Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA Programme on behalf of NICE as project number 07/64/01. The protocol was agreed in October 2007. The assessment report began editorial review in April 2008 and was accepted for publication in August 2008. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NCCHTA, Alpha House, Enterprise Road, Southampton Science Park, Chilworth, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
Chapter 1 Aims and background
Review question
What are the harmful health effects of taking 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) for recreational use?
Pharmacology
‘Ecstasy’ is the common street-name for drugs that contain – or purport to contain – 3,4-methylenedioxymethamphetamine (MDMA) as their active ingredient. Following the convention of Gowing et al. ,1 the term ecstasy is used here to denote the drug as it is sold on the street (with composition unknown), whereas MDMA refers to the known chemical substance.
MDMA is a synthetic chemical belonging to the amphetamine family. Several chemically closely related substances are also commonly used as recreational drugs:
-
amphetamine (‘speed’, ‘whizz’)
-
methamphetamine (MA; ‘crystal meth’)
-
paramethoxyamphetamine (PMA)
-
3,4-methylenedioxyamphetamine (MDA)
-
3,4-methylenedioxy-N-ethylamphetamine (MDEA; ‘Eve’)
-
3,4-methylenedioxy-phenyl-N-methylbutanamine (MBDB).
Drugs sold as ‘ecstasy’ frequently contain one or more of these substances, instead of or in addition to MDMA. 2 Another street-drug, gamma-hydroxybutyric acid (GHB) is colloquially known as ‘liquid ecstasy’, despite being pharmacologically very different from this group. GHB is outside the scope of this review.
The intended effects for which ecstasy users take the drug are described in terms of euphoria, exhilaration and a sense of increased intimacy and empathy with others,3 effects that have been reproduced by administration of MDMA in laboratory conditions. 4 The neuropharmacological mechanisms by which these effects are produced involve the release of extracellular serotonin (5-HT) and dopamine,5 neurotransmitters that are commonly associated with the mood and pleasure systems of the brain.
On ingestion, MDMA is rapidly absorbed and first effects are felt 30–60 minutes later, peaking at 60–120 minutes. 6,7 Psychoactive effects last for 2 to 4 hours although MDMA remains detectable in the blood much longer, with a half-life of 6 to 8 hours. 6
In controlled conditions in humans, cardiovascular effects are evident at doses of MDMA of 1.0 mg/kg or higher. 6 Heart-rate rises to a peak of an average of 20–30 beats per minute higher than baseline approximately an hour after consumption of doses similar to those taken recreationally. 8–10 Blood pressure increases over a similar period: systolic blood pressure rises by 25–40 mmHg and diastolic blood pressure by 10–20 mmHg. 8–10 Body temperature also rises (by 0.3–1.0°C), but this effect is less immediate, with a peak several hours after consumption. 8,10,11 Body temperature increase is related to ambient temperature, which may be more pronounced in club settings. 6 These responses mimic those of the sympathetic nervous system, and may be exacerbated by the environmental conditions under which ecstasy is typically taken – in clubs or parties, with loud music, flashing lights and long periods of dancing. 12 The apparently non-linear nature of MDMA pharmacokinetics has been emphasised; blood concentrations of MDMA rise disproportionately as dosage is increased. 13
History
The first documentary record of the synthesis of MDMA is the 1912 German patent application of Merck pharmaceuticals, but there is no record of MDMA being tested in humans until 1960, and no commercial application was identified for the substance by Merck, or any other manufacturer. 14 In the 1970s, some use was made by mental-health professionals in west coast USA to enhance empathy, lower defensive barriers and enhance intimacy among people in psychotherapy. 7 Following very sporadic reports in the 1970s, recreational use of MDMA became more widespread during the 1980s. 15 The term ‘ecstasy’ first appeared in print in reference to MDMA in 198516 and in the British media in 1987. 17
The US Drug Enforcement Administration classified MDMA as a Schedule 1 controlled substance with effect from 1 July 1985. 18 In the UK, it had already been criminalised; a statutory instrument of 1977, without naming MDMA in particular, categorised all ring-substituted phenethylamines as Class A substances under the Misuse of Drugs Act,19 a classification that has remained in place.
In the late 1980s and early 1990s, consumption of ecstasy became strongly associated with a widespread culture of dance parties (‘raves’),20 characterised by loud music, extensive light shows and marathon dancing sessions. 21 As the 1990s progressed, ecstasy retained its strong association with dance music, although the scene moved into nightclubs, partly as a result of legislation that sought to prevent raves taking place. 22
Administration, purity, dose and price
Ecstasy is usually taken orally in pill form. The price of ecstasy has reduced dramatically over recent years, from an average of more than £15 per tablet in 1993 to around £5 in 2003. 23 Most recent figures show that the trend is continuing, with a median price of £3 per tablet in 2006, although prices vary regionally and may be as little as £1. 24 Over a similar period, the average MDMA content of a tablet has also reduced – though not to the same degree – falling from 100 mg in 1993 to approximately 75 mg in 2001. 25
Most ecstasy used in the UK is sourced from the Netherlands or Belgium. 26 Ecstasy tablets as sold on the street contain a variable amount of MDMA, and tablets which look the same, sharing logos, may have very different compositions in terms of the amount and type of drug they contain. 27 Analysis of the content of drugs purporting to be ecstasy tablets seized by the police in 2006 showed the amount of MDMA ranging from none to around 120 mg. 27 MDMA was the main drug in the vast majority of cases, but other active substances were dominant in a small proportion of tablets (MDEA 0.04%, MDA < 0.01%, other amphetamines 0.2%, piperazines 1.5%). Some tablets also contain MDEA, MDA or amphetamine in addition to MDMA. Ecstasy tablets may also be ‘cut’ with unrelated substances. Some of these are pharmacologically weak (e.g. caffeine, paracetamol – 0.06% of tablets seized in 2006 contained no controlled drug26); however, there have also been reports of stronger psychoactive substances (e.g. atropine, opiates, phenylbutanamine and dextromethorphan). 2 In 2004, it was suggested that, following a period in the 1990s during which ecstasy tablets were relatively unlikely to contain MDMA as their sole active ingredient, tablets had become rather more ‘pure’ at around the turn of the millennium. 2 One US source suggests that any such effect may have been short lived: tablets analysed in 2005–7 appeared to have approximately a one-in-three chance of containing only MDMA, MDMA along with other active ingredients, or no MDMA at all. 27 Such variations in dose, along with difficulties in obtaining accurate self-reported consumption, cause difficulties in estimating lifetime use, although many studies attempt to do this.
Usage
In the UK, reported MDMA consumption has remained relatively stable over the past decade, with around 2% of 16–59-year-olds reporting ecstasy use in the preceding 12 months. 28 Use is higher among young people, with a 1996 meta-analysis of general population surveys about use among 16–24-year-olds suggesting that 7% [95% confidence interval (CI) 6.1–7.8] had used ecstasy in the previous year, and 3% (95% CI 2.4–3.6) had used it in the previous month. 29 This makes it the third most used illegal drug in the UK after cannabis and cocaine. Among people regularly attending raves and nightclubs, the number of people ever having used ecstasy may be as high as 80–90%. 30,31 It has been estimated that somewhere between 500,000 and 2 million doses of MDMA are consumed each week in the UK. 32
The overwhelming pattern of ecstasy usage is as part of polydrug consumption (use of more than one drug) and co-use (mixed consumption of two or more drugs on the same occasion). 31,33 In a 2003 survey of UK users (recruited through an advertisement in a dance music publication), ecstasy-using respondents also reported extensive concomitant use of alcohol (88% of users reported consumption on one or more occasions in conjunction with ecstasy), amphetamines (83%), cannabis (82%), cocaine (58%) and amyl nitrate (51%), and there was also some use of lysergic acid diethylamide (LSD), ketamine, fluoxetine, crack cocaine, herbal highs and sildenafil. In addition, various substances were used in the ‘comedown’ period following ecstasy consumption, most notably cannabis (82%), alcohol (60%), benzodiazepines (18%) and heroin (2%).
As a result of these factors, together with the unknown composition of pills bought as ecstasy, it is not possible to isolate exposure to MDMA in particular in any individual history or in characteristics across cohorts. Even if there were such a thing as an identifiable group of individuals whose ecstasy consumption alone distinguished them from the general population, it would still be impossible to ascertain to which chemicals they had been exposed, and at what dosage.
Safety
Reports from investigators assessing the psychotherapeutic potential of MDMA in 1986 suggested that the drug was ‘apparently physically safe’, despite some ‘undesirable’ effects. 34 Within a year of such claims, the first reports of ecstasy-related deaths appeared in the medical literature. 35 In the UK, the first reported fatalities came in 1991. 36,37 At around the same time, concerns about long-term neuropsychiatric sequelae of ecstasy use began to be expressed in the popular press. 38 The issue of ecstasy safety made a dramatic impression on the popular imagination with the death of Leah Betts, who died after taking a single ecstasy tablet during her eighteenth birthday party in late 1995. However, it has been suggested that fatalities related to ecstasy use receive a disproportionate amount of attention in the media, particularly if the victim is young and female. 39 An assessment of the number of newspaper reports of drug-related deaths in Scotland in the 1990s compared to Registrar General records of deaths approached a 1 : 1 ratio for ecstasy, while for other drugs the ratio was much higher (for example, for heroin there was one newspaper report for every five deaths; for cocaine 1 : 8; for amphetamines 1 : 3; and for paracetamol 1 : 265). 39
This review assesses the published evidence of the incidence and impacts of adverse health effects of recreational consumption of MDMA.
Chapter 2 Methods
Review methods
The review proceeded according to a prespecified protocol, which is reproduced in full as Appendix 2. Departures from the planned protocol are acknowledged in the following description of methods. Except where otherwise specified, the general methods of the review followed the guidance on the conduct of systematic reviews published by the Centre for Reviews and Dissemination. 40
Identification of evidence
The search strategy comprised the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
scrutiny of bibliographies of retrieved papers.
Search strategy for electronic databases
A comprehensive search syntax using indexed keywords (e.g. MeSH, EMTREE) and free-text terms was developed. The search strategy is shown in full in Appendix 3.
Databases searched
The following electronic databases were searched: MEDLINE, EMBASE and PsycINFO (all via Dialog DataStar); Web of Knowledge.
Inclusion of relevant evidence
The outputs of searches were considered against the prespecified inclusion/exclusion criteria, with a sample of citations screened by a second reviewer, to appraise the validity of assessment. Studies that could confidently be identified as not meeting eligibility criteria on the basis of title and abstract were excluded. The full texts of all other papers were obtained, and assessed to ascertain whether they fulfilled the inclusion criteria. As a result of the volume of material retrieved, it was not possible to satisfy our protocol requirement that each potentially relevant paper would be reviewed for inclusion by two reviewers; however, a sample of inclusion decisions was checked by a second reviewer, with good agreement.
Inclusion/exclusion criteria
The relevance of all evidence was appraised with respect to the following criteria:
Population
-
Users of recreational drugs in the UK or in populations relevant to the UK.
-
Animal studies.
-
Non-drug-using volunteers enrolled in prospective research.
Exposures
-
Recreational use of substances shown to or believed by the investigator(s) to contain MDMA.
-
Use of street drugs shown not to or believed by the investigator(s) not to contain MDMA, whether referred to as ‘ecstasy’ or not.
-
Therapeutic use of MDMA.
-
Generic drug-using populations in which it is not possible to isolate a subgroup with exposure to MDMA in particular.
Comparators
Where comparative evidence was reviewed, studies with comparator arm(s) meeting the following characteristics were considered eligible:
-
Recreational users of drugs other than MDMA.
-
Non-drug-users.
Outcomes
-
Death.
-
Acute, clinically observable health harms.
-
Long-term, clinically observable health harms.
-
Surrogate measures of harm (e.g. neuroimaging studies, biochemical markers), where there is no explicit correlation to observed effect.
-
Biochemical indices of MDMA consumption (e.g. testing for MDMA use in blood or hair samples).
-
Studies reporting therapeutic measures for adverse events without providing data on individuals suffering such complications.
-
Subjective measures of psychostimulation (i.e. studies of the drug’s intended short-term intoxicative effects).
-
Indirect harms, e.g.
-
accidental injury where ecstasy consumption is detected/implicated
-
health consequences of high-risk sexual behaviour contributed to by ecstasy consumption
-
birth defects secondary to maternal exposure to MDMA.
-
Papers in languages other than English
Only studies published in English were included in the review.
Meeting abstracts
Reports published as meeting abstracts wereincluded in the review only if sufficient methodological details were reported to allow critical appraisal of study quality.
Methods of analysis/synthesis
General approach
Initially, all included evidence was reviewed to establish a taxonomy of reported outcomes. For each outcome, the available evidence was categorised in a predefined hierarchy of research design:
-
Level I Pre-existing systematic research syntheses (systematic reviews, meta-analyses, syntheses of qualitative data)
-
Level II Controlled observational studies (cohort studies, case–control studies, etc.)
-
Level III Uncontrolled observational evidence (case reports and case series).
Where adequately designed and conducted, Level I evidence was preferred.
Where no adequate Level I evidence was identified for a given outcome, any Level II evidence was systematically reviewed. The quality of research was appraised and described, and findings were reported. Where possible and appropriate, quantitative synthesis of study outcomes was also undertaken (for methods, see Quantitative synthesis of Level II data: general approach, below).
Where neither Level I nor Level II evidence was available, Level III evidence was systematically surveyed.
Critical appraisal
Level I evidence
Level I evidence was appraised with reference to a bespoke quality-assessment instrument (Table 1), which was adapted from the recommendations of the MOOSE (meta-analysis of observational studies in epidemiology) proposal. 41
| Item | Possible responses | Notes |
|---|---|---|
| 1. Is study defined as a systematic review in title? | Yes | |
| No | ||
| 2. Are study aims clearly described and focused? | Yes | |
| No | ||
| 3. Do study objectives describe population, study design, exposure? | Completely | Full details of population, study design, exposure |
| Partially | Some details | |
| No | ||
| 4. Search strategy supplied (or available) and appropriate? | Yes | Details of databases searched and search terms used |
| No | ||
| 5. Additional sources used? | Yes | For example, author contact or hand searching |
| No | ||
| Can’t tell | ||
| 6. Double data extraction? | Yes | Either double-data entry or one reviewer recording data with second reviewer checking each datapoint |
| No | ||
| Can’t tell | ||
| 7. Assessment of study quality? | Yes | List instruments used in notes |
| No | ||
| Can’t tell | ||
| 8. Assessment of heterogeneity? | Appropriate | List methods used |
| Not appropriate | ||
| Not done | ||
| 9. Results pooled? | Yes | List methods used |
| No | ||
| 10. Pooling appropriate? | Yes | Assessment of synthesis methods (fixed- vs random-effects models, etc.) |
| No | ||
| NA | ||
| 11. Subgroups considered in pooling? | Yes | Either separate or stratified analyses |
| No | ||
| NA | ||
| 12. Results of pooling presented as forest plots? | All | |
| Some | ||
| None | ||
| NA | ||
| 13. Strengths and weaknesses of review discussed? | Yes | |
| No | ||
| 14. Potential biases of review discussed? | Yes | |
| No |
Level II evidence
Level II evidence was appraised with reference to a bespoke quality-assessment instrument (Table 2), which was constructed with reference to recommendations made by Levine and colleagues,42 Downs and Black,43 the NHS Centre for Reviews and Dissemination40 and Mallen and co-workers. 44
| Item | Possible responses | Notes |
|---|---|---|
| 1. Are study aims clearly described and focused? | Yes | |
| No | ||
| 2. Is study design (controlled, observational) appropriate to answer these aims? | Yes | |
| No | If Q1 and Q2 are both answered ‘No’, then stop here | |
| 3. Was study prospective? | Prospective | |
| Cross-sectional | ||
| Ambidirectional | ||
| 4. Exposure to MDMA | Quantified | Sufficient to analyse exposure history and estimate total lifetime exposure |
| Partial | Some details, but insufficient to quantify total lifetime exposure | |
| Inadequate | Not possible to ascertain exposure history | |
| 5. Exposure to other substances | Quantified | Sufficient to analyse exposure history and estimate total lifetime exposure |
| Reported | Some details, but insufficient to quantify total lifetime exposure | |
| Partial | Select if exposure to important substances is not reported, and list in notes | |
| NR | ||
| 6. Are there explicit inclusion and exclusion criteria for study? | Partial | Some indication of eligibililty criteria, but incomplete information |
| No | ||
| Can’t tell | ||
| Yes | ||
| 7. How has sample been recruited? | Advertising | Note where, if stated |
| Direct approach | For example, individuals approached in club | |
| Other | Describe | |
| Snowball | ||
| NR | ||
| 8. From where has MDMA cohort(s) (or cases in case–control studies) been recruited? | Club | |
| University | ||
| Community | ||
| Health-care system | ||
| Other | Please note | |
| Mixture | More than one of these categories | |
| 9. From where has control cohort(s) been recruited? | Club | |
| University | ||
| Community | ||
| Health-care system | ||
| Other | Please note | |
| Mixture | More than one of these categories | |
| 10. Are sample characteristics adequately described? | Partial | Some details, but important information missing |
| No | ||
| Yes | For example, age and gender; depending on outcome, others – e.g. intelligence – may be important; SDs for continuous variables | |
| 11. Are there significant differences between cohorts? | Yes | Significance testing should be undertaken, where possible, if authors have not reported this |
| No | ||
| Can’t tell | ||
| 12. Do analyses attempt to control for confounders? | Yes – matched cohorts | Cohorts are matched on important confounders |
| Yes – adjusted analyses | For example, exposure to other substance included as a covariate in effect size calculations (ANCOVA; other regression) | |
| Yes – stratified analyses | ||
| Partial | Note any shortcomings in approach adopted | |
| No | ||
| Can’t tell | ||
| NA | ||
| 13. Is there a power calculation? | Yes | |
| No | ||
| Can’t tell | ||
| 14. Is sample size sufficient? | Yes | Only answer ‘Yes’ if sample size fulfils criteria of explicit power calculation |
| No | Only answer ‘No’ if there is an explicit power calculation but sample size does not fulfil criteria | |
| Not analysed | All other cases | |
| 15. Is primary outcome measure objective? | Objective | |
| Subjective | Includes all self-reported measures; however, note if measured according to validated instrument | |
| 16. Are secondary outcome measures objective? | Objective | |
| Subjective | ||
| Mixed | ||
| 17. Were outcome assessors blind to exposure status? | Yes | |
| No | ||
| Can’t tell | ||
| NA | ||
| 18. Are dose–response relationships considered? | Yes | |
| No | ||
| Can’t tell | ||
| 19. Is temporal relationship correct? | No | Outcome precedes exposure |
| Can’t tell | ||
| Yes | Exposure shown to precede outcome, enabling causal inference | |
| 20. Are drop-out rates similar between MDMA cohort and controls? | Yes | |
| No | ||
| Can’t tell | ||
| NA | Will be the case for most retrospective study designs |
Level III evidence
Because a very large amount of Level III evidence was identified and there were few methodological characteristics with which it could be distinguished (i.e. all such evidence was, by definition, of a poor quality), no formal critical appraisal was undertaken.
Data extraction
Data were extracted using a bespoke database. Because of the very large volume of material retrieved, it was not possible to satisfy our protocol requirement that all data extraction would be double-checked by a second reviewer; however, the data extracted from the 20 studies on which our syntheses relied most heavily were checked by a second reviewer. There were no major errors, and minor errors were corrected. Data extraction tables have not been reproduced in this report because they would run to many hundreds of pages. Details are available from the authors.
Quantitative synthesis of Level II data: general approach
In deciding the approach to the meta-analysis of outcomes of the included studies, a number of aspects of this dataset need to be considered:
-
substantial heterogeneity in the design, risk of bias, population and definition of ecstasy and control exposures
-
the wide range and large number of outcome measures reported (in total, 915 different outcome measures were identified in the evidence-base)
-
substantial level of multiplicity:
-
multiple comparisons, i.e. inclusion of more than one ecstasy exposure (e.g. heavy ecstasy users versus light ecstasy users versus ecstasy-naïve controls; current ecstasy users versus former ecstasy users versus ecstasy-naïve controls) or more than one control arm (e.g. ecstasy users versus polydrug-using controls versus drug-naïve controls) in a single study
-
multiple outcomes, i.e. inclusion of more than one outcome measure assessing a given outcome domain within a single study, either through the reporting of several relevant subscales from a single instrument (e.g. individual immediate memory trials from the RAVLT) or through the reporting of several relevant measures (e.g. the RAVLT and the RBMT)
-
repeated measures, i.e. comparison between exposure and control over more than one time point (e.g. follow-up over a period of abstinence, with repeated measurements at regular intervals)
-
-
observational basis of comparisons.
Collectively these issues pose a substantial methodological challenge to the application of standard meta-analysis methods. Our methodological approach to each of these issues is discussed below.
Substantial (clinical) heterogeneity
Four strategies were employed to minimise the potential problem of heterogeneity. First, separate meta-analyses were conducted according to the types of control groups in included studies (ecstasy users versus polydrug-using controls without exposure to ecstasy; ecstasy users versus drug-naïve controls). Throughout this document, the term polydrug controls is used to refer to control groups in which some or all of the participants had a history of exposure to illegal drugs other than ecstasy. In contrast, drug-naïve controls are those who have no experience of illegal substances, although most will have a history of alcohol consumption and/or tobacco smoking. Three studies45–47 were excluded from analysis because they provided insufficient information on whether control participants had exposure to other substances; hence, it could not be ascertained to which of our analyses data should contribute. Several studies were designed to compare ecstasy-exposed participants with separate polydrug and drug-naïve control arms; in these instances, the relevant comparisons are included in each meta-analysis, as appropriate. Second, each meta-analysis was, where possible, stratified to distinguish between current ecstasy users and former users. Third, a random-effects meta-analysis was used throughout, thereby explicitly recognising that the separate studies may be estimating different effect sizes of ecstasy exposure. Last, study-level regression (‘metaregression’) was used to explore the statistical heterogeneity across studies. The association between the exposure effect size and population [e.g. mean age, sex and baseline intelligence quotient (IQ)] and ecstasy exposure characteristics (e.g. duration and frequency of usage) was examined univariately.
Range and number of outcomes
To rationalise the range and diversity of outcomes reported, a pre-hoc decision was made to focus and synthesise the results according to a series of domains, representing key areas of interest. The underlying principle was to maximise parsimony, i.e. to reduce the heterogeneous evidence-base to as few meta-outcomes as could be sensibly delineated. The categorisation of outcomes into domains was initially defined by the reviewers, with particular reference to the textbooks of Lezak et al. ,48 Hersen et al. 49 and Strauss et al. 50 In the particular areas of executive function and attention, we were guided by conceptual models – based on principal components analyses – proposed by Miyake et al. 51 and Mirsky et al. 52 respectively. These categories were reviewed and, where necessary, revised by our expert advisory group. Where outcome domains featured some objective measures and some self-reported measures, these were analysed separately.
To combine studies using different outcome measures within each domain, effect sizes were expressed as a standardised mean difference (SMD). The SMD expresses the size of the exposure effect of ecstasy in each study relative to the variability observed in that study. Accordingly, for a given study i,
where m1i and m2i represent the reported means in ecstasy-exposed and control cohorts, respectively, and si is the pooled standard deviation across both groups, estimated as,
where n1i, n2i and Ni represent the sample sizes of ecstasy-exposed, control and combined cohorts respectively, and the reported standard deviations of measurements in ecstasy-exposed and control groups are SD1i and SD2i. To pool SMDs, it is necessary to derive the standard error, which is estimated as follows:
The method assumes that the differences in standard deviations among studies reflect differences in measurement scales and not real differences in variability among study populations.
Multiplicity
To include studies with multiple comparison arms within a conventional meta-analysis, it is first necessary to decompose the data in question to a series of pairwise comparisons (so A versus B versus C becomes A versus C and B versus C, assuming C is the common comparator). However, it would be inappropriate to treat each such comparison as an independent unit of analysis, by entering all datapoints into a single meta-analysis, because to do so is effectively to double-count data from the shared comparator (that is to say: if A versus C and B versus C are entered into the same analysis, then the data representing C effectively appears twice) (see Section 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions49).
To minimise this unit-of-analysis error, we have adopted two different approaches:
-
Our primary approach was to include each pairwise comparison in our analyses, but to adjust the size of the shared comparator to reflect the number of comparisons in which it is involved. For example, if a trial compared 100 current ecstasy users and 100 former ecstasy users with 100 ecstasy-naïve controls, we assumed that half of the control group was committed to each comparison. Accordingly, two comparisons would be entered into the meta-analysis: 100 current ecstasy users versus 50 ecstasy-naïve controls and 100 former ecstasy users versus 50 ecstasy-naïve controls. For dichotomous outcomes, both the number of events and the total number of participants is halved; for continuous outcomes, it is only necessary to adjust the total number of participants (in turn, this decreases the precision of each effect estimate, because the sample size feeds into the calculation of standard error, and ensures that each individual comparison will have reduced weight in the meta-analysis).
-
Another approach to the same problem is to pool all relevant datapoints to provide a single unit of analysis for the pairwise comparison of interest. Using the same example as above, a meta-arm of 200 current and former ecstasy users would be compared to the 100 control participants. For dichotomous data, event numbers are simply added; for continuous outcomes, the mean for the combined arm is estimated as the weighted mean from the multiple separate arms (where the numbers in each arm provide the weights), and the standard deviation for the combined arm is calculated according to the usual formula (an extension of equation (2), above, accounting for a combination of more than two estimates):(4)sc=∑i=1k(ni−1)si2∑i=1k(ni−1),
where i indexes a total of k arms being combined, ni is the number of participants in each arm, and si is the standard deviation for that arm.
The disadvantage of this latter approach is that inter-arm heterogeneity – which, in itself, may be informative – is obscured. In particular, it is difficult to perform metaregression on analyses constructed in this way, because covariates of interest would also have to be pooled, with the likely effect that any influence of variables of interest on overall effect will be disguised. For example, in the case previously put forward, it would not make sense to investigate the effects of duration of abstinence on exposure effect, when two groups with very different profiles have been conflated.
In each instance, our primary analysis is based on the separate pairwise approach. However, we recognise that this method only partially overcomes the unit-of-analysis error (because the resulting comparisons remain correlated). 53 Therefore, we also performed sensitivity analyses, adopting the second aggregation method, to investigate whether our choice of approach had any notable influence on results.
Methods are available for synthesising multiple outcome measures in a single meta-analysis. 54–56 The benefit of such methods is that they take into account the level of correlation that exists between outcomes from the same study in the analysis. On the other hand, these methods are complex, and may obscure within-study heterogeneity, which may be important. For these reasons, this approach was not pursued.
Instead, we derived single units of analysis by pooling domain-related outcomes into a single ‘omnibus’ domain-specific outcome. Deriving these estimates was a four-stage procedure:
-
All potentially relevant outcome measures were screened to ensure no duplicate data content. For example, if a study reported a series of subtests along with an index score that had been categorised as relevant to the domain of interest, the index score only was included in our analysis. Wherever second-order manipulations of subscores were reported (e.g. a Stroop test in which interference effect was reported as time in interference trial minus time in simple naming), those measures were not included if the individual subscores on which they were based were already part of the dataset. In the event that such second-order measures were the only relevant datapoints extracted from a study (in the above example, where interference effect is reported without raw trial times), there would be no double-counting of data, so such datapoints were included.
-
Data for each individual outcome measure were adjusted to reflect the multiplicity of comparisons (as described in Multiple comparisons, above).
-
Each individual measure was expressed in terms of SMD (see Range and number of outcomes, above).
-
For each comparison, a weighted average of all SMDs was calculated, using the precision of the estimates as the weighting factor (this could be seen as a sub-meta-analysis, adopting a fixed-effects model with inverse variance weighting).
This method assumes that the correlation between outcomes is uninformative (as described above for multiple comparisons). However, assuming a relatively conservative correlation between outcomes of 0.5 and based on three or four domain-specific outcomes, it estimated that our method will overestimate the precision of the omnibus outcome estimate by only some 10 to 15%. 57
We believe this approach should provide a more informative – and less biased – estimate of effect than those available in some previous meta-analyses of the effects of ecstasy exposure which, when faced with a multiplicity of outcomes, have simply selected a single outcome as most representative of the domain in question. 58,59 This approach not only discards potentially informative data but also relies very heavily on the assumption that the reviewer’s choice of outcome is truly representative of the domain in question.
Other reviewers have adopted a similar approach to ours, basing their analyses on multiple outcomes ‘aggregated … to produce an average effect size’. 60,61 However, in each instance, the methods used to pool separate outcomes are not described.
A relatively small subset of studies reported repeated measurements of an outcome of interest (e.g. over a period of abstinence,62,63 or before and after an experimental procedure64). In such cases, we have entered only the first measurement taken into our quantitative syntheses. An exception to this principle was made for a few studies in which measurements had been taken in users experiencing the acute and/or subacute effects of ecstasy consumption, and then a subsequent measurement recorded when such effects had worn off. In these instances, the later measurement – which more properly captures the long-term effects of ecstasy exposure – was used. Previous meta-analyses have explicitly61 or presumably58–60 taken a similar approach. An alternative approach would have been to use an effect estimate based on time-to-event analysis (such as hazard ratio). However, no such analyses were reported.
Observational basis of comparisons
Because of the observational nature of the included studies, potential confounders (e.g. participant age, exposure to legal and illegal drugs other than ecstasy) are highly unlikely to be equally distributed across the exposure and control arms. Dependent on direction and magnitude, within-study confounder imbalances are likely either to overestimate or to underestimate any underlying exposure effect. This asymmetric distribution of confounders has not been explicitly considered in previous meta-analyses of the effects of ecstasy. Using an extension of an analytic approach recently described by Trowman et al. ,65 we used metaregression similar to analysis of covariance (ANCOVA) to explore the evidence for important confounding of effect, and to ‘adjust’ the exposure effect size for potential imbalance in confounder distribution between exposure and control groups:
The output of particular interest is the constant (‘exposure effect’), which represents the ‘true’ effect of the exposure after accounting for baseline differences in confounders between the arms of individual studies. When the difference in confounder is 0, this value is equivalent to unadjusted exposure effect size. This can be seen clearly when the relationship is plotted on a graph as the point at which the estimated regression line intersects the y-axis.
Quantitative synthesis of Level II data: technical approach
Primary meta-analyses
We used random-effects meta-analyses (DerSimonian and Laird model66) only, regardless of any statistical evidence of inter-study homogeneity. Heterogeneity was explored by visualisation of results and, in statistical terms, by calculation of both Cochran’s Q (compared to a chi-squared distribution)67 and the I2-statistic. 68,69 Small-study effects (including publication bias) were visualised using funnel plots and quantified using Egger’s test. 70 Analyses were conducted using bespoke software, written in Visual Basic for Applications and applied in both Microsoft Access and Microsoft Excel. Stata 9.1 was used to verify the accuracy of analyses ( command) and to assess small-study effects ( command).
Metaregression
Metaregression was undertaken using Stata 9.1 ( command). The method of moments model was used for all metaregressions because, although the restricted maximum likelihood estimator is generally recommended in this situation,71,72 our methods extended to using the outputs of metaregression analyses to calculate adjusted effect estimates (see Observational basis of comparisons, above). Therefore, it was important for us to compare the outputs of metaregressions with our original meta-analyses, and the method of moments model is identical to a classical random-effects meta-analysis when the effect of the covariate is zero. Because of inconsistencies in the evidence-base, it was not possible to undertake multivariate analyses, so regressions were conducted solely on a univariate basis.
The metaregression analyses presented in our results fall into three categories:
-
‘Classical’ metaregression, in which the covariate is a study-level characteristic (e.g. average age of all participants, average IQ of all participants).
-
Dose–response analyses, in which the covariate is one of several estimates of ecstasy exposure in the ecstasy arm [e.g. estimated total lifetime dose (ETLD), duration of use].
-
Exploration of inter-arm confounding, in which the covariate is a measure of the difference between cohorts in any one of several characteristics other than exposure to ecstasy (e.g. difference in age, difference in exposure to other substances). Two methods were used to quantify asymmetry in drug exposure. First, differences were calculated on an absolute scale: difference in ETLD of the substance in question, calculated according to uniform units (joints of cannabis, grams of amphetamine and cocaine, units of alcohol). In meta-analyses comparing ecstasy-exposed populations with drug-naïve controls (for whom the ETLD of illegal substances is, by definition, nil), this variable becomes a simple index of consumption in the ecstasy-using arm. Second, because ETLD is only reported by a minority of studies, the SMD between arms was calculated using any one of several drug exposure variables. Standardised difference scores for drug consumption were based on the highest ranking measure available in each study according to the following hierarchy:
-
ETLD (amount of the substance ever taken; any quantitative unit)
-
estimated total lifetime exposure (number of occasions on which the substance has ever been taken)
-
dose over a specified period (e.g. estimated amount taken in past 12 months)
-
frequency (e.g. number of occasions taken per month)
-
typical dose (amount of substance taken per occasion)
-
exposure score (average score on a bespoke ordinal scale)
-
duration of use (length of history of exposure to the substance).
Because single values cannot be manipulated in the same way as inter-arm differences, standardised differences in drug exposure were only calculated for meta-analyses comparing ecstasy-exposed populations with polydrug controls. In comparisons with drug-naïve controls, these covariates were omitted from analysis.
-
Throughout this document, the term ‘confounder’ is used to refer to any variable that, while unrelated to the outcomes of interest, may potentially have an influence on observed effect. In some cases, the assumption of independence may be an inaccurate one, and it may be more correct to use the term ‘effect modifier’, to emphasise that there is a causal interaction between the variable and the outcome. However, it is not possible for us to disentangle such relationships on the basis of the evidence-base available to us.
Chapter 3 Results
The papers identified by literature searches, screened against the inclusion criteria and finally included in the review are shown in Figure 1, together with the reasons for exclusion of the rest.
FIGURE 1.
Review flowchart. AEs, adverse events.
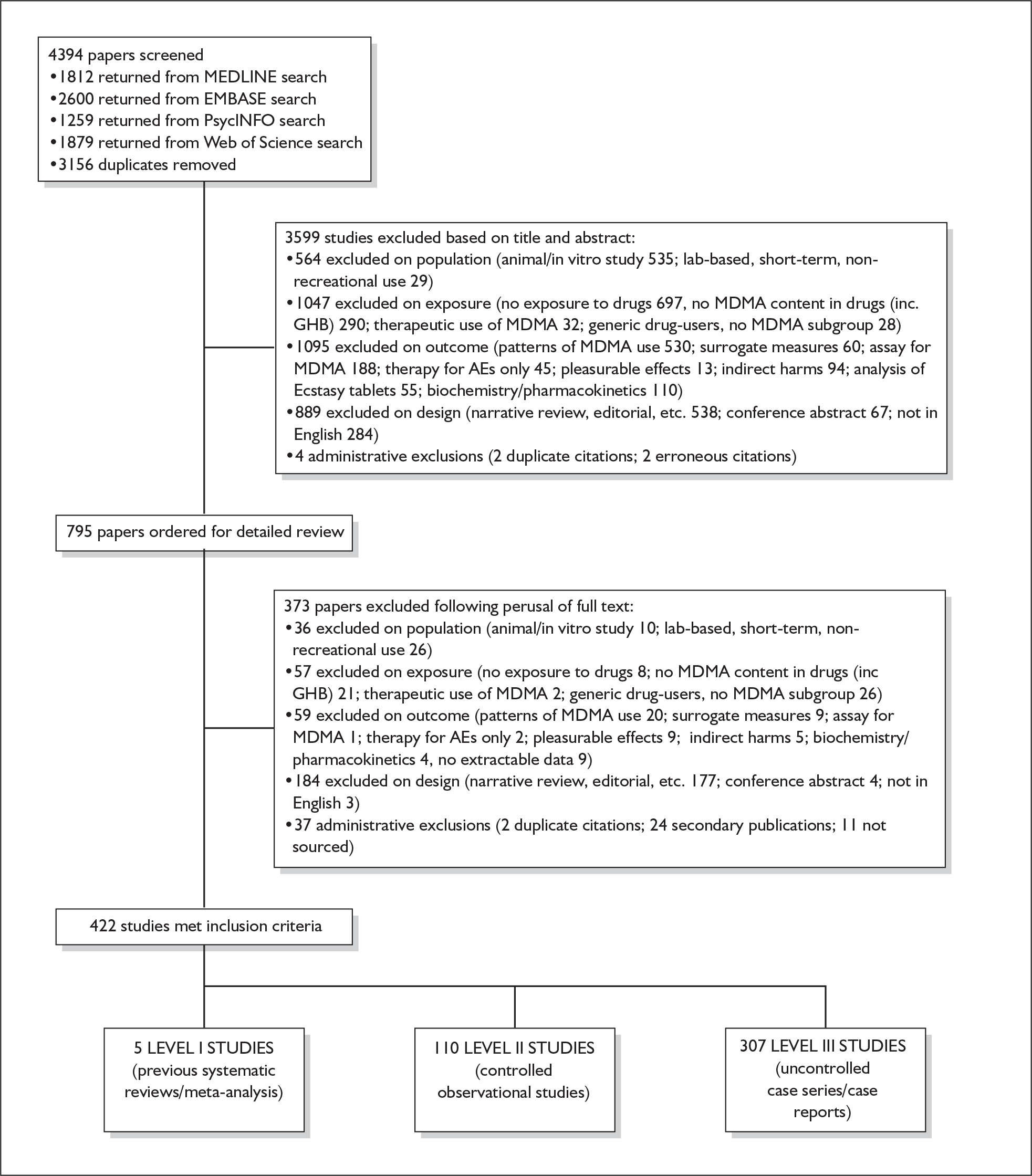
Although we were not able to integrate new findings in our review, we performed updated literature searches on 28 February 2008. Of 289 new citations returned, 44 appeared – on the basis of title or abstract alone – as though they might be relevant to the content of this project; these references are given in Appendix 4. We recommend that any future update of this review considers this evidence for inclusion.
Previous syntheses (Level I)
We identified five previous systematic reviews and/or meta-analyses. One reported on self-reported depressive symptomatology in ecstasy users56 and three were concerned with the chronic neurocognitive effects of ecstasy. 58–60,73,74 The fifth review discussed the acute subjective effects of ecstasy associated with intoxication and was not considered further. 74
Methods
The characteristics and methods of the identified studies are summarised in Table 3.
| Paper | Search strategy | Inclusion/exclusion criteria | Research question | Meta-analysis? |
|---|---|---|---|---|
| Sumnall and Cole 200554 |
Web of Knowledge, PsycINFO, MAPS MDMA databases searched 1914–2004 Ecstasy, MDMA, human, self-report, depressive, depression Reference lists of retrieved articles searched, experts consulted for unpublished data |
Inclusion: self-reported depressive symptomatology using validated measures in community samples of ecstasy users 25 studies identified |
To quantify self-reported depressive symptomatology in substance misusers reporting ecstasy use | Yes |
| Verbaten 200355 | PsycINFO and MEDLINE searched 1975–2002, search terms not mentioned |
Inclusion: n, mean and SD reported for all dependent variables; subjects drug free for at least a week 10 studies included |
Existence and strength of effect of neurocognitive damage from ecstasy use; evidence for a dose–response effect | Yes – regression for lifetime exposure |
| Laws and Kokkalis 200756 |
MEDLINE, Google Scholar, PsycINFO, National Institute on Drug Abuse, Erowid using MDMA, memory, ecstasy, cogniti*, neuropsych* Reference lists of retrieved articles and core on-line journals searched |
Inclusion: studies contained relevant memory subtest data for an appropriate non-MDMA-using control group that could be used to derive an effect size 28 studies identified |
Impact of recreational MDMA use on memory – updating Verbaten’s review | Yes – no forest plots presented, fixed- and random- effects models used, subgroup analyses for studies addressing confounders |
| Kalechstein et al. 200769 | PsycINFO and MEDLINE searched using MDMA, neurocognition, neuropsychology, cognition |
Lenient group inclusion: measures of neurocognition, matched controls 23 studies included Stringent group inclusion: as above plus controls similar in age, education/premorbid IQ, MDMA users not treatment-seeking and abstinent at time of assessment 11 studies included |
To quantify the association between neurocognition and MDMA misuse | Yes, but no forest plots, summary measures only reported in tables |
Findings
Depressive symptomatology
The meta-analysis by Sumnall and Cole 200558 of self-reported depressive symptomatology in community samples of ecstasy users found a significantly increased level of depressive symptoms in ecstasy users compared to a mix of polydrug and drug-naïve controls – 22 studies, effect size 0.31 (95% CI 0.18–0.44; p < 0.001). The authors state that they used polydrug controls where available rather than drug-naïve controls, but do not specify more detail. Weighted metaregression analysis showed that estimated lifetime ecstasy use, but not duration of use, dose per episode or abstention period, predicted effect size and that this effect remained after partially controlling for alcohol, amphetamine and cannabis. The effect size for studies was significant using the Beck Depression Inventory (BDI) (0.48; 95% CI 0.29–0.66; p < 0.001) and the Symptom Checklist-90-revised (SCL-90R) (0.26; 95% CI 0.02–0.50; p < 0.05), but using the original SCL-90 it was not. Metaregression also showed decreasing effect size as study size increased: only studies with fewer than 40 subjects produced a significant effect size (16/22). As the funnel plot was significantly asymmetrical, publication bias is likely in this review and is identified as an issue by the authors. There is no narrative synthesis or quality assessment of the studies and the methods of the included studies are unclear.
Memory and neurocognition
Three previous syntheses have conducted meta-analyses based on systematic identification of studies. 59,60,73 None provide a critique of the quality of the included studies.
Kalechstein et al. 73 reported that in their ‘lenient’ group of studies (n = 23), exposure to MDMA, was associated with poorer performance in each of the neurocognitive domains: attention [SMD (Cohen’s d) = 0.40], verbal learning and memory (0.73), non-verbal learning and memory (0.58), motor/psychomotor speed (0.55) and executive systems functioning (0.52) (p < 0.001 for each domain). It is not clear what the matched controls were in terms of other drug use. For the more stringent group of studies (n = 11), results were similar, with verbal learning and memory still showing the greatest effect (SMD = 0.85). No narrative synthesis of the studies was included, so no detail of the quality of the included studies is available.
The effect sizes of Verbaten59 are based on comparisons between the highest ecstasy-using group and a non-ecstasy-using control from each of the 10 included studies. For short-term memory, the mean effect size of – 1.15 remained significant after controlling for lifetime exposure to ecstasy (– 0.95; p < 0.01) and cannabis use (– 0.67; p < 0.01). For long-term memory, the mean effect size of – 1.25 remained significant after controlling for lifetime ecstasy consumption, but not after controlling for lifetime cannabis use (– 1.15; p > 0.05). For sustained attention-processing speed, the mean effect size of 0.41 (p < 0.01) remained significant after controlling for lifetime ecstasy consumption. For attention performance, the mean effect size of – 0.82 remained significant after controlling for lifetime ecstasy consumption and lifetime cannabis consumption.
Laws and Kokkalis60 provide an updated meta-analysis for Verbaten59 of 28 studies. On short-term memory, ecstasy users performed worse than controls in 22 of 25 studies (SMD – 0.63; 95% CI – 0.91 to – 0.41). For long-term memory, ecstasy users performed worse than controls in 17 of 19 studies (SMD – 0.87; 95%CI – 1.38 to – 0.45). Ecstasy users performed worse than controls on verbal memory (SMD – 1.00; 95% CI – 1.45 to – 0.59) and visual memory (SMD – 0.27; 95% CI – 0.55 to – 0.03). While the effect size was larger for long-term than short-term memory, this difference was not significant. Deficits were significantly greater for verbal than for visual memory. No significant differences in effect sizes were observed when comparing drug-naïve with non-naïve controls. There was no effect of lifetime exposure to ecstasy or cannabis use on effect sizes.
Conclusions
None of these studies was judged to have exactly the same focus as our review and, in each case, it was difficult to ascertain the exact methods adopted in the review. This lack of detail may be the result of constraints imposed by journals on article length. A particular problem was identifying what evidence had been included in quantitative syntheses. For these reasons, we concluded that it would not be appropriate to rely on these previous reviews alone for any outcomes of interest. Accordingly, our review of Level II evidence includes all the outcomes on which previous reviewers have reported. We compare our results with theirs in Chapter 4 (Strength and consistency of effect).
Controlled (Level II) evidence (chronic harms)
Assessment of the quality of studies
This section of the review uses data from 110 studies. Aside from data derived from the Netherlands XTC toxicity study (NeXT), which will be discussed separately, all studies assessed the effects of ecstasy in people who already had a history of ecstasy use. Virtually all studies, therefore, provide only cross-sectional data from a group of ecstasy-exposed subjects compared to a control group without or with minimal ecstasy exposure.
Recruitment
Recruiting users of illegal drugs for research studies is challenging and authors have used various methods. Some subjects have been recruited from those attending programmes in drug addiction centres or admitted to long-term rehabilitation programmes, which include urine monitoring for MDMA and other drug use. Other studies recruited active users at raves/dance parties, while others used advertising either in specialist media or via their research institution. The snowball technique has been used extensively: participants initially recruited are encouraged to recruit others by word of mouth. These methods are very likely to provide a non-representative sample of ecstasy users. The samples chosen could reflect subjects with a high proportion of problems associated with ecstasy use (in those already in drug addiction programmes) or those who share certain characteristics unrelated to ecstasy use, such as those who choose to respond to an advertisement. The extent to which results from any of these studies can be generalised to the whole ecstasy-using population is therefore uncertain.
Recruitment of the control group may also lead to bias in the result. Often the control group comprised individuals from the research establishment who reported no illicit drug use. These may be students at a university or health-care workers. Such individuals may be reluctant to report illegal drug use and are also likely to differ systematically from the ecstasy users in other ways, such as socioeconomic status and educational attainment. In some studies, urine samples were screened during the study period, so that self-reported recent drug use could be objectively validated.
Study size
In the majority of studies, a power calculation was not performed. Without a power calculation it is not known what chance the study had of detecting a difference between groups, if a true difference exists. Given the very small sample size of many of these studies, it has to be assumed that the chance of declaring false-negative findings (type 2 error) is high. This point is especially relevant where authors have reported that ecstasy-using groups did not differ from controls in terms of baseline characteristics.
Confounding
Given the lack of randomised and other prospective studies, a major issue for this review was the extent to which confounding variables could be identified and controlled for in the included studies. Some sought to control for potential confounding by matching of groups, stratifying patients according to variables thought to be important, such as amount of ecstasy use (e.g. Dafters et al. 75), or by conducting analyses using potentially important variables as covariates (e.g. Heffernan et al. 76). Many studies, however, did not control for the effect of differing prior exposure, or other confounders, in either the design or the analysis plan. Studies also varied in the extent to which they quantified prior exposure to ecstasy and other drugs. In some, an estimate of total lifetime exposure was made by the authors or sufficient data were presented to enable an estimate to be made.
A limitation around the use of studies describing matched groups is that there is no uniformity amongst the variables considered important to match. One study (Back-Madruga et al. 46), which describes groups as matched, actually uses historical archival controls in which ecstasy use was not questioned. In most cases, matching has been restricted to basic demographic variables, but some also include educational attainment, IQ, socioeconomic variables and concomitant drug use. However, in 27 studies, the analyses had not been adjusted to account for potential confounders. For example, Butler and Montgomery77 found that impulsivity and risk taking was greater in ecstasy users than in non-users and further that risk-taking scores were higher amongst high ecstasy users than low ecstasy users. However, there were significant differences in the use of cocaine, amphetamines and LSD between the groups which were not allowed for in the analysis and so the extent to which this result can be attributed to ecstasy use is uncertain. Similarly, another analysis of depressive symptomatology reports an ‘Ecstasy using’ cohort whose history, when compared to that of controls, featured significantly more consumption of alcohol, nicotine, cannabis, psilocybin, amphetamine, LSD, amyl nitrate, ketamine, cocaine and opiates78 but did not attempt to adjust the results to account for these differences. Attributing harmful health effects to MDMA use rather than to other drugs is therefore extremely difficult. 79
Using only cross-sectional data also limits the extent to which effects can be attributed to a possible cause, as the causal association, should there be one, can go in either direction. For example, a group of studies have noted that novelty-seeking behaviour is stronger among ecstasy users; in these cross-sectional studies the explanation could equally well be either that ecstasy leads to such behaviour or that individuals who already exhibit that behaviour are more likely to use ecstasy.
A small number of studies obtained cross-sectional data and then followed patients up for a period of days to several years to obtain further data. We have classified such studies as ‘ambidirectional’ because, although they have a prospective component (observing different groups over time), the original exposure precedes enrolment into the study and the results may be confounded by factors that were present on enrolment.
Disappointingly, we were compelled to exclude one of the very few prospective studies in this area (Lieb et al. 80) from our review because it only reports results from a cohort exposed to ‘ecstasy, amphetamine or related compounds’ (contact with the authors failed to elicit data limited to those exposed to ecstasy only). Similarly, the longitudinal follow-up study by Daumann et al. 81 conflates the use of ecstasy and amphetamine for follow-up measurements (though not for baseline data, which are included in our review). We appreciate that such classifications are more reflective of common usage patterns; additionally, this means that they are more practical to adopt from a study recruitment perspective. However, it is very difficult to make use of such data in a policy-making context because it is impossible to disentangle the contributions of the various substances to the reported results.
Dose-related effects
Determining any dose-related effects of MDMA is more problematic than with prescribed medication in clinical trials or other studies for a number of reasons. Illegal drugs are not produced with pharmaceutical quality assurance procedures and there is ample evidence of great variability in the dose of MDMA contained in available tablets. Consequently, there is no assurance of the dose taken by participants even if they can recall accurately the number of tablets they have taken. Aside from variability in content of the desired active drug there is also variability in content of contaminants, some of which may exert a pharmacological action. Participants in these studies are perhaps also more likely than patients in clinical trials to have inaccurate recall or to lie about their drug consumption. Any claims for a dose effect must therefore be interpreted very cautiously.
Despite this caution, a number of studies attempt to investigate the suggestion that long-term harm from ecstasy use is associated with heavy use rather than low episodic use. There is variation in the thresholds that different researchers have set for low and high use, but all estimates are based on self-reported use and are subject to recall bias, particularly where use over a number of years is recorded.
Abstinent period
To maximise a study’s ability to distinguish long-term effects from acute and subacute sequelae of drug consumption, it is important to ensure that participants are tested after a period of abstinence long enough to rule out any residual effects of their last dose(s). We did not routinely extract information about the extent of abstinence required by each study before testing, or the means by which compliance with such criteria was verified. However, we note that studies varied widely in this respect. For example, Gerra et al. 82 required participants to have ceased consumption of illegal drugs 3 weeks before testing, and used urine screening three times a week to ensure compliance. In another study, the same author ensured abstinence over a 12-month period by the same method. At the other end of the spectrum, Quednow et al. 83 relied upon subjects’ self-declaration that they were drug free for 3 days before participation in the study.
Blinding
Many studies do not state whether the researchers carrying out the assessments were blinded to the exposure status of the participant.
Outcome measures and reporting bias
A feature of the dataset for this review is the large number and diverse range of outcome measures that researchers have assessed. In many cases the outcomes assessed are subjective and rely on the participants’ self-report of a characteristic. In some cases well-established outcome measures are used, whereas in others the validation of the assessment tool is less clear. Studies assessing personality dimensions and mood tended to make use of subjective measures, while those assessing memory and cognitive function made greater use of objective measures. In many cases the studies did not identify a primary outcome measure but subjected the range of data to statistical analyses and hypothesis tests. In most cases no adjustment to significance level has been made for the multitude of hypothesis tests conducted, and the findings of such studies should be regarded as exploratory and hypothesis generating.
In addition, studies have not always reported all outcomes investigated, but have included only those which yielded positive results. Together with the uncertain, but often large, number of outcomes investigated, this selective reporting adds to the interpretation difficulties and increases the likelihood that many results are chance findings.
The Netherlands XTC Toxicity Study
The Netherlands XTC toxicity study is the only study meeting the inclusion criteria for this review that provided data which can truly be described as prospective. A number of objective tests were employed to assess different aspects of memory and visuospatial functioning, and although references are provided it is not clear to what extent the measurement tools used have been validated. Statistically significant differences between the groups were only observed for measures of verbal memory. A large number of statistical comparisons have been made and it would be a moot point to discuss whether the p-values used to declare significance should have been adjusted to reflect this. The authors also chose to use one-tailed tests as they hypothesised that ecstasy use could have been associated only with impaired performance and not with enhanced performance. It would have been more conservative to have used two-tailed tests, keeping p < 0.05 constant as the level at which to declare statistical significance. The conclusion that exposure to even a low dose of MDMA may impair verbal memory has recently been challenged. It was noted that the difference in scores between the groups arose because the increased performance on retest was greater in the ecstasy-naïve group than in the incident ecstasy-using group, i.e. verbal memory test scores numerically increased in both groups but to a lesser degree in the ecstasy group. The scores remained within the normal range. There is some debate as to whether the relative decline in scores is attributable to ecstasy affecting verbal memory in a way that serves to blunt the benefit of a retest some 18 months after the initial test. The conclusion that these effects are apparent even after a low cumulative dose has also been challenged as the range of ecstasy use was reported as 0.5–70 tablets. In response to this challenge the authors present some sensitivity analysis excluding four subjects (approximately 7% of the sample) who used in excess of 10 tablets, yielding a new group mean consumption of 1.95 tablets (range 0.5–6), which was found to have little effect on the results. Dose of ecstasy per occasion was also considered briefly with data presented showing that 95% of users took no more than two tablets per occasion and that during the period of study the mean dose of MDMA per tablet was 78 mg. The authors conducted logistic regression analysis which showed an increased risk of a decline in a verbal learning test with increased consumption.
The strength of the Netherlands XTC toxicity study is the prospective nature whereby a cohort of ecstasy-naïve subjects was followed up for around 2 years. The sampling methods resulted in a study population that is probably not representative of the general population of young people, but the varied situations from which recruitment occurred and the fact that both the eventual ecstasy-using and the control groups came from this same sample make this study stand out from many of the others. It presents a range of objective cognitive measures, and subjective mood and personality measures.
Although many potential confounders are possible, the authors attempt to identify these and adjust their analysis accordingly. The principal concerns are centred on the direction of results in both the active and control groups in one of only three measures out of a possible 12 that were statistically significant, and the relatively large p-values associated with these in the context of multiple one-tailed hypothesis tests.
Results: the Netherlands XTC Toxicity (NeXT) study
Methods
This study started in 2002 with the aims of examining:
-
the causality of ecstasy use in observed brain pathology in humans
-
the long-term course of brain pathology in ecstasy users
-
the clinical relevance of observed brain pathology in ecstasy users.
The study design included three arms:
-
a cross-sectional study of heavy users of ecstasy and controls using varying amounts of other drugs
-
a prospective cohort study of subjects who were ecstasy-naïve at recruitment but had a high risk for future first ecstasy use
-
a retrospective cohort study of lifetime ecstasy users with matched controls.
As this study is the only one we have identified that has included prospective data, we report its methodology and results separately from the rest of the Level II evidence that is purely cross-sectional in nature.
We have identified nine publications from the whole study. Two84,85 describe the methodology, including a detailed assessment of the recruitment techniques,85 particularly the possibility that the investigators’ approach encouraged the drug-naïve subjects to start using ecstasy. Two more publications86,87 report findings from the cross-sectional study; one presents qualitative data from older ecstasy users86 and the other presents neuroimaging data (functional magnetic resonance imaging),87 which are not included in this review. A third report from the cross-sectional arm, identified through an update search and also not fully included in this review, presents cognitive effects in 71 subjects with a spectrum of drug-using histories using a range of instruments. The remaining four publications present results from the prospective cohort arm; two of these report functional magnetic resonance imaging data and are not included in this review,88,89 whereas the others report cognitive90 and depression, impulsivity and sensation-seeking91 data. To date, no publications have been identified that report findings from the retrospective cohort study of lifetime ecstasy users and matched controls identified from a pre-existing longitudinal study in the Netherlands.
Recruitment
Subjects were recruited to both the cross-sectional and the prospective arms by website, an internet campaign, snowball sampling and site sampling at a variety of locations (dance events, youth fairs, universities, etc.). For the prospective arm, subjects were asked about their future intention to use ecstasy and included only if they had a high probability of intending to use ecstasy in the near future. Subjects were paid for their participation in the various assessments.
Follow-up
Subjects in the prospective arm completed further questionnaires on drug use at 3-monthly intervals for a year. The main outcomes were assessed at three time points: after recruitment (i.e. before first ecstasy use), shortly after first ecstasy use for those who started using ecstasy and 12–24 months after baseline assessment in all ecstasy-users and in a sample of those who remained ecstasy-naïve.
Measuring exposure to ecstasy
Ecstasy exposure was assessed initially by questionnaire. Subjects were asked to abstain from drug use for 2 weeks before testing and from alcohol for 1 week before testing. Abstinence was checked by urinalysis and prior exposure to ecstasy and other amphetamines was checked by hair analysis.
Neuropsychological and psychopathological outcomes
Included outcome measures were: working memory/executive functioning, verbal and visual memory, visuospatial functioning, verbal intelligence, depression (BDI), impulsivity (Barratt Impulsiveness Scale; BIS) and Spannings Behoefte Lijst (SBL; Dutch version of the Sensation-Seeking Scale).
Results of the prospective study
One hundred and eighty-eight ecstasy-naïve subjects who were considering ecstasy use in the near future and preferably had at least one friend currently using ecstasy, were recruited over a 2-year period from April 2002 to April 2004. All 188 underwent initial assessment; 158 completed all the follow-up questionnaires of whom 64 said they had started ecstasy use since inclusion in the study and 59 of these 64 participated in the follow-up assessment session, 16–19 months after the initial assessment, together with 61 of the 94 subjects who said they had not used ecstasy, matched for age, sex and IQ (Dutch Adult Reading Test). Subjects were young (average age 21 years) with slightly more women (57%).
At initial assessment, there were no significant differences between those who started using ecstasy and those who did not in terms of age, sex, IQ, educational status and the consumption of other drugs (alcohol, tobacco, amphetamine and cocaine) with the exception of cannabis (greater in those who started using ecstasy, mean joints per week 48.8 versus 17.2, p < 0.05 Mann–Whitney test). There were also no significant differences in any of the neuropsychological or psychopathological tests between the two groups at baseline. 86,87 The mean cumulative dose of ecstasy in those who started using it was three or six tablets, depending on which paper you read.
Baseline total scores for depression (BDI), impulsivity (BIS) and sensation-seeking (SBL) did not predict incident ecstasy use, even after controlling for years of education and alcohol, cannabis and cocaine use. 87 At follow-up, there were significant differences between those using ecstasy and the ecstasy-naïve subjects in three of the subscales of the SBL: experience-seeking (β-coefficient 1.76; 95% CI 0.09–3.42), disinhibition (β-coefficient 3.31; 95% CI 1.74–4.88) and general sensation-seeking (β-coefficient 0.54; 95% CI 0.20–0.87) even after correcting for baseline scores. After correcting for potential confounders, ecstasy use had a significant effect on only the SBL general score and the disinhibition subscale. Cannabis use in the last year had a positive predictive value on future ecstasy use [odds ratio (OR) 1.30; 95% CI 1.08–1.56]. The thrill- and adventure-seeking subscale unexpectedly had a negative predictive value on future first ecstasy use (OR 0.95; 95% CI 0.91–1.00).
At follow-up approximately a year later, there was a significant difference in the change in scores (follow-up minus initial) between those subjects stating that they had started using ecstasy (mean cumulative dose three tablets) and those who remained ecstasy-naïve for immediate and delayed verbal memory (0.86 versus 3.90, p = 0.03; – 0.52 versus 0.65, p = 0.03 respectively). A higher proportion of the ecstasy-using group showed a decline in verbal recognition (22.4% versus 6.7%, p = 0.02). The effect of ecstasy use on delayed verbal memory remained after controlling for cocaine and amphetamine use. All other neuropsychological tests showed no significant differences. The ecstasy-naïve subjects showed a normal retest effect, but this was not demonstrated in the ecstasy-using group even after controlling for other drug use. 92 Overall test performance for all subjects remained within the normal range of an age- and sex-comparable general population (indeed, all the RAVLT memory scores for which differences were found represent very high-functioning performance, when compared with norms).
In conclusion, the only prospective study we have identified for this review found that a low cumulative dose of ecstasy is associated with a (small) decline in verbal memory and may increase certain aspects of sensation seeking, but is not associated with depression or impulsivity.
Syntheses: individual outcome measures
In the first instance, we searched the assembled evidence-base for outcome data that had been reported by multiple studies using the same instruments and the same scales. We identified seven outcome measures that were reported with enough consistency to be meta-analysed without further transformation in a meaningful number of studies. With the exception of the National Adult Reading Test IQ, all of these outcomes were measures of verbal memory and could only be analysed in comparisons between ecstasy users and polydrug controls.
The results of these syntheses are summarised in Table 4. Note that effect measures are presented as weighted mean differences, meaning that the estimated effect reflects the difference between comparators on the original measurement scale.
| Current ecstasy users vs controls | Former ecstasy users vs controls | All ecstasy-exposed vs controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | MD | (95% CI) | p | n | MD | (95% CI) | p | n | MD | (95% CI) | p | |
| MDMA users vs polydrug controls | ||||||||||||
| RAVLT verbal recall (immediate) | 8 | –3.912 | (–7.124 to –0.700) | 0.017 | 2 | –5.497 | (–17.216 to 6.221) | 0.358 | 8 | –4.049 | (–6.994 to –1.105) | 0.007 |
| RAVLT verbal recall (delayed) | 7 | –3.727 | (–6.784 to –0.671) | < 0.001 | 1 | –3.000 | (–5.435 to –0.565) | – | 7 | –1.173 | (–1.770 to –0.575) | < 0.001 |
| RBMT prose recall (immediate) | 6 | –0.340 | (–1.198 to 0.518) | 0.437 | 4 | –1.436 | (–2.638 to –0.234) | 0.019 | 6 | –0.657 | (–1.308 to –0.006) | 0.048 |
| RBMT prose recall (delayed) | 6 | –0.441 | (–1.195 to 0.314) | 0.252 | 4 | –1.726 | (–2.890 to –0.563) | 0.004 | 6 | –0.769 | (–1.407 to –0.131) | 0.018 |
| Digit span (forwards) | 5 | –0.449 | (–0.817 to –0.080) | 0.017 | 1 | –0.270 | (–1.123 to 0.583) | – | 5 | –0.421 | (–0.759 to –0.082) | 0.015 |
| Digit span (backwards) | 6 | –0.626 | (–1.081 to –0.170) | 0.007 | 0 | – | – | – | 6 | – 0.626 | (–1.081 to –0.170) | 0.007 |
| IQ (National Adult Reading Test) | 13 | 0.002 | (–0.953 to 0.957) | 0.996 | 5 | –2.745 | (–5.299 to –0.191) | 0.035 | 13 | –0.321 | (–1.248 to 0.606) | 0.498 |
| MDMA users vs drug-naïve controls | ||||||||||||
| IQ (National Adult Reading Test) | 6 | –0.372 | (–1.657 to 0.913) | 0.570 | 2 | –1.174 | (–4.501 to 2.153) | 0.489 | 6 | –0.474 | (–1.618 to 0.670) | 0.417 |
Measures of verbal memory showed an average deficit for ecstasy-exposed populations of sufficient magnitude that the null hypothesis of no inter-cohort difference could be rejected at conventional levels of statistical significance (i.e. p < 0.05), with the exception of the immediate prose recall score from the Rivermead Behavioural Memory Test, which fell only marginally short (p = 0.052).
There was no detectable difference between populations in the National Adult Reading Test IQ, in comparisons between ecstasy users and drug-naïve controls or in comparisons between ecstasy users and polydrug controls.
Full details of these analyses are set out in the following section.
Rey Auditory Verbal Learning Test verbal recall (immediate) – MDMA users versus polydrug controls
The Rey Auditory Verbal Learning Test (RAVLT) is one of the most widely used neuropsychological assessment instruments in our evidence-base. Amongst a broad range of subscales reflecting immediate memory, the most commonly reported was the sum of items remembered across all five initial trials in the test. These data are shown and synthesised using a random-effects meta-analysis in Figure 2. We include one study91 for which reported data are based on the Dutch translation of the test.
FIGURE 2.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (immediate) (sum of trials 1–5) – ecstasy users versus polydrug controls: random-effects meta-analysis.
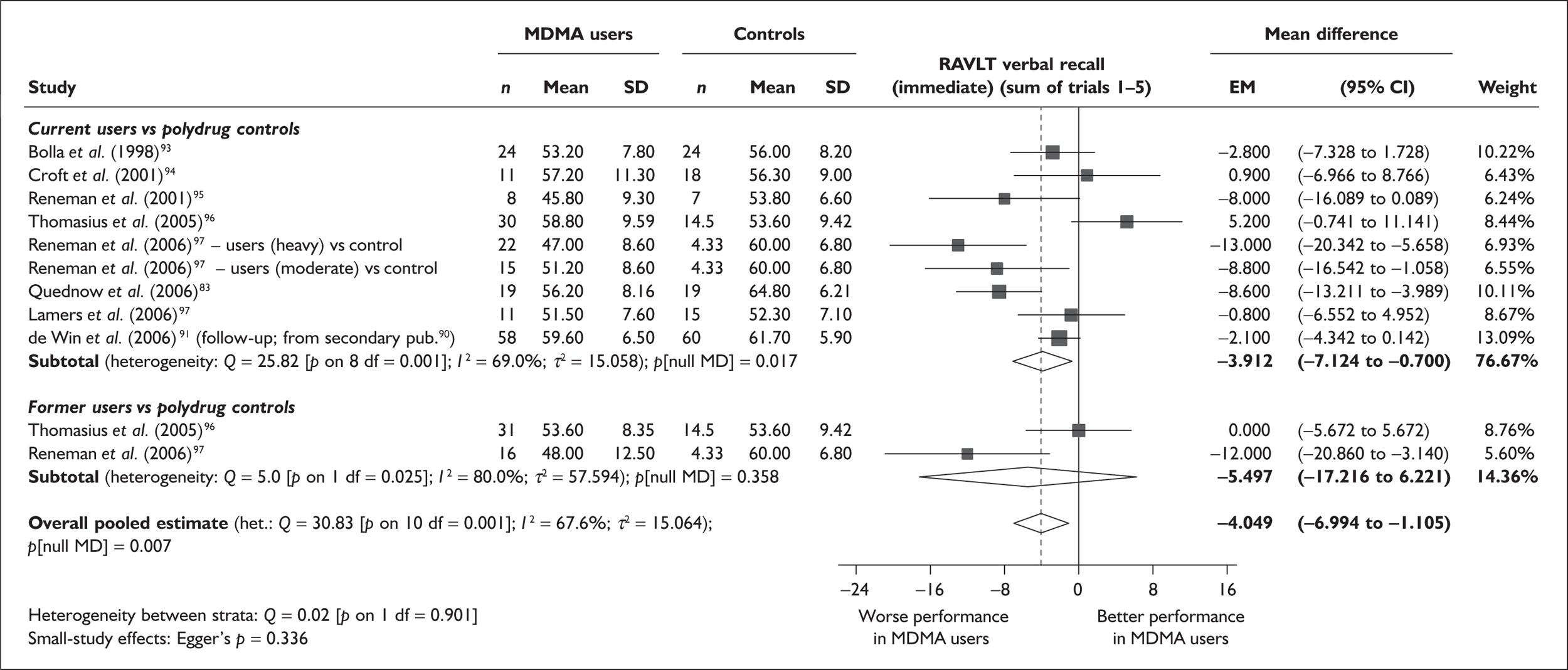
The evidence for worse performance in ecstasy-exposed populations is strong, with a mean difference of around four items. This difference equates to slightly more than half a standard deviation in the normative population (the norm for those aged 20–29 is 56.1 items; SD 7.3). 92
Sensitivity analysis with aggregated comparisons for each study provides a mean difference estimated at – 3.758 (95% CI – 7.126 to – 0.391), suggesting that our primary analysis may marginally overestimate the difference between populations. More notable than this slight reduction in effect estimate is the revised hypothesis test: whereas, in the primary analysis, evidence is strong for a difference between populations (p = 0.007), the sensitivity analysis provides a p-value that, while still comfortably within the bounds of conventional statistical significance, is somewhat less compelling (p = 0.029).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.336), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 15 covariates, shown in Table 5. There was no evidence of a dose–response effect per se (see Figure 88 in Appendix 7). There was the suggestion of an association between duration of use and extent of memory deficit, with those who had used ecstasy for the longest performing worst, relative to their respective controls. However, this trend was not strong enough to achieve conventional statistical significance.
| Covariate | Effect modification | Adjusted effect estimate | |||||
|---|---|---|---|---|---|---|---|
| n | β-coefficient | (95% CI) | p | WMD | (95% CI) | p | |
| Average values across all participants | |||||||
| Age (years) | 11 | –1.167 | (–2.466 to 0.132) | 0.078 | |||
| Sex (% male) | 10 | – 10.092 | (–31.899 to 11.715) | 0.364 | |||
| IQ | 5 | 0.418 | (–0.729 to 1.565) | 0.475 | |||
| Education (years) | 5 | 1.901 | (–0.208 to 4.010) | 0.077 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 9 | 0.003 | (–0.010 to 0.015) | 0.689 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 5 | 0.010 | (–0.028 to 0.048) | 0.599 | |||
| Duration of ecstasy use (days) | 7 | – 0.003 | (–0.007 to 0.000) | 0.074 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 11 | 1.617 | (0.028–3.206) | 0.046 | –2.958 | (–5.867 to –0.048) | 0.046 |
| Sex (% male) | 10 | – 20.519 | (–36.621 to –4.416) | 0.013 | –3.299 | (–5.887 to –0.710) | 0.013 |
| Baseline intelligence measures (SMD) | 6 | 4.105 | (–6.271 to 14.480) | 0.438 | –1.030 | (–6.432 to 4.373) | 0.709 |
| Education (years) | 5 | 1.375 | (–1.313 to 4.064) | 0.316 | –2.486 | (–6.560 to 1.588) | 0.232 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 10 | – 3.629 | (–7.875 to 0.618) | 0.094 | –3.022 | (–6.603 to 0.558) | 0.098 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 10 | 2.475 | (–10.178 to 15.127) | 0.701 | –5.773 | (–13.948 to 2.403) | 0.166 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 9 | – 6.003 | (–11.127 to –0.880) | 0.022 | –1.669 | (–5.294 to 1.955) | 0.367 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 9 | – 5.439 | (–16.520 to 5.643) | 0.336 | –4.424 | (–8.369 to –0.480) | 0.028 |
For metaregressions assessing the influence of confounding (inter-arm asymmetry), significant results were seen for age, gender and cocaine exposure. For age (Figure 3), a positive coefficient was estimated, suggesting that the younger the ecstasy users were in comparison to controls, the worse their relative performance in the memory test.
FIGURE 3.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (immediate) (sum of trials 1–5) – ecstasy users versu.polydrug controls: mean difference in score against inter-arm asymmetry in age.
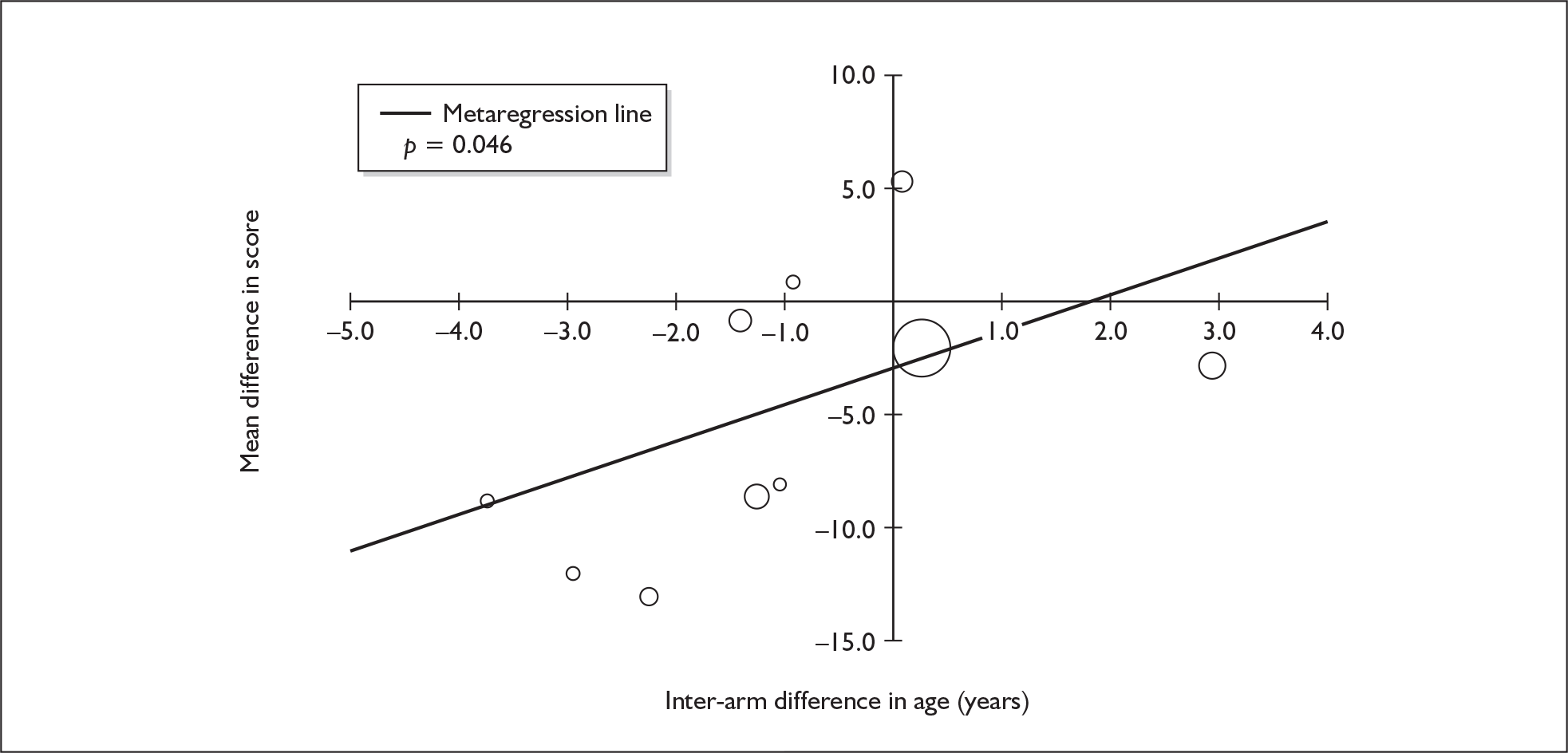
The effect of gender imbalance is shown in Figure 4. It can be seen that the plot is characterised by a number of studies in which gender distribution is well balanced (six of the datapoints appear on or close to the graph’s y-axis). Aside from these studies, it appears to be the case that a negative inter-arm gender difference (indicating that the proportion of males was lower in the ecstasy-exposed arm than in the polydrug controls) is associated with little or no difference between arms in memory performance. Conversely, those studies in which greatest difference was seen between populations were also those in which ecstasy-exposed arms had a greater proportion of men than their respective control groups. These findings may not be a surprise because women are often found to score more highly in the RAVLT than men. 48
FIGURE 4.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (immediate) (sum of trials 1–5) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in gender.
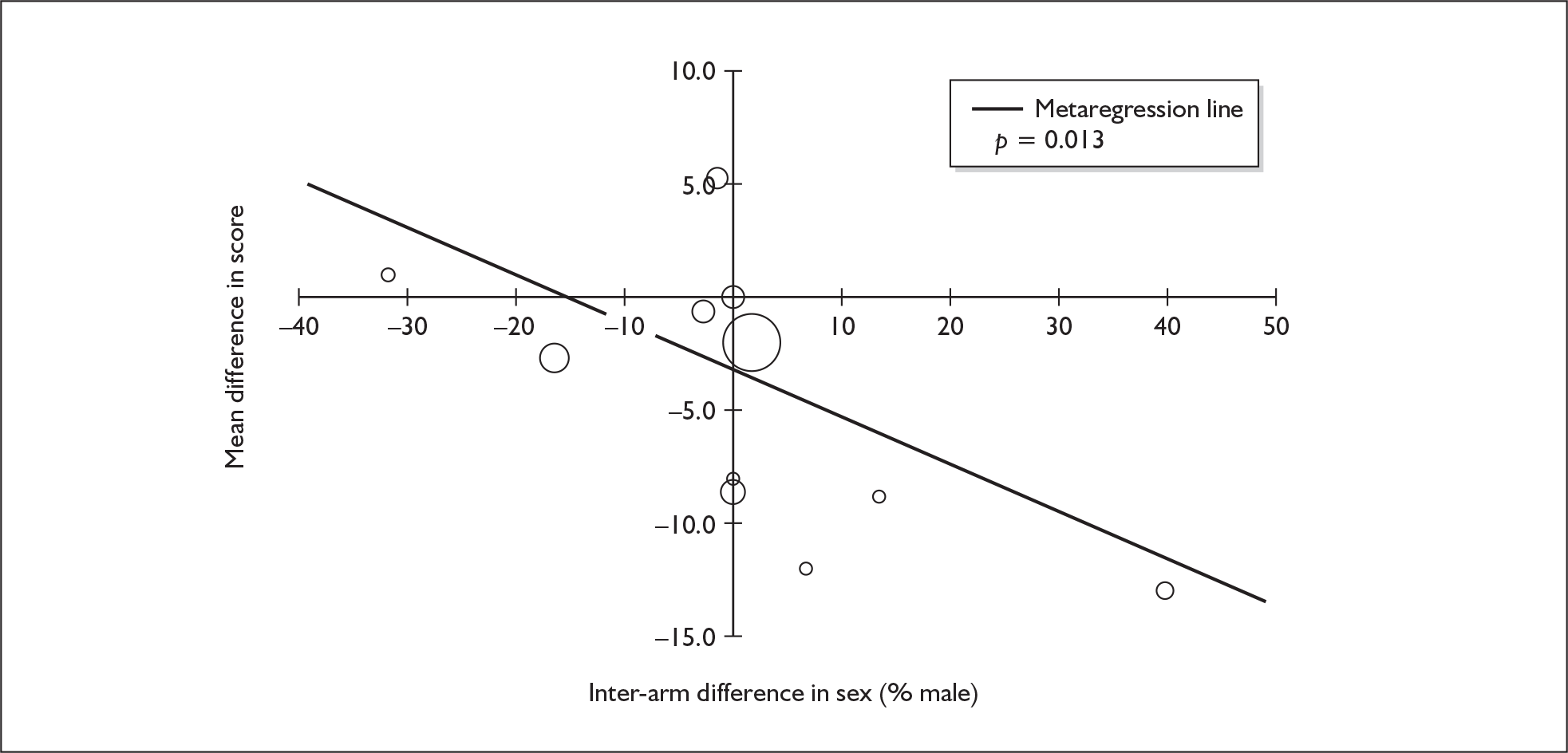
Figure 5 depicts the influence of imbalances in cocaine exposure on measured memory performance. If the model estimated in this analysis were to be accepted, confounding by exposure to cocaine would account for most of the difference between cohorts. The adjusted estimate of mean difference (i.e. the difference that would be expected if groups were perfectly matched for cocaine exposure) is – 1.669 (95% CI – 5.294 to 1.955). Under this model, the evidence for an underlying difference in populations appears weak (p = 0.367).
FIGURE 5.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (immediate) (sum of trials 1–5) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in cocaine exposure (standardised mean difference).
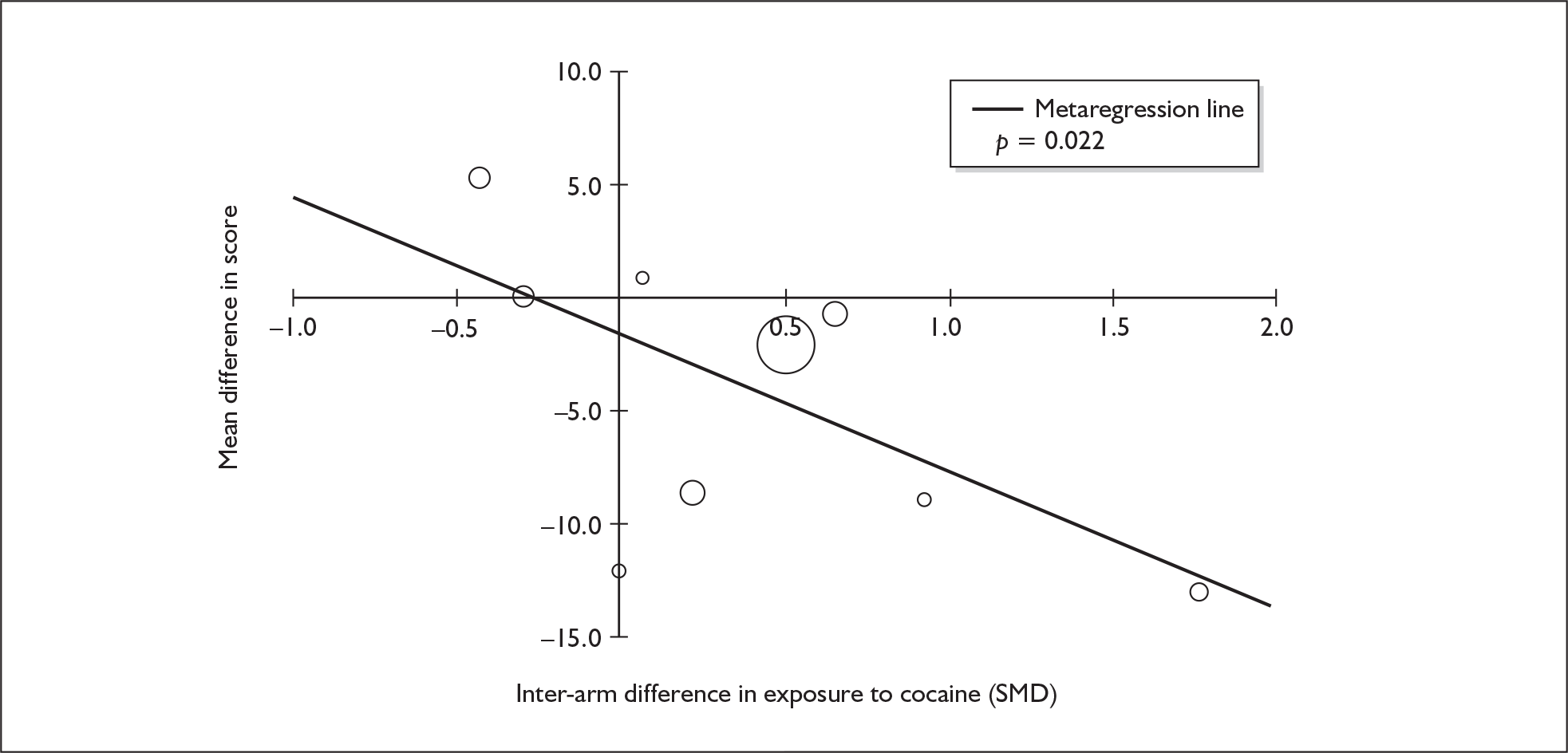
Rey Auditory Verbal Learning Test verbal recall (delayed) – MDMA users versus polydrug controls
In addition to the estimate of immediate memory, we were able to synthesise one RAVLT subscale reflecting delayed verbal memory: items remembered in trial 8. Seven studies provided data on this outcome measure. Details are presented, along with a random-effects meta-analysis, in Figure 6.
FIGURE 6.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (delayed) (trial 8) – ecstasy users versus polydrug controls: random-effects meta-analysis.
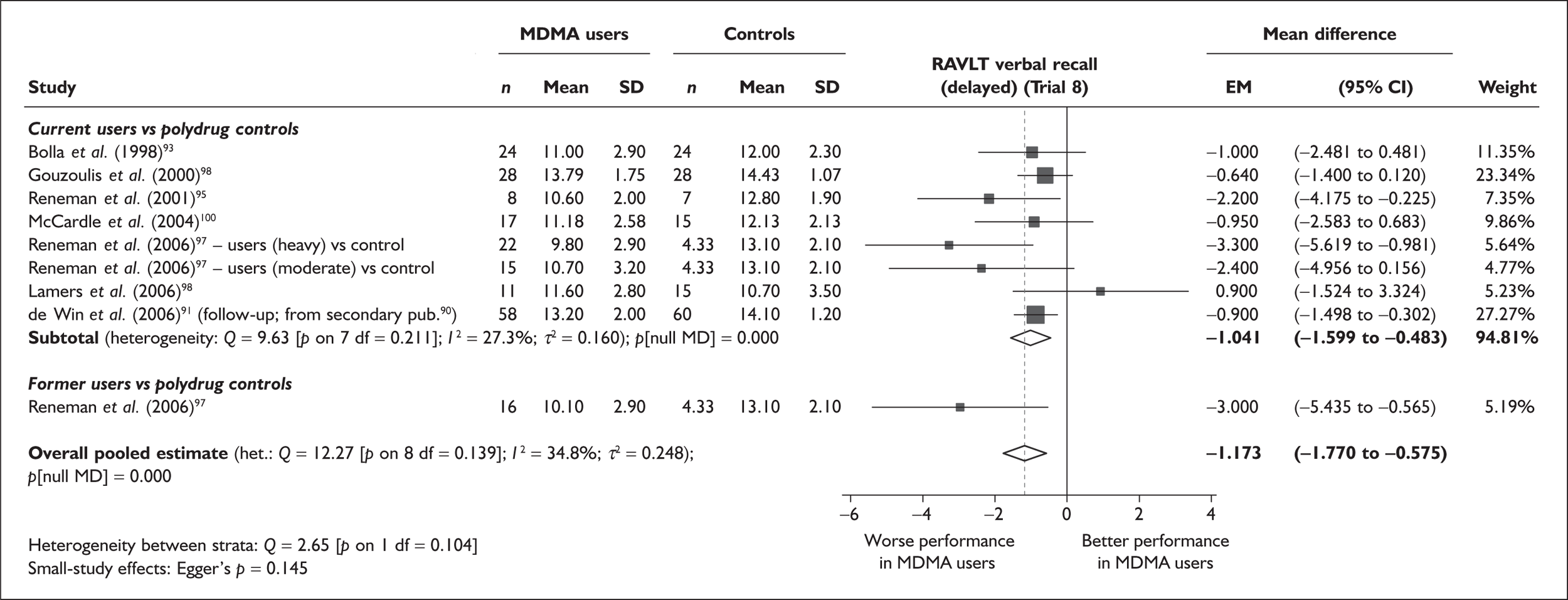
The results of this analysis reflect those obtained for RAVLT immediate memory (see Figure 2) fairly closely. Ecstasy-exposed individuals are estimated to recall a little over one item fewer than polydrug controls. Again, this difference equates to approximately half a standard deviation in the normative population (the norm for those aged 20–29 years is 11.3 items; SD 2.3). 92 The probability of such results occurring if there were no underlying difference between cohorts is very small (p < 0.001).
Sensitivity analysis with single, pooled comparisons for each study provides a mean difference estimated at – 1.134 (95% CI – 1.805 to – 0.463), which is extremely close to the primary analysis.
Egger’s test for small-study bias falls some way short of significance (p = 0.145); nevertheless, we note that the funnel plot for this dataset (Figure 7) shows that the most extreme effect estimates tended to come from the least precise studies.
FIGURE 7.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (delayed) (trial 8) – ecstasy users versus polydrug controls: funnel plot.
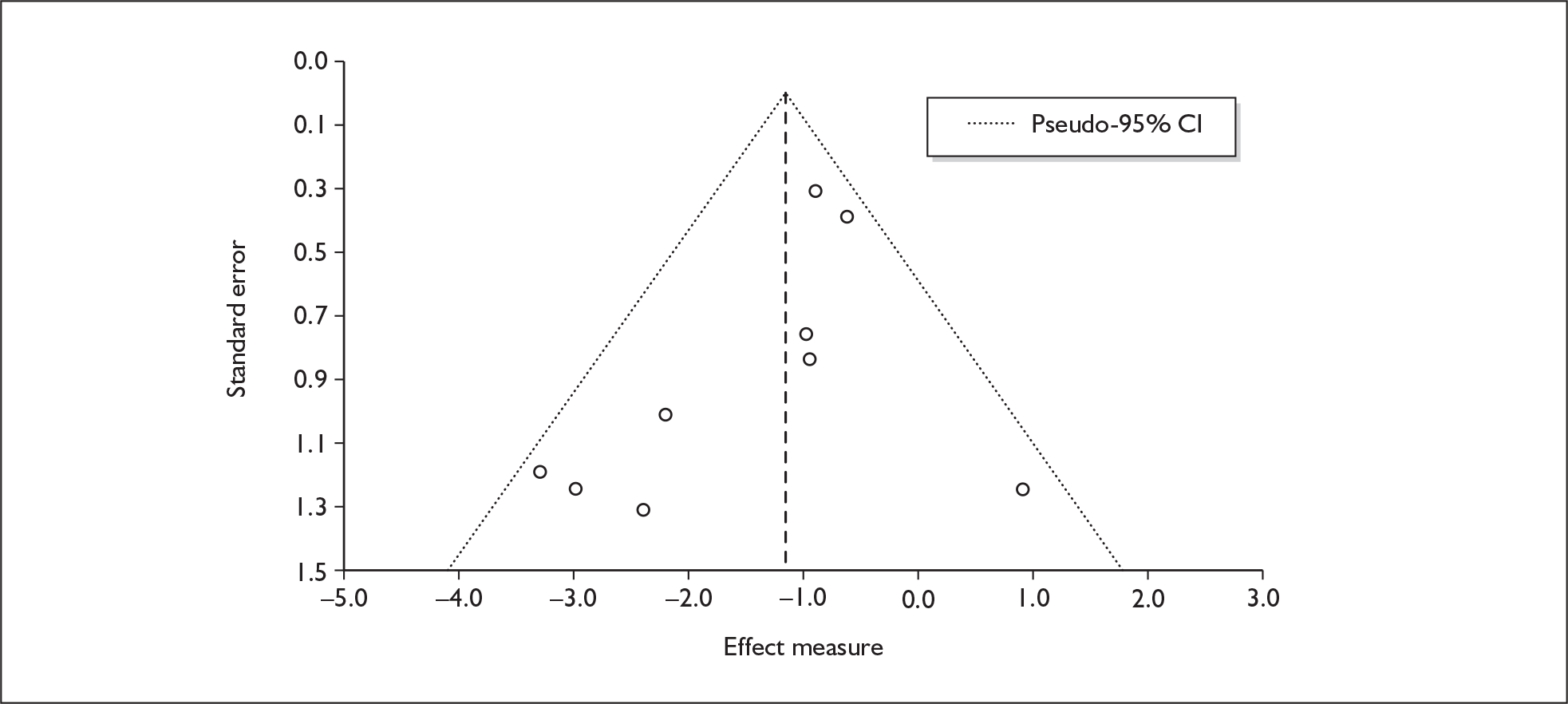
Sufficient data were available to attempt metaregression analyses for 13 covariates, shown in Table 6. There was a weak suggestion of a dose–response effect, with more extreme effects seen in participants with greater ETLD of ecstasy. However, this finding is based on a small and – visually, at least – not especially convincing dataset (see Figure 89 in Appendix 7).
| Covariate | Effect modification | Adjusted effect estimate | |||||
|---|---|---|---|---|---|---|---|
| n | β-coefficient | (95% CI) | p | WMD | (95% CI) | p | |
| Average values across all participants | |||||||
| Age (years) | 9 | –0.183 | (–0.398 to 0.032) | 0.084 | |||
| Sex (% male) | 9 | –1.138 | (–5.829 to 3.553) | 0.584 | |||
| IQ | 5 | 0.034 | (–0.420 to 0.488) | 0.826 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 6 | –0.002 | (–0.006 to 0.001) | 0.155 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 5 | 0.001 | (–0.017 to 0.018) | 0.931 | |||
| Duration of ecstasy use (days) | 8 | –0.001 | (–0.001 to 0.000) | 0.104 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 9 | 0.293 | (–0.123 to 0.710) | 0.140 | –1.090 | (–1.724 to –0.456) | 0.005 |
| Sex (% male) | 9 | –4.338 | (–10.024 to 1.347) | 0.114 | –1.055 | (–1.692 to –0.418) | 0.006 |
| Baseline intelligence measures (SMD) | 7 | –2.034 | (–3.977 to –0.091) | 0.043 | –2.082 | (–3.271 to –0.893) | 0.006 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 8 | –0.776 | (–1.850 to 0.297) | 0.127 | –0.743 | (–1.668 to 0.182) | 0.097 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 7 | 0.352 | (–2.677 to 3.381) | 0.777 | –1.770 | (–4.025 to 0.484) | 0.100 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 6 | –0.918 | (–3.963 to 2.128) | 0.450 | –0.796 | (–3.328 to 1.737) | 0.432 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 7 | 0.978 | (–1.469 to 3.425) | 0.351 | –1.768 | (–3.173 to –0.362) | 0.023 |
The one metaregression that did produce a p-value < 0.05 was that using asymmetry in baseline intelligence as covariate (Figure 8). However, the negative coefficient means that the direction of this effect is counterintuitive, suggesting that greater memory deficits can be expected whenever ecstasy-exposed cohorts are more intelligent than their comparators. It is difficult to explain this finding, so it is tempting to infer a Type I error, especially in the context of multiple testing.
FIGURE 8.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (delayed) (trial 8) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in baseline intelligence measures (standardised mean difference).
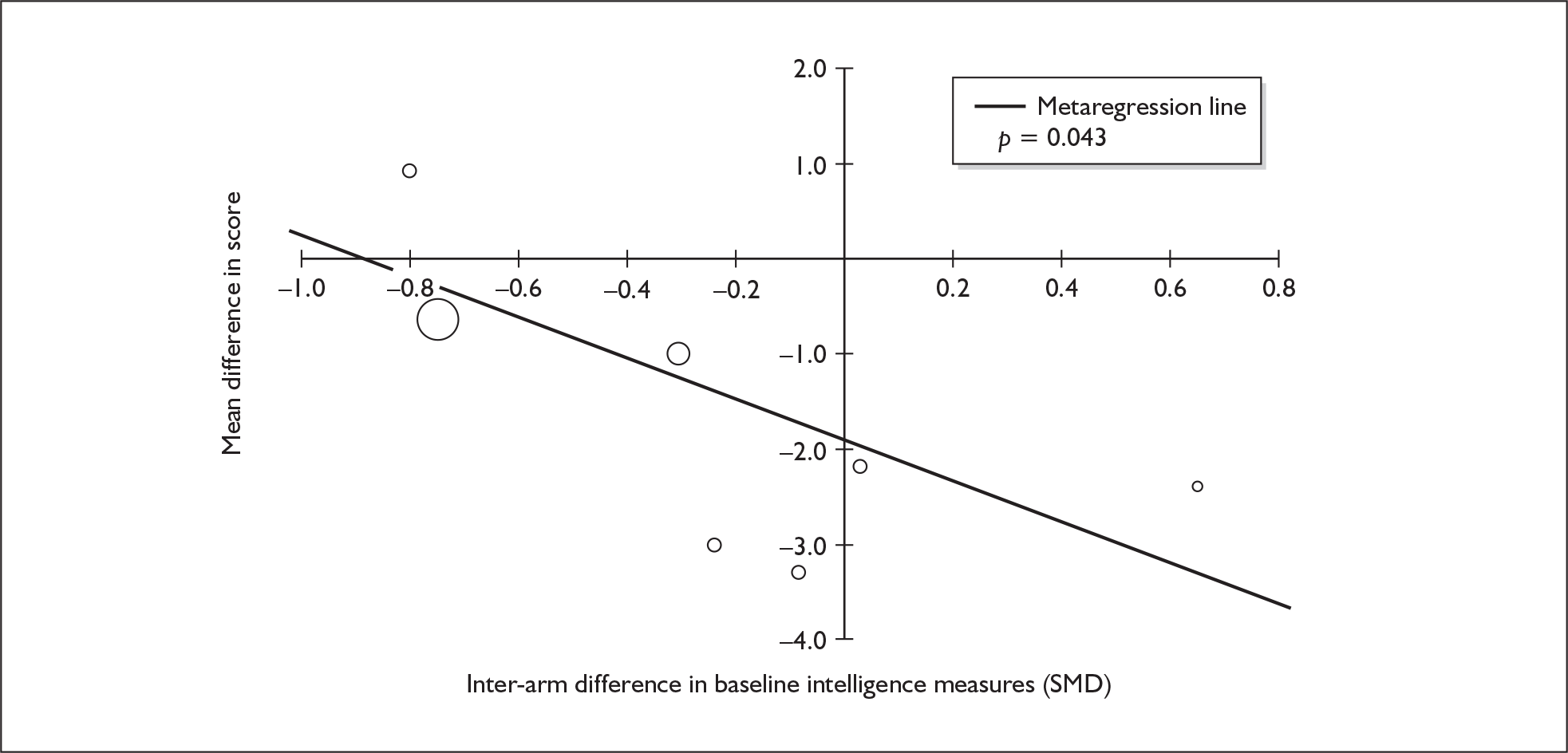
Rivermead Behavioural Memory Test prose recall (immediate) – MDMA users versus polydrug controls
The Rivermead Behavioural Memory Test (RBMT) is another instrument that is well represented in the assembled evidence-base. In particular, the prose (story) recall test was administered by enough investigators to make meta-analysis possible for both immediate and delayed memory (for the latter, see next section).
In total, this analysis includes 12 comparisons, drawn from six different studies (eight comparisons from six studies providing data for current ecstasy users and four comparisons from four studies providing data for former ecstasy users). One study was excluded from analysis because it presented only scaled scores. 101 We included data from the 2006 study by Reneman et al. ,97 which presents results as the sum of two consecutive administrations of the test, by halving the reported figures (although this may not provide an accurate estimate of dispersion).
When meta-analysed (Figure 9), these data suggest that ecstasy-exposed cohorts recall an average of two-thirds of an item fewer than polydrug controls. It should be noted that there is a fairly wide range of performance, with control group scores ranging from 4.3 to 9.5. Sensitivity analysis using the aggregated data approach generated a comparable effect estimate (MD – 0.720); however – because this approach was, in this instance, subject to greater uncertainty than the primary analysis – the evidence for an exposure effect has a less statistically robust appearance [95% CI – 1.572 to 0.133; p(null MD) = 0.098]. There is no evidence of small-study bias in this dataset (Egger’s p = 0.332), and the funnel plot (not shown) had an unremarkable appearance.
FIGURE 9.
Rivermead Behavioural Memory Test (RBMT) prose recall (immediate) – ecstasy users versus polydrug controls: random-effects meta-analysis.
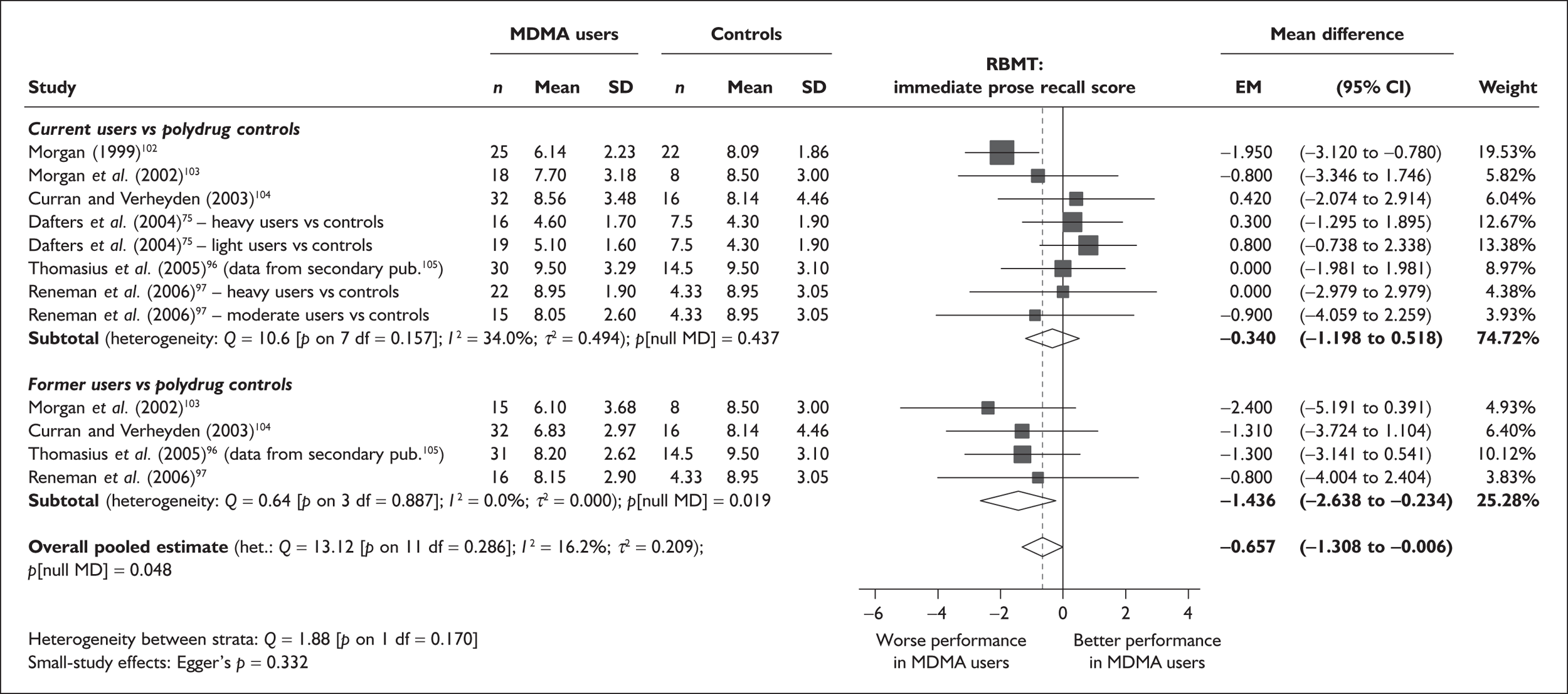
Sufficient data were available to attempt metaregression analyses for 12 covariates; details are shown in Table 7, with those analyses with results that achieved or approached conventional levels of significance discussed in detail below. There was no evidence of a dose–response effect (see Figure 90 in Appendix 7).
| Covariate | Effect modification | Adjusted effect estimate | |||||
|---|---|---|---|---|---|---|---|
| n | β-coefficient | (95% CI) | p | WMD | (95% CI) | p | |
| Average values across all participants | |||||||
| Age (years) | 12 | 0.007 | (–0.394 to 0.408) | 0.974 | |||
| Sex (% male) | 12 | 0.431 | (–3.658 to 4.520) | 0.836 | |||
| IQ | 9 | 0.077 | (–0.101 to 0.256) | 0.398 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 10 | 0.000 | (–0.003 to 0.003) | 0.995 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | 6 | 0.002 | (–0.004 to 0.009) | 0.470 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 12 | 0.088 | (–0.286 to 0.461) | 0.646 | –0.691 | (–1.379 to –0.002) | 0.049 |
| Sex (% male) | 12 | 4.170 | (–0.195 to 8.535) | 0.061 | –0.480 | (–1.099 to 0.139) | 0.128 |
| Baseline intelligence measures (SMD) | 10 | 1.656 | (0.522–2.792) | 0.004 | –0.471 | (–1.126 to 0.183) | 0.158 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 12 | –0.092 | (–1.336 to 1.152) | 0.885 | –0.615 | (–1.426 to 0.196) | 0.137 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 11 | 1.784 | (–0.074 to 3.642) | 0.060 | –1.848 | (–3.592 to –0.105) | 0.038 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 10 | 0.461 | (–0.943 to 1.864) | 0.520 | –0.730 | (–1.692 to 0.232) | 0.137 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 12 | 1.263 | (0.288–2.237) | 0.011 | –0.682 | (–1.257 to –0.107) | 0.020 |
The most statistically robust – and intuitively appealing – metaregression assesses the relationship between baseline intelligence imbalances and RBMT performance, as shown in Figure 10. The positive coefficient implies that, the greater the extent to which ecstasy users outperformed ecstasy-naïve participants on intelligence measures, the less they could be expected to suffer in comparison to controls when it came to the outcome of interest. This strongly suggests that the apparent exposure effect is confounded by this variable. The intercept of the metaregression – which, in an analysis of this type, provides an adjusted estimate of effect size accounting for the influence of the covariate – is, at – 0.471 (95% CI – 1.126 to 0.183), somewhat reduced compared to the primary analysis. More notably still, the hypothesis test assessing the evidence against a null effect appears much weaker (p = 0.158). According to this model, then, the apparent difference between cohorts is at least partially ascribable to unequal intelligence status.
FIGURE 10.
Rivermead Behavioural Memory Test (RBMT) prose recall (immediate) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in baseline intelligence measures (standardised mean difference).
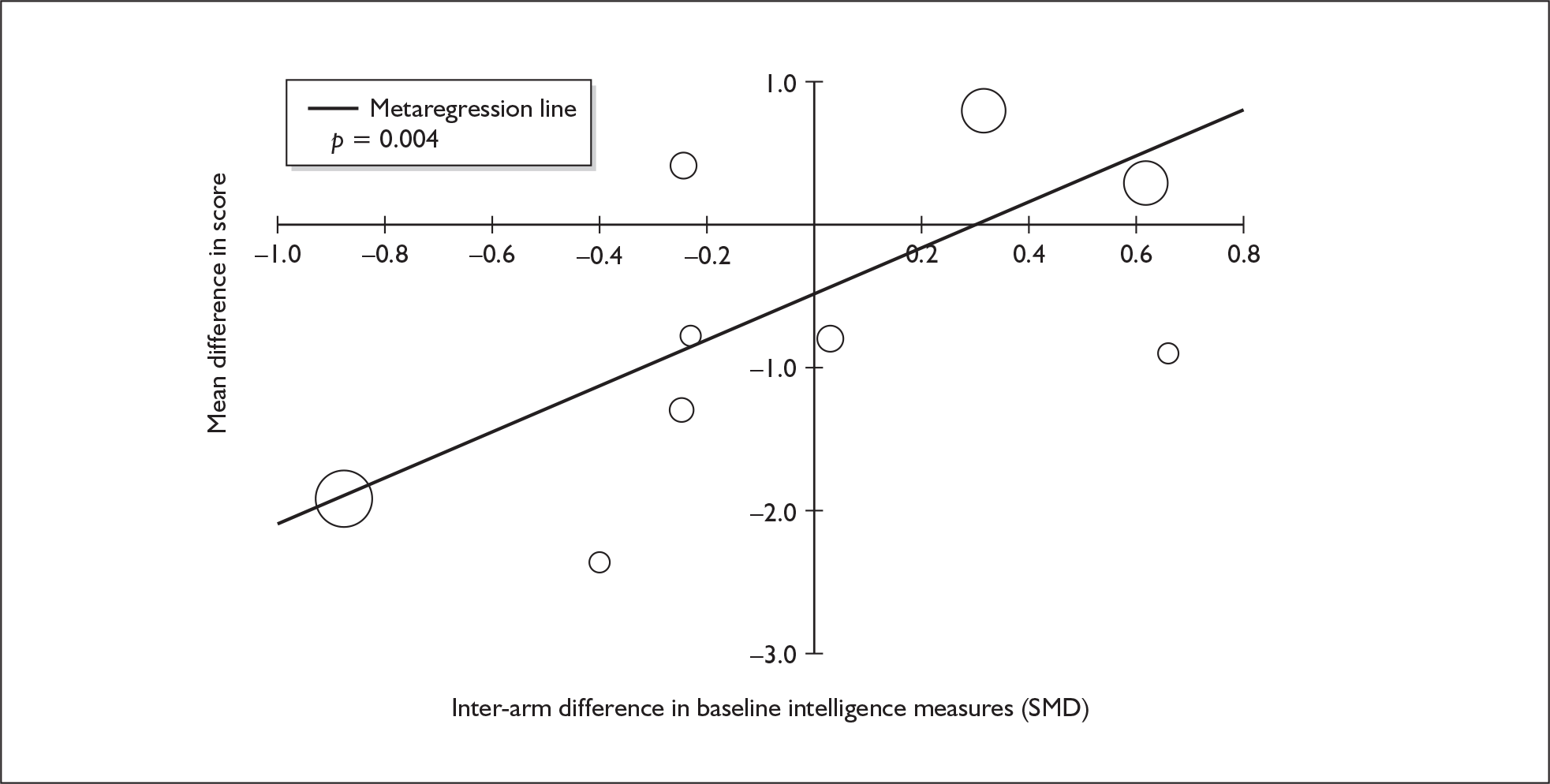
The second metaregression producing results that would, conventionally, be considered statistically significant covaries asymmetry in exposure to alcohol against the outcome of interest. The relationship between these variables is depicted in Figure 11. While, at first glance, this looks like a relatively strong association, it should be noted that a positive correlation is estimated, suggesting that those studies in which ecstasy-exposed participants exhibited better memory performance were those in which ecstasy users drank more alcohol than controls.
FIGURE 11.
Rivermead Behavioural Memory Test (RBMT) prose recall (immediate) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in exposure to alcohol (standardised mean difference).
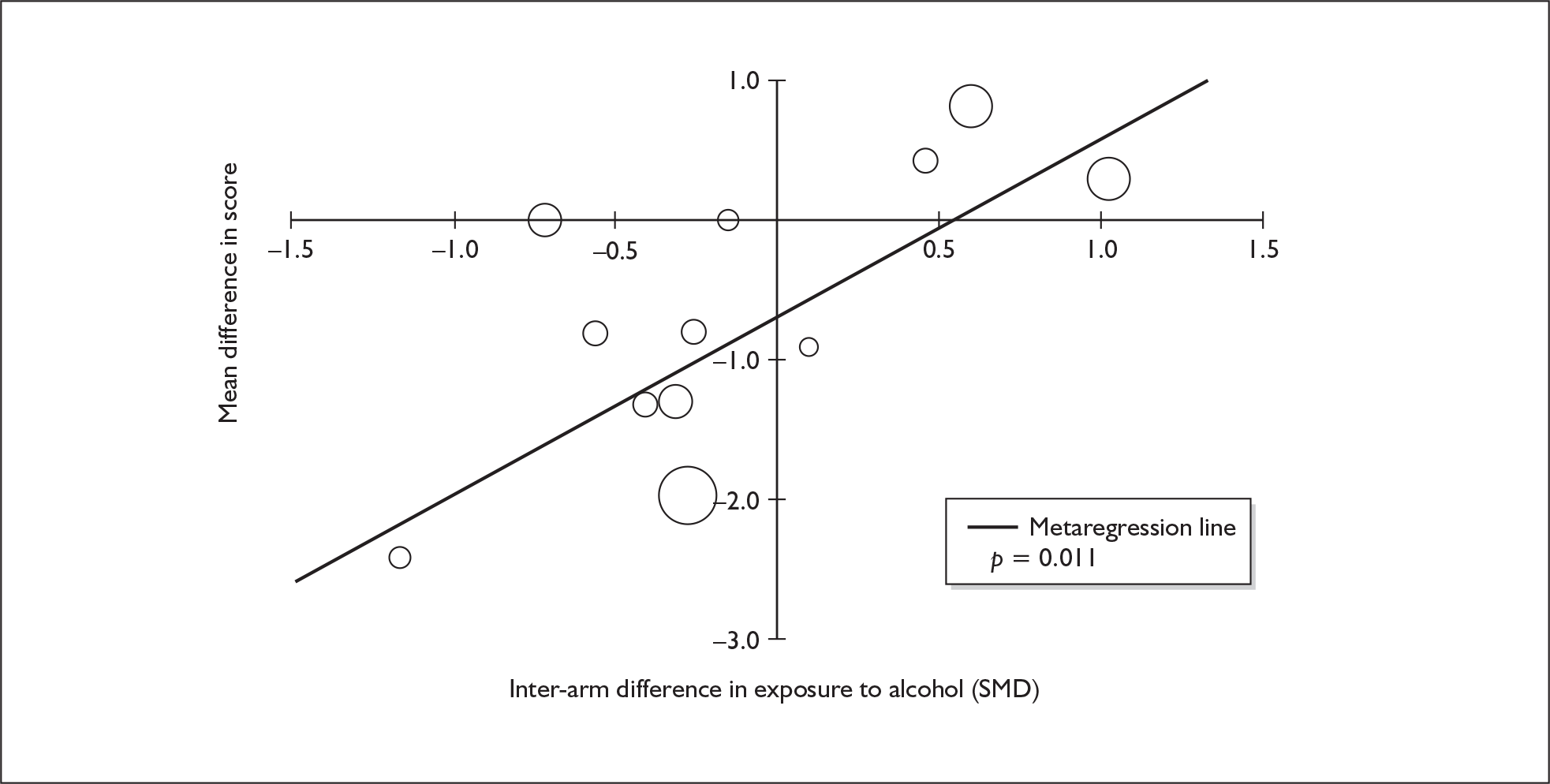
Rivermead Behavioural Memory Test prose recall (delayed) – MDMA users versus polydrug controls
This analysis includes the same 12 comparisons from six studies described for immediate recall, above. Once more, the study by Zakzanis et al. 101 was excluded, and the datapoints from Reneman et al. 97 were halved.
When meta-analysed (Figure 12), these data provide a very similar picture to that seen in the synthesis of immediate recall data (Figure 9), suggesting that ecstasy-exposed cohorts recall an average of around three-quarters of an item fewer than polydrug controls. Again, a fairly wide range of performance was seen, with control group scores ranging from 3.85 to 8.95. Sensitivity analysis using the aggregated data approach generated a similar effect estimate and, in this instance, the reanalysis retained an exposure effect that would conventionally be considered statistically significant [MD – 0.864; 95% CI – 1.688 to – 0.039; p(null MD) = 0.040].
FIGURE 12.
Rivermead Behavioural Memory Test (RBMT) prose recall (delayed) – ecstasy users versus polydrug controls: random-effects meta-analysis.
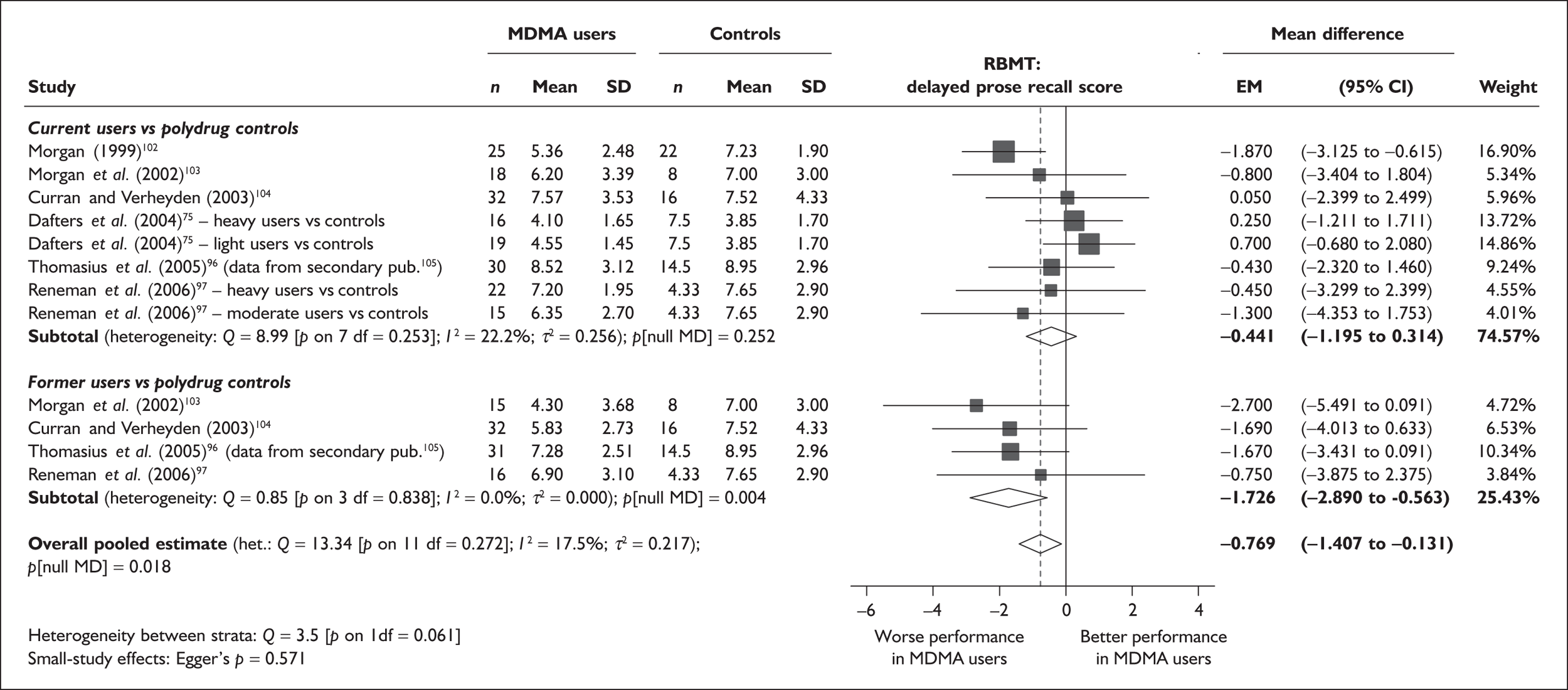
There is no evidence of small-study bias in this dataset (Egger’s p = 0.571), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 12 covariates; details are shown in Table 8, with those analyses with results that achieved or approached conventional levels of significance discussed in detail below. There was no evidence of a dose–response effect (see Figure 91 in Appendix 7).
| Covariate | Effect modification | Adjusted effect estimate | |||||
|---|---|---|---|---|---|---|---|
| n | β-coefficient | (95% CI) | p | WMD | (95% CI) | p | |
| Average values across all participants | |||||||
| Age (years) | 10 | –0.260 | (–0.805 to 0.285) | 0.350 | |||
| Sex (% male) | 12 | –0.084 | (–4.189 to 4.022) | 0.968 | |||
| IQ | 11 | 0.097 | (–0.021 to 0.215) | 0.108 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure: | |||||||
| ETLD (tablets) | 10 | –0.001 | (–0.004 to 0.002) | 0.646 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | 6 | 0.003 | (–0.008 to 0.015) | 0.569 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 10 | 0.306 | (–0.155 to 0.767) | 0.193 | –0.889 | (–1.642 to –0.136) | 0.021 |
| Sex (% male) | 12 | 4.119 | (–2.129 to 10.367) | 0.196 | –0.533 | (–1.280 to 0.214) | 0.162 |
| Baseline intelligence measures (SMD) | 12 | 1.511 | (0.366–2.657) | 0.010 | –0.668 | (–1.256 to –0.079) | 0.026 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 12 | –0.115 | (–1.686 to 1.456) | 0.886 | –0.783 | (–1.622 to 0.056) | 0.067 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 11 | 2.551 | (0.491–4.612) | 0.015 | –2.874 | (–4.926 to –0.822) | 0.006 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 10 | 0.661 | (–0.946 to 2.269) | 0.420 | –0.937 | (–1.904 to 0.029) | 0.057 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 12 | 1.379 | (0.447–2.311) | 0.004 | –0.838 | (–1.422 to –0.253) | 0.005 |
A pronounced positive correlation was found between baseline intelligence imbalances and RBMT delayed memory performance, as shown in Figure 13. This association – which closely reflects the results for RBMT immediate memory (see Figure 10) – suggests that results may be at least partially explained by asymmetry in intelligence. However, even when results are adjusted for this confounding, fairly strong evidence of an underlying exposure effect remains (p = 0.026).
FIGURE 13.
Rivermead Behavioural Memory Test (RBMT) prose recall (delayed) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in baseline intelligence measures (standardised mean difference).
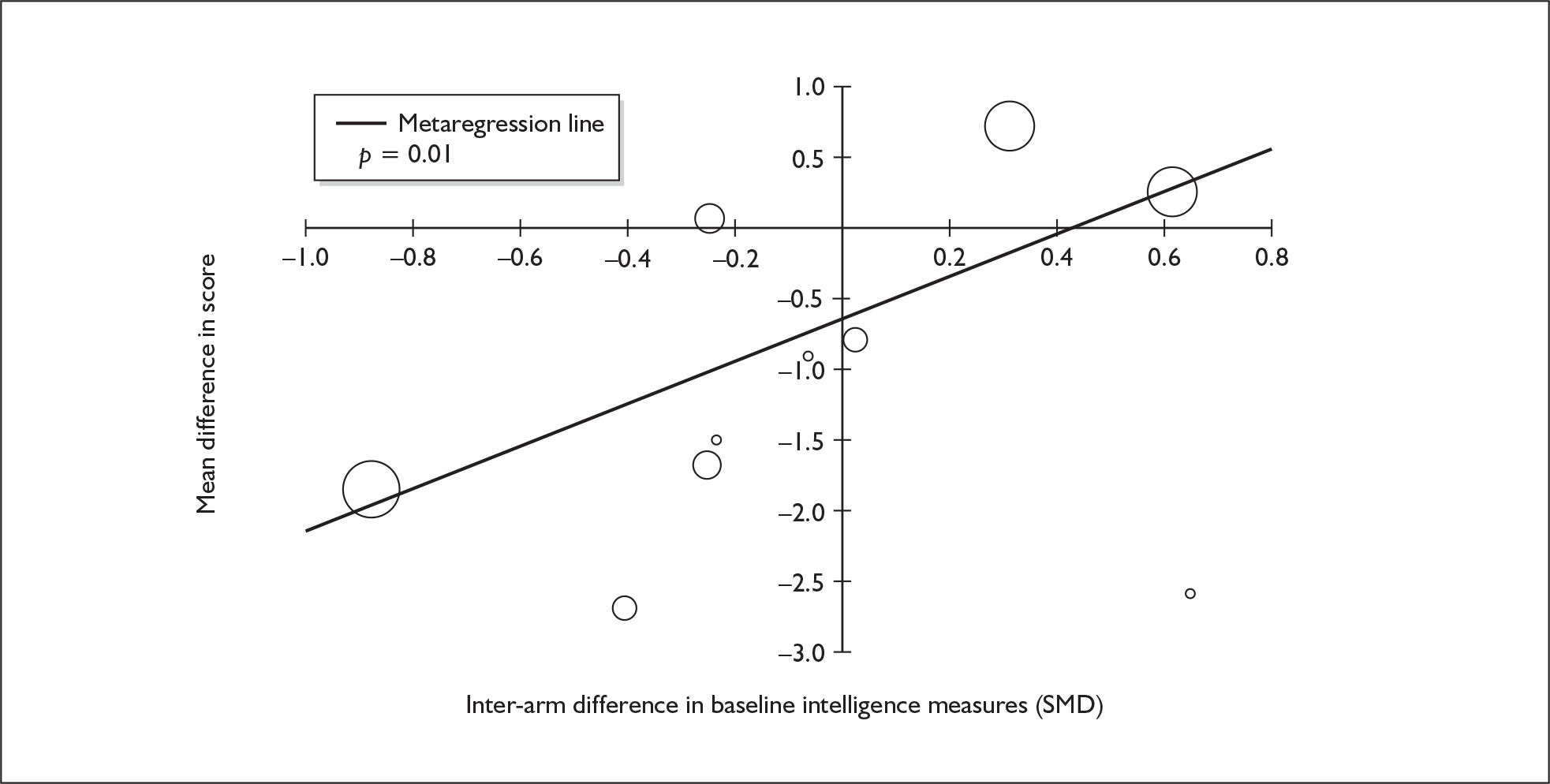
The effects of confounding in exposure to amphetamines and alcohol are shown in Figures 14 and 15 respectively. Once again, these findings are reminiscent of the results seen in equivalent metaregressions for RBMT immediate memory. In both cases, a fairly strong positive correlation is seen, again suggesting that the studies in which ecstasy users also had additional exposure to other substances, when compared to controls, were also those studies in which they performed best on the RBMT.
FIGURE 14.
Rivermead Behavioural Memory Test (RBMT) prose recall (delayed) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in exposure to amphetamines other than MDMA (standardised mean difference).
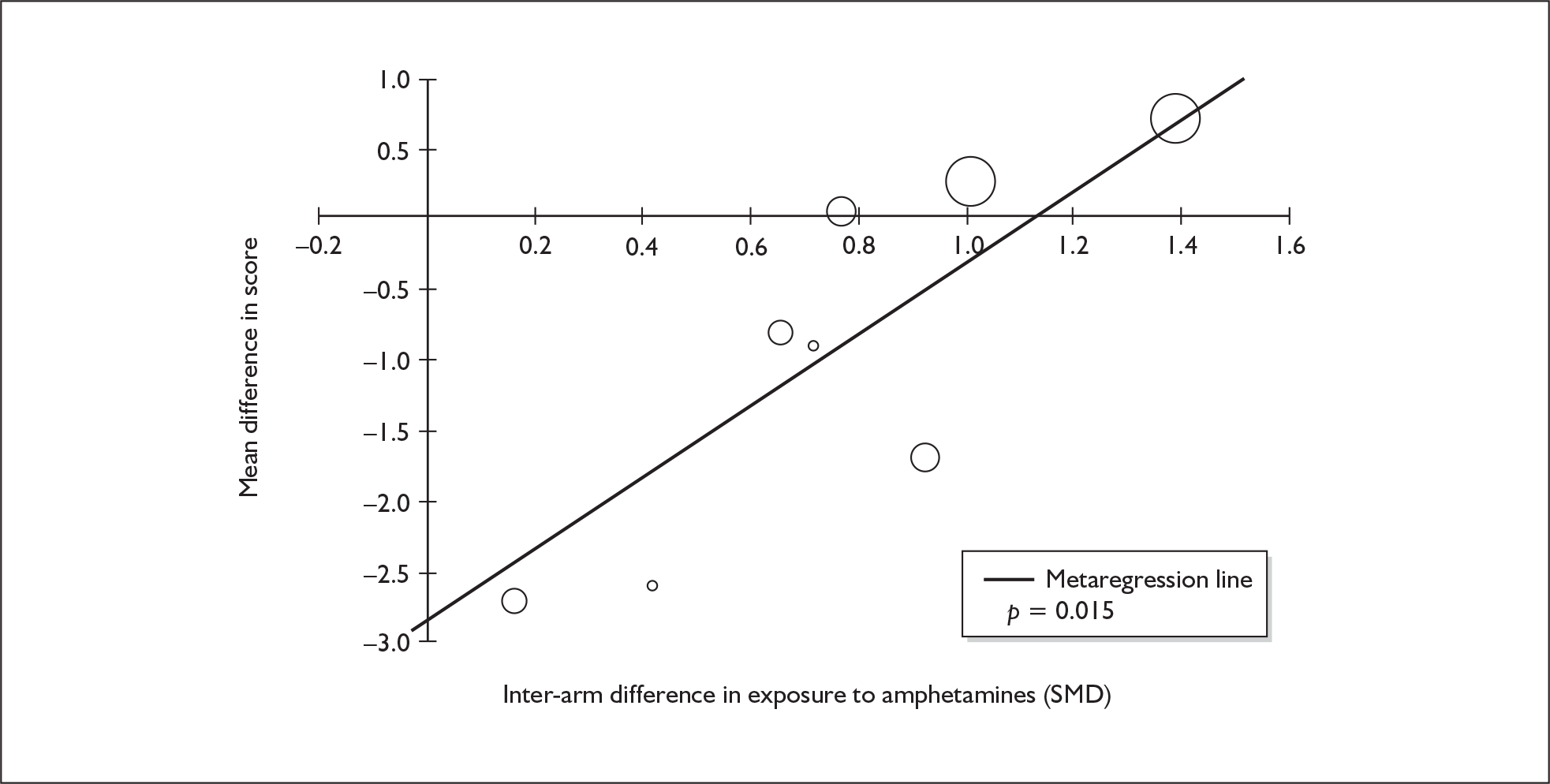
FIGURE 15.
Rivermead Behavioural Memory Test (RBMT) prose recall (delayed) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in exposure to alcohol (standardised mean difference).
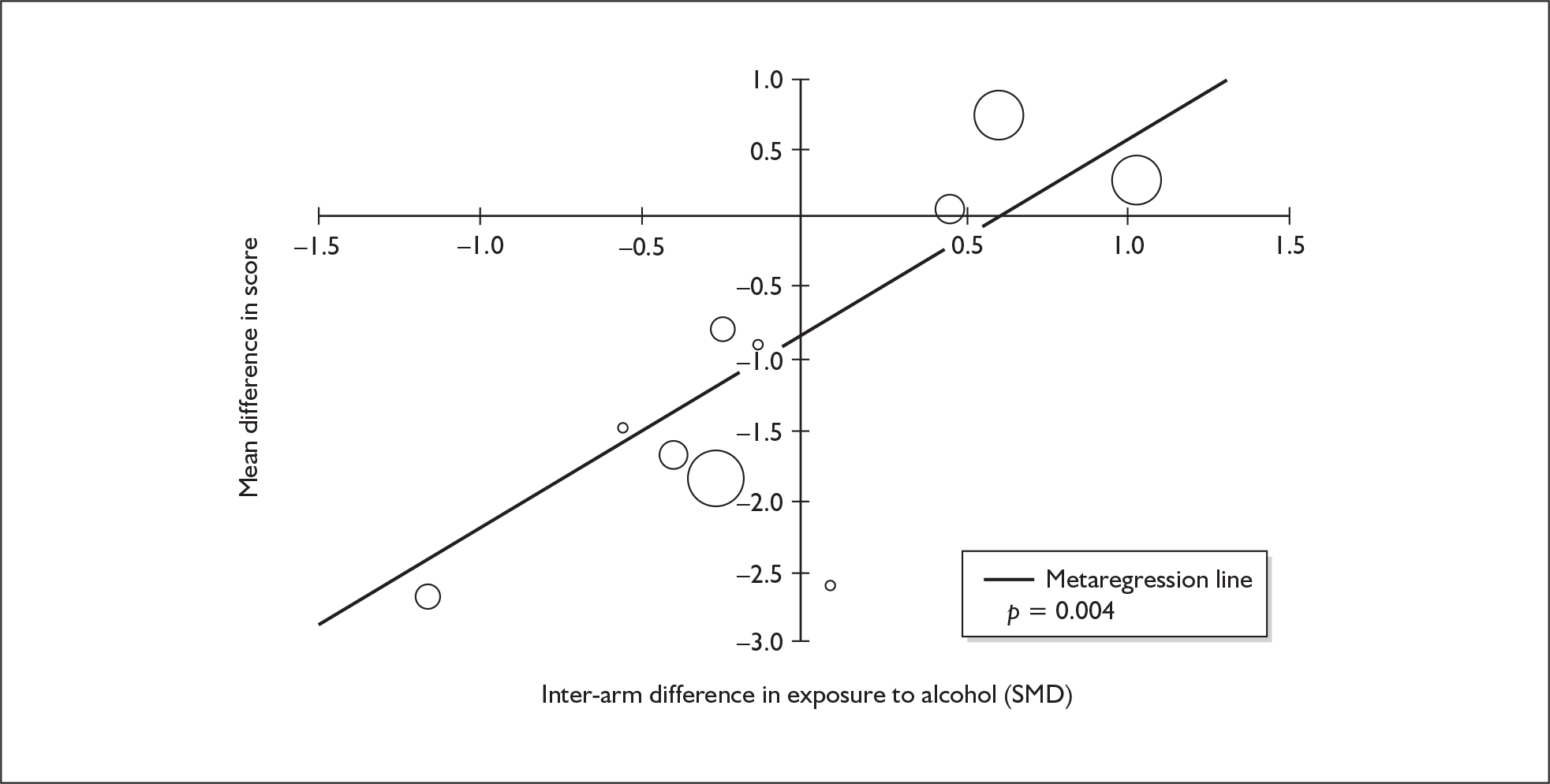
Because the analyses for immediate and delayed memory as measured by the RBMT are based on the same studies, and have very similar results, it is not a surprise to see analogous pictures emerging in metaregression analyses. With this in mind, it should be emphasised that the repetition of surprising results does not necessarily lend further credence to them. If the first paradoxical result-set is dismissed as a Type I error – that is to say, the apparently suggestive results have occurred by chance variation – then one might expect to see that artefactual pattern repeated in other analyses that are based on closely related data.
Digit span (forwards) – MDMA users versus polydrug controls
We identified seven comparisons, drawn from five studies, in which a forwards digit span was used to assess differences in verbal memory between ecstasy users and polydrug controls (six comparisons from five studies providing data for current ecstasy users and a single comparison providing data for former ecstasy users). The digit span data reported by de Win and colleagues90,91 was excluded from this analysis, because the investigators had used modified methods (with three instead of two series of digits per length), leading to scores that were not directly comparable with the other studies.
A random-effects meta-analysis of the identified data (Figure 16) suggests that ecstasy users have an average span of approximately 0.4 digits less than polydrug controls. This effect is just strong enough to achieve conventional statistical significance. Sensitivity analysis using the aggregated data approach generated a very similar effect estimate [MD – 0.412; 95% CI – 0.746 to – 0.078; p(null MD) = 0.016].
FIGURE 16.
Digit span (forwards) – ecstasy users versus polydrug controls: random-effects meta-analysis.
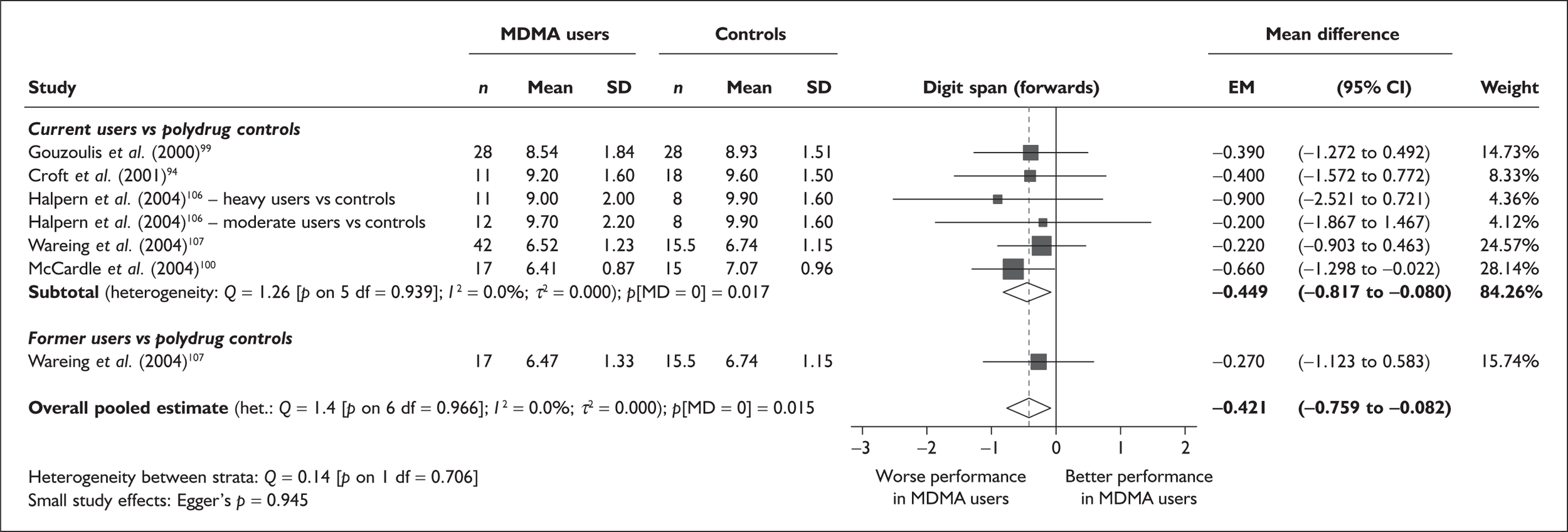
None of the average scores recorded by ecstasy users or controls are outside the normal range for this test (Lezak et al. 48 refer to any span of six or higher as ‘well within normal limits’).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.945), and the funnel plot (not shown) had an unremarkable appearance.
The small size of the dataset meant that we were able to attempt metaregression analyses for only five covariates, none of which provided any significant results. Details are shown in Table 9. There was no evidence of a dose–response effect (see Figure 92 in Appendix 7).
| Covariate | Effect modification | Adjusted effect estimate | |||||
|---|---|---|---|---|---|---|---|
| n | β-coefficient | (95% CI) | p | WMD | (95% CI) | p | |
| Average values across all participants | |||||||
| Age (years) | 5 | 0.059 | (–0.186 to 0.304) | 0.636 | |||
| Sex (% male) | < 5 | ||||||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | < 5 | ||||||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/months) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 5 | 0.020 | (–0.216 to 0.256) | 0.867 | –0.402 | (–0.762 to –0.041) | 0.029 |
| Sex (% male) | < 5 | ||||||
| Baseline intelligence measures (SMD) | 5 | 0.723 | (–1.860 to 3.306) | 0.583 | 0.049 | (–1.327 to 1.426) | 0.944 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
Digit span (backwards) – MDMA users versus polydrug controls
We identified eight comparisons, drawn from six studies, in which a backwards digit span was used to assess differences in verbal memory between ecstasy users and polydrug controls (all data related to current ecstasy users). Once more, we excluded data from the studies by de Win’s group,90,91 because of their inconsistent methods.
Meta-analysed results (Figure 17) are fairly similar to those seen in the forwards digit span, with a significant difference between ecstasy users and controls of about 0.6 digits. Again, all average scores appear to be within the normal range. Sensitivity analysis using the aggregated data approach generated a very similar effect estimate [MD – 0.638; 95% CI – 1.096 to – 0.181; p(null MD) = 0.006].
FIGURE 17.
Digit span (backwards) – ecstasy users versus polydrug controls: random-effects meta-analysis.
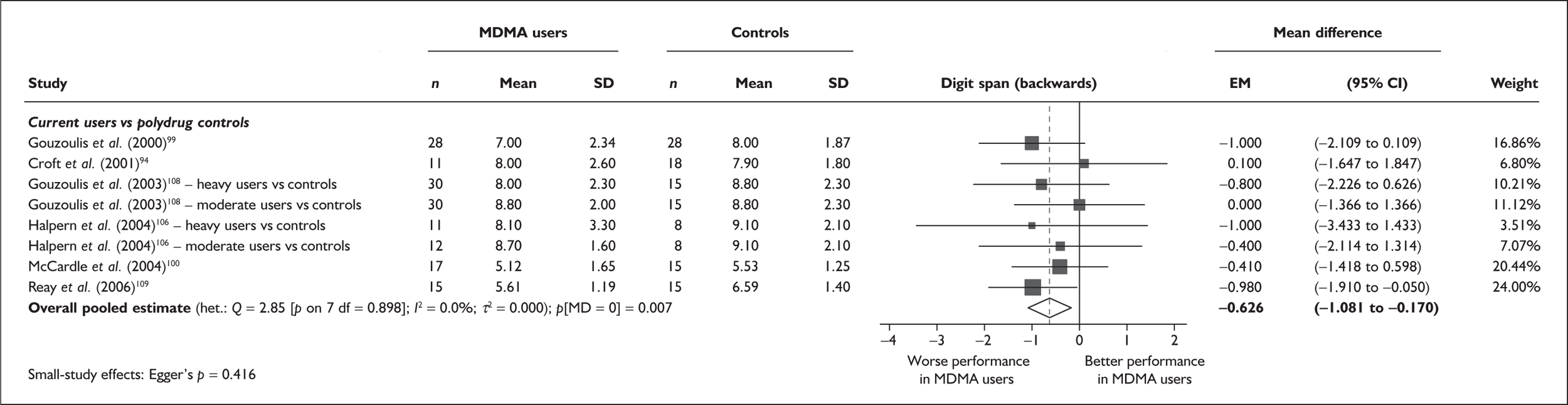
There is no evidence of small-study bias in this dataset (Egger’s p = 0.416), and the funnel plot (not shown) had an unremarkable appearance.
