Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/35/29. The contractual start date was in February 2014. The draft report began editorial review in June 2020 and was accepted for publication in August 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 O’Connell et al. This work was produced by O’Connell et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 O’Connell et al.
Chapter 1 Introduction
Parts of this report have been reproduced with permission from Harries et al. 1 © Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/
Parts of this report have been reproduced with permission from Cornish et al. 2 © 2016 The Author(s). Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Background
An incisional hernia (IH) is a common and potentially serious complication following abdominal surgery. An IH is a bulge in the abdomen through or close to a previously made incision caused by the patient’s intestines, organs and/or other tissue protruding through a weakening in the abdominal muscles as a result of surgery. The reported rates of IH range from 8.6% to 39.9% following open colorectal surgery and from 4.7% to 24.3% following laparoscopic surgery. 3–7 In a systematic review8 that included 14,618 patients, the incidence of IH was 12.8% at 2 years’ follow-up, with incidence rates as high as 35.6% among patients who had received a midline incision during surgery. In patients with colorectal cancer, the rate of IH has been reported to be as high as 39.9%. 5
A number of potential risk factors for IH have been identified, including male sex, increased age, increased BMI, history of chronic obstructive pulmonary disease, history of smoking and certain medications. Surgeon-modifiable risk factors include surgical technique and suture type for abdominal closure; however, although a number of studies have been conducted investigating different surgical methods, uncertainty remains around the impact of such surgeon-modifiable factors on IH rates, with several studies reporting conflicting results. For example, three meta-analyses9–11 concluded that non-absorbable stitches reduce the risk of IH, one meta-analysis12 reported that absorbable stitches are associated with a lower risk of IH and one meta-analysis8 reported no difference in IH rates when comparing absorbable and non-absorbable stitches.
Recent cost analyses have found that the treatment and repair of IH places a considerable strain on already-stretched health-care resources. Direct per-patient cost estimates range from €3497 to €16,367 in European countries13,14 and from US$6530 to US$16,889 in the USA,15–17 with hospitalisation and surgery costs, as well as complications, adverse events and recurrences, identified as the main cost drivers.
An IH can be diagnosed as a result of patient-reported symptoms, such as a lump, abdominal pain and symptoms of obstruction. If the hernia has become incarcerated or strangulated, then this can also lead to tissue necrosis.
Treatment can vary depending on the size and anatomy of the hernia, the general health of the patient and the desired level of physical activity post repair. However, it will generally require one of two types of surgery: an open or, sometimes, a laparoscopic hernia repair. In attempting to reduce the risk of hernia recurrence, there is an increasing reliance on the use of synthetic or biologic mesh to facilitate the repair.
Rationale
More than 30,000 patients are diagnosed with colorectal cancer in the UK each year. 18 Most of these patients will undergo surgery as part of their treatment, and the incidence of complications following surgery is high. 19 One common complication after abdominal surgery is the occurrence of an IH following the closure of the midline incision.
An IH may have a negative impact on a patient’s quality of life (QoL) and their overall experience. 20 The outcomes for patients with IHs are poor, and many will suffer with chronic pain or suffer a repeat hernia even after the first repair. This can, in turn, lead to increased NHS resource use as a result of additional or longer hospital stays. It is, therefore, important to identify surgical procedures and strategies that can reduce the risk of IHs in patients who undergo abdominal surgery.
The aim of this study was to assess the potential of Hughes abdominal repair, an alternative wound closure method, to prevent IHs. The study recruited colorectal cancer patients who were due to receive surgical treatment for their cancer. The care pathway followed its standard course, except at point of abdominal wall closure, when the patient was randomised to either Hughes abdominal closure or standard mass closure.
Literature update
A review of the literature was conducted in key databases, including MEDLINE, EMBASE, Cochrane Database of Systematic Reviews and Cochrane Register of Controlled Trials. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),21 and the PRISMA flow chart (Figure 1) details the amount of evidence sifted and excluded at each stage. Search strategies were developed in MEDLINE and adapted for other databases (see Appendix 1, Table 29).
FIGURE 1.
The PRISMA flow diagram. Reproduced with permission from Moher et al. 21 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
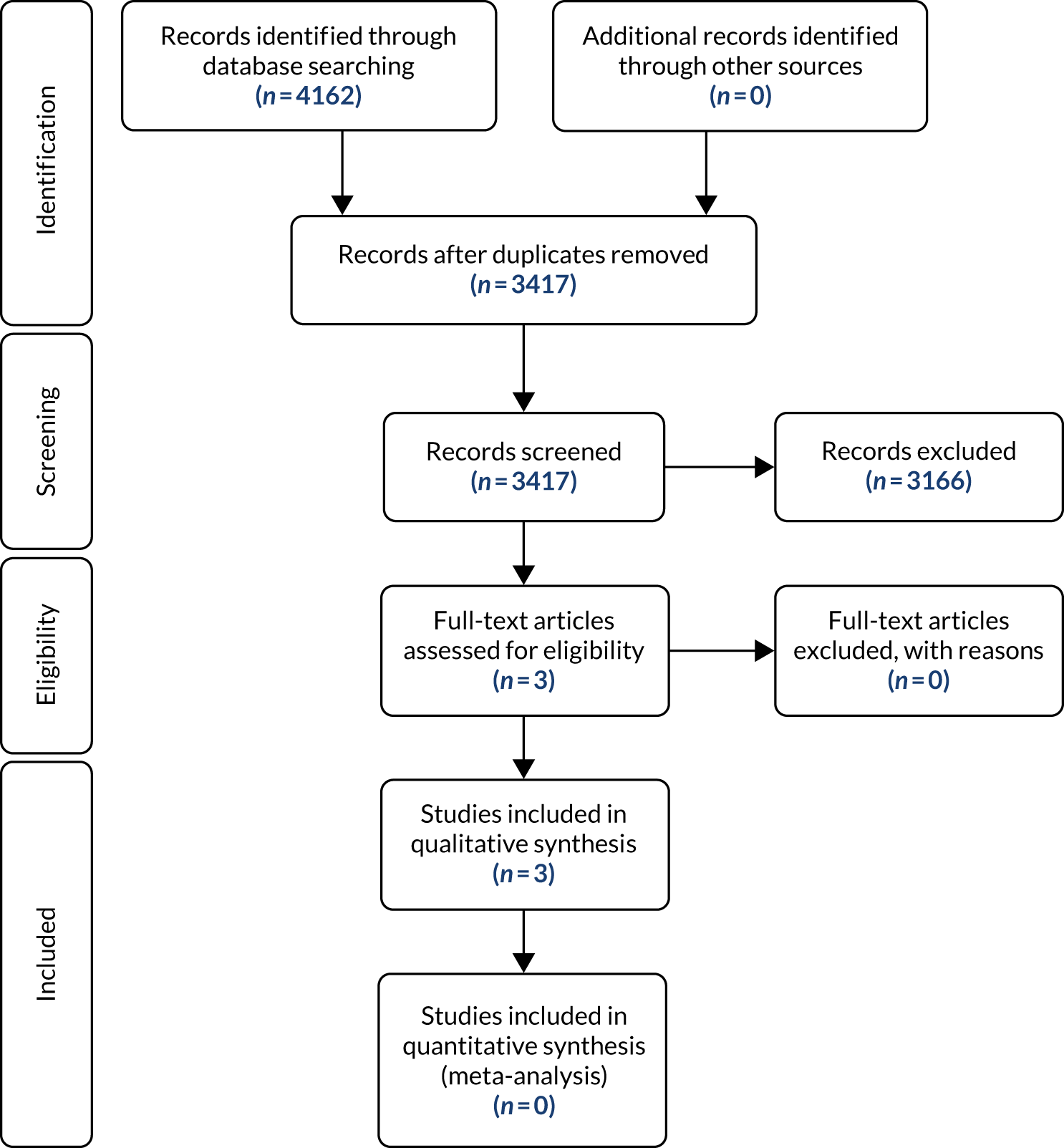
The study team was aware of a published systematic review and meta-regression that reported on factors affecting rates of midline IH in 14,618 patients from 56 individual studies, as well as the prevalence of IH at the 2-year follow-up. 8 The quality of the studies included was variable. All study types were considered for inclusion in the meta-analysis if they described a population of adult patients undergoing primary suture closure of a midline laparotomy wound. The review included a literature search up to March 2013. For this reason, it was decided to update the literature searches from this point. Initial database searches were carried out for the period from January 2013 to November 2018, with update searches carried out in September 2019 and again in April 2020 to ensure completeness. Following the removal of duplicate records, searches identified 3417 potentially relevant records (see Figure 1).
During the initial sifting of the updated literature search results, a Cochrane review published in 2017 was identified, entitled ‘Closure methods for laparotomy incisions for preventing incisional hernias and other wound complications’. 22 Assessment of the Cochrane review indicated that the searches were comprehensive and directly relevant to this study. As this provided a thorough review of relevant evidence up to February 2017, the authors decided that a full literature review was not required at this time. The decision was made to limit the evidence review for this report to new evidence and to include only relevant evidence published after the date of the searches in the Cochrane review.
Although an earlier systematic review8 had included all study types, the decision was also made to limit the searches to randomised controlled trials (RCTs) reporting the primary outcome, incidence of IH following abdominal surgery, in line with the Cochrane review,22 as it was considered that there were sufficient RCTs available.
As this was not a full systematic review of the literature, it was not registered on the PROSPERO database.
In addition, a review of clinical trial databases was conducted to identify any relevant ongoing clinical trials. Five additional relevant ongoing trials were identified, three of which have corresponding trial protocols published. 23–25 Ongoing trials include:
-
the ESTOIH study,23 investigating the influence of stitch length, using an elastic, extra-long-term absorbable monofilament suture, on the long-term clinical outcome of abdominal wall closure
-
the HULC trial,24 investigating whether or not a combination of small stitched fascial closure and onlay mesh augmentation after elective midline laparotomies reduces the risk of IH
-
the CONTINT trial,25 comparing continuous slowly absorbable sutures with interrupted rapidly absorbable sutures for abdominal wall closure after midline incisions for emergency laparotomy
-
the E-STITCH trial,26 comparing the small-tissue-bite technique with the large-bite technique for the closure of emergency midline laparotomy
-
the Rein4CeTo1 trial,27 comparing the IH incidence 1 year after planned colorectal cancer surgery performed through a midline incision that is closed either by a standardised small stitch 4 : 1 technique or with the same technique plus a reinforced tension-line suture.
Full details of these additional studies can be found in Appendix 2.
Based on the adjusted criteria for inclusion in the review, only two additional studies not covered by the Cochrane review were identified as relevant: the STITCH trial28 and the HART feasibility trial. 1
In total, three studies are included in the literature review. 1,22,28 The methods and results of each of the studies are detailed in the following sections.
STITCH trial28
A total of 540 patients were randomly assigned to large bites (1 cm every 1 cm) or small bites (5 mm every 5 mm). IH was reported in 21% (n = 57) of patients in the large-bites group and in 13% (n = 35) of patients in the small-bites group (p = 0.0131). Radiological (using ultrasound) and physical follow-up assessments were carried out in 62% (n = 338) of patients, and IH was identified in a total of 26% (n = 87) of patients. In 49% (n = 43) of patients, IH was identified by both radiological and physical examination, in 47% (n = 41) of patients it was identified by radiological examination only and in 3% (n = 3) of patients it was identified by physical examination only. Re-admission rates and the number and type of adverse events did not differ significantly between the groups.
No significant differences in pain scores, as measured with the visual analogue scale, were reported in the first week postoperatively, and no significant difference was observed between the arms for any patient-reported outcomes, as measured with the Short Form-36 items (SF-36) subdomain or the EuroQol-5 Dimensions (EQ-5D).
Patients who developed IH reported lower general health scores [mean 60.16 (SD 18.27) vs. mean 64.84 (SD 48.7); p = 0.0326] and reported more problems in the EQ-5D dimension of mobility [mean 1.46 (SD 1.06) vs. mean 1.36 (SD 0.46); p = 0.0318] than those who did not develop IH.
HART feasibility study1
A 30-patient feasibility trial demonstrated that a RCT comparing Hughes abdominal closure with standard mass closure would be acceptable to patients, achieve adequate recruitment and present no early safety concerns. Patient participation rates were high, with 69% of all eligible patients consenting to take part. The feasibility study recruited 30 patients over a 5-month period, suggesting that the proposed sample size of 800 patients for a full trial would be achievable.
The importance of having adequate numbers of approved consenting staff on the delegation log was highlighted, as nine consenting patients could not be randomised because of staff shortages.
Rates of serious adverse events (SAEs) were similar between the arms [34% for Hughes (10 SAEs in five patients) vs. 31% for mass closure (six SAEs in five patients); p = 1.00], and no suspected unexpected serious adverse reactions were reported.
Cochrane review main findings22
For the primary outcome, namely the proportion of participants who developed IH at ≥ 1 year of follow-up, the authors did not find evidence that suture (absorption moderate), closure method (very low-quality evidence) or closure technique (moderate-quality evidence) resulted in a difference in the risk of IH. They did, however, find evidence to suggest that monofilament sutures reduced the risk of IH when compared with multifilament sutures (moderate-quality evidence).
Considering the secondary outcomes, the authors reported that none of the interventions under investigation [suture absorption (moderate-quality evidence), closure method (low- to moderate-quality evidence) or closure technique (moderate-quality evidence)] reduced the risk of wound infection or wound dehiscence.
Absorbable sutures reduced the risk of sinus or fistula tract formation compared with non-absorbable sutures, but this was based on low-quality evidence. None of the other comparisons showed a difference in risk of sinus or fistula tract formation (very low- to low-quality evidence). Table 1 provides a summary of the results by comparison and outcome.
| Comparison | Risk ratio (95% CI) | |||
|---|---|---|---|---|
| IH | Wound infection | Wound dehiscence | Sinus/fistula tract formation | |
| Absorbable vs. non-absorbable sutures | 1.07 (0.86 to 1.32) | 0.99 (0.84 to 1.17) | 0.78 (0.55 to 1.10) | 0.49 (0.26 to 0.94) |
| Slow vs. fast absorbable sutures | 0.81 (0.63 to 1.06) | 1.16 (0.85 to 1.57) | 1.55 (0.92 to 2.61) | 0.88 (0.05 to 16.05) |
| Mass vs. layered closure | 1.92 (0.58 to 6.35) | 0.93 (0.67 to 1.30) | 0.69 (0.31 to 1.52) | 0.49 (0.15 to 1.62) |
| Continuous vs. interrupted | 1.01 (0.76 to 1.35) | 1.13 (0.96 to 1.34) | 1.21 (0.90 to 1.64) | 1.51 (0.64 to 3.61) |
| Monofilament sutures vs. multifilament sutures | 0.76 (0.59 to 0.98) | |||
In summary, the authors reported that, based on their review of the evidence, a number of factors, including closure type and suture material, may have an impact on patient outcomes, such as IH rate, wound complications and QoL, following a midline incision. However, in their conclusions the authors note that the quality of the evidence ranges from moderate to very low, and there is a need for larger, high-quality trials. The conclusions of the authors also recommend that future studies ensure that proper randomisation and allocation techniques are performed, wound assessors are blinded and the duration of follow-up is adequate. 22
The authors note that it is important that only one type of intervention is compared between arms. In addition, they suggest that a homogeneous patient population would allow for a more accurate assessment of the interventions. 22 The STITCH trial,28 conducted in the Netherlands, is one such trial, in which patients were randomly assigned to large bites (1 cm every 1 cm) or small bites (5 mm every 5 mm). This well-conducted, double-blind, randomised controlled trial included all patients scheduled to undergo midline incision for any condition, not just colorectal cancer. IH rates differed significantly between the two arms (p = 0.0131).
The HART study proposed a large, multicentre, pragmatic clinical trial comparing only one type of intervention, Hughes abdominal closure method (hereafter referred to as Hughes repair), with standard mass closure exclusively in colorectal cancer patients. Although the population was limited to patients having surgery for colorectal cancer and the intervention arm was tightly defined with no variance in approach allowed, it should be noted that, in the control arm, the approach to standard mass closure allowed surgeon preference and this may have introduced an element of heterogeneity.
Hypothesis
The null hypothesis states that, in patients having midline abdominal wall closure following elective or emergency colorectal cancer surgery, there is no difference in the rate of IH over 1 year between those undergoing Hughes repair and those undergoing standard mass closure.
The alternative hypothesis states that, in patients having midline abdominal wall closure following elective or emergency colorectal cancer surgery, Hughes repair alters the incidence of IH over 1 year when compared with standard mass closure.
Patient and public involvement
Patient and public involvement (PPI) was an integral part of the HART study. A minimum of two PPI representatives were involved at any given time throughout the study, one of whom had experience of colorectal surgery for a colorectal cancer. PPI representatives had previous experience of working with research groups and sat on the Trial Management Group for the study. In addition, a PPI representative sat on the Steering Committee for the trial. PPI began at the protocol development stage and continued right through to the interpretation, discussion and dissemination of results.
Patient and public involvement representatives were paid honoraria and out-of-pocket expenses in line with Health Care Research Wales and INVOLVE guidelines for attending meetings.
Although the impact of PPI was not an outcome of the study, feedback was sought from the patient representatives at all stages of the study and their experience of being part of a clinical study is reported.
Chapter 2 Methods
Trial design
This was a multicentre, single-blinded randomised controlled trial. The patients were randomised 1 : 1 to enable the comparison of two suture techniques for the closure of the midline abdominal wound following surgery for colorectal cancer.
Changes to trial design
The study was split into three phases: feasibility, pilot and main. The feasibility phase assessed recruitment, randomisation, deliverability and early safety of the surgical technique. Following a successful feasibility phase, the trial was approved by the independent Data Monitoring Committee (DMC) and progressed to the pilot and main phases. No changes were adopted in the trial design specified during the feasibility phase and the study finished its primary end point as per the published study protocol. 1,2
Conduct of the study: approvals and trial registration
The trial was conducted in compliance with the protocol, the Declaration of Helsinki as currently revised29 and the principles of Good Clinical Practice (GCP),30 and in accordance with all applicable regulatory guidance.
The study protocol and all subsequent amendments were reviewed and approved by the Wales 3 Research Ethics Committee (REC) (MREC 12/WA/0374) and the research and development offices of the participating NHS sites. A list of key protocol amendments can be found in Appendix 3.
Annual progress and safety reports were submitted to the REC.
The trial was registered in the ISRCTN registry and the trial registration number is ISRCTN25616490.
Participants
The study identified patients who were due to undergo abdominal surgery for the treatment of colorectal cancer. Patients undergoing emergency surgical treatment and patients receiving elective surgical treatment were screened. Patients were not excluded if they had undergone previous abdominal surgery for conditions other than the new colorectal cancer. To be eligible for inclusion, patients had to be considered suitable for either Hughes repair or standard mass closure. Patients had to have a midline incision at least 5 cm in length or they were excluded from the study.
Surgery was carried out as per standard surgical procedure in a general surgical unit within the NHS across the UK. Prior to commencing closure and provided that the patient still met the inclusion criteria, the closing surgeon accessed the telephone randomisation system and the patient was allocated to either Hughes repair or standard mass closure.
An initial feasibility phase in which 30 participants were randomised to Hughes repair or standard mass closure was conducted at University Hospital of Wales, Cardiff, only. Following the feasibility phase, which demonstrated that a RCT comparing Hughes repair with standard mass closure would be acceptable to patients,28 the study moved into the pilot and main phases. Patients were randomised to the pilot (n = 80) and main (n = 722) phases of this study (Hughes repair, n = 401; standard mass closure, n = 401) over a period of approximately 3 years and 5 months. No changes were made to the inclusion and exclusion criteria from the feasibility study to the full trial. Data from the pilot and main phases of the study were combined and are referred to as the main study phase in the rest of this report.
Inclusion and exclusion
Eligibility assessment
Eligible patients were identified through a screening process that identified whether or not patients met the initial pre-surgery inclusion criteria. Potentially eligible patients included both those undergoing elective surgery for colorectal cancer (identified via a multidisciplinary team meeting) and those undergoing emergency surgery for suspected colorectal cancer.
Eligibility for inclusion in the trial was assessed at two stages: first at screening and again at the point of surgical closure (which was the point of randomisation). The inclusion and exclusion criteria for the trial were as follows.
Inclusion criteria
At screening:
-
patients aged ≥ 18 years
-
able to give informed consent
-
both standard mass closure and Hughes repair were suitable closing techniques for the patient
-
patient was undergoing elective colorectal cancer surgery following full staging investigations including abdominal computed tomography (CT) scanning OR patient was undergoing emergency colorectal surgery because of a strong suspicion of colorectal cancer based on admission CT scanning.
At the point of surgical closure/randomisation:
-
midline abdominal incision (open or laparoscopic assisted/converted)
-
incision of ≥ 5 cm in length.
Exclusion criteria
At screening:
-
unable to provide informed consent.
At point of surgical closure/randomisation:
-
insertion of a mesh as part of abdominal closure
-
undergoing musculofascial flap closure of perineal defect in abdominoperineal wound closure.
Consent
Patients identified as eligible were approached by a member of the research team to discuss taking part in the study. Interested patients were given a patient information pack containing a letter of invitation, a consent form and a patient information sheet for the study. Patients were given time to review the information and ask questions. Patients who agreed to take part in the study were consented prior to their surgery (Figure 2). In 2018, an updated consent statement was implemented to meet with GDPR (General Data Protection Regulation) requirements. This was sent to each site for their records.
FIGURE 2.
Flow diagram of consent process. PIS, participant information sheet.
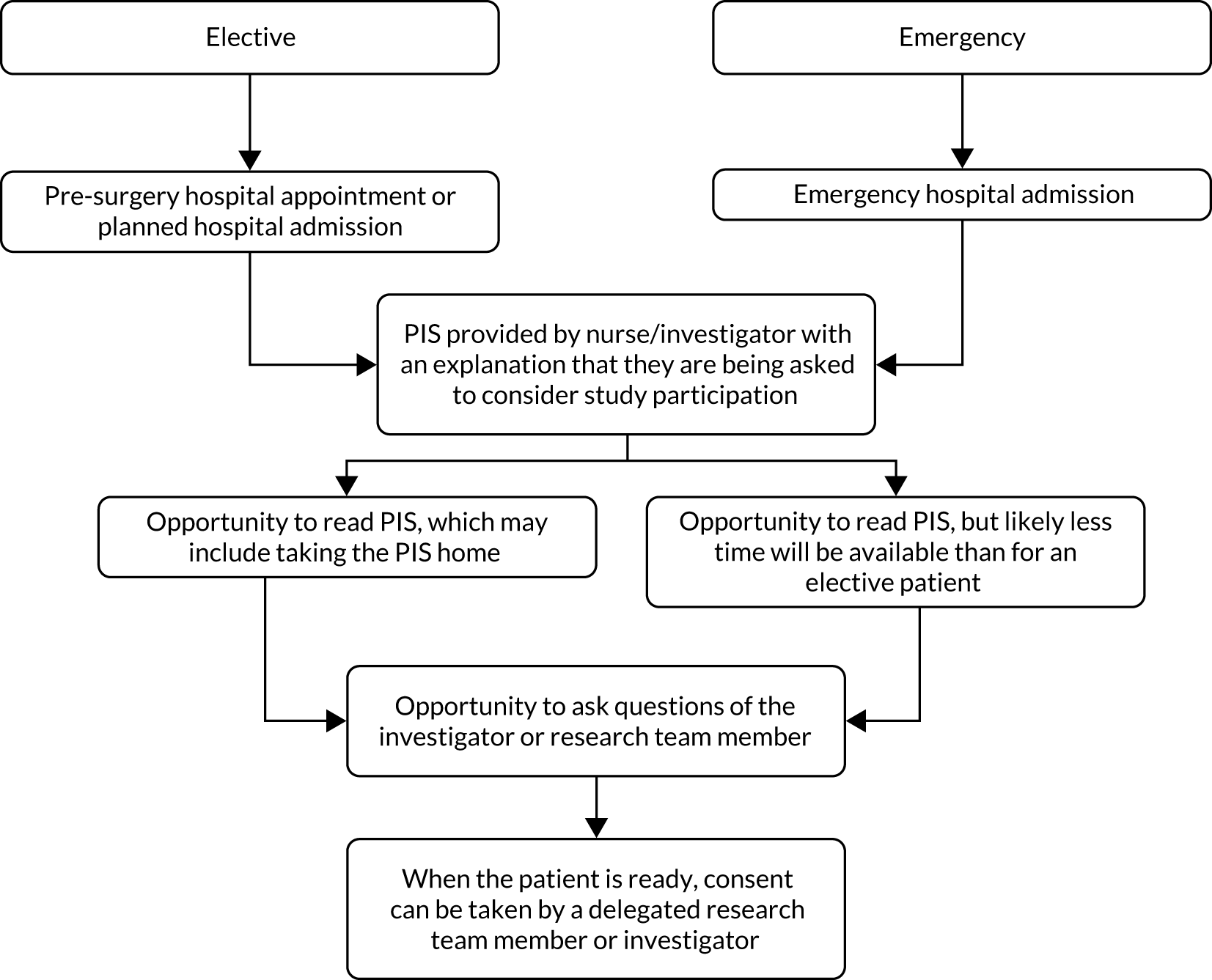
The mean time from signing the consent form to randomisation (surgery) was 2.5 days [standard deviation (SD) 7.2 days]. The maximum time was 91 days and the minimum time was 0 days. For patients undergoing elective surgery, the mean time from consent to randomisation (surgery) was 2.7 days, and for patients undergoing emergency surgery, the mean time from consent to randomisation was 0.4 days.
Study settings
The main study phase was conducted across 28 sites in England and Wales, with the University Hospital of Wales (Cardiff and Vale University Health Board) acting as the lead site and governance sponsor. Recruitment began in August 2014 and stopped in January 2018. A list of all participating sites and their level of recruitment can be found in Appendix 4, Table 30.
Interventions
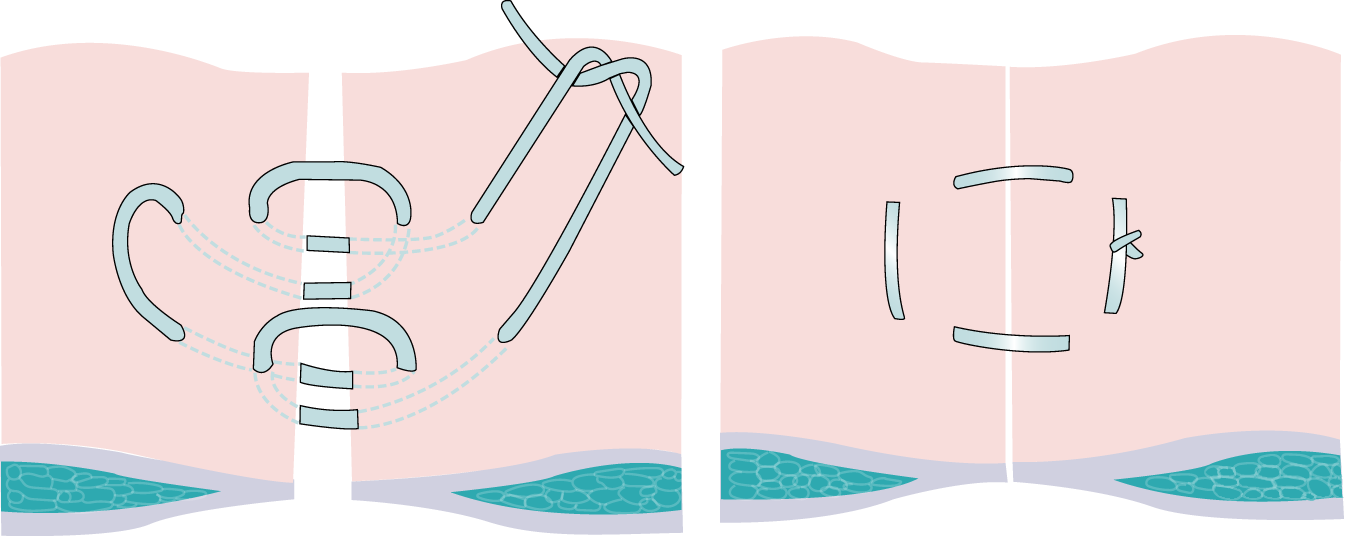
The Hughes abdominal repair technique involves the use of ‘near and far’ sutures to close the abdominal wall. The technique combines a standard mass closure [two-loop 1-polydioxanone (PDS) sutures] with a series of horizontal and two vertical mattress sutures within a single suture (1 nylon), theoretically distributing the load along the incision length as well as across it (Figure 3). Surgeons use loop 1-PDS for the mass closure element of the Hughes repair, with the multiple nylon sutures used for the ‘near and far’ sutures. The principles of the technique are to ensure that only sound normal tissues are used for repair, to use graduated tension for easy approximation and to use a monofilament nylon suture, which has the advantage of slipping easily through tissues to create a pulley system. 2 This was a standardised intervention arm with no variation permitted.
FIGURE 3.
Hughes repair. Reproduced with permission from Cornish et al. 2 © 2016 The Author(s). Open Access. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Standard care (control arm) comprised standard mass closure. This involves closing all layers of the abdominal wall (excluding the skin), usually using non-absorbable sutures, although ‘slow-resorbing’ sutures, such as PDS, are also widely used. Variation was allowed in this arm according to surgeon preference, but the technique used was recorded on the patient case report form (CRF).
All patients were given the same postoperative rehabilitation advice regardless of study arm.
Standardisation and training in intervention
Training in the Hughes repair (arm A) technique was undertaken by either the chief investigator or the co-investigator, both of whom were colorectal surgeons, as part of each site induction. This training involved both a 6-minute instructional video31 and a Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) presentation describing the background to the trial and the trial outline, followed by a practical clinical skills session with subsequent formal assessment. This practical clinical skills session involved either the chief investigator or the co-investigator demonstrating the Hughes repair technique on an abdominal wall simulation jig using the same sutures as used in the trial (loop 1-PDS and 1 nylon). Those being trained (surgical consultants and trainees) were then able to practise the technique until they were confident in it. A formal assessment in technique competence was then completed for each of those being trained at the site induction.
With regard to other members at each site who did not attend the site induction but were to be on the delegation log and performing abdominal wall closure as part of the trial, the site principal investigator was given responsibility for their training and formal assessment (once the principal investigator was deemed competent themselves).
Primary outcome
The primary outcome measure was the incidence of IH at the 1-year clinical examination.
The clinical presence of a hernia was assessed by an independent surgeon who was blinded to the closure technique wherever possible. In some centres, this examination was carried out by a nurse specialist who had appropriate accreditation for patient examination and was also blinded to the closure technique. The CRF included explicit details/instructions on how to carry out the examination. The presence of a hernia was detected as a reducible, palpable mass, usually with a cough impulse, which may cause pain or discomfort.
Secondary outcomes
-
Quality of life (QoL) measured using SF-12 and FACT-C. Questionnaires were administered at baseline and at 30 days, 6 months, 1 year and 2 years following randomisation. QoL was compared between arms (Hughes repair vs. standard mass closure) and between patients who developed IH and patients who did not develop IH.
-
Cost-effectiveness of Hughes repair compared with standard mass closure in the first year from the perspective of the NHS. Information regarding health-care resource use (including surgery-specific resources) was collected using a Client Service Receipt Inventory (CSRI) questionnaire at baseline, 6 months and 1 year.
-
Postoperative full thickness abdominal wall dehiscence (burst abdomen) within 30 days of surgery, as well as details of any repair surgery and closing sutures used.
-
Identification and characterisation of patient and surgical factors associated with an increased risk of developing an IH.
-
Prevalence of hernia at 1-year clinical examination.
Tertiary outcomes
-
Prevalence of clinically detectable IH at 5 years from surgery.
-
Effect of Hughes repair and standard mass closure over the 5 years from surgery.
-
Cost-effectiveness of Hughes repair compared with standard mass closure over 5 years from the perspective of health and social care.
-
Sensitivity and specificity of CT image identification over 2 years compared with clinical examination over 2–5 years from surgery.
-
Quality of life between patients with IH and patients with no IH in both arms over 5 years.
Sample size
A clinically important difference between the study arms was deemed to be a reduction in the IH rate from 30% in the standard mass closure arm to 20% in the Hughes repair arm. To detect this difference, it was calculated that a total of 640 patients would be required, providing 80% statistical power with a 5% level of significance. Assuming loss to follow-up of about 20% at 1 year, as seen in similar trials, HART aimed to recruit a total of 800 patients. A completed sample of 640 participants was calculated to yield 80% power of detecting (with a 5% significance level) a standardised difference (differences in means scaled by SD) of 0.225 in QoL (the principal secondary outcome).
Interim analyses and stopping guidelines
No interim analysis was planned in this study. Primary analysis was performed when all 1-year visits had been completed, data had been collated and the database had been locked. A separate analysis was carried out on data collected in year 2 of follow-up. The statistician received unblinded data after the database was locked for the final data analysis at year 1 and again at year 2.
