Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/154/04. The contractual start date was in April 2016. The draft report began editorial review in September 2019 and was accepted for publication in March 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Gumley et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health and Care Research, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Schizophrenia is a long-term and serious mental illness for which a wide variety of risk and protective factors have been evidenced. 1 It is a form of psychosis characterised by positive and negative symptoms. Positive symptoms relate to distortions of thinking, including fixed beliefs, delusions and paranoia, as well as auditory and visual hallucinations. Negative symptoms include deficits in cognitive functioning, low mood and blunted emotions. Schizophrenia has a lifetime prevalence rate of 4.0 (1.6–12.1) per 1000 people2 and is estimated to affect 21 million people worldwide. Onset of symptoms typically occurs in early adulthood, with the majority of people continuing to experience persisting or fluctuating symptoms. 2 Schizophrenia contributes 13.4 (9.9–16.7) million years of life lived with disability to burden of disease3 and is one of the top 15 leading global causes of disability. 4
People with a schizophrenia diagnosis die 14.5 (11.2–17.8) years earlier than those in the general population, with a mean age at death for men of 59.9 (55.5–64.3) years and 67.6 (63.1–72.1) years for women,5 meaning it is a major public health concern. In a recent longitudinal study,6 11.7% of 171 participants were deceased at follow-up 20 years after a first episode of psychosis, more than double the expected mortality. Worryingly, this differential mortality gap has widened over recent decades. 7 Although it is impossible to quantify the significant emotional distress and life disruption associated with schizophrenia and psychosis for people directly affected and for those closest to them, it is estimated that the overall costs to society are somewhere in the region of £11B in the UK8 and AU$4.9B annually in Australia. 9
Relapse in psychosis
Estimates suggest that around 80% of people with a schizophrenia diagnosis experience a relapse after 5 years. 10,11 A 2012 systematic review12 found pooled prevalence rates of relapse in positive symptoms following a first episode of 28% (range 12–47%) at 1-year follow-up, 43% (range 35–54%) at 18 months and 54% (range 40–63%) at 3 years, with similar relapse rates identified in more recent longitudinal studies. 13,14 The second Australian national survey of psychosis15 found that > 60% of respondents had experienced multiple episodes of psychosis symptoms interspersed with periods of full or partial remission, with 35% having experienced one or more hospital admissions in the previous year. Relapse and associated hospital admissions can be deeply distressing and traumatic experiences for people affected,16 and fear of future relapse can be disabling for service users17 and family members alike. 18
Direct treatment costs for people who experience a relapse are three times higher than they are for people who do not, with the majority of additional costs associated with unplanned hospital admissions. 19 Health-care costs for people characterised as unstable (defined as having had one or more admissions in the previous 2 years) have been found to be four times higher than for people who are stable, again with the vast majority of additional costs associated with hospital admissions. 20 Almost half (46%) of health sector costs for psychosis are generated by inpatient care in Australia, accounting for 96% of the total (AU$609M). 9 The greatest impact on these patterns of service use is relapsing psychosis. 15 Inpatient admissions are associated with suicidal ideation, younger age, poorer functioning and increased symptomology. 21
Relapse in psychosis is associated with a lifetime risk of functional and clinical deterioration,14 meaning that there is an urgency to identify and respond to valid predictors to inform personalised treatment responses. 22,23 A broad range of potential predictors of relapse have been investigated, with a small number consistently identified across the literature. 12,22 Non-adherence to treatment, including medication, increases the risk of relapse12,14 by potentially up to five times. 11 Medication non-adherence is, in turn, predicted by poorer insight of illness, previous involuntary treatment, poorer premorbid functioning, forensic service history and substance misuse, previous suicide attempts, embarrassment, wider issues with service engagement and poor working alliance. 24–28 Other predictors of relapse include substance misuse,12 cannabis use,13 younger age at onset of psychosis,14 poorer premorbid functioning, increased family criticism12 and fear of relapse itself. 17 Reduced social functioning, isolation11,12,29 and negative interpersonal style, possibly linked to poorer use of social support,30 may also predict relapse, with a potential dose–response effect noted between repeated relapses and poorer social functioning. 31
Evidence for the prevention of relapse
Recent review evidence suggests that it is possible to intervene to reduce the likelihood of psychosis relapses, with absolute risk reductions of between 13.6% and 37.0%. 32 At the same time, it has been argued that it is not possible to make specific recommendations about the predictors that should be included in a prognostic model, owing to a lack of high-quality evidence. 23 At present, oral antipsychotic medication in combination with psychological interventions is the recommended treatment for prevention of relapse in first-episode and recurrent psychosis. 33
Reviews and meta-analyses suggest that although antipsychotics are consistently more effective than placebo34 they tend to be of intermediate efficacy for relapse prevention, with limited differences noted between individual drugs. 35 Discontinuation of antipsychotics is common and, although discontinuation rates vary between drugs,36 one trial found that almost three-quarters of participants had an unplanned end to their antipsychotic treatment before the end of the 18-month study period. 37 The odds ratios of discontinuation compared with those for placebo have been found to range from 0.43 for the best-performing drug to 0.80 for the worst. 34 Discontinuation often relates to the intolerability of adverse effects of antipsychotics and, although the type and frequency of adverse effects vary considerably between medications, they can include weight gain, extrapyramidal side effects, sedation and prolactin increase. 34
Meta-analyses of cognitive–behavioural therapy (CBT) for psychosis have found small to moderate effect sizes in favour of CBT for psychosis for a variety of outcomes, including improvement in mental state,38 reduction in positive39,40 and negative symptoms,40 reduced hospital admissions41,42 and improved mood and functioning. 40 Some have urged caution in the interpretation of positive effects given an apparent inverse relationship between effect size and methodological rigour, particularly in relation to masking,40,43 and effects in favour of CBT for psychosis may also be less clear when trials use other psychological therapies as the comparator. 44 Reviews of family interventions, which take different forms but tend to be underpinned by cognitive–behavioural principles,45 have shown reductions in relapse and hospital admissions38,46 as well as improvements in treatment compliance. 46 However, the availability of both CBT for psychosis and family therapies is limited by poor implementation in adult mental health services. 8,47
In summary, the strongest evidence among treatment approaches for relapse prevention is to be found for antipsychotic medication, but its benefits are limited by high levels of discontinuation and it is often associated with prevalent and burdensome side effects. Evidence in support of psychological therapies for relapse prevention is limited, with questions over methodological rigour. Access to psychological therapies is also limited by poor implementation in services. This means that there is a pressing need to investigate alternative methods of relapse prevention.
Early warning signs monitoring and interventions
A further well-established approach for relapse prevention in research and practice is early warning signs (EWS) monitoring, established by Birchwood, Herz and colleagues. 48,49 EWS monitoring is based on the assumption that it is possible to intervene to reduce the likelihood of a relapse of psychosis through its timely prediction. This is generally achieved by comparing ongoing assessments of EWS indicators against an individual’s ‘baseline’ score. 50 Relapse in psychosis is now understood to be the end point of a process of change associated with early signs including changes in affect and incipient psychotic experiences. 50 EWS may be identifiable for as long as 5–8 weeks prior to a relapse,51 creating significant opportunity for early intervention. However, review evidence suggests considerable variation in the proportion of relapses correctly predicted by EWS (sensitivity 10–80%, median 60%) and non-relapses correctly predicted (specificity 38–100%, median 81%), with more frequent monitoring and the inclusion of both affective and psychosis symptoms found to improve prediction. 52
Eisner et al. 52 identified 17 papers involving early signs interventions in their 2013 review. Early signs interventions involve responding to EWS changes with a plan designed to prevent or minimise relapse. Just one of these studies used a purely psychological approach, with two or three sessions of relapse prevention focused CBT administered in response to an assessed increase in EWS from baseline. 53 People in the CBT arm had significantly fewer relapses than people in a treatment-as-usual arm, but the findings may have been influenced by the lack of masked assessment of relapse. Four studies included interventions in response to EWS increases that Eisner et al. described as multicomponent relapse prevention techniques. These included stress management and problem-solving methods, increased practitioner contact and antipsychotic medication increases,54–57 as well as three interventions that involved a relative or friend. 54,55,57 In two out of the three studies that assessed relapse, those in the intervention arm had significantly improved outcomes compared with those receiving treatment as usual,54,55 and the other study was potentially underpowered to show an effect on relapse. 57
The remaining studies in the Eisner et al. 52 review related to interventions in response to EWS that involved changes in medication alone. In one study, targeted medication or placebo was given in addition to maintenance medication on the emergence of EWS. However, no differences were observed between the arms in relation to time to symptom exacerbation at 2-year follow-up. 58 The remaining 11 studies temporarily used targeted medication on the emergence of EWS but in the absence of other ‘maintenance’ antipsychotic medication. 59–70 Although there were variations in the method of treatment and in comparators, in all but one65 of the seven studies of targeted medication approaches where relapse was assessed the outcomes were better for people in receipt of a moderate dose of maintenance medication than for people receiving targeted medication alone. 61,62,64,67–69 Two studies59,60 measured hospital admission alone, finding no significant differences in admission rates between people in receipt of maintenance medication regimes and those in receipt of targeted medication regimes.
Across all of the included studies, comparison was complicated by the heterogeneity of EWS assessment and the definition of relapse alongside a number of methodological weaknesses, including potential sampling bias and underpowered studies. Despite this, a number of conclusions were drawn about EWS-informed interventions. Targeted medication in response to EWS was found to be less effective than maintenance for relapse prevention, and replication with blinded assessment of relapse was recommended for the lone psychological intervention53 before firm conclusions could be drawn about its seeming potential. Multicomponent responses to EWS were described as showing promise for relapse prevention, but methodological weaknesses were highlighted, suggesting the need for further research.
Additional evidence of the potential for EWS interventions was provided in a 2013 Cochrane review71 that compared the effectiveness of EWS interventions with that of treatment as usual on time to relapse, hospitalisation, functioning and symptoms. The review found that significantly fewer people relapsed with EWS interventions than with usual care [23% vs. 43%; risk ratio 0.53, 95% confidence interval (CI) 0.36 to 0.79; 15 randomised controlled trials (RCTs), n = 1502] and significantly fewer people were readmitted to hospital (19% vs. 39%; risk ratio 0.48, 95% CI 0.35 to 0.66; 15 RCTs, n = 1457). There was, however, no effect on reducing time to relapse. However, the review found low overall quality of evidence, which limited generalisability to usual care, making it impossible to recommend the use of EWS interventions in routine practice until higher-quality evidence becomes available.
Service user barriers to relapse detection and prevention
Uncertainty about the prognostic validity of EWS52 brings with it a risk of unnecessary interventions from services, which may, in turn, heighten the fear of relapse in people with experiences of psychosis and their carers. 17 Feelings of fear, helplessness and depression are commonly experienced prior to full relapse. 72 Fear of illness or relapse is also associated with poorer insight,25 emotional dysfunction,73 suicide risk,74 more traumatic experiences of psychosis, and fear of psychosis symptoms and hospital. 16 It is also a barrier to coping and relapse prevention for family members. 18 Our 2015 RCT17 of relapse detection showed that fear of recurrence contributed independently to the prediction of relapse (sensitivity 72%, 95% CI 52% to 86%) compared with EWS (sensitivity 79%, 95% CI 62% to 89%) and should, therefore, be included in EWS monitoring. Fear of recurrence was also associated with increased depression and feelings of entrapment, self-blame and shame and was a significant predictor of time to relapse. This suggests that fear of recurrence may be a risk factor for relapse and for increased distress arising from psychosis experiences and that it may play some role in accelerating the process of relapse. All in all, this suggests that fear of recurrence is a key barrier to, and potential target for, interventions to predict and prevent relapse.
Concurrently, people affected by psychosis are more likely than those in the general population to adopt avoidant coping styles,75,76 which may be an attempt to prevent relapse and minimise the effects of public stigma. 73 Avoidant coping in psychosis has been associated with higher neuroticism and lower extraversion,77 reduced insight,78 negative early childhood experiences, insecure identity,79 cognitive deficits80 and a generally greater insecurity in relationships and reluctance to seek help in a crisis. 81,82 In the context of active psychosis symptoms, avoidance may represent a safety behaviour based on the perceived threat of other people. 83 Review evidence suggests that greater difficulties in forming relationships among people affected by psychosis is associated with poorer service engagement and poorer relationships with practitioners, as well as longer and more frequent inpatient admissions. 81 Reluctance to seek help may be an understandable response to fear generated through previous negative, and potentially traumatic, experiences of inpatient admission. 16 In totality, this suggests that identifying and responding to EWS may be constrained by a variety of factors. These include avoidant coping styles and an associated reluctance to seek help or disclose EWS, poor-quality relationships between people in receipt of services and those providing them, and a wider fear of relapse.
Service barriers to relapse detection and prevention
One potentially important means of improving the implementation of EWS approaches without necessarily increasing fear of relapse is using interventions to increase shared decision-making for relapse prevention and risk management. Joint crisis plans (JCPs), which allow for the expression of service user preferences in the event of a future relapse, are one such example in the UK. The CRIMSON study84 compared the effectiveness of JCPs with that of treatment as usual for people with a diagnosis of schizophrenia but found no significant impact on the reduction of compulsory treatment, raising questions about the wider application of JCPs. An associated process evaluation85 examined how stakeholders perceived JCPs and shared decision-making as well as the barriers to implementation. The main barriers identified related to practitioners and included a general ambivalence towards JCPs and the perception that shared decision-making was already happening, as well as concerns about service users’ expressed wishes for JCPs. These barriers led to poor clinician engagement, which in turn undermined service users’ contributions to the process. It was also noted that, in times of crisis, JCPs were largely ignored, with practitioners reverting to standard practice. As a result of feeling unable to influence practitioner views through JCPs, people using services felt that there was a lack of respect for their views and that they were not able to influence shared decisions. Clinicians themselves experienced their ongoing clinical interactions as ritualised, particularly in relation to risk, while wider evidence highlights the need to be conscious that EWS monitoring for relapse prevention is also contingent on the person receiving services initiating help-seeking from a position of vulnerability and perceived threat (linked, for example, to previous experiences of coercive treatment). 86
People affected by psychosis may have had difficult or traumatic experiences of relapse,16 and there can be many barriers to help-seeking,87 which reduce the possibility of early intervention at times of crisis. This may in turn increase reliance on coercive practices, potentially building on already negative expectations in a vicious cycle. This means that there is some urgency to develop and evaluate an intervention that can facilitate safe and honest disclosure of possible EWS while also encouraging collaborative and non-coercive responses from mental health practitioners.
Conceptual framework for improving relapse detection and prevention
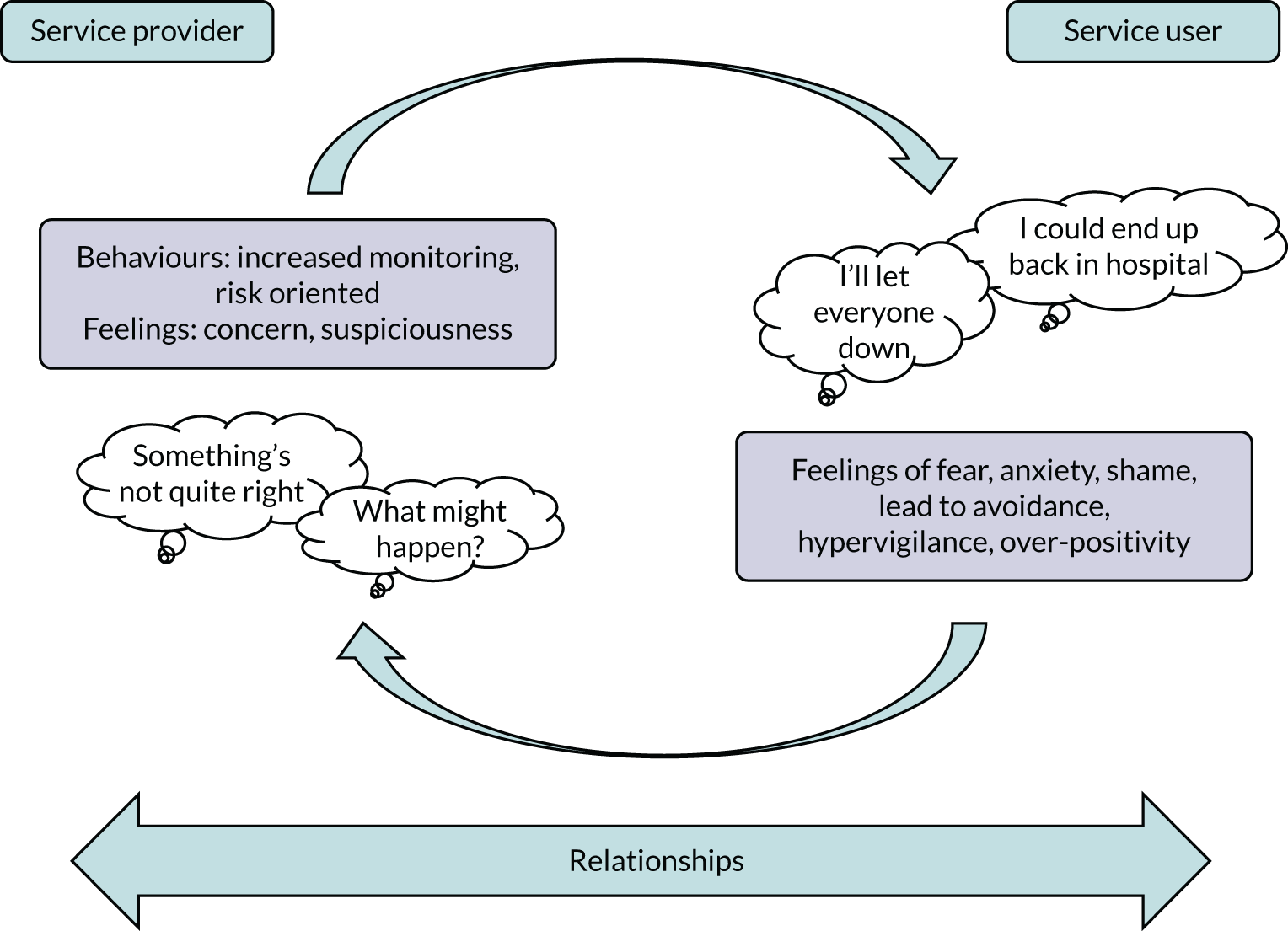
Our conceptual framework for improving relapse detection and prevention aims to understand the awareness of and response to EWS in the context of relationships. Figure 1 illustrates a cognitive–interpersonal model of EWS in which fear of recurrence drives negative emotions including fear, anxiety and shame. This distress may be regulated by coping strategies including avoidance and hypervigilance. Such responses may in turn influence practitioner responses at times of increased risk of relapse, for example interpreting avoidance (of appointments, telephone calls, etc.) as evidence of the need to focus more on risk management. For someone using services, such shifts in practice may in turn simply reaffirm existing negative expectations in a cyclical manner. Therefore, interventions that can enhance positive emotional awareness, choice and autonomy (through self-management promotion) and improved communication (through increased understanding) could provide a means to disrupt and change these negative interpersonal cycles. Given that the modal time window to intervene in the context of EWS is 2 weeks,52 interventions that can enhance access to data to inform help-seeking and shared decision-making could contribute to relapse detection and prevention. Digital technology may offer such an opportunity for this kind of timely intervention.
FIGURE 1.
Cognitive–interpersonal framework for EWS.
Digital technology for early warning signs monitoring and intervention
Mobile health technology, also known as mHealth, has the potential to make a variety of mental health interventions more widely available through a combination of computing power, portability and widespread ownership of mobile devices. There has been a rapid increase in internet-connected smartphone ownership with an estimated 3 billion smartphone users globally in 2019. 88 There are indications that a ‘digital divide’, restricting access to mobile technologies for people affected by psychosis in comparison with the general population, has diminished in recent years,89 with estimated rates of ownership ranging from 66.4% (95% CI 54.1% to 77.6%) to 81.4% in more recent studies. 90
The potential of mHealth to transform remote measurement of changes in health and well-being across health conditions has been recognised,91 and in mental health this includes the identification of its potential to improve EWS monitoring and relapse prevention. 92,93 Emerging evidence suggests that people with experiences of psychosis are generally comfortable with the application of digital mobile technologies to support self-management and enhance service engagement. 90,94 Many studies of mHealth for psychosis also show high levels of acceptability,95 although problems with inconsistent assessment and reporting of usability and acceptability in mHealth for psychosis studies need to be addressed. 96
Mobile technologies, in particular smartphones, provide an opportunity to overcome some of the known barriers to EWS implementation potentially through both ‘active’ and ‘passive’ data collection. Active data collection invites people to respond to intermittent EWS self-report assessments on their mobile device. 97 Passive monitoring relates to the assessment of relevant data routinely gathered on a mobile device, for example in relation to phone usage or location. 98 Both approaches may also be combined. 99
The mobile collection of data on repeated occasions in real-world circumstances represents a form of ecological momentary assessment (EMA). EMA, which is being applied increasingly in psychosis research,100 brings with it the potential to reduce the type of recall bias often associated with retrospectively gathered data. 101 Such routine monitoring can generate rich data about people’s experiences, which have the potential to support shared decision-making and to reduce the ambiguity within which practitioners may be required to reach clinical decisions, particularly at times of crisis. Bell et al. 100 reviewed nine EMA interventions for people with experiences of psychosis (n = 459),97,102–109 finding some evidence for improved clinical outcomes as well as reasonable feasibility and acceptability. Similarly, positive results have been reported in terms of feasibility, acceptability and adherence for a further 10 mHealth for psychosis studies that feature the prospective assessment of symptom course. Of particular relevance are studies of the Information Technology Aided Relapse Prevention Programme in Schizophrenia (ITAREPS)97,108,109 and ExPRESS110 early signs interventions.
The ITAREPS system, which was tested in Czech and Slovak mental health services, used text-based EWS data collection to notify clinicians when scores breached a specified threshold. Treating clinicians were expected to increase medication by 20% within 24 hours of an alert. Initial non-controlled mirror design testing suggested a 60% decrease in hospital admissions during ITAREPS participation over a mean of 283.3 days (± 111.9) when compared with the same time period before entering the programme. 108,109 However, a more rigorous follow-up double-blind randomised trial found no statistically significant differences in hospitalisation rates between people in an ITAREPS group and people in a control group. 97 However, there were significant problems with clinicians’ adherence to the treatment protocol, and a post hoc analysis suggested a ninefold reduction in the risk of hospitalisation for the subset of people among whom there was found to be high clinician adherence. A later study of the ITAREPS system, which removed the mHealth component, found that adherence to the protocol improved when mental health nurses were employed to triage EWS assessments. 111 More recently, Eisner et al. 110 tested the feasibility of combining conventional EWS monitoring with basic symptoms112 monitoring in an app (smartphone application), with scores above a specified threshold prompting a phone-based assessment of relapse. Participants completed 65% of app assessments over 6 months. App ratings also showed high concurrent validity with researcher assessments and there was preliminary evidence of the predictive validity of app ratings.
Although the overall results of mHealth for psychosis studies are promising, it should be noted that the vast majority of research in the field relates to small pilot or feasibility studies. Questions have been raised over the quality of reporting generally96 and the assessment and reporting of safety outcomes113 and also how end-user experience and engagement are measured114 and encouraged. 115 There is, however, emerging evidence to suggest that engagement with mHealth for psychosis interventions can be enhanced by providing social support, including peer support, as part of the interventions. 116,117
Peer support working
The creation of formalised peer support roles in mental health systems, where peer workers are employed and trained to work with people in receipt of services, in part based on a shared lived experience of mental distress, is considered to be an important component of recovery-oriented mental health systems. 118,119 Peer workers are theoretically well placed to reduce the power imbalances inherent in mental health services, drawing on their own experiences to build relationships with people using services that are reciprocal and founded on mutuality. 120 Although limitations in the conduct and reporting of research on peer support121 should be acknowledged, there is increasing evidence that peer supported interventions are at least as effective as non-peer-delivered equivalents122 and that peer workers are particularly well placed to deliver on recovery-related outcomes, including hope and empowerment. 119,121 Recent trial evidence also suggested that a peer-supported self-management intervention with people in receipt of crisis services was effective in reducing hospital re-admissions. 123 Fortuna et al. 124 have proposed a theoretical model of the role of peer support specific to digital health behaviour interventions. Central to this model are reciprocal accountability processes shared between peer supporters and people using digital interventions, which the authors argue build engagement through various mechanisms, including the promotion of autonomy, goal-setting, a strong peer-to-end-user working alliance and therapeutic bonding.
Conclusion
In summary, mHealth interventions, and smartphone interventions specifically, offer a significant opportunity to deliver EMA-based monitoring of changes in well-being that are ecologically valid and contextually sensitive. They also offer potential solutions to some of the implementation barriers that have hindered the routine application of EWS approaches. Although there is a need for more rigorous and consistent research, preliminary studies have shown encouraging levels of user acceptability and engagement, including those specific to EWS interventions, facilitating timely assessment and intervention to promote self-management and relapse prevention. However, there are important implementation challenges to ensure that these technologies can enhance relationships and shared decision-making, particularly during times of increased stress and crisis. Blending peer support with digital EWS interventions has the potential to improve engagement and user experience, contributing to the need to develop interventions that enhance positive emotional awareness, choice and autonomy.
Chapter 2 Methods
EMPOWER phase 1
Objective
The objective of phase 1 was to conduct task group interviews to (1) evaluate the acceptability and usability of mobile symptom reporting using smartphones among service users, carers and mental health staff; (2) identify incentives and barriers to use by service users and carers and to implementation by mental health staff; and (3) identify pathways to relapse identification and prevention. These interviews informed modifications to the EMPOWER mobile app, which was then subjected to a 5-week beta-testing phase.
The aims of the work packages (WPs) that constituted phase 1 are as follows.
-
WP 1: (1) evaluate the acceptability and usability of mobile symptom recording using smartphones among service users and their carers; and (2) identify incentives and barriers to use.
Deliverables: software and protocol updates in response to feedback from service users and carers.
-
WP 2: (1) evaluate the acceptability and usability of mobile symptom recording using smartphones among professional mental health care staff; (2) identify incentives and barriers to implementation by mental health staff; and (3) identify relapse prevention pathways and whole-team responses.
Deliverables: (1) carry out software and team protocol updates in response to feedback from professional care staff; (2) develop care pathways and identify operational barriers and enablers; and (3) identify the training needs of teams participating in the phase 2 pilot cluster randomised controlled trial.
-
WP 3: finalise the EMPOWER app for its implementation in phase 2.
Deliverables: (1) carry out software and protocol updates in response to feedback from service users, carers and staff; (2) agree on final modifications to the EMPOWER app to enhance its usability; (3) and finalise measurement methods for the assessment of self-reported acceptability and usability.
Settings
Parallel arms of data collection for phase 1 took place in NHS Greater Glasgow & Clyde, UK, and NorthWestern Adult Community Mental Health Services in Melbourne, Australia.
Methods
Work package 1: task groups with service users and carers
A task group is a type of focus group designed to generate qualitative data and the principles for action, which are grounded in the experience of group members. Task groups were used to elicit views about experiences of relapse, incentives and barriers to help-seeking and optimal responses to relapse or the threat of relapse. Task groups explored:
-
the utility of early signs monitoring
-
views about using self-management messages and which self-management messages would have greatest salience
-
the design parameters of the system that could best sustain their involvement
-
views about help-seeking and activating a relapse prevention pathway
-
the best way to involve carer stakeholders
-
the best way to contact mental health staff
-
how they would like to use their data from EMPOWER.
A copy of the task group guide can be found in Report Supplementary Material 1. These data informed the final design and beta testing of the EMPOWER app to optimise the usability, salience, applicability and overall coherence of the intervention. We also recognised that some participants would be unable to attend task groups (e.g. owing to time constraints or difficulties engaging in groups). Therefore, to maximise engagement and diversity of views, we offered participants who were unable to attend task groups the opportunity to participate in individual interviews.
Work package 2: task groups with professional mental health care staff
The aim of the task groups with mental health care staff was to clarify the existing support pathways and procedures, systems and policies in teams participating in usual care, and to clearly differentiate these from our experimental intervention. We focused on the following topic areas:
-
strengths and limitations of these existing pathways
-
relevant policies and procedures that guide treatment as usual
-
feasibility and risks of and incentives to incorporating mobile phone technology into the monitoring and detection of risk of relapse
-
best methods to deal with false positives
-
opportunities to optimise pathways to relapse prevention.
A copy of the task group guide can be found in Report Supplementary Material 1. We recognised that some staff members may have been unable attend task groups, for example because of the time required. Therefore, to maximise engagement and diversity of views, we offered participants who were unable to attend task groups the opportunity to participate in individual interviews.
Work package 3: software beta testing
Beta testing was conducted with service users over a 5-week period. We utilised beta testing as a form of software user experience (UX) testing and investigation conducted to provide stakeholders with information about the quality of the tested software product. 125 Following the software beta testing, we conducted in-depth interviews exploring service user participants’ experiences of using the app and their perspectives on its acceptability and utility.
Study population
Recruitment procedure
Work package 1
Potential service user participants were approached to participate via community mental health services (CMHS). We invited mental health care staff to identify potentially eligible participants and we also placed posters in local CMHS advertising the research. Potential participants were provided with a participant information sheet and a consent form. They were advised that participation was entirely voluntary and that refusal to participate would not affect the care provided by their local CMHS. Following the provision of written and informed consent, service user participants were invited to nominate a carer to participate. When insufficient carers were recruited via service users, task group participation was opened to any carer associated with, and made known through carer organisations at, each participating site. Following an amendment to the protocol (SA01–AM02: see Table 1) in Glasgow, we also engaged with the Mental Health Network (Greater Glasgow & Clyde) and ACUMEN to support the recruitment of potentially eligible participants. Both of these organisations work directly with NHS Greater Glasgow & Clyde to promote the wider involvement of service users and carers in shaping mental health services and facilitate collaboration through support and networking. In addition, we engaged with Support in Mind Scotland, which has strong engagement with carers of people diagnosed with schizophrenia. These organisations expressed a strong interest to engage with EMPOWER to highlight the study among members of their constituencies.
Work package 2
Professional mental health care staff were identified through service managers and through presentations at staff meetings. Staff members were invited to take part in a task group and provided with a participant information sheet and a consent form. They were advised that participation was entirely voluntary and that refusal to participate would not affect their employment.
Work package 3
In the first instance, service users who took part in WP 1 were invited to take part in the software beta testing. In addition, we recruited eligible participants from our local service user networks.
Eligibility criteria
Service users
Service users were eligible for participation in WP 1 if:
-
they were adults (≥ 16 years of age)
-
they were in contact with a local community-based service
-
they had either:
-
been admitted to a psychiatric inpatient service at least once in the previous 2 years for a relapse of psychosis or
-
received crisis intervention (e.g. via a crisis intervention service; re-engaged with a CMHS) in the previous 2 years for a relapse of psychosis
-
-
they had a diagnosis of a relevant DSM-5 schizophrenia-related disorder (i.e. schizophrenia, schizoaffective disorder or substance-/medication-induced psychotic disorder)
-
their current presentation did not include severe acute symptoms
-
they were able to provide informed consent as judged by their care co-ordinator/case manager or, if in doubt, the responsible consultant
-
they were able to manage the language requirement of participation.
Service users were eligible for participation in WP 3 if:
-
they were adults (≥ 16 years of age)
-
they were in contact with a local community-based service
-
they had a diagnosis of a relevant DSM-5 schizophrenia-related disorder (i.e. schizophrenia, schizoaffective disorder or substance-/medication-induced psychotic disorder)
-
their current presentation did not include severe acute symptoms
-
they were able to provide informed consent as judged by their care co-ordinator/case manager or, if in doubt, the responsible consultant
-
they were able to manage the language requirement of participation.
Carers
Carers were family members who were in regular (i.e. at least weekly) contact with an individual using services who had a diagnosis of a relevant DSM-5 schizophrenia-related disorder (i.e. schizophrenia, schizoaffective disorder or substance-/medication-induced psychotic disorder). The frequency of contact was the only eligibility criterion for carer participation. Carers nominated by eligible service users who provided informed consent were also approached for inclusion in the study.
Professional mental health care staff
Professional mental health care staff were eligible for participation if they had been working for the service for longer than 2 months, to ensure that they had an orientation to and were familiar with the local service system.
Sample size
The numbers projected for WPs 1 and 2 (i.e. 30 service users, 30 carers and 20–30 professional mental health care staff) and WP 3 (i.e. 10 service users) were to provide sufficient data to create the framework of analysis. No formal sample size calculation (e.g. power analysis) was considered appropriate for these WPs, as their aim was not to evaluate treatment effects.
Analytic methods
Task groups (WPs 1 and 2) and follow-up interviews (WP 3) were digitally recorded, transcribed and anonymised before being entered into NVivo version 12 (a computer-assisted qualitative software package; QSR International, Warrington, UK) to organise the data and enable progression to analysis. Analysis drew on framework analysis, which is a qualitative approach specialising in pragmatic, generalisable qualitative methods designed for real-world implementation. 126,127 This approach has been developed specifically for applied or policy-relevant qualitative research in which the objectives of the investigation are typically set in advance and shaped by the information requirements of the funding body. The time scales of applied research tend to be short and there is often a need to link the analysis with quantitative findings.
Participant safety and withdrawal
Risk identification
Risks associated with work packages 1 and 2
The potential risks of harm or discomfort to service users, carers and professional mental health care staff who participated in the task groups (i.e. WPs 1 and 2) included:
-
risks to personal privacy associated with the dissemination of personal information by other participants
-
distress from inappropriate, abusive or offensive interaction(s) with other participants
-
increased paranoia resulting from participation, especially in the event of deterioration of the mental well-being of service user participants
-
potential distress from talking about experiences of relapse.
The likelihood of these risks eventuating was considered low based on the past experiences of the investigators.
Risks associated with work package 3
The potential risks of harm or discomfort to service users who participated in the software beta testing (i.e. WP 3) included:
-
risk to personal privacy associated with the unlawful dissemination of personal information by hackers
-
risks to the clinical safety of service user participants (i.e. true- and false-positive detections of relapse).
The likelihood that personal privacy would be breached by the unlawful dissemination of personal information by hackers was considered low given the past experiences of the investigators. Specifically, this related to experiences developing and implementing the ClinTouch system that was the platform for EMPOWER.
The likelihood that clinical safety would be comprimised (i.e. service user participants’ well-being deteriorating and the system flagging a true-positive detection of relapse, or the system flagging a false-positive detection of relapse) was also considered low given the short duration of the software beta testing.
Risk management
The potential risks of harm or discomfort to service users, carers and professional mental health care staff who participated in the task groups (i.e. WPs 1 and 2) as outlined above were negated, minimised or managed through the following processes:
-
All task groups were co-facilitated by two individuals. Key facilitator responsibilities included advising participants of rules of engagement with the group (e.g. confidentiality, respectful communication), and upholding the same.
-
Facilitators also monitored participants’ degree of distress and took action accordingly. Participants who displayed or reported distress were offered a debriefing session.
The potential risks to system and personal privacy and clinical safety associated with WP 3 were negated/minimised/managed via a rigorous safety protocol developed by the research team and experts from the information systems discipline. The safety protocol comprised two levels of security: system and privacy protection and clinical safety.
Clinical safety
A range of measures were also in place to ensure participants’ clinical safety. Changes in EWS were observable by the researchers, and responses were manual rather than automated. Information related to clinical safety (i.e. EWS, idiosyncratic signs, etc.) was screened three times per week on the clinician interface, and specific attention was paid to the deterioration of EWS. Any detected increase meant that the study research assistant advised the clinical team of any significant change to the participant’s mental health.
In the case that a participant contacted the study research assistant, or another member of the research team, communicating distress, the study research assistant or member of the research team provided immediate support, and then contacted the participant’s treating team.
If a participant stopped using the system (i.e. they missed more than two scheduled prompts), the following protocol was adopted: (1) after two missed prompts, a SMS would be sent reminding the service user to use the app, and (2) after subsequent instances/missed prompts, the research team would follow up with a supportive telephone call encouraging participation. Information was to be passed on to the service user’s treating team if the service user stopped responding to the prompts to monitor their EWS, and if they missed the follow-up interview with the study research assistant.
Risk monitoring
Risks were monitored by the study research assistant. As part of their role as interviewer and group facilitator of the various work packages, the study research assistant monitored participants’ degree of distress and took action accordingly. All interviewees were invited to discuss any feelings of distress associated with participating in the interview, and task group participants were also invited to speak privately with the study research assistant and/or co-facilitator at the conclusion of the task group if they felt distressed. The study research assistant monitored the risks associated with the software beta testing; information about clinical safety was screened three times per week.
Risk reporting
The study research assistant reported all instances of distress that came to their attention and any potential clinical deterioration in participants’ mental health to the chief investigator and to the participant’s treating team. The study research assistant also recorded all instances in a database, and the chief investigator reported any serious adverse events that were related and unexpected according to the International Conference on Harmonisation Guidelines on reporting Serious Adverse Events (Section II B) to the sponsor and the Research Ethics Committee.
Handling of withdrawals
Procedures
Participants were free to withdraw from the study at any time. As a part of the informed consent procedure, participants were instructed to let a member of the research team know of their withdrawal ahead of time. Participants who chose to withdraw were offered debriefing as a matter of course. The treating team overseeing the care of service user participants was also advised of any withdrawals. Information collected from participants up to the point of withdrawal was stored in the databank.
Data security and management
The confidentiality of all study data was ensured using the security mechanisms outlined below.
The EMPOWER app
A range of measures were in place to ensure the security of the EMPOWER app and the data generated by its users. The app was hosted on the University of Manchester web server, which had standard measures in place to prevent unauthorised access. All data transmitted to and from EMPOWER servers were encrypted over https with strong ciphers as detailed in Approved Cryptographic Algorithms: Good Practice Guideline. 128 Cipher suites were implemented in compliance with section 6 (‘Preferred uses of cryptographic algorithms in security protocols’) of the Good Practice Guidelines. 128 In cases where participant data were downloaded from the EMPOWER site, these data were securely encrypted with a pass phrase of appropriate length and complexity. Data transfers were secured using standard web security protocols. Uploading study data to a central server in real time enabled them to be captured and so this protected against data loss, such as if a phone was lost or stolen. This removed the need for personal data to be stored on the device. The purpose of the server in this case was secure data storage. We also incorporated ISO 25010,129 which provides for safety-in-use and measures satisfaction with security. These security measures correspond closely to the NHS standards.
A number of technical measures were also employed to protect personally identifiable data. Any data stored on the phone by the participant were encrypted. We also recommended that service users set a passcode to access their smartphone. All service users recruited to the study gave their informed consent, and this included acknowledging risks to the security of their data.
Other study data
Each study participant was assigned a unique trial identification number at the start of the assessment process. This number was written on all clinical assessment forms/datasheets and databases used to record data on study participants. A securely stored and encrypted electronic copy of a record sheet linking patient identity, contact details and trial identification numbers for all participants was kept at each site.
The local study research assistant entered the data on to an electronic database, and all of these data were checked for errors before being transferred to the appropriate statistical package. All data were kept secure at all times and maintained in accordance with General Data Protection Regulation (GDPR)130 requirements.
Audio recordings of the task groups and participant interviews were also stored securely on a server at the University of Glasgow and were destroyed following transcription and analysis of the data. Digital recordings from Melbourne were sent by secure transfer and stored on secure servers at the University of Glasgow.
Protocol amendments
Protocol amendments are described in Table 1.
| Amendment number | Date | Protocol number | Main change summary |
|---|---|---|---|
| SA01 | 3 August 2016 | 1.2 |
|
| SA02 | 2 November 2016 | 1.3 |
|
| SA03 | 15 December 2016 | 1.4 |
|
Protocol breaches
The Post-Study System Usability Questionnaire was omitted from WP 3 follow-up interviews.
EMPOWER phase 2
Objectives
Our phase 2 protocol is published elsewhere. 131 The overall purpose of this study was to establish the feasibility of conducting a definitive cluster randomised controlled trial (cRCT) comparing EMPOWER with treatment as usual (TAU). We sought to establish the parameters of the feasibility, acceptability, usability, safety and outcome signals of an intervention as an adjunct to usual care that would be deliverable in the NHS and Australian CMHS settings. The EMPOWER intervention aimed to:
-
enhance the recognition of EWS among people using services and their carers
-
provide a stepped-care pathway that was either self-activated or in liaison with a carer and/or a community health-care professional, which then
-
triggered a relapse prevention strategy that could be stepped up to a whole-team response to reduce the likelihood of a psychotic relapse.
Specifically, we aimed to:
-
determine the rates of eligibility, consent and recruitment of potentially eligible participants (people using services, carers and care co-ordinators) to the study
-
assess the performance and safety of the EMPOWER class 1 medical device (CI/2017/0039)
-
assess the feasibility, acceptability and usability of the intervention, including feedback on suggested enhancements from people receiving the intervention, peer support workers and clinicians
-
assess primary and secondary outcomes to determine the preliminary signals of efficacy of the EMPOWER intervention as a basis for assessing the feasibility of collecting follow-up measures, determining primary and secondary outcomes, and determining probable sample size requirements for a future main trial
-
undertake a qualitative analysis of relapses to refine the intervention in the main trial
-
establish the study parameters and data-gathering frameworks required for a co-ordinated health economic evaluation of a full trial across the UK and Australia
-
enhance and tailor our mobile phone software app to deliver EWS monitoring, self-management interventions and access to a relapse prevention pathway that was embedded in whole-team protocols and action.
Trial design
We evaluated EMPOWER using a multicentre, two-arm, parallel-group cRCT involving eight purposively selected CMHS (two in Melbourne, Australia, and six in Glasgow, UK) with 12-month follow-up. The CMHS were the unit of randomisation (the cluster), with the intervention delivered by the teams to people using services and with the outcomes assessed within these clusters. The study was planned and implemented in concordance with the Consolidated Standards of Reporting Trials (CONSORT) cluster trial extension132 and the extension to randomised pilot and feasibility trials. 133 We chose a cluster design as the EMPOWER intervention aimed to enable a team-based response to people in receipt of services whose real-time EWS monitoring activates a relapse prevention pathway.
Ethics and governance
The study received approvals from the West of Scotland Research Ethics Service (GN16MH271 reference 16/WS/0225) and Melbourne Health Human Research Ethics Committee (HREC/15/MH/344). The study sponsors were NHS Greater Glasgow & Clyde in the UK and the Australian Catholic University in Australia. The trial also received approvals from the NHS Health Research Authority and a notice of no objection for a trial of a medical device (CI/2017/0039) from the UK Medicines and Healthcare products Regulatory Agency (MHRA), and was prospectively registered (ISRCTN99559262). The trial methods of enrolment, interventions and assessments are summarised in Table 2.
| Assessment | Enrolment: baseline | Allocation: 0 months | Post allocation | Close-out: 12 months | |
|---|---|---|---|---|---|
| 3 months | 6 months | ||||
| Enrolment | |||||
| Eligibility screen | ✗a | —b | — | — | — |
| Informed consent | ✗ | — | — | — | — |
| Allocation | — | ✗ | — | — | — |
| Intervention | |||||
| EMPOWER | — | — | ✗ | ✗ | ✗ |
| Service user assessments | |||||
| Feasibility | ✗ | — | ✗ | ✗ | ✗ |
| Acceptability and usability | — | ✗ | ✗ | ✗ | |
| Remission status | ✗ | — | ✗ | ✗ | ✗ |
| Relapse | — | ✗ | ✗ | ✗ | |
| PANSS | ✗ | — | ✗ | ✗ | ✗ |
| Personal and Social Performance Scale | ✗ | — | ✗ | ✗ | ✗ |
| CDSS | ✗ | — | ✗ | ✗ | ✗ |
| Timeline Followback | ✗ | — | ✗ | ✗ | ✗ |
| HADS | ✗ | — | ✗ | ✗ | ✗ |
| PBIQ-R | ✗ | — | ✗ | ✗ | ✗ |
| Service Attachment Questionnaire | ✗ | — | ✗ | ✗ | ✗ |
| Medication Adherence Rating Scale | ✗ | — | ✗ | ✗ | ✗ |
| EuroQol-5 Dimensions | ✗ | — | ✗ | ✗ | ✗ |
| Assessment of quality of life | ✗ | — | ✗ | ✗ | ✗ |
| Resource Use Questionnaire | ✗ | — | ✗ | ✗ | ✗ |
| Questionnaire about the Process of Recovery | ✗ | — | ✗ | ✗ | ✗ |
| General Self-Efficacy Scale | ✗ | — | ✗ | ✗ | ✗ |
| Psychosis Attachment Measure | ✗ | — | ✗ | ✗ | ✗ |
| Perceived Criticism and Warmth Measure | ✗ | — | ✗ | ✗ | ✗ |
| Carer assessments | |||||
| Feasibility | ✗ | — | ✗ | ✗ | ✗ |
| Carer Quality of Life-7 Dimensions | ✗ | — | ✗ | ✗ | ✗ |
| EuroQol-5 Dimensions, five-level version | ✗ | — | ✗ | ✗ | ✗ |
| Resource use questionnaire | ✗ | — | ✗ | ✗ | ✗ |
| Perceived Criticism and Warmth Measure | ✗ | — | ✗ | ✗ | ✗ |
| Involvement Evaluation Questionnaire | ✗ | — | ✗ | ✗ | ✗ |
| Care co-ordinator | |||||
| Feasibility | ✗ | — | ✗ | ✗ | ✗ |
| Service Engagement Scale | ✗ | — | ✗ | ✗ | ✗ |
A trial Study Steering Committee (SSC) and a Data Monitoring and Ethics Committee (DMEC) were constituted in accordance with National Institute for Health and Care Research (NIHR) guidance. The DMEC reported to the SSC and the SSC reported to NIHR. Protocol amendments were reviewed by the DMEC and SSC before being submitted to the relevant Research Ethics Committee (see Table 4). The study was conducted in accordance with the recommendations for physicians involved in research on human participants adopted by the 18th World Medical Assembly, Helsinki 1964,134 and later revisions.
Preliminary work: patient and public involvement
Phase 1, as described above, was included to ensure extensive consultation with key stakeholders, namely mental health care staff, people with lived experience and carers, in a series of task groups across Glasgow and Melbourne. Stakeholder consultation directly shaped the development of the EMPOWER intervention. The Scottish Recovery Network also played a key role in shaping further consultation with people with lived experience to further refine intervention development and planning.
Eligibility criteria
Participation was sought from CMHS in NHS Greater Glasgow & Clyde in the UK and NorthWestern Mental Health services in Melbourne, Australia. All participants (mental health care staff, service users and carers) were approached for their informed and written consent prior to assessment and randomisation. Research assistants were responsible for recruitment and taking informed consent. Written informed consent was obtained from all trial participants.
Community mental health services
We engaged CMHS that were likely to have five or more care co-ordinators willing to participate for a period of 12 months and had potential care co-ordinators with eligible service users on their caseload who were likely to consider participating.
Service users
Service users from participating CMHS were eligible for inclusion if they:
-
were adults (aged ≥ 16 years)
-
were in contact with a local CMHS
-
had either:
-
been admitted to a psychiatric inpatient service at least once in the previous 2 years for a relapse of psychosis or
-
received crisis intervention (e.g. via a crisis intervention service; re-engaged with a CMHS) in the previous 2 years for a relapse of psychosis.
-
-
had received a diagnosis of a schizophrenia-related disorder, specifically:
-
295.40 schizophreniform disorder [International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), F20.81]
-
295.70 schizoaffective disorder (ICD-10 F25)
-
295.90 schizophrenia (ICD-10 F20.9)
-
297.10 delusional disorder (ICD-10 F22).
-
-
were able to provide informed consent as judged by the care co-ordinator or, if in doubt, the responsible consultant.
Carers
Carers of people receiving support from participating CMHS were eligible for inclusion if they:
-
had been nominated by eligible participants
-
were in regular contact (weekly) with the person receiving services
-
provided informed consent to participate in the study.
Exclusion criteria
Individuals were ineligible for participation if they did not meet the inclusion criteria outlined above. In addition, participants were excluded if they had experienced a recent relapse, operationally defined as having been discharged from the care of a crisis team or psychiatric inpatient service within the previous 4 weeks.
Interventions
In describing the EMPOWER intervention we utilise the Template for Intervention Description and Replication (TIDieR) checklist. 135
EMPOWER intervention
Rationale
The rationale for the EMPOWER intervention was informed by the cognitive–interpersonal model outlined in Chapter 1 and was designed to enable participants to monitor changes in their well-being on a daily basis. In EMPOWER, we referred to changes in well-being as ‘ebb and flow’ as a means of moving away from risk-orientated monitoring that can sensitise individuals to increased fear of relapse. The terminology also attempted to convey a normalising framework for understanding changes in emotions and psychotic experiences in daily life. Clinical triage of changes in well-being that were suggestive of EWS was enabled through an EMPOWER algorithm that triggered a check-in prompt (ChIP). This enabled the prompt identification of any EWS and triggered a relapse prevention pathway if warranted.
The EMPOWER app was developed in part through consultation with people using services, their carers and mental health care professionals. Service user participants had access to the EMPOWER app for up to 12 months of the intervention period. EMPOWER was developed as a flexible tool for users to:
-
monitor daily the ‘ebb and flow’ of changes in well-being
-
incorporate personalised EWS items
-
receive automated EMPOWER (self-management) messages directly
-
review their own data and keep a diary of their experiences via a smartphone user interface.
Materials
Daily monitoring of well-being was initiated by pseudo-random mobile phone notifications to complete the EMPOWER questionnaire (see Report Supplementary Material 2). Notification reminders were sent after 5 minutes (when there had been no response), after which app users were allowed a 5-hour time window within which they could respond to questions. The questionnaire contains 22 items reflecting 13 domains (e.g. mood, anxiety, coping, psychotic experiences, self-esteem, connectedness to others, fear of relapse and personalised EWS). Items included both positive (e.g. ‘I’ve been feeling close to others’) and negative (e.g. ‘I’ve been worrying about relapse’) content. Each item was completed using a simple screen swipe, which enabled quick and efficient completion by users. Each item was automatically scored on a scale of 1–7. Where particular items scored > 3, users were invited to complete supplementary questions to enable a more fine-grained assessment of that domain. This provided up to a maximum of 56 questionnaire items.
Peer support working in EMPOWER
The EMPOWER intervention was blended with peer support. Peer support workers are people who bring lived experience of mental health problems and recovery to their work in mental health services, with practices underpinned by a set of values and principles. 120,136 The role is increasingly well established in mental health policy and practice in many countries, including the UK and Australia. 137 Peer support is also being increasingly integrated with digital mental health interventions. 124 Two peer support workers were employed in Glasgow and one was employed in Melbourne, all on a part-time basis, to work with people in the intervention arm of the study. Their work was underpinned by a values framework developed by the Scottish Recovery Network,138 with supervision available at both sites and also across sites.
Peer support worker roles initially focused on introducing participants to the ethos and principles of the EMPOWER stepped-care approach and setting up and personalising the app on provided or personal handsets. Following this set-up and familiarisation period, peer support workers maintained regular contact with app users, at least weekly in the baseline period and approximately fortnightly following the baseline. Contact was maintained primarily through telephone calls, although text messaging and in-person meetings also took place, with the type and amount of contact determined by personal preference and need. Peer support worker roles included:
-
Supporting engagement with the intervention. This included checking in with people to assess whether or not there were any blocks to their successful use of the app and to advise on how usage might be adapted to individual needs, for example encouraging people to take breaks from self-monitoring as required.
-
Offering technical advice and support in relation to participants’ use of the phone. This included supporting people in becoming familiar with mobile phone functions and ensuring that they had mobile phone data connectivity and adequate credit for data.
-
Encouraging the general exploration of well-being and of how data generated through the app reflected life experience and aided self-management.
-
Reviewing and supporting participants’ use of different app functions. This included supporting participants to access and make use of chart and diary functions. In particular, peer support workers encouraged participants to review charts and sought to prompt curiosity about chart data and the implications of these for self-management and well-being.
-
Aiding the interpretation of messages generated by the app. This included giving app users additional information on message content, as required, for example providing further information on how to access support to develop an advance statement.
-
Sharing personal experiences of app use. Peer support workers had personal experience of using the EMPOWER app and were able to reflect with research participants on their own experiences and, if required, offer practical advice and share aspects of their experience that they felt might support participants.
-
Monitoring and reporting performance and safety issues. This included making routine enquiries in relation to any possible adverse events and the extent to which these were associated with the intervention.
