Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA Programme as project number 04/38/03. The contractual start date was in July 2005. The draft report began editorial review in December 2006 and was accepted for publication in March 2008. As the funder, by devising a commissioning brief, the HTA Programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2008. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2008 Queen’s Printer and Controller of HMSO
Chapter 1 Aim of the review
The aim of the planned research is to assess the relative clinical effectiveness and cost utility of established and emerging interventional treatments for men suffering symptoms or complications caused by benign prostatic enlargement (BPE).
The specific objectives are:
-
To determine the clinical effectiveness of alternative procedures.
-
To determine the magnitude of risk of their short- and long-term side effects.
-
To rank the clinical effectiveness and risk profile of new interventional procedures against transurethral resection of the prostate (TURP), currently considered the gold standard of care.
-
To estimate the cost utility of the alternative procedures.
-
To assess the effects of skill and learning on cost-effectiveness.
-
To identify clinical indications and contraindications for specific procedures.
-
To assess the speed of development in the field.
-
To identify areas in which future research is required.
The research was based on four inter-related components:
-
Development of care pathways for the chosen treatment options for men presenting with symptoms or complications resulting from BPE.
-
A systematic review of the literature of the effects of the alternative procedures.
-
A systematic review of economic evaluations to inform (4) below.
-
Construction of a Markov model and cost-utility analysis of the treatment options.
Chapter 2 Background
Description of the underlying health problem
Introduction
Clinical BPE describes a condition affecting older men characterised by the combination of increased prostate size and urinary symptoms such as frequency and poor urinary flow that bother the patient. The pathophysiology of benign enlargement involves hyperplasia of the epithelial and stromal components of the prostate gland leading to progressive obstruction of urine flow, and increased activity of the bladder (detrusor) muscle. These secondary urodynamic changes of bladder outlet obstruction and detrusor dysfunction are thought to result in the typical bladder storage symptoms such as frequency and nocturia and voiding symptoms such as poor flow and intermittent stream. For simplicity, the variety of symptomatic effects are grouped together as lower urinary tract symptoms (LUTS). Although the precise relationship between symptoms, prostate enlargement and detrusor dysfunction can be debated, there is no doubt that removal of prostatic tissue in affected men results in improvement of symptoms, urodynamic parameters and quality of life.
Men are diagnosed as suffering from clinical BPE by documenting a combination of storage and voiding symptoms, finding a uniformly enlarged prostate gland on digital rectal examination and the measurement of a reduced peak urinary flow rate (Qmax). Qmax is normally used to predict response to surgery and acts as a proxy for urodynamic studies. Men with a Qmax of less than 10 ml/s are more likely to have urodynamically proven bladder outflow obstruction and as a result are more likely to have a good outcome after surgery. The usefulness of other indicators of lower urinary tract function, in particular the diagnosis of bladder outlet obstruction by invasive pressure flow studies, continues to be debated. In general, such testing before surgery will reduce the number of men having a poor outcome at the expense of denying a proportion of men classified as not obstructed successful surgery. Because Qmax was the only urodynamic inclusion criterion for the studies included in the systematic review, the utility of further testing has not been considered further. 1
The diagnosis also requires exclusion of other lower urinary tract disorders by urinalysis, prostate-specific antigen (PSA) level and use of a frequency/volume chart. The severity of the disorder is assessed using a validated symptom-scoring questionnaire, most commonly the International Prostate Symptom Score (IPSS). 2 This questionnaire asks the patient to rate voiding symptoms (poor stream, intermittent flow, incomplete emptying, straining) and storage symptoms (urgency, frequency, nocturia) on a scale from 0 (none) to 5 (very severe). Completion of the IPSS yields a total score ranging from 0 to 35 defining mild (score 0–7), moderate (score 8–19) and severe (score 20–35) symptomatic states. In addition, a single disease-specific quality of life question scores how bothersome symptoms are for each individual [range 0 (delighted) to 6 (terrible)]. This basic assessment is used to discuss management options with each patient, which may involve lifestyle changes alone, drug treatment or invasive therapy to remove or ablate prostate tissue. In some men the predominant clinical problem is characterised as a complication of BPE. This can be recurrent lower urinary tract infection (UTI), bleeding (haematuria) or urinary retention. Such complications are generally an indication for invasive treatment to remove prostate tissue. Other assessment instruments include the well-validated American Urological Association (AUA) symptom index, which uses seven questions that are identical to the IPSS questions with the exception of the disease-specific quality of life question, and the Madsen–Iversen index, which is no longer recommended for assessing symptoms as it was not designed to be self-administered by patients. The Madsen–Iversen index is usually completed by an interviewer and includes questions about stream, straining to void, hesitancy, intermittency, bladder emptying, incontinence, urgency, nocturia and frequency, with different symptoms attracting different scoring schemes. Although providing semi-objective symptom quantification, these questionnaires, including the currently favoured IPSS, have been criticised for giving undue weight to voiding symptoms at the expense of the sometimes more troublesome storage complaints.
Epidemiology and natural history
Clinical BPE is a common disorder, affecting 30% of those older than 60 years and 40% of those older than 70 years. 3 What is becoming increasingly clear is the generally progressive nature of BPE. 4,5 In a randomised comparison with TURP, 30% of men assigned to advice alone required prostate surgery for progressive symptoms during a 3-year period of surveillance. 6 Longitudinal community observational studies such as that performed in Olmsted County, USA7 have shown an increase in both symptom severity and adverse effects on quality of life associated with progressive prostate enlargement and deterioration in urine flow. This study followed 2115 randomly selected white male residents and found that 26% of men aged from 40 to 49 years and 46% of men aged from 70 to 79 years reported moderate to severe urinary symptoms. Longitudinal data also confirmed an annual increase in prostate volume of 1.6%, an overall annual increase in symptom score of 0.298 and a consistent annual decline in peak flow of 2% across all age groups. 9 In the same cohort of patients there was an increased risk of acute urinary retention with increasing age, with baseline age, symptom severity, prostate size and maximum flow rate identified as independent predictors. 10 A potential drawback of such community-based studies is the lack of histological confirmation of benign hyperplasia, which in other studies has been found to be present in 40% of men in their 50s and around 90% of men in their 80s. 11–13 Although the natural history of clinical BPE is more accurately determined using community-based cohorts such as in the Olmsted County study, further insights are gained from placebo arms of trials of drugs used to treat clinical BPE, such as the medical therapy of prostatic symptoms (MTOPS) study14 which documented that the risk of BPE progression averaged 17% at 4 years.
Significance in terms of ill health
The combination of improved life expectancy and reduction in birth rate has resulted in an actual or predicted progressive ageing of the population in most communities worldwide. For men, it is estimated that the population of those aged over 65 years reached 207 million in 2005, constituting 6.38% of the world’s male population. 15 These demographic changes inevitably result in an increased prevalence of chronic health problems associated with ageing. This has been shown for clinical BPE by a number of epidemiological studies. 16 The prevalence of moderate to severe symptoms progressively increases from 18% of men in their 40s to 56% of those in their 70s. 17 The bothersome nature of urinary symptoms is linked to adverse changes in quality of life and drives men to seek medical advice and treatment. In the past the range of treatment was limited to open or endoscopic removal of the prostate but now options include single or combination drug therapy, phytotherapy and the application of various energy sources to remove or ablate prostate tissue. The increased range of therapies has encouraged more men to seek help to alleviate their symptoms and has led to a widening of the indications for interventional treatments. Thus, although it is rarely a life-threatening problem, clinical BPE represents a major and increasing health condition that consumes a significant proportion of health-care expenditure. 18
The goals of treatment of clinical BPE are to reduce the severity of symptoms together with the bother that they cause, to normalise the dynamics of the lower urinary tract and to resolve or prevent complications. Treatment options balance likely benefits with possible occurrence and severity of side effects. Simple reassurance and lifestyle advice can be sufficient for those men without much bother but they incur the risk of later complications. Drug treatment can be effective for relief of symptoms and evidence suggests that long-term treatment with a drug combination may also lessen the risk of complications. 19 Drug treatment is, however, costly, of only moderate effectiveness and does not improve urodynamic status. Procedures that reduce prostate bulk combine higher effectiveness with the attraction of a single treatment, but they are associated with increasing severity of unwanted effects; open removal of the prostate (prostatectomy), for example, has the greatest effectiveness but results in the highest morbidity. Although still an option for larger glands, open prostatectomy is not commonly used for the treatment of BPE in the UK and will not be considered further in this review, which concentrates on newer interventions. TURP has been the mainstay of treatment for clinical BPE for many years because it combines high effectiveness with a previously acceptable side-effect profile. More recently, in the UK, men have tended to seek help earlier in the natural history of the disease and access to secondary health care has improved. This, together with increasing co-morbidities present in the ageing population at risk and the desire of health providers to contain costs, has fuelled the search for less morbid invasive treatments. There is also some evidence that men without complications or severe symptoms would prefer a less morbid method of prostate ablation with a shorter hospital stay. 20 Technological developments have allowed clinical investigators and medical device manufacturers to apply alternative energy sources with varying degrees of invasiveness to achieve reduction of prostate bulk without some of the side effects of TURP, such as bleeding, cardiovascular disturbance due to irrigation, incontinence and ejaculatory dysfunction. These interventions can be subdivided into surgical procedures that generally involve removal of prostate tissue requiring general or regional anaesthesia and minimally invasive options, which do not require general anaesthesia and can be carried out in an outpatient setting. 21 The former group are generally more efficacious than the latter group but have higher complication rates; however, estimates of beneficial and unwanted effects do vary between procedures within these two categories. 21
Description of new interventions
In this section we describe standard and newer interventions that will be compared in the review of clinical effectiveness and economic model. The UK government-funded health service (NHS) is fortunate in having comprehensive centralised data collection systems from which numbers of procedures and their costs can be extracted. 22 Unfortunately, current coding systems do not differentiate between energy sources used in prostate ablation, with all procedures coded as TURP. This makes it difficult to estimate the number of newer interventions being performed, and the occurrence rates for specific procedures given below should be considered as approximate. Considering the relevant OPCS-4 codes (M65.1, M65.2, M65.3, M65.8, M65.9, M66.2, M66.8, M66.9, M67.8, M67.9, M70.8), a total of 28,799 procedures were performed within NHS hospitals in England during the financial year 2004–2005 (main operation four-character codes 2004–2005), which tallies well with the count of 30,387 using the simplified Healthcare Resource Group codes L27, L28 and L29 (Healthcare Resource Group codes 2004–2005). 22 Given a total population of 49 million and a population at risk (men > 59 years) of 4.5 million, this gives crude incidence rates of 60 per 100,000 per year and 667 per 100,000 per year respectively for surgical treatment of clinical BPE. 23 Table 1 provides a summary of the main surgical procedures, detailing the main characteristics, number of operations performed by the NHS in 2006 and cost.
| Procedure | Hospital stay | Energy source | Method of tissue removal | Period of catheterisation | NHS procedures (per year)a | Cost (£)b |
|---|---|---|---|---|---|---|
| Minimally invasive | ||||||
| TUMT | Day case | Microwave | Coagulative necrosis | 1–2 weeks | 300 | 1800 |
| TUNA | Day case | Radio frequency | Coagulative necrosis | 3 days | 100 | 1600 |
| HIFU | Day case | Ultrasound | Coagulative necrosis | 2 weeks | 100 | 1000 |
| Laser coagulation | 1–2 days | Laser | Coagulative necrosis | 3–7 days | 500 | 750 |
| Ablative | ||||||
| TUIP | 1–2 days | Diathermy | None | 1–2 days | 2500 | 1800 |
| TURP | 3–5 days | Diathermy | Resection | 1–3 days | 20,000 | 2000 |
| Laser vaporisation | 1–2 days | Laser | Vaporisation | 1–2 days | 3000 | 2600 |
| TUVP | 2–3 days | Diathermy | Vaporisation | 1–2 days | 2000 | 1800 |
| HoLEP | 2–3 days | Laser | Enucleation | 1–2 days | 1500 | 1900 |
Minimally invasive treatments
Introduction
Minimally invasive treatments seek to ablate BPE using low-energy heating devices. Typically temperatures of 40–80°C are achieved, causing areas of coagulative necrosis, which either slough via the urethra or are reabsorbed during tissue repair. The resultant defect is usually visible on transrectal ultrasound scanning but is considerably smaller than for TURP. Provided energy delivery is kept low these treatments can be carried out in the office or outpatient clinic, whereas higher energy levels require anaesthesia and hence an operating theatre. Delayed necrosis means that relatively prolonged catheterisation is required to avoid urinary retention and painful micturition and, as a consequence, treatment benefit may not be realised for 2–3 months. 24 The use of urethral stents is also discussed in this section.
Interventions
Transurethral microwave thermotherapy
Microwave energy is used in transurethral microwave thermotherapy (TUMT), achieving temperatures of 45–70°C in the prostate depending on the device and power setting. Initially, energy was delivered at low power settings but variable higher energy delivery is now more usual. Microwaves induce oscillation of water molecules causing heat generation and inducing coagulative necrosis of prostatic tissue. 25 The procedure is typically performed using an antenna mounted within a transurethral catheter through which cooling fluid circulates. Temperature control is regulated by urethral and rectal thermometer probes to prevent collateral damage. The procedure lasts for 30–60 minutes and is performed using local anaesthesia and oral analgesia together with sedation for high-energy protocols. Requirement for postoperative catheterisation varies from 1 to 12 weeks depending on the protocol used. 26
Transurethral needle ablation of the prostate
Transurethral needle ablation (TUNA) of the prostate involves the delivery of radio frequency energy via a modified urethral catheter attached to a generator to ablate prostate tissue. Two adjustable needles located at the end of the catheter are inserted into the prostate under endoscopic control. The radio frequency waves generate ionic agitation of molecules within the prostate, which in turn produces a localised heating effect of up to 115°C resulting in areas of coagulative necrosis. Teflon sheaths are advanced over the needles following placement to a depth of 5–6 mm to protect the urethra. The radio frequency power is usually delivered at 2–15 W for 5 minutes per lesion. 27 Once the coagulative effect has been achieved the needles are placed in a different area of the prostate and the procedure repeated. Depending on prostate size, the procedure generally lasts between 30 and 60 minutes and is performed under local or regional anaesthesia. 28 An indwelling catheter is placed for up to 3 days and antibiotic therapy given. 29
Urethral stent
The rationale for stenting of the prostatic urethra in men with BPE is to nullify the compressive and constrictive obstructive effect of the adenomatous tissue and hence reduce the bladder pressure required to open the urethra. 30 The currently available device is made of woven braided wire mesh that can be delivered and expanded in the prostatic urethra under endoscopic or radiological control. The proximal end is engaged in the bladder neck and the distal end must lie above the external sphincter to prevent incontinence. The procedure can be accomplished using local anaesthesia. The inner aspect of the stent becomes lined with epithelium over a 3- to 12-week period. Unfortunately, device migration, ingrowth of fibrous stroma and encrustation are common longer-term sequelae leading to explantation in up to 50% of cases.
High-intensity focused ultrasound
High-intensity focused ultrasound (HIFU) uses ultrasound as the energy source, which, when tightly focused, can cause coagulative necrosis of tissue. It is delivered by a transrectal probe equipped with a transducer incorporating both imaging and ablative capabilities on the same ceramic crystal operating at 4 MHz. Ultrasound can be delivered to a precisely located focal zone of 2 × 10 mm leading to a rapid rise in temperature of up to 80–100°C using short exposure duration. Multiple lesions are then created throughout the prostate by moving the probe, with a treatment session lasting about 60 minutes. A catheter is placed to drain the bladder throughout the procedure and remains in place for about 2 weeks. 31,32 The high temperatures achieved necessitate general anaesthesia or sedoanalgesia with the procedure carried out as a day case.
Transurethral ethanol ablation of the prostate
Transurethral ethanol ablation of the prostate (TEAP) is chemical ablation of prostatic tissue using dehydrated ethanol. This results in the development of intraprostatic necrotic areas due to dehydration, protein degeneration and thrombotic closure of arterioles and venules. 33 Delivery of absolute ethanol into the prostate can be achieved by injection via a transperineal,34 transrectal35 or transurethral36 route. The transurethral route is the most commonly reported delivery route. Commercially available 0.5–2.0 ml injection of ethanol (99.5% v/v) is injected into the prostate using either an injection and aspiration set for periurethral injection (Richard Wolf GmbH, Knittlingen, Germany) or a cystourethroscopy injection system (Olympus Winter & Ibe GmbH, Hamburg, Germany). The sites of injection are about halfway between the bladder neck and the verumontanum at the 2, 4, 8 and 10 o’clock positions, at least 1.5 cm proximal to the external sphincter. The number of injections depends upon the size of the prostate gland. The requirement for postoperative catheterisation is longer than in standard TURP and the retreatment rates are higher. 37 There are no long-term outcome or cost-effectiveness reports.
Water-induced thermotherapy
Water-induced thermotherapy (WIT) destroys prostate tissue by way of heat energy delivered by hot water flowing through a urethral catheter made up of four contiguous sections – a urine drainage lumen, a positioning balloon, a treatment balloon and an insulated shaft. 38 The catheter is inserted into the urinary bladder and secured by inflating the positioning balloon. Hot water circulates through the treatment balloon, which lies in the prostatic urethra, and is precisely maintained at 60°C (140°F) by thermocouples located in the catheter and machine. The procedure takes approximately 45 minutes under local anaesthesia and analgesia. The treatment catheter is removed and replaced by a standard urethral drainage catheter, which remains for 4–17 days. 39
Transurethral laser coagulation of the prostate
Laser-induced coagulative necrosis of the prostatic tissue can be achieved either by surface application to the prostatic urethra in a technique termed visual laser ablation of the prostate (VLAP) or by inserting specially designed fibres into the prostatic tissue via the urethra, termed interstitial laser coagulation (ILC). VLAP uses a neodynium:yttrium-aluminium-garnet (Nd:YAG) laser to create areas of coagulative necrosis extending out from the prostatic urethra. This laser has a unique wavelength of 1064 nm and penetrates tissue for up to 1.7 cm leading to delayed necrosis and sloughing of tissue into the urethra over a period of 6–8 weeks. For ILC, a diode laser is transmitted through a fine fibre, which is inserted into the prostate under endoscopic control to a depth of 1 cm to create 3 cm3 lesions within 2–3 minutes at a temperature of 85°C. Typically, up to ten locations can be treated, with the procedure lasting for 30–60 minutes under local anaesthesia. Catheterisation is typically required for between 3 and 7 days. 40
Identification of patient subgroups and criteria for treatment
The one-off outpatient nature of minimally invasive therapy makes it an attractive option for men with moderate to severe LUTS who do not wish to have long-term medical treatment or who are concerned about the side effects of more invasive treatments. The reduced need for anaesthesia and lower morbidity make it suitable for men with extensive co-morbidity. 27 These procedures are generally not suitable for men with larger prostates (> 50 g) because of prolonged treatment time and high rates of post-treatment dysuria and urinary retention. In addition, they are not indicated for men with absolute indications for prostate surgery such as urinary retention, bleeding and recurrent urinary infection. The use of stents is restricted to men with urinary retention with extensive co-morbidity, which precludes prostate ablation techniques.
Personnel involved
Most of these treatments can be performed by a single physician, typically a urologist, in an office or clinic setting. The physician should have expertise in both the technique and the administration of local anaesthetic. A nurse assistant is also required together with appropriate reception and administration staff. Removal of the catheter can be performed at a subsequent office visit or by a community nurse.
Setting
These technologies are suitable for use in the office, clinic or ambulatory care facility with a typical stay of approximately 4–8 hours. For procedures performed under local anaesthetic a well-equipped clinic room with basic resuscitation facilities, appropriate utility supply and recovery area are all that are required; however, for some procedures a standard operating theatre set-up with anaesthetic support is required. High capital costs and concerns regarding effectiveness have led to low use of these procedures in the UK, with only a few centres using the technology. It is estimated that fewer than 1000 procedures in total are carried out per year, representing less than 4% of the total.
Equipment
In general, these technologies require a generator and a delivery device, which is typically a single-use modified urethral catheter. In addition, some require cooling circuits, endoscopic positioning and transrectal imaging for device placement and monitoring of effect. Drugs and delivery equipment for local anaesthesia and sedation are also required. Patients are generally discharged home shortly after completion of the procedure with an indwelling catheter. Different manufacturers offer competing devices, which differ mainly in power output and delivery system. For TUMT the main devices are Prolieve™ (Boston Scientific, USA), CoreTherm™ (Prostalund, Sweden), TherMatrx® (American Medical System, USA), and Prostatron® and Targis™ (Urologix, USA). TUNA is provided by Prostiva™ (Medtronic, USA), WIT by AquaTherm™ (WIT) (ACMI, USA) and HIFU by Sonablate® 500 (Focus Surgery, USA). The currently available interstitial laser device is Indigo Optimax (Indigo LaserOptic™(Johnson & Johnson, USA). 40,41 The available prostatic stent is marketed as Urolume® (American Medical System, USA).
Costs
The cost of a TUMT generator is approximately £14,000, with an additional cost of disposables of approximately £350 per case (Urologix, USA). The TUNA machine costs £5750 with an additional cost of £700 for the disposable cartridge (Medtronic, UK). The purchase cost of the Sonablate 500 HIFU system is around £300,000 (UK HIFU). Urolume stents cost £1365 (American Medical System, UK). The remaining devices are not marketed in the UK.
Transurethral resection of the prostate (TURP)
Introduction
TURP has been the standard method of surgical management of clinical BPE for 50 years and in recent times has accounted for more than 90% of prostatectomies performed for this indication,42 although in current practice this has been reduced to 60–80% by the advent of other ablative procedures detailed below. 42 The technology uses diathermy current for prostate resection via a loop electrode using a continuous flow endoscope passed down the urethra with non-ionic fluid irrigant, usually 1.5% glycine. Coagulative haemostasis is achieved during and at the end of the procedure with a ball diathermy electrode. For most men a skilled urologist can achieve complete resection of up to 100 g of tissue within 1 hour. Improvements in endoscope design, diathermy units and bladder irrigation have reduced both operating time and risk of major morbidity. Postoperatively the bladder is irrigated for 6–24 hours; the catheter is removed at 24–48 hours after surgery before discharge home. 43
Identification of patient subgroups
TURP is a versatile technique that can achieve effective relief for men with bothersome moderate or severe symptoms. It is also highly effective at treating other manifestations of BPE such as urinary retention, recurrent infection and haematuria. Blood loss and absorption of irrigant fluids are the main causes of operative morbidity, particularly in men with clotting disorders, those taking anticoagulant or antiplatelet medication and those with significant cardiovascular morbidity. Safety can be improved by use of preoperative drug treatment aimed at reducing both the size of the prostate and bleeding during the procedure and use of preoperative antibiotic prophylaxis. Improvements in spinal anaesthesia and better videoendoscopic equipment have resulted in shorter operation times, and more aggressive catheter removal policies have shortened hospital stay. 44
Personnel involved
TURP requires full operating room facilities with a urologist, scrub and circulating nurses and an anaesthetist. Standard inpatient pathways with experienced ward and recovery room staff and porters are also required.
Setting
Traditionally TURP was considered an inpatient procedure requiring admission the day before surgery and a 4-day postoperative stay in a urology hospital ward. In the UK, the last 1–2 years have seen the development of managed care pathways and a drive towards shortened hospital stay, stimulated partly by competing techniques and partly by cost containment and avoidance of hospital-related morbidity. This has meant that stay for straightforward TURP has been shortened to 2–3 days with discharge the morning following midnight catheter removal. 44
Equipment
A standard diathermy generator is required with cutting and coagulation outputs. The videoendoscopic equipment is also standardised with, typically, a 26Fr sheath, operating element, 30° telescope, xenon light source and ‘two-chip’ camera with appropriate monitor.
Costs
Multiple manufacturers compete for this market, which tends to keep actual purchase costs low although list prices are high. Most of the equipment would be considered standard operating department stock with multifunctionality for use in open surgery, endourology and laparoscopic surgery. Within the NHS the procedure has unique Healthcare Resource Group codes, L27 for men aged over 69 years and L28 for men aged under 70 years, with mean costs (2004–2005) set by providers of £2060 (interquartile range £1715–2429) and £1864 (interquartile range £1547–2198) respectively. 45
Transurethral incision of the prostate
Endoscopic incision of the prostate from bladder neck to verumontanum at the 7 o’clock position using cutting diathermy via a standard resectoscope is a relatively simple technique that is claimed to have short-term equivalence in effectiveness to TURP for men with smaller prostates (< 30 g). 46,47 The advantages of transurethral incision of the prostate (TUIP) are reduced bleeding with no need for postoperative irrigation and shortened catheterisation time together with a lower risk of developing retrograde ejaculation. 47 The disadvantage is that no prostatic tissue is removed leading to a high rate of symptom recurrence and need for further surgery. 43
Patient selection, personnel required, setting, equipment and costs are similar to those for TURP. 24,45 TUIP has a specific OPCS-4 code (M66.2) and data from the NHS suggest that 2464 procedures were carried out in England during 2005, representing 8.5% of the total (main operation four-character codes 2004–2005). 22
Other tissue ablative techniques
Vaporisation of the prostate
Introduction
Vaporisation of tissue requires rapid localised heating to temperatures of 100°C or more with minimal depth of penetration. The anatomy of the prostate and in particular the development of hyperplasia within the inner periurethral zones of the gland mean that transurethral delivery of energy for vaporisation is both feasible and desirable. At present two alternative sources of energy are available for transurethral vaporisation of the prostate (TUVP): laser and electrosurgical. 48
Interventions using laser technology
Basic research has enabled the identification of lasers with source, wavelength and absorption characteristics suitable for rapid heating with minimal tissue penetration that could be delivered by the transurethral route and cause vaporisation on contact with the prostate. 49 Initially, Nd:YAG was used at a power setting of 40 W. 50 This had a disadvantage for vaporisation purposes of relatively deep tissue penetration (4–18 mm) related to low absorption and a wavelength of 1064 nm in the invisible spectrum. 51 These characteristics were improved by passing the Nd:YAG-generated beam through a potassium-titanyl-phosphate (KTP) crystal, which doubles the frequency and halves the wavelength. By doing so, the light becomes visible in the green spectrum (532 nm), which encourages absorption by haemoglobin52 and results in a depth of penetration ranging from 0.8 to 3 mm. 49 In a highly vascular tissue such as BPE, this results in a high energy density and rapid vaporisation, which is further improved by the higher power source (80 W) that is currently available for this technology. 51 The holmium laser can also be used for transurethral prostate vaporisation by delivering energy at a wavelength of 2140 nm. 53 This laser has limited tissue penetration (0.4 mm), affords excellent haemostasis and is preferentially absorbed by water, enhancing the effectiveness of tissue ablation. Initially, moderate power (60 W) was used but this has now been increased to 80–100 W to improve efficiency. 49 Contact laser vaporisation is performed using an irrigating cystoscope but still requires similar anaesthesia and operating conditions to TURP, with the operating time increased by a factor of approximately 1.5. 54
Interventions using non-laser technology
This technique utilises a standard monopolar electrodiathermy device to deliver sufficient power, typically 180–300 W on the ‘cut’ setting, to vaporise tissue on contact. The procedure is performed using an irrigating sheath and telescope passed along the urethra, which allows continuous flow of a non-ionic solution such as 1.5% glycine to maintain a clear view. The current is delivered through a grooved ball or modified loop electrode giving a depth of penetration of 1–3 mm. 55,56 The procedure is similar to TURP in terms of requirement for spinal or general anaesthesia, operating time and aftercare. 55,57 More recently, further modification has allowed the use of bipolar current, which enables the use of physiological saline as a safer irrigant with tissue effects occurring at lower temperatures (ranging from 40°C to 70°C) than with monopolar electrosurgery (300–400°C). 58,59
Identification of patient subgroups and criteria for treatment
The requirement for general anaesthesia and standard operating room conditions and the degree of invasiveness mean that indications for vaporisation surgery in terms of symptom severity, symptom bother and degree of co-morbidity are similar to those for TURP. The simultaneous haemostatic coagulating effect of vaporisation techniques suggests additional usefulness for men on long-term anticoagulant or antiplatelet therapy who may have been previously advised against TURP. 60 The increased operating time compared with resection procedures, however, suggests that these techniques are most suited to small or medium-sized prostates up to approximately 60 ml. The lack of tissue samples means that prostate cancer should be excluded when necessary by preoperative investigation.
Personnel involved
Vaporisation of the prostate requires standard operating room preparation and facilities. Patients will be admitted to a hospital bed or ambulatory care facility and prepared for surgery by nursing and ancillary staff with preceding anaesthetic assessment. On transfer to the operating room, the anaesthetist and assistant will administer the appropriate anaesthetic. The urologist, supported by a scrub nurse and two circulating nurses, carries out the surgery. Following completion, the patient is transferred to a staffed recovery room and then back to the ward setting to complete the hospital stay, which is typically 2 days. If discharged with an indwelling catheter this will require planned removal by a hospital or community-based nurse.
Setting
In the UK the procedure will be carried out through an inpatient urology unit, typically with day of surgery admission and subsequent single overnight stay. Some units have set up US-style ambulatory care facilities to restrict the hospital stay to less than 24 hours if clinically and socially appropriate. It is difficult to give precise figures concerning the number of such procedures performed under the NHS because of imprecise coding but it is likely to be fewer than 5000, representing less than 17% of the total.
Equipment
For electrovaporisation, the only equipment that is required in addition to that used for TURP is the modified ball or loop electrode, which is currently designed for single patient use. For laser vaporisation, a source generator is required together with laser fibres, which are generally single patient use, and protective eyewear.
Costs
In comparison with TURP, electrovaporisation requires a more expensive modified electrode (Gyrus, UK), typically three times the cost of the standard loop and ball electrode (£40) used for TURP. The major cost for laser vaporisation is the capital purchase of the source generator, which ranges from £90,000 for the KTP laser (Laserscope, Cwmbran, UK) to £120,000 for the holmium laser (Sigmacon, Stanmore, UK), together with single-use fibre costs of £750 and £550 per patient respectively. The main cost saving (and associated gain in benefits) is reduced requirement for blood transfusion. With modern care pathways, hospital stay is likely to be 1 day less than for TURP.
Resection of the prostate
Introduction
These techniques seek to create a similar tissue ablative effect to TURP but with reduced bleeding and fluid absorption leading to lower perioperative morbidity. Modified irrigating cystoscopes or resectoscopes are used and the prostate is removed piecemeal as in TURP allowing subsequent histological examination. At present this can potentially be achieved either by holmium:YAG laser resection or by bipolar electroresection using normal saline.
Interventions using laser technology
Holmium laser prostatectomy used to be performed by resection of small pieces of prostate tissue down to the prostate capsule (HoLRP); however, this technique has largely been superseded by holmium laser enucleation of the prostate lobes (HoLEP). HoLEP uses the laser to dissect in the surgical planes and is conceptually the endoscopic equivalent of open prostatectomy. In this technique the holmium laser is used at a high power setting of 60–80 W with an end-firing fibre61. The procedure is performed using a continuous flow resectoscope with a video system and saline irrigation to maintain a clear view. The laser fibre is passed through a stabilising catheter with 5–10 cm of cladding stripped off at the distal end. Typically, the laser is set at an energy of 2 J and a frequency of 50 Hz, with minor variations depending on the preference of the surgeon. The procedure starts with bladder neck incisions at 5 and 7 o’clock to define surgical margins. The median and lateral lobes are then undermined and resected off the prostatic capsule in a retrograde direction until the bladder neck is reached. The resected lobes are pushed into the bladder, morcellated and removed. The procedure can be carried out under spinal or general anaesthesia, with slightly longer operating times than for TURP but with similar postoperative care. 51,62–64
Interventions using non-laser technology
The technique of bipolar electroresection requires a diathermy generator (200 W capability, a radio frequency range of 320–450 kHz and a voltage range of 254–350 V) and a cutting loop that is similar to a monopolar loop in shape but which has the active and return electrode on the same axis separated by a ceramic insulator. A chip in the loop automatically adjusts the power setting of the generator for the best cutting and coagulating parameters. 65 The underlying principle of this technique is the conversion of conductive solution into vapour (plasma) containing energy-charged particles that cause molecular dissociation of tissues. The electric arc (charged particles) takes the path of least resistance, the saline irrigant, thus controlling temperatures at the treatment site and reducing the risk of thermal damage to the surrounding tissue. 58,66 The procedure is performed using a continuous flow resectoscope with saline irrigation reducing the risks of fluid absorption and blood loss. 67
Transurethral vaporesection of the prostate (TUVRP) involves simultaneous resection and vaporisation with coagulation of prostatic tissue. The main differences between standard TURP and TUVRP are in the design of the loop and the level of electroenergy used. In TUVRP, a thick band-like loop is coupled with a high electrosurgery cutting energy. The perceived advantages of TUVRP are shorter duration of catheterisation and hospital stay, less blood loss, better visualisation during resection and reduced electrolyte disturbances. 68 The main disadvantage of TUVRP is longer duration of the procedure because of slower passage of the band electrode to allow for maximum coagulation and desiccation of the prostatic tissue, which remain central to this technique.
Identification of patient subgroups and criteria for treatment
The selection of patients, preoperative workup, informed consent, type of anaesthesia, postoperative care and clinical follow-up are similar to those of TURP. If appropriate, prostate cancer should be excluded by biopsy before proceeding with HoLEP. 69 Improved haemostasis with these techniques encourages their use for men with clotting abnormalities or those taking anticoagulant or antiplatelet drugs. There is some suggestion that this procedure is suitable for prostate enlargement of any size. 51,64 A long learning curve and 20–30% longer operative time than for standard TURP mean that increased surgeon expertise and operating room availability are required. 70,71
Personnel involved
Resection of the prostate requires standard operating room preparation and facilities. Protective eyewear is worn by surgeons, theatre personnel and patients to avoid eye damage from the laser. Before carrying out the procedures the laser machine is checked by trained theatre personnel according to the manufacturer’s instructions. Patients will be admitted to a hospital bed or ambulatory care facility and prepared for theatre by nursing and ancillary staff with preceding anaesthetic assessment. On transfer to the operating room, the anaesthetist and assistant will administer the appropriate anaesthetic. The urologist carries out the surgery supported by a scrub nurse and two circulating nurses. It is difficult to define how many procedures a surgeon must perform to become competent but it is generally agreed that about 30 cases are required for a urologist familiar with transurethral surgery to feel reasonably safe performing the HoLEP technique. Following completion, the patient is transferred to the staffed recovery room and then back to the ward setting to complete the hospital stay, which is typically 2–3 days. If discharged with an indwelling catheter this will require planned removal by a hospital or community-based nurse.
Setting
In the UK, laser resection and transurethral resection (in normal saline) procedures will be carried out through an inpatient urology unit, typically with day of surgery admission and subsequent single overnight stay. Some units have set up US-style ambulatory care facilities to restrict hospital stay to less than 24 hours if clinically and socially appropriate. It is unclear how many of these procedures are performed in the UK but it is likely to be fewer than 2500 per year, representing less than 9% of the total.
Equipment
For laser resection of the prostate using holmium:YAG lasers, in addition to a high-power machine (100 W VersaPulse; Lumenis, USA), a 550-μm end-firing fibre, 6Fr ureteric catheter, morcellator and eyewear are required. The resection is performed using a 27Fr continuous flow resectoscope with a modified inner sheath for the laser fibre channel. The irrigating solution is 0.9% saline. 61,72 For bipolar resection in saline, a source generator and bipolar resection system with special cutting loops are required (Gyrus, USA). 65,67
Costs
A HoLEP generator costs approximately £120,000, the tissue morcellator £20,000, laser fibre £550 and the morcellator blade £440. 51 However, a holmium:YAG laser can be efficiently used as a multifunctional endourological energy source in management of other conditions such as urinary stone disease, and the laser fibres and morcellator blades are designed for multipatient use. The main cost saving (and associated gain in benefits) is the reduced requirement for blood transfusion, possible shorter hospital stay and lower requirement for continuous postoperative irrigants.
Chapter 3 Description of care pathways
During the first half of the last century open prostatectomy was the only treatment option for BPE and because of significant mortality it was reserved for men with life-threatening problems such as urinary retention. The 1960s saw the advent of endoscopic transurethral techniques, particularly TURP, which allowed much safer surgery and widened treatment indications to include men with troublesome symptoms. Further improvements in perioperative care made TURP one of the most frequently performed operations towards the end of the twentieth century, particularly in the USA. Recent years have seen the increased use of drugs that can improve symptoms and possibly slow progression,19,73 which has led to a decreased rate of surgical intervention, this being reserved for those who fail drug treatment or suffer complications.
The treatment strategy of reassurance followed by drugs followed by surgery is now standard in clinical practice and has been explored in previous reviews of cost-effectiveness. 74 A parallel development has been the trial of differing energy delivery technologies to achieve varying degrees of surgical prostate tissue ablation, with the aim of high efficacy and low morbidity to challenge the standard of TURP. In this field there have been many false dawns, with technologies being introduced in a haphazard and uncontrolled manner and then being abandoned, as the hoped-for advantages over TURP have not been realised. In the last few years, however, the application of randomised controlled trial (RCT) methodology to surgical treatments has stimulated a more evidence-based approach, partly driven by tighter regulatory requirements.
One deficiency of the current evidence, however, is the assumption that surgical treatment of BPE involves a single treatment over a patient’s lifetime. This head-to-head comparative approach does not take into account the balance between short- or long-term effectiveness on one hand and morbidity and economic costs on the other, which differs between treatments, nor does it cater for the continued progression of the disease, which frequently results in the need for retreatment.
We therefore decided to formulate strategies consisting of sequences of escalating surgical intervention based on concepts underlying the ranking of particular treatments. A number of meetings were held between the clinical members of the research team to consider the likely place and use of each treatment modality in plausible strategies of management of BPE. These were then checked with colleagues within their respective urology units. Given funding constraints, formal consensus-building approaches such as the Delphi technique were not used. We first categorised treatments as being minimally invasive, typified by ambulatory care, reduced anaesthetic requirement and no tissue removal; tissue ablative, signifying the use of differing energy sources to remove prostate tissue; or standard, indicating TURP or TUIP. Again, using clinical consensus we defined plausible treatment sequences taking into account treatment mechanism and effect on the remaining prostate tissue. We similarly placed limits on the number of retreatments allowed based on current concepts of the use and effect of the differing procedures.
Figure 1 details plausible options of care informed by current clinical practice for a patient with BPE wanting surgery after a trial of drug therapy because the treatment has not resulted in symptomatic benefit or as a result of disease progression after initial benefit from drug treatment. The patient could be offered a minimally invasive intervention and if this results in symptomatic benefit no further treatment may be necessary. Should there be inadequate benefit or disease progression after initial benefit, the patient may be offered a choice of four other treatment options (drug therapy, repeat of minimally invasive intervention, a TURP or one of the other tissue ablative interventions such as KTP laser or TUVP). Should the patient have inadequate benefit or further disease progression after a second minimally invasive intervention, it was felt that the most plausible treatment option would be either a TURP or one of the other tissue ablative interventions. An alternative care pathway for a patient with BPE wanting surgery after a trial of drug therapy would be to have one of the other tissue ablative interventions first, such as KTP laser or TUVP. Should there be inadequate benefit or disease progression after initial benefit, the patient may be offered a choice of another tissue ablative intervention or a TURP. Should the patient have inadequate benefit or further disease progression, one further TURP was allowed in the pathway.
FIGURE 1.
Description of care pathways.
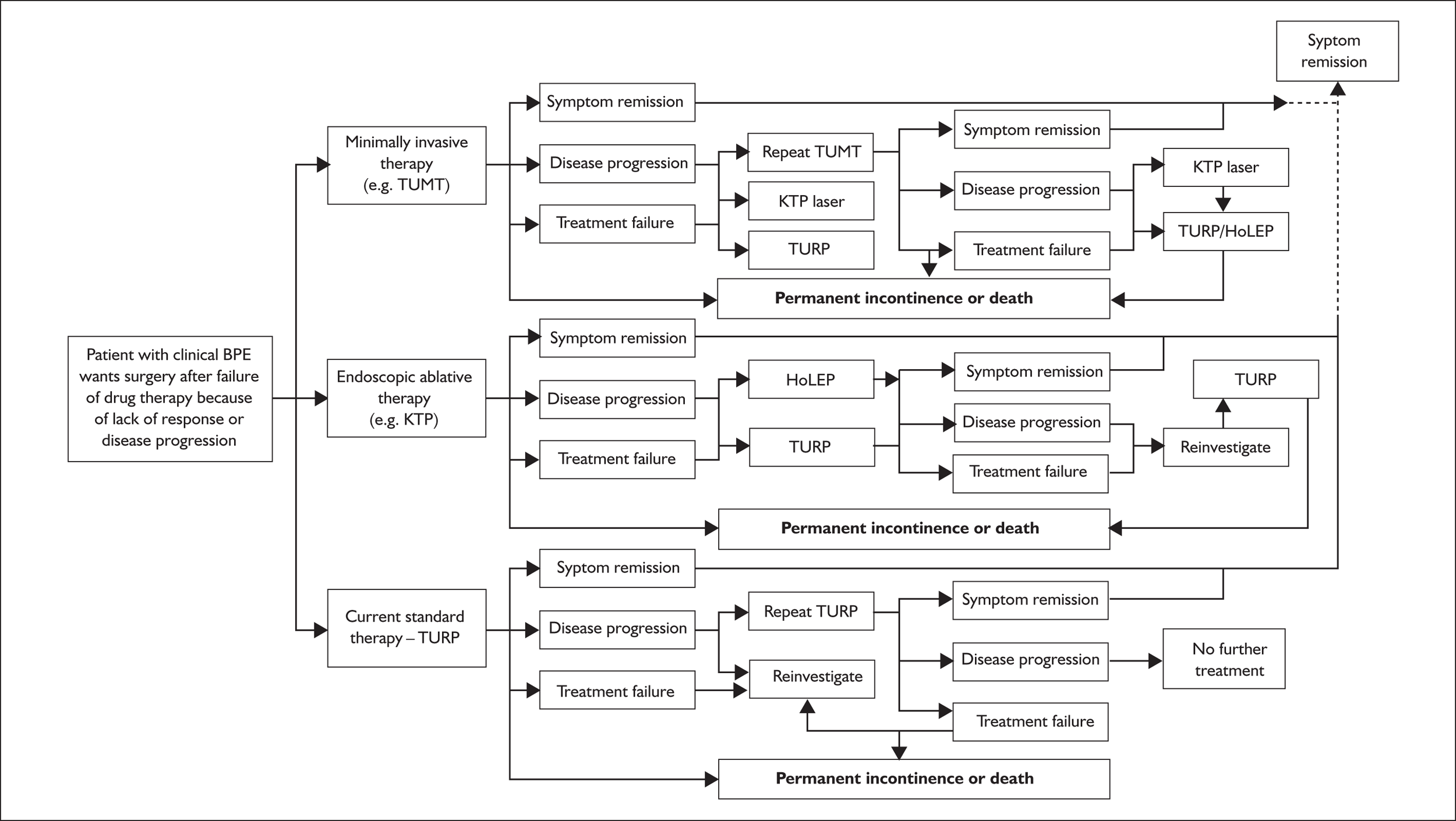
One exception to this rule occurs when HoLEP, one of the other tissue ablative interventions, is the choice of treatment, because it is felt to be equivalent to open prostatectomy and, as such, no further ablative procedures are allowed for in the care pathway. If, on the other hand, a patient with BPE wanting surgery after a trial of drug therapy chooses to have the gold standard, TURP, then the only option allowed for in the care pathway should there be inadequate benefit or disease progression is a repeat TURP. Based on current clinical practice, a repeat TURP would usually be carried out only after reinvestigation, usually in the form of urodynamic assessment.
Chapter 4 Systematic review of previous economic evaluations
A technology is defined as being ‘best’ if it is the one that maximises the benefits (achieves the goals) that are intended by the decision-maker(s) from a given budget. Economic evaluation involves the comparison of cost and benefit for any technology change and thus provides a means of informing decisions about which technology is best. 75
In this study the comparison between the different strategies depicted in the care pathways (see Figure 1) is made using a decision-analytic model (DAM). 75 The DAM is intended to show, first, the consequences in terms of costs and effects of each technology for the given population. These data are then used to inform the decision as to which technology or, when there is sufficient doubt, which technologies are the best, given current belief informed by evidence and judgement. Second, the DAM, in accounting for uncertainty, can be used to provide information about the likely value of conducting future research (evidence gathering) to reduce the uncertainty surrounding the decision about which technology or technologies are best. 75
Sensitivity analysis might be used to show the effect on the results of the model of plausible variation in model structure or parameter values. Deterministic sensitivity analysis seeks to identify what change in a parameter value is required to produce a decision change. However, to account for parameter uncertainty with many parameters, each of which could have many values, it can be very difficult to interpret such thresholds. A solution is to use probabilistic sensitivity analysis. 76 Probabilistic sensitivity analysis can also be used to estimate the value of information (VOI), which can be used to inform decisions about further research (details of this method are available elsewhere76,77).
How such an economic evaluation of alternative surgical treatments for BPE might be conducted can be informed by a review of the existing literature. The purpose of the review was, first, to show the extent and results of current literature and, second, via a critique, to learn lessons in order to conduct the most appropriate economic evaluation to aid decision-making.
The following is a list of the information requirements for all DAMs:
-
the population
-
the technologies to compare
-
the epidemiology: model structure (relationship between parameters)
-
the epidemiology: parameterisation of the model (effectiveness, complications, utilities and costs)
-
sensitivity analysis.
This list of requirements will form the framework used in this chapter to critique existing models and then in Chapter 11 the model used in this evaluation.
Because of deficiencies in any of the DAM information requirements, the results of existing economic evaluations were extremely unlikely to be sufficient to inform a decision now. Therefore, the only studies that were critiqued were those that considered at least some of the surgical treatments for men with moderate to severe symptoms of BPE and no complications, and which estimated outcomes using a DAM.
Search strategy
The following databases were searched for information on economic evaluations and quality of life: MEDLINE (1966–March Week 2 2006), EMBASE (1980–2006 Week 11), MEDLINE In–Process (20 March 2006), ISI Science Citation Index (1981–1 March 2006), Health Management Information Consortium Database (March 2006), NHS Economic Evaluation Database (March 2006) and HTA database (March 2006). In addition, recent conference proceedings of the European Association of Urology, American Urological Association and British Association of Urological Surgeons were searched. Reference lists of all included studies were scanned to identify additional potentially relevant studies. Full details of the search strategies used are documented in Appendix 1.
The results of the literature searches, after deduplication against the Ovid multifile search, are presented in Table 2.
| Database | Hits screened | Selected for full assessment |
|---|---|---|
| MEDLINE/EMBASE/MEDLINE Extra multifile search (after deduplication in Ovid) | 1213 | 65 |
| ISI Science Citation Index | 88 | 3 |
| NHS Economic Evaluation Database | 45 | 0 |
| HTA database | 21 | 12 |
| Health Management Information Consortium Database | 31 | 2 |
| Selected from conference abstracts | 6 | 0 |
| Total | 1404 | 82 |
Studies selected for critique
Three studies published in six papers that contained data relevant to formulation of the DAM were identified. One study by Ackerman and colleagues was published in three papers,78–80 and another by DiSantostefano and colleagues was published in two papers. 74,81 The third study by Howard and Wortley was published as a technology assessment report for the Australian Medical Services Advisory Committee (MSAC). 82
Population
All three studies considered essentially similar populations, although DiSantostefano and colleagues and Ackerman and colleagues, in considering drug treatment and watchful waiting, actually considered a broader population. Ackerman and colleagues considered a cohort aged 65 years, Howard and Wortley did not state age, and DiSantostefano and colleagues considered the effect of varying age from 45 to 85 years.
Technologies
DiSantostefano and colleagues and Ackerman and colleagues compared TUMT and TURP in addition to drugs whereas Howard and Wortley compared TUMT with TURP. None compared strategies, i.e. what is the best sequence of treatments if, on failure or relapse (judged in some way), another procedure is planned. Instead they all assumed that should the initial treatment fail then there would be some chance of further treatment, which for all three studies was TURP. However, if the choice of initial treatment is at all dependent on the outcome of any future treatments then there is a need to consider the outcome of these future treatments in the economic evaluation. Of course, there might also be reason to consider repeating a procedure such as TUMT instead of using TURP immediately on failure or switching to a different procedure such as TUVP.
The epidemiology: model structure
To find the best technology, costs and consequences (including utility) must be estimated for each technology. Individual variability for a given population and technology implies that the various health-related events (e.g. degree of symptom improvement, death) that can occur over time must be expressed as probabilities. Therefore, the model estimates the expected (‘average’) cost and utility for the population. However, the complexity of patient pathways prevents specification of a probability distribution for every pathway. One solution is a Markov model,83 in which events are reduced to a set of discrete health states of fixed duration (cycle length). An individual may only be in one health state at a time and at the end of each cycle they face the probability of making the transition to another health state. The individual will continue moving between health states until the prespecified number of cycles has been reached or until the individual moves into an absorbing health state (normally death) from where further transitions are not possible. This enables the calculation for each strategy of the expected value of cost and utility. These expected values are the sum of the value of the cost and utility for each state multiplied by the number of cycles spent in that state.
All three studies used a Markov model. The time horizon was 5 years for Ackerman and colleagues and 20 years for the other two studies. Cycle length was 3 months for Ackerman and colleagues, 6 months for Howard and Wortley and 1 year for DiSantostefano and colleagues, thus giving 20, 40 and 20 cycles respectively. The number of health states considered were 25, four and nine respectively.
The epidemiology: parameterisation of the model
No study claimed to have conducted a systematic review of the literature, although Ackerman and colleagues used the term ‘comprehensive review’.
Effectiveness
One advantage of the simple ‘chance’ approach to second treatments is that the probability of failure can be simply assumed to be the probability of reoperation. However, the decision-making criteria underlying reported reoperation probabilities are usually unknown and different criteria might mean different outcomes. DiSantostefano and colleagues derived estimates of treatment failure (‘no improvement’) from the 1994 Agency for Health Care Policy and Research (AHCPR) guideline19,84 and of reoperation for TURP from the AUA guideline19,85 for the period up to 2000. Reoperation rates for TUMT were derived from two RCTs. 86,87 However, the AHCPR guideline is over 10 years old and its authors admit that very few studies reported symptom scores and that those that did used many different methods. 84 Although this limitation is allowed for to some extent in the wide confidence interval (CI) for this estimate (see Accounting for uncertainty in Chapter 10, p.112), the relationship between degree of symptom improvement and probability of retreatment is unclear. For example, do those who are counted as successful and who thus receive no further treatment continue with, ‘on average’, almost complete symptom relief or was the change only just sufficient to warrant no further treatment? For those who fail but receive no further treatment, it was not clear to what extent this was because the clinician believed that further treatment would not work or because further treatment was refused by the patient. It was also not clear why those who receive TURP have an annual probability of relapse (‘disease progression’) of about 1%, but those who receive TUMT cannot relapse.
Howard and Wortley used a single RCT82 for TURP and several sources for TUNA to estimate ‘early treatment failure’ (within 6 months). Longer-term failure rates (equivalent to relapse) were stated to come from an RCT and a cohort study for TURP with a 10-year follow-up. For TUNA, data were derived from the percentage undergoing retreatment after 5 years.
Ackerman and colleagues used the same definition of treatment success for all treatments: ‘significant improvement, achieving a 50% or greater decrease in the AUA symptom score; moderate improvement, achieving a 30–49% decrease in the AUA symptom score; minimal improvement, a less than 30% decrease in the AUA symptom score’. They cited various publications, as well as the ‘multispeciality clinical panel’, as sources for their probability of each degree of success, although it is not clear how these sources were synthesised. These probabilities were stated to be time dependent, although not all estimates were shown: the 5-year probabilities of ‘success’ for TURP and TUMT were 0.85 and 0.65 respectively.
Ackerman and colleagues78 and DiSantostefano and colleagues74,81 also had health states with different degrees of symptoms. However, this refinement would be important only if the choice of states that have differential effects on outcome is contingent on the symptom level. For example, if on day one 90% have some success such that they receive no further treatment for the next 10 years, it makes no difference whether half of them spend that time in a state of ‘mild’ symptoms and half in a state of ‘no’ symptoms or whether all of them spend that time in a single state, as long as the outcome of that state is equal to the average of the outcome of ‘mild’ and ‘no’ symptoms, each weighted by 50%.
Complications
All models consider the possibility of complications, the most comprehensive being that of Ackerman and colleagues. 78 However, depending on the source of estimates, it is possible that there could be some unnecessary and perhaps misleading inclusions. For example, DiSantostefano and colleagues argue against the inclusion of differential mortality rates because either there is no difference between treatments or the difference is so small that to try to estimate would lead to bias. 74,81 This is backed up by long-term studies;85 the same argument can be made for life-threatening complications such as myocardial infarction (MI).
Retrograde ejaculation occurs as a result of removal of prostate tissue by whatever means and does not significantly lower the utility value of successful treatment and is not associated with any costs. Erectile dysfunction (ED) following prostate surgery is a difficult and controversial issue: the meta-analysis presented later and previous systematic reviews have shown no statistically significant difference in occurrence between types of surgery. For the purposes of the cost-effectiveness analysis modelled over a 10-year period, we chose not to include ED as a complication as it was more likely to be caused by other concurrent, randomly distributed disease processes than the interventions under consideration. In addition, there is increasing evidence of an association between ED and urinary symptoms that would also confound estimated rates.
Utilities
All three studies used cost-utility analysis (CUA) and each had a utility of 1 for some states reflecting either ‘significant improvement’ or ‘remission’ and of 0 for death. Only Ackerman and colleagues78 elicited preferences using the standard gamble approach75 to estimate utilities for each of their other health states; however, their sample was small (only n = 6 or n = 7 for each of the ‘risk averse’ and ‘non-risk averse’ groups). Such data may be unreliable as they are based on so few observations. They may also not be comparable with utilities calculated for other patient populations – a larger sample from the general public would have been better. DiSantostefano and colleagues74,81 used utilities from a variety of sources, including Ackerman and colleagues78 for incontinence. Howard and Wortley simply used opinion (they do not state the source) and values for treatment success (as full health, i.e. 1) for failure (0.9) or side effects (0.95). 82
Costs
All three studies estimated costs in at least the categories of ‘procedure’, ‘complications’ and ‘failure’ (implying the inclusion of reoperation costs). However, Howard and Wortley and Ackerman and colleagues simply used estimates for each category and provided no further breakdown. DiSantostefano and colleagues provided a slightly fuller breakdown by resource use for each procedure such as number of physician visits. However, none of the studies differentiated between procedure and hospital stay and none expressed cost of equipment as a function of its lifetime or reusability.
Sensitivity analysis
All three studies performed some deterministic sensitivity analyses. Only DiSantostefano and colleagues used probabilistic analysis for parameter uncertainty. 74,81 Their distributions for probability of treatment failure, reoperation and complications were estimated appropriately using beta distributions. They stated that they were parameterised using the 95% confidence intervals from various sources, for example the AUA meta-analysis,85 and presumably used the means from these sources. The distributions for their cost estimates were assumed to be normal and parameterised from US national databases for TURP and TUMT: they stated that the standard deviation was used, but the appropriate statistic is the standard error. Given the likely large sample size of these databases, the standard deviation would probably considerably overestimate the uncertainty, although this is a matter of judgement.
Conclusion
Previous studies have attempted to address the challenges of constructing a DAM for BPE surgical treatments. All of these studies had some limitations, which have been discussed. Taking these limitations into account it is suggested that a future DAM should:
-
include more single treatments and treatment strategies
-
develop methods to estimate the probability of failure using clinical criteria relevant to the UK, comparing the effect of this with simply using reoperation rates
-
develop methods to estimate utilities that more explicitly use the main outcome of effectiveness evidence, the IPSS
-
include relevant complications and mortality rates for the UK
-
provide a breakdown of costs that is sufficient to estimate the independent effects of procedure cost, hospital inpatient stay and purchase of any new equipment
-
conduct sensitivity analysis deterministically when appropriate and with probability distributions for all relevant parameters, obtained by explicit methods in accordance with theory and best practice.
When developing the economic model published in Chapter 10, consideration was given to how these limitations could best be avoided or minimised.
Chapter 5 Methods of, and studies included in, the systematic reviews of clinical effectiveness
Methods for reviewing effectiveness
Search strategy
Electronic searches were undertaken to identify published and unpublished reports of RCTs evaluating the effectiveness of established and new interventional treatments for the management of symptoms and complications subsequent to BPE. Searches were not restricted by publication year or language and included conference proceedings.
The databases searched were MEDLINE (1966–September Week 3 2006), EMBASE (1980–2006 Week 38), MEDLINE In–Process (27 September 2006), BIOSIS (1985–22 September 2006), ISI Science Citation Index (1981–23 September 2006), ISI Proceedings (1990–18 March 2006), Cochrane Controlled Trials Register (CENTRAL) (The Cochrane Library, Issue 1, 2006), Cochrane Database of Systematic Reviews (The Cochrane Library, Issue 1, 2006), Database of Abstracts of Reviews of Effectiveness (March 2006), HTA database (March 2006), National Research Register (Issue 1, 2006), Clinical Trials (March 2006) and Current Controlled Trials (March 2006). In addition, recent conference proceedings of the European Association of Urology, the American Urological Association and the British Association of Urological Surgeons were searched. Reference lists of all included studies were scanned to identify additional potentially relevant studies. Full details of the search strategies used are documented in Appendix 1.
All titles and abstracts identified in these ways were assessed to identify potentially eligible studies. Two reviewers independently assessed them for inclusion, using a study eligibility form developed for this purpose (see Appendix 2). Any disagreements were resolved by consensus or arbitration.
Inclusion and exclusion criteria
Types of studies
Individual RCTs were eligible for inclusion irrespective of publication language if they assessed interventional treatment options for the treatment of BPE. Initially, it was intended to include population-based observational studies with a minimum follow-up of 3 years but this was subsequently deemed not to be necessary as long-term follow-up data from RCTs was sufficient to provide more robust estimates of rare complications and effectiveness. Abstracts were considered only when no full-text RCTs were available for a particular intervention.
Types of participants
Trials of men with a clinical diagnosis of BPE who have undergone surgery were included. Patients undergoing conservative management (watchful waiting or medical therapy) were excluded.
Types of interventions
Methods of surgical intervention for BPE included:
-
minimally invasive techniques
-
transurethral microwave thermotherapy (TUMT)
-
transurethral needle ablation (TUNA) of the prostate
-
stents
-
high-intensity focused ultrasound (HIFU)
-
transurethral ethanol ablation of the prostate (TEAP)
-
water thermotherapy (WIT)
-
transurethral laser coagulation of the prostate
-
-
transurethral incision of the prostate (TUIP)
-
transurethral resection of the prostate (TURP)
-
reference standard
-
-
other tissue ablative techniques
-
transurethral laser prostatectomy – resection
-
transurethral laser prostatectomy – vaporisation
-
bipolar TURP
-
transurethral electrovaporisation of the prostate (TUVP)
-
bipolar TUVP
-
transurethral vaporesection of the prostate (TUVRP)
-
bipolar TUVRP.
-
Types of outcomes
Data were sought to describe both short-term and long-term outcomes. The following measures of outcomes were sought for different follow-up periods (3, 6 and 12 months or longer):
-
Primary outcome
-
symptom score.
-
-
Other outcomes
-
urodynamic
-
peak urine flow rate
-
mean urine flow rate
-
total voided volume
-
residual volume
-
detrusor pressure
-
-
complications
-
intraoperative complications
-
co-interventions
-
clot retention
-
cardiovascular events
-
transurethral resection (TUR) syndrome
-
blood transfusion
-
septicaemia
-
urinary retention
-
recatheterisation
-
urinary tract infection (including epididymitis)
-
irritative urinary symptoms
-
incontinence
-
retrograde ejaculation
-
erectile dysfunction
-
stricture
-
reoperation rate
-
mortality
-
-
other
-
prostate size
-
quality of life score.
-
-
Data extraction strategy
The titles and abstracts of all papers identified by the search strategy were screened. Full-text copies of all potentially relevant studies were obtained and two reviewers independently assessed them for inclusion. Reviewers were not blinded to the study authors, institutions or sources of the reports. Any disagreements were resolved by consensus or arbitration.
A data extraction form was developed to record details of trial methods, interventions, participants’ characteristics and outcomes (see Appendix 3). Two reviewers independently extracted data from the included studies. Any differences that could not be resolved through discussion were referred to an arbiter.
Quality assessment strategy
Two reviewers working independently assessed the methodological quality of the included full-text studies. Again, any disagreements were resolved by consensus or arbitration. Primary RCTs were assessed using an assessment tool, drawing on the schema suggested by the NHS Centre for Reviews and Dissemination,88 Verhagen and colleagues,89 Downs and Black90 and the Generic Appraisal Tool for Epidemiology (see Appendix 4).
Data synthesis
For trials with multiple publications, only the most up-to-date data for each outcome were included. Dichotomous outcome data were combined using the Mantel–Haenszel relative risk (RR) method and continuous outcomes were combined using the inverse variance weighted mean difference (WMD) method. The results are all reported using a fixed-effects model. Chi-squared tests and I-squared statistics were used to explore statistical heterogeneity across studies and, when present, random-effects methods were applied. Other possible reasons for heterogeneity were explored using sensitivity analyses. The meta-analyses were conducted using the standard Cochrane software RevMan 4.2. Because of the lack of uniformity of the data presented by many studies, a qualitative review looking for consistency between studies was also performed.
Symptoms assessed with the IPSS and the AUA symptom index were considered equivalent and therefore trials reporting symptoms in these ways were combined. Studies reporting symptoms as Madsen–Iversen symptom indexes were analysed separately. The IPSS/AUA scale ranges from 0 to 35. Scores ranging from 0 to 7 are equivalent to mild symptoms, from 8 to 19 are equivalent to moderate symptoms, and from 20 to 35 are equivalent to severe symptoms.
A large prostate was defined as having an estimated weight of more than 40 g, a moderate-sized prostate a weight of between 30 and 40 g and a small prostate a weight of less than 30 g (Professor James N’Dow, University of Aberdeen, 2006).
As some complications could not be confidently separated into those reported in the immediate postoperative period and those experienced over the course of the trial, all reports of the same complication were pooled together regardless of the timing of occurrence. Also, for the purposes of this review, ‘strictures’ included bladder neck stenosis and urethral stricture as it was difficult to distinguish between them given the information provided in the trials and because definitions of these complications were inconsistent from report to report. Only blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in the results section as these were felt to be the most important for the economic model. Other outcomes are presented in the appendices.
In terms of urodynamic outcomes, only the results for peak urine flow rate are presented in the body of this report because clinical experts consider this to be a more precise measure of a urodynamic outcome. Other urodynamic outcomes were also analysed and are presented in the appendices.
Quantity and quality of research available
Number of studies identified
The search strategies identified 3794 study reports after removing duplicates (Figure 2). Of these, 621 (466 full text, 155 abstracts) were selected for further assessment (Table 3).
FIGURE 2.
Study selection process.

| Database searched | Number selected |
|---|---|
| MEDLINE/EMBASE/MEDLINE In-Process multifile search (after deduplication in Ovid) | 370 |
| ISI Science Citation Index | 52 |
| BIOSIS | 118 |
| CENTRAL | 8 |
| Cochrane Database of Systematic Reviews | 0 |
| Database of Abstracts of Reviews of Effectiveness | 4 |
| HTA database | 7 |
| National Research Register | 10 |
| Current Controlled Trials | 7 |
| Clinical trials | 0 |
| Conference abstracts | 45 |
| Total selected | 621 |
Number and types of studies included
In total, 158 reports met the inclusion criteria for the review and these described 88 RCTs (Figure 2). Apart from one,91 which was an abstract, the primary reports of the studies were full-text papers. The included studies and associated references are listed in Appendix 5.
Number and types of studies excluded, with reasons for specific exclusions
In total, 178 reports were obtained but subsequently excluded because they failed to meet one or more of the inclusion criteria (see Figure 2). Of these, 145 were not RCTs. Of the 33 remaining reports, ten included comparisons involving other surgical management,90,92–100 four included comparisons involving medical management for BPE,101–104 two compared TURP with watchful waiting,6,105 and one compared different dosages of ethanol within an RCT of transurethral ethanol ablation of the prostate. 106 An additional 16 reports had no usable data. 107–122
Study quality
A summary of the quality assessment of the 88 full-text RCTs is presented in Table 4 and the detailed quality assessment score for each of the included studies is reported in Appendix 6. The method of randomisation was unclear in the majority of the studies (75%); however, in one (1%),123 an inadequate approach to sequence generation (alternation) was used. Suboptimal approaches to concealment of treatment allocation (serially numbered sealed envelopes) were used in 12 studies (14%). 57,124–134 It was unclear whether the groups were similar at baseline in seven studies (8%) with respect to the most important prognostic factors. 135–141 The eligibility criteria were clearly specified in all but one study. 142 In the majority of the studies (62%) the groups were treated in the same way apart from the intervention received, but this was unclear in 13 studies (15%). 136,138,139,143–151,167 In most studies (95%) follow-up was long enough to detect important effects on short-term outcomes (at least 3 months); however, only 69% of the studies followed up their participants for at least 1 year.
| Criteria | Yes | No | Unclear |
|---|---|---|---|
| 1. Was the assignment to the treatment groups really random? | 21 (24%) | 1 (1%) | 65 (75%) |
| 2. Was the treatment allocation concealed? | 10 (11%) | 12 (14%) | 65 (75%) |
| 3. Were the groups similar at baseline in terms of prognostic factors? | 65 (75%) | 15 (17%) | 7 (8%) |
| 4. Were the eligibility criteria specified? | 84 (97%) | 1 (1%) | 2 (2%) |
| 5. Was the intervention (and comparison) clearly defined? | 81 (93%) | 2 (2%) | 4 (5%) |
| 6. Were the groups treated in the same way apart from the intervention received? | 54 (62%) | 20 (23%) | 13 (15%) |
| 7. Was follow-up long enough to detect important effects on outcomes of interest? | |||
| (a) For short-term outcomes, at least 3 months | 83 (95%) | 3 (3%) | 1 (1%) |
| (b) For long-term outcomes, at least 1 year | 60 (69%) | 25 (29%) | 2 (2%) |
| 8. Were the outcome assessors blinded to the treatment allocation? | 13 (15%) | 5 (6%) | 69 (79%) |
| 9. Were the care providers blinded? | 3 (3%) | 7 (8%) | 77 (88%) |
| 10. Were the patients blinded? | 15 (17%) | 8 (9%) | 64 (74%) |
| 11. Were the point estimates and measures of variability presented for the primary outcome measures? | 77 (88%) | 7 (8%) | 3 (3%) |
| 12. Was the withdrawal/dropout rate likely to cause bias? | 1 (1%) | 12 (14%) | 74 (85%) |
| 13. Did the analyses include an intention to treat analysis? | 16 (18%) | 4 (5%) | 67 (77%) |
| 14. Was the operation undertaken by someone experienced in performing the procedure? | 11 (13%) | 6 (7%) | 70 (80%) |
In the majority of the studies it was unclear whether outcome assessors, care providers and patients were blinded. Point estimates and measures of variability were presented in 88% of the studies, although in three studies it was unclear whether means or medians were used as the point estimate measure. 134,152,153 The dropout rate was unlikely to cause bias in 12 studies124,138,150,154–162 but this information was unclear in 74 (85%) of the studies. Only 16 studies (18%) stated that an intention to treat analysis was performed; however, this seems questionable in 11 of these studies57,70,125,130,136,139,145,154,163–165 as they failed to include the total number of participants in each arm in the subsequent follow-up assessments and an additional study stated that patients failing to complete the treatment or failing to return for follow-up were substituted. 124 It was unclear whether 67 other studies (77%) included an intention to treat analysis. It was also unclear in some studies how many patients were assessed at each follow-up. In 11 studies (13%) it was stated that the interventions were undertaken by someone experienced in performing the procedure; however, another 70 studies (80%) failed to provide this information for both types of intervention being delivered to the participants.
Characteristics of included studies
Appendix 7 provides details of the characteristics of the included studies. There were 94 relevant comparisons in the 88 eligible RCTs (8494 randomised participants); one trial had four arms and four trials had three arms (Table 5). In the following chapters an overview of the characteristics of the included studies for each identified comparison is presented.
| Comparison | Number of trials | Participants | References |
|---|---|---|---|
| TUMT vs TURP | 6 | 549 | Ahmed et al., 1997;124 Wagrell et al., 2002;165 d’Ancona et al., 1998;166 Dahlstrand et al., 1993;167 Dahlstrand et al., 1995;168 de la Rosette et al., 2003169 |
| TUMT vs sham | 11 | 1159 | Bdesha et al., 1994;125 Blute et al., 1996;126 Ogden et al., 1993;133 Abbou et al., 1995;143 Larson et al., 1998;159 Nawrocki et al., 1997;160 Albala et al., 2002;170 Brehmer et al., 1999;171 de Wildt et al., 1996;172 Trachtenberg and Roehrborn, 1998;173 Zerbib et al., 1994174 |
| TUNA vs TURP | 4 | 450 | Hill et al., 2004;144 Kim et al., 2006;151 Cimentepe et al., 2003;175 Hindley et al., 2001176 |
| Stents vs TURP | 1 | 60 | Chapple et al., 199591 |
| TEAP vs TURP | 1 | 204 | Kim et al., 2006151 |
| Laser coagulation vs TURP | 13 | 1231 | Costello et al., 1995;123 Kursh et al., 2003;130 Liedberg et al., 2003;131 Donovan et al., 2000;136 Gujral et al., 2000;139 McAllister et al., 2000;145 Rodrigo Aliaga et al., 1998;149 Kim et al., 2006;151 Chacko et al., 2001;154 Cowles et al., 1995;163 Kabalin et al., 1995;177 Mårtenson et al., 1999;178 Suvakovic and Hindmarsh, 1996179 |
| TUIP vs TURP | 11 | 871 | Christensen et al., 1990;135 Rodrigo Aliaga et al., 1998;149 Riehmann et al., 1995;152 Hellström et al., 1986;157 Dørflinger et al., 1992;180 Jahnson et al., 1998;181 Li and Ng, 1987;182 Nielson, 1988;183 Saporta et al., 1996;184 Soonawalla and Pardanani, 1992;185 Tkocz and Prajsner, 2002186 |
| Laser resection vs TURP | 5 | 530 | Kuntz et al., 2004;64 Wilson et al., 2006;134 Gupta et al., 2006;187 Montorsi et al., 2004;188 Westenberg et al., 2004189 |
| Laser vaporisation vs TURP | 11 | 955 | Carter et al., 1999;127 Bouchier-Hayes et al., 2006;141 Shingleton et al., 2002;146 Zorn et al., 1999;148 Tuhkanen et al., 2003;153 Keoghane et al., 2000;164 Suvakovic and Hindmarsh, 1996;179 Mottet et al., 1999;190 Tuhkanen et al., 2001;191 Sengor et al., 1996;192 van Melick et al., 2003193 |
| Bipolar TURP vs TURP | 6 | 336 | de Sio et al., 2006;65 Singh et al., 2005;147 Kim et al., 2006;150 Seckiner et al., 2006;161 Nuhoğlu et al., 2006;194 Tefekli et al., 2005195 |
| TUVP vs TURP | 17 | 1449 | Kaplan et al., 1998;55 Fowler et al., 2005;57 Erdaği et al., 1999;128 Hammadeh et al., 2003;129 Gallucci et al., 1998;138 Patel et al., 1997;140 Ekengren et al., 2000;142 Gotoh et al., 1999;156 Kupeli et al., 1998;158 Nathan and Wickham, 1996;162 van Melick et al., 2003;193 Çetinkaya et al., 1996;196 Kupeli et al., 1998;197 Netto et al., 1999;198 Nuhoğlu et al., 2005;199 Shokeir et al., 1997;200 Wang et al., 2002201 |
| Bipolar TUVP vs TURP | 2 | 211 | Hon et al., 2006;70 Dunsmuir et al., 2003137 |
| TUVRP vs TURP | 5 | 429 | Talic et al., 2000;68 Liu et al., 2006;132 Gupta et al., 2006;187 Helke et al., 2001;202 Kupeli et al., 2001203 |
| Bipolar TUVRP vs TURP | 1 | 60 | Fung et al., 2005155 |
Assessment of effectiveness
The assessment of effectiveness is reported in the following chapters, beginning with the minimally invasive techniques. No studies involved a comparison with HIFU and water thermotherapy. Three direct comparisons reported in three RCTs204–206 between a minimally invasive intervention and other ablative interventions were identified. These are presented in Appendix 10.
Chapter 6 Clinical effectiveness of minimally invasive techniques
Transurethral microwave thermotherapy (TUMT) versus TURP
Characteristics of included studies
The characteristics of the included studies are summarised in Table 6. Six RCTs, reported in 19 papers,86,124,165–169,207–218 were eligible for this comparison, in which a total of 549 participants were randomised. The number of participants randomised to TUMT or TURP ranged from 2168 to 99. 165 The total number of participants allocated to TUMT was 314 and the total allocated to TURP was 235.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Ahmed et al., 1997124 | TUMT | 30 | 69 | 18.5 | 10.1 | 94 | 37 |
| TURP | 30 | 69 | 18.4 | 9.5 | 109 | 46 | |
| Dahlstrand et al., 1993167 | TUMT | 39 | 68 | 11.2b | 8.0 | 105 | 33 |
| TURP | 40 | 70 | 13.3b | 7.9 | 116 | 37 | |
| Dahlstrand et al., 1995168 | TUMT | 37 | 67 | 12.1b | 8.6 | 194 | 43c |
| TURP | 32 | 70 | 13.6b | 8.6 | 1104 | 45c | |
| d’Ancona et al., 1998166 | TUMT | 31 | 69 | 18.3 | 9.3 | 49 | 43 |
| TURP | 21 | 69 | 16.7 | 9.3 | 91 | 45 | |
| de la Rosette et al., 2003169 | TUMT | 78 | 67 | 20.0 | 9.2 | 65 | 51 |
| TURP | 66 | 66 | 20.0 | 8.0 | 91 | 52 | |
| Wagrell et al., 2002165 | TUMT | 99 | 67 | 21.0 | 7.6 | 106 | 49 |
| TURP | 46 | 69 | 20.4 | 7.8 | 94 | 53 |
Two studies each took place in the Netherlands166,169 and Sweden167,168 and one in the UK,124 and one was a multicentre study involving Sweden, Denmark and the US. 165 Only three studies gave details of the recruitment dates;165,166,169 recruitment dates ranged from January 1994 to November 1999.
Four out of the six RCTs reported baseline IPSS/AUA scores. The total number of participants who had moderate symptoms of BPE and underwent TUMT was 61 (26%), compared with 51 (31%) with moderate symptoms allocated to TURP. There were 177 (74%) patients with severe symptoms in the TUMT group and 112 (69%) with severe symptoms in the TURP group.
Of the studies reporting estimated prostate size, 69 (22%) and 245 (78%) patients allocated to TUMT had moderate-sized and large prostates respectively. Of the patients allocated to TURP, 40 (17%) had moderate-sized and 195 (83%) had large prostates.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8. The results of the meta-analyses are given in Appendix 9. Note that in terms of long-term evaluation, only the longest follow-up is presented.
Symptom scores
At 3 months
Of the six eligible studies, three165,166,213 (n = 290) provided information on IPSS/AUA scores (Appendix 8.1, Table 42). At 3 months IPSS was higher for TUMT than for TURP (Figure 3, comparison 01:01:01). Overall, the WMD was 4.08 (95% CI 2.78–5.39, p < 0.001). There was evidence of statistical heterogeneity, but the direction of effect was consistent even though the size of effect estimates varied. Using a random-effects model did not change this pattern. The cause of heterogeneity is unclear but in the study by d’Ancona and colleagues166 patients appear to have milder disease than in the other two studies.
FIGURE 3.
Symptom scores, TUMT vs TURP.
In total, four studies166,168,210,213 (n = 306) provided information on the improvement of Madsen–Iversen scores after surgery (Appendix 8.1, Table 42). Meta-analysis of the four trials showed heterogeneity, with results tending to favour TURP, but the difference was not statistically significant (Figure 3, comparison 01:02:01: WMD 0.63, 95% CI –0.08 to 1.33, p = 0.08). The direction and size of effect varied across studies, with Dahlstrand and colleagues168 reporting lower scores for TUMT. This study appears to be contributing much of the statistical heterogeneity that is present and this could be because patients allocated to the TURP group had higher residual volumes. Removal of this study from the analysis resulted in a substantial decrease in heterogeneity.
At 12 months
Meta-analysis of data from three trials165,166,169 reporting IPSS/AUA scores at 12 months after surgery showed a statistically significant worse score for TUMT compared with TURP (Figure 3, comparison 01:01:03: WMD 2.41, 95% CI 1.40–3.42, p < 0.001). Again, there was marked statistical heterogeneity between the three studies. When a random-effects model was applied the direction of effect remained the same but the difference between the groups was no longer statistically significant (WMD 2.26, 95% CI –0.38 to 4.91).
At 12 months, all four trials reporting Madsen–Iversen symptom scores166–168,213 reported higher (worse) scores following TUMT (Figure 3, comparison 01:02:03). Overall, the WMD was 1.97 (95% CI 1.27–2.66, p < 0.001).
Longer-term follow-up
Two studies reported data beyond 12 months. 166,169 These data also favoured TURP, but again with variation between trials in the estimated size of difference (Figure 3, comparison 01:01:05: WMD 8.90, 95% CI 6.65–11.15, p < 0.001). A similar trend was observed for the earlier follow-ups.
Complications
Data describing complications are tabulated in Appendix 8.1, Table 43. Information on 13 types of complications was identified across the six eligible studies for this comparison. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 4). Results for other complications are presented in Appendix 9.1, comparison 01:03. The results of these meta-analyses should be treated with caution as the length of follow-up of the RCTs varied.
FIGURE 4.
Complications, TUMT vs TURP.

Blood transfusion
Blood transfusion was reported in three studies. 124,166,168 None of the patients required a blood transfusion following TUMT compared with four (5%) patients following TURP (Figure 4, comparison 01:03:01: RR 0.11, 95% CI 0.01–1.98, p = 0.13).
Urinary retention
In both trials with data,165,169 urinary retention was reported more commonly in the patients undergoing TUMT than in those undergoing TURP (Figure 4, comparison 01:03:02: RR 1.64, 95% CI 0.77–3.50, p = 0.20).
Urinary tract infection
Meta-analysis of data from five studies124,165–168 showed no statistically significant differences between the two arms; the direction of effect varied across studies with two124,165 favouring TUMT (Figure 4, comparison 01:03:03: 16/237 versus 13/174, RR 1.05, 95% CI 0.53–2.08, p = 0.90).
Stricture
Only one stricture (urethral) was reported amongst 172 participants allocated to TUMT versus 11 (including five bladder neck stenoses) amongst 168 participants allocated to TURP (Figure 4, comparison 01:03:04: RR 0.20, 95% CI 0.05–0.75, p = 0.02). The direction and size of effect were consistent across the four studies reporting this outcome. The event rates in this meta-analysis should be treated with caution as the length of follow-up of the RCTs varied.
TUR syndrome
Out of the six included studies, only one reported data on this outcome. 165 One event was observed in the TURP arm amongst 100 patients as opposed to none in the TUMT arm. This difference does not reach statistical significance, but the confidence intervals were wide and therefore important clinical differences may exist (Figure 4, comparison 01:03:05: RR 5.83, 95% CI 0.24–140.55, p = 0.28).
Urinary incontinence
A total of 10 (4.9%) people were reported to have incontinence episodes amongst 205 allocated TUMT interventions compared with 13 (8.3%) people amongst 157 allocated TURP interventions (Figure 4, comparison 01:03:06: RR 0.61, 95% CI 0.30–1.26, p = 0.18). The direction and size of effect varied across studies and there was evidence of statistical heterogeneity across the three studies reporting this outcome. 165,167,169 This may be because some of the studies failed to report the type of incontinence and the length of follow-up varied.
Quality of life
Two studies165,169 used the IPSS QoL (0–6) questionnaire to measure quality of life of people undergoing TUMT or TURP (Appendix 8.1, Table 44 and Figure 5), where 0 is being delighted and 6 represents feeling terrible concerning urinary symptoms.
FIGURE 5.
Quality of life, TUMT vs TURP.

At 3 months
Both studies165,169 reported better quality of life scores at 3 months following TURP. The mean difference based on data from one study165 was 0.40 for TUMT versus TURP, but this result did not reach statistical significance (Figure 5: MD 0.40, 95% CI –0.17 to 0.97, p = 0.17).
At 12 months
Evidence from the two studies165,169 showed poorer quality of life scores following TUMT than following TURP (Figure 5: WMD 0.87, 95% CI 0.56–1.18, p < 0.001). The size of the estimated difference varied but the reasons for this were unclear.
Longer-term follow-up
The mean difference based on data from one study169 was 1.70 for TUMT versus TURP in terms of quality of life (Figure 5: 95% CI 1.22–2.18, p < 0.001). However, this same trial gave higher estimates of long-term IPSS/AUA differences than other trials, so the size of the difference in quality of life should be interpreted cautiously.
Urodynamic outcomes
Data on peak urine flow rate, voided volume, residual volume, detrusor pressure and prostate size were reported to a varying extent across the six studies. 124,165–169 Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 8.1, Table 45 and Appendix 9.1, comparisons 01:04–01:08.
At all time points considered, the peak urine flow rate was statistically significantly lower in the TUMT arm than in the TURP arm (Appendix 9.1, comparison 01:04); at both 3 and 12 months there was evidence of heterogeneity between studies included in the meta-analyses but there was consistency in the direction of effect. At 3 months, using the random-effects method, the WMD was 5.32 ml/s (95% CI –6.95 to –3.70, p < 0.001). The main source of heterogeneity appeared to be from the study by Wagrell and colleagues;165 however, the reasons for this remain unclear. When data from this study were excluded from the analysis, the trend towards TURP was maintained but the WMD increased (WMD 7.04, 95% CI 4.93–9.15, p < 0.001). At 12 months, fitting a random-effects model only increased the imprecision around the estimate of relative effectiveness.
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.1, Table 46. Information on length of hospital stay and reoperation rates was identified to a varying extent across the six eligible studies for this comparison.
Duration of operation
No studies reported duration of operation.
Length of hospital stay
Length of hospital stay was reported in two studies. 166,169 Note that those allocated to TUMT were treated as outpatients and therefore hospital stay was longer in the TURP arm (Appendix 8.1, Table 46; Appendix 9.1, comparison 01:11: WMD 5.30 days, 95% CI 4.48–6.12, p < 0.001).
Reoperation
Five studies124,166–169 provided details on reoperation rates. A total of 22 (10.2%) reoperations were reported amongst 215 participants allocated to TUMT compared with 9 (4.8%) amongst 189 participants allocated to TURP. Meta-analysis of the five trials just failed to reach statistical significance at the conventional 5% level (Appendix 9.1, comparison 01:12: RR 2.01, 95% CI 0.96–4.18, p = 0.06). This result should be treated with caution as the length of follow-up of the RCTs varied.
Summary and conclusions of the evidence for and against the intervention
This review considered data from 549 participants across six RCTs of generally moderate to poor quality (or poor reporting). Compared with TURP the data suggest that, after TUMT, improvement in IPSS/AUA symptom scores and quality of life is less, peak urine flow rate is lower, but length of hospital stay is shorter. Data describing blood transfusion, urinary retention, urinary tract infection, stricture, TUR syndrome, urinary incontinence and reoperation rates are too few to provide sufficiently precise estimates of differences but are consistent with fewer complications following TUMT, such as strictures and incontinence.
In this review the results for symptom scores, peak urine flow rate and urinary incontinence displayed statistically significant heterogeneity. Consistency in the direction and size of effect varied in the last outcome. Much of the heterogeneity might be due to differences in the characteristics of participants, particularly differences in prostate size. Moreover, it may in part have been due to differences in power delivery or other technical outputs of surgery across studies and to differences in the way that urinary incontinence is defined. Other likely sources of heterogeneity include differences in the length of follow-up.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 7. These should be interpreted in view of the comments mentioned earlier in this section.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 3 (3) | 4.08a | 2.78–5.39 | < 0.001 |
| 12 months | 3 (3) | 2.41a | 1.40–3.42 | < 0.001 |
| Longer term | 1 (1) | 8.90a | 6.65–11.15 | < 0.001 |
| Madsen–Iversen score | ||||
| 3 months | 4 (4) | 0.63a | –0.08 to 1.33 | 0.08 |
| 12 months | 4 (4) | 1.97a | 1.27–2.66 | < 0.001 |
| Longer term | 2 (2) | 1.32a | 0.20–2.44 | 0.02 |
| Blood transfusion | 3 (3) | 0.11b | 0.01–1.98 | 0.13 |
| Urinary retention | 2 (2) | 1.64b | 0.77–3.50 | 0.20 |
| Urinary tract infection | 5 (5) | 1.05b | 0.53–2.08 | 0.90 |
| Stricture | 4 (4) | 0.20b | 0.05–0.75 | 0.02 |
| TUR syndrome | 1 (1) | 0.65b | 0.03–15.62 | 0.28 |
| Incontinence | 3 (3) | 0.61b | 0.30–1.26 | 0.18 |
| Quality of life | ||||
| 3 months | 1 (2) | 0.40a | –0.17 to 0.97 | 0.17 |
| 12 months | 2 (2) | 0.87a | 0.56–1.18 | < 0.001 |
| Longer term | 1 (1) | 1.70a | 1.22–2.18 | < 0.001 |
| Qmax | ||||
| 3 months | 4 (4) | –5.35a | –7.09 to –3.62 | < 0.001 |
| 12 months | 4 (4) | –5.32a | –6.95 to –3.70 | < 0.001 |
| Longer term | 1 (1) | –11.10a | –15.50 to –6.70 | < 0.001 |
| Duration of operation | 0 (0) | NR | NR | NR |
| Length of hospital stay | 1 (2) | –5.30a | –6.12 to –4.48 | < 0.001 |
| Reoperation | 5 (5) | 2.01b | 0.96–4.18 | 0.06 |
Transurethral microwave thermotherapy (TUMT) versus sham
Characteristics of included studies
The baseline characteristics of the included studies are summarised in Table 8. A total of 1209 participants were randomised across 11 eligible RCTs reported in 21 papers. 125,126,133,143,159,160,170–174,219–228
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Abbou et al., 1995143 | TUMT | 66 | 65 | 10.9b | 10.4 | 66 | 45 |
| Sham | 31 | 66 | 12.8b | 9.9 | 61 | 44 | |
| Albala et al., 2002170 | TUMT | 125 | 65 | 22.5 | 8.9 | 58 | 50 |
| Sham | 65 | 65 | 22.7 | 8.4 | 53 | 47 | |
| Bdesha et al., 1994125 | TUMT | 22 | 64 | 19.2 | 12.3 | 104 | NR |
| Sham | 20 | 63 | 18.8 | 10.8 | 80 | NR | |
| Blute et al., 1996126 | TUMT | 78 | 67 | 19.9 | 7.3 | 140 | 37.4 |
| Sham | 37 | 67 | 20.8 | 7.4 | 145 | 36.1 | |
| Brehmer et al., 1999171 | TUMT 30 | 14 | NR | A/B – 58/40c | 8.7 | NR | ≤ 50 |
| TUMT 60 | 16 | NR | A/B – 49/36c | 7.0 | NR | ≤ 50 | |
| Sham | 14 | NR | A/B – 46/36c | 7.9 | NR | ≤ 50 | |
| de Wildt et al., 1996172 | TUMT | 46 | 64 | 12.9b | 9.6 | 85 | 49 |
| Sham | 47 | 66 | 13.7b | 9.2 | 94 | 49 | |
| Larson et al., 1998159 | TUMT | 125 | 66 | 20.8 | 7.8 | 99 | 38 |
| Sham | 44 | 66 | 21.3 | 7.8 | 104 | 45 | |
| Nawrocki et al., 1997160 | TUMT | 38 | NR | 19 | 8.8 | 252 | 86 |
| Sham | 40 | NR | 17.5 | 9.4 | 269 | 96 | |
| Ogden et al., 1993133 | TUMT | 22 | 68 | 14.5b | 8.5 | 147 | 38 |
| Sham | 21 | 67 | 14.2b | 8.6 | 118 | 35 | |
| Trachtenberg and Roehrborn, 1998173 | TUMT | 147 | 66 | 23.6 | 7.7 | 80 | 48 |
| Sham | 73 | 66 | 23.9 | 8.1 | 67 | 50 | |
| Zerbib et al., 1994174 | TUMT | 38 | NR | NR | 7.6 | 110 | NR |
| Sham | 30 | NR | NR | 10.6 | 84 | NR |
Three studies took place in the US,126,159,170 three in the UK,125,133,160 two in France143,174 and one each in Sweden171 and the Netherlands,172 and one was a multicentre trial that took place in the US and Canada. 173 Four studies provided details of recruitment dates;133,159,160,172 the earliest recruitment date was June 1994 and latest recruitment date was June 1996.
Although all 11 studies gave some details of the ages of participants, three gave only the mean or median age of the participant group as a whole, regardless of which intervention to which they were randomised. 160,171,174
Of the studies reporting IPSS/AUA scores at entry, 475 (74%) participants allocated to TUMT had severe symptoms of BPE and 60 (9%) had moderate symptoms. Of the participants randomised to sham, 219 (58%) had severe symptoms and 60 (16%) had moderate symptoms.
Two studies failed to report the prostate size of the enrolled participants125,174 and in one study authors reported that, to be included, patients had to have prostate sizes of less than 50 ml. The total numbers of participants who had moderate-sized and large prostates in the TUMT group were 225 (34%) and 438 (66%) respectively. The equivalent numbers allocated to a sham procedure were 58 (17%) and 314 (84%) respectively.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.2. The results of the meta-analyses are given in Appendix 9.2. Because of the nature of the comparator intervention (sham), the most useful information comes from short-term outcomes. The value of long-term assessment of outcomes is limited by a high dropout rate as most of the patients were judged to require a true TUMT procedure by 12 months; it is likely that only the least severe patients at baseline remained untreated at this time point, thus comparisons limited to untreated men are subject to selection bias.
Symptom scores
At 3 months
Of the 11 eligible studies, eight provided information on symptom scores at 3 months following surgery. 125,126,133,159,160,170,172,173 Six of those reported IPSS/AUA scores. 125,126,159,160,170,173 In all studies, IPSS/AUA scores were superior in the TUMT group and this was statistically significant in all six studies (p < 0.05). Only three studies125,126,159 presented data in a form that was sufficiently similar to allow quantitative synthesis (Figure 6, comparison 02:01:01). The WMD was –5.69 (95% CI –7.38 to –3.99, p < 0.00001) for TUMT versus sham surgery. This result is consistent with the data from those trials that provided data that were not amenable for meta-analysis.
FIGURE 6.
Symptom scores, TUMT vs sham.

Meta-analysis of three studies reporting Madsen–Iversen scores126,133,172 showed that TUMT resulted in a greater decrease in score than sham treatment (Figure 6, comparison 02:02:01: WMD –5.66, 95% CI –6.85 to –4.46, p < 0.000001). There was some evidence of heterogeneity and, when a random-effects model was fitted, the principal change was in the width of the confidence interval. The main source of heterogeneity appeared to be the study by Ogden and colleagues;133 however, the reasons for this are unclear. When data from Ogden and colleagues were removed from the analysis, the trend towards TUMT was maintained but the WMD decreased (WMD –5.10, 95% CI –6.40 to –3.79, p < 0.00001).
At 12 months
No studies reported IPSS/AUA symptom scores for patients at 12 months after the surgery.
One study reported Madsen–Iversen symptom scores at 12 months following surgery. 172 The WMD was –4.00 (Figure 6, comparison 02:02:03: 95% CI –5.81 to –2.19, p < 0.0001).
Longer-term follow-up
No longer-term follow-up data on symptom scores have been reported by any of the eligible studies.
Complications
Data describing complications by study are detailed in Appendix 8.2, Table 48. Out of the eligible studies, complications were reported in ten. 125,126,133,143,159,160,170–173 Ten categories of complications were identified. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures and urinary incontinence are presented in this section (Figure 7). Results for other complications are presented in Appendix 9.2, comparison 02:03. The results of these meta-analyses should be treated with caution as the length of follow-up of the RCTs varied.
FIGURE 7.
Complications, TUMT vs sham.

Blood transfusion
Only one study159 provided details on blood transfusion rates. There were no reports of blood transfusions amongst 125 and 44 patients allocated to TUMT or sham respectively.
Urinary retention
All seven trials with data showed higher rates of urinary retention after TUMT. This applied to a total of 77 (12%) patients amongst 644 allocated to TUMT compared with two (0.5%) amongst 360 patients allocated to a sham procedure (Figure 7, comparison 02:03:02: RR 10.57, 95% CI 4.11–27.20, p < 0.0001). This result should be treated with caution as the length of follow-up of the RCTs varied.
Urinary tract infection
Meta-analysis of data from four trials133,143,159,173 that reported urinary tract infections showed a higher number of infections following TUMT; however, this was not statistically significant (Figure 7, comparison 02:03:03: RR 1.49, 95% CI 0.84–2.67, p = 0.17).
Stricture
Urethral strictures were reported in two trials. 159,170 Three cases were reported in one trial following TUMT, with no cases following sham treatment (Figure 7, comparison 02:03:04: 3/246 versus 0/106, RR 2.50, 95% CI 0.13–47.46, p = 0.54).
Incontinence
Data on incontinence from one trial159 showed five cases (4%) out of a total of 125 patients following TUMT versus no cases after sham treatment. This result was not statistically significant and confidence intervals were wide (Figure 7, comparison 02:03:05: RR 3.93, 95% CI 0.22–69.63, p = 0.35).
Quality of life
Four studies,133,159,170,173 using a variety of instruments, reported the quality of life of people undergoing TUMT or a sham procedure (Appendix 8.2, Table 49). In two studies159,173 the quality of life was assessed using the IPSS QoL (0–6) questionnaire. This was evaluated by patients’ responses to the question of how they would feel if their current urinary symptoms were to continue indefinitely. In the third study170 quality of life index was used and in the final study133 quality of life was measured using a questionnaire derived from the Veterans’ Administration study of TURP versus watchful waiting. This questionnaire had five sections: A, perception of urinary difficulties; B, sexual performance; C, activities of daily living; D, general psychological well-being; and E, social activities. At the 3-month evaluation, two studies133,173 reported higher quality of life following TUMT. This difference was statistically significant in both studies (p < 0.05). Larson and colleagues159 report that the improvement in quality of life score remained at a comparable level in the 12-month evaluation in the TUMT group.
Urodynamic outcomes
Data on peak urine flow rate, voided volume and residual volume were reported across 11 studies. 125,126,133,143,159,160,170–174 Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 8.2, Table 50 and Appendix 9.2, comparisons 02:04–02:09.
A total of seven studies125,126,133,159,172–174 reported peak urine flow rate at 3 months after surgery. In all but one study174 the peak urine flow rate was higher in the TUMT group. Six studies125,126,133,159,172,174 presented data that were sufficiently similar to allow quantitative synthesis (Appendix 9.2, comparison 02:04:01: WMD 2.53 ml/s, 95% CI 1.69–3.37, p < 0.001). With regard to longer-term follow-up (12 months), only one study172 reported this outcome (WMD 2.90, 95% CI –0.24 to 6.04, p = 0.07).
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.2, Table 51. Information on reoperation rates was identified in five studies.
Duration of operation
No studies reported this outcome.
Length of hospital stay
No studies reported this outcome.
Reoperation
The percentage of patients requiring a reoperation in the TUMT group was 6% compared with 54% of patients in the sham group requiring surgery. Meta-analysis of five trials125,133,159,171,172 presents a RR of 0.14 in favour of TUMT (95% CI 0.09–0.23, p < 0.00001). 37,46,69,80,81 This result should be interpreted with caution as the length of follow-up varied.
Summary and conclusions of the evidence for and against the intervention
This review considered data from 1209 participants across 11 RCTs of generally moderate to poor quality (with respect to conduct and reporting). The data suggest that TUMT both reduces symptoms and increases peak urine flow rate at 3 months after the procedure. Reoperation rates for TUMT were lower than for sham. Patients who underwent TUMT had a high risk of developing urinary retention. Confidence intervals were wide. The meta-analyses failed to indicate differences in the incidence of blood transfusion, strictures and urinary incontinence, although the direction of effect was consistent with what would be expected after an operative procedure, again with wide confidence intervals.
In this review the data contributing to meta-analyses were too few to provide precise estimates of differences, particularly for the complications, and confidence intervals were so wide that clinically important differences could not be ruled out.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 9. Again, these should be interpreted in view of the comments mentioned above.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 3 (6) | –5.69a | –7.38 to –3.99 | < 0.001 |
| 12 months | 0 (1) | NR | NR | NR |
| Longer term | 0 (0) | NR | NR | NR |
| Madsen–Iversen score | ||||
| 3 months | 3 (4) | –5.66a | –6.85 to –4.46 | < 0.001 |
| 12 months | 1 (3) | –4.00a | –5.81 to –2.19 | < 0.001 |
| Longer term | 0 (0) | NR | NR | NR |
| Blood transfusion | 1 (1) | NE | NE | NE |
| Urinary retention | 8 (8) | 9.12b | 3.36–24.80 | < 0.001 |
| Urinary tract infection | 4 (4) | 1.49b | 0.84–2.67 | 0.17 |
| TUR syndrome | 0 (0) | NR | NR | NR |
| Stricture | 2 (2) | 2.50b | 0.13–47.46 | 0.54 |
| Incontinence | 1 (1) | 3.93b | 0.22–69.63 | 0.35 |
| Quality of life | ||||
| 3 months | 0 (2) | NR | NR | NR |
| 12 months | 0 (0) | NR | NR | NR |
| Longer term | ||||
| Qmax | ||||
| 3 months | 6 (9) | 2.53a | 1.69–3.37 | < 0.001 |
| 12 months | 1 (4) | 2.90a | –0.24 to 6.04 | 0.07 |
| Longer term | 0 (0) | NR | NR | NR |
| Duration of operation | 0 (0) | NR | NR | NR |
| Length of stay | 0 (0) | NR | NR | NR |
| Reoperation | 5 (5) | 0.14b | 0.09–0.23 | < 0.001 |
Transurethral needle ablation (TUNA) versus TURP
Characteristics of included studies
The characteristics of the included studies are summarised in Table 10. Four RCTs, reported in nine papers,144,151,175,176,229–233 were eligible for this comparison, in which a total of 450 participants were randomised. These trials took place in the US,144 Turkey,175 Korea151 and the UK. 176
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Cimentepe et al., 2003175 | TUNA | 26 | 60 | 22.9 | 9.8 | 67 | 46 |
| TURP | 33 | 63 | 24.1 | 9.2 | 76 | 49 | |
| Hill et al., 2004144 | TUNA | 65 | 66 | 23.9 | 8.8 | 92 | 36 |
| TURP | 56 | 66 | 24.1 | 8.8 | 83 | 36 | |
| Hindley et al., 2001176 | TUNA | 25 | 66b | 22b | 8.5 | 55 | NR |
| TURP | 25 | 71b | 20b | 9.0 | 74 | NR | |
| Kim et al., 2006151 | TUNA | 110 | 66 | 20.8 | 7.0 | 257 | 41 |
| TURP | 110 | 67 | 24.0 | 11.9 | 187 | 44 |
All four studies provided details of the participants’ baseline IPSS/AUA symptom scores, according to which all 450 participants had severe symptoms.
The studies presented variations in relation to prostate size. Of the studies reporting this characteristic, 65 (32%) and 136 (66%) participants randomised to TUNA had moderate-sized and large prostates respectively. Of the patients allocated to TURP, 56 (28%) had moderate-sized prostates and 143 (72%) had large prostates.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.3. The results of the meta-analyses are given in Appendix 9.3. Note that in terms of long-term evaluation, only the longest follow-up is presented.
Symptom scores
At 3 months
At 3 months after surgery, three out of the four eligible trials reported AUA/IPSS symptom scores. 144,151,175 Only two studies reported data that were amenable to meta-analysis. 144,175 Symptom scores were slightly lower following TURP than following TUNA (Figure 8, comparison 03:01:01: WMD 1.18, 95% CI –0.03 to 2.40, p = 0.06).
FIGURE 8.
Symptom scores, TUNA vs TURP

At 12 months
Three reports presented IPSS/AUA results at 12 months of follow-up. 144,151,176 Analysis of data from one report showed better symptom scores in patients undergoing TURP than in those following TUNA (Figure 8 comparison 03:01:03: MD 3.90, 95% CI 1.27–6.53, p = 0.004). This result is consistent with that observed in the studies by Hindley and colleagues176 and Kim and colleagues. 151
Longer-term follow-up
Only one trial144 reported 5-year IPSS/AUA scores. At this point in time, TUNA and TURP appeared to be equivalent in terms of improvement in symptoms, albeit with confidence intervals that included differences seen at earlier time points (Figure 8 comparison 03:01:07: MD 0.60, 95% CI –3.55 to 4.75, p = 0.78). The narrowing of the difference reflected better scores in the TUNA group. This should be interpreted cautiously as this follow-up included 33% of those who initially underwent surgery and so could reflect selection bias.
Data for other follow-up times (2 and 3 years) were also reported by Hill and colleagues144 and can be seen in Appendix 8.3, Table 52 and the respective forest plots in Figure 8.
Complications
Data describing complications are tabulated in Appendix 8.3, Table 53. Information on nine categories of complications was identified across the four eligible studies for this comparison. The data were too few to provide precise estimates of differences and all confidence intervals were wide such that clinically important differences could not be ruled out. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures and urinary incontinence are presented in this section (Figure 9). Results for other complications are presented in Appendix 9.3, comparison 03:03. The results of these meta-analyses should be treated with caution as the length of follow-up of the RCTs varied. Also, for urinary incontinence it was unclear whether the type of incontinence considered was the same across all studies.
FIGURE 9.
Complications, TUNA vs TURP.

Blood transfusion
There were no cases of blood transfusion in the TUNA arms amongst 146 patients across three studies. 144,151,175 Blood transfusion was required in 14% (n = 22) of the patients undergoing TURP (Figure 9, comparison 03:03:01: RR 0.05, 95% CI 0.01–0.32, p = 0.002).
Urinary retention
Urinary retention following surgery was reported in three studies. 151,175,176 Six cases (4.1%) of urinary retention were recorded amongst 146 patients in the TUNA arms. Four patients (2.6%) who underwent TURP exhibited urinary retention. The confidence intervals are wide and, therefore, this result should be interpreted with caution (Figure 9, comparison 03:03:02: RR 1.48, 95% CI 0.49–4.52, p = 0.49).
Urinary tract infection
Urinary tract infection occurred more frequently in the TUNA arms (10.2%) than in the TURP arms (7.0%), but again with wide confidence intervals (Figure 9, comparison 03:03:03 RR 1.42, 95% CI 0.69–2.91, p = 0.34).
Stricture
Across three trials, the incidence of strictures or bladder neck contractures was documented in one patient (0.5%) in the TUNA group and 13 (6.8%) in the TURP group. This difference was statistically significant (Figure 9, comparison 03:04: RR 0.14, 95% CI 0.03–0.62, p = 0.009).
TUR syndrome
No studies reported this outcome.
Urinary incontinence
All four studies reported urinary incontinence following surgery. The types of incontinence were not fully described across studies (Appendix 8.3, Table 53). The overall incidence of urinary incontinence was 0.9% (n = 2) in the TUNA group versus 8.0% (n = 17) in the TURP group (Figure 9, comparison 03:03:05: RR 0.16 95% CI 0.05–0.51, p = 0.002).
Quality of life
Four studies,144,151,175,176 using a variety of instruments, reported the quality of life of people following TUNA or TURP (Appendix 8.3, Table 54). In three studies151,175,176 the quality of life was assessed using the IPSS QoL (0–6) questionnaire. In one study144 the type of scale used to measure quality of life was unclear.
At 3 months
Two studies151,175 provided details on quality of life at 3 months after surgery. Only one study175 provided data that were amenable to meta-analysis. Quality of life was higher for TURP with a mean difference of 0.20 (95% CI –0.10 to 0.50, p = 0.19). This result was not statistically significant (Figure 10, comparison 03:08:01). This result should be treated with caution as the total number of participants available for this evaluation was unclear. This result is, however, consistent with that provided by Kim and colleagues. 151
FIGURE 10.
Quality of life, TUNA vs TURP.

At 12 months
Three studies144,151,176 provided details on quality of life at 12 months after surgery; however, only one was suitable for quantitative synthesis. The quality of life was higher for TURP with a WMD of 0.60 (Figure 10, comparison 03:08:02: 95% CI –1.08 to 2.28, p = 0.48). This result is consistent with those reported by Hindley and colleagues176 and Kim and colleagues. 151
Longer-term follow-up
Evidence from one study144 indicated that the quality of life of patients who underwent both TUNA and TURP decreased over time; however, it remained statistically significantly better compared with quality of life measured at baseline (p < 0.0001). Up to 5 years the two procedures appear to be comparable in terms of quality of life (Figure 10, comparison 03:08:03–03:08:07). The loss to follow-up is high and caution should be taken when interpreting the results of this meta-analysis.
Urodynamic outcomes
Data on peak urine flow rate, residual volume, detrusor pressure and prostate size were reported to a varying extent across four studies. 144,151,175,176 These are tabulated in Appendix 8.3, Table 55. Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 8.3, Table 55 and Appendix 9.3, comparisons 03:04–03:07.
Peak urine flow rate was statistically significantly lower in the TUNA arm than in the TURP arm at all time points (Appendix 9.3, comparison 03:04). At 12 months there was evidence of statistical heterogeneity in the results; however, the direction of effect is consistent across the two studies reporting data amenable to meta-analysis144,176 and with the results reported by Kim and colleagues. 151 Applying a random-effects model did not change this pattern. The total number of patients contributing to the measurement of this estimate is unclear and it should be noted that only 20% and 27% of those who underwent TUNA and TURP, respectively, were available for the 5-year follow-up assessment. Thus, these results should be treated with considerable caution.
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.3, Table 56. Information on duration of operation, length of hospital stay and reoperation rates was identified to a varying extent across the three eligible studies for this comparison.
Duration of operation
Two studies151,175 provided information on the duration of operation (Appendix 8.3, Table 56). Only one study provided data that were suitable for quantitative synthesis. The duration of operation in the TUNA group was on average 11.60 minutes longer than the duration of operation in the TURP group (Appendix 9.3, comparison 03:09: 95% CI 6.41–16.79, p < 0.001). This result was consistent with that reported by Kim and colleagues151 who reported that TUNA took 14 minutes more than TURP.
Length of hospital stay
Length of hospital stay appeared to be longer for patients undergoing TUNA than for those undergoing TURP in two studies. Hindley and colleagues176 reported that patients undergoing TUNA are discharged a few days following the procedure whereas patients undergoing TURP are discharged in the first postoperative day. Cimentepe and colleagues175 treated TURP patients as outpatients whereas patients allocated to TUNA would stay for at least 48 hours. On the other hand, Kim and colleagues151 reported a shorter length of hospital stay for those patients undergoing TUNA, with a mean difference of 5.2 days.
Reoperation
Across the four trials, reoperations were documented in 6.2% (13/211) of patients allocated to TUNA compared with 0.5% (1/212) of patients in the TURP group (RR 6.89, 95% CI 1.58–29.95). Although the difference is statistically significant, the confidence interval is wide and it should be noted that the follow-up of the three eligible studies varied from 12 months144,151 to 2 years. 176
Summary and conclusions of the evidence for and against the intervention
This review considered data from four RCTs of moderate quality. A total of 450 participants were randomised across the four studies and therefore the data were too few to provide precise estimates for all of the outcomes. A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 11.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 2 (3) | 1.18a | –0.03 to 2.40 | 0.06 |
| 12 months | 1 (3) | 3.90a | 1.27–6.53 | 0.004 |
| Longer term | 1 (1) | 0.60a | –3.55 to 4.75 | 0.78 |
| Blood transfusion | 3 (3) | 0.05b | 0.01–0.32 | 0.002 |
| Urinary retention | 3 (3) | 1.48b | 0.49–4.52 | 0.49 |
| Urinary tract infection | 3 (3) | 1.42b | 0.69–2.91 | 0.34 |
| Stricture | 3 (3) | 0.14b | 0.03–0.62 | 0.009 |
| TUR syndrome | 0 (0) | NR | NR | NR |
| Incontinence | 4 (4) | 0.16b | 0.05–0.51 | 0.002 |
| Quality of life | ||||
| 3 months | 1 (2) | 0.20a | –0.10 to 0.50 | 0.19 |
| 12 months | 1 (3) | 0.60a | –1.08 to 2.28 | 0.48 |
| Longer term | 1 (1) | 0.50a | –1.58 to 2.58 | 0.64 |
| Qmax | ||||
| 3 months | 1 (2) | –6.40a | –8.90 to –3.90 | < 0.001 |
| 12 months | 2 (3) | –8.12a | –10.85 to –5.40 | < 0.001 |
| Longer term | 1 (1) | –7.20a | –12.28 to –2.12 | 0.005 |
| Duration of operation | 1 (2) | 11.60a | 6.41–16.79 | < 0.001 |
| Length of hospital stay | 0 (3) | NR | NR | NR |
| Reoperation | 4 (4) | 6.89b | 1.58–29.95 | 0.01 |
Stents versus transurethral resection of the prostate (TURP)
Characteristics of included studies
No full-text reports of RCTs were identified in the searches. One abstract of an RCT presented as a conference proceeding was identified. 91 This UK study allocated 34 men to undergo prostatic stent insertion and 26 to undergo TURP.
The mean age of participants allocated to stent insertion was 73 years (range 63–86) compared with 72.6 years (range 63–86) for patients allocated to TURP.
On average, participants in the TURP arm had more severe symptoms (mean = 21.6) than those in the stents arm (mean = 19.0).
Participants in both arms presented equivalent mean peak urine flow rate measurements of 8.0 ml/s.
Assessment of effectiveness
Symptom scores
At 3 months
The mean IPSS scores observed at 3 months were 11.2 and 11.0 in the stents and TURP groups respectively.
Complications
The stents group exhibited a slight increase in irritative urinary symptoms compared with the TURP group.
Urodynamic outcomes
The only uroflowmetry data reported were peak urine flow rate. There was no statistically significant difference between the use of a Urolume stent and TURP (MD 0.00, 95% CI –5.84 to 5.84, p = 1.00).
Descriptors of care
The only descriptor of care observed was two reoperations in the stents group because of misplacement of the Urolume stent. The authors describe this as being due to technical reasons and therefore a TURP procedure was carried out.
Transurethral ethanol ablation of the prostate (TEAP) versus TURP
Characteristics of included studies
One RCT was identified. 151 In this Korean study, 94 men were allocated to undergo TEAP and 110 to undergo TURP.
The mean age of participants allocated to TEAP was 66 years (range 49–88) compared with 67 years (range 60–87) for patients allocated to TURP.
All participants had severe symptoms and the mean or median peak urine flow rate measurements were 7.2 and 11.9 ml/s for the TEAP and TURP groups respectively. All participants in the TEAP arm had moderate-sized prostates whereas those in the TURP arm had large prostates.
Assessment of effectiveness
Symptom scores
The mean IPSS scores observed at 3 months were 9.6 and 10.6 in the TEAP and TURP groups respectively. The mean difference was similar at 12 months with scores of 7.5 for TEAP and 8.8 for TURP.
Complications
Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 11). These results should be treated with caution as the time points at which the complications took place were uncertain.
FIGURE 11.
Complications, TEAP vs TURP

Blood transfusion
There were no blood transfusions reported across 94 patients in the TEAP arm compared with 19 (19%) reported in the TURP arm (Figure 11, comparison 26:01:01: RR 0.03, 95% CI 0.00–0.45, p = 0.01).
Urinary retention
Two cases (2%) of urinary retention were reported amongst the 94 patients allocated to TEAP compared with four cases (4%) across 101 patients allocated to TURP. This difference was not statistically significant (Figure 11, comparison 26:01:02: RR 0.54, 95% CI 0.10–2.87, p = 0.47).
Urinary tract infection
No statistically significant difference was observed between the two arms in terms of urinary tract infections (Figure 11, comparison 26:01:03: RR 0.77, 95% CI 0.25–2.34, p = 0.64).
Stricture
There were no cases of strictures or bladder neck stenosis in the TEAP arm as opposed to seven cases (7%) in the TURP arm. This result did not reach statistical significance (Figure 11, comparison 26:01:04: RR 0.07, 95% CI 0.00–1.24, p = 0.07).
Urinary incontinence
Urinary incontinence was observed in four patients following TURP amongst a total of 101 randomised patients. Again, this result did not reach statistical significance (Figure 11, comparison 26:01:05: RR 0.12, 95% CI 0.01–2.19, p = 0.15).
Quality of life
Quality of life was measured in terms of IPSS QoL scores and was found to be improved in both arms at both the 3- and 12-month assessments. Quality of life measurements at 3 and 12 months following surgery were 3.4 and 2.3, respectively, for those in the TEAP arm compared with 2.8 and 2.6, respectively, for those in the TURP arm.
Urodynamic outcomes
Mean differences in peak urine flow rates at 3 and 12 months were approximately 7.9 ml/s in favour of TURP.
Descriptors of care
Duration of operation and length of hospital stay were shorter in the TEAP arm than in the TURP arm. No reoperations were recorded in either arm.
Laser coagulation versus TURP
Laser coagulation of the prostate is a method that encompasses several techniques including interstitial laser coagulation, visual laser ablation and transurethral laser prostatectomy. It was not possible to confidently describe the actual method used from the information available from the trials. For analysis purposes these techniques have therefore been considered together.
Characteristics of included studies
The characteristics of the included studies are summarised in Table 12. Thirteen RCTs, reported in 17 papers,123,130,131,136,139,145,149,151,154,163,177–179,234–237 were eligible for this comparison, in which a total of 1231 participants were randomised. The total number allocated to laser coagulation was 612 and the total number allocated to TURP was 619.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Chacko et al., 2001;154 CLasP study | Laser coagulation | 74 | 74 | 17.6 | NR | NR | NR |
| TURP | 74 | 73 | 19.4 | NR | NR | NR | |
| Costello et al., 1995123 | Laser coagulation | 34 | 68 | NR | 8.76 | NR | 30 |
| TURP | 37 | 68 | NR | 9.48 | NR | 34 | |
| Cowles et al., 1995163 | Laser coagulation | 56 | 65 | 18.7 | 8.9 | 163 | 42 |
| TURP | 59 | 67 | 20.8 | 9.5 | 207 | 39 | |
| Donovan et al., 2000;136 CLasP study | Laser coagulation | 117 | 67 | 19.1 | 10.4 | 124 | 41 |
| TURP | 117 | 66 | 19.2 | 10.3 | 104 | 38 | |
| Gujral et al., 2000;139 CLasP study | Laser coagulation | 38 | 70 | 20.9 | 11.2 | 438 | 41c |
| TURP | 44 | 70 | 19.5 | 8.5 | 545 | 50c | |
| Kabalin et al., 1995177 | Laser coagulation | 13 | 65 | 20.9 | 8.5 | 236 | 24b |
| TURP | 12 | 69 | 18.8 | 9.0 | 291 | 17b | |
| Kim et al., 2006151 | Laser coagulation | 89 | 69 | 21.1 | 8.6 | 219 | 43 |
| TURP | 110 | 67 | 24.0 | 11.9 | 187 | 44 | |
| Kursh et al., 2003130 | Laser coagulation | 37 | 68 | 24.0c | 9.2c | 81c | 41c |
| TURP | 35 | 69 | 23.0c | 9.1c | 87c | 40c | |
| Liedberg et al., 2003131 | Laser coagulation | 20 | NR | 19c | 8c | 96c | 49c |
| TURP | 11 | NR | 17c | 8c | 117c | 47c | |
| Mårtenson and de la Rosette, 1999178 | Laser coagulation | 30 | > 45 | 21.7 | 7.3 | 116 | 46 |
| TURP | 14 | > 45 | 21.6 | 9.3 | 88 | 50 | |
| McAllister et al., 2000145 | Laser coagulation | 76 | 68 | 18.1 | 9.6 | 113 | NR |
| TURP | 75 | 68 | 18.2 | 10.0 | 120.7 | NR | |
| Rodrigo Aliaga et al., 1998149 | Laser coagulation | 18 | NR | 25.5 | 7.0 | 77 | 20–60b |
| TURP | 21 | NR | 24.2 | 8.3 | 89 | 20–60b | |
| Suvakovic and Hindmarsh, 1996179 | Laser coagulation | 10 | 67 | 15.7 | 10.5 | 47 | 24b |
| TURP | 10 | 66 | 18.8 | 11.1 | 162 | 22b |
Five studies took place in the UK,136,139,145,154,179 three in the US,130,163,177 and one each in Sweden,131 Australia,123 Spain,149 Korea151 and the Netherlands. 178 Six studies provided details on recruitment dates. 130,131,145,151,163,178 The earliest recruitment date was August 1991163 and the latest recruitment date was December 2002. 151
Overall, the total numbers of participants with moderate and severe symptoms allocated to receive laser coagulation were 353 (61%) and 225 (39%) respectively. There were 343 (58%) moderately and 239 (41%) severely symptomatic participants allocated to TURP.
In general, studies reported prostate size. Two studies154,179 failed to report prostate size of the enrolled participants and in one study149 authors reported that, to be included, patients had to have prostate sizes between 20 and 60 g. The total numbers of participants who had small, moderate-sized and large prostates in the laser coagulation group were 23 (5%), 34 (8%) and 387 (87%) respectively. Of those allocated to a TURP procedure, 22 (5%) had a small prostate, 176 (39%) had a moderate-sized prostate and 214 (48%) had a large prostate.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.4. The results of the meta-analyses are given in Appendix 9.4. Note that in terms of long-term evaluation, only the longest follow-up is presented.
Symptom scores
At 3 months
Of the 13 eligible RCTs, six provided information on the mean or median IPSS/AUA scores 3 months after surgery. 131,149,151,177–179 Two studies149,177 showed better scores in the laser group than in the TURP group, and four131,151,178,179 favoured TURP. This variation may be explained by the fact that trials included participants with various levels of prostate size. For example, in the trial by Kabalin and colleagues,177 participants had an average prostate size of 17 g (ml) in the TURP group, whereas in the study reported by Mårtenson and colleagues,178 participants randomised to TURP had on average a prostate size of 50 ml (g). Because of this heterogeneity we opted not to derive a pooled estimate (Figure 12, comparison 04:01:01).
FIGURE 12.
Symptom scores, laser coagulation vs TURP.