The study followed the principle of allowing for early stopping were there to be a safety concern. During the study, the DMC and the study sponsor were responsible for stopping the study early had continuation of the trial been considered not in the patients’ best interest. Study data were reviewed by the DMC approximately every 6 months and reports were made to the Trial Steering Committee (TSC). The sponsor was ultimately responsible for trial progression after the consideration of recommendations by the DMC and TSC. The TSC also monitored recruitment and study progress to inform any decision to halt the study were it to be considered that the study had failed to deliver its objective as a result of delayed recruitment or lack of data besides any safety concern for the patients.
Randomisation and treatment allocation
We used an adaptive randomisation design to allocate the eligible patients to arms of similar sizes using an allocation ratio of 1 : 1. The sequence was created by a computerised random number generator and the allocation of participants was balanced by controlling the stratification variables. This dynamic randomisation stratified by operation type (elective or emergency) and site. 1 The principles of the randomisation design can be found in Russell et al. 32 The customised randomisation process was hosted by Sealed EnvelopeTM (London, UK; www.sealedenvelope.com; accessed March 2021), an independent company registered with the Information Commissioner’s Office, which provided a validated and fully automated 24-hour access telephone service for this dynamic randomisation. Randomisation was performed by the closing surgeon and took place as close as possible to commencement of closure. HART study surgeons were provided with training on the use of the telephone randomisation system.
Blinding
Study participants were informed prior to surgery that they would be randomised to receive one of the two closing techniques. Both the study participants and the post-surgical clinical assessors were blinded to the closure techniques. To attempt to maintain blinding, the method of closure was not documented in the operating notes and/or the clinical assessor was asked to complete the hernia examination (CRF) before reviewing the patient notes. The surgeon could not be blinded to the arm allocation because they were to undertake the closure as per randomisation. The data entry staff, the trial manager and the data manager were not blinded because of their central role in data collection and collation. The trial statisticians were blinded until the point of data lock during the final analysis stage.
Data
All data, including those on screening, eligibility, randomisation, surgery and follow-up, were collected on a patient CRF and managed using the MACRO database (Elsevier, Amsterdam, the Netherlands; www.elsevier.com/en-gb/solutions/veridata/macro; accessed March 2021), a secure electronic data capture system recording the information collected on CRFs and allowing rapid data extraction.
Radiology data (CT scans and related data) were managed in REDCap (Research Electronic Data Capture; www.project-redcap.org; accessed March 2021), a secure web application for data collection.
The data collection comprised pre-surgery data, intraoperative data and post-surgery follow-up data. For detailed information of the data collected in the CRF, see Report Supplementary Material 1.
Patients were assessed for eligibility using the study inclusion and exclusion criteria, and eligible patients who gave informed consent were recruited to the trial. Once they were recruited, and prior to surgery, patients’ baseline demographic information, medical history, surgical history, hernia status and QoL were collected. Intraoperative data were collected immediately post surgery and included the surgery type, grade of surgeon, details on the materials used and surgical outcomes.
Quality-of-life data were collected using the validated SF-1233 and FACT-C questionnaires. 34 Questionnaires were given to patients to complete while they were waiting for follow-up visits or, where necessary, were sent by post. The SF-12 is a short version of the SF-36 item health survey. It is a general health questionnaire capturing information on both physical and mental health across eight domains. FACT-C is a 37-item colorectal cancer-specific tool that adds a subset of 10 colorectal cancer-specific items to the original 27-item FACT-G (which is used for any cancer population). FACT-C consists of five subscales: physical well-being, social and family well-being, emotional well-being, functional well-being and the Colorectal Cancer Subscale. Both SF-12 and FACT-C can be self-administered by the patient or completed in an interview with the patient. Both tools are used widely in research, and a review of available generic and colorectal cancer-specific patient-reported outcome measure tools35 suggests that the SF-12 and FACT-C were appropriate choices for this study.
The MACRO database was locked for year 1 data collection in March 2019 and for year 2 data collection in April 2020. The REDCap database was locked for year 1 data in September 2019. The later datalock for REDCap was to allow all CT scan images and data to be transferred from individual sites via the Picture Archiving and Communications System (PACS) and entered into the database to be reviewed independently by two radiologists.
Following data lock, data were checked for completeness. Any outstanding queries were raised with individual sites and resolved, and SAE coding was completed and reviewed.
Year 1 data analysis included data collected at 12 months ± 2 months (i.e. 10–14 months) and year 2 data analysis similarly included a 2-month window either side (24 months ± 2 months; range 22–26 months). If a patient’s treatment course and clinical requirements meant that no CT was undertaken during this window, the CT scan closest to the 1-year time point was used. In total, 86.5% of CT scans were recorded within 14 months, with 95% recorded within 16 months.
Follow-up
Patients consented to be followed up at 30 days, 6 months and 1 year and annually thereafter. Postoperative follow-up data included data from clinical examination, CT scanning, patient-completed SF-12 and FACT-C quality-of-life forms and patient diaries. Full details of the data collected at individual follow-up points are reported in Appendix 4, Table 31.
Safety
As part of the monitoring of adverse events, information related to surgical site infection (SSI) and postoperative burst abdomen was collected. Colorectal cancer stage information was collected within 30 days post surgery.
Statistical methods
The trial analysis was carried out in accordance with the statistical analysis plan (SAP) using treatment allocated [intention to treat (ITT)], with participants in the arm allocated at randomisation. QoL data were collected using recognised and validated patient-reported outcome measure tools, namely the generic SF-12 and the condition-specific FACT-C.
Continuous variables that follow an approximately normal distribution were summarised using n (non-missing sample size), means, SDs, minimums and maximums. Skewed continuous variables were summarised using medians and interquartile ranges. Categorical variables were summarised using frequencies and percentages. All hypothesis testing was planned to be two-tailed with a 5% significance level and no adjustment for multiple testing.
Binary logistic regression analysis for the outcome variable IH was adjusted for all of the important baseline covariates. As this was a variable selection method, we employed a stepwise backward selection search starting with the full model, considering all of the adjusting covariates and removing the least significant, and repeating until only statistically significant covariates and the arm indicator remained in the model. The initial list of variables was arm indicator, age, gender, ethnicity, BMI, diabetes, any chemotherapy, radiotherapy, history of high alcohol use, history of chronic obstructive pulmonary disease (COPD), any IH present clinically, American Society of Anesthesiologists (ASA) class, whether the patient was from a high (≥ 50 enrolled participants) or a low (< 50 enrolled participants) recruiting site, baseline QoL measures [i.e. the SF-12 Physical Component Summary (PCS) and Mental Component Summary (MCS) and FACT-C score] and baseline Physiological and Operative Severity Score for understanding Mortality and Morbidity (POSSUM) score.
Data processing and analyses were performed using Stata® version 16 (StataCorp LP, College Station, TX, USA).
Baseline data
Baseline characteristics, including demographics, medical history, specific conditions (e.g. COPD and diabetes), chemotherapy, radiotherapy, abdominal aortic aneurysm, abdominal surgery history, current hernia status (incisional, non-incisional), baseline POSSUM score and QoL measures (FACT-C and SF-12), were summarised by treatment arm using appropriate descriptive methods for all randomised participants. Full details can be found in the statistical and health economic analysis plan (see Report Supplementary Material 2).
Primary end point
The primary outcome was the incidence of IH, and we tested the null hypothesis that there is no difference between the two surgical procedures (i.e. Hughes repair and standard mass closure) at 1 year (the primary end point). Patient and surgical factors associated with a risk of IH were identified via binary logistic regression models.
Secondary end points
Quality of life
Patient-reported outcome measures measure a patient’s health status or health-related QoL at any specific time point. They are collected using patient self-reported questionnaires about their symptoms, conditions and overall QoL. We used SF-12 and FACT-C and measured patients’ QoL at baseline, 30 days, 6 months and 1 year, and annually thereafter. The PCS score and MCS score were calculated from the SF-12. Scores on both of these range from 0 to 100, with higher scores indicating better physical activity and better mental health, respectively. The FACT-C score ranges from 0 to 136, with higher scores indicating better health. In accordance with licence agreements, we followed the standard manuals to score these outcome measures and used standard methods for dealing with missing responses when scoring. We scored the summary statistics of the PCS, MCS and FACT-C scores at all time points by arm and have explored whether or not there was any statistically significant difference at any time point between the arms. Following this descriptive analysis, we performed an adjusted analysis using mixed-model repeated measures to explore the changes in PCS, MCS and FACT-C scores over time (baseline to 1 month, 3 months, 6 months, 1 year and 2 years). We used all of the covariates as confounding factors in this adjusted analysis [e.g. age, gender, ethnicity, BMI, COPD, any baseline chemotherapy, radiotherapy, ASA class 2 and ≥ 3 (reference: class 1), smoking history and visit time (1 month, 6 months, 1 year and 2 years; reference: baseline)]. The outcome of this analysis enabled us to explore whether or not any changes in score (e.g. PCS) from baseline were significantly different by arm after adjustment for all of the factors considered.
Postoperative wound dehiscence
The postoperative full thickness wound dehiscence rate at 30 days following surgery was calculated for each arm and compared using Fisher’s exact chi-squared test with 95% confidence interval (CI) for any significance difference.
Prevalence of incisional hernia
The prevalence of IH was calculated at 1 year following surgery for each arm as the proportion of IH cases at 1 year of the total number of patients.
Sensitivity and specificity of computed tomography image compared with clinical examination at 1 year
Abdominal CT images (both preoperative and postoperative) were independently reviewed by two radiologists who were blinded to both the type of abdominal wall closure and the IH clinical finding. All cases of disagreement were resolved by consensus. The sensitivity, specificity and positive and negative predictive values of detecting IH by CT image were calculated with respect to clinical examination. The index test was CT scanning and the reference standard was clinical examination. The receiver operating characteristic (ROC) curve was calculated to provide the area under the curve and detect the best combination of sensitivity and specificity of CT image identification of hernias over 1 year compared with clinical examination data following surgery.
Safety data
All enrolled patients were included in the safety analysis. Safety data included adverse events, SAEs, serious adverse reactions and suspected unexpected serious adverse reactions. A number of adverse events and SAEs were expected in patients in this study, including lower respiratory tract infection, urinary tract infection, anastomotic leak, intra-abdominal sepsis, deep-vein thrombosis, pulmonary embolus, wound infection, SSI, wound breakdown, paralytic ileus, bleeding, myocardial infarction and stoma complications. All SAEs within 30 days of surgery and all deaths (regardless of time after report) were reported by the site staff and reviewed weekly by the chief investigator. Events reportable to the REC were identified during the weekly review and submitted by the chief investigator and trial manager (or delegate). Six-monthly reports were also provided to the REC. SAEs were summarised by the total number and the number of patients involved in each arm. SAEs were graded using the Clavien–Dindo classification36 and SAE types were classified using MedDRA. 37
Patient and public involvement
Two PPI representatives were involved in this study at any given point. Both representatives had previous experience of working with research groups and sat on the Trial Management Group for the project. A PPI representative sat also on the Steering Committee for the trial.
The PPI representatives were involved throughout this study from protocol development stage to study conclusion.
Patient and public representatives co-participated with the study team in areas such as designing patient materials and promoting the study to patients. PPI representatives worked with study team members to produce a patient information pack introducing the trial and explaining the aims of the research. The PPI representatives were involved with the development of the patient information sheets, patient questionnaires and contributed extensively to the writing of the Plain English summary for the study.
Patient and public representatives were in attendance at all Trial Management Group meetings and actively contributed to any discussions and decision-making processes. Over the course of the study, PPI representatives joined the clinical team at a number of public events giving presentations about public involvement in research to nurses and medical students. They were also involved with providing feedback to trial participants throughout the study and keeping sites up to date with trial progress via a study newsletter and designed a thank-you card giving information about the trial and details of where the results would be posted, which was sent to all surviving participants following the completion of the trial.
The PPI representatives were paid honoraria and out-of-pocket expenses in line with Health Care Research Wales and INVOLVE guidelines for attending meetings.
Patient representatives will have further input into the dissemination of the final results.
Chapter 3 Results
Participant flow
Recruitment
The first study site opened for recruitment in August 2014 and the last site opened in December 2016. A total of 28 sites opened for recruitment; one proposed site did not open and one site, although open for recruitment, did not recruit any patients to the study. Recruitment was planned to be completed by March 2017. However, owing to slower than anticipated recruitment, the recruitment phase of the study was extended and recruitment was completed in January 2018 (Figure 4). Detailed recruitment by site is reported in Appendix 3, Table 30.
FIGURE 4.
Cumulative recruitment.
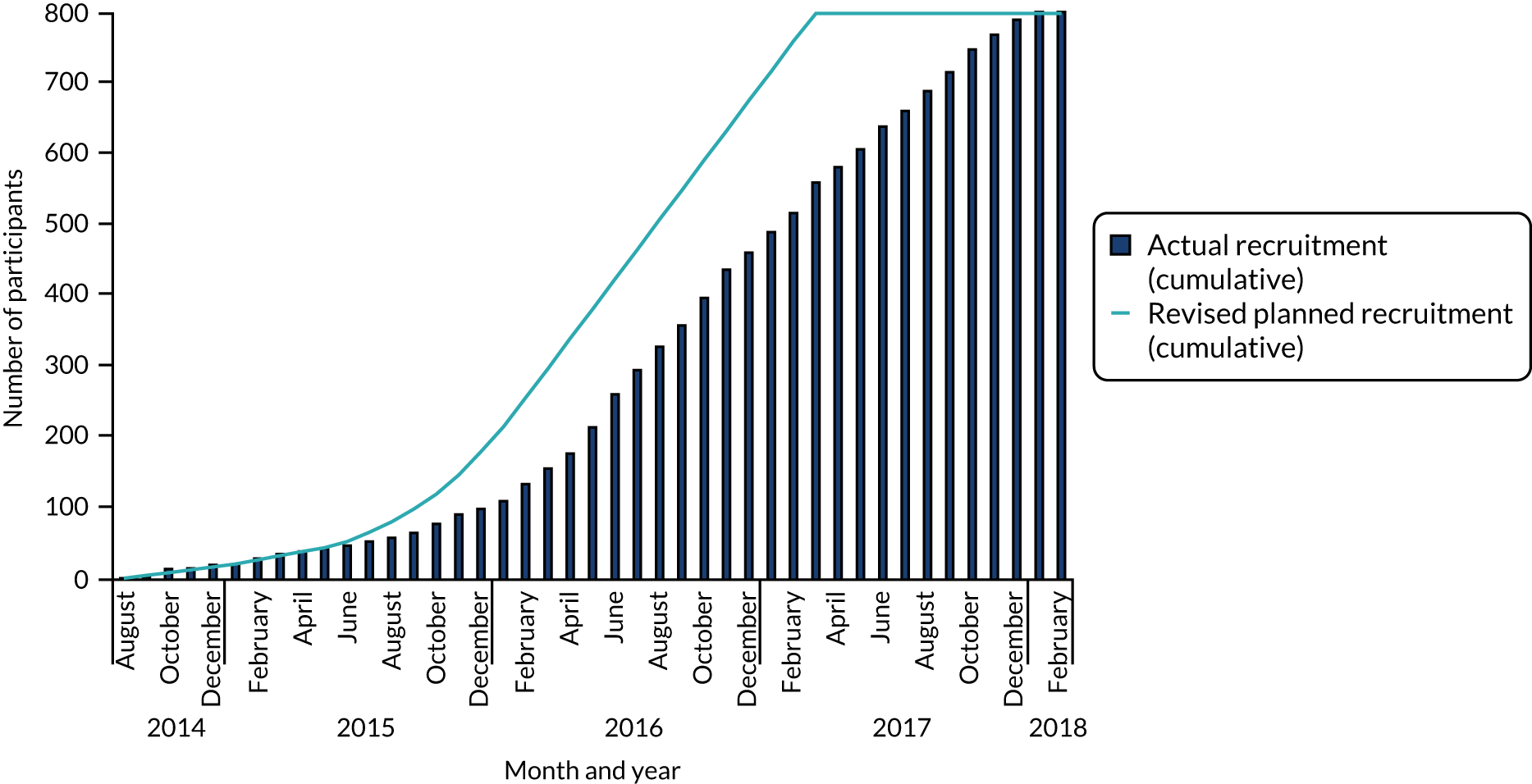
Screening and eligibility
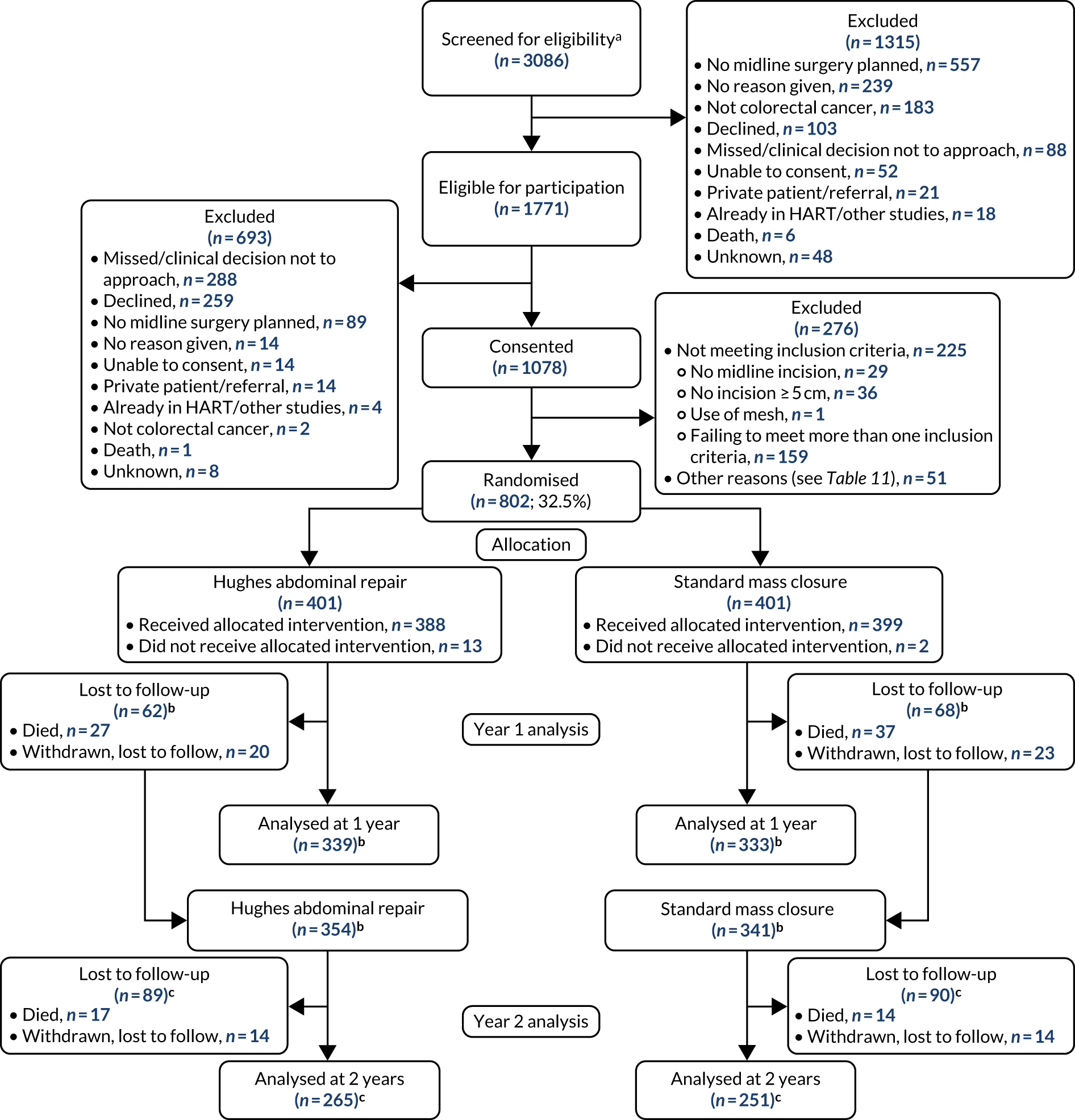
Screening logs showed that 3086 patients across 28 sites were screened for eligibility, with 1771 patients deemed to be eligible for study inclusion. Primary reasons for non-eligibility included not having a midline incision, not having colorectal cancer and declining to take part in the study. In a large number of cases, screening logs did not report a reason for non-eligibility (Figure 5).
FIGURE 5.
The CONSORT flow diagram of consented and randomised patients. a, Some sites included only eligible patients on their screening logs; therefore, the number screened is likely to be larger; b, a small number of patients (Hughes repair, n = 15; standard mass closure, n = 13) reported no year 1 data and could not be included in year 1 analysis, as no study discontinuation form had been completed. Of these 28 patients, eight were seen at year 2 (four in each arm), with five having no IH; c, 58 patients in Hughes repair arm and 62 patients in standard mass closure arm reported no year 2 data and could not be included in year 2 analysis. As no study discontinuation form had been completed, these patients could not be considered lost to follow-up in year 2.
Losses and exclusions
Eligible patients were asked to give their informed consent to take part in the study. Primary reasons for exclusion following eligibility assessment included not being approached for consent because of either nurse or physician unavailability, or declining to take part (see Figure 5).
Consent was obtained from 1078 patients and, of these, a total of 802 were randomised at the point of surgical incision closure (standard mass closure, n = 401; Hughes repair, n = 401). Most of the 276 participants excluded at this stage no longer satisfied the inclusion criteria, with 159 failing to meet more than one inclusion criterion (see Figure 5). Other reasons for the non-randomisation of consented patients are categorised in Table 2, with full details in Appendix 4, Table 32.
| Category | Count (n) |
|---|---|
| Logistical issues [e.g. operation took too long/complications in theatre/problems with surgery times (delayed/brought forward/busy)/equipment unavailable] | 18 |
| Surgeon decision | 17 |
| Patient eligibility | 14 |
| Reason not stated | 1 |
| Consent withdrawn | 1 |
| Total | 51 |
Following randomisation, 15 patients (Hughes repair, n = 13; standard mass closure, n = 2) did not receive their allocated treatment.
A total of 107 patients were lost to follow-up at year 1 (Hughes repair, n = 47; standard mass closure, n = 60). Of these, 64 died (Hughes repair, n = 27; standard mass closure, n = 37) and 43 withdrew (Hughes repair, n = 20; standard mass closure, n = 23).
Numbers analysed
A total of 672 patients were included in the year 1 analysis (Hughes repair, n = 339; standard mass closure, n = 333) and 516 patients were included in the year 2 analysis (Hughes repair, n = 265; standard mass closure, n = 251) (see Figure 5). For a number of patients (Hughes repair, n = 15; standard mass closure, n = 8), data were available for year 2 but no year 1 results were recorded and no discontinuation information was available and, therefore, these patients were not considered in the year 1 analysis but were included in the year 2 analysis.
Missing data
The study team made every effort to capture a full data set. The frequency of missing data is summarised for each variable. The statistical analysis plan and study protocol necessitated that if data were not missing completely at random, then the trial statistician and chief investigator would discuss the findings and agree on an approach to handle the missing data. The trial statistician identified no reason to indicate that data were not missing completely at random; therefore, protocol procedures for handling data not missing completely at random were not needed. For the key primary outcome, participants with no follow-up data were automatically excluded from the analysis. We scored the QoL measures (i.e. SF-12 and FACT-C) using their scoring algorithms. After this scoring, 20% of data were missing in the outcome scores of these QoL measures at baseline. For those missing, we adopted a multiple imputation method to impute the outcome scores outcome scores.
Short Form questionnaire-12 items questionnaire in the pilot phase
It was observed from SF-12 responses that 33 patients in the initial pilot phase were given a hybrid version of SF-12 version 1 and version 2 to complete for a total of 76 visits (33 visits at baseline, 26 at 1 month and 17 at 6 months). This affected four questions (3a, 3b, 4a and 4b), which offered two response categories from version 1 (i.e. yes and no) instead of five categories from version 2 (ranging from ‘all the time’ to ‘none of the time’). To resolve this issue, an imputation method for estimating the responses based on the distribution of their other responses was adopted.
The scoring software Procore 1.4 (https://support.procore.com; accessed March 2021) calculates SF-12 scores based on eight domains (i.e. general health perceptions, physical functioning, role limitations owing to physical health, role limitations owing to emotional problem, bodily pain, mental health, vitality and social functioning). For missing data, the maximum data recovery method (missing data estimation) from Procore was used. Under this missing data estimation, the software imputes a scale value if at least one item in the scale has valid data. PCS scores are calculated when the physical functioning scale and at least six of the remaining seven scale values are known or can be estimated. For MCS, the mental health scale and at least six of the remaining seven scale values must be known or able to be estimated.
FACT-C questionnaire
We followed the method for scoring FACT-C as per the FACT-C scoring manual, as described in their guidelines. 38 In accordance with the manual, FACT-C outcome scores were calculated only for patients who responded to at least 50% of the questions at a specific time point.
Baseline data
A summary of the baseline data for the study cohort is presented in Table 3 and a detailed summary of intraoperative characteristics is given in Table 4. The two arms were well balanced, with no differences observed between the arms in any of the baseline characteristics.
| Characteristic | Hughes repair (N = 401) | Standard mass closure (N = 401) | Total (N = 802) |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 69.0 (10.7) | 68.1 (12.7) | 68.5 (11.7) |
| Age (years) (categories), n (%) | |||
| < 40 | 3 (0.8) | 12 (3.0) | 15 (1.9) |
| 40–50 | 10 (2.5) | 25 (6.2) | 35 (4.4) |
| 50–60 | 63 (15.7) | 58 (14.5) | 121 (15.1) |
| 60–70 | 127 (31.7) | 96 (23.9) | 223 (27.8) |
| 70–80 | 129 (32.2) | 132 (32.9) | 261 (32.5) |
| > 80 | 69 (17.2) | 78 (19.5) | 147 (18.3) |
| Gender, n (%) | |||
| Male | 262 (65.3) | 247 (61.6) | 509 (63.5) |
| Female | 139 (34.7) | 154 (38.4) | 293 (36.5) |
| Ethnicity, n (%) | |||
| White | 385 (96.0) | 386 (96.3) | 771 (96.1) |
| Black | 7 (1.8) | 5 (1.3) | 12 (1.5) |
| Asian | 6 (1.5) | 7 (1.8) | 13 (1.6) |
| Other | 3 (0.8) | 3 (0.8) | 6 (0.8) |
| BMI (kg/m2) | |||
| Mean (SD) | 28.0 (5.3) | 27.7 (5.6) | 27.8 (5.4) |
| Missing | 4 (1.0) | 5 (1.3) | 9 (1.1) |
| BMI (kg/m2) (categories), n (%) | |||
| < 18.5 (low or underweight) | 10 (2.5) | 7 (1.8) | 17 (2.1) |
| ≥ 18.5 to < 25 (healthy) | 106 (26.4) | 122 (30.4) | 228 (28.4) |
| ≥ 25 to < 30 (overweight) | 157 (39.2) | 157 (39.2) | 314 (39.2) |
| ≥ 30 to < 40 (obese) | 114 (28.4) | 97 (24.2) | 211 (26.3) |
| ≥ 40 (severely obese) | 14 (3.5) | 18 (4.50) | 32 (4.0) |
| Diabetes mellitus, n (%) | |||
| Yes | 65 (16.2) | 68 (17.0) | 133 (16.6) |
| No | 336 (83.8) | 333 (83.0) | 669 (83.4) |
| Neoadjuvant chemotherapy, n (%) | |||
| Yes | 43 (10.7) | 34 (8.5) | 77 (9.6) |
| No | 358 (89.3) | 367 (91.5) | 725 (90.4) |
| Neoadjuvant radiotherapy, n (%) | |||
| Yes | 38 (9.5) | 32 (8.0) | 70 (8.7) |
| No | 363 (90.5) | 369 (92.0) | 732 (91.3) |
| COPD, n (%) | |||
| Yes | 50 (12.5) | 62 (15.5) | 112 (14) |
| No | 351 (87.5) | 339 (84.5) | 690 (86.0) |
| Abdominal aortic aneurysm, n (%) | |||
| Yes | 4 (1.0) | 2 (0.5) | 6 (0.8) |
| No | 397 (99.0) | 399 (99.5) | 796 (99.3) |
| Smoking, n (%) | |||
| Yes | 31 (7.7) | 37 (9.2) | 68 (8.5) |
| No | 228 (56.9) | 218 (54.4) | 446 (55.6) |
| Ex-smoker | 142 (35.4) | 144 (35.9) | 286 (35.7) |
| Missing | 0 | 2 (0.5) | 2 (0.3) |
| High alcohol use, n (%) | |||
| Yes | 25 (6.2) | 25 (6.2) | 50 (6.2) |
| No | 376 (93.8) | 375 (93.5) | 751 (93.6) |
| Missing | 0 | 1 (0.3) | 1 (0.1) |
| ASA class, n (%) | |||
| 1 | 52 (13.0) | 51 (12.7) | 103 (12.8) |
| 2 | 223 (55.6) | 233 (58.1) | 456 (56.9) |
| 3 | 121 (30.2) | 110 (27.4) | 231 (28.8) |
| 4 | 5 (1.3) | 5 (1.3) | 10 (1.3) |
| Missing | 0 | 2 (0.5) | 2 (0.3) |
| QoL measures | |||
| PCS score | |||
| Mean (SD) | 43.6 (5.5) | 43.7 (5.5) | 43.7 (5.5) |
| Missing | 78 (19.4) | 82 (20.4) | 160 (20.0) |
| MCS score | |||
| Mean (SD) | 52.5 (11.9) | 52.9 (12.1) | 52.7 (12.0) |
| Missing | 81 (20.2) | 78 (19.4) | 159 (19.8) |
| FACT-C | |||
| Mean (SD) | 70.5 (9.9) | 71.7 (10.0) | 71.1 (10.0) |
| Missing | 88 (22.0) | 90 (22.4) | 178 (22.2) |
| Abdominal surgery history, n (%) | |||
| Yes | 167 (41.7) | 169 (42.1) | 336 (41.9) |
| No | 234 (58.4) | 232 (57.9) | 466 (58.1) |
| Current hernia status | |||
| Incisional hernias, n (%) | |||
| Yes | 9 (2.2) | 3 (0.8) | 12 (1.5) |
| No | 392 (97.8) | 396 (98.8) | 788 (98.3) |
| Missing | 0 | 2 (0.5) | 2 (0.3) |
| Non-incisional hernias, n (%) | |||
| Yes | 35 (8.7) | 28 (7.0) | 63 (7.9) |
| No | 366 (91.3) | 371 (92.5) | 737 (91.9) |
| Missing | 0 | 2 (0.5) | 2 (0.3) |
| Characteristic | Hughes repair (N = 401) | Standard mass closure (N = 401) | Total (N = 802) |
|---|---|---|---|
| Grade of surgeon performing abdominal wall closure, n (%) | |||
| Surgical trainee (ST5 or below) | 31 (7.7) | 47 (11.7) | 78 (9.7) |
| Surgical trainee (ST6–8) | 105 (26.2) | 161 (40.2) | 266 (33.2) |
| Consultant | 262 (65.3) | 187 (46.6) | 449 (56.0) |
| Missing | 3 (0.8) | 6 (1.5) | 9 (1.1) |
| ASA class, n (%) | |||
| 1 | 52 (13.0) | 51 (12.7) | 103 (12.8) |
| 2 | 223 (55.6) | 233 (58.1) | 456 (56.9) |
| 3 | 121 (30.2) | 110 (27.4) | 231 (28.8) |
| 4 | 5 (1.3) | 5 (1.3) | 10 (1.3) |
| Missing | 0 | 2 (0.5) | (0.3) |
| Operation performed, n (%) | |||
| Abdominoperineal resection | 19 (4.7) | 13 (3.2) | 32 (4.0) |
| Anterior resection | 130 (32.4) | 122 (30.4) | 252 (31.4) |
| Hartmann’s procedure | 22 (5.5) | 26 (6.5) | 48 (6.0) |
| Left hemicolectomy | 19 (4.7) | 18 (4.5) | 37 (4.6) |
| Right hemicolectomy | 131 (32.7) | 147 (36.7) | 278 (34.7) |
| Extended right hemicolectomy | 24 (6.0) | 34 (8.5) | 58 (7.2) |
| Panproctocolectomy | 3 (0.8) | 1 (0.3) | 4 (0.5) |
| Subtotal colectomy | 9 (2.2) | 11 (2.7) | 20 (2.5) |
| Sigmoid colectomy | 17 (4.2) | 10 (2.5) | 27 (3.4) |
| Other | 27 (6.7) | 19 (4.7) | 46 (5.7) |
| Stoma formed, n (%) | |||
| No stoma | 259 (64.6) | 277 (69.1) | 536 (66.8) |
| End ileostomy | 8 (2.0) | 8 (2.0) | 16 (2.0) |
| Loop ileostomy | 71 (17.7) | 64 (16.0) | 135 (16.8) |
| End colostomy | 49 (12.2) | 49 (12.2) | 98 (12.2) |
| Loop colostomy | 3 (0.8) | 3 (0.8) | 6 (0.8) |
| Other | 11 (2.7) | 0 | 11 (1.4) |
| Mode of operation, n (%) | |||
| Open | 171 (42.6) | 151 (37.7) | 322 (40.2) |
| Laparoscopic | 125 (31.2) | 127 (31.7) | 252 (31.4) |
| Laparoscopic assisted | 35 (8.7) | 64 (16.0) | 99 (12.3) |
| Laparoscopic converted to open (midline incision) | 70 (17.5) | 59 (14.7) | 129 (16.1) |
| Colorectal cancer resected, n (%) | |||
| Yes | 399 (99.5) | 400 (99.8) | 799 (99.6) |
| No | 2 (0.5) | 1 (0.2) | 3 (0.4) |
| Intraoperative blood transfusion | |||
| Yes, n (%) (mean number of units transfused) | 21 (5.2) (2.0) | 14 (3.5) (1.6) | 35 (4.4) |
| No, n (%) | 380 (94.8) | 387 (96.5) | 767 (95.6) |
| Intraoperative complications, n (%) | |||
| Yes | 20 (5.0) | 10 (2.5) | 30 (3.8) |
| No | 381 (95.0) | 390 (97.3) | 771 (96.1) |
| Missing | 0 | 1 (0.3) | 1 (0.1) |
| Wound closed as per randomisation, n (%) | |||
| Yes | 388 (96.8) | 399 (99.5) | 787 (98.1) |
| No | 13 (3.2) | 2 (0.5) | 15 (1.9) |
| Anti-adhesive agent used intraoperatively, n (%) | |||
| Yes | 7 (1.8) | 6 (1.5) | 13 (1.6) |
| No | 391 (97.5) | 390 (97.3) | 781 (97.4) |
| Missing | 3 (0.8) | 5 (1.3) | 8 (1.0) |
| Final length of midline incision (cm) | |||
| Mean (SD) | 15.5 (8.4) | 14.7 (8.2) | 15.1 (8.3) |
| Missing | 9 (2.2) | 20 (5.0) | 29 (3.6) |
| Skin closure method, n (%) | |||
| Surgical clips | 162 (40.4) | 144 (35.9) | 306 (38.2) |
| Subcuticular absorbable suture(s) | 238 (59.4) | 250 (62.3) | 488 (60.9) |
| Interrupted sutures | 1 (0.2) | 2 (0.5) | 3 (0.4) |
| Other | 0 | 4 (1.0) | 4 (0.5) |
| Missing | 0 | 1 (0.3) | 1 (0.1) |
| Total time taken for the procedure (minutes) | |||
| Mean (SD) | 202.9 (90.9) | 183.4 (75.1) | 193.2 (83.9) |
| Missing | 4 (1.0) | 8 (2.0) | 12 (1.5) |
| Time taken for the fascial closure (minutes) | |||
| Mean (SD) | 22.0 (9.7) | 13.2 (6.7) | 17.6 (9.5) |
| Missing | 8 (2.0) | 19 (4.7) | 27 (3.4) |
| Postoperative length of stay (days) | |||
| Mean (SD) | 10.6 (11.5) | 9.8 (12.4) | 10.2 (11.9) |
| Missing | 9 (2.2) | 7 (1.7) | 16 (2.0) |
| Level of postoperative care, n (%) | |||
| ITU | 19 (4.7) | 9 (2.2) | 28 (3.5) |
| HDU | 96 (23.9) | 100 (24.9) | 196 (24.4) |
| Ward/PACU | 286 (71.3) | 292 (72.8) | 578 (72.1) |
| POSSUM score | |||
| Physiological score | |||
| Mean (SD) | 17.5 (4.2) | 17.5 (4.3) | 17.5 (4.3) |
| Missing | 8 (2.0) | 11 (2.7) | 19 (2.4) |
| Operative severity score | |||
| Mean (SD) | 13.0 (3.9) | 12.5 (3.3) | 12.7 (3.6) |
| Missing | 7 (1.8) | 11 (2.7) | 18 (2.2) |
| Overall POSSUM score | |||
| Mean (SD) | 8.3 (7.9) | 7.8 (7.8) | 8.0 (7.8) |
| Missing | 10 (2.5) | 17 (4.2) | 27 (3.4) |
Primary outcome
Incidence of incisional hernia
The incidence of IH detected by clinical examination alone, by study arm, is shown in Table 5. Although the primary outcome was incidence of IH at year 1, the results for year 2 are presented alongside for comparison. Hughes repair was associated with a lower IH rate on clinical examination at 1 year [14.8% (n = 50) in the Hughes repair arm vs. 17.1% (n = 57) in the standard mass closure arm]; however, this difference did not reach statistical significance [odds ratio (OR) 0.84, 95% CI 0.55 to 1.27; p = 0.40]. The incidence of IH was also lower in the Hughes repair arm in year 2, but, again, the difference was not statistically significant (OR 0.86, 95% CI 0.59 to 1.25; p = 0.43). Of the 107 patients who experienced IH in year 1, 20 (18.7%), underwent hernia repair in year 2 (nine in the Hughes repair arm and 11 in the standard mass closure arm).