Procedures
A peer support worker met with participants on an individual basis to introduce the rationale for using the app, to collaboratively set up the app on their participant’s mobile phone or a study phone, and to support the individual’s familiarisation with the handset and app functions. Participants were invited to choose up to three personalised EWS items to be included in the EMPOWER questionnaire and further personalisation of delusion-specific items could also be made. Where possible, an individual’s care co-ordinator and nominated carer were invited to contribute to this meeting. Participants were asked to undertake daily monitoring for an initial 4-week baseline period to help establish their personal baseline of the ‘ebb and flow’ of their well-being. During this period, additional support was provided by peer support workers through weekly telephone follow-up. This provided an opportunity to encourage use of the app, solve any technical problems and identify any adverse effects. At the end of the 4 weeks, a further meeting was arranged with the peer support worker or mental health nurse to review monitoring, encourage engagement with EMPOWER messages, agree participants’ preferences for actions in response to changes in well-being that were suggestive of EWS and encourage participants to continue utilising local CMHS for clinical care. All participants were offered ongoing fortnightly peer support worker support to encourage them to use the app, to support their reflection on changes in well-being and their broader context including, for example, stressful life events, and to encourage them to use self-management strategies prompted by EMPOWER messages.
Digital procedures
The analysis and handling of questionnaire data was governed by the EMPOWER algorithm. The EMPOWER algorithm is a class 1 medical device (CI/2017/0039) that forms one part of a broader system that was designed to identify and respond to changes in well-being that were suggestive of EWS. Figure 2 provides a graphical representation of the system’s high-level components and data flow.
FIGURE 2.
The EMPOWER system. Google and the Google logo are registered trademarks of Google LLC, used with permission.
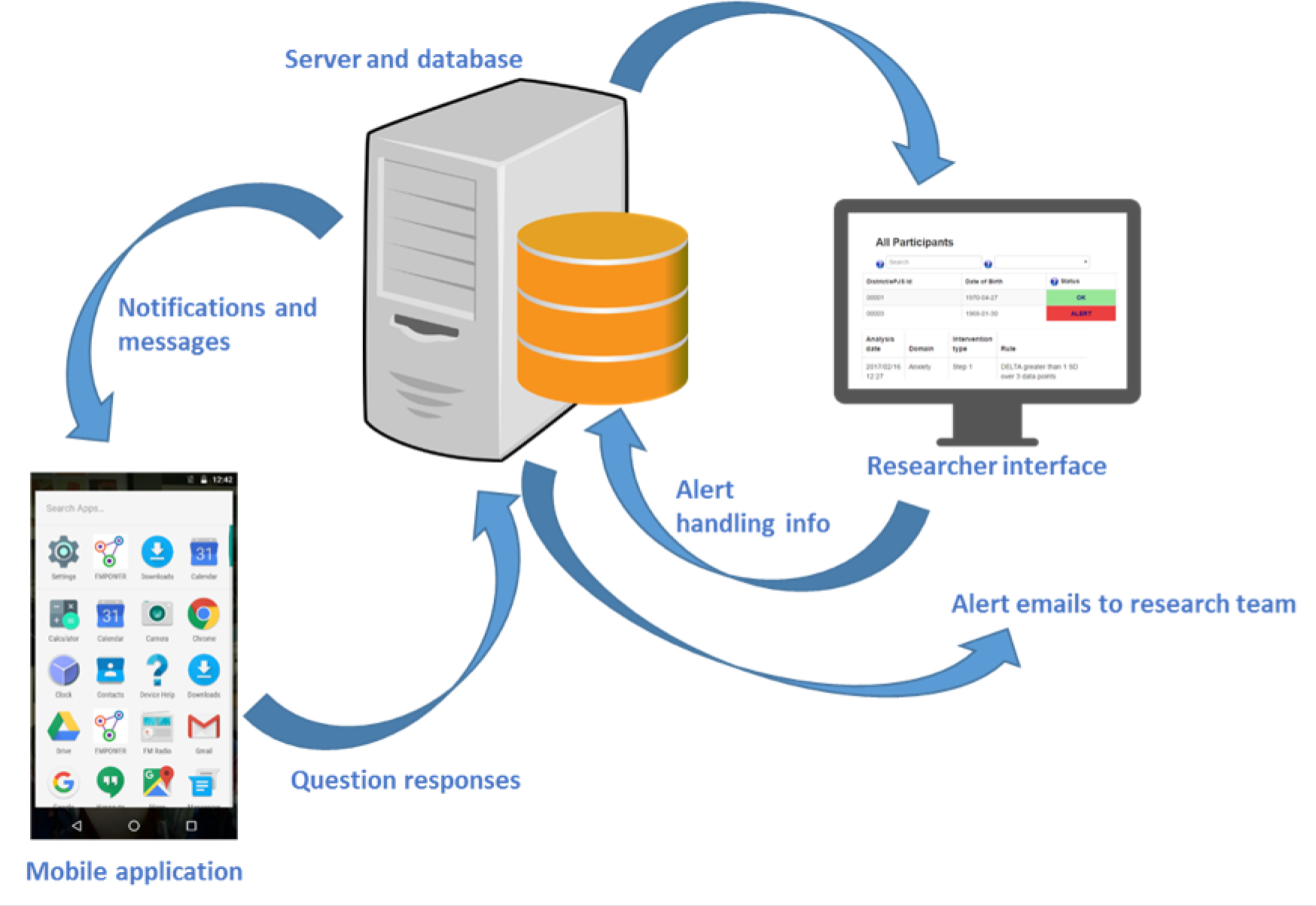
Participants used a mobile phone app that prompted them to answer a daily questionnaire about the potential EWS of psychosis. The data were then submitted to the EMPOWER server and analysed by the ChIP algorithm. The algorithm, which is summarised in Figure 3, compared a participant’s latest data entry against their personal baseline. If changes exceeded predefined thresholds, a ChIP was generated for the participant. The consequences of the ChIP were that the research team, which included a registered mental health nurse (in the UK), clinical psychologists (in the UK and Australia) and a general psychologist (in Australia only), were e-mailed about the participant and that participant’s status was set to ‘alert’ and was highly visible on a secure web-based researcher interface. In addition to viewing and handling ChIPs, researchers could view longitudinal graphs of their participants’ well-being and possible EWS, filtered by question or by domain (group of questions). ChIPs were initially screened by a member of the research team, followed by (1), in Australia, sharing a summary of relevant ChIP data with the clinical team, and (2), in the UK, a member of the research team checking in with the participant. Based on the outcome of this triage assessment the researcher could share an update with the participant’s care co-ordinator, who could, if indicated, escalate increased support to the participant from their local CMHS to reduce risk of relapse. We aimed to respond to ChIPs in some way within 24 hours or the next working day.
FIGURE 3.
EMPOWER algorithm responding.

The alert algorithm also ran a separate process scanning for EWS changes against the baseline. Based on these changes, the logic selected a message from the most appropriate of several content-based message pools (i.e. one message pool contained helpful messages about ‘mood’, another had messages about ‘anxiety and coping’, etc.). This message was delivered back to, and displayed on, the participant’s EMPOWER app. Messages were intended to help people have a greater sense of control over their mental health and well-being and to support self-management. We set up a specific patient and public involvement group to assist us in the co-design of the message function in the app. The group advised on the curation, content and delivery of messages. This group met on four occasions and had public representation from four people with direct lived experience of psychosis.
Training and support to community mental health service staff
After CMHS had been randomised to EMPOWER, we aimed to provide training to those mental health care staff in teams based on our model of relapse prevention, which emphasised:
-
therapeutic alliance
-
barriers to help-seeking
-
familiarisation with the app
-
developing an individualised formulation of risk of relapse
-
developing a collaborative relapse prevention plan.
Following this, we aimed to meet with care co-ordinators on a fortnightly basis to provide support in the implementation of EMPOWER. These meetings aimed to clarify and encourage formulation of any changes and participant responses within the model, and support clinicians to consider EMPOWER-consistent intervention options.
Background intellectual property
The background intellectual property and functionality was the pre-existing ClinTouch app developed in 2010 and funded by two grants from the UK Medical Research Council. The aims of the app were to help people with serious mental illnesses to manage their own symptoms and to prevent relapse. Software development and beta testing used an experience-driven design process in which service users with psychosis were involved in the design and development of the app, its functionality and its standard operating procedures. Health professionals were included in system design where it related to routine practice, and in the design of new, digitally enabled workflows. Randomised feasibility trials showed this method of active symptom monitoring to be safe, feasible and acceptable. 139–141 This personalised smartphone application triggers the collection of symptom self-ratings several times daily and wirelessly uploads these in real time to a secure central server. A semi-random beep (two to four times daily) alerts the user to complete a set of 12–14 branching items about current symptom severity using a touchscreen slider. A graphical summary of how symptoms fluctuate over time is assembled and displayed on the handset. A validation study compared face-to-face ratings on the gold-standard Positive and Negative Syndrome Scale (PANSS) with four-times-daily ClinTouch assessments over 1 week. This confirmed the validity of the self-reported items, with core psychotic symptom and mood items showing moderate to strong (r > 0.6) correlations between the two methods. 139 Non-core, behaviourally assessed items, such as negative symptoms, showed weaker correlations.
Based on this proof of concept, the standalone smartphone system was integrated via an application programming interface into NHS trust ICT (information and communication technology) platforms to allow the streaming of summary information into electronic health records, and to enable health professionals to track current symptoms on desktops at the team base and receive alerts when symptoms exceeded a pre-agreed personalised threshold. An open, randomised trial of ClinTouch active symptom monitoring compared with management as usual was conducted in NHS mental health trusts in Manchester and South London. 142 Recruitment to the trial took place between February 2014 and May 2015. The aims were to assess (1) the acceptability and safety of continuous monitoring over 3 months, (2) the impact of active self-monitoring on positive psychotic symptoms assessed at 6 and 12 weeks, (3) the feasibility of detecting EWS of relapse communicated to health-care staff via an application programming interface allowing data to be streamed into the electronic health record and (4) the impact of active self-monitoring on positive psychotic symptoms assessed at 6 and 12 weeks. Eligible participants with a DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) diagnosis of schizophrenia and related disorders and a history of relapse within the previous 2 years were enrolled from an early intervention team and a community mental health team.
The findings were that, of 181 eligible patients, 81 (45%) consented and were randomised to either active symptom monitoring or management as usual. Of those in the active symptom monitoring group, 90% continued to use the system at 12-week follow-up. In this group, adherence, defined as responding to > 33% of alerts, was 84%. At 12 weeks, active symptom monitoring compared with usual management was associated with no difference on the empowerment scale. The pre-planned intention-to-treat analysis of the primary outcome (i.e. positive symptom score on the PANSS scale) showed a significant reduction in the active symptom monitoring group over 12 weeks in the early intervention centre. Alerts for personalised EWS of relapse were able to be built into the workflows of the two NHS trusts, and 100% of health professional staff used the system in a new digital workflow. Qualitative analyses supported the acceptability of the system to participants and staff.
Treatment-as-usual control
Treatment as usual (TAU) was chosen as a control condition in both the Glasgow and Melbourne centres as this provided a fair comparison with routine clinical practice. In Glasgow and Melbourne, secondary care is delivered by adult CMHS, which largely involves regular (fortnightly or monthly) follow-up with a care co-ordinator and regular review by a psychiatrist.
Outcomes
All outcome measures were administered at baseline and subsequently at 3, 6 and 12 months by research assistants who were trained in the use of all of the instruments and scales and had achieved a satisfactory level of inter-rater reliability (see Appendix 7). Research assistants had, as a minimum, a strong honours degree in psychology or a related discipline. Regular inter-rater reliability assessments were conducted during the trial. Research assistants entered anonymised participant data from paper records onto an electronic case record form hosted by the University of Aberdeen, with the exception of relapse data, which were entered by the trial manager. In Glasgow, data were collected in participants’ homes or in health service facilities. In Melbourne, all data were collected on health service premises. Data quality and error checking were conducted at each time point, namely baseline and 3, 6 and 12 months post randomisation.
Primary outcomes
Feasibility: service users
For all participants, outcome assessment included:
-
proportion of eligible and willing service users who then consented and the proportion continuing for 3, 6 and 12 months to the end of the study
-
frequency of service user seeking help in relation to EWS
-
frequency of family member/carer seeking help in response to EWS
-
frequency of clinical care changes in response to EWS at 3, 6 and 12 months.
Feasibility: mental health care staff
We assessed:
-
self-reported frequency of discussing EWS with care co-ordinator
-
frequency of person seeking help in relation to EWS
-
frequency of care co-ordinator seeking help in response to EWS
-
frequency of clinical care changes in response to EWS at 3, 6 and 12 months (e.g. appointment brought forward, medication changed).
Feasibility: carers
We assessed:
-
self-reported frequency of discussing EWS with family member/carer
-
frequency of person seeking help in relation to EWS
-
frequency of family member/carer seeking help in response to EWS
-
frequency of clinical care changes in response to EWS at 3, 6 and 12 months (e.g. appointment brought forward, medication changed).
Acceptability and usability
For those randomised to EMPOWER, we assessed:
-
the length of time participants were willing to use the app
-
the number who completed > 33% EWS data sets
-
self-reported frequency of app use
-
frequency of sharing data with the key worker
-
frequency of sharing data with family member/carer
-
frequency of accessing charts at 3, 6 and 12 months
-
self-reported acceptability and usability using an adapted version of the Mobile App Rating Scale user version (uMARS;143 see Appendix 1).
Safety: adverse events
Details of recording and reporting of all adverse events complied with the Medical Devices Regulations 2002 (MEDDEV 2.1/6),144 ISO/FDIS 14155:2011145 and Standards for Good Clinical Practice. 146 An adverse event was defined as serious if it:
-
resulted in death
-
was a life-threatening illness or injury
-
required (voluntary or involuntary) hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity, or medical or surgical intervention was required to prevent any of the above
-
led to fetal distress or fetal death or consists of a congenital anomaly or birth defect, or
-
was otherwise considered medically significant by the investigator.
Our adverse event procedures involved recording all untoward medical occurrences or clinical indications, their relatedness to the investigational medical device, their seriousness and intensity and whether or not the event was anticipated. We separately monitored and reported device deficiencies that were inadequacies of the medical device (the algorithm) with respect to its identity, quality, reliability, safety or performance. In addition, we measured all adverse effects arising from study procedures including the use of the EMPOWER app. We prespecified (but did not limit) these as:
-
Increased fear of relapse or paranoia associated with responding to questions in the EMPOWER app.
-
Increased worries about surveillance by psychiatric services. We also assessed changes in fear of relapse using the Fear of Recurrence Scale. 17
The relationship between the investigational medical device and the occurrence of each adverse event was assessed and categorised. The chief investigator used clinical judgement to determine the relationship. Alternative causes, such as natural history of the participant’s underlying condition, concomitant therapy and other risk factors, were considered.
All serious adverse events were reported to the chairperson of the DMEC, the MHRA and the manufacturer (the University of Manchester) via the Glasgow study sponsor. Summaries of all adverse events were reported to and discussed by the DMEC and the SSC on a 6-monthly basis.
Performance
The following performance end points were identified:
-
Each participant had the app successfully uploaded on a smartphone.
-
Each participant had personalised EWS included in the EMPOWER questionnaire.
-
Each participant received a daily prompt to complete their questionnaire.
-
Participants received an EMPOWER message each time they completed the questionnaire.
-
Following 4 weeks of app use, the EMPOWER algorithm calculated each of the participant’s individualised baseline or variance of symptoms and experiences.
-
Participants could access charts of their symptoms and experiences covering 1-week and 1-month time intervals.
-
Following completion of the questionnaire, participants’ data were transferred to the secure server.
-
Researchers accessed participants’ questionnaire responses and generated charts to observe changes over time.
-
Researchers received a record of alerts for each participant and could record actions in relation to these alerts.
Candidate outcomes
Across measures, a lower score generally represents a positive or more desirable rating. Exceptions to this are the Service Attachment Questionnaire147 and the Medication Adherence Rating Scale,148 on which a lower score represents poorer engagement and poorer adherence, respectively.
Candidate primary outcome
Research assistants extracted data on symptom changes and possible relapse from electronic case notes. Relapse was defined as:
-
a return of or an exacerbation in psychotic symptoms of at least moderate degree
-
symptoms of at least 1 week in duration
-
evidence of a decline in functioning or an increase in risk to self or others
-
evidence of a clinical response from services.
The relapse criteria are summarised in Table 3.
| Criterion | Notes and definitions | |
|---|---|---|
| A return of or exacerbation in psychotic symptoms of at least moderate severity (if present, score 1) |
|
|
| AND | Where symptoms have lasted at least 1 week (if present, score 1) | |
| AND | Where there is evidence of a decline in functioning (if present, score 1) | Includes a decline in one or more of the role performance areas identified from the PSP scale:
|
| OR | An increase in risk to self or others (if present, score 1) |
|
| AND | There is evidence of a clinical response from services (if present, score 1 for each of these criteria; maximum score 3) |
|
Our assessment of relapse was blinded. Research assistants screened electronic case records to identify potential episodes of relapse and exacerbation. These episodes provided the basis for individual anonymised case vignettes that were submitted to our independent adjudication panel. All vignettes were fully anonymised, and any information relating to the EMPOWER intervention was concealed. This panel contained expert clinicians/researchers who had the necessary knowledge, experience and skills to make independent blinded judgements regarding relapse/exacerbation. We planned to report the number (%) of relapses and time to relapse between groups, and number (%) with (1) return or exacerbation of psychotic symptoms, (2) duration of at least 1 week, (3) reduction in functioning, (4) increase in risk, (5) change in clinical management, (6) admission to hospital and (7) use of the Mental Health Act. 149 For each relapse we determined a severity score by allocating a point for each of these criteria (score = 0–7). All participants were assessed at each follow-up point for the presence of any of the criteria described below, enabling calculation of a mean severity score across participants and allocated groups at each follow-up point.
Candidate secondary outcomes
We assessed changes in symptoms, substance use, emotional distress, carer burden, service engagement and adherence and health-related quality of life.
-
Mental health status: the PANSS,150 the Personal and Social Performance Scale,151 and the Calgary Depression Scale for Schizophrenia (CDSS)152 were completed with service user participants.
-
Substance use measures: the Timeline Followback for drugs and alcohol (over 28 days) was completed. 153
-
Emotional distress: the Hospital Anxiety and Depression Scale (HADS)154 and the Personal Beliefs About Illness Questionnaire-Revised (PBIQ-R) were completed. 155
-
Service engagement: the Service Attachment Questionnaire147 and the Medication Adherence Rating Scale148 were completed by service user participants.
-
Health economics: the EuroQol-5 Dimensions,156 the Assessment of Quality of Life–Eight Dimensions,157 the CarerQoL-7D158 and a resource use questionnaire (RUQ) were completed.
Candidate mechanisms
Measures were selected that map directly on to hypothesised mechanisms of change as well as known predictors of relapse. Mechanisms of service user benefit were operationalised as improvements in personal recovery, efficacy and utilisation of social supports.
-
Recovery and self-efficacy: the Questionnaire about the Process of Recovery159 and the General Self-Efficacy Scale160 were completed by service user participants.
-
Social and interpersonal context: the Psychosis Attachment Measure161 and the Perceived Criticism and Warmth Measure adapted from the Perceived Criticism Measure162 were completed by service user participants.
Carer outcomes
The Involvement Evaluation Questionnaire163 was completed as a measure of carers’ worrying, tension, urging and supervision. A carer-perceived criticism measure, adapted from the Perceived Criticism Measure, was used as a measure of carers’ perspectives on relationship quality.
We also assessed carer health economic outcomes using a purposively designed RUQ, the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) and the CarerQol-7D.
Care co-ordinator outcomes
Participants’ care co-ordinators completed the Service Engagement Scale. 164
Process evaluation
In line with Medical Research Council guidance on the process evaluation of complex interventions,165 we produced a logic model for the EMPOWER intervention and conducted a process evaluation. The process evaluation will be used to explore the ways in which EMPOWER may operate to produce outcomes. Specifically, it will focus on intervention fidelity, exposure, reach, context, recruitment, retention and contamination, as well as the acceptability of study procedures. We will interview service users, carers, mental health care staff and research staff to ensure a multiperspective understanding of the intervention. The process evaluation has been published elsewhere. 166
Sample size
No formal sample size calculation was appropriate for this pilot phase. The proposed sample size of up to 120 service users across 40 care co-ordinators in eight CMHS was deemed to be sufficient to establish feasibility and obtain parameters, including the relevant intracluster correlation coefficients for the cluster design, to inform the design and size of a future definitive, pragmatic, multicentre and multinational cRCT.
Recruitment and randomisation
The unit of randomisation was the CMHS (the cluster). Participating CMHS were randomised within stratified pairs to the EMPOWER relapse prevention intervention or to continue their usual approach to care.
Researchers approached each eligible care co-ordinator and sought their consent to participate in the trial. Prior to randomisation, consenting care co-ordinators provided an anonymised list of their current potentially eligible caseload of people using services. This list was then randomly ordered by the Centre for Healthcare Randomised Trials (CHaRT). Researchers then approached identified people sequentially in blocks of up to five potentially eligible participants and sought informed consent to participate in the study. If there were further participants eligible for inclusion at the end of this block, the researcher moved onto the next block of five (if applicable). Care co-ordinators provided participants with an easy-to-read information leaflet about the study to enable potential participants to express interest in finding out more information. In Australia, information posters were displayed in the staff areas of participating sites to inform care co-ordinators about the study and the contact details of research assistants were provided for those who wanted to participate.
We aimed to approach and consent, on average, three participants per care co-ordinator (giving a total of up to 120 potential participants). Following delays resulting from Medical Device Registration procedures and consultation with the independent DMEC and SSC, this recruitment target was amended to 86 participants. After completing baseline assessments on all consenting service users in care co-ordinators’ and CMHS’ caseloads, the clinical trials unit (CTU) at CHaRT conducted randomisation of the CMHS. For Australia, with just two clusters, this was by simple randomisation by the CTU. For Glasgow, with six clusters, the CTU created three pairs of teams based on similarity of the catchment area in terms of social deprivation (Carstairs) score or CMHS type (e.g. early intervention service). The CTU randomly allocated one member of the pair to the intervention and the remaining member to control.
In this pilot phase we explored the best method of randomly allocating the clusters in the full trial, specifically to establish what matching factors (if any, and/or if matching at all is appropriate, methodologically) were suitable. Any violations of the study protocol were recorded and reported to the Research Ethics Committee, the SSC and the independent DMEC.
Statistical analysis
A full statistical analysis plan was written (and published on the CHaRT website167) prior to any analysis being undertaken. All analyses were carried out using the intention-to-treat principle with data from all participants included in the analysis including those who did not complete the intervention. Every effort was made to follow up all participants in both arms for research assessments. The analysis followed the guidelines of the CONSORT statement for cluster randomised trials and recommendations for the analysis of cluster randomised trials when presenting and analysing the data. Here, we had potentially repeated measures on individual patients nested within care co-ordinators who were nested within teams (the unit of randomisation) who were nested within region (Australia and Scotland).
A full trial analysis may consider adjusting for these factors using appropriate random (service user, if relevant; care co-ordinator; and team) and fixed (region) effects. However, for this feasibility study, analysis models for all outcomes used a simplified approach whereby we analysed the unmatched data.
Baseline characteristics were also examined for dropouts and completers in each treatment arm. Treatment arms were described at baseline and follow-up using means [with standard deviations (SDs)], medians (with interquartile ranges) and numbers (with percentages), where relevant, for demographics and outcome measures. We analysed the main primary outcome, one or more relapses within the previous 12 months, using a generalised linear model, as a modified random-effects multilevel Poisson regression with a log-link function and robust error variance. 168 This facilitates the estimation of covariate adjusted relative risks (RRs) and the derivation of the absolute risk difference. This model was adjusted for a fixed country effect and accounted for possible CMHS clustering using a random-effects robust variance for centres. As relapse was not assessed formally at baseline, this could not be incorporated into the model. Time to the first relapse was assessed using Cox regression.
The repeated measures aspects of the secondary outcomes meant that we used mixed random-effects generalised linear models with appropriate distributional forms depending on the outcome being analysed. These allowed the estimation of the treatment effects over time by including a time-by-treatment interaction for fixed (nominal) time points of 3, 6 and 12 months from randomisation. These were adjusted for the baseline measures for the outcome being assessed as well as having a fixed country effect and random centre effect. Owing to the large number of secondary outcome measures and the feasibility nature of the study, although we examined p-values (but only those with < 1% significance) and effect size (using Cohen’s d), this was not to judge the treatment impact but rather to assess the worth of each scale for inclusion in any potential full trial. The trial statistician remained blind to the primary outcome until the final data cut.
A full health economic statistical analysis plan was written prior to analysis being undertaken. As part of the within-trial economic evaluation, we tested two health-related quality of life measures that can be used to assess quality-adjusted life-years (QALYs), the EQ-5D-5L, and the Assessment of Quality of Life (AQoL-8D), in the feasibility trial. Although the EQ-5D-5L is very commonly used in the UK and Australian contexts, its sensitivity in and appropriateness for people with schizophrenia has been seriously questioned. 169 The AQoL-8D is a newer health-related quality-of-life measure that was developed to be sensitive to the quality-of-life domains that are important to people with mental health problems. A RUQ to capture the costs incurred was also tested. This questionnaire needed to be appropriate to both the UK and the Australian contexts but would possibly require some system-specific modules for services that differ between the two.
Work package 6: widening stakeholder engagement
Following the cRCT, we aimed to engage with mental health services and local service user and carer organisations in the potential centres for a main trial (Edinburgh, Manchester and Birmingham). Our plan was to host three knowledge exchange events in Edinburgh, Manchester and Birmingham and invite key representatives of NHS services, professional staff and local service user and carer organisations. At these events we aimed to identify the key learning outcomes from the EMPOWER project and work with stakeholders to develop plans for the main study phase. We aimed to follow up these knowledge exchange events with active engagement with local NHS services, CMHS and management, local research and development (R&D) and information governance departments. We aimed to identify potential changes to services that would threaten cluster randomisation in a future trial. The outcomes of WP 6 are summarised in Appendix 11.
Protocol amendments
The protocol amendments are described in Table 4.
| Amendment number | Date | Protocol number | Main change summary |
|---|---|---|---|
| 1.0 | 7 August 2017 | 1.1 |
|
| 2.0 | 4 December 2017 | 1.2 |
|
| 3.0 | 4 June 2018 | 1.3 |
|
Protocol breaches
There were no protocol breaches.
Study amendments
In September 2018 we were granted a 6-month extension to the study by the Health Technology Assessment programme team on the basis of delays incurred as a result of medical device registration. Within the extension request we made the case, with the backing of our SSC and DMEC, for a reduction in the target sample from 120 service user participants to 86. This revised target was based on our recruitment progress and justified with reference to sample sizes of similar feasibility studies of mHealth for psychosis. 117,170
System safety and privacy protection
Three general principles of information security (confidentiality, integrity and availability) were followed in the design and implementation of EMPOWER, which applied to phases 1 and 2 (WPs 3 and 4). All data transmitted to and from EMPOWER servers were encrypted over https with strong ciphers as detailed in Approved Cryptographic Algorithms: Good Practice Guideline. 128 Cipher suites were implemented in compliance with section 6 (‘Preferred uses of cryptographic algorithms in security protocols’) of the Good Practice Guidelines. In cases where participant data were downloaded from the EMPOWER site, these data were securely encrypted with a pass phrase of appropriate length and complexity. Data transfers were secured using standard web security protocols. Uploading study data to a central server in real time enabled them to be captured and so this protected against data loss, such as if a phone was lost or stolen. This removed the need for personal data to be stored on the device. The purpose of the server in this case was secure data storage.
Chapter 3 Qualitative findings
Background
Digital interventions for psychosis have shown promising preliminary acceptability and efficacy in feasibility studies. 171 However, many digital interventions that report effectiveness have failed to generalise from clinical trials into clinical practice. 115,172 Given concerns about generalisation beyond trials, the UK Department of Health and Social Care173 now encourages systematic implementation research to increase understanding of how interventions become implemented or rejected. Embedding implementation research from the earliest stages of a trial appears key to understanding the emerging implementation process. Beyond psychosis research, there is evidence of the value of user involvement in implementation. For example, the early involvement of key stakeholders was noted to be important in developing implementable digital interventions to support caregivers of people with dementia. 174 Key implementation issues identified for digital interventions for psychosis that require further research include developing an understanding of the logistics of how a digital intervention might fit into clinical practice. 175 Additionally, developing knowledge of what factors are important in understanding and optimising user engagement is important,114 especially because user engagement with digital interventions for psychosis can be low. 115,176 Understanding implementation from the point of view of intended end-users may present a way forward in anticipating implementation logistics and user engagement.
However, understanding implementation from the point of view of end-users has not always been valued. Historically, mental health service users’ personal perspectives about interventions are rated low in the evidence hierarchy,177 with RCT evidence (especially in systematic reviews) rated highest. 178 However, even with strong RCT evidence, no relapse prediction system for schizophrenia will be useful if it is not able to be integrated into clinical care and actually used by clinicians and patients,175 making implementation key. Implementation research differs from most clinical research because it aims to understand why (historically) clinicians come to use interventions in routine care179 rather than to test for clinical efficacy. However, the range of interventions on offer continues to expand, and digital interventions in particular91 can be used outside clinical settings. The implementation (or implementation failure) of interventions emerges out of many decision-making processes that occur over a period of time. Therefore, it is important to conceptualise service users (and carers) as potential intervention implementers as well as end-users.
Decisions to use interventions are shaped by numerous factors, including individual preferences, professional roles and the environment in which decisions are made. 180 The inherent complexity of decisions to use interventions has been described as being a socially constructed process. 181 It is understood that the health-care system comprises dynamically interacting factors that are enmeshed in longstanding social practices, with which an intervention will come to interact. 182 As highlighted by Moore and Evans,183 adding something new to a complex system, such as adding a new part to a car, should be completed only once there is a clear understanding of the complex system (in their example, the car). Therefore, it makes sense to try to understand the clinical health-care system and its dynamically interacting factors, and longstanding social practices, before attempting to implement an intervention.
The Medical Research Council framework for evaluating complex interventions recommends that implementation research proactively include stakeholders because people who are expected to engage with an intervention are likely to have relevant experiential knowledge that is useful for understanding the implementation process during a trial. 165 Despite the value of mapping out complex systems prior to testing an intervention, the majority of implementation research on engagement with interventions has been retrospective184 and, therefore, not conducted in the early stages of feasibility or pilot trials, when researchers generally engage potential stakeholders for later trial activities, such as recruitment. However, there are notable exceptions. 185–187 Qualitative research carried out during a trial can aid understanding of why an intervention might work and how context affects implementation. 188 However, stakeholders may have pre-existing expectations about implementation that may be important for researchers to be aware of. To capture these, we incorporated qualitative research methods to understand the expectations held by mental health care staff, carers and service users in advance of intervention testing.
Theory
In understanding a problem in health care, it is important to be pragmatic and use methodologies that create interpretive insights for researchers to theorise about how a problem is sustained. 189 Moore and Evans183 state that implementation theories are useful because they help to explain what happens during clinical trials. 190 Incorporating theory can also enhance understandings of barriers to research translation and how these barriers might operate. The Medical Research Council guidelines on evaluating complex interventions165 recommend using theory to understand implementation processes. In line with this, questions asked of participants during phase 1 were designed using normalisation process theory (NPT),191 which is concerned with the work that groups and individuals do when interacting with an intervention and how they make sense of it within their everyday practices. To differentiate EMPOWER from current relapse prevention practice, we also used NPT to characterise stakeholder discussions about both current practice and the proposed intervention. In particular, we evaluated expectations of the EMPOWER intervention using the following NPT constructs: coherence (e.g. did EMPOWER make sense to service users, clinicians and carers?), cognitive participation (e.g. did stakeholders think it was a good idea?) and collective action (operational work that people expect they would need to do to implement EMPOWER). Our rationale for using theory to analyse the data was the pragmatic goal of explaining stakeholder views as discussed during the preliminary qualitative phase, rather than building or testing theories. 192 Therefore, the results will be reported in line with whether or not the EMPOWER approach made sense to participants and how the intervention was expected to ‘fit’ in terms of relationships and the everyday work of relapse prevention.
Methods
Aims
The aims have been detailed in Chapter 2. To summarise, there were three WPs.
Work package 1
The aims of WP 1 were to:
-
evaluate the acceptability and usability of mobile symptom recording using smartphones among service users and their carers
-
identify incentives and barriers to use.
Deliverables were software and protocol updates in response to feedback from service users and carers.
Work package 2
The aims of WP 2 were to:
-
evaluate the acceptability and usability of mobile symptom recording using smartphones among professional mental health care staff
-
identify incentives and barriers to implementation by mental health staff
-
identify relapse prevention pathways and whole-team responses.
Deliverables were:
-
Software and team protocol updates in response to feedback from professional care staff. We will operationalise protocols for dealing with false positives and activation of relapse prevention pathways.
-
The development of care pathways, identification of operational barriers and enablers.
-
Identification of training needs of teams participating in our future pilot cRCT.
Work package 3
The aim of WP 3 was to:
-
finalise the EMPOWER app for implementation in a pilot cRCT that will compare EMPOWER with treatment as usual.
Deliverables were:
-
software and protocol updates in response to feedback from service users, carers and staff
-
agreement of final modifications to the EMPOWER app to enhance usability
-
finalisation of measurement methods for assessment of self-reporting of acceptability and usability to be administered in our future pilot cRCT.
Work packages 1 and 2
The methods are reported in line with COREQ (Consolidated Criteria for Reporting Qualitative Research). 193 For WPs 1 and 2 we utilised a task group design to gain insight into participants’ perspectives, experiences and expectations. 194 Using task groups enabled respondents to interact with and respond to the ideas and comments of other participants. 195 Following best practice guidelines,165 we used a theoretical framework to guide our task group schedule. An interview schedule informed by NPT191 was developed to explore stakeholders’ expectations. A copy of the topic guide for each stakeholder task group is provided in Report Supplementary Material 1. We planned to use NPT to explore how mental health care staff, carers and service users made sense of EWS, how they responded to EWS, the actions they took in relation to EWS and how useful they thought EWS were in detecting and preventing relapse. We also explored with stakeholders their expectations for the implementation of the EMPOWER intervention in their context.
Work package 3
A range of different testing techniques were applied during the development of EMPOWER, including automated testing, manual testing, validation of the alert algorithm and beta testing. Automated testing consisted of unit testing to verify the functionality of specific sections of code and integration testing to test how parts of the system worked together. Manual testing included running through a full set of system tests and formally recording the results. The alert algorithm was validated by having an external validator produce an entirely separate implementation of the algorithm. Both implementations of the algorithm were then given the same input data and the outputs checked to ensure that they matched. For WP 3, we utilised beta testing as a form of software UX testing and an investigation conducted to provide stakeholders with information about the quality of the tested software product. 125 According to Bertolino,196 testing software involves its validation in order to assess the extent to which it behaves as predicted. This enables the systematic and independent identification of errors to understand the risks involved in subsequent implementation. 125 Test techniques include the process of executing a program or application with the intent of finding software bugs (errors and other defects) and verifying that a product is viable. 125
For WP 3, we utilised individual qualitative interviews to explore any software problems or defects, and to explore broader UX. First, the participants’ general experiences with using the app were reviewed. Thereafter, during the course of the interview the conversation moved towards more specific aspects such as experiences with the questions and generated messages. The final interview stage aimed for a more in-depth exploration of the EMPOWER app use in relation to recovery. In the course of that, participants were asked to consider if and how the app would enhance their well-being or could serve as a tool for self-management in their personal recovery journeys. Details of the interview are in Appendix 2.
Sampling and recruitment
Work packages 1 and 2
Staff who support people with psychosis were recruited from CMHS in Glasgow, UK, and Melbourne, Australia. Staff were invited to take part through the research team making contact with clinical team leads in all eligible CMHS in both health boards. Service users were recruited to take part in task groups through being directly approached by mental health staff and via posters placed in relevant support organisations. Service user participants were eligible if they were in contact with a local CMHS, had experienced a psychotic relapse in the previous 2 years, had a diagnosis of a schizophrenia spectrum condition and were able to provide informed consent. Self-identified carers of people with psychosis were recruited by the research team advertising on posters and by word of mouth in mental health services and support groups. Participants included 86 mental health care staff working either in the NHS in the UK (n = 54; 9 focus groups) or in NorthWestern Mental Health services in Australia (n = 32; 5 focus groups). Twenty-one service users were recruited from local mental health services in the UK (n = 5; 3 focus groups) and Australia (n = 16; 4 focus groups) and 40 carers were recruited in the UK (n = 20; 2 focus groups) and Australia (n = 20; 3 focus groups). To maximise participant anonymity, we did not collect any demographic data beyond whether the participant was a carer, a service user or a mental health clinician. Following a short presentation about EMPOWER, which covered the trial rationale and design and the key aspects of the intervention, researchers trained in qualitative methods conducted 25 task groups locally, following the topic guide, between 20 July 2016 and 6 September 2017. Task group length ranged from 57 minutes to 2 hours and 9 minutes (Table 5).
| Group | Location | Participants (n) |
|---|---|---|
| Carer 1 | UK | 11 |
| Carer 2 | UK | 9 |
| Carer 3 | Australia | 4 |
| Carer 4 | Australia | 9 |
| Carer 5 | Australia | 7 |
| Service user 1 | UK | 2 |
| Service user 2 | UK | 1 |
| Service user 3 | UK | 2 |
| Service user 4 | Australia | 5 |
| Service user 5 | Australia | 2 |
| Service user 6 | Australia | 8 |
| Service user 7 | Australia | 1 |
| Staff 1 | UK | 4 |
| Staff 2 | UK | 8 |
| Staff 3 | UK | 10 |
| Staff 4 | UK | 7 |
| Staff 5 | UK | 5 |
| Staff 6 | UK | 5 |
| Staff 7 | UK | 3 |
| Staff 8 | UK | 6 |
| Staff 9 | UK | 6 |
| Staff 10 | Australia | 7 |
| Staff 11 | Australia | 9 |
| Staff 12 | Australia | 10 |
| Staff 13 | Australia | 6 |
Work package 3
Participants were eligible irrespective of whether they owned a smartphone. Smartphones were made available to participants if they did not own an Android device or if they expressed a preference for using a study smartphone. Seven service users participated in a 5-week beta-testing phase. This time scale was chosen to adequately assess users’ experiences of the mobile app and to provide enough time to test the EMPOWER algorithm, which required a 4-week baseline phase to allow subsequent changes in well-being to be calculated in week 5. Interviews were conducted between 29 March 2017 and 19 April 2017. The interviews lasted between 15 and 40 minutes.
Analysis
Work package 1 and 2
All data from recorded task groups were transcribed, largely verbatim. As the focus of this research was establishing actions and expectations, it was decided that language features such as ‘uhms’ and ‘erms’ would not be transcribed. 197 Following transcription, the transcripts were read and inductive thematic coding198 was performed. Following the inductive coding stage, the data were deductively coded utilising key concepts from NPT as a framework for analysis. 127 The themes presented in this report were constructed as being the most salient in terms of establishing existing clinical practice around EWS-based monitoring and implementation expectations for the EMPOWER intervention. Strategies to improve rigour such as member-checking199 (i.e. presenting analysis to participants to check if it makes sense to them) were not used.
Work package 3
All interviews were digitally recorded, transcribed verbatim and anonymised. Data were analysed in line with interpretative phenomenological analysis200,201 because we wanted to specifically focus on participants’ experiences of using the app and how they made sense of these in terms of their experience of recovery and staying well. The process of analysis comprised the following stages:
-
multiple reading of each transcript
-
initial note-taking
-
development of emerging themes through close line-by-line analysis and identification of descriptive, linguistic and conceptual codes
-
establishment of inter-relationships across emerging themes
-
search for patterns across cases and clustering of emergent themes into superordinate and subordinate themes and creation of a coherent table. 201,202
Results
Task groups with service users (work package 1)
Coherence
Early signs monitoring-based approaches to relapse prevention seemed to make sense to service users. Service users reported that being aware of changes in their well-being was generally helpful and could help make them aware of potential signs of relapse:
And how helpful would you say it is to kind of monitor early warning signs?
It’s important. It’s important for your well-being. See how you feel the next morning. See how your health is, your mindset. It’s very important.
Service user group 6, Australia
Do you think it’s helpful to monitor early warning signs, or?
Yeah.
I think it helps to be aware of them.
Service user group 3, UK
However, there were barriers to the use of EWS monitoring-based approaches among service users in the task groups. For example, service users were concerned about potential over-reaction from mental health services, which could act as a block to their reporting of EWS:
I get a bit scared to tell people about the early signs. Because you don’t want people to blow it out of proportion and then they’re staring at you and watching your every move. I don’t like that; I like my privacy. And so, I like . . . I don’t know.
Participant 1, service user group 1, UK
Cognitive participation
EMPOWER as it was described to participants during task groups seemed to make sense to service user participants. Having access to data was perceived as potentially helpful, and service users remarked that the technology associated with the intervention and having easy access to a record could make self-monitoring of experiences easier, as evidenced by the following quotation:
I love the idea of being able to log, during the bad time, what exactly is bad about the day and my mood and the symptoms and seeing it in black and white, not just the graph but the questions that you are asked, to know that you don’t have to text in a couple of sentences or a paragraph, you just slide a slider up and down on the bar.
Participant 1, service user group 2, UK
Service users seemed aware that EWS monitoring-based approaches to relapse prevention were part of a social practice in a system that included actions by the whole mental health team. Relating this to the EMPOWER intervention, some service users were concerned about staff receiving their personal EWS-based data. It appeared that service users expected the relational work of EWS monitoring that exists in current practice to possibly act as a barrier to using EMPOWER because there was uncertainty about how staff may respond to the data if these were shared:
We know that nothing is essentially private, well I happen to know that nothing that you tell any counsellor or social worker, nurse, therapist, anything, everything you tell them can be transferred even if it’s just in the lounge in the kitchen during lunchtime ‘oh blah de blah de blah’. We know they share information about us. We know they . . . um there is no privacy. Well I know it.
Uh what was the question again?
It’s really about the security arrangements and confidentiality with app as we have explained it, if there is any concerns or comments about that?
Totally, it’s going to be sending information to the treating team.
Service user group 7, Australia
The well-being messages approach seemed to generally make sense to participants as an intervention component. For example, the message that generated information tailored to service users’ input appeared coherent as the first step of a stepped-care pathway:
I’m sure EMPOWER could step before . . . you’re not sick enough to go to the hospital, you’re having a setback but maybe just need a little bit of encouragement.
Participant 2, service user group 7, Australia
However, as can be seen in the following exchange, some participants raised caution that message content could have the potential to be perceived negatively, for example if a message seemed to downplay or minimise distress or to be inappropriate to the context of that distress. Therefore, message content would need to be portrayed in an appropriate manner that did not seem dismissive of people’s struggles:
Yeah. There’s a risk that it might be a wee bit patronising. Just a risk, I don’t know. I know that me personally if I was feeling down in the dumps and I got a message saying ‘go for a walk’ . . . [laughs].
Pull your socks up.
Yeah. It may infuriate me. But maybe if I had the option to read the message, I was choosing to read the message, it wouldn’t be so annoying.
Service user group 1, UK
Collective action
Although concerns about staff having access to the data in terms of privacy and personal service user autonomy were common, these were set among discussions that support from a human being was always going to be important in relapse management and that an app on its own would not be considered helpful:
Seems a poor substitute for seeing a person that knows you.
Participant 2, service user group 1, UK
Task groups with carers (work package 1)
Coherence
Throughout carer task groups, EWS monitoring approaches were described as having utility in relapse prevention. Moreover, monitoring EWS made sense to participants because being aware of EWS provided an opportunity to intervene early in relapse prevention:
You can stop it from escalating and into a full-blown episode. You can see when it’s coming on. They can increase the medication or encourage them to see the doctor or something like that. There’s lots of things you can do. But, you know, once they get too sick, then it gets more difficult, they get more suspicious.
Participant 6, carer group 4, Australia
One key issue highlighted by carers in both Australia and the UK was the perception that mental health staff did not always agree with carers’ own assessment of whether or not a service user was exhibiting EWS. Carers seemed to feel that they were on the outside in this process and were unable to influence clinician decision making:
Well I don’t like the fact that I get told ‘your son is doing really really well, really really really’ and I phone up and say ‘I’m really concerned’.
Participant 2, carer group 2, UK
Cognitive participation
The EMPOWER approach to EWS monitoring was described as making sense because it could help to identify patterns in well-being changes and highlight when additional support might be needed. In terms of cognitive participation, the EMPOWER data were expected to signal when help-seeking might need to be initiated:
If the chart was, you notice yourself it’s is [sic] negative, they are definitely going down the tube, you will encourage them, if they don’t see their doctor on a regular basis, that we should go and visit a doctor.
Participant 2, carer group 3, Australia
Collective action
Despite largely positioning themselves as on the outside of the mental health staff–service user relationship that forms current EWS monitoring approaches, carers did situate themselves as potentially in a position to encourage use of the app. Carers stated that they would require some basic knowledge of how EMPOWER works to be able to do this optimally. However, carers were acutely aware of the importance of their loved ones seeking their involvement in and support with using the app, something that could not be taken for granted:
If we [as carers] had a good working understanding of it [EMPOWER] I’d find it easier to say to her ‘oh how are you getting on with the app?’ and just encouraging her with it if she was happy to be encouraged, yeah. So, I think that’d be really good.
Participant 5, carer group 1, UK
Task groups with staff (work package 2)
Coherence
Mental health care staff reported that an EWS monitoring approach made sense as a standard clinical approach to relapse prevention. Mental health care staff appeared to value their clinical ability to identify, recognise and then act on changes in well-being suggestive of EWS:
I think it’s their behaviours; you know, if there’s a sudden change or you know, that if you’ve done your relapse prevention and they have identified relapse triggers, then the person’s starting to do them.
Participant 1, staff group 7, UK
In mapping out current relapse prevention in both the UK and Australia, staff responses to EWS depended on a triage-like process. If EWS were perceived to be lower risk, staff typically responded by getting in touch with the participant. However, if EWS appeared to be signalling a more imminent relapse, then escalation to input from the medical team and, potentially, coercive measures such as community treatment orders could be employed:
There will be steps to take to ensure the consumer isn’t getting to the point where they are really unwell – being able to prevent that pretty much – most of it, all of it, getting in contact, you know. Getting input from the medical team – even using legal measures such as a temporary community treatment order or a system those things . . . yeah. It’s different for everybody, but there are ways where we can really try and manage someone.
Participant 3, staff group 12, Australia
However, mental health care staff across the task groups said that they were not always aware of EWS because there were numerous barriers to the implementation of standard EWS-based approaches in current clinical practice. For example, identifying EWS was challenging as the signs were not necessarily clear-cut; common EWS are not always specific to relapse and may reflect other issues, such as reactions to life events and adverse circumstances. In addition, service users may downplay experiences or avoid appointments, further reducing the staff’s ability to detect EWS:
I think sometimes for ourselves, well partly how people might present, or like the content of what they’re maybe wanting to discuss in their sessions. But also people started to DNA [did not attend], or just not turning up to sessions. So something it’s that they’re not presenting at all, and you think, ‘Oh my goodness, what’s going on here?’
Participant 2, staff group 7, UK
Staff spoke about encountering barriers when trying to undertake relapse prevention work, even when they had identified EWS successfully:
You can only do what you can do as a key clinician in terms of you know those policy procedures we’ve just discussed and there’s a whole other gamut of influences that might impact on a consumer that are out of our scope to influence.
Participant 1, staff group 12, Australia
Examples of barriers to successfully using EWS monitoring approaches that staff identified as out of their control were typically factors in a service user’s life (often structural) that had the potential to impact on the their well-being. Staff appeared to feel powerless in the face of these challenges. Highlighting these barriers was important because it revealed the context in which the work of EWS-based monitoring is carried out in current practice. In the following quotation, a staff member reflects on the fact that participants can be socially isolated and may take either alcohol or drugs:
Isolation. Out bevvying that night and the next morning they felt awful. Whatever . . .
Participant 2, staff group 6, UK
In addition to sociodemographic factors, barriers to the implementation of EWS monitoring approaches could be systemic. For example, when discussing current EWS monitoring-based practice, it was very common for mental health staff (in both the UK and Australia) to refer to the fact that staff are under enormous time pressure. In the following quotation, a participant alludes to needing to almost ration their clinical time, with the result that not every patient receives what is perceived to be best practice. Therefore, it is important to note that staff are likely to interact with the EMPOWER intervention in a context of significant time constraints:
What we find difficult as nurses is massive caseloads and trying to maintain quality of care trying to make sure things like that are all up to date so it’s hard it’s nice in theory to say ‘oh this is what happens and this always happens’ but we’d love it to always happen but sometimes we don’t have time to do that and it’s there chasing you every day and you’re thinking oh my god. Best practice. Core standard. Every patient would have that, but reality is we don’t often get the time to do it for everybody.
Participant 1, staff group 1, UK
Cognitive participation
Associated with significant time constraints, staff reported that they may not have adequate time to allocate to using the EMPOWER intervention:
It definitely makes sense, in that my only worry about it is that thinking about my caseload at the moment and I just don’t know where we’d have the capacity to be working with it. [Sounds of agreement from other participants.] Particularly because it’s psychosis and schizophrenia illness and how disabling that is . . . erm, to people.
Participant 1, staff group 2, UK
Among the staff groups, the EMPOWER intervention was described as making sense and was generally expected to result in data that could be useful for staff to identify EWS and offer an opportunity for relapse prevention. Therefore, the EMPOWER intervention seemed to staff to be a coherent approach:
You see where the stressors are, what times, what the patterns are, the patterns would be so clear.
Participant 1, staff group 2, UK
Although the EMPOWER approach to EWS-based monitoring seemed coherent to staff, concerns were raised about how the intervention would interact with current practice. For example, staff expressed concern that, based on the data they had inputted to the app, patients might expect a response from staff that staff may not be able to provide:
Patients might have a higher expectation of seeing somebody if they pressed these buttons then that somebody’s going to come and see them a lot quicker.
Participant 4, staff group 8, UK
Beyond concerns that intervention would increase service users’ expectations of support, staff were concerned about the accuracy of data input and the subsequent clinical value of the data in decision-making. One barrier staff anticipated was if they perceived that the data entered by patients did not provide an accurate reflection of well-being; this was a common concern throughout the task groups:
What would possibly make you lose that confidence in its potential?
If it was you know people misusing the app for not the correct purpose – maybe if some of the data didn’t appear to be correct.
Staff group 10, Australia
Collective action
Although staff generally anticipated that EMPOWER data would add value to current EWS monitoring-based relapse prevention, they expected barriers to both mental health staff and service users using the app as intended. For example, the intervention was expected to be too burdensome for at least some service users. Therefore, although staff perceived the EMPOWER approach as coherent, they also expected that they would not choose to use EMPOWER with every patient:
It sounds like there’d be quite a specific group of patients that would benefit from this in terms of the people who are able to kind of reflect, who are you know, their lives aren’t so chaotic that they can’t keep hold of a mobile phone, you know, it doesn’t end up somewhere else or in someone else’s hands or whatever, and it’s – I think it will be really useful for people who are functioning at that level and are able to reflect on things like that, but I guess it’s – I suppose I’m just thinking it’s a shame because it’s often the people I suppose who I wonder might be at more risk of more kind of relapsing or being lost in the system somehow and becoming very unwell, are maybe already a bit too chaotic or functioning at too poor a level supposed to be able to make use of something as helpful potentially as this.
Participant 1, staff group 7, UK
Another potential barrier staff identified was that EMPOWER data, even if considered an accurate reflection of a service user’s mental state, lacked important contextual information. There was concern that it could become overwhelming for staff if EMPOWER data were presented in their ‘raw’ form. To reduce this risk, the task groups suggested that humans be involved in interpreting the clinical significance of the EMPOWER data:
A bit of an overload of information perhaps if we’re getting like you know three or whatever plus messages from the app a day and we’d need to do a management plan around . . . at presentation and a big limitation in that sort of context is that you don’t . . . it’s difficult to get a feel from the person about what is happening for the person . . .
Missing out on the interpersonal context.
Staff group 12, Australia
Beta testing (work package 3)
Participants had access to the app for an average of 36.7 days (range 32–49 days) and completed the app questionnaire on an average of 25.4 days (range 16–35 days), giving an overall rate of engagement of 68.9%. These data are summarised in Table 6. All service users described the app as easy to use, including three participants who were not familiar with smartphones. Participants’ experiences were generally positive, and the majority of participants felt that the EMPOWER app was an accessible and useful tool to enhance self-management.
| Participants (pseudonyms) | Gender | Age (years) | App beta-testing duration (days) | Number of completed days | Number of days with missed alert | Percentage responding days |
|---|---|---|---|---|---|---|
| (1) Robyn | Female | 35 | 36 | 32 | 4 | 88.9 |
| (2) Emma | Female | 25 | 34 | 26 | 8 | 76.5 |
| (3) Nancy | Female | 31 | 34 | 20 | 14 | 58.8 |
| (4) Paul | Male | 53 | 32 | 16 | 16 | 50.0 |
| (5) Susan | Female | 30 | 35 | 26 | 9 | 74.3 |
| (6) David | Male | 36 | 49 | 35 | 14 | 71.4 |
| (7) Kevin | Male | 53 | 37 | 23 | 14 | 62.2 |
Questionnaire
The questions were generally considered relevant to participants. One service user described the questionnaire as more ‘in depth’ when she was not so well and ‘shallow’ when she was doing OK. Another service user described the questions as being pertinent to indication of relapse. The personalised questions were appreciated, although it was suggested that the questions could be personalised further (e.g. not all participants hear voices).