At 12 months
IPSS/AUA scores were reported in a total of seven studies. 131,145,151,163,177–179 The direction and size of effect varied across the studies. The improvements in IPSS reported by Cowles and colleagues163 and Liedberg and colleagues131 were, however, consistently lower in the laser coagulation intervention group (Figure 12, comparison 04:01:03).
Complications
Complications listed by study are detailed in Appendix 8.4, Table 58. Seventeen types of complications were reported to varying extents across the 13 studies. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 13). Results for other complications are presented in Appendix 9.4, comparison 04:02. The results of these meta-analyses should be treated with caution as the length of follow-up of the RCTs varied. For urinary incontinence it was unclear whether the type of incontinence considered was the same across all studies.
FIGURE 13.
Complications, laser coagulation vs TURP.

Blood transfusion
One (0.2%) laser patient as opposed to 46 (7.8%) TURP patients required a blood transfusion (Figure 13, comparison 04:02:01: RR 0.11, 95% CI 0.04–0.26, p < 0.001). 123,130,136,139,145,149,151,154,163,177,178
Urinary retention
The pooling of data from three studies151,163,179 showed that 13% (n = 20) of the patients following laser coagulation had urinary retention compared with 5% (n = 9) of those following TURP (Figure 13, comparison 04:02:02: RR 2.31, 95% CI 1.11–4.80, p = 0.02).
Urinary tract infection
Meta-analysis of eight trials123,130,131,136,139,145,151,178 suggested that the incidence of urinary tract infection was higher following laser coagulation than after TURP (Figure 13, comparison 04:02:03: 65/439 versus 30/434, RR 1.84, 95% CI 1.22–2.79, p = 0.004). Note that three trials had particularly high rates of infection and that two and three of the infections in the laser and TURP groups, respectively, were actually epididymitis. These results should also be treated with caution as the length of follow-up of the RCTs varied.
Stricture
In five RCTs with data,123,131,151,163,177 a total of two (0.9%) strictures were reported amongst 212 participants allocated to laser procedures versus 19 (8.6%) strictures amongst 220 participants allocated to TURP procedures (Figure 13, comparison 04:02:04: RR 0.18, 95% CI 0.06–0.56, p = 0.003).
Quality of life
Six studies,130,136,139,151,154,178 using a variety of instruments, reported the quality of life of people following laser coagulation or TURP (Appendix 8.4, Table 59). In four studies136,139,151,154 the quality of life was assessed using the IPSS QoL (0–6) questionnaire. In one study130 the AUA quality of life questionnaire was used and another study178 used the quality of life index.
In three studies providing data,130,151,178 the quality of life scores were poorer following laser coagulation than following TURP at 3, 12 and 24 months (Figure 14, comparison 04:10). Meta-analysis of the change of quality of life from baseline reported in three trials136,139,154 was consistent with this although the difference between the groups was not statistically significant (Figure 14, comparison 04:11).
FIGURE 14.
Quality of life, laser coagulation vs TURP

Urodynamic outcomes
Data on peak urine flow rate, total voided volume, residual volume, detrusor pressure and prostate size were reported across ten studies. 130,131,139,145,149,151,163,177–179 These are tabulated in Appendix 8.4, Table 60. Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 9.4, comparisons 04:04–04:08.
At 3 months
All eight studies that provided information on peak urine flow rates at 3 months after operation reported lower mean or median flow rates in the laser coagulation group (Appendix 8.4, Table 60). Meta-analysis of five RCTs145,149,177–179 reporting data suitable for quantitative synthesis gave a WMD of –5.36 ml/s (95% CI –7.28 to –3.45, p < 0.001) favouring TURP. This result should be treated with caution as two studies failed to report how many patients contributed to the analysis.
At 12 months
A total of six studies131,145,163,177–179 provided details on peak urine flow rate at 12 months after operation. All but one study177 reported higher median or mean peak urine flow rates in the TURP group. In the four studies145,177–179 that presented means and standard deviations, the WMD was –4.57 ml/s (Appendix 9.4, comparison 04:04:03: 95% CI –6.55 to –2.59, p < 0.001). There was evidence of statistical heterogeneity amongst the studies included in the meta-analysis. Using a random-effects model did not change this result. Cowles and colleagues163 reported change from baseline rather than absolute rates. Their results were consistent with those of the meta-analysis.
Longer-term follow-up
Two studies177,178 reported peak urine flow rates at 2 years after laser coagulation and TURP. Meta-analysis of data from these studies did not show any statistically significant difference in peak urine flow rate between the two arms (Appendix 9.4, comparison 04:04:06: WMD –0.76, 95% CI –5.30 to 3.77, p = 0.74). Note that the number of patients available for this follow-up assessment is unclear.
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.4, Table 61. Information on duration of operation, length of hospital stay and reoperations was identified to a varying extent across the 13 eligible studies.
Duration of operation
Duration of operation was reported in five trials. 123,151,163,177,179 Combining data from two trials163,179 indicated that the duration of operation in the laser coagulation arm was statistically significantly shorter than that for the TURP arm (Appendix 9.4, comparison 04:12: WMD –12.24 minutes, 95% CI –16.78 to –7.69, p < 0.001). This result is consistent with findings from trials whose data were not amenable to meta-analysis. There was evidence of statistical heterogeneity. Using a random-effects model resulted in the difference no longer being significant (WMD –11.54, 95% CI –31.74 to 8.65, p = 0.29). The sources of heterogeneity were unclear. However, patients included in the trial by Suvakovic and Hindmarsh179 had considerably smaller prostates than those included in the trial by Cowles and colleagues. 163 In addition, there was a high degree of uncertainty surrounding the results from the former trial because of the small sample size.
Length of hospital stay
Nine out of ten studies providing information on length of hospital stay reported lower mean or median stay in the laser coagulation group. Two RCTs reported data suitable for quantitative synthesis. 145,163 Across them, the average length of stay was significantly shorter in the laser coagulation group than in the TURP group (Appendix 9.4, comparison 04:13: WMD –1.33; 95% CI –1.68 to –0.98, p < 0.001).
Reoperation
A total of nine RCTs123,130,139,145,151,154,163,177,178 provided information on reoperation rates. The results of the meta-analysis showed a statistically significant higher rate following laser coagulation (Appendix 9.4, comparison 04:02:16: RR 3.21, 95% CI 1.65–6.24, p < 0.001). As the length of follow-up ranged from 6 months123 to 5 years,145 the results of this meta-analysis should be treated with caution.
Summary and conclusions of the evidence for and against the intervention
Data from over 1000 participants randomised across 13 RCTs of generally moderate to poor quality (or reporting) were included. The data indicate that symptom scores at 12 months or more and quality of life and peak urine flow rate at 3 and 12 months are worse after laser coagulation than after TURP. The occurrence of blood transfusion, strictures and urinary incontinence was lower in the laser coagulation group but urinary retention and urinary tract infection appeared to be higher. TUR syndrome does not appear to differ between the two approaches. In terms of descriptors of care, the data suggest that duration of operation and length of hospital stay are likely to be shorter after laser coagulation than after TURP but that the reoperation rate is higher after laser coagulation than after TURP.
The results for symptom scores, peak urine flow rate and duration of operation displayed significant heterogeneity. There was consistency in the direction and size of effect across the studies for all except symptom scores. This heterogeneity may be due to variations in the characteristics of the randomised participants, particularly differences in baseline prostate size and symptom score. It may also be due to differences in the specific aims and objectives of the trials, which led to important differences in inclusion criteria.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 13.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 4 (6) | 0.70a | –1.28 to 2.68 | 0.49 |
| 12 months | 4 (7) | 2.69a | 1.24–4.14 | < 0.001 |
| Longer term | 2 (3) | 4.51a | 2.04–6.97 | < 0.001 |
| Blood transfusion | 10 (10) | 0.11b | 0.04–0.26 | < 0.001 |
| Urinary retention | 3 (3) | 2.31b | 1.11–4.80 | 0.02 |
| Urinary tract infection | 8 (8) | 1.84b | 1.22–2.79 | 0.004 |
| Stricture | 5 (5) | 0.18b | 0.06–0.56 | 0.003 |
| TUR syndrome | 3 (3) | 0.23b | 0.04–1.34 | 0.10 |
| Incontinence | 5 (5) | 0.16b | 0.04–0.71 | 0.02 |
| Quality of life | ||||
| 3 months | 1 (2) | 1.40a | 0.55–2.25 | 0.001 |
| 12 months | 1 (3) | 1.60a | 0.92–2.28 | < 0.001 |
| Longer term | 1 (3) | 1.50a | 0.79–2.21 | < 0.001 |
| Qmax | ||||
| 3 months | 5 (8) | –5.36a | –7.28 to –3.45 | < 0.001 |
| 12 months | 4 (7) | –4.57a | –6.55 to –2.59 | < 0.001 |
| Longer term | 2 (3) | –0.76a | –5.30 to 3.77 | 0.74 |
| Duration of operation | 2 (5) | –12.24a | –16.78 to –7.69 | < 0.001 |
| Length of hospital stay | 2 (10) | –1.33a | –1.68 to –0.98 | < 0.001 |
| Reoperation | 9 (9) | 3.21b | 1.63–6.32 | 0.0008 |
Chapter 7 Clinical effectiveness of transurethral incision of the prostate
Transurethral incision of the prostate (TUIP) versus TURP
Characteristics of included studies
The baseline characteristics of the included studies are summarised in Table 14. A total of 871 participants were randomised across 11 eligible RCTs and reported in 14 papers. 135,149,152,157,180–186,238–240 The total number of people allocated to TUIP was 430 and the total allocated to TURP was 441.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Rodrigo Aliaga et al., 1998149 | TUIP | 20 | NR | 24.2 | 8.7 | 89 | 20–60b |
| TURP | 21 | NR | 24.4 | 8.3 | 146 | 20–60b | |
| Christensen et al., 1990135 | TUIP | 38 | 63c | 16d | 7.8 | NR | ≤ 20 |
| TURP | 38 | 62c | 16d | 9.7 | NR | ≤ 20 | |
| Dørflinger et al., 1992180 | TUIP | 29 | 69 | 15d | 10 | NR | ≤ 20 |
| TURP | 31 | 71 | 15d | 8 | NR | ≤ 20 | |
| Hellström et al., 1986157 | TUIP | 11 | 63 | NR | 8.6 | 62 | ≤ 30 |
| TURP | 13 | 59 | NR | 7.5 | 43 | ≤ 30 | |
| Jahnson et al., 1998181 | TUIP | 42 | 71 | 15.8d | 8.5 | 109 | 20–40 |
| TURP | 43 | 70 | 15.4d | 9.0 | 139 | 20–40 | |
| Li and Ng, 1987182 | TUIP | 29 | 65 | NR | NR | NR | ≤ 30 |
| TURP | 30 | 70 | NR | NR | NR | ≤ 30 | |
| Nielsen, 1988183 | TUIP | 25 | 73c | NR | 5c | NR | NR |
| TUIP | 24 | 69c | NR | 5c | NR | NR | |
| Riehmann et al., 1995152 | TUIP | 56 | 64 | 15.0d | 11 | NR | NR |
| TURP | 61 | 65 | 15.5d | 9 | NR | NR | |
| Saporta et al., 1996184 | TUIP | 20 | 66.8 | NR | NR | NR | ≥ 40 |
| TURP | 20 | 71.4 | NR | NR | NR | ≥ 40 | |
| Soonawalla and Pardanani, 1992185 | TUIP | 110 | 65.0 | NR | NR | NR | NR |
| TURP | 110 | 62.2 | NR | NR | NR | NR | |
| Tkocz and Prajsner, 2002186 | TUIP | 50 | 63 | 17.1 | 7.6 | 75 | 27.0 |
| TURP | 50 | 63 | 17.1 | 6.9 | 68 | 28.2 |
Two studies took place in the US,135,152 two in Denmark,180,183 and one each in Spain,149 Finland,157 Sweden,181 Hong Kong,182 India,185 Israel184 and Poland. 186 Three studies provided details on recruitment dates,135,152,181 with the earliest recruitment being January 1985152 and the latest August 1990.
In terms of symptom scores, two studies reported IPSS/AUA scores149,186 and four reported Madsen–Iversen scores. 135,152,180,181 Of the studies reporting IPSS/AUA scores, 50 participants allocated to TUIP had moderate symptoms of BPE and 20 had severe symptoms compared with 21 with severe and 50 with moderate symptoms among those allocated to TURP.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.5. The results of the meta-analyses are given in Appendix 9.5. Note that in terms of long-term evaluation, only the longest follow-up is presented.
Symptom scores
At 3 months
Of the 11 eligible RCTs, five reported IPSS/AUA or Madsen scores at 3 months, although for only one of these the data were reported in a way that was potentially amenable to analysis and there was no evidence of a statistically significant difference. Two tended to favour TUIP, one TURP and two showed no difference.
At 12 months
Data describing IPSS/AUA scores at 12 months were available for six trials but, again, only one provided means and standard deviations. Again, no clear pattern emerged: three tended to favour TURP, one TUIP and two showed no difference.
Longer-term follow-up
Losses to follow-up were high in nearly all studies reporting long-term follow-up. Only one study181 reported Madsen scores at 5 years following operation. No significant differences were observed between the TUIP and TURP groups (Appendix 8.5, Table 62). Data for other follow-up times (2 and 3 years) were also reported by Christensen and colleagues,135 Jahnson and colleagues,181 Riehmann and colleagues152 and Saporta and colleagues. 184 These can be seen in Appendix 8.5 and the respective forest plots in Appendix 9.5, comparison 05:02.
Complications
Data describing 18 types of complications are tabulated in Appendix 8.5, Table 63. Although some data were estimated from the reports of ten trials, data describing individual complications were available from more than half of the 11 trials for only five of the 18 complications. The reliability and usefulness of data for the other 13 were therefore very limited. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 15). Results for other complications are presented in Appendix 9.5, comparison 05:03. The results of these meta-analyses should be treated with caution as the length of follow-up of the RCTs varied. For urinary incontinence it was unclear if the type of incontinence considered was the same across all studies.
FIGURE 15.
Complications, TUIP vs TURP.

Blood transfusion
Seven studies149,157,180–183,185 provided information on blood transfusions. There were fewer blood transfusions following TUIP in all except one trial, which reported no transfusions in either group (Figure 15, comparison 05:03:01: 3/266 (11%) versus 77/272 (28%), RR 0.06, 95% CI 0.03–0.16, p < 0.001), reflecting particularly high rates of transfusion following TURP in four trials.
Urinary retention
Meta-analysis of data from four trials181–183,185 reporting urinary retention showed no statistically significant difference between the TUIP and TURP groups and wide confidence intervals (Figure 15, comparison 05:03:02: 10/206 versus 5/207, RR 1.84, 95% CI 0.70–4.86, p = 0.22). The direction of effect varied across studies with one trial favouring TUIP,181 two favouring TURP183,185 and one showing no difference. 182
Urinary tract infection
Only one study reported the incidence of urinary tract infections (including epididymo-orchitis) following surgery. 185 A total of five (4.5%) infections were reported amongst 110 participants allocated to TUIP compared with two (1.8%) infections amongst 110 allocated to TURP (Figure 15, comparison 05:03:03: RR 2.50, 95% CI 0.50–12.61, p = 0.27).
Stricture
Six studies provided data on strictures. 152,157,180,182,183,185 There was marked heterogeneity across the studies, with no clear pattern of results (Figure 15, comparison 05:03:04: 23/263 versus 17/265, RR 1.33, 95% CI 0.77–2.31, p = 0.30). The source of heterogeneity was uncertain, although the lack of separation between urethral stricture and bladder neck contracture may have been a factor as definitions of these conditions varied across the trials. In addition, the length of follow-up varied across studies.
TUR syndrome
TUR syndrome was reported in two studies. 182,185 No cases of a TUR syndrome were recorded in patients randomised to the TUIP arm. On the other hand, 6.4% of the patients (all in one trial) allocated to TURP had TUR syndrome (Figure 15, comparison 05:03:05: 0/139 versus 7/140, RR 0.07, 95% CI 0.00–1.15, p = 0.06).
Urinary incontinence
Meta-analysis of three trials that reported urinary incontinence showed no statistically significant difference between the TUIP and the TURP groups even though there were fewer events in the TUIP group (Figure 15, comparison 05:03:06: 3/163 versus 7/165, RR 0.47, 95% CI 0.14–1.65, p = 0.24). This result should be interpreted with caution as the length of follow-up varied, the types of incontinence were not fully described across studies and the confidence interval is wide.
Quality of life
Only one study186 reported quality of life of patients following surgery using the IPSS QoL (0–6) questionnaire. At 2 years, quality of life appeared to be marginally higher for those patients who underwent TURP (Appendix 9.5, comparison 05:08:01: WMD 0.20, 95% CI 0.01–0.39, p = 0.04).
Urodynamic outcomes
Data on peak urine flow rate, mean urine flow rate, total voided volume, residual volume and detrusor pressure were reported to a varying extent across eleven studies. 135,149,152,157,180–186 These are tabulated in Appendix 8.5, Table 65. Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 9.5, comparisons 05:05–05:07.
At 3 months
Nine studies135,149,152,157,180–183,185 provided peak urine flow rate measurements at 3 months for patients treated with TUIP and TURP (Appendix 8.5, Table 65). Seven studies135,152,157,180,181,183,185 showed that patients in the TURP group achieved a higher mean or median peak urine flow rate than patients in the TUIP group, and two studies149,182 showed a higher value in the TUIP group. Only three RCTs149,157,182 presented data that were sufficiently similar to allow quantitative synthesis (Appendix 9.5, comparison 05:05:01). Meta-analysis showed no statistically significant difference between the groups (WMD –0.07 ml/s, 95% CI –3.53 to 3.39, p = 0.97).
At 12 months
All six studies135,180,181,183–185 that provided information on the mean or median peak urine flow rate for patients 12 months after surgery reported lower mean or median peak urine flow rates following TUIP (Appendix 8.5, Table 65). Only one study184 reported data that were suitable for analysis (Appendix 9.5, comparison 05:05:03: MD –2.71 ml/s, 95% CI –5.77 to 0.35, p = 0.08).
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.5, Table 66. Information on duration of operation, length of hospital stay and reoperation rates was identified to a varying extent across the 11 eligible studies for this comparison.
Duration of operation
Seven studies152,157,180–183,185 provided information on the duration of operation (Appendix 8.5, Table 66). In all studies the duration of operation was shorter in the TUIP group. Only two studies157,182 presented data in a sufficiently similar form to allow quantitative synthesis (Appendix 9.5, comparison 05:09]; a TUIP procedure was 18.9 minutes shorter than TURP (95% CI –24.13 to –13.67, p < 0.001). This result was consistent with the other five studies reporting medians.
Length of hospital stay
Eight studies135,149,152,157,180,182,183,185 provided information on length of hospital stay (Appendix 8.5, Table 66). Despite marked differences between studies in overall length of stay, in six135,149,152,157,182,185 they reported it to be shorter for TUIP and in two 180,183 there was no difference. Two RCTs157,182 reported data that were suitable for synthesis. Across them, the average length of stay was significantly shorter in the TUIP group than in the TURP group (Appendix 9.5, comparison 05:10: WMD –2.26 days, 95% CI –3.81 to –0.71, p = 0.004). The within-trial differences in medians tended to be smaller than this.
Reoperation
Reoperations were reported in seven trials. 135,149,152,180,181,183,184 Reoperation was more common in the TUIP groups (17.5%) than in the TURP groups (9%) (Appendix 9.5, comparison 05:04:18: RR 1.87, 95% CI 1.16–3.03, p = 0.01). It should be noted that differences between studies in timing and completeness of follow-up might have introduced bias.
Summary and conclusions of the evidence for and against the intervention
This review considered data from 871 randomised participants across 11 RCTs of moderate to poor quality (and reporting). There is no evidence that the two interventions are different in terms of symptomatic outcome as no clear pattern emerged. The data indicate that, after TUIP, improvements in peak urine flow rate and quality of life are lower than after TURP, whereas the rate of blood transfusion and occurrence of TUR syndrome are higher after TURP than after TUIP. Urinary retention, urinary tract infection, strictures and incontinence do not appear to differ between the two approaches, although clinically important differences could not be ruled out. TUIP appears to be associated with shorter duration of operation and length of hospital stay but the reoperation rate is higher. It is important to note that the latest recruitment date was August 1990 and so the TURP outcomes then and now would not be comparable given the improvements in TURP technology over the past 16 years, reflected best by the higher transfusion rates reported in the seven trials included in this review of TUIP versus TURP.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 15. These should be interpreted in view of the comments mentioned earlier in this chapter.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 1 (1) | –0.50a | –3.35 to 2.35 | 0.73 |
| 12 months | 1 (1) | –1.00a | –1.73 to –0.27 | 0.007 |
| Longer term | NR | NR | NR | NR |
| Madsen–Iversen score | ||||
| 3 months | 0 (3) | NR | NR | NR |
| 12 months | 1 (5) | 0.34a | –1.55 to 2.23 | 0.72 |
| Longer term | 1 (3) | 1.21a | –0.87 to 3.29 | 0.26 |
| Blood transfusion | 7 (7) | 0.06b | 0.03–0.16 | < 0.001 |
| Urinary retention | 4 (4) | 1.84b | 0.70–4.86 | 0.22 |
| Urinary tract infection | 1 (1) | 2.50b | 0.50–12.61 | 0.27 |
| Stricture | 6 (6) | 1.33b | 0.77–2.31 | 0.30 |
| TUR syndrome | 2 (2) | 0.07b | 0.00–1.15 | 0.06 |
| Incontinence | 4 (4) | 0.47b | 0.14–1.65 | 0.24 |
| Quality of life | ||||
| 3 months | NR | NR | NR | NR |
| 12 months | NR | NR | NR | NR |
| Longer term | 1 | 0.20a | 0.01–0.39 | 0.04 |
| Qmax | ||||
| 3 months | 3 (9) | –0.07a | –3.53 to 3.39 | 0.97 |
| 12 months | 1 (6) | –2.71a | –5.77 to 0.35 | 0.08 |
| Longer term | 1 (2) | –1.71a | –4.74 to 1.32 | 0.27 |
| Duration of operation | 2 (7) | –18.90a | –24.13 to –13.67 | < 0.001 |
| Length of hospital stay | 2 (8) | –2.26a | –3.81 to –0.71 | 0.004 |
| Reoperation | 7 (7) | 1.87b | 1.16–3.03 | 0.01 |
Chapter 8 Clinical effectiveness of other ablative techniques
Interventions using laser technology
Holmium laser resection versus TURP
Characteristics of included studies
The characteristics of the included studies are summarised in Table 16. Five RCTs, reported in 15 papers,63,64,69,134,187–189,241–248 were eligible for this comparison, in which a total of 580 participants were randomised.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Gupta et al., 2006187 | Laser resection | 50 | 66 | 23.4 | 5.1 | 112 | 58 |
| TURP | 50 | 66 | 23.3 | 4.5 | 84 | 60 | |
| Kuntz et al., 200464 | Laser resection | 100 | 68 | 22.1 | 4.9 | 238 | 53 |
| TURP | 100 | 69 | 21.4 | 5.9 | 216 | 50 | |
| Montorsi et al., 2004188 | Laser resection | 52 | 65 | 21.6 | 8.2 | 4 | 70 |
| TURP | 48 | 64 | 21.9 | 7.8 | 4 | 56 | |
| Westenberg et al., 2004189 | Laser resection | 61 | 67 | 21.9 | 8.9 | 88 | 44 |
| TURP | 59 | 67 | 23.0 | 9.1 | 85 | 45 | |
| Wilson et al., 2006134 | Laser resection | 30 | 71 | 26.0 | 8.4 | 113 | 78 |
| TURP | 30 | 70 | 23.7 | 8.3 | 126 | 70 |
Two trials took place in New Zealand134,189 and one trial each in India,187 Italy188 and Egypt. 64 Recruitment dates were reported in all five studies and ranged from April 1996 to December 2003.
All five studies provided details of the participants’ IPSS/AUA symptom scores and prostate size, showing that all 580 participants had severe symptoms and large prostates at trial entry.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.6 and also in Figure 16. The results of the meta-analyses are given in Appendix 9.6. Note that in terms of long-term evaluation, only the longest follow-up is presented.
FIGURE 16.
Symptom scores, laser resection vs TURP.

Symptom scores
Out of the five eligible studies for this comparison, only two reported IPSS/AUA symptom scores at 3 months after surgery. 189,248 No statistically significant differences were observed between the two groups (Figure 16, comparison 06:01:01: WMD –0.47, 95% CI –1.92 to 0.98, p = 0.53).
Five trials reported IPSS/AUA scores measured within 12 months. Pooling of the data displayed statistically significantly lower scores for laser resection (Figure 16, comparison 06:01:03) with a WMD of –0.42 (95% CI –0.52 to –0.32, p < 0.00001). As there appeared to be heterogeneity present in this comparison, a random-effects model was applied. The WMD still favoured laser resection; however, the difference was no longer statistically significant (WMD –0.80, 95% CI –1.70 to 0.10, p = 0.08).
Figure 16, comparison 06:01:05 shows data from the single trial that compared IPSS scores of patients who underwent laser resection and TURP at follow-up after 2 and 4 years. There were lower scores for laser resection technology as opposed to TURP at both follow-ups, although this was not statistically significant. However, losses to follow-up were high at both time periods (Figure 16, comparison 06:01:05: MD –1.40, 95% CI –3.91 to 1.11, p = 0.27).
Complications
Data describing complications by study are given in Appendix 8.6, Table 68. In total, 12 categories of complications were identified across the five studies. These data are difficult to interpret. For seven of the complications, data were only available for one or two trials. Even for those complications more consistently reported, confidence intervals are wide and tend to include clinically important differences. Furthermore, the length of follow-up varied across the trials. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 17). Results for other complications are presented in Appendix 9.6, comparison 06:02.
FIGURE 17.
Complications, laser resection vs TURP.

In a meta-analysis of five studies64,187–189,248 patients allocated to laser resection were less likely to have a blood transfusion than those allocated to TURP (Figure 17, comparison 06:02:01: 1/293 versus 9/287, RR 0.27, 95% CI 0.07–0.95, p = 0.04).
All five studies provided details on the incidence of urinary retention after surgery. There were 15 (5.1%) reports of urinary retention amongst 293 participants allocated laser resections versus 21 (7.3%) amongst 287 participants allocated to TURP. The direction of effect varied across studies and the difference was not statistically significant (Figure 17, comparison 06:02:02: RR 0.71, 95% CI 0.38–1.32, p = 0.28).
There were five reports of urinary tract infection in each arm across two studies. 189,248 The direction of effect varied and the difference was not statistically significant (Figure 17, comparison 06:02:03: 5/91 versus 5/89, RR 0.98, 95% CI 0.31–3.09, p = 0.97).
Strictures were reported in all five studies. There were no statistically significant differences between the two arms in terms of the incidence of strictures after surgery (Figure 17, comparison 06:02:04: 15/287 versus 17/273, RR 0.84, 95% CI 0.43–1.65, p = 0.61).
Out of the five eligible studies, only one reported TUR syndrome. There were no cases of a TUR syndrome amongst 52 patients randomised to laser resection. In the TURP arm, one event (2%) was recorded amongst 48 randomised patients (Figure 17, comparison 06:02:05: RR 0.31, 95% CI 0.01–7.39, p = 0.47).
Meta-analysis of four trials64,187–189 showed no difference in the risk of developing urinary incontinence following laser resection compared with the risk for those allocated to TURP (Figure 17, comparison 06:02:06: 55/252 versus 54/253, RR 0.97, 95% CI 0.72–1.31, p = 0.83). This result should be interpreted with caution as the length of follow-up varied and the type of incontinence was not fully described across studies.
Quality of life
Three studies134,188,189 reported quality of life of patients following surgery. The quality of life was assessed using the IPSS QoL (0–6) questionnaire (Figure 18).
FIGURE 18.
Quality of life, laser resection vs TURP.

Meta-analysis of data from two studies134,189 showed no statistically significant difference between holmium laser resection and TURP (Figure 18, comparison 06:08:01: WMD –0.19, 95% CI –0.68 to 0.30, p = 0.45).
At 12 months, evidence from three studies134,188,189 showed marked heterogeneity present in the meta-analysis and the direction of effect was not consistent. In two studies the total number of participants available for quality of life evaluation was unclear and therefore this result should be treated with further caution.
Based on only one trial,189 quality of life appeared to be similar in the laser group when compared with TURP at 2 and 4 years after surgery (Figure 18, comparison 06:08:06). A further caution is that the total number of participants available for this follow-up assessment was unclear.
Urodynamic outcomes
Data on peak urine flow rate, mean urine flow rate, residual volume, detrusor pressure and prostate size were reported to a varying extent across the five studies. 64,134,187–189 Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 8.6, Table 69 and Appendix 9.6. comparisons 06:03–06:07.
Out of the total of five studies, two134,189 reported peak urine flow rate at the 3-month follow-up. Laser resection was associated with a higher peak urine flow rate (Appendix 9.6, comparison 06:03:01: WMD 3.49 ml/s, 95% CI 0.63–6.35, p = 0.02).
Again, meta-analysis of five studies64,134,187–189 reporting peak urine flow rate showed higher peak urine flow rates for laser resection at 12 months after surgery (WMD 1.43, 95% CI 0.92–1.93, p < 0.001).
Only one study189 reported peak urine flow rates at 4 years after the initial operation and this was based on about 60% of the original participants. No statistically significant difference was observed in this outcome between the two groups but the confidence interval was wide (Appendix 9.6, comparison 06:03:06: WMD 3.80, 95% CI –1.36 to 8.96, p = 0.15).
Descriptors of care
Data describing selected aspects of care are tabulated in Appendix 8.6, Table 70. Information on duration of operation, length of hospital stay and reoperation rates was identified across five eligible studies for this comparison.
The duration of a laser resection intervention was found to be on average 17 minutes longer than a TURP intervention (Appendix 9.6, comparison 06:10: 95% CI 13.45–20.47, p < 0.001). The direction and size of effect were consistent across studies.
Across the five studies the average length of stay was significantly shorter in the laser resection group than in the TURP group (Appendix 9.6, comparison 06:11: WMD –1.05 days, 95% CI –1.20 to –0.89, p < 0.001). The direction and size of effect were also consistent across studies.
Reoperations were reported in four trials. 64,188,189,248 No statistically significant differences were observed (Appendix 9.6, comparison 06:02:12: 10/231 versus 15/232, RR 0.68, 95% CI 0.32–1.44, p = 0.31).
Summary and conclusions of the evidence for and against the intervention
Five RCTs of moderate quality involving 580 participants were available to compare laser resection with TURP. In terms of symptom scores, laser resection appeared to be better than TURP; however, this difference was only statistically significant at 12 months when a complete data set involving all 580 participants was available. The data also indicate that peak urine flow rate was better after laser resection than after TURP at 3 and 12 months after the interventions. Although these results are statistically significant, the difference is small and therefore may not be clinically relevant. The rate of blood transfusion for laser resection was lower. The occurrence of urinary retention, urinary tract infection, stricture, TUR syndrome, urinary incontinence and reoperation was similar but with wide confidence intervals. Quality of life does not appear to differ between the two groups and there is good evidence that laser resection is associated with longer duration of operation but shorter length of hospital stay.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 17. These should be interpreted in view of the comments mentioned earlier in this chapter.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 2 (2) | –0.47a | –1.92 to 0.98 | 0.53 |
| 12 months | 5 (5) | –0.42a | –0.5 to –0.32 | < 0.001 |
| Longer term | 1 (1) | –1.40a | –3.9 to 1.11 | 0.27 |
| Blood transfusion | 5 (5) | 0.27b | 0.07–0.95 | 0.04 |
| Urinary retention | 5 (5) | 0.71b | 0.38–1.31 | 0.28 |
| Urinary tract infection | 2 (2) | 0.98b | 0.31–3.09 | 0.97 |
| Stricture | 5 (5) | 0.84b | 0.43–1.65 | 0.61 |
| TUR syndrome | 1 (1) | 0.31b | 0.01–7.39 | 0.47 |
| Incontinence | 4 (4) | 0.97b | 0.72–1.31 | 0.83 |
| Quality of life | ||||
| 3 months | 2 (2) | –0.19a | –0.6 to 0.30 | 0.45 |
| 12 months | 3 (3) | 0.06a | –0.2 to 0.38 | 0.73 |
| Longer term | 1 (1) | –0.30a | –0.9 to 0.30 | 0.33 |
| Qmax | ||||
| 3 months | 2 (2) | 3.49a | 0.63–6.35 | 0.02 |
| 12 months | 5 (5) | 1.43a | 0.92–1.93 | < 0.001 |
| Longer term | 1 (1) | 3.80a | –1.3 to 8.96 | 0.15 |
| Duration of operation | 5 (5) | 16.96a | 13.45–20.47 | < 0.001 |
| Length of hospital stay | 4 (4) | –1.05a | –1.2 to –0.89 | < 0.001 |
| Reoperation | 4 (4) | 0.68b | 0.32–1.44 | 0.31 |
Laser vaporisation versus TURP
Characteristics of included studies
The baseline characteristics of the included studies are summarised in Table 18. A total of 854 participants were randomised across 11 eligible RCTs reported in 27 papers. 121,127,141,146,148,153,164,179,190–193,249–263 The total number of people allocated to laser vaporisation was 425 and the total allocated to TURP was 429.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Bouchier-Hayes et al., 2006141 | Laser vaporisation | 38 | 65 | NR | NR | NR | 42 |
| TURP | 38 | 66 | NR | NR | NR | 33 | |
| Carter et al., 1999127 | Laser vaporisation | 95 | 68 | 20.3 | 9.0 | 109 | 42 |
| TURP | 96 | 67 | 19.8 | 9.5 | 135 | 42 | |
| Keoghane et al., 2000164 | Laser vaporisation | 72 | 69 | 19.9 | 11.8 | NR | 55 |
| TURP | 79 | 70 | 19.4 | 11.4 | NR | 52 | |
| Mottet et al., 1999190 | Laser vaporisation | 17 | 64 | 21.7 | 8.8 | NR | 37 |
| TURP | 13 | 67 | 23.7 | 7.7 | NR | 34 | |
| Sengor et al., 1996192 | Laser vaporisation | 30 | 61 | 21.8 | 8.7 | 110 | NR |
| TURP | 30 | 66 | 22.1 | 8.4 | 155 | NR | |
| Shingleton et al., 2002146 | Laser vaporisation | 50 | 68 | 22 | NR | NR | 32 |
| TURP | 50 | 67 | 21 | NR | NR | 30 | |
| Suvakovic and Hindmarsh,1996179 | Laser vaporisation | 10 | 63 | 18.0 | 12.2 | 140 | 24 |
| TURP | 10 | 66 | 18.8 | 11.1 | 162 | 22 | |
| Tuhkanen et al., 2001191 | Laser vaporisation | 21 | 67b | 23b,c | 7.2 | 138 | 55 |
| TURP | 25 | 67b | 19b,c | 8.5 | 125 | 55 | |
| Tuhkanen et al., 2003153 | Laser vaporisation | 26 | 68b | 18b,c | 8.3b | 87b | 30b |
| TURP | 26 | 67b | 18b,c | 8.6b | 83b | 28b | |
| van Melick et al., 2003193 | Laser vaporisation | 45 | 67 | 18.9 | 12.0 | 300 | 37 |
| TURP | 50 | 66 | 16.8 | 11.0 | 350 | 37 | |
| Zorn et al., 1999148 | Laser vaporisation | 21 | 71 | 24.0 | 8.7 | NR | 30 |
| TURP | 12 | 69 | 24.7 | 9.0 | NR | 34 |
Three studies took place in the UK,127,164,179 two each in the US146,148 and Finland,153,191 and one each in Australia,141 France,190 Turkey192 and the Netherlands. 193 All but two studies146,179 provided details on recruitment dates, with the earliest being January 1993164 and the latest in January 2004. 141
In terms of symptom scores, all but three studies141,153,191 reported IPSS/AUA scores. Of the studies reporting baseline IPSS/AUA scores, 285 (84%) participants allocated to laser vaporisation had severe symptoms of BPE and 55 (16%) had moderate symptoms compared with 201 (59%) with severe and 155 (46%) with moderate symptoms allocated to TURP.
Of the studies reporting prostate size, 226 (57%) participants allocated to laser vaporisation had large prostates, 112 (28%) had moderate-sized prostates and 10 (2%) had small prostates. In the TURP arm, 200 (51%) had large prostates, 113 (29%) had moderate-sized prostates and 86 (22%) had small prostates.
Assessment of effectiveness
As discussed in Chapter 2 there are several laser devices that can be used to vaporise the prostate. The most commonly used are Nd:YAG, holmium:YAG and KTP lasers. These can be used either alone or in combination (hybrid laser). For analysis purposes, trials reporting a vaporisation technique were combined, regardless of the method/devices used.
Symptom scores
Of the 11 eligible studies, only five provided details on IPSS/AUA scores at 3 months following surgery. 146,164,179,190,192 Meta-analysis of three of these trials164,179,192 is marked by considerable heterogeneity in which the direction of effect and effect sizes vary across studies with one study favouring TURP. 164 The source of heterogeneity is unclear; however, it may be due to different levels of energy delivery across studies. Moreover, there is variation in the prostate size of patients measured before surgery. On average, patients included in the Oxford laser trial164 exhibited large prostates whereas those included in the trial by Suvakovic and Hindmarsh had small prostates. 179 Sengor and colleagues192 did not provide details on baseline prostate size.
At 12 months, all but one study193 out of eight favoured TURP. Pooling the data of three studies amenable to meta-analysis showed statistically significant better IPSS/AUA scores in support of TURP. However, confidence intervals were wide, there was evidence of heterogeneity and the trials included a small number of participants (Figure 19, comparison 07:01:03: WMD 1.30, 95% CI 0.12–2.47, p = 0.03).
FIGURE 19.
Symptom scores, laser vaporisation vs TURP.
At 5 years, combining data from three trials gave higher (poorer) scores for laser vaporisation than for TURP (Figure 19, comparison 07:01:06: WMD 2.42, 95% CI 0.08–4.75, p = 0.04).
Complications
Data describing complications by study are given in Appendix 8.7, Table 72. Information from one or more of the 11 trials was available for 17 complications. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 20). Results for other complications are presented in Appendix 9.7, comparison 07:02. The results of these meta-analyses should be treated with caution as the length of follow-up of the RCTs varied.
FIGURE 20.
Complications, laser vaporisation vs TURP.

In the ten studies127,141,146,148,153,164,190–193 that reported blood transfusion there was only one transfusion amongst 374 laser patients versus 24 amongst 415 TURP patients (Figure 20, comparison 07:02:01: RR 0.14, 95% CI 0.05–0.42, p = 0.0004).
In six studies 127,146,164,190,191,193 a total of 32 (10.5%) cases of urinary retention amongst 304 patients allocated to laser vaporisation versus 11 (3.6%) cases amongst 306 TURP patients were reported (Figure 20, comparison 07:02:02: RR 2.89, 95% CI 1.55–5.42, p = 0.0009).
Meta-analysis of data from four studies127,153,164,193 indicated fewer episodes of urinary tract infection following TURP with an RR of 1.63 (95% CI 0.99–2.69, p = 0.05). However, this result depends entirely on data from the study by Carter and colleagues,127 as epididymitis and prostatitis are reported as well as simple urinary tract infections that occurred in the early postoperative period. When only epididymitis and prostatitis are considered, the difference in rates observed in the laser vaporisation and TURP groups is no longer statistically significant (RR 1.17, 95% CI 0.60–2.26, p = 0.32).
The incidence of strictures for those who underwent laser vaporisation and TURP was available from nine studies. 127,141,146,153,164,190–193 The proportion of people who developed strictures appeared to be lower following laser vaporisation than following TURP. The pooled RR of strictures among laser patients compared with TURP patients was 0.54 (Figure 20, comparison 07:02:04: 13/350 versus 27/353, 95% CI 0.32–0.90, p = 0.02). It should be noted that eight of the 13 strictures and 11 of the 27 strictures observed in the laser vaporisation and TURP groups, respectively, were actually bladder neck contractures.
There were no cases of TUR syndrome amongst 161 patients allocated to laser vaporisation compared with one amongst 122 patients allocated to TURP (Figure 20, comparison 07:02:05: RR 0.33, 95% CI 0.01–7.93, p = 0.50).
Taken together, data from five trials suggest a higher rate of incontinence following laser vaporisation (Figure 20, comparison 07:02:06: 16/272 versus 7/285, RR 2.24, 95% CI 1.03–4.88, p = 0.04). However, this result depended on a single trial193 in which rates were high in both groups but particularly following laser vaporisation. This result should also be treated with caution because the length of follow-up varied and the definition of incontinence was not fully described in the studies.
Quality of life
Three studies193,249,250 using a variety of methods reported quality of life of patients following surgery (Appendix 8.7, Table 73). In one study193 the quality of life was assessed using the disease-specific IPSS QoL (0–6) questionnaire. In another study249 the generic quality of life measure Medical Outcomes Study 36-item Short Form Health Study (SF-36) was used. In the third study250 quality of life was measured using two distinct instruments: SF-36 and EuroQol Five Dimensions (EQ-5D) (scored using the UK tariffs).
At 3 months there appeared to be little change in quality of life as a consequence of either surgical intervention, irrespective of which quality of life tool was used. 250 No differences in quality of life were detected in two studies193,250 at 12 months.
Urodynamic outcomes
Data on peak urine flow rate, mean urine flow rate, residual volume, detrusor pressure and prostate size were reported to a varying extent across eight studies. 127,146,148,153,164,190–192 Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 8.7, Table 74 and Appendix 9.7, comparisons 07:04–07:08.
Six studies146,153,164,190–192 provided details on peak urine flow rate for patients at 3 months after surgery. Only four,146,153,164,192 however, presented data that were sufficiently similar to allow quantitative synthesis. The WMD was 1.76 ml/s, lower (worse) for laser vaporisation (Appendix 9.7, comparison 07:04:01: 95% CI 0.57–2.94, p = 0.004). This result was consistent with that reported by Tuhkanen and colleagues191 but not with the small study reported by Mottet and colleagues. 190
Five studies127,146,148,164,190 provided details on peak urine flow rate at 12 months after surgery. Only two,146,164 however, presented data that were sufficiently similar to allow quantitative synthesis. The WMD was 2.02 ml/s, lower (worse) for laser vaporisation (Appendix 9.7, comparison 07:04:03: 95% CI 0.71–4.75, p = 0.15). With regard to the studies in which data were not amenable to meta-analysis, two127,148 favoured TURP and one190 favoured laser vaporisation.
Meta-analysis of data from two146,164 studies reporting 5-year data showed no statistically significant difference between the two groups (Appendix 9.7, comparison 07:04:06: WMD 0.28, 95% CI 1.76–2.32, p = 0.79). Loss to follow-up was high in both trials.
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.7, Table 75. Information on duration of operation, length of hospital stay and reoperation rates was identified across the eligible studies for this comparison.
A total of nine studies127,148,153,164,179,190–193 provided information on duration of operation. In three studies164,179,192 the mean duration of operation was shorter in the laser group and in one193 there were no differences between the two groups. Meta-analysis of four studies with suitable data showed a non-statistically significant difference between laser vaporisation and TURP (Appendix 9.7, comparison 07:11: WMD 0.29, 95% CI –2.19 to 2.78, p = 0.82).
Length of hospital stay was reported in eight studies, with six favouring the laser vaporisation group and two favouring TURP. 153,191 Only one study reported means and standard deviations141,193 and meta-analysis suggested that there was no evidence of a difference between the two groups (Appendix 9.7, comparison 07:12).
Reoperations were reported in nine trials. 141,146,148,153,164,190,191,193,249 Reoperation was more common in the laser vaporisation group (9.3%) than in the TURP groupt (5.4%) (Appendix 9.7, comparison 07:03:17: RR 1.60, 95% CI 0.97–2.63, p = 0.06). It should be noted that differences between studies in timing and completeness of follow-up might have introduced bias.
Summary and conclusions of the evidence for and against the intervention
A total of 854 participants were randomised across 11 eligible studies of generally moderate quality. At 12 months or longer, the data indicated that symptom scores were worse after laser vaporisation than after TURP. There was a tendency for peak urine flow rate to favour TURP but this was only statistically significant at the 3- and 12-month follow-up assessments. The differences observed for both symptom scores and peak urine flow rate, although statistically significant, may not be clinically relevant or appreciable by patients. The occurrence of complications such as urinary retention, urinary tract infection and incontinence was higher for laser vaporisation than for TURP. However, blood transfusion and the incidence of strictures were lower. The duration of operation and length of hospital stay did not appear to differ between the two approaches.
The results for symptom scores displayed significant heterogeneity and there was a lack of consistency in the direction and size of effect across studies. Much of the variation might be due to differences in the specific aims and objectives of the trials.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 19. These should be interpreted in view of all of the comments mentioned earlier in this chapter.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 3 (8) | –0.01a | –1.39 to 1.36 | 0.98 |
| 12 months | 4 (9) | 1.30a | 0.12–2.47 | 0.03 |
| Longer term | 2 (3) | 2.42a | 0.08–4.75 | 0.04 |
| Blood transfusion | 10 (10) | 0.14b | 0.05–0.42 | < 0.001 |
| Urinary retention | 6 (6) | 2.89b | 1.55–5.42 | < 0.001 |
| Urinary tract infection | 4 (4) | 1.63b | 0.99–2.69 | 0.05 |
| Stricture | 9 (9) | 0.54b | 0.32–0.90 | 0.02 |
| TUR syndrome | 3 (3) | 0.33b | 0.01–7.93 | 0.50 |
| Incontinence | 5 (5) | 2.24b | 1.03–4.88 | 0.04 |
| Quality of life | ||||
| 3 months | 0 (2) | NR | NR | NR |
| 12 months | 1 (3) | 0.00a | –0.40 to 0.40 | 1.00 |
| Longer term | 1 (1) | 0.10a | –0.77 to 0.97 | 0.82 |
| Qmax | ||||
| 3 months | 4 (6) | –1.76a | –2.94 to –0.57 | 0.004 |
| 12 months | 2 (5) | –2.02a | –4.75 to 0.71 | 0.15 |
| Longer term | 2 (3) | –0.28a | –2.32 to 1.76 | 0.79 |
| Duration of operation | 4 (9) | 0.29a | –2.19 to 2.78 | 0.82 |
| Length of hospital stay | 2 (9) | –1.39a | –1.69 to –1.10 | < 0.001 |
| Reoperation | 9 (9) | 1.68b | 1.03–2.74 | 0.04 |
Interventions using non-laser technology
Transurethral vaporesection of the prostate (TUVRP) versus TURP
Characteristics of included studies
The characteristics of the included studies are summarised in Table 20. Five RCTs68,132,187,202,203 were eligible for this comparison, randomising a total of 271 men to TUVRP and 258 to TURP.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Helke et al., 2001202 | TUVRP | 93 | 69 | 17.3 | 10.8 | 76 | 49 |
| TURP | 92 | 67 | 18.3 | 8.5 | 102 | 50 | |
| Kupeli et al., 2001203 | TUVRP | 50 | 61 | 21.6 | 9.2 | NR | 57 |
| TURP | 50 | 59 | 19.4 | 7.9 | NR | 58 | |
| Gupta et al., 2006187 | TUVRP | 50 | 68 | 24.9 | 4.6 | 103 | 63 |
| TURP | 50 | 66 | 23.3 | 4.5 | 84 | 60 | |
| Liu et al., 2006132 | TUVRP | 44 | 66 | 25.6 | 6.9 | 131 | 58 |
| TURP | 32 | 65 | 26.8 | 6.9 | 142 | 60 | |
| Talic et al., 200068 | TUVRP | 34 | 71 | 24.9 | 7.5 | NR | 57 |
| TURP | 34 | 70 | 20.1 | 9.1 | NR | 52 |
Single studies took place in India,187 Taiwan,132 Turkey,203 Saudi Arabia68 and Germany. 202 Three studies provided details of recruitment dates132,187,203 with the earliest in November 1997203 and the latest in December 2003. 187
In terms of baseline IPSS/AUA scores, the total numbers of participants with moderate and severe symptoms who were allocated to TUVRP were 93 (34%) and 178 (66%) respectively. The equivalent figures in the TURP group were 142 (55%) and 116 (45%).
All studies reported prostate size, with all 529 participants having large prostates.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.8. The results of the meta-analyses are given in Appendix 9.8. Note that in terms of long-term evaluation, only the longest follow-up is presented.
Symptom scores
At 3 months after surgery, IPSS/AUA scores were reported in three of the five eligible studies. 132,202,203 Two of the three studies reported no statistically significant differences between TUVRP and TURP (Appendix 8.8, Table 76), whereas in the third study, reporting means and standard deviations, the mean difference was 0.30 (Figure 21, comparison 08:01:01: 95% CI 0.63–1.23, p = 0.53).
FIGURE 21.
Symptom scores, TUVRP vs TURP.

Evidence from two studies showed no statistically significant differences in IPSS/AUA scores at 12 months after TUVRP and TURP (Figure 21, comparison 08:01:03: WMD –0.59, 95% CI –1.40 to 0.23, p = 0.16).
IPSS/AUA scores at 2 years after surgery were provided in one trial. Again, no statistically significant differences were observed between TUVRP and TURP (Figure 21, comparison 08:01:04: WMD 0.60, 95% CI –1.09 to 2.29, p = 0.49).
Complications
The list of complications by study is detailed in Appendix 8.8, Table 77. Data describing 12 types of complications were variably reported across the five studies. The data were too few to provide precise estimates of differences and all confidence intervals were wide, such that clinically important differences could not be ruled out. None of the complications proved to be significantly different between TUVRP and TURP. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 22). Results for other complications are presented in Appendix 9.8, comparison 08:02.
FIGURE 22.
Complications, TUVRP vs TURP.

All five trials provided information on blood transfusions. 68,132,187,202,203 A total of seven (2.3%) patients required a blood transfusion following TUVRP as opposed to 12 (4.6%) patients following TURP (Figure 22, comparison 08:02:01: RR 0.57, 95% CI 0.24–1.36, p = 0.20).
There were six cases of urinary retention amongst 144 patients randomised to TUVRP versus seven cases of urinary retention amongst 132 patients randomised to TURP (Figure 22, comparison 08:02:02: RR 0.72, 95% CI 0.26–2.05, p = 0.54). 132,187,203
No studies reported this outcome.
Four studies132,187,202,203 reported the incidence of strictures postoperatively. Meta-analysis showed no statistically significant differences between TUVRP and TURP (Figure 22, comparison 08:02:03: 9/229 versus 11/218, RR 0.75, 95% CI 0.32–1.77, p = 0.51).
Quality of life
Only one study132 reported quality of life of patients following TUVRP or TURP (Appendix 8.8, Table 78). Quality of life was assessed using the IPSS QoL (0–6) questionnaire. At 3 months and 2 years there appeared to be little difference in quality of life between the groups as a consequence of either surgical intervention (Appendix 9.8, comparison 08:05).
Urodynamic outcomes
Data on peak urine flow rate and prostate size were reported across five studies. 68,132,187,202,203 These are tabulated in Appendix 8.8, Table 79. Only peak urine flow rate is presented in this section.
Two studies132,202 provided details on peak urine flow rate for patients at 3 months after surgery. In one trial132 the mean difference was 0.90 ml/s for TUVRP versus TURP (Appendix 9.8, comparison 08:03:01: 95% CI –0.04 to 1.84, p = 0.06). Helke and colleagues202 reported a non-statistically significant difference between the two groups.
Two studies,187,202 provided details on peak urine flow rate at 12 months after surgery. The WMD was 0.10 ml/s for TUVRP versus TURP (Appendix 9.8, comparison 08:03:03: 95% CI –0.41 to 0.61, p = 0.70).
One study132 provided results beyond 12 months (2 years). At this time point there was a non-statistically significant difference between TUVRP and TURP (Appendix 9.8, comparison 08:03:04: WMD 1.60, 95% CI –0.30 to 3.50, p = 0.10).
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.8, Table 80. Information on duration of operation, length of hospital stay and reoperation rates was identified to a varying extent across the five eligible studies for this comparison.
Three studies68,132,187 provided information on the duration of operation. The results were not statistically significant (Appendix 9.8, comparison 08:06: WMD –1.91, 95% CI –8.80 to 5.07, p = 0.59).
Only one study132 provided information on length of hospital stay (Appendix 8.8, Table 80). The mean difference was less than a day (MD 0.41 days), favouring TUVRP. This was statistically significant (Appendix 9.8, comparison 08:07: 95% CI –0.54 to –0.28, p < 0.001).
Two studies132,202 provided information on reoperation rates. Reoperation rates appeared to be higher in the TUVRP group (11.6%) than in the TURP group (5.9%). This difference, however, did not reach statistical significance (Appendix 9.8, comparison 08:02:13: RR 1.90, 95% CI 0.80–4.52, p = 0.15).
Summary and conclusions of the evidence for and against the intervention
This review considered data from over 500 randomised participants across five RCTs of generally moderate to low quality (and reporting). The data suggest that symptom scores, quality of life and peak urine flow rate do not differ between TUVRP and TURP. The incidence of blood transfusion, urinary retention, strictures, TUR syndrome and urinary incontinence was also similar in the two groups. The duration of operation and reoperation rates were also statistically similar in both groups; however, length of hospital stay was slightly shorter for TUVRP than it was for TURP.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 21. These should be interpreted in view of the comments mentioned earlier in this chapter.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 1 (3) | 0.30a | –0.63 to 1.23 | 0.53 |
| 12 months | 2 (2) | –0.28a | –1.01 to 0.45 | 0.45 |
| Longer term | 1 (1) | 0.60a | –1.09 to 2.29 | 0.49 |
| Blood transfusion | 5 (5) | 0.57b | 0.24–1.36 | 0.20 |
| Urinary retention | 3 (3) | 0.72b | 0.26–2.05 | 0.54 |
| Stricture | 4 (4) | 0.75b | 0.32–1.77 | 0.51 |
| TUR syndrome | 3 (3) | 0.15b | 0.01–2.95 | 0.21 |
| Incontinence | 4 (4) | 0.85b | 0.45–1.61 | 0.62 |
| Quality of life | ||||
| 3 months | 1 (1) | 0.20a | –0.09 to 0.49 | 0.18 |
| Longer term | 1 (1) | 0.20a | –0.19 to 0.59 | 0.31 |
| Qmax | ||||
| 3 months | 1 (2) | –0.90a | –1.84 to 0.04 | 0.06 |
| 12 months | 2 (2) | 0.10a | –0.41 to 0.61 | 0.70 |
| Longer term | 1 (1) | –1.60a | –3.50,0.30 | 0.10 |
| Duration of operation | 2 (2) | 1.06a | –8.70 to 10.83 | 0.83 |
| Length of hospital stay | 1 (1) | –0.41a | –0.54 to –0.28 | < 0.001 |
| Reoperation | 2 (2) | 1.90b | 0.80–4.52 | 0.15 |
Bipolar transurethral resection of the prostate (B-TURP) versus TURP
Characteristics of included studies
The characteristics of the included studies are summarised in Table 22. Six RCTs65,147,150,161,194,195 were eligible for this comparison, in which a total of 386 participants were randomised, 192 to B-TURP and 194 to conventional TURP.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| de Sio et al., 200665 | B-TURP | 35 | 59 | 24.2 | 7.1 | 80 | 52 |
| TURP | 35 | 61 | 24.3 | 6.3 | 75 | 47 | |
| Kim et al., 2006150 | B-TURP | 25 | 68 | 19.0 | 6.5 | NR | 53 |
| TURP | 25 | 71 | 18.6 | 6.1 | NR | 52 | |
| Nuhoğlu et al., 2006194 | B-TURP | 27 | 65 | 17.6 | 6.9 | 96 | 47 |
| TURP | 30 | 65 | 17.3 | 7.3 | 88 | 49 | |
| Seckiner et al., 2006161 | B-TURP | 24 | 61 | 24.1 | 8.5 | 88 | 49 |
| TURP | 24 | 64 | 23.2 | 8.3 | 138 | 41 | |
| Singh et al., 2005147 | B-TURP | 30 | 69 | 20.5 | 5.8 | 124 | NR |
| TURP | 30 | 68 | 21.6 | 5.1 | 136 | NR | |
| Tefekli et al., 2005195 | B-TURP | 51 | 69 | NR | NR | NR | 54 |
| TURP | 50 | 69 | NR | NR | NR | 50 |
Three trials took place in Turkey161,194,195 and one each took place in India,147 Korea150 and Italy. 65 Five studies provided details of recruitment dates147,150,161,194,195 with the earliest in 2001194,195 and the latest in October 2004. 150
All but one study195 provided details of participants’ IPSS/AUA scores at baseline, showing that 89 were severely symptomatic in each arm and 52 and 55 moderately symptomatic in the B-TURP and conventional TURP arms respectively.
Of the studies reporting prostate size,65,147,161,194 all participants had large prostates.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.9. The results of the meta-analyses are given in Appendix 9.9. Note that in terms of long-term evaluation, only the longest follow-up is presented here.
Symptom scores
Data were available for only one161 of five eligible trials. No differences in IPSS/AUA scores were observed between B-TURP and conventional TURP 3 months after surgery (Figure 23, comparison 09:01:01).
FIGURE 23.
Symptom scores, B-TURP vs TURP.

Of the three trials65,161,194 providing information on IPSS/AUA scores, two161,194 provided data that were suitable for meta-analysis. The improvement in symptoms in patients undergoing B-TURP was similar to that observed in conventional TURP patients (Figure 23; comparison 09:01:03: WMD 0.29, 95% CI –1.12 to 1.71, p = 0.69). This result is consistent with that observed in the study by de Sio and colleagues. 65
Complications
The list of complications by study is detailed in Appendix 8.9, Table 82. Data describing nine complications were reported for one or more studies. The data were too few to provide precise estimates of differences and all confidence intervals were wide, such that clinically important differences could not be ruled out. Meta-analyses of the complications showed non-statistically significant differences between B-TURP and conventional TURP. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 24). Results for other complications are presented in Appendix 9.9, comparison 09:02.
FIGURE 24.
Complications, B-TURP vs TURP.

Quality of life
Three studies65,147,161 reported quality of life of patients following surgery using the IPSS QoL (0–6) questionnaire, but only one study161 presented data in a form that would allow quantitative synthesis. No statistically significant differences in quality of life were observed between B-TURP and conventional TURP at either the 3- or the 12-month follow-up (Appendix 9.9, comparison 09:07). This result is consistent with that reported by the studies that were not amenable to analysis.
Urodynamic outcomes
Data on peak urine flow rate, mean urine flow rate, residual volume and prostate size were reported to a varying extent across four studies150,161,194,195 and are tabulated in Appendix 8.9, Table 84. Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 9.9, comparisons 09:03–09:06.
Two studies161,195 provided details on peak urine flow rate for patients at 3 months after surgery. Across them, the average peak urine flow rate in the bipolar arm was not statistically significantly different from that observed in the conventional TURP arm (Appendix 9.9, comparison 09:03:01: WMD –0.98, 95% CI –2.25 to 0.29, p = 0.13).
Three studies161,194,195 provided details on peak urine flow rate for patients at 12 months after surgery. There were no statistically significant differences between the two groups (Appendix 9.9, comparison 09:03:03: WMD 0.01, 95% CI –1.10 to 1.08, p = 0.98).
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.9, Table 85. Information on duration of operation and reoperations was identified to a varying extent across the eligible studies for this comparison.
Five studies provided information on duration of operation, with the results being inconsistent (highly significant heterogeneity). Two161,194 suggested no difference between the groups whereas, in another three,65,150,195 a 3-, 5- and 17-minute difference in the mean length of operation, respectively, was observed, favouring B-TURP (Appendix 8.9, Table 85). Four studies provided data that were amenable to quantitative synthesis. Fitting a random-effects model resulted in a WMD of –4.56 minutes in favour of B-TURP. This result was not statistically significant (95% CI –15.36 to 6.23, p = 0.41).
Evidence from two studies65,150 suggests that the average length of stay following B-TURP was shorter than the average length of stay following TURP. The mean difference was –0.7 days (Appendix 8.9, Table 85; Appendix 9.9, comparison 09:10: 95% CI –01.37 to –0.03, p = 0.04).
Three studies65,194,195 provided information on reoperation rates. No differences were observed between the two groups (Appendix 9.9, comparison 09:02:09: 3/111 versus 2/112, RR 1.46, 95% CI 0.25–8.57, p = 0.67). The time point of reoperation was unclear and the length of follow-up across studies ranged from 12 to 18 months.
Summary and conclusions of the evidence for and against the intervention
A total of 386 participants randomised to undergo B-TURP or conventional TURP across six RCTs of moderate to low quality were considered for this review. The data were too few to provide precise estimates for all of the outcomes considered and statistically significant differences could not be detected, with the exception of duration of operation, which bears significant heterogeneity and is of doubtful clinical or economic importance. A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 23. These should be interpreted in view of the comments mentioned earlier in this chapter.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 1 (3) | –1.30a | –4.26 to 1.66 | 0.39 |
| 12 months | 2 (3) | 0.29a | –1.12 to 1.71 | 0.69 |
| Longer term | NR | NR | NR | NR |
| Blood transfusion | 3 (3) | 1.03b | 0.24–4.49 | 0.96 |
| Urinary retention | 2 (2) | 1.71b | 0.24–12.38 | 0.60 |
| Urinary tract infection | 1 (1) | 1.00b | 0.07–15.12 | 1.00 |
| Stricture | 4 (4) | 1.38b | 0.45–4.26 | 0.33 |
| TUR syndrome | 2 (2) | NE | NE | NE |
| Incontinence | 2 (2) | 0.59b | 0.08–4.31 | 0.60 |
| Quality of life | ||||
| 3 months | 1 (3) | –0.30a | –0.92 to 0.32 | 0.35 |
| 12 months | 1 (1) | –0.20a | –0.67 to 0.27 | 0.41 |
| Longer term | NR | NR | NR | NR |
| Qmax | ||||
| 3 months | 2 (4) | 0.98a | –0.29 to 2.25 | 0.13 |
| 12 months | 3 (4) | 0.01a | –1.08 to 1.10 | 0.98 |
| Longer term | NR | NR | NR | NR |
| Duration of operation (minutes) | 4 (5) | –4.56a | –15.36 to 6.23 | 0.41 |
| Length of hospital stay (days) | 1 (2) | –0.70a | –1.37 to –0.03 | 0.04 |
| Reoperation | 3 (3) | 1.46b | 0.25 to 8.57 | 0.67 |
Bipolar transurethral vaporesection of the prostate (B-TUVRP) versus TURP
Characteristics of included studies
Only one RCT155 making this comparison was identified in the searches (Table 24). This study, which was carried out in Hong Kong, allocated 29 men to undergo B-TUVRP and 31 to undergo TURP. Participants in both groups appeared to have moderate symptoms. No information was available to judge prostate size.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Fung et al., 2005155 | B-TUVRP | 29 | 72 | 15.8 | NR | NR | NR |
| TURP | 31 | 73 | 19.4 | NR | NR | NR |
Assessment of effectiveness
This study reported outcomes on symptom scores, intraoperative and postoperative complications and quality of life. No urodynamic data were reported. These results are tabulated in Appendix 8.10, Tables 86–89.
Symptom scores
IPSS at 3 months were slightly better for B- TUVRP than for TURP. Patients in the B-TUVRP group showed a 54% improvement in mean scores from baseline compared with 39% in the TURP arm.
Complications
There was no difference in the incidence of urinary tract infections or clot retention after B-TUVRP and conventional TURP, with wide confidence intervals. There were no cases of TUR syndrome in either arm of the trial (Table 25 and Appendix 9.10, comparison 10:01).
| Outcome | Number of trials | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| Urinary tract infection | 1 | 1.43 | 0.40–5.08 | 0.58 |
| TUR syndrome | 1 | NE | NE | NE |
Quality of life
Quality of life in patients following B-TUVRP was comparable to that in the TURP group at the 3-month follow-up (Appendix 8.10, Table 88). 155
Descriptors of care
There was no statistically significant difference between B-TUVRP and TURP in terms of duration of operation or length of hospital stay (Appendix 8.10, Table 89).
Transurethral vaporisation of the prostate (TUVP) versus TURP
Characteristics of included studies
The characteristics of the included studies are summarised in Table 26. Seventeen RCTs reported in 27 papers55,57,128,129,138,140,142,156,158,162,193,196–201,262–271 randomised a total of 1449 participants.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Çetinkaya et al., 1996196 | TUVP | 23 | 68 | NR | NR | NR | 48.4 |
| TURP | 23 | 62 | NR | NR | NR | 48.8 | |
| Ekengren et al., 2000142 | TUVP | 26 | 70 | 25b | NR | NR | NR |
| TURP | 28 | 71 | 22b | NR | NR | NR | |
| Erdaği et al., 1999128 | TUVP | 20 | 66 | 21.5 | 4.6 | 122.8 | 37.0 |
| TURP | 20 | 64 | 20.6 | 5.1 | 68.0 | 32.5 | |
| Fowler et al., 200557 | TUVP | 115 | 70 | 20.7 | 10.1 | 181.0 | 54.3 |
| TURP | 120 | 70 | 20.7 | 10.5 | 171.0 | 51.1 | |
| Gallucci et al., 1998138 | TUVP | 70 | NR | 18.8 | 7.3 | 84.7 | 36.6 |
| TURP | 80 | NR | 18.2 | 8.8 | 64.6 | 36.6 | |
| Gotoh et al., 1999156 | TUVP | 23 | 70 | 19.6 | 7.3 | 56.7 | 56.7 |
| TURP | 28 | 66 | 18.9 | 9.4 | 41.9 | 44.7 | |
| Hammadeh et al., 2003129 | TUVP | 52 | 67 | 26.5 | 8.9 | 131.0 | 32.0 |
| TURP | 52 | 70 | 26.6 | 8.6 | 101.0 | 27.0 | |
| Kaplan et al., 199855 | TUVP | 32 | 67 | NR | NR | NR | NR |
| TURP | 32 | 73 | NR | NR | NR | NR | |
| Kupeli et al., 1998158 | TUVP | 30 | 60 | 21.6 | 9.2 | NR | 51.7 |
| TURP | 30 | 62 | 19.4 | 7.9 | NR | 48.9 | |
| Kupeli et al., 1998197 | TUVP | 30 | 66 | 13.7 | 8.3 | NR | 41.6 |
| TURP | 36 | 62 | 14.6 | 8.8 | NR | 43.6 | |
| Nathan and Wickham, 1996162 | TUVP | 20 | 65 | 21.9 | 10.2 | 132.0 | 53.5 |
| TURP | 20 | 69 | 17.0 | 7.2 | 120.0 | 53.4 | |
| Netto et al., 1999198 | TUVP | 40 | 67 | 19.6 | 7.9 | 73.0 | 46.9 |
| TURP | 38 | 65 | 24.3 | 6.8 | 88.6 | 44.7 | |
| Nuhoğlu et al., 2005199 | TUVP | 37 | 64 | 17.6 | 6.3 | 88.0 | 39.0 |
| TURP | 40 | 65 | 17.3 | 5.9 | 95.0 | 39.0 | |
| Patel et al., 1997140 | TUVP | 6 | 66 | 23.3 | 7.5 | NR | 64.6 |
| TURP | 6 | 67 | 29.6 | 10.0 | NR | 54.0 | |
| Shokeir et al., 1997200 | TUVP | 35 | 68 | 26.3 | 7.8 | 75.2 | 44.6 |
| TURP | 35 | 68 | 25.1 | 6.9 | 77.1 | 48.8 | |
| Wang et al., 2002201 | TUVP | 97 | 71 | 20 | 7.0 | 1231 | NR |
| TURP | 109 | 72 | 20 | 7.0 | 120 | NR | |
| van Melick et al., 2003193 | TUVP | 46 | 64 | 20.2 | 11.0 | 290.0 | 35.0 |
| TURP | 50 | 66 | 16.8 | 11.0 | 350.0 | 37.0 |
Four studies took place in Turkey,128,158,196,197 three in the UK,57,129,162 three in the US,55,140,199 and one each in Sweden,142 Italy,138 Japan,156 the Netherlands,193 Brazil,198 Saudi Arabia200 and China. 201 Eight studies gave details of the recruitment dates,57,129,158,193,196,197,199,200 which ranged from 1995 to 2003.
All but two RCTs55,196 reported participants’ IPSS/AUA scores. The total number of participants who had severe symptoms of BPE and underwent TUVP was 487 (75%) compared with 408 (59%) with severe symptoms allocated to TURP. There were 160 (25%) participants with moderate symptoms allocated to TUVP and 284 (41%) with moderate symptoms allocated to TURP patients.
In the studies reporting prostate size, 322 (59%) and 225 (41%) participants allocated to TUVP had large and moderate-sized prostates, respectively, compared with 336 (58%) with large and 242 (42%) with moderate-sized prostates allocated to TURP.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.11. The results of the meta-analyses are given in Appendix 9.11. Note that in terms of long-term evaluation, only the longest follow-up is presented here.
Symptom scores
Of the 18 eligible studies, 13 provided information on IPSS/AUA scores at 3 months after surgery (Appendix 8.11, Table 90). Meta-analysis of seven studies55,57,138,156,162,199,200 showed no difference between TUVP and TURP in terms of symptom scores (Figure 25, comparison 11:01:01: WMD 0.09, 95% CI –0.42 to 0.61, p = 0.72). This result is consistent with the data from those trials that were not amenable to meta-analysis.
FIGURE 25.
Symptom scores, TUVP vs TURP.

Eight studies provided information on the mean or median IPSS/AUA scores at 12 months (Appendix 8.11, Table 90). A meta-analysis involving five studies55,129,138,193,200 reporting data that were suitable for synthesis again showed no statistically significant difference between the groups (Figure 24, comparison 11:01:03: WMD 0.34, 95% CI –0.19 to 0.86, p = 0.21). This result is consistent with the other three studies that were not amenable to meta-analysis.
Complications
Information about complications is detailed in Appendix 8.11, Table 91. Data describing 15 types of complications were reported to a varying extent across 15 studies. Results regarding blood transfusion, urinary retention, urinary tract infection, strictures, TUR syndrome and urinary incontinence are presented in this section (Figure 26). Results for other complications are presented in Appendix 9.11, comparisons 11:02 and 11:03. The results of these meta-analyses should be treated with caution as the length of follow-up of the RCTs varied and the confidence intervals were wide.
FIGURE 26.
Complications, TUVP vs TURP.
A total of 13 studies55,57,128,129,138,140,156,158,162,193,196,197,199 reported blood transfusions. Meta-analysis suggested a lower rate of blood transfusion following TUVP than following TURP (Figure 26, comparison 11:02:01: 2/504 versus 29/537, RR 0.19, 95% CI 0.08–0.44, p = 0.0001).
Pooling data from 11 studies55,129,138,142,156,158,162,193,196,197,199 reporting this outcome showed a higher risk of urinary retention amongst those who underwent TUVP than amongst those who underwent TURP (Figure 26, comparison 11:02:02: 33/389 versus 15/419, RR 2.12, 95% CI 1.23–3.68, p = 0.007).
Eight studies55,128,129,138,156,162,193,197 provided details on the incidence of urinary tract infections after operation. A total of 21 (7.0%) urinary tract infections were reported amongst 298 participants allocated to TUVP compared with 33 (10.4%) amongst 318 participants allocated to TURP (Figure 26, comparison 11:02:03: RR 0.65, 95% CI 0.40–1.08, p = 0.09).
Evidence from 11 studies showed no statistically significant difference between TUVP and TURP in terms of incidence of strictures or bladder neck contractures (Figure 26, comparison 11:02:04: 12/418 versus 14/446, RR 0.91, 95% CI 0.45–1.85, p = 0.80).
A total of three (0.9%) patients suffered a TUR syndrome following surgery amongst 314 randomised to TUVP as opposed to six (1.8%) amongst 329 patients randomised to TURP (Figure 26, comparison 11:02:05: RR 0.59, 95% CI 0.17–2.12, p = 0.42).
Urinary incontinence was reported in nine studies (Figure 26). Urinary incontinence occurred less frequently in the TUVP arm than in the TURP arm. This difference was not statistically significant (Figure 26, comparison 11:02:06: 57/489 versus 64/533, RR 0.92, 95% CI 0.69–1.21, p = 0.53).
Quality of life
Five studies57,129,142,162,193 reported quality of life of patients following surgery. In three studies, quality of life was assessed using the IPSS QoL (0–6) questionnaire. In the other two studies, authors did not provide further information on the measure used and later it was assumed that it was the IPSS QoL. One of the studies also assessed quality of life using the EQ-5D (using the UK tariff) and the SF-36 measures and the authors concluded that any change in general health-related quality of life resulting from their intervention was not detectable by either the EQ-5D or the SF-36 tools (the ranges and standard deviations were large).
There was no statistically significant difference in IPSS QoL at 3 or 12 months or for any longer-term follow-ups between TUVP and TURP following surgery (Appendix 9.11, comparison 11:10).
Urodynamic outcomes
Data on peak urine flow rate, mean urine flow rate, total voided volume, residual volume, detrusor pressure and prostate size were reported across 16 studies. 55,57,128,129,138,140,142,156,158,187,193,197–201 Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 8.11, Table 93 and Appendix 9.11, comparisons 11:04–11:09.
A total of 12 studies55,57,128,129,138,140,156,158,187,193,199,200 provided details of peak urine flow rate at 3 months after surgery. In five studies128,156,158,187,199 the average peak urine flow rate was higher in the TUVP group. A total of eight studies55,57,138,156,158,193,199,200 presented data that were sufficiently similar to allow quantitative synthesis (Appendix 9.11, comparison 11:04:01). The WMD was 0.10 ml/s for TUVP versus TURP (95% CI –0.53 to 0.73, p = 0.76).
Nine studies55,129,138,142,193,197,198,200,201 provided details of mean or median peak urine flow rate at 12 months after surgery. Five of the studies55,142,197,198,201 reported lower rates in the TUVP group. Data from five trials55,129,193,198,200 reporting data that were amenable to meta-analysis showed no statistically significant difference between TUVP and TURP (Appendix 9.11, comparison 11:04:03: WMD –0.11, 95% CI –0.97 to 0.74, p = 0.80).
Three studies129,193,199 reported peak urine flow rate measurements 5 years after surgery. Meta-analysis of data from these trials showed no statistically significant difference between the two groups (Appendix 9.11, comparison 11:04:06: WMD 0.60, 95% CI –1.06 to 2.26, p = 0.31).
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.11, Table 94. Information on duration of operation, length of hospital stay and reoperation rates was identified to a varying extent across the 17 eligible studies for this comparison.
A total of 14 studies55,57,129,140,142,156,158,162,193,196,198–201 reported duration of operation (Appendix 8.11, Table 94). In half of the studies55,57,129,158,162,199,200 this was longer in the TUVP group whereas in the other half140,142,156,193,196,198,201 it was shorter in the TUVP group. Eight studies presented data in a form sufficiently similar to allow quantitative synthesis (Appendix 9.11, comparison 11:11). The WMD was –1.62 minutes, favouring TUVP, although this was not statistically significant (95% CI –12.23 to 8.99, p = 0.76).
Ten studies55,57,129,138,140,162,193,197,198,200 provided information on length of hospital stay (Appendix 8.11, Table 94). In all but two studies57,193 length of hospital stay was reported to be shorter for TUVP. In two studies there were no differences. Eight RCTs55,57,129,138,193,197,198,200 reported data that were suitable for synthesis. Across them, the average length of stay was 1.00 day less following TUVP (Appendix 9.11, comparison 11:12: WMD –1.00, 95% CI –1.25 to –0.75, p < 0.001).
Only seven studies129,142,162,193,197,199,270 out of a possible 17 reported reoperation rates. The risk of having a reoperation following TUVP was no different from that following TURP. However, the confidence intervals were wide enough for clinically important differences to exist between the two groups (Appendix 9.11, comparison 11:03:15: 14/326 versus 14/346, RR 1.04, 95% CI 0.53–2.07, p = 0.90).
Summary and conclusions of the evidence for and against the intervention
This review considered data from 1449 randomised participants across 17 RCTs of moderate to low quality. The data suggest that the rates of blood transfusion and urinary tract infection are lower and the rate of urinary retention is higher after TUVP than after TURP. The length of hospital stay for TUVP was shorter in the trials. There were no statistically significant differences in IPSS/AUA symptom scores, quality of life and peak urine flow rate at 3 or 12 months or at any longer-term follow-up between TUVP and TURP. The incidence of complications such as strictures, TUR syndrome and urinary incontinence was similar. Duration of operation and reoperation rates also did not appear to differ between the two groups.
There was evidence of high statistical heterogeneity in the results for peak urine flow rate, quality of life, duration of operation and length of hospital stay. There was no consistency in the direction of effect and clinically important differences could not be ruled out. Much of the variation might be due to differences in participants’ characteristics or the ways in which the technologies were used. In addition, differences in the specific aims and objectives of the studies might have led to important differences in their inclusion criteria. In the case of duration of operation, the variation may be explained by differences in operator experience and baseline prostate size, which can be considered as a proxy for duration of operation.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 27.
| Outcome | Number of trials MA (total) | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 3 months | 7 (13) | 0.09a | –0.42 to 0.61 | 0.72 |
| 12 months | 5 (8) | 0.34a | –0.19 to 0.86 | 0.21 |
| Longer term | 3 (3) | –0.32a | –1.95 to 1.31 | 0.70 |
| Blood transfusion | 13 (13) | 0.19b | 0.08–0.44 | < 0.001 |
| Urinary retention | 11 (11) | 2.12b | 1.23–3.68 | 0.007 |
| Urinary tract infection | 8 (8) | 0.65b | 0.40–1.08 | 0.09 |
| Stricture | 11 (11) | 0.91b | 0.45–1.85 | 0.80 |
| TUR syndrome | 8 (8) | 0.59b | 0.17–2.12 | 0.42 |
| Incontinence | 9 (9) | 0.92b | 0.69–1.21 | 0.53 |
| Quality of life | ||||
| 3 months | 1 (2) | 0.30a | –0.18 to 0.78 | 0.22 |
| 12 months | 2 (3) | 0.47a | –0.23 to 0.32 | 0.73 |
| Longer term | 1 (1) | –0.60a | –1.30 to 0.10 | 0.09 |
| Qmax | ||||
| 3 months | 8 (12) | 0.10a | –0.53 to 0.73 | 0.78 |
| 12 months | 5 (10) | –0.11a | –0.97 to 0.74 | 0.80 |
| Longer term | 3 (3) | 0.60a | –1.06 to 2.26 | 0.48 |
| Duration of operation | 8 (14) | –1.62a | –12.23 to 8.99 | 0.76 |
| Length of hospital stay | 8 (11) | –1.00a | –1.25 to –0.75 | < 0.001 |
| Reoperation | 7 (7) | 1.04b | 0.53–2.07 | 0.90 |
Bipolar transurethral vaporisation of the prostate (B-TUVP) versus TURP
Characteristics of included studies
Only two RCTs making this comparison were identified by the searches (Table 28). 70,137,272 One study took place in Australia137,272 and the other in the UK. 70 A total of 111 men were allocated to undergo B-TUVP and 100 to undergo TURP. Participants in the bipolar group appeared to have severe symptoms whereas 21 (21%) of those in the TURP group had moderate symptoms and 79 (79%) had severe symptoms preoperatively. Participants in the bipolar group had moderate-sized prostates whereas those in the TURP group had large prostates.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Dunsmuir et al., 2003137,272 | B-TUVP | 30 | 63 | 24 | 9.6 | 112 | 39 |
| TURP | 21 | 60 | 17 | 10.4 | 96 | 42 | |
| Hon et al., 200670 | B-TUVP | 81 | 66 | 21 | 12 | 147 | 38 |
| TURP | 79 | 68 | 21 | 12 | 182 | 40 |
Assessment of effectiveness
Symptom scores
Data reported in the study by Dunsmuir and colleagues137,272 showed better improvement in mean AUA scores at 3 months for bipolar TUVP than for TURP (Appendix 8.12, Table 95). In contrast, Hon and colleagues70 did not find any statistically significant difference between the two groups at 9 months following surgery (Appendix 9.12, comparison 12:01:01: MD 0.80, 95% CI –1.23 to 2.83, p = 0.44).
Complications
Four types of complication were identified, with no statistically significant differences between the groups (Appendix 9.12, comparison 12:02).
Quality of life
Both studies reported quality of life of patients following surgery. The IPSS QoL scale was used in one study70 and the AUA QoL in the other. 137,272 AUA QoL was taken from section C of the AUA7 system. It comprises five questions to give a maximum score of 19. No statistically significant differences were observed between the two groups at 3, 9 and 12 months following surgery (Appendix 8.12, Table 97; Appendix 9.12, comparison 12:04:01).
Urodynamic outcomes
Data on peak urine flow rate, mean flow rate and residual volume were reported across the two studies. Results for these outcomes are presented in Appendix 8.12, Table 98 and Appendix 9.12, comparisons 12:05–12:07. No statistically significant differences were identified between the two groups.
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.12, Table 99. Information on duration of operation, length of hospital stay and reoperation rates were identified across the two eligible studies for this comparison.
Duration of operation was found to be longer in the B-TUVP arm than in the TURP arm in the two studies reporting this outcome (Appendix 8.12, Table 99; Appendix 9.12, comparison 12:08).
Both studies reported this outcome (Appendix 8.12, Table 99). In one study137,272 length of hospital stay in the B-TUVP arm was no different from that observed in the TURP arm (p = 0.78). The other study reported a higher mean length of hospital stay in the B-TUVP arm than in the TURP arm (Appendix 9.12, comparison 12:09: MD –0.40, 95% CI –0.71 to –0.01, p = 0.01).
Evidence based on one study showed that there was no statistically significant difference in reoperation rates between the two arms (Appendix 9.12, comparison 12:02:05: 1/81 versus 2/79, RR 0.49, 95% CI 0.05–5.27, p = 0.55).
Chapter 9 Most promising intervention(s) for benign prostatic enlargement
Because of the lack of RCTs comparing minimally invasive interventions with ablative methods other than TURP, a narrative review investigating trends across the interventions was performed to identify the most promising minimally invasive and ablative methods. For all comparisons considered, symptom scores are reported on the same forest plot (Figures 27 and 28). Plots for other outcomes can be seen in Appendix 9.14. These forest plots can be used to illustrate the differences between interventions.
FIGURE 27.
IPSS/AUA scores by comparison at 3 months.

FIGURE 28.
IPSS/AUA scores by comparison at 12 months.

There does not appear to be a clear winner in terms of which intervention is the most promising to treat BPE. Some interventions perform better when assessed in terms of one outcome than others. Interpretation is difficult because of the paucity of data and the multitude of comparators. However, in summary, there seems to be little evidence that any treatment is more effective than TURP in terms of resolution of symptoms of BPE. What evidence there is relates to improvement in peak urine flow rate (laser resection better) with doubtful translation to clinically significant benefit. Several procedures appear to perform better than TURP, at least in terms of one measure of complications. The performance of the different interventions relative to TURP is detailed in Table 29.
| Outcome | Relative to TURP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUMT | TUNA | Stents | TEAP | Laser coagulation | TUIP | HoLEP | Laser vaporisation | TUVRP | B-TURP | B-TUVRP | TUVP | B-TUVP | |
| IPSS, 12 months | W | W | U | U | W | U | S | W | S | U | U | S | U |
| Blood transfusion | U | B | U | U | B | B | B | B | S | S | U | B | U |
| Urinary retention | U | U | U | U | W | U | S | W | U | S | U | W | U |
| Urinary tract infection | S | S | U | U | W | S | S | S | U | S | U | B | U |
| Stricture | B | B | U | U | B | S | S | B | S | S | U | S | U |
| TUR syndrome | U | U | U | U | U | U | U | U | U | U | U | S | U |
| Incontinence | U | B | U | U | B | S | S | W | S | U | U | S | U |
| Quality of life, 12 months | W | S | U | U | W | U | U | S | S | S | U | S | U |
| Qmax, 12 months | W | W | U | U | W | W | B | U | U | S | U | S | U |
| Duration of operation | U | W | U | U | B | B | W | S | S | S | U | S | U |
| Length of stay | B | U | U | U | B | B | B | B | U | B | U | B | U |
| Reoperation | U | W | U | U | W | W | S | U | S | S | U | S | U |
Given that the results indicate that there are trade-offs between the outcomes provided by different treatments, patient preference becomes more important. However, these choices might be informed by the synthesis of the different clinical outcomes into a single measure, as performed in Chapter 10.
Chapter 10 Economic analysis
The economic perspective was that of the English NHS and, in the base case, the time horizon was 10 years, which was chosen because it was believed a priori that this would be sufficient to show the difference between technologies. A discount rate of 3.5% for costs and benefits was used in the base case, but this was varied in sensitivity analysis. The price year was 2006 and the currency was UK pounds sterling.
Multiple versus single cohort analysis
A time horizon of ‘until death’ was planned for a sensitivity analysis. However, the need to incorporate capital costs (e.g. for HoLEP) led to a change in model structure from one that is ‘individual based’ [i.e. estimating the expected cost and quality-adjusted life-years (QALYs) per individual] to one that is ‘population based’. The standard individual-based model structure is identical to averaging across a cohort of identical individuals all starting treatment at the same time. The approach used in this analysis allowed for new individuals to enter over a time period, which we assumed was the approximate lifetime of the technologies of 10 years. This is equivalent to having multiple versus single cohort analysis. This means that there is a ‘mixing’ of individuals over time such that at 1-year post technology change there will be equal numbers of those receiving their first treatment (0 years post treatment) and those who are 1-year post first treatment. After 10 years there will thus be equal numbers from year 0 to year 10 post treatment. This allows for the incorporation of capital equipment costs over the time horizon as required for strategies in which equipment is not used as the first-line treatment but rather to manage subsequent failure of treatment or relapse. For example, in the strategy TUVP/HoLEP, HoLEP is never used as the first-line treatment and it is clear that, over time, as more new individuals are treated, the amount of HoLEP equipment required will increase.
Such a model involves greater complexity in that, as described below, the model must ‘keep track’ of time post technology introduction (for the whole population) as well as time post first treatment (for the individual, including age-dependent mortality). However, this approach allows the simulation of the purchasing of new equipment as required over the time horizon and produces a more accurate estimation of costs and effectiveness and thus cost-effectiveness.
The rest of this chapter is subdivided in the same way as in the review of economic evaluation reported in Chapter 4 except that the section on sensitivity analysis deals only with the probabilistic analysis. As already explained, deterministic sensitivity analysis is reserved for testing the effect of parameter variability or model structure uncertainty and is therefore dealt with under the subheadings below.
Population
The population is men with a specified mean start age with a diagnosis of BPE (no other size criteria), presence of LUTS (with a measure of IPSS > 7), no complications and TURP indicated. This implies that medical treatment is either contraindicated or has failed. In the base-case analysis the mean start age was 70. This value was chosen because it lies approximately in the middle of the current range of age at treatment. In the sensitivity analysis the mean start age has been varied between 50 and 90 because this represents approximate ranges for the defined population.
The technologies to compare
The strategies chosen were those that the clinical experts believed to be clinically appropriate and were designed to adequately capture events that were likely to incur costs and health changes over the 10-year time horizon of the model. The strategies were formulated over the course of several meetings of clinicians involved in the study supplemented by discussion with the health economist and with additional input from other urologist colleagues to resolve differences in opinion. A time horizon of 10 years was chosen because it was believed a priori that this would be sufficient to show the differences between technologies.
The problem with using strategies is that the comparison of each possible sequence of treatments is costly in terms of building and estimating the model and, until the model is completed, it cannot be used to eliminate any sequences. However, the guiding principle at each step of the research process is the perceived value of information analysis, based on the current evidence. Before undertaking any calculation of value of information based on the model itself, judgement is required as to whether a particular sequence is feasible for the given setting and is sufficiently important to warrant the additional research cost of adding that sequence. Therefore, treatment sequences were reduced according to a set of clinical rules regarding treatments in the minimally invasive (M), TURP (T) and other tissue ablative (A) categories, and:
-
Always proceed from less to more invasive.
-
Never repeat one of the other tissue ablative procedures.
-
Repeat a minimally invasive procedure no more than once.
-
Repeat TURP only once and only after performing a pressure test.
-
Never change to another treatment from the same category.
The basis for (1) is the belief that if a more invasive treatment is ineffective then the less invasive one will also be ineffective. The second assumption is tantamount to saying that any change due to tissue ablative treatments renders the prostate ‘immune’ to further benefit from this class of treatments. The third assumption is based on the belief that additional structural change to the prostate is extremely unlikely to occur given two previous attempts. The fourth is based on standard practice within the UK. The fifth is based on the belief that if one procedure in a category has failed then another from the same category is unlikely to be more successful than repeating the same procedure and that no more than one treatment from the same category is likely to be available in any given institution.
The strategies compared in the DAM were:
-
One treatment only: M, A, T.
-
Two treatments: MM, MA, MT, AT, TT.
-
Three treatments: MMA, MMT, MAT, MTT.
-
Four treatments: MMAT, MMTT, MATT.
-
Five treatments: MMATT.
Out of all possible treatments in each of the categories, a representative was chosen based on the one most likely to be used in the UK: TUMT and laser coagulation in the minimally invasive category; and TUVP in the tissue ablative category. Two further treatments were added: laser resection, as exemplified by HoLEP; and laser vaporisation, as exemplified by KTP. HoLEP was treated as a TURP substitute but without the possibility that it could be repeated as it was believed that it removes so much tissue that there can be no subsequent treatment. KTP was treated as a substitute for TUVP.
The epidemiology: model structure
A Markov model was used in which health states and order of transitions were determined a priori according to a logical sequence of events (e.g. treatment cannot follow death) and expert clinical judgement (e.g. permanent urinary incontinence contraindicates further treatment). The cycle length was set at 3 months. This was based on the advice by the clinical expert group that there was unlikely to be any difference between treatments over a shorter time period. Given the length of follow-up of 10 years this meant that costs and effects were estimated over 40 cycles. As described above, as well as estimating the consequences (cost and QALYs) accruing to each individual over time, these consequences were summed over a population over 10 years. This was operationalised by creating another ‘state’ from which new individuals could enter the model in each cycle. For simplicity this state is not included in the diagram below. The number of new individuals was assumed to be 25,000 per year, which is approximately the number of first TURP procedures per year in the UK. This was estimated from the NHS reference costs, 2005–6,45 assuming that approximately 5% of all TURPs in a year are reoperations.
Figure 29 shows a generic component of the Markov model, which represents the care pathway shown in Figure 1, in which each box corresponds to a health state. Given survival (i.e. no death) there are two main dimensions of outcome (incontinence or not and remission or not), which, being independent, imply four possible health state transitions following treatment. The state of death, which is not shown, is an absorbing state in that it cannot be left and it can also be entered from any of the other states. In keeping with the argument presented in Chapter 4, the only long-term complication that needed to be included in the DAM was incontinence. The states of ‘no remission’ (whether with incontinence or not) are entered with the probability of failure for that particular treatment. This defines failure as a lack of change of original symptoms. If there is incontinence then no further treatment for BPE can occur and the only transitions possible are to death and from the ‘remission, incontinence’ state to the ‘no remission, incontinence’ state. If there is no incontinence and no remission and there are further treatments in the sequence then transition to the next treatment will occur. If the end of the treatment sequence is reached then the only transition that is possible is to the state of death. If there is no incontinence and remission then transition to ‘no remission, no incontinence’ can occur with the probability of relapse. This defines relapse as return to the original symptoms following an initially successful treatment.
FIGURE 29.
Schematic of Markov model component.

All other complications are short term in that they are assumed to have resolved within the first 3 months post operation and are therefore included in the treatment state. The events considered are acute urinary retention (AUR), bladder neck contracture or urethral stricture (labelled BNC), blood transfusion, TUR syndrome and UTI.
All parameter values used to estimate the transition probabilities and probabilities of adverse short-term complications are given as expected values. Separate tables are presented for cost and utility estimation in the relevant sections. Parameter distributions that are used in the Monte Carlo simulation can be seen in Table 30.
| Treatment | Event | Source | Expected value | SE | 95% CI (except for uniform distributions) | Distribution type | |
|---|---|---|---|---|---|---|---|
| Low | High | ||||||
| Baseline risk with TURP | |||||||
| TURP | AUR | AUA meta-analysis (2003) (Francisca et al., 199986) | 0.05 | 0.01 | 0.04 | 0.08 | Beta |
| TURP | BNC | AUA meta-analysis (2003) (Francisca et al., 199986) | 0.07 | 0.01 | 0.05 | 0.08 | Beta |
| TURP | Incontinence | AUA meta-analysis (2003) (Francisca et al., 199986) | 0.03 | 0.01 | 0.02 | 0.05 | Beta |
| TURP | Transfusion | AUA meta-analysis (2003) (Francisca et al., 199986) | 0.08 | 0.02 | 0.05 | 0.11 | Beta |
| TURP | TUR syndrome | AUA meta-analysis (2003) (Francisca et al., 199986) | 0.03 | 0.01 | 0.01 | 0.05 | Beta |
| TURP | UTI | AUA meta-analysis (2003) (Francisca et al., 199986) | 0.06 | 0.01 | 0.05 | 0.09 | Beta |
| TURP | Failure at 12 months | Individual level data | 0.06 | 0.02 | 0.03 | 0.09 | Beta |
| Relative risks (TURP/treatment) | |||||||
| HoLEP | AUR | Meta-analysis | 0.71 | NA | 0.38 | 1.32 | Lognormal |
| HoLEP | BNC | Meta-analysis | 0.84 | NA | 0.43 | 1.65 | Lognormal |
| HoLEP | Incontinence | Meta-analysis | 0.82 | NA | 0.53 | 1.27 | Lognormal |
| HoLEP | Transfusion | Meta-analysis | 3.70 | NA | 1.05 | 14.29 | Lognormal |
| HoLEP | TUR syndrome | Meta-analysis | 3.24 | NA | 0.14 | 77.79 | Lognormal |
| HoLEP | UTI | Meta-analysis | 1.02 | NA | 0.32 | 3.23 | Lognormal |
| HoLEP | Failure at 12 months (model 2) | Meta-analysis | 1.47 | NA | 0.69 | 3.13 | Lognormal |
| KTP | AUR | Meta-analysis | 2.89 | NA | 1.55 | 5.42 | Lognormal |
| KTP | BNC | Meta-analysis | 0.54 | NA | 0.32 | 0.90 | Lognormal |
| KTP | Incontinence | Meta-analysis | 1.17 | NA | 0.60 | 2.26 | Lognormal |
| KTP | Transfusion | Meta-analysis | 2.24 | NA | 1.03 | 4.88 | Lognormal |
| KTP | TUR syndrome | Meta-analysis | 0.14 | NA | 0.05 | 0.42 | Lognormal |
| KTP | UTI | Meta-analysis | 0.33 | NA | 0.01 | 7.93 | Lognormal |
| KTP | Failure at 12 months (model 2) | Meta-analysis | 1.68 | NA | 1.03 | 2.74 | Lognormal |
| TUMT | AUR | Meta-analysis | 1.64 | NA | 0.77 | 3.50 | Lognormal |
| TUMT | BNC | Meta-analysis | 0.20 | NA | 0.05 | 0.75 | Lognormal |
| TUMT | Incontinence | Meta-analysis | 1.64 | NA | 0.79 | 3.33 | Lognormal |
| TUMT | Transfusion | Meta-analysis | 9.09 | NA | 0.51 | 100.00 | Lognormal |
| TUMT | TUR syndrome | Meta-analysis | 5.83 | NA | 0.24 | 140.50 | Lognormal |
| TUMT | UTI | Meta-analysis | 0.95 | NA | 0.48 | 1.89 | Lognormal |
| TUMT | Failure at 12 months (model 2) | Meta-analysis | 0.50 | NA | 0.24 | 1.04 | Lognormal |
| TUVP | AUR | Meta-analysis | 2.12 | NA | 1.23 | 3.68 | Lognormal |
| TUVP | BNC | Meta-analysis | 0.91 | NA | 0.45 | 1.85 | Lognormal |
| TUVP | Incontinence | Meta-analysis | 0.92 | NA | 0.69 | 1.21 | Lognormal |
| TUVP | Transfusion | Meta-analysis | 0.19 | NA | 0.08 | 0.44 | Lognormal |
| TUVP | TUR syndrome | Meta-analysis | 0.59 | NA | 0.17 | 2.12 | Lognormal |
| TUVP | UTI | Meta-analysis | 0.65 | NA | 0.40 | 1.08 | Lognormal |
| TUVP | Failure at 12 months (model 2) | Meta-analysis | 1.04 | NA | 0.53 | 2.07 | Lognormal |
| Other risks | |||||||
| TUMT | Failure of second treatment relative to first | Expert opinion | 0.75 | NA | 0.50 | 1.00 | Uniform |
| TURP | Failure of second treatment relative to first | Expert opinion | 0.75 | NA | 0.50 | 1.00 | Uniform |
| TURP | Pressure test positive | Expert opinion | 0.75 | NA | 0.65 | 0.85 | Uniform |
| Reoperation | |||||||
| Any but TUMT and laser | Reoperation at 8 years | Madersbacher et al., 200587 | 0.08 | 0.00 | 0.07 | 0.08 | Beta |
| TUMT | Reoperation at 5 years | Francisca et al., 199986 | 0.36 | 0.01 | 0.33 | 0.39 | Beta |
| Baseline and relative mean IPSS scores | |||||||
| Any | Pretreatment mean | Individual level data | 22.08 | 0.47 | 21.16 | 23.01 | Normal |
| Any | Successful treatment mean | Individual level data | 6.61 | 0.38 | 5.86 | 7.35 | Normal |
| HoLEP | WMD | Meta-analysis | 0.42 | 0.05 | 0.32 | 0.52 | Normal |
| KTP | WMD | Meta-analysis | –1.30 | 0.60 | –0.12 | –2.47 | Normal |
| TUMT | WMD | Meta-analysis | –2.41 | 0.52 | –3.42 | –1.40 | Normal |
| TUVP | WMD | Meta-analysis | –0.18 | 0.23 | –0.63 | 0.26 | Normal |
| Utilities | |||||||
| Any | AUR | Ackerman et al., 200078 | 0.88 | 0.00 | 0.22 | 0.23 | Beta |
| Any | BNC | Ackerman et al., 200078 | 0.92 | 0.00 | 0.24 | 0.24 | Beta |
| Any | Incontinence | Ackerman et al., 200078 | 0.88 | 0.00 | 0.22 | 0.23 | Beta |
| Any | TUR syndrome | Ackerman et al., 200078 | 0.80 | 0.01 | 0.19 | 0.21 | Beta |
| Any | UTI | Ackerman et al., 200078 | 0.92 | 0.00 | 0.23 | 0.23 | Beta |
| Any | No remission | Kok et al., 2002273 | 0.96 | 0.00 | 0.23 | 0.24 | Beta |
| Any | Remission | Kok et al., 2002273 | 1.00 | 0.00 | NA | NA | Beta |
| Costing | |||||||
| Any | Risk of TURP after AUR | Expert opinion | 0.50 | 0.05 | 0.40 | 0.60 | Beta |
| Any | Risk that incontinence is urge type | Expert opinion | 0.95 | 0.02 | 0.91 | 0.99 | Beta |
| Any | Baseline treatment (including mean LOS) | NHS reference costs, 200545 | 1862.34 | NA | 1546.32 | 2195.54 | Lognormal |
| Any | Urology ward bed day | NHS reference costs, 200545 | 250.00 | NA | 141.00 | 443.26 | Lognormal |
| Any | LOS of TUR syndrome | Expert opinion | 2.00 | 0.51 | 1.00 | 3.00 | Normal |
| Any | LOS of UTI | Expert opinion | 3.00 | 1.40 | 0.25 | 5.75 | Normal |
| Any | Pressure test | NHS reference costs, 200545 | 125.10 | 16.70 | 92.37 | 157.83 | Normal |
| Any | Transfusion | Expert opinion | 1270.00 | 323.98 | 635.00 | 1905.00 | Normal |
| Any | Oxybutynin | Assumption | 166 | NA | 65 | 267 | Uniform |
| HoLEP | Life of machine | Expert opinion | 10.00 | NA | 5.00 | 15.00 | Uniform |
| HoLEP | Number of uses of HoLEP blade | Expert opinion | 7.50 | NA | 5.00 | 10.00 | Uniform |
| HoLEP | Number of uses of HoLEP fibre | Expert opinion | 25.00 | NA | 20.00 | 30.00 | Uniform |
| KTP | Life of machine | Expert opinion | 10.00 | NA | 5.00 | 15.00 | Uniform |
The epidemiology: parameterisation of the model
Effectiveness
Probability of failure (1)
In this subsection the method used to estimate the probability of failure (defined above as the transition from a treatment state to the ‘no remission, no incontinence’ or ‘no remission, incontinence’ states) of any treatment used on the first occasion is described. Its modification for repeating the same procedure in a strategy is described later in this chapter.
The challenge was to find a definition of failure that would be consistent for all treatments and a reliable method to estimate its probability. Therefore, given that the aim of treatment is to improve symptoms (LUTS), treatment failure was defined as ‘insufficient improvement’ in symptom score. ‘Insufficiency’ might be variably defined but, according to the clinical experts (R Pickard, University of Newcastle, J N’Dow, S McClinton, University of Aberdeen, 2006, personal communication), in clinical practice a percentage change of less than 10% in IPSS is most often used.
Formally:
Let f(p) be the probability distribution of p in the population where p represents the relative change in symptom score pre and post surgery such that:
where Ipost is the IPSS score post surgery and Ipre is the IPSS score pre surgery.
Therefore, the probability of failure is given by:
where x is the minimum percentage change in symptom score between pre and post surgery that would be considered sufficient (= 0.1).
Substituting (1) into (2) gives the probability of failure as:
where the probability of failure, P(fail), is equal to the probability that the percentage change in symptom score between pre and post surgery, ((Ipost–Ipre)/Ipre), is less than x.
The main problem in estimating P(p < x) from any effectiveness evidence is that these data are not reported and the IPSS scores are reported only as means of the whole sample at various points in time. Ideally, individual level data (ILD) for each treatment would be used but such data are unavailable. ILD were available, however, for two time points, pre treatment (baseline) and 4-month follow-up, for a sample of men from a study population (in particular, pretreatment IPSS > 7) who had received TURP (R Pickard, 2006, personal communication). Details of the characteristics of the patient population can be found in Appendix 11. The most reliable data comparing the effectiveness of TURP with other procedures should come from the estimates of the WMD derived from the meta-analysis (see Chapters 6–8). Therefore, the challenge was to estimate P(fail) for the other procedures using the ILD for TURP and the WMD for the comparison with TURP for each other procedure. This constituted ‘model 1’ for estimating the probability of failure. A second model was also developed. In model 2 the relative risks of retreatment obtained from the meta-analysis were used to estimate the relative risk of failure of each treatment compared with TURP. The results obtained from these two models were compared in a sensitivity analysis.
Model 1 (base case)
To estimate Pt(fail) for each treatment t, it is known that the mean IPSS score post treatment (as reported in a study) is equal to the average score of the mean of those who are successful and those who fail. This can be represented as:
This formula can be rearranged to give the probability of failure for each treatment, Pt(fail):
If it is assumed that the trial sample is similar to the ILD sample then the mean IPSS score post treatment for treatment t (meant(Ipost)) can be calculated as:
where meanTURP(Ipost) is the mean IPSS post treatment for TURP, and WMDtpost is the weighted mean difference in IPSS post treatment for the comparison of treatment t with TURP.
Substituting equation (6) into equation (5) gives the following:
In this equation meanTURP(Ipost) can be estimated from the ILD and WMD can be estimated from the meta-analysis. However, it is not known what the mean IPSS is for those who, by some definition, fail or have success. To solve this problem it was first assumed that a percentage change in IPSS of less than 10% (x = 0.1), given sample uncertainty, is equivalent to no change in symptoms, i.e. a proportion of individuals who are treated will be considered to have failed insofar as ‘on average’ they do not show any improvement in symptoms and this is independent of the initial IPSS.
The first assumption is, therefore, that the mean post-treatment IPSS of those who fail (mean(Ipost)fail) is the same as the mean IPSS pre treatment (mean(Ipre)) and is constant across all treatments, i.e.:
Substituting equation (8) into equation (7) gives:
If it is further assumed that the mean IPSS post treatment for those for whom treatment was a success (mean(Ipost)success)) is constant across treatments then:
Both of these assumptions imply that the difference in mean IPSS between treatments (i.e. the WMD) is due only to a difference in the probability of failure and not to a difference in mean IPSS of those who are successful or mean IPSS of those who fail. This is convenient for the Markov model because it also means that the utility [which is a function of IPSS (see Utilities)] for the states of ‘remission’ and ‘no remission’ also does not vary between treatments.
When t is defined as TURP then the probability of TURP failing, PTURP(fail), can be defined as:
Rearranging and substituting equation (11) into equation (10) leads to a definition of the probability of treatment t failing, Pt(fail), as:
In the DAM, PTURP(fail), mean(Ipre) and mean(Ipost)success were estimated from the ILD, and WMDtpost was estimated from the meta-analyses reported in Chapters 6–8. Because IPSS values in the meta-analysis continued to decline for up to 12 months post operation, it was assumed that this represented continued improvement. Therefore, the WMD at 12 months was used as the estimate of WMDtpost.
Model 2
As for model 1 it was assumed that the treatments only differed by probability of failure and that those who failed had a mean IPSS post treatment that was the same as the pre treatment score and that those who were successful had identical post-treatment IPSS regardless of the treatment that they received. Probability of failure of TURP was also still estimated from the ILD. However, in model 2 the probability of failure of the other treatments was estimated from the retreatment relative risks estimate obtained as part of the review of effectiveness reported in Chapters 6–8. Of course, how the decisions to retreat were made in the trials is not known and they were perhaps not made according to the rule given above with regard to percentage change in IPSS.
Probability of failure (2): repeat and subsequent procedures
Given no other available evidence it was decided to estimate the probability of failure of subsequent, but different, procedures as if there was no previous history of treatment and subsequent repeat procedures according to a relative risk (RR):
where Ptfail2 is the probability of failure of a second (repeat) procedure and the relative risk was estimated by clinical expert opinion (R Pickard, 2006, personal communication).
Probability of relapse
Relapse has already been defined in terms of the transition from the ‘remission’ state to the ‘no remission’ state. Again there is a lack of long-term data for all types of treatment and the data that are available are only in the form of the rate of retreatment. Also, because long-term retreatment is the sum of retreatment following relapse and retreatment following failure (as defined above), each relapse rate was calculated as the remainder from the total retreatment rate once the failure rate had been deducted, i.e.:
where Pd(relapse) is the probability of relapse, Ptd(retreatment) is the total probability of retreatment (including that following failure) over the time period d (obtained from the literature) and Pt(fail) is the probability of failure (estimated by either model 1 or 2).
All long-term probabilities were converted to transition probabilities by assuming a constant rate over the time period. Thus:
Long-term data on retreatment were obtained for TURP for d = 5 years and for TUMT for d = 8 years. The other treatments were assumed to be identical to TURP or TUMT depending on their short-term similarity as shown by the WMD in IPSS at 12 months. Thus, TUVP and HoLEP are the same as TURP and KTP the same as TUMT.
Complications (long and short term)
These are the probabilities for those complications occurring in the treatment state (AUR, BNC, transfusion, TUR syndrome and UTI) and incontinence. All non-TURP treatment complication probabilities were expressed in terms of a relative risk with respect to TURP and were based on data from the meta-analyses reported in Chapters 6–8. The baseline values for TURP were estimated by summing events across all TURP treatment arms of this meta-analysis. In the base case those from the UK were used and then compared with all studies. 85 Given the variability in reporting, the DAM has not attempted to differentiate between the different levels of severity of these events.
Utilities
The following equation expresses how the model calculates the discounted expected number of QALYs:
where EUcycle is the expected utility of each cycle, i.e. the sum of the utilities of each state weighted by their probabilities, and ‘0.25’ indicates that each cycle was a quarter of a year. Of course, the population total was estimated by multiplying the probability of each state counted post first treatment by the number of individuals from each cohort for each cycle and summing across all cohorts and all cycles. For example, during year 1 (1–2 years post technology introduction) there will be 25,000 new entrants, whose state transition probabilities are those of their first year post first treatment, plus 25,000 entrants who entered during year 0, whose transition probabilities will therefore be those of their second year post first treatment.
To estimate the utility of each health state it was necessary to express utility as a function of both LUTS and complications. As stated already, the states of ‘no remission’ and ‘remission’ are already defined in terms of IPSS, i.e. ‘no remission’ is the study population that have an IPSS greater than 7 and ‘remission’ is the mean IPSS of those who do not fail, as estimated from the ILD. Only one study273 that maps IPSS to utility values could be found from the search for economic evaluations (which included studies reporting utility values).
However, although the clinical experts believed that by far the most important factor in making a treatment choice is LUTS, it was necessary to modify the utility values in the presence of any complications, for the incontinence states and for the within-treatment state complications (AUR, BNC, TUR syndrome and UTI). Therefore, what is first shown is how utility values are calculated for the states of ‘no remission, no incontinence’ and ‘remission, no incontinence’.
Utility as a function of IPSS
Kok and colleagues273 elicited preferences using an accepted method of time trade-off. 75 The sample was also fairly large (n = 170) and was composed of members of the general public (around Rotterdam in the Netherlands), which facilitates comparability with the use of utilities to calculate QALYs in other populations. In their analysis they mapped IPSS scores on to utility values such that (Lo, Li)1 is preferred to (Lo, Li)2 if and only if U(Lo, Li)1 > U(Lo, Li)2, where (Lo, Li) is a set of levels, Lo referring to obstructive and Li to irritative, each defined according to a range of the sum of the scores on either the obstructive or irritative domains of the IPSS measure. For example, Lo = 1 if Io <= 4. The complete set of levels (derived in the Kok and colleagues study from factor analysis of the IPSS of 1414 patients over the age of 50 years newly referred in 13 hospitals in the Netherlands) is given in Table 31.
| Domain | Summary score | Level |
|---|---|---|
| Obstructive | ||
| Seldom/never | ≤ 4 | Obstructive 1 |
| About half of the time/sometimes | ≥ 5 and ≤ 16 | Obstructive 2 |
| Almost always | ≥ 17 | Obstructive 3 |
| Irritative | ||
| Seldom/never | ≤ 3 | Irritative 1 |
| About half of the time/sometimes | ≥ 4 and ≤ 9 | Irritative 2 |
| Almost always | ≥ 10 | Irritative 3 |
The resulting utility values are given in Table 32.
| Obstructive score | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Irritative score | 1 | 1.00 | 0.97 (0.11) | 0.95 (0.09) |
| 2 | 0.97 (0.10) | 0.94 (0.12) | 0.92 (0.11) | |
| 3 | 0.92 (0.15) | 0.90 (0.14) | 0.87 (0.14) | |
Therefore, each combination of obstructive and irritative scores can be mapped to a mean utility score.
Unfortunately, the IPSS values are only reported in the literature in the form of mean total scores, which are the sum of the irritative and obstructive domain scores. Therefore, an assumption is required as to the relative contributions of each of these domains to the total. In the absence of evidence it could be assumed that the observed proportion of the total IPSS of each of the domains is the same as the proportion of the maximum score, i.e. because Itotal = Io + Ii, where Itotal is the total IPSS, Io is the sum of the scores on the obstructive domains and Ii is the sum of the scores on the irritative domains, and because out of seven domains there are four obstructive to three irritative, each with the same maximum score, then Io = 4 · Itotal/7 and Ii = 3 · Itotal/7.
From this the utility of the state of ‘no remission, no incontinence’ can be estimated, as it is known that the mean IPSS estimated from the ILD is approximately 22. Therefore, if Itotal = 22, Io = (4 × 22)/7 and Ii = (3 × 22)/7, i.e. approximately Io = 13 and Ii = 9, which, using the table of Kok and colleagues, maps to 2 on both the obstructive and the irritative domains. Using the algorithm provided by Kok and colleagues273 these give a utility of 0.90, i.e. the utility of the preoperative state, which is also the state of ‘no remission, no incontinence’, is ‘on average’ 0.94. Similarly, for ‘remission, no incontinence’ the mean IPSS is estimated from the ILD to be about 6, which maps to a utility of 1, i.e. ‘on average’ successful treatment restores individuals to a state equivalent to full health. The ILD provided some support that Io and Ii can be treated in the way described above in that they had a correlation coefficient of 0.4 and they occurred in approximately the same ratio preoperatively. Also, the mean utility estimated from the ILD both pre- and postoperatively was found to differ by less than 0.005 when estimated according to the assumption or when estimated using the actual data. Nevertheless, a sensitivity analysis tested the effect of using the minimum utility consistent with an IPSS of 7 for the state of remission. This corresponds to Io = 1 and Ii = 2 (or vice versa) and, thus, using the data provided by Kok and colleagues a utility of 0.97 was estimated instead of 1.
Utility as a function of IPSS and non-LUTS factors
Only one study was found, by Ackerman and colleagues78 (see Chapter 4), that estimated utility as a function of both LUTS and complications. The challenge was to ‘map’ these values to the Kok and colleagues utilities. 273 This was achieved by ‘anchoring’ to the state without complications by assuming that the state of ‘moderate to severe’ BPE described by Ackerman and colleagues was equivalent to the mean IPSS pre treatment (estimated from the ILD). This assumption can be justified because the definition of ‘moderate to severe’ is an IPSS > 7, which is also the IPSS in the study population. The Ackerman utilities for complications were then used in one of two forms, compared in a sensitivity analysis, either unadjusted or adjusted, by calculating them as:
This, therefore, allows the estimation of the utility of a treatment state as the sum of the utility of the short-term complications (AUR, BNC, transfusion, TUR syndrome and UTI) that occur within this state weighted by their probabilities. Because incontinence can occur with or without remission from LUTS, there are two utilities: one for the state ‘incontinence, no remission’ and one for the state ‘incontinence, remission’. It was assumed that the utility of ‘incontinence, no remission’ was equal to that for ‘incontinence, remission’ reduced by the ‘disutility’ (1–utility) of the ‘no incontinence, no remission’ health state. The effect of assuming that the utility of these states was the same was tested in a sensitivity analysis.
Costs
In keeping with the economic perspective, only costs applicable to the NHS in England and Wales have been included. The following formula expresses the discounted cost function estimated for each individual (thus excluding capital costs) for each treatment strategy:
As for QALYs, the total population costs were estimated by multiplying the probability of each state at each time post first treatment by the number of individuals from each cohort and summing across all cohorts and cycles. However, in addition, capital costs were included, which were the purchase of equipment that could be used by more than one individual and over several years. This category was assumed to apply only to HoLEP, TUMT and KTP. Also, as for utility, the cost of each state needs to be estimated. The states of ‘no incontinence, remission’ and ‘no incontinence, no remission’ incur no costs. The states including ‘incontinence’ (‘incontinence, no remission’ and ‘incontinence, remission’) incur the cost of treating incontinence. The treatment states incur the procedure cost and the cost of treating the short-term complications of AUR, BNC, transfusion, TUR syndrome and UTI. The cost of the procedures was also distinguished by:
-
the length of stay of the procedure (LOSprocedure), which was taken to be separate from any extra LOS due to complications and which had a cost, costLOSprocedure
-
the procedure cost (costOP excluding hospital stay but including perioperative ward time, investigations and theatre costs)
-
complication costs (costcomp)
-
the cost of purchase of equipment for each individual (costequipment).
Therefore, the cost of the treatment state for treatment t is:
Procedure cost
CosttOP was assumed to be the same for all procedures. It was estimated by assuming that the 2005 NHS reference cost45 [Health Care Resource Group (HRG) code L28 (without complications)] for the surgical treatment of BPE was the total treatment cost (including LOS due to initial procedure and complications). CosttOP was calculated by netting out the cost of LOS from NHS reference costs using the formula below:
where cost per bed day, costday, was estimated for a urological surgery ward from the NHS reference costs with HRG code L09 (‘treatment of kidney or urinary tract infection’) as this typically does not involve surgery. This cost was confirmed by estimating the difference in cost between HRG L27 (with complications) and L28 (without complications) and assuming that this difference was due mostly to the difference in LOS. LOSreference is the mean LOS given with the reference cost data for codes L27 and L28.
Although LOS estimates were retrieved from the meta-analysis it was the opinion of the experts that these largely reflected local practice and therefore the LOS of each procedure (LOStprocedure) was based on expert opinion of standard UK practice. Therefore, LOStprocedure was assumed to be 3 days for TURP or TUVP, 2 days for holmium laser resection or laser vaporisation, and 0 days (day-case procedure) for TUMT. These values were varied in a sensitivity analysis.
In the absence of direct evidence the day unit cost of TUMT was estimated using expert opinion and evidence from several sources, including the lowest NHS reference costs for a day case and a local estimate (with cost elements removed to prevent the double counting of ‘operation cost’). The cost was estimated to be between £200 and £400, with an expected value of £250 and most likely to be no more than £250 (the probability of being no more than £250 was 0.75).
TURP and TUVP were assumed to incur no additional equipment costs. For KTP, TUMT and HoLEP, additional costs of blades/fibres/probes were included. Costs of laser equipment were estimated from manufacturers (R Pickard, 2006, personal communication). The fibre/blade/probe costs per individual were calculated by assuming that for KTP and TUMT they are not reusable but for HoLEP they are. The number of reuses is expressed as a distribution based on expert opinion and manufacturers estimates (R Pickard, 2006, personal communication). All of the data used to calculate these costs are reported in Table 30.
Capital costs were those of the purchase of the machines and were estimated in the base-case model assuming efficient use at 250 uses per year with a lifetime consistent with that of the model of 10 years. Sensitivity analysis was conducted to reduce the number of uses per year. Travel costs were not included, as too few data were available on the siting of equipment.
Short-term complication costs
All costs of short-term complications were estimated based on expert opinion (R Pickard, personal communication 2006). More specifically, the cost of AUR was calculated as the cost of an additional day of LOS for ‘trial without catheter’ plus, for the proportion of patients who fail this trial (probability of TURP after AUR), the cost of TURP. The cost of BNC was assumed to be the cost of an additional TUIP. The cost of transfusion was calculated based on the cost of a unit of blood of £635 (R Pickard, 2006, personal communication) multiplied by the number of units (two on average). The cost of UTI was estimated as the cost of an additional LOS (3 days on average). The cost per bed day was costday as estimated by the method described above.
Cost of incontinence
The cost of incontinence was calculated partly as a recurring cost of oxybutynin [from the British National Formulary (www.bnf.org/bnf/) on 3 November 2006; from 2.5 mg twice a day (£8.98 for 56-tablet pack) to 5 mg twice a day (£3.26 for 84-tablet pack)] multiplied by the proportion who would have urge incontinence, estimated as 0.95 by expert opinion. For the remaining 5%, incontinence was assumed to be cured by artificial sphincter, which incurred a one-off cost of £6000 (R Pickard, 2006, personal communication).
Accounting for uncertainty
Given that a systematic review and meta-analysis were included as part of this project, in estimating the parameter distributions for the Monte Carlo simulation, the starting point for all parameters was always an estimate of the expected value from the sample and a sampling distribution, which is equivalent to the likelihood. The clinical experts were asked to examine all of the estimates from the meta-analysis that informed the parameters in the DAM to see how credible the mean was as an estimate of population expected value and whether the size of the 95% confidence interval was a suitable estimate of the magnitude of uncertainty. When there was other sample evidence, such as from the ILD to estimate the probability of failure of treatment, the sampling distribution was also used. When no such data existed, the posterior used in the model was essentially a prior, estimated by expert opinion (R Pickard, 2006, personal communication) and checked by further expert opinion (J N’Dow, S McClinton, 2006, personal communication). The distribution was then estimated using an expected value and range, which implied an approximate 95% confidence interval, or, where there was greatest uncertainty, only a range, which implied a uniform distribution.
Table 30 contains a list of all parameters, their expected values, the standard errors and the confidence intervals along with a note of the distribution used and the source of data. All distribution shapes were chosen according to standard practice. 77 All relative risk estimates from the meta-analysis for complications and retreatment and for cost from the NHS reference cost data on procedure cost and LOS were log transformed to parameterise a symmetrical normal distribution. Beta distributions were parameterised from sample-based means and standard errors and used to estimate the uncertainty of parameters bounded by 0 and 1 (baseline probabilities and utilities). The normal distribution was parameterised from sample data using sample-based means and standard errors. This approach was used for IPSS estimation for the WMDs from the meta-analysis; the mean IPSS preoperatively (‘no remission, no incontinence’ state) and following successful treatment (‘remission, no incontinence’ state), both from the ILD; and the cost of the pressure test following the first TURP, from NHS reference costs.
When there were no sample data, the shape of the parameter distribution depended on some judgement as to the degree of uncertainty. Therefore, the normal distribution was parameterised by assuming that the expert opinion of upper and lower bounds corresponded to the 95% confidence interval. This approach was used to estimate the uncertainty surrounding the LOS for TUR syndrome and UTI and the cost of transfusion. The beta distribution was similarly parameterised for estimating the uncertainty of the probability of requiring TURP because of AUR. A uniform distribution was used for the number of reuses of the HoLEP and laser fibres/blades and the lifetime of each of the machines as well as the probability of the pressure test showing obstruction.
The Monte Carlo simulation was run with 10,000 samples. The number of samples was chosen by trialling the Monte Carlo simulation with increasing numbers of samples to determine at which point the addition of further samples resulted in no changes in the strategies that were non-dominated and non-extendedly dominated as well as little effect on the incremental cost-effectiveness ratios (ICERs). Because the analysis was carried out at the ‘population level’, the expected value of perfect information (EVPI) was calculated immediately for an incidence of 25,000 per year over 10 years at a discount rate of 3.5%.
Results
The cost-effectiveness analysis
Table 33 shows the results of a Monte Carlo simulation with 10,000 samples.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness (QALYS) | Incremental effectiveness (QALYS) | ICER |
|---|---|---|---|---|---|
| TUVP | £380,774,844 | 917,082 | |||
| TUMT | £387,042,593 | £6,267,749 | 906,333 | –10,749 | (Dominated) |
| HoLEP | £400,549,783 | £19,774,939 | 919,656 | 2574.1 | £7682 |
| TUVP/HoLEP | £413,712,972 | £13,163,189 | 921,041 | 1384.8 | £9505 |
| TUVP/TURP | £416,466,605 | £2,753,633 | 920,931 | –109.3 | (Dominated) |
| TUVP/TURP × 2 | £418,264,231 | £4,551,258 | 921,091 | 50.2 | £90,576 |
| TURP | £435,632,543 | £17,368,313 | 918,222 | –2868.7 | (Dominated) |
| TURP × 2 | £457,866,096 | £39,601,866 | 920,340 | –751.3 | (Dominated) |
| TUMT/TUVP | £502,437,525 | £84,173,294 | 919,219 | –1871.9 | (Dominated) |
| TUMT × 2 | £504,459,471 | £86,195,241 | 915,639 | –5451.6 | (Dominated) |
| TUMT/HoLEP | £509,607,654 | £91,343,423 | 919,893 | –1197.7 | (Dominated) |
| TUMT/TUVP/HoLEP | £512,222,250 | £93,958,020 | 920,231 | –860.0 | (Dominated) |
| TUMT/TUVP/TURP | £512,936,161 | £94,671,930 | 920,203 | –887.7 | (Dominated) |
| TUMT/TUVP/TURP × 2 | £513,448,707 | £95,184,476 | 920,243 | –848.0 | (Dominated) |
| TUMT/TURP | £519,051,244 | £100,787,013 | 919,281 | –1810.1 | (Dominated) |
| TUMT/TURP × 2 | £525,599,769 | £107,335,538 | 920,059 | –1031.5 | (Dominated) |
| TUMT × 2/TUVP | £543,805,485 | £125,541,255 | 919,592 | –1498.7 | (Dominated) |
| TUMT × 2/HoLEP | £546,577,726 | £128,313,496 | 919,798 | –1292.5 | (Dominated) |
| TUMT × 2/TUVP/HoLEP | £547,091,377 | £128,827,147 | 919,896 | –1195.2 | (Dominated) |
| TUMT × 2/TUVP/TURP × 2 | £547,469,842 | £129,205,611 | 919,899 | –1191.8 | (Dominated) |
| TUMT × 2/TUVP/TURP | £549,476,915 | £131,212,685 | 918,172 | –2919.0 | (Dominated) |
| TUMT × 2/TURP × 2 | £551,652,179 | £133,387,949 | 919,846 | –1244.7 | (Dominated) |
| TUMT × 2/TURP | £556,354,850 | £138,090,619 | 919,684 | –1406.5 | (Dominated) |
| KTP | £557,310,731 | £139,046,500 | 907,708 | –13,382.6 | (Dominated) |
What is clear from the results presented in this table is that effectiveness increases (in terms of QALYs) when moving from performing only one treatment to repeating treatments or adding treatments on initial failure or later relapse in a strategy.
The strategy that would be considered cost-effective depends upon society’s willingness to pay for a QALY. For example, if the threshold is £20,000 per QALY, then TUVP/TURP × 2 would not be cost-effective. However, if current practice is TURP × 2, i.e. TURP followed by another TURP as required, then TUVP/HoLEP and TUVP/TURP × 2 are both less costly and more effective. Therefore, a move from current practice to TUVP/HoLEP at such a threshold would follow from these results.
The cost-effectiveness acceptability curve (CEAC) (Figure 30) gives an indication of the amount of uncertainty surrounding point estimates of cost-effectiveness. Most of the strategies have a zero probability of being cost-effective. Assuming that society’s willingness to pay for a QALY is £20,000, it is clear that not only is TUVP/HoLEP cost-effective ‘on average’ but also that it has a probability of about 0.8 of being cost-effective. If society’s willingness to pay for a QALY is £80,000 then ‘on average’ TUVP/TURP × 2 would be most likely to be cost-effective. However the probability of being cost-effective is 0.5, similar to that of TUVP/HoLEP (Figure 30). Such uncertainty might affect the decision as to which strategy to implement. However, the CEAC should be interpreted with caution in that it does not reveal for each sample what the size of the differences in cost and effectiveness are.
FIGURE 30.
Cost-effectiveness acceptability curve (Monte Carlo simulation with 10,000 samples).