| IH | Hughes repair arm, n (%) | Standard mass closure arm, n (%) | Odds ratio (95% CI) | p-value |
|---|---|---|---|---|
| 1 year | ||||
| Sample size | 339 | 333 | ||
| Yes | 50 (14.8) | 57 (17.1) | 0.84 (0.55 to 1.27) | 0.402 |
| No | 289 (85.3) | 276 (82.9) | ||
| Over 2 years | ||||
| Sample size | 272 | 264 | ||
| Yes | 78 (28.7) | 84 (31.8) | 0.86 (0.59 to 1.25) | 0.429 |
| No | 194 (71.3) | 180 (68.2) | ||
Identification of risk factors for incisional hernia
Logistic regression analysis (Table 6), adjusted for baseline characteristics, suggests that increased age, male sex, increased BMI, higher POSSUM score, preoperative radiotherapy and lower physical activity (i.e. SF-12 PCS) are indicators of an increased odds of IH. The odds of IH were significantly increased with increasing age at year 1 and over year 2. The OR for age at year 1 was 1.03, indicating a 3% increased odds of an IH with a 1-year increase in age. Similar outcomes can be observed at year 2 (see Table 6).
| Parameter | Year 1 | Year 2 | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Arms: Hughes repair | 0.73 (0.48 to 1.12) | 0.165 | 0.79 (0.54 to 1.17) | 0.235 |
| Age | 1.03 (1.01 to 1.05) | 0.009 | 1.02 (1.00 to 1.04) | 0.023 |
| Sex: male | 1.72 (1.07 to 2.77) | 0.027 | 1.48 (0.98 to 2.27) | 0.070 |
| BMI | 1.05 (1.01 to 1.10) | 0.053 | 1.07 (1.02 to 1.10) | 0.002 |
| Radiotherapy use | 3.80 (1.37 to 9.45) | 0.010 | 3.30 (1.20 to 9.02) | 0.020 |
| POSSUM score | 0.99 (0.85 to 1.03) | 0.692 | 1.03 (1.00 to 1.08) | 0.034 |
| SF-12: PCS (baseline) | 0.96 (0.92 to 1.00) | 0.054 | 0.97 (0.93 to 1.01) | 0.096 |
| Emergency admission | 2.46 (1.07 to 5.68) | 0.034 | 2.16 (0.90 to 5.19) | 0.084 |
Male sex was found to be associated with significantly increased odds of IH at year 1 (p = 0.0266) but not at year 2 (p = 0.077). At year 1, male patients were 72% more likely than female patients to have IH.
At year 1, BMI was not significantly associated with increased odds of IH (p = 0.05); however, it strongly affected the outcome of IH at year 2 (p = 0.00202).
Radiotherapy at baseline was a strong indicator of the odds of IH at year 1 and over year 2. The odds of having IH for someone having baseline radiotherapy were 3.88 (p = 0.01) times higher at year 1 and 3.33 (p = 0.02) times higher at year 2. The results relating to radiotherapy should be considered cautiously because of the wider CIs with the estimates (see Table 6).
The SF-12 PCS score remained in the final model of the stepwise regression at both year 1 and year 2 but with a non-significant outcome. The POSSUM score did not remain in the final step of the logistic regression model step for year 1; however, in the year 2 model, baseline POSSUM score seems to significantly affect the outcome of IH over 2 years. With a 1% increase in risk of death or POSSUM score at baseline, there was a 33% increase in odds of IH over 2 years.
Admission type also seems to have a statistically significant effect on the risk of having IH at year 1. The odds of having an IH at year 1 was 2.46 (p = 0.034) higher for patients admitted via emergency route. However, the effect of admission type on having an IH at year 2 is not statistically significant.
There were 662 observations in the final stepwise logistic regression model at year 1 and 529 observations in the final model at year 2.
Secondary outcomes
Quality of life
All of the QoL measures (i.e. SF-12 PCS, SF-12 MCS and FACT-C scores) were well balanced between the arms at baseline. No significant difference in score was observed between the arms for either SF-12 or FACT-C at any time point (Tables 7 and 8).
| QoL measure | Hughes repair arm | Standard mass closure arm | Total | Mean difference (I – C) (95% CI) | p-value (t-test) |
|---|---|---|---|---|---|
| SF-12: PCS | |||||
| Baseline | |||||
| Mean (SD) | 43.6 (5.5) | 43.7 (5.5) | 43.7 (5.5) | –0.09 (–0.93 to 0.76) | 0.8 |
| Missing, n (%) | 82 (20.5) | 78 (19.5) | 160 (20.0) | ||
| 30 days | |||||
| Mean (SD) | 41.5 (5.7) | 40.8 (5.8) | 41.2 (5.7) | 0.73 (–0.19 to 1.64) | 0.1 |
| Missing, n (%) | 108 (26.9) | 108 (26.9) | 216 (26.9) | ||
| 6 months | |||||
| Mean (SD) | 42.8 (5.0) | 42.9 (4.8) | 42.8 (4.9) | –0.08 (–0.88 to 0.72) | 0.8 |
| Missing, n (%) | 101 (25.2) | 121 (30.2) | 222 (27.7) | ||
| 1 year | |||||
| Mean (SD) | 43.1 (4.4) | 43.7 (4.5) | 43.4 (4.4) | –0.56 (–1.30 to 0.18) | 0.1 |
| Missing, n (%) | 112 (27.9) | 134 (33.4) | 246 (30.7) | ||
| 2 years | |||||
| Mean (SD) | 43.0 (4.3) | 43.2 (4.4) | 43.1 (4.3) | –0.13 (–0.95 to 0.68) | 0.75 |
| Missing, n (%) | 178 (44.4) | 187 (46.6) | 365 (45.5) | ||
| SF-12: MCS | |||||
| Baseline | |||||
| Mean (SD) | 52.5 (11.9) | 52.9 (12.1) | 52.7 (12.0) | –0.41 (–2.2 to 1.50) | 0.7 |
| Missing, n (%) | 81 (20.2) | 78 (19.4) | 159 (19.8) | ||
| 30 days | |||||
| Mean (SD) | 46.5 (13.2) | 48.2 (12.8) | 47.30 (13.0) | –1.77 (–3.87 to 0.33) | 0.1 |
| Missing, n (%) | 107 (26.7) | 106 (26.4) | 213 (26.6) | ||
| 6 months | |||||
| Mean (SD) | 51.50 (13.3) | 51.7 (12.3) | 51.6 (12.8) | –0.15 (–2.23 to 1.94) | 0.9 |
| Missing, n (%) | 101 (25.2) | 121 (30.2) | 222 (27.7) | ||
| 1 year | |||||
| Mean (SD) | 53.9 (12.1) | 53.7 (11.7) | 53.8 (11.9) | 0.21 (–1.77 to 2.18) | 0.8 |
| Missing, n (%) | 112 (27.9) | 133 (33.2) | 245 (30.5) | ||
| 2 years | |||||
| Mean (SD) | 54.0 (12.0) | 54.0 (12.8) | 54 (12.4) | 0.08 (–2.25 to 2.42) | 0.95 |
| Missing, n (%) | 178 (44.4) | 187 (46.6) | 365 (45.5) | ||
| Time point | Hughes repair arm | Standard mass closure arm | Total | Mean difference (I – C) (95% CI) | p-value (t-test) |
|---|---|---|---|---|---|
| Baseline | |||||
| Mean (SD) | 70.6 (9.9) | 71.7 (10.0) | 71.08 (10.0) | –1.21 (–2.78 to 0.35) | 0.13 |
| Missing, n (%) | 88 (21.9) | 90 (22.4) | 178 (22.2) | ||
| 30 days | |||||
| Mean (SD) | 66.8 (8.9) | 65.9 (8.8) | 66.3 (8.9) | 0.86 (–0.59 to 2.32) | 0.25 |
| Missing, n (%) | 116 (28.9) | 116 (28.9) | 232 (28.9) | ||
| 6 months | |||||
| Mean (SD) | 66.3 (9.9) | 66.6 (9.3) | 66.5 (9.7) | –0.33 (–1.92 to 1.25) | 0.68 |
| Missing, n (%) | 107 (26.7) | 125 (31.2) | 232 (28.9) | ||
| 1 year | |||||
| Mean (SD) | 67.6 (8.7) | 67.3 (9.7) | 67.4 (9.2) | 0.34 (–1.21 to 1.89) | 0.66 |
| Missing, n (%) | 124 (30.9) | 133 (33.2) | 257 (32.0) | ||
| 2 years | |||||
| Mean (SD) | 67.0 (8.9) | 68.2 (8.9) | 67.6 (8.9) | –1.14 (–2.83 to 0.54) | 0.18 |
| Missing, n (%) | 171 (42.6) | 195 (48.6) | 366 (45.6) | ||
Tables 9 and 10 outline the QoL scores in patients with and patients without IH (regardless of their allocated arm). Patients with an IH at year 1 had significantly lower mean PCS scores than patients without an IH (p = 0.03) at baseline. However, this difference was not observed at other time points. No statistically significant differences were observed in mean SF-12 MCS (see Table 7) or FACT-C (see Table 8) scores when comparing patients with and patients without an IH at any time point.
| QoL measure | Patients with IH (N = 107) | Patients without IH (N = 565) | Total (N = 672) | Mean difference (IH – no IH) (95% CI); p-value (t-test) |
|---|---|---|---|---|
| SF-12: PCS | ||||
| Baseline | ||||
| Mean (SD) | 42.6 (5.6) | 44.0 (5.4) | 43.8 (5.4) | –1.40 (–2.70 to –0.11); 0.03 |
| Missing, n (%) | 27 (25.2) | 96 (17.0) | 123 (18.3) | |
| 30 days | ||||
| Mean (SD) | 41.2 (5.9) | 41.1 (5.6) | 41.1 (5.7) | 0.13 (–1.18 to 1.44); 0.8 |
| Missing, n (%) | 21 (19.6) | 117 (20.7) | 138 (20.5) | |
| 6 months | ||||
| Mean (SD) | 42.4 (4.9) | 43.0 (4.9) | 42.9 (4.9) | –0.59 (–1.73 to 0.54); 0.3 |
| Missing, n (%) | 23 (21.5) | 111 (19.7) | 134 (19.9) | |
| 1 year | ||||
| Mean (SD) | 43.8 (4.6) | 43.3 (4.4) | 43.4 (4.4) | 0.51 (–0.50 to 1.52); 0.3 |
| Missing, n (%) | 19 (17.8) | 108 (19.1) | 127 (18.90) | |
| SF-12: MCS | ||||
| Baseline | ||||
| Mean (SD) | 53.7 (11.4) | 53.2 (11.6) | 53.3 (11.6) | 0.48 (–2.30 to 3.23); 0.7 |
| Missing, n (%) | 27 (25.2) | 95 (16.8) | 122 (18.1) | |
| 30 days | ||||
| Mean (SD) | 47.3 (12.2) | 47.7 (12.9) | 47.6 (12.8) | –0.46 (3.42 to 2.50); 0.8 |
| Missing, n (%) | 21 (19.6) | 114 (20.2) | 135 (20.1) | |
| 6 months | ||||
| Mean (SD) | 52.1 (12.7) | 52.2 (12.4) | 52.2 (12.4) | –0.11 (–3.01 to 2.78); 0.9 |
| Missing, n (%) | 23 (21.5) | 111 (19.6) | 134 (19.9) | |
| 1 year | ||||
| Mean (SD) | 52.8 (12.1) | 53.9 (11.8) | 53.7 (11.9) | –1.01 (–3.73 to 1.70); 0.5 |
| Missing, n (%) | 19 (17.8) | 107 (18.9) | 126 (18.8) | |
| Time point | Patients with IH (N = 107) | Patients without IH (N = 565) | Total (N = 672) | Mean difference (I – C), 95% CI; p-value (t-test) |
|---|---|---|---|---|
| Baseline | ||||
| Mean (SD) | 70.9 (8.5) | 71.6 (9.7) | 71.5 (9.5) | –0.74 (–30.2 to 1.55); 0.5 |
| Missing, n (%) | 29 (27.1) | 110 (19.5) | 139 (20.7) | |
| 30 days | ||||
| Mean (SD) | 66.7 (7.7) | 66.2 (9.1) | 66.3 (8.9) | 0.42 (–1.65 to 2.50); 0.7 |
| Missing, n (%) | 22 (20.6) | 132 (23.4) | 154 (22.9) | |
| 6 months | ||||
| Mean (SD) | 65.9 (9.8) | 66.7 (9.6) | 66.6 (9.6) | –0.85 (–3.13 to 1.42); 0.5 |
| Missing, n (%) | 25 (23.4) | 119 (21.1) | 144 (21.4) | |
| 1 year | ||||
| Mean (SD) | 67.5 (7.8) | 67.4 (9.5) | 67.4 (9.2) | 0.08 (–2.10 to 2.25); 0.9 |
| Missing, n (%) | 24 (22.4) | 113 (20.0) | 137 (20.4) | |
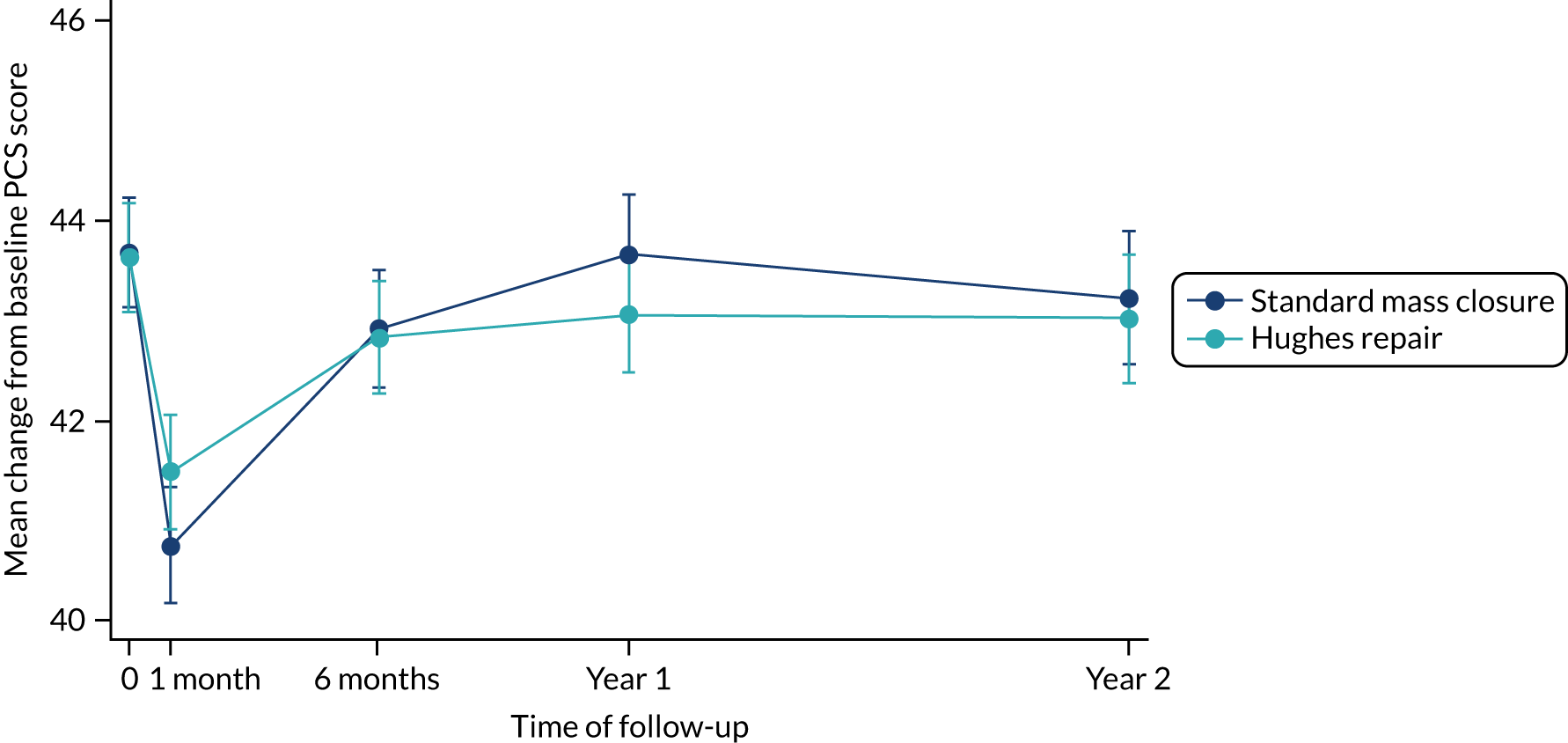
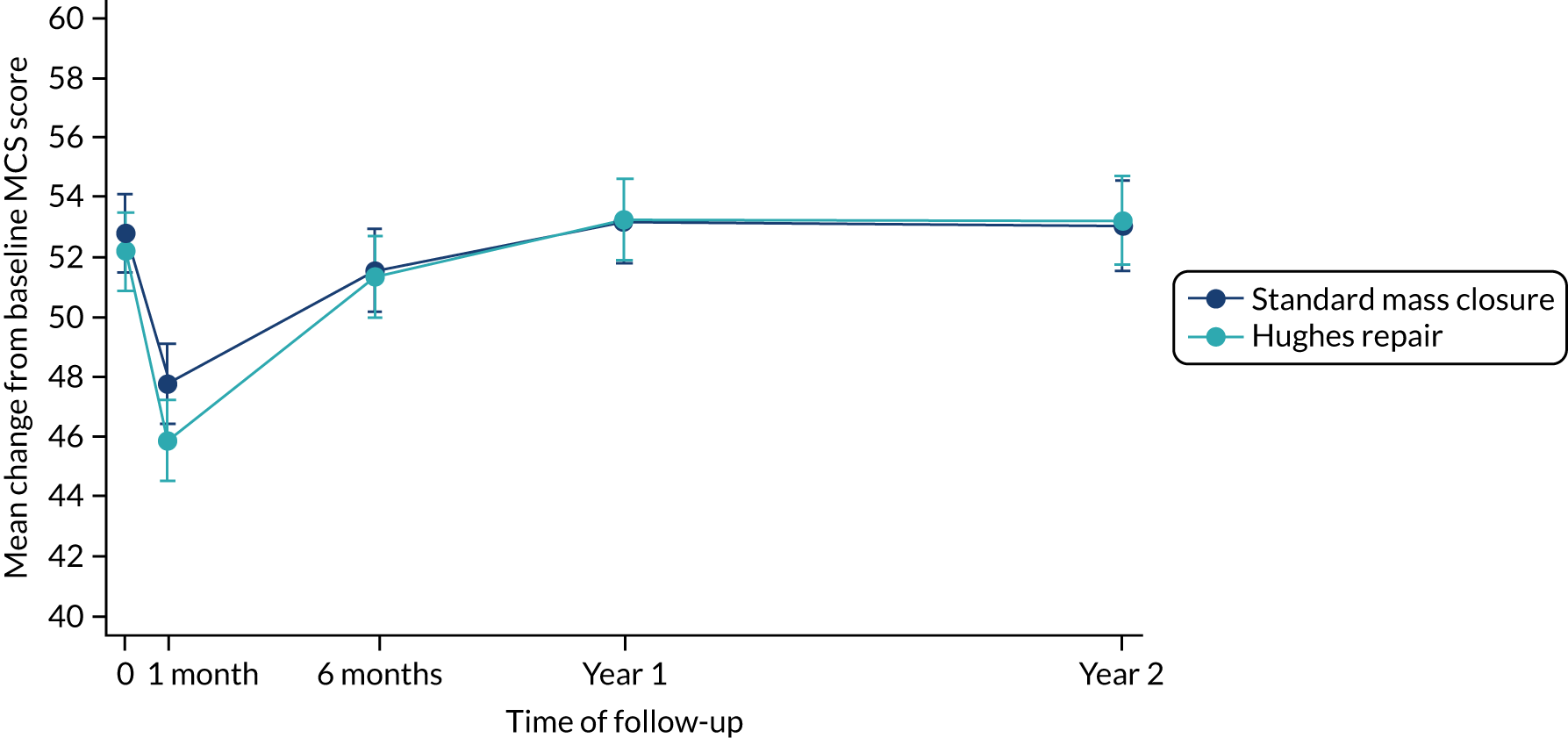
The results of the mixed-model repeated-measures analysis of the PCS scores showed no statistically significant differences in the between-group difference mean scores at any time point (Table 11 and Figure 6). Similar results were observed from the mixed-model repeated-measures analysis of the MCS scores (Table 12 and Figure 7). Following an initial sharp decline in MCS score in the month immediately following their index operation in both arms, patients reported improvement over time, with similar scores in both arms from 6 months onwards. In terms of FACT-C scores, the between-arm differences of the mean change from baseline to first month and to year 1 are statistically significant. Despite that the actual difference in the FACT-C score is not clinically relevant, the score in the standard mass closure arm seems to decrease steeply when compared with the Hughes repair arm at these two time points. The detailed mixed-model repeated analysis for the FACT-C score can be found in Appendix 5, Table 41 and Figure 13.
| Variable | Mean baseline PCS score (SD) | Mean change in PCS score from baseline (95% CI);a p-value | |||
|---|---|---|---|---|---|
| Month 1 | Month 6 | Year 1 | Year 2 | ||
| Standard mass closure | 43.7 (5.5) | –2.9 (–3.65 to –2.21); 0.00 | –0.8 (–1.51 to –0.04); 0.04 | 0.0 (–0.76 to 0.73); 0.9 | 0.5 (–1.31 to 0.29); 0.2 |
| Hughes repair | 43.6 (5.5) | –2.2 (–2.91 to –1.46); 0.00 | –0.8 (–1.53 to –0.10); 0.03 | –0.6 (–1.35 to 0.10); 0.09 | –0.6 (–1.40 to 0.17); 0.13 |
| Between-arm difference in means | 0.8 (–0.27 to 1.77); 0.15 | –0.0 (–1.07 to 0.99); 0.94 | –0.6 (–1.64 to 0.43); 0.25 | –0.1 (–1.22 to 1.01); 0.86 | |
FIGURE 6.
Mean PCS scores over time by arm.
| Variable | Mean baseline MCS score (SD) | Mean change in MCS score from baseline (95% CI);a p-value | |||
|---|---|---|---|---|---|
| Month 1 | Month 6 | Year 1 | Year 2 | ||
| Standard mass closure | 52.9 (12.1) | –5.2 (–6.58 to –3.87); 0.00 | –1.5 (–2.97 to –0.19); 0.03 | 0.0 (–1.4 to 1.4); 0.99 | 0.0 (–1.5 to 1.51); 0.99 |
| Hughes repair | 52.5 (11.9) | –6.2 (–7.57 to –4.86); 0.00 | –0.9 (–2.25 to 0.45); 0.2 | 0.8 (–0.54 to 2.2); 0.24 | 0.9 (–0.56 to 2.4); 0.22 |
| Between-arm difference in means | –1.0 (–2.91 to 0.93); 0.31 | 0.7 (–1.25 to 2.62); 0.49 | 0.8 (–1.13 to 2.78); 0.41 | 0.9 (–1.19 to 3.02); 0.40 | |
FIGURE 7.
Mean MCS scores over time by arm.
Wound dehiscence (burst abdomen)
There were three occurrences of full thickness wound dehiscence at 30 days post surgery – one in the Hughes repair arm and two in the standard mass closure arm – giving an overall rate of 0.79% for the whole study. The cumulative incidence of wound dehiscence at 30 days post surgery was 0.25% for Hughes repair and 0.50% for standard mass closure.
Prevalence of incisional hernia at 1 year
Twelve patients (Hughes repair arm, n = 9; standard mass closure arm, n = 3) had IH at baseline (see Table 22). By the 1-year follow-up, IH had been repaired in nine patients (Hughes repair arm, n = 6; standard mass closure arm, n = 3); in the remaining three patients (all of whom were in the Hughes repair arm), the IH had not been repaired at year 1 or this information was missing.
Sensitivity and specificity of computed tomography scanning compared with clinical examination for identification of incisional hernia at 1 year
The rate that IH was diagnosed by clinical examination or by CT scan at 1 year is reported in Table 13. Clinical examination was considered the reference test, and the results from this were considered the ‘true’ results. In total, 630 patients had both a 1-year clinical examination result and a CT scan available for analysis and are included in the analysis for sensitivity and specificity.
| Hughes repair arm, n | Standard mass closure arm, n | Total study (both arms), n | |
|---|---|---|---|
| IH on CE only | 7 | 9 | 16 |
| IH on CT only | 114 | 103 | 217 |
| IH on both CE and CT | 37 | 47 | 84 |
| Total IH | 158 | 159 | 317 |
| Negative for IH on both | 156 | 157 | 313 |
| Total | 314 | 316 | 630 |
In total, across both study arms, 317 IHs were diagnosed by clinical examination and/or CT scan, of which 84 were identified by both modalities: 37 in the Hughes repair arm and 47 in the standard mass closure arm. Clinical examination identified 44 IHs in the Hughes repair arm and 56 IHs in the standard mass closure arm, that is clinical examination identified a further seven IHs in the Hughes repair arm and nine in the standard mass closure arm that were not detected on the patient’s CT scan. Conversely, CT scan alone identified an additional 114 IHs in the Hughes repair arm and an additional 103 IHs in the standard mass closure arm (see Table 13).
From the 2 × 2 tables (Table 14) and the analysis of these results, it appears that CT scanning has high sensitivity and moderate specificity when compared with clinical examination. The sensitivity of CT scanning was 84%, with 301 scans indicating the presence of an IH, and the specificity was 59%, with 313 patients without a hernia having a negative CT scan across both arms (Table 15). Of the 301 patients with a positive CT scan, only 84 had an IH on clinical examination, indicating that CT scanning had a low positive predictive value (27.9%). Similarly, of 329 patients with a negative CT scan, 313 did not have an IH on clinical examination, indicating that CT scanning had a very high negative predictive value of 95% (see Table 15). The corresponding ROC curve (Figure 8) or area under the curve was 72%.
| IH by clinical examination | IH by CT scan, n (%) | ||
|---|---|---|---|
| Positive | Negative | Total | |
| All patients | |||
| Positive | 84 (84.0) | 16 (16.0) | 100 |
| Negative | 217 (40.9) | 313 (59.1) | 530 |
| Total | 301 | 329 | 630 |
| Hughes repair arm | |||
| Positive | 37 (84.1) | 7 (15.9) | 44 |
| Negative | 114 (42.2) | 156 (57.8) | 270 |
| Total | 151 | 163 | 314 |
| Standard mass closure arm | |||
| Positive | 47 (83.9) | 9 (16.1) | 56 |
| Negative | 103 (39.6) | 157 (60.4) | 260 |
| Total | 150 | 166 | 316 |
| Hughes repair | Standard mass closure | All patients | |
|---|---|---|---|
| Sensitivity (%) | 84.1 | 83.9 | 84 |
| Specificity (%) | 57.8 | 60.4 | 59.1 |
| Positive predictive value (%) | 24.5 | 31.3 | 27.9 |
| Negative predictive value (%) | 95.7 | 94.6 | 95.1 |
| ROC areas (95% CI) | 0.71 (0.65 to 0.77) | 0.72 (0.67 to 0.78) | 0.72 (0.67 to 0.76) |
FIGURE 8.
The ROC curve (reference test: clinical examination). Area under ROC curve = 0.7153.