There were sufficient data to attempt metaregression analyses for seven covariates; details are shown in Table 10. None of the analyses provided significant results, and there was no evidence of a dose–response effect (see Figure 93 in Appendix 7).
| Covariate | Effect modification | Adjusted effect estimate | |||||
|---|---|---|---|---|---|---|---|
| n | β-coefficient | (95% CI) | p | WMD | (95% CI) | p | |
| Average values across all participants | |||||||
| Age (years) | 6 | 0.082 | (–0.250 to 0.414) | 0.628 | |||
| Sex (% male) | 6 | 2.416 | (–1.974 to 6.806) | 0.281 | |||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | < 5 | ||||||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (day) | < 5 | ||||||
| Duration of ecstasy use (day) | 5 | –0.001 | (–0.002 to 0.001) | 0.309 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 6 | –0.149 | (–0.395 to 0.098) | 0.237 | –0.541 | (–1.044 to –0.038) | 0.035 |
| Sex (% male) | 6 | –2.492 | (–6.390 to 1.406) | 0.210 | –0.642 | (–1.125 to –0.160) | 0.009 |
| Baseline intelligence measures (SMD) | 5 | 1.172 | (–0.737 to 3.081) | 0.229 | –0.127 | (–1.191 to 0.937) | 0.815 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 6 | 0.220 | (–0.558 to 0.998) | 0.580 | –0.826 | (–1.672 to 0.021) | 0.056 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
IQ (National Adult Reading Test) – MDMA users versus polydrug controls
Of all the measures in the assembled evidence-base, the most frequently reported was IQ as measured by the National Adult Reading Test (it should be noted that we include here studies using foreign-language translations of the test). In the majority of cases, investigators did not present these data as outcomes of interest in their own studies, but rather used them to estimate the underlying intelligence of their participants (the most notable reason for doing so being to ensure a reasonable balance between cohorts). Nevertheless, we have included the National Adult Reading Test as an outcome measure of interest in our analyses because we believed it was reasonable to look for differences between populations with regard to this measure. Of course, if the assumptions underpinning most investigators’ use of the test are correct, then we would hope to see no difference between ecstasy-exposed and ecstasy-naïve populations.
Figure 18 shows our random-effects meta-analysis of these data. It suggests that, while the IQs of ecstasy-exposed individuals were rated an average of 0.321 points lower than those of polydrug controls, this is unlikely to represent a significant difference (p = 0.498). Interestingly, the evidence of lower IQ scores among ecstasy users was somewhat stronger in the ex-users’ stratum: former users had IQs an average of 2.75 points lower than controls, with reasonable evidence against a null effect (p = 0.035). It should be noted that this finding is based on a relatively small number of studies, so high susceptibility to Type I error may be inferred. In comparisons between current ecstasy users and polydrug controls, the difference between groups was very nearly zero.
FIGURE 18.
IQ (National Adult Reading Test) – ecstasy users versus polydrug controls: random-effects meta-analysis.
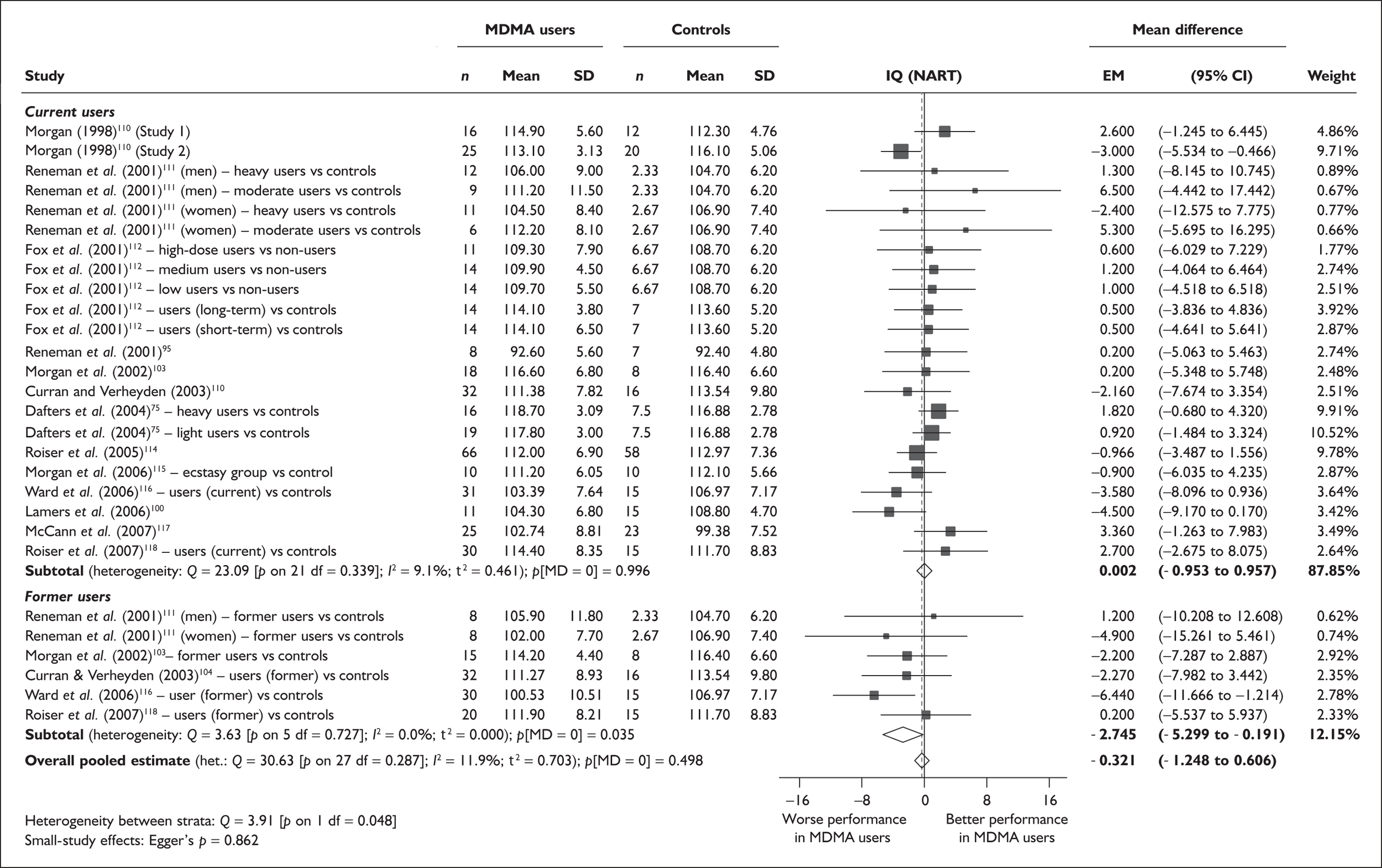
Sensitivity analysis with single, pooled comparisons for each study suggests that our primary analysis may very slightly underestimate the discrepancy between cohorts (by less than 0.1 of an IQ point: MD 0.418; 95% CI – 1.614 to 0.778). As might be expected, the evidence of a difference between cohorts is equally weak in this analysis (p = 0.493).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.862), and the funnel plot (not shown) had an unremarkable appearance.
In total, sufficient data were available to attempt metaregression analyses for 16 covariates; details are shown in Table 11. It should be noted that, in other analyses in this review, we have used baseline intelligence measures as an explanatory variable in metaregression analyses. In this instance, where the intelligence of study participants is the response variable of interest, these covariates have been excluded.
| Covariate | Effect modification | Adjusted effect estimate | |||||
|---|---|---|---|---|---|---|---|
| n | β-coefficient | (95% CI) | p | WMD | (95% CI) | p | |
| Average values across all participants | |||||||
| Age (years) | 28 | 0.080 | (–0.401 to 0.561) | 0.744 | |||
| Sex (% male) | 28 | –2.720 | (–8.875 to 3.434) | 0.386 | |||
| IQ | |||||||
| Education (years) | 12 | –1.017 | (–3.443 to 1.410) | 0.411 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 19 | 0.001 | (–0.003 to 0.005) | 0.646 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 21 | –0.001 | (–0.005 to 0.003) | 0.643 | |||
| Duration of ecstasy use (days) | 17 | –0.001 | (–0.004 to 0.002) | 0.620 | |||
| Frequency of ecstasy use (occasions/month) | 6 | –0.107 | (–1.440 to 1.227) | 0.875 | |||
| Inter-arm differences | |||||||
| Age (years) | 28 | –0.152 | (–0.574 to 0.271) | 0.481 | –0.303 | (–1.254 to 0.647) | 0.532 |
| Sex (% male) | 28 | 0.715 | (–4.922 to 6.353) | 0.804 | –0.364 | (–1.374 to 0.645) | 0.479 |
| Baseline intelligence measures (SMD) | |||||||
| Education (years) | 12 | –0.780 | (–2.641 to 1.082) | 0.412 | –2.343 | (–5.793 to 1.108) | 0.183 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 21 | 0.691 | (–0.106 to 1.488) | 0.089 | –0.414 | (–1.442 to 0.614) | 0.430 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 20 | 0.285 | (–1.616 to 2.185) | 0.769 | –0.227 | (–1.720 to 1.266) | 0.766 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 17 | –0.103 | (–1.710 to 1.503) | 0.900 | –0.210 | (–2.006 to 1.585) | 0.818 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 21 | 1.918 | (0.142–3.694) | 0.034 | –0.506 | (–1.540 to 0.529) | 0.338 |
There was no evidence of a dose–response effect: cohorts with high exposure to ecstasy were no more disadvantaged against controls than those who had consumed comparatively little (see Figure 94 in Appendix 7).
Figure 19 compares mean difference in IQ with asymmetry in the amount of alcohol exposure between study arms. It should be noted that this analysis generates a positive coefficient, suggesting that those studies in which higher IQs were found in the ecstasy-exposed participants were those in which ecstasy users drank more alcohol than controls. However, because this dataset shows a reasonable balance across the spectrum of imbalance of alcohol exposure, this variable has little influence on the estimated average effect of ecstasy exposure: the adjusted mean difference is less than 0.1 IQ points greater (– 0.506; 95% CI – 1.540 to 0.529), and just as consistent with a null difference (p = 0.338).
FIGURE 19.
IQ (National Adult Reading Test) – Ecstasy users versus polydrug controls: mean difference in IQ against inter-arm asymmetry in exposure to alcohol (standardised mean difference).
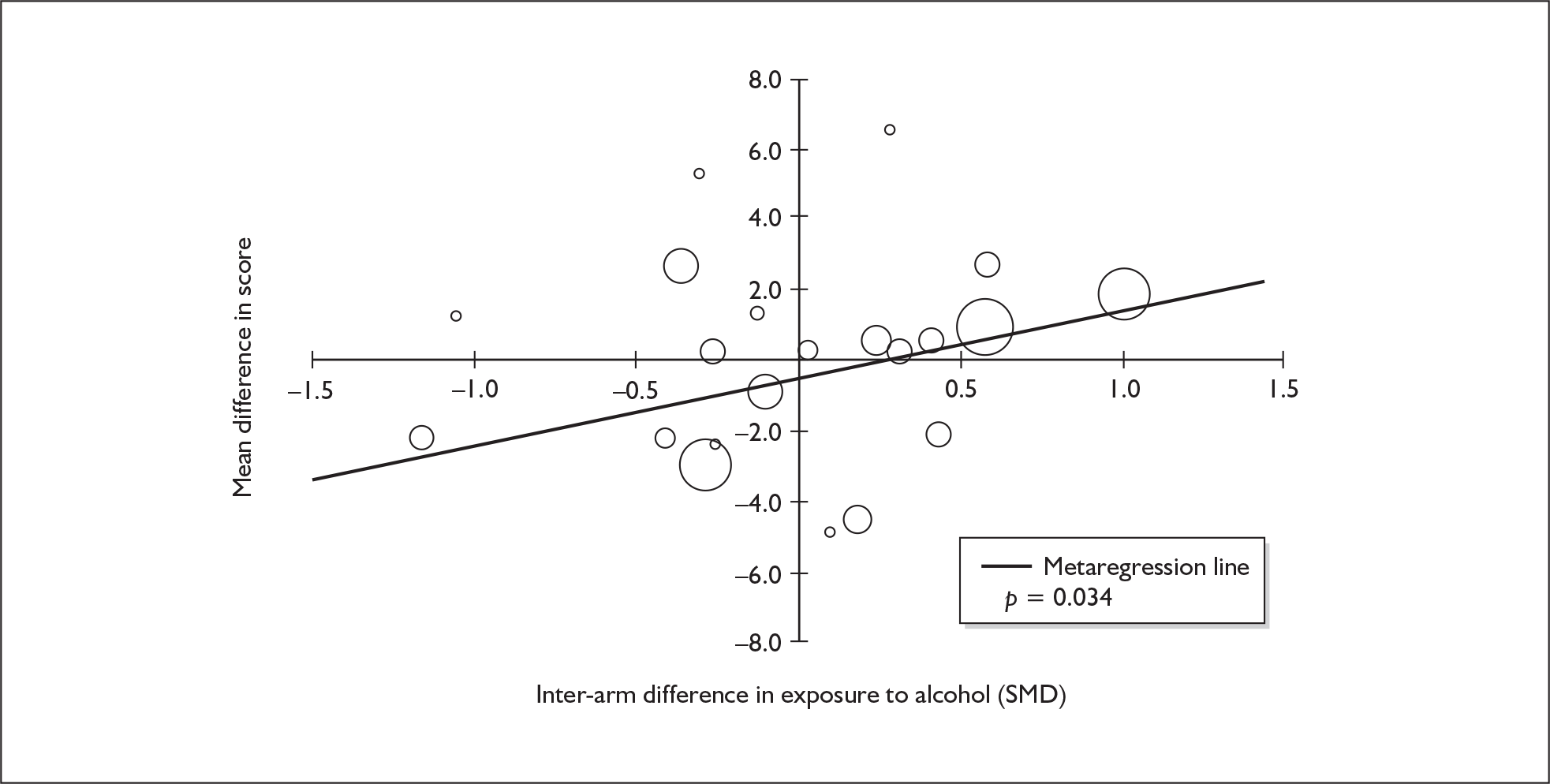
IQ (National Adult Reading Test) – MDMA users versus drug-naïve controls
As in the comparison with polydrug controls, we included studies using foreign-language translations of the test.
Figure 20 shows our random-effects meta-analysis of these data. It shows an extremely similar picture to that seen in the comparison with polydrug controls. Ecstasy-exposed individuals’ IQs rated an average of 0.474 points lower than those of polydrug controls, but this is unlikely to represent a significant difference (p = 0.417). Again, ex-users appear more disadvantaged than current users although, in this case, the former users’ stratum is even more underpowered (comprising only two datapoints). In the comparison between current ecstasy users and controls, a non-significant average difference of less than 0.4 IQ points was seen.
FIGURE 20.
IQ (National Adult Reading Test) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
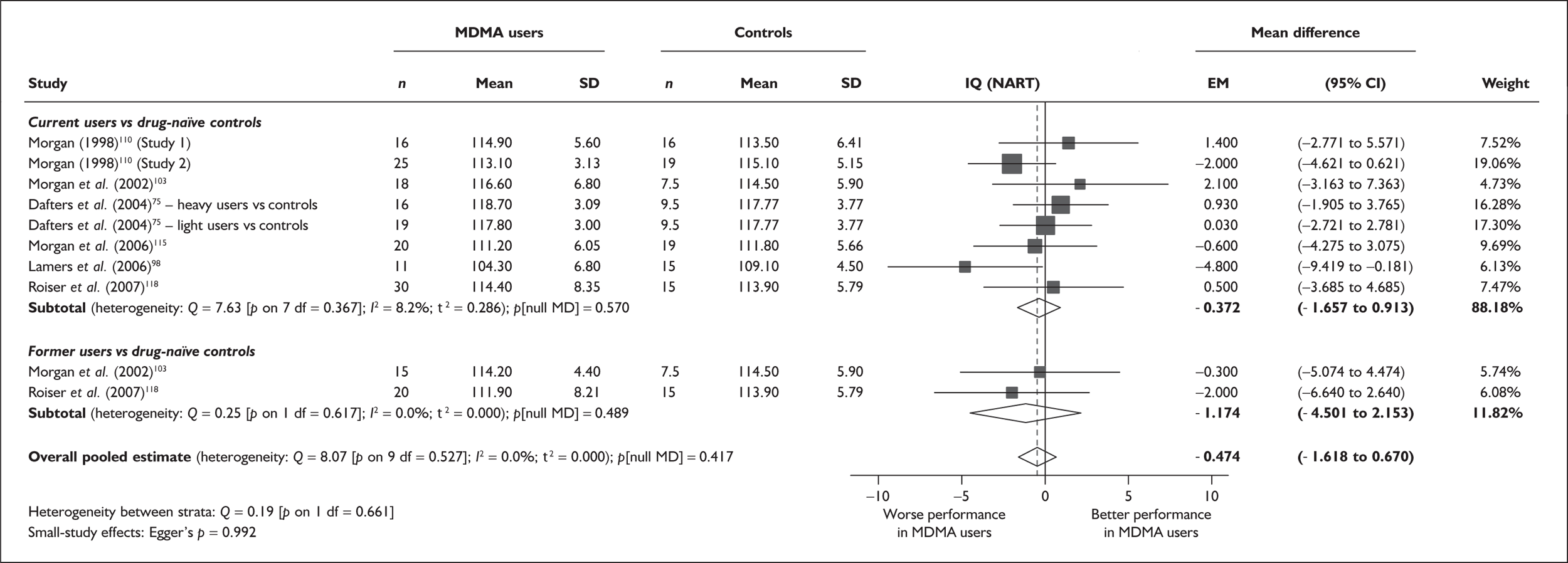
Sensitivity analysis with single, pooled comparisons for each study generated results very similar to those of the primary analysis (MD – 0.491; 95% CI – 1.755 to 0.772; null effect p = 0.446).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.992), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for nine covariates; details are shown in Table 12. Once more, we excluded intelligence measures as explanatory variables. None of the analyses were able to provide a statistically convincing explanation of the heterogeneity seen amongst base-case effect estimates. There was no evidence of a dose–response effect (see Figure 95, in Appendix 7).
| Covariate | Effect modification | Adjusted effect estimate | |||||
|---|---|---|---|---|---|---|---|
| n | β-coefficient | (95% CI) | p | WMD | (95% CI) | p | |
| Average values across all participants | |||||||
| Age (years) | 8 | –0.610 | (–1.953 to 0.734) | 0.374 | |||
| Sex (% male) | 10 | –12.053 | (–31.529 to 7.424) | 0.225 | |||
| IQ | |||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 7 | 0.000 | (–0.006 to 0.006) | 0.963 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 5 | –0.002 | (–0.008 to 0.004) | 0.588 | |||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/months) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 8 | 0.350 | (–0.506 to 1.206) | 0.423 | –0.995 | (–2.540 to 0.549) | 0.207 |
| Sex (% male) | 10 | –3.601 | (–17.401 to 10.200) | 0.609 | –0.329 | (–1.601 to 0.942) | 0.612 |
| Baseline intelligence measures (SMD) | |||||||
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 8 | –0.682 | (–2.828 to 1.463) | 0.533 | 0.012 | (–1.591 to 1.616) | 0.988 |
Syntheses: controlled (Level II) evidence – composite measures
We identified a total of 915 discrete outcome measures, measured according to 135 different instruments, among the Level II evidence. These were mapped into a series of 38 domains (‘meta-outcomes’). Full details of the mapping, along with abbreviations by which instruments are referred to in this section, are provided in Appendix 5.
Of the 38 outcome domains, 16 represented small collections of data that were not amenable to any form of synthesis, either because they comprised measures that were too general to fit among our domains (e.g. measures that sought to tap ‘memory’ as a single construct) or because they examined single, specific factors that could not be combined with other items in the evidence-base (e.g. ‘orientation’). These data were not analysed further. A further six meta-outcomes were identified as sensible units of analysis, but provided insufficient data for meaningful quantitative synthesis; these are considered in Other Level II outcome measures (see p.133).
The remainder of available data – mapped into a total of 16 composite domains – was sufficiently complete to make meta-analysis possible. It was possible to derive an effect estimate for ecstasy users compared to polydrug controls in all 16 cases, and for ecstasy users compared to drug-naïve controls in 11 of the domains. These analyses are summarised in Tables 13 and 14, respectively.
| Current ecstasy users versus controls | Former ecstasy users versus controls | All ecstasy exposed versus controls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | EM | (95% CI) | p | Studies | EM | (95%CI) | p | Studies | EM | (95% CI) | p | ||
| Verbal memory (immediate) | Figure 21 | 35 | –0.342 | (–0.468 to –0.217) | < 0.001 | 5 | –0.269 | (–0.638 to 0.101) | 0.154 | 40 | –0.332 | (–0.451 to –0.214) | < 0.001 |
| Verbal memory (delayed) | Figure 27 | 27 | –0.357 | (–0.495 to –0.220) | < 0.001 | 5 | –0.468 | (–0.720 to –0.216) | < 0.001 | 32 | –0.377 | (–0.498 to –0.257) | < 0.001 |
| Visual memory (immediate) | Figure 31 | 19 | –0.151 | (–0.295 to –0.007) | 0.040 | 3 | –0.064 | (–0.277 to 0.149) | 0.557 | 22 | –0.143 | (–0.270 to –0.016) | 0.027 |
| Visual memory (delayed) | Figure 38 | 12 | –0.180 | (–0.327 to –0.034) | 0.016 | 2 | –0.213 | (–0.647 to 0.221) | 0.336 | 14 | –0.184 | (–0.323 to –0.045) | 0.010 |
| Working memory | Figure 41 | 19 | –0.361 | (–0.579 to –0.144) | 0.001 | 3 | –0.649 | (–0.960 to –0.337) | < 0.001 | 22 | –0.391 | (–0.589 to –0.192) | < 0.001 |
| Attention (focus–execute) | Figure 46 | 26 | –0.240 | (–0.351 to –0.128) | < 0.001 | 4 | –0.157 | (–0.324 to 0.010) | 0.065 | 30 | –0.226 | (–0.323 to –0.130) | < 0.001 |
| Attention (sustain) | Figure 49 | 8 | –0.086 | (–0.288 to 0.115) | 0.401 | 3 | 0.136 | (–0.608 to 0.880) | 0.719 | 11 | –0.029 | (–0.238 to 0.180) | 0.784 |
| Executive function (planning) | Figure 54 | 10 | –0.150 | (–0.291 to –0.010) | 0.036 | 0 | – | – | – | 11 | –0.176 | (–0.324 to –0.028) | 0.020 |
| Executive function (response inhibition) | Figure 57 | 17 | –0.133 | (–0.360 to 0.093) | 0.247 | 3 | 0.120 | (–0.238 to 0.477) | 0.512 | 20 | –0.103 | (–0.303 to 0.097) | 0.314 |
| Executive function (shifting) | Figure 61 | 12 | –0.199 | (–0.516 to 0.118) | 0.218 | 0 | – | – | – | 13 | –0.184 | (–0.483 to 0.115) | 0.228 |
| Perceptual organisation | Figure 62 | 19 | –0.151 | (–0.295 to –0.007) | 0.040 | 3 | –0.064 | (–0.277 to 0.149) | 0.557 | 22 | –0.143 | (–0.270 to –0.016) | 0.027 |
| Depression (self-rated) | Figure 64 | 33 | –0.247 | (–0.361 to –0.133) | < 0.001 | 5 | –0.503 | (–0.804 to –0.202) | 0.001 | 38 | –0.272 | (–0.377 to –0.167) | < 0.001 |
| Memory (self-rated) | Figure 70 | 8 | –0.509 | (–0.690 to –0.328) | < 0.001 | 0 | – | – | – | 8 | –0.509 | (–0.690 to –0.328) | < 0.001 |
| Anxiety (self-rated) | Figure 72 | 27 | –0.249 | (–0.401 to –0.096) | 0.001 | 5 | –0.380 | (–0.673 to –0.086) | 0.011 | 32 | –0.263 | (–0.396 to –0.130) | < 0.001 |
| Impulsivity (objective measures) | Figure 76 | 9 | –0.247 | (–0.495 to 0.001) | 0.051 | 0 | – | – | – | 10 | –0.200 | (–0.417 to 0.017) | 0.071 |
| Impulsivity (subjective measures) | Figure 81 | 12 | –0.387 | (–0.643 to –0.130) | 0.003 | 2 | –0.437 | (–0.889 to 0.015) | 0.058 | 14 | –0.394 | (–0.616 to –0.173) | < 0.001 |
| Current ecstasy users versus controls | Former ecstasy users versus controls | All ecstasy exposed versus controls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | EM | (95% CI) | p | Studies | EM | (95% CI) | p | Studies | EM | (95% CI) | p | ||
| Verbal memory (immediate) | Figure 25 | 14 | –0.852 | (–1.031 to –0.672) | < 0.001 | 4 | –0.792 | (–1.053 to –0.531) | < 0.001 | 18 | –0.840 | (–0.990 to –0.690) | < 0.001 |
| Verbal memory (delayed) | Figure 29 | 14 | –1.114 | (–1.994 to –0.233) | 0.013 | 4 | –0.732 | (–1.044 to –0.421) | < 0.001 | 18 | –1.037 | (–1.734 to –0.341) | 0.004 |
| Visual memory (immediate) | Figure 37 | 6 | –0.177 | (–0.489 to 0.135) | 0.266 | 0 | – | – | – | 7 | –0.173 | (–0.418 to 0.071) | 0.165 |
| Visual memory (delayed) | Figure 39 | 6 | –0.409 | (–1.244 to 0.426) | 0.337 | 2 | –0.283 | (–0.705 to 0.139) | 0.189 | 8 | –0.366 | (–1.014 to 0.283) | 0.269 |
| Working memory | Figure 45 | 6 | –0.459 | (–0.862 to –0.056) | 0.025 | 0 | – | – | – | 7 | –0.505 | (–0.868 to –0.143) | 0.006 |
| Attention (focus–execute) | Figure 48 | 14 | –0.254 | (–0.422 to –0.085) | 0.003 | 2 | –0.436 | (–0.852 to –0.019) | 0.040 | 16 | –0.272 | (–0.424 to –0.120) | < 0.001 |
| Attention (sustain) | 4 | 0.159 | (–0.180 to 0.498) | 0.358 | |||||||||
| Executive function (planning) | |||||||||||||
| Executive function (response inhibition) | Figure 59 | 8 | –0.137 | (–0.348 to 0.074) | 0.204 | 2 | 0.123 | (–0.265 to 0.511) | 0.534 | 10 | –0.088 | (–0.282 to 0.105) | 0.371 |
| Executive function (shifting) | |||||||||||||
| Perceptual organisation | |||||||||||||
| Depression (self-rated) | Figure 66 | 27 | –0.538 | (–0.785 to –0.292) | < 0.001 | 4 | –0.853 | (–1.211 to –0.494) | < 0.001 | 31 | –0.573 | (–0.803 to –0.343) | < 0.001 |
| Memory (self-rated) | |||||||||||||
| Anxiety (self-rated) | Figure 74 | 22 | –0.323 | (–0.425 to –0.222) | < 0.001 | 3 | –0.571 | (–0.977 to –0.165) | 0.006 | 25 | –0.338 | (–0.437 to –0.239) | < 0.001 |
| Impulsivity (objective measures) | Figure 79 | 9 | –0.392 | (–0.682 to –0.102) | 0.008 | 0 | – | – | – | 10 | –0.333 | (–0.594 to –0.072) | 0.012 |
| Impulsivity (subjective measures) | Figure 83 | 8 | –0.780 | (–1.096 to –0.465) | < 0.001 | 0 | – | – | – | 9 | –0.778 | (–1.058 to –0.499) | < 0.001 |
Ecstasy users compared to polydrug controls
In 12 of 16 domains analysed, a significant effect of ecstasy exposure was seen (p < 0.05 against the null hypothesis of no exposure effect). Estimated effect sizes ranged from 0.143 to 0.509, with most estimates falling between 0.15 and 0.4. According to Cohen’s rule of thumb,119 such differences can be considered to fall in the range of ‘small’ effects, with some approaching ‘medium’ effect sizes.
The only domain in which an effect greater than 0.5 SD was found was that of self-rated memory. This is based on a small sample of studies (n = 5) reporting a collection of subjective outcome measures, both factors that would tend to increase uncertainty in the finding. Self-rated measures of impulsivity and anxiety also suggested a comparatively pronounced effect.
Among objective measures, the largest effects were seen in the domains of working memory (SMD – 0.391; 95% CI – 0.589 to – 0.192), delayed verbal memory (SMD – 0.377; 95% CI – 0.498 to – 0.257) and immediate verbal memory (SMD – 0.332; 95% CI – 0.451 to – 0.214). For the outcomes we have categorised as relating to attention, we identified a significant inter-population difference in the ‘focus–execute’ component, but not for the ‘sustain’ component. Amongst our executive function meta-outcomes, an exposure effect was seen for the ‘planning’ component, but not for ‘response inhibition’ or ‘shifting’.
Ecstasy users compared to drug-naïve controls
Eight of 12 domains analysed suggested a significant effect of ecstasy exposure, with estimated effect sizes ranging from 0.272 to 1.037. As in the polydrug-controlled comparisons, self-rated measures generated some of the most sizeable effect estimates, while the largest effects in objective measures were seen in the domains of immediate verbal memory (SMD – 0.840; 95% CI – 0.990 to – 0.690) and delayed verbal memory (SMD – 1.037; 95% CI – 1.734 to – 0.341).
Verbal memory (immediate) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 100 datapoints, representing a total of 40 pairwise comparisons, drawn from 27 different studies (35 comparisons from 27 studies providing data for current ecstasy users and five comparisons from five studies providing data for former ecstasy users). For data published in multiple studies originating from Liverpool John Moores University, data from a single publication120 only were included in this analysis, because it was not possible to deduce the extent of duplicate reporting across the full range of papers. In total, 46 different outcome measures are included, the most common being RBMT: prose recall (10 datapoints), RAVLT: sum of trials 1–5 (10 datapoints) and digit span – backwards (five datapoints). The complete dataset is detailed in Table 51, in Appendix 6.
The meta-analysis (Figure 21) suggests that ecstasy-exposed cohorts tended to perform worse than polydrug controls by around one-third of a standard deviation, with strong evidence against the null hypothesis of no difference between groups (p < 0.001). The stratified analysis identified no difference in exposure effect between current and former ecstasy users.
FIGURE 21.
Verbal memory – immediate (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
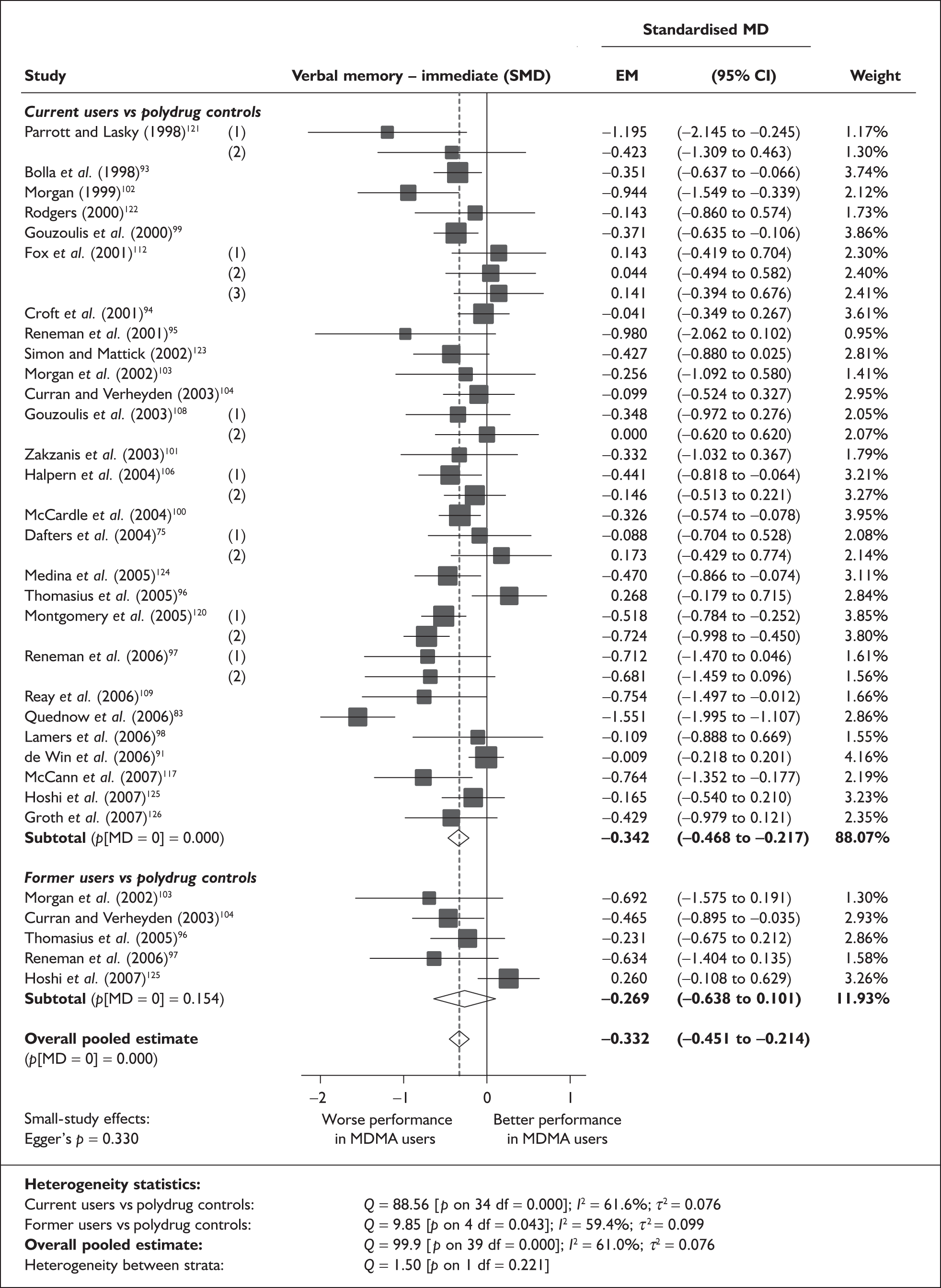
To contextualise the magnitude of this difference, we note that, in the Zakzanis et al. study,101 a standardised mean difference of precisely – 0.332 SD was seen between arms as a result of current ecstasy users scoring 0.2 less than controls on the RBMT immediate prose recall test (1.5 versus 1.7; scaled scores).
A sensitivity analysis in which all individual arms were aggregated to provide single, study-level estimates of effect for each outcome measure before meta-analysis revealed a very similar result (SMD – 0.339; 95% CI – 0.444 to – 0.234). This suggests that our primary analysis is robust to the assumptions underpinning the pooling of data.
There is little evidence of small-study bias, as indicated by Egger’s test (p = 0.330); similarly, the funnel plot (Figure 22) shows no clear trend, although there may be a slight tendency for the least precise studies to produce the most extreme effect estimates.
FIGURE 22.
Verbal memory – immediate (composite measure) – ecstasy users versus polydrug controls: funnel plot.
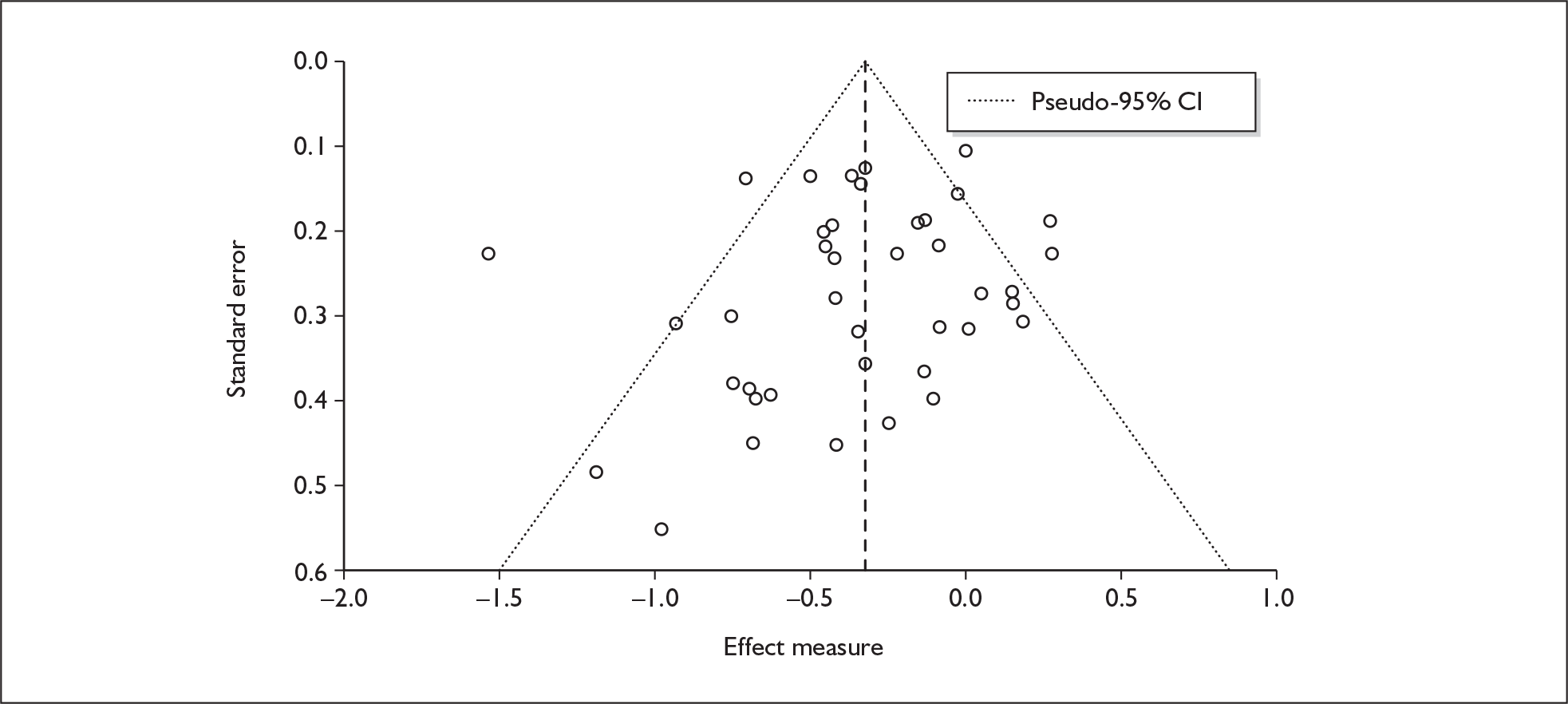
Sufficient data were available to attempt metaregression analyses for 17 covariates; details are shown in Table 15. There was no evidence of a dose–response effect (see Figure 96 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (year) | 36 | 0.022 | (–0.029 to 0.074) | 0.395 | |||
| Sex (% male) | 36 | –0.304 | (–0.924 to 0.317) | 0.337 | |||
| IQ | 18 | 0.032 | (–0.011 to 0.076) | 0.145 | |||
| Education (years) | 12 | 0.295 | (0.085–0.506) | 0.006 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 20 | 0.000 | (–0.001 to 0.001) | 0.586 | |||
| ETLE (occasions) | 6 | 0.001 | (–0.001 to 0.003) | 0.190 | |||
| Period since last consumption (days) | 17 | 0.000 | (0.000–0.001) | 0.244 | |||
| duration of ecstasy use (days) | 24 | 0.000 | (0.000–0.000) | 0.874 | |||
| Frequency of ecstasy use (occasions/month) | 10 | 0.102 | (–0.044 to 0.249) | 0.171 | |||
| Inter-arm differences | |||||||
| Age (years) | 36 | 0.034 | (–0.025 to 0.093) | 0.256 | –0.321 | (–0.452 to –0.189) | 0.000 |
| Sex (% male) | 36 | –0.191 | (–1.109 to 0.728) | 0.684 | –0.310 | (–0.441 to –0.178) | 0.000 |
| Baseline intelligence measures (SMD) | 30 | 0.356 | (0.067–0.645) | 0.016 | –0.240 | (–0.384 to –0.096) | 0.001 |
| Education (years) | 12 | –0.028 | (–0.309 to 0.253) | 0.847 | –0.402 | (–0.833 to 0.029) | 0.067 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 26 | –0.166 | (–0.344 to 0.011) | 0.066 | –0.184 | (–0.315 to –0.053) | 0.006 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 23 | –0.135 | (–0.331 to 0.062) | 0.180 | –0.116 | (–0.272 to 0.040) | 0.146 |
| Exposure to cocaine (ETLD) | 6 | –0.001 | (–0.003 to 0.001) | 0.210 | –0.112 | (–0.318 to 0.094) | 0.287 |
| Exposure to cocaine (SMD) | 19 | –0.242 | (–0.555 to 0.070) | 0.129 | –0.131 | (–0.307 to 0.046) | 0.147 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 23 | –0.005 | (–0.232 to 0.222) | 0.966 | –0.250 | (–0.390 to –0.111) | 0.000 |
Figure 23 plots estimated effect size against average education level, showing that there is a tendency for differences in performance to diminish as education level increases. Notably, the four comparisons in this dataset estimating the greatest deficit for ecstasy users are also those in which participants have the lowest average education levels. If this model were to be believed, one would not expect to see a difference between cohorts if it could be assumed that a study’s participants had received around 15½ years of education. However, the dataset is a restricted one: only 12 of the 40 pairwise comparisons available in the full meta-analysis provide covariate data (although the estimated effect size in this subgroup is comparable to that seen in the full analysis: SMD – 0.371; 95% CI – 0.659 to – 0.083).
FIGURE 23.
Verbal memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against education of participants (average value across all cohorts).
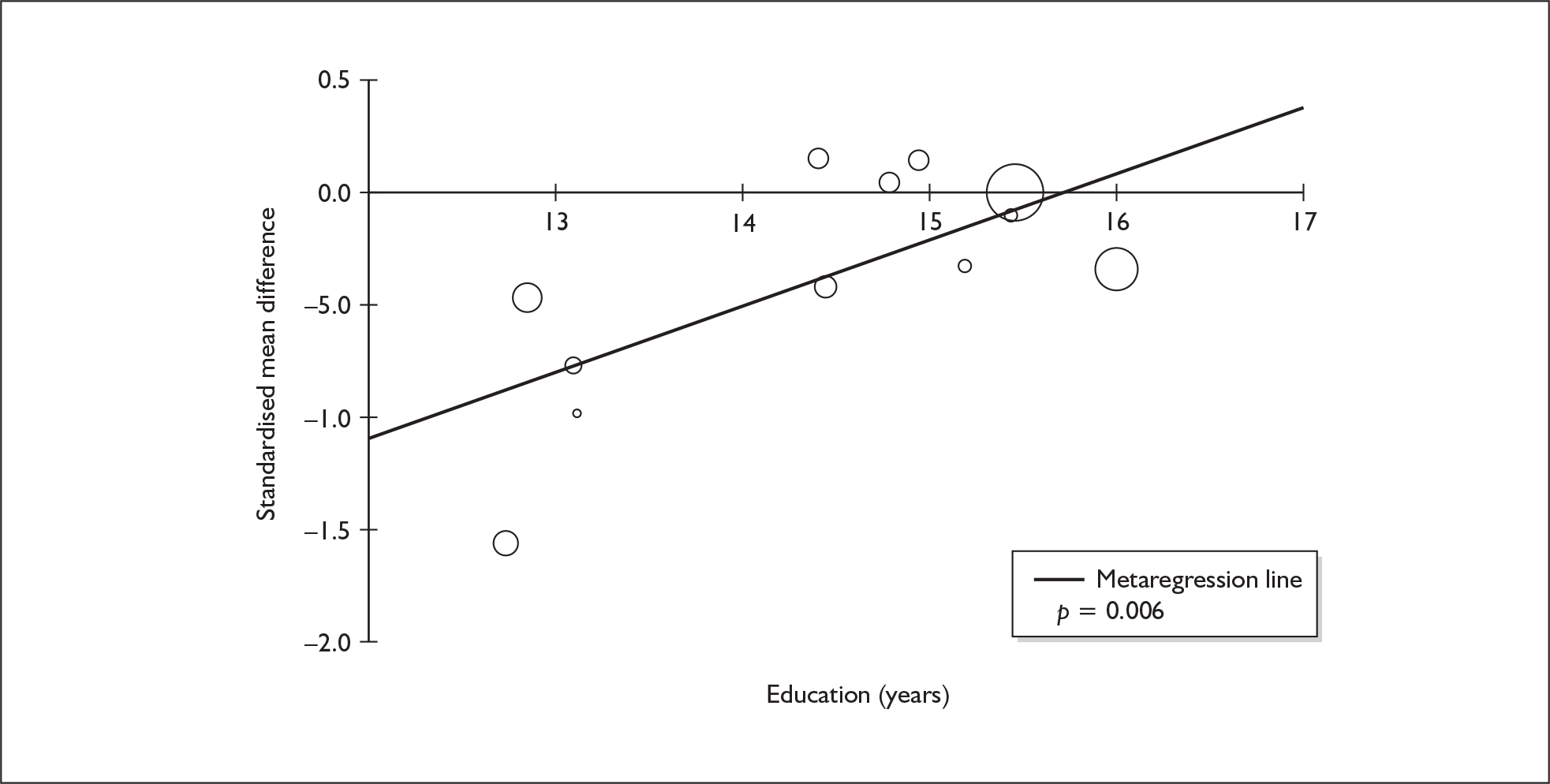
Figure 24 plots estimated effect size against inter-arm asymmetry in intelligence. The fact that most comparisons are located in the ‘south-west’ quadrant of the plot shows that, in the majority of studies, ecstasy-exposed participants not only performed worse in the memory tasks but also were less intelligent than controls. Conversely, the effect size is smaller (indeed, in several cases suggesting an advantage for the ecstasy users), when intelligence measures favour those cohorts. The regression analysis suggests that there may be a general trend for worse performance in those studies in which ecstasy users had lower intelligence scores than controls. However, even if this model is to be believed, asymmetry in intelligence does not explain differences between cohorts entirely, and the evidence for worse performance in ecstasy-exposed cohorts remains strong (adjusted effect estimate: SMD – 0.240; 95% CI – 0.384 to – 0.096; p = 0.003).
FIGURE 24.
Verbal memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in baseline intelligence measures (standardised mean difference).
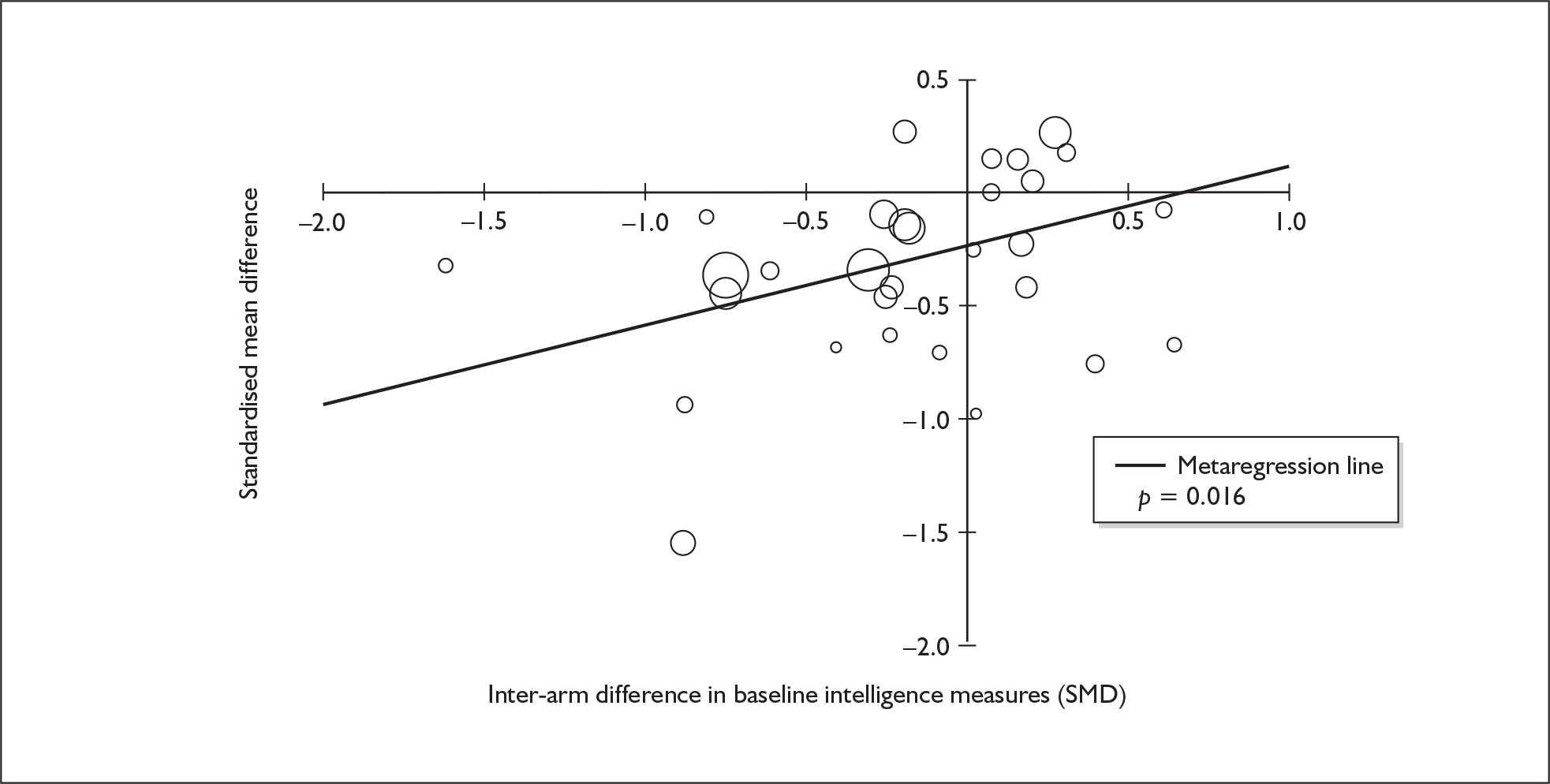
Verbal memory (immediate) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 41 datapoints, representing a total of 18 pairwise comparisons, drawn from 12 different studies (14 comparisons from 12 studies providing data for current ecstasy users and four comparisons from four studies providing data for former ecstasy users). Twenty different outcome measures are included, the most common being RBMT: prose recall (seven datapoints), digit span – backwards (six datapoints) and RAVLT: sum of trials 1–5 (five datapoints). The complete dataset is detailed in Table 52 in Appendix 6.
When this dataset was meta-analysed (Figure 25), both current and former ecstasy users tended to perform worse than drug-naïve controls by around 0.8 of a standard deviation, with strong evidence against the null hypothesis of no difference between groups (p < 0.001). According to Cohen’s rule of thumb, this would qualify as a ‘large’ inter-population difference. To give an indication of the magnitude of this standardised difference in real terms, the datapoint from Morgan’s 1999 study102 appears relatively typical of the pattern of results seen here. In this study, current ecstasy users recalled an average of 1.95 fewer items than drug-naïve controls in the immediate prose recall task of the RBMT (SMD – 0.852).
FIGURE 25.
Verbal memory – immediate (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
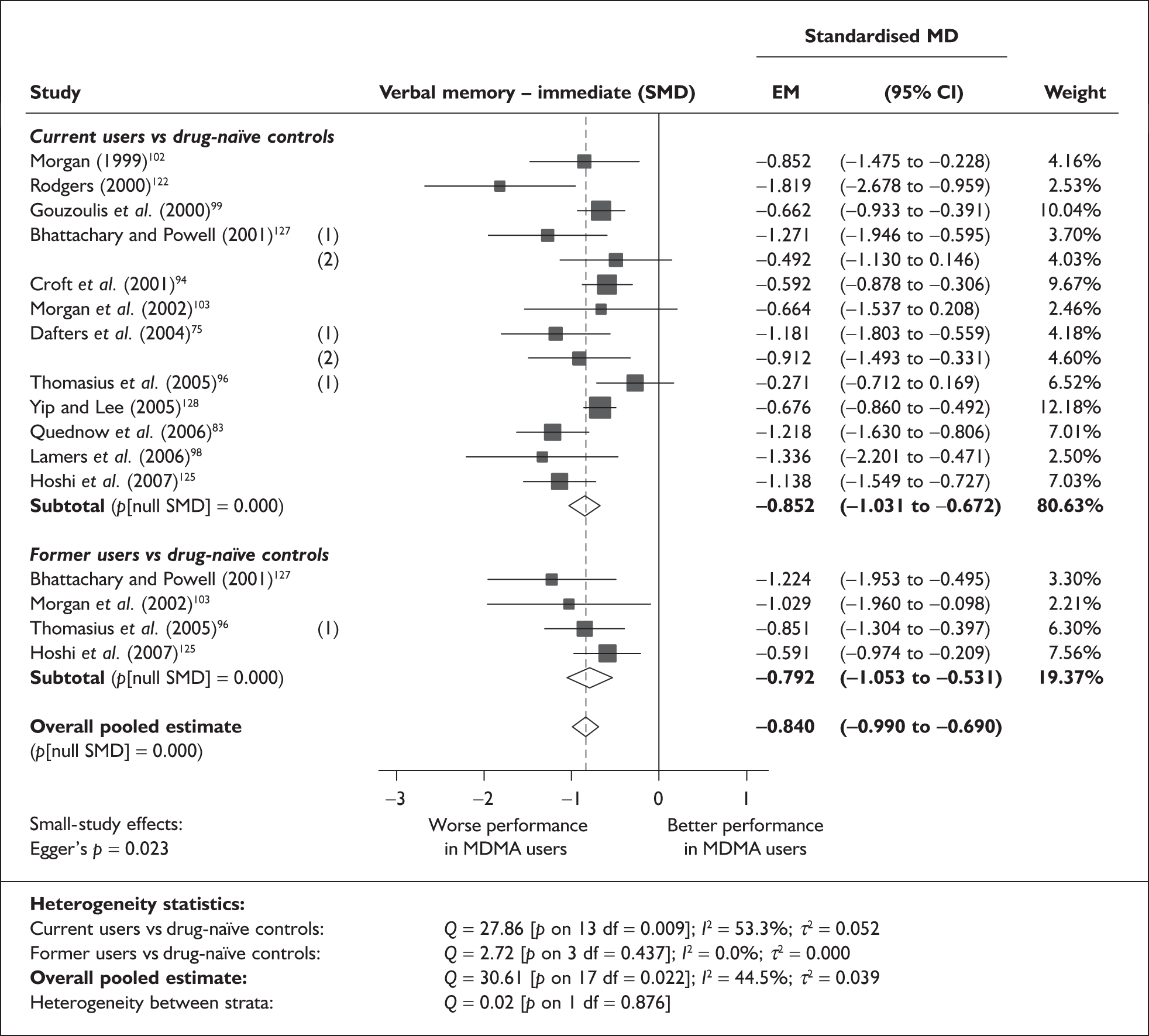
The stratified analysis identified no difference in exposure effect between current and former ecstasy users.
Sensitivity analysis with aggregated comparisons for each study suggested that our primary analysis may underestimate the difference between populations by around 0.1 SD [revised SMD – 0.959; 95% CI – 1.285 to – 0.633; p(null SMD) < 0.001].
There is some evidence of small-study bias (Egger’s p = 0.023). The funnel plot for this dataset (Figure 26) shows that the four estimates with the highest precision provide a smaller-than-average estimate of exposure effect and, conversely, that those datapoints suggesting greatest difference between cohorts tend to be amongst those that are subject to the greatest uncertainty. Accordingly, one might conclude that, had every relevant test ever undertaken been available to this meta-analysis, the estimated exposure effect may have been somewhat lower.
FIGURE 26.
Verbal memory – immediate (composite measure) – ecstasy users versus drug-naïve controls: funnel plot.
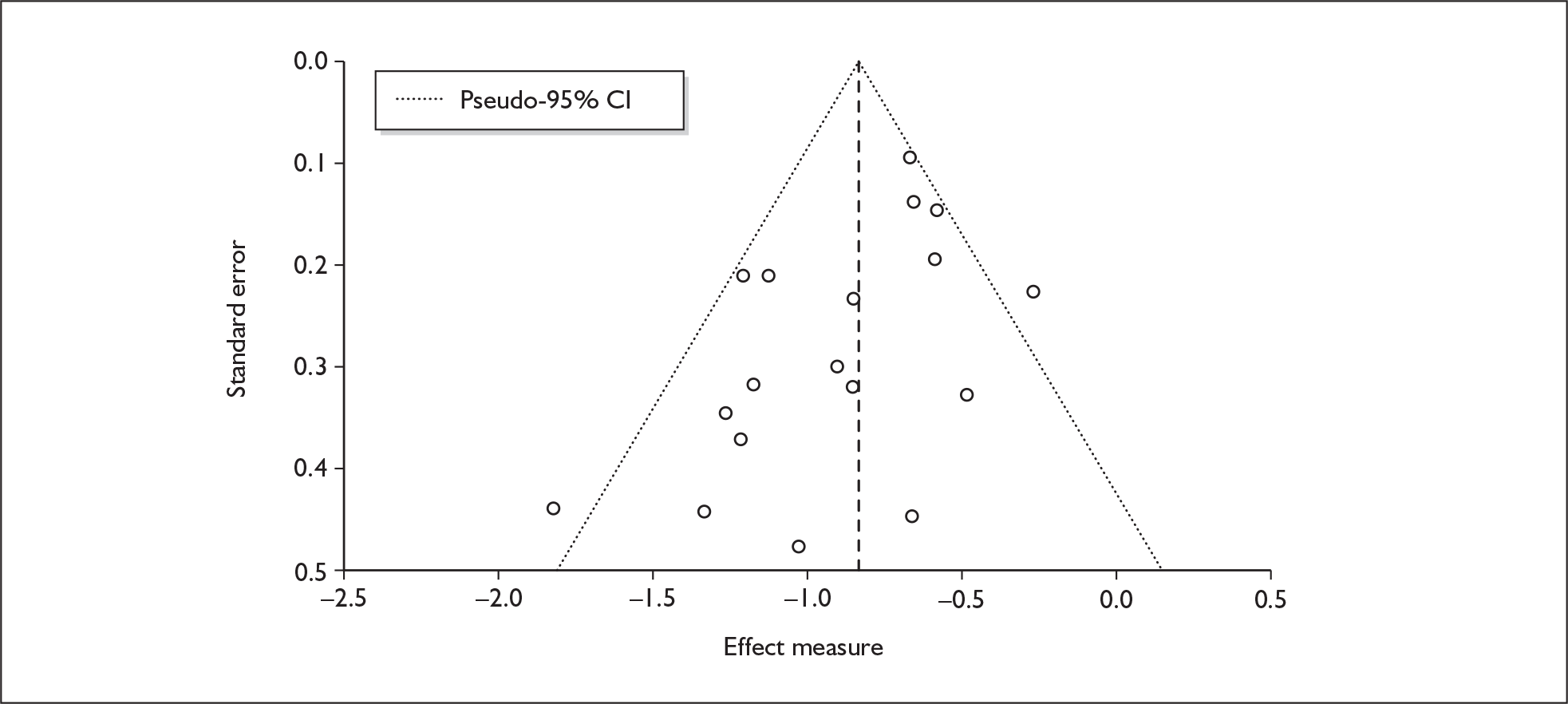
Sufficient data were available to attempt metaregression analyses for 10 covariates; details are shown in Table 16. None of the metaregressions generated results that achieved or approached conventional levels of significance, and there was no evidence of a dose–response effect (see Figure 97 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 18 | –0.009 | (–0.060 to 0.042) | 0.733 | |||
| Sex (% male) | 17 | –0.269 | (–1.053 to 0.515) | 0.501 | |||
| IQ | 11 | –0.003 | (–0.034 to 0.029) | 0.855 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 11 | 0.000 | (–0.001 to 0.001) | 0.969 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 7 | 0.000 | (0.000–0.001) | 0.352 | |||
| Duration of ecstasy use (days) | 7 | 0.000 | (0.000–0.000) | 0.096 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 18 | 0.011 | (–0.079 to 0.102) | 0.810 | –0.849 | (–1.009 to –0.690) | 0.000 |
| Sex (% male) | 17 | –2.024 | (–5.506 to 1.459) | 0.255 | –0.870 | (–1.037 to –0.702) | 0.000 |
| Baseline intelligence measures (SMD) | 16 | 0.047 | (–0.439 to 0.533) | 0.850 | –0.828 | (–1.012 to –0.645) | 0.000 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | 5 | –0.003 | (–0.007 to 0.001) | 0.139 | –0.525 | (–0.854 to –0.196) | 0.002 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 14 | –0.182 | (–0.544 to 0.180) | 0.325 | –0.747 | (–0.962 to –0.532) | 0.000 |
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 30 | –0.019 | (–0.069 to 0.031) | 0.454 | |||
| Sex (% male) | 30 | –0.253 | (–0.852 to 0.346) | 0.407 | |||
| IQ | 18 | 0.033 | (–0.007 to 0.072) | 0.103 | |||
| Education (years) | 12 | 0.177 | (0.011–0.344) | 0.037 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 17 | 0.000 | (0.000 – 0.001) | 0.649 | |||
| ETLE (occasions) | 6 | –0.001 | (–0.004 to 0.002) | 0.600 | |||
| Period since last consumption (days) | 17 | 0.000 | (–0.001 to 0.001) | 0.599 | |||
| Duration of ecstasy use (days) | 21 | 0.000 | (0.000 – 0.000) | 0.994 | |||
| Frequency of ecstasy use (occasions/month) | 7 | 0.045 | (–0.184 to 0.273) | 0.702 | |||
| Inter-arm differences | |||||||
| Age (years) | 30 | 0.042 | (–0.015 to 0.099) | 0.152 | –0.395 | (–0.524 to –0.267) | 0.000 |
| Sex (% male) | 30 | –0.201 | (–1.238 to 0.837) | 0.705 | –0.378 | (–0.508 to –0.248) | 0.000 |
| Baseline intelligence measures (SMD) | 28 | 0.082 | (–0.222 to 0.385) | 0.598 | –0.323 | (–0.480 to –0.167) | 0.000 |
| Education (years) | 12 | 0.027 | (–0.207 to 0.260) | 0.824 | –0.374 | (–0.707 to –0.040) | 0.028 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 23 | –0.134 | (–0.374 to 0.106) | 0.275 | –0.365 | (–0.518 to –0.213) | 0.000 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 21 | 0.078 | (–0.258 to 0.414) | 0.649 | –0.417 | (–0.677 to –0.158) | 0.002 |
| Exposure to cocaine (ETLD) | 5 | 0.000 | (–0.003 to 0.002) | 0.763 | –0.100 | (–0.541 to 0.341) | 0.657 |
| Exposure to cocaine (SMD) | 18 | –0.121 | (–0.494 to 0.252) | 0.525 | –0.327 | (–0.557 to –0.096) | 0.005 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 21 | 0.086 | (–0.173 to 0.344) | 0.517 | –0.358 | (–0.507 to –0.210) | 0.000 |
Verbal memory (delayed) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 49 datapoints, representing a total of 32 pairwise comparisons, drawn from 22 different studies (27 comparisons from 22 studies providing data for current ecstasy users and five comparisons from five studies providing data for former ecstasy users). Twenty-two different outcome measures are included, the most common being RBMT: prose recall (10 datapoints), RAVLT: trial 8 (seven datapoints) and Buschke: overall score (four datapoints). The complete dataset is detailed in Table 53 in Appendix 6.
The meta-analysis, shown in Figure 27, suggests that ecstasy-exposed individuals’ long-term verbal memory is worse than that of polydrug controls by a little under 0.4 SD. According to Cohen’s guidelines, this would probably be thought of as somewhere between a ‘small’ and a ‘medium’ difference. The effect might appear to be greater in former ecstasy users, whom controls outperformed by almost 0.5 SD (a ‘medium’ difference, according to Cohen). However, there is insufficient evidence to reject a null hypothesis of homogeneous strata (p = 0.533). Sensitivity analysis with single, pooled comparisons for each study provides a SMD estimated at –0.402 [95% CI – 0.515 to – 0.288; p(null SMD) < 0.001], which is extremely close to the primary analysis.
FIGURE 27.
Verbal memory – delayed (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
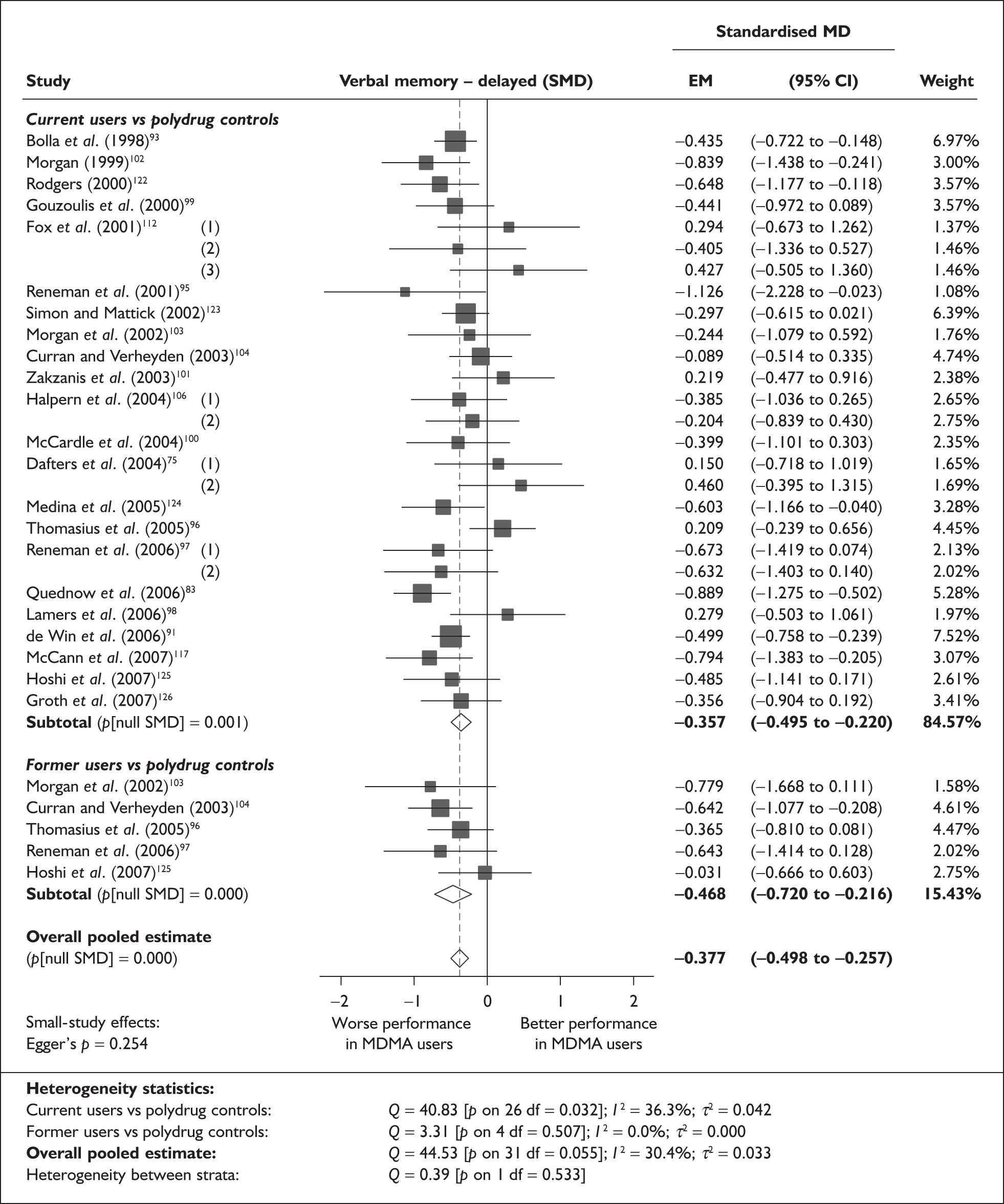
To translate these findings back into a more easily interpretable scale, it may be useful to return to the raw data on which the analysis was based, to see which individual datapoints are closest to the calculated average. For the comparison between current users and controls, a relatively typical datapoint is the WMS-III delayed memory index score from the study by Groth et al. ,126 in which the ecstasy-using cohort registered lower scores than polydrug controls by an average of 3.8 points (108.4 versus 112.2; SMD – 0.356). Where former users were compared to controls, the most representative datapoint was that from Curran and Verheyden,104 where the difference between cohorts was 1.69 items on the RBMT delayed prose recall test (5.825 versus 7.515; SMD – 0.506).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.254), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 15 covariates; details are shown in Table 15. There was no evidence of a dose–response effect (see Figure 98 in Appendix 7).
Figure 28 plots estimated effect size against average education level, showing that there is a tendency for differences in performance to diminish as education level rises. This is a very similar picture to that seen for immediate verbal memory (see Figure 23). It should be noted, however, that both analyses are based on fairly restricted datasets.
FIGURE 28.
Verbal memory – delayed (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against education of participants (average value across all cohorts).
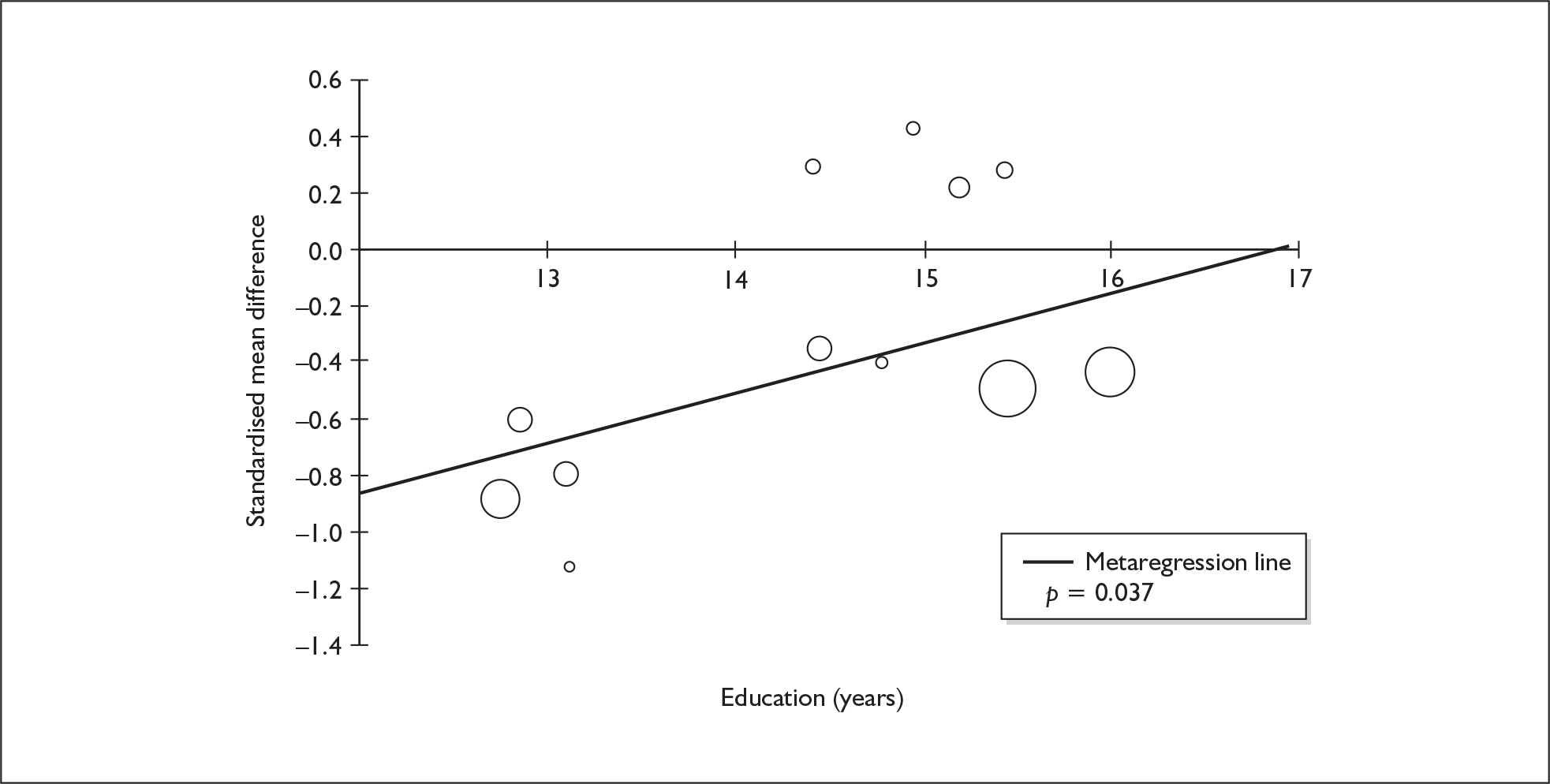
Verbal memory (delayed) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 28 datapoints, representing a total of 20 pairwise comparisons, drawn from 12 different studies (15 comparisons from 12 studies providing data for current ecstasy users and five comparisons from four studies providing data for former ecstasy users). Fifteen different outcome measures are included, the most common being RBMT: prose recall (seven datapoints), prose retained (three datapoints) and prose recall (three datapoints). The complete dataset is detailed in Table 54 in Appendix 6.
In the meta-analysis (Figure 29), ecstasy-exposed individuals’ delayed verbal memory is estimated to be inferior to that of drug-naïve controls by very nearly 1 SD. However, the forest plot shows very clearly that one effect estimate – that from Yip and Lee’s study128 – is entirely atypical of results from other studies. If this single datapoint is excluded from the meta-analysis, the estimated SMD falls to – 0.717 (95% CI – 0.915 to – 0.518); however, the evidence for an overall exposure effect remains strong (p < 0.001).
FIGURE 29.
Verbal memory – delayed (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
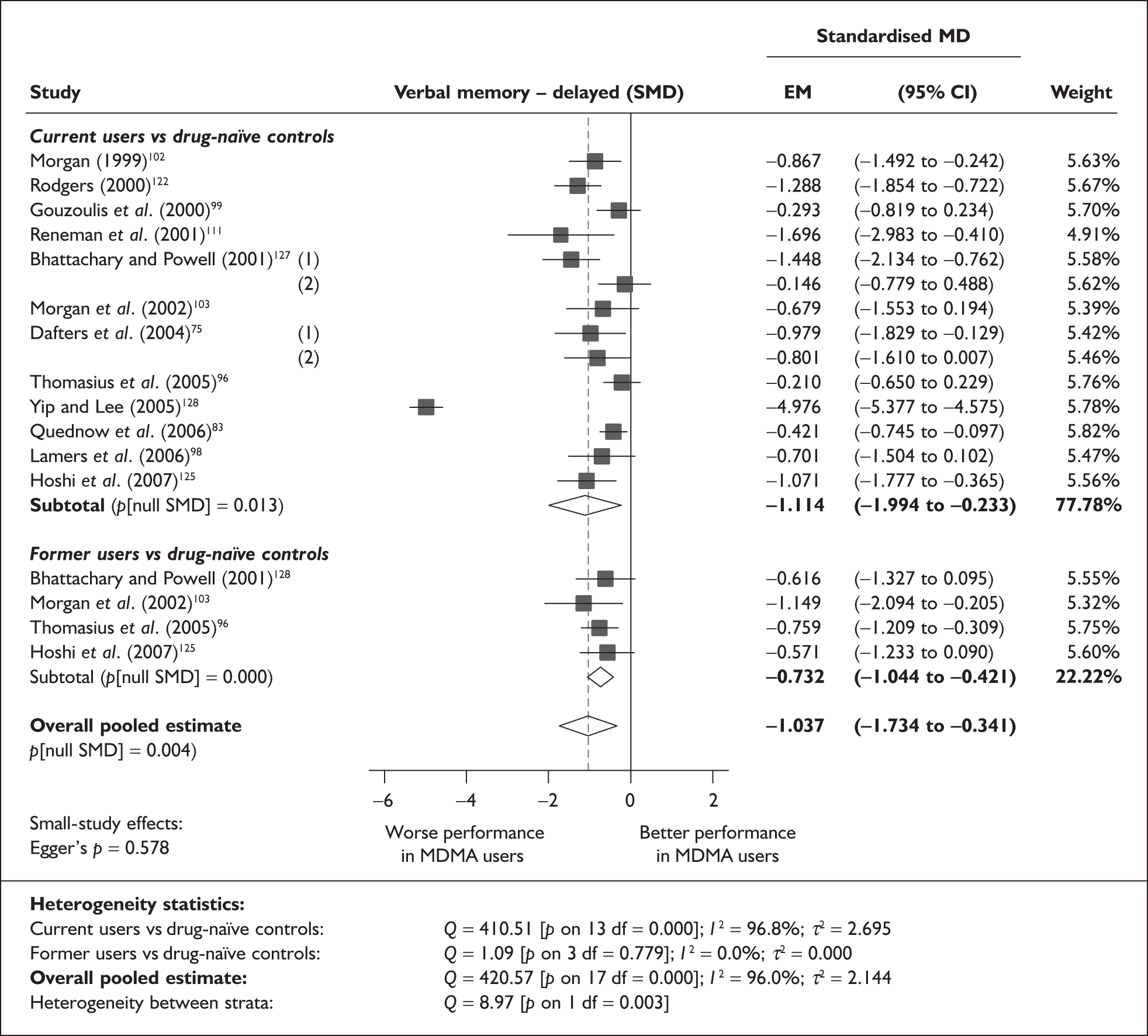
Yip and Lee’s anomalous datapoint represents a composite of two subtests from the RAVLT, in both of which the performance of ecstasy-exposed participants was less than half the standard achieved by drug-naïve controls. There are a number of possible explanations for this extreme result. First, it should be noted that the outlying datapoints are those based on the Chinese version of the RAVLT; this is the only study in the evidence-base to rely on this instrument, the validity and characteristics of which are unclear to us. Second, it is possible that there are environmental and/or genetic factors that make ecstasy exposure effects unusual – or, at least, difficult to generalise to a UK context – in a Hong Kong Chinese population. Third, the authors’ description of the population from which their cohorts were drawn implies that Hong Kong clubbers use ecstasy and other drugs in a markedly different way to the patterns seen elsewhere. They claim to have recruited relatively uncontaminated ecstasy-using and control cohorts, excluding participants with exposure to other substances, including tobacco and alcohol (more than one drink per week). The fact that nearly two-thirds of potential participants were excluded for violating these criteria would tend to enhance such claims; most other included studies had broad eligibility rules, and appear to have included most or all prospective participants. Accordingly, it could be argued that – although it remains subject to all the limitations of the observational paradigm – Yip and Lee’s study overcomes some of the confounding seen in other research, with exposure to ecstasy providing the only clearly observable difference between cohorts. Nevertheless, it would be a substantial step to extend this argument to the suggestion that Yip and Lee’s estimate provides a ‘true’ exposure effect, while the additional confounding inherent in other studies serves drastically to underestimate the real difference.
Unsurprisingly, this outlying estimate has a substantial effect on calculated heterogeneity statistics. With Yip and Lee’s data included, tests reveal an extremely heterogeneous dataset (p < 0.001; I2 = 96.0%), whereas reanalysis without the anomalous estimate reveals a picture that suggests a much more homogeneous dataset (p = 0.047; I2 = 39.6%). Similarly, initial tests are strongly suggestive of interstratum heterogeneity (p = 0.003) but, on closer inspection, it becomes clear that this result is driven entirely by the single atypical estimate from Yip and Lee’s study: the reanalysis without this datapoint is wholly consistent with a homogeneous effect across strata (p = 0.595).
Sensitivity analysis with single, pooled comparisons for each study provides a mean difference estimated at –1.253 (95% CI – 1.936 to – 0.571). This may appear to be a relatively substantial discrepancy from the primary analysis; however, further analysis reveals that this is because the aggregated approach is affected to an even greater extent by Yip and Lee’s outlying estimate (without this datapoint, the sensitivity analysis generates a pooled estimate of – 0.745; 95% CI – 0.991 to – 0.499, which is close to the primary analysis).
Returning to the raw data on which the analysis was based, the individual datapoints that are closest to the calculated averages are – for the full dataset including Yip and Lee – the RBMT prose recall subscore reported by Dafters and colleagues75 [in which heavy ecstasy users scored an average of 1.85 less than controls (SMD – 0.979)], and – for the restricted dataset without the outlying estimate – the delayed (trial 8) RAVLT recall score from Lamers et al. 98 [in which the deficit for ecstasy users is estimated at 1.5 items (SMD – 0.701)].
When applied to the full dataset, Egger’s test suggested that there was no evidence of small-study bias in this dataset (p = 0.578). Once more, however, this result is substantially affected by the single outlying estimate: if Yip and Lee’s data are excluded, then Egger’s test returns a p-value of 0.021, suggesting that the null hypothesis of no small-study effect is difficult to support. The trend for more precise studies to estimate a smaller difference can be clearly visualised in the funnel plot for this dataset (Figure 30), as can the distorting influence of Yip and Lee’s study.
FIGURE 30.
Verbal memory – delayed (composite measure) – ecstasy users versus drug-naïve controls: funnel plot.
Sufficient data were available to attempt metaregression analyses for 13 covariates (Table 18); none provided significant results, and there was no evidence of a dose–response effect (see Figure 99 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 18 | –0.118 | (–0.319 to 0.082) | 0.246 | |||
| Sex (% male) | 17 | 0.396 | (–0.563 to 1.355) | 0.419 | |||
| IQ | 11 | –0.014 | (–0.047 to 0.018) | 0.387 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 11 | 0.002 | (–0.002 to 0.005) | 0.290 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 8 | 0.001 | (–0.004 to 0.005) | 0.684 | |||
| Duration of ecstasy use (days) | 7 | 0.001 | (0.000–0.003) | 0.126 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 18 | 0.085 | (–0.343 to 0.513) | 0.697 | –1.083 | (–1.814 to –0.352) | 0.004 |
| Sex (% male) | 17 | –2.030 | (–5.560 to 1.499) | 0.260 | –0.703 | (–0.898 to –0.509) | 0.000 |
| Baseline intelligence measures (SMD) | 16 | –0.545 | (–2.812 to 1.722) | 0.638 | –1.073 | (–1.926 to –0.219) | 0.014 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 13 | 0.057 | (–0.364 to 0.477) | 0.792 | –0.740 | (–1.021 to –0.460) | 0.000 |
Visual memory (immediate) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 66 datapoints, representing a total of 22 pairwise comparisons, drawn from 16 different studies (19 comparisons from 16 studies providing data for current ecstasy users and three comparisons from three studies providing data for former ecstasy users). Forty-one different outcome measures are included, the most common being Corsi Block: span (six datapoints), Corsi Block: span plus one (five datapoints) and WMS-R: visual reproduction (four datapoints). The complete dataset is detailed in Table 55 in Appendix 6.
The meta-analysis (Figure 31) suggests that ecstasy-exposed cohorts performed worse than controls by a small, but nonetheless significant, margin. Sensitivity analysis using the aggregated data approach generated very similar results [SMD – 0.126; 95% CI – 0.233 to – 0.020; p(null SMD) = 0.020]. The inter-population difference appears to be even smaller in the former-ecstasy-using stratum; however, the hypothesis test for interstratum heterogeneity provides no statistical justification for supposing the participants belong to different distributions.
FIGURE 31.
Visual memory – immediate (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
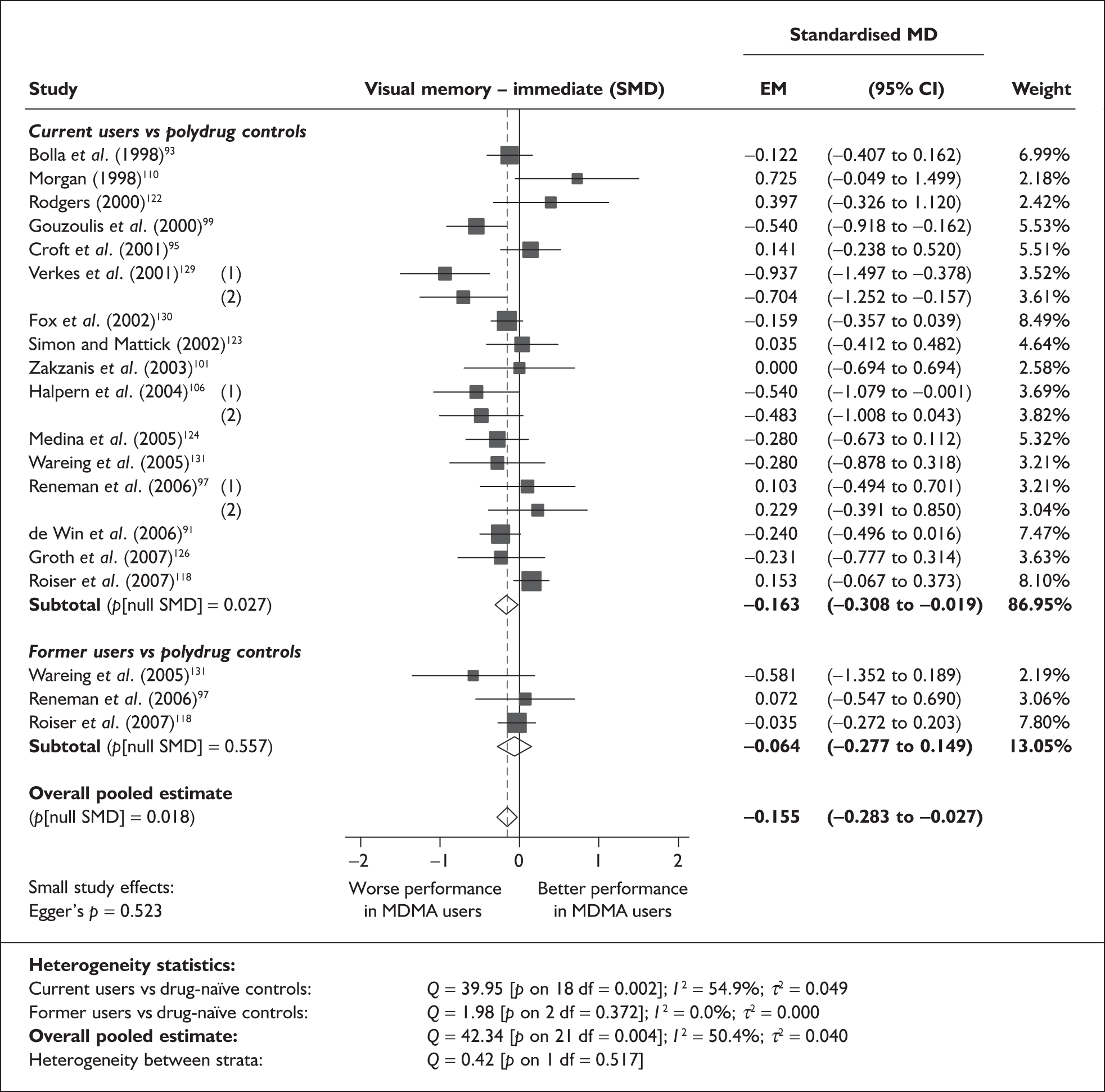
The small magnitude of this standardised difference becomes apparent when one compares the pooled estimate with the raw data on which the meta-analysis is based. For example, in Bolla et al. ,93 ecstasy users scored an average of 0.2 less than controls in WMS-R figural memory (7.3 versus 7.5; SMD – 0.166) and, in the spatial recognition task in Fox et al. ,130 there was an additional response latency of 110 milliseconds in the ecstasy-exposed cohort (2.4 seconds versus 2.29 seconds; SMD – 0.168).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.523), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 17 covariates; details are shown in Table 19. There was no evidence of a dose–response effect (see Figure 100 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 20 | 0.062 | (0.011–0.113) | 0.017 | |||
| Sex (% male) | 18 | –1.002 | (–1.851 to –0.152) | 0.021 | |||
| IQ | 6 | 0.010 | (–0.049 to 0.068) | 0.740 | |||
| Education (years) | 7 | 0.028 | (–0.117 to 0.173) | 0.705 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 17 | 0.000 | (–0.001 to 0.000) | 0.382 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 11 | 0.000 | (–0.001 to 0.001) | 0.782 | |||
| Duration of ecstasy use (days) | 15 | 0.000 | (0.000–0.000) | 0.986 | |||
| Frequency of ecstasy use (occasions/month) | 6 | 0.015 | (–0.077 to 0.107) | 0.752 | |||
| Inter-arm differences | |||||||
| Age (years) | 20 | –0.060 | (–0.117 to –0.003) | 0.040 | –0.124 | (–0.247 to –0.002) | 0.047 |
| Sex (% male) | 18 | –0.250 | (–1.314 to 0.814) | 0.645 | –0.109 | (–0.249 to 0.032) | 0.129 |
| Baseline intelligence measures (SMD) | 15 | 0.422 | (0.174–0.670) | 0.001 | –0.028 | (–0.137 to 0.081) | 0.614 |
| Education (years) | 7 | –0.020 | (–0.177 to 0.138) | 0.808 | –0.237 | (–0.463 to –0.012) | 0.039 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 15 | –0.028 | (–0.360 to 0.304) | 0.868 | –0.117 | (–0.321 to 0.086) | 0.259 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 12 | –0.215 | (–0.426 to –0.004) | 0.046 | 0.022 | (–0.092 to 0.136) | 0.704 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 10 | –0.091 | (–0.402 to 0.219) | 0.564 | 0.002 | (–0.216 to 0.221) | 0.984 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 14 | 0.013 | (–0.344 to 0.371) | 0.941 | –0.096 | (–0.258 to 0.066) | 0.246 |
Figure 32 plots memory performance against average age (classical metaregression, with covariate measured across all participants). It appears that the most marked deficit for ecstasy users may be found when populations with lower average age are assessed (it is notable that the eight lowest effect estimates appear amongst the youngest cohorts). In contrast, inter-arm differences, apparently, tend to be minimal in older cohorts.
FIGURE 32.
Visual memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against average age (all participants).
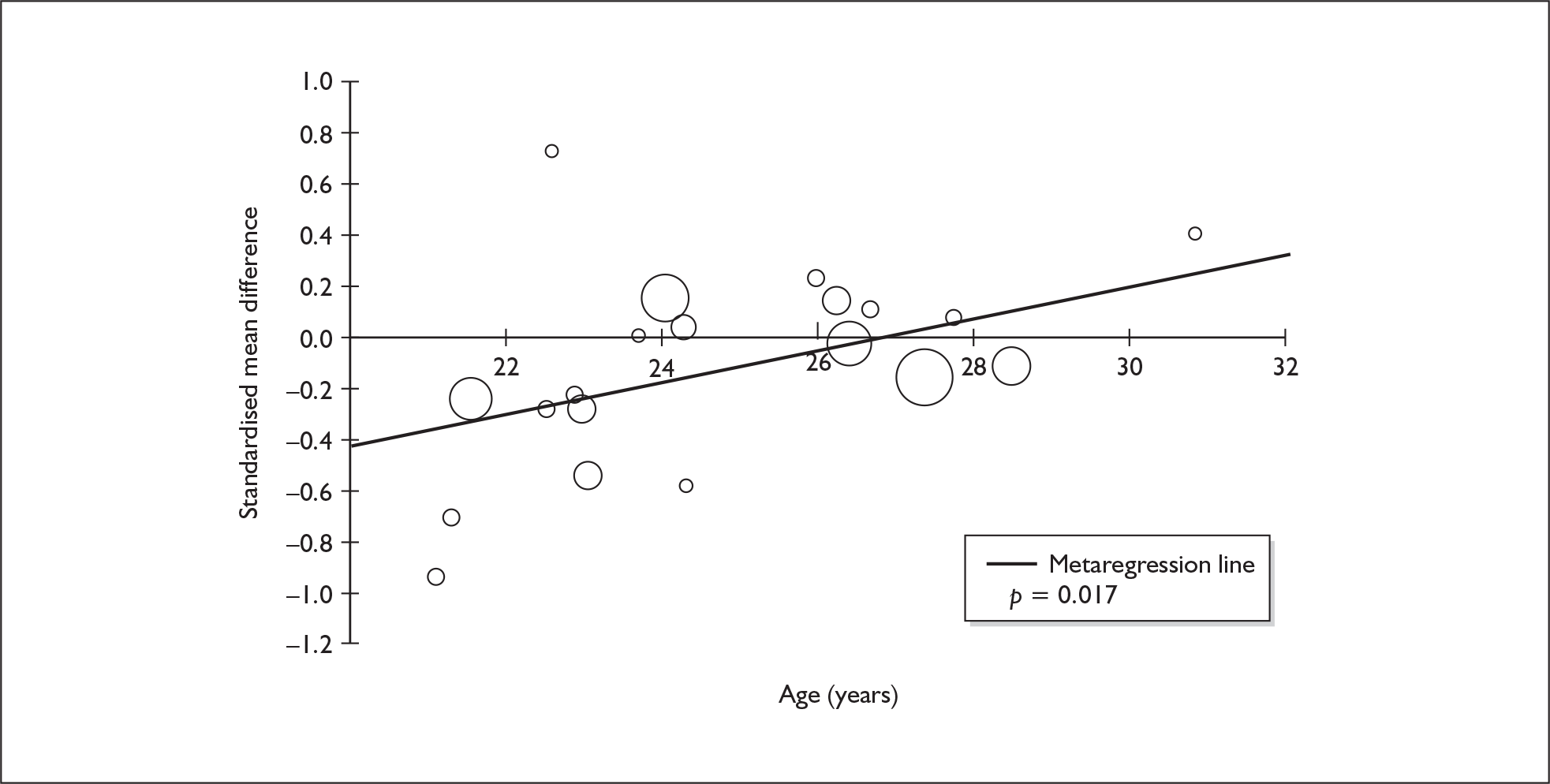
There may also be a gender effect in evidence: Figure 33 plots the outcome of interest against the gender composition of the populations under analysis (classical metaregression, with covariate measured across all participants). It shows that deficits were greatest in ecstasy-using cohorts that were predominantly made up of men. It is noticeable that the two datapoints contributed by comparisons of 100% male populations are those suggesting the greatest underperformance in ecstasy users.
FIGURE 33.
Visual memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against gender (across all participants).
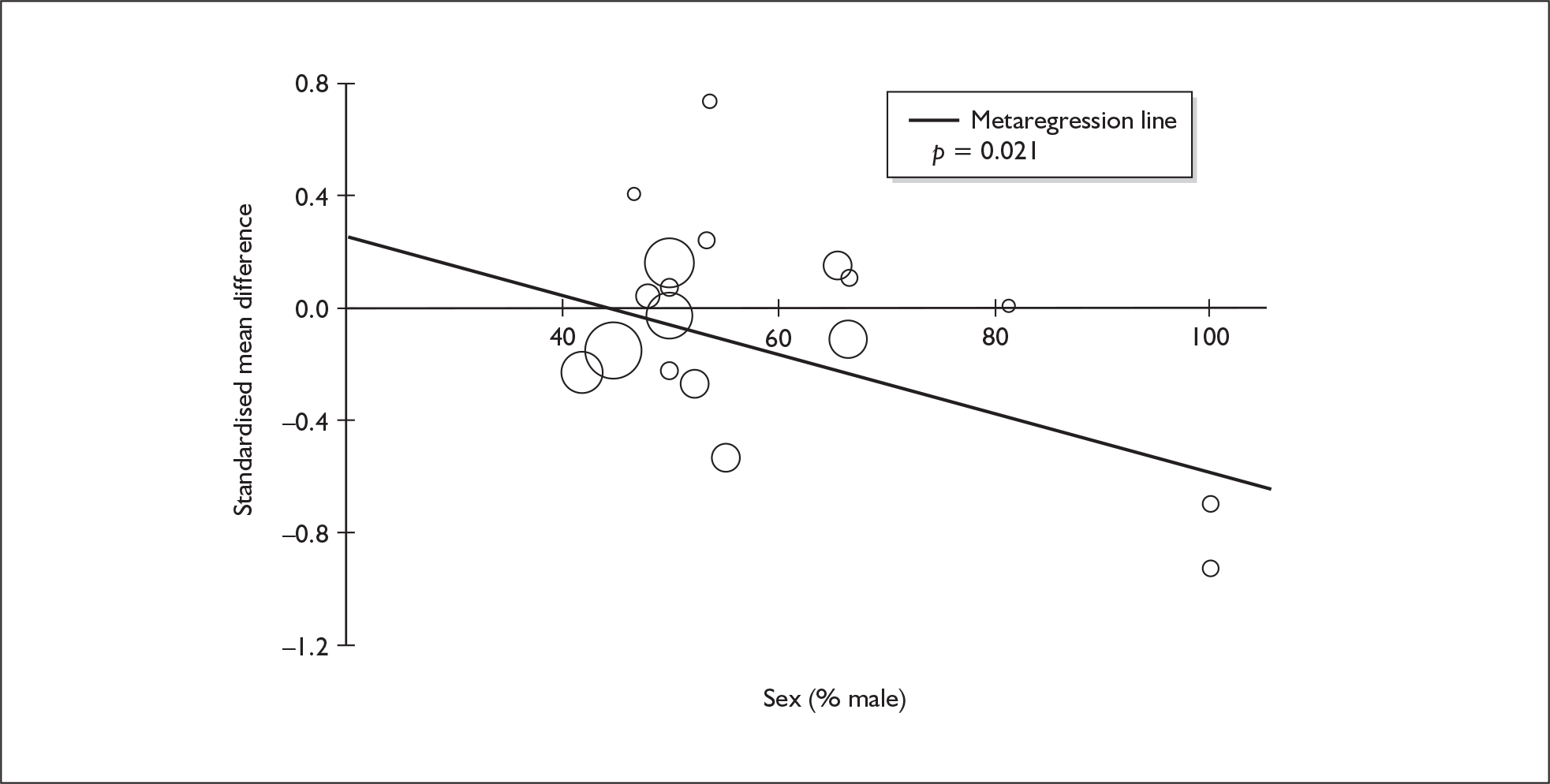
For differential covariates, a very strong positive correlation was found between immediate visual memory outcomes and baseline asymmetry in intelligence, suggesting that good performance in these tests can be expected wherever one cohort has an advantage over the other in intelligence. This relationship is clear in Figure 34, which plots the variables against each other. It can be seen that datapoints representing worst performance in ecstasy users tend to be those in which they were less intelligent than controls whereas, in studies in which ecstasy users were more intelligent than controls, they could be expected to match or outperform controls in the memory tests. We note that a similar – albeit slightly less compelling – picture was seen in the equivalent metaregression for the analogous measure of verbal memory (see Figure 24).
FIGURE 34.
Visual memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in baseline intelligence measures.
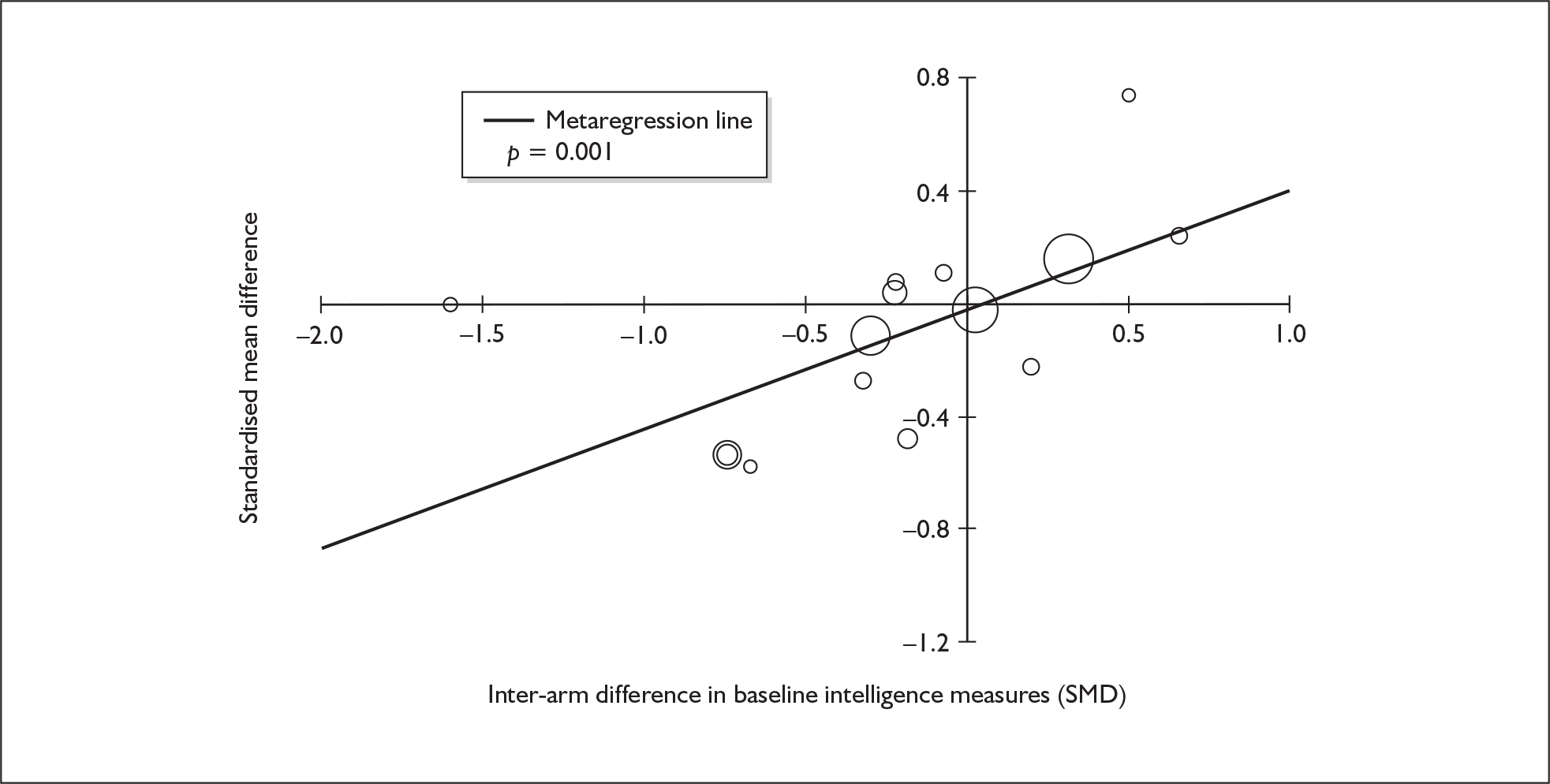
This model suggests that the small exposure effect seen in the primary analysis is ascribable entirely to baseline imbalances in intelligence: when accounting for this confounding, the adjusted SMD is estimated at – 0.028 (95%CI – 0.148 to 0.092), which is consistent with a null effect (p = 0.623). This can be clearly seen in Figure 34, because the metaregression line passes almost directly through the origin of the graph.
In addition to the absolute effect of age (see Figure 32), inter-population asymmetry in age may also have an effect on observed results. Figure 35 shows that this effect has a negative coefficient, suggesting that worse performance by ecstasy-exposed cohorts is seen when they are older than their control groups.
FIGURE 35.
Visual memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in age.
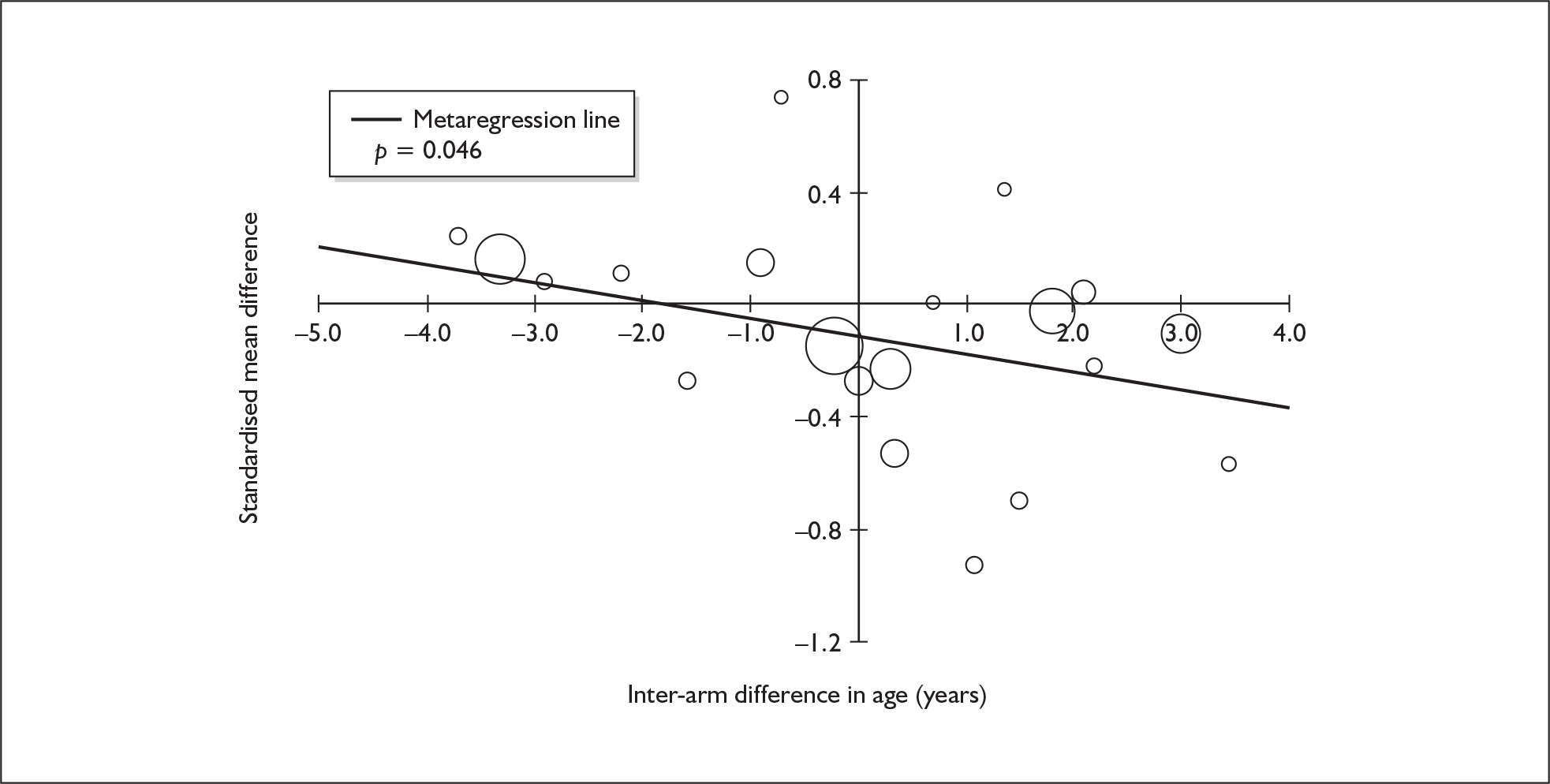
Figure 36 shows the effect of differential amphetamine exposure on observed results. Although there appears to be a trend associating poorer performance with increased exposure asymmetry (i.e. ecstasy users taking more amphetamines than controls), it should be noted that a single datapoint is exerting considerable leverage on this analysis. The bubble on the left-hand side of the plot represents the study by Roiser et al. ,118 in which there was substantially greater exposure to amphetamines in the control group than in the current ecstasy users. If this single datapoint is excluded from the evidence-base, the apparent association with outcome disappears entirely (p = 0.379).
FIGURE 36.
Visual memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in exposure to amphetamines other than MDMA.
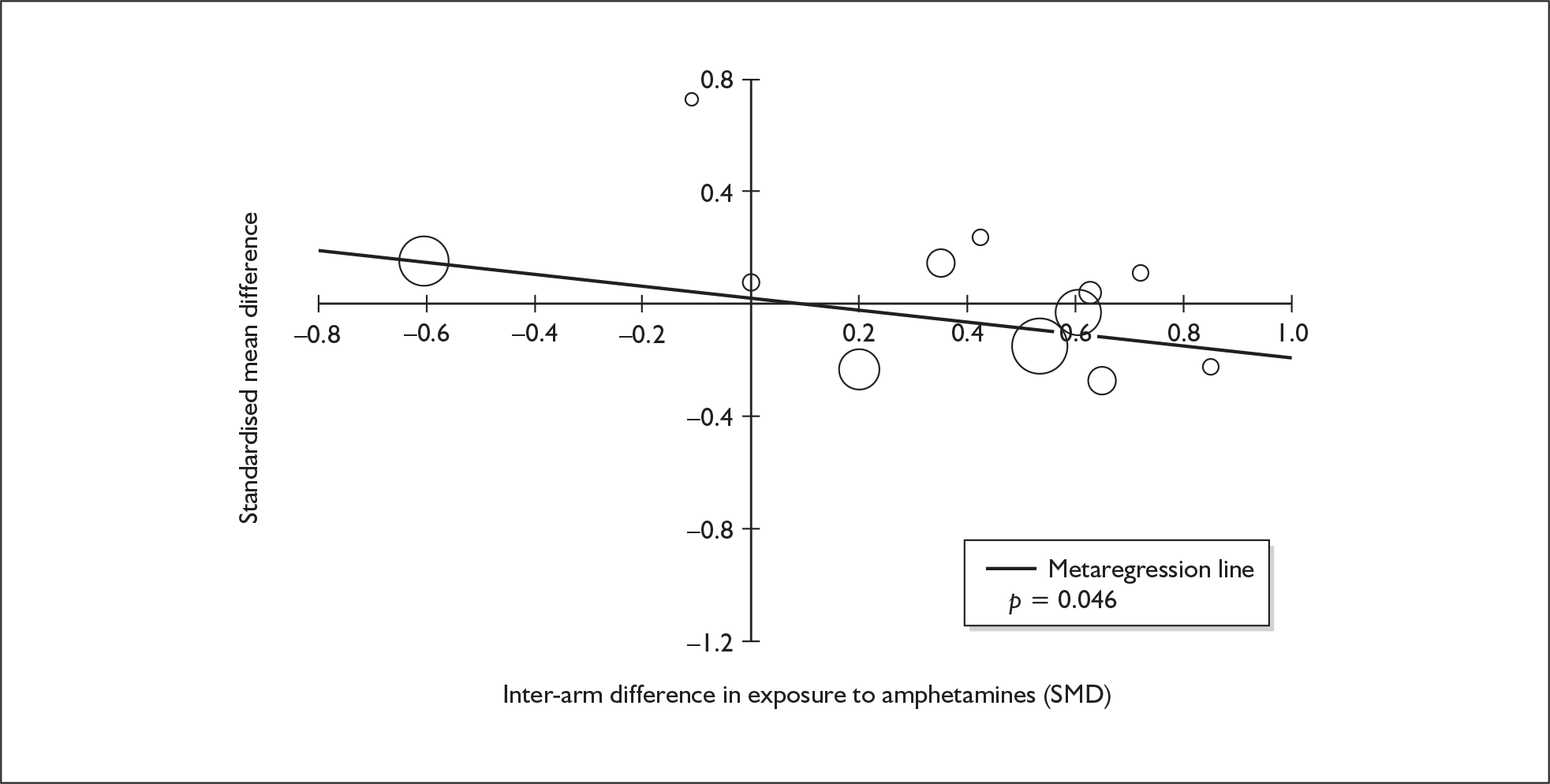
Visual memory (immediate) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 25 datapoints, representing a total of seven pairwise comparisons, drawn from six different studies (six comparisons from six studies providing data for current ecstasy users and one comparison from one study providing data for former ecstasy users). Seventeen different outcome measures are included, the most common being PRM: latency (two datapoints), PRM: correct (two datapoints) and CANTAB DMTS: simultaneous–latency (two datapoints). The complete dataset is detailed in Table 56 in Appendix 6.
When meta-analysed (Figure 37), these data suggest that there is little evidence of an exposure effect in this area. The effect estimate is noticeably similar to that seen in the comparison with polydrug controls (see Figure 31) but, in this instance, the analysis is based on a smaller dataset, and is subject to greater uncertainty. Sensitivity analysis using the aggregated data approach did not produced markedly different findings [SMD – 0.132; 95% CI – 0.294 to 0.029; p(null SMD) = 0.107].
FIGURE 37.
Visual memory – immediate (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
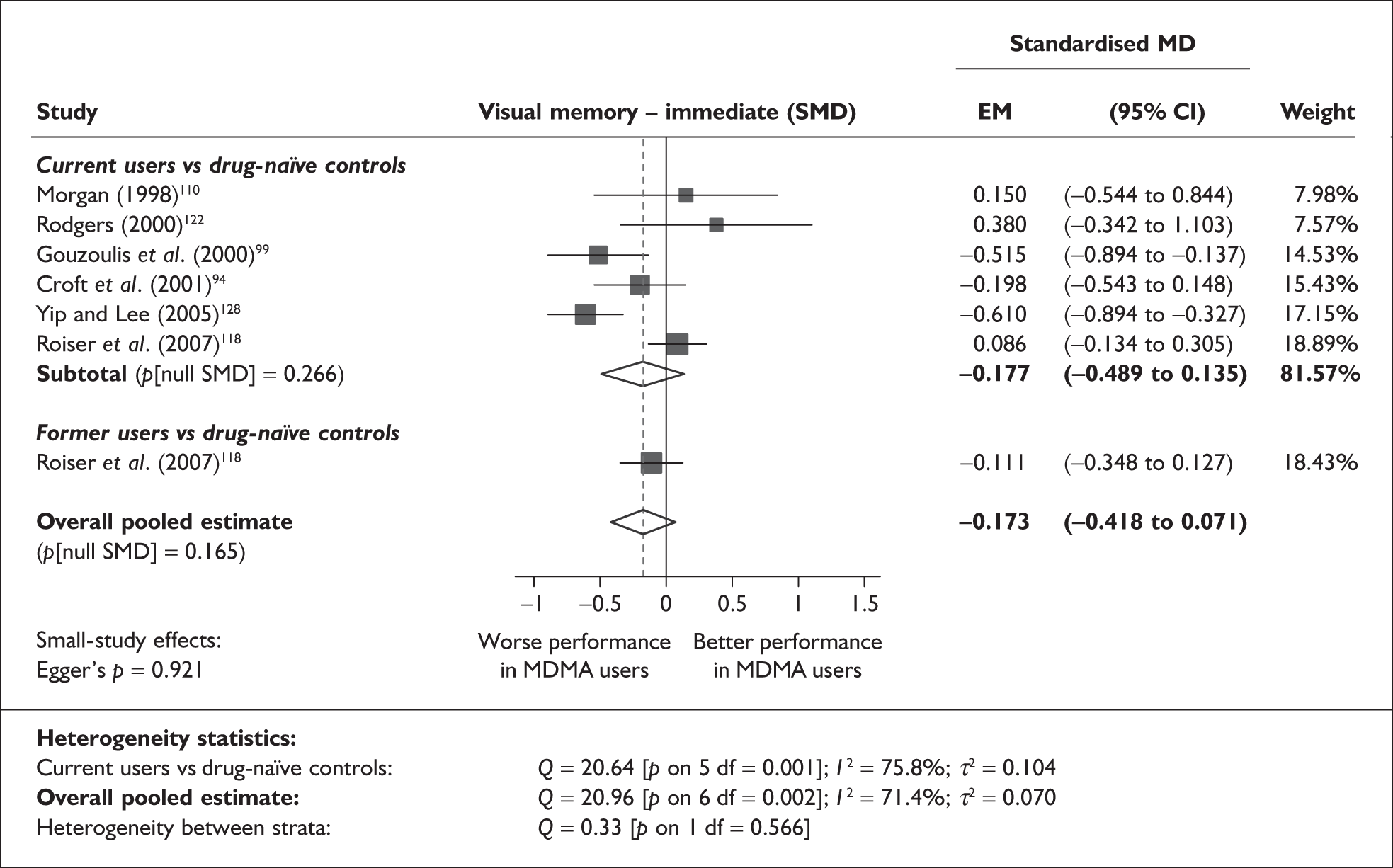
There is no evidence of small-study bias in this dataset (Egger’s p = 0.921), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for six covariates; details are shown in Table 20. None of the analyses were able to provide a statistically convincing explanation of the heterogeneity seen amongst base-case effect estimates. Both covariates relating to gender distribution generated p-values approaching 0.05; however, little credence can be given to these findings, in the context of multiple testing with very limited (n = 6) datasets. There was no evidence of a dose–response effect (see Figure 101 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 7 | –0.009 | (–0.098 to 0.080) | 0.842 | |||
| Sex (% male) | 6 | –3.563 | (–7.306 to 0.180) | 0.062 | |||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 6 | 0.000 | (–0.001 to 0.001) | 0.470 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 7 | –0.008 | (–0.171 to 0.154) | 0.921 | –0.160 | (–0.461 to 0.141) | 0.297 |
| Sex (% male) | 6 | 4.394 | (–0.407 to 9.194) | 0.073 | –0.112 | (–0.282 to 0.059) | 0.200 |
| Baseline intelligence measures (SMD) | 5 | 0.538 | (–0.398 to 1.474) | 0.260 | –0.122 | (–0.471 to 0.227) | 0.493 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
Visual memory (delayed) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 22 datapoints, representing a total of 14 pairwise comparisons, drawn from 10 different studies (12 comparisons from 10 studies providing data for current ecstasy users and two comparisons from two studies providing data for former ecstasy users). Ten different outcome measures are included, the most common being WMS-R: visual reproduction (five datapoints), R-OCFT: total score (four datapoints) and WMS-R: visual paired associates (two datapoints). The complete dataset is detailed in Table 57 in Appendix 6.
When meta-analysed (Figure 38), these data are strongly reminiscent of the immediate visual memory findings discussed above (see Figure 31), with ecstasy-exposed individuals apparently subject to a small but significant deficit in performance. Once more, sensitivity analysis using the aggregated data approach generated extremely similar results [SMD – 0.186; 95% CI – 0.325 to – 0.047; p(null SMD) = 0.009].
FIGURE 38.
Visual memory – delayed (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
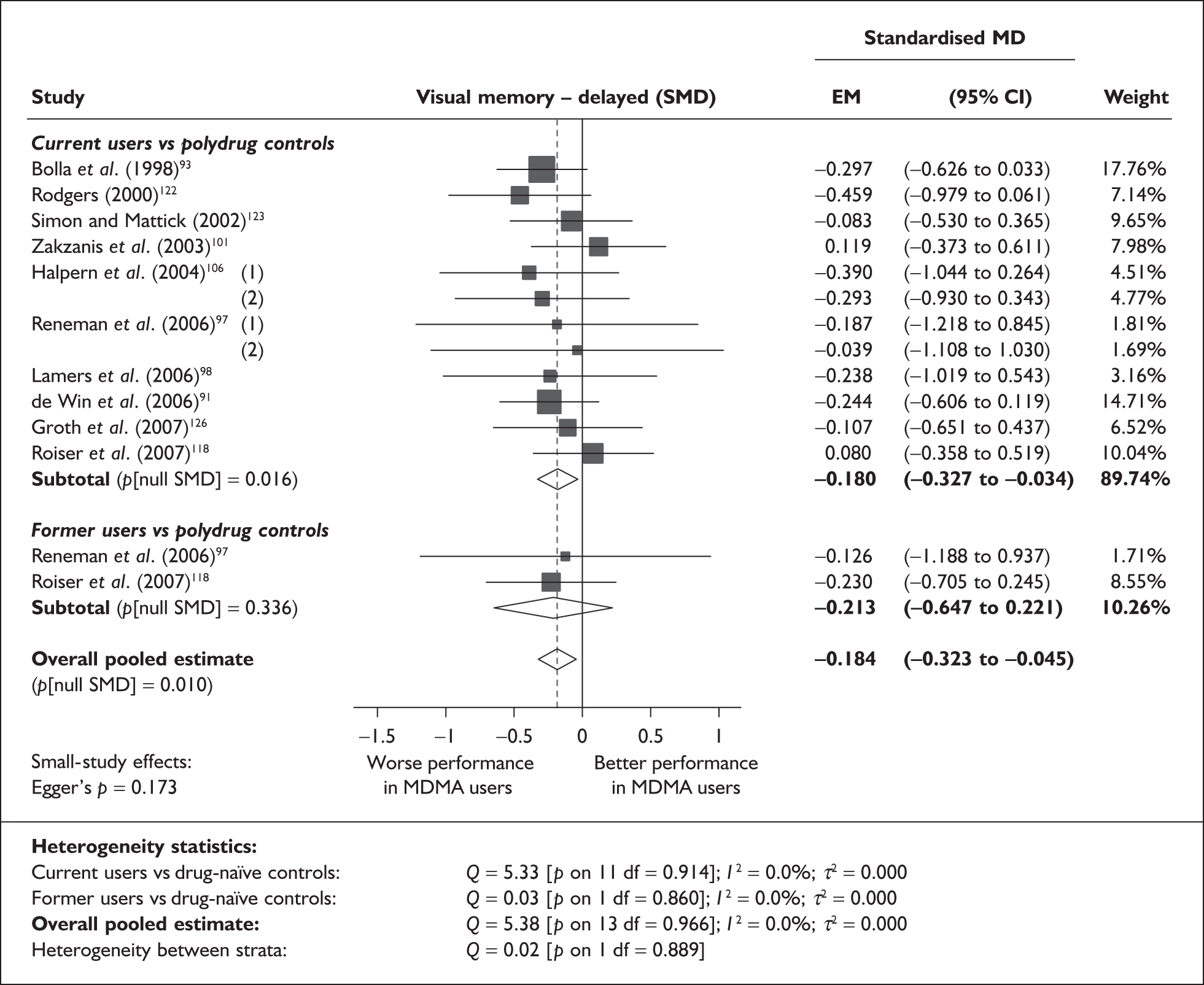
A typical datapoint feeding this analysis is found in Reneman et al. ,93 in which the WMS-R visual reproduction score was a single point lower in the ecstasy-exposed arm than in the controls (35.4 versus 36.4; SMD – 0.187).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.173), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for nine covariates; details are shown in Table 21. None of the analyses were able to provide a statistically convincing explanation of the heterogeneity seen amongst base-case effect estimates, and there was no evidence of a dose–response effect (see Figure 102 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 12 | –0.032 | (–0.084 to 0.020) | 0.223 | |||
| Sex (% male) | 12 | 0.351 | (–0.886 to 1.588) | 0.578 | |||
| IQ | 6 | 0.025 | (–0.068 to 0.118) | 0.599 | |||
| Education (years) | 5 | –0.180 | (–0.574 to 0.215) | 0.372 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 8 | 0.000 | (–0.001 to 0.001) | 0.952 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 6 | 0.000 | (–0.001 to 0.000) | 0.749 | |||
| duration of ecstasy use (days) | 8 | 0.000 | (0.000–0.000) | 0.799 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 12 | –0.035 | (–0.101 to 0.031) | 0.302 | –0.144 | (–0.297 to 0.009) | 0.065 |
| Sex (% male) | 12 | 0.371 | (–1.191 to 1.934) | 0.641 | –0.157 | (–0.310 to –0.003) | 0.045 |
| Baseline intelligence measures (SMD) | 12 | –0.030 | (–0.321 to 0.260) | 0.838 | –0.156 | (–0.332 to 0.021) | 0.084 |
| Education (years) | 5 | 0.047 | (–0.191 to 0.284) | 0.700 | –0.134 | (–0.465 to 0.196) | 0.426 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 9 | –0.025 | (–0.415 to 0.365) | 0.901 | –0.126 | (–0.329 to 0.077) | 0.224 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 9 | –0.146 | (–0.533 to 0.242) | 0.461 | –0.088 | (–0.305 to 0.129) | 0.428 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 8 | –0.033 | (–0.748 to 0.682) | 0.928 | –0.124 | (–0.560 to 0.312) | 0.578 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 9 | 0.094 | (–0.283 to 0.471) | 0.626 | –0.158 | (–0.370 to 0.053) | 0.143 |
Visual memory (delayed) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 11 datapoints, representing a total of seven pairwise comparisons, drawn from five different studies (five comparisons from five studies providing data for current ecstasy users and two comparisons from two studies providing data for former ecstasy users). Six different outcome measures are included, the most common being R-OCFT: total score (three datapoints), PRM: correct (two datapoints), and PRM: latency (two datapoints). The complete dataset is detailed in Table 58 in Appendix 6.
Meta-analysis (Figure 39) suggests that, although ecstasy exposure is associated with worse performance in the majority of cases, pooled results do not provide convincing evidence against the null hypothesis of no exposure effect. This is true of the two strata individually and of the overall pooled estimate.
FIGURE 39.
Visual memory – delayed (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
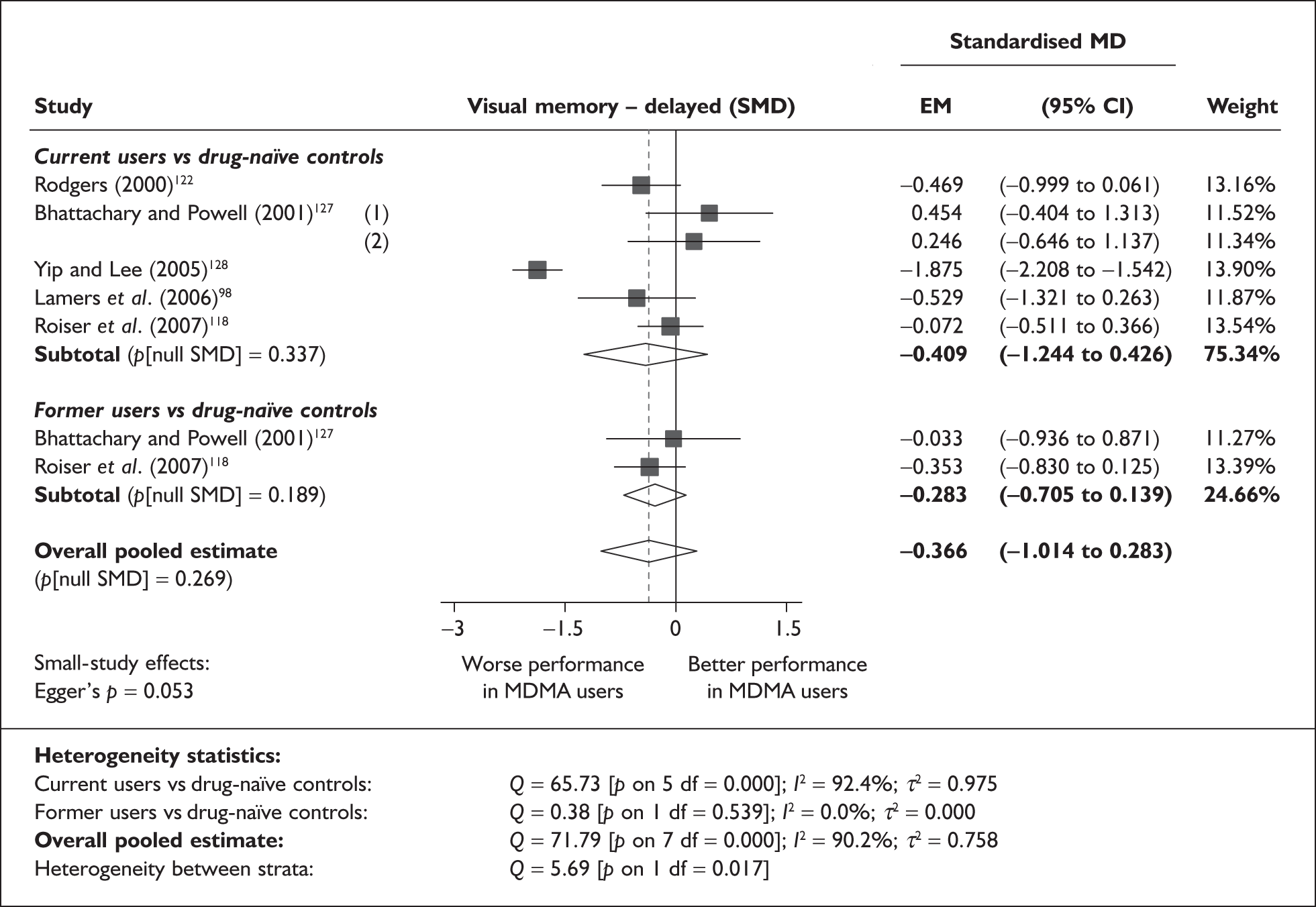
As in the analogous measure of delayed verbal memory (see Figure 29), the forest plot shows that the effect estimate from Yip and Lee’s study128 is markedly atypical of results from other studies. If this single datapoint is excluded from the meta-analysis, results become much more suggestive of a homogeneous dataset (Q = 5.68; p on 6 df = 0.460; I2 = 0.0%). Without Yip and Lee’s study,128 the estimated SMD falls somewhat to – 0.191 but, because the heterogeneity term in the random-effects model is much reduced, the estimate appears rather more precise (95% CI – 0.423 to 0.041). The evidence for an overall exposure effect remains weak (p = 0.460).
Our initial sensitivity analysis, adopting single, aggregated comparisons for each study, generated an SMD estimated at – 0.520 (95% CI – 1.239 to 0.198), which is noticeably higher than that seen in the primary analysis. However, as previously, this discrepancy appears to be an artefact of the distortions of Yip and Lee’s study: repeated sensitivity analysis excluding the outlier is closely comparable to the primary analysis using the restricted dataset [SMD – 0.234; 95% CI – 0.605 to 0.137; p(null SMD) = 0.216].
When applied to the full dataset, Egger’s test suggested that evidence of small-study bias approached significance (p = 0.053). However, Yip and Lee’s study is exerting considerable leverage in this analysis; reanalysis with the datapoint excluded is much more suggestive of an unbiased dataset (p = 0.338). The funnel plot for this analysis (Figure 40) is unlikely to cause concern about publication bias, though it reinforces the outlying nature of Yip and Lee’s effect estimate.
FIGURE 40.
Visual memory – delayed (composite measure) – ecstasy users versus drug-naïve controls: funnel plot.
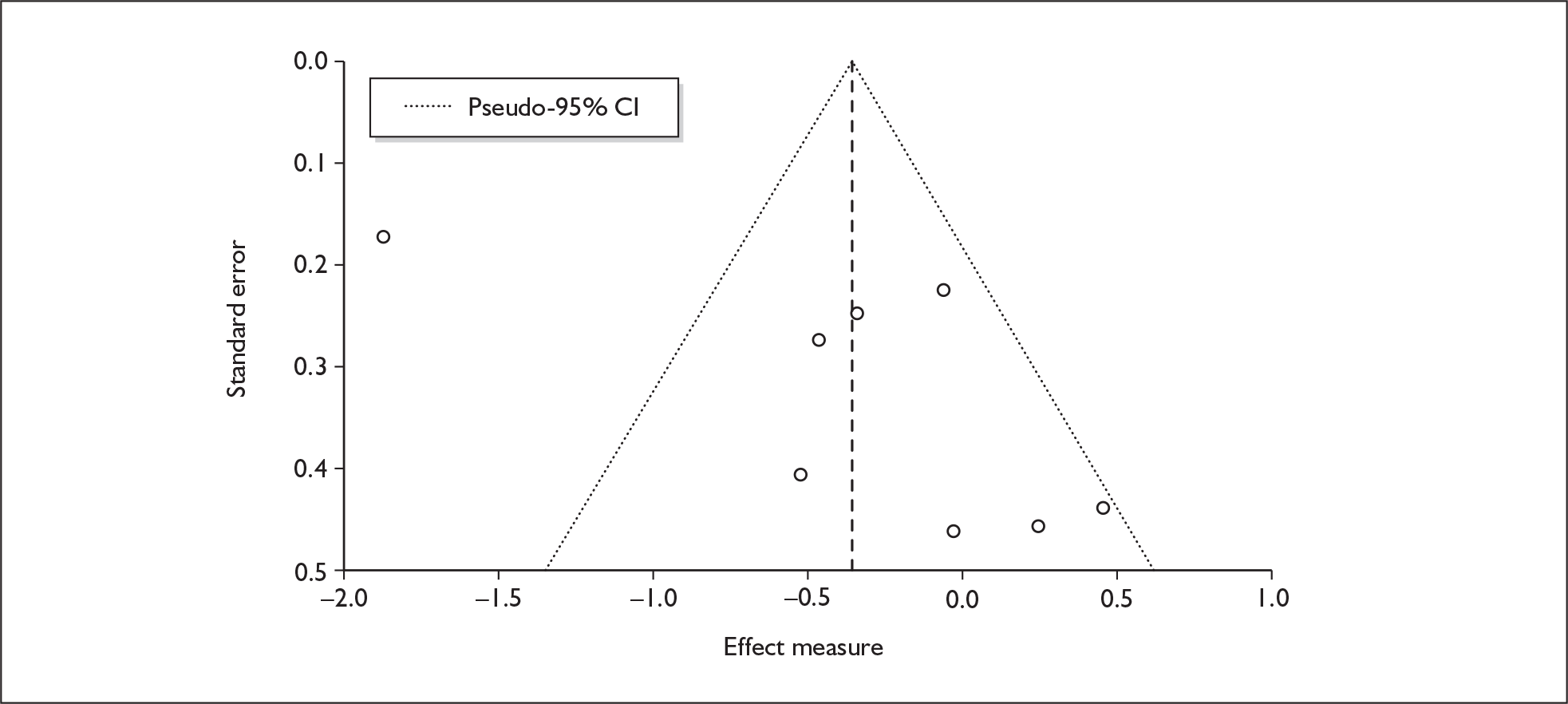
Sufficient data were available to attempt metaregression analyses for seven covariates; details are shown in Table 22. None of the analyses were able to provide a statistically convincing explanation of the heterogeneity seen amongst base-case effect estimates, and there was no evidence of a dose–response effect (see Figure 103 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 8 | –0.133 | (–0.312 to 0.046) | 0.145 | |||
| Sex (% male) | 7 | 2.027 | (–2.256 to 6.310) | 0.354 | |||
| IQ | 6 | –0.022 | (–0.058 to 0.013) | 0.213 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | < 5 | ||||||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 8 | 0.133 | (–0.265 to 0.531) | 0.512 | –0.447 | (–1.188 to 0.294) | 0.237 |
| Sex (% male) | 7 | –1.630 | (–8.268 to 5.008) | 0.630 | –0.150 | (–0.438 to 0.138) | 0.308 |
| Baseline intelligence measures (SMD) | 7 | 0.535 | (–1.958 to 3.028) | 0.674 | –0.250 | (–1.159 to 0.659) | 0.590 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 6 | –0.206 | (–0.866 to 0.455) | 0.541 | –0.006 | (–0.468 to 0.457) | 0.981 |
Working memory – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 47 datapoints, representing a total of 23 pairwise comparisons, drawn from 15 different studies (20 comparisons from 15 studies providing data for current ecstasy users and three comparisons from three studies providing data for former ecstasy users). Twenty-nine different outcome measures are included, the most common being computation span (three datapoints), spatial recall (three datapoints) and reading span (two datapoints). The complete dataset is detailed in Table 59 in Appendix 6.
When meta-analysed (Figure 41), these data reflect an inter-population difference of approximately 0.4 SD. This effect size approaches a ‘medium’-sized difference, according to Cohen’s rule of thumb. Sensitivity analysis with data pooled at study level produced a closely comparable result [SMD – 0.406; 95% CI – 0.587 to – 0.225; p(null SMD) < 0.001]. There is evidence of interstratum heterogeneity: former users performed less well, in comparison to controls, than current users. For current users, the average inter-arm difference was of the order of one-third of an SD, while ex-users’ scores showed an effect size approaching two-thirds of an SD.
FIGURE 41.
Working memory (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
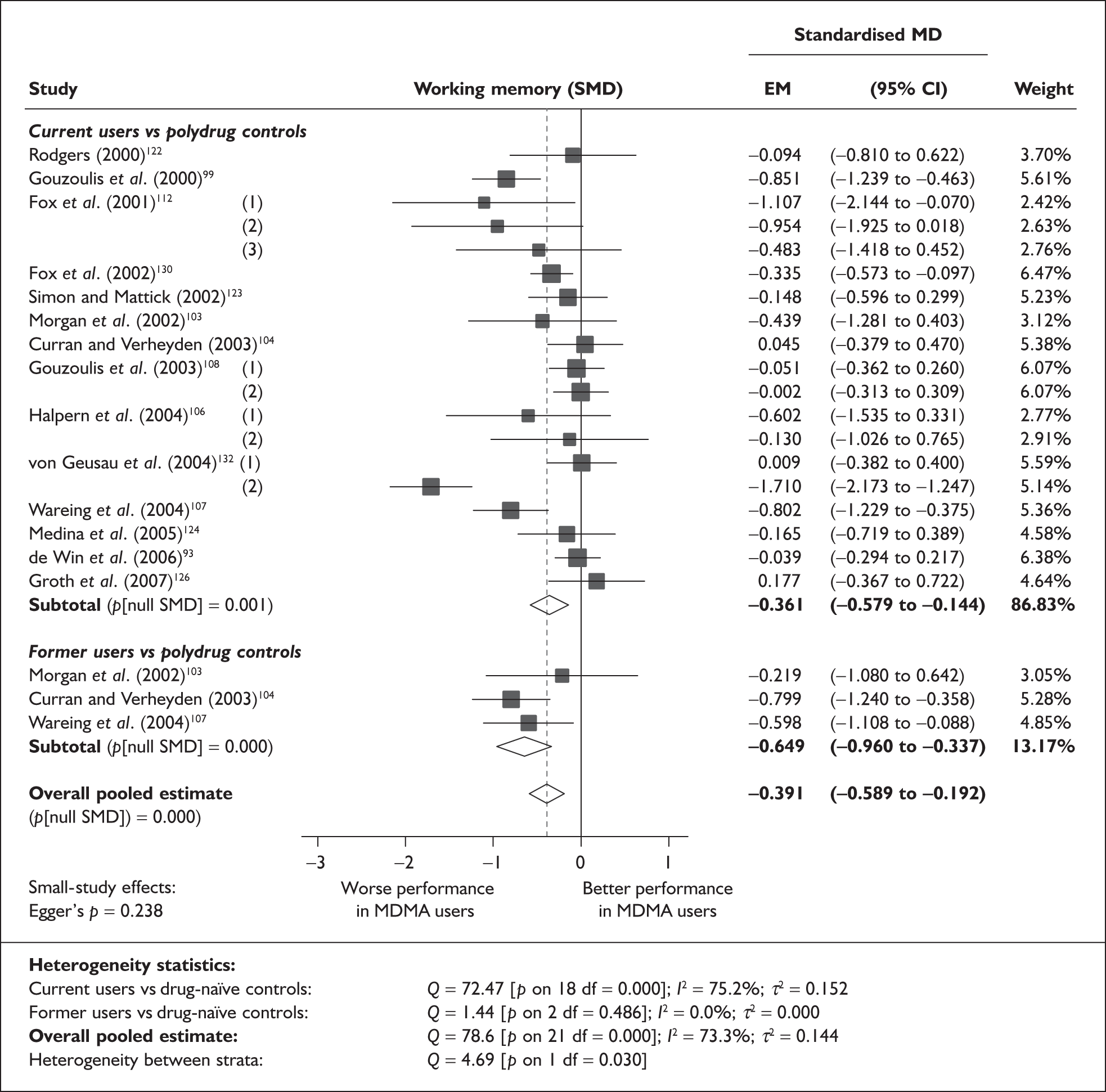
A representative datapoint from the underlying dataset is found in the 2002 study by Morgan et al. ,103 in which the ecstasy-using cohort made an average of 0.675 more errors than controls in the serial sevens subtraction task (1.725 versus 1.05; SMD – 0.439).
There is no evidence of small-study bias (Egger’s p = 0.238), and the funnel plot for this dataset (not shown) showed no pronounced trend, although there was a cluster of more powerful studies around the null effect point.
Sufficient data were available to attempt metaregression analyses for 15 covariates, shown in Table 23. There was no evidence of a dose–response effect (see Figure 104 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 20 | 0.036 | (–0.066 to 0.139) | 0.490 | |||
| Sex (% male) | 18 | 0.244 | (–0.975 to 1.464) | 0.695 | |||
| IQ | 7 | 0.074 | (–0.069 to 0.217) | 0.312 | |||
| Education (years) | 8 | –0.088 | (–0.524 to 0.348) | 0.692 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 14 | 0.000 | (–0.001 to 0.001) | 0.908 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 10 | 0.000 | (–0.001 to 0.001) | 0.790 | |||
| Duration of ecstasy use (days) | 17 | 0.000 | (–0.001 to 0.000) | 0.526 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 20 | –0.053 | (–0.185 to 0.078) | 0.426 | –0.342 | (–0.588 to –0.096) | 0.006 |
| Sex (% male) | 18 | –1.922 | (–3.550 to –0.294) | 0.021 | –0.239 | (–0.465 to –0.013) | 0.038 |
| Baseline intelligence measures (SMD) | 16 | 0.356 | (–0.319 to 1.032) | 0.301 | –0.292 | (–0.561 to –0.024) | 0.033 |
| Education (years) | 8 | 0.365 | (0.036–0.694) | 0.030 | –0.101 | (–0.471 to 0.269) | 0.592 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 14 | 0.014 | (–0.518 to 0.546) | 0.959 | –0.324 | (–0.671 to 0.024) | 0.068 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 13 | –0.082 | (–1.233 to 1.069) | 0.889 | –0.220 | (–1.010 to 0.571) | 0.586 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 8 | –0.461 | (–1.533 to 0.612) | 0.400 | 0.041 | (–0.948 to 1.030) | 0.935 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 9 | 0.383 | (0.089–0.677) | 0.011 | –0.224 | (–0.355 to –0.092) | 0.001 |
Figure 42 plots working memory performance against inter-arm asymmetry in gender. The most immediately noticeable feature of the graph is the dense cluster of datapoints around the origin; this suggests that those studies that were well matched for gender tended to show no difference in working memory between arms. Otherwise, the preponderance of data appears in the ‘south-east’ quadrant of the graph, showing that, where ecstasy-using participants were more likely to be men than controls, they tended to record worse test scores.
FIGURE 42.
Working memory (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in gender.
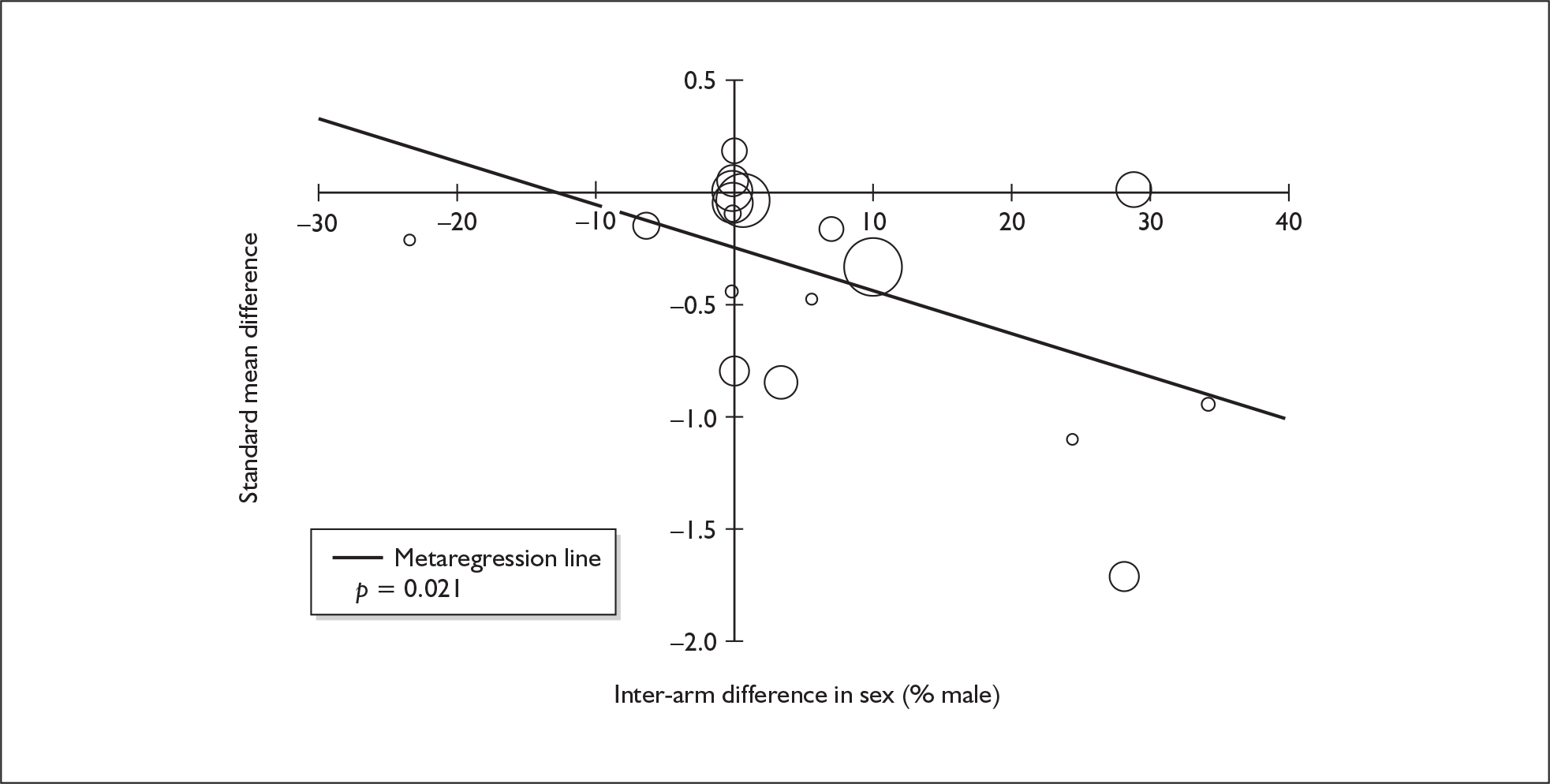
The relationship between inter-arm asymmetry in education and the response variable is visualised in Figure 43. The positive coefficient suggests that, in the various tasks synthesised here, worse performance tends to be seen amongst those ecstasy-exposed cohorts who had also received less education, on average, than their respective controls. There was limited availability of covariate data so this analysis is based on a fairly small subset of the full dataset; however, if the model were to be accepted, it would entirely explain the inter-population difference that might otherwise be ascribed to exposure to ecstasy.
FIGURE 43.
Working memory (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in education.
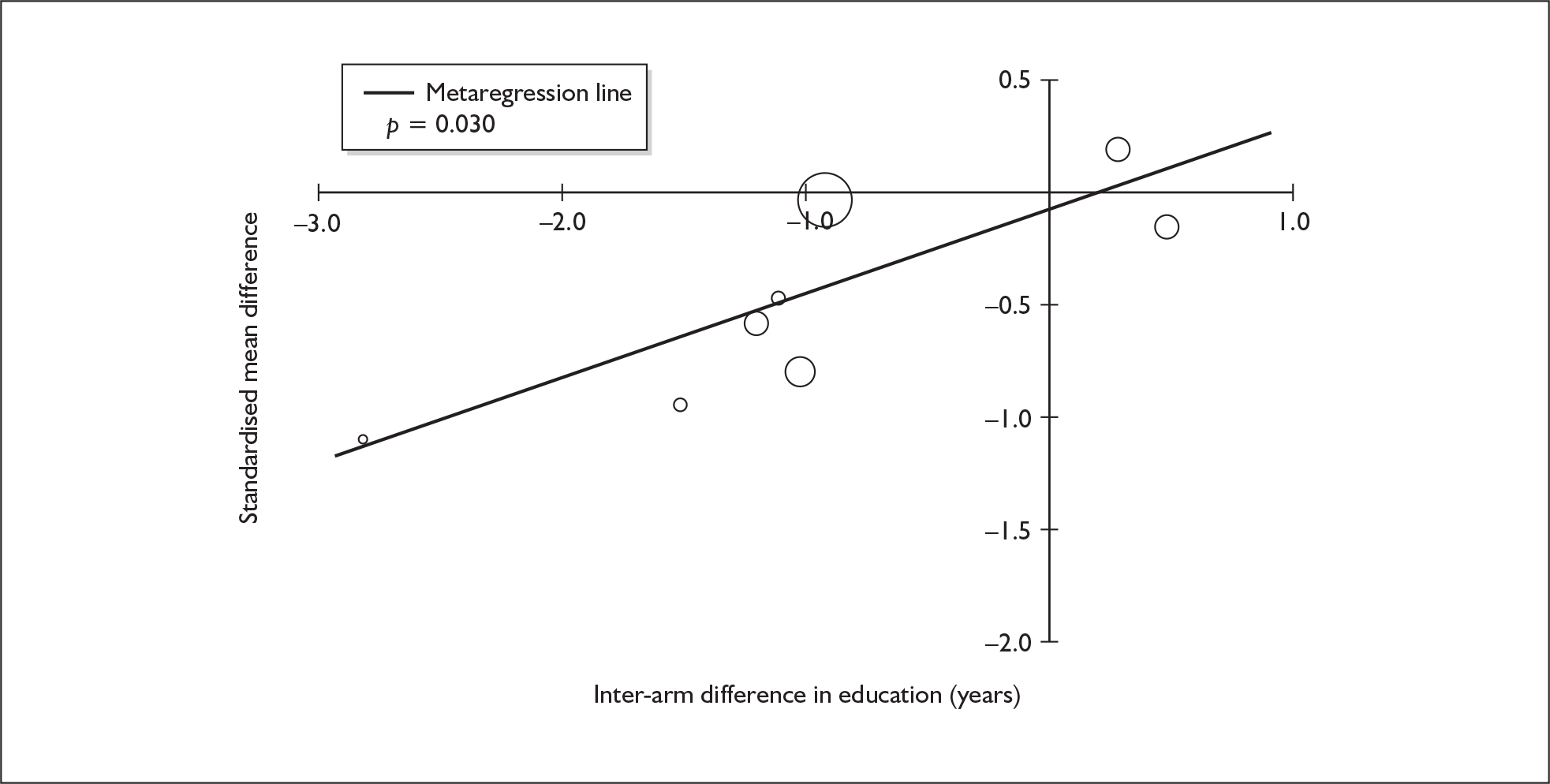
Figure 44 plots inter-arm asymmetry in exposure to alcohol against working memory performance. It shows that, once more, greater exposure to alcohol appears to be associated with better relative performance in the ecstasy-exposed cohort. The adjusted effect estimate from this analysis is, at – 0.224, a fair amount lower than that calculated in the primary analysis; however, because the regression gradient is relatively shallow, the overall exposure effect remains significant (p = 0.001).
FIGURE 44.
Working memory (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in exposure to alcohol (standardised mean difference).
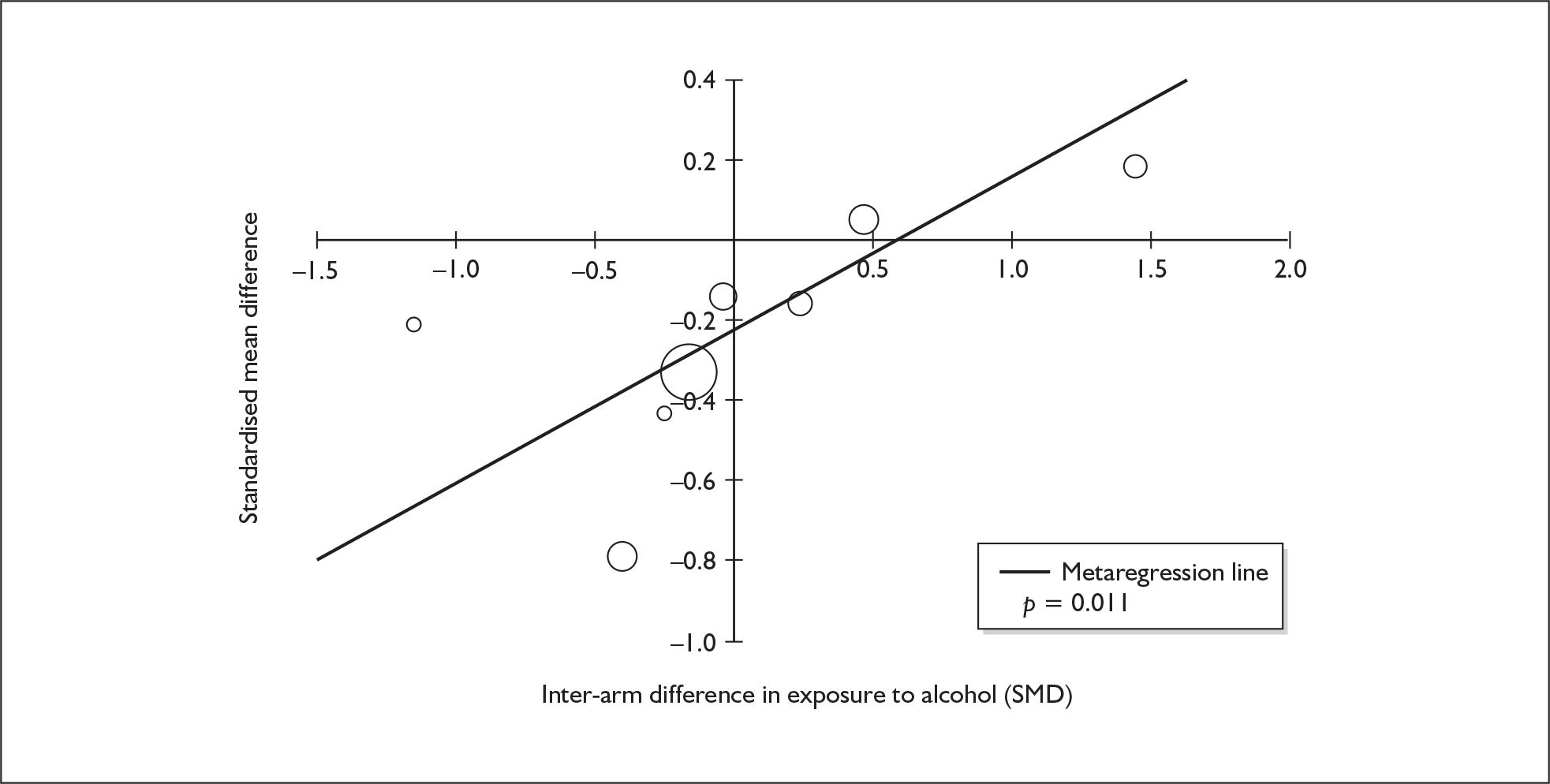
Working memory – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 12 datapoints, representing a total of seven pairwise comparisons, drawn from five different studies (six comparisons from five studies providing data for current ecstasy users and one comparison from one study providing data for former ecstasy users). Ten different outcome measures are included, none of which is adopted in more than one study. The complete dataset is detailed in Table 62 in Appendix 6.
When meta-analysed (Figure 45), this dataset suggests an exposure effect in the order of 0.5 SD, which slightly exceeds that seen in the comparison with polydrug controls and would be classified as a ‘medium’-sized effect in Cohen’s schema.
FIGURE 45.
Working memory (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
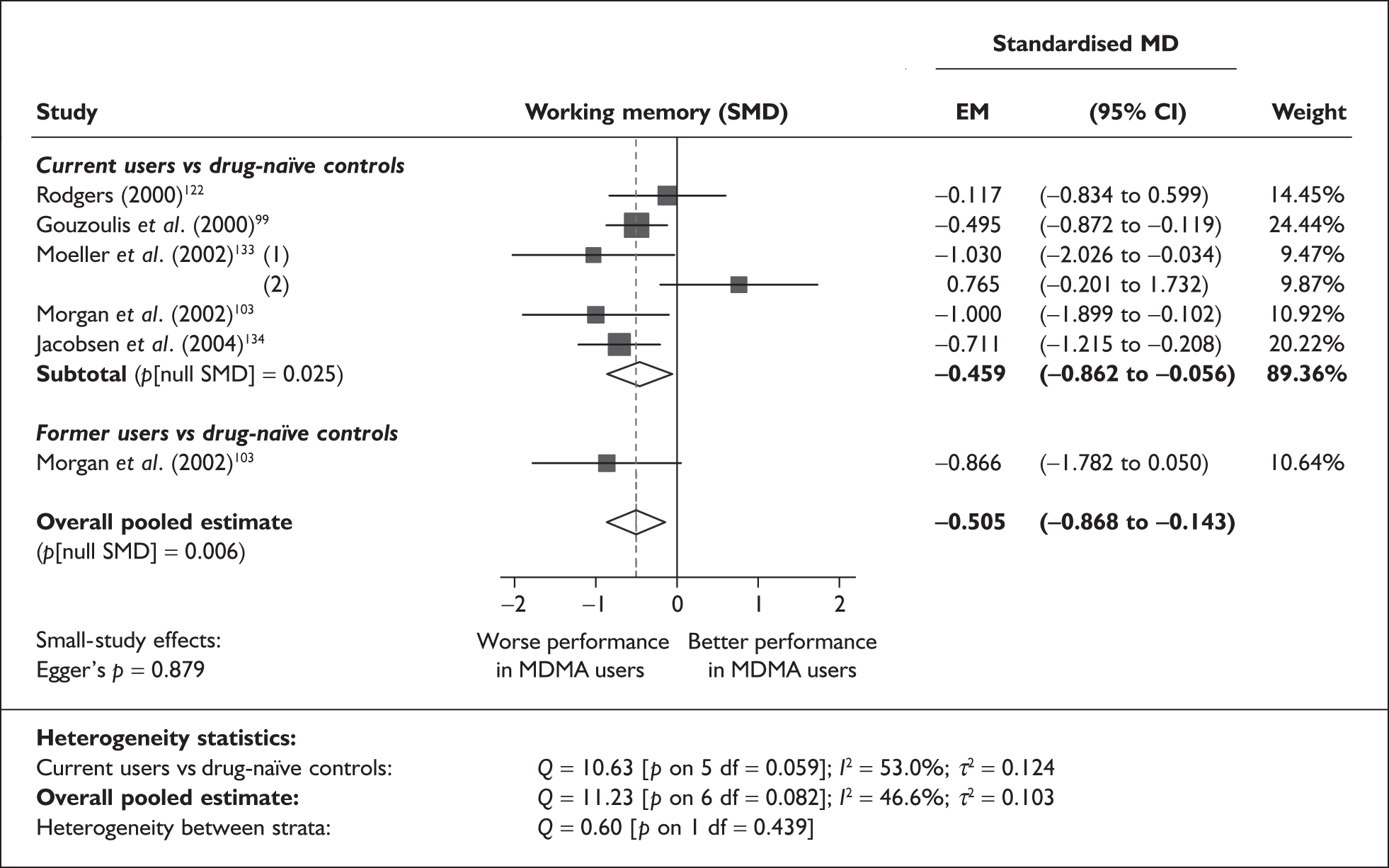
There is no evidence of small-study bias in this dataset (Egger’s p = 0.879), and the funnel plot (not shown) had an unremarkable appearance.
Because of the very small size of this dataset, it was only possible to perform metaregressions on four covariates; none was significant (Table 24), and there was no evidence of a dose–response effect (see Figure 105 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 7 | 0.049 | (–0.034 to 0.132) | 0.248 | |||
| Sex (% male) | 5 | 0.819 | (–1.421 to 3.060) | 0.474 | |||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 5 | –0.002 | (–0.005 to 0.001) | 0.150 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 7 | –0.195 | (–0.434 to 0.044) | 0.109 | –0.557 | (–0.884 to –0.230) | 0.001 |
| Sex (% male) | 5 | 0.699 | (–3.873 to 5.272) | 0.764 | –0.569 | (–0.839 to –0.298) | 0.000 |
| Baseline intelligence measures (SMD) | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
Attention (focus–execute) – MDMA users versus polydrug controls
The dataset assembled for this measure is the largest in this review. It comprises 119 datapoints, representing a total of 30 pairwise comparisons, drawn from 19 different studies (26 comparisons from 19 studies providing data for current ecstasy users and four comparisons from four studies providing data for former ecstasy users). In total, 49 different outcome measures are included, the most common being TMT: Part A – time (seven datapoints), TMT: Part B – time (seven datapoints) and Stroop: colour reading – time (six datapoints). The complete dataset is detailed in Table 61 in Appendix 6.
When synthesised in a random-effects meta-analysis (Figure 46), these data suggest that ecstasy-exposed populations tend to perform worse than polydrug controls by a little over 0.2 SD. This would be considered a ‘small’ inter-population difference, according to Cohen’s schema. There is no evidence of interstratum heterogeneity. Sensitivity analysis using study-level aggregated data produced similar results [SMD – 0.256; 95% CI – 0.360 to – 0.153; p(null SMD) < 0.001].
FIGURE 46.
Attention – focus–execute (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
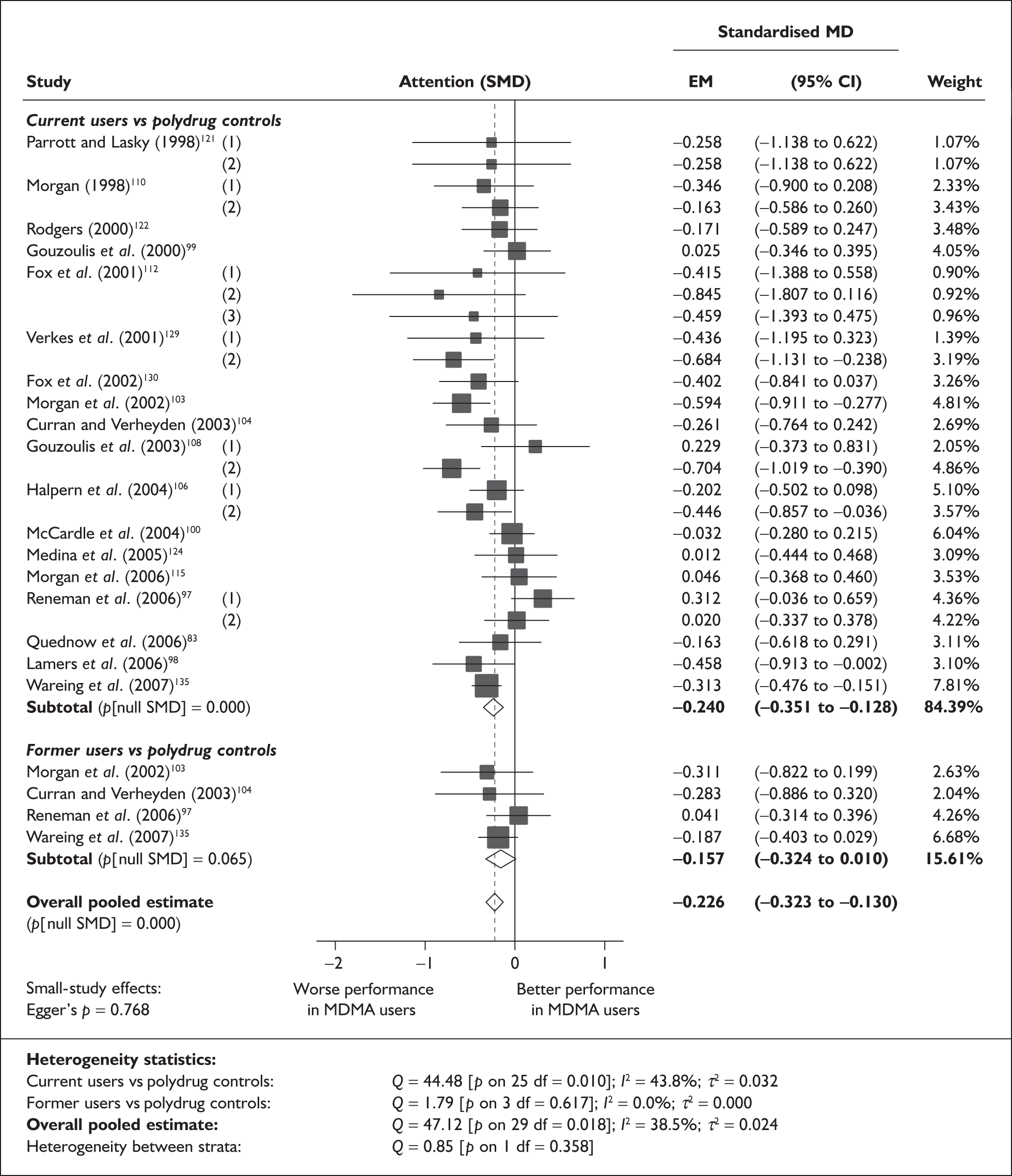
To compare the pooled estimate with a typical datapoint from a well-known instrument from the underlying dataset, a good example would be the WAIS digit–symbol test reported by McCardle et al. ,100 in which current ecstasy users scored 2.01 points less than controls (64.06 versus 66.07; SMD – 0.205).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.768), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 17 covariates; details are shown in Table 25. There was no evidence of a dose–response effect (see Figure 106 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 28 | 0.024 | (–0.016 to 0.065) | 0.235 | |||
| Sex (% male) | 26 | –0.091 | (–0.650 to 0.468) | 0.750 | |||
| IQ | 14 | –0.028 | (–0.063 to 0.006) | 0.105 | |||
| Education (years) | 8 | –0.075 | (–0.162 to 0.013) | 0.094 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 18 | 0.000 | (–0.001 to 0.000) | 0.642 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 16 | 0.000 | (0.000–0.000) | 0.416 | |||
| Duration of ecstasy use (days) | 18 | 0.000 | (0.000–0.000) | 0.698 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 28 | –0.049 | (–0.092 to –0.006) | 0.025 | –0.211 | (–0.300 to –0.122) | 0.000 |
| Sex (% male) | 26 | 0.655 | (–0.102 to 1.411) | 0.090 | –0.206 | (–0.312 to –0.100) | 0.000 |
| Baseline intelligence measures (SMD) | 19 | 0.146 | (–0.155 to 0.446) | 0.341 | –0.151 | (–0.297 to –0.004) | 0.044 |
| Education (years) | 8 | 0.025 | (–0.103 to 0.153) | 0.703 | –0.223 | (–0.365 to –0.081) | 0.002 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 16 | –0.227 | (–0.458 to 0.004) | 0.054 | –0.183 | (–0.319 to –0.047) | 0.008 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 15 | –0.068 | (–0.475 to 0.340) | 0.745 | –0.124 | (–0.387 to 0.138) | 0.353 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 13 | 0.094 | (–0.125 to 0.313) | 0.399 | –0.204 | (–0.454 to 0.046) | 0.110 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 18 | 0.019 | (–0.277 to 0.316) | 0.898 | –0.192 | (–0.337 to –0.048) | 0.009 |
Figure 47 depicts the influence of inter-arm asymmetry in age on the outcome of interest. It shows that, in studies in which ecstasy users were younger than controls, inter-population differences tended to be relatively slight but, where they were older, the exposure effect had a tendency to be larger. However, because this dataset is relatively well balanced on this variable, this gradient has no notable effect on the overall pooled effect estimate (the adjusted value is only 0.01 SD lower than the base-case estimate).
FIGURE 47.
Attention – focus-execute (composite measure) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in age.
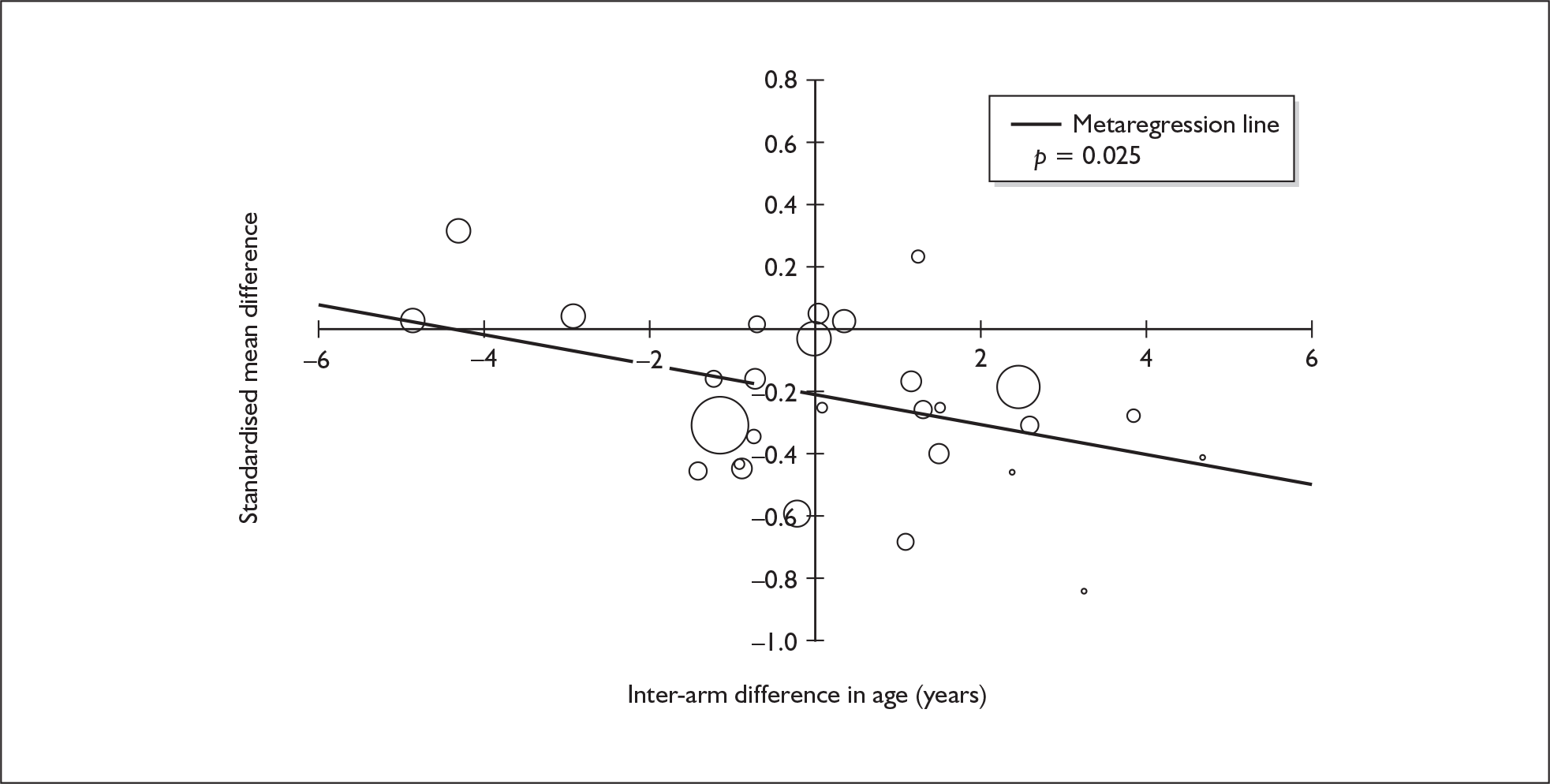
Attention (focus–execute) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 36 datapoints, representing a total of 16 pairwise comparisons, drawn from 12 different studies (14 comparisons from 12 studies providing data for current ecstasy users and two comparisons from two studies providing data for former ecstasy users). A total of 21 different outcome measures are included, the most common being MFFT-20: total errors (six datapoints), MFFT-20: latency to first response (six datapoints) and TMT: Part B – errors (two datapoints). The complete dataset is detailed in Table 62 in Appendix 6.
Our random-effect meta-analysis of these data (Figure 48) suggests that ecstasy users tended to perform worse than controls by a little over one-quarter of an SD. This result is comparable to that seen in the comparison between ecstasy users and polydrug controls (see Figure 46). Sensitivity analysis using the aggregated data approach generated similar – though slightly more uncertain – results [SMD – 0.295; 95% CI – 0.538 to – 0.052; p(null SMD) = 0.017]. In the dataset on which this meta-analysis is based, the most typical datapoint is the nine-letter comparison speed task reported by Wareing et al. ,136 in which former ecstasy users achieved 0.8 fewer correct items than controls (11.7 versus 12.5; SMD – 0.288).
FIGURE 48.
Attention – focus–execute (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
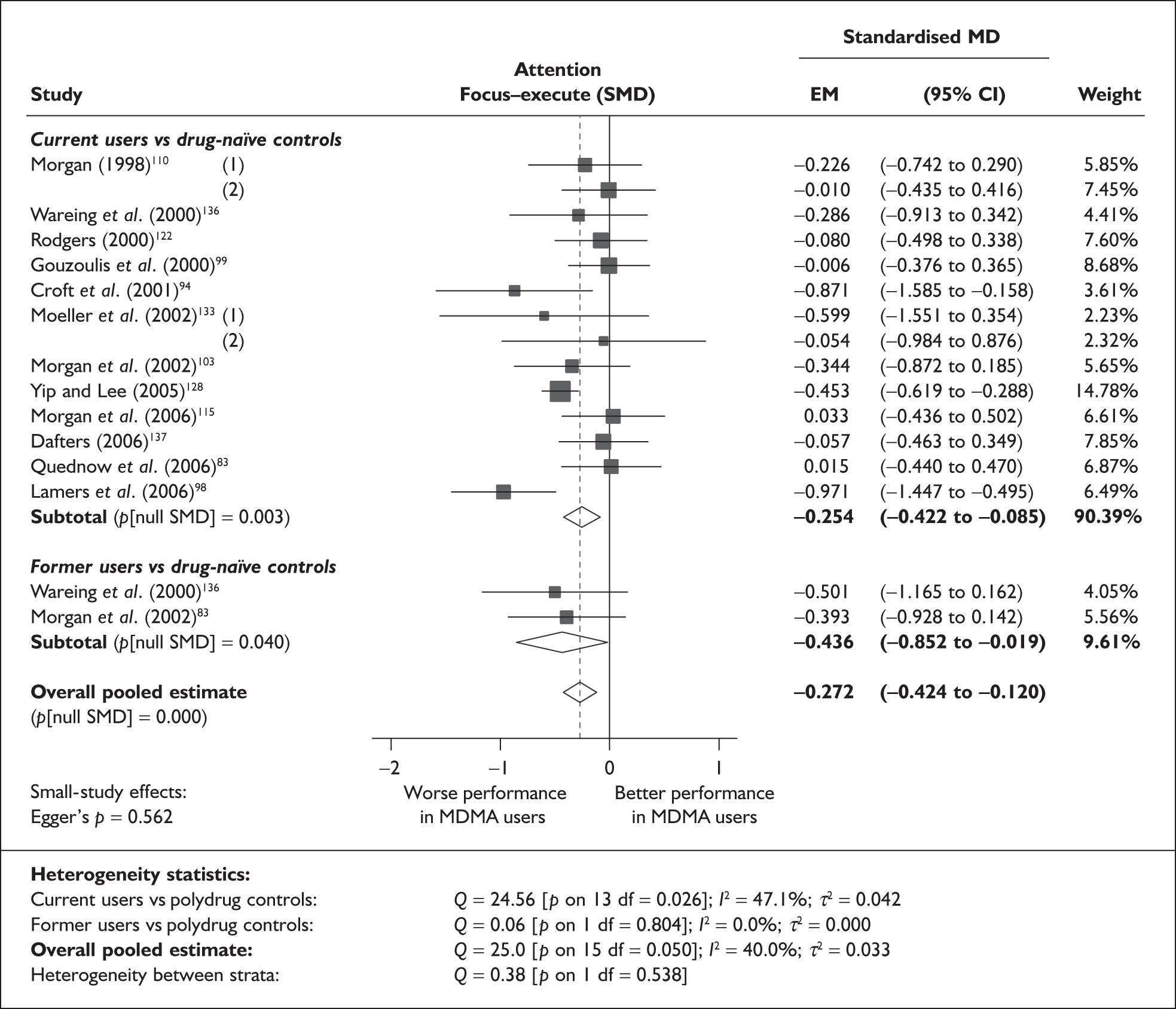
There is no evidence of small-study bias in this dataset (Egger’s p = 0.562); the funnel plot (not shown) suggests a slight trend towards lower exposure effects in higher-precision studies, but all datapoints appear within the expected range.
Sufficient data were available to attempt metaregression analyses for 13 covariates; details are shown in Table 26. None of the analyses were able to provide a statistically convincing explanation of the heterogeneity seen amongst base-case effect estimates, and there was no evidence of a dose–response effect (see Figure 107 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 16 | –0.010 | (–0.063 to 0.042) | 0.699 | |||
| Sex (% male) | 13 | 0.413 | (–0.690 to 1.515) | 0.463 | |||
| IQ | 6 | 0.077 | (–0.013 to 0.167) | 0.093 | |||
| Education (years) | 5 | –0.103 | (–0.271 to 0.064) | 0.227 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 12 | 0.000 | (0.000–0.001) | 0.405 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 9 | 0.000 | (–0.001 to 0.001) | 0.395 | |||
| Duration of ecstasy use (days) | 8 | 0.000 | (0.000–0.000) | 0.109 | |||
| Frequency of ecstasy use (occasions/month) | 5 | –0.055 | (–0.151 to 0.041) | 0.260 | |||
| Inter-arm differences | |||||||
| Age (years) | 16 | 0.017 | (–0.132 to 0.167) | 0.819 | –0.274 | (–0.430 to –0.118) | 0.001 |
| Sex (% male) | 13 | 0.493 | (–1.970 to 2.955) | 0.695 | –0.257 | (–0.454 to –0.060) | 0.011 |
| Baseline intelligence measures (SMD) | 8 | 0.107 | (–0.491 to 0.704) | 0.727 | –0.270 | (–0.548 to 0.007) | 0.056 |
| Education (years) | 5 | 0.580 | (–0.140 to 1.300) | 0.114 | –0.316 | (–0.607 to –0.025) | 0.033 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 6 | –0.184 | (–0.475 to 0.108) | 0.217 | –0.451 | (–0.778 to –0.124) | 0.007 |
Attention (sustain) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 27 datapoints, representing a total of 11 pairwise comparisons, drawn from seven different studies (eight comparisons from seven studies providing data for current ecstasy users and three comparisons from three studies providing data for former ecstasy users). Sixteen different outcome measures are included, the most common being G/N-G: correct responses (four datapoints), visual scanning: non-critical trials – time (three datapoints) and visual scanning: critical trials – time (three datapoints). The complete dataset is detailed in Table 63 in Appendix 6.
Our random-effects meta-analysis of these data (Figure 49) suggests that there is essentially no difference between populations, with no evidence of interstratum heterogeneity. However, for the only occasion in this review, our sensitivity analysis with data aggregated at study level generated markedly different results from our primary analysis, with a significant negative exposure effect estimated [SMD – 0.157; 95% CI – 0.304 to – 0.009; p(null SMD) = 0.037]. This borderline-significant estimate of an exposure effect may represent a more accurate synthesis of the available data, although, even if it is preferred, it remains a very small difference.
FIGURE 49.
Attention – sustain (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
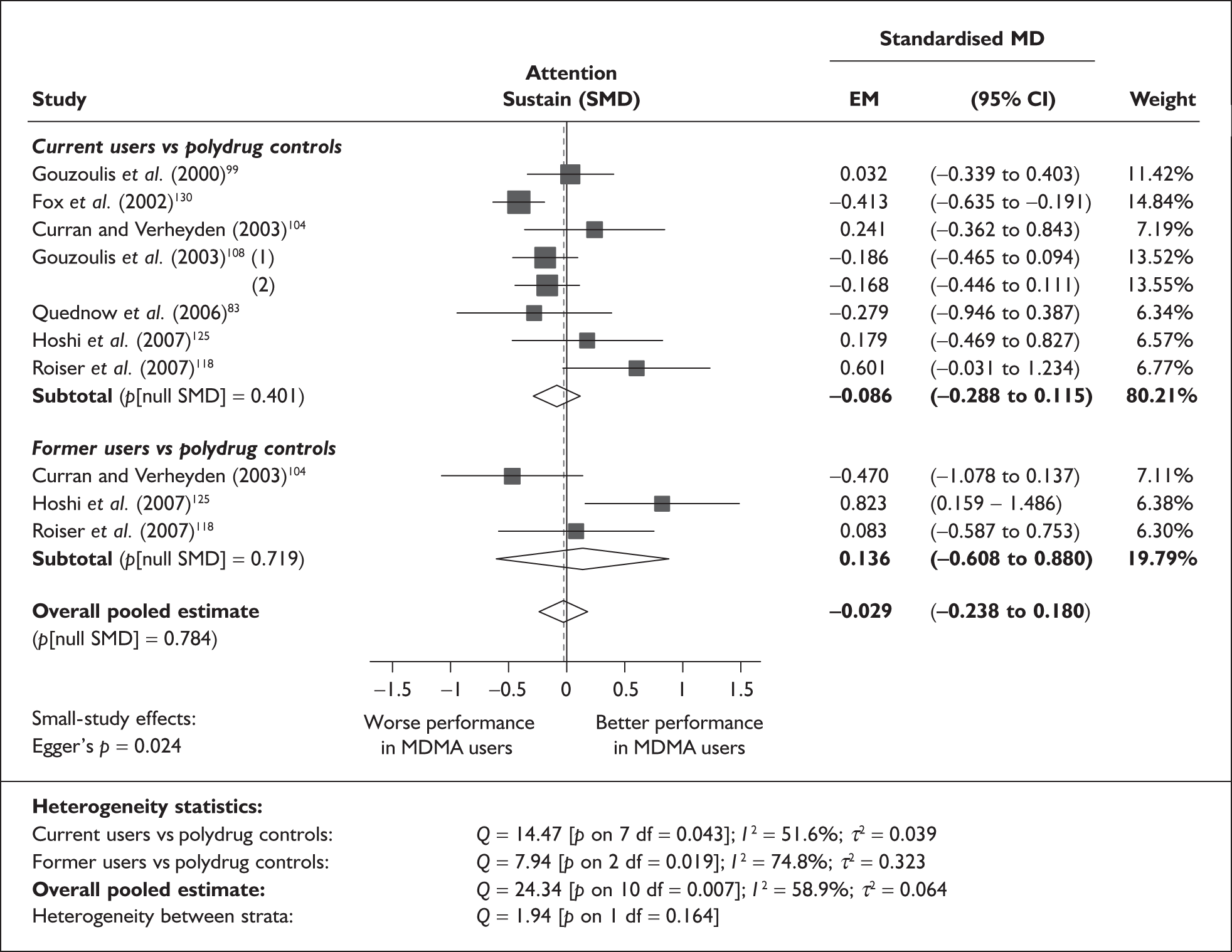
Although Egger’s test did achieve conventional levels of significance (p = 0.024), a positive coefficient is estimated by the test, which suggests that a greater negative exposure effect is associated with high precision estimates. This trend is clearly seen in the funnel plot for this dataset (Figure 50).
FIGURE 50.
Attention – sustain (composite measure) – ecstasy users versus polydrug controls: funnel plot.
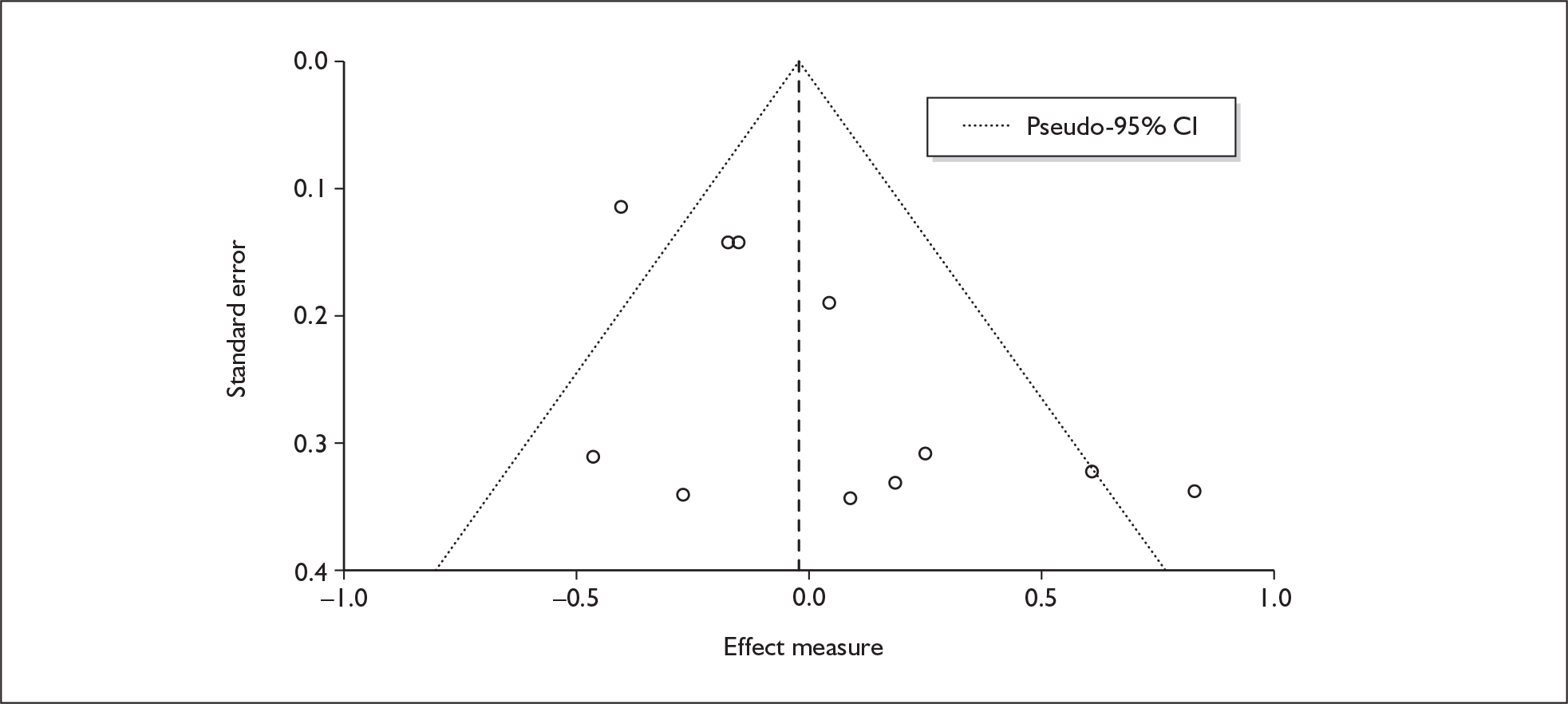
Sufficient data were available to attempt metaregression analyses for 17 covariates; details are shown in Table 27. There was no evidence of a dose–response effect (see Figure 108 in Appendix 7). A significant coefficient was estimated for one covariate: inter-arm asymmetry in exposure to amphetamines other than MDMA. This analysis is plotted in Figure 51. A relatively polarised picture can be seen: where ecstasy users had taken fewer amphetamines than controls, their performance was superior, and the opposite is the case where amphetamine consumption was greater in the ecstasy-exposed arms. The adjusted estimate of effect size remains consistent with a null hypothesis of no exposure effect.
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 11 | 0.038 | (–0.062 to 0.139) | 0.455 | |||
| Sex (% male) | 11 | 0.333 | (–0.623 to 1.288) | 0.495 | |||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 7 | 0.000 | (–0.001 to 0.001) | 0.774 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 6 | 0.000 | (0.000–0.001) | 0.421 | |||
| Duration of ecstasy use (days) | 7 | 0.000 | (0.000–0.001) | 0.547 | |||
| Frequency of ecstasy use (occasions/months) | 8 | 0.043 | (–0.066 to 0.151) | 0.443 | |||
| Inter-arm differences | |||||||
| Age (years) | 11 | –0.098 | (–0.207 to 0.010) | 0.076 | –0.078 | (–0.280 to 0.124) | 0.450 |
| Sex (% male) | 11 | –4.220 | (–9.602 to 1.162) | 0.124 | 0.045 | (–0.178 to 0.269) | 0.691 |
| Baseline intelligence measures (SMD) | 7 | 0.364 | (–0.395 to 1.124) | 0.347 | 0.107 | (–0.227 to 0.441) | 0.529 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 10 | –0.327 | (–0.710 to 0.057) | 0.095 | 0.099 | (–0.153 to 0.350) | 0.441 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 9 | –0.560 | (–0.936 to –0.184) | 0.004 | 0.168 | (–0.074 to 0.410) | 0.174 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 7 | –0.624 | (–1.440 to 0.192) | 0.134 | 0.414 | (–0.122 to 0.950) | 0.130 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 7 | 0.881 | (–0.038 to 1.800) | 0.060 | 0.000 | (–0.314 to 0.314) | 0.999 |
FIGURE 51.
Attention – sustain (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in exposure to amphetamines other than MDMA.
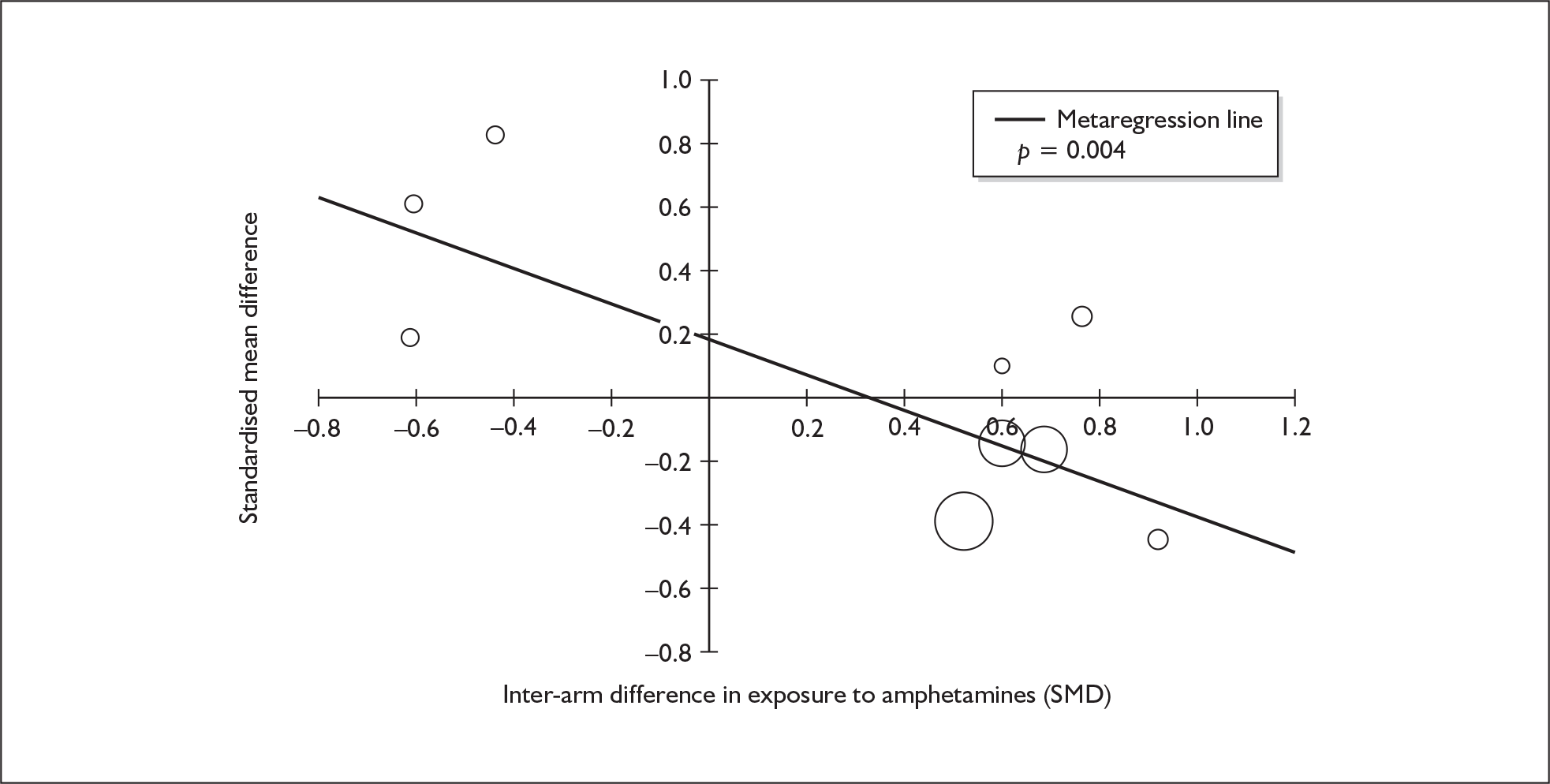
Although neither achieves a conventional level of statistical significance, two further metaregressions are worthy of note. First, the relationship between asymmetry in alcohol consumption and test performance appears to show quite a strong trend (Figure 52). As has been seen in other analyses, increased alcohol exposure appears to result in a lesser degree of underperformance in the ecstasy-exposed arms.
FIGURE 52.
Attention – sustain (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in exposure to alcohol (standardised mean difference).
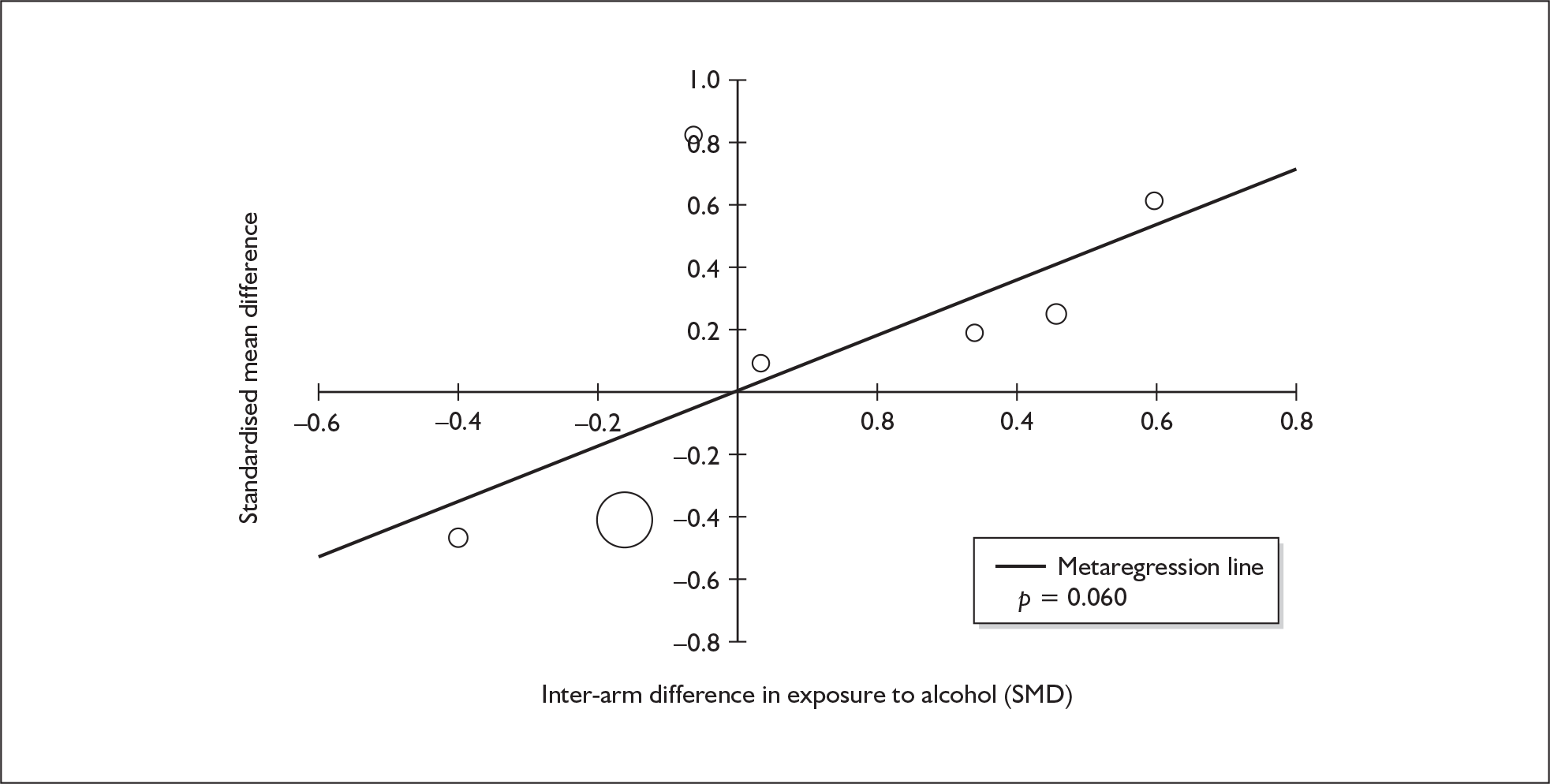
Second, Figure 53 shows the relationship between exposure effect and inter-arm imbalance in participant age. Although this metaregression did not reveal a statistically significant relationship, it is worth emphasising the strong similarity between this graph and the analogous analysis for attention focus–execute (see Figure 47). The coefficient estimated in that case suggests that, for every year by which ecstasy users were older than controls, the exposure effect can be expected to grow by 0.049 SD. For sustained attention, a coefficient of – 0.098 SD is estimated.
FIGURE 53.
Attention – sustain (composite measure) – ecstasy users versus polydrug controls: mean difference in score against inter-arm asymmetry in age.
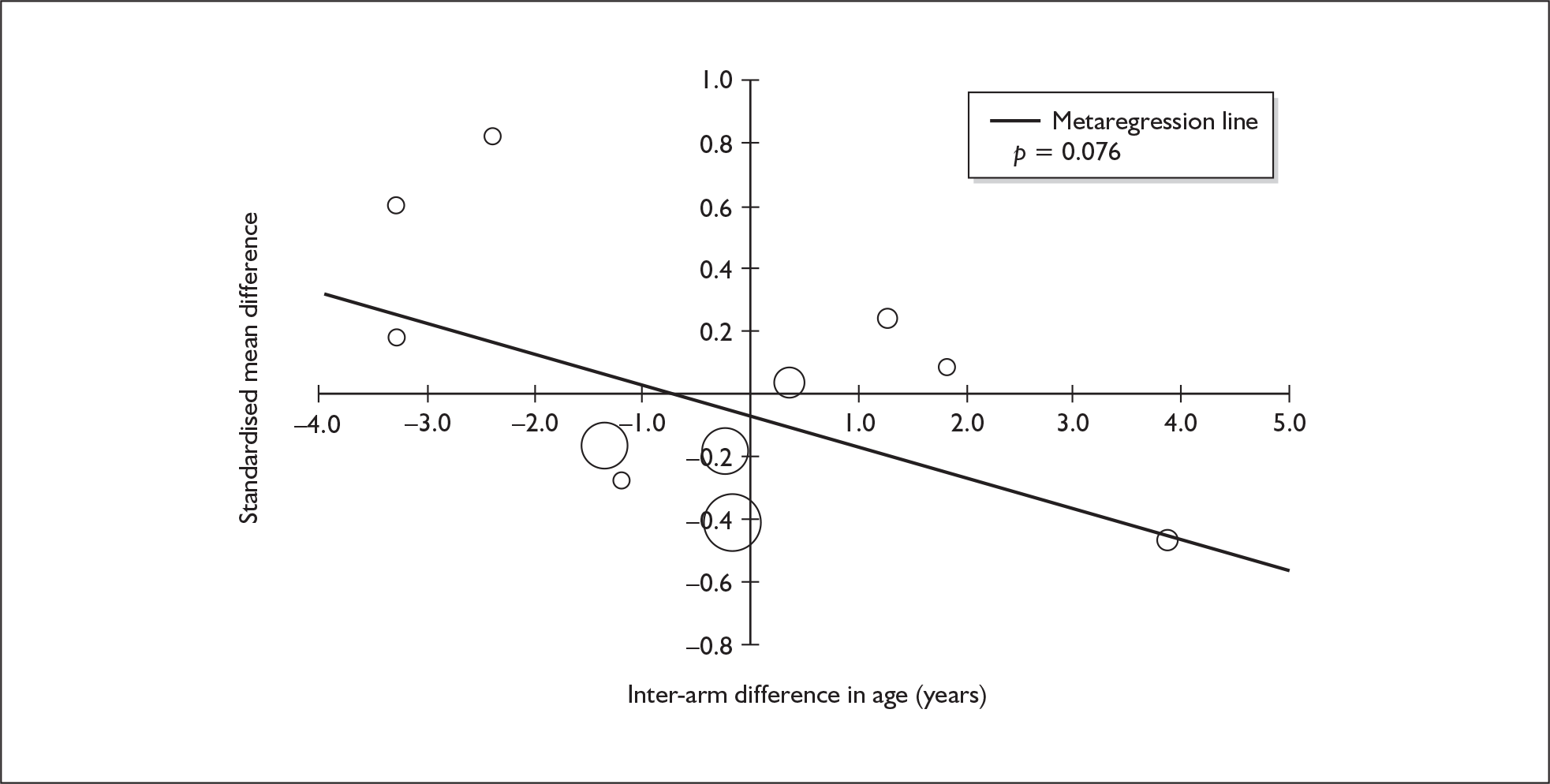
Attention (sustain) – MDMA users versus drug-naïve controls
Only four studies in the evidence-base reported measures of sustained attention in comparisons between ecstasy users and drug-naïve controls,61,83,99,125 so we did not pursue extensive analysis of this dataset. When meta-analysed according to the model used in other analyses, these data generate a non-significant SMD of 0.159 [95% CI – 0.180 to 0.498; p(null SMD) = 0.358].
Executive function (planning) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 40 datapoints, representing a total of 11 pairwise comparisons, drawn from five different studies (10 comparisons from five studies providing data for current ecstasy users and one comparison from one study providing data for former ecstasy users). Fourteen different outcome measures are included, the most common being ToL: Planning time (five datapoints). The complete dataset is detailed in Table 64 in Appendix 6.
Random-effects meta-analysis (Figure 54) estimates a pooled effect size of under 0.2 SD, i.e. less than a ‘small’ difference, in Cohen’s schema. The dataset appears to be relatively homogeneous. Sensitivity analysis with data aggregated at study level generated a very similar result [SMD – 0.179; 95% CI – 0.497 to 0.140; p(null SMD) = 0.271].
FIGURE 54.
Executive function – planning (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
There is no evidence of small-study bias in this dataset (Egger’s p = 0.525), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 13 covariates; details are shown in Table 28. There was no evidence of a dose–response effect (see Figure 109 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 11 | –0.042 | (–0.102 to 0.018) | 0.175 | |||
| Sex (% male) | 11 | 0.075 | (–0.801 to 0.950) | 0.867 | |||
| IQ | 5 | 0.097 | (0.027–0.167) | 0.006 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 6 | 0.000 | (–0.001 to 0.000) | 0.337 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 7 | –0.001 | (–0.002 to 0.000) | 0.043 | |||
| Duration of ecstasy use (days) | 11 | 0.000 | (0.000–0.000) | 0.694 | |||
| Frequency of ecstasy use (occasions/month) | 6 | –0.047 | (–0.255 to 0.161) | 0.656 | |||
| Inter-arm differences | |||||||
| Age (years) | 11 | –0.053 | (–0.123 to 0.017) | 0.141 | –0.166 | (–0.305 to –0.026) | 0.020 |
| Sex (% male) | 11 | –0.519 | (–1.475 to 0.438) | 0.288 | –0.138 | (–0.299 to 0.023) | 0.093 |
| Baseline intelligence measures (SMD) | 9 | –0.232 | (–0.638 to 0.173) | 0.261 | –0.224 | (–0.401 to –0.048) | 0.013 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 8 | –0.156 | (–0.487 to 0.176) | 0.357 | 0.003 | (–0.272 to 0.278) | 0.983 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 8 | –0.098 | (–0.463 to 0.268) | 0.600 | –0.066 | (–0.283 to 0.151) | 0.553 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 5 | –0.035 | (–0.510 to 0.440) | 0.885 | –0.023 | (–0.478 to 0.431) | 0.920 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
Significant coefficients were estimated for two covariates: study-level average IQ (classical metaregression, Figure 55) and duration of abstinence from ecstasy (Figure 56). For the former, a clear shape is seen amongst the five datapoints, with lower average IQ associated with a larger disadvantage for ecstasy users in the outcomes of interest. However, it is easy to conclude that the neatness of the correlation is dependent on the small number of contributing datapoints. For the latter, a less visually arresting significant association is seen. It may be that, as this picture suggests, the ecstasy users that have been abstinent for the longest are those that perform least well in comparison to controls but, given the small sample and less-than-unequivocal p-value, a Type I error is a real danger, so a degree of scepticism is probably appropriate.
FIGURE 55.
Executive function – planning (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against IQ (across all participants).
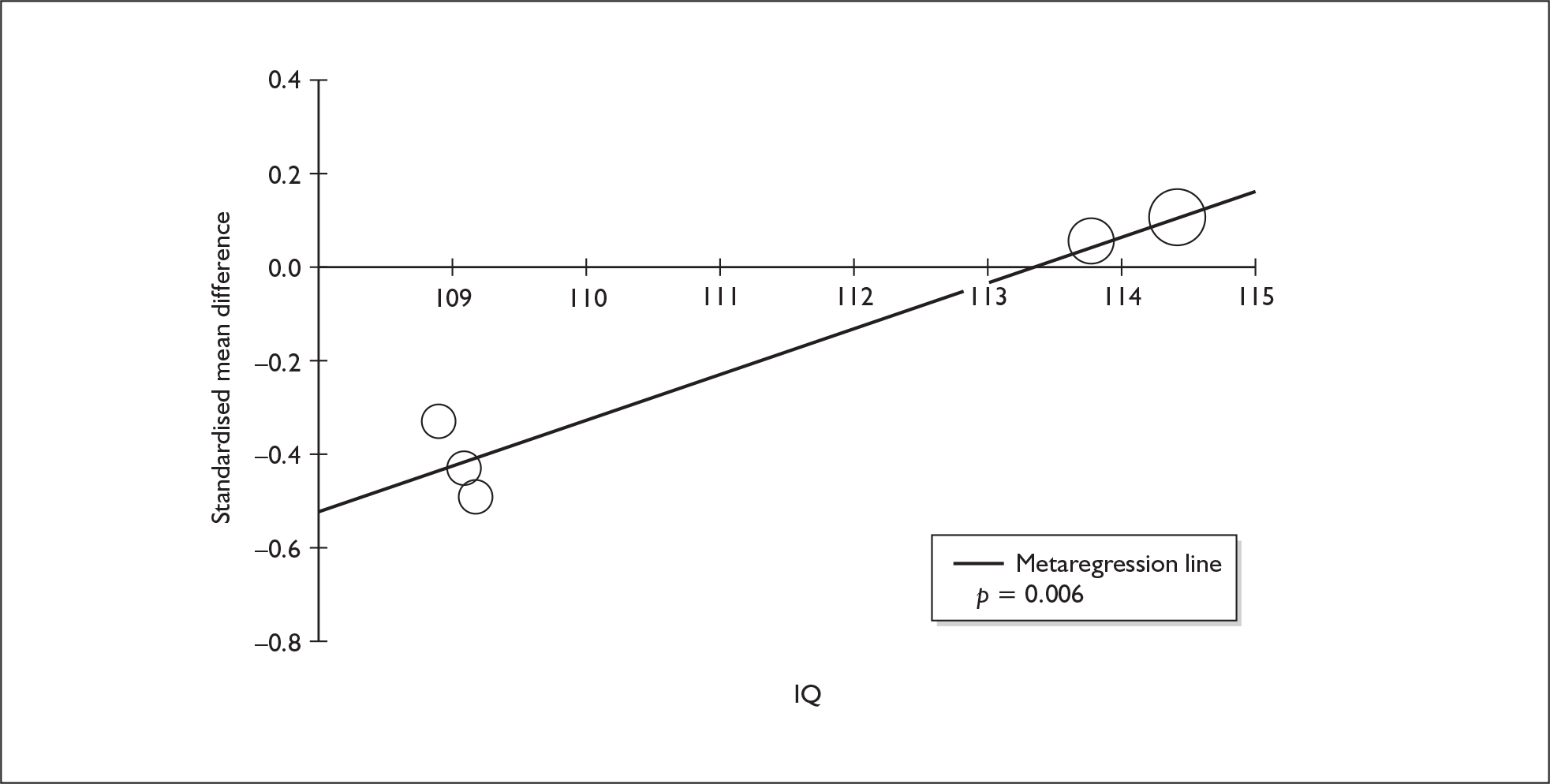
FIGURE 56.
Executive function – planning (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against duration of abstinence in ecstasy users.
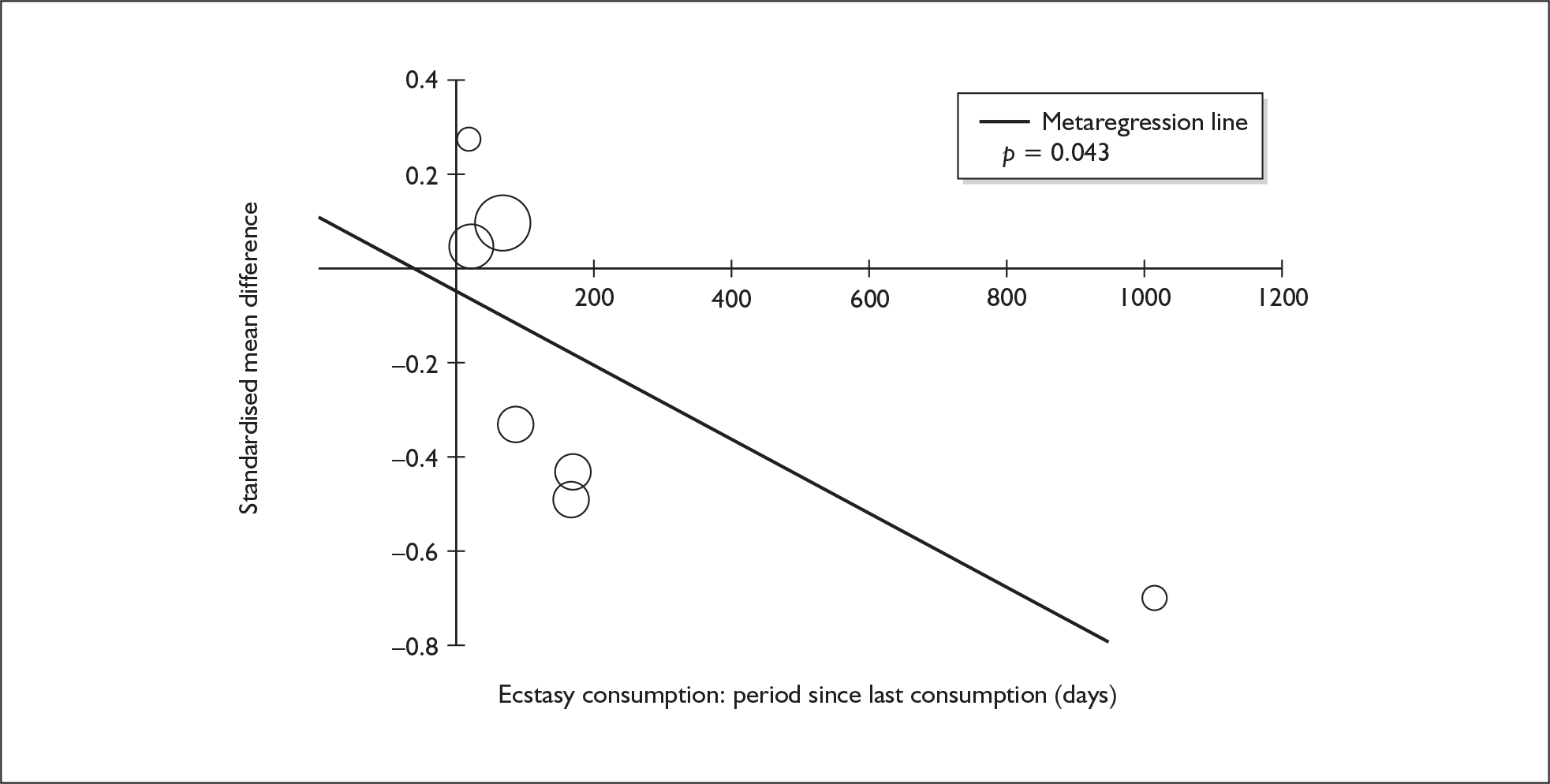
Executive function (planning) – MDMA users versus drug-naïve controls
We were only able to identify two studies reporting the appropriate comparison for this outcome. 110,125 When meta-analysed according to the model used elsewhere in this review, a small, non-significant SMD of – 0.170 (95% CI – 0.484 to 0.144) was estimated.
Executive function (response inhibition) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 34 datapoints, representing a total of 21 pairwise comparisons, drawn from 13 different studies (18 comparisons from 13 studies providing data for current ecstasy users and three comparisons from three studies providing data for former ecstasy users). Eighteen different outcome measures are included, the most common being Stroop: interference – time (seven datapoints) and G/N-G: reaction time (four datapoints). The complete dataset is detailed in Table 65 in Appendix 6.
When synthesised in a random-effects meta-analysis (Figure 57), these data suggest that there is no difference between ecstasy users and polydrug controls in this domain. The estimated SMD of approximately 0.1 SD would be considered very small, even if the difference was certain enough to meet conventional levels of significance. Sensitivity analysis with study-level aggregate data reveals a similar picture [SMD – 0.090; 95% CI – 0.338 to 0.159; p(null SMD) = 0.480]. We note that one datapoint in the forest plot appears atypical: the comparison between female ecstasy users and controls in von Geusau et al. 132 Above all, this extreme value is driven by performance in the HvdM Eriksen–Flankers test, in which ecstasy users outperformed controls by 3.7 SD (99.3% correct versus 96.7% correct; it should be noted that although this may seem to be a relatively small discrepancy, both estimates are subject to very small variance, so the difference between them is strongly significant and, when standardised, it becomes substantial). If the entire comparison for the female subgroup in this investigation is removed from analysis, then the estimated overall difference between populations does become borderline-significant, although the effect size remains small [SMD – 0.172; 95% CI – 0.336 to – 0.008; p(effect = 0) = 0.040].
FIGURE 57.
Executive function – response inhibition (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
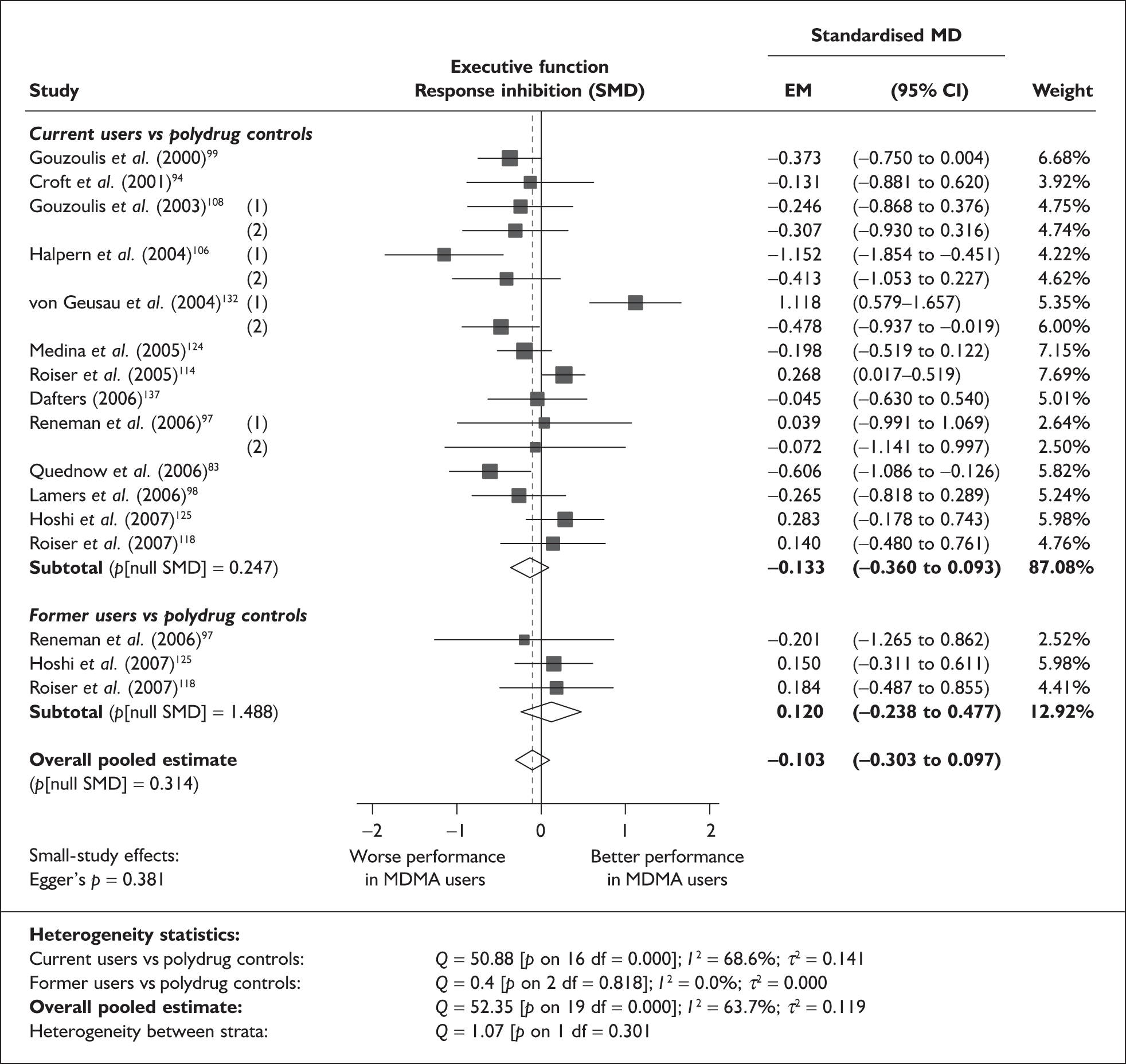
There is no evidence of small-study bias in this dataset (Egger’s p = 0.381), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 14 covariates, shown in Table 29. There was no evidence of a dose–response effect (see Figure 110 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| age (years) | 18 | 0.019 | (–0.056 to 0.093) | 0.626 | |||
| Sex (% male) | 18 | –0.219 | (–1.334 to 0.897) | 0.701 | |||
| IQ | 5 | 0.073 | (–0.008 to 0.153) | 0.077 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 11 | 0.000 | (–0.002 to 0.001) | 0.773 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | 10 | 0.000 | (0.000–0.001) | 0.570 | |||
| Frequency of ecstasy use (occasions/month) | 5 | 0.098 | (–0.122 to 0.317) | 0.383 | |||
| Inter-arm differences | |||||||
| Age (years) | 18 | –0.013 | (–0.140 to 0.114) | 0.838 | –0.052 | (–0.292 to 0.188) | 0.668 |
| Ssex (% male) | 18 | 0.815 | (–0.488 to 2.117) | 0.220 | –0.087 | (–0.291 to 0.117) | 0.404 |
| Baseline intelligence measures (SMD) | 12 | 0.540 | (0.020 to 1.060) | 0.042 | 0.018 | (–0.227 to 0.263) | 0.887 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 14 | –0.010 | (–0.370 to 0.349) | 0.955 | –0.048 | (–0.324 to 0.228) | 0.734 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 13 | –0.042 | (–0.568 to 0.484) | 0.876 | –0.001 | (–0.322 to 0.320) | 0.994 |
| Exposure to cocaine (ETLD) | 5 | 0.000 | (–0.005 to 0.004) | 0.830 | –0.041 | (–0.458 to 0.376) | 0.848 |
| Exposure to cocaine (SMD) | 11 | 0.150 | (–0.337 to 0.638) | 0.545 | –0.063 | (–0.484 to 0.357) | 0.767 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 9 | 0.053 | (–0.773 to 0.880) | 0.899 | –0.050 | (–0.251 to 0.152) | 0.628 |
Only one covariate generated a significant coefficient: inter-arm asymmetry in baseline intelligence. Figure 58 plots this variable against the outcome of interest. The preponderance of datapoints in the ‘south-west’ quadrant of the graph indicates that, in the majority of cases, ecstasy-exposed cohorts scored lower than controls on both the explanatory and response variables (i.e. they had lower baseline measures of intelligence and also performed worse on tests of response inhibition). The fact that the regression line passes through the graph’s origin suggests that, when one corrects for this imbalance, no inter-population difference would be expected at all.
FIGURE 58.
Executive function – response inhibition (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in baseline intelligence measures.
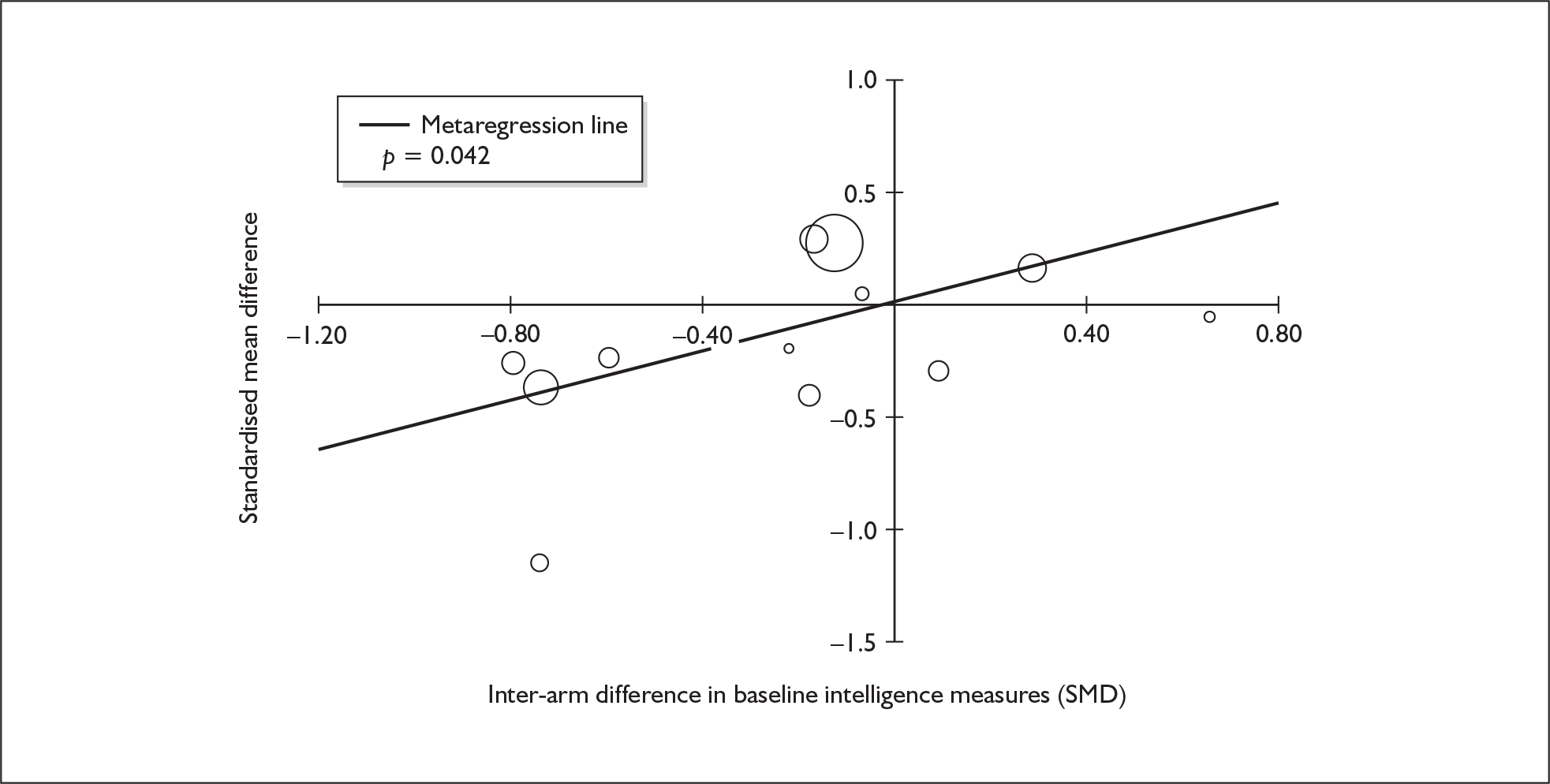
Executive function (response inhibition) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 15 datapoints, representing a total of 10 pairwise comparisons, drawn from eight different studies (eight comparisons from eight studies providing data for current ecstasy users and two comparisons from two studies providing data for former ecstasy users). Ten different outcome measures are included, the most common being Stroop: interference – time difference (three datapoints) and G/N-G (two datapoints). The complete dataset is detailed in Table 66 in Appendix 6.
When meta-analysed (Figure 59), this dataset is closely analogous to the results seen when comparing ecstasy users with polydrug-using controls (see Figure 57). As in that analysis, a small, non-significant difference is seen between cohorts, and inter-study heterogeneity is not especially pronounced. Sensitivity analysis with data aggregated at study level is comparable [SMD – 0.107; 95% CI – 0.364 to 0.151; p(null SMD) = 0.416].
FIGURE 59.
Executive function – response inhibition (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
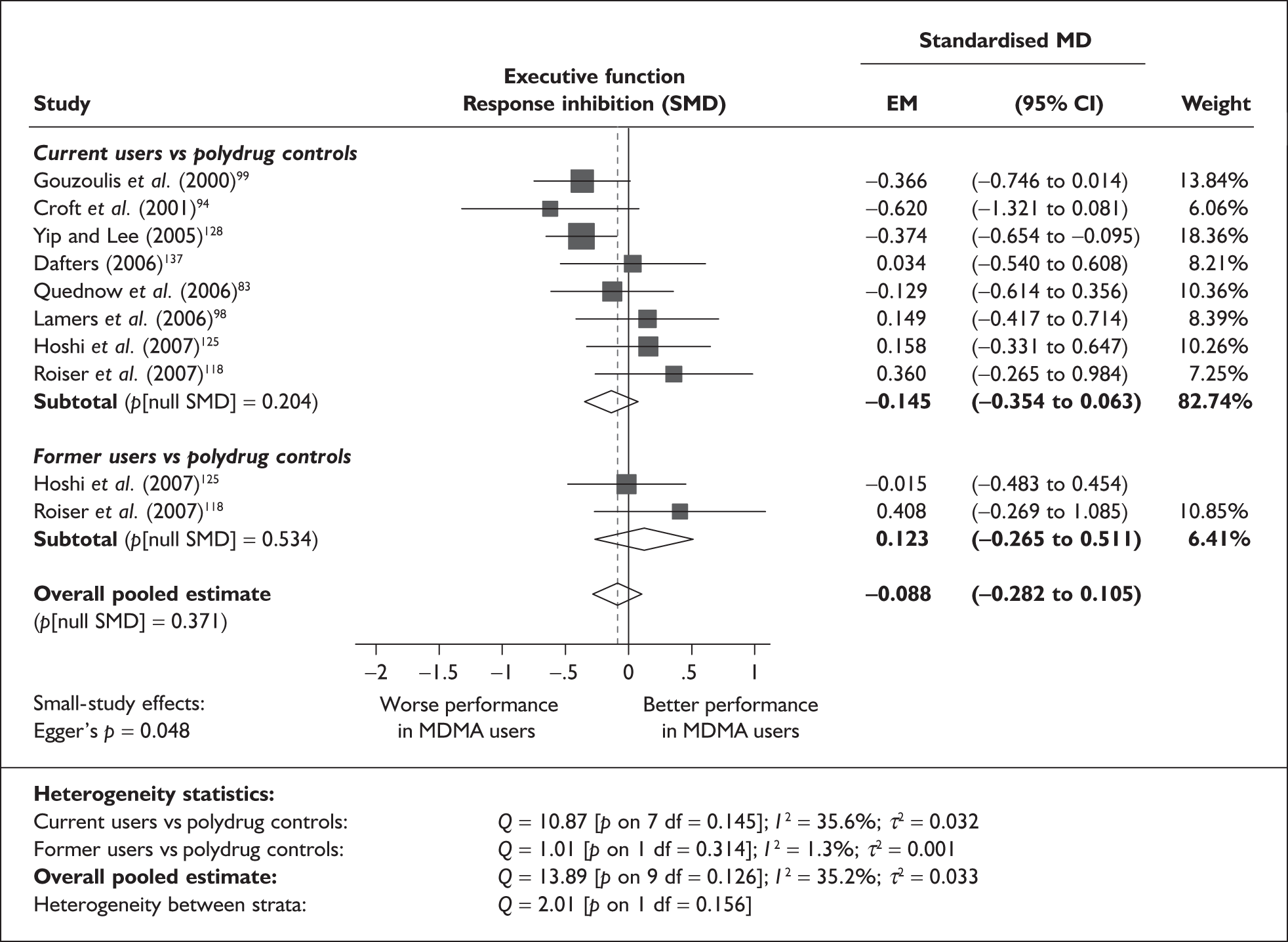
Although Egger’s test did achieve conventional levels of significance (p = 0.048), it seems unlikely that this analysis is biased by small-study effects. A positive coefficient is estimated by the test, which suggests that a greater exposure effect is associated with high precision estimates. This trend is clearly seen in the funnel plot for this dataset (Figure 60).
FIGURE 60.
Executive function – response inhibition (composite measure) – ecstasy users versus drug-naïve controls: funnel plot.
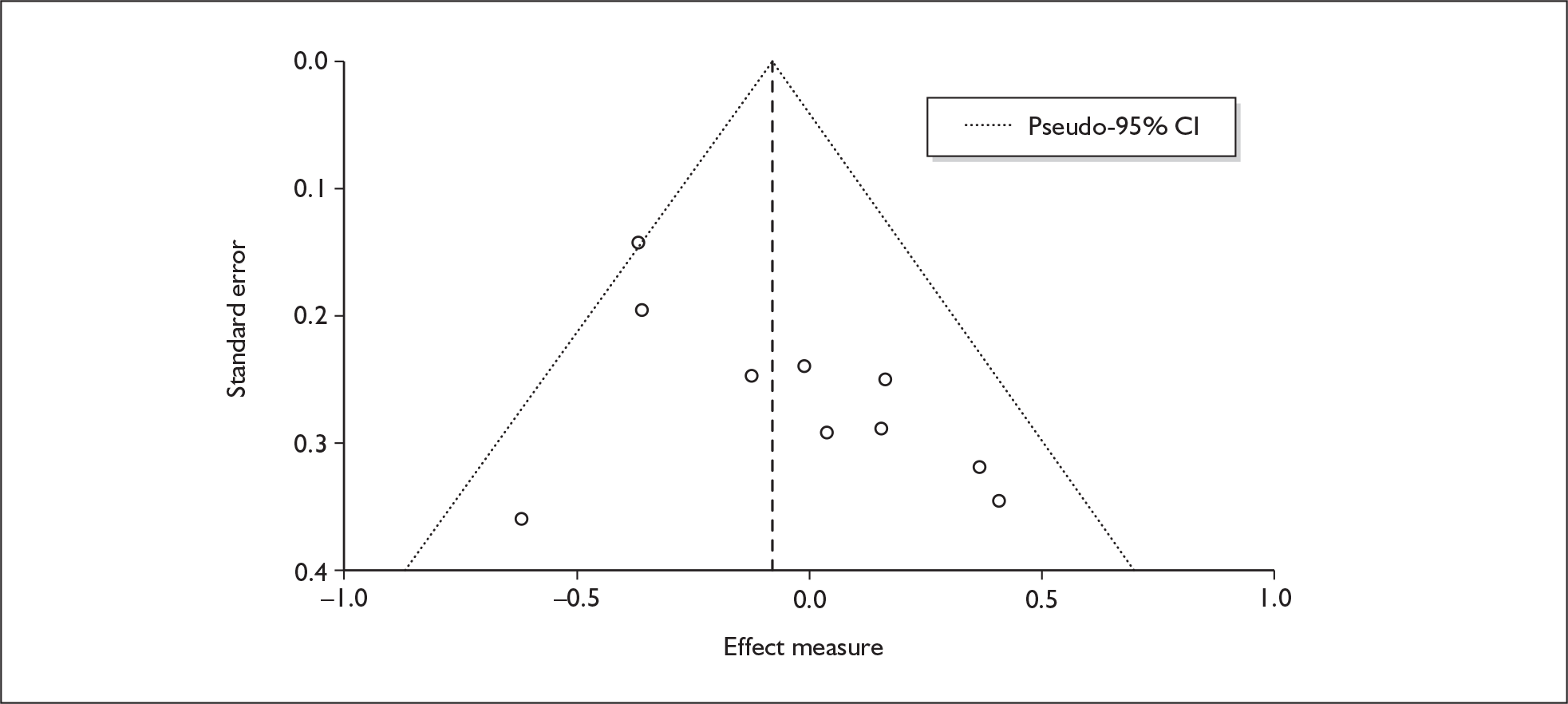
Sufficient data were available to attempt metaregression analyses for eight covariates; details are shown in Table 30. There was fairly good evidence of a dose–response effect (see Figure 111 in Appendix 7). It should be noted, however, that a positive coefficient is estimated, implying that those ecstasy-exposed cohorts that had taken most ecstasy were those that performed best in comparison to their respective controls.
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 10 | 0.000 | (–0.068 to 0.068) | 0.992 | |||
| Sex (% male) | 9 | 0.105 | (–0.851 to 1.060) | 0.830 | |||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 7 | 0.001 | (0.000–0.002) | 0.008 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 7 | 0.000 | (0.000–0.001) | 0.473 | |||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasons/months) | 5 | 0.084 | (–0.019 to 0.188) | 0.111 | |||
| Inter-arm differences | |||||||
| Age (years) | 10 | –0.033 | (–0.144 to 0.078) | 0.557 | –0.099 | (–0.304 to 0.106) | 0.342 |
| Sex (% male) | 9 | 2.262 | (–2.848 to 7.373) | 0.386 | –0.048 | (–0.250 to 0.155) | 0.645 |
| Baseline intelligence measures (SMD) | 5 | –0.115 | (–0.879 to 0.649) | 0.768 | –0.183 | (–0.581 to 0.215) | 0.368 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
Executive function (shifting) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 41 datapoints, representing a total of 13 pairwise comparisons, drawn from seven different studies (12 comparisons from seven studies providing data for current ecstasy users and one comparison from one study providing data for former ecstasy users). Fifteen different outcome measures are included, the most common being WCST: categories (eight datapoints), WCST: Total no. errors (four datapoints) and WCST: perseverative errors (four datapoints). The complete dataset is detailed in Table 67, in Appendix 6.
The meta-analysis for this data-set (Figure 61) is reminiscent of the two analyses seen for response inhibition (see Figures 57 and 59 respectively). Although a small exposure effect (with ecstasy users performing worse than polydrug controls) is estimated, the dataset is also entirely consistent with a null result. Sensitivity analysis with study-level aggregated data generates a similar result [SMD – 0.158; 95% CI – 0.635 to 0.319; p(null SMD) = 0.516]. Much as in the response inhibition analysis (see Figure 57), the good performance of the ecstasy-exposed participants in the female subgroup of von Geusau et al. 132 makes the datapoint appear to be somewhat of an outlier in the forest plot. If this comparison is excluded from the overall analysis, a significant exposure effect is estimated [SMD – 0.281; 95% CI – 0.509 to –0.054; p(null SMD) = 0.015].
FIGURE 61.
Executive function – shifting (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
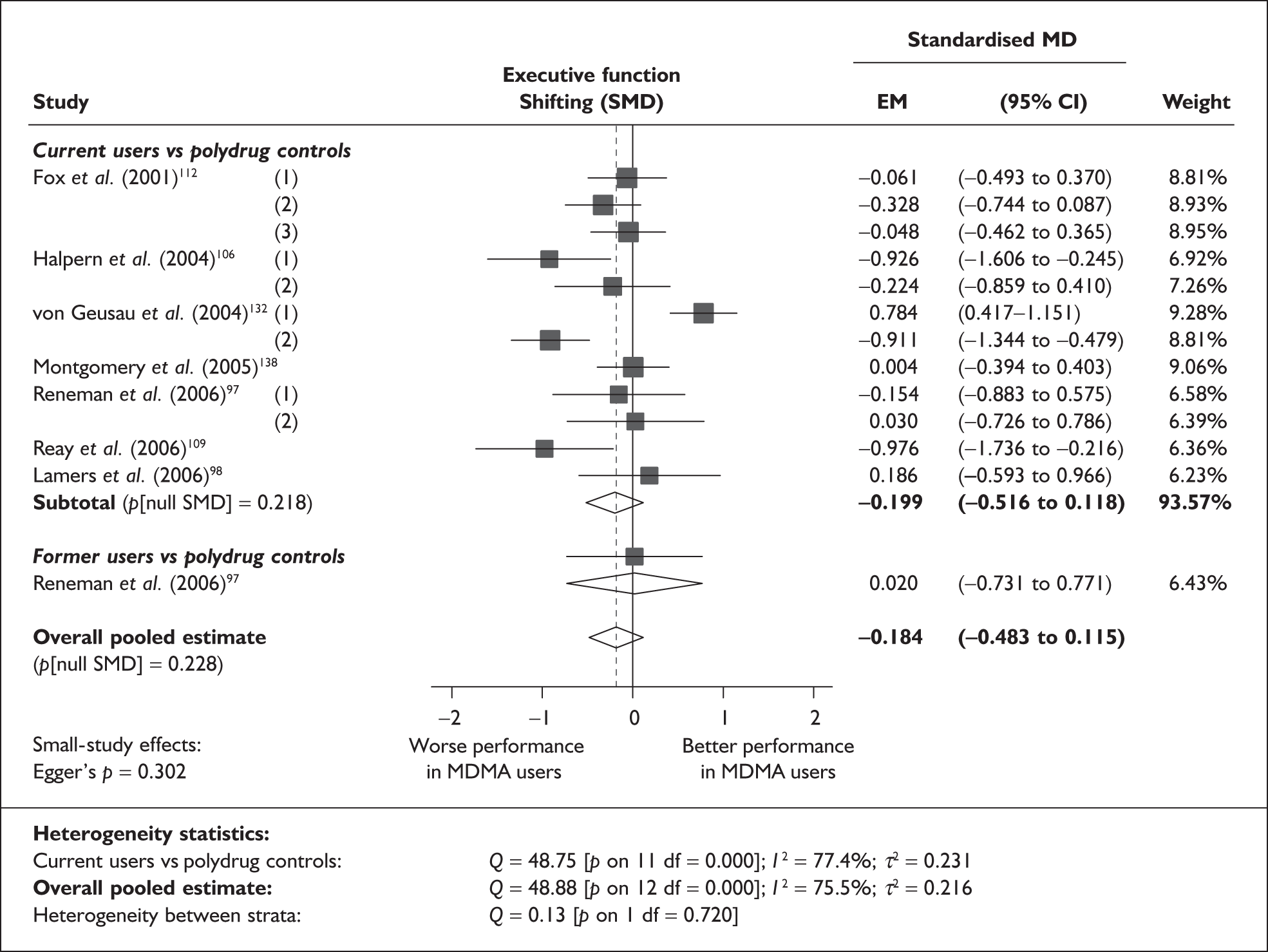
There is no evidence of small-study bias in this dataset (Egger’s p = 0.302), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 15 covariates; details are shown in Table 31. None of these analyses generated results that achieved conventional levels of significance, and there was no evidence of a dose–response effect (see Figure 112 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 11 | 0.030 | (–0.181 to 0.241) | 0.755 | |||
| Sex (% male) | 11 | –0.109 | (–4.049 to 3.830) | 0.951 | |||
| IQ | < 5 | ||||||
| Education (years) | 5 | 0.193 | (–0.713 to 1.098) | 0.546 | |||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 6 | 0.000 | (–0.005 to 0.005) | 0.914 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 5 | 0.000 | (–0.008 to 0.009) | 0.931 | |||
| Duration of ecstasy use (days) | 8 | 0.000 | (0.000–0.001) | 0.282 | |||
| Frequency of ecstasy use (occasions/months) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 11 | –0.031 | (–0.132 to 0.071) | 0.514 | –0.139 | (–0.532 to 0.254) | 0.446 |
| Sex (% male) | 11 | –0.261 | (–1.966 to 1.444) | 0.737 | –0.098 | (–0.522 to 0.327) | 0.615 |
| Baseline intelligence measures (SMD) | 7 | 0.368 | (–0.450 to 1.187) | 0.299 | –0.079 | (–0.426 to 0.267) | 0.582 |
| Education (years) | 5 | 0.052 | (–0.216 to 0.320) | 0.582 | –0.033 | (–0.463 to 0.397) | 0.823 |
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 8 | –0.099 | (–1.060 to 0.862) | 0.809 | –0.034 | (–1.055 to 0.987) | 0.937 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 6 | 0.445 | (–2.564 to 3.453) | 0.702 | –0.260 | (–2.193 to 1.673) | 0.728 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 7 | 0.304 | (–0.921 to 1.529) | 0.551 | –0.414 | (–1.774 to 0.946) | 0.469 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 5 | –0.232 | (–2.982 to 2.517) | 0.805 | –0.195 | (–0.957 to 0.567) | 0.476 |
Executive function (shifting) – MDMA users versus drug-naïve controls
Only one datapoint was found comparing ecstasy-exposed individuals with drug-naïve controls for this outcome. 98 The data reported in this study equate to a SMD of – 0.03 (95% CI – 0.81 to 0.75).
Perceptual organisation – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 31 datapoints, representing a total of six pairwise comparisons, drawn from four different studies (five comparisons from four studies providing data for current ecstasy users and one comparisons from one studies providing data for former ecstasy users). Sixteen different outcome measures are included, the most common being WAIS-R: Block design (three datapoints). The complete dataset is detailed in Table 68, in Appendix 6.
When meta-analysed (Figure 62), these data provide little evidence of an exposure effect in this area, with a non-significant SMD of only 0.05 SD.
FIGURE 62.
Perceptual organisation (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
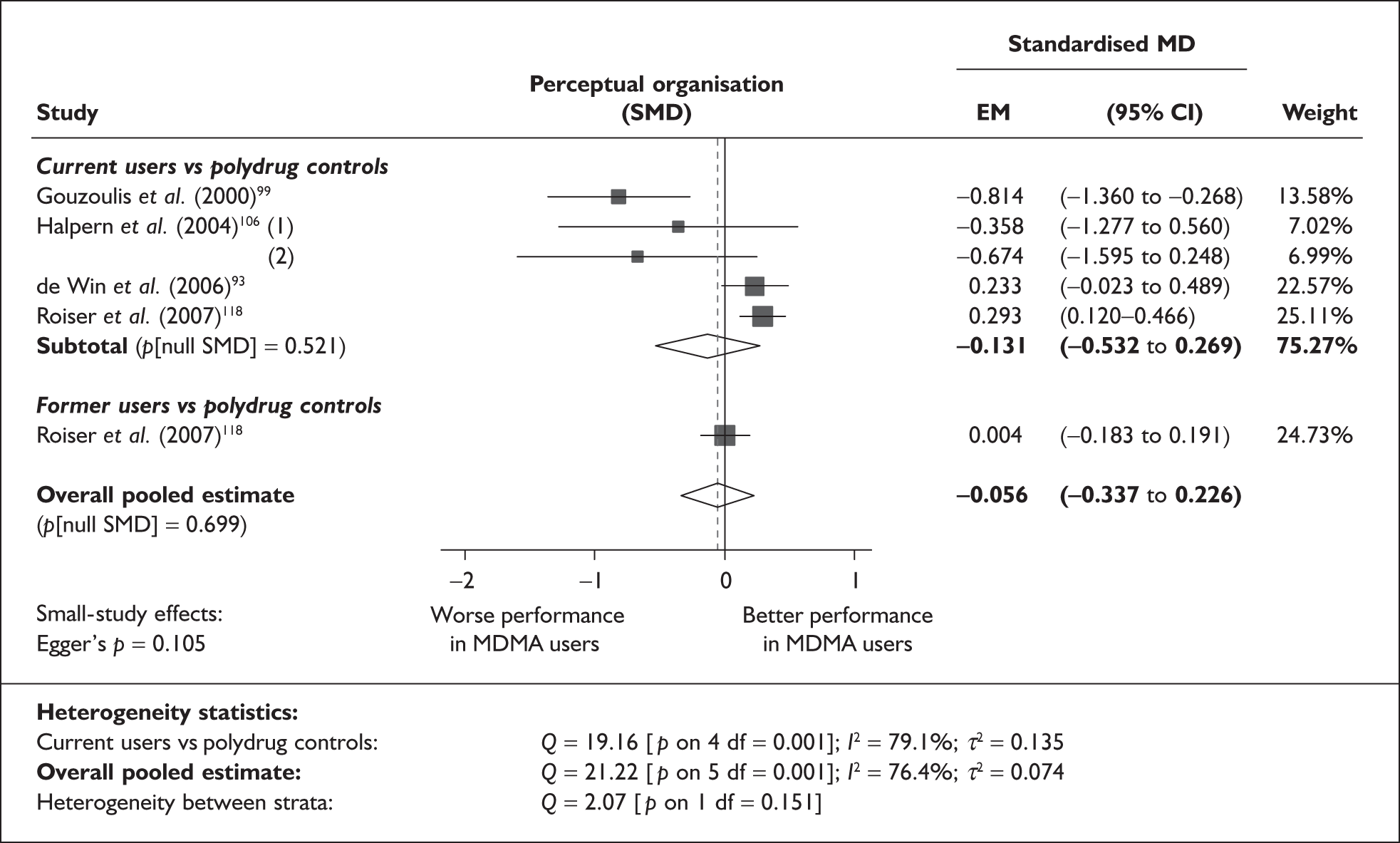
There is no evidence of small-study bias in this dataset (Egger’s p = 0.105), and the funnel plot (not shown) was not especially illuminating, because of the very small sample under analysis, although it did highlight that the three datapoints suggesting a negative exposure effect are those that are subject to the greatest uncertainty.
Sensitivity analysis with aggregated comparisons for each study provided a rather different effect estimate, with ecstasy-exposed individuals estimated to perform better than controls (SMD 0.114; 95% CI – 0.010 to 0.238). However, this reanalysis remained consistent with a null effect (p = 0.072). The discrepancy between primary and secondary analysis is explained by the very large number of datapoints contributing to the omnibus outcome in Roiser et al. 118
Because of the small size of this dataset, it was possible to perform metaregression on a single covariate – standardised mean difference in intelligence measures – only (Table 32). Nevertheless, this analysis generated significant results, suggesting that any difference between populations in the studies under analysis may be ascribable entirely to baseline imbalances in intelligence (see Figure 63). There was no evidence of a dose–response effect (see Figure 113 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | < 5 | ||||||
| Sex (% male) | < 5 | ||||||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | < 5 | ||||||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | < 5 | ||||||
| Sex (% male) | < 5 | ||||||
| Baseline intelligence measures (SMD) | 5 | 0.985 | (0.255–1.715) | 0.023 | –0.019 | (–0.231 to 0.194) | 0.795 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
FIGURE 63.
Perceptual organisation (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in baseline intelligence measures.
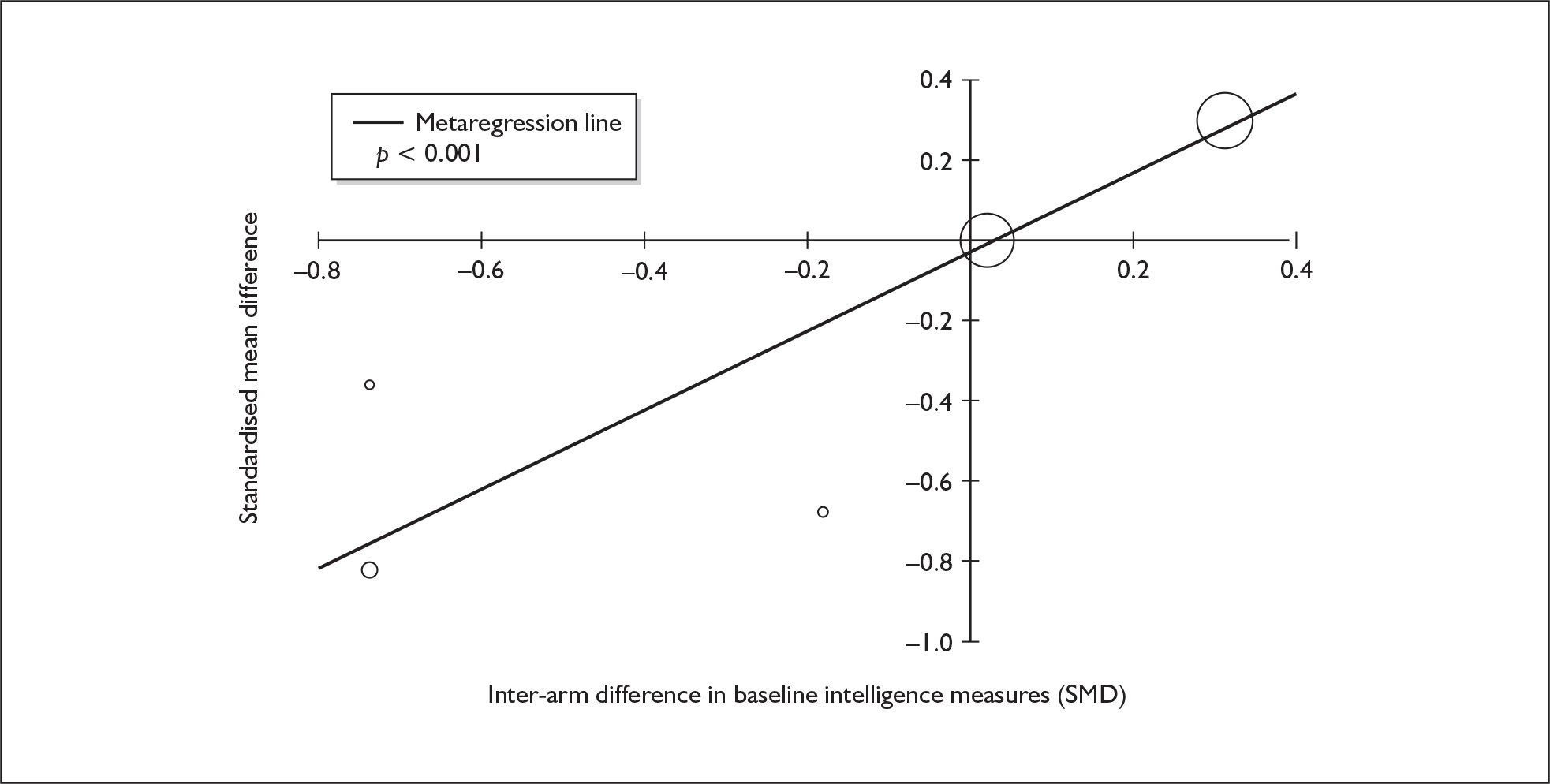
Perceptual organisation – MDMA users versus drug-naïve controls
Only two studies in our evidence-base provided data relevant to this comparison. 99,118 When meta-analysed according to the model used elsewhere in this review, a non-significant SMD of – 0.204 (95% CI – 0.501 to 0.093) is estimated.
Depression (self-rated) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 38 datapoints, representing a total of 38 pairwise comparisons, drawn from 20 different studies (33 comparisons from 20 studies providing data for current ecstasy users and five comparisons from five studies providing data for former ecstasy users). Five different outcome measures are included, the most common being SCL-90: depression score (15 datapoints), BDI: overall score (nine datapoints) and BDI-II: overall score (six datapoints). The complete dataset is detailed in Table 69, in Appendix 6.
The meta-analysis, shown in Figure 64, suggests that ecstasy-exposed individuals tend to exhibit more depression than polydrug controls by a little over one-quarter of an SD. According to Cohen’s guidelines, this would probably be thought of as a ‘small’ difference. The effect might appear to be greater in former ecstasy users, whom controls outperformed by 0.5 SD (a ‘medium’ difference, according to Cohen), but the hypothesis test for interstratum heterogeneity provides no statistical justification for supposing the participants belong to different distributions.
FIGURE 64.
Depression – self-rated (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
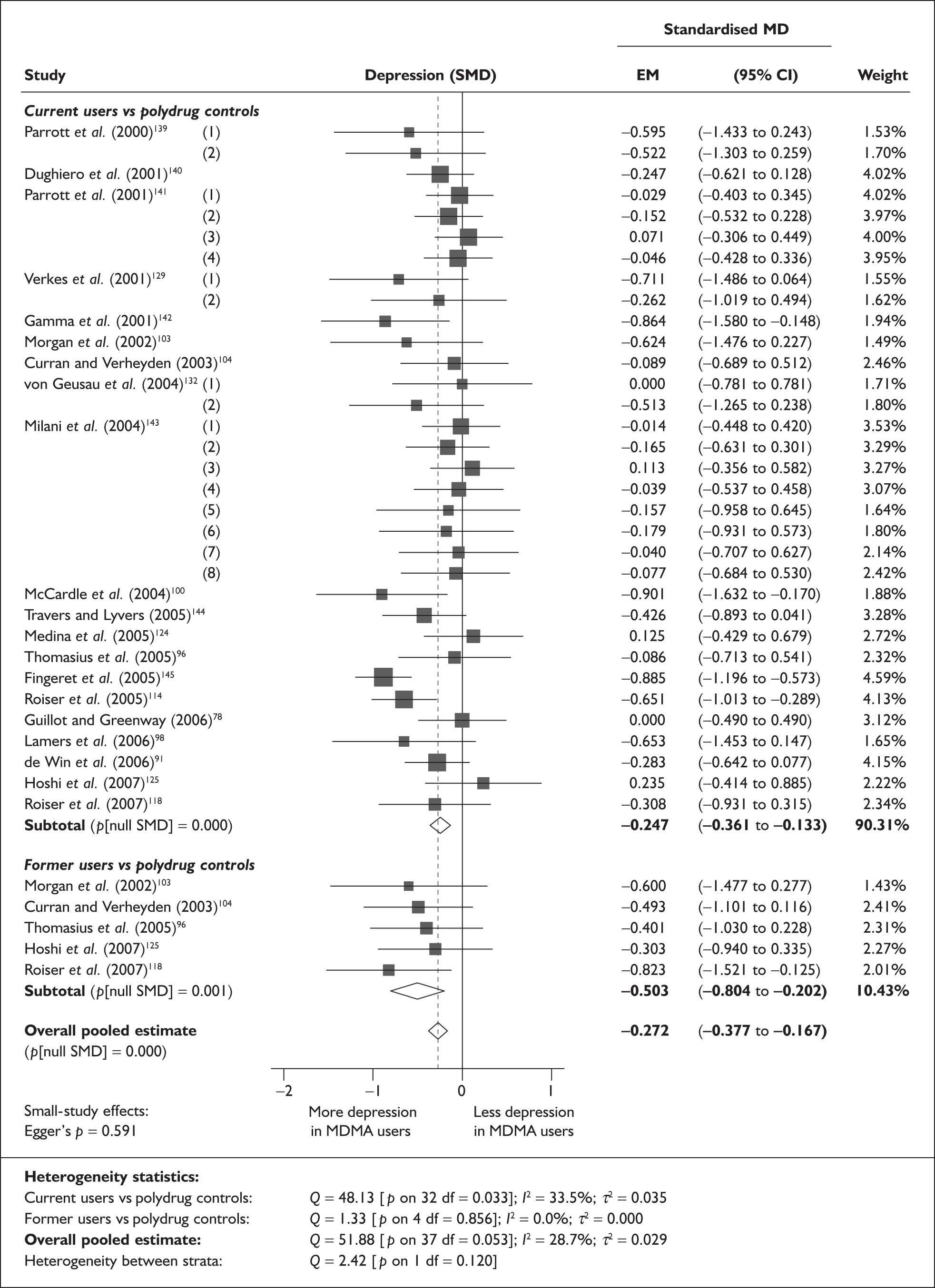
Sensitivity analysis with single, pooled comparisons for each study provides a SMD estimated at – 0.340 [95% CI – 0.478 to – 0.202; p(null SMD) < 0.001], which is close to the primary analysis. There is no evidence of small-study bias in this dataset (Egger’s p = 0.591), and the funnel plot (not shown) had an unremarkable appearance.
For the comparison between current users and controls, a relatively typical datapoint is the SCL-90 depression score reported by Dughiero et al. 140 Ecstasy-exposed participants rated 0.15 points higher on the subscale, although both cohorts averaged well below 1.0, which is considered the upper threshold for normality in this test (0.78 versus 0.63; SMD – 0.247). Where former users were compared to controls, the most representative datapoint was that from Curran and Verheyden’s 2003 study,104 in which ecstasy users scored a little less than three points more on the BDI (overall score: 8.48 versus 5.59; SMD – 0.493).
Sufficient data were available to attempt metaregression analyses for 15 covariates; details are shown in Table 33. There was no evidence of a dose–response effect (see Figure 114 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 38 | –0.030 | (–0.075 to 0.016) | 0.201 | |||
| Sex (% male) | 30 | 0.273 | (–0.418 to 0.965) | 0.438 | |||
| IQ | 8 | –0.028 | (–0.082 to 0.026) | 0.307 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 23 | 0.000 | (–0.001 to 0.000) | 0.699 | |||
| ETLE (occasions) | 6 | 0.002 | (0.000–0.005) | 0.079 | |||
| Period since last consumption (days) | 12 | 0.000 | (–0.001 to 0.001) | 0.882 | |||
| Duration of ecstasy use (days) | 12 | 0.000 | (0.000–0.000) | 0.393 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 38 | 0.068 | (0.030–0.105) | 0.000 | –0.284 | (–0.372 to –0.195) | 0.000 |
| Sex (% male) | 30 | 0.011 | (–0.936 to 0.958) | 0.982 | –0.332 | (–0.475 to –0.189) | 0.000 |
| Baseline intelligence measures (SMD) | 11 | 0.372 | (–0.317 to 1.061) | 0.290 | –0.303 | (–0.496 to –0.110) | 0.002 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 17 | –0.178 | (–0.445 to 0.089) | 0.191 | –0.266 | (–0.445 to –0.088) | 0.003 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 17 | –0.244 | (–0.553 to 0.065) | 0.121 | –0.097 | (–0.310 to 0.116) | 0.370 |
| Exposure to cocaine (ETLD) | 7 | 0.000 | (–0.002 to 0.002) | 0.749 | –0.303 | (–0.612 to 0.005) | 0.054 |
| Exposure to cocaine (SMD) | 15 | –0.214 | (–0.556 to 0.128) | 0.220 | –0.131 | (–0.328 to 0.065) | 0.190 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 26 | 0.092 | (–0.228 to 0.411) | 0.574 | –0.158 | (–0.268 to –0.048) | 0.005 |
The only apparently strong explanatory variable is inter-arm difference in age, which is plotted against the outcome of interest in Figure 65. This dataset looks surprisingly heterogeneous, given the strongly significant p-value, and further analysis shows that disproportionate leverage is being exerted by the single datapoint provided by Fingeret et al. 145 (appearing in the bottom-left of the graph). When this study is excluded from analysis, the association between variables becomes substantially weaker (β = 0.031; p = 0.288).
FIGURE 65.
Depression – self-rated (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in age.
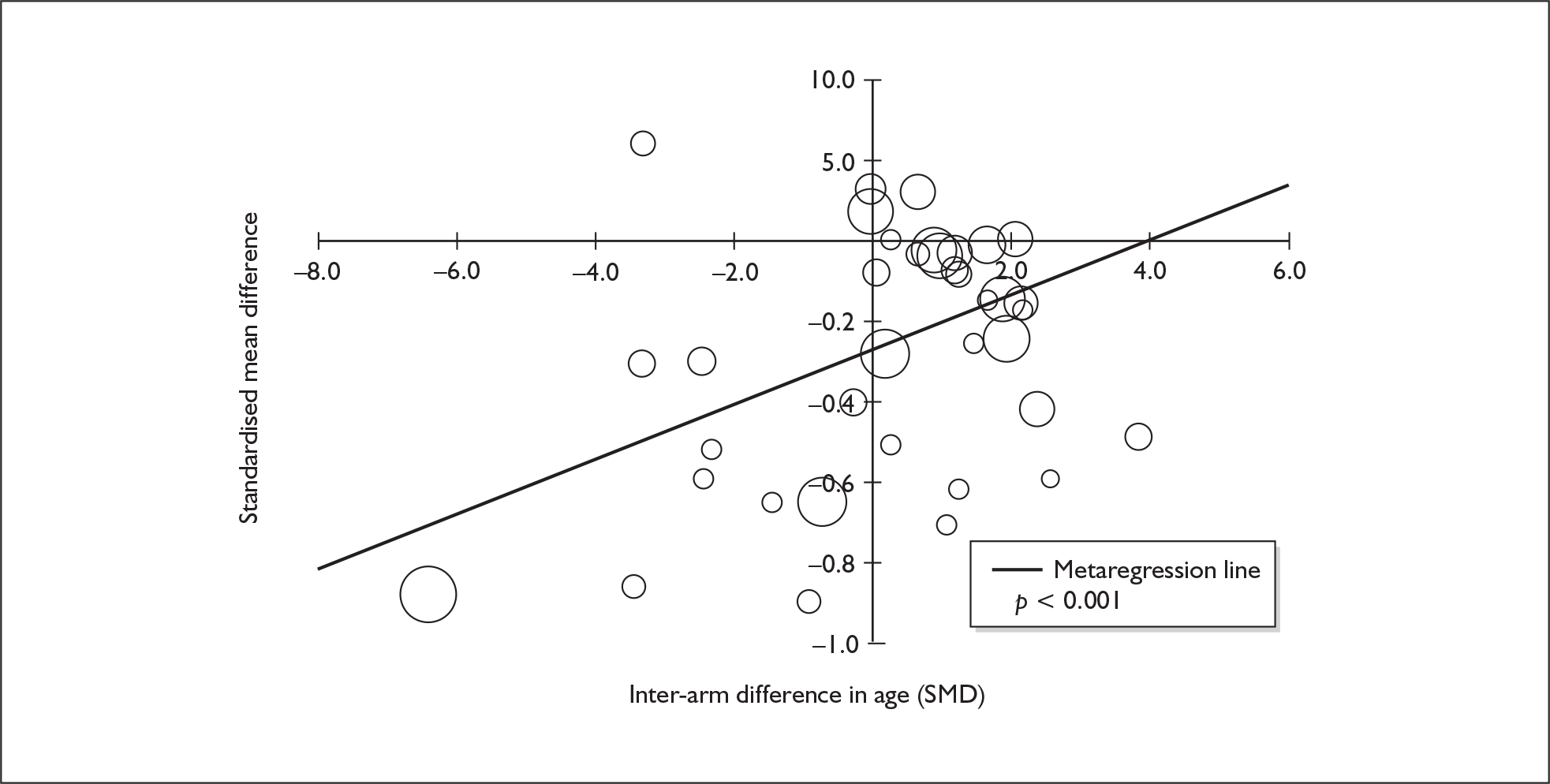
Depression (self-rated) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 35 datapoints, representing a total of 31 pairwise comparisons, drawn from 13 different studies (27 comparisons from 13 studies providing data for current ecstasy users and four comparisons from four studies providing data for former ecstasy users). Eight different outcome measures are included, the most common being SCL-90: depression score (12 datapoints), SCL-BSI: depression score (five datapoints) and SCL-90-R: depression score (four datapoints). The complete dataset is detailed in Table 70 in Appendix 6.
A random-effects meta-analysis of these data is shown in Figure 66. It suggests that ecstasy-exposed cohorts tend to exhibit more depression than drug-naïve controls; in current users, the size of effect is approximately 0.5 SD (a ‘medium’ difference, according to Cohen) while, in former users, the difference is a little over 0.8 SD (which would be considered ‘large’).
FIGURE 66.
Depression – self-rated (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
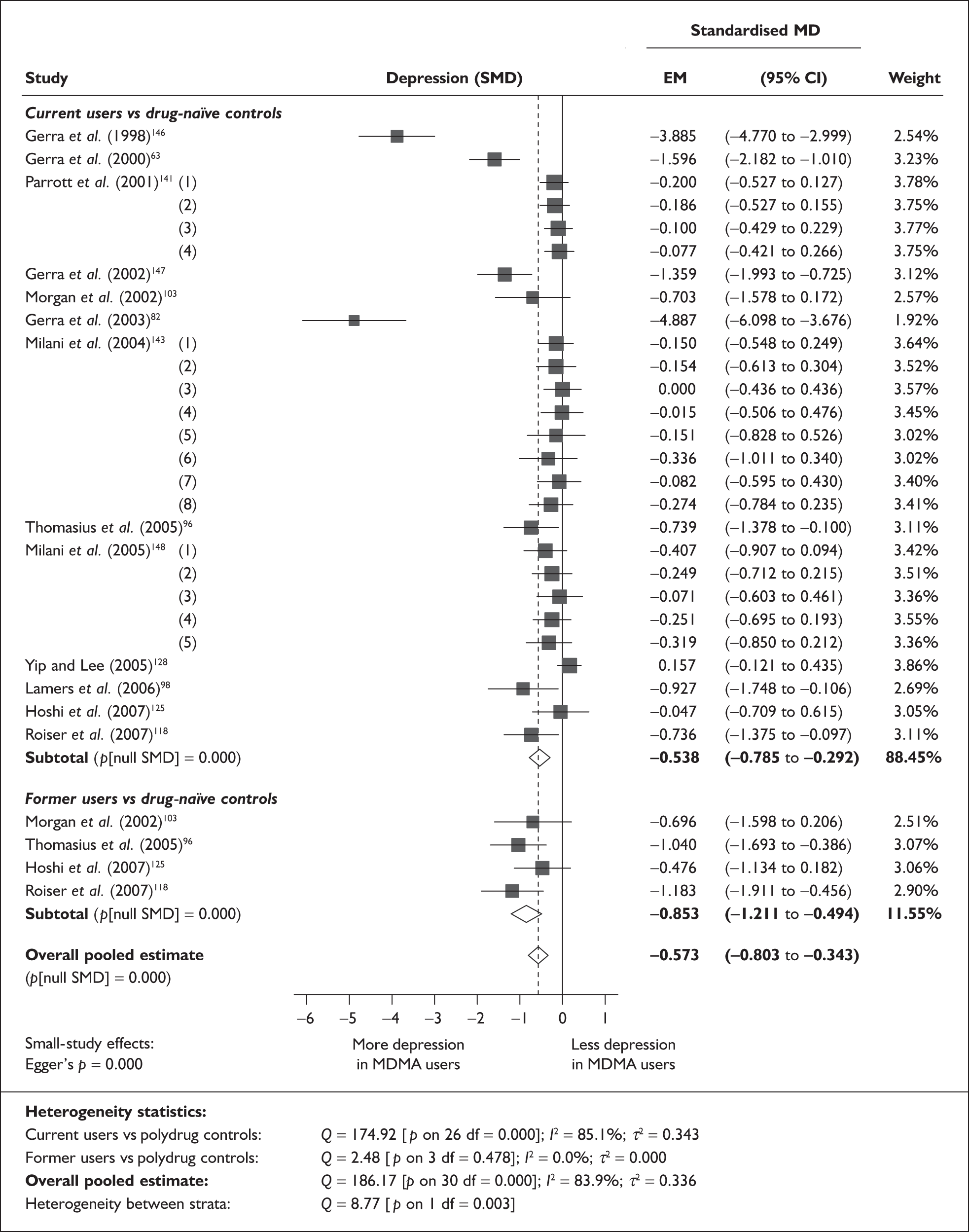
The most notable feature of the forest plot is the outlying status of four datapoints, all of which are drawn from studies published by an Italian research collaboration headed, in each case, by Gilberto Gerra. 63,82,146,147 In comparisons between current users and controls, these are the only datapoints with an estimated effect size greater than 0.8. It is not clear why these studies should have produced such disparate findings, although we note that they rely on an instrument – the Hamilton Depression Rating Scale – that is not used by other investigators.
When these extreme datapoints are excluded from analysis, a clearer picture emerges. There is strong evidence of within-stratum homogeneity in both current users (Q = 19.72; p on 22 df = 0.600; I2 = 0.0%) and former users (unchanged from primary analysis), but there is equally forceful evidence of between-stratum heterogeneity (Q = 13.14; p < 0.001). Current users are seen to display additional depressive symptoms to a small but significant degree [SMD – 0.167; 95% CI – 0.261 to – 0.072; p(null SMD) = 0.001], whereas the difference between former users and drug-naïve controls is much more pronounced [SMD – 0.853; 95% CI – 1.211 to – 0.494; p(null SMD) < 0.001]. The revised overall effect size is estimated at – 0.245 [95% CI – 0.356 to – 0.134; p(null SMD) < 0.001].
Initial sensitivity analysis with single, pooled comparisons for each study provided an SMD of – 1.173 (95% CI – 1.524 to – 0.822). The fairly large size of the discrepancy between this estimate and that from the primary analysis arises because the aggregated approach is affected to an even greater extent by Gerra’s team’s outlying estimates (these studies comprise 34.3% of total weight in the sensitivity analysis, compared to 10.8% in primary analysis). Without the anomalous datapoints, the aggregate approach estimates an effect size of – 0.330 (95% CI – 0.520 to – 0.139), which is comparable to that generated in our primary reanalysis.
Returning to the raw data on which the analysis was based, several individual datapoints could be cited as providing a reasonable example of the calculated average effect sizes:
-
For the comparison between current users and controls in the restricted dataset excluding Gerra’s team’s publications, the most typical datapoint is the SCL-90 depression score reported for the comparison of heavy ecstasy users and drug-free controls by Milani et al. 143 where users scored an average of 0.17 points higher than controls (0.91 versus 0.74; SMD – 0.154).
-
For the comparison between former users and controls, no individual datapoint provides an especially good approximation of the estimated pooled effect. It falls somewhere between two estimates using the SCL-90-R depression score: those from the studies of Morgan et al. 103 [in which ecstasy users scored 0.57 points higher than controls (0.92 versus 0.35); SMD – 0.696] and Thomasius et al. 96,105 [in which ecstasy users scored 0.56 points higher than controls (0.98 versus 0.42)]; SMD – 1.040]. It will be noted that the absolute differences are very similar in these two studies; however the greater variability in the paper by Morgan et al. 103 leads to a lower SMD.
There is strong evidence of small-study bias in this dataset (Egger’s p < 0.001). The funnel plot (Figure 67) shows a clear trend for the effect estimate to decrease as the precision of the study increases, and emphasises the outlying nature of the datapoints discussed above. However, excluding all four of the studies by Gerra et al. did nothing to diminish the suggestion of bias, with Egger’s p remaining less than 0.001. Similarly, even if one overlooks extreme observations, the funnel plot has the typical appearance of a dataset with substantial small-study bias. In particular, we note that all of the studies with the highest precision cluster on or around the point of null effect.
FIGURE 67.
Depression – self-rated (composite measure) – ecstasy users versus drug-naïve controls: funnel plot.
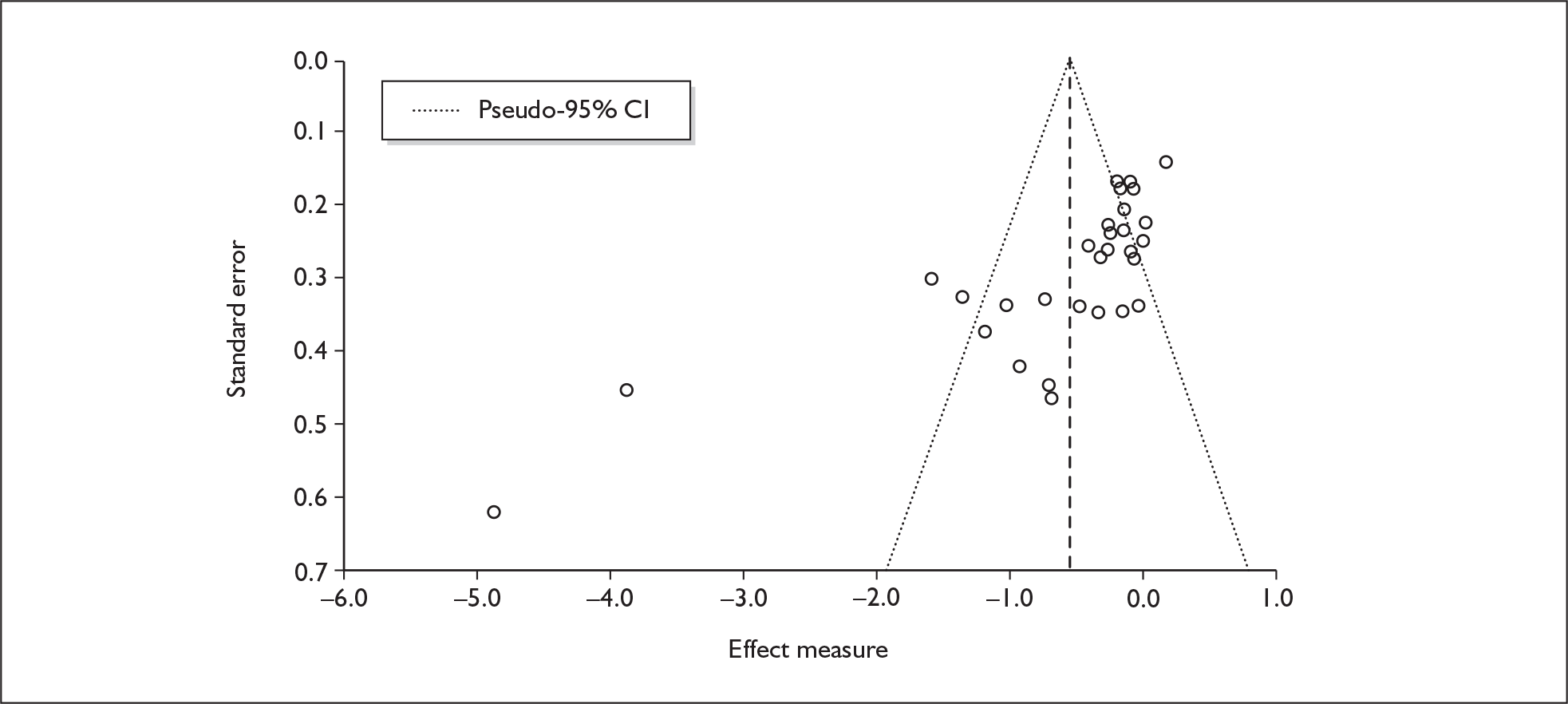
Sufficient data were available to attempt metaregression analyses for 12 covariates, shown in Table 34. There was some evidence of a dose–response effect, with studies in which the participants had a higher average ETLD more likely to report increased depression amongst users (see Figure 115, in Appendix 7). In view of this finding, it might be seen as paradoxical that the metaregression in which duration of ecstasy use is the covariate (Figure 68) produces a significant positive coefficient, suggesting that the largest depression effects are seen in those who have been using ecstasy for the shortest time.
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 26 | 0.027 | (–0.077 to 0.131) | 0.612 | |||
| Sex (% male) | 15 | –2.671 | (–4.647 to –0.696) | 0.008 | |||
| IQ | 5 | 0.014 | (–0.060 to 0.088) | 0.706 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 19 | –0.001 | (–0.002 to 0.000) | 0.004 | |||
| ETLE (occasions) | 5 | 0.012 | (0.000–0.025) | 0.057 | |||
| Period since last consumption (days) | 11 | –0.001 | (–0.001 to 0.000) | 0.075 | |||
| Duration of ecstasy use (days) | 13 | 0.001 | (0.000–0.002) | 0.043 | |||
| Frequency of ecstasy use (occasions/month) | 5 | –0.744 | (–1.517 to 0.030) | 0.060 | |||
| Inter-arm differences | |||||||
| Age (years) | 26 | 0.108 | (–0.013 to 0.229) | 0.079 | –0.808 | (–1.134 to –0.482) | 0.000 |
| Sex (% male) | 15 | 3.063 | (–0.232 to 6.359) | 0.068 | –1.163 | (–1.661 to –0.665) | 0.000 |
| Baseline intelligence measures (SMD) | 8 | –0.018 | (–1.294 to 1.258) | 0.978 | –0.514 | (–0.965 to –0.063) | 0.025 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 17 | 0.093 | (–0.162 to 0.349) | 0.474 | –0.316 | (–0.540 to –0.093) | 0.005 |
FIGURE 68.
Depression – self-rated (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against duration of ecstasy use.
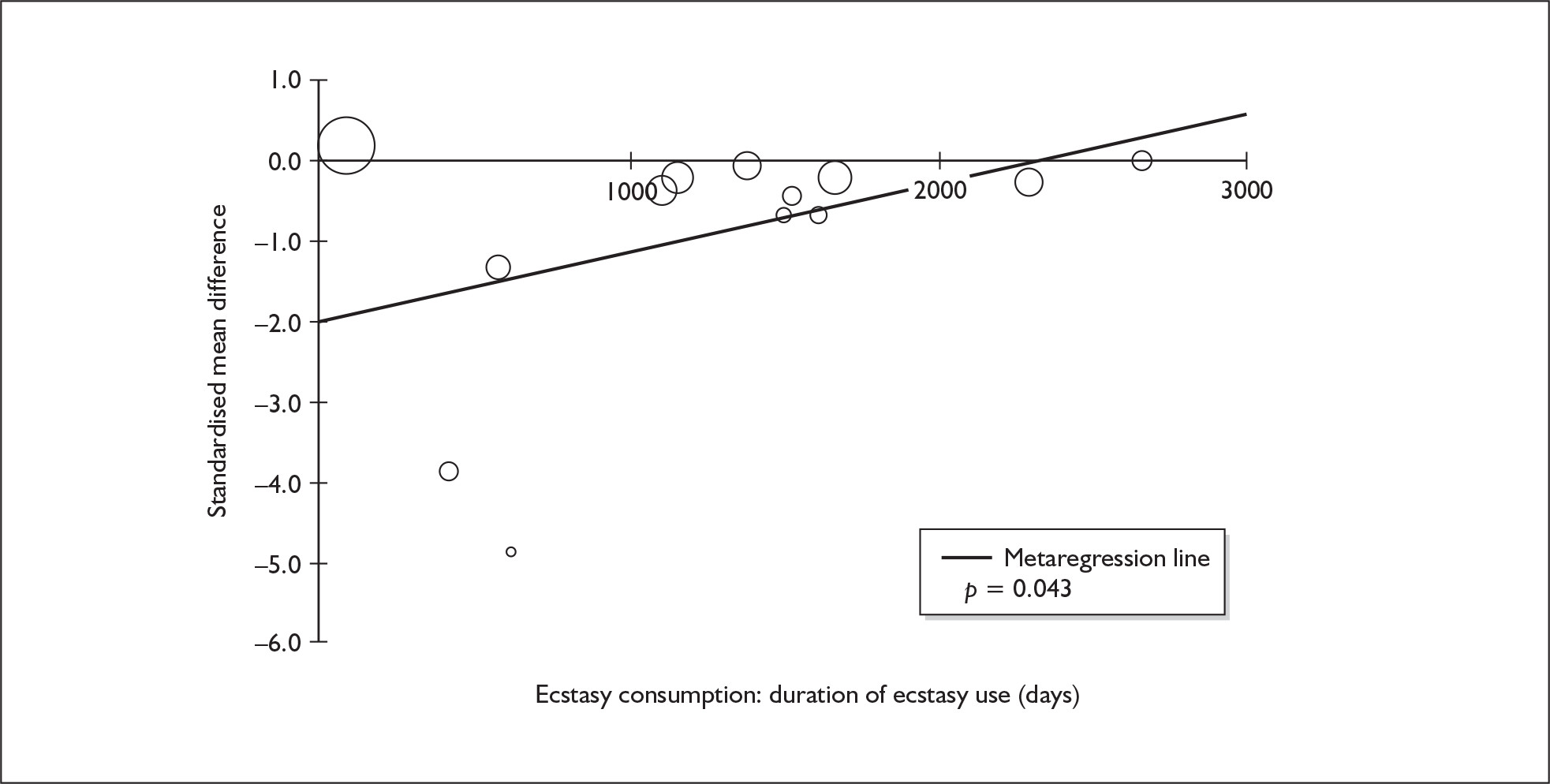
A significant regression coefficient was also calculated for the association between depression and study-level gender distribution (Figure 69). This suggests that the greater the extent to which men outnumbered women in studies, the higher the relative level of depression that could be expected to be seen amongst ecstasy-exposed arms.
FIGURE 69.
Depression – self-rated (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against gender (across all participants.
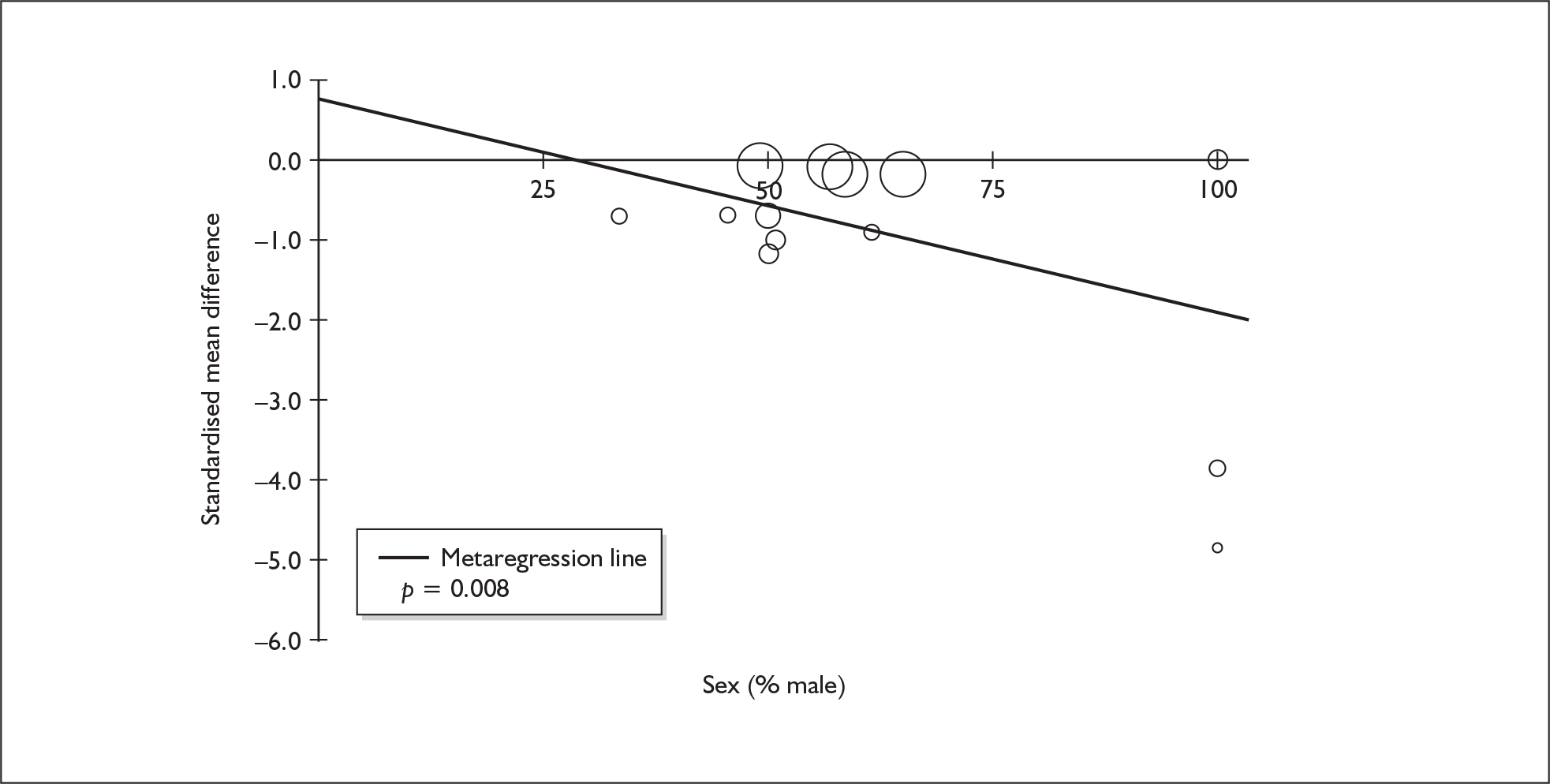
Memory (self-rated) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 20 datapoints, representing a total of eight pairwise comparisons, drawn from five different studies (all providing data for current ecstasy users only). Eleven different outcome measures are included, the most common relating to the PMQ and CFQ. The complete dataset is detailed in Table 71 in Appendix 6.