Comparisons of all treatment strategies against a TURP alone as a common comparator
The data reported in Table 33 were used to compare each individual treatment strategy with the strategy of TURP alone (i.e. patients all initially receive a TURP but should the procedure subsequently be deemed to have failed then the patient is managed non-surgically).
Table 34 shows the comparison of treatment strategies involving only a single surgery with TURP alone. For the comparison of TUMT or TUVP with TURP, TURP is more costly but more effective. The incremental costs per QALY for these two comparisons suggest that the savings obtained from a move from TURP to TUMT are probably not worth the loss of QALYs. Conversely, the savings that may be obtained from moving from TURP to TUVP may be worth the loss of benefits (the incremental cost per additional QALY provided by TURP compared with TUVP is greater than £30,000). HoLEP appears to be on average less costly and more effective than TURP alone (i.e. HoLEP is dominant) and KTP is less effective and more costly than TURP (TURP is dominant).
| Comparison with TURP | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY | ||
|---|---|---|---|---|---|---|---|
| Alternative | TURP | Alternative | TURP | ||||
| TUMT | £387,042,593 | £435,632,543 | 90,6333 | 91,8222 | –£48,589,950 | –11,890 | £4087 |
| HoLEP | £400,549,783 | £435,632,543 | 91,9656 | 91,8222 | –£35,082,760 | 1434 | HoLEP dominant |
| KTP | £557,310,731 | £435,632,543 | 90,7708 | 91,8222 | £121,678,188 | –10,514 | TURP dominant |
| TUVP | £380,774,844 | £435,632,543 | 91,7082 | 91,8222 | –£54,857,699 | –1141 | £48,100 |
A similar comparison was made for those strategies involving a second surgery for those people for whom a first surgery was deemed to have failed (Table 35). TUMT × 2 is more costly and less effective than TURP (TURP is dominant). Other strategies involving TUMT as a first-line surgery are on average unlikely to be considered cost-effective.
| Comparison with TURP | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY | ||
|---|---|---|---|---|---|---|---|
| Alternative | TURP | Alternative | TURP | ||||
| TURP × 2 | £457,866,096 | £435,632,543 | 920,340 | 918,222 | £22,233,553 | 2117 | £10,500 |
| TUMT × 2 | £504,459,471 | £435,632,543 | 915,639 | 918,222 | £68,826,928 | –2583 | TURP dominant |
| TUMT/HoLEP | £509,607,654 | £435,632,543 | 919,893 | 918,222 | £73,975,111 | 1671 | £44,267 |
| TUMT/TUVP | £502,437,525 | £435,632,543 | 919,219 | 918,222 | £66,804,982 | 997 | £67,019 |
| TUMT/TURP | £519,051,244 | £435,632,543 | 919,281 | 918,222 | £83,418,701 | 1059 | £78,801 |
| TUVP/HoLEP | £413,712,972 | £435,632,543 | 921,041 | 918,222 | –£21,919,571 | 2819 | TUVP/HoLEP dominant |
| TUVP/TURP | £416,466,605 | £435,632,543 | 920,931 | 918,222 | –£19,165,938 | 2709 | TUVP/TURP dominant |
Strategies involving TUVP as a first-line intervention were found to be less costly and more effective than TURP, continuing the trend started with the comparison of TUVP with TURP.
The final set of comparisons was for those strategies that allow more than one subsequent surgery if necessary (Table 36). The only strategies considered in this comparison were those in which the initial surgery was TUMT or TUVP. For all those strategies starting with TUMT, the incremental cost per QALY is at best on the borderline of what society might consider to be worthwhile, as would be expected given the analyses reported in Tables 34 and 35. The one strategy starting with TUVP is more effective and less costly than TURP alone.
| Comparison with TURP | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY | ||
|---|---|---|---|---|---|---|---|
| Alternative | TURP | Alternative | TURP | ||||
| TUMT/TUVP/HoLEP | £418,264,231 | £435,632,543 | 921,091 | 918,222 | –£17,368,312 | 2869 | TUMT/TUVP/HoLEP dominant |
| TUMT/TUVP/TURP | £512,222,250 | £435,632,543 | 920,231 | 918,222 | £76,589,707 | 2009 | £38,129 |
| TUMT/TUVP/TURP × 2 | £512,936,161 | £435,632,543 | 920,203 | 918,222 | £77,303,618 | 1981 | £39,023 |
| TUMT/TURP × 2 | £513,448,707 | £435,632,543 | 920,243 | 918,222 | £77,816,164 | 2021 | £38,510 |
| TUMT × 2/TUVP | £525,599,769 | £435,632,543 | 920,059 | 918,222 | £89,967,226 | 1837 | £48,970 |
| TUMT × 2/HoLEP | £543,805,485 | £435,632,543 | 919,592 | 918,222 | £108,172,942 | 1370 | £78,958 |
| TUM × 2/TUVP/HoLEP | £546,577,726 | £435,632,543 | 919,798 | 918,222 | £110,945,183 | 1576 | £70,388 |
| TUMT × 2/TUVP/TURP | £547,091,377 | £435,632,543 | 919,896 | 918,222 | £111,458,834 | 1674 | £66,602 |
| TUMT × 2/TUVP/TURP × 2 | £549,476,915 | £435,632,543 | 918,172 | 918,222 | £113,844,372 | –50 | TURP dominant |
| TUMT × 2/TURP | £547,469,842 | £435,632,543 | 919,899 | 918,222 | £111,837,299 | 1677 | £66,693 |
| TUMT × 2/TURP × 2 | £556,354,850 | £435,632,543 | 919,684 | 918,222 | £120,722,307 | 1462 | £82,562 |
| TUVP/TURP × 2 | £551,652,179 | £435,632,543 | 919,846 | 918,222 | £116,019,636 | 1624 | £71,441 |
Sensitivity analyses
Table 37 shows the results of one-way sensitivity analysis on a series of predetermined parameters. Varying the values for these parameters did not affect the set of non-dominated or non-extendedly dominated strategies. The exception to this was when the probability of treatment failure was based on the risks of reoperation and not changes in symptom scores. In this situation the use of HoLEP as a single treatment was excluded as it was extendedly dominated by the other treatment strategies considered. The reason for this is that the probabilities of failure all improved when the probability of treatment failure was based on the risks of reoperation and not changes in symptom scores. However, the probability of cost-effectiveness for HoLEP improved the least.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness (QALYs) | Incremental effectiveness (QALYs) | ICER |
|---|---|---|---|---|---|
| Base caseb | |||||
| TUVP | £380,774,844 | 91,7082 | |||
| HoLEP | £400,549,783 | £19,774,939 | 91,9656 | 2574 | £7682 |
| TUVP/HoLEP | £413,712,972 | £13,163,189 | 92,1041 | 1385 | £9505 |
| TUVP/TURP × 2 | £418,264,231 | £4,551,258 | 92,1091 | 50 | £90,576 |
| Start age 90 | |||||
| TUVP | £376,991,192 | 541,771 | |||
| HoLEP | £397,495,122 | £20,503,931 | 543,268 | 1497 | £13,695 |
| TUVP/HoLEP | £405,702,102 | £8,206,980 | 543,703 | 435 | £18,872 |
| TUVP/TURP × 2 | £409,475,528 | £3,773,426 | 543,715 | 12 | £309,087 |
| Start age 50 | |||||
| TUVP | £381,248,895 | 1,002,040 | |||
| HoLEP | £400,940,948 | £19,692,053 | 100,4857 | 2818 | £6988 |
| TUVP/HoLEP | £414,850,642 | £13,909,693 | 100,6451 | 1594 | £8727 |
| TUVP/TURP × 2 | £419,518,524 | £4,667,882 | 100,6511 | 59 | £78,771 |
| Utility of ‘incontinence, no remission’ the same as utility of ‘incontinence, remission’ | |||||
| TUVP | £380,774,844 | 917,131 | |||
| HoLEP | £400,549,783 | £19,774,939 | 919,679 | 2548 | £7762 |
| TUVP/HoLEP | £413,712,972 | £13,163,189 | 921,092 | 1413 | £9315 |
| TUVP/TURP × 2 | £418,264,231 | £4,551,258 | 921,144 | 52 | £88,045 |
| Utility of IPSS < 8 is 0.97 | |||||
| TUVP | £380,774,844 | 893,516 | |||
| HoLEP | £400,549,783 | £19,774,939 | 894,844 | 1328 | £14,889 |
| TUVP/HoLEP | £413,712,972 | £13,163,189 | 895,584 | 740 | £17,791 |
| TUVP/TURP × 2 | £418,264,231 | £4,551,258 | 895,611 | 28 | £163,682 |
| BPE risk data from all studies | |||||
| TUVP | £380,774,844 | 917,082 | |||
| HoLEP | £400,549,783 | £19,774,939 | 919,656 | 2574 | £7682 |
| TUVP/HoLEP | £413,712,972 | £13,163,189 | 921,041 | 1385 | £9505 |
| LOS TURP = LOS TUVP = 2 days | |||||
| TUVP | £376,715,152 | 917,082 | |||
| TURP | £380,679,392 | £3,964,240 | 918,222 | 1140 | £3476 |
| TURP × 2 | £400,362,758 | £19,683,366 | 920,340 | 2117 | £9296 |
| TUVP/TURP × 2 | £409,495,593 | £9,132,834 | 921,091 | 751 | £12,156 |
| Probability of failure (model 2) | |||||
| TUVP | £380,793,296 | 918,558 | |||
| TUVP/HoLEP | £404,008,222 | £23,214,926 | 921,217 | 2659 | £8731 |
| TUVP/TURP × 2 | £406,972,673 | £2,964,451 | 921,269 | 52 | £56,845 |
| Test for obstruction after TUVPc | |||||
| TUVP | £380,774,844 | 917,082 | |||
| HoLEP | £400,549,783 | £19,774,939 | 919,656 | 2574 | £7682 |
| TUVP/HoLEP | £405,478,440 | £4,928,657 | 920,051 | 395 | £12,475 |
| TUVP/TURP × 2 | £409,175,523 | £3,697,083 | 920,128 | 78 | £47,659 |
| TURP × 2 | £457,866,096 | £48,690,573 | 920,340 | 211 | £230,608 |
In all sensitivity analyses the ICERs are reported in Table 37. Any changes in ICERs are intuitively sensible. Whether these changes are sufficient to affect the choice of strategy depends again on society’s willingness to pay for a QALY. However, in all cases but two a change from the status quo of TURP × 2 would be cost-effective. One case is if the LOS of TURP (exclusive of complications) were to be reduced from 3 to 2 days in line with that of TUVP. Here the decision would depend on the opportunity cost of moving to the more expensive but more effective TUVP/TURP × 2. In the other case, pressure testing is applied after TUVP as well as after TURP, which, although not standard practice, might be plausible and would thus makes TURP × 2 the most effective strategy. Although the ICER for TURP × 2 would be extremely high, given that it is already current practice it might be difficult to cancel the most effective although perhaps rather costly treatment.
Multiple cohort (population-based) versus single cohort (individual-based) model comparison
Table 38 shows the effect of estimating costs and QALYs for the entire population of men presenting for surgery at the rate of 25,000 per year for the next 10 years versus the effect of estimating costs and QALYs per individual from that population over 10 years, each starting now, discounted at 3.5%. To make the comparison clearer, capital costs have been excluded. It has already been argued that the former (population-based) approach is the appropriate model for dealing with capital costs and therefore this sensitivity analysis is intended to show that there is also difference between the models excluding such costs because of the ‘mixing’ effect described above (see the beginning of Chapter 11).
| Cost (£) | Incremental cost (£) | Effectiveness (QALYs) | Incremental effectiveness (QALYs) | ICER | |
|---|---|---|---|---|---|
| Individual based (single cohort model) | |||||
| TUVP | £1794 | 7.119357 | |||
| HoLEP | £1819 | £25 | 7.139511 | 0.020154 | £1242 |
| TUVP/HoLEP | £1958 | £139 | 7.152449 | 0.012938 | £10,755 |
| TUVP/TURP × 2 | £1990 | £31 | 7.152964 | 0.000515 | £60,896 |
| Population based (multiple cohort model) | |||||
| TUVP | £380,774,844 | 917,081.6 | |||
| HoLEP | £386,049,783 | £5,274,939 | 919,655.7 | 2574.14 | £2049 |
| TUVP/HoLEP | £412,403,965 | £26,354,182 | 921,040.6 | 1384.83 | £19,031 |
| TUVP/TURP × 2 | £418,264,231 | £5,860,266 | 921,090.8 | 50.24794 | £116,627 |
It can be seen that the model does make a difference to the precise ICERs but that TUVP/HoLEP and TUVP/TURP × 2 are still more effective and less costly (not shown) than TURP × 2 (assumed to be current practice) and therefore the choice of strategy is between these strategies.
Expected value of perfect information (EVPI)
As described in Chapter 4 it is possible to use the DAM to estimate the value of reducing the uncertainty within the model and hence reduce the probability of making a wrong decision. Uncertainty can be reduced by obtaining further information and Table 39 provides an indication of the value of reducing all uncertainty in the model (i.e. our choice about which treatment or sequence of treatments is most cost-effective is based on perfect information). Also included in this table is the value of removing all uncertainty surrounding estimates of specific groups of parameters (the expected value of partial perfect information; EVPPI). The EVPI and the EVPPIs reported in Table 39 are calculated at a threshold value for society’s willingness to pay for a QALY of £20,000, given uncertainty as to its value.
| Parameter group | EVPI (£) |
|---|---|
| All parameters (expected value of perfect information) | 5,269,869 |
| Expected value of partial perfect information (EVPPI) | |
| TUVP epidemiology | 4,187,062 |
| HoLEP epidemiology | 1,652,886 |
What Table 39 provides is an indication of the cost of the uncertainty, either overall (the EVPI) or in specific groups of parameters (EVPPI), and, therefore, the maximum value of future research that might be conducted to reduce this uncertainty. Parameter groupings such as utilities are not included because their EVPI was either extremely low or zero, i.e. their uncertainty had little or no effect on which strategy was cost-effective. It should be noted that this analysis does not reflect the value of improving model structure, for example the method of mapping IPSS on to utilities. It also assumes that the distributions around all of the parameters identified are accurate representations of the real uncertainty surrounding these parameter estimates.
Given an annual number of men undergoing TURP in the UK of 25,000, a discount rate of 3.5% and a £20,000 per QALY threshold, this places an upper limit on all future research investment of about £5.3 million over 10 years. If it is assumed that the sizes of the EVPPIs are directly proportional to the value of conducting further research then research focusing on improving the estimates of TUVP epidemiology (i.e. estimates of relative risks of complications and estimates of the WMD in IPSS relative to TURP) would have by far the highest priority. This could be achieved by undertaking more research comparing TUVP with TURP, perhaps within an RCT setting, with an upper limit on spending of about £4.1 million. The EVPI is highly sensitive to the willingness to pay for a QALY in that it almost doubles to £10.2 million on moving from £20,000 to £10,000 per QALY. This can be understood by observing that on the CEAC there is no clear ‘front runner’ at £10,000, which implies greatest uncertainty.
Consequences (disaggregated)
The cost-effectiveness analysis reported above aggregates the time spent in the various states of the model by the quality of life associated with these states. Although this has been carried out using the best evidence available and using explicit methods, further insight can be gained by considering the time spent in each of the states within the model for each treatment and treatment strategy considered (Table 40).
| Operation | Remission | No remission | Incontinence | Death | Total | |
|---|---|---|---|---|---|---|
| TUMT | 0.25 | 5.28 | 2.74 | 0.06 | 1.67 | 10 |
| TUVP | 0.25 | 7.27 | 0.72 | 0.09 | 1.67 | 10 |
| KTP | 0.25 | 5.65 | 2.21 | 0.21 | 1.67 | 10 |
| TURP | 0.25 | 7.44 | 0.55 | 0.09 | 1.67 | 10 |
| HoLEP | 0.25 | 7.65 | 0.33 | 0.09 | 1.67 | 10 |
| TUMT × 2 | 0.35 | 6.82 | 1.08 | 0.08 | 1.67 | 10 |
| TUMT/TUVP | 0.35 | 7.56 | 0.33 | 0.08 | 1.67 | 10 |
| TUMT/TURP | 0.35 | 7.61 | 0.27 | 0.09 | 1.67 | 10 |
| TUVP/TURP | 0.27 | 7.89 | 0.07 | 0.09 | 1.67 | 10 |
| TURP × 2 | 0.26 | 7.78 | 0.18 | 0.10 | 1.67 | 10 |
| TUMT × 2/TUVP | 0.40 | 7.62 | 0.23 | 0.08 | 1.67 | 10 |
| TUMT × 2/TURP | 0.40 | 7.63 | 0.21 | 0.09 | 1.67 | 10 |
| TUMT/TUVP/TURP | 0.36 | 7.75 | 0.13 | 0.09 | 1.67 | 10 |
| TUMT/TURP × 2 | 0.36 | 7.71 | 0.16 | 0.09 | 1.67 | 10 |
| TUVP/TURP × 2 | 0.27 | 7.92 | 0.04 | 0.09 | 1.67 | 10 |
| TUMT × 2/TUVP/TURP | 0.40 | 7.68 | 0.16 | 0.09 | 1.67 | 10 |
| TUMT × 2/TURP × 2 | 0.40 | 7.67 | 0.17 | 0.09 | 1.67 | 10 |
| TUMT/TUVP/TURP × 2 | 0.36 | 7.75 | 0.12 | 0.09 | 1.67 | 10 |
| TUMT × 2/TUVP/TURP × 2 | 0.40 | 7.68 | 0.16 | 0.09 | 1.67 | 10 |
| TUMT/HoLEP | 0.35 | 7.68 | 0.21 | 0.09 | 1.67 | 10 |
| TUMT × 2/HoLEP | 0.40 | 7.66 | 0.19 | 0.09 | 1.67 | 10 |
| TUVP/HoLEP | 0.27 | 7.91 | 0.05 | 0.09 | 1.67 | 10 |
| TUMT/TUVP/HoLEP | 0.36 | 7.75 | 0.12 | 0.09 | 1.67 | 10 |
| TUMT × 2/TUVP/HoLEP | 0.40 | 7.68 | 0.16 | 0.09 | 1.67 | 10 |
Table 40 shows that each strategy is associated with the same risk of death and hence the average time spent in that state is the same. The majority of time for each strategy is spent in the state of remission, although the average number of years spent in this state varies between 5.28 years for TUMT only and 7.92 years for TUVP/TURP × 2. Except for the strategy of KTP (0.21 years), the time spent in the state of incontinence is approximately a tenth of a year or less. Finally, the time spent in the state of no remission also varies considerably, with patients receiving TUVP/TURP × 2 and TUVP/HoLEP spending on average 0.05 of a year or less in this state and patients receiving a single TUMT spending on average over 2.74 years in this state.
In Table 41 the different strategies are ranked in order of the time spent in two particular states: remission from LUTS and incontinence (the highest ranked strategy for remission is the strategy associated with the longest time spent in remission and the highest ranked strategy for incontinence is the one in which the least time is spent with incontinence). These two states are included as they are key determinants of the QALY estimates presented above. As this table illustrates there is no clear winning strategy. However, the CUA presented above suggests that the greater time spent in remission tends to be more important than the shorter time spent in the state of incontinence. Therefore, the findings of the CUA that TUVP/TURP × 2 is the most effective in terms of QALYs are perhaps to some extent validated by this analysis.
| Rank | Remission | Incontinence |
|---|---|---|
| 1 | TUVP/TURP × 2 | TUMT |
| 2 | TUVP/HoLEP | TUMT × 2 |
| 3 | TUVP/TURP | TUMT/TUVP |
| 4 | TURP × 2 | TUMT × 2/TUVP |
| 5 | TUMT/TUVP/TURP × 2 | TUMT × 2/HoLEP |
| 6 | TUMT/TUVP/HoLEP | TUMT × 2/TUVP/HoLEP |
| 7 | TUMT/TUVP/TURP | TUMT × 2/TUVP/TURP |
| 8 | TUMT/TURP × 2 | TUMT × 2/TURP |
| 9 | TUMT × 2/TUVP/TURP × 2 | TUMT × 2/TUVP/TURP × 2 |
| 10 | TUMT × 2/TUVP/HoLEP | TUMT/HoLEP |
| 11 | TUMT × 2/TUVP/TURP | TUMT × 2/TURP × 2 |
| 12 | TUMT/HoLEP | TUVP |
| 13 | TUMT × 2/TURP × 2 | TUMT/TUVP/HoLEP |
| 14 | TUMT × 2/HoLEP | TUMT/TURP |
| 15 | HoLEP | TUMT/TUVP/TURP |
| 16 | TUMT × 2/TURP | TUMT/TUVP/TURP × 2 |
| 17 | TUMT × 2/TUVP | TUMT/TURP × 2 |
| 18 | TUMT/TURP | HoLEP |
| 19 | TUMT/TUVP | TURP |
| 20 | TURP | TUVP/HoLEP |
| 21 | TUVP | TUVP/TURP |
| 22 | TUMT × 2 | TUVP/TURP × 2 |
| 23 | KTP | TURP × 2 |
| 24 | TUMT | KTP |
Summary
In this chapter our DAM has been presented, which responded to the issues raised by the critique of previous DAMs reported in Chapter 4. The results show that the least costly treatment is TUVP followed by TUMT and then HoLEP but that TUMT is less effective than TUVP and HoLEP is more effective than TUVP. However, HoLEP might not be considered to be the most cost-effective when balancing all relevant complications with LUTS improvement as shown by the use of QALYs. This is because no treatment is 100% effective and the use of the most effective single treatment of HoLEP is believed to preclude any further treatment that might otherwise ‘mop up’ those who fail. Therefore, treating with a less effective, but nonetheless still very effective, treatment that allows further treatment should there be failure might be the best option. This approach has the advantage of most men achieving effective symptom relief with reduced complications and lower cost, although a few men would be disadvantaged by needing a further, more invasive treatment.
Whether this is indeed the case and what sequence of treatments is optimal depends on two major factors, the ‘true’ outcomes of the procedures and society’s willingness to pay for a QALY. What is the appropriate level of society’s willingness to pay for a QALY is unclear as it depends upon the opportunity cost of the resources required to obtain an additional QALY, which is unknown. As for the first factor, this study has attempted, through economic and statistical methods, to represent the beliefs of decision-makers, informed by the best evidence, regarding the relationship between the outcomes and each strategy. As stressed earlier in this report, there are considerable limitations in the current evidence base for estimating effects and so the values used in the DAM may be subject to considerable uncertainty. Nevertheless, the base-case results should provide a basis to inform the current decision as to which technology should be implemented. Should it be shown that it is affordable then the model suggests that the best strategy would be TUVP followed, if necessary, by up to two TURPs. In practice, however, these results should be interpreted with caution and the data on which they are based are probably not strong enough to warrant a change in NHS practice from the TURP × 2 strategy. However they do indicate that strategies of HoLEP alone and TUVP followed by HoLEP (TUVP/HoLEP) might be worthy of further consideration.
The value of perfect information results indicate that it might be worth considering further research to better inform a decision in the future and also to determine the relative priorities of the types of evidence that need to be gathered. It should be noted that the results presented depend upon the imprecision around estimates being fully incorporated into the model. Nevertheless, the results indicate that it may be worthwhile gathering further evidence to compare TUVP and TURP.
Chapter 11 Discussion
In common with other areas of medicine the surgical treatment of BPE has undergone rapid technological change in recent years. Routine application of such new technology is dependent on many factors but is ideally governed by demonstration of benefit over existing standard treatment, in this case TURP. A systematic review of interventions with meta-analysis of available data and an economic evaluation was undertaken to determine whether any of the currently available newer technologies provide greater effectiveness, fewer complications and greater cost-effectiveness than TURP.
Summary of results
In respect of symptoms associated with BPE we found that TURP provides a consistently high level of improvement, which persists in the long term. This is associated with significant improvement in quality of life and peak urine flow rate. Of the newer technologies, minimally invasive options such as TUMT and TUNA result in less symptom improvement and a smaller increase in peak urine flow rate. Ablative procedures such as TUVP and laser resection (HoLEP) give similar symptom and quality of life improvements to TURP, and HoLEP additionally results in a greater improvement in flow rate (WMD + 1.43 ml/s at the 12-month follow-up). Purely in terms of effectiveness, HoLEP would appear to be unique amongst the newer technologies in offering an advantage over TURP, although, based on the current short-term outcome data available, this is confined to the urodynamic outcome, which may not be of importance to patients. Longer-term outcome data are keenly awaited. Reduction in hospital stay for elective surgery is currently considered to provide benefit to the patient in terms of avoiding complications and to the care provider in terms of reducing costs. Some of the newer technologies take longer to carry out but in the UK and the US context may result in a reduction in stay of up to 1 day, although this may be associated with a more prolonged period of catheterisation at home. It should also be noted that hospital stay for TURP is also shortening, from 5–6 days in the older trials to 3 days in the more contemporary ones. The impact of increased operating time and reduced hospital stay will vary between care providers and different health-care systems.
The search for alternative methods of prostate ablation has been fuelled largely by the risk of adverse consequences of bleeding during and after conventional TURP. This is a particular issue because excessive blood loss and the requirement for irrigation during the procedure may contribute to perioperative risk, particularly for elderly men who often have pre-existing cardiovascular disease. Our review confirmed that severe blood loss, as indicated by the need for blood transfusion, was more common amongst men randomised to TURP than amongst those undergoing most, if not all, other interventions. It should be noted, however, that contemporary studies such as those involving HoLEP show much lower rates of transfusion after TURP than older studies, suggesting beneficial changes to the performance of standard surgery over time.
The situation regarding complications that cause continued disability and hence that can be assumed to have an adverse effect on quality of life with associated ongoing health-care costs is much less clear. Sexual side effects of surgery, particularly loss of ejaculation and erectile dysfunction, are also of concern to men undergoing prostate surgery. The risk of retrograde ejaculation is significantly lower for minimally invasive procedures and TUIP, presumably indicating relative preservation of the preprostatic sphincter. For ablative procedures, perhaps not surprisingly, the risk is similar to that of TURP. Reassuringly, the occurrence of ejaculatory dysfunction does not seem to cause much in the way of quality of life impairment following prostate surgery. Rates of erectile dysfunction were similar across all procedures although lack of baseline data is a likely source of bias. The lack of effect of prostate surgery on this aspect of sexual function is supported by data from trials including a no intervention arm. 6 The rate of incontinence, the adverse effect most feared by men undergoing surgery for BPE, was similar across all interventions with the exception of TUNA and laser coagulation (for which reported rates were lower), although comparative analysis was hampered by variability in definition. This finding is perhaps expected because all of the tissue ablative procedures follow the concept of removing prostate tissue to achieve benefit and therefore have the same risk of sphincter damage or pre-existing bladder dysfunction. The other most pertinent long-term adverse effect is the need for further treatment as a result of stricture formation, urinary retention or disease relapse. Unfortunately, as is frequently the case, these were not primary outcome measures in any of the RCTs and the necessary long-term follow-up data were either missing or incomplete. Difficulty passing urine after surgery reflected by the complication of acute retention together with the later need for reoperation was, however, more frequently seen with newer technologies, especially the minimally invasive interventions, which probably reflects the generally smaller amount of tissue removed or ablated by these procedures. This contention is supported by results from trials using HoLEP, in which the extent of prostate removal is similar to that of TURP, which is reflected in equivalent rates of retention and reoperation.
The results of the review of effectiveness were, along with other relevant data (e.g. on costs and utilities), combined in an economic model (the DAM). The purpose of the DAM was to determine which single surgical treatment or sequence of surgical treatments for BPE would be considered most likely to be cost-effective. The DAM can be thought of as a further level of evidence synthesis as it sought to combine the best available evidence to provide estimates of costs, effectiveness (measured in terms of QALYs) and cost-effectiveness. The results of the DAM suggest that the treatment or sequence of treatments that would be considered cost-effective is dependent upon what value we think society would be willing to pay to obtain an additional QALY. The most effective single treatment was HoLEP. However, the most effective strategy was TUVP/TURP × 2. The difference between these appears to be small on average, but the crucial issue is whether society is willing to pay for this gain in effectiveness.
HoLEP as a single treatment was found to be cost-effective for a willingness to pay of up to about £4556 per QALY. Up to £47,221 per QALY, TUVP followed by HoLEP would be considered cost-effective. Only at higher values for society’s willingness to pay would one choose the most effective strategy, i.e. TUVP/TURP × 2. However, the story does not end there because, even if we believe that these results reflect our beliefs as informed by the best available evidence, there remains uncertainty. This was represented in a probabilistic way and can be observed partly in the CEAC and also in the EVPI and EVPPI. The CEAC shows that, at a willingness to pay of about £20,000, there is little doubt that TUVP/HoLEP is cost-effective. However, there are peaks of uncertainty at about £5000 and £50,000 and at these values for society’s willingness to pay for a QALY, EVPI and EVPPI are highest, particularly at a threshold of £5000 per QALY. If one believes that the current threshold for the NHS is about £20,000, which is probably conservative, then it would seem reasonable to recommend changing from the current practice of a single TURP to TUVP/HoLEP. However, the economic model should be interpreted cautiously because of the assumptions and uncertainties that underpin it as well as the threshold value for society’s willingness to pay for a QALY.
These results are consistent with the finding of the systematic review of effectiveness. It is important to note that even relatively modest changes in the parameter estimates used in the DAM might change these results because there are few data available for many of the comparisons and, as a result, estimates of effectiveness (and hence cost-effectiveness) will change as new data become available.
Strengths and weaknesses of the review of clinical effectiveness
The strength of the study is the systematic approach taken to review the evidence (published and unpublished data without language restrictions). Exhaustive systematic searches were made of the major electronic databases. All potentially eligible studies were reviewed for eligibility and the study quality assessed. Outcome parameters were predetermined and data were extracted using standard forms. Despite these efforts it is possible that some relevant and usable data remained hidden as a result of non-publication.
Moreover, more than half of the available evidence was reported in abstract form rather than in full-text published studies. The difficulties in accessing raw or summarised data from studies reported only in abstract form are well recognised and the process was beyond the funding limits of our review. The exclusion of these studies prevents us from estimating the impact of this form of publication bias on the results. The reasons why so many trials were only reported as abstracts were unclear and ideally should be investigated because publication bias has been shown to account for up to 45% of an observed association, which may change the direction of effect. 274
Empirical research in other fields has shown that unpublished reports tend to show less positive results than published reports, and so exclusion of these could introduce publication bias. In total, 88 full-text primary RCTs were identified. Although this haul of relevant trials is impressive, the majority of studies recruited small numbers of patients and covered many different comparisons, diluting the opportunities for meta-analysis. The confidence intervals around estimates of differences were often wide and this problem may result in a failure to demonstrate statistical significance for a clinically important effect or a failure to rule out an effect when it does not exist. 275,276
Another major limitation resulted from the fact that the majority of comparisons were made against TURP, with few head-to-head comparisons of the newer technologies. Study inclusion criteria also varied considerably between the trials, which calls into question the generalisability of the findings on meta-analysis to ‘everyday practice’. This was exacerbated by variation in operative technique and treatment protocols between studies investigating the same technology. These variations were of particular concern in studies involving laser technology, in which there was variation in power settings and temperature, together with site and duration of laser application. The limited descriptions of technologies in some study reports made it hard to determine whether they were minimally invasive or tissue ablative. This is an important possible explanation for the statistical heterogeneity that was common in the analysis. The long time base of the studies reviewed (20 years) in the context of rapidly changing and evolving technology also presents difficulties in interpretation of the findings. To overcome this we categorised interventions conceptually according to the mechanism of treatment of BPE between standard, minimally invasive and tissue ablative. Despite this the ablative group does have a range of tissue effects from partial vaporisation to complete resection. In addition, the standard of conventional TURP has not been static over this time frame. Developments in camera and televisual display and diathermy generators, improvements in perioperative care and concentration of the procedure in the hands of specialist urologists have all served to make the operation more uniform in outcome and less morbid in terms of adverse effects. All of these factors are likely to influence the findings. Although the review attempted to identify and explore sources of variability, for many outcomes it remained unclear as to whether any conclusions should be drawn from the results given the high statistical heterogeneity that was present.
The role of quality assessment in the conduct of a systematic review is important. For this review a robust combined checklist assessing different sources of bias was produced. We avoided using a scoring scale approach as this has been reported to be inaccurate concerning the direction of bias277,278 and can include items that are unrelated to the internal validity of a study. 279 In this review we found that the majority of included RCTs were poorly reported, which may be associated with low levels of methodological quality. 279 There are a number of mitigating factors such as space limitations in the publishing journals but it is a generally held view that if necessary information is not provided then the quality will always be inadequate. 280 Without adequate reporting, assessing quality becomes impossible,281 and the drive to ensure adherence to standardised conduct and reporting guidelines for RCTs has much to commend it from the point of view of the systematic reviewer. 88 It is also of concern that reporting of allocation concealment was unclear in 74% of the included studies and 14% used an inadequate approach to concealment of randomisation. This increases the risk of selection bias by disrupting the assignment sequence and may result in loss of the advantages of randomisation. 282 The main consequence of this is thought to be the generation of larger estimates of treatment effects. 281 An observational study that assessed methodological quality of 250 RCTs from 33 meta-analyses found that odds ratios were exaggerated by 30% for trials with unclear concealment protocols. 281 There were also differences between trials with regard to baseline characteristics. For example, studies comparing the efficacy and safety of laser resection with the efficacy and safety of TURP included patients with large prostate glands, whereas those assessing laser vaporisation included patients with a wide variation in prostate size. Variations such as these make the results difficult to interpret.
Blinding of patients, outcome assessors and care providers is another important methodological issue and reporting of this was unclear in more than 70% of the studies. For the present review, obvious differences in the technologies make blinding of the patient and operator difficult, but the outcome assessor could be blind to the allocated treatment and trial reports should include a description of the attempts made to prevent ascertainment bias.
Many studies failed to report point estimates and measures of variability, which hinders calculation of the precision of the overall pooled estimate and calculation of weighted mean differences when standard deviations are required. 283 In this review of effectiveness, when an appropriate measure of variability was not reported for continuous outcomes, consistency across studies reporting the outcome was investigated. Methods to derive an estimate of standard deviation have been described, based on the imputation of plausible values, but doubts as to their validity exist as many have not been theoretically derived or empirically tested. 283 It is possible that if means and standard deviations were reported more consistently, effect sizes would be different. This is another reason why adherence to CONSORT guidelines for reporting of clinical trials greatly aids the conduct of robust meta-analyses.
A more specific methodological limitation that frustrated pooled analysis was the use of differing measures of symptomatic outcome in the older studies. We did attempt to convert the older and now little-used Madsen–Iversen symptom score to the present standard of AUA/IPSS using a method suggested by Barry and colleagues284 but found that the results lacked reliability. This problem forced us to analyse studies using the Madsen–Iversen index separately, so reducing the power of the meta-analyses.
In summary, we believe that we have used the best available techniques to identify, review and meta-analyse the data that were available to us. This approach has enabled us to make robust broad conclusions concerning the relative beneficial and adverse effects of new technologies for the invasive treatment of symptomatic BPE compared with the standard of TURP. Our ability to consider infrequent complications and achieve precise separation of the different procedures according to relative effectiveness was limited by the small numbers of patients studied, inadequate reporting of trials, the use of differing outcome measures and the pace of technological development.
Strengths and limitations of the DAM
The DAM chapter provides an explicit and detailed description of the method used. It sought to use a set of criteria to identify which treatments and strategies were clinically plausible for the UK and these were then compared in terms of their costs and consequences. The pathways, developed following detailed discussions with the clinical experts involved in the study, were used to structure the economic model and identify which data would be required to parameterise the model. The methods used to obtain the parameter estimates were explicit and systematic and sought to identify the best data available. When assumptions were made about which data to use or how they would be used in the model, these have been described and justified, and, when necessary, they were tested in sensitivity analyses. This sensitivity analysis was conducted deterministically when appropriate, along with probabilistic sensitivity analysis. The probabilistic sensitivity analysis and probability distributions for all relevant parameters were obtained using explicit methods that met current guidelines for best practice in economic modelling. 285
Despite our best efforts to conduct a rigorous economic evaluation using the best methods and data available, the results of the economic model should be interpreted cautiously because of the uncertainties and assumptions that underpin it. In particular, as described in the previous subsection, the evidence on effectiveness is limited because of the paucity of the available evidence base. As these data formed many of the input parameters of the DAM, this leads to uncertainties in the results obtained from the DAM. As indicated above, when possible, data inputs to the DAM and assumptions were tested in sensitivity analyses. In addition, when appropriate, parameters were estimated as distributions. These distributions were based on the available data and on guidelines for best practice and attempted to account for the imprecision surrounding the point estimates used within the DAM. It is still, however, contestable whether the parameter estimates and their associated distributions are an accurate measure of the true values of the parameters. However, although the data used were the best available and all distributions were examined in terms of summary statistics (expected value and confidence intervals) by the clinical experts to test their face validity, it is possible that the available data are biased. This is because, as described earlier, the data contributing to the pooled estimates of effectiveness were incomplete and heterogeneous. When sampling data were not available, distributions were constructed in a pragmatic way; however, expert opinion was always sought. Extensive one-way sensitivity analysis was also used to reveal parameters when the decision was sensitive to variability within the range of a distribution.
In addition to the limitations caused by the evidence base, the economic evaluation suffers from a number of other limitations. First, conclusions about cost-effectiveness are sensitive to the value that we think society might be willing to pay for an additional QALY. Although there have been some attempts to define what this value might be,286 in this report we do not explicitly identify the opportunity cost (i.e. the benefits forgone) of redeploying resources to provide a more costly but more beneficial procedure.
The model attempted to compare many different strategies, indeed many more than any previous evaluation in this area. Nevertheless, it was not possible to include every permutation of treatments. Therefore, a series of judgements had to be made about which strategies to present. This judgement was informed by discussions with the surgeons involved in the project team. Thus, twenty strategies were compared within the model and the reasoning behind including these strategies was explicit, with justification based on expert opinion and logic.
One of the determinants of cost-effectiveness was the probability of treatment failure. Within the model, assumptions had to be made as to how best to define treatment failure. For the base-case analysis, the definition used for treatment failure was based on clinical criteria relevant to the UK (the percentage change in IPSS). However, this created problems in terms of estimating probabilities of failure from the literature in cases in which only reoperation rates were available and the criteria for reoperation used in the different studies were either unknown or variable. A method was found to solve this problem whereby the best available evidence, i.e. weighted mean differences from the meta-analysis, was used, but it necessitated the use of observational individual-level data and the use of certain contestable assumptions. Therefore, the results of this analysis were compared with the results obtained when the failure was defined using reoperation rates. The results using these two different approaches were reassuringly consistent.
IPSS scores were also central to the estimation of QALYs. More specifically it was believed that utility scores that underpin the QALY estimates should be related to IPSS as well as to the presence or absence of complications. However, no single reliable source could be found that would allow us to do this. Thus, a set of assumptions to synthesise data from various sources were made. Some of these assumptions are contestable but they were tested in the sensitivity analysis and were found, on the whole, not to affect outcome. Perhaps more importantly, the estimation of QALYs relied on a mapping exercise from IPSS on to utility scores. There was no alternative source of such data and there may be concerns over the validity and usefulness of the estimates it produced. The estimates of EVPI did not capture the effect of removing this uncertainty as no probability distribution was specified. However, it is likely that further research into the mapping of IPSS on to health state utilities would be warranted.
Estimates of cost were not always easy to obtain; however, this study provided a breakdown of costs that was sufficient to estimate the independent effects of procedure cost, hospital inpatient stay and purchase of any new equipment. Nevertheless, costing by all resource categories was not possible. Therefore, a judgement was made as to those resource categories that were most likely to produce a difference in the decision. These judgements were informed by data that were relevant to the UK including the NHS reference costs. However, the NHS reference costs are not provided for all relevant treatments and, indeed, largely refer to TURP only. They also include a length of stay component that not only is a function of the complications of treatment but also reflects variations in practice. Therefore, methods were used to replace the length of stay component of these costs with length of stay costs based on typical length of stay (based on clinical opinion) and the cost of a day in hospital on a urology ward. It was also assumed, on the basis of availability in a typical institution, that TURP and TUVP would incur no additional equipment costs but that TUMT, HoLEP and KTP would. All of these assumptions were tested in sensitivity analyses.
The incorporation of complications into the model was also problematic. For example, there were no standard reference costs available for the management of complications and, therefore, expert opinion was used to inform the cost of these events. The likelihood of complications occurring (i.e. the event probabilities) was also important for the model. These probabilities were estimated using the best available source, i.e. relative risks and pooled baseline TURP probabilities from the meta-analysis. They are limited, nevertheless, by the imprecision of the estimates, the possibility of population heterogeneity, variability in reporting and uncertainty in the time frame over which these events might occur. Thus, it was assumed that all complications except incontinence were short term and that all cases of incontinence were of urge incontinence (although it is possible that some cases may in fact be stress urinary incontinence), which was assumed to be permanent. Again, these assumptions were explicit and justification was provided.
In summary, the DAM has sought to use the best available data relevant to the UK and combined it within an explicit model that was again structured to reflect the costs and consequences of treatments and treatment strategies potentially relevant to the UK. Although the results of the DAM should be treated cautiously, we believe that the results provide the best evidence on cost-effectiveness of surgical treatments for BPE available to the UK.
Chapter 12 Conclusions
Implications for practice
Based on current evidence it is not possible to reliably identify the most promising minimally invasive intervention, although, as a group, these interventions are less effective than TURP but are associated with fewer adverse effects. It is similarly not possible to reliably identify the most promising tissue ablative intervention for the reasons described above. TURP continues to be effective although is associated with potentially significant morbidity. Each of the surgical interventions for BPE has advantages and disadvantages. Irrespective of the choice of intervention, the true cost to patients and society in terms of quality of life has not been quantified to date. Given that there are broad similarities in clinical effectiveness of the minimally invasive and tissue ablative interventions, perhaps the most important issue is whether patients would prefer to have a minimally invasive procedure if they were aware that the intervention, albeit with fewer adverse effects, would be less effective than a tissue ablative intervention and would have a higher chance of requiring a second intervention.
Current UK clinical practice suggests a preference for oral medication using an alpha-blocker or 5-alpha-reductase inhibitor, alone or in combination, rather than the use of minimally invasive interventions. If oral medication fails to improve symptoms or if side effects develop, a tissue ablative intervention is offered. There is some evidence to suggest that the benefits offered by minimally invasive interventions are equivalent to those gained from oral medication287–289 and so this could be a popular option for some men.
The economic model should be interpreted cautiously because of the assumptions and uncertainties that underpin it. The model suggests that TURP alone or repeated is amongst the more effective strategies although it is not cost-effective in the Markov model. The model reveals that other strategies are possibly less costly and slightly more effective. Should it be judged affordable then the results of the model suggest that a strategy of TUVP followed by up to two more TURPs (should a previous procedure fail) would be most likely to be considered cost-effective. At lower levels of willingness to pay, a policy of TUVP followed by HoLEP for failure might be worthwhile.
For the NHS, increased use of TUVP and/or holmium laser prostatectomy would lead to an increased requirement for training, which may be costly. Because of the limited number of surgeons currently providing these treatments it will take time to establish an adequate level of provision. It is unclear how long this will take as no evidence was found to indicate the speed at which surgeons may progress up the learning curves for these procedures. If interventions such as these are to be used as second-line procedures, it would be important that their use is limited to specialist centres only. However, in the absence of strong evidence in favour of newer methods, TURP remains clinically effective and cost-effective. The use of minimally invasive technologies in the NHS is not appropriate until a more effective and/or less costly technology is available.
Implications for future research
Research efforts in the management of clinical BPE should now be concentrated on the performance of higher-quality, more rigorous studies. As a minimum, these should be RCTs using predefined, ideally standardised, measures of outcome, and be multicentre to ensure sufficiently precise estimates of the various outcomes. Such trials should be protocol driven and a detailed protocol of how the project is to be conducted should be agreed before commencement of the study. The protocol should state the research objectives, reasons for the study, issues related to study recruitment (inclusion and exclusion criteria), information to be collected at entry to the study, interventions of interest and arrangements for follow-up. A crucial stage in the development of a study protocol is agreement on the definition of outcome measures of interest so that outcomes/complications reported in different collaborating centres share the same meaning. Although all outcome measures should be predefined, this is most important for specific outcome measures such as urinary incontinence, urinary tract infection and failure of procedure. It is also essential that the reasons for reoperation be clearly stated, including when this decision is largely driven by patient choice. Future trials should also include direct measures of health state utilities.
In the context of the NHS and the patient, it is highly likely that choices based on strategies of management are more important than choices based on individual interventions. Areas in which further research would be important include:
-
For men who might currently be managed medically, a systematic review including modelling to determine how many years of medical treatment are necessary to offset the cost of treatment with a minimally invasive or ablative intervention in the first instance.
-
The true costs of the different interventions as a critical driver of economic evaluations.
-
Consensus work in partnership with governing bodies such as the British Association of Urological Surgeons to agree parameters for conducting future trials, such as standardising definitions and reporting of outcome measures.
-
For men judged to need ablative therapy, is there an alternative to TURP that is more effective, safe or cost-effective? A well-conducted head-to-head trial of treatment strategies – TUVP followed by either TURP or HoLEP versus HoLEP versus TURP × 2 – would be the most desirable to establish the gold standard. Such a trial should take prostate size into account and should also include direct measures of utility. Newer technologies could then be compared against this gold standard and, given the rapid developments in this area, a tracker trial approach may be appropriate.
-
Trials of different strategies aimed at improving outcomes and minimising adverse effects after TURP, particularly bleeding (the main serious adverse effect).
It should be stated clearly how data are to be collected and processed, what the primary and secondary outcome measures are and how statistical analysis will be conducted. The early involvement of trialists, statisticians and health economists is important to ensure that proposed trial designs and methods are appropriate, including sample size calculations. Consideration should be given to establishing a steering committee and a data monitoring committee to guide the conduct of the study.
In addition to any future RCT, a further area of research relevant to estimating cost-effectiveness, which might be performed as part of an RCT or as a parallel study, would consider in more detail how estimates of IPSS map on to estimates of utility and how utility, measured by a generic instrument, would change as IPSS changes. Such work would facilitate any modelling that may be required to extrapolate from the results of a future RCT.
Acknowledgements
This report would not have been possible without the support of Ailsa Snaith, Linda McIntyre and Sian Thomas who provided data abstraction of the included studies and Bronwyn Davidson for secretarial assistance.
The Health Services Research Unit is core funded by the Chief Scientist Office of the Scottish Government Health Directorate.
Contributions of authors
James N’Dow (Professor of Urology, clinical expert) led and co-ordinated all aspects of the project. Tania Lourenco (Research Fellow) reviewed the effectiveness of the technologies with the assistance of Angela Coutts (Research Assistant) and Susan Wong (Clinical Research Fellow), and wrote the executive summary with the assistance of James N’Dow. Nigel Armstrong (Research Fellow), with the assistance of Mark Deverill (Research Fellow) and Luke Vale (Senior Research Fellow), conducted the economic evaluation. Graham Mowatt (Research Fellow) commented on drafts of the report. Cynthia Fraser (Information Officer) developed and ran the search strategies and was responsible for obtaining papers and for reference management. Graeme MacLennan (Statistician) provided statistical support and advice. Robert Pickard (Senior Lecturer and Consultant Urological Surgeon, clinical expert), Samuel McClinton (Consultant Urological Surgeon, clinical expert), James N’Dow and Ghulam Nabi (Clinical Lecturer in Urology) wrote the background, developed the care pathways and provided clinical advice and critical comments. Adrian Grant (Director, methodology adviser) provided clinical and methodological advice and commented on drafts of the report. James N’Dow, Nigel Armstrong, Tania Lourenco, Luke Vale, Adrian Grant and Robert Pickard wrote the discussion and conclusions of the report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA Programme or the Department of Health.
References
- Homma Y. Pressure-flow studies in benign prostatic hyperplasia: to do or not to do for the patient?. BJU Int 2001;87:19-23.
- Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549-57.
- Boyle P, Robertson C, Mazzetta C, Keech M, Hobbs FD, Fourcade R, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int 2003;92:409-14.
- Fitzpatrick JM. The natural history of benign prostatic hyperplasia. BJU Int 2006;97:3-6.
- Fong YK, Milani S, Djavan B. Natural history and clinical predictors of clinical progression in benign prostatic hyperplasia. Curr Opin Urol 2005;15:35-8.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995;332:75-9.
- Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology 2001;58:5-16.
- Sarma AV, Jacobsen SJ, Girman CJ, Jacobson DJ, Roberts RO, Rhodes T, et al. Concomitant longitudinal changes in frequency of and bother from lower urinary tract symptoms in community dwelling men. J Urol 2002;168:1446-52.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000;163:107-13.
- Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997;158:481-7.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984;132:474-9.
- Guess HA. Epidemiology and natural history of benign prostatic hyperplasia. Urol Clin North Am 1995;22:247-61.
- Wei JT, Calhoun EA, Jacobsen SJ, Litwin MS, Saigal CS. Urologic diseases in America. Washington DC National Institutes for Health, National Institute of Diabetes and Digestive and Kidney Diseases: US Government Publishing Office; 2004.
- McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998;338:557-63.
- United Nations . World Population Prospects: The 2004 Revision Population Database 2004. http://esa.un.org/unpp (accessed August 2006).
- Chute CG, Panser LA, Girman CJ, Oesterling JE, Guess HA, Jacobsen SJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol 1993;150:85-9.
- Emberton M, Andriole GL, de la RJ, Djavan B, Hoefner K, Vela NR, et al. Benign prostatic hyperplasia: a progressive disease of aging men. Urology 2003;61:267-73.
- Foley CL, Taylor C, Kirby RS. Counting the cost of treating benign prostatic hyperplasia. BJU Int 2004;93:250-2.
- McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387-98.
- Barry MJ, Fowler FJ, Mulley AG, Henderson JV, Wennberg JE. Patient reactions to a program designed to facilitate patient participation in treatment decisions for benign prostatic hyperplasia. Med Care 1995;33:771-82.
- AUA Practice Guidelines Committee . AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol 2003;170:530-47.
- NHS Health and Social Care Information Centre . Hospital Episode Statistics, 2006 n.d. www.hesonline.nhs.uk (accessed August 2006).
- Office for National Statistics . United Kingdom Census 2001 n.d. www.statistics.gov.uk/census/ (accessed August 2006).
- de la Rosette JJ, Alivizatos G, Madersbacher S, Rioja Sanz C, Nordling J, Emberton M. European Association of Urology . Guidelines on Benign Prostatic Hyperplasia n.d. www.uroweb.nl/files/uploaded_files/guidelines/11%20BPH.pdf (accessed March 2006).
- Naspro R, Salonia A, Colombo R, Cestari A, Guazzoni G, Rigatti P, et al. Update of the minimally invasive therapies for benign prostatic hyperplasia. Curr Opin Urol 2005;51:49-53.
- Rubenstein J, McVary KT. Transurethral microwave thermotherapy of the prostate (TUMT). Emedicine; 2005.
- Boyle P, Robertson C, Vaughan ED, Fitzpatrick JM. A meta-analysis of trials of transurethral needle ablation for treating symptomatic benign prostatic hyperplasia. BJU Int 2004;94:83-8.
- Heaton JP. Radiofrequency thermal ablation of the prostate: the TUNA technique. Tech Urol 1995;1:3-10.
- Muruve NA, Steinbecker K, Willard TB. Transurethral needle ablation of the prostate (TUNA). Emedicine; 2005.
- Ogiste JS, Cooper K, Kaplan SA. Are stents still a useful therapy for benign prostatic hyperplasia?. Curr Opin Urol 2003;13:51-7.
- Madersbacher S, Marberger M. High-energy shockwaves and extracorporeal high-intensity focused ultrasound. J Endourol 2003;17:667-72.
- Sullivan LD, McLoughlin MG, Goldenberg LG, Gleave ME, Marich KW. Early experience with high-intensity focused ultrasound for the treatment of benign prostatic hypertrophy. Br J Urol 1997;79:172-6.
- Gelczer RK, Charboneau JW, Hussain S, Brown DL. Complications of percutaneous ethanol ablation. J Ultrasound Med 1998;17:531-3.
- Chiang PH, Chuang YC, Huang CC, Chiang CP. Pilot study of transperineal injection of dehydrated ethanol in the treatment of prostatic obstruction. Urology 2003;61:797-801.
- Levy DA, Cromeens DM, Evans R, Stephens LC, von Eschenbach AC, Pisters LL. Transrectal ultrasound-guided intraprostatic injection of absolute ethanol with and without carmustine: a feasibility study in the canine model. Urology 1999;53:1245-51.
- Mutaguchi K, Matsubara A, Kajiwara M, Hanada M, Mizoguchi H, Ohara S, et al. Transurethral ethanol injection for prostatic obstruction: an excellent treatment strategy for persistent urinary retention. Urology 2006;68:307-11.
- Goya N, Ishikawa N, Ito F, Kobayashi C, Tomizawa Y, Toma H. Transurethral ethanol injection therapy for prostatic hyperplasia: 3-year results. J Urol 2004;172:1017-20.
- Muschter R. Conductive heat: hot water-induced thermotherapy for ablation of prostatic tissue. J Endourol 2003;17:609-16.
- Muschter R, Schorsch I, Danielli L, Russel C, Timoney A, Yachia D, et al. Transurethral water-induced thermotherapy for the treatment of benign prostatic hyperplasia: a prospective multicenter clinical trial. J Urol 2000;164.
- Brosman SA. Interstitial laser coagulation of the prostate. Emedicine; 2006.
- The buyer’s guide for medical professionals. Prostate Management 2006. www.medcompare.com/ (accessed June 2006).
- Emberton M, Neal DE, Black N, Harrison M, Fordham M, McBrien MP, et al. The National Prostatectomy Audit: the clinical management of patients during hospital admission. Br J Urol 1995;75:301-16.
- Reich O, Gratzke C, Stief CG. Techniques and long-term results of surgical procedures for BPH. Eur Urol 2006;49:970-8.
- Lynch M, Anson K. Time to rebrand transurethral resection of the prostate?. Curr Opin Urol 2006;16:20-4.
- NHS reference costs. London: UK Department of Health; 2005.
- Riehmann M, Bruskewitz R. Transurethral incision of the prostate and bladder neck. J Androl 1991;12:415-22.
- Yang Q, Peters TJ, Donovan JL, Wilt TJ, Abrams P. Transurethral incision compared with transurethral resection of the prostate for bladder outlet obstruction: a systematic review and meta-analysis of randomized controlled trials. J Urol 2001;165:1526-32.
- Kaplan SA, Te AE. Transurethral electrovaporization of the prostate: a novel method for treating men with benign prostatic hyperplasia. Urology 1995;45:566-72.
- Wilson LC, Gilling PJ. From coagulation to enucleation: the use of lasers in surgery for benign prostatic hyperplasia. Nat Clin Pract Urol 2005;2:443-8.
- Daughtry JD, Rodan BA. Transurethral laser prostatectomy: a comparison of contact tip mode and lateral firing free beam mode. J Clin Laser Med Surg 1993;11:21-8.
- Kuntz RM. Current role of lasers in the treatment of benign prostatic hyperplasia (BPH). Eur Urol 2006;49:961-9.
- Te AE. The development of laser prostatectomy. BJU Int 2004;93:262-5.
- Gilling PJ, Fraundorfer MR. Holmium laser prostatectomy: a technique in evolution. Curr Opin Urol 1998;8:11-5.
- Sandhu JS, Ng C, Vanderbrink BA, Egan C, Kaplan SA, Te AE. High-power potassium-titanyl-phosphate photoselective laser vaporization of prostate for treatment of benign prostatic hyperplasia in men with large prostates. Urology 2004;64:1155-9.
- Kaplan SA, Laor E, Fatal M, Te AE. Transurethral resection of the prostate versus transurethral electrovaporization of the prostate: a blinded, prospective comparative study with 1-year followup. J Urol 1998;159:454-8.
- Tewari A, Narayan P. Electrovaporization of the prostate. Br J Urol 1996;78:667-76.
- Fowler C, McAllister W, Plail R, Karim O, Yang Q. Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia. Health Technol Assess 2005;9:1-30.
- Botto H, Lebret T, Barre P, Orsoni JL, Herve JM, Lugagne PM. Electrovaporization of the prostate with the Gyrus device. J Endourol 2001;15:313-16.
- Smith D, Khoubehi B, Patel A. Bipolar electrosurgery for benign prostatic hyperplasia: transurethral electrovaporization and resection of the prostate. Curr Opin Urol 2005;15:95-100.
- Sandhu JS, Ng AS, Gonzalez RR, Kaplan SA, Te AE. Photoselective laser vaporization prostatectomy in men receiving anticoagulants. J Endourol 2005;19:1196-8.
- Kuo RL, Paterson RF, Kim SC, Siqueira TM, Elhilali MM, Lingeman JE. Holmium laser enucleation of the prostate (HoLEP): a technical update. World J Surg Oncol 2003;1.
- Aho TF, Gilling PJ, Kennett KM, Westenberg AM, Fraundorfer MR, Frampton CM. Holmium laser bladder neck incision versus holmium enucleation of the prostate as outpatient procedures for prostates less than 40 grams: a randomized trial. J Urol 2005;174:210-14.
- Gilling PJ, Kennett KM, Fraundorfer MR. Holmium laser resection v transurethral resection of the prostate: results of a randomized trial with 2 years of follow-up. J Endourol 2000;14:757-60.
- Kuntz RM, Ahyai S, Lehrich K, Fayad A. Transurethral holmium laser enucleation of the prostate versus transurethral electrocautery resection of the prostate: a randomized prospective trial in 200 patients. J Urol 2004;172:1012-16.
- de Sio M, Autorino R, Quarto G, Damiano R, Perdona S, di Lorenzo G, et al. Gyrus bipolar versus standard monopolar transurethral resection of the prostate: a randomized prospective trial. Urology 2006;67:69-72.
- Eaton AC, Francis RN. The provision of transurethral prostatectomy on a day-case basis using bipolar plasma kinetic technology. BJU Int 2002;89:534-7.
- Starkman JS, Santucci RA. Comparison of bipolar transurethral resection of the prostate with standard transurethral prostatectomy: shorter stay, earlier catheter removal and fewer complications. BJU Int 2005;95:69-71.
- Talic RF, El Tiraifi A, El Faqih SR, Hassan SH, Attassi RA, Abdel-Halim RE. Prospective randomized study of transurethral vaporization resection of the prostate using the thick loop and standard transurethral prostatectomy. Urology 2000;55:886-90.
- Gilling PJ, Mackey M, Cresswell M, Kennett K, Kabalin JN, Fraundorfer MR. Holmium laser versus transurethral resection of the prostate: a randomized prospective trial with 1-year follow-up. J Urol 1999;162:1640-4.
- Hon NHY, Brathwaite D, Hussain Z, Ghiblawi S, Brace H, Hayne D, et al. A prospective, randomized trial comparing conventional transurethral prostate resection with plasmakinetic vaporization of the prostate: physiological changes, early complications and long-term follow-up. J Urol 2006;176:205-9.
- Kuntz RM, Lehrich K. Transurethral holmium laser enucleation versus transvesical open enucleation for prostate adenoma greater than 100 gm: a randomized prospective trial of 120 patients. J Urol 2002;168:1465-9.
- Kabalin JN, Gilling PJ, Fraundorfer MR. Application of the holmium:YAG laser for prostatectomy. J Clin Laser Med Surg 1998;16:21-7.
- MacDonald D, McNicholas TA. Drug treatments for lower urinary tract symptoms secondary to bladder outflow obstruction: focus on quality of life. Drugs 2003;63:1947-62.
- DiSantostefano RL, Biddle AK, Lavelle JP. The long-term cost-effectiveness of treatments for benign prostatic hyperplasia. Pharmacoeconomics 2006;24:171-91.
- Drummond MF, Sculpher M, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997.
- Briggs A, McGuire DM, McGuire A. Economic evaluation in health care: merging theory with practice. Oxford: Oxford University Press; 2001.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27.
- Ackerman SJ, Rein AL, Blute M, Beusterien K, Sullivan EM, Tanio CP, et al. Cost-effectiveness of microwave thermotherapy in patients with benign prostatic hyperplasia: I: Methods. Urology 2000;56:972-80.
- Blute M, Ackerman SJ, Rein AL, Beusterien K, Sullivan EM, Tanio CP, et al. Cost-effectiveness of microwave thermotherapy in patients with benign prostatic hyperplasia: II: Results. Urology 2000;56:981-7.
- Manyak MJ, Ackerman SJ, Blute ML, Rein AL, Buesterien K, Sullivan EM, et al. Cost-effectiveness of treatment for benign prostatic hyperplasia: an economic model for comparison of medical, minimally invasive, and surgical therapy. J Endourol 2002;16:51-6.
- DiSantostefano RL, Biddle AK, Lavelle JP. An evaluation of the economic costs and patient-related consequences of treatments for benign prostatic hyperplasia. BJU Int 2006;97:1007-16.
- Howard K, Wortley S. Transurethral needle ablation (TUNA) for the treatment of benign prostatic hyperplasia. MSAC application 1014. Medical Services Advisory Committee; 2002.
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397-409.
- Agency for Health Care Policy and Research . Benign Prostatic Hyperplasia: Diagnosis and Treatment 1994. www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat6.chapter.17571 (accessed August 2005).
- American Urological Association . Guideline on the Management of Benign Prostatic Hyperplasia (BPH) 2003. www.auanet.org/guidelines/bph.cfm (accessed August 2005).
- Francisca EA, d’Ancona FC, Meuleman EJ, Debruyne FM, de la Rosette JJ. Sexual function following high energy microwave thermotherapy: results of a randomized controlled study comparing transurethral microwave thermotherapy to transurethral prostatic resection. J Urol 1999;161:486-90.
- Madersbacher S, Lackner J, Brossner C, Rohlich M, Stancik I, Willinger M, et al. Reoperation, myocardial infarction and mortality after transurethral and open prostatectomy: a nation-wide, long-term analysis of 23,123 cases. Eur Urol 2005;47:499-504.
- NHS Centre for Reviews and Dissemination . Undertaking Systematic Reviews of Research on Effectiveness. CRD’s Guidance for Those Carrying Out or Commissioning Reviews 2001.
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84.
- Chapple CR, Rosario DJ, Wasserfallen M, Woo HH. A randomised study of the urolume stent vs prostatic surgery. J Urol 1995;153.
- Gilling PJ, Kennett K, Aho T, Westenberg A, Fraundorfer M. Holmium bladder neck incision vs holmium laser enucleation of the prostate (HOLEP) as outpatient clinic procedures for small prostate glands (%3C40 g): a randomised trial with one year follow-up. J Urol 2004;171.
- Ho H, Tatt K, Cheng C. A prospective randomized controlled trial comparing bipolar transurethral resection in saline (TURIS) system and conventional monopolar transurethral resection of prostate in men with benign prostate hyperplasia: a 1 year’s clinical efficacy and safety. J Endourol 2005;19.
- Chiou RK, Binard JE, Ebersole ME, Horan JJ, Chiou YK, Lynch B. Randomized comparison of balloon dilation and transurethral incision for treatment of symptomatic benign prostatic hyperplasia. J Endourol 1994;8:221-4.
- D’Addessi A, Porreca A, Foschi N, Racioppi M. Thick loop prostatectomy in the endoscopic treatment of benign prostatic hyperplasia: results of a prospective randomised study. Urol Int 2005;74:114-17.
- Holmes M, Cox J, Stewart J, King D, Bary P, Wright W. Thick vs thin loop transurethral resection of the prostate: a double-blind prospective trial of early morbidity. BJU Int 2002;89:197-201.
- Leskinen MJ, Kilponen A, Lukkarinen O, Tammela TL. Transurethral needle ablation for the treatment of chronic pelvic pain syndrome (category III prostatitis): a randomized, sham-controlled study. Urology 2002;60:300-4.
- Talic RF, El Tiraifi A, El Faqih SR, Abdel-Halim RE. A prospective randomized study of the thick loop and standard wire-loop in transurethral prostatectomy: one year follow up. J Endourol 2000;14.
- Tsukada O, Murakami M, Kosuge T, Yokoyama H. Treatment of benign prostatic hyperplasia using transurethral microwave thermotherapy and dilatation with double-balloon catheter. J Endourol 2005;19:1016-20.
- Yeni E, Unal D, Verit A, Gulum M. Minimal transurethral prostatectomy plus bladder neck incision versus standard transurethral prostatectomy in patients with benign prostatic hyperplasia: a randomised prospective study. Urol Int 2002;69:283-6.
- Hansen BJ, Flyger H, Mortensen S, Mensink HJ, Meyhoff HH. Symptomatic outcome of transurethral prostatectomy, alpha-blockade and placebo in the treatment of benign prostatic hyperplasia. Evaluation of treatment with the Danish Prostatic Symptom Score (DAN-PSS-1) system. The ALFECH Study Group. Scand J Urol Nephrol 1996;30:103-7.
- Petas A, Isotalo T, Talja M, Tammela TL, Valimaa T, Tormala P. A randomised study to evaluate the efficacy of a biodegradable stent in the prevention of postoperative urinary retention after interstitial laser coagulation of the prostate. Scand J Urol Nephrol 2000;34:262-6.
- Roehrborn CG, Rhee EY, Miller SD, Brawer MK, Heron SP, Ruckle HC, et al. Initial results of a randomized multi-center trial comparing the efficacy and safety of Indigo Optima laser treatment with tamsulosin in men with LUTS and BPH. J Urol 2005;173.
- Yamamoto M, Hibi H, Miyake K. A comparison of transurethral resection of the prostate and medical treatment for the patient with moderate symptoms of benign prostatic hyperplasia. Nagoya J Med Sci 1996;59:11-6.
- A comparison of quality of life with patient reported symptoms and objective findings in men with benign prostatic hyperplasia. The Department of Veterans Affairs Cooperative Study of transurethral resection for benign prostatic hyperplasia. J Urol 1993;150:1696-700.
- Plante M. A safety comparison of a randomized mutli-centre study evaluating transurethral ethanol ablation of the prostate (TEAP) for the treatment of benign prostatic hyperplasia (BPH). Eur Urol Suppl 2004;3.
- Abbou C-C, Colombel M, Payan C, Viens-Bitker C, Richard F, Boccon L, et al. Transrectal and transurethral hyperthermia versus sham to treat benign prostatic hyperplasia: a double blind, randomized, multicentric study. J Urol 1994;151.
- Billebaud T, Mechali P, Astier L, Monneins F, Savatovsky I, Coloby P. Transurethral microwave hyperthermia for benign prostatic hyperplasia: is it effective? Results of a multicentric randomized prospective study versus placebo with six month follow-up. J Urol 1994;151.
- Brookes S, Peters T, Campbell R, Featherstone K, Neal D, Abrams P, et al. Including a ‘no active intervention’ arm in surgical trials is possible: evidence from the CLasP randomised trial. J Health Serv Res Policy 2003;8:209-14.
- Çetinkaya M, Ozturk B, Akdemir O, Aki FT. A comparison of fluid absorption during transurethral resection and transurethral vaporization for benign prostatic hyperplasia. BJU Int 2000;86:820-3.
- de la Rosette JJ, Floratos DL, Severens JL, Laguna MP, Kiemeney LA, Debruyne FM. Cost-consequences analysis of TUMT compared to TURP. Results of a randomized study. J Urol 2001;165.
- Holtgrewe HL. An American Urological Association prospective, randomized clinical trial in the treatment of benign prostatic hyperplasia. Cancer 1992;70:351-4.
- Kuntz RM, Lehrich K, Fayad A. Transurethral holmium laser enucleation of the prostate (HoLEP) compared to traditional transurethral resection of the prostate (TURP) for prostates smaller than 100 grams: a randomized prospective clinical study. J Endourol 2001;15.
- Lawrence WT. Ablation of the prostate by laser (ELAP). Journal d’ Urologie 1993;99.
- Norby B, Nielsen HV, Frimodt-Moller PC. Cost-effectiveness of new treatments for benign prostatic hyperplasia: results of a randomized trial comparing the short-term cost-effectiveness of transurethral interstitial laser coagulation of the prostate, transurethral microwave thermotherapy and standard transurethral resection or incision of the prostate. Scand J Urol Nephrol 2002;36:286-95.
- Patel A, Fuchs GJ, Gutierrez-Aceves J, Ryan TP. A prospectively randomized study of energy utilization during transurethral electrosurgical resection (TURP) and electro-vaporization (TUEVAP). J Endourol 1996;10.
- Patel A, Fuchs GJ, Gutierrez-Aceves J. A pilot study of energy utilization patterns during different transurethral electrosurgical treatments of the prostate. Urology 1997;50:138-41.
- Patankar SB, Dobhada SH. Superpulse bipolar prostate resection vs conventional TURP: a randomized prospective trial with 3 months follow-up – and Indian experience. J Endourol 2004;18.
- Eaton AC, de Silva B, Duncan NH, Francis RN. A randomised prospective study comparing the performance of the 4mm plasma kinetic loop against the standard monopolar TURP in prostates 40–100 gm size. J Urol 2004;171.
- Lloyd SN. A multicentre comparative study of the Gyrus plasmakinetic bipolar electrosurgical system and conventional monopolar loop TURP in BPH. BJU Int 2002;90.
- Tuhkanen K, Heino A, Aaltoma S, Ala-Opas M. Sexual function of LUTS patients before and after neodymium laser prostatectomy and transurethral resection of prostate. A prospective, randomized trial. Urol Int 2004;73:137-42.
- Patel A, Fuchs GJ, Gutierrez-Aceves J, Andrade-Perez F. Completeness and efficiency of prostate tissue removal: loop resection compared with a new operative technique of transurethral electrovaporization. BJU Int 1999;84:43-9.
- Costello AJ, Crowe HR, Jackson T, Street A. A randomised single institution study comparing laser prostatectomy and transurethral resection of the prostate. Ann Acad Med 1995;24:700-4.
- Ahmed M, Bell T, Lawrence WT, Ward JP, Watson GM. Transurethral microwave thermotherapy (Prostatron version 2.5) compared with transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: a randomized, controlled, parallel study. Br J Urol 1997;79:181-5.
- Bdesha AS, Bunce CJ, Snell ME, Witherow RO. Sham controlled trial of transurethral microwave therapy with subsequent treatment of the control-group. J Urol 1994;152:453-8.
- Blute ML, Patterson DE, Segura JW, Tomera KM, Hellerstein DK. Transurethral microwave thermotherapy v sham treatment: double-blind randomized study. J Endourol 1996;10:565-73.
- Carter A, Sells H, Speakman M, Ewings P, MacDonagh R, O’Boyle P. A prospective randomized controlled trial of hybrid laser treatment or transurethral resection of the prostate, with a 1-year follow-up. BJU Int 1999;83:254-9.
- Erdaği U, Akman RY, Sargin SY, Yazicioglu A. Transurethral electrovaporization of the prostate versus transurethral resection of the prostate: a prospective randomized study. Arch Ital Urol Androl 1999;71:125-30.
- Hammadeh MY, Madaan S, Hines J, Philp T. 5-year outcome of a prospective randomized trial to compare transurethral electrovaporization of the prostate and standard transurethral resection. Urology 2003;61:1166-71.
- Kursh ED, Concepcion R, Chan S, Hudson P, Ratner M, Eyre R. Interstitial laser coagulation versus transurethral prostate resection for treating benign prostatic obstruction: a randomized trial with 2-year follow-up. Urology 2003;61:573-8.
- Liedberg F, Adell L, Hagberg G, Palmqvist IB. Interstitial laser coagulation versus transurethral resection of the prostate for benign prostatic enlargement – a prospective randomized study. Scand J Urol Nephrol 2003;37:494-7.
- Liu CK, Lee WK, Ko MC, Chiang HS, Wan KS. Transurethral electrovapor resection versus standard transurethral resection treatment for a large prostate: a 2-year follow-up study conducted in Taiwan. Urol Int 2006;76:144-9.
- Ogden C, Reddy P, Johnson H, Carter S. Sham vs TUMT: a randomized study with cross over. J Urol 1993;149.
- Wilson LC, Gilling P, Williams A, Kennett KM, Frampton CM, Westenberg AM, et al. A randomised trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40 grams: results at 2 years. Eur Urol 2006;50:569-73.
- Christensen MM, Aagaard J, Madsen PO. Transurethral resection versus transurethral incision of the prostate. A prospective randomized study. Urol Clin North Am 1990;17:621-30.
- Donovan JL, Peters TJ, Neal DE, Brookes ST, Gujral S, Chacko KN, et al. A randomized trial comparing transurethral resection of the prostate, laser therapy and conservative treatment of men with symptoms associated with benign prostatic enlargement: the CLasP study. J Urol 2000;164:65-70.
- Dunsmuir WD, McFarlane JP, Tan A, Dowling C, Downie J, Kourambas J, et al. Gyrus bipolar electrovaporization vs transurethral resection of the prostate: a randomized prospective single-blind trial with 1 y follow-up. Prostate Cancer Prostatic Dis 2003;6:182-6.
- Gallucci M, Puppo P, Perachino M, Fortunato P, Muto G, Breda G, et al. Transurethral electrovaporization of the prostate vs. transurethral resection. Results of a multicentric, randomized clinical study on 150 patients. Eur Urol 1998;33:359-64.
- Gujral S, Abrams P, Donovan JL, Neal DE, Brookes ST, Chacko KN, et al. A prospective randomized trial comparing transurethral resection of the prostate and laser therapy in men with chronic urinary retention: the CLasP study. J Urol 2000;164:59-64.
- Patel A, Fuchs GJ, Gutierrez-Aceves J, Ryan TP. Prostate heating patterns comparing electrosurgical transurethral resection and vaporization: a prospective randomized study. J Urol 1997;157:169-72.
- Bouchier-Hayes DM, Anderson P, Van Appledorn S, Bugeja P, Costello AJ. KTP laser versus transurethral resection: early results of a randomized trial. J Endourol 2006;20:580-5.
- Ekengren J, Haendler L, Hahn RG. Clinical outcome 1 year after transurethral vaporization and resection of the prostate. Urology 2000;55:231-5.
- Abbou C-C, Payan C, Viens-Bitker C, Richard F, Boccon-Gibod L, Jardin A, et al. Transrectal and transurethral hyperthermia versus sham treatment in benign prostatic hyperplasia: a double-blind randomized multicentre clinical trial. Br J Urol 1995;76:619-24.
- Hill B, Belville W, Bruskewitz R, Issa M, Perez-Marrero R, Roehrborn C, et al. Transurethral needle ablation versus transurethral resection of the prostate for the treatment of symptomatic benign prostatic hyperplasia: 5-year results of a prospective, randomized, multicenter clinical trial. J Urol 2004;171:2336-40.
- McAllister WJ, Absalom MJ, Mir K, Shivde S, Anson K, Kirby RS, et al. Does endoscopic laser ablation of the prostate stand the test of time? Five-year results from a multicentre randomized controlled trial of endoscopic laser ablation against transurethral resection of the prostate. BJU Int 2000;85:437-9.
- Shingleton WB, Farabaugh P, May W. Three-year follow-up of laser prostatectomy versus transurethral resection of the prostate in men with benign prostatic hyperplasia. Urology 2002;60:305-8.
- Singh H, Desai MR, Shrivastav P, Vani K. Bipolar versus monopolar transurethral resection of prostate: randomized controlled study. J Endourol 2005;19:333-8.
- Zorn BH, Bauer JJ, Ruiz HE, Thrasher JB. Randomized trial of safety and efficacy of transurethral resection of the prostate using contact laser versus electrocautery. Tech Urol 1999;5:198-201.
- Rodrigo Aliaga M, Valls Blasco F, Jimenez Cruz JF. Lasers as an alternative to the endoscopic surgery in BPH. Actas Urol Esp 1998;22:17-22.
- Kim JY, Moon KH, Yoon CJ, Park TC. Bipolar transurethral resection of the prostate: a comparative study with monopolar transurethral resection. Korean J Urol 2006;47:493-7.
- Kim TS, Choi S, Rhew HY, Ahn JH, Jang JH, Cho MH. Comparative study on the treatment outcome and safety of TURP, ILC, TUNA and TEAP for patients with benign prostatic hyperplasia. Korean J Urol 2006;47:13-9.
- Riehmann M, Knes JM, Heisey D, Madsen PO, Bruskewitz RC. Transurethral resection versus incision of the prostate: a randomized, prospective study. Urology 1995;45:768-75.
- Tuhkanen K, Heino A, Aaltomaa S, Ala-Opas M. Long-term results of contact laser versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia with small or moderately enlarged prostates. Scand J Urol Nephrol 2003;37:487-93.
- Chacko KN, Donovan JL, Abrams P, Peters TJ, Brookes ST, Thorpe AC, et al. Transurethral prostatic resection or laser therapy for men with acute urinary retention: the CLasP randomized trial. J Urol 2001;166:166-70.
- Fung BT, Li SK, Yu CF, Lau BE, Hou SS. Prospective randomized controlled trial comparing plasmakinetic vaporesection and conventional transurethral resection of the prostate. Asian J Surg 2005;28:24-8.
- Gotoh M, Okamura K, Hattori R, Nishiyama N, Kobayashi H, Tanaka K, et al. A randomized comparative study of the Bandloop versus the standard loop for transurethral resection of the prostate. J Urol 1999;162:1645-7.
- Hellström P, Lukkarinen O, Kontturi M. Bladder neck incision or transurethral electroresection for the treatment of urinary obstruction caused by a small benign prostate? A randomized urodynamic study. Scand J Urol Nephrol 1986;20:187-92.
- Küpeli S, Baltaci S, Soygur T, Aytac S, Yilmaz E, Budak M. A prospective randomized study of transurethral resection of the prostate and transurethral vaporization of the prostate as a therapeutic alternative in the management of men with BPH. Eur Urol 1998;34:15-8.
- Larson TR, Blute ML, Bruskewitz RC, Mayer RD, Ugarte RR, Utz WJ. A high-efficiency microwave thermoablation system for the treatment of benign prostatic hyperplasia: results of a randomized, sham-controlled, prospective, double-blind, multicenter clinical trial. Urology 1998;51:731-42.
- Nawrocki JD, Bell TJ, Lawrence WT, Ward JP. A randomized controlled trial of transurethral microwave thermotherapy. Br J Urol 1997;79:389-93.
- Seckiner I, Yesilli C, Akduman B, Altan K, Mungan NA. A prospective randomized study for comparing bipolar plasmakinetic resection of the prostate with standard TURP. Urol Int 2006;76:139-43.
- Nathan MS, Wickham JEA. TVP: a cheaper and effective alternative to TURP. Minim Invasive Ther Allied Technol 1996;5:292-6.
- Cowles RS, Kabalin JN, Childs S, Lepor H, Dixon C, Stein B, et al. A prospective randomized comparison of transurethral resection to visual laser ablation of the prostate for the treatment of benign prostatic hyperplasia. Urology 1995;46:155-60.
- Keoghane SR, Lawrence KC, Gray AM, Doll HA, Hancock AM, Turner K, et al. A double-blind randomized controlled trial and economic evaluation of transurethral resection vs contact laser vaporization for benign prostatic enlargement: a 3-year follow-up. BJU Int 2000;85:74-8.
- Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, Schain M, et al. Feedback microwave thermotherapy versus TURP for clinical BPH – a randomized controlled multicenter study. Urology 2002;60:292-9.
- d’Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, de la Rosette JJ. Transurethral resection of the prostate vs high-energy thermotherapy of the prostate in patients with benign prostatic hyperplasia: long-term results. Br J Urol 1998;81:259-64.
- Dahlstrand C, Geirsson G, Fall M, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for benign prostatic hyperplasia: preliminary results of a randomized study. Eur Urol 1993;23:292-8.
- Dahlstrand C, Walden M, Geirsson G, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for symptomatic benign prostatic obstruction: a prospective randomized study with a 2-year follow-up. Br J Urol 1995;76:614-8.
- de la Rosette JJ, Floratos DL, Severens JL, Kiemeney LA, Debruyne FM, Pilar LM. Transurethral resection vs microwave thermotherapy of the prostate: a cost-consequences analysis. BJU Int 2003;92:713-8.
- Albala DM, Fulmer BR, Turk TM, Koleski F, Andriole G, Davis BE, et al. Office-based transurethral microwave thermotherapy using the TherMatrx TMx-2000. J Endourol 2002;16:57-61.
- Brehmer M, Wiksell H, Kinn A. Sham treatment compared with 30 or 60 min of thermotherapy for benign prostatic hyperplasia: a randomized study. BJU Int 1999;84:292-6.
- de Wildt MJ, Hubregtse M, Ogden C, Carter SS, Debruyne FM, de la Rosette JJ. A 12-month study of the placebo effect in transurethral microwave thermotherapy. Br J Urol 1996;77:221-7.
- Trachtenberg J, Roehrborn CG. Updated results of a randomized, double-blind, multicenter sham-controlled trial of microwave thermotherapy with the Dornier Urowave in patients with symptomatic benign prostatic hyperplasia. Urowave Investigators Group. World J Urol 1998;16:102-8.
- Zerbib M, Steg A, Conquy S, Debre B. Hyperhermia: a randomized prospective study applying hyperthermia or a sham procedure in obstructive benign hyperplasia of the prostate. Prog Clin Biol Res 1994;386:439-48.
- Cimentepe E, Unsal A, Saglam R. Randomized clinical trial comparing transurethral needle ablation with transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: results at 18 months. J Endourol 2003;17:103-7.
- Hindley RG, Mostafid AH, Brierly RD, Harrison NW, Thomas PJ, Fletcher MS. The 2-year symptomatic and urodynamic results of a prospective randomized trial of interstitial radiofrequency therapy vs transurethral resection of the prostate. BJU Int 2001;88:217-20.
- Kabalin JN, Gill HS, Bite G, Wolfe V. Comparative study of laser versus electrocautery prostatic resection: 18-month followup with complex urodynamic assessment. J Urol 1995;153:94-7.
- Mårtenson AC, de la Rosette JJMC. Interstitial laser coagulation in the treatment of benign prostatic hyperplasia using a diode laser system: results of an evolving technology. Prostate Cancer Prostatic Dis 1999;2:148-54.
- Suvakovic N, Hindmarsh JR. A step towards day case prostatectomy. Br J Urol 1996;77:212-14.
- Dørflinger T, Jensen FS, Krarup T, Walter S. Transurethral prostatectomy compared with incision of the prostate in the treatment of prostatism caused by small benign prostate glands. Scand J Urol Nephrol 1992;26:333-8.
- Jahnson S, Dalen M, Gustavsson G, Pedersen J. Transurethral incision versus resection of the prostate for small to medium benign prostatic hyperplasia. Br J Urol 1998;81:276-81.
- Li MK, Ng AS. Bladder neck resection and transurethral resection of the prostate: a randomized prospective trial. J Urol 1987;138:807-9.
- Nielsen HO. Transurethral prostatotomy versus transurethral prostatectomy in benign prostatic hypertrophy. A prospective randomised study. Br J Urol 1988;61:435-8.
- Saporta L, Aridogan IA, Erlich N, Yachia D. Objective and subjective comparison of transurethral resection, transurethral incision and balloon dilatation of the prostate. A prospective study. Eur Urol 1996;29:439-45.
- Soonawalla PF, Pardanani DS. Transurethral incision versus transurethral resection of the prostate. A subjective and objective analysis. Br J Urol 1992;70:174-7.
- Tkocz M, Prajsner A. Comparison of long-term results of transurethral incision of the prostate with transurethral resection of the prostate, in patients with benign prostatic hypertrophy. Neurourol Urodyn 2002;21:112-16.
- Gupta N, Sivaramakrishna, Kumar R, Dogra PN, Seth A. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of > 40 g. BJU Int 2006;97:85-9.
- Montorsi F, Naspro R, Salonia A, Suardi N, Briganti A, Zanoni M, et al. Holmium laser enucleation versus transurethral resection of the prostate: results from a 2-center, prospective, randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol 2004;172:1926-9.
- Westenberg A, Gilling P, Kennett K, Frampton C, Fraundorfer M. Holmium laser resection of the prostate versus transurethral resection of the prostate: results of a randomized trial with 4-year minimum long-term followup. J Urol 2004;172:616-19.
- Mottet N, Anidjar M, Bourdon O, Louis JF, Teillac P, Costa P, et al. Randomized comparison of transurethral electroresection and holmium: YAG laser vaporization for symptomatic benign prostatic hyperplasia. J Endourol 1999;13:127-30.
- Tuhkanen K, Heino A, Ala-Opas M. Two-year follow-up results of a prospective randomized trial comparing hybrid laser prostatectomy with TURP in the treatment of big benign prostates. Scand J Urol Nephrol 2001;35:200-4.
- Sengör F, Kose O, Yucebas E, Beysel M, Erdogan K, Narter F. A comparative study of laser ablation and transurethral electroresection for benign prostatic hyperplasia: results of a 6-month follow-up. Br J Urol 1996;78:398-400.
- van Melick HH, van Venrooij GE, Boon TA. Long-term follow-up after transurethral resection of the prostate, contact laser prostatectomy, and electrovaporization. Urology 2003;62:1029-34.
- Nuhoğlu B, Ayyildiz A, Karaguzel E, Cebeci O, Germiyanoglu C. Plasmakinetic prostate resection in the treatment of benign prostate hyperplasia: results of 1-year follow up. Int J Urol 2006;13:21-4.
- Tefekli A, Muslumanoglu AY, Baykal M, Binbay M, Tas A, Altunrende F. A hybrid technique using bipolar energy in transurethral prostate surgery: a prospective, randomized comparison. J Urol 2005;174:1339-43.
- Çetinkaya M, Ulusoy E, Adsan O, Saglam H, Ozturk B, Basay S. Comparative early results of transurethral electroresection and transurethral electrovaporization in benign prostatic hyperplasia. Br J Urol 1996;78:901-3.
- Küpeli B, Yalcinkaya F, Topaloglu H, Karabacak O, Gunlusoy B, Unal S. Efficacy of transurethral electrovaporization of the prostate with respect to standard transurethral resection. J Endourol 1998;12:591-4.
- Netto NR, de Lima ML, Lucena R, Lavoura NS, Cortado PL, Netto MR. Is transurethral vaporization a remake of transurethral resection of the prostate?. J Endourol 1999;13:591-4.
- Nuhoğlu B, Ayyildiz A, Fidan V, Ersoy E, Huri E, Germiyanogu C. Transurethral electrovaporization of the prostate: is it any better than standard transurethral prostatectomy? 5-year follow-up. J Endourol 2005;19:79-82.