Charts
One service user described the charts as transferring a sense of ownership of their data to them. A few participants found that the charts did not work (as they were using an earlier version) or that when the charts did work they were not as clear as they could be, indicating that some improvements could be made in this area. One participant suggested the app was useful because it gathered a lot of data that could be used in appointments with mental health professionals (e.g. being able to share charts would provide a useful memory aid).
Diary
The use of the diary varied; it was not used by all participants, and when it was used it was used occasionally rather than every day. One participant mentioned that he would like to be able to share his diary (as well as the other data) with the EMPOWER research nurse and mental health care professionals.
EMPOWER messages
Not all participants were able to receive the EMPOWER messages. However, participants who did receive the messages described them as helpful and, in some cases, empowering. After reading messages about getting back into work, two service users began considering returning to work for themselves. One service user described how he had not previously given much consideration to returning to work, but reading about how other people with severe mental health problems were getting back to work had sparked his curiosity and hope. Another participant found that messages gave him perspective to think about himself beyond his mental health issues.
Interpretative phenomenological analysis (work package 3)
As part of the analysis of the transcribed interviews with seven participants, three inter-related superordinate themes were constructed: (1) generating evidence, (2) awareness and learning and (3) acceptability and feasibility. Figure 4 illustrates these three superordinate themes and their subordinate themes.
FIGURE 4.
Superordinate and subordinate themes.
Generating evidence
The first superordinate theme, ‘generating evidence’, was closely linked to ‘awareness and learning’. However, this superordinate theme focused on the meaning of using the app as a tool for self-monitoring. The vast majority of participants recognised the ability to track their well-being and link responses to experiences as one of the most beneficial elements of the EMPOWER app:
[Most beneficial element for recovery] Being able to track it on an app, (. . .) having a record on your phone which is easily accessed; that would be the best thing for me.
Emma
The creation of evidence also led to significant opportunities for relapse prevention and early intervention. Most of the informants felt that being able to access their data enhanced self-reflection as it enabled them to explore their own judgement of their well-being and to use the graphs as visible proof of their improvement or deterioration. EMPOWER was therefore considered a useful tool for self-monitoring and could heighten the chances of receiving support in the early stages of a possible recurrence, as well as reinforcing well-being:
You can see – well I am actually recovering, I am doing quite well.
Emma
Red flags if you scored high at a certain question; are a warning sign. Something you need to deal with; reach out and get help from your team, your family.
David
The consistency among participants’ data-sharing preferences was striking. All of the participants stated that to varying degrees they were willing to share data with professionals such as their general practitioner (GP) or psychiatrist, but not with family or friends:
I probably wouldn’t share it with friends or family but I might use it as evidence for myself or evidence for professionals.
Nancy
[Long-term monitoring] Might be very useful for if I am seeing my psychiatrist cause I don’t see her very often and sometimes I forget how I am over the longer term.
Susan
Awareness and learning
The second superordinate theme encompassed what was described as ‘awareness and learning’. Participants saw the questions in the app as a tool for self-reflection. The following quotations illustrate how the questions enhanced the reflexivity of two participants, Robyn and Paul:
It made me think of things which I hadn’t really considered but which had been in the background.
Robyn
Got my feelings out and you know, getting that therapy type thing.
Paul
Moreover, several of the participants noted the benefits of the personalised questions as these were tailored specifically to users’ own experiences:
I liked that they could be personalised ’cause of course psychosis is such a broad construct and means lots of different things to different people.
Susan
After the questionnaire was completed, the app generated a message that aimed to prompt users to explore and reflect on different approaches to recovery and self-management. Several participants described how the messages gave them access to relevant and useful information:
I do feel the utility of the messages is – it’s giving people access to information and self-management they might not typically get from the NHS.
Susan
Took me to some interesting links – like a newspaper with a mental health newsletter; they had quite interesting articles.
Kevin
The subordinate theme of self-reflection and self-management also included discussions of how participants incorporated new learning strategies through their use of the app:
The app was a bit of a revolution in how to deal with you – how to manage your health.
David
[Most useful element for future usage] Facing up to reality. Realising your problems and that.
Paul
Two of the participants described the usefulness of the app in the social context, suggesting that awareness of one’s own emotions and coping strategies could influence interpersonal relationships:
It’s a good gauge of how I am gonna respond for a day – how I am gonna respond to other people.
Robyn
If it’s hard to talk about something you could just show them.
Emma
It appeared that self-awareness increased through the recognition of subjective triggers through the questions and charts facility. Ease of access to evidence in the graphs could promote reflection on and recognition of individuals’ own triggers. This was particularly evident in relation to hospitalisation, which had a strong negative association. Therefore, fear of relapse and subsequent hospitalisation was an element that some participants brought up. One participant recognised the benefits of early intervention opportunities fostered by ongoing mood-tracking:
Having something to kind of track moods and also have someone at the end of the phone if I needed it is a good idea as well – rather than going at a full-blown relapse before I get help (. . .) That’s what happens a lot – you have to be really, really ill in order to get help and then it’s too late basically and you are back to square one.
Emma
Acceptability and feasibility
The final superordinate theme, ‘acceptability and feasibility’, encapsulated the participants’ experiences and assessment of the app with regard to simplicity of use, relevance of questions, randomisation and issues of disclosure, timing of use and, finally, aspects of commitment, trust and honesty. All participants, including one who had no previous experience with smartphones, felt that the app was straightforward and easy to use. Moreover, the majority of participants viewed the questions as relevant:
I can’t really put my finger on anything which wasn’t useful. Everything that was there was relevant.
Robyn
There were some contrary opinions about disclosing mental illness as a result of using the app. One participant valued the app more than innovations that relied on paper, such as psychosis diaries, because it increased their feelings of privacy and anonymity. Another informant was interested in changing the app diary function into a journal with unrestricted access given to the treating team; that, in turn, could offer professionals some context for the given data. One participant was critical about the randomised timing of alerts to complete the questionnaire at different times of the day:
If you are not open about mental health anyway; I think it would be very difficult to use the app in public.
Nancy
Participants reflected about the most favourable time to use the app. Some felt that they would gain most benefits from using it in the recovery period after a relapse:
I probably wouldn’t want to do it if I was very unwell but if I was recovering from coming unwell I might want to do it again.
Nancy
Several participants who considered themselves recovered felt that the app would have greater utility at times when they were less well:
I have recovered you know, but it has only been a few years ago when I was still in the hospital. It would have been more useful you know – when I wasn’t quite recovered fully, you know. So when I came out of the hospital for the last time; it would have been very useful then I think.
Kevin
Most of the participants were unsure about the benefits of the randomised timing of alerts to complete the questionnaire and its feasibility in day-to-day life, for example the alarm going off while they were at work. The beta-testing version had a reduced response window, and one participant was disappointed that they had ‘just one chance’ to complete the questionnaire. Based on this, giving users the chance to respond to the questions over a longer period of time might improve acceptability and feasibility:
I would have preferred if you could just – if there was a flexible time; you can just, you know, just tap one and go into it at any time.
Kevin
Moreover, participants reflected on important issues of commitment, trust and honesty. For example, commitment was identified as an important basis for longer-term app use:
You have to be quite on the ball to use that thing by the way (. . .) You know what I mean, you got to be with it.
Paul
It really takes for you to embrace it for it to work for you. You know it can’t just be a casual thing, it is a lifestyle thing.
David
The aspects of trust and honesty were closely related. Previous negative experiences with services could decrease participants’ trust in the app and reduce the likelihood of their disclosing EWS because of fear of rehospitalisation:
Not everybody in my shoes will be trusting enough to pour their heart out into this app and it just really – the success of it just depends upon being honest.
David
Altogether, the data gathered in this study indicate that the EMPOWER app contributed to participants’ enhanced self-reflection on the ‘ebb and flow’ of emotions and experiences in daily life. The app also promoted participants’ access to new channels of learning. Overall acceptability was high, and suggestions for improvements were focused primarily on technical aspects of the app. Even though these results cannot be generalised to the broader population of potentially eligible service users, a closer look at the data suggests that most participants regarded the app as a useful tool for exploring aspects of recovery and self-management:
I was quite happy to use it – quite happy to be part of its trial.
Robyn
Discussion
The objective of this study was to conduct task groups to:
-
evaluate the acceptability and usability of mobile symptom reporting using smartphones among service users, carers and mental health care staff
-
identify the incentives and barriers to use by service users and carers and to implementation by mental health care staff
-
identify pathways to relapse identification and prevention.
Our further aim was to finalise the EMPOWER app for implementation in the phase 2 feasibility cRCT comparing EMPOWER with treatment as usual. This was assessed over a 5-week beta-testing phase plus follow-up interviews with service user participants to identify technical issues that needed to be resolved prior to phase 2, and to explore participants’ experiences of using the app. This phase was an important precursor to establishing the key parameters of the EMPOWER intervention, that is to enable the recognition of EWS by service users and their carers and to support a care pathway towards relapse prevention. We utilised NPT as a framework to understand the work required to optimise the EMPOWER intervention ahead of conducting the feasibility trial. 203
Coherence
In WPs 1 and 2, it was important to understand if the EMPOWER app made sense, had a clear purpose, was distinct from existing practices, would bring benefits that stakeholders would value, and would fit with the goals and activities of stakeholders. In NPT, this is referred to as coherence: the extent to which participants make sense of an intervention. During WPs 1 and 2, all participants described relapse as a negative event leading to significant distress and/or disruption in their spheres and contexts. There was a clear and shared understanding of the function of monitoring EWS to identify changes in well-being suggestive of relapse. The EMPOWER app seemed to offer something distinctive from routine care and was valued by participants. Anticipated benefits included being able learn from the data and develop a better understanding of well-being, providing a memory aid to support personal decision-making or shared decision-making, providing a tool to enable carers to encourage learning, monitoring and help-seeking, and offering a means of developing greater self-reflection.
Cognitive participation
It was also important to understand if stakeholders thought that the app was a good idea, the extent to which they saw the app as having utility, and whether or not they would be prepared to invest time and effort in the EMPOWER app. In NPT this is referred to as cognitive participation, or the extent of necessary commitment and engagement by participants. Although the app was distinctive and made sense to mental health care staff, they also raised important and distinctive concerns, especially in relation to high caseloads and time pressures that already had an impact on their ability to spend time working with service users. Staff perceived that if the app increased their workload then they would not have capacity to work with it. Staff also were concerned that many of their service users would not use the intervention because of the severity of illness, cognitive impairments, the impacts of digital exclusion or other stresses arising from poverty and deprivation. These are common concerns that have resulted in digital triallists conducting research focusing on digital exclusion in the context of psychosis. 204 Staff reflected that often it was these same service users who were at highest risk of relapse, and therefore those most in need of relapse prevention would be least likely to utilise the intervention. Berry et al. 205 previously reported that mental health care staff have conflicting views about the use of the internet and mobile phones for self-management by people with psychosis. The authors found that mental health care staff were cautiously optimistic about using mobile phone apps for monitoring. However, they also found that staff had concerns about their responsibility when receiving symptom reports from service users as they were worried about missing important risk information. Therefore, staff expressed a preference for receiving reports on monitoring during sessions with service users. In other research,170 staff have found the number of data from digital interventions overwhelming. These findings resonate with our own finding that concerns about lack of staff time and capacity are key logistical barriers to the implementation of digital technologies in psychosis care175 and should be considered when designing interventions. By contrast, both service users and carers felt that the app provided an important opportunity to keep track of and identify changes in well-being as a way of improving self-management and control. In addition, service users saw messages as a potential means of additional support and encouragement to access the knowledge and skills to stay well, as long as those messages were salient and meaningful.
Collective action
In NPT, collective action relates to the work that participants need to engage in to make an intervention function in its context. This includes questions regarding how the intervention may promote or impede existing practices, and what impact the intervention might have on the division of resources or allocations of responsibilities among stakeholders. 206 Mental health care staff spoke about the importance of data from the EMPOWER app being reliable and valid in order to be useful for incorporation into routine practice and clinical decision-making. This included reflections on threats to the reliability and validity of data. Staff were concerned that service users may use the app to increase access to support and crisis care by exaggerating the severity of their experiences and that service users might lack the insight to reliably enter data that would be helpful to clinical decision-making. Staff were also concerned about data being viewed without broader awareness of the context of either the person (e.g. their access to sources of support, coping) or the service (e.g. its knowledge and expertise in delivering care to that individual). All of these factors were seen as potentially having an impact on existing practice in unhelpful ways, such as by increasing workload. Service users expressed concerns about help-seeking in the context of early signs of relapse, in particular about their data being shared and utilised to initiate unwanted interventions, including changes to medication and rehospitalisation. At the same time, service users said that the app should not be seen as a replacement for routine support from mental health services (a concern also expressed by mental health care staff and carers). In mapping out standard EWS-based monitoring from the task groups, it appeared that carers felt they were on the outside of the service user–clinician relationship, and many reported that clinicians could fail to take their concerns about their loved one’s well-being on board. These concerns manifested in relation to the app, and carers expressed a desire to be involved, where agreed with their loved one, in encouraging use of the app. In summary, different stakeholder groups had unique perspectives and concerns about current relapse management that appeared to act as a barrier to shared decision-making, echoing the qualitative work conducted following the CRIMSON study. 207 In terms of collective action, predictions about EMPOWER implementation barriers appeared to be clustered around whether or not the intervention was expected to have an impact on existing issues and concerns. For example, if the intervention increased workload, staff would be likely to resist using it, a phenomenon sometimes observed when novel digital interventions are implemented in clinical settings. 172
Translation to EMPOWER phase 2
Task groups identified that the proposed EMPOWER intervention (including the app) made sense to all of our stakeholder groups and was relevant to either existing mental health services practice or the concerns and values of service users and carers. We identified and theoretically framed important barriers to implementation in routine clinical practice. These barriers included the current context of practice, as staff reported carrying large and busy caseloads and were concerned that the app might create additional and unnecessary work as a result of the lack of reliability and validity of service users’ self-reported data. In addition, staff saw themselves as having strengths in their knowledge about and expertise in managing relapse and in their ability to tailor their approach to relapse detection and prevention to individual service users based on their knowledge of that person. Therefore, based on the NPT framework,190 there exists in current clinical practice potential structural barriers (e.g. lack of staff time) but also cognitive barriers (e.g. how staff think about their own role and skills in relation to relapse prevention). In response, we were careful to consider the role of triage in the design of the broader EMPOWER intervention. That is, rather than alerts triggered by changes in well-being going directly to mental health care staff, these alerts would be triaged by members of the research team and shared with staff members as part of a broader discussion incorporating relevant changes having an impact on the service user and relevant staff interactions with the service user. Sekhon et al. 208 stated that decisions about whether or not an intervention is appropriate for end-users are made through anticipated or actual cognitive and emotional responses to that intervention. Although service users and carers in this study valued relapse prevention as an important goal, they also raised important concerns around fears of help-seeking in the event of a crisis and the risk of exposure to unwanted interventions, particularly rehospitalisation. Mapping out the extent to which emotional responses such as fear may factor into hypothetical intervention acceptability was a key part of this research. The triage by research team members of changes in well-being was an important component of the EMPOWER intervention as a means of making sense of changes in well-being over time, linking changes to broader contextual factors and identifying patterns of change over time, all of which it was hoped would create an enriched understanding of changes in well-being. Therefore, the aim of the triage role was to reduce the fear and uncertainty of using a novel self-monitoring approach situated within existing fears about help-seeking that are inherent in current EWS-based monitoring approaches to relapse prevention. In addition, an important part of the EMPOWER intervention was the role of peer support. The results from task groups and beta testing also enabled the research team to carefully consider and refine the role of peer support workers in the intervention. We further emphasised the peer support workers’ role in helping service users (and, where relevant, carers) learn about the mobile app and its functions, providing support with using the app and reflecting on app-generated messages, prompting engagement and curiosity in these messages as a basis for developing greater autonomy and self-management. As part of this, peer support workers were also app users, enabling them to share their own experiences of mental health more broadly or their experiences of using the app more specifically.
Beta testing
User experience research in digital interventions for psychosis has been important in understanding and anticipating potential implementation barriers for interventions addressing smoking,209 paranoia185 and symptom management. 210 Conducting UX testing directly with end-users in advance of the trial was a further important step in anticipating specific implementation issues with the EMPOWER intervention. Through conducting beta testing, we identified a number of relevant and important technical issues. Fixes for these technical issues were included in software updates successfully delivered before phase 2. Technical problems resulting in poor UX, such as apps crashing, have been identified as key implementation barriers in clinical trials of digital interventions for psychosis,211 highlighting the importance of conducting short-scale UX testing to identify technical issues. There were a number of important findings in relation to participants’ experience of the app, including learning from the experiences of those who had never used a smartphone previously, themselves an under-researched subgroup. 204 Participants noted that the app was simple to use and that the questions were relevant to their well-being. Participants felt that the app was a useful tool that they would be able to use to self-monitor their own well-being, and this was viewed as an important basis for staying well. Having used the app, all participants in the beta-testing phase expressed a preference for being able to share data with others, particularly mental health care professionals. In this regard, the app was seen as a tool for generating evidence to support personal awareness and learning or, indeed, for sharing evidence with mental health care professionals. The well-being messages generated in response to user data were understood as credible and a helpful prompt for self-management, providing access to broader information about experiences relevant to staying well.
Although the hypothetical acceptability of the well-being messages appeared reasonable in both the task groups and the UX testing phase, service user participants nonetheless expressed that if they perceived the messages to be patronising they would not be likely to engage with them. Furthermore, previous research has demonstrated framing effects (e.g. a willingness to even continue to read messages) of the words that are used in well-being messages. 212 To ensure that the messages were most relevant and salient to service users, we set up a patient and public involvement group to steer the development of principles for the curation and refinement of messages, to provide advice and good sources of user-friendly information and also to review the content and delivery of messages for EMPOWER. In recognition of how participants spoke about how current relapse prevention practice operates within an overarching structure of fear and uncertainty, we also changed the language around EWS monitoring. The language of EWS monitoring used by the researchers during task groups suggested that all stakeholders identified strong associations with risk. By using the app as a way to monitor the ‘ebb and flow’ of well-being, the language took on a more normalising and engaging tone. This was reflected in changes to the ‘clinician interface’ (where app user data were monitored by the research team), where changes identified by the algorithm generated ‘alerts’ that also continued to evoke the language of risk. We modified this to the term ‘check-in prompts’ to reflect the shift towards triage as a basis for learning and recognising that there was significant uncertainty about the clinical relevance of changes detected by the algorithm and the extent to which these may reflect true or false positives.
Chapter 4 Baseline, feasibility, acceptability and safety results
Recruitment and participant flow
Eight CMHS were recruited to the study, six from NHS Greater Glasgow & Clyde and two from NorthWestern Mental Health in Melbourne. In each team we drew from a reservoir of potentially eligible participants who were approached for consent prior to the allocation of treatment condition. After initially screening caseloads for diagnosis and the presence of a designated care co-ordinator, we fully screened 1140 potentially eligible service users across all teams. From these, we excluded a further 66 people on diagnostic grounds, 187 people who were being transferred or discharged and nine people who were deceased. Among the remaining 878 potentially eligible service users, we excluded 491 for whom there was no evidence of a relapse in the previous 2 years, 31 who were currently experiencing a relapse and 93 for other reasons including lack of capacity, communication needs and being in a first episode of psychosis. From the remaining group of 263 service users, we were able to approach 129 people between January and August 2018 in order to seek their informed consent. Of this group, 86 service users initially provided informed consent across the two sites: 56 in Glasgow and 30 in Melbourne. Figure 5 describes the recruitment of these 86 service users to the study over time.
FIGURE 5.
Service user recruitment over time.
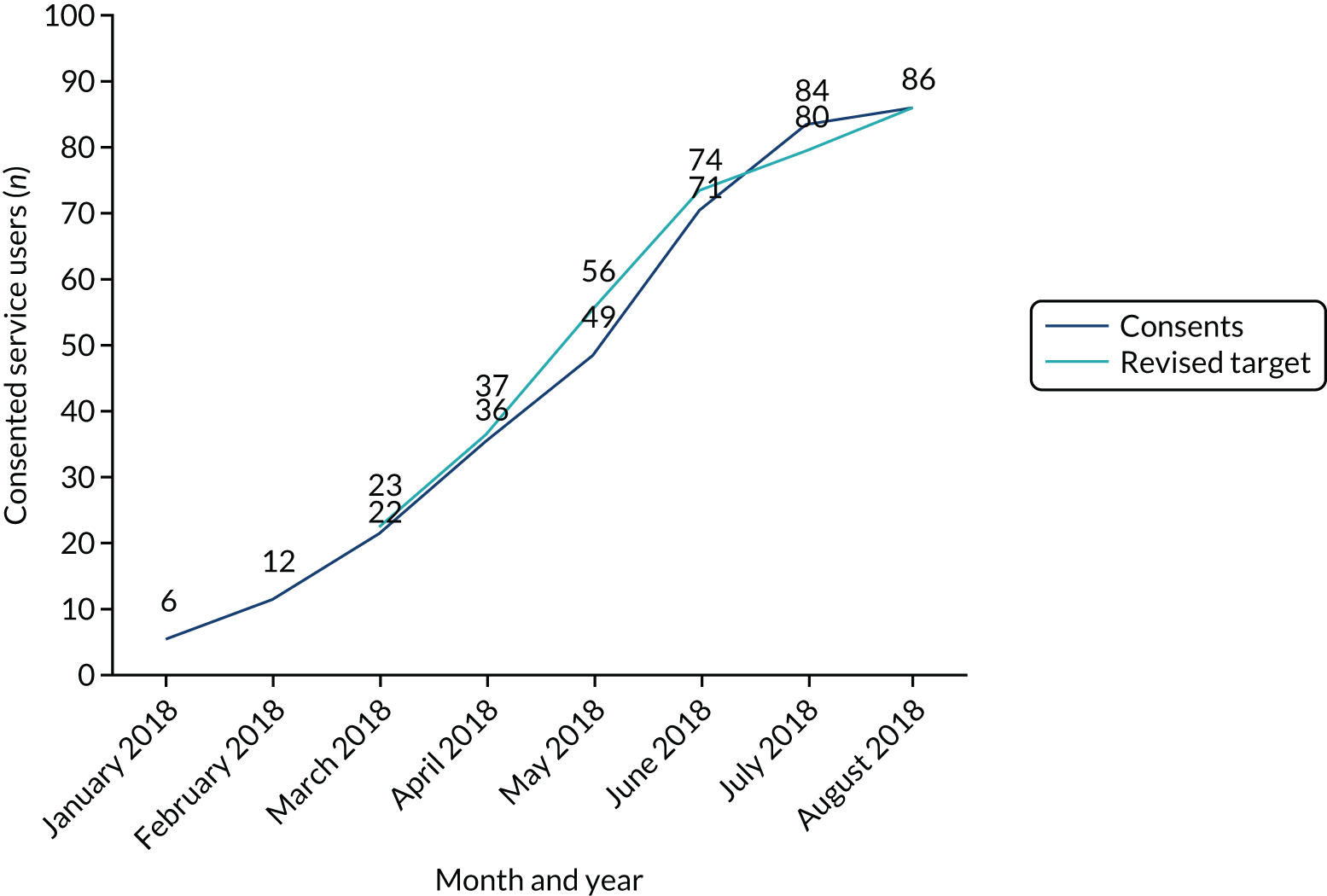
Thirteen service user participants withdrew before the treatment allocation was revealed to them or to their service. Reasons for withdrawal at this stage were available for six people. Three people cited a change in life circumstances after consent that meant that they were no longer able to commit to the study. Two people described being worried after providing consent about the implications of their involvement and one participant withdrew because they did not want to use the app (despite assurances that they might be allocated to the TAU arm). This left 73 service user participants who took part in the trial. Among this group, 27 (37%) participants had a nominated carer: 10 (24%) in EMPOWER and 17 (55%) in TAU. Of these 27 carers, 17 (63%) gave their informed consent to participate: seven (70%) in EMPOWER and 10 (59%) in TAU.
The flow of participants through the study is summarised in a CONSORT flow diagram (Figure 6). Four teams were randomised to each arm of the study: three in Glasgow and one in Melbourne. Within those teams, 42 service users were allocated to the EMPOWER arm and 31 were allocated to TAU. The small imbalance in the allocated number of participants in each arm was due to randomising a small number of clusters of varying size. In the EMPOWER arm all but one service user participant had the EMPOWER app installed. The remaining service user participant received part of the intervention (peer support). Participant follow-up took place between March 2018 and July 2019. At 12 months, primary outcome data were collected for 32 service user participants in the EMPOWER arm (76%) and for 28 (90%) in the TAU arm. During the study seven people withdrew from the EMPOWER arm, two moved out of the area and there was one death. There was a clear association between withdrawing from the EMPOWER arm and low engagement with the app. Four out of the seven participants who withdrew had the app installed but failed to complete an adequate number of baseline observations. The other three participants did not use the app at all. Additional information on withdrawals is as follows. In two instances people withdrew from the EMPOWER arm of the study directly after an app-related adverse event (see Table 10). Another person withdrew for reasons that were less clear but may have been influenced by a technical issue with their phone that had caused some distress at that time. One person described the reason as being related to stress and their general mental health. One participant was withdrawn as a result of their behaviour towards a member of the research team. In two instances the reasons for withdrawal were not clear. In the TAU arm, one service user participant withdrew but it was not clear why. Of those who withdrew from the EMPOWER arm, four were male and three were female. The mean age of those who withdrew appeared to be slightly higher, at 50 years (SD 12 years), than the mean age of the sample as a whole, at 43 years (SD 12 years).
FIGURE 6.
The CONSORT flow diagram. Values for those lost or withdrawn are cumulative.
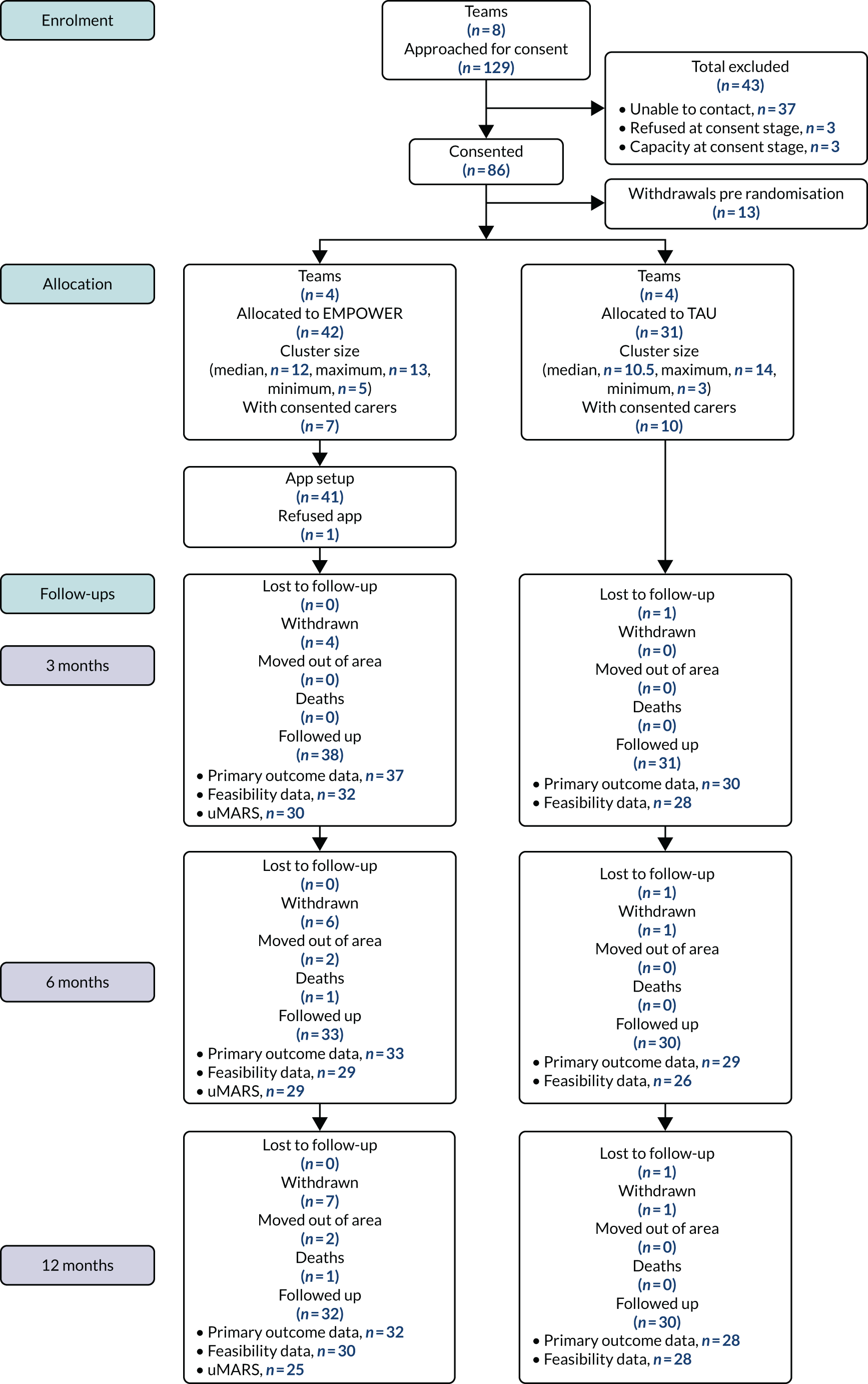
Baseline characteristics
Service user baseline demographic characteristics
Baseline characteristics are summarised in Table 7. Men and women were similarly represented in the sample, and the mean age of service user participants was 43 years. Thirty-seven per cent of participants described themselves as having an informal carer. We noted a higher proportion of service users in the TAU arm stating that they had a carer (n = 17, 55%) than in the EMPOWER arm (n = 10, 24%). On average, service users had been in contact with mental health services for around 12 years.
| Characteristic | EMPOWER (n = 42) | TAU (n = 31) | Total (N = 73) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 21 (50) | 16 (52) | 37 (51) |
| Female | 21 (50) | 15 (48) | 36 (49) |
| Age (years), n: mean (SD) | 42: 42 (13) | 31: 43 (12) | 73: 43 (12) |
| Years of education, n: mean (SD) | 35: 12 (3) | 30: 13 (3) | 65: 12 (3) |
| First contact with mental health services (months), n: mean (SD) | 38: 154 (121) | 30: 134 (92) | 68: 145 (109) |
| Have a carer: yes, n (%) | 10 (24) | 17 (55) | 27 (37) |
| UK ethnicity, n (%) | 30 (71) | 19 (61) | 49 (67) |
| Scottish | 21 (70) | 16 (84) | 37 (76) |
| Other British | 1 (3) | 1 (5) | 2 (4) |
| Other white ethnic group | 1 (3) | – | 1 (2) |
| Mixed or multiple ethnic group | 1 (3) | 1 (5) | 2 (4) |
| Pakistani | 2 (7) | – | 2 (4) |
| Indian | 1 (3) | – | 1 (2) |
| African | 3 (10) | – | 3 (6) |
| Unknown | – | 1 (5) | 1 (2) |
| Australian ethnicity, n (%) | 12 (29) | 12 (39) | 24 (33) |
| Born in Australia | 7 (58) | 12 (100) | 19 (79) |
| Born elsewhere | 5 (42) | – | 5 (21) |
| Aboriginal/Torres Strait Islander | – | 1 (8) | 1 (4) |
Clinical characteristics of service users at baseline
Clinical characteristics of service users at baseline are described in Table 8. Ninety-five per cent of service users were assessed as being in full or partial remission at baseline. The total scores on the PANSS indicate that this group of individuals had a moderate level of illness severity,213 and Personal and Social Performance (PSP) scale scores suggest a marked impairment of functioning across the group. 151 Levels of depression were also high, with 56% of the sample scoring ≥ 6 on the CDSS, suggesting the presence of major depression. 214 Levels of alcohol and other drug use were low at baseline.
| Assessment | EMPOWER (N = 42) | TAU (N = 31) | Total (N = 73) |
|---|---|---|---|
| Remission at baseline, n (%) | |||
| Full remission | 20 (48) | 12 (39) | 32 (44) |
| Partial remission | 19 (45) | 18 (58) | 37 (51) |
| Inadequate evidence | 2 (5) | 1 (3) | 3 (4) |
| Missing | 1 (2) | 1 (1) | |
| PANSS, n: mean (SD) | |||
| Positive | 42: 14.83 (5.92) | 30: 15.43 (6.68) | 72: 15.08 (6.21) |
| Negative | 42: 13.90 (5.45) | 30: 12.47 (4.08) | 72: 13.31 (4.95) |
| Disorganisation | 42: 15.86 (7.17) | 30: 14.63 (4.67) | 72: 15.35 (6.24) |
| Excitement | 42: 4.95 (1.65) | 30: 4.33 (0.55) | 72: 4.69 (1.34) |
| Emotional distress | 42: 11.95 (4.40) | 30: 12.07 (3.24) | 72: 12.00 (3.93) |
| Total | 42: 61.50 (18.14) | 30: 58.93 (13.73) | 72: 60.43 (16.39) |
| PSP scale, n: mean (SD) | |||
| PSP total | 42: 56.86 (16.02) | 30: 59.37 (19.29) | 72: 57.90 (17.37) |
| CDSS, n: mean (SD) | |||
| CDSS total | 42: 6.93 (5.34) | 30: 6.97 (4.15) | 72: 6.94 (4.85) |
| Timeline Followback for drugs and alcohol, n (%) | |||
| In last 28 days, n (%) | |||
| Alcohol | 15 (36) | 12 (39) | 27 (37) |
| Not used | 26 (62) | 18 (58) | 44 (60) |
| Missing | 1 (2) | 1 (3) | 2 (3) |
| Cannabis | 7 (17) | 5 (16) | 12 (16) |
| Not used | 34 (81) | 25 (81) | 59 (81) |
| Missing | 1 (2) | 1 (3) | 2 (3) |
| Other main drug | 4 (10) | 5 (16) | 9 (12) |
| Not used | 37 (88) | 25 (81) | 62 (85) |
| Missing | 1 (2) | 1 (3) | 2 (3) |
| HADS, n: mean (SD) | |||
| Anxiety | 41: 9.76 (5.11) | 30: 10.70 (4.88) | 71: 10.15 (5.00) |
| Depression | 40: 7.38 (4.98) | 29: 8.03 (4.66) | 69: 7.65 (4.83) |
| PBIQ-R, n: mean (SD) | |||
| Control | 41: 10.29 (2.52) | 30: 9.83 (2.61) | 71: 10.10 (2.55) |
| Shame | 41: 10.10 (2.96) | 30: 10.67 (3.07) | 71: 10.34 (2.99) |
| Entrapment | 41: 10.73 (2.88) | 30: 10.80 (3.38) | 71: 10.76 (3.08) |
| Loss | 41: 9.84 (2.82) | 30: 10.20 (2.54) | 71: 9.99 (2.69) |
| Socially marginalised | 41: 11.64 (2.89) | 30: 11.87 (2.75) | 71: 11.74 (2.82) |
Carer and care co-ordinator baseline characteristics
Carer and care co-ordinator baseline characteristics are summarised in Tables 30 and 31 (see Appendix 3). Carers were predominantly female (71%) and had a mean age of 49 years. Care co-ordinators were also predominantly female (74%) and had been qualified for 11 years on average.
General feasibility
Service users, carers and care co-ordinators in both arms of the trial were asked questions at all time points about general feasibility. Novel measures were developed that assessed their use of health and well-being apps and help-seeking related to EWS and their consequent changes in clinical management. Measures are available in Appendix 1. Table 32 (see Appendix 4) shows that, among those randomised to the EMPOWER arm, we observed greater self-reported use of well-being apps (rated as ‘sometimes’) over 12 months than among those randomised to TAU (rated as between ‘not at all’ and ‘rarely’). In both groups, help-seeking for EWS, carer help-seeking for EWS and changes in clinical management were rated as between ‘not at all’ and ‘sometimes’. Tables 33 and 34 (see Appendix 4) show these ratings from the perspective of carers and care co-ordinators, respectively. These show a similar pattern of results as observed in relation to help-seeking and changes in clinical management for service users. We asked carers and care co-ordinators to rate how often service users discussed EWS with them. We found that, in both arms of the trial, carers and care co-ordinators rated these discussions as happening between ‘rarely’ and ‘sometimes’.
Feasibility and acceptability of the EMPOWER app
The feasibility and acceptability results relate to the 41 service users in the EMPOWER intervention arm who had the EMPOWER app installed, and these are summarised in Table 9. Self-reported app usage was consistently reported as high at all time points, but the self-reported sharing of information from the app in the form of charts was more limited, both with care co-ordinators and with carers. At all time points, people were more likely to share information with a care co-ordinator than with a carer, but this may in part reflect that not all app users were identified as having a carer. However, people reported accessing the charts for their own use on a regular basis.
| Assessment | 3 months | 6 months | 12 months |
|---|---|---|---|
| App feasibility | |||
| Roughly how often use app?a | |||
| Not at all,b n (%) | 1 (3) | ||
| n: mean (SD) | 30: 4.63 (0.96) | 26: 4.65 (0.75) | 23: 4.65 (0.57) |
| Roughly how often share with key worker?c | |||
| Not sure, n (%) | 3 (10) | 1 (4) | 1 (4) |
| n: mean (SD) | 26: 2.04 (1.00) | 25: 2.24 (1.16) | 22: 2.45 (1.30) |
| Roughly how often share with family?c | |||
| Not sure, n (%) | 1 (3) | 1 (4) | |
| n: mean (SD) | 28: 1.71 (0.98) | 26: 1.96 (1.15) | 22: 1.91 (1.19) |
| Roughly how often access charts?c | |||
| Not sure, n (%) | 1 (3) | ||
| n: mean (SD) | 28: 3.04 (0.74) | 26: 2.54 (1.14) | 23: 3.00 (1.04) |
| uMARSa | |||
| Is the app interesting to use?, n: mean (SD) | 29: 3.93 (0.88) | 26: 3.92 (0.98) | 23: 3.52 (0.99) |
| Is it easy to learn?, n: mean (SD) | 29: 4.14 (0.64) | 26: 4.12 (0.91) | 23: 4.17 (0.78) |
| Moving/links between screens work?, n: mean (SD) | 29: 3.97 (0.87) | 26: 4.12 (0.71) | 23: 4.17 (0.78) |
| Is app content correct/well written/relevant?, n: mean (SD) | 29: 4.07 (0.70) | 26: 4.04 (0.77) | 23: 4.13 (0.69) |
| Is app information from a credible source?, n: mean (SD) | 29: 4.45 (0.78) | 26: 4.58 (0.58) | 23: 4.57 (0.66) |
| Would you recommend the EMPOWER app?, n: mean (SD) | 29: 3.83 (1.07) | 26: 3.85 (1.12) | 23: 3.83 (1.34) |
| How do you rate the app? | |||
| NA, n (%) | 10 (33) | 10 (38) | 6 (26) |
| n: mean (SD) | 19: 4.26 (0.81) | 16: 4.31 (0.79) | 17: 4.06 (0.75) |
| App has increased awareness, n: mean (SD) | 29: 3.97 (0.98) | 26: 4.46 (0.71) | 23: 4.22 (1.04) |
| App has increased knowledge/understanding, n: mean (SD) | 29: 3.76 (1.09) | 26: 4.19 (0.80) | 23: 3.96 (1.02) |
| App changed attitudes towards improvement, n: mean (SD) | 29: 3.72 (0.88) | 26: 4.04 (0.96) | 23: 3.83 (1.03) |
| App increased my intentions/motivation, n: mean (SD) | 29: 3.97 (0.87) | 26: 4.35 (0.75) | 23: 3.87 (0.97) |
| App encourages me to seek help, n: mean (SD) | 29: 4.14 (0.88) | 26: 4.42 (0.70) | 23: 4.09 (1.04) |
Acceptability ratings from the adapted uMARS measure suggested that the app was interesting to use and was consistently rated as easy to learn. Content was rated as being well written and the credibility of information was rated particularly positively throughout, peaking at 4.58 out of a possible 5 at 6 months. There was also a consistently positive overall rating for the app, ranging between 3.83 and 3.85 out of 5.
In terms of perceived mental health impact, positive ratings were given for awareness, attitudes, motivation and help-seeking (with a range of between 3.72 and 4.46 out of 5 across these indicators at all time points).
App engagement
Of the 41 participants who had the app set up, 33 (80.5%) completed the 4-week baseline. These participants used the app for a mean of 31.5 weeks (SD 14.5 weeks; range 44 weeks). During that period, participants used the app for a mean of 64.1% (SD 22.5%; range 76.3%) of days. Therefore, according to our a priori criterion for acceptable engagement of 33% daily use, 30 (91%) participants met our criterion for adherence. Among those randomised to the EMPOWER arm, this represents 71% meeting the criterion. This range of 71–91% provides a broader estimate of overall engagement.
Fourteen participants (42.4%) were still using the app at their final follow-up assessment. Survival analysis methods are recommended for understanding attrition in digital interventions. 215 Attrition represents the amount of time to a relevant event occurring; in this case, the criterion was 4 sequential weeks of not meeting the intended adherence criterion of 33%. The analysis was completed using the survfit function in the survival package in R, with bootstrapping performed using the bootkm function from the hmisc package in R. Overall, the median survival for not missing 4 sequential weeks of 33% use was 32 weeks (bootstrapped 95% CI 14 weeks to ∞). We noted that the upper limit returned an infinite value, likely to be a result of the skewed data. In other words, for 50% of participants who had completed a baseline it took 33 weeks before missing 4 sequential weeks of intended intervention usage. The width of the CIs suggest that some level of uncertainty is appropriate when interpreting the result of this test (Figure 7). To summarise, the length of time at which 50% of participants no longer meet the intended adherence criterion is hard to predict within this sample (especially in terms of an upper limit) but it is likely not to fall below 14 weeks. Supplementary information on our approach to describing engagement with the EMPOWER app is provided in Appendix 5.
FIGURE 7.
Kaplan–Meier curve of time to 4 weeks’ app use < 33%.

We explored the number of peer support worker sessions delivered over the course of the study. A total of 522 peer support worker sessions were offered to 42 participants in the EMPOWER arm (mean 12.4, SD 6.8; median 11.0, interquartile range 10.0). We also explored the number of ChIPs triggered by the algorithm during the study. There were 558 ChIPs during the study across 37 participants (mean 15.1 per participant, SD 10.5 per participant; median 13.0, interquartile range 15).
Provision of study phones
A total of 28 mobile phones were provided to and used by participants: 22 in Glasgow and six in Melbourne. Six provided phones were either lost or stolen, four in Glasgow and two in Melbourne, and handsets were replaced on three of those occasions. The remaining participants in the EMPOWER arm chose to have the app installed on a personal handset. We did not ask for provided phones to be returned.
Safety
Adverse events
Adverse events monitoring and reporting included serious and non-serious adverse events, in line with the registration of the study as the Investigation of a Class 1 Medical Device. The medical device was specifically the algorithm that reviewed app data and determined responses. Across both arms there was a total of 54 adverse events, affecting 29 people, during the study. Around half of all events across arms were classified as serious adverse events, and the vast majority of these were anticipated. There was one death during the study. In terms of relatedness of adverse events, six events were assessed as being related to a study procedure, one of which was serious. This involved a threat made to a member of research staff in Melbourne in relation to the use of a study phone.
Adverse events are summarised in Table 10. There were no adverse events of any type related to the EMPOWER Class 1 Medical Device. However, to ascertain a fuller picture of possible harms than would have been available from focusing solely on medical device-related events, we additionally monitored adverse events related to people’s experience of the EMPOWER app more generally. There were 13 app-related adverse events, affecting 11 people, one of which was serious. This involved a brief hospital admission for a physical health complaint, which the service user described as being in part related to feeling overwhelmed by the recent installation of the app. The service user, who had not yet used the app, appeared to be experiencing depression based on their contemporaneous CDSS assessment, and subsequently withdrew from the study. Examples of non-serious app-related adverse events included four instances in which the app caused unhelpful rumination. In one of these instances, where the self-monitoring approach was described as counter to the service user’s usual coping strategy of ‘burying things’, the participant withdrew from the study. Other participants described feeling forced to think about being unwell because of questions in the app, with one person suggesting less frequent monitoring in future iterations. Unhelpful rumination of this type was identified by one participant as an issue when they were well, whereas a second person was affected when they felt more depressed. Two participants specifically cited increased paranoia as a result of the app. On one occasion this related to the timing of question alarms and on another it related to the specific content of personalised questions. A further participant identified that personalised question content unhelpfully triggered traumatic memories of psychosis. One participant reported experiencing increased anxiety after being asked a question that was new to them as a result of branching rules in the question set. In one case a participant reported increased worry as a result of losing their provided mobile phone, and, in a further event, a participant experienced distress as a result of a technical fault arising from a conflict between the app’s software and their personal phone. App-related adverse events and our responses to them are described in more detail elsewhere. 113
| Event characteristics | EMPOWER (N = 42) | TAU (N = 31) | Total (N = 73) | |||
|---|---|---|---|---|---|---|
| Adverse events | 29 | 25 | 54 | |||
| People affected | 19 | 10 | 29 | |||
| Adverse event type, n (%) | ||||||
| Serious adverse event | Yes, 11 (38) | No, 18 (62) | Yes, 15 (60) | No, 10 (40) | Yes, 26 (48) | No, 28 (52) |
| Anticipated | 7 (64) | – | 14 (93) | – | 21 (81) | – |
| Death | 1 (9) | 1 (4) | ||||
| Event relatedness, n (%) | ||||||
| Study procedurea | 1 (9) | 3 (17) | 2 (20) | 1 (4) | 5 (18) | |
| App | 1 (9) | 12 (67) | 1 (4) | 12 (43) | ||
| Medical device | – | – | ||||
| Event intensity, n (%) | ||||||
| Mild | 1 (9) | 3 (17) | 4 (40) | 1 (4) | 7 (25) | |
| Moderate | 1 (9) | 12 (67) | 3 (30) | 1 (4) | 15 (54) | |
| Severe | 9 (82) | 3 (17) | 15 (100) | 3 (30) | 24 (92) | 6 (21) |
Device deficiencies
Two device deficiencies of the investigational medical device were identified during the study. The first was categorised as a manufacturing defect and was related to the following performance end point:
-
Following 4 weeks of usage, the EMPOWER algorithm calculates participants’ individualised baseline of symptoms and experiences.
One participant’s initial baseline observations showed no variation. That is, they scored the same for each item on each day of the baseline period. As the result of an anomaly in the algorithm’s calculation of standard deviation, every time the participant responded to questions following the baseline period, a ChIP was automatically generated regardless of any relevant change in scores.
A second device deficiency, which was categorised as a device malfunction, involved an unexpected server shutdown at the University of Manchester. This lasted for around 5 hours, affecting the following performance end points during that time:
-
Researcher accesses participants’ questionnaire responses and generates charts to observe changes over time.
-
Researcher receives a record of alerts for each participant and is able to record actions in relation to these alerts.
Chapter 5 Candidate outcomes of the main trial
Candidate service user outcomes
Candidate primary outcomes
We collected complete relapse data on 67 (92%) participants at 3 months and on 61 (84%) participants at every follow-up time point. Research assistants screened electronic case records to identify potential episodes of relapse and exacerbation at 3, 6 and 12 months post randomisation. These episodes provided the basis for individual anonymised case vignettes that were reviewed by an independent and blinded adjudication panel, who determined what, if any, criteria for relapse were met, the date of relapse and the type of relapse. Following calibration on four relapse assessments, inter-rater reliability testing was undertaken on a further 12 assessments for which we noted substantial agreement (k = 0.80). These findings demonstrate the feasibility of collecting data relating to relapses and exacerbations from routine clinical data.
During the study, we identified 27 relapses over 12 months, with a larger number in the TAU arm (n = 19) than in the EMPOWER arm (n = 8). Among these relapses were 11 hospital admissions, nine in the TAU arm and two in the EMPOWER arm. Five relapses involved the use of mental health legislation, all of which were in the TAU arm. Details of the relapse characteristics collected at each follow-up time point are in Appendix 6, Table 37.
Our main approach was to include participants with complete 12-month follow-up data; there were 8 out of 33 (24%) relapses in EMPOWER and 13 out of 28 (46%) in TAU (Table 11). Using all available follow-up data in a time-to-first relapse analysis showed that relapse was less likely in the EMPOWER arm (hazard ratio 0.32, 95% CI 0.14 to 0.74). This is also illustrated in the Kaplan–Meier plot in Figure 8.
| Outcomea | Summary | EMPOWER | TAU | Risk rate estimate (95% CI) |
|---|---|---|---|---|
| Relapse over 12 monthsb | n/N (%) | 8/33 (24) | 13/28 (46) | ARD –0.24 (–0.43 to –0.04) |
| RR 0.50 (0.26 to 0.98) | ||||
| Time to first relapse (months)c | n: mean (SD) | 8: 5.20 (2.99) | 13: 3.63 (3.88) | HR 0.32 (0.14 to 0.74) |
| Median (IQR) | 4.8 (2.7, 7.6) | 1.4 (0.7, 7.1) | ||
| Minimum, maximum | 2, 10 | 0, 10 |
FIGURE 8.
Kaplan–Meier plot of time to first relapse.
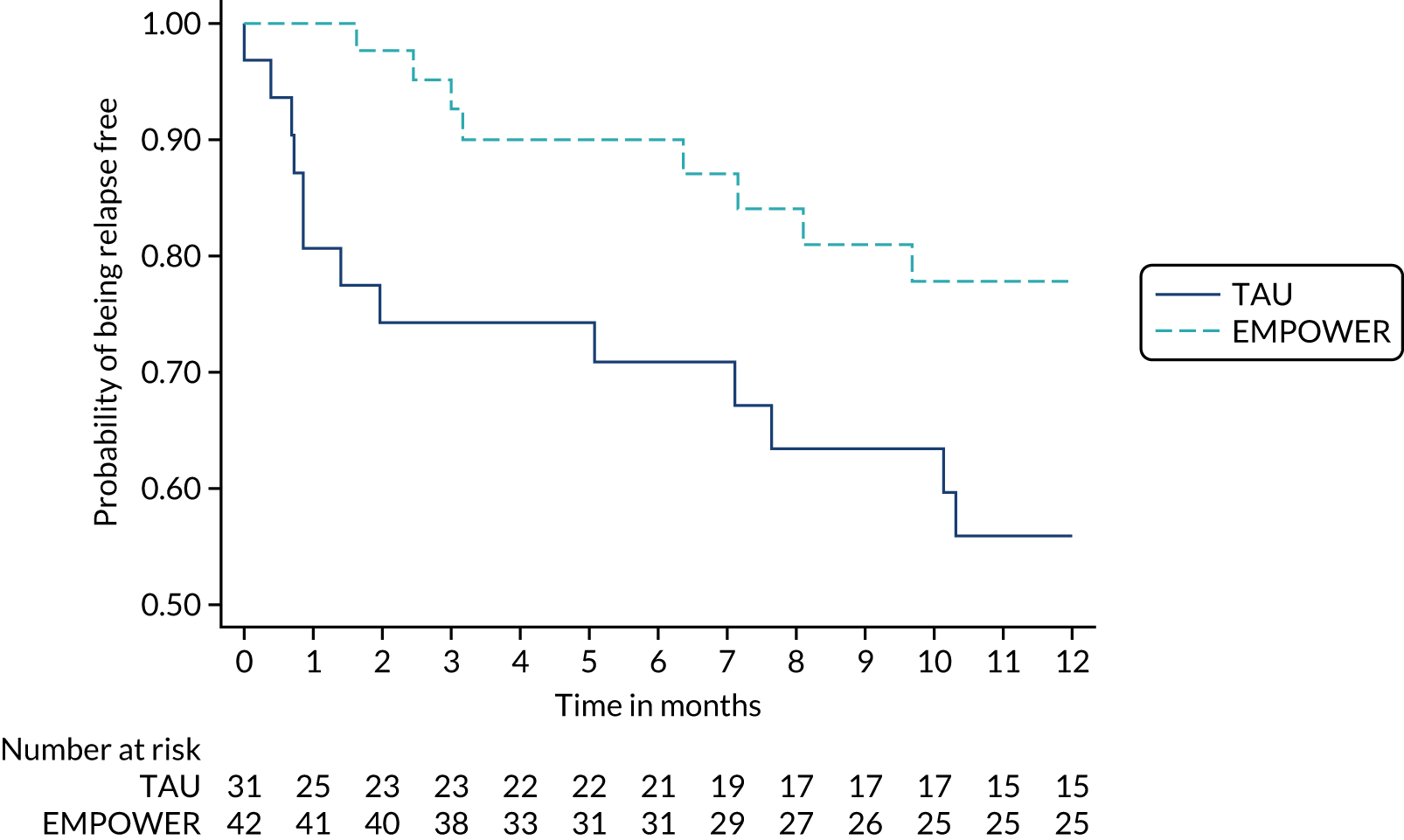
Table 11 also shows the time to first relapse, with indications that this was longer for those in the EMPOWER arm (median 4.8 months) than for those in the TAU arm (median 1.4 months).