When synthesised in a random-effects meta-analysis (Figure 70), this dataset suggests that ecstasy users report significantly more memory problems than controls, with an average effect size of around 0.5 SD (a ‘medium’ difference). Sensitivity analysis with single, aggregated comparisons for each study provides an SMD estimated at – 0.549 [95% CI – 0.756 to – 0.343; p(null SMD) < 0.001], which is closely comparable to the primary analysis.
FIGURE 70.
Memory – self-rated (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
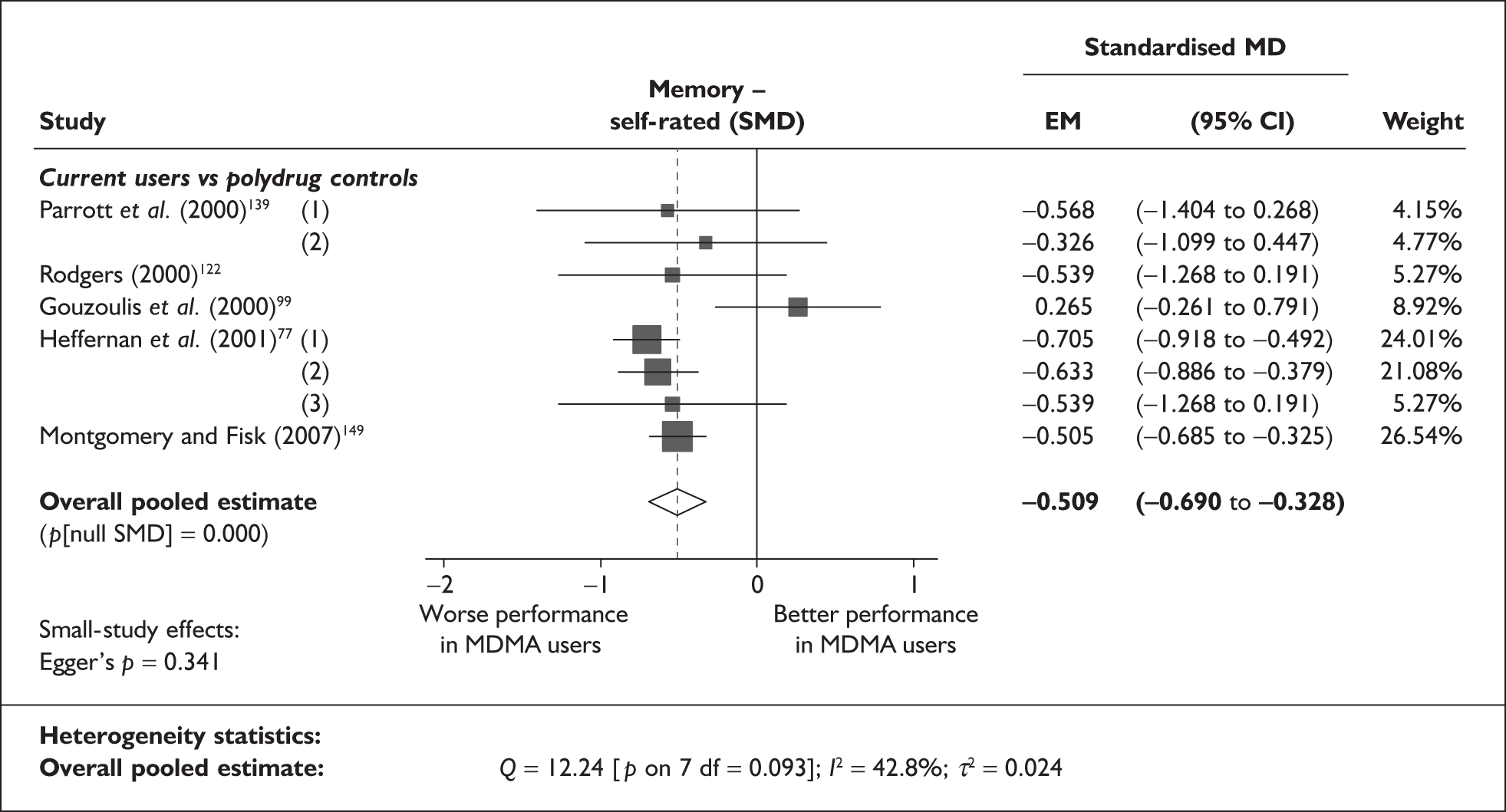
There is no evidence of small-study bias in this dataset (Egger’s p = 0.341), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for four covariates, shown in Table 35. There was no evidence of a dose–response effect (see Figure 116 in Appendix 7). The bubble-plot comparing study-level gender distribution with the outcome of interest (Figure 71) shows an apparently convincing association between these variables, with those studies in which men were outnumbered by women being more likely to report a sizeable deficit for ecstasy users. However, with very few datapoints contributing to the analysis, it is easy to imagine such an appearance occurring by chance.
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 8 | –0.034 | (–0.117 to 0.049) | 0.420 | |||
| Sex (% male) | 8 | 4.439 | (1.598–7.280) | 0.002 | |||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | < 5 | ||||||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 8 | 0.063 | (–0.043 to 0.169) | 0.243 | –0.422 | (–0.654 to –0.189) | 0.000 |
| Sex (% male) | 8 | –0.797 | (–3.099 to 1.506) | 0.498 | –0.418 | (–0.721 to –0.115) | 0.007 |
| Baseline intelligence measures (SMD) | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
FIGURE 71.
Memory – self-rated (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against gender (across all participants).
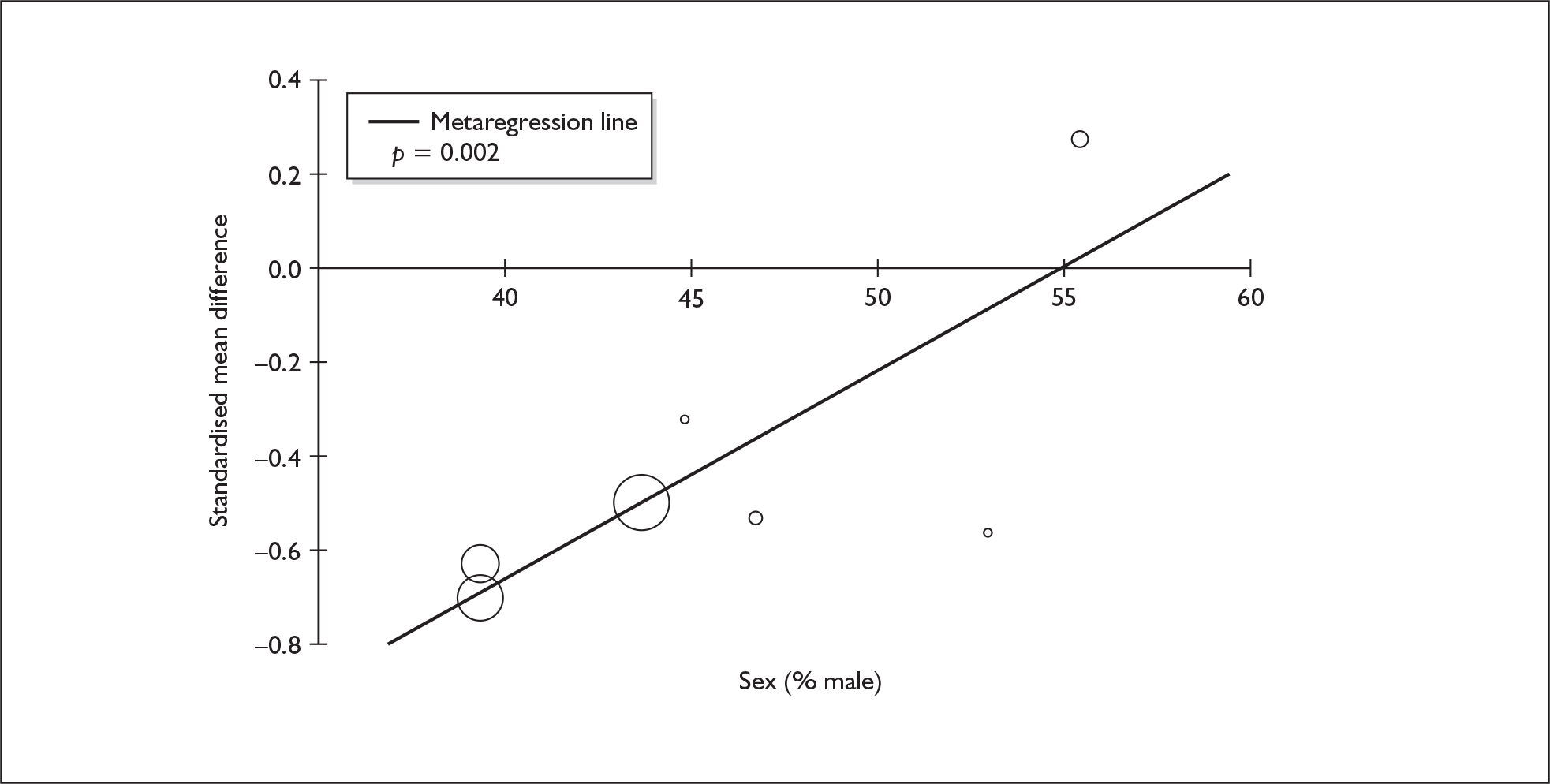
Memory (self-rated) – MDMA users versus drug-naïve controls
Only three studies in the evidence-base reported measures of self-rated memory in comparisons between ecstasy users and drug-naïve controls,76,99,122 so we did not pursue extensive analysis of this dataset. When meta-analysed according to the model used in other analyses, these data generate a non-significant SMD of 0.156 (95% CI – 0.210 to 0.521).
Anxiety (self-rated) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 32 datapoints, representing a total of 32 pairwise comparisons, drawn from 14 different studies (27 comparisons from 14 studies providing data for current ecstasy users and five comparisons from five studies providing data for former ecstasy users). Six different outcome measures are included, the most common being SCL-90: anxiety score (14 datapoints), SCL-90-R: anxiety score (seven datapoints) and STAI: trait anxiety (five datapoints). Measures of in-test state anxiety (e.g. those reported by Medina et al. 124) were excluded. The complete dataset is detailed in Table 72 in Appendix 6.
When analysed in a random-effects meta-analysis (Figure 72), these data suggest that ecstasy users display significantly greater symptoms of anxiety than controls, with the magnitude of difference in the order of one-quarter of an SD (which Cohen would label a ‘small’ difference). No substantial differences were seen between strata, although, on face value, former users showed a larger effect size.
FIGURE 72.
Anxiety – self-rated (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
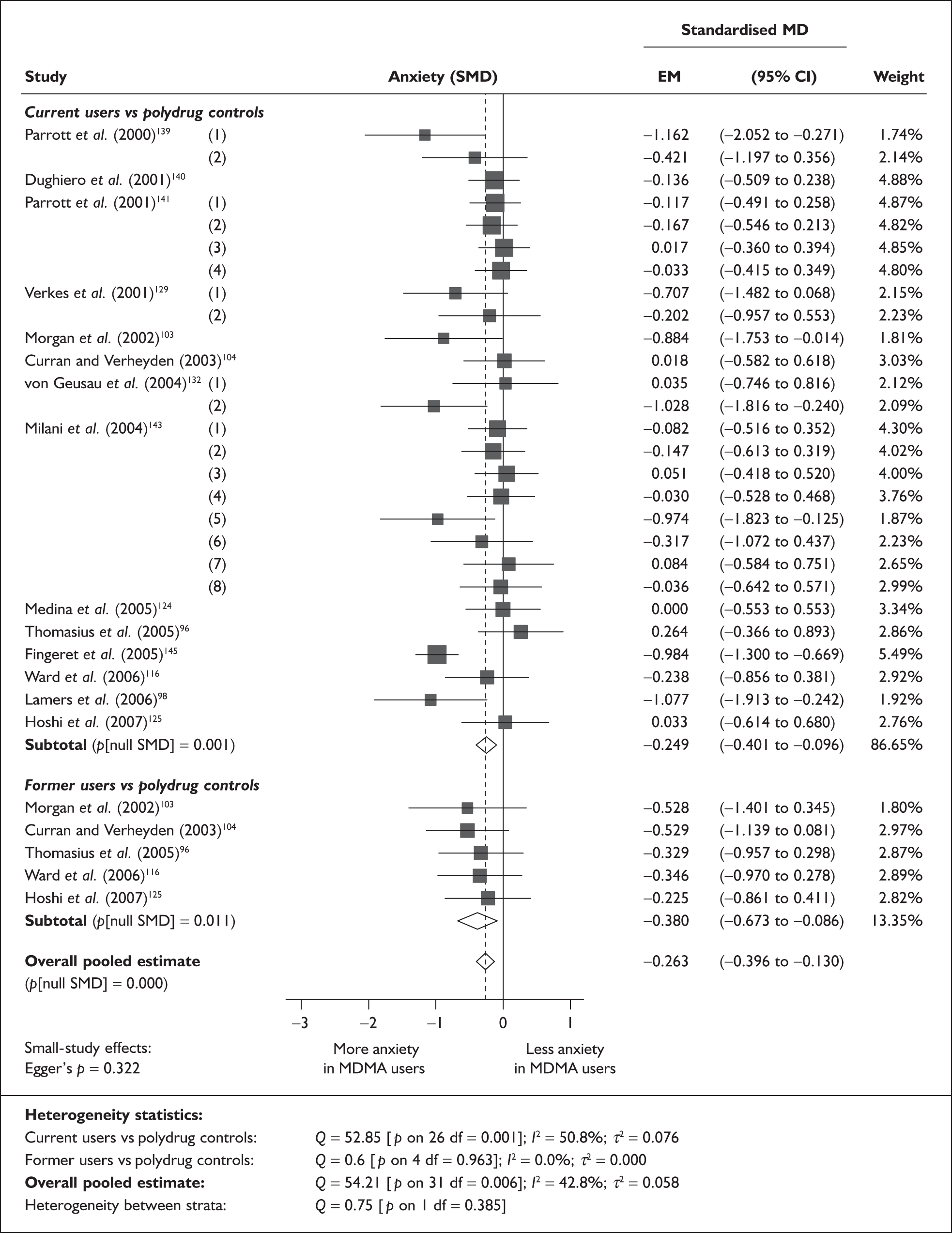
Sensitivity analysis with aggregated comparisons for each study suggests that our primary analysis is relatively robust, but may slightly underestimate the inter-population difference, with the alternative estimate equating to exactly one-third of an SD [SMD – 0.333; 95% CI – 0.514 to – 0.152; p(null SMD) < 0.001].
Using the calculated pooled value to identify a typical datapoint in the raw data on which the analysis was based, the most representative appears to be the BAI overall score from Ward et al. ,116 in which ecstasy-exposed participants scored 2.07 points higher than controls (10.1 versus 8.03; SMD – 0.238).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.322), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 14 covariates; details are shown in Table 36. There was no evidence of a dose–response effect (see Figure 117 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 32 | –0.021 | (–0.075 to 0.034) | 0.460 | |||
| Sex (% male) | 24 | 0.309 | (–0.504 to 1.121) | 0.457 | |||
| IQ | 9 | –0.031 | (–0.085 to 0.023) | 0.264 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 20 | 0.000 | (–0.001 to 0.000) | 0.557 | |||
| ETLE (occasions) | 6 | 0.001 | (–0.002 to 0.004) | 0.352 | |||
| Period since last consumption (days) | 8 | 0.000 | (–0.001 to 0.001) | 0.985 | |||
| Duration of ecstasy use (days) | 10 | 0.000 | (0.000–0.001) | 0.318 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 32 | 0.073 | (0.024–0.121) | 0.004 | –0.278 | (–0.390 to –0.166) | 0.000 |
| Sex (% male) | 24 | –0.216 | (–1.371 to 0.940) | 0.714 | –0.299 | (–0.488 to –0.111) | 0.002 |
| Baseline intelligence measures (SMD) | 11 | 0.284 | (–0.406 to 0.973) | 0.420 | –0.222 | (–0.493 to 0.049) | 0.108 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 13 | –0.221 | (–0.660 to 0.219) | 0.325 | –0.315 | (–0.549 to –0.082) | 0.008 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 13 | –0.115 | (–0.554 to 0.325) | 0.609 | –0.201 | (–0.497 to 0.094) | 0.181 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 11 | –0.290 | (–0.667 to 0.088) | 0.133 | –0.098 | (–0.335 to 0.138) | 0.414 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 23 | 0.233 | (–0.144 to 0.610) | 0.225 | –0.169 | (–0.286 to –0.053) | 0.004 |
The only covariate for which a significant regression coefficient was estimated was inter-arm asymmetry in age. The positive coefficient suggests that the extent to which ecstasy-exposed cohorts were younger than controls was associated with the extent to which they exhibited more anxiety. This is a very similar picture to that seen for self-rated measures of depression (see Figure 65). In common with that analysis, disproportionate leverage is being exerted by the single datapoint provided by Fingeret et al. 145 (appearing in the bottom-left of Figure 73). When this study is excluded from the analysis, the association between variables disappears entirely (β = 0.005; p = 0.897).
FIGURE 73.
Anxiety – self-rated (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in age.
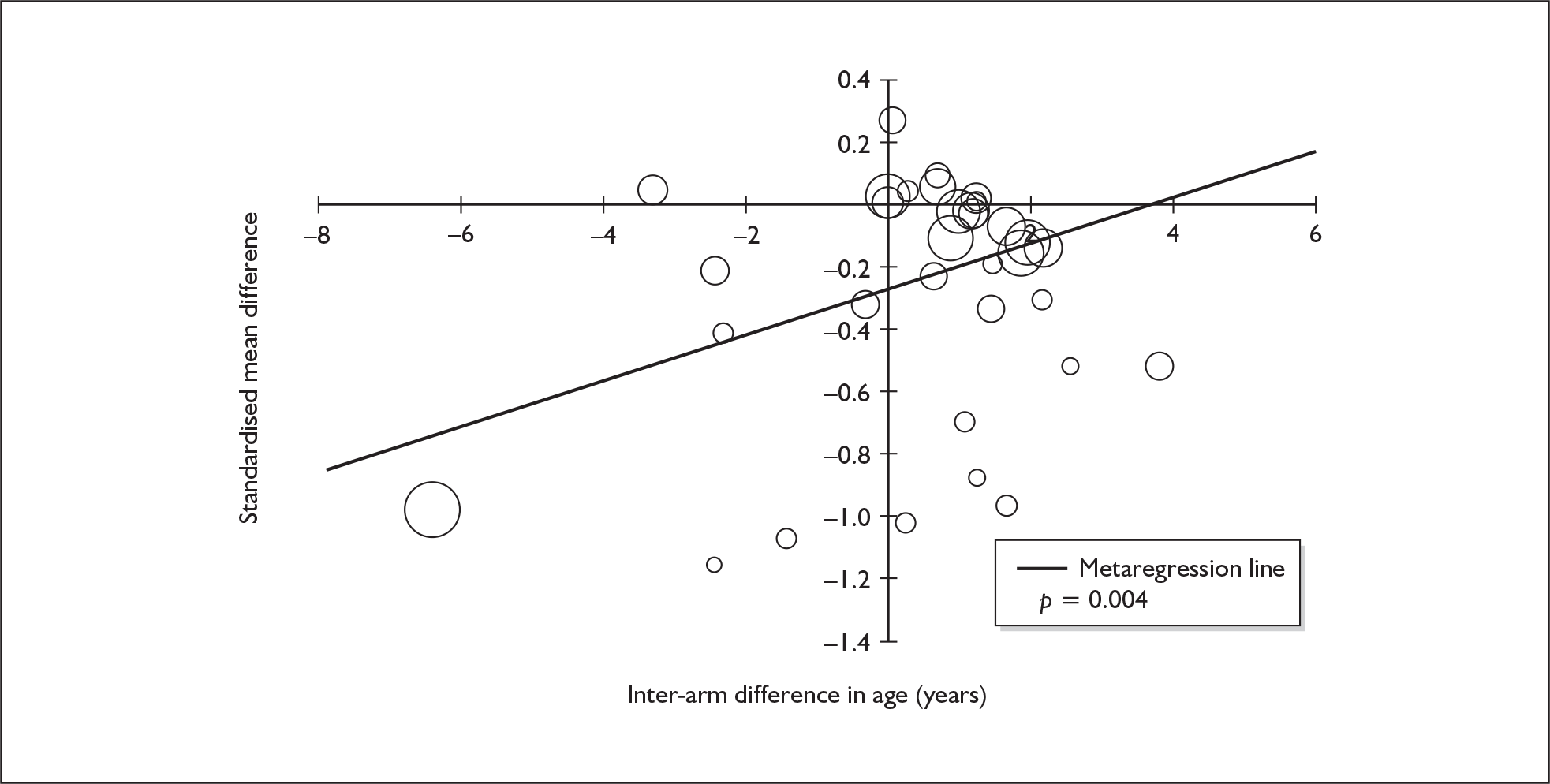
Anxiety (self-rated) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 25 datapoints, representing a total of 25 pairwise comparisons, drawn from eight different studies (22 comparisons from eight studies providing data for current ecstasy users and three comparisons from three studies providing data for former ecstasy users). Six different outcome measures are included, the most common being SCL-90: anxiety score (12 datapoints), SCL-BSI: anxiety score (five datapoints) and SCL-90-R: anxiety score (four datapoints). As before, measures of in-test state anxiety (e.g. those reported by Wareing et al. 136) were excluded. The complete dataset is detailed in Table 73 in Appendix 6.
The random-effects meta-analysis (Figure 74) is similar to that seen in the comparison with polydrug controls (Figure 72), with a slightly larger effect size estimated at all levels of the analysis. The overall difference between populations is approximately one-third of an SD (this would probably fall into the category of a ‘small’ effect size), a similar effect was seen in the current users stratum, while a ‘medium’ difference a little over one-half of an SD was estimated amongst former users. Sensitivity analysis with single, pooled comparisons for each study provides a mean difference estimated at – 0.340 [95% CI – 0.438 to – 0.242; p(null SMD) ≤ 0.001], which is extremely close to the primary analysis.
FIGURE 74.
Anxiety – self-rated (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
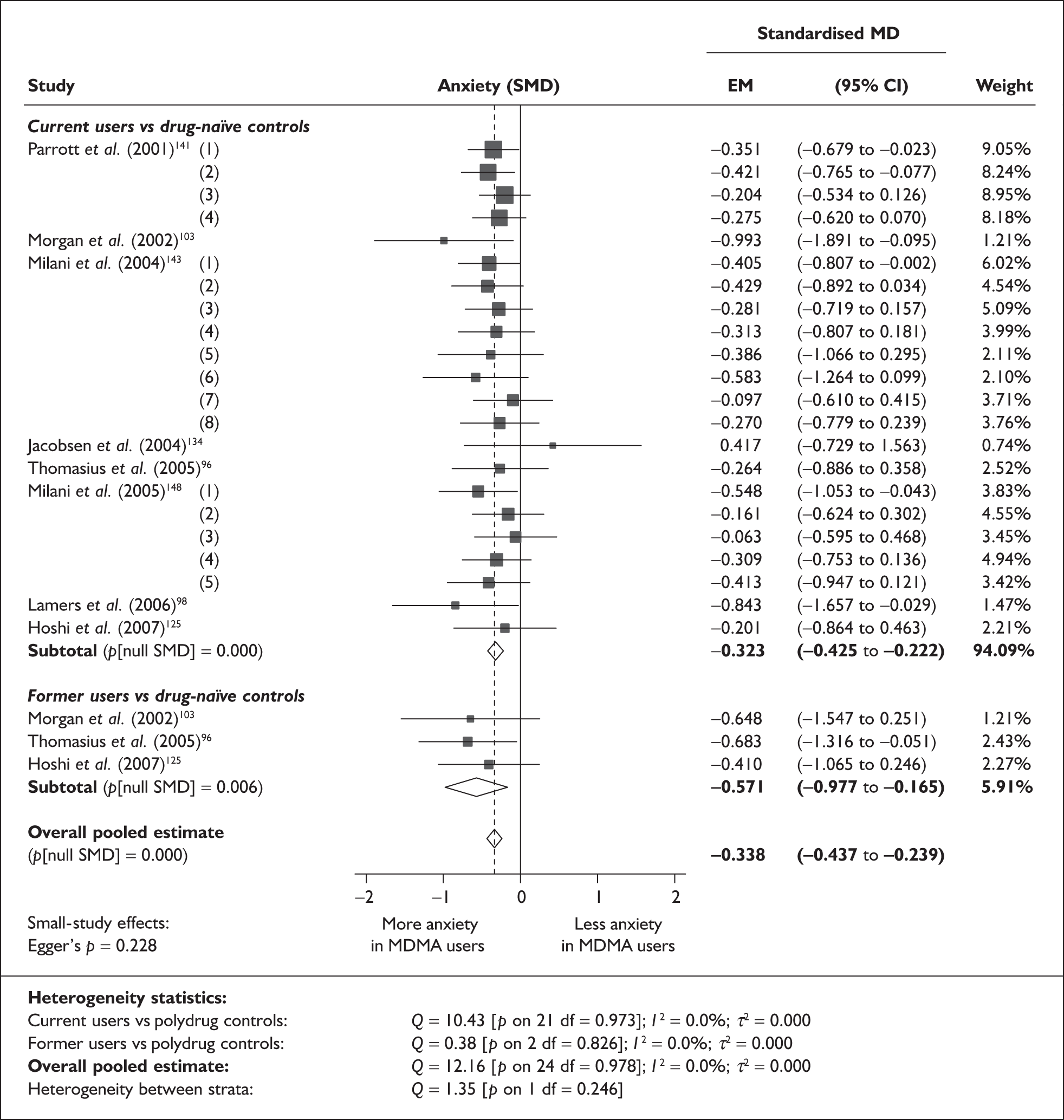
In the raw data underpinning this analysis, the datapoint that most closely reflects the meta-analysed effect size is the comparison by Parrott et al. 141 between heavy ecstasy users and alcohol–tobacco controls, in which users scored 0.19 points higher on the SCL-90 anxiety score (0.88 versus 0.69; SMD – 0.351).
Statistical testing provided no evidence of small-study bias (Egger’s p = 0.228), although the funnel plot for this dataset (Figure 75) appears to show a trend towards larger effect sizes in the least precise comparisons.
FIGURE 75.
Anxiety – self-rated (composite measure) – ecstasy users versus drug-naïve controls: funnel plot.
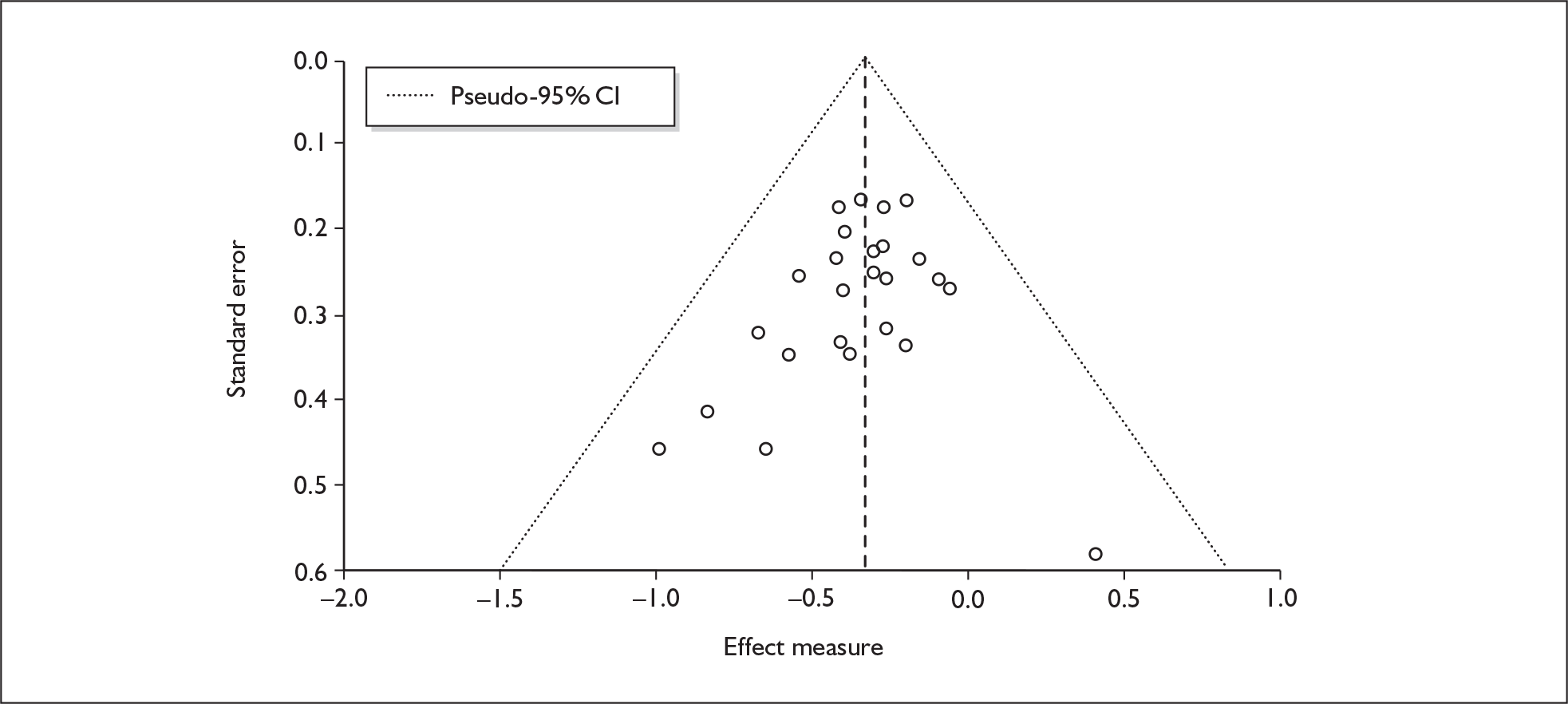
Sufficient data were available to attempt metaregression analyses for 11 covariates; details are shown in Table 37. None of these analyses generated results that achieved conventional levels of significance, and there was no evidence of a dose–response effect (see Figure 118 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 20 | –0.013 | (–0.062 to 0.036) | 0.602 | |||
| Sex (% male) | 12 | 0.070 | (–0.890 to 1.029) | 0.887 | |||
| IQ | 5 | –0.038 | (–0.112 to 0.036) | 0.310 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 17 | 0.000 | (–0.001 to 0.000) | 0.230 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 10 | 0.000 | (–0.001 to 0.001) | 0.924 | |||
| Duration of ecstasy use (days) | 9 | 0.000 | (0.000–0.000) | 0.985 | |||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 20 | –0.010 | (–0.065 to 0.045) | 0.721 | –0.325 | (–0.493 to –0.158) | 0.000 |
| Sex (% male) | 12 | –0.053 | (–0.997 to 0.892) | 0.913 | –0.349 | (–0.558 to –0.140) | 0.001 |
| Baseline intelligence measures (SMD) | 8 | –0.102 | (–0.911 to 0.707) | 0.805 | –0.486 | (–0.775 to –0.196) | 0.001 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | 5 | –0.392 | (–1.270 to 0.487) | 0.382 | –0.211 | (–1.203 to 0.781) | 0.676 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 17 | 0.008 | (–0.240 to 0.257) | 0.947 | –0.363 | (–0.576 to –0.151) | 0.001 |
Impulsivity (objective measures) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 20 datapoints, representing a total of 10 pairwise comparisons, drawn from five different studies (nine comparisons from five studies providing data for current ecstasy users and one comparison from one study providing data for former ecstasy users). Seven different outcome measures are included, the most common relating to the RGT and MFFT. The complete dataset is detailed in Table 74 in Appendix 6.
The meta-analysis of these data (Figure 76) generates a pooled estimate which, at 0.2 SD (precisely matching Cohen’s definition of a ‘small’ effect size), falls just short of conventional statistical significance. Sensitivity analysis with single, pooled comparisons for each study provides a very similar effect estimate [SMD – 0.181; 95% CI – 0.367 to 0.006; p(null SMD) = 0.058].
FIGURE 76.
Impulsivity – objective measures (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
There is no evidence of small-study bias in this dataset (Egger’s p = 0.249), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for nine covariates; details are shown in Table 38. There was no evidence of a dose–response effect (see Figure 119 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 10 | 0.094 | (–0.039 to 0.226) | 0.165 | |||
| Sex (% male) | 10 | –0.402 | (–1.881 to 1.077) | 0.594 | |||
| IQ | 5 | –0.153 | (–0.451 to 0.146) | 0.316 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 5 | 0.001 | (0.000–0.002) | 0.144 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 10 | 0.014 | (–0.122 to 0.151) | 0.835 | –0.206 | (–0.448 to 0.036) | 0.096 |
| Sex (% male) | 10 | 4.229 | (1.077–7.380) | 0.009 | –0.094 | (–0.257 to 0.069) | 0.258 |
| Baseline intelligence measures (SMD) | 5 | 0.285 | (–0.483 to 1.054) | 0.467 | –0.197 | (–0.468 to 0.074) | 0.154 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 5 | –1.004 | (–2.447 to 0.438) | 0.172 | –0.211 | (–0.487 to 0.066) | 0.135 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 5 | 0.653 | (0.131–1.174) | 0.014 | –0.223 | (–0.402 to –0.044) | 0.015 |
Significant coefficients were estimated for two covariates: inter-arm asymmetry in gender distribution and inter-arm asymmetry in exposure to alcohol. The impact of imbalances in gender is visualised in Figure 77. It appears that greater impulsivity is seen amongst ecstasy users in those studies in which the proportion of men is smaller in the exposed arm than in controls. A positive coefficient was also estimated for confounding by alcohol (Figure 78), suggesting that greatest additional impulsivity was found in those studies where ecstasy users drank more than polydrug controls. Because this model runs in a counterintuitive direction, it suggests that imbalances in alcohol exposure are masking a greater effect than is seen in the primary analysis (the adjusted effect estimate provides reasonably strong evidence against the null hypothesis of no effect). Both these analyses are based on very restricted datasets.
FIGURE 77.
Impulsivity – objective measures (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in gender.
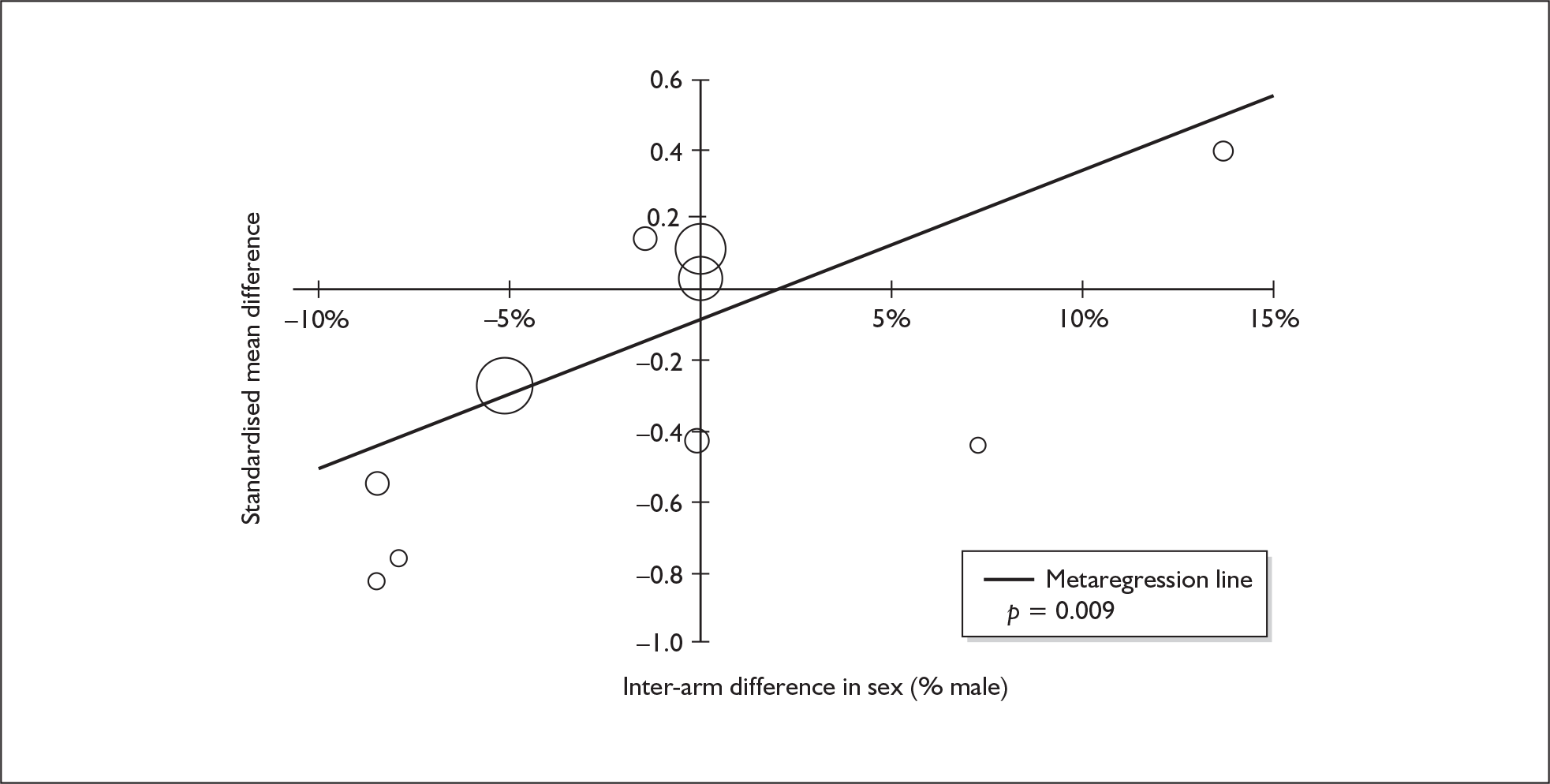
FIGURE 78.
Impulsivity – objective measures (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in exposure to alcohol.
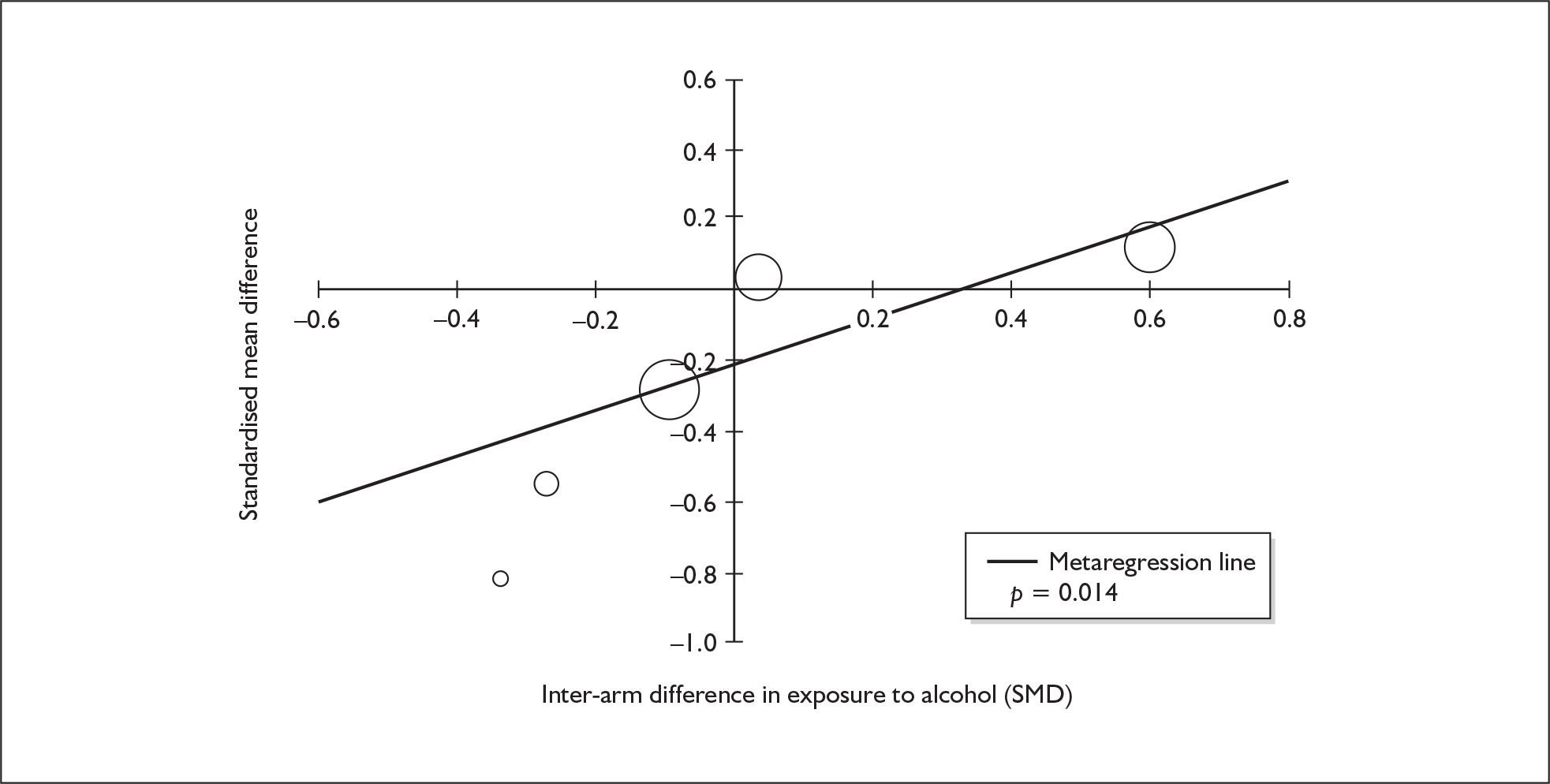
Impulsivity (objective measures) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 23 datapoints, representing a total of 10 pairwise comparisons, drawn from six different studies (nine comparisons from six studies providing data for current ecstasy users and one comparison from one study providing data for former ecstasy users). Ten different outcome measures are included, the most common relating to the RGT and MFFT. The complete dataset is detailed in Table 75 in Appendix 6.
Figure 79 shows a random-effects meta-analysis of these data. The estimated effect size is exactly one-third of an SD (which would probably be a ‘small’ difference, in Cohen’s schema). The evidence against the null hypothesis of no inter-population difference is sufficiently weak to meet conventional definitions of statistical significance.
FIGURE 79.
Impulsivity – objective measures (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
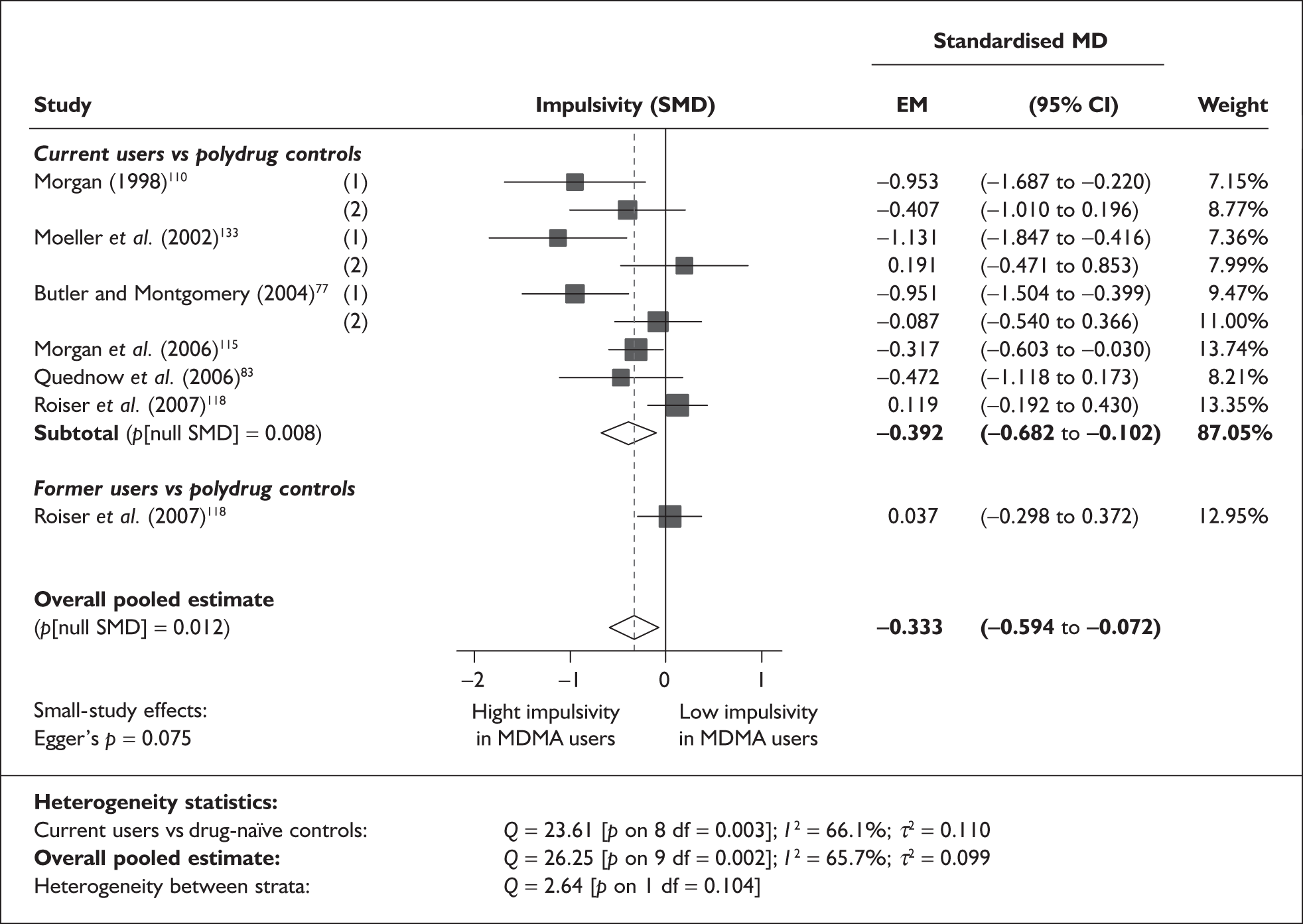
Sensitivity analysis with aggregated, study-level estimates of effect generated a slightly lower effect estimate than that seen in the primary analysis, but shared the key feature of a small but significant difference [SMD – 0.264; 95% CI – 0.460 to – 0.068; p(null SMD) = 0.008].
A representative datapoint from the underlying dataset is found in the 2006 study by Morgan et al. ,115 in which the ecstasy-using cohort responded more swiftly than controls by 677 milliseconds in the gains-only trial of the RGT (3589 milliseconds versus 4266 milliseconds; SMD – 0.337).
There may be a tendency towards small-study bias in this dataset (Egger’s p = 0.075). This suspicion is strengthened by scrutiny of the funnel plot (Figure 80), in which a trend with a negative coefficient – suggesting high study precision is associated with lower exposure effects – is discernible.
FIGURE 80.
Impulsivity – objective measures (composite measure) – ecstasy users versus drug-naïve controls: funnel plot.
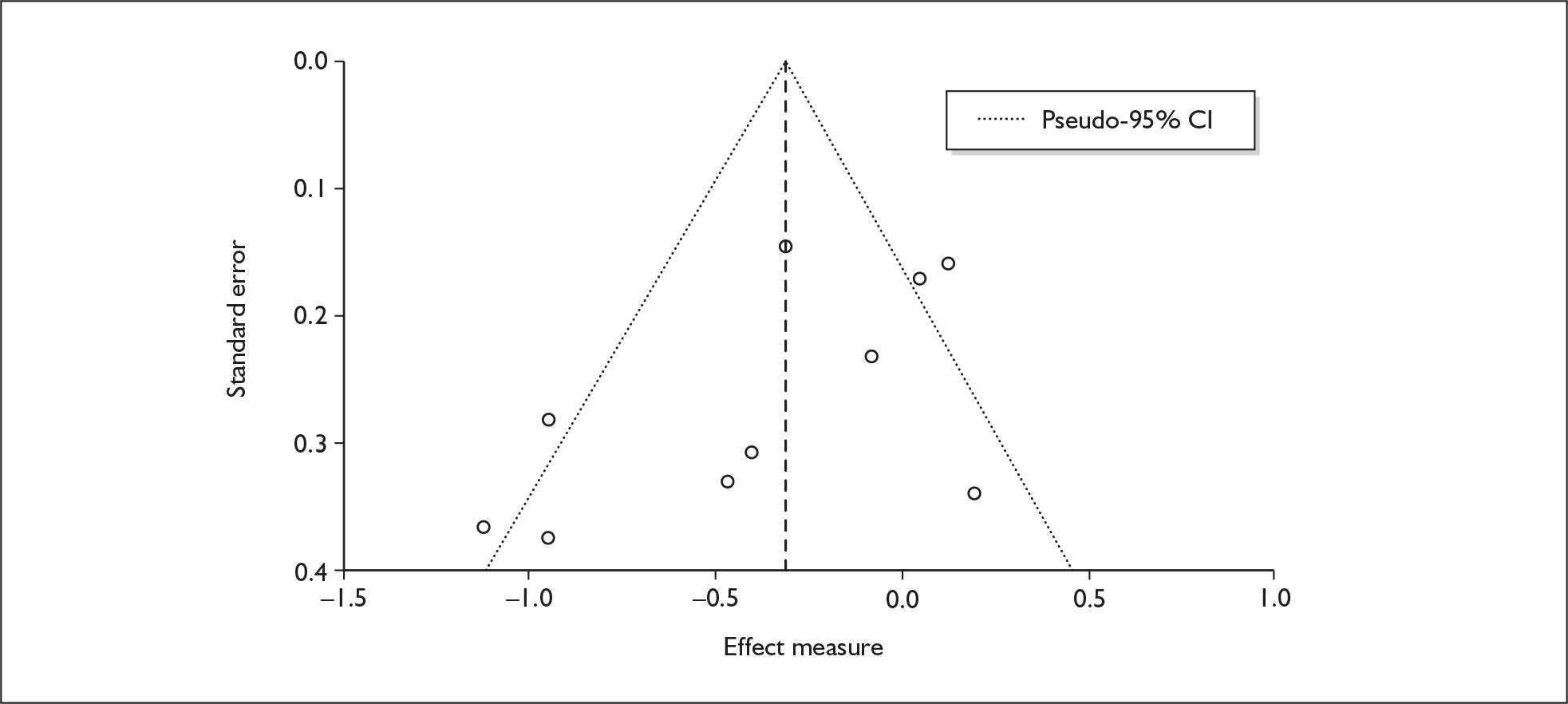
Sufficient data were available to attempt metaregression analyses for seven covariates; details are shown in Table 39. None of the analyses were able to provide a statistically convincing explanation of the heterogeneity seen amongst base-case effect estimates, and there was no evidence of a dose–response effect (see Figure 120 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 10 | 0.068 | (–0.106 to 0.243) | 0.442 | |||
| Sex (% male) | 8 | –0.515 | (–1.913 to 0.883) | 0.470 | |||
| IQ | 5 | –0.003 | (–0.323 to 0.318) | 0.988 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 7 | 0.001 | (–0.001 to 0.002) | 0.283 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 10 | 0.028 | (–0.125 to 0.181) | 0.717 | –0.350 | (–0.635 to –0.064) | 0.016 |
| Sex (% male) | 8 | –0.248 | (–4.208 to 3.712) | 0.902 | –0.309 | (–0.583 to –0.035) | 0.027 |
| Baseline intelligence measures (SMD) | 5 | –0.317 | (–1.799 to 1.165) | 0.675 | –0.261 | (–0.644 to 0.122) | 0.181 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
Impulsivity (subjective measures) – MDMA users versus polydrug controls
The dataset assembled for this measure comprises 14 datapoints, representing a total of 14 pairwise comparisons, drawn from eight different studies (12 comparisons from eight studies providing data for current ecstasy users and two comparisons from two studies providing data for former ecstasy users). Only two different outcome measures are included: IVE: overall score (10 datapoints) and BIS-II: total (four datapoints). The complete dataset is detailed in Table 76 in Appendix 6.
When synthesised in a random-effects meta-analysis (Figure 81), these data suggest that ecstasy users report significantly more impulsive behaviour than controls, with the size of the difference estimated at approximately 0.4 SD. There is no evidence of differential effects among current and former users of ecstasy. Sensitivity analysis with data aggregated at study level generates results that are very close to the primary analysis [(SMD – 0.387; 95% CI – 0.660 to – 0.115; p(null SMD) = 0.005].
FIGURE 81.
Impulsivity – subjective measures (composite measure) – ecstasy users versus polydrug controls: random-effects meta-analysis.
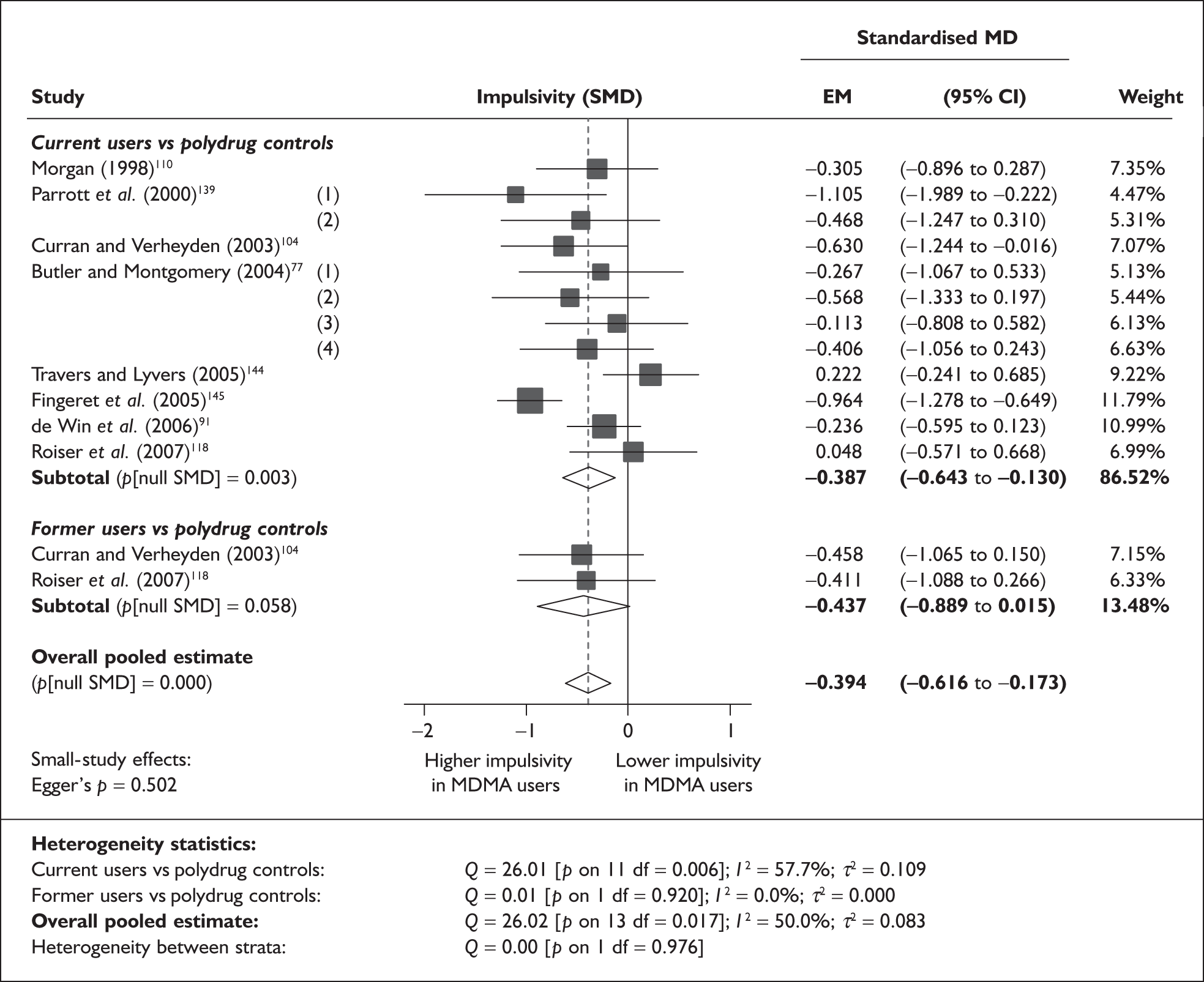
Of all the observations in the raw dataset on which the meta-analysis is based, the IVE impulsivity score from Butler and Montgomery’s 2004 study78 – in which light ecstasy users scored 1.6 points higher than cannabis-using controls (10.3 versus 8.7; SMD – 0.406) – is closest to the estimated pooled overall effect size.
There is no evidence of small-study bias in this dataset (Egger’s p = 0.502), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 12 covariates; details are shown in Table 40. There was no evidence of a dose–response effect (see Figure 121 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 14 | –0.095 | (–0.193 to 0.003) | 0.056 | |||
| Sex (% male) | 14 | –0.193 | (–1.449 to 1.062) | 0.763 | |||
| IQ | 6 | –0.010 | (–0.063 to 0.043) | 0.701 | |||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 7 | 0.000 | (–0.002 to 0.001) | 0.420 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | 6 | 0.000 | (–0.001 to 0.001) | 0.465 | |||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 14 | 0.068 | (0.013–0.123) | 0.015 | –0.365 | (–0.540 to –0.190) | 0.000 |
| Sex (% male) | 14 | 0.361 | (–1.954 to 2.676) | 0.760 | –0.408 | (–0.657 to –0.158) | 0.001 |
| Baseline intelligence measures (SMD) | 6 | 0.287 | (–0.505 to 1.078) | 0.478 | –0.249 | (–0.521 to 0.023) | 0.073 |
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to cannabis (SMD) | 6 | 0.231 | (–0.731 to 1.193) | 0.638 | –0.335 | (–0.584 to –0.087) | 0.008 |
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to amphetamines (SMD) | 6 | –0.384 | (–0.889 to 0.121) | 0.136 | –0.157 | (–0.453 to 0.138) | 0.297 |
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to cocaine (SMD) | 6 | –1.125 | (–2.960 to 0.711) | 0.230 | 0.239 | (–0.680 to 1.158) | 0.610 |
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | 6 | 0.186 | (–0.508 to 0.879) | 0.600 | –0.326 | (–0.556 to –0.096) | 0.005 |
The only apparently strong explanatory variable is inter-arm difference in age, which is plotted against the outcome of interest in Figure 82. This graph shows a very similar picture to that seen for previous self-rated measures of depression (Figure 65) and anxiety (Figure 73). In common with those analyses, disproportionate leverage is being exerted by the single datapoint provided by Fingeret et al. 145 (appearing in the bottom-left of the graph) and, when this study is excluded from analysis, the association between variables disappears entirely (β = 0.010; p = 0.819).
FIGURE 82.
Impulsivity – subjective measures (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against inter-arm asymmetry in age.
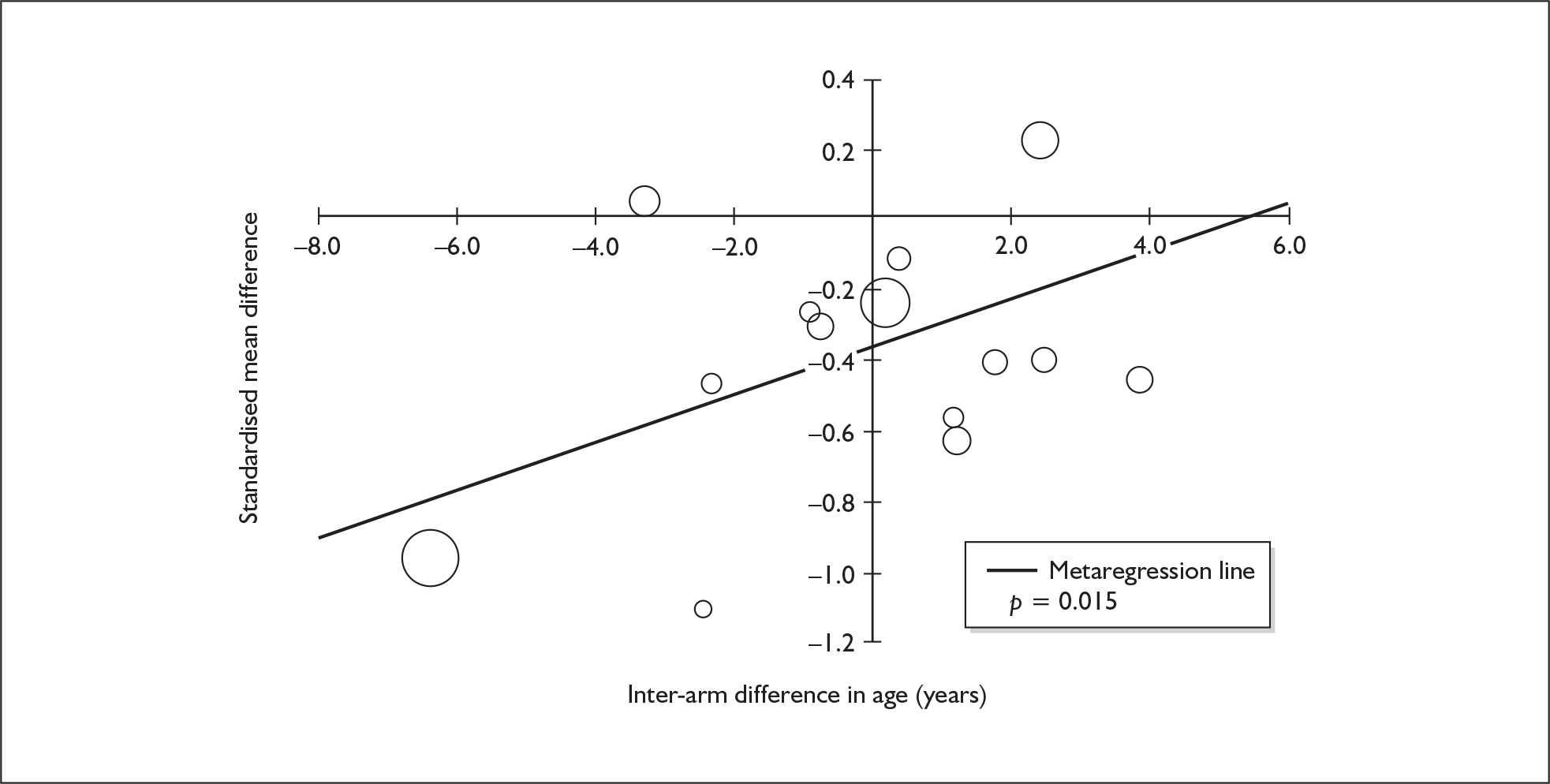
Impulsivity (subjective measures) – MDMA users versus drug-naïve controls
The dataset assembled for this measure comprises 11 datapoints, representing a total of nine pairwise comparisons, drawn from five different studies (eight comparisons from five studies providing data for current ecstasy users and one comparison from one study providing data for former ecstasy users). Only two different outcome measures are included: IVE: overall score (seven datapoints) and BIS-II: total (four datapoints). The complete dataset is detailed in Table 77, in Appendix 6.
A random-effects meta-analysis of these data (Figure 83) suggests that there is a ‘large’ difference of just under 0.8 SD between cohorts, with ecstasy users reporting significantly more impulsive behaviour than controls. Sensitivity analysis with study-level aggregated data generated results that were extremely close to the primary analysis [SMD – 0.784; 95% CI – 1.041 to – 0.528; p(null SMD) < 0.001].
FIGURE 83.
Impulsivity – subjective measures (composite measure) – ecstasy users versus drug-naïve controls: random-effects meta-analysis.
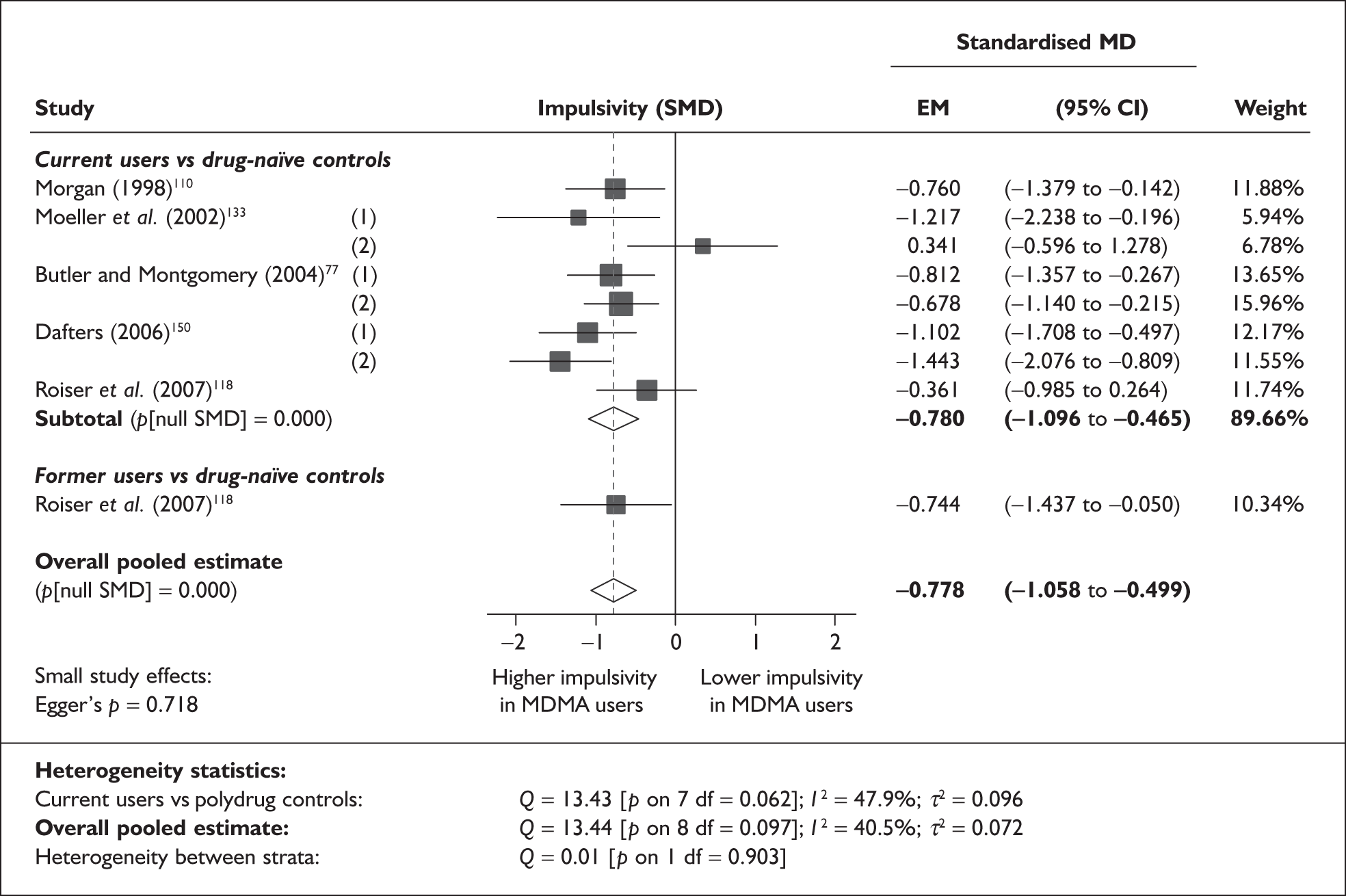
The most typical datapoint in the raw dataset underlying the meta-analysis is the IVE impulsivity score from Morgan’s study,110 in which the ecstasy-exposed arm averaged 3.53 points higher than drug-naïve controls (12.00 versus 8.47; SMD – 0.760).
There is no evidence of small-study bias in this dataset (Egger’s p = 0.718), and the funnel plot (not shown) had an unremarkable appearance.
Sufficient data were available to attempt metaregression analyses for 17 covariates; details are shown in Table 41. None of the analyses could provide a statistically meaningful explanation of the intercomparison heterogeneity seen in the meta-analysis, and there was no evidence of a dose–response effect (see Figure 122 in Appendix 7).
| Covariate | n | Effect modification | Adjusted effect estimate | ||||
|---|---|---|---|---|---|---|---|
| β-coefficient | (95% CI) | p | SMD | (95% CI) | p | ||
| Average values across all participants | |||||||
| Age (years) | 9 | 0.080 | (–0.132 to 0.293) | 0.458 | |||
| Sex (% male) | 7 | –0.156 | (–1.931 to 1.620) | 0.864 | |||
| IQ | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Characteristics of ecstasy exposure | |||||||
| ETLD (tablets) | 7 | 0.000 | (–0.002 to 0.001) | 0.551 | |||
| ETLE (occasions) | < 5 | ||||||
| Period since last consumption (days) | < 5 | ||||||
| Duration of ecstasy use (days) | < 5 | ||||||
| Frequency of ecstasy use (occasions/month) | < 5 | ||||||
| Inter-arm differences | |||||||
| Age (years) | 9 | –0.078 | (–0.243 to 0.087) | 0.355 | –0.746 | (–1.039 to –0.452) | 0.000 |
| Sex (% male) | 7 | –1.628 | (–3.926 to 0.671) | 0.165 | –0.792 | (–1.020 to –0.563) | 0.000 |
| Baseline intelligence measures (SMD) | < 5 | ||||||
| Education (years) | < 5 | ||||||
| Exposure to cannabis (ETLD) | < 5 | ||||||
| Exposure to amphetamines (ETLD) | < 5 | ||||||
| Exposure to cocaine (ETLD) | < 5 | ||||||
| Exposure to alcohol (ETLD) | < 5 | ||||||
| Exposure to alcohol (SMD) | < 5 | ||||||
Summary of quantitative syntheses of Level II evidence
The key findings of our quantitative syntheses are shown in Table 42. These results may be further summarised as follows:
-
Ecstasy-using populations performed worse than their controls in all except one of our meta-analyses, and the effect was strong enough to meet conventional definitions of statistical significance in six out of eight individual measures and 20 out of 28 composite meta-outcomes.
-
The magnitude of difference between ecstasy users and polydrug controls tended to be no more than 0.5 SD, with many falling in the range 0.15–0.4 SD. When drug-naïve control groups are considered, evidence becomes slightly more heterogeneous, with effect sizes ranging from very small to relatively large (the greatest SMD was a little over 1 SD).
-
The largest, most consistent exposure effects were seen in meta-analyses of memory domains. Deficits appear to be greatest in verbal and working memory, with less marked effects seen in visual memory. The focus–execute component of attention also appears to be affected, though sustained attention may not be. A significant exposure effect was seen in the planning but not in the response-inhibition or shifting components of executive function.
-
There was a fair degree of inter-study heterogeneity in most of the meta-analyses we performed. In some cases, the heterogeneity was substantially ascribable to single studies (or groups of studies from the same research centres), with a much more homogeneous picture emerging when outlying estimates were excluded from analysis [for examples, see sections on Verbal memory (delayed) – MDMA users versus drug-naïve controls, Visual memory (delayed) – MDMA users versus drug-naïve controls, and Depression (self-rated) – MDMA users versus drug-naïve controls].
-
In our stratified meta-analyses, former ecstasy users frequently showed deficits that matched or exceeded those seen among current users. A significant difference between strata, with a greater exposure effect seen in ex-users, was found in three instances (with a further case very close to conventional levels of significance). In contrast, none of the analyses showed a significant advantage for former over current users, when compared to controls. Most of the analyses showed no difference between strata.
-
Significant evidence of small-study bias was found in a few analyses, but only in comparisons between ecstasy users and drug-naïve controls. There is strong evidence that the meta-analysis of depression in ecstasy users versus drug-naïve controls may be distorted by this bias [see Depression (self-rated) – MDMA users versus drug-naïve controls].
-
Our metaregression analyses sought to explain heterogeneity in estimated exposure effects with reference to study-level and arm-level characteristics, as well as inter-arm differences. Most results were inconsistent and, in the context of multiple testing, should be seen as uncertain. For two covariates, a more uniform pattern emerged:
-
Several meta-analyses appeared to be biased by asymmetry in the baseline intelligence of participants in the studies. In these cases, a preponderance of studies in which ecstasy users were less intelligent than their respective controls appeared to have an influence on the estimated inter-population effect.
-
In the 25 separate analyses for which sufficient data were available to perform metaregression analyses with asymmetry in exposure to alcohol as the explanatory variable, 19 (76%) estimated a positive coefficient and, in five of these cases, a significant p-value (< 0.05) was generated. This suggests that effects were least in studies in which ecstasy users had greater exposure to alcohol than their controls.
-
| Outcome | Result | Inter-study heterogeneity | Interstratum heterogeneity | SS bias | Significant effect-modifiers |
|---|---|---|---|---|---|
| Individual outcome measures – polydrug controls | |||||
| RAVLT verbal recall (immediate) | Ecstasy users sig. < controls by 4.0 items | High | No | No |
+ Inter-arm asymmetry in age – Inter-arm asymmetry in sex (% male) – Inter-arm asymmetry in exposure to cocaine |
| RAVLT verbal recall (delayed) | Ecstasy users sig. < controls by 1.2 items | Moderate | No | No | – Inter-arm asymmetry in intelligence |
| RBMT prose recall (immediate) | Ecstasy users sig. < controls by 0.66 items | Low | No | No |
+ Inter-arm asymmetry in intelligence + Inter-arm asymmetry in exposure to alcohol |
| RBMT prose recall (delayed) | Ecstasy users sig.< controls by 0.77 items | Low | Former?< current | No |
+ Inter-arm asymmetry in intelligence + Inter-arm asymmetry in exposure to amphetamines + Inter-arm asymmetry in exposure to alcohol |
| Digit span (forwards) | Ecstasy users sig. < controls by 0.42 | None | No | No | None |
| Digit span (backwards) | Ecstasy users sig. < controls by 0.63 | None | NA | No | None |
| IQ (National Adult Reading Test) | Ecstasy users sig. < controls by 0.32 points | None | Former sig. < current | No | + Inter-arm asymmetry in exposure to alcohol |
| Individual outcome measures – drug-naïve controls | |||||
| IQ (National Adult Reading Test) | Ecstasy users sig. < controls by 0.47 points | None | No | No | None |
| Composite outcome measures – polydrug controls | |||||
| Verbal memory – immediate | Ecstasy users sig. < controls by 0.33 SD | High | No | No |
+ Education + Inter-arm asymmetry in intelligence |
| Verbal memory – delayed | Ecstasy users sig. < controls by 0.38 SD | Low | No | No | + Education |
| Visual memory – immediate | Ecstasy users sig. < controls by 0.15 SD | Moderate | No | No |
+ Age – Sex (% male) – Inter-arm asymmetry in age + Inter-arm asymmetry in intelligence – Inter-arm asymmetry in exposure to amphetamines |
| Visual memory – delayed | Ecstasy users sig. < controls by 0.18 SD | None | No | No | None |
| Working memory | Ecstasy users sig.< controls by 0.39 SD | High | Former sig. < current | No |
– Sex (% male) + Inter-arm asymmetry in education + Inter-arm asymmetry in exposure to alcohol |
| Attention – focus–execute | Ecstasy users sig. < controls by 0.23 SD | Moderate | No | No | – Inter-arm asymmetry in age |
| Attention – sustain | No significant difference | High | No | Maybe | –Inter-arm asymmetry in exposure to amphetamines |
| Executive function – planning | Ecstasy users sig. < controls by 0.32 SD | Low | No | No |
+ IQ –Period since last consumption of ecstasy |
| Executive function – response inhibition | No significant difference | High | No | No | + Inter-arm asymmetry in intelligence |
| Executive function – shifting | No significant difference | High | No | No | None |
| Perceptual organisation | No significant difference | High | No | No | + Inter-arm asymmetry in intelligence |
| Depression – self-rated | Ecstasy users sig. < controls by 0.27 SD | Moderate | No | No | + Inter–arm asymmetry in age |
| Memory – self-rated | Ecstasy users sig. < controls by 0.51 SD | Moderate | NA | No | + Sex (% male) |
| Anxiety – self-rated | Ecstasy users sig. < controls by 0.26 SD | Moderate | No | No | + Inter-arm asymmetry in age |
| Impulsivity – objective measures | No significant difference | Moderate | No | No |
+ Inter-arm asymmetry in sex (% male) + Inter-arm asymmetry in exposure to alcohol |
| Impulsivity – subjective measures | Ecstasy users sig. < controls by 0.39 | Moderate | No | No | + Inter-arm asymmetry in age |
| Composite outcome measures – drug-naïve controls | |||||
| Verbal memory – immediate | Ecstasy users sig. < controls by 0.84 SD | Moderate | No | Yes | None |
| Verbal memory – delayed | Ecstasy users sig. < controls by 1.04 SD | High | No | No | None |
| Visual memory – immediate | No significant difference | High | No | No | None |
| Visual memory – delayed | No significant difference | High | No | Maybe | None |
| Working memory | Ecstasy users sig. < controls by 0.50 SD | Moderate | No | No | None |
| Attention – focus–execute | Ecstasy users sig. < controls by 0.27 SD | Moderate | No | No | None |
| Attention – sustain | – | ||||
| Executive function – planning | – | ||||
| Executive function – response inhibition | No significant difference | Moderate | No | Maybe | + ETLD of ecstasy |
| Executive function – shifting | – | ||||
| Perceptual organisation | – | ||||
| Depression – self-rated | Ecstasy users sig. < controls by 0.57 SD | High | Former sig. < current | Yes |
– Sex (% male) – ETLD of ecstasy + Duration of ecstasy use |
| Memory – self-rated | – | ||||
| Anxiety – self-rated | Ecstasy users sig. < controls by 0.34 SD | None | No | No | None |
| Impulsivity – objective measures | No significant difference | Moderate | No | Maybe | None |
| Impulsivity – subjective measures | Ecstasy users sig. < controls by 0.78 SD | Moderate | No | No | None |
Additional description of metaregressions
The results of these analyses (encompassing both individual and composite outcome measures) are discussed in the following section.
Average values across all participants
Our first category of metaregressions was the ‘classical’ type, in which covariates representing a characteristic of all participants were investigated, to ascertain the extent to which study-level factors may influence outcomes.
Sufficient information about participant age was provided to enable metaregression on this covariate in most cases. The resulting picture was ambiguous: only one of 34 analyses was significant (immediate verbal memory in ecstasy users versus polydrug controls), and there was an even split between positive and negative coefficients (17 : 17).
Again, most studies reported this variable, so we were able to perform metaregressions in 33 cases. Three analyses generated significant results: immediate visual memory (polydrug), self-rated memory (polydrug) and self-rated depression (drug-naïve). The first and last of these had negative coefficients, suggesting that deficits were greatest in ecstasy cohorts when the proportion of males was higher, but there was a positive coefficient for the remaining variable, indicating the opposite relationship. It is hard to draw any conclusions from these ostensibly contradictory findings.
Baseline IQ was reported with insufficient frequency to enable many metaregressions to be performed; where they were possible, they appear uninformative.
Sufficient study-level covariate data for years of education was available for only 10 meta-analyses. In two cases, a significant, positive coefficient was estimated (immediate and delayed memory in comparisons with polydrug controls), suggesting that reported exposure effects diminished as study-level education values rose. However, this was not a universal finding.
Characteristics of ecstasy exposure
Our metaregressions suggested that very little of the heterogeneity in reported exposure effects could be explained by aggregate measurements of ecstasy exposure. A significant coefficient was estimated for ETLD of ecstasy in two instances – executive function (response inhibition) and self-rated depression (both drug-naïve). However, a positive coefficient was estimated in the former case and a negative one in the latter, which suggests that any apparent differences may well have developed by chance. None of the other ecstasy exposure variables for which we collected and analysed data provided informative results. We conclude that – at aggregated study level, at least – there is no reliable evidence of a dose–response effect between exposure to ecstasy and long-term neurocognitive deficit.
Inter-arm differences
These analyses sought to examine the extent to which heterogeneity in reported effects could be explained by imbalances between the ecstasy-exposed cohort(s) and their controls.
Although this variable appears to be an influential one, with five statistically significant metaregressions, the direction of results is inconsistent. In two cases – immediate visual memory (polydrug) and attention (focus–execute) (polydrug) – a negative coefficient suggests that worse performance is seen in ecstasy-exposed cohorts who are older than their controls. In contrast, the remaining three significant analyses – self-rated depression, self-rated anxiety and subjective measures of impulsivity (all with polydrug controls) – have positive coefficients, which indicates that the studies in which ecstasy users were younger than their controls were those in which the greatest deficits were seen. As explained in the description of each analysis, there are good statistical reasons to be sceptical about these findings because they are very heavily influenced by a single datapoint. Aside from this, the fact that all three of these meta-outcomes are based on self-reported measures may be significant.
Another speculative explanation is that this variable could, in fact, be expected to act in different directions, with increasing age representing a disadvantage in measures of cognitive function, whereas the opposite applies for measures of mood.
This is another variable which produced inconsistent results in our metaregressions. Three significant coefficients were estimated – two negative (immediate RAVLT verbal recall and working memory) and one positive (objective measures of impulsivity) – all in comparisons with polydrug controls. The equivocal nature of these analyses, together with a similar lack of consistency in non-significant metaregressions, suggests that this variable does not have any detectable, uniform effect on reported exposure effects.
In 30 separate analyses, sufficient data were available to perform metaregression analyses with asymmetry in baseline intelligence (standardised difference across various measures) as the explanatory variable. Of these analyses, 21 (70%) estimated a positive coefficient and, in six of these cases, a significant p-value (< 0.05) was generated. In contrast, a negative coefficient was estimated in nine instances, of which only one was significant by conventional standards. These results suggest that baseline imbalance in this area could have an adverse influence on the ability of a study to detect and quantify inter-population differences that could be ascribed to the exposure of interest.
There were very few instances in which sufficient studies reported ETLD of substances of interest in standard units in a way that would permit metaregression analyses. As a result, we were unable to draw any conclusions about the influence of these variables.
No significant coefficients were estimated in metaregressions in which inter-arm asymmetry in exposure to cannabis was the covariate of interest.
No clear pattern appeared in analyses in which the explanatory variable was inter-arm asymmetry in exposure to amphetamines other than ecstasy. In 11 of 18 cases (61%), a negative coefficient was estimated (suggesting that greater exposure effects were estimated in those studies in which the ecstasy-using arms also had greater exposure to amphetamines than their respective controls). In one instance (delayed RAVLT verbal recall), the association was statistically significant. On the other hand, there were seven metaregressions (39%) in which the opposite relationship was suggested.
In 13 of the 18 (72%) metaregressions for which there were sufficient covariate data to analyse the potential influence of inter-arm asymmetry in exposure to cocaine, a negative coefficient was estimated, implying that greater exposure effects were estimated in those studies in which the ecstasy-using arms also had greater exposure to cocaine than their respective controls. However, in only one instance (immediate RAVLT verbal recall) was the association statistically significant.
In 25 separate analyses, sufficient data were available to perform univariate metaregression analyses with a standardised difference in exposure to alcohol as the explanatory variable. Of these, 19 (76%) estimated a positive coefficient and, in five of these cases, a significant p-value (< 0.05) was generated. In contrast, a negative coefficient was estimated in only six instances, none of them significant by conventional standards. These results are relatively clear, but somewhat counterintuitive, because they suggest that effects were least in studies in which ecstasy users had greater exposure to alcohol than their controls. Nevertheless, these findings may be explicable. Early experimental research suggests that there is a complex pharmacological interaction between MDMA and alcohol, which may include some degree of attenuation of the hyperthermic effect of MDMA,151 so it is possible that alcohol consumption is, to some degree, neuroprotective to ecstasy users. Alternatively, it is possible that there are differences between ecstasy users who drink alcohol and those who tend not to. One Australian study has found that ecstasy users who do not drink alcohol tend to be more disadvantaged, with greater levels of unemployment, less education, higher rates of drug-user treatment and prison history, as well as being more likely to be drug injectors and to be positive for hepatitis C virus, in comparison with those who use ecstasy and alcohol together. 152 Whether or not these findings can be generalised to a UK context, they can be interpreted as indicative of radically distinct populations of ecstasy–alcohol and ecstasy-only consumers. A difference such as this – with low alcohol consumption characteristic of high-risk ecstasy users, and heavier drinking associated with a more casual approach to ecstasy – could easily explain the results seen in our analyses.
Other Level II outcome measures
We found a number of reported outcomes in the Level II evidence-base which could not be combined into pools that were amenable to full-scale quantitative synthesis. This evidence is described in the following section.
Psychopathology
A small number of included studies reported measures of long-term psychiatric harm using the SCL-90. This instrument measures self-reported symptom severity on a number of psychological subscales for 90 items using a Likert scale. There are nine primary symptom dimensions (Somatisation, Obsessive–Compulsive, Interpersonal Sensitivity, Depression, Anxiety, Hostility, Phobic Anxiety, Paranoid Ideation and Psychoticism) and three global indices (the Global Severity Index, The Positive Symptom Distress Index and the Positive Symptom Total). A revised edition also exists (SCL-90-R) which replaces some items on the Anxiety and Obsessive–Compulsive dimensions that were considered psychometrically flawed.
We were able to provide pooled estimates for the global severity index, domains of obsessive–compulsion, somatisation, sensitivity, psychoticism and hostility. For these pooled analyses we have used scores generated from both revised and unrevised checklists and, because scores have been reported differently in different studies, we have used standardised mean differences.
Ecstasy users versus polydrug controls
For most analyses, including the global severity index, pooled data shows no difference between ecstasy users and polydrug using controls. Pooled data for one domain, Obsessive–Compulsive, suggests this is greater in ecstasy users (see Table 43). Only one of the studies pooled used the revised SCL-90. Tests for heterogeneity were not significant.
| SCL-90 measure | SMD | (95% CI) | p(null SMD) | p (heterogeneity) | Studies included in analysis |
|---|---|---|---|---|---|
| GSI | 0.187 | (–0.039 to 0.413) | 0.106 | 0.41 | Thomasius et al. 2005;92 Morgan et al. 2002;99 Dughiero et al. 2001136 |
| Somatisation | 0.194 | (–0.048 to 0.255) | 0.181 | 0.78 | Thomasius et al. 2006;58 von Geusau et al. 2004;128 Parrott et al. 2000;135 Parrott et al. 2001137 |
| Sensitivity | 0.132 | (–0.061 to 0.325) | 0.181 | 0.21 | Thomasius et al. 2005;92 von Geusau et al. 2004;128 Parrott et al. 2000;135 Parrott et al. 2001137 |
| Hostility | 0.079 | (–0.076 to 0.234) | 0.318 | 0.47 | von Geusau et al. 2004;128 Parrott et al. 2000;135 Parrott et al. 2001137 |
| Psychoticism | 0.233 | (–0.012 to 0.478) | 0.063 | 0.04 | Thomasius et al. 2006;58 Parrott et al. 2000;135 Parrott et al. 2001137 |
| Obsessive–compulsive | 0.264 | (0.092–0.435) | 0.003 | 0.29 | Thomasius et al. 2005;92 Parrott et al. 2000;135 Parrott et al. 2001137 |
Ecstasy users versus drug-naïve controls
Data from fewer studies were available for comparisons of ecstasy users and drug-naïve comparators, with only two studies reporting on each of the outcomes (one study each using the original and revised scales). For the global severity index, pooled analysis of psychoticism and obsessive–compulsive domains shows higher scores, meaning worse outcome, in ecstasy users (Table 44). No significant difference was seen between exposure groups in the measure of sensitivity.
| SCL-90 measure | SMD | (95% CI) | p (null SMD) | p (heterogeneity) | Studies included in analysis |
|---|---|---|---|---|---|
| GSI | 0.908 | (0.538–1.281) | < 0.001 | 0.90 | Thomasius et al. 2005;92 Morgan et al. 200299 |
| Sensitivity | 0.164 | (–0.080 to 0.407) | 0.19 | 0.05 | Thomasius et al. 2005;92 Parrott et al. 2001137 |
| Psychoticism | 0.367 | (0.204–0.531) | < 0.001 | 0.85 | Thomasius et al. 2006;58 Parrott et al. 2001137 |
| Obsessive–compulsive | 0.670 | (0.420–0.921) | < 0.001 | 0.05 | Thomasius et al. 2005;92 Parrott et al. 2001137 |
Aggression/anger
We found 14 studies that provided data assessing measures of aggression/anger/hostility. Seven studies assessed subacute effects with measures recorded between 0 and 15 days after an exposure to ecstasy. 62,125,153–158 These were excluded from analysis because the data were not judged to represent either an acute health harm or a long-term, clinically observable health harm. Data recorded after a minimum abstinence period of 21 days were available from the remaining seven studies;61,82,104,125,147,148,158,159 this time period was judged sufficiently long after exposure that any effects noticed might represent a long-term effect.
Two studies of subjects with a minimum abstinence period of 21 days provided data derived from objective measures; however, they were considered unsuitable to be pooled for further analysis because one experimental design used an interpretative paradigm158 while the other used a behavioural measure. 159 The study using a behavioural paradigm found aggressive-responding behaviour more frequent among a predominantly ecstasy-using group compared to non-drug users, whereas the interpretive paradigm study found an angry cognitive bias among three groups of substance misusers including current and ex-ecstasy users, but this study lacked a non-drug-using control group.
The remaining studies provided data from subjective measurement tools. One was considered to use control groups (ex-users and polydrug-using controls) that were too dissimilar from the other studies to permit pooling (non-drug-using controls verified by urine screens). Subjective measures were available from five studies (all originating from the same research group)63,82,147,158,159 which were similar in key aspects of design relevant to this outcome domain. They all assessed the same measure of aggression (BDHI direct subscale) at 3–4 weeks after discontinuing ecstasy and compared the results with those obtained from a control group of non-drug-using hospital workers and high school students. Two of the papers63,158 appeared to present data from the same or substantially the same cohort as part of a longitudinal study, so we had data from four studies that were potentially suitable to be pooled for further analysis. Throughout this work we have considered that five or more datapoints would be required for meaningful meta-analysis to be carried out and we therefore decided not to present the results as a table with subsequent analyses. Nevertheless, we subjected these data to some statistical analysis and found a weighted mean difference in BDHI direct hostility score of 16.58 (95% CI 15.08–18.08; p < 0.001) with no evidence of heterogeneity in the data (I2 = 0%). These data from four studies with little heterogeneity suggest that ecstasy users have significantly higher levels of subjectively-rated aggression than non-drug-using controls. This finding is limited by all the comparisons being made between ecstasy users who were seeking treatment or advice regarding their drug use and non-drug-using hospital workers and high school students. We note that this research group produced results that were markedly divergent from those reported in other centres for self-rated depression [see Depression (self-rated) – MDMA users versus drug-naïve controls, above]. The wider generalisability of these findings, therefore, is not clear.
Motor function
We found three studies reporting data for the outcome domain of motor function. These studies did not provide sufficient datapoints considered suitable for meta-analysis but brief summaries of findings are presented here. Two outcome measures were used to assess motor function – finger tap and pegboard – which were assessed in dominant and non-dominant hands in one study,47 the non-dominant hand only in one study,160 and left and right without defining dominance in the third study. 94 Unsurprising findings were that motor function speed and fine dexterity were greater in dominant hands. Finger tap speed was found to be faster in the dominant hand only in drug-naïve controls compared to current ecstasy users in the first study. This contrasts with the second study, which found no differences in non-dominant hands between ex-users, current users and drug-naïve controls. However, this study probably lacked statistical power because this was one measure contributing to a composite ‘cognitive battery’ assessment. Finger tap scores decreased numerically in the order ex-ecstasy users, drug-naïve controls, and finally current ecstasy users.
Pegboard test speed and fine control (number of drops) using either hand did not differ significantly between groups in the second study. The third study found that pegboard speed using the right hand (controlled by the left hemisphere of the brain as reported) was significantly faster in ecstasy users than in polydrug controls.
Given the small number of studies, the unsuitability of the data for pooling and the contrasting results, it is not possible to draw even tentative conclusions on the effects of ecstasy exposure on measures of motor control.
Sleep disturbance
We found 11 papers reporting outcome measures assessing various aspects of sleep. Four of these emanated from the same research group, reporting five studies, and we could not be sure that these were reporting mutually exclusive cohorts. As a result, we decided not to consider these for pooling with others for meta-analysis. The paper including the largest number of participants120 found no significant difference between ecstasy users and controls (around a quarter of whom used cannabis) on either the Epworth Sleepiness Scale or the average amount of sleep per night. This finding was in accordance with the results reported by the same group in three out of four of their other papers. 138,149,161 Five other papers were found reporting self-reported measures of sleep. In three of these,132,139,162 no significant difference was found between ecstasy users and controls (polydrug-using and drug-naïve). In two papers140,163 ecstasy users reported poorer sleep than did polydrug controls.
Two papers reported the results from polysomnographic sleep studies. One of these investigated the effect of pharmacologically induced inhibition of monoamine synthesis and the direct clinical relevance of the differences in sleep architecture observed are not clear. 117 The other study found differences in sleep architecture between ecstasy users and controls with less total sleep time amongst ecstasy users, primarily because of less time in REM sleep. 164
These studies provided insufficient data that were suitable for meta-analysis. An effect on sleep is suggested from both objective sleep measures in polysomnographic studies and self-reported sleep quality. It is not clear if this results in daytime sleepiness or other clinical sequelae.
Dental damage/oral health
We found two papers assessing aspects of oral health. These provided insufficient data for meta-analysis. One study165 reports significantly increased wear of molar teeth in a group of 30 ecstasy users compared to 28 polydrug controls. There was no difference in wear of front teeth. The authors attribute these findings to reports of teeth clenching by the ecstasy users. The second study166 compared responses to an oral sensation questionnaire amongst 119 polydrug users. Those who used ecstasy reported grinding of teeth, the desire to chew something and temporomandibular joint tenderness significantly more frequently than non-ecstasy drug users.
Loneliness
A single researcher has published two studies comparing the experience of ecstasy-exposed individuals with controls measured according to a self-created ‘Loneliness Questionnaire’. 167,168 Results suggest that ecstasy users may experience more loneliness (including ‘Unfulfilling Intimate Relationships’ and ‘Social Marginality’) than non-users. The relevance and robustness of these findings is unclear.
Uncontrolled (Level III) evidence (acute harms)
The Level II evidence we identified covered most chronic harms of interest, so our review of Level III evidence is dominated by the acute harms of ecstasy.
There are a number of fatal and non-fatal acute harms that may result from the use of ecstasy. These harms may be direct (for example as the result of toxicity) or indirect (relating to accidents while under the influence of a drug, for example.) We are primarily concerned with direct harms. Information about acute harms may be gleaned from a number of sources – registry data records, and case series or case reports in the medical literature, none of which is without problems. We have focused on datasets that are drawn from coherent sampling frames, for example registry data relating to death certificates and coroners’ reports for fatalities related to ecstasy (see Deaths related to ecstasy use) and audits of consecutive cases presenting at emergency rooms for non-fatal harms (see Acute harms reported in retrospective case series from hospital emergency departments). While these registries give an indication of the scale of fatalities associated with ecstasy use, clinical causes of death are not well described, so data available from other case series in the literature are also surveyed (see Acute harms of ecstasy reported in case series and case reports).
Given the large number of papers identified and their study design, we did not assess the quality of individual study reports as originally planned. Case reports and case series of acute harms suffer from a number of well-recognised problems. They are unlikely to be representative of the population under study, and there is no comparison group from which to draw inferences. Further, publication bias is a problem, as case reports on any particular condition are more likely when these are first reported, or are reported in novel circumstances. Later, as effects become recognised by clinicians and therefore become well described and researched, they are less likely to be reported in the literature as worthy of note. This means that the information found in such reports cannot be used to indicate the prevalence of any particular adverse effect, or cause of death, but is restricted to providing a catalogue of events as reported in the literature. Even in this there were limitations. We found acute outcomes difficult to catalogue accurately because there are overlapping outcomes in many cases that are the result of an initiating event such as hyperthermia. We found that there was poor and inconsistent reporting and indexing of outcomes, with symptomatic and clinical sequelae not always clear and missing data about the nature of drug-taking history and co-used substances common. Our reporting of these data sources remains necessarily brief and impressionistic.
Audit data based on all those presenting at emergency rooms having taken ecstasy are generally of too short a duration to provide enough cases to enable an accurate picture of the frequency with which different adverse effects are experienced. Only one such study comes from the UK, and presents a series of 48 cases, none of which were fatal. However, these studies suggest that, even among those experiencing adverse effects serious enough to present at Accident and Emergency (A&E), fatal instances are rare.
It is difficult to assess what might be a fatal dose of ecstasy. Fatalities have occurred from doses that are the same as a normally active dose which others tolerate. It is difficult to know how much MDMA has been ingested because self-reports may be unreliable, and the composition of any taken substance may vary. Most studies use (femoral) blood following a postmortem to assess the levels of MDMA concentration; however, levels of MDMA in the blood are known to rise following death because MDMA is released from body tissues. 169 Conversely, in a few studies, the levels of MDMA in blood were from the time of admission to A&E, leading to an underestimation of levels in a comparison.
The case series of non-fatal acute harms were heterogeneous, selective in their reporting of outcomes and unlikely to be generalisable to the whole population of recreational ecstasy users. We have made no attempt to report or calculate frequencies of individual health harms and have confined ourselves to simply listing the main effects documented.
Deaths related to ecstasy use
Deaths associated with ecstasy use are recorded in national and regional database studies (retrospective case series), as well as in case series or in individual case reports in the literature. In this section we report registry data which give an indication of the incidence of death in England and Wales, information about whether ecstasy was the sole drug involved or whether other drugs were co-used and some demographic information. There are two main national sources for information on number of drug-related deaths (DRDs) for England and Wales from which those involving ecstasy can be identified:23
-
General Mortality Register (GMR), collated by the Office of National Statistics
-
Special Mortality Register collated by the National Programme on Substance Abuse Deaths (np-SAD) St George’s Hospital, London.
This section will describe the number of deaths related to ecstasy use (alone or in combination with other substances) from available registry data. Data will be presented to allow an overview of trends and comparison with deaths related to other illicit drugs.
General Mortality Register data
The GMR is a database maintained by the Office for National Statistics based on information from death certificates and coroner’s reports. 170,171 For registry data, accuracy relies on the information recorded on the death certificate by the coroner. About 10% of deaths on the GMR relate to a general description only (such as ‘drug overdose’), limiting its use in the data, while in others it is not always possible to determine which is the primary drug involved where more than one is identified. 170
Included on the GMR are deaths as a result of illegal drugs, prescription drugs (such as antidepressants) and over-the-counter medications (such as paracetamol). Deaths from accidents and suicides, as well as poisoning as the result of abuse and drug dependence, are reported. As no detailed information is recorded on toxicology,23 a death may be categorised as ecstasy related without MDMA (or its metabolites) being reported on postmortem forms. 172 The GMR therefore combines deaths related to substances known to be MDMA and those related to reported ingestion of a substance believed to be ecstasy. In the case of multiple substances (co-drug use) being present, the GMR records all those mentioned on the death certificate. This was the case in 31% of DRDs recorded in 2006.
Between 1993 and 2006, the average annual number of DRDs in England and Wales according to the GMR was 2727, about two-thirds of which were in men. Trends are shown in Figure 84. In men, 30% of deaths were accidental, while this was the case in 24% of deaths in women. (Other drug-related deaths are recorded as intentional, undetermined, mental and behavioural disorders due to drug use or due to assault.) There were 1102 records annually of illicit (and related prescription) drugs over the same time period. These include heroin, morphine, methadone, cocaine, amphetamines (including MDMA/ecstasy), cannabis and GHB. Because the GMR records all co-use drugs mentioned, this figure will be higher than the number of people dying from these drugs.
FIGURE 84.
General Mortality Register all drug-related deaths 1993–2006.
Table 45 shows the average annual number of deaths where illicit drugs were recorded by the GMR either as the sole drug or as one of the drugs involved. The category ‘all amphetamines’ includes those related to ecstasy/MDMA. For 1993 to 2006, an annual average of 681 deaths related to a single illicit drug is recorded, of which heroin and morphine account for two-thirds, and methadone a further 22%. Similar numbers are attributable solely to cocaine or amphetamines (4.6%, 4.9%) and half of all amphetamine deaths are attributed to ecstasy (n = 17; 2.5% of the annual average of sole illicit DRDs).
| Mean annual deaths (%) – sole drug | Mean annual deaths – co-use drug mentions | |
|---|---|---|
| Heroin and morphine | 447 (65.6) | 622 |
| Methadone | 150 (22.0) | 276 |
| Cocaine | 31 (4.6) | 86 |
| All amphetamines | 34 (4.9) | 70 |
| MDMA/ecstasy | 17 (2.5) | 33a |
| Cannabis | 1 (0.2) | 14 |
| Gamma-hydroxybutyrate (GHB) | 1 (0.2) | 2 |
Figure 85 shows trends in deaths related to illicit drug use (and methadone) for 1993–2006. Cocaine deaths appear to be increasing year on year, while amphetamine deaths generally, and ecstasy specifically, appear to have increased to 2001 but stabilised thereafter.
FIGURE 85.
General Mortality Register drug-related deaths 1993–2006 (including co-drug mentions).
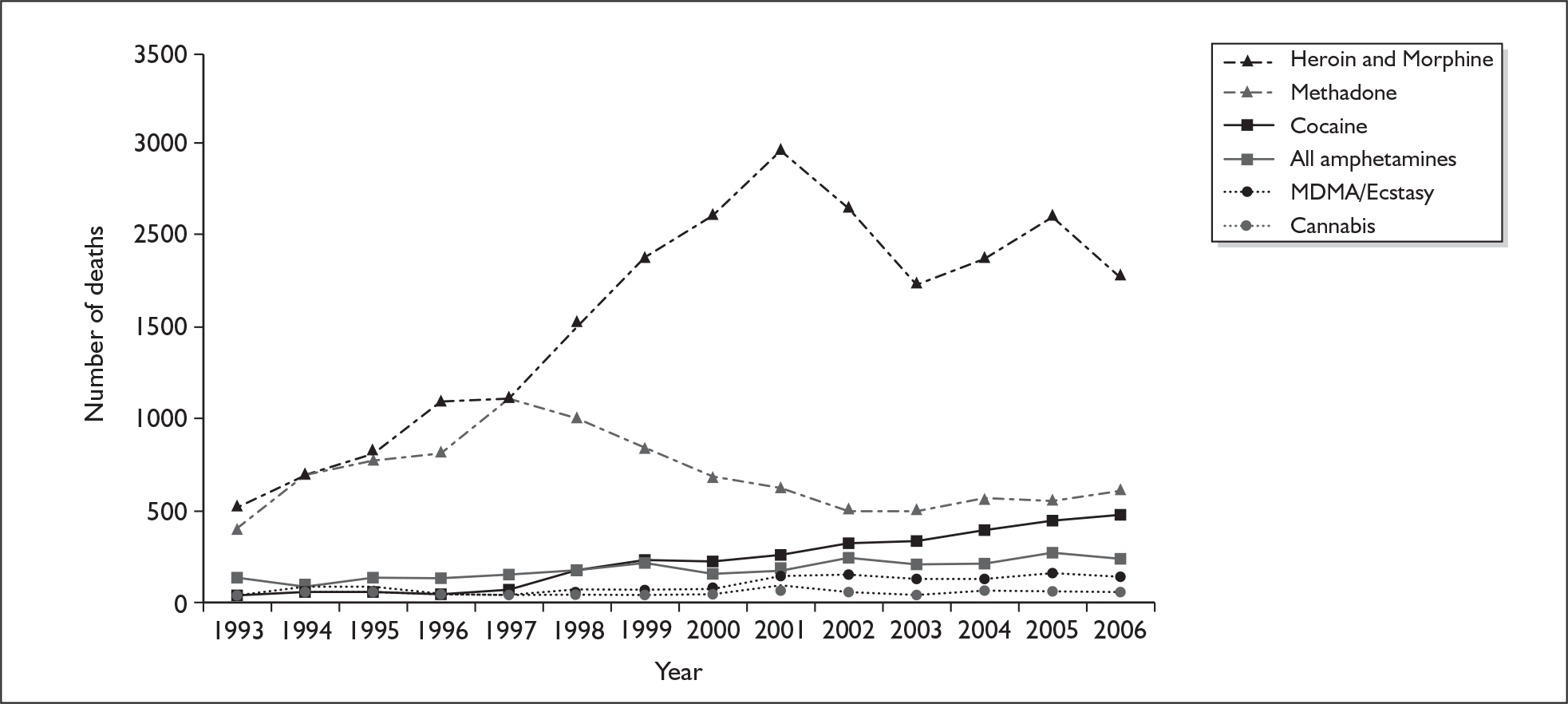
The much higher fatal impact of heroin, morphine and methadone masks the detail of stimulant trends. We therefore excluded these substances from Figure 86. In addition, we separated out amphetamine deaths that were related to MDMA/ecstasy and other amphetamine deaths. Given that the absolute number of deaths due to sole drugs is small, there may be natural variations in deaths which appear as large fluctuations when presented graphically. To ameliorate the impact of these fluctuations, 3-year rolling averages were calculated. Figure 86 shows 3-year rolling averages in relation to deaths which are attributable to a sole drug only. Amphetamines data in Figure 86 have been calculated by the reviewers based on all amphetamine deaths less those recorded as MDMA/ecstasy. These are not cleanly distinct categories so some misclassification is likely. There was a relatively rapid rise in ecstasy deaths between 1999–2001, where it overtook deaths from other amphetamines which were falling at the same time. Thereafter, the number of ecstasy deaths plateau while other amphetamine deaths rise, so that these two appear to be converging. Deaths from cocaine continue to rise steeply.
FIGURE 86.
General Mortality Register drug related deaths 1993–2006 (sole drug mentioned): three-year rolling averages for cocaine, MDMA/ecstasy and amphetamines (excluding MDMA/ecstasy).
National Programme on Substance Abuse Deaths (np-SAD) data
The National Programme on Substance Abuse Deaths (np-SAD) maintains the Special Mortality Register (SMR) at St George’s Hospital, London. This records voluntary submissions of coroners’ reports for England and Wales, including post mortem and toxicological reports. 173 Records implicating ecstasy will rely on evidence and reports from the scene as well as toxicology reports. As this database relies on coroners voluntarily returning their reports, it is unlikely to be a complete record. 173,174 Comprehensiveness is also limited by differences in the way coroners, or their pathologists, incorporate findings. 174 Despite this, it also has advantages over the GMR data in that it relates to greater detail recorded on the coroners’ reports, including toxicology, which may not have been available at the time of the death certificates being filed. While returns from coroners’ reports were low initially (13% in 1997), they rose to over 90% by 1999. In addition, the np-SAD database records greater contextual and social demographic information than the GMR. 23
For the np-SAD, MDMA, MDA, MDEA, PMA and methylthioamphetamine (MTA) are classed as ecstasy. It should be noted that this definition is broader than that adopted elsewhere in this review. To emphasise this distinction, the term ecstasy-related substances (ERS) has been used in the following discussion. The amphetamine category includes amphetamine sulphate and methamphetamine.
In 2006, there were 1366 drug-related deaths recorded by the np-SAD database, of which 69 (5.0%) mentioned ERS as present. In the same year, 78 (5.7%) mentioned amphetamines.
Figure 87 shows deaths recorded by GMR and np-SAD over the same time period, 1997–2006. These are deaths in which ERS were mentioned (GMR) or implicated (np-SAD), meaning that other substances may be co-implicated or causal in the fatality. After an initial lower count (when fewer coroners returned their reports), the np-SAD has consistently shown more deaths in which ERS were involved. For 1997 to 2005, over which period data are available from both databases, the np-SAD recorded 426 deaths in which ERS were implicated, compared to 343 in the GMR. Both sources show similar trends.
FIGURE 87.
Comparison of deaths in which ecstasy was present for General Mortality Register (GMR) and the National Programme on Substance Abuse Deaths ( np-SAD) 1997–2006.
Between 1997 and 2006, 495 deaths were recorded by np-SAD in which ERS were implicated. This compares to 689 in which other amphetamines were implicated, 1917 in which cocaine was implicated and 6643 in which heroin/morphine was implicated. Table 46 shows whether these drugs were considered to be the sole drug implicated, to have contributed to the death together with another substance or, although present, were not considered to have contributed to the death according to the np-SAD. In 14% of cases ERS were not believed to have contributed to the death although it was present, while they were the sole drug implicated in 20% of cases. ERS were considered to have contributed, together with another substance, in 67% of cases. Where other drugs were also implicated, these results are broken down in Table 46. In 62% of cases relating to ERS, three or more drugs were identified at post mortem and all the drugs implicated are recorded, meaning that the percentages presented in the table cannot be summed.
| Drug implicated in deaths | Ecstasy-related substances (n = 495) | Other amphetamines (n = 689) | Cocaine (n = 1917) | Heroin/morphine (n = 6643) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Annual mean = 49.5 | n (%) | Annual mean = 68.9 | n (%) | Annual mean = 191.7 | n (%) | Ann. mean = 664.3 | |
| Sole drug | 97 (20) | 9.7 | 103 (15) | 10.3 | 218 (11) | 21.8 | 1866 (28) | 186.6 |
| Other drug implicated (drug of interest present) | 67 (14) | 6.7 | 113 (16) | 11.3 | 396 (21) | 39.6 | 195 (3) | 19.5 |
| Drug of interest and another drug implicated | 331 (67) | 33.1 | 473 (69) | 47.3 | 1303 (68) | 13.03 | 4582 (69) | 458.2 |
| Other drugs co-implicated in death | (n = 331) | (n = 473) | (n = 1303) | (n = 4582) | ||||
| Alcohol | 145 (44) | 137 (20) | 558 (29) | 2389 (36) | ||||
| Heroin/morphine | 110 (33) | 260 (38) | 1002 (52) | |||||
| Other opiates | 44 (13) | 94 (14) | 299 (16) | 1193 (18) | ||||
| Methadone | 41 (12) | 110 (16) | 320 (17) | 790 (12) | ||||
| Cannabis | 60 (18) | 101 (15) | 188 (10) | 432 (6) | ||||
| Cocaine | 95 (29) | 76 (11) | 935 (14) | |||||
| Ecstasy-related substances | 85 (12) | 96 (5) | 113 (2) | |||||
| Amphetamines | 78 (24) | 69 (4) | 237 (4) | |||||
| Gamma-hydroxybutyrate (GHB) | 9 (3) | 5 (1) | 8 (< 1) | 2 (< 1) | ||||
| Hypnotics/sedatives | 74 (22) | 116 (24) | 409 (21) | 1603 (24) | ||||
| Antidepressants | 31 (9) | 63 (9) | 124 (7) | 435 (7) | ||||
| Antiepileptics | 3 (1) | 6 (1) | 16 (1) | 46 (1) | ||||
| Antipsychotics | 5 (2) | 15 (2) | 20 (1) | 81 (1) | ||||
| Antiparkinsonism drugs | 1 (< 1) | 6 (1) | 6 (< 1) | 23 (< 1) | ||||
Data about amphetamines are also shown in Table 46. Amphetamine was thought to be the sole drug or was not implicated in the death in proportions similar to those in ERS-related deaths. Again, in more than two-thirds of cases, co-use of drugs was implicated. Amphetamine fatalities are less likely than ecstasy fatalities to have co-used cocaine or alcohol.
Table 47 shows the characteristics of people with ERS-related deaths from 1997 to 2006. Similar data are presented for other amphetamines, cocaine and heroin/morphine. This updated analysis of data kept by np-SAD was undertaken for this review. The cohorts are similar, although fewer ERS users are known drug addicts and more are employed. A picture of the usual ERS-related fatality emerges as an employed white male in his twenties, who is a registered drug addict and who has co-used a number of other substances.
| Characteristic | Ecstasy-related substances (n = 495) | Amphetamines (n = 689) | Cocaine (n = 1917) | Heroin (n = 6643) | |
|---|---|---|---|---|---|
| Sex | Male (%) | 83 | 80 | 84 | 86 |
| Age (years) | Mean | 29 | 32 | 33 | 34 |
| Mode | 24 | 27 | 31 | 29 | |
| Range | 14–71 | 15–62 | 16–83 | 1–95 | |
| Employment status | Employed (%) | 52 | 32 | 38 | 26 |
| Unemployed (%) | 34 | 55 | 52 | 64 | |
| Student/pupil (%) | 6 | 3 | 2 | 1 | |
| Other (%) | 9 | 5 | 8 | 9 | |
| Ethnicity | White (%) | 84 | 84 | 79 | 84 |
| Not known (%) | 11 | 14 | 13 | 13 | |
| Other (%) | 5 | 2 | 8 | 3 | |
| Known drug addicts (%) | 76a | 86a | 87a | 89a | |
| Place of death | Private residence (%) | 50 | 59 | 63 | 66 |
| Hospital (%) | 40 | 32 | 28 | 25 | |
| Other (%) | 10 | 9 | 9 | 9 | |
| No. of drugs found at postmortem | 0 (%) | 7 | 4 | < 1 | 4 |
| 1 (%) | 11 | 11 | 9 | 17 | |
| 2 (%) | 20 | 22 | 22 | 28 | |
| 3 (%) | 24 | 26 | 29 | 26 | |
| 4 + (%) | 38 | 36 | 39 | 25 |
Nearly half of the ERS-related deaths (49%) occurred on Saturday or Sunday night, whereas this was the case for about a third (36%) of amphetamine fatalities. This could indicate different patterns of use.
Identified studies reporting database and registry data
Our searches identified 16 studies which were based on national and regional registries and databases (retrospective case series). Seven studies are not related to the UK and were not considered in detail (two from the USA,175,176 and one each from Belgium,177 Spain,178 Greece,179 Slovenia180 and the Netherlands. 181)
Nine UK studies were reviewed in detail and these are summarised in Table 48. Three of these relate to data from the np-SAD over different time periods up to 2002,182–184 and one to GMR for the UK 1994–2003. 23 A further two studies audited death certificates in Scotland in the 1990s (using Registrar General data)39 or in Scotland 1995–7 (using Registrar General data) and England in 1995–6 (using death certificates). 29 Three studies audited regional data, one in Sheffield 1997–9,185 one in London 2003186 and one in Strathclyde 1995–8. 187
| Study | Location | Year of deaths | Total no. of DRDs | No. of ecstasy deaths | Deaths ecstasy sole drug (% all ecstasy deaths) | Age (mean years) | Male (%) | Data source |
|---|---|---|---|---|---|---|---|---|
| UK national | ||||||||
| Schifano et al. 2003178 | England and Wales | August 1996–April 2002 | NA | 202, 36 annual mean 2.9/month | 34 (17%), 6 annual average, 0.5/month | <30 years 73% | 80 | np-SAD database |
| Schifano et al. 2003179 | England and Wales | July 1997–June 2000 | NA | 81, 27 annual mean | 6 (7%), 2 annual average, 0.2/month | 27.2 | 81 | np-SAD database |
| Webb et al. 2003180 | England and Wales | 2000 | 1037 | 26 implicated 30, on post mortem | NR | 37.7 (all DRD) | 80 (all DRD) | np-SAD database |
| Gore 199929 | England and Scotland | 1995–6 (England) | NA | 18, 9 annual mean | NR | Only those aged 15–24 included | NR | Death certificates (England) |
| 1995–7 (Scotland) | 11, 3.7 annual mean | Audit of Registrar General (Scotland) | ||||||
| Forsyth 200139 | Scotland | 1990–9 | 2255 | 28, 4.0 annual meana | 7 (33%) | NR | 68 | Audit of Registrar General (Scotland) |
| Schifano et al. 200623 | UK | 1994–2003 | NA | 394 | 165 (42%) | NR | NR | GMR for England, Scotland, N. Ireland |
| UK regional | ||||||||
| Oliver et al. 2002181 | Sheffield | 1997–9 | 82 | 2 (contributory cause of death) | NR | 29.4 (all DRD) | 92 (all DRD) | Audit of Sheffield’s coroner’s reports |
| Hickman et al. 2007182 | London | 2003 | 148 | 16 (combined total for all amphetamines) | 1 (6%) | 35.8 | 80 | Audit of 7/8 London coroners |
| Seymour et al. 2001183 | Strathclyde | 1995–8 | 443 | 12, 3.0 annual mean | NR | 28 (all DRD) | 81 (all DRD) | Audit of toxicological database, University of Glasgow |
Given the lack of solid information about the number of people taking ecstasy, the amount they take (in terms of the number of tablets taken, the composition of those tablets and their purity), it is very difficult to make sensible estimates about the risk to any individual taking any particular pill. In the literature, estimates of the death rates from ecstasy are few. Gore estimates that the ecstasy-related death rate in those aged 15–24 years in 1995–6 in England was between 0.2 and 5.3 per 10,000 (all users), i.e. between 1 in 2000 to 1 in 50,000. 29 She compares this with a death rate of 1.0 per 10,000 from road traffic accidents. More specifically, the death rate for first-time users was estimated to be approximately two to four times (1.29/0.38 and 0.70/0.38) that of sporadic users – defined as having used ecstasy in the past year for more than 1 year – depending on the method of calculation. 29 (Gore argues that use in the previous month is assumed to reflect regular user, and use in the previous year, but not in the previous month, reflects sporadic use. So some sporadic users will be first-time users. 29) However, this calculation does not take into consideration the number of exposures (or dose and purity) within the previous year (excluding the previous month). Three death rates were estimated for Slovenia,180 the Netherlands181 and USA,175 where population size was provided. Rates were 0.15, 0.73 and 0.88 per million population per year respectively. However, these estimates did not take into consideration the number of users, dose or purity, while the Dutch study also included deaths in the presence of amphetamine and other phenethylamines.
Cause of death data from registries
It is not possible to identify causes of death in the np-SAD registry data. The data are presented for all ERS-related deaths, whether the drug was present or causal, or a single or co-used substance. In addition, the majority of cases are recorded as accidental poisonings – which do not give an indication of the clinical picture. For example, 69% of ecstasy deaths are categorised as ICD code X42 (accidental poisoning by and exposure to narcotics and psychodysleptics) or X41 (accidental poisoning by and exposure to antiepileptic, sedative–hypnotic, antiparkinsonism and psychotropic drugs). Suicide was recorded as the cause of death in 7% of cases and traumatic injury (such as that due to a traffic accident or to drowning) accounted for another 7%.
As the information about cause of death was limited, we turned to case series and case reports in the literature. The following sections report first on retrospective case series which are based on consecutive data about people presenting with ecstasy-related harms at hospital emergency rooms, both fatal and non-fatal, and second on other case series and case reports in the literature.
Acute harms reported in retrospective case series from hospital emergency departments
We identified three retrospective case series that were based on audits of hospital emergency department databases of admissions due to ecstasy use. These are based on self-reported use, clinical signs and toxicologically confirmed cases (Table 49). These papers record a death rate between 0 and 2%, suggesting that most acute adverse effects resolve either spontaneously or with treatment, even among those serious enough to present at hospital. 188–192
| Author | Country | Sample | Common presentations (all) | Other substances | Outcomes |
|---|---|---|---|---|---|
| Liechti et al. 2005184 | Zurich, Switzerland | All 52 self-reported cases of ecstasy intoxication at emergency department 2001–3; aged 16–44 years (mean 26); 79% men |
Collapse/loss of consciousness 36%, palpitations 19%, dizziness/weakness 15%, anxiety 14% Five severe presentations – symptoms included cardiac arrest, hyperthermia, rhabdomyolysis, DIC, renal insufficiency, liver failure, seizures |
90% co-used other substances (commonly alcohol, cocaine, GHB, amphetamines, cannabis); 72% had co-used illicit drugs | Most managed in A&E. 11% to ICU; one fatality. |
| Williams et al. 1998186 | London, UK | All cases treated at one A&E department where case notes recorded MDMA involvement; n = 48; aged 16–30 years (mean 23); 67% men |
Feeling unwell/dizzy/strange 31%, collapse/loss of consciousness 23%, palpitations 21%, dizziness/weakness 23%, anxiety 23% Six severe presentations – symptoms included delirium, seizure, coma |
67% had co-used other substances; 50% had co-used other illicit substances (commonly other amphetamines, cocaine) | Most managed in A&E; 15% admitted (one ecstasy use alone); no fatalities. |
| Sanuro et al. 2004a | Barcelona, Spain | All cases of self-reported or toxicologically confirmed amphetamine ingestion 2000–2 no. of ecstasy cases 135; aged 16–47 years (mean 24); 68% men |
Anxiety, agitation, cognitive disturbances, loss of consciousness, fits. Three severe presentations |
67% co-used other drugs or alcohol | One admitted to ICU; two fatalities |
Only one such audit was identified from the UK. This report, by Williams et al. ,192 uses all cases treated over a 15-month period at one London A&E department where case notes recorded MDMA involvement (n = 48). (This involved triage notes on arrival relating to ecstasy, substance misuse/ingestion, intoxication, overdose or a number of other key clinical symptoms.) Cases were aged 16–30 years (mean 23 years) and 67% were men. One-third had taken solely ecstasy, with the remainder co-using alcohol and/or other illicit drugs. Differences in symptoms and clinical signs at presentation between those solely using ecstasy and those co-using additional substances are reproduced in Table 50. Numbers are too small to permit meaningful statistical comparisons; however, some possible differences between those who have only taken ecstasy and those who have co-used other drugs are collapse/loss of consciousness (6% versus 31%), hyperventilation (18% versus 13%) and hyperthermia (32% versus 13%). Other emergency room studies have also noted that coma was only present in those who co-used other substances (specifically GHB and opiates188).
| Complaint |
Ecstasy alone (n = 16) No. (%) |
Ecstasy and other drugs/alcohol (n = 32) No. (%) |
Clinical findings |
Ecstasy alone (n = 16) No. (%) |
Ecstasy and other drugs/alcohol (n = 32) No. (%) |
|---|---|---|---|---|---|
| Strange/unwell/dizzy/weak | 7 (44) | 8 (25) | High pulse rate (> 100 bpm) | 13 (81) | 19 (59) |
| Collapse/loss of consciousness | 1 (6) | 10 (31) | Dilated pupils | 6 (38) | 12 (38) |
| Nausea/vomiting | 5 (31) | 6 (19) | Hyperventilation (> 20 breaths/minute | 6 (38) | 4 (13) |
| Panic/anxiety/restlessness | 5 (31) | 4 (13) | Anxiety/agitation/disturbed behaviour | 4 (25) | 6 (19) |
| Palpitations | 6 (38) | 6 (19) | High temperature (> 37.1°C) | 5 (32) | 4 (13) |
| Feeling feverish/shivering | 4 (25) | 3 (9) | High blood pressure (> 160/95 mmHg) | 0 | 6 (19) |
| Sweating | 3 (19) | 3 (9) | Drowsiness | 0 | 3 (9) |
| Shaking | 2 (13) | 4 (13) | Dehydration | 1 (6) | 1 (3) |
| Headache | 2 (13) | 4 (13) | Shivering | 1 (6) | 1 (3) |
| Chest pain | 1 (6) | 3 (9) | Seizure | 0 | 2 96) |
| Difficulty breathing | 2 (13) | 2 (6) | Nystagmus | 2 (13) | 0 |
| Abdominal pain | 3 (19) | 1 (3) | Hallucination | 0 | 1 (3) |
| Muscle aches/pains | 1 (6) | 3 (9) | Sweating | 1 (6) | 0 |
| Visual disturbance | 2 (13) | 1 (3) | Unconscious | 0 | 1 (3) |
| Thirst | 2 (13) | 1 (3) | Tremulousness | 0 | 1 (3) |
| Seizure | 0 | 3 (9) | No abnormality found | 0 | 3 (9) |
| Twitching | 0 | 1 (3) | Other | 3 (19) | 6 (19) |
| Other | 4 (25) | 1(3) | Missing data | 0 | 1 (3) |
All three emergency room audits showed very high proportions of presentation at the weekend (67–75%). In the UK, 40% of cases reported previous ecstasy use whereas the Swiss study reported 87% had a previous history of drug use. 188
Acute harms of ecstasy reported in case series and case reports
We identified a total of 57 case series or case study papers reporting on fatalities related to the use of ecstasy. Six of these did not report causes of death or preceding symptoms, leaving 51 papers that reported a number of adverse effects of ecstasy that were associated with fatalities:
-
hyperthermia
-
cardiovascular dysfunction
-
disseminated intravascular coagulopathy (DIC) and other haematological disorders
-
seizures
-
rhabdomyolysis (and other muscular dysfunction)
-
kidney failure
-
brain haemorrhage/other organic brain damage
-
hyponatraemia
-
liver failure
-
suicide/attempted suicide
-
neurological dysfunction
-
respiratory dysfunction
-
psychotic episodes
-
hypoglycaemia
-
immunological dysfunction (aplastic anaemia, etc.)
-
movement disorder (dystonia)
-
diabetic complications
-
vascular abnormalities.
For an evidence map showing the number of and references for case series which reported these outcomes, please see Appendix 8. Note that this list includes symptoms that were reported in the same case (for example, hyperthermia-related DIC, rhabdomyolysis and organ failure).
We also identified 236 case series and case reports which reported on non-fatal acute harms of ecstasy. In descending order of the frequency with which they are reported, these harms are (note again that one case may be subject to multiple negative outcomes, particularly where a major syndrome is involved):
-
hyperthermia
-
seizures
-
acute central nervous system abnormalities
-
liver failure
-
hyponatraemia
-
rhabdomyolysis (and other muscular dysfunction)
-
pneumomediastinum, pneumothorax and similar
-
acute psychotic episodes
-
DIC and other haematological disorders
-
brain haemorrhage/other organic brain damage
-
kidney failure
-
acute movement disorders
-
acute cardiac events
-
sensorineural dysfunction (auditory, optical)
-
urogenital dysfunction (including urinary retention)
-
dental damage/other oral injury
-
dermatological disorders
-
stroke
-
ocular injury
-
vascular abnormalities
-
movement disorders (including parkinsonism)
-
attention deficit disorder
-
dependency
-
diabetic complications
-
hypoglycaemia
-
attempted suicide
-
asthma exacerbation/other respiratory distress
-
hyperkalaemia
-
hypothermia
-
immunological dysfunction (aplastic anaemia, etc.).
For an evidence map showing the number of and references for case series which reported these outcomes, please see Appendix 8.
This chapter now outlines what is known about the major syndromes associated with ecstasy use – hyperthermia and hyponatraemia – and their sequelae in fatal and non-fatal cases. It then moves on to consider other major acute harms in cardiovascular dysfunction, neurological dysfunction, respiratory dysfunction, liver failure, kidney failure, suicide and other psychiatric effects.
Major syndromes
The most critical acute complications of ecstasy consumption are, in a majority of cases, related to two well-recognised syndromes, each involving serious derangement of homeostasis leading to multiple organ failure: hyperthermia and hyponatraemia.
Hyperthermia
Derangements of thermoregulation are a widely reported feature of MDMA toxicity,191 repeatedly demonstrated in experimental settings,192,193 and commonly observed in humans. The mechanism by which body temperature is increased is still debated; activation of the sympathetic nervous system and the hypothalamic–pituitary–thyroid axis might be involved. 194 The susceptibility of a small minority of ecstasy users to dangerous degrees of hyperthermia is idiosyncratic, and probably multifactorial, involving factors such as co-ingestants, ambient room temperature, physical activity and fluid intake. 194,195 It is also possible that underlying medical or genetic conditions affect either thermoregulation or fatal susceptibility to hyperthermia. 194–196
The physiological manifestations of MDMA-induced hyperthermia are similar to those seen in severe heatstroke. 197 The most noteworthy effects are:
-
Rhabdomyolysis Rapid breakdown of skeletal muscle, leading to tissue necrosis, with intracellular constituents (most notably myoglobin) leaking into the circulation.
-
Disseminated intravascular coagulopathy (DIC) Serious derangement of blood coagulation, which results in both excessive clotting (leading to localised ischaemia and tissue necrosis) and widespread bleeding.
-
Acute renal failure (ARF) Kidney dysfunction can develop as a consequence of either of the above. ARF secondary to rhabdomyolysis is caused by myoglobinuria (meaning the renal filtration system becomes obstructed by a build-up of the myoglobin that has leaked into the circulation). ARF secondary to DIC occurs when microvascular thrombosis causes renal ischaemia.
-
Acute liver failure Hepatic necrosis is a primary effect of hyperthermia,198,199 and it is possible that such injury may be exacerbated in the presence of amphetamines, which may impair the liver’s natural thermotolerance. 200 DIC and ARF may both contribute to and be exacerbated by liver failure.
These changes are often accompanied by a variety of symptoms, the most commonly reported being palpitations, anxiety, agitation and confusion (and to a lesser extent tremors, myoclonus and seizures). 188–190,201 In some cases, these result in rapid presentation (i.e. within hours) to A&E departments. 188–190 Collapse or loss of consciousness is reported in a significant proportion of admissions (8.8–36.5%). 188–190
We found hyperthermia to be the most commonly reported adverse effect of ecstasy use for both fatal and non-fatal cases. There were 41 fatalities relating to hyperthermia reported in the literature between 1991 and 2007. 36,37,169,181,188,194–196,201–216 However, these numbers should be treated with caution given the nature of case series evidence, and because there were also a number of other cases where the cause of death or the precursors of fatal organ failure were not clear. In addition to the fatal cases, we identified 43 case series or case reports giving details of non-fatal hyperthermia and related conditions.
The association between ecstasy use and prolonged dancing may be important for hyperthermia because core temperature also rises during intensive exercise. 217 Furthermore, ambient temperature is believed to influence MDMA-impaired thermoregulation, and ecstasy is commonly consumed in environments that are likely to be crowded, hot and poorly ventilated. In rats, MDMA induces an exaggerated degree of hyperthermia when ambient temperature is high but, conversely, hypothermia is engendered in cold conditions. 218–220 However, recent research suggests that the latter effect may not be reproduced in humans. 11 In the literature, slightly more than half of reported fatal cases occurred after the subject had been at a club, nightclub or rave (22/41).
Peak body temperature varied from 38.5 to 46.1°C. The average of the 31 cases that reported a temperature was 41.5°C, with only five fatal cases reporting a temperature below 40°C. 176,204,211,213 Note, however, that such very high temperatures have also proved non-fatal with medical intervention. 221
Two studies based on retrospective A&E databases suggest that hyperthermia is more common among those solely ingesting ecstasy, compared to those co-using other drugs. 195,211 Substances such as GHB and opiates when ingested with MDMA are reported to reduce body temperature, sometimes resulting in hypothermia. 198–200 However, co-use of PMA may compound the problem. 211 In 13 of the cases, co-use of other drugs was noted; however, this was not reported in all of the studies. Other drugs noted (some cases had multiple drug use) were alcohol (n = 4), PMA (n = 2), MDA (n = 6, although this is a metabolite of MDMA and may not have been ingested separately), LSD (n = 1) and cannabis (n = 1).
Four deaths were known to be in first-time or second-time users of MDMA,36,202,205 although the type of user was not reported in all studies, and deaths for first-time users may also be more ‘newsworthy’ in terms of publishing case reports. In 14 deaths, the number of MDMA tablets was reported, with an average of 2.9 (range 1–10), and in 26 deaths, the concentration of MDMA in the blood was reported, with an average of 1.1 mg/l (0.02–7.15 mg/l) (although see the note in the earlier section, Cause of death data from registries, about measuring MDMA concentrations).
Hyponatraemia
When the hyperthermogenic properties of MDMA are combined with intense physical activity (such as dancing) substantial amounts of sodium can be lost in perspiration. This problem is significantly compounded by the tendency of ecstasy users to drink large quantities of water, for which there are several reasons: ‘dry mouth’ is a common subjective response to MDMA;3 users exposed to publicity regarding the dangers of MDMA-induced hyperthermia may overcompensate by consuming excessive amounts of water;222 and MDMA may induce compulsive repetitive behaviour such as obsessive drinking of water. 223
The combination of sodium loss and excessive water consumption may also be exacerbated by excess fluid retention (as the result of inappropriate secretion of antidiuretic hormones and/or impairment of renal function224). The resultant hyponatraemic state sees a fall in serum osmolar pressure, allowing intracellular displacement of water, the most hazardous result of which is cerebral oedema. 225
The early clinical manifestations of hyponatraemic cerebral oedema are headache and nausea, progressing to confusion and seizures,224–227 although such altered states may be difficult to distinguish from the ‘normal’ intoxicative effects of MDMA or alcohol. 222 If not corrected, the syndrome will commonly progress to tentorial herniation, respiratory arrest, cerebral hypoxia and death.
Retrospective studies of A&E admissions suggest that hyponatraemia and associated brain oedema is a severe but rare complication of MDMA toxicity. 188 We identified 10 deaths resulting from hyponatraemia reported in the eight case reports and case series. 206,224–233 Most were reported between 1997 and 2002, with one in 2006. 232 Only three were in England and Wales. 206,224 Twenty-four case series or case reports involving non-fatal hyponatraemia were also identified. 234–254
All fatal cases were women aged between 16 and 21 years (average 18.4 years), which could support suggestions that women (and children) are more prone to hyponatraemia. 230,255 A retrospective study of enquiries to the London Centre of the National Poisons Information Service from December 1993 to March 1996 identified 17 such enquiries regarding hyponatraemia associated with ecstasy use, two of which were fatal, both in women. Seven non-fatal cases were in men. 224
The clinical pattern reported in the literature was remarkably uniform, with initial vomiting, disturbed behaviour, followed by seizures, drowsiness, a mute state of disorientation,224 loss of consciousness,206,224–230 tentorial herniation,230 respiratory arrest,228 pulmonary and cerebral oedema,224,229,231,232,256 hypoxia,224,230,232 and finally brain death. 224,228,230 Four cases were identified as hypothermic (i.e. body temperature < 35°C). 224,230,232 Levels of MDMA intoxication were relatively low (0.03–0.4 mg/l, with an average 0.13 mg/l, where reported).
Of the 10 deaths, there were seven cases in which prior dancing or party attendance was reported,206,224,229,230,232,233 in four of which the consumption of large amounts of water and consumption of alcohol were also recorded. 224,229,232 Although not all reported on co-use of other drugs, three reported that alcohol (n = 3), cannabis (n = 1) and other amphetamines (n = 1) were used.
Isolated acute harms
Cardiovascular dysfunction
Tachycardia is an invariable response to MDMA consumption, and is the most frequently reported clinical symptom in series detailing acute admissions in A&E departments. 188,192 There are reports of MDMA-related myocardial infarction157–260 and sudden cardiac death. 261 The importance of excluding concomitant use of other drugs (especially cocaine, which is well known to induce critical cardiovascular dysfunction) has been emphasised. 262
We identified seven deaths due to cardiovascular dysfunction that appeared unrelated to the major syndromes described in the previous section. 35,178,179,201,261–265 All were in men, aged 17–39 years (mean 27 years). Where reported, levels of MDMA intoxication were 0.2–4.56 mg/l (mean 1.65 mg/l). Co-use of other drugs was reported in four cases, for alcohol (n = 3) and MDEA (n = 1).
One man had a history of Wolff–Parkinson–White syndrome261 and another was human immunodeficiency virus-positive and his death was thought to be the result of interaction between ecstasy and ritonavir medication. 263 In one case, the victim fell down stairs and hit his head, although it is not clear if this caused, or was the result of, the cardiac arrest. 265
Fourteen case series and case reports reporting non-fatal cardiac events were also identified. 90,258–260,266–275
Neurological dysfunction
Seizures are a recognised manifestation of both hyponatraemia and hyperthermia as discussed earlier. Cases have also been reported of MDMA-induced seizures which apparently do not involve either of these underlying causes. 276 We identified three studies that reported seizures without hyperthermia or hyponatraemia. However, it has been emphasised that concomitant administration of other substances may be an important element in such findings, with the conclusion that, for those who have taken MDMA alone, central nervous system (CNS) dysfunction appears rare in the absence of hyponatraemia or hyperthermia. 1
Most of the CNS problems reported in the literature were non-fatal – we identified 63 case series reporting 88 non-fatal cases and 66 fatal cases, 20 of which were the result of indirect causes such as road traffic accidents. 176,177,179,190,202,222,244,250,254,277–299 In the majority of cases (n = 58) other drugs had also been ingested, most commonly other amphetamines (n = 28) and cannabis (n = 14), but also opiates, cocaine, LSD, benzatropines and ketamine.
Nearly three-quarters of patients were aged under 25 years and 70% were men. Most made a full recovery (63 cases, 72%). However, in 18 cases (12% of patients with brain disorders), a more severe course of CNS disorders led to complications which may be the result of chronic cerebral/cerebellar damage: four papers reported psychological personality disorders,290,293,300,301 two reported epilepsy,291,292 three reported chronic movement disorders,284,285,302 and three reported serious neurological disorders (such as vegetative state). 213,282,295
Brain haemorrhage/haematoma related to ecstasy use has been reported in 21 cases, six of which were fatal. 176,202,230,276,277,292 Such events are commonly associated with pre-existing cerebrovascular vulnerabilities (e.g. aneurysm278,288 or arteriovenous malformation283,292); however, cases have also been reported in which no such features were identified. 292,303 It has been postulated that long-term MDMA use may expose individuals to a higher risk of cerebrovascular accident, either through vasculitis292,303,304 or as a consequence of arterial damage sustained through repeated episodes of vasoconstriction and hypertension (‘surge’). 288 Of the 15 patients who recovered, 12 recovered fully278–280,283,288,292,305,306 while three experienced ongoing effects such as hemiparesis and seizures. 284,285,292
We identified two reports in the literature of non-fatal cerebral ischaemic stroke, both of whom recovered fully. 289,307 One study reported a lesion of the spinal cord C1–C4 causing residual mild hemiparesis. 302
Respiratory dysfunction
Pneumomediastinum is an abnormal presence of air in the mediastinal tissues. The main symptoms include chest pain, dyspnoea and neck pain. 308 Chest pain secondary to pneumomediastinum may be reported by those presenting for medical attention following MDMA consumption. 309–321 Less frequently, pneumothorax313,317 and pneumopericardium316 have also been reported. Pneumomediastinum is believed not to be a direct pharmacological effect of MDMA, but rather the result of physical activity over a prolonged period accompanied by episodes of forced expiratory effort against a closed airway, such as that through screaming, whistle blowing, coughing or vomiting. 322–325 Onset of symptoms is mostly reported within 12 hours of taking ecstasy, and some symptoms, such as shortness of breath or chest pain, may appear even sooner. 316 In most cases, symptoms resolve spontaneously in few days.
We identified 29 cases of pneumomediastinum in 22 studies. In six cases pneumothorax was also present. 269,308,319–322 Most (24/25) were under 25 years old and two-thirds were men. Only five were known to be smokers and one was asthmatic, both of which are known risk factors for pneumomediastinum. In one case, symptoms were experienced after taking only half a tablet of ecstasy. 312 Most studies (23/29) do not report whether other substances were co-used.