- Shokeir AA, al Sisi H, Farage YM, el Maaboud MA, Saeed M, Mutabagani H. Transurethral prostatectomy: a prospective randomized study of conventional resection and electrovaporization in benign prostatic hyperplasia. Br J Urol 1997;80:570-4.
- Wang ZL, Wang XF, Li B, Ji JT, Hou SC, Shao SX, et al. Comparative study of transurethral electrovaporization of prostate versus transurethral resection of prostate on benign prostatic hyperplasia. Zhong Hua Nan Ke Xue 2002;8:428-30.
- Helke C, Manseck A, Hakenberg OW, Wirth MP. Is transurethral vaporesection of the prostate better than standard transurethral resection?. Eur Urol 2001;39:551-7.
- Küpeli S, Yilmaz E, Soygur T, Budak M. Randomized study of transurethral resection of the prostate and combined transurethral resection and vaporization of the prostate as a therapeutic alternative in men with benign prostatic hyperplasia. J Endourol 2001;15:317-21.
- Abdel-Khalek M, El Hammady S, Ibrahiem E. A 4-year follow-up of a randomized prospective study comparing transurethral electrovaporization of the prostate with neodymium:YAG laser therapy for treating benign prostatic hyperplasia. BJU Int 2003;91:801-5.
- Narayan P, Tewari A, Aboseif S, Evans C. A randomized study comparing visual laser ablation and transurethral evaporation of prostate in the management of benign prostatic hyperplasia. J Urol 1995;154:2083-8.
- Shingleton WB, Renfroe LD, Kolski JM, Fowler JE. A randomized prospective study of transurethral electrovaporization vs laser ablation of the prostate in men with benign prostatic hypertrophy. Scand J Urol Nephrol 1998;32:266-9.
- d’Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, de la Rosette JJ. High energy thermotherapy versus transurethral resection in the treatment of benign prostatic hyperplasia: results of a prospective randomized study with 1 year of follow-up. J Urol 1997;158:120-5.
- Dahlstrand C, Fall M, Geirsson G, Petterson DE. Transurethral microwave thermotherapy versus transurethral resection for benign prostatic hyperplasia: results of a randomized study. J Urol 1993;149.
- Dahlstrand C, Geirsson G, Walden M. Prospective randomized study between transurethral resection (TURP) and transurethral microwave treatment (TUMT) for benign prostatic hyperplasia. Scand J Urol Nephrol Suppl 1993;151:32-3.
- Dahlstrand C, Walden M, Geirsson G, Sommar S, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for BPH. Prog Clin Biol Res 1994;386:455-61.
- Duelund J, Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, et al. Prostalund feedback treatment versuis TURP: a prospective randomized multicenter study with 36 month follow-up. Scand J Urol Nephrol 2003;37:34-5.
- Floratos DL, Kiemeney LA, Rossi C, Kortmann BB, Debruyne FM, de la Rosette JJ. Long-term follow-up of randomized transurethral microwave thermotherapy versus transurethral prostatic resection study. J Urol 2001;165:1533-8.
- Francisca EA, d’Ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, de la Rosette JJ. A randomized study comparing high-energy TUMT to TURP: quality-of-life results. Eur Urol 2000;38:569-75.
- Kobelt G, Spangberg A, Mattiasson A. The cost of feedback microwave thermotherapy compared with transurethral resection of the prostate for treating benign prostatic hyperplasia. BJU Int 2004;93:543-8.
- Nordling J, Wagrell L, Larson T, Duelund J, Kroyer K, Mattiasson A. ProstaLund feedback treatment (PLFT) vs TURP: a prospective randomized muliticenter study with 36 month follow up. BJU Int 2005;95.
- Wagrell L, Schelin S, Nordling J, Larsson T, Mattiasson A. Prostalund microwave feedback treatment compared with TURP for treatment of BPH: a prospective randomized multicentre study. Aust NZ J Surg 2003;73.
- Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, Schain M, et al. ProstaLund microwave feedback treatment compared with TURP for treatment of BHP: a prospective randomized multicenter study with 24 months folllow up. J Urol 2003;169.
- Walden M, Acosta S, Carlsson P, Pettersson S, Dahlstrand C. A cost-effectiveness analysis of transurethral resection of the prostate and transurethral microwave thermotherapy for treatment of benign prostatic hyperplasia: two-year follow-up. Scand J Urol Nephrol 1998;32:204-10.
- Albala DM, Koleski F, Nuzzarello J, Davis BE, Eure GR, Andriole G, et al. Periurethral prostatic microwave thermotherapy using the Thermatrx TMX-2000TM: follow-up of a randomized, blinded, sham-controlled study in patients with BPH. J Urol 2000;163.
- Albala DM, Andriole G, Davies B. Transurethral Microwave Thermotherapy (TUMT) Using the Thermatrx TMX-2000: Durability Exhibited in a Study Comparing TUMT With a Sham Procedure in Patients With Benign Prostatic Hyperplasia 2003.
- Albala DM, Andriole G, Davies B, Eure GR, Kabalin JN, Lingeman JE, et al. Transurethral Microwave Thermotherapy (TUMT) Using the Thermatrx TMX-2000: Long-Term Results in a Study Comparing TUMT With a Sham Procedure in Patients With Benign Prostatic Hyperplasia 2005.
- Bdesha AS, Bunce CJ, Kelleher JP, Snell ME, Vukusic J, Witherow OR. Transurethral microwave treatment for benign prostatic hypertrophy: a randomised controlled clinical trial. BMJ 1993;306:1293-6.
- de la Rosette JJ, de Wildt MJ, Alivizatos G, Froeling FM, Debruyne FM. Transurethral microwave thermotherapy (TUMT) in benign prostatic hyperplasia: placebo versus TUMT. Urology 1994;22:58-63.
- Francisca EA, d’Ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, de la Rosette JJ. Quality of life assessment in patients treated with lower energy thermotherapy (Prostasoft 2.0): results of a randomized transurethral microwave thermotherapy versus sham study. J Urol 1997;158:1839-44.
- Kabalin JN, Albala DM, Koleski F, Andriole G, Sundaram C, Davis BE, et al. Office-based transurethral microwave thermotherapy for benign prostatic hyperplasia (BPH) using the TherMatrxTM TMx-2000TM: results of a multi-center prospective randomized sham-controlled trial. J Urol 2001;165:367-8.
- Roehrborn C, Sech S, Preminger G, Cohen T, Perlmutter A, Razvi H, et al. A randomized, blinded study comparing microwave thermotherapy (Dornier Urowave) with a sham procedure in patients with clinical benign prostatic hyperplasia (BPH). J Endourol 1997;11.
- Roehrborn CG, Preminger G, Newhall P, Denstedt J, Razvi H, Chin LJ, et al. Microwave thermotherapy for benign prostatic hyperplasia with the Dornier Urowave: results of a randomized, double-blind, multicenter, sham-controlled trial. Urology 1998;51:19-28.
- Tan AH, Nott L, Hardie WR, Chin JL, Denstedt JD, Razvi H. Long-term results of microwave thermotherapy for symptomatic benign prostatic hyperplasia. J Endourol 2005;19:1191-5.
- Bruskewitz R, Issa MM, Roehrborn CG, Naslund MJ, Perez-Marrero R, Shumaker BP, et al. A prospective, randomized 1-year clinical trial comparing transurethral needle ablation to transurethral resection of the prostate for the treatment of symptomatic benign prostatic hyperplasia. J Urol 1998;159:1588-93.
- Mostafid AH, Harrison NW, Thomas PJ, Fletcher MS. A prospective randomized trial of interstitial radiofrequency therapy versus transurethral resection for the treatment of benign prostatic hyperplasia. Br J Urol 1997;80:116-22.
- Naslund M, Oesterling JE, Issa M, Roehrborn CG, Bruskewitz R, Perez-Marrero R, et al. Long term follow-up of a prospective, randomized clinical trial comparing transurethral needle ablation (TUNA) to transurethral resection of the prostate (TURP) for the treatment of benign prostatic hyperplasia (BPH). J Endourol 1997;11.
- Naslund M, Perez-Marrero R, Roehrborn C, Bruskewitz R, Issa M. Intermediate term outcomes of TUNA for BPH: 36 month results of the TUNA vs TURP US randomized study. J Urol 1999;161.
- Roehrborn CG, Burkhard FC, Bruskewitz RC, Issa MM, Perez-Marrero R, Naslund MJ, et al. The effects of transurethral needle ablation and resection of the prostate on pressure flow urodynamic parameters: analysis of the United States randomized study. J Urol 1999;162:92-7.
- Anson K, Nawrocki J, Buckley J, Fowler C, Kirby R, Lawrence W, et al. A multicenter, randomized, prospective study of endoscopic laser ablation versus transurethral resection of the prostate. Urology 1995;46:305-10.
- Brookes ST, Donovan JL, Peters TJ, Abrams P, Neal DE. Sexual dysfunction in men after treatment for lower urinary tract symptoms: evidence from randomised controlled trial. BMJ 2002;324:1059-61.
- Donovan JL, Brookes ST, Kennedy LG, Abrams P, Peters TJ, Neal DE. The CLasP randomised controlled trial: comparing laser therapy, conservative management and TURP for men with lower urinary tract symptoms. J Urol 1998;159.
- Kabalin JN. Laser prostatectomy performed with a right angle firing neodymium:YAG laser fiber at 40 watts power setting. J Urol 1993;150:95-9.
- Aagaard J, Chopin D, Knes J, Madsen PO. Transurethral resection TURP vs incision TUIP of the prostate: a prospective randomized study. J Urol 1990;143.
- Riehmann M, Knes J, Madaan S, Bruskewitz R. Transurethral resection (TURP) versus incision (TUIP) of the prostate: a prospective randomized study. J Urol 1993;149.
- Sparwasser C, Riehmann M, Knes J, Madsen PO. Long-term results of transurethral prostate incision (TUIP) and transurethral prostate resection (TURP). A prospective randomized study. Urologe (Ausg A) 1995;34:153-7.
- Briganti A, Naspro R, Vavassori I, Suardi N, Salonia A, Mazzoccoli B, et al. Impact of holmium laser enucleation (HoLEP) versus transurethral resection (TURP) of the prostate on sexual function: results of a prospective multicentric randomized trial. J Urol 2004;171.
- Briganti A, Naspro R, Vavassori I, Suardi N, Salonia A, Mazzoccoli B, et al. Impact of holmium laser enucleation (HoLEP) versus transurethral resection (TURP) of the prostate on sexual function: results of a prospective multicentric randomized trial. Eur Urol Suppl 2004;3.
- Fraundorfer MR, Gilling PJ, Kennett KM, Dunton NG. Holmium laser resection of the prostate is more cost-effective than transurethral resection of the prostate: results of a randomized prospective study. Urology 2001;57:454-8.
- Gilling P, Westenberg A, Kennett KM, Fraundorfer MR. Holmium laser resection of the prostate (HOLRP) vs transurethral resection of the prostate (TURP): results at 4 years. Aust NZ J Surg 2003;73.
- Gilling PJ, Fraundorfer MR, Westenberg AM, Neill MG, Frampton CM, Kennett KM. Relief of bladder outflow obstruction (BOO) following HoLEP and TURP: a pooled analysis of data from 4 randomized trials. BJU Int 2005;95.
- Kuntz RM, Ahyai S, Lehrich K. Holmium laser enucleation vs TURP: a randomized prospective study with 2 years of follow-up. BJU Int 2003;91.
- Montosori F, Naspro R, Salonia A, Suardi N, Briganti A, Zanoni M. Holmium laser enucleation versus transurethral resection of the prostate: results from a two-centre prospective randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol 2004;171.
- Tan AH, Gilling PJ, Kennett KM, Frampton C, Westenberg AM, Fraundorfer MR. A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams). J Urol 2003;170:1270-4.
- Carter A, Sells H, Speakman M, Ewings P, O’Boyle P, MacDonagh R. Quality of life changes following KTP/Nd:YAG laser treatment of the prostate and TURP. Eur Urol 1999;36:92-8.
- Jenkinson C, Gray A, Doll H, Lawrence K, Keoghane S, Layte R. Evaluation of index and profile measures of health status in a randomized controlled trial. Comparison of the Medical Outcomes Study 36-Item Short Form Health Survey, EuroQol, and disease specific measures. Med Care 1997;35:1109-18.
- Keoghane S, Cranston D, Lawrence K, Doll H. The Oxford laser prostate trial: a prospective randomised controlled trial of contact vaporisation of the prostate versus TURP. J Urol 1995;153.
- Keoghane SR, Doll HA, Lawrence KC, Jenkinson CP, Cranston DW. The Oxford Laser Prostate Trial: sexual function data from a randomized controlled clinical trial of contact laser prostatectomy. Eur Urol 1996;30:424-8.
- Keoghane SR, Lawrence KC, Jenkinson CP, Doll HA, Chappel DB, Cranston DW. The Oxford Laser Prostate Trial: sensitivity to change of three measures of outcome. Urology 1996;47:43-7.
- Keoghane SR, Cranston DW, Lawrence KC, Doll HA, Fellows GJ, Smith JC. The Oxford Laser Prostate Trial: a double-blind randomized controlled trial of contact vaporization of the prostate against transurethral resection; preliminary results. Br J Urol 1996;77:382-5.
- Keoghane SR, Sullivan ME, Doll HA, Kourambas J, Cranston DW. Five-year data from the Oxford Laser Prostatectomy Trial. BJU Int 2000;86:227-8.
- Mottet N, Anidjar M, Costa P, Louis JF, Teillac P, Duc A. A randomized study comparing the holmium-YAG vaporisation and the transurethral resection of symptomatic BPH. J Endourol 1997;11.
- Pearcy R, Carter A, Sells H, O’Boyle P, MacDonagh R, Speakman M, et al. Long term follow up of hybrid KITP/ND:YAG laser treatment of the prostate versus TURP: a prospective randomised trial, 18 month results. J Urol 1999;161.
- Shingleton WB, Terrell F, Renfroe DL, Kolski JM, Fowler JE. A randomized prospective study of laser ablation of the prostate versus transurethral resection of the prostate in men with benign prostatic hyperplasia. Urology 1999;54:1017-21.
- Shingleton WB, Farabaugh P. Prospective randomized study of laser prostatectomy and transurethral resection of the prostate in men with benign prostatic hyperplasia: 3 year follow-up. J Urol 2001;165.
- Tuhkanen K, Heino A, Ala-Opas M. Hybrid laser treatment compared with transurethral resection of the prostate for symptomatic bladder outlet obstruction caused by a large benign prostate: a prospective, randomized trial with a 6-month follow-up. BJU Int 1999;84:805-9.
- Tuhkanen K, Heino A, Ala-Opas M. Contact laser prostatectomy compared to TURP in prostatic hyperplasia smaller than 40 ml. Six-month follow-up with complex urodynamic assessment. Scand J Urol Nephrol 1999;33:31-4.
- van Melick HH, van Venrooij GE, Eckhardt MD, Boon TA. A randomized controlled trial comparing transurethral resection of the prostate, contact laser prostatectomy and electrovaporization in men with benign prostatic hyperplasia: urodynamic effects. J Urol 2002;168:1058-62.
- van Melick HH, van Venrooij GE, Eckhardt MD, Boon TA. A randomized controlled trial comparing transurethral resection of the prostate, contact laser prostatectomy and electrovaporization in men with benign prostatic hyperplasia: analysis of subjective changes, morbidity and mortality. J Urol 2003;169:1411-16.
- Hammadeh MY, Madaan S, Singh M, Philp T. Two-year follow-up of a prospective randomised trial of electrovaporization versus resection of prostate. Eur Urol 1998;34:188-92.
- Hammadeh MY, Madaan S, Singh M, Philp T. Two years follow up of a prospective randomised trial of electro-vaporisation of the prostate vs standard TURP. J Endourol 1998;12.
- Hammadeh MY, Fowlis GA, Singh M, Philp T. Transurethral electrovaporization of the prostate – a possible alternative to transurethral resection: a one-year follow-up of a prospective randomized trial. Br J Urol 1998;81:721-5.
- Hammadeh MY, Madaan S, Singh M, Philp T. Three years follow up of a prospective randomised trial comparing transurethral electrovaporization of the prostate to standard TURP. J Endourol 1999;13.
- Hammadeh MY, Madaan S, Singh M, Philp T. A 3-year follow-up of a prospective randomized trial comparing transurethral electrovaporization of the prostate with standard transurethral prostatectomy. BJU Int 2000;86:648-51.
- Hammadeh MY, Madaan S, Hines J, Philp T. The efficacy and durability of transurethral electrovaporisation of the prostate: 5 year result of a prospective randomised trial. Eur Urol Suppl 2003;2.
- McAllister WJ, Karim O, Plail RO, Samra DR, Steggall MJ, Yang Q, et al. Transurethral electrovaporization of the prostate: is it any better than conventional transurethral resection of the prostate?. BJU Int 2003;91:211-14.
- Puppo P, Perachino M, Breda G, Boccafoschi C, Comeri G, Francesca F, et al. Transurethral electrovaporization of the prostate (T.V.P.): a multicentric randomized comparative study vs TURP. J Urol 1996;155.
- Love CJ, Dowling C, Pham T, Tan A, McFarlane JP, Dunsmuir WD. Gyrus (R) bipolar electrovaporization versus transurethral resection of the prostate: a randomized prospective trial with 1-year follow-up. J Urol 2003;169.
- Kok ET, McDonnell J, Stolk EA, Stoevelaar HJ, Busschbach JJV. The valuation of the international prostate symptom score (IPSS) for use in economic evaluations. Eur Urol 2002;42:491-7.
- Tweedie RL, Scott DJ, Biggerstaff BJ, Mengersen KL. Bayesian meta-analysis, with application to studies of ETS and lung cancer. Lung Cancer 1996;14:S171-94.
- Newcombe RG. Towards a reduction in publication bias. BMJ 1987;295:656-9.
- Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol 2000;53:207-16.
- Greenland S. Quality scores are useless and potentially misleading. [Reply to “Re: A critical look at some popular analytical methods”.]. Am J Epidemiol 1994;140:300-1.
- Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchial view of proposed solutions. Biostatistics 2001;2:463-71.
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42-6.
- Soares HP, Daniels S, Kumar A, Clarke M, Scott C, Swann S, et al. Bad reporting does not mean bad methods for randomised trials: observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. BMJ 2004;328:22-4.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12.
- Schulz KF. Assessing allocation concealment and blinding in randomised controlled trials: why bother?. Evid Based Nurs 2001;4:4-6.
- Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. J Clin Epidemiol 2006;59:342-53.
- Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK. Correlation of the American Urological Association symptom index with self-administered versions of the Madsen–Iversen, Boyarsky and Maine Medical Assessment Program symptom indexes. Measurement Committee of the American Urological Association. J Urol 1992;148:1558-63.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8:1-158.
- National Institute for Clinical Excellence . Guide on the Methods of Technology Appraisal (reference N0515) 2004. www.nice.org.uk/page.aspx?o=201973 (accessed September 2006).
- Djavan B, Roehrborn CG, Shariat S, Ghawidel K, Marberger M. Prospective randomized comparison of high energy transurethral microwave thermotherapy versus alpha-blocker treatment of patients with benign prostatic hyperplasia. J Urol 1999;161:139-43.
- Djavan B, Seitz C, Roehrborn CG, Remzi M, Fakhari M, Waldert M, et al. Targeted transurethral microwave thermotherapy versus alpha-blockade in benign prostatic hyperplasia: outcomes at 18 months. Urology 2001;57:66-70.
- Bouchier-Hayes DM, Anderson P, Van Appledom S, Bugeja P, Costello AJ. A randomised trial comparing photoselective vaporization of the prostate (PVP) and transurethral resection of the prostate (TURP) in treatment of LUTS. J Urol 2006;175.
Appendix 1 Search strategies
Clinical effectiveness
MEDLINE (1966–September Week 3 2006), EMBASE (1980–2006 Week 38), MEDLINE In-Process (27 September 2006)
Ovid Multifile Search. URL: http://gateway.ovid.com/athens/
-
prostatic hyperplasia/su use mesz
-
prostate hypertrophy/su use emez
-
prostatic hyperplasia/ use mesz
-
prostate hypertrophy/ use emez
-
bladder neck obstruction/ use mesz
-
bladder obstruction/ use emez
-
(benign prostat$ adj1 (hyperplas$ or hypertroph$ or obstruct$ or enlarge$ or disease)).tw.
-
(bph or bpo or bpe).tw.
-
bladder neck obstruct$.tw.
-
bladder outlet obstruct$.tw.
-
bladder outflow obstruct$.tw.
-
or/3-11
-
exp prostatectomy/ use mesz
-
exp prostate surgery/ use emez
-
(transurethral adj3 (resect$ or electroresect$ or incision$ or diatherm$)).tw.
-
(transurethral adj3 (vapori$ or electrovapori$ or evapori$)).tw.
-
(transurethral adj3 (ablat$ or thermo$ or inject$ or coagulat$)).tw.
-
exp electrosurgery/
-
laser surgery/
-
laser coagulation/
-
holmium laser/ use emez
-
yag laser/ use emez
-
(laser adj3 (resect$ or ablat$ or coagulat$ or incision$ or vapori$)).tw.
-
(laser adj3 (enucleat$ or prostatect$)).tw.
-
((holmium or yag or nd or green light) adj3 laser$).tw.
-
(photoselectiv$ adj1 vapori$).tw.
-
(gyrus or (plasma adj3 (electrovapori$ or vapori$))).tw.
-
(needle adj3 ablat$).tw.
-
(microwave adj3 thermo$).tw.
-
(coretherm or prostatron or targis or thermatrx or prolieve).tw.
-
(high intensity adj3 ultrasound).tw.
-
(ethanol adj3 inject$).tw.
-
((water or cooled) adj3 thermotherapy).tw.
-
ultrasound, high-intensity focused, transrectal/
-
high intensity focused ultrasound/
-
stents/
-
(prostat$ adj3 (stent$ or spiral$)).tw.
-
(turp or tuvp or tevap or tvp or tuevap).tw.
-
(tuip or vlap or holrp or holep or tuna or tumt).tw.
-
(ilc or tulip or hifu).tw.
-
or/18-23,25-29,31-36,40
-
12 and 41
-
or/1-2,13-17,24,30,37-39,42
-
prostate cancer/ or bladder cancer/ use emez
-
prostatic neoplasms/ or bladder neoplasms/ use mesz
-
(cancer$ or carcinoma$ or neoplasm$).tw.
-
or/44-46
-
47 not 12
-
43 not 48
-
animal/ not human/ use mesz
-
(animal/ or nonhuman/) not human/ use emez
-
49 not (50 or 51)
-
clinical trial.pt. use mesz
-
exp controlled clinical trials/ use mesz
-
randomised controlled trial/ use emez
-
clinical trial/ use emez
-
random allocation/ use mesz
-
randomization/ use emez
-
random$.tw.
-
meta analysis.tw.
-
meta analysis.pt. use mesz
-
meta analysis/ use emez
-
review.ab.
-
review.pt. use mesz
-
systematic review/ use emez
-
or/53-65
-
52 and 66
-
remove duplicates from 67
Science Citation Index (1981–23 September 2006), ISI Proceedings (1990–18 March 2006)
Web of Knowledge. URL: http://wok.mimas.ac.uk/
#1 TS=(benign prostat* SAME (hyperplas* OR hypertroph* or obstruct* or enlarge* or disease*))
#2 TS=(bph OR bpo OR bpe)
#3 TS=bladder neck obstruct*
#4 TS=bladder outlet obstruct*
#5 TS=bladder outflow obstruct*
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 TS=prostatectomy
#8 TS=(prostat* SAME (surgery or surgical))
#9 TS=transurethral
#10 TS=electrosurg*
#11 TS=(laser SAME (surgery OR resect* OR ablat* or coagulat*))
#12 TS=(laser SAME (incision* OR enucleat* OR prostatect*))
#13 TS=(laser SAME (holmium OR yag OR nd OR green light))
#14 TS=vapori*
#15 TS=electrovapori*
#16 TS=(needle same ablat*)
#17 TS=(microwave SAME thermo*)
#18 TS=(high intensity SAME ultrasound)
#19 TS=(ethanol SAME inject*)
#20 TS=(prostat* SAME ( stent* OR spiral*))
#21 #7 or #8 OR #9 OR #10 OR #11 or #12 or #13 or #14 OR #15 or #16 or #17 or #18 OR #19 OR #20
#22 #6 AND #21
#23 TS=(turp OR tuvp OR tevap OR tvp OR tuevap)
#24 TS=(tuip OR vlap OR holrp OR holep OR tuna OR tumt)
#25 TS=(ilc OR tulip OR hifu)
#26 #22 OR #23 OR #24 OR #25
#27 TS=prostat* cancer*
#28 TS=prostat* neoplasm*
#29 TS=prostat* carcinoma*
#30 #27 or #28 or #29
#31 #30 NOT #6
#32 #26 not #31
#33 TS=randomised
#34 TS=randomized
#35 TS=randomly
#36 TS=clinical trial*
#37 TS=controlled trial*
#38 #33 or #34 or #35 or #36 or #37
#39 #32 and #38#
BIOSIS (1985–22 September 2006)
Edina. URL: http://edina.ac.uk/
((al: (random*) or al: (trial*)) and ((((((((((al: (tuna) and al: (prostat*)) or (al: (tumt))) or (al: (vlap) or al: (holrp) or al: (holep))) or (al: (tvp) or al: (tuevap) or al: (tuip))) or (al: (turp) or al: (tuvp) or al: (tevap))) or (al: (thermatrx) or al: (prolieve))) or (al: (coretherm) or al: (prostatron) or al: (targis)))) or ((((((((al: (transurethral)) or (al: (tulip) or al: (ilc))) or (al: (hifu) or al: ((ultrasound n1 high intensity))))) and ((((al: (bladder n1 obstruct*)) or (al: (bph) or al: (bpe) or al: (bpo))) or (al: ((benign n1 prostat*)) or al: ((prostat* n1 hyperplasia)) or al: ((prostat* n1 hypertroph*))))))) or (((((((al: (ablat*) or al: (thermo*)) or (al: (electrovapor* ) or al: (vapor*))) or (al: ((laser n3 coagulat*)) or al: ((holmium n1 laser*)) or al: ((yag n1 laser*)))) or (al: ((minimal* n1 invasiv*)) or al: (electrosurg*) or al: ((laser n3 surg*))))) and ((((al: (bladder n1 obstruct*)) or (al: (bph) or al: (bpe) or al: (bpo))) or (al: ((benign n1 prostat*)) or al: ((prostat* n1 hyperplasia)) or al: ((prostat* n1 hypertroph*))))))))))))
Cochrane Library (2006 Issue 1)
URL: www3.interscience.wiley.com/
#1 MeSH descriptor Prostatic Hyperplasia explode all trees with qualifier: SU in MeSH
#2 MeSH descriptor Prostatic Hyperplasia explode all trees in MeSH products
#3 MeSH descriptor Bladder Neck Obstruction explode all trees in MeSH products
#4 prostate hypertrophy in Keywords or bladder obstruction in Keywords or benign prostat* in All Fields or bladder near/3 obstruct* in All Fields in all products
#5 bph in All Fields or bpo in All Fields or bpe in All Fields in all products
#6 (#2 OR #3 OR #4 OR #5)
#7 MeSH descriptor Prostatectomy explode all trees in MeSH products
#8 MeSH descriptor Electrosurgery explode all trees in MeSH products
#9 MeSH descriptor Laser Surgery, this term only in MeSH products
#10 MeSH descriptor Laser Coagulation, this term only in MeSH products
#11 holmium laser in Keywords or yag laser in Keywords or laser in All Fields in all products
#12 electrovaporis* in All Fields or vaporis* in All Fields or ablat* in All Fields or thermo* in All Fields in all products
#13 MeSH descriptor Ultrasound, High-Intensity Focused, Transrectal, this term only in MeSH products
#14 hifu in All Fields or tulip in All Fields or ilc in All Fields in all products
#15 MeSH descriptor Stents explode all trees in MeSH products
#16 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15)
#17 (#6 AND #16)
#18 (#1 OR #17)
#19 coretherm in All Fields or prostatron in All Fields or targis in All Fields or thermatrx in All Fields or prolieve in All Fields in all products
#20 turp in All Fields or tuvp in All Fields or tevap in All Fields or tvp in All Fields or tuevap in All Fields in all products
#21 tuip in All Fields or vlap in All Fields or holrp in All Fields or holep in All Fields in all products
#22 tuna in All Fields or tumt in All Fields in all products
#23 (#18 or #19 OR #21 OR #22)
DARE and HTA databases (March 2006)
NHS Centre for Reviews and Dissemination. URL: www.york.ac.uk/inst/crd/crddatabases.htm
benign hyperplasia/ or bph or bpe
or
benign and prostate
or
transurethral and prostate
or
turp or tuip or tuvp or tuna or tumt or hifu
National Research Register (2006 Issue 1)
URL: www.nrr.nhs.uk/
#1 Prostatic Hyperplasia [su] explode all trees (MeSH)
#2 Prostatic Hyperplasia explode all trees (MeSH)
#3 Bladder Neck Obstruction explode all trees (MeSH)
#4 (benign prostat*)
#5 (bladder near obstruct*)
#6 (bph or bpo or bpe)
#7 (#2 OR #3 OR #4 OR #5 or #6)
#8 Prostatectomy explode all trees (MeSH)
#9 Electrosurgery explode all trees (MeSH)
#10 Laser Surgery single term (MeSH)
#11 Laser Coagulation single term (MeSH)
#12 laser*
#13 (electrovaporis* or vaporis* or ablat* or thermo*)
#14 Ultrasound, High-Intensity Focused, Transrectal single term (MeSH)
#15 (hifu or tulip or ilc)
#16 Stents explode all trees (MeSH)
#17 (#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16)
#18 (#7 AND #17)
#19 (#1 OR #18)
#20 (coretherm or prostatron or targis or thermatrx or prolieve)
#21 (turp or tuvp or tevap or tvp or tuevap)
#22 (tuip or vlap or holrp or holep)
#23 (tuna or tumt)
#24 (#19 OR #21 OR #22 or #23)
Clinical Trials (March 2006)
URL: http://clinicaltrials.gov/ct/gui/c/r
Benign prostatic hyperplasia or bph or turp or tuip or tuvp or tuna or tnmt or hifu
Current Controlled Trials (March 2006)
URL: www.controlled-trials.com/
(prostat% and hyperplasia)or (prostat% and transurethral) or bph or bpe oor turp or tuip or tuvp or tuna or tnmt or hifu
Cost-effectiveness and quality of life
MEDLINE (1966–March Week 2 2006), EMBASE (1980–2006 Week 11), MEDLINE In-Process (20 March 2006)
Ovid Multifile Search. URL: http://gateway.ovid.com/athens/
-
prostatic hyperplasia/su use mesz
-
prostate hypertrophy/su use emez
-
prostatic hyperplasia/ use mesz
-
prostate hypertrophy/ use emez
-
bladder neck obstruction/ use mesz
-
bladder obstruction/ use emez
-
(benign prostat$ adj1 (hyperplas$ or hypertroph$ or obstruct$ or enlarge$ or disease)).tw.
-
(bph or bpo or bpe).tw.
-
bladder neck obstruct$.tw.
-
bladder outlet obstruct$.tw.
-
bladder outflow obstruct$.tw.
-
or/3-11
-
exp prostatectomy/ use mesz
-
exp prostate surgery/ use emez
-
(transurethral adj3 (resect$ or electroresect$ or incision$ or diatherm$)).tw.
-
(transurethral adj3 (vapori$ or electrovapori$ or evapori$)).tw.
-
(transurethral adj3 (ablat$ or thermo$ or inject$ or coagulat$)).tw.
-
exp electrosurgery/
-
laser surgery/
-
laser coagulation/
-
holmium laser/ use emez
-
yag laser/ use emez
-
(laser adj3 (resect$ or ablat$ or coagulat$ or incision$ or vapori$)).tw.
-
(laser adj3 (enucleat$ or prostatect$)).tw.
-
((holmium or yag or nd or green light) adj3 laser$).tw.
-
(photoselectiv$ adj1 vapori$).tw.
-
(gyrus or (plasma adj3 (electrovapori$ or vapori$))).tw.
-
(needle adj3 ablat$).tw.
-
(microwave adj3 thermo$).tw.
-
(coretherm or prostatron or targis or thermatrx or prolieve).tw.
-
(high intensity adj3 ultrasound).tw.
-
(ethanol adj3 inject$).tw.
-
((water or cooled) adj3 thermotherapy).tw.
-
ultrasound, high-intensity focused, transrectal/
-
high intensity focused ultrasound/
-
stents/
-
(prostat$ adj3 (stent$ or spiral$)).tw.
-
(turp or tuvp or tevap or tvp or tuevap).tw.
-
(tuip or vlap or holrp or holep or tuna or tumt).tw.
-
(ilc or tulip or hifu).tw.
-
or/18-23,25-29,31-36,40
-
12 and 41
-
or/1-2,13-17,24,30,37-39,42
-
prostate cancer/ or bladder cancer/ use emez
-
prostatic neoplasms/ or bladder neoplasms/ use mesz
-
(cancer$ or carcinoma$ or neoplasm$).tw.
-
or/44-46
-
47 not 12
-
43 not 48
-
animal/ not human/ use mesz
-
(animal/ or nonhuman/) not human/ use emez
-
49 not (50 or 51)
-
exp “costs and cost analysis”/
-
economics/
-
exp economics,hospital/
-
exp economics,medical/
-
economics,pharmaceutical/
-
exp budgets/
-
exp models, economic/
-
exp decision theory/
-
ec.fs. use mesz
-
monte carlo method/
-
markov chains/
-
exp quality of life/
-
“Value of Life”/
-
cost of illness/
-
exp health status indicators/
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
-
economics model$.tw.
-
(economics$ or pharmacoeconomic$ or pharmo-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(value adj2 (money or monetary)).tw.
-
quality adjusted life.tw.
-
disability adjusted life.tw.
-
(qaly? or qald? or qale? or qtime? or daly?).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
(hye or hyes).tw.
-
(health adj3 (indicator? or status or utilit?)).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
or/53-84
-
52 and 85
-
remove duplicates from 86
Science Citation Index (1981–1 March 2006)
Web of Knowledge. URL: http://wok.mimas.ac.uk/
#1 TS=(benign prostat* SAME (hyperplas* OR hypertroph* or obstruct* or enlarge* or disease*))
#2 TS=(bph OR bpo OR bpe)
#3 TS=bladder neck obstruct*
#4 TS=bladder outlet obstruct*
#5 TS=bladder outflow obstruct*
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 TS=prostatectomy
#8 TS=(prostat* SAME (surgery or surgical))
#9 TS=transurethral
#10 TS=electrosurg*
#11 TS=(laser SAME (surgery OR resect* OR ablat* or coagulat*))
#12 TS=(laser SAME (incision* OR enucleat* OR prostatect*))
#13 TS=(laser SAME (holmium OR yag OR nd OR green light))
#14 TS=vapori*
#15 TS=electrovapori*
#16 TS=(needle same ablat*)
#17 TS=(microwave SAME thermo*)
#18 TS=(high intensity SAME ultrasound)
#19 TS=(ethanol SAME inject*)
#20 TS=(prostat* SAME ( stent* OR spiral*))
#21 #7 or #8 OR #9 OR #10 OR #11 or #12 or #13 or #14 OR #15 or #16 or #17 or #18 OR #19 OR #20
#22 #6 AND #21
#23 TS=(turp OR tuvp OR tevap OR tvp OR tuevap)
#24 TS=(tuip OR vlap OR holrp OR holep OR tuna OR tumt)
#25 TS=(ilc OR tulip OR hifu)
#26 #22 OR #23 OR #24 OR #25
#27 TS=prostat* cancer*
#28 TS=prostat* neoplasm*
#29 TS=prostat* carcinoma*
#30 #27 or #28 or #29
#31 #30 NOT #6
#32 #26 not #31
#33 TS=(cost* SAME (effective* OR utility* OR benefit* OR minimis*))
#34 TS=( economic* same evaluat*)
#35 TS=(price OR pricing)
#36 TS=(financial OR finance OR finances OR financed)
#37 TS=(value SAME (money OR monetary))
#38 #33 or #34 or #35 or #36 or #37
#39 #32 and #38
#40 TS=quality of life
#41 TS=quality adjusted life
#42 TS=disability adjusted life
#43 TS=(qaly* OR qald* OR qale* OR qtime* OR daly)
#44 TS=(euroqol* OR euro qol* OR eq5d OR eq 5d)
#45 TS=(hql OR hqol OR h qol OR hrqol OR hr qol)
#46 TS=health* year* equivalent*
#47 TS=(hye OR hyes OR hui OR hui1 OR hui2 OR hui3)
#48 TS=(health utilit* OR disutilit*)
#49 #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48
#50 #32 and #49
#51 TS=willingness to pay
#52 TS=standard gamble
#53 TS=(markov OR monte carlo)
#54 TS=(decision SAME (tree* OR analy* OR model*))
#55 #51 or #52 or #53 or #54
#56 #32 and #55
#57 #39 or #50 or #56
NHS Economic Evaluation Database (March 2006)
NHS Centre for Reviews and Dissemination. URL: www.york.ac.uk/inst/crd/crddatabases.htm
benign hyperplasia/ or bph or bpe
or
benign and prostate
or
transurethral and prostate
or
turp or tuip or tuvp or tuna or tumt or hifu
Health Management Information Consortium (March 2006)
Ovid. URL: http://gateway.ovid.com/athens/
-
benign prostat$.tw
-
(bph or bpo or bpe).tw
-
(bladder adj3 obstruct$).tw
-
or/1-3
-
prostatectomy/
-
prostatectomy.yw
-
(coretherm or prostatron or targis or thermatrx or prolieve).tw
-
(turp or tuvp or tevap or tvp or tuevap).tw
-
(tuip or vlap or holrp or holep).tw
-
(tuna or tumt).tw
-
(prostat$ adj3 (laser$ or electro$ or vapor$ or ablat$ or thermo$ or resect$)).tw
-
(prostat$ adj3 transurethral).tw
-
or/5-12
-
4 or 13
-
(tuna and fish).mp
-
14 not 15
Conference proceedings
European Association of Urology
17th Congress, 2002. Eur Urol Suppl January 2002;1(1).
18th Congress, 2003. Eur Urol Suppl February 2003;2(1).
19th Congress, 2004. Eur Urol Suppl January 2004;3(2).
20th Congress, 2005. Eur Urol Suppl March 2005;4(3).
British Association of Urological Surgeons
Annual Scientific Meeting, 2001. BJU Int June 2001;88(Suppl1).
Annual Scientific Meeting, 2002. BJU Int July 2002;90(Suppl1).
Annual Scientific Meeting, 2003. BJU Int June 2003;91(Suppl2).
Annual Scientific Meeting, 2004. BJU Int June 2004;93(Suppl4).
Annual Scientific Meeting, 2005. BJU Int June 2005;95(Suppl5).
URL: www.blackwell-synergy.com/
BPH or bengn prostatic or benign hyperplasia turp or tuip or tuvp or tuna or tnmt or hifu
Websites consulted
American Urological Association. URL: www.auanet.org/. Accessed July 2005 and January 2006.
British Association of Urological Surgeons. URL: www.baus.org.uk/. Accessed July 2005 and January 2006.
Clinical Evidence. URL: www.clinicalevidence.com/. Accessed July 2005 and January 2006.
European Association of Urology. URL: www.uroweb.org/. Accessed July 2005 and January 2006.
Laserscope. URL: www.laserscope.com/. Accessed July 2005 and January 2006.
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Urologic Diseases in America. URL: http://kidney.niddk.nih.gov/statistics/uda/. Accessed July 2005 and January 2006.
TriP database. URL: www.tripdatabase.com/index.html. Accessed July 2005 and January 2006.
Urologix. URL: www.urologix.com/. Accessed July 2005 and January 2006.
Appendix 2 Study eligibility form
| Study eligibility form | |||
|---|---|---|---|
| Assessor initials: ____________ Date assessed: ____________ | |||
|
Study identifier (surname of first author and year of publication) |
Yes ⇩ Go to Next Question ⇩ |
Unclear ⇩ |
No ⇩ Exclude |
|
Type of study Q1. Is the study an RCT with follow-up of at least 3 months? |
Yes ⇩ Go to Next Question ⇩ |
Unclear ⇩ |
No ⇩ Exclude |
|
Participants in the study Q2. Are the participants in the study men with BPE? |
Yes ⇩ Go to Next Question ⇩ |
Unclear ⇩ |
No ⇩ Exclude |
|
Interventions in the study Q3. Does the study compare two or more of the following interventions: TURP; TUVP; TUIP; transurethral laser prostatectomy; TUNA; TUMT; HIFU; gyrus bipolar electrovaporisation; stents; water thermotherapy? |
Yes ⇩ Go to Next Question ⇩ |
Unclear ⇩ |
No ⇩ Exclude |
|
Outcomes in the study Q4. Does the study report one or more of the following outcomes: symptom score; quality of life; flow rate? |
Yes ⇩ Include, subject to clarification of ‘unclear’ points |
Unclear ⇩ |
No ⇩ Exclude |
| Final decision | Include | Unclear | Exclude |
Appendix 3 Data extraction form
Effectiveness of surgical treatments for men with benign prostatic enlargement
Reviewer ID: □ Date: □
| Study | |||
|---|---|---|---|
| Study ID: | Country: |
RCT □ Quasi-RCT □ Cohort study □ Unclear □ |
|
| Funding: government/private/manufacturer/pharmaceutical/not funded/other (specify) | |||
| Duration of study (recruitment dates): | Length of follow-up: | ||
| Intervention groups | |||
| Intervention 1 | Intervention 2 | Intervention 3 | |
| Participants | |||
| Criteria for inclusion: | |||
| Peak urine flow rate (voiding volume): __________________ Other: | |||
| Mean urine flow rate: __________________ | |||
| Residual urine volume: __________________ | |||
| Symptom score (list type of scale used): __________________ | |||
| (report only if used as a diagnostic tool) | |||
| Global physician assessment: ____________________________ | |||
| Criteria for exclusion (circle all that apply and describe) | |||
| Age (> 75 years for example): | Prostate size (> denoted size): | ||
| Prostate cancer: | Medications (current/contraindicated): | ||
| Previous treatment: | Infections: | ||
| Urinary retention (definition): | Co-morbidity/limited life expectancy: | ||
| Other: | |||
| Participant characteristics | |||
|---|---|---|---|
| Intervention 1 | Intervention 2 | Intervention 3 | |
| Eligible | |||
| Enrolled | |||
| No completed trial | |||
| Lost to follow-up | |||
|
Age (mean, SD): < 60 60–80 > 80 |
|||
| Ethnicity | |||
|
Symptom score: Mean ± SD Mild/mod/severe No. patients with score: 0–7 8–19 20–35 Write name of scale |
|||
| Peak urine flow (PUF) Qmax (ml/s), mean ± SD | |||
| Mean urine flow (MUF) (ml/s), mean ± SD | |||
| Total voided volume (ml), mean ± SD | |||
| Residual volume (ml), mean ± SD | |||
| Prostate size (ml), mean ± SD | |||
| Quality of life score, mean ± SD | |||
|
Sexually active/not Sexual function score, mean ± SD |
|||
|
Comments (other indications – retention, recurrent UTIs, bleeding, stones) |
|||
| Intervention characteristics | |||
|---|---|---|---|
| Intervention 1 | Intervention 2 | Intervention 3 | |
| Frequency (MHz) | |||
| Temperature | |||
| Power (W) | |||
| Duration of procedure (minutes) | |||
|
Intervention performed by consultant/trainee/not mentioned No. of years of experience of operating surgeon |
|||
|
Catheter protocol Yes/no/unclear If yes, mention duration |
|||
|
Preprocedural antibiotics Yes/no/unclear |
|||
| Others | |||
| Comments: | |||
| Complications | |||
|---|---|---|---|
|
Periprocedural (intraoperative and immediate postoperative) |
Intervention 1 N = |
Intervention 2 N = |
Intervention 3 N = |
| Intraoperative complications (bladder perforation, aborted procedure/device failure/rectal perforation, haemorrhage), number n/N (%) | |||
| Blood transfusion, number n/N (%) | |||
| Urinary tract infection (including epididymitis), number n/N (%) | |||
| Urinary retention, number n/N (%) | |||
| Catheter duration (days) | |||
| Re-catheterization, number n/N (%) | |||
| Co-interventions, number n/N (%) | |||
| Clot retention, number n/N (%) | |||
| TUR syndrome, number n/N (%) | |||
| Cardiovascular events | |||
| Mortality, number n/N (%) | |||
| Mortality, number n/N (%) | |||
| Incontinence, number n/N (%) | |||
| Septicaemia, number n/N (%) | |||
| Other (list if > 1%) | |||
| Complications | |||
|---|---|---|---|
|
Postoperative (at 3–12 months) |
Intervention 1 N = |
Intervention 2 N = |
Intervention 3 N = |
| Irritative urinary symptoms, number n/N (%) | |||
| Stricture (urethral/bladder neck), number n/N (%) | |||
| Urinary incontinence, number n/N (%) | |||
| Retrograde ejaculation, number n/N (%) | |||
| Erectile dysfunction, number n/N (%) | |||
| Re-operation rate, number n/N (%) | |||
| Urinary tract infections, number n/N (%) | |||
| Retention, number n/N (%) | |||
| Other (list if > 1%) | |||
|
Postoperative (> 12 months; mention follow-up period) |
|||
| Irritative urinary symptoms, number n/N (%) | |||
| Stricture (urethral/bladder neck), number n/N (%) | |||
| Urinary incontinence, number n/N (%) | |||
| Retrograde ejaculation, number n/N (%) | |||
| Erectile dysfunction, number n/N (%) | |||
| Re-operation rate, number n/N (%) | |||
| Urinary tract infections, number n/N (%) | |||
| Retention, number n/N (%) | |||
| Other (list if > 1%) | |||
| Outcomes and results – SYMPTOM SCORES | |||
|---|---|---|---|
| At 3 months |
Intervention 1 N = |
Intervention 2 N = |
Intervention 3 N = |
|
Symptom score, mean ± SD #1 write name of scale |
|||
|
Change in mean symptom score, mean ± SD #1 write name of scale |
|||
| Quality of life score (disease specific), mean ± SD | |||
| Change in quality of life score (disease specific), mean ± SD | |||
|
Any other quality of life score (such as SF-36), mean ± SD Name of QOL instrument |
|||
|
Change in any other quality of life score (such as SF-36), mean ± SD Name of QOL instrument |
|||
|
Global assessment ‘improvement in symptoms: subject rating’ (% and # improved/total) Follow-up duration at time of assessment: |
|||
|
Global assessment ‘improvement in symptoms: MD rating’ (% and # improved/total) Follow-up duration at time of assessment: |
|||
| Hospital length of stay, mean ± SD | |||
| Rehospitalization, number and % | |||
| At 12 months or more (follow-up ______) |
Intervention 1 N = |
Intervention 2 N = |
Intervention 3 N = |
|
Symptom score, mean ± SD #1 write name of scale |
|||
|
Change in mean symptom score, mean ± SD #1 write name of scale |
|||
| Quality of life score (disease specific), mean ± SD | |||
| Change in quality of life score (disease specific), mean ± SD | |||
|
Any other quality of life score (such as SF-36), mean ± SD Name of QOL instrument |
|||
|
Change in any other quality of life score (such as SF-36), mean ± SD Name of QOL instrument |
|||
|
Global assessment ‘improvement in symptoms: subject rating’ (% and # improved/total) Follow-up duration at time of assessment: |
|||
|
Global assessment ‘improvement in symptoms: MD rating’ (% and # improved/total) Follow-up duration at time of assessment: |
|||
| Outcomes and results – UROFLOWMETRY | |||
|---|---|---|---|
| At 3 months |
Intervention 1 N = |
Intervention 2 N = |
Intervention 3 N = |
| Peak urine flow (PUF) maximum flow rate (Qmax) (ml/s), mean ± SD | |||
| Change in peak urine flow (PUF) maximum flow rate (Qmax) (ml/s), mean ± SD | |||
| Mean urine flow (MUF) (ml/s), mean ± SD | |||
| Change in mean urine flow (MUF) (ml/s), mean ± SD | |||
| Total voided volume (ml), mean ± SD | |||
| Change in total voided volume (ml), mean ± SD | |||
| Residual volume (ml), mean ± SD | |||
| Change in residual volume (ml), mean ± SD | |||
| Mean detrusor pressure | |||
| Change in detrusor pressure | |||
| Prostate size (ml), mean ± SD | |||
| At 12 months or more (follow-up ______ ) | |||
| Peak urine flow (PUF) maximum flow rate (Qmax) (ml/s), mean ± SD | |||
| Change in peak urine flow (PUF) maximum flow rate (Qmax) (ml/s), mean ± SD | |||
| Mean urine flow (MUF) (ml/s), mean ± SD | |||
| Change in mean urine flow (MUF) (ml/s), mean ± SD | |||
| Total voided volume (ml), mean ± SD | |||
| Change in total voided volume (ml), mean ± SD | |||
| Residual volume (ml), mean ± SD | |||
| Change in residual volume (ml), mean ± SD | |||
| Mean detrusor pressure | |||
| Change in detrusor pressure | |||
| Prostate size (ml), mean ± SD | |||
| Additional information/other comments |
|---|
| Contact with author |
|---|
Date: ………/………/……… Signature: ………………………………
Appendix 4 Quality assessment form: randomised controlled trials
Study identifier: Date completed: Assessor initials:
| Criteria | Yes | No | Unclear | Comments |
|---|---|---|---|---|
|
1. Was the assignment to the treatment groups really random? Adequate approaches to sequence generation Inadequate approaches to sequence generation |
||||
|
2. Was the treatment allocation concealed? Adequate approaches to concealment of randomisation Inadequate approaches to concealment of randomisation |
||||
| 3. Were the groups similar at baseline in terms of prognostic factors? | ||||
| 4. Were the eligibility criteria specified? | ||||
| 5. Was the intervention (and comparison) clearly defined? | ||||
| 6. Were the groups treated in the same way apart from the intervention received? | ||||
| 7. Was follow-up long enough to detect important effects on outcomes of interest? | ||||
| a. For short-term outcomes, at least 3 months | ||||
| b. For long-term outcomes, at least 1 year | ||||
| 8. Were the outcome assessors blinded to the treatment allocation? | ||||
| 9. Were the care providers blinded? | ||||
| 10. Were the patients blinded? | ||||
| 11. Were the point estimates and measures of variability presented for the primary outcome measures? | ||||
| 12. Was the withdrawal/dropout rate likely to cause bias? | ||||
| 13. Did the analyses include an intention to treat analysis? | ||||
| 14. Was the operation undertaken by someone experienced in performing the procedure? |
Appendix 5 Included studies
Abbou 1995143
Primary reference
17. Abbou C-C, Payan C, Viens-Bitker C, Richard F, Boccon-Gibod L, Jardin A, et al. Transrectal and transurethral hyperthermia versus sham treatment in benign prostatic hyperplasia: a double-blind randomized multicentre clinical trial. Br J Urol 1995;76:619–24.
Ahmed 1997124
Primary reference
18. Ahmed M, Bell T, Lawrence WT, Ward JP, Watson GM. Transurethral microwave thermotherapy Prostatron version 2.5) compared with transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: a randomized, controlled, parallel study. Br J Urol 1997;79:181–5.
Albala 2002170
Primary reference
19. Albala DM, Fulmer BR, Turk TM, Koleski F, Andriole G, Davis BE, et al. Office-based transurethral microwave thermotherapy using the TherMatrx TMx-2000. J Endourol 2002;16:57–61.
Secondary references
20. Albala DM, Koleski F, Nuzzarello J, Davis BE, Eure GR, Andriole G, et al. Periurethral prostatic microwave thermotherapy using the Thermatrx TMX-2000TM: follow-up of a randomized, blinded, sham-controlled study in patients with BPH. J Urol 2000;163:269.
21. Albala DM, Andriole G, Davis B. Transurethral microwave thermotherapy (TUMT) using the thermatrx TMX-2000: durability exhibited in a study comparing TUMT with a sham procedure in patients with benign prostatic hyperplasia. Abstract no. 1746. 2003. Annual Meeting of the American Urological Association.
22. Albala DM, Andriole G, Davis B, Eure GR, Kabalin JN, Lingeman JE, et al. Transurethral microwave thermotherapy (TUMT) using the thermatrx TMX-2000: long-term results in a study comparing TUMT with a sham oricedure in patients with benign prostatic hyperplasia. Abstract no. 1551. 2005. Annual Meeting of the American Urological Association.
23. Kabalin JN, Albala DM, Koleski F, Andriole G, Sundaram C, Davis BE, et al. Office-based transurethral microwave thermotherapy for benign prostatic hyperplasia (BPH) using the TherMatrxTM TMx-2000TM: results of a multi-center prospective randomized sham-controlled trial. J Urol 2001;165:367–8.
Bdesha 1994125
Primary reference
24. Bdesha AS, Bunce CJ, Snell ME, Witherow RO. Sham controlled trial of transurethral microwave therapy with subsequent treatment of the control-group. J Urol 1994;152:453–8.
Secondary reference
25. Bdesha AS, Bunce CJ, Kelleher JP, Snell ME, Vukusic J, Witherow OR. Transurethral microwave treatment for benign prostatic hypertrophy: a randomised controlled clinical trial. BMJ 1993;306:1293–6.
Blute 1996126
Primary reference
26. Blute ML, Patterson DE, Segura JW, Tomera KM, Hellerstein DK. Transurethral microwave thermotherapy v sham treatment: double-blind randomized study. J Endourol 1996;10:565–73.
Bouchier-Hayes 2006141
Primary reference
27. Bouchier-Hayes DM, Anderson P, Van Appledorn S, Bugeja P, Costello AJ. KTP laser versus transurethral resection: early results of a randomized trial. J Endourol 2006;20:580–5.
Brehmer 1999171
Primary reference
28. Brehmer M, Wiksell H, Kinn A. Sham treatment compared with 30 or 60 min of thermotherapy for benign prostatic hyperplasia: a randomized study. BJU Int 1999;84:292–6.
Carter 1999127
Primary reference
29. Carter A, Sells H, Speakman M, Ewings P, MacDonagh R, O’Boyle P. A prospective randomized controlled trial of hybrid laser treatment or transurethral resection of the prostate, with a 1-year follow-up. BJU Int 1999a;83:254–9.
Secondary references
30. Carter A, Sells H, Speakman M, Ewings P, O’Boyle P, MacDonagh R. Quality of life changes following KTP/Nd:YAG laser treatment of the prostate and TURP. Eur Urol 1999b;36:92–8.
31. Pearcy R, Carter A, Sells H, O’Boyle P, MacDonagh R, Speakman M, et al. Long term follow up of hybrid KITP/ND:YAG laser treatment of the prostate versus TURP: a prospective randomised trial, 18 month results. J Urol 1999;161:390.
Çetinkaya 1996196
Primary reference
32. Çetinkaya M, Ulusoy E, Adsan O, Saglam H, Ozturk B, Basay S. Comparative early results of transurethral electroresection and transurethral electrovaporization in benign prostatic hyperplasia. Br J Urol 1996;78:901–3.
Chacko 2001154
Primary reference
33. Chacko KN, Donovan JL, Abrams P, Peters TJ, Brookes ST, Thorpe AC, et al. Transurethral prostatic resection or laser therapy for men with acute urinary retention: the CLasP randomized trial. J Urol 2001;166:166–70.
Chapple 199591
Primary reference
34. Chapple CR, Rosario DJ, Wasserfallen M, Woo HH. A randomised study of the urolume stent vs prostatic surgery. J Urol 1995;153:436A.
Christensen 1990135
Primary reference
35. Christensen MM, Aagaard J, Madsen PO. Transurethral resection versus transurethral incision of the prostate. A prospective randomized study. Urol Clin North Am 1990;17:621–30.
Secondary reference
36. Aagaard J, Chopin D, Knes J, Madsen PO. Transurethral resection TURP vs incision TUIP of the prostate: a prospective randomized study. J Urol 1990;143:411A.
Cimentepe 2003175
Primary reference
37. Cimentepe E, Unsal A, Saglam R. Randomized clinical trial comparing transurethral needle ablation with transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: results at 18 months. J Endourol 2003;17:103–7.
Costello 1995123
Primary reference
38. Costello AJ, Crowe HR, Jackson T, Street A. A randomised single institution study comparing laser prostatectomy and transurethral resection of the prostate. Ann Acad Med 1995;24:700–4.
Cowles 1995163
Primary reference
39. Cowles RS, III, Kabalin JN, Childs S, Lepor H, Dixon C, Stein B, et al. A prospective randomized comparison of transurethral resection to visual laser ablation of the prostate for the treatment of benign prostatic hyperplasia. Urology 1995;46:155–60.
d’Ancona 1998166
Primary reference
40. d’Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, de la Rosette JJ. Transurethral resection of the prostate vs high-energy thermotherapy of the prostate in patients with benign prostatic hyperplasia: long-term results. Br J Urol 1998;81:259–64.
Secondary reference
41. d’Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, de la Rosette JJ. High energy thermotherapy versus transurethral resection in the treatment of benign prostatic hyperplasia: results of a prospective randomized study with 1 year of followup. J Urol 1997;158:120–5.
Dahlstrand 1993167
Primary reference
42. Dahlstrand C, Geirsson G, Fall M, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for benign prostatic hyperplasia: preliminary results of a randomized study. Eur Urol 1993;23:292–8.
Secondary references
43. Dahlstrand C, Fall M, Geirsson G, Petterson DE. Transurethral microwave thermotherapy versus transurethral resection for benign prostatic hyperplasia: results of a randomized study. J Urol 1993;149:250A.
44. Dahlstrand C, Geirsson G, Walden M. Prospective randomized study between transurethral resection (TURP) and transurethral microwave treatment (TUMT) for benign prostatic hyperplasia. Scand J Urol Nephrol Suppl 1993;151:32–3.
45. Dahlstrand C, Walden M, Geirsson G, Sommar S, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for BPH. Prog Clin Biol Res 1994;386:455–61.
Dahlstrand 1995168
Primary reference
46. Dahlstrand C, Walden M, Geirsson G, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for symptomatic benign prostatic obstruction: a prospective randomized study with a 2-year follow-up. Br J Urol 1995;76:614–18.
Secondary reference
47. Walden M, Acosta S, Carlsson P, Pettersson S, Dahlstrand C. A cost-effectiveness analysis of transurethral resection of the prostate and transurethral microwave thermotherapy for treatment of benign prostatic hyperplasia: two-year follow-up. Scand J Urol Nephrol 1998;32:204–10.
de la Rosette 2003169
Primary reference
48. de la Rosette JJ, Floratos DL, Severens JL, Kiemeney LA, Debruyne FM, Pilar LM. Transurethral resection vs microwave thermotherapy of the prostate: a cost-consequences analysis. BJU Int 2003;92:713–18.
Secondary references
49. Floratos DL, Kiemeney LA, Rossi C, Kortmann BB, Debruyne FM, de la Rosette JJ. Long-term followup of randomized transurethral microwave thermotherapy versus transurethral prostatic resection study. J Urol 2001;165:1533–8.
50. Francisca EA, d’Ancona FC, Meuleman EJ, Debruyne FM, de la Rosette JJ. Sexual function following high energy microwave thermotherapy: results of a randomized controlled study comparing transurethral microwave thermotherapy to transurethral prostatic resection. J Urol 1999;161:486–90.
51. Francisca EA, d’Ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, de la Rosette JJ. A randomized study comparing high-energy TUMT to TURP: quality-of-life results. Eur Urol 2000;38:569–75.
de Sio 200665
Primary reference
52. de Sio M, Autorino R, Quarto G, Damiano R, Perdona S, di Lorenzo G, et al. Gyrus bipolar versus standard monopolar transurethral resection of the prostate: a randomized prospective trial. Urology 2006;67:69–72.
de Wildt 1996172
Primary reference
53. de Wildt MJ, Hubregtse M, Ogden C, Carter SS, Debruyne FM, de la Rosette JJ. A 12-month study of the placebo effect in transurethral microwave thermotherapy. Br J Urol 1996;77:221–7.
Secondary references
54. de la Rosette JJ, de Wildt MJ, Alivizatos G, Froeling FM, Debruyne FM. Transurethral microwave thermotherapy (TUMT) in benign prostatic hyperplasia: placebo versus TUMT. Urology 1994;22:58–63.
55. Francisca EA, d’Ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, de la Rosette JJ. Quality of life assessment in patients treated with lower energy thermotherapy (Prostasoft 2.0): results of a randomized transurethral microwave thermotherapy versus sham study. J Urol 1997;158:1839–44.
Donovan 2000136
Primary reference
56. Donovan JL, Peters TJ, Neal DE, Brookes ST, Gujral S, Chacko KN, et al. A randomized trial comparing transurethral resection of the prostate, laser therapy and conservative treatment of men with symptoms associated with benign prostatic enlargement: the CLasP study. J Urol 2000;164:65–70.
Secondary references
57. Brookes ST, Donovan JL, Peters TJ, Abrams P, Neal DE. Sexual dysfunction in men after treatment for lower urinary tract symptoms: evidence from randomised controlled trial. BMJ 2002;324:1059–61.
58. Donovan JL, Brookes ST, Kennedy LG, Abrams P, Peters TJ, Neal DE. The CLasP randomised controlled trial: comparing laser therapy, conservative management and TURP for men with lower urinary tract symptoms. J Urol 1998;159:248.
Dørflinger 1992180
Primary reference
59. Dørflinger T, Jensen FS, Krarup T, Walter S. Transurethral prostatectomy compared with incision of the prostate in the treatment of prostatism caused by small benign prostate glands. Scand J Urol Nephrol 1992;26:333–8.
Dunsmuir 2003137
Primary reference
60. Dunsmuir WD, McFarlane JP, Tan A, Dowling C, Downie J, Kourambas J, et al. Gyrus bipolar electrovaporization vs transurethral resection of the prostate: a randomized prospective single-blind trial with 1 y follow-up. Prostate Cancer Prostatic Dis 2003;6:182–6.
Secondary reference
61. Love CJ, Dowling C, Pham T, Tan A, McFarlane JP, Dunsmuir WD. Gyrus (R) bipolar electrovaporization versus transurethral resection of the prostate: a randomized prospective trial with 1-year follow-up. J Urol 2003;169:390.
Ekengren 2000142
Primary reference
62. Ekengren J, Haendler L, Hahn RG. Clinical outcome 1 year after transurethral vaporization and resection of the prostate. Urology 2000;55:231–5.
Erdaği 1999128
Primary reference
63. Erdaği U, Akman RY, Sargin SY, Yazicioglu A. Transurethral electrovaporization of the prostate versus transurethral resection of the prostate: a prospective randomized study. Arch Ital Urol Androl 1999;71:125–30.
Fowler 200557
Primary reference
64. Fowler C, McAllister W, Plail R, Karim O, Yang Q. Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia. Health Technol Assess 2005;9:1–30.
Secondary reference
65. McAllister WJ, Karim O, Plail RO, Samra DR, Steggall MJ, Yang Q, et al. Transurethral electrovaporization of the prostate: is it any better than conventional transurethral resection of the prostate? BJU Int 2003;91:211–14.
Fung 2005155
Primary reference
66. Fung BT, Li SK, Yu CF, Lau BE, Hou SS. Prospective randomized controlled trial comparing plasmakinetic vaporesection and conventional transurethral resection of the prostate. Asian J Surg 2005;28:24–8.
Gallucci 1998138
Primary reference
67. Gallucci M, Puppo P, Perachino M, Fortunato P, Muto G, Breda G, et al. Transurethral electrovaporization of the prostate vs. transurethral resection. Results of a multicentric, randomized clinical study on 150 patients. Eur Urol 1998;33:359–64.
Secondary reference
68.Puppo P, Perachino M, Breda G, Boccafoschi C, Comeri G, Francesca F, et al. Transurethral electrovaporization of the prostate (TVP): a multicentric randomized comparative study vs TURP. J Urol 1996;155(5Suppl):408A
Gotoh 1999156
Primary reference
69. Gotoh M, Okamura K, Hattori R, Nishiyama N, Kobayashi H, Tanaka K, et al. A randomized comparative study of the Bandloop versus the standard loop for transurethral resection of the prostate. J Urol 1999;162:1645–7.
Gujral 2000139
Primary reference
70. Gujral S, Abrams P, Donovan JL, Neal DE, Brookes ST, Chacko KN, et al. A prospective randomized trial comparing transurethral resection of the prostate and laser therapy in men with chronic urinary retention: the CLasP study. J Urol 2000;164:59–64.
Gupta 2006187
Primary reference
71. Gupta N, Sivaramakrishna, Kumar R, Dogra PN, Seth A. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of > 40 g. BJU Int 2006;97:85–9.
Hammadeh 2003129
Primary reference
72. Hammadeh MY, Madaan S, Hines J, Philp T. 5-year outcome of a prospective randomized trial to compare transurethral electrovaporization of the prostate and standard transurethral resection. Urology 2003;61:1166–71.
Secondary references
73. Hammadeh MY, Fowlis GA, Singh M, Philp T. Transurethral electrovaporization of the prostate – a possible alternative to transurethral resection: a one-year follow-up of a prospective randomized trial. Br J Urol 1998a;81:721–5.
74. Hammadeh MY, Madaan S, Singh M, Philp T. Two-year follow-up of a prospective randomised trial of electrovaporization versus resection of prostate. Eur Urol 1998b;34:188–92.
75. Hammadeh MY, Madaan S, Singh M, Philp T. Two years follow up of a prospective randomised trial of electro-vaporisation of the prostate vs standard TURP. J Endourol 1998c;12:S175.
76. Hammadeh MY, Madaan S, Singh M, Philp T. Three years follow up of a prospective randomised trial comparing transurethral electrovaporization of the prostate to standard TURP. J Endourol 1999;13:A29.
77. Hammadeh MY, Madaan S, Singh M, Philp T. A 3-year follow-up of a prospective randomized trial comparing transurethral electrovaporization of the prostate with standard transurethral prostatectomy. BJU Int 2000;86:648–51.
78. Hammadeh MY, Madaan S, Hines J, Philp T. The efficacy and durability of transurethral electrovaporisation of the prostate: 5 years result of a prospective randomised trial. Eur Urol Suppl 2003;2:168.
Helke 2001202
Primary reference
79. Helke C, Manseck A, Hakenberg OW, Wirth MP. Is transurethral vaporesection of the prostate better than standard transurethral resection? Eur Urol 2001;39:551–7.
Hellström 1986157
Primary reference
80. Hellström P, Lukkarinen O, Kontturi M. Bladder neck incision or transurethral electroresection for the treatment of urinary obstruction caused by a small benign prostate? A randomized urodynamic study. Scand J Urol Nephrol 1986;20:187–92.
Hill 2004144
Primary reference
81. Hill B, Belville W, Bruskewitz R, Issa M, Perez-Marrero R, Roehrborn C, et al. Transurethral needle ablation versus transurethral resection of the prostate for the treatment of symptomatic benign prostatic hyperplasia: 5-year results of a prospective, randomized, multicenter clinical trial. J Urol 2004;171:2336–40.
Secondary references
82. Bruskewitz R, Issa MM, Roehrborn CG, Naslund MJ, Perez-Marrero R, Shumaker BP, et al. A prospective, randomized 1-year clinical trial comparing transurethral needle ablation to transurethral resection of the prostate for the treatment of symptomatic benign prostatic hyperplasia. J Urol 1998;159:1588–93.
83. Naslund M, Oesterling JE, Issa M, Roehrborn CG, Bruskewitz R, Perez-Marrero R, et al. Long term follow-up of a prospective, randomized clinical trial comparing transurethral needle ablation (TUNA) to transurethral resection of the prostate (TURP) for the treatment of benign prostatic hyperplasia (BPH). J Endourol 1997;11:S188.
84. Naslund M, Perez-Marrero R, Roehrborn C, Bruskewitz R, Issa M. Intermediate term outcomes of TUNA for BPH: 36 month results of the TUNA vs TURP US randomized study. J Urol 1999;161:389.
85. Roehrborn CG, Burkhard FC, Bruskewitz RC, Issa MM, Perez-Marrero R, Naslund MJ, et al. The effects of transurethral needle ablation and resection of the prostate on pressure flow urodynamic parameters: analysis of the United States randomized study. J Urol 1999;162:92–7.
Hindley 2001176
Primary reference
86. Hindley RG, Mostafid AH, Brierly RD, Harrison NW, Thomas PJ, Fletcher MS. The 2-year symptomatic and urodynamic results of a prospective randomized trial of interstitial radiofrequency therapy vs transurethral resection of the prostate. BJU Int 2001;88:217–20.
Secondary reference
87. Mostafid AH, Harrison NW, Thomas PJ, Fletcher MS. A prospective randomized trial of interstitial radiofrequency therapy versus transurethral resection for the treatment of benign prostatic hyperplasia. Br J Urol 1997;80:116–22.
Hon 200670
Primary reference
88. Hon NHY, Brathwaite D, Hussain Z, Ghiblawi S, Brace H, Hayne D, et al. A prospective, randomized trial comparing conventional transurethral prostate resection with plasmakinetic vaporization of the prostate: physiological changes, early complications and long-term follow-up. J Urol 2006;176:205–9.
Jahnson 1998181
Primary reference
89. Jahnson S, Dalen M, Gustavsson G, Pedersen J. Transurethral incision versus resection of the prostate for small to medium benign prostatic hyperplasia. Br J Urol 1998;81:276–81.
Kabalin 1995177
Primary reference
90. Kabalin JN, Gill HS, Bite G, Wolfe V. Comparative study of laser versus electrocautery prostatic resection: 18-month followup with complex urodynamic assessment. J Urol 1995;153:94–7.
Secondary reference
91. Kabalin JN. Laser prostatectomy performed with a right angle firing neodymium:YAG laser fiber at 40 watts power setting. J Urol 1993;150:95–9.
Kaplan 199855
Primary reference
92. Kaplan SA, Laor E, Fatal M, Te AE. Transurethral resection of the prostate versus transurethral electrovaporization of the prostate: a blinded, prospective comparative study with 1-year followup. J Urol 1998;159:454–8.
Keoghane 2000164
Primary reference
93. Keoghane SR, Lawrence KC, Gray AM, Doll HA, Hancock AM, Turner K, et al. A double-blind randomized controlled trial and economic evaluation of transurethral resection vs contact laser vaporization for benign prostatic enlargement: a 3-year follow-up. BJU Int 2000;85:74–8.
Secondary references
94. Jenkinson C, Gray A, Doll H, Lawrence K, Keoghane S, Layte R. Evaluation of index and profile measures of health status in a randomized controlled trial. Comparison of the Medical Outcomes Study 36-Item Short Form Health Survey, EuroQol, and disease specific measures. Med Care 1997;35:1109–18.
95. Keoghane S, Cranston D, Lawrence K, Doll H. The Oxford laser prostate trial: a prospective randomised controlled trial of contact vaporisation of the prostate versus TURP. J Urol 1995;153:230A.
96. Keoghane SR, Doll HA, Lawrence KC, Jenkinson CP, Cranston DW. The Oxford Laser Prostate Trial: sexual function data from a randomized controlled clinical trial of contact laser prostatectomy. Eur Urol 1996a;30:424–8.
97. Keoghane SR, Cranston DW, Lawrence KC, Doll HA, Fellows GJ, Smith JC. The Oxford Laser Prostate Trial: a double-blind randomized controlled trial of contact vaporization of the prostate against transurethral resection; preliminary results. Br J Urol 1996b;77:382–5.
98. Keoghane SR, Lawrence KC, Jenkinson CP, Doll HA, Chappel DB, Cranston DW. The Oxford Laser Prostate Trial: sensitivity to change of three measures of outcome. Urology 1996c;47:43–7.
99. Keoghane SR, Sullivan ME, Doll HA, Kourambas J, Cranston DW. Five-year data from the Oxford Laser Prostatectomy Trial. BJU Int 2000;86:227–8.
Kim 2006a151
Primary reference
100. Kim TS, Choi S, Rhew HY, Ahn JH, Jang JH, Cho MH. Comparative study on the treatment outcome and safety of TURP, ILC, TUNA and TEAP for patients with benign prostatic hyperplasia. Korean J Urol 2006;47:13–19.
Kim 2006b150
Primary reference
101. Kim JY, Moon KH, Yoon CJ, Park TC. Bipolar transurethral resection of the prostate: a comparative study with monopolar transurethral resection. Korean J Urol 2006;47:493–7.
Kuntz 200464
Primary reference
102. Kuntz RM, Ahyai S, Lehrich K, Fayad A. Transurethral holmium laser enucleation of the prostate versus transurethral electrocautery resection of the prostate: a randomized prospective trial in 200 patients. J Urol 2004;172:1012–16.
Secondary reference
103. Kuntz RM, Ahyai S, Lehrich K. Holmium laser enucleation vs TURP: a randomized prospective study with 2 years of follow-up. BJU Int 2003;91(Suppl2):71.
Küpeli 1998a158
Primary reference
104. Küpeli S, Baltaci S, Soygur T, Aytac S, Yilmaz E, Budak M. A prospective randomized study of transurethral resection of the prostate and transurethral vaporization of the prostate as a therapeutic alternative in the management of men with BPH. Eur Urol 1998;34:15–18.
Küpeli 1998b197
Primary reference
105. Küpeli B, Yalcinkaya F, Topaloglu H, Karabacak O, Gunlusoy B, Unal S. Efficacy of transurethral electrovaporization of the prostate with respect to standard transurethral resection. J Endourol 1998;12:591–4.
Küpeli 2001203
Primary reference
106. Küpeli S, Yilmaz E, Soygur T, Budak M. Randomized study of transurethral resection of the prostate and combined transurethral resection and vaporization of the prostate as a therapeutic alternative in men with benign prostatic hyperplasia. J Endourol 2001;15:317–21.
Kursh 2003130
Primary reference
107. Kursh ED, Concepcion R, Chan S, Hudson P, Ratner M, Eyre R. Interstitial laser coagulation versus transurethral prostate resection for treating benign prostatic obstruction: a randomized trial with 2-year follow-up. Urology 2003;61:573–8.
Larson 1998159
Primary reference
108. Larson TR, Blute ML, Bruskewitz RC, Mayer RD, Ugarte RR, Utz WJ. A high-efficiency microwave thermoablation system for the treatment of benign prostatic hyperplasia: results of a randomized, sham-controlled, prospective, double-blind, multicenter clinical trial. Urology 1998;51:731–42.
Li 1987182
Primary reference
109. Li MK, Ng AS. Bladder neck resection and transurethral resection of the prostate: a randomized prospective trial. J Urol 1987;138:807–9.
Liedberg 2003131
Primary reference
110. Liedberg F, Adell L, Hagberg G, Palmqvist IB. Interstitial laser coagulation versus transurethral resection of the prostate for benign prostatic enlargement – a prospective randomized study. Scand J Urol Nephrol 2003;37:494–7.
Liu 2006132
Primary reference
111. Liu CK, Lee WK, Ko MC, Chiang HS, Wan KS. Transurethral electrovapor resection versus standard transurethral resection treatment for a large prostate: a 2-year follow-up study conducted in Taiwan. Urol Int 2006;76:144–9.
Mårtenson 1999178
Primary reference
112. Mårtenson AC, de la Rosette JJMC. Interstitial laser coagulation in the treatment of benign prostatic hyperplasia using a diode laser system: results of an evolving technology. Prostate Cancer Prostatic Dis 1999;2:148–54.
McAllister 2000145
Primary reference
113. McAllister WJ, Absalom MJ, Mir K, Shivde S, Anson K, Kirby RS, et al. Does endoscopic laser ablation of the prostate stand the test of time? Five-year results from a multicentre randomized controlled trial of endoscopic laser ablation against transurethral resection of the prostate. BJU Int 2000;85:437–9.
Secondary reference
114. Anson K, Nawrocki J, Buckley J, Fowler C, Kirby R, Lawrence W, et al. A multicenter, randomized, prospective study of endoscopic laser ablation versus transurethral resection of the prostate. Urology 1995;46:305–10.
Montorsi 2004188
Primary reference
115. Montorsi F, Naspro R, Salonia A, Suardi N, Briganti A, Zanoni M, et al. Holmium laser enucleation versus transurethral resection of the prostate: results from a 2-center, prospective, randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol 2004;172:1926–9.
Secondary references
116. Briganti A, Naspro R, Vavassori I, Suardi N, Salonia A, Mazzoccoli B, et al. Impact of holmium laser enucleation (HoLEP) versus transurethral resection (TURP) of the prostate on sexual function: results of a prospective multicentric randomized trial. J Urol 2004;171:398.
117. Briganti A, Naspro R, Vavassori I, Suardi N, Salonia A, Mazzoccoli B, et al. Impact of holmium laser enucleation (HoLEP) versus transurethral resection (TURP) of the prostate on sexual function: results of a prospective multicentric randomized trial. Eur Urol Suppl 2004;3:144.
118. Montosori F, Naspro R, Salonia A, Suardi N, Briganti A, Zanoni M. Holmium laser enucleation versus transurethral resection of the prostate: results from a two-centre prospective randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol 2004;171:409.
Mottet 1999190
Primary reference
119. Mottet N, Anidjar M, Bourdon O, Louis JF, Teillac P, Costa P, et al. Randomized comparison of transurethral electroresection and holmium: YAG laser vaporization for symptomatic benign prostatic hyperplasia. J Endourol 1999;13:127–30.
Secondary reference
120. Mottet N, Anidjar M, Costa P, Louis JF, Teillac P, Duc A. A randomized study comparing the holmium-YAG vaporisation and the transurethral resection of symptomatic BPH. J Endourol 1997;11(Suppl 1):S160.
Nathan 1996162
Primary reference
121. Nathan MS, Wickham JEA. TVP: a cheaper and effective alternative to TURP. Minim Invasive Ther Allied Technol 1996;5:292–6.
Nawrocki 1997160
Primary reference
122. Nawrocki JD, Bell TJ, Lawrence WT, Ward JP. A randomized controlled trial of transurethral microwave thermotherapy. Br J Urol 1997;79:389–93.
Netto 1999198
Primary reference
123. Netto NR, Jr, de Lima ML, Lucena R, Lavoura NS, Cortado PL, Netto MR. Is transurethral vaporization a remake of transurethral resection of the prostate? J Endourol 1999;13:591–4.
Nielsen 1988183
Primary reference
124. Nielsen HO. Transurethral prostatotomy versus transurethral prostatectomy in benign prostatic hypertrophy. A prospective randomised study. Br J Urol 1988;61:435–8.
Nuhoğglu 2005199
Primary reference
125. Nuhoğlu B, Ayyildiz A, Fidan V, Ersoy E, Huri E, Germiyanogu C. Transurethral electrovaporization of the prostate: is it any better than standard transurethral prostatectomy? 5-year follow-up. J Endourol 2005;19:79–82.
Nuhoğlu 2006194
Primary reference
126. Nuhoğlu B, Ayyildiz A, Karaguzel E, Cebeci O, Germiyanoglu C. Plasmakinetic prostate resection in the treatment of benign prostate hyperplasia: results of 1-year follow up. Int J Urol 2006;13:21–4.
Ogden 1993133
Primary reference
127. Ogden C, Reddy P, Johnson H, Carter S. Sham vs TUMT: a randomized study with cross over. J Urol 1993;149(Suppl 4):250A.
Patel 1997140
Primary reference
128. Patel A, Fuchs GJ, Gutierrez-Aceves J, Ryan TP. Prostate heating patterns comparing electrosurgical transurethral resection and vaporization: a prospective randomized study. J Urol 1997;157:169–72.
Riehmann 1995152
Primary reference
129. Riehmann M, Knes JM, Heisey D, Madsen PO, Bruskewitz RC. Transurethral resection versus incision of the prostate: a randomized, prospective study. Urology 1995;45:768–75.
Secondary references
130. Riehmann M, Knes J, Madaan S, Bruskewitz R. Transurethral resection (TURP) versus incision (TUIP) of the prostate: a prospective randomized study. J Urol 1993;149(Suppl 4):323A.
131. Sparwasser C, Riehmann M, Knes J, Madsen PO. Long-term results of transurethral prostate incision (TUIP) and transurethral prostate resection (TURP). A prospective randomized study. Urologe (Ausg A) 1995;34:153–7.
Rodrigo Aliaga 1998149
Primary reference
132. Rodrigo Aliaga M, Valls Blasco F, Jimenez Cruz JF. Lasers as an alternative to the endoscopic surgery in BPH. Actas Urol Esp 1998;22:17–22.
Saporta 1996184
Primary references
133. Saporta L, Aridogan IA, Erlich N, Yachia D. Objective and subjective comparison of transurethral resection, transurethral incision and balloon dilatation of the prostate. A prospective study. Eur Urol 1996;29:439–45.
Seckiner 2006161
Primary reference
134. Seckiner I, Yesilli C, Akduman B, Altan K, Mungan NA. A prospective randomized study for comparing bipolar plasmakinetic resection of the prostate with standard TURP. Urol Int 2006;76:139–43.
Sengör 1996192
Primary reference
135. Sengör F, Kose O, Yucebas E, Beysel M, Erdogan K, Narter F. A comparative study of laser ablation and transurethral electroresection for benign prostatic hyperplasia: results of a 6-month follow-up. Br J Urol 1996;78:398–400.
Shingleton 2002146
Primary reference
136. Shingleton WB, Farabaugh P, May W. Three-year follow-up of laser prostatectomy versus transurethral resection of the prostate in men with benign prostatic hyperplasia. Urology 2002;60:305–8.
Secondary references
137. Shingleton WB, Terrell F, Renfroe DL, Kolski JM, Fowler JE, Jr. A randomized prospective study of laser ablation of the prostate versus transurethral resection of the prostate in men with benign prostatic hyperplasia. Urology 1999;54:1017–21.
138. Shingleton WB, Farabaugh P. Prospective randomized study of laser prostatectomy and transurethral resection of the prostate in men with benign prostatic hyperplasia: 3 year follow-up. J Urol 2001;165(Suppl 5):297.
Shokeir 1997200
Primary reference
139. Shokeir AA, al Sisi H, Farage YM, el Maaboud MA, Saeed M, Mutabagani H. Transurethral prostatectomy: a prospective randomized study of conventional resection and electrovaporization in benign prostatic hyperplasia. Br J Urol 1997;80:570–4.
Singh 2005147
Primary reference
140. Singh H, Desai MR, Shrivastav P, Vani K. Bipolar versus monopolar transurethral resection of prostate: randomized controlled study. J Endourol 2005;19:333–8.
Soonawalla 1992185
Primary reference
141. Soonawalla PF, Pardanani DS. Transurethral incision versus transurethral resection of the prostate. A subjective and objective analysis. Br J Urol 1992;70:174–7.
Suvakovic 1996179
Primary reference
142. Suvakovic N, Hindmarsh JR. A step towards day case prostatectomy. Br J Urol 1996;77:212–14.
Talic 200068
Primary reference
143. Talic RF, El Tiraifi A, El Faqih SR, Hassan SH, Attassi RA, Abdel-Halim RE. Prospective randomized study of transurethral vaporization resection of the prostate using the thick loop and standard transurethral prostatectomy. Urology 2000;55:886–90.
Tefekli 2005195
Primary reference
144. Tefekli A, Muslumanoglu AY, Baykal M, Binbay M, Tas A, Altunrende F. A hybrid technique using bipolar energy in transurethral prostate surgery: a prospective, randomized comparison. J Urol 2005;174:1339–43.
Tkocz 2002186
Primary reference
145. Tkocz M, Prajsner A. Comparison of long-term results of transurethral incision of the prostate with transurethral resection of the prostate, in patients with benign prostatic hypertrophy. Neurourol Urodyn 2002;21:112–16.
Trachtenberg 1998173
Primary reference
146. Trachtenberg J, Roehrborn CG. Updated results of a randomized, double-blind, multicenter sham-controlled trial of microwave thermotherapy with the Dornier Urowave in patients with symptomatic benign prostatic hyperplasia. Urowave Investigators Group. World J Urol 1998;16:102–8.
Secondary references
147. Roehrborn C, Sech S, Preminger G, Cohen T, Perlmutter A, Razvi H, et al. A randomized, blinded study comparing microwave thermotherapy (Dornier Urowave) with a sham procedure in patients with clinical benign prostatic hyperplasia (BPH). J Endourol 1997;11(Suppl1):S155.
148. Roehrborn CG, Preminger G, Newhall P, Denstedt J, Razvi H, Chin LJ, et al. Microwave thermotherapy for benign prostatic hyperplasia with the Dornier Urowave: results of a randomized, double-blind, multicenter, sham-controlled trial. Urology 1998;51:19–28.
149. Tan AH, Nott L, Hardie WR, Chin JL, Denstedt JD, Razvi H. Long-term results of microwave thermotherapy for symptomatic benign prostatic hyperplasia. J Endourol 2005;19:1191–5.
Tuhkanen 2001191
Primary reference
150. Tuhkanen K, Heino A, Ala-Opas M. Two-year follow-up results of a prospective randomized trial comparing hybrid laser prostatectomy with TURP in the treatment of big benign prostates. Scand J Urol Nephrol 2001;35:200–4.
Secondary reference
151. Tuhkanen K, Heino A, Ala-Opas M. Hybrid laser treatment compared with transurethral resection of the prostate for symptomatic bladder outlet obstruction caused by a large benign prostate: a prospective, randomized trial with a 6-month follow-up. BJU Int 1999;84:805–9.
Tuhkanen 2003153
Primary reference
152. Tuhkanen K, Heino A, Aaltomaa S, Ala-Opas M. Long-term results of contact laser versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia with small or moderately enlarged prostates. Scand J Urol Nephrol 2003;37:487–93.
Secondary references
153. Tuhkanen K, Heino A, Ala-Opas M. Contact laser prostatectomy compared to TURP in prostatic hyperplasia smaller than 40 ml. Six-month follow-up with complex urodynamic assessment. Scand J Urol Nephrol 1999;33:31–4.
154. Tuhkanen K, Heino A, Aaltoma S, Ala-Opas M. Sexual function of LUTS patients before and after neodymium laser prostatectomy and transurethral resection of prostate. A prospective, randomized trial. Urol Int 2004;73:137–42.
van Melick 2003193
Primary reference
155. van Melick HH, van Venrooij GE, Boon TA. Long-term follow-up after transurethral resection of the prostate, contact laser prostatectomy, and electrovaporization. Urology 2003;62:1029–34.
Secondary references
156. van Melick HH, van Venrooij GE, Eckhardt MD, Boon TA. A randomized controlled trial comparing transurethral resection of the prostate, contact laser prostatectomy and electrovaporization in men with benign prostatic hyperplasia: urodynamic effects. J Urol 2002;168:1058–62.
157. van Melick HH, van Venrooij GE, Eckhardt MD, Boon TA. A randomized controlled trial comparing transurethral resection of the prostate, contact laser prostatectomy and electrovaporization in men with benign prostatic hyperplasia: analysis of subjective changes, morbidity and mortality. J Urol 2003;169:1411–16.
Wagrell 2002165
Primary reference
158. Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, Schain M, et al. Feedback microwave thermotherapy versus TURP for clinical BPH – a randomized controlled multicenter study. Urology 2002;60:292–9.
Secondary references
159. Duelund J, Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, et al. Prostalund feedback treatment versuis TURP: a prospective randomized multicenter study with 36 month follow-up. Scand J Urol Nephrol 2003;37(Suppl 214):34–5.
160. Kobelt G, Spangberg A, Mattiasson A. The cost of feedback microwave thermotherapy compared with transurethral resection of the prostate for treating benign prostatic hyperplasia. BJU Int 2004;93:543–8.
161. Nordling J, Wagrell L, Larson T, Duelund J, Kroyer K, Mattiasson A. ProstaLund feedback treatment PLFT) vs TURP: a prospective randomized muliticenter study with 36 month follow up. BJU Int 2005;95(Suppl 5):75.
162. Wagrell L, Schelin S, Nordling J, Larsson T, Mattiasson A. Prostalund microwave feedback treatment compared with TURP for treatment of BPH: a prospective randomized multicentre study. Aust NZ J Surg 2003;73:A146.
163. Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, Schain M, et al. ProstaLund microwave feedback treatment compared with TURP for treatment of BHP: a prospective randomized multicenter study with 24 months folllow up. J Urol 2003;169(Suppl 4):1748.
Wang 2002201
Primary reference
164. Wang ZL, Wang XF, Li B, Ji JT, Hou SC, Shao SX, et al. Comparative study of transurethral electrovaporization of prostate versus transurethral resection of prostate on benign prostatic hyperplasia. Zhong Hua Nan Ke Xue 2002;8:428–30.
Westenberg 2004189
Primary reference
165. Westenberg A, Gilling P, Kennett K, Frampton C, Fraundorfer M. Holmium laser resection of the prostate versus transurethral resection of the prostate: results of a randomized trial with 4-year minimum long-term followup. J Urol 2004;172:616–19.
Secondary references
166. Fraundorfer MR, Gilling PJ, Kennett KM, Dunton NG. Holmium laser resection of the prostate is more cost-effective than transurethral resection of the prostate: results of a randomized prospective study. Urology 2001;57:454–8.
167. Gilling PJ, Mackey M, Cresswell M, Kennett K, Kabalin JN, Fraundorfer MR. Holmium laser versus transurethral resection of the prostate: a randomized prospective trial with 1-year follow-up. J Urol 1999;162:1640–4.
168. Gilling PJ, Kennett KM, Fraundorfer MR. Holmium laser resection v transurethral resection of the prostate: results of a randomized trial with 2 years of follow-up. J Endourol 2000;14:757–60.
169. Gilling P, Westenberg A, Kennett KM, Fraundorfer MR. Holmium laser resection of the prostate (HOLRP) vs transurethral resection of the prostate (TURP): results at 4 years. Aust NZ J Surg 2003;73:A145.
170. Gilling PJ, Fraundorfer MR, Westenberg AM, Neill MG, Frampton CM, Kennett KM. Relief of bladder outflow obstruction (BOO) following HoLEP and TURP: a pooled analysis of data from 4 randomized trials. BJU Int 2005;95(Suppl5):74.
Wilson 2006134
Primary reference
171. Wilson LC, Gilling P, Williams A, Kennett KM, Frampton CM, Westenberg AM, et al. A randomised trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40 grams: results at 2 years. Eur Urol 2006;50:569–73.
Secondary reference
172. Tan AH, Gilling PJ, Kennett KM, Frampton C, Westenberg AM, Fraundorfer MR. A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams). J Urol 2003;170:1270–4.
Zerbib 1994174
Primary reference
173. Zerbib M, Steg A, Conquy S, Debre B. Hyperhermia: a randomized prospective study applying hyperthermia or a sham procedure in obstructive benign hyperplasia of the prostate. Prog Clin Biol Res 1994;386:439–48.
Zorn 1999148
Primary reference
174. Zorn BH, Bauer JJ, Ruiz HE, Thrasher JB. Randomized trial of safety and efficacy of transurethral resection of the prostate using contact laser versus electrocautery. Tech Urol 1999;5:198–201.
Appendix 6 Detailed quality assessment for each of the included studies
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7a | Q7b | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbou 1995 | Y | U | Y | Y | Y | U | Y | Y | U | U | Y | N | U | Y | U |
| Ahmed 1997 | U | N | N | Y | Y | N | Y | N | U | U | U | Y | N | Y | U |
| Albala 2002 | U | U | Y | Y | Y | Y | Y | Y | Y | U | Y | N | U | U | U |
| Bdesha 1994 | U | N | Y | Y | Y | Y | Y | N | Y | U | Y | Y | U | Y | U |
| Blute 1996 | Y | N | Y | Y | U | Y | Y | U | Y | U | Y | Y | U | U | U |
| Brehmer 1999 | U | U | N | Y | Y | Y | Y | Y | U | U | Y | N | U | U | U |
| Bouchier-Hayes 2006 | U | U | U | Y | Y | Y | U | U | U | U | U | Y | U | U | N |
| Carter 1999 | Y | N | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| Çetinkaya 1996 | U | U | Y | Y | Y | Y | Y | N | U | U | U | Y | U | U | U |
| Cimentepe 2003 | U | U | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| Chacko 2001 | Y | Y | Y | Y | Y | N | Y | N | N | N | N | Y | N | Y | U |
| Christensen 1990 | U | U | U | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Costello 1995 | N | U | Y | Y | Y | N | Y | N | U | U | U | Y | U | U | U |
| Cowles 1995 | Y | Y | N | Y | Y | Y | Y | Y | U | U | U | Y | U | Y | U |
| d’Ancona 1998 | U | U | N | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| Dahlstrand 1993 | U | U | N | Y | Y | U | Y | Y | U | U | Y | Y | U | U | U |
| Dahlstrand 1995 | U | U | Y | Y | Y | N | Y | Y | U | U | Y | Y | U | U | U |
| de la Rosette 2003 | U | U | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| de Sio 2006 | Y | U | Y | Y | Y | Y | Y | Y | U | U | U | U | U | U | U |
| de Wildt 1996 | U | U | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | U | U | U |
| Donovan 2000 | Y | Y | U | Y | Y | U | Y | N | U | U | N | Y | U | Y | U |
| Dørflinger 1992 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | N | U | U | U |
| Dunsmuir 2003 | U | Y | U | Y | Y | Y | Y | Y | N | Y | N | Y | U | N | U |
| Ekengren 2000 | U | U | N | N | Y | Y | N | Y | U | U | U | Y | U | U | Y |
| Erdaği 1999 | U | N | N | Y | Y | Y | Y | N | U | U | U | Y | U | U | U |
| Fowler 2005 | Y | N | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Fung 2005 | Y | U | Y | Y | Y | Y | Y | N | Y | N | Y | Y | N | Y | U |
| Galluci 1998 | U | U | U | Y | Y | U | Y | Y | U | U | U | Y | N | Y | U |
| Gotoh 1999 | U | U | Y | Y | Y | Y | Y | N | U | U | U | Y | N | U | Y |
| Gujral 2000 | Y | Y | U | Y | Y | U | Y | N | U | U | N | Y | U | Y | U |
| Gupta 2006 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | N |
| Hammadeh 2003 | U | N | N | Y | Y | N | Y | Y | Y | N | N | Y | U | U | N |
| Helke 2001 | U | U | N | Y | Y | Y | Y | Y | U | U | U | Y | U | U | Y |
| Hellström 1986 | U | U | Y | Y | Y | Y | Y | N | U | U | U | Y | N | U | U |
| Hill 2004 | Y | U | Y | Y | Y | U | Y | Y | U | U | U | Y | U | U | U |
| Hindley 2001 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Hon 2006 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | U | Y | U |
| Jahnson 1998 | U | U | N | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Kabalin 1995 | U | U | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| Kaplan 1998 | U | U | Y | Y | Y | Y | Y | Y | Y | U | N | Y | U | N | U |
| Keoghane 2000 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | N |
| Kim 2006a | U | U | Y | U | U | U | Y | Y | U | U | U | Y | U | N | U |
| Kim 2006b | U | U | Y | U | U | U | Y | N | U | U | U | Y | N | Y | U |
| Kuntz 2004 | Y | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Küpeli 1998a | U | U | Y | Y | Y | Y | Y | N | U | U | U | Y | N | U | U |
| Küpeli 1998b | Y | U | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| Küpeli 2001 | U | U | Y | Y | Y | Y | Y | N | U | U | U | Y | U | U | U |
| Kursh 2003 | Y | N | Y | Y | Y | N | Y | Y | U | U | U | Y | U | Y | U |
| Larson 1998 | U | U | N | Y | Y | Y | Y | N | Y | U | Y | Y | N | U | U |
| Li 1987 | U | Y | Y | U | Y | Y | Y | N | U | U | U | Y | U | U | Y |
| Liedberg 2003 | U | N | Y | Y | Y | Y | Y | Y | N | U | U | Y | U | U | U |
| Liu 2006 | U | N | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | Y |
| Mårtenson | U | U | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| McAllister 2000 | Y | U | Y | Y | Y | U | Y | Y | U | U | U | Y | U | Y | U |
| Montorsi 2004 | U | U | N | Y | Y | Y | Y | Y | N | N | N | Y | U | U | N |
| Mottet 1999 | Y | U | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | Y |
| Nathan 1996 | U | U | Y | Y | Y | Y | Y | N | U | U | U | Y | N | Y | U |
| Nawrocki 1997 | U | U | Y | Y | Y | Y | Y | N | U | U | Y | Y | N | U | U |
| Netto 1999 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Nielsen 1998 | Y | U | Y | Y | Y | Y | N | Y | U | U | U | N | U | U | U |
| Nuhoğlu 2005 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Nuhoğlu 2006 | U | U | Y | Y | Y | Y | N | Y | U | U | U | Y | U | U | U |
| Ogden 1993 | U | N | Y | Y | Y | Y | Y | N | N | N | Y | Y | U | U | U |
| Patel 1997 | U | U | U | Y | Y | Y | Y | N | U | U | U | Y | U | U | U |
| Riehmann 1994 | U | U | N | Y | Y | Y | Y | Y | U | U | U | U | Y | U | U |
| Rodrigo Aliaga 1998 | U | U | N | Y | N | U | Y | N | U | U | U | Y | U | U | U |
| Saporta 1996 | U | U | Y | Y | Y | N | U | Y | U | U | U | Y | U | U | U |
| Seckiner 2006 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | N | U | U |
| Sengor 1996 | U | U | Y | Y | Y | Y | Y | U | U | U | U | Y | U | U | U |
| Shingleton 2002 | Y | U | Y | Y | Y | U | Y | Y | U | U | U | Y | U | U | U |
| Shokeir 1997 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Singh 2005 | U | U | Y | Y | Y | U | Y | N | U | U | U | Y | U | U | Y |
| Soonwalla1992 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Suvakovic 1996 | U | U | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| Talic 2000 | U | U | Y | Y | Y | Y | Y | N | U | U | U | U | U | U | U |
| Tefekli 2005 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | N | Y |
| Trachtenberg 1998 | U | Y | Y | Y | Y | Y | Y | N | Y | U | Y | N | U | U | U |
| Tkocz 2002 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Tuhkanen 2001 | U | U | Y | Y | Y | N | Y | Y | U | U | U | N | U | U | Y |
| Tuhkanen 2003 | U | U | Y | Y | U | N | Y | Y | U | U | U | U | U | U | Y |
| van Melick 2003 | U | Y | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | N |
| Wagrel 2002 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | Y | U |
| Wang 2002 | U | U | Y | Y | Y | Y | Y | Y | U | U | U | Y | U | U | U |
| Westenberg 2004 | U | U | Y | Y | Y | N | Y | Y | U | U | U | Y | U | U | U |
| Wilson 2006 | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | Y | U | U | U |
| Zerbib 1994 | U | U | N | Y | Y | Y | Y | N | Y | N | Y | Y | U | U | U |
| Zorn 1999 | U | U | Y | Y | Y | U | Y | Y | U | U | U | Y | U | U | Y |
Appendix 7 Characteristics of included studies
Abbreviations used throughout this appendix are as follows: APSA, American Physicians Scientist Association; AUA, American Urological Association; AUA SI, American Urological Association symptom index; ASA, American Society of Anaesthesiologists; BOO, bladder outlet obstruction; BPH, benign prostate hyperplasia; BSFQ, brief sexual function questionnaire; B-TURP, bipolar transurethral resection of the prostate; B-TUVP, bipolar transurethral vaporisation of the prostate; B-TUVRP, bipolar transurethral vaporection of the prostate; CLVP, contact laser vaporisation; DRE, digital rectal exmination; ICS, International Continence Society; IPSS, International Prostate Symposium Score; ISC, intermittent self catheterisation; IQR, interquartile range; LUT, lower urinary tract; MI, myocardial infaction; MUF, mean urine flow; NSAID, non-steroidal anti-inflammatory drugs; PCAR, presumed circle area ratio; Pdetmax; maximal detrusor pressure; PSA, prostate-specific antigen; PVR, post-voiding residual urine volume; Qmax, peak flow rate; QoL, quality of life; RCT, randomised controlled trial, TEAP, transurethral ethanol ablation of the prostate; TRUS, transrectal ultrasound; TUIP, transurethral incision of the prostate;TUMT, transurethral microwave thermotherapy; TUNA, transurethral needle ablation; TUR, transurethral resection; TURP, transurethral resection of the prostate; TUVP, transurethral electrovaporisation of the prostate; TUVRP, transurethral vaporesection of the prostate; URA, urethral resistance factor; US, ultrasound; UT, urinary tract; urinary tract infection; VLAP, visual laser ablation of prostate; WHO, World Health Organization.
| TUMT vs TURP | |||||
|---|---|---|---|---|---|
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Ahmed 1997124 Study design: RCT Location: UK Length follow-up: 6 months |
Inclusion criteria: residual urine volume ≤ 300 ml; AUA score ≥ 12; urine flow rate < 15 ml/s; prostate volume 25–100 ml by TRUS; symptomatic uncomplicated BPH > 1 year; Pdetmax > 70 cmH2O; informed consent; obstructed on Abrams–Griffiths nomogram; suitable for either treatment Exclusion criteria: < 55 years; prostate cancer (by TRUS scan); previous prostatic surgery; acute or chronic retention; mental incapacity; severe cardiovascular disease; rectal surgery or disease (except haemorrhoids); pelvic mass surgery; cardiac pacemaker; metallic implants; uncontrolled coagulation disorder; meatal stricture; upper U-tract dilatation; obstructive uropathy; serum creatinine > 150 mmol/l; bladder calculi; bladder diverticula; recurrent prostatic haematuria; ‘active’ drugs; previous medication for BPH; prostatic abscess; active UTI; recurrent UTI; prominent middle lobe; < 25 mm between bladder neck and verumontanum Number of eligible patients: 60 Number of patients randomised: 60 |
TUMT (n = 30) vs TURP (n = 30) |
Mean age (range) (years): 69.36 (56–88) Mean prostate size (95% CI) (mm): 36.6 (31.8–41.4) (TRUS) Mean AUA score (95% CI): 18.5 (17.1–20.1) Mean Qmax (95% CI) (ml/s): 10.1 (9.2–10.9) Mean residual volume (95% CI) (ml): 94.4 (70.0–112.8) Sexually active: 19/30 Mean Pdetmax (95% CI) (cmH2O): 98.5 (70.1–116.9) Intervention performed by M Ahmed Temperature: 43.5°C Power: 70 W Other: anaesthesia: topical anaesthesia with Instillagel® (CliniMed; 3/30 had parenteral pethidine); antibiotics: gentamicin, oral trimethoprim 200 mg twice a day for 5 days; Prostanec™ (TechnoMed, Lyon, France) treatment catheter |
Mean age (range) (years): 69.45 (58–82) Mean prostate size (95% CI) (mm): 46.1(38.1–54.1) (TRUS) Mean AUA score (95% CI): 18.4 (16.7-20.1) Mean Qmax (95% CI) (ml/s): 9.5 (8.9–10.1) Mean residual volume (95% CI) (ml): 109.1 (88.2–130) Sexually active: 18/30 Mean Pdetmax (95% CI) (cmH2O): 96.7 (85.5–103.9) Intervention performed by surgeon of senior registrar grade or above Other: standard technique |
Symptom score (AUA) Qmax Detrusor pressure Residual volume Prostate volume Blood transfusion UTI Catheter duration Septicaemia Stricture Retrograde ejaculation Erectile dysfunction Reoperation |
|
Dahlstrand 1993 Study design: RCT Location: Sweden Length follow-up: 12 months Links with: Dahlstrand 1994; Dahlstrand 1993 and 1993 (abstracts) |
Inclusion criteria: residual urine volume ≤ 350 ml; Madsen score ≥ 8; prostate length 35–50 mm from TRUS; Qmax < 15 m/s (twice); BPH; anaesthetic risk group 1–3; obstructive symptoms > 3 months. Exclusion criteria: < 45 years; suspicion of or known prostate cancer or bladder cancer; previous surgery for cancer of prostate or radiotherapy; rectal surgery; previous surgery or heat treatment for BPH; large median lobe; neurogenic bladder disorder; mental incapacity, dementia or inability to give informed consent; neurological disorders that may affect bladder function; peripheral arterial disease (intermittent claudication or Leriche’s syndrome); disorder of haemostasis or serum creatinine > 2 mg/dl; uncontrolled cardiac dysrhythmias or cardiac pacemaker; total hip replacement or other metallic implants; indwelling or condom catheter; post-void residual urine > 350 ml; urethral stricture; bladder stones; α-adrenergic blockers (within 4 weeks); antiandrogen medication within 1 year or other medication that might affect prostate or bladder; bacterial prostatitis or UTI at time of treatment; prostatic urethral length of > 50 mm or < 35 mm by TRUS; anaesthesia risk category 4 or 5 (ASA class 4 or 5). Number of eligible patients: 83 Number of patients randomised: 79 |
TURP (n = 40) vs TUMT (n = 39) Additional information: position of treatment catheter checked with TRUS |
Mean age (years): 68 Mean Madsen ± SD (range): 11.2 ± 3.1 (8–18) Mean Qmax ± SD (range) (ml/s): 8.0 ± 2.8 (3–14) Mean residual volume ± SD (range) (ml): 105 ± 88 (10–380) Mean prostate size (ml): 33 Intervention performed by single physician with Prostatron® Power: 60 W Temperature: urethral, 44.5°C; rectal, 42.5°C Catheter protocol: if no voiding, indwelling catheter for 3–5 days Other: no general anaesthesia but intraurethral topical lidocaine HCl jelly 2% and NSAIDs; postoperative: oral norfloxacin 400 mg twice a day for 5 days |
Mean age (years): 70 Mean Madsen ± SD (range): 13.3 ± 4.2 (8–22) Mean Qmax ± SD (range) (ml/s): 7.9 ± 3.2 (1–14) Mean residual volume ± SD (range) (ml): 116 ± 97 (15–346) Mean prostate size (ml): 37 Sexually active 16/40 Intervention performed by urologists at senior registrar level or above Other: resectoscope with Charrière of 24–48, down to surgical capsule and extended from bladder neck to verumontanum |
Symptom score (Madsen) Qmax Residual volume Prostate size Retrograde ejaculation UTI Retention Catheter duration Reoperation Incontinence Length of hospital stay |
|
Dahlstrand 1995 Study design: RCT Location: Sweden Length follow-up: 2 years Links with: Walden 1998 |
Inclusion criteria: residual urine volume ≤ 350 ml; Madsen score ≥ 8; prostate length 35–50 mm from TRUS Exclusion criteria: prostate cancer or bladder cancer; previous surgery for cancer of prostate; previous treatment for BPH; indwelling catheter; urethral stricture; large median lobe; neurogenic bladder disorder; metallic hip implant Number of eligible patients: 72 Number of patients randomised: 69 |
TUMT (n = 37) vs TURP (n = 32) Additional information: it is unclear whether this study is the same as Dahlstrand 1993. Several attempts were made to contact the authors |
Mean age ± SD (years): 67.9 ± 9 Mean Madsen ± SD: 12.1 ± 3 Mean prostate size ± SD (mm): 43.4 ± 4.4 (TRUS) Mean Qmax ± SD (ml/s): 8.6 ± 32.5 Mean residual volume ± SD (ml): 194 ± 78 Catheter protocol: yes Temperature: maximum 44.5°C; minimum 42.5°C Power: 60 W Other: nafloxacin 400 mg; local anaesthetic; Prostatron (TechnoMed) with Prostasoft version 2.0 |
Mean age ± SD (years): 70 ± 6 Mean Madsen ± SD: 13.6 ± 3.9 Mean prostate size ± SD (mm): 44.8 ± 5.9 (TRUS) Mean Qmax ± SD (ml/s): 8.6 ± 3.0 Mean residual volume ± SD (ml): 1104 ± 95 |
UTI Urinary retention Catheter duration Clot retention Symptom score (Madsen) Length of hospital stay Qmax Residual volume |
|
d’Ancona 1998 Study design: RCT Location: Netherlands Recruitment dates: January 1994–August 1995 Median length follow-up: 2.5 years Links with: d’Ancona 1997 |
Inclusion criteria: unequivocal BPH candidates for TURP; Qmax 15 ml/s; residual volume < 350 ml; Madsen score ≥ 8; prostate length 25–50 mm; prostate volume 30–100 ml; minimum voided volume 100 ml; ≥ 45 years old Exclusion criteria: prostate cancer; previous prostate surgery; urinary retention requiring catheterisation; medications prescribed for prostate/bladder treatment; neurogenic disorders affecting bladder function; diabetic neuropathy; possible microwave-sensitive implants (pacemaker, hip prosthesis); renal impairment or obstructed bladder neck due to enlarged median lobe of prostate Number of patients randomised: 52 |
TUMT (n = 31) vs TURP (n = 21) Additional information: performed by two experienced urologists |
Mean age ± SD (years): 69.6 ± 8.5 Mean Madsen score ± SD: 13.8 ± 4.2 Mean IPSS score ± SD: 16.7 ± 5.6 Mean Qmax ± SD (ml/s): 9.3 ± 3.4 Mean residual volume ± SD (ml): 91 ± 105 Mean prostate size ± SD (ml): 45 ± 15 Mean total voided volume ± SD (ml): 178 ± 84.1 Mean PdetQmax ± SD (cmH2O): 65.4 ± 24.9 Power: 70 W Catheter protocol: catheter with leg bag immediately after treatment Intervention performed by two experienced urologists Other: mean total energy (kJ) 151.8 (SD 45.5) with Prostatron (EDAP) v.2.5; no anaesthesia but sedative given intravenously if required; diclofenac suppository (100 mg) and midazolam (2 mg) given |
Mean age ± SD (years): 69.3 ± 5.9 Mean Madsen score ± SD: 13.3 ± 4.2 Mean IPSS score ± SD: 18.3 ± 6.3 Mean Qmax ± SD (ml/s): 9.3 ± 3.9 Mean residual volume ± SD (ml): 49.5 ± 69.9 Mean prostate size ± SD (ml): 43 ± 12 Mean total voided volume ± SD (ml): 193.5 ± 85.7 Mean PdetQmax ± SD (cmH2O): 77.7 ± 40.0 Intervention performed by two experienced urologists |
Symptom score (IPSS/Madsen) Improvement > 50% Qmax Total voided volume Residual volume Prostate size PdetQmax Blood transfusion Catheter duration Mortality Irritative urinary symptoms Reoperation UTI Length of hospital stay |
|
de la Rosette 2003 Study design: RCT Location: Netherlands Recruitment dates: January 1996–March 1997 Median length follow-up: 33 months Links with: Francisca 1999; Francisca 2000; Floratos 2001 |
Inclusion criteria: peak urine flow rate ≤ 15 ml/s; residual urine volume ≤ 350 ml; Madsen score ≥ 8; urethral length ≥ 25 mm. Exclusion criteria: age < 45 years; prostate size < 30 ml; prostate carcinoma; previous prostatic surgery; acute prostatitis; UTI; severe co-morbidity; symptoms < 3 months; neurological disorders affecting lower UT function; isolated prostate middle lobe protruding in bladder; urethral stricture Number of eligible patients: 155 Number of patients randomised: 144 |
TUMT (n = 78) vs TURP (n = 66) |
Mean age ± SD (years): 67 ± 8.3 Mean prostate size ± SD (ml): 51 ± 20 Mean IPSS ± SD: 20 ± 6.7 Mean QoL ± SD: 4 ± 0.9 Mean Qmax ± SD (ml/s): 9.2 ± 3.1 Mean residual volume ± SD (ml): 65 ± 84 Catheter protocol: yes |
Mean age ± SD (years): 66 ± 8.2 Mean prostate size ± SD (ml): 52 ± 19 Mean IPSS ± SD: 20 ± 6.3 Mean QoL ± SD: 4 ± 1.1 Mean Qmax ± SD (ml/s): 8.0 ± 2.9 Mean residual volume ± SD (ml): 91 ± 98 Catheter protocol: yes |
Catheter duration Mortality Symptom score (IPSS) Quality of life score Prostate volume Length of hospital stay Qmax Residual volume |
|
Wagrell 2002 Study design: RCT Location: Sweden, Denmark and USA Recruitment dates: November 1998–November 1999 Median length follow-up: 24 months Links with: Kobelt 2004; Nordling 2005 (abstract); Wagrell 2003 and 2003 (abstracts) |
Inclusion criteria: peak urine flow rate ≤ 13 ml/s; IPSS score ≥ 13; prostate volume 30–100 ml Number of eligible patients: 154 Number of patients randomised: 146 |
TUMT (n = 99) vs TURP (n = 46) |
Mean age ± SD (years): 67 ± 8 Mean IPSS ± SD: 21 ± 5.4 Mean Qmax ± SD (ml/s): 7.6 ± 2.7 Mean residual volume ± SD (ml): 106 ± 77 Mean prostate size ± SD (ml): 48.9 ± 15.8 Mean Pdetmax ± SD (cmH2O): 73.7 ± 29.7 Temperature: 55°C Catheter protocol: indwelling Foley catheter Intervention performed by clinicians with average age and experience Other: given as outpatient procedure requiring sedoanalgesic ± local anaesthetic; diazepam, ketorolac, or ketobemidone or combinations of these |
Mean age ± SD (years): 69 ± 8 Mean IPSS ± SD: 20.4 ± 5.9 Mean Qmax ± SD (ml/s): 7.9 ± 2.7 Mean residual volume ± SD (ml): 94 ± 82 Mean prostate size ± SD (ml) by TRUS: 52.7 ± 17.3 Mean Pdetmax ± SD (cmH2O): 79.4 ± 35.3 Catheter protocol: catheter for 3–4 days Intervention performed by clinicians with average age and experience |
Symptom score (IPSS) Quality of life Qmax Residual volume Detrusor pressure Prostate size UTI Retention Clot retention TUR syndrome Erectile dysfunction Incontinence Mortality Septicaemia Catheter duration |
| TUMT vs sham | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Abbou 1995 Study design: RCT Location: France Length follow-up: 12 months |
Inclusion criteria: peak urine flow rate < 15 ml/s with voided volume of 150 ml (twice); residual urine volume measured by suprapubic ultrasound on 2 days < 300 ml; symptomatic prostatism symptoms for ≥ 3 months; serum creatinine level 160 μmol/l; written informed consent; prostate weight 30–80 g by TRUS; intravenous pyelogram (IVP) with cysturethrogram; APSA < 10 ng/ml or PSA < 15 ng/ml for a prostate weight ≥ 60 g Exclusion criteria: < 50 years; urinary bacterial infection; confirmed or suspicion of cancer by DRE; previous prostate or bladder surgery; PSA 4–10 ng/ml entered after transrectal biopsy of prostate for cancer; mental incapacity; any chronic disease potentially hindering follow-up; diabetes; participating in any clinical protocol in last 3 months; any other urological disease; any medical treatment for voiding disorders within 15 days of inclusion; diuretics in previous 3 months; anticoagulant therapy; allergy to lidocaine or colorectal disease Number of patients randomised: 97 |
TUMT (n = 66) vs sham (n = 31) |
Mean age ± SD (years): 65 ± 8 Mean Madsen score ± SD: 10.9 ± 4.3 Mean Qmax ± SD (ml/s): 10.4 ± 2.7 Mean total voided volume ± SD (ml): 249 ± 82 Mean residual volume ± SD (ml): 66 ± 60 Mean prostate size ± SD (g): 45 ± 15 Temperature: 45°C Other: used three devices for TURP: Thermex II (Israel), Prostcare (France), BSD-50 (USA) |
Mean age ± SD (years): 66 ± 7 Mean Madsen score ± SD: 12.8 ± 4.5 Mean Qmax ± SD (ml/s): 9.9 ± 2.5 Mean total voided volume ± SD (ml): 242 ± 89 Mean residual volume ± SD (ml): 41 ± 42 Mean prostate size ± SD (g): 44 ± 11 Temperature: 37°C Other: used three devices: Prostathermer system (Israel), Prostcare (France), Primus (Belgium) |
Duration of operation Acute retention UTI Intraoperative complications Symptom score (Madsen) |
|
Albala 2002 Study design: RCT Location: USA Length follow-up: 12 months Links with: Albala 2000,2003,2005 (abstracts); Kabalin 2001 (abstract) |
Inclusion criteria: peak urine flow rate < 12 ml/s; age 50–80 years; residual volume < 125 ml; AUA SI score > 13; bother score > 11; prostate volume 30–100 ml without significant intravesical middle lobe Exclusion criteria: PSA 4–10 ng/ml entered after transrectal biopsy of prostate for cancer Number of patients randomised: 190 |
TUMT (n = 125) vs sham (n = 65) Additional information: performed in urology offices or clinics |
Mean age ± SD (years): 65.2 ± 7.3 Mean AUA SI score ± SD: 22.2 ± 5.0 Mean Qmax ± SD (ml/s): 8.9 ± 3.0 Mean residual volume ± SD (ml): 57.9 ± 53.9 Mean prostate size ± SD (ml): 50.5 ± 18.6 (TRUS) Mean QoL index: 11.4 Temperature: 50–55°C; power cut-off at rectal temperature > 42.5°C Power: 60–90 W Catheter protocol: Foley catheter left in place 2–4 days postopoperatively Other: Toradol® (Roche; 10 mg) and lorazepam (2 mg) given orally before treatment; lidocaine jelly for 15 minutes preoperatively |
Mean age ± SD (years): 64.6 ± 7.1 Mean AUA SI score ± SD: 22.7 ± 5.7 Mean Qmax ± SD (ml/s): 8.4 ± 2.0 Mean residual volume ± SD (ml): 52.6 ± 51.9 Mean prostate size ± SD (ml): 47.1 ± 17.9 (TRUS) Other: procedure same as TUMT but without microwave energy |
Symptom score (AUA SI) Qmax Urinary retention Recatheterisation |
|
Bdesha 1994 Study design: RCT Location: UK Length follow-up: 12 months Links with: Bdesha 1993 |
Inclusion criteria: prostatism of at least 6 months; peak urine flow rate < 15 ml/s; residual volume < 200 ml but > 50 ml; AUA score > 14 Exclusion criteria: acute retention; prostatic surgery; upper UT dilatation; impaired renal function; significant median lobe hypertrophy; UT pathology; bladder neck to verumontanum > 40 mm Number of eligible patients: 42 Number of patients randomised: 42 |
TUMT (n = 22) vs sham (n = 20) |
Mean age (years): 63.7 Mean AUA score (95% CI): 19.2 (16.3–22.1) Mean Qmax (95% CI) (ml/s): 12.3 (10.7–13.9) Mean residual volume (95% CI) (ml): 104 (85–125) (TRUS) Temperature: < 42.5°C rectal temperature cut-off Frequency: 915 MHz Power: 20 W Catheter protocol: 18Fr catheter |
Mean age (years): 62.6 Mean AUA score (95% CI): 18.8 (16.0–21.7) Mean Qmax (95% CI) (ml/s): 10.8 (9.2–12.4) Mean residual volume (95% CI) (ml): 80 (57–103) (TRUS) Temperature: < 42.5°C rectal temperature Other: procedure as TUMT but without active microwave |
Duration of operation Symptom score (AUA) Qmax Residual volume Retrograde ejaculation Erectile dysfunction Retention Length of hospital stay |
|
Blute 1996 Study design: RCT Location: US Length follow-up: 12 months |
Inclusion criteria: symptomatic BPH; peak urine flow rate < 10 ml/s; residual volume 100–200 ml; Madsen score > 8; prostate length 35–50 mm from TRUS Exclusion criteria: prostate cancer; transurethral or rectal surgery; urinary retention; any medications that affect prostate symptoms; antiandrogen therapy; upper UT pathology shown by ultrasound; metallic implants; symptoms suggesting neuropathological bladder; serum creatinine > 2 mg/dl; bladder stones; uncontrolled dysrhythmias or cardiac pacemaker; asymmetric median lobe enlargement; patients at high risk from prostatic disease Number of patients randomised: 115 |
TUMT (n = 78) vs sham (n = 37) Additional information: sham group offered TUMT at 3 months |
Mean age ± SD (years): 66.9 ± 7.8 Mean Madsen score ± SD: 13.9 ± 3.4 Mean AUA score ± SD: 19.9 ± 7.2 (n = 37) Mean Qmax ± SD (ml/s): 7.3 ± 1.6 Mean MUF ± SD (ml/s): 4.0 ± 1.1 Mean residual volume ± SD (ml): 140.4 ± 35.8 Mean prostate size ± SD (ml): 37.4 ± 14.2 (TRUS) Antibiotics: given pretherapy Other: procedure: rectal thermometry probe inserted and treatment catheter with Foley balloon located by transabdominal ultrasound and TRUS; anaesthesia: 71/78 (89%) had only local anaesthetic (lidocaine), 7/78 had midazolam–fentanyl intravenously; blood pressure, pulse and temperature monitored every 15 minutes during treatment; observation for 2 hours |
Mean age ± SD (years): 66.9 ± 7.1 Mean Madsen score ± SD: 14.9 ± 3.1 Mean AUA score ± SD: 20.8 ± 6.7 Mean Qmax ± SD (ml/s): 7.4 ± 1.7 (n = 36) Mean MUF ± SD (ml/s): 3.9 ± 1.1 (n = 36) Mean residual volume ± SD (ml): 145.2 ± 35.6 Mean prostate size ± SD (ml): 36.1 ± 13.4 (TRUS) Antibiotics: given pretherapy Other: procedure: no sedation; urethral coolant circulated; NSAIDs given pretherapy |
Duration of operation Symptom score (Madsen, AUA) Global assessment Qmax Residual volume Intraoperative complications Retention Retrograde ejaculation Erectile dysfunction |
|
Brehmer 1999 Study design: RCT Location: Sweden Length follow-up: 12 months |
Inclusion criteria: LUTS dominated by hesitancy, slow urination and an enlarged prostate; peak urine flow rate < 15 ml/s Exclusion criteria: suspected prostate cancer; surgery for prostate disease; prostate size > 350 ml; indwelling catheter; median prostatic lobe; neurological disease Number of patients randomised: 30 |
TUMT (n = 16) vs sham (n = 14) | Mean age (range) (years): 70.4 (53–83) all patients. | Mean age (range) (years): 70.4 (53–83) all patients. |
Reoperation Symptom score (ICS) Global assessment Qmax |
|
de Wildt 1996 Study design: RCT Location: the Netherlands and UK (two centres) Recruitment dates: June 1991–December 1992 Length follow-up: 12 months Links with: de la Rosette 1994; Francisca 1997 |
Inclusion criteria: peak urine flow rate < 15 ml/s during two voids of > 150 ml; > 45 years; residual volume < 300 ml; Madsen > 8; symptom duration > 3 months Exclusion criteria: prostate cancer; history of TURP or TUIP; isolated enlargement of middle lobe; length of prostate urethra < 35 mm; prostate size < 30 ml; drugs affecting bladder function; bacterial prostatitis; UTI; urethral stricture; neurogenic bladder dysfunction; diabetes mellitus; intravesical pathology (stones, neoplasm); metallic pelvic implants; disorders of blood flow or coagulation; mental incapacity or inability to give informed consent Number of patients randomised: 93 |
TUMT (n = 47) vs sham (n = 46) Additional information: if patient saw no improvement in 3 months after sham or TUMT, a second TUMT was performed on request |
Mean age ± SD (years): 66.3 ± 8.1 Mean Madsen score ± SD (95% CI): 13.7 ± 3.4 (12.7–14.7) Mean Qmax ± SD (ml/s): 9.2 ± 2.5 Mean residual volume ± SD (ml): 93.9 ± 75.4 Mean prostate size ± SD (ml): 48.6 ± 16.6 Mean voided fraction ± SD (%): 74.9 ± 16.6 Other: interventions described previously in Carter 1991 |
Mean age ± SD (years): 63.9 ± 6.0 Mean Madsen score ± SD (95% CI): 12.9 ± 3.1 (11.9–13.9) Mean Qmax ± SD (ml/s): 9.6 ± 2.7 Mean residual volume ± SD (ml): 84.7 ± 66.1 Mean prostate size ± SD (ml): 49 ± 20.0 Mean voided fraction ± SD (%): 77.3 ± 15.7 Other: procedure as TUMT but without microwave activation |
Symptom score (Madsen) Qmax Residual urine Voided fraction Mortality Urinary retention Catheterisation Reoperation |
|
Larson 1998 Study design: RCT Location: USA (five centres) Recruitment dates: September 1994–June 1996 Length follow-up: 12 months |
Inclusion criteria: peak urine flow rate < 12 ml/s during two voids of > 125 ml within 30 days of enrolment; 45–85 years; AUA score > 9; preprostatic urethral length 3–5 cm from cystoscopy or TRUS; life expectancy ≥ 1 year Exclusion criteria: prostate or bladder cancer; enlarged or prominent prostatic middle lobe on cystoscopy; prostate size > 100 ml by TRUS; alpha-antagonists within 4 weeks or antiandrogens within 3 months; acute UTI within 1 week of enrolment as determined by positive urine culture; acute urinary retention; previous prostate surgery or non-medical treatment for BPH other than balloon dilatation < 12 months; gross haematuria not due to BPH; concomitant medications that could affect outcome study measures; co-existing disease that could mimic obstructive bladder neck syndrome; co-existing illness or specific obstructive symptoms caused by neurogenic bladder; bladder stones; renal failure; cardiac failure; urethral stricture (inability to pass 22Fr urethroscope easily); severe bladder neck contracture; urinary sphincter abnormalities; prostatitis or hepatic failure; continuous or intermittent catheterisation within 2 weeks of procedure; penile implant or artificial urinary sphincter; previous pelvic or rectal surgery that would increase patient risk or render study procedures more difficult; metallic pelvic implants; cardiac pacemaker; desire for future offspring; likely non-compliance with study follow-up evaluation requirements Number of patients randomised: 169 |
TUMT (n = 125) vs sham (n = 44) Additional information: 27/44 sham patients (61%) elected to undergo microwave treatment within 12 months of sham procedure. |
Mean age (95%CI) (years): 65.9 (63.4–68.3) Mean AUA score (95% CI): 20.8 (19.8–21.9) (n = 124); 50/124 (42%) AUA 9–19, 69/124 (58%) AUA > 20 Mean Qmax (95% CI) (ml/s): 7.8 (7.4–8.2) (n = 106) Mean residual volume (95% CI) (ml): 99.1 (82.0–116.1) (n = 105) Mean prostate volume (95% CI) (ml): 38.1 (35.1–41.2) Mean QoL score (95% CI): 4.2 (4.0–4.4) Frequency: 902–928 MHz Temperature: target urethral temperature 40 ± 1°C Catheter protocol: catheter for 30–60 hours Antibiotics: preprocedural antibiotics at discretion of investigator Other: Targis™ (formerly T3) thermoablation system used; did not use heated pad on lower abdomen to reduce awareness of heating procedure |
Mean age (95%CI) (years): 66.0 (64.7–67.4) Mean AUA score (95% CI): 21.3 (19.3–23.3) (n = 42); 15/42 (43%) AUA 9–19, 20/42 (57%) AUA > 20 Mean Qmax (95% CI) (ml/s): 7.8 (7.0–8.6) (n = 39) Mean residual volume (95% CI) (ml): 103.6 (79.4–127.8) (n = 39) Mean prostate volume (95% CI) (ml): 44.7 (38.8–50.5) Mean QoL score (95% CI): 4.0 (3.6–4.3) Temperature: urethral 8–20°C Catheter protocol: catheter for 30–60 hours Antibiotics: preprocedural antibiotics at discretion of investigator Other: procedure same as TUMT but without microwave activity |
Duration of operation Symptom score (AUA) Qmax Prostate size Residual volume Urinary retention Incontinence Epididymitis Stricture Retrograde ejaculation Reoperation Rehospitalisation Quality of life |
|
Nawrocki 1997 Study design: RCT Location: UK Recruitment dates: June 1991–December 1992 Length follow-up: 6 months |
Inclusion criteria: peak urine flow rate < 15 ml/s; residual volume ≤ 350 ml; voided volume ≥ 150 ml; symptoms of LUT dysfunction due to BPH and meriting surgical treatment; Pdetmax ≥ 70 cm water Exclusion criteria: complications of BOO; previous prostate or pelvic surgery or radiotherapy; urinary retention; recurrent UTI; treatment or medication that might affect UT function; renal failure; bladder calculus; bladder diverticulum; suspicion of malignancy from DRE; abnormal PSA level or other clinical features; short prostate (< 30 mm on TRUS); prominent prostate middle lobe projecting asymmetrically into bladder; urethral stricture; presence of metal within lower trunk or upper legs; uncontrolled cardiac dysrhythmias or presence of cardiac pacemaker; neurological disorders that might affect lower body; inability to understand treatment procedure, investigations or to give informed consent Number of patients randomised: 78 |
TUMT (n = 38) vs sham (n = 40) Additional information: an additional control group had no treatment; all procedures as outpatients |
Median age (range) (years): 70 (56–80) Median AUA score (range): 19 (7–31) Mean Qmax ± SD (ml/s): 8.83 ± 2.32 Mean total voided volume ± SD (ml): 252.1 ± 64.79 Mean residual volume ± SD (ml): 85.7± 56.6 Mean prostate size ± SD (ml): 41.2 ± 14.6 Mean Pdetmax ± SD (cmH20): 99.7 ± 27.09 Other: used Prostasoft version 2.0 with local anaesthesia |
Median age (range) (years): 70 (56–80) Median AUA score (range): 17.5 (7–28) Mean Qmax ± SD (ml/s): 9.44 ± 2.78 Mean total voided volume ± SD (ml): 269.1 ± 72.29 Mean residual volume ± SD (ml): 96.5 ± 56.3 Mean prostate size ± SD (ml): 46.7 ± 16.8 Mean Pdetmax ± SD (cmH20): 103.9 ± 33.24 Other: as for TUMT but without microwave activity; used heat pad (as can be purchased for rheumatic complaints) on lower abdomen |
Duration of operation Symptom score (AUA) Qmax Total voided volume Residual volume Detrusor pressure Acute retention Prostate size |
|
Ogden 1993 Study design: RCT Location: UK Recruitment dates: from September 1991 Length follow-up: 3 months |
Inclusion criteria: peak urine flow rate < 15 ml/s on two occasions; residual volume ≤ 350 ml; Madsen score > 8 for 6 months, prostate urethral length 35–50 mm Exclusion criteria: prostate cancer from DRE; heat to prostate or pelvic surgery/radiotherapy; urinary retention requiring catheterisation; alpha-blockers within 4 weeks; antiandrogens within 1 year; anything affecting prostate or bladder; prostatitis or UTI; renal dysfunction; peripheral arterial disease with intermittent claudication or Leriche’s syndrome; diabetic neuropathy; UT disease; bladder disease; mental incapacity, dementia, inability to give informed consent; neurological disorders affecting bladder function; disorders of blood flow or coagulation; history of uncontrolled cardiac arrhythmias or cardiac pacemaker; metallic pelvic implant; prominent isolated median lobe (from cystoscopy); intravesical pathology (stones, neoplasm or diverticula); renal impairment due to chronic retention; urethral stricture inhibiting catheterisation Number of eligible patients: 43 Number of patients randomised: 43 |
TUMT (n = 22) vs sham (n = 21) Additional information: if patient saw no improvement in 3 months after sham or TUMT, a second TUMT was performed on request |
Mean age (95% CI): 68.3 (64.1–72.5) Mean Madsen score (95% CI): 14.5 (12.9–16.1) Mean Qmax score (95% CI) (ml/s): 8.5 (7.5–9.5) Mean total voided volume (95% CI) (ml): 267 (235–297) Mean residual volume (95% CI) (ml): 147 (116–177) Mean prostate size (95% CI) (ml): 38.1 (32.4–43.8) (TRUS) Mean QoL score (95% CI): 13.4 (10.7–16.1) Catheter protocol: catheter inserted for retention for 1 week |
Mean age (95% CI): 67.1 (63.7–70.3) Mean Madsen score (95% CI): 14.2 (12.7–15.7) Mean Qmax score (95% CI) (ml/s): 8.6 (7.6–9.6) Mean total voided volume (95% CI) (ml): 285 (235–334) Mean residual volume (95% CI) (ml): 118 (84.8–151) Mean prostate size (95% CI) (ml): 35.4 (27.4–43.4) (TRUS) Mean QoL score (95% CI): 13.3 (9.2–17.4) Catheter protocol: catheter inserted for retention for 1 week |
Symptom score (Madsen) Quality of life Qmax Total voided volume Residual volume UTI Urinary retention |
|
Trachtenberg 1998 Study design: RCT Location: USA and Canada Length follow-up: 6 months Links with: Roehrborn 1998;Tan 2005;Roehrborn 1997 (abstract) |
Inclusion criteria: peak urine flow rate < 1 ml/s; > 55 years; AUA > 13; voided volume > 125 ml; PSA level < 10 ng/ml; prostate size 25–100 ml Exclusion criteria: prostate cancer; bladder neck to verumontanum measurement > 30 mm Number of patients randomised: 220 |
TUMT (n = 147) vs sham (n = 73) Additional information: at 6 months postoperatively patients unblended and sham-treated patients given choice to receive active treatments; sham was identical to TUMT but without power |
Mean age (range) (years): 66.2 (54.4–82.7) Mean AUA score (range): 23.6 (12–35) Mean AUA SI bother score (range): 18.5 (0–28) Mean Qmax (range) (ml/s): 7.7 (4.0–11.5) Mean urine flow (ml/s): 4.3 Mean total voided volume (ml): 254 Mean residual volume (range) (ml): 79.7 (0.0–248) Mean prostate size (range) (ml): 48.1 (25.2–96.5) Mean QoL score (0–21) (range): 14.3 (4.0–21.0) Frequency: 915 MHz Temperature: max of 50°C in urethra and 42.5°C in rectum Power: 90 W Intervention performed by physician and assistant Other: Dornier Uroware used; antibiotics at investigators choice; interstitial intraprostatic temperature monitoring |
Mean age (range) (years): 66 (55.1–78.1) Mean AUA score (range): 23.9 (13–35) Mean AUA SI bother score (range): 18.6 (0–28) Mean Qmax (range) (ml/s): 8.1 (4.0–11.9) Mean urine flow (ml/s): 4.5 Mean total voided volume (ml): 251 Mean residual volume (range) (ml): 67.5 (0.0–241) Mean prostate size (range) (ml): 50.5 (24.9–99.6) Mean QoL score (0–21) (range): 14.4 (2.0–21.0) Intervention performed by physician and assistant Other: sham procedure was identical to TUMT but without power; antibiotics at investigators choice; no interstitial intraprostatic temperature monitoring in sham group |
Duration of operation Intraoperative complications Symptom score (AUA) Qmax Mean urine flow rate Irritative urinary symptoms Stricture Retrograde ejaculation Erectile dysfunction UTI Retention |
|
Zerbib 1994 Study design: RCT Location: France Length follow-up: 3 months |
Inclusion criteria: candidates for prostatectomy. All had failed one conservative treatment, e.g. alpha-blockers Exclusion criteria: anterior rectal wall thickness > 10 mm or < 2 mm; anterior to posterior thickness of prostate > 55 mm Number of patients randomised: 68 |
TUMT (n = 38) vs sham (n = 30) Additional information: mean age for all patients 69.5 ± 10.44 (53–88) |
Mean Qmax ± SD (ml/s): 7.6 ± 3.8 Mean total voided volume ± SD (ml): 151 ± 92.0 Mean residual volume ± SD (ml): 110 ± 88.8 Mean prostate size for all patients ± SD (ml): 41.5 ± 15.6 (15–90) Temperature: intraprostatic temperature maintained at 43 ± 0.5°C Other: 1-hour session per week for 5 consecutive weeks |
Mean Qmax ± SD (ml/s): 10.6 ± 5.8 Mean total voided volume ± SD (ml): 145 ± 86.3 Mean residual volume ± SD (ml): 84.2 ± 76.6 Mean prostate size for all patients ± SD (ml): 41.5 ± 15.6 (15–90) Temperature: intraprostatic temperature maintained at 37 ± 0.5°C by radiofrequency power Other: 1-hour session per week for 5 consecutive weeks |
Qmax Residual volume |
| TUNA vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Cimentepe 2003 Study design: RCT Location: Turkey Recruitment dates: May 1999–May 2000 Length follow-up: 18 months |
Inclusion criteria: peak urine flow rate < 15 ml/s; IPSS score > 13; prostate weight 20–70 g; LUT symptoms due to BPH; > 40 years Exclusion criteria: previous prostate surgery; suspicion of cancer due to DRE and PSA levels; urethral stricture; bladder neck contracture; bladder stones or tumours; neurogenic bladder; prominent median lobe Number of patients randomised: 59 |
TUNA (n = 26) vs TURP (n = 33) Additional information: all patients sexually active. TUNA: temperature ≤ 50°C; maximum 100°C. Anaesthetic: spinal/epidural for both procedures |
Mean age ± SD years 60.1 ± 7.3 Mean IPSS score ± SD: 22.9 ± 3.8 Mean Qmax ± SD (ml/s): 9.8 ± 3.6 Mean residual volume ± SD (ml): 67.4 ± 294 Mean QoL score ± SD: 4.8 ± 0.75 Mean prostate size ± SD (g): 46.1 ± 11.2 Frequency: 465 kHz Temperature: ≤ 50°C, maximum 100°C Same urologists for both Antibiotic and analgesic therapy given to both groups postoperatively Catheter duration: 12–24 hours Other: anaesthetic: spinal or epidural |
Mean age ± years 63.3 ± 5.9 Mean IPSS symptom score ± SD: 24.1 ± 3.8 Mean Qmax ± SD (ml/s): 9.2 ± 3.4 Mean residual volume ± SD (ml): 76.1 ± 50.1 Mean QoL score ± SD: 5.2 ± 0.65 Mean prostate size ± SD (g): 49.1 ± 17.1 Catheter duration: 48–72 hours Other: anaesthetic: spinal or epidural |
Symptom score (IPSS) Qmax Quality of life Residual volume Prostate size Blood transfusion Incontinence Irritative symptoms Reoperation Stricture Retrograde ejaculation Erectile dysfunction Length of hospital stay |
|
Hill 2004 Study design: RCT Location: USA Recruitment dates: November 1994–July 1995 Length follow-up: 12 months Links with: Bruskewitz 1998; Roehrborn 1999; Naslund 1997,1999 (abstracts) |
Inclusion criteria: peak urine flow rate < 12 ml/s; AUA > 12; residual urine ≤ 350 ml; voided volume ≥ 125 ml; BPH with LUT symptom for > 3 months; prostate 20–100 g; > 50 years Exclusion criteria: prostate cancer (by biopsy); therapy affecting prostate physiology; urinary retention; UTI; compromised renal function; abnormal DRE; PSA > 10 ng/ml; significant prostate median lobe; medical conditions posing risk for these procedures Number of eligible patients: 121 Number of patients randomised: 121 |
TUNA (n = 65) vs TURP (n = 56) |
Mean age ± SD or SE (years): 66 ± 1 Mean AUA score ± SD or SE: 23.9 ± 0.8 Mean Qmax ± SD or SE (ml/s): 8.8 ± 0.3 Mean residual volume ± SD or SE (ml): 91.8 ± 10.0 Mean QoL score ± SD or SE: 12.8 ± 0.5 Mean prostate size ± SD or SE (ml): 36.2 ± 1.5 (TRUS) Frequency: 460 kHz Power: 2–15 W for 5 minutes/lesion; coagulation mode: 40–70 W Temperature: minimum of 50°C after 4 minutes; 100°C at needle tip. Temperature maintained for a minimum of 1 minute Catheter protocol: not usually left in; 40% had catheter 24–48 hours Antibiotics: broad-spectrum before treatment; postoperative antibiotics and oral anti-inflammatories Other: anaesthesia: 2% lidocaine jelly intraurethrally for 10 minutes preoperatively or oral or intravenous sedation; 7% required spinal or general anaesthesia |
Mean age ± SD or SE (years): 66 ± 1 Mean AUA score ± SD or SE: 24.1 ± 0.8 Mean Qmax ± SD or SE (ml/s): 8.8 ± 0.3 Mean residual volume ± SD or SE (ml): 82.6 ± 9.5 Mean QoL score ± SD or SE: 12.8 ± 0.5 Mean prostate size ± SD or SE (ml): 35.7 ± 1.9 (TRUS) Catheter protocol: indwelling catheter for 24–48 hours Intervention performed by one urologist at each centre (minimum of 100 operations) Other: general or spinal anaesthesia |
Blood transfusion Catheter duration Symptom score (AUA) Quality of life score Qmax Residual volume Prostate size Intraoperative complications UTI Stricture Incontinence Retrograde ejaculation Erectile dysfunction Length of hospital stay |
|
Hindley 2001 Study design: RCT Location: UK Length follow-up: 2 years Links with: Mostafid 1997 |
Inclusion criteria: age > 50 years; confirmed BOO due to BPH with fall in PdetQmax within obstructed area of Abrams–Griffiths nomogram; IPSS score > 13; residual urine volume ≤ 250 ml measured by US; IPSS QoL ≥ 3; written informed consent Exclusion criteria: previous illness/surgery that might confound results of the study or pose additional risk to patient; confirmed or suspected malignancy of prostate by DRE or biopsy; PSA > 4 ng/ml unless cancer excluded by biopsy; previous prostatic surgery or thermotherapy; pharmacological treatment for BPH in last 6 months; confirmed or suspected bladder cancer; previous rectal surgery other than haemorrhoidectomy; previous pelvic irradiation; history of cystolithiasis, haematuria or bladder pathology, urethral strictures, bladder neck contracture, active UTI or prostatitis; previous history of neurogenic disorder including Parkinson’s disease, multiple sclerosis, stroke and diabetic neuropathy; patients wishing to maintain potential fertility; PVR > 250 ml; (measured by US); compromised renal function with serum creatinine > 180 mg/l or radiological evidence of UT dilatation; inability to provide at least one voided volume of > 150 ml; inability to give informed consent Number of patients randomised: 50 |
TUNA (n = 25) vs TURP (n = 25) |
Median age (IQR) (years): 66 (56–82) Median IPSS score (IQR): 20 (15–23) Mean Qmax ± SD (ml): 8.5 ± 3.7 Mean residual volume ± SD (ml): 55 ± 44 Mean Pdetmax ± SD (cmH2O): 92 ± 12 Power: 10 W coagulation for 3 minutes Catheter protocol: after treatment, catheterised and allowed home on first postoperative day; catheter removed 7 days postoperatively Other: 7Fr RF needle electrode inserted into lateral prostate lobes with a catheterising endoscope; gentamicin (120 mg intravenously) |
Median age (IQR) (years): 71 (56–88) Median IPSS score (IQR): 22 (18–25) Mean Qmax ± SD (ml): 9.0 ± 3.6 Mean residual volume ± SD (ml): 74 ± 53 Mean Pdetmax ± SD (cmH2O): 99 ± 10 Intervention performed by experienced surgeon Catheter protocol: a 22Fr three-way urethral catheter inserted for bladder irrigation Other: standard procedure; gentamicin (120 mg intravenously); allowed home after successful voiding |
Symptom score (IPSS) Quality of life Residual volume Detrusor pressure Qmax Blood transfusion UTI Clot retention Mortality Incontinence Irritative urinary symptoms Reoperation |
|
Kim 2006a Study design: RCT Location: Korea Recruitment dates: January 1998–December 2002 Length follow-up: 12 months |
Inclusion criteria: patients with symptomatic BPE Number of eligible patients: 235 Number of patients randomised: 220 |
TUNA (n = 110) vs TURP (n = 110) |
Mean or median age (range) (years): 66.4 (48–80) Mean or median IPSS score: 20.8 Mean or median Qmax (ml/s): 7.0 Mean or median residual volume (ml): 257 Mean or median prostate size (ml): 40.6 Mean or median QoL score: 4.3 Other: VidaMed TUNA® system (VidaMed Inc.) |
Mean or median age (range) (years): 67.4 (60–87) Mean or median IPSS score: 24.0 Mean or median Qmax (ml/s): 11.9 Mean or median residual volume (ml): 187 Mean or median prostate size (ml): 44.2 Mean or median QoL score: 4.7 |
Duration of operation Blood transfusion UTI Recatheterisation Incontinence Stricture Retrograde ejaculation Erectile dysfunction Reoperation Symptom score (IPSS) Quality of life Length of hospital stay Qmax Residual volume Prostate size |
| Stents vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Chapple 1995 Study design: RCT (abstract) Location: UK Length follow-up: 3 months |
Inclusion criteria: patients with obstructive BPH; symptomatic patients with urodynamic evidence of bladder outflow obstruction Number of patients randomised: 60 |
Stents (n = 34) vs TURP (n = 26) |
Mean age (range) (years): 73 (65–90) Mean IPSS score: 19 (n = 27) Mean peak urine flow ± SD or SE (ml/s): 8.4 ± 0.5 (n = 20) |
Mean age (range) (years): 72.6 (63–86) Mean IPSS score: 21.6 (n = 20) Mean peak urine flow ± SD or SE (ml/s): 8.0 ± 0.6 (n = 14) |
Duration of operation Reoperation Symptom score (IPSS) Length of hospital stay Peak urine flow rate |
| TEAP vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Kim 2006a Study design: RCT Location: Korea Recruitment dates: January 1998–December 2002 Length follow-up: 12 months |
Inclusion criteria: patients with symptomatic BPE Number of eligible patients: 223 Number of patients randomised: 204 |
TEAP (n = 94) vs TURP (n = 110) |
Mean or median age (range) (years): 66.2 (49–88) Mean or median IPSS score: 19.5 Mean or median Qmax (ml/s): 7.2 Mean or median residual volume (ml): 126.1 Mean or median prostate size (ml): 36.4 Mean or median QoL score: 4.4 Other: Prostajec™ device (American Medical Systems, Minnetonka, MN, USA) |
Mean or median age (range) (years): 67.4 (60–87) Mean or median IPSS score: 24.0 Mean or median Qmax (ml/s): 11.9 Mean or median residual volume (ml): 187 Mean or median prostate size (ml): 44.2 Mean or median QoL score: 4.7 |
Duration of operation Blood transfusion UTI Recatheterisation Incontinence Stricture Retrograde ejaculation Erectile dysfunction Reoperation Symptom score (IPSS) Quality of life Length of hospital stay Qmax Residual volume Prostate size |
| Laser coagulation vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Chacko 2001 CLasP study Study design: RCT Location: UK Length follow-up: 7.5 months |
Inclusion criteria: acute painful urinary retention Exclusion criteria: prostate cancer or surgery; prostate size > 120 ml; life expectancy < 6 months; urinary retention associated with recent operation, constipation, neurogenic bladder dysfunction; drugs; serum creatinine > 250 μmol/l Number of eligible patients: 155 Number of patients randomised: 148 |
Laser coagulation (n = 74) vs TURP (n = 74) |
Mean age ± SD (years): 74.2 ± 7.9 Mean IPSS score ± SD: 20.3 ± 9.3 Ethnicity (% white): 97.3 Median IPSS QoL score (IQR): 5 (4–6) Power: 30 W KTP; 60 W Nd:YAG for 60 seconds; depends on prostate size Energy: 33.93 kJ Catheter protocol: suprapubic voiding trial 1–2 weeks after discharge Other: anti-inflammatory suppository given; procedure: Nd:YAG/non-contact VLAP, side-firing fibre |
Mean age ± SD (years): 72.7 ± 7.3 Mean IPSS score ± SD: 19.4 ± 7.6 Ethnicity (% white): 97.3 Median IPSS QoL score (IQR): 5 (4–6) Catheter protocol: suprapubic; duration depends on success of voiding after urine is clear Other: anti-inflammatory suppository given; procedure: standard electroresection |
Blood transfusion Catheter duration Quality of life Length of hospital stay Reoperation TUR syndrome Cardiovascular events Intraoperative complications Septicaemia Mortality Incontinence |
|
Costello 1995 Study design: RCT Location: Australia Length follow-up: 6 months |
Inclusion criteria: candidates for TURP from documented prostatism Exclusion criteria: < 50 years; anticoagulant therapy; prostate cancer; chronic urinary retention Number of patients randomised: 71 |
Laser coagulation (n = 34) vs TURP (n = 37) |
Mean age (range) (years): 67.9 (55–88) Mean Qmax (ml/s): 8.76 Mean prostate volume (range) (ml): 30.0 (13.9–77) (TRUS) Number sexually active: 16/34 (47%) Power: 60 W for 60 seconds; 21.7 kJ (9.7–33.9 kJ) Catheter protocol: two-way non-irrigating catheter Other: Nd:YAG; preoperative TRUS-guided six-sector biopsies; cephalosporin (500 mg) perioperatively |
Mean age (range) (years): 68.2 (50–84) Mean Qmax (ml/s): 9.48 Mean prostate volume (range) (ml): 38.9 (12–70) (TRUS) Number sexually active: 11/37 (30%) Catheter protocol: 22Fr three-way catheter Other: conventional with 24Fr resectoscope and glycine irrigation; cephalosporin (500 mg) perioperatively; saline irrigation for 12–24 hours |
Symptom score (AUA) Qmax MUF Residual volume Blood transfusion UTI Incontinence Stricture Retrograde ejaculation Reoperation Erectile dysfunction Length of hospital stay |
|
Cowles 1995 Study design: RCT Location: US Recruitment dates: August 1991–June 1992 Length follow-up: 12 months |
Inclusion criteria: candidates for surgical treatment for BOO from BPH Exclusion criteria: < 50 years; life expectancy < 6 months; prostate cancer; hormonal therapy; alpha-blockers; finasteride; physical status exceeding category III of American Society of Anesthesiologists; bladder neck to verumontanum < 2.4 cm; recent MI; coagulopathy; recent stroke; sepsis; clinically significant illnesses Number of patients randomised: 115 |
Laser coagulation (n = 56) vs TURP (n = 59) |
Mean age ± SD (range) (years): 65.8 ± 6.7 (51–84) Mean AUA-6 score ± SD (range): 18.7 ± 6.0 (6–29) Mean Qmax ± SD (range) (ml/s): 8.9 ± 3.6 (2–18.1) Mean total voided volume ± SD (range) (ml): 206.7 ± 181.9 (2–800) Mean prostate volume ± SD (range) (ml): 42.2 ± 19.0 (7.7–93.9) Energy: mean 10.2 kJ (5.76–11.5); dependent on prostate size Power: 40 W Other: previous BPH 9/56 (16/1%); Nd:YAG laser (Trimedyne, CA) directed with Urolase™ fibre, 5.5 ± 2.1 laser applications; general or regional anaesthesia |
Mean age ± SD (range) (years): 67 ± 7.8 (50–83) Mean AUA-6 score ± SD (range): 20.8 ± 4.8 (7–30) Mean Qmax ± SD (range) (ml/s): 9.5 ± 5.2 (3–37.4) Mean total voided volume ± SD (range) (ml): 206.7 ± 181.9 (2–800) Mean prostate volume ± SD (range) (ml): 38.6 ± 20.2 (11.2–108.2) Other: previous BPH 17/59 (28.8%); standard prostate resection using wire loop electrocautery instruments under direct vision; general or regional anaesthesia |
Symptom score (AUA-6) Quality of life Qmax Residual volume Length of hospital stay Blood transfusion UTI Urinary retention Clot retention Cardiovascular events Incontinence Stricture Erectile dysfunction Reoperation TUR syndrome |
|
Donovan 2000 CLasP study Study design: RCT Location: UK Length follow-up: 7.5 months Links with: Brooks 2002; Donovan 1998 (abstract) |
Inclusion criteria: LUT symptoms associated with BPH; urine flow rate < 15 ml/s but related to voided volume; IPSS ≥ 8. Exclusion criteria: prostate cancer; prostate size > 120 ml; life expectancy < 6 months; neurogenic bladder dysfunction; drugs; serum creatinine > 250 μmol/l Number of patients randomised: 340 |
Laser coagulation (n = 117) vs TURP (n = 117) |
Mean age ± SD (years): 67.4 ± 8.1 Mean IPSS score ± SD: 19.1 ± 6.6 Mean Qmax ± SD (ml/s): 10.4 ± 2.9 Mean residual volume ± SD (ml): 123.7 ± 91.8 Mean prostate size ± SD (ml): 40.7 ± 21.4 Median IPSS QoL score (range): 5 (2–6) Number of patients obstructed: 90/115 (78.3%) Energy: mean total energy delivered 28.7 kJ Power: 40 W Other: Nd:YAG/non-contact VLAP, side-firing fibre |
Mean age ± SD (years): 66.4 ± 7.9 Mean IPSS score ± SD: 19.2 ± 6.7 Mean Qmax ± SD (ml/s): 10.3 ± 2.7 Mean residual volume ± SD (ml): 104.2 ± 69.5 Mean prostate size ± SD (ml): 38.1 ± 19.1 Median IPSS QoL score (range): 4 (0–6) Number of patients obstructed: 91/116 (78.4%) Other: standard electroresection; anti-inflammatory suppository given |
Symptom score (IPSS) Quality of life Qmax Residual volume Blood transfusion UTI Catheter duration Mortality Septicaemia Length of hospital stay |
|
Gujral 2000 CLasP study Study design: RCT Location: UK Length follow-up: 7.5 months |
Inclusion criteria: LUT symptoms associated with BPH with chronic retention; urine flow rate < 15 ml/s but related to voided volume; IPSS ≥ 8; residual urine volume ≥ 3000 on US Exclusion criteria: prostate cancer; previous prostate surgery; prostate size > 120 ml; long-term drugs affecting LUT; serum creatinine > 250 μmol/l; abnormal upper UT on renal tract US Number of eligible patients: 72 Number of patients randomised: 72 |
Laser coagulation (n = 38) vs TURP (n = 44) Additional information: all in chronic retention |
Mean age ± SD (years): 70.2 ± 6.8 Ethnicity: all white males Mean IPSS score ± SD: 120.9 ± 6.4 Mean Qmax ± SD (ml/s): 11.2 ± 5.3 Mean residual volume ± SD (ml): 438 ± 151 Mean prostate size ± SD (ml): 40.7 ± 19.9 Median IPSS QoL score (range): 5.0 ± 2.6 Mean energy: 33.8 kJ Power: median lobe 30 seconds at 60 W to each side Other: Nd:YAG source via Urolase™ (Bard, GA) right-angle laser fibre using standard fixed-spot technique; laser duration depends on prostate length; NSAIDs and prophylactic antibiotics given |
Mean age ± SD (years): 70.6 ± 5.8 Mean IPSS score ± SD: 19.5 ± 7.2 Mean Qmax ± SD (ml/s): 8.5 ± 3.6 Mean residual volume ± SD (ml): 545 ± 275 Mean prostate size ± SD (ml): 49.7 ± 21.8 Median IPSS QoL score (range): 4.5 ± 2.6 Other: standard electroresection; NSAIDs and prophylactic antibiotics given |
Intraoperative complications Blood transfusion UTI Catheter duration Mortality Septicaemia Reoperation Length of hospital stay Symptom score (IPSS) Qmax Residual volume |
|
Kabalin 1995 Study design: RCT Location: USA Length follow-up: 18 months Links with: Kabalin 1993 |
Inclusion criteria: symptomatic BOO due to BPH or patients request for surgery; urine flow rate < 15 ml/s; age > 50 years Exclusion criteria: prostate cancer or suspicion of; severe medical problems; coagulant disorder requiring anticoagulants; ASA class 4 or 5 Number of patients randomised: 25 |
Laser coagulation (n = 13) vs TURP (n = 12) |
Mean age (years): 65 Mean AUA score ± SE: 20.9 ± 1.9 Mean Qmax ± SE (ml/s): 8.5 ± 1.1 Mean residual volume ± SE (ml): 236 ± 74 Mean prostate size (range) (g): 24 (15–45), p < 0.02 Mean Pdetmax ± SE (cmH2O): 91.3 ± 5.2 Mean energy: 11.5 kJ (7.2–19.2) Power: 40 W Catheter protocol: Foley catheter with no postoperative irrigation; 18Fr 5-ml balloon Foley urethral catheter Other: Nd:YAG continuous-firing laser fibre; direct vision under 21Fr Storz parendoscope; Urolase right-angle firing; urinary retention: one patient |
Mean age (years): 69 Mean AUA score ± SE: 18.8 ± 1.8 Mean Qmax ± SE (ml/s): 9.0 ± 1.1 Mean residual volume ± SE (ml): 291 ± 8.8 Mean prostate size (range) (g): 17 (10–30) Mean Pdetmax ± SE (cmH2O): 92.3 ± 3.4 Catheter protocol: three-way Foley catheter left for ≥ 24 hours postoperatively Other: standard TURP electroresection with 26Fr Storz or 28Fr continuous flow resectoscope; gentamicin 3 days perioperatively; postoperative bladder irrigation; urinary retention: one patient |
Duration of operation Symptom score (AUA) Global (patient) assessment Qmax Residual volume Detrusor pressure Prostate size Blood transfusion Catheter duration Mortality TUR syndrome Stricture Retrograde ejaculation Erectile dysfunction Reoperation |
|
Kim 2006a Study design: RCT Location: Korea Recruitment dates: January 1998–December 2002 Length follow-up: 12 months |
Inclusion criteria: patients with symptomatic BPE Number of eligible patients: 212 Number of patients randomised: 199 |
Laser coagulation (n = 89) vs TURP (n = 110) |
Mean or median age (range) (years): 68.7 (50–89) Mean or median IPSS score: 21.1 Mean or median Qmax (ml/s): 8.6 Mean or median residual volume (ml): 219 Mean or median prostate size (ml): 42.7 Mean or median QoL score: 4.7 Other: procedure: Indigo 830e™ laser optic system (Ethicon Endosurgery) |
Mean or median age (range) (years): 67.4 (60–87) Mean or median IPSS score: 24.0 Mean or median Qmax (ml/s): 11.9 Mean or median residual volume (ml): 187 Mean or median prostate size (ml): 44.2 Mean or median QoL score: 4.7 |
Duration of operation Blood transfusion UTI Recatheterisation Incontinence Stricture Retrograde ejaculation Erectile dysfunction Reoperation Symptom score (IPSS) Quality of life Length of hospital stay Qmax Residual volume Prostate size |
|
Kursh 2003 Study design: RCT Location: USA (multicentre) Recruitment dates: November 1997–February 1999 Length follow-up: 24 months |
Inclusion criteria: urine flow rate < 15 ml/s; prostatic length of 15 mm or more; residual urine 30–300 ml; AUA ≥ 13 Exclusion criteria: prostate cancer or suspected by DRE or PSA level > 4 ng/ml, excluded by biopsy; urinary retention; prostate < 75 ml; any condition/surgery/history of illness that (in the opinion of the investigator) might pose additional risk to the patient, e.g. unstable angina, significant renal impairment (creatinine > 1.8 mg/dl) or poorly controlled diabetes mellitus; acute urinary retention; cystolithiasis; neurogenic bladder; bladder neck contracture; anticholinergic medications, finasteride or phytotherapy within 1 month of enrolment; terazosin, doxazosin, tamsulosin within 14 days of enrolment Number of eligible patients: 73 Number of patients randomised: 72 |
Laser coagulation (n = 37) vs TURP (n = 35) |
Mean age (range) (years): 67.6 (50–81) Ethnicity: 30 white (81%); 5 Asian (14%) 2 African American (5%) Median AUA score: 24 Median Qmax (ml/s): 9.2 (n = 35) Median residual volume (ml): 81.0 Median prostate size (ml): 41.5 Median QoL score: 11.0 Median sexual function score: 18.0 Problems from Symptom Index score (0–28): 17 Power: 20 W Catheter protocol: catheter for 1 week. Other: procedure: interstitial laser coagulation, Indigo 830e (830 nm); general, topical or spinal anaesthesia; prophylactic antibiotics |
Mean age (range) (years): 69.3 (50–81) Ethnicity: 29 white (83%); 6 Asian (17%) Median AUA score: 23 Median Qmax (ml/s): 9.1 Median residual volume (ml): 87.5 Median prostate size (ml): 40.0 Median QoL score: 11.0 Median sexual function score: 17.0 Problems from Symptom Index score (0–28): 19 Catheter protocol: catheter 1 day postoperatively before discharge Other: procedure: standard radiofrequency monopolar loop procedure; general or spinal anaesthesia; prophylactic antibiotics |
Symptom score (AUA) Quality of life Sexual function Length of hospital stay Qmax Residual volume Prostate size Blood transfusion UTI Mortality Incontinence Reoperation Incontinence |
|
Liedberg 2003 Study design: RCT Location: Sweden Recruitment dates: December 1997–February 2000 Length follow-up: 12 months |
Inclusion criteria: urine flow rate < 15 ml/s but related to voided volume; IPSS ≥ 12 Exclusion criteria: prostate cancer; indwelling urinary catheter or suspicion of neurogenic bladder disturbance Number of eligible patients: 38 Number of patients randomised: 31 |
Laser coagulation (n = 20) vs TURP (n = 11) |
Median IPSS score (IQR): 17 (17–24) Median Qmax (IQR) (ml/s): 8 (7–10) Median residual volume (IQR) (ml): 96 (64–190) Median prostate volume (IQR) (ml): 49 (41–61) Power: 20 W Temperature: target temperature 85°C for 3 minutes at each site Catheter duration: catheter for 1 week; removed when PVR < 150 ml Other: procedure: interstitial laser coagulation, Indigo 830e (830 nm); general or spinal anaesthesia; norfloxacin (400 mg) while suprapubic tube in situ |
Median IPSS score (IQR): 17 (17–24) Median Qmax (IQR) (ml/s): 8 (6–9) Median residual volume (IQR) (ml): 117 (67–200) Median prostate volume (IQR) (ml): 47 (37–61) Other: procedure: complete circumferential resection to prostatic capsule |
IPSS score Qmax Residual volume Prostate volume Catheter duration Length of hospital stay Intraoperative complications UTI Stricture Retrograde ejaculation |
|
Mårtenson 1999 Study design: RCT Location: Netherlands Recruitment dates: October 1994–April 1996 Length follow-up: 12 months |
Inclusion criteria: Qmax < 15 ml/s; residual volume < 350 ml; age > 45; IPSS > 12 for > 3 months Exclusion criteria: prostate cancer; prostate size < 25 ml; urethral stricture; neurogenic bladder dysfunction; diabetes mellitus; UTI; bacterial prostatitis; use of drugs influencing bladder function Number of patients randomised: 44 |
Laser coagulation (n = 30) vs TURP (n = 14) |
Mean IPSS score ± SD: 21.7 ± 6.1 Mean Qmax ± SD (ml/s): 7.3 ± 3.8 Mean total voided volume ± SD (ml): 185 ± 84 Mean residual volume ± SD (ml): 116 ± 146 Mean prostrate size ± SD (ml): 46 ± 20 Mean QoL index ± SD: 4.1 ± 1.4 Normal erectile function (%): 92% Intervention performed by one of the authors using video imaging technique Power: 10 W gradually reduced to 5 W Temperature: 85°C Catheter protocol: yes; catheter removed when adequate voiding demonstrated at one of scheduled follow-up visits (1–2 or 4 weeks) |
Mean IPSS score ± SD: 21.6 ± 7.7 Mean Qmax ± SD (ml/s): 9.3 ± 3.2 Mean total voided volume ± SD (ml): 230 ± 107 Mean residual volume ± SD (ml): 88 ± 126 Mean prostrate size ± SD (ml): 50 ± 16 Mean QoL index ± SD: 4.0 ± 1.3 Normal erectile function: 89% Intervention performed by one of the authors using video imaging technique Catheter protocol: yes; catheter removed according to individual’s needs Other: perioperative prophylaxis with co-trimoxazole–Sulfatrim for 7 days (960 mg twice a day) |
IPSS score Quality of life index Qmax Total voided volume Residual volume Prostate size URA value Linn-PURR grade Blood transfusion UTI Catheter duration Clot retention Recatheterisation Irritative voiding complaints Transient haematuria Incontinence Reoperation rate |
| Other: procedure: diode laser system (Indigo 830, Indigo Medical, USA); perioperative prophylaxis with co-trimoxazole–Sulfatrim® for 7 days (960 mg twice a day) | |||||
|
McAllister 2000 Study design: RCT Location: UK Recruitment dates: March 1992 Length follow-up: 5 years Links with: Anson 1995 |
Inclusion criteria: suitable candidates for TURP; ASA class 1–3; urinary flow rates consistent with BOO; prostatic urethral length > 2.4 cm Exclusion criteria: prostate cancer by DRE; ASA class > 3; age ≤ 50; inability to provide informed consent; known history or suspicion of prostate carcinoma; renal impairment; life expectancy < 6 months; precluded from study by medications, e.g. anticoagulants Number of eligible patients: 151 Number of patients randomised: 151 |
Laser coagulation (n = 76) vs TURP (n = 75) |
Mean age (95% CI) (years): 67.9 (66.3–69.5) AUA score (95% CI): 18.1 (17.1–19.1) Mean Qmax (95% CI) (ml/s): 9.6 (8.8–10.4) Total voided volume (95% CI) (ml): 234.1 (211.5–256.7) Residual volume (95% CI) (ml): 113 (91.4–134.6) Number sexually active: 27/76 Power: 60 W Catheter protocol: left at the discretion of the individual consultant; some received suprapubic catheter, others received urethral catheter Preprocedural antibiotics: yes Other: Nd:YAG laser energy |
Mean age (95% CI) (years): 68.3 (66.5–70.1) AUA score (95% CI): 18.2 (17.1–19.3) Mean Qmax (95% CI) (ml/s): 10.0 (9.1–10.9) Total voided volume (95% CI) (ml): 234.3 (208.2–260.4) Residual volume (95% CI) (ml): 120.7 (93.0–148.4) Number sexually active: 24/75 Intervention performed by experienced consultant Catheter protocol: left at the discretion of the individual consultant; some received suprapubic catheter, others received urethral catheter Preprocedural antibiotics: yes |
AUA score Qmax Residual volume Secondary haemorrhage Blood transfusion UTI Catheter duration Clot retention Cardiovascular events Reoperation rate Retrograde ejaculation Length of hospital stay |
|
Rodrigo Aliaga 1998 Study design: RCT Location: Spain Length follow-up: 6 months |
Inclusion criteria: patients with BPH; prostate size 20–60 g; symptom score ≤ 12 ml/s; IPSS score ≥ 15 Exclusion criteria: age < 50 years Number of patients randomised: 39 |
Laser coagulation (n = 18) vs TURP (n = 21) Additional information: patients left hospital 24–72 hours postoperatively if no complications |
Mean IPSS score ± SD: 24.2 ± 7.7 Mean Qmax ± SD (ml/s): 8.3 ± 4.5 Mean residual volume ± SD (ml): 89 ± 92 Intervention performed by two surgeons who were different from those who performed the TURP procedure Catheter protocol: yes |
Mean IPSS score ± SD: 25.5 ± 10.1 Mean Qmax ± SD (ml/s): 7.0 ± 8.1 Mean residual volume ± SD (ml): 77 ± 63 Intervention performed by two surgeons who were different from those who performed the laser procedure Catheter protocol: yes |
Blood transfusion Irritative symptoms Retrograde ejaculation Quality of life Reoperation Symptom score (IPSS) Length of hospital stay Catheter duration Qmax Residual volume |
|
Suvakovic 1996 Study design: RCT Location: UK Length follow-up: 12 months |
Inclusion criteria: symptomatic BPH; Qmax < 15 ml/s for voided volume of ≥ 150 ml; age > 50; PSA level > 2.5 ng/ml; prostate volume < 40 ml; AUA > 15; prostate urethral length > 4 cm Exclusion criteria: prostate cancer Number of eligible patients: 20 Number of patients randomised: 20 |
Laser coagulation (n = 10) vs TURP (n = 10) |
Mean age ± SD (years): 67.5 ± 8.7 Mean AUA score ± SD: 15.7 ± 5.1 Mean Qmax ± SD (ml/s): 10.5 ± 3.7 Mean residual volume ± SD (ml): 47.4 ± 48.1 Mean prostate size ± SD (g): 23.6 ± 6.4 Power: 60 W Catheter protocol: catheter for 24 hours Other: procedure: side-firing Nd:YAG laser; prophylactic antibiotics |
Mean age ± SD (years): 66.1 ± 5.1 Mean AUA score ± SD: 18.0 ± 6.0 Mean Qmax ± SD (ml/s): 11.1± 6.4 Mean residual volume ± SD (ml): 161.8 ± 104 Mean prostate size ± SD (g): 22 ± 5 Catheter protocol: catheter for 48 hours Other: prophylactic antibiotics |
Symptom score (AUA) Qmax Residual volume Duration of operation Catheter duration Retention Length of hospital stay |
| TUIP vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Rodrigo Aliaga 1998 Study design: RCT Location: Spain Length follow-up: 6 months |
Inclusion criteria: patients with BPH; prostate size 20–60 g; symptom score ≤ 12 ml/s; IPSS score ≥ 15 Exclusion criteria: age < 50 years Number of patients randomised: 31 |
TUIP (n = 20) vs TURP (n = 21) Additional information: patients left hospital 24–72 hours postoperatively if no complications |
Mean IPSS score ± SD: 24.2 ± 7.7 Mean Qmax ± SD (ml/s): 8.7 ± 5.5 Mean residual volume ± SD (ml): 89 ± 92 |
Mean IPSS score ± SD: 24.4 ± 10.3 Mean Qmax ± SD (ml/s): 8.3 ± 4.5 Mean residual volume ± SD (ml): 146 ± 133 |
Blood transfusion Irritative symptoms Retrograde ejaculation Quality of life score (WHO) Reoperations Symptom score (IPSS) Length of hospital stay Catheter duration Qmax Residual volume |
|
Christensen 1990 Study design: RCT Location: US Recruitment dates: February 1985–August 1989 Length follow-up: 48 months Links with: Aagaard 1990 (abstract) |
Inclusion criteria: BPH with LUT symptoms Exclusion criteria: prostate size > 20 g; previous prostatic or pelvic surgery; suspected prostate cancer; median lobe > 2 g; prostatic urethra > 3 cm; surgical or anaesthetic risk; overt neurological or psychiatric disease; UTI or urethral stricture excluded until condition had been corrected Number of eligible patients: 93 Number of patients randomised: 76 |
TUIP (n = 38) vs TURP (n = 38) |
Median age (range) (years): 63 (51–77) Median symptom score (range): 16 (8–23) Median Qmax (range): 7.8 (2.8–28) Catheter protocol: 24Fr three-way catheter to closed drainage system, irrigation with normal saline; removed when urine was clear Other: guiding finger in rectum, Collings knife used for deep incision through interureteric ridge and bladder neck to verumontanum at 6 o’clock position |
Median age (range) (years): 62 (42–78) Median symptom score (range): 16 (7–23) Median Qmax (range): 9.7 (1.7–29.4) Catheter protocol: 24Fr three-way catheter to closed drainage system, irrigation with normal saline; removed when urine was clear Other: complete resection, circumferentially the anatomic capsule from bladder neck to verumontanum; resected tissue examined histologically |
Symptom scores Qmax Intraoperative complications Retrograde ejaculation Erectile dysfunction Length of hospital stay Mortality |
|
Dørflinger 1992 Study design: RCT Location: Denmark Length follow-up: 12 months |
Inclusion criteria: bladder neck to seminal crest < 2 cm Exclusion criteria: prostatic cancer; previous prostatic or major pelvic surgery; high operative risk or overt neurological or psychiatric disease; patients with urethral stricture; prostate size > 20 g Number of patients randomised: 60 |
TUIP (n = 29) vs TURP (n = 31) |
Median age (years): 69 Median Madsen score: 15 Median Qmax (ml/s): 10 Median total voided volume (ml): 200 Other: urinary retention, 9 (31%); 24Fr resectoscope and Collings knife used |
Median age (years): 71 Median Madsen score: 15 Median Qmax (ml/s): 8 Median total voided volume (ml): 176 Other: urinary retention, 5 (16%); 24Fr resectoscope used and prostatic tissue resected in a standard fashion |
Duration of operation Blood transfusion Catheter duration Reoperation rate Symptom score (Madsen) Qmax Total voided volume Length of hospital stay |
|
Hellström 1986 Study design: RCT Location: Finland Length follow-up: 6 months |
Exclusion criteria: prostate size > 30 g Number of patients randomised: 24 |
TUIP (n = 11) vs TURP (n = 13) |
Mean age ± SD (range) (years): 63 ± 7.04 (54–77) Mean Qmax ± SD (range) (ml/s): 8.6 ± 4.5 (2–16) Mean residual volume ± SD (range) (ml): 62 ± 74.5 (5–230) based on 9 participants Mean detrusor pressure ± SD (range) (cmH2O): 35 ± 18.8 (7–68) Intervention performed by authors Catheter protocol: yes, 3 days Other: mean amount of prostatic tissue removed (range) (g): 0 |
Mean age ± SD (range) (years): 59 ± 2.92 (54–63) Mean Qmax ± SD (range) (ml/s): 7.5 ± 3.8 (1–14) Mean residual volume ± SD (range) (ml): 43 ± 49.6 (0–145) based on 12 participants Mean detrusor pressure ± SD (range) (cmH2O): 58 ± 34.1 (14–149) Intervention performed by authors Catheter protocol: yes, 3 days Other: mean amount of prostatic tissue removed (range) (g): 7.9 (4.9–14) |
Duration of operation Blood transfusion Stricture Retrograde ejaculation Length of hospital stay Qmax Residual volume Detrusor pressure |
|
Jahnson 1998 Study design: RCT Location: Sweden Recruitment dates: February–September 1991 Length follow-up (range): 2–60 months |
Inclusion criteria: patients admitted from the waiting list for surgical treatment of BPH; no previous treatment for BPH; prostate weight at DRE 20–40 g; distance from verumontanum to bladder neck < 4 cm; informed consent obtained from the patient Exclusion criteria: bladder stone; bladder cancer; prostatitis; chronic cystitis; clinical prostatic cancer; prominent median lobe of the prostate; adequate follow-up difficult for geographical, psychological or social reasons Number of patients randomised: 85 |
TUIP (n = 43) vs TURP (n = 42) |
Mean age (range) (years): 70.2 (52–87) Mean Madsen–Iversen score (range): 15.4 (6–27) Mean Qmax (95%CI): 9 (7.5–11) Mean residual volume (range) (ml): 139 (0–650) Catheter protocol: overnight Other: perioperative heparin, 13; mean resection weight (range (g): 18.8 (8–45); antibiotics, 17 |
Mean age (range) (years): 70.8 (56–85) Mean Madsen–Iversen score (range): 15.8 (5–28) Mean Qmax (95%CI): 8.5 (7.5–9.5) Mean residual volume (range) (ml): 109 (0–400) Catheter protocol: overnight Other: perioperative heparin, 17; antibiotics, 14 |
Duration of operation Intraoperative complications Blood transfusion Catheter duration Reoperation rate Symptom score (Madsen) Qmax Residual volume |
|
Li 1987 Study design: RCT Location: China (Hong Kong) Length follow-up: 3 months |
Inclusion criteria: patients catheterised for retention; prostate size ≤ 30 g Exclusion criteria: renal impairment; ischaemic heart disease; stroke; diabetes mellitus Number of patients randomised: 59 |
TUIP (n = 29) vs TURP (n = 30) |
Mean age ± SE (years): 65 ± 1.4 Catheter protocol: yes, three-way catheter removed on days 2–3 Preprocedural antibiotics: gentamicin (1 mg/kg) for 1 day preoperatively |
Mean age ± SE (years): 70 ± 1.7 Catheter protocol: yes, three-way catheter removed on days 2–3 Preprocedural antibiotics: gentamicin (1 mg/kg) for 1 day preoperatively |
Duration of operation Intraoperative complications Blood transfusion Recatheterisation Clot retention TUR syndrome Cardiovascular events Mortality Incontinence Septicaemia Stricture UTI Retention Length of hospital stay Qmax |
|
Nielsen 1998 Study design: RCT Location: Denmark Length follow-up: 2 and 12 months |
Inclusion criteria: patients with BPH aged 60; informed consent obtained from the patient Number of patients randomised: 49 |
TUIP (n = 24) vs TURP (n = 25) |
Median age (range) (years): 69 (60–85) Prostate weight (g): < 30 = 3 patients; 30–50 = 14 patients; > 50 = 7 patients Median Qmax (range) (ml/s): 5 (5–10) Catheter protocol: yes, as long as the urine became clear Other: acute retention: 8/24 |
Median age (range) (years): 73 (61–83) Prostate weight (g): <30 = 7 patients; 30–50 = 14 patients; >50 = 4 patients Median Qmax (range) (ml/s): 5 (5–13) Catheter protocol: yes, as long as the urine became clear Other: acute retention: 7/25 |
Duration of operation Blood transfusion Catheter duration Septicaemia Urine flow rate Incontinence Length of hospital stay |
|
Riehmann 1995 Study design: RCT Location: US Recruitment dates: January 1985–August 1990 Mean length follow-up: 34 (7–82 months) Links with: Sparwasser 1995; Riehmann 1993 (abstract) |
Inclusion criteria: patients with bladder outlet obstruction symptoms Exclusion criteria: prostatic urethra > 3 cm; median lobe > 2g; previous prostatic or major pelvic surgery; high operative risk or overt neurological or psychiatric disease Number of eligible patients: 120 Number of patients randomised: 117 |
TUIP (n = 61) vs TURP (n = 56) |
Mean age (range) (years): 65 (51–77) Mean Madsen score: 15.5 Mean Qmax (ml/s): 9 (n = 52) |
Mean age (range) (years): 64 (42–78) Mean Madsen score: 15 Mean Qmax (ml/s): 11 (n = 50) |
Duration of operation Catheter duration Reoperation rate Symptom score (Madsen) Qmax Obstructive symptom score Overall subjective assessment Length of hospital stay Mortality Erectile dysfunction |
|
Saporta 1996 Study design: RCT Location: Israel Length follow-up: up to 36 months |
Inclusion criteria: patients with obstructive BPH symptoms; prostate weight at DRE ≥ 40 g Exclusion criteria: chronic urinary retention; urethral stricture, bladder cancer, prostatitis; clinical and suspicion of prostatic cancer; prominent median lobe of prostate; neurogenic bladder Number of patients randomised: 40 |
TUIP (n = 20) vs TURP (n = 20) |
Mean age ± SD (years): 66.85 ± 2.28 Catheter protocol: 14Fr Foley through trocar cystostomy channel and 20Fr Foley through urethra; irrigated for 18–24 hours; 14Fr Foley removed next day, 20Fr 48 hours after procedure Other: anaesthesia: spinal, epidural or general; procedure: low pressure continuous flow with trocar cystostomy |
Mean age ± SD (years): 71.45 ± 1.15 Catheter protocol: 20Fr Foley for 18–24 hours Other: anaesthesia: spinal, epidural, general or local; procedure: incision with Collings knife from interureteric ridge to verumontanum as deep as fat layer |
Catheter duration Reoperation rate Symptom score (Madsen) Qmax Patient’s global assessment |
|
Soonawalla 1992; Saporta 1996 Study design: RCT Location: India Length follow-up: ≤ 24 months |
Inclusion criteria: patients with prostatic hypertrophy Exclusion criteria: prostatic cancer or suspicion of malignancy Number of patients randomised: 220 |
TUIP (n = 110) vs TURP (n = 110) |
Mean age (years): 62.2 Catheter protocol: 24Fr Foley; 24–48 hours Other: anaesthesia: general anaesthesia/local |
Mean age (years): 65.0 Catheter protocol: 24Fr Foley; ≤ 48 hours Other: anaesthesia: general anaesthesia/spinal/epidural |
Duration of operation Blood transfusion Catheter duration Reoperation rate Qmax Mean urine flow Subjective evaluation UTI Urinary retention TUR syndrome Mortality Length of hospital stay Retrograde ejaculation |
|
Tkocz 2002 Study design: RCT Location: Poland |
Inclusion criteria: prostate size < 30 g Exclusion criteria: median enlargement Number of patients randomised: 100 |
TUIP (n = 50) vs TURP (n = 50) Additional information: subarachnoid anaesthesia with hyperbaric lidocaine |
Mean age ± SD (years): 3 ± 6.7 Mean IPSS ± SD: 17.1 ± 2.2 Mean QoL ± SD: 4.6 ± 0.5 Prostate size ± SD (g): 27 ± 2 Mean Qmax ± SD: 7.6 ± 1.8 Mean residual volume ± SD (ml): 75 ± 22 Mean Pdetmax ± SD (cmH2O): 84 ± 10 Catheter protocol: Foley 18Fr for 24 hours |
Mean age ± SD (years): 63 ± 6.7 Mean IPSS ± SD: 17.1 ± 1.9 Mean QoL ± SD: 4.4 ± 0.3 Prostate size ± SD (g): 28.2 ± 2 Mean Qmax ± SD: 6.9 ± 1.5 Mean residual volume ± SD (ml): 68 ± 21 Mean Pdetmax ± SD (cmH2O): 85 ± 8 Catheter protocol: Foley 18Fr for 24 hours Other: procedure: bilateral incisions 24Fr resectoscope or Collings blade |
Symptom score (IPSS) Quality of life score Qmax Detrusor pressure Retrograde ejaculation Residual volume |
| Laser vaporisation vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Bouchier-Hayes 2006 Study design: RCT Location: Australia Recruitment date: January 2004 Length follow-up: 12 months |
Inclusion criteria: peak urine flow rate ≤ 15 ml/s; IPSS ≥ 12; referred by family physician for LUTS; gland 15–85 ml (TRUS); obstructed on Abrams–Griffiths nomogram; able to complete quality of life, bother score and BSFQ questionnaires; able to give fully informed consent Exclusion criteria: age ≤ 50 years; known or suspected prostate cancer; neurogenic bladder; chronic retention; taking alpha-blocker or herbal medication believed active in prostate; permanently on anticoagulant; taking finasteride or dutasteride Number of eligible patients: 95 Number of patients randomised: 76 |
Laser vaporisation (n = 38) vs TURP (n = 38) |
Mean age (range) (years): 65.2 (51–81) Mean prostate size (range) (ml): 42. 4 (16.5–82.6) Power: 80 W Intervention performed by registrars in training or fellows in the department, all of whom had performed < 5 laser prostatectomies each Catheter protocol: at the discretion of the operating surgeon Other: GreenLight™ laser system (KTP) (American Medical Systems, Minnetonka, MN, USA) |
Mean age (range) (years): 66.2 (55–801) Mean prostate size (range) (ml): 33.2 (15.4–67.5) Intervention performed by registrars in training or fellows in the department, all of whom had performed between 35 and 325 TURPs Catheter protocol: at the discretion of the operating surgeon |
Duration of operation Blood transfusion Catheter duration Recatheterisation Clot retention TUR syndrome Stricture Reoperation Length of hospital stay Rehospitalisation |
|
Carter 1999 Study design: RCT Location: UK Recruitment dates: June 1995–July 1996 Length follow-up: 12 months Links with: Carter 1999; Pearcy 1999 (abstract) |
Inclusion criteria: based on British Laser Urological Evaluation Society (BLUES); Qmax ≤ 15 ml/s; voided volume > 150 ml; PVR < 300 ml; IPSS ≥ 12 Exclusion criteria: prostate cancer (histological diagnosis); prostate size (TRUS) > 100 ml; history of urinary retention; neurogenic bladder dysfunction Number of eligible patients: 204 Number of patients randomised: 191 |
Laser vaporisation (n = 95) vs TURP (n = 96) |
Mean age ± SD (years): 67.9 ± 7.8 Mean IPSS score: 20.3 Mean Qmax (ml/s): 9.0 Mean PSA (ng/ml): 3.8 ± 2.7 Mean residual volume (ml): 109 (estimated from graph) Mean prostate volume (ml): 41.6 ± 17.3 (TRUS) Intervention performed by one of three consultants, two senior registrars, one clinical research fellow or one staff-grade urologist Power: 30 W KTP, mean energy 12.3 ± 9.5 kJ; 60 W Nd:YAG, mean energy 26.1 ± 16.3 kJ Antibiotics: single-dose gentamicin at operation and catheter removal Catheter protocol: urethral catheter removed either 1 or 2 days or 1–2 weeks postoperatively Other: continued any prophylactic antithrombolytic therapy |
Mean age ± SD (years): 67± 7.5 Mean IPSS score: 19.8 Mean Qmax (ml/s): 9.5 Mean PSA (ng/ml): 3.2± 2.4 Mean residual volume (ml): 135 (estimated from graph) Mean prostate volume (ml): 41.7 ± 19.4 (TRUS) Intervention performed by one of three consultants, two senior registrars, one clinical research fellow or one staff-grade urologist Catheter protocol: catheter removed postoperatively when clinically indicated Antibiotics: single-dose gentamicin at operation and catheter removal Other: conventional methods through 24- or 26Fr resectoscopes Discontinued any prophylactic antithrombolytic therapy 1 week before surgery |
Duration of operation Symptom scores (IPSS) Quality of life Length of hospital stay Qmax Residual volume Blood transfusion UTI Urinary retention Catheter duration TUR syndrome Stricture Reoperation |
|
Keoghane 2000 Study design: RCT Location: UK Recruitment dates: January 1993–January 1995 Length follow-up: 5 years Links with: Jenkinson 1997; Keoghane 1996a; Keoghane 1996b; Keoghane 1996c; Keoghane 2000; Keoghane 1995 (abstract) |
Inclusion criteria: BPE Exclusion criteria: surgery or instrumentation for BPE; prostate cancer; inability to understand English or refused consent forms or questionnaires Number of eligible patients: 152 Number of patients randomised: 148 |
Laser vaporisation (n = 72) vs TURP (n = 76) |
Mean age (range) (years): 69 (51–95) Mean AUA score ± SD: 19.9 ± 7.7 (n = 54) Mean Qmax ± SD (ml/s): 11.8 ± 4.5 Mean prostate size ± SD (ml): 54.2 ± 26.3 (n = 44) Sexually active: 9/38 (24%) Mean SF-36 (physical) ± SD: 43.69 ± 12.58 (n = 51) Mean SF-36 (mental) ± SD: 47.07 ± 11.2 (n = 51) Mean EQ-5D ± SD: 0.81 ± 0.18 (n = 62) Mean bothersome score ± SD: 5.90 ± 3.03 (n = 59) Thermometer score: 75.8 ± 17.1 (n = 69) Intervention performed by five surgeons with limited experience with laser procedure before study; consultant, senior registrar or registrar Energy (range) (kJ): 33.47 (18.74–44.96) Catheter protocol: three-way catheter left in place regardless of blood loss Antibiotics: oral ciprofloxacin 2 hours before surgery Other: Nd:YAG/SLT MD60; general or spinal anaesthesia determined by patients’ medical condition and preference |
Mean age (range) (years): 70 (47–84) Mean AUA score ± SD: 19.4 ± 6.5 (n = 63) Mean Qmax ± SD (ml/s): 11.4 ± 5.0 Mean prostate size ± SD (ml): 51.9 ± 24.1 (n = 48) Sexually active: 20/50 (40%) Mean SF-36 (physical) ± SD: 44.66 ± 12.12 (n = 57) Mean SF-36 (mental) ± SD: 47.75 ± 10.47 (n = 57) Mean EQ-5D ± SD: 0.81 ± 0.18 (n = 65) Mean bothersome score ± SD: 5.99 ± 2.40 (n = 68) Thermometer score: 78.3 ±3.2 (n = 66) Antibiotics: oral ciprofloxacin 2 hours before surgery Other: standard TURP with Storz equipment, continuous irrigation; general or spinal anaesthesia determined by patients’ medical condition and preference |
Symptom score (AUA) Quality of life Bothersome score Qmax Reoperation Mortality Intraoperative complications Blood transfusion UTI Urinary retention Catheter duration Cardiovascular events Incontinence Stricture Erectile dysfunction Retrograde ejaculation |
|
Mottet 1999 Study design: RCT Location: France Recruitment dates: February 1995–February 1996 Length follow-up: 12 months Links with: Mottet 1997 (abstract) |
Inclusion criteria: peak urine flow rate < 12 ml/s; age > 45 years; residual volume < 250 ml; AUA > 13; PSA < 10 ng/ml: informed consent Exclusion criteria: history of prostatic or urethral surgery; prostate > 60 g; diabetes, bladder or neurogenic disease Number of patients randomised: 36 |
Laser vaporisation (n = 17) vs TURP (n = 13) Additional information: one laser patient converted to TURP because of bleeding and endoscope malfunction |
Mean age (range) (years): 67 (50–77) (n = 23) Mean Madsen score: 15 Mean IPSS score: 21.7 Mean Qmax (ml/s): 8.8 Mean prostate size (ml): 36.7 Intervention performed by same surgeons experienced in both techniques Power: 80 W holmium energy in pulsed mode; wavelength 2140 nm; 25 pulses at 60 W or 30 pulses at 80 W; energy 103.6 kJ Catheter protocol: 24Fr or 20Fr Foley catheter without irrigation, removed next day Other: saline irrigation through 24Fr cystoscope |
Mean age (range) (years): 64 (50–77) Mean Madsen score: 17 Mean IPSS score: 23.7 Mean Qmax (ml/s): 7.7 Mean prostate size (ml): 34 Intervention performed by same surgeons experienced in both techniques Catheter protocol: catheter removed next day Other: glycine irrigation during procedure, saline irrigation until urine clears; spinal anaesthesia |
Duration of operation Symptom scores (IPSS, Madsen) Length of hospital stay Qmax Prostate size Intraoperative complications Blood transfusion Catheter duration Stricture Reoperation Incontinence Retrograde ejaculation Erectile dysfunction |
|
Sengör 1996 Study design: RCT Location: Turkey Recruitment dates: June 1994–April 1995 Length follow-up: 6 months |
Inclusion criteria: significant voiding symptoms to request therapy; Qmax < 15 ml/s; Qave ≤ 10 ml/s; age > 50 years Exclusion criteria: prostate cancer; infections; induration or nodularity of prostate on DRE or PSA > 4.0 mg/ml examined for cancer Number of patients randomised: 60 |
Laser vaporisation (n = 30) vs TURP (n = 30) Additional information: patients with UTI treated with preoperative antibiotics |
Mean age (range) (years): 61 (55–70) Mean AUA score ± SD: 21.8 ± 7.6 Mean Qmax ± SD (ml/s): 8.7 ± 2.3 Mean urine flow ± SD (ml/s): 4.6 ± 1.8 Mean residual volume ± SD (ml): 110 ± 68 Number sexually active: 23/30 (77%) Mean prostate volume (range) (ml): 55 (30–80) Power: 60 W continuous firing; 12.5–110 kJ energy (mean 46.6 kJ). Catheter protocol: suprapubic catheter |
Mean age (range) (years): 66 (50–85) Mean AUA score ± SD: 22.1 ± 2.6 Mean Qmax ± SD (ml/s): 8.4 ± 2.8 Mean urine flow ± SD (ml/s): 4.7 ± 2.1 Mean residual volume ± SD (ml): 155 ±40 Number sexually active: 27/30 (90%) Mean prostate volume (range) (ml): 47 (30–50) Catheter protocol: yes |
Duration of operation Symptom score (AUA) Length of hospital stay Qmax Mean urine flow rate Residual volume Blood transfusion Retrograde ejaculation |
|
Shingleton 2002 Study design: RCT Location: US Length follow-up: 72 months Links with: Shingleton 1999;Shingleton 2001 (abstract) |
Inclusion criteria: peak urine flow rate < 15 ml/s; age > 45 years; failure of medical therapy (α-blockers); able to undergo regional/general anaesthesia; medical therapy discontinued 1 month before surgery Exclusion criteria: presence or history of cancer Number of patients randomised: 100 |
Laser vaporisation (n = 50) vs TURP (n = 50) |
Mean age ± SD (years): 68.2 ± 7.9 Ethnicity: 38/50 (76%) white Mean AUA-6 score ± SD: 22.5 ±6 Mean Qmax ± SD (ml/s): 7.6 ± 3.4 Mean urine flow ± SD (ml/s): 8.2 ± 3.2 Other: KTP/Nd:YAG/PVP |
Mean age ± SD (years): 67.4 ± 7.3 Ethnicity: 34/50 (68%) white Mean AUA-6 score ± SD: 21 ± 6.1 Mean Qmax ± SD (ml/s): 6.5 ± 4.0 Mean urine flow ± SD (ml/s): 7.3 ± 3.7 |
Intraoperative complications Blood transfusion Urinary retention Stricture Incontinence Retrograde ejaculation Cardiovascular events Reoperation Symptom score (AUA) Global assessment Qmax Prostate size |
|
Suvakovic 1996 Study design: RCT Location: UK Length follow-up: 12 months |
Inclusion criteria: symptomatic BPH; Qmax < 15 ml/s for voided volume of ≥ 150 ml; age > 50; PSA > 2.5 ng/ml; prostate volume < 40 ml; AUA > 15; prostate urethral length > 4 cm Exclusion criteria: prostate cancer Number of eligible patients: 20 Number of patients randomised: 20 |
Laser vaporisation (n = 10) vs TURP (n = 10) |
Mean age ± SD (years): 62.6 ± 5.8 Mean AUA score ± SD: 18.8 ± 4.5 Mean Qmax ± SD (ml/s): 12.2 ± 3.8 Mean residual volume ± SD (ml): 139.6 ± 103 Mean prostate size ± SD (g): 24 ± 5.8 Catheter protocol: catheter for 24 hours Power: 40 W Other: contact Nd:YAG laser; prophylactic antibiotics |
Mean age ± SD (years): 66.1 ± 5.1 Mean AUA score ± SD: 18.0 ± 6.0 Mean Qmax ± SD (ml/s): 11.1 ± 6.4 Mean residual volume ± SD (ml): 161.8 ± 104 Mean prostate size ± SD (g): 22 ± 5 Catheter protocol: catheter for 48 hours Other: prophylactic antibiotics |
Symptom score (AUA) Qmax Residual volume Duration of operation Catheter duration Retention Length of hospital stay |
|
Tuhkanen 2001 Study design: RCT Location: Finland Recruitment dates: January 1995–November 1997 Length follow-up: 24 months Links with: Tuhkanen 1999 |
Inclusion criteria: BPH and BOO; obstructed if min. voiding pressure > 40 cm water Exclusion criteria: prostate cancer or surgery; urinary retention; prostate size (TRUS) < 40 ml or > 100 ml Number of patients randomised: 46 |
Laser vaporisation (n = 21) vs TURP (n = 25) |
Mean age (range) (years): 67 (46–77) Mean Dan PSSI score (range): 23 (5–69) Mean Qmax (range) (ml/s): 7.2 (3.7–14.8) Mean urine flow (range) (ml/s): 3.6 (1.4–7.0) Mean residual volume (range) (ml): 144 (0–450) Mean prostate size (range) (ml): 55 (40–94) (TRUS) Mean Pdetmax (range) (cmH2O): 83 (47–137) Intervention performed by experienced urologist Catheter protocol: 24Fr urethral catheter inserted until urine was clear; no suprapubic catheter Other: 28Fr Storz (Wolf) resectoscope with glycine irrigant; spinal anaesthesia |
Mean age (range) (years): 67 (55–78) Mean Dan PSSI score (range): 18.6 (5–40) Mean Qmax (range)(ml/s): 8.5 (2.3–17.2) Mean urine flow (range) (ml/s): 4.2 (1.0–9.2) Mean residual volume (range)(ml): 125 (0–350) Mean prostate size (range) (ml): 55 (42–83) (TRUS) Mean Pdetmax (range) (cmH2O): 79 (47–131) Intervention performed by experienced urologist Catheter protocol: 20Fr urethral catheter for 1 day; suprapubic catheter removed when patient could void and residual urine was < 150 ml Power: 40 W for 90 seconds; energy 1011 J/ml (519–1785) of tissue Other: non-contact Nd:YAG coagulation then contact Nd:YAG vaporisation; urethrocystoscopy with 25Fr Storz 30 wide-angle cystoscope and 14Fr suprapubic cystostomy catheter with saline irrigant; spinal anaesthesia |
Duration of operation Symptom score (Dan PSS-1 score) Length of hospital stay Qmax Residual volume Prostate volume Detrusor pressure Mean urine flow rate Blood transfusion Catheter duration Clot retention Stricture Retrograde ejaculation Reoperation Retention Mortality |
|
Tuhkanen 2003 Study design: RCT Location: Finland Recruitment dates: September 1994–January 1998 Length follow-up: 4 years Links with: Tuhkanen 1999;Tukhanen 2004 |
Inclusion criteria: LUT with confirmed BOO; minimum volume ≥ 120 ml; minimum voiding detrusor pressure > 40 cmH2O Exclusion criteria: prostate cancer; prostate surgery or history of TUIP or TURP; prostate size > 40 ml; urethral stricture; neurogenic bladder dysfunction; residual volume > 300 ml Number of patients randomised: 52 |
Laser vaporisation (n = 26) vs TURP (n = 26) |
Median age (range) (years): 68 (56–82) Median Dan IPSS (range): 18 (5–54) Median Qmax (range) (ml/s): 8.6 (5.0–15.9) Median residual volume (range) (ml): 87(0–331) Median prostate size (range) (ml): 30 (15–37) Median Pdetmax (range) (cmH2O): 57 (40–137) Number of patients sexually active: 16 Intervention performed by experienced urologist Power: 40 W Catheter protocol: 20Fr urethral catheter inserted for 1 day Other: Nd:YAG/contact with SLT MTRL 10 contact probe, 25Fr Storz port for 90 seconds, 15Fr suprapubic catheter introduced at beginning of operation with free saline irrigant; ciproflavin (250 mg) evening and morning of operation |
Median age (range) (years): 67 (55–77) Median Dan IPSS (range): 18 (4–46) Median Qmax (range) (ml/s): 8.6 (5.0–15.9) Median residual volume (range) (ml): 83 (8–350) Median prostate size (range) (ml): 28 (15–38) Median Pdetmax (range) (cmH2O): 57 (40–137) Number of patients sexually active: 16 Intervention performed by experienced urologist Other: standard TURP through 28Fr Storz resectoscope; ciproflavin (250 mg) evening and morning of operation |
Duration of operation Symptom score (Dan IPSS) Length of hospital stay Qmax Residual volume Prostate size Detrusor pressure Mortality UTI Catheter duration Clot retention Intraoperative complications Stricture Retrograde ejaculation Reoperation |
|
van Melick 2003 Study design: RCT Location: Netherlands Recruitment dates: 1996–2001 Mean length follow-up: up to 7 years Links with: van Melick 2002; van Melick 2003 |
Inclusion criteria: patients with lower UT symptoms suggestive of BPH; met ISC criteria for BPH; Schafer obstruction score ≥ 2; prostate size 20–65 ml Exclusion criteria: age ≤ 45 years Number of patients randomised: 95 |
Laser vaporisation (n = 45) vs TURP (n = 50) |
Mean age ± SD (years): 67 ± 9 Mean IPSS ± SD: 18.9 ± 6.8 Mean Qmax ± SD ml/s: 12 ± 4 Mean residual volume ± SD: 300 ± 135 Mean prostate size ± SD (ml): 37 ± 11 Quality of life score (IPSS) ± SD: 3.7 ± 1.6 Detrusor pressure ± SD (cmH2O): 69 ± 24 Intervention performed by mostly experienced urologist and trainees Catheter protocol: 20Fr transurethral catheter postoperatively Preprocedural antibiotics: yes, intravenously Other: Nd:YAG with SLT MTRL 10 probe |
Mean age ± SD (years): 66 ± 8 Mean IPSS ± SD: 11 ± 4 Mean Qmax ± SD ml/s: 10.8 ± 4.76 Mean residual volume ± SD: 350 ± 140 Mean prostate size ± SD (ml): 37 ± 11 Quality of life score (IPSS) ± SD: 3.8 ± 1.5 Detrusor pressure ± SD (cmH2O): 76 ± 27 Intervention performed by alternate experienced urologist and trainees Catheter protocol: suprapubic if required perioperatively Preprocedural antibiotics: yes, intravenously Other: standard 24Fr resectoscope |
Duration of operation Intraoperative complications Blood transfusion UTI Catheter duration Clot retention Cardiovascular events Mortality Incontinence Stricture Reoperation Retention Symptom score (AUA) Quality of life Qmax Residual volume |
|
Zorn 1999 Study design: RCT Location: US (multicentre) Recruitment dates: June 1995–June 1996 Length follow-up: 12 months |
Inclusion criteria: symptomatic BPH; Qmax < 15 ml/s; age > 50; AUA score ≥ 13; PVR > 125 ml Exclusion criteria: previous surgical therapy for BPH; known prostate, bladder, urethral or neurological conditions that could affect the bladder Number of patients randomised: 33 |
Laser vaporisation (n = 21) vs TURP (n = 12) |
Mean age (years): (a) 70.6; (b) 69.6 Mean AUA score: (a) 24.0; (b) 24.2 Mean Qmax (ml/s): (a) 8.7; (b) 6.2 Mean prostate size (ml): (a) 29.9; (b) 67.4 Power: CLVP 50–60 W Performed under general or regional anaesthesia |
Mean age (years): 69.0 Mean AUA score: 24.7 (n = 11) Mean Qmax (ml/s): 9.0 (n = 11) Mean prostate size (ml): 33.9 Performed under general or regional anaesthesia |
Duration of operation Symptom score (AUA) Residual volume Qmax Blood transfusion Catheter duration Recatheterisation Stricture Reoperation Length of hospital stay |
| TUVRP vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Helke 2001 Study design: RCT Location: Germany Length follow-up: 1 year |
Inclusion criteria: residual volume > 60 ml; IPSS > 10; BPH; moderate/severe LUT symptoms Exclusion criteria: prostate cancer or other untreated malignancies; previous prostatic surgery; neurogenic bladder; urethral strictures; severe neurological disease or psychiatric abnormalities Number of patients randomised: 185 |
TUVRP (n = 93) vs TURP (n = 92) |
Mean age ± SD (range) (years): 67.3 ± 7.73 (47–85) Mean IPSS ± SD (range): 17.29 ± 6.06 (10–35) Mean Qmax ± SD (range) (ml/s): 10.8 ± 4.76 (4.2–28.4) Mean residual volume ± SD (range) (ml): 76.0 ± 60.50 (0–330) Mean prostate size ± SD (range) (ml): 48.8 ± 21.21 (13–110) by suprapubic US Power: 250 W (cutting) Catheter protocol: catheter removed on second or third day after surgery; discharged day after removal Other: preoperative indwelling catheter, n/N (%): 28/93 (30.1); preoperative UTI, n/N (%): 27/92 (29.3); anaesthesia: general or epidural; antibiotics: ciprofloxamin (200 mg twice a day) or co-trimoxazole 160/80 twice a day for 2 days |
Mean age ± SD (range) (years): 68.7 ± 8.38 (53–89) Mean IPSS ± SD (range): 18.29 ± 7.49 (10–35) Mean Qmax ± SD (range) (ml/s): 8.5 ± 5.19 (5.2–29.0) Mean residual volume ± SD (range) (ml): 101.8 ± 84.1 (0–410) Mean prostate size ± SD (range) (ml): 49.9 ± 22.1 (20–140) Power: 150 W (cutting) Catheter protocol: catheter removed on second or third day after surgery; discharged day after removal Other: preoperative indwelling catheter, n/N (%): 32/92 (34.4); preoperative UTI, n/N (%): 29/91 (32); anaesthesia: general or epidural; antibiotics: ciprofloxamin (200 mg twice a day) or co-trimoxazole 160/800 mg twice a day for 2 days |
Symptom score (IPSS) Qmax Residual volume Incontinence Blood transfusion Stricture Reoperation |
|
Küpeli 2001 Study design: RCT Location: Turkey Recruitment dates: November 1997–not reported Length follow-up: 6 months |
Inclusion criteria: peak urine flow rate < 15 ml/s; IPSS score ≥ 85 Exclusion criteria: prostate cancer; history of prostate surgery; neurogenic bladder Number of patients randomised: 100 |
TUVRP (n = 50) vs TUVP (n = 50) |
Mean age ± SD (years): 61.4 ± 3.2 Mean IPSS: 19.4 Mean Qmax ± SD (ml/s): 7.9 ± 2.1 Prostate size ± SD (range) (ml): 57.8 ± 4.1 (34–95) (TRUS) Sexually active: 36 Power: cutting current: 250–300 W Other: anaesthesia: general in 14 patients and spinal or epidural in 36; Wing (Wolf) gold-plated one-system electrode with pure cutting diathermy |
Mean age ± SD (years): 58.9 ± 3.6 Mean IPSS: 21.6 Mean Qmax ± SD (ml/s): 9.2 ± 2.6 Prostate size ± SD (range) (ml): 56.7 ± 6.3 (34–110) (TRUS) Sexually active: 25 Power: cutting current: 80–120 W Other: anaesthesia: general in 16 patients and spinal or epidural in 34; Karl Storz 24Fr cutting loop with the Valleylab Force 40 electrical current generator |
Duration of operation Blood transfusion Retention Catheter duration Recatheterisation TUR syndrome Incontinence Stricture Retrograde ejaculation Erectile dysfunction Symptom score (IPSS) Length of hospital stay Qmax Prostate size |
|
Gupta 2006 Study design: RCT Location: India Recruitment dates: July 2002–December 2003 Length follow-up: 1 year |
Inclusion criteria: BPH candidates for TURP Exclusion criteria: prostate cancer; prostate size < 40 g; prostatic or urethral surgery; neurovesical dysfunction Number of patients randomised: 150 |
TUVRP (n = 50) vs TURP (n = 50) |
Mean age ± SD (range) (years): 67.98 ± 9.8 (48–92) Mean IPSS score ± SD (range): 24.9 ± 3.9 (17–32) Mean Qmax ± SD (range) (ml/s): 4.65 ± 3.6 (0–12) Mean residual volume ± SD (range) (ml): 103 ± 174.1 (0–881) Mean prostate size ± SD (range) (ml): 62.6 ± 14.8 (42–133) (TRUS) Intervention performed by experienced surgeons Catheter protocol: 22Fr double-lumen Foley catheter with continuous saline irrigation until effluent clear; removed 6 hours after urine is clear then discharged 6–12 hours later Power: cutting current: 180 W Coagulation current: 80 W Other: 27Fr continuous flow resectoscope with Wing (Wolf) loop; spinal subarachnoid anaesthesia with 0.5% bupivacaine; perioperative antibiotics; number of catheterised patients (%): 19/50 (38) Co-morbidities: ischaemic heart disease (%): 7/50 (14); hypertension (%): 14/50 (28); chronic airway disease (%): 3/50 (6); diabetes mellitus (%): 5/50 (10); cerebrovascular accident (%): 1/50 (2) |
Mean age ± SD (range) (years): 65.67 ± 7.5 (48–84) Mean IPSS score ± SD (range): 23.3 ± 3.9 (17–31) Mean Qmax ± SD (range) (ml/s): 4.5 ±4.7 (0–13) Mean residual volume ± SD (range) (ml): 84.0 ± 129.7 (0–600) Mean prostate size ± SD (range) (ml): 59.8 ± 16.5 (40–110) (TRUS) Intervention performed by experienced surgeons Catheter protocol: 22Fr double-lumen Foley catheter with continuous saline irrigation until effluent clear; removed 6 hours after urine is clear then discharged 6–12 hours later Power: cutting current: 80 W Coagulation current: 50 W Other: 27Fr continuous flow resectoscope (Wolf) with standard tungsten wire loop; standard technique, bladder neck to surgical capsule as far as clear transverse fibres; spinal subarachnoid anaesthesia with 0.5% bupivacaine; perioperative antibiotics; number of catheterised patients (%): 16/50 (32) Co-morbidities: ischaemic heart disease (%): 5/50 (10); hypertension (%): 14/50 (28); chronic airway disease (%): 1/50 (2); diabetes mellitus (%): 7/50 (14); cerebrovascular accident (%): 0/50 (0) |
Duration of operation Symptom score (IPSS) Qmax Residual volume Intraoperative complications Blood transfusion Catheter duration Recatheterisation Mortality Incontinence Stricture |
|
Liu 2006 Study design: RCT Location: Taiwan Recruitment dates: July 1999–June 2002 Length follow-up: 2 years |
Inclusion criteria: bladder outlet obstruction due to BPH and on waiting list for elective surgery at their institution; prostate size > 50 ml; QoL score ≥ 3; peak urine flow rate ≤ 12 ml/s; IPSS score ≥ 15 Exclusion criteria: prostate cancer; PSA 4 ng/ml or higher; signs of a neurogenic bladder; bladder stones; previous urethral or prostatic surgery; undergoing any anticoagulant therapy Number of patients randomised: 76 |
TUVRP (n = 44) vs TURP (n = 32) |
Mean age ± SD (range) (years): 66 ± 6.6 (54–90) Mean IPSS ± SD: 26.8 ± 4.7 Mean Qmax ± SD (ml/s): 6.9 ± 2.1 Mean residual volume ± SD (ml): 142 ± 48 Prostate size ± SD (range) (ml): 60.5 ± 10.9 (51–116) QoL score ± SD: 4.1 ± 0.6 Sexually active: 17 Power: cutting current: 200 W; coagulating current: 60 W Intervention performed by three staff urologists having equivalent experience and after each had undergone a surgical experience ‘learning curve’ training program for the TUVRP procedure, which encompassed performing surgery in at least 10 patients with an enlarged prostate Catheter protocol: 22Fr three-way Foley catheter Other: wedge resection loop |
Mean age ± SD (range) (years): 64.7 ± 6.3 (55–88) Mean IPSS ± SD: 25.6 ± 3.5 Mean Qmax ± SD (ml/s): 6.9 ± 1.9 Mean residual volume ± SD (ml): 131 ± 41 Prostate size ± SD (range) (ml): 58.4 ±4 (52–109) QoL score ± SD: 4.0 ± 0.7 Sexually active: 13 Power: cutting current: 110 W; coagulating current: 60 W Intervention performed by three staff urologists having equivalent experience and after each had undergone a surgical experience ‘learning curve’ training program for the TUVRP procedure, which encompassed performing surgery in at least 10 patients with an enlarged prostate Catheter protocol: 22Fr three-way Foley catheter Other: standard wire loop |
Duration of operation Intraoperative complications Blood transfusion Catheter duration Recatheterisation Clot retention TUR syndrome Stricture Incontinence Retrograde ejaculation Erectile dysfunction Reoperation Symptom score (IPSS) Quality of life score Length of hospital stay Rehospitalisation Qmax Residual volume |
|
Talic 2000 Study design: RCT Location: Saudi Arabia Mean length follow-up (range): 9 (1–15) months |
Inclusion criteria: peak urine flow rate < 15 ml/s, IPSS score > 15; some patients with retention Exclusion criteria: previous prostate surgery; neurogenic bladder Number of patients randomised: 68 |
TUVRP (n = 34) vs TURP (n = 34) |
Mean age ± SD (range) (years): 70.9 ± 9.3 (55–94) Mean IPSS ± SD (range): 24.9 ± 6 (15–31) Mean Qmax ± SD (range) (ml/s): 7.5 ± 3.5 (2–14.6) Mean prostate size (ml) (range): 52.4 ± 18.7 (20–100) (TRUS) Power: cutting current: 250 W; coagulation current: 80 W Intervention performed by consultant Catheter protocol: yes, unless haematuric; urinary catheters removed the morning after operation Other: Wing (thick-loop) electrode (Wolf); urinary retention preoperatively: 15/34 (44%); symptomatic BPH: 19/34 (56%) |
Mean age ± SD (range) (years): 70.4 ± 8.8 (53–86) Mean IPSS ± SD (range): 20.1 ± 6.8 (11–30) Mean Qmax ± SD (range) (ml/s): 9.1 ± 6.3 (1–15) Mean prostate size (ml) (range): 57.2 ± 22.5 (20–105) (TRUS) Power: cutting current: 250 W; coagulation current: 80 W Intervention performed by consultant. Catheter protocol: yes, unless haematuric; urinary catheters removed the morning after operation Other: Wing (thick-loop) electrode (Wolf); urinary retention preoperatively: 18/34 (53%); symptomatic BPH: 16/34 (47%) |
Duration of operation Catheter duration Clot retention TUR syndrome Stricture Retrograde ejaculation Erectile dysfunction Symptom score (IPSS) Qmax |
| B-TURP vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
de Sio 2006 Study design: RCT Location: Italy Follow-up (range): 9 (6–18) months |
Inclusion criteria: Qmax < 15 ml/s; IPSS > 18; good performance status; acute urinary retention if the removal of catheter failed after therapy with alpha-blockers or chronic urinary retention unresponsive to medical therapy Exclusion criteria: age ≤ 50 years; documented or suspected prostate cancer; previous prostatic surgery; prostate size < 30 ml; neurogenic bladder, bladder stone or diverticula; urethral stricture; maximal bladder capacity > 500 ml; warfarin therapy Number of patients randomised: 70 |
B-TURP (n = 35) vs TURP (n = 35) |
Mean age ± SD (years): 59 ± 5.9 Mean IPSS score ± SD: 24.18 ± 4 Mean Qmax ± SD (ml/s): 7.1 ± 2 Mean residual volume ± SD (ml): 80 ± 22.5 Mean prostate size ± SD (ml): 51.6 ± 3.9 Mean QoL score ± SD: 4.2 ± 1 Catheter protocol: yes, 22Fr three-way Fufour catheter until the effluent was completely clear Other: Storz 26Fr continuous flow resectoscope; spinal anaesthesia |
Mean age ± SD (years): 61 ± 5.9 Mean IPSS score ± SD: 24.3 ± 5 Mean Qmax ± SD (ml/s): 6.3 ± 3 Mean residual volume ± SD (ml): 75 ± 35.5 Mean prostate size ± SD (ml): 47.5 ± 5.1 Mean QoL score ± SD: 3.9 ± 1 Catheter protocol: yes, 22Fr three-way Fufour catheter until the effluent was completely clear Other: Storz 26Fr continuous flow resectoscope; spinal anaesthesia |
Duration of operation Blood transfusion Catheter duration Clot retention TUR syndrome Stricture Reoperation Symptom score (IPSS) Quality of life Qmax Residual volume |
|
Kim 2006b Study design: RCT Location: Korea Recruitment dates: August 2003–October 2004 Length follow-up: 6 months |
Inclusion criteria: patients suffering from BPE Number of eligible patients: 50 Number of patients randomised: 50 |
B-TURP (n = 25) vs TURP (n = 25) |
Mean age ± SD (years): 68.1 ± 8.9 Mean IPSS score ± SD: 19.0 ± 6 Mean Qmax ± SD (ml/s): 6.5 ± 2.2 Mean prostate size ± SD (ml): 53.2 ± 14.9 |
Mean age ±SD (years): 70.6 ± 7.5 Mean IPSS score ± SD: 18.6 ± 3.3 Mean Qmax ± SD (ml/s): 6.1 ± 1.7 Mean prostate size ± SD (ml): 51.7 ± 19.1 |
Duration of operation Intraoperative complications UTI Catheter duration TUR syndrome Incontinence Stricture Symptom score (IPSS) Length of hospital stay Qmax |
|
Nuhoğlu 2006 Study design: RCT Location: Turkey Recruitment dates: 2001–2003 |
Inclusion criteria: patients with symptoms of the lower urinary system; Qmax < 10 ml/s; IPSS > 15 Exclusion criteria: suspicion of prostate cancer by DRE and PSA examination; previous surgery of the prostate and urethra; neurogenic bladder Number of patients randomised: 57 |
B-TURP (n = 27) vs TURP (n = 30) |
Mean age ± SD (years): 64.6 ± 8.8 Mean IPSS score ± SD: 17.6 ± 6.1 Mean Qmax ± SD (ml/s): 6.9 ± 2.8 Mean urine flow ± SD (ml/s): 2.6 ± 1.3 Mean residual volume ± SD (ml): 96 ± 27 Mean prostate size ± SD (ml): 47 ± 7.7 Catheter protocol: yes, 22Fr three-way removed after macroscopic haematuria disappeared Preprocedural antibiotics: yes Other: general or local anaesthesia; 27Fr Sheet (Gyrus Medical, Bourne End, UK) and plasma electrode |
Mean age ± SD (years): 65.2 ± 9.3 Mean IPSS score ± SD: 17.3 ± 5.8 Mean Qmax ± SD (ml/s): 7.3 ± 2.1 Mean urine flow ± SD (ml/s): 2.8 ± 1.2 Mean residual volume ± SD (ml): 88 ± 20 Mean prostate size ± SD (ml): 49 ± 8.1 Catheter protocol: yes, 22Fr three-way removed after their macroscopic haematuria disappeared Preprocedural antibiotics: yes Other: general or local anaesthesia; 25Fr Sheet Storz resectoscope and glycine solution |
Duration of operation Blood transfusion Urinary retention Catheter duration Recatheterisation TUR syndrome Reoperation rate Symptom score (IPSS) Qmax Mean urine flow rate Residual volume Prostate size |
|
Seckiner 2006 Study design: RCT Location: Turkey Recruitment dates: January 2003–October 2003 Mean follow-up ± SD: 13.9 ± 4.1 months |
Inclusion criteria: Qmax < 15 ml/s; IPSS ≥ 8; prostate volume 50–70 g on TRUS Exclusion criteria: age < 50 years; prostate or bladder cancer; history of prostate surgery; known neurogenic bladder; currently on medication known to affect voiding function Number of patients randomised: 48 |
B-TURP (n = 24) vs TURP (n = 24) |
Mean age ± SD (years): 61.2 ± 9.3 Mean IPSS score ± SD: 24.1 ± 5.2 Mean Qmax ± SD (ml/s): 8.5 ± 2.9 Mean residual volume ± SD (ml): 88 ± 74 Prostate size ± SD (ml): 49.4 ± 18.9 Quality of life score ± SD: 4.4 ± 0.6 Power: 160 W (cutting); 80 W (desiccate mode) Intervention performed by the same surgeon Other: plasmakinetic tissue management system (Gyrus Medical); 27Fr continuous flow resectoscope is used for PL resections; spinal or general anaesthesia |
Mean age ± SD (years): 63.9 ± 10.9 Mean IPSS score ± SD: 23.2 ± 4.9 Mean Qmax ± SD (ml/s): 8.3 ± 3.1 Mean residual volume ± SD (ml): 138 ± 115 Prostate size ± SD (ml): 41.4 ± 14.5 Quality of life score ± SD: 4.7 ± 0.9 Power: 120 W (cutting); 80 W (coagulation) Intervention performed by the same surgeon Other: 26Fr continuous flow resectoscope and Karl Storz 27040 electrodes under spinal or general anaesthesia |
Duration of operation Intraoperative complications Catheter duration Stricture Symptom score (IPSS) Quality of life Qmax Prostate size |
|
Singh 2005 Study design: RCT Location: India Recruitment dates: September 2003–May 2004 Follow-up: 3 months |
Inclusion criteria: men with BPE requiring surgical intervention; Qmax ≤ 12 ml/s; IPSS ≥ 7; Schäfer obstruction grade 2 Exclusion criteria: age ≤ 50 years; PCAR of < 0.75 on TRUS; neurological illness; renal insufficiency; bladder stone, urethral stricture or taking finasteride Number of patients randomised: 60 |
B-TURP (n = 30) vs TURP (n = 30) |
Mean age ± SD (years): 68.9 ± 9.8 Mean IPSS score ± SD: 20.5 ± 4.8 Mean Qmax ± SD (ml/s): 5.8 ± 3.0 Mean residual volume ± SD (ml): 124 ± 58 Quality of life score ± SD (ml): 4.4 ± 1.0 Intervention performed by single experienced surgeon Preprocedural antibiotics: yes Other: 25.6Fr ACMI Elite system continuous flow resectoscope with Vista CTR™ dual-loop electrode and generator (ACMI Corporation, Southborough, MA, USA) |
Mean age ± SD (years): 67.9 ± 9.8 Mean IPSS score ± SD: 21.6 ± 6.3 Mean Qmax ± SD (ml/s): 5.1 ± 2.0 Mean residual volume ± SD (ml): 136 ± 52 Quality of life score ± SD (ml): 4.4 ± 1.0 Intervention performed by single experienced surgeon Catheter protocol: yes, 20Fr three-way removed once urine completely clear for 24 hours and the patient had passed stool; in patients with large prostates (> 40 g of resected tissue) the catheter was removed at 72 hours per protocol Preprocedural antibiotics: yes Other: Wolf 25.5F resectoscope and Force FX™ electrosurgical generator (Valleylab, Boulder, CO, USA) |
Duration of operation Intraoperative complications Catheter duration TUR syndrome Incontinence Stricture Symptom score (IPSS) Length of hospital stay Qmax |
|
Tefekli 2005 Study design: RCT Location: Turkey Recruitment dates: 2001–2002 Mean follow-up ± SD: 18.3 ± 6.7 months |
Inclusion criteria: BPH-related lower UT symptoms Exclusion criteria: history of prostate surgery; abnormal DRE; increased serum PSA; evidence of neurogenic bladder (i.e. history of diabetes, cerebrovascular accident, etc.); urethral stricture disease or bladder stone or tumour Number of patients randomised: 101 |
B-TURP (n = 51) vs TURP (n = 50) |
Mean age ± SD (years): 68.7 ± 6.3 Mean IPSS score ± SD: 21.3 ± 3.2 Mean Qmax ± SD (ml/s): 7.8 ± 3.7 Prostate size ± SD (g): 50.1 ± 17.3 Frequency: 320–450 kHz Power: 200 W (maximum) Intervention performed by consultant urologists with equivalent experience Catheter protocol: yes, 22Fr three-way Foley catheter 12–24 hours after the urine became clear Other: especially designed Plasmakinetic® 27Fr continuous flow resectoscope (Gyrus ACMI, Southborough, MA, USA); spinal anaesthesia or general anaesthesia depending on patient cardiovascular status |
Mean age ± SD (years): 69.4 ± 5.9 Mean IPSS score ± SD: 20.4 ± 3.5 Mean Qmax ± SD (ml/s): 8.3 ± 3.6 Prostate size ± SD (g): 54.0 ± 15.2 Power: 80–100 W (cutting); 50–70 W (coagulation) Intervention performed by consultant urologists with equivalent experience Catheter protocol: yes, 22Fr three-way Foley catheter 12–24 hours after the urine became clear Other: 26Fr continuous flow resectoscope and standard loop electrode Martine ME 401 electrosurgical generator Gebruder Martin, Germany); spinal anaesthesia or general anaesthesia depending on patient cardiovascular status |
Duration of operation Blood transfusion Retention Catheter duration Recatheterisation Stricture Incontinence Retrograde ejaculation Reoperation Symptom score (IPSS) Qmax |
| B-TUVRP vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Fung 2005 Study design: RCT Location: China (Hong Kong) Recruitment dates: August 2001–January 2002 Follow-up: 3 months |
Inclusion criteria: men admitted from the waiting list for surgery for BPH; Qmax < 10 ml/s; IPSS > 20; acute retention of urine and failure to remove catheter; chronic retention of urine due to obstruction causing renal impairment and severe lower UT symptoms Exclusion criteria: known/suspected prostate cancer; previous prostatic surgery; known neurogenic bladder, urethral stricture, bladder stone or warfarin therapy Number of patients eligible: 60 Number of patients randomised: 51 |
B-TUVRP (n = 21) TURP (n = 30) |
Mean age (range) (years): 72.5 (59–91) Mean IPSS score: 15.82 Mean QoL: 3.55 Power: 60 W (coagulation); 240 W (vaporisation) Intervention performed by a consultant, senior medical officer or senior registrar experienced in performing TURP Catheter protocol: yes, 22Fr three-way Foley catheter was inserted and removed in the morning Other: Gyrus plasmakinetic 27Fr resectoscope with a plasmakinetic loop electrode; spinal anaesthesia and the surgical technique was similar |
Mean age (range) (years): 73 (59–88) Mean IPSS score: 19.36 Mean QoL score: 3.64 Power: 60 W (coagulation); 120 W (cutting) Intervention performed by a consultant, senior medical officer or senior registrar experienced in performing TURP Catheter protocol: yes, 22Fr three-way Foley catheter was inserted and removed in the morning Other: Wolf 27Fr continuous flow resectoscope with loop electrode; spinal anaesthesia and the surgical technique was similar |
UTI Retention Catheter duration Clot retention TUR syndrome Symptom score (IPSS) Quality of life Qmax |
| TUVP vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Çetinkaya 1996 Study design: RCT Location: Turkey Recruitment dates: September–December 1995 Length follow-up: 3 months |
Inclusion criteria: peak urine flow rate < 15 ml/s; AUA moderate to severe Exclusion criteria: patients who had previously undergone a prostate operation or who had any abnormality of kidney and liver function, urethral strictures, neurogenic deficits or bladder stones or those with confirmed or suspected prostate cancer Number of patients randomised: 46 |
TUVP (n = 23) vs TURP (n = 23) |
Mean age ± SD (years): 68.4 ± 8.3 Mean prostate size ± SD (ml): 48.4 ± 9.7 (TRUS) Catheter protocol: yes Power: cutting mode: 240–300 W; coagulation mode: 40–70 W |
Mean age ± SD (years): 62.5 ± 10.1 Mean prostate size ± SD (ml): 48.8 ± 15.4 (TRUS) Catheter protocol: yes Other: conducted by conventional electroresection |
Duration of operation Blood transfusion Catheter duration Symptom score (AUA) Qmax Residual volume |
|
Ekengren 2000 Study design: RCT Location: Sweden Length follow-up: 12 months |
Inclusion criteria: peak urine flow rate < 15 ml/s; AUA moderate to severe Exclusion criteria: patients who had previously undergone a prostate operation or who had any abnormality of kidney and liver function, urethral strictures, neurogenic deficits or bladder stones or those with confirmed or suspected prostate cancer Number of patients randomised: 54 |
TUVP (n = 26) vs TURP (n = 28) |
Mean age (range) (years): 71 (49–82) Median IPSS (range): 22 (1–100) Median urine flow rate (range) (ml/s): 2 (0–10) (or peak) Median QoL (range): 4.5 (2–6) Intervention performed by consultant |
Mean age (range) (years): 70 (48–83) Median IPSS (range): 25 (13–100) Median urine flow rate (range) (ml/s): 4 (0–8) (or peak) Median QoL (range): 5.5 (3–6) Intervention performed by consultant |
Duration of operation Symptom score (IPSS) Change in urine flow Quality of life Mortality Reoperation Stricture |
|
Erdaği 1999 Study design: RCT Location: Turkey Length follow-up: up to 6 months |
Inclusion criteria: symptomatic BPH Exclusion criteria: patients who had previously undergone a prostate operation; urethral strictures; neurogenic bladder; those with confirmed or suspected prostate cancer Number of patients randomised: 40 |
TUVP (n = 20) vs TURP (n = 20) |
Mean age (range) (years): 64.2 (56–82) Mean prostate size (range) (ml): 32.5 (20–48) (TRUS) Mean IPSS (range): 20.6 (12–27) Mean Qmax (range): 5.1 (0–11.27) Mean urine flow (range) (ml/s): 2.5 (0–5.3) Mean residual volume (range) (ml): 68 (20–150) Power: cutting 240 W; coagulation 40 W Other: anaesthesia: general anaesthesia, 6/20; spinal or epidural, 14/20; VaporTrode® rollerball Storz electrode. Middle lobe vaporised from bladder neck to verumontanum; lateral lobe vaporised to prostatic capsule |
Mean age (range) (years): 66.1 (58–75) Mean prostate size (range): 37 (15–60) (TRUS) Mean IPSS (IPSS): 21.5 (11–30) Mean Qmax (range): 4.6 (0–9.6) Mean urine flow rate (range) (ml/s): 2.3 (0–5.5) Mean residual volume (range) (ml): 122.8 (0–600) Other: anaesthesia: general anaesthesia, 10/20; spinal or epidural, 10/20; 26Fr continuous flow resectoscope with mannitol irrigation |
Duration of operation Blood transfusion Intraoperative complications Catheter duration Symptom score (IPSS) Qmax Residual volume Stricture Retrograde ejaculation |
|
Fowler 2005 Study design: RCT Location: UK Recruitment dates: March 1997–March 1999 Length follow-up: 2 years Links with: McAllister 2003 |
Inclusion criteria: candidate for surgical treatment for BOO; must have completed pretreatment evaluation with current criteria for prostate surgery; able to give written informed consent to randomisation and treatment Exclusion criteria: previous bladder outlet surgery; clinical evidence of prostate cancer; physical status > ASA class 3; medications that (in investigators opinion) would preclude entry into trial; clinically significant acute illness; known disease of central or peripheral nervous system; prostate cancer Number of eligible patients: 445 Number of patients randomised: 235 |
TUVP (n = 115) TURP (n = 120) Additional information: irrigation fluid varied with different centres Conventional Circon-ACMI 24.5 Fr continuous–flow resectoscope with new wire loop for each patient Catheter protocol: three-way catheter with irrigation or forced diuresis with frusemide Antibiotics: at surgeon’s request |
Mean age (years): 70.2 Mean prostate size (ml): 54.3 (n = 103) (TRUS) Mean IPSS ± SD: 20.7 ± 7.3 Qmax ± SD: 10.10 ± 4.35 Residual volume (ml): 181 (n = 94) Mean QoL score ± SD (95% CI): 4.9 ± 0.98 (4.7–5) Normal sexual function: 75/109 Power: cutting current: 180 W; coagulation: 55 W Other: Valleylab Force FX electrosurgical generator; erectile dysfunction: 70/103 |
Mean age (years): 69.7 Mean prostate size (ml): 51.1 (n = 100) (TRUS) Mean IPSS ± SD: 20.7 ± 6.9 Mean Qmax ± SD: 10.52 ± 5.04 Residual volume (ml): 171 (n = 91) Mean QoL score ± SD (95% CI): 4.6 ± 1.17 (4.4–4.8) Normal sexual function: 62/110 Power: cutting current: 120–140 W; coagulation: 55 W Other: conventional TURP manner; erectile dysfunction: 65/101 |
Symptom score (IPSS) Quality of life Qmax Sexual function Catheter duration Intraoperative complications Cardiovascular events Length of hospital stay |
|
Galluci 19981 Study design: RCT Location: Italy Length follow-up: 1 year Links with: Puppo 1996 (abstract) |
Inclusion criteria: diagnosis of symptomatic BPE with urodynamically assessed obstruction Exclusion criteria: prostate cancer or suspected prostate cancer; prostate size > 70 g; complete urinary retention; bladder calculi; neurogenic bladder; bladder cancer; mental or psychological illness Number of patients randomised: 150 |
TUVP (n = 70) vs TURP (n = 80) Additional information: the operations were carried out by video endoscopy; Before the actual operation a urethrocystoscopy was carried out |
Mean prostate size ± SE: 36.59 ± 1.37 (TRUS) Mean IPSS ± SE: 18.19 ± 0.66 Mean Qmax ± SE: 8.78 ± 1.16 Mean residual volume ± SE: 64.61 ± 8.65 Catheter protocol: 22Fr three-way Foley catheter Other: standard 22.5Fr resectoscope with a standard diathermic loop |
Mean prostate size ± SE: 36.61 ± 1.52 (TRUS) Mean IPSS ± SE: 18.84 ± 0.68 Mean Qmax ± SE: 7.26 ± 0.37 Mean residual volume ± SE: 84.7 ± 11.39 Power: 200–250 W Catheter protocol: 22Fr three-way Foley catheter Other: standard 22.5Fr resectoscope with a VaporTrode electrode (Circon ACMI) |
Intraoperative complications Blood transfusion UTI Urinary retention Catheter duration Urinary incontinence Length of hospital stay Symptom score (IPSS) Qmax Residual volume |
|
Gotoh 1999 Study design: RCT Location: Japan Length follow-up: 3 months |
Inclusion criteria: Qmax ≤ 15 ml/s; IPSS ≥ 10; normal specific antigen Exclusion criteria: prostate size < 30 ml Number of eligible patients: 53 Number of patients randomised: 51 |
TUVP (n = 23) vs TURP (n = 28) |
Mean age ± SD (years): 69.7 ± 6.3 Mean IPSS score ± SD (range): 19.6 ± 7.5 Mean Qmax ± SD (ml/s): 7.3 ± 2.8 Mean residual volume ± SD (ml): 56.7 ± 51.4 Mean prostate size ± SD (ml): 47.8 ± 16.4 Power: cutting current: 230–250 W Intervention performed by one urologist with ≥ 10 years experience at each of the seven hospitals Other: epidural anaesthesia |
Mean age ± SD (years): 66.5 ± 15.7 Mean IPSS score ± SD: 18.9 ± 7.3 Mean Qmax ± SD (ml/s): 9.4 ± 2.8 Mean residual volume ± SD (ml): 41.9 ± 25.5 Mean prostate size ± SD (ml): 41.9 ± 25.5 Power: cutting current: 120 W Intervention performed by one urologist with ≥ 10 years experience at each of the seven hospitals Other: number of catheterised patients (%): 16/50 (32) |
Duration of operation Blood transfusion Catheter duration Recatheterisation TUR syndrome Stricture IPSS Qmax Residual volume |
|
Hammadeh 2003 Study design: RCT Location: UK Recruitment dates: June 1995–January 1996 Length follow-up: 5 years Links with: Hammadeh 1998b; Hammadeh 1998a; Hammadeh 2000; and Hammadeh 1998c,1999,2003 (abstracts) |
Inclusion criteria: men with bladder outflow obstruction due to BPH and on the waiting list expecting TURP; peak urine flow rate < 15 ml/s; IPSS ≥ 13; QoL index ≥ 3 Exclusion criteria: prostate cancer; previous prostatic or urethral surgery; neurogenic bladder; patients who were appropriate for bladder neck incision Number of eligible patients: 109 Number of patients randomised: 104 |
TUVP (n = 52) vs TURP (n = 52) Additional information: none of the patients included was on retention |
Mean age ± SD (range) (years): 67.5 ± 6.7 (52–82) Mean prostate size ± SD (range): 32 ± 9.1 (15–60) (by TRUS) Mean IPSS ± SD: 26.5 ± 4.5 Prostate > 50 g: 4 Mean Qmax ± SD: 8.9 ± 3.2 Mean residual volume ± SD (ml): 131 ± 78.5 Power: cutting current: 240 W; coagulation: 60 W Intervention performed by consultants (44%); registrars (56%) Catheter protocol: 22Fr three-way Foley catheter, until urine became clear Other: used VaporTrode resectoscope |
Mean age ± SD (range) (years): 70.2 ± 7.2 (52–87) Mean prostate size ± SD (range): 27 ± 12.2 (10–60) (by TRUS) Prostate > 50 g: 4 Mean IPSS ± SD: 26.6 ± 4.8 Mean Qmax ± SD: 8.6 ± 3.2 Mean residual volume ± SD (ml): 101 ± 87.9 Power: cutting current: 145 W; coagulation: 60 W Intervention performed by consultants (46%); registrars (54%) Catheter protocol: 22Fr three-way Foley catheter, until urine became clear Other: standard 27Fr resectoscope with a Force 2 electrical current generator |
Duration of operation Blood transfusion UTI Urinary retention Catheter duration Clot retention TUR syndrome Cardiovascular events Mortality Stricture Incontinence Retrograde ejaculation Reoperation Symptom score (IPSS) Quality of life Length of hospital stay Qmax Residual volume |
|
Kaplan 1998 Study design: RCT Location: USA Mean length of follow-up 10.4 months |
Inclusion criteria: peak urine flow rate ≤ 15 ml/s; AUA score ≥ 10; prostate volume 15–60 g on TRUS Exclusion criteria: age < 50 years; known neurogenic bladder, cancer of the prostate or bladder or history of prostate surgery; currently on medications known to affect voiding function Number of patients randomised: 64 |
TUVP (n = 32) vs TURP (n = 32) |
Mean age ± SD (years): 68.9 ± 8.7 Mean IPSS ± SD: 19.4 ± 3.5 Mean Qmax ± SD (ml/s): 7.2 ± 2.8 Mean residual volume ± SD (ml): 77.8 ± 20.3 Mean prostate size ± SD (ml): 47.8 ± 22.3 Power: cutting current: 240–270 W Other: fluted roller electrode. General anaesthesia: 2/32 (6%); spinal/epidural anaesthesia: 28/32 (88%); intravenous sedation: 2/32 (6%) |
Mean age ± SD (years): 72.8 ± 6.9 Mean IPSS ± SD: 18.3 ± 4.7 Mean Qmax ± SD (ml/s): 8.3 ± 3.6 Mean residual volume ± SD (ml): 66.9 ± 15.7 Mean prostate size ± SD (ml): 41.5 ± 19.7 Power: cutting current: 25–45% lower than TUVP Other: loop electrode. General anaesthesia: 6/32 (18%); spinal epidural anaesthesia: 26/32 (82%) |
Duration of operation Blood transfusion UTI Catheter duration Recatheterisation Clot retention TUR syndrome Incontinence Irritative urinary symptoms Stricture Retrograde ejaculation Erectile dysfunction Symptom score (AUA) Length of hospital stay Qmax Total voided volume |
|
Küpeli 1998a Study design: RCT Location: Turkey Recruitment dates: July–October 1995 Mean length follow-up: 4.2 months |
Inclusion criteria: patients with symptomatic BPH; peak urine flow rate < 15 ml/s; IPPS > 8 Exclusion criteria: prostate cancer; previous treatment for prostate; neurogenic bladder Number of patients randomised: 60 |
TUVP (n = 30) vs TURP (n = 30) |
Mean age ± SD (years): 62.4 ± 3.2 Mean IPSS: 19.4 Mean Qmax ± SD (ml/s): 7.9 ± 2.1 Prostate size ± SD (g): 48.9 ± 8.7 (TRUS) Power: cutting current: 250–300 W Catheter protocol: unclear Other: 24Fr resectoscope, Storz Spike 24Fr; anaesthesia: epidural, 14 |
Mean age ± SD (years): 59.8 ± 2.6 Mean IPSS: 21.6 Mean Qmax ± SD (ml/s): 9.2 ± 2.6 Prostate size ± SD (g): 51.7 ± 9.1 (TRUS) Power: cutting current: 180–120 W Catheter protocol: unclear Other: anaesthesia: general, 8; spinal, 18; epidural, 4 |
Duration of operation Blood transfusion Recatheterisation Clot retention Urinary retention Symptom score (IPSS) Qmax Prostate size Erectile dysfunction Retrograde ejaculation |
|
Küpeli 1998b Study design: RCT Location: Turkey Recruitment dates: July–October 1995 Length follow-up: 12 months |
Inclusion criteria: patients with symptomatic BPH; peak urine flow rate < 15 ml/s; AUA score ≥ 7 Exclusion criteria: prostate cancer; prostate size > 60 g from TRUS Number of patients randomised: 66 |
TUVP (n = 30) vs TURP (n = 36) |
Mean age (range) (years): 65.7 (52–72) Mean AUA (range): 13.7 (7–29) Mean Qmax (range) (ml/s): 8.3 (2.7–11.8) Prostate size ± SD (g): 41.46 ± 10.7 (TRUS) Power: cutting current: 180–250 W (average: 220 W) Visible sites of bleeding coagulation: 60 W (range: 40–70 W) Other: 24Fr resectoscope |
Mean age (range) (years): 62.4 (56–70) Mean AUA (range): 14.6 (8–32) Mean Qmax (range) (ml/s): 8.8 (3–12.4) Prostate size ± SD (g): 43.57 ± 12.01 (TRUS) Other: 24Fr resectoscope |
Duration of operation Intraoperative complications Blood transfusion UTI Urinary retention Catheter duration Irritative urinary symptoms Stricture Incontinence Reoperation Symptom score (AUA) Length of hospital stay Qmax Recatheterisation |
|
Nathan 1996 Study design: RCT Location: UK Length of follow-up: 12 weeks |
Inclusion criteria: men requiring TURP Exclusion criteria: previous prostate operation; indwelling catheter; on anticoagulant therapy Number of patients randomised: 40 |
TUVP (n = 20) vs TURP (n = 20) |
Mean age (range) (years): 65.4 (57–77) Mean IPSS ± SD: 21.9 ± 4.2 (13–27) Mean Qmax ± SD: 10.2 ± 4.4 (3.1–21.8) Mean urine flow ± SD (ml): 5.5 ± 2.5 (1.2–10) Mean residual volume (ml): 132 (0–300) Mean prostate size ± SD (g): 53.5 ± 28 (30–130) (TRUS) Mean QoL ± SD (IPSS): 4.0 ± 0.7 (3–6) Power: cutting: 200 W; coagulation: 40 W Duration of procedure: 39.2 minutes Catheter protocol: three-way 20Fr Porges catheter at the end of the procedure Other: 25Ch ACMI resectoscope and the VaporTrode™ (Gyrus ACMI, Southborough, MA, USA) |
Mean age (range) (years): 69.2 (57–81) Mean IPSS ± SD: 17 ± 4.3 (9–24) Mean Qmax ± SD: 7.2 ± 3.5 (2.5–15) Mean urine flow ± SD (ml): 3.5 ± 1.2 (1.5–7.1) Mean residual volume (ml): 120 (0–380) Mean prostate size ± SD (g): 53.4 ± 21 (17–91) (TRUS) Mean QoL ± SD (IPSS): 4.9 ± 0.7 (4–6) Power: cutting: 120 W; coagulation: 60 W Duration of procedure: 37.4 minutes Catheter protocol: three-way 20Fr Porges catheter at the end of the procedure Other: JEAW using a 24Ch continuous resectoscope and its loop |
Duration of operation Blood transfusion UTI Urinary retention Catheter duration Clot retention TUR syndrome Reoperation Symptom score (IPSS) Quality of life score Length of hospital stay Qmax Mean urine flow Residual volume |
|
Netto 1999 Study design: RCT Location: Brazil Mean length follow-up (range): 17 (11–23) months |
Inclusion criteria: patients with more than 1 year symptomatic and uncomplicated BPH; peak urine flow rate < 15 ml/s; residual urine volume < 250 ml; voided volume ≥ 250 ml; IPSS score > 12; prostate volume between 25 and 90 ml Exclusion criteria: history or evidence of prostate cancer; patients who have been exposed to drugs such as alpha-antagonists, anticholinergics, cholinergics, diuretics, estrogens, androgens, antihypertensive medications or other agents within the previous 2 weeks; pelvic irradiation, urethral stricture or surgery for BPH or evidence of UT stone disease; neurogenic bladder dysfunction; hydronephrosis or a UTI within 3 months before the study Number of patients randomised: 78 |
TUVP (n = 40) vs TURP (n = 38) |
Mean age (range) (years): 66.8 (52–80) Mean IPSS ± SD: 19.65 ± 6.14 Mean Qmax ± SD (ml/s): 7.88 ± 2.51 Mean residual volume ± SD (ml): 73.0 ± 5.81 Mean prostate size ± SD (ml): 46.88 ± 17.1 Power: cutting current: 250–300 W Catheter protocol: 22Fr Foley catheter Other: spinal anaesthesia; the cautery mode was not used for haemostasis in this group; all received antibiotics for a week after the procedure; resection loop was used for one to three patients (mean 2.3) |
Mean age (range) (years): 65 (51–82) Mean IPSS ± SD: 24.29 ± 6.48 Mean Qmax ± SD (ml/s): 6.77 ± 3.08 Mean residual volume ± SD (ml): 88.64 ± 8.43 Mean prostate size ± SD (ml): 44.68 ± 8.50 (by TRUS) Power: cutting current: 50–80 W Haemostasis: 50 W Catheter protocol: 22Fr Foley catheter Other: spinal anaesthesia; all received antibiotics for a week after the procedure; resection loop was used for one to five patients (mean 3.2) |
Catheter duration TUR syndrome Irritative urinary symptoms Retention Stricture Retrograde ejaculation Erectile dysfunction Symptom score (IPSS) Length of hospital stay Qmax Residual volume |
|
Nuhoğlu 2005 Study design: RCT Location: US Recruitment dates: 1996–2003 Length follow-up: 5.6 years |
Inclusion criteria: BPH with LUT symptoms; Qmax < 10 ml/s; IPSS > 15 Exclusion criteria: prostate cancer or suspected; history of prostate or urethral surgery; neurogenic bladder Number of patients randomised: 77 |
TUVP (n = 37) vs TURP (n = 40) Additional information: balloon inflated with 35 ml normal saline. No difference between groups |
Mean age ± SD (years): 64.5 ± 8.7 Mean IPSS ± SD: 17.6 ± 7.2 Mean Qmax ± SD (ml): 56.3 ± 2.1 Mean urine flow ± SD (ml): 2.6 ± 1.2 Mean residual volume ± SD (ml): 88 ± 20 Mean prostate size ± SD (ml): 39 ± 8.1 (TRUS) Power: cutting current: 250 W; coagulation current: 100 W Catheter protocol: three-way catheter without traction inserted at end of procedure Other: Spike loop (Storz) electrode; antibiotics according to surgeon’s normal practice; all patients discharged after urethral catheter removed and first micturition observed |
Mean age ± SD (years): 65.1 ± 9.4 Mean IPSS ± SD: 17.3 ± 6.8 Mean Qmax ± SD (ml): 5.9 ± 2.6 Mean urine flow ± SD (ml): 2.4 ± 1.3 Mean residual volume ± SD (ml): 95 ± 26 Mean prostate size ± SD (ml): 39 ± 7.7 (TRUS) Catheter protocol: three-way catheter without traction inserted at end of procedure Other: standard 24Fr Storz resectoscope, antibiotics according to surgeon’s normal practice; all patients discharged after urethral catheter removed and first micturition observed |
Symptom score (IPSS) Qmax Mean urine flow Residual volume Prostate size Blood transfusion Catheter duration Retention Retrograde ejaculation Erectile dysfunction Reoperation UTI |
|
Patel 1997 Study design: RCT Location: USA Mean length follow-up: 6 months: |
Inclusion criteria: acute urinary retention; moderate to severe symptoms of bladder outlet obstruction Exclusion criteria: UTI; co-existent neurological cause of voiding dysfunction Number of patients randomised: 12 |
TUVP ( 6) vs TURP ( 6) |
Mean age (range) (years): 67 (60–85) Mean IPSS (range): 29.6 (28–31) Mean Qmax (range) (ml/s): 10 (7.3–13.1) Mean prostate volume (range) (ml): 54 (25–90) Frequency: 10 MHz Power: cutting current: 150 W; coagulation current: 40 W |
Mean age (range) (years): 65.8 (59–71) Mean IPSS (range): 23.3 (17–29) Mean Qmax (range) (ml/s): 7.5 (5.1–11) Mean prostate volume (range) (ml): 64.6 (31.5–119) Frequency: 10 MHz Power: cutting current: 150 W; coagulation current: 40 W |
Duration of operation Catheter duration Erectile dysfunction Symptom score (IPSS) Qmax |
|
Shokeir 1997 Study design: RCT Location: Saudi Arabia Recruitment dates: February 1996–February 1998 Length follow-up: 24 months |
Inclusion criteria: peak urine flow rate < 12 ml/s; AUA score > 15; prostate size < 60 g measured by TRUS Exclusion criteria: previous prostate surgery Number of patients randomised: 70 |
TUVP ( 35) vs TURP ( 35) Additional information: TUVP: mean follow-up ± SD (range): 14.5 ± 1.8 (12–17) months; TURP: mean follow-up ± SD (range): 14.3 ± 2.1 (12–17) months |
Mean age ± SD (range) (years): 68.4 ± 9.5 (54–85) Mean AUA ± SD (range): 26.3 ± 5.2 (16–29) Mean Qmax ± SD (ml/s): 7.8 ± 2.1 (4.1–11.4) Mean residual volume ± SD (range) (ml): 75.2 ± 21.2 (40–120) Mean prostate size ± SD (range) (g): 44.6 ± 10.1 (30–60) Power: cutting current: 240 W (200–300); coagulation current: 70 W (50–80) Other: spinal/epidural anaesthesia, 28; general anaesthesia, 7 |
Mean age ± SD (range) (years): 68.4 ± 9.6 (51–86) Mean AUA ± SD (range): 25.1 ± 5.5 (18–30) Mean Qmax ± SD (ml/s): 6.9 ± 1.7 (3.4–10) Mean residual volume ± SD (range) (ml): 77.1 ± 20.3 (46–110) Mean prostate size ± SD (range) (g): 48.8 ± 10.6 (28–60) Catheter protocol: 22Fr three-way catheter Other: spinal/epidural anaesthesia, 30; general anaesthesia, 5 |
Duration of operation Catheter duration Recatheterisation Irritative urinary symptoms Symptom score (AUA) Length of hospital stay Qmax Residual volume |
|
van Melick 2003 Study design: RCT Location: Netherlands Recruitment dates: 1996–2001 Mean length follow-up: up to 7 years Links with: van Melick 2002; van Melick 2003 |
Inclusion criteria: patients with lower UT symptoms suggestive of BPH; met ISC criteria for BPH; Schafer obstruction score ≥ 2; prostate size between 20 and 65 ml Exclusion criteria: age ≤ 45 years Number of patients randomised: 96 |
TURP (n = 50) vs TUVP (n = 46) |
Mean age ± SD (years): 64 ± 10 Mean IPSS ± SD: 20.2 ± 6.6 Mean Qmax ± SD (ml/s): 11 ± 4 Mean residual volume ± SD: 290 ± 145 Mean prostate size ± SD (ml): 35 ± 11 Quality of life score (IPSS) ± SD: 4.1 ± 1.4 Detrusor pressure ± SD (cmH2O): 75 ± 26 Intervention performed by alternate experienced urologist and trainees Catheter protocol: 20Fr transurethral catheter Preprocedural antibiotics: type IV perioperatively Other: VaporTrode element (Circon ACMI) |
Mean age ± SD (years): 66 ± 8 Mean IPSS ± SD: 11 ± 4 Mean Qmax ± SD (ml/s): 10.8 ± 4.76 Mean residual volume ± SD: 350 ± 140 Mean prostate size ± SD (ml): 37 ± 11 Quality of life score (IPSS) ± SD: 3.8 ± 1.5 Detrusor pressure ± SD (cmH2O): 76 ± 27 Intervention performed by alternate experienced urologist and trainees Catheter protocol: suprapubic if required Preprocedural antibiotics: type IV perioperatively Other: standard 24Fr resectoscope |
Duration of operation Intraoperative complications Blood transfusion UTI Catheter duration Clot retention Cardiovascular events Mortality Incontinence Stricture Reoperation Retention Symptom score (AUA) Quality of life score Qmax Residual volume |
|
Wang 2002 Study design: RCT Location: China Length follow-up: 24 months |
Exclusion criteria: prostate cancer: suspicious, confirmed or investigated with biopsy; neurogenic bladder; urethral stricture Number of patients randomised: 206 |
TUVP (n = 97) vs TURP (n = 109) |
Mean age (range) (years): 72 (62–85) Mean IPSS (range): 20 (8–30) Mean Qmax (range) (ml/s): 7 (2–13) Mean residual volume (range) (ml): 120 (60–400) Power: 240–260 W Other: epidural anaesthesia/general anaesthesia |
Mean age (range) (years): 71 (61–84) Mean IPSS (range): 20 (9–31) Mean Qmax (range) (ml/s): 7 (3–12) Mean residual volume (range) (ml): 1231 (60–380) Power: 100–140 W Other: spinal/epidural anaesthesia |
Duration of operation Stricture Symptom score (IPSS) Qmax Residual volume TUR syndrome Mortality |
| B-TUVP vs TURP | |||||
| Study details | Participant characteristics | Intervention/comparator | Intervention population characteristics | Comparator population characteristics | Outcomes |
|
Dunsmuir 2003 Study design: RCT Location: Australia Follow-up range: 1 year Links with: Love 2003 (abstract) |
Inclusion criteria: patients presenting to the outpatient clinic with lower UT symptoms, secondary to BPH and considered to be appropriate for TURP Exclusion criteria: age > 80 years; suspicion of prostate cancer (men with PSA > 4 ng/ml unless biopsies were negative); previous prostate surgery; acute urinary retention; prostate > 80 ml; on anticoagulant therapy Number of patients randomised: 40 |
B-TUVP (n = 20) vs TURP (n = 20) |
Mean age ± SD (range) (years): 63 ± 7.1 (59–79) Mean IPSS score ± SD (range): 24 ± 6.9 (9–30) Mean Qmax ± SD (range) (ml/s): 9.6 ± 3 (8–14) Mean residual volume ± SD (range) (ml): 112 ± 13.3 (42–188) Mean prostate size ± SD (range) (ml): 39 ± 19 (16–56) Mean QoL (AUA) score ± SD (range): 12 ± 3.4 (9–18) Catheter protocol: yes, 22Fr three-way removed after macroscopic haematuria disappeared |
Mean age ± SD (range) (years): 60 ± 6.5 (60–78) Mean IPSS score ± SD (range): 17 ± 6.2 (10–29) Mean Qmax ± SD (range) (ml/s): 10.4 ± 3.1 (7–14) Mean residual volume ± SD (range) (ml): 96 ± 11.4 (40–167) Mean prostate size ± SD (range) (ml): 42 ± 21 (22–60) Mean QoL (AUA) score ± SD (range): 11 ± 3.2 (7–17) Catheter protocol: yes, 22Fr three-way removed after macroscopic haematuria disappeared |
|
|
Hon 2006 Study design: RCT Location: UK Follow-up range: 9 months |
Inclusion criteria: men with BOO undergoing elective transurethral prostatectomy Exclusion criteria: prostate size > 80 ml by TRUS; confirmed or suspected prostate cancer; previous myocardial infarction within the 6 months preceding surgery; previous TURP; serum creatine > 200 mmol/l Number of patients randomised: 160 |
B-TUVP (n = 81) vs TURP (n = 79) |
Mean age ± SD (years): 66.1 ± 8.5 Mean IPSS score ± SD: 21.3 ± 6.2 Mean Qmax ± SD (ml/s): 12.0 ± 6.4 Mean urine flow rate ± SD (ml/s): 5.9 ± 3.3 Mean residual volume ± SD (ml): 147 ± 156 Mean prostate size ± SD (ml): 38 ± 17.5 Mean QoL score ± SD: 4.2 ± 1.1 Power: 160 W cutting; 80 W coagulation Intervention performed by consultant in 69% of cases |
Mean age ± SD (years): 68.1 ± 7.5 Mean IPSS score ± SD: 20.6 ± 7.0 Mean Qmax ± SD (ml/s): 11.9 ± 6.0 Mean urine flow rate ± SD (ml/s): 6.1 ± 2.9 Mean residual volume ± SD (ml): 182 ± 180 Mean prostate size ± SD (ml): 40 ± 17.1 Mean QoL score ± SD: 4.3 ± 1.3 Intervention performed by consultant in 57% of cases |
Duration of operation Intraoperative complications Blood transfusion Stricture Reoperation Symptom score (IPSS) Quality of life Length of hospital stay Rehospitalisation Qmax Mean urine flow rate Residual volume |
Appendix 8 Data tables
Abbreviations used throughout this appendix are as follows: B-TURP, bipolar transurethral resection of the prostate; B-TUVP, bipolar transurethral vaporisation of the prostate; B-TUVRP, bipolar transurethral vaporection of the prostate; TEAP, transurethral ethanol ablation of the prostate; TUIP, transurethral incision of the prostate; TUMT, transurethral microwave thermotherapy; TUNA, transurethral needle ablation; TUR, transurethral resection; TURP, transurethral resection of the prostate; TUVP, transurethral electrovaporisation of the prostate; TUVRP, transurethral vaporesection of the prostate.
Appendix 8.1 TUMT versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | 24 months | 3 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUMT | TURP | TUMT | p-value | TURP | TUMT | p-value | TURP | TUMT | p-value | TURP | TUMT | p-value | TURP | TUMT | p-value | |
| Ahmed 1997 (AUA) |
n = 30 18.4 (16.7–20.1)a |
n = 30 18.5 (17.1–20.1)a |
n = 30 5.2 (3.9–6.5)a p < 0.001 |
n = 30 5.3 (3.9–6.4)a p = 0.001 |
|||||||||||||
| Dahlstrand 1993 (Madsen) |
n = 39 13.3, SD 4.2 (8–22) |
n = 39 11.2, SD 3.1 (8–18) |
n = 39 1.6, SD 2.5 (0–11) |
n = 37 2.3, SD 2.7 (0–13) |
NS |
n = 23 0.9, SD 1.6 (0–6) |
n = 28 3.1, SD 3.0 (0–11) |
n = 22 0.9, SD 2.2 (0–9) |
n = 25 2.7, SD 2.9 (0–10) |
< 0.05 | |||||||
| Dahlstrand 1995 (Madsen) |
n = 32 13.6, SD 3.9 (12.1–15)a |
n = 37 12.1, SD 3.0 (11.1–13.1)a |
n = 32 1.7, SD 2.6 (0.7–2.6)a p < 0.01 |
n = 36 2.9, SD 3.0 (1.9–3.9)a p < 0.01 |
n = 32 1.1, SD 1.8 (0.4–1.7)a p < 0.01 |
n = 37 2.6, SD 2.6 (1.8–3.5)a p < 0.01 |
n = 31 0.6, SD 1.4 (0.1–1.1)a p < 0.01 |
n = 33 2.2, SD 2.4 (1.3–3.0)a p < 0.01 |
n = 30 1.2, SD 1.9 (0.4–1.9)a p < 0.01 |
n = 31 2.3, SD 3.0 (1.2–3.4)a p < 0.01 |
|||||||
| d’Ancona 1998 (Madsen) |
n = 21 13.8, SD 4.2 |
n = 31 13.3, SD 4.2 |
n = 21 3.6, SD 3.2 |
n = 31 5.2, SD 4.1 |
n = 20 2.5, SD 2.3 |
n = 28 4.4, SD 4.4 |
n = 17 2.7, SD 4.0 |
n = 27 4.2, SD 4.6 |
n = 12b 3.6, SD 3.1 |
n = 17b 5.8, SD 3.8 |
> 0.05 | ||||||
| d’Ancona 1998 (IPSS) |
n = 21 16.7, SD 5.6 |
n = 31 18.3, SD 6.3 |
n = 21 5.1, SD 3.1 |
n = 31 15.1, SD 8.2 |
n = 20 4.0, SD 2.1 |
n = 38 6.7, SD 5.5 |
n = 17 3.4, SD 2.2 |
n = 27 5.0, SD 2.7 |
n = 12b 6.3, SD 4.8 |
n = 17b 7.9, SD 6.3 |
> 0 0.05 |
||||||
| de la Rosette 2003; in Francisca 2000 (Madsen) |
n = 70 15.1, SD 4.1 (8–24) |
n = 69 14.9, SD 4.0(8–22) |
n = 48 3.5, SD 3.7 |
n = 54 6.4, SD 5.7 |
< 0.01 |
n = 35 2.1, SD 2.1 |
n = 38 5.5, SD 4.6 |
< 0.01 | |||||||||
| de la Rosette 2003 (IPSS) |
n = 68 20.8, SD 6.2 (13–29) |
n = 68 20.1, SD 6.5 (10–27) |
n = 55 5.3, SD 5.2 |
n = 57 10.5, SD 7.9 |
n = 48 3.2, SD 3.0 |
n = 58 8.1, SD 6.0 |
n = 38 3.7, SD 4.9 |
n = 46 9.3 SD 7.3 |
n = 33 2.6 SD 2.2 |
n = 35 11.5 SD 6.4 |
|||||||
| Wagrell 2002 (IPSS) |
n = 46 20.4, SD 5.9 |
n = 99 21.0, SD 5.4 |
n = 41 6.7, SD 4.3 |
n = 85 8.4, SD 5.5 |
n = 43 5.9, SD 5.0 |
n = 95 7.4, SD 6.2 |
n = 43 7.1, SD 6.6 |
n = 93 7.2, SD 6.2 |
0.0603 |
n = ? 5.0 |
n = ? 7.0 |
||||||
| Complication | Study | TURP | TUMT | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Blood loss (ml) | Dahlstrand 1995 | 32 | 282, SD 102 | 37 | ||||
| Blood transfusion | Ahmed 1997 | 30 | 4 | 13.3 | 30 | 0 | 0 | |
| Dahlstrand 1995 | 32 | 0 | 0 | 37 | ||||
| d’Ancona 1998 | 21 | 0 | 0 | 31 | 0 | 0 | ||
| Clot retention | Wagrell 2002 | 51 | 1 | 1.9 | 100 | Serious | ||
| Incontinence | Dahlstrand 1993 | 40 | 4 | 10 | 39 | 7 | 18 | |
| Mortality | Dahlstrand 1993 | 40 | 1 | 2.5 | 39 | |||
| Dahlstrand 1995 | 32 | 1 | 3.1 | 37 | Brain tumour at 6 months | |||
| d’Ancona 1998 | 21 | – | – | 31 | 1 | 3.2 | Death, unrelated disease | |
| de la Rosette 2003 | 66 | 2 | 3 | 66 | 2 | 3 | At 36 months | |
| Wagrell 2002 | 51 | 1 | 1.9 | 100 | 27 days after treatment | |||
| TUR syndrome | Wagrell 2002 | 51 | 1 | 1.9 | 100 | Serious | ||
| Urinary tract infection | Ahmed 1997 | 30 | 3 | 13.3 | 30 | 1 | 3.3 |
TURP: urinary tract infection leading to septicaemia in 1 and 2 were Escherichia coli infection TUMT: epididymo-orchitis and haematuria. This patient had urethral catheter for 6 weeks |
| Dahlstrand 1993 | 40 | 4 | 10 | 39 | 5 | 12.8 | ||
| Dahlstrand 1995 | 32 | 4 | 12.5 | 37 | 5 | 13.5 | At > 1 week | |
| Wagrell 2002 | 51 | 1 | 1.9 | 100 | Serious | |||
| Urinary retention | de la Rosette 2003 | 66 | 66 | 2 | 3 | |||
| Wagrell 2002 | 51 | 100 | 1 | 1 | Serious retention | |||
| Postoperative (3–12 months) | ||||||||
| Erectile dysfunction | Ahmed 1997 | 19 | 4 | 21 | 18 | 0 | 0 | |
| Dahlstrand 1995 | 32 | 0 | 0 | 37 | 0 | 0 | Unclear as to whether all patients were sexually active | |
| de la Rosette 2003; in Francisca 1999 | 53 | 9 | 70 | 35 | 7 | 20 | Problem with erection, premature loss of erection | |
| 48 | 10 | 21 | 50 | 14 | 28 | |||
| Wagrell 2002 | 51? | 6 | 100? | 13 | Impotence (up to 12 months) | |||
| Incontinence | Dahlstrand 1993 | 40 | 1 | 2.5 | 39 | Urinary leakage | ||
| Wagrell 2002 | 51 | 7 | 13 | 100 | 3 | 3 | Transient non-serious up to 12 months | |
| Irritative urinary symptoms | d’Ancona 1998 | 21 | 4 | 19 | 31 | 9 | 29 | |
| Retention | Wagrell 2002 | 51 | 7 | 13 | 100 | 19 | 19 | Non-serious |
| Retrograde ejaculation | Ahmed 1997 | 19 | 12 | 63.2 | 18 | 4 | 22.2 | |
| Dahlstrand 1993 | 16 | 4 | 25 | 0 | 0 | |||
| Dahlstrand 1995 | 32 | 37 | TURP: half with prograde ejaculation became retrograde after TURP | |||||
| de la Rosette 2003 | 42 | 32 | 76 | 46 | 14 | 30 | ||
| Stricture | Ahmed 1997 | 30 | 1 | 3.3 | 30 | Bladder neck stenosis; two urethral and one was bladder neck | ||
| Dahlstrand 1993 | 40 | 3 | 7.5 | 39 | – | Urethral stricture requiring internal urethomy | ||
| Dahlstrand 1995 | 32 | 2 | 6.2 | 37 | ||||
| de la Rosette 1998 | 66 | 2 | 3 | 66 | 1 | 1.5 | Urethral; total for 36 months | |
| Urinary tract infection | d’Ancona 1998 | 21 | 1 | 4.7 | 31 | 5 | 16 | At 12 months |
| Postoperative (> 12 months) | ||||||||
| Erectile dysfunction | Wagrell 2002 | 51? | 15 | 100? | 11 | Impotence at 24 months | ||
| Incontinence | de la Rosette 2003; in Floratos 2001 | 66 | 1 | 1.5 | – | – | – | At 36 months |
| Stricture | de la Rosette 1998 | 66 | 2 | 3 | 66 | 1 | 1.5 | Urethral; total for 36 months |
| Study | Baseline | 3 months | 12 months | 24 months | 3 years | Comments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUMT | TURP | TUMT | p-value | TURP | TUMT | p-value | TURP | TUMT | p-value | TURP | TUMT | p-value | ||
| de la Rosette 2003 |
n = 66 4, SD 1.1 |
n = 78 4, SD 0.9 |
n = 48 1.3 (0–5); change from baseline 69% |
n = 54 2.1 (0–6); change from baseline 50% |
n = 48 0.6, SD 0.7 (0–2); change from baseline 86% |
n = 58 1.9, SD 1.3 (0–5); change from baseline 55% |
n = 38 0.9, SD 1.1 |
n = 46 1.9, SD 1.0 |
n = 33 0.6, SD 0.8 |
n = 35 2.3, SD 1.2 |
IPSS QoL (0–6); quality of life data obtained from Francisca 1999 and de la Rosette 2003 | ||||
| Wagrell 2003 |
n = 46 4.2, SD 1.1 |
n = 99 4.3, SD 1.0 |
n = 41 1.1, SD 1.6 |
n = 84 1.5, SD 1.4 |
n = 43 1.4, SD 1.3 |
n = 93 1.5, SD 1.7 |
0.972 | IPSS QoL (0–6) | |||||||
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 24 months | 3 years | Comments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUMT | TURP | TUMT | p-value | TURP | TUMT | p-value | TURP | TUMT | p-value | TURP | TUMT | p-value | TURP | TUMT | p-value | |||
| Peak urine flow rate (ml/s) | Ahmed 1997 |
n = 30 9.5 (16.7–20.1)a |
n = 30 10.1 (9.2–10.9)a |
n = 30 14.6 (13.4–15.8)a |
n = 30 9.1 (8–10.2)a |
||||||||||||||
| Dahlstrand 1994 |
n = 32 8.3, SD 3.2 |
n = 38 8.4, SD 2.6 |
n = 32 18.1, SD 7.1 p < 0.001 |
n = 37 11.5, SD 4.2 p < 0.001 |
< 0.001 |
n = 31 18.6, SD 5.2 p < 0.01 |
n = 38 11.7, SD 3.9 p < 0.001 |
< 0.001 |
n = 31 18.9, SD 6.0 p < 0.001 |
n = 33 12.3, SD 4.1 p < 0.001 |
< 0.001 |
n = 14 19.7, SD 5.5 p < 0.001 |
n = 13 12.8, SD 4.8 p < 0.01 |
< 0.01 | It is suspected that these two studies are the same. Uncertainty exists because, in terms of symptoms scores, patients appear to be different. Attempts to contact authors were made with no success | ||||
| Dahlstrand 1995 |
n = 32 8.6, SD 3.0 (12.1–15.0)a |
n = 37 8.6, SD 2.5 (7.7–9.4)a |
n = 32 18.1, SD 7.1 p < 0.001 |
n = 36 11.6, SD 4.2 p < 0.001 |
< 0.001 |
n = 31 18.6, SD 5.2 p < 0.001 |
n = 37 11.8, SD 3.9 p < 0.001 |
n = 31 18.9, SD 6.0 p < 0.01 |
n = 33 12.6, SD 3.9 p < 0.01 |
n = 29 17.6, SD 5.9 p < 0.001 |
n = 30 12.3, SD 4.4 p < 0.001 |
||||||||
| d’Ancona 1998 |
n = 21 9.3, SD 3.4 |
n = 31 9.3, SD 3.9 |
n = 21 19.6, SD 11.2 |
n = 31 15.5, SD 8.0 |
>0.05 |
n = 20 15.3, SD 5.9 |
n = 28 17.0, SD 7.5 |
> 0.05 |
n = 17 19.3, SD 10.7 |
n = 27 17.1, SD 7.8 |
>0.05 |
n = 12 19.1, SD 8.2b |
n = 17 15.1, SD 9.6b |
> 0.05 | |||||
| de la Rosette 2003 |
n = 66 7.8, SD 2.8 |
n = 78 9.2, SD 3.1 |
n = 47 25.0, SD 7.5 |
n = 54 15.5, SD 12.1 |
<0.01 |
n = 48 23.8, SD 10.4 |
n = 58 14.9, SD 7.2 |
<0.01 |
n = 38 22.5, SD 11.4 |
n = 46 13.7, SD 6.4 |
n = 33 22.8, SD 11.6 |
n = 35 11.7, SD 5.8 |
|||||||
| Wagrell 2002 |
n = 35 7.9, SD 2.7 |
n = 79 7.6, SD 2.7 |
n = 41 14.6, SD 9.0 |
n = 81 12.8, SD 6.1 |
n = 43 13.8, SD 6.8 |
n = 91 13.5, SD 6.1 |
n = 31 15.2, SD 7.8 |
n = 73 13.3, SD 6.0 |
0.944 |
n = ? 15.6 |
n = ? 12.4 |
From Wagrell 2002 abstract | |||||||
| Total voided volume (ml) | de la Rosette 2003; in Francisca 2000 |
n = 68 223, SD 117 |
n = 68 222, SD 85 |
n = 47 263, SD 141 |
n = 52 249, SD 116 |
0.77 | |||||||||||||
| d’Ancona 1998 |
n = 21 178, SD 84.1 |
n = 31 193.5, SD 85.7 |
n = 21 234.1, SD 95.5 |
n = 31 244.4, SD 161 |
n = 20 219.3, SD 107.7 |
n = 28 258.9, SD 130 |
n = 17 272.4, SD 151.3 |
n = 27 274.9, SD 145.2 |
n = 12 272.7, SD 133.4b |
n = 17 249.7, SD 182b |
|||||||||
| Residual volume (ml) | Ahmed 1997 |
n = 30 109.1 (88.2–130)a |
n = 30 94.4 (70.0–112.8)a |
n = 30 32.5 (22.5–40.5)a p = NS |
n = 30 104.9 (78.9–130.9)a p < 0.001 |
||||||||||||||
| d’Ancona 1998 |
n = 21 91.1, SD 104.7 |
n = 31 49.5, SD 69.9 |
n = 21 10.5, SD 24.5 |
n = 31 25.5, SD 58.1 |
n = 20 52.7, SD 70.7 |
n = 28 30.6, SD 41.0 |
n = 17 23.6, SD 29.8 |
n = 27 70.4, SD 81.3 |
n = 12 9.3, SD 14.6b |
n = 17 27.4, SD 49.1b |
|||||||||
| Wagrell 2002 |
n = 45 94, SD 82 |
n = 99 106, SD 77 |
n = 38 54, SD 77 |
n = 86 49, SD 70 |
0.680 |
n = ? 40 |
n = ? 55 |
From the abstract Wagrell 2002 | |||||||||||
| Dahlstrand 1995 |
n = 32 1104, SD 95 (70-139)a |
n = 37 194, SD (68-120)a |
n = 32 134, SD 32 (22-45)a p < 0.001 |
n = 36 147, SD 45 (32-62)a p < 0.001 |
n = 32 134, SD 30 (23-44)a p < 0.001 |
n = 37 166, SD 64 (44-87)a p < 0.001 |
n = 31 123, SD 18 (16-29)a p < 0.001 |
n = 33 152, SD 64 (30-75)a p < 0.01 |
n = 30 127, SD 2 (14–39)a p < 0.001 |
n = 31 148, SD 44 (32–64)a p < 0.001 |
|||||||||
| de la Rosette 2003 |
n = 66 97, SD 99 |
n = 78 68, SD 85 |
n = 53 14, SD 21 |
n = 57 64, SD 76 |
< 0.01; data in Francisca 2000 |
n = 48 20, SD 49 |
n = 54 55, SD 69 |
n = 38 29, SD 39 |
n = 46 91, SD 116 |
n = 33 35, SD 56 |
n = 35 94, SD 114 |
||||||||
| Detrusor pressure (cmH2O) | Ahmed 1997 |
n = 30 96.7 (85.5–103.9)a |
n = 30 98.5 (70.1–116.9)a |
n = 30 48.8 (44.3–52.7)a p < 0.001 |
n = 30 105.6 (73.7–117.5)a p = NS |
||||||||||||||
| Dahlstrand 1995 |
n = 32 75, SD 31 |
n = 37 70, SD 29 |
n = 32 36, SD 8 p < 0.001 |
n = 37 67, SD 29 p = NS |
|||||||||||||||
| d’Ancona 1998 |
n = 21 65.4, SD 24.9 |
n = 31 77.7, SD 40.0 |
n = 20 38.5, SD 24.5 |
n = 28 54.0, SD 15.9 |
|||||||||||||||
| Wagrell 2002 |
n = 45 79.4, SD 35.3 |
n = 99 73.7, SD 29.7 |
n = 39 41.8, SD 16.6 |
n = 82 48.5, SD 25.0 |
|||||||||||||||
| Prostate size (ml) | Ahmed 1997 |
n = 30 46.1 (38.1-54.1)a |
n = 30 36.6 (31.8-54.1)a |
n = 30 25.4 (19.4-31.4)a p = 0.001 |
n = 30 34.5 (29.7-39.3)a p = NS |
||||||||||||||
| Dahlstrand 1995 |
n = 32 36.8, SD 16 |
n = 37 33.9, SD 11.9 |
n = 30? 22.5, SD 10.9 p = NS |
n = 31? 30.3, SD 9.6 p < 0.001 |
|||||||||||||||
| d’Ancona 1998 |
n = 21 44.9, SD 15.3 |
n = 31 43.4, SD 11.8 |
n = 21 23.0, SD 8.8 |
n = 31 36.6, SD 10.0 |
|||||||||||||||
| de la Rosette 2003 |
n = 66 52, SD 19.2 |
n = 78 51, SD 20 |
n = 48 23, SD 7.4 |
n = 54 41, SD 16.1 |
p < 0.01; data in Francisca 2000 |
n = 35 25, SD 10.7 |
n = 38 48, SD 18.4 |
p < 0.01; data in Francisca 2000 | |||||||||||
| Wagrell 2002 |
n = 46 52.7, SD 17.3 |
n = 99 48.9, SD 15.8 |
n = 41 26, SD 13 |
n = 90 34, SD 16 |
n = ? 25 |
n = ? 37 |
|||||||||||||
| Outcome | Study | TURP | TUMT | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Length of hospital stay (days) | d’Ancona 1998 |
N = 21 4.1 (4–5) |
N = 31 Outpatient |
Mean (range) | ||||
| de la Rosette 2003 |
N = 66 5.3, SD 3.4 |
N = 78 0, SD 0.16 |
Mean, SD; TUMT: 2/78 patients for 1 day | |||||
| N | n | % | N | n | % | |||
| Reoperation | Ahmed 1997 | 30 | – | – | 30 | 1 | 3.3 | Reoperation with TURP |
| Dahlstrand 1993 | 40 | 1 | 2.5 | 39 | 4 | 10.2 | TURP: one repeat TURP because of bladder neck sclerosis. TUMT: two had TURP at 6 months and two had repeat TUMT. One of those did not improve after TURP | |
| Dahlstrand 1995 | 32 | 4 | 12.5 | 37 | 4 | 10.8 | TURP: three had repeat TURP because of bleeding or clots at 1 week after treatment; one had repeat TURP because of bladder neck stenosis at 1 year. TUMT: two had TURP at 6 months; two had repeat TUMT at 6 months but had to receive TURP at 1 year | |
| d’Ancona 1998 | 21 | 1 | 4.8 | 31 | 2 | 6.4 | TURP: at 12 months. TUMT: TURP at 6 months | |
Appendix 8.2 TUMT versus sham
| Study | Baseline | 3 months | 6 months | 12 months | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUMT | Sham | TUMT | Sham | p-value | TUMT | Sham | p-value | TUMT | Sham | p-value | ||
| Albala 2002 (AUA) |
n = 125 22.2, SD 5.0 |
n = 65 22.7, SD 5.7 |
n = 124 12.4 |
n = 63 17 |
< 0.05 |
n = 115 12.1 |
n = 119 11.9 p < 0.05 |
|||||
| Bdesha 1994 (AUA) |
n = 22 19.2 (16.3–22.1)a |
n = 20 18.8 (16.0–21.7)a |
n = 22 7.1 (5.0–9.2)a |
n = 18 16.2 (12.8–19.6)a |
< 0.001 | |||||||
| Blute 1996 (Madsen) |
n = 78 13.9, SD 3.4 |
n = 37 14.9, SD 3.1 |
n = 75 6.3, SD 5.0 |
n = 35 10.8, SD 4.4 |
< 0.00001 |
n = 68 5.74 |
n = 63 5.75 |
|||||
| Blute 1996 (AUA) |
n = 37 19.9, SD 7.2 |
n = 37 20.8, SD 6.7 |
n = 64 11.3, SD 6.3 |
n = 31 16.3, SD 7.6 |
||||||||
| Brehmer 1999 (ICS) |
n = 13 A: 49; B: 36 |
n = 13 A: 46; B: 36 |
32 questions about the symptom (A) and bother related to symptom (B). Max A and B: 124 and 92 respectively. High score indicates worse symptoms | |||||||||
| de la Rosette 1994; Francisca 1997 (Madsen) |
n = 25 13.2, SD 3.4 |
n = 25 12.1, SD 2.9 |
n = 24 5.9, SE 0.8 p = 0.0001 |
n = 23 7.9, SE 0.9 p = 0.001 |
< 0.025 |
n = 24 5.3, SE 0.9 |
n = 23 9.1, SE 0.9 |
0.001 |
n = 12 3.3 |
n = 7 9.1 |
||
| de Wildt 1996 (Madsen) |
n = 47 13.7, SD 3.4 (12.7–14.7)a |
n = 46 12.9, SD 3.1 (11.9–13.9)a |
n = 45 4.7 (3.6–5.9)a p < 0.003 |
n = 43 10.4 (8.9–11.8)a p = 0.001 |
n = 33 4.2 (3.0–5.3)a p < 0.001 |
n = 13 8.2 (5.5–11.0)a p < 0.001 |
||||||
| Larson 1998 (AUA) |
n = 124 20.8 (19.8–21.9)a |
n = 42 21.3 (19.3–23.3)a |
n = 123 9.6 (8.6–10.7)a |
n = 40 14.5 (12.4–16.6)a |
< 0.01 |
n = 120 10.5 (9.2–11.8)a |
n = 352 14.3 (12.2–16.4)a |
< 0.05 | ||||
| Nawrocki 1997 (AUA) |
n = 38 19b (7–31) |
n = 40 17.5b (7–28) |
n = 38? 9.5b (1–27) |
n = 40? 9.5b (0–30) |
0.81 | |||||||
| Ogden 1993 (Madsen) |
n = 21 14.5 (12.9–16.1)a |
n = 19 14.2 (12.5–15.7)a |
n = 21 4.3 (2.4–6.2)a |
n = 19 12.8 (10.5–15)a |
0.001 |
n = 19 3.6 |
n = 5 7.7 |
|||||
| Roehrborn 1998 (AUA) |
n = 147 23.6, SD 5.6 |
n = 73 23.9, SD 5.6 |
n = 147? 11.7 |
n = 73? 16.2 |
< 0.05 |
n = 124? 12.7 |
n = 65? 18.0 |
< 0.05 | Same study as Trachtenberg 1998 | |||
| Trachtenberg 1998 (AUA) |
n = 147 23.6 (12–35) |
n = 73 23.9 (13–35) |
n = 142 11.6 |
n = 70 16.4 |
< 0.05 |
n = 142 12.6 |
n = 70 17.9 |
< 0.05 | Same study as Roehrborn 1998 | |||
| Complication | Study | TUMT | Sham | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Blood loss (> 50 ml) | Larson 1998 | 125 | 1 | 0.8 | 44 | 0 | 0 | |
| Blood transfusion | Larson 1998 | 125 | 0 | 0 | 44 | 0 | 0 | |
| Urinary retention | Albala 2002 | 121 | 20 | 16.8 | 62 | 0 | 0 | Number who were recatheterised |
| Abbou 1995 | 66 | 0 | 0 | 37 | 0 | 0 | Acute retention | |
| Blute 1996 | 78 | 20 | 25.6 | 37 | 0 | 0 | Up to 3 months, requiring catheterisation | |
| de Wildt 1996 | 47 | 10 | 21.3 | 46 | 1 | 2.17 | ||
| Larson 1998 | 125 | 10 | 8 | 44 | 1 | 2 | 1 week after procedure | |
| Nawrocki 1997 | 38 | 4 | 10.5 | 40 | 0 | 0 | Acute retention in the first 24 hours after treatment requiring catheterisation for up to 1 week | |
| Ogden 1993 | 22 | 5 | 22.8 | 21 | 0 | 0 | Immediate post treatment | |
| Urinary tract infection | Abbou 1995 | 66 | 12 | 18 | 31 | 6 | 19 | Cystitis |
| 66 | 1 | 1.5 | 31 | 1 | 3 | Prostatitis | ||
| 66 | 0 | 0 | 31 | 1 | 3 |
Urinary tract infection Up to 4 weeks |
||
| Larson 1998 | 125 | 8 | 6 | 44 | 22 | 5 | ||
| 125 | 3 | 2 | 44 | 0 | 0 | Epididymitis | ||
| Ogden 1993 | 22 | 5 | 22.8 | 21 | 1 | 4.8 | Up to 3 months | |
| Recatheterisation | Albala 2002 | 121 | 20 | 16.8 | 62 | 0 | 0 | |
| Postoperative (3–12 months) | ||||||||
| Incontinence | Larson 1998 | 125 | 5 | 4 | 44 | Transient incontinence; overflow incontinence defined as dripping or wetting of clothing involving a urine volume greater than one table spoon; time point is unclear | ||
| Irritative urinary symptoms | Trachtenberg 1998; in Roehrborn 1998 | 147 | 32 | 21.8 | 73 | 6 | 8.2 | p = 0.019; at up to 6 months; mostly patients with dysuria and urgency |
| Mortality | de Wildt 1996 | 47 | 1 | 2.1 | 46 | 0 | 0 | Unrelated to treatment |
| Stricture/bladder neck | Albala 2002 | 121 | 0 | 0 | 62 | 0 | 0 | Urethral stricture |
| Larson 1998 | 125 | 3 | 2 | 44 | 0 | 0 | Urethral stricture; time point is unclear (total follow-up is 1 year) | |
| Erectile dysfunction | Bdesha 1994 | 22 | 0 | 0 | 20 | 0 | 0 | Of the ones that had normal sexual function before the surgery |
| Blute 1996 | 78 | 37 | No reports of sexual dysfunction | |||||
| Trachtenberg 1998; in Roehrborn 1998 | 147 | 44 | 28.9 | 73 | 1 | 1.4 | Sexual dysfunction includes mostly patients with hematospermia and other ejaculatory abnormalities as well as one patient with impotence due to corporeal fibrosis | |
| Retention | Trachtenberg 1998; in Roehrborn 1998 | 147 | 8 | 5.4 | 73 | 0 | 0 | p = 0.055; developed after the catheter was removed |
| Retrograde ejaculation | Albala 2002 | 121 | 0 | 0 | 62 | 0 | 0 | Ejaculatory dysfunction; total follow-up 1 year |
| Bdesha 1994 | 22 | 0 | 0 | 20 | 0 | 0 | ||
| Blute 1996 | 78 | 37 | No reports of sexual dysfunction | |||||
| Larson 1998 | 125 | 5 | 4 | 44 | 0 | 0 | Loss of ejaculation; time point is unclear (total follow-up 1 year) | |
| Trachtenberg 1998; in Roehrborn 1998 | 147 | 21 | 14.3 | 73 | 1 | 4.4 | Ejaculatory dysfunctions including hematospermia, abnormal ejaculation, painful ejaculation | |
| Urinary tract infection | Trachtenberg 1998; in Roehrborn 1998 | 147 | 11 | 7.5 | 73 | 2 | 2.7 | p = 0.228 |
| Study | Baseline | 3 months | 6 months | 12 months | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUMT | Sham | TUMT | Sham | p-value | TUMT | Sham | p-value | TUMT | Sham | p-value | ||
| Albala 2002 |
n = 125 11.4 |
n = 65 NR |
n = 124 6.2 |
n = ? NR |
n = 115 5.8 |
n = ? NR |
n = 119 5.8 |
n = ? NR |
Mean; quality of life index | |||
| Larson 1998 |
n = 125 4.2 (4.0–4.4) |
n = 44 4.0 (3.6–4.3) |
n = 120 2.2 (1.9–2.4) p < 0.0005 48% lower |
n = 35 2.9 (2.5–3.3) 28% lower |
Mean, 95% CI; quality of life score was evaluated by patient responses to the question of how they would feel if their current urinary symptoms were to continue indefinitely (no information was provided about the scale used). The improvement in QoL score remained at a comparable level at both the 9- and 12-month follow-up evaluations in the TUMT group | |||||||
| Ogden 1993 |
n = 22 13.4 (10.7–16.1) |
n = 21 13.3 (9.2–17.4) |
n = 21 5.1 (2.3–7.9) |
n = 19 10.2 (6.9–13.5) |
Mean, 95% CI; the quality of life questionnaire was derived from the Veterans’ Administration study of TURP vs watchful waiting. It had five sections: A = perception of urinary difficulties; B = sexual performance: C = activities of daily living; D = general psychological well-being; and E = social activities (A–D, high score = deterioriation; opposite for E) | |||||||
| Trachtenberg 1998; in Roehrborn 1998 |
n = 147 4.3, SD 1.0 |
n = 73 4.3, SD 1.1 |
n = 142 2.2 |
n = 70 3.1 |
< 0.05 |
n = 142 2.2 |
n = 70 3.2 |
< 0.05 | The observations made regarding the single QoL question, with a scale from 0 to 6, were nearly identical. At baseline the patients reported unfavorable and high QoL scores, representing strong dissatisfaction with urinary symptoms on the part of the participants. At 3 and 6 months there was a statistically significant difference in the improvement of QoL in the TUMT and sham-treated patients | |||
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUMT | Sham | TUMT | Sham | p-value | TUMT | Sham | p-value | TUMT | Sham | p-value | ||
| Peak urine flow rate (ml/s) | Abbou 1995 |
n = 66 10.4, SD 2.7 |
n = 31 9.9, SD 2.5 |
|||||||||
| Albala 2002 |
n = 125 8.9, SD 3.0 |
n = 65 8.4, SD 2.0 |
n = 119 13.9 p < 0.05 |
|||||||||
| Bdesha 1994 |
n = 22 12.3 (10.7–13.9)a |
n = 20 10.8 (9.2–12.4)a |
n = 22 14.6 (12.1–17.1)a |
n = 18 9.8 (8.5–11.1)a |
NS | |||||||
| Blute 1996 |
n = 78 7.3, SD 1.6 |
n = 36 7.4, SD 1.7 |
n = 74 11.5, SD 4.0 p < 0.0001 |
n = 34 9.4, SD 3.7 p < 0.001 |
< 0.01 |
n = 64 11.89 |
n = 53 12.14 |
|||||
| Brehmer 1999 |
n = 16 7.0b |
n = 14 7.9b |
n = 16 9.9b |
n = 13 8.3b |
4-month data | |||||||
| de Wildt 1996 |
n = 47 9.2, SD 2.5 |
n = 46 9.6, SD 2.7 |
n = 45 13.4 (11.7–15.3)a p = 0.846 |
n = 43 9.6 (8.7–10.7)a p < 0.001 |
n = 33 13.4 (11.6–15.1)a p < 0.001 |
n = 13 10.5 (7.9–13.1)a p = 0.657 |
||||||
| Larson 1998 |
n = 106 7.8 (7.4–8.2)a |
n = 39 7.8 (7.0–8.6)a |
n = 102 11.7 (10.7–12.8)a |
n = 37 9.2 (8–10.4)a |
< 0.005 |
n = 101 11.8 (10.7–13)a |
n = 31 9.8 (8.4–11.2)a |
< 0.05 |
n = 107 11.6 (10.5–12.7)a |
|||
| Nawrocki 1997 |
n = 38 8.83, SD 2.32 |
n = 40 9.44, SD 2.78 |
n = 38 9.94, SD 3.08 |
n = 40 9.49, SD 2.88 |
||||||||
| Ogden 1993 |
n = 22 8.5 (7.5–9.5)a |
n = 21 7.4 (7.6–9.6)a |
n = 21 13 (10.4–15.4)a |
n = 19 9.2 (7.2–11.2)a |
n = 19 13.5 |
n = 5 11.5 |
From abstract 1993 | |||||
| Trachtenberg 1998; in Roehrborn 1998 |
n = 147 7.7, SD 2.0 (3.5–11.5) |
n = 73 8.1, SD 2.0 (4.0–11.9) |
n = 142 11 |
n = 70 9.7 |
< 0.05 |
n = 142 10.6 |
n = 70 9.6 |
< 0.05 | ||||
| Zerbib 1994 |
n = 38 7.6, SD 3.8 |
n = 30 10.6, SD 5.8 |
n = 38 9.6, SD 5.8 p = 0.002 |
n = 30 10.8, SD 5.4 p = 0.4 |
||||||||
| Mean urine flow rate (ml/s) | Blute 1996 |
n = 78 4.0, SD 1.1 |
n = 36 3.9, SD 1.1 |
|||||||||
| Trachtenberg 1998; in Roehrborn 1998 |
n = 147 4.3, SD 1.3 |
n = 73 4.5, SD 1.3 |
n = 142 6.0 |
n = 70 5.3 |
< 0.05 |
n = 142 6.0 |
n = 70 5.3 |
< 0.05 | ||||
| Voided volume (ml) | Abbou 1995 |
n = 66 249, SD 82 |
n = 31 242, SD 89 |
|||||||||
| Nawrocki 1997 |
n = 38 252.1, SD 64.79 |
n = 40 269.1, SD 72.29 |
n = 38 229.6, SD 71.80 |
n = 40 239.8, SD 67.05 |
||||||||
| Ogden 1993 |
n = 22 267 (235–297)a |
n = 21 285 (235–334)a |
n = 21 269 (224–313)a |
n = 19 258 (218–297) |
||||||||
| Trachtenberg 1998; in Roehrborn 1998 |
n = 147 254, SD 82 |
n = 73 251, SD 92 |
||||||||||
| Zerbib 1994 |
n = 38 151, SD 92.0 |
n = 30 145, SD 86.3 |
n = 38 154, SD 90.0 p = 0.7 |
n = 30 166, SD 91.3 p = 0.2 |
||||||||
| Residual volume (ml) | Abbou 1995 |
n = 66 66, SD 60 |
n = 31 61, SD 42 |
n = 19 11.8 |
n = 5 127 |
From abstract 1993 | ||||||
| Albala 2002 |
n = 125 57.9, SD 53.9 |
n = 65 52.6, SD 51.9 |
||||||||||
| Bdesha 1994 |
n = 22 104 (85–125)a |
n = 20 80 (57–103)a |
n = 22 52 (34–70)a |
n = 18 94 (71–117)a |
<0.05 | |||||||
| Blute 1996 |
n = 78 140.4, SD 35.8 |
n = 36 145.2, SD 1.7 |
n = 71 145.5, SD 126.1 |
n = 33 147.2, SD 107.2 |
||||||||
| de Wildt 1996 |
n = 47 93.9, SD 75.4 |
n = 46 84.7, SD 66.1 |
n = 45 34.2 (19.4–46.8)a p = 0.002 |
n = 43 104.1 (74.7–133.4)a p = 0.433 |
n = 33 49.7 (33.0–66.3)a |
n = 13 56.3 (16.9–95.7)a |
||||||
| Larson 1998 |
n = 105 99.1 (82.0–116.1)a |
n = 39 103.6 (79.4–127.8)a |
n = 103 68.4 (52.9–83.8)a |
n = 37 93.0 (57.6–128.4)a |
NS |
n = 101 84.5 (67.8–101.2)a |
n = 31 84.4 (58.3–110.6)a |
NS | ||||
| Nawrocki 1997 |
n = 38 85.7, SD 56.6 |
n = 40 96.5, SD 56.3 |
n = 38 85.8, SD 51.2 |
n = 40 106.3, SD 84.5 |
||||||||
| Ogden 1993 |
n = 22 147 (116–117)a |
n = 21 118 (84.8–151)a |
n = 21 12 (–2.0–26)a |
n = 19 171 (121–220)a |
||||||||
| Trachtenberg 1998; in Roehrborn 1998 |
n = 147 79.7, SD 20.1 (0–248) |
n = 73 67.5, SD 63.4 (0–241) |
||||||||||
| Zerbib 1994 |
n = 38 110, SD 88.8 |
n = 30 84.2, SD 76.6 |
n = 38 67, SD 101.6 p = 0.006 |
n = 30 81.2, SD 66.8 |
||||||||
| Outcome | Study | TUMT | Sham | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Reoperation | Bdesha 1994 | 22 | – | – | 20 | 16 | 80 | Sham: had TUMT |
| Brehmer 1999 | 16 | 3 | 18.7 | 14 | 7 | 50 | TUMT: all had TURP. Sham: three had TURP and four had TUMT | |
| de Wildt 1996 | 47 | 8 | 17 | 46 | 27 | 58.7 | TUMT: three had a TURP at 3 months, 6 months and 1 year; four had a TUMT at 6 months. Sham: 23 had a TUMT at 6 months and four had a TUMT at 1 year | |
| Larson 1998 | 125 | 2 | 1.6 | 44 | 27 | 61.4 | Further treatment: either therapeutic procedure or medication. Sham: 20 had a TUMT procedure after 6 months; seven had TUMT before 6 months | |
| Ogden 1993 | 22 | 1 | 4.5 | 21 | 1 | 4.8 | TUMT: chose to have TURP because of retention that had not resolved at 10 days. Sham: underwent TUMT. Total follow-up: 3 months | |
Appendix 8.3 TUNA versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | 18 months | 24 months | 3 years | 5 years | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUNA | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | |
| Cimentepe 2003 (IPSS) |
n = 33 24.1, SD 3.8 |
n = 26 22.9, SD 3.8 |
n = 33? 8.3, SD 2.9 p = 0.00 |
n = 26? 9.7, SD 2.8 p = 0.00 |
0.248 |
n = 33? 8.6, SD 1.8 p = 0.00 |
n = 26? 8.5, SD 3.2 p = 0.00 |
0.899 | |||||||||||||||
| Hill 2004 (AUA) |
n = 55 24.1, SE 0.8 |
n = 65 24.0, SE 0.8 |
n = 47 9.4, SE 0.7 p < 0.001 |
n = 59 10.1, SE 0.9 p < 0.001 |
0.75 |
n = 47 8.4, SE 0.8 p < 0.001 |
n = 59 11.0, SE 1.0 p < 0.001 |
0.4513 |
n = 44 7.8, SE 0.9 p < 0.001 |
n = 56 11.7, SE 1.0 p < 0.001 |
0.0047 |
n = 35 9.5, SE 1.1 p < 0.001 |
n = 43 15.0, SE 1.3 p < 0.001 |
0.0028 |
n = 31 10.1, SE 1.4 p < 0.001 |
n = 38 15.2, SE 1.3 p < 0.001 |
0.0079 |
n = 22 10.8, SE 1.6 p < 0.001 |
n = 18 10.7, SE 1.4 p < 0.001 |
0.9813 | |||
| Hindley 2001 (IPSS) |
n = 25 |
n = 25 |
n = 22 |
n = 20 |
n = 19 |
n = 19 |
n = 19 |
n = 19 8 (5–13)b |
|||||||||||||||
| Kim 2006a (IPSS) | n = 110 24.0 | n = 110 20.8 | n = 110 10.6 | n = 110 10.8 | n = 110 8.9 | n = 110 11.4 | n = 110 8.8 | n = 110 11.6 | |||||||||||||||
| Complication | Study | TURP | TUNA | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Blood transfusion | Cimentepe 2003 | 33 | 0 | 0 | 26 | 0 | 0 | |
| Hindley 2001 | 22 | 3 | 13.6 | 20 | 0 | 0 | Patients received 2 units each | |
| Kim 2006a | 101 | 19 | 19 | 100 | 0 | 0 | ||
| Clot retention | Hindley 2001 | 22 | 1 | 4.5 | 20 | 0 | 0 | |
| Mortality | Hindley 2001 | 22 | 0 | 0 | 20 | 0 | 0 | |
| Recatheterisation | Cimentepe 2003 | 33 | 26 | 1 | 3.8 | For 73 hours | ||
| Hindley 2001 | 22 | 0 | 0 | 20 | 1 | 5 | Failed trial of voiding | |
| Kim 2006a | 101 | 4 | 4 | 100 | 4 | 4 | ||
| Urinary retention | Cimentepe 2003 | 33 | 26 | 1 | 3.8 | In this patient the catheter had been removed 12 hours after the procedure and required recatheterisation for 72 hours | ||
| Urinary tract infection | Cimentepe 2003 | 33 | 26 | 1 | 3.8 | |||
| Hindley 2001 | 22 | 4 | 18.1 | 20 | 4 | 20 | ||
| Kim 2006a | 101 | 7 | 7 | 100 | 10 | 10 | ||
| Postoperative (≥ 3 months) | ||||||||
| Irritative urinary symptoms | Cimentepe 2003 | 33 | 26 | TURP: most but more prominent (solved 2–3 weeks); TUNA: most (solved after 7–10 days) | ||||
| Hindley 2001 | 22 | 0 | 0 | 20 | 4 | 20 | ||
| Retrograde ejaculation | Cimentepe 2003 | 33 | 16 | 48.5 | 26 | 0 | 0 | At 18 months |
| Hill 2004 | 56 | 23 | 41.1 | 65 | 0 | 0 | At the end of 5 years | |
| Kim 2006a | 101 | 39 | 39 | 100 | 5 | 5 | Up to 12 months | |
| Stricture | Cimentepe 2003 | 33 | 2 | 6 | 26 | 0 | 0 | Urethral at 18-month follow-up (cumulative) |
| Hill 2004 | 56 | 4 | 7.1 | 65 | 1 | 1.5 | Stricture formation or scar tissue at the end of a 5-year follow-up (cumulative) | |
| Kim 2006a | 101 | 5 | 5 | 100 | 0 | 0 | Urethral stricture | |
| 101 | 2 | 2 | 100 | 0 | 0 | Bladder neck contracture | ||
| Up to 12 months | ||||||||
| Urinary incontinence | Cimentepe 2003 | 33 | 1 | 0.3 | 26 | 0 | 0 | Stress |
| Hill 2004 | 56 | 12 | 21.4 | 2 | 65 | 3.1 | TUNA: one stress, one urge at 5 years | |
| Kim 2006a | 101 | 4 | 4 | 100 | 4 | 4 | Up to 12 months | |
| Erectile dysfunction | Kim 2006a | 101 | 13 | 13 | 100 | 1 | 1 | Up to 12 months |
| Cimentepe 2003 | 33 | 4 | 12 | 26 | 0 | 0 | At 18 months | |
| Hill 2004 | 56 | 12 | 21.4 | 65 | 2 | 3.1 | At the end of 5 years | |
| Study | Baseline | 3 months | 6 months | 12 months | 18 months | 2 years | Comments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUNA | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | ||
| Cimentepe 2003 |
n = 33 5.2, SD 0.65 |
n = 26 4.8, SD 0.75 |
n = 33? 1.9, SD 0.5 p = 0.000 |
n = 26 ? 2.1, SD 0.5 p = 0.000 |
0.296 |
n = 33 ? 1.7, SD 0.5 p = 0.000 |
n = 26 ? 1.8, SD 1.3 p = 0.000 |
0.351 | Quality of life score reported (mean, SD) | |||||||||
| Hill 2004 |
n = 56 12.6, SE 0.5 |
n = 64 11.8, SE 0.5 |
n = 45 3.7, SE 0.7 p < 0.0001 |
n = 55 4.3, SE 0.5 p < 0.0001 |
0.4814 |
n = 33 3.7, SE 0.7 p < 0.0001 |
n = 43 4.3, SE 0.7 p < 0.0001 |
0.0309 | Quality of life score, but no further information has been reported. The type of scale used is unclear. (mean, SE) | |||||||||
| Hindley 2001 |
n = 25 5 (4-5) |
n = 25 4 (3-5) |
n = 22 1 (0-2) |
n = 20 2 (1-3) |
n = 19 1 (0-2) |
n = 19 1 (1-3) |
n = 19 1 (0-2) |
n = 19 2 (1-3) |
Quality of life score reported (median, IQR) | |||||||||
| Kim 2006a |
n = 110 4.7 |
n = 110 4.3 |
n = 110 2.8 |
n = 110 3.3 p < 0.05 |
n = 110 2.6 |
n = 110 2.5 |
n = 110 2.6 |
n = 110 2.6 |
IPSS QoL (mean or median) | |||||||||
| Study | Baseline | 3 years | 4 years | 5 years | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUNA | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | ||
| Hill 2004 |
n = 56 12.6, SE 0.5 |
n = 64 11.8, SE 0.5 |
n = 32 4.7, SE 1.0 p < 0.0001 |
n = 40 5.4, SE 0.7 p < 0.0001 |
0.5275 |
n = 21 3.7, SE 1.0 p < 0.0001 |
n = 22 5.2, SE 0.9 p < 0.0001 |
0.2316 |
n = 22 3.8, SE 0.7 p < 0.0001 |
n = 18 4.3, SE 0.8 p < 0.0001 |
0.8419 | Quality of life score, but no further information has been reported. The type of scale used is unclear (mean, SE) |
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 18 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUNA | TURP | TUNA | p-value | TURP | TUNA | TURP | TUNA | p-value | TURP | TUNA | p-value | ||
| Detrusor pressure (cmH2O) | Hindley 2001 |
n = 25 99, SD 10 |
n = 25 92, SD 12 |
n = 22 44, SD 11 |
n = 20 70, SD 12 |
|||||||||
| Peak urine flow rate (ml/s) | Cimentepe 2003 |
n = 33 9.2, SD 3.4 |
n = 26 9.8, SD 3.6 |
n = 33? 23.1, SD 5.3 p = 0.000 |
n = 26? 16.7, SD 4.5 p = 0.00 |
0.002 |
n = 33? 23.3, SD 4.9 p = 0.00 |
n = 26? 17.7, SD 4.2 p = 0.00 |
0.004 | |||||
| Hill 2004 |
n = 56 8.8, SE 0.3 |
n = 65 8.8, SE 0.3 |
n = 43 21.1, SE 1.3 < 0.0001 |
n = 53 14.6, SE 1.0 < 0.0001 |
< 0.0001 | |||||||||
| Hindley 2001 |
n = 25 9.0, SD 3.6 |
n = 25 8.5, SD 3.7 |
n = 22 18.4, SD 7.7 |
n = 20 9.8, SD 4.0 |
n = 19 22, SD 10.3 |
n = 19 9.7, SD 5.0 |
||||||||
| Kim 2006a |
n = 110 11.9 |
n = 110 7.0 |
n = 110 22.6 |
n = 110 15.4 |
n = 110 22.2 |
n = 110 18.0 |
n = 110 22.9 |
n = 110 17.8 |
||||||
| Prostate size (ml) | Cimentepe 2003 |
n = 33 49.1, SD 17.7 |
n = 26 46.1, SD 11.2 |
n = 33? 34.3, SD 10.4 |
n = 26? 41.9, SD 10.9 |
0.079 | ||||||||
| Kim 2006a |
n = 110 44.2 |
n = 110 40.6 |
n = 110 24.9 |
n = 110 29.6 |
n = 110 25.1 |
n = 110 28.5 |
n = 110 25.3 |
n = 110 28 |
||||||
| Residual volume (ml) | Cimentepe 2003 |
n = 33 76.1, SD 50.1 |
n = 26 67.4, SD 29.4 |
n = 33? 32.4, SD 17.4 p = 0.003 |
n = 26? 45.3, SD 16.7 p = 0.003 |
0.065 |
n = 33? 30.3, SD 18.7 p = 0.001 |
n = 26? 46.4, SD 17.5 p = 0.001 |
0.031 | |||||
| Hill 2004 |
n = 56 81.9, SE 9.3 |
n = 65 91.8, SE 10.0 |
n = 43 47.1, SE 7.0 p = 0.0014 |
n = 52 80.3, SE 11.0 p = 0.1161 |
||||||||||
| Hindley 2001 |
n = 25 74, SD 53 |
n = 25 55, SD 44 |
n = 22 87, SD 74 |
n = 20 50, SD 44 |
n = 19 21, SD 36 |
n = 19 104, SD 109 |
||||||||
| Kim 2006a |
n = 110 187 |
n = 110 257 |
n = 110 23 |
n = 110 33 |
n = 110 12 |
n = 110 29 |
n = 110 14 |
n = 110 32.6 |
||||||
| Urodynamic outcome | Study | 2 years | 3 years | 4 years | 5 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | TURP | TUNA | p-value | ||
| Detrusor pressure (cmH2O) | Hindley 2001 |
n = 9 36, SD 8 |
n = 12 71, SD 36 |
||||||||||
| Peak urine flow rate (ml/s) | Hill 2004 |
n = 33 21.3, SE 1.4 < 0.0001 |
n = 40 12.5, SE 0.7 < 0.0001 |
< 0.001 |
n = 26 19.1, SE 2.0 < 0.001 |
n = 33 13.0, SE 1.3 0.025 |
0.01 |
n = 17 18.9, SE 2.5 p = 0.006 |
n = 18 11.7, SE 1.4 p = 0.0358 |
0.0142 |
n = 15 18.6, SE 2.3 p = 0.0005 |
n = 13 11.4, SE 1.2 p = 0.0162 |
0.0143 |
| Hindley 2001 |
n = 19 18.1, SD 7.1 |
n = 19 8.6, SD 3.5 |
|||||||||||
| Residual volume (ml) | Hill 2004 |
n = 31 34.6, SE 5.6 p = 0.0005 |
n = 40 74.1, SE 12.6 p = 0.3788 |
0.0114 |
n = 26 50.7, SE 10.4 p = 0.0095 |
n = 32 78.2, SE 13.7 p = 0.102 |
0.1285 |
n = 17 39.5, SE 13.1 p = 0.0058 |
n = 19 138.2, SE 45.7 p = 0.4019 |
0.0564 |
n = 17 27.4, SE 7.9 p = 0.0031 |
n = 13 60.4, SE 21.8 p = 0.0872 |
0.1281 |
| Hindley 2001 |
n = 19 32, SD 42 |
n = 19 89, SD 81 |
|||||||||||
| Outcome | Study | TURP | TUNA | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Cimentepe 2003 |
N = 33 55.9, SD 12.4 |
N = 26 44.3, SD 7.8 |
p = 0.06 | ||||
| Kim 2006a |
N = 110 51 (20–85) |
N = 110 37 (25–60) |
Mean (range) | |||||
| Length of hospital stay (days) | Hindley 2001 |
N = 25 First postoperative day |
N = 25 Few days later |
|||||
| Cimentepe 2003 |
N = 33 Patients discharged on the same day |
N = 26 Stay for at least 48 hours |
||||||
| Kim 2006a |
N = 110 6.5 (6–8) |
N = 110 1.3 (1–3) |
Mean (range) | |||||
| N | n | % | N | n | % | |||
| Reoperation | Hill 2004 | 56 | 1 | 1.8 | 65 | 9 | 13.8 | TURP: reoperated with TUIP; TUNA: reoperated with TURP; at end of 5 years |
| Hindley 2001 | 22 | 0 | 0 | 20 | 1 | 5 | Reoperated with TURP up to 2 years | |
| Cimentepe 2003 | 33 | 0 | 0 | 26 | 2 | 7 | Reoperation with TURP at follow-up 18 months | |
| Kim 2006a | 101 | 0 | 0 | 100 | 0 | 0 | Up to 12 months | |
Appendix 8.4 Laser coagulation versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | 18 months | 2 years | 5 years | Comments | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | Laser coagulation | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | ||
| Chacko 2001; CLasP study (IPSS) |
n = 74 19.4, SD 7.6 |
n = 74 17.6, SD 9.3 |
n = 48a |
n = 48a |
Acute retention patients; non-contact VLAP, side-firing fibre | ||||||||||||||||
| Costello 1995 (AUA) |
n = 37 4.43 |
n = 34 9.27 |
0.01 | Nd:YAG laser coagulation | |||||||||||||||||
| Cowles 1995 (AUA) |
n = 59 20.8, SD 4.8 (7–30) |
n = 56 18.7, SD 6.0 (6–29) |
n = 57 –13.3,b SD 7.5 (–29–8) |
n = 55 –9.0,b SD 7.5 (–27–8) |
NS | Nd:YAG laser; non-contact VLAP | |||||||||||||||
| Donovan 2000; CLasP study (IPSS) |
n = 117 19.2 SD 6.7 |
n = 117 19.1 SD 6.6 |
n = 89a |
n = 89a |
Symptomatic patients; Nd:YAG laser; non-contact VLAP; side-firing laser | ||||||||||||||||
| Gujral 2000; CLasP study (IPSS) |
n = 44 19.5, SD 7.2 |
n = 44 20.9, SD 6.4 |
n = 33a |
n = 29a |
Chronic retention patients; Nd:YAG laser; VLAP side-firing laser | ||||||||||||||||
| Kabalin 1995 (AUA) |
n = 12 18.8, SE 1.8 |
n = 13 20.9, SE 1.9 |
n = 12 9.9, SE 2.6 |
n = 13 7.2, SE 1.7 |
n = 10 5.7, SE 1.2 |
n = 11 4.6, SE 0.7 |
n = 10 6.3, SE 1.1 |
n = 10 4.3, SE 1.3 |
n = 10 6.4, SE 1.3 |
n = 9 6.0, SE 1.3 |
n = 9 6.8, SE 1.7 |
n = 9 4.7, SE 1.7 |
Nd:YAG laser; non-contact VLAP | ||||||||
| Kim 2006a (IPSS) |
n = 110 24.0 |
n = 89 21.1 |
n = 110 10.6 |
n = 89 7.8 |
n = 110 8.9 |
n = 89 7.1 |
n = 110 8.8 |
n = 89 7.9 |
Mean or median; ILC | ||||||||||||
| Kursh 2003 (AUA) |
n = 35 23.0d |
n = 37 24.0d |
n = 35? 6.0d |
n = 35? 7.0d |
n = 35? 7.0d |
n = 35? 9.0d |
ILC | ||||||||||||||
| Mårtenson 1999 (IPSS) |
n = 14 21.6, SD 7.7 |
n = 30 21.7, SD 6.1 |
n = 14? 4.7, SD 4.0 |
n = 30? 11.8, SD 6.9 |
n = 14? 3.8, SD 2.4 |
n = 30? 10.3, SD 5.4 |
n = 14? 3.5, SD 2.9 |
n = 30? 12.4, SD 7.7 |
n = 14? 5.0, SD 4.4 |
n = 30? 12.0, SD 4.9 |
Contact ILC with diode laser system (indigo) | ||||||||||
| Liedberg 2003 (IPSS) |
n = 11 17,d IQR (17–24) |
n = 20 19,d IQR (16–24) |
n = 11 4,d IQR (2–7) p < 0.05 |
n = 20 10,d IQR (4–15) p < 0.05 |
<0.05 |
n = 9 6,d IQR (3–10) p < 0.05 |
n = 19 11,d IQR (6–14) p < 0.05 |
<0.05 | Contact ILC with indigo 830e laser system | ||||||||||||
| McAllister 2000 (AUA-6) |
n = 75 18.2 (17.1–19.3)c |
n = 76 18.1 (17.1–19.1)c |
n = 75 5.9 (4.6–7.2)c |
n = 76 7.9 (6.4–9.4)c |
n = 75 5.1 (3.8–6.4)c |
n = 75 7.7 (6.3–9.1)c |
0.046 |
n = 39 6.5 |
n = 28 6.3 |
ELAP: endoscopic laser, Nd:YAG | |||||||||||
| Rodrigo Aliaga 1998 (IPSS) |
n = 21 24.2, SD 7.7 |
n = 18 25.5, SD 10.1 |
n = 21? 8.6, SD 4.2 |
n = 18? 4.8, SD 4.8 |
n = 21? 3.7, SD 3.8 p < 0.05 |
n = 18? 7.4, SD 4.2 p < 0.005 |
|||||||||||||||
| Suvakovic 1996 (AUA) |
n = 10 18.8, SD 4.5 |
n = 10 15.7, SD 5.1 |
n = 10 12.8, SD 5.9 |
n = 10 16.8, SD 15.0 |
n = 9 8.5, SD 3 |
n = 9 8, SD 5.7 |
n = 9? 7.2, SD 6.1 |
n = 9? 10, SD 4.9 |
Sd Nd:YAG non-contact | ||||||||||||
| Complication | Study | TURP | Laser coagulation | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Procedural (intraoperative or immediate postoperative) | ||||||||
| Anaemia | Kursh 2003 | 35 | 2 | 5.7 | 35 | 0 | 0 | Hematocrit < 30% |
| Blood loss (ml) | Liedberg 2003 | 11 | 350 (200–514) | 20 | 0 (0–50) | Median (IQR); p < 0.001 | ||
| Blood transfusion | Chacko 2001 | 74 | 4 | 5.4 | 74 | 0 | 0 | |
| Costello 1995 | 37 | 3 | 8.2 | 34 | 0 | 0 | ||
| Cowles 1995 | 59 | 2 | 3.4 | 56 | 0 | 0 | p = 0.50 | |
| Donovan 2000 | 117 | 1 | 0.85 | 117 | 1 | 0.85 | ||
| Gujral 2000 | 44 | 3 | 6.8 | 38 | 0 | 0 | ||
| Kabalin 1995 | 12 | 1 | 8.3 | 13 | 0 | 0 | ||
| Kim 2006a | 101 | 19 | 19 | 89 | 0 | 0 | ||
| Kursh 2003 | 35 | 0 | 0 | 35 | 0 | 0 | ||
| Mårtenson 1999 | 14 | 0 | 0 | 30 | 0 | 0 | ||
| McAllister 2000 | 75 | 12 | 16 | 76 | 0 | 0 | Three of the transfusions were carried out perioperatively; the mean transfusion requirement was 2.7 units | |
| Cardiovascular events | Chacko 2001 | 74 | 2 | 2.7 | 74 | 0 | 0 | Myocardial infarction/cardiac failure during hospital stay |
| Cowles 1995 | 59 | 1 | 3.4 | 56 | 0 | 0 | DVT; p = 1.00 | |
| McAllister 2000 | 75 | 4 | 5.3 | 76 | 2 | 2.6 | TURP: two DVTs; one pulmonary embolism, one myocardial infarction. Laser: one DVT; one CVA | |
| Clot retention | Chacko 2001 | 74 | 2 | 2.7 | 74 | 0 | 0 | During hospital stay |
| Cowles 1995 | 59 | 2 | 3.4 | 56 | 0 | 0 | p = 0.50 | |
| Cowles 1995 | 59 | 3 | 5.1 | 56 | 0 | 0 | p = 0.24 | |
| Kabalin 1995 | 12 | 1 | 8.3 | 13 | ||||
| Liedberg 2003 | 11 | 0 | 0 | 20 | 1 | 5 | ||
| Mårtenson 1999 | 14 | 0 | 0 | 30 | 0 | 0 | ||
| McAllister 2000 | 75 | 5 | 6.6 | 76 | 1 | 1.3 | ||
| Heavy bleeding | Chacko 2001 | 74 | 3 | 4 | 74 | 2 | 2.7 | During operation |
| Gujral 2000 | 44 | 6 | 13.3 | 38 | 0 | 0 | ||
| Incontinence | Chacko 2001 | 74 | 3 | 4 | 74 | 0 | 0 | During hospital stay |
| Mortality | Chacko 2001 | 74 | 4 | 5.4 | 74 | 2 | 2.7 | Not treatment related |
| Donovan 2000 | 117 | 0 | 0 | 117 | 5 | 4.3 | Not treatment related | |
| Gujral 2000 | 44 | 1 | 2.3 | 38 | 0 | 0 | Not treatment related | |
| Kabalin 1995 | 12 | – | – | 13 | 1 | 7.7 | Cardiovascular event | |
| Kursh 2003 | 35 | 2 | 5.7 | 35 | By 6 months; unrelated cause | |||
| Perforation | Donovan 2000 | 117 | 2 | 1.7 | 117 | – | – | |
| Gujral 2000 | 44 | 1 | 2.3 | 38 | 0 | 0 | ||
| Secondary haemorrhage | Costello 1995 | 37 | 1 | 2.7 | 34 | 0 | 0 | |
| McAllister 2000 | 75 | 3 | 4 | 76 | 0 | 0 | ||
| Septicaemia | Chacko 2001 | 74 | 4 | 5.4 | 74 | 3 | 4 | During hospital stay |
| Donovan 2000 | 117 | 2 | 1.7 | 117 | 0 | 0 | Time point unclear (follow-up 7.5 months) | |
| Gujral 2000 | 44 | 3 | 6.8 | 38 | 1 | 2.63 | During hospital stay | |
| Urinary retention | Suvakovic 1996 | 10 | 0 | 0 | 10 | 1 | 10 | Failure to void on removal of catheter after 24 hours |
| Urinary tract infection | Gujral 2000 | 44 | 2 | 4.5 | 38 | 1 | 2.63 | Symptomatic urinary tract infection during hospital stay |
| Kim 2006a | 101 | 7 | 7 | 89 | 7 | 7.9 | ||
| Kursh 2003 | 35 | 4 | 11 | 35 | 7 | 20 | ||
| Liedberg 2003 | 11 | 1 | 9 | 20 | 13 | 65 | Laser: treated with antibiotics; however, two were resistant to it; p = 0.007 | |
| 11 | 0 | 0 | 20 | 3 | 15 | Bacterial prostatitis. These patients necessitated long-term antibiotic treatment | ||
| Mårtenson 1999 | 14 | 4 | 28 | 30 | 10 | 33 | No cases of epididymitis in either group | |
| McAllister 2000 | 75 | 1 | 1.3 | 76 | 2 | 2.6 | Epididymo-orchitis | |
| 75 | 5 | 6.6 | 76 | 18 | 23.7 | Positive urinary cultures in the first 4 weeks postoperatively | ||
| Recatheterisation | Kim 2006a | 101 | 4 | 4 | 89 | 2 | 2.2 | |
| Postoperative (3–12 months) | ||||||||
| Erectile dysfunction | Costello 1995 | 11 | 0 | 0 | 16 | 0 | 0 | Impotence (follow-up 6 months) |
| Cowles 1995 | 59 | 2 | 3.4 | 56 | 3 | 5.4 | Impotence. Time point is unclear (12 months total follow-up). Article does not specify whether patients were all sexually active before the operation | |
| Kim 2006a | 101 | 13 | 13 | 89 | 0 | 0 | Up to 12 months | |
| Mårtenson 1999 | 12 | 3 | 25 | 28 | 0 | 0 | At 12 months | |
| Retention | Cowles 1995 | 59 | 5 | 8.5 | 56 | 17 | 30.3 | Time point is unclear (total follow up was 12 months); p < 0.01 |
| van Melick 2003a | 50 | 0 | 0 | 45 | 5 | 11.1 | Up to 12 months | |
| Retrograde ejaculation | Costello 1995 | 11 | 8 | 72.7 | 16 | 2 | 12.3 | Did not retain ejaculatory function (follow-up 6 months) |
| Kabalin 1995 | 10 | 9 | 90 | 12 | 0 | 0 | At 3 months | |
| 10 | – | – | 12 | 1 | 8.3 | At 6 months | ||
| Kim 2006a | 101 | 39 | 39 | 89 | 4 | 4.5 | Up to 12 months | |
| Liedberg 2003 | 11 | 3 | 27 | 20 | 1 | 5 | p = 0.084 | |
| McAllister 2000 | 24 | 15 | 63 | 27 | 9 | 33 | At 12 months | |
| Stricture | Costello 1995 | 37 | 2 | 5.4 | 34 | 2 | 5.8 | Bladder neck stenosis – were reoperated by BNI (follow-up 6 months) |
| Cowles 1995 | 59 | 6 | 10.2 | 56 | 0 | 0 | Urethral stricture; p = 0.0 | |
| 59 | 3 | 5.1 | 56 | 0 | 0 | Bladder neck contracture; p = 0.24 | ||
| Time point is unclear (total follow-up 12 months) | ||||||||
| Kim 2006a | 101 | 5 | 5 | 89 | 0 | 0 | Urethral stricture | |
| 101 | 2 | 2 | 89 | 0 | 0 | Bladder neck | ||
| Up to 12 months | ||||||||
| Kabalin 1995 | 12 | 1 | 8.3 | 13 | 0 | 0 | At 6 months and was successfully treated with visual internal urethrotomy (the initial TURP had lasted 55 minutes, using a 28Fr resectoscope sheath) | |
| Liedberg 2003 | 11 | 0 | 0 | 20 | 0 | 0 | At 1 year | |
| Urinary tract infection | Costello 1995 | 37 | 2 | 5.4 | 34 | 1 | 2.9 | Urinary tract infection |
| 37 | 2 | 5.4 | 34 | 0 | 0 | Epididymitis | ||
| Follow-up 6 months | ||||||||
| Donovan 2000 | 117 | 2 | 1.7 | 117 | 3 | 2.6 | Time point unclear (total follow-up 7.5 months) | |
| McAllister 2000 | 75 | 7 | 9.3 | 76 | 28 | 36.8 | Positive urinary cultures (cumulative up to 12 months) | |
| Incontinence | Cowles 1995 | 59 | 2 | 3.4 | 56 | 0 | 0 | p = 0.50. Time point is unclear (total follow-up was 12 months) |
| Kim 2006a | 101 | 4 | 4 | 89 | 0 | 0 | Up to 12 months | |
| Kursh 2003 | 35 | 2 | 5.7 | 35 | 0 | 0 | One urgency; one stress requiring pads. Time point is unclear (total follow-up was 24 months) | |
| Mårtenson 1999 | 14 | 0 | 0 | 30 | 0 | 0 | Time point is unclear (total follow-up was 12 months) | |
| Study | Baseline | 3 months | 6 months | 12 months | 2 years | 3 years | Comments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | Laser coagulation | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | ||
| Chacko 2001; CLasP trial |
n = 74 5 (4–6)a |
n = 74 5 (4–6)a |
n = 45 –3.42 (–3.89 to –2.95)b |
n = 49 –3.10 (–3.65 to –2.55)b |
Acute urinary retention patients: IPSS QoL. All changes are statistically significant | |||||||||||||
| Donovan 2000; CLasP trial |
n = 117 4 (0–6)c |
n = 117 4 (2–6)c |
n = 85 –2.2 (–2.5 to –1.8)b |
n = 93 –1.9 (–2.3 to –1.6)b |
Symptomatic patients: IPSS QoL. All changes are statistically significant | |||||||||||||
| Gujral 2000; CLasP trial |
n = 44 4.5, SD 2.6 |
n = 38 5.0, SD 2.6 |
n = 33 –3.2 (–3.9 to –2.6)b |
n = 30 –2.8 (–3.4 to –2.1)b |
Chronic retention patients: IPSS QoL. All changes are statistically significant | |||||||||||||
| Kim 2006a |
n = 110 4.7 |
n = 89 4.7 |
n = 110 2.8 |
n = 89 3.5 p < 0.05 |
n = 110 2.6 |
n = 89 2.5 |
n = 110 2.6 |
n = 89 2.4 |
IPSS QoL; mean or median | |||||||||
| Kursh 2003 |
n = 35 11.0 |
n = 35 11.0 |
n = 35 2.0 |
n = 35 2.0 |
n = 35 2.0 |
n = 35 3.0 |
AUA QoL; median | |||||||||||
| Mårtenson 1999 |
n = 14 4.0, SD 1.3 |
n = 30 4.1, SD 1.4 |
n = 14? 0.9, SD 1.3 |
n = 30? 2.3, SD 1.4 |
n = 14? 0.5, SD 0.7 |
n = 30? 2.2, SD 1.4 |
n = 14? 0.6, SD 0.8 |
n = 30? 2.2, SD 1.5 |
n = 14? 0.7, SD 0.9 |
n = 30? 2.2, SD 1.5 |
QoL index | |||||||
| van Melick 2003a |
n = 50 3.9, SD 1.6 |
n = 45 3.6, SD 1.6 |
n = 37 0.5, SD 0.5 |
n = 33 0.8, SD 1.0 |
n = 41 0.6, SD 0.9 |
n = 37 0.6, SD 0.9 |
n = 15d 1.1, SD 1.2 |
n = 10d 2.0, SD 1.0 |
n = 15 1.3, SD 1.3 |
n = 17 1.4, SD 1.2 |
IPSS QoL | |||||||
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 18 months | 24 months | 5 years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | Laser coagulation | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | TURP | Laser coagulation | p-value | ||
| Peak urine flow rate (ml/s) | Roberto Aliaga 1998 |
n = 21 5.7, SD 7.7 |
n = 18 7.0, SD 8.1 |
n = 21? 18.6, SD 8.5 |
n = 18? 10.5, SD 5.0 |
n = 21? 20.6, SD 10.1 |
n = 18? 10.5, SD 4.6 |
||||||||||||||
| Costello 1995 |
n = 37 9.48 |
n = 34 8.76 |
n = 37? 19.1 |
n = 34? 15.7 |
|||||||||||||||||
| Cowles 1995 |
n = 59 9.5, SD 5.2 (3.0–37.4) |
n = 56 8.9, SD 3.6 (2.0–18.1) |
n = 57 7.0, SD 9.5 (–16.8–27.8) |
n = 55 5.3, SD 6.9 (–5.9–26.6) |
Change from baseline | ||||||||||||||||
| Donovan 2000; CLasP study; symptomatic patients |
n = 117 10.3 SD 2.7 |
n = 117 10.4 SD 2.9 |
|||||||||||||||||||
| Gujral 2000; CLasP study; chronic retention patients |
n = 44 8.5, SD 3.6 |
n = 38 11.2, SD 5.3 |
n = 40 9.4 (6.5-12.2)a |
n = 40 5.70 (2.6-8.8)a |
At 7.5 months; change in Qmax | ||||||||||||||||
| Kabalin 1995 |
n = 12 9.0, SE 1.1 |
n = 13 8.5, SE 1.1 |
n = 12 21.7, SE 2.9 |
n = 13 18.7, SE 2.6 |
n = 10 22.9, SE 2.8 |
n = 11 20.5, SE 1.8 |
n = 10 21.6, SE 2.2 |
n = 10 21.6, SE 1.5 |
n = 10 21.2, SE 2.9 |
n = 9 20.0, SE 1.9 |
n = 9 18.8, SE 1.7 |
n = 9 23.6, SE 2.4 |
|||||||||
| Kim 2006a |
n = 110 11.9 |
n = 89 8.6 |
n = 110? 22.6 |
n = 89? 16.8 |
n = 110? 22.2 |
n = 89? 19.7 |
n = 110? 22.9 |
n = 89? 19.6 |
|||||||||||||
| Kursh 2003 |
n = 35 9.1b |
n = 35 9.2 b |
n = 35 16.6 b |
n = 35 14.3 b |
n = 35 16.5b |
n = 35 13.9b |
|||||||||||||||
| Liedberg 2003 |
n = 11 8,b IQR (6–9) |
n = 20 8,b IQR (7–10) |
n = 10 12,b IQR (9–18) |
n = 19 11,b IQR (8–15) |
< 0.05 |
n = 9 14,b IQR (10–19) |
n = 18 11,b IQR (6–12) |
< 0.05 | |||||||||||||
| Mårtenson 1999 |
n = 14 9.3, SD 3.2 |
n = 30 7.3, SD 3.8 |
n = 14? 25.8, SD 9.7 |
n = 30? 12.5, SD 5.4 |
n = 14? 18.2, SD 6.6 |
n = 30? 11.1, SD 4.5 |
n = 14? 25.7, SD 11.1 |
n = 30? 11.9, SD 5.5 |
n = 14? 20.1, SD 13.7 |
n = 30? 10.3, SD 4.4 |
|||||||||||
| McAllister 2000 |
n = 75 10.0 (9.1–10.9)a |
n = 76 9.6 (8.8–10.4)a |
n = 75 21.3 (19.0–23.6)a |
n = 76 15.9 (13.6–18.2)a |
0.0081 |
n = 75 19.9 (17.4–22.4)a |
n = 76 15.6 (13.7–17.5)a |
n = 75 21.8 (18.5–25.1)a |
n = 76 15.4 (13.6–17.2)a |
n = 36 20.0 |
n = 24 17.8 |
||||||||||
| Suvakovic 1996 |
n = 10 11.1, SD 6.4 |
n = 10 10.5, SD 3.7 |
n = 10 17.8, SD 3.8 |
n = 10 14.8, SD 5.4 |
n = 10 16.2, SD 4.2 |
n = 10 19.0, SD 0.8 |
n = 9? 15.2, SD 2.7 |
n = 10? 12.6, SD 3.7 |
|||||||||||||
| Mean urine flow rate (ml/s) | Costello 1995 |
n = 37? 10.07 |
n = 34? 7.99 |
||||||||||||||||||
| Voided volume (ml) | Mårtenson 1999 |
n = 14 230, SD 107 |
n = 30 185, SD 84 |
n = 14? 309, SD 163 |
n = 30? 206, SD 115 |
n = 14? 210, SD 151 |
n = 30? 244, SD 121 |
n = 14? 358, SD 225 |
n = 30? 218, SD 110 |
n = 14? 266, SD 146 |
n = 30? 237, SD 158 |
||||||||||
| McAllister 2000 |
n = 75 234.3 (208.2–260.4)a |
n = 76 234.1 (211.5–256.7)a |
n = 75 266.4 (234.6–298.2)a |
n = 76 236.3 (206.1–266.5)a |
n = 75 261.8 (227.1–296.5)a |
n = 76 274.7 (244.1–305.3)a |
n = 75 294.7 (252.8–336.6)a |
n = 76 247.2 (219.4–279.0)a |
|||||||||||||
| Residual volume (ml) | Aliaga 1998 |
n = 21 89, SD 92 |
n = 18 77, SD 63 |
n = 21? 76, SD 97 |
n = 18? 54, SD 47 |
n = 21? 38, SD 51 |
n = 18? 62, SD 50 |
||||||||||||||
| Gujral 2000; CLasP study; chronic retention patients |
n = 44 545, SD 275 |
n = 38 438, SD 151 |
n = 40 –464 (–553,–374)a |
n = 33 –329 (–377,–281)a |
At 7.5 months; change in residual volume | ||||||||||||||||
| Donovan 2000; CLasP study; symptomatic patients |
n = 117 104.2, SD 69.5 |
n = 117 123.7, SD 91.8 |
n = 98 –74.0 (–89.2,–58.8)a |
n = 100 –73.4 (–91.3,–55.5)a |
At 7.5 months; change in residual volume | ||||||||||||||||
| Costello 1995 |
n = 37? 28.1 |
n = 34? 88.6 |
NS | ||||||||||||||||||
| Kabalin 1995 |
n = 12 291, SE 8.8 |
n = 13 236, SE 74 |
n = 12 145, SE 27 |
n = 13 185, SE 52 |
n = 10 121, SE 24 |
n = 11 112, SE 27 |
n = 10 152, SE 48 |
n = 10 140, SE 36 |
n = 10 143, SE 43 |
n = 10 154, SE 38 |
n = 9 103, SE 22 |
n = 9 148, SE 28 |
|||||||||
| Kim 2006a |
n = 110 18 |
n = 89 219 |
n = 110? 23 |
n = 89? 28 |
n = 110? 12 |
n = 89? 22 |
n = 110? 14 |
n = 89? 17 |
|||||||||||||
| Suvakovic 1996 |
n = 10 161.8, SD 104 |
n = 10 47.4, SD 48.1 |
n = 10 21.5, SD 176 |
n = 10 51.9, SD 50.8 |
|||||||||||||||||
| Cowles 1995 |
n = 59 266.7, SD 181.9 (2–800) |
n = 56 162.7, SD 126.6 (19–700) |
|||||||||||||||||||
| McAllister 2000 |
n = 75 120.7 (93.0–148.4)a |
n = 76 113 (91.4–134.6)a |
n = 75 62.1 (43.9–80.3)a |
n = 76 70.3 (51.3–89.3)a |
n = 75 56.8 (41.4–72.2)a P |
n = 76 90.1 (61.6–118.0)a |
0.0403 |
n = 75 45.9 (30.5–61.3)a |
n = 76 69.2 (48.1–90.3)a |
0.040 |
n = 35 55 |
n = 24 76 |
|||||||||
| Kursh 2003 |
n = 35 87.5b |
n = 37 81.0b |
n = 35 46.0b |
n = 35 42.4b |
n = 35 44.0b |
n = 35 57.7b |
|||||||||||||||
| Liedberg 2003 |
n = 11 117,b IQR (61–200) |
n = 20 96,b IQR (64–190) |
n = 10 0,b IQR (0–53) |
n = 19 74,b IQR (38–110) |
<0.05 |
n = 8 22,b IQR (3–62) |
n = 19 126,b IQR (25–190) |
<0.05 | |||||||||||||
| Mårtenson 1999 |
n = 14 88, SD 126 |
n = 30 116, SD 146 |
n = 14? 12, SD 19 |
n = 30? 58, SD 103 |
n = 14? 14, SD 27 |
n = 30? 60, SD 56 |
n = 14? 14, SD 21 |
n = 30? 59, SD 77 |
n = 14? 63, SD 100 |
n = 30? 94, SD 128 |
|||||||||||
| Detrusor pressure (cmH2O) | Kabalin 1995 |
n = 12 92.3, SE 3.4 |
n = 13 91.3, SE 5.2 |
n = 10 58.7, SE 4.9 |
n = 10 54.6, SE 6.9 |
||||||||||||||||
| Prostate size (ml) | Costello 1995 |
n = 37 33.89 (12–70) |
n = 34 29.96 (13.9–77) |
||||||||||||||||||
| Cowles 1995 |
n = 59 38.6, SD 20.2 (11.2–108.2) |
n = 56 42.2, SD 19 (7.7–93.9) |
|||||||||||||||||||
| Donovan 2000; CLasP study; symptomatic patients |
n = 117 38.1, SD 19.1 |
n = 117 40.7, SD 21.4 |
|||||||||||||||||||
| Gujral 2000; CLasP study |
n = 44 49.7, SD 21.8 |
n = 38 40.7, SD 19.9 |
|||||||||||||||||||
| Kabalin 1995 |
n = 12 34.2, SE 2.2 |
n = 13 38.9, SE 4.5 |
n = 10 13.7, SE 4.5 |
n = 13 28.8, SE 4.5 |
|||||||||||||||||
| Kim 2006a |
n = 110 44.2 |
n = 89 42.7 |
n = 110? 24.9 |
n = 89? 26.6 |
n = 110? 25.1 |
n = 89? 24.6 |
n = 110? 25.3 |
n = 89? 27.7 |
|||||||||||||
| Kursh 2003 |
n = 35 40.0b |
n = 35 41.5b |
n = 35 27.0b |
n = 35 35.1b |
n = 35 18.6b |
n = 35 38.4b |
|||||||||||||||
| Liedberg 2003 |
n = 11 47,b IQR (37–61) |
n = 20 49,b IQR (41–75) |
n = 11 22,b IQR (15–28) |
n = 20 37,b IQR (30–49) |
<0.05 |
n = 9 27,b IQR (20–35) |
n = 19 35,b IQR (26–42) |
< 0.05 | |||||||||||||
| Mårtenson 1999 |
n = 14 50, SD 16 |
n = 30 46, SD 20 |
n = 14? 28, SD 11 |
n = 30? 40, SD 21 |
|||||||||||||||||
| Suvakovic 1996 |
n = 10 22 g, SD 5 |
n = 10 23.6 g, SD 6.4 |
|||||||||||||||||||
| Outcome | Study | TURP | Laser coagulation | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Costello 1995 | N = 37 |
N = 34 6.42 (2.3–13.5) |
Lasing time | ||||
| Cowles 1995 |
N = 59 45.2, SD 21.5 |
N = 56 23.4, SD 11.1 |
p < 0.01 | |||||
| Kabalin 1995 |
N = 58.3 (45–85) |
N = 24.2 (15–40) |
p = NS | |||||
| Kim 2006a |
N = 110 51 (20–85) |
N = 89 38 (20–60) |
Mean or median (range) | |||||
| Suvakovic 1996 |
N = 10 20.1, SD 6.2 |
N = 10 18.9, SD 8.8 |
p = NS | |||||
| Length of hospital stay (days) | Chacko 2001 |
N = 74 5.8, 95% CI 5.2,6.5 |
N = 74 3.4, 95% CI 2.8,4.0 |
Geometric mean; p < 0.0001 | ||||
| Costello 1995 |
N = 37 5.8 (3–31) |
N = 34 6.2 (3–21) |
||||||
| Cowles 1995 |
N = 59 3.1, SD 0.9 |
N = 56 1.8, SD 1.1 |
p < 0.01 | |||||
| Donovan 2000 |
N = 117 3.9, 95% CI 3.7,4.2 |
N = 117 2.2, 95% CI 1.9,2.4 |
Geometric mean; p < 0.0001 | |||||
| Gujral 2000 |
N = 44 4.4, 95% CI 3.9,4.9 |
N = 36 2.2, 95% CI 1.7,2.8 |
Geometric mean; p < 0.0001 | |||||
| Kim 2006a |
N = 110 6.5 (5–8) |
N = 89 1.2 (1–3) |
Mean or median (range) | |||||
| Kursh 2003 |
N = 35 1.40 (0.42–5) |
N = 35 Outpatient |
Median | |||||
| Liedberg 2003 |
N = 11 3 (3–4) |
N = 20 2.5 (0.25–3.8) |
Median (IQR, interquartile range) | |||||
| McAllister 2000 |
N = 75 4.3, 95% CI 3.3,5.3 |
N = 76 2.7, 95% CI 2.2,3.2 |
p = NS | |||||
| Suvakovic 1996 |
N = 10 3.5 |
N = 10 1.25 |
p < 0.05 | |||||
| Outcome | Study | TURP | Laser coagulation | |||||
| N | n | % | N | n | % | |||
| Reoperation | Chacko 2001 | 74 | 1 | 1.3 | 74 | 7 | 9.4 | Because of unacceptable symptoms or failure to void (follow-up 7.5 months); p = 0.008 |
| Costello 1995 | 37 | 2 | 5.4 | 34 | 5 | 14.7 | TURP: underwent BNI because of bladder neck stenosis. Laser: two underwent BNI because of bladder neck stenosis; three TURP. Follow-up 6 months | |
| Cowles 1995 | 59 | 0 | 0 | 56 | 2 | 5.74 | Both received VLAP. Time point is unclear (total follow-up 12 months) | |
| Gujral 2000 | 44 | 0 | 0 | 38 | 3 | 7.9 | Received TURP. Total follow-up 7.5 months | |
| Kabalin 1995 | 12 | 1 | 8.3 | 13 | 2 | 15.4 | TURP: at 6 months visual internal urethrotomy because of stricture. Laser: same procedure and have improved voiding | |
| Kim 2006a | 101 | 0 | 0 | 89 | 1 | 1.1 | Up to 12 months | |
| Kursh 2003 | 35 | 0 | 0 | 35 | 6 | 17 | Two had ILC/TURP before 6 months and four had TURP before 1 year. In a 2-year follow-up there was a total of six reoperations. The retreated group still remained obstructed | |
| Mårtenson 1999 | 14 | 1 | 7.1 | 30 | 6 | 20 | Within 24 months. TURP: underwent urethrotomy at 5.5 months. Laser: underwent TURP at 8.5–24 months | |
| McAllister 2000 | 75 | 0 | 0 | 76 | 3 | 3.9 | At 3 months (TURP) | |
| 75 | 0 | 0 | 76 | 2 | 2.6 | At 10 and 11 months (BNI) | ||
| 51 | 8 | 15.7 | 47 | 18 | 38 | Total reoperation up to 5 years | ||
| N = number of patients participating in the 5-year review; p = 0.006 | ||||||||
Appendix 8.5 TUIP versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | 24 months | 3 years | 5 years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUIP | TURP | TUIP | TURP | p-value | TUIP | TURP | p-value | TUIP | TURP | p-value | TURP | TUIP | p-value | TUIP | TURP | p-value | TUIP | TURP | p-value | |
| Christensen 1990 (score unclear) |
n = 38 16a (8–23) |
n = 38 16a (7–23) |
n = 35 4a (0–19) |
n = 38 4a (0–12) |
NS |
n = 26 4.5a (0–15) |
n = 26 4a (0–12) |
NS |
n = 22 5a (0–16) |
n = 11 3a (0–22) |
NS |
n = 9 8a (2–12) |
n = 11 4a (1–13) |
NS | ||||||
| Dørflinger 1992 (Madsen) |
n = 29 15a |
n = 29 16a |
n = 22 2.5a p < 0.05 |
n = 29 1a p < 0.05 |
NS |
n = 21 2a |
n = 26 2a |
NS | ||||||||||||
| Jahnson 1998 (Madsen) |
n = 43 15.4 (6–27) |
n = 42 15.8 (5–28) |
n = 41 3.5b (0–21) p < 0.05 |
n = 39 3.8b (0–16) p < 0.05 |
NS |
n = 36 4.3 (0–21) p < 0.05 |
n = 34 3.5 (0–18) p < 0.05 |
NS |
n = 31 3.6 (0–15) p < 0.05 |
n = 32 2.8 (0–11) p < 0.05 |
NS |
n = 33 3.0 (0–16) p < 0.05 |
n = 31 3.4 (0.15) p < 0.05 |
NS |
n = 22 4.5 (0–14) p < 0.05 |
n = 24 4.7 (0.17) p < 0.05 |
NS | |||
| Riehmann 1994 (Madsen) |
n = 61 15.5c |
n = 56 15.0c |
n = 51 5c |
n = 52 5c |
NS |
n = 60 6.0c |
n = 46 5.5c |
NS |
n = 41 7c |
n = 40 5c |
n = 22 8c |
n = 19 6.3c |
n = 8 9c |
n = 15 9.1c |
||||||
| Roberto Aliaga 1998 (AUA) |
n = 20 24.4, SD 10.3 |
n = 21 24.2, SD 7.7 |
n = 20 ? 4.3 ± 4.5 |
n = 21 ? 4.8, SD 4.8 |
n = 20 ? 5.7, SD 6.2 p < 0.05 |
n = 21 ? 3.7, SD 3.8 p < 0.05 |
||||||||||||||
| Saporta 1996 (Madsen) |
n = 20 14.7, SE 0.96 (7–21) |
n = 20 14.3, SE 0.93 (6–22) |
n = 17 5.29, SE 0.62 (2–13) p < 0.05 |
n = 20 4.95, SE 0.74 (1–14) p < 0.05 |
p<0.05 |
n = 17 7.0, SE 0.64 (3–14) p < 0.05 |
n = 19 5.79, SE 0.85 (1–18) p < 0.05 |
p>0.05 | ||||||||||||
| Trocz 2002 (IPSS) |
n = 50 17.1, SD 2.2 |
n = 50 17.1, SD 1.9 |
n = 50 4.1, SD 1.8 p < 0.001 |
n = 50 5.1, SD 1.9 p < 0.001 |
||||||||||||||||
| Complication | Study | TURP | TUIP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Bleeding at home | Hellström 1986 | 13 | 2 | 15 | 11 | 0 | 0 | One was catheterised and the other was hospitalised for two days |
| Blood loss (ml) | Christensen 1990 | 38 | 150 (25–350) | 38 | 25 (5–300) | Median (range); p = 0.0001 | ||
| Dørflinger 1992 | 31 | 65 | 29 | 10 | Median; p < 0.001 | |||
| Jahnson 1998 | 42 | 211 (0–700) | 43 | 37 (0–300) | Mean (range); p < 0.05 | |||
| Nielsen 1998 | 25 | 170 (75–500) | 24 | 70 (10–300) | Median (range); p < 0.01 | |||
| Riehmann 1994 | 56 | 190 (25–700) | 60 | 54 (5–300) | Mean (range); p < 0.0001 | |||
| Blood transfusion | Rodrigo Aliaga 1998 | 21 | 1 | 4.8 | 20 | 0 | 0 | |
| Dørflinger 1992 | 31 | 4 | 13 | 29 | 0 | 0 | p = 0.11, Fisher’s exact test | |
| Hellström 1986 | 13 | 0 | 0 | 11 | 0 | 0 | ||
| Jahnson 1998 | 42 | 1 | 2.4 | 43 | 0 | 0 | ||
| Li 1987 | 30 | 13 | 43.3 | 29 | 2 | 6.9 | p = 0.004 | |
| Nielsen 1998 | 25 | 20 | 80 | 24 | 1 | 4.2 | p < 0.02 | |
| Soonawalla 1992 | 110 | 38 | 34.5 | 110 | 0 | 0 | ||
| Cardiovascular events | Christensen 1990 | 38 | 0 | 0 | 38 | 1 | 2.6 | Pulmonary oedema due to malfunction of the transurethral catheter |
| Li 1987 | 30 | 0 | 0 | 29 | 0 | 0 | ||
| Clot retention | Li 1987 | 30 | 0 | 0 | 29 | 0 | 0 | |
| Nielsen 1998 | 25 | 1 | 4 | 24 | 1 | 4.2 | Both needed to return to the operating theatre | |
| Haemorrhage | Li 1987 | 30 | 0 | 0 | 29 | 0 | 0 | |
| Soonawalla 1992 | 110 | 3 | 2.7 | 110 | 0 | 0 | Haemorrhage requiring open surgery | |
| Incontinence | Li 1987 | 30 | 2 | 6.7 | 29 | 1 | 3.4 | Mild transient for 2 weeks |
| Soonawalla 1992 | 110 | 4 | 3.6 | 110 | 2 | 1.8 | ||
| Talic 2002 | 50 | 0 | 0 | 50 | 0 | 0 | Follow-up unclear | |
| Mortality | Christensen 1990 | 38 | 2 | 5.3 | 38 | 2 | 5.3 | TURP: one died 24 days after TURP as a result of pulmonary saddle embolism; one died from causes unrelated to genitourinary disease before 3 months. TUIP: two died before 3-month follow-up from causes unrelated to genitourinary disease |
| Li 1987 | 30 | 0 | 0 | 29 | 0 | |||
| Nielsen 1998 | 25 | 1 | 4 | 24 | 1 | 4.2 | TURP: ischaemic heart disease. TUIP: colonic cancer | |
| Jahnson 1998 | 42 | 1 | 2.4 | 43 | Cardiovascular lesion | |||
| Riehmann 1994 | 56 | 8 | 14.3 | 60 | 14 | 23 | Mortality within total observational period (follow-up of up to 82 months); p = NS. Causes of death TURP: laryngeal cancer, brain tumor, pulmonary embolism, five unknown. Causes of death TUIP: lung cancer, suicide, car accident, unknown in 11 | |
| Soonawalla 1992 | 110 | 2 | 1.8 | 110 | 1 | 0.9 | ||
| Perforation | Soonawalla 1992 | 110 | 3 | 2.7 | 110 | 2 | 0.27 | Perforation requiring open surgery |
| Recatheterisation | Hellström 1986 | 13 | 1 | 7.5 | 11 | |||
| Li 1987 | 30 | 2 | 6.7 | 29 | 0 | 0 | Second haemorrhage requiring recatheterisation and irrigation | |
| Septicaemia | Nielsen 1998 | 25 | 2 | 8 | 24 | 1 | 4.2 | p = NS |
| TUR syndrome | Li 1987 | 30 | 0 | 0 | 29 | 0 | 0 | |
| Soonawalla 1992 | 110 | 7 | 6.4 | 110 | 0 | 0 | ||
| Urinary retention | Jahnson 1998 | 42 | 1 | 2.4 | 43 | At 3 weeks and a bladder neck stricture was incised 3 weeks later | ||
| Li 1987 | 30 | 0 | 0 | 29 | 0 | 0 | ||
| Nielsen 1998 | 25 | 0 | 0 | 24 | 3 | 12.5 | These three patients were reoperated by TURP | |
| Soonawalla 1992 | 110 | 4 | 3.6 | 110 | 7 | 6.4 | Failed to void after catheter removal | |
| Urinary tract infection | Soonawalla 1992 | 110 | 2 | 1.8 | 110 | 5 | 4.5 | Epididymo-orchitis |
| Postoperative (3–12 months) | ||||||||
| Erectile dysfunction | Christensen 1990 | 20 | 1 | 5 | 24 | 1 | 4.2 | Did not retain sexual activity with loss of ejaculation |
| Dørflinger 1992 | 24 | 4 | 16.6 | 19 | 1 | 5.3 | Worse potency at 12 months | |
| Saporta 1996 | 10 | 1 | 10 | 16 | 2 | 12.5 | Impotence at end of first year | |
| Soonawalla 1992 | 49 | 0 | 0 | 60 | 0 | 0 | Erectile capacity (loss of) | |
| Irritative urinary symptoms | Rodrigo Aliaga 1998 | 21 | 1 | 4.8 | 20 | 1 | 5 | More than 15 days |
| Retrograde ejaculation | Christensen 1990 | 19 | 7 | 37 | 23 | 3 | 13 | p > 0.1; loss of ejaculation; it is unclear whether data are for follow-up 3–12 months or cumulative for 4 years |
| Dørflinger 1992 | 24 | 12 | 50 | 19 | 1 | 5.2 | At 12 months | |
| Hellström 1986 | 13 | 8 | 62 | 7 | 0 | 0 | ||
| Riehmann 1994 | 22 | 15 | 68 | 23 | 8 | 35 | p < 0.02 | |
| Rodrigo Aliaga 1998 | 21 | 15 | 71.4 | 20 | 14 | 70 | Follow-up 6 months; unclear whether all participants are sexually active | |
| Saporta 1996 | 10 | 9 | 90 | 16 | 3 | 18.7 | At end of first year | |
| Soonawalla 1992 | 49 | 13 | 26.5 | 60 | 14 | 23.3 | Loss of ejaculation | |
| Talic 2002 | 50 | 16 | 32 | 50 | 6 | 12 | Unclear whether all participants are sexually active | |
| Stricture/bladder neck | Dørflinger 1992 | 31 | 0 | 0 | 29 | 1 | 3.4 | Urethral stricture within 3 months; p = 0.2 |
| Hellström 1986 | 13 | 11 | 1 | 9 | ||||
| Li 1987 | 30 | 2 | 6.7 | 29 | 0 | 0 | One was at bladder neck and the other was at bulbous urethra at 3 months (both were asymptomatic); p = 0.48 | |
| Nielsen 1998 | 25 | 4 | 16 | 24 | At 12 months | |||
| Riehmann 1944 | 56 | 8 | 14 | 60 | 0 | 0 | Bladder neck | |
| Soonawalla 1992 | 110 | 3 | 2.7 | 110 | 5 | 4.5 | ||
| Urinary incontinence | Nielsen 1998 | 25 | 1 | 4 | 24 | |||
| Study | Baseline | 24 months | Comments | ||||
|---|---|---|---|---|---|---|---|
| TURP | TUIP | p-value | TURP | TUIP | p-value | ||
| Tkocz 2002 |
n = 50 4.4, SD 0.3 |
n = 50 4.6, SD 0.5 |
n = 50 1.9, SD 0.6 p < 0.001 |
n = 50 2.1, SD 0.3 p < 0.001 |
IPSS QoL (0–6); no other details were given about the scale | ||
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 24 months | 3 years | 5 years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUIP | TURP | TUIP | p-value | TURP | TUIP | p-value | TURP | TUIP | p-value | TURP | TUIP | p-value | TURP | TUIP | p-value | TURP | TUIP | p-value | ||
| Peak urine flow rate (ml/s) | Rodrigo Aliaga 1998 |
n = 21 8.3, SD 4.5 |
n = 20 8.7, SD 5.5 |
n = 21? 18.6, SD 8.5 |
n = 20? 22.0, SD 12.2 |
n = 21? 20.6, SD 10.1 |
n = 20? 20.6, SD 8.7 |
||||||||||||||
| Christensen 1990 |
n = 34 9.7a (1.7–29.4) |
n = 35 7.8a (2.8–28.0) |
n = 35 16.6a (5.6–46.4) |
n = 31 12.7a (2.6–34.5) |
0.07 |
n = 22 18.5a (4.0–48.5) |
n = 23 13.5a (5.3–45.3) |
0.31 |
n = 15 16.6a (6.2–30.2) |
n = 17 12.6a (7.3–20.7) |
0.04 |
n = 9 14.6a (7.4–38.0) |
n = 9 10.9a (7.8–18.5) |
0.38 36 months = 3–4yrs |
|||||||
| Dørflinger 1992 |
n = 31 8a |
n = 29 10a |
n = 29 18.8a p < 0.05 |
n = 22 15.2a p < 0.05 |
0.025 |
n = 26 20.2a p < 0.05 |
n = 21 14.5a p < 0.05 |
0.025 | |||||||||||||
| Hellström 1986 |
n = 13 7.5, SD 3.8 (1–14) |
n = 11 8.6, SD 4.5 (2–16) |
n = 13 16.5, SD 6.0 (7–25) p < 0.001 |
n = 11 12.9, SD 6.04 (4–21) p = 0.073 |
0.163 | ||||||||||||||||
| Jahnson 1998 |
n = 34 |
n = 36 |
n = 39 |
n = 41 |
< 0.05 |
n = 34 |
n = 36 |
<0.05 |
n = 32 |
n = 31 |
< 0.05 |
n = 31 |
n = 33 |
< 0.05 |
n = 24 |
n = 22 |
|||||
| Li 1987 | NR | NR |
n = 30 19, SE 2.7 (6–68) |
n = 29 23, SE 2.9 (8–50) |
0.09 | ||||||||||||||||
| Nielsen 1988 |
n = 25 5a (5–13) |
n = 24 5a (5–10) |
n = 25 17a (6–32) |
n = 24 10a (7–18) |
< 0.02; 2 months not 3 months |
n = 23 12a (5–28) |
n = 22 9a (5–25) |
||||||||||||||
| Riehmann 1994 |
n = 50 11.2c |
n = 52 9.1c |
n = 44 20c |
n = 42 15c |
0.015 |
n = 8 19e |
n = 4 13e |
6 yearse | |||||||||||||
| Saporta 1996 |
n = 20 6.5, SE 0.43 (3.2–11.9) |
n = 20 7.35, SE 0.56 (3.7–12) |
n = 20 17.29, SE 1.16 (8.2–7.1) |
n = 17 14.58, SE 1.05 (5.3–5.7) |
n = 19 14.36, SE 1.14 (5.5–25.5) |
n = 17 12.65, SE 1.04 (4.1–23.3) |
|||||||||||||||
| Soonawalla 1992 |
n = 110 8.04 |
n = 110 7.91 |
n = 110 20.69 |
n = 110 19.38 |
n = 67 20.1 |
n = 70 19.45 |
n = 26 19.86 |
n = 21 18.91 |
|||||||||||||
| Trocz 2002 |
n = 50 6.9, SD 1.5 |
n = 50 7.6, SD 1.8 |
n = 50 17.6, SD 1.7 p < 0.01 |
n = 50 16.9, SD 1.9 p < 0.01 |
|||||||||||||||||
| Mean urine flow (ml/s) | Soonawalla 1992 |
n = 110 3.99 |
n = 110 3.82 |
n = 110 10.61 |
n = 110 11.32 |
n = 67 10.61 |
n = 70 11.21 |
n = 21 11.04 |
n = 26 11.94 |
||||||||||||
| Total voided volume (ml) | Dørflinger 1992 |
n = 31 176a |
n = 29 200a |
n = 29 166a |
n = 22 183a |
n = 26 273a |
n = 29 207a |
||||||||||||||
| Riehmann 1994 | No significant differences were observed (range: 170–344 ml) | ||||||||||||||||||||
| Residual volume (ml) | Aliaga 1998 |
n = 21 89, SD 92 |
n = 20 146, SD 133 |
n = 21? 76, SD 97 |
n = 20? 61, SD 95 |
n = 21? 38, SD 51 |
n = 20? 60, SD 82 |
||||||||||||||
| Hellström 1986 |
n = 12 43, SD 49.6 (0–145) |
n = 9 62, SD 74.5 (5–230) |
n = 12 28.5, SD 38.5 (0–140) |
n = 9 94, SD 133.1 (0–380) |
0.163 | ||||||||||||||||
| Jahnson 1998 |
n = 42 109 (0–400) |
n = 43 139 (0–660) |
n = 39 29d (0–125) |
n = 41 75d (0–310) |
< 0.05 | ||||||||||||||||
| Soonawalla 1992 |
n = 110 8.04 |
n = 110 7.91 |
Number of patients with high residual volume = 9 (6.2%)f | Number of patients with high residual volume = 8 (7.3%)f | Number of patients with high residual volume = 9 (6.2%)f | Number of patients with high residual volume = 8 (7.3%)f | Number of patients with high residual volume = 9 (6.2%)f | Number of patients with high residual volume = 8 (7.3%)f | |||||||||||||
| Detrusor pressure (cmH2O) | Hellström 1986 |
n = 13 58, SD 34.1 (14–149) |
n = 11 35, SD 18.8 (7–68) |
n = 13 26, SD 11.4 (7–54) |
n = 11 35, SD 10.8 (20–54) |
0.04 | |||||||||||||||
| Trocz 2002 |
n = 50 8.5, SD 8 |
n = 50 8.4, SD 10 |
n = 50 44, SD 6 p < 0.001 |
n = 50 45, SD 6 p < 0.001 |
|||||||||||||||||
| Outcome | Study | TURP | TUIP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Dørflinger 1992 |
N = 31 30 |
N = 29 15 |
Median ; p < 0.01 | ||||
| Hellström 1986 |
N = 13 36, SD 10.1 (20–55) |
N = 11 16, SD 5.59 (15–30) |
p < 0.001 | |||||
| Jahnson 1998 |
N = 42 32 (15–60) |
N = 43 15 (5–40) |
||||||
| Li 1987 |
N = 30 35.5, SE 3.6 (15–75) |
N = 29 18.8, SE 2.9(10–60) |
p = 0.0002 | |||||
| Nielsen 1998 |
N = 25 45 (20–80) |
N = 24 18 (10–35) |
||||||
| Riehmann 1994 |
N = 56 55 (5–135) |
N = 61 23 (7–95) |
p < 0.0001 | |||||
| Soonawalla 1992 |
N = 110 59.2 (30–95) |
N = 110 20.4 (10–40) |
||||||
| Length of hospital stay (days) | Aliaga 1998 |
N = 21 4.9 |
N = 20 4.8 |
|||||
| Christensen 1990 |
N = 34 4 (2–10) |
N = 35 3 (1–8) |
Median (range); p = 0.0000 | |||||
| Dørflinger 1992 |
N = 29 3 |
N = 22 3 |
Median; p = NS | |||||
| Hellström 1986 |
N = 13 8.4, SD 2.69 (5–13) |
N = 11 6.2, SD 1.94 (4–10) |
p = 0.05 | |||||
| Li 1987 |
N = 30 8.0, SE 1.3 (2–39) |
N = 29 5.6, SE 0.6 (3–14) |
p = 0.08 | |||||
| Nielsen 1988 |
N = 25 3 (2–13) |
N = 24 3(2–18) |
Median (range); p = NS | |||||
| Riehmann 1995 |
N = 52 4.3 (2–14) |
N = 57 3.0 (1–8) |
Mean (range) | |||||
| Soonawalla 1992 |
N = 110 7.16 |
N = 110 6.03 |
Mean | |||||
| N | n | % | N | n | % | |||
| Reoperation | Aliaga 1998 | 21 | 1 | 4.8 | 20 | 1 | 5 | One TURP was reoperated because of bladder neck sclerosis. One TUIP was reoperated because of a Retzius abscess in a participant who had a prostate size of 60 g |
| Dørflinger 1992 | 31 | 1 | 3.2 | 29 | 296 | 20.7 | Within the first month | |
| Nielsen 1998 | 25 | – | – | 24 | 3 | 12.5 | At 2 months, underwent TURP | |
| Riehmann 1994 | 56 | 9 | 16 | 60 | 13 | 21.6 | TURP: eight TURBNC or TUIP and one TURP at mean follow-up of 18 months (6–30). TUIP: 12 TURP and one TUIP at mean follow-up of 31 months (1–61); p = 0.908 | |
| Saporta 1996 | 20 | 0 | 0 | 20 | 3 | 15 | Two underwent TURP and one underwent TUIP | |
| Christensen 1990 | 38 | 7 | 18.4 | 38 | 5 | 13.1 | TURP patients underwent BNI for bladder neck contracture at 6, 10, 13, 20, 25, 26 and 30 months after TURP. TUIP patients underwent TURP at 25, 27, 32 and 36 months after TUIP | |
| Jahnson 1998 | 42 | 3 | 7.1 | 43 | 10 | 23.2 | TURP: bladder neck stricture was incised 3 weeks after the operation; total reoperation at a mean follow-up of 11 months (2–25). TUIP: two TURPs at 6 weeks; total reoperation at a mean follow-up of 16 months (1–38); p = 0.039 | |
Appendix 8.6 Laser resection versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | 24 months | 5 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | Laser resection | TURP | Laser resection | p-value | TURP | Laser resection | p-value | TURP | Laser resection | p-value | TURP | Laser resection | p-value | TURP | Laser resection | p-value | |
| Gupta 2006 (IPSS) |
n = 50 23.3, SD 3.9 (17–31) |
n = 50 23.4, SD 4.5 (13–34) |
n = 50? 6.1, SD 0.42 (0–16) |
n = 50? 5.2, SD 0.31 (0–14) |
n = 50? 5.6, SD 0.32 (0–9) |
n = 50? 5.2, SD 0.17 (0–8) |
|||||||||||
| Kuntz 2004 (AUA) |
n = 100 21.4, SD 5.5 (9–32) |
n = 100 21.4, SD 5.5 (9–32) |
n = 89 3.7, SD 3.7 (3–4) |
n = 94 2.2, SD 1.6 (0–9) |
0.006 |
n = 86 3.9, SD 3.9 (0–19) |
n = 89 1.7, SD 1.8 (0–9) |
0.0001 | |||||||||
| Montorsi 2004 (IPSS) |
n = 52 21.6, SD 6.7 |
n = 48 21.9, SD 7.2 |
n = 52? 3.9, SD 2.9 |
n = 48? 2.9, SD 2.6 |
0.72 |
n = 52? 4.1, SD 2.3 |
n = 48? 3.9, SD 3.6 |
0.58 | |||||||||
| Westenberg 2004 (AUA) |
n = 59 23.0, SD 5.9 (9–35) |
n = 61 21.9, SD 6.2 (10–35) |
n = 59 5.7, SD 5.2 (0–30) |
n = 61 5.6, SD 5.1 (0–25) |
0.88 |
n = ? 5.0, SD 4.5 (0–23) |
n = ? 3.8, SD 3.8 (0–24) |
0.17 |
n = ? 4.3, SD 4.1 (0–16) |
n = ? 4.2, SD 6.0 (0–29) |
0.92 |
n = 41 3.7, SD 4.9 (0–21) |
n = 45 3.4, SD 4.9 (0–23) |
0.84 |
n = 30 6.6. a SD 5.0 (1–20) |
n = 43 5.2,a SD 5.9 (0–21) |
0.32 |
| Wilson 2006 (Tan 2003) (AUA) |
n = 30 23.7, SE 1.2 (9–35) |
n = 30 26.0, SE 1.1 (14–35) |
n = 29 3.4, SE 0.9 (0–24) |
n = 28 4.8, SE 0.8 (0–18) |
NS |
n = 29 4.8, SE 0.7 (0–18) |
n = 26 6.0, SE 1.0 (0–17) |
NS |
n = 27 5.0, SE 0.9 (0–21) |
n = 25 4.3, SE 0.7 (1–14) |
NS |
n = 26 5.2, SE 0.8 |
n = 25 6.1, SE 1.0 |
||||
| Complication | Study | TURP | Laser resection | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Blood loss | Montorsi 2004 | 48 | 1.29, SD 2.1 | 52 | 1.32, SD 1.8 | g/dl (mean, SD); p = NS | ||
| Gupta 2006 | 50 | 140.5, SD 60.7 (60–315) | 50 | 40.6, SD 37.3 (30–240) | ml (mean, SD) | |||
| Capsular perforation | Gupta 2006 | 50 | 0 | 0 | 50 | 1 | 2 | |
| Blood transfusion | Kuntz 2004 | 100 | 2 | 2 | 100 | 0 | 0 | p = 0.50 |
| Montorsi 2004 | 48 | 1 | 2 | 52 | 0 | 0 | ||
| Tan 2003 | 30 | 1 | 3.3 | 30 | 0 | 0 | ||
| Westenberg 2004 | 59 | 4 | 6.8 | 61 | 0 | 0 | ||
| Gupta 2006 | 50 | 1 | 2 | 50 | 0 | 0 | ||
| Urinary tract infection | Tan 2003 | 30 | 2 | 6.6 | 30 | 0 | 0 | |
| Urinary retention | Montorsi 2004 | 48 | 1 | 2.1 | 52 | 3 | 5.8 | Acute urinary retention at 1 month |
| Recatheterisation | Kuntz 2004 | 100 | 5 | 5 | 100 | 0 | 0 | p = 0.06 |
| Tan 2003 | 30 | 4 | 13.3 | 30 | 5 | 16.6 | ||
| Westenberg 2004 | 59 | 8 | 13.1 | 61 | 5 | 8.2 | p = NS | |
| Gupta 2006 | 50 | 3 | 6 | 50 | 2 | 6 | For 24–72 hours | |
| TUR syndrome | Montorsi 2004 | 48 | 1 | 2.1 | 52 | 0 | 0 | At 1 month |
| Cardiovascular events | Westenberg 2004 | 59 | 1 | 1.7 | 61 | 0 | 0 | |
| Westenberg 2004 | 59 | 1 | 1.7 | 61 | 1 | 1.6 | TURP: at 3 months. Laser resection: because of myocardial infarction at 8 days postoperatively | |
| Gupta 2006 | 50 | 0 | 0 | 50 | 0 | 0 | ||
| Incontinence | Montorsi 2004 | 48 | 17 | 35.4 | 52 | 25 | 48 | Transitory urge at 1 month |
| Postoperative (3–12 months) | ||||||||
| Stricture | Kuntz 2004 | 88 | 2 | 2.2 | 95 | 6 | 6.3 | TURP: one urethral at 6 months and three bladder neck at 12 months. Laser: one urethral at 6 months and three bladder neck at 12 months; p = 0.62 |
| Montorsi 2004 | 48 | 4 | 8.3 | 52 | 1 | 1.9 | ||
| Urinary incontinence | Tan 2003 | 28 | 3 | 10.7 | 29 | 1 | 3.4 | TURP: one submeatal, one bulbar. Laser resection: submeatal |
| Gupta 2006 | 50 | 2 | 4 | 50 | 1 | 2 | Treated with internal urethrotomy. Time point is unclear (follow-up 12 months) | |
| Kuntz 2004 | 53 | 1 | 1.9 | 62 | 1 | 1.6 | Stress incontinence at 12 months | |
| Montorsi 2004 | 48 | 1 | 2.1 | 52 | 1 | 1.9 | Stress incontinence at 6–12 months | |
| Westenberg 2004 | 59 | 2 | 3.4 | 61 | 1 | 1.6 | Required pads | |
| Gupta 2006 | 50 | 1 | 2 | 50 | 1 | 2 | Transient incontinence. Time point is unclear (follow-up 12 months) | |
| Retrograde ejaculation | Westenberg 2004 | 37 | 32 | 86 | 25 | 24 | 96 | At 12 months |
| Mortality | Tan 2003 | 30 | 1 | 3.3 | 30 | 0 | 0 | Mortality at 6 months as a result of cardiovascular disease |
| Postoperative (> 12 months) | ||||||||
| Stricture | Westenberg 2004 | 59 | 6 | 10.2 | 61 | 6 | 9.8 | One required operative intervention for stricture and the rest were treated with urethral dilation; at 48 months; p = NS |
| Urinary incontinence | Westenberg 2004 | 59 | 17 | 61 | 20 | At 48 months | ||
| Retrograde ejaculation | Wilson 2006 | 13 | 8 | 61.5 | 16 | 12 | 75 | At 24 months |
| Erectile dysfunction | Westenberg 2004 | 17 | 8 | |||||
| Wilson 2006 | 26 | 2 | 7.7 | 22 | 2 | 9 | At 24 months | |
| Urinary tract infection | Westenberg 2004 | 59 | 3 | 5.1 | 61 | 5 | 8.1 | p = NS |
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 18 months | 24 months | 48 months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | Laser resection | TURP | Laser resection | p-value | TURP | Laser resection | p-value | TURP | Laser resection | p-value | TURP | Laser resection | p-value | TURP | Laser resection | p-value | TURP | Laser resection | p-value | ||
| Peak urine flow rate (ml/s) | Gupta 2006 |
n = 50 4.5, SD 4.7 (0–13) |
n = 50 5.15, SD 4.4 (0–12) |
n = 50? 20.7, SD 1.32 (10–39) |
n = 50? 23.1, SD 1.2 (15–40) |
0.33 |
n = 50? 23.7, SD 1.58 (9–41) |
n = 50? 25.1, SD 1.06 (12–45) |
0.62 | ||||||||||||
| Montorsi 2004 |
n = 48 7.8, SD 3.6 |
n = 52 8.2, SD 3.2 |
n = 48? 26.5, SD 15.5 |
n = 52? 23.1, SD 8.6 |
0.007 |
n = 48? 24.7, SD 10 |
n = 52? 25.1, SD 7.2 |
0.25 | |||||||||||||
| Wilson 2006; in Tan 2003 |
n = 30 8.3, SE 0.4 (3–12) |
n = 30 8.4, SE 0.5 (2–14) |
n = 29 18.9, SE 1.9 (6–41) |
n = 28 24.2, SE 1.7 (11–52) |
n = 29 20.8, SE 2.3 (7–48) |
n = 26 26.4, SE 1.8 (13–65) |
NS |
n = 27 18.4, SE 2.8 (2–40) |
n = 25 21.8, SE 2.1 (8–36) |
n = 26 19.3, SE 2.2 |
n = 22 21.0, SE 2.0 |
||||||||||
| Kuntz 2004 |
n = 100 5.9, SD 3.9 (0–12) |
n = 100 4.9, SD 3.8 (0–11) |
n = 89 25.1, SD 9.4 (8–47) |
n = 94 25.1, SD 6.9 (10–49) |
0.72 |
n = 86 27.7, SD 12.2 (8–56) |
n = 89 27.9, SD 9.9 (5–53) |
0.76 | |||||||||||||
| Westenberg 2004 |
n = 59 9.1, SD 3.2 (3–14) |
n = 61 8.9, SD 3.0 (3–14) |
n = 59? 20.2, SD 9.5 (6–50) |
n = 61? 22.8, SD 10.0 (6–50) |
0.16 |
n = 59? 22.4, SD 9.0 (8–43) |
n = 61? 23.9, SD 8.7 (7–50) |
0.35 |
n = 59? 20.4, SD 8.5 (6–44) |
n = 61? 25.2, SD 11.9 (6–63) |
< 0.05 |
n = 59? 19.2, SD 9.3 (7–41) |
n = 61? 25.1, SD 9.3 (10–44) |
< 0.01 |
n = 41 20.9, SD 11.1 (6–39) |
n = 45 25.0, SD 11.0 (3–74) |
0.14 |
n = 30 18.5, SD 8.2 (3–43) |
n = 43 22.3, SD 14.2 (5–58) |
0.23 | |
| Mean urine flow rate (ml/s | Montorsi 2004 |
n = 48 4.3, SD 2.3 |
n = 52 4.3, SD 2.0 |
n = 48? 9.1, SD 3.6 |
n = 52? 13.3, SD 5.7 |
0.01 |
n = 48? 12.1, SD 3.3 |
n = 52? 15.5, SD 4.2 |
0.01 | ||||||||||||
| Wilson 2006 |
n = 30 85.8, SE 5.4 (46–156) |
n = 30 76.2, SE 4.4 (44–137) |
n = 29 40.7, SE 2.7 (10–97) p < 0.01 |
n = 26 20.8, SE 2.8 (4–41) p < 0.01 |
< 0.001 | ||||||||||||||||
| Kuntz 2004 |
n = 100 87.3, SD 31.4 (46–150) |
n = 100 83.5, SD 34.9 (50–197) |
|||||||||||||||||||
| Westenberg 2004 |
n = 59 83.4, SD 27.9 (43–143) |
n = 61 75.9, SD 26.2 (39–149) |
n = 59? 39.2 (13–77) |
n = 61? 35.2 (43–143) |
NS | ||||||||||||||||
| Residual volume (ml) | Gupta 2006 |
n = 50 84.0, SD 129.7 (0–600) |
n = 50 112.0, SD 155.9 (0–780) |
< 20 | < 20 | < 20 | < 20 | ||||||||||||||
| Tan 2003 |
n = 30 126, SE 21.32 (1–394) |
n = 30 113.5, SE 15.5 (19–380) |
n = 29 51.8, SE 14.5 (0–324) |
n = 26 33.7, SE 5.5 (0–105) |
NS | ||||||||||||||||
| Kuntz 2004 |
n = 100 216, SD 177 (50–800) |
n = 100 238, SD 163 (50–1000) |
n = 89 16.7, SD 16.9 (0–130) |
n = 94 4.8, SD 12.5 (0–60) |
< 0.0001 |
n = 86 26.6, SD 60.4 (0–150) |
n = 89 5.3, SD 15.3 (0–70) |
< 0.0001 | |||||||||||||
| Westenberg 2004 |
n = 59 84.7, SD 81.7 (0–373) |
n = 61 87.8, SD 88.4 (0–346) |
n = 59? 34.3 (0–295) |
n = 61? 26.7 (0–245) |
NS | ||||||||||||||||
| Prostate size (ml) | Gupta 2006 |
n = 50 59.8, SD 16.5 (40–100) |
n = 50 57.9, SD 17.6 (41–125) |
||||||||||||||||||
| Montorsi 2004 |
n = 48 56.2, SD 19.4 |
n = 52 70.3, SD 36.7 |
|||||||||||||||||||
| Wilson 2006 |
n = 30 70.0, SE 5.0 (46–156) |
n = 30 77.8, SE 5.6 (42–152) |
n = 29 46.6, SE 4.4 (26–96) < 0.001 |
n = 26 28.4, SE 1.8 (13–43) p < 0.001 |
< 0.001 | ||||||||||||||||
| Kuntz 2004 |
n = 100 49.9, SD 21.1 (20–99) |
n = 100 53.5, SD 20.0 (20–95) |
|||||||||||||||||||
| Westenberg 2004 |
n = 59 44.6, SD 20.7 (11.5–93) |
n = 61 44.3, SD 19.0 (11–92) |
n = 59? 27.3 (10–75) |
n = 61? 29.3 (11–61) |
NS | ||||||||||||||||
| Outcome | Study | TURP | Laser resection | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Kuntz 2004 |
N = 100 73.8, SD 24.0 (30–40) |
N = 100 94.6, SD 35.1 (39–209) |
p < 0.0001 | ||||
| Montorsi 2004 |
N = 48 57, SD 15 |
N = 52 74, SD 19.5 |
p < 0.05 | |||||
| Wilson 2006 |
N = 29 33.1, SE 3.7 |
N = 28 62.1, SE 5.9 (11–76) |
||||||
| Westenberg 2004 |
N = 59 25.3, SD 14.7 |
N = 61 41.5, SD 23.1 |
p < 0.001 | |||||
| Gupta 2006 |
N = 50 64, SD 13.1 (40–110) |
N = 50 75.4, SD 22.8 (40–145) |
||||||
| Length of hospital stay (days) | Kuntz 2004 |
N = 100 3.58, SD 1.63 |
N = 100 2.22, SD 0.58 |
p < 0.0001 | ||||
| Montorsi 2004 |
N = 48 3.58, SD 0.79 |
N = 52 2.46, SD 0.83 |
p < 0.001 | |||||
| Tan 2003 |
N = 29 2.08, SE 0.23 |
N = 28 1.15, SE 0.11 |
p < 0.001 | |||||
| Westenberg 2004 |
N = 59 1.98, SD 0.73 |
N = 61 1.08, SD 0.49 |
p < 0.001 | |||||
| Reoperation | N | n | % | N | n | % | ||
| Kuntz 2004 | 88 | 3 | 3.4 | 95 | 1 | 1.05 | Urethral stricture incision at 6 months | |
| 88 | 1 | 1.1 | 95 | 3 | 3.1 | Bladder contracture incision | ||
| Montorsi 2004 | 48 | 1 | 2.1 | 52 | 1 | 1.9 | Reintervention because of bleeding at 1 month | |
| Tan 2003 | 30 | 2 | 6.6 | 30 | 0 | 0 | One underwent HoLEP after 1 month | |
| Westenberg 2004 | 59 | 8 | 13.5 | 61 | 5 | 8.2 | At 48 months follow-up; p = NS | |
Appendix 8.7 Laser vaporisation versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | 18 months | 24 months | 3 years | 5 years | Comments | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | Laser vaporisation | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | ||
| Carter 1999a (IPSS) |
n = 96 19.8 |
n = 95 20.3 |
n = 89 6.4 |
n = 90 6.7 |
NS |
n = 84 5.9 |
n = 86 6.6 |
NS | KTP laser for vaporisation; Nd:YAG laser for coagulation | |||||||||||||||
| Keoghane 2003 (AUA) |
n = 63 19.4, SD 6.5 |
n = 54 19.9, SD 7.7 |
n = 62 6.5, SD 5.1 |
n = 55 9.6, SD 7.5 |
0.029 |
n = 60 5.77, SD 5.4 p = 0.006 |
n = 52 8.87, SD 6.51 p = 0.006 |
< 0.01 |
n = 52 5.7, SD 6.0 |
n = 45 7.8, SD 6.6 |
0.018 |
n = 41 6.5, SD 6.5 |
n = 37 8.9, SD 6.6 |
0.001 |
n = 32 7.0, SD 5.7 |
n = 25 9.7, SD 7.5 |
NS | Nd:YAG SLT MD 60 | ||||||
| van Melick 2003 (IPSS) |
n = 50 16.8, SD 6.0 |
n = 37 3.2, SD 2.7 |
n = 33 5.9, SD 5.5 |
n = 41 4.1, SD 4.8 |
n = 37 3.6, SD 3.4 |
n = 15 5.8, SD 7.5a |
n = 10 9.3, SD 5.2a |
n = 15 7.3, SD 7.1b |
n = 17 8.3, SD 6.4b |
Contact Nd:YAG laser | ||||||||||||||
| Mottet 1999 (IPSS) |
n = 13 23.7 |
n = 17 21.7 |
n = 12 7.5 |
n = 17 8.6 |
n = 11 7.7 |
n = 15 7.3 |
n = 7 4.7 |
n = 8 6.5 |
Holmium:YAG laser | |||||||||||||||
| Mottet 1999 (Madsen) |
n = 13 17 |
n = 17 15 |
n = 12 4.5 |
n = 17 5.2 |
n = 11 4.4 |
n = 15 3.4 |
n = 7 3 |
n = 8 5.1 |
Holmium:YAG laser | |||||||||||||||
| Sengör 1996 (AUA) |
n = 30 22.1, SD 2.6 |
n = 30 21.8, SD 7.6 |
n = 30? 9.8, SD 3.1 |
n = 30? 8.5, SD 4.2 |
n = 30? 9.3, SD 4.2 |
n = 30? 7.8, SD 2.6 |
Non-contact VLAP Nd:YAG laser | |||||||||||||||||
| Shingleton 2002 (AUA) |
n = 50 21.2, SD 6.1 |
n = 50 22.5, SD 6.0 |
n = 48 4 |
n = 48 7 |
0.011 |
n = 48 4 |
n = 46 7 |
0.011 |
n = 33 3.8, SD 4.1 |
n = 40 6.0, SD 6.0 |
n = 19 4.6, SD 4.2c |
n = 23 5.9, SD 5.7c |
n = 33 7.7, SD 5.6d |
n = 29 9.9, SD 6.7d |
NS | KTP/Nd:YAG laser | ||||||||
| Zorn 1999 (AUA) |
n = 11 24.7 |
n = 21 24.0 |
n = 10 8.2 |
n = 19 9.1 |
n = 7 4.7 |
n = 18 8.4 |
Significant | Contact Nd:YAG laser | ||||||||||||||||
| Suvakovic 1996 (AUA) |
n = 10 18.8, SD 4.5 |
n = 10 18, SD 6.6 |
n = 10 12.8, SD 5.9 |
n = 10 9.7, SD 2.6 |
n = 10 8.5, SD 3 |
n = 9 8.7, SD 5.4 |
n = 10? 7.2, SD 6.1 |
n = 10? 8.7, SD 4.9 |
Contact | |||||||||||||||
| Tuhkanen 2001 (Dan PSSI) |
n = 25 23 (5–69) |
n = 21 19 (5–40) |
n = 22 5.6 |
n = 21 10.0 |
NS |
n = 24 4.7 |
n = 21 5.5 |
NS |
n = ? 3.7e |
n = ? 5.5e |
NS |
n = ? 3.4 (0–21) p < 0.001 |
n = ? 7.2 (0–25) p < 0.01 |
NS | Hybrid laser technique; non-contact Nd:YAG laser; large prostates | |||||||||
| Tuhkanen 2003 (Dan PSS I) |
n = 26 18f (4–46) |
n = 26 18f (5–54) |
n = 25 5, SD 6 p < 0.001 |
n = 25 6, SD 7 p < 0.001 |
NS |
n = 26? 3,e SE 1 p < 0.01 |
n = 26 ? 5.5,e SE 2 p < 0.01 |
NS |
n = 20 4f (0–18) p < 0.01 |
n = 22 5f (0–34) p < 0.001 |
Contact Nd:YAG laser; SLT MTRL 10 probe; last is 4 years | |||||||||||||
| Complication | Study | TURP | Laser vaporisation | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative and immediate postoperative) | ||||||||
| Blood loss (ml) | Keoghane 2000 | 76 | 200 | 72 | 39 | Median | ||
| Tuhkanen 2001 | 24 | 332 (50–1000) | 21 | 68 (20–200) | Mean (range); p < 0.001 | |||
| Tuhkanen 2003 | 25 | 175 (30–520) | 25 | 51 (20–75) | p < 0.001 | |||
| Capsular perforation | van Melick 2003 | 50 | 5 | 10 | 45 | 3 | 6.6 | |
| Urethral injury | van Melick 2003 | 50 | 1 | 2 | 45 | 0 | 0 | |
| Haemorrhage | Keoghane 2000 | 76 | 7 | 9.2 | 72 | 3 | 4.1 | Haemorrhage requiring recatheterisation |
| Mottet 1999 | 13 | 0 | 0 | 23 | 1 | 4.3 | Converted to TURP because of bleeding and endoscopic malfunctions | |
| Blood transfusions | Bouchier-Hayes 2006 | 38 | 1 | 2.6 | 38 | 0 | 0 | |
| Keoghane 2000 | 76 | 13 | 17 | 72 | 0 | 0 | p < 0.0001 | |
| Shingleton 2002 | 50 | – | – | 50 | 0 | 0 | ||
| Mottet 1999 | 13 | 0 | 0 | 23 | 0 | 0 | ||
| Tuhkanen 2003 | 26 | 0 | 0 | 26 | 0 | 0 | ||
| Zorn 1999 | 12 | 0 | 0 | 12 | 0 | 0 | ||
| Tuhkanen 2001 | 24 | 2 | 8.3 | 21 | 1 | 4.8 | ||
| Carter 1999a | 96 | 5 | 5.2 | 95 | – | – | ||
| Sengör 1996 | 30 | 2 | 6.7 | 30 | 0 | 0 | ||
| van Melick 2003 | 50 | 1 | 2 | 45 | 0 | 0 | ||
| Irritative urinary symptoms | Mottet 1999 | 13 | 0 | 0 | 23 | 0 | 0 | At 1 month |
| Urinary tract infection | Keoghane 2000 | 76 | 3 | 3.9 | 72 | 1 | 1.3 | Proven (0–3 months) |
| 76 | – | – | 72 | 1 | 1.3 | Prostatitis (0–3 months) | ||
| Carter 1999a | 92 | 5 | 5.2 | 93 | 17 | 18.3 | Simple | |
| 92 | 0 | 0 | 93 | 5 | 5.4 |
Epididymitis These are all for up to 6 weeks |
||
| Tuhkanen 2003 | 26 | 1 | 3.8 | 26 | – | – | Epididymitis (treated) | |
| Urinary retention | Carter 1999a | 92 | 2 | 2.2 | 93 | 5 | 5.4 | Acute retention at 6 weeks |
| 96 | 5 | 5.2 | 95 | 26 | 27.4 | Failure to void | ||
| Keoghane 2000 | 76 | 8 | 12 | 72 | 17 | 28 | p < 0.05 (0–3 months) | |
| van Melick 2003 | 50 | 5 | 10 | 45 | 4 | 9 | At 1 week postoperatively | |
| 50 | 0 | 0 | 45 | 5 | 11.1 | Up to 12 months | ||
| Recatheterisation | Bouchier-Hayes 2006 | 38 | 6 | 15.8 | 38 | 4 | 10.5 | |
| Keoghane 2000 | 76 | 7 | 9.2 | 72 | 3 | 4.1 | Because of haemorrhage | |
| Tuhkanen 2001 | 24 | 1 | 4.1 | 21 | 1 | 4 | ||
| Mottet 1999 | 13 | 0 | 0 | 23 | 0 | 0 | ||
| Zorn 1999 | 12 | 3 | 25 | 21 | 3 | 14 | Because of clot retention or inability to void | |
| Clot retention | Bouchier-Hayes 2006 | 38 | 10 | 26.3 | 38 | 0 | 0 | |
| Tuhkanen 2001 | 24 | 2 | 8.3 | 21 | 1 | 4 | Required bladder irrigation under spinal anaesthesia | |
| Tuhkanen 2003 | 26 | 1 | 3.8 | 26 | 0 | 0 | Removed by syringe irrigation | |
| van Melick 2003 | 50 | 1 | 2 | 45 | 2 | 4.4 | ||
| TUR syndrome | Bouchier-Hayes 2006 | 38 | 1 | 2.6 | 38 | 0 | 0 | |
| Sengör 1996 | 30 | 0 | 0 | 30 | 0 | 0 | ||
| Carter 1999a | 92 | 0 | 0 | 93 | 0 | 0 | ||
| Cardiovascular events | Keoghane 2000 | 76 | 1 | 1.3 | 72 | 1 | 1.4 | TURP: heart disease at 2 months, died. Laser: myocardial infarction but had pre-existing heart disease at 3 months |
| Tuhkanen 2001 | 24 | – | – | 21 | 1 | 4.8 | Myocardial infarction on the day of treatment (patient had history of angina pectoris) | |
| van Melick 2003 | 50 | 1 | 2 | 45 | 0 | 0 | Myocardial infarction | |
| Mortality | Keoghane 2000 | 76 | 5 | 6.6 | 72 | 5 | 6.9 | Overall mortality at 3 years |
| Tuhkanen 2001 | 24 | 1 | 4.2 | 21 | 1 | 4.8 | TURP: at 13 months; unknown cause. Laser: cardiac infarct at 5 months | |
| Tuhkanen 2003 | 26 | 1 | 3.8 | 26 | 3 | 11.5 | Overall mortality at 13 months for TURP. BPH unrelated causes | |
| van Melick 2003 | 50 | 4 | 8 | 45 | 3 | 6.6 | Cardiac failure | |
| Stricture | Bouchier-Hayes 2006 | 38 | 8 | 21 | 38 | 5 | 13 | At 6 weeks |
| Incontinence | Keoghane 2000 | 76 | 1 | 1.3 | 72 | – | – | 0–3 months |
| van Melick 2003 | 50 | 4 | 8 | 45 | 18 | 40 | Up to 12 months | |
| Postoperative (>3 months) | ||||||||
| Irritative urinary symptoms | Zorn 1999 | 12 | 0 | 0 | 21 | 0 | 0 | Time point is unclear (total follow-up is 1 year) |
| Stricture | Shingleton 2002 | 50 | 1 | 2 | 50 | 4 | 8 | TURP: urethral stricture. Laser: three bladder neck contractures; one urethral stricture. Time point unclear (could it be cumulative for up to 72 months?) |
| Tuhkanen 2001 | 25 | 1 | 4 | 21 | – | – | Urethral stricture at 3 months (underwent internal urethrotomy at 5 months) | |
| Carter 1999a | 85 | 9 | 10.6 | 84 | 2 | 2.4 | Urethral | |
| 85 | 6 | 7.0 | 84 | 5 | 5.9 |
Bladder neck At 1 year |
||
| Keoghane 2000 | 76 | 5 | 6.6 | 72 | – | – | TURP: three urethral strictures and two bladder neck contractures (0–3 months) | |
| Mottet 1999 | 13 | 2 | 15.4 | 23 | – | – | Bladder neck contractures at 2 and 6 months; treated by cold knife incision | |
| Sengör 1996 | 30 | 0 | 0 | 30 | 0 | 0 | Follow-up 6 months | |
| van Melick 2003 | 50 | 2 | 4 | 45 | 2 | 4.4 | Urethral stricture; up to 12 months | |
| Tuhkanen 2003 | 25 | 1 | 4 | 25 | 0 | 0 | Bladder neck contracture at 6-month follow-up; treated by TURP 2 months later | |
| Urinary incontinence | Shingleton 2002 | 50 | 1 | 2 | 50 | 1 | 2 | Stress; time point unclear (up to 72 months) |
| Tuhkanen 2001 | 24 | 1 | 4.2 | 21 | – | – | Overflow incontinence at 13 months | |
| Carter 1999a | 85 | 0 | 0 | 84 | 1 | 1.2 | At 7 months | |
| Retrograde ejaculation | Shingleton 2002 | 21 | 2 | 9.5 | 22 | 2 | 9.1 | Time point unclear (up to 72 months) |
| Mottet 1999 | 13 | – | 50 | 23 | – | 50 | At 1 year; article does not report whether all patients were sexually active | |
| Tuhkanen 2001 | 14 | 12 | 85.7 | 16 | 3 | 18.8 | Loss of ejaculate; p < 0.001 | |
| Sengör 1996 | 27 | 24 | 89 | 23 | 1 | 4.3 | Follow-up 6 months | |
| Tuhkanen 2003 | 16 | 13 | 81 | 16 | 1 | 6 | At 3-month follow-up; p < 0.0001 | |
| Erectile dysfunction | Shingleton 2002 | 21 | – | – | 22 | 8 | 37.5 | At 6 months; no erections. TURP: minimal change after 6 months |
| Mottet 1999 | 13 | – | 0 | 23 | 10 | At 1 year; article does not report whether all patients were sexually active | ||
| Urinary tract infection | Carter 1999a | 85 | 6 | 7.0 | 84 | 2 | 2.4 | Simple |
| 85 | 0 | 0 | 84 | 2 | 2.4 | Epididymitis | ||
| 85 | 1 | 1.2 | 84 | 2 | 2.4 |
Prostatitis All at 1 year |
||
| Urinary retention | Tuhkanen 2001 | 25 | – | – | 21 | 2 | 9.5 | At 17 months and underwent TURP |
| Shingleton 2002 | 50 | 1 | 2 | 50 | 3 | 6 | Time point unclear (up to 12 months) | |
| Study | Baseline | 3 months | 6 months | 12 months | 18 months | 2 years | Comments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | Laser vaporisation | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | ||
| Carter 1999b | Role physical domain score was significantly worse than the preoperative levela | Patients reported worse mean scores in all health domains. The differences reached statistical significance in the domains of bodily pain and social functiona | Improvement in the vitality domain score at 1 year and mental health domain | No significant differences were seen between the groups in any domain. General health domain was significantly worse than the preoperative score | SF-36 HRQL | |||||||||||||
| van Melick 2003 |
n = 50 3.9, SD 1.6 |
n = 45 3.6 , SD 1.6 |
n = 37 0.5, SD 0.5 |
n = 33 0.8, SD 1.0 |
n = 41 0.6, SD 0.9 |
n = 37 0.6, SD 0.9 |
n = 15 1.1, SD 1.2b |
n = 10 2.0, SD 1.0b |
n = 15 1.3, SD 1.3c |
n = 17 1.4, SD 1.2c |
IPSS QoL | |||||||
| Keoghane 2000; in Jenkinson 1997 |
n = 65 0.81, SD 0.18 |
n = 62 0.81, SD 0.18 |
n = 55 0.85, SD 0.17 |
n = 58 0.85, SD 0.20 |
n = 58 0.82, SD 0.22 |
n = 51 0.82, SD 0.21 |
EQ-5D health states, weighted tariffs | |||||||||||
|
n = 66 78.3, SD 13.2 |
n = 59 75.8, SD 17.1 |
n = 58 79.9, SD 16.3 |
n = 49 74.2, SD 19.5 |
n = 58 77.2, SD 16.9 |
n = 51 46.5, SD 18.1 |
Thermometer score | ||||||||||||
| No statistically significant changes with time were detected for patients in either group. Both the mean EQ-5D health states valued by the results of the tariffs and self-assessment EuroQoL thermometer score suggested only minimal change for both groups from baseline to follow-up at 3 months and 1 year | ||||||||||||||||||
|
n = 57 44.66, SD 12.12 |
n = 51 43.69, SD 12.58 |
n = 60 41.85, SD 12.17 |
n = 52 42.96, SD 11.24 |
n = 56 43.37, SD 13.46 |
n = 52 42.35, SD 14.12 |
Physical score | ||||||||||||
|
n = 57 47.75, SD 10.47 |
n = 51 47.07, SD 11.20 |
n = 60 46.21, SD 11.56 |
n = 52 46.28, SD 11.16 |
n = 56 45.71, SD 13.21 |
n = 52 45.57, SD 14.11 |
Mental | ||||||||||||
| Little change as a consequence of either surgical intervention. Quality of life: SF-36 in Jenkinson 1997 is reporting eight different dimensions of the SF-36 questionnaire (physical functioning, role physical, pain, general health perception, energy/vitality, social functioning, role emotional, mental health). Scale goes from 0 to 100, in which 0 is worst possible health state and 100 is best possible health state. Also a short form health survey is reported: PCS – physical component summary score; MCS – mental component summary score | ||||||||||||||||||
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 24 months | 3 years | 4 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | Laser vaporisation | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | p-value | TURP | Laser vaporisation | TURP | Laser vaporisation | p-value | ||
| Peak urine flow rate (ml/s) | Zorn 1999 |
n = 11 9.0 |
n = 21 8.7 |
n = 10 23.1 |
n = 18 20.0 |
n = 6 26.9 |
n = 18 20.0 |
|||||||||||||
| Tuhkanen 2004 |
n = 51 7.6,a SD 3.0 |
n = 47 8.3,a SD 3.6 |
||||||||||||||||||
| Tuhkanen 2003 |
n = 26 8.6a (5.0–15.9) |
n = 26 8.3a (4.8–19.6) |
n = 25 19, SD 9.0 |
n = 25 15.0, SD 5.2 |
NS |
n = 25 21.1, SD 9.7 |
n = 25 17.9, SD 7.1 |
NS; from 1999 paper |
n = 20 16.1a (7.2–39.6) p < 0.01 |
n = 22 14.3a (10.1–33.6) p < 0.001 |
NS | |||||||||
| Shingleton 2002 |
n = 50 7.3, SD 3.7 |
n = 50 8.2, SD 3.2 |
n = 48? 16.0, SD 8.0 |
n = 48? 15.0, SD 5.7 |
0.60 |
n = 48? 16.3, SD 6.4 |
n = 48? 15.8, SD 6.9 |
0.77 |
n = 33 16.7, SD 7.6 |
n = 40 15.4, SD 5.9 |
0.42 |
n = 19 14.3, SD 6.3 |
n = 23 14.9, SD 5.4 |
18–24 months |
n = 33? 12.8, SD 5.6 |
n = 29? 12.3, SD 5.3 |
Follow-up 36–72 months | |||
| Keoghane 2000 |
n = 54 11.4, SD 5.0 |
n = 48 11.8, SD 4.5 |
n = 52 21.8, SD 12.2 |
n = 46 21.3, SD 11.6 |
NS |
n = 45 21.2, SD 12.4 |
n = 42 17.1, SD 13.2 |
n = 31 15.9, SD 8.0 |
n = 27 14.2, SD 7.4 |
n = 24 12.7, SD 6.4 |
n = 24 13.4, SD 7.3 |
n = 32 14.0, SD 5.2 |
n = 25 14.0, SD 6.4 |
Follow-up 5 years | ||||||
|
Change: n = 40 9.6, SD 2.4 (5.8–13.4)b |
Change: n = 38 10.7, SD 1.4 (7.1–14.3)b |
NS |
Change: n = 45 9.4, SD 12.5 (5.2–13.6)b |
Change: n = 42 6.2, SD 15 (0.8–11.6)b |
NS |
Change: n = 26 4.9, SD 7.5 (1.9–7.9)b |
Change: n = 18 5.2, SD 7.0 (1.7–8.7)b |
Change: n = 24 2.1, SD 6.9 (–0.8–5.0)b |
Change: n = 24 1.8, SD 6.2 (–0.8–4.4)b |
|||||||||||
| Mottet 1999 |
n = 13 7.7 |
n = 17 8.8 |
n = 12 18.3 |
n = 17 23.5 |
n = 11 16.6 |
n = 15 18.6 |
n = 7 17.6 |
n = 8 19.9 |
||||||||||||
| Tuhkanen 2001 |
n = 25 7.2 (3.7–14.8) |
n = 21 8.5 (2.3–17.2) |
n = 22 21.0 |
n = 21 13.7 |
< 0.01 |
n = 21 19.6 |
n = 19 14.4 |
n = ? 20.6 p < 0.001 |
n = ? No significant increase |
< 0.001 | ||||||||||
| Carter 1999a |
n = 96 9.5 |
n = 95 9 |
n = 89 19 |
n = 90 18 |
NSc |
n = 85 20 |
n = 84 18.5 |
NSc | ||||||||||||
| Sengör 1996 |
n = 30 8.4, SD 2.8 |
n = 30 8.7, SD 2.3 |
n = 30? 20.7, SD 2.6 |
n = 30? 18.9, SD 3.1 |
n = 30? 19.8, SD 2.5 |
n = 30? 18.2, SD 2.1 |
||||||||||||||
| Mean urine flow rate (ml/s) | Sengör 1996 |
n = 30 4.7, SD 2.1 |
n = 30 4.6, SD 1.8 |
n = 30? 10.6, SD 1.7 |
n = 30? 10.7, SD 1.7 |
n = 30? 10.3, SD 1.3 |
n = 30? 10.9, SD 2.7 |
|||||||||||||
| Tuhkanen 2001 |
n = 24 3.6 (1.4–7.0) |
n = 21 4.2 (1–9.2) |
n = 22 11.0 |
n = 21 6.5 |
< 0.01 | |||||||||||||||
| Residual volume (ml) | Sengör 1996 |
n = 30 155, SD 40 |
n = 30 110, SD 68 |
n = 30? 70, SD 27 |
n = 30? 50.4, SD 30 |
n = 30? 68, SD 22 |
n = 30? 47, SD 19 |
|||||||||||||
| Zorn 1999 | 39 decreased | – | ||||||||||||||||||
| Tuhkanen 2004 |
n = 51 100,a SD 115 |
n = 47 96,a SD 93 |
||||||||||||||||||
| Tuhkanen 2003 |
n = 26 83a (8–350) |
n = 26 87a (0–331) |
n = 25 36, SD 39 |
n = 25 44, SD 39 |
From 1999 paper |
n = 25 32, SD 37 p < 0.001 |
n = 25 50, SD 64 p < 0.001 |
NS; from 1999 paper |
n = 20 10a (0–90) |
n = 22 60a (0–380) |
||||||||||
| Tuhkanen 2001 |
n = 24 138 (0–450) |
n = 21 125 (0–350) |
n = ? 58 |
n = ? 114 |
< 0.01 | |||||||||||||||
| Carter 1999a |
n = 96 135c |
n = 95 109c |
n = 89 35 |
n = 90 22 |
NSc |
n = 85 40 |
n = 86 30 |
NSc | ||||||||||||
| Detrusor pressure (cmH2O) | Tuhkanen 2003 |
n = 26 57a (40–137) |
n = 26 64a (32–112) |
n = 25 31.3, SD 9.9 p < 0.001 |
n = 25 38.3, SD 9.7 p < 0.001 |
NS; from 1999 paper |
n = 20 28a (9–44) p < 0.001 |
n = 22 38a (18–65) p < 0.001 |
< 0.001 | |||||||||||
| Tuhkanen 2001 |
n = 24 83 (47–137) |
n = 21 79 (47–131) |
n = 21 36 (7–63) |
n = 19 53 (26–109) |
< 0.01 | |||||||||||||||
| Prostate size (ml) | Zorn 1999 |
n = 12 33.9 |
n = 21 29.9 |
|||||||||||||||||
| Tuhkanen 2004 |
n = 51 38,a SD 16 |
n = 47 36,a SD 17 |
||||||||||||||||||
| Tuhkanen 2003 |
n = 26 28a (15–38 |
n = 26 30a (15–37) |
n = 20 22 p < 0.05 |
n = 22 30 |
< 0.05c | |||||||||||||||
| Shingleton 2002 |
n = 50 29.6, SD 15.4 |
n = 50 33.9, SD 24.2 |
n = 48? 22, SD 12.1 |
n = 48? 29.1, SD 20.0 |
n = 33 21.5, SD 15.4 |
n = 40 28.4, SD 22.7 |
n = 19 20.5, SD 13.3 |
n = 23 27.5, SD 19.9 |
18–24 months |
n = 33? 26.3, SD 20.2 |
n = 29? 32.9, SD 26.5 |
Follow-up 36–72 months | ||||||||
| Keoghane 2000 |
n = 48 51.9, SD 24.1 |
n = 44 54.2, SD 26.3 |
||||||||||||||||||
| Mottet 1999 |
n = 13 34 |
n = 17 36.7 |
n = 11 17.7 |
n = 15 28 |
n = 7 18 |
n = 8 22.5 |
||||||||||||||
| Tuhkanen 2001 |
n = 25 55 (40–95) |
n = 21 55 (42.83) |
n = 21 29 |
n = 19 49 |
||||||||||||||||
| Carter 1999a |
n = 96 41.7, SD 19.4 |
n = 95 41.6, SD 17.3 |
||||||||||||||||||
| Outcome | Study | TURP | Laser vaporisation | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Carter 1999a |
N = 96 35.7, SD 10.8 |
N = 95 37.4, SD 12.1 |
|||||
| Tuhkanen 2001 |
N = 24 44 (24–80) |
N = 21 75 (45–108) |
Mean (range) | |||||
| van Melick 2003 |
N = 50 58, SD 26 (25–150) |
N = 45 58, SD 11 (30–80) |
||||||
| Sengör 1996 |
N = 30 56 (45–90) |
N = 30 43 (15–70) |
||||||
| Keoghane 2000 |
N = 69 39 SD 20 |
N = 69 36 SD 15 |
NS | |||||
| Mottet 1999 |
N = 13 56 |
N = 17 75 |
0.041 | |||||
| Suvakovic 1996 |
N = 10 20.1, SD 6.2 |
N = 10 18.9, SD 8.8 |
NS | |||||
| Tuhkanen 2003 |
N = 25 34 (15–71) |
N = 25 51 (20–75) |
< 0.001 | |||||
| Zorn 1999 |
N = 11 68 |
N = 19 70 |
||||||
| Length of hospital stay (days) | Tuhkanen 2001 |
N = 25 3.5 (1–8) |
N = 21 4.0 (2–9) |
Mean (range) | ||||
| Carter 1999a |
N = 92 3.7 |
N = 93 2.7 |
Median | |||||
| van Melick 2003 |
N = 50 3.9, SD 0.9; 4.0 (3.0–5.9) |
N = 45 3.8, SD 1.3; 3.5 (2.0–6.0) |
Median (percentiles) | |||||
| Sengör 1996 |
N = 30 5.9 (4–7) |
N = 30 1.6 (1–3) |
||||||
| Keoghane 2000 |
N = 76 4 |
N = 72 3 |
Median; p < 0.005 | |||||
| Tuhkanen 2003 |
N = 25 2.9 (2–5) |
N = 25 3.4 (2–7) |
Median; p = NS | |||||
| Mottet 1999 |
N = 13 3.1 |
N = 17 1.7 |
||||||
| Zorn 1999 |
N = 10 2.5 |
N = 19 1.9 |
||||||
| Outcome | Study | TURP | Laser vaporisation | Comments | ||||
| N | n | % | N | n | % | |||
| Reoperation | Bouchier-Hayes 2006 | 38 | 0 | 0 | 38 | 2 | 5.3 | At 6-week follow-up |
| Shingleton 2002 | 50 | 0 | 0 | 50 | 3 | 6 | Time point unclear (up to 72 months) | |
| Keoghane 2000 | 76 | 7 | 9.2 | 72 | 13 | 18 | Up to 3 years. TURP: six reoperated with TURP and one reoperated with contact laser vaporisation. Laser: 12 reoperated with TURP and one reoperated with contact laser vaporisation | |
| Mottet 1999 | 13 | – | – | 23 | 1 | 4.3 | Repeat laser procedure after 7 days. This patient is included in further analysis (maybe not to include as no reference is given of which group within the lasers this is for) | |
| Tuhkanen 2002 | 25 | 1 | 4 | 25 | 0 | 0 | TURP: reoperated by TURP at 8 months because of bladder neck contracture. Laser: follow-up 6 months | |
| Zorn 1999 | 12 | 0 | 0 | 21 | 0 | 0 | Time point is unclear (up to 1 year) | |
| van Melick 2003 | 50 | 2 | 4 | 45 | 1 | 2.2 | Up to 12 months. All underwent TURP | |
| Tuhkanen 2001 | 25 | 1 | 4 | 21 | 3 | 14.3 | TURP: repeat TURP at 13 months because of overflow incontinence. Laser: received TURP; one at 7 months because of gross haematuria, residual adenoma and bladder stones; two at 17 months postoperatively because of urinary retention | |
| Carter 1999a | 85 | 1 | 1.2 | 84 | 2 | 2.4 | TURP: at 7 months. Laser: one was at 12 weeks. Reoperations as a result of poor symptom resolution | |
Appendix 8.8 TUVRP versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | 24 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUVRP | TURP | TUVRP | p-value | TURP | TUVRP | p-value | TURP | TUVRP | p-value | TURP | TUVRP | p-value | |
| Helke 2001 (IPSS) |
n = 92 18.29, SD 7.49 (10–35) |
n = 93 17.29, SD 6.06 (10–35) |
n = 69 6.8a |
n = 80 7.4a |
NS |
n = 74 5.8a |
n = 80 5.3a |
NS |
n = 73 5.21, SD 5.1 (0–31) |
n = 79 4.66, SD 4.3 (0–19) |
NS | |||
| Liu 2006 (IPSS) |
n = 32 25.6, SD 3.5 |
n = 44 26.8, SD 4.7 |
n = 30 7.9, SD 1.8 |
n = 42 8.2, SD 2.2 |
0.53 |
n = 21 8.4, SD 2.6 |
n = 23 9.0, SD 3.1 |
0.45 | ||||||
| Kupeli 2001 (IPSS) |
n = 50 21.6 |
n = 50 19.4 |
n = 50? 5.0 |
n = 50? 4.0 |
n = 50? 5.0 |
n = 50? 4.0 |
||||||||
| Talic 2000 (IPSS) |
n = 34 20.1, SD 6.8 (11–30) |
n = 34 24.9, SD 6 (15–31) |
n = 34? 5.6, SD 3.1 |
n = 34? 4, SD 3.4 |
0.03 | |||||||||
| Gupta 2006 (IPSS) |
n = 50 23.3, SD 3.9 (17–31) |
n = 50 24.9, SD 3.9 (17–32) |
n = 50? 6.1, SD 0.42 (0–16) |
n = 50? 5.9, SD 0.25 (0–10) |
n = 50? 5.6, SD 0.32 (0–9) |
n = 50? 5.4, SD 0.28 (0–9) |
||||||||
| Complication | Study | TURP | TUVRP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Haemorrhage | Helke 2001 | 92 | 3 | 3.3 | 93 | 1 | 1.1 | Hemorrhage requiring surgical intervention |
| Liu 2006 | 32 | 0 | 0 | 44 | 1 | 2.3 | Serious haemorrhage | |
| Talic 2000 | 34 | 1 | 3 | 34 | 1 | 3 | Delayed haemorrhage | |
| Blood transfusion | Gupta 2006 | 50 | 1 | 2 | 50 | 0 | 0 | |
| Helke 2001 | 92 | 9 | 9.8 | 93 | 6 | 6.4 | p = NS | |
| Liu 2006 | 32 | 2 | 6.2 | 44 | 1 | 2.3 | p = 0.38 | |
| Kupeli 2001 | 50 | 0 | 0 | 50 | 0 | 0 | ||
| Talic 2000 | 34 | 0 | 0 | 34 | 0 | 0 | ||
| Urinary retention | Kupeli 2001 | 50 | 0 | 0 | 50 | 0 | 0 | |
| Recatheterisation | Kupeli 2001 | 50 | 0 | 0 | 50 | 0 | 0 | |
| Liu 2006 | 32 | 4 | 12.5 | 44 | 3 | 6.8 | p = 0.33 | |
| Gupta 2006 | 50 | 3 | 6 | 50 | 3 | 6 | For 24–72 hours | |
| Clot retention | Liu 2006 | 32 | 2 | 6.2 | 44 | 2 | 4.5 | Causing readmission |
| Talic 2000 | 34 | 1 | 3 | 34 | 1 | 3 | ||
| TUR syndrome | Kupeli 2001 | 50 | 0 | 0 | 50 | 0 | 0 | |
| Liu 2006 | 32 | 2 | 6.2 | 44 | 0 | 0 | p = 0.17 | |
| Talic 2000 | 34 | 0 | 0 | 34 | 0 | 0 | ||
| Incontinence | Kupeli 2001 | 50 | 0 | 0 | 50 | 0 | 0 | Sphincteric |
| Mortality | Gupta 2006 | 50 | 0 | 0 | 50 | 1 | 2 | Death due to pneumonia after the surgery; died 2 weeks later (87-year-old patient) |
| Postoperative (3–12 months) | ||||||||
| Stricture | Helke 2001 | 92 | 7 | 7.6 | 93 | 5 | 5.4 | Urethral |
| Kupeli 2001 | 50 | 0 | 0 | 50 | 0 | 0 | Up to 6 months | |
| Liu 2006 | 26 | 2 | 7.7 | 36 | 2 | 5.6 | Urethral or bladder neck; up to 6 months; p = 0.56 | |
| Gupta 2006 | 50 | 2 | 4 | 50 | 1 | 2 | Urethral stricture. Time point is unclear (follow-up up to 12 months). All were treated with internal urethrotomies | |
| Urinary incontinence | Gupta 2006 | 50 | 1 | 2 | 50 | 0 | 0 | Transient incontinence. Time point is unclear (follow-up 12 months) |
| Helke 2001 | 93 | 6 | 6.4 | 92 | 5 | 5.4 | Transient urge incontinence at 12 months | |
| 93 | 8 | 8.6 | 92 | 9 | 1.08 | Grade 1 stress incontinence at 12 months | ||
| Liu 2006 | 26 | 1 | 3.8 | 36 | 1 | 2.8 | Up to 6 months; p = 0.67 | |
| Retrograde ejaculation | Küpeli 2001 | 50 | 26 | 52 | 44 | 27 | 61 | Up to 6 months |
| Liu 2006 | 13 | 7 | 53.8 | 17 | 10 | 58.8 | Up to 6 months; p = 0.54 | |
| Erectile dysfunction | Liu 2006 | 13 | 3 | 23 | 17 | 4 | 23.5 | Impotence; up to 6 months; p = 0.66 |
| Küpeli 2001 | 36 | 12 | 33 | 31 | 11 | 35 | Up to 6 months | |
| Talic 2000 | 18 | 0 | 0 | 18 | 0 | 0 | ||
| Postoperative (> 12 months) | ||||||||
| Stricture | Liu 2006 | 21 | 0 | 0 | 26 | 1 | 4.3 | At 24 months; urethral or bladder neck; p = 0.52 |
| Liu 2006 | 21 | 0 | 0 | 26 | 1 | 4.3 | At 24 months; p = 0.52 | |
| Study | Baseline | 3 months | 2 years | Comments | |||||
|---|---|---|---|---|---|---|---|---|---|
| TURP | TUVRP | TURP | TUVRP | p-value | TURP | TUVRP | p-value | ||
| Liu 2006 |
n = 32 4.0, SD 0.7 |
n = 44 4.1, SD 0.6 |
n = 30 1.5, SD 0.7 |
n = 42 1.7, SD 0.5 |
0.57 |
n = 21 1.4 , SD 0.7 |
n = 23 1.6, SD 0.6 |
0.48 | IPSS QoL |
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 24 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUVRP | TURP | TUVRP | p-value | TURP | TUVRP | p-value | TURP | TUVRP | p-value | TURP | TUVRP | p-value | ||
| Peak urine flow rate (ml/s) | Gupta 2006 |
n = 50 4.5, SD 4.7 (0–13) |
n = 50 4.6 , SD 3.6 (0–12) |
n = 50? 20.7, SD 1.32 (10–39) |
n = 50? 22.5, SD 0.95 (13–35) |
NS |
n = 50? 23.7, SD 1.58 (9–41) |
n = 50? 423.6, SD 0.96 (16–37) |
NS | ||||||
| Helke 2001 |
n = 92 8.5, SD 5.19 (5.2–29) |
n = 93 10.8, SD 4.76 (4.2–28.4) |
n = 69 21a |
n = 74 21a |
NS |
n = 80 23a |
n = 80 22.5a |
NS |
n = 73 22.12, SD 10.6 (5–50) p < 0.001 |
n = 79 22.19, SD 12.3 (5.2–41.5) p < 0.01 |
|||||
| Kupeli 2001 |
n = 50 9.2, SD 2.6 |
n = 50 7.9, SD 2.1 |
n = 50? 24.6, SD 3.4 p < 0.01 |
n = 50? 26.7, SD 3.7 p < 0.01 |
|||||||||||
| Liu 2006 |
n = 32 6.9, SD 1.9 |
n = 44 6.9, SD 2.1 |
n = 30 21.6, SD 2.0 |
n = 42 20.7, SD 2.0 |
0.20 |
n = 21 21.2, SD 2.7 |
n = 23 19.6, SD 3.7 |
0.12 | |||||||
| Talic 2000 |
n = 34 9.1, SD 6.3 (1–15) |
n = 34 7.5, SD 3.5 (2–14.6) |
n = 34? 15.2, SD 10 |
n = 34? 19, SD 6.5 |
|||||||||||
| Residual volume (ml) | Gupta 2006 |
n = 50 84.0, SD 129.7 (0–600) |
n = 50 103, SD 174.1 (0–881) |
< 20 | < 20 | < 20 | < 20 | ||||||||
| Prostate size (ml) | Küpeli 2001 (g) |
n = 50 56.7, SD 6.3 (34–110) |
n = 50 57.8, SD 4.1 (34–95) |
n = 50? 20.8, SD 3.1 p < 0.05 |
n = 50? 20.6, SD 3.6 p < 0.05 |
||||||||||
| Gupta 2006 |
n = 50 59.8, SD 16.5 (40–100) |
n = 50 62.6, SD 14.8 (42–133) |
|||||||||||||
| Outcome | Study | TURP | TUVRP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Liu 2006 |
N = 32 52.9, SD 6.0 |
N = 44 49.4, SD 8.0 |
p = 0.04 | ||||
| Talic 2000 |
N = 34 35.9, SD 12.8 |
N = 34 42.4, SD 15 |
p = 0.02 | |||||
| Length of hospital stay (days) | Liu 2006 |
N = 32 2.06, SD 0.35 |
N = 44 1.65, SD 0.20 |
p < 0.0001 | ||||
| N | n | % | N | n | % | |||
| Reoperation | Helke 2001 | 92 | 5 | 5.4 | 93 | 9 | 9.7 | Four of the TURPs and two of the TUVPs underwent radical prostatectomy |
| Liu 2006 | 26 | 2 | 7.7 | 36 | 5 | 5.6 | Up to 6 months; p = 0.56 | |
| 21 | 1 | 4.8 | 26 | 0 | 0 | At 24 months; p = 0.48 | ||
Appendix 8.9 B-TURP versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | B-TURP | TURP | B-TURP | p-value | TURP | B-TURP | p-value | TURP | B-TURP | p-value | ||
| de Sio 2006 (IPSS) |
n = 35 24.3, SD 5 |
n = 35 24.18, SD 4 |
n = 35? |
n = 35? |
n = 35? |
n = 35? |
n = 35? |
n = 35? |
||||
| Kim 2006b (IPSS) |
n = 25 18.6, SD 3.3 |
n = 25 19.0, SD 6.0 |
n = 25 5.6, SD 1.4 |
n = 25 6.0, SD 1.0 |
||||||||
| Nuhoğlu 2006 (IPSS) |
n = 30 17.3, SD 5.8 |
n = 27 17.6, SD 6.1 |
n = 30 4.7, SD 3.1c |
n = 27 4.8, SD 3.4c |
n = 26 5.2, SD 3.2 |
n = 24 5.4, SD 3.7 |
||||||
| Seckiner 2006 (IPSS) |
n = 24 23.2, SD 4.9 |
n = 24 24.1, SD 5.2 p < 0.01 |
n = 24 10.6, SD 6.3 p < 0.01 |
n = 24 9.3, SD 3.9 p < 0.01 |
n = 23 6.0, SD 6.7 |
n = 24 7.4, SD 2.2 |
n = 21 8.3, SD 2.9 |
n = 23 8.7, SD 4.1 |
||||
| Complication | Study | TURP | B-TURP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Blood transfusion | Tefekli 2005 | 47 | 1 | 2.1 | 49 | 1 | 2 | |
| Nuhoğlu 2006 | 30 | 2 | 6.7 | 27 | 1 | 3.7 | ||
| de Sio 2006 | 35 | 0 | 0 | 35 | 1 | 2.8 | p = NS | |
| Urinary tract infection | Kim 2006b | 25 | 1 | 4 | 25 | 1 | 4 | |
| Urinary retention | Tefekli 2005 | 47 | 1 | 2.1 | 49 | 1 | 2 | Early |
| Nuhoğlu 2006 | 30 | 27 | 1 | 3.7 | On the tenth day postoperatively | |||
| Recatheterisation | Tefekli 2005 | 47 | 1 | 2.1 | 49 | 3 | 6 | For 1 week |
| Nuhoğlu 2006 | 27 | 1 | 3.7 | 30 | For 1 week | |||
| Clot retention | Nuhoğlu 2006 | 30 | 0 | 0 | 27 | 0 | 0 | |
| de Sio 2006 | 35 | 4 | 11.4 | 35 | 2 | 5.7 | p = NS | |
| TUR syndrome | de Sio 2006 | 35 | 0 | 0 | 35 | 0 | 0 | |
| Kim 2006b | 25 | 0 | 0 | 25 | 0 | 0 | ||
| Postoperative (3–12 months) | ||||||||
| Irritative urinary symptoms | Tefekli 2005 | 47 | 2 | 4.3 | 49 | 6 | 12.2 | Early and severe |
| Stricture | Tefekli 2005 | 47 | 1 | 2.1 | 3 | 49 | 6.1 | Long term (time point is unclear: mean follow-up 18 months); p = 0.002 |
| Kim 2006b | 25 | 2 | 8 | 25 | 1 | 4 | Urethral; up to 6 months | |
| de Sio 2006 | 35 | 1 | 2.9 | 35 | 1 | 2.9 | Required a reoperation at 6 months | |
| Seckiner 2006 | 24 | 1 | 4.2 | 24 | 2 | 8.3 | Urethral stricture (time point is unclear; up to 12 months) treated with visual optic urethrotomy | |
| Urinary incontinence | Tefekli 2005 | 47 | 1 | 2.1 | 49 | 0 | 0 | Long term (time point is unclear; mean follow-up 18 months) |
| Kim 2006b | 25 | 1 | 4 | 25 | 1 | 4 | Up to 6 months | |
| Retrograde ejaculation | Tefekli 2005 | 47 | 30 | 63.8 | 49 | 29 | 59.2 | Long term (time point is unclear; mean follow-up 18 months); article doesn’t report the number of patients who were sexually active |
| Study | Baseline | 3 months | 6 months | 12 months | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | B-TURP | TURP | B-TURP | p-value | TURP | B-TURP | p-value | TURP | B-TURP | p-value | ||
| de Sio 2006 |
n = 35 3.9, SD 1 |
n = 35 4.2, SD 1 |
n = 35 1.4 |
n = 35 2.1 |
n = 35 1.0 |
n = 35 1.1 |
Quality of life score | |||||
| Seckiner 2006 |
n = 24 4.4, SD 0.6 |
n = 24 4.7, SD 0.9 |
n = 24 2.1, SD 1.2 p < 0.01 |
n = 24 1.8, SD 1.0 p <0.01 |
n = 23 1.6, SD 1.3 |
n = 24 1.6, SD 0.7 |
n = 21 2.0, SD 0.8 |
n = 23 1.8, SD 0.8 |
IPSS QoL | |||
| Singh 2005 |
n = 30 4.4, SD 1.0 |
n = 30 4.6, SD 0.9 |
n = 30? 1.0 |
n = 30? 1.1 |
Quality of life score | |||||||
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 24 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | B-TURP | TURP | B-TURP | p-value | TURP | B-TURP | p-value | TURP | B-TURP | p-value | TURP | B-TURP | p-value | ||
| Peak urine flow rate (ml/s) | Seckiner 2006 |
n = 24 8.3, SD 3.1 |
n = 24 8.5, SD 2.9 |
n = 24 18.6, SD 9.1 |
n = 24 17.7, SD 9.1 |
n = 23 16.2, SD 12.0 |
n = 24 23.4, SD 10.6 |
n = 21 15.7, SD 6.3 |
n = 23 18.8, SD 6.9 |
||||||
| Singh 2005 |
n = 30 5.1, SD 2.0 |
n = 30 5.8, SD 3.0 |
n = 30? 17.8 |
n = 30? 19.0 |
|||||||||||
| de Sio 2006 |
n = 35 6.3, SD 3 |
n = 35 7.1, SD 2 |
n = 35 20.5a |
n = 35 21.5a |
n = 35 20.0a |
n = 35 20.5a |
n = ? 22 |
n = ? 20.5 |
|||||||
| Kim 2006b |
n = 25 6.1, SD 1.7 |
n = 25 6.5, SD 2.2 |
n = 25 20.5, SD 4.2 |
n = 25 20.6, SD 4.5 |
|||||||||||
| Nuhoğlu 2006 |
n = 30 7.3, SD 2.1 |
n = 27 6.9, SD 2.8 |
n = 26 17.9, SD 3.1 |
n = 24 17.1, SD 2.7 |
|||||||||||
| Tefekli 2005 |
n = 50 8.3, SD 3.6 |
n = 51 7.8, SD 3.7 |
n = 49 15.8, SD 3.7 |
n = 47 16.9, SD 2.8 |
n = 47 17. SD 4.3 |
n = 49 18.3, SD 3.5 |
n = 47 16.9, SD 4.1 |
n = 49 17.2, SD 3.9 |
< 0.05 | ||||||
| Mean urine flow rate (ml/s) | Nuhoğlu 2006 |
n = 30 2.8, SD 1.2 |
n = 27 2.6, SD 1.3 |
n = 26 9.9, SD 2.3 |
n = 24 10.4, SD 2.9 |
||||||||||
| Residual volume (ml) | Seckiner 2006 |
n = 24 138, SD 115 |
n = 24 88, SD 74 |
||||||||||||
| Singh 2005 |
n = 30 136, SD 52 |
n = 30 124, SD 58 |
|||||||||||||
| de Sio 2006 |
n = 35 75, SD 35.5 |
n = 35 80, SD 22.5 |
n = 35? 36 |
n = 35? 44 |
n = 35? 40 |
n = 35? 34 |
n = ? 22 |
n = ? 20.5 |
|||||||
| Nuhoğlu 2006 |
n = 30 88, SD 20 |
n = 27 96, SD 27 |
n = 26 35, SD 15 |
n = 24 33, SD 19 |
|||||||||||
| Prostate size (ml) | Seckiner 2006 |
n = 24 41.4, SD 14.5 |
n = 24 49.4, SD 18.9 |
n = 23 28.5, SD 7.5 p < 0.05 |
n = 24 25.3, SD 8.1 p < 0.001 |
||||||||||
| de Sio 2006 |
n = 35 47.5, SD 5.1 |
n = 35 51.6, SD 3.9 |
|||||||||||||
| Kim 2006b |
n = 25 51.7, SD 19.1 |
n = 25 53.2, SD 14.9 |
|||||||||||||
| Nuhoğlu 2006 |
n = 30 49, SD 18.1 |
n = 27 47, SD 7.7 |
n = 26 24, SD 7.1 |
n = 24 22, SD 6.8 |
|||||||||||
| Tefekli 2005 |
n = 50 54.0, SD 15.2 |
n = 51 50.1, SD 17.3 |
|||||||||||||
| Outcome | Study | TURP | B-TURP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | de Sio 2006 |
N = 35 53 |
N = 35 49 |
|||||
| Kim 2006b |
N = 25 57, SD 15.4 |
N = 25 54, SD 13.6 |
||||||
| Nuhoğlu 2006 |
N = 30 52, SD 13.2 |
N = 27 55, SD 9.7 |
||||||
| Seckiner 2006 |
N = 24 52.9, SD 16.3 |
N = 24 52.9, SD 12.8 |
p = 0.835 | |||||
| Tefekli 2005 |
N = 50 57.8, SD 13.4 (30–60) |
N = 51 40.3, SD 11.4 (30–60) |
p < 0.001 | |||||
| Length of hospital stay (days) | de Sio 2006 |
N = 35 107 |
N = 35 78.2 |
Hours; p < 0.05 | ||||
| Kim 2006b |
N = 25 4.0, SD 1.3 |
N = 25 3.3, SD 1.1 |
p < 0.05 | |||||
| N | n | % | N | n | % | |||
| Reoperation | de Sio 2006 | 35 | 1 | 2.9 | 35 | 1 | 2.9 | Because of bladder neck contractures |
| Nuhoğlu 2006 | 30 | 0 | 0 | 27 | 0 | 0 | At 1 year | |
| Tefekli 2005 | 47 | 1 | 2.1 | 49 | 2 | 4.1 | Because of obstructive symptoms; long term (time point is unclear; mean follow-up 18 months) | |
Appendix 8.10 B-TUVRP versus TURP
| Study | Baseline | 3 months | |||
|---|---|---|---|---|---|
| TURP | B-TUVRP | TURP | B-TUVRP | p-value | |
| Fung 2005 (IPSS) |
n = 30 15.82 |
n = 21 19.36 |
n = 30 9.63a |
n = 21 8.81a |
0.862 |
| Complication | Study | TURP | B-TUVRP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Urinary tract infection | Fung 2005 | 30 | 4 | 13.3 | 21 | 4 | 19 | Up to 3 months follow-up; p = 0.7 |
| Urinary retention | Fung 2005 | 30 | 3 | 10 | 21 | 4 | 19 | On postoperative day 1 |
| Clot retention | Fung 2005 | 30 | 5 | 16.7 | 21 | 1 | 4.8 | Requiring removal in ward or recovery room; p = 0.2 |
| TUR syndrome | Fung 2005 | 30 | 0 | 0 | 30 | 0 | 0 | |
| Study | Baseline | 3 months | Comments | |||
|---|---|---|---|---|---|---|
| TURP | B-TURP | TURP | B-TURP | p-value | ||
| Fung 2005 |
n = 30 3.64 |
n = 21 3.55 |
n = 30 1.54a |
n = 21 0.55a |
0.169 | Quality of life score |
| Outcome | Study | TURP | TUVRP | Comments |
|---|---|---|---|---|
| Duration of operation (minutes) | Fung 2005 |
n = 30 32.9 (12–105) |
n = 21 36.6 (12–76) |
Resection time; p = 0.48 |
| Length of hospital stay | Fung 2005 |
n = 30 NR |
n = 30 NR |
No significant differences reported by the authors in the text |
Appendix 8.11 TUVP versus TURP
| Study | Baseline | 3 months | 6 months | 12 months | 18 months | 24 months | 3 years | 5 years | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUVP | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | |
| Çetinkaya 1996 (AUA) | n = 23 | n = 23 |
n = 23? –21.31a p < 0.001 |
n = 23? –20.89a p < 0.001 |
|||||||||||||||||||
| Ekengren 2000 (IPSS) |
n = 28 25b (13–100) |
n = 26 22b (1–100) |
n = 28 ? 4.0b (0–100) |
n = 26? 4.5b (0–24) |
NS | ||||||||||||||||||
| Erdaği 1999 (IPSS) |
n = 20 21.5c (11–30) |
n = 20 20.6c (12–27) |
n = 20? 5.3c (1–12) |
n = 20? 0.9c (0–4) |
NS |
n = 20? 3.9c (1–9) p < 0.01 |
n = 20? 0.9c (0–3) p < 0.001 |
NS | |||||||||||||||
| Fowler 2005 (IPSS) |
n = 114 20.7, SD 6.9 (19.4–22)d |
n = 107 20.7, SD 7.3 (19.3–22.1)d |
n = 110 |
n = 105 |
n = 108 6.9, SD 5.5 (5.8–7.9)d |
n = 106 8.5, SD 7.4 (7.1–10)d |
n = 77 7.5, SD 5.8 (6.2–8.8)d |
n = 90 8.6, SD 7.2 (7.1–10.1)d |
NS | ||||||||||||||
| Galluci 1998 (IPSS) |
n = 80 18.19, SE 0.66 |
n = 70 18.84, SE 0.68 |
n = 80 5.52, SE 0.46 |
n = 70 5.50, SD 0.57 |
NS |
n = 80 3.77, SE 0.37 |
n = 70 4.94, SD 0.56 |
NS |
n = 80 3.52, SE 0.34 |
n = 70 4.04, SE 0.51 |
NS | ||||||||||||
| Gotoh 1999 (IPSS) |
n = 28 18.9, SD 7.3 |
n = 23 19.6, SD 7.5 |
n = 28? 3.8, SD 2.3 |
n = 23? 3.7, SD 2.4 |
|||||||||||||||||||
| Hammadeh 2003 (IPSS) |
n = 52 26.6, SD 4.8 |
n = 52 26.5, SD 4.5 |
n = 52 5.9f |
n = 52 7.3f |
NS |
n = 52 4.9f |
n = 52 5.9f |
n = 51 5.9, SD 5.2 |
n = 51 4.4, SD 3.8 |
0.3 |
n = 47 6.3, SD 4.6 |
n = 47 4.3, SD 3.5 |
0.02 |
n = 40 7.1, SD 6.2 |
n = 40 4.1, SD 3.3 |
0.01 |
n = 27 8.6, SD 7.1 |
n = 26 5.9, SD 6.3 |
0.16 | ||||
| Kaplan 1998 (AUA) |
n = 32 18.3, SD 4.7 |
n = 32 19.4, SD 3.5 |
n = 32 8.6, SD 2.5 p < 0.02 |
n = 32 9.2, SD 2.7 p < 0.02 |
NS |
n = 32 7.9, SD 3.1 p < 0.02 |
n = 32 7.4, SD 2.9 p < 0.02 |
NS |
n = 31 6.1, SD 1.9 p < 0.02 |
n = 30 6.6, SD 2.4 p < 0.02 |
NS | ||||||||||||
| Küpeli 1998a (IPSS) |
n = 30 21.6 |
n = 30 19.4 |
n = 30 ? 5.2 |
n = 30 ? 4.1 |
|||||||||||||||||||
| Küpeli 1998b (AUA) |
n = 36 14.6 (8–32) |
n = 30 13.7 (7–29) |
n = 33 7.3 (1–12) |
n = 27 7.9 (0–12) |
n = 30 7.0 (0–14) |
n = 26 6.1 (0–11) |
|||||||||||||||||
| McAllister 2003 (IPSS) |
n = 114 20.7 (19.4–22.0)d |
n = 107 20.7 (19.3–22.1)d |
n = 110 |
n = 105 |
>0.12 |
n = 108 6.9 (5.8–7.9)d |
n = 106 8.5 (7.1–10.0)d |
NS | |||||||||||||||
| Netto 1999 (IPSS) |
n = 38 24.29, SD 6.48 |
n = 40 19.65, SD 6.14 |
n = 38? 8.68, SD 2.3 |
n = 40? 3.83, SD 4.62 |
0.88 | ||||||||||||||||||
| Nuhoğlu 2005 (IPSS) |
n = 40 17.6, SD 7.2 |
n = 37 17.3, SD 6.8 |
n = 38 4.8, SD 4.2 |
n = 35 4.7, SD 3.1 |
NS |
n = 23 6.1, SD 3.5 |
n = 21 6.5, SD 3.2 |
NS | |||||||||||||||
| Patel 1997 (IPSS) |
n = 6 23.3 (17–29)g |
n = 6 29.6 (28–31)g |
n = 6 3.2 (1–5) |
n = 6 3.5 (2–4) |
|||||||||||||||||||
| Shokeir 1997 (AUA) |
n = 35 25.1, SD 5.5 (18–30) |
n = 35 26.3, SD 5.2 (16–29) |
n = 35? 4.8, SD 2.2 (5–14) p < 0.01 |
n = 35? 4.5, SD 1.9 (6–15) p < 0.01 |
NS |
n = 35? 4.5, SD 1.3 (3–8) p < 0.01 |
n = 35? 4.6, SD 1.2 (3–7) p < 0.01 |
NS |
n = 35? 4.7, SD 1.5 (4–9) p < 0.01 |
n = 35? 5.2, SD 1.4 (4–8) p < 0.01 |
NS | ||||||||||||
| Wang 2002 (IPSS) |
n = 109 20 (9–31) |
n = 97 20 (8–30) |
n = 109 3 (1–17) p < 0.01 |
n = 96 4 (4–20) p < 0.01 |
n = 43 4 (2–21) p < 0.01 |
n = 38 5 (4–23) p < 0.01 |
> 0.05 | ||||||||||||||||
| van Melick 2003 (IPSS) |
n = 50 16.8, SD 6.0 |
n = 46 20.2, SD 6.6 |
n = 37 3.2, SD 2.7 |
n = 33 3.8, SD 2.7 |
n = 41 4.1, SD 4.8 |
n = 34 4.8, SD 4.9 |
n = 15 5.8, SD 7.5h |
n = 12 8.4, SD 8.7h |
n = 15 7.3, SD 7.1i |
n = 12 7.0, SD 5.6i |
|||||||||||||
| Nathan 1996 (IPSS) |
n = 20 17, SD 4.3 (9–24) |
n = 20 21.9, SD 4.2 (13–27) |
n = 20 3.1, SD 2.3 (0–8) |
n = 20 2.86, SD 2.8 (0–10) |
|||||||||||||||||||
| Complication | Study | TURP | TUVP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Blood loss (ml) | Ekengren 2000 | 28 | 150 (8–400) | 26 | 75 (8– 400) | Median (range); p < 0.04 | ||
| Küpeli 1998b | 36 | 340 (210–830) | 30 | 60 (32–235) | Mean (range) p < 0.001 | |||
| Erdaği 1999 | 20 | 491 (35–950) | 20 | 117.6 (10–358) | Mean or median (range); p < 0.001 | |||
| Haemorrhage | Ekengren 2000 | 28 | 0 | 0 | 26 | 2 | 7.7 | |
| Fowler 2005 | 120 | 7 | 5.8 | 115 | 1 | 0.8 | Heavy bleeding; p = 0.06 | |
| Hammadeh 2003 | 52 | 3 | 5.8 | 5–6 hours postoperatively; bleeding | ||||
| Netto 1999 | 38 | 1 | 2.6 | 40 | 2 | 5 | At 3 weeks; bleeding | |
| Perforation | Küpeli 1998 b | 36 | 0 | 0 | 30 | 1 | 3.3 | Bladder perforation |
| Galluci 1998 | 80 | 0 | 0 | 70 | 1 | 1.4 | Requiring surgical drainage; perforation (capsular) | |
| van Melick 2003 | 50 | 5 | 10 | 46 | 2 | 4 | Capsule perforation | |
| Urethral injury | van Melick 2003 | 50 | 1 | 2 | 46 | 0 | 0 | |
| Blood transfusion | Küpeli 1998a | 30 | 0 | 0 | 30 | 0 | 0 | |
| Küpeli 1998b | 36 | 2 | 5.6 | 30 | 0 | 0 | ||
| Fowler 2005 | 120 | 9 | 7.5 | 115 | 2 | 1.74 | One TUVP patient underwent TURP instead through operator error; p = 0.04 | |
| Galluci 1998 | 80 | 0 | 0 | 70 | 0 | 0 | ||
| Hammadeh 2003 | 52 | 1 | 1.9 | 52 | 0 | 0 | p = 0.3 | |
| Kaplan 1998 | 32 | 1 | 3 | 32 | ||||
| van Melick 2003 | 50 | 1 | 2 | 46 | 0 | 0 | ||
| Nuhoğlu 2005 | 40 | 2 | 5 | 37 | On day 1 | |||
| Patel 1997 | 6 | 0 | 0 | 6 | 0 | 0 | ||
| Çetinkaya 1996 | 23 | 2 | 8.7 | 23 | 0 | 0 | ||
| Erdaği 1999 | 20 | 9 | 45 | 20 | ||||
| Gotoh 1999 | 28 | 0 | 0 | 23 | 0 | 0 | ||
| Nathan 1996 | 20 | 2 | 10 | 20 | 0 | 0 | ||
| Urinary tract infection | Küpeli 1998b | 36 | 3 | 8.3 | 30 | 4 | 13 | |
| Galluci 1998 | 80 | 4 | 5 | 1 | 70 | 1.4 | Epididymitis | |
| Hammadeh 2005 | 52 | 2 | 4 | 52 | 3 | 6 | p = 0.7 | |
| Kaplan 1998 | 32 | 4 | 13 | 32 | 5 | 16 | ||
| Erdaği 1999 | 20 | 5 | 25 | 20 | 1 | 5 | ||
| van Melick 2003 | 50 | 5 | 10 | 46 | 2 | 4.3 | At 1 week postoperatively | |
| Gotoh 1999 | 28 | 0 | 0 | 23 | 0 | 0 | Up to 3 months | |
| Nathan 1996 | 20 | 10 | 50 | 20 | 5 | 25 | Up to 3 months | |
| Urinary retention | Küpeli 1998a | 30 | 0 | 0 | 30 | 0 | 0 | |
| Küpeli 1998b | 36 | 30 | 1 | 3.3 | On day 7 after catheter removal | |||
| Galluci 1998 | 80 | 3 | 3.75 | 70 | 12 | 17.1 | Transient | |
| Hammadeh 2003 | 52 | 4 | 7.7 | 52 | 12 | 23 | p = 0.04 | |
| Nuhoğlu 2005 | 40 | 37 | 1 | 2.7 | On day 10 for 1 week | |||
| Nathan 1996 | 20 | 5 | 25 | 20 | 0 | 0 | Up to 3 months | |
| Recatheterisation | Küpeli 1998a | 30 | 0 | 0 | 30 | 0 | 0 | |
| Kaplan 1998 | 32 | 2 | 6 | 32 | 3 | 9 | ||
| Çetinkaya 1996 | 23 | 23 | 4 | 17.4 | ||||
| Gotoh 1999 | 28 | 0 | 0 | 23 | 0 | 0 | ||
| Liu 2006 | 32 | 4 | 12.5 | 44 | 3 | 6.8 | p = 0.33 | |
| Clot retention | Hammadeh 2003 | 52 | 4 | 7.7 | 52 | 0 | 0 | p = 0.05 |
| Kaplan 1998 | 32 | 2 | 6 | 32 | 3 | 9 | ||
| McAllister 2003 | 120 | 2 | 1.7 | 107 | 1 | 0.9 | Causing readmission | |
| Erdaği 1999 | 20 | 5 | 25 | 20 | – | – | ||
| van Melick 2003 | 50 | 1 | 2 | 46 | 0 | 0 | ||
| Nathan 1996 | 20 | 1 | 5 | 20 | 0 | 0 | Up to 3 months | |
| TUR syndrome | Küpeli 1998a | 30 | 0 | 0 | 30 | 0 | 0 | |
| Netto 1999 | 38 | 0 | 0 | 40 | 0 | 0 | ||
| Hammadeh 2003 | 52 | 0 | 0 | 52 | 0 | 0 | ||
| Kaplan 1998 | 32 | 1 | 3 | 32 | – | – | ||
| Wang 2002 | 109 | 5 | 4.6 | 97 | 3 | 3.1 | ||
| Erdaği 1999 | 20 | 0 | 0 | 20 | 0 | 0 | ||
| Gotoh 1999 | 28 | 0 | 0 | 23 | 0 | 0 | ||
| Nathan 1996 | 20 | 0 | 0 | 20 | 0 | 0 | Up to 3 months | |
| Cardiovascular events | Fowler 2005 | 120 | 1 | 0.83 | 115 | 1 | 0.86 | Cardiovascular problem |
| van Melick 2003 | 50 | 1 | 2 | 46 | 0 | 0 | Myocardial infarction | |
| Mortality | Ekengren 2000 | 28 | 0 | 0 | 26 | 1 | 0 | Myocardial infarction |
| Hammadeh 2003 | 52 | 6 | 11.5 | 52 | 3 | 5.76 | Cardiopulmonary disease | |
| Wang 2002 | 109 | – | – | 97 | 1 | 1.03 | Myocardial infarction | |
| van Melick 2003 | 50 | 1 | 2 | 46 | 0 | 0 | Cardiac failure | |
| 50 | 4 | 8 | 46 | 2 | 4 | Total mortality at 4.3 years | ||
| Incontinence | Küpeli 1998b | 36 | 1 | 2.8 | 30 | 1 | 3.3 | |
| Küpeli 1998a | 30 | 0 | 0 | 30 | 0 | 0 | Sphincteric | |
| Galluci 1998 | 80 | 3 | 3.75 | 70 | 13 | 18.6 | Transient stress incontinence at 1 month | |
| 2 | 2.5 | 0 | 0 | Urge incontinence | ||||
| Hammadeh 2003 | 52 | 0 | 0 | 52 | 0 | 0 | ||
| Kaplan 1998 | 32 | 0 | 0 | 32 | 0 | 0 | ||
| Wang 2002 | 109 | 2 | 1.8 | 97 | 4 | 4.1 | p = 0.05 | |
| van Melick 2003 | 50 | |||||||
| Gotoh 1999 | 28 | 0 | 0 | 23 | 0 | 0 | Up to 3 months | |
| Postoperative (3–12 months) | ||||||||
| Irritative urinary symptoms | Ekengren 2000 | 28 | 0 | 0 | 26 | 1 | 0 | Long-standing irritation; many had irritative symptoms but only one was longstanding |
| Küpeli 1998b | 36 | 3 | 8.3 | 30 | 10 | 33.3 | Longer than 7 days after catheter removal; p = 0.0123 | |
| Küpeli 1998a | 30 | – | 30 | + | +, greater; –, less; after catheter removal; lasted an average of 10 days | |||
| Hammadeh 2003 | 52 | 18 | 35 | 52 | 13 | 25 | p = 0.36 | |
| Kaplan 1998 | 32 | 4 | 13 | 32 | 5 | 16 | ||
| Shokeir 1997 | 35 | 2 | 5.7 | 35 | 3 | 8.6 | 6–12 weeks postoperatively | |
| Stricture | Ekengren 2000 | 28 | 0 | 0 | 26 | 2 | 0 | Urethral |
| Küpeli 1998b | 36 | 0 | 0 | 30 | 0 | 0 | Urethral | |
| Küpeli 1998a | 30 | 0 | 0 | 30 | 0 | 0 | Urethral and bladder neck | |
| Netto 1999 | 38 | 0 | 0 | 40 | 0 | 0 | Bladder neck stenosis | |
| Hammadeh 2003 | 52 | 2 | 4 | 52 | 2 | 4 | Urethral | |
| 51 | 2 | 4 | 51 | 0 | 0 |
Bladder neck stenosis; p = 0.16 These two are the same for the 5-year follow-up |
||
| Kaplan 1998 | 32 | 1 | 3 | 32 | 1 | 3 | ||
| Wang 2002 | 109 | 1 | 0.92 | 96 | 4 | 4.2 | ||
| Çetinkaya 1996 | 23 | 0 | 0 | 23 | 1 | 4.3 | Urethral stenosis | |
| Erdaği 1999 | 20 | 1 | 5 | 20 | – | – | Urethral stricture | |
| van Melick 2003 | 50 | 2 | 4 | 46 | 1 | 2.4 | Urethral stricture; up to 12 months | |
| Gotoh 1999 | 28 | 0 | 0 | 23 | 0 | 0 | Either bladder neck or urethral; up to 3 months | |
| Urinary incontinence | Hammadeh 2003 | 52 | 0 | 0 | 52 | 0 | 0 | Same for the 5-year follow-up |
| McAllister 2003 | 120 | 1 | 0.8 | 107 | 1 | 0.9 | At 6 months; neither required a surgical intervention | |
| van Melick 2003 | 50 | 4 | 8 | 46 | 7 | 15 | Up to 12 months | |
| Retrograde ejaculation | Küpeli 1998a | 24 | 13 | 54 | 30 | 7 | 23 | |
| Fowler 2005 | 94 | 24 | 94 | 23 | 24 | Failed ejaculation at 2 months | ||
| 98 | 23 | 23.4 | 98 | 33 | 33.7 | Failed ejaculation at 6 months | ||
| 94 | 37 | 37 | 94 | 37 | 39.4 | No ejaculation at 2 months | ||
| 98 | 40 | 40.8 | 92 | 36 | 39.1 | No ejaculation at 6 months | ||
| Hammadeh 2003 | 28 | 25 | 89 | 29 | 21 | 72 | p = 0.47; same for the 5-year follow-up | |
| Kaplan 1998 | 17 | 13 | 76 | 20 | 17 | 85 | p = NS | |
| McAllister 2003 | 59 | 35 | 59 | 64 | 31 | 48 | p = NS with chi square test | |
| Nuhoğlu 2005 | 24 | 4 | 17 | 35 | 5 | 14 | At 3 months | |
| Shokeir 1997 | 15 | 15 | 100 | 18 | 18 | 100 | All potent men had retrograde ejaculation | |
| Erdaği 1999 | 17 | 12 | 70.5 | 16 | 2 | 12.5 | p < 0.001 | |
| Erectile dysfunction | Fowler 2005 | 59 | 4 | 6.8 | 70 | 11 | 15.7 | At 2 months |
| 58 | 5 | 8.6 | 69 | 12 | 17.4 |
At 6 months p = NS |
||
| Küpeli 1998a | 30 | 19 | 63 | 30 | 16 | 53 | ||
| Netto 1999 | 38 | 0 | 0 | 40 | 0 | 0 | ||
| Hammadeh 2003 | 28 | 3 | 11 | 29 | 5 | 17 | Postoperative impotence; none of the patients had any improvement in their sexual function at 1 year; p = 0.49 | |
| Kaplan 1998 | 18 | 0 | 0 | 20 | 1 | 5 | ||
| McAllister 2003 | 58 | 5 | 9 | 69 | 12 | 17 | Impotence | |
| Nuhoğlu 2005 | 25 | 4 | 16 | 20 | 2 | 10 | At 3 months | |
| Patel 1997 | 6 | 0 | 0 | 6 | 0 | 0 | Potency loss | |
| Shokeir 1997 | 15 | 0 | 0 | 18 | 2 | 11.1 | Impotence | |
| Erdaği 1999 | 16 | 0 | 0 | |||||
| Retention | Ekengren 2000 | 28 | 1 | 3.6 | 26 | 0 | 0 | Acute retention |
| Van Melick 2003 | 50 | 0 | 0 | 46 | 0 | 0 | Up to 12 months | |
| Postoperative (> 12 months) | ||||||||
| Stricture | Wang 2002 | 43 | 1 | 2.3 | 38 | 1 | 2.63 | At 24 months |
| Retrograde ejaculation | Fowler 2005 | 68 | 15 | 22.1 | 83 | 16 | 19.3 | Failed ejaculation at 2 years |
| 68 | 22 | 32.3 | 83 | 31 | 37.3 |
No ejaculation at 2 years p = NS |
||
| Erectile dysfunction | Fowler 2005 | 43 | 8 | 18.6 | 64 | 12 | 18.7 | At 2 years; p = NS |
| Study | Baseline | 2 months | 6 months | 12 months | 2 years | Comments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUVP | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | ||
| Fowler 2005 |
n = 114 4.9, SD 0.98 (4.7–5.0) |
n = 109 4.6, SD 1.17 (4.4–4.8) |
n = 109 2.3, SD 1.73 (2.0–2.7) |
n = 105 2.6, SD 1.82 (2.2–2.9) |
n = 108 1.6, SD 1.34 (1.4–1.9) |
n = 107 2.0, SD 1.63 (1.6–2.2) |
n = 80 1.8, SD 1.34 (1.4–1.2) |
n = 89 1.9, SD 1.62 (1.3–2.0) |
IPSS quality of life score. The Wilcoxon signed rank–rank test showed a significant difference in the IPSS QoL assessment, which was sustained to 2 years from randomisation. The improvement at all data points was similar in both groups. We conclude that TURP and TUVP produce a significant improvement in IPSS QoL score, which is sustained to 2 years from randomisation | ||||||
|
n = 116 0.74, SD 0.25 (0.68–0.78) |
n = 112 0.78, SD 0.23 (0.73–0.82) |
n = 110 0.75, SD 0.26 (0.79–0.80) |
n = 108 0.79, SD 0.25 (0.74–0.84) |
n = 108 0.79, SD 0.24 (0.74–0.83) |
n = 105 0.77, SD 0.28 (0.71–0.82) |
n = 82 0.74, SD 0.25 (0.69–0.80) |
n = 90 0.78, SD 0.27 (0.72–0.83) |
Quality of life (EuroQoL health scale scores). There was no difference between baseline EuroQoL scores and scores at all points after randomisation | |||||||
|
n = 116 71.3, SD 17.6 (68.1–74.6) |
n = 109 75.8, SD 16.0 (72.7–78.8) |
n = 108 71.4, SD 18.9 (67.8–75.0) |
n = 107 77.2, SD 16.1 (74.2–80.3) |
n = 109 72.9, SD 18.3 (69.4–76.4) |
n = 108 76.9, SD 19.4 (73.2–80.6) |
n = 77 70.4, SD 19.5 (66.0–74.9) |
n = 87 75.6, SD 20.1 (71.3–79.9) |
Quality of life (EuroQoL health scale scores). In this part of the EuroQoL questionnaire, the man is asked to mark a level on a 1–100 analogue scale to indicate health status, where 100 indicates perfect health. There was no significant change at any data point | |||||||
| Results are not provided; however, there was little or no change in domains of the SF-36 and no significant difference between the two groups in this respect | Quality of life (SF-36). The authors conclude that any change in general health-related QoL resulting from either intervention is not detectable by EuroQoL or SF-36 | ||||||||||||||
| Ekengren 2000 |
n = 28 5.5 (3–6) |
n = 26 4.5 (2–6) |
n = 28 1.0 (0–6) |
n = 26 1.5 (0–6) |
NS | IPSS QoL (0–6) | |||||||||
| Hammadeh 2003 |
n = 52 5.0, SD 0.7 |
n = 52 4.9, SD 0.9 |
n = 52 1.5a |
n = 52 1.7a |
n = 52 1.4a |
n = 52 1.5a |
n = 51 1.5, SD 1 |
n = 51 1.2, SD 1 |
0.3 |
n = 47 1.7, SD 1.1 |
n = 47 1.1, SD 1 |
0.004 | Quality of life score | ||
| van Melick 2003 |
n = 50 3.9, SD 1.6 |
n = 46 4.3, SD 1.3 |
n = 50 0.5, SD 0.5 |
n = 46 1.0, SD 0.8 |
n = 41 0.6, SD 0.8 |
n = 34 1.0, SD 0.9 |
n = ? 1.1, SD 1.2 |
n = ? 1.0, SD 1.2 |
Data is for 1–4 years | Quality of life reported (IPSS QoL) | |||||
| Study | 3 years | 4 years | 5 years | Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | ||
| Hammadeh 2003 |
n = 40 1.6, SD 1.4 |
n = 40 1, SD 0.9 |
0.04 |
n = 27 1.7, SD 1.4 |
n = 26 1.1, SD 1.2 |
0.09 | Quality of life score | |||
| van Melick 2003 |
n = ? 1.3, SD 1.3 |
n = ? 1.4, SD 0.8 |
Quality of life reported (IPSS QoL); 4- to 7-year data | |||||||
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 24 months | 3 years | 5 years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | TUVP | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | TURP | TUVP | p-value | ||
| Peak urine flow rate (ml/s) | Ekengren 2000 |
n = 28 2a (0–10) |
n = 26 4a (0–8) |
n = 28 11a (0–19) |
n = 26 10a (4–19) |
NS | |||||||||||||||
| Erdaği 1999 |
n = 20 4.6 (4–9.6) |
n = 20 5.1 (0–11.3) |
n = 20? 17 |
n = 20? 21 |
n = 20? 17.7 p < 0.001 |
n = 20? 21.4 p < 0.001 |
NS | ||||||||||||||
| Fowler 2005 |
n = 97 10.5, SD 5.04 (9.5–11.5)b |
n = 94 10.1, SD 4.35 (9.2–11.0)b |
n = 111 21.23, SD 10.2 (19.3–23.1)b |
n = 108 19.12, SD 11.76 (16.9–21.4)b |
n = 109 22.29, SD 10.25 (20.3–24.2)b |
n = 109 19.6, SD 11.04 (17.5–21.7)b |
NS | ||||||||||||||
| Galluci 1998 |
n = 80 8.78, SE 1.16 |
n = 70 7.26, SE 0.37 |
n = 80 19.21, SE 0.91 |
n = 70 18.18, SE 0.92 |
NS |
n = 80 20.77, SE 0.95 |
n = 70 20.13, SE 1.15 |
n = 80 20.3, SE 0.71 |
n = 70 20.31, SE 0.72 |
NS | |||||||||||
| Hammadeh 2003; 3- and 6-month data taken from Hammadeh 1998 |
n = 52 8.6, SD 3.2 |
n = 52 8.9, SD 3.2 |
n = 52 22.3c |
n = 52 20c |
NS |
n = 52 21.1c |
n = 52 19.8c |
NS |
n = 51 20.8, SD 7.7 |
n = 51 22.5, SD 9 |
0.4 |
n = 47 21.2, SD 8.5 |
n = 47 22.4, SD 7.7 |
0.5 |
n = 40 18, SD 7.1 |
n = 40 22.2, SD 8.5 |
0.02 |
n = 27 17.9, SD 13.1 |
n = 26 21.0, SD 9 |
0.17 | |
| Kaplan 1998 |
n = 32 8.3, SD 3.6 |
n = 32 7.2, SD 2.8 |
n = 32 16.8, SD 3.6 |
n = 32 14.8, SD 3.9 |
n = 32 18.1, SD 4.2 |
n = 32 15.6, SD 3.2 |
n = 31 19.6, SD 4.9 |
n = 30 16.9, SD 4.1 |
|||||||||||||
| Küpeli 1998a |
n = 30 7.9, SD 2.1 |
n = 30 9.2, SD 2.6 |
n = 30? 17.7, SD 3.6 p < 0.01 |
n = 30? 19.7, SD 3.2 p < 0.01 |
|||||||||||||||||
| Küpeli 1998b |
n = 36 8.8 (3–12.4) |
n = 30 8.3 (2.7–11.8) |
n = 33 14.3 (7.2–17.5) |
n = 27 13.8 (8.2–16.4) |
n = 30 19.6 (9.4–24.5) |
n = 26 17.3 (11.5–23.8) |
|||||||||||||||
| Netto 1999 |
n = 38 6.77 , SD 3.08 |
n = 40 7.88 , SD 2.51 |
n = 38? 16.16 SD 2.48 |
n = 40? 15.43 SD 3.40 |
< 0.02 | ||||||||||||||||
| Nuhoğlu 2005 |
n = 40 5.9, SD 2.6 |
n = 37 6.3, SD 2.1 |
n = 38 17.5, SD 3.3 |
n = 35 17.7, SD 2.3 |
NS |
n = 23 13.8, SD 2.9 |
n = 21 12.9, SD 3.1 |
NS | |||||||||||||
| Patel 1997 |
n = 6 7.5 (5.1–11) |
n = 6 10 (7.3–13.1) |
n = 6 22.6 (19.3–25.2) |
n = 6 21.4 (17.2–25.3) |
|||||||||||||||||
| Shokeir 1997 |
n = 35 6.9, SD 1.7 (3.4–10) |
n = 35 7.8, SD 2.1 (4.1–11.4) |
n = 35? 19.4, SD 2.1 (16–26) |
n = 35? 19.4, SD 2.2 (15–24) |
n = 35? 19.3, SD 2 (16–24) |
n = 35? 19.2, SD 2 (16–23) |
NS |
n = 35? 18.2, SD 3 (15–25) |
n = 35? 20.1, SD 3.2 (18–25) |
||||||||||||
| Wang 2002 |
n = 109 7 (3–12) |
n = 97 7 (2–13) |
n = 109 20 (3–24) p < 0.01 |
n = 96 17 (4–25) p < 0.01 |
n = 43 15 (4–21) |
n = 38 16 (3–24) |
|||||||||||||||
| Gotoh 1999 |
n = 28 9.4, SD 2.8 |
n = 23 7.3, SD 2.8 |
n = 28 21.2, SD 9.4 |
n = 23 23.6, SD 13.9 |
|||||||||||||||||
| van Melick 2003; in van Melick 2002 |
n = 46 11, SD 4 |
n = 41 11, SD 4 |
n = 15 25, SD 11 |
n = 19 20, SD 10 |
n = 37 24, SD 7 |
n = 33 23, SD 10 |
n = 11 21, SD 8 |
n = 9 23, SD 8 |
n = 15d 20, SD 5 |
n = 12d 23, SD 6 |
n = 15e 17, SD 8 |
n = 12e 16, SD 11 |
|||||||||
| Mean urine flow rate (ml/s) | Nuhoğlu 2005 |
n = 40 2.4, SD 1.3 |
n = 37 2.6, SD 1.2 |
n = 38 10.5, SD 2.4 |
n = 35 9.8, SD 1.7 |
NS | |||||||||||||||
| Erdaği 1999 |
n = 20 2.3 (0–5.5) |
n = 20 2.5 (0–5.3) |
n = 20 13.1 p < 0.001 |
n = 20 11.3 p < 0.001 |
NS |
n = 23 8.3, SD 2.9 |
n = 21 7.9, SD 2.3 |
NS | |||||||||||||
| Total voided volume (ml) | Kaplan 1998 |
n = 32? 34.2, SD 19.6 |
n = 32? 43.6, SD 22.4 |
0.11 | |||||||||||||||||
| Residual volume (ml) | Netto 1999 |
n = 38 88.64, SD 8.43 |
n = 40 73.0, SD 5.81 |
n = 38? 11.20, SD 1.30 p < 0.05 |
n = 40? 12.3, SD 1.90 p < 0.05 |
0.78 | |||||||||||||||
| Nuhoğlu 2005 |
n = 40 95, SD 26 |
n = 38 88, SD 20 |
n = 38 23, SD 12 |
n = 35 25, SD 13 |
NS |
n = 23 38, SD 17 |
n = 21 35, SD 15 |
NS | |||||||||||||
| Shokeir 1997 |
n = 35 77.1, SD 20.3 (46–110) |
n = 35 75.2, SD 4.2 (40–112) |
n = 35? 30, SD 16 (18–72) |
n = 35? 31.4, SD 17 (18–64) |
n = 35? 22.1, SD 10.4 (10–50) |
n = 35? 20.3, SD 9.6 (10–53) |
NS |
n = 35? 25.3, SD 11.5 (12–55) |
n = 35? 23.4, SD 10.1 (18–25) |
||||||||||||
| Hammadeh 2003 |
n = 52 101, SD 87.9 |
n = 52 131, SD 78.5 |
n = 52 22PφP |
n = 52 18PφP |
NS |
n = 52 20PφP |
n = 52 19PφP |
NS |
n = 51 25.8, SD 25.6 |
n = 51 24.3, SD 33.1 |
0.1 |
n = 47 22.8, SD 29.8 |
n = 47 18.8, SD 21.2 |
0.5 |
n = 40 21.9, SD 26.2 |
n = 40 30, SD 38 |
0.27 |
n = 27 10.7, SD 13.1 |
n = 26 27.3, SD 44.3 |
||
| Fowler 2005 |
n = 94 171 |
n = 91 181 |
n = 109 71.8, SD 87.4 (54.7–87.8)b |
n = 109 71.0, SD 72.0 (47.8–70.8)b |
NS | ||||||||||||||||
| Galluci 1998 |
n = 80 64.6, SE 8.62 |
n = 70 84.7, SE 11.39 |
n = 80 7.27, SE 2.76 |
n = 70 8.69, SE 2.58 |
NS |
n = 80 1.71, SE 0.79 |
n = 70 11.86, SE 4.40 |
NS |
n = 80 3.15, SE 1.96 |
n = 70 5.24, SE 2.44 |
NS | ||||||||||
| Erdaği 1999 |
n = 20 122.8 (0–600) |
n = 20 68 (20–150) |
n = 20? 8.0 |
n = 20? 7.2 |
n = 20? 6 p < 0.001 |
n = 20? 3.6 p < 0.001 |
NS | ||||||||||||||
| Ekengren 2000 |
n = 28 100a (0–3000) |
n = 26 55a (0–3000) |
n = 28 57a (0–766) |
n = 26 17a (0–300) |
NS | ||||||||||||||||
| Wang 2002 |
n = 109 131 (60–380) |
n = 97 120 (60–400) |
n = 109 31 (0–240) p < 0.01 |
n = 67 36 (0–165) p < 0.01 |
n = 43 31 (0–266) |
n = 38 42 (4–263) |
|||||||||||||||
| Gotoh 1999 |
n = 28 41.9, SD 25.5 |
n = 23 56.7, SD 51.4 |
n = 28? 9.3, SD 22.1 |
n = 23? 8.1, SD 12.9 |
|||||||||||||||||
| Detrusor pressure (cmH2O) | Galluci 1998 |
n = 80 75.89, SE 3.49 |
n = 70 73.41, SE 3.89 |
n = 80 41.53, SE 1.71 |
n = 70 38.23, SE 1.81 |
NS | |||||||||||||||
| van Melick 2003 | Decreased | Decreased | |||||||||||||||||||
| Prostate size (ml) | Nuhoğlu 2005 |
n = 40 39, SD 7.7 |
n = 37 39, SD 8.1 |
n = 38 19, SD 6.4 |
n = 35 21, SD 6.8 |
NS |
n = 23 23, SD 6.9 |
n = 21 24, SD 7.1 |
NS | ||||||||||||
| Küpeli 1998a |
n = 30 51.7, SD 9.1 |
n = 30 48.9, SD 8.7 |
n = 30 26.2, SD 3.4 p < 0.05 |
n = 30 27.8, SD 4.1 p < 0.05 |
|||||||||||||||||
| Fowler 2005 |
n = 103 51.1 |
n = 100 54.3 |
n = 98 24.8f (19.9–29.7) |
n = 97 21.5f (16.8–26.2) |
NS | ||||||||||||||||
| Ekengren 2000 |
n = 28 39a (20–80) |
n = 26 55a (25–90) |
n = 28 28a (15–57) |
n = 26 33a (15–84) |
|||||||||||||||||
| Outcome | Study | TURP | TUVP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Çetinkaya 1996 |
N = 23 52.4, SD 20.0 |
N = 23 41.6, SD 22.1 |
p = 0.20 | ||||
| Ekengren 2000 |
N = 28 33 (10–90) |
N = 26 30 (15–80) |
Median (range) | |||||
| Hammadeh 2003 |
N = 54 21.6, SD 8.4 (10–60) |
N = 55 25.9, SD 8.3 (10–50) |
p = 0.01 | |||||
| Kaplan 1998 |
N = 32 34.6, SD 11.2 (25–55) |
N = 32 47.6, SD 17.6 (23–76) |
p < 0.01 | |||||
| Küpeli 1998a |
N = 30 41.3 |
N = 30 47.3 |
||||||
| Fowler 2005; in McAllister 2003 |
N = 114 44.7 |
N = 107 49.0 |
||||||
| Netto 1999 |
N = 38 56.32, SD 8.36 |
N = 40 29.78, SD 11.78 |
p = 0.001 | |||||
| Nuhoğlu 2005 |
N = 40 42, SD 9.5 |
N = 37 45, SD 13.2 |
p < 0.09 | |||||
| Patel 1997 |
N = 6 66 (27–95) |
N = 6 64.3 (40–120) |
p = 0.34; median (range) | |||||
| Shokeir 1997 |
N = 35 39.7, SD 8.8 (25–60) |
N = 35 52, SD 12.5 (30–76) |
p < 0.001 | |||||
| Wang 2002 |
N = 109 50 (30–90) |
N = 97 35 (25–70) |
p < 0.05 | |||||
| Gupta 2006 |
N = 50 64, SD 13.1 (40–110) |
N = 50 55.9, SD 18.1 (35–115) |
||||||
| van Melick 2003 |
N = 50 58, SD 26 (25–150) |
N = 46 50, SD 16 (20–90) |
p = 0.09 | |||||
| Gotoh 1999 |
N = 28 61.1, SD 29.0 |
N = 23 60.0, SD 28.0 |
||||||
| Nathan 1996 |
N = 20 37.4 |
N = 20 39.2 |
Mean | |||||
| Length of hospital stay (days) | Fowler 2005; in McAllister 2003 |
N = 120 ? 4.6, 95%CI 3.9–5.4 |
N = 115? 4.4, 95% CI 3.8–5.1 |
Data available for all but three patients; p < 0.0000 | ||||
| Hammadeh 2003 |
N = 52 3.1, SD 0.76 (1.6–5.7) |
N = 52 2.2, SD 0.59 (1.7–3.8) |
p < 0.001 | |||||
| Kaplan 1998 |
N = 32 2.6, SD 0.9 |
N = 32 1.3, SD 0.5 |
p = 0.03 | |||||
| Küpeli 1998a |
N = 30 4.16, SD 1.46 |
N = 30 1.92, SD 0.89 |
p < 0.0001 | |||||
| Küpeli 1998b |
N = 36 4.16, SD 1.46 |
N = 30 1.92, SD 0.89 |
p < 0.0001 | |||||
| Galluci 198 |
N = 80 4.69, SE 0.22 |
N = 70 3.9, SE 0.24 |
||||||
| Netto 1999 |
N = 38 2.63, SD 0.63 |
N = 40 1.55, SD 0.75 |
p < 0.001 | |||||
| Patel 1997 |
N = 6 2.6 (2–4) |
N = 6 1.8 (1–2) |
||||||
| Shokeir 1997 |
N = 35 2.5, SD 1 (1–4) |
N = 35 1.5, SD 0.7 (1–4) |
p < 0.001 | |||||
| van Melick 2003 |
N = 50 3.9, SD 0.9 4.0 (3.0–5.9) |
N = 46 3.4, SD 0.9 3.0 (2.0–5.0) |
Median (2.5–97.5 percentiles) | |||||
| Nathan 1996 |
N = 20 3.45 |
N = 20 1.85 |
Mean; p < 0.0001 | |||||
| N | n | % | N | n | % | |||
| Reoperation | Ekengren 2000 | 28 | 1 | 3.6 | 26 | 2 | 7.7 | Late reoperation |
| Küpeli 1998b | 36 | – | – | 30 | 1 | 3 | Reoperated with TURP after 3 months | |
| Hammadeh 2003 | 51 | 2 | 4 | 51 | 2 | 4 | At 1 year | |
| 47 | 2 | 4.3 | 47 | 2 | 4.3 | At 2 years | ||
| 40 | 2 | 5 | 40 | 2 | 5 | At 3 years | ||
| 27 | 1 | 3.7 | 26 | 1 | 3.8 | At 5 years | ||
| 52 | 7 | 13.4 | 52 | 7 | 13.4 | Total | ||
| Four TUVP patients underwent TURP (the surgeon had no experience in TUVP). Three TUVP patients had a repeat TUVP | ||||||||
| McAllister 2003 | 120 | 4 | 3.3 | 115 | 1 | 0.9 | At 6 months; all underwent TURP | |
| van Melick 2003 | 50 | 2 | 4 | 46 | 2 | 4.3 | Up to 12 months; all underwent TURP | |
| Nathan 1996 | 20 | 0 | 0 | 20 | 0 | 0 | Up to 3 months | |
| Nuhoğlu 2005 | 40 | – | – | 37 | 1 | 2.7 | During the fourth year postoperatively | |
Appendix 8.12 B-TUVP versus TURP
| Study | Baseline | 3 months | 9 months | 12 months | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | B-TUVP | TURP | B-TUVP | p-value | TURP | B-TUVP | p-value | TURP | B-TUVP | p-value | ||
| Dunsmuir 2003 (AUA-7) |
n = 21 17, SD 6.2 (10–29) |
n = 30 24, SD 6.9 (9–30) |
n = 21 |
n = 30 |
n = 20 |
n = 20 |
Graph readings not very accurate | |||||
| Hon 2006 (IPSS) |
n = 79 20.6, SD 7.0 |
n = 81 21.3, SD 6.2 |
n = 73 6.9, SD 5.8 |
n = 76 7.7, SD 6.8 |
0.44 | |||||||
| Complication | Study | TURP | B-TUVP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Blood transfusion | Hon 2006 | 79 | 4 | 5.3 | 81 | 0 | 0 | p = 0.02 |
| Recatheterisation | Dunsmuir 2003 | 21 | 1 | 4.7 | 30 | 10 | 33 | p < 0.005 |
| Clot retention | Dunsmuir 2003 | 21 | 4 | 19 | 30 | 0 | 0 | Clot evacuation; p < 0.001 |
| Postoperative (3–12 months) | ||||||||
| Stricture | Hon 2006 | 79 | 1 | 1.3 | 81 | 0 | 0 | Urethral stricture |
| 79 | 2 | 2.5 | 81 | 1 | 1.2 | Bladder neck stenosis | ||
| Follow-up unclear (up to 9 months) | ||||||||
| Study | Baseline | 3 months | 9 months | 12 months | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | B-TUVP | TURP | B-TUVP | p value | TURP | B-TUVP | p value | TURP | B-TUVP | p value | ||
| Dunsmuir 2003 |
n = 21 11, SD 3.1 (7–17) |
n = 30 12, SD 3.4 (8–14) |
n = 21 5.5a |
n = 30 5.5a |
n = 20 8.5a |
n = 20 7a |
AUA QoL taken from section C of the AUA-7 system. It comprises five questions to give a maximum score of 19 | |||||
| Hon 2006 |
n = 79 4.3, SD 1.3 |
n = 81 4.2, SD 1.1 |
n = 79 1.5, SD 1.5 |
n = 81 1.7, SD 1.5 |
0.64 | IPSS QoL score | ||||||
| Urodynamic outcome | Study | Baseline | 3 months | 9 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TURP | B-TUVP | TURP | B-TUVP | p-value | TURP | B-TUVP | p-value | TURP | B-TUVP | p-value | ||
| Peak urine flow rate (ml/s) | Dunsmuir 2003 |
n = 21 10.4, SD 3.1 (7–14) |
n = 30 9.6, SD 3.0 (8–14) |
n = 21 21.5a |
n = 30 19a |
n = 20 15.5a |
n = 20 17a |
|||||
| Hon 2006 |
n = 79 11.9, SD 6.0 |
n = 81 12.0, SD 6.4 |
n = 73 23.5, SD 15.2 |
n = 76 25.6, SD 15.6 |
0.41 | |||||||
| Mean urine flow rate (ml/s) | Hon 2006 |
n = 79 6.1, SD 2.9 |
n = 81 5.9, SD 3.3 |
n = 73 11.9, SD 7.9 |
n = 76 15.0, SD 9.4 |
0.03 | ||||||
| Residual volume (ml) | Dunsmuir 2003 |
n = 21 96, SD 11.4 (40–167) |
n = 30 112, SD 13.3 (42–188) |
n = 21 62.5a |
n = 30 87.5a |
n = 20 80a |
n = 20 87.5a |
|||||
| Hon 2006 |
n = 79 182, SD 180 |
n = 81 147, SD 156 |
n = 73 69, SD 67 |
n = 76 64, SD 65 |
0.68 | |||||||
| Prostate size (ml) | Dunsmuir 2003 |
n = 21 42, SD 21 (22–60) |
n = 21 39, SD 19 (16–56) |
|||||||||
| Hon 2006 |
n = 79 40, SD 17.1 |
n = 81 38, SD 17.5 |
||||||||||
| Outcome | Study | TURP | B-TUVP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Dunsmuir 2003 |
N = 21 26 |
N = 30 33 |
Mean or median; p = 0.78 | ||||
| Hon 2006 |
N = 79 28.5, SD 15.2 |
N = 81 32.6, SD 13.4 |
Resection time; p = 0.08 | |||||
| Length of hospital stay (days) | Dunsmuir 2003 |
N = 21 1.45 |
N = 30 1.5 |
Mean or median; p = 0.88 | ||||
| Hon 2006 |
N = 79 3.4, SD 1.1 |
N = 81 3.0, SD 0.9 |
p = 0.04 | |||||
| N | n | % | N | n | % | |||
| Reoperation | Hon 2006 | 79 | 2 | 2.5 | 81 | 1 | 1.2 | BNI for stenosis. Follow-up is unclear (up to 9 months) |
Appendix 8.13 Laser coagulation versus TUVP
| Study | Baseline | 3 months | 6 months | 12 months | 2 years | 3 years | 4 years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laser coagulation | TUVP | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | |
| Abdel-Khalek 2003 (IPSS) |
n = 90 27.9, SD 5.3 |
n = 90 26.0, SD 5.8 |
n = 89 13.3, SD 6 |
n = 89 5.6, SD 3.5 |
0.003 |
n = 86 12.2, SD 5.6 |
n = 84 5.2, SD 3.3 |
0.006 |
n = 73 13.1, SD 5.7 |
n = 82 4.8, SD 2.6 |
0.002 |
n = 62 11.9, SD 6.1 |
n = 78 3.7, SD 1.3 |
< 0.001 | ||||||
| Shingleton 1998 (AUA) |
n = 11 19 (13–26) |
n = 20 22.1 (8–31) |
n = 11 5.9 (2–24) |
n = 20 5.2 (1–12) |
NS |
n = 11 5.0 (0–10) |
n = 20 5.2 (1–19) |
NS | ||||||||||||
| Narayan 1995 (AUA) |
n = 32 22.1 (15–30) |
n = 32 22.4 (14–35) |
n = 32? 8.4 |
n = 32? 7.0 |
n = 32? 5.1 |
n = 32? 5.0 |
n = 32? 5.2 |
n = 32? 5.3 |
||||||||||||
| Complication | Study | Laser coagulation | TUVP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Periprocedural (intraoperative or immediate postoperative) | ||||||||
| Bleeding | Abdel-Khalek 2003 | 90 | 0 | 0 | 90 | 1 | 1 | At surgery |
| Blood transfusion | Narayan 1995 | 32 | 0 | 0 | 32 | 0 | 0 | p > 0.05 |
| Urinary retention | Abdel-Khalek 2003 | 90 | 9 | 10 | 90 | 2 | 2 | |
| Narayan 1995 | 32 | 8 | 25 | 32 | 2 | 6.2 | Retention > 7 days; p = 0.039. Follow-up uncertain (< 3 months) | |
| Shingleton 1998 | 11 | 3 | 27.3 | 20 | 1 | 5 | ||
| Incontinence | Narayan 1995 | 32 | 0 | 0 | 32 | 0 | 0 | Follow-up uncertain (< 3 months) |
| Irritative urinary symptoms | Narayan 1995 | 32 | 10 | 32 | 32? | 11 | 39 | p = 0.056. Follow-up uncertain (< 3 months) |
| Stricture | Narayan 1995 | 32 | 0 | 0 | 32 | 0 | 0 | Follow-up uncertain (< 3 months) |
| Shingleton 1998 | 11 | 1 | 9 | 20 | 0 | 0 | Time not reported (total follow-up: 6 months) | |
| Erectile dysfunction | Narayan 1995 | 32? | 0 | 0 | 32? | 0 | 0 | Follow-up uncertain (< 3 months) |
| Shingleton 1998 | 11 | 1 | 9 | 20 | 2 | 10 | Impotence. Timing not reported (total follow-up: 6 months) | |
| Urinary tract infection | Narayan 1995 | 32 | 2 | 6.2 | 32 | 1 | 3.1 | Epididymitis; p > 0.05 |
| 32 | 0 | 0 | 32 | 0 | 0 | |||
| Cardiovascular events | Abdel-Khalek 2003 | 90 | 1 | 1.1 | 90 | 2 | 2.2 | |
| Postoperative (3–12 months)) | ||||||||
| Stricture | Abdel-Khalek 2003 | 90? | 2 | 2.2 | 90? | 4 | 4.4 | At 1 year |
| 90? | 2 | 2.2 | 90? | 2 | 2.2 | Bladder neck stenosis | ||
| Retrograde ejaculation | Abdel-Khalek 2003 | 90? | 16 | 17.8 | 90? | 57 | 63.3 | At 1 year; p < 0.001 |
| Erectile dysfunction | Abdel-Khalek 2003 | 49 | 0 | 0 | 53 | 4 | 7.5 | Impotence; p = 0.04 |
| Study | Baseline | 12 months | 2 years | 3 years | 4 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laser coagulation | TUVP | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | |
| Abdel-Khalek 2003 (scale not defined) |
n = 90 5, SD 0.8 |
n = 90 4.8, SD 0.9 |
n = 89 3.4, SD 0.4 |
n = 89 1.4, SD 0.5 |
0.008 |
n = 84 3.2, SD 0.5 |
n = 86 1.4, SD 0.4 |
0.009 |
n = 73 3.3, SD 0.6 |
n = 82 1.4, SD 0.5 |
0.009 |
n = 62 3.1, SD 1.0 |
n = 78 1.3, SD 0.5 |
|
| Urodynamic outcome | Study | Baseline | 3 months | 6 months | 12 months | 24 months | 3 years | 4 years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laser coagulation | TUVP | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | Laser coagulation | TUVP | p-value | ||
| Peak urine flow rate (ml/s) | Abdel-Khalek 2003 |
n = 90 6.9, SD 2.8 |
n = 90 6.4, SD 2.5 |
n = 89 15.1, SD 6.0 |
n = 89 20.8, SD 7.4 |
0.029 |
n = 89 15.1, SD 6.0 |
n = 89 20.8, SD 7.4 |
0.029 |
n = 84 14.2, SD 6.3 |
n = 86 20.2, SD 4.4 |
0.007 |
n = 73 13.9, SD 5.3 |
n = 82 20.5, SD 6.2 |
0.002 |
n = 62 13.6, SD 3.6 |
n = 78 21.4, SD 4.1 |
< 0.001 | |||
| Narayan 1995 |
n = 32 7.0 (0–14) |
n = 32 6.4 (0–15) |
n = 32? 16.3 |
n = 32 19.7 |
n = 32? 16.9 |
n = 32? 19.9 |
|||||||||||||||
| Shingleton 1998 |
n = 11 9.2 (0–12) |
n = 20 7.7 (6–13.2) |
n = 11? 17.6 (6.2–22) |
n = 20? 17.5 (7.8–27) |
NS |
n = 11? 16.5 (7.1–24.9) |
n = 20? 14.3 (7.8–27.1) |
NS | |||||||||||||
| Residual volume (ml) | Abdel-Khalek 2003 |
n = 90 120, SD 97.5 |
n = 90 125, SD 97.5 |
n = 89 61.3, SD 49.2 |
n = 89 22.1, SD 22 |
< 0.001 |
n = 84 73.2, SD 56.2 |
n = 86 33.4, SD 29 |
< 0.001 |
n = 73 56.8, SD 47 |
n = 82 21.4, SD 18 |
< 0.001 |
n = 62 64.6, SD 29.8 |
n = 78 25.1, SD 12.8 |
< 0.001 | ||||||
| Narayan 1995 |
n = 32 210 (0–250) |
n = 32 276.6 (20–960) |
n = 32? 20 |
n = 32? 31 |
n = 32? 28 |
n = 32? 26 |
|||||||||||||||
| Prostate size (ml) | Abdel-Khalek 2003 |
n = 78 35.9, SD 11.0 |
n = 62 27.9, SD 8.6 |
n = 89 33, SD 12.8 |
n = 89 25.8, SD 8.7 |
0.001 |
n = 78 35.9, SD 11.0 |
n = 62 27.9, SD 8.6 |
< 0.001 | ||||||||||||
| Shingleton 1998 |
n = 11 34.6 (9.2–87.8) |
n = 20 34.6 (13.7–66.4) |
|||||||||||||||||||
| Narayan 1995 |
n = 32 41.43 (20–62) |
n = 32 51.67 (16–120) |
|||||||||||||||||||
| Outcome | Study | Laser coagulation | TUVP | Comments | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of operation (minutes) | Shingleton 1998 |
N = 11 27.5 |
N = 20 46 |
p < 0.05 | ||||
| Abdel-Khalek 2003 |
N = 90 36.6, SD 16.4 |
N = 90 37.5, SD 15 |
p = 0.786 | |||||
| Length of hospital stay (days) | Narayan 1995 | 40% discharged within 24 hours; 37% discharged within 36 hours; all within 48 hours | 50% discharged within 24 hours; 40% discharged within 36 hours; all within 48 hours | |||||
| Abdel-Khalek 2003 |
N = 90 1.1, SD 0.5 |
N = 90 2.2, SD 0.8 |
p = 0.107 | |||||
| N | n | % | N | n | % | |||
| Reoperation rate | Narayan 1995 | 32 | 5 | 16 | 32 | 0 | 0 | p = 0.0199 |
| Abdel-Khalek 2003 | 90 | 35 | 39 | 90 | 11 | 12 | ||
Appendix 9 Results of meta-analyses
Appendix 9.1 TUMT versus TURP
Abbreviations used throughout this appendix are as follows: TEAP, transurethral ethanol ablation of the prostate; TUIP, transurethral incision of the prostate; TUMT, transurethral microwave thermotherapy; TUNA, transurethral needle ablation; TUR, transurethral resection; TURP, transurethral resection of the prostate; TUVP, transurethral electrovaporisation of the prostate; TUVRP, transurethral vaporesection of the prostate.
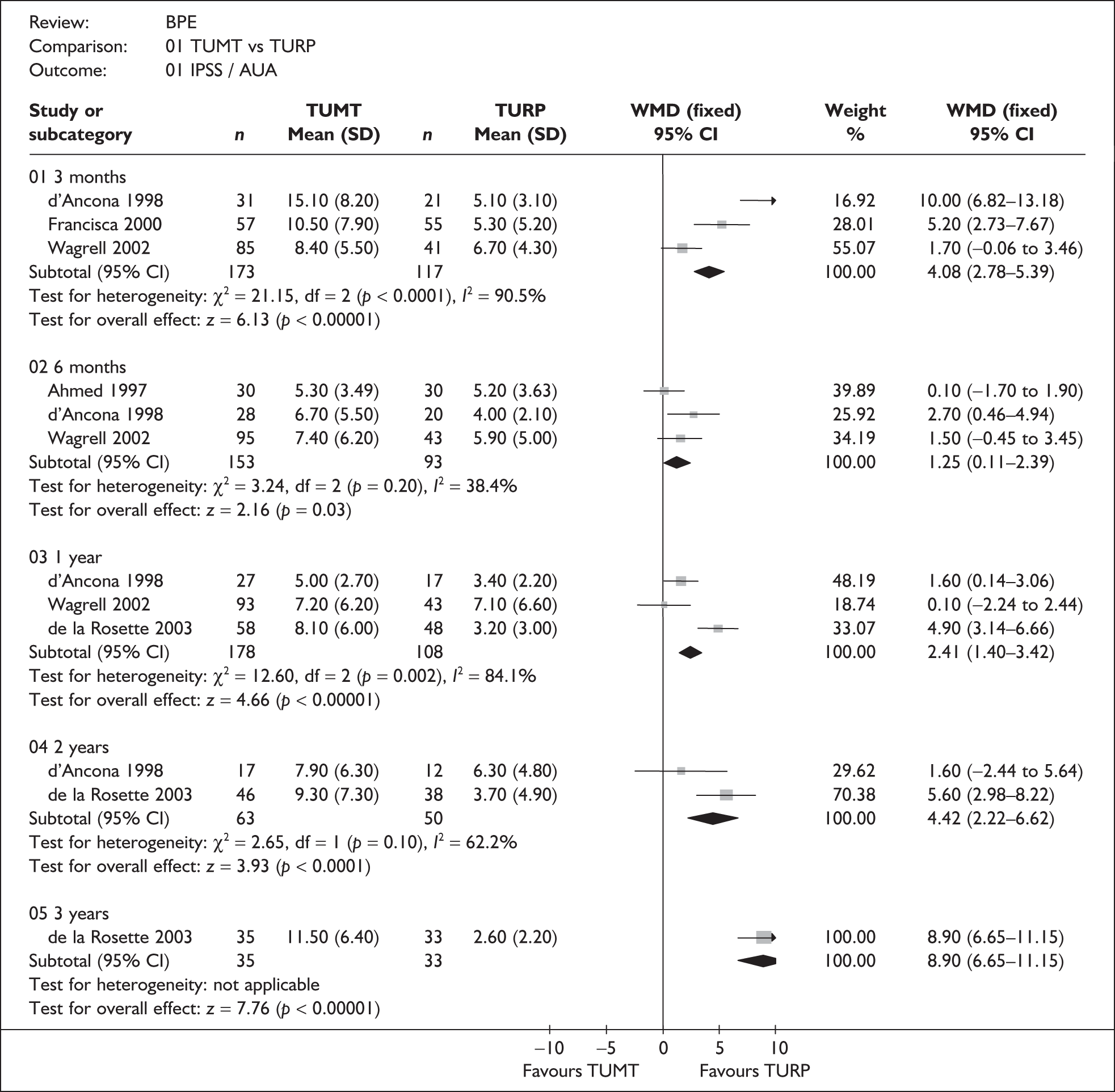




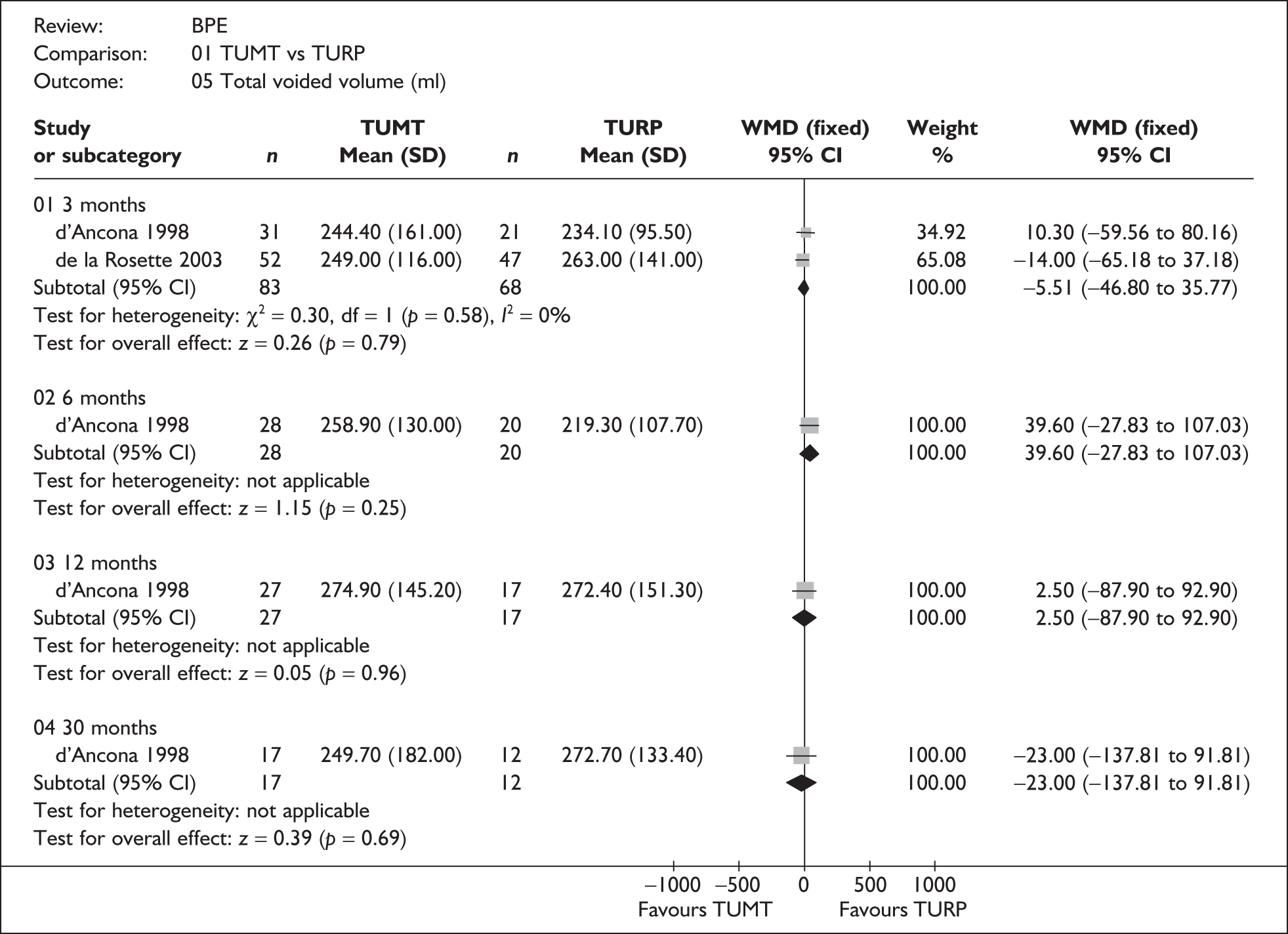






Appendix 9.2 TUMT versus sham

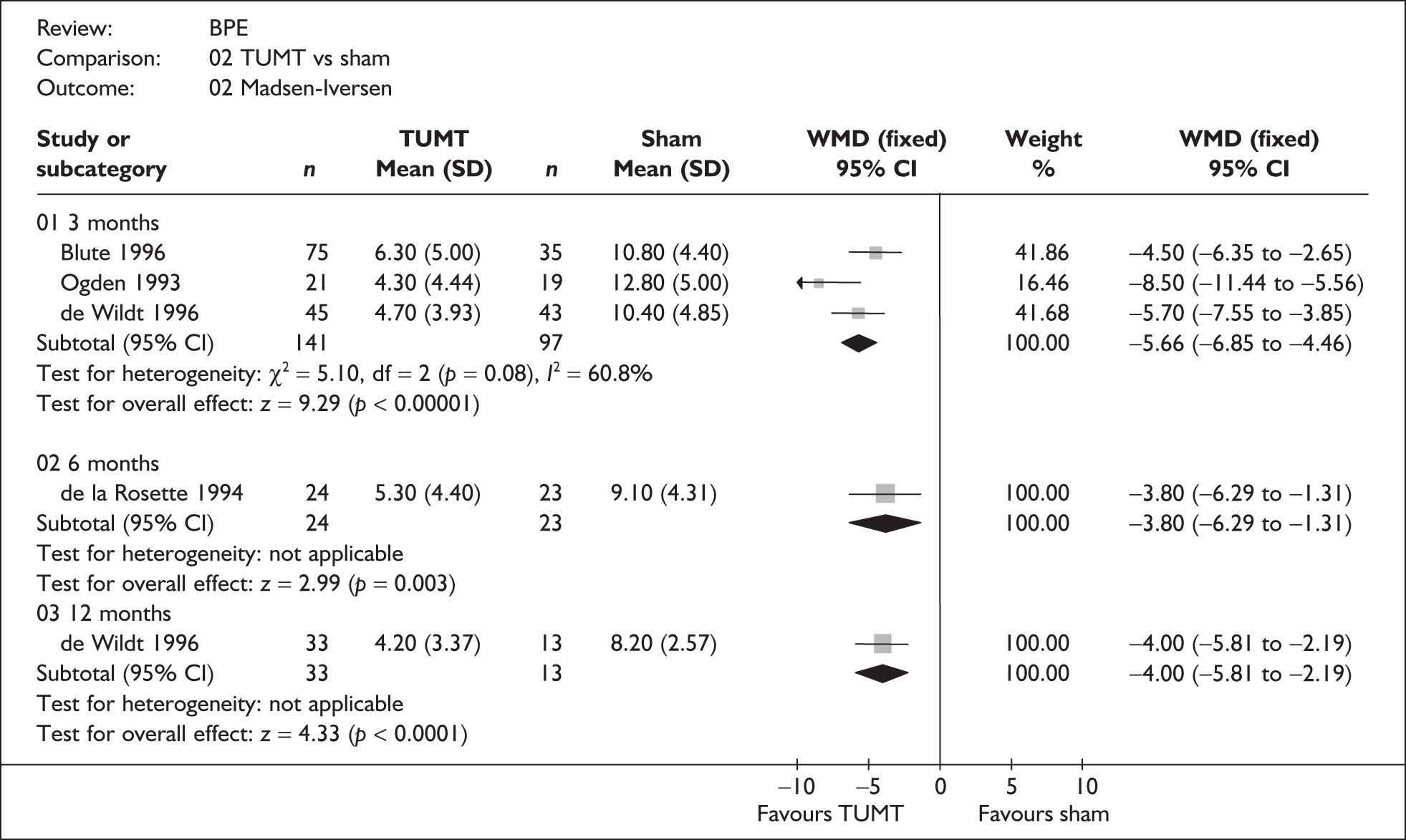

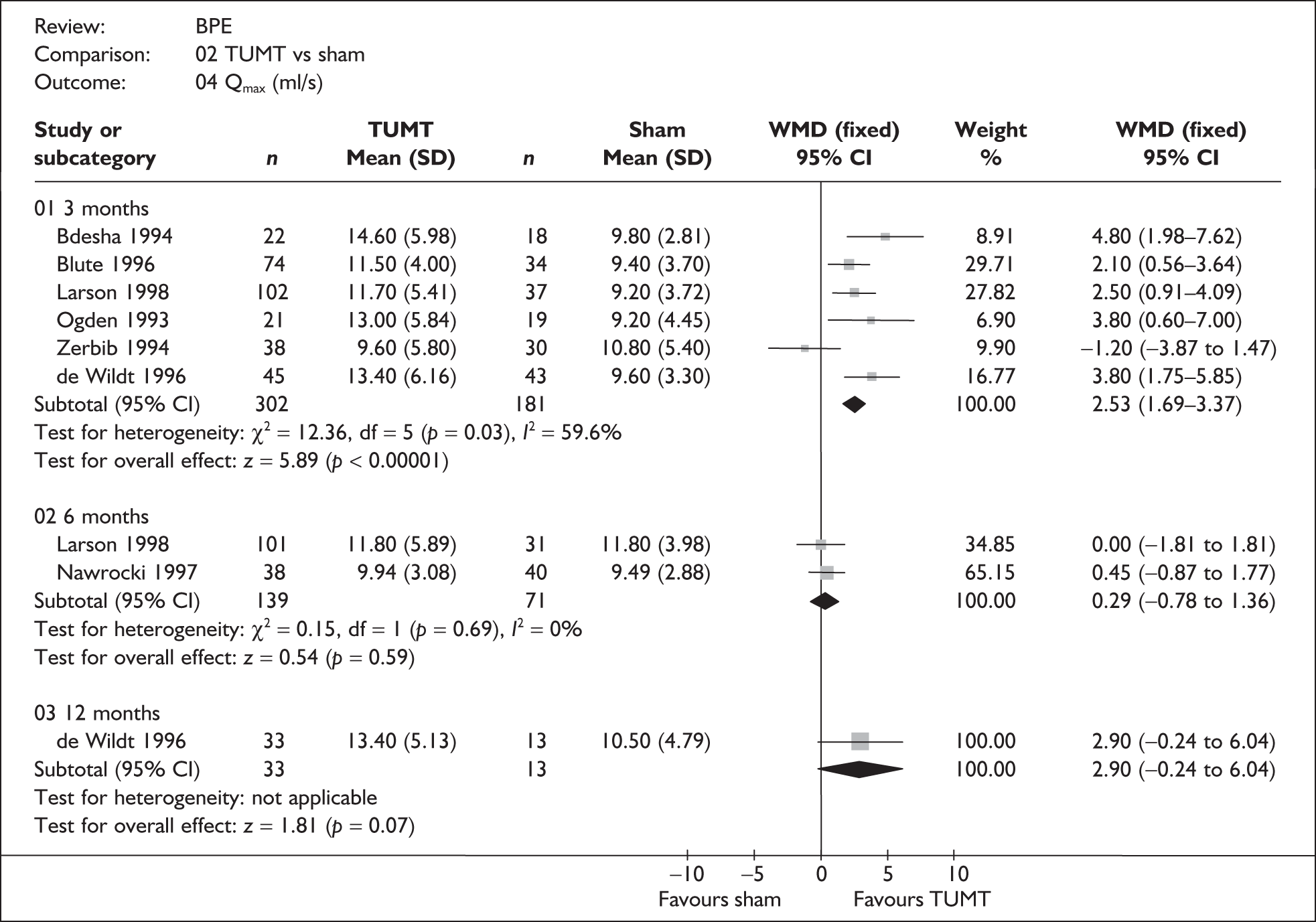

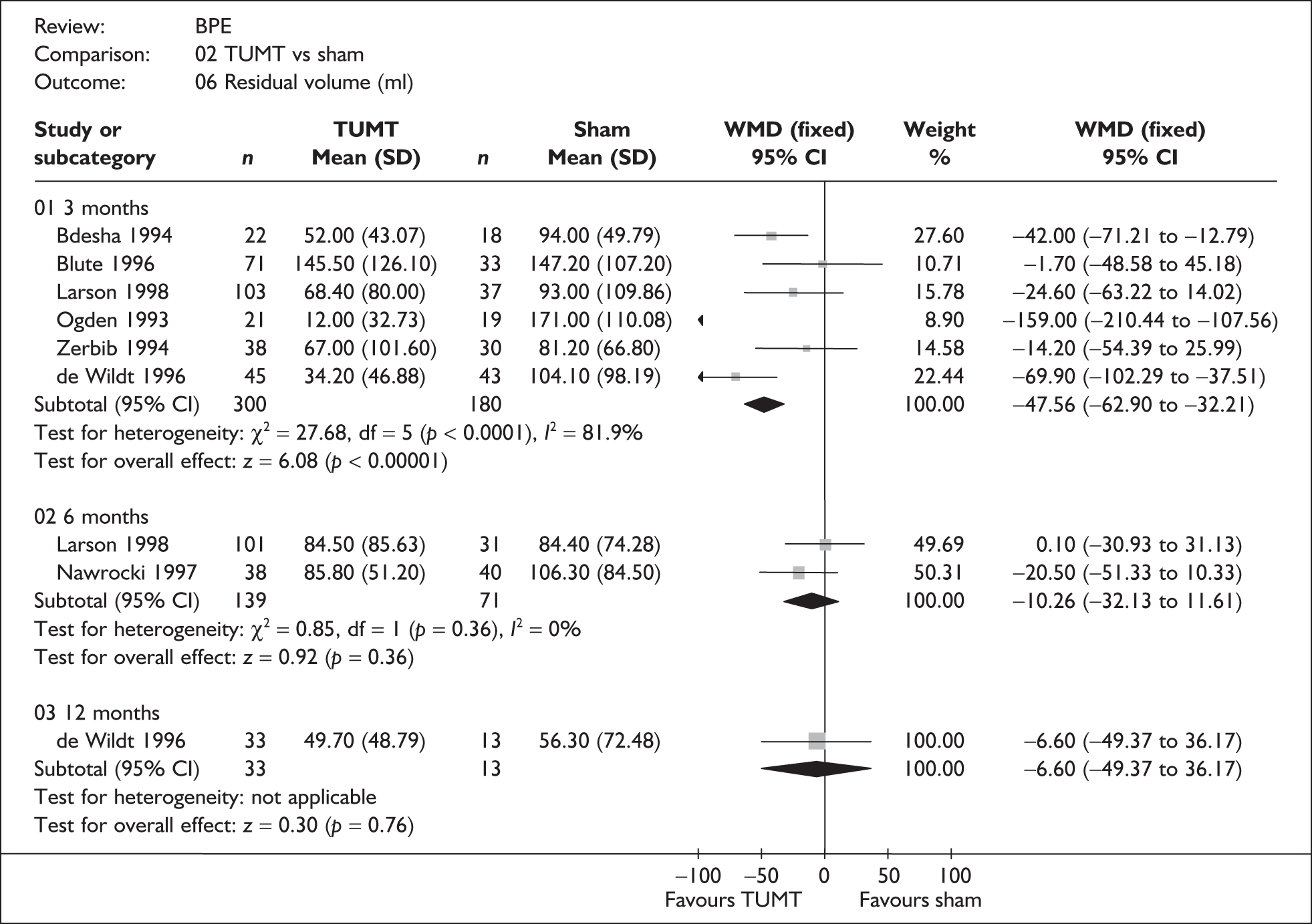


Appendix 9.3 TUNA versus TURP




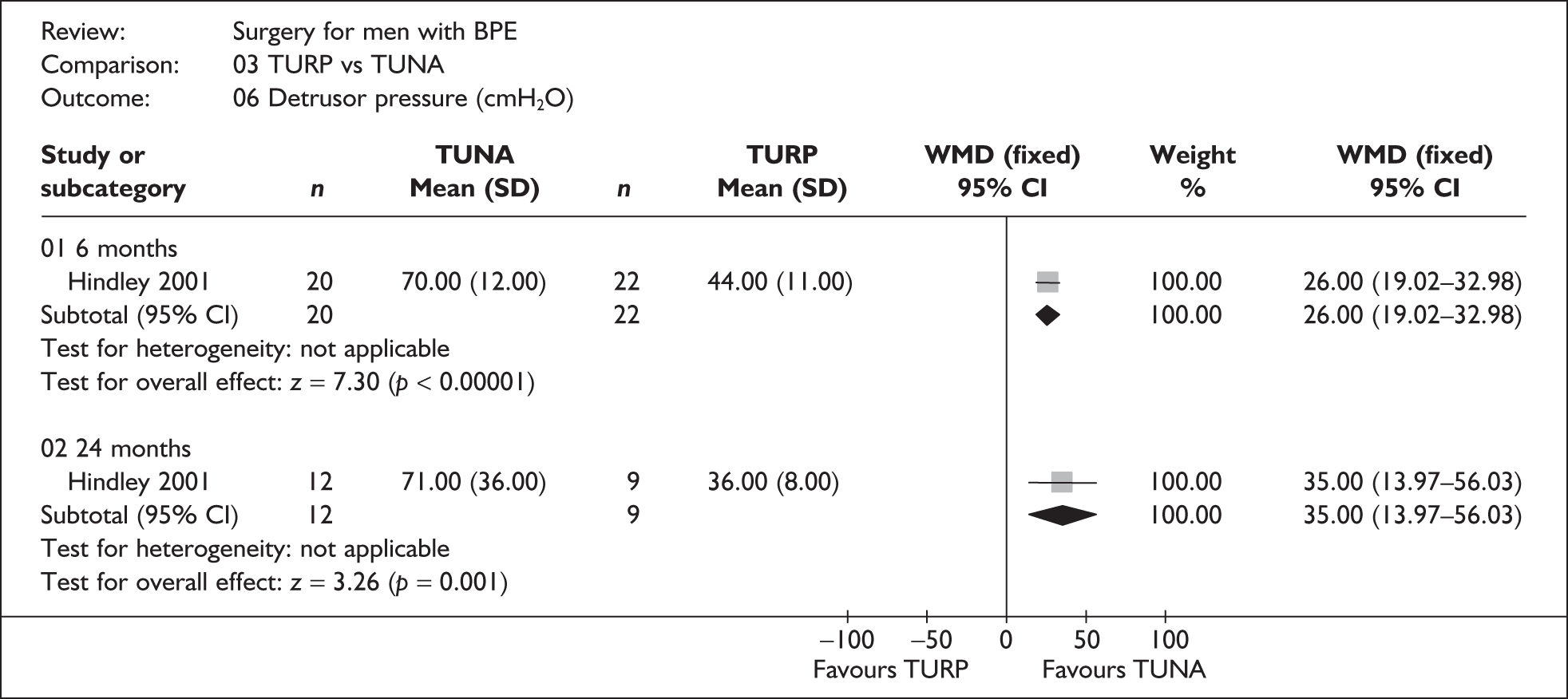



Appendix 9.4 Laser coagulation versus TURP








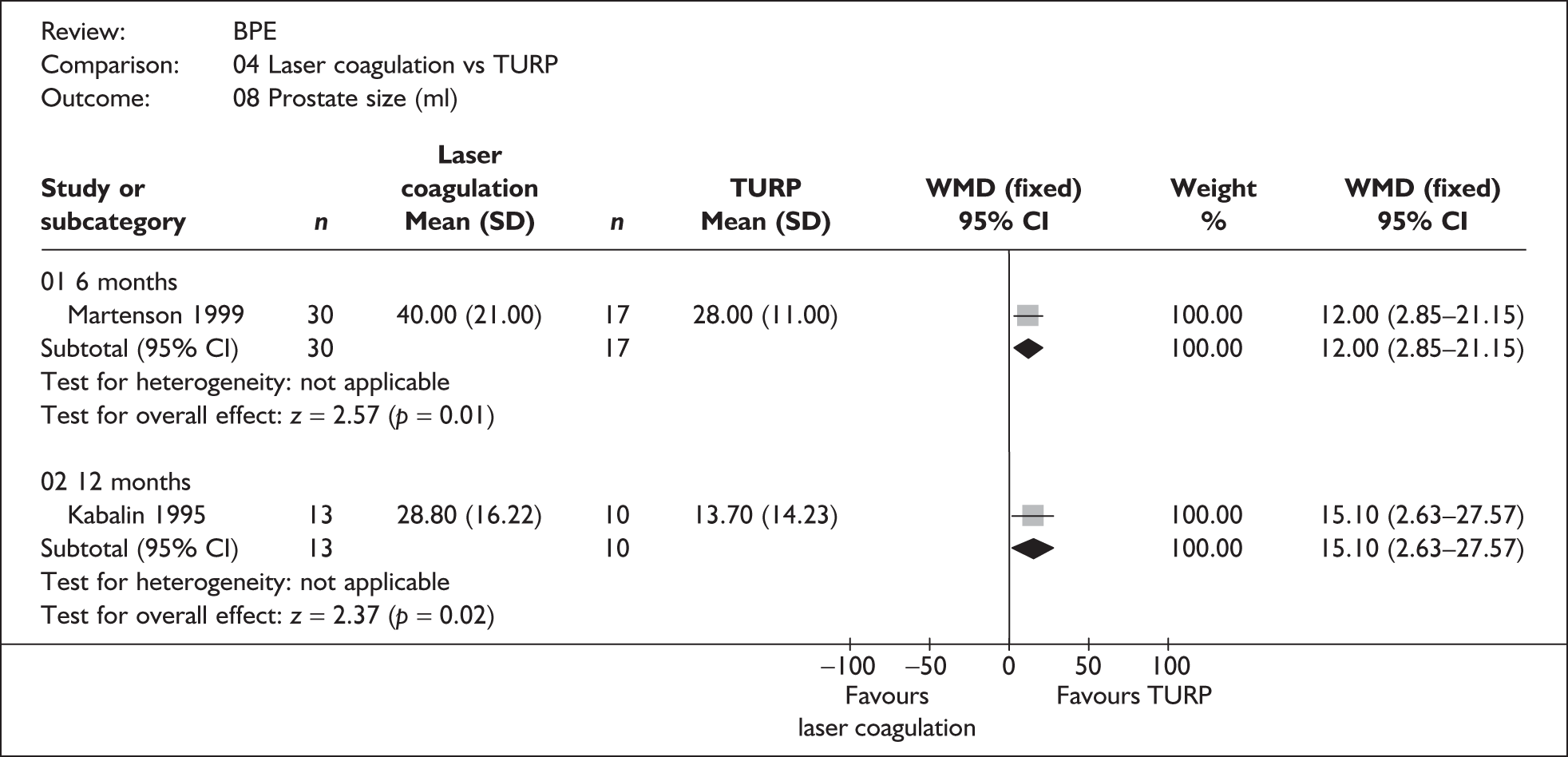
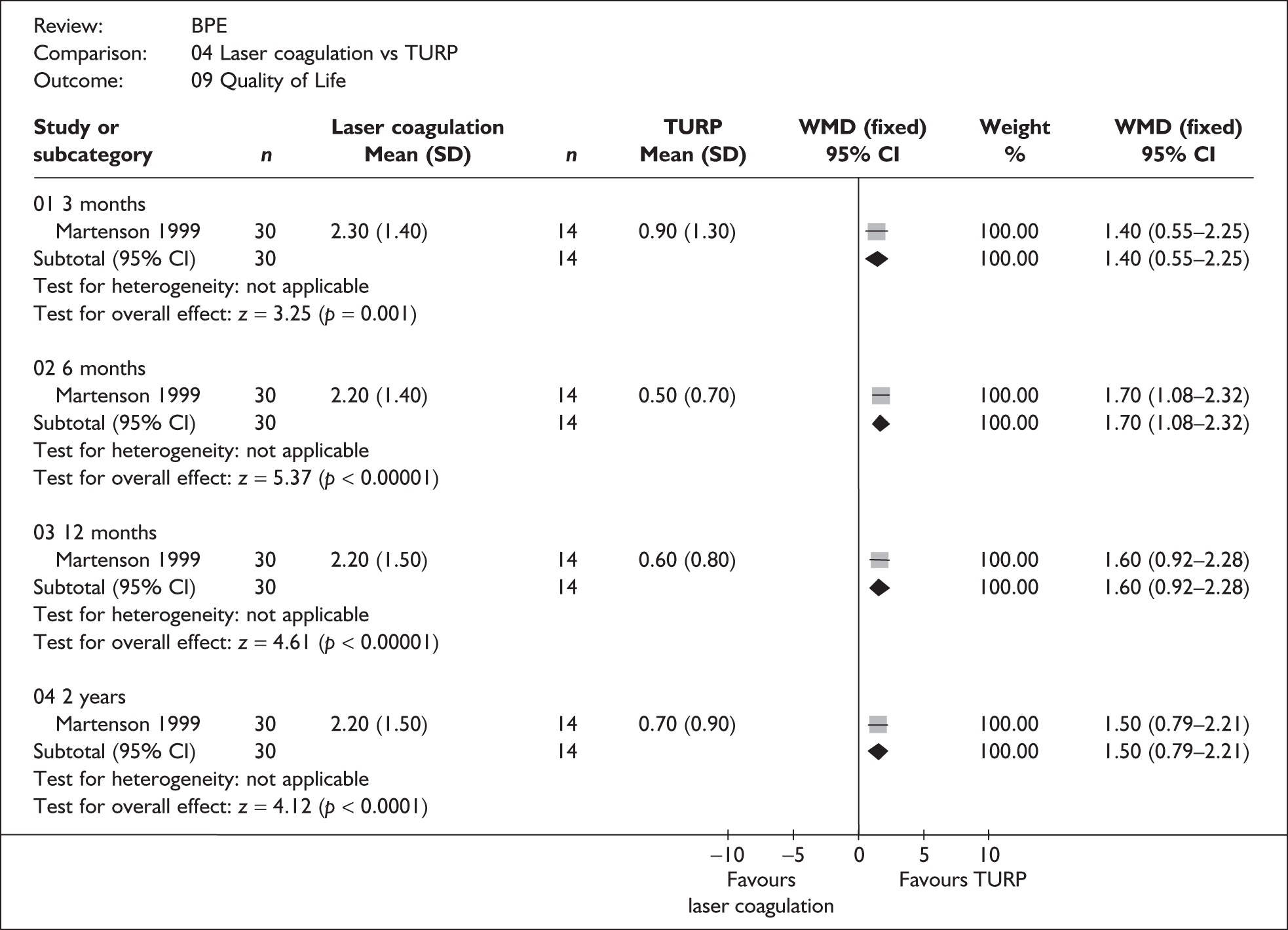



Appendix 9.5 TUIP versus TURP



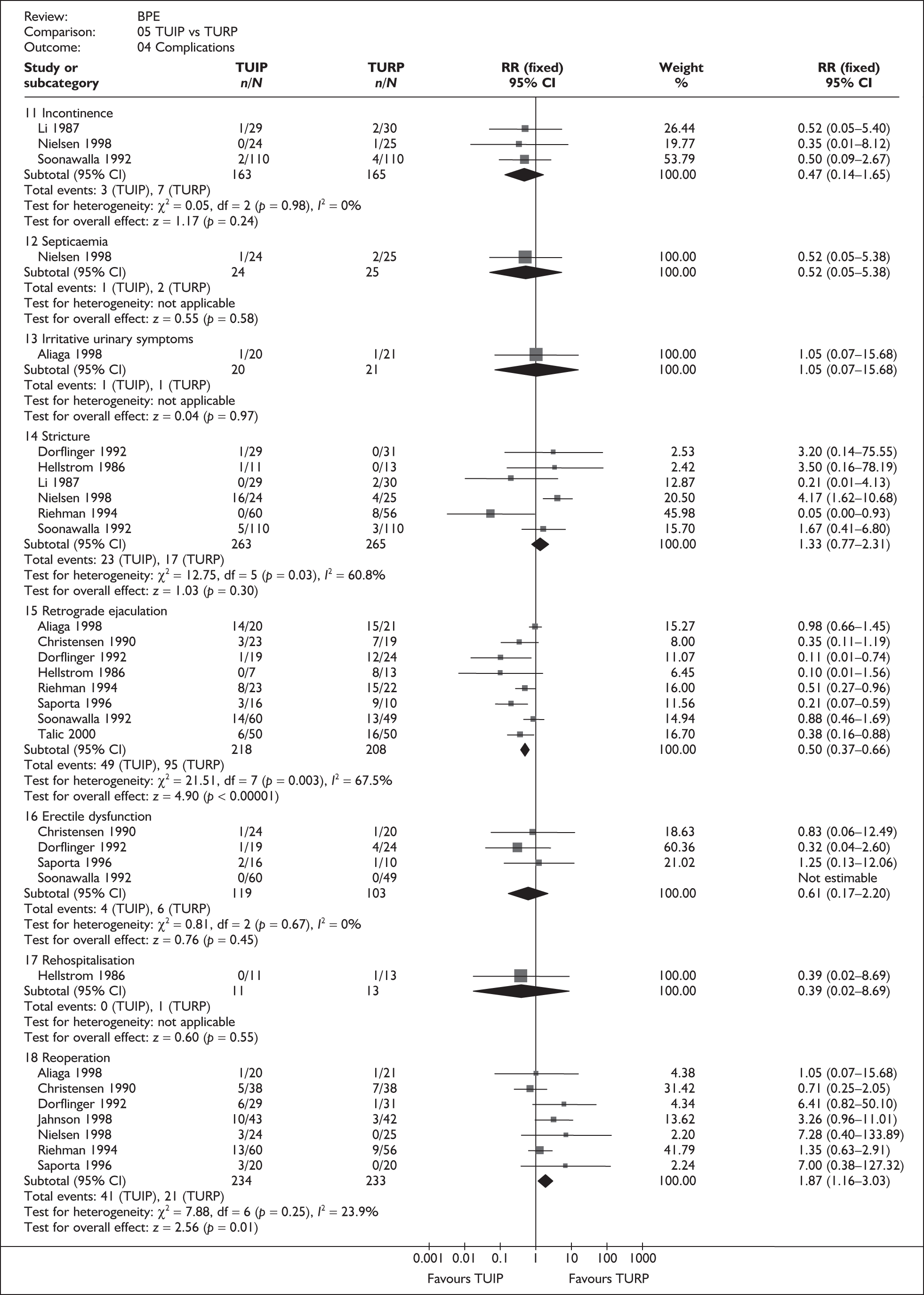






Appendix 9.6 Laser resection versus TURP






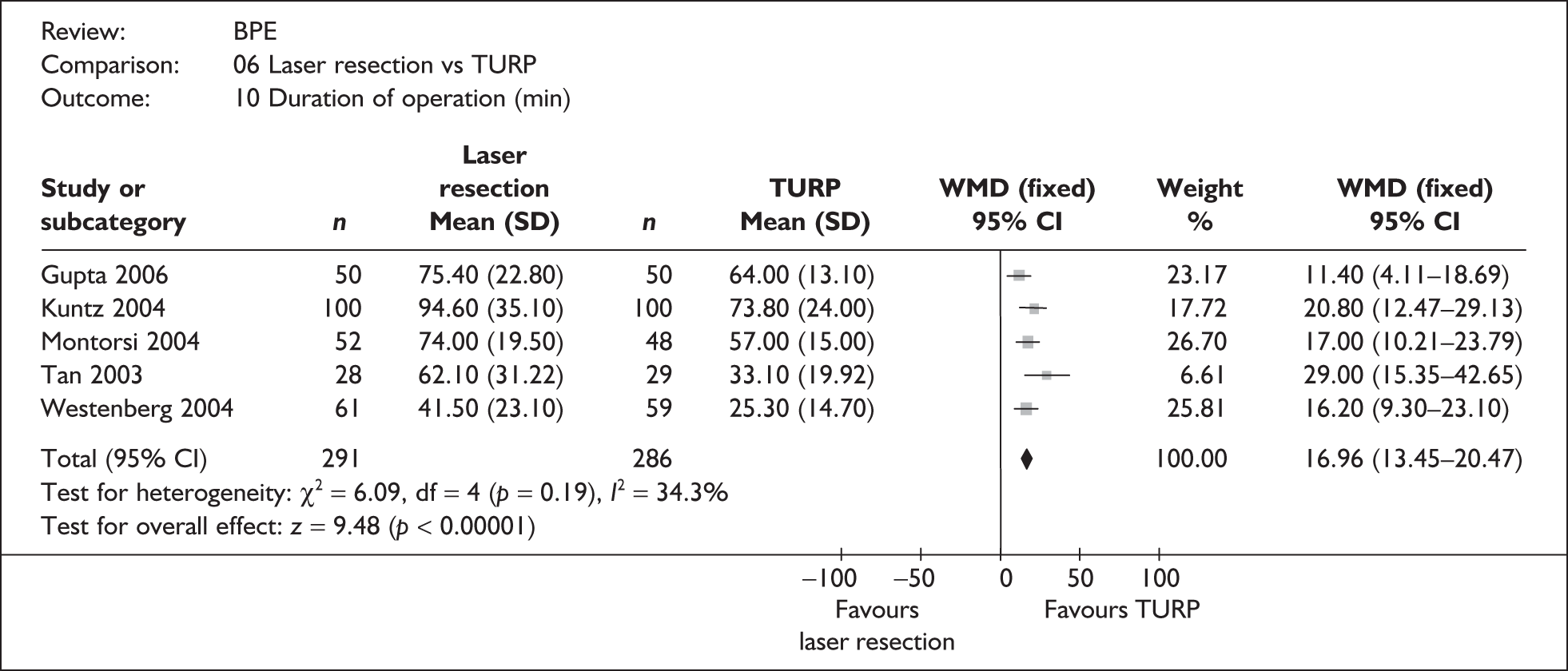

Appendix 9.7 Laser vaporisation versus TURP










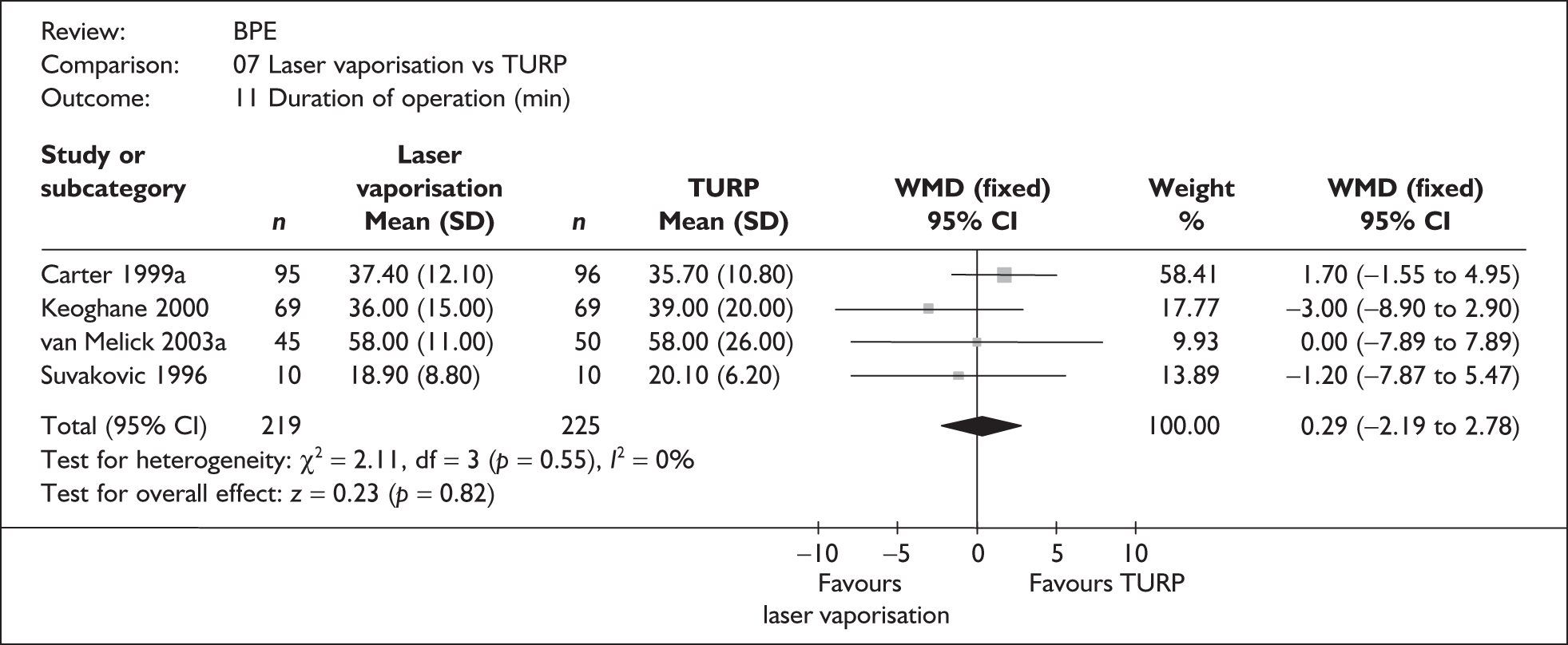

Appendix 9.8 TUVRP versus TURP
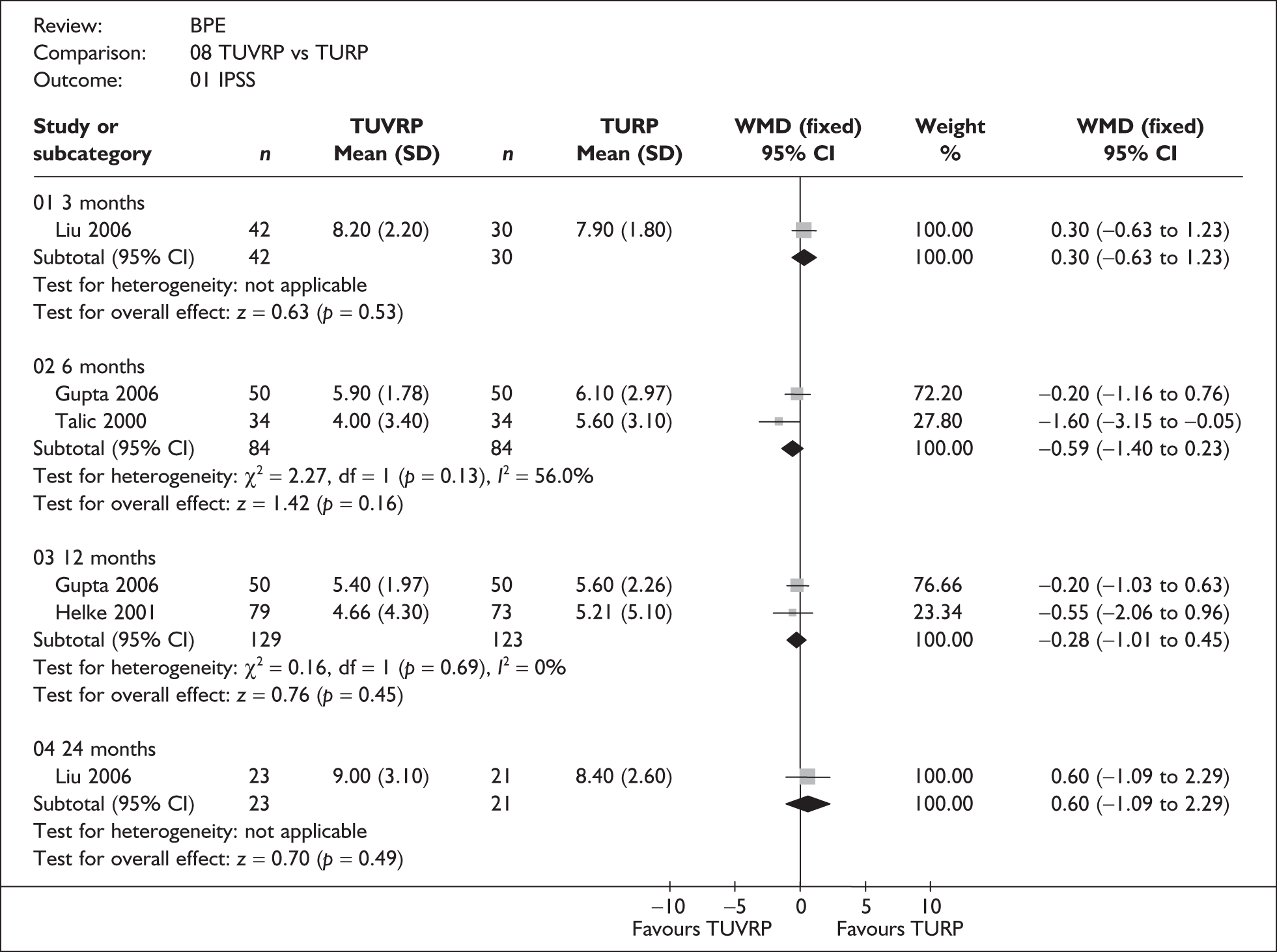
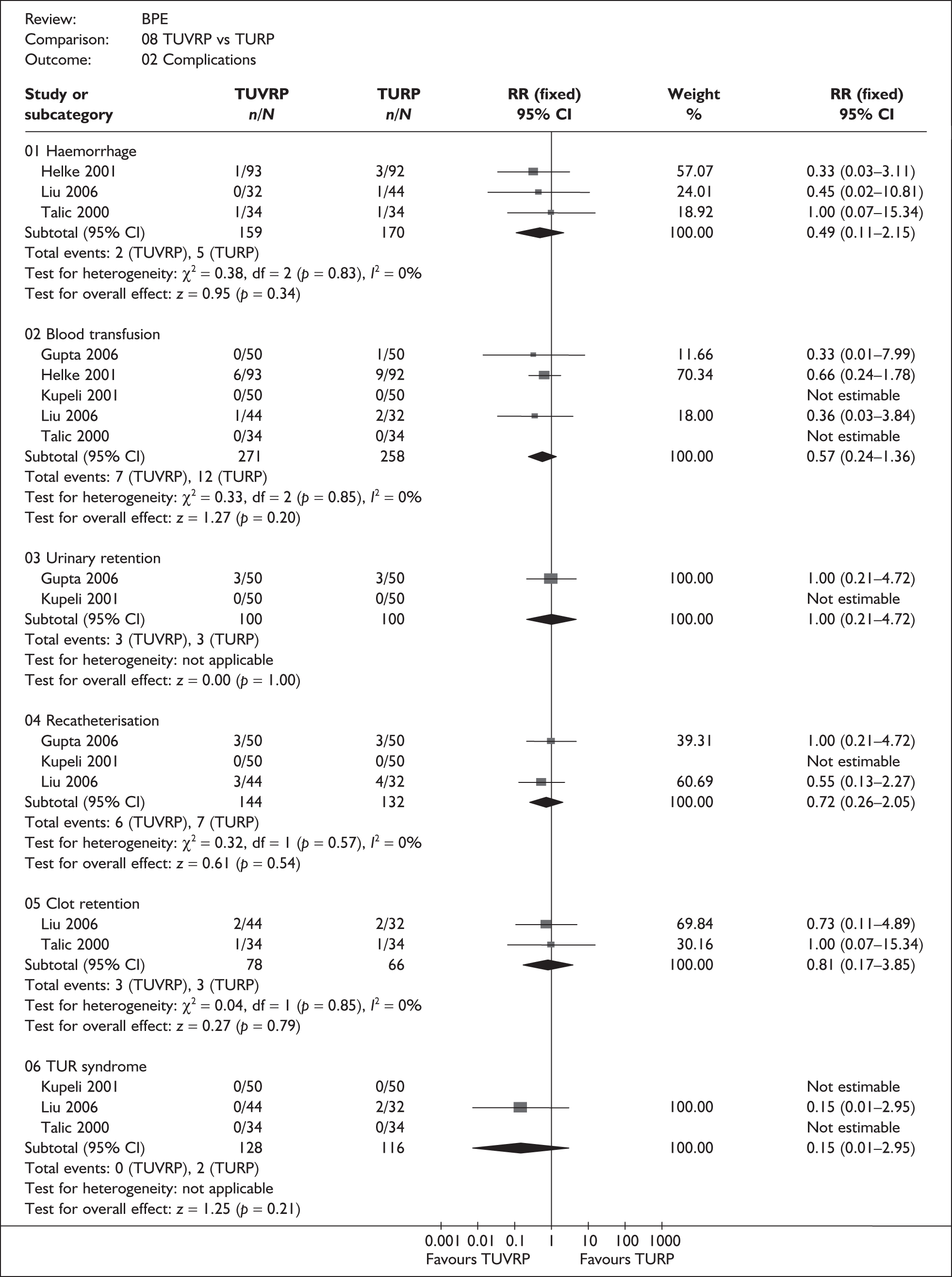
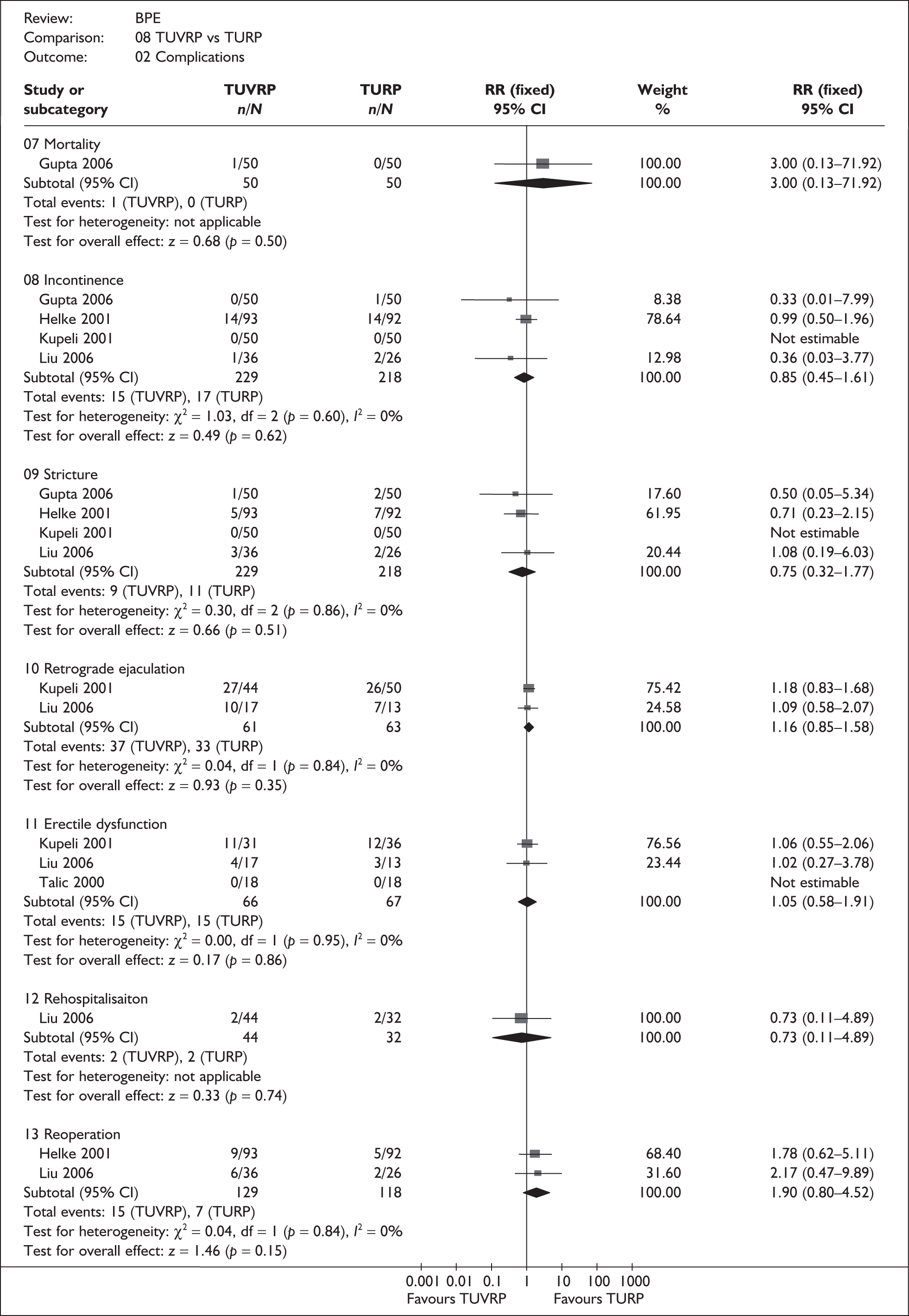





Appendix 9.9 B-TURP versus TURP
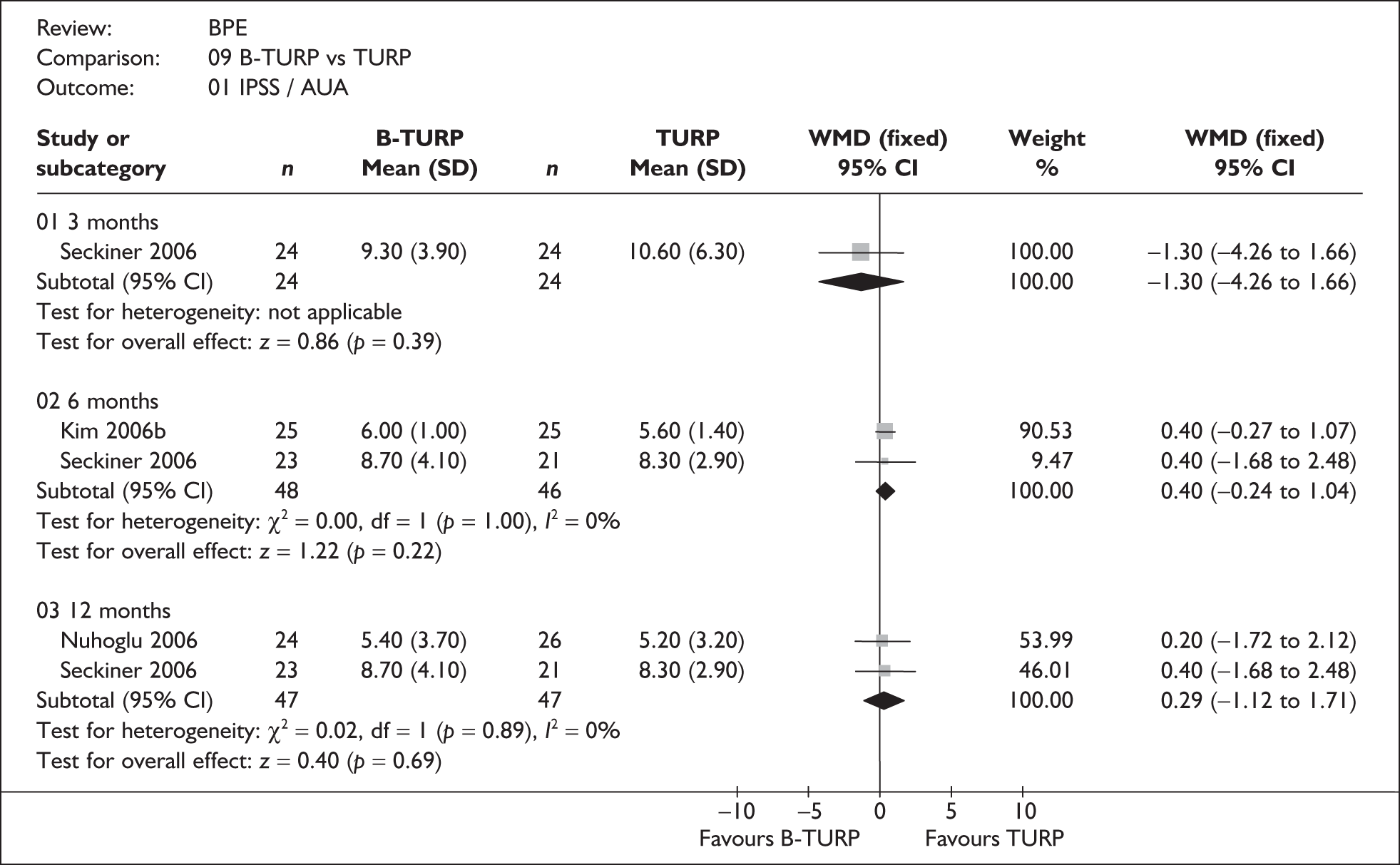

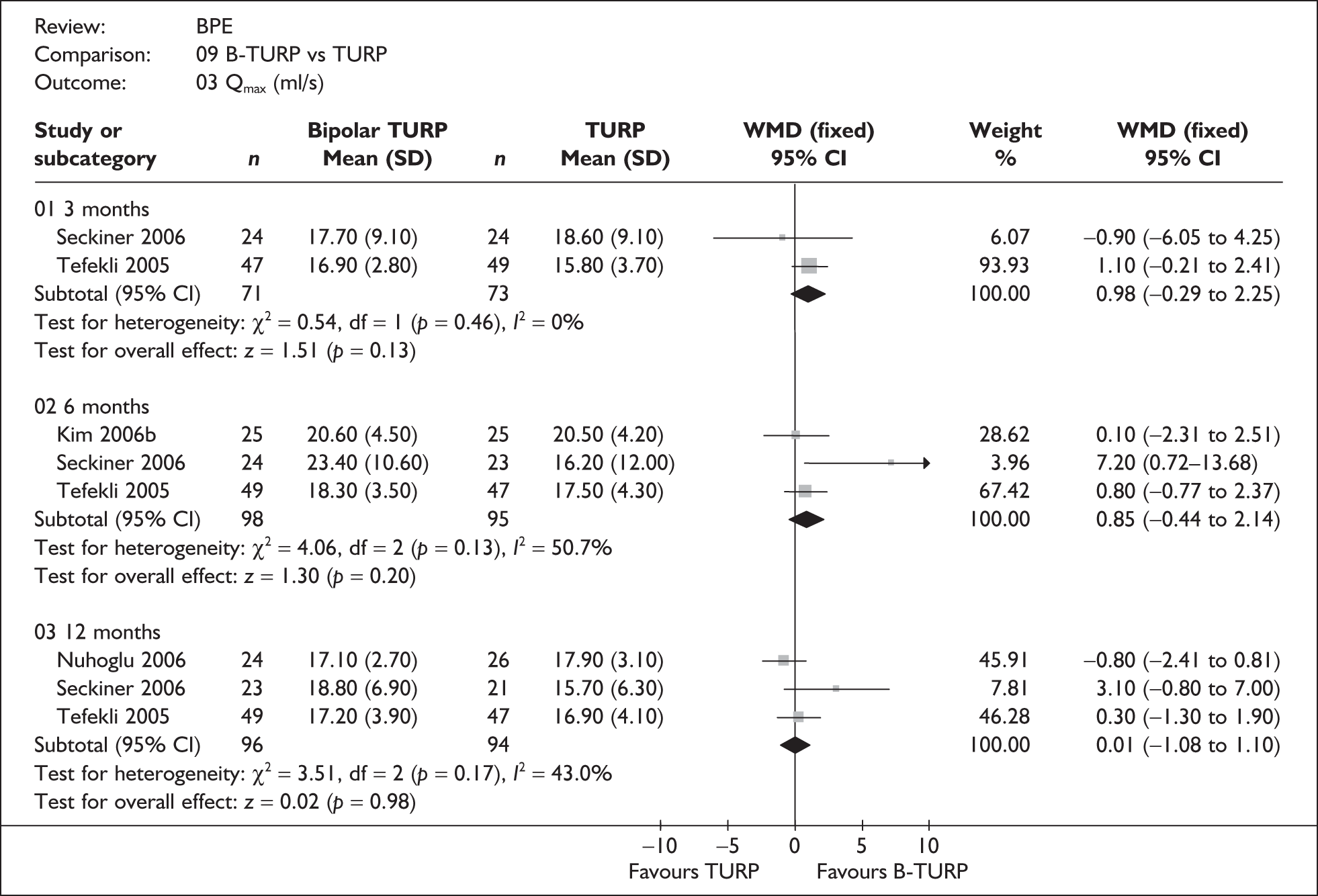
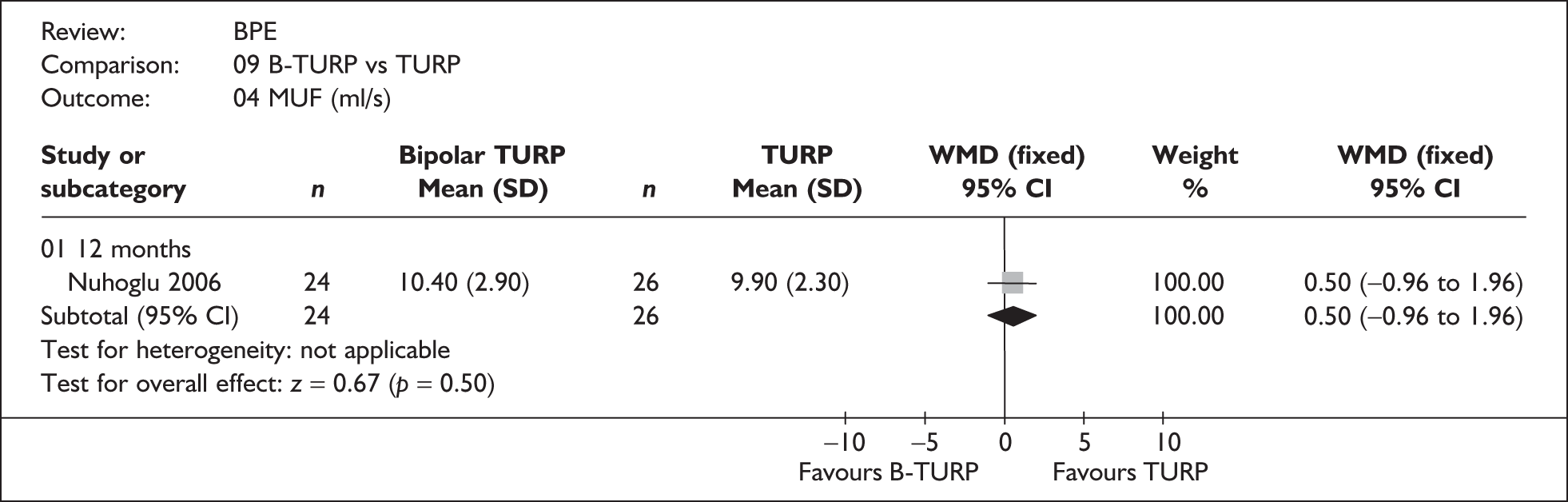




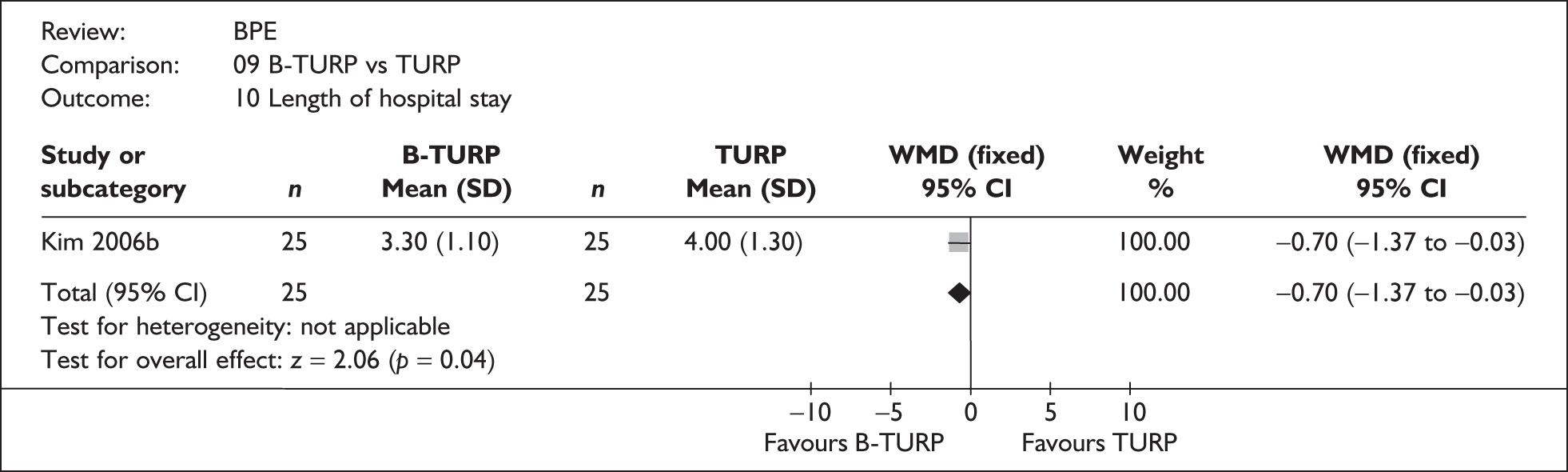
Appendix 9.10 B-TUVRP versus TURP

Appendix 9.11 TUVP versus TURP


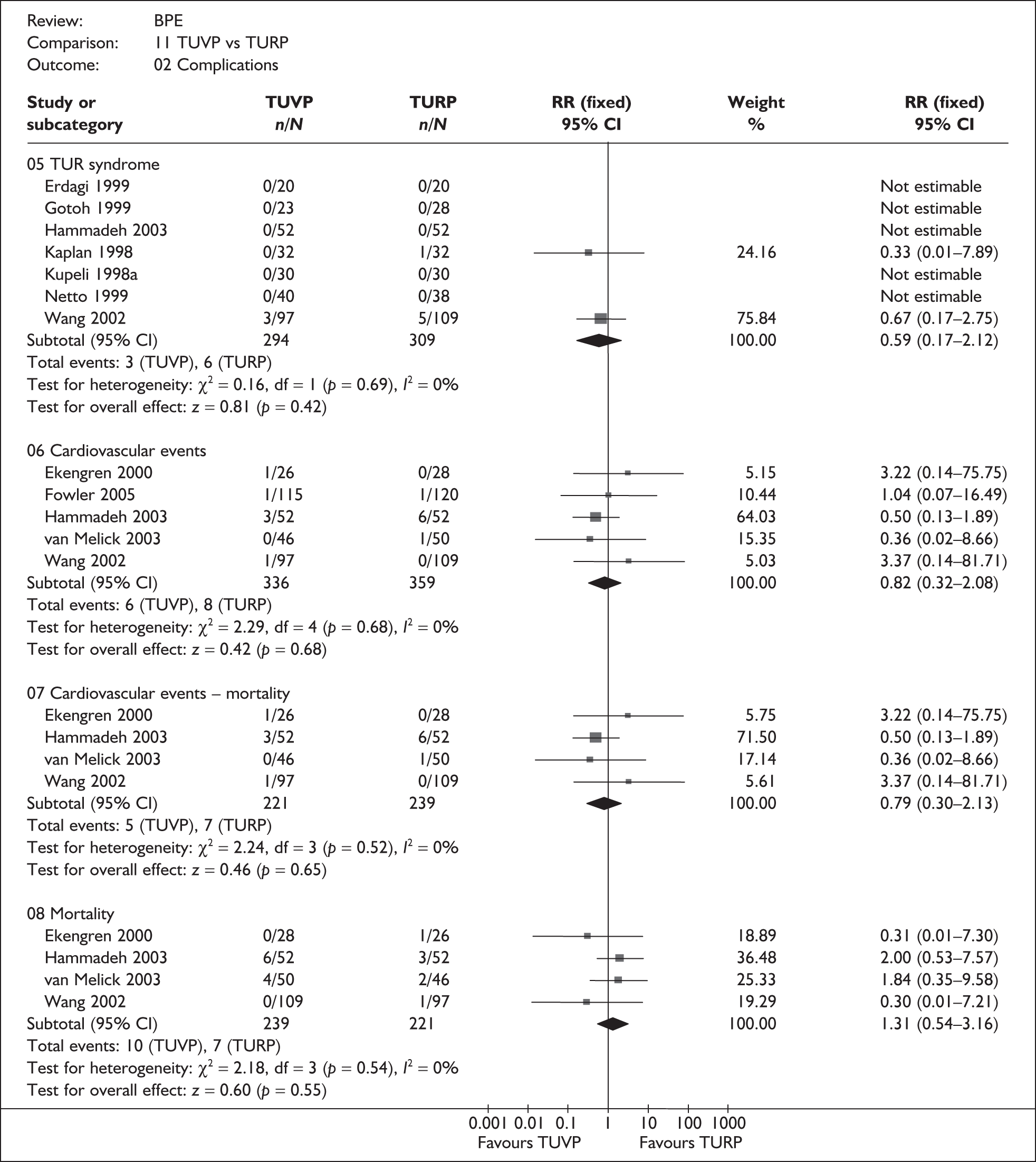


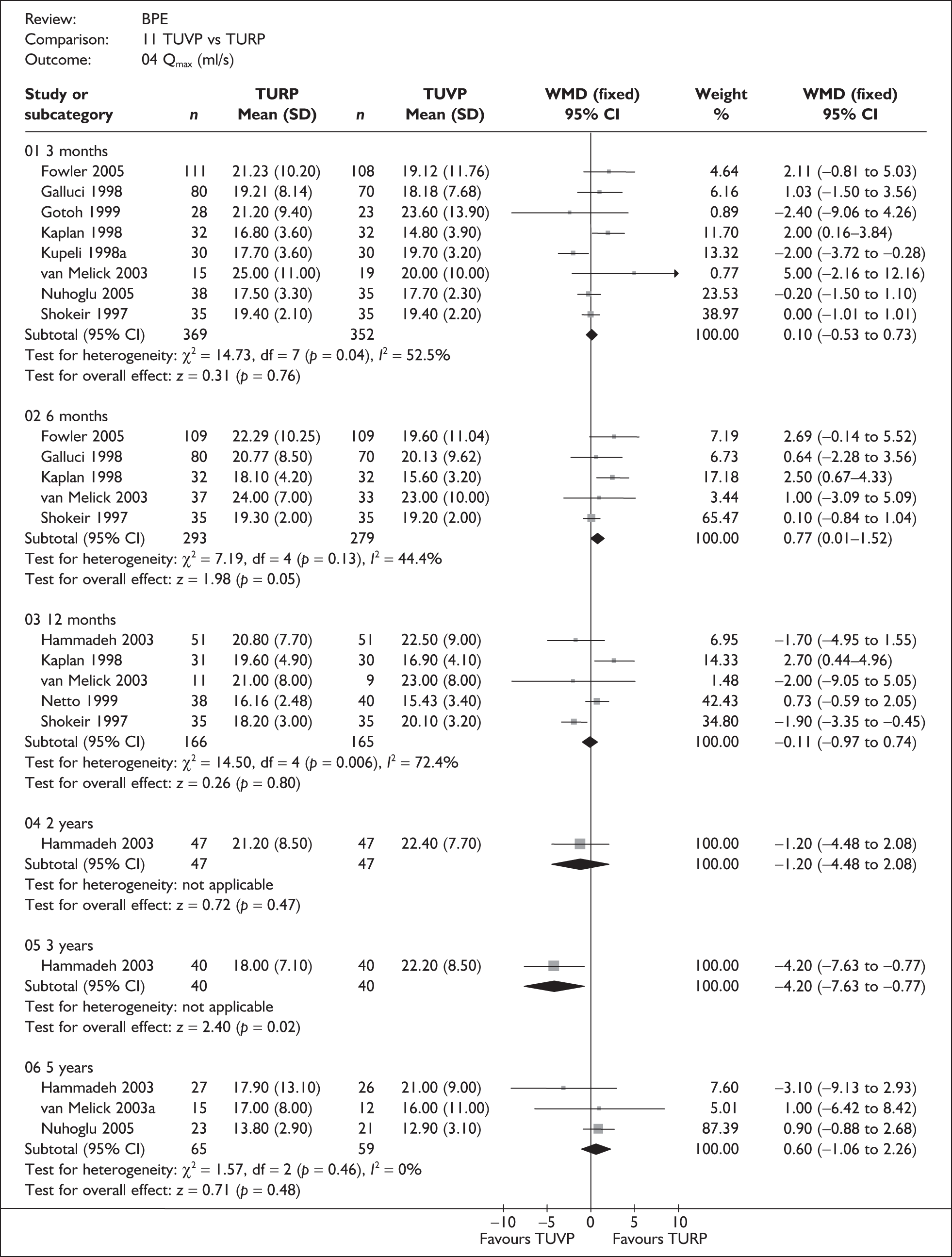






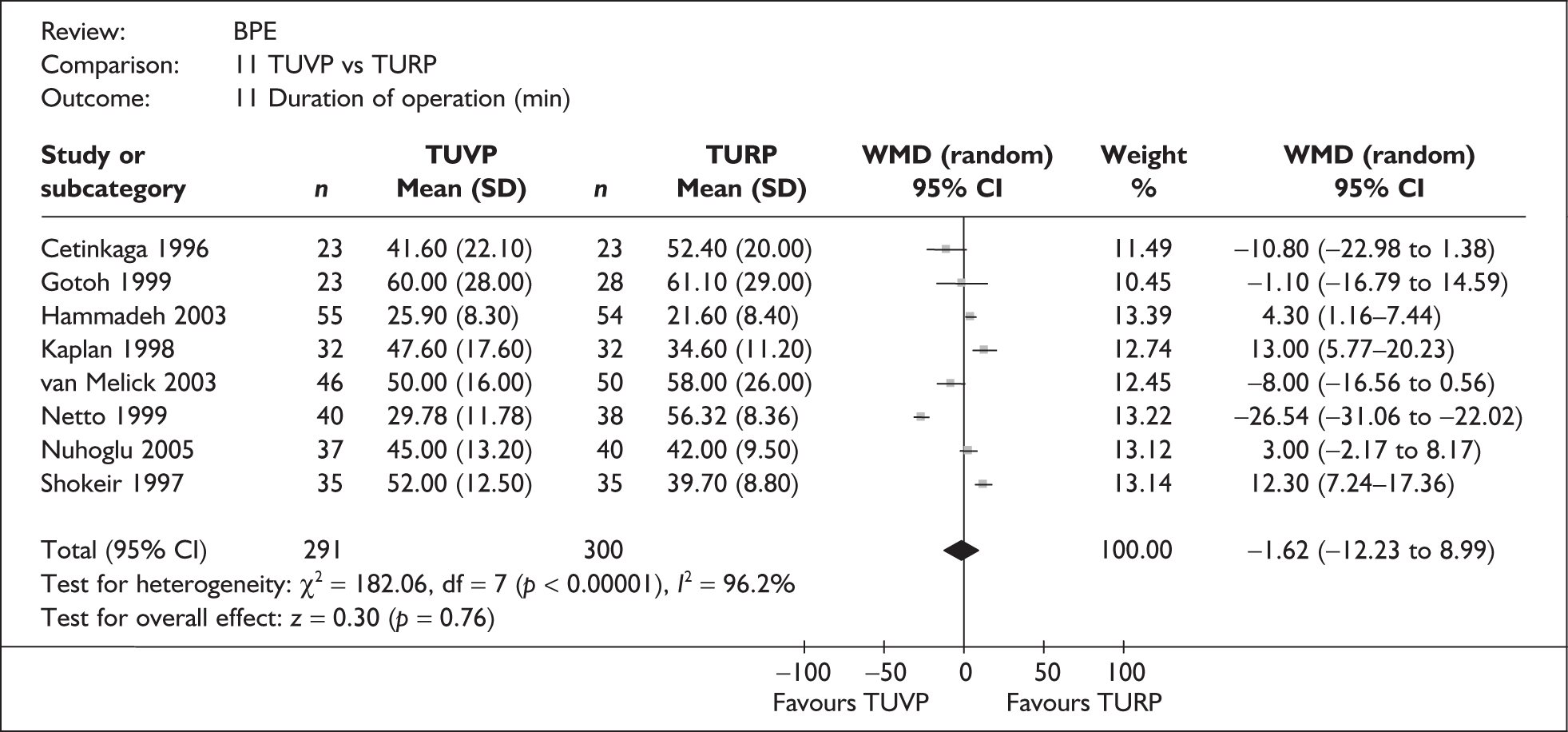

Appendix 9.12 B-TUVP versus TURP








Appendix 9.13 Laser coagulation versus TUVP
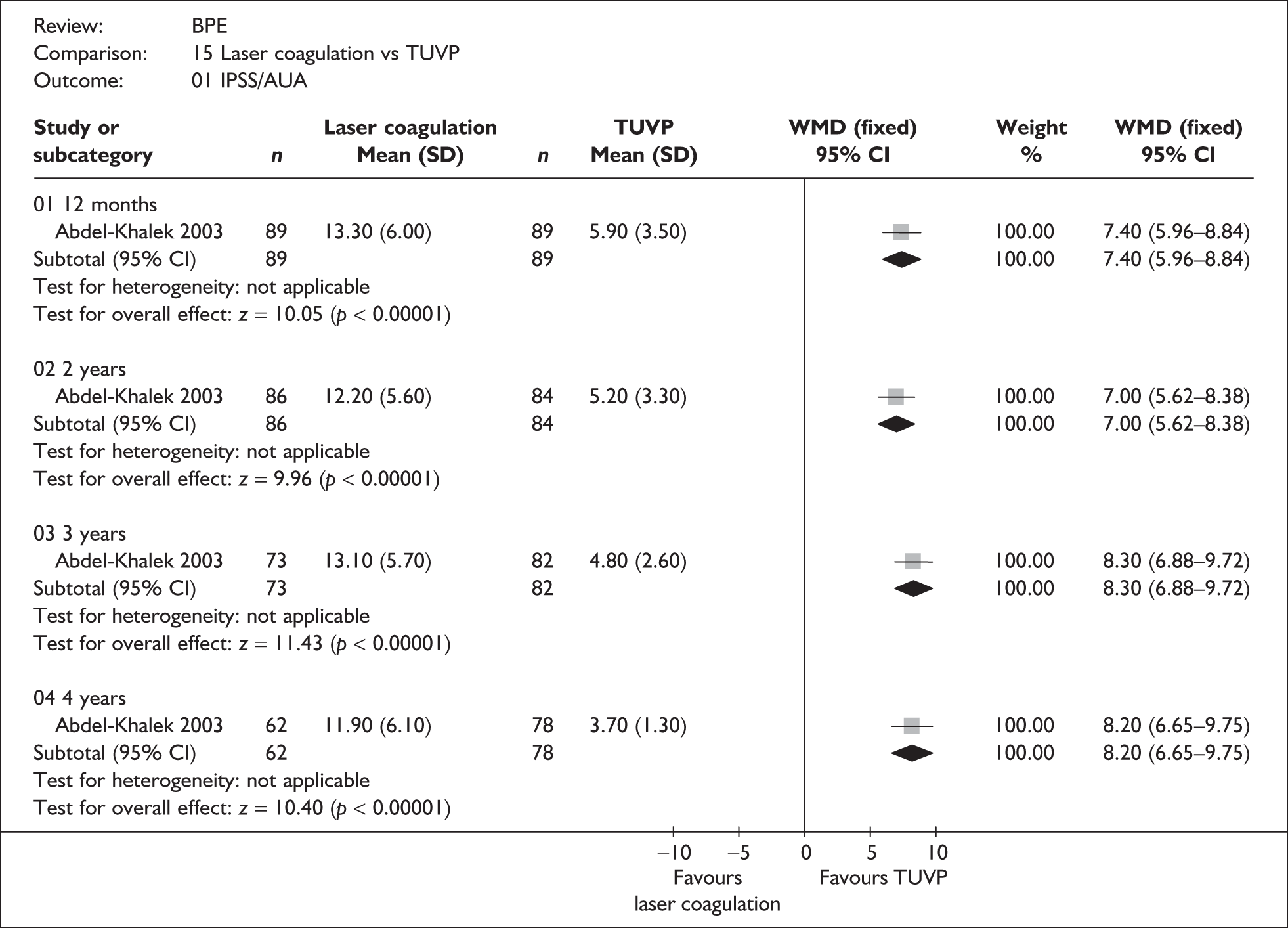








Appendix 9.14 All interventions

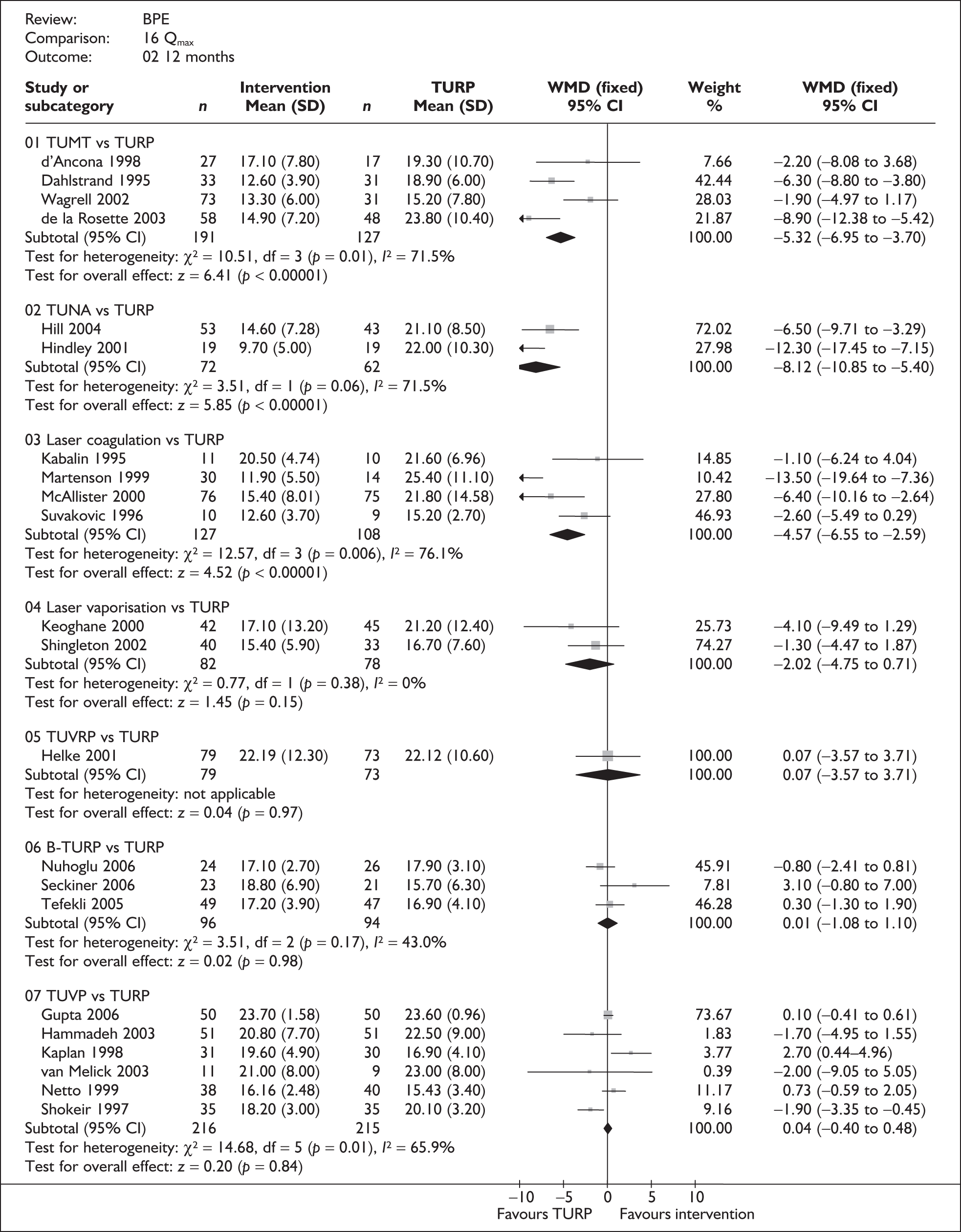
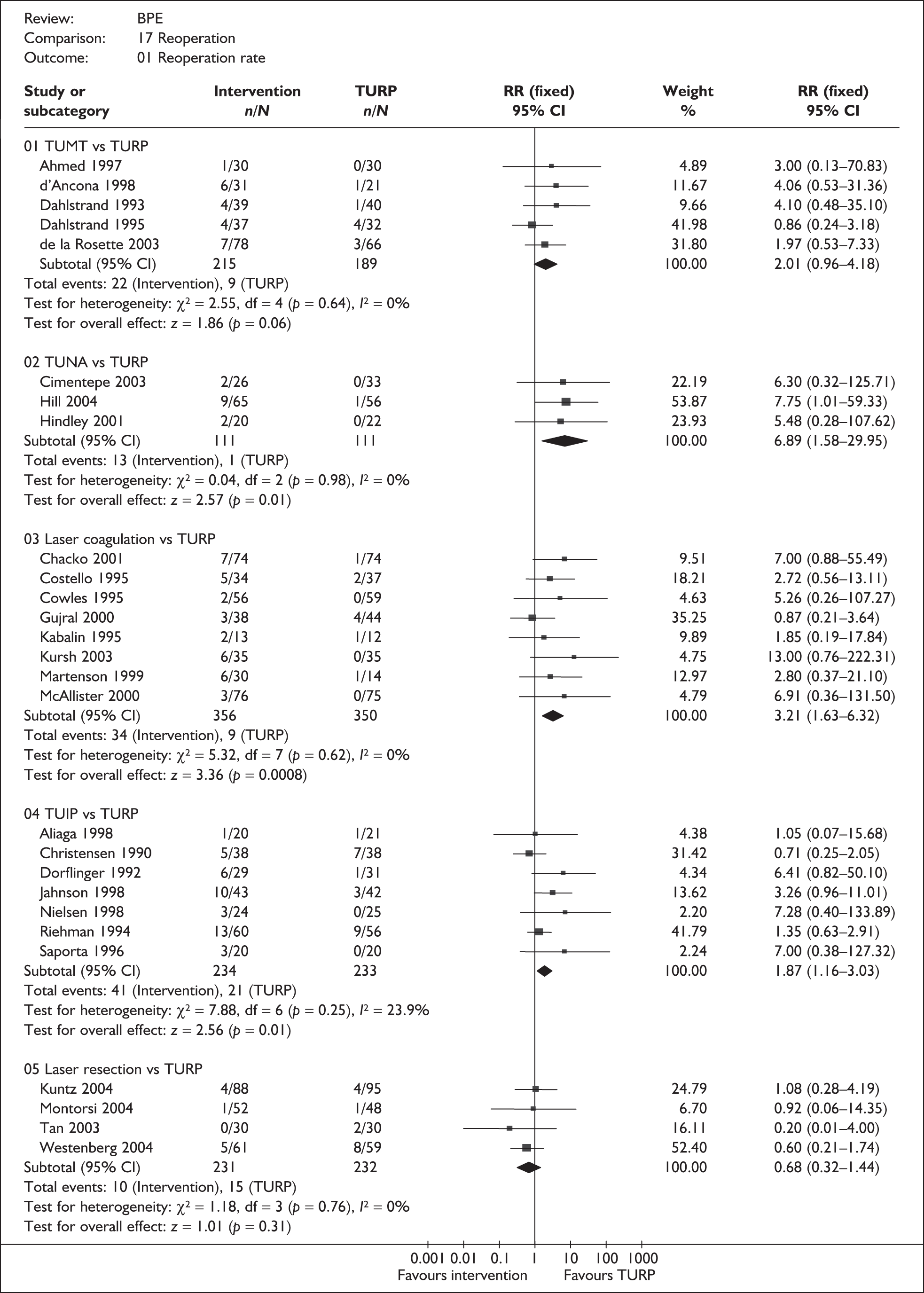

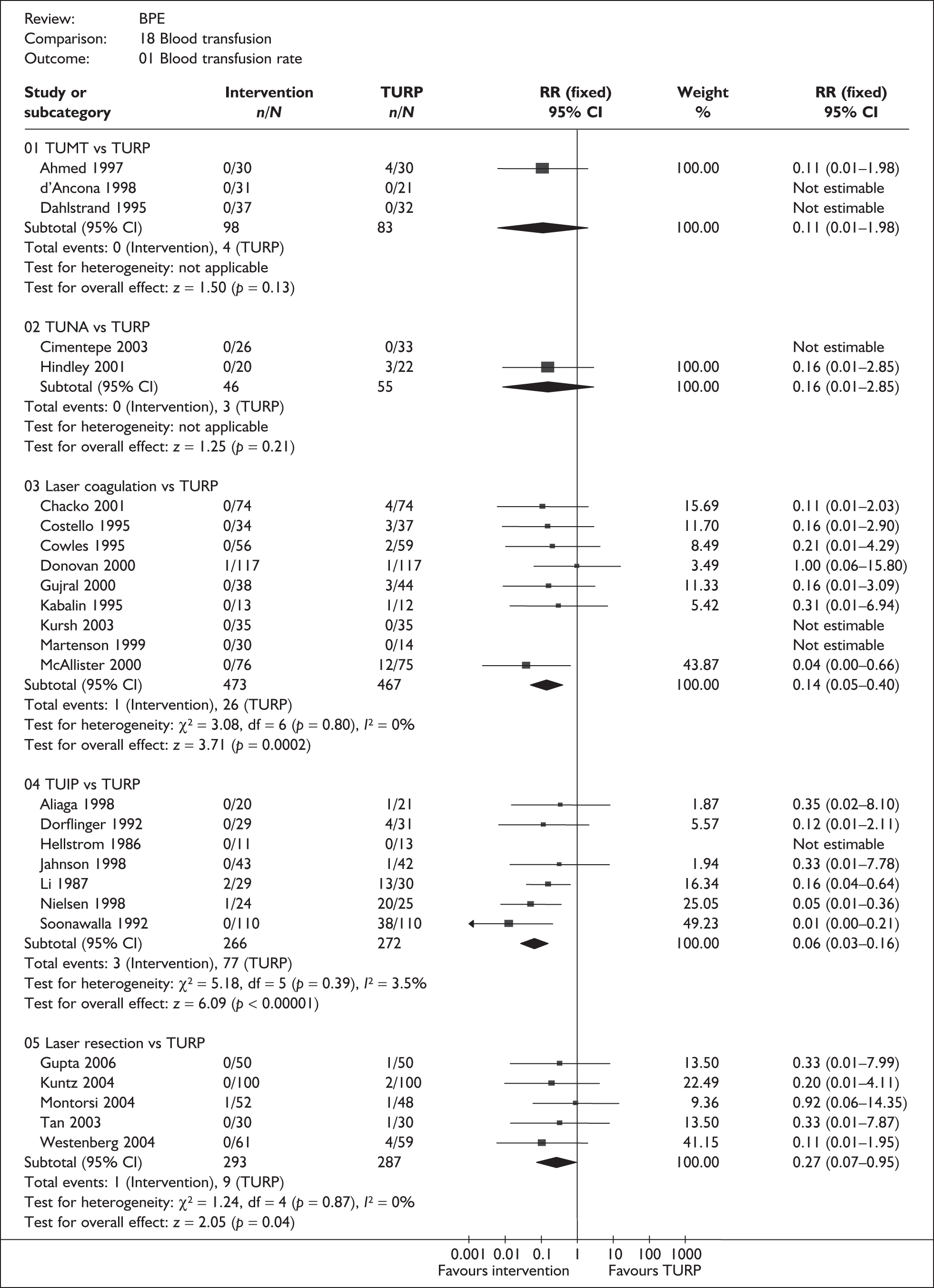








Appendix 10 Direct comparisons between minimally invasive and other ablative methods
Laser coagulation versus TUVP
Characteristics of included studies
The characteristics of the included studies are summarised in Table 105. Three RCTs204–206 were eligible for this comparison, in which a total of 275 participants were randomised. There were 133 and 142 participants allocated to laser coagulation and TUVP respectively.
| Study | Comparators | Number of participants | Age (years) | Symptom scorea | Qmax (ml/s) | Residual volume (ml) | Prostate size (ml) |
|---|---|---|---|---|---|---|---|
| Abdel-Khalek 2003 | Laser coagulation | 90 | 50 | 27.9 | 6.9 | 120 | 44 |
| TUVP | 90 | 55 | 26.0 | 6.4 | 125 | 47 | |
| Narayan 1995 | Laser coagulation | 32 | 64 | 22.1 | 7.0 | 210 | 41 |
| TUVP | 32 | 66 | 22.4 | 6.4 | 277 | 52 | |
| Shingleton 1998 | Laser coagulation | 11 | 67 | 19.0 | 9.2 | NR | 35 |
| TUVP | 20 | 67 | 21.1 | 7.7 | NR | 35 |
Two of the RCTs took place in the US205,206 and one took place in Egypt. 204 Only one study provided details of recruitment dates,204 with a recruitment period between March 1995 and May 2004.
All studies provided details of participants’ IPSS/AUA symptom scores showing that all had severe symptoms.
Prostate size was also reported by all studies. There were 122 participants in each arm with large prostates, and 11 and 20 participants with moderate-sized prostates in the laser coagulation and TUVP arms respectively.
Assessment of effectiveness
Tables giving a detailed description for all outcomes can be found in Appendix 8.13. The results of the meta-analyses are given in Appendix 9.13. Note that in terms of long-term evaluation, only the longest follow-up is presented.
Symptom scores
At 3 months
Data were available for two trials. 205,206 There were no differences in AUA symptom scores between laser coagulation and TUVP (Appendix 8.13).
At 12 months
Of the three trials, two provided details on symptom scores at 12 months after surgery. 204,205 Only one, however, reported data that were suitable for meta-analysis. 204 IPSS/AUA scores were worse following laser coagulation than following TUVP (Appendix 9.13), comparison 15:01:01: MD 7.40, 95% CI 5.96–8.84, p < 0.001). This result is not consistent with that reported by Narayan and colleagues. 205 In this study there were no differences between the two arms in terms of symptom scores at 12 months.
Longer-term follow-up
Data from one study204 reporting IPSS scores at 4 years showed better scores following TUVP than after laser coagulation (Appendix 9.13), comparison 15:01:04: MD 8.20, 95% CI 6.65–9.75, p < 0.001).
Complications
Data describing complications by study are given in Appendix 8.13, Table 101. Eleven categories of complications were identified across the three studies. These data are hard to interpret. For seven of the complications, data were only available for one trial (Figure 29). Even for those complications more consistently reported, confidence intervals are wide and include both clinically important and clinically insignificant differences. Furthermore, the length of follow-up varies across the trials. Only complications such as urinary retention and strictures were reported across the three trials. There were more patients with retention in the laser coagulation arm (15%) than in the TUVP arm (3.5%) following surgeries. In terms of strictures, there were no statistically significant differences between the two arms (Figure 31).
FIGURE 31.
Complications, laser coagulation vs TUVP.
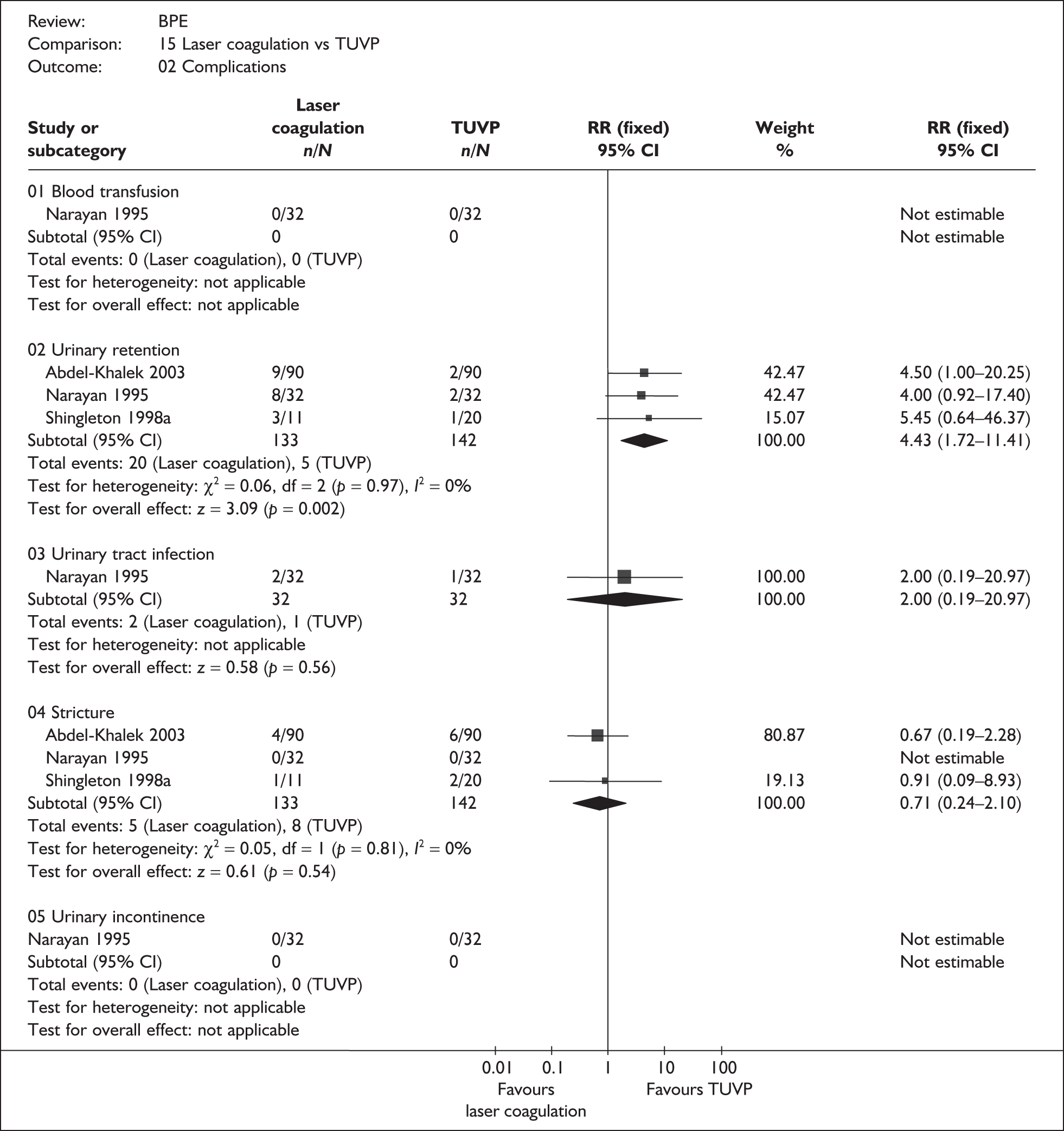
Quality of life
One study reported quality of life of patients following surgery (Appendix 8.13). 204 The scale used was unclear and was later assumed to be IPSS QoL. At all time points considered, the quality of life was significantly better following TUVP than following laser coagulation.
Urodynamic outcomes
Data on peak urine flow rate, residual volume and prostate size were reported to a varying extent across the three studies. Only peak urine flow rate is presented in this section. Results for the other urodynamic outcomes are presented in Appendix 8.13, Table 103 and Appendix 9.13, comparison 15:05–07.
Peak urine flow rate
At 3 months
Two studies205,206 provided details on peak urine flow rates in patients measured at 3 months following surgery. There was no consistency in the results. One study205 favoured TUVP and the other206 showed no differences between the two arms.
At 12 months
At 12 months, two studies204,205 showed that peak urine flow rate is worse following laser coagulation than following TUVP. The mean differences in the two studies were 5.7 and 3.0 ml/s, respectively, favouring TUVP (Appendix 8.13, Table 103).
Descriptors of care
Data describing descriptors of care are tabulated in Appendix 8.13, Table 104. Information on duration of operation, length of hospital stay and reoperation rates was identified across the three eligible studies for this comparison.
Duration of operation
Two studies reported duration of operation (Appendix 8.13, Table 104). The evidence is not consistent: in one study206 the mean difference was 18.5 minutes in favour of laser coagulation; in the other study204 duration of operation in the laser arm was equivalent to that in the TUVP arm.
Length of hospital stay
Evidence from two studies204,205 suggests that the average length of stay following laser coagulation is similar to that following TUVP (Appendix 8.13, Table 104).
Reoperation
Two studies provided information on reoperation rates. 204,205 A total of 40 (33%) reoperations were recorded amongst 122 laser patients compared with 11 (9.0%) amongst 122 TUVP patients (Appendix 9.13, comparison 15:08: RR 3.52, 95% CI 1.94–6.40, p < 0.001). This result should be interpreted with caution as the length of follow-up varied across the two studies.
Summary and conclusions of the evidence for and against the intervention
Three RCTs of moderate quality involving 275 participants were available to compare laser coagulation with TUVP. The data indicate that, at any follow-up assessment, symptoms, quality of life and peak urine flow rate are worse after laser coagulation than after TUVP. Following laser coagulation, the incidence of urinary retention and the reoperation rate are higher than after TUVP. The occurrence of strictures, urinary incontinence and urinary tract infection was similar, but with wide confidence intervals.
Clinical effect size
A summary of the clinical effect sizes for all outcomes derived from the meta-analyses for which data were available is given in Table 106.
| Outcome | Number of trials | Effect size | 95% CI | p-value |
|---|---|---|---|---|
| IPSS/AUA score | ||||
| 12 months | 1 | 7.40a | 5.96–8.84 | < 0.001 |
| Longer term | 1 | 8.20a | 6.65–9.75 | < 0.001 |
| Blood transfusion | 1 | NE | NE | NE |
| Urinary retention | 3 | 4.43b | 1.72–11.41 | 0.002 |
| Urinary tract infection | 1 | 2.00b | 0.19–20.97 | 0.56 |
| Stricture | 3 | 0.71b | 0.24–2.10 | 0.54 |
| Incontinence | 1 | NE | NE | NE |
| Quality of life | ||||
| 12 months | 1 | 2.00a | 1.87–2.13 | < 0.001 |
| Longer term | 1 | 1.80a | 1.53–2.07 | < 0.001 |
| Qmax | ||||
| 12 months | 1 | 5.70a | 3.72–7.68 | < 0.001 |
| Longer term | 1 | 7.80a | 6.52–9.08 | < 0.001 |
| Reoperation | 2 | 3.52b | 1.94–6.40 | < 0.001 |
Appendix 11 Characteristics of patient population used for individual-level data in the economic model
| Variable | Descriptive |
|---|---|
| Number of patients, n | 179 |
| Age (years), mean (range) | 69 (47–88) |
| Preoperative IPSS (0–35), mean (SD) | 22 (7) |
| Preoperative IPSS QoL (0–5), mean (SD) | 4 (1) |
| Preoperative Qmax (ml/s), mean (SD) | 10.8 (4.7) |
| Preoperative residual volume (ml), mean (SD) | 130 (123) |
| Preoperative invasive PFS performed, n (%) | 49 (27) |
| Resected weight (g), mean (SD) | 16.7 (12) |
| Men with prostate cancer in resected prostate, n (%) | 19 (11) |
| Surgical success rate, (%) | 77 |
List of abbreviations
- AUA
- American Urological Association
- AUR
- acute urinary retention
- BNC
- bladder neck contracture or urethral stricture
- BPE
- benign prostatic enlargement
- B-TURP
- bipolar transurethral resection of the prostate
- B-TUVP
- bipolar transurethral vaporisation of the prostate
- B-TUVRP
- bipolar transurethral vaporesection of the prostate
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CUA
- cost-utility analysis
- DAM
- decision-analytic model
- ED
- erectile dysfunction
- EQ-5D
- EuroQol Five Dimensions
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- HoLEP
- holmium laser enucleation of the prostate
- HIFU
- high-intensity focused ultrasound
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- ILC
- interstitial laser coagulation
- ILD
- individual level data
- IPSS
- International Prostate Symptom Score
- KTP
- potassium-titanyl-phosphate
- LOS
- length of stay
- LUTS
- lower urinary tract symptoms
- MI
- myocardial infarction
- MTOPS
- medical therapy of prostatic symptoms
- NICE
- National Institute for Health and Clinical Excellence
- OPCS
- Office for Population Censuses and Surveys
- PSA
- prostate-specific antigen
- QALYs
- quality-adjusted life-years
- RCT
- randomised controlled trial
- RR
- relative risk
- SF-36
- Medical Outcomes Study 36-item Short Form Health Study
- TEAP
- transurethral ethanol ablation of the prostate
- TUIP
- transurethral incision of the prostate
- TUMT
- transurethral microwave thermotherapy
- TUNA
- transurethral needle ablation
- TUR
- transurethral resection
- TURP
- transurethral resection of the prostate
- TUVP
- transurethral vaporisation of the prostate
- TUVRP
- transurethral vaporesection of the prostate
- UTI
- urinary tract infection
- VLAP
- visual laser ablation of the prostate
- VOI
- value of information
- WIT
- water-induced thermotherapy
- WMD
- weighted mean difference
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS) or it has been used only once or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
Health Technology Assessment Programme
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NCCHTA
-
Dr Andrew Cook, Consultant Advisor, NCCHTA
-
Dr Peter Davidson, Director of Science Support, NCCHTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NCCHTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NCCHTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NCCHTA
HTA Commissioning Board
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programmes, Research and Development Directorate, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington