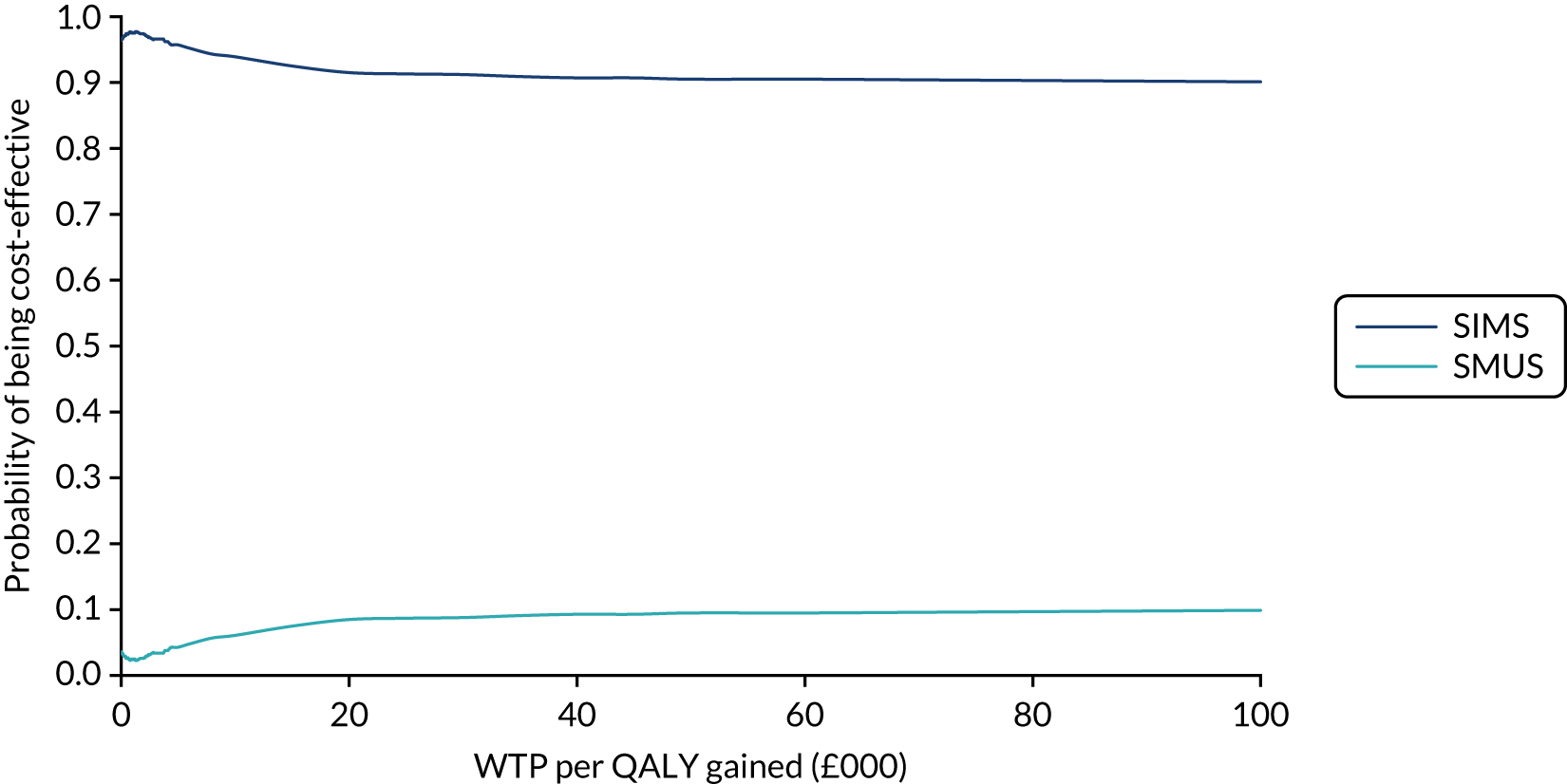
FIGURE 37.
Scatterplot of incremental costs and QALYs for SIMSs, compared with SMUSs: societal perspective – using both NHS and patient time and travel costs.
FIGURE 38.
Cost-effectiveness acceptability curve: SIMSs vs. SMUSs – societal perspective: using both NHS and patient time and travel costs.
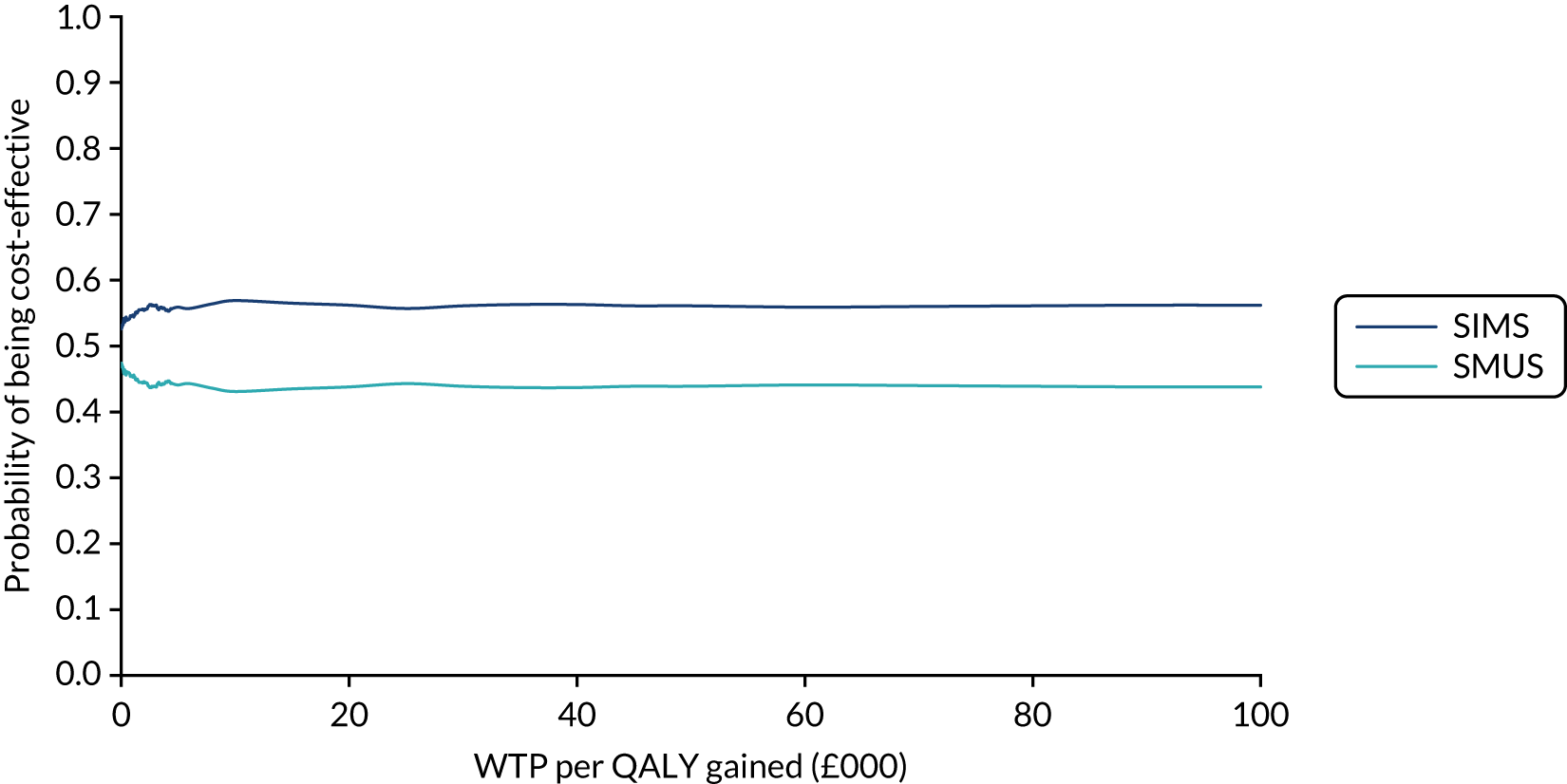
List of abbreviations
- AE
- adverse event
- ASC
- alternative specific constant
- BC
- Burch colposuspension
- BMI
- body mass index
- BSUG
- British Society of Urogynaecology
- CEAC
- cost-effectiveness acceptability curve
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CISC
- clean intermittent self-catheterisation
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CST
- cough stress test
- DCE
- discrete choice experiment
- DMC
- Data Monitoring Committee
- DO
- detrusor overactivity
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-VAS
- EuroQol Visual Analogue Scale
- E-TOT
- Evaluation of Transobturator Tapes
- GA
- general anaesthetic
- GLM
- generalised linear model
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICIQ-FLUTS
- International Consultation on Incontinence Questionnaire-Female Lower Urinary Tract Symptoms
- ICIQ-FLUTSsex
- International Consultation on Incontinence Questionnaire-Female Sexual Matters Associated with Lower Urinary Tract Symptoms
- ICIQ-LUTSqol
- International Consultation on Incontinence Questionnaire-Lower Urinary Tract Symptoms-Quality of Life
- ICIQ-UI-SF
- International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form
- IPG
- interventional procedures guidance
- IQR
- interquartile range
- ITT
- intention to treat
- KHQ
- King’s Health Questionnaire
- LA
- local anaesthetic
- LC
- laparoscopic colposuspension
- LUT
- lower urinary tract
- MUI
- mixed urinary incontinence
- MUS
- mid-urethral sling
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OAB
- overactive bladder
- OR
- odds ratio
- PFMT
- pelvic floor muscle training
- PGI-I
- Patient Global Impression of Improvement
- PI
- principal investigator
- PIL
- patient information leaflet
- PISQ-12
- Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire-12 items
- PISQ-IR
- Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire, International Urogynecological Association-Revised
- PMG
- Project Management Group
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RD
- risk difference
- REC
- Research Ethics Committee
- RN
- research nurse
- RP-TVT
- retropubic tension-free vaginal tape
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SIMS
- single-incision mini-sling
- SMUS
- standard mid-urethral sling
- SUI
- stress urinary incontinence
- TFS
- Tissue Fixation System
- TOMUS
- Trial Of Mid-Urethral Slings
- TO-TVT
- transobturator tension-free vaginal tape
- TSC
- Trial Steering Committee
- TVT
- tension-free vaginal tape
- TVT-O
- tension-free vaginal tape – obturator
- UDI-6
- Urogenital Distress Inventory-6 items
- UI
- urinary incontinence
- UPS
- Urgency Perception Scale
- UTI
- urinary tract infection
- UUI
- urgency urinary incontinence
- VD
- voiding dysfunction
- WMD
- weighted mean difference
- WTP
- willingness to pay