Other types of isolated respiratory failure appear to be uncommon following MDMA consumption. One case of acute pulmonary oedema326 and one asthma-related death35 have been reported (although, in the latter case, the causal relationship between the exposure and the outcome is unclear). Two other instances of respiratory complication that have been cited elsewhere1 appear to be related to consumption of MDEA. 329,330
Liver failure
Critical hepatic dysfunction is a notable consequence of hyperthermia and extensive hepatic necrosis is an invariable postmortem finding in MDMA deaths. 206 In addition, it is well established that MDMA-induced acute liver failure can also occur without thermoregulatory dysfunction. 199,231,331–338 This type of acute hepatic failure (the term fulminant liver failure is also used, either synonymously or in reference to the most rapidly symptomatic cases339) develops over a slightly longer period than in hyperthermic liver failure, with cases becoming symptomatic a matter of days, rather than hours, after MDMA ingestion. In most reported cases, acute hepatitis appears to develop following a history of repeated MDMA use. However, cases involving very limited exposure (including, ostensibly, consumption of a single tablet) have also been described. 199,332,333,335
Spontaneous resolution of symptoms can be expected in some cases; however, failure can also be irreversible, leading to death or requiring salvage liver transplantation. 334 It has been emphasised that, in common with other hepatotoxic substances, MDMA could be expected to cause silent liver damage in a number of cases over and above those that are clinically evident. 200
One retrospective case series reported acute liver failure in the absence of hyperthermia. This was based on consecutive non-paracetamol-induced fulminant hepatic failure presenting at the Scottish Liver Transplant Unit (which serves the whole of Scotland) in 1992–2004. 340 Of 30 cases, six were related to ecstasy use and had not been preceded by hyperthermia, and two of these proved fatal. Of the four survivors, two had a liver transplant. 231 One other study reported on a successful liver transplant in a 25-year-old woman with liver and kidney failure. 231
Kidney failure and other urinary tract abnormalities
It is thought that MDMA-induced kidney dysfunction can occur in the absence of hyperthermia or hyponatraemia. A similar causative mechanism to that postulated in respect of cerebral vasculitis (see above) may be implicated because renal arteritis has been demonstrated in postmortem examinations. 341 However, we did not identify any fatal cases with acute renal failure that did not also record hyperthermia. One fatal case study of chronic renal failure reported on a 30-year-old man in the UK who presented 1 week after having taken ecstasy and other amphetamines. 341 He was reportedly apyrexial although no temperature is given, was hypertensive and had pulmonary oedema. Postmortem revealed necrotising angiitis confined to the kidney.
Transient urinary retention is a relatively common characteristic of the 24 hours following MDMA consumption, with catheterisation occasionally required to resolve symptoms. 244,342–344 All four cases we identified were under 20 years (mean 18, range 17–19) and three were men.
Suicide
It is postulated that impaired serotoninergic function as a result of ecstasy use could lead to depression and suicide. 345 We identified 10 cases of suicide related to ecstasy use in the literature either as a means of overdose, or reported as having been taken in the run-up to suicide by other means. 177,210,214,345–350 Eight cases were in men, aged 17–53 years (mean 31), and two were in women, whose ages were not recorded. One woman hanged herself in jail after a 3-day session of injecting ecstasy. The other woman committed suicide having been admitted to a psychiatric ward as the result of paranoid delusions after ingesting an unknown quantity of ecstasy. 345 She is reported as having a long history of undefined ‘drug abuse’.
In three cases, MDMA was deliberately taken as the means of suicide, following a personal crisis or imprisonment. 210,214,349 In two cases, MDMA was found at autopsy but no further details about its use are provided – in one of these cases, heroin and antidepressants were also found and, in the other, suicide was assumed after the man died under a train. 177 In five cases, MDMA had been consumed before expression of suicidal intention. 345–347,350 In three of these cases, ecstasy use seemed to precipitate a psychotic episode leading to suicide (although there was also some reporting of prior emotional distress or depression345,346), in one case within 3 hours, in another within 8 days, this latter following admission to and discharge from psychiatric hospital. One case was in the UK, in a 17-year-old boy, who was apparently a first-time user. 347
Other psychiatric effects
We identified one retrospective case series based on audit data from a psychiatric admission ward in Cardiff. Over a 12-month period, this records that 50 out of 390 admissions were drug related, and that ecstasy was implicated in 35 cases (70% of all drug-related admissions). 351 Usual presentations included panic attacks, restlessness and psychotic behaviour. Most were initially treated with tranquillisers with behaviour change seen 48 to 72 hours later. The authors report that eight (23%) of this sample were still receiving treatment from psychiatric services 8 months after admission, including two as inpatients. None of these eight had any previous history of mental illness. 351
We also identified 25 cases of acute psychiatric episodes in 15 case reports and case series. 296,298,301,352–362 Four cases in two series were from the UK. 358,359 Reports were published fairly evenly from 1986 to 2005 and cases were among those aged 17–52 years (mean 25.4), of whom 18 were men (72%). No prior history of psychiatric disorder was recorded in 22/25 cases. Two cases were reported after first-time use of ecstasy353,362 and, in a further two, ecstasy was taken unintentionally for the first time following friends ‘spiking’ drinks. 350,354
Commonly reported presentations were panic attacks (reported in 12/25 cases), auditory and/or visual hallucinations (11/25) and paranoid delusions or psychosis (7/25); other symptoms included delirium, aggression, obsessional behaviour, self-harm and suicide ideation. Additional physical symptoms such as palpitations, hyperthermia and seizures were also reported.
In only two cases was ecstasy the only substance taken, although this is not reported in four papers. Reported co-used substances included alcohol (3/21), cannabis (9/21), cocaine (4/21), heroin (1/21, with a further two having a prior history of heroin addiction), methadone (2/21), LSD (2/21), other amphetamines (1/21), benzodiazepine (1/21), citalopram (1/21), valium (1/21) and opioid-based painkillers (1/21) – in six cases multiple substances were co-used.
Symptoms manifested from minutes to days after ingesting ecstasy and persisted for hours, days or months with treatment. Most papers report full recovery, after 3 hours to 7 months of treatment, but five papers report symptoms remaining at 1–9 months. 301,359,360,363
Chapter 4 Discussion
Statement of principal findings
This systematic review assesses the health harms of the recreational use of MDMA. We adopted a much broader remit than previous syntheses, encompassing and expanding on previous areas of interest. In addition, our review provides greater detail about the methods used for meta-analysis and we use innovative methods to pool data and to examine possible confounders between study arms. We also distinguish between polydrug-using and drug-naïve controls, which was not the case in all previous meta-analyses.
We include a large number of studies that have investigated a wide range of possible chronic harmful effects of ecstasy on recreational users of the drug. There is good agreement in the results of these studies, whether they emanate from previous meta-analyses or from meta-analyses undertaken for this review of either individual outcome measures or composite outcomes. Ecstasy users consistently perform worse than controls across a wide range of neurocognitive tests and psychopathological instruments. The effects are most consistent and marked for memory, particularly measures of verbal and working memory, but are also seen particularly strongly in self-rated measures of depression, memory, anxiety and impulsivity. While the commonest comparison made in studies is that of current recreational users of ecstasy with polydrug-using controls (subjects who use other legal and illegal drugs but not ecstasy), similar results are seen when current ecstasy users are compared to controls naïve to illegal drugs and when former ecstasy users are compared to either control group. Substantial caution, however, should be taken in interpreting these results, the key reasons for which are outlined below.
Key registry data about drug-related deaths is available from the np-SAD and GMR registry databases. These data are not directly comparable because of differences in data sources and recording of drug use. In the 10 years to 2006, the np-SAD recorded an average of 50 drug-related deaths in which ecstasy was mentioned as present (69 in 2006): 5% of the total for the year (see Figure 87). Ecstasy was the sole drug implicated in an average of 10 deaths annually over the same time period (other amphetamines implicated as the sole drug in an annual average of 10 deaths, cocaine in 22 and heroin in 187).
The GMR reports an average annual number of all drug-related deaths between 1993 and 2006 of 2727, about two-thirds of which were in men. There were, on average, 17 deaths a year in this period where ecstasy was recorded as the sole drug involved (2.5% of all deaths ascribed to a single drug) and an additional 33 per year where it was reported as co-drug use (see Table 45). Ecstasy deaths appear to have increased up to 2001, but to have stabilised thereafter, while cocaine deaths are increasing year on year. Heroin and morphine, as expected, account for the great majority of drug-related deaths (65.6%).
The typical victim of an ecstasy death is an employed white male in his twenties, who is a known drug addict co-using a number of other substances (see Table 46). Given the paucity of data about scale and patterns of use, the risk associated with taking an ecstasy tablet is very difficult to assess.
Methodological considerations
The controlled observational studies (Level II evidence) included in the report investigated the chronic harmful effects of recreational ecstasy use, largely neurocognitive effects and depressive symptomatology. All these studies, apart from one with a prospective design, are cross-sectional in nature and compare ecstasy users either with users of other legal and illegal drugs or with users who were naïve to illegal drugs.
We did not identify any Level II evidence concerning the acute harmful effects of ecstasy: all the included literature on this aspect of the review consisted of Level III evidence, either case series or case reports.
Chronic harms
Our systematic review has included many more studies of controlled observational data than previous meta-analyses: 110 compared to 28 in the largest of the previous relevant meta-analyses we have included. The range of health outcomes considered is also broader, with previous reviews focused on self-reported depression or neurocognitive damage generally, and memory specifically. In addition, we have provided more detailed critical appraisal of the included studies, which are all cross-sectional in design, except one prospectively conducted study, and have numerous significant methodological flaws, which are discussed in detail (see Chapter 3, Assessment of the quality of studies).
Inclusion/exclusion criteria
With the time constraints of this project, we had to confine our review to studies published in the English language, which may have led to the exclusion of relevant studies published in other languages. It is difficult to predict what effect this exclusion may have on our results: papers in other languages may be more likely to report negative findings which would weaken the associations we have found. We have found some evidence of publication bias in the outcomes we have assessed (especially in comparisons between ecstasy users and drug-naïve controls), suggesting that there may be other unpublished studies reporting negative findings, which would also weaken our findings.
We excluded laboratory-based studies for two main reasons: recreational use of ecstasy means that the dangers of pills taken as ecstasy need to be considered, regardless of their actual purity or dose; and the impact of ecstasy, in terms of both acute and chronic harms, is influenced by the environment in which it is taken. While these are strengths in interpreting the data, they also cause limitations in that the actual impact of MDMA, as opposed to other substances, may not be being measured. Many laboratory studies also focus on the acute pleasurable effects of taking ecstasy, which are beyond the scope of this review.
We excluded studies which assess the health harms of the recreational use of amphetamines generally if it was not possible to identify which results specifically assessed the harms of ecstasy.
Outcome measures
We identified a huge number of wide-ranging outcome measures: 915 different outcome measures were used in the included studies, many of which were ostensibly measuring the same attribute, sometimes in the same study. In addition, some papers used subscales while others used the full scale of the same instrument. Some scales have revised or amended versions, and a mixture of the original and the revised scales was used in the included studies. It is not possible to determine what the impact of pooling across these scales might be. In addition, it is unclear what we should be trying to measure. It is possible that some understanding of impact on total brain function is important, rather than the specific domains (such as memory, cognition or behaviour) on which studies tend to focus.
We identified only eight outcome measures for which a meaningful number of studies had used the same instrument and the same scales, all of which were measures of verbal memory or intelligence; all except one compared ecstasy-using groups to polydrug-using controls.
Where several different outcome measures were used to measure the same attribute, we categorised and collapsed these into similar domains to allow meta-analysis. These domains and the identification of outcomes that were appropriate to include within them were based initially on reviewers’ classification and then validated by our expert advisory group. Necessarily, some of these classifications are matters of judgment and other investigators may have chosen to group outcomes differently.
Many of the outcomes used in the studies, especially those assessing personality dimensions and mood, rely on self-reports of a characteristic rather than objective measurement. This may be a particular problem in self-selected study groups, who may participate because of preconceived notions of the effect of ecstasy. In pooled analyses, self-rated measures showed the greatest impact of ecstasy use in comparison to both polydrug-using and drug-naïve controls.
To pool data from different studies using disparate scales to measure the same outcome, effect sizes were converted to a standardised mean difference. One consequence of this strategy is to complicate the interpretation of the analyses further, because it is unclear how to decipher the clinical meaning of a difference of any magnitude.
There was substantial heterogeneity in study design, which may have implications for the meta-analyses, although we used random-effects models for all analyses to account for the expected heterogeneity.
There were a number of outcome domains for which data were not sufficient to pool for meta-analysis. Narrative synthesis only was possible for some of these outcomes while other individual outcomes have not been considered in the review.
Confounding
As all the included studies were observational, it is unlikely that potential confounders (such as age, exposure to other drugs, educational status, etc.) are equally distributed in the study arms. We used metaregression techniques to explore the impact of such potential confounders, in univariate analyses only. Multivariate analyses would be desirable in future syntheses, but availability of covariate data was too patchy to enable such an approach in this case. In addition to standard metaregression techniques, we examined the effect of imbalances in arm characteristics on the exposure effect estimated in studies (a technique that, as far as we are aware, has not been used previously). The benefit of this approach is that it should enable us to identify the extent to which observed differences in outcomes may be confounded by factors other than exposure to ecstasy.
Despite these analyses, it has not been possible to explore or control for all possible confounders, because of the variable and incomplete documentation of possible confounders in the literature. In addition, confounders are measured at population levels rather than individual levels, and attempts to extrapolate to individuals may lead to ecological fallacy.
The small size of many of the studies together with the suggestion of publication bias in several analyses suggest that caution is needed in interpreting the results, which may be subject to Type I errors (false rejection of the null hypothesis). We found that imbalances in baseline intelligence had a significant impact on observed outcomes in a number of cases: where ecstasy-using groups were, on average, less intelligent than their control arms, they tended to perform worse in neurocognitive tests. Other drug use, mainly amphetamines, cocaine and cannabis, may affect the results in either direction, with no consistent pattern, suggesting that these findings may be artefacts. It is possible, however, that these drugs may act to ameliorate the impact of ecstasy by lessening its hyperthermic effects. This is seen in some studies of acute harms (see Chapter 3, Hyperthermia) and chronic effects could also be influenced by increases in body temperature. In addition, metaregression in 25 analyses found that increased co-use of alcohol was associated with reduced negative effects. As discussed earlier (see Chapter 3, Inter-arm differences), it is possible that alcohol use is a marker of different patterns of drug use, or that alcohol consumption may beneficially attenuate the hyperthermic effects of ecstasy, leading to less long-term damage. These are speculative suggestions which should be treated with caution.
One Hong Kong study, by Yip and Lee,128 indicated a much bigger impact of ecstasy use for delayed verbal and visual memory outcomes than other studies included in these meta-analyses. The characteristics of this study’s participants might mean that this represents a unique insight into the pure effects of ecstasy, as this study was able to recruit clubbing cohorts of ecstasy-only users and drug-naïve controls, neither of which were exposed to other substances, including alcohol and tobacco. It would be very useful to have more studies with comparisons between such groups; however, these have proved very difficult to recruit in European settings. Other qualities might also account for these outlying results, as this is the only study to use the Chinese version of the RAVLT measure. In addition, ketamine contamination was reportedly common in pills sold as ecstasy in Hong Kong at that time.
Acute harms
We did not identify any controlled observational studies concerning acute health harms of ecstasy that met our inclusion criteria. There was, however, a large number of uncontrolled case series and case reports which met our inclusion criteria, including several case series of deaths and hospital admissions, based on data from death registers, coroners’ reports, emergency department databases and hospital statistics. We obtained additional information from authors who maintain the np-SAD in the UK, to bring the data on UK deaths from ecstasy as up-to-date as possible. Establishing cause of death caused particular difficulties because death registers record the underlying cause of death only as due to poisoning, rather than stating the immediate cause or mode of death, such as hyperthermia or renal failure.
Inclusion/exclusion criteria
As outlined in the protocol, we did not consider indirect harms of ecstasy, for example the role of ecstasy in accidental deaths due to road traffic accidents, or users’ vulnerability to acquiring sexually transmitted infections following unsafe sex. An assessment of such outcomes would contribute to a more complete picture of all possible harms relating to ecstasy use.
Confounding
Three-quarters of deaths recorded in registries relate to ecstasy use among known drug users. From the data, it is not possible to ascertain whether the minority who die with ecstasy as the sole drug in their system were also known drug users. In any event, it seems that those most at risk of death related to ecstasy are also co-using other drugs or have a history of polydrug abuse.
Analysis
The weak nature of the evidence-base, in terms of both study design and poor and incomplete reporting of outcomes and confounders, made a detailed synthesis of the acute harms unfeasible. We have therefore confined ourselves to describing the case series of deaths from ecstasy in a narrative way. As the case series and case reports of non-fatal acute harms were so heterogeneous, selective in their reporting of outcomes and unlikely to be generalisable to the whole population of recreational ecstasy users, we have made no attempt to report or calculate frequencies of individual health harms and have confined ourselves simply to listing the main effects documented.
Strengths and limitations of the evidence: chronic harms
As outlined earlier in this chapter (see Statement of principal findings), there was a small but consistent negative effect of ecstasy use across a large number of studies. The fact that this effect was seen across so many different outcome measures suggests that there is a real association of ecstasy with impairment of neurocognitive function, particularly some aspects of memory, and with increased psychopathological symptomatology. There are, however, very substantial cautions attached to such an interpretation. With one exception, the evidence on which these findings are drawn is based on cross-sectional studies, so that causation cannot be inferred. There are also significant methodological flaws in many of the studies. The weakness in the study designs is also apparent in the difficulty in controlling for the many possible confounders in these studies.
In assessing whether the association between ecstasy and poor neurocognitive function and increased psychopathological symptomatology (such as anxiety, depression and impulsivity) is real and attributable to the recreational use of ecstasy, the quality of the evidence is discussed below according to relevant criteria for assessing causality:364
-
strength and consistency of the effect
-
dose–response effect
-
temporal relationship
-
plausibility and coherence.
Strength and consistency of effect
The detrimental effects of ecstasy on memory, depression, anxiety and impulsivity are consistently identified in previous meta-analyses and the meta-analyses undertaken for this review, for both individual outcome measures and composite measures derived by pooling outcomes measuring the same domain. This is despite different focus, outcome groupings and inclusion criteria between this systematic review and those previously published. The three previous meta-analyses of neurocognitive function all found that ecstasy users performed worse than controls in all domains: verbal learning and memory, attention, non-verbal learning and memory, psychomotor speed, executive systems function, short-term memory, long-term memory and visual memory. 59,60,73 The greatest deficits (‘moderate ’to ‘large’ using Cohen’s guidelines) were seen for verbal learning in all three reviews. Effect sizes were not modified by lifetime exposure to ecstasy, but former users were not analysed in these studies so further exploration of dose–response is not possible. It is worth noting that Verbaten confined his analysis to heavy users of ecstasy where possible and his effect sizes were larger than other meta-analyses. Our analyses do not suggest the presence of such a dose–response effect.
Sumnall and Cole’s previous review of depressive symptoms also found very similar results to our analyses of such outcomes, including suggestions of publication bias. 58 Again, this review found a positive association with lifetime exposure to ecstasy and, while we found a weak association between ETLD and depression effect size when ecstasy users were compared with drug-naïve controls, there was no such evidence in the comparison with polydrug-using controls.
The commonest comparison made in our analyses is between current ecstasy users and polydrug-using controls. Polydrug-using controls are those who use legal and illegal drugs, but not ecstasy, and have generally been recruited in the same way as ecstasy users. While, given the observational nature of all the evidence available, it is not possible to be certain that both groups come from the same population, it seems reasonable to assume that polydrug-using controls and ecstasy users are fairly similar in most respects apart from ecstasy use. This assumption is generally borne out by the reported levels of other drug use and sociodemographic variables in the individual studies.
Drug-naïve controls, on the other hand, are a more heterogeneous group and while, in some cases, they may have been recruited in a similar way to the ecstasy users, there are also instances in which they have been drawn from very different populations, such as researchers and hospital workers. While ecstasy users also perform worse than this control group on neurocognitive tests and have more psychopathological symptomatology, it is likely that unidentified (and therefore uncontrolled) confounders may be modifying this effect to a greater extent than with polydrug-using controls. It is also impossible to disentangle the effects of ecstasy from those of the other drugs to which the ‘ecstasy’ arms of these trials have been exposed.
Although consistent in direction, the size of all identified effects is generally small. For individual outcome measures, the mean effects in both user groups (current and former ecstasy users) and both control groups (polydrug users and drug-naïve controls) are within the normal range of the tests used. For the composite outcome domains, the effect sizes generally fall in the range classified as ‘small’ according to the Cohen guidelines. 119 This statement is true for all comparisons of ecstasy users with polydrug-using controls, with the exception of self-rated memory, where the difference is 0.51 SD (a ‘moderate’ effect according to Cohen). The range of differences is 0.15–0.51 SD.
Differences between users and drug-naïve controls are larger, with those for immediate and delayed verbal memory, 0.84 and 1.04 SD, being classified as ‘large’ (range of differences 0.27–1.04 SD). Self-rated depression, memory and impulsivity also produced ‘moderate’ to ‘large’ differences for this comparison. Less weight should be attached to self-rated measures than to objective outcomes, even though the effect is consistent.
We remain uncertain of the clinical meaning or relevance of any of these identified differences between ecstasy users and control groups. It is not clear what, if any, impact the ‘deficits’ described might have on everyday living or quality of life. None of the included studies collected data directly reflecting the quality of life of participants. Similarly, we found no attempts to assess the clinical meaningfulness of any inter-cohort differences, and it is difficult for us to assess this on the basis of aggregate level data alone. As we are not aware that ecstasy users present in any great numbers to drug services, unlike other drug misusers, it seems unlikely that the differences described cause major clinical or functional problems for the majority of consumers.
Methodological flaws in the included studies may also partially explain the effects seen, particularly because the effects are generally small. None of the included studies stated whether the researchers were blind to the ecstasy-using status of each subject; while some of the outcomes are sufficiently objective to make this weakness unlikely to lead to significant observer bias, other outcomes are more open to interpretation. Observer bias cannot therefore be excluded as a partial explanation of our findings. In addition, it not clear what information on the nature of the study was given to subjects at recruitment. As subjects in these studies cannot be blind to their own ecstasy-using status, they may have prior beliefs or expectations about its effects that could influence their performance. The effect of such beliefs could affect our results in either direction: subjects may accept that ecstasy causes memory problems or may be keen to demonstrate that ecstasy use has no effect, or a beneficial effect, on their brain function.
Our metaregression analyses did not consistently identify confounders, although other drug use and differences between study arms in age, sex and intelligence do affect some analyses, in some cases in a counterintuitive direction. Such inconsistent findings weaken the associations identified and strengthen the methodological concerns about the included studies. The apparently consistent, positive effect of additional alcohol consumption may be explained either as a direct chemical effect or as an indicator of more casual modes of ecstasy consumption, as discussed above.
Many of the included studies were very small, which means that they are subject to substantial uncertainty. However, in common with all conventional meta-analyses, our syntheses give weight to contributing studies according to their precision, which is directly influenced by the size of the sample on which they are based. These methods were developed for synthesising the results of randomised controlled trials, where it can be assumed that, as long as they are well conducted, larger studies are more likely to provide an accurate estimate of treatment effect. This assumption does not necessarily hold true in observational studies: a large study may very well be more biased than a small one. 72 As a result our meta-analyses can only reflect the biases inherent in underlying evidence.
While the total number of outcome measures reported in the studies is very large, it is not clear to what extent they are truly independent measures. The consistency of the effects seen may be artefactually strengthened by the interdependence of the outcomes. In addition, we are aware that many studies do not report results for all of the outcomes they state have been measured. Selective reporting of outcomes may also apparently strengthen any effects, as negative findings are perhaps less likely to be reported.
Significant evidence of small-study bias was found in a few analyses, but only in comparisons between ecstasy users and ecstasy-naïve controls. This may be a chance finding, or it may reflect a lower level of methodological rigour in such studies, leading to biased findings. Selective outcome reporting – which one would expect to find in low-quality studies – might be a contributory factor. There appears to be especially strong evidence that the meta-analysis of depression in ecstasy users compared to drug-naïve controls may be distorted by this bias (see Chapter 3, Depression (self-rated) – MDMA users versus drug-naïve controls).
Finally, subjects have been recruited for the individual studies in a number of ways, none of which suggests that they can be considered truly representative of the ecstasy-using population as a whole. Those participating are self-selected populations, often recruited through snowball methods which may exaggerate any specific, local qualities of the sample, particularly because individual study sizes are often very small. Generalising these effects to the total population of ecstasy users is therefore problematic.
Dose–response
If the associations seen are real and causally linked, we would expect to find greater effects in cases where more ecstasy has been consumed. In addition, the effects in former ecstasy users might diminish the longer they abstain from ecstasy use. In fact, we found very little evidence that studies in which subjects were exposed to more ecstasy reported greater deficits in neurocognitive function or psychopathological symptomatology. Most metaregression analyses showed no impact of exposure, and of the two that did show a relationship, one showed a positive effect and the other a negative effect; chance findings are therefore a possibility.
Ecstasy exposure has been defined in various ways: as total lifetime exposure measured as number of tablets consumed, as duration of ecstasy use, as frequency of ecstasy consumption and as number of tablets consumed on each occasion. Measuring exposure to ecstasy is difficult. All our included studies rely on self-reported consumption of tablets sold as ecstasy, sometimes over a period of several years. Such information is highly subject to recall bias, with both overestimates and underestimates of consumption likely. Compounding this is the lack of knowledge of the exact composition of any tablet sold as ecstasy. Amount of MDMA present varies and other psychoactive and non-psychoactive substances may also be present. All estimates of ecstasy use are likely to be inaccurate indications of MDMA consumption. As a result, it may not be surprising that we cannot demonstrate a dose–response effect in current ecstasy users.
As most ecstasy users co-use other drugs or use other drugs at other times, isolation of an effect particular to ecstasy is very difficult. Use of other drugs is clearly an important potential confounder; however, details about the frequency, volume and combinations of consumptions are varied and subject to the same difficulties of accurate estimate seen for ecstasy use. The importance of co-use, as opposed to poly-use on separate occasions, is not known.
Contrary to expectation when looking for a dose–response effect, former ecstasy users appear to have a disadvantage comparable to – and, in some instances, significantly greater than – current users. We suggest that this may be an artefact of the self-selection process, with people worried about the impact of former drug use more likely to volunteer to participate. Additionally, negative experiences with ecstasy use may cause people to stop taking it. In all cases, the number of studies providing data for pooling about former ecstasy users is smaller than for current users and so the sample of studies may be subject to greater chance variation.
Similarly, we have not demonstrated any effect of length of abstinence on effect size. While it is possible that any effect of ecstasy is permanent and does not improve after exposure ceases, a number of methodological explanations should also be considered. Some studies established quit status objectively, while others relied on self-report, and both methods could result in inaccuracies of measurement over longer periods of time. In other studies, it is not clear how long subjects had abstained from ecstasy consumption. The sampling biases discussed in the previous paragraph may also apply.
On the other hand, the nature of our analysis, combining data at study level rather than individual level, means that any large effects seen in a small number of individuals would not be identified. It is possible that a minority of ecstasy users are substantially affected by the drug. Such idiosyncratic responses have been noted in the acute effects of ecstasy with some people experiencing severe, even fatal, responses to doses tolerated by others. Unfortunately, such subgroup analysis is not possible with the aggregate-level data identified for this review.
Temporal relationship
Cross-sectional studies, which make up the bulk of available evidence about possible chronic health harms of ecstasy, do not permit causal relationships to be inferred, as it is never possible to ascertain if exposure preceded outcome. The one prospective study that we identified for this review found small deficits in memory and increased self-rated depression in a group of subjects who started using ecstasy in the year after they were recruited to a longitudinal study (although test results for all participants were comfortably within the range of normal function). 84,90,91 The comparison group for this study comprised matched controls that had not started using ecstasy. The reported cumulative dose of ecstasy in this group is small (averaging only three to six tablets), which makes their findings important if such a small exposure to ecstasy can result in defects of measurable magnitude, even if their clinical significance appears to be extremely minor. Controls and ecstasy users did not differ at baseline and were recruited at the same time and in the same way without investigators knowing which would become ecstasy users. However, it is not clear whether researchers at the follow-up testing were aware of the ecstasy-using status of the subjects, so observer bias is a possibility that cannot be excluded. Nevertheless, the methodological quality of this study is good, and we should give more weight to its findings than to those of other studies. We can cautiously suggest, therefore, that a small causal effect of ecstasy on neurocognitive function is possible. The fact that the results from this study support those from the meta-analyses in this review adds to the consistency of findings (see Strength and consistency of effect).
Other longitudinal studies have investigated the performance of ecstasy users over time in comparison to controls. Most of them, however, started by recruiting pre-existing users and controls using the same sort of methods as the cross-sectional studies, making it as difficult to establish causation as in the rest of the literature, despite their subsequent longitudinal nature. 365–369 All five studies are small, but did follow up subjects for between 1 and 2 years after recruitment, noting those who became abstinent during follow-up. The largest study was subject to substantial drop-out (only 38/60 users were tested at the 18-month follow-up), a finding likely to substantially bias the results, as the authors acknowledge. 368 Overall, the results from these longitudinal studies are conflicting: one reported no change over time in task performance for current or ex-users,81 another showed no difference between ecstasy users and ecstasy abstainers at follow-up,368 another showed that ex-users failed to improve over time while current users did not deteriorate366 and two showed that scores remained static or improved for ex-users while they declined for current users. 366,369 These studies are also as subject to confounding as the rest of the literature and their small size makes their evidence very weak. Overall, they cannot be taken as providing any evidence of a causal link between ecstasy use and neurocognitive deficits.
One final longitudinal study provides substantially better evidence that mental disorders are more likely to precede illegal drug use than develop as a consequence. 80 This study used a pre-existing population-based longitudinal study of young people being followed for the early development of mental disorders to investigate symptomatology with and without exposure to ecstasy and other amphetamines. As it is not possible to separate out results relating to ecstasy use, we have not included the study’s results in our meta-analyses. It did not include any neurocognitive testing in its methods. Nevertheless, the size and methodological quality of this study suggest that we should give weight to these results, which show outcome preceding exposure in the majority of cases (that is to say, participants started using ecstasy after the onset of psychopathological symptoms). These findings suggest that amphetamines generally do not cause mental disorders, but rather that their use follows the onset of such disorders.
Plausibility and coherence
The link between ecstasy and neurocognitive deficits in particular is plausible and can be predicted from animal, pharmacological and experimental studies which have not been reviewed for this report.
The consistent confounding effect of alcohol on the associations was initially surprising, but may be explained in two ways (either as a direct neuroprotective effect or via the existence of different populations with different consumption patterns), as outlined in Chapter 3, Inter-arm differences.
Strengths and limitations of the evidence: acute harms
Registry data for the UK provides an indication of the scale of ecstasy-related deaths; however, without detailed understandings of the scale and nature of ecstasy use, it is not possible to assess the risk of taking any ecstasy tablet. This uncertainty is compounded by apparent idiosyncratic responses in some people which cause acute harms including, in some cases, death, after ingesting doses that are tolerated by others. We identified no studies that offered ways of identifying those most at risk of fatal effects. The variable content of ecstasy tablets is also an issue.
Two-thirds of those deaths recorded in registry data as having ecstasy as a contributory factor were in individuals who were also found to have consumed other drugs. In nearly 60% of these cases, heroin, methadone or other opiates were also found. In populations recruited for the comparative studies about chronic harms of ecstasy use, heroin was not a commonly co-used drug. It is possible that the majority of those fatal cases involving ecstasy use are in those who are opiate abusers who also use other drugs, a subgroup which is not representative of the majority of ecstasy users.
There are few audits of presentations in emergency rooms related to ecstasy use and only one of these was from the UK, in which no fatal cases were recorded. In other such hospital-based audits, fatalities are seen in between 0 and 2% of presentations, suggesting that most adverse effects resolve spontaneously or with treatment, even where they are severe enough to result in presentation at A&E.
Given the lack of information about cause of death in registries and the small size of hospital audit samples, we had to use other case series literature to explore the nature of acute harms. Such data are subject to a number of well-known limitations [see Chapter 3, Uncontrolled (Level III) evidence (acute harms)] Most fatal and non-fatal acute harms reported appear to be related to the main syndromes of hyperthermia and hyponatraemia – women may be more susceptible to the latter.
The scope of our review was such that transient subacute health harms of ecstasy consumption have not been reviewed. This may be particularly relevant where short-term disturbance of mood is concerned, as this phenomenon may be related to long-term depressive outcomes. 591
Further research
Our recommendations for future research are as follows:
-
Large, population-based, prospective studies are required to examine the time relationship between ecstasy exposure and neurocognitive deficits and psychopathological symptoms.
-
Further research synthesis of the social and other indirect health harms of ecstasy would provide a more complete picture. Similar synthesis of the health harms of amphetamines generally would provide a useful comparison.
-
Future cross-sectional studies will only add to the evidence-base if they are large, as representative as possible of the ecstasy-using population, use well-validated outcome measures, measure outcomes as objectively as possible with researchers blind to the ecstasy-using status of their subjects, report on all outcomes used, and provide complete documentation of possible effect modifiers. Cohorts should be matched for baseline factors, including IQ and exposure to alcohol.
-
The heterogeneity of outcome measures used by different investigators is unhelpful: consensus on the most appropriate instruments to use should be sought. Investigators should collect data directly reflecting the quality of life of participants and/or attempt to assess the clinical meaningfulness of any inter-cohort differences.
-
A registry of adverse events related to illegal intoxicants presenting to medical services (akin to the ‘yellow card’ system for prescription medicines) would enable useful estimation of the incidence of harmful effects of ecstasy in comparison to other substances.
-
Future case reports of acute harms of ecstasy are unlikely to contribute valuable information to the evidence-base. Where novel findings are presented, care should be taken to report toxicological findings confirming the precise identity of the substance(s) consumed by the individual(s) in question.
Conclusions
Chronic harms
The one prospective study identified for this review (the Netherlands XTC study) found that subjects using ecstasy had a poorer performance on neurocognitive testing of various aspects of memory and reported increased depressive symptomatology, when compared to subjects who had not used ecstasy.
Previous meta-analyses of cross-sectional studies report small to medium decreases in performance on various neurocognitive outcomes concerned with memory, and an increase in depressive symptomatology.
Meta-analyses undertaken for this review also find the same deficits, whether for individual outcome measures or for pooled outcomes measuring the same function (e.g. immediate or delayed memory). These neurocognitive deficits remain largely within the normal range for individual measures or are classified mostly as small effects, with some verging on medium-sized effects. Slightly larger effect differences are generally (but not universally) seen when current ecstasy users are compared to users naïve to illegal drugs rather than users of other illegal drugs. The differences are frequently slightly larger for former ecstasy users compared to both control groups than for current ecstasy users.
As all the data for these meta-analyses are derived from cross-sectional studies, no causal inference can be made. Metaregression shows that differences in baseline intelligence and consumption of other drugs, particularly alcohol, partially explain the difference between groups, although not necessarily in the expected direction (e.g. higher consumption of alcohol amongst ecstasy-using cohorts appears to be associated with better relative performance in neurocognitive tests). Level of education, intelligence, age and gender do not consistently explain the differences seen between studies.
No dose–response effect for ecstasy is seen in most of the analyses, whether dose is measured as duration or frequency of use, size of dose at each use or lifetime exposure. Period of abstinence from ecstasy before testing also has no identifiable effect. In some cases (notably the NeXT study), neurocognitive effects are demonstrable after apparently very low doses of only a few tablets. Our lack of identified dose–response effect is, perhaps, surprising. It certainly might be expected that differences exist between novice users taking only a few tablets and those who have taken hundreds of tablets over many years. The lack of identified dose–response effect may relate to the difficulties in measuring exposure, which include both recall bias in subjects and variable quantities of MDMA plus possible other psychoactive ingredients in tablets consumed as ecstasy.
Other explanations include methodological ones, such as the lack of blinding of investigators to the drug-using status of subjects: the differences tend to be greater when subjective outcomes are measured. The artificiality of separating illegal drug users into those declaring that they use ecstasy and those who do not may also contribute. Polydrug-using controls are likely to be the population of subjects from which ecstasy users are drawn, while drug-naïve controls may be quite different and more heterogeneous, making it likely that they differ markedly from the recreational ecstasy users in many unmeasured ways. In addition, our analyses will not pick up individual severe effects, as there is a big variation in exposure within most studies and the large effects in a few individuals will be averaged out (i.e. subject to the ecological fallacy).
Estimated exposure effects are consistently small or within normal ranges, suggesting that effects are unlikely to have serious clinical implications for the average user. However, there are no long-term follow-up data to monitor any persistent effects over time or any rate of comparative neurocognitive decline in these groups.
Acute harms
Death remains a rare event following exposure to ecstasy. Documentation is inconsistent and incomplete, but, such as it is, suggests that death usually occurs within a few hours of ingestion of ecstasy and is associated mainly with hyperthermia and its consequences or hyponatraemia. Occasionally, it is associated with isolated liver failure occurring over a period of days or weeks rather than hours. It is not possible to calculate a risk of death from taking ecstasy, not least because many victims have been exposed to other drugs, both alcohol and other illegal drugs, and reporting of this is not always complete. Women appear to bemore susceptible to fatal hyponatraemia, but this phenomenon is extremely rare and is likely to be reported more completely and thoroughly than other deaths.
Acknowledgements
We particularly acknowledge the invaluable help of the Expert Advisory Group for this project (Appendix 1), who provided advice and comments on the protocol and drafts of this report. Any errors that remain are the responsibility of the authors.
We would like to thank the Advisory Council on the Misuse of Drugs for their guidance throughout the project, especially Professor David Nutt, Professor Les Iversen and other members of the Technical Committee, and Will Reynolds and Mohammed Ali of the secretariat. We were also assisted by Professor Val Curran (Professor of Psychopharmacology, University College London), who commented on a draft of this report, on behalf of ACMD.
We are extremely grateful to John Corkery at the National Programme on Substance Abuse Deaths (np-SAD), who was very generous in the provision of data and expertise. Gary Wallace (Plymouth Drug and Alcohol Action Team) provided useful orientation at the beginning of the project.
At PenTAG, Jo Perry and Sue Whiffin provided exemplary administrative project support, and Ken Stein offered useful insights. We are also grateful to Tiffany Moxham, who performed update searches.
Contribution of authors
Gabriel Rogers co-ordinated the project, drafted the protocol, contributed to devising the search strategy, contributed to the review (study selection; data extraction and checking; critical appraisal of studies; data synthesis), contributed to drafting the report (all sections) and compiled and edited the report. Julian Elston contributed to the review (study selection; data extraction and checking; critical appraisal of studies; data synthesis) and contributed to drafting the report (results; discussion). Ruth Garside was co-responsible for project direction, contributed to the review (study selection; data extraction and checking; critical appraisal of studies; data synthesis) and contributed to drafting the report (all sections). Chris Roome contributed to the review (data extraction and checking; critical appraisal of studies; data synthesis) and contributed to drafting the report (results). Rod Taylor contributed to the design, implementation and checking of quantitative synthesis, contributed to the review (data checking), and contributed to drafting the report (methods). Paula Younger devised the search strategy, conducted the literature searches and contributed to drafting the report (methods; results). Anna Zawada contributed to the review (critical appraisal of studies; data synthesis) and contributed to drafting the report (results). Margaret Somerville was co-responsible for project direction, contributed to the review (data checking; critical appraisal of studies; data synthesis) and contributed to drafting the report (all sections).
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA Programme or the Department of Health.
References
- Gowing LR, Henry-Edwards SM, Irvine RJ, Ali RL. The health effects of ecstasy: a literature review. Drug Alcohol Rev 2002;21:53-6.
- Parrott AC. Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology 2004;173:234-41.
- Baylen CA, Rosenberg H. A review of the acute subjective effects of MDMA/ecstasy. Addiction 2006;101:933-47.
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002;162:396-405.
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol 1996;49:455-79.
- Dumont GJH, Verkes RJ. A review of acute effects of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol 2006;20:176-87.
- Outslay M. Understanding ecstasy. JAAPA 2006;19:42-7.
- Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 1999;290:136-45.
- Lester SJ, Baggott M, Welm S, Schiller NB, Jones RT, Foster E, et al. Cardiovascular effects of 3,4-methylenedioxymethamphetamine. A double-blind, placebo-controlled trial. Ann Intern Med 2000;133:969-73.
- de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, et al. Pharmacology of MDMA in humans. Ann N Y Acad Sci 2000;914:225-37.
- Freedman RR, Johanson CE, Tancer ME. Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2005;183:248-56.
- Schifano F. A bitter pill. Overview of ecstasy (MDMA, MDA) related fatalities. Psychopharmacology 2004;173:242-8.
- de la Torre R, Farre M, Ortuno J, Mas M, Brenneisen R, Roset PN, et al. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol 2000;49:104-9.
- Freudenmann RW, Oxler F, Bernschneider-Reif S. The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents. Addiction 2006;101:1241-5.
- Seymour RB. MDMA: another view of Ecstasy. Pharmchem Newsletter 1985;14:1-4.
- Los Angeles Times 1985.
- Six held after drug seizure. The Times 1987.
- DEA will ban hallucinogen known to users as ‘Ecstasy’. The Washington Post 1985.
- Misuse of Drugs Act 1971 (Modification) Order 1977.
- Randall T. Ecstasy-fueled ‘rave’ parties become dances of death for English youths. JAMA 1992;268:1505-6.
- Schwartz RH, Miller NS. MDMA (ecstasy) and the rave: a review. Pediatrics 1997;100:705-8.
- Collin M. Mad for it. The Guardian 1997.
- Schifano F, Corkery J, Deluca P, Oyefeso A, Ghodse AH. Ecstasy (MDMA, MDA, MDEA, MBDB) consumption, seizures, related offences, prices, dosage levels and deaths in the UK (1994–2003). J Psychopharmacol 2006;20:456-63.
- Street drug prices 2006. Druglink 2006;20.
- Greater London Authority . London: The Highs and the Lows 2. A Report from the Greater London Alcohol and Drug Alliance 2007. www.london.gov.uk/mayor/health/drugs_and_alcohol/docs/highs-lows2.rtf.
- Drugs Intelligence Unit . Data for Police Seizures Examined by the Forensic Science Service in 2006 2007.
- Summary Statistics for EcstasyData.Org Lab Testing Results 2007. www.ecstasydata.org/datastats.php.
- Nicholas S, Kershaw C, Walker A. Crime in England and Wales 2006/07. Statistical Bulletin – Home Office Research Development And Statistics Directorate 2007.
- Gore SM. Fatal uncertainty: death-rate from use of ecstasy or heroin. Lancet 1999;354:1265-6.
- Cole J, Sumnall HR, Grob CS. Sorted: ecstasy. Psychologist 2002;15:464-7.
- Riley S, Hayward E. Patterns, trends, and meanings of drug use by dance-drug users in Edinburgh, Scotland. Drug Educ 2004;11:243-62.
- Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth 2006;96:678-85.
- Schifano F, Di Furia L, Forza G, Minicuci N, Bricolo R. MDMA (‘ecstasy’) consumption in the context of polydrug abuse: a report on 150 patient. Drug Alcohol Depend 1998;52:85-90.
- Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs 1986;18:319-27.
- Dowling GP, McDonough ET, Bost RO. ‘Eve’ and ‘Ecstasy’. A report of five deaths associated with the use of MDEA and MDMA. JAMA 1987;257:1615-17.
- Chadwick IS, Curry PD, Linsley A, Freemont AJ, Doran B. Ecstasy, 3-4 methylenedioxymethamphetamine (MDMA), a fatality associated with coagulopathy and hyperthermia. J R Soc Med 1991;84.
- Campkin NT, Davies UM. Another death from Ecstasy. J R Soc Med 1992;85.
- Lafferty F. Ecstasy?. Sunday Times 1992.
- Forsyth AJM. Distorted? A quantitative exploration of drug fatality reports in the popular press. Int J Drug Policy 2001;12:435-53.
- NHS Centre for Reviews and Dissemination . Undertaking Systematic Reviews of Research on Effectiveness 2001.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008-12.
- Levine M, Walter S, Lee H, Haines T, Holbrook A, Moyer V. Users’ guides to the medical literature. IV. How to use an article about harm. Evidence-Based Medicine Working Group. JAMA 1994;271:1615-19.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Hlth 1998;52:377-84.
- Mallen C, Peat G, Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. J Clin Epidemiol 2006;59:765-9.
- Smith R, Tivarus M, Campbell H, Hillier A, Beversdorf D. Apparent transient effects of recent “ecstasy” use on cognitive performance and extrapyramidal signs in human subjects. Cogn Behav Neurol 2006;19:157-64.
- Back-Madruga M, Boone K, Chang L, Grob C, Lee A, Nations H, et al. Neuropsychological effects of 3,4-methylenedioxymethamphetamine (MDMA or Ecstasy) in recreational users. Clin Neuropsychol 2003;17:446-59.
- Hanson K, Luciana M. Neurocognitive function in users of MDMA: the importance of clinically significant patterns of use. Psychol Med 2004;34:229-46.
- Lezak M, Howieson D, Loring D, Hannay H, Fisher J. Neuropsychological assessment. Oxford: Oxford University Press; 2004.
- Hersen M, Goldstein G, Beers SR. Comprehensive handbook of psychological assessment: Volume 1 Intellectual and neuropsychological assessment. Chichester: Wiley; 2004.
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press; 2006.
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cogn Psychol 2000;41:49-100.
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: a neuropsychological approach. Neuropsychol Rev 1991;2:109-45.
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Oxford: Cochrane Collaboration; 2008.
- Berkey CS, Hoaglin DC, Antczak-Bouckoms A, Mosteller F, Colditz GA. Meta-analysis of multiple outcomes by regression with random effects. Stat Med 1998;17:2537-50.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Thompson JR. Bivariate random-effects meta-analysis and the estimation of between-study correlation. BMC Med Res Methodol 2007;7.
- Riley RD, Thompson JR, Abrams KR. An alternative model for bivariate random-effects meta-analysis when the within-study correlations are unknown. Biostat 2007.
- Pocock SJ, Geller NL, Tsiatis AA. The analysis of multiple endpoints in clinical trials. Biometrics 1987;43:487-98.
- Sumnall H, Cole J. Self-reported depressive symptomatology in community samples of polysubstance misusers who report ecstasy use: a meta-analysis. J Psychopharmacol 2005;19:84-92.
- Verbaten M. Specific memory deficits in ecstasy users? The results of a meta-analysis. Hum Psychopharmacol Clin Exp 2003;18:281-90.
- Laws K, Kokkalis J. Ecstasy MDMA and memory function: a meta-analytic update. Hum Psychopharmacol 2007;22:381-8.
- Zakzanis KK, Campbell Z, Jovanovski D. The neuropsychology of ecstasy (MDMA) use: a quantitative review. Hum Psychopharmacol 2007;22:427-35.
- Thomasius R, Zapletalova P, Petersen K, Buchert R, Andresen B, Wartberg L, et al. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol 2006;20:211-25.
- Gerra G, Zaimovic A, Ferri M, Zambelli U, Timpano M, Neri E, et al. Long-lasting effects of (±)3,4-methylenedioxymethamphetamine (Ecstasy) on serotonin system function in humans. Biolog Psychiatry 2000;47:127-36.
- McCann U, Mertl M, Eligulashvili V, Ricaurte G. Cognitive performance in (±)3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users: a controlled study. Psychopharmacology 1999;143:417-25.
- Trowman R, Dumville JC, Torgerson DJ, Cranny G. The impact of trial baseline imbalances should be considered in systematic reviews: a methodological case study. J Clin Epidemiol 2007;60:1229-33.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34.
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693-708.
- Egger M, Smith GD, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ Books; 2001.
- Kalechstein A, De La Garza R, Mahoney J, Fantegrossi W, Newton T. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology 2007;189:531-7.
- Gouzoulis M, Daumann J. Neurotoxicity of methylenedioxyamphetamines (MDMA; ecstasy) in humans: how strong is the evidence for persistent brain damage?. Addiction 2006;101:348-61.
- Dafters R, I, Hoshi R, Talbot A. Contribution of cannabis and MDMA (“ecstasy”) to cognitive changes in long-term polydrug users. Psychopharmacology 2004;173:405-10.
- Heffernan T, Jarvis H, Rodgers J, Scholey AB, Ling J. Prospective memory, everyday cognitive failure and central executive function in recreational users of Ecstasy. Hum Psychopharmacol Clin Exp 2001;16:607-12.
- Butler GKL, Montgomery AMJ. Impulsivity, risk taking and recreational ‘ecstasy’ (MDMA) use. Drug Alcohol Depend 2004;76:55-62.
- Guillot C, Greenway D. Recreational ecstasy use and depression. J Psychopharmacol 2006;20:411-16.
- Maxwell J. The response to club drug use. Curr Opin Psychiatry 2003;16:279-89.
- Lieb R, Schuetz C, Pfister H, von Sydow K, Wittchen H. Mental disorders in ecstasy users: a prospective-longitudinal investigation. Drug Alcohol Depend 2002;68:195-207.
- Daumann J, Fischerman T, Heekeren K, Thron A, Gouzoulis M. Neural mechanisms of working memory in ecstasy (MDMA) users who continue or discontinue ecstasy and amphetamine use: evidence from an 18-month longitudinal functional magnetic resonance imaging study. Biol Psychiatry 2004;56:349-55.
- Gerra G, Bassignana S, Zaimovic A, Moi G, Bussandri M, Caccavari R, et al. Hypothalamic–pituitary–adrenal axis responses to stress in subjects with 3,4-methylenedioxy-methamphetamine (‘ecstasy’) use history: correlation with dopamine receptor sensitivity. Psychiatry Res 2003;120:115-24.
- Quednow B, Jessen F, Kuehn K, Maier W, Daum I, Wagner M. Memory deficits in abstinent MDMA (ecstasy) users: neuropsychological evidence of frontal dysfunction. J Psychopharmacol 2006;20:373-84.
- de Win MM, Jager G, Vervaeke H, Schilt T, Reneman L, Booij J, et al. The Netherlands XTC Toxicity (NeXT) study: objectives and methods of a study investigating causality, course, and clinical relevance. Int J Meth Psychiatr Res 2005;14:167-85.
- Vervaeke H, Korf D, Benschop A, van den Brink W. How to find future ecstasy-users: targeted and snowball sampling in an ethically sensitive context. Addictive Behav 2007;32:1705-13.
- Vervaeke H, Korf D. Long-term ecstasy use and the management of work and relationships. Int J Drug Policy 2006;17:484-93.
- Jager G, de Win MM, van der Tweel I, Schilt T, Kahn R, van den Brink W, et al. Assessment of cognitive brain function in ecstasy users and contributions of other drugs of abuse: results from an fMRI study. Neuropsychopharmacology 2008;33:247-58.
- Jager G, de Win MM, Vervaeke H, Schilt T, Kahn R, van den Brink W, et al. Incidental use of ecstasy: no evidence for harmful effects on cognitive brain function in a prospective fMRI study. Psychopharmacology 2007;193:403-14.
- de Win MM, Reneman L, Jager G, Vlieger E, Olabarriaga S, Lavini C, et al. A prospective cohort study on sustained effects of low-dose ecstasy use on the brain in new ecstasy users. Neuropsychopharmacology 2007;32:458-70.
- Schilt T, de Win MM, Koeter M, Jager G, Korf D, van den Brink W, et al. Cognition in novice ecstasy users with minimal exposure to other drugs: a prospective cohort study. Arch Gen Psychiatry 2007;64:728-36.
- de Win MM, Schilt T, Reneman L, Vervaeke H, Jager G, Dijkink S, et al. Ecstasy use and self-reported depression, impulsivity, and sensation seeking: a prospective cohort study. J Psychopharmacol 2006;20:226-35.
- Groth-Marnat G. Handbook of psychological assessment. Hoboken, NJ: Wiley; 2003.
- Bolla KI, McCann U, Ricaurte G. Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology 1998;51:1532-7.
- Croft R, Mackay A, Mills A, Gruzelier J. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology 2001;153:373-9.
- Reneman L, Majoie C, Schmand B, van den Brink W, den Heeten GJ. Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol Psychiatry 2001;50:550-4.
- Thomasius R, Petersen KU, Zapletalova P, Wartberg L, Zeichner D, Schmoldt A. Mental disorders in current and former heavy ecstasy (MDMA) users. Addiction 2005;100:1310-19.
- Reneman L, Schilt T, de Win MM, Booij J, Schmand B, van den Brink W, et al. Memory function and serotonin transporter promoter gene polymorphism in ecstasy (MDMA) users. J Psychopharmacol 2006;20:389-99.
- Lamers CTJ, Bechara A, Rizzo M, Ramaekers JG. Cognitive function and mood in MDMA/THC users, THC users and non-drug using controls. J Psychopharmacol 2006;20:302-11.
- Gouzoulis M, Daumann J, Tuchtenhagen F, Pelz S, Becker S, Kunert H, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol 2000;68:719-25.
- McCardle K, Luebbers S, Carter JD, Croft RJ, Stough C. Chronic MDMA (ecstasy) use, cognition and mood. Psychopharmacology 2004;173:434-9.
- Zakzanis K, Young D, Campbell Z. Prospective memory impairment in abstinent MDMA (“Ecstasy”) users. Cogn Neuropsychiatry 2003;8:141-53.
- Morgan M. Memory deficits associated with recreational use of “ecstasy” (MDMA). Psychopharmacology 1999;141:30-6.
- Morgan MJ, McFie L, Fleetwood LH, Robinson JA. Ecstasy (MDMA): are the psychological problems associated with its use reversed by prolonged abstinence?. Psychopharmacology 2002;159:294-303.
- Curran HV, Verheyden S. Altered response to tryptophan supplementation after long-term abstention from MDMA (ecstasy) is highly correlated with human memory function. Psychopharmacology 2003;169:91-103.
- Thomasius R, Petersen K, Buchert R, Andresen B, Zapletalova P, Wartberg L, et al. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology 2003;167:85-96.
- Halpern J, Pope H, Sherwood A, Barry S, Hudson J, I, Yurgelun T. Residual neuropsychological effects of illicit 3,4-methylenedioxymethamphetamine (MDMA) in individuals with minimal exposure to other drugs. Drug Alcohol Depend 2004;75:135-47.
- Wareing M, Fisk J, Murphy P, Montgomery C. Verbal working memory deficits in current and previous users of MDMA. Hum Psychopharmacol: Clin Exp 2004;19:225-34.
- Gouzoulis M, Thimm B, Rezk M, Henson G, Daumann J. Memory impairment suggests hippocampal dysfunction in abstinent ecstasy users. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:819-27.
- Reay JL, Hamilton C, Kennedy DO, Scholey AB. MDMA polydrug users show process-specific central executive impairments coupled with impaired social and emotional judgement processes. J Psychopharmacol 2006;20:385-8.
- Morgan M. Recreational use of “ecstasy” (MDMA) is associated with elevated impulsivity. Neuropsychopharmacology 1998;19:252-64.
- Reneman L, Booij J, de Bruin K, Reitsma J, de Wolff FA, Gunning W, et al. Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet 2001;358:1864-69.
- Fox HC, Parrott AC, Turner JJD. Ecstasy use: cognitive deficits related to dosage rather than self- reported problematic use of the drug. J Psychopharmacol 2001;15:273-81.
- Fox HC, Toplis AS, Turner JJD, Parrott AC. Auditory verbal learning in drug-free Ecstasy polydrug users. Hum Psychopharmacol Clin Exp 2001;16:613-18.
- Roiser J, Cook L, Cooper J, Rubinsztein D, Sahakian B. Association of a functional polymorphism in the serotonin transporter gene with abnormal emotional processing in ecstasy users. Am J Psychiatry 2005;162:609-12.
- Morgan M, Impallomeni L, Pirona A, Rogers R. Elevated impulsivity and impaired decision-making in abstinent ecstasy (MDMA) users compared to polydrug and drug-naive controls. Neuropsychopharmacology 2006;31:1562-73.
- Ward J, Hall K, Haslam C. Patterns of memory dysfunction in current and 2-year abstinent MDMA users. J Clin Exp Neuropsychol 2006;28:306-24.
- McCann U, Peterson S, Ricaurte G. The effect of catecholamine depletion by alpha-methyl-para-tyrosine on measures of cognitive performance and sleep in abstinent MDMA users. Neuropsychopharmacology 2007;32:1695-706.
- Roiser J, Rogers R, Sahakian B. Neuropsychological function in ecstasy users: a study controlling for polydrug use. Psychopharmacology 2007;189:505-16.
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
- Montgomery C, Fisk J, Newcombe R. The nature of ecstasy-group related deficits in associative learning. Psychopharmacology 2005;180:141-9.
- Parrott AC, Lasky J. Ecstasy (MDMA) effects upon mood and cognition: before, during and after a Saturday night dance. Psychopharmacology 1998;139:261-8.
- Rodgers J. Cognitive performance amongst recreational users of “ecstasy”. Psychopharmacology 2000;151:19-24.
- Simon N, Mattick R. The impact of regular ecstasy use on memory function. Addiction 2002;97:1523-9.
- Medina K, Shear P, Corcoran K. Ecstasy (MDMA) exposure and neuropsychological functioning: a polydrug perspective. J Int Neuropsychol Soc 2005;11:753-65.
- Hoshi R, Mullins K, Boundy C, Brignell C, Piccini P, Curran HV. Neurocognitive function in current and ex-users of ecstasy in comparison to both matched polydrug-using controls and drug-naive controls. Psychopharmacology 2007;194:371-9.
- Groth M, Howchar H, Marsh A. Memory performance in abstinent 3,4-methylendeioxymethamaphetamine (MDMA, “ecstasy”) users. Percept Mot Skills 2007;104:43-55.
- Bhattachary S, Powell J. Recreational use of 3,4-methylenedioxymethamphetamine (MDMA) of “ecstasy’: evidence for cognitive impairment. Psychol Med 2001;31:647-58.
- Yip J, Lee T. Effect of ecstasy use on neuropsychological function: a study in Hong Kong. Psychopharmacology 2005;179:620-8.
- Verkes R, Gijsman H, Pieters M, Schoemaker R, de Visser S, Kuijpers M, et al. Cognitive performance and serotonergic function in users of ecstasy. Psychopharmacology 2001;153:196-202.
- Fox HC, McLean A, Turner JJD, Parrott AC, Rogers R, Sahakian BJ. Neuropsychological evidence of a relatively selective profile of temporal dysfunction in drug-free MDMA (“ecstasy”) polydrug users. Psychopharmacology 2002;162:203-14.
- Wareing M, Fisk J, Murphy P, Montgomery C. Visuo-spatial working memory deficits in current and former users of MDMA (‘ecstasy’). Hum Psychopharmacol Clin Exp 2005;20:115-23.
- von Geusau NA, Stalenhoef P, Huizinga M, Snel J, Ridderinkhof K. Impaired executive function in male MDMA (“ecstasy”) users. Psychopharmacology 2004;175:331-41.
- Moeller F, Dougherty D, Steinberg J, Swann A, Silverman P, Ruiz P, et al. Heavy “ecstasy” use is associated with increased impulsivity. Addict Disord Treat 2002;1:47-52.
- Jacobsen L, Mencl W, Pugh K, Skudlarski P, Krystal J. Preliminary evidence of hippocampal dysfunction in adolescent MDMA (“ecstasy”) users: possible relationship to neurotoxic effects. Psychopharmacology 2004;173:383-90.
- Wareing M, Fisk J, Montgomery C, Murphy P, Chandler M. Information processing speed in ecstasy (MDMA) users. Hum Psychopharmacol Clin Exp 2007;22:81-8.
- Wareing M, Fisk J, Murphy P. Working memory deficits in current and previous users of MDMA (‘ecstasy’). Br J Psychol 2000;91:181-8.
- Dafters RI. Chronic ecstasy (MDMA) use is associated with deficits in task-switching but not inhibition or memory updating executive functions. Drug Alcohol Depend 2006;83:181-4.
- Montgomery C, Fisk J, Newcombe R, Murphy P. The differential effects of ecstasy/polydrug use on executive components: shifting, inhibition, updating and access to semantic memory. Psychopharmacology 2005;182:262-76.
- Parrott AC, Sisk E, Turner JJD. Psychobiological problems in heavy “ecstasy’ (MDMA) polydrug users. Drug Alcohol Depend 2000;60:105-10.
- Dughiero G, Schifano F, Forza G. Personality dimensions and psychopathological profile of Ecstasy users. Hum Psychopharmacol Clin Exp 2001;16:635-9.
- Parrott A, Milani R, Parmar R, Turner J. Recreational ecstasy/MDMA and other drug users from the UK and Italy: psychiatric symptoms and psychobiological problems. Psychopharmacology 2001;159:77-82.
- Gamma A, Buck A, Berthold T, Vollenweider FX. No difference in brain activation during cognitive performance between ecstasy (3,4-methylenedioxymethamphetamine) users and control subjects: A (H215O)-positron emission tomography study. J Clin Psychopharmacol 2001;21:66-71.
- Milani R, Parrott A, Turner J, Fox H. Gender differences in self-reported anxiety, depression, and somatization among ecstasy/MDMA polydrug users, alcohol/tobacco users, and nondrug users. Addict Behav 2004;29:965-71.
- Travers K, Lyvers M. Mood and impulsivity of recreational ecstasy users in the week following a “rave”. Addiction Res Theory 2005;13:43-52.
- Fingeret M, Moeller F, Stotts A. Gender differences among MDMA users on psychological and drug history variables. Addict Disord Treat 2005;4:43-8.
- Gerra G, Zaimovic A, Giucastro G, Maestri D, Monica C, Sartori R, et al. Serotonergic function after (±)3,4-methylene-dioxymethamphetamine (“Ecstasy”) in humans. Int Clin Psychopharmacol 1998;13:1-9.
- Gerra G, Zaimovic A, Moi G, Giusti F, Gardini S, Delsignore R, et al. Effects of (±) 3,4-methylene-dioxymethamphetamine (ecstasy) on dopamine system function in humans. Behav Brain Res 2002;134:403-10.
- Milani RM, Parrott AC, Schifano F, Turner JJD. Pattern of cannabis use in ecstasy polydrug users: moderate cannabis use may compensate for self-rated aggression and somatic symptoms. Hum Psychopharmacol Clin Exp 2005;20:249-61.
- Montgomery C, Fisk J. Everyday memory deficits in ecstasy-polydrug users. J Psychopharmacol 2007;21:709-17.
- Dafters RI. Impulsivity, inhibition and negative priming in ecstasy users. Addict Behav 2006;31:1436-41.
- Ben Hamida S, Plute E, Bach S, Lazarus C, Tracqui A, Kelche C, et al. Ethanol-MDMA interactions in rats: the importance of interval between repeated treatments in biobehavioral tolerance and sensitization to the combination. Psychopharmacology 2007;192:555-69.
- Breen C, Degenhardt L, Kinner S, Bruno R, Jenkinson R, Matthews A, et al. Alcohol use and risk taking among regular ecstasy users. Subst Use Misuse 2006;41:1095-109.
- Hoshi R, Cohen L, Lemanski L, Piccini P, Bond A, Curran HV. Ecstasy (MDMA) does not have long-term effects on aggressive interpretative bias: a study comparing current and ex-ecstasy users with polydrug and drug-naive controls. Exp Clin Psychopharmacol 2007;15:351-8.
- Curran HV, Rees H, Hoare T, Hoshi R, Bond A. Empathy and aggression: two faces of ecstasy? A study of interpretative cognitive bias and mood change in ecstasy users. Psychopharmacology 2004;173:425-33.
- Hoshi R, Pratt H, Mehta S, Bond A, Curran HV. An investigation into the sub-acute effects of ecstasy on aggressive interpretative bias and aggressive mood – are there gender differences?. J Psychopharmacol 2006;20:291-30.
- Hoshi R, Bisla J, Curran HV. The acute and sub-acute effects of ‘ecstasy’ (MDMA) on processing of facial expressions: preliminary findings. Drug Alcohol Depend 2004;76:297-304.
- Verheyden S, Hadfield J, Calin T, Curran HV. Sub-acute effects of MDMA (±3,4-methylenedioxymethamphetamine, “ecstasy”) on mood: evidence of gender differences. Psychopharmacology 2002;161:23-31.
- Bond A, Verheyden S, Wingrove J, Curran HV. Angry cognitive bias, trait aggression and impulsivity in substance users. Psychopharmacology 2004;171:331-9.
- Gerra G, Zaimovic A, Ampollini R, Giusti F, Delsignore R, Raggi M, et al. Experimentally induced aggressive behavior in subjects with 3,4- methylenedioxy-methamphetamine (“Ecstasy”) use history: psychobiological correlates. J Subst Abuse 2001;13:471-91.
- Golding JF, Groome DH, Rycroft N, Denton Z. Cognitive performance in light current users and ex-users of ecstasy (MDMA) and controls. Am J Drug Alcohol Abuse 2007;33:301-7.
- Montgomery C, Fisk J, Newcombe R, Wareing M, Murphy P. Syllogistic reasoning performance in MDMA (Ecstasy) users. Exp Clin Psychopharmacol 2005;13:137-45.
- Pavarin R. Substance use and related problems: a study on the abuse of recreational and not recreational drugs in Northern Italy. Ann 1st Sup Sanita 2006;42:477-84.
- Rendell P, Gray T, Henry J, Tolan A. Prospective memory impairment in “ecstasy” MDMA users. Psychopharmacology 2007;194:497-504.
- Allen RP, McCann UD, Ricaurte GA. Persistent effects of ± 3,4-methylenedioxymethamphetamine MDMA, ecstasy on human sleep. Sleep 1993;16:560-4.
- Redfearn PJ, Agrawal N, Mair LH. An association between the regular use of 3,4 methylenedioxy- methamphetamine ecstasy and excessive wear of the teeth. Addiction 1998;93:745-8.
- McGrath C, Chan B. Oral health sensations associated with illicit drug abuse. Br Dent J 2005;198:159-62.
- Rokach A. Determinants of loneliness of young adult drug users. J Psychol Interdisc Appl 2002;136:613-30.
- Rokach A. Loneliness and drug use in young adults. Int J Adolesc Youth 2002;10:237-54.
- Garcia R, Moreno E, Soriano T, Jurado C, Gimenez MP, Menendez M. Tissue concentrations of MDMA and its metabolite MDA in three fatal cases of overdose. Forensic Sci Int 2003;135:110-14.
- Health Statistics Quarterly and Population Trends. London: Palgrave Macmillan; 2007.
- Office for National Statistics (ONS) . Deaths related to drug poisoning: England and Wales, 2002–2006. Health Stat Quart Population Trends 2007;36:66-72.
- Ramsey JD, Johnston A, Holt DW, Lind J, Oyefeso A, Pollard M, et al. Death rate from use of ecstasy or heroin (multiple letters) (6). Lancet 1999;354:2166-7.
- Ghodse H, Corkery J, Oyefeso A, Schifano F, Tonia T. National Programme on Substance Abuse Deaths (np-SAD): Drug-Related Deaths in the UK. Annual Report 2007 n.d.
- Forrest ARW. Data set on deaths related to taking ecstasy looks incomplete. BMJ 2003;326.
- Patel M, Wright D, Ratcliff J, Miller M. Shedding new light on the safe club drug: methylenedioxymethamphetamine ecstasy-related fatalities. Acad Emerg Med 2004;11:208-10.
- Gill J, Hayes J, deSouza I, Marker E, Stajic M. Ecstasy MDMA deaths in New York City: a case series and review of the literature. J Forensic Sci 2002;47:121-6.
- De Letter EA, Piette M, Lambert W, Cordonnier J. Amphetamines as potential inducers of fatalities: a review in the district of Ghent from 1976–2004. Med Sci Law 2006;46:37-65.
- LoraTamayo C, Tena T, Rodriguez A. Amphetamine derivative related deaths. Forensic Sci Int 1997;85:149-57.
- Raikos N, Tsoukali H, Psaroulis D, Vassiliadis N, Tsoungas M, Njau S. Amphetamine derivative related deaths in northern Greece. Forensic Sci Int 2002;128:31-4.
- Karlovsek M. Illegal drugs-related fatalities in Slovenia. Forensic Sci Int 2004;146:S71-5.
- Verschraagen M, Maes A, Ruiter B, Bosman I, Smink B, Lusthof K. Post mortem cases involving amphetamine-based drugs in the Netherlands: comparison with driving under the influence cases. Forensic Sci Int 2007;170:163-70.
- Schifano F, Oyefeso A, Corkery J, Cobain K, Jambert G, Martinotti G, et al. Death rates from ecstasy (MDMA, MDA) and polydrug use in England and Wales 1996–2002. Hum Psychopharmacol Clin Exp 2003;18:519-24.
- Schifano F, Oyefeso A, Webb L, Pollard M, Corkery J, Ghodse A. Review of deaths related to taking ecstasy, England and Wales, 1997–2000. BMJ 2003;326:80-1.
- Webb L, Oyefeso A, Schifano F, Cheeta S, Pollard M, Ghodse AH. Cause and manner of death in drug-related fatality: an analysis of drug-related deaths recorded by coroners in England and Wales in 2000. Drug Alcohol Depend 2003;72:67-74.
- Oliver P, Keen J, Mathers N. Deaths from drugs of abuse in Sheffield 1997–1999: what are the implications for GPs prescribing to heroin addicts?. Fam Pract 2002;19:93-4.
- Hickman M, Carrivick S, Paterson S, Hunt N, Zador D, Cusick L, et al. London audit of drug-related overdose deaths: characteristics and typology, and implications for prevention and monitoring. Addiction 2007;102:317-23.
- Seymour A, Black M, Oliver JS. Drug related deaths in the Strathclyde region of Scotland, 1995–1998. Forensic Sci Int 2001;122:52-9.
- Liechti M, Kunz I, Kupferschmidt H. Acute medical problems due to Ecstasy use: case-series of emergency department visits. Swiss Med Weekly 2005;135:652-7.
- Sanjurjo E, Nogue S, Miro O, Munne P. Analysis of patients attended in an emergency department due to ecstasy consumption. Med Clin 2004;123:90-2.
- Williams H, Dratcu L, Taylor R, Roberts M, Oyefeso A. Saturday night fever: ecstasy related problems in a London accident and emergency department. J Accident Emerg Med 1998;15:322-6.
- Green AR, O’Shea E, Colado MI. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur J Pharmacol 2004;500:3-13.
- Nash JF, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther 1988;245:873-9.
- O’Shea E, Granados R, Esteban B, Colado MI, Green AR. The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy’). Neuropharmacology 1998;37:919-26.
- Martin T, Chiasson D, Kish S. Does hyperthyroidism increase risk of death due to the ingestion of ecstasy?. J Forensic Sci 2007;52:951-3.
- Patel M, Belson M, Longwater A, Olson K, Miller M. Methylenedioxymethamphetamine ecstasy-related hyperthermia. J Emerg Med 2005;29:451-4.
- Fineschi V, Centini F, Mazzeo E, Turillazzi E. Adam MDMA and Eve MDEA misuse: an immunohistochemical study on three fatal cases. Forensic Sci Int 1999;104:65-74.
- Kalant H. The pharmacology and toxicology of ‘ecstasy’ (MDMA) and related drugs. CMAJ 2001;165:917-28.
- Kew M, Bersohn I, Seftel H, Kent G. Liver damage in heatstroke. Am J Med 1970;49:192-20.
- Ellis AJ, Wendon JA, Portmann B, Williams R. Acute liver damage and ecstasy ingestion. Gut 1996;38:454-8.
- Jones AL, Simpson KJ. Review article: mechanisms and management of hepatotoxicity in ecstasy (MDMA) and amphetamine intoxications. Aliment Pharmacol Ther 1999;13:129-33.
- Cregg MT, Tracey JA. Ecstasy abuse in Ireland. Irish Med J 1993;86:118-20.
- Screaton GR, Singer M, Cairns HS, Thrasher A, Sarner M, Cohen SL. Hyperpyrexia and rhabdomyolysis after MDMA ecstasy abuse. Lancet 1992;339:677-8.
- Jones C, Dickinson P. Substance abuse: from ecstasy to agony. Nursing Times 1992;88:27-30.
- Squier MV, Jalloh S, Hilton J, Series H. Death after ecstasy ingestion: Neuropathological findings. J Neurol 1995;58.
- Coore JR. A fatal trip with ecstasy: a case of 3,4-methylenedioxymethamphetamine/3,4-methylenedioxyamphetamine toxicity. J R Soc Med 1996;89:P51-52.
- Milroy CM, Clark JC, Forrest AR. Pathology of deaths associated with ecstasy and eve misuse. J Clin Pathol 1996;49:149-53.
- Cox DE, Williams KR. ‘ADAM’ or ‘EVE’? – a toxicological conundrum. Forensic Sci Int 1996;77:101-8.
- Dar KJ, McBrien ME. MDMA induced hyperthermia: report of a fatality and review of current therapy. Intens Care Med 1996;22:995-6.
- Byard RW, Gilbert J, James R, Lokan RJ. Amphetamine derivative fatalities in South Australia – is ecstasy the culprit?. Am J Forensic Med Pathol 1998;19:261-5.
- Walubo A, Seger D. Fatal multi-organ failure after suicidal overdose with MDMA, ‘ecstasy’: case report and review of the literature. Hum Exp Toxicol 1999;18:119-25.
- Byard R, Rodgers N, James R, Kostakis C, Camilleri A. Death and paramethoxyamphetamine – an evolving problem. Med J Aust 2002;176.
- Borowiak KS, Waloszczyk P, Michalska K, Machoy M, Jasionowicz P, Janus T, et al. Death of a teenaged girl due to MDMA (ecstasy) overdose. Z Zagadnien Nauk Sadowych 2003;55:9-19.
- Greene S, Dargan PI, Connor N, Jones A, Kerins M. Multiple toxicity from 3,4-methylenedioxymethamphetamine ecstasy. Am J Emerg Med 2003;21:121-4.
- Karlovsek M, Alibegovic A, Balazic J. Our experiences with fatal ecstasy abuse two case reports. Forensic Sci Int 2005;147:S77-80.
- Ravina P, Quiroga J, Ravina T. Hyperkalemia in fatal MDMA ‘ecstasy’ toxicity. Int J Cardiol 2004;93:307-8.
- Mueller PD, Korey WS. Death by ecstasy: the serotonin syndrome?. Ann Emerg Med 1998;32:377-80.
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol 1966;21:1757-62.
- Gordon CJ, Watkinson WP, O’Callaghan JP, Miller DB. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav 1991;38:339-44.
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 1998;18:5086-94.
- Dafters RI. Effect of ambient temperature on hyperthermia and hyperkinesis induced by 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy’) in rats. Psychopharmacology (Berl) 1994;114:505-8.
- Ferrie R, Loveland RC. Bilateral gluteal compartment syndrome after ‘ecstasy’ hyperpyrexia. J R Soc Med 2000;93.
- Matthai SM, Davidson DC, Sills JA, Alexandrou D. Cerebral oedema after ingestion of MDMA (‘ecstasy’) and unrestricted intake of water. BMJ 1996;312.
- Finch E, Sell L, Arnold D. Cerebral oedema after MDMA (‘ecstasy’) and unrestricted water intake. Drug workers emphasise that water is not an antidote to drug. BMJ 1996;313.
- Hartung TK, Schofield E, Short AI, Parr MJ, Henry JA. Hyponatraemic states following 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) ingestion. QJM 2002;95:431-7.
- Giorgi FS, Lazzeri G, Natale G, Iudice A, Ruggieri S, Paparelli A, et al. MDMA and seizures: a dangerous liaison?. Ann N Y Acad Sci 2006;1074:357-64.
- Sue YM, Lee YL, Huang JJ. Acute hyponatremia, seizure, and rhabdomyolysis after ecstasy use. J Toxicol Clin Toxicol 2002;40:931-2.
- Magee C, Staunton H, Tormey W, Walshe JJ. Hyponatraemia, seizures and stupor associated with ecstasy ingestion in a female. Irish Med J 1998;91.
- Donohoe A, Flynn K, Shields K, Hawi Z, Gill M. MDMA toxicity: no evidence for a major influence of metabolic genotype at CYP2D6. Addiction Biol 1998;3:309-14.
- Connor A, Cluroe A, Couch R, Galler L, Lawrence J, Synek B. Death from hyponatraemia-induced cerebral oedema associated with MDMA ecstasy use. N Z Med J 1999;112:255-6.
- Parr MJ, Low HM, Botterill P. Hyponatraemia and death after ecstasy ingestion. Med J Aust 1997;166:136-7.
- Caballero F, Lopez N, Cotorruelo J, Txoperena G. Ecstasy-induced brain death and acute hepatocellular failure: multiorgan donor and liver transplantation. Transplantation 2002;74:532-7.
- Kalantar Z, Nguyen M, Chang R, Kurtz I. Fatal hyponatremia in a young woman after ecstasy ingestion. Nat Clin Pract Nephrology 2006;2:283-8.
- Hoorn EJ. A fatal case of ecstasy poisoning. Paediatr Child Health 2001;6.
- Rosenson J, Smollin C, Sporer K, Blanc P, Olson K. Patterns of ecstasy-associated hyponatremia in California. Ann Emerg Med 2007;49:164-71.
- Hsu Y, Chiu J, Lu K, Chau T, Lin S. Biochemical and etiological characteristics of acute hyponatremia in the emergency department. J Emerg Med 2005;29:369-74.
- Hoorn EJ, Halperin ML, Zietse R. Diagnostic approach to a patient with hyponatraemia: traditional versus physiology-based options. QJM 2005;98:529-40.
- Rukskul P. Ecstasy MDMA ingestion related with severe hyponatremia in patients with mild head injury. J Med Assoc Thailand: Chotmaihetthangphaet 2005;88:41-4.
- Brvar M, Kozelj G, Osredkar J, Mozina M, Gricar M, Bunc M. Polydipsia as another mechanism of hyponatremia after ‘ecstasy’ 3,4 methyldioxymethamphetamine ingestion. Eur J Emerg Med 2004;11:302-4.
- Budisavljevic M, Stewart L, Sahn S, Ploth D. Hyponatremia associated with 3,4-methylenedioxymethylamphetamine (“ecstasy”) abuse. Am J Med Sci 2003;326:89-93.
- Kwon C, Zaritsky A, Dharnidharka V. Transient proximal tubular renal injury following ecstasy ingestion. Pediatr Nephrol (Berl) 2003;18:820-2.
- Traub S, Hoffman R, Nelson L. The ecstasy hangover: hyponatremia due to 3,4- methylenedioxymethamphetamine. J Urban Health 2002;79:549-5.
- Demirkiran M, Jankovic J, Dean J. Ecstasy intoxication: an overlap between serotonin syndrome and neuroleptic malignant syndrome. Clin Neuropharmacol 1996;19:157-64.
- Halachanova V, Sansone RA, McDonald S. Delayed rhabdomyolysis after ecstasy use. Mayo Clin Proc 2001;76:112-13.
- Gomez B, Pena H, Morillas C, Hernandez A. Syndrome of inappropriate antidiuretic hormone secretion and designer drugs ecstasy. J Pediatr Endocrinol Metab 2000;13:437-8.
- Holmes SB, Banerjee AK, Alexander WD. Hyponatraemia and seizures after ecstasy use. Postgrad Med J 1999;75:32-3.
- Ajaelo I, Koenig K, Snoey E. Severe hyponatremia and inappropriate antidiuretic hormone secretion following ecstasy use. Acad Emerg Med 1998;5:839-40.
- Watson ID, Serlin M, Moncur P, Tames F. Acute hyponatraemia. Postgrad Med J 1997;73:443-4.
- Nuvials X, Masclans JR, Peracaula R, de Latorre FJ. Hyponatraemic coma after ecstasy ingestion. Intensive Care Med 1997;23.
- Box SA, Prescott LF, Freestone S. Hyponatraemia at a rave. Postgrad Med J 1997;73:53-4.
- Holden R, Jackson MA. Near-fatal hyponatraemic coma due to vasopressin over-secretion after ecstasy 3,4-MDMA. Lancet 1996;347.
- Lehmann ED, Thom CH, Croft DN. Delayed severe rhabdomyolysis after taking ‘ecstasy’. Postgrad Med J 1995;71:186-7.
- Satchell SC, Connaughton M. Inappropriate antidiuretic hormone secretion and extreme rises in serum creatinine kinase following MDMA ingestion. Br J Hosp Med 1994;51.
- Kessel B. Hyponatraemia after ingestion of ecstasy. BMJ 1994;308.
- Maxwell DL, Polkey MI, Henry JA. Hyponatraemia and catatonic stupor after taking ecstasy. BMJ 1993;307.
- Arieff AI. Management of hyponatraemia. BMJ 1993;307:305-8.
- McCann UD, Slate SO, Ricaurte GA. Adverse reactions with 3,4-methylene dioxymethamphetamine MDMA; ‘ecstasy’. Drug Safety 1996;15:107-15.
- Sadeghian S, Darvish S, Shahbazi S, Mahmoodian M. Two ecstasy-induced myocardial infarctions during a three month period in a young man. Arch Iran Med 2007;10:409-12.
- Lai TI, Hwang JJ, Fang CC, Chen WJ. Methylene 3, 4 dioxymethamphetamine-induced acute myocardial infarction. Ann Emerg Med 2003;42:759-62.
- Qasim A, Townend J, Davies MK. Ecstasy induced acute myocardial infarction. Heart 2001;85.
- Duflou J, Mark A. Aortic dissection after ingestion of ‘ecstasy’ (MDMA). Am J Forensic Med Pathol 2000;21:261-3.
- Suarez RV, Riemersma R. ‘Ecstasy’ and sudden cardiac death. Am J Forensic Med Pathol 1988;9:339-41.
- Rella JG, Murano T. Ecstasy and acute myocardial infarction. Ann Emerg Med 2004;44:550-1.
- Henry JA, Hill IR. Fatal interaction between ritonavir and MDMA. Lancet 1998;352:1751-2.
- De Letter EA, Lambert W, Bouche M, Cordonnier J, Van Bocxlaer EF, Piette M. Postmortem distribution of 3,4-methylenedioxy-N,N-dimethyl-amphetamine MDDM or MDDA in a fatal MDMA overdose. Int J Legal Med 2007;121:303-7.
- Klys M, Rojek S, Wozniak K, Rzepecka W. Fatality due to the use of a designer drug MDMA Ecstasy. Legal Med (Tokyo) 2007;9:185-91.
- Jacobs W. Fatal amphetamine-associated cardiotoxicity and its medicolegal implications. Am J Forensic Med Pathol 2006;27:156-60.
- Elliott S. MDMA and MDA concentrations in antemortem and postmortem specimens in fatalities following hospital admission. J Analyt Toxicol 2005;29:296-300.
- Bassi S, Rittoo D. Ecstasy and chest pain due to coronary artery spasm. Int J Cardiol 2005;99:485-7.
- Mortelmans L, Bogaerts P, Hellemans S, Volders W, Van Rossom P. Spontaneous pneumomediastinum and myocarditis following Ecstasy use: a case report. Eur J Emerg Med 2005;12:36-8.
- Madhok A, Boxer R, Chowdhury D. Atrial fibrillation in an adolescent--the agony of ecstasy. Pediatr Emerg Care 2003;19:348-9.
- Kramer L, Bauer E, Schenk P, Steininger R, Vigl M, Mallek R. Successful treatment of refractory cerebral oedema in ecstasy/cocaine-induced fulminant hepatic failure using a new high-efficacy liver detoxification device FPSA-Prometheus. Wiener Klin Wochenschr 2003;115:599-603.
- Drake WM, Broadhurst PA. QT-interval prolongation with Ecstasy. South Afr Med J 1996;86:180-1.
- Bedford R, Schwartz RH, Dawling S. Accidental ingestion of ‘Ecstasy’ 3,4- methylenedioxymethylamphetamine. Arch Dis Child 1992;67:1114-15.
- Harry RA, Sherwood R, Wendon J. Detection of myocardial damage and cardiac dysfunction following ecstasy ingestion. Clin Intensive Care 2001;12:85-7.
- De Meester A, Thys F, Jacques JM, Chaudron JM. Symptomatic non-sustained ventricular tachycardia after one ‘Ecstasy’ tablet ingestion. Clin Intensive Care 1994;5:311-12.
- Sawyer J, Stephens WP. Misuse of ecstasy. BMJ 1992;305.
- Ho M, Tsai J, Wong Y. Subarachnoid hemorrhage and death following coingestion of MDMA with other drugs. JCMA 2004;67:640-3.
- Auer J, Berent R, Weber T, Lassnig E, Eber B. Subarachnoid haemorrhage with ecstasy abuse in a young adult. Neurol Sci 2002;23:199-201.
- Agaba E, Lynch R, Baskaran A, Jackson T. Massive intracerebral hematoma and extradural hematoma in amphetamine abuse. Am J Emerg Med 2002;20:55-7.
- McEvoy AW, Kitchen ND, Thomas DG. Intracerebral haemorrhage and drug abuse in young adults. Br J Neurosurg 2000;14:449-54.
- Gagajewski A, Apple FS. Amphetamines: role of toxicology laboratory for assisting in medical examiner cases. J Clin Ligand Assay 2003;26:25-9.
- Bertram M, Egelhoff T, Schwarz S, Schwab S. Toxic leukencephalopathy following ecstasy ingestion. J Neurol 1999;246:617-18.
- Selmi F, Davies KG, Sharma RR, Neal JW. Intracerebral haemorrhage due to amphetamine abuse: report of two cases with underlying arteriovenous malformations. Br J Neurosurg 1995;9:93-6.
- Hughes JC, McCabe M, Evans RJ. Intracranial haemorrhage associated with ingestion of ‘ecstasy’. Arch Emerg Med 1993;10:372-4.
- Manchanda S, Connolly MJ. Cerebral infarction in association with Ecstasy abuse. Postgrad Med J 1993;69:874-5.
- Watson JD, Ferguson C, Hinds CJ, Skinner R, Coakley JH. Exertional heat stroke induced by amphetamine analogues. Does dantrolene have a place?. Anaesthesia 1993;48:1057-60.
- Nimmo SM, Kennedy BW, Tullett WM, Blyth AS, Dougall JR. Drug-induced hyperthermia. Anaesthesia 1993;48:892-5.
- Gledhill JA, Moore DF, Bell D, Henry JA. Subarachnoid haemorrhage associated with MDMA abuse. J Neurol Neurosurg Psychiatry 1993;56:1036-7.
- Rothwell PM, Grant R. Cerebral venous sinus thrombosis induced by ‘ecstasy’. J Neurol Neurosurg Psychiatry 1993;56.
- Webb C, Williams V. Ecstasy intoxication: appreciation of complications and the role of dantrolene. Anaesthesia 1993;48:542-3.
- Cadier MA, Clarke JA. Ecstasy and Whizz at a rave resulting in a major burn plus complications. Burns 1993;19:239-40.
- Harries DP, De Silva R. ‘Ecstasy’ and intracerebral haemorrhage. Scott Med J 1992;37:150-2.
- Singarajah C, Lavies NG. An overdose of ecstasy. A role for dantrolene. Anaesthesia 1992;47:686-7.
- Cosentino C. Ecstasy and acute dystonia. Movement Disord 2004;19:1386-7.
- Kopelman MD, Reed LJ, Marsden P, Mayes AR, Jaldow E, Laing H, et al. Amnesic syndrome and severe ataxia following the recreational use of 3,4-methylene-dioxymethamphetamine (MDMA, ‘ecstasy’) and other substances. Neurocase 2001;7:423-32.
- Lauerma H, Wuorela M, Halme M. Interaction of serotonin reuptake inhibitor and 3,4- methylenedioxymethamphetamine?. Biol Psychiatry 1998;43.
- Priori A, Bertolasi L, Berardelli A, Manfredi M. Acute dystonic reaction to ecstasy. Movement Disord 1995;10.
- Brown C, Osterloh J. Multiple severe complications from recreational ingestion of MDMA ‘Ecstasy’. JAMA 1987;258:780-1.
- Parks K, Kennedy C. Club drugs: reasons for and consequences of use. J Psychoact Drugs 2004;36:295-302.
- Granato P, Weill S, Revillon JJ. Ecstasy and dementia in a young subject. Eur Psychiatry 1997;12:369-71.
- Hayner G, McKinney H. MDMA: the dark side of ecstasy. J Psychoact Drugs 1986;18:341-7.
- Goldstein L, Mordish Y, Abu K, I, Toledano M, Berkovitch M. Acute paralysis following recreational MDMA Ecstasy use. Clin Toxicol 2006;44:339-41.
- Yin Foo LG, Wooi Kee GG, Vrodos N, Patrick BB. ‘Ecstasy’-induced subarachnoid haemorrhage: an under-reported neurological complication?. J Clin Neurosci 2003;10:705-7.
- McEvoy AW, Kitchen ND, Thomas DGT. Lesson of the week: Intracerebral haemorrhage in young adults: the emerging importance of drug misuse. BMJ 2000;320:1322-4.
- Lee GYF, Gong GWK, Vrodos N, Brophy BP. ‘Ecstasy’-induced subarachnoid haemorrhage: An under-reported neurological complication?. J Clin Neurosci 2003;10:705-7.
- Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine ecstasy. Lancet 1992;340:384-7.
- Vallee J, Crozier S, Guillevin R, Obadia M, Lo D, Barragan C, et al. Acute basilar artery occlusion treated by thromboaspiration in a cocaine and ecstasy abuser. Neurology 2003;61:839-41.
- Harris R, Joseph A. Spontaneous pneumomediastinum – ‘ectasy’: a hard pill to swallow. Aust N Z J Med 2000;30:401-3.
- Mutlu H, Silit E, Pekkafali Z, Incedayi M, Basekim C, Kizilkaya E. ‘Ecstasy’(MDMA)-induced pneumomediastinum and epidural pneumatosis. Diagn Interv Radiol 2005;11:150-1.
- Hutchison RP, Burgess B. Spontaneous pneumomediastinum – a right pain in the neck?. Injury 2005;36:801-3.
- Bernaerts A, Verniest T, Vanhoenacker F, Van den BP, Petre C, De Schepper AM. Pneumomediastinum and epidural pneumatosis after inhalation of ‘ecstasy’. Eur Radiol 2003;13:642-3.
- Rejali D, Glen P, Odom N. Pneumomediastinum following Ecstasy (methylenedioxymetamphetamine, MDMA) ingestion in two people at the same ‘rave’. J Laryngol Otol 2002;116:75-6.
- Mazur S, Hitchcock T. Spontaneous pneumomediastinum, pneumothorax and ecstasy abuse. Emerg Med (Fremantle) 2001;13:121-3.
- Ryan J, Banerjee A, Bong A. Pneumomediastinum in association with MDMA ingestion. J Emerg Med 2001;20:305-6.
- Quin GI, McCarthy GM, Harries DK. Spontaneous pneumomediastinum and ecstasy abuse. J Accid Emerg Med 1999;16.
- Ahmed JM, Salame MY, Oakley GD. Chest pain in a young girl. Postgrad Med J 1998;74:115-16.
- Pittman JA, Pounsford JC. Spontaneous pneumomediastinum and Ecstasy abuse. J Accid Emerg Med 1997;14:335-6.
- Rezvani K, Kurbaan AS, Brenton D. Ecstasy induced pneumomediastinum. Thorax 1996;51:960-1.
- Onwudike M. Ecstasy induced retropharyngeal emphysema. J Accid Emerg Med 1996;13:359-61.
- Levine AJ, Drew S, Rees GM. ‘Ecstasy’ induced pneumomediastinum. J R Soc Med 1993;86:232-3.
- Ng CP, Chau LF, Chung CH. Massive spontaneous haemopneumothorax and ecstasy abuse. Hong Kong J Emerg Med 2004;11:94-7.
- Marasco S, Lim H. Ecstasy-associated pneumomediastinum. Ann R Coll Surgeons Engl 2007;89:389-93.
- Badaoui R, El K, Fikri M, Ouendo M, Canova B, Ossart M. Spontaneous cervical and mediastinal air emphysema after ecstasy abuse. Anesth Analg 2002;95.
- Persaud R, Roberts D, Gleeson M. Ectasy abuse linked to extensive cervical emphysema. CME Bull Otorhinolaryngol Head Neck Surg 2003;7:48-9.
- Levine AJ, Drew S, Rees GM. Ecstasy induced pneumomediastinum. J R Soc Med 1993;86:232-3.
- Chang S, Lai T, I, Chen W, Fang C. MDMA-induced acute pulmonary edema in a patient without other organ dysfunction. Am J Emerg Med 2006;24:734-6.
- Burghart S, Armbruster C. Ecstasy induced pneumomediastinum and pneumothorax new causes, new questions. Respir Med Extra 2005;1:53-5.
- Chung CH. X-ray quiz: a young man with chest pain. Hong Kong J Emerg Med 2004;11:91-3.
- Weinmann W, Bohnert M. Lethal monointoxication by overdosage of MDEA. Forensic Sci Int 1998;91:91-101.
- Forrest AR, Galloway JH, Marsh ID, Strachan GA, Clark JC. A fatal overdose with 3,4-methylenedioxyamphetamine derivatives. Forensic Sci Int 1994;64:57-9.
- Brncic N, Kraus I, Viskovic I, Mijandrusic-Sincic B, Vlahovic-Palcevski V. 3,4-methylenedioxymethamphetamine (MDMA): an important cause of acute hepatitis. Med Sci Monit 2006;12:CS107-9.
- De Carlis L, De Gasperi A, Slim AO, Giacomoni A, Corti A, Mazza E, et al. Liver transplantation for ecstasy-induced fulminant hepatic failure. Transplant Proc 2001;33:2743-4.
- Andreu V, Mas A, Bruguera M, Salmeron JM, Moreno V, Nogue S, et al. Ecstasy: a common cause of severe acute hepatotoxicity. J Hepatol 1998;29:394-7.
- Brauer RB, Heidecke CD, Nathrath W, Beckurts KT, Vorwald P, Zilker TR, et al. Liver transplantation for the treatment of fulminant hepatic failure induced by the ingestion of ecstasy. Transplant Int 1997;10:229-33.
- Fidler H, Dhillon A, Gertner D, Burroughs A. Chronic ecstasy (3,4-methylenedioxymetamphetamine) abuse: a recurrent and unpredictable cause of severe acute hepatitis. J Hepatol 1996;25:563-6.
- Dykhuizen RS, Brunt PW, Atkinson P, Simpson JG, Smith CC. Ecstasy induced hepatitis mimicking viral hepatitis. Gut 1995;36:939-41.
- Shearman JD, Chapman RW, Satsangi J, Ryley NG, Weatherhead S. Misuse of ecstasy [letter]. BMJ 1992;305.
- Gorard DA, Davies SE, Clark ML. Misuse of ecstasy [letter]. BMJ 1992;305.
- Sass DA, Shakil AO. Fulminant hepatic failure. Gastroenterol Clin North Am 2003;32:1195-211.
- Smith I, Simpson K, Garden O, Wigmore S. Non-paracetamol drug-induced fulminant hepatic failure among adults in Scotland. Eur J Gastroenterol Hepatol 2005;17:161-7.
- Bingham C, Beaman M, Nicholls AJ, Anthony PP. Necrotizing renal vasculopathy resulting in chronic renal failure after ingestion of methamphetamine and 3,4-methylenedioxymethamphetamine (‘ecstasy’). Nephrol Dial Transplant 1998;13:2654-5.
- Delgado JH, Caruso MJ, Waksman JC, Honigman B, Stillman D. Acute, transient urinary retention from combined ecstasy and methamphetamine use. J Emerg Med 2004;26:173-5.
- Inman DS, Greene D. The agony and the ecstasy: acute urinary retention after MDMA abuse. BJU Int 2003;91.
- Bryden AA, Rothwell PJ, O’Reilly PH. Urinary retention with misuse of ‘ecstasy’. BMJ 1995;310.
- Cox DE. ‘Rave’ to the grave. Forensic Sci Int 1993;60:5-6.
- Rohrig TP, Prouty RW. Tissue distribution of methylenedioxymethamphetamine. J Analyt Toxicol 1992;16:52-3.
- Cohen R. Adverse symptomatology and suicide associated with the use of methylenedioxymethamphetamine (MDMA; “Ecstasy”). Biol Psychiatry 1996;39:819-20.
- Liu R, Liu H, Lin D. Distribution of methylenedioxymethamphetamine MDMA and methylenedioxyamphetamine MDA in postmortem and antemortem specimens. J Analyt Toxicol 2006;30:545-50.
- Libiseller K, Pavlic M, Grubwieser P, Rabl W. An announced suicide with ecstasy. Int J Legal Med 2007;121:40-3.
- Ellis P, Schimmel P. Ecstasy abuse. N Z Med J 1989;102.
- Lehane M, Rees C. When Ecstasy means agony. Nursing Standard 1996;10:24-5.
- Semiz U, Basoglu C, Cetin M, Ebrinc S. Panic disorder due to ingestion of single dose ecstasy. Int J Psychiatry Clin Pract 2005;9:138-41.
- Vecellio M, Schopper C, Modestin J. Neuropsychiatric consequences (atypical psychosis and complex-partial seizures) of ecstasy use: possible evidence for toxicity-vulnerability predictors and implications for preventative and clinical care. J Psychopharmacol 2003;17:342-5.
- Vaiva G, Boss V, Bailly D, Thomas P, Lestavel P, Goudemand M. An “accidental” acute psychosis with ecstasy use. J Psychoact Drugs 2001;33:95-8.
- Tuwir I, Chako E, Brosnahan D, Cassidy L. Drug induced autoenucleation with resultant chiasmal damage (2). Br J Ophthalmol 2005;89.
- Alciati A, Scaramelli B, Fusi A, Butteri E, Cattaneo ML, Mellado C. Three cases of delirium after “ecstasy” ingestion. J Psychoact Drugs 1999;31:167-70.
- Windhaber J, Maierhofer D, Dantendorfer K. Panic disorder induced by large doses of 3,4- methylenedioxymethamphetamine resolved by paroxetine. J Clin Psychopharmacol 1998;18:95-6.
- Cassidy G, Ballard C. Psychiatric sequelae of MDMA (ecstasy) and related drugs. Irish J Psychol Med 1994;11:132-3.
- Series H, Boeles S, Dorkins E, Peveler R. Psychiatric complications of “Ecstasy” use. J Psychopharmacol 1994;8:60-1.
- Keenan E, Gervin M, Dorman A, Connor J. Psychosis and recreational use of MDMA (“Ecstasy”). Irish J Psychol Med 1993;10:162-3.
- Pallanti S, Mazzi D. MDMA (ecstasy) precipitation of panic disorder. Biol Psychiatry 1992;32:91-5.
- Whitaker A, Aronson TA. Ecstasy MDMA-induced panic. Am J Psychiatry 1989;146.
- Spatt J, Glawar B, Mamoli B. A pure amnestic syndrome after MDMA ecstasy ingestion. J Neurol Neurosurg Psychiatry 1997;62:418-19.
- Hill A. The environment and disease: association or causation?. Proc R Soc Med 1965;58:295-300.
- Cole JC, Michailidou K, Jerome L, Sumnall HR. The effects of stereotype threat on cognitive function in ecstasy users. J Psychopharmacol 2006;20:518-25.
- Zakzanis K, Campbell Z. Memory impairment in now abstinent MDMA users and continued users: a longitudinal follow-up. Neurology 2006;66:740-1.
- Blowers J. 3,4-Methylenedioxymethamphetamine (Ecstasy) and symptoms of depression in the collegiate population. J Alcohol Drug Educ 2004;48:5-12.
- Daumann J, Hensen G, Thimm B, Rezk M, Till B, Gouzoulis M. Self-reported psychopathological symptoms in recreational ecstasy (MDMA) users are mainly associated with regular cannabis use: further evidence from a combined cross-sectional/longitudinal investigation. Psychopharmacology 2004;173:398-404.
- Zakzanis KK, Young DA. Memory impairment in abstinent MDMA Ecstasy users: a longitudinal investigation. Neurology 2001;56:966-9.
- Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol 1992;63:452-9.
- Bond A, Lader M. A method to elicit aggressive feelings and behaviour via provocation. Biol Psychol 1986;22:69-7.
- Gernsbacher MA, Goldsmith HH, Robertson RRW. Do readers mentally represent characters’ emotional states. Cognit Emot 1992;6:89-111.
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol 1957;21:343-9.
- Copello AG, Tata PR. Violent behaviour and interpretative bias: an experimental study of the resolution of ambiguity in violent offenders. Br J Clin Psychol 1990;29:417-28.
- Siegel J. The multidimensional Anger inventory. J Personal Soc Psychol 1986;51:191-200.
- Cherek DR. Point subtraction aggression paradigm (PSAP). Houston: University of Texas; 1992.
- Derogatis LR. SCL-90-R: administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems; 1994.
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety. J Consult Clin Psychol 1988;56:893-7.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 1994.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatry Scand 1983;67:361-70.
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959;32:50-5.
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol 1974;47.
- Little K, Penman E. Measuring subacute mood changes using the profile of mood states and visual analogue scales. Psychopathology 1989;22:42-9.
- Derogatis LR. The brief symptom inventory (BSI). SCL-90: Administration, scoring and procedures manual for the revised version and other instruments of the psychopathology rating scales series. Baltimore, MD: Clinical Psychometric Research; 1982.
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1970.
- van der Ploeg HM, Defares PB. Handleiding bij de Zelf-Beoordelings Vragenlijst ZBV: een nederlandstalige bewerking van de Spielberger State-Trait Anxiety Inventory STAI-DY. Lisse: Swets & Zeitlinger; 1980.
- Baddeley AD, Emslie H, Nimmo-Smith I. The speed and capacity of language-processing test. Bury St Edmunds: Thames Valley Test Company; 1992.
- Wechsler D. WMS-R: Wechsler memory scale – revised: manual. San Diego: Psychological Corporation/ Harcourt Brace Jovanovich; 1987.
- Hodgson M, Golding J. Psychometric evaluation of divers performing a series of heliox non-saturation dives. Aviation Space Environm Med 1991;62:407-13.
- Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: evidence for a specific attentional dysfunction. Neuropsychologia 1989;27:1329-43.
- Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol 1996;11:391-400.
- Alpherts W, Aldenkamp AP. FePsy, The Iron Psyche Manual. Heemstede, the Netherlands: Het Instituut voor Epilepsiebestrijding; 1995.
- Cairns E, Commock T. Development of a more reliable version of the matching familiar figures Test. Dev Psychol 1978;14:555-60.
- Ruff RM, Allen CC. Ruff 2 & 7 selective attention test: professional manual. Lutz, FL: Psychological Assessment Resources; 1996.
- Smith A. Symbol digit modalities test manual (revised). Los Angeles: Western Psychological Services; 1982.
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643-62.
- Zimmermann P, Fimm B. Test for attentional performance (TAP). Herzogenrath, Germany: PsyTest; 1995.
- Robertson IH. Test of everyday attention (TEA). Bury St Edmunds: Thames Valley Test Company; 1994.
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 1958;8.
- Reitan RM. Trail making test: manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992.
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Neurol Sci 1996;17:305-9.
- D’Elia LF, Satz P, Lyons-Uchiyama C, White T. Color trail test: professional manual. Odessa, FL: Psychological Assessment Resources Inc; 1996.
- Wechsler D. Wechsler adult intelligence scale. San Antonio, TX: The Psychological Corporation; 1997.
- Wechsler D. Wechsler adult intelligence scale – revised: manual. San Diego: Harcourt Brace Jovanovich; 1981.
- Thorne DR, Genser SG, Sing HC, Hegge FW. The Walter Reed performance assessment battery. Neurobehav Toxicol Teratol 1985;7:415-18.
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med 1999;29:1307-21.
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J Abnorm Psychol 1986;95:252-6.
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology 1983;9:223-9.
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 2001;39:376-89.
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000;123:2189-202.
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology 2003;28:153-62.
- Levine B, Dawson D, Boutet I, Schwartz ML, Stuss DT. Assessment of strategic self-regulation in traumatic brain injury: its relationship to injury severity and psychosocial outcome. Neuropsychology 2000;14:491-500.
- Beck AT, Steer RA. BDI, Beck depression inventory: manual. San Diego: Psychological Corporation/Harcourt Brace Jovanovich; 1987.
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Personal Assessm 1996;67:588-97.
- World Health Organization . Composite International Diagnostic Interview, Version 2.1 1997.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62.
- Hathaway SR. Minnesota multiphasic personality inventory: manual. San Diego: Psychological Corp.; 1951.
- Hathaway SR, McKinley JC. MMPI-2: Minnesota multiphasic personality inventory-2. Minneapolis: University of Minnesota Press; 1989.
- Grace J, Malloy PF. Frontal systems behavior scale (FrSBe). Odessa, FL: Psychological Assessment Resources, Inc; 1999.
- Wilson B, Alderman N, Burgess PW, Emslie H, Evans J. Manual for the behavioural assessment of the dysexecutive syndrome. Bury St Edmunds: Thames Valley Test Company; 1996.
- Baddeley A. Exploring the central executive. Q J Exp Psychol A 1996;49:5-28.
- Baddeley AD. The capacity for generating information by randomization. Q J Exp Psychol 1966;18:119-29.
- Funke J, Kruger T, Funke J, Fritz A. Neue Konzepte und Instrumente zur Planungsdiagnostik. Bonn: Deutscher Psychologen Verlag; 1995.
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 1995;33:1-24.
- Shallice T. Specific impairments of planning. Phil Trans R Soc London Ser B, Biol Sci 1982;298:199-20.
- Ridderinkhof KR, van der Molen MW. A psychophysiological analysis of developmental differences in the ability to resist interference. Child Dev 1995;66:1040-56.
- Eriksen CW, Schultz DW. Information processing in visual search: a continuous flow conception and experimental results. Percept Psychophys 1979;25:249-63.
- van Boxtel GJM, van der Molen MW, Jennings JR, Brunia CHM. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biol Psychol 2001;58:229-62.
- Burgess PW, Shallice T. The Hayling and Brixton tests. Bury St Edmunds: Thames Valley Test Company; 1997.
- Heaton RK. A manual for the Wisconsin card sorting Test. Los Angeles: Western Psychological Services; 1981.
- Delis DC, Kaplan E. Delis–Kaplan executive function System: D-KEFS. San Antonio, TX: PsychCorp; 2001.
- Ruff RM. RFFT, Ruff figural fluency test: professional manual. Lutz, FL: Psychological Assessment Resources; 1996.
- Rey A. L’examen clinique psychologique dans les cas d’encéphalopathie traumatique. Arch Psychol 1941;28:286-340.
- Osterrieth P. Le test de copie d’une figure complexe: Contribution a l’étude de la perception et de la mémoire. Arch Psychol 1944;30:286-35.
- Barratt ES, Patton JH, Zucherman M. Biological bases of sensation feeling, impulsivity, and anxiety. Hillsdale, NJ: Erlbaum; 1983.
- Barratt ES, Spence J, Izard C. Motivation, emotion, and personality. North Holland: Elsevier; 1985.
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995;51:768-74.
- Eysenck HJ, Eysenck SBG. Adult impulsiveness, venturesomeness and empathy scale. London: Hodder Stoughton; 1991.
- Kagan J. Information processing in the child: significance of analytic and reflective attitudes. Washington DC: American Psychological Association; 1964.
- Kaufman AS. K-BIT: Kaufman brief intelligence test. Circle Pines, MN: American Guidance Service; 1990.
- Court J, Raven JC. The Mill Hill vocabulary scale: 1988 revision. London: HK Lewis; 1988.
- Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest. Manual. Balingen: Medizinische Verlagsgesellschaft; 1999.
- Lehrl S. Manual. Balingen: Perimed-Spitta; 1995.
- Nelson HE. National adult reading test (NART). Windsor: NFER-Nelson; 1982.
- Nelson HE, Willison J. The revised national adult reading test: manual. Windsor: NFER-Nelson; 1991.
- Schmand B, Lindeboom J, Van Harskamp F. Nederlandse Leestest voor Volwassenen (Dutch Adult Reading Test). Lisse, the Netherlands: Swets & Zeitlinger; 1992.
- Ammons RB, Ammons CH. The Quick test (qt): provisional manual. Psychol Rep 1962;11:111-61.
- Raven J, Court J. Manual for Raven’s progressive matrices and vocabulary scales. Oxford: Oxford Psychologists Press; 1998.
- Zachary RA. Estimating WAIS-R IQ from the Shipley institute of living scale. J Clin Psychol 1985;41:532-40.
- Weiss J, Schell R. Estimating WAIS-R IQ from the Shipley institute of living scale: a replication. J Clin Psychol 1991;47:558-62.
- Baddeley A, Emslie H, Nimmo-Smith I. The spot-the-word test: a robust estimate of verbal intelligence based on lexical decision. Br J Clin Psychol 1993;32:55-6.
- Brown L, Sherbenou R, Johnsen S. Test of non-verbal intelligence: a language-free measure of cognitive ability. Austin, TX: Pro-Ed; 1997.
- Woodcock RW. Woodcock–Johnson tests of achievement – revised. Allen, TX: DLM Teaching Resources; 1989.
- Wilson B, Cockburn J, Baddeley A. The Rivermead behavioural memory test: manual. Bury St Edmunds: Thames Valley; 1985.
- Wechsler D. WMS-III: Wechsler Memory Scale: Manual. San Antonio, TX: Psychological Corporation; 1997.
- Bäumler G. Lern-und Gedächtnistest. Göttingen, Germany: Hogrefe Verlag für Psychologie; 1974.
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982;21:1-16.
- Sunderland A, Harris J, Baddeley AD. Do laboratory tests predict everyday memory? A neuropsychological study. J Verbal Learn Verbal Behav 1983;22:341-57.
- von Cramon D, Mai N, Ziegler W. Neuropsychologische Diagnostik. Weinheim: VCH; 1993.
- Hannon R, Adams P, Harrington S, Fries-Dias C, Gibson MT. Effects of brain injury and age on prospective memory self-rating and performance. Rehab Psychol 1995;40:289-98.
- Parrott AC, Kaye FJ. Daily uplifts, hassles, stresses and cognitive failures: in cigarette smokers, abstaining smokers, and non-smokers. Behav Pharmacol 1999;10:639-46.
- Rendell PG, Craik FIM. Virtual week and actual week: age-related differences in prospective memory. Appl Cogn Psychol 2000;14:S43-62.
- Buschke H, Fuld P. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974;24.
- Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1964.
- Helmstaedter C, Durwen H. VLMT: Verbaler Lern- und Merkfähigkeitstest: ein praktikables und differenziertes Instrumentarium zur Prüfung der verbalen Gedächtnisleistungen. Schweiz Arch Neurol Psychiatry 1990;141:21-30.
- Hänsgen K, Merten T. Leistungsdiagnostisches Labor (LEILA). Göttingen: Hogrefe Verlag; 1995.
- Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test-II. New York, NY: Psychological Corporation; 2000.
- Van den Burg W, Saan RJ, Deelman BG. 15-Woordentest. Provisional manual. Groningen, Netherlands: University Hospital Groningen; 1985.
- Mitrushina MN, Boone KB, D’Elia L. Handbook of normative data for neuropsychological assessment. New York: Oxford University Press; 1999.
- Calev A. Recall and recognition in mildly disturbed schizophrenics: the use of matched tasks. Psychol Med 1984;14:425-9.
- Warrington EK. Recognition memory test. Windsor: NFER-Nelson; 1984.
- Majdan A, Sziklas V, Jones-Gotman M. Performance of healthy subjects and patients with resection from the anterior temporal lobe on matched tests of verbal and visuoperceptual learning. J Clin Exp Neuropsychol 1996;18:416-30.
- Graham F, Kendall B. Memory-for-designs test: revised general manual. Percept Mot Skills 1960;11:147-88.
- Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry 1989;154.
- Sivan AB. Benton Visual Retention Test. San Antonio, TX: The Psychological Corporation; 1992.
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, et al. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain 1988;111:695-718.
- Fray P, Robbins TW, Sahakian B. Neuropsychiatric applications of CANTAB. Int J Geriatr Psychiatry 1996;11:329-36.
- Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull 1971;27:272-7.
- Trahan DE, Larrabee GJ. Continuous visual memory test. Odessa, FL: Psychological Assessment Resources; 1988.
- Larson GE. Information processing and intelligence: some implications of task complexity. Intelligence 1988;12:131-47.
- Gronwall DMA. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills 1977;44:367-73.
- Janke W, Debus G. Die Eigenschaftswörterliste EWL. Göttingen: Hogrefe; 1978.
- Nowlis V, Tomkins S, Izard C. Affect, cognition, and personality. New York: Springer; 1965.
- Kløve H. Clinical neuropsychology. Med Clin North Am 1963;47:1647-58.
- Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test. Arch Neurol 1978;35:364-7.
- Shepard RN, Metzler N. Mental rotation of three dimensional objects. Science 1971;171:701-3.
- Eysenck HJ. Manual of the Eysenck personality questionnaire. London: Hodder & Stoughton; 1975.
- Gerris JRM, Houtmans MJM, Kwaaitaal-Roosen EMG, De Schipper JC, Vermulst AA, Janssens JMAM. Parents, adolescents and young adults in Dutch families: a longitudinal study. Nijmegen, Netherlands: Institute of Family Studies; 1998.
- Zuckerman M, Link K. Construct validity for the sensation-seeking scale. J Consult Clin Psychol 1968;32:420-6.
- Feij JA, van Zuilen RW, Gazendam A. De ontwikkeling van een Nederlandse vragenlijst voor sensation seeking: de Spanningsbehoeftelijst (SBL). Gedrag 1982;10:364-83.
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry 1987;44:573-88.
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview according to DSM-III-R. Washington, DC: American Psychiatric Press; 1990.
- Hyler S, Rieder R, Williams JBW, Spitzer RL, Hendler J, Lyons M. The personality diagnostic questionnaire: development and preliminary results. J Personal Disord 1988;2:229-37.
- Horn W. Leistungsprufsystem LPS. Gottingen, Germany: Hogrefe; 1983.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5.
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Research Institute, UCLA; 1968.
- Kaplan E, Goodglass H, Weintraub S. Boston naming test. Philadelphia: Lea & Febiger; 1983.
- Thurstone LL, Thurstone TG. Primary mental abilities – revised. Chicago: Science Research Associates; 1962.
- Reneman L, Booij J, Schmand B, van den Brink W, Gunning B. Memory disturbances in “Ecstasy” users are correlated with an altered brain serotonin neurotransmission. Psychopharmacology 2000;148:322-4.
- Quednow B, Kuehn K, Hoppe C, Westheide J, Maier W, Daum I, et al. Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (“Ecstasy”). Psychopharmacology 2007;189:517-30.
- Roiser J, Sahakian B. Relationship between ecstasy use and depression: a study controlling for poly-drug use. Psychopharmacology 2004;173:411-17.
- Medina K, Shear P. Anxiety, depression, and behavioral symptoms of executive dysfunction in ecstasy users: contributions of polydrug use. Drug Alcohol Depend 2007;87:303-11.
- Libiseller K, Pavlic M, Grubwieser P, Rabl W. Ecstasy – deadly risk even outside rave parties. Forensic Sci Int 2005;153:227-30.
- Forrest ARW. “Review of deaths related to taking ecstasy, England and Wales, 1997–2000”: Comment. BMJ 2003;326:823-4.
- Karch SB, Stephens BG, Nazareno GV. GHB Club drug or confusing artifact?. Am J Forens Med Pathol 2001;22:266-9.
- Galloway JH, Forrest ARW. Caveat emptor: Death involving the use of 4-methoxyamphetamine. Vet J 2002;164.
- Moore KA, Mozayani A, Fierro MF, Poklis A. Distribution of 3,4-methylenedioxymethamphetamine MDMA and 3,4- methylenedioxyamphetamine MDA stereoisomers in a fatal poisoning. Forensic Sci Int 1996;83:111-19.
- Bentley AJ, Busuttil A. Deaths among drug abusers in south-east Scotland (1989–1994). Med Sci Law 1996;36:231-6.
- Falck R, Carlson R, Wang J, Siegal H. Psychiatric disorders and their correlates among young adult MDMA users in Ohio. J Psychoact Drugs 2006;38:19-2.
- Jovanovski D, Zakzanis K. Donepezil in a chronic drug user-a potential treatment?. Hum Psychopharmacol: Clin Exp 2003;18:561-4.
- Bryant S, Kolodchak J. Serotonin syndrome resulting from an herbal detox cocktail. Am J Emerg Med 2004;22:625-6.
- Finsterer J, Stoellberger C, Steger C, Kroiss A. Long lasting impaired cerebral blood flow after ecstasy intoxication. Psychiatry Clin Neurosci 2003;57:221-5.
- Masi G, Mucci M, Floriani C. Acute catatonia after a single dose of ecstasy. J Am Acad Child Adolesc Psychiatry 2002;41.
- Van Kampen J, Katz M. Persistent psychosis after a single ingestion of ‘ecstasy’. Psychosomatics 2001;42:525-7.
- Lora T, Tena T, Rodriguez A, Sancho JR, Molina E. Intoxication due to 1,4-butanediol. Forensic Sci Int 2003;133:256-9.
- Kaskey G. Possible interaction between MAOI and “Ecstasy.”. Am J Psychiatry 1992;149:411-12.
- Harrington RD, Woodward JA, Hooton TM, Horn JR. Life-threatening interactions between HIV-1 protease inhibitors and the illicit drugs MDMA and gamma-hydroxybutyrate. Arch Intern Med 1999;159:2221-4.
- Regenthal R, Krueger M, Rudolph K, Trauer H, Preiss R. Survival after massive ecstasy MDMA ingestion. Intensive Care Med 1999;25:640-41.
- Rochester JA, Kirchner JT. Ecstasy 3,4-methylenedioxymethamphetamine: history, neurochemistry, and toxicology. J Am Board Family Pract 1999;12:137-42.
- Mallick A, Bodenham AR. MDMA induced hyperthermia: a survivor with an initial body temperature of 42.9 degrees C. J Accid Emerg Med 1997;14:336-8.
- Murthy BV, Wilkes RG, Roberts NB. Creatine kinase isoform changes following Ecstasy overdose. Anaesth Intensive Care 1997;25:156-9.
- Bitsch A, Thiel A, Rieckmann P, Prange H. Acute inflammatory CNS disease after MDMA ‘ecstasy’. Eur Neurol 1996;36:328-9.
- Fahal IH, Sallomi DF, Yaqoob M, Bell GM. Acute renal failure after ecstasy. BMJ 1992;305.
- Smilkstein MJ, Smolinske SC, Rumack BH. A case of MAO inhibitor/MDMA interaction: agony after ecstasy. J Toxicol Clin Toxicol 1987;25:149-59.
- Theron L, Jansen K, Miles J. Benzylpiperizine-based party pills’ impact on the Auckland City Hospital Emergency Department Overdose Database 2002–2004 compared with ecstasy MDMA or methylene dioxymethamphetamine, gamma hydroxybutyrate GHB, amphetamines, cocaine, and alcohol. N Z Med J 2007;120.
- Brazier WJ, Dhariwal DK, Patton DW, Bishop K. Ecstasy related periodontitis and mucosal ulceration – a case report. Br Dent J 2003;194:197-9.
- See SJ, Tan EK. Severe amphethamine-induced bruxism: treatment with botulinum toxin. Acta Neurol Scand 2003;107:161-3.
- Murray MO, Wilson NH. Ecstasy related tooth wear. Br Dental J 1998;185.
- Davidson JA, Patel SB, Emin A, Bennett AMD. Minerva. BMJ 2007;335:216-21.
- Cottler L, Womack S, Compton W, Ben A. Ecstasy abuse and dependence among adolescents and young adults: applicability and reliability of DSM-IV criteria. Hum Psychopharmacol Clin Exp 2001;16:599-606.
- Jansen K. Ecstasy (MDMA) dependence. Drug Alcohol Depend 1999;53:121-4.
- Ahmed M, Islam S, Hoffman GR. Widespread oral and oropharyngeal mucosal oedema induced by ecstasy MDMA: a case for concern. Br J Oral Maxillofac Surg 2007;45:496-8.
- Tan B, Foley P. Guttate psoriasis following Ecstasy ingestion. Aust J Dermatol 2004;45:167-9.
- Wollina U, Kammler HJ, Hesselbarth N, Mock B, Bosseckert H. Ecstasy pimples – a new facial dermatosis. Dermatology 1998;197:171-3.
- Clark AD, Butt N. Ecstasy-induced very severe aplastic anaemia complicated by invasive pulmonary mucormycosis treated with allogeneic peripheral blood progenitor cell transplant. Clin Lab Haematol 1997;19:279-81.
- Lee P, Nicoll AJ, McDonough M, Colman PG. Substance abuse in young patients with type I diabetes: easily neglected in complex medical management. Intern Med J 2005;35:359-61.
- Seymour HR, Gilman D, Quin JD. Severe ketoacidosis complicated by ‘ecstasy’ ingestion and prolonged exercise. Diabet Med 1996;13:908-9.
- Goorney BP, Scholes P. Transient haemolytic anaemia due to ecstasy in a patient on HAART. Int J STD AIDS 2002;13.
- Schirren CA, Berghaus TM, Sackmann M. Thrombotic thrombocytopenic purpura after Ecstasy-induced acute liver failure. Ann Intern Med 1999;130.
- Montgomery H, Myerson S. 3,4-methylenedioxymethamphetamine (MDMA, or ecstasy) and associated hypoglycemia. Am J Emerg Med 1997;15.
- Hall AP, Lyburn ID, Spears FD, Riley B. An unusual case of Ecstasy poisoning. Intensive Care Med 1996;22:670-1.
- Barrett PJ, Taylor GT. ‘Ecstasy’ ingestion: a case report of severe complications. J R Soc Med 1993;86:233-4.
- Barrett PJ. ‘Ecstasy’ misuse – overdose or normal dose?. Anaesthesia 1993;48.
- Logan S, Stickle B, Keefe N, Hewitson H. Survival following ‘Ecstasy’ ingestion with a peak temperature of 42°C. Anaesthesia 1993;48:1017-18.
- Moon J, Cros J. Role of dantrolene in the management of the acute toxic effects of Ecstasy (MDMA). Br J Anaesth 2007;99.
- Connolly E, Callaghan G. MDMA toxicity presenting with severe hyperpyrexia: a case report. Crit Care Resusc 1999;1:368-70.
- Melian A, Burillo P, Campo C, Padron A, Ramos C. Accidental ecstasy poisoning in a toddler. Pediatr Emerg Care 2004;20:534-5.
- Bordo D, Dorfman M. Ecstasy overdose: rapid cooling leads to successful outcome. Am J Emerg Med 2004;22:326-7.
- Weinbroum A. Importance of early identification of methylenedioxymethamphetamine ‘ecstasy’ ingestion in victims of motor vehicle accidents. Eur J Emerg Med 2003;10:19-22.
- Sharma A. A case of sensorineural deafness following ingestion of Ecstasy. J Laryngol Otol 2001;115:911-15.
- Ling LH, Marchant C, Buckley NA, Prior M, Irvine RJ. Poisoning with the recreational drug paramethoxyamphetamine death. Med J Aust 2001;174:453-5.
- Hamilton J, Balint P, Field M, Sturrock RD. A concealed cause of recurrent renal failure in a patient with juvenile chronic arthritis. Ann Rheum Dis 1999;58:396-8.
- Ramcharan S, Meenhorst PL, Otten JM, Koks CH, de Boer D, Maes RA, et al. Survival after massive ecstasy overdose. J Toxicol Clin Toxicol 1998;36:727-31.
- Cooper AJ, Egleston CV. Accidental ingestion of ecstasy by a toddler: unusual cause for convulsion in a febrile child. J Accid Emerg Med 1997;14:183-4.
- Williams A, Unwin R. Prolonged elevation of serum creatine kinase (CK) without renal failure after ingestion of ecstasy. Nephrol Dial Transplant 1997;12:361-2.
- McCoy EP, Renfrew C, Johnston JR, Lavery G. Malignant hyperpyrexia in an MDMA (Ecstasy) abuser. Ulster Med J 1994;63:103-7.
- Wake D. Ecstasy overdose: a case study. Intensive Crit Care Nurs 1995;11:6-9.
- Roberts L, Wright H. Survival following intentional massive overdose of ‘Ecstasy’. J Accid Emerg Med 1994;11:53-4.
- Walsh T, Carmichael R, Chestnut J. A hyperthermic reaction to ‘ecstasy’. Br J Hosp Med 1994;51.
- Marsh JC, Abboudi ZH, Gibson FM, Scopes J, Daly S, Shaunnessy D, et al. Aplastic anaemia following exposure to 3,4-methylenedioxymethamphetamine (‘Ecstasy’). Br J Haematol 1994;88:281-5.
- Riva N, Morana P, Cerri F, Gerevini S, Amadio S, Formaglio F, et al. Acute myelopathy selectively involving lumbar anterior horns following intranasal insufflation of ecstasy and heroin. J Neurol Neurosurg Psychiatry 2007;78:908-9.
- Hurault D, El H, Comoz F, Lobbedez T, Pujo M, Griveau A, et al. Early loss of two renal grafts obtained from the same donor: role of ecstasy?. Transplantation 2005;80:153-6.
- Woodrow G, Harnden P, Turney JH. Acute renal failure due to accelerated hypertension following ingestion of 3,4-methylenedioxymethamphetamine (‘ecstasy’). Nephrol Dial Transplant 1995;10:399-400.
- Muddu AK, Wright M, Sheron N. ecstasy: an important cause of acute liver failure. Acute Med 2006;5:93-5.
- Kocak Z, Bulut C, Kinikli S, Irmak H, Yilmaz GR, Demiroez AP. A case report of ecstasy-induced acute hepatic failure. Turk J Med Sci 2006;36:319-21.
- Hwang I, Daniels A, Holtzmuller K. “Ecstasy”-induced hepatitis in an active duty soldier. Military Med 2002;167:155-6.
- Heneghan MA, Portmann BC, Norris SM, Williams R, Muiesan P, Rela M, et al. Graft dysfunction mimicking autoimmune hepatitis following liver transplantation in adults. Hepatology (Baltimore MD) 2001;34:464-70.
- Lawler LP, Abraham S, Fishman EK. 3,4-Methylenedioxymethamphetamine ecstasy-induced hepatotoxicity: multidetector CT and pathology findings. J Comput Assist Tomogr 2001;25:649-52.
- Garbino J, Henry JA, Mentha G, Romand JA. Ecstasy ingestion and fulminant hepatic failure: liver transplantation to be considered as a last therapeutic option. Vet Hum Toxicol 2001;43:99-102.
- Case records of the Massachusetts General Hospital Weekly clinicopathological exercises Case 6–2001 . A 17-year-old girl with marked jaundice and weight loss. N Engl J Med 2001;344:591-9.
- Jones C, Little K. Hepatorenal problems presented in an urban high dependancy unit in a user of ecstasy and cocaine. Accid Emerg Nurs 2000;8:20-3.
- Pereira SP, McCarthy M, Ellis AJ, Wendon J, Portmann B, Rela M, et al. Auxiliary partial orthotopic liver transplantation for acute liver failure. J Hepatol 1997;26:1010-17.
- Chenard N, Boudjema K, Bernuau J, Degott C, Belghiti J, Cherqui D, et al. Auxiliary liver transplantation: regeneration of the native liver and outcome in 30 patients with fulminant hepatic failure – a multicenter European study. Hepatology (Baltimore MD) 1996;23:1119-27.
- Khakoo SI, Coles CJ, Armstrong JS, Barry RE. Hepatotoxicity and accelerated fibrosis following 3,4-methylenedioxymetamphetamine (ecstasy) usage. J Clin Gastroenterol 1995;20:244-7.
- Oranje WA, von Pol P, van der Wurff A, Zeijen RN, Stockbruegger RW, Arends JW. XTC-induced hepatitis. Neth J Med 1994;44:56-9.
- Salacz M, Williams D, Mueller CV, Warm E. Ecstasy induced hepatic failure. J Gen Intern Med 2002;17.
- Rodgers J, Buchanan T, Pearson C, Parrott A, Ling J, Heffernan T, et al. Differential experiences of the psychobiological sequelae of ecstasy use: quantitative and qualitative data from an internet study. J Psychopharmacol 2006;20:437-46.
- Huizink A, Ferdinand R, van der Ende J, Verhulst F. Symptoms of anxiety and depression in childhood and use of MDMA: prospective, population based study. BMJ 2006;332:825-8.
- Gouzoulis M, Fischermann T, Rezk M, Thimm B, Hensen G, Daumann J. Memory performance in polyvalent MDMA (ecstasy) users who continue or discontinue MDMA use. Drug Alcohol Depend 2005;78:317-23.
- Verdejo G, Lopez T, Aguilar d, Perez G. Differential effects of MDMA, cocaine, and cannabis use severity on distinctive components of the executive functions in polysubstance users: a multiple regression analysis. Addict Behav 2005;30:89-101.
- Parrott AC, Buchanan T, Scholey AB, Heffernan T, Ling J, Rodgers J. ecstasy/MDMA attributed problems reported by novice, moderate and heavy recreational users. Hum Psychopharmacol Clin Exp 2002;17:309-12.
- Rodgers J, Buchanan T, Scholey AB, Heffernan TM, Ling J, Parrott A. Differential effects of ecstasy and cannabis on self-reports of memory ability: a web-based study. Hum Psychopharmacol Clin Exp 2001;16:619-25.
- Curran HV, Travill R. Mood and cognitive effects of ± 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”): week-end “high” followed by mid-week low. Addiction 1997;92:821-31.
- Krystal J, Price L. Chronic 3,4-methylenedioxymethamphetamine (MDMA) use: effects on mood and neuropsychological function?. Am J Drug Alcohol Abuse 1992;18:331-41.
- McCann U, Ricaurte G. Lasting neuropsychiatric sequelae of (±)methylenedioxymethamphetamine (“ecstasy”) in recreational users. J Clin Psychopharmacol 1991;11:302-5.
- Cohen RS, Cocores J. Neuropsychiatric manifestations following the use of 3,4- methylenedioxymethamphetamine MDMA: Ecstasy. Prog Neuro-Psychopharmacol Biol Psychiatry 1997;21:727-34.
- Marchesi C, Tonna M, Maggini C. Obsessive-compulsive disorder followed by psychotic episode in long-term ecstasy misuse. World J Biol Psychiatry 2007:1-4.
- Falck R, Wang J, Carlson R, Siegal H. Prevalence and correlates of current depressive symptomatology among a community sample of MDMA users in Ohio. Addict Behav 2006;31:90-101.
- Fetter J. Mirtazepine for MDMA-induced depression [letter]. Am J Addict 2005;14:300-1.
- Verheyden S, Maidment R, Curran HV. Quitting ecstasy: an investigation of why people stop taking the drug and their subsequent mental health. J Psychopharmacol 2003;17:371-8.
- Verheyden S, Henry J, Curran HV. Acute, sub-acute and long-term subjective consequences of ‘ecstasy’ (MDMA) consumption in 430 regular users. Hum Psychopharmacol Clin Exp 2003;18:507-17.
- Lerner A, Shufman E, Kodesh A, Kretzmer G, Sigal M. LSD-induced hallucinogen persisting perception disorder with depressive features treated with reboxetine: Case report. Isr J Psychiatry Related Sci 2002;39:100-3.
- Passie T, Schneider U, Emrich H. Persisting continuous visual perception disorder in a chronic MDMA (“ecstasy”) user. Aust N Z J Psychiatry 2002;36:266-7.
- Daumann J, Pelz S, Becker S, Tuchtenhagen F, Gouzoulis M. Psychological profile of abstinent recreational Ecstasy (MDMA) users and significance of concomitant cannabis use. Hum Psychopharmacol Clin Exp 2001;16:627-33.
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend 1997;44:87-94.
- Landabaso MA, Iraurgi I, Jimenez L, Calle R, Sanz J, Gutierrez F. Ecstasy-induced psychotic disorder: six-month follow-up study. Eur Addict Res 2002;8:133-40.
- Cohen R. Subjective reports on the effects of the MDMA (“ecstasy”) experience in humans. Prog Neuro-Psychopharmacol Biol Psychiatry 1995;19:1137-45.
- Schifano F, Magni G. MDMA (“Ecstasy”) abuse: psychopathological features and craving for chocolate: a case series. Biol Psychiatry 1994;36:763-7.
- McGuire P, Cope H, Fahy T. Diversity of psychopathology associated with use of 3,4-methylenedioxymethamphetamine (“Ecstasy”). Br J Psychiatry 1994;165:391-5.
- Teggin AF. Ecstasy – a dangerous drug. South Afr Med J 1992;81:431-2.
- Benazzi F, Mazzoli M. Psychiatric illness associated with ecstasy. Lancet 1991;338.
- Suilleabhain P, Giller C. Rapidly progressive parkinsonism in a self-reported user of ecstasy and other drugs. Movement Disord 2003;18:1378-81.
- Mintzer S, Hickenbottom S, Gilman S. Parkinsonism after taking ecstasy. New Engl J Med 1999;340.
- Trittibach P, Frueh B, Goldblum D. Bilateral angle-closure glaucoma after combined consumption of ecstasy and marijuana. Am J Emerg Med 2005;23:813-14.
- Michael J, Pak J, Pulido J, de Venecia G. Central serous chorioretinopathy associated with administration of sympathomimetic agents. Am J Ophthalmol 2003;136:182-5.
- Jacks AS, Hykin PG. Retinal haemorrhage caused by ecstasy. Br J Ophthalmol 1998;82:842-3.
- Reid L, Elifson K, Sterk C. Hug drug or thug drug? Ecstasy use and aggressive behavior. Violence and Victims 2007;22:104-19.
- Miller JM, Vorel SR, Tranguch AJ, Kenny ET, Mazzoni P, Van G, et al. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry 2006;163:786-8.
- Liester M, Grob C, Bravo G, Walsh R. Phenomenology and sequelae of 3,4-methylenedioxymethamphetamine use. J Nerv Ment Dis 1992;180:345-52.
- Barnett J, Werners U, Secher S, Hill K, Brazil R, Masson K, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry 2007;190:515-20.
- Creighton FJ, Black DL, Hyde CE. “Ecstasy” psychosis and flashbacks. Br J Psychiatry 1991;159:713-15.
- McCann U, Ricaurte G. MDMA (“Ecstasy”) and panic disorder: induction by a single dose. Biol Psychiatry 1992;32:950-3.
- Williams H, Meagher D, Galligan P. MDMA Ecstasy; a case of possible drug-induced psychosis. Irish J Med Sci 1993;162:43-4.
- McGuire P, Fahy T. Chronic paranoid psychosis after misuse of MDMA ecstasy. BMJ 1991;302.
- Shah HV, Irvine GH, Bradley M. Rhabdomyolysis of the masseter muscle: case report. Br J Oral Maxillofac Surg 2007.
- Swan M, Lam D, Giele H. Intravascular ecstasy: an unusual cause of thigh compartment syndrome. J Trauma 2006;60:1129-31.
- Verdone F. Rhabdomyolysis and 3,4 methylenedioxymethamphetamine in rheumatological practice. J Rheumatol 2001;28:1936-7.
- Behan WMH, Madigan M, Clark BJ, Goldberg J, McLellan DR. Muscle changes in the neuroleptic malignant syndrome. J Clin Pathol 2000;53:223-7.
- Carmondy J, Delanty N. Juvenile myoclonic epilepsy masquerading as ecstasy withdrawal. Irish Med J 2005;98.
- Campbell S, Qureshi T. Taking Ecstasy… it’s child’s play!. Paediatr Anaesth 2005;15:257-9.
- Cardaioli E, Da Pozzo P, Gallus G, Franceschini R, Rufa A, Dotti M, et al. Leber’s hereditary optic neuropathy associated with cocaine, ecstasy and telithromycin consumption. J Neurol 2007;254:255-6.
- Kumar RS, Grigg J, Farinelli AC. Ecstasy induced acute bilateral angle closure and transient myopia (5). Br J Ophthalmol 2007;91:693-5.
- Schroeder B, Brieden S. Bilateral sixth nerve palsy associated with MDMA ecstasy abuse. Am J Ophthalmol 2000;129:408-9.
- Neill D, Dart JK. Methylenedioxyamphetamine ‘Ecstasy’ associated keratopathy. Eye 1993;7:805-6.
- Hassan L, Carvalho C, Yannuzzi LA, Iida T, Negrao S. Central serous chorioretinopathy in a patient using methylenedioxymethamphetamine (MDMA) or “ecstasy”. Retina 2001;21:559-61.
- Tokcuoglu F, Celebisoy M, Oezdemirkiran T, Ruksen S. Brain stem infarction due to basilar artery occlusion as a complication of sympathomimetic drugs containing MDMA. Turk Serebrovaskuler Hast Derg 2007;13:25-7.
- Hanyu S, Ikeguchi K, Imai H, Imai N, Yoshida M. Cerebral infarction associated with 3,4-methylenedioxymethamphetamine ‘ecstasy’ abuse. Eur Neurol 1995;35.
- Zwick OM, Fischer DH, Flanagan JC. Ecstasy induced immunosuppression and herpes zoster ophthalmicus. Br J Ophthalmol 2005;89:923-24.
- Dubin NN, Razack AH. Priapism: ecstasy related?. Urology 2000;56.
- Leithaeuser B, Langheinrich A, Rau W, Tillmanns H, Matthias F. A 22-year-old woman with lower limb arteriopathy Buerger’s disease, or methamphetamine- or cannabis-induced arteritis?. Heart Vessels 2005;20:39-43.
- Williams A, Segal O, Andrews B. Short gastric artery perforation after use of ‘ecstasy’. J R Soc Med 1998;91:541-2.
Appendix 1 Expert Advisory Group
Dr Lewis Jones, Consultant in Emergency Medicine, Royal Devon and Exeter Hospital
Dr Michael Morgan, Senior Lecturer in Experimental Psychology, Department of Psychology, University of Sussex
Dr Jacqui Rodgers, Senior Academic Tutor and Lecturer in Clinical Psychology, Institute of Neuroscience, University of Newcastle
Appendix 2 Review protocol
Title of the project
The harmful health effects of recreational ecstasy: a systematic review of observational evidence
Name of TAR team and project lead
Group: Peninsula Technology Assessment Group (PenTAG)
Host institution: Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
Project co-ordinator: Mr Gabriel Rogers
Post held: Associate Research Fellow
Address: Noy Scott House Barrack Road Exeter EX2 5DW, UK
Telephone: 00 44 (0)1392 406971 [group administrator: 00 44 (0)1392 406966]
Fax: 00 44 (0)1392 406401
E-mail: gabriel.rogers@pms.ac.uk
Other staff
Dr Julian Elston, Academic Specialist Trainee in Public Health/Honorary Research Fellow
Ms Paula Younger, Electronic Resources Librarian
Ms Ruth Garside, Research Fellow
Dr Margaret Somerville, Senior Lecturer and Consultant in Public Health
Plain English summary
Street-drugs known as ecstasy have been sold for about 20 years in the UK. The active substance that such tablets contain – or purport to contain – is 3,4-methylenedioxymethamphetamine (MDMA). MDMA does not exist in nature; it can only be made chemically. Shortly after consumption, MDMA releases chemicals in the brain that tend to bring about a sense of euphoria, exhilaration and increased intimacy with others. In the UK, MDMA has been a Class A illegal substance for 30 years. This means that it is classified among the most dangerous drugs, and serious penalties are imposed for possession or supply. Most people who take ecstasy also use other legal and illegal drugs, sometimes at the same time. Ecstasy is commonly taken in nightclubs and at parties, and is very often associated with extended sessions of dancing.
Along with the pleasurable effects sought by users of MDMA, it has become clear that the drug can cause a range of unintended harms. In the short term, the most serious dangers arise when MDMA interferes with the body’s ability to maintain a constant temperature. In severe cases, multiple organ failure can develop, and this can prove swiftly fatal. To counteract this danger, ecstasy users are advised to drink plenty of fluid. However, some people overcompensate, drinking excessive amounts, and a condition can result in which the excess fluid leaks into the brain, causing it to swell, often with fatal consequences. A variety of other adverse events have been reported in the immediate aftermath of MDMA consumption, including heart failure, brain haemorrhage, and liver failure.
Consumption of MDMA may also have long-term consequences, especially as regards users’ mental health. People who have taken ecstasy in the past may have increased susceptibility to depression, and their memory may also be affected. There are other possible psychiatric effects, some of them serious.
This project will systematically review the medical literature detailing the harms to human health from ecstasy. Electronic databases will be searched for journal articles describing the incidence and impact of adverse events. The identified material will be analysed and summarised. Consideration will be given to the features of the evidence that may make its interpretation complex (for example: to what extent is it possible to isolate the long-term harms of MDMA from those of the other substances that users have almost always taken?) If several papers report the same kind of numerical information, these data will be combined by meta-analysis. An effort will also be made to analyse factors that might make some types of user more or less likely to suffer an adverse event.