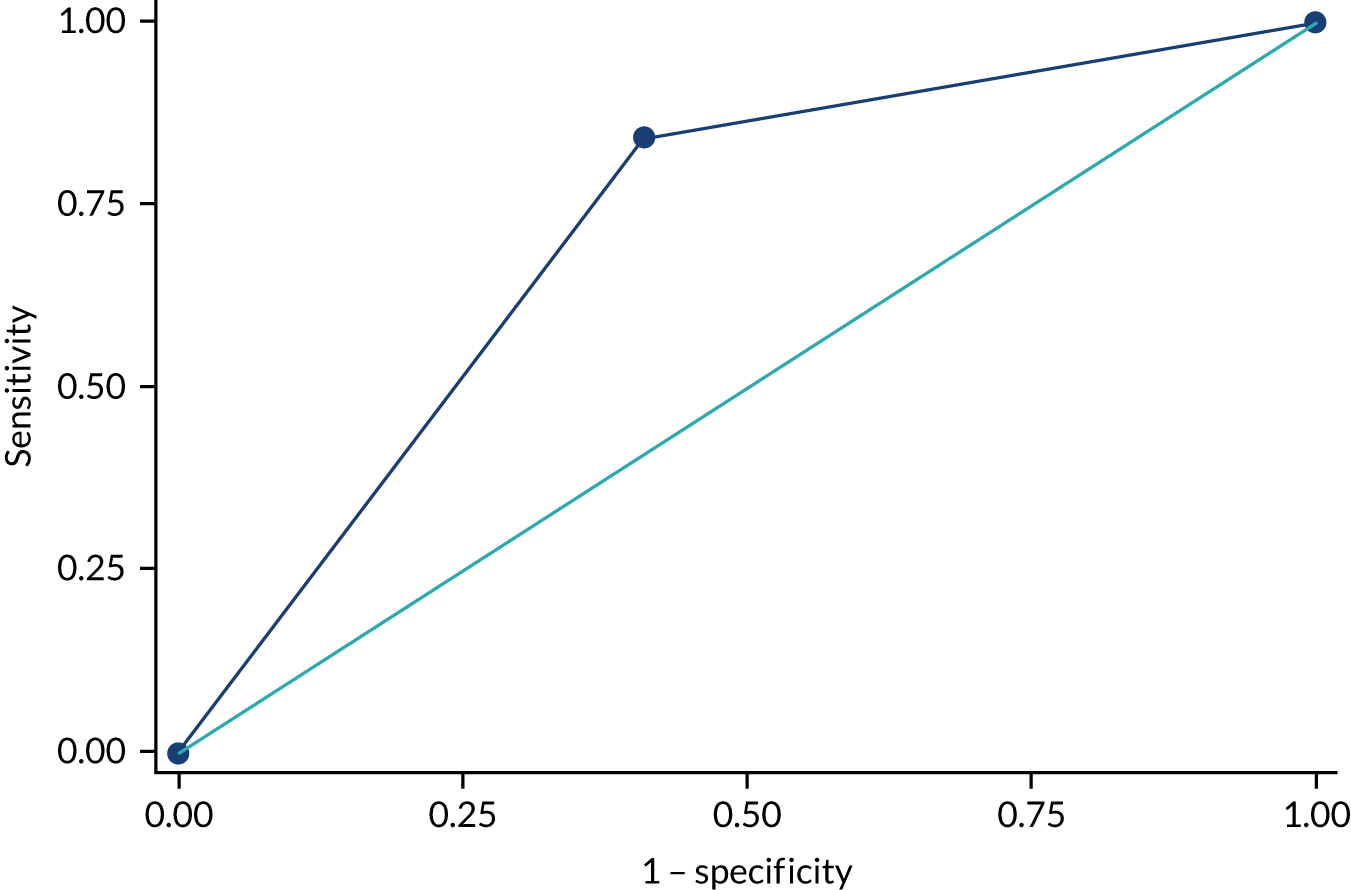
Safety and harms (adverse events)
Safety reporting accounted for any event within 30 days of the index operation plus death at any time during follow-up. The summary of the safety reporting shows that a total of 281 events (including deaths) were reported in 221 patients (Table 16). Although the numbers of events per arm were well balanced, the number of patients experiencing an adverse event was smaller in the Hughes repair arm than in the standard mass closure arm. Fewer deaths were reported in the Hughes repair arm than in the standard mass closure arm. Similarly, fewer life-threatening events were recorded in the Hughes repair arm (Table 17). Hospitalisation and extended hospital stays occurred more frequently in the Hughes repair arm (see Table 17). The numbers of SAEs of each type were mostly well balanced between the arms (Table 18). Broadly, the results show no difference between the arms in terms of the grade of SAE using the Clavien–Dindo classification. The most notable difference was that there were more grade II events and fewer grade V events in the Hughes repair arm than in the standard mass closure arm (Table 19).
| Hughes repair arm, n (%) | Standard mass closure arm, n (%) | Number of SAEs and patients | |
|---|---|---|---|
| SAE | 139 (49.5) | 142 (50.5) | 281 |
| Patients | 104 (47.1) | 117 (53.9) | 221 |
| Seriousness criteria | Hughes repair arm, n (%) | Standard mass closure arm, n (%) | Total, n | |||
|---|---|---|---|---|---|---|
| SAEs (N = 131) | Patients (N = 122) | SAEs (N = 142) | Patients (N = 134) | SAEs (N = 281) | Patients (N = 256) | |
| Death | 53 (45.7) | 53 (45.7) | 63 (54.3) | 63 (54.3) | 116 | 116 |
| Life-threatening | 9 (47.4) | 9 (47.4) | 10 (52.6) | 10 (52.6) | 19 | 19 |
| Hospitalisation or extended hospital stay | 67 (57.8) | 54 (54.5) | 49 (42.2) | 45 (45.5) | 116 | 99 |
| Other medically important condition | 10 (33.3) | 6 (27.3) | 20 (66.7) | 16 (72.7) | 30 | 22 |
| SAE type | Hughes repair, n | Standard mass closure, n | Total, n |
|---|---|---|---|
| Abnormal laboratory results | 0 | 1 | 1 |
| Cardiac disorders | 4 | 8 | 12 |
| Death | 53 | 63 | 116 |
| Dermatological disorders | 1 | 0 | 1 |
| Gastrointestinal disorders | 44 | 42 | 86 |
| Genitourinary disorders | 7 | 5 | 12 |
| Haematological disorders | 1 | 0 | 1 |
| Immune disorder | 0 | 3 | 3 |
| Non-wound infection | 1 | 3 | 4 |
| Respiratory disorders | 14 | 8 | 22 |
| Vascular disorders | 1 | 1 | 2 |
| Wound complications | 13 | 8 | 21 |
| Total | 139 | 142 | 281 |
| SAE/surgical complications | Hughes repair, n (%) | Standard mass closure, n (%) | Total, n |
|---|---|---|---|
| Grade I | 21 (46.7) | 24 (53.3) | 45 |
| Grade II | 38 (56.7) | 29 (43.3) | 67 |
| Grade IIIa | 6 (42.9) | 8 (57.1) | 14 |
| Grade IIIb | 11 (57.9) | 8 (42.1) | 19 |
| Grade IVa | 8 (47.1) | 9 (52.9) | 17 |
| Grade IVb | 2 (66.3) | 1 (33.3) | 3 |
| Grade V | 53 (45.7) | 63 (54.3) | 114 |
| Total | 139 | 142 | 281 |
The number of suspected unexpected serious adverse reactions was well balanced between arms; however, the number of related events was larger in the Hughes arm, although the difference was not significant (Table 20). The reported suspected unexpected serious adverse reactions consisted mainly of abnormal laboratory results, deaths, cardiac disorders, gastrointestinal disorders and immune disorders.
| Hughes repair arm, n (%) | Standard mass closure arm, n (%) | Total, n | |
|---|---|---|---|
| Number of SUSARs | 52 (47.3) | 58 (52.7) | 110 |
| Number of related events | 6 (85.7) | 1 (14.3) | 7 |
Chapter 4 Cost-effectiveness
A within-trial health economic analysis was undertaken from a health service perspective (NHS), reflecting the trial follow-up period of 12 months. The evaluation addressed the secondary trial objective of assessing the costs and cost-effectiveness of using Hughes abdominal repair compared with standard mass closure in patients undergoing colorectal cancer surgery. Analyses included cost-effectiveness, cost–utility and cost–consequences analyses.
Methods of the health economic evaluation
Before the analysis commenced, a health economic analysis plan was produced, reviewed by the trial team and incorporated into the SAP. The health economic team followed this analysis plan during the conduct of the economic evaluation without deviation.
Costs included in the health economic analysis
The health economic analysis considered the following costs:
-
intervention implementation cost of Hughes repair
-
post-surgery costs, including costs of hospital stay, critical care, complications, adverse events and reoperations
-
cost of subsequent health-care resource use (primary, secondary and social care, including any cancer treatment).
Costs are based on the ITT population in accordance with the SAP in the base case and all available cases (for the most complete overview) in sensitivity analysis and for descriptive purposes. All costs are expressed as 2017/18 Great British pounds and were inflated and converted appropriately39 when no relevant UK 2017/18 unit costs were available. Neither costs nor outcomes were discounted, as the trial follow-up period did not exceed 1 year.
Missing cost data
Mean imputation was used to account for missing data. The problems concerning missing data are particularly relevant to health economic analysis because the main outcomes are cumulative measures collected over the trial period. Missing items relating to health-care service use may underestimate the total costs, while missing outcome data may be correlated to effects as those individuals without information may be systematically different from those for whom all information is observed. 35 For this reason, using complete-case assessments and available-cases analysis only could result in meaningful data being excluded. 40 Multiple imputation would be the most appropriate technique for providing a comprehensive investigation of the impact of missing data on the estimations of cost-effectiveness. However, health-care resource use was not collected for the patients in the internal pilot at baseline, and data could not be considered missing at random. Based on the overall small number of missing cases, mean imputation was deemed to be feasible and was adopted for the cost data.
Intervention implementation cost
We considered the initial investigations and the main surgical procedure to be equal for both arms given that neither of these was influenced by the intervention.
Intervention costs considered in the health economic analysis were:
-
costs associated with staff training in the Hughes abdominal repair technique
-
any additional resources required for Hughes repair compared with standard mass closure
-
any increase in theatre time if required post randomisation only.
Costs of staff training
Resources required for training surgeons at all sites in the Hughes repair technique were collected through discussions with the trial team, surgeons and through review of study notes.
Training costs included staff costs (based on time spent in training) for all trainer and trainee surgeons (assuming a 50/50 split of consultants and registrars in the base case) and materials used for training. The unit costs of materials and consumables were obtained directly from the manufacturers. Staff costs were estimated using published unit costs. 40 Any assumptions were based on clinical expert opinion from the HART study team and the impact on the results was tested as part of the sensitivity analysis.
During the trial, training was undertaken as part of the site induction. For this reason, travel costs were excluded from the overall training costs. This was assumed to more accurately reflect routine practice, as in-hospital cascade training would be adopted if Hughes repair was implemented routinely in the NHS. All surgeons who were trained in the Hughes repair technique were expected to watch a 5-minute instructional video in their own time. This was assumed to be incorporated into the overall training time and was not costed separately.
Intraoperative costs
Intraoperative costs associated with the Hughes repair technique included only those directly associated with the intervention. Details on the sutures required to perform the Hughes repair (suture type and quantity used) in addition to the sutures used as standard to close the abdomen, and the time surgeons required to perform the two different methods of closure were collected routinely on the intraoperative CRF. Consultant time was costed at £1.80 per minute and registrar time was costed at £0.72 per minute. 41
Information regarding the type and quantity of sutures used in any reoperations (if required) was obtained from the reoperation CRF. The different closure methods were compared to evaluate the differences in time and cost. The surgical team was assumed to consist of a consultant, a registrar and an anaesthetist.
Post-surgical care costs
Inpatient costs following the index operation were considered to be post-surgical care costs. The duration of the index hospital stay was calculated as the time from randomisation to the date of hospital discharge, as recorded on the discharge CRF. The level of postoperative care (intensive care unit, high-dependency unit or ward), requirement for post-surgical blood transfusions and re-operation, as well as nasogastric or total parenteral feeding and the occurrence of SSI, burst abdomen and other SAEs, were obtained from the discharge CRF and SSI diaries. These parameters were taken into account by adding the number of complications per patient and adjusting the unit cost of the inpatient stay according to the complexity and comorbidities (CC) score reported as part of NHS Reference Costs 2017 to 2018 (see the unit costs in Appendix 6, Table 42). 42,43 Standard reference unit costs were adjusted according to actual length of stay by adding the cost of an excess bed-day or subtracting daily inpatient costs (based on mean length of stay stipulated in the unit cost) as required to account for the likely higher cost at the beginning of a hospital stay. Where the discharge date was missing, a mean of 10 days was assumed. For patients who discontinued the study before discharge from hospital, but consented to their surgery data being used, the hospital stay was censored at the withdrawal date. As this could underestimate the true costs, it was addressed in the sensitivity analysis. Costs were allocated using published sources. 41,43
Cost of subsequent health-care resource use
This included the costs of all health-care resource use accrued after hospital discharge following the index operation. Health-care resource use (including primary care consultations, emergency department visits, outpatient appointments and inpatient stays) was established using patient diaries at 30-day follow-up and an adapted Client Service Receipt Inventory (based on Beecham and Knapp;44 see Appendix 6, Tables 43–49). The CSRI was adapted specifically for health-care resource use collection in colorectal surgery patients and administered at baseline and at the 6-month and 1-year follow-ups. If one or more items in any health-care consultations section of the CSRI had been completed (values of ≥ 0), the CSRI was assumed to have been fully completed and any missing items were imputed with a zero. If the CRF was marked as ‘not done’ or was otherwise fully incomplete, data were considered missing. Costs were based on the ITT population for the cost-effectiveness analysis (CEA) and cost–utility analysis (CUA) base cases and all available cases in descriptives and sensitivity analyses. Missing costs in the ITT population were imputed with the mean value of each missing cost component (e.g. mean cost of emergency department visits for Hughes repair arm or standard mass closure arm patients, respectively).
Information about health-care resource use 6 months prior to randomisation was collected retrospectively, based on patient recall, using the CSRI at baseline and used to check for baseline imbalances. The mean total health-care costs before the randomisation of patients to the Hughes repair and standard mass closure arms were compared with the view to adjusting for statistically significant baseline imbalances.
Each questionnaire asked for health-care resource use in the previous 6 months. Therefore, the costs at both time points were summed to produce a total cost over the 12-month follow-up period post randomisation. CSRI data were cross-checked with data on recurrences (taken from post-surgery CRFs), the 30-day patient diary (for general practice visits, as well as antibiotic prescriptions and wound swabs), the 1-year post-surgery CRF (for local cancer recurrences, radiotherapy and chemotherapy, additional abdominal operations and stitch sinuses as well as length of stay of readmissions) and costs related to the index hospital stay. This was necessary to ensure that all health-care resource use was recorded while avoiding double-counting. Costs were assigned using published unit costs, which are summarised in Appendix 6.
The health-care costs in primary, secondary and social care for both Hughes repair and standard mass closure in the 12 months post randomisation were then summated and the mean cost difference per patient (including 95% CIs and p-values) was calculated using SPSS version 26 (IBM SPSS Statistics, Armonk, NY, USA). Independent-sample t-tests were used for comparison, with a 5% significance level. Bonferroni–Holm sequential corrections were used to adjust for type I error rate inflation in multiple comparisons. 45
Primary care costs
Primary care costs included the costs of general practitioner (GP) visits in surgery, home visits and telephone consultations, as well as the costs of practice nurse appointments in surgery, district nurse visits at patients’ homes, counsellor appointments, health visitor contacts, NHS Direct consultations and other primary care contacts. Other contacts were costed as specified (e.g. phlebotomist, dietitian and diabetic nurse specialist). When no details on the nature of other contacts were available, these were assumed to be interactions with a receptionist, as this was the most common other primary care surgery contact.
The CSRI data were cross-checked with the 30-day patient diaries. Patient diary entries for primary care contacts were assumed to be GP visits unless otherwise indicated. If the number of GP visits recorded in patient diaries was larger than the number reported in the 6-month CSRI, the CSRI data were overwritten with the patient diary, as the latter was completed daily and recall was assumed to be more reliable. If the number of GP visits was smaller in the patient diaries than reported in the CSRI, patient diary visits were assumed to be included in the CSRI report and the CSRI-reported number of GP visits was used.
If patient diaries indicated that antibiotics were prescribed, the antibiotics were assumed to be flucloxacillin or co-amoxiclav, based on clinical expert advice. They were costed as a mean daily cost calculated from all available strengths for 7-day courses. Wound swabs reported in the 30-day patient diaries were costed as directly accessed pathology services via primary care (microbiology). 43
All unit costs used for costing primary care resource use can be found in Appendix 6.
Social care costs
Social care costs included social worker, home help and care assistant visits, day-centre visits and contacts with social services. Social worker visits were assumed to be at the patient’s home unless otherwise indicated. Social service contacts were costed as community occupational health appointments when information on the nature of the contact was missing. All unit costs used for costing social care resource use can be found in Appendix 6.
Secondary care costs
Secondary care costs were divided into surgery-related care and other secondary health care. All research-related contacts were excluded.
Surgery-related health-care contacts comprised consultant surgeon appointments, specialist nurse visits at home and in hospital, stoma nurse visits and other surgical department staff contacts. Other contacts were assigned unit costs individually according to the reported nature of the contact. When details were missing, other contacts were assumed to be an anaesthetist. If two other contacts were reported but not specified, these were assumed to be an anaesthetist and a specialist nurse.
Other secondary health care included day hospital visits, emergency department visits, outpatient consultations and inpatient stays. Furthermore, occupational health appointments, ambulance call-outs and other secondary care contacts were recorded. When details of other contacts were missing, these were assumed to be specialist nurse appointments. Hospital contacts reported in the 30-day patient diaries were assumed to be emergency department visits based on clinical expert opinion. The number of CSRI-reported emergency department visits was overwritten by the number from the patient’s diary if the latter was larger.
Inpatient stay unit costs were based on a weighted mean across all NHS reference cost entries for elective excess bed-days and multiplied by the number of bed-days recorded. Outpatient visit costs were calculated as the mean consultant-led outpatient appointment, weighted across all departments. All unit costs used for costing secondary care resource use are reported in Appendix 6.
Cancer treatment cost
Data on the administration of adjuvant chemotherapy, as well as radiotherapy and chemotherapy treatment received for local recurrences and metastatic disease, were taken from the 1-year post-surgery visit CRF. No information on the regimen or number of chemotherapy cycles or radiotherapy fractions was available. Therefore, the analysis assumed five fractions of radiotherapy at a per-fraction cost of £83.06 for same day radiotherapy admission or attendance (excluding brachytherapy), taken from NHS Reference Costs 2017 to 2018. 43 Long-course radiotherapy (25 fractions) was considered in sensitivity analyses. The cost of chemotherapy was obtained from the SCOT trial,46 assuming 9 months of treatment at a total cost of £3816.36.
Total costs
By adding up the implementation costs of Hughes repair (in the intervention group only), post-surgical inpatient costs (including adverse events such as SSI and burst abdomen) and subsequent primary, secondary and social care for all patients in both trial groups, the total and mean costs per patient (including 95% CIs) of the two closure methods were calculated to derive the incremental costs (or cost savings) of the intervention at the 12-month follow-up.
Health outcomes
Clinical examination identified 50 (14.8%) IHs in the Hughes repair arm and 57 (17.1%) IHs in the standard mass closure arm (see Chapter 3).
Quality-adjusted life-years (QALYs) required for the cost–utility analysis were derived from SF-12 responses. We used the SPSS syntax algorithm for SF-12 version 2 to map SF-12 responses to SF-6D scores and patient utilities based on UK value sets for baseline and the 1-, 6- and 12-month follow-up points. 47 During this procedure, missing SF-12 items were replaced with the trial population mean of each item if at least half of the items were complete. 48 When more than six items were missing, the questionnaire was considered ‘not completed’ and no utility value was calculated. We reported utility values for all available cases, which were defined as those with at least a complete baseline questionnaire and at least a utility score at 12 months. Utilities for participants who died during the follow-up period were set to 0 between the point of death and the end of follow-up. The cost–utility analysis used the ITT population, with missing utility values imputed as the mean of each time point, in the base case. Sensitivity analyses used last observation carried forward/backward (LOCF) imputation and available cases (available data for all patients at each time point) to test the impact of missingness on the analysis results. Once the analysis populations were defined, and imputation performed where required, QALYs for each individual patient were calculated according to an area-under-the-curve approach and linear interpolation. This used all four time points to estimate the overall QALYs as a combined measure of patients’ QoL over 12 months.
Cost-effectiveness analysis
The CEA compared the incremental costs with the primary outcome (number of IHs) at the 1-year follow-up to calculate the incremental cost-effectiveness ratio (ICER) for Hughes repair reported as cost per IH avoided.
The base-case analysis was based on the ITT population for the primary outcome of number of IHs identified at clinical examination. The total costs at 12 months for the ITT population (after mean imputation) only were considered. The results of the comparative analysis of incremental costs and effects were summarised in terms of incremental cost-effectiveness ratios (ICERs). An ICER can be represented as:
where C1 and E1 are the costs and effects of the intervention arm and C0 and E0 are the costs and effects of the control arm, with ΔC and ΔE the incremental costs and effects of the intervention compared with the control.
The ICER is reported to determine the cost-effectiveness of the intervention compared with competing alternatives and to aid decision-making. No established willingness-to-pay threshold for the cost-effectiveness in reduction of IHs is available. Furthermore, cost-effectiveness is a spectrum rather than a dichotomy, with the maximum threshold increasing depending on the circumstances. The reported ICERs from our analysis are presented to assist the decision-making process and are not an absolute statement on whether or not the intervention can be deemed to be cost-effective.
Cost–utility analysis
A within-trial CUA was undertaken to assess the incremental costs per QALY gained as a result of the use of Hughes repair at 12 months. The total costs at 12 months and QALYs for the ITT population were used to calculate the incremental cost per QALY gained.
Quality-adjusted life-years incorporate quantity of life (additional life-years) and QoL in one measure. Thus, by dividing the difference in costs by the difference in QALYs, a cost per QALY can be calculated for each comparison to establish whether the new intervention is less or more cost-effective than, or similarly cost-effective to, the comparator treatment. The ICER resulting from the CUA was compared with the willingness-to-pay threshold of £20,000 per QALY gained as standardised by NICE. No conditions for non-inferiority were applied in this analysis. The results are reported as ICERs showing the extra cost of producing one extra QALY or the extra savings achieved by sacrificing one additional QALY.
Sensitivity analysis
Sensitivity analyses were undertaken to test the robustness of the results of both CEA and CUA considering the uncertainty in input parameters, such as costs and outcomes, and in different scenarios. Deterministic, univariate sensitivity analysis changed surgery, complication and health-care costs and outcomes individually within plausible ranges (using 10%, 20% and 30% of the mean value). Scenario analyses tested different assumptions and recalculated the ICER. Furthermore, probabilistic sensitivity analysis used non-parametric bootstrapping to address joint parameter uncertainty and assess the impact on the ICER during 1000 simulations that were undertaken using random sampling of the distributions of costs and outcomes with results presented on cost-effectiveness planes and as cost-effectiveness acceptability curves (CEACs). The cost-effectiveness plane is a scatterplot of the point estimates obtained as a result of the 1000 simulations depicted in four quadrants representing the probability of the intervention being more or less costly and more or less effective than the control. A CEAC is a curve that describes the probability that the intervention will be cost-effective at different willingness-to-pay-thresholds based on the probabilistic sensitivity analysis. We also undertook scenario analyses to test for the potential impact of baseline imbalances, training costs and imputation method.
Cost–consequences analysis
A cost–consequences table was created to present all of the relevant primary and secondary outcomes alongside the costs in tabular form (without combining them into ICERs) to leave decision-makers the option to form their own view of relative importance.
Results of the health economic evaluation
Intervention implementation costs
Training costs
A detailed breakdown of all training costs is presented in Table 21. The total training cost associated with the Hughes repair technique was estimated to be £20,409.52, or £60.20 per patient who underwent Hughes repair, and was included in the ITT population (n = 339). This cost comprises £14,302.00 for staff training (£42.19 per Hughes repair patient) and £6107.52 for materials and consumables (£18.02 per trial participant undergoing Hughes repair).
| Cost component | Unit cost (£) | Total cost (£) |
|---|---|---|
| Training staff costs | ||
| Trainee costs (total of 162 trainees at 27 sites, 50/50 consultant-to-registrar split assumed) | ||
| 81 consultant-grade surgeons for a 1-hour session each (three per site) | 108 per houra | 8748 |
| 81 surgical trainees ST5 or below or ST6–8 for a 1-hour session each (three per site) | 43 per houra | 3483 |
| Trainer costs (two trainers, trial consultant and trial registrar, covering 27 sites) | ||
| 14 × 1-hour training sessions by consultant-grade surgeon | 108 per houra | 1512 |
| 13 × 1-hour training sessions by registrar-grade surgeon | 43 per houra | 559 |
| Training material and consumable cost | ||
| Suture type: 120 Ethicon 747 nylon sutures (single use) | 1.57 per sutureb | 890.19 |
| Three used per trainee: 3 × 162 trainees = 486 | ||
| Three used per trainer: 3 × 27 sessions = 81 | ||
| Suture type: 36 loop PDS sutures (single use) | 6.19 per sutureb | 3509.73 |
| Three used per trainee: 3 × 162 trainees = 486 | ||
| Three used per trainer: 3 × 27 sessions = 81 | ||
| Abdominal wall pad (synthetic foam/plastic pad to mimic the abdominal wall, single use) | 15 per padc | 1620 |
| One wall pad per two trainees × 162 = 81 | ||
| One wall pad used per trainer per 27 sessions = 27 | ||
| Jig (plastic frame to hold the synthetic pad for trainee to suture); HART used eight jigs for 3 years (reusable, lifespan of 20 years) | 73 per jigc | 87.60 |
| Total training cost | 20,409.52 | |
| Total cost per patient (n = 339) | 60.20 | |
All hospital sites taking part in the HART study held a 1-hour training session in the Hughes repair technique. For each of the 27 sites, it was assumed that three consultants and three registrars were trained by either the trial chief investigator consultant or the co-investigator surgical trainee. In total, 162 surgeons were trained, and any training of further surgeons occurred in-house during surgery as standard at no additional cost.
Intrasurgical costs
Hughes repair and standard mass closure require different types of sutures. The unit costs of sutures were obtained from the trial team and can be found in Appendix 6.
Based on all available cases, the results in Table 22 show that, compared with standard mass closure, Hughes repair uses significantly more sutures (4.16 more per patient) at a significantly higher cost (£12.52 more per patient).
| Hughes repair arm (n = 368) | Standard mass closure arm (n = 364–368) | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Mean number of sutures (SD) | 6.29 (2.80); minimum = 1; maximum = 18 | 2.13 (1.63); minimum = 1; maximum = 22 | 4.16 (3.83 to 4.50) | < 0.000 |
| Mean cost of sutures (SD) | £18.17 (£6.83); minimum = £1.57; maximum = £46.74 | £5.65 (£3.91); minimum = £2.11; maximum = £45.36 | £12.52 (£11.71 to £13.32) | < 0.000 |
| Mean surgery time post randomisation in minutes (SD) | 30.20 (38.44) | 21.38 (38.64) | 8.82 (3.39 to 14.26) | 0.001 |
| Mean surgeon cost post randomisation (SD) | £112.26 (£123.02) | £78.72 (£122.58) | £33.54 (£16.22 to £50.86) | 0.001 |
The costs used to calculate the cost difference include all sutures as specified during the index operation and any reoperations required in both trial arms.
In the standard mass closure arm, 368 reports of sutures were available. However, in four cases, suture size was not recorded and, therefore, a unit cost could not be applied. For two patients, five or more sutures were indicated in the intrasurgical CRF. A conservative approach was taken to interpret this as five sutures for costing purposes.
A breakdown of the types of sutures used during the index operation and any reoperations in which resuturing of the original surgical wound was required for each arm is detailed in Appendix 6. Of the 49 reported reoperations, 34 required resuturing of the surgical wound. For the Hughes repair arm, 17 surgical wounds were resutured, of which 10 used the standard mass closure method and seven the Hughes repair method. For the control group, 17 reoperations were reported using standard mass closure in 11 instances and Hughes repair for six closures.
The Hughes repair technique took, on average, 8.82 minutes longer per patient at an extra cost of £33.54 (see Table 22). This extra time and cost includes the initial index operation and all necessary reoperations. However, although the index operation required extra surgical time to be carried out, there was no significant difference between the two arms in surgical time for any reoperations (p = 0.806).
Overall, Hughes repair cost an additional £106.26 compared with standard mass closure, comprising training (£60.20), sutures (£12.52) and additional surgery time (£33.54).
Cost of immediate post-surgical care
Based on all available cases, patients in the Hughes repair arm (n = 401) remained in hospital for 11.41 days (SD 14.65 days) following their index operation. This is 1.60 days (95% CI –0.28 to 3.47 days; p = 0.095) longer than standard mass closure patients (n = 401), who stayed in hospital a mean of 9.81 days (SD 12.30 days). Although the numbers of adverse events (blood infusions, nasogastric and total parenteral nutrition feeding, burst abdomen and other adverse events) were non-significantly smaller in the Hughes repair arm, the number of days in the intensive therapy unit and high-dependency unit were slightly higher, and SSIs were significantly more common (0.13 per patient in the Hughes repair arm, compared with 0.08 per patient in the standard mass closure arm; mean difference 0.055, 95% CI 0.0126 to 0.0979; p = 0.01).
This affected the CC score and resulted in a total cost of post-surgical care per patient of £9613.49 (SD £6465.94) in the Hughes repair arm compared with £8999.16 (SD £5610.75) in the standard mass closure arm. The mean difference of £614.33 (95% CI –£224.85 to £1453.50) was not statistically significant (p = 0.151).
Cost of subsequent health-care resource use
The results reported in this chapter represent the mean cost per patient based on all available cases. A breakdown of health-care resource use can be found in Appendix 6.
All costs are based on available cases for individual time points. All costs for the total period between baseline and 12 months are calculated for 268 patients in the Hughes repair arm and 256 patients in the standard mass closure arm for whom health-care data were available for both the 6-month and the 12-month follow-up points. The cost differences calculated for the entire follow-up period (baseline to 12 months) are not equal to the sum of the two time periods between baseline and 6 months and between 6 and 12 months, as they are calculated from different populations of available cases.
Based on all available cases, the total health-care costs in the 6 months before randomisation amounted to £1997.09 (SD £3208.01) in the Hughes repair arm (n = 296) and £1748.41 (SD £2521.88) in the standard mass closure arm (n = 295). As the difference of £248.67 (95% CI –£217.51 to £714.84) was not statistically significant (p = 0.295), baseline imbalance was not adjusted for in the base case of the CEA and CUA. However, baseline imbalance in the ITT population was accounted for as part of sensitivity analysis.
Primary care costs
A summary of the available primary care costs for the follow-up periods between baseline and 6 months and between 6 and 12 months, respectively, can be found in Table 23.
| Primary care resource type | Mean (SD) cost (£) per patient | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Hughes repair arm | Standard mass closure arm | |||
| Between baseline and 6 months | ||||
| Sample size (n) | 311 | 296 | ||
| GP visits at surgery | 90.55 (111.11) | 86.17 (116.95) | 4.38 (–13.80 to 22.56) | 0.637 |
| Nurse visits at surgery | 16.48 (42.83) | 18.40 (81.20) | –1.92 (–12.64 to 8.51) | 0.717 |
| GP visits at home | 25.38 (113.23) | 22.56 (70.51) | 2.82 (–12.35 to 17.94) | 0.712 |
| District nurse visits at home | 167.52 (375.34) | 183.21 (468.26) | –15.69 (–83.56 to 52.17) | 0.650 |
| GP telephone consultations | 13.30 (23.43) | 14.79 (24.58) | –1.49 (–5.32 to 2.34) | 0.445 |
| Counsellor | 1.84 (16.66) | 1.64 (18.58) | 0.20 (–2.61 to 3.02) | 0.887 |
| NHS Direct telephone call | 2.64 (8.52) | 3.42 (12.16) | –0.79 (–2.47 to 0.89) | 0.350 |
| Health visitor at home | 2.21 (20.06) | 5.37 (28.09) | –3.15 (–7.06 to 0.75) | 0.114 |
| Other contacts | 1.60 (9.19) | 3.24 (23.96) | –1.64 (–4.56 to 1.28) | 0.271 |
| Total cost of primary care use per patient | 321.52 (446.24) | 338.80 (524.42) | –17.28 (–95.09 to 60.52) | 0.663 |
| Between 6 and 12 months | ||||
| Sample size (n) | 313 | 303 | ||
| GP visits at surgery | 70.26 (110.09) | 61.84 (87.91) | 8.42 (–7.38 to 24.22) | 0.296 |
| Nurse visits at surgery | 9.50 (24.83) | 14.72 (63.25) | –5.21 (–12.87 to 2.45) | 0.182 |
| GP visits at home | 10.25 (45.17) | 8.59 (36.34) | 1.66 (–4.83 to 8.17) | 0.615 |
| District nurse visits at home | 37.81 (176.12) | 61.34 (303.57) | –23.53 (–62.99 to 15.93) | 0.242 |
| GP telephone consultations | 11.58 (28.71) | 8.52 (19.98) | 3.06 (–0.87 to 6.98) | 0.127 |
| Counsellor | 2.25 (21.02) | 3.49 (51.02) | –1.24 (–7.38 to 4.90) | 0.693 |
| NHS Direct telephone call | 1.14 (5.73) | 1.42 (6.31) | –0.28 (–1.24 to 0.67) | 0.559 |
| Health visitor at home | 2.88 (16.45) | 2.10 (14.14) | 0.78 (–1.65 to 3.21) | 0.529 |
| Other contacts | 1.49 (8.95) | 3.22 (19.71) | –1.72 (–4.37 to 0.68) | 0.165 |
| Total cost of primary care use per patient | 147.17 (261.46) | 165.21 (383.19) | –18.04 (–70.13 to 34.04) | 0.494 |
The total primary care costs between baseline and the 12-month follow-up amounted to £435.00 (SD £541.17) per patient in the Hughes repair arm and £434.04 (SD £602.35) per patient in the standard mass closure arm. The mean difference of £1.95 (95% CI –£96.48 to £100.38) was not statistically significant (p = 0.969).