A key concern was that the EMPOWER intervention would lead to an increased fear of relapse because of the potential that monitoring possible early signs of relapse would lead to increased anxiety and hypervigilance. Table 12 summarises the outcomes of all subscales and the total scale of the Fear of Recurrence Scale (FoRSe) at all time points, including baseline. At 12 months, FoRSe data were available for 58 (79.5%) randomised participants. We did not observe any increase in the FoRSe subscale fear of relapse over time in the EMPOWER group. however, we did observe that fear of relapse appeared to be lower in the EMPOWER arm than in the TAU arm at 12 months (mean difference –4.29, 95% CI –7.29 to –1.28; Cohen’s d = –0.76). For intrusiveness the mean difference was –1.16 (95% CI –3.84 to 1.52; Cohen’s d = –0.18) and for awareness the mean difference was –2.17 (95% CI –4.47 to 0.12; Cohen’s d = –0.36); the FoRSe total mean difference was –7.53 (95% CI –14.45 to 0.60; Cohen’s d = –0.53).
| Time point | EMPOWER | TAU | Effecta (95% CI) | Cohen’s d | ||
|---|---|---|---|---|---|---|
| N | n: mean (SD) | N | n: mean (SD) | |||
| Intrusiveness | ||||||
| Baseline | 42 | 42: 16.93 (6.01) | 31 | 30: 16.60 (6.87) | ||
| 3 months | 38 | 32: 14.41 (5.84) | 31 | 28: 16.82 (6.93) | –2.57 (–5.22 to 0.09) | –0.41 |
| 6 months | 33 | 30: 15.60 (4.95) | 30 | 25: 15.24 (7.61) | –0.10 (–2.82 to 2.62) | –0.02 |
| 12 months | 32 | 30: 14.96 (6.02) | 30 | 28: 15.82 (6.08) | –1.16 (–3.84 to 1.52) | –0.18 |
| Awareness | ||||||
| Baseline | 42 | 42: 22.63 (6.33) | 31 | 30: 19.73 (5.06) | ||
| 3 months | 38 | 32: 20.66 (4.81) | 31 | 28: 20.36 (5.66) | –1.17 (–3.44 to 1.09) | –0.20 |
| 6 months | 33 | 30: 22.00 (5.44) | 30 | 25: 19.58 (6.50) | 0.83 (–1.52 to 3.18) | 0.14 |
| 12 months | 32 | 30: 19.31 (5.47) | 30 | 28: 20.02 (6.47) | –2.17 (–4.47 to 0.12) | –0.36 |
| Fear of relapse | ||||||
| Baseline | 42 | 42: 16.25 (5.39) | 31 | 30: 16.12 (6.16) | ||
| 3 months | 38 | 32: 13.63 (4.53) | 31 | 28: 16.23 (6.12) | –2.89 (–5.88 to 0.09) | –0.51 |
| 6 months | 33 | 30: 13.97 (4.55) | 30 | 25: 15.88 (6.11) | –2.68 (–5.71 to 0.35) | –0.47 |
| 12 months | 32 | 30: 13.24 (4.73) | 30 | 28: 16.93 (5.99) | –4.29 (–7.29 to –1.28) | –0.76 |
| Total | ||||||
| Baseline | 42 | 42: 55.81 (12.89) | 31 | 30: 52.45 (15.69) | ||
| 3 months | 38 | 32: 48.69 (11.05) | 31 | 28: 53.40 (16.42) | –6.50 (–13.36 to 0.36) | –0.46 |
| 6 months | 33 | 30: 51.57 (10.49) | 30 | 25: 50.70 (18.78) | –1.86 (–8.87 to 5.14) | –0.13 |
| 12 months | 32 | 30: 47.51 (12.90) | 30 | 28: 52.77 (16.09) | –7.53 (–14.45 to -0.60) | –0.53 |
We undertook an analysis of the eight relapses in the EMPOWER arm. Of these, seven occurred during exposure to the EMPOWER intervention, and six of these were associated with a ChIP. Among the seven relapses, help-seeking was initiated by a service user on four occasions and by a carer on one occasion. In the remaining two events, the relapse was identified by a care co-ordinator during a routine follow-up appointment.
In summary, fewer participants in the EMPOWER arm had a relapse and time to first relapse was longer, and at 12 months the EMPOWER participants were less fearful of having a relapse than participants receiving TAU. For a feasibility study, these are encouraging results.
Candidate secondary outcomes
Clinical outcomes
Mental health outcomes were assessed using the PANSS, the PSP scale and the CDSS at baseline and at 3, 6 and 12 months. Our approach to establishing and monitoring rater reliability is detailed in Appendix 7. Overall rater agreement was as follows: PANSS (84%), CDSS (96%) and PSP scale (86%). Table 13 shows that, at 12 months, PANSS data were available for 55 (75%), PSP scale for 55 (75%) and CDSS for 53 (73%) of randomised participants. Outcomes at 12 months on the PANSS scales were as follows: PANSS positive (mean difference 0.67, 95% CI –1.56 to 2.91; Cohen’s d = 0.11), PANSS negative (mean difference –2.82, 95% CI –4.75 to –0.89; Cohen’s d = –0.57), PANSS disorganisation (mean difference –0.48, 95% CI –2.01 to 1.06; Cohen’s d = –0.08), PANSS excitement (mean difference –0.04, 95% CI –0.45 to 0.37; Cohen’s d = –0.03), PANSS emotional distress (mean difference –0.65, 95% CI –2.26 to 0.95; Cohen’s d = –0.17), and PANSS total (mean difference –3.75, 95% CI –8.31 to 0.81; Cohen’s d = –0.23). The mean difference was –6.09 for the PSP scale (95% CI –0.23 to 12.41; Cohen’s d = 0.35) and –1.20 for the CDSS (95% CI –2.83 to 0.44; Cohen’s d = –0.25).
| Time point | EMPOWER | TAU | Effecta (95% CI) | Cohen’s d | ||
|---|---|---|---|---|---|---|
| N | n: mean (SD) | N | n: mean (SD) | |||
| PANSS positive | ||||||
| Baseline | 42 | 42: 14.83 (5.92) | 31 | 30: 15.43 (6.68) | ||
| 3 months | 38 | 35: 13.80 (5.76) | 31 | 28: 14.46 (6.61) | 0.33 (–1.80 to 2.46) | 0.053 |
| 6 months | 33 | 30: 13.47 (6.55) | 30 | 25: 13.56 (7.03) | 0.84 (–1.39 to 3.07) | 0.136 |
| 12 months | 32 | 30: 13.27 (6.41) | 30 | 25: 13.32 (5.80) | 0.67 (–1.56 to 2.91) | 0.109 |
| PANSS negative | ||||||
| Baseline | 42 | 42: 13.90 (5.45) | 31 | 30: 12.47 (4.08) | ||
| 3 months | 38 | 35: 10.51 (3.50) | 31 | 28: 12.64 (5.03) | –2.82 (–4.68 to –0.95) | –0.57 |
| 6 months | 33 | 30: 11.27 (4.21) | 30 | 25: 12.40 (4.90) | –1.71 (–3.63 to 0.21) | –0.35 |
| 12 months | 32 | 30: 10.57 (3.95) | 30 | 25: 12.64 (5.07) | –2.82 (–4.75 to –0.89) | –0.57 |
| PANSS disorganisation | ||||||
| Baseline | 42 | 42: 15.86 (7.17) | 31 | 30: 14.63 (4.67) | ||
| 3 months | 38 | 35: 14.20 (5.87) | 31 | 28: 14.25 (3.96) | –0.38 (–1.85 to 1.09) | –0.06 |
| 6 months | 33 | 30: 13.60 (6.62) | 30 | 25: 13.56 (4.74) | 0.24 (–1.29 to 1.77) | 0.04 |
| 12 months | 32 | 30: 13.20 (5.18) | 30 | 25: 13.44 (4.23) | –0.48 (–2.01 to 1.06) | –0.08 |
| PANSS excitement | ||||||
| Baseline | 42 | 42: 4.95 (1.65) | 31 | 30: 4.33 (0.55) | ||
| 3 months | 38 | 35: 4.26 (0.44) | 31 | 28: 4.50 (1.07) | –0.36 (–0.75 to 0.02) | –0.27 |
| 6 months | 33 | 30: 4.30 (0.84) | 30 | 25: 4.64 (0.99) | –0.42 (–0.83 to –0.01) | –0.31 |
| 12 months | 32 | 30: 4.37 (0.96) | 30 | 25: 4.32 (0.63) | –0.04 (–0.45 to 0.37) | –0.03 |
| PANSS emotional distress | ||||||
| Baseline | 42 | 42: 11.95 (4.40) | 31 | 30: 12.07 (3.24) | ||
| 3 months | 38 | 35: 10.60 (3.84) | 31 | 28: 11.57 (3.62) | –0.68 (–2.21 to 0.85) | –0.17 |
| 6 months | 33 | 30: 10.47 (3.69) | 30 | 25: 10.20 (3.04) | 0.14 (–1.46 to 1.75) | 0.04 |
| 12 months | 32 | 30: 10.17 (3.97) | 30 | 25: 10.48 (3.79) | –0.65 (–2.26 to 0.95) | –0.17 |
| PANSS total | ||||||
| Baseline | 42 | 42: 61.50 (18.14) | 31 | 30: 58.93 (13.73) | ||
| 3 months | 38 | 35: 53.37 (11.72) | 31 | 28: 57.43 (13.43) | –4.45 (–8.78 to –0.12) | –0.27 |
| 6 months | 33 | 30: 53.10 (15.78) | 30 | 25: 54.36 (12.60) | –1.34 (–5.89 to 3.22) | –0.08 |
| 12 months | 32 | 30: 51.57 (12.87) | 30 | 25: 54.20 (11.13) | –3.75 (–8.31 to 0.81) | –0.23 |
| PSP scale score | ||||||
| Baseline | 42 | 42: 56.86 (16.02) | 31 | 30: 59.37 (19.29) | ||
| 3 months | 38 | 33: 55.70 (14.82) | 31 | 28: 55.32 (16.74) | 1.89 (–4.16 to 7.94) | 0.11 |
| 6 months | 33 | 30: 60.97 (14.44) | 30 | 25: 57.08 (18.13) | 3.42 (–2.89 to 9.72) | 0.20 |
| 12 months | 32 | 30: 62.07 (15.15) | 30 | 25: 58.48 (20.14) | 6.09 (–0.23 to 12.41) | 0.35 |
| CDSS total | ||||||
| Baseline | 42 | 42: 6.93 (5.34) | 31 | 30: 6.97 (4.15) | ||
| 3 months | 38 | 33: 5.67 (4.30) | 31 | 28: 5.93 (3.76) | 0.01 (–1.55 to 1.57) | 0.00 |
| 6 months | 33 | 30: 4.70 (3.30) | 30 | 25: 5.52 (4.02) | –0.87 (–2.49 to 0.75) | –0.18 |
| 12 months | 32 | 28: 5.75 (4.53) | 30 | 25: 6.60 (5.05) | –1.20 (–2.83 to 0.44) | –0.25 |
Substance use outcomes
Substance use outcomes were assessed using the Timeline Followback measure at baseline and at 3-, 6- and 12-month follow-up (Table 14). At 12 months, data on substance use were available for 62 (85%) randomised participants. At 12 months, the RR indices were as follows: alcohol (RR 1.09, 95% CI 0.94 to 1.16), cannabis (RR 0.96, 95% CI 0.91 to 1.02) and other drugs (RR 0.97, 95% CI 0.93 to 1.02).
| Time point | EMPOWER, n/N (%) | TAU, n/N (%) | RRa (95% CI) |
|---|---|---|---|
| Timeline Followback for drugs and alcohol: alcohol in last 28 days | |||
| Baseline | 15/42 (36) | 12/31 (39) | |
| 3 months | 15/38 (36) | 13/31 (42) | 0.98 (0.88 to 1.10) |
| 6 months | 13/33 (31) | 12/30 (39) | 1.04 (0.82 to 1.32) |
| 12 months | 12/32 (29) | 15/30 (48) | 1.09 (0.94 to 1.26) |
| Timeline Followback for drugs and alcohol: cannabis in last 28 days | |||
| Baseline | 7/42 (17) | 5/31 (16) | |
| 3 months | 6/38 (14) | 4/31 (13) | 0.98 (0.92 to 1.04) |
| 6 months | 4/33 (10) | 2/30 (6) | 0.98 (0.90 to 1.06) |
| 12 months | 4/32 (10) | 3/30 (10) | 0.96 (0.91 to 1.02) |
| Timeline Followback for drugs and alcohol: other main drug in last 28 days | |||
| Baseline | 4/42 (10) | 5/31 (16) | |
| 3 months | 3/38 (7) | 3/31 (10) | 0.98 (0.93 to 1.03) |
| 6 months | 1/33 (2) | 2/30 (6) | 1.00 (0.98 to 1.02) |
| 12 months | 2/32 (5) | 2/30 (6) | 0.97 (0.93 to 1.02) |
Emotional distress outcomes
Emotional distress outcomes were assessed using the HADS and the PBIQ-R at baseline and at the 3-, 6- and 12-month follow-ups (Table 15). At 12 months, data were available for 58 (80%) randomised participants. At 12 months, the mean differences were –1.40 for HADS anxiety (95% CI –3.53 to –0.47; Cohen’s d = –0.28) and –2.00 for HADS depression (95% CI –3.05 to 0.26; Cohen’s d = –0.41). For PBIQ-R, mean difference was –1.07 for control (95% CI –2.12 to –0.02; Cohen’s d = –0.42), –0.60 for shame (95% CI –1.85 to 0.50; Cohen’s d = –0.23), –1.06 for entrapment (95% CI –2.28 to 0.16; Cohen’s d = –0.34), –0.45 for loss (95% CI –1.45 to 0.55; Cohen’s d = –0.17) and 0.00 for social marginalisation (95% CI –1.41 to 1.42; Cohen’s d = –0.00).
| Time point | EMPOWER | TAU | Effecta (95% CI) | Cohen’s d | ||
|---|---|---|---|---|---|---|
| N | n: mean (SD) | N | n: mean (SD) | |||
| HADS anxiety | ||||||
| Baseline | 42 | 41: 9.76 (5.11) | 31 | 30: 10.70 (4.88) | ||
| 3 months | 38 | 32: 9.34 (5.42) | 31 | 27: 11.19 (4.70) | –1.00 (–2.64 to 0.65) | –0.20 |
| 6 months | 33 | 30: 9.30 (4.70) | 30 | 25: 9.48 (4.70) | 0.44 (–1.25 to 2.14) | 0.09 |
| 12 months | 32 | 30: 8.80 (4.75) | 30 | 28: 10.64 (4.13) | –1.40 (–3.05 to 0.26) | –0.28 |
| HADS depression | ||||||
| Baseline | 42 | 40: 7.38 (4.98) | 31 | 29: 8.03 (4.66) | ||
| 3 months | 38 | 32: 6.22 (4.59) | 31 | 27: 8.85 (4.27) | –1.81 (–3.33 to –0.29) | –0.38 |
| 6 months | 33 | 29: 7.07 (4.92) | 30 | 25: 8.68 (3.58) | –1.07 (–2.64 to 0.49) | –0.22 |
| 12 months | 32 | 30: 7.13 (4.75) | 30 | 28: 9.54 (3.87) | –2.00 (–3.53 to –0.47) | –0.41 |
| PBIQ-R control | ||||||
| Baseline | 42 | 41: 10.29 (2.52) | 31 | 30: 9.83 (2.61) | ||
| 3 months | 38 | 32: 9.66 (2.46) | 31 | 28: 9.43 (2.77) | –0.08 (–1.11 to 0.96) | –0.03 |
| 6 months | 33 | 30: 9.37 (2.24) | 30 | 26: 9.62 (2.59) | –0.55 (–1.62 to 0.52) | –0.22 |
| 12 months | 32 | 30: 9.08 (3.01) | 30 | 28: 9.79 (1.93) | –1.07 (–2.12 to –0.02) | –0.42 |
| PBIQ-R shame | ||||||
| Baseline | 42 | 41: 10.10 (2.96) | 31 | 30: 10.67 (3.07) | ||
| 3 months | 38 | 32: 9.97 (2.97) | 31 | 28: 10.42 (3.11) | –0.14 (–1.30 to 1.03) | –0.05 |
| 6 months | 33 | 30: 10.06 (2.76) | 30 | 25: 10.32 (2.56) | 0.02 (–1.18 to 1.22) | 0.01 |
| 12 months | 32 | 30: 9.57 (3.21) | 30 | 28: 10.36 (2.33) | –0.68 (–1.85 to 0.50) | –0.26 |
| PBIQ-R entrapment | ||||||
| Baseline | 42 | 41: 10.73 (2.88) | 31 | 30: 10.80 (3.38) | ||
| 3 months | 38 | 32: 10.00 (3.10) | 31 | 28: 10.00 (3.37) | –0.07 (–1.28 to 1.13) | –0.024 |
| 6 months | 33 | 30: 9.47 (2.73) | 30 | 26: 10.46 (2.32) | –1.05 (–2.28 to 0.19) | –0.341 |
| 12 months | 32 | 30: 9.70 (3.15) | 30 | 28: 10.62 (2.48) | –1.06 (–2.28 to 0.16) | –0.344 |
| PBIQ-R loss | ||||||
| Baseline | 42 | 41: 9.84 (2.82) | 31 | 30: 10.20 (2.54) | ||
| 3 months | 38 | 32: 9.46 (3.14) | 31 | 28: 10.32 (2.99) | –0.64 (–1.62 to 0.35) | –0.24 |
| 6 months | 33 | 30: 9.93 (2.74) | 30 | 26: 10.67 (2.26) | –0.47 (–1.48 to 0.54) | –0.17 |
| 12 months | 32 | 30: 9.91 (2.76) | 30 | 28: 10.54 (2.56) | –0.45 (–1.45 to 0.55) | –0.17 |
| PBIQ-R socially marginalised | ||||||
| Baseline | 42 | 41: 11.64 (2.89) | 31 | 30: 11.87 (2.75) | ||
| 3 months | 38 | 32: 11.13 (3.63) | 31 | 28: 11.79 (3.61) | –0.50 (–1.90 to 0.90) | –0.18 |
| 6 months | 33 | 30: 11.13 (2.75) | 30 | 26: 11.46 (2.10) | –0.09 (–1.53 to 1.35) | –0.03 |
| 12 months | 32 | 30: 11.33 (3.46) | 30 | 28: 11.61 (2.25) | 0.00 (–1.41 to 1.42) | 0.00 |
Service engagement and adherence
Service engagement outcomes were measured using the Service Attachment Questionnaire. Medication adherence was measured used the Medication Adherence Rating Scale. Both measures were administered at baseline and at the 3-, 6- and 12-month follow-ups (Table 16). At 12 months, data were available for 58 (80%) randomised participants. The outcomes at 12 months were mean difference of 0.04 on the Service Attachment Questionnaire (95% CI –0.09 to 0.16; Cohen’s d = 0.15) and mean difference of –0.84 on the Medication Adherence Rating Scale (95% CI –1.66 to –0.01; Cohen’s d = 0.50).
| Time point | EMPOWER | TAU | Effecta (95% CI) | Cohen’s d | ||
|---|---|---|---|---|---|---|
| N | n: mean (SD) | N | n: mean (SD) | |||
| Service Attachment Questionnaire | ||||||
| Baseline | 42 | 41: 2.36 (0.24) | 31 | 30: 2.35 (0.25) | ||
| 3 months | 38 | 32: 2.29 (0.30) | 31 | 28: 2.30 (0.31) | –0.05 (–0.17 to 0.07) | –0.21 |
| 6 months | 33 | 30: 2.29 (0.27) | 30 | 26: 2.31 (0.30) | –0.11 (–0.23 to 0.02) | –0.44 |
| 12 months | 32 | 30: 2.37 (0.28) | 30 | 28: 2.27 (0.37) | 0.04 (–0.09 to 0.16) | 0.15 |
| Medication Adherence Rating Scale | ||||||
| Baseline | 42 | 42: 3.83 (1.69) | 31 | 30: 4.04 (1.64) | ||
| 3 months | 38 | 32: 3.77 (1.52) | 31 | 27: 4.44 (1.78) | –0.50 (–1.31 to 0.32) | –0.30 |
| 6 months | 33 | 30: 4.23 (1.92) | 30 | 26: 4.35 (1.69) | –0.08 (–0.92 to 0.75) | –0.05 |
| 12 months | 32 | 30: 3.47 (1.56) | 30 | 28: 4.43 (1.89) | –0.84 (–1.66 to -0.01) | –0.50 |
Candidate mechanism outcomes
Table 17 summarises the outcomes for the Questionnaire for Personal Recovery, the General Self Efficacy Scale and the Psychosis Attachment Measure at baseline and at 3, 6 and 12 months. At 12 months, data were available for 62 (85%) randomised participants. The outcomes at 12 months were mean difference of 3.41 for the Questionnaire for Personal Recovery (95% CI –0.51 to 7.32; Cohen’s d = 0.32), 1.91 for the General Self Efficacy Scale (95% CI –0.40 to 4.21, Cohen’s d = 0.33), –0.13 for the Psychosis Attachment Measure Avoidance (95% CI –0.36 to 0.09; Cohen’s d = –0.22) and –0.05 for the Psychosis Attachment Measure Anxiety (95% CI –0.31 to 0.20; Cohen’s d = –0.07).
| Time point | EMPOWER | TAU | Effecta (95% CI) | Cohen’s d | ||
|---|---|---|---|---|---|---|
| N | n: mean (SD) | N | n: mean (SD) | |||
| Questionnaire for Personal Recovery score | ||||||
| Baseline | 42 | 41: 37.66 (11.09) | 31 | 30: 36.67 (10.31) | ||
| 3 months | 38 | 32: 39.81 (10.10) | 31 | 28: 37.39 (10.66) | 2.45 (–1.42 to 6.32) | 0.23 |
| 6 months | 33 | 30: 40.12 (11.12) | 30 | 26: 37.35 (7.62) | 2.29 (–1.69 to 6.26) | 0.21 |
| 12 months | 32 | 30: 38.35 (12.01) | 30 | 28: 35.34 (9.70) | 3.41 (–0.51 to 7.32) | 0.33 |
| General Self Efficacy Scale | ||||||
| Baseline | 42 | 42: 27.12 (6.20) | 31 | 30: 26.10 (5.44) | ||
| 3 months | 38 | 32: 27.09 (7.28) | 31 | 28: 26.68 (5.33) | –0.20 (–2.48 to 2.09) | –0.03 |
| 6 months | 33 | 30: 28.23 (7.30) | 30 | 25: 26.45 (5.17) | 1.08 (–1.28 to 3.43) | 0.18 |
| 12 months | 32 | 30: 27.40 (6.77) | 30 | 28: 25.05 (5.75) | 1.91 (–0.40 to 4.21) | 0.33 |
| Psychosis Attachment Measure avoidance | ||||||
| Baseline | 42 | 41: 1.52 (0.59) | 31 | 30: 1.44 (0.60) | ||
| 3 months | 38 | 32: 1.40 (0.73) | 31 | 28: 1.60 (0.56) | –0.31 (–0.53 to –0.09) | –0.52 |
| 6 months | 33 | 30: 1.37 (0.62) | 30 | 26: 1.46 (0.56) | –0.18 (–0.41 to 0.05) | –0.31 |
| 12 months | 32 | 30: 1.48 (0.67) | 30 | 28: 1.50 (0.50) | –0.13 (–0.36 to 0.09) | –0.22 |
| Psychosis Attachment Measure anxiety | ||||||
| Baseline | 42 | 41: 1.26 (0.74) | 31 | 30: 1.27 (0.73) | ||
| 3 months | 38 | 32: 1.28 (0.81) | 31 | 28: 1.32 (0.73) | 0.01 (–0.25 to 0.26) | 0.01 |
| 6 months | 33 | 30: 1.09 (0.68) | 30 | 26: 1.18 (0.75) | –0.08 (–0.34 to 0.18) | –0.11 |
| 12 months | 32 | 30: 1.24 (0.87) | 30 | 28: 1.26 (0.83) | –0.05 (–0.31 to 0.20) | –0.07 |
Carer and care co-ordinator outcomes
Carers
Descriptions of carer outcomes using the Involvement Evaluation Questionnaire at baseline and at 3, 6 and 12 months are presented in Table 18. At 12 months, data were available for eight (47%) consented carers. Only descriptive data are provided for information and transparency purposes.
| Time point | EMPOWER | TAU | ||
|---|---|---|---|---|
| N | n: mean (SD) | N | n: mean (SD) | |
| Involvement Evaluation Questionnaire tension | ||||
| Baseline | 7 | 7: 7.32 (4.30) | 10 | 10: 5.24 (3.62) |
| 3 months | 6 | 5: 8.95 (5.72) | 10 | 6: 4.83 (3.60) |
| 6 months | 6 | 5: 5.00 (1.87) | 10 | 6: 6.00 (4.15) |
| 12 months | 6 | 4: 7.69 (2.22) | 10 | 4: 5.00 (2.16) |
| Involvement Evaluation Questionnaire supervision | ||||
| Baseline | 7 | 7: 4.07 (2.86) | 10 | 10: 4.60 (4.55) |
| 3 months | 6 | 5: 7.80 (6.30) | 10 | 6: 4.33 (5.09) |
| 6 months | 6 | 5: 3.80 (3.27) | 10 | 6: 6.50 (7.23) |
| 12 months | 6 | 4: 5.75 (3.86) | 10 | 4: 4.25 (2.50) |
| Involvement Evaluation Questionnaire worrying | ||||
| Baseline | 7 | 7: 11.29 (7.06) | 10 | 10: 7.30 (3.43) |
| 3 months | 6 | 5: 12.60 (6.27) | 10 | 6: 6.83 (3.37) |
| 6 months | 6 | 5: 8.60 (5.50) | 10 | 6: 7.00 (2.45) |
| 12 months | 6 | 4: 10.95 (7.20) | 10 | 4: 6.25 (2.06) |
| Involvement Evaluation Questionnaire urging | ||||
| Baseline | 7 | 7: 13.43 (9.40) | 10 | 10: 10.30 (3.30) |
| 3 months | 6 | 5: 14.20 (6.10) | 10 | 6: 9.17 (3.87) |
| 6 months | 6 | 5: 10.80 (6.22) | 10 | 6: 10.00 (5.18) |
| 12 months | 6 | 3: 8.67 (1.41) | 10 | 4: 8.75 (2.22) |
| Involvement Evaluation Questionnaire total | ||||
| Baseline | 7 | 7: 34.88 (19.35) | 10 | 10: 26.39 (9.53) |
| 3 months | 6 | 5: 41.98 (22.10) | 10 | 6: 24.08 (12.18) |
| 6 months | 6 | 5: 27.50 (13.66) | 10 | 6: 28.33 (15.76) |
| 12 months | 6 | 4: 32.67 (12.05) | 10 | 4: 23.13 (6.47) |
Care co-ordinators
Data for care co-ordinators’ assessment of service user engagement with services using the Service Engagement Scale at baseline and at 3, 6 and 12 months are summarised in Table 19. At 12 months, data were available for 31 (42%) randomised service user participants. Lower scores on this scale are indicative of better service engagement. The outcomes at 12 months were mean difference of –0.16 for availability (95% CI –1.20 to 0.88; Cohen’s d = 0.59), 0.54 for collaboration (95% CI –0.77 to 1.85; Cohen’s d = 0.26), 1.03 for help-seeking (95% CI –0.49 to 2.55; Cohen’s d = 0.33), 1.15 for treatment adherence (95% CI –0.21 to 2.09; Cohen’s d = 0.58) and 2.50 for treatment total (95% CI –0.79 to 5.79; Cohen’s d = 0.37).
| Time point | EMPOWER | TAU | Effecta (95% CI) | Cohen’s d | ||
|---|---|---|---|---|---|---|
| N | n: mean (SD) | N | n: mean (SD) | |||
| Service Engagement Scale availabilityb | ||||||
| Baseline | 42 | 39: 0.74 (1.37) | 31 | 25: 1.52 (2.14) | ||
| 3 months | 38 | 30: 0.83 (1.72) | 31 | 23: 0.87 (1.46) | 0.41 (–0.45 to 1.28) | 0.24 |
| 6 months | 33 | 23: 0.83 (1.87) | 30 | 13: 0.46 (0.66) | 0.28 (–0.71 to 1.27) | 0.16 |
| 12 months | 32 | 19: 0.74 (1.66) | 30 | 12: 1.00 (1.28) | –0.16 (–1.20 to 0.88) | 0.09 |
| Service Engagement Scale collaborationb | ||||||
| Baseline | 42 | 39: 2.62 (2.07) | 31 | 25: 2.72 (2.15) | ||
| 3 months | 38 | 30: 1.77 (1.87) | 31 | 23: 2.26 (2.07) | –0.58 (–1.63 to 0.48) | 0.28 |
| 6 months | 33 | 23: 1.65 (1.67) | 30 | 13: 2.00 (1.91) | –0.64 (–1.88 to 0.61) | –0.31 |
| 12 months | 32 | 19: 3.00 (1.83) | 30 | 12: 2.67 (2.71) | 0.54 (–0.77 to 1.85) | 0.26 |
| Service Engagement Scale help-seekingb | ||||||
| Baseline | 42 | 39: 3.68 (3.11) | 31 | 25: 3.89 (3.22) | ||
| 3 months | 38 | 29: 3.45 (3.16) | 31 | 23: 2.70 (2.57) | 0.61 (–0.58 to 1.79) | 0.19 |
| 6 months | 33 | 23: 2.57 (2.11) | 30 | 13: 2.26 (2.97) | 0.19 (–1.23 to 1.62) | 0.06 |
| 12 months | 32 | 19: 2.95 (2.86) | 30 | 12: 2.42 (3.00) | 1.03 (–0.49 to 2.55) | 0.33 |
| Service Engagement Scale treatment adherenceb | ||||||
| Baseline | 42 | 39: 1.44 (1.74) | 31 | 25: 1.52 (2.35) | ||
| 3 months | 38 | 29: 0.90 (1.59) | 31 | 23: 1.00 (1.83) | –0.16 (–0.96 to 0.65) | –0.08 |
| 6 months | 33 | 23: 0.83 (1.34) | 30 | 13: 0.69 (0.75) | 0.09 (–0.81 to 1.00) | 0.05 |
| 12 months | 32 | 19: 1.42 (1.80) | 30 | 12: 0.67 (1.23) | 1.15 (0.21 to 2.09) | 0.58 |
| Service Engagement Scale treatment totalb | ||||||
| Baseline | 42 | 39: 8.47 (6.13) | 31 | 25: 9.65 (7.76) | ||
| 3 months | 38 | 29: 7.03 (6.28) | 31 | 23: 6.83 (6.09) | 0.56 (–2.08 to 3.20) | 0.08 |
| 6 months | 33 | 23: 5.87 (5.04) | 30 | 13: 5.41 (4.69) | –0.17 (–3.28 to 2.95) | –0.03 |
| 12 months | 32 | 19: 8.11 (5.94) | 30 | 12: 6.75 (6.28) | 2.50 (–0.79 to 5.79) | 0.37 |
Sensitivity analyses
Small numbers of clusters
Despite the use of random-effects models, the estimates from these models may not be robust because of the small number of clusters. To investigate the impact of this, the models presented above for continuous (or pseudo-continuous) outcomes were compared with those using the Kenward–Roger correction216 in Stata® version 15 (Stata Corp LP, College Station, TX, USA). The point estimates were similar to the original analyses, as were the Cohen’s d estimates. These were the measures used to assess the outcomes. Therefore, the interpretation of the original models remains unchanged, although this should be done with caution given that the models are based on a feasibility study.
Impact of possible informative withdrawal
The possibility of informative withdrawal was also investigated, with several scenarios considered. There are too few data points to conduct the usual type of pattern mixture with multiple imputed data sets using the full model. To get an initial feel of the impact, a simple pattern mixture investigation using a basic logistic model was used. This indicated that the point estimates were stable if around 50% of those who withdrew were set to ‘relapse’. Next, we examined several different scenarios using the more complex full model. This included (1) the best outcome – all who withdrew (without primary outcome data) were set as ‘no relapse’; (2) the worst outcome – all who withdrew were set as ‘relapse’; and (3) all points in between.
A conservative rate of ‘relapse’ for these withdrawals would be the rate observed in the TAU arm (46%) implying that no more than three might have been expected to ‘relapse’ had they remained in the study. However, a RR of 0.70 is a clinically significant effect for relapse reduction. Using the pattern mixture scenarios and the full models indicated that while standard errors increased there had to be 6 or more of these withdrawals set to ‘relapse’ for the RR to increase beyond 0.70. The original intention-to-treat (ITT) estimate was 0.50. Using the scenarios if 5 withdrawals were set to ‘relapse’ this still only increased the estimate to 0.69.
Chapter 6 Health economic analysis
Introduction
The aim of the health economic analysis was to test the feasibility, acceptability and usability of collecting economic measures in the EMPOWER trial for service users and carers, and to inform the design of an economic evaluation alongside a full trial of the EMPOWER intervention. The objectives of the health economic analysis included the development and testing of an economic resource use questionnaire (one each for service users and carers), collecting quality-of-life data using the EQ-5D-5L217 and AQoL-8D157 for service users and the EQ-5D-5L217 and CarerQoL-7D218 for carers. We planned to report missingness for all measures, test the data for usability in carrying out an economic evaluation, make recommendations for economic data collection in a full trial, identify key cost drivers and establish the cost of the EMPOWER intervention.
Methods
Overview
The report of this health economic evaluation followed the Consolidated Health Economics Evaluation Reporting Standards (CHEERS) guidance. 219 The economic evaluation was conducted using individual participant cost and effect data collected alongside the EMPOWER trial. The time horizon of the analysis was 12 months with four assessment points: baseline and 3-, 6- and 12-month follow-up.
Study perspective
Different perspectives were adopted for the economic analysis, reflecting the national health technology assessment guidelines in Australia and the UK. 220,221 The base-case scenario adopted three perspectives. First, a health-care payer perspective was adopted; this perspective was limited to costs borne directly by the UK NHS or Australian government (e.g. primary, secondary and community services) and outcomes related to service users (EQ-5D-5L217 and AQoL-8D157). Second, a health-care sector perspective was adopted; this perspective extended the health-care payer perspective to include medical costs paid for by patient out-of-pocket expenses and third-party payers. These additional costs are of particular relevance to the Australia health-care system. The final perspective adopted was societal; this includes all of the costs considered in the health-care sector perspective plus additional relevant costs outside the health-care sector. These additional costs include service user time for using the app, informal care, productivity loss, costs of online self-help services and contacts with the legal, criminal and justice departments. The societal perspective also includes additional outcomes; in the case of EMPOWER, this included outcomes for carers (EQ-5D-5L217 and CarerQoL-7D218).
Identification, measurement and valuation of resource use
Guided by the different perspectives adopted in this study, different cost components were identified: costs related to the intervention, health service use costs, costs outside the health-care sector and costs directly incurred by service users and their carers. All costs were collected based on the 2017 reference year and were expressed as Great British pounds. Australian unit costs were converted to pounds using the CCEMG–EPPI-Centre Cost Converter. 222 As the costs were collected over a 1-year period, discounting was not applied.
Intervention cost
A micro-costing approach was undertaken that allowed for a precise assessment of the economic costs of the EMPOWER app. Costing consisted of two parts: collecting detailed data on resources utilised (i.e. measuring quantities of resource use) and assigning unit costs or prices. Unlike the gross-costing approach that uses aggregate-level estimates, micro-costing is particularly useful for estimating the costs of new interventions when there is no established estimate of their aggregate costs. In the calculations of the costs of the EMPOWER app, detailed cost information was provided by the principal investigators.
The cost of the intervention included initial R&D costs, ongoing maintenance costs and costs of delivering the intervention. Costs can be categorised into direct costs, such as costs incurred in implementing and running the intervention (e.g. labour, consumable, capital), and indirect costs, which refer to productivity loss in paid and unpaid economic activity as a result of premature death or disability. Direct costs can be incurred in the health sector or outside the health-care sectors. A further distinction can be made between fixed costs, which do not change with an increase in additional users of the intervention, and variable costs, which do vary with the quantity of users. It is important to separate fixed costs from variable costs, as variable costs are the important drivers of scaling up the intervention. The intervention costs are summarised in Appendix 8, Table 40, and further discussed in the following paragraphs.
Research and development costs comprised the costs associated with the development of the EMPOWER content, the actual app development and the registration of the app as a medical device. Although R&D costs are generally considered as a ‘sunk cost’ in economics, which would not need to be repeated if the intervention were adopted on a broader scale,223 these still constitute important opportunity costs, as the resources could have been spent on other health interventions and services. These costs, generally, are an important component of the overall costs224 and previous economic evaluations have included development costs in their analyses. 225 However, allocating these costs to study service users in the intervention arm is inappropriate and does not accurately reflect the true cost per service user once the intervention has been disseminated to a wider audience. Therefore, the R&D costs were attributed to all potential users of EMPOWER. Thereby, we estimated the number of people likely to receive the intervention when it is implemented in the Australian and UK contexts using assumptions based on the published literature.
The intervention pathway starts with adults aged ≥ 16 years living in Australia (n = 20,290,033)226 and the UK (n = 53,257,957). 227 Published evidence has found a 0.4% prevalence among the population for schizophrenia-related disorder,228 which was estimated to be 294,192 for the combined population across Australia and the UK. The rate of relapse was based on a previous review of relapse rates among people with a diagnosis of schizophrenia in receipt of maintenance antipsychotic medication, which was estimated to be 27%. 229 This reduces the number of potential users to 79,432 individuals. For this cohort, a conservative assumption was made that approximately half of those would be interested in using EMPOWER. This leads to an average R&D cost per person of £3.27.
Ongoing maintenance costs included app hosting and labour costs for app maintenance. Both cost categories can be considered fixed costs that do not change if additional service users are enrolled in the intervention. Similar to R&D costs, these costs need to be allocated to all potential users of EMPOWER. The average ongoing maintenance costs per person was estimated to be £0.37.
The costs of the intervention delivery were the most relevant component that can be broken down into capital costs, consumables and labour costs.
Capital costs are costs associated with the purchase of the major capital assets required that represent investments at a single point in time. This comprises the costs of smartphone devices for using the EMPOWER app. An Android operating system is required to use the EMPOWER app. Some study service users owned their own smartphone and only for some service users was the purchase of a new smartphone required. In costing the intervention, these costs that occurred for some service users were distributed to all service users in the intervention arm, with an average price of £109.52 per service user. Data connection, which represents consumable costs, was costed at £69.30 per service user for the 12-month study period.
Labour costs were associated with:
-
the training of care co-ordinators
-
the training of research assistants (Australia) and research nurses (UK) for monitoring EWS
-
peer support workers’ engagement with service users
-
the time spent using the app intervention by service users
-
the routine monitoring of EWS
-
the ongoing supervision of CMHS staff.
-
Despite some variations between countries, the training of care co-ordinators generally consisted of a half-day workshop (lasting around 3 hours) provided by a consultant clinical psychologist. Each workshop consisted, usually, of six care co-ordinators, a peer support worker and a research assistant (in Australia) or a research mental health nurse (in the UK) who was responsible for monitoring EWS. The training cost of EMPOWER was determined based on 28 care co-ordinators recruited for the EMPOWER study.
-
Monitoring training consisted of approximately 4 hours of training provided by a consultant clinical psychologist to a research mental health nurse in Glasgow and a research assistant in Melbourne.
-
The role of the peer support worker in EMPOWER involved introducing the rationale for using the app to the service user, setting up the app on the phone and supporting the individual’s familiarisation with the handset and app functions. During the initial 4-week period, the peer support worker provided additional support through weekly telephone follow-up. After this period, the peer support worker provided ongoing fortnightly support by encouraging the service user to use the app and by checking on the service user’s well-being.
-
The time spent using the EMPOWER app is an important opportunity cost from the service user perspective and needs to be considered as part of the societal perspective. 223 As it was not possible to track the actual time service users spent on the app, the minimum amount of 2 minutes per day was estimated that was necessary to answer a daily questionnaire about potential EWS of psychosis. The time cost was estimated based on the opportunity cost method, using 25% of the average national income that reflected the value of the leisure activity forgone.
-
The routine monitoring of potential EWS of psychosis was undertaken by a research mental health nurse in Glasgow and a research assistant in Melbourne.
-
The final component was the ongoing supervision of CMHS staff. The supervision was provided by a consultant clinical psychologist from the study team. The peer support worker and the research co-ordinator also attended those sessions. During the 12-month study period, there were nine 1-hour supervision sessions, with an average of three CMHS staff attending each session. Importantly, these supervision sessions were conducted in Australia only, as in the UK the research nurse liaised with CMHS staff.
Depending on the different perspective adopted, the final intervention costs were £2202 from a health-care payer perspective, £2380 from a health-care sector perspective and £2447 from a societal perspective. These intervention costs were assigned to everyone in the intervention arm according to the perspective under consideration.
Health service use costs
We worked with different health service systems in the UK and Australia to produce resource use questionnaires applicable to both contexts for service users and carers (available on request from the corresponding author). We used the feasibility study to refine the capture and measurement of economic data.
Payers and services in the two countries are different and some services have different names. Health services included publicly funded services and voluntary sector services in both countries, as well as private services in Australia (specialised mental health clinical services). Data on service user and carer resource use were collected at baseline and at 3, 6 and 12 months for the period prior to the last data collection (except the baseline questionnaire, which collected information for the previous 3 months).
Health service use costs in the service user resource use questionnaire included primary health-care services (e.g. GP and mental health nurse), hospital services, non-hospital mental health care (e.g. respite and residential care), diagnostic tests, mental health-related medication and non-mental health-related medication. A carer-specific resource use questionnaire was also developed, which covered health professional visits, hospital services, diagnostic tests, medication and informal care (available on request from the corresponding author).
There is little guidance on how to value resource use in multicountry trials. 230,231 Given the size of this feasibility study, no attempt was made to quantitatively adjust for differences in each country’s resource use. Country-specific results are presented by pooling all resource use and applying jurisdiction-specific unit cost estimates. Therefore, this study employed a multicountry costing approach, whereby unit cost estimates were applied from individual countries. 232
The UK context unit costs were taken from NHS Reference Costs233 and Personal Social Services Research Unit234 for health-care costs, from the British National Formulary235 for medication costs and from the literature for other costs. A detailed listing of UK unit costs is provided in Appendix 9, Table 41. For Australia, the unit costs (see Table 42) for different resources used were obtained from the Medicare Benefits Schedule (MBS) book,236 the Pharmaceutical Benefits Scheme,237 the National Hospital Cost Data Collection Cost Report (Round 21),238 the Australian Institute of Health and Welfare Health Expenditure Report,239 and the Victoria Mental Health Services Annual Report 2016/17. 240
Costs directly incurred by service users and their carers
Costs directly incurred by service users consisted of out-of-pocket costs for health-care services as well as out-of-pocket costs for (online) self-help services that included formal online therapy, smartphone self-help apps, other self-help materials such as books or DVDs, gym memberships and other services used for mental health. Costs directly incurred by carers included out-of-pocket costs for health-care services. Costs directly incurred by service users and their carers were considered only when adopting a health-care sector or societal perspective (i.e. not when adopting a health-care payer perspective).
Costs outside the health-care sector
Costs outside the health-care sector included criminal justice, productivity (absenteeism and presenteeism) and informal care, and were considered only when adopting a societal perspective. In terms of criminal justice, this included the number of contacts with the police, the number of nights spent in a police cell or prison, psychiatric assessment while in custody and the number of court appearances. Costs of contact with police and criminal court were sourced from the Bedfordshire/Cambridgeshire/Hertfordshire Fees & Charges Handbook (in the UK)241 and the Australian Government Productivity Commission (in Australia),242 and Costs of the Criminal Justice System in Scotland Dataset 2018 (in the UK)243 and the Australian Bureau of Statistics (in Australia). 244 Productivity costs included both absenteeism and presenteeism. Absenteeism for paid work was valued at the national average hourly rate in Australia and the UK, plus 25% on-costs. Presenteeism was measured using a modified version of the Work Productivity and Activity Impairment Questionnaire. 245 The costs of productivity loss as a result of presenteeism were calculated by multiplying the national average hourly rate (+25% on-costs) by the formula [(1 – X/10) × lost hours], where X denotes the score on the Likert scale included in the Work Productivity and Activity Impairment Questionnaire. The Likert scale ranges from 0 to 10, with higher scores indicating better work capacity. Informal care was measured from the perspective of service users who reported the number of hours of extra help they received from friends or relatives (unpaid help) with different tasks (hours spent on childcare, household activities, help outside the home, personal care and other help). Informal care hours were valued using the opportunity cost method, by multiplying the hours of informal care by the national minimum wage in Australia and the UK. While the resource use questionnaire completed by carers also contained questions related to informal care, the informal care cost for the base-case analysis was based on service users’ self-report of informal care. Finally, service users were asked to report the number of hours of work their friends or relatives took off because of their mental or physical health. Carers’ productivity loss was valued at the national average hourly rate, plus 25% on-costs.
Identification, measurement and valuation of outcome measures
For service users, we tested two health-related quality-of-life measures, both of which can be used to estimate QALYs: the EQ-5D-5L217 and the AQoL-8D. 157
The EQ-5D-5L is used frequently in the UK and Australian contexts. It is a generic questionnaire with five questions covering mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each of these questions has a possible five levels of response. However, a previous review has indicated mixed findings in terms of the measure’s responsiveness to change and convergent validity. 169 Therefore, the EQ-5D-5L was complemented with the newer AQoL-8D, which was designed to be sensitive to the aspects of quality of life important to people with mental health problems. The AQoL-8D contains 35 items, each of which has between four and six possible responses. It has eight dimensions, which are further summarised as super-dimension physical and super-dimension psychosocial.
The outcome measures were valued using preference-based value sets. Although a value set has been published for the EQ-5D-5L for England,156 currently the National Institute for Health and Care Excellence (NICE) does not recommend the use of this measure. 246 Therefore, the EQ-5D-5L utility scores were mapped on to the EQ-5D-3L using the EQ5Dmap command in Stata. 247 As there is currently no Australian value set available for the EQ-5D-5L, the UK value set was used and then also mapped on to the EQ-5D-3L, reflecting the preferences of members of the public in the UK. Finally, the AQoL-8D responses were converted to utilities using the scoring algorithm developed by Richardson et al. 157
With regard to carer outcomes, two outcome measures were included in the carer survey to capture the impact of EMPOWER on carers’ health-related quality of life, using the EQ-5D-5L, and informal care, using the CarerQoL-7D. 218 Carers’ EQ-5D-5L responses were also mapped on to the EQ-5D-3L, whereas the UK utility weights were used for the CarerQoL-7D. 248
All outcome measures were administered at baseline and at 3, 6 and 12 months by trained research assistants.
Missing data
The extent of missing data was examined and assessed to establish the completion rates of the RUQ and outcome measures. Missing data are a well-known issue in cost-effectiveness analyses alongside clinical trials. Instead of conducting a complete-case analysis that is likely to result in bias in economic evaluation, multiple imputation by chained equations was applied to impute missing values for cost and effects. The analysis was performed in Stata using the ‘ice’ package and Stata codes developed by Faria et al. 249 In total, 34 data sets were imputed that reflected the percentage of cumulative missing values. The costs and outcome analysis models were then run across the 34 imputed data sets and combined using Rubin’s rules250 to produce a single set of results.
Analysis
The analysis carried out was according to the ITT principle, which implies that service users will be analysed in accordance with their allocated treatment arm, irrespective of the treatment they received. The differences in resource use between the groups is presented without costs attached to exclude any issues with valuing resource use. The results are unadjusted and include available cases to make use of all data. ‘Available cases’ include all complete data collected during the trial, irrespective of whether, for a particular participant, this included all follow-up points. All outcome measures were assessed for their suitability for collection during the definitive trial. The utility scores derived from the outcome measures are presented for available cases for each arm without adjustment to make use of all data.
Resource use and unit costs were then combined to give a total cost for each participant. The primary type of analysis was a cost–utility analysis, using QALYs as a measure of outcome derived from the EQ-5D-5L measure. For both arms, the mean values of costs and outcomes are reported, as well as the mean differences between the groups. Generalised linear models were used to assess the mean difference in costs and outcomes between the arms, adjusted for baseline characteristics (i.e. baseline utility, baseline costs, country, age and gender). For mean differences in total costs, a gamma distribution and an identity link were applied, whereas for the incremental effect in QALYs, a Gaussian distribution and identity link were used. The Glass’s Delta effect size was calculated (i.e. the difference between the mean scores of the EMPOWER and TAU arms divided by the standard deviation of the TAU arm) for all costs and outcomes, with the recommended minimum effect size defined as Δ = 0.41, moderate Δ = 1.15, and strong Δ = 2.70. 251
Once the difference in cost and QALYs had been estimated, a joint assessment of the two was reported using an incremental approach, with differences in mean costs and mean outcome scores expressed using a ratio of incremental cost per QALY [i.e. an incremental cost-effectiveness ratio (ICER)]:
The non-parametric ‘bootstrapping’ technique was then used with 1000 iterations of incremental cost and incremental effect so that sampling uncertainty could be graphically plotted on a cost-effectiveness plane. Cost-effectiveness planes are graphical displays of cost-effectiveness results plotted on a two-by-two-dimension graph where the horizontal axis represents the differences in outcomes (in this case QALYs) and the vertical axis represent the differences in costs. Results in the north-east quadrant show that the intervention costs more but also has greater benefits than the comparator, whereas results in the south-east quadrant show that the intervention costs less but has greater benefits than the comparator (this is referred to as the intervention being ‘dominant’). Results in the south-west quadrant show that the intervention costs less but also has fewer health benefits than the comparator and results in the north-west quadrant show that the intervention costs more but also has fewer health benefits than the comparator (this is referred to as the intervention being ‘dominated’). The proportions of ICERs falling in each quadrant of the cost-effectiveness plane were calculated, as were the proportions falling underneath the threshold of £20,000 per QALY (for results falling in the top right-hand quadrant and the bottom right-hand quadrant).
An additional cost-effectiveness analysis was carried out that combined the costs with within-trial relapse data (primary outcome) to present a cost per relapse case avoided.
Owing to the small number of carers recruited, carer costs and effects are presented descriptively and not as a formal incremental analysis or combined with service user costs and effects.
Sensitivity analysis
To explore the robustness of the results, sensitivity analyses were carried out. First, owing to the use of multiple imputation, we conducted a complete-case analysis. Secondly, the AQoL-8D questionnaire results were used as the outcome, rather than the results from the EQ-5D-5L.