Scope of the review
Review question
What are the harmful health effects of taking 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) for recreational use?
Background
Ecstasy is the common street-name for drugs that contain – or purport to contain – 3,4-methylenedioxymethamphetamine (MDMA) as their active ingredient. Following the convention of Gowing et al. ,P1 the term ecstasy is used here to denote the drug as it is sold on the street, whereas MDMA refers to the known chemical substance.
Pharmacology
MDMA is an entirely synthetic chemical belonging to the amphetamine family, a group of phenethylamines. Several substances that are closely related in chemical structure are also commonly used as recreational drugs:
-
amphetamine (‘speed’, ‘whizz’)
-
methamphetamine (MA; ‘crystal meth’)
-
paramethoxyamphetamine (PMA)
-
3,4-methylenedioxyamphetamine (MDA)
-
3,4-methylenedioxy-N-ethylamphetamine (MDEA; ‘Eve’)
-
3,4-methylenedioxy-phenyl-N-methylbutanamine (MBDB).
Drugs sold as ‘ecstasy’ frequently contain one or more of these substances, instead of or in addition to MDMA. P2 The intended effects for which ecstasy users take the drug are described in terms of euphoria, exhilaration and a sense of increased intimacy and empathy with others. P3 The neuropharmacological mechanisms by which these effects are produced involve the release of extracellular serotonin and dopamine,P4 neurotransmitters that are commonly associated with the mood and pleasure systems of the brain.
The physiological effects of MDMA in humans have been studied in controlled conditions. Heart rate rises to a peak an average of 20–30 beats per minute higher than baselineP5–P7 approximately an hour after consumption of doses similar to those taken recreationally. Blood pressure increases over a similar period (systolic by 25–40 mmHg, diastolic by 10–20 mmHg). P5–P7 Body temperature also rises (by 0.3–1.0°C), but this effect is less immediate, with a peak several hours after consumption. P5,P7,P8 The apparently non-linear nature of MDMA pharmacokinetics has been emphasised; blood concentrations of MDMA rise disproportionately as dosage is increased. P9
History
The first documentary record of the synthesis of MDMA is the 1912 German patent application of Merck pharmaceuticals in relation to haemostasis,P10 but it was not tested on humans until 1960. P10 Following very sporadic reports in the 1970s, recreational use of MDMA became more widespread during the 1980s,P11 with discussion proliferating in the popular press in 1985. P12 The term ecstasy first appeared in print in reference to MDMA in 1985P13 and in the British media in 1987. P14
The US Drug Enforcement Administration classified MDMA as a Schedule 1 controlled substance with effect from 1 July 1985,P15 In the UK, it had already been criminalised; a statutory instrument of 1977, without naming MDMA in particular, categorised all ring-substituted phenethylamines as Class A substances under the Misuse of Drugs Act. P16
In the UK, reported ecstasy consumption has remained relatively stable over the past decade, with somewhere in the region of 2% of 16- to 59-year-olds reporting ecstasy use in the preceding 12 months (Office for National Statistics crime survey). This makes it the third most-used illegal drug in the UK. It has been estimated that somewhere between 500000 and 2 million doses of ecstasy are consumed each week in the UK. P17
Usage
The overwhelming pattern of ecstasy usage is as a part of polydrug consumption. P18 A 2003 survey of UK ecstasy-using respondents also reported extensive concomitant use of alcohol (88%), amphetamines (83%), cannabis (82%), cocaine (58%) and amyl nitrate (51%), and there was also some use of LSD, ketamine, fluoxetine, crack cocaine, herbal highs and sildenafil. In addition, various substances were used in the ‘comedown’ period following ecstasy consumption, most notably cannabis (82%), alcohol (60%), benzodiazepines (18%) and heroin (2%).
Ecstasy tablets as sold on the street contain a variable amount of MDMA, ranging from none to around 150 mg. P2 As noted above, some tablets contain MDEA, MDA and/or amphetamine in addition to or instead of MDMA. Ecstasy tablets may also be ‘cut’ with unrelated substances. Many of these are pharmacologically weak (e.g. caffeine, paracetamol); however, there are reports of stronger psychoactive substances (e.g. atropine, opiates, phenylbutanamine and dextromethorphan). P2 One US source analysed tablets in 2005–7 and found them to have approximately a one in three chance of containing only MDMA, MDMA along with other active ingredients, or no MDMA at all. P19
As a result of these factors, it is not possible to isolate exposure to MDMA in particular in any individual history or in characteristics across cohorts. Even if there were such a thing as an identifiable group of individuals whose ecstasy consumption alone distinguished them from the general population, it would still be impossible to ascertain to which chemicals they had been exposed, and at what dosage.
Safety
Reports from investigators assessing the psychotherapeutic potential of MDMA in 1986 suggested that the drug was ‘apparently physically safe’, despite some ‘undesirable’ effects. P20 Within a year of such claims, the first reports of ecstasy-related deaths appeared in the medical literature. P21 In the UK, the first reported fatalities came in 1991. P22,P23 Over the past 20 years, a wide variety of fatal and non-fatal complications have been ascribed to consumption of ecstasy.
Acute harms
Major syndromes
The most critical acute complications of MDMA consumption are, in a majority of cases, related to two well recognised syndromes, each involving serious derangement of homeostasis leading to multiple organ failure: hyperthermia and hyponatraemia.
Derangements of thermoregulation are a widely reported feature of MDMA toxicity,P24 with temperatures as high as 43°C reported in some cases. P25–P29 In this context, the association between MDMA use and prolonged dancing may be important because core temperature rises during intensive exercise. P30
The physiological manifestations of MDMA-induced hyperthermia are similar to those seen in severe heatstroke. P31 The most noteworthy effects are rhabdomyolysis, disseminated intravascular coagulopathy (DIC), acute renal failure (ARF) and acute liver failure.
When the hyperthermogenic properties of MDMA are combined with intense physical activity, profuse sweating inevitably results, and substantial amounts of sodium can be lost in perspiration. This problem is significantly compounded by the tendency of MDMA users to drink large quantities of water. The combination of sodium loss and excess water consumption may also be exacerbated by excess fluid retention (as the result of inappropriate secretion of antidiuretic hormones and/or impairment of renal functionP32). The resultant hyponatraemic state sees a fall in serum osmolar pressure, allowing intracellular displacement of water, the most hazardous result of which is cerebral oedema. P32
The early clinical manifestations of hyponatraemic cerebral oedema are headache and nausea, progressing to confusion and seizures. P32–P35 If not corrected the syndrome will commonly progress to tentorial herniation, respiratory arrest, cerebral hypoxia and death.
Subgroup effects may be an issue. Regardless of cause, hyponatraemia is known to be most hazardous in women, especially during menstruation. P36
Isolated acute harms
Tachycardia is an invariable response to MDMA consumption, and is the most frequently reported clinical symptom in series detailing acute admissions in accident and emergency departments. P37,P38 There are reports of MDMA-related myocardial infarctionP39–P42 and sudden cardiac death. P43 The importance of excluding concomitant use of other drugs (especially cocaine, which is well known to induce critical cardiovascular dysfunction) has been emphasised. P44
Seizures are a recognised manifestation of both hyponatraemia and hyperthermia (see above). Cases have also been reported of MDMA-induced seizures which apparently do not involve either of these underlying causes. P45
There are several reports of intracranial haemorrhage following consumption of MDMA. Such events are commonly associated with pre-existing cerebrovascular vulnerabilities (e.g. aneurysmP46,P47 or arteriovenous malformationP48,P49); however, cases have also been reported in which no such features were identified. P49,P50
Chest pain secondary to pneumomediastinum (leakage from the airways into the mediastinum; also known as mediastinal emphysema) is a relatively commonly reported condition in those presenting for medical attention following MDMA consumption. P51–P63 Less frequently, pneumothoraxP55,P59 and pneumopericardiumP58 have also been reported.
Critical hepatic dysfunction is a notable consequence of hyperthermia (see above), and extensive hepatic necrosis is an invariable post mortem finding in MDMA deaths. P64 In addition, it is well established that MDMA-induced acute liver failure can also occur without thermoregulatory dysfunction. P28,P65–P74 This type of acute hepatic failure develops over a slightly longer period than in hyperthermic liver failure, with cases becoming symptomatic a matter of days, rather than hours, after MDMA ingestion.
Occasionally, MDMA-induced kidney dysfunction can occur in the absence of hyperthermia or hyponatraemia. Such cases are frequently associated with severe hypertension. P75–P77
Long-term harms
For all potential long-term harms, it is extremely difficult to disentangle the long-term effects of MDMA use from those of the other legal and illegal substances with which the histories of users are invariably confounded. P85
Neuropsychiatric sequelae
While short-term depression of mood in the few days following MDMA use is a common finding in qualitativeP86,P87 and observationalP88 literature, the long-term neuropsychiatric effects of MDMA use are the subject of much research and are widely believed to be irreversible. P89 Some biochemical analyses have shown depletion of serotonin metabolites in the cerebrospinal fluid of human MDMA users, from which permanent impairment of serotonergic function is inferred. P90 The impact of MDMA consumption on dopamine activity has been a more controversial topic. A variety of clinical manifestations may result. Studies have most commonly examined the impact of MDMA use on depression, neurocognitive impairment (with a particular focus on both short- and long-term memory), psychomotor dysfunction and psychotic symptomatology.
It is hypothesised that, if MDMA use compromises serotonergic function, long-term consumption can be expected to result in chronic depression of mood. P91
It is suggested that recreational MDMA use is associated with deficits in general neurocognitive function,P92 with particularly strong evidence of short- and long-term memory impairment. P92–P94
Other long-term harms
Trismus and bruxism are very frequent characteristics of MDMA intoxication,P3 and excessive toothwear can result. The problem may be exacerbated by consumption of carbonated drinks, which is common. P102
There is some discussion about whether long-term exposure to MDMA predisposes users to epilepsy. P33
Methods for systematic review of evidence
Inclusion/exclusion criteria
The relevance of all evidence will be appraised with respect to the following criteria.
Population
Included
-
Users of recreational drugs in the UK or in populations relevant to the UK.
Excluded
-
Animal studies.
-
Non-drug-using volunteers enrolled in prospective research.
-
Gamma-hydroxybutyric acid (GHB, ‘liquid ecstasy’).
Exposures
Included
-
Recreational use of substances shown or believed by the investigator(s) to contain MDMA.
Excluded
-
Use of street-drugs shown or believed by the investigator(s) not to contain MDMA, whether referred to as ‘ecstasy’ or not.
-
Therapeutic use of MDMA.
-
Generic drug-using populations in which it is not possible to isolate a subgroup with exposure to MDMA in particular.
Comparators
Some uncontrolled evidence will be considered in the review, where appropriate (see below). Where comparative evidence is reviewed, studies with comparator arm(s) meeting the following characteristics will be eligible.
Included
-
Recreational users of drugs other than MDMA.
-
Non-drug-users.
Outcomes
Included
-
Death
-
Acute, clinically observable health harms, including (but not limited to)
-
hyperpyrexia
-
hyponatraemia
-
acute cardiovascular dysfunction
-
acute neurological dysfunction (seizures)
-
acute renal failure/anuria
-
acute liver failure (including ‘subacute’ liver failure and hepatitis)
-
intracranial haemorrhage
-
respiratory dysfunction (including pneumomediastinum and pneumothorax)
-
rhabdomyolysis
-
disseminated intravascular coagulopathy
-
acute ophthalmic injury (including retinal haemorrhage, keratopathy, glaucoma, diplopia, myopia).
-
-
Long term, clinically observable health harms, including (but not limited to)
-
neuropsychiatric sequelae (including depression, psychosis, memory impairment, disorders of neurocognition, psychomotor symptoms)
-
-
Dental damage.
Excluded
-
Surrogate measures of harm (e.g. neuroimaging studies, biochemical markers), where there is no explicit correlation to observed effect
-
Biochemical indices of MDMA consumption (e.g. testing for MDMA use in blood or hair samples)
-
Studies reporting therapeutic measures for adverse events without providing data on individuals suffering such complications
-
Subjective measures of psychostimulation (i.e. studies of the drug’s intended short-term intoxicative effects)
-
Indirect harms
-
accidental injury where ecstasy consumption is detected/implicated
-
health consequences of high-risk sexual behaviour contributed to by ecstasy consumption
-
-
Birth defects secondary to maternal exposure to MDMA.
Methods
Except where otherwise specified, the general methods of the review will follow the guidance on the conduct of systematic reviews published by the Centre for Reviews and Dissemination. P103
Identification of evidence
The search strategy will comprise the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
scrutiny of bibliographies of retrieved papers.
Search strategy for electronic databases
A comprehensive search syntax using indexed keywords (e.g. MeSH, EMTREE) and free-text terms will be developed. This will build upon the search syntax devised and used for the scoping searches (Preliminary search strategy).
Databases to be searched
The electronic databases that will be searched include: MEDLINE, EMBASE and PsycINFO (all via Dialog DataStar); PubMed (limited to recent publications and in-process citations); Web of Knowledge; the Cochrane Library (including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register); DARE; NHS HTA database. Simple keywords (e.g. ‘Ecstasy’; ‘MDMA’) will also be used to consult research registers, to identify any relevant prospective studies.
Inclusion of relevant evidence
The outputs of searches will be considered against the prespecified inclusion/exclusion criteria, with a sample of citations screened by a second reviewer, to appraise validity of assessment. Studies that can confidently be identified as not meeting eligibility criteria on the basis of title and abstract will be excluded. The full texts of all other papers will be obtained. Two reviewers will independently assess whether these studies fulfil the inclusion criteria, with disagreements resolved by consensus.
Papers in languages other than English
As a result of the time restraints on this project, only studies published in English will be included in the review.
Meeting abstracts
Reports published as meeting abstracts will only be included in the review if sufficient methodological details are reported to allow critical appraisal of study quality.
Methods of analysis/synthesis
General approach
Initially, all included evidence will be reviewed to establish a taxonomy of reported outcomes. For each outcome, the available evidence will be categorised in a predefined hierarchy of research design:
-
Level I Pre-existing systematic research syntheses (systematic reviews, meta-analyses, syntheses of qualitative data)
-
Level II Controlled observational studies (cohort studies, case–control studies, etc.)
-
Level III Uncontrolled observational evidence (case reports and case series).
Where it is adequately designed and conducted (see below for methods of critical appraisal), Level I evidence will be preferred. Any such synthesis of primary research can be expected to include consideration of all relevant Level II evidence, if it is appropriately comprehensive. Accordingly, where reasonable-quality Level I evidence is available for a given outcome, Level II evidence will only be considered to the extent that it supplements the pre-existing syntheses. For example, Level II studies that post-date the higher-level evidence will be reviewed and appraised. Where possible and appropriate, attempts will be made to extend any quantitative analyses contained in Level I evidence to include such additional evidence. Where no adequate Level I evidence is identified for a given outcome, any Level II evidence will be systematically reviewed. The quality of research will be appraised and described, and findings reported. Where possible and appropriate, quantitative synthesis of study outcomes will also be undertaken (for methods, see below). A brief tabulation and/or summary of Level III evidence will be provided.
Where neither Level I nor Level II evidence is available, Level III evidence will be systematically surveyed.
Critical appraisal of evidence
The internal validity of included studies will be assessed using methods appropriate to study design.
Systematic reviews of observational evidence will be appraised with reference to a quality assessment instrument adapted from the recommendations of the MOOSE (Meta-analysis of Observational Studies in Epidemiology) proposal. P104
Cohort studies and case–control studies will be appraised using a bespoke quality assessment instrument, which will be constructed with reference to recommendations made by Levine et al. ,P105 Downs and Black,P106 the NHS Centre for Reviews and Dissemination (2004)P103 and Mallen et al. P107
Case series and case reports will be appraised using a bespoke quality assessment instrument, which will be constructed with reference to the findings of Dalziel et al. P108
Data extraction
Data will be extracted using a bespoke database. Recorded information, where available, will include:
-
study design (e.g. design, country, setting, dates, length of follow-up)
-
details of study participants, including
-
baseline demographics (e.g. age, gender)
-
previous exposure to ecstasy and other legal and illegal substances)
-
-
details of exposure, including
-
details of ecstasy consumed (e.g. number of tablets, MDMA content, other substances contained in tablets)
-
other substances consumed (e.g. alcohol, other recreational drugs)
-
-
outcome data, including
-
quantitative data describing key study outcomes
-
-
inter-cohort comparisons.
All extracted data will be checked by a second reviewer. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
Quantitative synthesis
Where it is possible and appropriate, meta-analysis will be carried out using random-effects models by default. If there is statistical evidence of inter-study homogeneity and no reason to suspect clinical heterogeneity, sensitivity analyses using fixed-effects models will be undertaken. stata software will be used to pool results and estimate an overall effect measure. Heterogeneity will be explored through consideration of the study populations, methods and exposures, by visualisation of results and, in statistical terms, by the chi-squared test for homogeneity and the I2 statistic. Small-study effects (including publication bias) will be assessed and quantified.
Subgroup effects
For all outcomes, consideration will be given to the possibility of differential effects existing in subgroups (e.g. by age group, by gender, by exposure to other substances, etc.) Where quantitative synthesis is undertaken, stratified analyses and metaregression, using potential predictors of effect size as covariates, will be considered.
Expertise in the review team
Peninsula Technology Assessment Group
The Peninsula Technology Assessment Group is part of the Institute of Health and Social Care Research at the Peninsula Medical School. PenTAG was established in 2000 and carries out independent Health Technology Assessments for the UK HTA Programme and other local and national decision-makers. The group is multi-disciplinary and draws on individuals’ backgrounds in public health, health services research, computing and decision analysis, systematic reviewing, statistics and health economics. The Peninsula Medical School is part of the Peninsula College of Medicine and Dentistry within the Universities of Plymouth and Exeter.
Team members
The PenTAG team members who will undertake the project have previously produced reports for NICE, the Health Technology Assessment Programme and the Department of Health. These projects have included Technology Assessment Reports, National Guidelines, and short reports. The members of the project team and their role in the project are listed below.
| Mr Gabriel Rogers, Associate Research Fellow |
Responsible for project coordination Responsible for drafting the protocol Contributor to devising the search strategy Contributor to review (study selection; data extraction and checking; critical appraisal of studies; data synthesis) Contributor to drafting report (all sections) Responsible for compiling and editing report |
| Dr Julian Elston, Academic Specialist Trainee in Public Health/Honorary Research Fellow |
Contributor to review (study selection; data extraction and checking; critical appraisal of studies; data synthesis) Contributor to drafting report (results; discussion) |
| Ms Paula Younger, Electronic Resources Librariana |
Responsible for devising the search strategy Responsible for conducting the literature searches Contributor to drafting report (methods; results) |
| Ms Ruth Garside, Research Fellow |
Co-responsible for project direction Contributor to drafting report (executive summary; discussion) |
| Dr Margaret Somerville, Principal Lecturer and Consultant in Public Health |
Co-responsible for project direction Contributor to drafting report (executive summary; discussion) |
Competing interests of authors
None.
Timetable
The report will be delivered to NCCHTA by 20 December 2007.
References
- Gowing LR, Henry-Edwards SM, Irvine RJ, Ali RL. The health effects of ecstasy: a literature review. Drug Alcohol Rev 2002;21:53-6.
- Parrott AC. Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology 2004;173:234-41.
- Baylen CA, Rosenberg H. A review of the acute subjective effects of MDMA/ecstasy. Addiction 2006;101:933-47.
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol 1996;49:455-79.
- Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 1999;290:136-45.
- Lester SJ, Baggott M, Welm S, Schiller NB, Jones RT, Foster E, et al. Cardiovascular effects of 3,4-methylenedioxymethamphetamine. A double-blind, placebo-controlled trial. Ann Intern Med 2000;133:969-73.
- de la Torre R, Farre M, Roset PN, Lopez CH, Mas M, Ortuno J, et al. Pharmacology of MDMA in humans. Ann N Y Acad Sci 2000;914:225-37.
- Freedman RR, Johanson CE, Tancer ME. Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2005;183:248-56.
- de la Torre R, Farre M, Ortuno J, Mas M, Brenneisen R, Roset PN, et al. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol 2000;49:104-9.
- Freudenmann RW, Oxler F, Bernschneider-Reif S. The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents. Addiction 2006;101:1241-5.
- Seymour RB. MDMA: another view of Ecstasy. Pharmchem Newsletter 1985;14:1-4.
- Shulgin AT. The background and chemistry of MDMA. J Psychoactive Drugs 1986;18:291-304.
- Los Angeles Times 1985;Mar 28.
- Six held after drug seizure. The Times 1987;May 14.
- DEA Will Ban Hallucinogen Known to Users as ‘Ecstasy’. The Washington Post 1985;Jun 1.
- Misuse of Drugs Act 1971 (Modification) Order 1977.
- Hall AP, Henry JA. Acute toxic effects of ‘ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth 2006;96:678-85.
- Schifano F, Di Furia L, Forza G, Minicuci N, Bricolo R. MDMA (‘ecstasy’) consumption in the context of polydrug abuse: a report on 150 patients. Drug Alcohol Depend 1998;52:85-90.
- Summary Statistics for EcstasyData.Org Lab Testing Results n.d. URL: http://www.ecstasydata.org/datastats.php.
- Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. J Psychoactive Drugs 1986;18:319-27.
- Dowling GP, McDonough ET, Bost RO. ‘Eve’ and ‘Ecstasy’. A report of five deaths associated with the use of MDEA and MDMA. JAMA 1987;257:1615-17.
- Chadwick IS, Curry PD, Linsley A, Freemont AJ, Doran B. Ecstasy, 3–4 methylenedioxymethamphetamine (MDMA), a fatality associated with coagulopathy and hyperthermia. J R Soc Med 1991;84.
- Campkin NT, Davies UM. Another death from Ecstasy. J R Soc Med 1992;85.
- Green AR, O’Shea E, Colado MI. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur J Pharmacol 2004;500:3-13.
- Connolly E, O’Callaghan G. MDMA toxicity presenting with severe hyperpyrexia: a case report. Crit Care Resusc 1999;1:368-70.
- Greene SL, Dargan PI, O’Connor N, Jones AL, Kerins M. Multiple toxicity from 3,4-methylenedioxymethamphetamine (‘ecstasy’). Am J Emerg Med 2003;21:121-4.
- Ferrie R, Loveland RC. Bilateral gluteal compartment syndrome after ‘ecstasy’ hyperpyrexia. J R Soc Med 2000;93.
- Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (‘ecstasy’). Lancet 1992;340:384-7.
- Screaton GR, Singer M, Cairns HS, Thrasher A, Sarner M, Cohen SL. Hyperpyrexia and rhabdomyolysis after MDMA (‘ecstasy’) abuse. Lancet 1992;339:677-8.
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol 1966;21:1757-62.
- Kalant H. The pharmacology and toxicology of ‘ecstasy’ (MDMA) and related drugs. CMAJ 2001;165:917-28.
- Hartung TK, Schofield E, Short AI, Parr MJ, Henry JA. Hyponatraemic states following 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) ingestion. QJM 2002;95:431-7.
- Giorgi FS, Lazzeri G, Natale G, Iudice A, Ruggieri S, Paparelli A, et al. MDMA and seizures: a dangerous liaison?. Ann N Y Acad Sci 2006;1074:357-64.
- Sue YM, Lee YL, Huang JJ. Acute hyponatremia, seizure, and rhabdomyolysis after ecstasy use. J Toxicol Clin Toxicol 2002;40:931-2.
- Magee C, Staunton H, Tormey W, Walshe JJ. Hyponatraemia, seizures and stupor associated with ecstasy ingestion in a female. Ir Med J 1998;91.
- Arieff AI. Management of hyponatraemia. BMJ 1993;307:305-8.
- Liechti ME, Kunz I, Kupferschmidt H. Acute medical problems due to Ecstasy use. Case-series of emergency department visits. Swiss Med Weekly 2005;135:652-7.
- Williams H, Dratcu L, Taylor R, Roberts M, Oyefeso A. ‘Saturday night fever’: ecstasy related problems in a London accident and emergency department. J Accid Emerg Med 1998;15:322-6.
- Sadeghian S, Darvish S, Shahbazi S, Mahmoodian M. Two ecstasy-induced myocardial infarctions during a three month period in a young man. Arch Iran Med 2007;10:409-12.
- Lai TI, Hwang JJ, Fang CC, Chen WJ. Methylene 3, 4 dioxymethamphetamine-induced acute myocardial infarction. Ann Emerg Med 2003;42:759-62.
- Qasim A, Townend J, Davies MK. Ecstasy induced acute myocardial infarction. Heart 2001;85.
- Duflou J, Mark A. Aortic dissection after ingestion of ‘ecstasy’ (MDMA). Am J Forensic Med Pathol 2000;21:261-3.
- Suarez RV, Riemersma R. ‘Ecstasy’ and sudden cardiac death. Am J Forensic Med Pathol 1988;9:339-41.
- Rella JG, Murano T. Ecstasy and acute myocardial infarction. Ann Emerg Med 2004;44:550-1.
- Sawyer J, Stephens WP. Misuse of ecstasy. BMJ 1992;305.
- Auer J, Berent R, Weber T, Lassnig E, Eber B. Subarachnoid haemorrhage with ‘Ecstasy’ abuse in a young adult. Neurol Sci 2002;23:199-201.
- Gledhill JA, Moore DF, Bell D, Henry JA. Subarachnoid haemorrhage associated with MDMA abuse. J Neurol Neurosurg Psychiatry 1993;56:1036-7.
- Selmi F, Davies KG, Sharma RR, Neal JW. Intracerebral haemorrhage due to amphetamine abuse: report of two cases with underlying arteriovenous malformations. Br J Neurosurg 1995;9:93-6.
- Harries DP, De SR. ‘Ecstasy’ and intracerebral haemorrhage. Scott Med J 1992;37:150-2.
- Yin Foo LG, Wooi Kee GG, Vrodos N, Patrick BB. ‘Ecstasy’-induced subarachnoid haemorrhage: an under-reported neurological complication?. J Clin Neurosci 2003;10:705-7.
- Mutlu H, Silit E, Pekkafali Z, Incedayi M, Basekim C, Kizilkaya E. ‘Ecstasy’(MDMA)-induced pneumomediastinum and epidural pneumatosis. Diagn Interv Radiol 2005;11:150-1.
- Hutchison RP, Burgess B. Spontaneous pneumomediastinum – a right pain in the neck?. Injury 2005;36:801-3.
- Bernaerts A, Verniest T, Vanhoenacker F, Van den BP, Petre C, De Schepper AM. Pneumomediastinum and epidural pneumatosis after inhalation of ‘Ectasy’. Eur Radiol 2003;13:642-3.
- Rejali D, Glen P, Odom N. Pneumomediastinum following ecstasy (methylenedioxymetamphetamine, MDMA) ingestion in two people at the same ‘rave’. J Laryngol Otol 2002;116:75-6.
- Mazur S, Hitchcock T. Spontaneous pneumomediastinum, pneumothorax and ecstasy abuse. Emerg Med (Fremantle) 2001;13:121-3.
- Ryan J, Banerjee A, Bong A. Pneumomediastinum in association with MDMA ingestion. J Emerg Med 2001;20:305-6.
- Quin GI, McCarthy GM, Harries DK. Spontaneous pneumomediastinum and ecstasy abuse. J Accid Emerg Med 1999;16.
- Ahmed JM, Salame MY, Oakley GD. Chest pain in a young girl. Postgrad Med J 1998;74:115-16.
- Pittman JA, Pounsford JC. Spontaneous pneumomediastinum and Ecstasy abuse. J Accid Emerg Med 1997;14:335-6.
- Rezvani K, Kurbaan AS, Brenton D. Ecstasy induced pneumomediastinum. Thorax 1996;51:960-1.
- Onwudike M. Ecstasy induced retropharyngeal emphysema. J Accid Emerg Med 1996;13:359-61.
- Levine AJ, Drew S, Rees GM. ‘Ecstasy’ induced pneumomediastinum. J R Soc Med 1993;86:232-3.
- Ng CP, Chau LF, Chung CH. Massive spontaneous haemopneumothorax and ecstasy abuse. Hong Kong J Emerg Med 2004;11:94-7.
- Milroy CM, Clark JC, Forrest AR. Pathology of deaths associated with ‘ecstasy’ and ‘eve’ misuse. J Clin Pathol 1996;49:149-53.
- Brncic N, Kraus I, Viskovic I, Mijandrusic-Sincic B, Vlahovic-Palcevski V. 3,4-methylenedioxymethamphetamine (MDMA): an important cause of acute hepatitis. Med Sci Monit 2006;12:CS107-9.
- Caballero F, Lopez-Navidad A, Cotorruelo J, Txoperena G. Ecstasy-induced brain death and acute hepatocellular failure: multiorgan donor and liver transplantation. Transplantation 2002;74:532-7.
- De Carlis L, De Gasperi A, Slim AO, Giacomoni A, Corti A, Mazza E, et al. Liver transplantation for ecstasy-induced fulminant hepatic failure. Transplant Proc 2001;33:2743-4.
- Andreu V, Mas A, Bruguera M, Salmeron JM, Moreno V, Nogue S, et al. Ecstasy: a common cause of severe acute hepatotoxicity. J Hepatol 1998;29:394-7.
- Brauer RB, Heidecke CD, Nathrath W, Beckurts KT, Vorwald P, Zilker TR, et al. Liver transplantation for the treatment of fulminant hepatic failure induced by the ingestion of ecstasy. Transpl Int 1997;10:229-33.
- Fidler H, Dhillon A, Gertner D, Burroughs A. Chronic ecstasy (3,4-methylenedioxymetamphetamine) abuse: a recurrent and unpredictable cause of severe acute hepatitis. J Hepatol 1996;25:563-6.
- Ellis AJ, Wendon JA, Portmann B, Williams R. Acute liver damage and ecstasy ingestion. Gut 1996;38:454-8.
- Dykhuizen RS, Brunt PW, Atkinson P, Simpson JG, Smith CC. Ecstasy induced hepatitis mimicking viral hepatitis. Gut 1995;36:939-41.
- Shearman JD, Chapman RW, Satsangi J, Ryley NG, Weatherhead S. Misuse of ecstasy. BMJ 1992;305.
- Gorard DA, Davies SE, Clark ML. Misuse of ecstasy. BMJ 1992;305.
- Woodrow G, Harnden P, Turney JH. Acute renal failure due to accelerated hypertension following ingestion of 3,4-methylenedioxymethamphetamine (‘ecstasy’). Nephrol Dial Transplant 1995;10:399-400.
- Woodrow G, Turney JH. Ecstasy-induced renal vasculitis. Nephrol Dial Transplant 1999;14.
- Bingham C, Beaman M, Nicholls AJ, Anthony PP. Necrotizing renal vasculopathy resulting in chronic renal failure after ingestion of methamphetamine and 3,4-methylenedioxymethamphetamine (‘ecstasy’). Nephrol Dial Transplant 1998;13:2654-5.
- Verdone F. Rhabdomyolysis and 3,4 methylenedioxymethamphetamine in rheumatological practice. J Rheumatol 2001;28:1936-7.
- Halachanova V, Sansone RA, McDonald S. Delayed rhabdomyolysis after ecstasy use. Mayo Clin Proc 2001;76:112-13.
- Jacks AS, Hykin PG. Retinal haemorrhage caused by ‘ecstasy’. Br J Ophthalmol 1998;82:842-3.
- O’Neill D, Dart JK. Methylenedioxyamphetamine (‘Ecstasy’) associated keratopathy. Eye 1993;7:805-6.
- Trittibach P, Frueh BE, Goldblum D. Bilateral angle-closure glaucoma after combined consumption of ‘ecstasy’ and marijuana. Am J Emerg Med 2005;23:813-14.
- Schroeder B, Brieden S. Bilateral sixth nerve palsy associated with MDMA (‘ecstasy’) abuse. Am J Ophthalmol 2000;129:408-9.
- Kumar RS, Grigg J, Farinelli AC. Ecstasy induced acute bilateral angle closure and transient myopia. Br J Ophthalmol 2007;91:693-5.
- Gouzoulis-Mayfrank E, Daumann J. The confounding problem of polydrug use in recreational ecstasy/MDMA users: a brief overview. J Psychopharmacol 2006;20:188-93.
- Rodgers J, Buchanan T, Pearson C, Parrott AC, Ling J, Hefferman TM, et al. Differential experiences of the psychobiological sequelae of ecstasy use: quantitative and qualitative data from an internet study. J Psychopharmacol 2006;20:437-46.
- Davison D, Parrott AC. Ecstasy (MDMA) in recreational users: self-reported psychological and physiological effects. Hum Psychopharmacol Clin Exp 1997;12:221-6.
- Parrott AC, Lasky J. Ecstasy (MDMA) effects upon mood and cognition: before, during and after a Saturday night dance. Psychopharmacology (Berl) 1998;139:261-8.
- Ricaurte GA, Yuan J, McCann UD. (+/–)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 2000;42:5-10.
- McCann UD, Eligulashvili V, Ricaurte GA. (+/–)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: clinical studies. Neuropsychobiology 2000;42:11-6.
- Montoya AG, Sorrentino R, Lukas SE, Price BH. Long-term neuropsychiatric consequences of ‘ecstasy’ (MDMA): a review. Harv Rev Psychiatry 2002;10:212-20.
- Smilkstein MJ, Smolinske SC, Rumack BH. A case of MAO inhibitor/MDMA interaction: agony after ecstasy. J Toxicol Clin Toxicol 1987;25:149-59.
- Verbaten MN. Specific memory deficits in ecstasy users? The results of a meta-analysis. Hum Psychopharmacol 2003;18:281-90.
- Laws KR, Kokkalis J. Ecstasy (MDMA) and memory function: a meta-analytic update. Hum Psychopharmacol 2007;22:381-8.
- Lamers CT, Bechara A, Rizzo M, Ramaekers JG. Cognitive function and mood in MDMA/THC users, THC users and non-drug using controls. J Psychopharmacol 2006;20:302-11.
- Topp L, Hando J, Dillon P, Roche A, Solowij N. Ecstasy use in Australia: patterns of use and associated harm. Drug Alcohol Depend 1999;55:105-15.
- Parrott AC, Rodgers J, Buchanan T, Scholey AB, Heffernan T, Ling J. The reality of psychomotor problems, and the possibility of Parkinson’s disorder, in some recreational ecstasy/MDMA users: a rejoinder to Sumnall et al. (2003). Psychopharmacology (Berl) 2004;171:231-3.
- O’Suilleabhain P, Giller C. Rapidly progressive parkinsonism in a self-reported user of ecstasy and other drugs. Mov Disord 2003;18:1378-81.
- Kuniyoshi SM, Jankovic J. MDMA and Parkinsonism. N Engl J Med 2003;349:96-7.
- Mintzer S, Hickenbottom S, Gilman S. Parkinsonism after taking ecstasy. N Engl J Med 1999;340.
- Winstock AR. Chronic paranoid psychosis after misuse of MDMA. BMJ 1991;302:1150-1.
- Milosevic A, Agrawal N, Redfearn P, Mair L. The occurrence of toothwear in users of Ecstasy (3,4-methylenedioxymethamphetamine). Community Dent Oral Epidemiol 1999;27:283-7.
- Undertaking Systematic Reviews of Research on Effectiveness. York: NHS Centre for Reviews and Dissemination; 2001.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008-12.
- Levine M, Walter S, Lee H, Haines T, Holbrook A, Moyer V. Users’ guides to the medical literature. IV. How to use an article about harm. Evidence-Based Medicine Working Group. JAMA 1994;271:1615-19.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84.
- Mallen C, Peat G, Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. J Clin Epidemiol 2006;59:765-9.
- Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L. Do the findings of case series studies vary significantly according to methodological characteristics?. Health Technol Assess 2005;9.
Preliminary search strategy
The following search was run in MEDLINE only (via PubMed) on 15 August 2007, with 2204 hits identified. The review’s final search strategy will build upon this approach and syntax.
No study design filters or language restrictions applied.
Appendix 3 Literature search: strategy and results
Dialog DataStar (MEDLINE; EMBASE; PsycINFO); run 19 September 2007
| No. | Database | Search term | Results |
|---|---|---|---|
| 1 | MEDLINE | (N-METHYL–3–4-METHYLENEDIOXYAMPHETAMINE-AE OR N-METHYL–3–4-METHYLENEDIOXYAMPHETAMINE-PO OR N-METHYL–3–4-METHYLENEDIOXYAMPHETAMINE-TO).DE. | 928 |
| 2 | MEDLINE | N-METHYL–3–4-METHYLENEDIOXYAMPHETAMINE#.DE. OR (methylenedioxymethamphetamine OR MDMA OR ecstasy OR ecstasy OR ectasy OR ectacy).TI,AB. | 3251 |
| 3 | MEDLINE | 2 AND ((DESIGNER-DRUGS-AE OR DESIGNER-DRUGS-PO OR DESIGNER-DRUGS-TO OR STREET-DRUGS-AE OR STREET-DRUGS-PO OR STREET-DRUGS-TO).DE. OR (adverse OR harm OR harms OR harmful OR safety OR consequence$OR outcome$OR sequel$).TI,AB.) | 643 |
| 4 | MEDLINE | 2 AND (DEATH#.DE. OR (death OR deaths OR fatal$OR mortal$).TI,AB.) | 290 |
| 5 | MEDLINE | 2 AND ((FEVER# OR HEAT-STROKE#).DE. OR (hyperthermi$OR pyrexi$OR hyperpyrexia$OR fever OR febrile OR heatstroke OR heat ADJ stroke).TI,AB.) | 299 |
| 6 | MEDLINE | 2 AND (WATER-ELECTROLYTE-IMBALANCE#.DE. OR (hyponatraemia OR hyponatremia OR water ADJ intoxication).TI,AB.) | 55 |
| 7 | MEDLINE | 2 AND ((CARDIOVASCULAR-SYSTEM# OR CARDIOVASCULAR-DISEASES#).DE. OR (heart OR cardiovascular OR cardiac).TI,AB.) | 270 |
| 8 | MEDLINE | 2 AND ((RESPIRATORY-SYSTEM# OR RESPIRATORY-TRACT-DISEASES# OR MEDIASTINAL-EMPHYSEMA# OR PNEUMOTHORAX#).DE. OR (respiratory OR respiration OR lung OR lungs OR pulmonary OR pneumomediastin$OR pneumothora$).TI,AB.) | 99 |
| 9 | MEDLINE | 2 AND ((LIVER# OR LIVER-DISEASES#).DE. OR (liver OR hepatic OR hepatitis OR hepatotox$).TI,AB.) | 187 |
| 10 | MEDLINE | 2 AND ((KIDNEY# OR KIDNEY-DISEASES#).DE. OR (kidney OR renal).TI,AB.) | 97 |
| 11 | MEDLINE | 2 AND (RHABDOMYOLYSIS#.DE. OR (rhabdomyoly$OR myoglobinur$).TI,AB.) | 70 |
| 12 | MEDLINE | 2 AND ((NEUROLOGIC-MANIFESTATIONS# OR EPILEPSY# OR SEIZURES#).DE. OR (seizure OR seizures OR fit OR fits OR fitting OR convuls$).TI,AB.) | 271 |
| 13 | MEDLINE | 2 AND (INTRACRANIAL-HEMORRHAGES#.DE. OR ((brain OR cerebral OR intracerebral OR intracranial OR subarachnoid) ADJ (haemorrhage OR hemorrhage OR bleed OR bleeds OR bleeding)).TI,AB.) | 19 |
| 14 | MEDLINE | 2 AND (DISSEMINATED-INTRAVASCULAR-COAGULATION.DE. OR (disseminated ADJ intravascular ADJ (coagulation OR coagulopathy OR clotting) OR DIC).TI,AB.) | 31 |
| 15 | MEDLINE | 2 AND ((EYE# OR EYE-DISEASES#).DE. OR (eye OR ophthalmic OR ophthalmol$OR retina OR retinas OR retinal OR cornea OR corneas OR corneal OR keratopath$OR glaucoma OR diplopi$OR myopi$).TI,AB.) | 58 |
| 16 | MEDLINE | 2 AND (WOUNDS-AND-INJURIES#.DE. OR (accident$OR trauma OR traumas OR traumatic).TI,AB.) | 88 |
| 17 | MEDLINE | 2 AND (MENTAL-DISORDERS#.DE. OR (neuropsychi$OR neuropsycho$OR psychology OR psychologic$OR psychiatric OR psychiatry OR psychopatholog$OR neurocogniti$OR cognitive OR cognition OR psychosis OR psychoses OR depression OR depressive OR depressed OR panic OR delus$OR hallucinat$OR memory OR mood OR impulsiv$OR motor OR psychomotor OR parkinson OR parkinsons OR parkinsonism).TI,AB.) | 1380 |
| 18 | MEDLINE | 2 AND ((TOOTH# OR TOOTH-DISEASES#).DE. OR (tooth OR teeth OR toothgr$OR teethgr$OR toothwear OR dental OR Bruxism).TI,AB.) | 23 |
| 19 | MEDLINE | 1 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 17 OR 18 | 2297 |
| 20 | MEDLINE | 19 NOT (ANIMAL=YES NOT HUMAN=YES) | 1812 |
| 21 | EMBASE | (3–4-METHYLENEDIOXYMETHAMPHETAMINE-CO OR 3–4-METHYLENEDIOXYMETHAMPHETAMINE-SI OR 3–4-METHYLENEDIOXYMETHAMPHETAMINE-AE OR 3–4-METHYLENEDIOXYMETHAMPHETAMINE-TO).DE. | 1368 |
| 22 | EMBASE | 3–4-METHYLENEDIOXYMETHAMPHETAMINE#.DE. OR (methylenedioxymethamphetamine OR MDMA OR ecstasy OR ecstasy OR ectasy OR ectacy).TI,AB. | 4109 |
| 23 | EMBASE | 22 AND ((DESIGNER-DRUG-CO OR DESIGNER-DRUG-SI OR DESIGNER-DRUG-AE OR DESIGNER-DRUG-TO OR STREET-DRUG-CO OR STREET-DRUG-SI OR STREET-DRUG-AE OR STREET-DRUG-TO).DE. OR (adverse OR harm OR harms OR harmful OR safety OR consequence$OR outcome$OR sequel$).TI,AB.) | 650 |
| 24 | EMBASE | 22 AND (DEATH#.DE. OR (death OR deaths OR fatal$OR mortal$).TI,AB.) | 448 |
| 25 | EMBASE | 22 AND (BODY-TEMPERATURE-DISORDER#.DE. OR (hyperthermi$OR pyrexi$OR hyperpyrexia$OR fever OR febrile OR heatstroke OR heat ADJ stroke).TI,AB.) | 429 |
| 26 | EMBASE | 22 AND ((DISORDERS-OF-MINERAL-ELECTROLYTE-AND-METAL-METABOLISM# OR ABNORMAL-SUBSTRATE-CONCENTRATION-IN-BLOOD#).DE. OR (hyponatraemia OR hyponatremia OR water ADJ intoxication).TI,AB.) | 171 |
| 27 | EMBASE | 22 AND ((CARDIOVASCULAR-SYSTEM# OR CARDIOVASCULAR-DISEASE#).DE. OR (heart OR cardiovascular OR cardiac).TI,AB.) | 540 |
| 28 | EMBASE | 22 AND ((RESPIRATORY-TRACT-DISEASE# OR PNEUMOMEDIASTINUM#).DE. OR (respiratory OR respiration OR lung OR lungs OR pulmonary OR pneumomediastin$OR pneumothora$).TI,AB.) | 243 |
| 29 | EMBASE | 22 AND ((LIVER# OR LIVER-DISEASE#).DE. OR (liver OR hepatic OR hepatitis OR hepatotox$).TI,AB.) | 312 |
| 30 | EMBASE | 22 AND ((KIDNEY# OR KIDNEY-DISEASE#).DE. OR (kidney OR renal).TI,AB.) | 159 |
| 31 | EMBASE | 22 AND (RHABDOMYOLYSIS#.DE. OR (rhabdomyoly$OR myoglobinur$).TI,AB.) | 116 |
| 32 | EMBASE | 22 AND (SEIZURE-EPILEPSY-AND-CONVULSION#.DE. OR (seizure OR seizures OR fit OR fits OR fitting OR convuls$).TI,AB.) | 234 |
| 33 | EMBASE | 22 AND (BRAIN-HEMORRHAGE#.DE. OR ((brain OR cerebral OR intracerebral OR intracranial OR subarachnoid) ADJ (haemorrhage OR hemorrhage OR bleed OR bleeds OR bleeding)).TI,AB.) | 43 |
| 34 | EMBASE | 22 AND (DISSEMINATED-INTRAVASCULAR-CLOTTING.DE. OR (disseminated ADJ intravascular ADJ (coagulation OR coagulopathy OR clotting) OR dic).TI,AB.) | 46 |
| 35 | EMBASE | 22 AND ((EYE# OR EYE-DISEASE#).DE. OR (eye OR ophthalmic OR ophthalmol$OR retina OR retinas OR retinal OR cornea OR corneas OR corneal OR keratopath$OR glaucoma OR diplopi$OR myopi$).TI,AB.) | 152 |
| 36 | EMBASE | 22 AND (INJURY#.DE. OR (accident$OR trauma OR traumas OR traumatic).TI,AB.) | 543 |
| 37 | EMBASE | 22 AND ((MENTAL-DISEASE# OR MENTAL-FUNCTION#).DE. OR (neuropsychi$OR neuropsycho$OR psychology OR psychologic$OR psychiatric OR psychiatry OR psychopatholog$OR neurocogniti$OR cognitive OR cognition OR psychosis OR psychoses OR depression OR depressive OR depressed OR panic OR delus$OR hallucinat$OR memory OR mood OR impulsiv$OR motor OR psychomotor OR parkinson OR parkinsons OR parkinsonism).TI,AB.) | 2140 |
| 38 | EMBASE | 22 AND (MOUTH-AND-TEETH#.DE. OR (tooth OR teeth OR toothgr$OR teethgr$OR toothwear OR dental OR Bruxism).TI,AB.) | 18 |
| 39 | EMBASE | 21 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 | 3253 |
| 40 | EMBASE | 39 NOT (ANIMAL=YES NOT HUMAN=YES) | 2600 |
| 41 | PsycINFO | METHYLENEDIOXYMETHAMPHETAMINE#.DE. OR (methylenedioxymethamphetamine OR MDMA OR ecstasy OR ecstasy OR ectasy OR ectacy).TI,AB. | 1614 |
| 42 | PsycINFO | 41 NOT (PO=ANIMAL NOT PO=HUMAN) | 1259 |
| 43 | combined sets 20, 40, 42 | 5671 | |
| 44 | dropped duplicates from 43 | 1840 | |
| 45 | unique records from 43 | 3831 |
Web of Science; run 7 October 2007
| No. | Search term | Results |
|---|---|---|
| # 1 | TS=(Methylenedioxymethamphetamine OR MDMA OR ecstasy OR ecstasy OR ectasy OR ectacy) | 4424 |
| # 2 | #1 AND (TS=(adverse OR harm OR harms OR harmful OR safety OR consequence* OR outcome* OR sequel*)) | 518 |
| # 3 | #1 AND (TS=(death OR deaths OR fatal* OR mortal*)) | 369 |
| # 4 | #1 AND (TS=(hyperthermi* OR pyrexi* OR hyperpyrexia* OR fever OR febrile OR heatstroke OR (heat ADJ stroke))) | 329 |
| # 5 | #1 AND (TS=(hyponatraemia OR hyponatremia OR (water ADJ intoxication))) | 44 |
| # 6 | #1 AND (TS=(heart OR cardiovascular OR cardiac)) | 156 |
| # 7 | #1 AND (TS=(respiratory OR respiration OR lung OR lungs OR pulmonary OR pneumomediastin* OR pneumothora*)) | 76 |
| # 8 | #1 AND (TS=(liver OR hepatic OR hepatitis OR hepatotox*)) | 182 |
| # 9 | #1 AND (TS=(kidney OR renal)) | 68 |
| # 10 | #1 AND (TS=(rhabdomyoly* OR myoglobinur*)) | 65 |
| # 11 | #1 AND (TS=(seizure OR seizures OR fit OR fits OR fitting OR convuls*)) | 113 |
| # 12 | #1 AND (TS=((brain OR cerebral OR intracerebral OR intracranial OR subarachnoid) SAME (haemorrhage OR hemorrhage OR bleed OR bleeds OR bleeding))) | 24 |
| # 13 | #1 AND (TS=((disseminated SAME intravascular SAME (coagulation OR coagulopathy OR clotting)) OR DIC)) | 23 |
| # 14 | #1 AND (TS=(eye OR ophthalmic OR ophthalmol* OR retina OR retinas OR retinal OR cornea OR corneas OR corneal OR keratopath* OR glaucoma OR diplopi* OR myopi*)) | 18 |
| # 15 | #1 AND (TS=(accident* OR trauma OR traumas OR traumatic)) | 72 |
| # 16 | #1 AND (TS=(neuropsychi* OR neuropsycho* OR psychology OR psychologic* OR psychiatric OR psychiatry OR psychopatholog* OR neurocogniti* OR cognitive OR cognition OR psychosis OR psychoses OR depression OR depressive OR depressed OR panic OR delus* OR hallucinat* OR memory OR mood OR impulsiv* OR motor OR psychomotor OR Parkinson OR Parkinsons OR Parkinsonism)) | 909 |
| # 17 | #1 AND (TS=(tooth OR teeth OR toothgr* OR teethgr* OR toothwear OR dental OR bruxism)) | 20 |
| # 18 | #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 | 1879 |
| unique additional citations after de-duplication against Dialog DataStar results | 563 |
Appendix 4 Updated literature search: results
Our updated literature searches identified the following potentially relevant studies, which should be considered for inclusion in any update of this review.
Ahmed M, Islam S, Hoffman GR. Widespread oral and oropharyngeal mucosal oedema induced by ecstasy (MDMA): A case for concern. Br J Oral Maxillofac Surg 2007;45:496–8.
Brown J, Edwards M, McKone E, Ward J. A long-term ecstasy-related change is visual perception. Psychopharmacology 2007;193:437–46.
de Win, Reneman L, Jager G, Vlieger E, Olabarriaga S, Lavini C, et al. A prospective cohort study on sustained effects of low-dose ecstasy use on the brain in new ecstasy users. Neuropsychopharmacology 2007;32:458–70.
Droogmans S, Cosyns B, D’haenen H, Creeten E, Weytjens C, Franken PR, et al. Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am J Cardiol 2007;100:1442–5.
Eifinger F, Roth B, Kröner L, Rothschild MA. Life-threatening ecstasy ingestion in a young infant. Notarzt 2007;23:165–6.
Falck RS, Jichuan W, Carlson RG. Depressive symptomatology in young adults with a history of MDMA use: a longitudinal analysis. J Psychopharmacol 2008;22:47–54.
Feldman KW, Mazor S. Ecstasy ingestion causing heatstroke-like, multiorgan injury in a toddler. Pediatr Emerg Care 2007;23:725–6.
Golding JF, Groome DH, Rycroft N, Denton Z. Cognitive performance in light current users and ex-users of ecstasy (MDMA) and controls. Am J Drug Alcohol Abuse 2007;33:301–7.
Groth M, Howchar H, Marsh A. Memory performance in abstinent 3,4-methylendeioxymethamaphetamine (MDMA, ‘ecstasy’) users. Percept Mot Skills 2007;104:43–55.
Guillot C. Is recreational ecstasy (MDMA) use associated with higher levels of depressive symptoms? J Psychoactive Drugs 2007;39:31–9.
Hanson K. Neurocognitive and personality function in MDMA and polydrug users: evidence of nonspecific impairments. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;67:4105.
Hoshi R, Cohen L, Lemanski L, Piccini P, Bond A, Curran HV. Ecstasy (MDMA) does not have long-term effects on aggressive interpretative bias: A study comparing current and ex-ecstasy users with polydrug and drug-naive controls. Exp Clin Psychopharmacol 2007;15:351–8.
Hoshi R, Mullins K, Boundy C, Brignell C, Piccini P, Curran HV. Neurocognitive function in current and ex-users of ecstasy in comparison to both matched polydrug-using controls and drug-naïve controls. Psychopharmacology 2007;194:371–9.
Jager G, de Win MM, Vervaeke H, Schilt T, Kahn R, van den Brink W, et al. Incidental use of ecstasy: No evidence for harmful effects on cognitive brain function in a prospective fMRL study. Psychopharmacology 2007;193:403–14.
Jager G, de Win MML, van der Tweel I, Schilt T, Kahn RS, van den Brink W, et al. Assessment of cognitive brain function in ecstasy users and contributions of other drugs of abuse: results from an fMRI study. Neuropsychopharmacology 2008;33:247–58.
Kalechstein A, De La Garza R II, Mahoney J, Fantegrossi W, Newton T. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology 2007;189:531–7.
Krebs T, Johansen P. No evidence of decrease in cognitive function in users of low-dose ecstasy. Arch Gen Psychiatry 2008;65:236.
Kuypers K, Ramaekers J. Acute dose of MDMA (75 mg) impairs memory for location but leaves contextual processing of visuospatial information unaffected. Psychopharmacology 2007;189:557–63.
Laws K, Kokkalis J. Ecstasy (MDMA) and memory function: a meta-analytic update. Hum Psychopharmacol Clin Exp 2007;22:381–8.
Mathias S, Lubman D, Hides L. Substance-induced psychosis: a diagnostic conundrum. J Clin Psychiatry 2008;69:358–67.
McCann U, Peterson S, Ricaurte G. The effect of catecholamine depletion by alpha-methyl-para-tyrosine on measures of cognitive performance and sleep in abstinent MDMA users. Neuropsychopharmacology 2007;32:1695–706.
Medina K, Shear P. Anxiety, depression, and behavioral symptoms of executive dysfunction in ecstasy users: contributions of polydrug use. Drug Alcohol Depend 2007;87:303–11.
Montgomery C, Fisk JE. Everyday memory deficits in ecstasy-polydrug users. J Psychopharmacol 2007;21:709–17.
Montgomery C, Fisk JE, Wareing M, Murphy P. Self reported sleep quality and cognitive performance in ecstasy users. Hum Psychopharmacol 2007;22:537–48.
Nicolato R, Pacheco J, Boson L, Leite R, Salgado JV, Romano S, et al. Cotard’s syndrome induced by ecstasy. J Bras Psiquiatr 2007;56:64–6.
Quednow B, Kühn K, Hoppe C, Westheide J, Maier W, Daum I, Wagner M. Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (‘Ecstasy’). Psychopharmacology 2007;189:517–30.
Reid L, Elifson K, Sterk C. Hug drug or thug drug? Ecstasy use and aggressive behavior. Violence and Victims 2007;22:104–19.
Rendell PG, Gray TJ, Henry JD, Tolan A. Prospective memory impairment in ‘ecstasy’ (MDMA) users. Psychopharmacology 2007;194:497–504.
Roiser J, Rogers R, Sahakian B. Neuropsychological function in ecstasy users: a study controlling for polydrug use. Psychopharmacology 2007;189:505–16.
Sadeghian S, Darvish S, Shahbazi S, Mahmoodian M. Two ecstasy-induced myocardial infarctions during a three month period in a young man. Arch Iran Med 2007;10:409–12.
Sauvageau A. Death from a possible anaphylactic reaction to ecstasy. Clin Toxicol (Philadelphia PA) 2008;46:156.
Schilt T, de Win MM, Koeter M, Jager G, Korf D, van den Brink W, et al. Cognition in novice Ecstasy users with minimal exposure to other drugs: a prospective cohort study. Arch Gen Psychiatry 2007;64:728–36.
Schilt T, de Winn MM, Koeter M, Jager G, Korf Den Brink W, et al. ‘Cognition in novice Ecstasy users with minimal exposure to other drugs: a prospective cohort study’: reply. Arch Gen Psychiatry 2008;65:236–7.
Shah HV, Irvine GH, Bradley M. Rhabdomyolysis of the masseter muscle: case report. Br J Oral Maxillofac Surg 2008;46:138–40.
Silins E, Copeland J, Dillon P. Qualitative review of serotonin syndrome, ecstasy (MDMA) and the use of other serotonergic substances: hierarchy of risk. Aust NZ J Psychiatry 2007;41:649–55.
Stull BW. Spontaneous pneumomediastinum following ecstasy ingestion and sexual intercourse. Emerg Med J 2008;25:113–14.
Wareing M, Fisk J, Montgomery C, Murphy P, Chandler M. Information processing speed in ecstasy (MDMA) users. Hum Psychopharmacol Clin Exp 2007;22:81–8.
Yen CF, Hsu SY. Symptoms of ecstasy dependence and correlation with psychopathology in Taiwanese adolescents. J Nerv Mental Dis 2007;195:866–9.
Yucel M, Lubman DI, Solowij N, Brewer WJ. Understanding drug addiction: a neuropsychological perspective. Aust NZ J Psychiatry 2007;41:957–68.
Zakzanis K, Campbell Z, Jovanovski D. The neuropsychology of ecstasy (MDMA) use: a quantitative review. Hum Psychopharmacol Clin Exp 2007;22:427–35.
Appendix 5 Mapping of outcome measures into composite domains
| Domain | Instrument | Abbreviation | Outcome measure |
|---|---|---|---|
| Aggression/anger | Aggression questionnaire370 | AQ | Anger |
| Hostility | |||
| Physical | |||
| Total | |||
| Verbal | |||
| Aggression Rating Scale371 | ARS | Overall score | |
| Angry Stories Task372 | AST | Reading time – angry endings – ms | |
| Reading time – non-angry endings – ms | |||
| Buss–Durkee Hostility Inventory373 | BDHI | Direct | |
| Guilty | |||
| Irritability | |||
| Total | |||
| Interpretative Bias test154,374 | IB | Reaction time | |
| Reaction time – aggressive – ms | |||
| Reaction time – neutral – ms | |||
| Sentences correctly identified | |||
| Time to endorse as seen | |||
| Time to endorse as seen – aggressive – ms | |||
| Time to endorse as seen – neutral – ms | |||
| Multidimensional Anger Inventory375 | MAI | Anger–arousal | |
| Anger–in | |||
| Anger–out | |||
| Hostile outlook | |||
| Range | |||
| Total | |||
| Point Subtraction Aggression Paradigm376 | PSAP | Aggressive responding – study end | |
| Symptom Check List (SCL-90-R)377 | SCL-90-R | Aggression/hostility score | |
| Anxiety | Beck Anxiety Inventory378 | BAI | Overall score |
| DSM-IV379 | DSM-IV | Current anxiety disorder | |
| Lifetime anxiety disorder | |||
| Hospital Anxiety and Depression scale380 | HADS | Anxiety score | |
| Hamilton Anxiety Rating Scale381 | HARS | Overall score | |
| Mood Rating Scale (visual analogue scale)382 | MRS–VAS | Anxiety vs calmness score | |
| NS | NS | In-test state anxiety | |
| Medication for anxiety disorder | |||
| Profile of Mood States (visual analogue scale)383 | POMS | Anxiety score | |
| Symptom Check List (SCL-90) | SCL-90 | Anxiety score | |
| Phobic anxiety score | |||
| Symptom Check List (SCL-90-R)377 | SCL-90-R | Anxiety score | |
| Phobic anxiety score | |||
| Symptom Check List – Brief Symptom Inventory384 | SCL– BSI | Anxiety | |
| Anxiety score | |||
| Phobic anxiety | |||
| Phobic anxiety score | |||
| Self-rated | S-R | Anxiety | |
| State–Trait Anxiety Inventory385 | STAI | State anxiety | |
| Trait anxiety | |||
| State–Trait Anxiety Inventory (Dutch)385,386 | STAI-DY | Trait anxiety | |
| Attention (general) | Speed of Comprehension Test387 | SCT | Sentences correct |
| Wechsler Memory Scale – Revised388 | WMS-R | Attention and concentration index score | |
| Attention–focus-execute | Automated Performance Test System389 | APTS | AREACT |
| CANTAB intradimensional/extra-dimensional test390 | CANTAB 3D-ID/ED | Errors – simple dimensional | |
| Errors – simple dimensional – reversal | |||
| Latency – simple dimensional | |||
| Latency – simple dimensional – reversal | |||
| Cognitive Drug Research battery391 | CDR | Choice 1 – correct [%] | |
| Choice 1 – reaction time [ms] | |||
| Choice 2 – correct [%] | |||
| Choice 2 – reaction time [ms] | |||
| Simple reaction time [ms] | |||
| FePsy392 | FePsy | Auditive reaction time – dominant hand [ms] | |
| Auditive reaction time – non-dominant hand [ms] | |||
| Binary choice – errors [n] | |||
| Binary choice – reaction time [ms] | |||
| Visual reaction time – dominant hand [ms] | |||
| Visual reaction time – non-dominant hand [ms] | |||
| Matching Familiar Figures Task-20393 | MFFT-20 | Latency to first response [s] | |
| Total errors [n] | |||
| NS | NS | Binary choice task – reaction time [ms] | |
| Complex reaction time [ms] | |||
| Double digit cancellation – time – s | |||
| Immediate memory task – correct [n] | |||
| Letter cancellation – commission errors | |||
| Letter cancellation – omission errors | |||
| Letter cancellation – time – s | |||
| Letter comparison speed task – three-letter – correct [%] | |||
| Letter comparison speed task – three-letter – correct [n] | |||
| Letter comparison speed task – three-letter – errors [n] | |||
| Letter comparison speed task – six-letter – correct [%] | |||
| Letter comparison speed task – six-letter – correct [n] | |||
| Letter comparison speed task – six-letter – errors [n] | |||
| Letter comparison speed task – nine-letter – correct [%] | |||
| Letter comparison speed task – nine-letter – correct [n] | |||
| Letter comparison speed task – nine-letter – errors [n] | |||
| Pattern comparison speed task – three-pattern – correct [n] | |||
| Pattern comparison speed task – three-pattern – errors [n] | |||
| Pattern comparison speed task – six-pattern – correct [n] | |||
| Pattern comparison speed task – six-pattern – errors [n] | |||
| Pattern comparison speed task – nine-pattern – correct [n] | |||
| Pattern comparison speed task – nine-pattern – errors [n] | |||
| Simple auditory reaction time [ms] | |||
| Simple visual reaction time [ms] | |||
| Visual reaction time [ms] | |||
| Visual search – time [s] | |||
| Ruff 2 and 7 Selective Attention Test394 | Ruff 2 and 7 | Controlled search accuracy | |
| Controlled search speed | |||
| Total accuracy | |||
| Total speed | |||
| Symbol Digit Modalities test395 | SDMT | Correct [n] | |
| Overall score | |||
| Stroop test396 | Stroop | Colour reading – errors [n] | |
| Colour reading – time [ms] | |||
| Colour reading – time [s] | |||
| Word reading – errors [n] | |||
| Word reading – time [ms] | |||
| Word reading – time [s] | |||
| Test for Attentional Performance397 | TAP | 1 – phasic reaction time [ms] | |
| 1 – tonic reaction time [ms] | |||
| Test of Everyday Attention398 | TEA | Map search 1 | |
| Map search 2 | |||
| Telephone search | |||
| Trailmaking Test399–401 | TMT | Part A – errors | |
| Part A – time | |||
| Part B – errors | |||
| Part B – part A – time | |||
| Part B – time | |||
| Part B – T-score | |||
| Colour trails test402 | TMT-C | Part 1 – time | |
| Part 2 – time | |||
| Wechsler Adult Intelligence Scale – Third Edition403 | WAIS-III | Digit symbol [standard score units] | |
| Wechsler Adult Intelligence Scale – Revised404 | WAIS-R | Digit symbol | |
| Digit symbol [age-corrected scaled score] | |||
| Walter Reed Army Institute of Research Performance Assessment Battery405 | WRAIR PAB | Code substitution | |
| Attention–sustain | CANTAB intradimensional/extra-dimensional test390 | CANTAB 3D-ID/ED | Errors – compound dimensional |
| Errors – compound dimensional – reversal | |||
| Errors – intradimensional | |||
| Errors – intradimensional – reversal | |||
| Latency – compound dimensional | |||
| Latency – compound dimensional – reversal | |||
| Latency – intradimensional | |||
| Latency – intradimensional – reversal | |||
| Affective Go/No-go task406 | CANTAB A-G/N-G | Omission errors [n] | |
| Cognitive Drug Research battery391 | CDR | Number vigilance – correct [%] | |
| Number vigilance – reaction time [ms] | |||
| Go/No-Go task397,407 | G/N-G | Correct responses | |
| Punishment-reward – omission errors | |||
| Reward-punishment – omission errors | |||
| Summed conditions – omission errors | |||
| Rapid visual information processing408 | RVIP | 10-minute task – correct [n] | |
| Test for Attentional Performance397 | TAP | Visual scanning – accuracy/speed correlation [z-score] | |
| Visual scanning – critical trials – correct [n] | |||
| Visual scanning – critical trials – time [ms] | |||
| Visual scanning – non-critical trials – correct [n] | |||
| Visual scanning – non-critical trials – time [ms] | |||
| Visual scanning – time/accuracy correlation [z-score] | |||
| Test of Everyday Attention398 | TEA | Elevator counting | |
| Elevator counting with distraction | |||
| Elevator counting with reversal | |||
| Decision-making | Iowa Gambling Task409,410 | IGT | Block 1 |
| Block 2 | |||
| Block 3 | |||
| Block 4 | |||
| Block 5 | |||
| Net score | |||
| Rogers Gambling Task411 | RGT | High loss – choices | |
| High loss – latency – ms | |||
| High probability – choices | |||
| High probability – latency – ms | |||
| High win – choices | |||
| High win – latency – ms | |||
| Low loss – choices | |||
| Low loss – latency – ms | |||
| Low probability – choices | |||
| Low probability – latency – ms | |||
| Low win – choices | |||
| Low win – latency – ms | |||
| Overall – choices | |||
| Overall – latency – ms | |||
| Revised Strategy Applications Test412 | R-SAT | Total 1 – all pages | |
| Total 2 – not including first two pages | |||
| Depression | Beck Depression Inventory413 | BDI | Median |
| Overall score | |||
| Beck Depression Inventory II414 | BDI-II | Cognitive subscale | |
| Cognitive–affective subscale | |||
| Overall score | |||
| Somatic subscale | |||
| Composite International Diagnostic Interview415 | CIDI | Current diagnosis [n] | |
| Hospital Anxiety and Depression Scale380 | HADS | Depression score | |
| Hamilton Depression Rating Scale416 | HDRS | Overall score | |
| Minnesota Multiphasic Personality Inventory417 | MMPI | Overall score | |
| Minnesota Multiphasic Personality Inventory – 2418 | MMPI 2 | Overall score | |
| NS | NS | Medication for depression | |
| Symptom Check List (SCL-90) | SCL-90 | Depression score | |
| Symptom Check List (SCL-90-R)377 | SCL-90-R | Depression score | |
| Symptom Check List – Brief Symptom Inventory385 | SCL–BSI | Depression | |
| Depression score | |||
| Self-rated | S-R | Depression | |
| Disinhibition | Frontal Systems Behavioral scale419 | FrSBe | Overall score |
| Executive function (general) | Behavioural Assessment of the Dysexecutive Syndrome – Dysexecutive questionnaire420 | DEX | Dysexecutive function score |
| Frontal Systems Behavioral scale419 | FrSBe | Executive dysfunction | |
| Random letter generation421,422 | Random letter generation | Alphabetical sequences – 1 s | |
| Alphabetical sequences – 2 s | |||
| Alphabetical sequences – 4 s | |||
| Alphabetical sequences [standardised score] | |||
| Composite score [standardised score] | |||
| Letters [standardised score] | |||
| Number of letters – 1 s | |||
| Number of letters – 2 s | |||
| Number of letters – 4 s | |||
| Redundancy – 1 s –% | |||
| Redundancy – 2 s –% | |||
| Redundancy – 4 s –% | |||
| Redundancy [standardised score] | |||
| Repeated sequences – 1 s | |||
| Repeated sequences – 2 s | |||
| Repeated sequences – 4 s | |||
| Repeated sequences [standardised score] | |||
| Vowels – 1 s – % | |||
| Vowels – 2 s – % | |||
| Vowels – 4 s – % | |||
| Executive function – inhibition of return | NS | NS | Mean slowing [ms] |
| Executive function – planning | Behavioural Assessment of the Dysexecutive Syndrome420 | BADS | Action program test |
| Key search test | |||
| Modified six elements test | |||
| Temporal judgement test | |||
| Total profile score | |||
| Zoo map test | |||
| Plan-A-Day simulation423 | Plan-A-Day | End score | |
| Peak – end score | |||
| Peak score | |||
| Sequences of deletions | |||
| Single deletions | |||
| Use of F2 key | |||
| CANTAB Stockings of Cambridge424 | SOC | Initial thinking time [ms] | |
| Tower of London425 | ToL | Errors – n | |
| Excess moves | |||
| Excess moves – % | |||
| Initial thinking time – ms | |||
| Perfect solutions | |||
| Planning time – s | |||
| Solution time – s | |||
| Subsequent thinking time – ms/move | |||
| Total moves | |||
| Total time – s | |||
| Trials completed – n | |||
| Executive function – processing speed | NS | NS | Letters – correct [n] |
| Patterns – correct [n] | |||
| Total errors [n] | |||
| Executive function – response inhibition | Affective Go/No-go task408 | CANTAB A-G/N-G | Commission errors – non-shift block |
| Commission errors – shift block | |||
| Commission errors [n] | |||
| Go/No-Go task397,407 | G/N-G | Commission errors | |
| Punishment–reward – commission errors | |||
| Punishment–reward – gain | |||
| Reaction time – ms | |||
| Reward–punishment – commission errors | |||
| Reward–punishment – gain | |||
| Summed conditions – Σ commission errors | |||
| Summed conditions – Σ gain | |||
| Huizinga and van der Molen – Eriksen Flankers test426,427 | HvdM EF | EF – Eriksen Flankers – correct – % | |
| EF – Eriksen Flankers – reaction time – ms | |||
| Huizinga and van der Molen – stop signal428 | HvdM SS | Stop signal – reaction time – ms | |
| Stroop test396 | Stroop | Colour naming – time [s] | |
| Inhibition/switching contrast [s] | |||
| Interference – errors [n] | |||
| Interference – negative priming – time [ms] | |||
| Interference – no negative priming – time [ms] | |||
| Interference – switching time difference [s] | |||
| Interference – time [ms] | |||
| Interference – time [s] | |||
| Interference – time difference [s] | |||
| Interference + switching – errors [n] | |||
| Interference + switching – time [s] | |||
| Switching – time [ms] | |||
| Test for Attentional Performance397 | TAP | Selective visual attention – sustain – time [ms] | |
| Executive function – shifting | Behavioural Assessment of the Dysexecutive Syndrome420 | BADS | Rule shift cards test |
| Brixton Spatial Anticipation task429 | BSA | Errors [n] | |
| CANTAB intradimensional/extra-dimensional test390 | CANTAB 3D–ID/ED | Errors – extra-dimensional | |
| Errors – extradimensional – reversal | |||
| Latency – extra-dimensional | |||
| Latency – extra-dimensional – reversal | |||
| Huizinga and van der Molen – dots–triangles47 | HvdM DT | Dots–triangles – correct – % | |
| Dots–triangles – response time – ms | |||
| Huizinga and van der Molen – local–global47 | HvdM LG | Local–global – correct – % | |
| Local–global – response time – ms | |||
| NS | NS | Number/letter switch cost | |
| Plus/minus task switch cost | |||
| Test of Everyday Attention398 | TEA | Telephone search with counting | |
| Wisconsin Card-Sorting Test430 | WCST | Categories | |
| Conceptual level responses [%] | |||
| Failure to maintain set | |||
| Learning-to-learn score | |||
| No. ambig. error | |||
| No. correct ambig. | |||
| Non-perseverative errors | |||
| Non-perseverative errors – % | |||
| Perseverative errors | |||
| Perseverative errors –% | |||
| Perseverative responses | |||
| Total no. correct | |||
| Total no. errors | |||
| Total no. trials | |||
| Trials to first category | |||
| Executive function – updating | Keep Track Test51 | Keep Track Test | Words correct [n] |
| NS | NS | Consonant updating – score | |
| Non-spatial associative learning | |||
| Executive function – visual fluency | Delis–Kaplan Executive Function System431 | D-KEFS | Closed |
| Open | |||
| Switching | |||
| Total accuracy | |||
| Total score | |||
| Ruff Figural Fluency Test432 | RFFT | Repeated designs [n] | |
| Unique designs – total [n] | |||
| Rey–Osterrieth Complex Figure Test433,434 | R-OCFT | Copy score | |
| Impulsivity | Barratt Impulsiveness Scale435,436 | BIS | Total |
| Barratt Impulsiveness Scale-II437 | BIS-II | Attentional | |
| Cognitive | |||
| Motor | |||
| Non-planning | |||
| Total | |||
| Impulsivity self-rating scale382 | ISRS | Overall score | |
| Adult impulsiveness, venturesomeness and empathy scale438 | IVE | Overall score | |
| Matching Familiar Figures Task439 | MFFT | Efficiency score | |
| Impulsivity score | |||
| Matching Familiar Figures Task–20393 | MFFT-20 | Impulsivity score | |
| NS | NS | Bets16 – risk-taking score | |
| Delayed memory task – adjusted commision errors [n] | |||
| Immediate memory task – adjusted commission errors [n] | |||
| Rogers Gambling Task411 | RGT | Gains only – latency [ms] | |
| Gains only – latency – ms | |||
| Gains only – risk-averse choices | |||
| Losses only – latency [ms] | |||
| Losses only – latency – ms | |||
| Losses only – risk-averse choices | |||
| Losses only – risk-seeking choices | |||
| Intelligence | Kaufman Brief Intelligence Test440 | K-BIT | Overall score |
| Mill Hill Vocabulary Scale441 | Mill Hill | Vocabulary | |
| Mehrfachwahl-Wortschatz-Intelligenztest (Multiple Choice Verbal Intelligence Test)442,443 | MWT-B | Verbal IQ | |
| National Adult Reading Test 444,445 | NART | 0 | |
| IQ | |||
| Overall score | |||
| National Adult Reading Test (Dutch version)444–446 | NART-D | IQ | |
| Quick Test447 | Quick | Verbal IQ | |
| Raven’s Progressive Matrices448 | RPM | D | |
| E | |||
| Total correct – C+D+E | |||
| Total correct – D+E | |||
| Total score | |||
| Shipley Institute of Living Scale449,450 | SILS | Abstraction | |
| IQ | |||
| Verbal | |||
| Spot the Word451 | STW | Overall score | |
| Test of non-verbal intelligence (TONI-3)452 | TONI-3 | Overall score | |
| Wechsler Adult Intelligence Scale – Third Edition403 | WAIS-III | Full-scale IQ | |
| Performance IQ | |||
| Similarities | |||
| Verbal IQ | |||
| Vocabulary | |||
| Wechsler Adult Intelligence Scale – Revised404 | WAIS-R | Full-scale IQ | |
| General knowledge [information] | |||
| Performance IQ | |||
| Verbal IQ | |||
| Vocabulary | |||
| Vocabulary [median] | |||
| Wechsler Abbreviated Scale of Intelligence403 | WASI | Vocabulary | |
| Woodcock–Johnson Revised Test of Achievement453 | WJR | Letter-word identification | |
| Letter-word identification – standard score | |||
| Word attack | |||
| Word attack – standard score | |||
| Memory (general) | Rivermead Behavioural Memory Test454 | RBMT | Total score |
| Wechsler Memory Scale – III455 | WMS-III | General index score | |
| Wechsler Memory Scale – Revised388 | WMS-R | General index score | |
| Memory (general) – delayed | Lern- und Gedächtnis-test456 | LGT-3 | City map test |
| German–Turkish test | |||
| Library test | |||
| Logos test | |||
| Wechsler Memory Scale – III455 | WMS-III | Auditory index score | |
| Wechsler Memory Scale – Revised388 | WMS-R | Index score | |
| Walter Reed Army Institute of Research Performance Assessment Battery405 | WRAIR PAB | Overall score | |
| Memory – self-rated | Cognitive Failures Questionnaire457 | CFQ | Other-rated – slips reported [%] |
| Other-rated – total score | |||
| Self-rated – slips reported [%] | |||
| Self-rated – total score | |||
| Everyday Memory Questionnaire458 | EMQ | Overall score | |
| Fragebogen zum Alltagsgedächtnis (questionnaire on everyday memory)459 | FZ–EMQ | Overall score | |
| Prospective Memory Questionnaire460 | PMQ | Internally cued | |
| Long-term | |||
| Long-term episodic | |||
| Short-term | |||
| Short-term habitual | |||
| Strategies | |||
| Rivermead Behavioural Memory Test454 | RBMT | Appointment | |
| Belonging | |||
| First/second name | |||
| Message | |||
| Uplifts/hassles questionnaire461 | Uplifts/hassles | Cognitive failures | |
| Virtual Week462 | VW | All tasks – correct | |
| All tasks – correct – frequent ecstasy users | |||
| All tasks – correct – infrequent ecstasy users | |||
| All tasks – late | |||
| All tasks – missed | |||
| All tasks – wrong | |||
| Irregular task – correct | |||
| Irregular task – late | |||
| Irregular task – missed | |||
| Irregular task – wrong | |||
| Regular task – correct | |||
| Regular task – late | |||
| Regular task –missed | |||
| Regular task – wrong | |||
| Time-check task – correct | |||
| Time-check task – late | |||
| Time-check task – missed | |||
| Time-check task – wrong | |||
| Memory (general) – immediate | Automated Performance Test System389 | APTS | Sternberg numbers – correct – n |
| Sternberg numbers – speed [s] | |||
| Cognitive Drug Research battery391 | CDR | Sternberg numbers – speed [ms] | |
| FePsy392 | FePSY | Sternberg figures – serial | |
| Sternberg figures – simultaneous | |||
| Sternberg words – serial | |||
| Sternberg words – simultaneous | |||
| Lern- und Gedächtnis-test456 | LGT-3 | City map test | |
| German–Turkish test | |||
| Library test | |||
| Logos test | |||
| Wechsler Memory Scale – III455 | WMS-III | Auditory index score | |
| Index score | |||
| Memory – learning performance | Buschke selective reminding task463 | Buschke | Trial 3 – trial 1 |
| Rey Auditory Verbal Learning Test464 | RAVLT | Learning – trial 5 – trial 1 | |
| Rey Auditory Verbal Learning Test – German version464,465 | RAVLT-G | Learning – trial 5 – trial 1 | |
| Repetitions required for learning – n | |||
| VIG: visuospatial memory466 | VIG | Learning – trial 5 – trial 1 | |
| Repetitions required for learning – n | |||
| Wechsler Memory Scale – III455 | WMS-III | Logical memory – verbal learning slope | |
| Memory – verbal (general) | California Verbal Learning Test – Second Edition467 | CVLT-II | Total recognition – z-score |
| Memory – verbal delayed | Automated Performance Test System389 | APTS | Overall score |
| Buschke selective reminding task463 | Buschke | Overall score | |
| Cognitive Drug Research battery391 | CDR | Word recall [n] | |
| California Verbal Learning Test – Second Edition467 | CVLT-II | Long-delay cued recall – z-score | |
| Long-delay cued recall correct | |||
| Long-delay false positives | |||
| Long-delay free recall – z-score | |||
| Long-delay free recall correct | |||
| Long-delay recognition hits | |||
| NS | NS | Prose recall | |
| Prose retained – % | |||
| Rey Auditory Verbal Learning Test464 | RAVLT | Overall score | |
| Recognition | |||
| Recognition – errors – list A | |||
| Recognition – errors – list B | |||
| Recognition – list A | |||
| Recognition – list B | |||
| Trial 8 | |||
| Rey Auditory Verbal Learning Test – Chinese version464 | RAVLT-C | Overall score | |
| Recognition | |||
| Trial 8 | |||
| Rey Auditory Verbal Learning Test – Dutch version464,468 | RAVLT-D | Recognition | |
| Trial 8 | |||
| Rey Auditory Verbal Learning Test – German version464,465 | RAVLT-G | Trial 8 | |
| Rivermead Behavioural Memory Test454 | RBMT | Prose recall | |
| Prose recall (est) | |||
| Prose recall (sum of two tests) | |||
| Wechsler Memory Scale – adapted388 | WMS adapted | Logical memory | |
| Wechsler Memory Scale – III455 | WMS-III | Auditory index score | |
| Index score | |||
| Logical memory | |||
| Logical memory – story A recall unit score | |||
| Logical memory – verbal % ret | |||
| Verbal paired associates | |||
| Wechsler Memory Scale – Revised388 | WMS-R | Logical memory | |
| Verbal paired associates | |||
| Verbal reproduction | |||
| Memory – verbal immediate | Auditory Consonant Trigrams469 | ACT | Score |
| Automated Performance Test System389 | APTS | Overall score | |
| Buschke selective reminding task465 | Buschke | Trial 1 | |
| Trial 2 | |||
| Trial 3 | |||
| Cognitive Drug Research battery391 | CDR | Word recall [n] | |
| California Verbal Learning Test – Second Edition467 | CVLT-II | Short-delay cued recall – z-score | |
| Short-delay cued recall correct | |||
| Short-delay free recall – z-score | |||
| Short-delay free recall correct | |||
| Total intrusions | |||
| Total list B correct | |||
| Total list B plus trial 1 correct | |||
| Total repetitions | |||
| Total trials 1–5 correct – n | |||
| Trial 1 correct – n | |||
| Trial 5 correct – n | |||
| Trial B correct – n | |||
| Matched verbal recall/recognition470 | MRR | Recall – hits – intrusions | |
| Recognition – hits – false alarms | |||
| NS | NS | Computation span | |
| Digit span – backwards | |||
| Digit span – forwards | |||
| Free recall | |||
| Letter span – forwards | |||
| Prose recall | |||
| Verbal paired associates – perseverative responses – n | |||
| Verbal paired associates – total forgotten – n | |||
| Verbal paired associates – trials to completion | |||
| Verbal paired associates – errors trial 1 | |||
| Verbal paired associates – errors trial 2 | |||
| Verbal paired associates – errors trial 3 | |||
| Verbal paired associates – errors trial 4 | |||
| Verbal paired associates – trial 1- correct – n | |||
| Word span | |||
| Rey Auditory Verbal Learning Test464 | RAVLT | Adjusted list A | |
| Adjusted list B | |||
| Interference – trial 5 – trial 7 | |||
| List B | |||
| Proactive interference – trial 1 – trial 6 | |||
| Recall consistency –% | |||
| Retroactive interference – trial 5 – trial 6 | |||
| Sum of trials 1–5 | |||
| Trial 1 | |||
| Trial 1 – errors | |||
| Trial 2 | |||
| Trial 2 – errors | |||
| Trial 3 | |||
| Trial 3 – errors | |||
| Trial 4 | |||
| Trial 4 – errors | |||
| Trial 5 | |||
| Trial 5 – errors | |||
| Trial 6 | |||
| Trial 6 – errors | |||
| Trial 6 – interference list | |||
| Trial 7 | |||
| Trial 7 – errors | |||
| Trial 7 – post-interference | |||
| Rey Auditory Verbal Learning Test – Chinese version464 | RAVLT-C | Items recalled in all trials 1–5 | |
| Overall score | |||
| Rey Auditory Verbal Learning Test – Dutch version464,468 | RAVLT-D | Sum of trials 1–5 | |
| Rey Auditory Verbal Learning Test – German version464,465 | RAVLT-G | Interference – trial 5 – trial 7 | |
| Trial 1 | |||
| Rivermead Behavioural Memory Test454 | RBMT | Prose recall | |
| Prose recall (est) | |||
| Prose recall (sum of two tests) | |||
| Recognition memory tests (Warrington)471 | RMT | Recognition | |
| Wechsler Adult Intelligence Scale – Third Edition403 | WAIS-III | Digit span – forwards | |
| Wechsler Adult Intelligence Scale – Revised404 | WAIS-R | Digit span – backwards | |
| Digit span – forwards | |||
| Wechsler Memory Scale – adapted388 | WMS adapted | Logical memory | |
| Wechsler Memory Scale – III455 | WMS-III | Auditory index score | |
| Index score | |||
| Logical memory | |||
| Logical memory – story A | |||
| Logical memory – story B | |||
| Logical memory 1 – 1st recall total score – stories A and B1 | |||
| LOGICAL memory 1 – recall total score – sum recall unit scores stories A, B1, B2 | |||
| Verbal paired associates | |||
| Wechsler Memory Scale – Revised388 | WMS-R | Digit span – total | |
| Index score | |||
| Logical memory | |||
| Verbal paired associates | |||
| Memory – visual delayed | Aggie figures learning test472 | AFLT | Overall score |
| Recognition | |||
| Memory for Designs473 | MFD | Correct – n | |
| CANTAB Pattern recognition memory474 | PRM | Correct – % | |
| Latency – ms | |||
| Rivermead Behavioural Memory Test454 | RBMT | Face recognition | |
| Picture recognition | |||
| Route | |||
| Rey–Osterrieth Complex Figure Test433,434 | R-OCFT | Retained –% | |
| Total score | |||
| Wechsler Memory Scale – III455 | WMS-III | Visual | |
| Visual reproduction | |||
| Wechsler Memory Scale – Revised388 | WMS-R | Visual paired associates | |
| Visual reproduction | |||
| Memory – visual immediate | Aggie figures learning test472 | AFLT | Overall score |
| Automated Performance Test System389 | APTS | ACODES – correct – n | |
| ACODES – speed – s | |||
| Benton Visual Retention Test – Fifth edition475 | BVRT | Correct – n | |
| Errors – n | |||
| CANTAB Delayed match to sample476 | CANTAB DMTS | All delayed – latency – ms | |
| Delayed – 0s – correct –% | |||
| Delayed – 12s – correct –% | |||
| Delayed – 4s – correct –% | |||
| Delayed – latency – ms | |||
| Simultaneous – correct –% | |||
| Simultaneous – latency – ms | |||
| CANTAB Spatial Span test477 | CANTAB SS | Spatial span | |
| Corsi Block Tapping Test478 | Corsi Block | Span | |
| Span plus one | |||
| Continuous visual memory test479 | CVMT | d′ | |
| False alarms | |||
| Hits | |||
| Recognition | |||
| Total | |||
| Memory for Designs473 | MFD | Correct – n | |
| Trials to completion – n | |||
| NS | NS | Paired associates – memory score – six-box trial | |
| Paired associates – memory score – eight-box trial | |||
| Pattern recognition – correct [%] | |||
| Pattern recognition – latency [s] | |||
| Spatial recognition – correct [%] | |||
| Spatial recognition – latency [s] | |||
| Spatial span | |||
| Visual paired associates – six-box trial – errors [n] | |||
| Visual paired associates – six-box trial – trials to completion | |||
| Visual paired associates – eight-box trial – errors [n] | |||
| Visual paired associates – eight-box trial – trials to completion | |||
| CANTAB Pattern recognition memory474 | PRM | Correct – % | |
| Latency – ms | |||
| Rey Auditory Verbal Learning Test464 | RAVLT | List B | |
| Sum of trials 1–5 | |||
| Trial 6 – interference list | |||
| Rivermead Behavioural Memory Test454 | RBMT | Route | |
| Recognition memory tests (Warrington)471 | RMT | Recognition | |
| Rey–Osterrieth Complex Figure Test433,434 | R-OCFT | Total score | |
| VIG: visuospatial memory466 | VIG | Recall | |
| Wechsler Memory Scale – III455 | WMS-III | Spatial span – visual backwards | |
| Spatial span – visual forwards | |||
| Spatial span – visual total | |||
| Visual | |||
| VISUAL reproduction | |||
| Wechsler Memory Scale – Revised388 | WMS-R | Figural memory | |
| Index score | |||
| Visual memory span | |||
| Visual paired associates | |||
| Visual reproduction | |||
| Visual reproduction% ret. | |||
| Visual reproduction 1 | |||
| Memory – working | Huizinga and van der Molen – mental counters480 | HvdM MC | MC – mental counters – correct – % |
| MC – mental counters – reaction time – ms | |||
| Huizinga and van der Molen – tic-tac-toe480 | HvdM TTT | Tic-tac-toe – correct – % | |
| Tic-tac-toe – reaction time – ms | |||
| n-back test (NS) | n-back | 0-back – correct responses – n | |
| 0-back – reaction time – ms | |||
| 1-back – correct responses – n | |||
| 1-back – reaction time – ms | |||
| 1-back and 2-back – auditory – correct responses – simple – % | |||
| 1-back and 2-back – auditory – reaction time – simple – ms | |||
| 1-back and 2-back – divided – correct responses – % | |||
| 1-back and 2-back – divided – reaction time – ms | |||
| 1-back and 2-back – divided – reaction time – ms | |||
| 1-back and 2-back – visual – correct responses – selective – % | |||
| 1-back and 2-back – visual – correct responses – simple – % | |||
| 1-back and 2-back – visual – reaction time – selective – % | |||
| 1-back and 2-back – visual – reaction time – selective – ms | |||
| 1-back and 2-back – visual – reaction time – simple – ms | |||
| 2-back – correct responses – n | |||
| 2-back – figures – correct responses – n | |||
| 2-back – figures – reaction time – ms | |||
| 2-back – letters – correct responses – n | |||
| 2-back – letters – reaction time – ms | |||
| 2-back – reaction time – ms | |||
| NS | NS | Affective – correct – 500 ms delay – % | |
| Affective – correct – 8000 ms delay – % | |||
| Affective – latency – 500 ms delay – ms | |||
| Affective – latency – 8000 ms delay – ms | |||
| Computation span | |||
| Delayed memory task – correct [n] | |||
| Reading span | |||
| Serial subtraction – SS7 – correct – n | |||
| Serial subtraction – SS7 – errors [n] | |||
| Spatial recall – correct – n | |||
| Spatial task – between errors–four-box trial | |||
| Spatial task – between errors–six-box trial | |||
| Spatial task – between errors–eight-box trial | |||
| Spatial task – error score – 4000 ms delay | |||
| Spatial task – error score – 4000–500 ms difference | |||
| Spatial task – error score – 500 ms delay | |||
| Spatial task – error score – 8000 ms delay | |||
| Spatial task – error score – 8000–500 ms difference | |||
| Spatial task – latency – 4000 ms delay – ms | |||
| Spatial task – latency – 4000–500 ms difference – ms | |||
| Spatial task – latency – 500 ms delay – ms | |||
| Spatial task – latency – 8000 ms delay – ms | |||
| Spatial task – latency – 8000–500 ms difference – ms | |||
| Spatial task – search strategy score | |||
| Spatial task – within errors–four-box trial | |||
| Spatial task – within errors–six-box trial | |||
| Spatial task – within errors–eight-box trial | |||
| Visuospatial span | |||
| Visuospatial span – alphabetic generation | |||
| Visuospatial span – control – no dual task | |||
| Visuospatial span – overall mean | |||
| Visuospatial span with random letter generation | |||
| Paced Auditory Serial Addition Test481 | PASAT | Hits – 1.6 s | |
| Hits – 2.4 s | |||
| Rapid visual information processing408 | RVIP | 5-minute task | |
| Test for Attentional Performance397 | TAP | 5 – divided attention – time [ms] | |
| 8 – intermodal integration – time [ms] | |||
| Wechsler Adult Intelligence Scale – Third Edition403 | WAIS-III | Letter number sequencing – scaled score | |
| Wechsler Memory Scale – III455 | WMS-III | Index score | |
| Mental control | |||
| Wechsler Memory Scale – Revised388 | WMS-R | Mental control | |
| Walter Reed Army Institute of Research Performance Assessment Battery405 | WRAIR PAB | Matching to sample task | |
| Serial add and subtract test | |||
| Mood | Affective Go/No-go task406 | CANTAB A-G/N-G | Affective bias [ms] |
| EWL Mood Rating Scale482 | EWL | Activity | |
| Depressiveness | |||
| Emotional excitability | |||
| Extro-/introversion | |||
| Inactivation | |||
| Well-being | |||
| Frontal Systems Behavioral Scale416 | FrSBe | Apathy | |
| Mood Rating Scale (visual analogue scale)382 | MRS | Discontentedness | |
| Sedation | |||
| Nowlis Mood Adjective Checklist483 | NMAC | Overall score | |
| Profile Of Mood States (Visual Analogue Scale)383 | POMS | Anger–hostility | |
| Confusion | |||
| Depression–dejection | |||
| Fatigue | |||
| Friendliness | |||
| Tension | |||
| Vigour | |||
| Symptom Check List (SCL-90) | SCL-90 | Positive moods | |
| Self-rated | S-R | Abnormal | |
| Calm | |||
| Clearheaded | |||
| Depressed | |||
| Drowsy | |||
| Energetic | |||
| Good tempered | |||
| Ill | |||
| Interested | |||
| Quick witted | |||
| Sad | |||
| Sober | |||
| Steady | |||
| Unpleasant | |||
| Unsociable | |||
| Well co-ordinated | |||
| Motor function | Automated Performance Test System389 | APTS | ATAP – finger tapping test – non-dominant hand |
| BTAP – finger tapping test – non-dominant hand | |||
| Grooved pegboard484 | Grooved pegboard | Time – dominant hand | |
| Time – left hand | |||
| Time – non-dominant hand | |||
| Time – right hand | |||
| NS | NS | Finger tapping test – dominant hand | |
| Finger tapping test – non-dominant hand | |||
| Orientation | Rivermead Behavioural Memory Test454 | RBMT | Date |
| Orientation | |||
| Perceptual organisation | Automated Performance Test System389 | APTS | PATRNC correct – n |
| PATRNC speed – s | |||
| Judgment of Line Orientation485 | JOLO | Pairs | |
| Mental Rotation test486 | Mental rotation test | Completely perfect [n] | |
| Mirror – errors [n] | |||
| Mirror – latency [ms] | |||
| Reaction time [ms] | |||
| Standard – errors [n] | |||
| Standard – latency [ms] | |||
| NS | NS | Heading task – angle 1 – correct [%] | |
| Heading task – angle 2 – correct [%] | |||
| Heading task – angle 4 – correct [%] | |||
| Heading task – angle 8 – correct [%] | |||
| Wechsler Adult Intelligence Scale – Revised404 | WAIS-R | Block design | |
| Block test – tile manipulation – copy – moves per problem | |||
| Block test – tile manipulation – copy – no. completely perfect | |||
| Block test – tile manipulation – copy – reaction time | |||
| Block test – tile manipulation – copy – thinking time | |||
| Block test – tile manipulation – mental rotation – moves per problem | |||
| Block test – tile manipulation – mental rotation – no. completely perfect | |||
| Block test – tile manipulation – mental rotation – thinking time | |||
| Block test – tile manipulation – mirror – errors | |||
| Block test – tile manipulation – mirror – latency – ms | |||
| Block test – tile manipulation – mirror – moves per problem | |||
| Block test – tile manipulation – mirror – no. completely perfect | |||
| Block test – tile manipulation – mirror – reaction time | |||
| Block test – tile manipulation – mirror – thinking time | |||
| Walter Reed Army Institute of Research Performance Assessment Battery405 | WRAIR PAB | Manikin task | |
| Time wall task | |||
| Personality | Eysenck Personality Questionnaire487 | EPQ | Extroversion |
| Lies | |||
| Neuroticism | |||
| Psychoticism | |||
| Goldberg’s Big Five questionnaire – Dutch version488 | GB5 | Agreeableness | |
| Conscientiousness | |||
| Emotional stability | |||
| Extroversion | |||
| Open experiences | |||
| Adult impulsiveness, venturesomeness and empathy scale438 | IVE | Empathy | |
| Venturesomeness | |||
| Sensation-Seeking Scale 489 | SSS | Sensation-seeking – boredom susceptibility | |
| Sensation-seeking – disinhibition | |||
| Sensation-seeking – experience seeking | |||
| Sensation-seeking – overall | |||
| Sensation-seeking – thrill and adventure seeking | |||
| Sensation-Seeking Scale – Dutch version489,490 | SSS-D | Sensation-seeking – boredom susceptibility | |
| Sensation-seeking – disinhibition | |||
| Sensation-seeking – experience seeking | |||
| Sensation-seeking – general | |||
| Sensation-seeking – thrill and adventure seeking | |||
| Tridimensional Personality Questionnaire491 | TPQ | Harm avoidance | |
| Novelty seeking | |||
| Reward dependence | |||
| Psychopathology | DSM-III-R – Structured Clinical Interview492 | DSM-III-R SCI | Axis 1 disorders |
| Axis 2 disorders | |||
| DSM-IV379 | DSM-IV | ADHD – current | |
| ADHD – lifetime | |||
| Adjustment disorder – current | |||
| Adjustment disorder – lifetime | |||
| Affective disorder – current | |||
| Affective disorder – lifetime | |||
| Eating disorder – current | |||
| Eating disorder – lifetime | |||
| SIDP – axis II disorders | |||
| ICD-10 | ICD-10 | Psychosis | |
| Personality Diagnostic Questionnaire – Revised493 | PDQ-R | Overall score | |
| Symptom Check List (SCL-90) | SCL-90 | Agoraphobia | |
| Anger–hostility | |||
| Appetite | |||
| Death cognitions | |||
| Early waking | |||
| Global score index | |||
| Guilt | |||
| Hostility | |||
| Insomnia | |||
| Insufficency | |||
| Interpersonal sensitivity | |||
| MDMA side effects | |||
| Negative psychobiology | |||
| Obessionality | |||
| Obsession–compulsion | |||
| Obsessive–compulsive | |||
| Overeating | |||
| Paranoid ideation | |||
| Positive life experiences | |||
| Positive psychobiology | |||
| Psychoticism | |||
| Sensitivity | |||
| Sociability | |||
| Somatisation | |||
| Total | |||
| Total negative | |||
| Total positive | |||
| Symptom Check List (SCL-90-R)377 | SCL-90-R | Anger-hostility | |
| GSI | |||
| Interpersonal sensitivity | |||
| Obsessive–compulsive | |||
| Overall score | |||
| Paranoid ideation | |||
| PSDI score | |||
| Psychoticism | |||
| Sensitivity | |||
| Somatisation | |||
| Symptom Check List – Brief Symptom Inventory384 | SCL–BSI | Anger–hostility | |
| Global severity index | |||
| Global severity index – moderate | |||
| Global severity index – severe | |||
| Hostility – moderate | |||
| Hostility – severe | |||
| Interpersonal sensitiveness | |||
| Obsessive–compulsive | |||
| Obsessive–compulsive – moderate | |||
| Obsessive–compulsive – severe | |||
| Paranoid ideation | |||
| Paranoid ideation – moderate | |||
| Paranoid ideation – severe | |||
| Positive symptom total | |||
| Positive symptoms distress index | |||
| Psychoticism | |||
| Psychoticism – moderate | |||
| Psychoticism – severe | |||
| Somatic complaints – moderate | |||
| Somatic complaints – severe | |||
| Somatisation | |||
| Reasoning | Automated Performance Test System389 | APTS | AREASON – correct – n |
| AREASON – speed – s | |||
| Leistungsprüfsystem–4466,494 | LPS–4 | Logical thinking/problem solving | |
| NS | NS | Syllogistic reasoning – correct – NVC – n | |
| Syllogistic reasoning – correct – one model – n | |||
| Syllogistic reasoning – correct – three model – n | |||
| Syllogistic reasoning – correct – three model/NVC – n | |||
| Syllogistic reasoning – correct – total – % | |||
| Syllogistic reasoning – correct – two/three model – n | |||
| Syllogistic reasoning – incorrect – NVC – n | |||
| Syllogistic reasoning – incorrect – one model – n | |||
| Syllogistic reasoning – incorrect – three model – n | |||
| Syllogistic reasoning – incorrect – total – s | |||
| Syllogistic reasoning – no response – NVC – n | |||
| Syllogistic reasoning – no response – one model – n | |||
| Syllogistic reasoning – no response – three model – n | |||
| Syllogistic reasoning – no response – total – n | |||
| Wechsler Adult Intelligence Scale – Third Edition403 | WAIS-III | Matrix reasoning – scaled score | |
| Wechsler Abbreviated Scale of Intelligence403 | WASI | Matrix reasoning | |
| Walter Reed Army Institute of Research Performance Assessment Battery405 | WRAIR PAB | Logical reasoning task | |
| Sleep | Epworth sleepiness scale495 | Epworth SS | Total score |
| NS | NS | Hours/night | |
| Morning/evening type | |||
| Quality | |||
| Refreshed | |||
| REM latency [min] | |||
| Sleep efficiency [%] | |||
| Sleep latency – min | |||
| Sometimes miss out a night | |||
| Total sleep time – min | |||
| Wake time after sleep onset – min | |||
| Rechtschaffen and Kales sleep rating procedures496 | Rechtschaffen and Kales | NREM – min | |
| REM – min | |||
| Stage 1 – min | |||
| Stage 2 – min | |||
| Stage 3/4 – min | |||
| Stage REM – min | |||
| TST – min | |||
| Symptom Check List (SCL-90) | SCL-90 | Self-reported sleep disturbances | |
| Self-rated | S-R | Scale 1–5 | |
| Sleep disorder | |||
| Verbal skills | Boston naming test497 | BNT | Naming fluency |
| Controlled Oral Word Association (‘FAS’ test) (NS) | COWA | Fluency – category – animals [n] | |
| Fluency – category [n] | |||
| Fluency – errors [n] | |||
| Fluency – inappropriate words [n] | |||
| Fluency – letter – FAS [n] | |||
| Fluency – letter [n] | |||
| Fluency – perseverative errors [n] | |||
| Fluency – switching [n] | |||
| Fluency – total [n] | |||
| Fluency – total perseverations [n] | |||
| Chicago Word Fluency Test498 | CWF | Fluency – letter – C4 [n] | |
| Fluency – letter – S [n] | |||
| Delis–Kaplan Executive Function System431 | D–KEFS | Fluency – category | |
| Fluency – FAS | |||
| Fluency – switching | |||
| NS | NS | Anagrams – correct [n] | |
| Anagrams – ln – time – [s] | |||
| Anagrams – time [s] | |||
| Fluency – category [n] |
Appendix 6 Datasets used in meta-analyses of composite outcome measures
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs polydrug controls | |||||||||||
| Parrott and Lasky 1998121 | Free recall | (+) | (1) Users (regular) vs controls | 15 | 12 | 2.324 | 7.5 | 15.1 | 3.098 | –1.195 | (–2.145 to –0.245) |
| (2) Users (novice) vs controls | 15 | 13.9 | 2.711 | 7.5 | 15.1 | 3.098 | –0.423 | (–1.309 to 0.463) | |||
| Bolla et al. 199893 | RAVLT: sum of trials 1–5 | (+) | 24 | 53.2 | 7.8 | 24 | 56 | 8.2 | –0.350 | (–0.920 to 0.220) | |
| WMS-R: digit span – total | (+) | 24 | 18.1 | 3.2 | 24 | 18.8 | 3.9 | –0.196 | (–0.763 to 0.371) | ||
| WMS-R: logical memory | (+) | 24 | 26.2 | 6.1 | 24 | 29.9 | 6.3 | –0.597 | (–1.175 to –0.018) | ||
| WMS-R: verbal paired associates | (+) | 24 | 21.3 | 3.6 | 24 | 22.1 | 2.1 | –0.271 | (–0.840 to 0.297) | ||
| Morgan 1999102 | RBMT: prose recall | (+) | 25 | 6.14 | 2.23 | 22 | 8.09 | 1.86 | –0.944 | (–1.549 to –0.339) | |
| Rodgers 2000122 | WMS-R: index score | (+) | 15 | 86.4 | 12.8 | 15 | 88.07 | 10.46 | –0.143 | (–0.860 to 0.574) | |
| Gouzoulis et al. 200099 | RAVLT-G: interference – trial 5 – trial 7 | (–) | 28 | 2.14 | 2.07 | 28 | 1.5 | 1.77 | –0.332 | (–0.860 to 0.195) | |
| RAVLT-G: trial 1 | (+) | 28 | 7.82 | 1.93 | 28 | 8.71 | 2.03 | –0.449 | (–0.980 to 0.081) | ||
| WAIS-R: digit span – backwards | (+) | 28 | 7 | 2.34 | 28 | 8 | 1.87 | –0.472 | (–1.003 to 0.059) | ||
| WAIS-R: digit span – forwards | (+) | 28 | 8.54 | 1.84 | 28 | 8.93 | 1.51 | –0.232 | (–0.757 to 0.294) | ||
| Fox et al. 2001112 | MRR: recall (hits – intrusions) | (–) | (1) Users (high-dose) vs controls | 11 | 15.8 | 7.2 | 6.67 | 14.5 | 5.3 | –0.198 | (–1.162 to 0.767) |
| (2) Users (medium) vs controls | 14 | 14.9 | 5.6 | 6.67 | 14.5 | 5.3 | –0.073 | (–0.995 to 0.850) | |||
| (3) Users (low) vs controls | 14 | 15.4 | 4.3 | 6.67 | 14.5 | 5.3 | –0.195 | (–1.119 to 0.730) | |||
| MRR: recognition (hits – false alarms) | (–) | (1) Users (high-dose) vs controls | 11 | 11.9 | 6.8 | 6.67 | 16.3 | 5.2 | 0.702 | (–0.291 to 1.695) | |
| (2) Users (medium) vs controls | 14 | 13.1 | 5.4 | 6.67 | 16.3 | 5.2 | 0.599 | (–0.343 to 1.541) | |||
| (3) Users (low) vs controls | 14 | 14.9 | 5.4 | 6.67 | 16.3 | 5.2 | 0.262 | (–0.664 to 1.188) | |||
| WMS adapted: logical memory | (+) | (1) Users (high-dose) vs controls | 11 | 19.5 | 7.8 | 6.67 | 19.8 | 4.6 | –0.044 | (–1.006 to 0.918) | |
| (2) Users (medium) vs controls | 14 | 17.7 | 5.9 | 6.67 | 19.8 | 4.6 | –0.379 | (–1.309 to 0.551) | |||
| (3) Users (low) vs controls | 14 | 21.4 | 4.4 | 6.67 | 19.8 | 4.6 | 0.359 | (–0.571 to 1.288) | |||
| Croft et al. 200194 | Digit span – backwards | (+) | 11 | 8 | 2.6 | 18 | 7.9 | 1.8 | 0.047 | (–0.703 to 0.797) | |
| Digit span – forwards | (+) | 11 | 9.2 | 1.6 | 18 | 9.6 | 1.5 | –0.260 | (–1.013 to 0.493) | ||
| RAVLT: list B | (+) | 11 | 7.3 | 2.6 | 18 | 6.5 | 2.2 | 0.340 | (–0.416 to 1.095) | ||
| RAVLT: sum of trials 1–5 | (+) | 11 | 57.2 | 11.3 | 18 | 56.3 | 9 | 0.091 | (–0.660 to 0.841) | ||
| RAVLT: trial 6 – interference list | (+) | 11 | 12.2 | 2.7 | 18 | 11.8 | 3 | 0.138 | (–0.613 to 0.889) | ||
| RMT: recognition | (+) | 11 | 45.5 | 7 | 18 | 48.3 | 1.8 | –0.623 | (–1.391 to 0.145) | ||
| Reneman et al. 200195 | RAVLT: sum of trials 1–5 | (+) | 8 | 45.8 | 9.3 | 7 | 53.8 | 6.6 | –0.980 | (–2.062 to 0.102) | |
| Simon and Mattick 2002123 | WMS-III: auditory index score | (+) | 40 | 106.4 | 13.4 | 37 | 112.4 | 14.7 | –0.427 | (–0.880 to 0.025) | |
| Morgan et al. 2002103 | RBMT: prose recall | (+) | 18 | 7.7 | 3.182 | 8 | 8.5 | 3 | –0.256 | (–1.092 to 0.580) | |
| Curran and Verheyden 2003104 | Buschke: trial 1 | (+) | 32 | 6.155 | 1.93 | 16 | 6.785 | 2.245 | –0.309 | (–0.913 to 0.294) | |
| RBMT: prose recall | (+) | 32 | 8.56 | 3.483 | 16 | 8.14 | 4.455 | 0.110 | (–0.491 to 0.710) | ||
| Gouzoulis et al. 2003108 | Digit span – backwards | (+) | (1) Users (heavy) vs controls | 30 | 8 | 2.3 | 15 | 8.8 | 2.3 | –0.348 | (–0.972 to 0.276) |
| (2) Users (moderate) vs controls | 30 | 8.8 | 2 | 15 | 8.8 | 2.3 | 0.000 | (–0.620 to 0.620) | |||
| Zakzanis et al. 2003101 | RBMT: prose recall | (+) | 15 | 1.5 | 0.7 | 17 | 1.7 | 0.5 | –0.332 | (–1.032 to 0.367) | |
| Halpern et al. 2004106 | CVLT-II: total trials 1–5 correct – n | (+) | (1) Users (heavy) vs controls | 11 | 63 | 9.6 | 8 | 66.5 | 8.8 | –0.377 | (–1.297 to 0.542) |
| (2) Users (moderate) vs controls | 12 | 66.2 | 7.3 | 8 | 66.5 | 8.8 | –0.038 | (–0.933 to 0.857) | |||
| CVLT-II: trial B correct – n | (+) | (1) Users (heavy) vs controls | 11 | 7.9 | 3.2 | 8 | 8.9 | 2.6 | –0.337 | (–1.255 to 0.581) | |
| (2) Users (moderate) vs controls | 12 | 9.5 | 3.1 | 8 | 8.9 | 2.6 | 0.206 | (–0.691 to 1.103) | |||
| WAIS-R: digit span – backwards | (+) | (1) Users (heavy) vs controls | 11 | 8.1 | 3.3 | 8 | 9.1 | 2.1 | –0.349 | (–1.267 to 0.569) | |
| (2) Users (moderate) vs controls | 12 | 8.7 | 1.6 | 8 | 9.1 | 2.1 | –0.221 | (–1.118 to 0.677) | |||
| WAIS-R: digit span – forwards | (+) | (1) Users (heavy) vs controls | 11 | 9 | 2 | 8 | 9.9 | 1.6 | –0.488 | (–1.413 to 0.438) | |
| (2) Users (moderate) vs controls | 12 | 9.7 | 2.2 | 8 | 9.9 | 1.6 | –0.101 | (–0.996 to 0.795) | |||
| WMS-III: logical memory | (+) | (1) Users (heavy) vs controls | 11 | 49.1 | 11.5 | 8 | 52.8 | 6.9 | –0.375 | (–1.294 to 0.544) | |
| (2) Users (moderate) vs controls | 12 | 52 | 9.1 | 8 | 52.8 | 6.9 | –0.096 | (–0.991 to 0.799) | |||
| WMS-III: verbal paired associates | (+) | (1) Users (heavy) vs controls | 11 | 24.9 | 6.2 | 8 | 28.7 | 3.1 | –0.737 | (–1.681 to 0.207) | |
| (2) Users (moderate) vs controls | 12 | 25.2 | 6.4 | 8 | 28.7 | 3.1 | –0.653 | (–1.572 to 0.267) | |||
| McCardle et al. 2004100 | Digit span – backwards | (+) | 17 | 5.12 | 1.65 | 15 | 5.53 | 1.25 | –0.278 | (–0.975 to 0.420) | |
| Digit span – forwards | (+) | 17 | 6.41 | 0.87 | 15 | 7.07 | 0.96 | –0.723 | (–1.441 to –0.005) | ||
| RAVLT: trial 1 | (+) | 17 | 7.47 | 1.84 | 15 | 8.07 | 2.37 | –0.285 | (–0.983 to 0.413) | ||
| RAVLT: trial 2 | (+) | 17 | 9.94 | 2.05 | 15 | 10.53 | 2.5 | –0.260 | (–0.957 to 0.438) | ||
| RAVLT: trial 3 | (+) | 17 | 12 | 2.03 | 15 | 12.07 | 1.79 | 0.036 | (–0.731 to 0.658) | ||
| RAVLT: trial 4 | (+) | 17 | 12.76 | 1.89 | 15 | 13.4 | 1.3 | –0.390 | (–1.091 to 0.311) | ||
| RAVLT: trial 5 | (+) | 17 | 12.41 | 1.97 | 15 | 13.4 | 1.55 | –0.554 | (–1.263 to 0.154) | ||
| RAVLT: trial 7 | (+) | 17 | 11.65 | 2.42 | 15 | 11.93 | 2.46 | –0.115 | (–0.810 to 0.580) | ||
| Dafters et al. 200475 | Free recall | (+) | (1) Heavy users vs controls | 16 | 8.85 | 2.15 | 7.5 | 9.6 | 2.15 | –0.349 | (–1.222 to 0.525) |
| (2) Light users vs controls | 19 | 9.3 | 2.55 | 7.5 | 9.6 | 2.15 | –0.122 | (–0.968 to 0.723) | |||
| RBMT: prose recall | (+) | (1) Heavy users vs controls | 16 | 4.6 | 1.7 | 7.5 | 4.3 | 1.9 | 0.170 | (–0.699 to 1.039) | |
| (2) Light users vs controls | 19 | 5.1 | 1.6 | 7.5 | 4.3 | 1.9 | 0.475 | (–0.381 to 1.330) | |||
| Medina et al. 2005124 | CVLT-II: short-delay cued recall (z-score) | (+) | 48 | –0.57 | 0.95 | 17 | –0.03 | 0.8 | –0.591 | (–1.153 to –0.028) | |
| CVLT-II: short-delay free recall (z-score) | (+) | 48 | –0.38 | 0.99 | 17 | –0.06 | 0.61 | –0.352 | (–0.909 to 0.204) | ||
| Thomasius et al. 200596 | RAVLT: sum of trials 1–5 | (+) | Data from secondary pub.105 | 30 | 58.8 | 9.585 | 14.5 | 53.6 | 9.424 | 0.545 | (–0.092 to 1.183) |
| RBMT: prose recall | (+) | Data from secondary pub.105 | 30 | 9.5 | 3.286 | 14.5 | 9.5 | 3.096 | 0.000 | (–0.627 to 0.627) | |
| Montgomery et al. 2005120 | Verbal paired associates – perseverative responses – n | (–) | (1) High lifetime dose vs controls | 18 | 0.67 | 1.28 | 31 | 0.16 | 0.66 | –0.547 | (–1.138 to 0.045) |
| (2) Low lifetime dose vs controls | 17 | 0.71 | 1.05 | 31 | 0.16 | 0.66 | –0.673 | (–1.280 to –0.066) | |||
| Verbal paired associates – trials to completion | (–) | (1) High lifetime dose vs controls | 18 | 5.67 | 1.28 | 31 | 4.32 | 1.46 | –0.966 | (–1.579 to –0.353) | |
| (2) Low lifetime dose vs controls | 17 | 6.59 | 2.4 | 31 | 4.32 | 1.46 | –1.232 | (–1.875 to –0.589) | |||
| Verbal paired associates – errors Trial 1 | (–) | (1) High lifetime dose vs controls | 18 | 0.72 | 0.83 | 31 | 0.39 | 0.75 | –0.423 | (–1.010 to 0.164) | |
| (2) Low lifetime dose vs controls | 17 | 1 | 1.22 | 31 | 0.39 | 0.75 | –0.649 | (–1.255 to –0.042) | |||
| Verbal paired associates – errors Trial 2 | (–) | (1) High lifetime dose vs controls | 18 | 0.06 | 0.24 | 31 | 0.1 | 0.35 | 0.127 | (–0.454 to 0.708) | |
| (2) Low lifetime dose vs controls | 17 | 0.47 | 0.87 | 31 | 0.1 | 0.35 | –0.632 | (–1.237 to –0.026) | |||
| Verbal paired associates – Trial 1 – correct – n | (+) | (1) High lifetime dose vs controls | 18 | 2.67 | 1.81 | 31 | 4.32 | 2.01 | –0.850 | (–1.456 to –0.245) | |
| (2) Low lifetime dose vs controls | 17 | 3.29 | 2.2 | 31 | 4.32 | 2.01 | –0.496 | (–1.096 to 0.104) | |||
| Reneman et al. 200697 | RAVLT: sum of trials 1–5 | (+) | (1) Users (heavy) vs controls | 22 | 47 | 8.6 | 4.33 | 60 | 6.8 | –1.552 | (–2.671 to –0.433) |
| (2) Users (moderate) vs controls | 15 | 51.2 | 8.6 | 4.33 | 60 | 6.8 | –1.062 | (–2.188 to 0.064) | |||
| RBMT: prose recall (sum for 2 tests) | (+) | (1) Users (heavy) vs controls | 22 | 17.9 | 3.8 | 4.33 | 17.9 | 6.1 | 0.000 | (–1.030 to 1.030) | |
| (2) Users (moderate) vs controls | 15 | 16.1 | 5.2 | 4.33 | 17.9 | 6.1 | –0.334 | (–1.409 to 0.740) | |||
| Reay et al. 2006109 | Digit span – backwards | (+) | 15 | 5.61 | 1.19 | 15 | 6.59 | 1.4 | –0.754 | (–1.497 to –0.012) | |
| Quednow et al. 200683 | RAVLT: adjusted list A | (+) | 19 | 0.85 | 0.1 | 19 | 0.93 | 0.05 | –1.012 | (–1.689 to –0.334) | |
| RAVLT: adjusted list B | (+) | 19 | 0.74 | 0.03 | 19 | 0.84 | 0.02 | –3.922 | (–5.029 to –2.815) | ||
| RAVLT: sum of trials 1–5 | (+) | 19 | 56.2 | 8.16 | 19 | 64.8 | 6.21 | –1.186 | (–1.878 to –0.494) | ||
| Lamers et al. 200698 | RAVLT: sum of trials 1–5 | (+) | 11 | 51.5 | 7.6 | 15 | 52.3 | 7.1 | –0.109 | (–0.888 to 0.669) | |
| de Win et al. 200584 | RAVLT-D: sum of trials 1–5 | (+) | Follow-up. Data from secondary pub.90 | 58 | 59.6 | 6.5 | 60 | 61.7 | 5.9 | –0.339 | (–0.702 to 0.025) |
| WAIS-R: digit span – backwards | (+) | Follow-up. Data from secondary pub.90 | 58 | 11.8 | 2.4 | 60 | 11.4 | 2.9 | 0.150 | (–0.211 to 0.511) | |
| WAIS-R: digit span – forwards | (+) | Follow-up. Data from secondary pub.90 | 58 | 15.5 | 2.8 | 60 | 15.1 | 2.2 | 0.159 | (–0.202 to 0.521) | |
| McCann et al. 2007117 | WMS-III: logical memory (recall total score) | (+) | 25 | 39.72 | 10.52 | 23 | 46.56 | 6.84 | –0.764 | (–1.352 to –0.177) | |
| Hoshi et al. 2007125 | Buschke: trial 1 | (+) | 25 | 5.35 | 1.5 | 14.5 | 5.2 | 1.62 | 0.097 | (–0.550 to 0.744) | |
| Buschke: trial 2 | (+) | 25 | 7.6 | 2 | 14.5 | 8.1 | 2.69 | –0.220 | (–0.869 to 0.429) | ||
| Buschke: trial 3 | (+) | 25 | 9.1 | 2 | 14.5 | 10 | 2.96 | –0.377 | (–1.029 to 0.276) | ||
| Groth et al. 2007124 | WMS-III: index score | (+) | 26 | 107.4 | 15.2 | 26 | 113.2 | 11.6 | –0.429 | (–0.979 to 0.121) | |
| Former users vs polydrug controls | |||||||||||
| Morgan et al. 2002103 | RBMT: prose recall | (+) | 15 | 6.1 | 3.679 | 8 | 8.5 | 3 | –0.692 | (–1.575 to 0.191) | |
| Curran and Verheyden 2003104 | Buschke: trial 1 | (+) | 32 | 5.755 | 1.607 | 16 | 6.785 | 2.245 | –0.560 | (–1.171 to 0.051) | |
| RBMT: prose recall | (+) | 32 | 6.83 | 2.973 | 16 | 8.14 | 4.455 | –0.372 | (–0.976 to 0.233) | ||
| Thomasius et al. 200596 | RAVLT: sum of trials 1–5 | (+) | Data from secondary pub.105 | 31 | 53.6 | 8.352 | 14.5 | 53.6 | 9.424 | 0.000 | (–0.624 to 0.624) |
| RBMT: prose recall | (+) | Data from secondary pub.105 | 31 | 8.2 | 2.617 | 14.5 | 9.5 | 3.096 | –0.469 | (–1.100 to 0.163) | |
| Reneman et al. 200697 | RAVLT: sum of trials 1–5 | (+) | 16 | 48 | 12.5 | 4.33 | 60 | 6.8 | –1.028 | (–2.140 to 0.084) | |
| RBMT: prose recall | (+) | 16 | 16.3 | 5.8 | 4.33 | 17.9 | 6.1 | –0.273 | (–1.338 to 0.792) | ||
| Hoshi et al. 2007125 | Buschke: trial 1 | (+) | 28 | 6.15 | 2.12 | 14.5 | 5.2 | 1.62 | 0.483 | (–0.160 to 1.126) | |
| Buschke: trial 2 | (+) | 28 | 8.85 | 2.12 | 14.5 | 8.1 | 2.69 | 0.323 | (–0.316 to 0.961) | ||
| Buschke: trial 3 | (+) | 28 | 9.95 | 2.65 | 14.5 | 10 | 2.96 | –0.018 | (–0.652 to 0.616) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs drug-naïve controls | |||||||||||
| Morgan 1999102 | RBMT: prose recall | (+) | 25 | 6.14 | 2.23 | 19 | 8.29 | 2.87 | –0.852 | (–1.475 to –0.228) | |
| Rodgers 2000122 | WMS-R: index score | (+) | 15 | 86.4 | 12.8 | 15 | 109.7 | 12.79 | –1.819 | (–2.678 to –0.959) | |
| Gouzoulis et al. 200099 | RAVLT-G: interference – trial 5 – trial 7 | (–) | 28 | 2.14 | 2.07 | 28 | 0.89 | 1.55 | –0.684 | (–1.223 to –0.144) | |
| RAVLT-G: trial 1 | (+) | 28 | 7.82 | 1.93 | 28 | 9.82 | 2.28 | –0.947 | (–1.500 to –0.393) | ||
| WAIS-R: digit span – backwards | (+) | 28 | 7 | 2.34 | 28 | 9.11 | 2.67 | –0.840 | (–1.388 to –0.293) | ||
| WAIS-R: digit span – forwards | (+) | 28 | 8.54 | 1.84 | 28 | 8.89 | 1.29 | –0.220 | (–0.746 to 0.305) | ||
| Bhattachary and Powell 2001127 | Digit span – backwards | (+) | (1) Regular users vs controls | 26 | 5.5 | 0.99 | 6.67 | 5.85 | 1.04 | –0.350 | (–1.206 to 0.505) |
| (2) Novice users vs controls | 18 | 5.56 | 1.09 | 6.67 | 5.85 | 1.04 | –0.269 | (–1.161 to 0.623) | |||
| Prose recall | (+) | (1) Regular users vs controls | 26 | 10.58 | 1.93 | 6.67 | 15.8 | 1.55 | –2.798 | (–3.900 to –1.696) | |
| (2) Novice users vs controls | 18 | 14.44 | 1.97 | 6.67 | 15.8 | 1.55 | –0.726 | (–1.639 to 0.188) | |||
| Croft et al. 200194 | Digit span – backwards | (+) | 11 | 8 | 2.6 | 31 | 9.3 | 2.2 | –0.564 | (–1.262 to 0.135) | |
| Digit span – forwards | (+) | 11 | 9.2 | 1.6 | 31 | 10.5 | 1.8 | –0.742 | (–1.449 to –0.035) | ||
| RAVLT: list B | (+) | 11 | 7.3 | 2.6 | 31 | 8.5 | 2.7 | –0.449 | (–1.143 to 0.246) | ||
| RAVLT: sum of trials 1–5 | (+) | 11 | 57.2 | 11.3 | 31 | 62.4 | 7.7 | –0.595 | (–1.295 to 0.105) | ||
| RAVLT: trial 6 – interference list | (+) | 11 | 12.2 | 2.7 | 31 | 13.2 | 1.6 | –0.517 | (–1.214 to 0.180) | ||