Social care costs
A summary of the available social care costs for the follow-up periods between baseline and 6 months and between 6 and 12 months, respectively, is presented in Table 24.
| Social care resource type | Mean (SD) cost (£) per patient | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Hughes repair arm | Standard mass closure arm | |||
| Between baseline and 6 months | ||||
| Sample size (n) | 301 | 286 | ||
| Social worker | 31.89 (503.74) | 3.19 (18.37) | 28.70 (–29.84 to 87.25) | 0.336 |
| Home help | 42.39 (474.96) | 102.92 (1077.24) | –60.53 (–194.37 to 73.31) | 0.375 |
| Care assistant | 56.51 (591.06) | 112.34 (1193.60) | –55.83 (–207.38 to 95.72) | 0.470 |
| Day centre | 1.31 (16.10) | 5.18 (48.40) | –3.87 (–9.79 to 2.04) | 0.199 |
| Social services | 3.84 (26.59) | 2.34 (20.42) | 1.50 (–2.35 to 5.36) | 0.444 |
| Total cost of social care use per patient | 135.95 (1547.28) | 225.98 (2196.21) | –90.03 (–396.78 to 216.73) | 0.565 |
| Between 6 and 12 months | ||||
| Sample size (n) | 313 | 303 | ||
| Social worker | 0.92 (11.49) | 3.33 (26.14) | –2.41 (–5.62 to 0.81) | 0.142 |
| Home help | 7.03 (89.54) | 10.31 (135.79) | –3.28 (–21.43 to 14.86) | 0.723 |
| Care assistant | 80.40 (1138.67) | 66.30 (1132.33) | 14.10 (–165.63 to 193.83) | 0.878 |
| Day centre | 1.90 (33.55) | 2.29 (21.93) | –0.39 (–4.89 to 4.11) | 0.865 |
| Social services | 0.60 (6.49) | 5.17 (79.61) | –4.57 (–13.60 to 4.45) | 0.320 |
| Total cost of social care use per patient | 90.84 (1142.12) | 87.39 (1175.86) | 3.45 (–179.96 to 186.86) | 0.971 |
The total social care costs between baseline and the 12-month follow-up amounted to £145.81 (SD £1261.61) per patient in the Hughes repair arm and £290.92 (SD £3522.46) per patient in the standard mass closure arm. The mean difference of –£145.11 (95% CI –£604.03 to £313.81) was not statistically significant (p = 0.534).
Secondary care costs
A summary of the available surgical care costs for the follow-up periods between baseline and 6 months and between 6 and 12 months, respectively, can be found in Table 25.
| Surgical care resource type | Mean (SD) cost (£) per patient | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Hughes repair arm | Standard mass closure arm | |||
| Between baseline and 6 months | ||||
| Sample size (n) | 301 | 286 | ||
| Consultant surgeon | 173.62 (213.98) | 183.09 (215.48) | –9.47 (–44.29 to 25.35) | 0.593 |
| Specialist nurse at home | 16.76 (77.46) | 27.82 (123.41) | –11.06 (–27.68 to 5.56) | 0.192 |
| Specialist nurse at hospital | 36.14 (66.01) | 56.79 (180.21) | –20.65 (–42.91 to 1.60) | 0.069 |
| Stoma nurse | 48.43 (153.36) | 52.91 (118.96) | –4.48 (–26.81 to 17.85) | 0.694 |
| Other surgical | 33.51 (357.22) | 14.24 (48.44) | 19.27 (–22.58 to 61.11) | 0.366 |
| Total cost of surgical care per patient | 308.46 (514.89) | 334.86 (437.61) | –26.40 (–104.05 to 51.25) | 0.505 |
| Between 6 and 12 months | ||||
| Sample size (n) | 313 | 303 | ||
| Consultant surgeon | 105.85 (140.52) | 141.77 (280.90) | –35.92 (–71.27 to –0.57) | 0.046a |
| Specialist nurse at home | 6.31 (37.63) | 10.81 (69.78) | –4.50 (–13.42 to 4.42) | 0.322 |
| Specialist nurse at hospital | 31.44 (56.21) | 37.73 (81.85) | –6.29 (–17.44 to 4.86) | 0.265 |
| Stoma nurse | 19.03 (52.08) | 29.55 (118.48) | –10.52 (–25.10 to 4.06) | 0.157 |
| Other surgical | 7.16 (26.34) | 10.28 (40.89) | –3.11 (–8.58 to 2.35) | 0.263 |
| Total cost of surgical care per patient | 169.81 (207.35) | 230.15 (413.44) | –60.34 (–112.39 to –8.29) | 0.023 |
Total surgical care costs between baseline and the 12-month follow-up amounted to £461.80 (SD £598.03) per patient in the Hughes repair arm and £484.84 (SD £568.73) per patient in the standard mass closure arm. The mean difference of –£23.04 (95% CI –£123.17 to £77.09) was not statistically significant (p = 0.651).
A summary of all other secondary care costs between baseline and 6 months, and between 6 and 12 months, respectively, based on all available cases, is presented in Table 26.
| Secondary care resource type | Mean (SD) cost (£) per patient | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Hughes repair arm | Standard mass closure arm | |||
| Between baseline and 6 months | ||||
| Sample size (n) | 310 | 305 | ||
| Emergency department visits | 76.02 (172.86) | 94.61 (309.04) | –18.59 (–58.36 to 21.17) | 0.359 |
| Outpatient visits | 248.74 (451.98) | 301.30 (546.65) | –52.55 (–132.06 to 26.95) | 0.195 |
| Day surgery visits | 897.69 (2495.88) | 739.66 (2365.53) | 158.03 (–227.01 to 543.07) | 0.421 |
| Inpatient stays | 1856.51 (4207.74) | 1438.53 (3142.28) | 417.98 (–169.578 to 1005.53) | 0.163 |
| Occupational health visits | 4.74 (46.28) | 2.98 (18.15) | 1.76 (–3.80 to 7.32) | 0.534 |
| Ambulance | 15.13 (57.37) | 9.61 (34.10) | 5.52 (–1.95 to 12.98) | 0.147 |
| Other hospital care | 31.17 (142.59) | 20.18 (82.58) | 10.99 (–7.44 to 29.41) | 0.242 |
| Total other hospital cost per patient | 3130.00 (5154.99) | 2606.87 (4369.64) | 523.13 (–234.21 to 1280.47) | 0.175 |
| Between 6 and 12 months | ||||
| Sample size (n) | 315 | 306 | ||
| Emergency department visits | 25.96 (77.16) | 26.72 (82.36) | –0.76 (–13.35 to 11.82) | 0.905 |
| Outpatient visits | 176.80 (301.37) | 236.19 (563.92) | –59.39 (–130.99 to 12.21) | 0.101 |
| Day surgery visits | 372.22 (1670.29) | 448.65 (1518.55) | –76.43 (–327.86 to 175.01) | 0.551 |
| Inpatient days | 640.63 (1918.51) | 715.74 (2434.17) | –75.11 (–421.18 to 270.97) | 0.670 |
| Occupational health visits | 1.78 (15.70) | 1.83 (11.88) | –0.05 (–2.24 to 2.14) | 0.963 |
| Ambulance | 9.61 (68.84) | 8.30 (49.97) | 1.31 (–8.15 to 10.77) | 0.785 |
| Other hospital care | 17.63 (104.91) | 14.05 (55.83) | 3.58 (–9.62 to 16.77) | 0.595 |
| Total other hospital cost per patient | 1244.63 (2799.55) | 1451.48 (3176.99) | –206.85 (–678.39 to 264.68) | 0.389 |
The total other secondary care costs between baseline and the 12-month follow-up amounted to £4391.34 (SD £6219.10) per patient in the Hughes repair arm and £3751.22 (SD £5643.80) per patient in the standard mass closure arm. The mean difference of £640.12 (95% CI –£378.30 to £1658.54) was not statistically significant (p = 0.217).
Cost of cancer treatment
Based on the available cases, adjuvant chemotherapy was given to 125 patients in the Hughes repair arm (n = 359) following their colorectal surgery, compared with 135 patients in the standard mass closure arm (n = 348). Seven people in the Hughes repair arm suffered local recurrences, of whom one was treated with radiotherapy and four were treated with chemotherapy. In the standard mass closure arm, nine patients with local recurrences were recorded, of whom three received radiotherapy and six received chemotherapy. Metastatic disease was diagnosed in 36 Hughes repair patients, with two radiotherapy and 16 chemotherapy treatments recorded. In the standard mass closure arm, 38 patients were diagnosed with metastatic diseases, with one treated with radiotherapy and 21 treated with chemotherapy.
The total mean cost of all cancer treatment (excluding surgery) was £1544.90 (SD £2295.63) per patient in the Hughes repair arm and £1781.35 (SD £2445.02) per patient in the standard mass closure arm. The mean difference of £236.46 (95% CI –£586.89 to 113.98) in favour of Hughes repair was not statistically significant (p = 0.186).
Total health-care costs
The total post-surgery health-care costs (based on available cases) included the cost of the index hospital stay post surgery, the cost of all primary, secondary and social care in the 12 months following discharge from hospital after the index operation, and the cost of any additional cancer treatment. Patients in the Hughes repair arm accrued mean per-patient post-surgery health-care costs of £15,295.24 (SD £9343.15), compared with £14,650.33 (SD £9092.01) in the standard mass closure arm. The incremental cost of Hughes repair was £644.91 (95% CI –£633.01 to 1922.83). The higher cost was caused by the longer index hospital stay and higher inpatient costs in the first 6 months following surgery, but the difference was not statistically significant (p = 0.322).
Adding the intervention mean per-patient implementation costs of £106.26 increases the cost difference to £751.17 (95% CI –£526.75 to £2029.10; p = 0.249) for the Hughes repair arm in the available-case population.
Total costs of the intention-to-treat population
Consistent with the statistical analysis, the cost-effectiveness and cost–utility analyses were based on the ITT population. Including all patients for whom primary outcome data were available at the 12-month follow-up point resulted in 339 patients in the Hughes repair arm and 333 patients in the standard mass closure arm.
Although these patients had complete primary outcome data, not all cost data were recorded at all time points for every patient. Missing data were spread relatively evenly between both trial arms, with 9.3% of cases with missing data in the standard mass closure arm and 7.7% in the Hughes repair arm. It was noted that 4.8% of patients in the standard mass closure arm and 3.2% of patients in the Hughes repair arm had systematically missing data because the first 37 patients entered into the trial had not been surveyed for health resource utilisation data at baseline. Therefore, as the data were not missing at random and the remaining missing data affected < 5% of the sample, the population mean health-care cost was used for imputation of missing data.
The mean total cost per patient for the ITT population was £15,490.32 (SD £8688.03) in the Hughes repair arm and £14,873.87 (SD £8685.73) in the standard mass closure arm, with a mean difference of £616.45 (95% CI –£699.56 to £1932.47; p = 0.358). This difference is made up of higher costs for the index hospital stay post surgery (mean £213.10, 95% CI –£527.05 to £953.26; p = 0.572) and subsequent health-care costs (mean £453.07, 95% CI –£498.64 to £1404.79; p = 0.350), lower costs for cancer treatment and adjuvant chemotherapy (mean –£155.98, 95% CI –£510.49 to £198.54; p = 0.388) plus an incremental intervention cost of £106.26 for all patients who underwent Hughes abdominal repair (Figure 9).
FIGURE 9.
Mean cost differences between Hughes repair and standard mass closure patients in the ITT population.
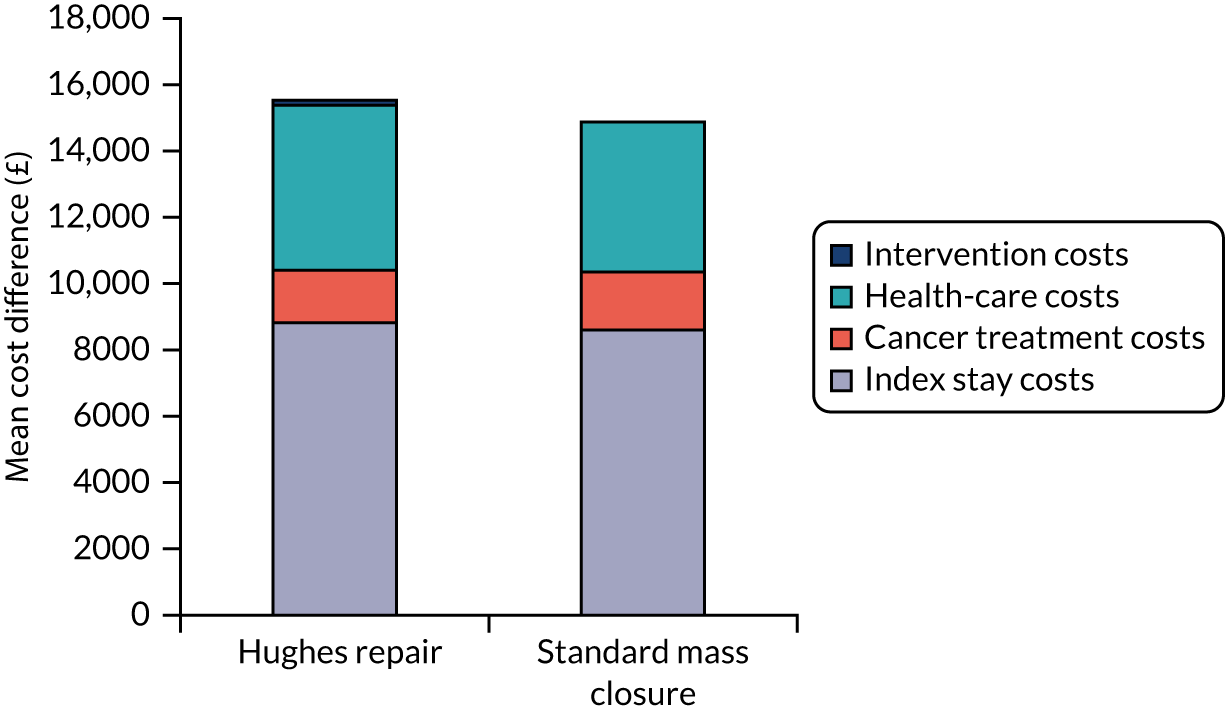
There was a statistically non-significant baseline imbalance in health-care costs for the ITT population. In the 6 months prior to randomisation, reported health-care resource use amounted to a mean total cost of £2065.30 (SD £3350.06) per patient in the Hughes repair arm (based on data for 256/339 patients available) compared with £1756.59 (SD £2623.74) in the standard mass closure arm (based on data for 247/333 patients), with a mean difference of £308.71 (95% CI –£217.47 to £834.10; p = 0.250) per patient. The difference was mainly attributed to the higher secondary care costs incurred by patients in the Hughes repair arm prior to surgery and fell slightly when mean imputation was applied to all missing cases to £231.02 (95% CI –£163.30 to £625.33; p = 250).
Health outcomes
The incidence of IH at 1-year clinical examination was 14.75% in the Hughes repair arm compared with 17.12% in the standard mass closure arm (OR 0.84, 95% CI 0.55 to 1.27; p = 0.40).
Using the ITT population (with mean imputation), there was no difference in QALYs between the arms at 12 months (p = 0.979). In the 12 months post randomisation, patients accumulated, on average, 0.6902 (SD 0.0725) QALYs in the Hughes repair arm compared with 0.6901 (SD 0.0673) QALYs in the standard mass closure arm. The QALY difference was 0.0001 (95% CI –0.0105 to 0.0107). The QALY outcomes for available cases and the LOCF imputation of missing data are summarised in Appendix 6.
Cost-effectiveness analysis
The unadjusted mean incremental cost for the ITT population (n = 672) at the 12-month follow-up point was £616.45 higher in the Hughes repair arm. As the number of patients who experienced IH following surgery was 57 (17.12%) in the standard mass closure arm, compared with 50 (14.75%) in the Hughes repair arm (see Table 6), the mean ICER is £26,034 per clinical hernia avoided.
Sensitivity analyses
One-way deterministic sensitivity analyses showed that results are sensitive to changes in costs and effects, with ICERs ranging from £7784 to £81,538 per hernia avoided. The ICERs are most affected by changes in health-care costs and the between-arm difference in hernias.
The majority of results of the 1000 bootstrap iterations during probabilistic sensitivity analysis indicate that Hughes repair is more costly but also more effective than standard mass closure in preventing hernias. However, the results are distributed across all sectors, with cases in which Hughes repair both dominates and is dominated by standard mass closure (Figure 10). This is a result of the small differences in cost and effect.
FIGURE 10.
Cost-effectiveness plane (ITT analysis) for the base case (incremental cost per hernia avoided).
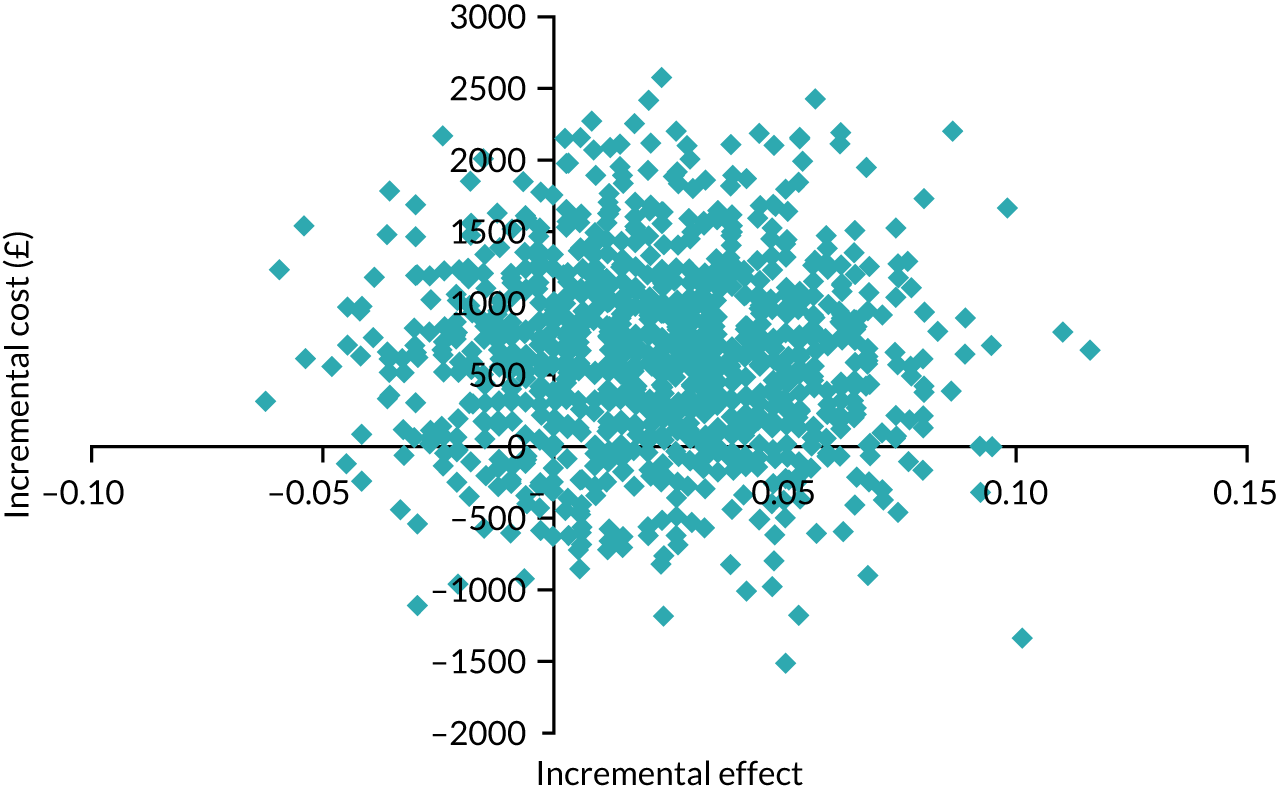
No willingness-to-pay threshold exists for cost-effectiveness analysis. However, the incremental cost of a hernia could be used as a proxy. During the trial, 107 patients experienced an IH and 565 did not. The mean total cost per hernia patient was £15,968.60 (SD £10,411.67). Patients who did not experience IH had a mean total cost of £15,036.42 (SD £8321.39). This means that the costs of patients with hernias were, on average, £932.18 (95% CI –£1175.71 to £3040.08; p = 0.383) higher than those of patients without hernias.
Removing Hughes repair training costs from the total costs, as part of scenario analysis, did not alter the ICER. However, after adjusting for the baseline imbalance in the health-care costs (£231.02 higher in the Hughes repair arm), the ICER was reduced to £16,278 per hernia avoided.
Cost–utility analysis
Based on the unadjusted incremental cost of Hughes repair compared with standard mass closure and the marginal QALY increase, the base-case ICER is £4,359,353 per QALY gained.
Sensitivity analyses
The direction of the results does not change when inputs are altered during deterministic sensitivity analysis (Table 27). Owing to the small between-arm differences in QALY gain, the results are sensitive to changes in this variable without the cost-effectiveness conclusions being affected.
| Parameter | Change | Most cost-effective option |
|---|---|---|
| N/A | N/A | Standard mass closure |
| Costs | 100% registrar or 100% consultant at training | Standard mass closure |
| Costs | Training costs removed | Standard mass closure |
| Costs | –10% | Standard mass closure |
| Costs | –20% | Standard mass closure |
| Costs | –30% | Standard mass closure |
| Costs | + 10% | Standard mass closure |
| Costs | + 20% | Standard mass closure |
| Costs | + 30% | Standard mass closure |
| Costs | Long-course radiotherapy (25 fractions) | Standard mass closure |
| Costs | Baseline imbalance adjusted | Standard mass closure |
| Costs | Patients who withdrew removed instead of censoring at withdrawal date | Standard mass closure |
| Utilities | –10% | Standard mass closure |
| Utilities | –20% | Standard mass closure |
| Utilities | –30% | Standard mass closure |
| Utilities | + 10% | Standard mass closure |
| Utilities | + 20% | Standard mass closure |
| Utilities | + 30% | Standard mass closure |
| All parameters | Last observation carried forward | Standard mass closure |
| All parameters | All available cases used | Standard mass closure |
Probabilistic sensitivity analysis shows that results are distributed across all quadrants of the CE plane when all analysis inputs are altered simultaneously within predefined ranges. Possible scenarios range from Hughes repair being dominated to it dominating standard mass closure, as a result of the small differences in costs and QALYs between the arms (Figure 11). Overall, the probability that Hughes repair is more cost-effective at a willingness-to-pay threshold of £20,000 per QALY gained is 18.9% (Figure 12).
FIGURE 11.
Cost-effectiveness plane (ITT analysis) for the base-case CUA (incremental cost per QALY gained).
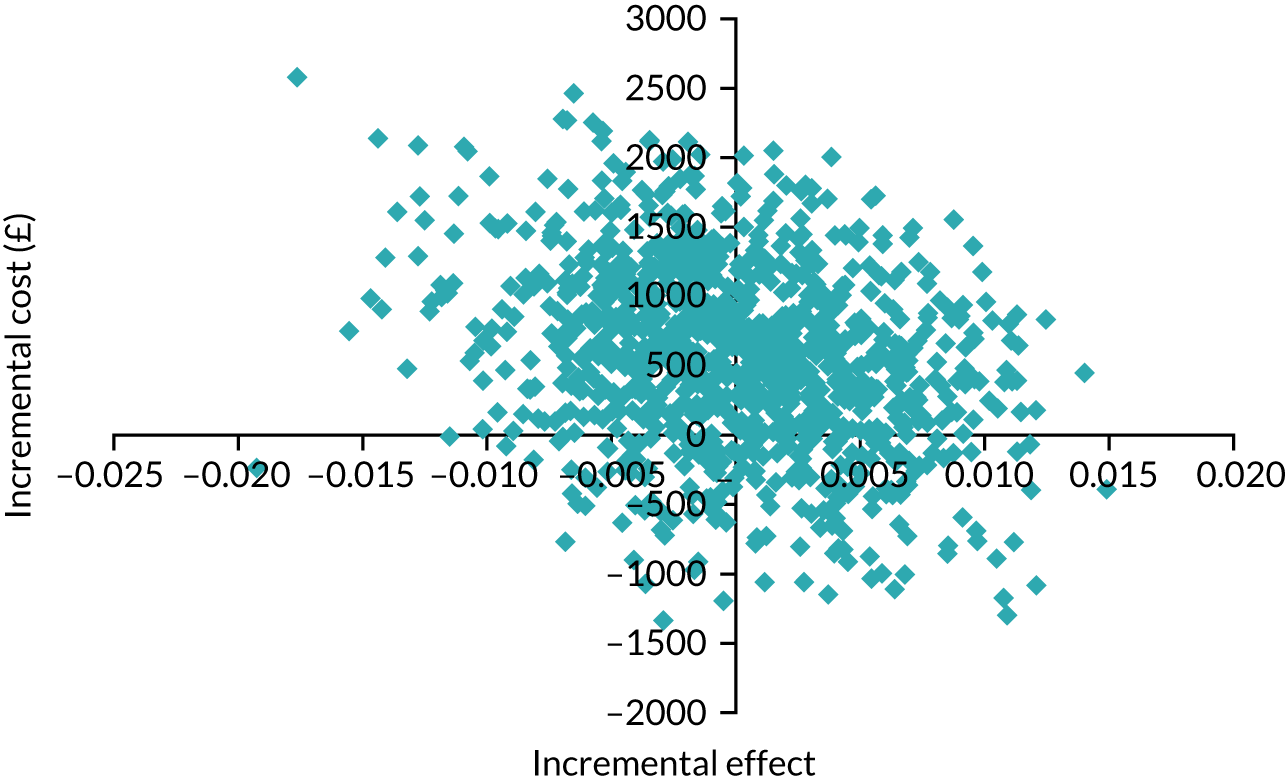
FIGURE 12.
Cost-effectiveness acceptability curve (ITT analysis) for the base-case CUA (incremental cost per QALY gained).
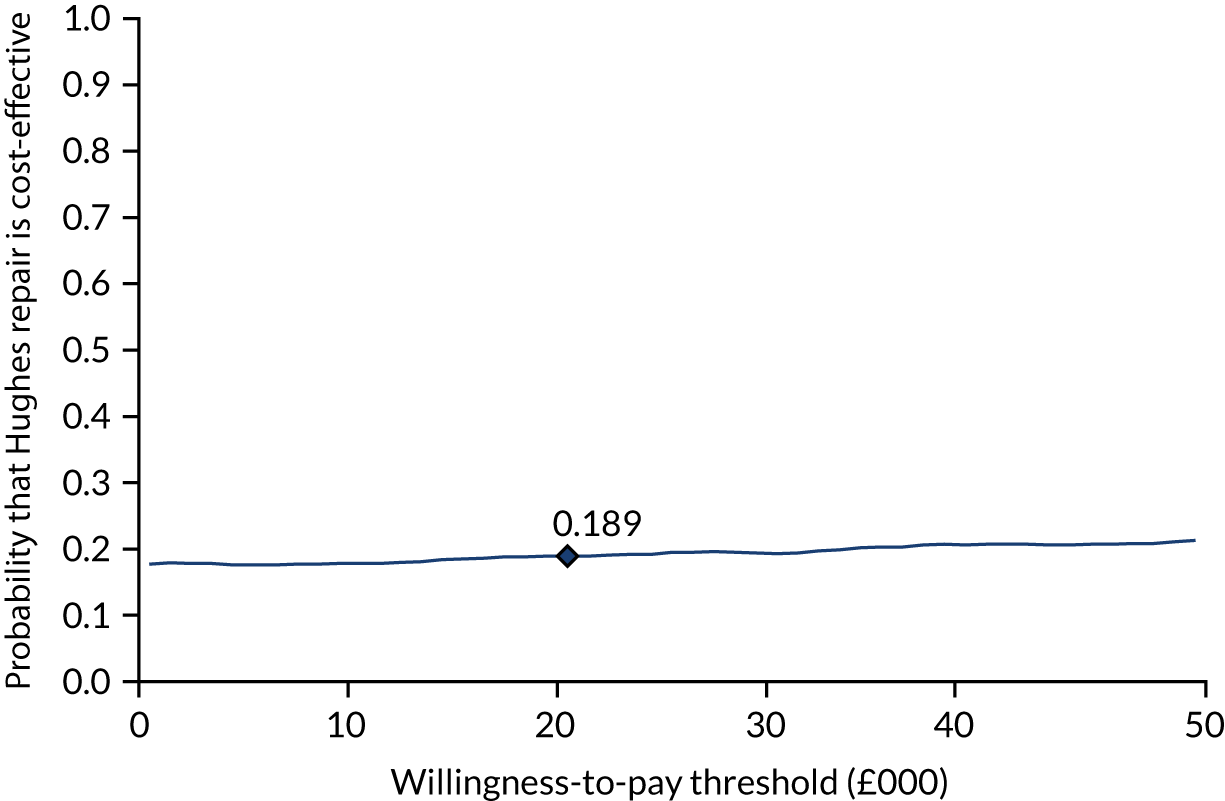
Removing all staff training costs as part of scenario analysis did not affect the ICER. Using LOCF as imputation method instead of mean imputation in the base case gives a mean QALY gain over 12 months of 0.6856 for Hughes repair patients and 0.6844 for standard mass closure patients. The slightly higher QALY difference of 0.0012 (95% CI –0.0119 to 0.0143) reduces the ICER to £516,421 per QALY gained.
Any utility imbalances at baseline were adjusted automatically as QALYs are calculated as relative gains. However, when adjusting for the baseline imbalance in health-care costs, the ICER was reduced to £2,725,658 per QALY gained, with a probability of 29.4% that Hughes repair is cost-effective.
Cost–consequences analysis
Table 28 summarises the results of the cost–consequences analysis.
| Hughes repair arm (n = 339) | Standard mass closure arm (n = 333) | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Cost impact (per patient, ITT population) | ||||
| Mean implementation cost | 106.26 | – | 106.26 | – |
| Mean index operation cost (SD) | 8818.92 (4282.83) | 8605.82 (5431.15) | 213.10 (–527.05 to 953.26) | 0.572 |
| Mean cancer treatment cost (SD) | 1591.01 (2306.38) | 1746.99 (2373.98) | –155.98 (–510.49 to 198.54) | 0.388 |
| Mean health resource use cost (SD) | 4974.13 (6420.69) | 4521.06 (6138.00) | 453.07 (–498.64 to 1404.79) | 0.350 |
| Total cost of all NHS resource use (SD) | 15,490.32 (8688.03) | 14,873.87 (8685.73) | 616.45 (–699.56 to 1932.47) | 0.358 |
| Health impact | ||||
| IHs at 1 year (primary end point), n (%) | n = 339; 50 (14.75) | n = 333; 57 (17.12) | 0.84 (0.55 to 1.27)a | 0.40 |
| SSI, n (%) | n = 401; 53 (13.2) | n = 401; 31 (7.7) | 1.82 (1.14 to 2.91)a | 0.01 |
| SF-12 PCS at 12 months | n = 289; 43.1 (4.4) | n = 267; 43.7 (4.5) | –0.56 (–1.3 to 0.19) | 0.10 |
| SF-12 MCS at 12 months | n = 289; 53.9 (12.1) | n = 268; 53.7 (11.7) | 0.21 (–1.77 to 2.18) | 0.83 |
| QALYs at 12 months | n = 339; 0.6902 (0.07) | n = 333; 0.6901 (0.07) | 0.0001 (–0.010 to 0.011) | 0.98 |
| FACT-C at 12 months | n = 277; 67.6 (8.7) | n = 268; 67.3 (9.7) | –0.34 (–1.89 to 1.21) | 0.66 |
Chapter 5 Discussion
The primary outcome of the HART study was the incidence of IH, which was diagnosed by clinical examination at 1 year postoperatively, in a cohort of colorectal cancer patients.
The results have demonstrated no statistically significant difference between the Hughes method of abdominal wall closure and standard closure methods in terms of this primary outcome.
Secondary outcomes defined in the protocol included:
-
QoL between the arms and QoL between those who did and those who did not develop an IH at 1 year – the results showed no significant difference between Hughes repair and standard mass closure for any QoL measure. However, a significantly lower PCS score (SF-12) was identified in patients at baseline who went on to develop IH regardless of surgical technique.
-
Prevalence of IH at 1 year between arms – the results showed no difference in prevalence.
-
Risk factors for IH – the identification and characterisation of patient and surgical factors associated with an increased risk of developing an IH yielded significant results. First, they confirmed the known risk factors of age, male sex and increasing BMI, and, second, they suggested potential additional risk factors, including preoperative radiotherapy and high POSSUM scoring.
-
Cost-effectiveness – Hughes repair was more costly and marginally more effective (in terms of preventing clinically diagnosed hernias and QoL measured as QALYs) and was found to be less cost-effective than standard mass closure.
A number of tertiary outcomes were defined in the protocol; however, as the majority of these were related to 5-year follow-up and were dependent on funding, they were not included in the scope of the NIHR Health Technology Assessment grant, which provided funding for 2 years. These outcomes will be evaluated as part of planned extended follow-up using routinely collected NHS data sets. In the case of one tertiary outcome – the sensitivity and specificity of CT imaging compared with that of clinical examination over 5 years – the results at 1 year are included in this report. As part of follow-up and data collection, CT scanning was carried out at 1 year to identify IHs that might have been missed by clinical examination, and the results are discussed further below. The study protocol included CT scanning at 2 years; however, although CT scans were collected by individual sites, the onset of the COVID-19 pandemic resulted in a reorganisation of many key services, including radiology. This meant that the study radiologists were unable to read and reconcile the 2-year scans in time for inclusion in this report.
The primary outcome of the study was the incidence of IH as diagnosed by clinical examination. Clinical examination is a standard part of follow-up for patients who undergo surgery for colorectal cancer and was chosen as the primary method of detection of IH to give a clearly defined single end point. The incidence of IH at 1 year using this method of detection was 14.8% in the Hughes arm compared with 17.1% in the standard mass closure arm. This difference was not statistically significant (p = 0.402). A lower, but also non-significant, rate of IH in the Hughes repair arm was seen using the same method of detection at 2 years (28.7% vs. 31.8%; p = 0.429).
As this was a pragmatic study, with surgeons in the standard mass closure arm allowed to use their individual preference for closure, inevitably there was variation in factors such as suture material and suture type in the standard mass closure arm, which was not the case in the Hughes repair arm. In sites that contributed more than 10 patients to the study, the rate of IH in standard mass closure arm ranged from 10% to 47%, indicating a potentially wide variation in the techniques used (see Appendix 5, Table 33). However, logistic regression analysis found no significant difference in the rate of incisional hernia when comparing high- and low-recruiting sites between the arms (see Table 7).
Overall, the study reported a much lower incidence of IH with clinical examination than had been anticipated. The lower rate of clinically detectable IH in the study as a whole may have been anticipated from the tendency for patients enrolled in trials to have better outcomes than those in real-world scenarios, as a result of the focused attention on wound closure and also an inherent learning effect as a result of trial participation by surgeons. The high standard of care given is also suggested by the overall low rate of complete abdominal wall dehiscence (0.79%) and also the relatively low rate of SSIs (13.2% vs. 7.7% in the standard mass closure arm). It is always possible, even in a pragmatic design such as this, that self-selection of surgeons and sites occurs, with those more likely to take part having a particular interest in one or both of the interventions being assessed.
However, when considering the findings from using CT scanning to identify IH, it appears likely that the true incidence of IH was underestimated as a result of the decision to use clinical examination as the method of detection. The study was powered to show a reduction in rate of IH between the arms from 30% for standard mass closure to 20% for Hughes repair. This was based on a retrospective internal audit carried out at the University Hospital of Wales, which suggested that the difference in IH rates between Hughes repair and standard mass closure could be as high as 18%. Crucially, this audit used CT scanning as the assessment tool, with two groups of surgeons using different techniques.
The use of clinical examination as the primary assessment method has proven to be the poorer technique for detecting IH. Logically, it may make smaller hernias, particularly if they are asymptomatic, more difficult to diagnose. A systematic review and meta-analysis48 reported that between 15% and 48% of reported IH diagnoses were established by CT scanning alone. In the HART study, using CT scanning alone as a detection method resulted in no significant difference in the rates of IH at 1 year. CT scanning alone identified an additional 114 (36.3%) IHs in the Hughes arm and 103 (32.6%) IHs in the standard mass closure arm at 1 year (see Table 14). The proportion of IHs identified using CT scanning was 48% (n = 151) in the Hughes arm (compared with 14.8% using clinical examination) and 47.5% (n = 150) in the standard mass closure arm (compared with 17.1% using clinical examination). The results from the systematic review show that there is currently no objective gold standard for the diagnosis of IH. 49 Ongoing trials, such as the HULC trial24 (clinical or imaging), the ESTOIH study23 (ultrasound) and the E-STITCH trial (clinical examination or ultrasound), are all utilising a method of radiological examination for detection either alone or in addition to clinical examination, supporting the theory that clinical examination alone is not sufficient to accurately diagnose IH. In addition, the authors of the systematic review reported that there was no consensus definition of IH across the included studies, potentially contributing to interobserver disagreement rates, which ranged from 11.2% to 14.2%. However, it should be noted that only one study using CT scanning reported interobserver disagreement rates. In the HART study, CT images were independently read by two blinded radiologists and any disagreements were discussed and resolved by consensus.
One subject of ongoing work is whether or not there is a difference between the size of hernias and the symptoms that they cause. The clinical relevance of IHs detected solely by imaging methods and the proportion that require treatment are unclear. 49 It is hoped that long-term follow-up will allow an understanding of whether or not there is clinical value in identifying asymptomatic IHs that are detectable only on a scan and whether or not these would benefit from early repair. Long-term follow-up will, therefore, aim to detect and identify those hernias that require treatment and repair and find if there is a difference between the groups (i.e. a difference in ‘clinically significant’ incisional hernias).
Age, male sex, high BMI and emergency surgery are established and accepted risk factors for IH, which the HART study confirmed. The identification of potential new risk factors merits discussion. The use of preoperative radiotherapy in the treatment of rectal cancer is an established adjunct to surgery. The radiotherapy aims to shrink the tumour to facilitate its removal or at least to sterilise the surrounding tissue to minimise the risk of local recurrence. Damage to healthy tissue is a recognised limitation of radiotherapy, and the inclusion of the lower part of a midline incision provides a compelling hypothesis for mechanism of action in the increased risk for IH that we have found. Collagen damage as a result of incidental irradiation inhibits healing and, thus, leads to an increased risk of IH. This aspect merits further investigation in future trials, particularly given the small number of patients overall included in the HART study who had received radiotherapy.
POSSUM scoring was also shown to be a risk factor for IH at 2 years, and we are unaware that this has been reported previously. The POSSUM score is a measure of physiological and operative risk assessment for morbidity and mortality predictions; our findings suggest that the POSSUM score might be used to identify patients at higher risk of developing IH in the long term.
At baseline, patients with IH had a significantly lower mean PCS score on the SF-12 (mean difference –1.40, 95% CI –2.70 to –0.11; p = 0.03), regardless of the closure technique received. This is indicative of a lower level of physical activity prior to surgery in those patients who develop an IH. This finding supports the current increasing attention given by the medical profession to prehabilitation: optimising preoperative conditions and health to minimise postoperative complications wherever possible. Enhanced Recovery after Surgery (ERAS) guidelines50 state that the evidence currently suggests promising results for multimodal prehabilitation in the recovery of functional capacity and may reduce complications after colorectal surgery, with less fit patients possibly more likely to benefit. The guidelines state that further research is required before this is considered a mandatory item in an ERAS protocol. To this end, a clinical trial is currently investigating the impact of multimodal prehabilitation on patients’ functional capacity and postoperative complications. 51 One question raised by the findings from the HART study is whether a low PCS score is a marker of poor health in general or indicative of other factors, such as motivation or social deprivation. The reason for the low PCS score at baseline should be investigated further, as the success of any prehabilitation programme is likely to be impacted by the reasons for that low score. It should be noted that approximately 20% of values were missing at baseline for all of the patient-reported outcomes (see Tables 8 and 9). The missing data occurred primarily at the largest study sites during the busy recruitment period, suggesting that time pressures on research staff may have had an impact on data collection and completeness.
There was no significant difference between the arms when comparing elective with emergency surgery (see Appendix 5, Tables 34 and 35) or when comparing stoma with no stoma (see Appendix 5, Tables 36 and 37). There was a significant difference between the arms in terms of SSI (see Appendix 5, Table 38) (13.2% in the Hughes group vs. 7.7% in the standard mass closure arm; p = 0.01), but not when SSI was considered by the presence or absence of IH (see Appendix 5, Table 39). There was no significant difference in the severity of SSI (see Appendix 5, Table 40). The Hughes technique required the use of interrupted non-absorbable nylon sutures in addition to the absorbable continuous component. The higher incidence of SSI in the Hughes arm may be explained by the bulkier knots required for the nylon sutures and their non-absorbable nature giving rise to a nidus for infection. Other plausible causative factors might include a longer closure time or simply the use of more suture material. There appears to be no evidence to preclude these interrupted sutures being placed using a non-absorbable suture to reduce this risk in the future use of the technique.
The study also included a health economic evaluation exploring the implementation costs of Hughes repair and its effect on subsequent health-care resources, and calculated the ICERs as part of a cost-effectiveness and a cost–utility analysis. To our knowledge, this is the first study reporting the cost-effectiveness of the use of Hughes repair compared with standard mass closure to reduce the incidence of IH.
The total incremental cost of the Hughes repair technique was £616.45 per patient at 12 months, driven mainly by the higher inpatient cost, both post surgery and subsequently, and the additional cost of the Hughes repair (£106.26 for surgeon training, sutures and additional surgery time). The ICERs were £26,034 (range in sensitivity analysis: £7784 to £81,538) per hernia avoided and £4,359,353 (range in sensitivity analysis: £57,505 to £13,649,859) per QALY gained, with a probability of 18.9% that Hughes repair is cost-effective at a willingness-to-pay threshold of £20,000. The high ICERs are caused by the increased cost but marginal incremental effect, especially on QALYs, of the Hughes repair technique compared with standard mass closure.
The increased total health-care costs in the Hughes repair arm were largely attributed to higher hospital inpatient costs. The longer index hospital stay post surgery in the Hughes repair arm may be associated with the significantly higher incidence of SSIs, although this seems unlikely given the low overall SSI incidence. However, no reasons for subsequent hospitalisations were recorded as part of the trial and it is unknown why Hughes repair patients had, on average, £417.98 higher inpatient costs and £158.03 higher day-case costs than standard mass closure patients in the first 6 months following surgery. These increased costs may still be linked to the initial SSIs. However, the statistically non-significant baseline imbalance in mean total health-care costs of £231.02 in the Hughes repair arm suggests that they may also be an artefact of sampling variation or the small number of events rather than an indication of an underlying issue related to the intervention itself.
Limitations
The pragmatic nature of the study, chosen to maximise study engagement by surgeons and sites, and patient recruitment nonetheless led to a wider variation in the incidence of IH in the standard mass closure arm than had been anticipated and may have affected the results. Shortly after the HART study commenced, the STITCH trial28 was published, showing reduced IH rates using the small-bites technique. This led to the significant promotion of this technique, which coincided with the early part of the HART study. This introduced two issues likely to have had an impact on our findings. First, surgeons were learning a second new technique (small stitch) in a non-controlled fashion, which potentially confounded the control arm. Second, it led to some surgeons losing equipoise, as they may have considered the issue of IH prevention resolved and felt that there was no role for the Hughes technique.
Intraoperative randomisation also highlighted the issue of equipoise. Hughes repair took longer to perform, with a mean time for fascial closure of 22 minutes in the Hughes repair arm, compared with 13.2 minutes in the standard mass closure arm. It is possible that at the end of difficult procedures surgeons chose not to randomise and so this important cohort of patients were lost to the study. This is hinted at by considering those who were randomised but did not receive their allocated treatment. A total of 15 patients fell into this category, 13 of whom were in the Hughes arm. Of these 13 patients, three had an IH at 1 year by clinical examination (i.e. 23%, compared with 14.8% in the Hughes arm) (analysed on an ITT basis). In addition, among patients who had consented but were subsequently not randomised, reasons for non-randomisation included ‘ran out of time’. These arguments around equipoise may also explain the large numbers of patients who were screened but did not enter the trial. In addition, screening logs do not report the route (emergency or elective) through which patients considered eligible but not consented were identified. Only 59 patients (standard mass closure arm, n = 29; Hughes repair arm, n = 30) underwent emergency surgery, representing 7.3% of the study population. In the UK, up to 20% of patients with colorectal cancer present in the emergency setting,18 suggesting that a number of potentially eligible patients may not have been identified. The mean time from consent to randomisation was only 0.4 days for emergency patients (compared with 2.7 days for elective patients), suggesting an increased urgency in treating patients in the emergency setting, and so surgeons may have had concerns around factors such as giving eligible patients enough time to decide to take part in the study.
One further limitation of this trial is considered to be the lack of follow-up time. European Hernia Society guidelines published after the commencement of the HART study recommend a minimum of 2-year follow-up and also imaging as a method of detection rather than clinical examination alone. 52 The 1-year follow-up suggests that rates of IH do not differ significantly between the two methods of closure. Further analysis of 2-year data similarly shows no significant difference in the incidence of IH detected by clinical examination. The lack of long-term follow-up in clinical trials is a common limitation, and outcomes such as IH can develop at any time after surgery, lending support to the idea that a minimum follow-up for trials should be considered to ensure that there is enough follow-up time for primary outcomes to be observed.
A limitation of the health economic evaluation is the use of mean imputation rather than multiple imputation to account for missing data in line with the analysis plan and protocol. Although this will cause CIs to be narrower than when using multiple imputation, considering that the number of missing cases was small, and also the overwhelming evidence pointing to the intervention being less cost-effective, it is unlikely that the approach to address missing data and the missingness will have had an impact on the findings. In addition, the health economic evaluation uses independent samples t-tests to derive incremental costs and QALYs. This approach does not account for any potential imbalances at baseline or the likely skewed nature of the data, particularly costs, which are often gamma or log-normal distributed. However, although the simple analysis does not adjust for baseline covariates, no significant baseline imbalances were found and undertaking regression modelling would not change the direction of the results. The results of the cost–utility analysis are sensitive to changes in patient QoL, which is caused by the small differences in utility scores between the two arms. Considering that disease-specific questionnaires have shown significantly reduced QoL in patients with IH after colonic cancer resection,53 this could be caused by an insensitivity and lack of responsiveness of the generic SF-12 instrument to small changes in health status with specific conditions. However, the SF-36 questionnaire, a longer version of the SF-12, was found to be suitable for picking up changes in QoL in hernia patients,54 and no significant difference was recorded in this trial in FACT-C scores. Therefore, the difference in hernia incidence of seven cases in year 1 could be too small to affect the mean QoL of the trial population.
Generalisability
The HART study was consciously designed as a pragmatic study to ensure that the results were as generalisable as possible across the UK and beyond in similar health-care systems. With its simple design and the addressing of a common clinical problem with a low-tech solution, it had the potential to be practice changing.
All patients with colorectal cancer were eligible, including elective and emergency patients. This was important because up to 20% of patients with colorectal cancer present as emergencies. A relatively small number of emergency patients was recruited in the end, reflecting the difficulties of trial recruitment in that setting. However, the broad demographic picture is representative of the colorectal cancer population in age, sex, ethnicity and comorbidity, allowing the results to be extrapolated across this group. The wide range of sites, from large university hospitals to smaller district general hospitals, also enhances this. In relation to ethnicity specifically, statistics published in 2009 from the National Cancer Intelligence Network55 report that age-standardised rates for colorectal cancer in England are lower in all ethnic groups, including black, Asian and Chinese ethnic groups, for both male and female patients than in the white ethnic group, and the authors consider this to be borne out in the lower proportion of patients recruited to the trial from these ethnic groups. The authors acknowledge, however, that understanding when risk may differ for different ethnic groups and ensuring that these groups are adequately represented in clinical trials is crucial to generating results that are of benefit to those most at risk.
This trial included only colorectal cancer patients and it is unclear whether patients undergoing surgery for colorectal cancer are different in any way from patients undergoing midline incisional surgery for other reasons. It is certainly appreciated that patients with abdominal aortic aneurysms are at very high risk for IH following surgery and, from the CT scan results presented here, it is clear that colorectal cancer patients are also a vulnerable group.
Informing future work
The HART study has identified some important learning points for further work in this field and a number of research recommendations can be made.
Key learning points
-
Incisional hernia remains a significant clinical postoperative problem for patients undergoing midline incisional surgery and merits future investment in research, particularly in relation to factors such as long-term follow-up, diagnostic accuracy of clinical examination, identification of high-risk patients and effectiveness of other techniques, such as small-stitch techniques.
-
Colorectal cancer patients have a high incidence of IH and represent a good study group for future trials.
-
The identification of high-risk patients may enable the number of participants required for future trials to be reduced.
Research recommendations
-
What is the long-term risk of IH in patients who have had a midline incision?
-
Long-term follow-up should be built into study design and funding to enable more understanding of the natural history of IH formation.
-
-
What is the diagnostic accuracy of clinical examination and imaging in the identification of IH?
-
Include imaging modalities such as CT scanning, PET (positron emission tomography) scanning, PET-CT scanning and ultrasound.
-
Include analysis of inter-reader variability and the impact on diagnostic accuracy.
-
-
Are there subgroups of patients who may be more at risk of developing IH following midline incision?
-
Consider factors such as previous radiotherapy and/or chemotherapy.
-
-
What is the effectiveness of small-stitch technique compared with Hughes abdominal repair in the prevention of IH?
-
Small stitch technique as the control arm.
-
The control arm technique should be standardised to prevent variation in that group.
-
-
Decision-analytic modelling based on longer-term (2- and 5-year) follow-up data should be considered in future, as the short time horizon in the current analysis may not be sufficient to adequately address the cost-effectiveness of the intervention.
Patient and public involvement
Patient and public involvement representatives understood that involvement in the HART study was a long-term commitment. The study lasted over 7 years from inception to the 1-year results point and, as a consequence, there was some turnover in the representatives involved at various points in the study. The HART study has been very fortunate to have had PPI representatives who have been actively involved from the very beginning and remain involved in working on the dissemination of the study results. PPI representatives were asked to attend monthly trial management meetings and took part in a number of public events, giving presentations and providing information about the HART study. This long-term commitment to an individual study has proven rewarding and given our PPI members a sense of ownership.
Patient and public involvement in the HART study resulted in a number of important developments that helped the clinical team to engage with study participants in a positive way. For example, one PPI representative suggested that trial packs be produced that could be given patients who were recruited to the study. Later in the process, the PPI representative also suggested that a thank-you card could be sent to all patients who had been recruited to the study to acknowledge the importance of the role of patients who agree to take part in clinical studies. Some study participants took the time to e-mail the study team to say how much they appreciated that gesture. It is perhaps important for study teams to remember that, although patients may be happy to take part in clinical trials and may well have a personal interest in taking part, it is nonetheless important to acknowledge the role that their participation plays, and small gestures can mean a lot.
Listening to the PPI representatives can provide a number of learning opportunities for clinical team members. Although the PPI representatives reported that they were treated with respect and felt that the study team always listened to their point of view and involved them in decision-making processes throughout the study, the patient representative did also indicate they felt a little out of their depth at the start of the process and conscious of the time taken by study team members in helping them to understand their role in the study. It is, therefore, important that study team members maintain an awareness of the needs of their PPI representatives, provide the relevant support and ensure that the representatives understand the value of their contribution. As a result of their positive experience of being involved in the HART study, one PPI representative stated they had gained so much confidence that they are now involved in training others, including research staff, in PPI. Another PPI representative has gone on to become the lead lay research partner for the Wales Cancer Research Centre and participated in the NICE Colorectal Guideline Committee. One patient representative reported that, although they found it stimulating to be part of the HART study, they had concerns about the possible benefit that they could bring, as they were not a colorectal cancer patient. The other patient representative had undergone major surgery for colorectal cancer but had not had an IH, and it is possible that having a patient representative with experience of both colorectal cancer and IH would have brought additional benefit and insight. In future studies, consideration could be given to the inclusion of a patient representative with direct experience of the outcome under investigation (IH) as well as a patient representative with more broadly relevant experiences.
Lessons learned
Data collection and management for such a large, multicentre study posed some logistical issues over the course of the study. Making CT images available to the study radiologists for review presented a particular issue. The study protocol stated that CT images should be transferred to the lead site over the course of the study, but this did not happen. Image files could not be stored in the study databases as part of the patient CRF; therefore, all CT images had to be transferred to the lead site via PACS at the end of the data collection period. In addition, once transferred to the lead site, the CT images remained on the system for only a short time before they were automatically deleted, presenting an additional difficulty. Not resolving this issue until close to the end of the study did create a time pressure that might have been avoided and, for future studies that require images, due consideration should be given to the most efficient way for the study team to access data, including any image files that might not easily be transferred between sites or made available on study databases.
Although all sites kept screening logs, some inconsistencies were noted in the way that sites recorded information. Some sites kept detailed screening logs only from the point at which patients were considered eligible, meaning that the number of people actually screened for eligibility might have been larger than the 3098 reported. Sites were given standard templates to record screening information; however, the use of the template was not mandatory and, as a result, there was variation from site to site in the level of detail recorded.
According to the study screening logs, 700 patients who had been initially considered eligible for inclusion in the trial were not consented to the study. In 259 cases, the reason given was that the patient declined to take part. No further information is available about the reasons for declining and, although patients are not required to give any reason why they do not want to take part in a study, it may be useful, where possible and a patient is willing, to ask for a reason so that researchers might better understand why people do not want to take part in studies. In the case of the HART study, the follow-up requirements may have been considered burdensome, with patients required to consent to 5 years of follow-up along with commitments to completing questionnaires and attending clinic visits at numerous points throughout the 5 years.
Although the study recruited participants from both the elective and the emergency settings, the number of patients recruited from the emergency setting was very small. This might be reflected in the fact that the study screening logs reported that in 288 cases the reason for not consenting an eligible patient was the surgeon’s decision, suggesting that consideration could be given to whether specific recruitment and consent processes may be required that account for the difference between elective and emergency routes. This might help to ensure that the proportion of patients recruited from the emergency setting reflects the current UK situation.
The HART study was a large trial conducted over 7 years. Changes in the make-up of the research team both at individual study sites and within trial management had an inevitable impact on the day-to-day running of the study. Changes in personnel at study sites can have an impact on recruitment rates and follow-up data collection, and changes in the trial management team can result in delays in identifying and responding to problems.
Conclusions
The HART study has provided a rich data set to enhance the understanding of the common and frustrating problem of IH following colorectal surgery. This will continue to yield more insights going forward. In considering a novel closure method using interrupted sutures to reinforce the abdominal wall (the Hughes technique), the trial has failed to show that this confers a statistically significant benefit. It has provided the most definitive evidence for the high incidence of IH after colorectal cancer surgery that will become the reference point in the surgical literature and will encourage research investment in future prevention methods, including the use of mesh.
The HART study has identified new risk factors for IH formation, including preoperative radiotherapy, that will inform future research.
The study has demonstrated the important clinical finding that IH cannot be confidently excluded by clinical examination alone, and this will inform future clinical practice.
The finding of a lower PCS score in patients who go on to develop IH strongly supports the concept of prehabilitation that is being widely promoted to improve postoperative outcomes.
Finally, the HART study has demonstrated that it is possible to carry out high-quality multicentre interventional surgical research in the UK across a wide variety of hospital settings, fostering collaboration and engagement with surgeons and research departments and, crucially, patients and the public and their representatives.
Acknowledgements
The authors wish to thank the patients who took part in the study.
We would like to thank the principal investigators and their teams at all participating sites for their valuable contribution and support.
Thanks to PPI representatives Julie Hepburn, Sian Jones and Colin Thomson for their valuable contributions over the course of the study.
Thanks to Professor Ian Russell who was the Director of WWORTH (the forerunner to STU) and helped to design the trial and secure funding and oversaw the development of the protocol, obtaining regulatory approvals and initiating study.
The authors also wish to thank Dr Buddug Rees (trial manager from September 2014 to May 2018).
Thanks to Professor Andrew Bradbury (chairperson of TSC), Professor Pete Wall (chairperson of DMC), Professor Jim Hill (chairperson of DMC) and Kim Cocks (DMC statistician) for their contributions.
Thanks to the members of the TSC (Tim Rockall, Alastair Windsor, Victoria Allgar, William Hollingworth, Candy Lovell-Smith, Julie Hepburn, Mark Gudgeon, Rhian Gabe and Colin Thomson) and the Trial Management Group (Dave Bosanquet, Heidi Cole, Rwth Ellis Owen, Hayley Hutchings, Craig Parry, Heather Stevens and Zoe Hilton) for their contributions over the course of the study.
Additional thanks go to the research administration team of Natalia Zbrzyzna and Natasha Fiera and to Rhian Jeremy (Radiology) for co-ordinating data collection from individual sites.
Contributions of authors
Susan O’Connell (https://orcid.org/0000-0002-5887-2771) (Healthcare Research Scientist/Triallist) led the writing of the Health Technology Assessment report and contributed to the interpretation of the trial findings. She contributed to the day-to-day trial management.
Saiful Islam (https://orcid.org/0000-0003-3182-8487) (Trial Statistician) co-wrote the statistical and health economic analysis plan and carried out all of the study data analyses. He contributed to the interpretations of the outcomes and drafting of the report.
Bernadette Sewell (https://orcid.org/0000-0001-5471-922X) (Health Economist) designed the health economic evaluation, co-wrote the statistical and health economic analysis plan, carried out the analysis and wrote the health economics sections of the report. She reviewed the final report for consistency.
Angela Farr (https://orcid.org/0000-0002-2087-9310) (Health Economics Analyst) was involved in the health economic analysis and drafting and checking the health economics sections of the report.
Laura Knight (https://orcid.org/0000-0003-2726-8026) (Healthcare Research Scientist/Trial Manager) was responsible for the day-to-day trial management from May 2018. She was responsible for the co-ordination and checking of safety data and contributed to the literature review and writing of the report.
Nadim Bashir (https://orcid.org/0000-0003-0501-1342) (Senior Data Manager at the Swansea Trials Unit) built and maintained the MACRO database for the collection of the data, developed checks and corresponded with sites to ensure data quality and completeness and assisted the statistician with preparation of analytical data sets. He reviewed the final report for accuracy.
Rhiannon Harries (https://orcid.org/0000-0001-7095-3673) (MD FRCS) (Consultant Colorectal Surgeon based at Swansea Bay University Health Board) was a co-investigator for the HART study and was involved in the grant application and the pilot and feasibility studies. She undertook most of the surgeon training and contributed to the writing of the final report.
Sian Jones (Public and Patient Involvement) contributed to the design of the trial. She contributed substantially to the PPI sections of the report and reviewed the final report for accuracy.
Andrew Cleves (https://orcid.org/0000-0003-0431-3138) (Healthcare Research Scientist/Triallist) contributed to the day-to-day trial management. He was responsible for the acquisition of radiology data from individual sites. He reviewed the final report for accuracy.
Greg Fegan (https://orcid.org/0000-0002-2663-2765) (Director of the Swansea Trials Unit) was involved with the statistical and health economic analysis plan, was a conduit between the data manager and statistician to maintain the latter’s blinding prior to data lock, assisted the trial statistician with the analysis, interpreted the trial findings and supported the drafting of the report.
Alan Watkins (https://orcid.org/0000-0003-3804-1943) (Professor, Health Data Science) contributed to study management and design modification, represented the study team on its oversight committees, co-wrote the statistical and health economic analysis plan, and supported the clinical effectiveness analysis. He contributed to the drafting of the report.
Jared Torkington (https://orcid.org/0000-0002-3218-0574) (Professor of Colorectal Surgery) was the chief investigator. He was involved with the original grant application, co-designed the trial, co-wrote the statistical and health economic analysis plan, interpreted the trial findings and supported the drafting of the report.
Publications
Cornish J, Harries RL, Bosanquet D, Rees B, Ansell J, Frewer N, et al. Hughes Abdominal Repair Trial (HART) – abdominal wall closure techniques to reduce the incidence of incisional hernias: study protocol for a randomised controlled trial. Trials 2016;17:454.
HART Collaborative. Incisional hernia following colorectal cancer surgery according to suture technique: the Hughes Abdominal Repair Randomised Trial (HART). Br J Surg 2022; in press.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Harries RL, Cornish J, Bosanquet D, Rees B, Horwood J, Islam S, et al. Hughes Abdominal Repair Trial (HART)–abdominal wall closure techniques to reduce the incidence of incisional hernias: feasibility trial for a multicentre, pragmatic, randomised controlled trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-017235.
- Cornish J, Harries RL, Bosanquet D, Rees B, Ansell J, Frewer N, et al. Hughes Abdominal Repair Trial (HART) – abdominal wall closure techniques to reduce the incidence of incisional hernias: study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1573-0.
- Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Di Carlo V. Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum 2005;48:2217-23. https://doi.org/10.1007/s10350-005-0185-7.
- Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long-term outcome of laparoscopic surgery for colorectal cancer: a Cochrane systematic review of randomised controlled trials. Cancer Treat Rev 2008;34:498-504. https://doi.org/10.1016/j.ctrv.2008.03.011.
- Pereira JA, Pera M, Grande L. Incidence of incisional hernia after open and laparoscopic colorectal cancer resection. Cir Esp 2013;91:44-9. https://doi.org/10.1016/j.cireng.2012.05.003.
- Skipworth JR, Khan Y, Motson RW, Arulampalam TH, Engledow AH. Incisional hernia rates following laparoscopic colorectal resection. Int J Surg 2010;8:470-3. https://doi.org/10.1016/j.ijsu.2010.06.008.
- Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparoscopic vs. open colectomy. Surg Endosc 2002;16:1420-5. https://doi.org/10.1007/s00464-002-8837-3.
- Bosanquet D, Ansell J, Abdelrahman T, Cornish J, Harries R, Stimpson A, et al. Systematic review and meta-regression of factors affecting midline incisional hernia rates: analysis of 14618 patients. PLOS ONE 2015;10.
- Hodgson NC, Malthaner RA, Ostbye T. The search for an ideal method of abdominal fascial closure: a meta-analysis. Ann Surg 2000;231:436-42. https://doi.org/10.1097/00000658-200003000-00018.
- van ‘t Riet M, Steyerberg EW, Nellensteyn J, Bonjer HJ, Jeekel J. Meta-analysis of techniques for closure of midline abdominal incisions. Br J Surg 2002;89:1350-6. https://doi.org/10.1046/j.1365-2168.2002.02258.x.
- Weiland DE, Bay RC, Del Sordi S. Choosing the best abdominal closure by meta-analysis. Am J Surg 1998;176:666-70. https://doi.org/10.1016/S0002-9610(98)00277-3.
- Diener MK, Voss S, Jensen K, Büchler MW, Seiler CM. Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg 2010;251:843-56. https://doi.org/10.1097/SLA.0b013e3181d973e4.
- Gillion JF, Sanders D, Miserez M, Muysoms F. The economic burden of incisional ventral hernia repair: a multicentric cost analysis. Hernia 2016;20:819-30. https://doi.org/10.1007/s10029-016-1480-z.
- Soliani G, De Troia A, Portinari M, Targa S, Carcoforo P, Vasquez G, et al. Laparoscopic versus open incisional hernia repair: a retrospective cohort study with costs analysis on 269 patients. Hernia 2017;21:609-18. https://doi.org/10.1007/s10029-017-1601-3.
- Earle D, Seymour N, Fellinger E, Perez A. Laparoscopic versus open incisional hernia repair: a single-institution analysis of hospital resource utilization for 884 consecutive cases. Surg Endosc 2006;20:71-5. https://doi.org/10.1007/s00464-005-0091-z.
- Finan KR, Kilgore ML, Hawn MT. Open suture versus mesh repair of primary incisional hernias: a cost-utility analysis. Hernia 2009;13:173-82. https://doi.org/10.1007/s10029-008-0462-1.
- Plymale MA, Ragulojan R, Davenport DL, Roth JS. Ventral and incisional hernia: the cost of comorbidities and complications. Surg Endosc 2017;31:341-51. https://doi.org/10.1007/s00464-016-4977-8.
- NBOCA 2019 . National Bowel Cancer Audit 2019: An Audit of the Care Received by People With Bowel Cancer in England and Wales v2.0 n.d. www.nboca.org.uk/reports/annual-report-2019/ (accessed June 2020).
- Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventative strategies. Patient Saf Surg 2010;4. https://doi.org/10.1186/1754-9493-4-5.
- van Ramshorst GH, Eker HH, Hop WC, Jeekel J, Lange JF. Impact of incisional hernia on health-related quality of life and body image: a prospective cohort study. Am J Surg 2012;204:144-50. https://doi.org/10.1016/j.amjsurg.2012.01.012.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30. https://doi.org/10.1371/journal.pmed.1000097.
- Patel SV, Paskar DD, Nelson RL, Vedula SS, Steele SR. Closure methods for laparotomy incisions for preventing incisional hernias and other wound complications. Cochrane Database Syst Rev 2017;11. https://doi.org/10.1002/14651858.CD005661.pub2.
- Fortelny RH, Baumann P, Thasler WE, Albertsmeier M, Riedl S, Steurer W, et al. Effect of suture technique on the occurrence of incisional hernia after elective midline abdominal wall closure: study protocol for a randomized controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0572-x.
- Heger P, Feißt M, Krisam J, Klose C, Dörr-Harim C, Tenckhoff S, et al. Hernia reduction following laparotomy using small stitch abdominal wall closure with and without mesh augmentation (the HULC trial): study protocol for a randomized controlled trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3921-3.
- Rahbari NN, Knebel P, Kieser M, Bruckner T, Bartsch DK, Friess H, et al. Design and current status of CONTINT: continuous versus interrupted abdominal wall closure after emergency midline laparotomy – a randomized controlled multicenter trial [NCT00544583.]. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-72.
- Small Versus Large Bite Closure of Emergency Midline Laparaotomy (E-STITCH) 2019. https://clinicaltrials.gov/ct2/show/NCT04098380 (accessed September 2019).
- Hernia After Colorectal Cancer Surgery: An RCT Comparing 4 : 1-Technique With or Without a Reinforced Tension Line Suture (Rein4CeTo1) 2018. https://clinicaltrials.gov/ct2/show/NCT03390764 (accessed September 2019).
- Deerenberg EB, Harlaar JJ, Steyerberg EW, Lont HE, van Doorn HC, Heisterkamp J, et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet 2015;386:1254-60. https://doi.org/10.1016/S0140-6736(15)60459-7.
- Declaration of Helsinki (1964) . BMJ 1996;313. https://doi.org/10.1136/bmj.313.7070.1448a.
- National Institute for Health and Care Research . Good Clinical Practice (GCP) n.d. www.nihr.ac.uk/health-and-care-professionals/learning-and-support/good-clinical-practice.htm (accessed September 2019).
- Hughes Repair Instructional Video n.d. www.youtube.com/watch?v=pn1Yq_ID3w0 (accessed March 2021).
- Russell D, Hoare ZS, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34. https://doi.org/10.1002/sim.4175.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res 1999;8:181-95. https://doi.org/10.1023/A:1008821826499.
- Hadi M. A Structured Review of Patient Reported Outcome Measures for Colorectal Cancer. Oxford: Patient Reported Outcome Measurement Group; 2010.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
- Medical Dictionary for Regulatory Activities (medDRA) n.d. www.meddra.org/ (accessed March 2021).
- FACIT Group . FACT-C n.d. www.facit.org/measures/FACT-C (accessed March 2021).
- Campbell and Cochrane Economics Methods Group and EPPI-Centre . CCEMG-EPPI-Centre Cost Converter 2019. http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed July 2019).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- Barker J. Interactive complexity and comorbidity splits in Health Resource Group 4+. Br J Healthc Manag 2015;21:433-9. https://doi.org/10.12968/bjhc.2015.21.9.433.
- Department of Health and Social Care . NHS Reference Costs 2017 to 2018 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed September 2019).
- Beecham J, Knapp M. Client Service Receipt Inventory 1997. www.dirum.org/assets/downloads/634462388066137028-CSRI.pdf (accessed September 2019).
- Eichstaedt KE, Kovatch K, Maroof DA. A less conservative method to adjust for familywise error rate in neuropsychological research: the Holm’s sequential Bonferroni procedure. NeuroRehabilitation 2013;32:693-6. https://doi.org/10.3233/NRE-130893.
- Robles-Zurita J, Boyd KA, Briggs AH, Iveson T, Kerr RS, Saunders MP, et al. SCOT: a comparison of cost-effectiveness from a large randomised phase III trial of two durations of adjuvant Oxaliplatin combination chemotherapy for colorectal cancer. Br J Cancer 2018;119:1332-8. https://doi.org/10.1038/s41416-018-0319-z.
- Brazier JE, Roberts JR. The estimation of a preference-based index from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Perneger TV, Burnand B. A simple imputation algorithm reduced missing data in SF-12 health surveys. J Clin Epidemiol 2005;58:142-9. https://doi.org/10.1016/j.jclinepi.2004.06.005.
- Kroese LF, Sneiders D, Kleinrensink GJ, Muysoms F, Lange JF. Comparing different modalities for the diagnosis of incisional hernia: a systematic review. Hernia 2018;22:229-42. https://doi.org/10.1007/s10029-017-1725-5.
- Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019;43:659-95. https://doi.org/10.1007/s00268-018-4844-y.
- van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019;19. https://doi.org/10.1186/s12885-018-5232-6.
- Muysoms FE, Antoniou SA, Bury K, Campanelli G, Conze J, Cuccurullo D, et al. European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia 2015;19:1-24. https://doi.org/10.1007/s10029-014-1342-5.
- Jensen KK, Emmertsen KJ, Laurberg S, Krarup PM. Long-term impact of incisional hernia on quality of life after colonic cancer resection. Hernia 2020;24:265-72. https://doi.org/10.1007/s10029-019-01978-w.
- Rogmark P, Petersson U, Bringman S, Ezra E, Österberg J, Montgomery A. Quality of life and surgical outcome 1 year after open and laparoscopic incisional hernia repair: PROLOVE: a randomized controlled trial. Ann Surg 2016;263:244-50. https://doi.org/10.1097/SLA.0000000000001305.
- National Cancer Intelligence Network . Cancer Incidence and Survival by Major Ethnic Group, England, 2002–2006 n.d. www.ncin.org.uk/publications/reports/reports_archive (accessed March 2021).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed September 2019).
Appendix 1 Study summaries
| Study details | Population and setting | Method of allocation to intervention/control | Objectives, outcomes and methods of analysis | Results |
|---|---|---|---|---|
|
Harries et al. , 20171 Randomised feasibility trial |
University Hospital of Wales, Cardiff Inclusion criteria:Exclusion criteria: |
Adaptive randomisation based on independent, computer-based sequence generated from an implementation of dynamic algorithm using operative category (elective or emergency) and surgeon as stratifying variables Randomisation was 1 : 1 Took place during surgery as close as possible to the time when surgeon commenced closure Patients were blinded to treatment allocation |
To assess the ability of the trial to recruit and consent patients over a 5-month period and the deliverability and safety of the Hughes repair Surgical quality assuranceRadiological evaluation of IH:Adverse events:Statistical analysis: |
A total of 62 patients were screened and assessed for eligibility between October 2013 and February 2014 A total of 43 patients consented to take part and a total of 30 patients were randomised in the operating theatre (14 to Hughes repair and 16 to mass closure) A total of 16 SAEs (10 in the Hughes repair arm and six in the mass closure arm) were reported in 10 patients (five patients in each arm) Rate of SAEs was 34% in the Hughes repair arm and 31% in the mass closure arm (p = 1.0000) No suspected unexpected SAEs in either arm One superficial SSI and two organ-space SSIs in the Hughes arm and two superficial SSIs and one complete wound dehiscence requiring a return to theatre in the standard mass closure arm |
|
Patel et al. , 201722 Cochrane systematic review and meta-analysis |
Inclusion criteria:
|
The majority of the included studies had poorly reported methods sections. Many trials did not specify the methods of randomisation and allocation concealment Randomisation was adequate in 15 out of 55 included studies. Of these, nine studies had an unclear risk of bias for allocation concealment Allocation concealment was adequate in 16 out of 55 studies. Of these, nine had either unclear or high risk of bias in randomisation |
The following comparisons were assessed:
|
Fifty-five RCTs were included, with a total of 19,174 participants Absorbable vs. non-absorbable sutures: IH at ≥ 1 years 4720 patients in 17 RCTs RR 1.07 (95% CI 0.86 to 1.32) Quality of evidence = moderate Wound infection 8457 patients in 29 RCTs RR 0.99 (95% CI 0.84 to 1.17) Quality of evidence = moderate Wound dehiscence 9004 patients in 34 RCTs RR 0.78 (95% CI 0.55 to 1.10) Quality of evidence = moderate Sinus or fistula formation 5470 patients in 19 RCTs RR 0.49 (95% CI 0.26 to 0.94) Quality of evidence = low Mass vs. layered closure IH at ≥ 1 year 1176 patients in five RCTs RR 1.92 (95% CI 0.58 to 6.35) Quality of evidence = very low Wound infection 2926 patients in 11 RCTs RR 0.96 (95% CI 0.67 to 1.30) Quality of evidence = low Wound dehiscence 2863 patients in 11 RCTs RR 0.69 (95% CI 0.31 to 1.52) Quality of evidence = moderate Sinus or fistula formation 1076 patients in six RCTs RR 0.49 (95% CI 0.15 to 1.62) Quality of evidence = low Continuous vs. interrupted closure techniques IH at ≥ 1 year 3854 patients in 11 RCTs RR 1.01 (95% CI 0.76 to 1.35) Quality of evidence = moderate Wound infection 10,039 patients in 23 RCTs RR 1.13 (95% CI 0.96 to 1.34) Quality of evidence = moderate Wound dehiscence 9228 patients in 21 RCTs RR 1.21 (95% CI 0.90 to 1.64) Quality of evidence = moderate Sinus or fistula formation 5082 patients in 10 RCTs RR 1.51 (95% CI 0.64 to 3.61) Quality of evidence = very low Monofilament vs. multifilament sutures IH at ≥ 1 year 4520 patients in 16 RCTs RR 0.76 (95% CI 0.59 to 0.98) Quality of evidence = moderate Wound infection 6557 patients in 23 RCTs RR 1.08 (95% CI 0.91 to 1.28) Quality of evidence = moderate Wound dehiscence 6199 patients in 22 RCTs RR 1.24 (95% CI 0.93 to 1.67) Quality of evidence = moderate Sinus or fistula formation 2285 patients in eight RCTs RR 1.91 (95% CI 0.77 to 4.73) Quality of evidence = very low Slow absorbable vs. fast absorbable sutures IH at ≥ 1 year 3643 patients in 10 RCTs RR 0.81 (95% CI 0.63 to 1.06) Quality of evidence = moderate Wound infection 4100 patients in 11 RCTs RR 1.16 (95% CI 0.85 to 1.57) Quality of evidence = moderate Wound dehiscence 3440 patients in eight RCTs RR 1.55 (95% CI 0.92 to 2.61) Quality of evidence = moderate Sinus or fistula formation 911 patients in two RCTs RR 0.88 (95% CI 0.05 to 16.05) Quality of evidence = very low |
|
Deerenberg et al. , 201528 Prospective, multicentre, double-blind randomised controlled trial; October 2009–March 2012 |
Ten hospitals in the Netherlands Inclusion criteria:Exclusion criteria: |
Patients were registered in an online database and assigned a unique trial code Patients were randomly assigned 1 : 1 during surgery (approximately 15 minutes before closure) using a computer-generated randomisation sequence to receive small tissue bites (5 mm every 5 mm) or large bites (1 cm every 1 cm) for fascial closure Randomisation was stratified by centre and between surgeons and residents with a minimisation procedure Patients and study investigators were blinded to group allocation |
To compare large-bites suture technique with small-bites technique for fascial closure of midline laparotomy incisions Primary outcome: Secondary outcomes: t-tests were used to analyse the difference between the groups for continuous variables and chi-squared tests were used for categorical variables Baseline covariates were predefined potential predictors of IH: abdominal aneurysm aorta, BMI, diabetes mellitus, corticosteroid usage, preoperative chemotherapy, preoperative radiotherapy, chronic obstructive pulmonary disease, smoking, age, collagen disorders, non-incisional hernias and cardiovascular disease Subgroup effects assessed by tests of interaction to prevent over interpretation of apparent differences in effectiveness for all baseline characteristics Quality-of-life differences assessed by multilevel analysis (linear mixed-effects model with random effect for each patient) |
A total of 560 patients were randomised (large bites, n = 248; small bites, n = 276) Follow-up ended on 30 August 2013, with 545 patients (97%) completing follow-up and included in the primary analysis Baseline characteristics were similar between groups (slightly more COPD patients in the small-bites group) Perioperative complications were similar between groups Blood loss and number of drains were similar between groups Follow-up assessments were carried out by clinical and radiological examination in 338 (62%) patients, by radiological examination in 76 (14%) and by physical examination in 131 (24%) patients IH at 1 year postoperatively In total, 57/277 (21%) of patients had IH in the large-bites group compared with 35/268 (13%) in the small-bites group (p = 0.022) (adjusted OR 0.52, 95% CI 0.31 to 0.87; p = 0.0131) No subgroup effects were identified In patients followed up by both radiological and physical examination, IH was identified in 43 out of 87 patients on both physical and radiological examination, 41 out of 87 by radiological examination only and 3 out of 87 solely by physical examination Mean fascial defect in patients with IH was 3.4 cm (SD 4.4 cm). The size of the hernia defects did not differ significantly between groups IH identified by radiological examination alone were not significantly smaller than those identified by both physical and radiological examination (mean 2.4 cm, SD 4.0 cm, vs. mean 4.2 cm, SD 0.5 cm; p = 0.065) Re-admission rates and adverse events did not differ significantly between the groups No significant differences in pain scores as measured with the visual analogue scale in the first week postoperatively In total, 452 out of 483 (94%) patients completed the SF-36 questionnaire and the EQ-5D 12 months postoperatively No significant difference was observed between the groups for any SF-36 subdomain or EQ-5D dimensions Patients who developed IH reported lower general health scores than those who did not (mean 60.16, SD 18.27, vs. mean 64.84, SD 48.7; p = 0.0326) and reported more problems in the EQ-5D dimensions of mobility (mean 1.46, SD 1.06, vs. mean 1.36, SD 0.46; p = 0.0318) |
Appendix 2 Ongoing clinical trials
| Trial name; trial reference; first author, year | Title | Trial design | Aim | Interventions | Outcomes | Current status |
|---|---|---|---|---|---|---|
| ESTOIH study; NCT01965249; Fortelny et al., 201523 | Effect of suture technique on the occurrence of incisional hernia after elective midline abdominal wall closure: study protocol for a randomized controlled trial |
Prospective, multicentre, international, double-blinded, randomised trial Countries: Austria and Germany Population: elective primary median laparotomy, with an incision length of ≥ 15 cm and an expected survival time of > 1 year |
To analyse the influence of stitch length, using an elastic, extra-long-term absorbable monofilament suture, on the long-term clinical outcome of abdominal wall closure |
Intervention: short-stitch technique Comparator: long-stitch technique |
Primary:
|
Primary completion date: January 2021 Study completion date: January 2026 |
| HULC trial; DRKS00017517; Heger et al., 201924 | Hernia reduction following laparotomy using small-stitch abdominal wall closure with and without mesh augmentation (the HULC trial): study protocol for a randomized controlled trial |
Multicentre, randomised controlled, observer- and patient-blinded surgical effectiveness trial with two parallel study groups Country: Germany Population: patients scheduled for elective clean or clean contaminated abdominal surgery as defined by the Centers for Disease Control and Prevention via a midline laparotomy for any indication |
To investigate whether or not a combination of small-stitched fascial closure and onlay mesh augmentation after elective midline laparotomies reduces the risk of IH |
Intervention: closure of the midline incision with a slowly absorbable monofilament suture augmented with a lightweight polypropylene mesh in onlay technique Comparator: closure of the midline incision with a slowly absorbable monofilament suture |
Primary:
|
Currently recruiting to the study |
| CONTINT trial; NCT00544583; Rahbari et al., 201225 | Design and current status of CONTINT: continuous versus interrupted abdominal wall closure after emergency midline laparotomy – a randomized controlled multicenter trial |
Multicentre, open-label, randomised controlled trial Country: Germany Population: patients undergoing a primary emergency midline laparotomy for an emergency surgical intervention with a suspected septic focus in the abdominal cavity |
To compare continuous slowly absorbable sutures with interrupted rapidly absorbable sutures for abdominal wall closure after midline incisions for emergency laparotomy |
Intervention: continuous closure with PDS II equivalent sutures (USP 1, 150-cm loops) Comparator: interrupted closure with Vicryl equivalent sutures (USP 2, 45 cm) |
Primary:
|
Unknown Study completion date was scheduled to be April 2012 No updates since January 2010 |
| E-STITCH trial; NCT04098380 | Effect of Stitch Technique on the Occurrence of Incisional Hernia After Abdominal Wall Closure (ESTOIH) |
Triple-blinded randomised trial Country: Egypt Population: patients undergoing emergency midline laparotomy |
To compare the small tissue bite technique with the large bite technique for closure of emergency midline laparotomy |
Active comparator: small bite The needle bites will be applied with a bite width of 5 mm and intersuture spacing of 5 mm Intervention: procedure – small bite Active comparator: large bite The needle bites will be applied with a width of 10 mm and intersuture spacing of 10 mm Intervention: procedure – larger bite |
Primary:
|
Currently recruiting Anticipated date of study completion is December 2021 |
| Rein4CeTo1 Trial; NCT03390764 | Hernia After Colorectal Cancer Surgery: an RCT Comparing 4 : 1-technique With or Without a Reinforced Tension Line Suture (Rein4CeTo1) |
Multicentre randomised controlled trial Country: Sweden Population: patients planned for colorectal surgery owing to cancer |
To compare the IH incidence 1 year after planned colorectal cancer surgery performed through a midline incision that is closed either by a standardised small stitch 4 : 1 technique or with the same technique plus a reinforced tension-line suture |
Active comparator: 4 : 1 closure group Patients randomised to and receiving the intervention small-stitch 4 : 1 technique for closure of the abdominal wall Active comparator: RTL plus 4 : 1 closure group Patients randomised to and receiving the intervention reinforced tension-line suture plus small-stitch 4 : 1 technique for closure of the abdominal wall |
Primary:
|
Currently recruiting Anticipated study completion date is December 2022 |
Appendix 3 Protocol amendments
Amendments to the study protocol
The HART study protocol was amended on six occasions. A list of the protocol sections impacted by changes made at each amendment is given below. Full details of specific changes are recorded in change logs that are held in the study archive.
HART protocol amendment 1 (April 2013): summary of changes
The purpose of the amendment was to make minor alterations. General administrative changes related to protocol cover page, synopsis and page headers/footers.
The version number of the study protocol was changed to version 2.7.
Summary of changes from HART protocol version 2.5 to 2.7
-
Section 3.1: addition of extra text.
-
Section 3.1: addition of extra text.
-
Sections 3.2: addition of extra text.
-
Section 4: aims and objectives.
-
Section 4.1: aims.
-
Section 4.1: aims.
-
Section 4.2: primary outcomes.
-
Section 4.3: secondary outcomes.
-
Section 5.2: setting.
-
Section 5.10: sample size estimation.
-
Section 6: references.
-
Section 7: Appendix 1.
HART protocol amendment 2 (April 2014): summary of changes
The purpose of the amendment was to make a substantial amendment affecting:
-
patient information leaflet
-
consent form
-
GP letter
-
SSI diary.
General administrative changes related to protocol cover page, synopsis and page headers/footers.
The version number of the study protocol was changed to version 2.8.
Summary of changes from HART protocol version 2.7 to 2.8
-
Contact details.
-
Section 2.2: rationale.
-
Section 3.3: secondary outcomes.
-
Section 4.4: inclusion criteria.
-
Section 4.5: exclusion criteria.
-
Section 4.8 management, safety and quality assurance.
Minor amendments:
-
minor grammatical wording changes and spelling corrections
-
removal of signature page
-
defined authorship model
-
addition of timeline to section 4.2.
HART protocol amendment 3 (November 2014): summary of changes
The purpose of the amendment was to make a substantial amendment affecting the randomisation process. General administrative changes related to protocol cover page, synopsis and page headers/footers.
The version number of the study protocol was changed to version 2.9.
Summary of changes from HART protocol version 2.8 to 2.9
The randomisation process changed from a web-based randomisation system to a fully automated, 24-hour telephone randomisation system. This allowed for easier access to randomisation from the surgical theatre where internet access is limited, but a telephone line is always available. Protocol v2.8, 18 April 2014, described the process of web-based randomisation in appendix 1; therefore, this appendix and any reference to it has been removed. An updated phone-based randomisation guidance will not be included within the protocol, but separate instructions will be made available to site as part of the site file.
Details of trial manager added.
HART protocol amendment 4 (March 2015): summary of changes
The purpose of the amendment was to make substantial amendments to multiple sections of the protocol. A number of minor amendments were also made. General administrative changes related to protocol cover page, synopsis and page headers/footers.
The version number of the study protocol was changed to version 3.0.
Summary of changes from HART protocol version 2.9 to 3.0
-
Abbreviations.
-
Contact details.
-
Funders and committees.
-
Section 1.1: literature review (section 2.1 in v2.9).
-
Section 1.2: rationale (section 2.2 in v2.9).
-
Section 1.3: hypothesis (section 2.3 in v2.9).
-
Section 2: aims, objectives and outcomes (section 3 in v2.9).
-
Section 2.1: objectives.
-
Section 2.1.1: primary objective.
-
Section 2.1.2: secondary objective.
-
Section 2.1.3: tertiary objective.
-
Section 2.2.1: primary outcome (section 3.2 in v2.9).
-
Section 2.2.2: secondary outcomes (section 3.3 in v2.9).
-
Section 2.2.3: tertiary outcomes (section 3.4 in v2.9).
-
Section 2.3: qualitative assessment.
-
Section 3.1: study design (section 4.1 in v2.9).
-
Section 3.2: study population.
-
Section 3.3: setting (section 4.2 in v2.9).
-
Section 4.1: inclusion (section 4.4 in v2.9).
-
Section 4.2: exclusion (section 4.5 in v2.9).
-
Section 5: study schedule.
-
Section 5.1: screening.
-
Section 5.2: consent (section 4.3: recruitment in v2.9).
-
Section 5.3: baseline visit.
-
Section 5.4: day of surgery.
-
Section 5.4.1: re-operation.
-
Section 5.5: discharge from hospital.
-
Section 5.6: 30-day visit.
-
Section 5.7: 6-month visit.
-
Section 5.8: 1-year visit.
-
Section 5.9: follow-up visits.
-
Section 6.1: Hughes closure technique.
-
Section 6.1.1: training in Hughes closure technique (section 4.6 in v2.9).
-
Section 6.1.2: mass closure technique.
-
Section 6.2: clinical evaluation of incisional hernia (section 4.7: evaluation of CT scanning in v2.9).
-
Section 6.3.1: CT imaging.
-
Section 6.3.2: transfer of CT images (section 4.7: evaluation of CT scanning in v2.9).
-
Section 7: safety (section 4.8: management, quality and safety assurance in v2.9).
-
Section 7.2.1: adverse events definition (section 4.8.1: adverse events in v2.9).
-
Section 7.3: list of adverse events.
-
Section 7.4: adverse events of special interest.
-
Section 7.5: adverse event recording and reporting (section 4.8.2 in v2.9).
-
Section 8.1: randomisation (section 4.9 in v2.9).
-
Section 8.3: analysis (section 4.11 in v2.9).
-
Section 10: ethical considerations and regulatory approvals (section 4.13: ethical considerations in v2.9).
-
Section 10.1: quality assurance.
-
Section 10.2: data handling and record keeping.
-
Section 11: end of trial.
-
Section 11.1: archiving.
-
Section 12: protocol amendment log.
HART protocol amendment 5 (November 2015): summary of changes
The purpose of the amendment was to make substantial amendments to multiple sections of the protocol. General administrative changes related to protocol cover page, synopsis and page headers/footers.
The version number of the study protocol was changed to version 4.0.
Summary of changes from HART protocol version 3.0 to 4.0
-
Abbreviations.
-
Key roles and contact details.
-
Study sites list removed.
-
Section 2.1.3: tertiary objectives.
-
Section 2.2.2: secondary outcomes.
-
Section 2.2.3: tertiary outcomes.
-
Section 5.6: 30-day visit.
-
Section 5.8: 1-year visit.
-
Section 5.9.1: 2-year follow-up.
-
Section 6.2: clinical evaluation of incisional hernia, primary end-point measure.
-
Section 6.3: radiological evaluation of incisional hernia, tertiary end-point measure.
-
Section 6.3.2: transfer of CT images.
-
Section 7.3: Clavien–Dindo classification.
-
Section 7.4: list of expected adverse events.
-
Section 10: ethical considerations and regulatory approvals.
-
References.
HART protocol amendment 5 (October 2015): summary of changes
The purpose of the amendment was to make substantial amendments to multiple sections of the protocol. General administrative changes related to protocol cover page, synopsis and page headers/footers.
The version number of the study protocol was changed to version 5.0.
Summary of changes from HART protocol version 4.0 to 5.0
-
Key roles and contact details.
-
Section 3.1: design.
-
Section 5.1: screening.
-
Section 5.2: consent.
-
Section 12: protocol amendment log.
Approval of amendments to the study protocol
The amendments were reviewed and approved by the sponsor, the chief investigator, the REC and the research and development offices of the NHS trusts at which recruitment of patients took place.
Appendix 4 Additional recruitment information
| # | Hospital site | Opening date | Standard mass closure (N = 401), n (%) | Hughes repair (N = 401), n (%) | Total (N = 802), n (%) |
|---|---|---|---|---|---|
| 1 | University Hospital of Wales | 27 August 2014 | 89 (22.19) | 87 (21.7) | 176 (21.95) |
| 2 | Singleton Hospital, Swansea | 19 May 2015 | 35 (8.73) | 35 (8.73) | 70 (8.73) |
| 3 | Princess of Wales Hospital, Bridgend | 9 July 2015 | 7 (1.75) | 5 (1.25) | 12 (1.5) |
| 4 | Queen’s Hospital, Burton on Trent | 26 October 2015 | 8 (2) | 7 (1.75) | 15 (1.87) |
| 5 | Royal Glamorgan Hospital, Llantrisant | 20 October 2015 | 12 (2.99) | 11 (2.74) | 23 (2.87) |
| 6 | Glan Clwyd Hospital, Rhyl | 25 November 2015 | 6 (1.5) | 9 (2.24) | 15 (1.87) |
| 7 | Ysbyty Maelor, Wrexham | 25 November 2015 | 9 (2.24) | 9 (2.24) | 18 (2.24) |
| 8 | Yeovil District Hospital | 4 January 2016 | 12 (2.99) | 13 (3.24) | 25 (3.12) |
| 9 | Royal Blackburn Hospital | 17 February 2016 | 9 (2.24) | 8 (2) | 17 (2.12) |
| 10 | Royal United Hospital, Bath | 12 February 2016 | 16 (3.99) | 17 (4.24) | 33 (4.11) |
| 11 | Churchill Hospital, Oxford | 5 January 2016 | 13 (3.24) | 12 (2.99) | 25 (3.12) |
| 12 | Weston General Hospital | 26 January 2016 | 1 (0.25) | 2 (0.5) | 3 (0.37) |
| 13 | Macclesfield General Hospital | 28 January 2016 | 23 (5.74) | 22 (5.49) | 45 (5.61) |
| 14 | St Mary’s Hospital, Imperial | 29 February 2016 | 14 (3.49) | 14 (3.49) | 28 (3.49) |
| 15 | Ealing Hospital, London | 19 February 2016 | 3 (0.75) | 4 (1) | 7 (0.87) |
| 16 | Royal Bolton Hospital, Bolton | 7 March 2016 | 17 (4.24) | 17 (4.24) | 34 (4.24) |
| 17 | Queen Elizabeth II Hospital, Birmingham | 12 May 2016 | 16 (3.99) | 15 (3.74) | 31 (3.87) |
| 18 | Countess of Chester, Chester | 23 March 2016 | 37 (9.23) | 37 (9.23) | 74 (9.23) |
| 19 | Maidstone Hospital, Maidstone | 14 March 2016 | 1 (0.25) | 2 (0.5) | 3 (0.37) |
| 20 | St Mark’s Hospital, London | 14 March 2016 | 7 (1.75) | 7 (1.75) | 14 (1.75) |
| 21 | St Peter’s Hospital, Chertsey | 15 April 2016 | 19 (4.74) | 20 (4.99) | 39 (4.86) |
| 22 | Royal Devon & Exeter Hospital | 19 April 2016 | 6 (1.5) | 6 (1.5) | 12 (1.5) |
| 23 | Doncaster Royal Infirmary | 21 April 2016 | 8 (2) | 7 (1.75) | 15 (1.87) |
| 24 | Hillingdon Hospital | 16 June 2016 | 5 (1.25) | 5 (1.25) | 10 (1.25) |
| 25 | Royal Derby Hospital | 7 July 2016 | 12 (2.99) | 12 (2.99) | 24 (2.99) |
| 26 | Derriford Hospital, Plymouth | 21 July 2016 | 12 (2.99) | 12 (2.99) | 24 (2.99) |
| 27 | Queen’s Medical Centre, Nottingham | 19 August 2016 | 4 (1) | 6 (1.5) | 10 (1.25) |
| 28 | Frimley Park, Frimley | 12 December 2016 | – | – | – |
| Study procedures | Outcome measures | Pre-index admission activity | Intraoperative activity | Day of discharge | Day 30 post surgery | Month 6 post surgery | Month 12 post surgery | Month 24 post surgery |
|---|---|---|---|---|---|---|---|---|
| Consent | Enrolment/consent (procedure) | ✗ | ||||||
| CT scan | ✗ | ✗ | ✗ | |||||
| Patient history | For example, sex, age, diagnosis and disease severity | ✗ | ||||||
| Clinical examinations | Hernia clinical examination | ✗ | ✗ | ✗ | ||||
| QoL questionnaires | FACT-C and SF-12 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| CSRI | Client Service Receipt Inventory | ✗ | ✗ | ✗ | ✗ | |||
| Pre-existing conditions | POSSUM questionnaire | ✗ | ||||||
| Surgical information | ✗ | |||||||
| SAE monitoring | Patient SSI diary and clinician reported – all except death | ✗ | ✗ | ✗ | ||||
| SAE monitoring | Patient SSI diary and clinician reported – death only | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Reasons | Count (n) |
|---|---|
| Operation went on late into evening; randomisation office closed | 1 |
| Surgeon not undertaken GCP update training | 1 |
| Surgeon not taking part | 1 |
| Surgeon: no HART training | 1 |
| Operation took too long | 1 |
| Palliative only: consultant felt that it was not appropriate | 1 |
| Clinician’s decision | 1 |
| Clinician’s decision: needed a quick finish to the operation as patient unstable | 1 |
| Patient done urgently on different theatre list | 1 |
| Missed in theatre | 1 |
| Missed in theatre | 1 |
| Consultant lost equipoise | 1 |
| Long, difficult operation, high blood loss, loss of equipoise from consultant surgeon | 1 |
| Consultant chose Hughes closure as previous hernia | 1 |
| Consultant surgeon’s choice of closure | 1 |
| Surgery was open and close | 1 |
| Surgeon declined to randomise | 1 |
| Registrar forgot | 1 |
| Procedure took longer than expected | 1 |
| Complications in theatre | 1 |
| Procedure time extended in surgery, unable to randomise | 1 |
| Open-and-shut case | 1 |
| Deteriorated in theatre: difficult operation resulting in ICU admission | 1 |
| Laparoscopic procedure (not midline) | 1 |
| Inoperable | 1 |
| Patient became unwell on table | 1 |
| Consultant decision made not to randomise as patient’s condition deteriorated | 1 |
| Patient’s surgery was brought forward and research team was not informed | 1 |
| Once in theatre, it was found that the patient had advanced metastatic disease | 1 |
| Surgery was brought forward, but research team were not informed, and surgeon forgot that patient was in the HART study | 1 |
| Extensive theatre delays; therefore, patient not randomised | 1 |
| Not able to access randomisation procedure as unable to get PIN code | 1 |
| There was an oversight because of distraction from a very difficult cancer operation | 1 |
| This patient was ineligible due to non-oncology pathology, realised by randomising clinician | 1 |
| No sutures available | 1 |
| No available trial-trained personnel to suture | 1 |
| Reason not stated | 1 |
| Patient had bleeding on table at end of procedure; we had delayed start we were running over, hence could not randomise | 1 |
| Clinician’s decision | 1 |
| Patient withdrew prior to randomisation | 1 |
| Surgery finished late, unable to randomise | 1 |
| Surgery finished late | 1 |
| Flap | 1 |
| Complicated surgery: unexpected | 1 |
| Complex case: decided not appropriate | 1 |
| Patient converted to transverse incision | 1 |
| Incision type: defunctioning | 1 |
| Surgeon decided not appropriate | 1 |
| Surgery too busy | 1 |
| [Surgeon] forgot and completed surgery before randomisation | 1 |
| Surgeon choice | 1 |
Appendix 5 Additional data tables
| # | Hospital site | Standard mass closure (N = 333), n | Hughes repair (N = 339), n | Total (N = 672), n | ||
|---|---|---|---|---|---|---|
| IH | Site total | IH | Site total | |||
| 1 | University Hospital of Wales | 14 | 80 | 14 | 74 | 154 |
| 2 | Singleton Hospital, Swansea | 3 | 24 | 7 | 30 | 54 |
| 3 | Princess of Wales Hospital, Bridgend | 0 | 6 | 0 | 5 | 11 |
| 4 | Queen’s Hospital, Burton on Trent | 2 | 5 | 1 | 6 | 11 |
| 5 | Royal Glamorgan Hospital, Llantrisant | 2 | 9 | 1 | 10 | 19 |
| 6 | Glan Clwyd Hospital, Rhyl | 2 | 5 | 2 | 4 | 9 |
| 7 | Ysbyty Maelor, Wrexham | 0 | 7 | 0 | 8 | 15 |
| 8 | Yeovil District Hospital | 1 | 10 | 1 | 11 | 21 |
| 9 | Royal Blackburn Hospital | 1 | 8 | 0 | 8 | 16 |
| 10 | Royal United Hospital, Bath | 1 | 16 | 2 | 16 | 32 |
| 11 | Churchill Hospital, Oxford | 2 | 7 | 1 | 11 | 18 |
| 12 | Weston General Hospital | 1 | 1 | 0 | 2 | 3 |
| 13 | Macclesfield General Hospital | 3 | 23 | 2 | 20 | 43 |
| 14 | St Mary’s Hospital, Imperial | 1 | 10 | 2 | 11 | 21 |
| 15 | Ealing Hospital, London | 0 | 3 | 0 | 4 | 7 |
| 16 | Royal Bolton Hospital, Bolton | 3 | 17 | 4 | 16 | 33 |
| 17 | Queen Elizabeth II Hospital, Birmingham | 2 | 10 | 0 | 9 | 19 |
| 18 | Countess of Chester, Chester | 7 | 31 | 5 | 35 | 66 |
| 19 | Maidstone Hospital, Maidstone | 0 | 0 | 0 | 1 | 1 |
| 20 | St Mark’s Hospital, London | 0 | 6 | 1 | 7 | 13 |
| 21 | St Peter’s Hospital, Chertsey | 8 | 17 | 5 | 16 | 33 |
| 22 | Royal Devon & Exeter Hospital | 0 | 5 | 0 | 6 | 11 |
| 23 | Doncaster Royal Infirmary | 2 | 7 | 0 | 6 | 13 |
| 24 | Hillingdon Hospital | 1 | 5 | 0 | 5 | 10 |
| 25 | Royal Derby Hospital | 1 | 10 | 2 | 10 | 20 |
| 26 | Derriford Hospital, Plymouth | 0 | 8 | 0 | 7 | 15 |
| 27 | Queen’s Medical Centre, Nottingham | 0 | 3 | 0 | 1 | 4 |
| Operation type | Standard mass closure, n (%) (N = 401) | Hughes repair, n (%) (N = 401) | ORa (95% CI) | p-value |
|---|---|---|---|---|
| Elective | 371 (95.5) | 372 (92.8) | ||
| Emergency | 30 (7.5) | 29 (7.2) | 0.96 (0.57 to 1.64) | 0.9 |
| Missing |
| Operation type | Patients with IH, n (%) (N = 107) | Patients without IH, n (%) (N = 565) | ORa (95% CI) | p-value |
|---|---|---|---|---|
| Elective | 98 (91.6) | 539 (95.4) | ||
| Emergency | 9 (8.4) | 26 (4.6) | 1.90 (0.86 to 4.20) | 0.1 |
| Missing |
| Operation type | Standard mass closure, n (%) (N = 401) | Hughes repair, n (%) (N = 401) | ORa (95% CI) | p-value |
|---|---|---|---|---|
| No stoma | 277 (69.10) | 259 (64.6) | ||
| Stoma | 124 (30.9) | 142 (35.4) | 1.22 (0.91 to 1.65) | 0.2 |
| Missing |
| Operation type | Patients with IH, n (%) (N = 107) | Patients without IH, n (%) (N = 565) | ORa (95% CI) | p-value |
|---|---|---|---|---|
| No stoma | 72 (67.3) | 385 (68.1) | ||
| Stoma | 35 (32.7) | 180 (31.9) | 1.04 (0.67 to 1.61) | 0.9 |
| Missing | 0 | 0 |
| SSI | Standard mass closure, n (%) (N = 401) | Hughes repair, n (%) (N = 401) | ORa (95% CI) | p-value |
|---|---|---|---|---|
| No | 367 (91.5) | 345 (86.0) | 1.82 (1.14 to 2.91) | 0.01 |
| Yes | 31 (7.7) | 53 (13.2) | ||
| Missing | 3 (0.8) | 3 (0.8) |
| SSI | Patients with IH, n (%) (N = 107) | Patients without IH, n (%) (N = 565) | ORa (95% CI) | p-value |
|---|---|---|---|---|
| No | 92 (86.0) | 517 (91.5) | ||
| Yes | 15 (14.0) | 48 (8.5) | 1.8 (0.94 to 3.27) | 0.07 |
| Severity of SSI | Standard mass closure, n (%) (N = 31) | Hughes repair, n (%) (N = 53) | Fisher’s exact test (p-value) |
|---|---|---|---|
| Superficial | 25 (23.3) | 38 (39.8) | |
| Deep | 3 (4.1) | 8 (6.9) | 0.7 |
| Organ/space (peritoneal cavity) | 3 (3.7) | 7 (6.3) | |
| Missing | 0 | 0 |
| Variable | Mean baseline FACT-C score (SD) | Mean change in FACT-C score from baseline (95% CI);a p-value | |||
|---|---|---|---|---|---|
| Month 1 | Month 6 | Year 1 | Year 2 | ||
| Standard mass closure | 71.69 (9.97) | –6.08 (–7.30 to –4.87); 0.00 | –5.33 (–6.6 to –4.1); 0.00 | –4.89 (–6.13 to –3.66); 0.00 | –4.27 (–5.6 to –2.9); 0.00 |
| Hughes repair | 70.58 (9.94) | –3.88 (–5.10 to –2.69); 0.00 | –4.11 (–5.3 to –2.9); 0.00 | 2.92 (–4.1 to –1.7); 0.00 | 3.43 (–4.7 to –2.1); 0.00 |
| Between-arm difference in means | 2.18 (0.47 to 3.9); 0.01 | 1.22 (–0.49 to 2.94); 0.16 | 1.97 (0.23 to 3.71); 0.02 | 0.84 (–1.02 to 2.7); 0.4 | |
FIGURE 13.
Mean FACT-C scores over time by arm.
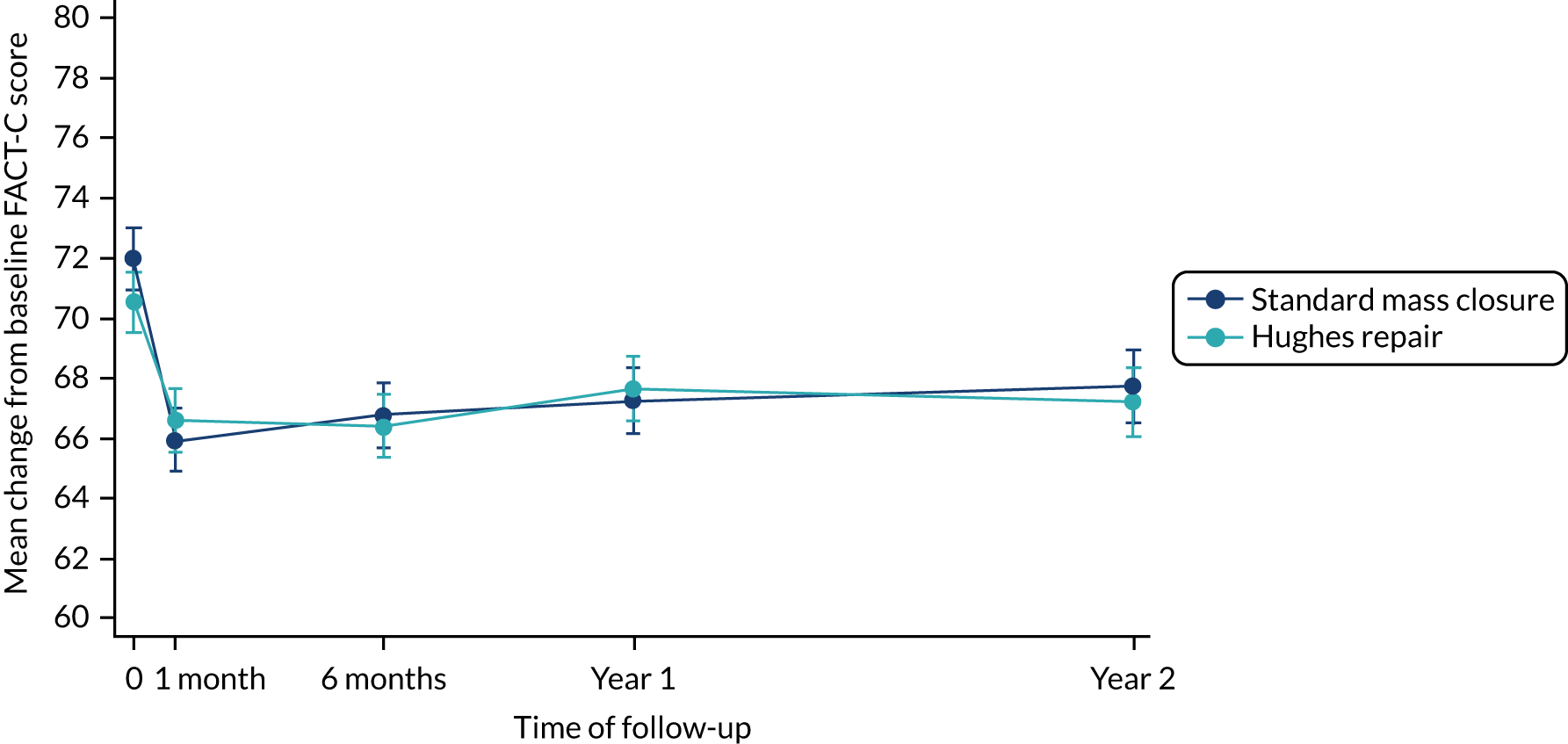
Appendix 6 Additional cost-effectiveness tables
| Resource | Currency code/HRG | Unit cost (£) | Notes |
|---|---|---|---|
| Post-surgical inpatient care | |||
| Post-surgery inpatient stay (CC score 0–2) | FF30–FF34 | 7157.79a | Weighted across all colon and large intestine procedures with CC score 0–2 |
| Post-surgery inpatient stay (CC score 3–5) | FF30–FF34 | 8305.65a | Weighted across all colon and large intestine procedures with CC score 3–5 |
| Post-surgery inpatient stay (CC score 6+) | FF30–FF34 | 13,252.30a | Weighted across all colon and large intestine procedures with CC score 6+ |
| Post-surgery excess bed-day | FF32–FF36 | 424.90a | Weighted across all colon and large intestine procedures, 19 years and over (added to inpatients stays exceeding average length of stay) |
| Post-surgery bed-day (CC score 0–2) | FF30–FF34 | 1405.69a | Weighted across all colon and large intestine procedures with CC score 0–2 (subtracted from inpatient stays shorter than average length of stay) |
| Post-surgery bed-day (CC score 3–5) | FF30–FF34 | 1171.23a | Weighted across all colon and large intestine procedures with CC score 3–5 (subtracted from inpatient stays shorter than average length of stay) |
| Post-surgery bed-day (CC score 6+) | FF30–FF34 | 917.36a | Weighted across all colon and large intestine procedures with CC score 6+ (subtracted from inpatient stays shorter than average length of stay) |
| Primary care | |||
| GP consultation at surgery | N/A | 37.40b | 9.22 minutes’ duration including direct care staff and qualifications |
| GP consultation at home | N/A | 86.73b | GP home visit (9.22 minutes) + 20 minutes’ travel time (indirect, £148 per hour) |
| GP consultation by telephone | N/A | 15.10b | 4 minutes’ duration |
| Practice nurse consultation at surgery | N/A | 7.00b | £42.00 per hour (including qualifications), 10-minute appointment assumed |
| District nurse consultation at home | N/A | 27.33b | Band 6; 10 minutes’ home visit (£74 per hour) + 20 minutes’ travel (indirect, £45 per hour) |
| Counsellor | N/A | 44.00b | Band 6; scientific and professional; £44 per hour; 1 hour assumed |
| Wound swab | DAPS07 | 7.59a | Microbiology |
| Antibiotics (wound infection) | N/A | 0.66c | Per day, weighted for flucloxacillin and augmentin |
| Phlebotomist | N/A | 5.09b | Including £1.93† (weighted average blood test cost) |
| Specialist nurse | N/A | 37.00b | Band 6; 30-minute visit assumed (£74 per hour) |
| Out-of-hours GP | N/A | 108.80b | Including qualifications |
| Receptionist | N/A | 0.58b | Band 2; £7 per hour; 5 minutes assumed |
| Physiotherapist | A08A1 | 57.26a | Physiotherapist, adult, one to one |
| Podiatrist | A09A–A09F | 43.39a | Weighted across all options |
| Health visitor | N03F | 52.97a | Health visitor, other clinical intervention |
| NHS Direct consultation | N/A | 14.90b | Costed as telephone triage (GP led) |
| Secondary care | |||
| Surgical care | |||
| Consultant surgeon | WF01A+B/104 | 119.24a | Colorectal surgery outpatient consultant-led; weighted for first and follow-up attendance |
| Consultant surgeon follow-up | WF01A/104 | 104.52a | Colorectal surgery outpatient consultant-led; weighted for first and follow-up attendance |
| Specialist nurse home visit | N/A | 52.00b | Band 6; £111 per hour of patient contact; £45 per working hour; 20 minute + 20 minute travel assumed |
| Specialist nurse at hospital | N/A | 37.00b | Band 6; £111 per hour of patient contact; 20-minute appointment assumed |
| Stoma nurse | N/A | 37.00b | Band 6; specialist nurse; 20-minute appointment assumed |
| Anaesthetist | WF01A+B/190 | 135.42a | Anaesthetics outpatient; weighted for first and follow-up attendance |
| Anaesthetist follow-up | WF01A/190 | 123.87a | Anaesthetics outpatient; non-admitted face-to-face attendance, follow-up |
| General surgeon | WF01A+B/100 | 149.24a | General surgery outpatient consultant-led; weighted for first and follow-up attendance |
| Surgical registrar | N/A | 7.17b | Based on registrar; £43 per working hour; 10-minute appointment assumed |
| Other secondary care | |||
| Day hospital | N/A | 742.09a | Weighted across all day-case entries |
| Emergency department attendance | N/A | 160.31a | Weighted across all A&E entries (patient dead on arrival excluded) |
| Ambulance callout | N/A | 97.68a | Weighted across all options |
| Inpatient day | N/A | 412.28a | Weighted across all elective excess bed-days (paediatrics excluded) |
| Occupational health | N/A | 35.00b | Band 5; £35 per hour; 1 hour assumed |
| Outpatient attendances | |||
| Outpatient appointment, unspecified | N/A | 138.19a | Weighted across all consultant-led outpatient (paediatrics excluded) |
| Anticoagulant service | 324 | 20.65a | Weighted for first and follow-up attendance |
| Audiology | 840 | 93.95a | Weighted for first and follow-up attendance |
| Cardiology | 320 | 143.32a | Weighted for first and follow-up attendance |
| Clinical genetics | 311 | 458.37a | Weighted for first and follow-up attendance |
| Dermatology | 330 | 114.27a | Weighted for first and follow-up attendance |
| Diabetic medicine | 307 | 155.91a | Weighted for first and follow-up attendance |
| Dietetics | 654 | 107.73a | Weighted for first and follow-up attendance |
| Gastroenterology | 301 | 159.53a | Weighted for first and follow-up attendance |
| Haematology | 303 | 173.68a | Weighted for first and follow-up attendance |
| Hepatobiliary and pancreatic surgery | 105 | 167.00a | Weighted for first and follow-up attendance |
| Medical oncology | 370 | 97.07a | Weighted for first and follow-up attendance |
| Neurology | 400 | 172.89a | Weighted for first and follow-up attendance |
| Physiotherapy | 650 | 55.30a | Weighted for first and follow-up attendance |
| Radiology | 811 | 177.30a | Weighted for first and follow-up attendance |
| Respiratory medicine | 340 | 165.36a | Weighted for first and follow-up attendance |
| Sport and exercise medicine | 325 | 71.04a | Follow-up, not consultant-led |
| Trauma and orthopaedics | 110 | 128.82a | Weighted for first and follow-up attendance |
| Urology | 101 | 112.70a | Weighted for first and follow-up attendance |
| Other secondary care | |||
| Dentist (NHS) | M01A-C | 149.06a | Weighted across all relevant options |
| Optician | N/A | 31.44b | |
| Mental health specialist | MHST | 169.22a | Weighted across all relevant options |
| Pharmacist (consultant) | N/A | 15.17b | Band 8C; £91 per hour; 10 minutes assumed |
| Phlebotomist (hospital) | DAPS08 | 3.00a | |
| Radiographer | N/A | 16.00b | Band 6; £48 per hour; 20 minutes assumed |
| Rehabilitation | VC03Z-VC40Z | 204.67a | Weighted across all options (other) |
| Specialist nurse telephone call | N/A | 9.25b | Band 6; £111 per hour of patient contact; 5 minutes assumed |
| Procedures and imaging | |||
| Ablation of kidney lesion | YL01Z–YL02Z | 2968.60a | Weighted across all relevant options |
| Adjuvant chemotherapy | N/A | 3816.36 | Based on SCOT trial;46 9 months assumed |
| Blood transfusion (day case) | SA44A | 499.00a | Single plasma exchange or other intravenous blood transfusion, 19 years and over |
| Bowel screening | WH15Z | 32.00a | Special screening, examinations or other genetic disorders |
| Cardiopulmonary exercise testing | DZ31Z | 178.52a | |
| Colonoscopy | FE31Z–FE36Z | 186.96a | Weighted for all general surgery options |
| CT scan | RD20A–RD28Z | 103.95a | Weighted for all options an number of areas |
| Electrocardiogram monitoring or stress testing | EY51Z | 119.00a | |
| Endoscopy | FE21Z, FE22Z, FE50A | 329.74a | Weighted across all relevant options |
| Hernia repair | FF60A–FF61Z | 4304.87a | Weighted across all relevant options |
| Ileostomy reversal | FF34C | 4257.30a | Major large intestine procedures, 19 years and over, with CC score 0 |
| Liver resection | GA03C–GA07E | 7723.59a | Weighted across all relevant options |
| Magnetic resonance imaging scan | RD01A–RD07Z | 145.56a | Weighted across all options |
| Radiotherapy | SC96Z | 83.06a | Same-day radiotherapy admission or attendance (excluding brachytherapy) |
| Social care | |||
| Social worker | N/A | 48.00b | £84 per hour of client-related work; 20-minutes- visit; 20 minutes’ travel assumed (£60/hour) |
| Home help | N/A | 44.00b | £22 per hour; 2 hours assumed (based on home care worker, non-face to face) |
| Care assistant | N/A | 54.00b | £27 per hour; 2 hours assumed (based on home care worker, face to face) |
| Day centre | DCF20 + DCF30 | 98.93a | Day-care facilities, regular attendances, weighted |
| Support and outreach worker | N/A | 23.00b | £23 per hour; 1 hour assumed |
| Community occupational therapist | N/A | 47.00b | £47 per hour; 1 hour assumed |
| Suture type | Unit cost (including VAT) (£) | Cost per individual suture (£) |
|---|---|---|
| Hughes repair | ||
| Interrupted 1-nylon sutures | 18.90 per pack of 12 sutures | 1.57 |
| Continuous loop PDS sutures | 148.46 per pack of 24 sutures | 6.19 |
| Standard mass closure | ||
| Absorbable (Vicryl); size 2–0/2o | 25.34 per pack of 12 sutures | 2.11 |
| Absorbable (Vicryl); size 0 | 27.28 per pack of 12 sutures | 2.27 |
| Absorbable (Vicryl); size 1 | 28.34 per pack of 12 sutures | 2.36 |
| Non-absorbable or PDS; size 2–0/2o | 97.04 per pack of 36 sutures | 2.70 |
| Non-absorbable or PDS; size 0 | 77.74 per pack of 24 sutures | 3.24 |
| Non-absorbable or PDS; size 1 | 109.56 per pack of 24 sutures | 4.56 |
| Non-absorbable or PDS; size 2 | 97.04 per pack of 36 sutures | 2.70 |
| Suture type | Hughes repair | Standard mass closure | ||
|---|---|---|---|---|
| Mean number of sutures (SD) | Mean cost of sutures (SD) | Mean number of sutures (SD) | Mean cost of sutures (SD) | |
| Index operation | ||||
| Interrupted 1-nylon sutures (n = 364) | 4.40 (2.33) | Mean = £6.91 (£3.65) | N/A | N/A |
| Minimum = 0; maximum = 14 | ||||
| Minimum = £0; max = £21.98 | ||||
| Continuous loop PDS sutures (n = 355) | 1.83 (0.52) | £11.33 (£3.22) | N/A | N/A |
| Minimum = 0; maximum = 4 | ||||
| Minimum = £0; maximum = £24.76 | ||||
| Absorbable sutures (all sizes; n = 290–294) | N/A | N/A | 1.86 (0.49) | £4.35 (£1.15) |
| Minimum = 1; maximum = 5 | Minimum = £2.11; maximum = £11.80 | |||
| Non-absorbable sutures (all sizes; n = 74) | N/A | N/A | 1.95 (0.52) | £7.83 (£2.32) |
| Minimum = 1; maximum = 5 | Minimum = £3.24; maximum = £16.20 | |||
| Reoperation | ||||
| Interrupted 1-nylon sutures | n = 364 | n = 364 | n = 6 | n = 6 |
| 0.09 (0.78) | £0.14 (£1.23) | 9.5 (4.37) | £14.92 (£6.86) | |
| Minimum = 0; maximum = 10 | Minimum = £0; maximum = £15.70 | Minimum = 6; maximum = 18 | Minimum = £9.42; maximum = £28.26 | |
| Continuous loop PDS sutures (n = 355) | n = 355 | n = 355 | n = 6 | n = 6 |
| 0.03 (0.22) | £0.17 (£1.38) | 1.50 (0.84) | £9.29 (£5.18) | |
| Minimum = 0; maximum = 2 | Minimum = £0; maximum = £12.38 | Minimum = 0; maximum = 2 | Minimum = £0; maximum = £12.38 | |
| Absorbable sutures (all sizes) | n = 6 | n = 6 | n = 294 | n = 294 |
| 2.0 (0) | £4.61 (£0.20) | 0.07 (0.43) | £0.18 (£1.13) | |
| Minimum = 2; maximum = 2 | Minimum = £4.22; maximum = £4.72 | Minimum = 0; maximum = 4 | Minimum = £0; maximum = £9.44 | |
| Non-absorbable sutures (all sizes) | n = 4 | n = 4 | n = 74 | n = 74 |
| 2.5 (1.29) | £10.74 (£6.36) | 0.08 (0.40) | £0.25 (£1.29) | |
| Minimum = 1; maximum = 4 | Minimum = £4.56; maximum = £18.24 | Minimum = 0; maximum = 2 | Minimum = £0; maximum = £9.12 | |
| Health-care resource | Hughes repair | Standard mass closure | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Primary care: visits per patient between baseline and 6-month follow-up | n = 311 | n = 296 | ||
| GP visits at surgery (SD) | 2.42 (2.97) | 2.30 (3.13) | 0.12 ( –0.37 to 0.60) | 0.637 |
| Nurse visits at surgery (SD) | 2.35 (6.12) | 2.63 (11.60) | –0.27 (–1.76 to 1.22) | 0.717 |
| GP visits at home (SD) | 0.29 (1.31) | 0.26 (0.81) | 0.03 (–0.14 to 0.21) | 0.712 |
| District nurse visits at home (SD) | 6.13 (13.73) | 6.70 (17.13) | –0.57 (–3.06 to 1.91) | 0.650 |
| GP telephone consultations (SD) | 0.88 (1.55) | 0.98 (1.63) | –0.10 (–0.35 to 0.16) | 0.445 |
| Counsellor (SD) | 0.04 (0.38) | 0.04 (0.42) | 0.00 (–0.06 to 0.07) | 0.887 |
| NHS Direct telephone call (SD) | 0.18 (0.57) | 0.23 (0.82) | –0.05 (–0.16 to 0.06) | 0.350 |
| Health visitor at home (SD) | 0.04 (0.38) | 0.10 (0.53) | –0.06 (–0.13 to 0.14) | 0.114 |
| Other contacts (SD) | 0.29 (1.33) | 0.35 (1.51) | –0.07 (–0.29 to 0.16) | 0.271 |
| Primary care: visits per patient between 6 and 12 months | n = 313 | n = 303 | ||
| GP visits at surgery (SD) | 1.88 (2.94) | 1.65 (2.35) | 0.23 (–0.20 to 0.65) | 0.296 |
| Nurse visits at surgery (SD) | 1.36 (3.55) | 2.10 (9.04) | –0.74 (–1.84 to 0.35) | 0.182 |
| GP visits at home (SD) | 0.12 (0.52) | 0.10 (0.42) | 0.02 (–0.06 to 0.09) | 0.615 |
| District nurse visits at home (SD) | 1.38 (6.44) | 2.24 (11.11) | –0.86 (–2.30 to 0.58) | 0.242 |
| GP telephone consultations (SD) | 0.77 (1.90) | 0.56 (1.32) | –0.20 (–0.06 to 0.46) | 0.127 |
| Counsellor (SD) | 0.05 (0.48) | 0.08 (1.16) | –0.03 (–0.17 to 11) | 0.693 |
| NHS Direct telephone call (SD) | 0.08 (0.38) | 0.10 (0.42) | –0.02 (–0.08 to 0.05) | 0.559 |
| Health visitor at home (SD) | 0.05 (0.31) | 0.04 (0.27) | 0.01 (–0.03 to 0.06) | 0.529 |
| Other contacts (SD) | 0.19 (0.77) | 0.30 (1.44) | –0.11 (–0.30 to 0.07) | 0.207 |
| Health-care resource | Hughes repair | Standard mass closure | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Social care: visits per patient between baseline and 6-month follow-up | n = 301 | n = 286 | ||
| Social worker (SD) | 0.67 (10.50) | 0.07 (0.38) | 0.60 (–0.62 to 1.82) | 0.333 |
| Home help (SD) | 0.96 (10.80) | 2.34 (24.48) | –1.38 (–4.42 to 1.67) | 0.375 |
| Care assistant (SD) | 1.05 (10.95) | 2.08 (22.10) | –1.03 (–3.84 to 1.77) | 0.470 |
| Day centre (SD) | 0.01 (0.16) | 0.05 (0.49) | –0.04 (–0.10 to 0.02) | 0.199 |
| Social services (SD) | 0.12 (0.90) | 0.32 (4.63) | –0.20 (–0.73 to 0.34) | 0.465 |
| Social care: visits per patient between 6 and 12 months | n = 313 | n = 303 | ||
| Social worker (SD) | 0.02 (0.24) | 0.07 (0.55) | –0.05 (–0.12 to 0.02) | 0.142 |
| Home help (SD) | 0.16 (2.03) | 0.23 (3.09) | –0.07 (–0.49 to 0.338) | 0.723 |
| Care assistant (SD) | 1.49 (21.09) | 1.23 (20.97) | 0.26 (–3.07 to 3.59) | 0.878 |
| Day centre (SD) | 0.02 (0.34) | 0.02 (0.22) | 0.00 (–0.05 to 0.4) | 0.865 |
| Social services (SD) | 0.01 (0.14) | 0.21 (3.45) | –0.20 (–0.59 to 0.19) | 0.318 |
| Health-care resource | Hughes repair | Standard mass closure | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Surgical care: visits per patient between baseline and 6-month follow-up | n = 301 | n = 286 | ||
| Consultant surgeon (SD) | 1.66 (2.05) | 1.75 (2.06) | –0.09 (–0.42 to 0.24) | 0.593 |
| Specialist nurse at home (SD) | 0.32 (1.49) | 0.53 (2.37) | –0.21 (–0.53 to 0.11) | 0.192 |
| Specialist nurse at hospital (SD) | 0.98 (1.78) | 1.54 (4.87) | –0.56 (–1.15 to 0.03) | 0.069 |
| Stoma nurse (SD) | 1.31 (4.14) | 1.43 (3.22) | –0.12 (–0.72 to 0.48) | 0.694 |
| Other surgical (SD) | 0.48 (2.21) | 0.40 (1.58) | 0.08 (–0.24 to 0.39) | 0.630 |
| Surgical care: visits per patient between 6 and 12 months | n = 313 | n = 303 | ||
| Consultant surgeon (SD) | 1.01 (1.34) | 1.36 (2.69) | –0.34 (–0.68 to –0.005) | 0.046 |
| Specialist nurse at home (SD) | 0.12 (0.72) | 0.21 (1.34) | –0.09 (–0.26 to 0.09) | 0.322 |
| Specialist nurse at hospital (SD) | 0.85 (1.52) | 1.02 (2.21) | –0.17 (–0.47 to 0.13) | 0.265 |
| Stoma nurse (SD) | 0.51 (1.41) | 0.80 (3.20) | –0.28 (–0.68 to 0.11) | 0.157 |
| Other surgical (SD) | 0.18 (0.59) | 0.22 (0.71) | –0.05 (–0.15 to 0.06) | 0.263 |
| Health-care resource | Hughes repair | Standard mass closure | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Hospital services: visits per patient between baseline and 6-month follow-up | n = 310 | n = 305 | ||
| Emergency department visits (SD) | 0.48 (1.08) | 0.59 (1.93) | –0.11 (–0.36 to 0.13) | 0.372 |
| Outpatient visits (SD) | 1.80 (3.27) | 2.18 (3.96) | –0.38 (–0.96 to 0.20) | 0.195 |
| Day surgery visits (SD) | 1.21 (3.36) | 1.00 (3.19) | 0.21 (–0.31 to 0.73) | 0.421 |
| Inpatient days (SD) | 4.30 (10.10) | 3.25 (7.42) | 1.05 (–0.35 to 2.45) | 0.141 |
| Occupational health visits (SD) | 0.14 (1.32) | 0.09 (0.52) | 0.05 (–0.11 to 0.21) | 0.534 |
| Ambulance (SD) | 0.15 (0.59) | 0.10 (0.35) | 0.06 (–0.03 to 0.13) | 0.147 |
| Other hospital care (SD) | 0.62 (3.17) | 0.42 (1.53) | 0.19 (–0.02 to 0.13) | 0.335 |
| Hospital services: cost per patient between 6 and 12 months | n = 315 | n = 306 | ||
| Emergency department visits (SD) | 0.16 (0.48) | 0.17 (0.51) | –0.01 (–0.08 to 0.07) | 0.905 |
| Outpatient visits (SD) | 1.28 (2.18) | 1.71 (4.08) | –0.43 (–0.95 to 0.09) | 0.101 |
| Day surgery visits (SD) | 0.50 (2.25) | 0.60 (2.05) | –0.10 (–0.44 to 0.24) | 0.551 |
| Inpatient days (SD) | 1.13 (3.74) | 1.31 (5.36) | –0.18 (–0.91 to 0.55) | 0.670 |
| Occupational health visits (SD) | 0.05 (0.45) | 0.05 (0.34) | 0.00 (–0.06 to 0.06) | 0.963 |
| Ambulance (SD) | 0.10 (0.70) | 0.09 (0.51) | 0.01 (–0.08 to 0.11) | 0.785 |
| Other hospital care (SD) | 0.23 (0.93) | 0.28 (1.26) | –0.05 (–0.22 to 0.13) | 0.595 |
| Population | Hughes repair | Standard mass closure | Difference (95% CI) | p-value |
|---|---|---|---|---|
| Available cases | ||||
| Sample size, n | 244 | 248 | – | – |
| Mean QALYs gained (SD) | 0.6938 (0.0856) | 0.6872 (0.0855) | 0.00659 (–0.00769 to 0.02171) | 0.392 |
| ITT (mean imputation) | ||||
| Sample size, n | 339 | 333 | – | – |
| Mean QALYs gained (SD) | 0.6902 (0.0725) | 0.6901 (0.0673) | 0.00014 (–0.0054 to 0.0107) | 0.979 |
| ITT (LOCF) | ||||
| Sample size, n | 332 | 326 | – | – |
| Mean QALYs gained (SD) | 0.6856 (0.0874) | 0.6844 (0.0854) | 0.00119 (–0.00674 to 0.01442) | 0.859 |
List of abbreviations
- ASA
- American Society of Anesthesiologists
- BMI
- body mass index
- CC
- complexity and comorbidities
- CE
- cost-effectiveness
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CRF
- case report form
- CSRI
- Client Service Receipt Inventory
- CT
- computed tomography
- CUA
- cost–utility analysis
- DMC
- Data Monitoring Committee
- EQ-5D
- EuroQol-5 Dimensions
- FACT-C
- Functional Analysis of Cancer Therapy – Colorectal
- GCP
- Good Clinical Practice
- GP
- general practitioner
- HART
- Hughes Abdominal Repair Trial
- ICER
- incremental cost-effectiveness ratio
- IH
- incisional hernia
- ITT
- intention to treat
- LOCF
- last observation carried forward
- MCS
- Mental Component Summary
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PCS
- Physical Component Summary
- PDS
- polydioxanone
- POSSUM
- Physiological and Operative Severity Score for understanding Mortality and Morbidity
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- REC
- Research Ethics Committee
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- SA
- sensitivity analysis
- SAE
- serious adverse event
- SAP
- Statistical Analysis Plan
- SD
- standard deviation
- SF-12
- Short Form-12 items
- SF-36
- Short Form-36 items
- SSI
- surgical site infection
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/CMWC8368).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