Results
Missing data
The number (and percentages) of completed economic measures are presented in Table 20 for both randomised service users and carers. For the resource use questionnaire at baseline, 97% (n = 71) of service users and 100% (n = 17) of carers had completed measures. At 3 months post randomisation, this fell to 81% (n = 59) and 65% (n = 11). At 6 months, the percentage of completed measures for service users reduced to 77% (n = 56), but for carers it remained at 65% (n = 11). At the final follow-up at 12 months, 75% (n = 55) of service users and 47% (n = 8) of carers completed measures.
| Economic measure | Time point, n (%) | Complete case, n (%) | |||
|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | ||
| Service users | |||||
| RUQ | 71 (97.3) | 59 (80.8) | 56 (76.7) | 55 (75.3) | 46 (63.0) |
| EQ-5D-5L | 70 (95.9) | 56 (76.7) | 56 (76.7) | 54 (74.0) | 45 (61.6) |
| AQoL-8D | 69 (94.5) | 57 (78.1) | 56 (76.7) | 55 (75.3) | 45 (61.6) |
| Carers | |||||
| RUQ | 17 (100.0) | 11 (64.7) | 11 (64.7) | 8 (47.1) | 7 (41.2) |
| EQ-5D-5L | 16 (94.1) | 11 (64.7) | 11 (64.7) | 8 (47.1) | 7 (41.2) |
| CarerQoL-7D | 17 (100.0) | 11 (64.7) | 11 (64.7) | 8 (47.1) | 7 (41.2) |
Outcome measures
Service users
Outcome measure results for service user available cases are presented in Tables 21 and 22. In the EMPOWER arm, EQ-5D-5L-derived utilities increased at each follow-up point; the utility score was 0.644 at baseline (SD 0.258) and 0.732 at the 12-month follow-up (SD 0.231). In the TAU arm, EQ-5D-5L utilities decreased slightly over the follow-up period; at baseline they were 0.620 (0.268), at 3-month follow-up they decreased to 0.593 (0.285), but at 6-month follow-up they increased to 0.657 (0.209) and they finally decreased to 0.607 (0.254) at 12-month follow-up. These trends are illustrated in Figure 9.
| Time point | EMPOWER | TAU | ||
|---|---|---|---|---|
| Mean (SD) | n (%) | Mean (SD) | n (%) | |
| Baseline | 0.644 (0.258) | 40 (95.2) | 0.620 (0.268) | 30 (96.8) |
| 3 months | 0.666 (0.234) | 30 (71.4) | 0.593 (0.285) | 26 (83.9) |
| 6 months | 0.694 (0.197) | 30 (71.4) | 0.657 (0.209) | 26 (83.9) |
| 12 months | 0.732 (0.231) | 28 (66.7) | 0.607 (0.254) | 26 (83.9) |
| Time point | EMPOWER | TAU | ||
|---|---|---|---|---|
| Mean (SD) | n (%) | Mean (SD) | n (%) | |
| Psychosocial | ||||
| Baseline | 0.234 (0.162) | 40 (95.2) | 0.221 (0.160) | 29 (93.5) |
| 3 months | 0.271 (0.207) | 30 (71.4) | 0.216 (0.149) | 27 (87.1) |
| 6 months | 0.269 (0.179) | 30 (71.4) | 0.207 (0.156) | 26 (83.9) |
| 12 months | 0.299 (0.176) | 28 (66.7) | 0.201 (0.132) | 27 (87.1) |
| Physical | ||||
| Baseline | 0.595 (0.250) | 40 (95.2) | 0.553 (0.190) | 29 (93.5) |
| 3 months | 0.576 (0.226) | 30 (71.4) | 0.551 (0.216) | 27 (87.1) |
| 6 months | 0.600 (0.227) | 30 (71.4) | 0.582 (0.245) | 26 (83.9) |
| 12 months | 0.649 (0.226) | 28 (66.7) | 0.569 (0.226) | 27 (87.1) |
| Total | ||||
| Baseline | 0.522 (0.228) | 40 (95.2) | 0.500 (0.190) | 29 (93.5) |
| 3 months | 0.545 (0.233) | 30 (71.4) | 0.491 (0.200) | 27 (87.1) |
| 6 months | 0.557 (0.220) | 30 (71.4) | 0.489 (0.212) | 26 (83.9) |
| 12 months | 0.609 (0.210) | 28 (66.7) | 0.485 (0.200) | 27 (87.1) |
FIGURE 9.
The EQ-5D-5L service user results, with 95% CIs.
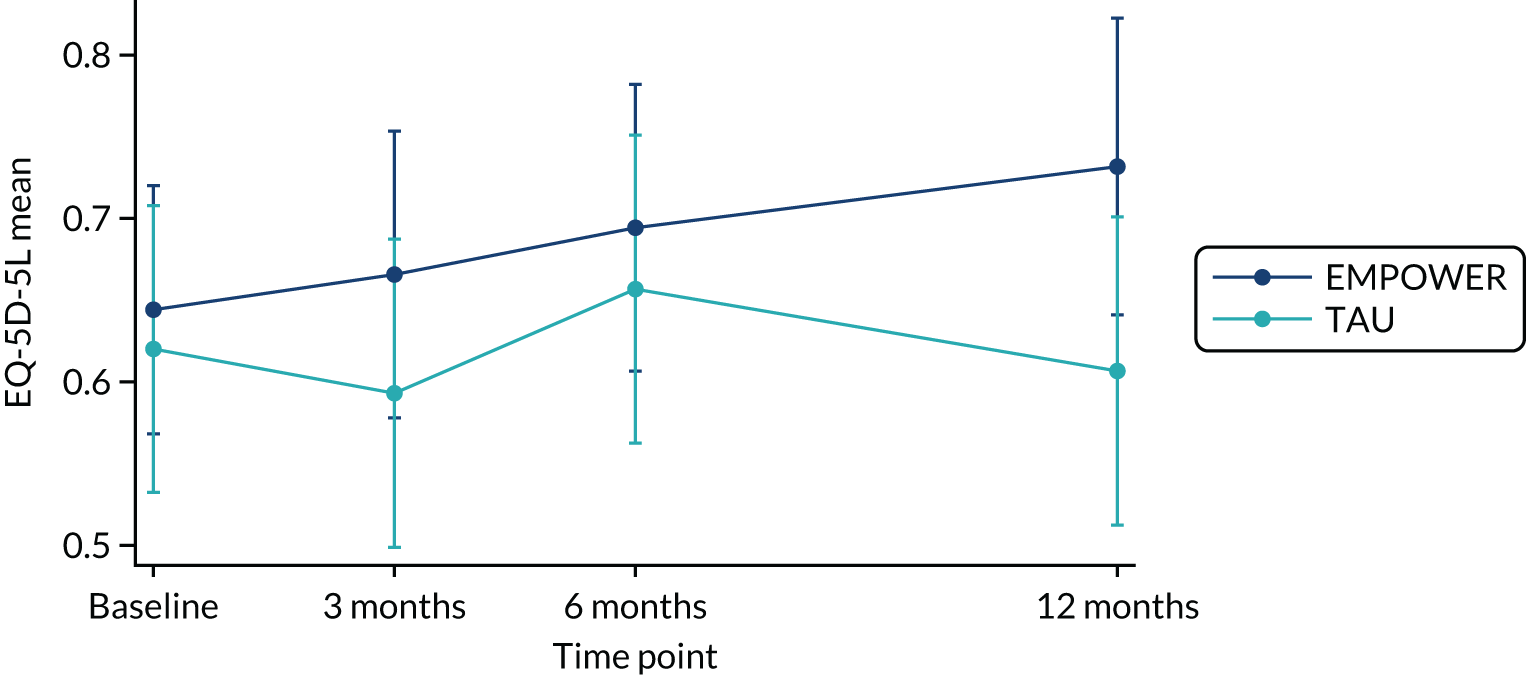
Total AQoL-8D utilities show a similar trend to EQ-5D-5L utilities; in the EMPOWER arm AQoL-8D utilities increased at each follow-up point from 0.522 (SD 0.228) at baseline to 0.609 (SD 0.210) at 12-month follow-up. In the treatment-as-usual arm, the utilities decreased at each follow-up point, from 0.500 (0.190) at baseline to 0.485 (SD 0.200) at the 12-month follow-up point. These trends are illustrated in Figure 10.
FIGURE 10.
The AQoL-8D service user results, with 95% CIs.
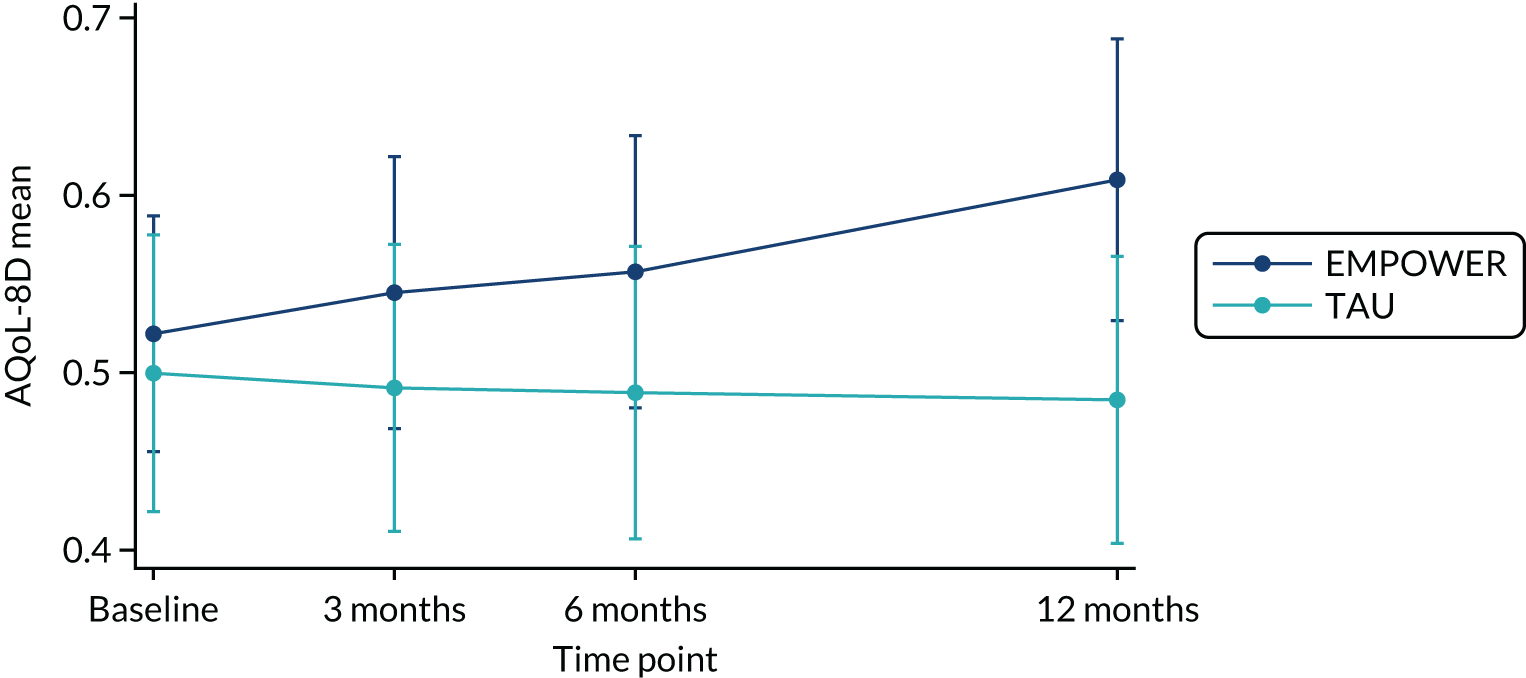
The AQoL-8D psychosocial dimension scores increased over the follow-up period, from 0.234 at baseline (SD 0.162) to 0.299 at the 12-month follow-up (SD 0.176), but decreased between the 3- and 6-month follow-up points. In the TAU arm, the psychosocial dimension decreased at each follow-up point, from 0.221 (0.160) at baseline to 0.201 (SD 0.132) at the 12-month follow-up. In terms of the physical dimension, scores in the EMPOWER arm increased during the follow-up period, from 0.595 at baseline (SD 0.250) to 0.649 (SD 0.266) at the 12-month follow-up, with a decrease between baseline and 3-month follow-up points. In the treatment-as-usual arm the physical dimension score also increased between baseline and 12 months follow-up; 0.553 (SD 0.190) to 0.569 (SD 0.226), with decreases between baseline and 3 months and 6 and 12 months, and an increase between 6- and 12-month follow-up. Again, overall AQoL-8D completion was higher in the TAU arm (93.5–83.9%, n = 29–26) than in the EMPOWER arm (95.2–66.7%, n = 40–28).
Carers
Outcome measures for carer available cases are presented in Tables 23 and 24. In the EMPOWER arm, EQ-5D-5L utilities decreased over the follow-up period from 0.815 (SD 0.107) at baseline to 0.798 (SD 0.099) at 12-month follow-up, with an increase between 3 and 6 months and 6 and 12 months. In the TAU arm, EQ-5D-5L utilities decreased over the follow-up period, from 0.748 (SD 0.195) at baseline to 0.695 (SD 0.156) at 12-month follow-up. These trends are illustrated in Figure 11.
| Time point | EMPOWER | TAU | ||
|---|---|---|---|---|
| Mean (SD) | n (%) | Mean (SD) | n (%) | |
| Baseline | 0.815 (0.107) | 7 (100) | 0.748 (0.195) | 9 (90.0) |
| 3 months | 0.775 (0.184) | 5 (71.4) | 0.678 (0.283) | 6 (60.0) |
| 6 months | 0.797 (0.169) | 5 (71.4) | 0.686 (0.233) | 6 (60.0) |
| 12 months | 0.798 (0.099) | 4 (57.1) | 0.695 (0.156) | 4 (40.0) |
| Time point | EMPOWER | TAU | ||
|---|---|---|---|---|
| Mean (SD) | n (%) | Mean (SD) | n (%) | |
| Baseline | 85.9 (15.8) | 7 (100) | 78.7 (14.3) | 10 (100) |
| 3 months | 86.1 (12.9) | 5 (71.4) | 79.5 (21.4) | 6 (60.0) |
| 6 months | 86.0 (5.08) | 5 (71.4) | 77.8 (19.0) | 6 (60.0) |
| 12 months | 91.8 (5.24) | 4 (57.1) | 91.5 (2.67) | 4 (40.0) |
FIGURE 11.
The EQ-5D-5L carer results, with 95% CIs.

For the CarerQoL-7D results, scores in the EMPOWER arm increased over the 12 months’ follow-up from 85.9 (SD 15.8) at baseline to 91.8 (SD 5.24) at 12 months, with a decrease between 6 and 12 months. This overall increase is mirrored in the TAU arm: 78.7 (SD 14.3) at baseline compared with 91.5 (SD 2.67) at 12 months, with a decrease between 3- and 6-month follow-up. These trends are illustrated in Figure 12. Similar to the EQ-5D-5L completion, numbers were greater in the EMPOWER arm (100–57.1%, n = 7–5) than in the TAU arm (100–40%, n = 9–6).
FIGURE 12.
The CarerQoL-7D results, with 95% CIs.

Resource use
Service users
The mean frequency of resource use and the numbers (and percentages) of service users reporting resource use are presented in Appendix 10, Table 43. Direct health-care resources reported as used by the greatest number of service users included GP visits, psychiatrist, mental health nurse and mental health medication, with a general trend for more people in the TAU arm to use these. Nobody reported using formal online therapy, and small numbers reported using counsellors, alcohol workers and speech therapists, and having had mental health inpatient stays or using other mental health resources such as Facebook groups and leaflets. The largest number of contacts with direct health care reported (a mean of ≥ 4) at any time point in either arm was for GP visit, psychologist, counsellor, mental health nurse, alcohol worker, case manager, speech therapist and occupational therapist. However, as mentioned above, small numbers of service users reported contact with these resources, suggesting that the larger mean number of visits were skewed by a small number of service users. A small number of service users reported having contact with the criminal justice system and with the police and making court appearances. Nobody reported spending nights in a police cell, having psychiatric assessments in custody or making civil court appearances.
The largest number of service users reporting receiving a specific benefit at a given time point was 17 for sickness/disability benefits [although a larger number of service users reported receiving ‘other benefits’ (33 at 3 months)]. The high use of the ‘other’ category was because researchers in the UK felt that it was a better fit for the large numbers of people in receipt of the new Employment Support Allowance and Personal Independence Payments benefits.
The most frequently reported employment status was unemployed, followed by employed, retired/unpaid work and school/studying. No service users reported being self-employed, a housewife/househusband or ‘other’. Of those service users reporting employed status, none reported being a manager or professional; the largest number reported ‘other’ as their occupation. Service users reported working between 16.4 and 26.3 hours per week on average, and between 2.04 and 4.33 days per week, with no trend towards differences between the arms. In terms of presenteeism, between two and six service users reported working when they felt bothered by physical or mental health problems over all time points; on average, the number of days worked while feeling bothered ranged from 5.0 (over 3 months) to 84.0 (over 6 months). On a Likert scale of 0–10, where 10 is worked at full capacity, the mean score was between 6.0 and 9.3, suggesting that, although the service user was bothered by physical or mental health problems, they were still able to work at a high capacity.
Small numbers of service users reported reducing their number of days and hours of unpaid work because of physical or mental health reasons; a large number of missing data for these variables means that the results for reducing unpaid work should be interpreted with caution.
In terms of receiving extra informal care for health reasons, a small number of service users reported needing extra help with child care, the greatest number of service users reporting receiving informal care was for help with household tasks (18 to 23) with no trend in differences between arms, and help outside the home (13 to 24) where there was a trend for more service users receiving help in the EMPOWER arm. A small number of service users (two to four) reported friends or relatives taking time off work because of the service user’s mental or physical health across all time points; the mean number of hours taken off by the friend or relative, reported across all time points and both arms, was between 18 and 268.3 hours.
A small number of participants in Glasgow reported feeling distress as a result of questions in the RUQ being similar to those asked during Department of Work and Pensions benefits assessments.
Carers
The mean frequency of resource use and the numbers (and percentages) of carers reporting resource use are presented in Appendix 10, Table 44. No carers reported using a speech therapist, occupational therapist, physiotherapist or inpatient for mental health. GP visits, care nurse and medication were reported at each time point.
Mean costs and quality-adjusted life-years
The multiple imputation base-case mean costs and QALYs are presented in Table 25. From the health-care sector and the health-care payer perspectives, the mean total costs for the EMPOWER arm were lower than those for the TAU arm, whereas the mean total societal costs for the EMPOWER arm were marginally higher than those for the TAU arm: £12,991 (SD £2622) compared with £12,820 (SD £2892), a difference of £170 (95% CI –£7783 to £8124). Key cost drivers include the intervention cost, health professional visits (mean of £2419 for EMPOWER and £2824 for TAU), hospital admission (mean of £2286 for EMPOWER and £3388 for TAU), stays at on-site accommodation (non-acute specialist residential mental health care in Melbourne only: mean of £2734 for EMPOWER and £3300 for TAU) and mental health medication (£836 for EMPOWER and £1106 for TAU). These cost drivers are illustrated in Figure 13, with a complete breakdown of total mean costs per arm. References to hospital admission costs and to ‘on-site’ costs reflect the differences in reporting of costs according to jurisdiction.
| Cost | EMPOWER (n = 42) | TAU (n = 31) | Treatment effect coefficientb | SE | 95% CI | Effect sizec | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Intervention costa | 2202.00 | 0 | 0.00 | 0 | NA | NA | NA | NA |
| 2380.00 | ||||||||
| 2447.00 | ||||||||
| Health professionals’ visits | 2419.12 | 224.59 | 2824.18 | 406.79 | –405.06 | 440.39 | –1270.41 to 460.29 | –1.00 |
| Ambulance | 94.00 | 72.82 | 70.93 | 51.54 | 23.07 | 97.65 | –175.13 to 221.28 | 0.45 |
| Emergency department | 89.88 | 50.04 | 158.42 | 62.90 | –68.54 | 97.17 | –259.49 to 122.41 | –1.09 |
| Day treatment at hospital | 50.09 | 21.65 | 117.10 | 37.59 | –67.01 | 50.82 | –166.90 to 32.89 | –1.78 |
| Hospital admission | 2285.98 | 1667.44 | 3388.42 | 1597.31 | –1102.43 | 2919.98 | –6837.78 to 4632.92 | –0.69 |
| Accommodation | 2734.09 | 1642.28 | 3299.65 | 2281.12 | –565.56 | 2808.36 | –6077.17 to 4946.05 | –0.25 |
| Diagnostic tests | 70.23 | 19.30 | 115.17 | 27.70 | –44.96 | 36.28 | –116.22 to 26.34 | –1.62 |
| Medication (mental health) | 835.82 | 194.59 | 1105.54 | 267.65 | –269.73 | 352.93 | –964.15 to 424.70 | –1.01 |
| Medication (non-mental health) | 108.11 | 38.62 | 61.02 | 31.73 | 47.09 | 52.52 | –56.17 to 150.35 | 1.48 |
| Total health-care payer costs | 10,899.30 | 2609.83 | 11,140.40 | 2802.01 | –1587.11 | 2593.92 | –6688.38 to 3514.17 | –0.09 |
| Online self-help | 10.81 | 4.38 | 10.07 | 4.91 | 0.74 | 6.92 | –12.89 to 14.34 | 0.15 |
| Out of pocket | 544.99 | 189.71 | 854.22 | 294.10 | –309.23 | 365.15 | –1026.98 to 408.52 | –1.05 |
| Total health-care sector costs | 11,623.10 | 2622.91 | 12,004.70 | 2903.02 | –1275.84 | 2612.94 | –6418.38 to 3866.71 | –0.13 |
| Criminal justice | 168.16 | 74.15 | 40.74 | 51.97 | 127.43 | 111.80 | –92.36 to 347.21 | 2.45 |
| Absenteeism (paid) | 37.17 | 37.32 | 117.12 | 65.76 | –79.96 | 87.73 | –252.25 to 92.33 | –1.22 |
| Presenteeism | 7.00 | 7.72 | 26.33 | 16.26 | –19.33 | 20.61 | –59.84 to 21.18 | –1.19 |
| Carer productivity loss | 430.67 | 342.03 | 126.35 | 202.06 | 304.32 | 434.61 | –551.11 to 1159.75 | 1.51 |
| Informal care | 657.48 | 182.29 | 505.01 | 160.50 | 152.46 | 252.93 | –345.51 to 650.44 | 0.95 |
| Total societal costs | 12,990.60 | 2622.58 | 12,820.30 | 2891.64 | –1625.09 | 262,679.26 | –6893.12 to 3642.95 | 0.06 |
| QALYs | 0.684 | 0.027 | 0.628 | 0.035 | 0.056 | 0.044 | –0.014 to 0.118 | 1.60 |
| EQ-5D-5L baseline | 0.642 | 0.041 | 0.618 | 0.049 | 0.024 | 0.063 | –0.100 to 0.147 | 0.49 |
| EQ-5D-5L 3 months | 0.662 | 0.041 | 0.600 | 0.054 | 0.062 | 0.066 | –0.050 to 0.192 | 1.15 |
| EQ-5D-5L 6 months | 0.694 | 0.033 | 0.659 | 0.040 | 0.353 | 0.053 | –0.065 to 0.140 | 0.87 |
| EQ-5D-5L 12 months | 0.711 | 0.040 | 0.613 | 0.049 | 0.098 | 0.061 | –0.014 to 0.220 | 2.00 |
FIGURE 13.
Breakdown of the total costs of EMPOWER and TAU (societal perspective).
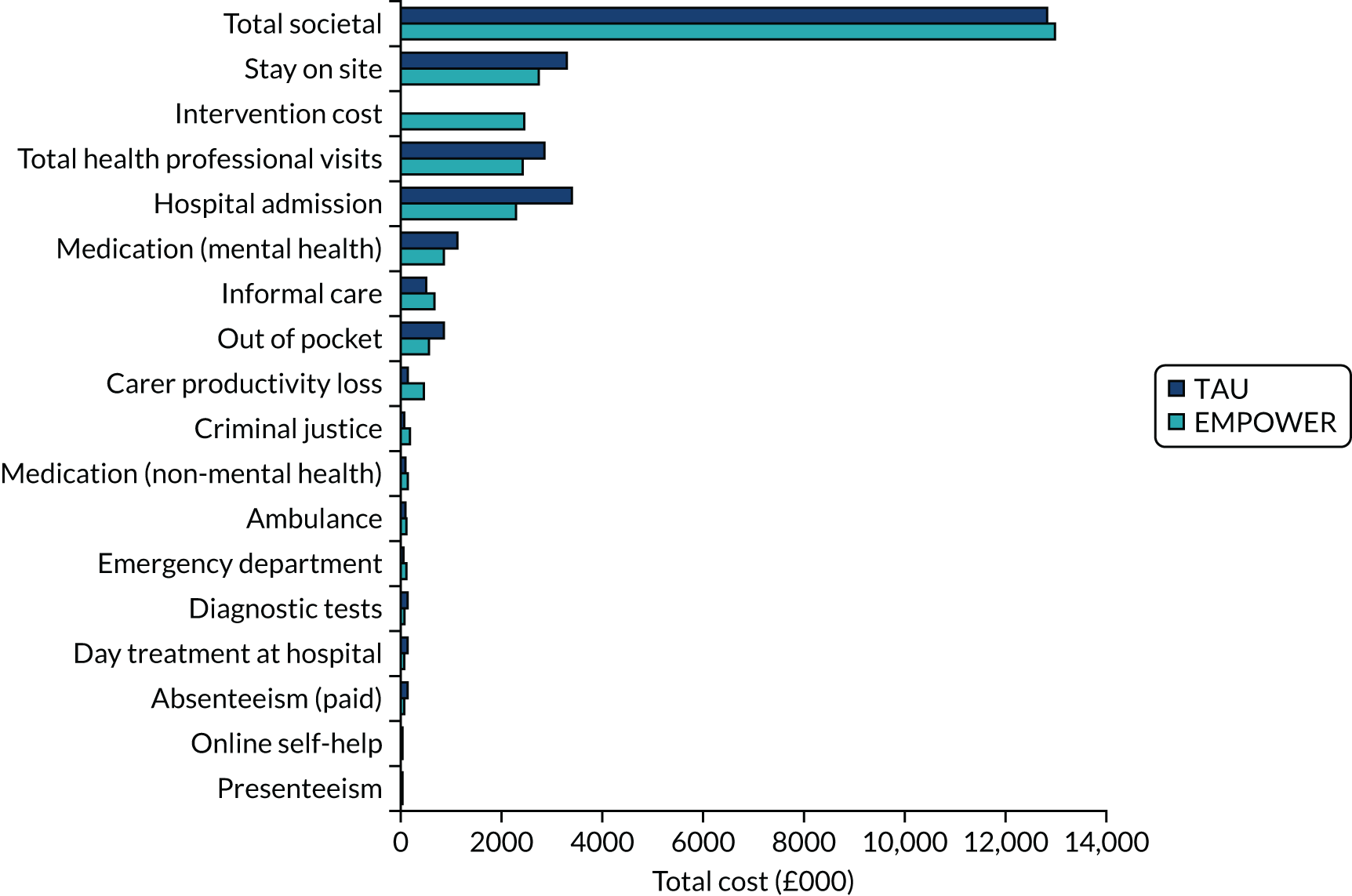
As discussed in Results, Outcome measures, there is a trend for the QALYs to be higher in the EMPOWER arm; this result is reflected in the multiple imputation results, with higher QALYs in the EMPOWER arm.
The results of the base-case cost–utility analysis are in Table 26. From both the health-care payer and the health-care sector perspectives, EMPOWER is dominant; it is both less costly and more effective than TAU. From the societal perspective, EMPOWER is more costly and more effective, resulting in an ICER of £3041. This ICER would be considered cost-effective using the NICE threshold of £20,000 per QALY gained. 220
| Arm | Cost (£) | QALY | Incremental costs (£) (95% CI) | Incremental QALYs (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Health-care payer perspective | |||||
| EMPOWER | 10,889.30 | 0.684 | –251.10 (–8073.34 to 7571.15) | 0.056 (–0.031 to 0.143) | Dominant |
| TAU | 11,140.40 | 0.628 | |||
| Health-care sector perspective | |||||
| EMPOWER | 11,623.10 | 0.684 | –381.60 (–8346.56 to 7583.39) | 0.056 (–0.031 to 0.143) | Dominant |
| TAU | 12,004.70 | 0.628 | |||
| Societal perspective | |||||
| EMPOWER | 12,990.60 | 0.684 | 170.34 (–7783.36 to 8124.03) | 0.056 (–0.031 to 0.143) | 3089.29 |
| TAU | 12,820.30 | 0.628 | |||
A cost-effectiveness plane for the health-care payer perspective is presented in Figure 14. It shows that the majority of cost and effect pairs fall beneath the £20,000 threshold. A minority of pairs fall into the north-west and south-west quadrants, which would indicate that EMPOWER would be considered less effective than TAU. However, the majority of pairs fall into the north-east and south-east quadrants, where EMPOWER is more effective than TAU. Although the cost-effectiveness plane shows uncertainty around whether EMPOWER is more or less costly than TAU, there is less certainty around whether EMPOWER is more effective in terms of QALYs calculated using the EQ-5D-5L. At a willingness-to-pay threshold of £20,000 per QALY gain, there is around a 70% probability that EMPOWER is cost-effective. The probability that EMPOWER dominates TAU is 58%.
FIGURE 14.
Cost-effectiveness plane (health-care payer perspective).
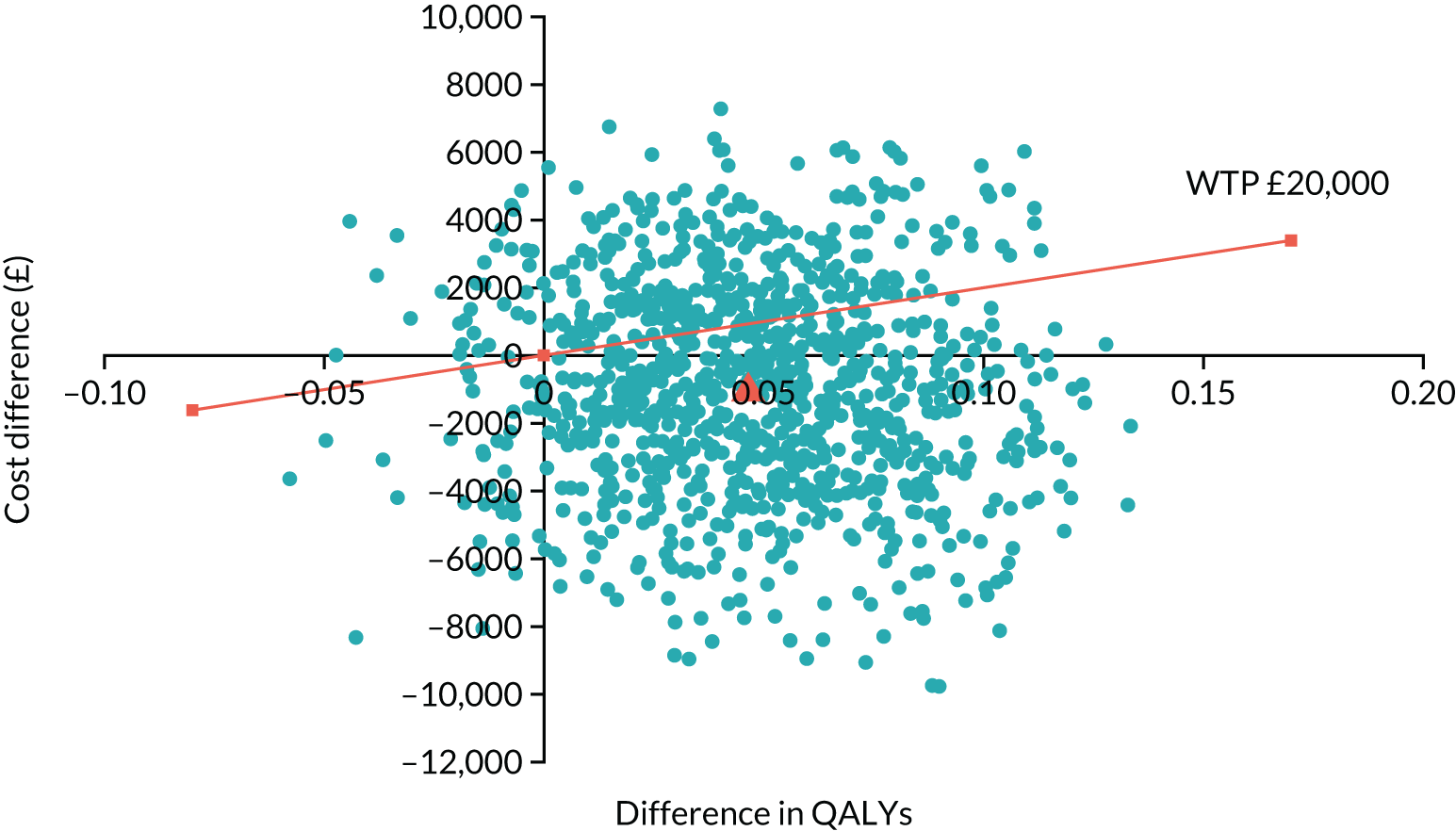
Table 27 shows the results for the two sensitivity analyses for the health-care payer perspective. When including complete-case costs and QALYs only, the ICER is £2817, indicating cost-effectiveness. Including QALYs from the AQoL-8D instead of the EQ-5D-5L results in EMPOWER dominating TAU.
| Arm | Cost (£) | QALY | Incremental costs (£) (95% CI) | Incremental QALYs (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Base-case analysis | |||||
| EMPOWER (n = 42) | 10,889.30 | 0.684 | –251.10 (–8073.34 to 7571.15) | 0.056 (–0.031 to 0.143) | Dominant |
| TAU (n = 31) | 11,140.40 | 0.628 | |||
| Sensitivity analysis 1: complete case | |||||
| EMPOWER (n = 23) | 12,305.53 | 0.722 | 236.63 (–10,278.12 to 10,751.38) | 0.084 (–0.028 to 0.197) | 2817.02 |
| TAU (n = 24) | 12,068.90 | 0.638 | |||
| Sensitivity analysis 2: AQoL-8D-QALYs | |||||
| EMPOWER (n = 42) | 10,889.30 | 0.554 | –251.10 (–8073.34 to 7571.15) | 0.059 (–0.028 to 0.146) | Dominant |
| TAU (n = 31) | 11,140.40 | 0.495 | |||
Mean costs and relapse cases
The results of the cost-effectiveness analysis are presented in Table 28. The number of relapse cases avoided is greater in the EMPOWER arm than in the TAU arm. The cost-effectiveness analysis results show that when using relapse cases avoided as the outcome, both the health-care payer and the societal perspective are dominant and when using the health-care sector perspective the ICER is £12 per relapse case avoided.
| Arm | Cost (£) | Relapse cases avoided | Incremental costs (£) | Incremental relapse cases avoided | ICER (£) |
|---|---|---|---|---|---|
| Health-care payer perspective | |||||
| EMPOWER | 10,889.30 | 32.35 | –251.10 | 14.76 | Dominant |
| TAU | 11,140.40 | 17.59 | |||
| Health-care sector perspective | |||||
| EMPOWER | 11,623.10 | 32.35 | –381.60 | 14.76 | Dominant |
| TAU | 12,004.70 | 17.59 | |||
| Societal perspective | |||||
| EMPOWER | 12,990.60 | 32.35 | 170.00 | 14.76 | 11.53 |
| TAU | 12,820.30 | 17.59 | |||
Carer costs and outcomes
Table 29 presents the complete-case costs and outcomes for carers. Owing to the small number of carers it was not possible to carry out a formal analysis and so the results should be interpreted with caution. There is a trend for the total societal costs to be higher in the TAU arm. However, the SDs are wide, indicating a lot of variation in the resources reported for carers.
| Cost | EMPOWER | TAU | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Health professionals | 4 | 194.95 | 121.97 | 4 | 312.83 | 206.78 |
| Hospital admission | 0 | NA | NA | 4 | 842.50 | 842.50 |
| Medication | 4 | 42.75 | 42.75 | 4 | 35.63 | 13.93 |
| Out of pocket | 4 | 190.00 | 113.36 | 4 | 95.75 | 94.75 |
| Informal care | 4 | 43,168.13 | 15,008.21 | 4 | 37,074.38 | 16,454.42 |
| Total societal costs | 3 | 36,049.83 | 18,412.3 | 4 | 38,361.08 | 17,036.62 |
| QALYs | 4 | 0.797 | 0.078 | 3 | 0.631 | 0.159 |
| EQ-5D-5L baseline | 7 | 0.815 | 0.040 | 9 | 0.748 | 0.05 |
| EQ-5D-5L 3 months | 5 | 0.775 | 0.082 | 6 | 0.678 | 0.116 |
| EQ-5D-5L 6 months | 5 | 0.797 | 0.076 | 6 | 0.686 | 0.095 |
| EQ-5D-5L 12 months | 4 | 0.798 | 0.049 | 4 | 0.695 | 0.078 |
| CarerQol-7D baseline | 7 | 85.871 | 5.987 | 10 | 78.680 | 4.518 |
| CarerQol-7D 3 months | 5 | 86.100 | 5.751 | 6 | 79.500 | 8.724 |
| CarerQol-7D 6 months | 5 | 85.980 | 2.274 | 6 | 77.767 | 7.756 |
| CarerQol-7D 12 months | 4 | 91.825 | 2.621 | 4 | 91.475 | 1.333 |
Chapter 7 Discussion
In this study we sought to establish the feasibility of conducting a definitive cRCT comparing the EMPOWER intervention with treatment as usual. Specifically, the EMPOWER intervention aimed to (1) enhance the recognition of EWS by people using services and their carers and (2) provide a stepped-care pathway that was either self-activated or in liaison with a carer and/or a care co-ordinator, which, if indicated, (3) triggered a relapse prevention strategy that could be stepped up to a whole-team response to reduce the likelihood of relapse. Each of the specific aims detailed in Chapter 2 is now considered in turn.
Eligibility, consent and recruitment
We aimed to determine the rates of eligibility, consent and recruitment of potential participants, including people using services, carers and care co-ordinators. During the study we successfully recruited and retained eight CMHS: six in Glasgow and two in Melbourne. We approached and screened the caseloads of care co-ordinators across these CMHS and identified 1140 potentially eligible service user participants. Following the screening of care co-ordinators’ caseloads, we found that 263 (23%) service users met our eligibility criteria. Of these potentially eligible participants, we were able to approach 129 (49%) and over an 8-month period then recruit 86 (67%), giving a recruitment rate of just under 11 participants per month from across the eight CMHS. We also successfully recruited 47 care co-ordinators to participate in the study. We found that service user participants identified a small number of carers, which meant that there was a small pool of carers to potentially participate in the study.
A challenge that we had not anticipated was the loss of eligible participants between informed consent and randomisation. Of the 86 participants who gave their informed consent, we randomised 73. This finding is worthy of further discussion. Prior to conducting the trial we noted that a key criticism of cRCTs is the chronology of allocation concealment, where initially clusters are recruited and randomised and then participants are recruited. This can then induce differential recruitment and so imbalances between groups can occur as a consequence of clusters knowing the outcome of randomisation before recruitment. 252–254 In this study we sought to avoid this problem by identifying and consenting eligible participants from each CMHS before the clinical trials unit revealed the allocation. We learned in phase 1 (see Chapter 3) that staff were concerned that many of their eligible service users would not use a digital intervention. Perceived barriers included older age, greater severity of symptoms, more cognitive impairments, digital exclusion, lack of insight, social problems and stresses related to poverty and deprivation. Maintaining allocation concealment during recruitment was an important means of reducing the potential bias from these staff expectations. In addition, research staff stressed the feasibility nature of the study to overcome these negative expectations, highlighting that a key aim of the study was to examine service user engagement with the trial and the intervention. Before randomisation and team allocation were revealed, 13 of the participants who had given their informed consent decided to withdraw. Of these participants, six received services from teams subsequently allocated to EMPOWER and seven received services from teams allocated to TAU. Reasons for withdrawal were available for six people, with most citing changes in their personal circumstances. We were unable to get reasons for withdrawal from the remaining seven participants. However, the extended period between informed consent and the start of the study, which had been designed to reduce the potential for bias, may well have increased the likelihood of participant withdrawal. In a future trial using these methods, retention might be improved by providing participants with clearer information about the anticipated gap between consent and the start of research procedures and by increasing efforts to maintain contact with consented participants and provide them with regular updates on progress. Alternatively, individual randomisation, as opposed to cluster randomisation, would minimise the early loss of consented participants.
Feasibility, acceptability and usability of the EMPOWER intervention
After being introduced to the app, 80% of participants completed the 4-week baseline self-monitoring period. This baseline period was necessary to enable the algorithm to calculate changes in well-being over time to both tailor self-management messages to changes in participants’ well-being and also to deliver ChIPs to research clinicians to triage and assess EWS. We found that these participants used the app for an average of 32 weeks (SD 14.5 weeks) and that, during this period, the app was used on 64% of days. Prior to conducting the study, we determined that the intended usage would be the period during which users complete at least 33% of daily prompts because this would result in data that are of maximum benefit in being reliable for detecting clinical changes. 255 We found that 91% of those who completed baseline met this criterion. If we include those who did not complete baseline, this estimate falls to 71%. Based on these figures, we could estimate that between 71% and 91% of participants would produce sufficient data to be considered reliable for the intervention in a main trial.
Trying to develop an accurate estimation of intended usage over time is challenging in a feasibility trial. We explored when participants stopped inputting data at least 3 days per week (meeting 33% intended usage criteria) for 4 sequential weeks. Overall, the median survival for not missing 4 sequential weeks of intended usage was 32 weeks (bootstrapped 95% CI –14 weeks to ∞). In other words, for 50% of participants who had completed a baseline, it took 33 weeks before they missed 4 sequential weeks of intended intervention usage. The width of the CI suggests that it is difficult to predict when participants stop adhering to use of the app (especially in terms of an upper limit), but it is probable that this time does not fall below 14 weeks.
Engagement with digital interventions can be conceptualised in multiple ways, including subjective experiences of how using an intervention makes a participant feel alongside purely behavioural measures of actual intervention usage. 256 Consistent with the behavioural data, participants reported using the app between ‘weekly’ to ‘daily’ during the 12-month follow-up. Participants themselves reported accessing their own data (using the charts function) ‘sometimes’. They reported sharing their data with carers and care co-ordinators ‘rarely’. We also observed signals of high levels of acceptability. Participants found the app interesting to use and easy to learn and navigate, and felt that the app content was well written, relevant and credible. They also said that they would recommend the app, and, overall, they rated the app very highly. Participants reported that the app increased their awareness, knowledge and understanding, improved their attitudes and intentions around staying well and encouraged help-seeking.
When we compared the use of well-being apps (more generally) between groups, we observed, as expected, higher levels of well-being app use in the EMPOWER arm than in the TAU arm. In terms of frequency, participants in EMPOWER reported using well-being apps ‘sometimes’ to ‘often’, compared with those in TAU, who reported using them either ‘not at all’ or ‘rarely’. Interestingly, we did not observe differences between the two groups in terms of frequency of help-seeking for EWS, frequency of carer help-seeking for EWS, or frequency of changes in clinical management being initiated following help-seeking for EWS. In the main these were reported as occurring ‘rarely’ in both arms of the trial. We observed a similar pattern of reporting among carers and care co-ordinators. These findings ran counter to our expectations. In our cognitive–interpersonal model of EWS, help-seeking and relapse described in Chapter 1 (see Figure 1), we expected that self-monitoring using the app would increase the availability of data to enhance shared decision-making. Consequently, we expected to observe an increase in reporting EWS in the EMPOWER arm (by service users and carers) and an increase in changes in clinical management in response to EWS. We did not observe this. There are a number of plausible reasons for these observations. It is possible that this lack of difference between groups was because EWS events were relatively infrequent and there were insufficient EWS events (at least from the perspectives of service users, carers and care co-ordinators) to generate a signal from the data. It is also possible that the response categories from ‘not at all’ to ‘often’ were insensitive to relatively infrequent events. Alternatively, the behavioural and self-reported acceptability data do suggest that people were using the app regularly, reporting increased awareness and knowledge and improved attitudes and intentions around staying well and help-seeking. These findings may therefore suggest that the app increased users’ self-management and autonomy in relation to staying well. Our findings in phase 1 (see Figure 4) suggested that users experienced the app as a tool to enhance self-awareness and reflection, enabling them to recognise changes in well-being that would trigger self-management and self-care. Previous qualitative studies that have explored experiences of mobile app monitoring have tended to focus on usability and acceptability. 140,257,258 They have shown that service users express a preference for utilising their data to enhance access to mental health care and for sharing with mental health staff257,258 or in relation to help-seeking. 259 These studies did not explore how participants themselves made sense of their data in relation to personal well-being and staying well, and the extent to which they experienced or utilised an increased sense of self-awareness as a result of self-monitoring.
In our study, self-monitoring was implemented differently from in these earlier studies. 140,257,258 In EMPOWER, self-monitoring was linked to the delivery of tailored messages that were designed to promote curiosity about changes in well-being and to encourage greater autonomy and self-management. Self-monitoring was also integrated with peer support, which aimed to enable service users to engage with the app. Peer support workers also provided technical support and encouraged service users to make sense of changes in their well-being in the broader context of other changes in their life and circumstances. We also observed sharing of experiences between peer workers and app users in relation to their experiences of self-monitoring but also more widely in relation to coping, self-management and recovery. Over the course of the study, participants were offered an average of 12.4 sessions of peer support, and the vast majority of these sessions were delivered over the telephone. We also established clinical triage in response to ChIPs generated by the algorithm. Over the course of the study, the algorithm generated 558 ChIPs across 37 app users, with each person having, on average, 15 ChIPs. Thus, it is possible that the increase in shared decision-making that we expected to see (and was predicted by our theoretical model) happened at the point of triage, rather than with the care co-ordinator. That is, when the research clinician made contact with a participant to check in about changes observed on the clinical interface, these discussions involved coming to a shared decision about whether or not to escalate these changes to the care co-ordinator. At the outset of the study we expected ChIPs to generate risk information that would require routine clinical triage to escalate changes detected by the algorithm to the relapse pathway. Thus, we did not expect that peer support workers would be involved in responding to ChIPs. We also felt that there was some risk in peer support workers assuming what might be construed as a clinical, and, therefore, a non-peer, role. However, we learned that the majority of these ChIPs were not specific to EWS or relapse but did frequently relate to changes that were of personal significance to service users. We learned that it was indeed possible for peer support workers to integrate information about ChIPs into their routine contacts with service users. Our experience was that this enabled more focused conversations around changes in well-being and often encouraged the mutual sharing of experiences, while not diminishing the distinctiveness of the peer support role.
To summarise, we demonstrated the feasibility, acceptability and usability of the EMPOWER intervention. The feasibility trial enabled us to develop the intervention to combine a number of key components: (1) digital self-monitoring of well-being, (2) an algorithm that tailored self-management messages to changes in well-being and triggered clinical triage of changes in well-being to assess for EWS and (3) regular follow-up with peer support. We did not find evidence that using the app increased reporting of EWS based on the perspectives of participants, carers or mental health professionals. Nor did use of the app increase help-seeking in relation to relapse. Therefore, the intervention has two key points of human interaction: first, a clinician who monitored changes in the service user’s well-being during the course of the study and responded to ChIPs generated by the algorithm; and, second, a peer support worker who delivered technical support to help service users use the app but also provided a context for the interpretation of the ‘ebb and flow’ of well-being in daily life. We noted the overall high rates of usage during the trial that were well above our a priori expectations. It has been shown that participant use of digital interventions for psychosis is higher when there is an increased level of human contact. 117 In this study it is likely that the high rates of use were linked to the blending of human contact with app use, not least because we did not offer financial incentives for engagement, as has been used in other digital psychosis studies. 170 In our preliminary work this blending was an important priority for service users, who expressed concerns that a mobile app should not be a replacement for clinical contact but rather should enhance or augment mental health support. 257,260
Performance and safety of the EMPOWER app
Very few studies of digital interventions for psychosis utilising EMA methods have reported methods and protocols for monitoring adverse events,113 and where this has been explicitly referenced it has been in relation to serious adverse events, such as hospitalisation or death. 170 Thus, adverse events or unwanted experiences have been a neglected area of study. Prior to starting the study we anticipated that fear of relapse was an important potential risk of introducing self-monitoring by digital technology. Relapse itself is associated with a range of distressing experiences including the return or exacerbation of distressing symptoms of psychosis, experiences of rehospitalisation and experiences of coercion into treatment. 16 Therefore, we expected that it was plausible that monitoring EWS may increase fear of relapse, especially given that early signs and fear of relapse are highly correlated. 17 Prior to undertaking the trial, we were required to register the EMPOWER app as a class 1 medical device with the UK MHRA (CI/2017/0039). It is our understanding that the EMPOWER app was the first mental health app to be regulated by the MHRA in this way. Medical device regulation requires detailed monitoring and reporting of performance and safety. In relation to performance, two device deficiencies were identified, which can be addressed in future versions of the app. Both events were identified quickly and did not result in any adverse effects on the participants or on the trial more broadly. No adverse events were related to the EMPOWER class 1 medical device (i.e. the EMPOWER algorithm). However, we identified 13 app-related adverse events (one serious adverse event) affecting 11 participants. For some people the app triggered unhelpful rumination by increasing their awareness of symptoms or anxiety or prompting distressing memories of previous relapses. Many of these adverse events occurred early in the course of using the app, and in two instances these led the participant to withdraw from the study. These findings have important implications for optimising the EMPOWER intervention. Before monitoring is initiated, it would be helpful to have a preliminary engagement phase for service users with the peer support worker to discuss and clarify the rationale for self-monitoring using the EMPOWER app, explore the advantages and disadvantages of self-monitoring, and identifying valued goals linked to self-monitoring and the broader EMPOWER intervention. Based on our experiences of adverse events, this phase would help address the triggers for adverse events and reduce the likelihood of their occurrence. In addition, this could possibly contribute to the informed and proactive withdrawal from the intervention of participants who express this preference (potentially without withdrawing more broadly from the main trial).
To summarise, a key learning point from this trial was that carefully monitoring adverse experiences of interacting with a digital intervention represents an important opportunity for learning and optimising digital interventions. Broader uptake of adverse event monitoring in digital health trials has clear potential to improve the experiences of end-users and reduce the risk of adverse events, while improving the overall design and acceptability of interventions. Our safety findings suggest that the design of the EMPOWER intervention could be improved to include an engagement phase prior to initiating self-monitoring, which would include sharing our cognitive–interpersonal model of relapse, exploring advantages and disadvantages of self-monitoring, and normalising monitoring ‘ebb and flow’ of well-being as a rationale for strengthening self-management, self-efficacy and empowerment.
Assessment of candidate primary and secondary outcomes
Candidate primary outcomes
A further aim of the feasibility trial was to assess primary and secondary outcomes to determine the preliminary signals of efficacy of the EMPOWER intervention as a basis for assessing the feasibility of collecting follow-up measures, determining primary and secondary outcomes, and determining probable sample size requirements for a future main trial. Our candidate co-primary outcomes were relapse and fear of relapse. Overall, the findings indicated that over 12 months, compared with TAU, EMPOWER appeared to reduce the number of relapses, increase the time to first relapse, and reduce fear of relapse. We successfully collected relapse data for 84% of participants at every follow-up point during the trial. We theorised that relapse events involve factors related to the service user (change in positive symptoms, duration of more than 1 week and reduction in functioning and/or increase in risk) and factors related to service responses (change in clinical management, hospitalisation and coercion into treatment). We successfully operationalised these criteria and measured relapse from routinely collected electronic patient record data. We subjected these data to blinded reliability assessments, finding substantial agreement between raters. Finally, all of these data were subject to a blinded outcome assessment.
In studies of interventions for relapse prevention in schizophrenia, there has been a lack of clear consensus about the definitions of relapse. For example, in a meta-analysis of antipsychotic drugs compared with placebo,229 the trial definitions of relapse varied and included clinical judgement (n = 26), need for medication (n = 17), rating scale-based definitions (n = 15), admission to hospital (n = 3) and dropout because symptoms worsened (n = 2). This lack of consensus is present in other meta-analyses. 261,262 In the Kishimoto et al. meta-analysis262 of first- compared with second-generation antipsychotics, nine of the authors’ included studies did not define relapse. A key strength of our study was that our definition was theoretically informed by our intervention model, was defined a priori, was capable of being extracted from routine health service data and was assessed blind with reliability data reported.
Relapse was less likely among participants in the EMPOWER arm, and we identified a RR of 0.50 (95% CI 0.14 to 0.74) over 12 months. This result, although encouraging, needs some cautionary interpretation given that the feasibility study had a small number of clusters, each with small numbers of participants, and that there were more withdrawals from the EMPOWER arm. The impact of the small numbers of clusters was examined and deemed not to be a major concern, although the overall small numbers prevent certainty about this. In terms of the potential impact of the withdrawals on the primary outcome, the number needed to change the benefit of the EMPOWER intervention would have to be set at six or more, a level felt to be unrealistically high. Sim263 and Eldridge et al. 264 have cautioned against the use of effect sizes derived from feasibility and pilot trials to be used as the basis for sample size estimation for a main trial. The imprecision arising from the smaller sample sizes in feasibility or pilot studies can increase uncertainty. Although our sample size (n = 73) is relatively good for a feasibility/pilot study,265 we note the wide 95% CI around our estimate for reduced relapse. Sim263 has recommended that effect size estimates for main trials be based on clinical judgement rather than on statistics. Our RR is comparable with meta-analytic evidence for early signs monitoring for relapse prevention71 (RR 0.53, 95% CI 0.36 to 0.79), family interventions for schizophrenia46 (RR 0.55, 95% CI 0.48 to 0.62: 12 months) and antipsychotic drugs compared with placebo over 12 months229 (RR 0.40, 95% CI 0.33 to 0.49: 12 months). Recently, Johnson et al. 123 has shown that peer-supported self-management reduced readmissions to acute care (odds ratio 0.66, 95% CI 0.43 to 0.99).
In a future main trial we envisage randomising between 300 and 500 participants. With a trial of this size, we would have 90% power to detect a range of important but potentially more realistic effects across a range of outcomes. In terms of our candidate primary outcome, if we assume a relapse at 1 year of 50% in the control group, we would have 90% power to detect a RR of 0.7 (i.e. 35% in the EMPOWER arm) and if we make allowance for a 20% dropout rate with a sample size of 500. For continuous outcomes we would have 90% power to detect effect sizes in the range of 0.3–0.4 SDs, or 80% power for 0.25 SD, which are worthwhile differences and are conservative in comparison with published data,46,71,229 reflecting high-quality findings on self-management interventions for schizophrenia. 123 Our choice of a RR of 0.7 also takes into account our analysis of the impact of the seven withdrawals in TAU and allows for the scenario in which five out of these seven withdrawals experienced a relapse. This is a conservative estimate given that if we allowed for the number of relapses in the withdrawal group to resemble our findings in TAU this number would be set at 3.
Our finding that fear of relapse was reduced more in the EMPOWER arm than in the TAU arm is consistent with our earlier empirical work in relation to fear of recurrence. 17 In that study we found that, controlling for baseline psychiatric symptoms and EWS, fear of relapse was the only predictor of time to relapse, in that greater fear of relapse was linked to shorter time to relapse. We also found that fear of relapse increased in the weeks preceding relapse and had the same sensitivity and specificity to relapse prediction as traditional measures of EWS. 17 In our theory, fear of relapse drives avoidance of help-seeking and potentially unhelpful coping strategies, thereby increasing vulnerability to relapse (see Figure 1). The finding that fear of relapse was reduced in those in the EMPOWER arm signals that this variable is likely to be an important co-primary outcome of a main trial.
Secondary and mechanism outcomes
Across our candidate secondary outcomes the rates of data completion among service user participants ranged from 73% to 85% (median 80%), demonstrating that collecting these outcomes is feasible. Again, although the impact of the small numbers of clusters was deemed not to be of major concern, as this was a feasibility study all of the results require cautionary interpretation. The profile of clinical and emotional distress outcomes was generally in favour of the EMPOWER arm, with some notable signals in relation to negative symptoms at 12 months (Cohen’s d = –0.57), self-rated depression (Cohen’s d = –0.41) and PBIQ-R control over illness (Cohen’s d = –0.42). We also noted that medication adherence was lower in the EMPOWER arm at 12 months (Cohen’s d = –0.50), signalling that the lower rate of relapse observed in the study may not be due to increased medication adherence. Although we would not rely on these outcome signals to infer efficacy, the measures provide important data that are relevant to the intervention and are feasible to collect in a main trial. We did note that rates of substance misuse were low and similar to those reported by Bucci et al. 170 We were successful in achieving good rates of follow-up using the Timeline Followback measure (85%) but, given the time-consuming nature of this measure, the low rates of substance misuse in the sample and the absence of any signals, we will carefully consider its inclusion in a main trial.
Across our candidate mechanism outcomes, rates of data completion were 85%. The profile of outcomes were generally in favour of the EMPOWER arm. We observed notable signals in relation to personal recovery at 12 months (Cohen’s d = 0.33) and general self-efficacy at 12 months (Cohen’s d = 0.33). These data indicate that these measures not only are feasible to collect but may provide important signals in a main trial in terms of mechanisms of action. In a future main trial we would also include fear of relapse as a mechanism of change, as discussed above.
As has been already noted, a minority of service users had a nominated carer and, overall, a small number of carers participated in the study. At 12-month follow-up, data were available for 47% of carer participants. Twelve-month data on care co-ordinators’ ratings of service user engagement with services were available for 42% of service user participants. During the trial we observed high rates of staff turnover and absence and changes to care co-ordinators. We also observed that care co-ordinators would often be unavailable when we were arranging appointments to collect data from them. In Chapter 3, care co-ordinators raised a number of important service-related factors including high caseloads and time pressures that had an impact on their ability to spend time with service users. These observations regarding the small number of nominated carers and the high attrition of carers and care co-ordinators have important implications for a main trial.
In terms of carers, this group expressed clear needs in relation to wanting to support family members with a diagnosis of schizophrenia and experiencing both satisfaction and stress in relation to their caring responsibilities. The EMPOWER intervention did not address the needs of carers directly, only through providing a resource to service users that they could choose (or not) to share with carers or friends and family. A recent systematic review of digital interventions for people with psychosis or bipolar disorder115 found that none of the 26 included studies targeted family or friends whereas the vast majority of digital interventions utilised support from staff or peers in delivery. There is clearly a need to develop interventions that focus on the needs of carers, families and friends. There are a number of emerging studies showing that online interventions that specifically target needs of carers are feasible, and future research aimed at involving relatives and friends should target their needs specifically. 266–268
In terms of staff, we have noted (see Chapters 1 and 3) the demands of routine practice, constraints of high caseloads and a focus on risk as constraints on joint crisis care plans and shared decision-making approaches. 84,85 In phase 1 of this trial (see Chapter 3), staff reported that the proposed EMPOWER intervention made sense but that if the app increased workload then they would not have the capacity to work with it. Based on our findings, and to scale up the intervention for a main trial, triage of EWS could be better placed within CMHS, rather than within the research team, thereby facilitating team-wide implementation in local services and better accommodating staff turnover and absences.
Health economics parameters
We aimed to establish the study parameters and data-gathering frameworks required for a co-ordinated health economic evaluation of a full trial across the UK and Australia. Overall, the findings indicated that, from a health-care payer and a health-care sector perspective, EMPOWER resulted in fewer costs and greater outcomes than TAU in terms of both QALYs gained and relapses avoided. When adopting a societal perspective, the EMPOWER arm resulted in higher costs but also in greater QALYs and more relapses avoided. Although there is no established willingness-to-pay threshold for a ‘relapse case avoided’, the resulting ICER of £3089 per QALY gained is below the current threshold of £20,000 recommended by NICE. The uncertainty analysis indicated that at a willingness-to-pay threshold of £20,000 per QALY gained there is around a 70% probability that EMPOWER is cost-effective from the health-care payer perspective. The results remained consistent after undertaking sensitivity analyses, using the AQoL-8D as a measure to derive QALYs and re-running the analyses using complete cases. In terms of carer costs and outcomes, the results indicate that carers in the EMPOWER arm had lower costs and greater outcomes than those in the TAU arm. However, the small number of carers included in the study mean that the results need to be interpreted with significant caution.
One economic element of the feasibility study explored developing a RUQ and the feasibility, acceptability and usability of the resulting measure. The RUQ for service users and carers was developed to include potential resource use in both the UK and Australian contexts. Some questions in the RUQ resulted in participant distress, which was associated with current approaches to benefits assessments in the UK. This suggests that questions on resource use should be carefully considered in any full trial and that people with lived experience should be involved in refining the RUQ to improve service users’ understanding of the importance of health economic evaluations in clinical trials and the acceptability of measures. Completion rates varied from 97% (n = 71) and 100% (n = 17) for service users and carers, respectively, at baseline, to 75% (n = 55) and 41% (n = 7), respectively, at 12-month follow-up.
The economic component also explored the feasibility, acceptability and usability of the chosen quality-of-life questionnaires. There were similar levels of completeness for the quality-of-life data collection as for the RUQ. No issues were reported with the acceptability of these measures and there were few partially completed questionnaires. The inclusion of two preference-based health-related quality-of-life measures also enabled the further exploration of the measures in terms of acceptance and suitability. The AQoL-8D has a greater focus on psychosocial aspects of quality of life and, as a result, it was assumed that this measure would be more sensitive to changes. However, both the EQ-5D-5L and the AQoL-8D scores improved over the 12-month trial period, highlighting the need for further validation work. In addition to this, the ReQoL-10 or ReQoL-20269 (a new recovery-focused outcome measure that can also be used to calculate QALYs) could be tested and considered in a final trial.
The economic component also estimated the costs of intervention and cost-effectiveness, and identified key cost drivers. The cost of the intervention was challenging to calculate as it comprised different elements between the UK and Australian settings; however, the micro-costing approach taken to the intervention produced an accurate cost of the EMPOWER intervention. Although previous studies have excluded the R&D costs of mobile apps, we have included these, although these costs were attributed to all future potential users of EMPOWER. The cost-effectiveness results presented indicate that EMPOWER would be cost-effective; while there is some uncertainty in the results, the two sensitivity analyses and the cost-effectiveness analysis indicate either that EMPOWER would be dominant or that the corresponding ICER would be considered well below the current NICE cost-effectiveness threshold. 220
The key cost drivers identified in the feasibility study included hospital admissions, health professional visits, medication for mental health and staying on site at an organisation. It should be noted that the question ‘staying on site at an organisation’ was completed only by service users in Australia, and this included adult prevention and recovery care services and community care units. The adoption of a broader societal perspective has also enabled us to examine other costs outside the health care sector. Although these were relatively low in comparison with costs in the health-care sector, criminal justice costs and the cost of informal care as well as carer productivity losses were notable and, therefore, should remain in a definitive trial. Costs of online self-help and absenteeism (paid) were low. Limitations include the number of missing data and the fact that, owing to an error in the RUQ, we were not able to investigate absenteeism from unpaid work. Most of the trial service users were unemployed, highlighting the importance of assessing absenteeism from unpaid work in a definitive trial.
From a societal perspective, ideally all costs and benefits should be taken into account regardless of who experiences these. However, although we collected costs and outcomes from service users and their carers, owing to the small number of carers we were not able to combine carers’ data with service users’ data. Combining outcome data from carers and service users poses another challenge, as QALYs need to be established that combine the utility of both carers and the service user.
Strengths of the feasibility study include collecting data on carers’ resource use and quality of life. Having these data enhanced the analysis and is best practice from a societal perspective. The analysis was further enhanced by the inclusion of sensitivity analyses, different perspectives and two different outcomes, one each for service users and carers. The inclusion of different outcome measures allowed the calculation of QALYs using the EQ-5D-5L as well as the AQoL-8D for service users. It is reassuring that, regardless of which measure was used, the EMPOWER intervention remained cost-effective.
A further strength of this economic analysis was the comprehensive design of the RUQ that enabled us to investigate which services were most commonly used to inform the design of the RUQ for the definitive trial.
Recommendations for data collection in a full trial include working with participants to include resource use that is not distressing or a burden to complete. Some work should also be completed to obtain the experiences of the researchers who filled in the questionnaires with the service users and carers, in order to understand why some variables were completed and others were not. Efforts should be made to minimise the number of missing data and to focus on obtaining good-quality data on resource use, particularly on the key cost drivers. Further involvement of people with lived experience of psychosis in developing methods to convey the rationale for the health economic evaluation, the collection of such detailed data and the refinement of the RUQ may be an important step towards improving the uptake of health economic measures and reducing missingness. Some resources were not reported as being used during this study and these could be omitted from the RUQ in a full trial. In our analysis we estimated the time service users spent on the EMPOWER app; in a full trial it would be preferable to also collect this information through the software.
This study has shown that it is feasible to collect data for an economic evaluation for a trial of the EMPOWER app. However, attempts should be made to reduce the number of missing data, particularly as the trial progresses. If the main causes of missing data can be addressed, a robust economic evaluation could be included as part of a full trial.
Enhance and tailor the EMPOWER mobile phone app
We aimed to enhance and tailor our mobile phone app to deliver EWS monitoring and self-management interventions and provide access to a relapse prevention pathway. We have already highlighted further opportunities for blending the app with peer support. We encountered a number of technical challenges. Installation would greatly be improved by making the app available from the Google Play store (Google Inc., Mountain View, CA, USA) or a similar online facility. We found for a number of individuals that the 4-week baseline used to enable the algorithm to function was not representative of the users’ usual ‘ebb and flow’ of well-being. This meant that there were a number of occasions where baseline monitoring had to be restarted and this meant having to uninstall and reinstall the app. Usability and scalability would be greatly improved by having the flexibility to restart or recalibrate baselines based on app user feedback. For some app users there were individual items that were not relevant to their experiences, however we did not have the flexibility to modify item content, beyond a limited number of personalised items. Future versions of the technology would benefit from greater flexibility of item content over time. Service users were prompted daily on a pseudo-random basis to complete questionnaires within a 5-hour window. User experience could be substantially improved by increased flexibility to modify the frequency and time of day of self-monitoring. Finally, the ChIP algorithm conveyed information to research clinicians and later to peer support workers via the clinician interface (see Figure 2). Exploring ways of enabling app users to engage with ChIP data could increase access to changes in their ‘ebb and flow’ of well-being, increase the sense of ownership and encourage them to reflect on changes. However, the direct delivery of ChIPs to app users would also need to be carefully considered given its potential to generate fear of relapse or hypervigilance. Finally, use of passively collected data could enhance the development of a broader range of social, behavioural, smartphone and geolocation-sensing data to inform changes in well-being using digital phenotyping. 270–272
Considerations for a larger trial of EMPOWER
Based on our learning from this feasibility study and on learning derived from our associated process evaluation,166 the findings of which will be published separately from this report, a number of considerations are needed in designing a larger multisite trial.
We designed the feasibility study as a cluster trial because we anticipated that potential intervention effects would, in part, be contingent on changes in clinical teams’ responses as a result of information generated through app usage. We anticipated that clinicians would be able to improve clinical decision-making based on app-generated data and that more collaborative and shared decision-making with app users would be encouraged as a result of the intervention. However, in practice we observed that, although most practitioners were interested in service users’ participation in the study and, to varying degrees, their use of the app, evidence of changes in practice as a result of app usage or improved access to data to inform decision-making was very limited. Consistent with our observations, our feasibility data showed us that participants, carers and mental health care staff did not report an increase in reporting EWS or help-seeking in relation to relapse. Rather, we observed that using the app-based monitoring and peer support led to increased self-management and autonomy.
The logic of using cluster randomisation was that we anticipated that the intervention would have a greater impact on the care team environment. However, our experience was that custom and practice prevailed in most cases and that participants did not increase their reporting of EWS to care teams or increase help-seeking for relapse. Our findings highlight a lack of ‘contamination’ within the care team environment, negating the need to use cluster randomisation in a future evaluation. In addition to reducing the required sample size, individual randomisation could bring other advantages over cluster randomisation. We believe that individual randomisation will lead to fewer withdrawals between consent and allocation. As a result of needing to wait until target recruitment levels were reached in each paired cluster before randomisation, some people faced a considerable gap between consenting to the study and being allocated a treatment. We believe that this may have contributed to the relatively large number of withdrawals between consent and allocation (n = 13). Individual randomisation would allow us to start people more promptly in either arm of the study, potentially reducing the general feelings of uncertainty that seemed to contribute to a number of pre-randomisation withdrawals in the feasibility study.
The peer support worker role in the intervention developed and changed over the course of the feasibility study, and we would like to enhance and more fully embed the role in a larger trial. Initially, peer workers were primarily concerned with setting up and providing technical support for the app, but over time they became more involved in encouraging service users to reflect on app data and in supporting self-management and recovery. Peer workers also assumed more of a role in discussing ChIPs with app users, given that they had the most regular contact with app users. Initially the research team felt that playing a role in reviewing and responding to app-generated data might negatively influence the peer relationship but, counter to this, we found that access to data actually created the foundation for richer discussions about wellness management and opportunities for reflection with app users. We would anticipate developing this practice in a main trial in tandem with a greater focus on self-management in app users.
We would also anticipate continuing to develop our practice in relation to adverse event monitoring, which we believe was a strength of the feasibility study. We would like to improve our monitoring and reporting of general deteriorations in mental health as adverse events and their relatedness to the intervention. As a result of data collected in the feasibility study, we will also be more able to anticipate the type, frequency and temporal likelihood of certain intervention-related adverse responses and to prepare participants and the research team accordingly. For example, we are aware that the start of app usage is a risk period and that better support may be needed for people who are adjusting to routine self-monitoring, particularly when it is an entirely new concept. There are clear indications to include an initial engagement phase to support people in developing an understanding of the rationale for monitoring their well-being, anticipating the costs and benefits of this approach, having a less fearful response to changes in early signs, and developing greater self-management in response to changes in well-being.
Strengths and limitations
Strengths
The trial had a number of important strengths. We established a priori our theory of EWS and relapse; we clearly defined our outcome variable of relapse and established the feasibility of utilising routine clinical data to inform classification of relapse; and we demonstrated reliability in the blind rating of these data to classify relapse events. Linked to our theory of EWS, we established clear mechanisms of relapse detection and prevention, including fear of relapse, personal recovery and self-efficacy. Across these outcomes we demonstrated signals to suggest that improved outcomes were associated with the EMPOWER arm compared to TAU.
Our study also adds to the growing literature on digital technology for people with psychosis. We demonstrated the feasibility, acceptability and usability of a blended intervention combining digital technology with peer support and clinical triage to assess changes in well-being indicative of EWS, which then activated a relapse prevention pathway to local CMHS.
We delivered a feasibility trial of a digital intervention across two distinct health-care systems, in Scotland and Australia, demonstrating the potential scalability of the EMPOWER intervention across health-care systems internationally.
We overcame important limitations in relation to selection bias in cluster randomised trials by identifying, approaching, consenting and assessing participants prior to revealing allocation. Although this led to some participants dropping out prior to allocation, we learned important lessons for retaining participants in future studies.
This was the first digital technology mental health trial to be regulated by the MHRA. The trial developed significant strengths in the monitoring and detection of adverse events in digital technology trials. In addition, these adverse events have been important in helping shape the future development of the intervention.
Limitations
The study had a number of important limitations. This was a feasibility trial and the outcome signals detected during the study cannot be taken as evidence of efficacy or effectiveness. We require a larger-scale definitive trial to determine effectiveness.
There appeared to be a differential rate of attrition from the EMPOWER arm of the study. This appeared to be related to the initial burden and effects of the intervention in terms of the daily monitoring. In addition, installation of the app could have been improved. More time could also have been spent at the outset supporting engagement with peer support, exploring the benefits and difficulties of self-monitoring and the model underpinning the intervention and linking this to the valued goals of participants. This might have reduced the rate of withdrawal or, at the very least, facilitated planned withdrawal and improved opportunities for participants to consider remaining in the study to complete follow-up assessments or to provide explicit consent for their routine data to be collected from case notes to allow relapse assessment. In retrospect, we do not feel that our participant information sheet was clearly worded in a way that made continued collection of these data possible following withdrawal.
The psychometric properties of the EMPOWER questionnaire require investigation, particularly the sensitivity and specificity to relapse. Our finding was that ChIPs were not specific to relapse, and while this was helpful to enable blending of the monitoring with peer support, we cannot use the measure to reliably predict relapse. In addition, the questionnaire would benefit from further patient and public involvement to help shape the content and range of questions in order to improve its salience for service users.
A further limitation of the study is that we did not measure participants’ engagement with self-management interventions. Although we have an ongoing process evaluation, which included in-depth interviews with a purposive sample of just over 40% of participants in the EMPOWER arm, the lack of a quantitative measure of self-management limits how confidently we can understand how the EMPOWER intervention might work. Such a measure would be valuable to include in a main trial.
Conclusions and recommendations
We demonstrated the feasibility of recruiting and retaining service user participants into the trial. In addition, the rates of data completeness for candidate primary, secondary and mechanistic outcomes over the 12 months were excellent. However, we did identify problems with the completeness of health economic measures data. We demonstrated that we can deliver the EMPOWER intervention blending our mobile app with peer support and an algorithm that supports the delivery of tailored messaging and clinical triage of possible EWS of relapse. In addition, we learned how to integrate ChIPs generated by the algorithm into peer support to promote increased awareness and motivation to engage in self-management. It is likely that EMPOWER may reduce relapse over 12 months and reduce fear of relapse. The intervention may improve other outcomes including negative symptoms, depression, personal recovery and self-efficacy. It is unlikely that EMPOWER improves medication adherence. It is likely that overall the costs of EMPOWER are higher than those of TAU, but the intervention also results in improved QALYs and reduced relapse. The ICER of £3089 per QALY gained is below the current £20,000 threshold recommended by NICE, and there is a 70% probability that EMPOWER is cost-effective from the health-care payer perspective. A further main trial seems merited by these overall findings. We estimate that in a main trial (assuming 90% power and 20% dropout) we would require a sample size of 500 service users to detect a RR of 0.7 for reduction of relapse and for continuous variables effect sizes of between 0.3 and 0.4.
More broadly, this trial has raised a number of important findings for research in digital interventions for psychosis and, potentially, for other conditions. The monitoring of adverse events tends to be poor, and future trials should establish transparent and robust frameworks for the monitoring of adverse events linked to digital interventions. Researchers and clinicians should utilise learning from these events to improve intervention delivery and user experience. Programme theory is critical to driving intervention development and evaluation. This trial raises important issues about the lack of theory underpinning relapse definition and the need to develop better measurements of relapse. Further research is required to incorporate the views of people with lived experience about the definitions of relapse, their preferences for intervention development to support staying well and the development of international consensus on relapse outcomes. More research is needed on peer support to facilitate personal recovery for people with a diagnosis of schizophrenia and in particular the opportunities provided by the integration of digital technology to support and optimise these interventions.
Acknowledgements
We are grateful to all of the service users, carers and mental health staff and community mental health services who gave their time and resources to contribute to the development of this study during the consultation phase. This was critical in shaping the refinement of the EMPOWER intervention and outcomes. We are grateful to all of the service users, carers and mental health staff and community mental health services who gave their time and resources to contribute to the cluster randomised controlled trial. We would like to acknowledge and recognise the contribution of members of the research team not listed as authors. These include Jayne Artis, Imogen Bell, Emily Castagnini, Andrea Clark, Amy Hood, Maria Lambrou, Casey Lynch, Casey McCrae, Ishani Majmudar, Claire Matrunola, Giada Micolucci, Sophie Norman, Lesley Smith, Suzy Syrett, David Thomson, Helen Whitehill and Alison Wilson Kay. We also express our gratitude to our Study Steering Committee members: Professor David Kingdon (chairperson), Professor Daniel Freeman (independent member), Professor Fiona Lobban (independent member), David Kavanagh (independent member), Frances Simpson (independent public and patient involvement representative) and Graham Morgan (independent public and patient involvement representative). We also express our gratitude to our DMEC members: Professor Emmanuelle Peters (chairperson), Dr Alison Brabban (independent clinician), Professor Rod Taylor (independent statistician) and Professor Greg Murray (independent statistician).
Contributions of authors
Professor Andrew I Gumley (https://orcid.org/0000-0002-8888-938X) (Professor of Psychological Therapy) was the chief investigator, with responsibility for all aspects of the study. He was extensively involved in preparing results for, and writing, the monograph.
Dr Simon Bradstreet (https://orcid.org/0000-0002-0339-1882) (Lecturer in Digital Health Interventions) was the trial manager, with managerial responsibilities across the study. He was extensively involved in preparing the results for, and writing, the monograph.
Professor John Ainsworth (https://orcid.org/0000-0002-2187-9195) (Professor of Health Informatics) was a principal investigator on the study. He was involved in the design of the study, software development and validation, and final approval of the monograph.
Stephanie Allan (https://orcid.org/0000-0002-1016-0708) (PhD Candidate) was involved in the analysis of phase 1 and app usage data and contributed to the preparation of the monograph.
Professor Mario Alvarez-Jimenez (https://orcid.org/0000-0002-3535-9086) (Director, eOrygen) was a principal investigator on the study. He contributed to the design and oversight of the study, and approved the monograph.
Professor Maximillian Birchwood (https://orcid.org/0000-0002-7476-0171) (Professor of Youth Mental Health) was a principal investigator on the study. He contributed to the design and oversight of the study, and approved the monograph.
Professor Andrew Briggs (https://orcid.org/0000-0002-0777-1997) (Professor of Health Economics) was a principal investigator on the study. He contributed to the design and oversight of the study, designed and oversaw the health economic analysis plan and approved the monograph.
Professor Sandra Bucci (https://orcid.org/0000-0002-6197-5333) (Professor of Clinical Psychology) was a principal investigator on the study. She contributed to the design and oversight of the study, was a member of the independent blind panel for relapse assessments, and approved the monograph.
Professor Sue Cotton (https://orcid.org/0000-0002-9386-8348) (Head of Health Services and Outcomes Research, Orygen) was a principal investigator on the study. She contributed to the design and oversight of the study and approved the monograph.
Dr Lidia Engel (https://orcid.org/0000-0002-7959-3149) (Research Fellow) was a principal investigator on the study. She was involved in the design, analysis and write-up of health economic data for the monograph.
Professor Paul French (https://orcid.org/0000-0003-4300-387X) (Associate Director for Early Intervention Service) was a principal investigator on the study. He contributed to the design and oversight of the study and approved the monograph.
Associate Professor Reeva Lederman (https://orcid.org/0000-0003-4005-329X) was a principal investigator on the study. She contributed to the design and oversight of the study and approved the monograph.
Professor Shôn Lewis (https://orcid.org/0000-0003-1861-4652) (Clinical Professor in Adult Psychiatry) was a principal investigator on the study. He contributed to the design and oversight of the study and approved the monograph.
Matthew Machin (https://orcid.org/0000-0001-6600-9508) was a principal investigator on the study. He was involved in the design of the study and software development and validation and approved the monograph.
Professor Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Director, CHaRT) was a principal investigator on the study. He was involved in the design of the study, offering expert methodological input. He was involved in the preparation of trial results for publication in the final monograph. He approved the monograph.
Professor Hamish McLeod (https://orcid.org/0000-0002-4225-1815) (Professor of Clinical Psychology) was a principal investigator on the study. He was involved in the design of the study, oversaw researcher calibration and inter-rater reliability procedures and approved the monograph.
Nicola McMeekin (https://orcid.org/0000-0003-2918-8820) (Research Assistant) was a health economist on the study. She was involved in the design, analysis and write-up of health economic data for the monograph. She approved the monograph.
Professor Cathy Mihalopoulos (https://orcid.org/0000-0002-7127-9462) (Chairperson and Head, Deakin Health Economics) was a principal investigator on the study. She contributed to the design and oversight of the study, designed and oversaw the health economic analysis plan and approved the monograph.
Dr Emma Morton (https://orcid.org/0000-0001-6179-1983) was a research co-ordinator on the study, with managerial responsibilities for the study in Melbourne. She was extensively involved in assessments and other research procedures and approved the monograph.
Professor John Norrie (https://orcid.org/0000-0001-9823-9252) (Director, Chairperson of Medical Statistics and Trial Methodology, University of Edinburgh) was a principal investigator on the study. He was involved in the design of the study, offering expert methodological input, and approved the monograph.
Frank Reilly (https://orcid.org/0000-0002-3742-6668) (Director, Scottish Recovery Network) was a principal investigator on the study. He employed the research assistant in Glasgow and approved the monograph.
Professor Matthias Schwannauer (https://orcid.org/0000-0002-4683-2596) (Professor of Clinical Psychology) was a principal investigator on the study. He was involved in the design and oversight of the study, was a member of the independent blind panel for relapse assessments and approved the monograph.
Professor Swaran P Singh (https://orcid.org/0000-0003-3454-2089) (Professor of Social and Community Psychiatry) was a principal investigator on the study. He was involved in the design and oversight of the study and approved the monograph.
Professor Suresh Sundrum (https://orcid.org/0000-0002-9674-0227) (Head, Department of Psychiatry, Monash University) was a principal investigator on the study. He was involved in the design and oversight of the study and approved the monograph.
Associate Professor Andrew Thompson (https://orcid.org/0000-0002-0567-6013) (Associate Clinical Professor in Psychiatry) was a principal investigator on the study. He was involved in the design and oversight of the study and approved the monograph.
Professor Chris Williams (https://orcid.org/0000-0001-5387-2863) (Honorary Senior Research Fellow) was a principal investigator on the study. He was involved in the design of the study and approved the monograph.
Professor Alison Yung (https://orcid.org/0000-0002-0401-9791) (Professor of Psychiatry) was a principal investigator on the study. She was involved in the design and oversight of the study and approved the monograph.
Dr Lorna Aucott (https://orcid.org/0000-0001-6277-7972) (Statistician) was a statistician on the study. She was involved in the analysis and preparation of trial results for publication in the final monograph.
Associate Professor John Farhall (https://orcid.org/0000-0003-2439-2830) (Psychology and Counselling) was co-chief investigator for the study in Australia, with responsibility for all aspects of the study there. He was involved in preparing results for, and writing, the monograph.
Professor John Gleeson (https://orcid.org/0000-0001-7969-492X) (Professor of Psychology) was co-chief investigator for the study in Australia, with responsibility for all aspects of the study there. He was involved in preparing results for, and writing, the monograph.
Publications
Allan S, Bradstreet S, McLeod H, Gleeson J, Farhall J, Lambrou M, et al. Service Users, Carers and Mental Health Staff understandings of early warning signs of relapse in psychosis: a qualitative investigation. BJPsych Open 2019;6:1–7.
Allan S, McLeod H, Bradstreet S, Beedie S, Moir B, Gleeson J, et al. Understanding implementation of a digital self-monitoring intervention for relapse prevention in psychosis: protocol for a mixed-methods process evaluation. JMIR Res Protoc 2019;8:e15634.
Allan S, Bradstreet S, McLeod H, Farhall J, Lambrou M, Gleeson J, et al. Monitoring early signs of psychosis relapse using a mobile app: developing a hypothetical implementation framework of expectations for staff, carers and service users using qualitative methods. J Med Internet Res 2019;21:e14366.
Bradstreet S, Allan S, Gumley A. Adverse event monitoring in mHealth for psychosis interventions provides an important opportunity for learning. J Ment Health 2019;28:461–6.
Gumley A, Bradstreet S, Ainsworth J, Allan S, Alvarez-Jinenez M, Beattie L, et al. Early signs Monitoring to Prevent relapse in psychosis and prOmote Wellbeing, Engagement and Recovery (EMPOWER): protocol for a feasibility cluster randomized controlled trial harnessing mobile phone technology blended with peer support. JMIR Res Protoc 2020;9:e15058.
Allan S, McLeod H, Bradstreet S, Bell I, Whitehall H, Wilson-Kay A, et al. Perspectives of trial staff on the barriers recruitment in a digital intervention for psychosis and how to work around them: qualitative study within a trial. JMIR Hum Factors 2021;8:e24055.
Gumley AI, Bradstreet S, Ainsworth J, Allan S, Alvarez-Jimenez M, Aucott L, et al. The EMPOWER blended digital intervention for relapse prevention in schizophrenia: a feasibility cluster randomised controlled trial in Scotland and Australia. Lancet Psychiatry 2022;9:477–86.
Data-sharing statement
We shall make data available to the scientific community with as few restrictions as feasible, while retaining exclusive use until the publication of major outputs. The data will be made available via the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Radua J, Ramella-Cravaro V, Ioannidis JPA, Reichenberg A, Phiphopthatsanee N, Amir T, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry 2018;17:49-66. https://doi.org/10.1002/wps.20490.
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLOS Med 2005;2. https://doi.org/10.1371/journal.pmed.0020141.
- Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr Bull 2018;44:1195-203. https://doi.org/10.1093/schbul/sby058.
- Vos T, Abajobir AA, Abbafati C, Abbas KM, Abate KH, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59. https://doi.org/10.1016/S0140-6736(17)32154-2.
- Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 2017;4:295-301. https://doi.org/10.1016/S2215-0366(17)30078-0.
- Doyle R, O’Keeffe D, Hannigan A, Kinsella A, Behan C, Kelly A, et al. The iHOPE-20 study: mortality in first episode psychosis-a 20-year follow-up of the Dublin first episode cohort. Soc Psychiatry Psychiatr Epidemiol 2019;54:1337-42. https://doi.org/10.1007/s00127-019-01721-x.
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia. Arch Gen Psychiatry 2007;64. https://doi.org/10.1001/archpsyc.64.10.1123.
- Rethink . The Abandoned Illness: A Report by the Schizophrenia Commission 2012.
- Neil AL, Carr VJ, Mihalopoulos C, Mackinnon A, Morgan VA. Costs of psychosis in 2010: findings from the second Australian National Survey of Psychosis. Aust N Z J Psychiatry 2014;48:169-82. https://doi.org/10.1177/0004867413500352.
- Hui CLM, Honer WG, Lee EHM, Chang WC, Chan SKW, Chen ESM, et al. Long-term effects of discontinuation from antipsychotic maintenance following first-episode schizophrenia and related disorders: a 10 year follow-up of a randomised, double-blind trial. Lancet Psychiatry 2018;5:432-42. https://doi.org/10.1016/S2215-0366(18)30090-7.
- Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999;56:241-7. https://doi.org/10.1001/archpsyc.56.3.241.
- Alvarez-Jimenez M, Priede A, Hetrick SE, Bendall S, Killackey E, Parker AG, et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res 2012;139:116-28. https://doi.org/10.1016/j.schres.2012.05.007.
- Bergé D, Mané A, Salgado P, Cortizo R, Garnier C, Gomez L, et al. Predictors of relapse and functioning in first-episode psychosis: a two-year follow-up study. Psychiatr Serv 2016;67:227-33. https://doi.org/10.1176/appi.ps.201400316.
- Pelayo-Terán JM, Gajardo Galán VG, de la Ortiz-García de la Foz V, Martínez-García O, Tabarés-Seisdedos R, Crespo-Facorro B, et al. Rates and predictors of relapse in first-episode non-affective psychosis: a 3-year longitudinal study in a specialized intervention program (PAFIP). Eur Arch Psychiatry Clin Neurosci 2017;267:315-23. https://doi.org/10.1007/s00406-016-0740-3.
- Morgan VA, Waterreus A, Jablensky A, Mackinnon A, McGrath JJ, Carr V, et al. People living with psychotic illness in 2010: the second Australian national survey of psychosis. Aust N Z J Psychiatry 2012;46:735-52. https://doi.org/10.1177/0004867412449877.
- White RG, Gumley AI. Postpsychotic posttraumatic stress disorder. J Nerv Ment Dis 2009;197:841-9. https://doi.org/10.1097/NMD.0b013e3181bea625.
- Gumley AI, MacBeth A, Reilly JD, O’Grady M, White RG, McLeod H, et al. Fear of recurrence: results of a randomized trial of relapse detection in schizophrenia. Br J Clin Psychol 2015;54:49-62. https://doi.org/10.1111/bjc.12060.
- Lal S, Malla A, Marandola G, Thériault J, Tibbo P, Manchanda R, et al. ‘Worried about relapse’: family members’ experiences and perspectives of relapse in first-episode psychosis. Early Interv Psychiatry 2019;13:24-9. https://doi.org/10.1111/eip.12440.
- Ascher-Svanum H, Zhu B, Faries DE, Salkever D, Slade EP, Peng X, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry 2010;10. https://doi.org/10.1186/1471-244X-10-2.
- Zeidler J, Slawik L, Fleischmann J, Greiner W. The costs of schizophrenia and predictors of hospitalisation from the statutory health insurance perspective. Health Econ Rev 2012;2. https://doi.org/10.1186/2191-1991-2-9.
- Raudino A, Carr VJ, Bush R, Saw S, Burgess P, Morgan VA. Patterns of service utilisation in psychosis: findings of the 2010 Australian national survey of psychosis. Aust N Z J Psychiatry 2014;48:341-51. https://doi.org/10.1177/0004867413511996.
- Gaebel W, Riesbeck M. Are there clinically useful predictors and early warning signs for pending relapse. Schizophr Res 2014;152:469-77. https://doi.org/10.1016/j.schres.2013.08.003.
- Sullivan S, Northstone K, Gadd C, Walker J, Margelyte R, Richards A, et al. Models to predict relapse in psychosis: a systematic review. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0183998.
- Subotnik KL, Nuechterlein KH, Ventura J, Gitlin MJ, Marder S, Mintz J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry 2011;168:286-92. https://doi.org/10.1176/appi.ajp.2010.09010087.
- Day JC, Bentall RP, Roberts C, Randall F, Rogers A, Cattell D, et al. Attitudes toward antipsychotic medication. Arch Gen Psychiatry 2005;62. https://doi.org/10.1001/archpsyc.62.7.717.
- Lambert M, Conus P, Cotton S, Robinson J, McGorry PD, Schimmelmann BG. Prevalence, predictors, and consequences of long-term refusal of antipsychotic treatment in first-episode psychosis. J Clin Psychopharmacol 2010;30:565-72. https://doi.org/10.1097/JCP.0b013e3181f058a0.
- Novick D, Haro JM, Suarez D, Perez V, Dittmann RW, Haddad PM. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res 2010;176:109-13. https://doi.org/10.1016/j.psychres.2009.05.004.
- Rummel-Kluge C, Schuster T, Peters S, Kissling W. Partial compliance with antipsychotic medication is common in patients with schizophrenia. Aust N Z J Psychiatry 2008;42:382-8. https://doi.org/10.1080/00048670801961107.
- Rooke O, Birchwood M. Loss, humiliation and entrapment as appraisals of schizophrenic illness: a prospective study of depressed and non-depressed patients. Br J Clin Psychol 1998;37:259-68. https://doi.org/10.1111/j.2044-8260.1998.tb01384.x.
- Gleeson JF, Rawlings D, Jackson HJ, McGorry PD. Agreeableness and neuroticism as predictors of relapse after first-episode psychosis: a prospective follow-up study. J Nerv Ment Dis 2005;193:160-9. https://doi.org/10.1097/01.nmd.0000154841.99550.d3.
- Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15-year followup of a Dutch incidence cohort. Schizophr Bull 1998;24:75-8. https://doi.org/10.1093/oxfordjournals.schbul.a033315.
- Vigod SN, Kurdyak PA, Dennis CL, Leszcz T, Taylor VH, Blumberger DM, et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry 2013;202:187-94. https://doi.org/10.1192/bjp.bp.112.115030.
- National Institute for Health and Care Excellence . Psychosis and Schizophrenia in Adults: Prevention and Management 2014.
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382:951-62. https://doi.org/10.1016/S0140-6736(13)60733-3.
- Zhao YJ, Lin L, Teng M, Khoo AL, Soh LB, Furukawa TA, et al. Long-term antipsychotic treatment in schizophrenia: systematic review and network meta-analysis of randomised controlled trials. BJPsych Open 2016;2:59-66. https://doi.org/10.1192/bjpo.bp.115.002576.
- Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry 2019;18:208-24. https://doi.org/10.1002/wps.20632.
- Swartz MS, Stroup TS, McEvoy JP, Davis SM, Rosenheck RA, Keefe RS, et al. What CATIE found: results from the schizophrenia trial. Psychiatr Serv 2008;59:500-6. https://doi.org/10.1176/ps.2008.59.5.500.
- Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Orbach G, et al. Psychological treatments in schizophrenia: I. Meta-analysis of family intervention and cognitive behaviour therapy. Psychol Med 2002;32:763-82. https://doi.org/10.1017/s0033291702005895.
- Zimmermann G, Favrod J, Trieu VH, Pomini V. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr Res 2005;77:1-9. https://doi.org/10.1016/j.schres.2005.02.018.
- Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008;34:523-37. https://doi.org/10.1093/schbul/sbm114.
- Lincoln TM, Suttner C, Nestoriuc Y. [Effects of cognitive interventions for schizophrenia: a meta-analysis.]. Psychol Rundschau 2008;59:217-32. https://doi.org/10.1026/0033-3042.59.4.217.
- Jones C, Cormac I, Silveira daMota Neto J, Campbell C. Cognitive-behaviour therapy for schizophrenia. Cochrane Database Syst Rev 2004;4:159-63. https://doi.org/10.1002/14651858.CD000524.pub2.
- Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry 2014;204:20-9. https://doi.org/10.1192/bjp.bp.112.116285.
- Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia. Cochrane Database Syst Rev 2012;4. https://doi.org/10.1002/14651858.CD008712.pub2.
- Haddock G, Spaulding W. Family interventions for psychosis. Prog Neurol Psychiatry 2013;17:18-9. https://doi.org/10.1002/pnp.273.
- Pharoah F, Mari J, Rathbone J, Wong W. Family intervention for schizophrenia. Cochrane Database Syst Rev 2010;12. https://doi.org/10.1002/14651858.CD000088.pub2.
- Harvey C, Lewis J, Farhall J. Receipt and targeting of evidence-based psychosocial interventions for people living with psychoses: findings from the second Australian national survey of psychosis. Epidemiol Psychiatr Sci 2019;28:613-29. https://doi.org/10.1017/S2045796018000288.
- Birchwood M, Smith J, Macmillan F, Hogg B, Prasad R, Harvey C, et al. Predicting relapse in schizophrenia: the development and implementation of an early signs monitoring system using patients and families as observers, a preliminary investigation. Psychol Med 1989;19:649-56. https://doi.org/10.1017/s0033291700024247.
- Herz MI. Prodromal symptoms and prevention of relapse in schizophrenia. J Clin Psychiatry 1985;46:22-5.
- Birchwood M, Spencer E, McGovern D. Schizophrenia: early warning signs. Adv Psychiatr Treat 2000;6:93-101. https://doi.org/10.1192/apt.6.2.93.
- Spaniel F, Bakstein E, Anyz J, Hlinka J, Sieger T, Hrdlicka J, et al. Relapse in schizophrenia: definitively not a bolt from the blue. Neurosci Lett 2018;669:68-74. https://doi.org/10.1016/j.neulet.2016.04.044.
- Eisner E, Drake R, Barrowclough C. Assessing early signs of relapse in psychosis: review and future directions. Clin Psychol Rev 2013;33:637-53. https://doi.org/10.1016/j.cpr.2013.04.001.
- Gumley A, O’Grady M, McNay L, Reilly J, Power K, Norrie J. Early intervention for relapse in schizophrenia: results of a 12-month randomized controlled trial of cognitive behavioural therapy. Psychol Med 2003;33:419-31. https://doi.org/10.1017/s0033291703007323.
- Herz MI, Lamberti JS, Mintz J, Scott R, O’Dell SP, McCartan L, et al. A program for relapse prevention in schizophrenia: a controlled study. Arch Gen Psychiatry 2000;57:277-83. https://doi.org/10.1001/archpsyc.57.3.277.
- Lee SH, Choi TK, Suh S, Kim YW, Kim B, Lee E, et al. Effectiveness of a psychosocial intervention for relapse prevention in patients with schizophrenia receiving risperidone via long-acting injection. Psychiatry Res 2010;175:195-9. https://doi.org/10.1016/j.psychres.2008.06.043.
- Stenberg JH, Jääskeläinen IP, Röyks R. The effect of symptom self-management training on rehospitalization for chronic schizophrenia in Finland. Int Rev Psychiatry 1998;10:58-61. https://doi.org/10.1080/09540269875113.
- van Meijel B, van der Gaag M, Kahn RS, Grypdonck M. The practice of early recognition and early intervention to prevent psychotic relapse in patients with schizophrenia: an exploratory study. Part 1. J Psychiatr Ment Health Nurs 2002;9:347-55. https://doi.org/10.1046/j.1365-2850.2002.00500.x.
- Marder SR, Wirshing WC, Van Putten T, Mintz J, McKenzie J, Johnston-Cronk K, et al. Fluphenazine vs placebo supplementation for prodromal signs of relapse in schizophrenia. Arch Gen Psychiatry 1994;51:280-7. https://doi.org/10.1001/archpsyc.1994.03950040024003.
- Carpenter WT, Heinrichs DW. Early intervention, time-limited, targeted pharmacotherapy of schizophrenia. Schizophr Bull 1983;9:533-42. https://doi.org/10.1093/schbul/9.4.533.
- Carpenter WT, Heinrichs DW, Hanlon TE. A comparative trial of pharmacologic strategies in schizophrenia. Am J Psychiatry 1987;144:1466-70. https://doi.org/10.1176/ajp.144.11.1466.
- Wiedemann G, Hahlweg K, Müller U, Feinstein E, Hank G, Dose M. Effectiveness of targeted intervention and maintenance pharmacotherapy in conjunction with family intervention in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2001;251:72-84. https://doi.org/10.1007/s004060170056.
- Wunderink L, Nienhuis FJ, Sytema S, Slooff CJ, Knegtering R, Wiersma D. Guided discontinuation versus maintenance treatment in remitted first-episode psychosis: relapse rates and functional outcome. J Clin Psychiatry 2007;68:654-61. https://doi.org/10.4088/jcp.v68n0502.
- Carpenter WT, Hanlon TE, Heinrichs DW, Summerfelt AT, Kirkpatrick B, Levine J, et al. Continuous versus targeted medication in schizophrenic outpatients: outcome results. Am J Psychiatry 1990;147:1138-48. https://doi.org/10.1176/ajp.147.9.1138.
- Gaebel W, Riesbeck M, Wölwer W, Klimke A, Eickhoff M, von Wilmsdorff M, et al. Relapse prevention in first-episode schizophrenia – maintenance vs intermittent drug treatment with prodrome-based early intervention: results of a randomized controlled trial within the German Research Network on Schizophrenia. J Clin Psychiatry 2011;72:205-18. https://doi.org/10.4088/JCP.09m05459yel.
- Herz MI, Glazer WM, Mostert MA, Sheard MA, Szymanski HV, Hafez H, et al. Intermittent vs. maintenance medication in schizophrenia. Two-year results. Arch Gen Psychiatry 1991;48:333-9. https://doi.org/10.1001/archpsyc.1991.01810280049007.
- Prytys M, Garety PA, Jolley S, Onwumere J, Craig T. Implementing the NICE guideline for schizophrenia recommendations for psychological therapies: a qualitative analysis of the attitudes of CMHT staff. Clin Psychol Psychother 2011;18:48-59. https://doi.org/10.1002/cpp.691.
- Jolley AG, Hirsch SR, McRink A, Manchanda R. Trial of brief intermittent neuroleptic prophylaxis for selected schizophrenic outpatients: clinical outcome at one year. BMJ 1989;298:985-90. https://doi.org/10.1136/bmj.298.6679.985.
- Pietzcker A, Gaebel W, Köpcke W, Linden M, Müller P, Müller-Spahn F, et al. Intermittent versus maintenance neuroleptic long-term treatment in schizophrenia-2-year results of a German multicenter study. J Psychiatr Res 1993;27:321-39. https://doi.org/10.1016/0022-3956(93)90059-B.
- Ruskin PE, Bland W, Feldman S. Continuous vs. targeted medication in older schizophrenic outpatients. Am J Geriatr Psychiatry 1994;2:134-43. https://doi.org/10.1097/00019442-199405000-00007.
- Schooler NR, Keith SJ, Severe JB, Matthews SM, Bellack AS, Glick ID, et al. Relapse rehospitalization schizophrenia. Arch Gen Psychiatry 1997;54:453-63. https://doi.org/10.1001/archpsyc.1997.01830170079011.
- Morriss R, Vinjamuri I, Faizal MA, Bolton CA, McCarthy JP. Training to recognise the early signs of recurrence in schizophrenia. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD005147.pub2.
- van Os J, Kapur S. Schizophrenia. Lancet 2009;374:635-45. https://doi.org/10.1016/S0140-6736(09)60995-8.
- Birchwood M. Pathways to emotional dysfunction in first-episode psychosis. Br J Psychiatry 2003;182:373-5. https://doi.org/10.1192/bjp.182.5.373.
- Hawton K, Sutton L, Haw C, Sinclair J, Deeks JJ. Schizophrenia and suicide: systematic review of risk factors. Br J Psychiatry 2005;187:9-20. https://doi.org/10.1192/bjp.187.1.9.
- Phillips LJ, Francey SM, Edwards J, McMurray N. Strategies used by psychotic individuals to cope with life stress and symptoms of illness: a systematic review. Anxiety Stress Coping 2009;22:371-410. https://doi.org/10.1080/10615800902811065.
- Horan WP, Ventura J, Mintz J, Kopelowicz A, Wirshing D, Christian-Herman J, et al. Stress and coping responses to a natural disaster in people with schizophrenia. Psychiatry Res 2007;151:77-86. https://doi.org/10.1016/j.psychres.2006.10.009.
- Lysaker PH, Taylor AC. Personality dimensions in schizophrenia: associations with symptoms and coping concurrently and 12 months later. Psychopathology 2007;40:338-44. https://doi.org/10.1159/000105532.
- Lysaker PH, Wilt MA, Plascak-Hallberg CD, Brenner CA, Clements CA. Personality dimensions in schizophrenia: associations with symptoms and coping. J Nerv Ment Dis 2003;191:80-6. https://doi.org/10.1097/01.NMD.0000050936.81128.5B.
- Tait L, Birchwood M, Trower P. Adapting to the challenge of psychosis: personal resilience and the use of sealing-over (avoidant) coping strategies. Br J Psychiatry 2004;185:410-15. https://doi.org/10.1192/bjp.185.5.410.
- Lysaker PH, Bryson GJ, Marks K, Greig TC, Bell MD. Coping style in schizophrenia: associations with neurocognitive deficits and personality. Schizophr Bull 2004;30:113-21. https://doi.org/10.1093/oxfordjournals.schbul.a007056.
- Gumley AI, Taylor HE, Schwannauer M, MacBeth A. A systematic review of attachment and psychosis: measurement, construct validity and outcomes. Acta Psychiatr Scand 2014;129:257-74. https://doi.org/10.1111/acps.12172.
- Bucci S, Roberts NH, Danquah AN, Berry K. Using attachment theory to inform the design and delivery of mental health services: a systematic review of the literature. Psychol Psychother 2015;88:1-20. https://doi.org/10.1111/papt.12029.
- Freeman D, Garety PA, Kuipers E. Persecutory delusions: developing the understanding of belief maintenance and emotional distress. Psychol Med 2001;31:1293-306. https://doi.org/10.1017/s003329170100455x.
- Thornicroft G, Farrelly S, Szmukler G, Birchwood M, Waheed W, Flach C, et al. Clinical outcomes of joint crisis plans to reduce compulsory treatment for people with psychosis: a randomised controlled trial. Lancet 2013;381:1634-41. https://doi.org/10.1016/S0140-6736(13)60105-1.
- Farrelly S, Brown G, Rose D, Doherty E, Henderson RC, Birchwood M, et al. What service users with psychotic disorders want in a mental health crisis or relapse: thematic analysis of joint crisis plans. Soc Psychiatry Psychiatr Epidemiol 2014;49:1609-17. https://doi.org/10.1007/s00127-014-0869-1.
- Gumley AI, Park C, French P, Reed M, Rayne M, Shiers D, et al. Promoting Recovery in Early Psychosis: A Practice Manual. Chichester: Wiley-Blackwell; 2010.
- Eisner E, Barrowclough C, Lobban F, Drake R. Qualitative investigation of targets for and barriers to interventions to prevent psychosis relapse. BMC Psychiatry 2014;14. https://doi.org/10.1186/1471-244X-14-201.
- Takahashi D. Newzoo: Smartphone Users Will Top 3 Billion in 2018, Hit 3.8 Billion by 2021 2018. https://venturebeat.com/2018/09/11/newzoo-smartphone-users-will-top-3-billion-in-2018-hit-3-8-billion-by-2021/ (accessed 26 June 2019).
- Robotham D, Satkunanathan S, Doughty L, Wykes T. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res 2016;18. https://doi.org/10.2196/jmir.6511.
- Firth J, Cotter J, Torous J, Bucci S, Firth JA, Yung AR. Mobile phone ownership and endorsement of ‘mHealth’ among people with psychosis: a meta-analysis of cross-sectional studies. Schizophr Bull 2016;42:448-55. https://doi.org/10.1093/schbul/sbv132.
- Simblett S, Greer B, Matcham F, Curtis H, Polhemus A, Ferrão J, et al. Barriers to and facilitators of engagement with remote measurement technology for managing health: systematic review and content analysis of findings. J Med Internet Res 2018;20. https://doi.org/10.2196/10480.
- Gleeson JF, Alvarez-Jimenez M, Lederman R. Moderated online social therapy for recovery from early psychosis. Psychiatr Serv 2012;63. https://doi.org/10.1176/appi.ps.20120p719.
- O’Hanlon P, Aref-Adib G, Fonseca A, Lloyd-Evans B, Osborn D, Johnson S. Tomorrows world: current developments in the therapeutic use of technology for psychosis. BJPsych Adv 2016;22:301-10. https://doi.org/10.1192/apt.bp.115.014654.
- Ben-Zeev D, Davis KE, Kaiser S, Krzsos I, Drake RE. Mobile technologies among people with serious mental illness: opportunities for future services. Adm Policy Ment Health 2013;40:340-3. https://doi.org/10.1007/s10488-012-0424-x.
- Naslund JA, Marsch LA, McHugo GJ, Bartels SJ. Emerging mHealth and eHealth interventions for serious mental illness: a review of the literature. J Ment Health 2015;24:321-32. https://doi.org/10.3109/09638237.2015.1019054.
- Torous J, Firth J, Mueller N, Onnela JP, Baker JT. Methodology and reporting of mobile heath and smartphone application studies for schizophrenia. Harv Rev Psychiatry 2017;25:146-54. https://doi.org/10.1097/HRP.0000000000000133.
- Španiel F, Hrdlička J, Novák T, Kožený J, Höschl C, Mohr P, et al. Effectiveness of the information technology-aided program of relapse prevention in schizophrenia (ITAREPS): a randomized, controlled, double-blind study. J Psychiatr Pract 2012;18:269-80. https://doi.org/10.1097/01.pra.0000416017.45591.c1.
- Barnett I, Torous J, Staples P, Sandoval L, Keshavan M, Onnela JP. Relapse prediction in schizophrenia through digital phenotyping: a pilot study. Neuropsychopharmacology 2018;43:1660-6. https://doi.org/10.1038/s41386-018-0030-z.
- Cella M, He Z, Killikelly C, Okruszek Ł, Lewis S, Wykes T. Blending active and passive digital technology methods to improve symptom monitoring in early psychosis. Early Interv Psychiatry 2019;13:1271-5. https://doi.org/10.1111/eip.12796.
- Bell IH, Lim MH, Rossell SL, Thomas N. Ecological momentary assessment and intervention in the treatment of psychotic disorders: a systematic review. Psychiatr Serv 2017;68:1172-81. https://doi.org/10.1176/appi.ps.201600523.
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol 2008;4:1-32. https://doi.org/10.1146/annurev.clinpsy.3.022806.091415.
- Ben-Zeev D, Brenner CJ, Begale M, Duffecy J, Mohr DC, Mueser KT. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull 2014;40:1244-53. https://doi.org/10.1093/schbul/sbu033.
- Ben-Zeev D, Wang R, Abdullah S, Brian R, Scherer EA, Mistler LA, et al. Mobile behavioral sensing for outpatients and inpatients with schizophrenia. Psychiatr Serv 2016;67:558-61. https://doi.org/10.1176/appi.ps.201500130.
- Depp CA, Mausbach B, Granholm E, Cardenas V, Ben-Zeev D, Patterson TL, et al. Mobile interventions for severe mental illness: design and preliminary data from three approaches. J Nerv Ment Dis 2010;198:715-21. https://doi.org/10.1097/NMD.0b013e3181f49ea3.
- Granholm E, Ben-Zeev D, Link PC, Bradshaw KR, Holden JL. Mobile Assessment and Treatment for Schizophrenia (MATS): a pilot trial of an interactive text-messaging intervention for medication adherence, socialization, and auditory hallucinations. Schizophr Bull 2012;38:414-25. https://doi.org/10.1093/schbul/sbr155.
- Pijnenborg GHM, Withaar FK, Brouwer WH, Timmerman ME, Van Den Bosch RJ, Evans JJ. The efficacy of SMS text messages to compensate for the effects of cognitive impairments in schizophrenia. Br J Clin Psychol 2010;49:259-74. https://doi.org/10.1348/014466509X467828.
- Sablier J, Stip E, Jacquet P, Giroux S, Pigot H, Franck N. Mobus Group. Ecological assessments of activities of daily living and personal experiences with Mobus, an assistive technology for cognition: a pilot study in schizophrenia. Assist Technol 2012;24:67-7. https://doi.org/10.1080/10400435.2012.659324.
- Spaniel F, Vohlídka P, Hrdlicka J, Kozený J, Novák T, Motlová L, et al. ITAREPS: information technology aided relapse prevention programme in schizophrenia. Schizophr Res 2008;98:312-17. https://doi.org/10.1016/j.schres.2007.09.005.
- Spaniel F, Vohlídka P, Kozený J, Novák T, Hrdlicka J, Motlová L, et al. The Information Technology Aided Relapse Prevention Programme in Schizophrenia: an extension of a mirror-design follow-up. Int J Clin Pract 2008;62:1943-6. https://doi.org/10.1111/j.1742-1241.2008.01903.x.
- Eisner E, Bucci S, Berry N, Emsley R, Barrowclough C, Drake RJ. Feasibility of using a smartphone app to assess early signs, basic symptoms and psychotic symptoms over six months: a preliminary report. Schizophr Res 2019;208:105-13. https://doi.org/10.1016/j.schres.2019.04.003.
- Komatsu H, Sekine Y, Okamura N, Kanahara N, Okita K, Matsubara S, et al. Effectiveness of Information Technology Aided Relapse Prevention Programme in Schizophrenia excluding the effect of user adherence: a randomized controlled trial. Schizophr Res 2013;150:240-4. https://doi.org/10.1016/j.schres.2013.08.007.
- Schultze-Lutter F, Klosterkötter J, Picker H, Steinmeyer EM, Ruhrmann S. Predicting first-episode psychosis by basic symptom criteria. Clin Neuropsychiatry 2007;4:11-22.
- Bradstreet S, Allan S, Gumley A. Adverse event monitoring in mHealth for psychosis interventions provides an important opportunity for learning. J Ment Health 2019;28:461-6. https://doi.org/10.1080/09638237.2019.1630727.
- Ng MM, Firth J, Minen M, Torous J. User engagement in mental health apps: a review of measurement, reporting, and validity. Psychiatr Serv 2019;70:538-44. https://doi.org/10.1176/appi.ps.201800519.
- Aref-Adib G, McCloud T, Ross J, O’Hanlon P, Appleton V, Rowe S, et al. Factors affecting implementation of digital health interventions for people with psychosis or bipolar disorder, and their family and friends: a systematic review. Lancet Psychiatry 2019;6:257-66. https://doi.org/10.1016/S2215-0366(18)30302-X.
- Biagianti B, Hidalgo-Mazzei D, Meyer N. Developing digital interventions for people living with serious mental illness: perspectives from three mHealth studies. Evid Based Ment Health 2017;20:98-101. https://doi.org/10.1136/eb-2017-102765.
- Killikelly C, He Z, Reeder C, Wykes T. Improving adherence to web-based and mobile technologies for people with psychosis: systematic review of new potential predictors of adherence. JMIR Mhealth Uhealth 2017;5. https://doi.org/10.2196/mhealth.7088.
- Chinman M, McInnes DK, Eisen S, Ellison M, Farkas M, Armstrong M, et al. Establishing a research agenda for understanding the role and impact of mental health peer specialists. Psychiatr Serv 2017;68:955-7. https://doi.org/10.1176/appi.ps.201700054.
- Repper J, Carter T. A review of the literature on peer support in mental health services. J Ment Health 2011;20:392-411. https://doi.org/10.3109/09638237.2011.583947.
- Gillard S, Foster R, Gibson S, Goldsmith L, Marks J, White S. Describing a principles-based approach to developing and evaluating peer worker roles as peer support moves into mainstream mental health services. Ment Heal Soc Incl 2017;21:133-43. https://doi.org/10.1108/MHSI-03-2017-0016.
- Lloyd-Evans B, Mayo-Wilson E, Harrison B, Istead H, Brown E, Pilling S, et al. A systematic review and meta-analysis of randomised controlled trials of peer support for people with severe mental illness. BMC Psychiatry 2014;14. https://doi.org/10.1186/1471-244X-14-39.
- Pitt V, Lowe D, Hill S, Prictor M, Hetrick SE, Ryan R, et al. Consumer-providers of care for adult clients of statutory mental health services. Cochrane Database Syst Rev 2013;3. https://doi.org/10.1002/14651858.CD004807.pub2.
- Johnson S, Lamb D, Marston L, Osborn D, Mason O, Henderson C, et al. Peer-supported self-management for people discharged from a mental health crisis team: a randomised controlled trial. Lancet 2018;392:409-18. https://doi.org/10.1016/S0140-6736(18)31470-3.
- Fortuna KL, Brooks JM, Umucu E, Walker R, Chow PI. Peer support: a human factor to enhance engagement in digital health behavior change interventions. J Technol Behav Sci 2019;4:152-61. https://doi.org/10.1007/s41347-019-00105-x.
- Yadav P, Kr Yadav U, Verma S. Software testing: approach to identify software bugs. Int J Res Rev Eng Sci Technol 2012;1:26-30.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- NHS Digital . Approved Cryptographic Algorithms: Good Practice Guideline 2016.
- International Organization for Standardization . ISO/IEC/25010:2011./Systems/and/Software/Engineering/—/Systems/and/Software/Quality/Requirements/and/Evaluation/(SQuaRE)/—/System/and/Software/Quality/Models 2017.
- European Parliament and Council of the European Union . General Data Protection Regulation 2016.
- Gumley A, Bradstreet S, Ainsworth J, Allan S, Alvarez-Jimenez M, Beattie L, et al. Early signs monitoring to prevent relapse in psychosis and promote well-being, engagement, and recovery: protocol for a feasibility cluster randomized controlled trial harnessing mobile phone technology blended with peer support. JMIR Res Protoc 2020;9. https://doi.org/10.2196/15058.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345. https://doi.org/10.1136/bmj.e5661.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. PAFS consensus group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0105-8.
- World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. Gesundheitswesen 2016;78:175-88. https://doi.org/10.1055/s-0041-111066.
- Solomon P. Peer support/peer provided services underlying processes, benefits, and critical ingredients. Psychiatr Rehabil J 2004;27:392-401. https://doi.org/10.2975/27.2004.392.401.
- George LC, O’Hagan M, Bradstreet S, Burge M, Smith M, Jury A. Workforce Development Theory and Practice in the Mental Health Sector. Hershey, PA: IGI Global; 2016.
- Scottish Recovery Network . Experts by Experience: Values Framework for Peer Working 2012.
- Palmier-Claus JE, Ainsworth J, Machin M, Barrowclough C, Dunn G, Barkus E, et al. The feasibility and validity of ambulatory self-report of psychotic symptoms using a smartphone software application. BMC Psychiatry 2012;12. https://doi.org/10.1186/1471-244X-12-172.
- Palmier-Claus JE, Rogers A, Ainsworth J, Machin M, Barrowclough C, Laverty L, et al. Integrating mobile-phone based assessment for psychosis. BMC Psychiatry 2013;13. https://doi.org/10.1186/1471-244X-13-34.
- Ainsworth J, Palmier-Claus JE, Machin M, Barrowclough C, Dunn G, Rogers A, et al. A comparison of two delivery modalities of a mobile phone-based assessment for serious mental illness: native smartphone application vs text-messaging only implementations. J Med Internet Res 2013;15. https://doi.org/10.2196/jmir.2328.
- Lewis SW, Ainsworth J, Sanders C, Stockton-Powdrell C, Machin M, Whelan P, et al. Smartphone-enhanced symptom management in psychosis: an open, randomized controlled trial. J Med Internet Res 2020;22.
- Stoyanov SR, Hides L, Kavanagh DJ, Wilson H. Development and validation of the user version of the Mobile Application Rating Scale (uMARS). JMIR Mhealth Uhealth 2016;4. https://doi.org/10.2196/mhealth.5849.
- Great Britain . The Medical Devices Regulations 2002 2002.
- International Organization for Standardization . Clinical Investigation of Medical Devices for Human Subjects — Good Clinical Practice 2011.
- World Health Organization . Handbook for Good Clinical Research Practice (GCP): Guidance for Implementation 2002.
- Goodwin I, Holmes G, Cochrane R, Mason O. The ability of adult mental health services to meet clients’ attachment needs: the development and implementation of the service attachment questionnaire. Psychol Psychother 2003;76:145-61. https://doi.org/10.1348/147608303765951186.
- Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr Res 2000;42:241-7. https://doi.org/10.1016/S0920-9964(99)00130-9.
- Great Britain . Mental Health Act 1983 1983.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. https://doi.org/10.1093/schbul/13.2.261.
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000;101:323-9. https://doi.org/10.1034/j.1600-0447.2000.101004323.x.
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res 1990;3:247-51. https://doi.org/10.1016/0920-9964(90)90005-R.
- Sobell LC, Sobell MB, Rush J. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Birchwood M, Jackson C, Brunet K, Holden J, Barton K. Personal beliefs about illness questionnaire-revised (PBIQ-R): reliability and validation in a first episode sample. Br J Clin Psychol 2012;51:448-58. https://doi.org/10.1111/j.2044-8260.2012.02040.x.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Richardson J, Sinha K, Iezzi A, Khan MA. Modelling utility weights for the Assessment of Quality of Life (AQoL)-8D. Qual Life Res 2014;23:2395-404. https://doi.org/10.1007/s11136-014-0686-8.
- Hoefman RJ, van Exel NJ, Looren de Jong S, Redekop WK, Brouwer WB. A new test of the construct validity of the CarerQol instrument: measuring the impact of informal care giving. Qual Life Res 2011;20:875-87. https://doi.org/10.1007/s11136-010-9829-8.
- Neil ST, Kilbride M, Pitt L, Nothard S, Welford M, Sellwood W, et al. The questionnaire about the process of recovery (QPR): a measurement tool developed in collaboration with service users. Psychosis 2009;1:145-55. https://doi.org/10.1080/17522430902913450.
- Schwarzer R, Jerusalem M, Weinman J, Wright S, Johnston M. Measures in Health Psychology: A User’s Portfolio. Windsor: NFER-Nelson; 1995.
- Berry K, Wearden A. Attachment styles, interpersonal relationships and psychotic phenomena in a non-clinical student sample. Pers Individ Dif 2006;41:707-18. https://doi.org/10.1016/j.paid.2006.03.009.
- Chambless DL, Blake KD. Construct validity of the perceived criticism measure. Behav Ther 2009;40:155-63. https://doi.org/10.1016/j.beth.2008.05.005.
- van Wijngaarden B, Schene AH, Koeter M, Vázquez-Barquero JL, Knudsen HC, Lasalvia A, et al. Caregiving in schizophrenia: development, internal consistency and reliability of the Involvement Evaluation Questionnaire – European Version. EPSILON Study 4. European Psychiatric Services: inputs linked to outcome domains and needs. Br J Psychiatry Suppl 2000;39:s21-7. https://doi.org/10.1192/bjp.177.39.s21.
- Tait L, Birchwood M, Trower P. A new scale (SES) to measure engagement with community mental health services. J Ment Health 2002;11:191-8. https://doi.org/10.1080/09638230020023570-2.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Allan S, McLeod H, Bradstreet S, Beedie S, Moir B, Gleeson J, et al. Understanding implementation of a digital self-monitoring intervention for relapse prevention in psychosis: protocol for a mixed method process evaluation. JMIR Res Protoc 2019;8. https://doi.org/10.2196/15634.
- University of Aberdeen . Statistical Analysis Plans n.d. www.abdn.ac.uk/hsru/what-we-do/trials-unit/statistical-analysis-plans-611.php (accessed 13 September 2019).
- Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6. https://doi.org/10.1093/aje/kwh090.
- Brazier J, Connell J, Papaioannou D, Mukuria C, Mulhern B, Peasgood T, et al. A systematic review, psychometric analysis and qualitative assessment of generic preference-based measures of health in mental health populations and the estimation of mapping functions from widely used specific measures. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18340.
- Bucci S, Barrowclough C, Ainsworth J, Machin M, Morris R, Berry K, et al. Actissist: proof-of-concept trial of a theory-driven digital intervention for psychosis. Schizophr Bull 2018;44:1070-80. https://doi.org/10.1093/schbul/sby032.
- Torous J, Woodyatt J, Keshavan M, Tully LM. A new hope for early psychosis care: the evolving landscape of digital care tools. Br J Psychiatry 2019;214:269-72. https://doi.org/10.1192/bjp.2019.8.
- Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.8775.
- Department of Health and Social Care . A Framework for Mental Health Research 2017.
- Christie HL, Martin JL, Connor J, Tange HJ, Verhey FRJ, de Vugt ME, et al. eHealth interventions to support caregivers of people with dementia may be proven effective, but are they implementation-ready?. Internet Interv 2019;18. https://doi.org/10.1016/j.invent.2019.100260.
- Torous J, Firth J. Bridging the dichotomy of actual versus aspirational digital health. World Psychiatry 2018;17:108-9. https://doi.org/10.1002/wps.20464.
- Arnold C, Villagonzalo KA, Meyer D, Farhall J, Foley F, Kyrios M, et al. Predicting engagement with an online psychosocial intervention for psychosis: exploring individual- and intervention-level predictors. Internet Interv 2019;18. https://doi.org/10.1016/j.invent.2019.100266.
- Greenhalgh T, Snow R, Ryan S, Rees S, Salisbury H. Six ‘biases’ against patients and carers in evidence-based medicine. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0437-x.
- Faulkner A. Randomised Controlled Trials: The Straitjacket of Mental Health Research?. London: McPin Foundation; 2015.
- Gray-Burrows KA, Willis TA, Foy R, Rathfelder M, Bland P, Chin A, et al. Role of patient and public involvement in implementation research: a consensus study. BMJ Qual Saf 2018;27:858-64. https://doi.org/10.1136/bmjqs-2017-006954.
- Turner S, D’Lima D, Hudson E, Morris S, Sheringham J, Swart N, et al. Evidence use in decision-making on introducing innovations: a systematic scoping review with stakeholder feedback. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0669-6.
- Brocklehurst PR, Williams L, Burton C, Goodwin T, Rycroft-Malone J. Implementation and trial evidence: a plea for fore-thought. Br Dent J 2017;222:331-5. https://doi.org/10.1038/sj.bdj.2017.213.
- Braithwaite J, Churruca K, Long JC, Ellis LA, Herkes J. When complexity science meets implementation science: a theoretical and empirical analysis of systems change. BMC Med 2018;16. https://doi.org/10.1186/s12916-018-1057-z.
- Moore GF, Evans RE. What theory, for whom and in which context? Reflections on the application of theory in the development and evaluation of complex population health interventions. SSM Popul Health 2017;3:132-5. https://doi.org/10.1016/j.ssmph.2016.12.005.
- Sutcliffe K, Thomas J, Stokes G, Hinds K, Bangpan M. Intervention component analysis (ICA): a pragmatic approach for identifying the critical features of complex interventions. Syst Rev 2015;4. https://doi.org/10.1186/s13643-015-0126-z.
- Hardy A, Wojdecka A, West J, Matthews E, Golby C, Ward T, et al. How inclusive, user-centered design research can improve psychological therapies for psychosis: development of SlowMo. JMIR Ment Health 2018;5. https://doi.org/10.2196/11222.
- Huerta-Ramos E, Escobar-Villegas MS, Rubinstein K, Unoka ZS, Grasa E, Hospedales M, et al. measuring users’ receptivity toward an integral intervention model based on mHealth Solutions for Patients With Treatment-Resistant Schizophrenia (m-RESIST): a qualitative study. JMIR Mhealth Uhealth 2016;4. https://doi.org/10.2196/mhealth.5716.
- Lobban F, Appleton V, Appelbe D, Barraclough J, Bowland J, Fisher NR, et al. IMPlementation of A Relatives’ Toolkit (IMPART study): an iterative case study to identify key factors impacting on the implementation of a web-based supported self-management intervention for relatives of people with psychosis or bipolar experiences in a National Health Service: a study protocol. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0687-4.
- Bonell C, Fletcher A, Morton M, Lorenc T, Moore L. Realist randomised controlled trials: a new approach to evaluating complex public health interventions. Soc Sci Med 2012;75:2299-306. https://doi.org/10.1016/j.socscimed.2012.08.032.
- Greenhalgh T. What have the social sciences ever done for equity in health policy and health systems?. Int J Equity Health 2018;17:1-3. https://doi.org/10.1186/s12939-018-0842-9.
- May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0758-1.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Qual Saf 2015;24:228-38. https://doi.org/10.1136/bmjqs-2014-003627.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Braun V, Clarke V. Novel insights into patients’ life-worlds: the value of qualitative research. Lancet Psychiatry 2019;6:720-1. https://doi.org/10.1016/S2215-0366(19)30296-2.
- Krueger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research. London: SAGE Publications Ltd; 2008.
- Bertolino A. Software Testing Research: Achievements, Challenges, Dreams 2007:85-103. https://doi.org/10.1109/FOSE.2007.25.
- Bucholtz M. The politics of transcription. J Pragmat 2000;32:1439-65. https://doi.org/10.1016/S0378-2166(99)00094-6.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Doyle S. Member checking with older women: a framework for negotiating meaning. Health Care Women Int 2007;28:888-90. https://doi.org/10.1080/07399330701615325.
- Smith JA. Beyond the divide between cognition and discourse: using interpretative phenomenological analysis in health psychology. Psychol Heal 1996;11:261-71. https://doi.org/10.1080/08870449608400256.
- Smith JA, Flowers P, Larkin M. Interpretative Phenomenological Analysis: Theory, Method and Research. London: SAGE; 2009.
- Smith JA, Osborn M, Smith JA. Qualitative Psychology: A Practical Guide to Research Methods. London: SAGE Publications Ltd; 2003.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- Greer B, Robotham D, Simblett S, Curtis H, Griffiths H, Wykes T. Digital exclusion among mental health service users: qualitative investigation. J Med Internet Res 2019;21. https://doi.org/10.2196/11696.
- Berry N, Bucci S, Lobban F. Use of the internet and mobile phones for self-management of severe mental health problems: qualitative study of staff views. JMIR Ment Health 2017;4. https://doi.org/10.2196/mental.8311.
- May CR, Johnson M, Finch T. Implementation, context and complexity. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0506-3.
- Farrelly S, Lester H, Rose D, Birchwood M, Marshall M, Waheed W, et al. Barriers to shared decision making in mental health care: qualitative study of the Joint Crisis Plan for psychosis. Health Expect 2016;19:448-58. https://doi.org/10.1111/hex.12368.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- Vilardaga R, Rizo J, Kientz JA, McDonell MG, Ries RK, Sobel K. User experience evaluation of a smoking cessation app in people with serious mental illness. Nicotine Tob Res 2016;18:1032-8. https://doi.org/10.1093/ntr/ntv256.
- Ben-Zeev D, Kaiser SM, Brenner CJ, Begale M, Duffecy J, Mohr DC. Development and usability testing of FOCUS: a smartphone system for self-management of schizophrenia. Psychiatr Rehabil J 2013;36:289-96. https://doi.org/10.1037/prj0000019.
- Kumar D, Tully LM, Iosif AM, Zakskorn LN, Nye KE, Zia A, et al. A mobile health platform for clinical monitoring in early psychosis: implementation in community-based outpatient early psychosis care. JMIR Ment Health 2018;5. https://doi.org/10.2196/mental.8551.
- Suka M, Yamauchi T, Yanagisawa H. Comparing responses to differently framed and formatted persuasive messages to encourage help-seeking for depression in Japanese adults: a cross-sectional study with 2-month follow-up. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020823.
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophr Res 2005;79:231-8. https://doi.org/10.1016/j.schres.2005.04.008.
- Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl 1993;163:39-44. https://doi.org/10.1192/S0007125000292581.
- Eysenbach G. The law of attrition. J Med Internet Res 2005;7. https://doi.org/10.2196/jmir.7.1.e11.
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997;53:983-97. https://doi.org/10.2307/2533558.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Brouwer WB, van Exel NJ, van Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res 2006;15:1005-21. https://doi.org/10.1007/s11136-005-5994-6.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346:1-6. https://doi.org/10.1136/bmj.f1049.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Department of Health . Pharmaceutical Benefits Advisory Committee. Guidelines for Preparing Submissions to the Pharmaceutical Benefits Advisory Committee (Version 5.0) 2016.
- Campbell and Cochrane Economics Methods Group, Evidence for Policy and Practice Information and Coordinating Centre . CCEMG– EPPI Centre Cost Converter 2019. https://eppi.ioe.ac.uk/costconversion/default.aspx (accessed 9 September 2019).
- Tate DF, Finkelstein EA, Khavjou O, Gustafson A. Cost effectiveness of internet interventions: review and recommendations. Ann Behav Med 2009;38:40-5. https://doi.org/10.1007/s12160-009-9131-6.
- Johns B, Baltussen R, Hutubessy R. Programme costs in the economic evaluation of health interventions. Cost Eff Resour Alloc 2003;1. https://doi.org/10.1186/1478-7547-1-1.
- Donker T, Blankers M, Hedman E, Ljótsson B, Petrie K, Christensen H. Economic evaluations of Internet interventions for mental health: a systematic review. Psychol Med 2015;45:3357-76. https://doi.org/10.1017/S0033291715001427.
- Australian Bureau of Statistics . Australian Demographic Statistics, Dec 2018 2019.
- Office for National Statistics . Overview of the UK Population: July 2017 2017.
- Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R. An evaluation of variation in published estimates of schizophrenia prevalence from 1990–2013: a systematic literature review. BMC Psychiatry 2015;15:1-14. https://doi.org/10.1186/s12888-015-0578-7.
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet 2012;379:2063-71. https://doi.org/10.1016/S0140-6736(12)60239-6.
- Oppong R, Coast J, Hood K, Nuttall J, Smith RD, Butler CC. GRACE-01 Study Team . Resource use and costs of treating acute cough/lower respiratory tract infections in 13 European countries: results and challenges. Eur J Health Econ 2011;12:319-29. https://doi.org/10.1007/s10198-010-0239-1.
- Rivero-Arias O, Gray A. The multinational nature of cost-effectiveness analyses alongside multinational clinical trials. Value Health 2010;13:34-41. https://doi.org/10.1111/j.1524-4733.2009.00582.x.
- Oppong R, Jowett S, Roberts TE. Economic evaluation alongside multinational studies: a systematic review of empirical studies. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0131949.
- NHS Improvement . Reference Costs 2017 18: Highlights, Analysis and Introduction to the Data 2018. https://webarchive.nationalarchives.gov.uk/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 17 February 2021).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- Joint Formulary Committee . British National Formulary 2017.
- Department of Health . 2017 MBS File Downloads n.d. www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads-2017-PDF (accessed 18 February 2021).
- Department of Health, Australian Government . Pharmaceutical Benefits Scheme (PBS) n.d. https://pbs.gov.au/pbs/home;jsessionid=1rhrjqs83awc412dygpt5trt0o (accessed 16 September 2019).
- Independent Hospital Pricing Authority . National Hospital Cost Data Collection Report, Public Sector, Round 21 (Financial Year 2016–17) n.d. www.ihpa.gov.au/sites/default/files/publications/national_hospital_cost_data_collection_australian_public_hospitals_cost_report_round_21_2016-17.pdf (accessed 18 February 2021).
- Australian Institute for Health and Welfare . Mental Health Services in Australia n.d. www.aihw.gov.au/reports/mental-health-services/mental-health-services-in-australia/report-contents/expenditure-on-mental-health-related-services/specialised-mental-health-services-expenditure (accessed 18 February 2021).
- State of Victoria . Department of Health and Human Services Policy and Funding Guidelines 2015. Volume 2: Health Operations 2015–16. Chapter 2: Pricing and Funding Arrangements for Victoria’s Health System n.d. www.dhhs.vic.gov.au/policy-and-funding-guidelines-health-services (accessed 18 February 2021).
- Bedfordshire Police, Cambridgeshire Constabulary and Hertfordshire Constabulary (Strategic Alliance) . 2017/2018/Fees/and/Charges/Handbook 2017. www.herts.police.uk/assets/Information-and-services/About-us/AboutUs-FeesChargesHandbook-201718.pdf (accessed 17 February 2021).
- Productivity Commission . Report on Government Services 2019. www.pc.gov.au/research/ongoing/report-on-government-services (accessed 9 September 2019).
- Scottish Government . Costs of the Criminal Justice System in Scotland Dataset 2018 2018.
- Australian Bureau of Statistics . 4513.0 – Criminal Courts, Australia, 2017–18 2019. www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/4513.0Main+Features12017-18?OpenDocument= (accessed 9 September 2019).
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 1993;4:353-65. https://doi.org/10.2165/00019053-199304050-00006.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated November 2018) 2018. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 3 September 2019).
- Hernández-Alava M, Pudney S. eq5dmap: a command for mapping between EQ-5D-3L and EQ-5D-5L. Stata J 2018;18:395-41. https://doi.org/10.1177/1536867X1801800207.
- Hoefman RJ, van Exel J, Brouwer WBF. Measuring care-related quality of life of caregivers for use in economic evaluations: CarerQol tariffs for Australia, Germany, Sweden, UK, and US. PharmacoEconomics 2017;35:469-78. https://doi.org/10.1007/s40273-016-0477-x.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley and Sons; 2004.
- Ferguson CJ. An effect size primer: a guide for clinicians and researchers. Prof Psychol Res Pract 2009;40:532-8. https://doi.org/10.1037/a0015808.
- Jordhøy MS, Fayers PM, Ahlner-Elmqvist M, Kaasa S. Lack of concealment may lead to selection bias in cluster randomized trials of palliative care. Palliat Med 2002;16:43-9. https://doi.org/10.1191/0269216302pm523oa.
- Togerson DJ, Togerson CJ. Designing Randomised Controlled Trials in Health, Education and Social Sciences. An Introduction. New York, NY: Palgrave Macmillan; 2008.
- Giraudeau B, Ravaud P. Preventing bias in cluster randomised trials. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000065.
- Kelders SM, Kok RN, Ossebaard HC, Van Gemert-Pijnen JE. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J Med Internet Res 2012;14. https://doi.org/10.2196/jmir.2104.
- Perski O, Blandford A, West R, Michie S. Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. Transl Behav Med 2017;7:254-67. https://doi.org/10.1007/s13142-016-0453-1.
- Bucci S, Morris R, Berry K, Berry N, Haddock G, Barrowclough C, et al. Early psychosis service user views on digital technology: qualitative analysis. JMIR Ment Health 2018;5. https://doi.org/10.2196/10091.
- Eisner E, Drake RJ, Berry N, Barrowclough C, Emsley R, Machin M, et al. Development, usability and long-term acceptability of ExPRESS, a smartphone app to monitor basic symptoms and early signs of psychosis relapse. JMIR MHealth UHealth 2019;7. https://doi.org/10.2196/11568.
- Aref-Adib G, O’Hanlon P, Fullarton K, Morant N, Sommerlad A, Johnson S, et al. A qualitative study of online mental health information seeking behaviour by those with psychosis. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-0952-0.
- Allan S, Bradstreet S, McLeod H, Farhall J, Gleeson J, Clark A, et al. Monitoring early signs of psychosis relapse using a mobile app: developing a hypothetical implementation framework of expectations for staff, carers and service users using qualitative methods. J Med Internet Res 2019;21. https://doi.org/10.2196/14366.
- Gleeson JF, Alvarez-Jimenez M, Cotton SM, Parker AG, Hetrick S. A systematic review of relapse measurement in randomized controlled trials of relapse prevention in first-episode psychosis. Schizophr Res 2010;119:79-88. https://doi.org/10.1016/j.schres.2010.02.1073.
- Kishimoto T, Agarwal V, Kishi T, Leucht S, Kane JM, Correll CU. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol Psychiatry 2013;18:53-66. https://doi.org/10.1038/mp.2011.143.
- Sim J. Should treatment effects be estimated in pilot and feasibility studies?. Pilot Feasibility Stud 2019;5:1-7. https://doi.org/10.1186/s40814-019-0493-7.
- Eldridge SM, Costelloe CE, Kahan BC, Lancaster GA, Kerry SM. How big should the pilot study for my cluster randomised trial be?. Stat Methods Med Res 2015;25:1039-56. https://doi.org/10.1177/0962280215588242.
- Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 2016;25:1057-73. https://doi.org/10.1177/0962280215588241.
- Honary M, Fisher NR, McNaney R, Lobban F. A web-based intervention for relatives of people experiencing psychosis or bipolar disorder: design study using a user-centered approach. JMIR Ment Health 2018;5. https://doi.org/10.2196/11473.
- Lobban F, Robinson H, Appelbe D, Barraclough J, Bedson E, Collinge L, et al. Protocol for an online randomised controlled trial to evaluate the clinical and cost-effectiveness of a peer-supported self-management intervention for relatives of people with psychosis or bipolar disorder: Relatives Education And Coping Toolkit (REACT). BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016965.
- Lederman R, Gleeson J, Wadley G, D’alfonso S, Rice S, Santesteban-Echarri O, et al. Support for carers of young people with mental illness. ACM Trans Comput Interact 2019;26:1-33. https://doi.org/10.1145/3301421.
- Keetharuth AD, Brazier J, Connell J, Bjorner JB, Carlton J, Taylor Buck E, et al. Recovering Quality of Life (ReQoL): a new generic self-reported outcome measure for use with people experiencing mental health difficulties. Br J Psychiatry 2018;212:42-9. https://doi.org/10.1192/bjp.2017.10.
- Ben-Zeev D, Brian R, Wang R, Wang W, Campbell AT, Aung MSH, et al. CrossCheck: integrating self-report, behavioral sensing, and smartphone use to identify digital indicators of psychotic relapse. Psychiatr Rehabil J 2017;40:266-75. https://doi.org/10.1037/prj0000243.
- Buck B, Scherer E, Brian R, Wang R, Wang W, Campbell A, et al. Relationships between smartphone social behavior and relapse in schizophrenia: a preliminary report. Schizophr Res 2019;208:167-72. https://doi.org/10.1016/j.schres.2019.03.014.
- Fraccaro P, Beukenhorst A, Sperrin M, Harper S, Palmier-Claus J, Lewis S, et al. Digital biomarkers from geolocation data in bipolar disorder and schizophrenia: a systematic review. J Am Med Inform Assoc 2019;26:1412-20. https://doi.org/10.1093/jamia/ocz043.
- Kimhy D, Delespaul P, Corcoran C, Ahn H, Yale S, Malaspina D. Computerized experience sampling method (ESMc): assessing feasibility and validity among individuals with schizophrenia. J Psychiatr Res 2006;40:221-30. https://doi.org/10.1016/j.jpsychires.2005.09.007.
- Myin-Germeys I, Krabbendam L, Delespaul PAEG, Van Os J. Do life events have their effect on psychosis by influencing the emotional reactivity to daily life stress?. Psychol Med 2003;33:327-33. https://doi.org/10.1017/S0033291702006785.
- Wykes T, Schueller S. Why reviewing apps is not enough: Transparency for Trust (T4T) principles of responsible health app marketplaces. J Med Internet Res 2019;21. https://doi.org/10.2196/12390.
- Scherer EA, Ben-Zeev D, Li Z, Kane JM. Analyzing mHealth engagement: joint models for intensively collected user engagement data. JMIR Mhealth Uhealth 2017;5. https://doi.org/10.2196/mhealth.6474.
- Murray JM, French DP, Patterson CC, Kee F, Gough A, Tang J, et al. Predicting outcomes from engagement with specific components of an internet-based physical activity intervention with financial incentives: process analysis of a cluster randomized controlled trial. J Med Internet Res 2019;21. https://doi.org/10.2196/11394.
- Torous J, Nicholas J, Larsen ME, Firth J, Christensen H. Clinical review of user engagement with mental health smartphone apps: evidence, theory and improvements. Evid Based Ment Health 2018;21:116-19. https://doi.org/10.1136/eb-2018-102891.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Information Services Division (NHS National Services Scotland) . Scottish Health Service Costs: Year Ended 31 March 2017 2018. https://beta.isdscotland.org/find-publications-and-data/healthcare-resources/finance/scottish-health-service-costs/20-november-2018/ (accessed 17 February 2021).
- Office for National Statistics . Average Hourly Pay 2018. www.ethnicity-facts-figures.service.gov.uk/work-pay-and-benefits/pay-and-income/average-hourly-pay/latest (accessed 9 September 2019).
- Australian Government . Report on Government Services 2019 – Chapter 11 Ambulance Services n.d. www.pc.gov.au/research/ongoing/report-on-government-services/2019/health/ambulance-services (accessed 18 February 2021).
- Australian Government Fair Work Ombudsman . Minimum Wages n.d. www.fairwork.gov.au/how-we-will-help/templates-and-guides/fact-sheets/minimum-workplace-entitlements/minimum-wages (accessed 18 February 2021).
- Australian Bureau of Statistics . Characteristics of Employment, Australia, August 2018 n.d. www.abs.gov.au/statistics/labour/earnings-and-work-hours/characteristics-employment-australia/latest-release (accessed 18 February 2021).
Appendix 1 Novel or adapted measures
Appendix 2 Work package 3 semistructured interview
Appendix 3 Additional baseline tables
| Characteristic | EMPOWER (N = 42) | TAU (N = 31) | Total (N = 73) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 2 (29) | 3 (30) | 5 (29) |
| Female | 5 (71) | 7 (70) | 12 (71) |
| Age (years), n: mean (SD) | 7: 56 (14) | 9: 58 (9) | 16: 57 (11) |
| Years of education, n: mean (SD) | 5: 16.20 (3.03) | 8: 15.00 (4.87) | 13: 15.46 (4.16) |
| UK ethnicity, n (%) | 5 (71) | 8 (80) | 13 (76) |
| Scottish | 5 (100) | 6 (75) | 11 (85) |
| Other British | – | 1 (13) | 1 (8) |
| UK unknown | – | 1 (13) | 1 (8) |
| Australian ethnicity, n (%) | 2 (29) | 2 (20) | 4 (24) |
| Born in Australia | 2 (100) | 2 (100) | 4 (100) |
| Characteristic | EMPOWER (N = 42) | TAU (N = 31) | Total (N = 73) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 6 (27) | 3 (12) | 9 (19) |
| Female | 16 (73) | 19 (76) | 35 (74) |
| Missing | – | 3 (12) | 3 (6) |
| Time with team (years), n: mean (SD) | 13: 3.49 (4.52) | 12: 6.57 (5.81) | 25: 4.97 (5.30) |
| Years since qualified, n: mean (SD) | 13: 7.22 (5.39) | 12: 15.10 (9.57) | 25: 11.01 (8.52) |
Appendix 4 General feasibility tables
| EMPOWER | TAU | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |
| Have you used any well-being apps?, n (%) | ||||||||
| Not sure | 1 (2) | 3 (7) | 1 (2) | 1 (3) | 2 (6) | 1 (3) | ||
| Yes | 4 (10) | 23 (55) | 19 (45) | 20 (48) | 5 (16) | 7 (23) | 5 (16) | 4 (13) |
| No | 37 (88) | 6 (14) | 10 (24) | 10 (24) | 24 (77) | 19 (61) | 20 (65) | 24 (77) |
| Missing | 10 (24) | 12 (29) | 12 (29) | 1 (3) | 3 (10) | 5 (16) | 3 (10) | |
| How often have you used wellbeing apps?, n: mean (SD) | 36: 1.25 (0.77) | 26: 3.42 (1.14) | 27: 3.11 (1.22) | 27: 2.93 (1.33) | 28: 1.29 (0.66) | 24: 1.63 (1.10) | 22: 1.41 (0.96) | 25: 1.24 (0.66) |
| How often have you sought help with EWS?, n: mean (SD) | 36: 2.19 (1.12) | 26: 1.96 (1.00) | 27: 2.04 (1.16) | 27: 1.85 (1.06) | 28: 2.39 (1.20) | 24: 1.75 (1.15) | 22: 2.14 (1.13) | 25: 1.76 (1.05) |
| How often has your carer sought help for EWS?, n: mean (SD) | 35: 1.80 (1.02) | 26: 1.69 (1.05) | 27: 1.48 (0.94) | 27: 1.33 (0.73) | 28: 1.46 (0.79) | 24: 1.54 (0.88) | 22: 1.45 (0.86) | 25: 1.52 (0.87) |
| How often has this changed clinical management, n: mean (SD) | 36: 2.42 (1.02) | 26: 1.85 (1.01) | 27: 1.96 (1.16) | 27: 1.52 (0.94) | 28: 2.25 (1.21) | 24: 1.79 (1.14) | 22: 1.95 (1.09) | 25: 1.64 (1.11) |
| EMPOWER, n: mean (SD) | TAU, n: mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |
| How often has service user discussed EWS with you? | 6: 3.17 (0.75) | 5: 2.40 (1.14) | 5: 2.40 (0.55) | 4: 3.25 (0.96) | 10: 2.90 (0.99) | 6: 2.67 (1.03) | 5: 2.40 (0.55) | 4: 2.25 (1.26) |
| How often have they sought help with EWS? | 6: 3.17 (1.17) | 5: 2.80 (1.10) | 5: 2.00 (0.71) | 4: 2.00 (1.41) | 10: 2.60 (0.97) | 6: 2.33 (1.21) | 5: 2.80 (1.30) | 4: 2.00 (1.41) |
| How often have you sought help for their EWS? | 6: 2.33 (1.03) | 5: 2.20 (1.30) | 5: 2.00 (1.00) | 4: 1.75 (1.50) | 10: 1.70 (0.82) | 6: 1.50 (0.55) | 5: 1.40 (0.55) | 4: 1.75 (1.50) |
| Has this changed clinical management | 6: 2.00 (1.10) | 5: 2.00 (1.00) | 5: 1.60 (0.89) | 4: 1.50 (1.00) | 10: 1.90 (0.88) | 6: 2.00 (1.10) | 5: 2.20 (1.30) | 4: 1.50 (1.00) |
| EMPOWER, n: mean (SD) | TAU, n: mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |
| How often has service user discussed EWS with you? | 39: 2.97 (1.01) | 30: 2.83 (0.79) | 22: 2.91 (0.92) | 13: 2.85 (0.99) | 25: 3.32 (0.85) | 21: 3.05 (0.67) | 13: 2.54 (0.97) | 10: 2.40 (0.70) |
| How often have they sought help with EWS? | 39: 2.36 (1.16) | 30: 2.13 (1.04) | 22: 2.27 (1.16) | 13: 2.69 (1.03) | 24: 2.50 (1.35) | 22: 2.32 (1.29) | 13: 2.31 (0.95) | 11: 2.45 (0.93) |
| How often has carer sought help for service user for EWS? | 39: 1.85 (1.11) | 30: 1.50 (0.86) | 22: 1.32 (0.65) | 13: 1.38 (0.65) | 25: 1.76 (1.05) | 22: 1.64 (1.00) | 13: 1.31 (0.75) | 11: 1.73 (1.10) |
| Has this changed clinical management | 39: 2.21 (1.22) | 30: 2.03 (1.03) | 22: 1.82 (0.91) | 13: 1.85 (0.99) | 25: 2.28 (1.17) | 21: 2.33 (1.11) | 13: 1.77 (0.93) | 11: 1.91 (1.04) |
Appendix 5 Describing engagement with the EMPOWER app
Exploratory user engagement
Engagement with digital interventions can be conceptualised in multiple ways, including subjective experiences of how using an intervention makes a participant feel, as well as purely behavioural measures of actual intervention usage. 256 However, Kelders et al. 255 propose delineating user engagement into actual usage (i.e. how much participants use an intervention) and intended usage (i.e. how much participants must use an intervention to obtain a maximum benefit of some kind). Previous digital research in schizophrenia273,274 used an EMA response rate of 33% for data to be considered reliable, while acknowledging that the criteria for determining EMA response rate feasibility vary in the literature. 100 Using the terminology developed by Kelders et al. ,255 intended usage would be the period during which users complete at least 33% of daily prompts because this would result in data that are of maximum reliability for detecting clinical change.
The proportion of users still using an app in its intended manner after 2 weeks has been suggested as a suitable metric for assessing intervention engagement. 275 However, this metric was developed for apps that are delivered remotely and is therefore less suitable for EMPOWER, given that it is a blended intervention. Additionally, participant usage of digital interventions for psychosis is higher in interventions that involve a high level of human contact. 117 Furthermore, trying to develop an accurate estimation of intended usage over time is especially challenging in a feasibility trial. Therefore, it was decided to explore when participants stopped inputting data at least 3 days per week (meeting 33% intended usage criteria) for 4 sequential weeks.
Methods
Survival analysis methods are recommended for understanding attrition in digital interventions. 215 Attrition represents the amount of time to some sort of relevant event occurring, in this case 4 sequential weeks of not meeting the intended adherence criterion of completing 33% of daily prompts. In survival analysis, an event can be fully observed for some participants, for example participants who stop using the app for 4 weeks in a row. However, some participants might have used the app continuously during the observation period. In this case, it is known that these participants did not have an event during the observation period, but it is unknown if their usage would have dropped off had we observed them for longer. In survival analysis, participants such as these are said to be ‘censored’ for the purposes of analysis. Therefore, all participants who did not have an event during the observation period (n = 14, 42.4%; see below) were censored for the purposes of this analysis. Usage data were obtained from our secure server after the end of the trial.
Only data from participants who had completed a full baseline were eligible for this engagement analysis (n = 33). If participants had restarted using the app for any reason, only their final usage period was used in this analysis as long as they had completed a full baseline. Fourteen participants (42.4%) were still using the app at their final follow-up assessment, so their usage period was cut to the week during which that follow-up assessment fell. The analysis was completed using the survfit function in the survival package in R, with bootstrapping performed using the bootkm function from the hmisc package in R.
Results and discussion
Overall, the median survival for not missing 4 sequential weeks of intended usage was 32 weeks, with bootstrapped 95% CI 14 weeks; the upper limit returned an infinite value probably because of the skewed data. The large CIs can be seen in the Kaplan–Meier curve of time to 4 weeks’ app use of < 33% (see Figure 7). In other words, for 50% of participants who had completed a baseline it took 33 weeks until they missed 4 sequential weeks of intended intervention usage. The width of the CIs suggests that some degree of uncertainty is appropriate in interpreting the result from this test. To summarise, the time to 50% of participants having 4 sequential weeks of no longer meeting the intended adherence criterion is hard to predict within this sample (especially in terms of an upper limit) but it is likely not to fall below 14 weeks.
Levels of participant engagement with interventions may change over time and may have implications for understanding what successful engagement would look like. 276 Therefore, it is important to report actual usage in addition to intended usage. 255 Five participants (15.1%) who had an event still continued to use the intervention afterwards for short periods, with a few cases of participants then re-meeting the 33% criteria, which suggests that recovery to intended usage may be possible following a 4-week period of discontinuation. Descriptive statistics of usage for these participants are provided in Table 35.
| Participant | Weeks using post event | Mean days per week |
|---|---|---|
| 10302 | 4 | 1.25 |
| 40202 | 3 | 3 |
| 40301 | 4 | 3 |
| 60801 | 2 | 2 |
| 80601 | 1 | 1 |
There is debate about whether future intervention studies should measure levels of engagement, with a view to making recommendations for retaining groups of participants who are at the highest risk of non-usage277 or trying to understand what optimal engagement would look like from the end view of users,256 rather than assuming that increased usage is good in some way. Although participants may need to respond to a certain number of prompts for the data to be valid for clinical use,274 they may have their own views on optimal usage. To that end, actual raw usage (in weeks and the mean days across the observation period) is reported for all participants in Table 36. Please note that these usage levels are the actual usage in weeks (as reported from the direct server data) and do not reflect the usage period being cut to the week number during the follow-up assessment. One exception to this is participant 80503, who restarted the intervention; their final assessment was during week 1 of their usage period. Following a conversation among team members, the decision was made not to censor the participant at this point but to use their entire usage period in the analysis. These data may reflect user preference and so are being shared here in the interests of transparency.
| Participant | Actual weeks used | Mean days per week | Percentage use |
|---|---|---|---|
| 10301 | 6 | 5.00 | 71.42 |
| 10302 | 25 | 1.44 | 20.57 |
| 10501 | 17 | 3.35 | 47.90 |
| 10701 | 13 | 2.69 | 38.46 |
| 40103 | 49 | 5.75 | 82.21 |
| 40105 | 45 | 6.24 | 89.21 |
| 40201 | 42 | 3.28 | 46.94 |
| 40202 | 21 | 3.09 | 44.22 |
| 40301 | 15 | 1.80 | 25.71 |
| 40402 | 48 | 6.66 | 95.24 |
| 40601 | 43 | 5.60 | 80.07 |
| 40701 | 37 | 4.35 | 62.16 |
| 40702 | 43 | 4.53 | 64.78 |
| 40703 | 45 | 5.88 | 84.13 |
| 40704 | 50 | 6.46 | 92.29 |
| 60104 | 42 | 3.23 | 46.26 |
| 60109 | 30 | 3.86 | 55.24 |
| 60201 | 44 | 6.43 | 91.88 |
| 60202 | 39 | 5.17 | 73.99 |
| 60203 | 44 | 5.93 | 84.74 |
| 60206 | 11 | 4.63 | 66.23 |
| 60208 | 37 | 6.29 | 89.96 |
| 60210 | 40 | 5.47 | 78.21 |
| 60403 | 7 | 2.57 | 36.73 |
| 60801 | 12 | 2.41 | 34.52 |
| 80307 | 42 | 3.35 | 47.96 |
| 80402 | 34 | 5.00 | 71.43 |
| 80503 | 6 | 3.83 | 54.76 |
| 80601 | 14 | 2.07 | 29.59 |
| 80606 | 21 | 3.38 | 48.30 |
| 80803 | 45 | 6.02 | 86.03 |
| 81103 | 37 | 6.78 | 96.91 |
| 81901 | 37 | 5.45 | 77.99 |
| Mean (SD) | 31.5 (14.5) | 4.5 (1.6) | 64.1 (22.5) |
| Median (IQR) | 37.0 (16.0–43.5) | 4.6 (3.3–5.9) | 66.2 (46.6–84.4) |
| Range | 44.0 | 5.3 | 76.3 |
Limitations
There are key limitations to these analyses. In taking such a data-driven approach we have not considered any predictors, including levels of negative symptoms, that have been shown to be linked to lower engagement with digital interventions,278 or the level of engagement with peer support workers, which may increase engagement. 117 Cox proportional hazards models that include theoretically justified predictors may be helpful. Additionally, only participants who completed a baseline have been considered for analysis; six participants were excluded for this reason. Furthermore, these findings may reflect engagement with a research trial rather than engagement with EMPOWER as a blended intervention in itself in a non-research context. Importantly, these are all behavioural measures of engagement and do not consider subjective user experiences or the broader context of intervention usage.
Appendix 6 Additional relapse outcome table
| Relapse characteristics | EMPOWER, n (%) | TAU, n (%) | ||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | 3 months | 6 months | 12 months | |
| Return of or exacerbation of psychotic symptoms | ||||||
| Yes | 10 (24) | 6 (14) | 9 (21) | 10 (32) | 5 (16) | 11 (35) |
| No | 27 (64) | 27 (64) | 23 (55) | 20 (65) | 24 (77) | 17 (55) |
| Missing | 5 (12) | 9 (21) | 10 (24) | 1 (3) | 2 (6) | 3 (10) |
| Duration of at least 1 week | ||||||
| Yes | 5 (12) | 5 (12) | 7 (17) | 8 (26) | 4 (13) | 11 (35) |
| No | 32 (76) | 28 (67) | 25 (60) | 22 (71) | 25 (81) | 17 (55) |
| Missing | 5 (12) | 9 (21) | 10 (24) | 1 (3) | 2 (6) | 3 (10) |
| Reduction in functioning | ||||||
| Yes | 7 (17) | 7 (17) | 7 (17) | 14 (45) | 7 (23) | 8 (26) |
| No | 30 (71) | 26 (62) | 25 (60) | 16 (52) | 22 (71) | 20 (65) |
| Missing | 5 (12) | 9 (21) | 10 (24) | 1 (3) | 2 (6) | 3 (10) |
| Increased risk | ||||||
| Yes | 1 (2) | 0 (0) | 2 (5) | 7 (23) | 1 (3) | 8 (26) |
| No | 36 (86) | 33 (79) | 30 (71) | 23 (74) | 28 (90) | 20 (65) |
| Missing | 5 (12) | 9 (21) | 10 (24) | 1 (3) | 2 (6) | 3 (10) |
| Change in clinical management | ||||||
| Yes | 7 (17) | 7 (17) | 6 (14) | 12 (39) | 4 (13) | 10 (32) |
| No | 30 (71) | 26 (62) | 26 (62) | 18 (58) | 25 (81) | 18 (58) |
| Missing | 5 (12) | 9 (21) | 10 (24) | 1 (3) | 2 (6) | 3 (10) |
| Hospital admission | ||||||
| Yes | 2 (5) | 0 (0) | 0 (0) | 3 (10) | 1 (3) | 5 (16) |
| No | 35 (83) | 33 (79) | 32 (76) | 27 (87) | 28 (90) | 23 (74) |
| Missing | 5 (12) | 9 (21) | 10 (24) | 1 (3) | 2 (6) | 3 (10) |
| Mental Health Act used | ||||||
| Yes | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 4 (13) |
| No | 37 (88) | 33 (79) | 32 (76) | 29 (94) | 29 (94) | 24 (77) |
| Missing | 5 (12) | 9 (21) | 10 (24) | 1 (3) | 2 (6) | 3 (10) |
| Type of relapse | ||||||
| Type I | 0 (0) | 2 (5) | 2 (5) | 1 (3) | 0 (0) | 3 (10) |
| Type II | 1 (2) | 0 (0) | 2 (5) | 7 (23) | 2 (6) | 5 (16) |
| Type III | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| No relapse | 36 (86) | 31 (74) | 28 (67) | 22 (71) | 27 (87) | 19 (61) |
| Missing | 5 (12) | 9 (21) | 10 (24) | 1 (3) | 2 (6) | 3 (10) |
Appendix 7 Clinical assessment calibration and reliability
Research assistant training and calibration in the EMPOWER trial was governed by a standard operating procedure designed to ensure that raters were well prepared for their role and provided with regular feedback on their rating fidelity throughout the trial. Oversight of the training, calibration and monitoring process was led by Hamish McLeod (UK), with additional input from John Farhall and John Gleeson (Australia) as required.
Reliability assessment of symptom and functioning measures occurred in two phases. First, all research assistants were calibrated to PANSS, CDSS and PSP scale measures by completing a structured training programme. Feedback on ratings was anchored to a set of training recordings and videos, with reference ratings provided by an expert rater and then checked for consensus agreement with at least three other raters. The majority of these training cases were conducted between April and May 2018, and research assistants did not progress to independently assessing study participants until they had been consistently rating recorded cases with no scoring deviations of 2 points or more from the reference rating. Up to the point of calibration being established for any rater, the scores they returned on assessed trial patients were adjusted to consensus scores if a deviation of ≥ 2 points was observed during co-rating checks, and the electronic record for that service user was adjusted.
Once raters had been calibrated and were providing consistently accurate ratings, the standard operating procedure moved into the reliability phase and no further adjustment of recorded scores occurred. In this phase, if a rater showed a deviation of more than 2 points on any item, they received feedback and additional supervision, but the recorded score was unchanged. The majority of data from this phase of reliability recording were acquired between June 2018 and May 2019. Nine separate cases were recorded and rated during this period, with input from at least one of the trial principal investigators (Table 38).
| June 2018 | July 2018 | August 2018 | August 2018 | October 2018 | December 2018 | January 2019 | February 2019 | May 2019 | |
|---|---|---|---|---|---|---|---|---|---|
| Service user | 70801 | 50302 | 71507 | 60403 | 40201 | 40103 | 80397 | 51003 | 71507 |
| Number of raters | 8 | 8 | 6 | 8 | 4 | 6 | 4 | 3 | 5 |
Data from the reliability phase were used to minimise rater drift and to ensure that research assistants were gaining regular feedback on their rating judgements. These data were analysed in two main ways. First, the rate at which ratings showed a deviation of 2 points or more was used to estimate how much the reported scale total scores may have been affected by unreliable rating. The results suggest that all three of the assessed scales were rated reliably for most items, a pattern that suggests that the training and calibration phase of rater training worked effectively. However, the data do suggest that rater disagreement affected a greater proportion of items on the PSP scale than on the CDSS and PANSS (Table 39).
| Measure | Frequency of score deviations of ≥ 2 points | Percentage of overall pool of scale items |
|---|---|---|
| PANSS | ||
| ED4 tension | 6 | 2.22 |
| P6 somatic concern | 5 | 1.85 |
| P3 unusual thought content | 3 | 1.11 |
| D1 stereotyped thinking | 3 | 1.11 |
| D2 poor attention | 3 | 1.11 |
| D5 difficulty in abstract thinking | 3 | 1.11 |
| E1 poor impulse control | 3 | 1.11 |
| P2 hallucinatory behaviour | 2 | 0.74 |
| N2 blunted affect | 2 | 0.74 |
| N3 emotional withdrawal | 2 | 0.74 |
| D8 disturbance of volition | 2 | 0.74 |
| ED2 depression | 2 | 0.74 |
| P1 delusions | 1 | 0.37 |
| P4 suspiciousness/persecution | 1 | 0.37 |
| N4 passive/apathetic social withdrawal | 1 | 0.37 |
| N6 poor rapport | 1 | 0.37 |
| N7 active social avoidance | 1 | 0.37 |
| D4 conceptual disorganisation | 1 | 0.37 |
| D6 mannerisms and posturing | 1 | 0.37 |
| D7 lack of judgement and insight | 1 | 0.37 |
| E2 excitement | 1 | 0.37 |
| ED3 guilt feelings | 1 | 0.37 |
| CDSS | ||
| CDSS4 guilty ideas of reference | 1 | 1.23 |
| CDSS7 early wakening | 2 | 2.47 |
| PSP scale | ||
| PSP scale A – socially useful activity | 2 | 5.56 |
| PSP scale C – self-care | 2 | 5.56 |
| PSP scale B – social relationships | 1 | 2.78 |
Appendix 8 Intervention costs
| Resource | Unit | Amount | Unit cost (£) | Cost (£) | Cost type | Unit cost source and notes | Perspective | ||
|---|---|---|---|---|---|---|---|---|---|
| Health-care payer | Health-care sector | Societal | |||||||
| R&D | |||||||||
| App development | Per app development | 1 | 101,493 | 101,493 | Sunk cost | Final grant application; year 1 software developer costs, 3 posts: £33,332 + £38,616 + £29,545 | ✓ | ✓ | ✓ |
| Content development (staff cost) | Per app development | 1 | 4552 | 4552 | Sunk cost | University of Glasgow salary; 3 weeks’ salary each band 6 and band 7. Band 6 2016, £35,296/52 × 3 = £2036; band 7 2016, £43,617/5 × 3 = £2516; total £4552 | ✓ | ✓ | ✓ |
| Medical device registration | Per app | 1 | 23,674 | 23,674 | Sunk cost | Trial team; MHRA costs £3200 plus £13,424 Trial manager’s time and £7050 Manchester time preparing application | ✓ | ✓ | ✓ |
| Ongoing maintenance | |||||||||
| App hosting | Per year | 1 | 1667 | 1667 | Fixed | Final grant application; 3-year trial period for servers to host EMPOWER app; £5000/3 years = £1667 for 1 year | ✓ | ✓ | ✓ |
| App maintenance – staffing | Per year | 1 | 12,947 | 12,947 | Fixed | Final grant application; year 2 and 3 software engineer, 1 post: £20,611 + £5282 = £25,893 | ✓ | ✓ | ✓ |
| Intervention delivery | |||||||||
| Capital costs | |||||||||
| Phones | Per device | 42 | 109.52 | 4600 | Variable |
Australia: 8 project phones AU$2454 (or £1380) UK: 28 project phones + 2 repairs £3220 Total £4600 |
✗ | ✓ | ✓ |
| Consumables | |||||||||
| Internet | Per device per year | 42 | 69.30 | 2910.63 | Variable |
Australia: total cost over 12 months AU$810 (or £455.63) UK: total cost over 12 months £2455 Total £2910.63 |
✗ | ✓ | ✓ |
| Labour costs | |||||||||
| Training CMHS staff | Per workshop | 5 | 833 | 4165 | Variable |
Australia: 1 senior clinical psychologist: AU$108 (including 30% on-costs) × 3 hours = AU$324 6 care co-ordinators: AU$46 (average wage of psychologist, nurse, social worker, occupational therapist; including 30% on-costs) × 3 hours × 6 = AU$828 1 research assistant: AU$56 (including 30% on-costs) × 3 hours = AU$168 1 peer support worker: AU$56 (including 30% on-costs) × 3 hours = AU$168 Total per workshop AU$1488 (or £833) |
✓ | ✓ | ✓ |
| Monitoring training of research assistants in reviewing ChIP information | Per training | 2 | 367 | 34 | Variable |
Australia: 1 senior clinical psychologist: AU$108 (including 30% on-costs) × 4 hours = AU$432 1 research assistant: AU$56 (including 30% on-costs) × 4 hours = AU$224 Total AU$656 (or £367) |
✓ | ✓ | ✓ |
| Peer support workers’ engagement with service user | Per service user | 42 | 1039 | 43,633 | Variable |
Australia: peer support worker (FTE 0.4) AU$86,205 × 0.4 = AU$34,482 (or £19,303) UK: peer support worker band 3 £24,330 Total £43,633/42 = £1039 per service user |
✓ | ✓ | ✓ |
| Time spent using the app | Per service user | 42 | 67 | 2814 | Variable |
In Australia, the average hourly wage in 2018 was AU$39.10 25% of $39.10 = $9.775 2 minutes × 365 days = 730 minutes (12 hours) during the 12-month period 12 hours × AU$9.775 = AU$119 (or £67) |
✗ | ✗ | ✓ |
| Routine monitoringa | Per service user | 42 | 1003 | 42,117 | Variable |
Australia: 1 research assistant (FTE 0.05) = AU$69,081 × 0.05 = AU$3454 (2 hours per week) 1 project co-ordinator (FTE 0.16) = AU$77,242 × 0.16 = AU$12,359 (6 hours per week) Total AU$15,813 (or £8897) UK: Research nurse band 6 = £33,220 Total £42,117/42 = £1003 per service user |
✓ | ✓ | ✓ |
| Ongoing supervision of CMHS staff | Per supervision session | 9 | 201.33 | 1812 | Variable |
Australia: 1 senior clinical psychologist: AU$108 (including 30% on-costs) × 1 hour = AU$108 3 care co-ordinators: AU$46 (average wage of psychologist, nurse, social worker, occupational therapist; including 30% on-costs) × 1 hour × 3 = AU$138 1 research assistant: AU$56 (including 30% on-costs) × 1 hour = $56 1 peer support worker: AU$56 (including 30% on-costs) × 1 hour = AU$56 Per session = AU$358 × 9 sessions = AU$3222 (or £1812) |
✓ | ✓ | ✓ |
| Total costs (£) | |||||||||
| R&D | 3.27 | 3.27 | 3.27 | ||||||
| Ongoing maintenance | 0.37 | 0.37 | 0.37 | ||||||
| Intervention delivery | 92,461 | 99,972 | 102,786 | ||||||
| Total | 92,465 | 99,976 | 102,790 | ||||||
| Cost per service userb | 2202 | 2380 | 2447 | ||||||
Appendix 9 Unit costs
| Service | Location | Sector | Cost in Great British pounds (£) | Source |
|---|---|---|---|---|
| Health professional costs | ||||
| GP | Clinic | Public | 28 | Unit Costs of Health and Social Care 2018 clinic excluding qualification and direct care staff 9.22 minutes @ £3 per minute234 |
| Phone | Public | 14.90 | Unit Costs of Health and Social Care 2018 GP telephone triage234 | |
| Psychiatrist | Clinic | Public | 391 | PSSRU 2015 plus uplift279 |
| Psychologist | Clinic | Public | 96 | PSSRU 2015 plus uplift279 |
| Counsellor | Clinic | Public | 25 | PSSRU 2018 counsellor band 691 |
| Primary care nurse | Clinic | Public | 9.30 | PSSRU 2018 GP nurse (excluding qualifications) |
| Mental health nurse | Clinic | Public | 79 | NHS Reference Costs 2017/18 Other specialist nursing adult face to face233 |
| Drug and alcohol worker | Clinic | Public | 118 | NHS Reference Costs 2017/18 DAS alcohol services adults community contacts233 |
| Case manager/social worker | Clinic | Public | 61 | NHS Reference Costs 2017/18 social worker adult services233 |
| Speech therapist | Clinic | Public | 99 | NHS Reference Costs 2017/18 Speech therapist A13A1 adult one to one233 |
| Occupational therapist | Clinic | Public | 81 | NHS Reference Costs 2017/18 Occupational therapist A06A1 adult one to one |
| Dentist | Clinic | Public | 21.60 | Unit Costs of Health and Social Care 2018 NHS dental charge band 1234 |
| Physiotherapist | Clinic | Public | 57 | NHS Reference Costs 2017/18 physiotherapist A08A1 adult one to one233 |
| Optician | Clinic | Public | 31.88 | PSSRU 2016/17 with uplift280 |
| Podiatrist | Clinic | Public | 44.48 | PSSRU 2016/17 with uplift280 |
| Dietitian | Clinic | Public | 77.04 | PSSRU 2016/17 with uplift280 |
| Support worker | Clinic | Public | 23 | NHS Reference Costs 2017/18 Mental health cluster 12 ongoing or recurrent psychosis233 |
| Other professional | Clinic | Public | 125 | NHS Reference Costs 2017/18 ‘Total outpatient attendances’, average outpatient attendance excluding costs for professions above233 |
| Other health-care costs | ||||
| Ambulance | Public | 120 | Unit Costs of Health and Social Care 2018 Ambulance service average of all attendances234 | |
| Emergency room | Public | 160 | NHS Reference Costs 2017/18 overall average A&E attendance233 | |
| Day treatment | Public | 134 | PSSRU 2017/18 outpatient service average of all attendances234 | |
| Inpatient mental health | Public | 410 | PSSRU 2017/18 Mental health cluster – bed-day234 | |
| Inpatient: general | Public | 337 | NHS Reference Costs 2017/18 non elective bed-day233 | |
| Diagnostic test | ||||
| Blood test | Public | 14.80 | ISD 2017/18 lab costs R130X plus nurse visit (above)281 | |
| CT scan | Public | 90 | NHS reference costs 2017/18 RD20A233 | |
| X-ray | Public | 61 | ISD 2017/18 R120X Other radiology281 | |
| MRI | Public | 141 | NHS Reference Costs 2017/18 MRI scan of one area RD01A233 | |
| Ultrasound | Public | 54 | NHS Reference Costs 2017/18 Ultrasound scan duration less than 20 minutes233 | |
| Urine test | Public | 10.18 | ISD 2017/18 R130X clinical chemistry plus one nurse visit (above)281 | |
| ECG | Public | 108 | NHS Reference Costs 2017/18 Simple echo 19 years and over233 | |
| Endoscopy | Public | 227 | NHS Reference Costs 2017/18 average of all ‘Wireless capsule endoscopy233 | |
| Blood pressure | Public | 9.30 | PSSRU 2017/18 Nurse visit as above234 | |
| Biopsy | Public | 228 | NHS Reference Costs 2017/18 average of all ‘Biopsy . . .’233 | |
| Heart rate monitor | Public | 18.60 | PSSRU 2017/18 Assume two nurse visits, one to attach and one to remove the monitor234 | |
| Productivity costs | ||||
| Minimum hourly wage | 7.50 | UK 2017 minimum wage ≥ 25 years282 | ||
| National average hourly rate | 14.04 | Office for National Statistics282 | ||
| National average hourly rate plus on-costs | 17.55 | Adding 25% on-costs to national average hourly rate | ||
| Unpaid work | 3.51 | 25% of national average hourly rate | ||
| Justice costs | ||||
| Contact with police | 268.40 | Statement and interview241 | ||
| Criminal court | 441 | Sheriff court, judge alone243 | ||
| Services | Location | Sector | Cost in Australian dollars (AU$) | Cost in Great British pounds (£) | Source |
|---|---|---|---|---|---|
| Health professional costs | |||||
| GP | Clinic | Public | 44.20 | 21.46 | MBS item number 3, 23, 36, 44, 2700, 2701, 2712, 2713, 2715, 2717, 2721, 2725236 |
| Psychiatrist | Clinic | Public | 159.39 | 77.37 | MBS item number 291, 293, 296, 300, 302, 304, 306, 308, 310, 312, 314, 316, 318, 319236 |
| Psychologist | Clinic | Public | 105.39 | 51.16 | MBS item number 10968, 80000, 80010, 80100, 80110, 81355236 |
| Clinic | Private | 372.16 | 180.66 | NHCDC Round 21 Tier 2 – non-admitted service events, item 4029 (psychology)238 | |
| Counsellor | Clinic | Public | 71.12 | 34.52 | Average of GP, psychologist, social worker, mental health worker236 |
| Phone | Public | 71.12 | 34.52 | Average of GP, psychologist, social worker, mental health worker236 | |
| Primary care nurse | Clinic | Public | 30.32 | 14.72 | MBS item number 82200, 82205, 82210, 82215236 |
| Mental health nurse | Clinic | Public | 30.72 | 14.91 | MBS item number 81325, 10956, 82200, 82205, 82210, 82215236 |
| Drug and alcohol worker | Clinic | Public | 170.91 | 82.97 | NHCDC Round 21 Tier 2 – non-admitted service events, item 4030 (alcohol and other drugs)238 |
| Case manager/social worker | Clinic | Public | 77.04 | 37.40 | MBS item number 80150, 80160236 |
| Home | Public | 95.74 | 46.48 | MBS item number 80155, 80165236 | |
| Phone | Public | 77.04 | 37.40 | MBS item number 80150, 80160236 | |
| Speech therapist | Clinic | Public | 57.54 | 27.93 | MBS item number 10970, 81360236 |
| Phone | Public | 57.54 | 27.93 | MBS item number 10970, 81360236 | |
| Occupational therapist | Clinic | Public | 141.45 | 68.67 | MBS item number 10985, 80125, 80130, 80135, 80140, 80145, 81330236 |
| Phone | Public | 101.75 | 49.39 | MBS item number 80130, 80140236 | |
| Dietitian | Clinic | Public | 53.44 | 25.94 | MBS item number 10954, 81320236 |
| Mental health service provider/mental health worker | Clinic | Public | 57.85 | 28.08 | MBS item number 10956, 81325236 |
| Physiotherapy | Clinic | Public | 53.35 | 25.90 | MBS item number 10960, 81335236 |
| Clinic | Private | 156.75 | 76.09 | NHCDC Round 21 Tier 2 – non-admitted service events, item 4009 (physiotherapy)238 | |
| Podiatry | Clinic | Public | 53.09 | 25.77 | MBS item number 10962, 81340236 |
| Chiropractor | Clinic | Public | 52.96 | 25.71 | MBS item number 10964, 81345236 |
| Osteopathy | Clinic | Public | 53.73 | 26.08 | MBS item number 10966236 |
| Dentist | Clinic | Public | 70.42 | 34.18 | MBS item number 75800236 |
| Clinic | Private | 396.56 | 192.50 | NHCDC Round 21 Tier 2 – non-admitted service events, 1004 (dental)238 | |
| Ophthalmologist | Clinic | Public | 62.43 | 30.31 | MBS item number 106236 |
| Clinic | Private | 62.43 | 30.31 | MBS item number 106236 | |
| Optometrist | Clinic | Public | 29.50 | 14.32 | MBS item number 10905, 10907236 |
| Massage therapist | Clinic | Public | 24.80 | 12.04 | www.payscale.com (accessed 16 September 2019) |
| Endocrinologist | Clinic | Public | 78.77 | 38.24 | MBS item number 104, 105, 110, 132, 133236 |
| Specialist | Clinic | Public | 115.60 | 56.12 | Average of all specialist |
| Other health-care costs | |||||
| Ambulance | 927.56 | 450.27 | Report on Government Services 2019283 | ||
| Emergency room | 532.81 | 258.65 | Non-admitted emergency department. NHCDC Round 21, sheet 15. Emergency department by jurisdiction238 | ||
| Day treatment | 309.00 | 150.00 | Independent Hospital Pricing Authority238 | ||
| Inpatient acute psychiatric unit | 1206.00 | 585.44 | Australian Institute of Health and Welfare Expenditure on mental health services (exp. 19)239 | ||
| Inpatient general | 2338.33 | 1135.11 | NHCDC Round 19 to 21 admitted acute overnight and same day, actual, by jurisdiction238 | ||
| On site at an organisation | |||||
| PARC | 480.78 | 233.39 | Victorian health policy and funding guidelines 2015–16, part 2: pricing and funding arrangements240 | ||
| Community care unit | 389.60 | 189.13 | Victorian health policy and funding guidelines 2015–20, part 2: pricing and funding arrangements240 | ||
| Diagnostic test | |||||
| ECG | 80.45 | 39.05 | MBS item number 11700, 11702, 11712, 55113, 55116, 55117236 | ||
| Ultrasound | 113.55 | 55.12 | MBS group I1: ultrasound236 | ||
| Blood test | 16.69 | 8.10 | MBS group P1: haematology236 | ||
| Urine test | 21.41 | 10.39 | MBS group P1: haematology236 | ||
| X-ray | 52.70 | 25.58 | MBS group: I3236 | ||
| MRI | 391.59 | 190.09 | MBS group: I5236 | ||
| CT scan | 324.41 | 157.48 | MBS group: I2236 | ||
| Mammography | 73.46 | 35.66 | MBS item number 59300, 59301, 59303, 59304, 59306, 59309, 59312, 59314, 59315, 59318, 59319236 | ||
| Spirometry | 21.75 | 10.56 | MBS item number 11506236 | ||
| Productivity costs | |||||
| Minimum hourly wage | 18.69 | 9.07 | Australian Government Fair Work Commission284 | ||
| National average hourly rate | 30.69 | 6.82 | Australian Bureau of Statistics, Characteristics of Employment, Australia, August 2018285 | ||
| National average hourly rate plus on-costs | 38.36 | 8.52 | Adding 25% to national average hourly rate | ||
| Unpaid work | 7.67 | 1.70 | 25% of national average hourly rate | ||
| Justice costs | |||||
| Contact with police | 459.00 | 222.82 | Australian Government Productivity Commission, Report on Government Services 2017242 | ||
| Criminal court | 1469.77 | 713.48 | Australian Bureau of Statistics, Criminal Courts, Australia, 2016–17244 | ||
Appendix 10 Resource use outcomes
| Resource type | 3 months | 6 months | 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMPOWER (n = 42) | TAU (n = 31) | EMPOWER (n = 42) | TAU (n = 31) | EMPOWER (n = 42) | TAU (n = 31) | |||||||
| n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | |
| Direct health care | ||||||||||||
| GP visits | 21 (50.0) | 2.57 (1.60) | 19 (61.3) | 2.63 (2.77) | 18 (42.9) | 1.94 (0.998) | 18 (58.1) | 2.72 (1.71) | 16 (38.1) | 2.38 (1.31) | 19 (61.3) | 5.63 (6.53) |
| Psychiatrist | 21 (50.0) | 1.55 (0.800) | 26 (83.9) | 2.31 (2.35) | 16 (38.1) | 1.94 (1.34) | 21 (67.7) | 1.55 (1.14) | 22 (52.4) | 2.18 (1.26) | 23 (74.2) | 3.0 (2.65) |
| Psychologist | 4 (9.5) | 2.0 (1.15) | 5 (16.1) | 2.4 (1.52) | 3 (7.1) | 1.67 (0.577) | 2 (6.5) | 2.5 (0.707) | 3 (7.1) | 3.33 (2.08) | 3 (9.7) | 7.67 (10.7) |
| Counsellor | 2 (4.8) | 3.0 (0) | 1 (3.2) | 1.0 (NA) | 1 (2.4) | 9.0 (NA) | 0 | NA | 0 | NA | 1 (3.2) | 4.0 (NA) |
| Care nurse | 4 (9.5) | 2.25 (1.26) | 1 (3.2) | 1.0 (NA) | 5 (11.9) | 3.8 (5.72) | 3 (9.7) | 1.33 (0.577) | 2 (4.8) | 1.5 (0.707) | 4 (12.9) | 1.75 (0.957) |
| Mental health nurse | 21 (50.0) | 5 (2.43) | 16 (51.6) | 4.75 (3.59) | 21(50.0) | 4.57 (3.49) | 16 (51.6) | 5.63 (4.92) | 19 | 6.47 (4.96) | 14 (45.2) | 6.14 (5.65) |
| Alcohol worker | 1 (2.4) | 3.0 (NA) | 1 (3.2) | 2.0 (NA) | 1 (2.4) | 3.0 (NA) | 2 (6.4) | 2.5 (0.707) | 1 (2.4) | 4.0 (NA) | 2 (6.4) | 3.0 (1.41) |
| Case manager | 9 (21.4) | 4.0 (2.40) | 12 (38.7) | 3.27 (3.35) | 11 (26.2) | 3.91 (3.11) | 10 (32.3) | 3.1 (3.03) | 12 (28.6) | 6.08 (6.30) | 12 (38.7) | 3.0 (3.13) |
| Speech therapist | 1 (2.4) | 1 (NA) | 1 (3.2) | 6 (NA) | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| Occupational therapist | 5 (11.9) | 2.0 (0.707) | 7 (22.6) | 3.86 (2.61) | 3 (7.1) | 2.0 (1.0) | 1 (3.2) | 3.5 (3.54) | 2 (4.8) | 4.0 (2.83) | 3 (9.7) | 3.67 (2.08) |
| Other health professionalsa | 4 (9.5) | 1.0 (0) | 9 (29.0) | 1.11 (0.333) | 3 (7.1) | 1.67 (0.577) | 5 (16.1) | 1.2 (0.447) | 1 (2.4) | 1.0 (NA) | 7 (22.6) | 1.43 (0.535) |
| Ambulance | 2 (4.8) | 1.5 (0.707) | 2 (6.4) | 1.0 (0) | 1 (2.4) | 1 (NA) | 0 | NA | 2 (4.8) | 2.0 (0) | 3 (9.7) | 2.0 (1.0) |
| Emergency room | 3 (7.1) | 1.33 (0.577) | 6 (19.4) | 1.67 (0.816) | 2 (4.8) | 1.0 (0) | 3 (9.7) | 2.33 (1.53) | 2 (4.8) | 1.5 (0.707) | 7 (22.6) | 1.43 (0.787) |
| Day treatment (outpatient) | 3 (7.1) | 1.0 (0) | 4 (12.9) | 1.75 (1.5) | 5 (11.9) | 1.4 (0.894) | 4 (12.9) | 1.75 (0.5) | 1 (2.4) | 1 (NA) | 6 (19.4) | 2.0 (1.10) |
| Inpatient: mental health units number of stays | 0 | NA | 4 (12.9) | 1.25 (0.5) | 1 (2.4) | 1.0 (NA) | 1 (3.2) | 1.0 (NA) | 0 | NA | 3 (9.7) | 1.33 (0.577) |
| Inpatient: non-mental health units number of stays | 2 (4.8) | 1.0 (0) | 3 (9.7) | 1.33 (0.577) | 2 (4.8) | 1.5 (0.707) | 1 (3.2) | 2.0 (NA) | 2 (4.8) | 1.0 (0) | 2 (6.4) | 1.5 (0.707) |
| Inpatient – mental health units,b length of stay (days) | 0 | NA | 4 (12.9) | 14.0 (12.12) | 1 (2.4) | 14.0 (NA) | 1 (3.2) | 20.0 (NA) | 0 | NA | 3 (9.7) | 28.8 (26.4) |
| Inpatient – non-mental health units, length of stay (days) | 2 (4.8) | 7.5 (9.19) | 3 (9.7) | 4.75 (6.24) | 2 (4.8) | 11.0 (14.7) | 1 (3.2) | 4.5 (2.12) | 2 (4.8) | 14.5 (19.1) | 2 (6.4) | 12.0 (1.73) |
| Non-hospital on-site stay | 1 (2.4) | 1.0 (NA) | 2 (6.4) | 1.0 (0) | 3 (7.1) | 1.0 (0) | 2 (6.4) | 1.0 (0) | 3 (7.1) | 1.0 (0) | 2 (6.4) | 1.5 (0.707) |
| Non-hospital on-site length of stay (nights/days) | 1 (2.4) | 90.0 (NA) | 2 (6.4) | 87.0 (4.24) | 3 (7.1) | 86.0 (3.46) | 2 (6.4) | 87.0 (4.24) | 3 (7.1) | 69.3 (35.8) | 2 (6.4) | 88.0 (3.46) |
| Diagnostic tests | 15 (35.7) | 1.2 (0.561) | 17 (54.8) | 2.18 (2.70) | 9 (21.4) | 1.22 (0.441) | 16 (51.6) | 2.06 (1.61) | 12 (28.6) | 1.58 (0.793) | 16 (51.6) | 1.63 (1.02) |
| Mental health (hours searching internet for information) | 19 (45.2) | 10.96 (16.9) | 9 (29.0) | 3.43 (2.53) | 23 (54.8) | 15.0 (26.9) | 12 (28.6) | 7.01 (12.3) | 18 (42.9) | 27.0 (50.9) | 9 (29.0) | 23.3 (25.0) |
| Formal online therapy (hours) | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| Smartphone self-help app (hours) – excludes EMPOWER | 4 (9.5) | 4.64 (2.46) | 2 (6.5) | 2.25 (1.06) | 7 (16.7) | 19.6 (17.9) | 2 (6.5) | 20.3 (26.5) | 6 (14.3) | 54.0 (49.8) | 2 (6.5) | 65.0 (18.4) |
| Self-help materials (books, etc.) (hours) | 1 (2.4) | 26 | 0 | NA | 3 (7.1) | 33.5 (26.3) | 3 (9.7) | 7.67 (6.83) | 4 (9.5) | 52.0 (48.5) | 2 (6.5) | 9.03 (12.7) |
| Gym attendance (hours) | 3 (7.1) | 47.7 (15.0) | 2 (6.5) | 71.5 (9.19) | 3 (7.1) | 30.3 (7.51) | 2 (6.5) | 52.8 (72.5) | 2 (4.8) | 32.0 (28.3) | 3 (9.7) | 61.5 (82.7) |
| Other mental health resourcesc | 0 | NA | 1 (3.2) | 1.0 (NA) | 1 (2.4) | 1.0 (NA) | 0 | NA | 0 | NA | 1 (3.2) | 1.0 (NA) |
| Medication (mental health) | 29 (69.0) | 1.62 (0.820) | 28 (90.3) | 1.79 (0.787) | 27 (64.3) | 1.78 (0.847) | 27 (87.1) | 2.19 (0.786) | 26 (61.9) | 2.12 (0.816) | 26 (83.9) | 1.92 (0.796) |
| Criminal justice services | ||||||||||||
| Contact with criminal justice services (yes/no) | 4 (9.5) | 0 | 3 (7.1) | 0 | 3 (7.1) | 2 (6.5) | ||||||
| Contacts with the police | 2 (4.8) | 1.5 (0.707) | 0 | NA | 3 (7.1) | 3.0 (0) | 0 | NA | 3 (7.1) | 2.33 (1.15) | 2 (6.5) | 1.0 (0) |
| Nights spent in police cell or prison | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| Number of psychiatric assessments while in custody | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| Number of court appearances (criminal) | 2 (4.8) | 1.0 (0) | 0 | NA | 1 (2.4) | 2.0 (0) | 0 | NA | 1 (2.4) | 1.0 (NA) | 0 | NA |
| Number of court appearances (civil) | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
| Benefits | ||||||||||||
| Receiving unemployment income support (yes/no) | 5 (11.9) | 1 (3.2) | 3 (7.1) | 1 (3.2) | 3 (7.1) | 1 (3.2) | ||||||
| Receiving sickness/disability benefit (yes/no) | 9 (21.4) | 7 (22.6) | 10 (23.8) | 7 (22.6) | 6 (14.3) | 7 (22.6) | ||||||
| Receiving housing benefit (yes/no) | 4 (9.5) | 1 (3.2) | 3 (7.1) | 1 (3.2) | 1 (2.4) | 2 (6.5) | ||||||
| Receiving other benefits (yes/no)d | 17 (40.5) | 16 (51.6) | 16 (38.1) | 15 (48.4) | 17 (40.5) | 14 (45.2) | ||||||
| Employment status | ||||||||||||
| School/studying | 1 (2.4) | 0 | 3 (7.1) | 1 (3.2) | 1 (2.4) | 2 (6.5) | ||||||
| Employed | 6 (14.3) | 8 (25.8) | 4 (9.5) | 5 (16.1) | 3 (7.1) | 4 (12.9) | ||||||
| Self-employed | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Housewife/househusband | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Unemployed | 19 (45.2) | 13 (41.9) | 18 (42.9) | 16 (51.6) | 19 (45.2) | 15 (48.4) | ||||||
| Unpaid work | 3 (7.1) | 3 (9.7) | 3 (7.1) | 1 (3.2) | 2 (4.8) | 3 (9.7) | ||||||
| Retired/pre-pension plan | 1 (2.4) | 4 (12.9) | 2 (4.8) | 3 (9.7) | 3 (7.1) | 3 (9.7) | ||||||
| Other | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Manager | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Profession | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Associate professional | 0 | 0 | 1 (2.4) | 0 | 1 (2.4) | 0 | ||||||
| Clerical worker | 0 | 1 (3.2) | 0 | 1 (3.2) | 0 | 1 (3.2) | ||||||
| Services and sales workers | 1 (2.4) | 2 (6.4) | 1 (2.4) | 0 | 0 | 0 | ||||||
| Skilled labourer | 0 | 1 (3.2) | 0 | 1 (3.2) | 0 | 0 | ||||||
| Plant and machine assemblers | 0 | 1 (3.2) | 0 | 1 (3.2) | 0 | 2 (6.4) | ||||||
| Elementary occupations | 1 (2.4) | 1 (3.2) | 1 (2.4) | 0 | 1 (2.4) | 0 | ||||||
| Othere | 4 (9.5) | 2 (6.4) | 2 (4.8) | 2 (6.4) | 1 (2.4) | 1 (3.2) | ||||||
| Number of paid hours per week | 6 (14.3) | 16.4 (14.8) | 8 (25.8) | 24.8 (22.9) | 5 (11.9) | 25.8 (11.1) | 5 (16.1) | 26.0 (13.5) | 3 (7.1) | 26.3 (15.9) | 4 (12.9) | 22.5 (15.5) |
| Number of days per week worked | 6 (14.3) | 2.04 (1.62) | 8 (25.8) | 3.25 (2.12) | 5 (11.9) | 3.6 (1.82) | 5 (16.1) | 4.0 (1.87) | 3 (7.1) | 4.33 (2.08) | 4 (12.9) | 3.75 (2.22) |
| Number of missed work days as a result of sickness | 2 (4.8) | 19.0 (15.6) | 6 (19.4) | 25.3 (34.7) | 3 (7.1) | 14.0 (14.4) | 3 (9.7) | 21.3 (30.1) | 1 (2.4) | 1.0 (NA) | 1 (3.2) | 30.0 (NA) |
| Monthly gross personal income (£) | 4 (9.5) | £1085 (£653) | 1 (3.2) | £850 (NA) | 3 (7.1) | £1267 (£651) | 0 | NA | 1 (2.4) | £900 (NA) | 0 | NA |
| Monthly gross personal income (AU$) | 2 (4.8) | $125 ($106) | 6 (19.4) | $1399 (£885) | 2 (4.8) | $1100 ($1273) | 2 (6.4) | $2000 ($1414) | 2 (4.8) | $1776 ($2438) | 4 (12.9) | $2068 ($1309) |
| At work but bothered by physical or mental problems (yes/no) | 2 (4.8) | 6 (19.4) | 4 (9.5) | 4 (12.9) | 3 (7.1) | 2 (6.4) | ||||||
| At work but bothered by physical or mental problems (days) | 2 (4.8) | 18.5 (9.19) | 7 (22.6) | 37.1 (40.4) | 4 (9.5) | 5.0 (4.83) | 3 (9.7) | 36.0 (42.5) | 2 (4.8) | 17.0 (4.24) | 1 (3.2) | 84.0 (NA) |
| How much of normal work capacity achieved on days bothered by health problems (scale 0–10, where 0 is none and 10 is full capacity) | 2 (4.8) | 9.0 (1.41) | 6 (19.4) | 7.0 (1.90) | 4 (9.5) | 8.75 (2.5) | 4 | 6.0 (2.58) | 3 (7.1) | 9.3 (1.15) | 2 (6.4) | 8.5 (2.12) |
| Reduce unpaid work because of physical/mental health reasons (days) | 6 (14.3) | 27.2 (32.3) | 3 (9.7) | 52.0 (42.6) | 1 (2.4) | 20.0 (NA) | 2 (6.4) | 17.0 (18.4) | 2 (4.8) | 55.0 (49.5) | 1 (3.2) | 3 (NA) |
| Reduce unpaid work because of physical/mental health reasons (average hours per day missed) | 6 (14.3) | 2.58 (1.36) | 3 (9.7) | 9.67 (12.5) | 1 (2.4) | 110.0 (NA) | 1 (3.2) | 25.0 (NA) | 2 (4.8) | 2.5 (0.707) | 1 (3.2) | 6.0 (NA) |
| Informal care | ||||||||||||
| Extra help with child care because of health problems (hours) | 1 (2.4) | 168.0 (NA) | 1 (3.2) | 5.0 (NA) | 1 (2.4) | 210.0 (NA) | 0 | NA | 2 (4.8) | 197.0 (264.5) | 2 (6.4) | 63.0 (46.7) |
| Extra help with household activities because of health problems (hours) | 13 (31.0) | 123.7 (170.3) | 10 (32.3) | 28.1 (29.3) | 10 (23.8) | 77.6 (94.2) | 11 (35.5) | 40.9 (98.4) | 9 (21.4) | 38.9 (11.7) | 9 (29.0) | 26.9 (15.0) |
| Extra help with shopping, transport, etc. because of health problems (hours) | 14 (33.3) | 62.2 (153.2) | 10 (32.3) | 11.1 (11.7) | 8 (19.0) | 38.7 (29.6) | 5 (16.1) | 8.6 (12.1) | 9 (21.4) | 55.3 (106.1) | 8 (25.8) | 15.1 (10.1) |
| Extra help with personal care because of health problems (hours) | 1 (2.4) | 144.0 (NA) | 3 (9.7) | 6.0 (4.0) | 2 (4.8) | 3.0 (1.41) | 1 (3.2) | 1.0 (NA) | 1 (2.4) | 30.0 (NA) | 1 (3.2) | 1.0 (NA) |
| Other extra help because of health problems (hours)f | 1 (2.4) | 288.0 (NA) | 0 | NA | 1 (2.4) | 2.0 (NA) | 0 | NA | 0 | NA | 0 | NA |
| Friends/relatives took time off work because of mental/physical health (hours) | 1 (2.4) | 48.0 (NA) | 1 (3.2) | 76.0 (NA) | 2 (4.8) | 65.5 (64.3) | 2 (6.4) | 18.0 (24.0) | 3 (7.1) | 268.3 (331.1) | 0 | NA |
| Resource type | 3 months | 6 months | 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EMPOWER (n = 42) | TAU (n = 31) | EMPOWER (n = 42) | TAU (n = 31) | EMPOWER (n = 42) | TAU (n = 31) | |||||||
| n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | n (%) | Mean visits (SD) | |
| Direct health care | ||||||||||||
| GP visits | 2 (40) | 1 (0) | 5 (83.33) | 1.2 (0.45) | 4 (80) | 1.67 (0.58) | 6 (100) | 2 (1) | 3 (75) | 1.5 (0.71) | 3 (75) | 1.67 (0.58) |
| Psychiatrist | 0 | 1 (16.67) | 1 | 0 | 2 (33.33) | 2 | 0 | 0 | ||||
| Psychologist | 0 | 0 | 0 | 0 | 0 | 1 (25) | Not stated | |||||
| Counsellor | 0 | 0 | 2 (40.0) | 1 | ||||||||
| Care nurse | 1 (20) | 1 (16.67) | 1 | 1 (20) | 2 | 1 (16.67) | 1 | 2 (50) | 1 (0) | |||
| Mental health nurse | 1 (20) | 0 | 0 | 0 | 0 | 0 | ||||||
| Alcohol worker | 1 (20) | 0 | 0 | 0 | 0 | 0 | ||||||
| Case manager | 0 | 0 | 1 (20) | 2 | 0 | 0 | 0 | |||||
| Speech therapist | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Occupational therapist | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Physiotherapist | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Other health professional | 2 | 2.5 (0.71) | 3 | 1.67 (0.58) | 2 | 1.5 (0.71) | 0 | 1 | 1 | |||
| Admitted to hospital (mental health) | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Admitted to hospital (not mental health) | 0 | 0 | 0 | 2 | 0 | 1 | ||||||
| Medication | ||||||||||||
| General medication (not specific to mental health) | 1 (20) | 4 (4.24) | 5 (83.3) | 3.8 (1.1) | 3 (60) | 3.33 (4.04) | 4 (66.67) | 3.5 (0.58) | 1 (25) | 7 | 4 (100) | 2.75 (1.26) |
Appendix 11 Work package 6 public engagement
We held three knowledge exchange events, one each in Coventry, Edinburgh and Glasgow, during August and September 2019. These events were titled ‘Digital mental health: what’s happening now? EMPOWER and beyond’ and each featured presenters and group discussions on the EMPOWER intervention.
A combined total of 225 delegates registered for these knowledge exchange events and, from those, 144 were able to attend and take part in discussions and feedback sessions. These delegates came from a variety of backgrounds and included academics, service users, carers and service providers.
After the EMPOWER team had presented a breakdown of how the intervention had been conceived, developed and then tested in the feasibility trial, we asked delegates to form discussion groups of about eight people and to share and write down their views on two separate flipchart sheets. One flipchart sheet was titled ‘EMPOWER now?’ to gauge their views on what they had heard during the presentations. The second was titled ‘EMPOWER future?’ and we asked delegates to tell us their thoughts and ideas on how EMPOWER might develop going forward.
After all of the events had concluded, we gathered all of the responses and used thematic analysis198 to construct themes to synthesise delegate perspectives.
‘EMPOWER now?’ feedback
Figure 15 illustrates the themes garnered from the ‘EMPOWER now?’ feedback (themes are not placed in any order of importance or priority). A summary of the main points raised by delegates in each theme follows.
FIGURE 15.
The ‘EMPOWER now?’ themes.

User–clinician interaction
There was considerable discussion around how EMPOWER would aid the monitoring of EWS for both users and their clinicians and could similarly be a useful source of additional information for clinicians to better understand service users. Delegates saw the app’s potential for opening up shared dialogue between app users and care teams in a way that would help the user feel more ‘heard’. They felt that it would offer the opportunity for people to feed back their experiences between appointments with their team, with increased options for how to communicate that feedback and better reflect on them together.
Inclusion/accessibility
Questions arose about whether or not service users would have access to a smartphone capable of hosting the EMPOWER app, and this was followed by discussion about how EMPOWER might not be accessible for those users with poor literacy skills, non-English speakers, ‘low-functioning’ service users, people with learning disabilities and people with cultural barriers. There was also a suggestion that a ‘very explicit introduction/guide’ would need to be developed to ensure that all users understood how to fully use the app. The app was also seen as a means to ‘break down barriers’ and would offer ‘high engagement’ among some users, in particular among young people.
Personalisation
A factor seen as central to the EMPOWER app’s success from the service user perspective was the app’s capacity to be personalised to individual needs and preferences. This led to discussions around making the app ‘individualistic’, ‘person centred’ and ‘recovery focused’, as well as having the capacity to incorporate ‘flexibility’, with one delegate suggesting being able to add in and accommodate ‘life events’ as they arise. There was positive feedback on how EMPOWER allows some personalisation of its ‘questions’ and that its approach is ‘not a one size fits all’.
Benefits
Comments on the benefits of using the EMPOWER app ranged from potentially increasing ‘self-awareness’ and being able to ‘see your pattern’ when logging data, to the app ‘supporting a positive recovery style’, ‘normalising “ebb and flow” of wellness’ and ‘boosting coping strategies and signposting to them’. The opportunity to improve app users’ insight and understanding of EWS, triggers, moods, experiences and ‘acceptance of their diagnosis’ was also mentioned.
Peer support
The peer support aspect of EMPOWER was viewed positively by delegates across all events. Peer support workers of EMPOWER were described as being able to ‘help balance potential fear or paranoia’ around the app as well as being ‘very important regarding hope and stigma’. Peer support workers were viewed as a central ‘human element’ of EMPOWER and there was much discussion about how peer support would work in current NHS practice.
General positives
This theme encompasses a collection of non-specific but positive comments delegates made on the flipchart sheets. Overall, the comments showed that a number of delegates felt positively about the EMPOWER intervention and told us that they viewed it as a ‘positive initiative’ with ‘great potential’. In terms of the development of EMPOWER to its current stage, delegates offered the following insights: ‘Reassured that it is not a substitute for relationships’; ‘initially thought it would be a standalone app. Positive to know it is supported via mental health services and peer support’; ‘Good consideration of adverse events’.
‘EMPOWER future?’ feedback
Themes we derived from ‘EMPOWER future?’ feedback are shown in Figure 16.
FIGURE 16.
The ‘EMPOWER future?’ themes.
After we thematically analysed the suggestions and views written on the flipcharts, we derived two main themes: ‘development of EMPOWER app’ and ‘NHS implementing EMPOWER’.
Development of the EMPOWER app themes
There were five subthemes, which are described below.
App format: ideas
Delegates were keen to discuss how improving the appearance of the EMPOWER app would make it easier to use as well as giving it a ‘more polished and modern-looking design’. They talked about whether the app could be made ‘available on a website’ to get around the issue of users needing a smartphone to use the app in its current form and to offer users more control over the content and when to respond to prompts to enter data. They also speculated about the self-management potential among users, which one delegate theorised could bring feelings of ‘treatment optimism’. Other discussions around reducing the demands the app places on the user also occurred with insights into offering users more control over when they used the app and including a ‘pause’ button to allow users to take a break and return later as suited them. The opportunity for users to use the app in conjunction with other users was also mentioned, with the idea of users feeling less isolated and more ‘connected to others’ being central to that discussion. Finally, there were contributions on how to make the app more dynamic to give users more options to interact with it in ways that that suited the app user as opposed to those set by the app itself.
User impact and accessibility: ideas
The vast majority of delegates’ comments on this theme underlined the perceived importance of making the app feel as personalised as possible. Delegates felt that personalisation should be ongoing throughout the app’s research development. They also suggested that app users collaborating with their care team might increase the app’s capacity to be personalised to the user’s preferences, in the hope of optimising its person-centred feel, especially in terms of language and prompt use.
Peer support
There was unanimous agreement across the discussion groups both that peer support is central to the effectiveness of the EMPOWER app and that every effort should be made to ensure this is a core factor of the intervention that must be retained going forward in its development.
Monitoring
On the whole, the comments on the subject of an app utilising both active (e.g. question responses) and passive (i.e. automatically collected) data were positive. That said, delegates were quick to underline the importance of ensuring that any app with monitoring capabilities is ‘optional’ for users and not something that would ever be mandatory or ‘forced’ on them. The idea that factors such as sleep, steps, time on social media and behaviour changes could be captured in real time was something delegates felt could be beneficial. Being able to identify patterns, better predict outcomes and receiving alerts about when it might be time to take ‘preventative action’ were all aspects that appealed to delegates. Fitbits (Fitbit, Inc., San Francisco, CA, USA) were mentioned as additional technology that could be incorporated to expand the EMPOWER app’s capacity to collect useful data from the user in a non-invasive way.
Future app development
Delegates discussed where the EMPOWER app could go next, in terms of both its next stage of development and its scope going forward and its capacity to engage with future app users by incorporating forms of media into its format. Both Skype (Microsoft Corporation, Redmond, WA, USA) and FaceTime (Apple Inc., Cupertino, CA, USA) were mentioned as apps that delegates wanted to see added to EMPOWER as ways for people to chat with their care or support teams over live video. Giving app users more flexibility by offering them access to other self-help apps within the EMPOWER app was another suggestion. There was some discussion about how the app might benefit inpatients in psychiatric wards if they used it as part of their discharge plan with their inpatient team. There were also ideas about expanding the app to offer access to things people might want to engage with more, ‘the app progressing with you’, in tandem with their recovery, and resources such as films, articles, music and a platform for creative writing were seen as positive additions.
Concerns
‘Who owns the data?’ was a concern commonly expressed by delegates and this led to broader discussions (noted elsewhere) about privacy, confidentiality and data protection. The app’s smartphone platform was seen as a barrier to those with certain disabilities, as was the use of the English language to those users in the UK who might not speak or read English fluently. There was some more discussion about whether people would feel obliged to use the app as part of a care package and that this was something to be avoided. In addition to this, and as some delegates were quick to point out, not everyone likes using mobile phones, and also the current ‘slide-scale’ way of answering questions on the EMPOWER app might not suit everyone. There was also a perceived risk that answering time-limited questions on the app might feel like an unwelcome pressure to some users. Further conversations were around whether adverts would become part of the app to help with costs and, if so, if there would be ways to ensure that these did not have a negative impact on ‘vulnerable patients’. Delegates also made it clear that if EMPOWER were to be implemented into the NHS it would need to have proven efficacy as ‘a real tool’. Finally, some discussion took place around EMPOWER and apps and technology more generally as a ‘threat to workforce’, as concerns were shared about not seeing the value of human roles as ‘tasks (are) replaced by technology’.
NHS implementing EMPOWER themes
Our second theme, ‘NHS implementing EMPOWER’, had four subthemes. The suggestions gathered from delegates around those themes are described in the following sections.
Effects on clinicians
Delegates had some concerns that the app would not be a viable option for those clinicians who work with ‘critical need’ service users, and they saw it as a way to stay more connected to GPs about service users’ needs. Interestingly, it was pointed out that ‘staff need to stay well too’ and that EMPOWER was an app that mental health staff might want to use to maintain their own well-being.
Implementation demands
There were concerns about the lack of peer support workers currently working in NHS mental health services. It was seen as desirable that both a recognition of the value of peer support workers and a financial commitment to funding such posts would have to take place for the EMPOWER intervention to be implemented in its current form. The importance of investing in training staff well so that they feel comfortable using the app with their service users was also highlighted to ‘help with enthusiasm’. Depending on the level of data being disclosed and shared, this might also enhance clinicians’ understanding of the contexts within which certain types of app responses take place. It was pointed out that ‘outpatient appointments, Care Programme Approach reviews and clinical reviews’ could be available to both service users and clinicians via the app and that if there was the potential to offer this information in a variety of formats it could increase shared understanding of the information and improve overall transparency.
Cost and data security
Points were raised about financial sustainability of the app within the NHS. Concerns around whether the NHS would be responsible for supplying the smartphones that host the EMPOWER app were expressed and also whether this would mean that the NHS would be expected to pay for software or data costs. Issues around the safety of storing data on mobile phones were raised often and, similarly, worries around data protection and confidentiality featured in the feedback.
Potential for broader implementation
Delegates had a wide variety of ideas in relation to the potential for EMPOWER to be implemented more broadly across the NHS. There was discussion around integrating it into current NHS systems and linking it with GPs, particularly as a resource for ‘medication changes and renewals’. The potential implementation of EMPOWER into the NHS was seen more broadly as a helpful way to ‘increase awareness and use across different populations’ and as a ‘part of prescribing’ and ‘watchful waiting’. Practical considerations such as how EMPOWER data could be linked to electronic clinical records were also mentioned. Overall, delegates were positive about EMPOWER being implemented into the NHS. The fact that the EMPOWER algorithm has been approved as a medical device by the MHRA led to a feeling that users and clinicians would view EMPOWER with a ‘reassurance of something that has an authority behind it’.
Glossary
We use the term ‘service user’ to refer to people with direct personal experiences of schizophrenia and psychosis who were recruited to the study. We are aware that the preferred term in Australia is ‘consumer’ and also that the term ‘service user’ can be criticised for, among other things, collectively describing a group of people based on their receipt of mental health services, which is limiting. However, we agreed this as our preferred option given its widespread adoption in related mental health research and also to be consistent with the language used in our protocol.
While service user eligibility criteria for different study elements related to schizophrenia and related diagnoses, we more routinely refer to psychosis when describing participants’ experiences and the intervention’s purpose. In doing so we are recognising that relapse relates specifically to psychosis experiences. We do, however, acknowledge that people with other diagnoses who would not have been eligible for this study, for example those with bipolar disorder, may also experience psychosis.
List of abbreviations
- app
- smartphone application
- AQoL-8D
- Assessment of Quality of Life
- CBT
- cognitive–behavioural therapy
- CDSS
- Calgary Depression Scale for Schizophrenia
- CHaRT
- Centre for Healthcare Randomised Trials
- ChIP
- check-in prompt
- CI
- confidence interval
- CMHS
- community mental health service
- CONSORT
- Consolidated Standards of Reporting Trials
- cRCT
- cluster randomised controlled trial
- CTU
- Clinical Trials Unit
- DMEC
- Data Monitoring and Ethics Committee
- EMA
- ecological momentary assessment
- EMPOWER
- Early signs Monitoring to Prevent relapse in psychosis and prOmote Wellbeing, Engagement, and Recovery
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EWS
- early warning signs
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- ICER
- incremental cost-effectiveness ratio
- ITAREPS
- Information Technology Aided Relapse Prevention Programme in Schizophrenia
- JCP
- joint crisis plan
- MBS
- Medicare Benefits Schedule
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NIHR
- National Institute for Health and Care Research
- NPT
- normalisation process theory
- PANSS
- Positive and Negative Syndrome Scale
- PBIQ-R
- Personal Beliefs About Illness Questionnaire-Revised
- PSP
- Personal and Social Performance
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- RR
- relative risk
- RUQ
- resource use questionnaire
- SD
- standard deviation
- SSC
- Study Steering Committee
- TAU
- treatment as usual
- UX
- user experience
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/HLZE0479).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