| RMT: recognition | (+) | 11 | 45.5 | 7 | 31 | 48.3 | 2.3 | –0.695 | (–1.400 to 0.009) | ||
| Morgan et al. 2002103 | RBMT: prose recall | (+) | 18 | 7.7 | 3.182 | 7.5 | 9.95 | 3.873 | –0.664 | (–1.537 to 0.208) | |
| Dafters et al. 200475 | Free recall | (+) | (1) Heavy users vs controls | 16 | 8.85 | 2.15 | 9.5 | 12.4 | 2.15 | –1.651 | (–2.582 to –0.720) |
| (2) Light users vs controls | 19 | 9.3 | 2.55 | 9.5 | 12.4 | 2.15 | –1.276 | (–2.128 to –0.425) | |||
| RBMT: prose recall | (+) | (1) Heavy users vs controls | 16 | 4.6 | 1.7 | 9.5 | 6.3 | 2.7 | –0.803 | (–1.638 to 0.032) | |
| (2) Light users vs controls | 19 | 5.1 | 1.6 | 9.5 | 6.3 | 2.7 | –0.594 | (–1.389 to 0.201) | |||
| Thomasius et al. 200596 | RAVLT: sum of trials 1–5 | (+) | Data from secondary pub.105 | 30 | 58.8 | 9.585 | 15 | 60.3 | 8.216 | –0.164 | (–0.784 to 0.457) |
| RBMT: prose recall | (+) | Data from secondary pub.105 | 30 | 9.5 | 3.286 | 15 | 10.75 | 3.286 | –0.380 | (–1.005 to 0.245) | |
| Yip and Lee 2005128 | Digit span – backwards | (+) | 100 | 9.38 | 1.01 | 100 | 9.57 | 1.03 | –0.186 | (–0.464 to 0.092) | |
| Digit span – forwards | (+) | 100 | 6.66 | 0.96 | 100 | 6.75 | 1.02 | –0.091 | (–0.368 to 0.186) | ||
| RAVLT-C: items recalled in all trials 1–5 | (+) | 100 | 5.2 | 0.8 | 100 | 10.51 | 1.45 | –4.535 | (–5.060 to –4.009) | ||
| Quednow et al. 200683 | RAVLT: adjusted list A | (+) | 19 | 0.85 | 0.1 | 19 | 0.9 | 0.08 | –0.552 | (–1.201 to 0.096) | |
| RAVLT: adjusted list B | (+) | 19 | 0.74 | 0.03 | 19 | 0.81 | 0.03 | –2.333 | (–3.167 to –1.500) | ||
| RAVLT: sum of trials 1–5 | (+) | 19 | 56.2 | 8.16 | 19 | 64.7 | 5.72 | –1.206 | (–1.901 to –0.512) | ||
| Lamers et al. 200698 | RAVLT: sum of trials 1–5 | (+) | 11 | 51.5 | 7.6 | 15 | 60 | 5.3 | –1.336 | (–2.201 to –0.471) | |
| Hoshi et al. 2007125 | Buschke: trial 1 | (+) | 25 | 5.35 | 1.5 | 13.5 | 7.1 | 2.08 | –1.017 | (–1.719 to –0.315) | |
| Buschke: trial 2 | (+) | 25 | 7.6 | 2 | 13.5 | 10.45 | 2.6 | –1.282 | (–2.006 to –0.557) | ||
| Buschke: trial 3 | (+) | 25 | 9.1 | 2 | 13.5 | 11.6 | 2.6 | –1.124 | (–1.835 to –0.414) | ||
| Former users vs drug-naïve controls | |||||||||||
| Bhattachary and Powell 2001127 | Digit span – backwards | (+) | 16 | 5.44 | 0.96 | 6.67 | 5.85 | 1.04 | –0.417 | (–1.330 to 0.495) | |
| Prose recall | (+) | 16 | 11.09 | 1.86 | 6.67 | 15.8 | 1.55 | –2.646 | (–3.857 to –1.434) | ||
| Morgan et al. 2002103 | RBMT: prose recall | (+) | 15 | 6.1 | 3.679 | 7.5 | 9.95 | 3.873 | –1.029 | (–1.960 to –0.098) | |
| Thomasius et al. 200596 | RAVLT: sum of trials 1–5 | (+) | Data from secondary pub.105 | 31 | 53.6 | 8.352 | 15 | 60.3 | 8.216 | –0.806 | (–1.445 to –0.167) |
| RBMT: prose recall | (+) | Data from secondary pub.105 | 31 | 8.2 | 2.617 | 15 | 10.75 | 3.286 | –0.896 | (–1.540 to –0.251) | |
| Hoshi et al. 2007125 | Buschke: trial 1 | (+) | 28 | 6.15 | 2.12 | 13.5 | 7.1 | 2.08 | –0.451 | (–1.108 to 0.206) | |
| Buschke: trial 2 | (+) | 28 | 8.85 | 2.12 | 13.5 | 10.45 | 2.6 | –0.701 | (–1.368 to –0.033) | ||
| Buschke: trial 3 | (+) | 28 | 9.95 | 2.65 | 13.5 | 11.6 | 2.6 | –0.626 | (–1.290 to 0.038) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs polydrug controls | |||||||||||
| Bolla et al. 199893 | RAVLT: recognition | (+) | 24 | 13.8 | 1.3 | 24 | 14.3 | 0.9 | –0.447 | (–1.020 to 0.126) | |
| RAVLT: trial 8 | (+) | 24 | 11 | 2.9 | 24 | 12 | 2.3 | –0.382 | (–0.953 to 0.189) | ||
| WMS-R: logical memory | (+) | 24 | 22 | 7.1 | 24 | 26.9 | 7.3 | –0.680 | (–1.263 to –0.098) | ||
| WMS-R: verbal paired associates | (+) | 24 | 7.8 | 0.5 | 24 | 7.9 | 0.3 | –0.243 | (–0.810 to 0.325) | ||
| Morgan 1999102 | RBMT: prose recall | (+) | 25 | 5.36 | 2.48 | 22 | 7.23 | 1.9 | –0.839 | (–1.438 to –0.241) | |
| Rodgers 2000122 | WMS-R: logical memory | (+) | 15 | 18.4 | 12.3 | 15 | 20.1 | 8.9 | –0.158 | (–0.875 to 0.559) | |
| WMS-R: verbal paired associates | (+) | 15 | 8.5 | 7.8 | 15 | 16.5 | 4.8 | –1.235 | (–2.021 to –0.450) | ||
| Gouzoulis et al. 200099 | RAVLT-G: trial 8 | (+) | 28 | 13.79 | 1.75 | 28 | 14.43 | 1.07 | –0.441 | (–0.972 to 0.089) | |
| Fox et al. 2001112 | WMS adapted: logical memory | (+) | (1) High-dose users vs controls | 11 | 19.8 | 6.1 | 6.67 | 18.2 | 4 | 0.294 | (–0.673 to 1.262) |
| (2) Medium-dose users vs controls | 14 | 15.8 | 6.6 | 6.67 | 18.2 | 4 | –0.405 | (–1.336 to 0.527) | |||
| (3) Low-dose users vs controls | 14 | 20 | 4.3 | 6.67 | 18.2 | 4 | 0.427 | (–0.505 to 1.360) | |||
| Reneman et al. 200195 | RAVLT: trial 8 | (+) | 8 | 10.6 | 2 | 7 | 12.8 | 1.9 | –1.126 | (–2.228 to –0.023) | |
| Simon and Mattick 2002123 | WMS-III: auditory index score | (+) | 40 | 105.3 | 11.9 | 37 | 109.9 | 10.2 | –0.414 | (–0.866 to 0.038) | |
| WMS-R: verbal reproduction | (+) | 40 | 104.5 | 13.4 | 37 | 106.9 | 12.9 | –0.182 | (–0.630 to 0.266) | ||
| Morgan et al. 2002103 | RBMT: prose recall | (+) | 18 | 6.2 | 3.394 | 8 | 7 | 3 | –0.244 | (–1.079 to 0.592) | |
| Curran and Verheyden 2003104 | Buschke: overall score | (+) | 32 | 7.315 | 3.045 | 16 | 7.905 | 3.11 | –0.192 | (–0.794 to 0.409) | |
| RBMT: prose recall | (+) | 32 | 7.565 | 3.528 | 16 | 7.515 | 4.33 | 0.013 | (–0.587 to 0.613) | ||
| Zakzanis et al. 2003101 | RBMT: prose recall | (+) | 15 | 1.8 | 0.4 | 17 | 1.7 | 0.5 | 0.219 | (–0.477 to 0.916) | |
| Halpern et al. 2004106 | WMS-III: logical memory | (+) | (1) Heavy users vs controls | 11 | 32 | 8.4 | 8 | 34.8 | 5.8 | –0.376 | (–1.296 to 0.543) |
| (2) Moderate users vs controls | 12 | 34.1 | 4.5 | 8 | 34.8 | 5.8 | –0.139 | (–1.034 to 0.757) | |||
| WMS-III: verbal paired associates | (+) | (1) Heavy users vs controls | 11 | 7.5 | 1.3 | 8 | 7.9 | 0.3 | –0.394 | (–1.314 to 0.526) | |
| (2) Moderate users vs controls | 12 | 7.6 | 1.4 | 8 | 7.9 | 0.3 | –0.270 | (–1.169 to 0.629) | |||
| McCardle et al. 2004100 | RAVLT: trial 8 | (+) | 17 | 11.18 | 2.58 | 15 | 12.13 | 2.13 | –0.399 | (–1.101 to 0.303) | |
| Dafters et al. 200475 | RBMT: prose recall | (+) | (1) Heavy users vs controls | 16 | 4.1 | 1.65 | 7.5 | 3.85 | 1.7 | 0.150 | (–0.718 to 1.019) |
| (2) Light users vs controls | 19 | 4.55 | 1.45 | 7.5 | 3.85 | 1.7 | 0.460 | (–0.395 to 1.315) | |||
| Medina et al. 2005124 | CVLT-II: long-delay free recall (z-score) | (+) | 48 | –0.57 | 1.1 | 17 | 0.06 | 0.86 | –0.603 | (–1.166 to –0.040) | |
| Thomasius et al. 200596 | RAVLT: overall score | (+) | Data from secondary pub.105 | 30 | 12.65 | 2.465 | 14.5 | 11.15 | 2.962 | 0.570 | (–0.069 to 1.208) |
| RBMT: prose recall | (+) | Data from secondary pub.105 | 30 | 8.52 | 3.122 | 14.5 | 8.95 | 2.962 | –0.140 | (–0.768 to 0.488) | |
| Reneman et al. 200697 | RAVLT: trial 8 | (+) | (1) Heavy users vs controls | 22 | 9.8 | 2.9 | 4.33 | 13.1 | 2.1 | –1.177 | (–2.259 to –0.095) |
| (2) Moderate users vs controls | 15 | 10.7 | 3.2 | 4.33 | 13.1 | 2.1 | –0.795 | (–1.896 to 0.306) | |||
| RBMT: prose recall (sum for 2 tests) | (+) | (1) Heavy users vs controls | 22 | 14.4 | 3.9 | 4.33 | 15.3 | 5.8 | –0.214 | (–1.246 to 0.818) | |
| (2) Moderate users vs controls | 15 | 12.7 | 5.4 | 4.33 | 15.3 | 5.8 | –0.475 | (–1.555 to 0.606) | |||
| Quednow et al. 200683 | RAVLT: recognition – errors – list B | (–) | 19 | 4.3 | 3.1 | 19 | 2.4 | 2 | –0.728 | (–1.386 to –0.071) | |
| RAVLT: recognition – list A | (+) | 19 | 14 | 1.2 | 19 | 15 | 0.4 | –1.118 | (–1.804 to –0.432) | ||
| RAVLT: recognition – list B | (+) | 19 | 10.8 | 2.1 | 19 | 12.4 | 1.7 | –0.837 | (–1.502 to –0.173) | ||
| Lamers et al. 200698 | RAVLT: trial 8 | (+) | 11 | 11.6 | 2.8 | 15 | 10.7 | 3.5 | 0.279 | (–0.503 to 1.061) | |
| de Win et al. 200691 | RAVLT-D: recognition | (+) | Follow-up. Data from secondary pub.90 | 58 | 29.66 | 0.8 | 60 | 29.93 | 0.3 | –0.450 | (–0.815 to –0.084) |
| RAVLT-D: trial 8 | (+) | Follow-up. Data from secondary pub.90 | 58 | 13.2 | 2 | 60 | 14.1 | 1.2 | –0.548 | (–0.916 to –0.180) | |
| McCann et al. 2007117 | WMS-III: logical memory (story A recall unit score) | (+) | 25 | 10.24 | 4.8 | 23 | 13.74 | 3.94 | –0.794 | (–1.383 to –0.205) | |
| Hoshi et al. 2007125 | Buschke: overall score | (+) | 25 | 5.2 | 2.5 | 14.5 | 6.55 | 3.23 | –0.485 | (–1.141 to 0.171) | |
| Groth et al. 2007126 | WMS-III: index score | (+) | 26 | 108.4 | 12.1 | 26 | 112.2 | 9 | –0.356 | (–0.904 to 0.192) | |
| Former users vs polydrug controls | |||||||||||
| Morgan et al. 2002103 | RBMT: prose recall | (+) | 15 | 4.3 | 3.679 | 8 | 7 | 3 | –0.779 | (–1.668 to 0.111) | |
| Curran and Verheyden 2003104 | Buschke: overall score | (+) | 32 | 5.685 | 2.685 | 16 | 7.905 | 3.11 | –0.784 | (–1.406 to –0.163) | |
| RBMT: prose recall | (+) | 32 | 5.825 | 2.733 | 16 | 7.515 | 4.33 | –0.506 | (–1.115 to 0.103) | ||
| Thomasius et al. 200596 | RAVLT: overall score | (+) | Data from secondary pub.105 | 31 | 10.8 | 3.229 | 14.5 | 11.15 | 2.962 | –0.111 | (–0.735 to 0.513) |
| RBMT: prose recall | (+) | Data from secondary pub.105 | 31 | 7.28 | 2.505 | 14.5 | 8.95 | 2.962 | –0.629 | (–1.266 to 0.009) | |
| Reneman et al. 200697 | RAVLT: trial 8 | (+) | 16 | 10.1 | 2.9 | 4.33 | 13.1 | 2.1 | –1.082 | (–2.200 to 0.035) | |
| RBMT: prose recall (sum for 2 tests) | (+) | 16 | 13.8 | 6.2 | 4.33 | 15.3 | 5.8 | –0.245 | (–1.309 to 0.820) | ||
| Hoshi et al. 2007125 | Buschke: overall score | (+) | 28 | 6.45 | 3.17 | 14.5 | 6.55 | 3.23 | –0.031 | (–0.666 to 0.603) | |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs drug-naïve controls | |||||||||||
| Morgan 1999102 | RBMT: prose recall | (+) | 25 | 5.36 | 2.48 | 19 | 7.61 | 2.74 | –0.867 | (–1.492 to –0.242) | |
| Rodgers 2000122 | WMS-R: logical memory | (+) | 15 | 18.4 | 12.3 | 15 | 38.2 | 9.3 | –1.816 | (–2.675 to –0.957) | |
| WMS-R: verbal paired associates | (+) | 15 | 8.5 | 7.8 | 15 | 15.3 | 7.6 | –0.883 | (–1.635 to –0.131) | ||
| Gouzoulis et al. 200099 | RAVLT-G: trial 8 | (+) | 28 | 13.79 | 1.75 | 28 | 14.21 | 1.03 | –0.293 | (–0.819 to 0.234) | |
| Reneman et al. 2001111 | RAVLT: trial 8 | (+) | Data from secondary pub.499 | 5 | 8.14 | 3.4 | 9 | 12.3 | 1.8 | –1.696 | (–2.983 to –0.410) |
| Bhattachary and Powell 2001127 | Prose recall | (+) | (1) Regular users vs controls | 26 | 8.94 | 1.76 | 6.67 | 14.33 | 2.01 | –2.980 | (–4.111 to –1.848) |
| (2) Novice users vs controls | 18 | 13.31 | 1.98 | 6.67 | 14.33 | 2.01 | –0.513 | (–1.414 to 0.388) | |||
| Prose retained [%] | (+) | (1) Regular users vs controls | 26 | 84.96 | 10.59 | 6.67 | 90.67 | 8.46 | –0.558 | (–1.420 to 0.304) | |
| (2) Novice users vs controls | 18 | 92.22 | 6.84 | 6.67 | 90.67 | 8.46 | 0.213 | (–0.678 to 1.104) | |||
| Morgan et al. 2002103 | RBMT: prose recall | (+) | 18 | 6.2 | 3.394 | 7.5 | 8.6 | 3.873 | –0.679 | (–1.553 to 0.194) | |
| Dafters et al. 200475 | RBMT: prose recall | (+) | (1) Heavy users vs controls | 16 | 4.1 | 1.65 | 9.5 | 5.95 | 2.25 | –0.979 | (–1.829 to –0.129) |
| (2) Light users vs controls | 19 | 4.55 | 1.45 | 9.5 | 5.95 | 2.25 | –0.801 | (–1.610 to 0.007) | |||
| Thomasius et al. 200596 | RAVLT: overall score | (+) | (1) Data from primary pub. | 30 | 12.65 | 2.465 | 15 | 12.95 | 2.574 | –0.120 | (–0.740 to 0.500) |
| RBMT: prose recall | (+) | (2) Data from secondary pub.105 | 30 | 8.52 | 3.122 | 15 | 9.45 | 3.012 | –0.301 | (–0.924 to 0.322) | |
| Yip and Lee 2005128 | RAVLT-C: recognition | (+) | 100 | 5.64 | 1.93 | 100 | 12.8 | 1.22 | –4.435 | (–4.952 to –3.917) | |
| RAVLT-C: trial 8 | (+) | 100 | 5.28 | 1.57 | 100 | 13.52 | 1.26 | –5.789 | (–6.423 to –5.155) | ||
| Quednow et al. 200683 | RAVLT: recognition – errors – list A | (–) | 19 | 0.2 | 0.5 | 19 | 0.2 | 0.3 | 0.000 | (–0.636 to 0.636) | |
| RAVLT: recognition – errors – list B | (–) | 19 | 4.3 | 3.1 | 19 | 3.4 | 2.5 | –0.320 | (–0.960 to 0.321) | ||
| RAVLT: recognition – list A | (+) | 19 | 14 | 1.2 | 19 | 14.8 | 0.5 | –0.870 | (–1.537 to –0.203) | ||
| RAVLT: recognition – list B | (+) | 19 | 10.8 | 2.1 | 19 | 12.1 | 2.7 | –0.537 | (–1.185 to 0.110) | ||
| Lamers et al. 200698 | RAVLT: trial 8 | (+) | 11 | 11.6 | 2.8 | 15 | 13.1 | 1.5 | –0.701 | (–1.504 to 0.102) | |
| Hoshi et al. 2007125 | Buschke: overall score | (+) | 25 | 5.2 | 2.5 | 13.5 | 8.35 | 3.64 | –1.071 | (–1.777 to –0.365) | |
| Former users vs drug-naïve controls | |||||||||||
| Bhattachary and Powell 2001127 | Prose recall | (+) | 16 | 10.38 | 1.66 | 6.67 | 14.33 | 2.01 | –2.241 | (–3.373 to –1.108) | |
| Prose retained [%] | (+) | 16 | 93.89 | 6.83 | 6.67 | 90.67 | 8.46 | 0.440 | (–0.473 to 1.354) | ||
| Morgan et al. 2002103 | RBMT: prose recall | (+) | 15 | 4.3 | 3.679 | 7.5 | 8.6 | 3.873 | –1.149 | (–2.094 to –0.205) | |
| Thomasius et al. 200596 | RAVLT: overall score | (+) | (1) Data from primary pub. | 31 | 10.8 | 3.229 | 15 | 12.95 | 2.574 | –0.708 | (–1.342 to –0.074) |
| RBMT: prose recall | (+) | (2) Data from secondary pub.105 | 31 | 7.28 | 2.505 | 15 | 9.45 | 3.012 | –0.811 | (–1.450 to –0.171) | |
| Hoshi et al. 2007125 | Buschke: overall score | (+) | 28 | 6.45 | 3.17 | 13.5 | 8.35 | 3.64 | –0.571 | (–1.233 to 0.090) | |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs polydrug controls | |||||||||||
| Bolla et al. 199893 | WMS-R: figural memory | (+) | 24 | 7.3 | 1.1 | 24 | 7.5 | 1.3 | –0.166 | (–0.733 to 0.401) | |
| WMS-R: visual memory span | (+) | 24 | 18.6 | 2.2 | 24 | 17.9 | 2.5 | 0.297 | (–0.272 to 0.866) | ||
| WMS-R: visual paired associates | (+) | 24 | 15.2 | 3.9 | 24 | 16.2 | 1.8 | –0.329 | (–0.899 to 0.241) | ||
| WMS-R: visual reproduction | (+) | 24 | 36.8 | 2.2 | 24 | 37.6 | 3.2 | –0.291 | (–0.860 to 0.278) | ||
| Morgan 1998110 | CANTAB SS: spatial span | (+) | Study 1 | 16 | 6.87 | 1.02 | 12 | 6 | 1.41 | 0.725 | (–0.049 to 1.499) |
| Rodgers 2000122 | WMS-R: index score | (+) | 15 | 113.3 | 7.5 | 15 | 110.3 | 7.62 | 0.397 | (–0.326 to 1.120) | |
| Gouzoulis et al. 200099 | Corsi Block: span | (+) | 28 | 5.82 | 0.77 | 28 | 6.18 | 0.9 | –0.430 | (–0.960 to 0.100) | |
| VIG: recall | (+) | 28 | 4.54 | 1.62 | 28 | 5.57 | 1.53 | –0.654 | (–1.192 to –0.116) | ||
| Croft et al. 200194 | RAVLT: list B | (+) | 11 | 6.5 | 2.1 | 18 | 7.3 | 2.1 | –0.381 | (–1.138 to 0.376) | |
| RAVLT: sum of trials 1–5 | (+) | 11 | 40.4 | 4.2 | 18 | 34.9 | 8.6 | 0.755 | (–0.022 to 1.531) | ||
| RAVLT: trial 6 – interference list | (+) | 11 | 7.7 | 2.5 | 18 | 7.3 | 2.1 | 0.177 | (–0.574 to 0.929) | ||
| RMT: recognition | (+) | 11 | 40.9 | 5.6 | 18 | 40.7 | 3.7 | 0.044 | (–0.706 to 0.795) | ||
| Verkes et al. 2001129 | Corsi Block: span plus one | (+) | (1) Heavy users vs controls | 21 | 5.5 | 0.9 | 10 | 6.4 | 1.1 | –0.931 | (–1.721 to –0.141) |
| (2) Moderate users vs controls | 21 | 5.7 | 1 | 10 | 6.4 | 1.1 | –0.678 | (–1.451 to 0.095) | |||
| Corsi Block: span | (+) | (1) Heavy users vs controls | 21 | 5 | 1.1 | 10 | 6.1 | 1.3 | –0.944 | (–1.735 to –0.152) | |
| (2) Moderate users vs controls | 21 | 5.2 | 1.2 | 10 | 6.1 | 1.3 | –0.731 | (–1.507 to 0.046) | |||
| Fox et al. 2002130 | Paired associates – memory score (six-box trial) | (+) | 20 | 4.7 | 1.5 | 20 | 4.5 | 1.8 | 0.121 | (–0.500 to 0.741) | |
| Paired associates – memory score (eight-box trial) | (+) | 20 | 4.6 | 2.2 | 20 | 5 | 1.6 | –0.208 | (–0.830 to 0.414) | ||
| Pattern recognition – correct [%] | (+) | 20 | 87.5 | 8.9 | 20 | 95.2 | 4.5 | –1.092 | (–1.759 to –0.425) | ||
| Pattern recognition – latency [s] | (–) | 20 | 2.05 | 0.47 | 20 | 2.09 | 0.45 | 0.087 | (–0.533 to 0.707) | ||
| Spatial recognition – correct [%] | (+) | 20 | 85.8 | 6.5 | 20 | 84 | 8.2 | 0.243 | (–0.379 to 0.865) | ||
| Spatial recognition – latency [s] | (–) | 20 | 2.4 | 0.77 | 20 | 2.29 | 0.51 | –0.168 | (–0.789 to 0.453) | ||
| Visual paired associates – six-box trial – errors [n] | (–) | 20 | 1.7 | 2.1 | 20 | 2.2 | 2.9 | 0.197 | (–0.424 to 0.819) | ||
| Visual paired associates – six-box trial – trials to completion | (–) | 20 | 1.7 | 0.8 | 20 | 1.8 | 1 | 0.110 | (–0.510 to 0.731) | ||
| Visual paired associates – eight-box trial – errors [n] | (–) | 20 | 7.7 | 7 | 20 | 4.7 | 2.9 | –0.560 | (–1.192 to 0.073) | ||
| Visual paired associates – eight-box trial – trials to completion | (–) | 20 | 3.8 | 2.5 | 20 | 2.9 | 1.1 | –0.466 | (–1.095 to 0.163) | ||
| Simon and Mattick 2002123 | WMS-III: visual | (+) | 40 | 98.2 | 11.1 | 37 | 97.8 | 11.9 | 0.035 | (–0.412 to 0.482) | |
| Zakzanis et al. 2003101 | RBMT: route | (+) | 15 | 1.9 | 0.5 | 17 | 1.9 | 0.2 | 0.000 | (–0.694 to 0.694) | |
| Halpern et al. 2004106 | R-OCFT: total score | (+) | (1) Heavy users vs controls | 11 | 20.5 | 7.4 | 8 | 22.3 | 6 | –0.262 | (–1.177 to 0.653) |
| (2) Moderate users vs controls | 12 | 20.2 | 5.4 | 8 | 22.3 | 6 | –0.372 | (–1.275 to 0.531) | |||
| WMS-III: spatial span – visual total | (+) | (1) Heavy users vs controls | 11 | 18.4 | 3.7 | 8 | 21.6 | 2.9 | –0.943 | (–1.907 to 0.021) | |
| (2) Moderate users vs controls | 12 | 19.8 | 2.4 | 8 | 21.6 | 2.9 | –0.691 | (–1.613 to 0.232) | |||
| WMS-III: visual reproduction | (+) | (1) Heavy users vs controls | 11 | 94.9 | 3.7 | 8 | 96.8 | 4.8 | –0.454 | (–1.377 to 0.470) | |
| (2) Moderate users vs controls | 12 | 94.1 | 7.9 | 8 | 96.8 | 4.8 | –0.393 | (–1.297 to 0.510) | |||
| Medina et al. 2005124 | BVRT: correct – n | (+) | 48 | 7.8 | 1.5 | 17 | 8.2 | 1.3 | –0.276 | (–0.831 to 0.280) | |
| BVRT: errors – n | (–) | 48 | 2.8 | 2.2 | 17 | 2.2 | 1.8 | –0.285 | (–0.840 to 0.270) | ||
| Wareing et al. 2005131 | Spatial span | (+) | 36 | 4.56 | 0.94 | 15.5 | 4.84 | 1.13 | –0.280 | (–0.878 to 0.318) | |
| Reneman et al. 200697 | Corsi Block: span plus one | (+) | (1) Heavy users vs controls | 22 | 6 | 1.1 | 4.33 | 5.6 | 0.6 | 0.383 | (–0.653 to 1.418) |
| (2) Moderate users vs controls | 15 | 5.9 | 1 | 4.33 | 5.6 | 0.6 | 0.320 | (–0.754 to 1.395) | |||
| Corsi Block: span | (+) | (1) Heavy users vs controls | 22 | 5.6 | 1.3 | 4.33 | 5.2 | 0.7 | 0.324 | (–0.710 to 1.358) | |
| (2) Moderate users vs controls | 15 | 5.7 | 1.1 | 4.33 | 5.2 | 0.7 | 0.483 | (–0.598 to 1.564) | |||
| WMS-R: visual reproduction | (+) | (1) Heavy users vs controls | 22 | 38.4 | 2.6 | 4.33 | 39.4 | 1.9 | –0.398 | (–1.434 to 0.639) | |
| (2) Moderate users vs controls | 15 | 39.2 | 1.8 | 4.33 | 39.4 | 1.9 | –0.110 | (–1.179 to 0.960) | |||
| de Win et al. 200691 | MFD: correct – n | (+) | Follow-up. Data from secondary pub.90 | 58 | 94.4 | 7.8 | 60 | 96.8 | 6.6 | –0.333 | (–0.696 to 0.031) |
| MFD: trials to completion – n | (–) | Follow-up. Data from secondary pub.90 | 58 | 3.3 | 1.3 | 60 | 3.1 | 1.4 | –0.148 | (–0.509 to 0.213) | |
| Groth et al. 2007126 | WMS-III: visual | (+) | 26 | 97.1 | 13.6 | 26 | 99.9 | 10.4 | –0.231 | (–0.777 to 0.314) | |
| Roiser et al. 2007118 | CANTAB DMTS: delayed – latency – ms | (–) | 30 | 2418 | 488 | 15 | 2815 | 842.6 | 0.635 | (0.001–1.269) | |
| CANTAB DMTS: delayed (0 s) – correct [%] | (+) | 30 | 90.3 | 8.1 | 15 | 89 | 14.5 | 0.122 | (–0.498 to 0.743) | ||
| CANTAB DMTS: delayed (12 s) – correct [%] | (+) | 30 | 80.3 | 16.5 | 15 | 77.3 | 15.1 | 0.187 | (–0.434 to 0.808) | ||
| CANTAB DMTS: delayed (4 s) – correct [%] | (+) | 30 | 88.7 | 14.6 | 15 | 86.7 | 13.2 | 0.141 | (–0.479 to 0.762) | ||
| CANTAB DMTS: simultaneous – correct [%] | (+) | 30 | 96.7 | 6.1 | 15 | 95 | 7.3 | 0.261 | (–0.361 to 0.883) | ||
| CANTAB DMTS: simultaneous – latency – ms | (–) | 30 | 3020 | 818.8 | 15 | 3244 | 648.4 | 0.293 | (–0.330 to 0.916) | ||
| PRM: correct [%] | (+) | 30 | 88.9 | 15.1 | 15 | 91.4 | 10.7 | –0.181 | (–0.802 to 0.440) | ||
| PRM: latency – ms | (–) | 30 | 1753 | 372.4 | 15 | 1673 | 383.3 | –0.215 | (–0.836 to 0.407) | ||
| Former users vs polydrug controls | |||||||||||
| Wareing et al. 2005131 | Spatial span | (+) | 12 | 4.08 | 1.51 | 15.5 | 4.84 | 1.13 | –0.581 | (–1.352 to 0.189) | |
| Reneman et al. 200697 | Corsi Block: span plus one | (+) | 16 | 6 | 1.2 | 4.33 | 5.6 | 0.6 | 0.359 | (–0.709 to 1.426) | |
| Corsi Block: span | (+) | 16 | 5.7 | 1.3 | 4.33 | 5.2 | 0.7 | 0.412 | (–0.658 to 1.482) | ||
| WMS-R: visual reproduction | (+) | 16 | 37.7 | 3.2 | 4.33 | 39.4 | 1.9 | –0.566 | (–1.643 to 0.511) | ||
| Roiser et al. 2007118 | CANTAB DMTS: delayed – latency – ms | (–) | 20 | 2609 | 604 | 15 | 2815 | 842.6 | 0.289 | (–0.384 to 0.962) | |
| CANTAB DMTS: delayed (0 s) – correct [%] | (+) | 20 | 88 | 10.6 | 15 | 89 | 14.5 | –0.081 | (–0.750 to 0.589) | ||
| CANTAB DMTS: delayed (12 s) – correct [%] | (+) | 20 | 76.5 | 19 | 15 | 77.3 | 15.1 | –0.046 | (–0.715 to 0.624) | ||
| CANTAB DMTS: delayed (4 s) – correct [%] | (+) | 20 | 87.5 | 15.2 | 15 | 86.7 | 13.2 | 0.056 | (–0.614 to 0.725) | ||
| CANTAB DMTS: simultaneous – correct [%] | (+) | 20 | 94.5 | 8.9 | 15 | 95 | 7.3 | –0.061 | (–0.730 to 0.609) | ||
| CANTAB DMTS: simultaneous – latency – ms | (–) | 20 | 3165 | 1226 | 15 | 3244 | 648.4 | 0.078 | (–0.592 to 0.748) | ||
| PRM: correct [%] | (+) | 20 | 86.2 | 14.9 | 15 | 91.4 | 10.7 | –0.392 | (–1.068 to 0.285) | ||
| PRM: latency – ms | (–) | 20 | 1730 | 504.3 | 15 | 1673 | 383.3 | –0.125 | (–0.795 to 0.545) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs drug-naïve controls | ||||||||||
| Morgan 1998110 | CANTAB SS: spatial span | (+) | Study 1 | 6.87 | 1.02 | 16 | 6.69 | 1.35 | 0.150 | (–0.544 to 0.844) |
| Rodgers 2000122 | WMS-R: index score | (+) | 113.3 | 7.5 | 15 | 109.5 | 11.98 | 0.380 | (–0.342 to 1.103) | |
| Gouzoulis et al. 200099 | Corsi Block: span | (+) | 5.82 | 0.77 | 28 | 6.04 | 1.04 | –0.240 | (–0.766 to 0.285) | |
| VIG: recall | (+) | 4.54 | 1.62 | 28 | 5.71 | 1.24 | –0.811 | (–1.357 to –0.265) | ||
| Croft et al. 200194 | RAVLT: list B | (+) | 6.5 | 2.1 | 31 | 6.7 | 2 | –0.099 | (–0.787 to 0.589) | |
| RAVLT: sum of trials 1–5 | (+) | 40.4 | 4.2 | 31 | 39.9 | 6.5 | 0.083 | (–0.605 to 0.771) | ||
| RAVLT: trial 6 – interference list | (+) | 7.7 | 2.5 | 31 | 8.2 | 1.7 | –0.259 | (–0.949 to 0.431) | ||
| RMT: recognition | (+) | 40.9 | 5.6 | 31 | 43 | 3.3 | –0.525 | (–1.222 to 0.173) | ||
| Yip and Lee 2005128 | AFLT: overall score | (+) | 5.44 | 1.58 | 100 | 6.54 | 2 | –0.610 | (–0.894 to –0.327) | |
| Roiser et al. 2007118 | CANTAB DMTS: delayed – latency – ms | (–) | 2418 | 488 | 15 | 2609 | 610.3 | 0.360 | (–0.265 to 0.984) | |
| CANTAB DMTS: delayed (0 s) – correct [%] | (+) | 90.3 | 8.1 | 15 | 89.3 | 9.8 | 0.115 | (–0.505 to 0.735) | ||
| CANTAB DMTS: delayed (12 s) – correct [%] | (+) | 80.3 | 16.5 | 15 | 77.7 | 16.3 | 0.158 | (–0.462 to 0.779) | ||
| CANTAB DMTS: delayed (4 s) – correct [%] | (+) | 88.7 | 14.6 | 15 | 89 | 11.2 | –0.022 | (–0.642 to 0.598) | ||
| CANTAB DMTS: simultaneous – correct [%] | (+) | 96.7 | 6.1 | 15 | 94.7 | 7.3 | 0.307 | (–0.316 to 0.930) | ||
| CANTAB DMTS: simultaneous – latency – ms | (–) | 3020 | 818.8 | 15 | 3121 | 628.9 | 0.133 | (–0.488 to 0.753) | ||
| PRM: correct [%] | (+) | 88.9 | 15.1 | 15 | 92.2 | 7.3 | –0.252 | (–0.874 to 0.370) | ||
| PRM: latency – ms | (–) | 1753 | 372.4 | 15 | 1713 | 356.9 | –0.109 | (–0.729 to 0.511) | ||
| Former users vs drug-naïve controls | ||||||||||
| Roiser et al. 2007118 | CANTAB DMTS: delayed – latency – ms | (–) | 2609 | 604 | 15 | 2609 | 610.3 | 0.000 | (–0.669 to 0.670) | |
| CANTAB DMTS: delayed (0 s) – correct [%] | (+) | 88 | 10.6 | 15 | 89.3 | 9.8 | –0.127 | (–0.797 to 0.544) | ||
| CANTAB DMTS: delayed (12 s) – correct [%] | (+) | 76.5 | 19 | 15 | 77.7 | 16.3 | –0.067 | (–0.737 to 0.603) | ||
| CANTAB DMTS: delayed (4 s) – correct [%] | (+) | 87.5 | 15.2 | 15 | 89 | 11.2 | –0.110 | (–0.780 to 0.560) | ||
| CANTAB DMTS: simultaneous – correct [%] | (+) | 94.5 | 8.9 | 15 | 94.7 | 7.3 | –0.024 | (–0.694 to 0.645) | ||
| CANTAB DMTS: simultaneous – latency – ms | (–) | 3165 | 1226 | 15 | 3121 | 628.9 | –0.043 | (–0.713 to 0.626) | ||
| PRM: correct [%] | (+) | 86.2 | 14.9 | 15 | 92.2 | 7.3 | –0.489 | (–1.169 to 0.191) | ||
| PRM: latency – ms | (–) | 1730 | 504.3 | 15 | 1713 | 356.9 | –0.037 | (–0.706 to 0.633) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs polydrug controls | |||||||||||
| Bolla et al. 199893 | R-OCFT: total score | (+) | 24 | 20 | 6.6 | 24 | 20.3 | 5.6 | –0.049 | (–0.615 to 0.517) | |
| WMS-R: visual paired associates | (+) | 24 | 5.8 | 0.5 | 24 | 5.9 | 0.3 | –0.243 | (–0.810 to 0.325) | ||
| WMS-R: visual reproduction | (+) | 24 | 33.2 | 4.7 | 24 | 35.9 | 4.1 | –0.612 | (–1.192 to –0.033) | ||
| Rodgers 2000122 | WMS-R: visual paired associates | (+) | 15 | 6.3 | 7.6 | 15 | 12.1 | 4.3 | –0.939 | (–1.696 to –0.183) | |
| WMS-R: visual reproduction | (+) | 15 | 38.7 | 11.9 | 15 | 39 | 8.7 | –0.029 | (–0.744 to 0.687) | ||
| Simon and Mattick 2002123 | WMS-III: visual | (+) | 40 | 96 | 12.4 | 37 | 97 | 11.8 | –0.083 | (–0.530 to 0.365) | |
| Zakzanis et al. 2003101 | RBMT: picture recognition | (+) | 15 | 1.9 | 0.3 | 17 | 1.8 | 0.5 | 0.239 | (–0.458 to 0.936) | |
| RBMT: route | (+) | 15 | 1.9 | 0.5 | 17 | 1.9 | 0.2 | 0.000 | (–0.694 to 0.694) | ||
| Halpern et al. 2004106 | R-OCFT: total score | (+) | (1) Heavy users vs controls | 11 | 21.6 | 6.4 | 8 | 22.3 | 6.3 | –0.110 | (–1.022 to 0.801) |
| (2) Moderate users vs controls | 12 | 21 | 3.5 | 8 | 22.3 | 6.3 | –0.272 | (–1.171 to 0.627) | |||
| WMS-III: visual reproduction | (+) | (1) Heavy users vs controls | 11 | 80.5 | 16.5 | 8 | 90.6 | 11.6 | –0.688 | (–1.628 to 0.252) | |
| (2) Moderate users vs controls | 12 | 87.4 | 9.1 | 8 | 90.6 | 11.6 | –0.315 | (–1.216 to 0.585) | |||
| Reneman et al. 200697 | WMS-R: visual reproduction | (+) | (1) Heavy users vs controls | 22 | 35.4 | 5.6 | 4.33 | 36.4 | 3.4 | –0.187 | (–1.218 to 0.845) |
| (2) Moderate users vs controls | 15 | 36.2 | 5.5 | 4.33 | 36.4 | 3.4 | –0.039 | (–1.108 to 1.030) | |||
| Lamers et al. 200698 | R-OCFT: total score | (+) | 11 | 20.6 | 7.6 | 15 | 22.3 | 6.8 | –0.238 | (–1.019 to 0.543) | |
| de Win et al. 200691 | MFD: correct – n | (+) | Follow-up. Data from secondary pub.90 | 58 | 20.8 | 0.5 | 60 | 20.9 | 0.3 | –0.244 | (–0.606 to 0.119) |
| Groth et al. 2007126 | WMS-III: visual | (+) | 26 | 99.2 | 11.2 | 26 | 100.3 | 9.2 | –0.107 | (–0.651 to 0.437) | |
| Roiser et al. 2007118 | PRM: correct [%] | (+) | 30 | 88.3 | 8.4 | 15 | 87.9 | 12.4 | 0.040 | (–0.579 to 0.660) | |
| PRM: latency – ms | (–) | 30 | 1702 | 346.7 | 15 | 1749 | 472.9 | 0.120 | (–0.500 to 0.740) | ||
| Former users vs polydrug controls | |||||||||||
| Reneman et al. 200697 | WMS-R: visual reproduction | (+) | 16 | 35.9 | 4.1 | 4.33 | 36.4 | 3.4 | –0.126 | (–1.188 to 0.937) | |
| PRM: correct [%] | (+) | 20 | 83.8 | 16.5 | 15 | 87.9 | 12.4 | –0.275 | (–0.948 to 0.398) | ||
| Roiser et al. 2007118 | PRM: latency – ms | (–) | 20 | 1850 | 591.8 | 15 | 1749 | 472.9 | –0.186 | (–0.857 to 0.485) | |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs drug-naïve controls | |||||||||||
| Rodgers 2000122 | WMS-R: visual paired associates | (+) | 15 | 6.3 | 7.6 | 15 | 13.4 | 2.6 | –1.250 | (–2.037 to –0.463) | |
| WMS-R: visual reproduction | (+) | 15 | 38.7 | 11.9 | 15 | 36.9 | 7.6 | 0.180 | (–0.537 to 0.898) | ||
| Bhattachary and Powell 2001127 | R-OCFT: total score | (+) | (1) Regular users vs non-users | 26 | 24.92 | 3.38 | 6.67 | 23.38 | 3.44 | 0.454 | (–0.404 to 1.313) |
| (2) Novice users vs non-users | 18 | 24.11 | 2.8 | 6.67 | 23.38 | 3.44 | 0.246 | (–0.646 to 1.137) | |||
| Yip and Lee 2005128 | AFLT: overall score | (+) | 100 | 5.45 | 1.57 | 100 | 8.81 | 1.99 | –1.875 | (–2.208 to –1.542) | |
| Lamers et al. 200698 | R-OCFT: total score | (+) | 11 | 20.6 | 7.6 | 15 | 23.7 | 4.2 | –0.529 | (–1.321 to 0.263) | |
| Roiser et al. 2007118 | PRM: correct [%] | (+) | 30 | 88.3 | 8.4 | 15 | 89 | 9.4 | –0.080 | (–0.700 to 0.540) | |
| PRM: latency – ms | (–) | 30 | 1702 | 346.7 | 15 | 1678 | 392.9 | –0.065 | (–0.685 to 0.555) | ||
| Former users vs drug-naïve controls | |||||||||||
| Bhattachary and Powell 2001127 | R-OCFT: total score | (+) | 16 | 23.28 | 2.91 | 6.67 | 23.38 | 3.44 | –0.033 | (–0.936 to 0.871) | |
| Roiser et al. 2007118 | PRM: correct [%] | (+) | 20 | 83.8 | 16.5 | 15 | 89 | 9.4 | –0.373 | (–1.049 to 0.302) | |
| PRM: latency – ms | (–) | 20 | 1850 | 591.8 | 15 | 1678 | 392.9 | –0.332 | (–1.006 to 0.342) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | SMD | (95% CI) | ||||
| Current users vs polydrug controls | |||||||||||
| Rodgers 2000122 | WMS-R: mental control | (+) | 15 | 4.9 | 3.2 | 15 | 5.3 | 5.1 | –0.094 | (–0.810 to 0.622) | |
| Gouzoulis et al. 200099 | TAP: 5 – divided attention – time [ms] | (–) | 28 | 671.6 | 57 | 28 | 625 | 35.1 | –0.984 | (–1.540 to –0.429) | |
| TAP: 8 – intermodal integration – time [ms] | (–) | 28 | 412.2 | 80.7 | 28 | 364.9 | 44.8 | –0.725 | (–1.266 to –0.183) | ||
| Fox et al. 2001112 | Spatial recall – correct – n | (+) | (1) High-dose users vs controls | 11 | 24.5 | 3.7 | 6.67 | 28.3 | 2.9 | –1.107 | (–2.144 to –0.070) |
| (2) Medium users vs controls | 14 | 25.4 | 3.1 | 6.67 | 28.3 | 2.9 | –0.954 | (–1.925 to 0.018) | |||
| (1) Light users vs controls | 14 | 26.9 | 2.9 | 6.67 | 28.3 | 2.9 | –0.483 | (–1.418 to 0.452) | |||
| Fox et al. 2002130 | Spatial task – between errors – four-box | (–) | 20 | 0.6 | 2.1 | 20 | 0.1 | 0.3 | –0.333 | (–0.958 to 0.291) | |
| Spatial task – between errors – six-box | (–) | 20 | 5.8 | 4.7 | 20 | 3.8 | 4.8 | –0.421 | (–1.048 to 0.206) | ||
| Spatial task – between errors – eight-box | (–) | 20 | 17.1 | 14.2 | 20 | 8.3 | 5.8 | –0.811 | (–1.457 to –0.165) | ||
| Spatial task – search strategy score | (+) | 20 | 32.7 | 5.3 | 20 | 32 | 4.1 | 0.148 | (–0.473 to 0.768) | ||
| Spatial task – within errors – four-box | (+) | 20 | 0.1 | 0.2 | 20 | 0.1 | 0.2 | 0.000 | (–0.620 to 0.620) | ||
| Spatial task – within errors – six-box | (–) | 20 | 0.9 | 1.6 | 20 | 0.7 | 1.6 | –0.125 | (–0.745 to 0.495) | ||
| Spatial task – within errors – eight-box | (–) | 20 | 2.9 | 3.3 | 20 | 0.7 | 1.1 | –0.894 | (–1.546 to –0.243) | ||
| Simon and Mattick 2002123 | WMS-III: index score | (+) | 40 | 105.1 | 13.1 | 37 | 107 | 12.5 | –0.148 | (–0.596 to 0.299) | |
| Morgan et al. 2002103 | Serial subtraction (SS7) – errors [n] | (–) | 18 | 1.725 | 1.591 | 8 | 1.05 | 1.4 | –0.439 | (–1.281 to 0.403) | |
| Curran and Verheyden 2003104 | Serial subtraction (SS7) – correct – n | (+) | 32 | 28.5 | 13.9 | 16 | 27.7 | 11.7 | 0.061 | (–0.540 to 0.661) | |
| RVIP: 5-minute task – correct [n] | (+) | 32 | 19.9 | 6.7 | 16 | 19.7 | 6.4 | 0.030 | (–0.570 to 0.630) | ||
| Gouzoulis et al. 2003108 | n-back: 2-back – figures – correct responses – n | (+) | (1) Heavy users vs controls | 30 | 8.9 | 3.1 | 15 | 9.7 | 3 | –0.261 | (–0.883 to 0.361) |
| (2) Moderate users vs controls | 30 | 8.7 | 2.8 | 15 | 9.7 | 3 | –0.349 | (–0.973 to 0.275) | |||
| n-back: 2-back – figures – reaction time – ms | (–) | (1) Heavy users vs controls | 30 | 695.2 | 146.5 | 15 | 742.5 | 131.6 | 0.334 | (–0.290 to 0.957) | |
| (2) Moderate users vs controls | 30 | 723.4 | 153.4 | 15 | 742.5 | 131.6 | 0.130 | (–0.490 to 0.751) | |||
| n-back: 2-back – letters – correct responses – n | (+) | (1) Heavy users vs controls | 30 | 11.9 | 2.7 | 15 | 12.3 | 3.2 | –0.139 | (–0.760 to 0.481) | |
| (2) Moderate users vs controls | 30 | 12.9 | 1.7 | 15 | 12.3 | 3.2 | 0.261 | (–0.361 to 0.883) | |||
| n-back: 2-back – letters – reaction time – ms | (–) | (1) Heavy users vs controls | 30 | 641.8 | 149.4 | 15 | 622.2 | 140.9 | –0.134 | (–0.754 to 0.487) | |
| (2) Moderate users vs controls | 30 | 630.1 | 153.9 | 15 | 622.2 | 140.9 | –0.053 | (–0.673 to 0.567) | |||
| Halpern et al. 2004106 | WMS-III: mental control | (+) | (1) Heavy users vs controls | 11 | 25.9 | 5.6 | 8 | 28.8 | 3.4 | –0.602 | (–1.535 to 0.331) |
| (2) Moderate users vs controls | 12 | 28.3 | 4.1 | 8 | 28.8 | 3.4 | –0.130 | (–1.026 to 0.765) | |||
| von Geusau et al. 2004132 | HvdM MC: Mental counters – correct – % | (+) | (1) Female | 9 | 89.7 | 1.8 | 21 | 89.7 | 1.2 | 0.000 | (–0.781 to 0.781) |
| (2) Male | 17 | 89.6 | 1.2 | 12 | 92.9 | 1.6 | –2.396 | (–3.373 to –1.419) | |||
| HvdM MC: Mental counters – reaction time – ms | (–) | (1) Female | 9 | 544.7 | 50 | 21 | 540.3 | 33.1 | –0.114 | (–0.895 to 0.668) | |
| (2) Male | 17 | 586.2 | 34.2 | 12 | 413.6 | 44.1 | –4.478 | (–5.883 to –3.074) | |||
| HvdM TTT: Tic-tac-toe – correct – % | (+) | (1) Female | 9 | 93 | 2.7 | 21 | 93 | 1.8 | 0.000 | (–0.781 to 0.781) | |
| (2) Male | 17 | 91.1 | 2.2 | 12 | 94.9 | 2.4 | –1.664 | (–2.526 to –0.802) | |||
| HvdM TTT: Tic-tac-toe – reaction time – ms | (–) | (1) Female | 9 | 376.8 | 20 | 21 | 379.1 | 13.1 | 0.149 | (–0.632 to 0.931) | |
| (2) Male | 17 | 385.6 | 16.1 | 12 | 376.6 | 17.4 | –0.541 | (–1.294 to 0.212) | |||
| Wareing et al. 2004107 | Computation span | (+) | 42 | 3.12 | 1.85 | 15.5 | 5.23 | 2.1 | –1.100 | (–1.717 to –0.482) | |
| Reading span | (+) | 42 | 2.57 | 0.94 | 15.5 | 3.1 | 1.16 | –0.529 | (–1.120 to 0.062) | ||
| Medina et al. 2005124 | WAIS-III: letter number sequencing – scaled score | (+) | 48 | 10.1 | 2.2 | 17 | 10.5 | 3 | –0.165 | (–0.719 to 0.389) | |
| de Win et al. 200691 | PASAT: hits – 1.6 s | (+) | Follow-up. Data from secondary pub.90 | 58 | 45.5 | 8 | 60 | 46.3 | 8.4 | –0.097 | (–0.459 to 0.264) |
| PASAT: hits – 2.4 s | (+) | Follow-up. Data from secondary pub.90 | 58 | 55 | 5 | 60 | 54.9 | 5 | 0.020 | (–0.341 to 0.381) | |
| Groth et al. 2007126 | WMS-III: index score | (+) | 26 | 107.6 | 14.6 | 26 | 105.5 | 8.2 | 0.177 | (–0.367 to 0.722) | |
| Former users vs polydrug controls | |||||||||||
| Morgan et al. 2002103 | Serial subtraction (SS7) – errors [n] | (–) | 15 | 1.35 | 1.356 | 8 | 1.05 | 1.4 | –0.219 | (–1.080 to 0.642) | |
| Curran and Verheyden 2003104 | Serial subtraction (SS7) – correct – n | (+) | 32 | 22.8 | 5.4 | 16 | 27.7 | 11.7 | –0.611 | (–1.224 to 0.002) | |
| RVIP: 5-min task – correct [n] | (+) | 32 | 13.7 | 5.8 | 16 | 19.7 | 6.4 | –1.000 | (–1.634 to –0.366) | ||
| Wareing et al. 2004107 | Computation span | (+) | 17 | 2.82 | 1.63 | 15.5 | 5.23 | 2.1 | –1.290 | (–2.051 to –0.529) | |
| Reading span | (+) | 17 | 3.06 | 1.39 | 15.5 | 3.1 | 1.16 | –0.031 | (–0.719 to 0.657) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs drug-naïve controls | |||||||||||
| Rodgers 2000122 | WMS-R: mental control | (+) | 15 | 4.9 | 3.2 | 15 | 5.4 | 5.1 | –0.117 | (–0.834 to 0.599) | |
| Gouzoulis et al. 200099 | TAP: 5 – divided attention – time [ms] | (–) | 28 | 671.6 | 57 | 28 | 638.7 | 69.7 | –0.517 | (–1.050 to 0.016) | |
| TAP: 8 – intermodal integration – time [ms] | (–) | 28 | 412.2 | 80.7 | 28 | 380.5 | 49.2 | –0.474 | (–1.006 to 0.057) | ||
| Moeller et al. 2002133 | Delayed memory task – correct [n] | (+) | (1) Heavy users vs controls | 8 | 77.5 | 15.15 | 10 | 90 | 9.127 | –1.030 | (–2.026 to –0.034) |
| (2) Infrequent users vs controls | 8 | 96 | 5.772 | 10 | 90 | 9.127 | 0.765 | (–0.201 to 1.732) | |||
| Morgan et al. 2002103 | Serial subtraction (SS7) – errors [n] | (–) | 18 | 1.725 | 1.591 | 7.5 | 0.325 | 0.678 | –1.000 | (–1.899 to –0.102) | |
| Jacobsen et al. 2004134 | n-back: 1-back and 2-back (auditory) – correct responses (simple) [%] | (+) | 6 | 0.88 | 0.14 | 6 | 0.89 | 0.06 | –0.093 | (–1.225 to 1.039) | |
| n-back: 1-back and 2-back (auditory) – reaction time (simple) [ms] | (–) | 6 | 1500 | 141.9 | 6 | 1208 | 119.2 | –2.224 | (–3.717 to –0.730) | ||
| n-back: 1-back and 2-back (divided) – correct responses [%] | (+) | 6 | 0.77 | 0.15 | 6 | 0.78 | 0.07 | –0.085 | (–1.218 to 1.047) | ||
| n-back: 1-back and 2-back (visual) – correct responses (selective) [%] | (+) | 6 | 0.89 | 0.1 | 6 | 0.97 | 0.05 | –1.012 | (–2.227 to 0.203) | ||
| n-back: 1-back and 2-back (visual) – correct responses (simple) [%] | (+) | 6 | 0.92 | 0.03 | 6 | 0.92 | 0.09 | 0.000 | (–1.132 to 1.132) | ||
| n-back: 1-back and 2-back (visual) – reaction time (simple) [ms] | (–) | 6 | 1272 | 212.1 | 6 | 932.7 | 95.8 | –2.064 | (–3.512 to –0.615) | ||
| Former users vs drug-naïve controls | |||||||||||
| Morgan et al. 2002103 | Serial subtraction (SS7) – errors [n] | (–) | 15 | 1.35 | 1.356 | 7.5 | 0.325 | 0.678 | –0.866 | (–1.782 to 0.050) | |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95%CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Parrott and Lasky 1998121 | Visual search – time [s] | (–) | (1) Regular users vs controls | 15 | 2.7 | 0.775 | 7.5 | 2.5 | 0.775 | –0.258 | (–1.138 to 0.622) |
| (2) Novice users vs controls | 15 | 2.7 | 0.775 | 7.5 | 2.5 | 0.775 | –0.258 | (–1.138 to 0.622) | |||
| Morgan 1998110 | MFFT-20: latency to first response [s] | (–) | (1) Study 1 | 16 | 12.48 | 6.64 | 12 | 14.73 | 5.27 | 0.369 | (–0.386 to 1.124) |
| (2) Study 2 | 25 | 12 | 3.71 | 20 | 13.16 | 3.76 | 0.311 | (–0.281 to 0.902) | |||
| MFFT-20: total errors [n] | (–) | (1) Study 1 | 16 | 11.81 | 7.57 | 12 | 4.83 | 2.25 | –1.176 | (–1.990 to –0.363) | |
| (2) Study 2 | 25 | 11.73 | 5.88 | 20 | 8.25 | 4.44 | –0.658 | (–1.262 to –0.053) | |||
| Rodgers 2000122 | Complex reaction time [ms] | (–) | 15 | 1090 | 261.7 | 15 | 951.5 | 122.8 | –0.679 | (–1.416 to 0.058) | |
| Simple auditory reaction time [ms] | (–) | 15 | 272 | 77.49 | 15 | 292.3 | 95.47 | 0.233 | (–0.485 to 0.952) | ||
| Visual reaction time [ms] | (–) | 15 | 357.5 | 71.78 | 15 | 349.6 | 96.97 | –0.093 | (–0.809 to 0.623) | ||
| Gouzoulis et al. 200099 | TAP: 1 – phasic reaction time [ms] | (–) | 28 | 214.8 | 24.8 | 28 | 214 | 26.7 | –0.031 | (–0.555 to 0.493) | |
| TAP: 1 – tonic reaction time [ms] | (–) | 28 | 218.9 | 28.2 | 28 | 221.1 | 26.3 | 0.081 | (–0.443 to 0.605) | ||
| Fox et al. 2001112 | Simple visual reaction time [ms] | (–) | (1) High-dose users vs controls | 11 | 291.2 | 35.4 | 6.67 | 277.9 | 25.4 | –0.415 | (–1.388 to 0.558) |
| (2) Medium users vs controls | 14 | 301 | 28.2 | 6.67 | 277.9 | 25.4 | –0.845 | (–1.807 to 0.116) | |||
| (3) Low users vs controls | 14 | 292.2 | 33.5 | 6.67 | 277.9 | 25.4 | –0.459 | (–1.393 to 0.475) | |||
| Croft et al. 200194 | Stroop: word reading – time [s] | (–) | 11 | 19.5 | 4.9 | 18 | 17.9 | 2.7 | –0.436 | (–1.195 to 0.323) | |
| Verkes et al. 2001129 | Binary choice task – reaction time [ms] | (–) | (1) Heavy users vs controls | 21 | 364 | 72 | 10 | 326 | 42 | –0.592 | (–1.360 to 0.176) |
| (2) Moderate users vs controls | 21 | 347 | 64 | 10 | 326 | 42 | –0.362 | (–1.120 to 0.397) | |||
| Simple auditory reaction time [ms] | (–) | (1) Heavy users vs controls | 21 | 247 | 36 | 10 | 224 | 23 | –0.707 | (–1.482 to 0.068) | |
| (2) Moderate users vs controls | 21 | 239 | 33 | 10 | 224 | 23 | –0.496 | (–1.260 to 0.268) | |||
| Simple visual reaction time [ms] | (–) | (1) Heavy users vs controls | 21 | 273 | 39 | 10 | 246 | 27 | –0.756 | (–1.534 to 0.022) | |
| (2) Moderate users vs controls | 21 | 258 | 37 | 10 | 246 | 27 | –0.351 | (–1.109 to 0.408) | |||
| Fox et al. 2002130 | CANTAB 3D – ID/ED: errors – simple dimensional (reversal) | (–) | 20 | 2.5 | 3.3 | 20 | 1.5 | 1.2 | –0.403 | (–1.029 to 0.224) | |
| CANTAB 3D – ID/ED: errors – simple dimensional | (–) | 20 | 0.7 | 1.1 | 20 | 0.2 | 0.5 | –0.585 | (–1.219 to 0.048) | ||
| CANTAB 3D – ID/ED: latency – simple dimensional (reversal) | (–) | 20 | 3.02 | 1.97 | 20 | 2.03 | 0.73 | –0.666 | (–1.304 to –0.029) | ||
| CANTAB 3D – ID/ED: latency – simple dimensional | (–) | 20 | 3.91 | 2.27 | 20 | 2.56 | 1.3 | –0.730 | (–1.371 to –0.089) | ||
| Morgan et al. 2002103 | MFFT-20: latency to first response [s] | (–) | 18 | 8.5 | 4.667 | 8 | 13 | 5.2 | 0.932 | (0.058–1.806) | |
| MFFT-20: total errors [n] | (–) | 18 | 15.8 | 5.091 | 8 | 9.2 | 7.6 | –1.112 | (–2.003 to –0.222) | ||
| TMT: Part B – errors (n) | (–) | 18 | 0.95 | 1.57 | 8 | 0.13 | 0.32 | –0.615 | (–1.466 to 0.235) | ||
| Curran and Verheyden 2003104 | Double digit cancellation – time [s] | (–) | 32 | 81.34 | 17.52 | 16 | 86.25 | 27.83 | 0.229 | (–0.373 to 0.831) | |
| Halpern et al. 2004106 | Stroop: colour reading – errors [n] | (–) | (1) Heavy users vs controls | 11 | 1.7 | 1.3 | 8 | 0.9 | 1.3 | –0.615 | (–1.549 to 0.319) |
| (2) Moderate users vs controls | 12 | 1.5 | 1.5 | 8 | 0.9 | 1.3 | –0.421 | (–1.326 to 0.484) | |||
| Stroop: colour reading – time [s] | (–) | (1) Heavy users vs controls | 11 | 61.3 | 10.8 | 8 | 51.7 | 5.3 | –1.072 | (–2.052 to –0.093) | |
| (2) Moderate users vs controls | 12 | 53.7 | 8 | 8 | 51.7 | 5.3 | –0.283 | (–1.182 to 0.617) | |||
| Stroop: word reading – errors [n] | (–) | (1) Heavy users vs controls | 11 | 1.1 | 1.1 | 8 | 0.6 | 0.7 | –0.523 | (–1.451 to 0.404) | |
| (2) Moderate users vs controls | 12 | 1.1 | 1.3 | 8 | 0.6 | 0.7 | –0.452 | (–1.359 to 0.455) | |||
| Stroop: word reading – time [s] | (–) | (1) Heavy users vs controls | 11 | 47.1 | 7.8 | 8 | 41.3 | 5.8 | –0.823 | (–1.775 to 0.129) | |
| (2) Moderate users vs controls | 12 | 41.4 | 5.9 | 8 | 41.3 | 5.8 | –0.017 | (–0.912 to 0.878) | |||
| TMT: Part A – errors (n) | (–) | (1) Heavy users vs controls | 11 | 0.6 | 0.7 | 8 | 0 | 0.5 | –0.959 | (–1.925 to 0.007) | |
| (2) Moderate users vs controls | 12 | 0.4 | 1 | 8 | 0 | 0.5 | –0.475 | (–1.383 to 0.433) | |||
| TMT: Part A – time (s) | (–) | (1) Heavy users vs controls | 11 | 24.7 | 9.9 | 8 | 22.1 | 6.3 | –0.302 | (–1.219 to 0.614) | |
| (2) Moderate users vs controls | 12 | 21.8 | 4.9 | 8 | 22.1 | 6.3 | 0.055 | (–0.840 to 0.949) | |||
| TMT: Part B – errors (n) | (–) | (1) Heavy users vs controls | 11 | 0.7 | 0.6 | 8 | 0.3 | 0.4 | –0.759 | (–1.705 to 0.187) | |
| (2) Moderate users vs controls | 12 | 0.3 | 0.7 | 8 | 0.3 | 0.4 | 0.000 | (–0.895 to 0.895) | |||
| TMT: Part B – time (s) | (–) | (1) Heavy users vs controls | 11 | 58.5 | 13.6 | 8 | 48.2 | 15.4 | –0.717 | (–1.659 to 0.225) | |
| (2) Moderate users vs controls | 12 | 52.6 | 17.4 | 8 | 48.2 | 15.4 | –0.264 | (–1.163 to 0.635) | |||
| WAIS-R: digit symbol | (+) | (1) Heavy users vs controls | 11 | 62.4 | 10.3 | 8 | 69.6 | 12.5 | –0.640 | (–1.575 to 0.296) | |
| (2) Moderate users vs controls | 12 | 69.8 | 12.7 | 8 | 69.6 | 12.5 | 0.016 | (–0.879 to 0.910) | |||
| McCardle et al. 2004100 | ?WAIS: digit symbol | (+) | 17 | 64.06 | 8.74 | 15 | 66.07 | 10.89 | –0.205 | (–0.901 to 0.491) | |
| TMT: Part A – time (s) | (–) | 17 | 34.02 | 9.99 | 15 | 24.49 | 8.05 | –1.043 | (–1.786 to –0.300) | ||
| TMT: Part B – time (s) | (–) | 17 | 68.09 | 15.47 | 15 | 64.7 | 25.35 | –0.164 | (–0.859 to 0.532) | ||
| Medina et al. 2005124 | Ruff 2 and 7: total accuracy –T-score | (+) | 48 | 44.9 | 10.3 | 17 | 45.4 | 7.5 | –0.052 | (–0.605 to 0.502) | |
| Ruff 2 and 7: total speed –T-score | (+) | 48 | 46.3 | 8.9 | 17 | 48.6 | 7.9 | –0.266 | (–0.821 to 0.289) | ||
| Stroop: colour reading – time [s] | (–) | 48 | 10.2 | 2 | 17 | 10.6 | 2.3 | 0.192 | (–0.362 to 0.746) | ||
| Stroop: word reading – time [s] | (–) | 48 | 10.4 | 3.1 | 17 | 10.5 | 3.2 | 0.032 | (–0.521 to 0.585) | ||
| TMT: Part B –T-score | (+) | 48 | 52.7 | 10.9 | 17 | 53.5 | 13.3 | –0.069 | (–0.623 to 0.484) | ||
| Morgan et al. 2006115 | MFFT–20: latency to first response [s] | (–) | 20 | 13.1 | 5.814 | 20 | 17.9 | 6.037 | 0.810 | (0.164–1.456) | |
| MFFT–20: total errors [n] | (–) | 20 | 12.5 | 6.485 | 20 | 7.85 | 5.367 | –0.781 | (–1.425 to –0.137) | ||
| Dafters 2006137 | Stroop: colour reading – time [ms] | (–) | 33 | 713 | 134 | 17 | 724 | 120 | 0.085 | (–0.500 to 0.670) | |
| Stroop: word reading – time [ms] | (–) | 33 | 683 | 133 | 17 | 684 | 124 | 0.008 | (–0.577 to 0.593) | ||
| Reneman et al. 200697 | FePsy: auditive reaction time – dominant hand [ms] | (–) | (1) MDMA users (heavy) vs control | 22 | 245.2 | 30.2 | 4.33 | 242.5 | 22.1 | –0.092 | (–1.123 to 0.938) |
| (2) MDMA users (moderate) vs control | 15 | 246.7 | 28.3 | 4.33 | 242.5 | 22.1 | –0.154 | (–1.224 to 0.916) | |||
| FePsy: auditive reaction time – non-dominant hand [ms] | (–) | (1) MDMA users (heavy) vs control | 22 | 245.5 | 26.8 | 4.33 | 244.4 | 34.6 | –0.039 | (–1.069 to 0.991) | |
| (2) MDMA users (moderate) vs control | 15 | 250.1 | 24.1 | 4.33 | 244.4 | 34.6 | –0.216 | (–1.287 to 0.856) | |||
| FePsy: binary choice – errors [n] | (–) | (1) MDMA users (heavy) vs control | 22 | 5 | 7.2 | 4.33 | 2.5 | 3.4 | –0.367 | (–1.403 to 0.668) | |
| (2) MDMA users (moderate) vs control | 15 | 2.6 | 3.1 | 4.33 | 2.5 | 3.4 | –0.032 | (–1.101 to 1.037) | |||
| FePsy: binary choice – reaction time [ms] | (–) | (1) MDMA users (heavy) vs control | 22 | 353.7 | 67.9 | 4.33 | 382.9 | 112.6 | 0.386 | (–0.650 to 1.422) | |
| (2) MDMA users (moderate) vs control | 15 | 368.2 | 53 | 4.33 | 382.9 | 112.6 | 0.214 | (–0.857 to 1.286) | |||
| FePsy: visual reaction time – dominant hand [ms] | (–) | (1) MDMA users (heavy) vs control | 22 | 257.4 | 30.7 | 4.33 | 282.1 | 52.2 | 0.717 | (–0.333 to 1.767) | |
| (2) MDMA users (moderate) vs control | 15 | 287.7 | 55.2 | 4.33 | 282.1 | 52.2 | –0.102 | (–1.172 to 0.967) | |||
| FePsy: visual reaction time – non-dominant hand [ms] | (–) | (1) MDMA users (heavy) vs control | 22 | 268.7 | 31.7 | 4.33 | 316 | 92.8 | 1.045 | (–0.026 to 2.117) | |
| (2) MDMA users (moderate) vs control | 15 | 298.6 | 56.2 | 4.33 | 316 | 92.8 | 0.268 | (–0.804 to 1.341) | |||
| Stroop: colour reading – time [s] | (–) | (1) MDMA users (heavy) vs control | 22 | 53.2 | 9 | 4.33 | 53.9 | 9 | 0.078 | (–0.953 to 1.108) | |
| (2) MDMA users (moderate) vs control | 15 | 56.7 | 10.5 | 4.33 | 53.9 | 9 | –0.274 | (–1.347 to 0.799) | |||
| TMT: Part A – time (s) | (–) | (1) MDMA users (heavy) vs control | 22 | 19.9 | 3.7 | 4.33 | 24.8 | 7.5 | 1.109 | (0.033–2.185) | |
| (2) MDMA users (moderate) vs control | 15 | 20.6 | 6.5 | 4.33 | 24.8 | 7.5 | 0.627 | (–0.463 to 1.716) | |||
| TMT: Part B – time (s) | (–) | (1) MDMA users (heavy) vs control | 22 | 46.4 | 15.7 | 4.33 | 47.9 | 12.5 | 0.098 | (–0.932 to 1.128) | |
| (2) MDMA users (moderate) vs control | 15 | 49.7 | 14.5 | 4.33 | 47.9 | 12.5 | –0.127 | (–1.197 to 0.942) | |||
| Quednow et al. 200683 | MFFT–20: latency to first response [s] | (–) | Data from secondary pub.500 | 19 | 49.5 | 19.3 | 19 | 53.3 | 21.7 | 0.185 | (–0.452 to 0.822) |
| MFFT–20: total errors [n] | (–) | Data from secondary pub.500 | 19 | 8.16 | 4.09 | 19 | 5.95 | 4.36 | –0.523 | (–1.170 to 0.124) | |
| Lamers et al. 200698 | TMT: Part A – time (s) | (–) | 11 | 26.8 | 9.7 | 15 | 22.3 | 8.5 | –0.499 | (–1.290 to 0.292) | |
| TMT: Part B – part A – time (s) | (–) | 11 | 24.2 | 3.8 | 15 | 22.7 | 2.5 | –0.483 | (–1.272 to 0.307) | ||
| TMT: Part B – time (s) | (–) | 11 | 51 | 17.7 | 15 | 45 | 13.3 | –0.392 | (–1.178 to 0.393) | ||
| Wareing et al. 2007135 | Letter comparison speed task – three-letter – correct [n] | (+) | 29 | 26 | 3.7 | 23 | 26.91 | 4.95 | –0.212 | (–0.761 to 0.337) | |
| Letter comparison speed task – three-letter – errors [n] | (–) | 29 | 1.61 | 1.24 | 23 | 1.09 | 1.01 | –0.454 | (–1.009 to 0.100) | ||
| Letter comparison speed task – six-letter – correct [n] | (+) | 29 | 14.91 | 1.6 | 23 | 15.53 | 3.17 | –0.256 | (–0.806 to 0.293) | ||
| Letter comparison speed task – six-letter – errors [n] | (–) | 29 | 1.28 | 1.09 | 23 | 0.75 | 0.54 | –0.595 | (–1.154 to –0.035) | ||
| Letter comparison speed task – nine-letter – correct [n] | (+) | 29 | 10.72 | 2.04 | 23 | 10.96 | 2.29 | –0.111 | (–0.659 to 0.436) | ||
| Letter comparison speed task – nine-letter – errors [n] | (–) | 29 | 1.43 | 1.47 | 23 | 0.94 | 0.9 | –0.391 | (–0.944 to 0.161) | ||
| Pattern comparison speed task – three-pattern – correct [n] | (+) | 29 | 21.34 | 3.6 | 23 | 19.96 | 5.37 | 0.309 | (–0.242 to 0.860) | ||
| Pattern comparison speed task – three-pattern – errors [n] | (–) | 29 | 1.23 | 0.94 | 23 | 0.91 | 1.19 | –0.303 | (–0.853 to 0.248) | ||
| Pattern comparison speed task – six-pattern – correct [n] | (+) | 29 | 5.35 | 2.8 | 23 | 14.4 | 3.49 | –2.898 | (–3.687 to –2.110) | ||
| Pattern comparison speed task – six-pattern – errors [n] | (–) | 29 | 1.59 | 1.27 | 23 | 1.33 | 1.27 | –0.205 | (–0.753 to 0.344) | ||
| Pattern comparison speed task – nine-pattern – correct [n] | (+) | 29 | 13.74 | 1.74 | 23 | 13.08 | 3.03 | 0.276 | (–0.274 to 0.826) | ||
| Pattern comparison speed task – nine-pattern – errors [n] | (–) | 29 | 1.73 | 1.54 | 23 | 1.31 | 1.67 | –0.263 | (–0.812 to 0.287) | ||
| Former users vs polydrug controls | |||||||||||
| Morgan et al. 2002103 | MFFT-20: latency to first response [s] | (–) | 15 | 10.2 | 4.648 | 8 | 13 | 5.2 | 0.579 | (–0.297 to 1.454) | |
| MFFT-20: total errors [n] | (–) | 15 | 14.4 | 5.035 | 8 | 9.2 | 7.6 | –0.865 | (–1.762 to 0.032) | ||
| TMT: Part B – errors (n) | (–) | 15 | 0.64 | 0.891 | 8 | 0.13 | 0.32 | –0.680 | (–1.562 to 0.203) | ||
| Curran and Verheyden 2003104 | Double digit cancellation – time [s] | (–) | 32 | 93.62 | 25.07 | 16 | 86.25 | 27.83 | –0.283 | (–0.886 to 0.320) | |
| Reneman et al. 200697 | FePsy: auditive reaction time – dominant hand [ms] | (–) | 16 | 244.1 | 29.3 | 4.33 | 242.5 | 22.1 | –0.057 | (–1.118 to 1.005) | |
| FePsy: auditive reaction time – non-dominant hand [ms] | (–) | 16 | 254.3 | 32.3 | 4.33 | 244.4 | 34.6 | –0.302 | (–1.368 to 0.763) | ||
| FePsy: binary choice – errors [n] | (–) | 16 | 2.6 | 1.9 | 4.33 | 2.5 | 3.4 | –0.044 | (–1.106 to 1.017) | ||
| FePsy: binary choice – reaction time [ms] | (–) | 16 | 368.3 | 69.5 | 4.33 | 382.9 | 112.6 | 0.185 | (–0.879 to 1.248) | ||
| FePsy: visual reaction time – dominant hand [ms] | (–) | 16 | 270.3 | 46.6 | 4.33 | 282.1 | 52.2 | 0.248 | (–0.817 to 1.312) | ||
| FePsy: visual reaction time – non-dominant hand [ms] | (–) | 16 | 279.9 | 53.6 | 4.33 | 316 | 92.8 | 0.577 | (–0.501 to 1.655) | ||
| Stroop: colour reading – time [s] | (–) | 16 | 53.5 | 7.9 | 4.33 | 53.9 | 9 | 0.049 | (–1.012 to 1.111) | ||
| TMT: Part A – time (s) | (–) | 16 | 24 | 11.6 | 4.33 | 24.8 | 7.5 | 0.073 | (–0.989 to 1.135) | ||
| TMT: Part B – time (s) | (–) | 16 | 52.5 | 13.5 | 4.33 | 47.9 | 12.5 | –0.345 | (–1.413 to 0.722) | ||
| Wareing et al. 2007135 | Letter comparison speed task – three-letter – correct [n] | (+) | 10 | 23.1 | 5 | 23 | 26.91 | 4.95 | –0.767 | (–1.534 to –0.001) | |
| Letter comparison speed task – three-letter – errors [n] | (–) | 10 | 1.2 | 0.72 | 23 | 1.09 | 1.01 | –0.118 | (–0.861 to 0.625) | ||
| Letter comparison speed task – six-letter – correct [n] | (+) | 10 | 13.83 | 3.16 | 23 | 15.53 | 3.17 | –0.537 | (–1.291 to 0.218) | ||
| Letter comparison speed task – six-letter – errors [n] | (–) | 10 | 0.63 | 0.6 | 23 | 0.75 | 0.54 | 0.215 | (–0.529 to 0.959) | ||
| Letter comparison speed task – nine-letter – correct [n] | (+) | 10 | 10.1 | 2.34 | 23 | 10.96 | 2.29 | –0.373 | (–1.121 to 0.375) | ||
| Letter comparison speed task – nine-letter – errors [n] | (–) | 10 | 0.87 | 0.61 | 23 | 0.94 | 0.9 | 0.085 | (–0.658 to 0.827) | ||
| Pattern comparison speed task – three-pattern – correct [n] | (+) | 10 | 17.6 | 4.8 | 23 | 19.96 | 5.37 | –0.453 | (–1.204 to 0.298) | ||
| Pattern comparison speed task – three-pattern – errors [n] | (–) | 10 | 1.17 | 1.29 | 23 | 0.91 | 1.19 | –0.213 | (–0.957 to 0.531) | ||
| Pattern comparison speed task – six-pattern – correct [n] | (+) | 10 | 14.23 | 3.22 | 23 | 14.4 | 3.49 | –0.050 | (–0.792 to 0.693) | ||
| Pattern comparison speed task – six-pattern – errors [n] | (–) | 10 | 1.1 | 1.12 | 23 | 1.33 | 1.27 | 0.187 | (–0.557 to 0.931) | ||
| Pattern comparison speed task – nine-pattern – correct [n] | (+) | 10 | 13.53 | 3.7 | 23 | 13.08 | 3.03 | 0.139 | (–0.604 to 0.882) | ||
| Pattern comparison speed task – nine-pattern – errors [n] | (–) | 10 | 2.07 | 2.22 | 23 | 1.31 | 1.67 | –0.412 | (–1.161 to 0.338) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs drug-naïve controls | |||||||||||
| Morgan 1998110 | MFFT-20: latency to first response [s] | (–) | (1) Study 1 | 16 | 12.48 | 6.64 | 16 | 16.19 | 5.83 | 0.594 | (–0.115 to 1.303) |
| (2) Study 2 | 25 | 12 | 3.71 | 19 | 13.9 | 7.02 | 0.353 | (–0.248 to 0.954) | |||
| MFFT-20: total errors [n] | (–) | (1) Study 1 | 16 | 11.81 | 7.57 | 16 | 5.18 | 3.1 | –1.146 | (–1.897 to –0.395) | |
| (2) Study 2 | 25 | 11.73 | 5.88 | 19 | 9.68 | 4.93 | –0.373 | (–0.975 to 0.229) | |||
| Wareing et al. 2000136 | Letter comparison speed task – three-letter – correct [n] | (+) | 10 | 18.9 | 5.61 | 5 | 22.7 | 3.02 | –0.766 | (–1.879 to 0.347) | |
| Letter comparison speed task – six-letter – correct [n] | (+) | 10 | 14.6 | 4.62 | 5 | 15.5 | 2.17 | –0.223 | (–1.300 to 0.854) | ||
| Letter comparison speed task – nine-letter – correct [n] | (+) | 10 | 12.9 | 4.65 | 5 | 12.5 | 1.72 | 0.100 | (–0.974 to 1.175) | ||
| Rodgers 2000122 | Complex reaction time [ms] | (–) | 15 | 1090 | 261.7 | 15 | 953.2 | 120.9 | –0.673 | (–1.410 to 0.064) | |
| Simple auditory reaction time [ms] | (–) | 15 | 272 | 77.49 | 15 | 279.8 | 67.35 | 0.108 | (–0.608 to 0.824) | ||
| Visual reaction time [ms] | (–) | 15 | 357.5 | 71.78 | 15 | 379.3 | 75.96 | 0.295 | (–0.425 to 1.015) | ||
| Gouzoulis et al. 200099 | TAP: 1 – phasic reaction time [ms] | (–) | 28 | 214.8 | 24.8 | 28 | 214.7 | 25.2 | –0.004 | (–0.528 to 0.520) | |
| TAP: 1 – tonic reaction time [ms] | (–) | 28 | 218.9 | 28.2 | 28 | 218.7 | 27.5 | –0.007 | (–0.531 to 0.517) | ||
| Croft et al. 200194 | Stroop: word reading – time [s] | (–) | 11 | 19.5 | 4.9 | 31 | 16.7 | 2.4 | –0.871 | (–1.585 to –0.158) | |
| Moeller et al. 2002131 | Immediate memory task – correct [n] | (+) | (1) MDMA users (heavy) vs control | 8 | 81 | 15.87 | 10 | 88 | 6.845 | –0.599 | (–1.551 to 0.354) |
| (2) MDMA users (infrequent) vs control | 8 | 87.5 | 11.54 | 10 | 88 | 6.845 | –0.054 | (–0.984 to 0.876) | |||
| Morgan et al. 2002103 | MFFT–20: latency to first response [s] | (–) | 18 | 8.5 | 4.667 | 7.5 | 14.3 | 6.584 | 1.101 | (0.193–2.009) | |
| MFFT–20: total errors [n] | (–) | 18 | 15.8 | 5.091 | 7.5 | 7.8 | 3.486 | –1.701 | (–2.682 to –0.720) | ||
| TMT: Part B – errors (n) | (–) | 18 | 0.95 | 1.57 | 7.5 | 0.14 | 0.349 | –0.601 | (–1.470 to 0.268) | ||
| Yip and Lee 2005128 | SDMT: overall score | (+) | 100 | 60.97 | 4.43 | 100 | 66.3 | 3 | –1.409 | (–1.719 to –1.099) | |
| TMT-C: Part 1 – time (s) | (–) | 100 | 27.62 | 3.33 | 100 | 27.42 | 3.11 | –0.062 | (–0.339 to 0.215) | ||
| TMT-C: Part 2 – time (s) | (–) | 100 | 64.44 | 6.92 | 100 | 63.88 | 7.04 | –0.080 | (–0.358 to 0.197) | ||
| Morgan et al. 2006115 | MFFT-20: latency to first response [s] | (–) | 20 | 13.1 | 5.814 | 19 | 22 | 11.55 | 0.981 | (0.315–1.648) | |
| MFFT-20: total errors [n] | (–) | 20 | 12.5 | 6.485 | 19 | 6.75 | 6.32 | –0.898 | (–1.558 to –0.237) | ||
| Dafters 2006137 | Stroop: colour reading – time [ms] | (–) | 33 | 713 | 134 | 18 | 699 | 129 | –0.106 | (–0.681 to 0.469) | |
| Stroop: word reading – time [ms] | (–) | 33 | 683 | 133 | 18 | 682 | 117 | –0.008 | (–0.582 to 0.566) | ||
| Quednow et al. 200683 | MFFT-20: latency to first response [s] | (–) | Data from secondary pub.500 | 19 | 49.5 | 19.3 | 19 | 60.5 | 29.9 | 0.437 | (–0.207 to 1.081) |
| MFFT-20: total errors [n] | (–) | Data from secondary pub.500 | 19 | 8.16 | 4.09 | 19 | 6.47 | 4.23 | –0.406 | (–1.049 to 0.237) | |
| Lamers et al. 200698 | TMT: Part A – time (s) | (–) | 11 | 26.8 | 9.7 | 15 | 19 | 5.7 | –1.023 | (–1.853 to –0.193) | |
| TMT: Part B – part A – time (s) | (–) | 11 | 24.2 | 3.8 | 15 | 19.1 | 6.4 | –0.933 | (–1.754 to –0.111) | ||
| TMT: Part B – time (s) | (–) | 11 | 51 | 17.7 | 15 | 38.1 | 9.3 | –0.959 | (–1.783 to –0.135) | ||
| Former users vs drug-naïve controls | |||||||||||
| Wareing et al. 2000136 | Letter comparison speed task – three-letter – correct [n] | (+) | 8 | 20 | 2 | 5 | 22.7 | 3.02 | –1.115 | (–2.326 to 0.095) | |
| Letter comparison speed task – six-letter – correct [n] | (+) | 8 | 14.9 | 3.67 | 5 | 15.5 | 2.17 | –0.187 | (–1.307 to 0.933) | ||
| Letter comparison speed task – nine-letter – correct [n] | (+) | 8 | 11.7 | 3.23 | 5 | 12.5 | 1.72 | –0.288 | (–1.412 to 0.836) | ||
| Morgan et al. 2002103 | MFFT-20: latency to first response [s] | (–) | 15 | 10.2 | 4.648 | 7.5 | 14.3 | 6.584 | 0.768 | (–0.139 to 1.676) | |
| MFFT-20: total errors [n] | (–) | 15 | 14.4 | 5.035 | 7.5 | 7.8 | 3.486 | –1.435 | (–2.415 to –0.454) | ||
| TMT: Part B – errors (n) | (–) | 15 | 0.64 | 0.891 | 7.5 | 0.14 | 0.349 | –0.656 | (–1.556 to 0.243) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Gouzoulis et al. 200099 | TAP: visual scanning – critical trials – time [ms] | (–) | 28 | 1959 | 493.7 | 28 | 1921 | 445.7 | –0.079 | (–0.603 to 0.445) | |
| TAP: visual scanning – non-critical trials – time [ms] | (–) | 28 | 3281 | 1017 | 28 | 3416 | 868.2 | 0.143 | (–0.382 to 0.667) | ||
| Fox et al. 2002130 | CANTAB 3D – ID/ED: errors – compound dimensional (reversal) | (–) | 20 | 1.9 | 1.7 | 20 | 1.3 | 0.5 | –0.479 | (–1.108 to 0.150) | |
| CANTAB 3D – ID/ED: errors – compound dimensional | (–) | 20 | 2.2 | 3.7 | 20 | 1.4 | 1.7 | –0.278 | (–0.901 to 0.345) | ||
| CANTAB 3D – ID/ED: errors – intradimensional (reversal) | (–) | 20 | 1.4 | 0.9 | 20 | 1.1 | 0.3 | –0.447 | (–1.075 to 0.181) | ||
| CANTAB 3D – ID/ED: errors – intradimensional | (–) | 20 | 1.2 | 1 | 20 | 0.9 | 0.9 | –0.315 | (–0.939 to 0.308) | ||
| CANTAB 3D – ID/ED: latency – compound dimensional (reversal) | (–) | 20 | 2.37 | 0.91 | 20 | 1.85 | 0.52 | –0.702 | (–1.341 to –0.062) | ||
| CANTAB 3D – ID/ED: latency – compound dimensional | (–) | 20 | 3.42 | 3.01 | 20 | 3.17 | 3.59 | –0.075 | (–0.695 to 0.545) | ||
| CANTAB 3D – ID/ED: latency – intradimensional (reversal) | (–) | 20 | 1.92 | 0.77 | 20 | 1.57 | 0.49 | –0.542 | (–1.174 to 0.089) | ||
| CANTAB 3D – ID/ED: latency – intradimensional | (–) | 20 | 2.6 | 0.93 | 20 | 2.15 | 0.9 | –0.492 | (–1.121 to 0.138) | ||
| Curran and Verheyden 2003104 | RVIP: 10 min task – correct [n] | (+) | 32 | 32.4 | 7.8 | 16 | 30.5 | 8.1 | 0.241 | (–0.362 to 0.843) | |
| Gouzoulis et al. 2003108 | G/N-G: correct responses | (+) | (1) Heavy E users vs controls | 30 | 19.7 | 0.5 | 15 | 19.4 | 0.9 | 0.456 | (–0.171 to 1.084) |
| (2) Moderate E users vs controls | 30 | 19.5 | 0.7 | 15 | 19.4 | 0.9 | 0.130 | (–0.491 to 0.750) | |||
| TAP: visual scanning – critical trials – correct [n] | (+) | (1) Heavy E users vs controls | 30 | 44.8 | 4.1 | 15 | 45.5 | 3.2 | –0.183 | (–0.804 to 0.438) | |
| (2) Moderate E users vs controls | 30 | 44.8 | 3.5 | 15 | 45.5 | 3.2 | –0.206 | (–0.827 to 0.416) | |||
| TAP: visual scanning – critical trials – time [ms] | (–) | (1) Heavy E users vs controls | 30 | 2756 | 778.2 | 15 | 2445 | 729.8 | –0.408 | (–1.034 to 0.218) | |
| (2) Moderate E users vs controls | 30 | 2542 | 840.7 | 15 | 2445 | 729.8 | –0.120 | (–0.740 to 0.501) | |||
| TAP: visual scanning – non-critical trials – correct [n] | (+) | (1) Heavy E users vs controls | 30 | 48.2 | 1.1 | 15 | 48.5 | 1 | –0.281 | (–0.903 to 0.342) | |
| (2) Moderate E users vs controls | 30 | 48.1 | 1 | 15 | 48.5 | 1 | –0.400 | (–1.026 to 0.226) | |||
| TAP: visual scanning – non-critical trials – time [ms] | (–) | (1) Heavy E users vs controls | 30 | 4993 | 1192 | 15 | 4368 | 1269 | –0.513 | (–1.143 to 0.116) | |
| (2) Moderate E users vs controls | 30 | 4765 | 1753 | 15 | 4368 | 1269 | –0.246 | (–0.868 to 0.376) | |||
| Quednow et al. 200683 | G/N-G: summed conditions – Σ omission errors | (–) | Data from secondary pub.500 | 18 | 10.94 | 6.83 | 17 | 9.18 | 5.69 | –0.279 | (–0.946 to 0.387) |
| Hoshi et al. 2007125 | G/N-G: correct responses | (+) | 25 | 65 | 4 | 14.5 | 63.5 | 12.92 | 0.179 | (–0.469 to 0.827) | |
| Roiser et al. 2007118 | CANTAB A-G/N-G: omission errors [n] | (–) | Data from secondary pub.501 | 30 | 3 | 2.7 | 15 | 5.2 | 5.1 | 0.601 | (–0.031 to 1.234) |
| Former users vs polydrug controls | |||||||||||
| Curran and Verheyden 2003104 | RVIP: 10-min task – correct [n] | (+) | 32 | 26.4 | 9 | 16 | 30.5 | 8.1 | –0.470 | (–1.078 to 0.137) | |
| Hoshi et al. 2007125 | G/N-G: correct responses | (+) | 27 | 69.8 | 1.56 | 14.5 | 63.5 | 12.92 | 0.823 | (0.159–1.486) | |
| Roiser et al. 2007118 | CANTAB A-G/N-G: omission errors [n] | (–) | Data from secondary pub.501 | 20 | 4.8 | 4.6 | 15 | 5.2 | 5.1 | 0.083 | (–0.587 to 0.753) |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Morgan 1998110 | ToL: excess moves (n) | (–) | (1) Study 1 | 16 | 1.52 | 0.88 | 12 | 1.42 | 0.74 | –0.121 | (–0.871 to 0.628) |
| (2) Study 2 | 24 | 1.45 | 0.59 | 20 | 1.65 | 0.66 | 0.321 | (–0.276 to 0.919) | |||
| ToL: initial thinking time (ms) | (–) | (1) Study 1 | 16 | 3434 | 2106 | 12 | 4668 | 3686 | 0.428 | (–0.329 to 1.186) | |
| (2) Study 2 | 24 | 2337 | 1401 | 20 | 2521 | 1535 | 0.126 | (–0.468 to 0.720) | |||
| ToL: perfect solutions (%) | (+) | (1) Study 1 | 16 | 59.06 | 19.99 | 12 | 61.11 | 18.91 | –0.105 | (–0.854 to 0.644) | |
| (2) Study 2 | 24 | 57.51 | 13.19 | 20 | 56.83 | 14.22 | 0.050 | (–0.544 to 0.643) | |||
| ToL: subsequent thinking time (ms / move) | (–) | (1) Study 1 | 16 | 1561 | 1335 | 12 | 1541 | 1510 | –0.014 | (–0.7 63 to 0.734) | |
| (2) Study 2 | 24 | 817 | 951 | 20 | 710 | 838 | –0.119 | (–0.713 to 0.475) | |||
| Fox et al. 2001112 | ToL: errors – n | (–) | (1) High-dose users vs controls | 11 | 4.6 | 3.8 | 6.67 | 3.8 | 2.7 | –0.232 | (–1.198 to 0.733) |
| (2) Medium users vs controls | 14 | 5.1 | 3 | 6.67 | 3.8 | 2.7 | –0.446 | (–1.380 to 0.487) | |||
| (3) Low users vs controls | 14 | 4.1 | 2.2 | 6.67 | 3.8 | 2.7 | –0.127 | (–1.050 to 0.796) | |||
| ToL: planning time – s | (–) | (1) High-dose users vs controls | 11 | 15.3 | 11.6 | 6.67 | 6.5 | 2.9 | –0.933 | (–1.949 to 0.083) | |
| (2) Medium users vs controls | 14 | 9.8 | 5.4 | 6.67 | 6.5 | 2.9 | –0.690 | (–1.639 to 0.258) | |||
| (3) Low users vs controls | 14 | 8.9 | 4.7 | 6.67 | 6.5 | 2.9 | –0.567 | (–1.507 to 0.373) | |||
| ToL: solution time – s | (–) | (1) High-dose users vs controls | 11 | 6.2 | 1.8 | 6.67 | 5.8 | 1.3 | –0.244 | (–1.210 to 0.721) | |
| (2) Medium users vs controls | 14 | 6.5 | 1.5 | 6.67 | 5.8 | 1.3 | –0.485 | (–1.421 to 0.450) | |||
| (3) Low users vs controls | 14 | 6.8 | 1.5 | 6.67 | 5.8 | 1.3 | –0.693 | (–1.642 to 0.255) | |||
| ToL: trials completed – n | (+) | (1) High-dose users vs controls | 11 | 11.8 | 0.4 | 6.67 | 11.8 | 0.6 | 0.000 | (–0.962 to 0.962) | |
| (2) Medium users vs controls | 14 | 11.6 | 0.5 | 6.67 | 11.8 | 0.6 | –0.376 | (–1.306 to 0.554) | |||
| (3) Low users vs controls | 14 | 11.6 | 0.5 | 6.67 | 11.8 | 0.6 | –0.376 | (–1.306 to 0.554) | |||
| Gouzoulis et al. 2003108 | Plan-A-Day: end score | (+) | (1) Heavy users vs controls | 30 | 24 | 7.1 | 15 | 25.8 | 7.7 | –0.247 | (–0.869 to 0.375) |
| (2) Moderate users vs controls | 30 | 25.1 | 7.6 | 15 | 25.8 | 7.7 | –0.092 | (–0.712 to 0.528) | |||
| Plan-A-Day: sequences of deletions | (–) | (1) Heavy users vs controls | 30 | 10.8 | 7.4 | 15 | 8.4 | 5.2 | –0.355 | (–0.979 to 0.269) | |
| (2) Moderate users vs controls | 30 | 12.2 | 7.9 | 15 | 8.4 | 5.2 | –0.533 | (–1.163 to 0.097) | |||
| Plan-A-Day: single deletions | (–) | (1) Heavy users vs controls | 30 | 20.6 | 13.7 | 15 | 19.1 | 15.3 | –0.105 | (–0.726 to 0.515) | |
| (2) Moderate users vs controls | 30 | 24.1 | 19.1 | 15 | 19.1 | 15.3 | –0.279 | (–0.901 to 0.344) | |||
| Plan-A-Day: use of F2 key | (–) | (1) Heavy users vs controls | 30 | 21.1 | 9.1 | 15 | 19.3 | 6.1 | –0.218 | (–0.840 to 0.403) | |
| (2) Moderate users vs controls | 30 | 21.6 | 10.6 | 15 | 19.3 | 6.1 | –0.245 | (–0.867 to 0.377) | |||
| von Geusau et al. 2004132 | ToL: excess moves [%] | (–) | (1) Female | 9 | 55.6 | 5.1 | 21 | 55.2 | 3.3 | –0.103 | (–0.884 to 0.679) |
| (2) Male | 17 | 54.1 | 3.7 | 12 | 31.7 | 4.4 | –5.600 | (–7.266 to –3.934) | |||
| ToL: planning time – s | (–) | (1) Female | 9 | 8.8 | 2.3 | 21 | 10.1 | 1.5 | 0.736 | (–0.068 to 1.540) | |
| (2) Male | 17 | 7.7 | 1.7 | 12 | 14.5 | 2 | 3.720 | (2.483–4.957) | |||
| ToL: total moves | (–) | (1) Female | 9 | 34.7 | 1.8 | 21 | 33.1 | 1.2 | –1.145 | (–1.981 to –0.308) | |
| (2) Male | 17 | 31.9 | 1.3 | 12 | 27.3 | 1.5 | –3.321 | (–4.475 to –2.168) | |||
| ToL: total time – s | (–) | (1) Female | 9 | 33.5 | 2.8 | 21 | 33.4 | 1.9 | –0.046 | (–0.827 to 0.735) | |
| (2) Male | 17 | 26.5 | 2.1 | 12 | 30.7 | 2.5 | 1.849 | (0.961–2.737) | |||
| Hoshi et al. 2007125 | SOC: initial thinking time [ms] | (–) | 24 | 8400 | 7348 | 14.5 | 10200 | 5385 | 0.269 | (–0.386 to 0.924) | |
| Former users vs polydrug controls | |||||||||||
| Hoshi et al. 2007125 | SOC: initial thinking time [ms] | (–) | 27 | 15000 | 7408 | 14.5 | 10200 | 5385 | –0.707 | (–1.364 to –0.051) | |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Gouzoulis et al. 200099 | Stroop: interference – time difference [s] | (–) | 28 | –3.84 | 4.38 | 28 | –3.86 | 6.13 | –0.004 | (–0.528 to 0.520) | |
| TAP: selective visual attention – time [ms] | (–) | 28 | 532 | 65.4 | 28 | 484.4 | 57.9 | –0.771 | (–1.314 to –0.227) | ||
| Croft et al. 200194 | Stroop: interference – time [s] | (–) | 11 | 22.1 | 5.2 | 18 | 21.5 | 4.2 | –0.131 | (–0.881 to 0.620) | |
| Gouzoulis et al. 2003108 | G/N-G: reaction time – ms | (–) | (1) Heavy users vs controls | 30 | 398 | 66.1 | 15 | 382.6 | 54.8 | –0.246 | (–0.868 to 0.376) |
| (2) Moderate users vs controls | 30 | 403.8 | 75 | 15 | 382.6 | 54.8 | –0.307 | (–0.930 to 0.316) | |||
| Halpern et al. 2004106 | Stroop: interference – errors [n] | (–) | (1) Heavy users vs controls | 11 | 5.1 | 3.5 | 8 | 2.2 | 2.1 | –0.965 | (–1.932 to 0.001) |
| (2) Moderate users vs controls | 12 | 3 | 2 | 8 | 2.2 | 2.1 | –0.392 | (–1.296 to 0.511) | |||
| Stroop: interference – time [s] | (–) | (1) Heavy users vs controls | 11 | 115 | 18.9 | 8 | 91.1 | 15.5 | –1.360 | (–2.378 to –0.341) | |
| (2) Moderate users vs controls | 12 | 98.7 | 18.7 | 8 | 91.1 | 15.5 | –0.434 | (–1.339 to 0.472) | |||
| von Geusau et al. 2004132 | HvdM EF: Eriksen Flankers – correct [%] | (+) | (1) Female | 9 | 99.3 | 0.9 | 21 | 96.7 | 0.6 | 3.720 | (2.471–4.968) |
| (2) Male | 17 | 96.6 | 1.2 | 12 | 96.7 | 1.5 | –0.075 | (–0.814 to 0.664) | |||
| HvdM EF: Eriksen Flankers – reaction time – ms | (–) | (1) Female | 9 | 417.8 | 14 | 21 | 437.6 | 9.3 | 1.824 | (0.909–2.740) | |
| (2) Male | 17 | 445.6 | 12.4 | 12 | 414.6 | 15.4 | –2.262 | (–3.217 to –1.308) | |||
| HvdM SS: stop signal – reaction time – ms | (–) | (1) Female | 9 | 236.8 | 91.9 | 21 | 202.8 | 68.4 | –0.448 | (–1.238 to 0.341) | |
| (2) Male | 17 | 195.2 | 39 | 12 | 204.9 | 62.6 | 0.194 | (–0.547 to 0.935) | |||
| Medina et al. 2005124 | Stroop: interference – time [s] | (–) | 48 | 10.6 | 2.9 | 17 | 10 | 3.3 | –0.200 | (–0.754 to 0.355) | |
| Stroop: interference + switching – errors [n] | (–) | 48 | 10.1 | 1.9 | 17 | 9.3 | 2.3 | –0.398 | (–0.956 to 0.159) | ||
| Stroop: interference + switching – time [s] | (–) | 48 | 10.2 | 2.5 | 17 | 10.2 | 2.7 | 0.000 | (–0.553 to 0.553) | ||
| Roiser et al. 2005114 | CANTAB A-G/N-G: commission errors (non-shift) | (–) | 66 | 5.413 | 1.055 | 58 | 5.538 | 0.9 | 0.127 | (–0.226 to 0.480) | |
| CANTAB A-G/N-G: commission errors (shift) | (–) | 66 | 6.345 | 0.927 | 58 | 6.761 | 1.095 | 0.412 | (0.056–0.769) | ||
| Dafters 2006137 | Stroop: interference – time [ms] | (–) | 33 | 834 | 85 | 17 | 829 | 150 | –0.045 | (–0.630 to 0.540) | |
| Reneman et al. 200697 | Stroop: interference – time [s] | (–) | (1) Heavy users vs controls | 22 | 82 | 15.5 | 4.33 | 82.6 | 14.4 | 0.039 | (–0.991 to 1.069) |
| (2) Moderate users vs controls | 15 | 83.5 | 12 | 4.33 | 82.6 | 14.4 | –0.072 | (–1.141 to 0.997) | |||
| Quednow et al. 200683 | G/N-G: summed conditions – Σ commission errors | (–) | Data from secondary pub.500 | 18 | 22.11 | 9.44 | 17 | 17.29 | 6.75 | –0.585 | (–1.262 to 0.093) |
| G/N-G: summed conditions – Σ gain | (+) | Data from secondary pub.500 | 18 | 6.86 | 1.26 | 17 | 7.61 | 1.12 | –0.628 | (–1.308 to 0.052) | |
| Lamers et al. 200698 | Stroop: interference – switching time difference [s] | (–) | 11 | 40.5 | 9 | 15 | 39.5 | 9.1 | –0.110 | (–0.889 to 0.668) | |
| Stroop: interference – time difference [s] | (–) | 11 | 16.3 | 17.4 | 15 | 10.6 | 9.8 | –0.422 | (–1.209 to 0.365) | ||
| Hoshi et al. 2007125 | G/N-G: commission errors | (–) | 25 | 11.8 | 3.5 | 14.5 | 13.6 | 4.31 | 0.472 | (–0.183 to 1.128) | |
| G/N-G: reaction time – ms | (–) | 25 | 355 | 25 | 14.5 | 359 | 59.24 | 0.098 | (–0.549 to 0.745) | ||
| Roiser et al. 2007118 | CANTAB A-G/N-G: commission errors [n] | (–) | Data from secondary pub.501 | 30 | 10.1 | 7.2 | 15 | 11.1 | 7 | 0.140 | (–0.480 to 0.761) |
| Former users vs polydrug controls | |||||||||||
| Reneman et al. 200697 | Stroop: interference – time [s] | (–) | 16 | 85.5 | 14.4 | 4.33 | 82.6 | 14.4 | –0.201 | (–1.265 to 0.862) | |
| Hoshi et al. 2007125 | G/N-G: commission errors | (–) | 27 | 10.2 | 4.68 | 14.5 | 13.6 | 4.31 | 0.746 | (0.087–1.405) | |
| G/N-G: reaction time – ms | (–) | 27 | 382 | 51.96 | 14.5 | 359 | 59.24 | –0.422 | (–1.066 to 0.223) | ||
| Roiser et al. 2007118 | CANTAB A-G/N-G: commission errors [n] | (–) | Data from secondary pub.501 | 20 | 10 | 5.1 | 15 | 11.1 | 7 | 0.184 | (–0.487 to 0.855) |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs drug-naïve controls | |||||||||||
| Gouzoulis et al. 200099 | Stroop: interference – time difference [s] | (–) | 28 | –3.84 | 4.38 | 28 | –3.1 | 6 | 0.141 | (–0.384 to 0.665) | |
| TAP: selective visual attention – time [ms] | (–) | 28 | 532 | 65.4 | 28 | 478.6 | 48.4 | –0.928 | (–1.480 to –0.376) | ||
| Croft et al. 200194 | Stroop: interference – time [s] | (–) | 11 | 22.1 | 5.2 | 31 | 19.5 | 3.8 | –0.620 | (–1.321 to 0.081) | |
| Yip and Lee 2005128 | Stroop: interference – time difference [s] | (–) | 100 | 13.7 | 2.38 | 100 | 13.03 | 0.86 | –0.374 | (–0.654 to –0.095) | |
| Dafters 2006137 | Stroop: interference – time [ms] | (–) | 33 | 834 | 85 | 18 | 838 | 163 | 0.034 | (–0.540 to 0.608) | |
| Quednow et al. 200683 | G/N-G: summed conditions – Σ commission errors | (–) | Data from secondary pub.500 | 18 | 22.11 | 9.44 | 15 | 20.87 | 12.87 | –0.112 | (–0.797 to 0.574) |
| G/N-G: summed conditions – Σ gain | (+) | Data from secondary pub.500 | 18 | 6.86 | 1.26 | 15 | 7.11 | 2.12 | –0.147 | (–0.833 to 0.539) | |
| Lamers et al. 200698 | Stroop: interference – switching time difference [s] | (–) | 11 | 40.5 | 9 | 15 | 48.2 | 9.9 | 0.808 | (–0.003 to 1.618) | |
| Stroop: interference – time difference [s] | (–) | 11 | 16.3 | 17.4 | 15 | 10.6 | 5.4 | –0.476 | (–1.266 to 0.313) | ||
| Hoshi et al. 2007125 | G/N-G: commission errors | (–) | 25 | 11.8 | 3.5 | 13 | 9.8 | 4.08 | –0.540 | (–1.222 to 0.142) | |
| G/N-G: reaction time – ms | (–) | 25 | 355 | 25 | 13 | 385 | 45.89 | 0.897 | (0.195–1.598) | ||
| Roiser et al. 2007118 | CANTAB A-G/N-G: commission errors [n] | (–) | Data from secondary pub.501 | 30 | 10.1 | 7.2 | 15 | 13 | 9.6 | 0.360 | (–0.265 to 0.984) |
| Former users vs drug-naïve controls | |||||||||||
| Hoshi et al. 2007125 | G/N-G: commission errors | (–) | 27 | 10.2 | 4.68 | 13 | 9.8 | 4.08 | –0.089 | (–0.751 to 0.573) | |
| G/N-G: reaction time – ms | (–) | 27 | 382 | 51.96 | 13 | 385 | 45.89 | 0.060 | (–0.602 to 0.722) | ||
| Roiser et al. 2007118 | CANTAB A-G/N-G: commission errors [n] | (–) | Data from secondary pub.501 | 20 | 10 | 5.1 | 15 | 13 | 9.6 | 0.408 | (–0.269 to 1.085) |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Fox et al. 2001112 | WCST: categories | (+) | (1) High-dose users vs controls | 11 | 5.5 | 0.7 | 6.67 | 5.3 | 1.3 | 0.208 | (–0.757 to 1.173) |
| (2) Medium users vs controls | 14 | 4.6 | 2.1 | 6.67 | 5.3 | 1.3 | –0.370 | (–1.300 to 0.560) | |||
| (3) Low users vs controls | 14 | 5.3 | 1.8 | 6.67 | 5.3 | 1.3 | 0.000 | (–0.922 to 0.922) | |||
| WCST: failure to maintain set | (–) | (1) High-dose users vs controls | 11 | 0.9 | 1.3 | 6.67 | 0.6 | 1.2 | –0.237 | (–1.203 to 0.728) | |
| (2) Medium users vs controls | 14 | 0.8 | 0.8 | 6.67 | 0.6 | 1.2 | –0.213 | (–1.138 to 0.712) | |||
| (3) Low users vs controls | 14 | 0.9 | 1.2 | 6.67 | 0.6 | 1.2 | –0.250 | (–1.176 to 0.676) | |||
| WCST: non-perseverative errors – % | (–) | (1) High-dose users vs controls | 11 | 14.1 | 7.5 | 6.67 | 11.7 | 8.4 | –0.306 | (–1.274 to 0.662) | |
| (2) Medium users vs controls | 14 | 15.9 | 14.4 | 6.67 | 11.7 | 8.4 | –0.326 | (–1.254 to 0.602) | |||
| (3) Low users vs controls | 14 | 14.6 | 10.3 | 6.67 | 11.7 | 8.4 | –0.297 | (–1.224 to 0.630) | |||
| WCST: perseverative errors –% | (–) | (1) High-dose users vs controls | 11 | 11.8 | 5.3 | 6.67 | 12.6 | 7.3 | 0.131 | (–0.832 to 1.094) | |
| (2) Medium users vs controls | 14 | 15.8 | 10.2 | 6.67 | 12.6 | 7.3 | –0.340 | (–1.269 to 0.589) | |||
| (3) Low users vs controls | 14 | 11.4 | 6 | 6.67 | 12.6 | 7.3 | 0.187 | (–0.737 to 1.111) | |||
| WCST: trials to first category | (–) | (1) High-dose users vs controls | 11 | 15 | 9.8 | 6.67 | 14.1 | 6.2 | –0.104 | (–1.066 to 0.859) | |
| (2) Medium users vs controls | 14 | 27.6 | 40.8 | 6.67 | 14.1 | 6.2 | –0.395 | (–1.325 to 0.536) | |||
| (3) Low users vs controls | 14 | 13.6 | 3.2 | 6.67 | 14.1 | 6.2 | 0.115 | (–0.808 to 1.038) | |||
| Halpern et al. 2004106 | WCST: categories | (+) | (1) Heavy users vs controls | 11 | 7.4 | 1.7 | 8 | 8.9 | 1.2 | –0.991 | (–1.960 to –0.021) |
| (2) Moderate users vs controls | 12 | 8.7 | 1.4 | 8 | 8.9 | 1.2 | –0.151 | (–1.047 to 0.745) | |||
| WCST: perseverative errors | (–) | (1) Heavy users vs controls | 11 | 12.2 | 5.5 | 8 | 7.2 | 6.2 | –0.862 | (–1.818 to 0.093) | |
| (2) Moderate users vs controls | 12 | 8.7 | 4.1 | 8 | 7.2 | 6.2 | –0.299 | (–1.199 to 0.601) | |||
| von Geusau et al. 2004132 | HvdM DT: dots–triangles – correct – % | (+) | (1) Heavy users vs controls | 9 | 92.3 | 2.7 | 21 | 86 | 1.8 | 3.004 | (1.896–4.113) |
| (2) Moderate users vs controls | 17 | 94.9 | 2 | 12 | 89.2 | 2.5 | 2.571 | (1.563–3.579) | |||
| HvdM DT: dots–triangles – response time – ms | (–) | (1) Heavy users vs controls | 9 | 938.6 | 68.9 | 21 | 826.7 | 46.2 | –2.086 | (–3.039 to –1.133) | |
| (2) Moderate users vs controls | 17 | 910.4 | 50.1 | 12 | 697.8 | 62.2 | –3.841 | (–5.104 to –2.578) | |||
| HvdM LG: local–global – correct –% | (+) | (1) Heavy users vs controls | 9 | 97.1 | 0.9 | 21 | 96.2 | 0.6 | 1.288 | (0.437–2.138) | |
| (2) Moderate users vs controls | 17 | 97 | 0.6 | 12 | 95.3 | 0.7 | 2.645 | (1.624–3.667) | |||
| HvdM LG: local–global – response time – ms | (–) | (1) Heavy users vs controls | 9 | 440.3 | 21.9 | 21 | 457.2 | 13.9 | 1.019 | (0.194–1.844) | |
| (2) Moderate users vs controls | 17 | 459.2 | 15 | 12 | 412.1 | 17.9 | –2.900 | (–3.969 to –1.830) | |||
| WCST: perseverative errors | (+) | (1) Heavy users vs controls | 9 | 14.4 | 3 | 21 | 14.4 | 2 | 0.000 | (–0.781 to 0.781) | |
| (2) Moderate users vs controls | 17 | 18.8 | 2.2 | 12 | 11.3 | 2.6 | –3.163 | (–4.285 to –2.042) | |||
| WCST: total no. correct | (+) | (1) Heavy users vs controls | 9 | 75.7 | 3.6 | 21 | 69.2 | 2.3 | 2.376 | (1.378–3.375) | |
| (2) Moderate users vs controls | 17 | 70.8 | 2.6 | 12 | 77.1 | 3.1 | –2.238 | (–3.188 to –1.288) | |||
| Montgomery et al. 2005138 | Number/letter switch cost (s) | (–) | Study 2 | 42 | 39.27 | 18.14 | 17 | 38.52 | 18.98 | –0.041 | (–0.604 to 0.523) |
| Plus/minus task switch cost (s) | (–) | Study 2 | 42 | 28.63 | 19.46 | 17 | 29.58 | 18.18 | 0.050 | (–0.514 to 0.613) | |
| Reneman et al. 200697 | WCST: categories | (+) | (1) Heavy users vs controls | 22 | 4.4 | 1.6 | 4.33 | 4.6 | 1.5 | –0.126 | (–1.157 to 0.905) |
| (2) Moderate users vs controls | 15 | 4.8 | 1.7 | 4.33 | 4.6 | 1.5 | 0.120 | (–0.949 to 1.190) | |||
| WCST: total no. errors | (–) | (1) Heavy users vs controls | 22 | 38.8 | 18.3 | 4.33 | 35.3 | 24 | –0.182 | (–1.214 to 0.849) | |
| (2) Moderate users vs controls | 15 | 36.7 | 22.8 | 4.33 | 35.3 | 24 | –0.061 | (–1.130 to 1.008) | |||
| Reay et al. 2006109 | BSA: errors [n] | (–) | 15 | 17 | 3.82 | 15 | 13.57 | 3.18 | –0.976 | (–1.736 to –0.216) | |
| Lamers et al. 200698 | WCST: total no. errors | (–) | 11 | 11.5 | 7.8 | 15 | 12.9 | 7.3 | 0.186 | (–0.593 to 0.966) | |
| Former users vs polydrug controls | |||||||||||
| Reneman et al. 200697 | WCST: categories | (+) | 16 | 4.7 | 2.1 | 4.33 | 4.6 | 1.5 | 0.050 | (–1.012 to 1.111) | |
| WCST: total no. errors | (–) | 16 | 35.5 | 19.2 | 4.33 | 35.3 | 24 | –0.010 | (–1.071 to 1.051) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Gouzoulis et al. 200099 | WAIS-R: block design | (+) | 28 | 36.11 | 6.09 | 28 | 40.86 | 5.57 | –0.814 | (–1.360 to –0.268) | |
| Halpern et al. 2004106 | WAIS-R: block design | (+) | (1) Heavy users vs controls | 11 | 39.3 | 5.9 | 8 | 41.6 | 7.1 | –0.358 | (–1.277 to 0.560) |
| (2) Moderate users vs controls | 12 | 36.9 | 6.9 | 8 | 41.6 | 7.1 | –0.674 | (–1.595 to 0.248) | |||
| de Win et al. 200691 | JOLO: pairs | (+) | Follow-up. Data from secondary pub.90 | 58 | 24.1 | 3.5 | 60 | 23.3 | 3.8 | 0.219 | (–0.143 to 0.581) |
| Mental rotation test: completely perfect [n] | (+) | Follow-up. Data from secondary pub.90 | 58 | 26.2 | 8.1 | 60 | 24.4 | 6.4 | 0.247 | (–0.115 to 0.609) | |
| Roiser et al. 2007118 | Mental rotation test: standard – errors [n] | (–) | 30 | 5.2 | 4.4 | 15 | 6.4 | 6.4 | 0.234 | (–0.388 to 0.855) | |
| Mental rotation test: standard – latency [ms] | (–) | 30 | 694.2 | 105.7 | 15 | 732.8 | 135.7 | 0.332 | (–0.292 to 0.956) | ||
| Mental rotation test: mirror – errors [n] | (–) | 30 | 5.5 | 5 | 15 | 5.6 | 6.5 | 0.018 | (–0.602 to 0.638) | ||
| Mental rotation test: mirror – latency [ms] | (–) | 30 | 796.8 | 95.4 | 15 | 839.7 | 154.8 | 0.363 | (–0.261 to 0.988) | ||
| WAIS-R: block test – copy – moves per problem | (–) | 30 | 4.2 | 0.34 | 15 | 4.3 | 0.38 | 0.283 | (–0.340 to 0.906) | ||
| WAIS-R: block test – copy – no. completely perfect | (+) | 30 | 4.1 | 1.1 | 15 | 3.9 | 1.1 | 0.182 | (–0.439 to 0.803) | ||
| WAIS-R: block test – copy – thinking time | (–) | 30 | 1500 | 590.8 | 15 | 1928 | 1182 | 0.515 | (–0.114 to 1.144) | ||
| WAIS-R: block test – mental rotation – moves per problem | (–) | 30 | 4.8 | 0.76 | 15 | 4.8 | 0.69 | 0.000 | (–0.620 to 0.620) | ||
| WAIS-R: block test – mental rotation – no. completely perfect | (+) | 30 | 2.4 | 1.2 | 15 | 2.1 | 1.3 | 0.243 | (–0.379 to 0.865) | ||
| WAIS-R: block test – mental rotation – thinking time | (–) | 30 | 2387 | 1176 | 15 | 2983 | 1692 | 0.437 | (–0.190 to 1.063) | ||
| WAIS-R: block test – mirror – moves per problem | (–) | 30 | 4.5 | 0.56 | 15 | 4.8 | 0.79 | 0.466 | (–0.162 to 1.093) | ||
| WAIS-R: block test – mirror – no. completely perfect | (+) | 30 | 2.6 | 1.2 | 15 | 2.2 | 1.4 | 0.315 | (–0.308 to 0.939) | ||
| WAIS-R: block test – mirror – thinking time | (–) | 30 | 2416 | 1004 | 15 | 2979 | 1684 | 0.445 | (–0.182 to 1.071) | ||
| Former users vs polydrug controls | |||||||||||
| Roiser et al. 200718 | Mental rotation test: standard – errors [n] | (+) | 19 | 6.4 | 4.9 | 15 | 6.4 | 6.4 | 0.000 | (–0.677 to 0.677) | |
| Mental rotation test: standard – latency [ms] | (–) | 19 | 693.6 | 106.5 | 15 | 732.8 | 135.7 | 0.326 | (–0.355 to 1.008) | ||
| Mental rotation test: mirror – errors [n] | (–) | 19 | 7.4 | 6.9 | 15 | 5.6 | 6.5 | –0.268 | (–0.948 to 0.413) | ||
| Mental rotation test: mirror – latency [ms] | (–) | 19 | 824.2 | 105.6 | 15 | 839.7 | 154.8 | 0.120 | (–0.558 to 0.797) | ||
| WAIS-R: block test – copy – moves per problem | (–) | 20 | 4.4 | 0.33 | 15 | 4.3 | 0.38 | –0.284 | (–0.957 to 0.389) | ||
| WAIS-R: block test – copy – no. completely perfect | (+) | 20 | 3.8 | 0.95 | 15 | 3.9 | 1.1 | –0.098 | (–0.768 to 0.571) | ||
| WAIS-R: block test – copy – thinking time | (–) | 20 | 2050 | 897.7 | 15 | 1928 | 1182 | –0.119 | (–0.789 to 0.551) | ||
| WAIS-R: block test – mental rotation – moves per problem | (–) | 20 | 4.7 | 0.68 | 15 | 4.8 | 0.69 | 0.146 | (–0.524 to 0.817) | ||
| WAIS-R: block test – mental rotation – no. completely perfect | (+) | 20 | 2.2 | 1.1 | 15 | 2.1 | 1.3 | 0.084 | (–0.586 to 0.754) | ||
| WAIS-R: block test – mental rotation – thinking time | (–) | 20 | 2489 | 923.4 | 15 | 2983 | 1692 | 0.378 | (–0.297 to 1.054) | ||
| WAIS-R: block test – mirror – moves per problem | (–) | 20 | 4.9 | 0.85 | 15 | 4.8 | 0.79 | –0.121 | (–0.791 to 0.549) | ||
| WAIS-R: block test – mirror – no. completely perfect | (+) | 20 | 2 | 1.5 | 15 | 2.2 | 1.4 | –0.137 | (–0.807 to 0.533) | ||
| WAIS-R: block test – mirror – thinking time | (–) | 20 | 2932 | 1438 | 15 | 2979 | 1684 | 0.030 | (–0.639 to 0.700) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Parrott et al. 2000139 | SCL-90: depression score | (–) | (1) Heavy usersvs controls | 12 | 16.4 | 10.6 | 11 | 10.5 | 9.1 | –0.595 | (–1.433 to 0.243) |
| (2) Light users vs controls | 16 | 16 | 11.4 | 11 | 10.5 | 9.1 | –0.522 | (–1.303 to 0.259) | |||
| Dughiero et al. 2001140 | SCL-90: depression score | (–) | 43 | 0.78 | 0.67 | 77 | 0.63 | 0.57 | –0.247 | (–0.621 to 0.128) | |
| Parrott et al. 2001141 | SCL-90: depression score | (–) | (1) Heavy users vs polydrug controls | 59.5 | 0.93 | 0.7 | 51 | 0.91 | 0.7 | –0.029 | (–0.403 to 0.345) |
| (2) Heavy users vs cannabis controls | 59.5 | 0.93 | 0.7 | 48.5 | 0.83 | 0.6 | –0.152 | (–0.532 to 0.228) | |||
| (3) Light users vs polydrug controls | 57.5 | 0.86 | 0.7 | 51 | 0.91 | 0.7 | 0.071 | (–0.306 to 0.449) | |||
| (4) Light users vs cannabis controls | 57.5 | 0.86 | 0.7 | 48.5 | 0.83 | 0.6 | –0.046 | (–0.428 to 0.336) | |||
| Verkes et al. 2001129 | BDI: overall score | (–) | (1) Heavy users vs controls | 21 | 3 | 3.7 | 10 | 7 | 6.3 | 0.858 | (0.073–1.642) |
| (2) Moderate users vs controls | 21 | 3.9 | 3.3 | 10 | 7 | 6.3 | 0.696 | (–0.078 to 1.470) | |||
| Gamma et al. 2001142 | HDRS: overall score | (–) | 16 | 11.7 | 9.2 | 17 | 5 | 6.1 | –0.864 | (–1.580 to –0.148) | |
| Morgan et al. 2002103 | SCL-90-R: depression score | (–) | 18 | 1.06 | 1.146 | 8 | 0.44 | 0.44 | –0.624 | (–1.476 to 0.227) | |
| Curran and Verheyden 2003104 | BDI: overall score | (–) | 32 | 6.06 | 5.05 | 16 | 5.59 | 5.77 | –0.089 | (–0.689 to 0.512) | |
| von Geusau et al. 2004132 | SCL-90-R: depression score | (–) | (1) Female | 9 | 21.9 | 3.1 | 21 | 21.9 | 5.4 | 0.000 | (–0.781 to 0.781) |
| (2) Male | 17 | 21.1 | 5.5 | 12 | 18.8 | 2.3 | –0.513 | (–1.265 to 0.238) | |||
| Milani et al. 2004143 | SCL-90: depression score | (–) | (1) Men: heavy users vs polydrug controls | 47 | 0.91 | 1.357 | 36 | 0.89 | 1.527 | –0.014 | (–0.448 to 0.420) |
| (2) Men: heavy users vs cannabis controls | 47 | 0.91 | 1.357 | 28.5 | 0.72 | 0.679 | –0.165 | (–0.631 to 0.301) | |||
| (3) Men: light users vs polydrug controls | 34 | 0.75 | 0.825 | 36 | 0.89 | 1.527 | 0.113 | (–0.356 to 0.582) | |||
| (4) Men: light users vs cannabis controls | 34 | 0.75 | 0.825 | 28.5 | 0.72 | 0.679 | –0.039 | (–0.537 to 0.458) | |||
| (5) Women: heavy users vs polydrug controls | 10.5 | 1.07 | 0.321 | 14 | 1.01 | 0.423 | –0.157 | (–0.958 to 0.645) | |||
| (6) Women: heavy users vs cannabis controls | 10.5 | 1.07 | 0.321 | 19.5 | 0.99 | 0.5 | –0.179 | (–0.931 to 0.573) | |||
| (7) Women: light users vs polydrug controls | 22.5 | 1.03 | 0.537 | 14 | 1.01 | 0.423 | –0.040 | (–0.707 to 0.627) | |||
| (8) Women: light users vs cannabis controls | 22.5 | 1.03 | 0.537 | 19.5 | 0.99 | 0.5 | –0.077 | (–0.684 to 0.530) | |||
| McCardle et al. 2004100 | BDI-II: overall score | (–) | 17 | 12.35 | 9.41 | 15 | 5.53 | 4.64 | –0.901 | (–1.632 to –0.170) | |
| Travers and Lyvers 2005144 | BDI: overall score | (–) | 43 | 9.5 | 7.05 | 31 | 6.8 | 5.2 | –0.426 | (–0.893 to 0.041) | |
| Medina et al. 2005124 | BDI-II: overall score | (–) | Data from secondary pub.502 | 48 | 9 | 8.1 | 17 | 10 | 7.7 | 0.125 | (–0.429 to 0.679) |
| Thomasius et al. 200596 | SCL-90-R: depression score | (–) | Data from secondary pub.105 | 30 | 0.78 | 0.548 | 14.5 | 0.73 | 0.646 | –0.086 | (–0.713 to 0.541) |
| Fingeret et al. 2005145 | HDRS: overall score | (–) | 83 | 4.147 | 4.747 | 91 | 1.035 | 1.771 | –0.885 | (–1.196 to –0.573) | |
| Roiser et al. 2005114 | BDI: overall score | (–) | 66 | 8.759 | 6.763 | 58 | 4.99 | 4.428 | –0.651 | (–1.013 to –0.289) | |
| Guillot and Greenway 200678 | BDI-II: overall score | (–) | 32 | 10.3 | 8 | 32 | 10.3 | 8.8 | 0.000 | (–0.490 to 0.490) | |
| Lamers et al. 200698 | BDI-II: overall score | (–) | 11 | 9.82 | 12 | 15 | 4.4 | 3.9 | –0.653 | (–1.453 to 0.147) | |
| de Win et al. 200691 | BDI: overall score | (–) | Follow-up data | 59 | 4.6 | 4.9 | 61 | 3.4 | 3.5 | –0.283 | (–0.642 to 0.077) |
| Hoshi et al. 2007125 | BDI: overall score | (–) | 25 | 5.16 | 3.4 | 14.5 | 6.03 | 4.17 | 0.235 | (–0.414 to 0.885) | |
| Roiser et al. 2007118 | BDI-II: overall score | (–) | Data from secondary pub.500 | 30 | 7.9 | 6.5 | 15 | 6 | 5.4 | –0.308 | (–0.931 to 0.315) |
| Former users vs polydrug controls | |||||||||||
| Morgan et al. 2002103 | SCL-90-R: depression score | (–) | 15 | 0.92 | 0.93 | 8 | 0.44 | 0.44 | –0.600 | (–1.477 to 0.277) | |
| Curran and Verheyden 2003104 | BDI: overall score | (–) | 32 | 8.48 | 5.91 | 16 | 5.59 | 5.77 | –0.493 | (–1.101 to 0.116) | |
| Thomasius et al. 200596 | SCL-90-R: depression score | (–) | Data from secondary pub.105 | 31 | 0.98 | 0.612 | 14.5 | 0.73 | 0.646 | –0.401 | (–1.030 to 0.228) |
| Hoshi et al. 2007125 | BDI: overall score | (–) | 28 | 7.57 | 5.49 | 14.5 | 6.03 | 4.17 | –0.303 | (–0.940 to 0.335) | |
| Roiser et al. 2007118 | BDI-II: overall score | (–) | Data from secondary pub.500 | 20 | 12.6 | 9.5 | 15 | 6 | 5.4 | –0.823 | (–1.521 to –0.125) |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs drug-naïve controls | |||||||||||
| Gerra et al. 1998146 | HDRS: overall score | (–) | 15 | 14.9 | 3.4 | 15 | 5.1 | 2.2 | –3.422 | (–4.569 to –2.275) | |
| MMPI: overall score | (–) | 15 | 64.3 | 3.7 | 15 | 48.5 | 3.2 | –4.568 | (–5.962 to –3.174) | ||
| Gerra et al. 200063 | HDRS: overall score | (–) | 3 weeks abstinent | 15 | 16 | 8.443 | 15 | 5.07 | 4.648 | –1.604 | (–2.434 to –0.774) |
| MMPI 2: overall score | (–) | 3 weeks abstinent | 15 | 59.87 | 12.39 | 15 | 43.5 | 7.669 | –1.588 | (–2.416 to –0.761) | |
| Parrott et al. 2001141 | SCL-90: depression score | (–) | (1) Heavy users vs alcohol/tobacco controls | 59.5 | 0.93 | 0.7 | 92.5 | 0.79 | 0.7 | –0.200 | (–0.527 to 0.127) |
| (2) Heavy users vs drug-free controls | 59.5 | 0.93 | 0.7 | 75 | 0.81 | 0.6 | –0.186 | (–0.527 to 0.155) | |||
| (3) Light users vs alcohol/tobacco controls | 57.5 | 0.86 | 0.7 | 92.5 | 0.79 | 0.7 | –0.100 | (–0.429 to 0.229) | |||
| (4) Light users vs drug-free controls | 57.5 | 0.86 | 0.7 | 75 | 0.81 | 0.6 | –0.077 | (–0.421 to 0.266) | |||
| Gerra et al. 2002147 | HDRS: overall score | (–) | 12 | 17.1 | 10.74 | 12 | 5.1 | 8.66 | –1.230 | (–2.109 to –0.351) | |
| MMPI: overall score | (–) | 12 | 62.6 | 11.43 | 12 | 48.8 | 6.235 | –1.499 | (–2.413 to –0.584) | ||
| Morgan et al. 2002103 | SCL-90-R: depression score | (–) | 18 | 1.06 | 1.146 | 7.5 | 0.35 | 0.503 | –0.703 | (–1.578 to 0.172) | |
| Gerra et al. 200382 | HDRS: overall score | (–) | 15 | 12.6 | 2.3 | 15 | 4.1 | 1.5 | –4.378 | (–5.729 to –3.026) | |
| MMPI 2: overall score | (–) | 15 | 60 | 3 | 15 | 46.1 | 2.3 | –5.200 | (–6.739 to –3.662) | ||
| Milani et al. 2004143 | SCL-90: depression score | (–) | (1) Men: heavy users vs alcohol/tobacco controls | 47 | 0.91 | 1.357 | 50 | 0.75 | 0.7 | –0.150 | (–0.548 to 0.249) |
| (2) Men: heavy users vs drug-free controls | 47 | 0.91 | 1.357 | 30 | 0.74 | 0.465 | –0.154 | (–0.613 to 0.304) | |||
| (3) Men: light users vs alcohol/tobacco controls | 34 | 0.75 | 0.825 | 50 | 0.75 | 0.7 | 0.000 | (–0.436 to 0.436) | |||
| (4) Men: light users vs drug-free controls | 34 | 0.75 | 0.825 | 30 | 0.74 | 0.465 | –0.015 | (–0.506 to 0.476) | |||
| (5) Women: heavy users vs alcohol/tobacco controls | 10.5 | 1.07 | 0.321 | 42 | 0.98 | 0.642 | –0.151 | (–0.828 to 0.526) | |||
| (6) Women: heavy users vs drug-free controls | 10.5 | 1.07 | 0.321 | 44.5 | 0.85 | 0.708 | –0.336 | (–1.011 to 0.340) | |||
| (7) Women: light users vs alcohol/tobacco controls | 22.5 | 1.03 | 0.537 | 42 | 0.98 | 0.642 | –0.082 | (–0.595 to 0.430) | |||
| (8) Women: light users vs drug-free controls | 22.5 | 1.03 | 0.537 | 44.5 | 0.85 | 0.708 | –0.274 | (–0.784 to 0.235) | |||
| Thomasius et al. 200596 | SCL-90-R: depression score | (–) | Data from secondary pub.113 | 30 | 0.78 | 0.548 | 15 | 0.42 | 0.329 | –0.739 | (–1.378 to –0.100) |
| Milani et al. 2005148 | SCL–BSI: depression score | (–) | (1) MDMA polydrug (no cannabis) vs controls | 44 | 1.07 | 0.87 | 24.2 | 0.73 | 0.77 | –0.407 | (–0.907 to 0.094) |
| (2) MDMA polydrug (monthly cannabis) vs controls | 70 | 0.95 | 0.92 | 24.2 | 0.73 | 0.77 | –0.249 | (–0.712 to 0.215) | |||
| (3) MDMA polydrug (weekly cannabis) vs controls | 31 | 0.79 | 0.9 | 24.2 | 0.73 | 0.77 | –0.071 | (–0.603 to 0.461) | |||
| (4) MDMA polydrug (daily cannabis) vs controls | 103 | 0.94 | 0.85 | 24.2 | 0.73 | 0.77 | –0.251 | (–0.695 to 0.193) | |||
| (5) MDMA polydrug (former heavy cannabis) vs controls | 32 | 1.01 | 0.95 | 24.2 | 0.73 | 0.77 | –0.319 | (–0.850 to 0.212) | |||
| Yip and Lee 2005128 | BDI: overall score | (–) | 100 | 1.31 | 1.15 | 100 | 1.48 | 1.01 | 0.157 | (–0.121 to 0.435) | |
| Lamers et al. 200698 | BDI-II: overall score | (–) | 11 | 9.82 | 12 | 15 | 2.47 | 2.2 | –0.927 | (–1.748 to –0.106) | |
| Hoshi et al. 2007125 | BDI: overall score | (–) | 25 | 5.16 | 3.4 | 13.5 | 4.96 | 5.47 | –0.047 | (–0.709 to 0.615) | |
| Roiser et al. 2007118 | BDI-II: overall score | (–) | Data from secondary pub.501 | 30 | 7.9 | 6.5 | 15 | 3.8 | 2.8 | –0.736 | (–1.375 to –0.097) |
| Former users vs drug-naïve controls | |||||||||||
| Morgan et al. 2002103 | SCL-90-R: depression score | (–) | 15 | 0.92 | 0.93 | 7.5 | 0.35 | 0.503 | –0.696 | (–1.598 to 0.206) | |
| Thomasius et al. 200596 | SCL-90-R: depression score | (–) | Data from secondary pub.105 | 31 | 0.98 | 0.612 | 15 | 0.42 | 0.329 | –1.040 | (–1.693 to –0.386) |
| Hoshi et al. 2007125 | BDI: overall score | (–) | 28 | 7.57 | 5.49 | 13.5 | 4.96 | 5.47 | –0.476 | (–1.134 to 0.182) | |
| Roiser et al. 2007118 | BDI-II: overall score | (–) | Data from secondary pub.501 | 20 | 12.6 | 9.5 | 15 | 3.8 | 2.8 | –1.183 | (–1.911 to –0.456) |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Parrott et al. 2000139 | Uplifts/hassles: cognitive failures | (–) | (1) Heavy users vs controls | 12 | 8.3 | 3.6 | 11 | 6.2 | 3.8 | –0.568 | (–1.404 to 0.268) |
| (2) Light users vs controls | 16 | 7.4 | 3.6 | 11 | 6.2 | 3.8 | –0.326 | (–1.099 to 0.447) | |||
| Rodgers 2000122 | CFQ: self-rated – total score | (–) | 15 | 45.2 | 8.11 | 15 | 40.8 | 8.23 | –0.539 | (–1.268 to 0.191) | |
| Gouzoulis et al. 200099 | FZ–EMQ: overall score | (–) | 28 | 27 | 10.61 | 28 | 29.86 | 10.95 | 0.265 | (–0.261 to 0.791) | |
| Heffernan et al. 200176 | PMQ: internally cued | (–) | (1) Study 1 | 46 | 4.35 | 1.84 | 46 | 3.09 | 1.18 | –0.815 | (–1.241 to –0.390) |
| (2) Study 2 | 30 | 3.35 | 1.15 | 37 | 2.85 | 1.3 | –0.405 | (–0.891 to 0.082) | |||
| PMQ: long-term | (–) | (1) Study 1 | 46 | 4.17 | 1.62 | 46 | 2.72 | 1.25 | –1.002 | (–1.436 to –0.568) | |
| (2) Study 2 | 30 | 3.45 | 0.91 | 37 | 2.25 | 0.82 | –1.393 | (–1.931 to –0.855) | |||
| PMQ: short-term | (–) | (1) Study 1 | 46 | 2.37 | 1.03 | 46 | 1.47 | 0.59 | –1.072 | (–1.510 to –0.635) | |
| (2) Study 2 | 30 | 2.39 | 1.12 | 37 | 1.34 | 0.47 | –1.271 | (–1.800 to –0.743) | |||
| PMQ: strategies | (–) | (1) Study 1 | 46 | 3.79 | 1.64 | 46 | 3.75 | 2.15 | –0.021 | (–0.430 to 0.388) | |
| (2) Study 2 | 30 | 2.91 | 1.17 | 37 | 3.33 | 1.61 | 0.294 | (–0.191 to 0.778) | |||
| CFQ: self-rated – total score | (–) | (3) Study 3 | 15 | 45.2 | 8.11 | 15 | 40.8 | 8.23 | –0.539 | (–1.268 to 0.191) | |
| Montgomery and Fisk 2007149 | CFQ: other-rated – total score | (–) | 26 | 14.65 | 6.44 | 31 | 10.71 | 3.63 | –0.772 | (–1.313 to –0.231) | |
| CFQ: self-rated – total score | (–) | 43 | 46.95 | 15.28 | 51 | 39.68 | 12.93 | –0.517 | (–0.930 to –0.105) | ||
| EMQ: overall score | (–) | 43 | 97.24 | 35.34 | 51 | 77.28 | 28.07 | –0.632 | (–1.048 to –0.216) | ||
| PMQ: internally cued | (–) | 28 | 2.92 | 1.25 | 35 | 2.3 | 0.76 | –0.616 | (–1.125 to –0.107) | ||
| PMQ: long-term episodic | (–) | 28 | 3.06 | 1.52 | 35 | 2.52 | 0.76 | –0.466 | (–0.969 to 0.038) | ||
| PMQ: short-term habitual | (–) | 28 | 1.26 | 0.32 | 35 | 1.19 | 0.32 | –0.219 | (–0.717 to 0.280) | ||
| PMQ: strategies | (–) | 28 | 3.29 | 1.65 | 35 | 2.84 | 1.41 | –0.296 | (–0.796 to 0.204) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Parrott et al. 2000139 | SCL-90: anxiety score | (–) | (1) Heavy users vs controls | 12 | 13.8 | 9.8 | 11 | 4.9 | 4.2 | –1.162 | (–2.052 to –0.271) |
| (2) Light users vs controls | 16 | 7.5 | 7.2 | 11 | 4.9 | 4.2 | –0.421 | (–1.197 to 0.356) | |||
| Dughiero et al. 2001140 | SCL-90-R: anxiety score | (–) | 43 | 0.81 | 0.59 | 77 | 0.73 | 0.59 | –0.136 | (–0.509 to 0.238) | |
| Parrott et al. 2001139 | SCL-90: anxiety score | (–) | (1) Heavy users vs polydrug controls | 59.5 | 0.88 | 0.6 | 51 | 0.81 | 0.6 | –0.117 | (–0.491 to 0.258) |
| (2) Heavy users vs cannabis controls | 59.5 | 0.88 | 0.6 | 48.5 | 0.78 | 0.6 | –0.167 | (–0.546 to 0.213) | |||
| (3) Light users vs polydrug controls | 57.5 | 0.8 | 0.6 | 51 | 0.81 | 0.6 | 0.017 | (–0.360 to 0.394) | |||
| (4) Light users vs cannabis controls | 57.5 | 0.8 | 0.6 | 48.5 | 0.78 | 0.6 | –0.033 | (–0.415 to 0.349) | |||
| Verkes et al. 2001129 | STAI-DY: trait anxiety | (–) | (1) Heavy users vs controls | 21 | 38.8 | 10.4 | 10 | 32.1 | 7 | –0.707 | (–1.482 to 0.068) |
| (2) Moderate users vs controls | 21 | 33.5 | 6.9 | 10 | 32.1 | 7 | –0.202 | (–0.957 to 0.553) | |||
| Morgan et al. 2002103 | SCL-90-R: anxiety score | (–) | 18 | 1.01 | 0.891 | 8 | 0.33 | 0.32 | –0.884 | (–1.753 to –0.014) | |
| Curran and Verheyden 2003104 | STAI: trait anxiety | (–) | 32 | 37.09 | 8.91 | 16 | 37.25 | 9.28 | 0.018 | (–0.582 to 0.618) | |
| von Geusau et al. 2004132 | SCL-90-R: anxiety score | (–) | (1) Female | 9 | 13.7 | 1.6 | 21 | 13.8 | 3.2 | 0.035 | (–0.746 to 0.816) |
| (2) Male | 17 | 13.2 | 3.2 | 12 | 10.6 | 0.9 | –1.028 | (–1.816 to –0.240) | |||
| Milani et al. 2004143 | SCL-90: anxiety score | (–) | (1) Men: heavy users vs polydrug controls | 47 | 0.84 | 0.679 | 36 | 0.79 | 0.509 | –0.082 | (–0.516 to 0.352) |
| (2) Men: heavy users vs cannabis controls | 47 | 0.84 | 0.679 | 28.5 | 0.74 | 0.679 | –0.147 | (–0.613 to 0.319) | |||
| (3) Men: light users vs polydrug controls | 34 | 0.76 | 0.66 | 36 | 0.79 | 0.509 | 0.051 | (–0.418 to 0.520) | |||
| (4) Men: light users vs cannabis controls | 34 | 0.76 | 0.66 | 28.5 | 0.74 | 0.679 | –0.030 | (–0.528 to 0.468) | |||
| (5) Women: heavy users vs polydrug controls | 10.5 | 0.96 | 0.092 | 14 | 0.89 | 0.053 | –0.974 | (–1.823 to –0.125) | |||
| (6) Women: heavy users vs cannabis controls | 10.5 | 0.96 | 0.092 | 19.5 | 0.83 | 0.5 | –0.317 | (–1.072 to 0.437) | |||
| (7) Women: light users vs polydrug controls | 22.5 | 0.85 | 0.604 | 14 | 0.89 | 0.053 | 0.084 | (–0.584 to 0.751) | |||
| (8) Women: light users vs cannabis controls | 22.5 | 0.85 | 0.604 | 19.5 | 0.83 | 0.5 | –0.036 | (–0.642 to 0.571) | |||
| Medina et al. 2005124 | STAI: trait anxiety | (–) | Data from secondary pub.502 | 48 | 53 | 12.2 | 17 | 53 | 7.3 | 0.000 | (–0.553 to 0.553) |
| Thomasius et al. 200596 | SCL-90-R: anxiety score | (–) | Data from secondary pub.105 | 30 | 0.42 | 0.411 | 14.5 | 0.54 | 0.539 | 0.264 | (–0.366 to 0.893) |
| Fingeret et al. 2005145 | HARS: overall score | (–) | 83 | 5.282 | 5.751 | 91 | 1.216 | 1.57 | –0.984 | (–1.300 to –0.669) | |
| Ward et al. 2006116 | BAI: overall score | (–) | 31 | 10.1 | 8.88 | 15 | 8.03 | 8.3 | –0.238 | (–0.856 to 0.381) | |
| Lamers et al. 200698 | BAI: overall score | (–) | 11 | 10.3 | 10.4 | 15 | 2.9 | 1.9 | –1.077 | (–1.913 to –0.242) | |
| Hoshi et al. 2007125 | STAI: trait anxiety | (–) | 25 | 35.52 | 7.55 | 14.5 | 35.76 | 6.93 | 0.033 | (–0.614 to 0.680) | |
| Former users vs polydrug controls | |||||||||||
| Morgan et al. 2002103 | SCL-90-R: anxiety score | (–) | 15 | 0.71 | 0.852 | 8 | 0.33 | 0.32 | –0.528 | (–1.401 to 0.345) | |
| Curran and Verheyden 2003104 | STAI: trait anxiety | (–) | 32 | 42.87 | 11.22 | 16 | 37.25 | 9.28 | –0.529 | (–1.139 to 0.081) | |
| Thomasius et al. 200596 | SCL-90-R: anxiety score | (–) | Data from secondary pub.105 | 31 | 0.775 | 0.779 | 14.5 | 0.54 | 0.539 | –0.329 | (–0.957 to 0.298) |
| Ward et al. 2006116 | BAI: overall score | (–) | 30 | 11.87 | 12.21 | 15 | 8.03 | 8.3 | –0.346 | (–0.970 to 0.278) | |
| Hoshi et al. 2007125 | STAI: trait anxiety | (–) | 28 | 37.75 | 9.67 | 14.5 | 35.76 | 6.93 | –0.225 | (–0.861 to 0.411) | |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs drug-naïve controls | |||||||||||
| Parrott et al. 2001141 | SCL-90: anxiety score | (–) | (1) Heavy users vs alcohol/tobacco controls | 59.5 | 0.88 | 0.6 | 92.5 | 0.69 | 0.5 | –0.351 | (–0.679 to –0.023) |
| (2) Heavy users vs drug-free controls | 59.5 | 0.88 | 0.6 | 75 | 0.65 | 0.5 | –0.421 | (–0.765 to –0.077) | |||
| (3) Light users vs alcohol/tobacco controls | 57.5 | 0.8 | 0.6 | 92.5 | 0.69 | 0.5 | –0.204 | (–0.534 to 0.126) | |||
| (4) Light users vs drug-free controls | 57.5 | 0.8 | 0.6 | 75 | 0.65 | 0.5 | –0.275 | (–0.620 to 0.070) | |||
| Morgan et al. 2002103 | SCL-90-R: anxiety score | (–) | 18 | 1.01 | 0.891 | 7.5 | 0.24 | 0.31 | –0.993 | (–1.891 to –0.095) | |
| Milani et al. 2004143 | SCL-90: anxiety score | (–) | (1) Men: heavy users vs alcohol/tobacco controls | 47 | 0.84 | 0.07 | 50 | 0.6 | 0.05 | –3.966 | (–4.656 to –3.276) |
| (2) Men: heavy users vs drug-free controls | 47 | 0.84 | 0.07 | 30 | 0.57 | 0.07 | –3.857 | (–4.626 to –3.089) | |||
| (3) Men: light users vs alcohol/tobacco controls | 34 | 0.76 | 0.08 | 50 | 0.6 | 0.05 | –2.508 | (–3.089 to –1.927) | |||
| (4) Men: light users vs drug-free controls | 34 | 0.76 | 0.08 | 30 | 0.57 | 0.07 | –2.517 | (–3.178 to –1.856) | |||
| (5) Women: heavy users vs alcohol/tobacco controls | 10.5 | 0.96 | 0.02 | 42 | 0.8 | 0.05 | –3.487 | (–4.446 to –2.528) | |||
| (6) Women: heavy users vs drug-free controls | 10.5 | 0.96 | 0.02 | 44.5 | 0.71 | 0.05 | –5.425 | (–6.657 to –4.193) | |||
| (7) Women: light users vs alcohol/tobacco controls | 22.5 | 0.85 | 0.09 | 42 | 0.8 | 0.05 | –0.752 | (–1.280 to –0.223) | |||
| (8) Women: light users vs drug-free controls | 22.5 | 0.85 | 0.09 | 44.5 | 0.71 | 0.05 | –2.122 | (–2.747 to –1.498) | |||
| Jacobsen et al. 2004134 | POMS: anxiety score | (–) | 6 | 0.5 | 3.6 | 6 | 2.2 | 4.5 | 0.417 | (–0.729 to 1.563) | |
| Thomasius et al. 200596 | SCL-90-R: anxiety score | (–) | 30 | 0.42 | 0.411 | 15 | 0.32 | 0.301 | –0.264 | (–0.886 to 0.358) | |
| Milani et al. 2005148 | SCL–BSI: anxiety score | (–) | (1) MDMA polydrug (no cannabis) vs controls | 44 | 1.12 | 0.84 | 24.2 | 0.69 | 0.67 | –0.548 | (–1.053 to –0.043) |
| (2) MDMA polydrug (monthly cannabis) vs controls | 70 | 0.82 | 0.85 | 24.2 | 0.69 | 0.67 | –0.161 | (–0.624 to 0.302) | |||
| (3) MDMA polydrug (weekly cannabis) vs controls | 31 | 0.73 | 0.6 | 24.2 | 0.69 | 0.67 | –0.063 | (–0.595 to 0.468) | |||
| (4) MDMA polydrug (daily cannabis) vs controls | 103 | 0.93 | 0.8 | 24.2 | 0.69 | 0.67 | –0.309 | (–0.753 to 0.136) | |||
| (5) MDMA polydrug (former heavy cannabis) vs controls | 32 | 1.07 | 1.07 | 24.2 | 0.69 | 0.67 | –0.413 | (–0.947 to 0.121) | |||
| Lamers et al. 200698 | BAI: overall score | (–) | 11 | 10.3 | 10.4 | 15 | 4 | 4.3 | –0.843 | (–1.657 to –0.029) | |
| Hoshi et al. 2007125 | STAI: trait anxiety | (–) | 25 | 35.52 | 7.55 | 13.5 | 33.94 | 8.44 | –0.201 | (–0.864 to 0.463) | |
| Former users vs drug-naïve controls | |||||||||||
| Morgan et al. 2002103 | SCL-90-R: anxiety score | (–) | 15 | 0.71 | 0.852 | 7.5 | 0.24 | 0.31 | –0.648 | (–1.547 to 0.251) | |
| Thomasius et al. 200596 | SCL-90-R: anxiety score | (–) | 31 | 0.775 | 0.779 | 15 | 0.32 | 0.301 | –0.683 | (–1.316 to –0.051) | |
| Hoshi et al. 2007125 | STAI: trait anxiety | (–) | 28 | 37.75 | 9.67 | 13.5 | 33.94 | 8.44 | –0.410 | (–1.065 to 0.246) | |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Morgan 1998110 | MFFT-20: impulsivity score | (–) | (1) Study 1 | 16 | 1.04 | 2.19 | 12 | –0.49 | 1.16 | –0.838 | (–1.620 to –0.055) |
| (2) Study 2 | 25 | 0.51 | 1.72 | 20 | –0.4 | 1.48 | –0.562 | (–1.162 to 0.038) | |||
| Butler and Montgomery 200477 | Bets16 – risk-taking score | (–) | (1) Heavy users vs polydrug controls | 9 | 8.3 | 4 | 18 | 6.1 | 5.2 | –0.454 | (–1.264 to 0.356) |
| (2) Heavy users vs cannabis controls | 9 | 8.3 | 4 | 27 | 4.9 | 4.5 | –0.775 | (–1.551 to 0.002) | |||
| (3) Light users vs polydrug controls | 14 | 4.3 | 4.2 | 18 | 6.1 | 5.2 | 0.376 | (–0.329 to 1.080) | |||
| (4) Light users vs cannabis controls | 14 | 4.3 | 4.2 | 27 | 4.9 | 4.5 | 0.136 | (–0.510 to 0.782) | |||
| Morgan et al. 2006115 | MFFT-20: impulsivity score | (–) | 20 | 1.075 | 1.342 | 20 | –0.23 | 1.342 | –0.973 | (–1.630 to –0.315) | |
| RGT: gains only – latency – ms | (+) | 20 | 3589 | 1948 | 20 | 4266 | 2063 | –0.337 | (–0.962 to 0.287) | ||
| RGT: gains only – risky choices | (–) | 20 | 0.769 | 0.273 | 20 | 0.8 | 0.285 | 0.112 | (–0.508 to 0.732) | ||
| RGT: losses only – latency – ms | (+) | 20 | 2410 | 1373 | 20 | 2392 | 868 | 0.016 | (–0.604 to 0.635) | ||
| RGT: losses only – risky choices | (–) | 20 | 0.225 | 0.242 | 20 | 0.144 | 0.244 | –0.334 | (–0.958 to 0.290) | ||
| Quednow et al. 200683 | MFFT: impulsivity score | (–) | Data from secondary pub.500 | 19 | 0.76 | 1.43 | 19 | 0.12 | 1.49 | –0.438 | (–1.082 to 0.206) |
| Roiser et al. 2007118 | RGT: gains only – latency – ms | (+) | 30 | 2220 | 1318 | 15 | 2006 | 776.3 | 0.183 | (–0.438 to 0.804) | |
| RGT: gains only – risky choices | (–) | 30 | 0.8 | 0.27 | 15 | 0.73 | 0.32 | –0.244 | (–0.866 to 0.378) | ||
| RGT: losses only – latency – ms | (+) | 30 | 3834 | 1771 | 15 | 3579 | 1691 | 0.146 | (–0.474 to 0.767) | ||
| RGT: losses only – risky choices | (–) | 30 | 0.27 | 0.32 | 15 | 0.37 | 0.3 | 0.319 | (–0.305 to 0.942) | ||
| Former users vs polydrug controls | |||||||||||
| Roiser et al. 2007118 | RGT: gains only – latency – ms | (+) | 20 | 2280 | 1199 | 15 | 2006 | 776.3 | 0.263 | (–0.410 to 0.935) | |
| RGT: gains only – risky choices | (–) | 20 | 0.76 | 0.32 | 15 | 0.73 | 0.32 | –0.094 | (–0.764 to 0.576) | ||
| RGT: losses only – latency – ms | (+) | 20 | 3728 | 1704 | 15 | 3579 | 1691 | 0.088 | (–0.582 to 0.758) | ||
| RGT: losses only – risky choices | (–) | 20 | 0.42 | 0.29 | 15 | 0.37 | 0.3 | –0.170 | (–0.841 to 0.501) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs drug-naïve controls | |||||||||||
| Morgan 1998110 | MFFT-20: impulsivity score | (+) | (1) Study 1 | 16 | 1.04 | 2.19 | 16 | –0.67 | 1.28 | 0.953 | (0.220–1.687) |
| (2) Study 2 | 25 | 0.51 | 1.72 | 19 | –0.28 | 2.2 | 0.407 | (–0.196 to 1.010) | |||
| Moeller et al. 2002133 | Delayed memory task – adjusted commission errors [n] | (+) | (1) Heavy users vs controls | 8 | 0.4 | 0.245 | 10 | 0.21 | 0.169 | 0.923 | (–0.060 to 1.906) |
| (2) Infrequent users vs controls | 8 | 0.14 | 0.13 | 10 | 0.21 | 0.169 | –0.457 | (–1.401 to 0.486) | |||
| Immediate memory task – adjusted commission errors [n] | (+) | (1) Heavy users vs controls | 8 | 0.426 | 0.182 | 10 | 0.21 | 0.137 | 1.366 | (0.323–2.409) | |
| (2) Infrequent users vs controls | 8 | 0.22 | 0.159 | 10 | 0.21 | 0.137 | 0.068 | (–0.862 to 0.998) | |||
| Butler and Montgomery 200477 | Bets16 – risk-taking score | (+) | (1) Heavy users vs controls | 18 | 8.3 | 4 | 56.5 | 3.9 | 4.8 | 0.951 | (0.399–1.504) |
| (2) Light users vs controls | 28 | 4.3 | 4.2 | 56.5 | 3.9 | 4.8 | 0.087 | (–0.366 to 0.540) | |||
| Morgan et al. 2006115 | MFFT-20: impulsivity score | (+) | 20 | 1.075 | 1.342 | 19 | –0.85 | 1.962 | 1.151 | (0.471–1.832) | |
| RGT: gains only – latency – ms | (–) | 20 | 3589 | 1948 | 19 | 4528 | 2120 | 0.462 | (–0.175 to 1.098) | ||
| RGT: gains only – risky choices | (+) | 20 | 0.769 | 0.273 | 19 | 0.822 | 0.261 | –0.201 | (–0.830 to 0.429) | ||
| RGT: losses only – latency – ms | (–) | 20 | 2410 | 1373 | 19 | 3044 | 3717 | 0.229 | (–0.401 to 0.859) | ||
| RGT: losses only – risky choices | (+) | 20 | 0.225 | 0.242 | 19 | 0.211 | 0.191 | 0.066 | (–0.562 to 0.694) | ||
| Quednow et al. 200683 | MFFT: impulsivity score | (+) | Data from secondary pub.500 | 19 | 0.76 | 1.43 | 19 | 0 | 1.77 | 0.472 | (–0.173 to 1.118) |
| Roiser et al. 2007118 | RGT: gains only – latency – ms | (–) | 30 | 2220 | 1318 | 15 | 2353 | 1584 | 0.095 | (–0.525 to 0.715) | |
| RGT: gains only – risky choices | (+) | 30 | 0.8 | 0.27 | 15 | 0.81 | 0.24 | –0.038 | (–0.658 to 0.581) | ||
| RGT: losses only – latency – ms | (–) | 30 | 3834 | 1771 | 15 | 3566 | 1928 | –0.147 | (–0.768 to 0.473) | ||
| RGT: losses only – risky choices | (+) | 30 | 0.27 | 0.32 | 15 | 0.4 | 0.36 | –0.390 | (–1.015 to 0.235) | ||
| Former users vs drug-naïve controls | |||||||||||
| Roiser et al. 2007118 | RGT: gains only – latency – ms | (–) | 20 | 2280 | 1199 | 15 | 2353 | 1584 | 0.053 | (–0.616 to 0.723) | |
| RGT: gains only – risky choices | (+) | 20 | 0.76 | 0.32 | 15 | 0.81 | 0.24 | –0.173 | (–0.844 to 0.498) | ||
| RGT: losses only – latency – ms | (–) | 20 | 3728 | 1704 | 15 | 3566 | 1928 | –0.090 | (–0.760 to 0.580) | ||
| RGT: losses only – risky choices | (+) | 20 | 0.42 | 0.29 | 15 | 0.4 | 0.36 | 0.062 | (–0.607 to 0.732) | ||
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs polydrug controls | |||||||||||
| Morgan 1998110 | IVE: overall score | (–) | Study 2 | 25 | 12 | 4.25 | 20 | 10.7 | 4.29 | –0.305 | (–0.896 to 0.287) |
| Parrott et al. 2000139 | IVE: overall score | (–) | (1) Heavy users vs controls | 12 | 13.4 | 3.4 | 11 | 8.9 | 4.7 | –1.105 | (–1.989 to –0.222) |
| (2) Light users vs controls | 16 | 11.1 | 4.7 | 11 | 8.9 | 4.7 | –0.468 | (–1.247 to 0.310) | |||
| Curran and Verheyden 2003104 | BIS-II: total | (–) | Data from secondary pub.158 | 32 | 59.84 | 13.83 | 16 | 51.03 | 14.29 | –0.630 | (–1.244 to –0.016) |
| Butler and Montgomery 200477 | IVE: overall score | (–) | (1) Heavy users vs polydrug controls | 9 | 11.1 | 5.4 | 18.5 | 9.8 | 4.6 | –0.267 | (–1.067 to 0.533) |
| (2) Heavy users vs cannabis controls | 9 | 11.1 | 5.4 | 27.5 | 8.7 | 3.8 | –0.568 | (–1.333 to 0.197) | |||
| (3) Light users vs polydrug controls | 14 | 10.3 | 4.2 | 18.5 | 9.8 | 4.6 | –0.113 | (–0.808 to 0.582) | |||
| (4) Light users vs cannabis controls | 14 | 10.3 | 4.2 | 27.5 | 8.7 | 3.8 | –0.406 | (–1.056 to 0.243) | |||
| Travers and Lyvers 2005144 | IVE: overall score | (–) | 43 | 9.42 | 4.49 | 31 | 10.42 | 4.53 | 0.222 | (–0.241 to 0.685) | |
| Fingeret et al. 2005145 | BIS-II: total | (–) | 83 | 68.04 | 13.01 | 91 | 57.43 | 8.819 | –0.964 | (–1.278 to –0.649) | |
| de Win et al. 200693 | BIS-II: total | (–) | Follow-up | 59 | 71.3 | 9.8 | 61 | 68.9 | 10.5 | –0.236 | (–0.595 to 0.123) |
| Roiser et al. 2007118 | IVE: overall score | (–) | 30 | 8.4 | 4.4 | 15 | 8.6 | 3.5 | 0.048 | (–0.571 to 0.668) | |
| Former users vs drug-naïve controls | |||||||||||
| Curran and Verheyden to 2003104 | BIS-II: total | (–) | Data from secondary pub.158 | 32 | 57.63 | 14.49 | 16 | 51.03 | 14.29 | –0.458 | (–1.065 to 0.150) |
| Roiser et al. 2007118 | IVE: overall score | (–) | 20 | 10.5 | 5.3 | 15 | 8.6 | 3.5 | –0.411 | (–1.088 to 0.266) | |
| Study | Measure | (+/–) | Comparison | MDMA users | Controls | SMD | (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||
| Current users vs drug-naïve controls | |||||||||||
| Morgan 1998110 | IVE: overall score | (+) | Study 2 | 25 | 12 | 4.25 | 19 | 8.47 | 5.12 | –0.760 | (–1.379 to –0.142) |
| Moeller et al. 2002133 | BIS-II: total | (+) | (1) Heavy users vs controls | 8 | 73 | 7.937 | 10 | 62.5 | 9.127 | –1.217 | (–2.238 to –0.196) |
| (2) Infrequent users vs controls | 8 | 59 | 11.54 | 10 | 62.5 | 9.127 | 0.341 | (–0.596 to 1.278) | |||
| Butler and Montgomery 200477 | IVE: overall score | (+) | (1) Heavy users vs controls | 18 | 11.1 | 5.4 | 58 | 7.5 | 4.1 | –0.812 | (–1.357 to –0.267) |
| (2) Light users vs controls | 28 | 10.3 | 4.2 | 58 | 7.5 | 4.1 | –0.678 | (–1.140 to –0.215) | |||
| Dafters 2006150 | BIS-II: total | (+) | (1) Light users vs controls | 18 | 81.4 | 11.9 | 9 | 69.7 | 8.2 | –1.078 | (–1.932 to –0.224) |
| (2) Heavy users vs controls | 18 | 81.3 | 6.7 | 9 | 69.7 | 8.2 | –1.608 | (–2.524 to –0.692) | |||
| IVE: overall score | (+) | (1) Light users vs controls | 18 | 11.12 | 3.5 | 9 | 7.44 | 2.7 | –1.127 | (–1.986 to –0.268) | |
| (2) Heavy users vs controls | 18 | 11.47 | 3.3 | 9 | 7.44 | 2.7 | –1.291 | (–2.168 to –0.415) | |||
| Roiser et al. 2007118 | IVE: overall score | (+) | 30 | 8.4 | 4.4 | 15 | 6.8 | 4.5 | –0.361 | (–0.985 to 0.264) | |
| Former users vs drug-naïve controls | |||||||||||
| Roiser et al. 2007118 | IVE: overall score | (+) | 20 | 10.5 | 5.3 | 15 | 6.8 | 4.5 | –0.744 | (–1.437 to –0.050) | |
Appendix 7 Dose–response: estimated total lifetime dose of ecstasy plotted against effect estimates
FIGURE 88.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (immediate) (sum of trials 1–5) – ecstasy users versus polydrug controls: mean difference in score against estimated total lifetime dose (ETLD) of MDMA.
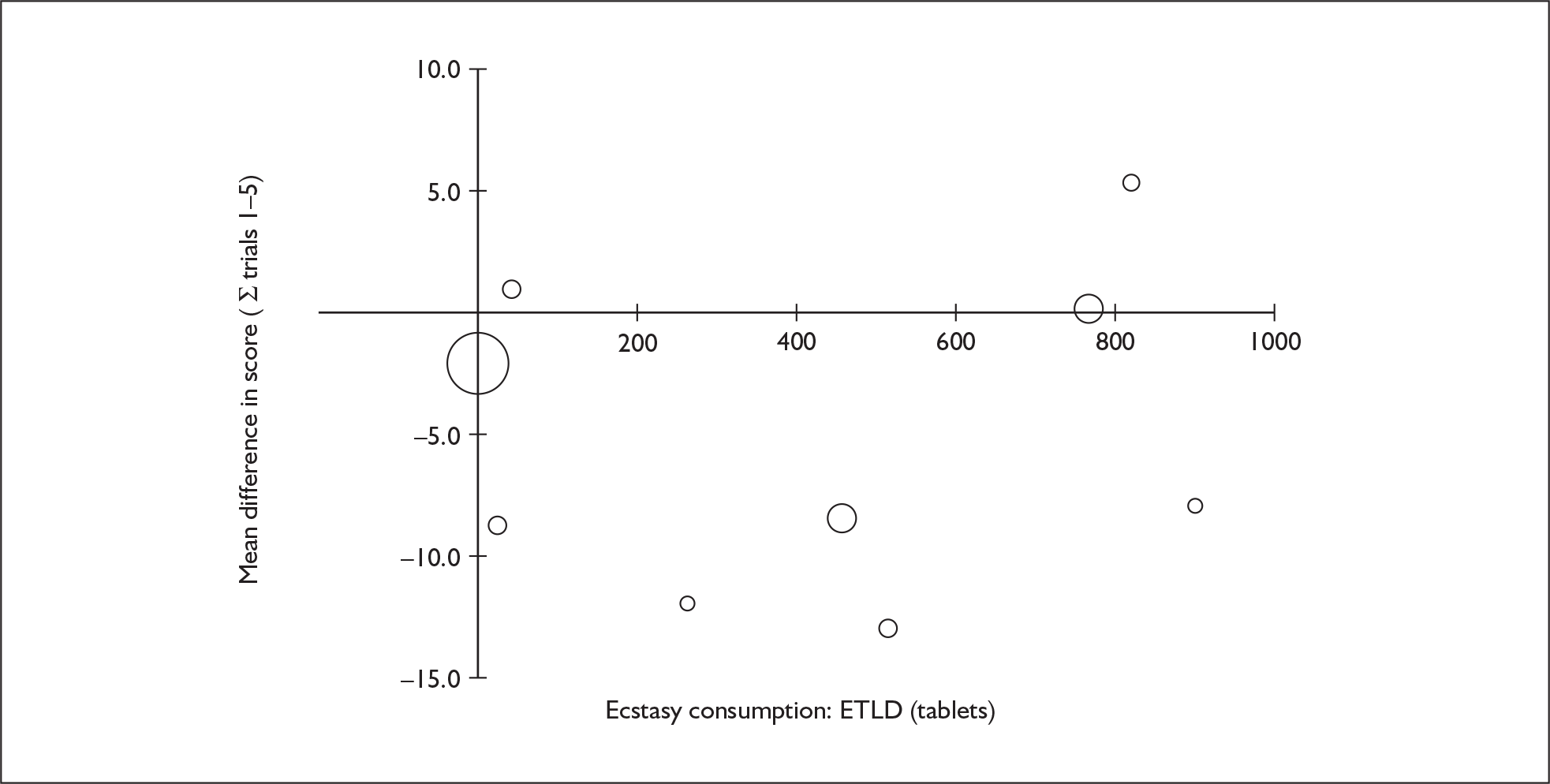
FIGURE 89.
Rey Auditory Verbal Learning Test (RAVLT) verbal recall (delayed) (trial 8) – ecstasy users versus polydrug controls: mean difference in score against estimated total lifetime dose (ETLD) of MDMA.
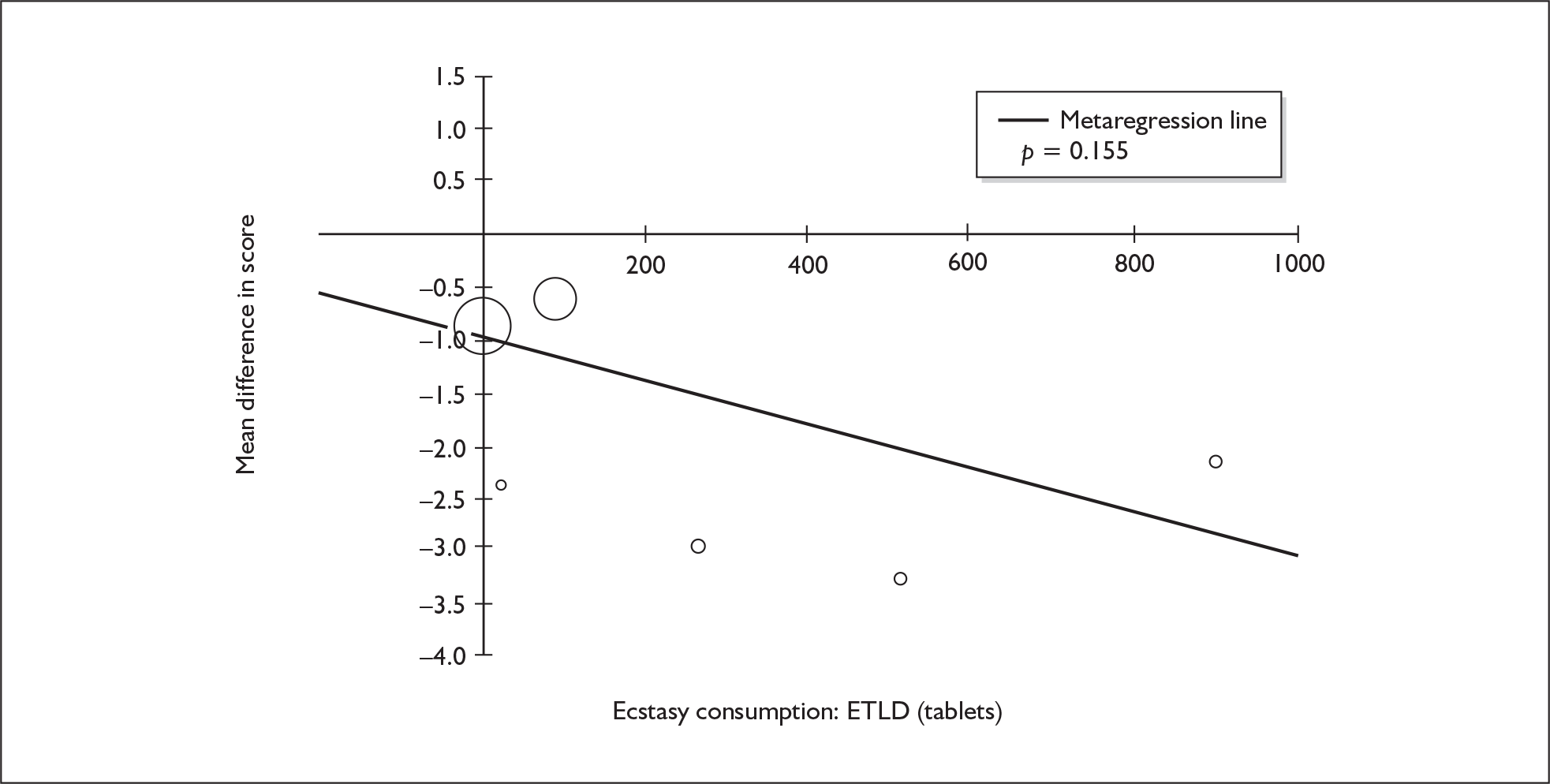
FIGURE 90.
Rivermead Behavioural Memory Test (RBMT) prose recall (immediate) – ecstasy users versus polydrug controls: mean difference in score against estimated total lifetime dose (ETLD) of MDMA.
FIGURE 91.
Rivermead Behavioural Memory Test (RBMT) prose recall (delayed) – ecstasy users versus. polydrug controls: mean difference in score against estimated total lifetime dose (ETLD) of MDMA.
FIGURE 92.
Digit span (forwards) – ecstasy users versus polydrug controls: mean difference in score against estimated total lifetime dose (ETLD) of MDMA.
FIGURE 93.
Digit span (backwards) – ecstasy users versus polydrug controls: mean difference in score against estimated total lifetime dose (ETLD) of MDMA.
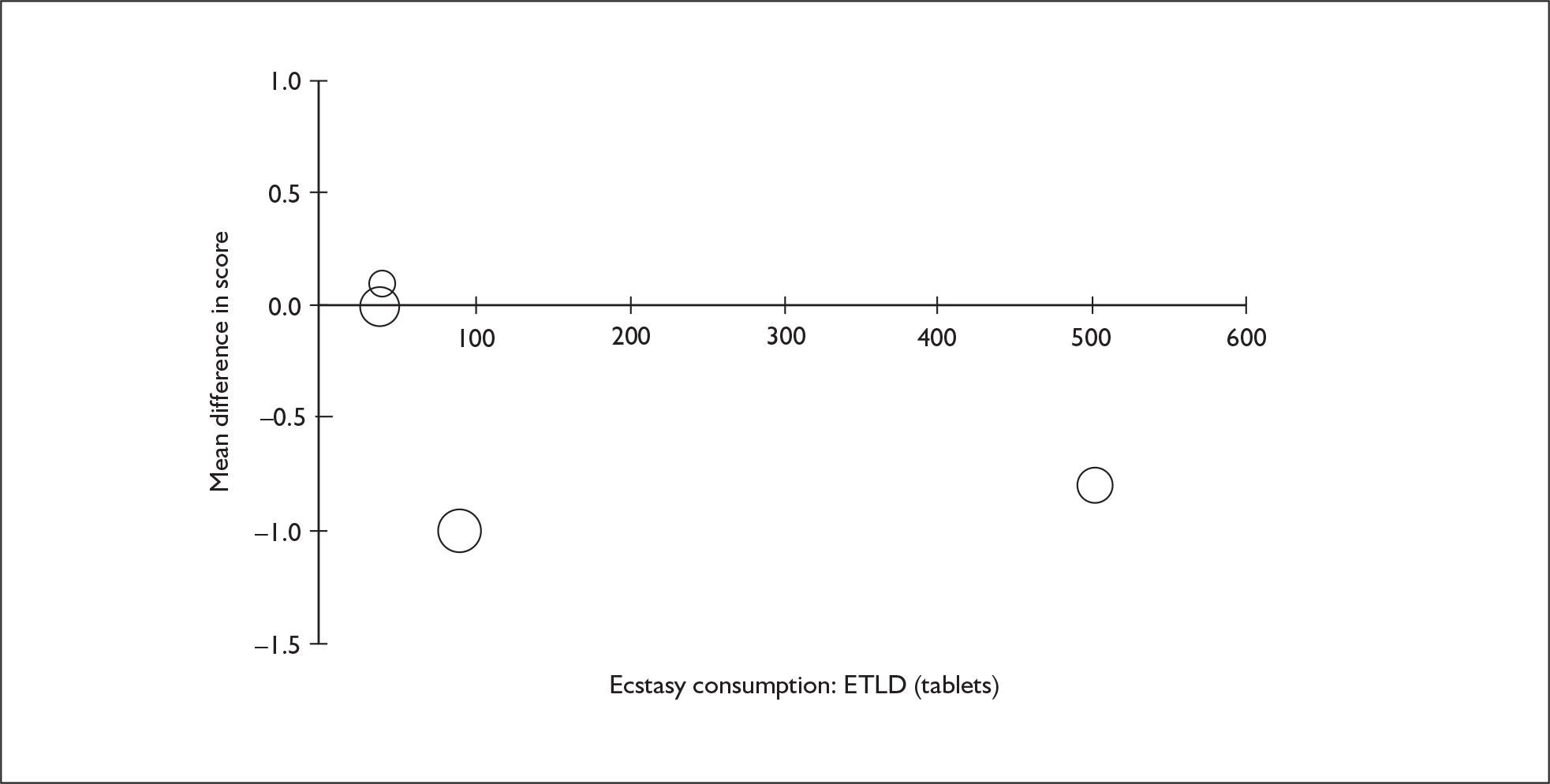
FIGURE 94.
IQ (National Adult Reading Test) – ecstasy users versus polydrug controls: mean difference in IQ against estimated total lifetime dose (ETLD) of MDMA.
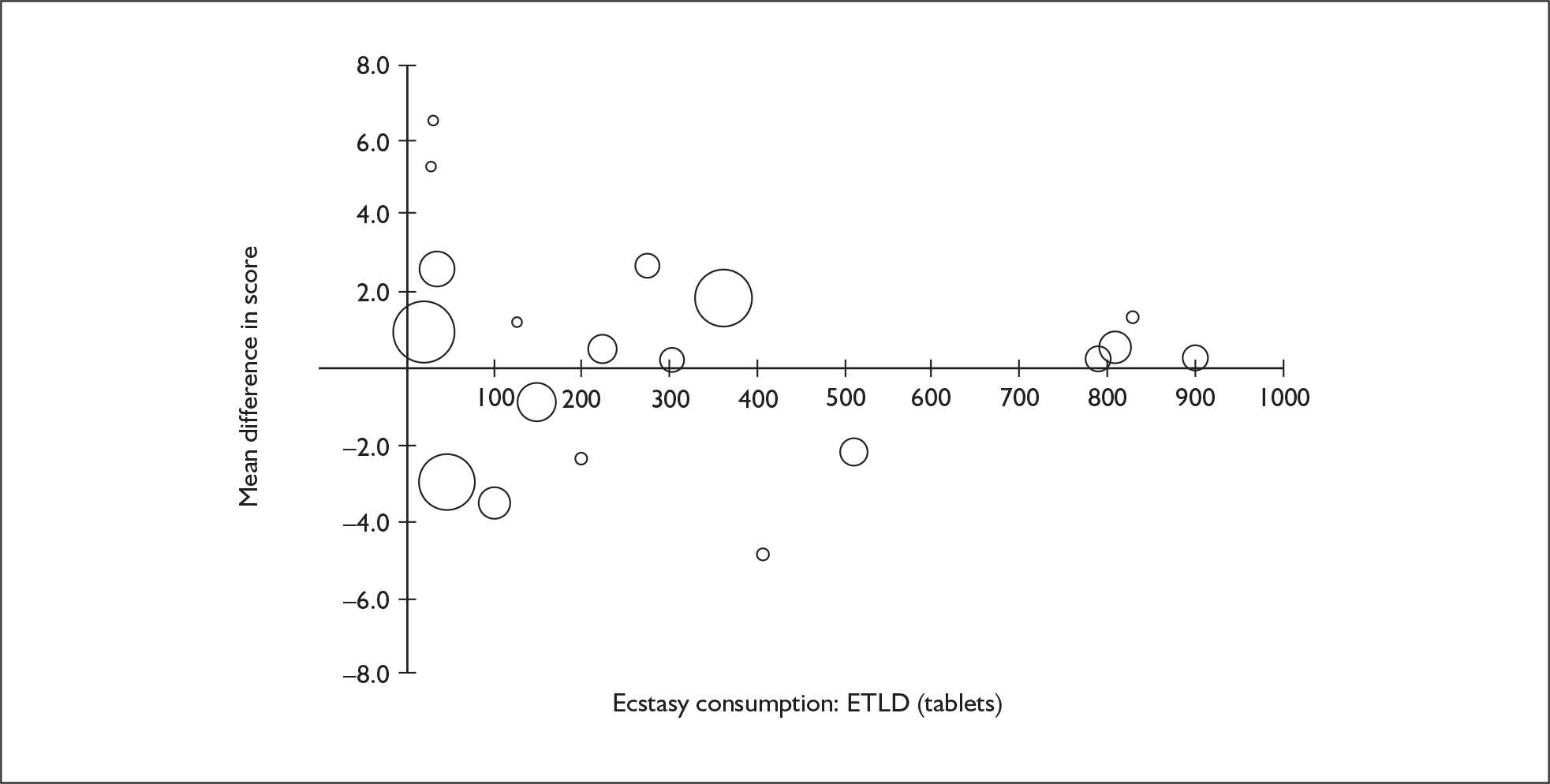
FIGURE 95.
IQ (National Adult Reading Test) – ecstasy users versus drug-naïve controls: mean difference in IQ against estimated total lifetime dose (ETLD) of MDMA.
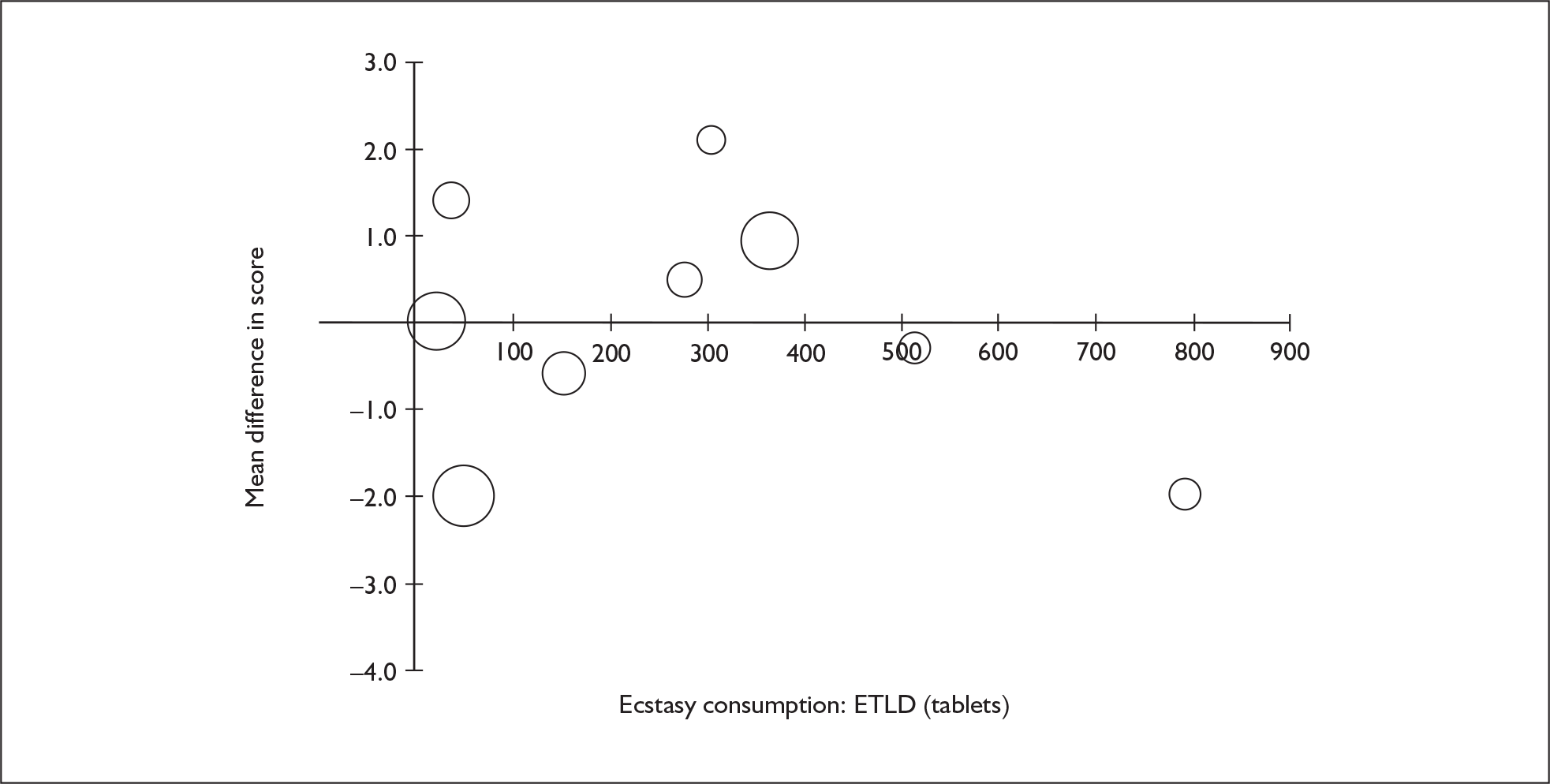
FIGURE 96.
Verbal memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
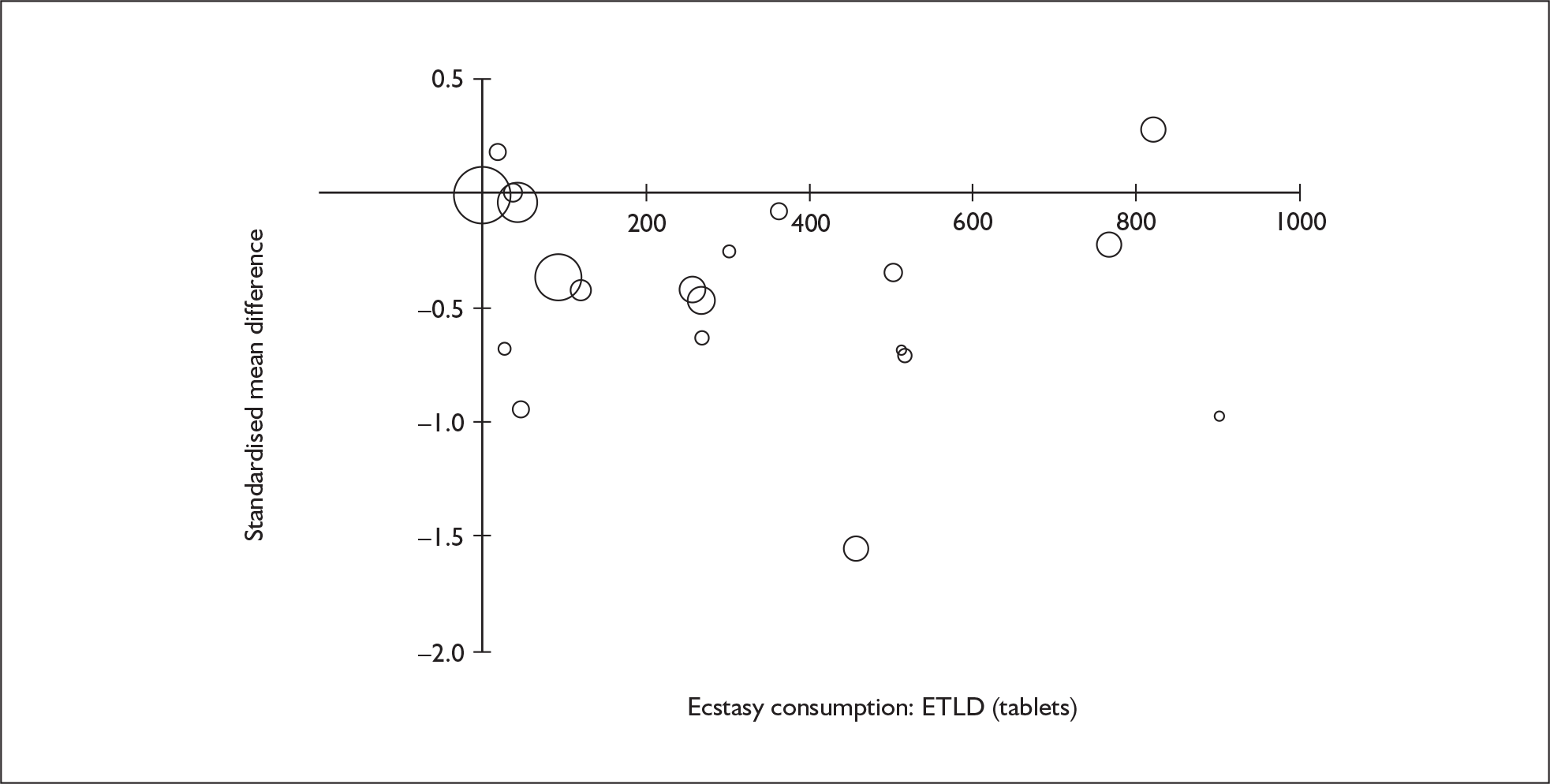
FIGURE 97.
Verbal memory – immediate (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
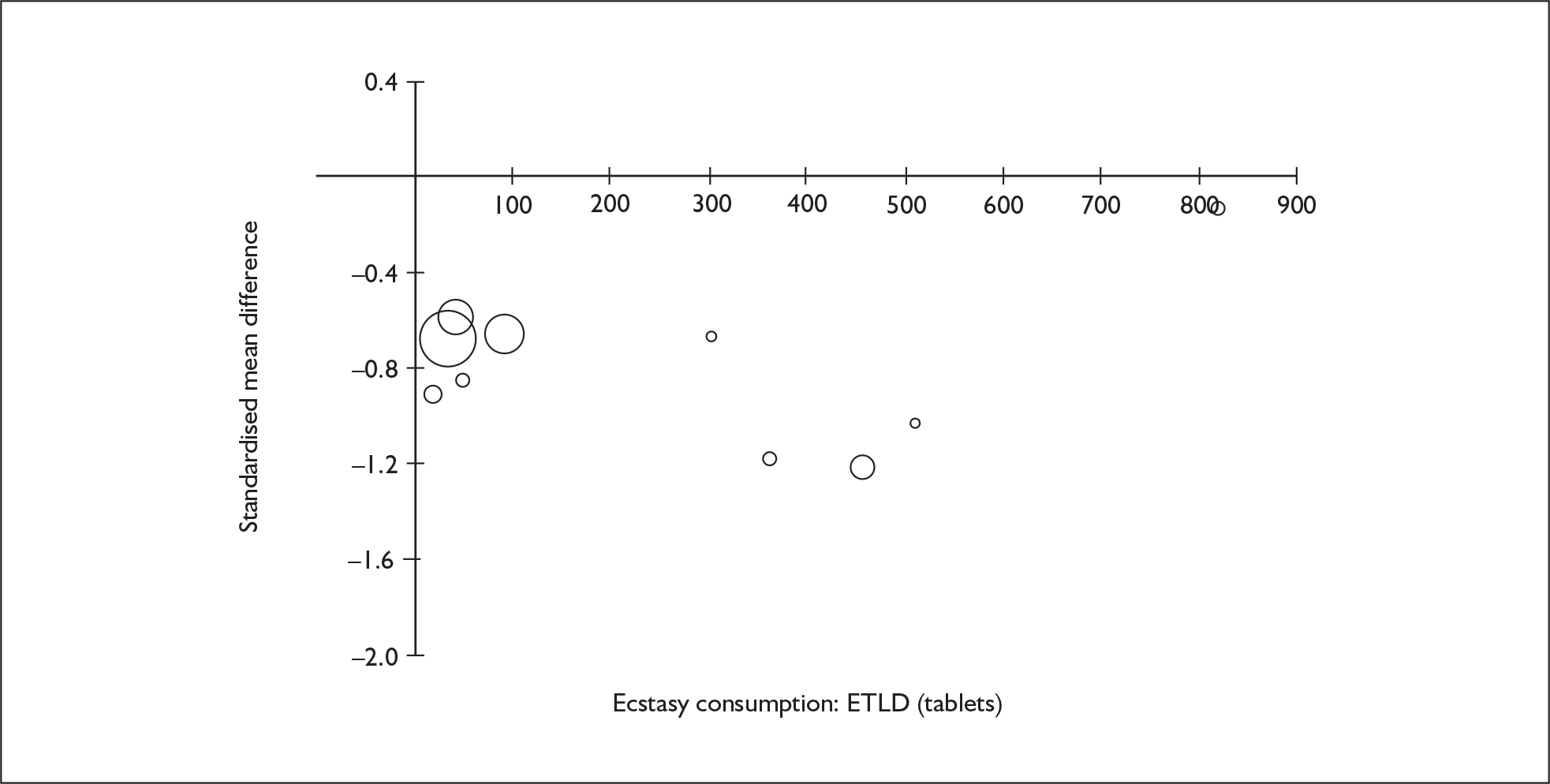
FIGURE 98.
Verbal memory – delayed (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
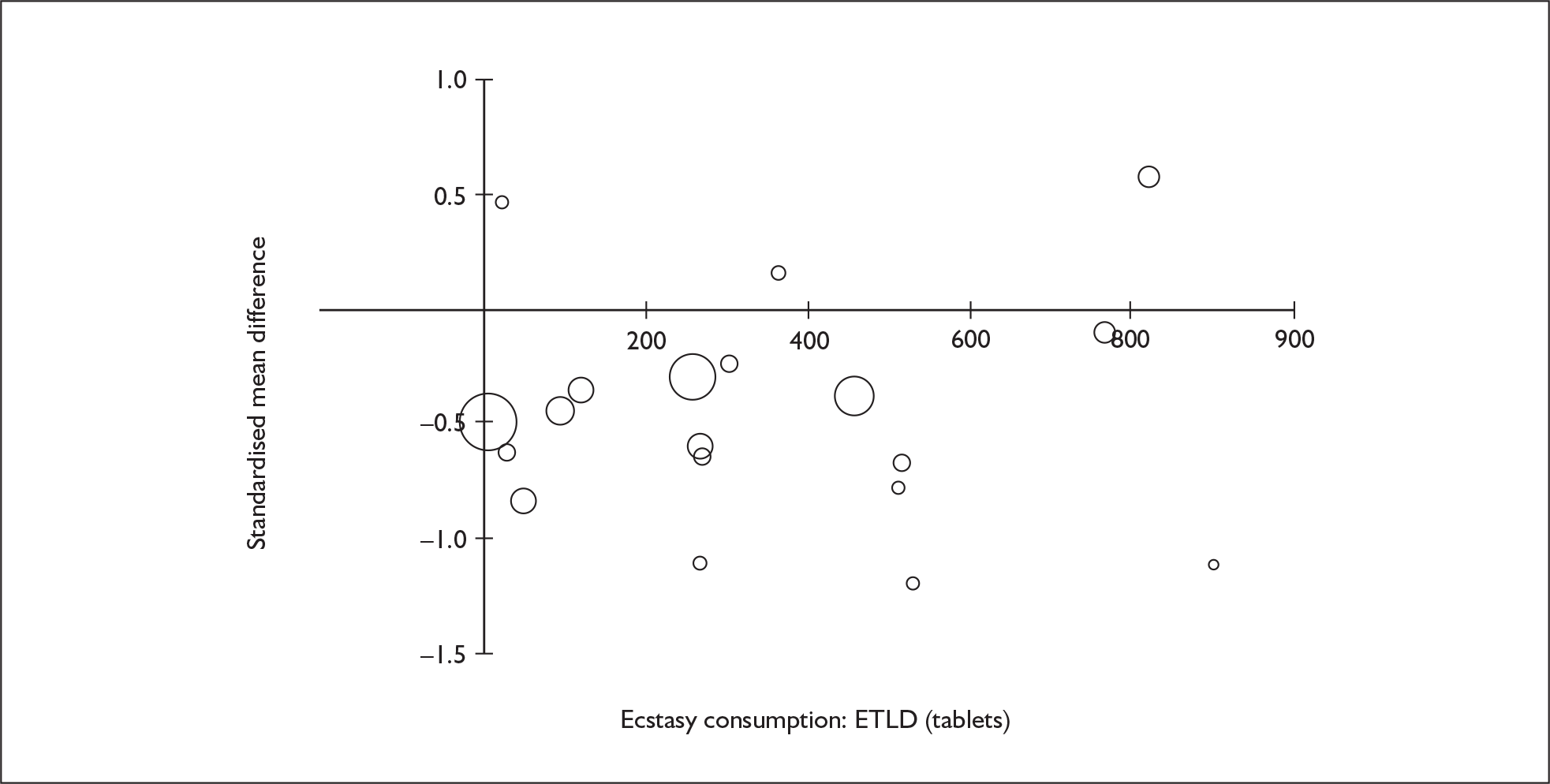
FIGURE 99.
Verbal memory – delayed (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
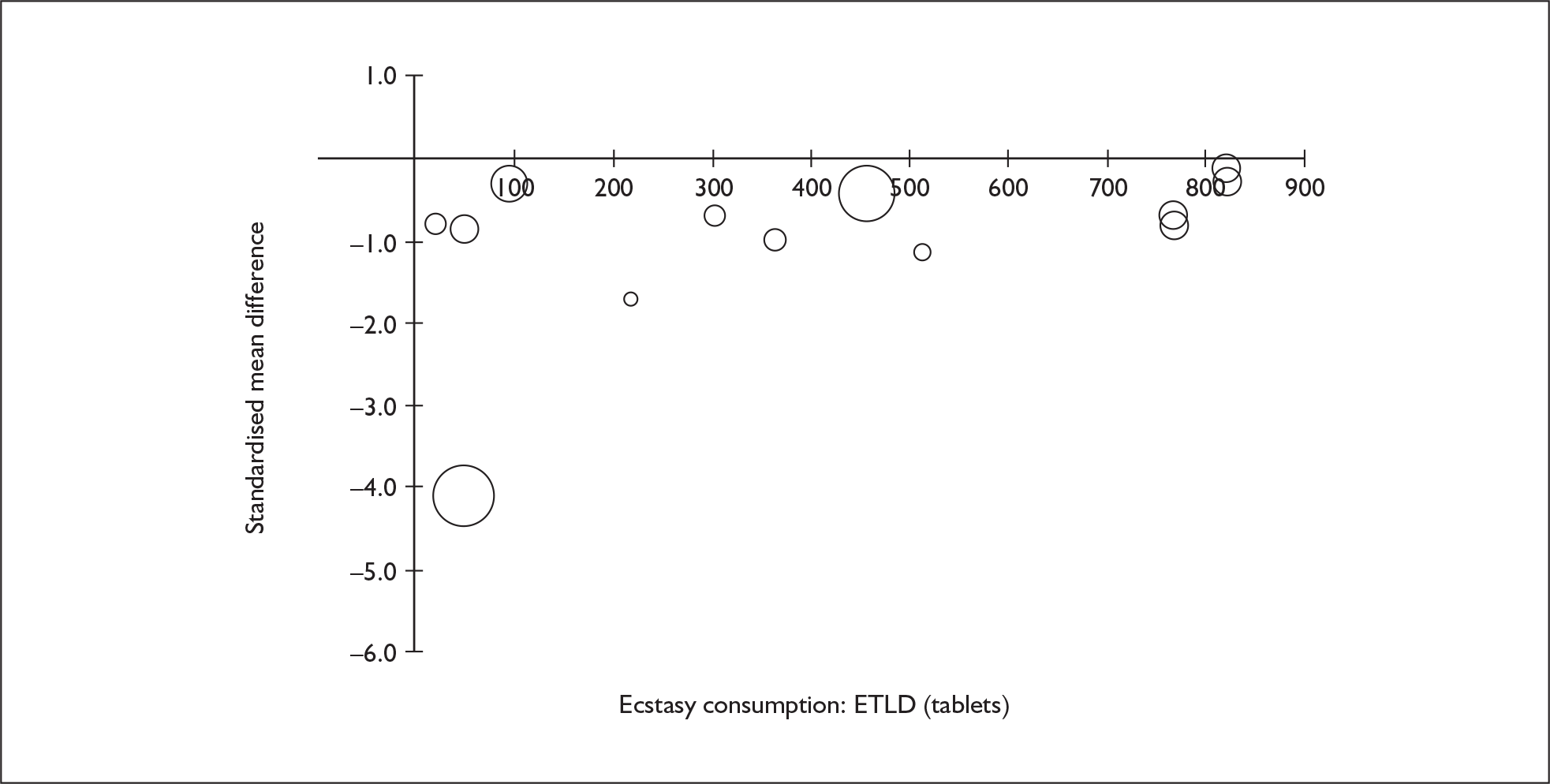
FIGURE 100.
Visual memory – immediate (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
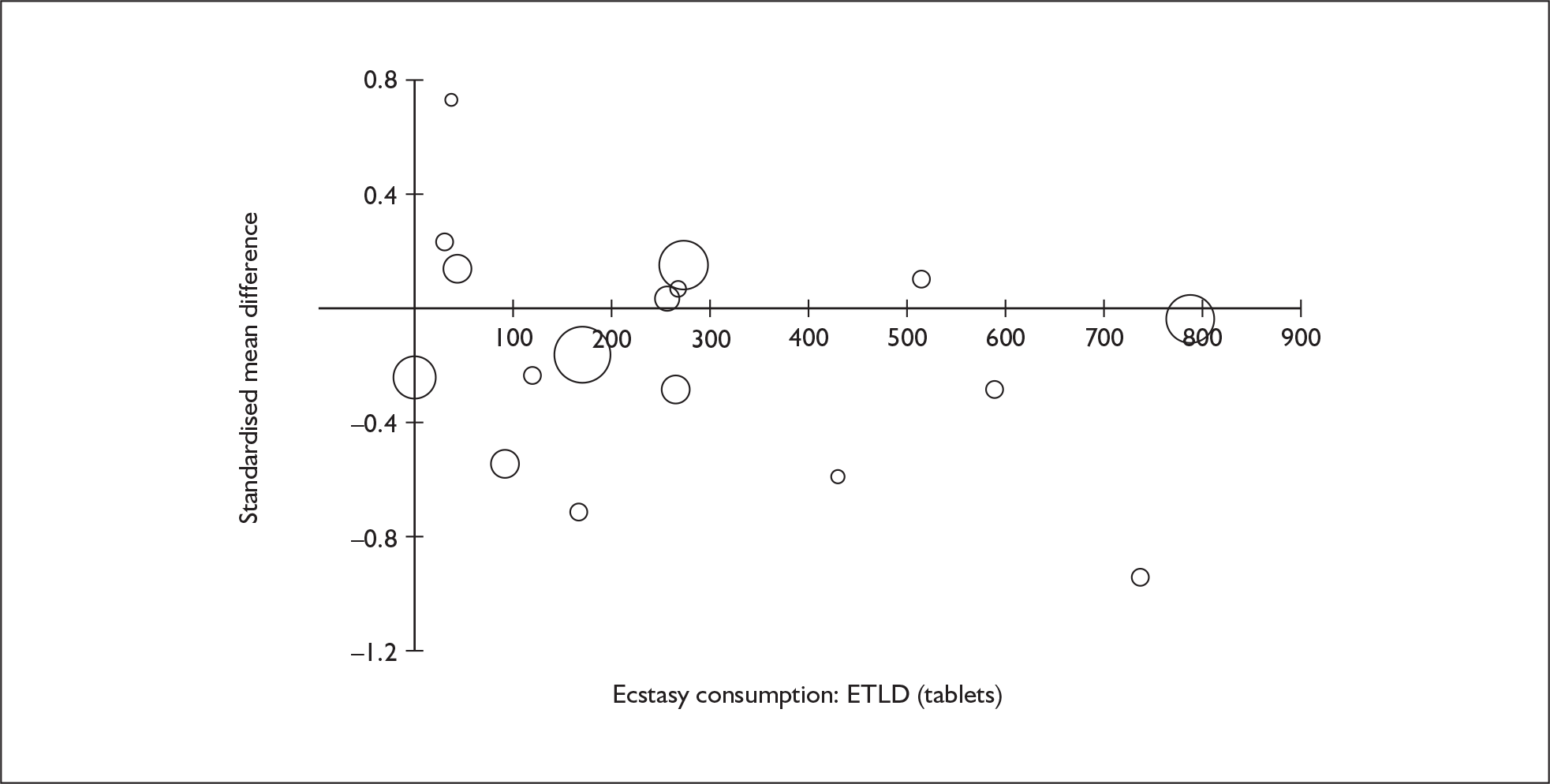
FIGURE 101.
Visual memory – immediate (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
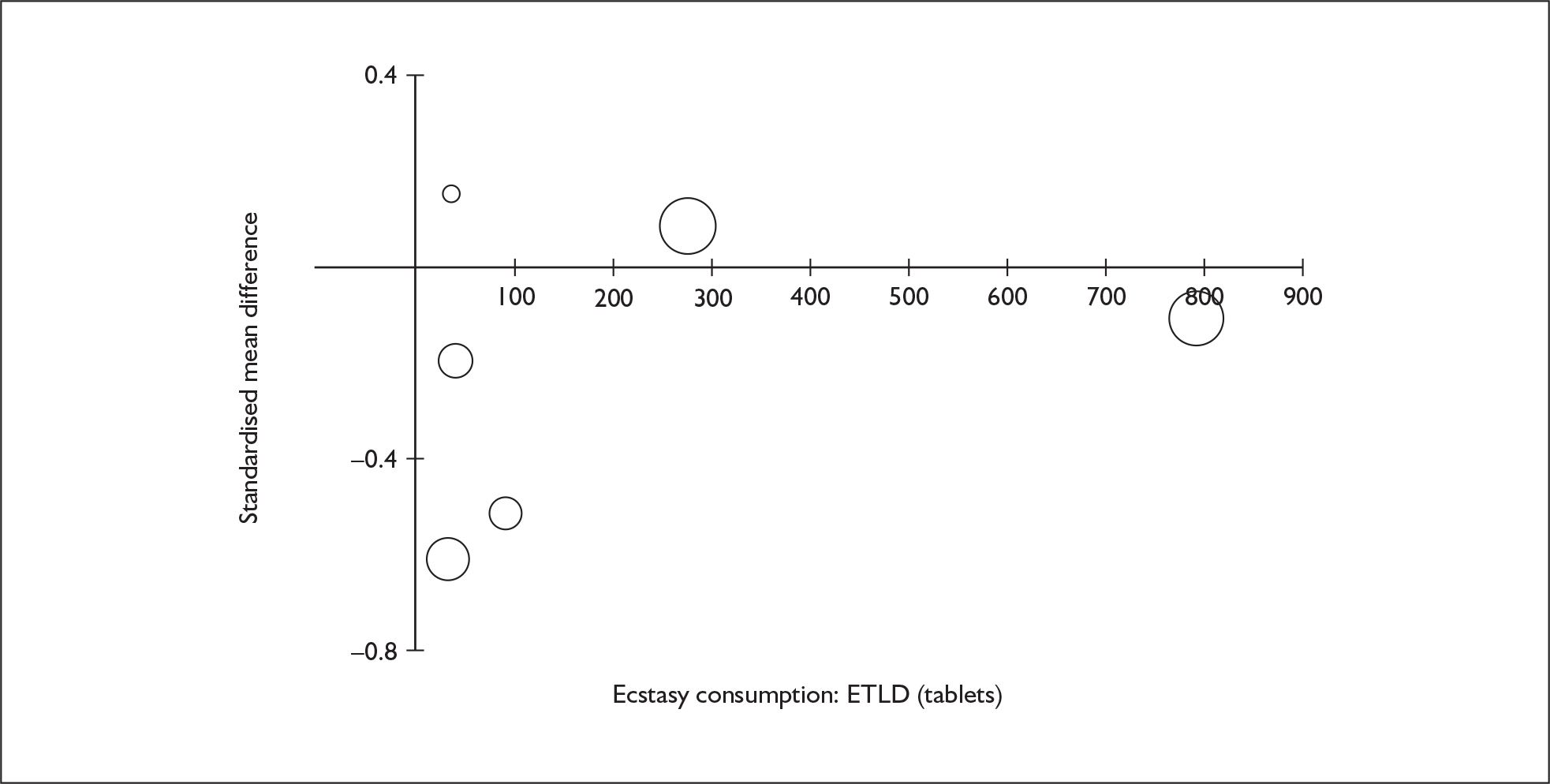
FIGURE 102.
Visual memory – delayed (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
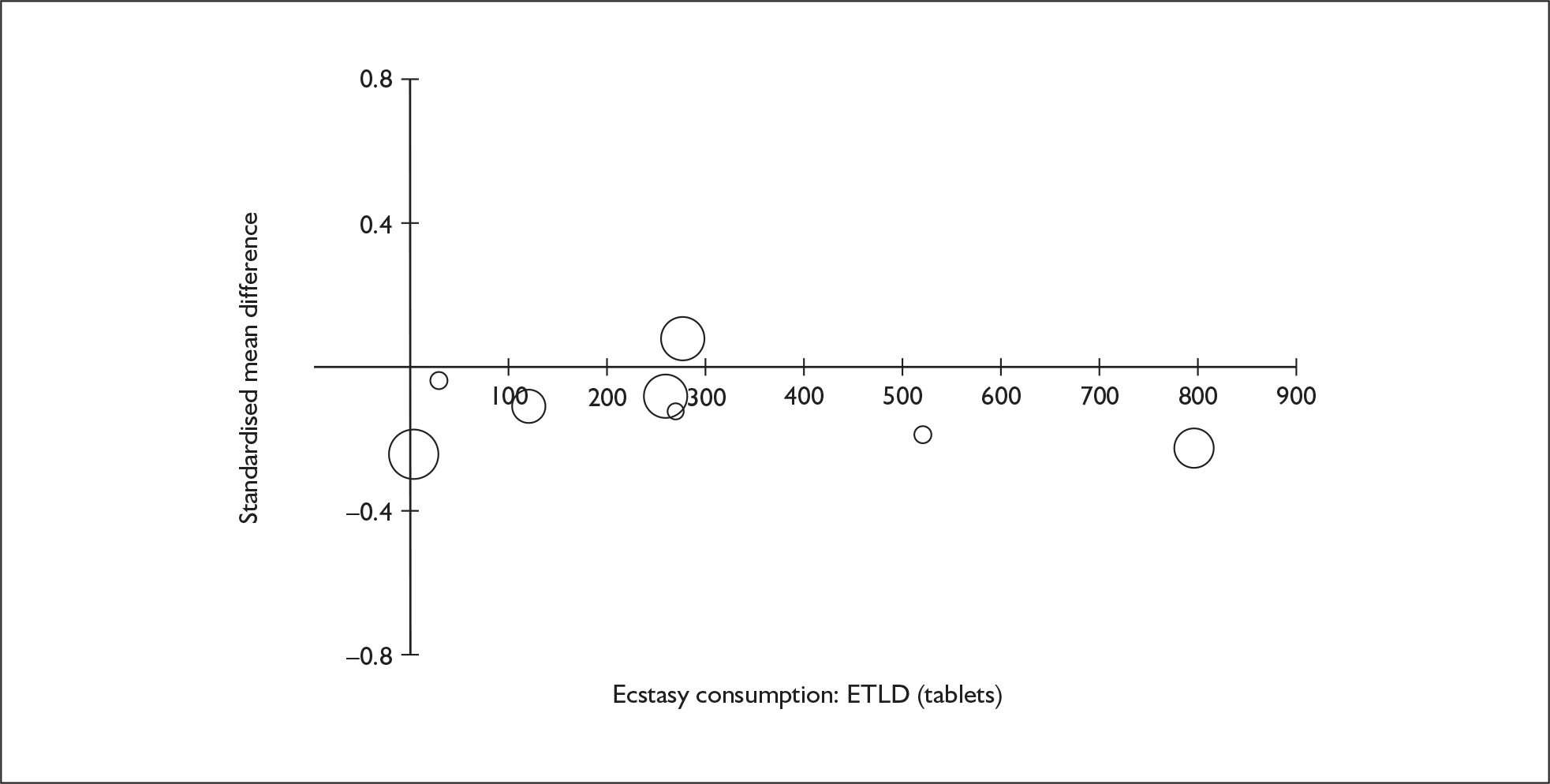
FIGURE 103.
Visual memory – delayed (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
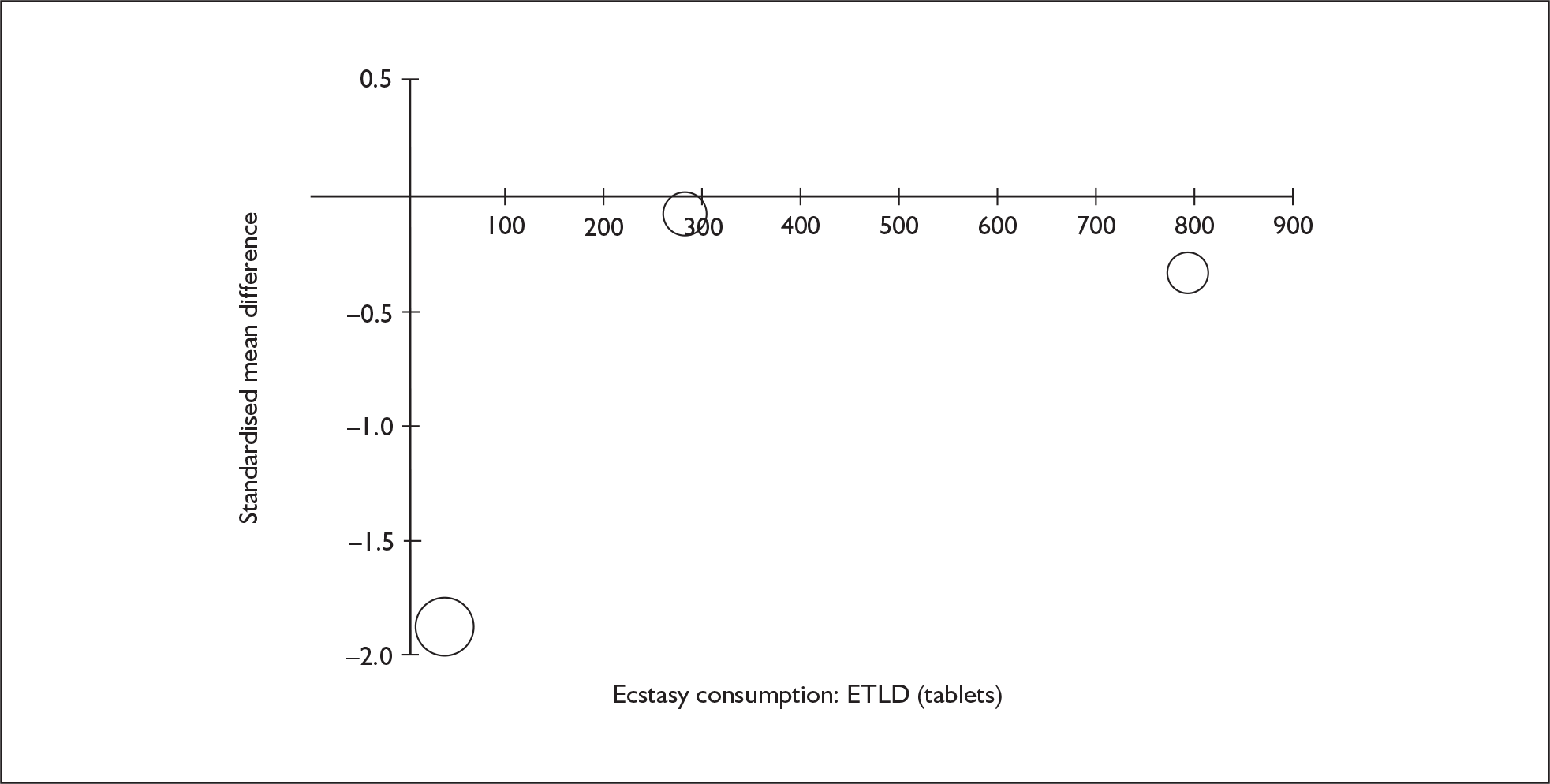
FIGURE 104.
Working memory (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
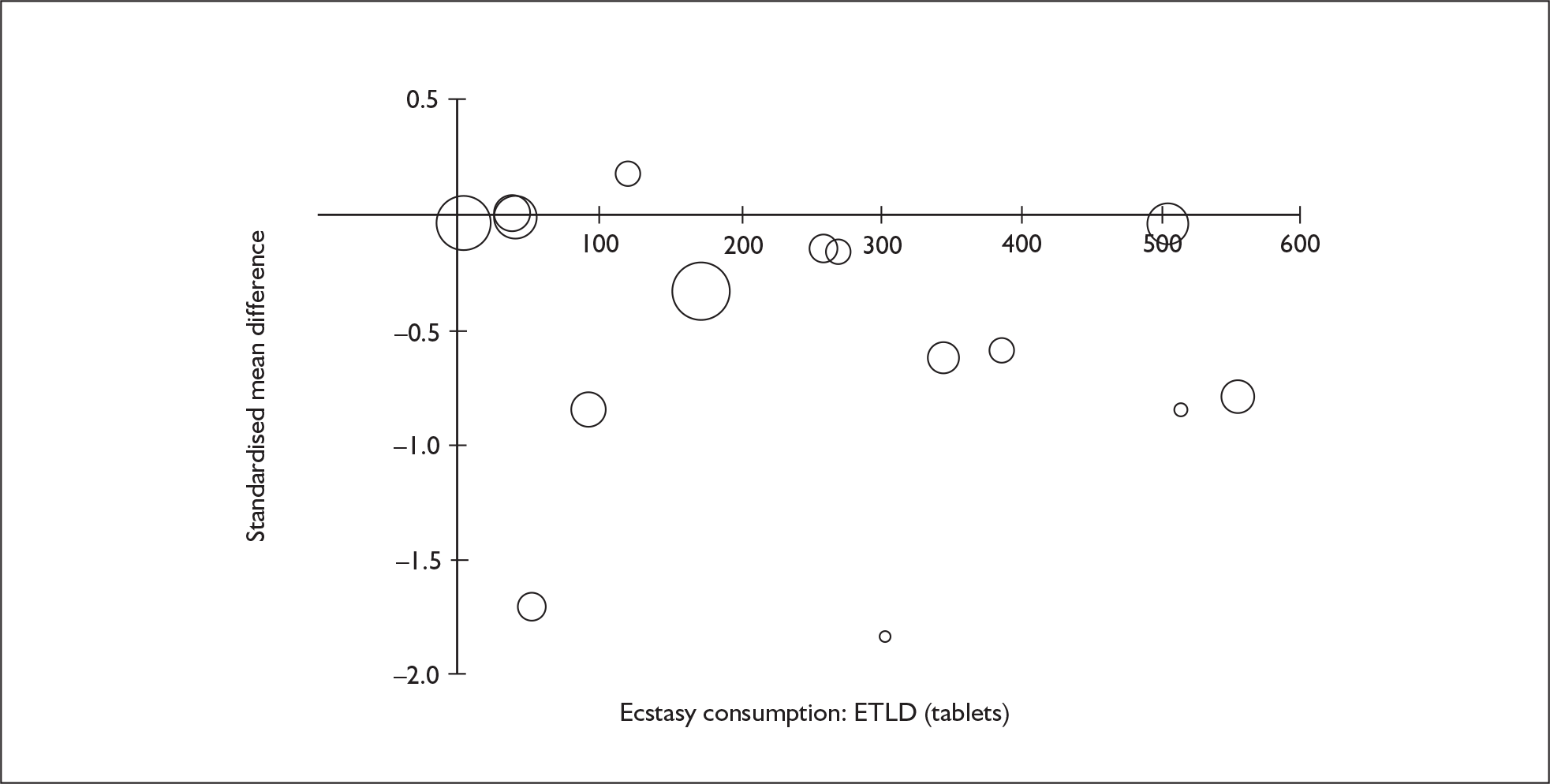
FIGURE 105.
Working memory (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
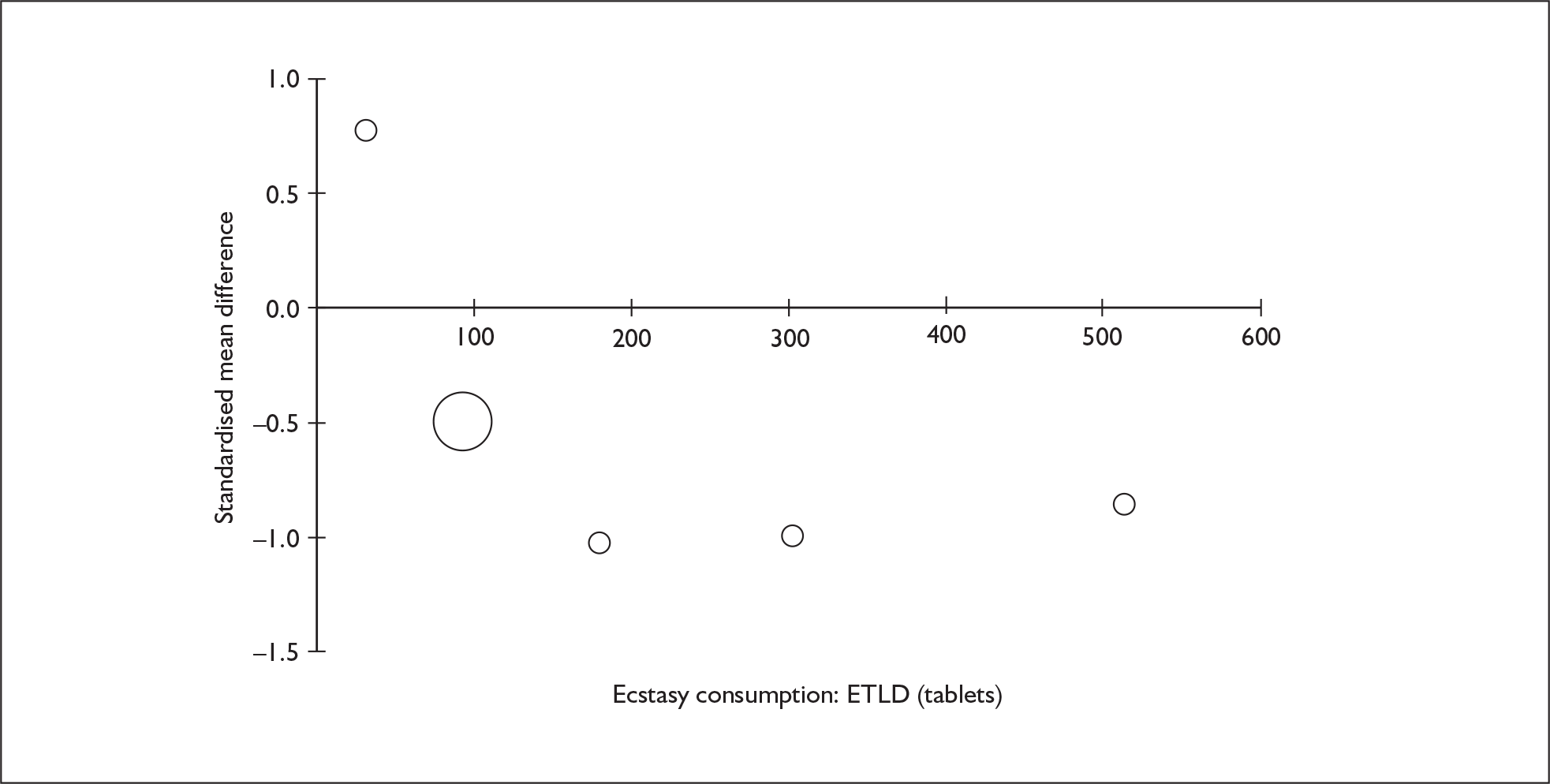
FIGURE 106.
Attention – focus–execute (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
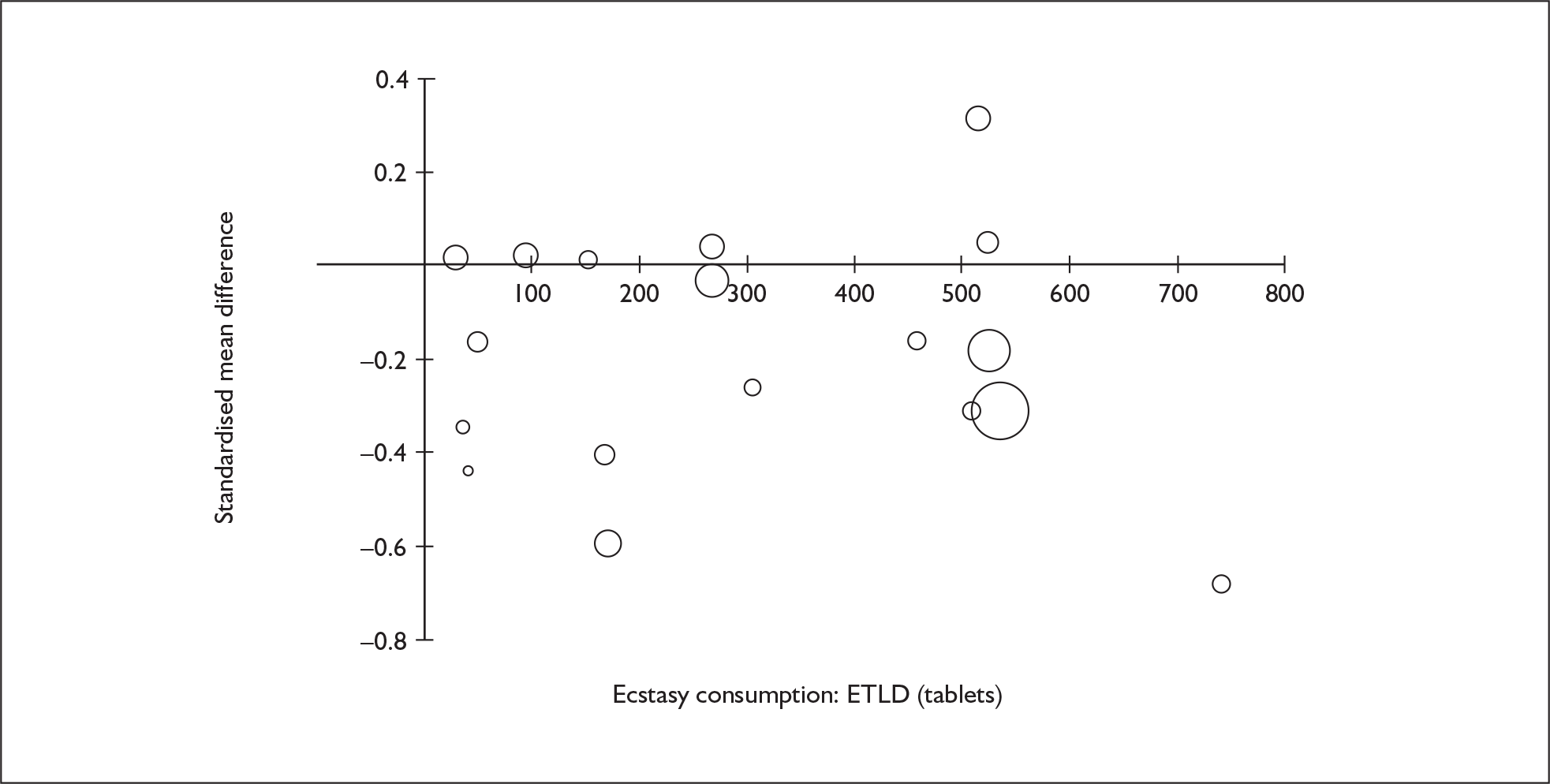
FIGURE 107.
Attention – focus–execute (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
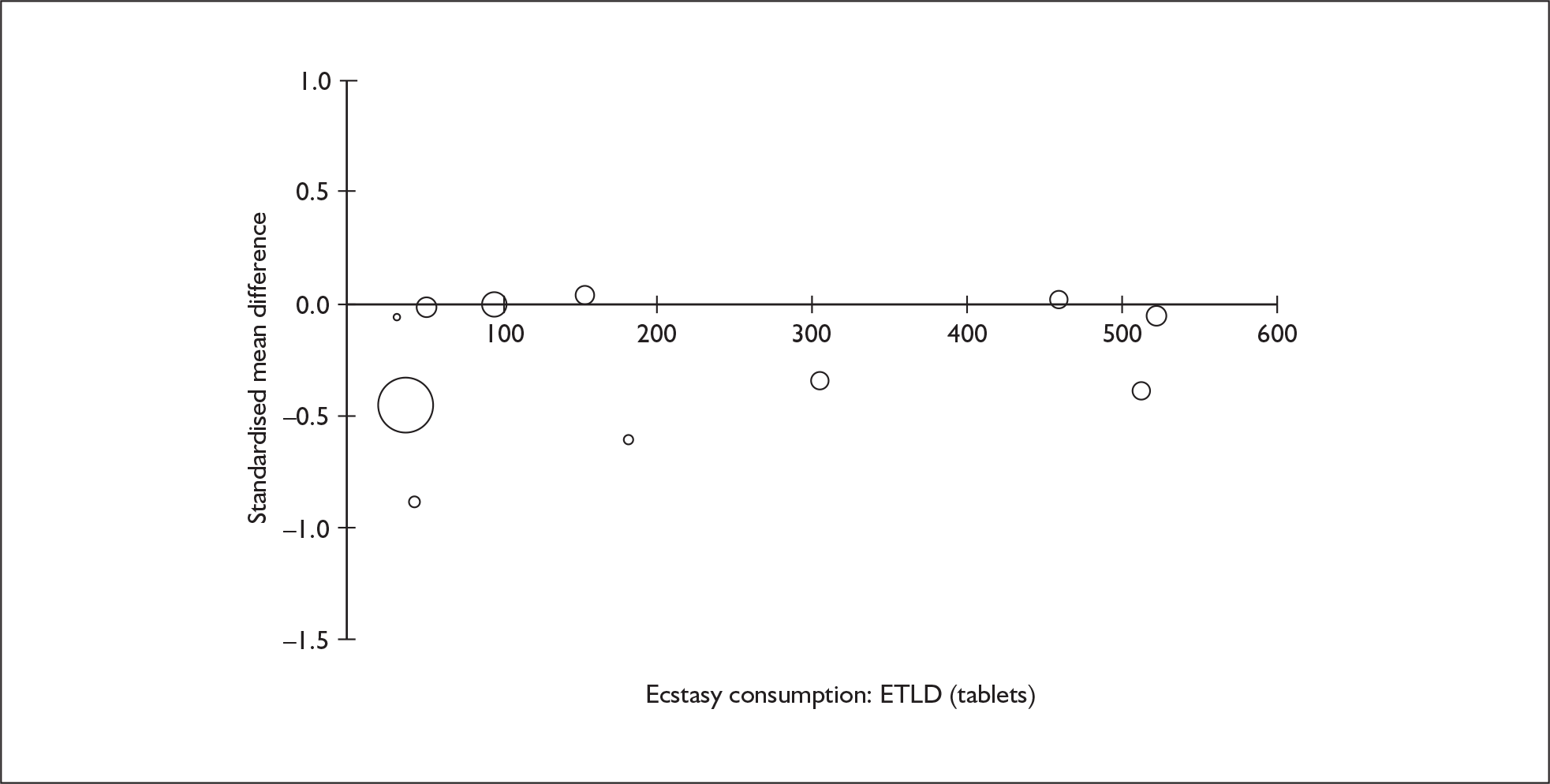
FIGURE 108.
Attention – sustain (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
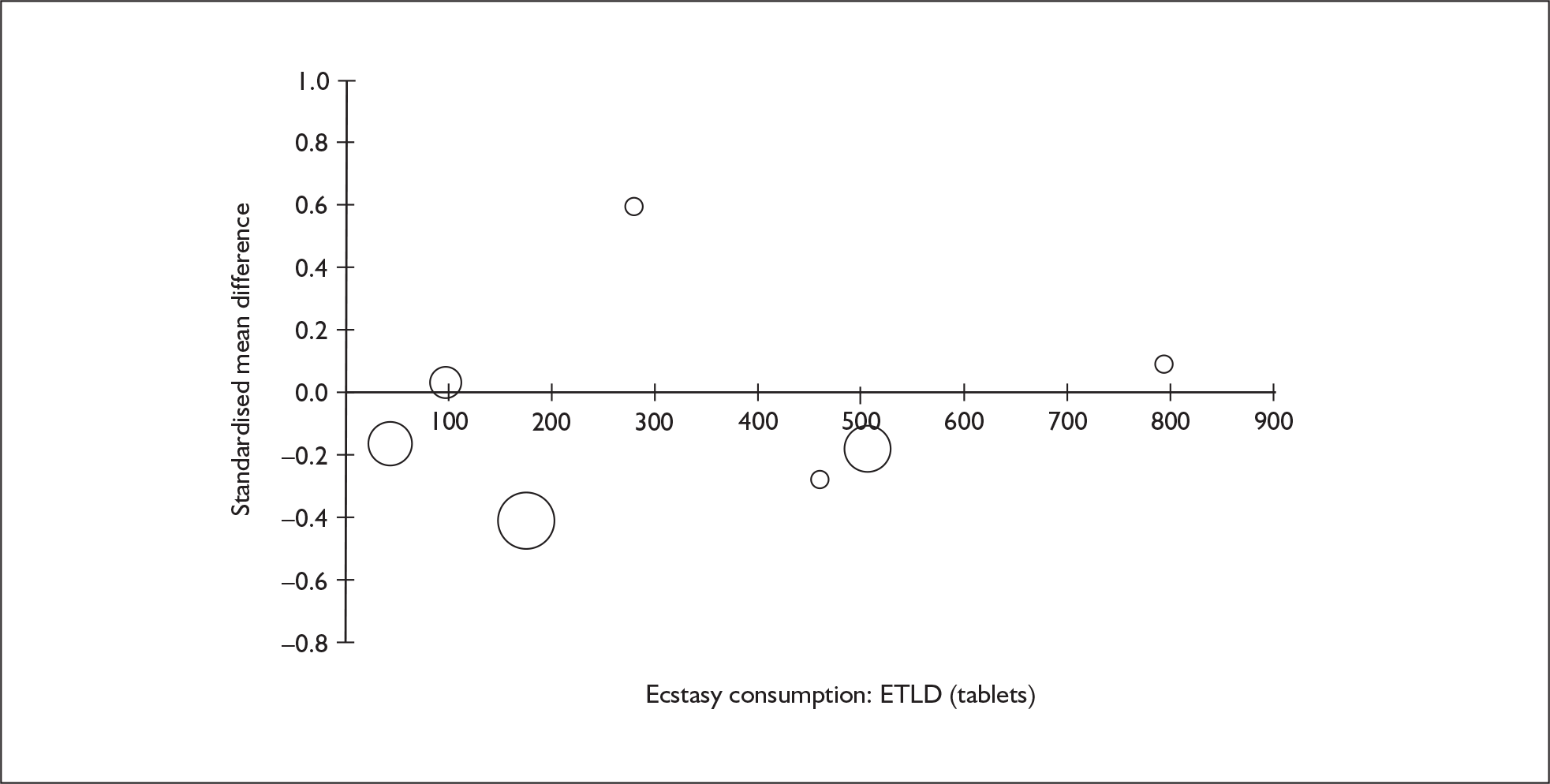
FIGURE 109.
Executive function – planning (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
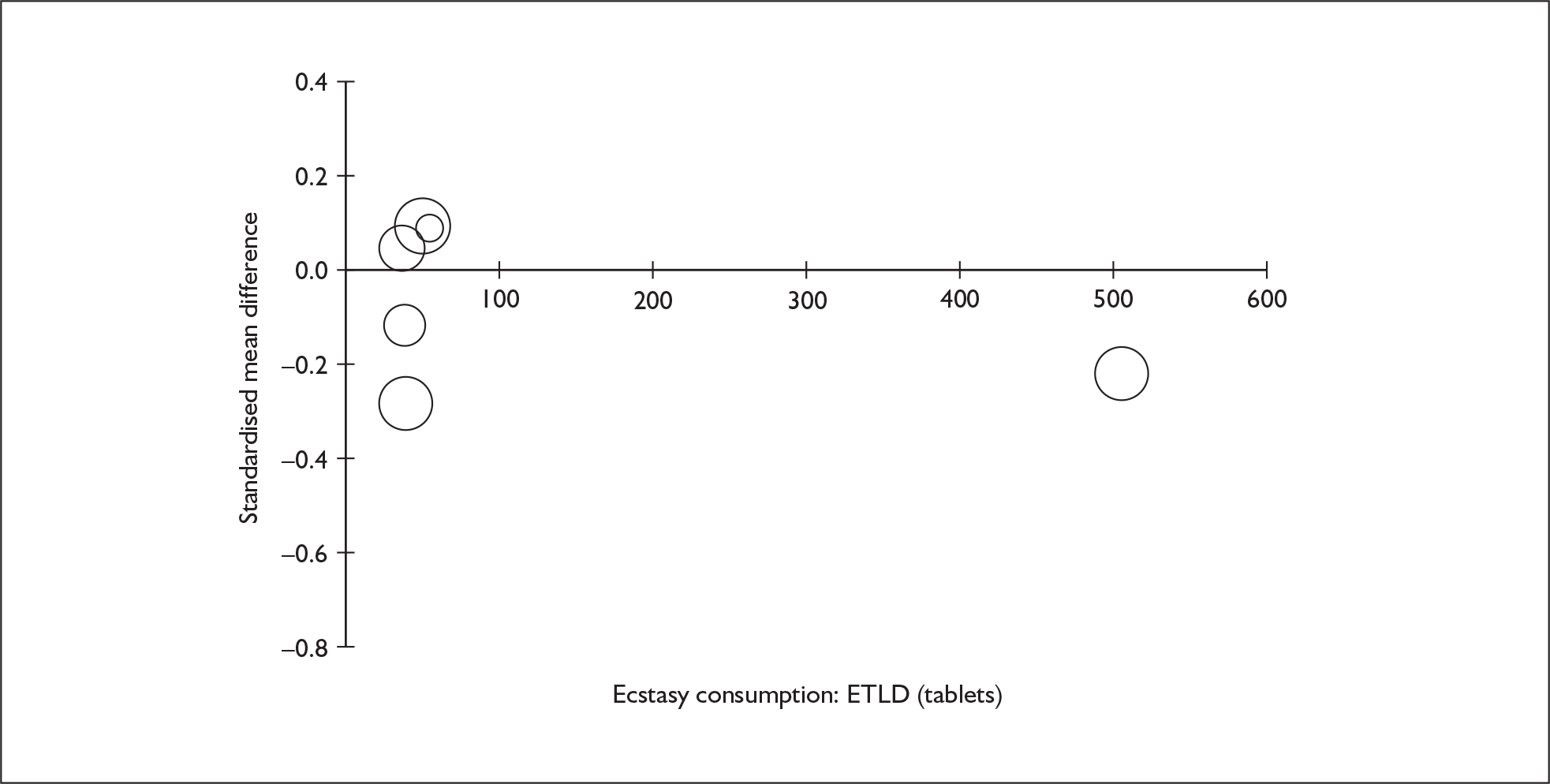
FIGURE 110.
Executive function – response inhibition (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
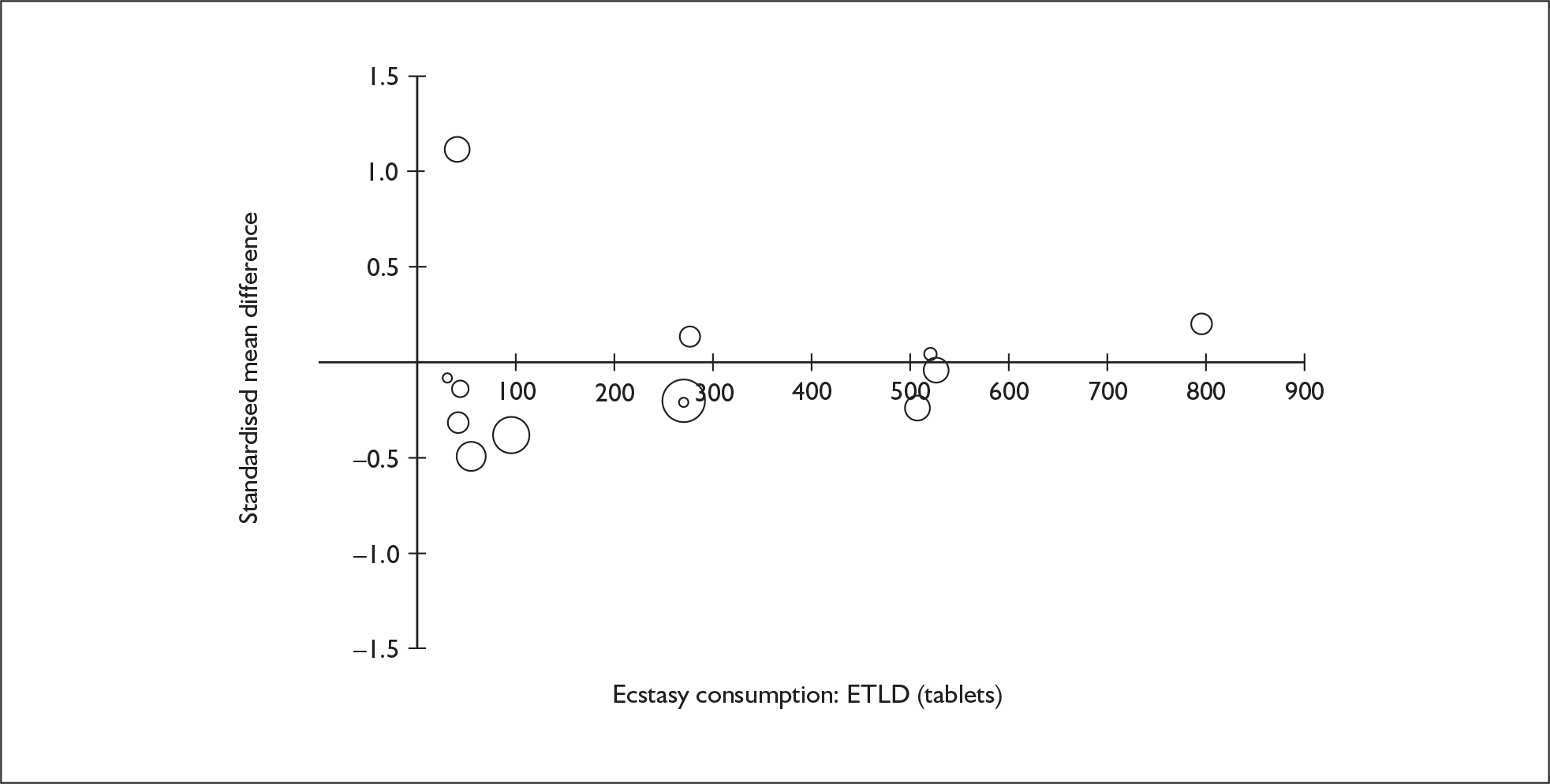
FIGURE 111.
Executive function – response inhibition (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
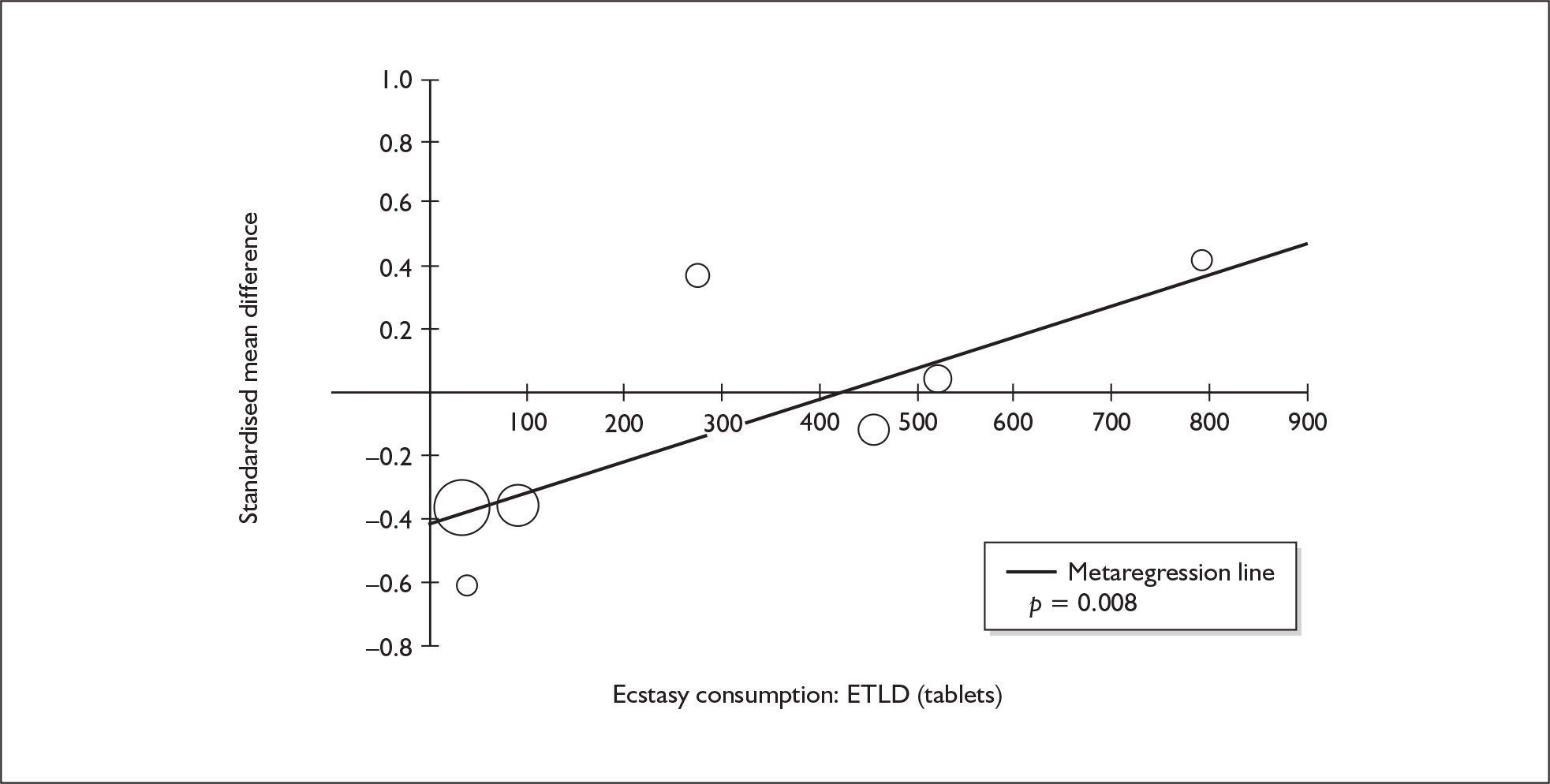
FIGURE 112.
Executive function – shifting (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
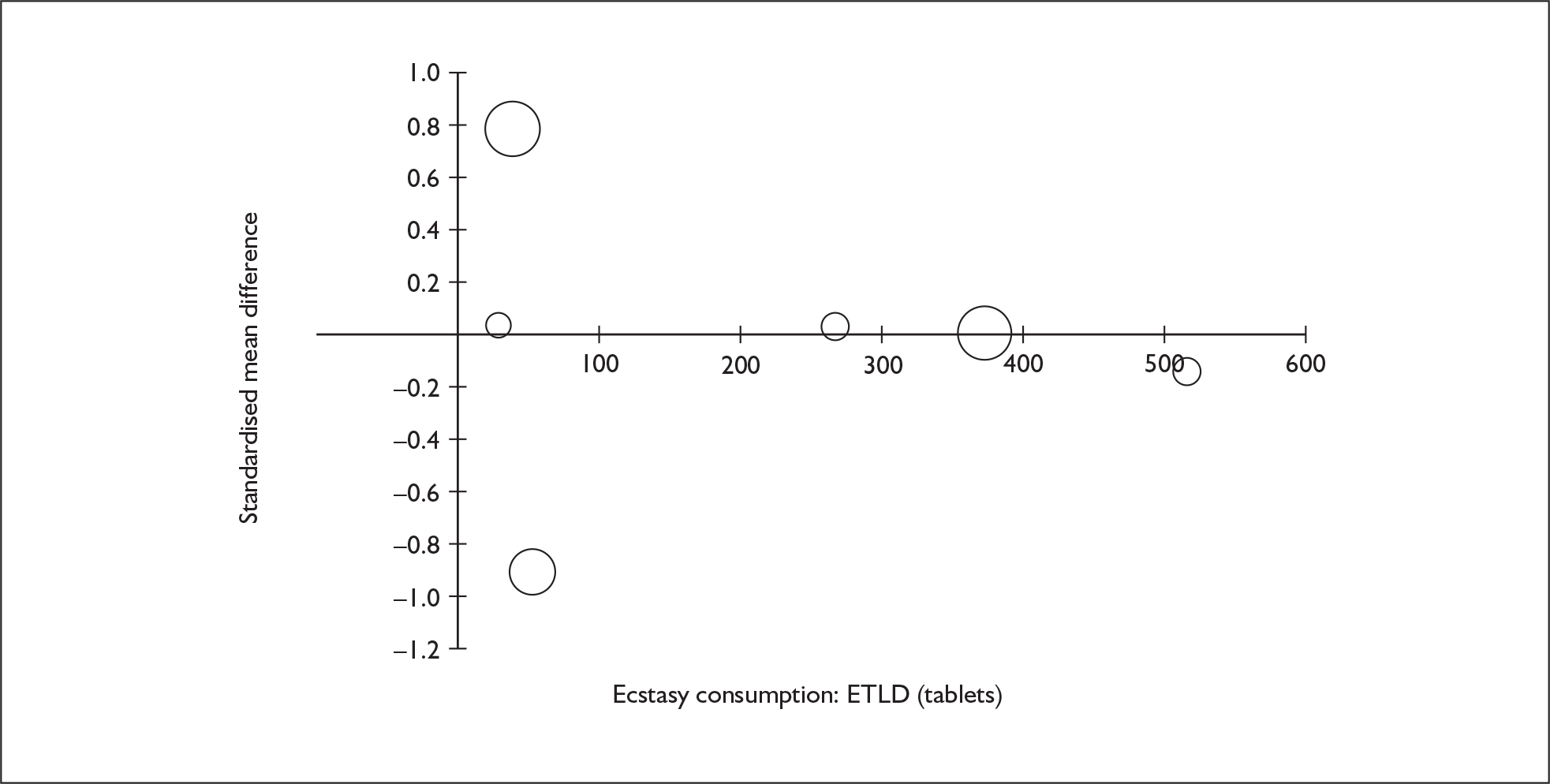
FIGURE 113.
Perceptual organisation (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
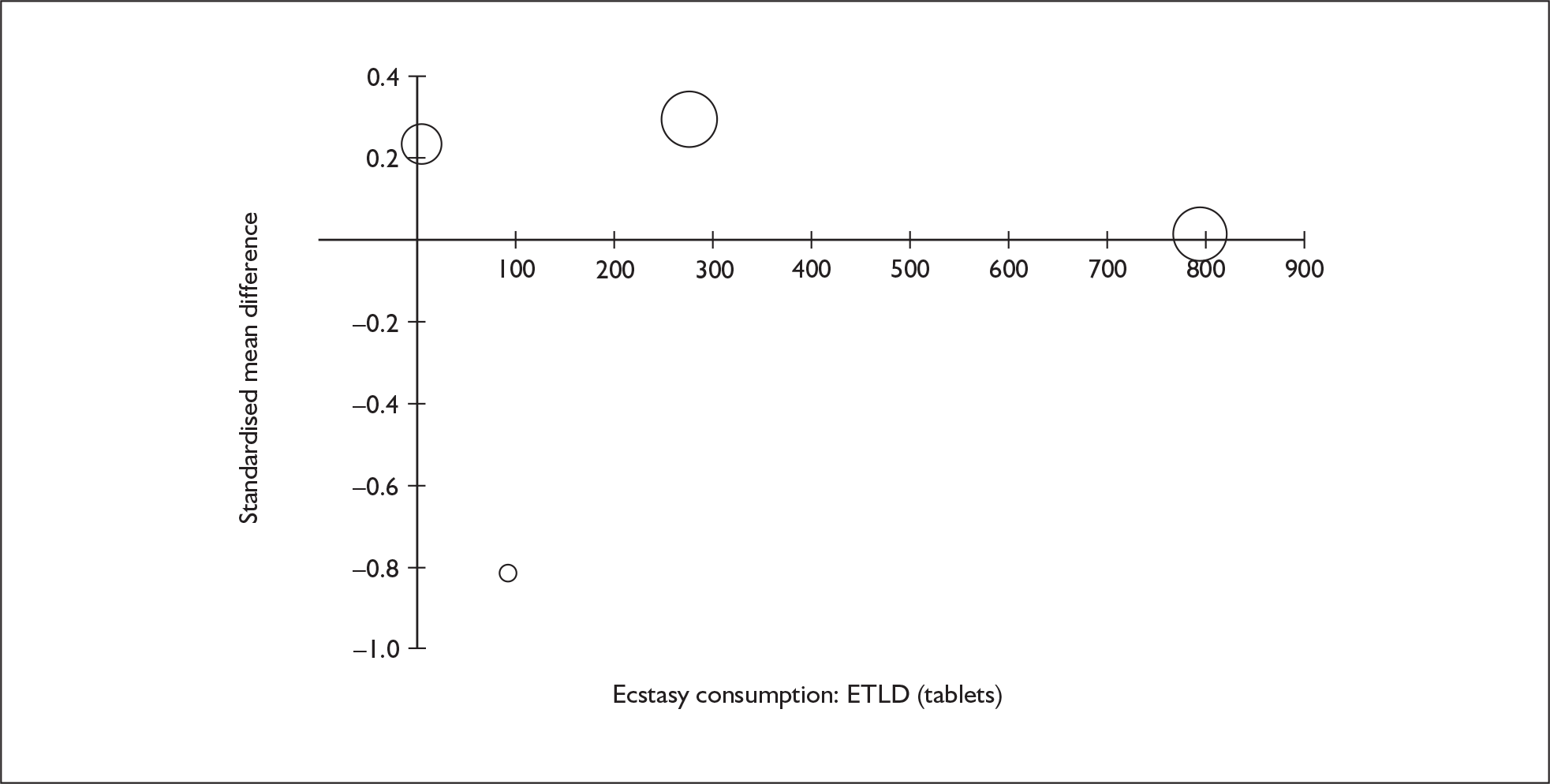
FIGURE 114.
Depression – self-rated (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
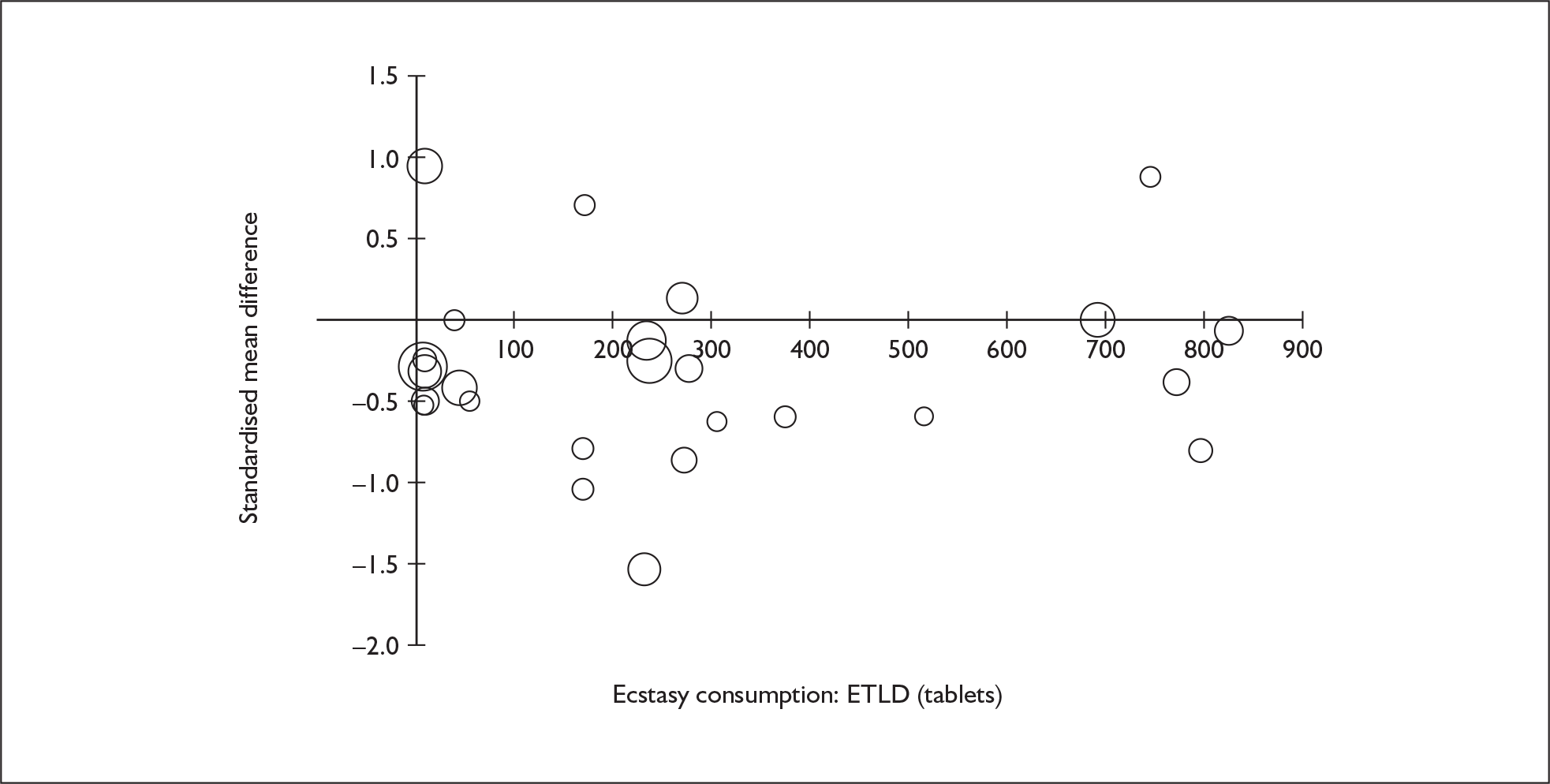
FIGURE 115.
Depression – self-rated (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
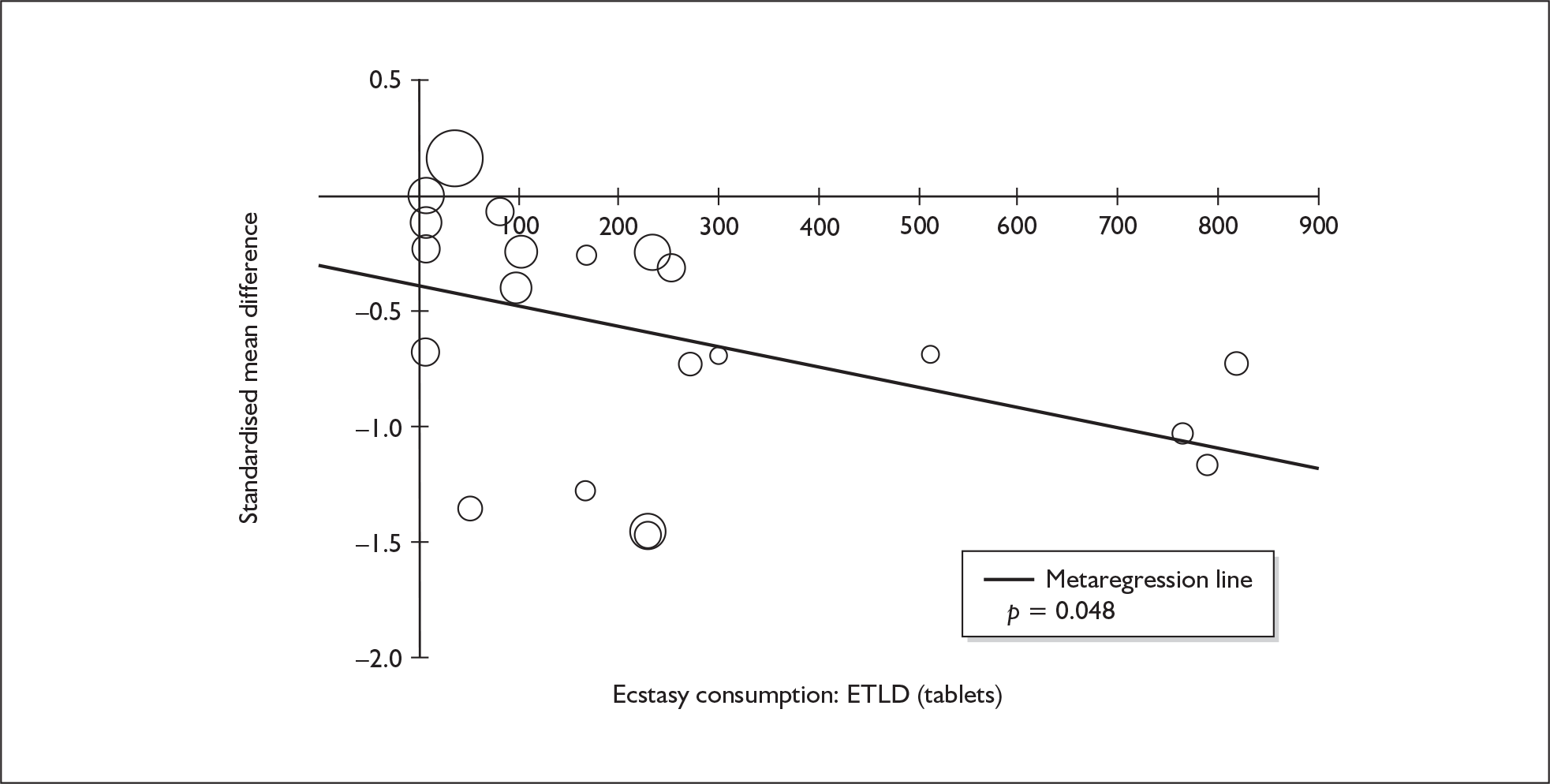
FIGURE 116.
Memory – self-rated (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
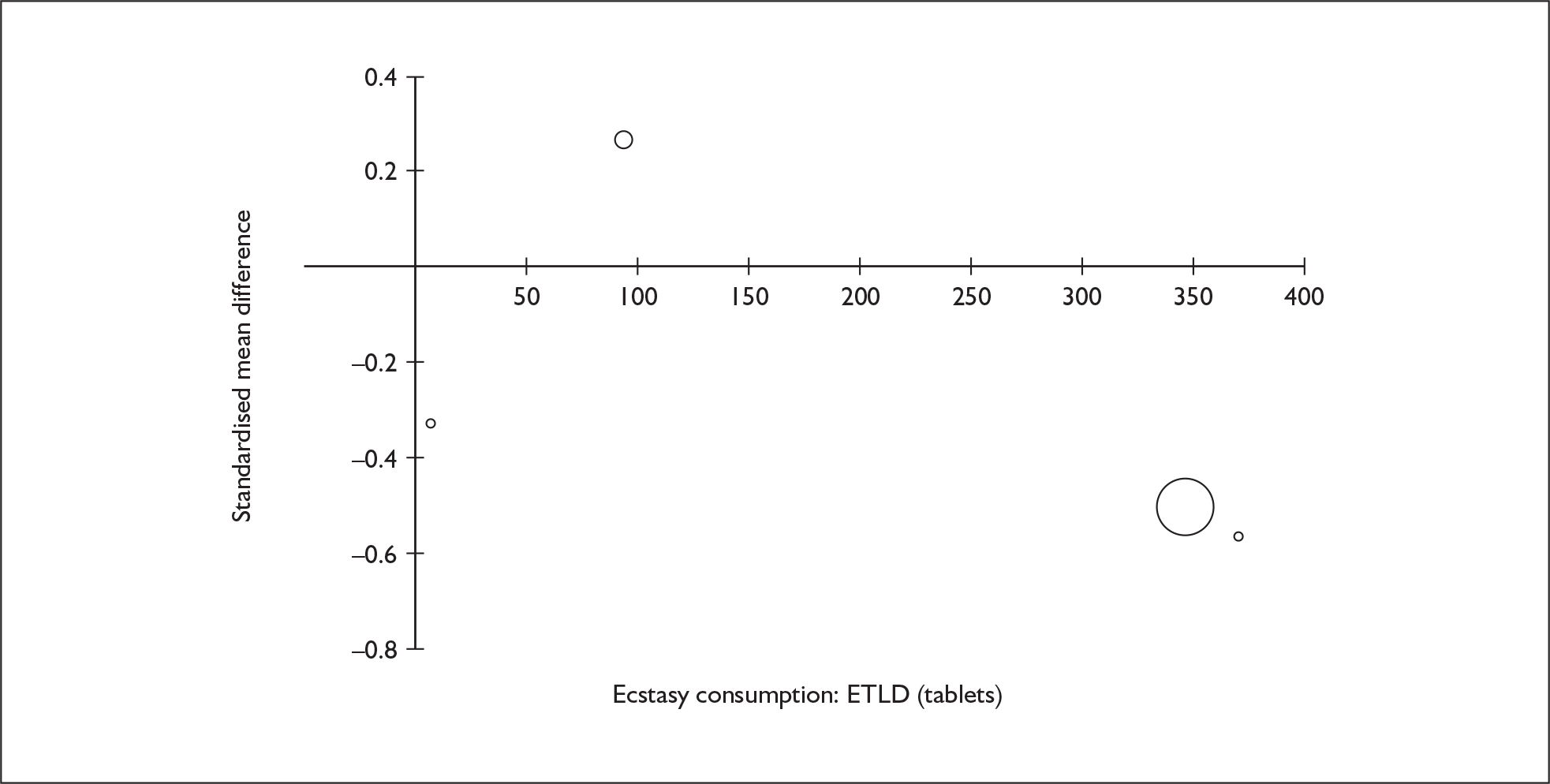
FIGURE 117.
Anxiety – self-rated (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
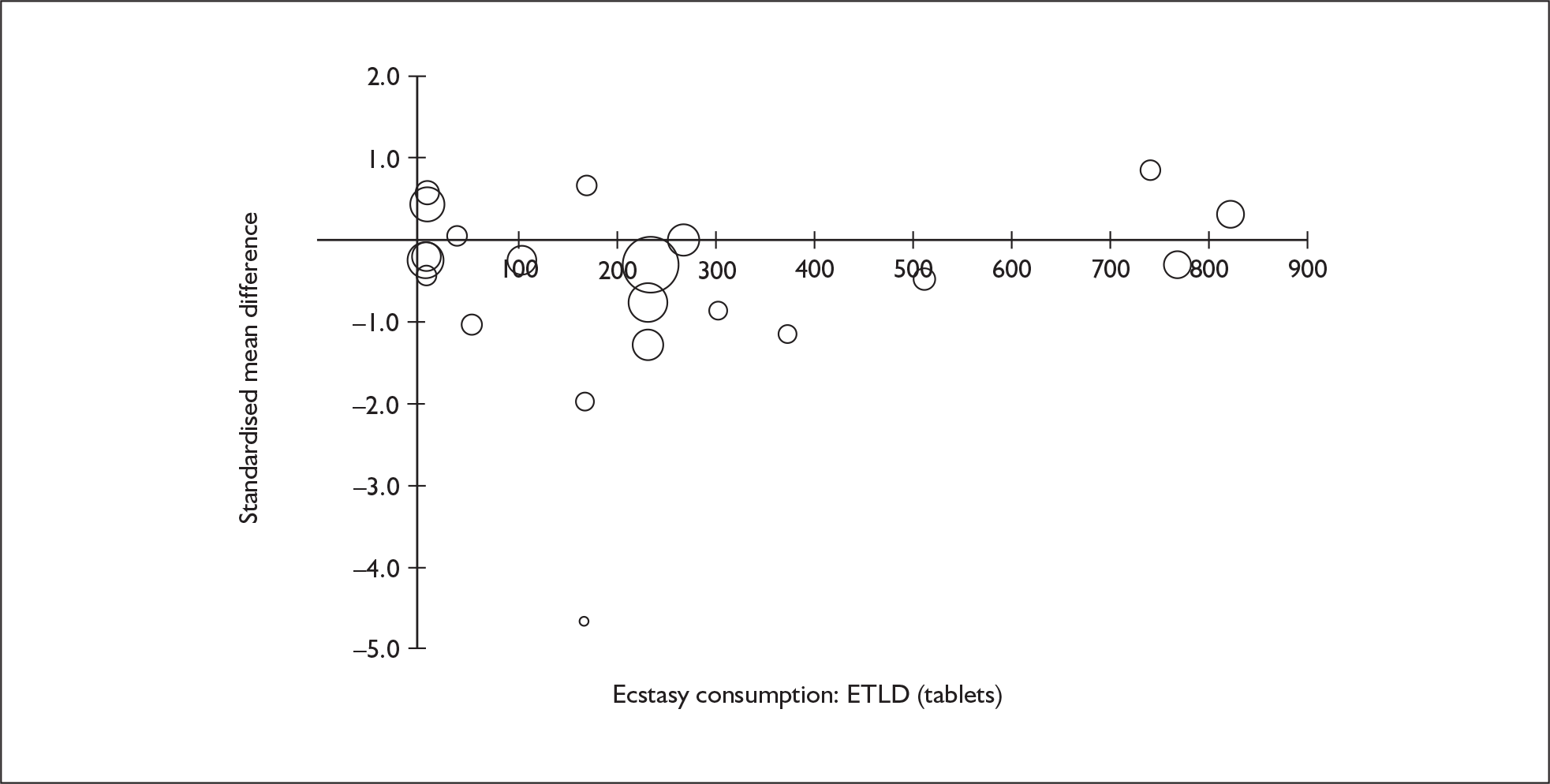
FIGURE 118.
Anxiety – self-rated (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
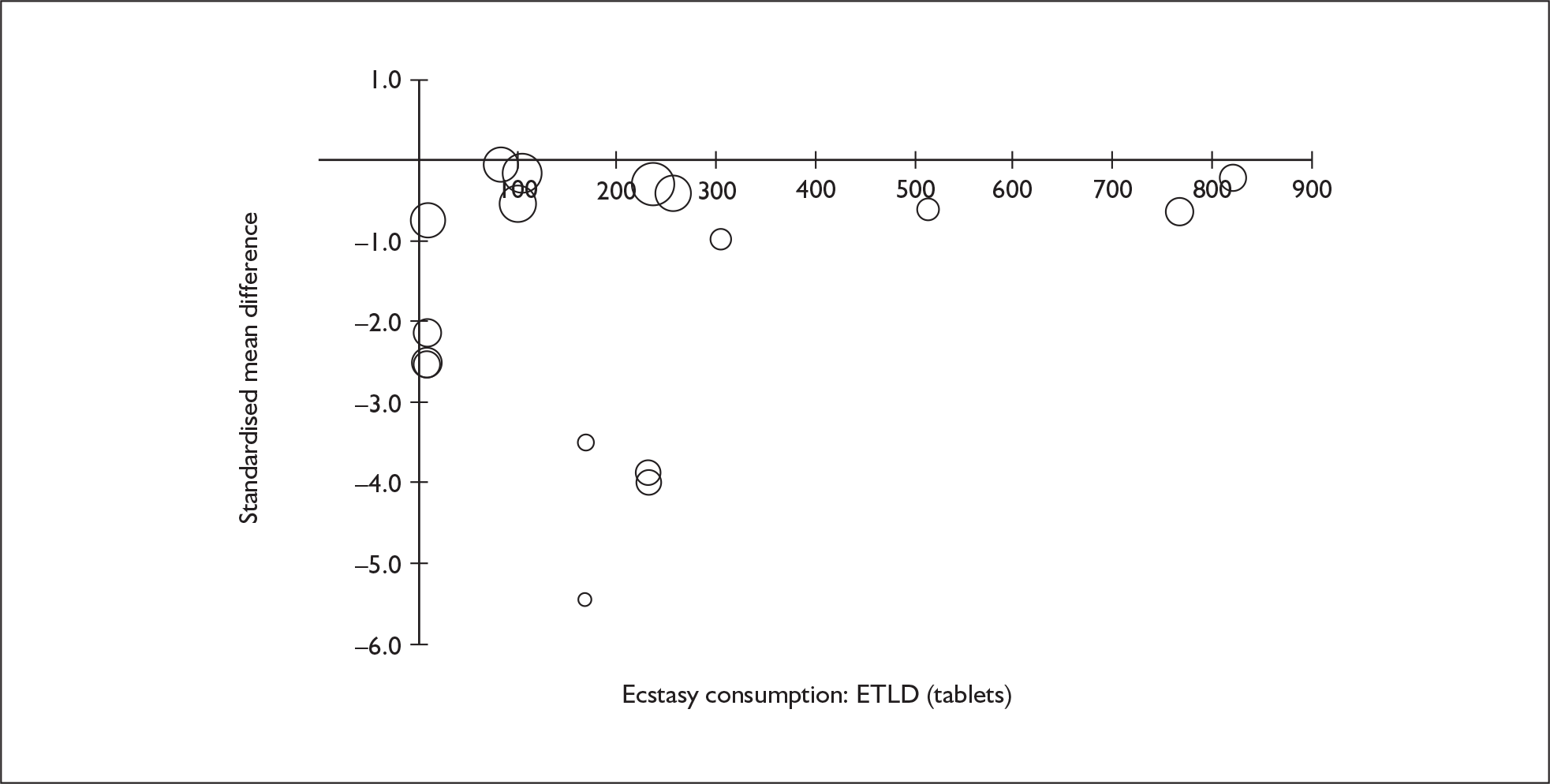
FIGURE 119.
Impulsivity – objective measures (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
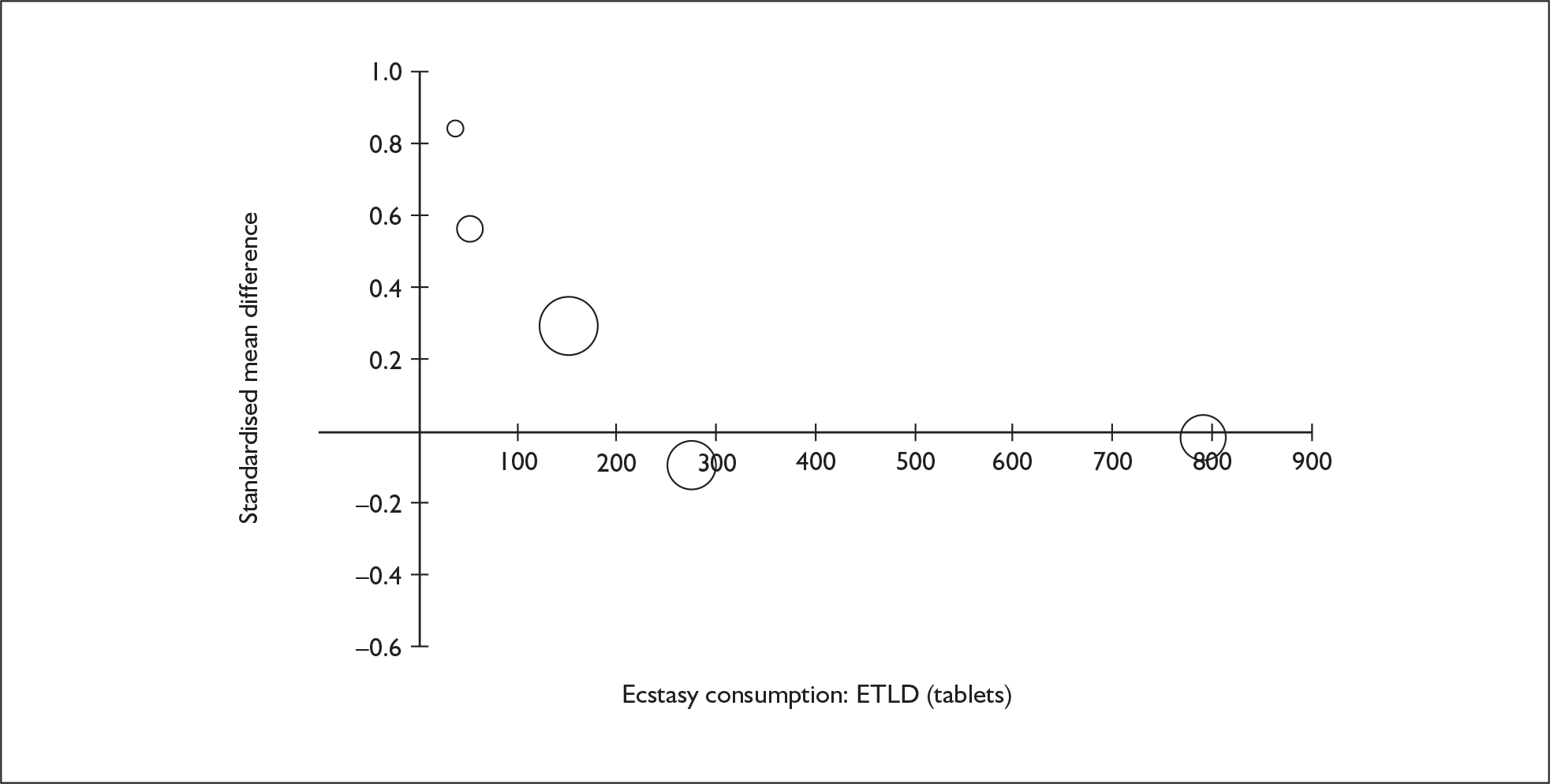
FIGURE 120.
Impulsivity – objective measures (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
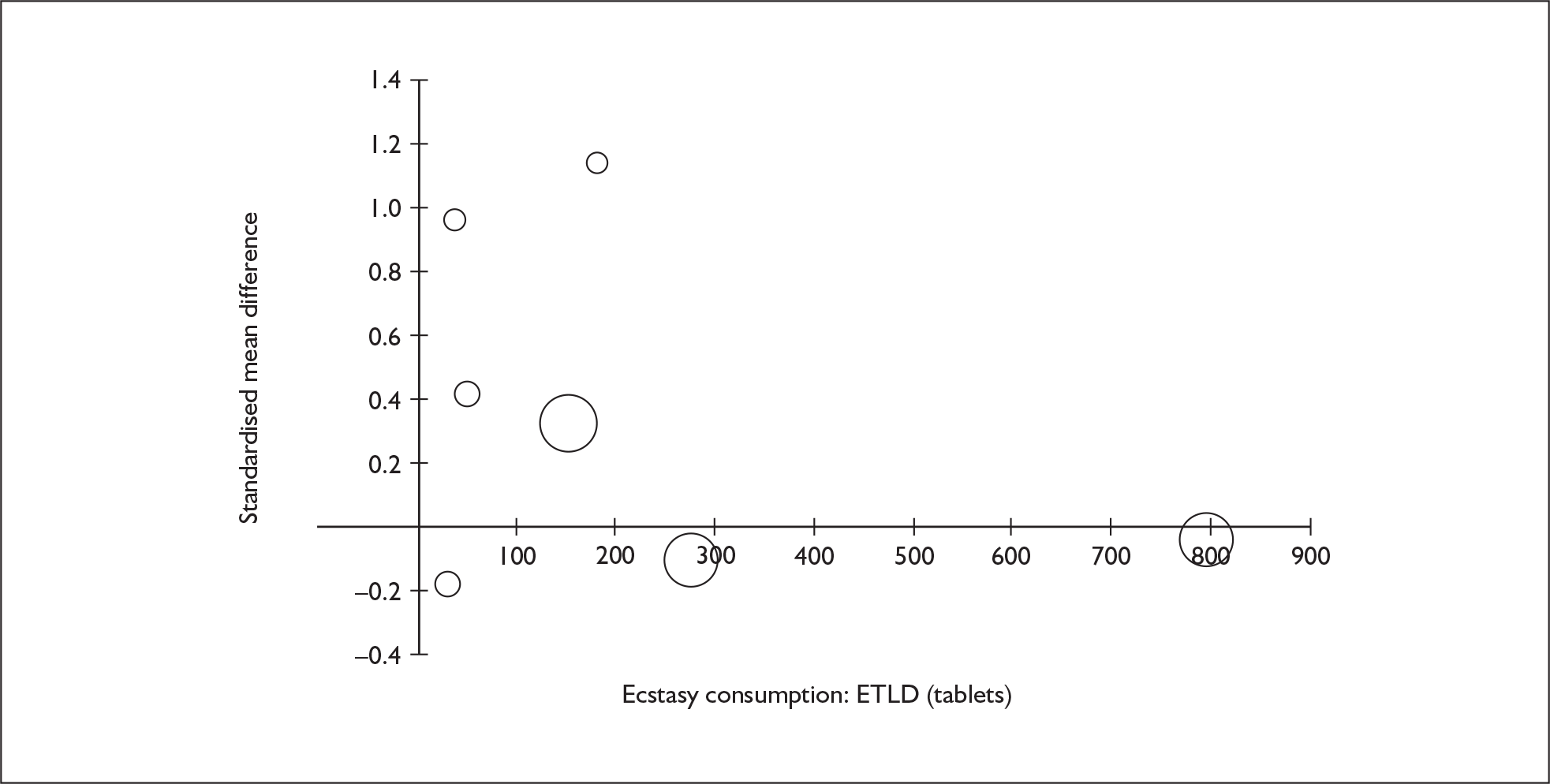
FIGURE 121.
Impulsivity – subjective measures (composite measure) – ecstasy users versus polydrug controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
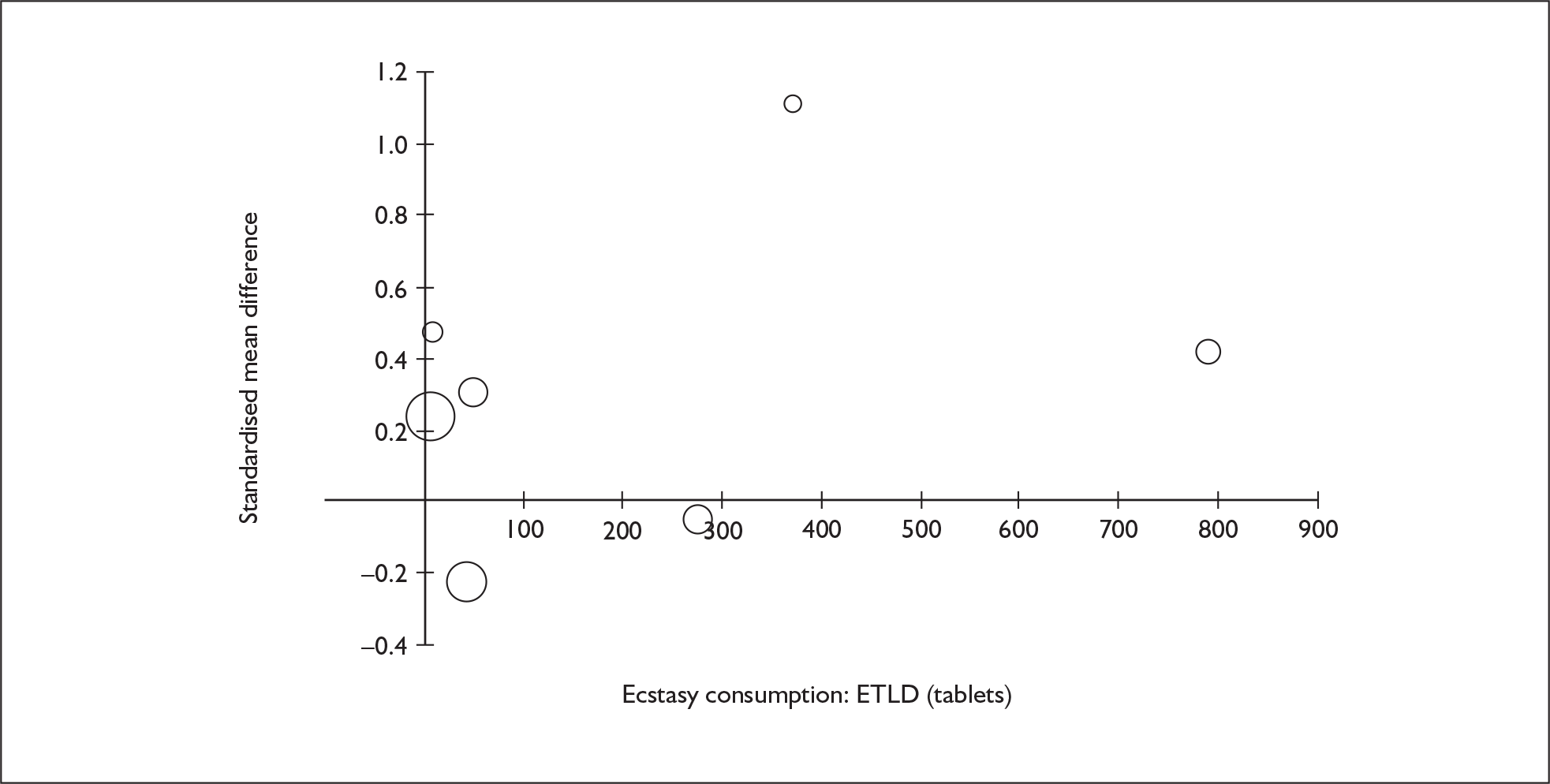
FIGURE 122.
Impulsivity – subjective measures (composite measure) – ecstasy users versus drug-naïve controls: standardised mean difference against estimated total lifetime dose (ETLD) of MDMA.
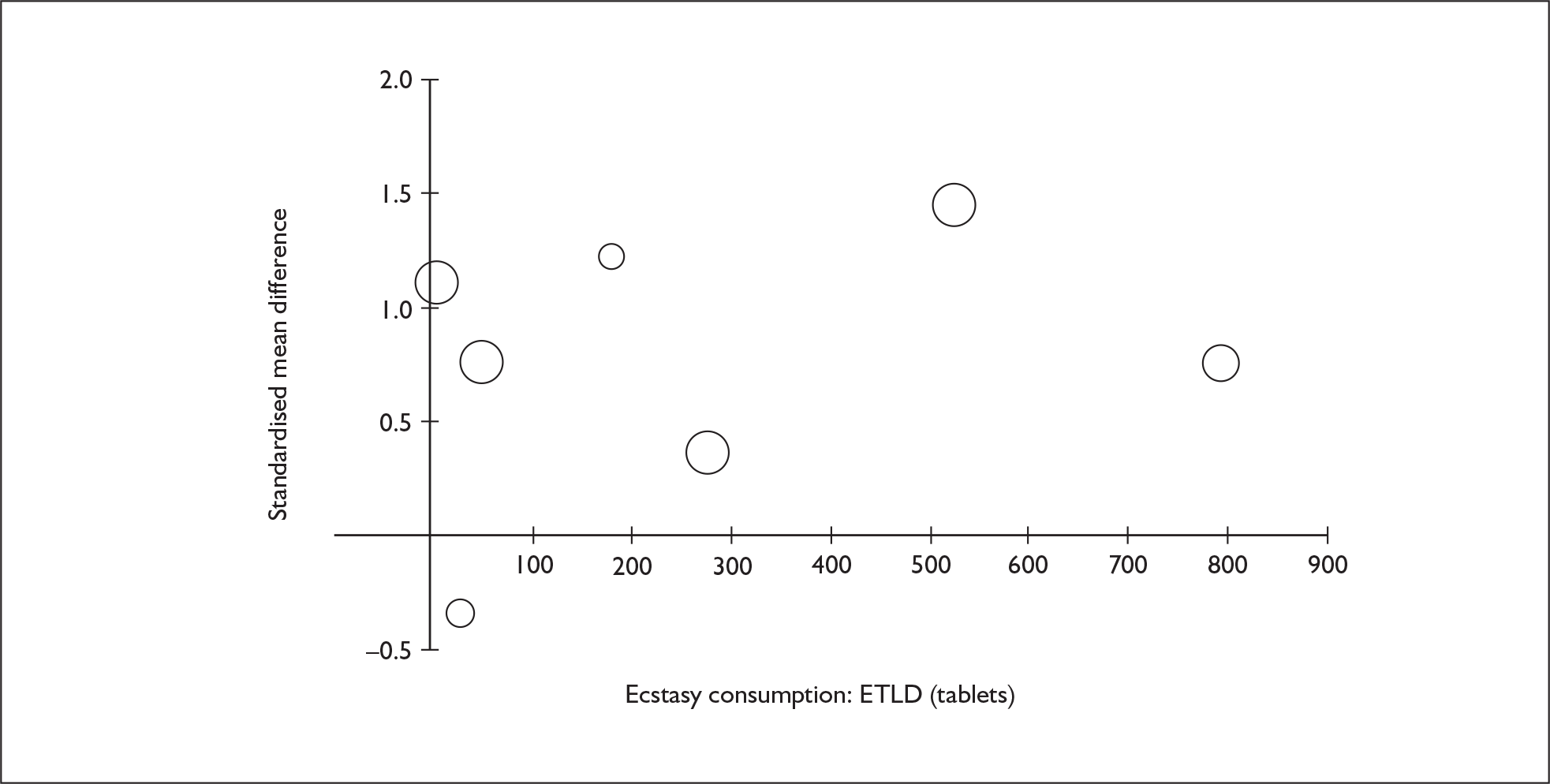
Appendix 8 Map of Level III evidence
Outcomes identified in case series and case reports containing fatal cases
| Outcome | n | References |
|---|---|---|
| Asthma exacerbation/other respiratory distress (see also: Pneumomediastinum) | 1 | 35 |
| Brain haemorrhage/other organic brain damage | 14 | 169,176,195,202,204,206,207,212,213,277,281,286,292,503 |
| Cardiac events (acute) (not sinus tachycardia) | 22 | 176–179,188,195,196,201,203,206,208,209,212,213,215,216,263,264,278,342,503 |
| Central nervous system abnormalities (acute) (see also: Seizures) | 5 | 208,213,229–231 |
| Death | 69 | 23,29,36,37,39,169,175–188,194–196,199,201,216,224–233,263–265,277,281,286,292,330,340,342,345–349,503–508 |
| Dental damage/other oral injury | 3 | 195,201,203 |
| Diabetic complications | 1 | 214 |
| Disseminated intravascular coagulopathy and other haematological disorders | 17 | 37,168,188,196,199,201–203,205–212,214 |
| Hyperkalaemia | 3 | 201,211,213,215 |
| Hyperthermia | 25 | 37,168,176,181,188,194–196,199,201–214,216,228 |
| Hypoglycaemia | 1 | 286 |
| Hyponatraemia | 8 | 206,224–233 |
| Hypothermia | 3 | 188,228,230 |
| Immunological dysfunction (aplastic anaemia, etc.) | 1 | 188 |
| Kidney failure | 13 | 168,188,194,196,199,201,205,207,210,211,213,214,345 |
| Liver failure | 12 | 188,194–196,199,205–207,213,214,340,503 |
| Memory (including learning) | 1 | 224 |
| Movement disorder (acute)(dystonia) | 1 | 292 |
| Psychoses/personality disorders (chronic) | 1 | 347 |
| Psychotic episode (acute) (including panic) | 2 | 345,347 |
| Rhabdomyolysis (and other muscular dysfunction) | 12 | 168,188,196,199,202,205,209–211,213,214 |
| Seizures | 15 | 37,168,188,195,199,203–205,207,209,216,228,230,263,292 |
| Suicide/attempted suicide | 8 | 177,210,214,345–349 |
| Vascular abnormalities | 1 | 181 |
Outcomes identified in case series and case reports containing non-fatal cases
| Outcome | n | References |
|---|---|---|
| Asthma exacerbation/other respiratory distress (see also: Pneumomediastinum) | 1 | 302 |
| Attention deficit disorder | 2 | 509,510 |
| Brain haemorrhage/other organic brain damage | 15 | 222,250,276,278–280,282–285,287–291 |
| Cardiac events (acute) (not sinus tachycardia) | 14 | 190,266–275 |
| Central nervous system abnormalities (acute) (see also: Seizures) | 30 | 190,241,244,248,271,273,276,293,295,300,305–307,511–524 |
| Dental damage/other oral injury | 5 | 525–529 |
| Dependency | 2 | 530,531 |
| Dermatological disorders | 5 | 298,532–538 |
| Diabetic complications | 2 | 536,537 |
| Disseminated intravascular coagulopathy and other haematological disorders | 16 | 221,274,295,298,306,521,523,535,538–544 |
| Hyperkalaemia | 1 | 276 |
| Hyperthermia | 43 | 221,226,262,249,251,267,260,273,275,290,291,293,295,298,301,306,511,512,516,519–522,540,541,544–559 |
| Hypoglycaemia | 2 | 290,540 |
| Hyponatraemia | 24 | 205–224 |
| Hypothermia | 1 | 220 |
| Immunological dysfunction (aplastic anaemia, etc.) | 1 | 560 |
| Kidney failure | 15 | 221,271,282,291,521,523,539,541–543,552,581,561–563 |
| Liver failure | 30 | 271,274,298,306,332,521,539,543,549,561,564–576 |
| Memory (including learning) | 18 | 221,295,365,368,371,510,525,577–602 |
| Mood (depression, anxiety, etc.) | 27 | 290,352,361,509,525,577,578,581,584–602 |
| Movement disorder (acute) (dystonia) | 15 | 190,222,244,254,280,283–285,295–299,525 |
| Movement disorder (long-term) (including parkinsonism) | 3 | 581,603,604 |
| Neurocognitive function (including decision-making, attention, learning) | 12 | 295,365,366,369,510,577–580,583,585,592 |
| Ocular injury | 4 | 355,605–607 |
| Personality traits (including impulsivity, aggression, loneliness, etc.) | 10 | 295,578,581,586,590,598,607–609 |
| Pneumomediastinum, pneumothorax and similar | 21 | 268,308,319,322 |
| Psychoses/personality disorders (chronic) | 32 | 251,280,293,300,301,351,353,357–369,509,513,514,578,581,585–587,592–599,610–614 |
| Psychotic episode (acute) (including panic) | 19 | 296,298,300,301,306,350–363 |
| Rhabdomyolysis (and other muscular dysfunction) | 24 | 220,240,242,248,250,271,274,282,293,298,520,523,541–543,546,549,550,555,561,615–618 |
| Seizures | 35 | 190,222,233–237,240,241,243,245,246,249,270,274,278,293,300,306,337,353,363,517,520,522,531,540,541,546,547,551,554,555,619,620 |
| Sensorineural dysfunction (auditory, optical) | 9 | 242,289,550,593,621–625 |
| Sexual dysfunction (chronic) | 2 | 581,598 |
| Sleep | 6 | 299,525,581,585,595,597 |
| Stroke | 5 | 300,307,600,606,627 |
| Suicide/attempted suicide | 2 | 350,611 |
| Susceptibility to infection (chronic) | 2 | 581,628 |
| Urogenital dysfunction (including urinary retention) | 6 | 244,344,552,629–631 |
| Vascular abnormalities | 4 | 308,548,625,626 |
Glossary
- Aneurysm
- Localised, blood-filled dilatation of a blood vessel.
- Angiitis
- Inflammation of blood vessels.
- Anuria
- Absence of urine output.
- Arteriovenous
- Relating to the blood vessels – arteries and veins.
- Bruxism
- Tooth grinding.
- Co-drug use
- Use of more than one drug on the same occasion.
- Diplopia
- Double vision.
- Disseminated intravascular coagulopathy
- A pathological process whereby systemic blood starts to coagulate throughout the body.
- Ecological fallacy
- A recognised error in the interpretation of statistical data, whereby inferences about the nature of individuals are based solely upon aggregate statistics collected for the group to which those individuals belong.
- Glaucoma
- Increased pressure within the eye.
- Hemiparesis
- Paralysis affecting one side of the body.
- Heterogeneity
- Difference in nature.
- Hyperpyrexia
- Exceptionally high fever.
- Hyperthermia
- Abnormally high body temperature, heat stroke.
- Hyponatraemia
- Decrease in blood sodium concentration below the normal range.
- Keratopathy
- Damage to, or dysfunction or abnormality of the cornea.
- Mediastinum
- The central compartment of the thoracic cavity, containing the heart.
- Myopia
- Short-sightedness.
- Necrosis
- Cell death.
- Neurocognitive deficit
- Reduction or impairment of mental processes relating to thinking, learning or judgement.
- Nystagmus
- Involuntary rapid eyeball movements.
- Oedema
- Excessive fluid in the tissue of the body causing swelling.
- Pneumomediastinum
- Air or gas in the mediastinum, usually resulting from a ruptured bleb on the surface of the lung.
- Pneumopericardium
- Air between the heart and the membrane around it (pericardium).
- Pneumothorax
- Air or gas in the space around the lungs, usually resulting from an air leak from the lungs and leading to lung collapse.
- Polydrug use
- Use of multiple types of drugs.
- Psychodysleptic
- Hallucinogenic.
- Psychopathology
- Behaviours or experiences that are indicative of psychological impairment.
- Psychosis
- Experience of loss of contact with reality which may be marked by hallucinations, agitated behaviour and delusions.
- Rhabdomyolysis
- The destruction of skeletal muscle cells.
- Snowball sampling
- A sampling method whereby initial contacts recruit others to take part in the study, and so on.
- Sympathomimetic
- Mimicking the effects of the sympathetic nervous system.
- Tachycardia
- Rapid heart beat.
- Tentorial herniation
- Brain tissue pushing through the tentorium as a result of brain swelling.
- Trismus
- Disturbance of nerves leading to spasm in jaw muscles and difficulty opening the mouth.
- Wolff–Parkinson–White syndrome
- A heart condition involving pre-excitement of the ventricles.
Abbreviations
- A&E
- accident and emergency
- ANCOVA
- analysis of covariance
- ARF
- acute renal failure
- CI
- confidence interval
- DIC
- disseminated intravascular coagulopathy
- DRD
- drug-related death
- EM
- effect measure
- ETLD
- estimated total lifetime dose
- ETLE
- estimated total lifetime exposure
- GHB
- gamma-hydroxybutyric acid
- GMR
- General Mortality Register
- HTA
- Health Technology Assessment
- IQ
- intelligence quotient
- LSD
- lysergic acid diethylamide, ‘acid’
- MA
- methamphetamine, ‘crystal meth’
- MBDB
- 3,4-methylenedioxy-phenyl-N-methylbutanamine
- MDA
- 3,4-methylenedioxyamphetamine
- MDEA
- 3,4-methylenedioxy-N-ethylamphetamine, ’Eve’
- MDMA
- 3,4-methylenedioxy-methamphetamine, ecstasy
- MOOSE
- meta-analysis of observational studies in epidemiology
- np-SAD
- National Programme on Substance Abuse Deaths
- OR
- odds ratio
- PMA
- paramethoxyamphetamine
- REM
- rapid eye movement
- SD
- standard deviation
- SMD
- standardised mean difference
- WMD
- weighted mean difference
- XTC
- ecstasy
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table. However, please note that, because of their large number, all abbreviations relating to outcome measures from contributing studies are defined in Appendix 5.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
Health Technology Assessment Programme
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NCCHTA
-
Dr Andrew Cook, Consultant Advisor, NCCHTA
-
Dr Peter Davidson, Director of Science Support, NCCHTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NCCHTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NCCHTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NCCHTA
HTA Commissioning Board
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programmes, Research and Development Directorate, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington