Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 06/34/02. The contractual start date was in May 2007. The draft report began editorial review in June 2008 and was accepted for publication in January 2009. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. 2009Queen’s Printer and Controller of HMSO
Chapter 1 Introduction and background
Infant feeding and health
Breastfeeding is a source of complete nutrition that changes to meet each infant’s growing needs, and confers active immunity to disease. The use of breastmilk substitutes is detrimental to the health and development of the infant and child, and to the health of the mother. Despite longstanding methodological challenges in this field, it is now recognised nationally and internationally that the public health implications of infant feeding are important in industrialised countries as well as in resource-poor countries. 1–3 Good-quality, large cohort studies, a large randomised controlled trial and a good-quality systematic review have shown that the absence of breastfeeding increases the risk of short-, medium- and long-term ill health in infants in industrialised countries (e.g. refs 4–8), and adversely affects long-term outcomes in mothers (e.g. ref. 9). Further, data from prospective studies and a large randomised controlled trial show that infants who are not breastfed have worse neurodevelopmental outcomes. 10–13
Particular care is needed in the interpretation of studies of health outcomes and infant feeding among infants in neonatal units. Studies of infant feeding can seldom be randomised, and there are confounding variables related to the socioeconomic factors associated with infant feeding behaviour. Outcomes measured are often short-term indicators of milk intake or time to discharge, and longer-term health and development outcomes are seldom measured. 14 Studies are often small, as there are relatively limited numbers of infants requiring care in these settings. Because formula feeding and bottle feeding have been standard care for many years, breastfeeding and breastmilk feeding and the techniques required to support them are novel and staff may be unfamiliar with them. Formula is not a standard product across time or across countries or hospitals. Neither can breastmilk be assumed to be standard; even when breastfed, infants can be supplemented with formula or other breastmilk substitutes, and breastmilk is often ‘fortified’ with commercial preparations depending on the nutritional status of the baby and the policy and practice of the country, unit or individual neonatologist. 15–18 Despite inconclusive evidence of effectiveness and safety,19 ‘fortification’ is such a common procedure in some countries (e.g. the USA) that not all studies report whether or not it is used. Breastmilk can also be enriched by maximising the intake of high-fat components of expressed breastmilk,20,21 although this is not common practice. Expressed breastmilk can also vary; fresh or stored mother’s milk differs from donor milk, which may be derived from one or more mothers at different stages of lactation. Expressed milk may be treated in different ways before being given to the baby. Each of these products is likely to have a different impact on outcomes. Further complicating interpretation is the use of different methods for the oral feeding of both formula and breastmilk. Gavage feeding, bottles and cups may each be associated with different outcomes regardless of the content of the feed. 22–24
Despite these methodological challenges, it has been shown that for preterm, growth-restricted and sick neonates including those requiring surgery, the use of breastmilk substitutes is associated with increased short- and long-term adverse outcomes including mortality and serious morbidity. Epidemiological studies,25,26 and randomised and quasi-randomised controlled trials18,27 in high-risk environments have found that the incidence of invasive infection is higher in low birthweight infants who are fed formula. A meta-analysis of randomised controlled trials28 has shown that formula-fed low birthweight infants have five times the risk of necrotising enterocolitis (NEC), a condition associated with a mortality of approximately 20% and significant long-term health-care costs amongst survivors. 29 In a UK randomised controlled trial, formula feeding resulted in later transition from parenteral nutrition,30 increasing the associated cost and infection risk. The studies of neurodevelopmental outcomes cited above indicate a larger deficit in low birthweight infants fed on formula (see, e.g., ref. 31). This finding is particularly important in this group where cognitive impairment is a frequent adverse outcome. 14
Deaths and serious morbidity as a result of infants in neonatal units consuming contaminated powdered formula have also been highlighted in the US press (www.cfsan.fda.gov/∼dms/inf-warn.html). In light of the epidemiological findings and the fact that powdered infant formulas are not commercially sterile products, the US Food and Drug Administration (FDA) now recommends that ‘powdered infant formulas not be used in neonatal intensive care settings unless there is no alternative available’ (www.cfsan.fda.gov/∼dms/inf-ltr3.html; 12 April 2002). The UK Food Standards Agency has informed consumers that powdered infant formula is a non-sterile product (www.food.gov.uk/news/newsarchive/2007/jul/nonsterile; 4 July 2007).
Following discharge from hospital, infants who are not breastfed continue to be exposed to hazards including contamination of feeds and feeding equipment, and errors of reconstitution of formula. 32–35
Feeding from the breast may facilitate other beneficial outcomes, for example a reduction in procedural pain36–38 and earlier discharge. 39,40
Finally, it has been argued that supporting mothers in breastfeeding and providing breastmilk is an essential part of a package of humane care, and assists in promoting attachment. 41 Such care includes gentle touch, decreased negative stimulation, exposure to the mother’s scent, skin-to-skin care and family involvement in care,42 all of which are inherent in breastfeeding. Breastfeeding/breastmilk feeding adds the important factor that the mother’s unique involvement in the nutrition and care of her infant may help to ease the inevitable shock, fear and grief following the birth, and decrease estrangement from her baby in the process of care in the high-tech environment of a neonatal unit. 43–46
Breastfeeding rates
Breastfeeding rates vary widely internationally. High incidence and prevalence are found in many resource-poor countries,2 although socioeconomic and geographic differences are apparent and these high rates can be disrupted by factors including conflict and displacement, maternal mortality and ill health. 47 Exclusive breastfeeding, which results in the biggest health gains, is far from universally practised; only 39% of infants are reported as being exclusively breastfed for 4 months following birth. 2 In industrialised countries, the first six or seven decades of the twentieth century saw breastfeeding rates decline steeply. Countries that have successfully reversed this decline include Sweden, Norway and Japan; Australia and Canada have also seen significant recent increases. Several industrialised countries have, however, not yet achieved such a reversal. These include the USA, France, Ireland and the UK, where low rates of initiation, duration and exclusivity have been observed for several decades. 48 Recent data suggest that initiation rates are increasing in the UK and the USA, although cessation rates have not improved. 49,50
There is also a marked contrast in breastfeeding rates across different socioeconomic groups in resource-poor countries compared with industrialised countries. The wider availability and promotion of formula is associated with increased formula feeding among the more affluent urbanised populations in resource-poor countries (e.g. ref. 51); recent developments in China, where tens of thousands of infants have become ill as a consequence of substandard formula use, demonstrate the potential adverse consequences of this (www.bmj.com/cgi/content/full/337/oct01_1/a1890). In industrialised countries, those most likely to formula feed are from the lowest income families (e.g. ref. 49).
Breastfeeding rates in the UK
Initiation rates in UK countries in 2005 were 78% in England, 70% in Scotland, 67% in Wales and 63% in Northern Ireland,49 indicating an increase in the previous 10 years. This increase is attenuated but not abolished if data are standardised for the age and socioeconomic composition of the survey sample.
In the same 2005 national survey, the rate of women breastfeeding at all in the UK at 6 weeks after birth was 48% (50% in England, 37% in Wales, 44% in Scotland and 32% in Northern Ireland), demonstrating that the rapid discontinuation of breastfeeding in the first few weeks persists. Exclusive breastfeeding rates are also very low; in 2000 only a quarter of those breastfeeding were breastfeeding exclusively at 2 months. In 2005, 27% of those breastfeeding were breastfeeding exclusively at 2 months, 17% at 3–5 months, 21% at 3 months and 5% at 5 months for the UK overall. 49
Incidence and prevalence are lowest amongst families from lower socioeconomic groups,49 particularly among white women compared with those of Asian, black or mixed ethnicity. 49,52 Teenage, young mothers and those least educated are also vulnerable groups, being half as likely as older mothers to initiate any breastfeeding. The increased prevalence of formula feeding in low-income families is an important contributor to inequalities in health. 53
Breastfeeding rates in neonatal units
One challenge in measuring breastfeeding rates in neonatal units is that, for these infants, feeding directly from the breast may not be possible. They may instead have breastmilk feeds, which can include fresh or stored mother’s own milk, or donor milk, and this milk may be fed by methods including bottle, cup and tube. It is important to distinguish between types of milk and methods of feeding, as they may each have a different impact on health outcomes. This is not always the case in surveys of rates, however, and the information available is limited in this respect. Throughout this report, we use the term ‘breastfeeding/breastmilk feeding’ whenever it is not possible to differentiate.
The absence of a definition of initiation of breastfeeding and breastmilk feeding specifically for infants admitted to neonatal units in the UK raises further difficulties for measurement of breastfeeding rates among this population. The UK definition for the initiation of breastfeeding is as follows: ‘The mother is defined as having initiated breastfeeding if, within the first 48 hours of birth, either she puts the baby to the breast or the baby is given any of the mother’s breast milk’. 54
In the case of infants admitted to neonatal units, it is particularly important to measure both of these components where they occur, namely, the baby receiving human milk (directly through breastfeeding, or with milk expressed by the mother or donor milk) and the mother having initiated breastfeeding or expression of breastmilk. Furthermore, the initiation of breastfeeding may be most usefully measured at the point at which the baby receives a nutritive breastfeed, an event which is likely to involve several occasions of the baby being put to the breast and may occur within days or weeks from birth. The time points for the most appropriate routine measurement of each of these components also require consideration to ensure consistency and to aid comparison with initiation rates among term, healthy infants.
Mothers responding to the 2005 national UK study of infant feeding49 reported that 5% of their infants were admitted to ‘special care’. No difference was found in initiation of breastfeeding/breastmilk feeding (the survey did not distinguish) according to whether or not the baby started life in a neonatal unit. Infants starting life in a neonatal unit were slightly more likely to be breastfed/have breastmilk both at 1 week (68% of neonatal unit infants compared with 64% of other infants) and at 2 weeks (63% compared with 60%), indicating that mothers were at least as motivated to produce breastmilk for these infants, or that staff encouraged them to do so, or both. This differential increased with the length of time spent in the neonatal unit, with 73% of infants spending at least 4 days in a neonatal unit being breastfed/having breastmilk at 1 week compared with 61% of infants spending only 1 day and 64% not in a neonatal unit at all. Similarly the prevalence of breastfeeding/breastmilk feeding at 2 weeks increased from 58% of infants spending up to 1 day in a neonatal unit to 67% spending 4 or more days. 49
Policy and infant feeding
The United Nations Global Strategy on Infant and Young Child Feeding2 recommends that all infants should be exclusively breastfed until 6 months, and that breastfeeding should continue at least until age 2 years. This report states that ‘Infants who are not breastfed, for whatever reason, should receive special attention from the health and social welfare system since they constitute a risk group.’ These international recommendations on duration and exclusivity of breastfeeding are supported by the governments of England, Wales and Northern Ireland, and there have been a series of policy developments intended to tackle low breastfeeding rates across the four countries in the UK (e.g. ref. 55). Breastfeeding is recognised as contributing to several Public Service Agreement targets and as an important part of the strategy to tackle inequalities in health. 53 Targets have been set to raise both initiation and duration rates. 56 Breastfeeding has been recognised as an important factor in reducing health inequalities in infant mortality,57 and infants in neonatal care are those most at risk of mortality and serious morbidity. Breastfeeding is also recognised as having a role to play in meeting the Every Child Matters agenda by improving children’s health. 58
Professional bodies and UK NHS organisations have long endorsed breastfeeding as appropriate for all infants (e.g. ref. 1), and recently the National Institute for Health and Clinical Excellence (NICE) recommended a series of interventions across the NHS to raise initiation and duration rates. 3,59 Statements on breastfeeding/breastmilk for preterm and sick infants are more limited. Strong support is given for breastfeeding/breastmilk for high-risk infants by the American Association of Pediatrics. 60 Recently, the Confidential Enquiry into Maternal and Child Health (CEMACH)61 specifically recommended that mothers with diabetes should be encouraged to breastfeed for both their own and their infants’ metabolic control; these infants are more likely to require care in a neonatal unit. Further, advice that mothers and infants in specific high-risk situations, such as human immunovirus (HIV)-positive mothers, those on antidepressants and substance users, should avoid breastfeeding is now being re-examined in the light of new evidence (e.g. refs 62,63).
There has never been a national UK policy initiative specifically intended to increase breastfeeding uptake and duration for infants in neonatal units, and information about such initiatives in other countries is lacking.
Factors affecting infant feeding rates in neonatal units
Reasons for the low prevalence of breastfeeding overall include the influence of societal and cultural norms, poor continuity of care in the health services, and a lack of effective care by health professionals in hospital and community. 64–66 These factors are likely to be amplified in the highly medicalised environment of neonatal units, making continuation of breastfeeding/breastmilk feeding difficult for those who do start. Specific factors examined here include the medical condition of infants, the health and well-being of mothers, the neonatal unit environment, the organisation of care, staff training, and the lack of consistent availability of care that would enable breastfeeding in this challenging environment.
Infants in neonatal units
Infants cared for in special care baby unit (SCBU)neonatal intensive care unit (NICU) settings include:
-
Infants born prematurely: these will range from very preterm births, down to 23 weeks, through to those born up to 36 completed weeks. These infants are likely to be low birthweight (LBW; birthweight < 2.5 kg). Some will be of appropriate birthweight for gestational age (AGA), others will be small for gestational age (SGA).
-
Infants born SGA: these are infants whose birthweight falls below a chosen threshold for gestation, most commonly the 10th centile. Some will be preterm infants who are also small for their gestational age, and some term or near term but who are growth restricted. Twins and multiple births will be over-represented in this group, and are more likely to be both preterm and SGA.
-
Infants born with or acquiring a health problem that requires additional care: this could include a variety of single or multiple system disorders, congenital malformations (particularly those requiring surgical intervention) and infections. It also includes infants of mothers with problems, for example, they may be infected with HIV or be substance users whose babies may exhibit neonatal abstinence syndrome,67 and infants admitted with feeding problems and/or weight loss.
As technology has allowed infants to survive at younger gestations, the very preterm, born before 28 weeks, have distinct challenges to their survival, health and development. These infants have had a major impact on the work of neonatal units as they have more complex problems, will require intensive care, and will have an increased length of stay in neonatal units. 68
There is a strong association between prematurity and multiple births; about 40–50% of twins and 90% of triplets are born prematurely. 69 With improvements in fertility treatment, the already established trend of increasing multiple pregnancies is likely to continue. The issues in relation to breastfeeding twins and multiples are complex and range from simple difficulties relating to the additional time it takes to breastfeed two or more infants, to more complex questions about fulfilling nutritional requirements.
The needs of infants born between 34+0 and 36+6 weeks’ gestation also require consideration. Although less physiologically and metabolically mature than term infants, they are usually well enough not to need admission to a neonatal unit and are often looked after on the postnatal ward in order to avoid maternal separation. These infants have relatively low oromotor tone and function so are more likely to have feeding difficulties, especially if breastfed, and to require readmission in the first month of life. 70,71
Mothers of infants in neonatal units and their families
Mothers of infants in neonatal units are more likely than mothers of term infants to have experienced a complicated labour and/or birth, to be prescribed medication, to have a range of pre-existing social and medical problems, and to be anxious about their children’s well-being and even survival. 72 They may have been prescribed antenatal corticosteroids if recognised to be at risk of preterm birth, and this may have a negative impact on milk production. 73 They may have health problems, such as HIV, that require careful consideration of feeding options. They are more likely than mothers of term infants to be from a low-income background74 and therefore less likely to choose to breastfeed; and, as their pregnancies may be curtailed by preterm labour, they are likely to miss out on the antenatal education that could influence their feeding decision. 75 Women with lifestyle challenges, such as smoking, use of non-prescription drugs and alcohol, will also be over-represented, as these factors predispose to preterm birth and to intrauterine growth retardation. 76 This group of mothers is less likely to have made a decision to breastfeed prior to the often unexpected early birth of their baby.
Mothers will be anxious and concerned about the health and survival of their infant or infants, and they may even be in a different hospital, or discharged home while the infant/infants remain in hospital. They may have to take care of older children, or even return to work if the infant’s stay is prolonged. For mothers of twins and multiples, there may be a healthy infant in addition to a sick infant/infants, and it is possible that one infant may be transferred to another hospital for specialist care, resulting in the mother having to choose which baby to spend most time with. Mothers of infants in neonatal units have described being exhausted, feeling insecure bonds with their infants, and experiencing unresolved grief,45 and the experience of having a small and preterm baby has been described as ‘a complete shock’, and ‘an unnerving experience’. 43 These experiences are likely to have an impact on trying to establish breastfeeding or breastmilk expression. 46
Family members, especially fathers and grandparents, are likely to be anxious and concerned about the baby, as well as the health and well-being of the mother. 77
Neonatal units: organisation of care, staff and ethos
Markedly improved survival rates at all gestations, as well as many more survivors at the extremes of prematurity, mean that there are increasing numbers of infants in neonatal units with complex problems. 14 In addition, the organisation of neonatal care in the UK has undergone substantial reorganisation in the last 5 years with the creation of neonatal networks and the centralisation of neonatal intensive care. 43 These factors combine to give rise to large populations of very small infants with complex needs in big tertiary neonatal units. Almost all units examined in a recent survey reported that they commonly exceeded their capacity, with three-quarters being closed to admissions at some time in the 6 months prior to the survey. 43 This system of care also requires transport of infants, with reported problems related to lack of specialised transport, and communication with parents.
Although the centralisation of care has delivered benefits including streamlining of care, shared meetings, staff training and shared protocols,43 the promotion of breastfeeding still requires attention. Staff working in neonatal units include neonatal nurses (who are likely to have diverse backgrounds including general nursing, adult intensive care and midwifery), paediatricians, speech therapists, nursery nurses and health-care assistants. Mothers will be cared for by a different set of staff in different settings, including hospital and community midwives, obstetricians, staff in critical and intensive care, health visitors and GPs. A recent learning needs assessment found that NHS staff were not adequately prepared to support breastfeeding among the general population,78 and that paediatricians were particularly ill-prepared to promote and support breastfeeding. 79 The problems of staff training for breastfeeding have been recognised recently, and NICE has recommended that the Baby Friendly Initiative becomes the minimum standard for care for NHS trusts. 3,59,80 However, although neonatal units are assessed to a limited degree as part of the Baby Friendly assessment of the maternity unit, there is as yet no Baby Friendly accreditation process for standards of care in neonatal units. Neonatal nurses and medical staff are therefore likely to be poorly trained in the complexities of supporting breastfeeding in this environment, including the skills needed to work with mothers of multiples;81 midwives and health visitors are unlikely to have the skills to support women to express breastmilk over long periods of time; and each discipline is likely to differ in their preparation for and approach to infant feeding, resulting in inconsistencies in approach.
These problems are compounded by understaffing. The national shortage of neonatal nurses means that the British Association of Perinatal Medicine guidelines82 of one nurse to one NICU patient are seldom adhered to; only 4% of neonatal units meet these standards, and the nurse workforce is understaffed by one-third. 43
Neonatal units are stressful for staff and students, as well as families. All the infants are ill or very small, parents are visibly anxious, staff are busy and concerned about the infants’ well-being and even survival. The atmosphere has been described as ‘stressful’, ‘frightening’ and ‘difficult’. 83 Parents need support from staff; some parents have described themselves as ‘completely overwhelmed’, and that ‘it felt like [their baby] belonged to the NHS and not to us’. 43 Equipment is essential and pervasive, including incubators, monitors and pumps.
Facilities may not be ideal for providing appropriate care. Finding space for parents to sit quietly with each other, to talk with staff, or to sit beside the baby and take in the fact of an unexpected preterm birth, a congenital problem, or an episode of worsened health status, can be problematic. In a recent survey, 25% of mothers reported that units had no facilities for them to stay in or close by the unit. 43 The lack of space beside incubators can make prolonged skin-to-skin care difficult or even impossible. The same report found that 25% of mothers ‘never’ had skin-to-skin care with their infants, and 60% sometimes felt they were ‘in the way’. 43
In such settings, the promotion of humane care becomes problematic. 41–44 It is widely accepted in the care of healthy, term infants that close contact between baby and mother is essential for breastfeeding, for attachment and for the well-being of the baby and the mother,84 and the lack of this contact adds to the vulnerability of mothers and infants in neonatal units. A system of care that includes reducing noise and light, minimal handling, and giving longer rest periods, known as developmental care (NIDCAP), has been instituted in some units internationally. Although the evidence base examining this form of care is limited, outcomes identified include decreased moderate–severe chronic lung disease and NEC, and improved family outcomes. 85
Breastfeeding/breastmilk feeding in neonatal units
Several factors influence breastfeeding/feeding with breastmilk in neonatal units.
Breastmilk production, and in particular the copious production of milk known as lactogenesis II, is delayed in women having a preterm birth, and this may be further complicated in women who have had antenatal corticosteroids. 73 Mammary growth may be incomplete, and the placental lactogen required for mammary development could also be impaired. 86 Establishing and sustaining lactation is much more complex than for mothers of healthy infants,87 expressing milk without the satisfaction of having a baby to feed can be demanding and disheartening,21 and expression often needs to be sustained over a prolonged period of time – the mean length of stay for infants in neonatal units in the UK is 55 days. 43
Mothers who have had a complicated birth including caesarean section, or who are themselves ill, will have major problems in expressing milk and visiting their infant, and will be unable to spend the close and intimate time needed to help establish breastfeeding/breastmilk supply. 88
It becomes increasingly hard for the mother to sustain milk production in the absence of direct feeding from the breast, often resulting in poor weight gain and growth. In order to promote growth clinicians either increase the volume of milk an infant is fed or supplement breastmilk. This is done by ‘fortification’ using a multinutrient breastmilk fortifier or individual supplements (protein, carbohydrate, fat and minerals) or supplementing intake with a preterm infant formula. Evidence on short- and long-term benefits and adverse effects of these practices is inconclusive. 19 Practice on this issue differs internationally and across different units in the UK, and there are concerns about the increased osmolality that results when breastmilk has commercial products added. 89 The psychological impact of implying to a mother that her milk is nutritionally inadequate is unknown but could be profound. Some units offer increased concentrations of hindmilk as a method of fortification,90 although evidence is lacking on the consequences for the infant in terms of growth, development and health.
Treatment and storage of mother’s own expressed breastmilk, whether fresh, frozen or pasteurised, is critical both for the baby’s intake and for the mother’s motivation to continue to express. Staff need to be trained and to have the facilities to ensure proper storage and use. 3
When mother’s own milk is not available or not sufficient, donor milk can provide a high-quality substitute. A recent unpublished study at Guy’s and St Thomas’ Hospital in London found that the establishment of a donor human milk bank was associated with a substantial increase in the provision of maternal breastmilk to infants with a birthweight of less than 1500 g at the time of discharge. Fifty per cent of infants received breastmilk at discharge before the milk bank opened, whereas 78% received breastmilk on discharge 18 months after their milk bank opened (Dr Camilla Kingdon, St Thomas’ Hospital and Association for Milk Banking, personal communication, 2008). An efficient milk bank system is not widely available in the UK;91 NICE is currently examining this issue (www.nice.org.uk/guidance/index.jsp?action=byID%26o=11973).
The transition to oral feeds is challenging as a result of the unco ordinated suck and swallow pattern of preterm and low birthweight infants. 92–94 Infants may not be able to tolerate oral feeds, will have problems of temperature control, may be difficult for parents to handle, and may have respiratory, cardiac, neurological or other problems that make oral feeding complicated. They may have nasogastric tubes and intravenous (i.v.) lines in place. Difficulties exist for all oral feeding methods,95 but breastfeeding is especially challenging if conducted in an environment where staff do not have the special skills needed, women are anxious about handling a fragile baby, facilities are not available for privacy, and milk supply may not be well established. 81,96 There is concern that giving the baby bottle teats or pacifiers may complicate the transition to feeding directly from the breast as the feeding action is different from breastfeeding,97,98 and alternatives including cups and nasogastric and orogastric tubes have been used to avoid this. 99 However, staff may find it more time consuming to help a mother to breastfeed or support her to express and store her milk than using formula or feeding from a bottle as these have been standard practice for some time.
Hospital protocols may interfere with breastfeeding/breastmilk feeding; these may relate to infants’ expected weight gain and growth, feeding frequency or mode of feeding. Such protocols are likely to be based on current standard care, which in the UK is more likely to be formula feeding.
A consistent strategy to promote breastfeeding/breastmilk feeding in neonatal units is lacking. 100 Without such a strategy at national and unit levels, the combination of the stressful environment and the lack of skills needed to support breastfeeding/breastmilk feeding in these vulnerable infants and their mothers is likely to result in inconsistent and ineffective care.
Conclusion
It is in this complex context that this review and economic analysis are set. The work is both timely and important, as this topic has the potential to have an impact on the mortality and morbidity of preterm and low birthweight infants, and the health and well-being of mothers, and to have considerable resource implications for the health service. Recognising the range of factors that affect the mother, infant, caregivers and the health service, and the potential of this topic to have an impact on inequalities in health, we sought to examine not only the clinical interventions that might work but also the public health context. This study includes work to examine both the effectiveness and cost-effectiveness of interventions.
Chapter 2 Aims and objectives of the study
Aims
This study, which includes a systematic review and a decision model, was commissioned by the NIHR Health Technology Assessment programme. The specific aim was to evaluate the effectiveness and cost-effectiveness of interventions that promote breastfeeding or feeding with breastmilk for infants admitted to neonatal units.
This study aimed to evaluate the impact of all types of breastfeeding promotion intervention among infants admitted to neonatal units. These could range from national policies that aim to support the mother in her role as prime carer, such as paid maternity leave, through to clinical interventions such as interim feeding methods, and education and support programmes that aim to increase women’s understanding of, and ability to, breastfeed their infants. The decision model focused on evaluating the impact of support, specifically enhanced staff support, on the long-term health of the infant.
Objectives
The specific objectives of this study were to:
-
identify and describe health promotion activity intended to increase breastfeeding or feeding with breastmilk for infants admitted to neonatal units
-
evaluate the effectiveness of any such health promotion activity, in terms of changing the number of women who breastfeed or feed with breastmilk, using the Ottawa Charter for Health Promotion framework101
-
analyse the cost-effectiveness of health promotion activity, specifically enhanced staff support, using a critical review of the existing cost-effectiveness literature and the development of a decision model
-
collate expert opinion on best practice, using the views of Advisory Group members and information from neonatal unit settings nationally and internationally where breastfeeding/breastmilk feeding rates are high
-
identify implications for policy, practice and education based on the findings of this study
-
identify an agenda for future research that will inform key gaps in knowledge.
This study was informed by an Advisory Group including academic, clinical and service user/consumer colleagues and a subgroup of clinical advisers (Appendix 1).
Chapter 3 Scope and methods of the effectiveness and health economics reviews
Effectiveness review
A systematic review of the literature was undertaken using guidelines published by the Centre for Reviews and Dissemination. 102
Inclusion and exclusion criteria for studies in the effectiveness review
Participants
This review included only studies that recruited infants or the mothers of infants who were admitted to neonatal units. The eligible infant population included preterm infants (both healthy and sick) and full-term infants who were growth restricted and/or sick. Twins and multiple births were eligible for inclusion, as were infants with congenital abnormalities, feeding problems, hypoglycaemia or jaundice, and those requiring surgery.
Studies recruiting population subgroups of mothers of eligible infants, such as mothers from low-income groups or different ethnic groups, were also eligible. Studies of interventions targeting other people were also considered: these participants included those linked to women who may breastfeed, such as partners, other family members or health professionals.
Interventions
This review included evaluations of any type of intervention that addressed breastfeeding/feeding with breastmilk in neonatal units, and studies that comprised a domiciliary care component following discharge from the unit. Control groups could receive standard or routine care or an alternative breastfeeding promotion intervention.
As the aim of this review was to examine breastfeeding-/breastmilk-related interventions in neonatal units, evaluations of interventions that were implemented during the antenatal period were excluded.
Studies that examined the effectiveness of breastmilk on clinical outcomes (e.g. studies that examined associations between breastmilk consumption and the incidence of necrotising enterocolitis, NEC), studies that evaluated the nutritional content of formula and breastmilk fortifiers, and studies of the establishment and maintenance of milk banking were outside the scope of this review of effectiveness.
Outcomes
A study must have reported a breastfeeding-/breastmilk-related outcome to be included in this review of effectiveness. These may have included breastmilk composition and volume, tasting dripped breastmilk, number of sucks, initiation of breastfeeding, any breastfeeding, exclusive breastfeeding and rates of breastfeeding at discharge and beyond. Studies that did not report a breastfeeding-/breastmilk-related outcome were excluded.
Secondary outcomes of interest included clinical/health outcomes (e.g. NEC, gastrointestinal disease, weight), process outcomes (e.g. time of hospital discharge, readmission, time spent by mother in contact with baby), psychosocial outcomes (e.g. views of mothers, fathers, families, health-care staff) and cost-effectiveness outcomes.
Outcomes were examined to assess the different gestational ages of the infant and/or ability to coordinate sucking and swallowing: for example, practice and outcomes for skin-to-skin care may be different for extremely low birthweight infants compared with low birthweight infants, and for infants with specific neurological problems.
Study designs
Randomised controlled trials (RCTs) and non-RCTs with concurrent controls were included in this review. For categories of interventions where evidence was limited and in recognition of the difficulties inherent in evaluating certain types of health promotion intervention, exceptions to this rule were considered. For example, multifaceted changes to organisation of care may have been conducted using a comparative study with retrospective controls or a before- and after-intervention design. Before/after studies that had utilised a cohort or cross-sectional study design were eligible for this review. It is important to note that results from these studies are likely to be less robust than those from RCTs and non-RCTs, and any reported effect on breastfeeding outcomes may not be solely attributable to the intervention(s). Studies without any form of control group (i.e. descriptive studies) and case studies were excluded.
We identified systematic reviews to assist with identification of eligible primary studies. Findings from identified systematic reviews were not included as a source of evidence for this review. This was due to differences in quality and methodological approaches across reviews for the analysis of primary studies, which may have been included in more than one review.
Identification of studies
The search strategies were devised in collaboration between the information officer (KM) and members of the research team familiar with the topic area. There was no limit by language or country of origin. Studies in this review were identified by searching a wide range of medical, nursing, psychological, sociological and grey literature databases. Each search strategy was developed for MEDLINE and adapted for use with other databases (see Appendix 2.1). In order to minimise potential publication bias for the effectiveness review, the search process aimed to identify published research, unpublished research or research reported in the grey literature, through the following four stages:
Search to identify systematic reviews
Searches were carried out to identify systematic review literature published in this field. Databases were searched for studies dated from 2006 to January 2008. Searches were limited to retrieve only systematic reviews. A total of 115 references were retrieved.
The following databases were searched:
-
MEDLINE and MEDLINE In-Process Citations
-
Cochrane Database of Systematic Reviews
-
Database of Abstracts of Reviews of Effectiveness
-
Health Technology Assessment Database
-
National Research Register
-
Scottish Intercollegiate Guidelines Network
-
National Guidelines Clearinghouse
-
Health Services/Technology Assessment Text
-
Turning Research into Practice
-
Health Evidence Bulletins Wales
-
Clinical Evidence.
Search to identify primary studies
All databases were systematically searched for primary studies dating from inception to August 2007. A pragmatic search of selected databases was undertaken in January 2008. The databases marked below (*) were identified for update searching based on yield of included studies. A total of 14,729 references were retrieved by both original and update searches.
The following databases were searched:
-
*MEDLINE and MEDLINE In-Process Citations
-
*EMBASE
-
*CINAHL
-
*Maternity and Infant Care
-
*PsycINFO
-
*British Nursing Index and Archive
-
*Health Management Information Consortium
-
*Cochrane Central Register of Controlled Trials
-
*Science Citation Index
-
*Pascal
-
*Inside Conferences
-
*Dissertation Abstracts
-
Sociological Abstracts
-
Latin American and Caribbean Health Sciences
-
Applied Social Sciences Index and Abstracts
-
Index to Theses
-
MetaRegister of Controlled Trials
-
National Research Register.
Search for studies evaluating galactagogues
As part of an iterative approach to searching, an additional search was undertaken to identify studies of galactagogues. Databases were searched for studies dated between 1991 and February 2008. Searches were not limited by study design. A total of 4045 references was retrieved.
The following databases were searched:
-
MEDLINE and MEDLINE In-Process Citations
-
Embase
-
CINAHL
-
Maternity and Infant Care
-
PsycINFO
-
British Nursing Index and Archive
-
Health Management Information Consortium
-
Cochrane Central Register of Controlled Trials
-
Science Citation Index.
To identify grey literature and unpublished studies and to check for completeness, bibliographies of studies retrieved were hand searched, and experts on the Advisory Group were asked to assist with the identification of other published or unpublished studies.
Data handling process
Titles and abstracts of bibliographic records were imported into endnote 9 bibliographic management software and duplicate records removed. Two reviewers independently screened titles and abstracts of identified records. Any disagreements were resolved by consensus. This process identified 138 potentially relevant studies. Full papers were ordered and assessed for inclusion using a prescreen form (see Appendix 3) by one reviewer and checked by a second. Any disagreement on whether a paper was relevant to the review was resolved by a third reviewer.
The five areas of health promotion action identified in the Ottawa Charter for Health Promotion101 were used as a framework to assist in classification of the different types of intervention to promote breastfeeding among infants in neonatal units. These were:
-
public policy such as legislation, fiscal measures (e.g. maternity leave)
-
supportive environments that protect natural resources and generate healthy living and working conditions (e.g. private rooms for expressing, provision of pumping equipment to express at home)
-
community action that uses existing human and material resources to enhance self-help and social support (e.g. social support through family, peers)
-
development of personal skills through the provision of information, education for health, and enhancing life skills (e.g. education programmes, clinical support)
-
reorientation of health services to promote health (e.g. staff training, the BFI).
Standardised data extraction and quality appraisal tables were adapted from the Centre for Reviews and Dissemination (CRD) Report 4. 102 Data were extracted and appraised for quality by one reviewer and checked by a second reviewer (see Tables 26–73 in Appendix 4.1). An overall quality rating was awarded to each study based on NICE guidance development methodology103 and the Cochrane Handbook (2008)104 (see Tables 74–87 in Appendix 5). Any disagreements in data extraction or quality appraisal were resolved by discussion or, if necessary, by a third reviewer. Details of studies that were excluded at either the prescreening or data extraction stage are shown in Appendix 6.1.
Five relevant systematic reviews were identified in the course of the searches. These were used to identify studies. Data extraction forms for these reviews are given in Appendix 7.
Analysis and presentation of results
Quality ratings for each study have been presented in the text (Chapter 4) using the following definitions:
-
Good quality – most or all criteria being fulfilled and where they were not met, the study conclusions were thought very unlikely to alter.
-
Moderate quality – some criteria being fulfilled and where they were not met, the study conclusions were thought unlikely to alter.
-
Poor quality – few criteria were fulfilled and the conclusions of the study were thought very likely to alter. Serious caution is warranted in interpretation of the results of these trials.
Details of the individual quality ratings for each study are provided in Appendix 5.
Within each topic area, results are presented first for RCTs and then for other study designs.
Results from all primary studies were assessed and summarised in a qualitative synthesis for each type of intervention (Chapter 4) and across types of intervention (Chapter 7). Meta-analysis was not considered appropriate due to the heterogeneity between studies for type of intervention, standard care, characteristics of participants, outcome measures, feeding intention and country settings. Relative risks for outcomes have been estimated for individual studies on an intention-to-treat (ITT) basis where appropriate outcome data were reported. Given the relatively high clinical risk among the target population, the ITT analysis was adjusted for legitimate postrandomisation exclusions. These were calculated as infants who were lost to the study due to death, not achieving predefined clinical stability to participate in the intervention, or other clearly defined inclusion or exclusion criteria such as discharge to original hospital. Data from studies rated as good or moderate quality have been presented in forest plots where appropriate. In the absence of meta-analyses, funnel plots and sensitivity analyses were not considered appropriate methods to assess publication bias.
Throughout the report, included studies are referred to using the name of the first author and the date, e.g. Jones 2001, or the citation number.
Results of the effectiveness review are presented in full in Chapter 4, and for the economic modelling in Chapter 5. Due to the inter-related nature of results from each topic area, results are summarized and discussed together in Chapter 7.
Methods of health economics literature review
Inclusion criteria
Studies were eligible if they were full economic evaluations (i.e. they included an explicit comparison of both costs and effects for an intervention and at least one comparator), and were considered to be useful in answering the research question relating to cost-effectiveness.
Identification of potential economic evaluations
The search strategies were devised in collaboration with an information specialist. There was no limitation by language or country of origin. A search strategy was developed for NHS Economic Evaluation Database (NHS EED) and adapted for use with other databases. Full details are presented in Appendix 2.1.
The search process was undertaken in three stages:
1. Searches of health economics resources The following resources were searched to identify economic evaluations:
-
NHS Economic Evaluation Database (NHS EED) (up to 2007/08/8) (internal CRD interface)
-
Health Economic Evaluations Database (HEED) (up to 2007/08/08) (internet)
-
Pediatric Economic Database Evaluation (PEDE) (1980–2003) (internet) http://pede.bioinfo.sickkids.on.ca/pede.search.jsp.
A total of 294 references were retrieved.
2. Subset search of Clinical Effectiveness Endnote Library An Endnote Library containing 10,262 references, identified by the search undertaken for the evidence of effectiveness review search detailed above, was searched to identify potentially relevant cost/economic studies. After deduplication, 1176 records were identified.
The following terms were entered line-by-line (_ indicates a space):
-
_cost_
-
_costs
-
_cost-
-
_costly
-
_costing
-
_econom
-
_budget
-
_price
-
_pricing
-
_expenditure
-
value for money
A total of 1176 references were retrieved and scanned for relevance.
3. Further searches to populate the decision model A series of focused supplementary searches were undertaken to identify data to populate the model. These searches were limited to a small collection of ‘core’ databases, as specified by the health economists:
-
NHS Economic Evaluation Database (NHS EED) (up to 2008/02/28 (internal CRD interface)
-
Health Economic Evaluations Database (HEED) (up to 2008/02/28) (internet)
-
MEDLINE and MEDLINE In-Process Citations (2003–2008/02/wk 2) (OVID)
-
Embase (2003–2008/wk 7) (OVID)
-
EconLit (2003–2008/01) (OVID).
Searches were undertaken for three supplementary topics:
-
long-term outcomes of NEC or sepsis
-
quality of life in infants with NEC, sepsis, meningitis, etc.
-
economic evaluations of NEC, sepsis, meningitis, etc. in preterms or neonatal units.
Totals of 713 (topic 1), 99 (topic 2) and 487 (topic 3) references were retrieved for the searches and scanned for relevance.
Results of health economics review
No economic evaluations that met the inclusion criteria were identified. Had suitable studies been identified, data would have been extracted (Appendix 4.2). Details of excluded studies are presented in Appendix 6.1, along with information on the planned quality appraisal process.
Framing recommendations
To inform implications for policy, practice and education, and to identify gaps in the evidence base and priorities for future research, two additional approaches were used:
-
Seven expert clinical advisors from neonatal units in Sweden, the USA and the UK (Appendix 1) were asked to identify key factors in their experiences of introducing successful breastfeeding-/breastmilk feeding-related change into their units (Chapter 6). This information was used to reflect on the findings of the study (Chapter 7).
-
After reading the findings of the study, Advisory Group members were asked to agree implications for policy, practice and education (Chapter 8) and to agree prioritisation of suggestions for future research studies (Chapter 9). Studies with methodological weaknesses, which were considered likely to have potentially misleading results, were not used in framing the implications for policy, practice and education.
Chapter 4 Results of the effectiveness review
Summary of review flow
The flowchart (Figure 1) is based on the QUOROM statement flow diagram105 to summarise the results of the methodology described in Chapter 3.
As detailed above, only 1% (119/10,184) of the total citations identified following deduplication were referred to a third reviewer for resolution regarding their potential relevance to this effectiveness review. Decisions regarding these citations were largely uncontroversial and, where any uncertainty remained, full papers were sought for further evaluation. Decisions regarding exclusions during the prescreening process were largely uncontroversial with the exception of one study (Sisk et al. 2006),110 which was excluded on the grounds that this study was not an evaluation of an intervention.
FIGURE 1.
QUOROM statement flow diagram summarising the methodology. *Reported in 51 papers.
Summary of evidence base
A total of 48 studies evaluating the effectiveness of interventions to promote breastfeeding in neonatal units met the inclusion criteria for this review. Of these studies, 65% (31/48) were randomised controlled trials (RCTs). The results of two studies were reported in two separate papers107–110 and Hill was reported in three separate papers. 111–113 One paper reported findings for two types of intervention presented in this review, with each intervention having been evaluated by a different study design method. 114 For the purposes of this review, this paper has been counted as two studies.
The five identified systematic reviews assisted with the identification of a total of 29 included primary studies including 22 RCTs,107–110,114–133 one randomised crossover study134 and six other forms of controlled studies. 135–139
A further 19 primary studies were identified through our independent search methods for inclusion in this review. These included nine RCTs,113,140–147 two randomised crossover studies114,148 and eight other forms of controlled studies. 20,81,149–154
Definitions of topic areas
The 48 included studies were grouped into nine topic areas, considered in detail in the following sections. Definitions of these topic areas and related issues are as follows:
Increased mother and infant contact interventions
Relevant interventions are those that promote warmth, developmental care, and early and successful breastfeeding for infants in need of special care. This includes skin-to-skin contact, which is defined as any contact between the mother’s and the infant’s skin over any period of time, usually from birth,155 and kangaroo mother care (KMC). KMC was originally developed in Colombia and comprises three components: (1) ongoing skin-to-skin contact in the kangaroo position, namely, between the mother’s breasts in an upright position; (2) kangaroo feeding policy, which is frequent and exclusive breastfeeding; (3) kangaroo discharge policy, which is early discharge from hospital regardless of weight or gestational age. 107 The use of KMC to optimise extrauterine transition in term154 and preterm157,158 infants is generally accepted as a safe intervention with multiple physiological advantages. 159 The potential of KMC to increase breastfeeding rates, resulting in associated physiological and emotional benefits, is, however, less established.
Interim feeding methods and related interventions
‘Interim feeding methods’ refers to the range of enteral feeding methods used to give babies either breastmilk or other fluids until feeding from the breast is possible. Enteral feeding is the administration of any feed into the gastrointestinal tract. 155 Feeding from vessels other than the breast may be necessary if the infant is too small or sick to take the breast directly or because the mother is unavailable; thus such methods may be used to replace or supplement feeding from the breast.
Interim feeding methods used include feeds given by nasogastric or orogastric tube or from vessels including bottles, cups, spoons and syringes. Some methods specifically aim to avoid the use of artificial teats on the rationale that learning to feed using a teat may make the transition to the breast more difficult. Such methods include cups, spoons and syringes, as well as ‘finger feeding’, where a nasogastric tube is attached to the finger of the carer and inserted in the infant’s mouth. Nipple shields are sometimes used with the aim of making feeding directly from the breast easier for small and sick babies.
‘Related interventions’ describes interventions used with the aim of enhancing feeding behaviours. This includes the use of pacifiers, which can be used for the purpose of enhancing non-nutritive sucking or in an effort to calm the infant. One alternative offered is the use of carers’ fingers.
Expressing breastmilk interventions
Relevant interventions include those that mothers may use to remove breastmilk from their breasts. The purpose of expression is normally twofold: to stimulate milk production, and to provide breastmilk for infants until they are able to satisfy their nutritional needs by feeding directly from the breast or until they are no longer receiving breastmilk. Variables of interest in the expression of breastmilk include both the equipment or technique that mothers may use for milk removal and the regimens for their use. Breastmilk may be expressed by hand and/or by pump; pumps may be hand- or foot-operated, or battery or electrically powered. Regimens may specify how, how often, how long or how much to express.
Additional interventions to enhance breastmilk production
Relevant interventions are those that mothers may use, usually in association with expressing breastmilk and/or breastfeeding, with the intention of increasing the volume of breastmilk produced. Such interventions include pharmacological (galactagogue medication) or dietary interventions, or interventions aimed at facilitating the mother’s let-down reflex with relaxation techniques or use of items such as photographs that she associates with her infant.
Interventions to support optimal nutritional intake from breastmilk
Relevant interventions include those that aim to optimise the quality and/or quantity of the breastmilk fed to infants in neonatal units and following discharge. Interventions may include test weighing infants before and after feeds, measuring the fat content of expressed breastmilk, and feeding hindmilk to increase the energy content of milk.
Breastfeeding education and support interventions
Relevant interventions include those that aim to offer support, education and/or counselling to parents of babies in neonatal care settings, and to take place either in hospital or at home during an infant’s hospital stay, or following discharge. Interventions may be offered by professionals or peers on a one-to-one or group basis and using a range of strategies including oral communication via face to face or telephone methods or written information via leaflets and other materials.
Staff training interventions
Interventions that aim to improve health-care professionals’ knowledge, skills and behaviour in relation to lactation and breastfeeding, and practices to support and promote breastfeeding and breastmilk production by mothers of infants in neonatal units.
Early hospital discharge with home support interventions
Early hospital discharge with home support intervention refers to discharge of infants prior to those infants having met standard weight gain criteria and/or having moved from gavage to full oral feeds. In most, but not necessarily all, cases, this intervention is conducted among clinically stable infants, defined as ones without cardiorespiratory compromise and maintaining normal body temperature when fully clothed in an open cot. Education and support of parents may be provided in the community setting following such discharge. Early discharge as part of comprehensive KMC is discussed below under ‘Increased mother and infant contact intervention’.
Organisation of care interventions
Relevant interventions are those that change care at the level of the individual unit (intra-unit) or between units (inter-unit). Both groups of intervention aim to improve the organisation of a single or allied health service or care within that service, to promote and support breastfeeding. These interventions are mostly, but not necessarily, conducted in hospital settings and may have several components implemented at one time. The changes to organisation of care may be implemented at the level of the hospital or the neonatal care unit or between hospitals or neonatal care units, as in the case of a managed clinical network.
Standard care
Standard or routine care was highly variable between studies and settings and often described in insufficient detail. Details of standard care or comparison group(s), where available, are provided for each study within each of the results sections below.
Initiation and duration of breastfeeding or feeding with breastmilk
The specific time points at which outcomes (such as initiation or duration of breastfeeding) were assessed varied between studies and in some cases were inadequately described. Definitions used by study authors are reported in the results sections below. These may or may not be consistent with the definitions adopted for the purposes of this review in accordance with the Department of Health definition for initiation of breastfeeding for the general population (see Glossary).
Included studies, the topic addressed, and whether or not they have been included in previous systematic reviews, are summarised in Table 1.
| Topic | Subgroups of intervention | No. of systematic reviews (SRs) | No. of studies in SRs (no of RCTs) | No. of extra primary studies (no of RCTs) | Total no. of primary studies (RCTs) |
|---|---|---|---|---|---|
| Increased mother and infant contact | Kangaroo care, skin-to-skin | 3 | 9a (7) | 3 (2) | 12 (9) |
| Interim feeding methods and related interventions | Nasogastric tube, bottle, cup, nipple shields, pacifiers | 3 | 6 (5) | 0 | 6 (5) |
| Expressing breastmilk | Electric and pedal pumps, manual, frequency of expressing | 1 | 4b (3) | 2 (2) | 6 (5) |
| Enhancing breastmilk production | Galactagogues, relaxation, therapeutic touch | 2 | 3 (3) | 4b (2) | 7 (5) |
| Supporting optimal nutritional intake from breastmilk | Mothers’ measures of creamatocrits, breastmilk intake weights, hindmilk feeds | 0 | 0 | 3 (2) | 3 (2) |
| Breastfeeding education and support | Peer or professional support, community or hospital based. Education for mothers | 2 | 3 (2) | 3 (1) | 6 (3) |
| Staff training | Training or education of health professionals | 0 | 0 | 2 (0) | 2 (0) |
| Early hospital discharge with home support | Home visits and support including home gavage feeding | 3 | 2c (2) | 0 | 2 (2) |
| Organisation of care | Policy, protocol-based care, BFI or non-BFI standard(s) | 1 | 2 (0) | 2 (0) | 4 (0) |
| TOTAL | 5d | 29 (22) | 19 (9) | 48 (31) |
Increased mother and infant contact interventions
A total of 12 primary studies evaluating mother and infant contact interventions were identified. As detailed in Table 2, nine primary studies were included in at least one of the three identified systematic reviews. Seven115,121,129,135,139,147,150 of the 12 studies were conducted in industrialised country settings including one in the UK. 147
| Primary paper | Study design No. analyseda | Inclusion in existing systematic review | Country |
|---|---|---|---|
| Cattaneo 1998131 | RCT | Conde-Agudelo 2003164 | Ethiopia, Indonesia and Mexico |
| n = 100 (site 1) | Edmond 2006155 | ||
| n = 104 (site 2) | McInnes 2006161 | ||
| n = 79 (site 3) | |||
| Charpakb 1997107 | RCT | Conde-Agudelo 2003164 | Colombia |
| n = 746 | Edmond 2006155 | ||
| McInnes 2006161 | |||
| Charpakb 2001108 | Edmond 2006155 | Colombia | |
| McInnes 2006161 | |||
| Sloan 1994132 | RCT | Conde-Agudelo 2003164 | Ecuador |
| n = 300 | Edmond 2006155 | ||
| McInnes 2006161 | |||
| Rojas 2003121 | RCT | McInnes 2006161 | USA |
| n = 57 | |||
| Blaymore Bier 1996115 | RCT | McInnes 2006161 | USA |
| n = 41 | |||
| Roberts 2000129 | RCT | McInnes 2006161 | Australia |
| n = 30 | |||
| Kadam 2005118 | Pilot RCT | McInnes 2006161 | India |
| n = 89 | |||
| Hurst 1997139 | Before/after | McInnes 2006161 | USA |
| n = 23 | |||
| Wahlberg 1992135 | Before/after | McInnes 2006161 | Sweden |
| n = 66 | |||
| Wilhelm 2005150 | Crossover | No | USA |
| n = 25 | |||
| Whitelaw 1988147 | RCT | No | UK |
| n = 63 | |||
| Boo 2007141 | RCT | No | Malaysia |
| n = 126 |
Results from randomised controlled trials
Nine randomised controlled trials of mother and infant contact interventions were identified107,108,115,118,121,129,131,132,141,147 (Tables 26–34 in Appendix 4.1).
Characteristics of participants
Four of the trials were conducted in industrialised country settings including one in the UK,147 two in the USA115,121 and one in Australia. 129 Of the remaining five trials, one was a multicentre trial conducted in Ethiopia, Indonesia and Mexico131 and one was a pilot RCT conducted in India. 118 The other three trials were conducted in Colombia,107,108 Ecuador132 and Malaysia. 141
All trials recruited infants according to criteria of birthweight or gestational age. Four trials115,121,141,147 focused on infants with the internationally recognised definition of very low birthweight (< 1500 g). The remaining trials used a variety of birthweight criteria, including infants with a birthweight of 2000 g or less,107,108,132 1000–1999 g131 and < 1800g. 118 The Colombian trial (Charpak 1997, 2001)107,108 included some infants [intervention (I):132/382; control (C): 155/364) who had a birthweight of 2000 g or less and were eligible to participate in the intervention but were not admitted to the neonatal unit. The remaining trial focused on infants at ≥ 30 weeks’ gestation. 129
All trials focused on infants who were clinically stable. Two trials included infants on minimal ventilatory support. 121,141 The remaining trials included infants who did not require oxygen equipment147 and were gavage fed,115 on oral feeds,118 tolerant of enteral feeds129,131,132 or demonstrating a satisfactory suck and swallow reflex. 107,108
Characteristics of maternal participants are limited and variable across trials. One of the nine trials focused on mothers from a range of social and economic settings. 131 Of the remaining trials that reported socioeconomic data, two trials comprised participants who were mostly high-level professionals115 or on medium to high incomes. 141 Maternal participants in the trial performed in the UK were mostly white (n = 50/63, 79%) with small numbers of Asian and Afro-Caribbean participants. 147
Two trials focused on mothers who intended to115 or had started to118 express breastmilk or breastfeed their infants. Two further trials reported participants’ intention to breastfeed147 and exclusive breastfeeding behaviour at enrolment131 by comparison groups.
Characteristics of interventions
Eight trials evaluated the skin-to-skin component of KMC where the infant is held upright between the mother’s breasts in a nappy and hat, usually covered by a blanket, and in a private setting, compared with traditional care where contact between mother and infant is fully clothed and privacy is not the norm. 115,118,121,129,131,132,141,147 The remaining trial evaluated a more comprehensive KMC intervention,107,108 namely, skin-to-skin contact together with early hospital discharge and regular, but not exclusive, breastfeeding. As characteristics of these studies vary, they are summarised in Table 3.
| Study | Country | Components of intervention | Total period of intervention | Duration of each daily contact | Inclusion criteria for infants by BW or GA | Other criteria for eligible infants |
|---|---|---|---|---|---|---|
| Boo 2007141 | Malaysia | Kangaroo skin-to-skin contact | Up to hospital discharge | 1 hour | < 1500 g | Minimal ventilatory support |
| Kadam 2005118 | India | Kangaroo skin-to-skin contact | Up to hospital discharge | 1 hour | < 1800 g | On oral feeds |
| Rojas 2003121 | USA | Kangaroo skin-to-skin contact | Up to hospital discharge | Up to 8 hours in two 4-hour periods | < 1500 g | Minimal ventilatory support |
| Roberts 2000129 | Australia | Kangaroo skin-to-skin contact | Up to hospital discharge | Not reported | ≥ 30 weeks | On enteral feeds |
| Cattaneo 1998131 | Mexico | Kangaroo skin-to-skin contact | Up to 40th week postnatal age | 20 hours | 1000–1999 g | On enteral feeds |
| Blaymore Bier 1996115 | USA | Kangaroo skin-to-skin contact | 10 days | 10 minutes | <1500g | Gavage fed |
| Sloan 1994132 | Ecuador | Kangaroo skin-to-skin contact | Up to hospital discharge | Not reported | < 2000 g | On enteral feeds |
| Whitelaw 1988147 | UK | Kangaroo skin-to-skin contact | Up to and beyond hospital discharge | At hospital visits (mean 2.1 hours daily visiting) | < 1500 g | No oxygen equipment |
| Charpak 1997, 2001107,108 | Colombia | Kangaroo skin-to-skin contact; early hospital discharge; regular breastfeeding | Up to 41 weeks corrected age | 24 hours | < 2000 g | Satisfactory suck and swallow reflex |
The total period during which kangaroo skin-to-skin contact was promoted varied considerably across trials, and between participants within trials; however, data on this were not reported in two trials. 129,132 Four trials reported the mean period of skin-to-skin contact during the hospital stay as 4.6 (range 0–40),107,108 8.5 (SD 4.4),118 11 (range 2–85), 17 (range not available)141 and 61 (SD 28)121 days. Two trials reported the median period of kangaroo skin-to-skin contact during the hospital stay as 11 (range 2–85)131 and 30 (range 5–83)147 days.
The large ranges around the average further highlight the heterogeneity of the total periods of kangaroo skin-to-skin contact between participants within an intervention group. This can be attributed in part to the range of birthweights of infants included in each trial and the inverse relationship between birthweight and length of hospital stay and in part to the diverse criteria for hospital discharge between trials.
The duration of each kangaroo skin-to-skin contact also varied considerably between trials. Three trials evaluated ‘short’ individual periods of contact of 10 minutes per weekday115 and one hour daily. 118,141 A further trial evaluated the promotion of kangaroo skin-to-skin contact at all visits during and beyond hospital stay,147 reporting a mean of 2.1 hours visiting per day. Data on actual contact time is not reported. 147
One trial evaluated a ‘medium’ level of skin-to-skin kangaroo care contact of up to 8 hours per day in two 4-hour periods. 121 Prolonged periods of contact were evaluated in two trials, defined as 24 hours per day until no longer tolerated by infant107,108 and reported as a mean of 20 hours per day during hospital stay. 131 Two trials did not define or report the duration of individual kangaroo skin-to-skin contacts. 129,132
The detail of standard care provided in control groups was limited. Incubators were available in all trials with the exception of one of the three study sites in the multicentre trial:131 standard care in Addis Ababa was open cribs in a warm room. 131 The Colombian trial specified visiting as restrictive for mothers in the control group107,108 while the UK trial specified open visiting practice as standard care. 147 Clothing of infants while being cuddled or fed was reported as standard care for the four trials conducted in industrialised countries. 115,121,129,147
Outcome assessment
One RCT reported the initiation of breastfeeding or receiving expressed breastmilk141 assessed between enrolment and hospital discharge. Another trial evaluated the timing of the initiation of breastfeeding118 assessed by the infant’s mean age in days when breastfeeding started.
Seven trials reported the duration of breastfeeding. 107,108,115,121,129,131,141,147 Two of these trials reported the duration of lactation where the mother, and not the infant, was the unit of allocation and analysis. 115,147 Exclusive rates of breastfeeding were reported in two trials. 107,108,132 One trial also evaluated the primary outcome of production of expressed breastmilk. 115 Interpretation of findings and further analysis are limited, however, due to lack of any numerical data to report this outcome (see Table 26 in Appendix 4.1).
Assessments of duration of breastfeeding varied between studies and included the following: 40–41 weeks’ corrected age,107,108 hospital discharge,115,121,129,141 1 month after hospital discharge,115 6 weeks,129 more than 6 weeks,147 3 months,107,108,129 6 months,107,108,129 9 months107,108 and 12 months107,108 and mean weeks’ lactation. 147 Exclusive breastfeeding was assessed at 40–41 weeks’ corrected age,107,108 at hospital discharge and 1 and 6 months. 132 With the exception of one trial,121 it is not clear whether the measures of breastfeeding started at the point of non-nutritive or nutritive breastfeeding.
All nine RCTs reported data on secondary outcomes including health,107,108,115,118,121,129,131,132,141 process,107,108,115,118,121,129,131,132,141,147 psychosocial 118,131,141 and cost-effectiveness outcomes. 108,109,131
One trial observed and monitored the predefined short duration of kangaroo skin-to-skin contact time. 115 This is unlikely to be possible for interventions promoting continuous or prolonged contact. The amount of enhanced kangaroo skin-to-skin contact is likely to be variable therefore between participants within each trial.
Methodological quality of included trials
No trials were rated as ‘good’ quality overall. Eight trials were rated as ‘moderate’ quality. 107,108,115,118,121,131,132,141,147 The remaining trial was considered to be ‘poor’ quality. 129 Serious caution is warranted in interpretation of the results of this trial. Details of the quality ratings for each trial are provided in Table 74 in Appendix 5.
Effectiveness of interventions
Primary outcomes
Complete outcome data for all those originally enrolled were provided in three trials,118,141,147 including data for postrandomisation exclusions where appropriate. 141 Relevant data have been sought and/or extracted to perform intention-to-treat analysis using postrandomisation exclusion data for five trials. 107,108,115,121,131,132 It was not possible to estimate the relative risk for primary outcome data for one study due to lack of denominator data. 129
Individual relative risk estimates for primary outcomes in trials that did not receive a poor overall quality rating are presented in forest plots. 107,108,115,118,121,131,132,147 These have been calculated on an ITT basis.
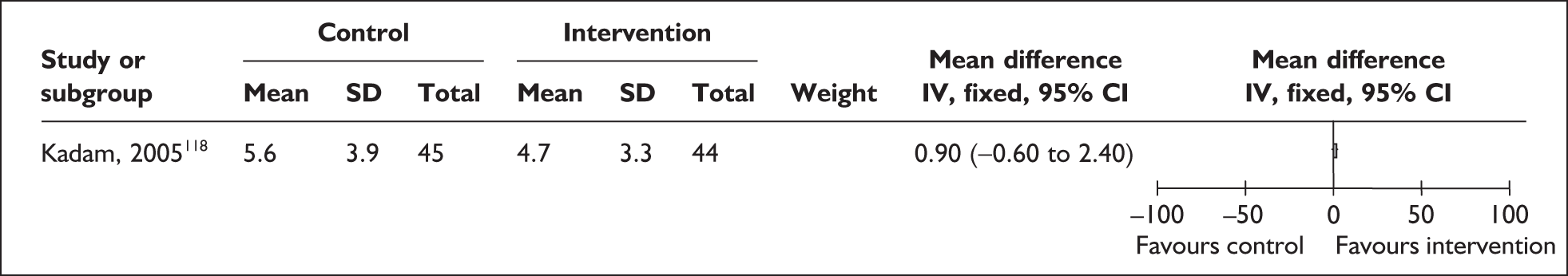
No trials evaluated the effect of mother and infant contact on the primary outcome of initiation of breastfeeding or oral feeding of expressed breastmilk. One small trial in India shows that kangaroo skin-to-skin contact for 1 hour a day has no effect on the age of initiation of breastfeeding among infants with birthweights of less than 1800 g118 (Figure 2). Some caution in interpretation of findings is required as age of the infants at enrolment is not reported by comparison group and between-group differences may affect this outcome. All infants in this study received only human milk and were either breastfed or spoon-fed.
FIGURE 2.
Kangaroo skin-to-skin contact vs standard care: age at initiation of breastfeeding (ITT).
Two trials that evaluated the effect of kangaroo skin-to-skin contact on the duration of any breastfeeding before hospital discharge in industrialised settings showed a positive effect of the intervention compared with standard care121,141 (Figure 3). Both trials were conducted among infants of very low birth weight and among populations with typically low breastfeeding rates (30% in 2000 census of study neonatal unit141 and 74% initiation in the USA nationally. 165
FIGURE 3.
Kangaroo skin-to-skin contact vs standard care: duration of any breastfeeding before hospital discharge (ITT).
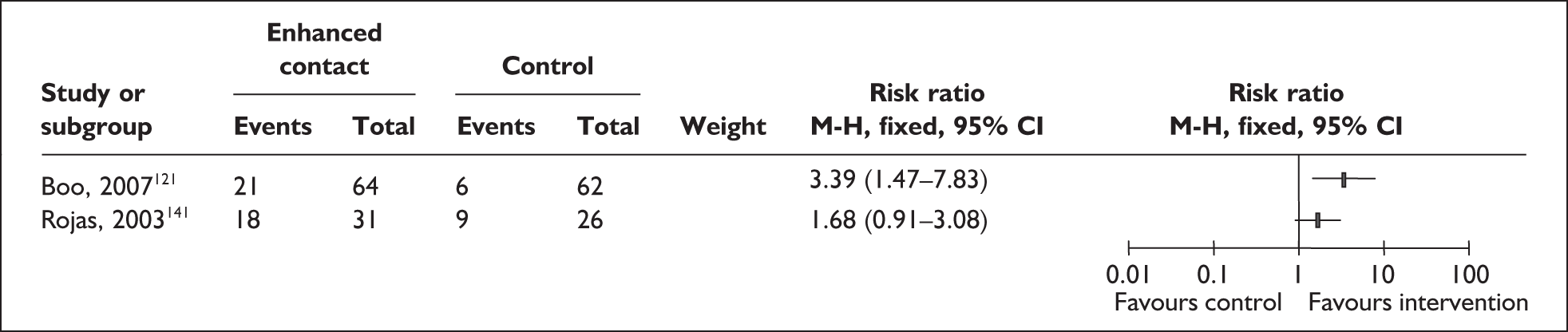
Results from one large trial have shown a statistically significant between-group difference (p = 0.004) as a result of the intervention. 141 This trial promoted kangaroo skin-to-skin contact for 1 hour a day with a mean hospital stay of 17 days (no range available). Some caution is required in interpretation of these findings as infants in the intervention group were of significantly later postmenstrual age, and intervention mothers had significantly more years of education, than their respective control groups. 141
A US-based trial reported no effect of the intervention. Findings of this trial are based on samples that are underpowered both as a total and as a subgroup sample for each comparison group and are not conducted on an ITT basis,121 and compliance was low in both groups.
Two trials that evaluated the effect of kangaroo skin-to-skin contact on the duration of any breastfeeding at hospital discharge showed a statistically significant increase (p = 0.05, Boo et al., 2007;141 p = 0.02, Blaymore Bier et al. , 1996115) in favour of the intervention compared with standard care (Figure 4). As stated above, some caution is required in interpretation of findings from this trial. 141
FIGURE 4.
Kangaroo mother care vs standard care: duration of any breastfeeding at hospital discharge or 40–41 weeks corrected age (ITT).
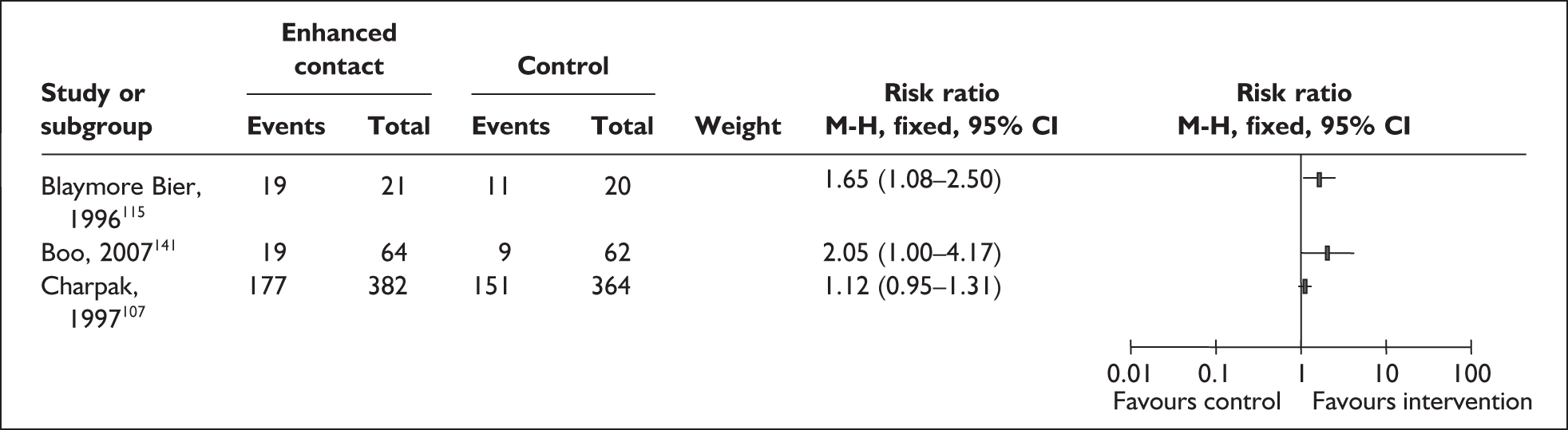
Two trials115,141 were conducted among infants of very low birthweight and among populations with low breastfeeding rates (as detailed above). Kangaroo skin-to-skin contact was for short periods in both trials, comprising 10 minutes per day for 10 days115 and 1 hour daily during a mean hospital stay of 17 days (no SD available). 141
One large trial evaluated a comprehensive intervention of KMC comprising prolonged kangaroo skin-to-skin contact, early discharge and regular breastfeeding. 107 No positive effect was found for duration rates of any breastfeeding at 40–41 weeks corrected age among infants of low birthweight in this resource-poor country setting.
Two small, moderate-quality trials evaluated the effect of kangaroo skin-to-skin contact on the duration of any breastfeeding for prolonged periods (more than 6 weeks;147 and 1 month after discharge115). Both trials took place in industrialised settings and demonstrated a statistically significant effect in favour of the intervention compared with standard care (p = 0.01, Blaymore Bier et al., 1996;115 p = 0.04 Whitelaw et al. , 1988147) (Figure 5). These interventions comprised relatively short daily periods of kangaroo skin-to-skin contact among infants of very low birthweight. Most of the mothers participating in the UK-based trial intended to breastfeed147 and all women in the US-based trial were expressing milk and planning to breastfeed. 115
FIGURE 5.
Kangaroo skin-to-skin contact vs standard care: duration of any breastfeeding for prolonged periods (ITT).
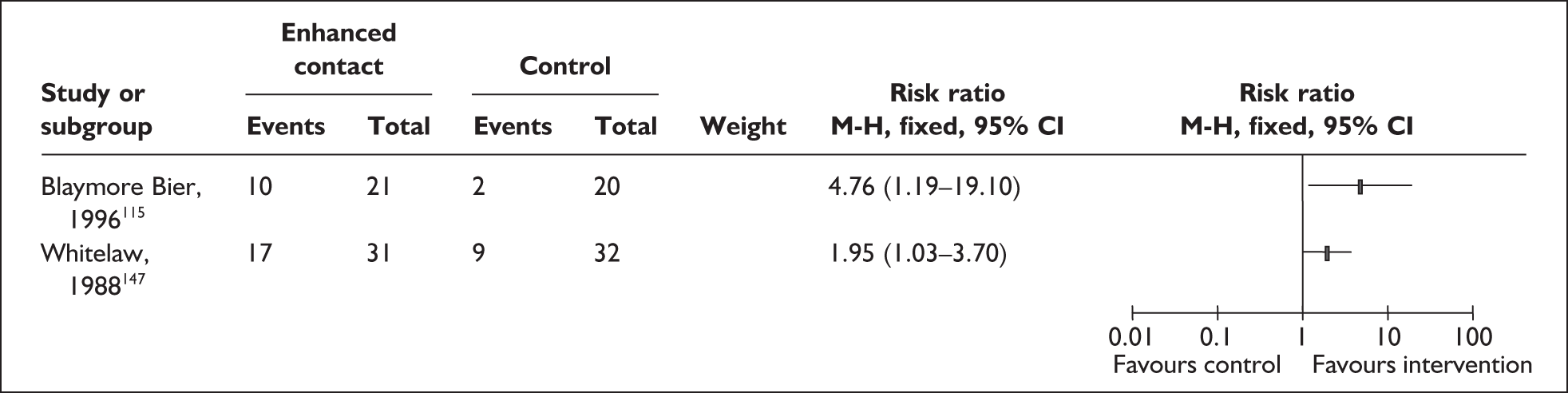
Significantly increased rates of any breastfeeding at 1 and 3 months corrected age were also reported as a result of a comprehensive kangaroo mother care intervention. 108 These findings are not based on an ITT analysis and outcome data are reported as percentages only. 108 These results are not presented in Figure 5.
Three trials evaluated the effect of kangaroo skin-to-skin contact on exclusive breastfeeding rates in resource-poor country settings. 107,108,131,132 A multicentred trial was conducted at three sites in three continents. 131 Results for each site have been presented separately to reflect between-site differences in characteristics of participants and standard care for control groups (Figure 6).
FIGURE 6.
Kangaroo skin-to-skin contact vs standard care: duration of exclusive breastfeeding at hospital discharge or 40–41 weeks corrected age.
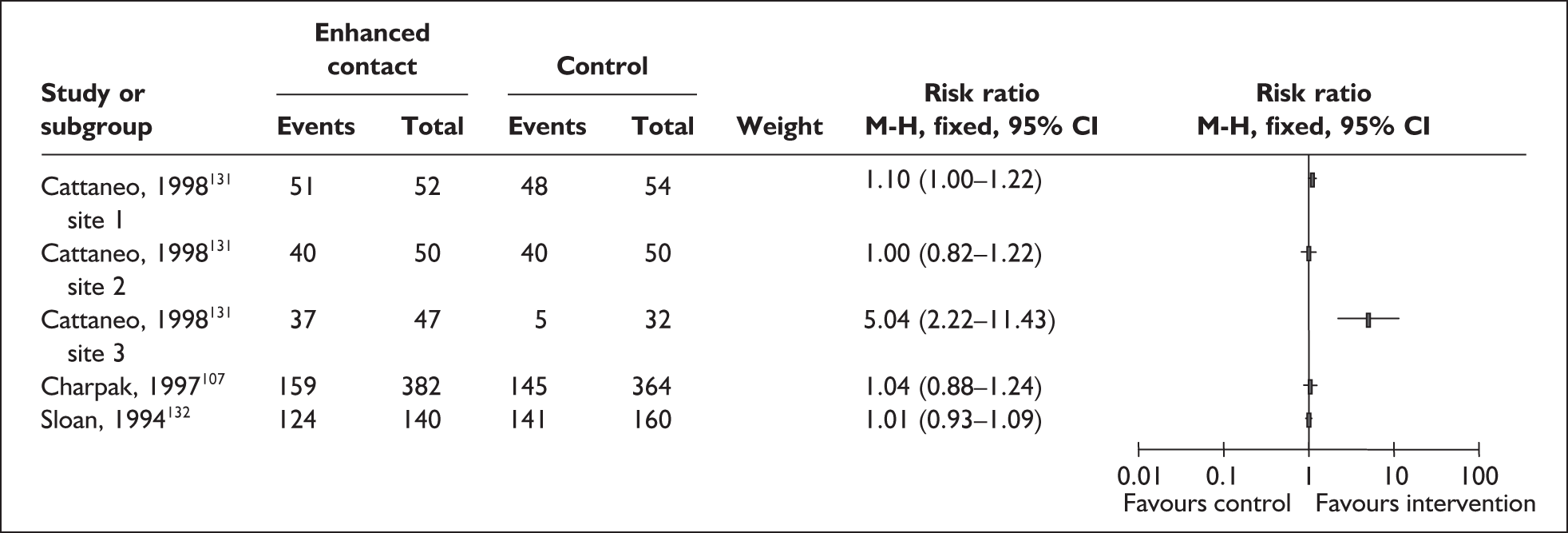
With the exception of one site in the multicentred trial, kangaroo skin-to-skin contact has been shown to have no effect on the duration of exclusive breastfeeding at hospital discharge or 40–41 weeks corrected age among infants of mainly low birthweight in resource-poor country settings (see Figure 6). Cattaneo et al. (1998)131 reported that exclusive breastfeeding rates at enrolment were significantly lower at the site that demonstrated a positive effect as a result of the intervention compared with sites one and two (p = 0.003). The differences in characteristics of participants, organisational and country setting and standard care between the three sites limit the ability to interpret the potential factors influencing the different findings reported for this site.
One trial (Sloan et al., 1994) also found that exclusive breastfeeding rates favoured the control group at 1 and 6 months. 132
Two of these trials were large, with a total of 300132 and 777107,108 participants across two arms in each trial. The three sites in the multicentre trial were of moderate size (n = 100, 104, 79, respectively) although an a priori sample size calculation had not been made. 131 The moderate quality of these larger trials, including between-group comparability at baseline, suggests these findings can be interpreted with some confidence. These trials suggest therefore that kangaroo skin-to-skin contact is not likely to increase the duration of exclusive breastfeeding rates at or beyond hospital discharge among mainly low birthweight infants in resource-poor country settings.
Secondary outcomes
Results for secondary outcomes are taken from available study data, including ITT analysis where available.
Two trials conducted among infants of very low birthweight and that employed ITT analysis for clinical outcome data reported a significantly greater mean weekly increase in head circumference (measured in centimetres) for the intervention group [intervention (I): 1.0 (SD 0.3); control (C): 0.7 (SD 0.3); p < 0.0001]141 and significantly greater rate of head growth (cm/day) (SD) for infants in intervention than control groups [I: 0.1 (0.03); C: 0.08 (0.02); p < 0.05]. 121 The third trial did not report raw data or conduct ITT analysis for health outcomes among study infants with a birthweight of 2000 g or less. 108 This study reported infants in the intervention group having a larger head circumference than control group infants at 12 months after estimated term (p = 0.0014). 108
Intention-to-treat analysis was employed by two small trials, which reported favourable outcomes for levels of oxygen saturation in the intervention groups. 118,121 Rojas et al. (2003)121 reported a significantly lower incidence of desaturation among very low birthweight infants in the intervention group than in the control group (I: 10/33; C: 15/27; p = 0.05). Mean oxygen saturation was found to be significantly higher for intervention than control groups (I: 95.7 ± 1.1; C: 94.8 ± 0.7; p < 0.01) and respiratory rates were significantly lower for the intervention group (I: 36.2 ± 3.3; C: 40.7 ± 2.9; p < 0.01). 118 One very small trial conducted in the USA among infants of very low birthweight found mean oxygen saturation was higher for intervention than for control groups (p < 0.001), with falls in haemoglobin oxygen saturation to < 90% occurring in 191/1716 (11%) recordings during skin-to-skin contact compared with 319/1334 (24%) during standard care (p < 0.001). 115 This study did not employ ITT analysis for assessment of clinical outcomes although losses to study were small (I: 0; C: 2). 115
Episodes of hypothermia (per 100 infants/day) were lower in one multicentred trial (I: 213 (10.8); C: 325 (14.6); p = 0.0005), particularly at the Mexican site (I: 82 (13.5); C: 141 (31.5); p = 0.00001). 131 These data were analysed on an available-case basis. A small trial that employed ITT analysis also reported fewer episodes of hypothermia in the intervention than in the control group (I: 10/44; C: 21/45; p < 0.01). 118 Both trials were conducted mainly among infants of low birthweight. 118,131
A trial conducted in Ecuador among low birthweight infants132 reported significant between-group differences for serious morbidity at 2 months (I: 7/131; C: 27/152; p = 0.002) and 6 months (p = 0.005). The difference at 2 months reduced slightly (p = 0.007) when controlled for pre-eligibility differences. 132 No significant differences were found between comparison groups for mild or moderate disorders. 132 These findings are not based on an ITT analysis although losses to study were less than 20% and the overall sample size was relatively large (n = 300).
No significant differences were found between comparison groups for incidence of sepsis,118,121,131,141 necrotising enterocolitis (NEC),118,121,131,141 apnoea,118 hyperthermia118 or growth indices including daily weight gain,132,141 weight at discharge,118 mean head circumference,141 total weight gain, total head circumference growth, total linear growth, rate of weight gain or rate of linear growth. 121
Process outcomes including median age at discharge, mean postmenstrual age at discharge141 and length of hospital stay118,121 were reported to be similar between comparison groups.
The one trial that evaluated a more comprehensive kangaroo mother care intervention including prolonged upright skin-to-skin contact, regular breastfeeding and early discharge found no differences between comparison groups for mortality or infectious morbidity, numbers of readmissions after discharge and psychomotor development up to 12 months. 107,108 These data were analysed on an available-case basis although losses to study were less than 20% and the overall sample size was large (n = 777).
A trial that evaluated mothers’ views of the intervention conducted among mothers of low birthweight infants in India reported that 86% of mothers were happy with kangaroo skin-to-skin contact and 14% preferred conventional care. 118 A UK-based study including very low birthweight infants147 found no significant differences between groups for levels of maternal self-reported confidence and positive feelings towards the infant at discharge and at age 6 months. One trial conducted in Malaysia examined mothers’ reasons for non-compliance with kangaroo skin-to-skin contact. 141 Reasons cited included mothers’ fear of handling their very low birthweight infants, mothers unable to visit regularly and fear that skin-to-skin contact would prevent their infant gaining weight. 141
Two trials that conducted a cost analysis of the intervention compared with standard neonatal care were conducted in resource-poor country settings among mainly low birthweight infants. 131,132 Both trials reported kangaroo skin-to-skin contact as less expensive overall than standardised incubator care, which was quantified in one study as $340 higher. 132 Both trials noted specific higher costs associated with the intervention including food for mothers and laundry131 and costs associated with increased contact time between mother, infant and siblings in the clinic. 132 Increased contact time was noted also to achieve improved capacity of mothers to care for their infants. 132
No adverse effects were reported as a result of kangaroo skin-to-skin contact, with or without early discharge from hospital, for infants of low or very low birthweight, when compared with standard care.
Results from other study designs
Three other forms of controlled studies were identified (Tables 35–37 in Appendix 4.1). 135,139,150 These include one repeated measures, crossover design150 and two cross-sectional before/after studies. 135,139 One before/after study collected retrospective data for the control group,139 the other for both comparison groups. 135
Characteristics of participants
All three studies were conducted in industrialised countries, including two in the USA139,150 and one in Sweden. 135 One study recruited all mothers in the neonatal unit, before and after introduction of a skin-to-skin holding policy in the neonatal unit. 139 It appears that all infants in the neonatal unit were ventilated and low birthweight. 139 Two studies included mother–infant pairs with stable infants according to predefined inclusion criteria: one study included infants aged 1–30 days when first taken out of the incubator,135 and another study included mothers of infants born < 33 weeks’ gestation and/or birthweight < 2000 g. 150 In this study, infants participated in the intervention during their first week of life and were not expected to breastfeed during the study period. 150 The study also recruited mothers who were already expressing breastmilk and intended to breastfeed for at least 3 months. 150 Maternal characteristics indicate these women were mostly white, not on very low incomes and held private insurance. 150
All infants included in these studies were preterm, gestational age ranging from a mean of 28 weeks150 to a mean of 32 weeks150. Mean birthweights were reported as 1490 g,135 1092 g139 and 1652 g. 150
Characteristics of interventions
All three studies evaluated skin-to-skin mother and infant contact. 135,139,150
One study defined the skin-to-skin component of kangaroo mother care with chest-to-chest contact for 1 hour of either 1 or 2 days depending on the comparison group within the first week of the infant’s life. 150 Mothers in the control group had no kangaroo care contact with unlimited visiting of their infant at the cotside. 150
Two studies promoted undefined skin-to-skin contact for either 30 minutes daily over a 2-week period compared with undefined standard care139 or as much as mother desired until hospital discharge, compared with mothers holding infants dressed and with blanket or heating pad. 135
Outcome assessment
One study reported the effect of kangaroo skin-to-skin contact on breastmilk production at 4–6 days150 and another reported the effect of skin-to-skin contact on the duration of breastfeeding at hospital discharge. 135 The remaining study reported breastmilk volume at 2, 3 and 4 weeks and rates of any and exclusive breastfeeding after hospital discharge. 139
Methodological quality of included studies
One study was rated as ‘moderate’ quality (Wahlberg et al. , 1992135), with some criteria being fulfilled, and where they were not met, the study conclusions are thought unlikely to alter.
Two studies were rated as ‘poor’ quality (Wilhelm, 2005;150 Hurst et al. , 1997139), in which few criteria were fulfilled and the conclusions of the study are thought very likely to alter.
Details of the quality ratings for each study are provided in Tables 75 and 76 in Appendix 5.
Effectiveness of interventions
Primary outcomes
It is not clear how outcome data have been derived and compared between comparison groups in the crossover study. 150 This limitation warrants extreme caution in interpretation of findings, and results have not been presented for this study. Some caution is warranted in interpretation of findings from the remaining studies due to methodological limitations of the study designs. 135,139
Wahlberg et al. (1992)135 report a retrospective comparison of records from a convenience sample of 66 mothers and infants in Sweden, half from the 18 months before the introduction of skin-to-skin contact and half from the 18 months following its introduction. The mothers and infants were well matched, but no comparisons with the population of mothers and infants in the unit are reported. This study reports higher rates of breastfeeding at hospital discharge [before (B): 15/33; after (A): 27/33; p value not reported in paper, calculated on an ITT basis p = 0.005].
The before/after study conducted in the USA reported a substantial increase in milk volume (ml) at 2 weeks for mothers in the ‘after’ group exposed to skin-to-skin contact compared with mothers in the non-exposed ‘before’ group (B: 462, SD 222; A: 574, SD 211). 139 The greater milk volume was maintained at 4 weeks (B: 421, SD 315; A: 851, SD 259) with the pattern of milk volume between groups from weeks 2 to 4 reported as significantly different (p = 0.01).
Secondary outcomes
One before/after study135 reported that infants in the exposed ‘after’ group were younger when they first came out of the incubator (p < 0.01), gained more weight per week (p < 0.05) and spent fewer days in the incubator (p < 0.05) and in hospital (p < 0.05). Authors note the methodological limitations of this study and the lack of generalisability of findings. 135
No lasting adverse incidents during skin-to-skin contact were reported in one before/after study. 139 Authors report the occurrence of an episode of mild oxygen desaturation in two infants exposed to the intervention. 139
Interim feeding methods and related interventions
A total of six primary studies and three systematic reviews examining interim feeding methods and related interventions were identified. As detailed in Table 4, all primary studies were included in at least one of three previous systematic reviews. All but one122 were conducted in industrialised country settings, including two in the UK. 120,124
| Primary paper | Study design (n analysed)a | Inclusion in existing systematic review | Country |
|---|---|---|---|
| Collins 2004119 | RCT (n = 303) | Edmond 2006155 | Australia |
| Flint 2007163 | |||
| McInnes 2006161 | |||
| Gilks 2004120 | RCT (n = 40) | Flint 2007163 | UK |
| McInnes 2006161 | |||
| Kliethermes 1999130 | RCT (n = 84) | McInnes 2006161 | US |
| Meier 2000136 | Retrospective crossover study (n = 34) | McInnes 2006161 | US |
| Mosley 2001124 | RCT (n = 16) | Flint 2007163 | UK |
| McInnes 2006161 | |||
| Rocha 2002122 | RCT (n = 78) | Edmond 2006155 | Brazil |
| Flint 2007163 | |||
| McInnes 2006161 |
Results from randomised controlled trials
Five RCTs of interim feeding methods and related interventions were identified119,120,122,124,130 (Tables 38–42 in Appendix 4.1).
Characteristics of participants
Four trials were conducted in industrialised country settings including two in the UK,120,124 one in the USA130 and one in Australia. 119 The fifth trial was conducted in Brazil. 122
All trials recruited infants in neonatal units using criteria of birthweight and/or gestational age at birth or study entry. Inclusion criteria comprised varying combinations of birthweight and gestational age: singleton or twin infants of less than 34 weeks’ gestation in Collins et al. (2004);119 over 30 weeks’ at time of study entry and up to 35 weeks’ gestation at birth in Gilks and Watkinson (2004);120 ‘preterm’ infants of 1000–2500 g in Kliethermes et al. (1999);130 30–37 weeks’ gestation in Mosley et al. (2001);124 and 32–36 weeks’ g estation and weighing less than 1700 g in Rocha et al. (2002). 122
All trials excluded infants with congenital abnormality that precluded enteral feeding. In addition, a variety of entry criteria were used that indicated that infants unable to tolerate enteral feeds would be excluded.
All trials were restricted to mothers who were breastfeeding or were planning to breastfeed. Limited information on socioeconomic or ethnic background of the mothers was given. The study of Kliethermes et al. (1999)130 was conducted in a private regional perinatal centre, probably indicating that poor women were unlikely to be included. Rocha et al. (2002)122 reported that in their trial in Brazil, over 60% had incomplete primary schooling and 70% were on a low annual income.
Breastfeeding rates in population
Background breastfeeding rates in the population groups from which these studies drew varied. Collins et al. (2004)119 noted that 85% of women in Australia started to breastfeed at that time, and the rate in their study unit was 45%. Gilks and Watkinson (2004)120 noted that 65% of women in their region of the UK started to breastfeed, and 40% of women in their study unit. Rocha et al. (2002)122 noted that only 20% of mothers of infants born weighing less than 1500 g breastfed at 3 months, although the population prevalence in Brazil is likely to be higher than in the UK. Neither Kliethermes et al. (1999)130 nor Mosley et al. (2001)124 gave information about breastfeeding rates in their populations, although it is likely that the rates for Mosley et al. (2001)124 are comparable with Gilks and Watkinson (2004). 120
Characteristics of interventions
Four trials examined the giving of oral enteral feeds by cup versus bottle. 119,120,122,124 One studied the use of a nasogastric tube versus bottle. 130 Two trials also examined the use of pacifiers as a co-intervention. 119,122 In one of these trials119 the use of pacifiers was randomised in a 2 · 2 design. In Rocha et al. (2002),122 the cup-feeding group also had pacifiers withdrawn and ‘non-nutritive sucking was provided by offering the little finger’. The implication is that the bottle-feeding group had pacifiers offered as part of standard care.
Although not explicitly stated, the implication in these trials was that the intervention continued until the infant was able to breastfeed exclusively, or as a means of complementary feeding once breastfeeding was established.
In all studies, it appeared that bottle feeding, and nasogastric tube feeding when it occurred, were standard care with which staff were familiar, with cup feeding being a novel or recently introduced technique.
Cup feeding
Use of, and staff training for, cup feeding varied between studies. In two studies, staff were trained in cup feeding before the trial started; Gilks and Watkinson (2004)120 indicated that it had been introduced to the unit 6 months before the start of the trial and supported by a teaching programme, and Rocha et al. (2002)122 indicated that staff were trained as part of a prior pilot project. Two other studies introduced cup feeding only in the context of and at the time of the trial; in Collins et al. (2004)119 participating hospitals received education, written instructions, and one-to-one support during the trial, and Mosley et al. (2001)124 reported that they gave an information sheet to all staff.
Rocha et al. (2002)122 described the technique of cup feeding; the infant was held in a slightly inclined position, having the cup touch the lower lip, allowing the infant to lick or sip the milk and avoiding pouring the milk into the child’s mouth. The cup they used was the protective cap of a feeding bottle. Collins et al. 119 noted that milk feeds were given via a small plastic medicine cup. In both of these trials, cup feeds were given when the mother was unable to breastfeed or additional milk was required after a breastfeed. Mosley et al. (2001)124 and Gilks and Watkinson (2004)120 do not describe the technique used.
The type of milk given by cup or bottle was not specified in Collins et al. (2004). 119 In the other studies, the milk used was expressed breastmilk.
Bottle feeding
No description of this technique is given, so no information is available on the type of teat or bottle used, the feeding technique used, or staff training.
Nasogastric tube feeding
This intervention involved the use of an indwelling nasogastric tube as an alternative to bottle feeding. 130 If a tube was already in place in infants allocated to the bottle-feeding group, it was removed ‘at the clinician’s discretion’.
Pacifiers
No study examined the use of pacifiers alone; they were used only as cointerventions. No information about the type of pacifier used in either study119,122 is available. Pacifiers were used during tube feeds, or when the infant was restless. 119 It was noted that in the group that did not receive pacifiers in Rocha et al. (2002)122 ‘non-nutritive sucking was provided by offering the little finger’, which in itself is an alternative intervention to the use of pacifiers. No information is provided, however, on the use of either pacifiers or fingers for non-nutritive sucking in either group.
Compliance
Only Collins et al. (2004)119 gave details of compliance rates. They were low; 56% of infants randomised to cup feeding had a bottle introduced, and 31% of those randomised to ‘no pacifier’ had one. It was noted that the hospital with the best compliance had used cup feeding before.
Collins et al. 119 reported a total of 16 exclusions, 10 from the cup-feeding group and 6 from the bottle-feeding group as a result of the mother’s decision or infant death. Gilks and Watkinson120 reported a differential withdrawal rate between their groups: 41% of mothers withdrew from the cup-feeding group, while only 11% withdrew from the bottle-feeding group. Reasons given were that mothers no longer wished to breastfeed or infants became ill or were on medication that contraindicated breastfeeding. Kliethermes et al. (1999)130 noted that six infants withdrew from the bottle-fed group and nine from the nasogastric tube group. Mosley et al. 124 noted two exclusions from the cup-feeding group. Three infants were excluded from the cup-feeding group and two from the bottle-feeding group in Rocha et al. ;122 reasons are given.
Outcome assessment
All studies examined breastfeeding duration, although reported it in different ways. Any breastfeeding at discharge was reported by all trials, and exclusive breastfeeding at discharge was also reported by Collins et al. (2004),119 Gilks and Watkinson (2004),120 Kliethermes et al. (1999)130 and Mosley et al. (2001). 124
Gilks and Watkinson120 reported exclusive and any breastfeeding rates at the equivalent of term and 6 weeks post-term gestational ages. Kliethermes et al. 130 reported age at first breastfeeding, and exclusive and any breastfeeding rates at 3 days post discharge. Rocha et al. 122 reported any breastfeeding rates at 5–15 days post follow-up, at 3 months and after 3 months. Collins et al. 119 and Kliethermes et al. 130 reported any breastfeeding at 3 and 6 months after discharge, and Kliethermes et al. 130 also reported exclusive breastfeeding at 3 months after discharge.
A range of other outcomes were reported including: length of stay and adverse events;119 time of nasogastric tube withdrawal;120 side effects of tube feeding;130 weight gain in the first week, time spent administering milk, and oxygen saturation. 122
Psychosocial and cost outcomes were not reported.
Methodological quality of included trials
Only one trial was rated as ‘good’ quality overall. 119 Compliance rates in this trial were low, however, which is likely to affect the results of the study.
Two trials were rated as ‘moderate’ quality. 122,124 Mosley et al. (2001)124 is a very small (n = 14) pilot study.
The remaining two trials were rated as ‘poor’ quality. 120,130 Serious caution is warranted in interpretation of the results of these trials. Details of the quality ratings for each trial are provided in Table 77 in Appendix 5.
Effectiveness of interventions
Primary outcomes
Relevant data have been extracted to perform ITT analyses for four trials. 119,120,122,124 It was not possible to conduct ITT analyses on the remaining trial. 130
Individual relative risk estimates, calculated on an ITT basis, are presented in forest plots for primary outcomes in the three trials that did not receive a poor overall quality rating. 119,122,124
No trials evaluated the effect of feeding methods on the primary outcome of initiation of breastfeeding.
No trials reported primary outcomes before the point of discharge.
Three trials gave results for the outcome of any breastfeeding at discharge. 119,120,122 None found a difference between the groups. Care is needed in interpreting this finding as rates of non-compliance were very high in Collins et al. (2004),119 the only trial whose design was rated as good quality (Figure 7).
FIGURE 7.
Cup feeding vs bottle feeding: any breastfeeding at discharge (ITT).
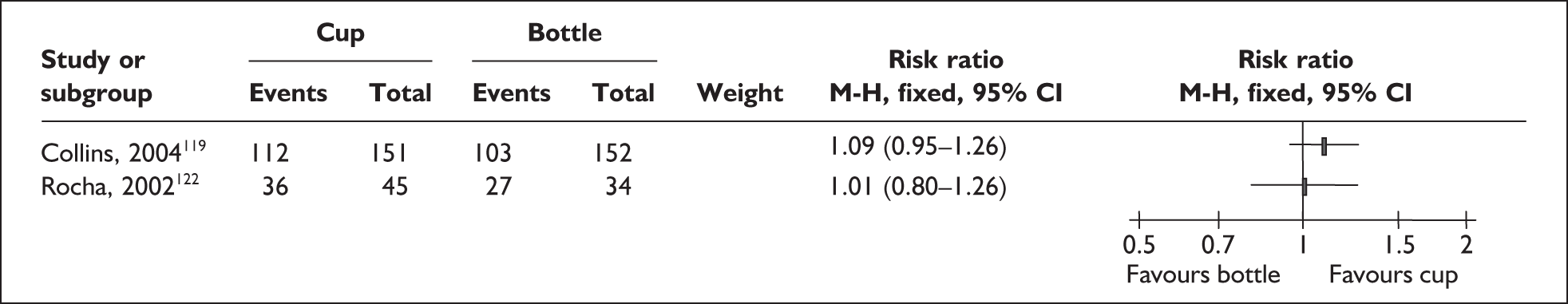
Three trials reported exclusive breastfeeding at discharge. 119,120,124 Mosley et al. 124 found no difference between the groups in their small pilot trial. Gilks and Watkinson120 found that 10/27 (37%) in the cup-feeding group were exclusively breastfeeding, versus 4/27 in the bottle-feeding group (15%). In the only good-quality trial, Collins et al. 119 found an increase in exclusive breastfeeding in the cup-feeding group [relative risk (RR) 1.29; confidence interval (CI) 1.04–1.59]. Care is needed in interpreting this finding as rates of non-compliance were very high119 (Figure 8).
FIGURE 8.
Cup feeding vs bottle feeding: exclusive breastfeeding at discharge (ITT).
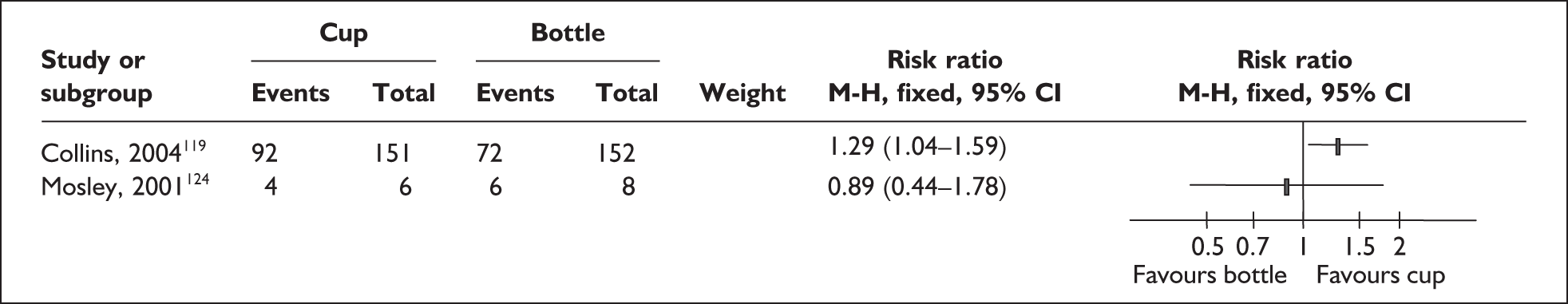
There were no differences in any breastfeeding after discharge when measured at term and 6 weeks post term,120 or at 5–15 days,122 3 months,119,122 or 6 months119 post discharge (Figures 9–11).
FIGURE 9.
Cup feeding vs bottle feeding: any breastfeeding 5–15 days after discharge (ITT).
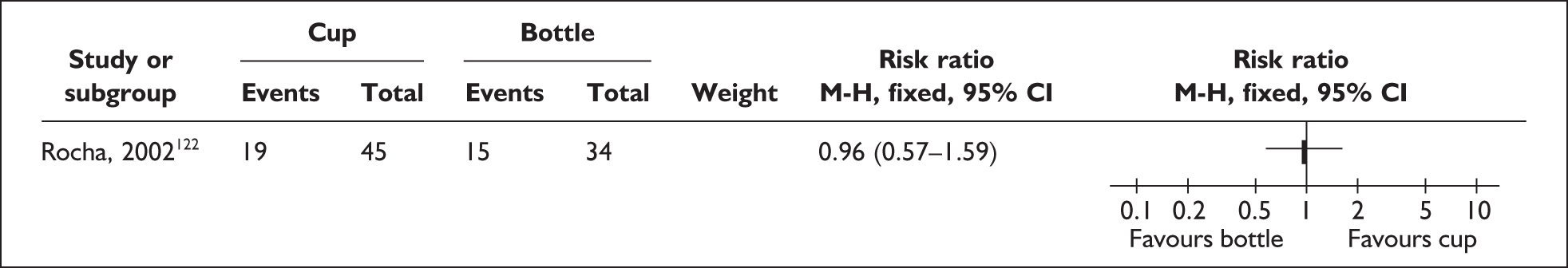
FIGURE 10.
Cup feeding vs bottle feeding: any breastfeeding 3 months after discharge (ITT).
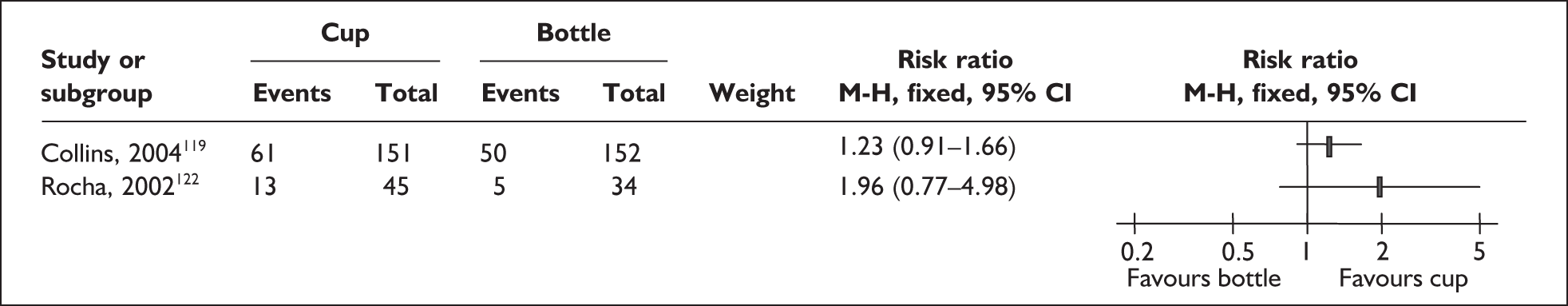
FIGURE 11.
Cup feeding vs bottle feeding: any breastfeeding at 6 months after discharge (ITT).
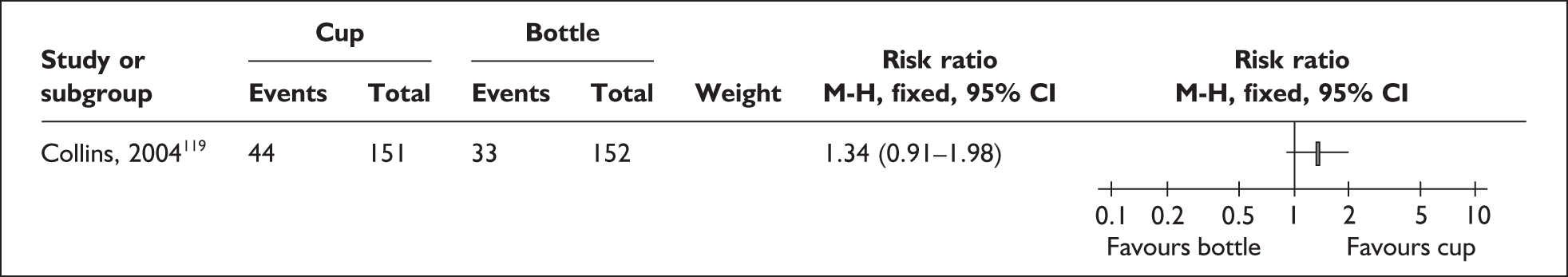
Only one trial, rated as poor quality, examined the use of nasogastric feeding as an alternative to bottle feeding. 130 It was not possible to report ITT results due to the way in which data were reported.
Two trials included pacifier use as part of the intervention,119,122 but results are only reported separately from feeding method by Collins et al. 119
No differences are reported in any or exclusive breastfeeding at discharge, or any breastfeeding at 3 and 6 months after discharge (Figures 12–15). Compliance was an important issue to consider; 31% of infants randomised to the ‘no pacifier’ group received one.
FIGURE 12.
Pacifier use: fully breastfeeding at discharge (ITT).
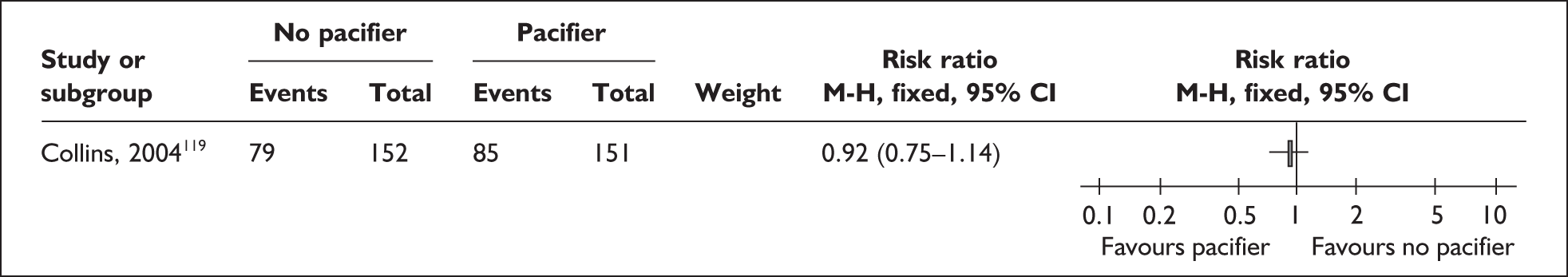
FIGURE 13.
Pacifier use: any breastfeeding at discharge (ITT).
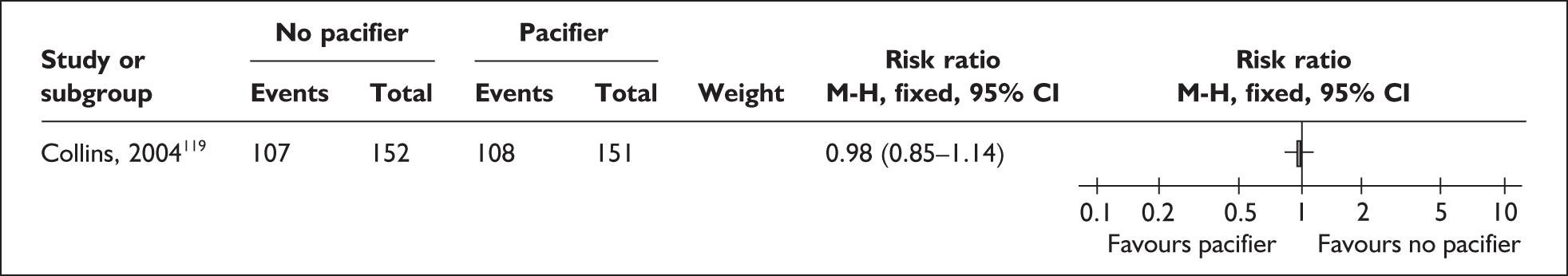
FIGURE 14.
Pacifier use: any breastfeeding at 3 months after discharge (ITT).
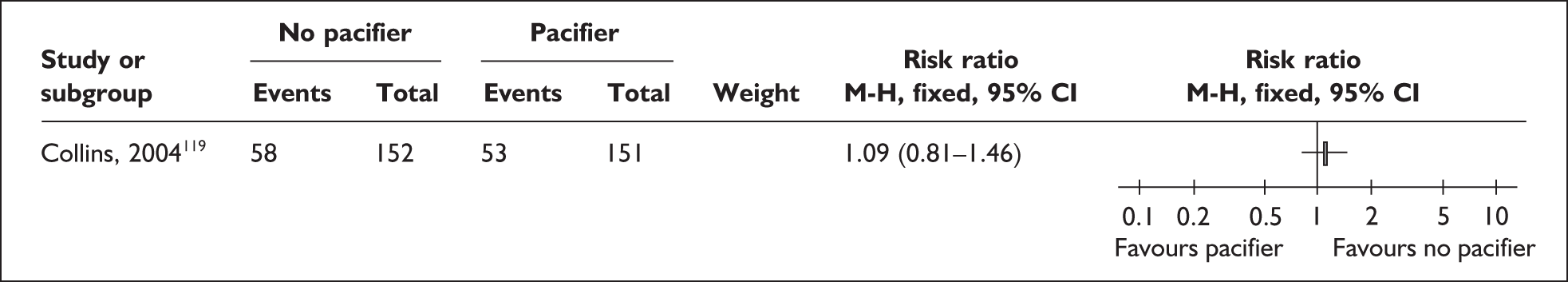
FIGURE 15.
Pacifier use: any breastfeeding at 6 months after discharge (ITT).
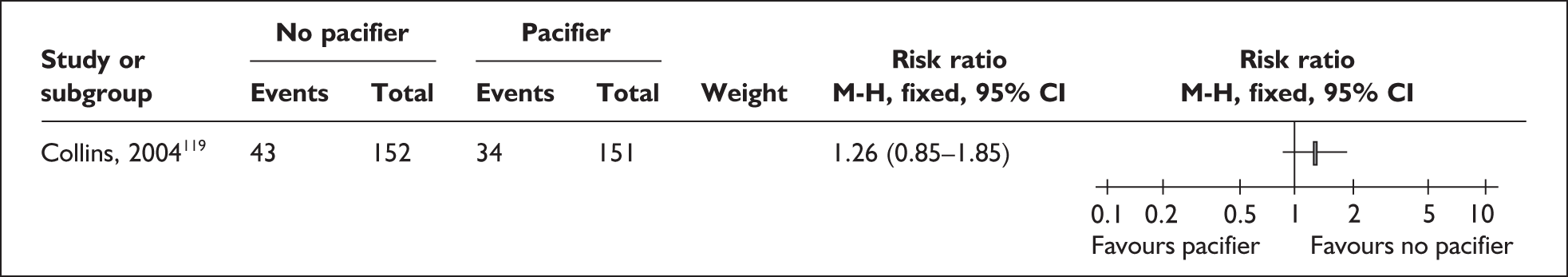
Secondary outcomes
Collins et al. (2004)119 reported that length of stay was significantly increased in infants who were cup fed (p = 0.01). This finding was confounded both by the fact that infants in the cup-feeding group took longer to satisfy the hospital criterion for discharge of taking all sucking feeds, and by the poor compliance rates. It was noted that the hospital with the best compliance had used cup feeding before. Some staff had strong feelings against cup feeding and the withholding of pacifiers and some parents did not like cup feeding.
No differences were reported in mean weight gain and time administering milk between the two groups in Rocha et al. (2002). 122 These authors did report a difference in severe oxygen desaturation (defined as < 85% during feeding: 35.3% in the bottle-fed group versus 13.6% in the cup-fed group, p = 0.02). Gilks and Watkinson (2004)120 reported no difference in time to withdrawal of the nasogastric tube, and Collins et al. (2004)119 reported no difference in occurrence of adverse events including incidence of NEC.
No difference in length of stay was found related to use of pacifiers,119 but poor compliance remains an important issue.
Results from other study types
A study was identified concerning the use of ultra-thin silicone nipple shields for mother–infant pairs experiencing problems with breastfeeding, using a crossover design with retrospective analysis of data136 (Table 43 in Appendix 4.1).
Characteristics of participants
Participants were mothers of preterm infants and their infants, already recruited to two trials of breastfeeding interventions. The 34 mother–infant pairs (14 infants were multiples, hence there were more infants than mothers) in the experimental groups in the trials, who experienced problems with attachment, sleepy infant and nipple pain, were included in this study. Mean birthweight (SD) was 1702 (521) g, mean weight at first breastfeeding (SD) was 1782 (403) g, gestational age at birth (SD) was 31.9 (3.0) weeks, and 70% were white, non-Hispanic ethnicity. No information is provided for breastfeeding rates in the population.
Characteristics of intervention
Mother and infant pairs with attachment problems (e.g. infant slipping off the breast) were given small, ultra-thin nipple shields by advanced nurse practitioners who continued to support the mothers. The feed immediately before the nipple shield was first introduced was used as the comparison for the first feed with the shield, hence mothers acted as their own controls. Mothers used the shields for a mean of 32.5 days. No information is reported about transfer from use of the shield to feeding without it.
There is no indication of any non-compliance and no indication of any exclusions.
Outcome assessment
The primary outcome was milk transfer, measured by test weighing of the infant before and after feeds. Duration of breastfeeding for the whole group (all of whom went on to use the shields) was measured. No longer-term milk transfer, clinical/health outcomes, process, psychosocial or cost outcomes are reported.
Methodological quality of included study
The quality of this crossover study was rated as ‘moderate’ (Table 78 in Appendix 5).
Effectiveness of intervention
Primary outcomes
The results136 indicate an increase in milk transfer with the use of the shield. Mean milk transfer (SD) with the shield was increased by 14.4 (9.1) ml, t = 9.25, p = 0.0001, paired Student’s t-test. The range was 2–41 ml and the SD is large, indicating a varied response to its use. It is reported that all infants consumed more milk in the feed with the shield than without. No information on breastfeeding outcomes is available.
Secondary outcomes
No secondary outcomes are reported.
Expressing breastmilk interventions
A total of six primary studies112,114,125,128,134,142 evaluating methods of expressing breastmilk were identified. As detailed in Table 5, four of the six were included in one systematic review. Four of the six studies were conducted in industrialised country settings, including two in the UK.
| Primary paper | Study design (n analysed)a | Inclusion in existing systematic review | Country |
|---|---|---|---|
| Fewtrell 2001125 | RCT (n = 167) (infants) | McInnes 2006161 | UK |
| Groh-Wargo 1995128 | RCT (n = 32) | McInnes 2006161 | USA |
| bHill 1999b 112 | RCT (n = 39 | No | USA |
| Jones 2001114 | RCT(n = 36) | McInnes 2006161 | UK |
| Paul 1996134 | Randomised crossover trial (two phases: n = 22, n = 14) | McInnes 2006161 | India |
| Slusher 2007142 | RCT (n = 65) | No | Kenya and Nigeria |
Results from randomised controlled trials and randomised crossover studies
Five RCTs112,114,125,128,142 and one randomised crossover study134 of methods of expressing breastmilk interventions were identified (see Tables 44–49 in Appendix 4.1).
Two of the six studies115,125 are linked with studies of additional interventions to enhance breastmilk production that appear in the next section of this review (see Additional interventions to enhance breastmilk production). Data from Fewtrell et al. (2001)125 are used in a later oxytocin trial144 for comparison purposes, and Jones et al. (2001)115 report both a breast pumping RCT and a randomised crossover trial of breast massage prior to pumping.
Characteristics of participants
Four of the six included trials were performed in industrialised country settings, two in the UK115,125 and two in the USA. 112,128 The remaining two trials took place in India134 and Kenya and Nigeria. 142
All trials recruited mothers providing expressed breastmilk for their infants hospitalised in neonatal units. None aimed to recruit mothers of a particular age, socioeconomic status or parity. The mothers included in three of the trials had a mean age in the late twenties112,125,128 and in a fourth trial the mothers from Kenya and Nigeria had a mean age in the mid-twenties. Age of the mothers was not reported for the other two trials. 115,134 On the basis of education, income, marital status and race details reported, the participants in the US studies112,128 and one of the UK studies125 were of mixed and not predominantly high or low socioeconomic status; these details are not reported for the other three studies. Around 60% of participants in three studies were multiparous,114,125,142 with smaller proportions of multiparous mothers in the two US studies112,128 and parity not reported in one study. 134 Previous preterm births were not reported in any of the six studies.
In the four studies from industrialised countries,112,114,125,128 20–40% of participants had previous breastfeeding experience, with 15% of participants in one of these studies125 having previous experience of expressing breastmilk. The studies from India134 and Kenya and Nigeria142 did not report breastfeeding or pumping experience. The percentage of mothers who had had multiple births ranged from 14% to 23% in three studies112,114,125; multiple births were not reported for the other three studies. With regard to providing breastmilk for their infants, participants in the two US studies had to be either expecting to provide breastmilk exclusively by mechanical expression (electric pump) for at least 6 weeks,112 or to have pumped for 4 weeks. 128 Mothers in the two UK studies had to have decided to provide milk for their infant125 or to be expressing at least five times a day before study entry. 114 The studies from India134 and Kenya and Nigeria142 specified only that participants had to be mothers of infants unable to breastfeed.
In all six studies, recruitment took place during the infant’s first week of life. The two US trials recruited only mothers of very low birthweight (VLBW) infants (≤ 1500 g). 112,128 The infants of mothers included in one UK study125 had a mean (SD) birthweight of 1357 (540) g in the intervention group and 1305 (565) g in the control group; in the other UK study114 mean birthweight was 1535 g (SD not reported). Mean gestational age of infants included in three of the four studies carried out in industrialised country settings was 28–30 weeks;112,114,125 gestational age was not reported for the fourth of these studies. 128 Birthweight and gestational age of infants in the studies from India and Kenya and Nigeria differed from those in the US and UK studies. The study from India134 included infants whose mean gestational age was 34 weeks (range 27–40 weeks, birthweight not reported) and the study from Kenya and Nigeria142 included infants with mean gestational age of 32 weeks (range 26–40 weeks) whose mean birthweight was 1709 g (range 907–4600 g). Three study reports state that no infant was breastfeeding. 112,134,142 One study report states that mothers left the study when their infant was ‘breastfeeding freely’; attempts to breastfeed before this point are not mentioned. 128 In one study, 70% of mothers attempted to breastfeed;125 results are reported separately for this subgroup of participants. One study report does not state whether or not infants were breastfeeding. 114
Characteristics of interventions
Interventions to assist mothers with breastmilk expression include both techniques/equipment and regimens for expressing breastmilk.
Techniques/equipment for expressing breastmilk
Hand expression was evaluated in the studies from India134 and Kenya and Nigeria,142 but techniques of hand expression were not described.
Hand-operated pumps were evaluated in two studies125,134 and a foot-operated pump in one study. 142 One of these studies acknowledges funding from the manufacturer;125 in the other two, funding sources are not reported. The hand-operated pump in the study by Fewtrell et al. 125 (Philips Avent ISIS, Philips, UK) was newly designed with petal cushions to simulate the infant’s compressive action on the areola during breastfeeding rather than simply operating by suction. The hand-operated pump in the study by Paul et al. 134 in India was a Medela cylindric pump made of plastic material, with a piston mechanism which, by in-and-out motion with one hand, produced suction at the breast cup as applied around the areola; the vacuum-sucked breastmilk then flowed into a receptacle-cum-bottle. A recent systematic review161 states this type of pump is not currently in use in the UK. The study in Kenya and Nigeria by Slusher et al. 142 tested a double collection non-electric pedal pump.
Five of the six studies112,114,125,128,142 evaluated electrically powered pumps. One of these studies acknowledges partial funding from the pump manufacturer;128 four acknowledge funding from other sources (research grant;128 university;112 a charity;114 the hand-pump manufacturer125) and funding sources are not reported for one study. 142 The two UK studies114,125 both used Egnell Ameda pumps (Egnell Ameda, Taunton, Somerset, UK). Fewtrell et al. 125 described the Egnell Ameda pump in their study as large, operating by suction, regarded as the gold standard and used in 94% of UK neonatal units. Jones et al. 114 state that the Egnell Ameda Electric Elite™ pump was used in their study because it created periodic and limited phases of negative pressure, and converted easily to simultaneous pumping mode. Jones et al. 114 selectively provided silastic shield inserts so that shield size and breast size could be matched. The two US studies and the study performed in Kenya and Nigeria used Medela pumps (Medela, Inc., McHenry, IL, USA). The pumps in the study by Groh-Wargo et al. (1995)128 are described as the Medela single electric pump and the Medela bilateral electric breast pump; those in Hill et al. 112 as Medela Lactina™; and that in Slusher et al. 142 as a double-collection Lactina electric breast pump.
The techniques and equipment used for expressing breastmilk in the six studies, and the mode of operation of the equipment, are summarised in Table 6.
| Study | What was used to express breastmilk?a | How did this technique/equipment work? |
|---|---|---|
| Paul 1996134 | Hand expression | Not described |
| Hand-operated pump (Medela cylindric) | Suction, vacuum | |
| Slusher 2007142 | Hand expression | Not described |
| Foot-operated pump (double-collection, non-electric) | Not described | |
| Electric pump (Double-collection Lactina) | Suctionb | |
| Fewtrell 2001125 | Hand-operated pump (ISIS) | Not simply by suction: had ‘petals’ to simulate the infant’s compressive action on the areola |
| Electric pump (Egnell Ameda) | Suction | |
| cJones 2001114 | Electric pump (Egnell Ameda) | Suction, negative pressure |
| Groh-Wargo 1995128 | Electric pump (Medela single, Medela bilateral) | Suctionb |
| Hill 1999b112 | Electric pump (Medela Lactina) | Suctionb |
Regimens for expressing breastmilk
Three of the six studies specifically compared single/unilateral/sequential pumping with double/bilateral/simultaneous pumping112,114,128 and this was a factor in another two of the studies. 125,142 Double pumping was not possible in the sixth study. 134 Comparisons for the six studies were as follows:
-
novel manual breast pump (Avent ISIS) (single mode only) versus standard electric breast pump (Egnell Ameda) in single or double mode according to the mother’s preference125
-
Egnell Ameda Electric Elite (with silastic shield inserts as appropriate) in sequential (single) mode versus the same pump in simultaneous (double) mode114 (this study also tested massage versus no massage before both modes of pumping in a crossover design – see Additional interventions to enhance breastmilk production)
-
Medela double pump versus Medela single pump128
-
Medela Lactina double pump versus Medela Lactina single pump112
-
double-collection Lactina electric breast pump versus double-collection non-electric pedal pump versus hand expression142
-
hand-operated Medela cylindric pump versus hand expression. 134
Protocols of all six trials prescribed how often mothers should express breastmilk. In descending order of frequency these were as follows: 2–3 hourly;142 eight sessions per day;112,114 six or more sessions per day;125 3-hourly except at night, with four or more sessions per day;128 and three per day at 10.00, 12.00 and 14.00. 134 In the study by Paul et al. 134 the three daily sessions were the ones at which study data were collected and it is not clear whether these were or were not the only expression sessions.
Protocols of all six trials prescribed the duration of the milk expression sessions. Two wanted mothers to express for given durations; 15 minutes134 and initially 5 minutes per breast then increasing time per breast. 125 Two trials wanted mothers to express until milk flow ceased: ‘until milk droplets cease flowing’142 or ‘until milk no longer appears in collection set’. 115 One trial combined these approaches,112 asking mothers to express for ‘a minimum of ten minutes per breast or until breast no longer dripping’ and one protocol changed during the study from 20 minutes to ‘as long as there is any flow of milk’. 128 None of the six trials prescribed an amount of milk to be expressed.
Studies varied in how long expression continued. Three studies were completed during the second week after the birth; two of these114,134 took place over two 48-hour periods and were completed by postnatal day 11, and in the third142 all mothers had completed the study by postnatal day 13. In contrast, the mothers in the study by Fewtrell et al. (2001)125 participated for a median of 15 days with a wide range; the criteria for ending study participation were if and when the mother stopped using the assigned pump, stopped filling in data forms, or the infant was fully breastfed, transferred, discharged or died. Mothers in the the two US studies112,128 contributed data for at least 4 weeks, until 4–7 weeks after giving birth.
Outcome assessment
Primary outcomes
All six trials reported breastmilk production, by volume125,128,134,142 or weight. 112,114 Two trials reported breastmilk feeding/breastfeeding, either at discharge125 or at term. 114 No other primary outcomes for this review were reported by intervention group, although some were presented in subgroup analyses. 112,125
Secondary outcomes
Two of the six trials reported clinical/health outcomes. Fewtrell et al. (2001)125 reported clinical outcomes both for infants (NEC, oxygen supplementation and ventilation) and for mothers (sore nipples, engorgement, mastitis and use of metoclopramide) and Groh-Wargo et al. (1995)128 reported serum prolactin. The other four trials did not report clinical/health outcomes. 112,114,134,142
Three of the six trials reported pumping frequency112,125,128 and two of the three125,128 also reported the time that mothers spent pumping. Jones et al. (2001)114 mention time spent pumping. The other two trials134,142 did not report process outcomes.
Mothers’ views of the methods of milk expression used in trials were reported for three studies. 114,125,134 Psychosocial outcomes were not reported for the other three studies. 112,128,142
None of the six trials reported cost-effectiveness outcomes.
Methodological quality of included trials
One trial was rated as ‘good’ quality overall. 125 Three trials were rated as ‘moderate’ quality. 114,128,142
The remaining two trials were considered to be ‘poor’ quality. 112,134 Serious caution is warranted in interpretation of the results of these trials. Details of the quality ratings for each trial are provided in Table 79 in Appendix 5.
Effectiveness of interventions
Primary outcomes
For maternal outcomes of four studies 112,114,125,142 estimations of effectiveness could not be calculated on an ITT/postrandomisation exclusions basis.
All six trials reported breastmilk production, by volume125,128,134,142 or weight. 112,114 The breastmilk production primary outcome was reported for all six studies using continuous data. To present individual estimated relative risks in forest plots we therefore needed to extract the number in each treatment group with the mean and standard deviation of the outcome. These numbers were reported for only one of the six studies;128 the other five did not report standard deviations. Breastmilk feeding was reported for two studies114,125 using categorical data. It was possible to work out relevant numbers from one study report125 but not from the other. 114
These results do not show any statistically significant difference in the weekly breastmilk volumes expressed by 16 mothers randomised to simultaneous (double) pumping (Medela) compared with 16 mothers randomised to sequential (single) pumping (Medela)128(Figure 16).
FIGURE 16.
Double vs single electric pumping: breastmilk production (ml/week, up to 6 weeks after the birth).
Two of the five remaining studies112,125 reported no significant differences between the groups for their breastmilk production outcomes. The other three115,134,142 reported significant differences in their breastmilk production outcomes. In Jones et al. (2001)115 (no time restrictions on pumping) weight of milk from a single expression (g) (95% CI) was 51 (46–56) without massage and 79 (73–85) with massage in the sequential pumping group versus 88 (79–97) without massage and 125 (110–140) with massage in the simultaneous pumping group (p < 0.01). Paul et al. (1996)134 reported a greater mean output per session (ml) (SD) in the hand-powered pump group than in the hand expression group both on days 4 and 5 [46.8 (26.3) versus 31.2 (15.5); t = 3.29 (Student’s t-test); p < 0.01] and on days 8 and 9 [50.4 (13.4) versus 38.4 (11.2); t = 4.42 (Student’s t-test); p < 0.01]. Slusher et al. (2007)142 reported mean (range) breastmilk volumes (ml/day) of 578 (135–1051) in the electric double-pump group versus 463 (85–1315) in the double-collection pedal pump group versus 323 (93–812) in the hand expression group (significantly different only between the electric double-pump group and the hand expression group, p < 0.01).
The numbers reported here (Figures 17 to 20) are based on all the infants of randomised mothers. The unit of allocation in this study125 was mothers, and the paper did not report any postrandomisation exclusions of infants. The study authors make the point that twins and triplets would be expected to reduce the proportion of infants receiving breastmilk, and to some extent this is shown above. However, none of these results show any statistically significant differences in breastmilk intake at discharge or transfer between the infants of mothers randomised to the Avent ISIS hand-powered pump and the infants of mothers randomised to the Egnell Ameda electrically powered pump (single or double pumping according to mothers’ preference), whether all infants or only singleton infants are included in the analysis.
FIGURE 17.
Hand pump vs electric pump: any breastmilk feedings at discharge or transfer (all infants).
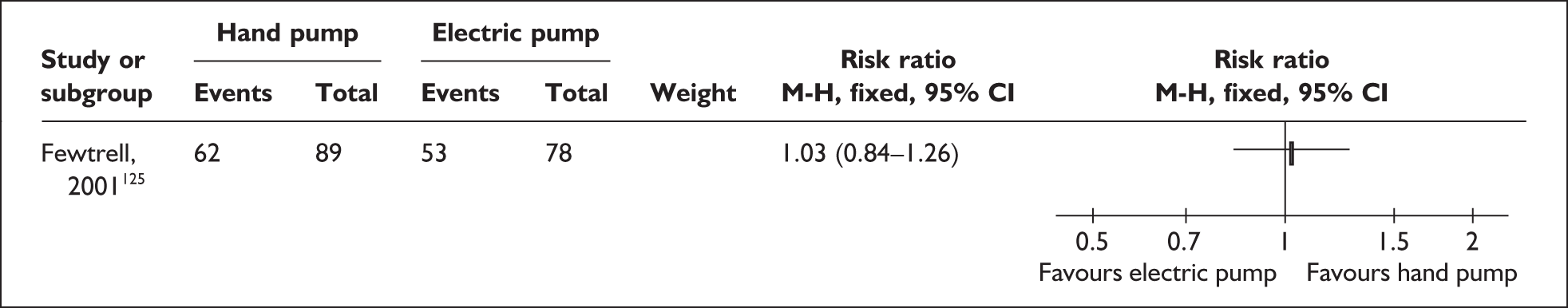
FIGURE 18.
Hand pump vs electric pump: any breastmilk feedings at discharge or transfer (singletons).
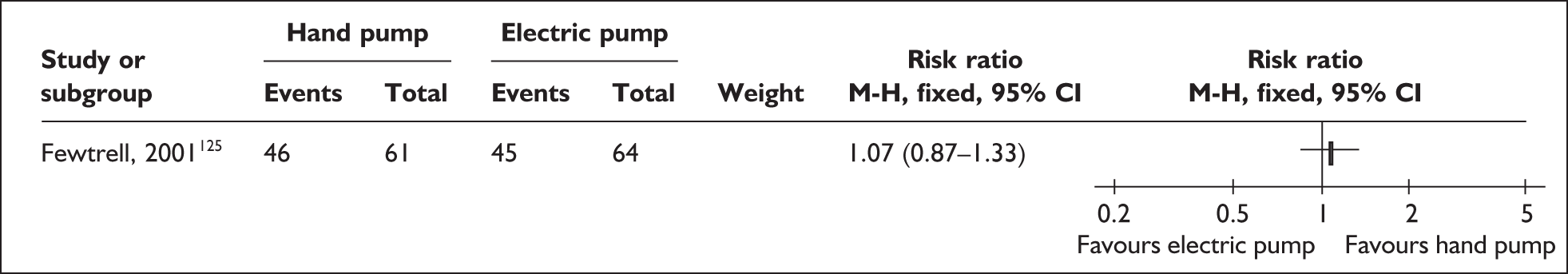
FIGURE 19.
Hand pump vs electric pump: > 50% intake as breastmilk at discharge or transfer (all infants).
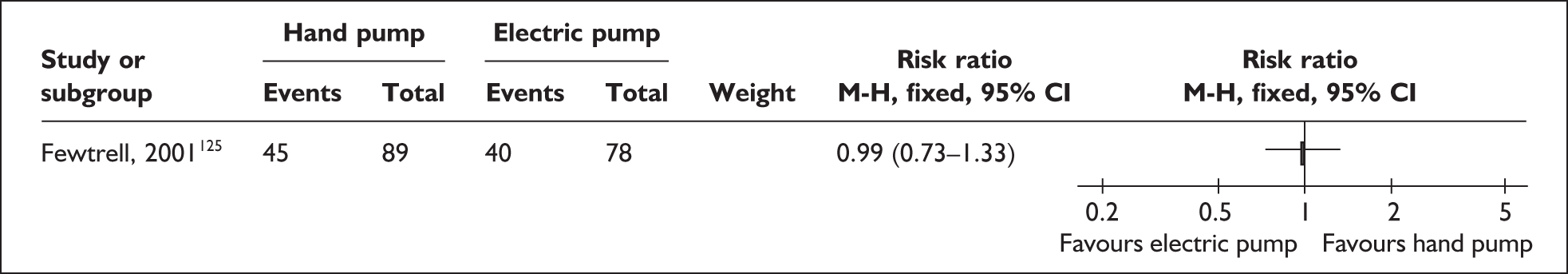
FIGURE 20.
Hand pump vs electric pump > 50% intake as breastmilk at discharge or transfer (singletons).
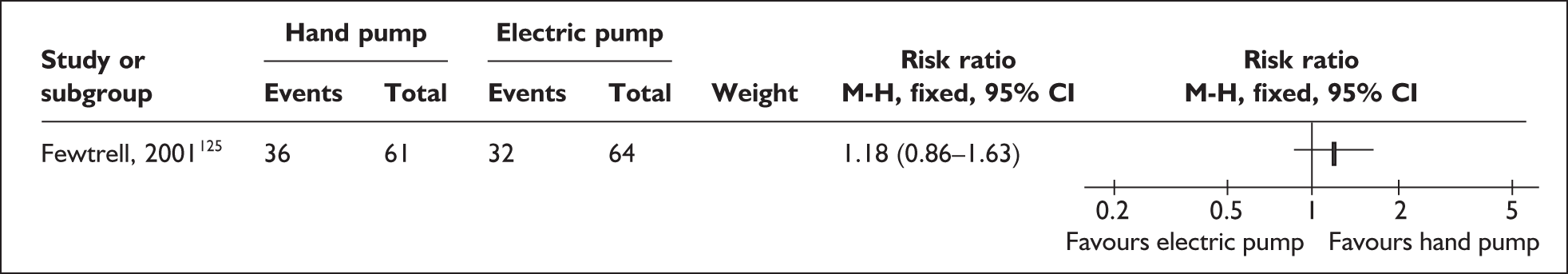
Secondary outcomes
Fewtrell et al. 125 found no significant differences between the groups for infants with NEC, oxygen supplementation or ventilation, and similar proportions of mothers from each group with sore nipples, engorgement and use of metoclopramide. Two mothers in the electric pump group developed mastitis. Fewtrell et al. 125 reported other psychosocial measurements during two days in the second postnatal week from a subsample of 45 mothers (31%). Groh-Wargo et al. 128 found no difference between groups in serum prolactin. Clinical/health outcomes were not reported for four studies. 112,114,134,142
Process outcomes were not reported in two of the six included trials. 134,142 Three trials reported that pumping took place less frequently than specified in study protocols. 112,125,128 Two of the three reported the time mothers spent pumping. Fewtrell et al. 125 found mothers randomised to the electric pump spent significantly less time per day expressing (median 51 minutes, 25th/75th centiles 38, 63) than mothers randomised to the manual pump (median 65 minutes, 25th/75th centiles 56, 85; p < 0.001). Electric pump users could express either simultaneously or sequentially, whereas manual pump users could only express sequentially. In a subgroup of 45 mothers (31%), however, Fewtrell et al. 125 found that mothers who used the manual pump took significantly less time to express a given volume of milk than mothers who were using the electric pump. In this subgroup Fewtrell et al. 125 also found that mothers who attempted to breastfeed had significantly higher total number of expressions and total volume expressed than mothers who did not attempt to breastfeed (both p < 0.001). Groh-Wargo et al. 128 found that 16 mothers allocated to sequential pumping spent a mean (SD) 11.1 (3.1)] hours per week pumping, compared with 7.6 (3.0) hours per week for 16 mothers allocated to simultaneous pumping (p = 0.003). In addition, Jones et al. 114 report that mothers allocated to simultaneous pumping took about 10–15 minutes per session compared with 25–40 minutes for women allocated to sequential pumping.
Mothers’ views of the methods of milk expression used were reported for three studies. Fewtrell et al. 125 reported that mothers gave the manual pump better ratings overall and for ‘comfort’ and ‘pleasant to use’ than the electric pump. Mothers in the study of Jones et al. 114 appreciated the time saved by simultaneous pumping and pointed out the need for pumps to have larger collection sets. Paul et al. 134 found that on days 4–5 mothers preferred the cylindric pump because it provided relief from engorgement; however, on days 8–9 they preferred hand expression because it was more gentle and convenient. Psychosocial outcomes were not reported for the other three studies. 112,128,142
Fewtrell et al. 125 reported that the hand-powered pump in their study cost ‘tens of pounds’ compared with ‘hundreds of pounds’ for the same study’s large electric pump. None of the six trials reported cost-effectiveness outcomes.
Results from other study designs
No studies of methods of expressing breastmilk using designs other than randomised trials were identified.
Additional interventions to enhance breastmilk production
A total of seven primary studies and two systematic reviews evaluating additional interventions to enhance milk production were identified. As detailed in Table 7, three primary studies were included in at least one of the two systematic reviews. All seven studies were conducted in industrialised country settings including two in the UK. 114,144
| Primary paper | Study design (n analysed)a | Inclusion in existing systematic review | Country |
|---|---|---|---|
| da Silva 2001123 | RCT (n = 16) |
Edmond 2006155 McInnes 2006161 |
Canada |
| Feher 1989146 | RCT (n = 55) | No | USA |
| Fewtrell 2006144 | RCT (n = 51) | No | UK |
| Gunn 1996133 | RCT (n = 18) | McInnes 2006161 | New Zealand |
| Hansen 2005116 | RCT (n = 57) |
Edmond 2006155 McInnes 2006161 |
USA |
| bJones 2001114 | Randomised crossover study (n = 36) | No | UK |
| Mersmann 1993148 | Randomised crossover study (n = 18) | No | USA |
Results from RCTs and randomised crossover studies
Seven studies of additional interventions to enhance milk production were identified; five were RCTs116,123,133,144,146 and two were randomised crossover studies114,148 (Tables 50–56 in Appendix 4.1).
Two of the seven studies114,144 are linked with studies of expressing milk interventions that appear in the previous section (see Expressing breastmilk interventions). Fewtrell et al. (2006)144 use data from the earlier breast pump study125 for comparison purposes, and Jones et al. 114 report both a breast pumping RCT and a randomised crossover study of breast massage prior to pumping.
Characteristics of participants
All seven studies were conducted in industrialised country settings including two in the UK,114,144 three in the USA,116,146,148 one in Canada123 and one in New Zealand. 132
All the studies included mothers who had given birth to preterm infants being cared for in neonatal units. None aimed to recruit mothers of a particular age, socioeconomic status or parity. The mothers included in three studies had a mean or median age in the twenties;116,123,146 in three studies the mean age of the mothers was in the thirties;132,144,148 and in one study age of the mothers was not reported. 114 Participants in three studies appeared to be of above average socioeconomic status,116,144,148 with fewer of these details reported by an older study from the USA146 and none in three study reports. 114,123,133 In one study133 3/18 participants (17%) were multiparous; the other six studies included higher proportions of multiparous mothers,114,116,123,144,146,148 with one of these116 reporting 21/60 participants (35%) having had a previous preterm birth. Forty-two percent of the mothers in one study (Jones et al., 2001),114 33% in another148 and 20% in a further two studies123,144 had previous breastfeeding experience. Two studies144,148 reported that around 18% of mothers had previous pumping experience. Only mothers who were expressing at least five times per day were enrolled into the study of Jones et al. ;114 none of the other studies report a minimum expression inclusion criterion. In five studies, around 18% of mothers had just had multiple births;114,116,123,144,148 the other two studies did not report multiple births.
Four of the seven studies reported birthweight. Mean birthweight was < 1500 g (VLBW) in two studies133,144 (standard deviations indicate wide ranges); in one study114 mean birthweight was 1535 g (standard deviation not reported) and in another,148 median birthweight was 1533 g and all but 2/21 infants were VLBW (n = 10) or LBW (n = 9). Three studies did not report birthweight. 116,123,146 Mean or median gestational age in six of the seven studies was between 28 and 30 weeks; in the seventh148 median gestational age was 32 weeks. In two studies116,144 randomisation was stratified by gestational age, and one study148 grouped infant gestational ages (< 30 weeks, 30–34 weeks and > 34 weeks). Two other studies reported mean gestational age with standard deviations,123,133 and in two study reports114,146 there was no indication of the range of gestational age of the infants.
Infant feeding at the breast is an important participant factor related to enhancing milk production. Two of the seven studies report that no infant was breastfeeding123,148 and two116,133 report that some infants were breastfeeding. One of these133 included older infants and calculated the amount of milk taken by weighing the infant before and after breastfeeds. The other116 included only recently born preterm infants and no measures of milk intake are reported. The three studies that did not report breastfeeding all reported short-term interventions with recently born preterm infants. 114,144,146
Characteristics of interventions
Of the seven studies, four RCTs116,123,133,144 investigated galactagogues. One RCT146 and two randomised crossover studies114,148 investigated other interventions to enhance milk production; a relaxation/imagery tape;146 breast massage prior to expression114 and therapeutic touch (TT). 148
Galactagogues
Four different galactagogues were each evaluated in one double-blind, placebo-controlled RCT. Two of these116,144 recruited mothers who had recently given birth to their preterm infants. The intervention in the study by Fewtrell et al. 144 was a five-day course of oxytocin nasal spray. Participants in this study also received extra support from the research nurse, who saw each mother daily and was available by telephone. This study was funded by a Medical Research Council (MRC) programme grant. The intervention in the study by Hansen et al. 116 was a 10-day course of oral metoclopramide 10 mg three times per day. This study was jointly supported by grants from the National Institutes of Health and Children’s Miracle Network. The other two galactagogue studies123,133 recruited mothers who had been expressing for varying lengths of time (mean length of time after the birth that the intervention started was over a month in both studies) and whose milk production was not meeting their infant’s needs. The intervention in the study by da Silva et al. 123 was a 7-day course of oral domperidone 10 mg three times per day, and the intervention adopted by Gunn et al. 133 was a 7-day course of recombinant human growth hormone (hGH) 0.2 IU/kg/day subcutaneously to a maximum of 16 IU/day. This latter study was jointly supported by the Health Research Council of New Zealand and Pharmacia AB, Stockholm, Sweden; the study by da Silva et al. 123 was funded by a grant from the Research and Education Foundation of the Canadian Society of Hospital Pharmacists.
Other interventions to enhance milk supply
A 20-minute audio cassette of relaxation/imagery techniques was evaluated in a RCT. 146 The tape consisted of a progressive relaxation exercise followed by a guided imagery section. Mothers were recommended to use the tape every time they wanted to express milk. This study was partially funded by a grant from the National Institutes of Health.
Two related interventions, a RCT of simultaneous versus sequential pumping (see Expressing breastmilk interventions) and a randomised crossover study of breast massage versus no breast massage prior to pumping, were reported in one paper. 114 Breast massage was carried out by the mother and consisted of gentle tactile stimulation of mammary and nipple tissue using a hand action that rolled the knuckles downwards over the breast, beginning at the ribs and working towards the areola. Breast massage did not involve manual expression of milk. This study by Jones et al. 114 took place over four days and all mothers included in the analyses had begun by day 7. The latter study was funded by Baby Lifeline.
Mersmann148 based her randomised crossover study on an association between relaxation and letdown, and literature on Therapeutic Touch (TT) that suggests TT decreases anxiety and impacts other physiological manifestations of relaxation. The TT group received therapeutic touch, which consisted of hand movements 2–4 inches above the whole of the mother’s body, with certain therapeutic awareness and intentionality on the part of the nurse. In the mimic therapeutic touch (MTT) group, hand movements were indistinguishable from those of TT, but MTT nurses counted instead of exercising the awareness and intentionality required in TT. A control group had neither treatment (NT). Mothers were required to have been expressing for at least 2 weeks, and at study entry half (9/18) of the included mothers had been expressing for 14–20 days, two (11%) for 21–27 days, three (17%) for 4–5 weeks and four (22%) for 6 weeks or more. 148 Funding for this study is not reported.
Standard care
Four of the seven studies were undertaken with mothers of recently born preterm infants, and in three of these,114,116,146 all the mothers appear at least to have received verbal and written instructions on how to use the electric breast pump, and had access to a pump. In addition, the UK unit in which one study114 was undertaken had an active breastfeeding policy and mothers had opportunity to view a video made by the researchers covering milk expression and preterm breastfeeding. In the fourth study with mothers of recently born preterm infants,144 undertaken in another UK unit, mothers were advised to express milk at least every 3 hours and instructed in the use of hand massage before pumping, with advice being provided by neonatal unit and postnatal ward staff.
In one study unit in the USA,148 the mothers had access to breastfeeding pamphlets and an electric breast pump that were stored in a newly built breastfeeding cubicle. This had an opaque, approximately 1.8 metre glass wall, offering mothers some degree of privacy for expression and breastfeeding. However, at the time of the study mothers of preterm infants who were not breastfeeding were neither encouraged to express nor discouraged from expressing milk; infants did not initiate breastfeeding until they were successful at bottle feeding; lactation educators were available on request but generally assisted with breastfeeding; and kangaroo care was not practised. In the study unit in Canada where da Silva et al. 123 performed the domperidone study, extensive teaching by lactation consultants for all women failing lactation was provided and only mothers continuing to have problems after this teaching were recruited to the study. Management before the point of lactation failure was not reported. Gunn et al. 133 did not describe standard care but stated that in the New Zealand unit where the hGH study was performed there was a well-established support system provided by hospital staff, and 90% of mothers were discharged breastfeeding, similar to the wider New Zealand data for preterm and term infants.
Outcome assessment
Primary outcomes
All seven studies reported the amount of milk expressed, either by volume116,123,133,146,148 or, in the two UK studies,114,144 by weight. Statistical challenges of reporting amounts of milk are discussed in two of the papers144,148 and addressed differently in different studies. Fat content of milk expressed was reported for the whole study sample by three studies114,146,148 and for a subgroup of participants by Fewtrell et al. 144 Breastfeeding at term (37 weeks) was reported by Jones et al. ,114 and Hansen et al. 116 reported median duration of breastfeeding.
Secondary outcomes
The four galactagogue studies116,123,133,144 discussed safety, and three reported drug/hormone levels. 116,123,133 In addition, one of these studies reported reasons mothers gave for stopping expressing. 116 Four studies reported rates of breastmilk expression114,133,144,148 and four reported mothers’ views. 114,144,146,148 None of the studies reported cost-effectiveness outcomes.
Methodological quality of included trials
One RCT was rated as ‘good’ quality overall. 144 Four RCTs116,123,133,146 and two randomised crossover studies114,148 were rated as ‘moderate’ quality (see Table 80 in Appendix 5).
Effectiveness of interventions
Primary outcomes
One double-blind, placebo-controlled RCT of oxytocin spray for mothers of recently born preterm infants144 found no significant difference between the groups in total milk weight over days 1–5. Milk production was significantly higher in the intervention group on day 2 only; results then converged. Individual plots presented for each mother on each day show the variability between mothers in both groups. In a subgroup analysis of fat content of milk expressed, no significant differences between the groups were found.
One double-blind, placebo-controlled RCT of metoclopramide for mothers of recently born preterm infants116 found no significant difference between the groups on any study day.
One double-blind, placebo-controlled RCT of domperidone that included mothers of older preterm infants123 compared the difference between mean milk volume at baseline and mean milk volume over study days 2–7 for seven mothers in the intervention group and nine in the placebo group. Mean volume increased significantly more in the intervention group. The sample was small and the standard deviations and confidence interval wide, as illustrated in the forest plot shown in Figure 21.
FIGURE 21.
Domperidone vs placebo: change in mean milk volume.
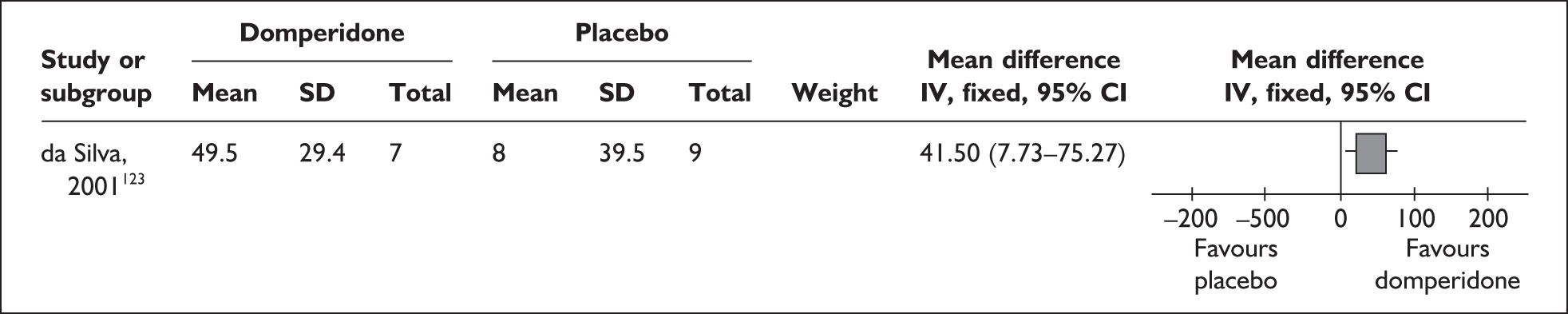
One double-blind, placebo-controlled RCT of human growth hormone (hGH) that included mothers of older preterm infants133 compared mean daily milk volume over study days 0–1 with mean milk volume on day 8 for nine mothers in each group. Mean milk production (SD) over 2 days at baseline was 139 (49) ml/day in the intervention group compared with 93 (50) ml/day in the control group. On study day 8 after the 7 days’ treatment, mean milk production increased to 175 (46) ml/day in the hGH group (p < 0.01) compared with 102 (69) ml/day (NS).
One RCT of a relaxation/imagery tape for mothers of recently born preterm infants146 compared mean milk volume at a single expression during the second postnatal week between 30 mothers in the intervention group and 25 mothers in the control group. Mean milk volume was significantly greater in the intervention group. The confidence interval and standard deviations were wide, as illustrated in the forest plot shown in Figure 22.
FIGURE 22.
Relaxation imagery tape vs no tape: mean milk volume.
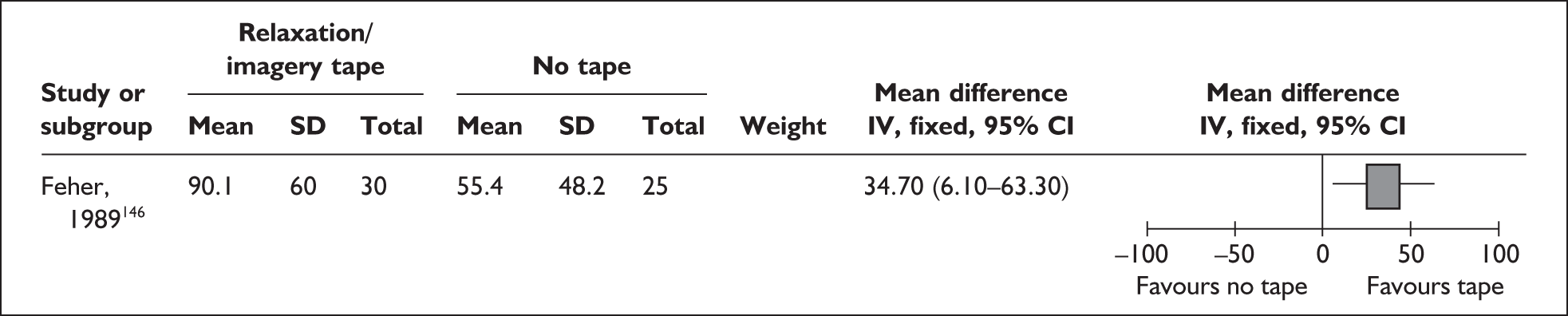
The fat content of a sample was analysed using a creamatocrit test. No statistically significant difference was found between the groups, as illustrated in the forest plot shown in Figure 23.
FIGURE 23.
Relaxation imagery tape vs no tape: fat content.
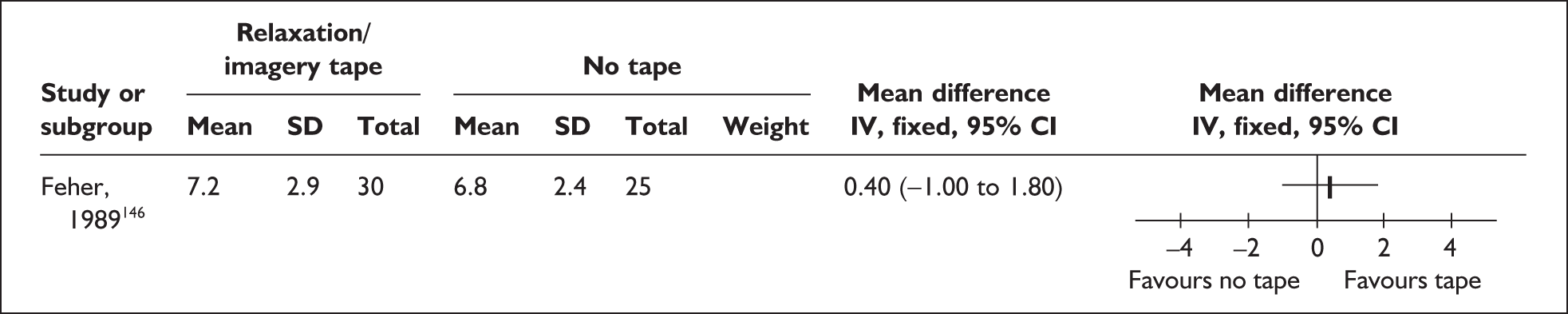
One randomised crossover study of breast massage prior to milk expression for mothers of recently born preterm infants114 randomised mothers to perform breast massage before pumping on either days 1 and 2 or days 3 and 4 of the study, and not to perform massage on the other pair of days. These mothers were taking part concurrently in a linked RCT of simultaneous versus sequential pumping (see Expressing breastmilk interventions). Complete data from 36 mothers on day 2 and day 4 were analysed and results of the two interventions are presented together as milk yield per expression in Table 8.
| Intervention | Milk yield per expression |
|---|---|
| Massage with sequential pumping | 78.71 (95% CI 85.19–72.24) |
| Massage with simultaneous pumping | 125.08 g (95% CI 140.43–109.74) |
| No massage with sequential pumping | 52.32 g (95% CI 56.57–46.07) |
| No massage with simultaneous pumping | 87.69 g (95% CI 96.80–78.57) |
The authors concluded that simultaneous pumping was more effective at producing milk than sequential pumping (p < 0.01) and that breast massage had an additive effect, improving milk production in both groups. It was reported that fat concentration in the milk was not affected by the increase in volume achieved by the interventions.
In a randomised crossover study148 of therapeutic touch (TT) that included mothers of older preterm infants, the author reported that five mothers experienced leaking of breastmilk during TT compared with one mother during mock TT (p < 0.05). The mean (SD), median and range of volumes of milk expressed after study treatments (not adjusted for milk leaked) by the 18 mothers are reported in Table 9.
| Intervention | Milk volume (ml) [SD], (median) and range |
|---|---|
| TT | 59.9 [53.9] (47) 5–200 |
| Mock TT | 49.6 [49.0] (38) 4–220 |
| No treatment | 47.3 [52.6] (32) 4–220 |
Milk volume was greater after TT than after mock TT or no treatment (p < 0.05).
The author noted that the large standard deviations reflect the large inter-participant variability. There were no significant differences in the fat content of the breastmilk expressed after each treatment.
Secondary outcomes
In the study of oxytocin nasal spray,144 no adverse effects were recorded. In the metoclopramide study116 the mean metoclopramide level found in milk from 14 intervention group mothers was 44.8 ± 20.4 ng/ml, stated to be similar to levels found in studies of term women. The authors calculated maximum exposure to metoclopramide would be about 3% of the recommended daily dosage for children. In the domperidone study123 domperidone levels were tested on day 5 and the milk:serum ratio was found to be 0.4. The authors state this is relatively low and much lower than metoclopromide. Mean (SD) serum prolactin on day 5 (μg/l) was 119.3 (97.3) in the intervention group versus 18.1 (14.7) in the control group (p = 0.008). Differences found between the groups in serum prolactin at baseline and 3 days after the last dose were not statistically significant. No side effects of domperidone were detected for mothers or infants in this small study. In the study of human growth hormone (hGH),133 plasma concentrations of growth hormone, measured 24 hours after the last hGH injection, did not change significantly after hGH therapy or placebo. In this small study, no adverse effects with hGH treatment were seen in mothers or infants.
In the two UK studies with mothers of recently born preterm infants, the mean number of expression sessions per day was 3.5 in Fewtrell et al. 144 and 5.2 in Jones et al. 114 In both studies this was below the rate specified in the study protocol.
Two of the studies that included older preterm infants also report this outcome. The mean frequency of pumping (SD) was 4.5 (2.2) times per day before and during Mersmann’s study. 148 In the study of hGH,133 all mothers in both the groups were consistently expressing from both breasts 5–6 times daily before and during the study.
Twenty-eight mothers in the oxytocin study,144 55% of those randomised, expressed a strong opinion on whether they had the active spray or placebo. Of twenty-two who were convinced they had the active spray, nine had the placebo; of six who thought they had the placebo, one had the active spray.
Nine of the 69 mothers (13%) randomised in the metoclopramide study116 stopped breastfeeding in the first week. Their reasons included ‘too little milk’, ‘too much stress’ and ‘too busy’.
In the study of the relaxation/imagery tape,146 mothers expressed many positive responses to the tape and no strong negative reactions. In the crossover study of breast massage,114 all the mothers valued massage and many of those who were randomised to use massage on days 1and 2 were reluctant about not using it on days 3 and 4. In the crossover study of TT, mothers were asked whether the first or second treatment they received was better. There were no differences between the groups.
Results from other study designs
No studies of additional interventions to enhance milk production that used other study designs were identified.
Interventions to support optimal nutritional intake from breastmilk
Three primary studies examining interventions related to optimising nutritional intake from breastmilk were identified. As detailed in Table 10, two studies were undertaken in the USA,20,145 and one in Nigeria. 140 Two were RCTs,140,145 and one was a concurrent comparison. 20
| Primary paper | Study design (n analysed)a | Country |
|---|---|---|
| Amali-Adekwu 2007140 | RCT (n = 68) | Nigeria |
| Hurst 2004143 | RCT (n = 31) | USA |
| Griffin 200020 | Concurrent comparison (n = 26) | USA |
Results from RCTs
Two RCTs were identified140,145 (Tables 57 and 58 in Appendix 4.1).
Characteristics of participants
One US trial145 recruited English-speaking mothers having a preterm infant, 31–36 weeks’ gestation, maintaining lactation during their stay in NICU and with the intention of breastfeeding post discharge. The Nigerian trial140 recruited healthy preterm infants of gestational age < 37 weeks, with birthweights between 1000 and 1500 g.
Mothers were mainly older, primiparous, married and Caucasian in the US study. 145 Amali-Adekwu et al. 140 reported that infants of mothers who were HIV positive were excluded. In both trials infants were defined as ‘healthy’.
Characteristics of interventions
One trial assessed the effect of selective hindmilk feeding on the growth of very low birthweight preterm infants. 140 The infants were stratified by birthweight and gestational age, and received either hindmilk or composite milk (both foremilk and hindmilk), via intermittent gavage feeds following 4 days of established enteral feeds. Each infant was fed for 14 days after which infants in the experimental group reverted to composite milk until discharge.
The other trial145 assessed infant milk intake by performing in-home measurement of weight pre- and post-feed measured at four time points: at discharge and at 1, 2 and 4 weeks post discharge. Extra milk feeds were given if required but type was not specified. Data were collected via questionnaire on breastfeeding history, infant feeding patterns up to 4 weeks post discharge, concerns about breastfeeding post discharge, and breastfeeding goals. A final questionnaire was completed on mothers’ perceptions of the in-home measurement of milk intake.
Outcome assessment
One study140 reported outcomes for milk production and milk quality (lipid concentration and calorific value). Fat concentration was estimated daily by creamatocrit measurement and the corresponding calorific values measured. Daily weight and weekly occipitofrontal circumference and recumbent length were measured. Peripheral blood samples were taken weekly to assess serum bicarbonate.
In Hurst,145 a breastfeeding history outcome was obtained from routinely collected data. Infant weight gain was measured at discharge from neonatal care, and 1, 2 and 4 weeks post discharge. Feeding patterns, urination and stooling were recorded from time of discharge to 4 weeks post discharge. For the intervention group the volume of milk intake was measured by test weighing. Milk intake for the control group was assessed using clinical indexes and infant behaviour. Concerns about breastfeeding were assessed at discharge and at 1, 2, and 4 weeks post discharge, and a breastfeeding goal questionnaire was completed at discharge. At the final post discharge visit mothers completed a questionnaire.
Methodological quality of included trials
Both RCTs140,145 were considered to be ‘poor’ quality. Serious caution is warranted in interpretation of the results of these trials. Details of the quality rating for each trial are provided in Table 81 in Appendix 5.
Results from RCTs
Primary outcomes
In the study of hindmilk feeding, energy values for hindmilk were reported as 3.73 ± 0.5 kJ/ml versus composite milk 2.8 ± 0.38 kJ/ml (p = 0.001), with creamatocrit (%) 9.23 ± 1.89 for hindmilk versus 5.73 ± 1.4 for composite milk. 140 Caution is required in interpretation of these findings due to the lack of reporting of complete data.
There were no differences in breastfeeding outcomes in this trial. 145
Findings should be interpreted with caution due to the poor quality of the trial.
Secondary outcomes
Amali-Adekwu et al. 140 reported that weight gain was significantly higher in both SGA and appropriate for gestational age (AGA) infants fed on hindmilk than in controls fed with composite milk. Based on mean and SD data reported, SGA infants receiving hindmilk were calculated to have experienced significantly higher daily weight gain compared with SGA infants receiving composite milk (I: 12.9 ± 11.0 g/kg, C: 5.0 ± 17.4 g/kg; p = 0.001).
There was also a positive correlation between rate of weight gain and lipid measurements of the breastmilk in both the SGA and AGA groups. Authors reported no significant differences in the occipitofrontal head circumference and length increments of any groups but presented no data. 140
Hurst145 reported no significant differences for change in weight or weight gain at any time points. Both comparison groups expressed concerns related to infant weight gain, quantity of milk consumed at each feed and concerns of infant getting enough from the breast. By week 1 post discharge, the main concerns were the adequacy of milk (intervention group) and the quantity of milk consumed (control group). The majority of the intervention group reported that they found it helpful to undertake the pre- and post-feed weighing of their infants although one-third of the mothers stated it made them feel nervous.
No trials reported cost-effectiveness data for the intervention.
Results from other study designs
One concurrent comparative study was identified evaluating interventions related to mothers’ involvement in measuring breastmilk quality20 (Table 59 in Appendix 4.1).
Characteristics of participants
This study was conducted in the USA20 among mothers who were expressing and when creamatocrit measurements were clinically indicated in the management of the infant’s nutritional plan. There was a diverse distribution of mothers in relation to maternal age, occupation and income level reported. Of the 26 participants, 12 (46%) were white, 9 (35%) African American, and 5 (19%) Hispanic.
No information was reported for infant characteristics.
Characteristics of intervention
Mothers were taught to measure the fat content of their own expressed breastmilk using the creamatocrit (CRCT) technique20 by one of two instructional registered nurses using a standardised teaching tool. The comparison group was formed of registered nurses who conducted the same procedure using a centrifuge located in an adjacent room. All CRCTs were performed within 30 minutes of milk expression.
Outcome assessment
Griffin et al. 20 reported the length of time taken to teach mothers the technique and the accuracy of the mothers’ measurements compared with registered nurses. After practising the CRCT for approximately 72 hours, one of two validating registered nurses performed simultaneous CRCTs on the same breastmilk sample. To standardise these differences with respect to the actual CRCT value the percentage error in each mother’s measurement was calculated by dividing the absolute difference between the registered nurse’s and mother’s CRCT by the registered nurse’s mean CRCT.
Methodological quality of the included study
This study was rated ‘good’ quality overall. 20 Details of the quality rating for this study are provided in Table 82 in Appendix 5.
Effectiveness of the intervention
Primary outcomes
This comparison study20 reported that mothers’ CRCT measurements were highly accurate when compared with the validating registered nurses. The differences between the mothers’ and registered nurses’ measures were compared using mean, standard deviation, minimum and maximum differences, mean absolute differences, the percentage of difference of 0.5% or < 1% and the percentage of error. The percentage error values between the two groups were: ≤ 0.5% error, 50%; < 1.0% error, 84.6%, with a percentage error between mothers and registered nurses of 6.8%, showing a high degree of accuracy between the mothers’ and registered nurses’ measures. When standardised, the percentage of error in the mothers’ measurements was lower than those between the two validating registered nurses performed during a pilot study (6.8% errors for mothers compared with 10.51% of error by the validating registered nurses). No association was found between magnitude of error and maternal age, years of formal education, or household income.
Secondary outcomes
The mean time for teaching this technique was reported as 23.6 minutes. 20 Mothers reported that performing CRCTs was easy to learn and simple to carry out, and they felt they were making a difference and influencing their infant’s outcomes.
Cost-effectiveness data were not reported.
Breastfeeding education and support interventions
A total of six primary studies and two systematic reviews evaluating breastfeeding support interventions were identified. As detailed in Table 11, three primary studies were included in at least one of the three systematic reviews. Four of the six studies were conducted in the USA, one in Canada126 and one in the Philippines. 117
| Primary paper | Study design (n analysed)a | Inclusion in existing systematic review | Country |
|---|---|---|---|
| Agrasada 2005117 | RCT (n = 204) | Edmond 2006155 | Philippines |
| Gonzalez 2003166 | Before/after (n = 350) | McInnes 2006161 | USA |
| Merewood 2006143 | RCT (n = 85) | No | USA |
| Pereira 1984153 | Before/after (n = 402) | No | USA |
| Pinelli 2001126 | RCT (n = 128) |
Edmond 2006155 McInnes 2006161 |
Canada |
| Senn 2004152 | Before/after (n = 50) | No | USA |
Results from RCTs
Three RCTs of breastfeeding education and support interventions were identified (see Tables 60–62 in Appendix 4.1).
Characteristics of participants
Two of the studies were performed in industrialised country settings including one in the USA143 and one in Canada. 126 The remaining study was conducted in the Philippines. 117
One study143 took place and another117 began in hospitals that had received Baby Friendly accreditation; the report of the third study does not mention Baby Friendly accreditation. 126
All three studies included mothers who intended to breastfeed, with that of Merewood et al. 143 requiring in addition that mothers should be eligible to breastfeed in accordance with American Academy of Pediatrics Work Group on Breastfeeding (1997)60 guidelines. Age and parity of the mothers in the study by Merewood et al. 143 were not reported. All spoke English or Spanish, over 70% were not US-born, 69% were African American and over 50% were on Medicaid. Five hundred and seventy-seven mother–infant pairs were assessed for eligibility and 452 (78%) were excluded for not meeting eligibility criteria, ‘many’ because of illicit maternal drug use. The study in the Ph ilippines117 specified that mothers should be primiparous and at least 18 years old, and excluded mothers taking medications that may compromise breastfeeding and mothers who were not going to stay in the study area with their infants for 6 months. The included mothers had a mean age in the early twenties; almost all had secondary or college education; 70% were living with a partner and just over 30% worked or studied outside the home. The mothers in Canada126 were older, with a mean age just under 30 years, and 40% had other children. All were English speaking and almost all were classified as social class I or II. Seventy percent were living with a partner, and fathers were included in this study.
Infant characteristics varied widely between the three studies. Only VLBW infants (< 1500 g) were included in the Canadian study;126 they were born at a mean (SD) of 29 (3) weeks. The study by Merewood et al. in the USA143 included infants with a mean birthweight of 1877 g (range 682–3320 g) and a mean gestational age of 32.7 weeks (range 26–37 weeks); 30% of the infants were born at < 32 weeks’ gestational age and 70% at ≥ 32 weeks. Agrasada’s study in the Philippines117 included LBW infants (< 2500 g) born vaginally at term (37–42 weeks). All the studies included only singleton infants and two specifically excluded infants with congenital abnormalities. 126,143
Characteristics of interventions
The intervention in the study of Pinelli et al. in Canada126 was supplemented structured breastfeeding counselling (SSBC) for both parents, from within 72 hours of the birth of their VLBW infant (n = 64). SSBC involved viewing a video on breastfeeding for preterm infants, individual counselling by the research lactation consultant (not a member of the hospital staff), weekly personal contact in the hospital and frequent postdischarge contact (type of contact unspecified) through the infant’s first year or until breastfeeding was discontinued. The control group (n = 64) were allocated to Conventional Hospital Breastfeeding Support (CHBS), standard care at the time of the study. CHBS included contact with the regular hospital staff (nurses, nutritionists, neonatal nurse practitioners, physicians), during the period of hospitalisation only. Only a limited number of staff had any formal education in lactation or breastfeeding support, and no specialised breastfeeding clinic was available to parents in the hospital. Standard care in the community was not described.
The intervention in the study of Merewood et al. in the USA143 was peer counsellor support for mothers, from within 72 hours of the birth of their preterm (26–37 weeks’ gestation), otherwise healthy infant (n = 53). The five peer counsellors were women with breastfeeding experience, drawn from the local community of mixed ethnic backgrounds; two had been teenage mothers. They were employed for this work, and two were also employed as lay childbirth assistants at the study hospital. Peer counsellors were trained at a 5-day breastfeeding course. They also had training at the hospital about NICU procedures and breastfeeding techniques, and attended regular, mandatory breastfeeding training days for maternity staff throughout their employment. After initial contact, the peer counsellor was in contact with the mother on a weekly basis for 6 weeks. After the infant was discharged from hospital, contact was by telephone unless the mother went to hospital. The control group (n = 55) was allocated to standard care. The study hospital had Baby Friendly accreditation, and standard care included staff who were highly trained in lactation management, access to three breastfeeding classes per week, referrals to the lactation consultant when needed, use of a breast pump in the hospital and a free, high-grade electric breast pump for home use if the mother’s insurance did not pay for a pump. No other community support components of standard care were reported.
In Agrasada’s study in the Philippines117 there were three study groups; breastfeeding counselling from community-based peer counsellors, childcare counselling from community-based peer counsellors, and no peer counselling. In all three groups, mothers who vaginally delivered term LBW infants were sent to the rooming-in ward. Term LBW infants with birthweights < 2 kg were observed in the NICU for 12–24 hours. The paper is not explicit about management of term LBW infants with birthweights of 2–2.5 kg. While separated from their mothers, the infants received (by dropper) fresh expressed breastmilk (EBM) donated by lactating mothers on the ward. As soon as their condition was stable, the infants joined their mothers on the rooming-in ward. Although the authors state the study hospital had been assessed as ‘Baby Friendly’, they also indicate that in this rooming-in ward, no hospital staff or volunteers were tasked to educate or assist mothers with breastfeeding. Mothers were discharged 24–72 hours post partum, breastfeeding exclusively. They were informed of their group assignment as they were leaving hospital and were asked to come to the hospital clinic for the usual seven infant visits, at 2 and 4 weeks then monthly until 6 months, at which study measures were taken. No other community support components of standard care were reported.
The peer counsellors in Agrasada’s study117 were 14 women health volunteers (age 22–50 years) with similar formal education to the mothers, who were willing to do home visits. They undertook 40 hours counselling training. Six were trained by a maternal child health-care specialist and became childcare counsellors (for CC group), and eight with positive personal breastfeeding experience were trained by a certified lactation specialist and became breastfeeding counsellors (for BC group). They were not salaried; they received local transport costs during training and home visits. Eight home visits were scheduled, at infant age 3–5, 7–10 and 21 days, then monthly up to 5.5 months. In the breastfeeding counselling intervention (BC, n = 68), peer counsellors informed mothers of the benefits of exclusive breastfeeding to 6 months, and assisted mothers in preventing and managing breastfeeding problems. In the childcare counselling intervention (CC, n = 67), peer counsellors assisted mothers on infant care and increasing mother–infant interaction using activities such as infant massage and smile therapy. In the control group (C, n = 69), there was no input from peer counsellors.
Outcome assessment
The main outcome measure in the Canadian study of VLBW infants126 was duration of breastfeeding. Proportions of intake from breastmilk/breastfeeding at term and up to 1 year are reported. It is implied but not stated that the time points up to 1 year are post term and not post birth. This study also reported aspects of mothers’ breastfeeding experiences in NICU (age after birth that pumping started, frequency and duration of pumping, amount pumped, age when infant first put to the breast, frequency of breastfeeding) and at home (breastfeeding problems, what resources mothers used to solve breastfeeding problems, reasons for discontinuing breastfeeding).
The main outcome measure of the study conducted in the USA143 was any breastmilk feeding at 12 weeks post partum. Odds of all the mothers, and of African American mothers, giving any, mostly or all breastmilk are presented. This study also reported process outcomes (percentage of documented cases in which the peer counsellor discussed pumping, helped the mother to pump, breastfeed, perform kangaroo care; accompanied the mother to NICU; and whether contact with the mother took place in person by telephone).
The main outcome measure of the study of term LBW infants in the Philippines117 was the proportion of mothers breastfeeding exclusively from 2 weeks to 6 months. This study also reported any breastfeeding, infants’ mean weight-for-age, infants’ rates of diarrhoea, and mothers’ views of the programme, as well as mothers’ breastfeeding knowledge and intentions as assessed at the start of the study.
Methodological quality of included trials
Two studies117,126 were rated as ‘good’ quality overall. One study141 was rated as ‘moderate’ quality (see Table 83 in Appendix 5).
Effectiveness of interventions
Primary outcomes
Complete outcome data for all those originally enrolled were provided in or could be calculated from two papers. 117,126 Individual relative risk estimates, calculated on an ITT basis, are presented in forest plots for primary outcomes from these two studies, which both received a ‘good’ overall quality rating (Figures 24 and 25). The report of the study by Merewood et al. ,143 which was quality-rated moderate, lacked the data needed for estimating relative risks.
FIGURE 24.
Counselling/support by professional or peer counsellor in hospital/at home: breastfeeding at term.
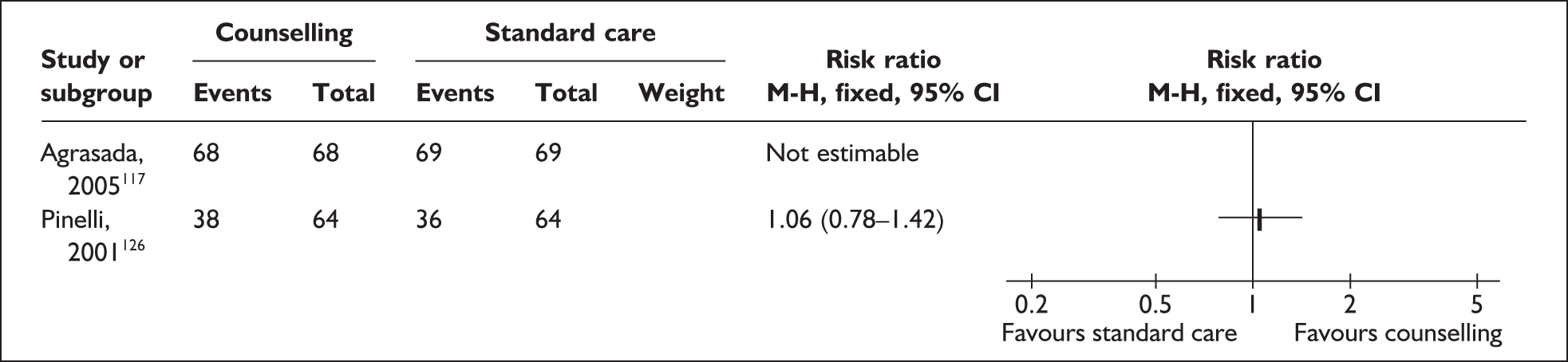
FIGURE 25.
Counselling/support by professional or peer counsellor in hospital/at home: any breastfeeding at 12 weeks.
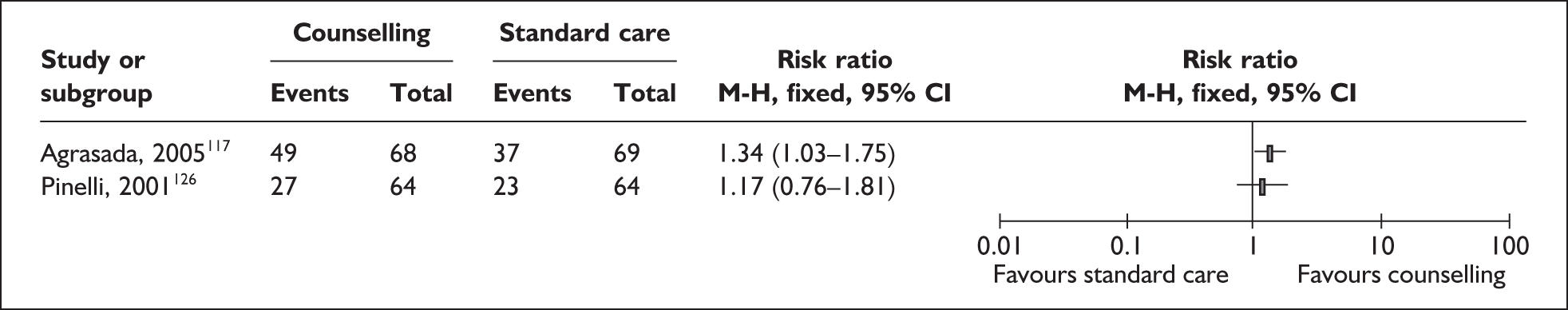
In the study of breastfeeding peer support vs childcare peer support vs no peer support (Agrasada 2005117) all the participants were exclusively breastfeeding at term, when the peer support interventions began. In the study of professional support vs standard care (Pinelli et al 2001126) there were no differences in intake of breastmilk between the groups at term (see Figure 24).
Any breastfeeding at 12 weeks
In Agrasada’s study, significantly more mothers were breastfeeding at 12 weeks in the breastfeeding peer support group than in the no peer support group (see Figure 25). The numbers of mothers in the childcare peer support group who were breastfeeding at 12 weeks was 36/67, very close to the numbers in the no peer support group.
FIGURE 26.
Counselling/support by professional or peer counsellor in hospital/at home: any breastfeeding at 24 weeks.
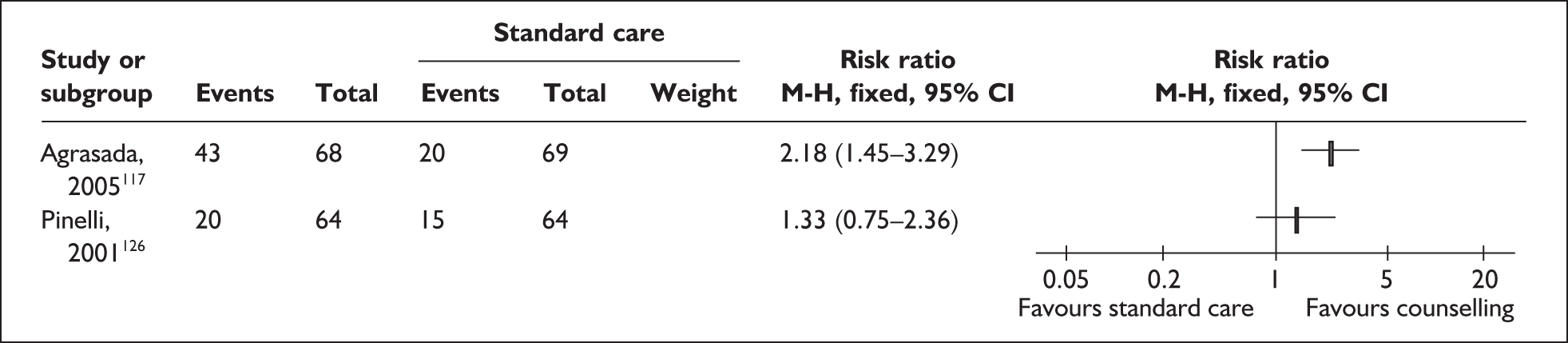
In the study of professional support vs standard care (Pinelli et al. 2001126), there was no statistically significant difference between the groups in numbers of infants with any intake from breastfeeding or breastmilk at 12 weeks (see Figure 25).
In the study of peer support vs Baby Friendly standard care (Merewood et al. 2006143), the numbers randomised, excluded post randomisation and lost to follow-up for each study group were reported. A total of 23 were lost from the 108 randomised in this study (21.3%), with more lost from the intervention group (15/63, 24%) than from the control group (8/55, 14%). Relative risks might therefore have been different if calculated on an ITT/postrandomisation exclusions basis as we planned, compared with what they would have been if calculated on an available case basis, as were the odds ratios reported in the paper. However, numerators were not reported so relative risks could not be calculated.
Merewood et al. 143 reported on outcome for 38/53 participants in the peer counselling group and 47/55 in the Baby Friendly standard care group. At 12 weeks post partum, mothers with a peer counsellor were significantly more likely to provide milk than mothers without a peer counsellor (odds ratio 2.81; 95% CI 1.11–7.14; p = 0.01). Among the subgroup of African American participants (30 in the peer counselling group and 29 in the Baby Friendly standard care group), Merewood et al. 143 reported that at 12 weeks, African American mothers with a peer counsellor were significantly more likely to provide milk than African American mothers without a peer counsellor (odds ratio 3.59; 95% CI 1.16–11.03; p = 0.03).
It should be noted that the postconception age of the infants at 12 weeks’ post partum varied in this study.
Any breastfeeding at 24 weeks
In Agrasada’s study, significantly more mothers were breastfeeding at 24 weeks in the breastfeeding peer support group than in the no peer support group (Figure 26). The numbers of mothers in the childcare peer support group who were breastfeeding at 24 weeks was 21/67, very close to the numbers in the no peer support group.
In Pinelli’s study there was no significant difference between the groups in numbers of infants with any intake from breastfeeding or breastmilk at 24 weeks (see Figure 26).
Exclusive breastfeeding from birth to 6 months
In Agrasada’s study117, significantly more mothers breastfed exclusively from birth to 6 months in the breastfeeding peer support group (32/68) than in the no peer support group (0/69) (Figure 27). Two of the 67 mothers in the childcare peer support group breastfed exclusively from birth to 6 months.
FIGURE 27.
Counselling/support by peer counsellor at home: exclusive breastfeeding from birth to 6 months.
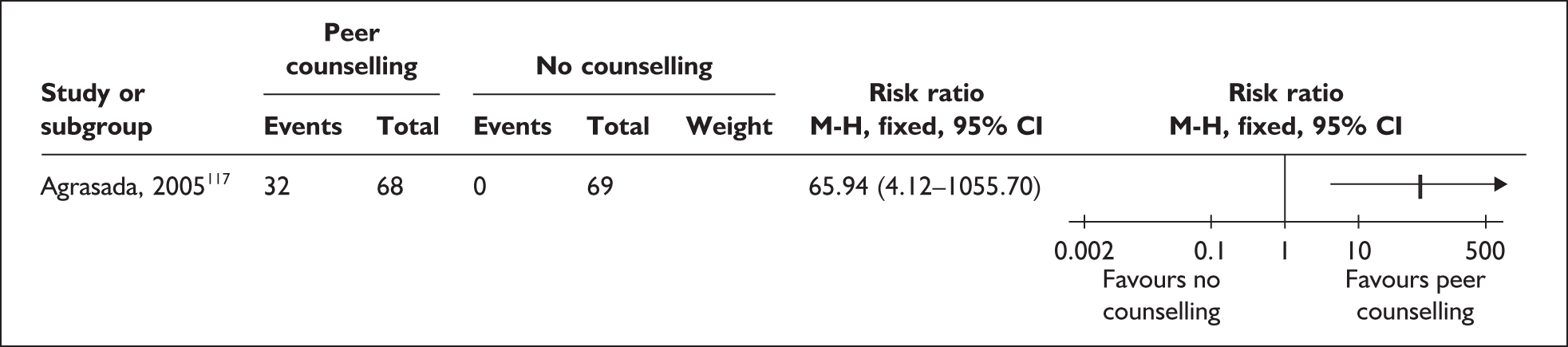
Amount of milk pumped for hospitalised infants
There was no significant difference between the groups in mean amount of milk pumped by mothers for their hospitalised infants in Pinelli’s study (Figure 28). The range of milk volumes obtained was wide (4–350 ml in the breastfeeding counselling group and 6–200 ml in the standard care group).
FIGURE 28.
Counselling/support by professional counsellor in hospital: amount of milk pumped (ml).
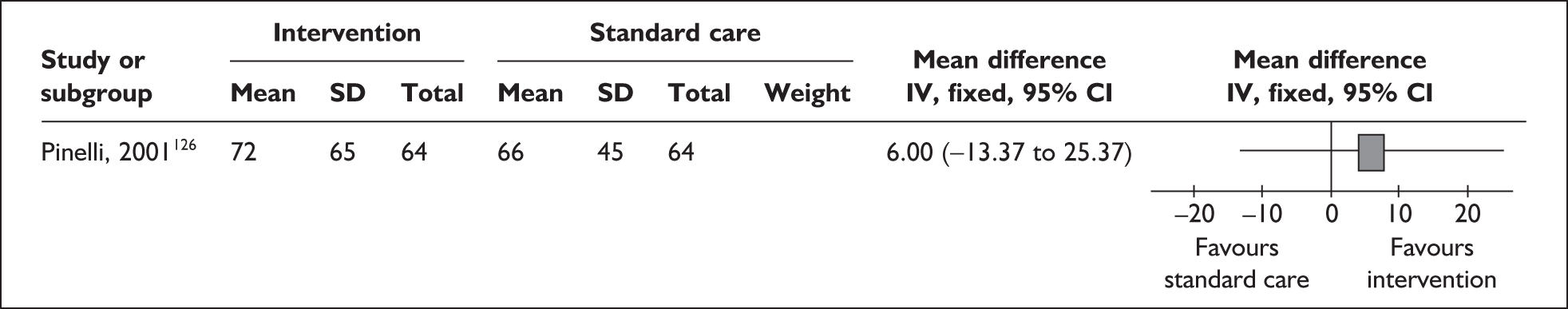
Secondary outcomes
Agrasada (2001)117 found no significant differences between the groups in mean weight for age at birth or 6 months. Rates of diarrhoea were halved in the breastfeeding peer counselling group (15%) compared with the other two groups. There were no infant deaths among the study participants. Clinical/health outcomes were not reported by Merewood et al. (2006)143 or Pinelli et al. (2001). 126
Pinelli et al. 126 found no significant differences between the groups in age after birth when pumping started (mean 28 days, range 1–72 days); frequency of pumping in NICU [mean 6 (SD 2) per 24 hours, range 1–20]; length of pumping sessions in NICU (mean 18.5 minutes, range 5–45); age when infant first put to the breast (mean 25 days, range 0–102) or frequency of breastfeeding per day in NICU (mean 3.5, range 1–9). Post discharge, more than 50% of mothers in both groups experienced breastfeeding problems including sore nipples, fatigue, not enough milk, infant not gaining weight and infant not interested in breastfeeding. The only significant difference between the groups was that more mothers in the intervention group reported the infant was not gaining weight (p = 0.05). At 6 months the main reason for stopping breastfeeding was ‘not enough milk’; at 1 year the main reason was ‘infant not interested in breastfeeding’. Merewood et al. 143 reported on peer counsellor’s field records for 43/48 (90%) of the intervention group. The peer counsellor discussed pumping in all documented cases; accompanied the mother to NICU in 72.1%; helped the mother to pump in 72.1%; and helped the mother to breastfeed, perform kangaroo care or both in 30.2%. After 4 weeks, 37.2% of the infants remained in NICU, and 81.3% of mothers of these infants were seen in person by the peer counsellor in NICU. Other contacts were by telephone. Agrasada117 did not report process outcomes.
Pinelli et al. 126 reported mothers using a wide variety of breastfeeding resources for solving breastfeeding problems at home, including health professionals, books, friends and family. The most used resource over all time periods was the lactation consultant. This included the research lactation consultant in the study group and community lactation consultants in both groups. At exit interview, mothers in Agrasada’s study115 who had counsellors stated they were satisfied with the programme. Mothers who had breastfeeding counsellors said the counsellor was the person who had influenced their feeding decisions the most. Mothers in the other two groups said the physician had influenced their feeding decisions the most. Merewood et al. 143 did not report psychosocial outcomes.
Results from other study designs
Three before/after studies were identified152,153,166 (see Tables 63–65 in Appendix 4.1). Two of these collected prospective data for the intervention group152,153 and one collected retrospective data for both groups. 166
All three studies were undertaken in the USA. That by Gonzalez et al. 166 took place in a NICU with approximately 700 admissions per year, with infants who were mainly preterm (< 37 weeks’ gestation) or low birthweight (≤ 2500 g). A survey prior to the study done by Pereira et al. 153 showed 17% of infants in that study unit were breastfed from birth and half of these were exclusively formula fed by the time of discharge. The rural NICU in which the study of Senn152 took place treated infants with birthweight ≥ 750 g who did not require surgery. In 1997–8, 49.7% of infants born ≤ 34 weeks’ gestation received breastmilk at least once.
Characteristics of participants
Gonzalez et al. 166 examined randomly selected records of infants in NICU during 6 months before (n = 175) and 6 months after (n = 175) initiation of the intervention. No significant differences between the groups were found for sociodemographic or clinical factors, including mother’s age, ethnicity, infant gender, Apgar scores at 5 minutes, or length of stay. Sixty-seven percent of the infants were preterm and/or low birthweight.
Pereira et al. 153 examined records of infants in NICU during two 6-month periods before (n= 192) and after (n = 210) initiation of the intervention, and gave a questionnaire to mothers who had received the intervention. The groups of mothers were comparable and mainly white, 20–30 years old and privately insured. Infant characteristics were not reported.
Senn152 recruited 25 mothers of infants in NICU and matched them with 25 historical controls. The mothers were on average in their mid- to late twenties, had public insurance or no insurance, more than a high-school education, and were married. Around half were multiparous, with breastfeeding experience not reported. Mothers of twins or triplets were invited to participate, with one infant chosen randomly for inclusion in the analyses. The only significant difference found between the groups was for race: all the intervention group were white, and seven of the 25 historical controls (28%) belonged to minority ethnic groups (p < 0.01). Infants were relatively healthy, born at around 33 weeks’ gestation and weighing around 2000 g at birth, with an average hospital stay of about 2.5 weeks.
Characteristics of interventions
The intervention in the study by Gonzalez et al. 166 was the introduction of an International Board Certified Lactation Consultant support service within the NICU. The lactation consultant contacted mothers within 24 hours of their infants’ admission to NICU and counselled mothers regarding the benefits and options for providing her milk to the infant. If the mother chose to provide her milk, a feeding plan was developed. Lactation consultants were available from 7 am to 6 pm to answer questions and assist with pumping. A telephone message service was available after hours. Standard care on the study unit was not described.
The intervention in Pereira’s study153 was the introduction of a programme of breastfeeding support by trained, community-based volunteer counsellors for mothers intending to breastfeed. Mothers were informed of the availability of counselling by NICU staff. The programme coordinator contacted the mother and assigned a counsellor living nearby. Seventeen peer counsellors who had successfully breastfed their sick infant and had received certification and orientation to the neonatal unit were available. Counselling included empathy and emotional support as well as collection, home storage and transport to hospital of expressed breastmilk; transition from tube feeding to breastfeeding; maternal diet during lactation and medications excreted in breastmilk. The paper implies but does not state that the counsellors made home visits. Telephone counselling was provided as needed. Standard care on the study unit and following discharge was not described.
The intervention in Senn’s study152 was the Lactation Education Breastfeeding Program. This programme had two core sessions, designed to be interesting, fun and interactive. Participants were given a $25 Wal-Mart gift card for each session they attended. In Session 1, mothers had an individual meeting with the lactation consultant shortly after the birth. Topics included pumping, storing and transporting breastmilk, hand washing, use and cleaning of the pump, how long and how frequently to pump and time for questions. Mothers who wanted to feed directly from the breast could meet the lactation consultant for an additional session when the infant was mature enough for this. In Session 2, mothers were invited to a weekly group educational session led by the lactation consultant, with group activities covering infant and maternal benefits of breastfeeding and social support for breastfeeding. Mothers created a list of people who could give them informational, tangible and emotional support with breastfeeding, and were given a list of community breastfeeding resources. Standard care on the study unit is not described.
Outcome assessment
Gonzalez et al. 166 reported percentages of infants given their own mother’s milk (OMM), and factors associated with receiving OMM feedings. Pereira et al. 153 reported the in-hospital breastfeeding rate, duration of breastfeeding and mothers’ views of the programme. Senn152 reported how many infants received breastmilk at least once; received breastmilk within 2 days of feeding initiation; the mean percentage of days on which infants received breastmilk; on days receiving breastmilk, the mean percentage of feedings of breastmilk they received; ever breastfed; the mean percentage of days breastfed; on days breastfed, the mean percentage of feedings breastfed; breastmilk at discharge; mean age at discharge; and the intervention group mothers’ perceptions of breastfeeding benefits, barriers and self-efficacy before and after the intervention.
Methodological quality of included studies
Two studies were rated as ‘moderate’ quality. 153,166 One study was rated as ‘poor’ quality. 152 Details of the quality ratings for each study are provided in Table 84 in Appendix 5.
Effectiveness of interventions
Primary outcomes
Gonzalez et al. 166 reported that 31% of infants before the intervention, compared with 47% in the lactation consultant group, ever received their own mother’s milk (p = 0.002, OR 2.0, 95% CI 1.3–3.0). At hospital discharge, 23% of infants before the intervention were receiving any breastmilk, compared with 37% in the lactation consultant group (p = 0.004, OR 2.0, 95% CI 1.2–3.2).
Pereira et al. 153 found the in-hospital breastfeeding rate before the intervention was 17%, compared with 30% after the intervention (p < 0.01). Duration of breastfeeding (mean, in days) was 41.6 (SEM 9.4) before the intervention, compared with 134 (SEM 12.9) after the intervention (p < 0.001).
Senn152 found no differences between the groups in infants receiving breastmilk at least once, those ever breastfed, and those receiving breastmilk at discharge.
Secondary outcomes
Using logistic regression analysis, Gonzalez et al. 166 found that factors significantly associated with giving own mother’s milk to infants in the NICU were lactation consultant care (p = 0.002), white ethnicity (p < 0.001), male gender of the infant (p = 0.04), higher 5-minute Agpar score (> 7) (p = 0.003), and a stay in NICU greater than 7 days (p = 0.007).
The questionnaire sent to mothers receiving the intervention in Pereira’s study153 had a 93% response rate (59/64). Overall, 61% of respondents ranked the programme ‘very beneficial’ and 39% found it ‘somewhat beneficial’. No respondent regarded counselling as ‘non-beneficial’.
None were reported from these studies.
Staff training interventions
Two studies evaluating staff training interventions were identified. As detailed in Table 12, one study was undertaken in the UK81 and one in the USA. Neither of these studies was included in a previous systematic review.
| Primary paper | Study design (n analysed)a | Country |
|---|---|---|
| Jones 200481 | Before/after | UK |
| Staff training (n = 88)a | ||
| Mothers (n = 135)a | ||
| Pineda 2006149 | Before/after | USA |
| Staff training (n = 34)a | ||
| Mothers (n = 262)a |
Results from RCTs
No randomised trials evaluating the effect of staff training on breastfeeding outcomes were identified.
Results from other study designs
Two before/after studies of training interventions for health-care professionals were identified81,149 (see Tables 66 and 67 in Appendix 4.1).
Characteristics of participants
The two studies were conducted in industrialised countries, one in the UK81 and one in the USA. 149
One study81 delivered an education intervention to health-care professionals working within the neonatal unit, including eight neonatal-trained midwives, eight neonatal-trained paediatric nurses, 12 registered nurses, three paediatric nurses, two paediatric house officers, and one paediatric registrar. No data were given related to health-care professional characteristics other than professional status. All mothers included in the evaluation intended to breastfeed. No infant characteristics were reported.
Pineda149 conducted an intervention that included an education package to health-care professionals including 75 nurses, three rehabilitation therapists, one nurse practitioner, two neonatologists, one respiratory therapist and five other health professionals. Mothers who received education and support in this intervention were comparable. There was a large proportion of mothers in both groups of low socioeconomic status (B: 77.5%; A: 70%). The infants were comparable for birthweight (mean birthweight B: 1074 g and A: 1114 g) and estimated gestational age (mean B: 28.57 weeks and A: 28.7 weeks). Mean length of stay in hospital (B: 50 days and A: 54 days) and numbers of transfers to other hospitals (B: 43.2% and A: 32.7%) were also comparable.
Characteristics of interventions
Jones et al. 81 designed an evidence-based programme of education describing preterm mammary physiology, milk expression and the establishment of preterm oral skills. This was taught in five separate modules, delivered by a neonatal breastfeeding coordinator, and took 10 hours to complete. Additional information was available via CD-ROM and video.
Pineda149 developed an evidence-based educational initiative addressing areas relevant to the neonatal unit setting. Topics covered included benefits of and barriers to breastfeeding, the physiology of lactation, use of breast pumps, prefeeding interventions and interventions related to the readiness of the preterm infant to feed. The programme was completed by health-care professionals working within the neonatal unit either through self-study or by attendance at taught in-service days in the neonatal unit. It incorporated a pathway of care for providing support for new mothers. Nurses documented the education and support given to mothers at predefined times. Critical time points were: within 6 hours of birth, to issue and instruct in proper pumping and breastmilk storage; within 24 hours of birth, to ensure proper pumping and storage technique; on days 3–5, to ensure milk has come in and troubleshoot any problems, foster continued pumping and skin-to-skin care; first oral feeding, to ensure it is a breastfeeding session; and at 10 days, to monitor milk supply and make referrals as appropriate. An educational pamphlet designed specifically for mothers whose infant was admitted to NICU was also devised. This contained information about the benefits of breastfeeding, the expressing and storage of milk, prebreastfeeding strategies and cue-based breastfeeding behaviour of the infant.
Outcome assessment
Jones et al. 81 reported educational outcomes for staff, as assessed by a pre- and post-test of knowledge. Following the development of a questionnaire, advice was sought from three experienced specialists and the package, including the pre- and post-test, was piloted on five trainee neonatal nurses. A total score of 85 was achievable. This study also reported changes in recording of milestones achieved through the transition phases towards breastfeeding at discharge, including actions to promote each transition, as assessed by case note review of infant records.
Pineda147 assessed outcomes by case note review and reported breastfeeding initiation, whether or not breastmilk was provided at discharge, and the proportion of hospital stay during which breastmilk was provided. The unit of allocation and analysis for this study was the mother and not the infant.
Methodological quality of included studies
Both studies were assessed as moderate quality. Details of the quality rating for each study are provided in Table 85 in Appendix 5.
Effectiveness of the intervention
Primary outcomes
Jones et al. 81 reported on retrospective analysis of infants’ notes for all admissions to the neonatal unit during a 3-month period prior to training and for an unspecified time after training (B: 135; A: 127). Expressed breastmilk was given to a higher proportion of infants following the intervention (B: 74/86, 86%; A: 72/74, 97%; p = 0.012). There was a significant increase in the documentation of a problem-solving plan for milk expression (B: 2/84, 2%; A: 57/66, 86%; p = < 0.0001) and skin-to-skin contact (B 15/46, 33%; A: 63/64, 98%; p = 0.001). There was also a significant increase in cup feed offered (B 53/82, 65%; A: 56/66, 85%; p < 0.006) and infants put to the breast (B: 57/76, 75%; A: 65/69, 94%; p = 0.002). A statistically significant increase in breastfeeding at discharge was not demonstrated (B: 49/73, 67%; A: 54/68, 79%; p = 0.1).
Pineda149 reported a comparison of VLBW infants admitted to the neonatal unit, 1 year prior to the intervention and 9 months after (B: 81; A: 54). There was a significant increase in the number of mothers who ever breastfed their infants while in hospital (B: 21/81; A: 24/54; OR 2.286; CI 1.1–4.75; p = 0.025). The provision of breastmilk at discharge did not increase, and the multifaceted staff training intervention did not demonstrate a significant increase in breastmilk provided during hospitalisation
Secondary outcomes
Jones et al. 81 conducted a pre- and post-test assessment to evaluate the effectiveness of the training package on health-care professionals’ knowledge related to the topics covered in the programme.
Data for the pre- and post-test assessment on staff knowledge was available for 34/42 health-care professionals. A statistically significantly increased (median) score was demonstrated between the pre- and post-test results (maximum score of 85) (B: 32.5, range 9–39; A: 44.6, range 34–60.5; p = 0.001). Authors have not adjusted for the independent effect of participating in the pre-test. The programme was positively evaluated by the participants. Skin-to-skin contact undertaken between the mother and infant increased significantly following the intervention (B: 5/46, 33%; A: 63/64, 98%; p = 0.0001).
Pineda149 reported that 88 (63%) of all neonatal health-care professionals (denominator data not provided) completed the educational programme. All achieved a post-test assessment of ≥ 80%. The independent effect of participating in the pre-test was not assessed. Nurses did not always comply with the established guidelines on the breastfeeding pathway and the educational booklet designed for the mother was not distributed consistently. Not all mothers were given their booklet on admission and some never received them (numbers not reported).
Cost-effectiveness data were not reported in either of these studies.
Early hospital discharge with home support interventions
Two primary studies reported in three papers109,110,127 evaluating early hospital discharge with home support interventions were identified. Both were conducted in industrialised countries, namely New Zealand and Sweden. As detailed in Table 13, both primary studies were included in at least one of the three previous systematic reviews. 155,160,161
| Primary paper | Study design (n analysed)a | Inclusion in existing systematic review | Country |
|---|---|---|---|
| Gunn 2000127 | RCT (n = 308) |
Edmond 2006155 McInnes 2006161 |
New Zealand |
| bOrtenstrand 2001110 | RCT (n = 88) | McInnes 2006161 | Sweden |
| bOrtenstrand 1999109 |
Collins 2003160 |
Sweden |
Results from RCTs
Both studies were RCTs109,110,127 (see Tables 68 and 69 in Appendix 4.1).
Characteristics of participants
Both trials took place in industrialised settings and recruited infants in neonatal care with a gestational age of less than 37 weeks. 109,110,127 Twins were included in both the trial conducted in New Zealand (I: 29/148; C: 33/160)127 and the trial conducted in Sweden (I: 5/45; C: 8/47). 109,110 The latter trial excluded triplets and quadruplets. 109,110
The mean gestational age (weeks) of infants recruited was slightly lower in the study conducted in Sweden by Ortenstrand et al. (I: 31.4, SD 2.8; C: 32.0, SD 2.3)109 than those recruited in the study conducted in New Zealand by Gunn et al. (I: 33.2, SD 2.3; C: 32.9, SD 2.5). 127 Similarly, mean birthweights (g) were slightly lower among infants in the Swedish study (I: 1677 ± 549; C: 1737 ± 486)109,110 than among infants in the New Zealand study (I: 2007, SD 503; C: 1970, SD 535). 127 Preterm infants of both low and very low birthweights were included.
The mean gestational age at discharge for infants in the New Zealand trial was 36.1 weeks (SD 1.5) for the intervention group and 36.4 (SD 1.2) for the control group. 127 It is important to note that statistically significant differences were reported between comparison groups for infants’ mean weight (g) at discharge (I: 2381 ± 315; C: 2460 ± 317; p = 0.05) and number of days breastfeeding in hospital (I: 2.5 ± 2.0; C: 4.4 ± 2.8; p < 0.0001). 127 These characteristics were significantly in favour of the control group.
Inclusion criteria for the Swedish trial specified that infants must be medically stable for more than 1 week, experience no apnoeic episodes and be able to maintain normal body temperature in an open crib. 109,110 Gavage feeding excluded infants in the control group from discharge. 109
Mothers of infants in both trials were reported to have a mean maternal age of approximately 30 years127 and 31 years109 across both groups, and data from both trials indicate they were not significantly socioeconomically deprived. Over 90% of women in each group intended to breastfeed in the New Zealand trial. 127 Infant feeding intention was not reported in the Swedish trial although breastfeeding rates have been maintained at around 98% of all women in Sweden since 1991. 167
Characteristics of interventions
Both trials evaluated the effectiveness of early discharge with domiciliary nursing support compared with standard neonatal care. 109,110,127
Infants receiving routine care in the New Zealand study unit were discharged when competent to feed orally by breast or bottle without cardiorespiratory compromise and they had a sustained pattern of weight gain after the establishment of full oral feeding and adequate maintenance of normal body temperature when fully clothed in an open cot. 127 Infants in the intervention group were discharged early with home support when meeting the same criteria but without the need for weight gain. 127
In the Swedish trial,109 discharge criteria did not include any weight limit for either group but all infants had to be clinically well and able to gain weight satisfactorily by breast or bottle feeding.
An individual care plan was developed in conjunction with a parent for participants in the intervention group in one study. 109,110 This included information on the care and safety of preterm infants and instructions on nasogastric tube feeding, including a strategy to bring gavage feeding to an end with professional confirmation. Domiciliary care was provided by the project nurse, specialised in paediatric and neonatal nursing, and with the support from the hospital-based neonatologist, nutritionist, social worker and psychiatric team. Consulting visits to the neonatologist on the ward were scheduled for infants with bronchopulmonary dysplasia or heart disease. Domiciliary nursing care was provided until each infant met the ordinary criteria of the ward for hospital discharge.
Home support for infants discharged early in the New Zealand trial127 comprised daily visits for the first 7–10 days, including weekends, by a team of visiting nurse specialists who were also available by telephone 24 hours a day. Routine care comprised contact of each family by a team of experienced home care nurses to enable visiting to occur in hospital and after discharge. The home care nurses made home visits or telephone contact during office hours on weekdays on a daily basis for the first five weekdays after discharge and thereafter depending on support required.
Outcome assessment
Both trials reported the duration of any and exclusive breastfeeding at various time points. One trial reported duration rates at discharge and 6 weeks and 6 months after discharge127 and one trial reported duration rates after completion of the domiciliary care programme and at 6 months. 109,110 This trial also reported the total duration of the lactation period.
Weight gain was reported in one trial as mean weight (g) 6 weeks after discharge and weight gain (g/kg) per day. 127 Both trials reported rates of readmission to hospital including at 6 weeks after discharge and 6 months of age127 and during the first year follow-up period. 109
Psychosocial outcomes were the primary focus of the Swedish trial,109,110 including parental anxiety at hospital discharge, on completion of the domiciliary care programme and at 1 year, and parental assessment of their infant’s health at 1 year. Parental satisfaction with duration of breastfeeding was also reported. 109,110 Examples of positive and adverse comments by parents participating in the New Zealand trial were reported. 127
Both trials reported a range of process outcomes potentially influencing the effectiveness of the intervention109,127 with one trial reporting numbers of home visits achieved according to the individual care plan. 110
Cost-effectiveness outcomes were not reported in either trial. 109,110,127
Methodological quality of included trials
One RCT127 was rated as moderate quality. This relatively large trial reported detailed participant characteristics at baseline and process outcomes to further interpret comparability of groups before and during the intervention. 127 Significant differences in some infant characteristics at baseline, however, warrant caution in interpretation of findings (see below for details). This trial conducted appropriate statistical analyses but failed to present supporting numerator, denominator or percentage data for comparison groups. Failure to report withdrawals further limits scope to assess whether analysis has been conducted on an ITT basis. 127
One RCT109,110 was rated as poor quality. This trial presented breastfeeding outcome data as an illustrative graph without supporting numerator, denominator or percentage data. 109,110 Primary outcomes of interest for this trial were reported appropriately, however, and using an ITT analysis. Inadequate methods of randomisation and concealment, the absence of an a priori sample size calculation, and the moderate size of the trial warrant caution in interpretation of findings reported in this trial. 109,110
Details of the quality ratings for both trials are provided in Table 86 in Appendix 5.
Effectiveness of interventions
Primary outcomes
Results are not presented in a forest plot due to lack of appropriate outcome data in both trials and the poor quality rating of one RCT. 109,110
Neither trial found statistically significant differences in duration rates of any or exclusive breastfeeding at various time points up to 6 months after discharge127 or 6 months (definition not specified). 110
Secondary outcomes
A significantly lower mean infant weight (g) was reported among the intervention group than the control group at 6 weeks after discharge (I: 4034 ± 592; C: 4189 ± 731; p < 0.04) in one trial. 127 Daily weight gain (g/kg) measured for an undefined period was comparable (I: 12.18 ± 2.98; C: 12.15 ± 3.61; NS). 127
The authors of one trial127 reported no significant effect on rates of readmission to hospital at 6 weeks (p = 0.37) after discharge or at 6 months of age (p = 0.96). This was consistent with findings from the other trial,109 which reported no statistically significant differences between the groups in terms of rehospitalisations during the first year after discharge (p = 1.0).
A significant reduction in hospital stay of infants in the intervention group was reported as a result of the intervention in both trials. 109,110,127 One trial reported a mean hospital stay that was 9.3 days shorter109,110 and one reported a reduction to 2.5 days in the intervention group compared with 4.4 days in the control group. 127
Personality trait anxiety scores (mean scores) among mothers in the early discharge group were not increased compared with the control group at discharge [I: 32.8 (SD 5.9); C: 33.3 (SD 7.9), p = 0.75] and on completion of their domiciliary care programme [I: 31.7 (SD 7.1); C: 31.1 (SD 7.8), p = 0.74]. 109,110 Mean personality trait anxiety scores of fathers were lower in the intervention than in the control group at discharge [I: 27.8 (SD 5.9); C: 33.5 (SD 7.7), p < 0.05] and on completion of the domiciliary care programme [I: 29.0 (SD 6.1); C: 32.3 (6.9), p < 0.05]. Anxiety levels and confidence in handling the infant were comparable between groups at 1 year for both mothers and fathers. 109,110 This same trial reported lower levels of maternal satisfaction with the duration of breastfeeding among the intervention than the control group (I: 59.5%; C: 72.7%; ·2(1) = 0.8; p = 0.36). 109,110
Results from other study designs
No studies other than those included in systematic reviews or RCTs were identified.
Organisation of care interventions
No studies were identified that evaluated changes to organisation of care between units, for example, introduction of a clinical network. A total of four primary studies137,138,151,154 evaluating organisation of intra-unit care interventions were identified. As detailed in Table 14, one systematic review (McInnes and Chambers 2006)161 included two of these primary studies138,143 although one was assessed in that review as a study of finger feeding138 and not organisation of care. Three137,138,152 of the four primary studies were conducted in industrialised country settings and one was conducted in Brazil. 151 No RCTs or concurrent comparisons evaluating organisation of care were identified.
| Primary paper | Study design (n analysed)a | Inclusion in a systematic review | Country |
|---|---|---|---|
| Bicalho-Mancini 2004151 | Before/after (n = 495) | No | Brazil |
| Bell 1995154 | Before/after (n = 117) | No | USA |
| Merewood 2003137 | Before/after (n = 227) | McInnes 2006161 | USA |
| Oddy 2003138 | Before/after (n = 35) | bMcInnes 2006161 | Australia |
Results from RCTs
No RCTs evaluating organisation of care were identified.
Results from other forms of controlled studies
Four before/after studies evaluating organisation of care compared with previous standard care were identified137,138,151,154 (see Tables 70–72 in Appendix 4.1).
Characteristics of participants
Two studies included all surviving infants with medical records who had been born at the study hospital and admitted to the neonatal care unit during the defined study periods. 137,151
In one of these studies,137 only 44% (117/264) of all infants in the unit met the inclusion criteria. Most were excluded on unspecified grounds; other reasons included medical records, lack of feeding data, ineligibility to breastfeed, adoption or custody issues.
Mothers in this study137 were mostly black American or Hispanic and over half were on low incomes based on receipt of Medicaid. Nearly two-thirds of included infants were preterm with a gestational age of 30–37 weeks (B: 51.8%; A: 61.5%) or < 30 weeks (B: 4.5%; A: 6%). The remaining infants had a gestational age of > 37 weeks. Mean birthweight (g) for all infants was 2619 (SD 987) for the before group and 2506 (SD 939) for the after group; details by gestational age or proportions across the range were not reported. All infants were receiving enteral feeds by 2 weeks of age. Breastfeeding initiation rates for black American women are typically lower (52%) than the general population (69%) based on 2001 data. 168 Baseline rates would be expected to be lower among mothers of infants in neonatal care.
The second study151 excluded one mother who had no desire to breastfeed although this was not specified as an inclusion criterion for this study. This may reflect the typically high breastfeeding rates experienced in Brazil – 92% of infants were reported as having been ever breastfed in 1996; thus it is very unlikely that a mother would express no desire to breastfeed [WHO Global Data Bank on Breastfeeding and Complementary Feeding (http://apps.who.int/research/iycf/bfcf/bfcf.asp?menu=11), accessed 20 December 2007].
The study did not report participant characteristics by group or comparability at baseline. 151 Nearly 80% of all included infants were preterm and nearly 80% had a birthweight of < 2500 g. Although this study took place in the high-risk ward, no details were provided on the proportions of infants with very low birthweight. Only 13% of all included infants were classified as small for gestational age and 77% of all infants were on enteral feeds. 151 Mothers’ mean age was 25 years (SD 6.7) with approximately 50% of mothers having < 4 or 5–12 years of education.
Another US study included only mothers of infants in the study unit who intended to breastfeed. 154 The study conducted in Australia included infants born at the study hospital at 34–35 weeks’ gestation. 138 No participants’ characteristics, comparability at baseline, numbers excluded and reasons for exclusions were reported for either study.
Characteristics of interventions
Three studies evaluated the effect of Baby Friendly accreditation for the maternity hospital on neonatal care, comparing this with standard care previously provided. 137,138,151 The remaining study154 was a multifaceted intervention that evaluated the effect of protocol-based care for breastfeeding for the preterm or ill infant combined with assessment of staff educational needs and patient/family teaching records. 154
Specific changes to practice following Baby Friendly accreditation were detailed in one study. 138 These included the need for a signed consent form for mothers if they requested a bottle feed or pacifier for their infant, maternal education, ongoing staff education, and a home visiting scheme. ‘Finger feeding’, which is feeding via a nasogastric tube attached to the carer’s finger in place of a bottle and teat, was also introduced as a result of a different staff training event at the same time. Standard care for introduction of suck feeds prior to Baby Friendly accreditation was either breast or bottle feeds. 138
The protocol-based care intervention was developed as standard for the paediatric nursing division, as guidelines for orientation of residents and staff physicians and as an educational tool and guide for parents. 154 It included initial education of mothers through written materials and a video supported by nurses facilitating initiation of pumping within 24 hours of birth. All mothers were provided with a kit for double pumping. The second stage of the protocol recommended use of skin-to-skin or kangaroo care for initiating non-nutritive time at the breast. Recognition of appropriate hunger cues and latching technique combined with use of the Systematic Assessment of the Infant at the Breast (SAIB) scale were recommended to assess appropriate progress through the transitional stages of non-nutritive and nutritive sucking and breastfeeding. Continued pumping and gavage feeding was advised until breastfeeding was established with no introduction of bottles until gavage supplements were not needed. The nurse should complete discharge teaching of breastfeeding and arrange for local breastfeeding support in the final stages. 154 Staff educational needs for implementation of the protocol were assessed followed by a programme of training by two certified lactation consultants. Staff received a resource manual and a pocket reference card for the SAIB scale. The final component of the intervention comprised a revised patient/family teaching record to include the protocol-based stages of breastfeeding.
Outcome assessment
One study reported the initiation of breastfeeding and/or receiving breastmilk by any means during the first week of enteral feeds. 137 This study also reported duration of any, most or exclusive breastfeeding at 2 and 6 weeks. 137
Two studies reported the duration of exclusive breastfeeding at hospital discharge138,151 and one study reported duration of any breastfeeding at discharge. 154
One study undertook multivariate logistic regression to assess independent risk factors associated with non-exclusive breastfeeding at discharge. 151
No secondary outcomes were reported in any studies.
Methodological quality of included trials
One study was rated as good quality. 137 It is worth noting, however, that study findings relate to 44% (117/264) of infants admitted to the unit; for details see Characteristics of participants (above); see also Chapter 7).
One study was rated as moderate quality. 151 This study was fairly large comprising a total of 495 participants across the before and after groups. Some caution is warranted in interpretation of findings from this study as the extensive list of characteristics of participants was not reported by group. Numerator and denominator data were not clearly reported for the primary outcome despite being detailed elsewhere in the paper.
The remaining two studies were rated as poor quality. 138,154 In addition, these studies had very small sample sizes in at least one of the comparison groups, and in Oddy and Glenn138 the intervention coincided with a clinical intervention, the use of ‘finger feeding’. Findings of these studies should be interpreted with serious caution.
All studies analysed complete data sets for participants according to their original comparison group. Losses post allocation do not apply for these studies where retrospective case-review methodology was applied according to defined inclusion criteria. Details of the quality ratings for both trials are provided in Table 87 in Appendix 5.
Effectiveness of interventions
Primary outcomes
Individual relative risk estimates have been calculated on an ITT basis for all four studies where relevant outcome data were reported. 137,138,151,154 These results are presented in forest plots for primary outcomes in the two studies that did not receive a poor overall quality rating. 137,151
However, some caution is required in interpreting the results even of the better quality studies due to the inherent methodological weaknesses of a before/after study design. However, before/after studies are considered an appropriate study design for evaluations of a unit-wide multifaceted organisation of care intervention such as the Baby Friendly Initiative (BFI).
One study137 reported a significant increase in the number of infants receiving any breastmilk (by any means) during the first week of enteral feeds as a result of changes to organisation of care to achieve BFI accreditation (p = 0.00001) (Figure 29).
FIGURE 29.
Baby Friendly accreditation vs previous care: any breastmilk during first week of enteral feeds (ITT).
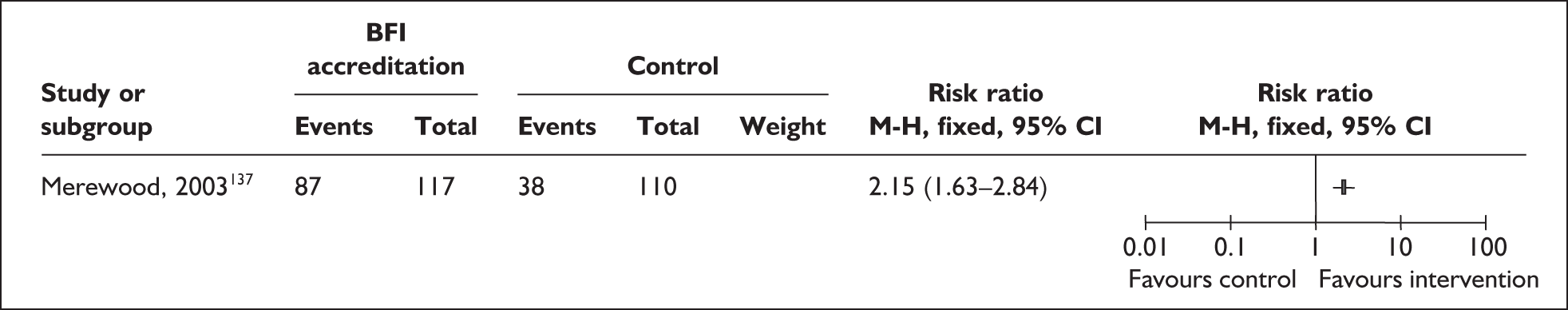
This US study also reported significant between-group increases (p = 0.005) in the duration of exclusive breastfeeding prior to discharge (at 2 weeks) after implementation of BFI accreditation137 (Figure 30). Significant increases in rates of exclusive breastfeeding were also demonstrated at hospital discharge in a large (n = 495) moderate quality before/after evaluation of BFI accreditation. 151
FIGURE 30.
Baby Friendly accreditation vs previous care: exclusive breastfeeding at or prior to hospital discharge (ITT).
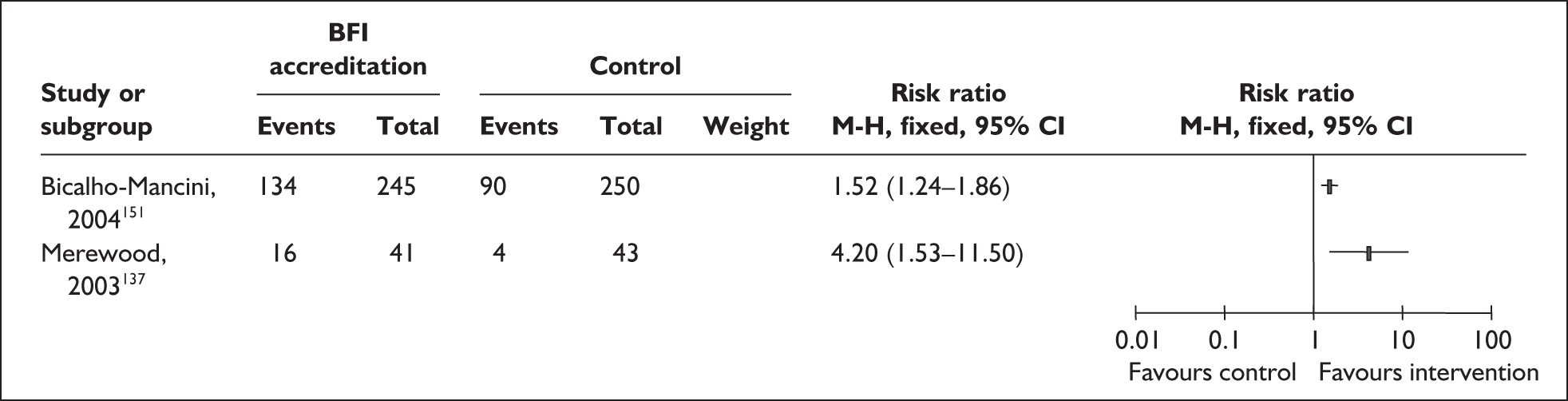
The US-based study137 found a statistically significant increase in the duration of any breastfeeding prior to discharge (at 2 weeks) (p = 0.001) among infants in neonatal intensive care (Figure 31). Numbers of infants remaining in the neonatal intensive care unit at 6 weeks were too small to provide meaningful results for the duration of any breastfeeding at this time point (B: 1/8; A: 6/9). 137
FIGURE 31.
Baby Friendly accreditation vs previous care: any breastfeeding prior to hospital discharge (ITT).
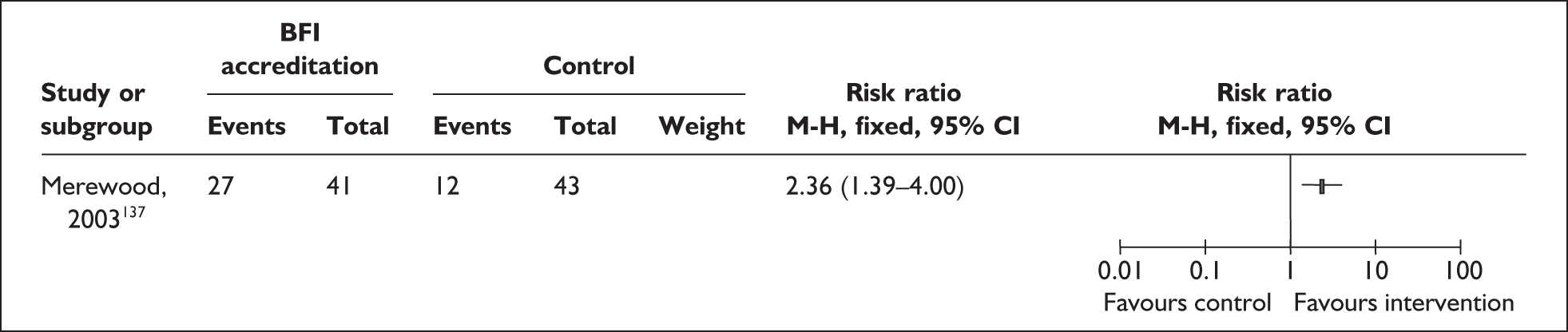
Neither of the two poor-quality studies identified differences in any or exclusive breastfeeding at hospital discharge or the percentage of women breastfeeding at discharge. 138,154 These findings warrant caution due to the study quality.
Chapter 5 Methods and results of economic modelling
Modelling background
Economic evaluations play a vital role in the allocation of health-care resources, and help to inform decisions about the efficient allocation of those resources. 169 The use of decision analysis allows the incorporation of all relevant evidence into a single framework. This evidence can be derived from a variety of sources including meta-analyses, clinical trials and national databases. The complexities of the issues being addressed through modelling mean that the evidence on the consequences and costs of interventions often cannot be derived from a single source.
Decision models provide an explicit framework for the implicit decisions that are already being made. 170 The issues regarding the treatment and care of preterm infants are extremely complex. The question of how to promote breastfeeding efficiently in neonatal units cannot easily be answered, and is certainly not answerable with reference to a single study. The use of an analytical framework, that is to say, a decision model, to allow the synthesis of all available evidence will provide decision-makers with a clearer picture of the meaning of the available evidence and where the uncertainty lies, and will assist in identifying crucial gaps in the available evidence.
The evidence base relating to breastfeeding in this particular population is scant, particularly in the area of economics. This is borne out by the lack of economic evaluations identified for inclusion within the review. However, it is important that all the available evidence is used to establish the long-term costs and benefits of increasing the uptake of breastfeeding in this population. Given the sparse nature of the evidence base the model used is likely to be an oversimplification of a complex problem. However, capturing all of the available evidence in a single framework will provide us with some useful evidence, while also generating some important and relevant questions and offering potential for use in future research.
Methods for health economics modelling
This work was informed throughout by input from clinical experts (see Appendix 1 and Acknowledgements) and by discussion within the whole research team. In the absence of any existing economic evidence, the decision was made to develop a decision model for one important topic, enhanced staff contact. This included both additional skilled support from staff and staff training. These interventions are discussed in the previous chapter (see Breastfeeding education and support interventions; and Staff training interventions). Only one study of support166 and one of staff training149 included all mothers, not just those intending to breastfeed, and therefore these studies were used to inform the concept of ‘enhanced staff contact’.
While other approaches to the promotion of breastfeeding were considered in the main effectiveness review, the issue of enhanced staff contact was deemed the most useful and applicable decision problem to evaluate in this part of the work. It was noted that the effectiveness of enhanced staff contact will also depend on the milk expression methods and incentives available in any particular centre, and the attraction of breastfeeding to the mother is likely to depend on privacy and ease of expression, storage and delivery of milk to the neonatal unit. Therefore, this model investigates the cost-effectiveness of enhanced staff contact given reasonable provision of privacy arrangements and free expression kits.
A decision tree was developed, using the software package data professional (TreeAge Software) to synthesise data on enhanced staff contact, breastmilk effectiveness, incidence of necrotising enterocolitis (NEC) and sepsis, resource use, survival and utilities. The objective of the model was to estimate the long-term costs and benefits of enhanced staff contact in promoting breastfeeding to mothers whose infants were admitted to neonatal units.
The premise of the model structure was that enhanced staff contact increases milk expression; in turn, it was assumed that this would lead to increased milk consumption by the infant. Milk consumption was then assumed to reduce the incidence of illness episodes thereby improving long-term health outcomes. The health benefits evaluated were quality-adjusted life-years (QALYs).
The population of interest was defined in accordance with the inclusion criteria for the review, that is to say, all mothers with infants in neonatal units. However, the evidence suggested that the greatest benefit could be achieved in those infants born earlier and smaller, therefore the population was limited to those infants < 2500 g. For the purpose of model development a hypothetical cohort of indeterminate size was divided into weight-based subgroups, which were modelled separately. The clinical rationale for these subgroups was that the incidence of diseases increases greatly as the birthweight decreases. These subgroups were: 500–999 g; 1000–1749 g; and 1750–2500 g. The perspective was that of the NHS, and costs and benefits were discounted at an annual rate of 3.5%, as recommended by the current NICE guidelines. 103
The structure of the model was determined by the evidence obtained during early stages of the effectiveness review, and by clinical studies identified in the additional modelling searches (see Appendix 2.3). It was then finalised by means of a series of meetings with clinical advisors. An illustration of the model structure is provided in Figures 32–34. A brief summary of the structure is provided in the following section; a number of subheadings have been used to aid understanding. A number of assumptions were made to facilitate modelling; these are outlined and explained in a later section.
FIGURE 32.
Model structure: intention to breastfeed.
FIGURE 33.
Model structure: breastmilk effectiveness and intermediate health outcomes. NEC, necrotising enterocolitis.
FIGURE 34.
Model structure: long-term outcomes. NDI, neurodevelopmental impairment

The two interventions evaluated in the base-case model were enhanced staff contact – the addition of specially trained staff, who would be available to advise and support mothers on milk expression and breastfeeding, compared with normal staff contact – that is to say, no addition of specially trained staff.
The model starts by dividing the population into those women who intend to breastfeed and those who do not intend to breastfeed prior to their infant’s birth. The literature suggested that the mother’s intention influenced the infant’s likely breastmilk consumption level171 (Figure 32).
The model was designed to capture the health effects for three different levels of milk consumption, namely:
-
all own mother’s milk
-
some mothers’ milk, which was supplemented by formula in the base case
-
formula alone.
The literature suggested that different levels of mothers’ milk consumption impacted on the health of the infant. In addition, the literature demonstrated that there were differences in effectiveness between donor breastmilk and preterm formula and, therefore, potential cost and benefit differences.
Currently in the UK donor breastmilk is neither widely nor readily available in the majority of units. In order to reflect this, two separate models were used in an attempt to capture the current situation where the use of donor breastmilk would be dictated by availability not choice. So, with the limited number of donor milk banks, the majority of infants would receive preterm formula due to a lack of any other option. By using two separate models, we hoped that we would be able to provide useful information regarding the long-term costs and benefits of supplementing with donor milk, alongside the long-term costs and benefits of supplementing with preterm formula, thereby reflecting the two separate situations that are current practice in the UK.
The in-hospital clinical outcomes of interest were sepsis, confirmed NEC (Bell stage II or greater) and mortality. These clinical outcomes, while not an exhaustive list, were deemed by our clinical advisors to be the most common and clinically relevant outcomes that are claimed to be linked to breastmilk intake. NEC was divided into medically treated NEC and surgically treated NEC, as both outcomes and resources varied depending on treatment. Suspected NEC was excluded from the analysis. As health benefits and potential resource use also varied dependent on the type of sepsis, this was subdivided into Gram-negative, Gram-positive and fungal infection. Mortality rates varied depending on the diagnosis (see Table 15). These subdivisions of clinical outcomes allowed the differences in resource use and utility outcomes to be captured (Figure 33).
| Data | Mean or odds ratio | SD or 95% CI | Source |
|---|---|---|---|
| Intention to breastfeed rate | 0.72 | Bolling 200749 | |
| For the enhanced staff contact intervention | |||
| For mothers intending to breastfeed | |||
| Probability of MM > 80% of total milk intake | 0.678 | cSisk 2006106 | |
| Probability of 80% > MM > 0.01% of total milk intake | 0.278 | cSisk 2006106 | |
| Probability of MM < 0.01% of total milk intake | 0.043 | cSisk 2006106 | |
| For mothers not intending to breastfeed | |||
| Probability of MM > 80% of total milk intake | 0.160 | cSisk 2006106 | |
| Probability of 80% > MM > 0.01% of total milk intake | 0.506 | cSisk 2006106 | |
| Probability of MM < 0.01% of total milk intake | 0.333 | cSisk 2006106 | |
| For the normal staff contact intervention | |||
| Odds of ever receiving own mother’s milk | |||
| Normal staff contact vs enhanced contact | 0.500 | 0.34–0.75 | Gonzalez 2003,166 Pineda 2006149 |
| Baseline incidences given MM and formula consumption | |||
| Incidence of sepsis by weight subgroup | |||
| 500–999 g | 0.272 | Fanaroff 1998177 | |
| 1000–1749 g | 0.082 | Fanaroff 1998177 | |
| 1750–2500 g | 0.047 | Fanaroff 1998177 | |
| Incidence of medical NEC by weight subgroup | |||
| 500–999 g | 0.035 | Guthrie 2003176 | |
| 1000–1749 g | 0.021 | Guthrie 2003176 | |
| 1750–2500 g | 0.005 | Guthrie 2003176 | |
| Incidence of surgical NEC by weight subgroup | |||
| 500–999 g | 0.033 | Guthrie 2003176 | |
| 1000–1749 g | 0.006 | Guthrie 20037176 | |
| 1750–2500 g | 0.001 | Guthrie 2003176 | |
| Odds of confirmed NEC (medical and surgical) | |||
| MM vs MM and donora | 0.885 | 0.69 | Schanler 2005173 |
| MM and donor vs MM and formulab | 0.465 | 0.656 | Lucas 1990174 |
| Formula vs MM and formula | 3.006 | 0.40 | Lucas 1990174 |
| Odds of sepsis | |||
| MM vs MM and donor | 0.709 | 0.38 | Schanler 2005173 |
| MM and donor vs MM and formula | 0.997 | 0.34 | Schanler 2005173 |
| Formula vs MM and formula | 0.803 | 0.15 | Vohr 200610 |
| Distribution of sepsis cultures | |||
| Gram-positve | 0.689 | Stoll 2002178 | |
| Gram-negative | 0.196 | Stoll 2002178 | |
| Fungal | 0.115 | Stoll 2002178 | |
| Baseline mortality rates given no disease | |||
| 500–999 g | 0.205 | Hintz 2005179 | |
| 1000–1749 g | 0.08 | Fanaroff 1998,177 Stoll 2002178 | |
| 1750–2500 g | 0.05 | dNHS, Scotland | |
| Odds of mortality | |||
| Gram-positive sepsis vs no disease | 1.609 | 0.12 | Stoll 2002178 |
| Gram-negative sepsis vs no disease | 7.263 | 0.14 | Stoll 2002178 |
| Fungal sepsis vs no disease | 5.969 | 0.18 | Stoll 2002178 |
| Medical NEC vs no disease | 2.055 | 0.14 | Hintz 2005179 |
| Surgical NEC vs no disease | 3.124 | 0.12 | Hintz 2005179 |
| Baseline incidence of NDI given no disease | |||
| 500–999 g | 0.485 | Larroque 2008180 | |
| 1000–1749 g | 0.413 | Larroque 2008180 | |
| 1750–2500 g | 0.343 | Larroque 2008180 | |
| Odds of NDI | |||
| Sepsis vs no disease | 2.282 | 0.07 | Stoll 2004181 |
| Medical NEC vs no disease | 1.187 | 0.19 | Hintz 2005179 |
| Surgical NEC vs no disease | 1.985 | 0.19 | Hintz 2005179 |
| Distribution of severity of disability given NDI | |||
| Severe disability | |||
| 500–999 g | 0.164 | Larroque 2008180 | |
| 1000–1749 g | 0.079 | Larroque 2008180 | |
| 1750–2500 g | 0.141 | Larroque 2008180 | |
| Moderate disability | |||
| 500–999 g | 0.297 | Larroque 2008180 | |
| 1000–1749 g | 0.236 | Larroque 2008180 | |
| 1750–2500 g | 0.209 | Larroque 2008180 | |
| Mild disability | |||
| 500–999 g | 0.538 | Larroque 2008180 | |
| 1000–1749 g | 0.685 | Larroque 2008180 | |
| 1750–2500 g | 0.650 | Larroque 2008180 | |
| Utilities | |||
| No disability | 0.940 | 0.12 | Colbourn 2007172 |
| Mild disability | 0.850 | 0.10 | Colbourn 2007172 |
| Moderate disability | 0.645 | 0.12 | Colbourn 2007172 |
| Severe disability | 0.470 | 0.25 | Colbourn 2007172 |
| Life expectancy | |||
| No disability | 78.5 | Colbourn 2007172 | |
| Mild disability | 78.5 | Colbourn 2007172 | |
| Moderate disability | 67.8 | Colbourn 2007172 | |
| Severe disability | 26.1 | Colbourn 2007172 | |
| Costs | |||
| Minutes of staff contact timee | |||
| Initial contact | 45 | Gonzalez 2003166 | |
| Further contact | 150 | Gonzalez 2003166 | |
| Unit costs (£) | |||
| Registered nurse (£/hour) | 41.12 | Curtis 2006183 | |
| Level 1 neonatal unit | 939.00 | 310.20 | DH 2008184 |
| Level 2 neonatal unit | 671.00 | 178.38 | DH 2008184 |
| Special Care Baby Unit | 405.00 | 99.80 | DH 2008184 |
| Major neonatal diagnosis | 1514.00 | 838.10 | DH 2008184 |
| Lifetime cost of disability (£) | |||
| Mild disability | 14,421 | Colbourn 2007172 | |
| Moderate disability | 13,959 | Colbourn 2007172 | |
| Severe disability | 364,005 | Colbourn 2007172 | |
Long-term outcomes
The selected clinical outcomes are considered to be intermediate outcomes and it was therefore necessary to link these to a final outcome.
In this instance, QALYs were used as the long-term outcome. This was achieved by a two-step process. First, the intermediate outcomes were linked to disability by means of neurodevelopmental impairment (NDI), which was reported in the clinical papers identified. NDI is a composite measure that captures many elements of disability including visual impairment, hearing impairment and mobility. The NDI scores were divided into four disability categories, namely: no disability, mild disability, moderate disability and severe disability. The probability of the infant ending up in each state depended on their clinical pathway. Utility values for each of the health states were then used to quality-weight life expectancy. Utilities are used as a means of representing the strength of individuals’ preferences for precise outcomes, in this instance disability states, under conditions of uncertainty. They fall between 0 and 1, with 1 representing perfect health. Life expectancy for infants in each of the four disability states was taken from Colbourn et al. (2007). 172 A combination of life expectancy and utilities were used to derive QALYs for each of the disability states. These were discounted at an annual rate of 3.5% (Figure 34). Discounting is a widely accepted practice in economic evaluations and allows future gains and losses to be weighted to reflect preferences for consumption now rather than in the future. 103
Modelling assumptions
In order to facilitate modelling a number of simplifying assumptions were required. These are outlined below.
-
It was assumed that clinical data from a number of countries, mainly the USA, were transferable to the UK setting.
-
The impact of fortification of mothers’ milk was assumed to be neutral. It can be assumed that all breastmilk data from the USA are based on fortified milk. However, this is not the case in the UK, where fortification is not routine practice. Data were derived from studies conducted in both settings.
-
In the base case, the effectiveness of enhanced staff contact was assumed to be equal regardless of the intent of the mother regarding breastfeeding. This assumption was tested in sensitivity analysis. (Details of the findings are presented below; see Intervention effectiveness estimate.)
-
The disability health state of the infant at diagnosis was assumed to remain constant throughout the lifetime of the infant. This may seem like a strong assumption; however, there was no evidence to suggest that an infant diagnosed as moderately disabled would either improve enough to change their classification to mild, or deteriorate sufficiently to change their classification to severe. Therefore, no sensitivity analysis was undertaken.
-
The issues of multiple births have not been considered.
-
The main possible health effects specific to this population of feeding mothers’ breastmilk to infants in neonatal units are on the reduction of NEC and sepsis. The negative effect of breastmilk through postnatal vertical transmission of cytomegalovirus has not been incorporated into the model. Other outcomes such as gastroenteritis, respiratory disease, and cognitive impairment have not been considered.
The search process to support the economic model was undertaken in a number of stages and included those searches already undertaken for the systematic review of economic evaluations (see Chapter 3). Full details of all searches relevant to the economics are presented in Appendix 2.2.
Input parameters
All model input parameters are presented in Table 15.
The intervention evaluated was enhanced staff contact, which consisted of additional specially trained staff. Two papers were considered appropriate for inclusion: Gonzalez et al. (2003)166 and Pineda (2006),149 which both included a suitable intervention, aimed at all mothers with infants in a neonatal unit. These two papers provided us with the probability of an infant receiving his or her own mother’s milk. Gonzalez et al. 166 evaluated the introduction of a lactation consultant, and Pineda149 the introduction of staff education and leaflets. For the purpose of the base case, we have assumed that both interventions lead to the same amount of enhanced specially trained staff contact for the mothers and infants. Therefore, a pooled odds ratio weighted by sample size was derived. This was then translated into a probability, which was used in the model. This assumption was tested in sensitivity analysis.
A baseline intention to breastfeed rate for England and Wales was taken from the Infant Feeding Survey 2005. 49 This rate was varied across plausible ranges, including the Northern Ireland rate, in sensitivity analysis.
Data from Sisk et al. (2006)106 were used to estimate incidence rates for the different types of milk consumption (all own mother’s milk, some own mother’s milk and formula) for mothers who intended to breastfeed prior to birth and those who did not. As the Sisk paper106 evaluated lactation counselling for mothers, the data obtained were used as the baseline for the enhanced staff contact branch of the model, as this seemed most appropriate.
The normal staff contact probabilities were obtained by adjusting the rate of receiving the different consumption levels by the relative effectiveness of the intervention.
The literature was searched for papers that reported two or more of the milk consumption categories of interest, namely: (1) formula only; (2) some mothers’ milk and formula supplement; (3) some mothers’ milk and donor supplement; and (4) mostly or all own mother’s milk (mostly was defined as > 80%) – and which evaluated the impact of breastmilk consumption on either the incidence of confirmed NEC (stage II or III) or sepsis. Priority was given to systematic reviews, RCTs and cohort studies. Studies with larger study populations were given greater weight.
The studies identified for NEC outcomes were Schanler et al. (2005)173 and Lucas and Cole (1990). 174 From the data provided in these papers a number of odds ratios were derived comparing different milk consumptions. In addition, studies identified for sepsis outcomes were Schanler et al. (2005)173 and Vohr et al. (2006). 10 Fortifier was added to mothers’ milk in the Schanler study, and in 75% of breastmilk feeds without parenteral nutrition in the Vohr study. It is not routine practice in the UK to add fortifier to mothers’ milk, and the impact of fortifier on outcomes is not clear but has been assumed neutral in this analysis.
A number of studies were identified through the additional modelling searches that reported the incidence of NEC and sepsis. In order to derive model inputs it was necessary to identify those studies that either reported the data for the relevant subpopulations or that would allow the data for the subpopulations to be calculated. The baseline incidence rates of NEC for the relevant weight bands were obtained by combining absolute numbers of NEC from Gilbert et al. (2003)175 with incidence rates per 250-g bands from Guthrie et al. (2003). 176 Discrete exponential growth of NEC prevalence numbers by birthweight bands was assumed. These data were deemed an appropriate baseline for the own mother’s milk plus formula branch. As the model subdivided NEC into medical and surgical, these were the incidence data that we extracted from Guthrie et al. 176
The baseline incidence of sepsis was derived from Fanaroff et al. (1998)177 and, like the Guthrie paper,176 the infants in the study had consumed a mix of own mother’s milk supplemented by formula. Hence, the incidence data derived seem most appropriate to the own mother’s milk plus formula branch. Sepsis data were presented as an aggregate rate and not for each of our subdivisions – Gram-positive, Gram-negative and fungal. Due to the differences in mortality rate and disability outcome that occur given each type of infection, it was important to ensure that the incidence of sepsis was further subdivided. The data for incidence of Gram-positive, Gram-negative and fungal were derived from Stoll et al. (2002)178 and were used to ascertain the probability of Gram-positive, Gram-negative and fungal, given a diagnosis of sepsis.
Mortality rates varied depending on the subpopulation being evaluated and the clinical outcome. Baseline rates for no NEC/no sepsis was derived from Hintz et al. (2005)179 for the 500–999 g group, from Fanaroff et al. (1998)177 and Stoll et al. (2002)178 for the 1000–1749 g group, and from a report Small Infants in Scotland (http://isd.scot.nhs.uk/isd/files/mat_bb_small_babies.pdf) from the Information and Statistics Division of NHS Scotland for the 1750–2500 g group. The odds ratio for mortality given medical NEC and surgical NEC was also derived from the Hintz et al. 179 paper and adjusted, where necessary, by the baseline mortality rate. Again, the probability of death given sepsis (Gram-positive, Gram-negative and fungal) was obtained from Stoll et al. 178 and adjusted for the baseline mortality rate in order to derive the probability of death given the clinical infection present in the infant.
Preterm infants who survive NEC and sepsis can be left with long-term disabilities. For the purpose of the model, we assumed that infants could have no neurodevelopmental problems, mild disability, moderate disability or severe disability. The NDI, a composite measure that captures many elements of disability including visual impairment, hearing impairment and mobility, was derived from Larroque et al. (2008). 180 This was the only paper identified that reported the relevant data for the three weight populations of interest and that provided sufficient data on the distribution of disabilities between the three severities, i.e. mild, moderate and severe. The incidence of severe disability for the 1750–2500 g infants appeared to be quite high, and this parameter was therefore considered in sensitivity analysis. The odds ratio for developing NDI from sepsis compared to no disease was obtained from Stoll et al. (2004). 181 The odds ratio for developing NDI from medical or surgical NEC compared to no disease was obtained from Hintz et al. 179
A published HTA report171 was used to obtain the utility estimates. As previously outlined, utilities allow life-years to be given health quality weights. The HTA report used a primary study, that by Oostenbrink et al. (2002),182 which had evaluated utilities using the EQ-5D to derive a valuation for preterm infants. The paper presented vignettes to 28 paediatricians in the Netherlands for seven case descriptions. In line with the approach taken by Colbourn et al.,172 utilities for mild, moderate and severe disabilities were derived from these seven case descriptions by grouping them into three clusters of severity and taking the average. Life expectancy given the final disability state of the infant was also taken from the HTA report. 172
Costing
The perspective adopted for the economic evaluation was that of the service provider (UK NHS). In accordance with this perspective, the costs included in the economic analysis were the direct costs incurred as a result of the interventions. These included: the intervention costs, treatment of NEC and sepsis, length of inpatient stay in level I, II or III units and the lifetime cost of disability. The price year was 2006/2007 and all prices were appropriately inflated using the health component of the consumer price index. Although the intervention will potentially increase breast pump resource use and donor milk consumption, and decrease formula consumption, these costs were excluded as independent cost items in the base-case model because the current practice of provision of breast pumps is not clear and therefore difficult to cost. It did not seem appropriate to include formula and not breast pumps or expression kits in the model, so both were left for sensitivity analyses to explore. Breast pumps and formula costs are likely to be included in the inpatient stay costs as part of hotel costs; however, this does not capture the incremental effect of the intervention on these resources.
The clinical effectiveness papers used to derive the model inputs concerned the provision of a lactation consultant to encourage the mothers to express milk, and to advise and help the mothers in expressing milk and answering general questions.
Resource use is represented by the time made available to the mothers by the lactation consultant. Using the paper by Gonzalez et al. ,166 this was assumed to be:
-
45 minutes of initial contact with each mother to encourage milk expressing
-
30 minutes developing milk expression plan
-
60 minutes helping milk expression at two sessions
-
60 minutes responding to questions and providing additional help.
For the purpose of our model, all mothers were initially assumed to use the full 45 minutes of specially trained staff time. However, only those mothers who decided to try to express milk were assumed to consume the extra time of the specially trained staff. Therefore, only those following the own mother’s milk pathway and the some mothers’ milk plus supplement pathway were assigned costs for the additional 2.5 hours of contact. This was an assumption on our part, as the paper did not provide sufficient detail to ascertain exactly how much time each mother or each pathway consumed. However, it is unlikely that if a mother decided not to express milk in order to feed her infant that further time would be spent initiating expression and establishing a milk plan.
The unit cost was based on the hourly rate of a registered sick children’s nurse, which was varied in sensitivity analysis to reflect the increase in cost if a midwife were used to provide the additional support.
The diagnosis of medical NEC and sepsis can include tests such as: microbiological culture tests for blood and urine; chest X-rays; cerebrospinal fluid; radiography; retinal examination; echocardiography; and renal ultrasonography. Treatments may include the use of broad-spectrum antibiotics, often intravenously administered; bowel rest; and regular monitoring of C-reactive protein and platelet count. These resources were not individually included as outlined below.
All hospitalised infants incur per patient/day costs as do infants without NEC/sepsis. The unit cost for an inpatient day was taken from the NHS Reference Costs for 2006/07184 for the following levels:
-
Neonatal Intensive Care Level 1 – unit cost of £939
-
Neonatal Intensive Care Level 2 – unit cost of £671
-
Special Care Baby Unit (Level 3) – unit cost of £405.
Supporting documents for the NHS Reference Costs indicate that the unit costs for these units include: hotel services, nursing, therapy services, medical staff, ward consumables, blood and blood products, drugs, diagnostics (e.g. pathology and radiology), and medical and surgical equipment [including specialist equipment, e.g. CPAP (continuous positive airway pressure) and NIPPV (non-invasive positive pressure ventilation) machines]. No theatre costs are included.
Identification of the volume and type of resources consumed was problematic and, as a result, the unit cost of an inpatient day for the three levels was used to reflect the different volumes consumed, and therefore costs incurred, by the infants. This assumption seemed reasonable given that the costs for the treatments and diagnostics appear to be included in the unit cost for an inpatient day. Additionally, if their costs had been derived separately, it was not clear if this would lead to an element of double counting.
Hall and Pierro (2004)185 suggest that there are three general approaches to surgery for NEC. These are: peritoneal drainage alone, peritoneal drainage followed by laparotomy (bowel resection, stoma formation, clip and drop); and laparotomy alone (bowel resection, stoma formation, clip and drop).
Correspondence with several clinical experts suggested that peritoneal drainage alone is rarely conducted in the UK setting. Therefore, it was agreed that the cost of laparotomy alone or with drainage would be included in the model. The same unit cost was used for both procedures, as it was assumed that drainage and laparotomy were both included in the average cost of treating a major neonatal diagnosis. The unit cost was derived from the NHS Reference Costs. 186
Average length of stay data for the UK setting were not available. In order to facilitate modelling, we combined length of stay data from Bisquera et al. ,29 Stoll et al. 178 and Fanaroff et al. ,177 all these containing US data. These data were then used to derive length of stay estimates for the different levels of care for infants divided into the three weight groups and infants divided into the following health episode groups: no NEC/sepsis; sepsis; confirmed medical NEC; and surgical NEC. This information was used to estimate the number of days that infants within a certain weight category, having had a particular health episode, would spend consuming the resources required from a level 1 NICU and from a level 2 NICU or a SCBU (level 3). The length of stay details are presented in Table 16. It should be noted that Table 16 shows the incrementallength of stay in a level 1 unit attributable to NEC and, hence, for the control group is zero. The unit cost for consuming resources from a level 2 NICU or a SCBU will be an average of the unit costs for the levels of care specified above.
| Surgical NEC | Medical NEC | Control | Sepsis | |
|---|---|---|---|---|
| 500–999 g | ||||
| Level 1 | 42 | 27 | 0 | 30 |
| Level 2 | 86 | 67 | 62 | 59 |
| Level 3 | 86 | 67 | 62 | 59 |
| 1000–1749 g | ||||
| Level 1 | 25 | 16 | 0 | 18 |
| Level 2 | 51 | 40 | 37 | 35 |
| Level 3 | 51 | 40 | 37 | 35 |
| 1750–2500 g | ||||
| Level 1 | 25 | 16 | 0 | 18 |
| Level 2 | 51 | 40 | 21 | 35 |
| Level 3 | 51 | 40 | 21 | 35 |
For each year of life it was necessary to include an annual cost that would be incurred solely as a result of the disability state. These costs were taken from Trotter and Edmunds (2002),187 who identified costs for mild, moderate and severe disability given survival of meningococcal disease, which can result in a variety of long-term sequelae. The authors derived their estimates from the Unit Costs of Health and Social Care, 2000. These costs adjusted for inflation are presented in Table 17.
| State | Annual cost (£) |
|---|---|
| No disability | 0 |
| Mild disability | 599.07 |
| Moderate disability | 599.07 |
| Severe disability | 25,759.91 |
Cost-effectiveness analysis
To compare the costs and consequences of the alternative intervention strategies, cost-effectiveness ratios were estimated as the cost per QALY gained. Those strategies with lower effectiveness and higher costs (dominated strategies) were eliminated from the analysis, and incremental cost-effectiveness ratios (ICERs) were estimated. An ICER is a ratio of the difference in cost between two interventions and the difference in effectiveness of the same two interventions. Its use allows the impact of switching from one intervention to the other to be evaluated.
A probabilistic sensitivity analysis (PSA) was performed on all three base-case models in order to incorporate statistical uncertainty into the analysis. This allowed some assessment of the effect on the results of simultaneously varying different parameters. Appropriate parameter distributions were selected, according to the nature of the data, for those input parameters for which suitable data were available. For probability parameters where only two categories of event were possible (i.e. NDI or no NDI) beta distributions were used. All odds ratios were given log-normal distributions and, finally, for those events for which more than two categories of event were possible, a Dirichlet distribution was used in order to account for the polychotomous nature of the variable. 188 The parameter distributions are fully reported in Appendix 8.
The unit costs per litre of donor milk and per 200 ml of preterm formula are presented in Table 18. These were not used in the base case, although a second model was evaluated incorporating donor milk costs. It was assumed that infants in the 500–999 g birthweight population commenced feeding on 60 ml/kg, then progressed to 80 ml/kg, then progressed to 120 ml/kg, and then to 150 ml/kg. It was assumed that, for the surgical NEC infants approximately 6.5 days were spent on each feed volume; for the medical NEC infants approximately 4.8 days; for the sepsis infants 4.2 days; and for the no NEC/no sepsis infants 4.4 days. Infants were then progressed to 200 ml/kg for the remainder of their estimated stay in the unit.
| Milk supplements | Unit cost (£) | Source |
|---|---|---|
| 200 ml of formula | 1.36 | BNFb |
| 1 litre of donor milk | 289.12 | BMBWGa |
It was assumed that infants in the 1000–1749 g population commenced feeding on 60 ml/kg, and then progressed to 120 ml/kg, followed by 150 ml/kg. It was assumed that, for the surgical NEC infants, approximately 4.4 days were spent on each feed volume; for the medical NEC infants approximately 3.4 days; for the sepsis infants 3 days; and for the no NEC/no sepsis infants 3 days. Infants were then progressed to 200 ml/kg for the remainder of their estimated stay in the unit.
For infants in the 1750–2500 g population it was assumed that they commenced feeding at 80 ml/kg and then progressed to 150 ml/kg. For the surgical NEC infants it was assumed that they spent approximately 6 days at each feeding rate; for the medical NEC 5 days; the sepsis group 4.5; and the no NEC/no sepsis 2.5 days.
These estimates are based on length of stay and weight multiplied by the number of ml/kg divided by the number of feeds.
Results
Base case
Three populations defined by birthweight were considered in the base-case models. The populations were: 500–999 g, 1000–1749 g and 1750–2500 g. The two alternative feeding supplements – donor milk and preterm formula – were evaluated in separate models for each birthweight population. It was felt that this best reflected the current situation regarding feeding supplementation for this population. Donor milk as a supplement was considered to be the situation in a minority of units in the UK. 91 However, a second model was created with the cost for donor breastmilk supplementation incorporated.
The base-case results of the cost-effectiveness analysis for each of the subpopulations defined by weight are reported in Table 19. In each of the subpopulations, the enhanced staff contact intervention was both less costly and more effective than the comparator, normal staff contact. Enhanced staff contact was the dominating intervention.
| Intervention | Cost (£) | Incremental cost | Benefits (QALY) | Incremental benefit | ICER (£/QALY) | |
|---|---|---|---|---|---|---|
| Base case | ||||||
| 500–999 g | Enhanceda | 86,759 | 14.70 | |||
| Normalb | 87,345 | 586 | 14.45 | – 0.251 | Dominated | |
| 1000–1749 g | Enhanced | 56,947 | 21.05 | |||
| Normal | 57,240 | 293 | 21.00 | – 0.056 | Dominated | |
| 1750–2500 g | Enhanced | 47,228 | 21.92 | |||
| Normal | 47,294 | 66 | 21.91 | – 0.009 | Dominated | |
| Donor milk supplements | ||||||
| 500–999 g | Enhanced | 88,029 | 14.75 | |||
| Normal | 88,107 | 78 | 14.46 | – 0.290 | Dominated | |
| 1000–1749 g | Enhanced | 58,195 | 21.06 | 3531 | ||
| Normal | 57,970 | – 225 | 21 | – 0.064 | ||
| 1750–2500 g | Enhanced | 48,145 | 21.92 | 34,905 | ||
| Normal | 47,816 | – 328 | 21.91 | – 0.010 | ||
The effectiveness of mothers’ milk in reducing both the incidence and severity of NEC and sepsis showed positive health impacts for the intervention arm of the model at a reduced cost. Given that the intervention was relatively cheap per infant, consuming only 45 to 195 minutes of staff time, this meant that the cost savings from reduced expenditure on treating NEC and sepsis were always greater than the cost of the intervention. A full breakdown of the cost results per infant are presented in Table 20.
| Intervention | Total cost (£) | Intervention cost (£) | Treatment costs (£) until discharge | Long-term disability costs (£) | Donor milk cost (£) | |
|---|---|---|---|---|---|---|
| Base case | ||||||
| 500–999 g | Enhanceda | 86,758 | 121 | 55,572 | 31,065 | – |
| Normalb | 87,344 | 0 | 56,405 | 30,939 | – | |
| 1000–1749 g | Enhanced | 56,947 | 121 | 38,159 | 18,666 | – |
| Normal | 57,240 | 0 | 38,527 | 18,712 | – | |
| 1750–2500 g | Enhanced | 47,228 | 121 | 22,648 | 24,458 | – |
| Normal | 47,294 | 0 | 22,816 | 24,478 | – | |
| Donor milk supplements | ||||||
| 500–999 g | Enhanced | 88,029 | 121 | 55,340 | 31,057 | 1512 |
| Normal | 88,107 | 0 | 56,304 | 30,942 | 862 | |
| 1000–1749 g | Enhanced | 58,195 | 121 | 38,072 | 18,649 | 1353 |
| Normal | 57,970 | 0 | 38,490 | 18,707 | 772 | |
| 1750–2500 g | Enhanced | 48,145 | 121 | 22,619 | 24,455 | 949 |
| Normal | 47,816 | 0 | 22,799 | 24,476 | 541 | |
The incidence of disease was inversely correlated with infant weight, and hence the health benefit and cost savings decreased as the birthweight increased. However, the base-case analysis showed that, despite declining incidence in the heavier populations, the intervention was found to be cost saving.
The cost estimate of a litre of donor milk was £289.12. This was the estimate for a milk banking set-up that represented a slight improvement over the milk bank system taken from the UK Breastmilk Banking Working Group report. 91 The model assumed that donor milk was only given to those infants who received some mothers’ milk. This may not reflect reality, where an infant whose mother was unable to provide milk may in fact receive 100% donor milk. However, to facilitate modelling, it was assumed that if the mother provided some milk, then in the donor milk supplementation model, the preterm infant would receive donor milk supplements. As the intervention increased the number of mothers expressing, and therefore the number of infants receiving some mothers’ milk, logically the results show an increase in cost due to the additional donor milk.
Nevertheless, in the 500–999 g population, the intervention still dominated normal contact. However, this was no longer the case for the heavier birthweight populations (see Table 19). For the 1000–1749 g population, the ICER was £3531 per QALY. For the 1750–2500 g population, the ICER was £34,905.
A PSA was run for the base case for each of the subpopulations defined by weight. PSA allows the explicit incorporation of parameter uncertainty, as each estimate is defined by an appropriate probability distribution rather than a point estimate. Incremental cost-effectiveness (ICE) scatter plots for each subpopulation are shown in Figures 35–37. The vast majority of the estimates were in the bottom-right quadrant, indicating that the intervention was more effective and cheaper than the comparator.
FIGURE 35.
Incremental cost-effectiveness scatterplot: 500–999 g population.
FIGURE 36.
Incremental cost-effectiveness scatterplot: 1000–1749 g population.
FIGURE 37.
Incremental cost-effectiveness scatterplot: 1750–2500 g population.
Cost-effectiveness acceptability curves have been produced for the three populations in Figures 38–40. These graphs illustrate the probability that the enhanced staff contact intervention has a cost-effectiveness ratio that is lower than the range of cost-effective threshold value values presented on the horizontal axis. For each population it is highly probable that the enhanced contact intervention is cost-effective even at a zero threshold value since the expected costs are expected to be lower for the enhanced intervention and the benefits are expected to be higher.
FIGURE 38.
Acceptability curve: 500–999 g population.
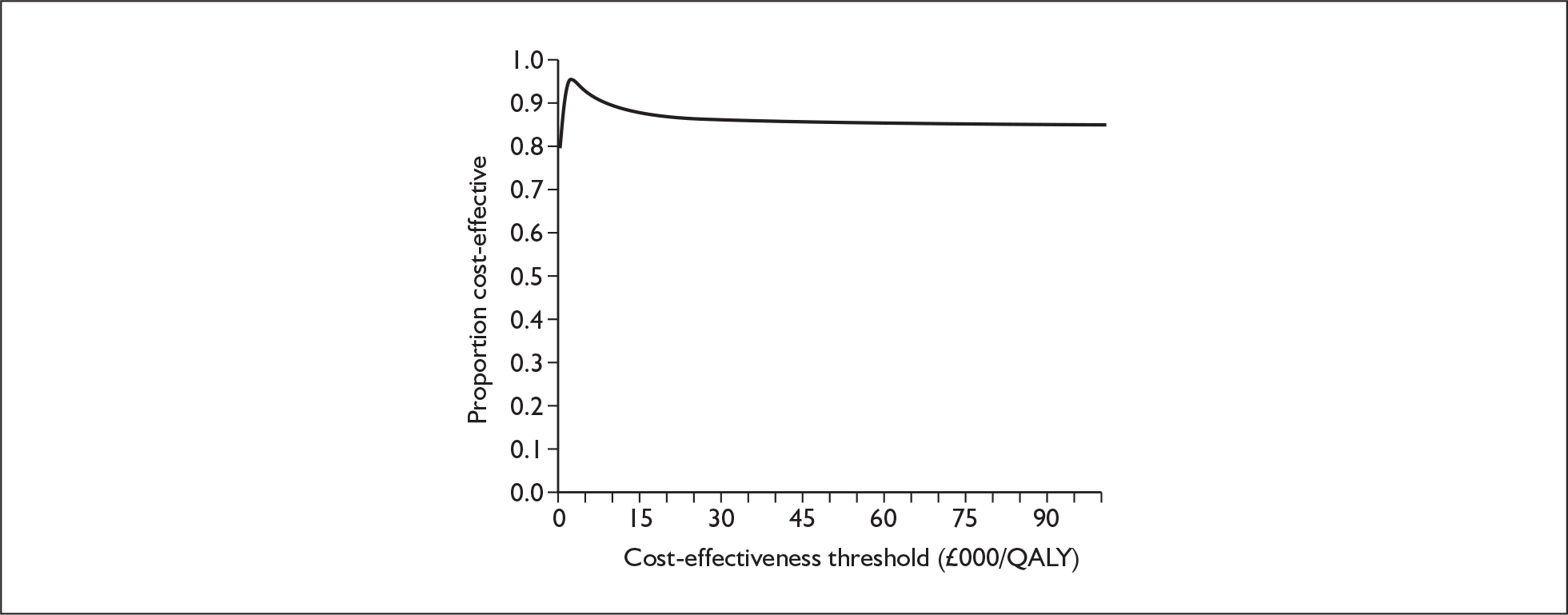
FIGURE 39.
Acceptability curve: 1000–1749 g population.
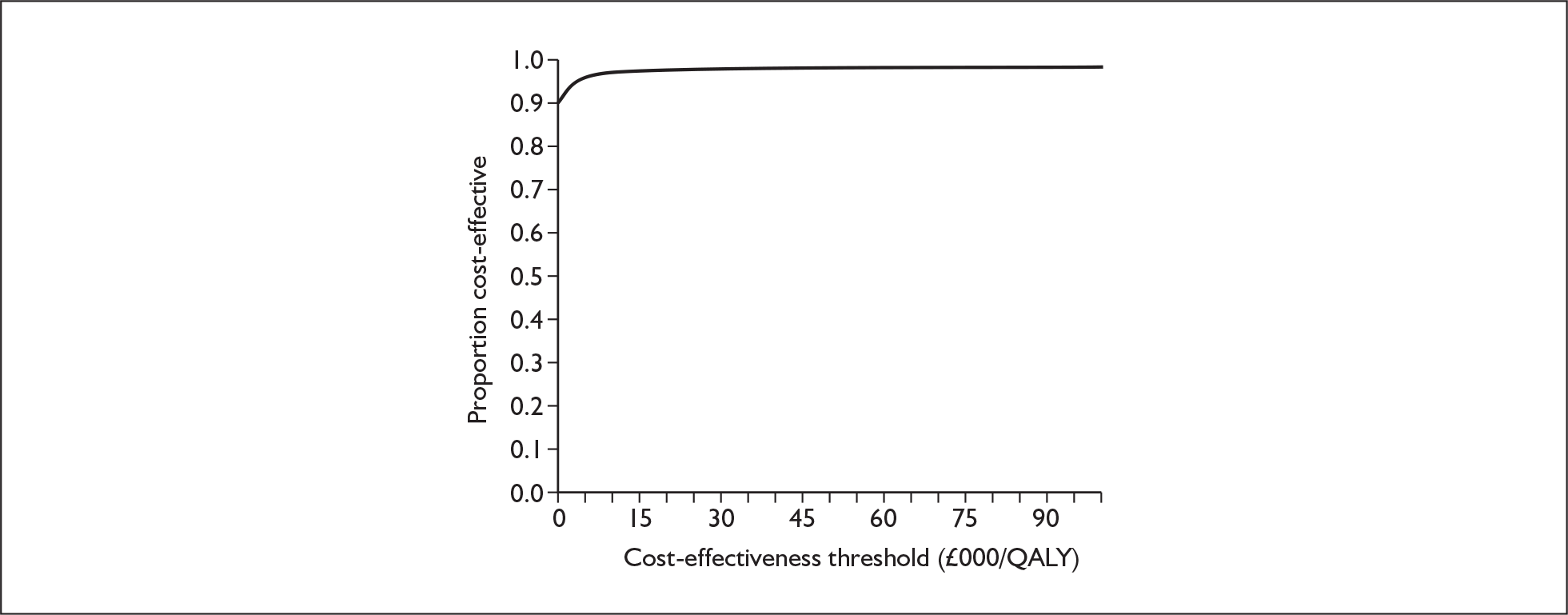
FIGURE 40.
Acceptability curve: 1750–2500 g population.
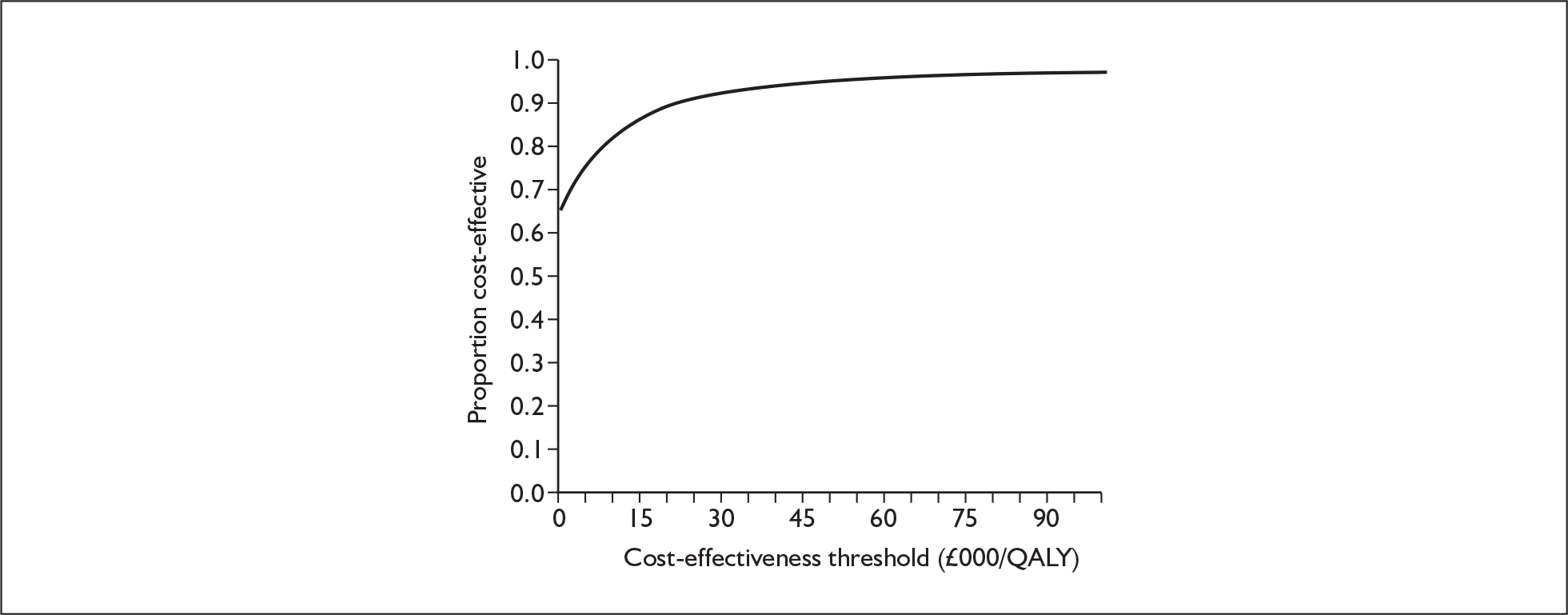
Sensitivity analyses
Summary
A summary of the main sensitivity analyses is presented here and, in addition, a table presenting the full results of all sensitivity analyses is provided in Appendix 9.
For all sensitivity analyses except for one, the results showed that, in this population, enhanced staff contact was always less costly and more effective than normal contact. The only scenario in which enhanced staff contact was not dominant was where donor milk was provided as a supplement to infants who partially received mother’s own milk and both formula and expression kit costs were included as additional costs. The cost-effectiveness ratio in this scenario was £354.68.
The only data input that caused the enhanced staff contact intervention to cease to be dominant was adding the cost of donor milk for those units that used it as a supplement to mothers’ milk only. The cost-effectiveness ratios were still very low, with the majority well within what is regarded as the acceptable threshold of £20,000.
For this population, with the exception of the cost of formula, all the cost inputs that were varied made the enhanced staff contact intervention less cost-saving and resulted in the intervention no longer dominating normal staff contact. The ICERs varied from as little as £663 up to £42,302, but the majority were well within the accepted threshold of £20,000.
Details of analyses undertaken
Intervention effectiveness estimate
The main intervention effect estimate was derived from two papers identified in the review. The results of the two papers149,166 were meta-analysed to produce the base-case intervention effectiveness estimate. As the interventions were not exactly the same and were based on feedback from our clinical advisors, it was decided to conduct the analysis again based only on the lactation consultants and the evidence from Gonzalez et al. 166 The resulting effectiveness estimate was only slightly worse in the Gonzalez et al. paper166 than the Pineda149 estimate, so the intervention was marginally less cost-saving and less effective. In addition, the intervention’s effectiveness was assumed to be the same in the base case regardless of the mothers’ intentions regarding breastfeeding. This assumption was tested in a one-way sensitivity analysis where the odds ratio of an infant receiving mothers’ milk was varied over plausible ranges. The results are presented in Table 21.
| Intervention | Cost (£) | Incremental cost | Benefits (QALY) | Incremental benefit | ICER (£/QALY) | |
|---|---|---|---|---|---|---|
| 0.6 ORc for ITB mothers and 0.4 OR for NITB mothers | ||||||
| 500–999 g | Enhanceda | 86,758.65 | 14.702 | |||
| Normalb | 87,275.22 | 516.57 | 14.485 | – 0.217 | Dominated | |
| 1000–1749 g | Enhanced | 56,946.67 | 21.051 | |||
| Normal | 57,193.47 | 246.8 | 21.001 | – 0.049 | Dominated | |
| 1750–2500 g | Enhanced | 47,227.50 | 21.92 | |||
| Normal | 47,272.71 | 45.21 | 21.912 | – 0.008 | Dominated | |
| 0.4 OR for ITB mothers and 0.6 OR for NITB mothers | ||||||
| 500–999 g | Enhanced | 86,758.65 | 14.702 | |||
| Normal | 87,464.03 | 705.38 | 14.398 | – 0.304 | Dominated | |
| 1000–1749 g | Enhanced | 56,946.67 | 21.051 | |||
| Normal | 57,315.95 | 369.28 | 20.985 | – 0.066 | Dominated | |
| 1750–2500 g | Enhanced | 47,227.50 | 21.92 | |||
| Normal | 47,328.00 | 100.50 | 21.909 | – 0.010 | Dominated | |
Breastmilk effectiveness estimate
In the base-case model, the odds ratio of getting confirmed NEC from formula feeding only compared with some mothers’ milk and formula was 3.01. 174 Vohr et al. 10 provided a significantly lower odds ratio of getting confirmed NEC, namely 1.48. When substituted into the model, this significantly reduced the effectiveness and cost-savings of the intervention, so much so that the intervention became dominated by the comparator for every subpopulation. The results are shown in Table 22.
| Intervention | Cost (£) | Incremental cost | Benefits (QALY) | Incremental benefit | ICER (£/QALY) | |
|---|---|---|---|---|---|---|
| 500–999 g | Enhanced staff contact | 86,514 | 14.82 | Dominated | ||
| Normal staff contact | 86,432 | – 82 | 14.90 | 0.08 | ||
| 1000–1749 g | Enhanced staff contact | 56,783 | 21.07 | Dominated | ||
| Normal staff contact | 56,631 | – 152 | 21.08 | 0.005 | ||
| 1750–2500 g | Enhanced staff contact | 47,154 | 22.36 | Dominated | ||
| Normal staff contact | 47,019 | – 135 | 22.36 | – 0.002 |
Intervention cost
The only intervention cost was the cost of staff time advising and supporting the mothers. In the base case, the unit cost per hour of registered nurse time was used. Registered nurses took the part of lactation consultants in the study of Gonzalez et al. 166 The unit cost used was £41.12. It is possible that a midwife might perform the role of lactation consultant, so the hourly unit cost of a hospital midwife was used in a sensitivity analysis, this being £65.57. 183 The intervention was still dominant for the two lower birthweight populations. In the 1750–2500 g population, the intervention no longer dominated. The ICER was £663 per QALY. The low incidence of disease in this group meant that the cost savings from reduced disease were less than the higher intervention cost.
Length of stay
In most of the models, the cost savings due to reduced disease outweighed the intervention costs. The length of stay was the main factor that in the model determined the cost implications of a disease, so the length of stay was halved for each clinical outcome (no disease, sepsis, medical NEC and surgical NEC) in each of the three levels of unit.
The intervention still dominated the comparator in the two lightest birthweight populations. However, it no longer dominated in the 1750–2500 g population. The ICER was £1639.
Expression kit costs
The current practice of the provision of breast pumps and expression kits in the UK is not clear. It is likely that, should breastfeeding rates increase, the need for more breast pumps will also increase, but we were unable to cost the impact of the intervention on the provision of breast pumps. Single-use expression kits used in addition to breast pumps are easier to cost, although the overall use of expression kits is not known. Ideally, both the cost of increased breast pump use and expression kit use would be costed, but in this sensitivity analysis only single-use expression kits have been costed.
Adding the cost of ‘single-use’ expression kits increased the cost of the enhanced staff contact. Enhanced contact still dominated in the lower two birthweight populations, but it no longer dominated in the 1750–2500 g population. The ICER of enhanced contact was £5591. The results are shown in Table 23.
| Intervention | Cost (£) | Incremental cost (£) | Benefits (QALY) | Incremental benefit | ICER (£/QALY) | |
|---|---|---|---|---|---|---|
| 500–999 g | Enhanced staff contact | 87,412 | 14.70 | |||
| Normal staff contact | 87,744 | 332.59 | 14.45 | – 0.251 | Dominated | |
| 1000–1749 g | Enhanced staff contact | 57,443 | 21.05 | |||
| Normal staff contact | 57,543 | 100.27 | 21.00 | – 0.056 | Dominated | |
| 1750–2500 g | Enhanced staff contact | 47,523 | 21.92 | |||
| Normal staff contact | 47,474 | – 48.64 | 21.91 | 0.009 | Dominated |
Lower donor milk costs
In the model for donor milk supplements, the cost estimate for a litre of milk was £289.12. This was the estimate for a milk banking set-up that was slightly improved in comparison with the existing milk bank system. It was estimated by the Breastmilk Banking Working Group91 that, if the milk banking system was significantly reformed and run in a manner similar to the Blood Bank Service, then the cost of producing a litre of donor milk would fall to £119.89. This excluded development costs. This cost was used to estimate the impact of such a reduction in donor milk costs. The reduction in the price greatly increased the cost-saving potential of the intervention. The lowest population became more cost-saving, the 1000–1749 g population became dominant from having a cost-effectiveness ratio of £3531 per QALY, and the ICER for the 1750–2500 g population reduced from £34,905 to £9500.
Formula and donor costs
The consumption of formula should reduce as breastfeeding increases. The cost of formula was excluded as an independent cost item from the base-case model because we were unable to cost the change in use of breast pumps, and both cost items should be included together. Formula costs are included in the unit cost of running a neonatal unit in that hotel services were included and food was a hotel service, but that does not capture the effect of the intervention specifically on the consumption of formula. The cost of formula was therefore included in a sensitivity analysis.
Adding the cost of formula made the intervention even more cost-saving as less formula was consumed as a result of increased milk expression. The intervention became more cost-saving for every population.
Adding formula and expression kit costs to the model had opposite effects and to some extent they neutralised each other, although the expression kits had the greatest impact. The intervention was no longer cost-saving in the 1000–1749 g group. When both were added to the model with donor milk supplied to infants who partially received their mother’s own milk, the enhanced staff contact intervention became less cost-effective due to the high cost of expression kits. In the 500–999 g group enhanced staff contact was no longer dominant, with a cost-effectiveness ratio of £355. In the 1000–1749 g group the cost-effectiveness ratio rose from £3531 to £5550. In the 1750–2500 g group the cost-effectiveness ratio rose from £34,905 to £42,301.
Both formula, at a cost of £1.36 per 200 ml, and supplementation with donor milk, at a price of £289.12 per litre, were included in the model together. This analysis was appropriate for the units that currently use donor milk as a supplement to mothers’ milk. The cost-saving effect of formula costs in the model caused the intervention to be more cost-effective with the ICER of enhanced contact reducing to £2533 from £3531 for the 1000–1749 g group and to £30,113 from £34,905 for the 1750–2500 g subgroups.
Adding formula costs to the model with the lower donor costs halved the cost-effectiveness ratio to £4690.
Incidence of mothers intending to breastfeed
In the base case the incidence of mothers intending to breastfeed prior to childbirth was 72%. This was the value for England in the Infant Feeding Survey 2005. In sensitivity analysis this was changed to 56%, the value for Northern Ireland. It was also varied from 50% to 90% to cover rates for different ethnic groups. An increase in the intention to breastfeed rate increased the health benefits of enhanced contact and increased cost-saving (see Table 24). This is the result of two factors. Firstly, the infants of mothers who intended to breastfeed would end up consuming more mothers’ milk than infants whose mothers did not intend to breastfeed given an enhanced contact intervention. Secondly, the effectiveness of an enhanced contact intervention was assumed to be the same for both mothers who intended to breastfeed and those who did not and, in reality, this may not be the case. This assumption was tested in sensitivity analysis and the results are also presented in Table 24.
| ITB incidence | Intervention | Cost (£) | Incremental cost (£) | Benefits (QALY) | Incremental benefit | ICER (£/QALY) |
|---|---|---|---|---|---|---|
| 50% | Enhanced staff contact | 57,033 | 21.04 | |||
| Normal staff contact | 57,305 | 271.97 | 20.99 | – 0.052 | Dominated | |
| 56% | Enhanced staff contact | 57,009 | 21.04 | |||
| Normal staff contact | 57,287 | 277.72 | 20.99 | – 0.053 | Dominated | |
| 72% | Enhanced staff contact | 56,947 | 21.05 | |||
| Normal staff contact | 57,240 | 293.04 | 21.00 | – 0.056 | Dominated | |
| 90% | Enhanced staff contact | 56,876 | 21.06 | |||
| Normal staff contact | 57,187 | 310.27 | 21.00 | – 0.059 | Dominated |
Lower disability rate for 1750–2500 g group
In the base case, the rate of severe disability was higher for the 1750–2500 g group than for the 1000–1749 g group. This was the result of the data in the Larroque paper. 180 However, as this appeared to be counterintuitive, on the advice from our clinical experts the probability of severe disability given some disability was reduced to 5% from 14.1% for the 1750–2500 g group. Correspondingly, the probability of mild disability given some disability was increased from 65% to 74.1%. This made the enhanced support only slightly less beneficial and cost-saving; the enhanced staff support still dominated normal support.
Chapter 6 Lessons from clinical practice
Background and methods
It is uncommon for neonatal units to have high rates of breastfeeding/breastmilk feeding. As a result, there is a possibility that important research questions have not been addressed at all, or that the use of specific interventions in practice may not be fully understood. To help set the findings of this evidence review in context, to inform the social and organisational context for generalisability of effective interventions to UK settings, and to inform discussion on research priorities, we sought the views of a group of clinical experts who work in neonatal units where breastfeeding/breastmilk feeding rates are high.
Seven experts who worked in neonatal units where breastfeeding/breastmilk feeding rates were known to be high were identified through professional networks, including reading published literature and recommendations by Advisory Group members; two were already members of the Advisory Group. They were approached informally using a range of methods, including face to face, telephone and email. They were asked for their responses to a standard set of questions, which included:
-
description of their neonatal unit and the population served
-
description of breastfeeding/breastmilk feeding-related practices, to include history of developments, obstacles to change, supportive factors, and practices that had worked
-
finally, they were asked what advice they would give to other units where staff wished to increase breastfeeding/breastmilk feeding rates.
Findings
Description of the units
Five units were in the UK, one in Sweden and one in the USA. The units included intensive care/high dependency, special care and transitional care settings. Units cared for populations that were mainly inner city, metropolitan or urban, and socially deprived, with only one unit serving a middle-class area. All had significant ethnic minority or refugee/asylum seeker populations. Five reported that most of their previous problems with breastfeeding/breastmilk feeding had been addressed and developments were now sustained; two UK units indicated that there were signs of improvement and there was the potential for sustained change. Five units had started work to improve breastfeeding in 1996 or before, with one indicating that they had started work on this issue since 2000. This information was not available for one unit.
The change process
All units reported that the work had been led by an individual specifically tasked to co-ordinate the changes. Five reported that this was an infant feeding specialist: in one case, this infant feeding specialist was working in conjunction with a consultant neonatologist, and in another the specialist was also a senior neonatal nurse with an academic background in breastfeeding. In one case, the change was led by a senior nurse.
The support of other staff was reported to be critically important. Units reported support and engagement by a range of staff, including clinical directors, general managers, service managers (medical and nursing), unit managers, senior neonatologists, neonatal nurses including nurse practitioners, midwives, the NHS trust breastfeeding co-ordinator, and the trust breastfeeding group. The US unit established a ‘Mother’s Milk Club’ to engage mothers themselves in the programme of change.
Five units reported that there was opposition to the changes, some of it transient, some longer lasting. This took the form of continued adherence to previous routines such as restrictions on feeding, routine supplementation, and bottles at night, also opposition to giving responsibility for feeding to mothers, and staff not liking the specialist role, thinking initially that it disempowered other staff. They also reported that neonatal nurses did not like consultants making changes, that it took a long time to change attitudes, that there was a perception that infants took longer to breastfeed, and that intensive care staff were rarely able to help with breastfeeding due to their work with critically ill infants. Some units reported that changes took several years. One unit reported that very rapid changes were possible, with the lactation initiation rate rising from 17% to 73% within 3 months of an integrated change programme.
Barriers to change
Several important barriers to change were reported. These included:
-
Lack of a coherent approach among staff:
-
– a lack of communication/co-ordination between staff groups, including neonatologists, neonatal nurses, and midwives
-
– an assumption among neonatal unit staff that postnatal and community midwives would deal with mothers and expression of breastmilk
-
– an assumption among medical staff that feeding was a nursing issue
-
– lack of support for change introduced by a nurse rather than by a doctor
-
– a lack of compliance with breastfeeding/breastmilk-feeding policy and apathy among staff.
-
-
Lack of knowledge and belief in breastfeeding/breastmilk feeding among staff:
-
– a lack of knowledge of the evidence base and clinical skills in helping mothers and infants
-
– a lack of knowledge of breastfeeding/breastmilk feeding, and associated belief in the mother and infant’s ability, including a belief that infants became exhausted by breastfeeding and a lack of belief in infants’ nutritive sucking ability.
-
-
Problems with facilities/the environment of the neonatal unit, including:
-
– lack of privacy/screens/comfortable chairs/space for parents
-
– lack of parent rooms for 24-hour stay
-
– lack of pumps and funnels for expressing, in hospital and at home
-
– infants being disturbed by the noise of other parents, staff
-
– workforce problems resulting in nurses looking after more then one sick infant, with no time for feeding/support for mothers.
-
-
Mothers being at a distance once they were discharged home.
Factors supporting change
The main factors identified that helped in creating the necessary change, and factors that the experts would advise other units to consider, fell into seven main categories. Units indicated that an integrated approach was needed across all of these. They were:
-
staff behaviour, training and co-ordination
-
audit and feedback of outcomes
-
involving parents
-
clinical practices
-
organisation of care
-
facilities
-
funds and resources.
Details of these categories are listed under the respective headings.
-
Leadership: a dedicated role was needed, supported by a core group with expert knowledge.
-
A consistent, evidence-based feeding policy and guidelines; including
-
– evidence-based information on the differences in lactation physiology between term and preterm mothers, and on the science of milk expression, milk composition, and infant feeding including unrestricted feeding, support for frequent feeding, and a gradual reduction of supplements
-
– focus on breastmilk as medicine, with evidence for improved health outcomes.
-
-
Effective evidence-based education and training for all staff, including senior staff, with mandatory training and annual updating and monitoring of consistent use of policy/guidelines.
-
Support from senior neonatalogists.
-
Good communication.
-
Using a team approach.
-
A gradual acceptance: nurses saw that it was possible and experienced success, then they convinced neonatologists.
-
Improvements valuable for staff morale.
-
Measuring dose and exposure period of breastmilk, as well as duration outcomes.
-
Measuring health outcomes for infants.
-
Responding to mothers’ requests for change.
-
Giving mothers evidence-based information on the importance of breastmilk for their infants, and on ways of maximising milk production.
-
Empowering mothers to believe in themselves and their infants.
-
Information and practical help for mothers.
-
Support for skin-to-skin care.
-
Bottles given only with mother’s permission.
-
Entrusting care to parents as soon as possible.
-
Supportive environment, screens for privacy, bed for parents beside the infant.
-
Training mothers who have been in neonatal unit as peer counsellors.
-
Supporting father’s presence.
-
Decreasing noise in parents’ rooms to encourage sleep.
-
Using creamatocrits and test weights to diagnose and manage problems and reinforce understanding of lactation for both staff and mothers.
-
Moving to total daily volume measurement instead of scheduled feeding, and giving mothers the milk volume records to keep themselves.
-
Focus on importance of providing breastmilk if mothers do not plan to breastfeed.
-
Programme for early discharge plus open-door policy if problems post discharge.
-
Training and appointment of peer counsellors.
-
Modifications to physical environment to enable ongoing contact between mothers and infants.
-
Freely available good-quality breast pumps.
-
Suitable environment in which to express milk.
-
Assistance with transport from home to hospital.
-
Access to milk bank.
-
Purchase of suitable clothing for mothers to enable skin-to-skin care.
-
Funds for resources and staff time to support all changes.
Chapter 7 Summary and discussion
This systematic review and economic analysis has examined the effectiveness and cost-effectiveness of interventions to enable women to breastfeed/give breastmilk to their infants despite the challenges of starting life in a neonatal unit. New evidence has been identified to inform care and future research, and the economic analysis conducted is the first in this complex and important field and offers a model for future decision analysis.
Effectiveness review: overview, strengths and limitations
The effectiveness review identified a total of 48 studies related to breastfeeding or feeding with breastmilk for infants in neonatal units. Studies were conducted from 1984 to 2007. They included 31 RCTs (65%), three randomised crossover studies (6%) and 14 other forms of controlled studies (29%); the inclusion of this range of study designs was considered important in a field where RCTs have not always been conducted and may not always be possible.
This work was intended primarily to inform those working in UK settings. However, it was recognised that there may be lessons to learn from those living in situations where breastfeeding is the norm and hence studies were not excluded on the basis of country. Included studies were conducted in 17 countries: 11 resource-poor countries, and six industrialised countries. Eight studies (16%) were conducted in the UK, including six RCTs – 26% of all the included RCTs. It is likely that the findings will be appropriate for those in countries with systems of care and breastfeeding rates similar to those in the UK, and findings from some studies may also have some value for those working in very different countries.
Strengths
This review was based on a comprehensive search, updated in January and February 2008, with additional studies identified by experts in the field. Through this process we identified 19 studies that were not already included in systematic reviews. We are aware that other studies may exist that have not been identified through this process.
All the included studies tested interventions with appropriate control groups. Each study was assessed for quality according to appropriate parameters for that design, and details of each study are presented accordingly in tables and in the text. The review has identified a wide range of studies, and the review team has worked to ensure appropriate analysis and conclusions. Details of included studies have been given in the data extraction and quality appraisal forms (Tables 26–73 in Appendix 4.1 and Tables 74–87 in Appendix 5). We have endeavoured to present methods and results in as transparent a manner as possible, avoiding findings that may be misleading. Wherever possible, results have been presented using intention to treat (ITT) analyses, adjusting for postrandomisation exclusions as appropriate. When results are shown in forest plots, studies rated as ‘poor’ have been omitted.
Studies included have examined a wide range of interventions, including clinical, health promotion and staff training interventions. This comprehensive perspective is essential in this topic area. Factors making breastfeeding difficult in neonatal units in the UK, as outlined in Chapter 1, are complex and multifaceted, and intervention to raise the rates is likely to require a range of different measures and an integrated approach.
The research team have backgrounds related to maternal and child nutrition including breastfeeding, public health nutrition, midwifery, neonatology, health economics, health services research and health policy, and we were supported throughout by expert academic, clinical and service user colleagues from the UK and internationally. This input was essential as breastfeeding remains a challenge in many neonatal units in the UK46 and abroad. Expertise and input from practitioners working in units where breastfeeding/breastmilk feeding rates are high, and from women with experience of having infants cared for in neonatal units, were needed to assist the questioning of current practice and in the interpretation of evidence. These views have been described in Chapter 6, and we have drawn on these experiences to reflect on the findings of this research, to inform the social and organisational context for generalisability of effective interventions to UK settings, and to help to shape the research agenda. We have also had important input from service user members of the Advisory Group, and we have used the literature that reports the views of women and families whose infants have been cared for in neonatal units in different settings (summarised in Chapter 1) to identify current experiences and wishes of families that might inform the research agenda.
Limitations
The main limitation of the review is the scarcity of high-quality research identified; only seven studies (15%) were assessed as good quality. In addition, studies were extremely heterogeneous in terms of characteristics of the interventions, controls or standard care, participants and outcomes reported. Meta-analyses have not been conducted on any topic as a result. This is discussed in relation to each section, and lessons learned for future research are discussed in Chapter 9.
Many studies were conducted in settings where breastfeeding rates in the population were low. Although this could support generalisability of such studies to a UK setting, this is a challenge for the interpretation of the findings of this review, as the interventions may have a different impact in settings where population rates are high, staff are familiar with and trained in supportive practices, families expect women to breastfeed, and infrastructure such as milk banking is in place. 190
An important eligibility criterion for this review was that infants were cared for in neonatal units at some stage, even if they were studied post discharge. There is inconsistency internationally in admission to neonatal units: some infants who are cared for in postnatal wards might, in other settings, be admitted to a neonatal unit, and vice versa. The same issue applies to admission to special versus intensive care, and for this reason we did not distinguish between infants admitted to these settings in our analysis. We may for this reason have excluded some relevant studies of preterm or low birthweight infants who were cared for outside a neonatal unit setting, including primary care settings.
A further limitation of the evidence base was the absence of studies that evaluated the effect of support from family members. One study reported fathers’ views of the intervention (Pinelli et al. 126) and another included fathers in the assessment of parental anxiety as a result of the intervention. 109,110 A further two studies121,141 included fathers as part of the intervention but results were reported for mothers only.
An important feature of this work was the iteration between the findings from the formal evidence base and the Advisory Group and clinical experts. This was considered to be necessary in a field where the evidence base was limited and heterogeneous, and where relevant skills and experience are limited in current practice. The intention was to use the strengths of the Advisory Group and clinical experts in both the topic area and study methodology. It is, however, possible that bias was introduced as a consequence. To address this, we have been both cautious and transparent in our use of information gained in this way.
Effectiveness review: summary and discussion
Increased mother and infant contact interventions
This section examined studies of additional contact between mother and infant, over and above standard care. ‘Standard care’ in these studies and in many neonatal units involved a high degree of separation between mothers and infants, and very limited opportunity for the intimate contact, including skin-to-skin contact of any form, that mothers of healthy infants would enjoy without the need for intervention. The evidence base was limited; none of these studies was rated as good quality overall.
All studies evaluating mother and infant contact were interventions of kangaroo skin-to-skin contact, kangaroo mother care (KMC) or skin-to-skin care, representing the largest category of breastfeeding promotion interventions examined in this review (12/48, 25%). The timing and duration of contact varied across the studies and between participants (see Table 3). All studies examined infants who were described as clinically stable. Several studies included twins and multiple births, but none reported analyses separately for these mothers and infants.
No studies reported rates of initiation of breastfeeding or oral feeding of expressed breastmilk.
Five studies115,121,135,141,147 evaluating kangaroo skin-to-skin contact showed increased duration of any breastfeeding prior to, at or up to 1 month after, hospital discharge. These studies were similar in several respects; a mostly short (i.e. including 10 minutes, 1 hour and at all visits) or medium (i.e. including two 4-hour periods) level of daily contact was implemented during the period of hospital stay among a population group of clinically stable infants of mainly very low birthweight in industrialised settings, including the UK. The limited psychosocial data available suggest that a medium level of contact may not be acceptable to mothers in the USA, although the findings of a recent trial, published outside the parameters of this review, question this. 159 The majority of mothers in a variety of industrialised settings, including the UK and USA, appeared to be willing to comply with short levels of kangaroo skin-to-skin contact. These findings may reflect cultural issues associated with countries with typically low breastfeeding rates and may vary in different cultural settings. They may also be modified with antenatal and postnatal education and support by staff. Further information is needed to explore the views and experiences of mothers and staff in regard to kangaroo skin-to-skin contact, as psychosocial data are limited in these studies.
Kangaroo skin-to-skin contact was effective among all mothers regardless of feeding intention, although greater gains may be achieved among women who intend to breastfeed their infants. 115,141,145 These findings of effectiveness were also demonstrated in a recent RCT published outside the search dates for this review (see Appendix 10).
One study found no positive benefit on duration of any breastfeeding at 40–41 weeks’ corrected age. 107 This study was different in two key respects: the intervention was more comprehensive including prolonged (i.e. including 20 or 24 hours or until the infant can no longer tolerate contact) kangaroo skin-to-skin contact, early discharge and regular breastfeeding; and it was conducted in a resource-poor country setting where breastfeeding rates in the population were high.
All studies that evaluated rates of exclusive breastfeeding at various time points were conducted in resource-poor country settings. Only one site in one study identified a positive effect on this outcome, and caution is required in interpretation of these findings due to methodological constraints. 131
Six studies conducted in both industrialised115,121,141 and resource-poor 107,118,131 country settings examined health outcomes. These included infants of very low birthweight in industrialised settings and primarily low birthweight infants in resource-poor country settings. They showed statistically significant improvements for the rate of head circumference growth, oxygen saturation, hypothermia and serious morbidity at 2 and 6 months.
All trials reported no adverse effects as a result of kangaroo skin-to-skin contact, with or without early discharge from hospital, for infants of low or very low birthweight when compared with standard care.
Mother and infant contact: conclusions
From the findings of our review, the effects of kangaroo skin-to-skin contact on breastfeeding in industrialised countries can be stated with some confidence. Short (i.e. including 10 minutes, 1 hour and at all visits) periods of skin-to-skin contact increase the duration of any breastfeeding up to 1 month after hospital discharge among clinically stable, very low and low birthweight infants. This effect is likely to be seen regardless of mothers’ feeding intentions prior to and at hospital discharge, although more prolonged increases in duration of any breastfeeding are likely among mothers who intend to breastfeed. Short exposure to kangaroo skin-to-skin contact during the hospital stay is feasible and acceptable to mothers in industrialised settings where breastfeeding is not the cultural norm. The provision of personal breastfeeding education and support by a skilled nurse as an integral part of the intervention is likely to increase the success of the intervention both in terms of breastfeeding outcomes and the acceptability of higher levels of skin-to-skin contact among women who intend to breastfeed their preterm infant.
Prolonged levels (20 or 24 hours per day) of kangaroo skin-to-skin contact are not likely to increase further the exclusive breastfeeding rates among clinically stable infants with birthweights of less than 2000 g in resource-poor country settings. Impact on the duration of any breastfeeding is inconclusive.
Daily use of kangaroo skin-to-skin contact, for short, medium or prolonged duration, is associated with improved health outcomes including the rate of head circumference growth, oxygen saturation, thermal stability and serious morbidity at 2 and 6 months for infants of less than 2000 g in both resource-poor countries and industrialised settings. Based on the limited data available from resource-poor country settings, medium and prolonged levels of skin-to-skin contact are less expensive than standard, incubator care.
These findings are supported by the views of the clinical experts subgroup as reported in Chapter 6. They reported that two important factors in increasing breastfeeding/breastmilk feeding rates in their neonatal units were close contact between mother and infant, and the involvement and empowerment of mothers in caring for their infants. This in turn is supported by the qualitative literature on mother–infant contact reported in Chapter 1, and by the views of consumer members of the Advisory Group. Skin-to-skin contact, and particularly kangaroo skin-to-skin contact, offers mothers and infants the experience of a close, intimate relationship, and mothers an opportunity to take responsibility for the care of their infant on a regular basis.
It is important to note that although parental contact with infants on neonatal units in the UK is less than many families wish, and facilities for ongoing, close contact are limited in many units43 there is relatively more visiting and parental involvement in the UK than in many other European units,191 offering greater potential for the introduction of interventions to promote skin-to-skin and other forms of breastfeeding promotion contact.
In the light of the potential for this intervention to have important positive consequences for mothers and infants, important questions remain about the transferability of this intervention to UK neonatal units, and this is discussed further in Chapters 8 and 9.
Interim feeding methods and related interventions
This section examined methods of feeding for the infant until feeding from the breast is possible, and included related interventions that may support or interfere with transition to the breast. Five RCTs,119,120,122,124,130 and one crossover study136 were identified that measured breastfeeding outcomes. Of these six studies, only one was rated as good quality,119 and that one had significant compliance problems.
All studies recruited infants who were well enough to tolerate enteral feeds, excluding those with congenital difficulties. Some studies included multiple births, but none presented analyses separately for these mothers and infants.
Findings from the four trials of cup versus bottle feeding119,120,122,124 indicate that cup feeding increases exclusive breastfeeding at discharge compared with standard bottle feeding. There is possibly a concomitant delay in discharge for cup-fed infants but these results are confounded by hospital policy and poor compliance. Episodes of severe oxygen saturation were increased in the bottle-feeding group in the sole trial to report this parameter. In addition to the compliance problems, confounding factors including the use of pacifiers and caregivers’ fingers for non-nutritive sucking, require consideration. More substantively, bottle feeding was the standard technique used in all included trials, and both staff and mothers were less familiar with cup feeding. Results are very likely to have been affected by the lack of familiarity with this novel intervention.
No good or moderate quality studies of nasogastric versus bottle feeding were identified, and no conclusions can be drawn. No effect of pacifier use on breastfeeding outcomes has been identified. There is no evidence that use of caregivers’ fingers in place of pacifiers improves or worsens breastfeeding outcomes. One small crossover study of moderate quality found that milk transfer was increased at the feed where mothers with breastfeeding problems used an ultra-thin nipple shield.
Interim feeding methods and related interventions: conclusions
One previous systematic review by Flint et al. 163 reached very different conclusions from the other two previous reviews155,161 in suggesting that cup feeding should not be used and further research be considered futile. On the basis of the findings from our review, we cannot support Flint’s conclusion. Our interpretation of the limited but important evidence is that cup feeding can be used in neonatal units as an alternative to bottle feeding for clinically stable infants who are ready for oral feeds, but only in settings where staff are adequately trained and mothers wish to use it. In such circumstances it is likely to increase exclusive breastfeeding at discharge and reduce the frequency of oxygen desaturation when compared with infants fed by bottle. This latter finding is supported. 192 A further consideration is the simplicity of cleaning cups as opposed to bottles and teats, potentially reducing the risk of nosocomial infection;193 this risk will be greater in resource-poor settings.
Questions remain, however, about two important issues: the optimum techniques to use for both bottle and cup feeding to promote appropriate feeding behaviour and optimise the transition to breastfeeding (with associated staff training implications); and whether or not cup feeding can sustain the nutritional intake needed – spillage of breastmilk from cups is an important factor that makes the measurement of nutritional intake difficult. 98
Encouraging oral feeding in preterms is dependent on the co-ordination of sucking, swallowing and breathing. 93 Oral feeding of infants requires caregivers to be aware of and responsive to infant cues related to this. No studies specifically examined this important issue.
There are safety concerns associated with the use of both nasogastric and orogastric tubes in preterm infants that should be considered in any future research of their use in this field. 194
Findings are inconclusive for the use of pacifiers and caregivers’ fingers for non-nutritive sucking. The fact that one study used caregivers’ fingers without noting this as a specific intervention122 indicates that this practice may be viewed as harmless. Related to this issue, authors of a study identified in another section of this review138 noted that they introduced ‘finger feeding’ – where a feeding tube leading from a container of milk is attached to the caregiver’s finger and inserted in the infant’s mouth – across both groups in their trial without citing experimental evidence to support its use. Theoretically, the insertion of fingers into the mouth of an infant could have at least as big an impact on future feeding behaviour as pacifiers, and in the absence of evidence should not be accepted as a more effective or safer intervention. Non-nutritive sucking is very important for infants who cannot feed directly from the breast, and important questions remain in relation to practices that might impact positively or negatively on future breastfeeding and other clinical outcomes.
Feeding infants effectively and in ways that encourage early transition to the breast is fundamental to enabling breastfeeding in this population. There is very little evidence to guide health professionals or parents in this area, and important research questions remain to be addressed. This suggests that enabling early feeding from the breast is important, and increased mother–infant contact may have a role to play in this. This is discussed further in Chapters 8 and 9.
Expressing breastmilk interventions
This section examined the use of different techniques, equipment and regimens to assist mothers with milk expression. Six studies contributed findings: five RCTs,112,114,124,128,142 and one randomised crossover study. 134 Four were conducted in industrialised countries, with two in the UK. 114,125 Participants were from socioeconomically mixed groups. Twins and multiples were included in some studies, but data are not reported separately for these. Only one trial was rated as good quality,125 and three were moderate. 114,128,142 Lack of compliance was reported to be an issue, even in the good-quality study. 125 Trials examined a wide range of techniques, equipment and regimens; each study tested a unique combination.
Double or simultaneous pumping was found to be more effective than single pumping or a hand pump in the first 2 weeks in the UK and in Nigeria. 114,142 In regard to later time periods, no differences in milk production were found between single versus double expression with an electric pump, or using a novel hand pump versus another electric pump. 125,128 Three studies 114,125,128 reported that simultaneous pumping took mothers less time than sequential pumping. This is likely to be welcomed by mothers whose time may be limited by other commitments, and who may be tired and stressed.
Only one study reported health outcomes for mothers, such as nipple damage and mastitis. 125 Such adverse events are likely to occur in these mothers, and can have a major negative impact on their motivation to continue with an already difficult task. It has been noted that pumps may not have different flange sizes to fit different breast sizes, and this can cause serious trauma. 21 No experimental studies of different types of flange sizes or inserts were identified. Data on mothers’ views are sparse.
Details of care of the mothers and infants including factors that may have acted as co-interventions were lacking. Cost-effectiveness outcomes were not identified.
Expressing breastmilk: conclusions
The expression of breastmilk is key for virtually all mothers of infants in neonatal units, and it is a practice that may have to be continued for weeks or even months by mothers of infants who are very preterm or sick, requiring high levels of motivation on the part of the mother and those supporting her. Identifying simple, comfortable, efficient and effective procedures could make a very important contribution to this aspect of care.
This evidence suggests that double pumping with a hospital-grade electric pump with suitable silastic inserts to prevent injury to mothers has advantages over other methods in the first 2 weeks. However, once the mother is discharged home, she may require pumps both at home and in hospital during her visits to the infant. At this stage, she may benefit from using other methods such as a hand-operated pump, or hand expression, for milk removal at home, as well as continuing to use the hospital pump when she is there. Hand pumps may have practical advantages for the mother as well as being cheaper, potentially increasing scope for more widespread provision by neonatal units within limited budgets.
No studies examined milk quality in relation to method of pumping; for example, the foremilk/hindmilk ratio may be affected by hand versus suction pumping. Most existing electric pumps work using a standard suction/release mechanism, which is unlike the pattern of sucking used by infants. Since the conduct of the trials included in this review, a novel type of pump that aims to mimic the sucking pattern of the infant has been developed (Medela Symphony® pump). Published experimental research on this pump is not yet available although a study by Meier et al. is forthcoming. Examination of the quality as well as the quantity of milk is important.
None of the trials prescribed an amount of milk to be expressed. The reported increases in milk production during the first 2 weeks as a result of double pumping may be important in order to build reserves of breastmilk and maternal capacity to continue producing milk in later weeks. Two papers195,196 have suggested setting a goal for daily milk volume of around 750 ml/day to encourage mothers to continue to express on a regular basis. This consideration further supports the use of double pumping during the first 2 weeks to promote sufficient mother’s own milk both to meet the needs of her newborn preterm infant and to establish lactation.
Only one study125 presented outcomes separately for mothers of twins and multiple births. Achieving optimum milk production is even more important for these mothers, whose milk is needed by more than one infant.
Expression may be used by different mothers for different purposes and this may confound study findings. Some mothers may wish to express to establish lactation and continue to breastfeed over time, while others may wish to provide milk only until the infant is discharged home. A subgroup analysis within one study125 found that mothers who attempted to breastfeed had a significantly higher number of expressions and volume expressed than mothers who did not attempt to breastfeed. This reflects the important issue, identified in other sections of this review, that increasing the number of women who want to start to breastfeed could have a positive impact on breastfeeding outcomes.
Issues related to regimens for milk removal, such as how often, how long and how much to express, are addressed further in the following section (see Additional interventions to enhance breastmilk production).
As described in Chapter 6, the clinical expert subgroup identified effective milk expression, starting from soon after birth, as fundamentally important both to provide mother’s own breastmilk for the infant and also to maximise milk supply for when the infant is able to take the breast.
It is evident that milk expression is a practice that is likely to be influenced by factors other than the use of a pump. Other interventions included in this review, including skin-to-skin contact, interventions to enhance milk production, support and staff training, are all likely to have an impact, as are attitudes of staff, time constraints and psychological factors. Only one included study examined co-interventions – i.e. breast massage before pumping. 114 As well as implementing the interventions identified here, therefore, there are important implications for the education and support of women who are expressing milk for prolonged periods both in hospital and at home, for staff training and for the organisation of care. Women need to be enabled to use appropriate equipment including pumps, correctly sized flanges and soft inserts to prevent trauma to their nipples and breasts, and to have suitable facilities in which to express. Such a multifaceted supportive system has been described by Meier et al. 21
Important questions remain about the optimum ways to enable women to produce adequate breastmilk for their infants, both to sustain the infant while in the neonatal unit, and to establish adequate lactation for the longer term. This is discussed in Chapter 9.
Additional interventions to enhance breastmilk production
Seven studies were identified that examined this topic. 114,123,133,144,146,148 Five were RCTs and two were randomised crossover studies. All were conducted in industrialised countries. One was rated as good quality,144 the others as moderate quality. No study intended to recruit women of a particular age, socioeconomic background or parity. Studies did recruit twins and multiple births, but results are not reported separately. Studies predominantly measured short-term milk volume outcomes, and there was limited assessment of breastfeeding/breastmilk feeding exclusivity or duration, or of breastmilk composition. There was no evidence of effects on breastmilk fat or breastfeeding duration of any intervention based on the findings of these seven studies.
There is no evidence that oxytocin spray or metoclopramide has an effect on breastfeeding/breastmilk feeding outcomes. There is very limited evidence that domperidone and human growth hormone may have a role to play. No adverse effects of galactagogues were reported, but studies may not have been large enough to detect them, or they may not have been measured.
Three studies114,146,148 of other interventions to enhance milk production were identified. These interventions are promising. A relaxation/imagery tape increased expressed milk volume without altering milk fat among mothers of recently born preterm infants. Breast massage increased the volumes of milk produced by mothers of recently born preterm infants using both simultaneous and sequential pumping, without altering fat content. Milk production was greater in mothers of non-breastfeeding preterm infants, including older preterm infants, after Therapeutic Touch (TT) than after mock TT or no treatment.
Cost-effectiveness outcomes were not reported in any of the seven studies.
Additional interventions to enhance breastmilk production: conclusions
The evidence base overall is very limited. Very few data are available on breastfeeding outcomes either in the short or longer term; most outcomes measured were short-term measures of milk volume.
Pharmaceutical galactagogues seem to have little role to play: there is no evidence to support the use of oxytocin spray or metoclopramide. Further research is needed before domperidone and human growth hormone are used. None of the drugs is licensed for this purpose. There is some evidence to support the use of relaxation-related interventions, which may counter the stress-related lactation problems that women experience in neonatal units. 197
Our search did not identify any studies investigating whether dietary customs or nutritional status of women in different ethnic groups and in different countries have any impact on breastmilk production. Participation of women from lower socioeconomic groups was limited, and there is no information available about the specific needs of mothers of twins and multiple births, who have increased needs for milk production.
As described above, enhancing breastmilk production is often a fundamentally important component of establishing and maintaining an adequate milk supply, especially when relying on milk expression before breastfeeding can be established. Milk expression is in itself a form of stimulating milk production, and these studies of additional interventions to enhance breastmilk production should not be viewed in isolation. Two studies114,144 did have a concurrent protocol for expression, but both studies reported that mothers had problems with adhering to the frequency of expression recommended. Whether or not infants are breastfeeding is also a factor in breastmilk production, as is support; there is very limited evidence on these co-interventions from these studies. Another factor is likely to be close contact between mother and infant, which in itself can stimulate the hormones needed for milk production and release. No studies of milk production reported the use of skin-to-skin contact. These limitations are important in the light of the experience of clinical experts reported in Chapter 6, who indicated that a supportive environment, and facilities for privacy and relaxation, were important for milk production.
Additional factors to consider in milk production also include the time since birth, as milk production is difficult to sustain over time, and the health and well-being of the mother.
Very sick mothers were unable to participate in these studies. Mothers of infants in neonatal units who are themselves critically ill are an important subgroup. In the light of concerns around their need to initiate and maintain a good breastmilk supply, they are likely to need specialised support for lactation, and issues related to excretion of drugs in breastmilk may need to be addressed. It is likely that infants of these mothers will need access to donor breastmilk at least at some stage in their care. In the case of mothers who are unable to communicate their wishes, for example, those who are unconscious or receiving respiratory support, prior knowledge of whether the mother intended to breastfeed would be valuable for staff. If women do indicate their feeding decision in pregnancy, noting the woman’s views in her record would be helpful in these circumstances.
Important questions remain about ways of enhancing breastmilk production. From the evidence in this review, effective interventions are likely to be multifaceted. This is discussed in Chapter 9.
Interventions to support optimal nutritional intake from breastmilk
This section examined interventions that aim to optimise the quality and/or quantity of the breastmilk fed to infants in neonatal units or following discharge. Three primary studies were identified;20,140,145 only one rated as good quality. No studies reported primary outcomes of initiation of breastfeeding or oral feeding of expressed breastmilk.
One US study found that mothers of all educational levels demonstrated the ability accurately to measure the lipid content of their expressed breastmilk when taught. 20 Performing this procedure increased maternal satisfaction, and mothers felt they had influenced and made a difference to their infants’ outcomes. Cost-effectiveness data were not reported though mothers taking responsibility for measuring lipid content of their expressed breastmilk may have the effect of reducing staff costs.
Optimal nutritional intake from breastmilk: conclusions
Optimising the nutritional intake of infants in neonatal units and following discharge is a dominant concern in practice, yet there is virtually no evidence to inform effective ways of doing this for breastfed/breastmilk-fed infants. Preterm mothers’ milk differs in composition from that of term mothers. 86 Optimising particularly the protein and lipid content of the milk is considered important to support growth, and, for this reason, fortification using artificial supplements has become a routine practice in many countries, although it has not been universally adopted in the UK. Enhancing the composition of mother’s own milk offers an apparently simple solution to this problem. Good quality experimental evidence is, however, lacking to examine the effectiveness of this. 198
Breastfeeding education and support interventions
This section examined the provision of education and support for mothers of infants admitted to neonatal units. It included all forms of professional and peer education and support. Six primary studies117,126,143,152,153,166 were identified. Two studies were rated as good quality117,126 and three as moderate. 143,153,166
All RCTs recruited women who wished to breastfeed. Merewood et al. 143 included a high proportion of mothers from low-income families; other studies appeared to recruit women from mixed socioeconomic backgrounds. Studies included both low birthweight and very low birthweight infants. Twins and multiples were included, but results were not reported separately.
There is good evidence that both hospital and community-based breastfeeding support from trained peer supporters in both industrialised and resource-poor settings improves breastfeeding outcomes, including in-hospital breastfeeding rates and a longer duration of breastfeeding and exclusive breastfeeding. Two study hospitals had Baby Friendly accreditation, suggesting that specific breastfeeding peer support for mothers in the community added effectiveness to standard Baby Friendly care, and one of these studies found that effectiveness was greater among the subgroup of African American participants.
In relation to hospital-based support from lactation consultants, one Canadian study found no effect, but in this study of relatively affluent women both groups had access to other lactation consultants in the community and mothers reported that lactation consultants were their most used source of breastfeeding support. One US study found that professional lactation support in the neonatal unit resulted in more infants receiving their own mother’s milk ever and at discharge.
No interventions to examine education for women or families on breastfeeding were identified.
Breastfeeding education and support interventions: conclusions
Provision of skilled support, both peer and professional, in hospital and at home, is fundamentally important for mothers of term, healthy infants. 199 It is potentially more important for mothers facing the additional challenges associated with a preterm or low birthweight infant. The studies examined here provide strong evidence for the effectiveness of peer support for mothers of preterm and low birthweight infants who wish to breastfeed; such support enhanced the effectiveness of standard Baby Friendly care and increased effectiveness in African American women. There is also evidence for the effectiveness of professional support from lactation consultants in neonatal units. None of these studies was conducted in the UK.
The results of the health economics analysis (see Chapter 5) found that professional support was potentially cost-effective, which adds weight to the need to examine the implementation of this intervention in UK neonatal units. This is discussed in Chapters 8 and 9.
All three RCTs recruited women who wished to breastfeed. Sisk et al. 106 have reported that offering support to women who want to breastfeed was more effective than offering the same support to women who do not plan to breastfeed. The health economics analysis (see Chapter 5) found that the cost-effectiveness of professional support is increased if more women intend to breastfeed. As in other sections of this review, there are important implications for the promotion of breastfeeding in pregnancy.
The key contribution of specialist breastfeeding/breastmilk-feeding support for mothers of infants in neonatal units has been emphasised by the clinical expert subgroup (see Chapter 6), who indicated that both peer and professional support is crucial. They have indicated that not only do women need support from a specialised member of staff to be available, but that all staff need to be trained to a minimum standard. This will be addressed in the next section.
The clinical experts subgroup (see Chapter 6) also indicated that an essential component of care for mothers of infants in neonatal units is evidence-based information on the importance of breastfeeding/feeding breastmilk for their infants, and on ways of enhancing this. Work is needed to examine information and education for mothers.
Staff training interventions
This section examined interventions that aimed to enhance staff training in breastfeeding/breastmilk feeding in neonatal units. Two moderate-quality studies were identified, both from industrialised countries. 81,149 Both studies examined a multifaceted and multidisciplinary staff training programme for staff in neonatal units, both with self-study components.
Breastfeeding outcomes improved following the educational teaching package developed and implemented in the UK,81 and also following a three-part intervention undertaken in the USA, which included a training component. 149 It is not possible to attribute these effects to individual components of these multifaceted interventions; it seems likely that the incremental effects of the different components all influenced breastfeeding rate. 200
Other outcomes affected included an increase in skin-to-skin contact and improved test scores. One programme was reported to be well received by participating staff.
Staff training interventions: conclusions
Lack of knowledge of breastfeeding/breastmilk feeding among health-care professionals is an important barrier to breastfeeding,78,79 especially in the context of neonatal unit settings and the specialist skills needed. Parents report that some staff in the UK are underprepared for, and at times resistant to, the promotion of breastfeeding/breastmilk feeding for infants in neonatal units;46 this includes some staff working in neonatal units, as well as midwifery, health visiting and medical staff in hospital and community settings. Neonatal unit staff themselves have recently reported that formula feeding is the norm in neonatal units, is easier, and indeed is necessary. 201 The limited evidence identified here suggests that educational interventions delivered to a multidisciplinary staff group may increase health-care professionals’ knowledge and can increase initiation rates and duration of breastfeeding, whether delivered alone or as part of a multifaceted intervention including maternal education and support.
The health economics analysis identified that staff training to provide supportive care was cost-effective (see Chapter 5), adding weight to the argument for implementing this in UK neonatal units.
Information from the clinical expert subgroup (see Chapter 6) indicates that educating all levels of staff on the neonatal unit is an essential component of changing practice. Their experience suggests that a dedicated specialist post is needed, but also that all staff need to be educated in the skills needed to support women and to promote breastfeeding/breastmilk intake for infants in neonatal units. It is unlikely that the effective interventions identified in other sections of this review will be implemented successfully in the absence of high-quality staff training.
Important questions remain about how to implement such training; these are addressed in Chapters 8 and 9.
Early hospital discharge with home support interventions
This section examined the provision of early discharge followed by home support for infants from neonatal units. One moderate-quality study from New Zealand127 was identified.
Early discharge with home support is not likely to have a positive effect, and may have an adverse effect, on duration rates of any and exclusive breastfeeding up to 6 months of age among clinically stable preterm infants. Readmission rates were found to remain unchanged or be slightly lower for the intervention group as a result of the early discharge intervention. 127 No outcomes related to cost-effectiveness of the early discharge and home support intervention were reported.
A high proportion of eligible mothers declined to participate and there were significantly more twins among the mothers who declined; the acceptability of early discharge for mothers may vary in different circumstances.
Early discharge with home support: conclusions
The evidence base for the effectiveness and acceptability of early discharge with home support is very limited. Findings suggest that this intervention is unlikely to improve and may adversely affect the duration of breastfeeding although some benefits for infection rates and readmission rates to hospital may occur. Prolonged hospitalisation of preterm or low birthweight infants is associated with an increased risk of contracting infections, delays in mother–child bonding, higher costs155,161,202 and adverse effects on family and functioning. 203–205
Improved breastfeeding rates cannot be considered a justification for early discharge from UK neonatal units.
Organisation of care interventions
No studies evaluating changes to the organisation of care between units, such as the introduction of a clinical network, met the inclusion criteria. One good-quality study137 and one moderate-quality before/after study151 examined organisation of care within a unit. 138,151,154 Both examined changes related to Baby Friendly accreditation of the associated maternity hospital.
Changes in the organisation of neonatal care driven by Baby Friendly accreditation of the host maternity hospital have significantly increased the numbers of infants receiving any breastmilk in the first week of enteral feeds, and the duration of any or exclusive breastfeeding prior to, and at, hospital discharge. 137,151 Both studies were conducted among infants of relatively lower risk within neonatal units. Mothers in one study were mostly black American and Hispanic women with typically low breastfeeding rates,137 whereas the other study was conducted among Brazilian women with typically high breastfeeding rates. 151
Organisation of care: conclusions
The evidence base for organisation of care interventions is restricted to two before/after studies of Baby Friendly accreditation of the associated maternity hospital in industrialised countries, including relatively low-risk infants. These studies identified improvements in several breastfeeding-related outcomes as a result of this multifaceted intervention.
National data show that Baby Friendly accreditation of maternity hospitals is effective in increasing initiation among all women in the UK, including those from disadvantaged and vulnerable groups,80,206 and limited data also support Baby Friendly accreditation in both the hospital and community207,208 in both industrialised and resource-poor country settings. This intervention has been shown to be cost-effective. 209 As a consequence, implementation of the Baby Friendly Initiative as the minimum standard in NHS trusts has been recommended in two recent NICE guidelines. 3,209
Several of the Ten Steps of the Baby Friendly Accreditation process may have a beneficial impact on neonatal units, and they inter-relate with much of the evidence presented in this review. These include:
-
Step 1: Have a written breastfeeding policy that is routinely communicated to all health-care staff (see Chapter 6).
-
Step 2: Train all health-care staff in the skills necessary to implement the breastfeeding policy (see in this chapter Breastfeeding education and support interventions).
-
Step 3: Inform all pregnant women about the benefits and management of breastfeeding (see Chapter 6).
-
Step 4: Help mothers initiate breastfeeding soon after birth (see Chapter 6; and in this chapter Expressing breastmilk interventions).
-
Step 5: Show mothers how to breastfeed and how to maintain lactation even if they are separated from their infants (see Chapter 6; and in this chapter Expressing breastmilk interventions).
-
Step 6: Give newborn infants no food or drink other than breastmilk, unless medically indicated.
-
Step 7: Practice rooming-in, allowing mothers and infants to remain together 24 hours a day (see Chapter 6; and in this chapter Increased mother and infant contact interventions).
-
Step 10: Foster the establishment of breastfeeding support groups and refer mothers to them on discharge from the hospital or clinic.
However, additional steps, or modifications of the existing steps, are needed in the neonatal unit environment. Baby Friendly neonatal standards have been developed (see Appendix 11.1). The way in which these relate to the interventions examined in this review is discussed in Chapter 4 (see Organisation of care interventions) and in Appendix 11.2.
Health economics and decision modelling: strengths, limitations and discussion
The economic model was developed with the aim of assessing the relative cost-effectiveness of interventions intended to promote/support breastfeeding in neonatal units. The model was developed with reference to the practices for decision modelling laid out in the Guidelines for Economic Evaluation in Health Technology Assessment. 210
Details of the sources, methods and assumptions used in the analysis have been presented to ensure the transparency and enhance the understanding and interpretability of the study results. As with any modelling analysis, the structure is a simplification of reality, the purpose of which is to allow the synthesis of different types of data. In this instance, due to the complexity of the issue and a lack of available evidence, it was necessary to oversimplify the model structure. There was a lack of available evidence, particularly UK evidence, suggesting that the processes and decisions that are currently being made in the UK are not based on published research. While this lack of evidence is a major limitation to conducting any secondary research, it is hoped that the analysis undertaken will be useful in helping to direct future research and identify data limitations.
The data requirements of modelling are normally beyond the scope of the accompanying systematic review, which is often concerned with establishing effectiveness. Effectiveness data are necessary, but not sufficient, to facilitate modelling. This is because the model must encompass all consequences and costs that are related to the intervention under evaluation. The systematic review has identified a number of interventions that have a positive impact on breastfeeding rates and volumes of milk expressed. Additional literature searches identified very few economic studies, limited cost and resource use data, limited evidence on incidence of necrotising enterocolitis (NEC) and sepsis, and scant utility data. All of these are required in modelling. Few of the studies providing relevant data were UK studies. As a consequence, it was necessary to assume that the clinical data obtained from studies conducted in the USA and Europe were transferable to the UK setting. In two of the studies providing effectiveness data for breastmilk on NEC and sepsis incidence, fortifier was added to mothers’ milk, which is not routine practice in the UK. The impact of fortifier on outcomes is unclear.
Potentially, numerous benefits and disadvantages are associated with different forms of infant feeding, both for the mother and the infant. Some are well established, some are theoretical and it is likely that some are as yet unknown. It was necessary for the model to identify and focus on those clinical outcomes that would enable a link to breastfeeding/breastmilk to be established. Discussions with clinical advisors led us to devise a model that would evaluate the impact of breastfeeding/breastmilk on NEC and sepsis and in turn associated long-term health benefits. Limiting the clinical outcomes of interest may underestimate or overestimate the benefits of breastfeeding/breastmilk. Hopefully, what we have captured are those clinical outcomes that have the greatest health impact on these infants; maternal outcomes were not included. Interestingly, the identified literature showed little effect of mothers’ milk consumption on sepsis, and it is likely that excluding sepsis from the analysis would have had limited impact on the results. Given the small number of outcomes incorporated it is extremely likely that several that might have impacted on the results have been excluded.
The evidence suggested that the intention of the mother regarding breastfeeding impacted on both initiation and duration of breastfeeding. Those mothers who had already decided to breastfeed their infant prior to entering hospital were more likely to express milk successfully for their infant. Intuitively, this would suggest that promotion of breastfeeding should occur at an early stage of pregnancy to reinforce lactation support in hospital.
Guidelines suggest that the time horizon of the model applied should be long enough to capture all relevant costs and benefit differences between the interventions. 211
In order to capture the costs and health benefits over the lifetime of the infants, it was assumed that the disability state of the infant once diagnosed would remain constant for the lifetime of the child. Each disability state incurred an annual cost, which was multiplied by the life expectancy to obtain a lifetime cost for each disability. These were then incorporated into the model. As costs and benefits were incurred over a lifetime, discounting was conducted. Data limitations meant that it was necessary to ignore readmission costs and the additional treatment costs that may be incurred. While this may lead to an underestimation of the costs, the relative difference between the two interventions is likely to remain the same. Additionally, given that the other potential health benefits and protective benefits of breastmilk have not been incorporated into the model, it is possible that the readmission and treatment rates would be higher in the formula-fed population, hence increasing the costs incurred in the normal staff contact arm. Further, no negative health effects of consumption of mothers’ milk were modelled. No adverse effects of breastfeeding were incorporated into the model and no searches were conducted to identify any. However, of those clinical papers that did inform the model none reported adverse effects from breastfeeding.
As has already been mentioned, the available evidence regarding resource use and costing for the UK setting was extremely poor. Despite conducting additional searches it was necessary to use national cost data at an aggregate level. This led to a number of different scenarios being evaluated in the sensitivity analysis. The reference costs indicated a number of resources that had already been included in the unit (daily) cost. To avoid double counting it was considered that all costs incurred in an inpatient day had been captured. However, surgical costs, which were explicitly stated to have been excluded from the unit cost, were included.
The inclusion of formula and breast pump provision in the hotel costs of an inpatient stay does not capture the incremental effect of the intervention on formula consumption and breast pump use. Due to uncertainty regarding the current practice of provision of breast pumps in the UK, it was difficult to measure and cost changes in breast pump use. However, various scenarios with formula costs, donor milk costs, expression kit costs and length of stay were evaluated in an attempt to ascertain the impact of these resource changes on the cost-effectiveness results. The results show that any variations in resources have an impact on the incremental cost-effectiveness ratio (ICER), particularly the introduction of donor milk, and this is to be expected. However, without a more detailed costing study, it is impossible to ascertain whether the actual resources that would be used in a real-life situation are reflected in the analysis outcomes.
Further, the costing of milk banking was problematic given the current situation in the UK. While donor milk is available in some units, it is not the norm. The report from the Donor Milk Working Group (2003) demonstrated that provision of regionally or nationally organised milk banking facilities could reduce the cost per litre and allow all preterm infants the opportunity to receive donor human milk rather than preterm formula. To demonstrate the impact of achieving the lower cost, two separate modelling scenarios were presented. The higher cost of donor milk did impact on the results and, while the two lower birthweight groups remained cost-effective options, the 1750–2500 g population obtained an ICER greater than the £20,000 threshold. However, donor milk would become cost-effective if the mechanisms by which it is provided were improved.
The enhanced staff contact intervention, which provided additional trained professional support in hospital, was more effective and less costly (due to reduced neonatal illness) than normal staff contact in most sensitivity analysis scenarios, and it is instructive to ask what, if anything, could prevent enhanced staff support from being cost-effective. In the sensitivity analyses, there were four data inputs that had more than a marginal impact on the cost-effectiveness of enhanced staff contact. These were: increasing the cost of the staff contact; reducing the length of stay in the units by half; including the cost of expression kits; and reducing the effect size of mothers’ milk relative to formula on incidence of confirmed NEC. Only the last had a major effect on the cost-effectiveness, and it was only important for the heaviest birthweight population.
The primary reason that enhanced staff contact is cost-effective in the model is that it is relatively cheap. The model assumes that other requirements for milk expression are already in place in neonatal units. The BLISS report46 and clinical advisors to this study suggest that private rooms for expressing milk should be provided, and that there are overnight rooms in some units, so it is possible that no extra facility costs need be incurred. However, this is uncertain. But what this analysis demonstrates is that by increasing the number of infants receiving breastmilk there would be a reduction in the severity and incidence of illness.
Overall, the outcomes of the model are fairly robust to the changes in the parameters varied in sensitivity analysis. We have made no attempt to address structural uncertainty and we are aware that the model structure may be too basic. In conclusion, the results demonstrate the likely cost-effectiveness of enhanced staff contact. However, we acknowledge that further research is required to describe fully the many other aspects of breastfeeding that could influence effectiveness. We hope that the model we have developed will contribute to future work in this field.
Conclusions
This review has identified effective and cost-effective interventions, few, if any, of which are widely practised in UK neonatal units. Limitations of the effectiveness review include the scarcity of high-quality evidence identified for most types of intervention, the heterogeneity of interventions and settings studied, and the fact that most studies examined predominantly clinically stable infants. Limitations of the economic analysis are similar, in that little existing evidence was identified. Although there are preliminary indications that enhanced staff contact may be cost-effective, further evidence is required to provide results with confidence.
We used the Advisory Group and clinical expert subgroup to inform the topic area, and study methodology and priorities for future research. It is possible that bias was introduced as a consequence. To address this, we have been both cautious and transparent in our use of information gained in this way.
Before considering implications for policy, practice and future research, it is important to note that one fundamentally important finding of this review, described in several sections and emphasised by the clinical expert subgroup in Chapter 6, is that many interventions inter-relate. It seems unlikely that specific clinical interventions such as double pumping or cup feeding will be effective if used alone, and particularly in the absence of staff training or of an environment in which mothers are encouraged and supported in having close and ongoing contact with their infants, and to breastfeed/express breastmilk. These issues are addressed in Chapters 8 and 9.
Chapter 8 Implications for policy, practice and education
Implications for policy, practice and education are presented here using the Ottawa Charter for health promotion,101 which we have used as a conceptual framework [see Chapter 3, Data handling process (p. 13)]. We first examined the findings of this study, then consulted with the Advisory Group and clinical expert subgroup to agree conclusions and recommendations as follows.
Public policy and public health
No studies related to public policy were identified. Although increasing breastfeeding initiation and duration rates for the population as a whole is a national policy priority, the profile of this vulnerable group of mothers and infants needs to be raised in relation to public health policy. Such a change could be accomplished by overtly including this group in the national targets for initiation and duration of breastfeeding, and recognising the contribution that breastfeeding/feeding with breastmilk could make to addressing inequalities in health and specifically to meeting Public Service Agreement targets on health inequalities and infant mortality. Other policy issues should be considered to enable breastfeeding/breastmilk feeding for these mothers and infants, including employment and financial protection for women whose infants require lengthy hospital stays and ongoing care at home, and the provision of support at home for mothers with other children.
The extensive changes needed to create sustained improvement in neonatal units will be difficult to implement with the current shortage of trained staff. The effectiveness and cost-effectiveness of staff support demonstrated in this study could encourage service commissioners to increase staffing levels in neonatal units and to promote staff training, as staff training and support for mothers is relatively inexpensive compared with the resources needed for neonatal care (see Chapter 4, Breastfeeding education and support interventions (p. 48); see also Chapter 5).
Interventions were more effective in women who wanted to breastfeed. This implies that antenatal interventions that increase initiation of breastfeeding among all women and families3,59 should be implemented or strengthened to increase the numbers of women who intend to breastfeed, including those who give birth prematurely [see Chapter 4: Increased mother and infant contact interventions (p. 19); Expressing breastmilk interventions (p. 34); Breastfeeding education and support interventions (p. 48)]. This includes support for the Baby Friendly Initiative as the minimum standard in NHS Trusts [see Chapter 4: Organisation of care interventions (p. 59)].
Facilities should be available for the safe transport, storage and feeding of breastmilk including own mother’s milk and donor milk [see Chapter 4: Expressing breastmilk interventions (p. 34); Chapter 5: Methods for health economics modelling (p. 63)]. The milk bank system in the UK is locally organised and neither uniformly available nor adequately monitored, leaving infants vulnerable to gaps in provision. 91 Donor milk banking is currently being reviewed by NICE and changes should await the outcome of that review (www.nice.org.uk/guidance/index.jsp?action=byID%26o=11973).
To inform policy developments and clinical care, improved monitoring is needed [see Chapter 4: Increased mother and infant contact interventions (p. 19); see also Chapter 5]. Consistent national data are currently lacking on disease and length of stay, individual infant treatment pathways and resource use for infants starting life in neonatal units in the UK; these data should be routinely collected. There is a need to develop consensus definitions of the initiation and duration of breastfeeding/breastmilk feeding with specific reference to infants admitted to neonatal units and their mothers. This should record both the stage at which the infant initially receives breastmilk and the stage at which nutritive breastfeeding is attained, as well as noting whether or not women initiated breastmilk expression. In addition, routine recording of breastfeeding/breastmilk feeding status at the point of hospital discharge and subsequently would aid comparison with breastfeeding rates for the general population.
A national infrastructure should be established to allow consistent records to be kept and collated in a manner that allows data analysis. Furthermore, feeding, health, educational, social and costs outcomes beyond discharge from neonatal unit care, for example from paediatric wards, hospital readmissions, GP and health visitor care, need to be incorporated. This is consistent with the Every Child Matters58 monitoring framework. Given the potential of standard and newly introduced feeding methods to impact on safety and on breastfeeding, exclusivity and duration of breastfeeding/breastmilk feeding and health outcomes should be monitored routinely for these infants. This should include details of breastfeeding/breastmilk feeding and supplemental feeding including both content and methods of feeding, using standardised and validated measurements.
We have proposed definitions [see Chapter 9: General issues of methodology and design (p. 108)] relevant to initiation and duration of breastfeeding/breastmilk feeding for infants who start life in neonatal units and their mothers.
ChiMat, the new national Child and Maternal Health Observatory, has an important role to play in identifying gaps in existing data and reporting systems; driving improvements in data and supporting implementation of improved systems for data collection and reporting; developing new indicators to support measurement and monitoring in relation to child and maternal health policy areas; and tackling health inequalities. These recommendations are relevant to that work programme.
Supportive environments
The views of parents should be fundamental to the organisation of care in neonatal units, and arrangements should be in place to ascertain and respond to those on a regular basis (see Chapter 6).
Avoiding non-essential separation of mothers and infants and offering all possible opportunities for engagement of the mother in caregiving for her infant should be priorities for care. Some neonatal units provide ‘transitional care’ areas in which the mother can be accommodated together with her clinically stable infant from about 32 weeks of gestation. The clinical expert subgroup noted that provision of such care is far from uniform, and this needs to be addressed [see Chapter 4: Increased mother and infant contact interventions (p. 19); Expressing breastmilk interventions (p. 34); see also Chapter 6].
This evidence base warrants increased implementation of a minimum of 1–2 hours daily kangaroo skin-to-skin contact for clinically stable infants of low and very low birthweight and their mothers. Core components of this intervention include kangaroo skin-to-skin contact during the hospital stay combined with personal breastfeeding education and support from a skilled neonatal nurse to assist mothers with initial breastfeeding experiences, recognition of subtle infant feeding cues and encouragement of self-regulated feeding in response to these cues. Routine implementation of early discharge as a component of kangaroo mother care is not supported [see Chapter 4: Increased mother and infant contact interventions (p. 19)].
Frequent effective expression of breastmilk is fundamentally important. Clean, comfortable, private facilities with appropriate breast pumps and equipment for pumping and for milk storage, should be freely available for mothers. In the absence of national guidance, mothers’ breastmilk should be handled in accordance with local guidelines approved by infection control specialists;212 national guidance would support local units in developing appropriate policies, and should be considered. Specialised support should be given to the mother wherever she is in the hospital, including the postnatal ward, transitional care, ICU, delivery suite or another specialist unit such as a mental health unit, and at home to encourage her to maximise her milk output. Mothers of twins and multiples are likely to require additional care and skilled support [see Chapter 4: Expressing breastmilk interventions (p. 34); see also Chapter 6].
There is evidence that a range of supportive/relaxation measures can help to enhance milk volume without reducing fat content. Mothers of infants who wish to initiate or maintain breastmilk production should be offered the opportunity for relaxation before and during expression. This will include facilities for expressing either away from the normal stresses of the neonatal unit or close to their infants, as preferred by the mother [see Chapter 4: Expressing breastmilk interventions (p. 34); Additional interventions to enhance breastmilk production (p. 40)].
The current system for the organisation of neonatal care can result in parents being at some distance from their infant(s). Support, including practical and financial support, is needed for the mother to visit frequently, and to express breastmilk both in hospital and home environments [see Chapter 4: Increased mother and infant contact interventions (p. 19); Expressing breastmilk interventions (p. 34); see also Chapter 6].
Enabling mothers and infants to have close and ongoing contact is likely to require changes to the built environment in neonatal units. Consideration of this should be part of the planning process for the design of all new neonatal units.
Community action
Hospital- and community-based peer support from women who have themselves had an infant in a neonatal unit and who are trained in breastfeeding counselling should be considered for all women with infants in neonatal units and following discharge. Suitable remuneration and standards of training and supervision will be required, with special consideration of the complex physical and social needs of mothers of infants who have started life in neonatal units [see Chapter 4: Breastfeeding education and support interventions (p. 48)].
Development of personal and professional skills
Trained professional breastfeeding support in the neonatal unit should be offered to all mothers, particularly those who are ill. This intervention has been shown to be clinically and potentially cost-effective [see Chapter 4: Breastfeeding education and support interventions (p. 48); see also Chapter 5].
Double pumping with a hospital-grade electric pump with suitable silastic inserts and well-fitting flanges to prevent injury to mothers has advantages over other methods in the first 2 weeks, and support should be available to enable mothers to do this. However, once the mother is discharged home, she may benefit from using other methods such as a hand-operated pump, or hand expression, for milk removal at home, as well as continuing to use the hospital pump when she is in hospital. Mothers should be encouraged to try a range of pumps at this stage to identify which works best for each individual. Mothers should be taught how to hand express, for use in circumstances where a pump is not available or not working [see Chapter 4: Expressing breastmilk interventions (p. 34)].
There is very limited evidence to support the use of prescribed medication to stimulate or maintain production. The use of such substances may divert attention from more fundamental aspects of care unless seen as an adjunct to other forms of supportive care [see Chapter 4: Additional interventions to enhance breastmilk production (p. 40)].
Mothers can be taught to separate foremilk and hindmilk accurately. Where this technique is used as part of infant care on the neonatal unit, this could support mothers’ involvement in the care of their infants [see Chapter 4: Interventions to support optimal nutritional intake from breastmilk (p. 46)].
Our interpretation of the limited but important evidence is that cup feeding can be used in neonatal units as an alternative to bottle feeding for clinically stable infants who are ready for oral feeds, but only in settings where staff are adequately trained and mothers wish to use it. Important questions remain about its use and more widespread adoption should await future research. Until such evidence is available, parents’ views should be respected, all equipment used should be appropriate to the developmental stage of the infant, care should be taken to optimise nutritional intake, staff should be adequately trained, and care taken to support the early transition to breastfeeding [see Chapter 4: Interim feeding methods and related interventions (p. 28)].
There is no evidence to support the removal of pacifier use in infants who are not able to suck feed, as non-nutritive sucking remains an important issue. 213 There is inadequate evidence to support the use of caregivers’ fingers in place of pacifiers, and this should be recognised as an intervention in its own right that should be fully tested before adoption into practice [see Chapter 4: Interim feeding methods and related interventions (p. 28)].
Reorientation of health services
All clinical experts reported that positive change requires strong and informed leadership within the neonatal unit. Planned change, engaging all professional groups, was reported as essential. The motivation for change was perceived as likely to be increased by knowledge and understanding of the evidence about health risks associated with formula feeding. An evidence-based policy for all staff was seen as essential, with audit of compliance and outcomes (see Chapter 6).
There is an urgent need to enhance the training of all staff working with families in neonatal units and following discharge in the skills needed to promote and protect breastfeeding, and support breastfeeding women. This should be covered during specialty training, and be seen as an aspect of continuing professional development. The design and delivery of training should be shared between trusts, universities, royal colleges and professional bodies, and the UNICEF Baby Friendly programme, and could include support from the NHS Institute for Innovation and Improvement [see Chapter 4: Increased mother and infant contact interventions (p. 19); Interim feeding methods and related interventions (p. 28); Expressing breastmilk interventions (p. 34); Additional interventions to enhance breastmilk production (p. 40); Breastfeeding education and support interventions (p. 48); Staff training interventions (p. 55); see also Chapters 5 and 6].
Communication between staff caring for the mother and infant in all settings in hospital, staff in community settings, and the mother and her family needs to be improved in order to maximise the mother’s milk production and increase her opportunity to be with her infant [see Chapter 4: Increased mother and infant contact interventions (p. 19); Interim feeding methods and related interventions (p. 28); Expressing breastmilk interventions (p. 34); Additional interventions to enhance breastmilk production (p. 40); Breastfeeding education and support interventions (p. 48); Staff training interventions (p. 55); see also Chapters 5 and 6].
The limited but relatively good quality of the evidence base for Baby Friendly accreditation further supports the existing evidence-based action59 and guidance3 for implementation of the UNICEF UK Baby Friendly Initiative for maternity services as the minimum standard in England and Wales [see Chapter 4: Organisation of care interventions (p. 59)].
Chapter 9 Research agenda
We formulated research questions arising from the current evidence base, and identified gaps in the evidence using the Ottawa Charter for Health Promotion101 as a framework. We then consulted with the Advisory Group and the clinical expert subgroup to agree on priorities. The list of priority research questions has resulted from this process. The methodological issues identified here should be considered in the design of all future research to improve the quality and relevance of studies.
Methodological issues
Conducting research with mothers and infants in neonatal units is complex and challenging. The inevitable distress experienced by parents complicates the process of information and consent, and the fragility of the infants requires the highest possible standards of care. Nevertheless there are studies in this review that have succeeded in conducting research to very high standards.
Some of the methodological problems identified in the existing evidence base are common in health-related research. Studies did not always report the details of interventions, standard care or characteristics of participants. Some results were not reported in ways that enabled checks on the quality of analysis. Compliance was a problem even in some studies of otherwise good quality. Many studies were too small to yield conclusive results. Outcomes measured tended to be short-term indicators such as milk intake or time to discharge. Longer-term breastfeeding, health, development and psychosocial outcomes were seldom, if ever, measured. Very few randomised controlled trials (RCTs) were identified, even in topic areas where this is a feasible approach. Of the six RCTs conducted in the UK, only two were assessed as good quality; both were related to milk production and expression. This has limited the confidence with which recommendations can be made, especially for changes to clinical practice.
Other problems were specific to the field being studied. Breastfeeding and breastmilk feeding behaviour was varied and seldom reported in detail. ‘Breastfeeding’ can describe both partial and exclusive feeding, and feeding with breastmilk as well as feeding from the breast. Infants may receive supplements of formula or other products, which are not standard across time, countries or hospitals. There is wide variation in the composition and use of artificial ‘fortifiers’, and, despite a lack of evidence informing their appropriate use, they are so widely accepted that their use is not always reported. Expressed breastmilk can vary in amount and composition; fresh or stored mothers’ milk differs from donor milk, which may be derived from one or more mothers at different stages of lactation and treated in different ways before being used. Methods of feed administration (bottle, tube or cup) were seldom reported in detail yet may differentially affect outcomes. Because formula feeding and bottle feeding have been widespread for so long in the UK, breastfeeding and breastmilk feeding and the techniques required to support them may be novel, and staff are likely to be unfamiliar with them.
Gaps in the evidence base: Ottawa Charter Framework
The evidence base is very limited, and it is especially limited in regard to the UK, both in quantity and in quality. In the two areas where evidence is strongest, increased mother–infant contact and support, only one UK study has been conducted and that was over 20 years ago. 147 Of the eight UK studies included in this review, only two were rated as good quality. 125,144 For many interventions, the main evidence was derived from US studies (20, 42% overall). This limits the transferability of findings and indicates the lack of investment to date in research in this field in the UK.
The gaps in the evidence base for each of the five health promotion categories of the Ottawa Charter Framework are summarised in Table 25 and discussed in the following section.
| Areas of health promotion action (Ottawa Charter) | Evidence of effectiveness for interventions to promote and support breastfeeding in neonatal units |
|---|---|
| Public policy – e.g. legislation, fiscal measures, inclusion in public health targets | No studies identified |
| Supportive environments (which protect natural resources and generate healthy living and working conditions) – e.g. public attitude and infrastructure to support breastfeeding for newborns in hospital (not separating mothers and infants, private room to express, etc.) or at home (equipment, home visits, etc.) |
Increased mother and infant contact via kangaroo skin-to-skin contact Early discharge with home support No studies identified on privacy or other aspects of humane care |
| Community action – e.g. self-help and social support through family, peer or other community support |
Peer support No studies identified on family or other community support |
| Development of personal skills – e.g. education programmes, clinical support |
Education and counselling Limited evidence for: |
| Reorientation of health services – e.g. managed clinical networks, breastfeeding policy, Baby Friendly Initiative, training of staff, transport service |
Baby Friendly Initiative Protocol-based care Staff training and education No studies identified examining effects of managed clinical networks or transport services |
Public policy and public health
No studies specifically related to public policy and public health either in the UK or internationally were identified. No studies of cost-effectiveness that could inform service commissioning were identified. Moreover, we had difficulty obtaining relevant data to populate the economic models developed. This indicates an urgent need to collect more routine data on infant feeding, growth and nutrition processes and outcomes in neonatal care.
An important finding of this review is that interventions, including skin-to-skin contact, expression and support, appeared to be more effective, and enhanced staff contact was more cost-effective, in women who wanted to breastfeed. The implication of this is that public health/health promotion interventions that increase initiation rates in the population may have an important part to play in raising rates among this group of mothers and infants.
Very few studies included mothers from subgroups with specific needs, who are over-represented in families with infants in neonatal units. This includes families from low-income backgrounds, minority ethnic and cultural groups, teenage and unsupported mothers, mothers using non-prescription drugs and other illegal substances, and mothers with serious illness including those requiring prescription drugs. For no studies were these subgroups a central concern, meaning that no studies were adequately powered to examine these issues.
Twelve studies reported that twins and multiples were included; all other studies did not report this or indicated that multiples were specifically excluded. Only one study125 reported any results separately for singletons and multiples, limiting the conclusions that can be drawn. No study examined twins and multiples and their mothers as a central concern, so all were underpowered in this regard. The needs of twins and multiples and their mothers are especially important in areas such as milk expression and mother–infant contact where having more than one infant presents specific challenges.
No studies examined the effect of marketing breastmilk substitutes on families of a preterm infant, nor on the attitudes and practices of staff caring for them.
Supportive environments
No studies examined ways to enhance breastfeeding/breastmilk feeding for mothers and infants in situations where they are separated by distance or by illness of the mother. Similarly no studies explored the effects on breastfeeding of the facilities that were provided. Relevant features of a supportive environment could include mechanisms to enable mothers to be with their infants, including assistance with transport; also a welcoming, private, comfortable, quiet environment for parents, and specifically one for mothers to express milk, to talk with staff, to consider bad news about their infant’s progress, and to talk with other parents. Clinical experts considered this to be very important (see Chapter 6), and an example of a resource designed for and by mothers has been identified. 21 It could be argued that empirical study of this issue is unnecessary since such facilities should be available as standard care, as a matter of human dignity and rights. In practice, however, it is reported that such facilities are not available in the UK,43,46 and it is feasible that cost-effectiveness data could contribute to provision of such an environment in the financially restricted environment of the NHS.
Similarly, no studies examined the effect on breastfeeding of broader aspects of humane care or of developmental care, where the environment of the whole neonatal unit offers quiet, supportive and developmentally appropriate facilities such as day-night lighting, appropriate handling, and close contact between mothers and infants. Kangaroo skin-to-skin contact is an important component of such care.
There were no studies of the impact of the provision of a comprehensive, quality-controlled, affordable milk bank service on breastfeeding/breastmilk and health outcomes.
Community action
Studies of peer support in the community were identified. None was conducted in the UK, however, and results require validation in UK settings, especially in regard to provision for women on low incomes, or with complex health and social needs, in urban or rural settings, and from different ethnic groups.
No studies of social support for these families were identified; this may be particularly important for mothers of multiples and for mothers with other children.
Only one study109 was identified that examined the involvement of fathers or other family members in education and/or support for breastfeeding.
Development of personal and professional skills
The majority of the studies identified included only clinically stable infants. Only one study reported outcomes separately for a specific group of infants (very low birthweight). 140 Little can be said about the skills needed to care for these particularly vulnerable mothers and infants. Developmentally appropriate interventions for infants of different gestational age or weight have not been examined in robust studies.
No studies specifically examined the ability of caregivers to assess and respond to infant cues on the coordination of sucking, swallowing and breathing to support oral feeding and transition to the breast.
Very few studies examined maternal complications such as painful nipples and breasts, engorgement, mastitis, problems with positioning and attachment, insufficient milk; mothers’ views and experiences (e.g. Fewtrell et al., 2001125); or measures of physical or emotional well-being such as exhaustion or depression. Only one study indicated that participating women had significant health problems,144 despite the fact that mothers of infants in neonatal units are themselves more likely to be ill. Breastfeeding/breastmilk feeding is demanding and difficult for mothers of infants in neonatal units, and maternal health and well-being is essential to its success. Skills to address some of these problems have been identified for mothers of term, healthy infants,3,59 and the specific care needed to help mothers of preterm and low birthweight infants needs to be examined. Maternal outcomes should be included in future research studies.
No studies of staff training used principles of adult learning or psychological models to underpin their approach. Behaviour change is challenging, and work is needed to identify theoretical approaches that might work to sustain such change.
No studies examined the current routine protocols for assessing weight and growth for infants in neonatal units and following discharge. The clinical expert subgroup noted that without an understanding of lactation and breastfeeding, or a supportive environment for lactating mothers, such protocols can have a negative impact on breastfeeding/breastmilk feeding. Ways of assessing infant growth, weight and development without undermining lactation and while also promoting breastmilk intake and breastfeeding should be examined.
No studies were identified in which infants in neonatal units had particular problems in feeding from the breast, such as cleft lip and palate, although studies involving infants in other settings have been conducted that may be relevant (e.g. Glenny et al., 2004;214 Darzi et al., 1996215).
Reorientation of health services
Very little research has been conducted on the current organisation of neonatal units, especially interunit care, clinical networks and transport, in particular to examine ways of using transport and effective links between units within a clinical network to reduce mother–infant separation and increase fathers’ contact time and involvement, particularly for families in rural settings or reliant on public transport.
Included studies did not examine the contribution the health service could make for mothers and infants from deprived groups or groups where breastfeeding rates were very low.
Recommendations for research
General issues of methodology and research design
Future trials should adhere to appropriate standards of reporting, for example the CONSORT statement for clinical trials (www.consort-statement.org/index.aspx?o=1030), and all trials should be registered from the start with the International Standard Randomised Controlled Trial Number Register (www.controlled-trials.com/isrctn).
Research designs should incorporate an understanding of lactation and breastfeeding, and should develop standardised measures of breastfeeding and breastmilk feeding, including measurement of dose and exposure period and noting both the content and mode of feeding. Suggested standardised measures are as follows:
-
Initiation of breastmilk feeding for infant When the infant receives first dose of breastmilk from any source by any method. Whether mother’s own or donor milk (or both) should be recorded, as should the method of feeding – breastfeeding, or expressed milk by bottle, cup or tube.
-
Initiation of breastmilk expression When the mother starts to express breastmilk by any method.
-
Initiation of breastfeeding When the mother provides the first nutritive feed directly from the breast.
-
Duration of breastfeeding/breastmilk feeding Any and exclusive intake of breastmilk from any source by any method; measured at weekly intervals until and including hospital discharge. Whether mother’s own or donor milk (or both) should be recorded, as should the method of feeding – breastfeeding, or expressed milk by bottle, cup or tube.
It will also be important to use standardised measures of intake of formula and fortifier, to include information about the amount and timing of feeds taken, the type of formula and fortifier used, and mode of feeding (i.e. bottle, tube, cup).
Future studies should provide clear sampling frames for target population groups including a priori sample size calculations and comparability of participants regarding demographic, socioeconomic and clinical characteristics at the points of enrolment and randomisation into comparison groups. Clear descriptions of ‘standard care’ (the comparator), admission and discharge policies, the nature of the intervention and standards of competence achieved by staff or peer supporters are critical to adapting interventions or replicating them in other settings.
Future studies should involve from the outset parents who have experienced the neonatal unit environment, and take account of the views of expert clinical staff in all relevant disciplines.
Experimental designs should also take into consideration the fact that it is rarely possible to conduct a single intervention; a recurring issue in this study has been the potential for confounding and the likelihood of a Hawthorne effect arising through associated change in parental and staff attitudes and practice. For many questions it will be more appropriate to randomise units rather than individual mothers or infants, as in the large RCT of Baby Friendly accreditation conducted by Kramer et al. (2001),4 or to conduct high-quality before/after studies in one or several units.
Although outcomes in the infants will usually be of primary interest we note a need for more information on other aspects such as maternal health and well-being, parents’ views and experiences, and staff views and behaviour. Outcomes in the infant may include short-, medium- and long-term measures of feeding, health and development. We observed a particular dearth of measurements of medium- or long-term outcomes, and psychosocial outcomes. Data to inform better cost-effectiveness research would be particularly valuable.
Studies should examine the specific needs and care for women with different feeding intentions and from different socioeconomic and ethnic backgrounds, and should include sick mothers, mothers of very low birthweight and very preterm infants, and mothers of multiple births and their infants. There is also likely to be a need either to stratify by birthweight at the randomisation stage or to explore the very different needs of infants at different gestational ages and birthweights, and their families, by subgroup analysis.
Where interventions may lead to differential feeding outcomes, for example exclusive or partial intake of breastmilk, it is important that research designs build in measurement of short-, medium- and long-term health outcomes. Such designs offer an unbiased opportunity to explore the effects of maternal milk provision on health and disease outcomes, as in Kramer et al. ;4 this information can otherwise be difficult to acquire.
Recommendations for future research studies
-
Research recommendations are grouped in the following categories:
-
Work to be considered when planning all future studies.
-
Urgent preliminary work to inform best practice for future intervention studies.
-
Recommended studies:
-
– priority level 1: intervention studies to address urgent gaps
-
– priority level 2: specific interventions and staff training.
-
Work to be considered when planning all future studies
Examining health and development outcomes and costs
Studies that examine the dose–response relationship between feeding and health and development outcomes would inform cost-effectiveness issues, which influence service commissioning decisions. Long-term studies are necessary to quantify more precisely the short-, medium- and long-term costs associated with not breastfeeding related to maternal and infant health and infant and child development. Resource use should be evaluated in any study of health and development outcomes to ensure that cost data applicable to the specific population can be obtained. This is true for both the long-term cost of treating disability and the cost of disease in neonatal units, especially NEC.
Such work could be conducted in the context of existing or planned cohort studies, or could be incorporated into large RCTs (such as those proposed below), or could use data available from routine monitoring, were an appropriate national system to be established. A large prospective cohort of 600 very low birthweight infants born to racially and economically diverse mothers has recently been funded by the National Institutes of Health (NIH) in the USA (Principal Investigator Dr Paula Meier). This study will examine health outcomes, health service issues and cost-effectiveness of different methods of infant feeding. Future research in this field should take this study into consideration. UK studies are required to determine the resource use and costs of disease and treatment in the UK.
Future modelling work should focus on capturing the multiple episodes of disease, adverse effects, complications at various intervals throughout the infant’s stay in the neonatal unit, and the impact of the history of these events on the long-term quality of life of the infant. Data for this type of model were not available at the time of this study. In addition, utilities and health outcomes for mothers related to infant feeding should be measured; although the health and development outcomes for infants are important, the outcomes for mothers should not be overlooked. 216
This work on outcomes should inform and support, and be incorporated in, large experimental studies such as those outlined below.
Parents’ health, views, experiences and needs
Within the context of each of the studies described below, nested qualitative and quantitative research should be conducted to examine a range of issues including the health and well-being of the mother, the infant feeding decisions and experiences of mothers of infants in neonatal units; the views and experiences of fathers and other family members and their role in infant feeding decisions; the educational needs of mothers and fathers to promote breastfeeding/breastmilk feeding; the feeding-related needs of parents of infants with specific challenges, including multiple births, extremely low birthweight and congenital anomalies; and the diverse needs of parents from different population subgroups.
Urgent preliminary work to inform best practice for future intervention studies
It is evident from the studies reviewed that an important contributor to the findings is the compliance or lack of compliance of staff and of women with the novel intervention. It is also evident that interventions to be tested in future studies are not in common practice in UK settings. Before best practice intervention studies can be conducted in accordance with the principles of the Medical Research Council (MRC) guidelines on testing complex interventions,217 the following pilot/preliminary studies are urgently needed.
Staff training
A staff training programme should be developed and tested to equip staff with the necessary knowledge, commitment and behaviour change to support appropriate and effective expressing of breastmilk, kangaroo skin-to-skin contact and access to peer support. This should address the needs of medical, neonatal nursing, midwifery, health visiting, speech therapy and related staff. The design of this programme should use psychological models of change and principles of adult learning (e.g. refs 218,219). A qualitative study on staff decision-making in neonatal units related to breastfeeding in Scotland has recently been completed, and these results will be relevant to inform study design (Dr Rhona McInnes, University of Stirling, personal communication, 2008).
Mother–infant contact
Adequate evaluation of kangaroo skin-to-skin contact in the UK would benefit from increased understanding of the views of staff and parents, improved staff training, and provision of facilities that enable mothers to remain with their infants. Qualitative research is needed to inform the development of a best practice model consistent with that practised in some units in Sweden, the USA and Colombia (e.g. ref. 220). This work also needs to reach consensus on the parameters of infant clinical stability within which kangaroo skin-to-skin contact can be used safely.
A pilot experimental evaluation of a best-practice model of kangaroo skin-to-skin contact could then be conducted in the UK. This could use a prospective before/after study design, to examine the impact of best-practice kangaroo skin-to-skin contact on breastfeeding and health outcomes among clinically stable infants of any gestational age. The evaluation should include core components of staff training and attitude and behaviour change, equipment, facilities, and staff and parental views as well as detailed description of the intervention components for potential replication or adaptation. Participants should include mothers and families from a range of ethnic and socioeconomic backgrounds and feeding intentions.
Peer support
Provision of peer support for mothers in neonatal units is complicated by the diversity of their problems and by the relatively high proportion who have complex health and social needs. The introduction of peer support into neonatal care will require qualitative research to examine the needs and wishes of mothers and their families, the views of hospital and community staff, and a workload analysis. This would inform the training of peer supporters and the design of an intervention study.
Together the qualitative research and the experimental evaluation would provide the necessary basis to inform the design of future intervention studies.
Recommended studies
Priority level 1: intervention studies to address urgent gaps
Based on the preliminary work described above, the following intervention studies are important priorities to address gaps in the current evidence base.
The evidence base on kangaroo skin-to-skin contact is limited to clinically stable infants. An RCT of kangaroo skin-to-skin contact for infants who are clinically less stable, possibly very preterm infants, has not been conducted in any country. Such a study would address the current evidence gap around the impact of this simple intervention in these particularly vulnerable mothers and infants. The design should take into consideration the methodological recommendations above.
It is evident that multifaceted interventions are required to create sustained change. The main elements of the intervention to be tested in this study would be: a breastfeeding promotion policy and associated implementation programme with identified leadership; skilled professional care and support; information and education for women; kangaroo skin-to-skin contact; facilities to enable initiation and maintenance of breastmilk production; care to enhance self-regulatory feeding; and community-based care following discharge. These elements are very similar to the Baby Friendly neonatal standards. Measuring their effectiveness and cost-effectiveness would be the aim of this study. The control group would receive standard care. Such a study would have significant training and resource implications, and would require the development work outlined above before it could be designed and implemented. Possible designs to be considered include a cluster RCT, or a high-quality before/after study. Measurement of short-, medium- and long-term feeding and health outcomes and resource use in the context of this study as recommended above would greatly contribute to the understanding of the contribution feeding has to make to the health and well-being of these infants and their mothers, and to cost-effectiveness, as demonstrated by Kramer et al. 4
The impact of peer support for mothers and families of infants in the neonatal unit has not been tested in the UK. It could be a more cost-effective intervention than professional support, and has the potential of offering consistent support across both hospital and community settings. Based on the preliminary research outlined above, a large RCT of peer support offered to mothers in both hospital and community settings could be designed, to include short-, medium- and longer-term breastfeeding/breastmilk feeding outcomes, infant health and well-being, women’s views and experiences, staff views, utilities and costs.
Priority level 2: specific interventions and staff training
In addition to inclusion of this aspect in the multifaceted supportive environment study, studies are needed to examine ways of maximising milk production. These studies would be smaller in scale and could be conducted over a shorter timescale than the large multifaceted study. Such studies should include examination of different pumps and pumping regimens, and the use of co-interventions of skin-to-skin contact, additional support, and relaxation. They should be informed by current understanding of the physiology of lactation and the specific physiological challenges for mothers who give birth preterm.
There is no evidence that pharmaceutical galactagogues are effective when used in isolation from supportive measures; studies that do not include provision of skilled lactation support for all participants should not be conducted. Where studies of galactagogues are conducted, they should examine their differential effectiveness in different subgroups (e.g. women separated from their infants for long periods, women with problems in producing adequate milk even when well supported and expressing frequently), and include a wide range of breastfeeding/breastmilk outcomes and assess women’s views.
High-quality research is needed to explore the appropriate mechanisms for feeding infants who are unable to breastfeed, both clinically stable and less stable ones, as there are problems associated with both bottle feeding and cup feeding. This requires an adequately sized RCT of bottle versus cup feeding, in which the bottles, teats and cups used are appropriate for the infant’s developmental stage. Outcomes should include short- and long-term measures of infant growth and health; and also breastfeeding/breastmilk feeding outcomes, including exclusivity, support needed post discharge to move from cup/bottle to breastfeeding, costs, utilities, and the views of parents and staff. This work should be conducted in an environment where early transition to the breast is encouraged by close contact between mother and infant and where staff are skilled in both techniques.
RCTs are needed to examine both short-term and longer-term outcomes of the use of nipple shields and other feeding aids for mothers of infants who are in neonatal units and have positioning and attachment problems, including infants with specific feeding challenges such as cleft lip and palate. These trials should be conducted in an environment in which the feeding aids are introduced to mothers by staff trained and experienced in breastfeeding, where ongoing support is available, and where the discontinuation of use of the feeding aids is also supported. Longer-term impact and any problems experienced by the mothers should be examined. Physiological research on milk transfer during feeding with and without shields would inform ways of enabling early transition to breastfeeding in this population.
Evidence is lacking on the effectiveness and safety of techniques to enhance the nutritional composition of human milk including commercial fortifiers and the use of components of human milk. In the light of the important short-, medium- and long-term health and development outcomes related to this question, high quality research is required.
Further good-quality research is required to evaluate the effect of Baby Friendly accreditation of maternity services on infants who are in neonatal units and have very low birthweight, gestational age of < 30 weeks and/or are small for gestational age. Good-quality concurrent evaluations should also be conducted to validate the existing evidence related to breastfeeding outcomes among all infants in neonatal units, and including preterm and low birthweight infants cared for in transitional care units and postnatal wards, in UK settings.
Research is needed to examine the most efficient and effective ways of promoting behaviour change and of conducting staff training – at continuing professional development level, to improve the knowledge and skills of current staff; and at preregistration/specialty training level, to ensure all new staff are educated in essential skills. Studies should examine staff attitudes and beliefs, barriers to change and strategies to support the implementation of change in stressful circumstances; there are examples of such work (e.g. ref. 221). This work will be informed by the preliminary work on staff training described above.
Acknowledgements
The authors are grateful for the support of the Advisory Group and the clinical experts subgroup (listed in Appendix 1) who gave generously of their time and expertise. We are also grateful to the following for additional input: Jake Abbas, Yorkshire and Humber Public Health Observatory; Sue Ashmore, UNICEF UK Baby Friendly Initiative; ND Embleton, Newcastle Neonatal Service, Royal Victoria Infirmary, Newcastle upon Tyne; Alan C Fenton, Newcastle Neonatal Service, Royal Victoria Infirmary, Newcastle upon Tyne; Kirsteen Macleod, Public Health Trainee, Cambridgeshire; Rhona McInnes, Nursing, Midwifery and Allied Health Professions Research Unit, University of Stirling; Paula Sisk, Department of Pediatrics, Wake Forest University School of Medicine, Winston-Salem, NC, USA; Gillian Weaver, United Kingdom Association for Milk Banking.
The Midwifery Department, School of Health Professions, Faculty of Health and Social Work, University of Plymouth, generously supported Elizabeth Stenhouse’s work on this study.
Administrative support was provided throughout by Jenny Brown.
Contribution of authors
Mary J Renfrew led this study and contributed to the concept and design, analysis and interpretation of data, drafting of the report and final approval of the version to be published.
Dawn Craig led the health economics component of the study and contributed to the concept and design, analysis and interpretation of data, drafting of the report and final approval of the version to be published.
Lisa Dyson contributed to the concept and design, analysis and interpretation of data, drafting of the report and final approval of the version to be published.
Felicia McCormick contributed to the concept and design, analysis and interpretation of data, drafting of the report and final approval of the version to be published.
Stephen JC Rice contributed to the concept and design, analysis and interpretation of data, drafting of the report and final approval of the version to be published.
Sarah King contributed to the concept and design, revising the report critically for important intellectual content and final approval of the version to be published.
Kate Misso contributed to the design of searches of the literature for evidence and references management, revising the report critically for important intellectual content and final approval of the version to be published.
Elizabeth Stenhouse contributed to the concept and design, revising the report critically for important intellectual content and final approval of the version to be published.
Anthony F Williams contributed to the concept and design, analysis and interpretation of data, revising the report critically for important intellectual content and final approval of the version to be published.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Scientific Advisory Committee on Nutrition . Infant Feeding Survey 2005: A Commentary on Infant Feeding Practices in the UK. 2008.
- World Health Organization . Global Strategy for Infant and Young Child Feeding 2003.
- National Institute for Health and Clinical Excellence . Improving the Nutrition of Pregnant and Breastfeeding Mothers and Children in Low-Income Households 2008.
- Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA 2001;285:413-20.
- Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Boston, MA: Agency for Healthcare Research and Quality, US Dept of Health and Human Services; 2007.
- Quigley MA, Cumberland P, Cowden JM, Rodrigues LC. How protective is breastfeeding against diarrhoeal disease in infants in 1990 England? A case–control study. Arch Dis Child 2005.
- Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom millennium cohort study. Pediatrics 2007;119:e837-42.
- Singhal A, Cole TJ, Lucas A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet 2001;357:413-9.
- Beral V. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50,302 women with breast cancer and 96,973 women without the disease. Lancet 2002;360:187-95.
- Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics 2006;118:e115-23.
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry 2008;65.
- Vohr BR, Poindexter NN, Dusick AM, McKinley LT, Higgins RD, Langer JC, et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics 2007;120:e953-9.
- Smith MM, Durkin M, Hinton VJ, Bellinger D, Kuhn L. Influence of breastfeeding on cognitive outcomes at age 6–8 years: follow-up of very low birth weight infants. Am J Epidemiol 2003;158:1075-82.
- Costeloe K. EPICure Study Group . EPICure: facts and figures; why preterm labour should be treated. BJOG 2006;113:10-2.
- Spatz DL. State of the science: use of human milk and breast-feeding for vulnerable infants. J Perinat Neonat Nurs 2006;20:51-5.
- Yu VYH. Extrauterine growth restriction in preterm infants: importance of optimizing nutrition in neonatal intensive care units. Croat Med J 2005;46:737-43.
- Canadian Medical Association . Nutrient needs and feeding of premature infants. Nutrition Commitee, Canadian Paediatric Society. CMAJ 1995;152:1765-85.
- Schanler RJ. The use of human milk for premature infants. Pediatr Clin North Am 2001;48:207-19.
- Kuschel CA, Harding JE. Multicomponent fortified human milk for promoting growth in preterm infants. Cochrane Database Syst Rev 2004;1. 101002/14651858CD000343pub2.
- Griffin TL, Meier PP, Bradford LP, Bigger HR, Engstrom JL. Mothers performing creamatocrit measures in the NICU: accuracy, reactions, and cost. J Obstet Gynecol Neonatal Nurs 2000;29:249-57.
- Meier PP, Engstrom JL, Mingolelli SS, Miracle DJ, Kiesling S. The Rush Mothers’ Milk Club: breastfeeding interventions for mothers with very-low-birth-weight infants. J Obstet Gynecol Neonatal Nurs 2004;33:164-74.
- Meier P. Bottle- and breast-feeding: effects on transcutaneous oxygen pressure and temperature in preterm infants. Nurs Res 1988;37:36-41.
- Chen C-H, Wang T-M, Chang H-M, Chi C-S. The effect of breast- and bottle-feeding on oxygen saturation and body temperature in preterm infants. J Hum Lact 2000;16:21-7.
- Blaymore Bier JA, Ferguson AE, Morales Y, Liebling JA, Oh W, Vohr BR. Breastfeeding infants who were extremely low birthweight. Pediatrics 1997;100.
- Furman L, Taylor G, Minich N, Hack M. The effect of maternal milk on neonatal morbidity of very low-birth-weight infants. Arch Pediatr Adolesc Med 2003;157:66-71.
- El-Mohandes AE, Picard MB, Simmens SJ, Keiser JF. Use of human milk in the intensive care nursery decreases the incidence of nosocomial sepsis. J Perinatol 1997;17:130-4.
- Narayanan I, Prakash K, Prabhakar AK, Gujral VV. A planned prospective evaluation of the anti-infective property of varying quantities of expressed breastmilk. Acta Paediatr Scand 1982;71:441-5.
- Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal 2007;92:F169-F175.
- Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics 2002;109:423-8.
- Lucas A, Morley R, Cole TJ, Gore SM. A randomised multicentre study of human milk versus formula and later development in preterm infants. BMJ 1994;70.
- Anderso JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr 1999;70:525-35.
- Department of Health . Department of Health and Food Standard Agency Revised Guidance on Preparation and Storage of Infant Formula Milk. 2006. www.dh.gov.uk/en/Healthcare/Maternity/Maternalandinfantnutrition/DH_4123674.
- Renfrew MJ, Ansell P, Macleod KL. Formula feed preparation – helping reduce the risks: a systematic review. Arch Dis Child 2003;88:855-8.
- World Health Organization . The Treatment of Diarrhoea. A Manual for Physicians and Other Senior Health Workers 2005. www.searo.who.int/LinkFiles/CAH_Publications_manual_physicians.pdf.
- World Health Organization . The World Health Report 2006 – Working Together for Health 2006.
- Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics (1) 2000;105.
- Shah PS, Aliwalas LL, Shah V. Breastfeeding or breast milk for procedural pain in neonates (Cochrane Review). Cochrane Database Syst Rev 2006. 101002/14651858CD004950.
- Carbajal R, Veerapen S, Couderc S, Jugie M, Ville Y. Analgesic effect of breast feeding in term neonates: randomised controlled trial. BMJ 2003;326.
- Callen J, Pinelli J, Atkinson S, Saigal S. Qualitative analysis of barriers to breastfeeding in very-low-birthweight infants in the hospital and postdischarge. Adv Neonat Care 2005;5:93-103.
- Johnson TS, Brennan RA, Flynn-Tymkow CD. A home visit program for breastfeeding education and support. J Obstet Gynecol Neonatal Nurs 1999;28:480-5.
- Chalmers B, Levin A, Van Pampus MG. Humane perinatal care. J Psychosom Obstet Gynecol 2003;24:53-4.
- Liu WF, Laudert S, Perkins B, MacMillan-York E, Martin S, Graven S. The development of potentially better practices to support the neurodevelopment of infants in the NICU. J Perinatol 2007;27:S48-S74.
- Redshaw M, Hamilton K. Networks, admissions and transfers: the perspectives of networks, neonatal units and parents. Oxford: National Perinatal Epidemiology Unit; 2006.
- Phillips SJ, Tooley GA. Improving child and family outcomes following complicated births requiring admission to neonatal intensive care units. Sexual Relationship Therapy 2005;20:431-42.
- Flacking R, Wallin L, Ewald U. Perinatal and socioeconomic determinants of breastfeeding duration in very preterm infants. Acta Paediatrica 2007;96:1126-30.
- BLISS . Breastfeeding or Expressing for a Sick or Premature Baby. An Overview of 500 women’s Experiences 2008.
- Patten T. Breastfeeding promotion: a vital emergency intervention disregarded?. Afr Health 1997;19.
- Cattaneo A, Yngve A, Koletzko B, Guzman LR. Protection, promotion and support of breast-feeding in Europe: current situation. Public Health Nutr 2007;8:39-46.
- Bolling K, Grant C, Hamlyn B, Thornton A. Infant Feeding Survey 2005. A survey conducted on behalf of The Information Centre for Health and Social Care and the UK Health Depts by BMRB Social Research. London: The Information Centre; 2007.
- Li R, Darling N, Maurice E, Barker L, Grummer-Strawn LM. Breastfeeding rates in the United States by characteristics of the child, mother, or family: the 2002 National Immunization Survey. Pediatrics 2005;115:e31-7.
- Villalpando S, Flores-Huerta S, López-Alarcón M, Cisneros-Silva I. Social and biological determinants in establishing lactation. Food Nutr Bull 1996;18:328-35.
- Griffiths LJ, Tate AR, Dezateux C, . The contribution of parental and community ethnicity to breastfeeding practices: evidence from the Millennium Cohort Study. Int J Epidemiol 2005;34:1378-86.
- Department of Health . Tackling Health Inequalities: 2007 Status Report on the Programme for Action 2007.
- Department of Health . Clarification of Definitions of Breastfeeding Initiation n.d. www.dh.gov.uk/en/Healthcare/Maternity/Maternalandinfantnutrition/DH_073254.
- Department of Health . Priorities and Planning Framework 2003–2006 2003.
- HM Government . PSA Agreement 12: Improve the Health and Wellbeing of Children and Young People 2007.
- Health Inequalities Unit DoH . Implementation Plan for Reducing Health Inequalities in Infant Mortality: A Good Practice Guide 2007.
- Department for Children Schools and Families and Department of Health . Every Child Matters: Change for Children 2008.
- Dyson L, Renfrew MJ, McFadden A, McCormick FM, Herbert G, Thomas J. Promotion of breastfeeding initiation and duration. Evidence into practice briefing. London: National Institute for Health and Clinical Excellence; 2006.
- Gartner LM, Morton J, Lawrence RA, Naylor AJ, O’Hare D, Schanler RJ, et al. Breastfeeding and the use of human milk. Pediatrics 2005;115:496-50.
- CEMACH . Diabetes in Pregnancy National Report 2007.
- Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1. JAMA 2000;283:1167-74.
- Hale T. Medications and mothers’ milk. Amarillo,TX: Hale Publications; 2008.
- Renfrew MJ, Dyson L, Wallace L, S’Souza L, McCormick FM, Spiby H. The effectiveness of health interventions to promote the duration of breastfeeding; systematic review. London: National Institute for Health and Clinical Excellence; 2005.
- Smale M, Renfrew MJ, Marshall JL, Spiby H. Turning policy into practice: more difficult than it seems. The case of breastfeeding education. Matern Child Nutr 2006;2:103-13.
- Dykes F. Breastfeeding in hospital: mothers, midwives and the production line. London: Routledge; 2006.
- Johnson K, Gerada C, Greenough A. Treatment of neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed 2003;88.
- Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure Study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 2000;106:659-71.
- Blondel B, Kaminski M. Trends in the occurrence, determinants, and consequences of multiple births. Semin Perinatol 2002;26:239-49.
- Tomashek KM, Shapiro-Mendoza CK, Weiss J, Kotelchuck M, Barfield W, Evans S, et al. Early discharge among late preterm and term newborns and risk of neonatal morbidity. Semin Perinatol 2006;30:61-8.
- Engle WA, Tomashek KM, Wallman C. ‘ Late-preterm’ infants: a population at risk. Pediatrics 2007;120.
- Furman L, Minich N, Hack M. Correlates of lactation in mothers of very low birth weight infants. Pediatrics 2002;109.
- Henderson JJ, Hartmann PE, Newnham JP, Simmer K. Effect of preterm birth and antenatal corticosteroid treatment on lactogenesis II in women. Pediatrics 2008;121.
- Macfarlane A, Mugford M. Birth counts: statistics of pregnancy and childbirth. Oxford: University of Oxford: National Perinatal Epidemiology Unit; 2000.
- Dyson LMF, Renfrew MJ. Interventions for promoting the initiation of breastfeeding. Cochrane Database Syst Rev 2005. 10.1002/14651858.CD001688.pub2.
- Alexander GR, Weiss J, Hulsey TC, Papiemik E. Preterm birth prevention: an evaluation of programs in the United States. Birth 1991;18:160-9.
- Carter JD, Mulder RT, Bartram AF, Darlow BA. Infants in a neonatal intensive care unit: parental response. Arch Dis Child Fetal Neonatal Ed 2005;90.
- Renfrew MJ. Time to get serious about educating health professionals. Matern Child Nutr 2006;2:193-5.
- Wallace LM, Kosmala-Anderson J. A training needs survey of doctors’ breastfeeding support skills in England. Matern Child Nutr 2006;2:217-31.
- UNICEF . Baby Friendly Initiative 2000.
- Jones E, Jones P, Dimmock P, Spencer A. Evaluating preterm breastfeeding training. Pract Midwife 2004;7:19-21.
- British Association of Perinatal Medicine . Standards for Hospitals Providing Neonatal Intensive and High Dependencey Care 2001.
- Bernaix LW, Schmidt CA, Jamerson PA, Seiter L, Smith J. The NICU experience of lactation and its relationship to family management style. Am J Matern Child Nurs 2006;2:95-100.
- Moore ER, Anderson GC, Bergman N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev 2007. 101002/14651858CD003519pub2.
- Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev 2006. 101002/14651858CD001814pub2.
- Hartmann PE, Ramsay DT. Mammary anatomy and physiology. Feeding and nutrition in the preterm infant. London: Elsevier Churchill Livingstone; 2006.
- Cregan MD, de Mello TR, Hartmann PE. Pre-term delivery and breast expression: consequences for initiating lactation. Adv Exp Biol 2000;478:427-8.
- Rowe-Murray HJ, Fisher JRW. Operative intervention in delivery is associated with compromised early mother-infant interaction. BJOG 2001;108:1068-75.
- Srinivasan L, Bokiniec R, King C, Weaver G, Edwards AD. Increased osmolality of breast milk with therapeutic additives. Arch Dis Child Fetal Neonatal Ed 2004;89.
- Meier PP, Engstrom JL, Murtaugh MA, Vasan U, Meier WA, Schanler RJ. Mothers’ milk feedings in the neonatal intensive care unit: accuracy of the creamatocrit technique. J Perinatol 2002;22:646-9.
- Breastmilk Banking Working Group 2005.
- Lau C, Sheena HR, Shulman RJ, Schanler RJ. Oral feeding in low birth weight infants. J Pediatr 1997;130:561-9.
- Lau C, Smith EO, Schanler RJ. Coordination of suck–swallow and swallow respiration in preterm infants. Acta Paediatrica 2003;92:721-7.
- Nyqvist KH, Strandell E. A cup feeding protocol for neonates: evaluation of nurses’ and parents’ use of two cups. J Neonatal Nurs 1999;5:31-6.
- Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatrica 2000;89:846-52.
- Jones E. Breastfeeding in the preterm infant. Modern Midwife 1994;4:22-6.
- Victora CG, Behague DP, Barros FC, Olinto MTA, Weiderpass E. Pacifier use and short breastfeeding duration: cause, consequence, or coincidence?. Pediatrics 1997;99:445-53.
- Dowling D, Thanattherakul W. Nipple confusion, alternative feeding methods and breastfeeding supplementation: state of the science. Newborn Infant Nurs Rev 2001;1:217-23.
- Lang S, Lawrence CJ, Orme RL. Cup feeding: an alternative method of infant feeding. Arch Dis Child 1994;71:365-9.
- Jones E, Spencer SA. Optimising the provision of human milk for preterm infants. BMJ 2007;92:236-8.
- World Health Organization . Ottawa Charter for Health Promotion. Health Promotion 1987;1:3-4.
- Centre for Reviews and Dissemination . Undertaking Systematic Reviews of Research on Effectiveness: CRD’s Guidance for Those Carrying Out or Commissioning Reviews n.d.
- National Institute for Health and Clinical Excellence . Guideline Development Methods Chapters and Appendices 2005. www.nice.org.uk.
- Higgins JPT, Green S. The Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions n.d.
- The Lancet n.d. www.biomedcentral.com/1471-2288/1/2.
- Sisk PM, Lovelady CA, Dillard RG, Gruber KJ. Lactation counseling for mothers of very low birth weight infants: effect on maternal anxiety and infant intake of human milk. Pediatrics. 2006;117:e67-75.
- Charpak N, RuizPelaez JG, Figueroa ZD, Charpak Y. Kangaroo mother versus traditional care for newborn infants ≤ 2000 grams: a randomized, controlled trial. Pediatrics 1997;100:682-8.
- Charpak N, Ruiz-Pelaez JG, Figueroa de CZ, Charpak Y. A randomized, controlled trial of kangaroo mother care: results of follow-up at 1 year of corrected age. Pediatrics 2001;108:1072-9.
- Ortenstrand A, Waldenström U, Winbladh B. Early discharge of preterm infants needing limited special care, followed by domiciliary nursing care. Acta Paediatrica 1999;88:1024-30.
- Ortenstrand A, Winbladh B, Nordstrom G, Waldenstrom U. Early discharge of preterm infants followed by domiciliary nursing care: parents’ anxiety, assessment of infant health and breastfeeding. Acta Paediatrica 2001;90:1190-5.
- Hill PD, Aldag JC, Chatterton RT. Breastfeeding experience and milk weight in lactating mothers pumping for preterm infants. Birth 1999;26:233-8.
- Hill PD, Aldag JC, Chatterton RT. Effects of pumping style on milk production in mothers of non-nursing preterm infants. J Human Lact 1999;15:209-16.
- Hill PD, Aldag JC, Chatterton RT. Initiation and frequency of pumping and milk production in mothers of non-nursing preterm infants. J Human Lact 2001;17:9-13.
- Jones E, Dimmock PW, Spencer SA. A randomised controlled trial to compare methods of milk expression after preterm delivery. Arch Dis Child Fetal Neonatal Ed 2001;85:F91-5.
- Blaymore Bier JA, Ferguson AE, Morales Y, Liebling JA, Archer D, Oh W, et al. Comparison of skin-to-skin contact with standard contact in low-birth-weight infants who are breast-fed. Arch Pediatr Adolesc Med 1996;150:1265-69.
- Hansen WF, McAndrew S, Harris K, Zimmerman MB. Metoclopramide effect on breastfeeding the preterm infant: a randomized trial. Obstet Gynecol 2005;105:383-9.
- Agrasada GV. Postnatal Peer Counselling on Exclusive Breastfeeding of Low-Birthweight Filipino Infants: Results of a Randomised Controlled Trial 2005.
- Kadam S, Binoy S, Kanbur W, Mondkar JA, Fernandez A. Feasibility of kangaroo mother care in Mumbai. Indian J Pediatr 2005;72:35-8.
- Collins CT, Ryan P, Crowther CA, . Effect of bottles, cups, and dummies on breast feeding in preterm infants: a randomised controlled trial. BMJ 2004;329:193-6.
- Gilks J, Watkinson M. Improving breast feeding rates in preterm babies: cup feeding versus bottle feeding. J Neonatal Nurs 2004;10:118-20.
- Rojas MA, Kaplan M, Quevedo M, Sherwonit E, Foster LB, Ehrenkranz RA, et al. Somatic growth of preterm infants during skin-to-skin care versus traditional holding: A randomized, controlled trial. J Dev Behav Pediatr 2003;24:163-8.
- Rocha NMN, Martinez FE, Jorge SM. Cup or bottle for preterm infants: effects on oxygen saturation, weight gain, and breastfeeding. J Human Lact 2002;18:132-8.
- da Silva OP, Knoppert DC, Angelini MM, Forret PA. Effect of domperidone on milk production in mothers of premature newborns: a randomized, double-blind, placebo-controlled trial. Can Med Assoc J 2001;164:17-21.
- Mosley C, Whittle C, Hicks C. A pilot study to assess the viability of a randomised controlled trial of methods of supplementary feeding of breast-fed pre-term babies. Midwifery 2001;17:150-7.
- Fewtrell MS, Lucas P, Collier S, Singhal A, Ahluwalia JS, Lucas A. Randomized trial comparing the efficacy of a novel manual breast pump with a standard electric breast pump in mothers who delivered preterm infants. Pediatrics 2001;107:1291-7.
- Pinelli J, Atkinson SA, Saigal S. Randomized trial of breastfeeding support in very low-birth-weight infants. Arch Pediatr Adolesc Med 2001;155:548-53.
- Gunn AJ, Gunn TR, Mitchell EA. Is changing the sleep environment enough? Current recommendations for SIDS. Sleep Med Rev 2000;4:453-69.
- Groh-Wargo S, Toth A, Mahoney K, Simonian S, Wasser T, Rose S. The utility of bilateral breast pumping system for mothers of premature infants. J Neonatal Nurs 1995;14:31-6.
- Roberts KL, Paynter C, McEwan B. A comparison of kangaroo mother care and conventional cuddling care. Neonat Netw 2000;19:31-5.
- Kliethermes PA, Cross ML, Lanese MG, Johnson KM, Simon SD. Transitioning preterm infants with nasogastric tube supplementation: increased likelihood of breastfeeding. J Obstet Gynecol Neonatal Nurs 1999;28:264-73.
- Cattaneo A, Davanzo R, Worku B, . Kangaroo mother care for low birthweight infants: a randomized controlled trial in different settings. Acta Paediatrica 1998;87:976-85.
- Sloan NL, Camacho LWL, Rojas EP, Stern C, Vasconez Roman F, Naranjo Castro C, et al. Kangaroo mother method: randomised controlled trial of an alternative method of care for stabilised low-birthweight infants. Lancet 1994;344:782-5.
- Gunn AJ, Gunn TR, Rabone DL, Breier BH, Blum WF, Gluckman PD. Growth hormone increases breast milk volumes in mothers of preterm infants. Pediatrics 1996;98:279-82.
- Paul VK, Singh M, Deorari AK, Pacheco J, Taneja U. Manual and pump methods of expression of breast milk. Indian J Pediatr 1996;63:87-92.
- Wahlberg V, Affonso DD, Persson B. A retrospective, comparative study using the Kangaroo method as a complement to the standard incubator care. Eur J Public Health 1992;2:34-7.
- Meier PP, Brown LP, Hurst NM, Spatz DL, Engstrom JL, Borucki LC, et al. Nipple shields for preterm infants: effect on milk transfer and duration of breastfeeding. J Human Lact 2000;16.
- Merewood A, Philipp BL, Chawla N, Cimo S. The baby-friendly hospital initiative increases breastfeeding rates in a US neonatal intensive care unit. J Human Lact 2003;19:166-71.
- Oddy WH, Glenn K. Implementing the Baby Friendly Hospital Initiative: the role of finger feeding. Breastfeed Rev 2003;11:5-10.
- Hurst NM, Valentine CJ, Renfro L, Burns P, Ferlic L. Skin-to-skin holding in the neonatal intensive care unit influences maternal milk volume. J Perinatol 1997;17:213-7.
- Amali-Adekwu O, Ogala W, Bode-Thomas F. Hindmilk and weight gain in preterm very low-birthweight infants. Pediatr Int 2007;49:156-60.
- Boo NY, Jamli FM. Short duration of skin-to-skin contact: Effects on growth and breastfeeding. J Paediatr Child Health 2007;43:831-6.
- Slusher T, Slusher IL, Biomdo M, Bode-Thomas F, Curtis BA, Meier P. Electric breast pump use increases maternal milk volume in African nurseries. J Trop Pediatr 2007;53:125-30.
- Merewood A, Chamberlain LB, Cook JT, Philipp BL, Malone K, Bauchner H. The effect of peer counselors on breastfeeding rates in the neonatal intensive care unit: results of a randomized controlled trial. Arch Pediatr Adolesc Med 2006;160:681-5.
- Fewtrell MS, Loh KL, Blake A, Ridout DA, Hawdon J. Randomised, double blind trial of oxytocin nasal spray in mothers expressing breast milk for preterm infants. Arch Dis Child Fetal Neonatal Ed 2006;91:F169-74.
- Hurst NM, Meier PP, Engstrom JL, Myatt A. Mothers performing in-home measurement of milk intake during breastfeeding of their preterm infants: Maternal reactions and feeding outcomes. J Human Lact 2004;20:178-87.
- Feher SD, Berger LR, Johnson JD, Wilde JB. Increasing breast milk production for premature infants with a relaxation/imagery audiotape. Pediatrics 1989;83:57-60.
- Whitelaw A, Heisterkamp G, Sleath K, Acolet D, Richards M. Skin to skin contact for very low birthweight infants and their mothers. Arch Dis Child 1988;63.
- Mersmann CA. Therapeutic Touch and Milk Letdown in Mothers of Non-Nursing Preterm Infants 1993.
- Pineda RG. Breastfeeding Practices in the Neonatal Intensive Care Unit before and After an Intervention Plan 2006.
- Wilhelm PA. The Effect of Early Kangaroo Care on Breast Skin Temperature, Distress, and Breastmilk Production in Mothers of Premature Infants 2005.
- Bicalho-Mancini PG, Velasquez-Melendez G. Exclusive breastfeeding at the point of discharge of high-risk newborns at a Neonatal Intensive Care Unit and the factors associated with this practice. J Pediatr (Rio J) 2004;80:241-8.
- Senn TE. Development and Evaluation of the Lactation Education Program to Increase Breastfeeding Rates in Hospitalized, Preterm Infants 2004.
- Pereira GR, Schwartz D, Gould P, Grim N. Breastfeeding in neonatal intensive care: beneficial effects of maternal counseling. Perinatol Neonatol 1984;8:35-42.
- Bell EH, Geyer J, Jones L. A structured intervention improves breastfeeding success for ill or preterm infants. MCN 1995;20:309-14.
- Edmond K, Bahl R. Optimal feeding of low-birth-weight infants: technical review. Geneva: World Health Organization; 2006.
- Anderson, Moore E, Bergman N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev 2007;3. 10.1002/14651858CD003519.pub2.
- Charpak N, Gabriel Ruiz J, Zupan J, Cattaneo A, Figueroa Z, Tessier R, et al. Kangaroo Mother Care: 25 years after. Acta Paediatrica 2005;94:514-22.
- Dodd VL. Implications of kangaroo care for growth and development in preterm infants. J Obst Gynecol Neonatal Nurs 2005;34:218-32.
- Hake-Brooks SJ, Anderson GC. Kangaroo care and breastfeeding of mother-preterm infant dyads 0–18 months, a randomised controlled trial. Neonat Netw 2008;27:1-9.
- Collins CT, Makrides M, McPhee AJ. Early discharge with home support of gavage feeding for stable preterm infants who have not established full oral feeds. Cochrane Database Syst Rev 2003. 101002/14651858CD003743.
- McInnes R, Chambers J. Breastfeeding in neonatal units: a review of breastfeeding publications between 1990 and 2005. Edinburgh: NHS Health Scotland; 2006.
- Collins A. Perinatal outcomes among US minority populations. J Nurse Practitioners 2006;2:165-9.
- Flint A, New K, Davies MW. Cup feeding versus other forms of supplemental enteral feeding for newborn infants unable to fully breastfeed. Cochrane Database Syst Rev 2007. 101002/14651858CD005092pub2.
- Conde-Agudelo A, Diaz-Rossello JL, Belizan JM. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants [update of Cochrane Database Syst Rev 2000; 4:CD002771; PMID: 11034759]. Cochrane Database Syst Rev 2003;2.
- Centers for Disease Control and Prevention . United States National Immunization Survey 2004.
- Gonzalez KA, Meinzen-Derr J, Burke BL, Hibler AJ, Kavinsky B, Hess S, et al. Evaluation of a lactation support service in a children’s hospital neonatal intensive care unit. J Human Lact 2003;19:286-92.
- World Health Organization Global Data Bank on Breastfeeding . Breastfeeding: The Best Start in Life 1996.
- Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics 2002;110:1103-9.
- Drummond M, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2007.
- Sisk PM. Lactation Counseling for Mothers of Very Low Birth Weight Infants: Effect on Maternal Anxiety, Infant Intake of Human Milk, and Infant Health in the Neonatal Intensive Care Unit 2005.
- Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al. Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost effectiveness and expected value of information analyses. Health Technol Assess 2007;11:1-226.
- Schanler RJ, Lau C, Hurst NM, Smith EOB. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 2005;116:400-6.
- Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. BMJ 1990;336:1519-23.
- Gilbert WM, Nesbitt TS, Danielson B. The cost of prematurity: quantity by gestational age and birth weight. Obstet Gynecol Clin N Am 2003;102:488-92.
- Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol 2003;23:278-85.
- Fanaroff AA, Korones SB, Wright LL, Verter J, Poland RL, Bauer CR, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J 1998;17:593-8.
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285-91.
- Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005;115:696-703.
- Larroque B, Ancel PY, Marret S, Marchand L, André M, Arnaud C, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet 2008;371:813-20.
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292:2357-65.
- Oostenbrink R, Molla HA, Essink-Botb ML. The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol 2002;55:791-9.
- Curtis L, Netten A. Unit costs of health and social care 2006. Canterbury: Personal Social Services Research Unit, University of Kent; 2006.
- Department of Health . Reference Costs 2006–2007 2008.
- Hall N, Pierro A. Necrotizing enterocolitis. Hospital Medicine 2004;65:220-5.
- Department of Health . NHS Reference Cost 2006: NHS Trust Reference Cost Index 2006 07 2006. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081094.
- Trotter CL, Edmunds WJ. Modelling cost effectiveness of meningococcal serogroup C conjugate vaccination campaign in England and Wales. BMJ 2002;324.
- Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making 2003;23:341-50.
- British National Formulary 56. London: BMA/RPSGB; 2008.
- Nyqvist KH. Breastfeeding support in neonatal care: an example of the integration of international evidence and experience. Newborn Infant Nurs Rev 2005;5:34-48.
- Cuttini M, Rebagliato M, Bortoli P, Hansen G, de Leeuw R, Lenoir S, et al. Parental visiting, communication, and participation in ethical decisions: a comparison of neonatal unit policies in Europe. Arch Dis Child Fetal Neonatal Ed 1999;81.
- Marinelli KA, Burke GS, Dodd VL. A comparison of the safety of cupfeedings and bottlefeedings in premature infants whose mothers intend to breastfeed. J Perinatol 2001;21:350-5.
- Renfrew MJ, McLoughlin M, McFadden A. Cleaning and sterilisation of infant feeding equipment: a systematic review. Public Health Nutr 2008;11:1188-99.
- Hawes J, McEwan P, McGuire W. Nasal versus oral route for placing feeding tubes in preterm or low birth weight infants. Cochrane Database Syst Rev 2004. 101002/14651858CD003952pub2.
- Slusher T, Hampton R, Bode-Thomas F, Pam S, Akor F, Meier P. Promoting the exclusive feeding of own mother’s milk through the use of hindmilk and increased maternal milk volume for hospitalized, low birth weight infants (%3C 1800 grams) in Nigeria: a feasibility study. J Human Lact 2003;19:191-8.
- Hurst NM, Meier PP, Riordan J, Sudbury M. Breastfeeding and human lactation. London: Jones & Bartlett; 2005.
- Lau C, Schanler RJ. Pediatr Clin North Am 2001;48:221-34.
- Quigley MA, Henderson G, Anthony MY, McGuire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2007. 101002/14651858CD002971pub2.
- Britton C, McCormick FM, Renfrew MJ, Wade A, King SE. Support for breastfeeding mothers. Cochrane Database Syst Rev 2007. 101002/14651858CD001141pub3.
- Fairbank L, O’Meara S, Renfrew MJ, Wooldridge M, Sowden AJ, Lister-Sharp D. A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding. Health Technol Assess 2000;4:1-171.
- McInnes RJ, Shepherd AJ, Cheyne H, Niven C. Breastfeeding in the neonatal unit (abstract) 2008.
- Freer Y. A comparison of breast and cup feeding in pre-term infants: effect on physiological parameters. J Neonatal Nurs 1998;5:16-21.
- Casiro OG, McKenzie ME, McFadyen L, Shapiro C, Seshia MM, MacDonald N, et al. Earlier discharge with community-based intervention for low birth weight infants: a randomized trial. Pediatrics 1993;92:128-34.
- Jeffcoate JA, Humphrey ME, Lloyd JK. Disturbance in parent–child relationship following preterm delivery. Dev Med Child Neurol 1979;21:344-52.
- Macey TJ, Harmon RJ, Esterbrooks MA. Impact of premature birth on the development of the infant in the family. J Consult Clin Psychol 1987;55:846-52.
- Bartington SE, Foster LJ, Dezateux C. G187 Evaluation of the UNICEF Baby Friendly Initiative for the Promotion of Breastfeeding: Findings from the Millennium Cohort Study n.d.;18.
- Wright AL, Naylor A, Wester R, Bauer M, Sutcliffe E. Using cultural knowledge in health promotion: breastfeeding among the Navajo. Health Educ Behav 1997;24.
- Coutinho SB, de Lira PIC, de Carvalho Lima M, Ashworth A. Comparison of the effect of two systems for the promotion of exclusive breastfeeding. Lancet 2005;366:1094-100.
- Dermott K, Bick D, Norman R, Ritchie G, Turnbull N, Adams C, et al. Clinical guidelines and evidence review for post natal care: routine post natal care of recently delivered women and their babies. London: National Collaborating Centre for Primary Care and Royal College of Physicians; 2006.
- Signatories to the consensus statement . Decision analytic modelling in the economic evaluation of health technologies: a consensus statement. Pharmacoeconomics 2000;17:443-4.
- National Institute for Clinical Excellence . Guide to the Methods of Technology Appraisal. 2004.
- Hunter PR. Application of Hazard Analysis Critical Control Point (HACCP) to the handling of expressed breast milk on a neonatal unit. J Hosp Infect 1991;17:139-46.
- Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 101002/14651858CD001071pub2.
- Glenny A, Hooper L, Shaw W, Reilly S, Kasem S, Reid J. Feeding interventions for growth and development in infants with cleft lip, cleft palate or cleft lip and palate. Cochrane Database Syst Rev 2004. 101002/14651858CD003315pub2.
- Darzi MA, Chowdri NA, Bhat AN. Breast feeding or spoon feeding after cleft lip repair: a prospective, randomised study. Br J Plast Surg 1996;49:24-6.
- Labbok M, Schanler RJ. Pediatr Clin North Am. 2001.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: new guidance. London: Medical Research Council; 2008.
- Hanbury A, Wallace LM, Clark M, Cushway D. Time for change: A way to increase staff adherence to guidelines in mental health. Clinical Governance Bulletin 2004;5:4-6.
- Michie S, Johnston M, Abraham C, Lawton RJ, Parker D, Walker A. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33.
- Chiu SH, Anderson GC, Burkhammer MD. Newborn temperature during skin-to-skin breastfeeding in couples having breastfeeding difficulties. Birth 2005;32:115-21.
- Bell RP, McGrath J. Implementing a research based kangaroo care program in the NICU. Nurs Clin North Am 1996;31:387-403.
- Study of maternal-milk feeding in neonatal units of the Secretariat of Health of the City of Rio de Janeiro . Pediatr Res 1999;45.
- Burke MG. Peer counseling for Mom fosters breastfeeding of premature infants. Contemp Pediatr 2006;23.
- Alexandre C, Bomy H, Bourdon E, Truffert P, Pierrat V. Lactation counselling support provided to mothers of preterm babies who intend to breastfeed. Evaluation of an educational intervention in a level III perinatal unit. Arch Pediatrie 2007;14:1413-9.
- Anderson GC, Goldson E. Nurturing the premature infant: developmental interventions in the neonatal intensive care nursery. New York: Oxford University Press; 1999.
- Bingham PM, Churchill D, Ashikaga T. Breast milk odor via olfactometer for tube-fed, premature infants. Behav Res Methods 2007;39:630-4.
- Blumenfeld H, Eisenfeld L. Does a mother singing to her premature baby affect feeding in the neonatal intensive care unit?. Clin Pediatr (Phila) 2006;45:65-70.
- Cabral IE, da Conceicao Rodrigues E. The Kangaroo Mother Method at one maternity center in Rio de Janeiro 2000–2002: children’s needs and parents’ demands for health education [Portuguese]. Texto Contexto Enfermagem 2006;15:629-36.
- Callen J, Pinelli J. A review of the literature examining the benefits and challenges, incidence and duration, and barriers to breastfeeding in preterm infants. Adv Neonatal Care 2005;5:72-88.
- Carfoot S, Williamson PR, Dickson R. A systematic review of randomised controlled trials evaluating the effect of mother/infant skin-to-skin care on successful breast feeding. Midwifery 2003;19:148-55.
- Castrucci BC, Hoover KL, Lim S, Maus KC. Availability of lactation counseling services influences breastfeeding among infants admitted to neonatal intensive care units. Am J Health Promot 2007;21:410-5.
- Charpak N, Ruiz JG. Breast milk composition in a cohort of pre-term infants’ mothers followed in an ambulatory programme in Colombia. Acta Paediatrica 2007;96:1755-9.
- Charpak N, Ruiz-Pelaez JG, Charpak Y. Rey-Martinez Kangaroo Mother Program: an alternative way of caring for low birth weight infants? One year mortality in a two cohort study. Pediatrics 1994;94:804-10.
- Cobb MAB. Promoting breastfeeding. A useful algorithm for nurses on a mother/baby unit. AWHONN Lifelines 2002;6:418-23.
- Cockerill J, Uthaya S, Dore CJ, Modi N. Accelerated postnatal head growth follows preterm birth. Arch Dis Child Fetal Neonatal Ed 2006;91:F184-7.
- Conseil d’Evaluation des Technologies de la Sante du Q . Evaluation of the Risks and Benefits of Early Postpartum Discharge – Systematic Review 1997.
- Dall’Oglio I, Salvatori G, Bonci E, Nantini B, D’Agostino G, Denta A. Breastfeeding promotion in neonatal intensive care unit: impact of a new program toward a BFHI for high-risk infants. Acta Paediatrica (Oslo) 2007;96:1626-31.
- Damanik R, Wahlqvist ML, Wattanapenpaiboon N. Lactagogue effects of Torbangun, a Bataknese traditional cuisine. Asia Pacific J Clin Nutr 2006;15:267-74.
- Ehrenkranz RA, Ackerman BA. Metoclopramide effect on faltering milk production by mothers of premature infants. Pediatrics 1986;78:614-20.
- Elliott S, Reimer C. Postdischarge telephone follow-up program for breastfeeding preterm infants discharged from a special care nursery. Neonat Netw 1998;17:41-5.
- Forsythe P. New practices in the transitional care center improve outcomes for babies and their families. J Perinatol 1998;18:13-7.
- Gilks J, Gould D, Price E. Decontaminating breast pump collection kits for use on a Neonatal Unit: review of current practice and the literature. J Neonatal Nurs 2007;13:191-8.
- Glazebrook C, Marlow N, Israel C, Croudace T, Johnson S, White IR, et al. Randomised trial of a parenting intervention during neonatal intensive care. Arch Dis Child-Fetal Neonatal Ed 2007;92:438-43.
- Gotsch C. One innovation helping breastfeeding mothers of preterm infants. J Human Lact 1991;7.
- Gray P, Flenady V. Cot-nursing versus incubator care for preterm infants. Cochrane Database Syst Rev 2001. 101002/14651858CD003062.
- Gupta A, Khanna K, Chattree S. Cup feeding: an alternative to bottle feeding in a neonatal intensive care unit. J Trop Pediatr 1999;45:108-10.
- Harding C, Law J, Pring J. The use of non-nutritive sucking to promote functional sucking skills in premature infants: an exploratory trial. Infant 2006;2:238-42.
- Hill PD, Aldag JC, Chatterton RT, Zinaman M. Comparison of milk output between mothers of preterm and term infants: the first 6 weeks after birth. J Human Lact 2005;21:22-30.
- Hurst NM. The 3 Ms of breast-feeding the preterm infant. J Perinatal Neonatal Nurs 2007;21:234-9.
- Jones E. Strategies to promote preterm breastfeeding. Modern Midwife 1995;5:8-11.
- Jones E, Spencer A. Promoting successful breast feeding for mothers of preterm infants – 1. Professional Care Mother Child 2000;10:145-7.
- Jones E, Spencer A. Successful breast feeding for mothers of preterm infants – 2. Professional Care Mother Child 2001;11:15-7.
- Lawrence RA, Pinelli J, Atkinson SA, Saigal S. Randomized trial of breastfeeding support in very low-birth-weight infants [editorial]. Arch Pediatr Adolesc Med 2001;155:543-4.
- Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: evidence and management strategies to protect breastfeeding. J Midwifery Womens Health 2007;52:579-87.
- Milsom SR, Rabone DL, Gunn AJ, Gluckman PD. Potential role for growth hormone in human lactation insufficiency. Hormone Res 1998;50:147-50.
- Narayanan I, Atkinson S, Hanson LA, Chandra RK. Human milk for low birth weight infants with special relevance to a developing country. Human Lact 1988;4:141-50.
- Narayanan I, Kumar H, Singhal PK, Dutta AK. Maternal participation in the care of the high risk infant: follow-up evaluation. Indian Pediatr 1991;28:161-7.
- Nyqvist KH, Ewald U. Successful breast feeding in spite of early mother–baby separation for neonatal care. Midwifery 1997;13:24-31.
- Page-Wilson G, Smith P, Welt C. Short-term prolactin administration causes expressible galactorrhea but does not affect bone turnover: pilot data for a new lactation agent. Int Breastfeeding J 2007;2.
- Patel AB, Shaikh S. Efficacy of breast milk gastric lavage in preterm neonates. Indian Pediatr 2007;44:199-203.
- Peterson KE, Sorensen G, Pearson M, Hebert JR, Gottlieb BR, McCormick MC. Design of an intervention addressing multiple levels of influence on dietary and activity patterns of low-income, postpartum women. Health Educ Res 2002;17:531-40.
- Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Syst Rev 2002. 101002/14651858CD001819.
- Preyde M. Mothers of very preterm infants:perspectives on their situation and a culturally sensitive intervention. Soc Work Health Care 2007;44:65-83.
- Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: Beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999;103:1150-7.
- Senarath U, Fernando DN, Rodrigo I. Effect of training for care providers on practice of essential newborn care in hospitals in Sri Lanka. J Obstet Gynecol Neonatal Nurs 2007;36:531-41.
- Sheppard JJ, Fletcher KR. Evidence-based interventions for breast and bottle feeding in the neonatal intensive care unit. Semin Speech Lang 2007;28:204-12.
- Spatz DL. Report of a staff program to promote and support breastfeeding in the care of vulnerable infants at a children’s hospital. J Perinatal Edu 2005;14:30-8.
- Thomas MR, Chan GM, Book LS. Comparison of macronutrient concentration of preterm human milk between two milk expression techniques and two techniques for quantitation of energy. J Pediatr Gastroenterol Nutr 1986;5:597-601.
- Toppare MF, Laleli Y, Senses DA, Kitapci F, Kaya IS, Dilmen U. Metoclopramide for breast-milk production. Nutr Res 1994;14:1019-29.
- Tosh K, McGuire W. Ad libitum or demand/semi-demand feeding versus scheduled interval feeding for preterm infants. Cochrane Database Syst Rev 2006. 101002/14651858CD005255pub2.
- Tyson JE, Kennedy KA. Trophic feedings for parenterally fed infants. Cochrane Database Syst Rev 2007. 10.1002/14651858.CD000504.pub2.
- VandenBerg KA, Samal L, Fleisher B, Spence S, Moragne E, Phan T, et al. Individualized developmental care for mothers of very low birthweight infants in the NICU enhances ability to support infants during feeding. J Invest Med 1999;47:20A-A.
- Wallis M, Harper M. Supporting breastfeeding mothers in hospital: part 2a. Paediatric Nursing 2007;19:31-5.
- Ward ML. A Study to Evaluate the Impact of Preterm Breastfeeding Information on a mother’s Experience of Milk Expression and Breastfeeding Following Preterm Delivery n.d.
- Warren I, Tan GC, Dixon PD, Ghaus K. Breast feeding success and early discharge for preterm infants: the result of a dedicated breast feeding programme. J Neonatal Nurs 2000;6:43-8.
- Weimers L, Svensson K, Dumas L, Naver L, Wahlberg V. Hands-on approach during breastfeeding support in a neonatal intensive care unit: a qualitative study of Swedish mothers’ experiences. Neonatal Intensive Care 2007;20:20-7.
- Wheeler JL, Johnson M, Collie L, Sutherland D, Chapman C. Promoting breastfeeding in the neonatal intensive care unit. Breastfeeding Rev 1999;7:15-8.
- Woldt EH. Breastfeeding support group in the NICU. Neonat Netw 1991;9:53-6.
- Young J. Positioning premature babies. Nursing preterm babies in intensive care: which position is best?. J Neonatal Nurs 1994;1:27-31.
- Zukowsky K. Breast-fed low-birth-weight premature neonates: developmental assessment and nutritional intake in the first 6 months of life. J Perinatal Neonatal Nurs 2007;21:242-9.
- Campbell-Yeo ML, Allen AC, Joseph KS, Ledwidge JM, Allen VM, Dooley KC. Study protocol: a double blind placebo controlled trial examining the effect of domperidone on the composition of breast milk [NCT00308334]. BMC Pregnancy Childbirth 2006;6.
- Smith MC. An Evaluation of Preterm Breastfeeding Information on a Mother’s Experience of Milk Expression and Breastfeeding Following Preterm Delivery n.d.
- Stola A. Feeding tolerance in preterm infants: randomized trial of bolus feeding every 4 hours versus every 3 hours. New York, USA: Weill Medical College of Cornell University; n.d.
- Welt C, Page-Wilson G, Smith P. Recombinant Human Prolactin for Lactation Induction (abstract) n.d.:4-8.
- Barton AJ, Danek G, Owens B. Clinical and economic outcomes of infants receiving breast milk in the NICU. J Specialists Pediatr Nurs 2001;6:5-10.
- Drane D. Breastfeeding and formula feeding: a preliminary economic analysis. Breastfeeding Rev 1997;5:7-15.
- Daga SR, Daga AS. Impact of breastmilk on the cost-effectiveness of the special care unit for the newborn. J Trop Pediatr 1985;31:121-3.
- Hake-Brooks SJ, Cranston Anderson G. Kangaroo care and breaastfeeding of mother–preterm infant dyads 0–18 months: a randomized controlled trial. Neonatal Netw 2008;27:151-8.
Appendix 1 Members of the research team, advisory group, and clinical expert subgroup
Research team
Dawn Craig, Research Fellow in Health Economics, Centre for Reviews and Dissemination, University of York
Lisa Dyson (from January 2008), Senior Researcher, Mother and Infant Research Unit, University of York
Sarah King (until December 2007), Senior Researcher, Mother and Infant Research Unit, University of York
Felicia McCormick, Research Fellow, Mother and Infant Research Unit, University of York
Kate Misso, Information Officer, Centre for Reviews and Dissemination, University of York
Mary Renfrew (Principal Investigator), Professor of Mother and Infant Health, Director, Mother and Infant Research Unit, University of York
Stephen Rice, Research Fellow in Health Economics, Centre for Reviews and Dissemination, University of York
Elizabeth Stenhouse, Senior Lecturer in Midwifery Research, University of Plymouth
Tony Williams, Reader in Child Nutrition and Consultant in Neonatal Paediatrics, St George’s, University of London
Advisory Group members
Gene Anderson, Edward J. and Louise Mellen Professor Emerita of Nursing, Case Western Reserve University; Professor Emerita, University of Florida
Rosie Dodds, Policy Researcher, National Childbirth Trust: service user/consumer representative member (Chair)
Sandra Lang, Consultant in Infant and Young Child Feeding and Newborn Care, Senior Teaching Fellow, Centre for International Health and Development, University College London Institute of Child Health, London
Shelley Mason, Family Support Co-ordinator, BLISS
Paula Meier, Professor and Director for Clinical Research and Lactation, Neonatal Intensive Care Unit, Rush University Medical Center, Chicago
Josephine Patterson, Breastfeeding Advisor, Winnicott Baby Unit, St Mary’s Hospital, Paddington
Mark Sculpher, Professor of Health Economics, University of York
Sarah O’Sullivan, Service user/consumer member
Amanda Sowden, Associate Director, Centre for Reviews and Dissemination, University of York
Louise Wallace, Professor and Director, Health Behaviour and Health Service Management Interventions programmes, Health and Lifestyles Interventions Research Centre, Coventry University
Clinical Expert Subgroup
Victoria Dugbartey, Infant Feeding Specialist, Queen Charlotte’s and Chelsea Hospital, London
Kerstin Hedberg Nyqvist, Department of Women’s and Children’s Health, Uppsala University
Elizabeth Jones, Infant Feeding Advisor, University Hospital of North Staffordshire
Caroline King, Paediatric Clinical Lead, Hammersmith Hospital, and Honorary Lecturer, Imperial College, London
Camilla Kingdon, Consultant Neonatologist, Queen Charlotte’s and Chelsea Hospital, London
Appendix 2 Search strategies
Appendix 2.1: Effectiveness review
1. Search strategy to identify systematic reviews
MEDLINE and MEDLINE In-Process Citations (OVID)
-
Breast Feeding/
-
breastfe$.ti,ab.
-
(breast$ adj2 (fed$ or feed$)).ti,ab.
-
Lactation/ or Milk, Human/
-
(breastmilk$ or lactat$).ti,ab.
-
(transitional care and (maternal$ or mother$ or baby or babies or infant$ or newborn$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).ti,ab.
-
((breast$ or mother$ or human or maternal$) adj2 milk).ti,ab.
-
(nursing adj2 (maternal$ or mother$ or baby or babies or infant$ or newborn$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).ti,ab.
-
((maintain$ or maintenance$ or establish$ or begin$ or start$ or commenc$ continu$ or sustain$ or prolong$ or extend$) adj2 (milk or breast$ fed$ or breast feed$ or lactat$ or nursing or suck$ or breastfed$ or breastfeed$)).ti,ab.
-
((milk or breastmilk) adj2 (donor$ or donat$ or bank$)).ti,ab.
-
((milk or breast$) adj2 express$).ti,ab.
-
(breast pump$ or breastpump$).ti,ab.
-
(hand$ adj2 express$).ti,ab.
-
kangaroo.ti,ab.
-
((skin$ adj2 contact) or skin-to-skin).ti,ab.
-
(suck$ adj2 breast$).ti,ab.
-
(tube adj2 (feed or fee$)).ti,ab.
-
(cup adj2 (fed or fee$)).ti,ab.
-
(bottle adj2 (fed or fee$)).ti,ab.
-
(transition adj2 breast$).ti,ab.
-
Lactation Disorders/nu, di, dh, pc, dt, th [Nursing, Diagnosis, Diet Therapy, Prevention & Control, Drug Therapy, Therapy]
-
Galactorrhea/di, dh, nu, pc, dt, th
-
(Galactagogue$ or Galactogogue$ or lactagogue$ or lactogogue$ or caffeine or hops or fenugreek or fennel seed$ or blessed thistle or domperidone or alfalfa).ti,ab.
-
Caffeine/tu [Therapeutic Use]
-
Humulus/ or Plants, Medicinal/tu or Metoclopramide/tu or Sulpiride/tu or Plant Extracts/tu or Chlorpromazine/tu or Dopamine Antagonists/tu or Oxytocin/tu or Thyrotropin-Releasing Hormone/tu or Human Growth Hormone/tu
-
Trigonella/ or (Sulpiride or metoclopramide or domeperidone or chlorpromazine or oxytocin or dopamine antagonist$ or thyrotropine releasing hormone$ or TRH or human growth hormone$).ti,ab.
-
Foeniculum/ or creamatocrit$.ti,ab.
-
Cnicus/
-
Domperidone/tu [Therapeutic Use]
-
Medicago sativa/
-
(nipple$ shield$ or breast$ shield$).ti,ab.
-
(dropper$ adj2 (fed or fee$)).ti,ab.
-
(spoon adj2 (fed or fee$)).ti,ab.
-
(syringe$ adj2 (fed or fee$)).ti,ab.
-
supplementer$.ti,ab.
-
Pacifiers/
-
(pacifier$ or dummy or dummies or soother$).ti,ab.
-
(non-nutritive suck$ or nonnutritive suck$).ti,ab.
-
Rooming-in Care/
-
(rooming-in or room-in or co-sleep$).ti,ab.
-
(bedshar$ or bed-shar$).ti,ab.
-
((bedside$ or bed side$) adj2 (cot or cots or cradle$ or crib or crib)).ti,ab.
-
or/1-42
-
Intensive Care Units, Neonatal/
-
Intensive Care, Neonatal/
-
(nicu or nicus).ti,ab.
-
(scbu or scbus).ti,ab.
-
((special or intensive or icu$) adj3 (newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).mp.
-
((newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies) adj2 unit$).mp.
-
or/44-49
-
43 and 50
-
Health Promotion/
-
(promotion$ or promoting).ti,ab.
-
promot$.ti,ab.
-
Inservice Training/
-
((staff or professional$ or nurse$ or doctor$ or physician$ or midwife$ or midwive$) adj2 training).ti,ab.
-
social support/
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 support$).ti,ab.
-
(antenatal educat$ or ante natal educat$ or neonatal educat$ or neo natal educat$ or prenatal educat$ or pre natal educat$ or preconception$ educat$ or pre conception$ educat$).ti,ab.
-
(postpartum educat$ or post partum educat$ or postnatal educat$ or post natal educat$).ti,ab.
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 involv$).ti,ab.
-
(early adj2 discharge$).ti,ab.
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 attitude$).ti,ab.
-
((staff or professional$ or nurse$ or doctor$ or physician$ or midwife$ or midwive$) adj2 attitude$).ti,ab.
-
Patient Education/
-
((peer$ or social$ or interpersonal$ or inter personal$ or midwife$ or midwive$ or profession$ or practitioner$ or nursing or lactation) adj2 (encourag$ or motivat$ or support$ or guid$ or counsel$ or consult$ or advic$ or advis$)).ti,ab.
-
(lactation adj2 (consultant$ or expert$ or adviser$ or specialist$ or advisor$)).ti,ab.
-
(humane adj2 (prematur$ or pre matur$ or premie or premies or perinat$ or peri nat$ or neonat$ or neo nat$) adj1 care).ti,ab.
-
((tallin or Levin) adj1 (method$ or approach$ or program$ or propos$ or unit$)).ti,ab.
-
((mother$ or parent$ or maternal$ or famil$) adj1 (led or focus$ or lead$ or direct$ or center$ or centre$) adj2 care).ti,ab.
-
(abm or lll or bfi or bfhi or nct).ti,ab.
-
(association of breastfeeding mothers or association of breast feeding mothers).ti,ab.
-
(la leche league or national childbirth trust).ti,ab.
-
(baby friendly or breaststart or breast start or beststart or best start or nidcap).ti,ab.
-
(support$ strateg$ or support$ system$ or support$ program$).ti,ab.
-
(Neonat$ Individuali?ed Developmental Care and Assessment Program$).ti,ab.
-
(Newborn$ Individuali?ed Developmental Care and Assessment Program$).ti,ab.
-
or/52-77
-
50 and 78
-
51 or 79
-
animal/
-
human/
-
81 not (81 and 82)
-
80 not 83
-
84 not (letter or comment or editorial).pt.
-
(200708$ or 200709$ or 200710$ or 200711$ or 200712$).ed.
-
2008$.ed.
-
86 or 87
-
85 and 88
-
review.ab.
-
review.pt.
-
meta-analysis.ab.
-
meta-analysis.pt.
-
meta-analysis.ti.
-
or/90-94
-
89 and 95
2. Search strategy to identify primary studies
MEDLINE and MEDLINE In-Process Citations (OVID)
-
Breast Feeding/ (18587)
-
breastfe$.ti,ab. (7049)
-
(breast$ adj2 (fed$ or feed$)).ti,ab. (11128)
-
Lactation/ or Milk, Human/ (37074)
-
(breastmilk$ or lactat$).ti,ab. (85491)
-
(transitional care and (maternal$ or mother$ or baby or babies or infant$ or newborn$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).ti,ab. (22)
-
((breast$ or mother$ or human or maternal$) adj2 milk).ti,ab. (12479)
-
(nursing adj2 (maternal$ or mother$ or baby or babies or infant$ or newborn$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).ti,ab. (1852)
-
((maintain$ or maintenance$ or establish$ or begin$ or start$ or commenc$ continu$ or sustain$ or prolong$ or extend$) adj2 (milk or breast$ fed$ or breast feed$ or lactat$ or nursing or suck$ or breastfed$ or breastfeed$)).ti,ab. (2789)
-
((milk or breastmilk) adj2 (donor$ or donat$ or bank$)).ti,ab. (400)
-
((milk or breast$) adj2 express$).ti,ab. (2623)
-
(breast pump$ or breastpump$).ti,ab. (159)
-
(hand$ adj2 express$).ti,ab. (888)
-
kangaroo.ti,ab. (1019)
-
((skin$ adj2 contact) or skin-to-skin).ti,ab. (1046)
-
(suck$ adj2 breast$).ti,ab. (44)
-
(tube adj2 (feed or fee$)).ti,ab. (3468)
-
(cup adj2 (fed or fee$)).ti,ab. (33)
-
(bottle adj2 (fed or fee$)).ti,ab. (1537)
-
(transition adj2 breast$).ti,ab. (27)
-
Lactation Disorders/nu, di, dh, pc, dt, th [Nursing, Diagnosis, Diet Therapy, Prevention & Control, Drug Therapy, Therapy] (627)
-
Galactorrhea/di, dh, nu, pc, dt, th (429)
-
(Galactagogue$ or caffeine or hops or fenugreek or fennel seed$ or blessed thistle or domperidone or alfalfa).ti,ab. (22466)
-
Caffeine/tu [Therapeutic Use] (836)
-
Humulus/ or Plants, Medicinal/tu or Metoclopramide/tu or Sulpiride/tu or Plant Extracts/tu or Chlorpromazine/tu or Dopamine Antagonists/tu or Oxytocin/tu or Thyrotropin-Releasing Hormone/tu or Human Growth Hormone/tu (19226)
-
Trigonella/ or (Sulpride or metoclopramide or domeperidone or chlorpromazine or oxytocin or dopamine antagonist$ or thyrotropine releasing hormone$ or TRH or human growth hormone$).ti,ab. (44536)
-
Foeniculum/ or creamatocrit$.ti,ab. (86)
-
Cnicus/ (0)
-
Domperidone/tu [Therapeutic Use] (306)
-
Medicago sativa/ (2584)
-
(nipple$ shield$ or breast$ shield$).ti,ab. (59)
-
(dropper$ adj2 (fed or fee$)).ti,ab. (2)
-
(spoon adj2 (fed or fee$)).ti,ab. (39)
-
(syringe$ adj2 (fed or fee$)).ti,ab. (12)
-
supplementer$.ti,ab. (5)
-
Pacifiers/ (136)
-
(pacifier$ or dummy or dummies or soother$).ti,ab. (3052)
-
(non-nutritive suck$ or nonnutritive suck$).ti,ab. (217)
-
Rooming-in Care/ (348)
-
(rooming-in or room-in or co-sleep$).ti,ab. (1572)
-
(bedshar$ or bed-shar$).ti,ab. (191)
-
((bedside$ or bed side$) adj2 (cot or cots or cradle$ or crib or crib)).ti,ab. (0)
-
or/1-42 (219727)
-
Intensive Care Units, Neonatal/ (5994)
-
Intensive Care, Neonatal/ (2696)
-
(nicu or nicus).ti,ab. (2642)
-
(scbu or scbus).ti,ab. (71)
-
((special or intensive or icu$) adj3 (newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).mp. (13714)
-
((newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies) adj2 unit$).mp. (7994)
-
or/44-49 (15629)
-
43 and 50 (908)
-
Health Promotion/ (29989)
-
(promotion$ or promoting).ti,ab. (82762)
-
promot$.ti,ab. (329719)
-
Inservice Training/ (13078)
-
((staff or professional$ or nurse$ or doctor$ or physician$ or midwife$ or midwive$) adj2 training).ti,ab. (7232)
-
social support/ (29285)
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 support$).ti,ab. (6551)
-
(antenatal educat$ or ante natal educat$ or neonatal educat$ or neo natal educat$ or prenatal educat$ or pre natal educat$ or preconception$ educat$ or pre conception$ educat$).ti,ab. (210)
-
(postpartum educat$ or post partum educat$ or postnatal educat$ or post natal educat$).ti,ab. (30)
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 involv$).ti,ab. (4806)
-
(early adj2 discharge$).ti,ab. (1901)
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 attitude$).ti,ab. (2157)
-
((staff or professional$ or nurse$ or doctor$ or physician$ or midwife$ or midwive$) adj2 attitude$).ti,ab. (4195)
-
Patient Education/ (48363)
-
((peer$ or social$ or interpersonal$ or inter personal$ or midwife$ or midwive$ or profession$ or practitioner$ or nursing or lactation) adj2 (encourag$ or motivat$ or support$ or guid$ or counsel$ or consult$ or advic$ or advis$)).ti,ab. (21378)
-
(lactation adj2 (consultant$ or expert$ or adviser$ or specialist$ or advisor$)).ti,ab. (168)
-
(humane adj2 (prematur$ or pre matur$ or premie or premies or perinat$ or peri nat$ or neonat$ or neo nat$) adj1 care).ti,ab. (6)
-
((tallin or Levin) adj1 (method$ or approach$ or program$ or propos$ or unit$)).ti,ab. (2)
-
((mother$ or parent$ or maternal$ or famil$) adj1 (led or focus$ or lead$ or direct$ or center$ or centre$) adj2 care).ti,ab. (601)
-
(abm or lll or bfi or bfhi or nct).ti,ab. (1267)
-
(association of breastfeeding mothers or association of breast feeding mothers).ti,ab. (0)
-
(la leche league or national childbirth trust).ti,ab. (53)
-
(baby friendly or breaststart or breast start or beststart or best start or nidcap).ti,ab. (323)
-
(support$ strateg$ or support$ system$ or support$ program$).ti,ab. (7123)
-
(Neonat$ Individuali?ed Developmental Care and Assessment Program$).ti,ab. (14)
-
(Newborn$ Individuali?ed Developmental Care and Assessment Program$).ti,ab. (23)
-
or/52-77 (471619)
-
50 and 78 (963)
-
51 or 79 (1702)
-
animal/ (4116476)
-
human/ (9822139)
-
81 not (81 and 82) (3119775)
-
80 not 83 (1691)
-
84 not (letter or comment or editorial).pt. (1651)
-
(200607$ or 200608$ or 200609$ or 200610$ or 200611$ or 200612$).ed. (339554)
-
2007$.ed. (400072)
-
86 or 87 (739626)
-
85 and 88 (133)
-
review.ab. (343906)
-
review.pt. (1295130)
-
meta-analysis.ab. (12712)
-
meta-analysis.pt. (15848)
-
meta-analysis.ti. (8155)
-
or/90-94 (1453608)
-
89 and 95 (32)
-
Breast Feeding/ (18587)
-
breastfe$.ti,ab. (7049)
-
(breast$ adj2 (fed$ or feed$)).ti,ab. (11128)
-
Lactation/ or Milk, Human/ (37074)
-
(breastmilk$ or lactat$).ti,ab. (85491)
-
(transitional care and (maternal$ or mother$ or baby or babies or infant$ or newborn$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).ti,ab. (22)
-
((breast$ or mother$ or human or maternal$) adj2 milk).ti,ab. (12479)
-
(nursing adj2 (maternal$ or mother$ or baby or babies or infant$ or newborn$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).ti,ab. (1852)
-
((maintain$ or maintenance$ or establish$ or begin$ or start$ or commenc$ continu$ or sustain$ or prolong$ or extend$) adj2 (milk or breast$ fed$ or breast feed$ or lactat$ or nursing or suck$ or breastfed$ or breastfeed$)).ti,ab. (2789)
-
((milk or breastmilk) adj2 (donor$ or donat$ or bank$)).ti,ab. (400)
-
((milk or breast$) adj2 express$).ti,ab. (2623)
-
(breast pump$ or breastpump$).ti,ab. (159)
-
(hand$ adj2 express$).ti,ab. (888)
-
kangaroo.ti,ab. (1019)
-
((skin$ adj2 contact) or skin-to-skin).ti,ab. (1046)
-
(suck$ adj2 breast$).ti,ab. (44)
-
(tube adj2 (feed or fee$)).ti,ab. (3468)
-
(cup adj2 (fed or fee$)).ti,ab. (33)
-
(bottle adj2 (fed or fee$)).ti,ab. (1537)
-
(transition adj2 breast$).ti,ab. (27)
-
Lactation Disorders/nu, di, dh, pc, dt, th [Nursing, Diagnosis, Diet Therapy, Prevention & Control, Drug Therapy, Therapy] (627)
-
Galactorrhea/di, dh, nu, pc, dt, th (429)
-
(Galactagogue$ or caffeine or hops or fenugreek or fennel seed$ or blessed thistle or domperidone or alfalfa).ti,ab. (22466)
-
Caffeine/tu [Therapeutic Use] (836)
-
Humulus/ or Plants, Medicinal/tu or Metoclopramide/tu or Sulpiride/tu or Plant Extracts/tu or Chlorpromazine/tu or Dopamine Antagonists/tu or Oxytocin/tu or Thyrotropin-Releasing Hormone/tu or Human Growth Hormone/tu (19226)
-
Trigonella/ or (Sulpride or metoclopramide or domeperidone or chlorpromazine or oxytocin or dopamine antagonist$ or thyrotropine releasing hormone$ or TRH or human growth hormone$).ti,ab. (44536)
-
Foeniculum/ or creamatocrit$.ti,ab. (86)
-
Cnicus/ (0)
-
Domperidone/tu [Therapeutic Use] (306)
-
Medicago sativa/ (2584)
-
(nipple$ shield$ or breast$ shield$).ti,ab. (59)
-
(dropper$ adj2 (fed or fee$)).ti,ab. (2)
-
(spoon adj2 (fed or fee$)).ti,ab. (39)
-
(syringe$ adj2 (fed or fee$)).ti,ab. (12)
-
supplementer$.ti,ab. (5)
-
Pacifiers/ (136)
-
(pacifier$ or dummy or dummies or soother$).ti,ab. (3052)
-
(non-nutritive suck$ or nonnutritive suck$).ti,ab. (217)
-
Rooming-in Care/ (348)
-
(rooming-in or room-in or co-sleep$).ti,ab. (1572)
-
(bedshar$ or bed-shar$).ti,ab. (191)
-
((bedside$ or bed side$) adj2 (cot or cots or cradle$ or crib or crib)).ti,ab. (0)
-
or/97-138 (219727)
-
Intensive Care Units, Neonatal/ (5994)
-
Intensive Care, Neonatal/ (2696)
-
(nicu or nicus).ti,ab. (2642)
-
(scbu or scbus).ti,ab. (71)
-
((special or intensive or icu$) adj3 (newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).mp. (13714)
-
((newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies) adj2 unit$).mp. (7994)
-
or/140-145 (15629)
-
139 and 146 (908)
-
Health Promotion/ (29989)
-
(promotion$ or promoting).ti,ab. (82762)
-
promot$.ti,ab. (329719)
-
Inservice Training/ (13078)
-
((staff or professional$ or nurse$ or doctor$ or physician$ or midwife$ or midwive$) adj2 training).ti,ab. (7232)
-
social support/ (29285)
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 support$).ti,ab. (6551)
-
(antenatal educat$ or ante natal educat$ or neonatal educat$ or neo natal educat$ or prenatal educat$ or pre natal educat$ or preconception$ educat$ or pre conception$ educat$).ti,ab. (210)
-
(postpartum educat$ or post partum educat$ or postnatal educat$ or post natal educat$).ti,ab. (30)
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 involv$).ti,ab. (4806)
-
(early adj2 discharge$).ti,ab. (1901)
-
((family or families or parent$ or mother$ or father$ or partner$) adj2 attitude$).ti,ab. (2157)
-
((staff or professional$ or nurse$ or doctor$ or physician$ or midwife$ or midwive$) adj2 attitude$).ti,ab. (4195)
-
Patient Education/ (48363)
-
((peer$ or social$ or interpersonal$ or inter personal$ or midwife$ or midwive$ or profession$ or practitioner$ or nursing or lactation) adj2 (encourag$ or motivat$ or support$ or guid$ or counsel$ or consult$ or advic$ or advis$)).ti,ab. (21378)
-
(lactation adj2 (consultant$ or expert$ or adviser$ or specialist$ or advisor$)).ti,ab. (168)
-
(humane adj2 (prematur$ or pre matur$ or premie or premies or perinat$ or peri nat$ or neonat$ or neo nat$) adj1 care).ti,ab. (6)
-
((tallin or Levin) adj1 (method$ or approach$ or program$ or propos$ or unit$)).ti,ab. (2)
-
((mother$ or parent$ or maternal$ or famil$) adj1 (led or focus$ or lead$ or direct$ or center$ or centre$) adj2 care).ti,ab. (601)
-
(abm or lll or bfi or bfhi or nct).ti,ab. (1267)
-
(association of breastfeeding mothers or association of breast feeding mothers).ti,ab. (0)
-
(la leche league or national childbirth trust).ti,ab. (53)
-
(baby friendly or breaststart or breast start or beststart or best start or nidcap).ti,ab. (323)
-
(support$ strateg$ or support$ system$ or support$ program$).ti,ab. (7123)
-
(Neonat$ Individuali?ed Developmental Care and Assessment Program$).ti,ab. (14)
-
(Newborn$ Individuali?ed Developmental Care and Assessment Program$).ti,ab. (23)
-
or/148-173 (471619)
-
146 and 174 (963)
-
147 or 175 (1702)
-
animal/ (4116476)
-
human/ (9822139)
-
177 not (177 and 178) (3119775)
-
176 not 179 (1691)
-
180 not (letter or comment or editorial).pt. (1651)
3. Search strategy to identify primary studies evaluating galactagogues
MEDLINE and MEDLINE In-Process Citations (OVID)
-
Caffeine/tu [Therapeutic Use]
-
Humulus/ or Plants, Medicinal/tu or Metoclopramide/tu or Sulpiride/tu or Plant Extracts/tu or Chlorpromazine/tu or Dopamine Antagonists/tu or Oxytocin/tu or Thyrotropin-Releasing Hormone/tu or Human Growth Hormone/tu
-
Trigonella/ or (Sulpiride or metoclopramide or domeperidone or chlorpromazine or oxytocin or dopamine antagonist$ or thyrotropine releasing hormone$ or TRH or human growth hormone$).ti,ab.
-
Foeniculum/
-
Cnicus/
-
Domperidone/tu [Therapeutic Use]
-
Medicago sativa/
-
Lactation Disorders/dh, dt
-
Galactorrhea/dh, dt
-
(Galactagogue$ or Galactogogue$ or lactagogue$ or lactogogue$ or caffeine or hops or fenugreek or fennel seed$ or blessed thistle or domperidone or alfalfa).ti,ab.
-
Metoclopramide/tu [Therapeutic Use]
-
Growth Hormone/tu [Therapeutic Use]
-
(maxalon or dolmatil or motilium or syntocinon or Sulpiride or metoclopramide or domeperidone or chlorpromazine or oxytocin or dopamine antagonist$ or thyrotropine releasing hormone$ or TRH or human growth hormone$).ti,ab.
-
or/1-13
-
Infant, Premature/
-
(premie or premies or pre term$ or preterm$ or prematur$).mp.
-
15 or 16
-
14 and 17
-
limit 18 to yr=’1991 - 2008’
Appendix 2.2: Health economics review
Searches of health economics resources
NHS Economic Evaluation Database (NHS EED) (internal CRD B system)
The NHS EED search was from inception up to 8 August 2007 and identified 38 references.
Limit e
-
S breastfe$ or breastmilk$ or lactat$
-
S breast$(w2)fed$ or breast$(w2)feed$
-
S transitional(w)care and (maternal$ or mother$ or baby or babies or infant$ or newborn$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies)
-
S (breast$ or mother$ or human or maternal$)(w)milk
-
S nursing(w)(maternal$ or mother$ or baby or babies or infant$ or newborn$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies)
-
S (maintain$ or maintenance$ or establish$ or begin$ or start$ or commenc$ continu$ or sustain$ or prolong$ or extend$)(w)(milk or breast$(w1)fed$ or breast(w1)feed$ or lactat$ or nursing or suck$ or breastfed$ or breastfeed$)
-
S (milk or breastmilk)(w)(donor$ or donat$ or bank$)
-
S (milk or breast$)(w)express$
-
S breast(w1)pump$ or breastpump$
-
S hand$(w)express$
-
S kangaroo or (skin$(w2)contact) or suck$(w)breast$
-
tube(w)(feed or fee$)
-
S cup(w)(fed or fee$)
-
S bottle(w)(fed or fee$)
-
S transition(w)breast$
-
S lactagogue$ or lactogogue$ or Galactagogue$ or caffeine or hops or fenugreek or (fennel(w1)seed$) or (blessed(w1)thistle) or domperidone or alfalfa
-
S Sulpiride or metoclopramide or domeperidone or chlorpromazine or oxytocin or (dopamine(w)antagonist$) or (thyrotropine(w)releasing(w)hormone$) or TRH or (human(w)growth(w)hormone$) or creamatocrit$
-
S (nipple$(w)shield$) or (breast$(w)shield$)
-
S (dropper$(w)fed$) or (dropper$(w)fee$)
-
S (spoon(w)fee$) or (spoon(w)fed$)
-
S (syringe$(w)fed$) or (syringe$(w)fee$)
-
s supplementer$ or pacifier$ or dummy or dummies or soother$
-
(non-nutritive(w)suck$) or (nonnutritive(w)suck$)
-
s rooming(w1)in or room(w1)in or co(w1)sleep$ or bedshar$ or bed(w1)shar$
-
s bedside$(w)(cot or cots or cradle$ or crib or crib)
-
s (bed(w)side$)(w)(cot or cots or cradle$ or crib or crib)
-
s s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16 or s17 or s18 or s19 or s20 or s21 or s22 or s23 or s24 or s25 or s26
-
s nicu or nicus or scbu or scbus
-
s (special or intensive or icu$)(w)(newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies)
-
s (newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies)(w)unit$
-
s s28 or s29 or s30
-
s 27 and s31
-
s promotion$ or promoting or promot$ or early(w)discharge$ or patient$(w)educat$
-
s (staff or professional$ or nurse$ or doctor$ or physician$ or midwife$ or midwive$)(w2)training
-
s (family or families or parent$ or mother$ or father$ or partner$)(w2)support$
-
s antenatal(w)educat$ or ante(w)natal(w)educat$ or neonatal(w)educat$ or neo(w)natal(w)educat$ or prenatal(w)educat$ or pre(w)natal(w)educat$ or preconception$(w)educat$ or pre(w)conception$(w)educat$
-
s postpartum(w)educat$ or post(w)partum(w)educat$ or postnatal(w)educat$ or post(w)natal(w)educat$
-
s (family or families or parent$ or mother$ or father$ or partner$)(w2)involv$
-
s (family or families or parent$ or mother$ or father$ or partner$)(w2)attitude$
-
s (staff or professional$ or nurse$ or doctor$ or physician$ or midwife$ or midwive$)(w2)attitude$
-
s (peer$ or social$ or interpersonal$ or inter(w)personal$ or midwife$ or midwive$ or profession$ or practitioner$ or nursing or lactation)(w2)(encourag$ or motivat$ or support$ or guid$ or counsel$ or consult$ or advic$ or advis$)
-
s lactation(w2)(consultant$ or expert$ or adviser$ or specialist$ or advisor$)
-
s humane(w2)(prematur$ or pre(w)matur$ or premie or premies or perinat$ or peri(w)nat$ or neonat$ or neo(w)nat$)(w)care
-
s (tallin or Levin)(w)(method$ or approach$ or program$ or propos$ or unit$)
-
s (mother$ or parent$ or maternal$ or famil$)(w)(led or focus$ or lead$ or direct$ or center$ or centre$)(w)care
-
s abm or lll or bfi or bfhi or nct
-
s association(w2)breastfeeding(w1)mothers or association(w2)breast(w1)feeding(w1)mothers
-
s la(w1)leche(w1)league or national(w1)childbirth(w1)trust
-
s baby(w1)friendly or breaststart or breast(w1)start or beststart or best(w1)start or nidcap
-
s support$(w)strateg$ or support$(w)system$ or support$(w)program$
-
s Neonat$(w1)Individualised(w1)Developmental(w1)Care(w2)Assessment(w1)Program$
-
s Neonat$(w1)Individualized(w1)Developmental(w1)Care(w2)Assessment(w1)Program$
-
s Newborn$(w1)Individualised(w1)Developmental(w1)Care(w2)Assessment(w1)Program$
-
s Newborn$(w1)Individualized(w1)Developmental(w1)Care(w2)Assessment(w1)Program$
-
s s33 or s34 or s35 or s36 or s37 or s38 or s39 or s40 or s41 or s42 or s43 or s44 or s45 or s46 or s47 or s48 or s49 or s50 or s51 or s52 or s53 or s54
-
s s31 and s55
-
s s32 or s56
HEED (Health Economic Evaluations Database) (internet)
The HEED searches were from inception up to 8 August 2007 and identified 214 references.
Search 1
-
TI=breastfe* or breastmilk* or lactat* or breast* fed* or breast* feed*
-
AB=breastfe* or breastmilk* or lactat* or breast* fed* or breast* feed*
-
KW=breastfe* or breastmilk* or lactat* or breast* fed* or breast* feed*
-
TI=transitional care AND (maternal* or mother* or baby or babies or infant* or newborn* or neonat* or neo nat* or perinat* or peri nat* or premie or premies)
-
AB=transitional care AND (maternal* or mother* or baby or babies or infant* or newborn* or neonat* or neo nat* or perinat* or peri nat* or premie or premies)
-
KW=transitional care AND (maternal* or mother* or baby or babies or infant* or newborn* or neonat* or neo nat* or perinat* or peri nat* or premie or premies)
-
TI=(breast* or mother* or human or maternal*) AND milk
-
AB=(breast* or mother* or human or maternal*) AND milk
-
KW=(breast* or mother* or human or maternal*) AND milk
-
cs=1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
4 hits
-
TI=nursing AND (maternal* or mother* or baby or babies or infant* or newborn* or neonat* or neo nat* or perinat* or peri-nat* or premie or premies)
-
AB=nursing AND (maternal* or mother* or baby or babies or infant* or newborn* or neonat* or neo nat* or perinat* or peri-nat* or premie or premies)
-
KW=nursing AND (maternal* or mother* or baby or babies or infant* or newborn* or neonat* or neo nat* or perinat* or peri-nat* or premie or premies)
-
TI=(maintain* or maintenance* or establish* or begin* or start* or commenc* continu* or sustain* or prolong* or extend*) AND (milk or breast* fed* or breast feed* or lactat* or nursing or suck* or breastfed* or breastfeed*)
-
AB=(maintain* or maintenance* or establish* or begin* or start* or commenc* continu* or sustain* or prolong* or extend*) AND (milk or breast* fed* or breast feed* or lactat* or nursing or suck* or breastfed* or breastfeed*)
-
KW=(maintain* or maintenance* or establish* or begin* or start* or commenc* continu* or sustain* or prolong* or extend*) AND (milk or breast* fed* or breast feed* or lactat* or nursing or suck* or breastfed* or breastfeed*)
-
TI=(milk or breastmilk) AND (donor* or donat* or bank*)
-
AB=(milk or breastmilk) AND (donor* or donat* or bank*)
-
KW=(milk or breastmilk) AND (donor* or donat* or bank*)
-
cs=1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
2 hits
-
TI=(milk or breast*) AND express*
-
AB=(milk or breast*) AND express*
-
KW=(milk or breast*) AND express*
-
TI=breast pump* or breastpump* or (hand* AND express*) or
-
kangaroo or (skin* AND contact) or (suck* AND breast*)
-
AB=breast pump* or breastpump* or (hand* AND express*) or
-
kangaroo or (skin* AND contact) or (suck* AND breast*)
-
KW=breast pump* or breastpump* or (hand* AND express*) or
-
kangaroo or (skin* AND contact) or (suck* AND breast*)
-
TI=transition AND breast*
-
AB=transition AND breast*
-
KW=transition AND breast*
-
cs=1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
3 hits
-
TI=tube or cup or bottle) OR (feed or fee*)
-
AB=tube or cup or bottle) OR (feed or fee*)
-
KW=tube or cup or bottle) OR (feed or fee*)
-
TI=lactagogue* or lactogogue* or Galactagogue* or caffeine or hops or fenugreek or ‘fennel seed*’ or ‘blessed thistle’ or domperidone or alfalfa
-
AB=lactagogue* or lactogogue* or Galactagogue* or caffeine or hops or fenugreek or ‘fennel seed*’ or ‘blessed thistle’ or domperidone or alfalfa
-
KW=lactagogue* or lactogogue* or Galactagogue* or caffeine or hops or fenugreek or ‘fennel seed*’ or ‘blessed thistle’ or domperidone or alfalfa
-
TI=Sulpiride or metoclopramide or domeperidone or chlorpromazine or oxytocin or ‘dopamine antagonist*’ or ‘thyrotropine releasing hormone*’ or TRH or ‘human growth hormone*’ or creamatocrit*
-
AB=Sulpiride or metoclopramide or domeperidone or chlorpromazine or oxytocin or ‘dopamine antagonist*’ or ‘thyrotropine releasing hormone*’ or TRH or ‘human growth hormone*’ or creamatocrit*
-
KW=Sulpiride or metoclopramide or domeperidone or chlorpromazine or oxytocin or ‘dopamine antagonist*’ or ‘thyrotropine releasing hormone*’ or TRH or ‘human growth hormone*’ or creamatocrit*
-
cs=1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
0 hits
-
TI=(nipple* shield*) or (breast* shield*) or (dropper* fed*) or (dropper* fee*)
-
AB=(nipple* shield*) or (breast* shield*) or (dropper* fed*) or (dropper* fee*)
-
KW=(nipple* shield*) or (breast* shield*) or (dropper* fed*) or (dropper* fee*)
-
TI=(spoon fee*) or (spoon fed*) or (syringe* fed*) or (syringe* fee*)
-
AB=(spoon fee*) or (spoon fed*) or (syringe* fed*) or (syringe* fee*)
-
KW=(spoon fee*) or (spoon fed*) or (syringe* fed*) or (syringe* fee*)
-
TI=supplementer* or pacifier* or dummy or dummies or soother* or (non-nutritive suck*) or (nonnutritive suck*)
-
AB=supplementer* or pacifier* or dummy or dummies or soother* or (non-nutritive suck*) or (nonnutritive suck*)
-
KW=supplementer* or pacifier* or dummy or dummies or soother* or (non-nutritive suck*) or (nonnutritive suck*)
-
cs=1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
0 hits
-
TI=rooming-in or room-in or co-sleep* or bedshar* or bed-shar*
-
AB=(rooming-in or room-in or co-sleep* or bedshar* or bed-shar*
-
KW=(rooming-in or room-in or co-sleep* or bedshar* or bed-shar*
-
TI=bedside* AND (cot or cots or cradle* or crib or crib)
-
AB=bedside* AND (cot or cots or cradle* or crib or crib)
-
KW=bedside* AND (cot or cots or cradle* or crib or crib)
-
TI=(bed-side*) AND (cot or cots or cradle* or crib or crib)
-
AB=(bed-side*) AND (cot or cots or cradle* or crib or crib)
-
KW=(bed-side*) AND (cot or cots or cradle* or crib or crib)
-
cs=1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
0 hits
-
TI=nicu or nicus or scbu or scbus
-
AB=nicu or nicus or scbu or scbus
-
KW=nicu or nicus or scbu or scbus
-
TI=(special or intensive or icu*) AND (newborn* or neonat* or baby or babies or infant* or neonat* or neo-nat* or perinat* or peri-nat* or premie or premies)
-
cs=1 or 2 or 3 or 4
86 hits
-
KW=(special or intensive or icu*) AND (newborn* or neonat* or baby or babies or infant* or neonat* or neo-nat* or perinat* or peri-nat* or premie or premies)
111 hits
-
AB=(special or intensive or icu*) AND (newborn* or neonat* or baby or babies or infant* or neonat* or neo-nat* or perinat* or peri-nat* or premie or premies)
-
TI=(newborn* or neonat* or baby or babies or infant* or neonat* or neo-nat* or perinat* or peri-nat* or premie or premies) AND unit*
-
AB=(newborn* or neonat* or baby or babies or infant* or neonat* or neo-nat* or perinat* or peri-nat* or premie or premies) AND unit*
-
KW=(newborn* or neonat* or baby or babies or infant* or neonat* or neo-nat* or perinat* or peri-nat* or premie or premies) AND unit*
-
cs=1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
104 hits
PEDE (Paediatric Economic Database Evaluation) (internet)
The PEDE search covered the date range 1980 to 31/12/2003 and retrieved 42 references. The database was searched on 9 August 2007.
http://pede.bioinfo.sickkids.on.ca/pede/search.jsp
-
Breast
-
Lactat
-
transitional care
-
milk
-
hand express
-
kangaroo
-
skin contact
-
skin-to-skin
-
bottle
-
tube
-
cup
-
dropper
-
spoon
-
syringe
-
lactagogue
-
lactogogue
-
Galactagogue
-
Caffeine
-
Hops
-
Fenugreek
-
fennel seed
-
blessed thistle
-
domperidone
-
alfalfa
-
Sulpiride
-
Metoclopramide
-
Domperidone
-
Chlorpromazine
-
Oxytocin
-
dopamine antagonist
-
thyrotropine releasing hormone
-
TRH
-
human growth hormone
-
creamatocrit
-
nipple
-
supplementer
-
pacifier
-
dummy
-
dummies
-
soother
-
non-nutritive
-
suck
-
nonnutritive
-
rooming-in
-
room-in
-
co-sleep
-
bedshar
-
bed-shar
-
bedside
-
bed-side
Supplementary searches to populate the decision model
Long-term outcomes of necrotising enterocolitis (NEC) or spsis
NHS Economic Evaluation Database (NHS EED) (up to 2008/02/28 (internal CRD interface)
The NHS EED search was from 2003 to 28/2/08 and identified 27 references.
-
51. s nec or (necroti$(w2)enterocol$)
-
52. s longterm(w2)outcome$ or cerebral$(w1)pals$ or cp or little$(w1)disease$ or spastic(w1)diplegia$
-
53. s (visual$ or vision or hearing)(w2)(loss or disability$ or impair$ or difficult$ or disorder$ or defect$ or deficit$ or deficienc$)
-
54. s Deaf$ or blind$ or hypoacusis or hypoacuses or macropsia$ or metamorphopsia$ or micropsia or dyslexi$ or dyspraxi$ or apraxi$ or disable$ or disability$
-
55. s Hemeralopia$ or Amblyopia$ or Amaurosis(w1)Fugax or Diplopia or Hemianopsia or Photophobia or Scotoma or Low(w1)Vision or mental$(w1)retard$
-
56. s (cognitive$ or development$ or intellectual$ or learning$)(w2)(loss or impair$ or disability$ or difficult$ or disorder$ or defect$ or delay$ or deficit$ or deficienc$)
-
57. s (psychomotor$ or physical$ or mobility$ or psycho(w1)motor$ or neurodevelopment$ or neuro(w1)development$)(w2)(loss or impair$ or disability$ or difficult$ or disorder$ or defect$ or delay$ or deficit$ or deficienc$)
-
58. s s2 or s3 or s4 or s5 or s6 or s7
-
59. s s1 and s8
-
60. S premie or premies or pre(w1)term$ or preterm$ or prematur$ or nicu or nicus or scbu or scbus
-
61. s (special or intensive or icu$)(w)(newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies)
-
62. s (newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies)(w)(unit$ or ward$)
-
63. s s10 or s11 or s12
-
64. s Meningit$ or meningococc$ or arachnoiditis or Meningoencephalitis or pachymeningit$ leptomeningit$
-
65. s (dura(w1)mater or mening$ or arachnoid(w1)membrane)(w2)(inflam$ or infect$)
-
66. s Bacteremia or Fungemia or septic(w1)shock or sepsis or septic$ or septicaemia
-
67. s Blood$(w1)(poison$ or infect$)
-
68. s (skin$ or pulmonary or Lung$ or soft(w1)tissue$)(w1)(infect$ or inflamm$)
-
69. s Bronchopneumonia$ or Pleuropneumonia$ or pneumonia$ or pneumonit$ or Streptococcal(w1)Infect$ or Staphylococc$(w1)Infect$ or Dermatomycos$ or Community(w1)Acquired(w1)Infection$ or Hospital$(w1)Acquired(w1)Infection$
-
70. s (Swan(w1)Ganz or indwelling or in(w1)dwelling or intrarterial or intra(w1)arterial or picc or hickman or subclavian or central or venous or arterial or peripheral or jugular)(w2)(line or lines or catheter$ port$)
-
71. s vascular(w1)access
-
72. s (joint or spine or spinal or bone$)(w1)(infect$ or inflamm$)
-
73. s Osteitis or osteomyelit$ or Discit$ or Periostit$ or Spondylit$
-
74. s (Osteoarticular or Osteo(w1)articular or bone$ or joint$ or spine or spinal)(w1)tuberculosis
-
75. s s14 or s15 or s16 or s17 or s18 or s19 or s20 or s21 or s22 or s23 or s24
-
76. s 13 and s25 and s8
-
77. s s9 or s26
Health Economic Evaluations Database (HEED) (up to 28/02/2008) (internet)
The HEED search was from 2003 to 28/2/08 and identified 110 references.
-
nec or necrotizing enterocolitis or necrotising enterocolitis
13 hits
Expert search
-
AX= pre term or preterm* or prematur* or nicu or nicus or scbu or scbus or newborn* or neonat* or baby or babies or infant* or neonat* or neo natal or neo nate or neo nates or neo natally or perinat* or peri nate or peri nates or peri natal or peri natally or premie or premies
-
AX= Meningit* or meningococc* or arachnoiditis or Meningoencephalitis or pachymeningit* or leptomeningit* or vascular access or inflam* or infect* or Bacteremia or Fungemia or septic shock or sepsis or septic* or septicaemia or osteomyelit* or tuberculosis or tubercular or Bronchopneumonia* or Pleuropneumonia* or pneumonia* or pneumonit* or Streptococcal Infection or Streptococcal or Staphylococcal or Staphylococcus or Dermatomycos* or Discit* or Periostit* or Spondylit*
-
AX=(Swan Ganz or indwelling or in-dwelling or intrarterial or picc or hickman or subclavian or central or venous or arterial or peripheral or jugular) within 2 (line or lines or catheter* or port*)
-
cs=2 or 3
-
cs=1 and 4
MEDLINE and MEDLINE In-Process Citations (2003–2008/02/wk 2) (OVID)
The Medline search was from 2003 to wk 2/02/2008 and identified 132 references.
-
exp Enterocolitis, Necrotizing/
-
(nec or necroti$ enterocol$).ti,ab.
-
1 or 2
-
Cerebral Palsy/
-
(longterm outcome$ or cerebral$ pals$ or cp or little$ disease$ or spastic diplegia$).ti,ab.
-
exp Hearing Disorders/
-
exp Vision Disorders/
-
((visual$ or vision or hearing) adj3 (loss or disabilit$ or impair$ or difficult$ or disorder$ or defect$ or deficit$ or deficienc$)).ti,ab.
-
exp Dyslexia/
-
exp Psychomotor Disorders/
-
(long term outcome$ or Deaf$ or blind$ or hypoacusis or hypoacuses or macropsia$ or metamorphopsia$ or micropsia or dyslexi$ or dyspraxi$ or apraxi$ or disabl$ or disabilit$).ti,ab.
-
(Hemeralopia$ or Amblyopia$ or Amaurosis Fugax or Diplopia or Hemianopsia or Photophobia or Scotoma or Low Vision or mental$ retard$).ti,ab.
-
exp Mental Retardation/
-
exp child development disorders, pervasive/ or developmental disabilities/ or exp learning disorders/ or motor skills disorders/ or stereotypic movement disorder/
-
((cognitive$ or development$ or intellectual$ or learning$) adj2 (loss or impair$ or disabilit$ or difficult$ or disorder$ or defect$ or delay$ or deficit$ or deficienc$)).ti,ab.
-
((psychomotor$ or physical$ or mobility$ or psycho motor$ or neurodevelopment$ or neuro development$) adj2 (loss or impair$ or disability$ or difficult$ or disorder$ or defect$ or delay$ or deficit$ or deficienc$)).ti,ab.
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
-
3 and 17
-
(premie or premies or pre term$ or preterm$ or prematur$ or nicu or nicus or scbu or scbus).ti,ab.
-
infant, premature/
-
intensive care units, neonatal/
-
intensive care, neonatal/
-
((special or intensive or icu$) adj3 (newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).mp.
-
((newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies) adj2 unit$).mp.
-
19 or 20 or 21 or 22 or 23 or 24
-
exp Meningitis/
-
exp sepsis/ or soft tissue infections/
-
exp Skin Diseases, Bacterial/
-
exp Staphylococcal Skin Infections/
-
exp Dermatomycoses/
-
Pseudomonas Infections/
-
exp Pneumonia, Bacterial/
-
soft tissue infections/ or exp urinary tract infections/
-
((urinar$ or urine or uret$) adj2 (infect$ or inflam$)).ti,ab.
-
exp Bone Diseases, Infectious/
-
(Osteitior osteomyelit$ or Discit$ or Periostit$ or Spondylit$).ti,ab.
-
((Osteoarticular or Osteo articular or bone$ or joint$ or spine or spinal) adj1 tuberculosis).ti,ab.
-
or/26-37
-
25 and 38 and 17
-
18 or 39
-
(‘2003’ or ‘2004’ or ‘2005’ or ‘2006’ or ‘2007’ or ‘2008’).yr.
-
40 and 41
Embase (2003–2008/wk 7) (OVID)
The Embase search was from 2003 to wk 7/2008 and identified 444 references.
-
Necrotizing Enterocolitis/
-
(nec or necroti$ enterocol$).ti,ab.
-
1 or 2
-
(longterm outcome$ or cerebral$ pals$ or cp or little$ disease$ or spastic diplegia$).ti,ab.
-
Cerebral Palsy/
-
exp Hearing Disorder/
-
exp Visual Disorder/
-
((visual$ or vision or hearing) adj3 (loss or disabilit$ or impair$ or difficult$ or disorder$ or defect$ or deficit$ or deficienc$)).ti,ab.
-
dyslexia/ or aphasia/
-
exp Psychomotor Disorder/
-
(long term outcome$ or Deaf$ or blind$ or hypoacusis or hypoacuses or macropsia$ or metamorphopsia$ or micropsia or dyslexi$ or dyspraxi$ or apraxi$ or disabl$ or disabilit$).ti,ab.
-
(Hemeralopia$ or Amblyopia$ or Amaurosis Fugax or Diplopia or Hemianopsia or Photophobia or Scotoma or Low Vision or mental$ retard$).ti,ab.
-
exp Mental Deficiency/
-
Developmental Disorder/
-
exp Learning Disorder/
-
exp Motor Dysfunction/
-
((cognitive$ or development$ or intellectual$ or learning$) adj2 (loss or impair$ or disabilit$ or difficult$ or disorder$ or defect$ or delay$ or deficit$ or deficienc$)).ti,ab.
-
((psychomotor$ or physical$ or mobility$ or psycho motor$ or neurodevelopment$ or neuro development$) adj2 (loss or impair$ or disability$ or difficult$ or disorder$ or defect$ or delay$ or deficit$ or deficienc$)).ti,ab.
-
or/4-18
-
(premie or premies or pre term$ or preterm$ or prematur$ or nicu or nicus or scbu or scbus).ti,ab.
-
((special or intensive or icu$) adj3 (newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies)).mp.
-
((newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo nat$ or perinat$ or peri nat$ or premie or premies) adj2 unit$).mp.
-
newborn intensive care/
-
newborn intensive care nursing/
-
or/20-24
-
exp Meningitis/
-
newborn sepsis/ or sepsis/ or septicemia/ or septic shock/
-
Soft Tissue Infection/
-
exp ‘bone and joint infections’/ or exp device infection/ or exp skin infection/
-
exp lung infection/ or exp pneumonia/
-
exp Urinary Tract Infection/
-
((urinar$ or urine or uret$) adj2 (infect$ or inflam$)).ti,ab.
-
(Osteitior osteomyelit$ or Discit$ or Periostit$ or Spondylit$).ti,ab.
-
((Osteoarticular or Osteo articular or bone$ or joint$ or spine or spinal) adj1 tuberculosis).ti,ab.
-
or/26-34
-
3 and 19
-
25 and 35 and 19
-
36 or 37
-
(‘2003’ or ‘2004’ or ‘2005’ or ‘2006’ or ‘2007’ or ‘2008’).yr.
-
38 and 39
EconLit (2003–2008/01) (OVID)
The EconLit search was from 2003 to 01/2008 and identified zero references.
-
#13 #5 and #12 0
-
#12 #6 and #11 and #4 0
-
#11 #7 or #7 or #8 or #9 or #10 330
-
#10 (Osteoarticular or Osteo articular or bone* or joint* or spine or spinal) adj tuberculosis 0
-
#9 ( (Swan Ganz or indwelling or in-dwelling or intrarterial or intra arterial or picc or hickman or subclavian or central or venouor arterial or peripheral or jugular) near (line or lines or catheter* or port*) )or( (joint or spine or spinal or bone* or urin* or urin* or uret*) near (infect* or inflamm*) )or( Osteitior osteomyelit* or Discit* or Periostit* or Spondylit* ) 287
-
#8 ( Blood* adj (poison* or infect*) )or( (skin* or pulmonary or Lung* or soft tissue*) adj (infect* or inflamm*) )or( Bronchopneumonia* or Pleuropneumonia* or pneumonia* or pneumonit* or Streptococcal Infect* or Staphylococc* Infect* or Dermatomycos* or Community Acquired Infection* or Hospital* Acquired Infection* ) 33
-
#7 ( Meningit* or meningococc* or arachnoiditis or Meningoencephalitis or pachymeningit* or leptomeningit* or vascular access )or( (dura mater or mening* or arachnoid membrane)near (inflam* or infect*) )or( Bacteremia or Fungemia or septic shock or sepsis or septic* or septicaemia ) 10
-
#6 ( premie or premies or pre term* or preterm* or prematur* or nicu or nicus or scbu or scbus )or( (special or intensive or icu*) near (newborn* or neonat* or baby or babies or infant* or neonat* or neo nat* or perinat* or peri nat* or premie or premies) )or( (newborn* or neonat* or baby or babies or infant* or neonat* or neo nat* or perinat* or peri nat* or premie or premies)near (unit* or ward*) ) 567
-
#5 #1 and #4 0
-
#4 #2 or #3 4620
-
#3 ( Hemeralopia* or Amblyopia* or Amaurosis Fugax or Diplopia or Hemianopsia or Photophobia or Scotoma or Low Vision or mental* retard* )or( (cognitive* or development* or intellectual* or learning*) near (loss or impair* or disabilit* or difficult* or disorder* or defect* or delay* or deficit* or deficienc*) )or( (psychomotor* or physical* or mobility* or psycho motor* or neurodevelopment* or neuro development*) near (loss or impair* or disability* or difficult* or disorder* or defect* or delay* or deficit* or deficienc*) ) 1259
-
#2 ( longterm outcome* or cerebral* pals* or cp or little* disease* or spastic diplegia* )or( (visual* or vision or hearing) near (loss or disabilit* or impair* or difficult* or disorder* or defect* or deficit* or deficienc*) )or( Deaf* or blind* or hypoacusis or hypoacuses or macropsia* or metamorphopsia* or micropsia or dyslexi* or dyspraxi* or apraxi* or disable* or disabilit* ) 3450
-
#1 nec or necroti* enterocol* 25
Quality of life in babies with NEC, sepsis, meningitis, etc.
NHS Economic Evaluation Database (NHS EED) (up to 2008/02/28 (internal CRD interface)
The NHS EED search was from inception to 28/2/08 and identified 39 references.
-
s nec or (necroti$(w2)enterocol$)
-
s Meningit$ or meningococc$ or arachnoiditis or Meningoencephalitis or pachymeningit$ or leptomeningit$
-
s (dura(w1)mater or mening$ or arachnoid(w1)membrane)(w2)(inflam$ or infect$)
-
s Bacteremia or Fungemia or septic(w1)shock or sepsis or septic$ or septicaemia
-
s Blood$(w1)(poison$ or infect$)
-
s (skin$ or pulmonary or Lung$ or soft(w1)tissue$)(w1)(infect$ or inflamm$)
-
s Bronchopneumonia$ or Pleuropneumonia$ or pneumonia$ or pneumonit$ or Streptococcal(w1)Infect$ or Staphylococc$(w1)Infect$ or Dermatomycos$ or Community(w1)Acquired(w1)Infection$ or Hospital$(w1)Acquired(w1)Infection$
-
s (Swan(w1)Ganz or indwelling or in(w1)dwelling or intrarterial or intra(w1)arterial or picc or hickman or subclavian or central or venous or arterial or peripheral or jugular)(w2)(line or lines or catheter$ or port$)
-
s vascular(w1)access
-
s (joint or spine or spinal or bone$)(w1)(infect$ or inflamm$)
-
s Osteitis or osteomyelit$ or Discit$ or Periostit$ or Spondylit$
-
s (Osteoarticular or Osteo(w1)articular or bone$ or joint$ or spine or spinal)(w1)tuberculosis
-
s s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 or s9 or s10 or s11 or s12
-
s quality(w2)life or qol or qaly$ or hrqol or eq5d or sf6d or sf36d or hui1 or hui or huis or hui2 or hui3 or health(w)utility$(w1)ind$
-
s baby or babies or infant$ or newborn$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies
-
s s13 and s14 and s15
Health Economic Evaluations Database (HEED) (up to 28/02/2008) (internet)
The HEED search was from inception to 28/2/08 and identified 60 references.
-
AX= pre term or preterm* or prematur* or nicu or nicus or scbu or scbus or newborn* or neonat* or baby or babies or infant* or neonat* or neo natal or neo nate or neo nates or neo natally or perinat* or peri nate or peri nates or peri natal or peri natally or premie or premies
-
AX= Meningit* or meningococc* or arachnoiditis or Meningoencephalitis or pachymeningit* or leptomeningit* or vascular access or inflam* or infect* or Bacteremia or Fungemia or septic shock or sepsis or septic* or septicaemia or osteomyelit* or tuberculosis or tubercular or Bronchopneumonia* or Pleuropneumonia* or pneumonia* or pneumonit* or Streptococcal Infection or Streptococcal or Staphylococcal or Staphylococcus or Dermatomycos* or Discit* or Periostit* or Spondylit*
-
AX=’quality of life’ or qol or qaly* or hrqol or eq5d or sf6d or sf36d or hui1 or hui or huis or hui2 or hui3 or ‘health utility’ or ‘health utilities’
-
cs=1 and 2 and 3
EconLit (2003–01/2008) (OVID)
The EconLit search was from inception to 1/2008 and identified zero references.
-
#8 #5 and #6 and #7 0
-
#7 Baby or babies or infant* or newborn* or neonat* or neo-nat* or perinat* or peri-nat* or premie or premies 1684
-
#6 ‘quality of life’ or qol or qaly* or hrqol or eq5d or sf6d or sf36d or hui1 or hui or huis or hui2 or hui3 or (health utility* ind*) 823
Searches and results below from saved search history scbu_lto_econ1b
-
#5 #1 or #1 or #2 or #3 or #4 332
-
#4 (Osteoarticular or Osteo articular or bone* or joint* or spine or spinal) adj tuberculosis 0
-
#3 ( (Swan Ganz or indwelling or in-dwelling or intrarterial or intra arterial or picc or hickman or subclavian or central or venouor arterial or peripheral or jugular) near (line or lines or catheter* or port*) )or( (joint or spine or spinal or bone*) near (infect* or inflamm*) )or( Osteitior osteomyelit* or Discit* or Periostit* or Spondylit* ) 288
-
#2 ( Blood* adj (poison* or infect*) )or( (skin* or pulmonary or Lung* or soft tissue*) adj (infect* or inflamm*) )or( Bronchopneumonia* or Pleuropneumonia* or pneumonia* or pneumonit* or Streptococcal Infect* or Staphylococc* Infect* or Dermatomycos* or Community Acquired Infection* or Hospital* Acquired Infection* ) 34
-
#1 ( Meningit* or meningococc* or arachnoiditis or Meningoencephalitis or pachymeningit* or leptomeningit* or vascular access )or( (dura mater or mening* or arachnoid membrane)near (inflam* or infect*) )or( Bacteremia or Fungemia or septic shock or sepsis or septic* or septicaemia ) 10
Economic evaluations of NEC, sepsis, meningitis, etc. in preterms or SCBUs
NHS Economic Evaluation Database (NHS EED) (up to 28/02/2008; internal CRD interface)
The NHS EED search was from inception to 28/2/08 and identified 43 references.
-
s (premie or premies or pre(w1)term$ or preterm$ or prematur$)
-
s nicu or nicus or scbu or scbus
-
s (special or intensive or icu$)(w)(newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies)
-
s (newborn$ or neonat$ or baby or babies or infant$ or neonat$ or neo(w1)nat$ or perinat$ or peri(w1)nat$ or premie or premies)(w)(unit$ or ward$)
-
s s1 or s2 or s3 or s4
-
s nec or (necroti$(w2)enterocol$)
-
s Meningit$ or meningococc$ or arachnoiditis or Meningoencephalitis or pachymeningit$ or leptomeningit$
-
s (dura(w1)mater or mening$ or arachnoid(w1)membrane)(w2)(inflam$ or infect$)
-
s Bacteremia or Fungemia or septic(w1)shock or sepsis or septic$ or septicaemia
-
s Blood$(w1)(poison$ or infect$)
-
s (skin$ or pulmonary or Lung$ or soft(w1)tissue$)(w1)(infect$ or inflamm$)
-
s Bronchopneumonia$ or Pleuropneumonia$ or pneumonia$ or pneumonit$ or Streptococcal(w1)Infect$ or Staphylococc$(w1)Infect$ or Dermatomycos$ or Community(w1)Acquired(w1)Infection$ or Hospital$(w1)Acquired(w1)Infection$
-
s (Swan(w1)Ganz or indwelling or in(w1)dwelling or intrarterial or intra(w1)arterial or picc or hickman or subclavian or central or venous or arterial or peripheral or jugular)(w2)(line or lines or catheter$ or port$)
-
s vascular(w1)access
-
s (joint or spine or spinal or bone$)(w1)(infect$ or inflamm$)
-
s Osteitis or osteomyelit$ or Discit$ or Periostit$ or Spondylit$
-
s (Osteoarticular or Osteo(w1)articular or bone$ or joint$ or spine or spinal)(w1)tuberculosis
-
s s6 or s7 or s8 or s9 or s10 or s11 or s12 or s13 or s14 or s15 or s16 or s17
-
s s5 and s18
Health Economic Evaluations Database (HEED) (up to 28/12/2008) (internet)
The HEED search was from inception to 28/2/08 and identified 443 references.
-
nec or necrotizing enterocolitis or necrotising enterocolitis
13 hits
Expert search
-
AX= pre term or preterm* or prematur* or nicu or nicus or scbu or scbus or newborn* or neonat* or baby or babies or infant* or neonat* or neo natal or neo nate or neo nates or neo natally or perinat* or peri nate or peri nates or peri natal or peri natally or premie or premies
-
AX= Meningit* or meningococc* or arachnoiditis or Meningoencephalitis or pachymeningit* or leptomeningit* or vascular access or inflam* or infect* or Bacteremia or Fungemia or septic shock or sepsis or septic* or septicaemia or osteomyelit* or tuberculosis or tubercular or Bronchopneumonia* or Pleuropneumonia* or pneumonia* or pneumonit* or Streptococcal Infection or Streptococcal or Staphylococcal or Staphylococcus or Dermatomycos* or Discit* or Periostit* or Spondylit*
-
AX=(Swan Ganz or indwelling or in-dwelling or intrarterial or picc or hickman or subclavian or central or venous or arterial or peripheral or jugular) within 2 (line or lines or catheter* or port*)
-
cs=2 or 3
-
cs=1 and 4
EconLit (2003–01/2008) (OVID)
The EconLit search was from inception to 01/2008 and identified one reference.
-
#9 #2 and #8 1
-
#8 #7 or #1 357
Searches and results below from saved search history scbu_lto_econ1b
-
3. #7 #3 or #3 or #4 or #5 or #6 332
-
4. #6 (Osteoarticular or Osteo articular or bone* or joint* or spine or spinal) adj tuberculosis 0
-
5. #5 ( (Swan Ganz or indwelling or in-dwelling or intrarterial or intra arterial or picc or hickman or subclavian or central or venouor arterial or peripheral or jugular) near (line or lines or catheter* or port*) )or( (joint or spine or spinal or bone*) near (infect* or inflamm*) )or( Osteitior osteomyelit* or Discit* or Periostit* or Spondylit* ) 288
-
6. #4 ( Blood* adj (poison* or infect*) )or( (skin* or pulmonary or Lung* or soft tissue*) adj (infect* or inflamm*) )or( Bronchopneumonia* or Pleuropneumonia* or pneumonia* or pneumonit* or Streptococcal Infect* or Staphylococc* Infect* or Dermatomycos* or Community Acquired Infection* or Hospital* Acquired Infection* ) 34
-
7. #3 ( Meningit* or meningococc* or arachnoiditis or Meningoencephalitis or pachymeningit* or leptomeningit* or vascular access )or( (dura mater or mening* or arachnoid membrane)near (inflam* or infect*) )or( Bacteremia or Fungemia or septic shock or sepsis or septic* or septicaemia ) 10
-
8. #2 ( premie or premies or pre term* or preterm* or prematur* or nicu or nicus or scbu or scbus )or( (special or intensive or icu*) near (newborn* or neonat* or baby or babies or infant* or neonat* or neo nat* or perinat* or peri nat* or premie or premies) )or( (newborn* or neonat* or baby or babies or infant* or neonat* or neo nat* or perinat* or peri nat* or premie or premies)near (unit* or ward*) ) 568
-
9. #1 nec or necroti* enterocol* 25
Appendix 2.3: Additional search strategies for economic modelling
Searches of health economics resources
The following resources were searched to identify economic evaluations for inclusion in the review and to inform the decision modelling:
NHS Economic Evaluation Database (NHS EED) (up to 2007/08/8) (internal CRD interface)
Health Economic Evaluations Database (HEED) (up to 2007/08/08) (internet)
Pediatric Economic Database Evaluation (PEDE) (1980–2003) (internet) (http://pede.bioinfo.sickkids.on.ca/pede.index.jsp).
A total of 294 references was retrieved.
Subset search of Clinical Effectiveness Endnote Library
An Endnote Library containing 10,262 references, identified by the search undertaken for the evidence of effectiveness review search detailed above, was searched to identify potentially relevant cost/economic studies. After deduplication, 1176 records were identified.
The following terms were entered line-by-line (_ indicates a space):
-
_cost_
-
_costs
-
_cost-
-
_costly
-
_costing
-
_econom
-
_budget
-
_price
-
_pricing
-
_expenditure
-
value for money.
A total of 1176 references was retrieved and scanned for relevance.
Further searches to populate the decision model
A series of focused supplementary searches were undertaken to identify data to populate the model. These searches were limited to a small collection of ‘core’ databases, as specified by the Health Economists:
-
NHS Economic Evaluation Database (NHS EED) (up to 2008/02/28) (internal CRD interface)
-
Health Economic Evaluations Database (HEED) (up to 2008/02/28) (internet)
-
MEDLINE and MEDLINE In-Process Citations (2003–2008/02/wk 2) (OVID)
-
Embase (2003–2008/wk 7) (OVID)
-
EconLit (2003–2008/01) (OVID).
Searches were undertaken for three supplementary topics:
-
long-term outcomes of necrotising enterocolitis (NEC) or sepsis
-
quality of life in babies with NEC, sepsis, meningitis, etc.
-
economic evaluations of NEC, sepsis, meningitis, etc. in preterms or SCBUs.
Totals of 713, 99 and 487 references, respectively, were retrieved for the searches and scanned for relevance.
Appendix 3 Pre-screen form – effectiveness review
1. Design
| Is this an evaluation of effectiveness of an intervention (SRs, RCTs, other study designs will be considered, case studies will not be included in the review) | No | Yes | ? |
| go to 5 | go to 2 | go to 2 |
2. Participants
| Are participants babies who are NOT both term and healthya, i.e. babies who need special careb? (e.g. preterm, growth-restricted and sick neonates, multiples, babies requiring surgery and babies with feeding problems, hypoglycaemia and jaundice) | No | Yes | ? |
| go to 5 | go to 3 | go to 3 |
-
a There may be studies of babies who have particular illnesses (e.g. cardiac) who have not been on NICU (they may have been on e.g. a specialised neonatal cardiac unit). At the trawling stage we will not exclude these studies – later we may decide to include only the ones where the baby was admitted to SCBU.
-
b We expect the babies in included studies will have been admitted to SCBU, but we will include studies about babies who needed special care and received (the intervention of) care on e.g. a transitional care ward.
3. Interventions
| Does the interventionc specifically addressd breastfeeding/feeding with breastmilk in SCBU/NICUs? | No | Yes | ? |
| go to 5 | go to 4 | go to 4 |
-
c A list of interventions from Table 1 of the final version of the protocol appears on the reverse of this sheet.
-
d Exclude (and mark as background) studies that evaluate:
-
the effectiveness of breastmilk on clinical outcomes (e.g. studies of associations between breastmilk consumption and the incidence of necrotising enterocolitis)
-
the nutritional content of formula and breastmilk fortifiers
-
the establishment and maintenance of milk banking will not be included in the review (include studies of availability of a milk bank/donor milk, see list over).
-
4. Outcomes
| Are breastfeeding/breastmilk-related outcomes reported, e.g. breastmilk composition and volume, licking mother’s nipple/ tasting dripped breastmilk, number of sucks, initiation of breastfeeding, any breastfeeding, exclusive breastfeeding, and rates of breastfeeding at discharge and beyond? | No | Yes | ? |
| go to 5 | go to 5 | go to 5 |
5. Decisions
If any 1–4 No – enter no (exclude)
If all 1–4 Yes – enter yes (paper to be ordered for data extraction)
If any 1–4 ? – enter ? (reviewer 1 and 2 discuss, involve reviewer 3 if no agreement)
Possible background papers – enter bg
6. Reference
list checked yes/no
Citations to follow up:
| Interventions (from Table 1 of final version of protocol) |
|---|
| Interventions to deliver breastmilk to babies: |
| Methods of feeding (tube, cup, spoon, supplementer, bottle, nipple shields) |
| Interventions that may affect breastfeeding behaviour: |
|
Pacifiers (or non-nutritive sucking): with and without use of breastmilk to taste Timing feeds according to cues/baby’s state |
| Interventions to support adequate nutritional intake (e.g. fat, protein) from breastmilk: |
|
Creamatocrits Hindmilk vs foremilk Morning expression vs later expression |
| Interventions involving physical contact: |
|
Skin-to-skin contact (mother and father) Kangaroo mother contact |
| Interventions involving access to and caring for the baby: |
|
Enabling mother to stay with and/or care for the baby (including rooming-in or 24-hour visitation) Involving family in aspects of baby care including feeding by tube/cup, etc. |
| Interventions involving breastfeeding education and/or support: |
|
Breastfeeding education to parents and families Breastfeeding support by the fathers/families Support from peers and/or professionals (antenatally and postnatally) |
| Interventions involving other aspects of organisation of care: |
|
Facilities for expression and storage Availability of milk bank/donor milk (not running of a milk bank) Feeding policies Policies for handling and testing breastmilk Early discharge Staffing levels and organisation |
| Interventions that affect breastmilk expression: |
|
Methods of breastmilk expression Teaching and support of breast expression Galactagogues |
| Interventions involving staff training: |
|
Staff training in breastfeeding support and in prescribing of drugs for breastfeeding women Staff training in baby weight gain |
Appendix 4 Data extraction tables for included studies
Appendix 4.1: Effectiveness review
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Blaymore Bier 1996 USA (Rhode Island) Research aim To evaluate the effect of maternal–infant skin-to-skin contact (SSC) vs standard contact (SC) on very low birthweight infants’ physiological profile, maternal milk production and duration of breastfeeding Study design Randomised controlled trial Method of group allocation Randomised by blindly picking a precoded label from a bin. Siblings were placed in same group – not clear if randomly allocated Unit of allocation Mother-infant dyads Unit of analysis Mother-infant dyads or infant depending on outcome Sample size calculation Not calculated Outcome measures Maternal milk production Breastfeeding at discharge and 1 & 6 month(s) after discharge Rate of infant weight gain Infant oxygen saturation, respiratory rate, heart rate and temperature maintenance Length of hospital stay Length of days in incubator |
Selection Fifty consecutive mother-infant dyads who met inclusion criteria from 3643 infants admitted to special care nursery during period 22 September 1993 to 14 November 1995 Inclusion criteria Mothers Expressing breastmilk Planning to breastfeed Infants Birthweight less than 1500 g Exclusion criteria Mothers History of drug use, mental illness, HIV infection or receiving any medications contraindicative to breastfeeding Infants Positive screen for cocaine or other illicit drugs or showing drug withdrawal symptoms at birth |
Mother-infant dyads SSC: n = 21 SC: n = 20 Infants SSC: n = 25 SC: n = 25 Mothers Mean [SD] (range) age (years) SSC: 29 [5] (20–37) SC: 30 [6] (17–38) Mean [SD] (range) socioeconomic status (Hollingshead score) SSC: 45 [14] (19–66) SC: 47 [15] (27–66) Previous live births SSC: 10 (48%) SC: 7 (35%) Previous experience in breastfeeding SSC: 8 (38%) SC: 6 (30%) Infants Mean weeks [SD] (range) gestational age SSC: 28 [2] (24–33) SC: 27 [2] (24–33) Mean birthweight [SD] (range) (g) SSC: 993 [275] (520–1470) SC: 942 [322] (350–1475) Mean days intubated [SD] (range) SSC 7 [10] (0–36) SC 8 [10] (0–42) Mean days oxygen requirement [SD] (range) SSC: 27 [29] (0–93) SC: 34 [28] (0–91) Number (%) with necrotising enterocolitis before the intervention SSC: 6 (24) SC: 2(8) Number (%) with sepsis before the intervention SSC: 3 (12) SC: 0 (0) No episodes of NEC or sepsis after sessions began Group comparability No significant differences between the groups were found in mother or infant characteristics |
I: n = 21 Skin-to-skin care (SSC) Infant held upright between the mother’s breasts. Infant clothed only in a diaper and hat and mother and infant covered with a blanket. SSC took place for 10 minutes per weekday for a maximum of 10 days C: n = 20 Standard contact (SC) Infant held cradled in mother’s arms. Infant fully clothed and wrapped in a blanket. SC was observed for 10 minutes per weekday for a maximum of 10 days. Both groups Study protocol began when enrolled infants considered medically stable to be held, i.e. not ventilator dependent, no chest tubes and not requiring continuous positive airway pressure (CPAP). During the study, mother-infant dyads were observed as detailed above until bottle and breastfeedings were initiated (i.e. all infants were gavage fed during the study) Data collection Maternal and neonatal characteristics were prospectively recorded. Mothers recorded the amount of milk they expressed daily. Duration of breastfeeding was monitored by face-to-face follow-up during infants’ stay in special care nursery and by telephone follow-up at 1, 3 and 6 months after discharge. Clinical outcomes were recorded each minute for 10 minutes of the 10 holding sessions and reported graphically in the paper |
Statistical techniques Unpaired t-test for baseline characteristics, analysis of variance for physiological data and chi-squared analysis for duration of breastfeeding data at discreet points in time Breastfeeding/breastmilk-related outcomes Milk production No difference in mean daily maternal milk expression by mothers of singleton infants during the 10-day period was noted between the groups Duration of breastfeeding for singleton mother-infant dyads Breastfeeding at:SSC (n = 21)SC (n = 18)Discharge p < 0.051911One month p < 0.011026 months20%10% (n not reported for either group) Duration of breastfeeding for multiple mother-infant dyads Breastfeeding at:SSC (n = 3)SC (n = 4)Discharge321 month106 months00 Clinical/health outcomes Infant weight gain, mean [SD], g/d SSC (n = 25)SC (n = 25)26 [6]25 [5] Mean oxygen saturation was reported as higher during SSC than SC (p < 0.001) Episodes of oxygen desaturation to < 90% occurred in 191/1716 (11%) recordings during SSC compared with 319/1334 (24%) recordings during SC (p < 0.001) Respiratory rate, heart rate and temperature were similar in both groups Process outcomes SSC (n = 25)SC (n = 25)Mean chronological age when study observations began29 days30 daysMean gestational age when observations began32 weeks31 weeksMean [SD] days hospitalised69 [25]73 [22]Mean [SD] days in incubator55 [24]59 [23] No statistically significant differences found Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
Breastfeeding at: | SSC (n = 21) | SC (n = 18) | Discharge p < 0.05 | 19 | 11 | One month p < 0.01 | 10 | 2 | 6 months | 20% | 10% | Breastfeeding at: | SSC (n = 3) | SC (n = 4) | Discharge | 3 | 2 | 1 month | 1 | 0 | 6 months | 0 | 0 | SSC (n = 25) | SC (n = 25) | 26 [6] | 25 [5] | SSC (n = 25) | SC (n = 25) | Mean chronological age when study observations began | 29 days | 30 days | Mean gestational age when observations began | 32 weeks | 31 weeks | Mean [SD] days hospitalised | 69 [25] | 73 [22] | Mean [SD] days in incubator | 55 [24] | 59 [23] |
41 mother-infant dyads were randomised (SSC 21, SC 20), comprising a total of 50 infants (25 per group) Total of four losses, all from SC group. Two mothers wanted to move to SSC so were excluded (including one mother of twins) and two mothers didn’t want to hold their infants each day. These mothers did participate in follow-up resulting in data for 18/20 SC group Numbers remaining in the study after 1 month not reported |
Data were not analysed using intention-to-treat model | ||||
| Breastfeeding at: | SSC (n = 21) | SC (n = 18) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discharge p < 0.05 | 19 | 11 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| One month p < 0.01 | 10 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 20% | 10% | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Breastfeeding at: | SSC (n = 3) | SC (n = 4) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discharge | 3 | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 month | 1 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 months | 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| SSC (n = 25) | SC (n = 25) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 26 [6] | 25 [5] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| SSC (n = 25) | SC (n = 25) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean chronological age when study observations began | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29 days | 30 days | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean gestational age when observations began | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 weeks | 31 weeks | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean [SD] days hospitalised | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 69 [25] | 73 [22] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean [SD] days in incubator | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 55 [24] | 59 [23] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Boo 2007 Malaysia (Kuala Lumpar) Research aim To compare the weight gain, head growth and breastfeeding rates in very low birthweight infants with or without exposure to short duration of skin-to-skin contact (STSC) while in a NICU Study design Randomised controlled trial Method of group allocation Stratified by multiplicity of pregnancy and birthweight prior to randomisation using serially numbered sealed envelopes prepared in blocks of eight Unit of allocation Infant Unit of analysis Infant Sample size calculation Minimal sample of 47 infants per group to detect a between-group difference of breastfeeding rate of 25% with 95% level of confidence and a power of 80%. Minimal sample of 13 infants per group to detect a difference of 5 g/day of weight gain Outcome measures Weight gain Head circumference Breastfeeding at discharge |
Selection Eligible infants admitted to NICU of a major teaching hospital (Hospital Universiti Kebangsaan Malyasia) from 1 January 2002 to 30 October 2004 Inclusion criteria Infants Birthweight less than 1501 g Stable and adapted to extrauterine life including: Exclusion criteria Infants Lethal or major malformations Severe perinatal asphyxia with evidence of hypoxic ischaemic encephalopathy Transfer to another hospital Parents (STSC group) Refusal to participate in study or STSC |
STSC; SC; p (n = 64); (n = 62) Mothers Mean [SD] age (years) 30.2 [2.9]; 31.1 [5.2]; NS Mean years education (%) 13.0 [2.9]; 12.1 [1.7]; 0.04 Household income/month (RM) (%) < 2501 22 (34); 19 (31); NS Parity > 2 (%) 5 (8); 7 (11); NS Malay ethnic group (%) 40 (63); 34 (55); NS Infants at enrolment Mean postmenstrual age [SD] in days 246 [15]; 240 [15]; 0.02 Mean bodyweight [SD] (g) 1515 [120]; 1492 [128]; NS Mean head circumference [SD]/cm 29.3[1.4]; 29.1[2.1]; NS Multiples (%) Twins: 12 (19); 8(13); NS Triplets: 3 (5); 6 (10); NS On expressed breastmilk (EBM) or any breastmilk (%) 20 (31); 13 (21); NS Group comparability Significant differences between the groups were found in maternal education and infants’ postmenstrual age |
I: n = 64 Skin-to-skin contact (STSC) plus standard care below STSC took place in infant’s cubicle with drawn curtain for at least 1 hour daily. Parents sat on sofa and wore front opening clothing. Mother removed bra, infants wore nappy and bonnet. Infant held upright between the breasts, covered with thermal blanket. If infant showed signs of searching for food, mother offered her breast. Infant’s vital signs monitored throughout. STSC terminated if severe adverse event occurred and resumed when stabilised. Parents were trained in STSC by a researcher using written instructions and photographs C: n = 62 Standard care (SC) Each infant was nursed in own cubicle with curtain at open end. On admission, mother was taught techniques for hand washing, handling their infants and expressing breastmilk Both groups Infants’ well-being reported weekly to parents by researcher. Mother counselled regularly on importance of providing EBM. Infants ≥ 1750 g with good sucking reflex started on oral feeds Mothers encouraged to breastfeed 2–2½ hourly Data collection Infants weighed naked using a calibrated, digital weighing scale by nurse in-charge each morning before first feed. Head circumferences measured weekly with disposable paper tapes. Nurses blind to aims or design of study. Clinical problems diagnosed using laboratory tests |
Statistical techniques Unpaired Student’s t test, Mann–Whitney U test, chi-squared or Fisher’s exact test and linear regression Breastfeeding/breastmilk-related outcomes STSC (n = 64)SC (n = 62)pOn EBM or any breastmilk between enrolment and discharge:21 (33%)6 (10%)0.0002Breastfeeding on discharge:19 (30%)9 (15%)0.004Clinical/health outcomesSTSC (n = 64)SC (n = 62)pDeveloped sepsis2 (3%)1 (1.6%)1.0Developed NEC00Mean [SD] increase in head circumference (cm/week):1.0 [0.3]0.7 [0.3]< 0.0001Mean [SD] head circumference at discharge (cm):31.5 [1.4]31.5 [1.6]0.9Mean [SD] weight gain (g/day):28.3 [11.3]27.5 [9.0]0.6Weight at discharge (g) median (IQR):1878 (160)1993 (452)0.001Process outcomesSTSC (n = 64)SC (n = 62)pReceived human milk fortifier (between enrolment and discharge):5 (8%)3 (5%)0.7Mean days postmenstrual age at discharge [SD]:253 [21]263 [16]1.0Median (IQR) days duration of hospital stay postrecruitment:13.5 [11.5]22.5 [14.0]< 0.0001 STSC infants received from a parent: Mean [SD] days 10 [5.6] Median hours/day (IQR) 1 (0) Mean [SD] total hours 11.3 [5.9] Psychosocial outcomes Eight STSC infants received STSC on < 50% of postrecruitment hospital stay. Mothers’ reasons: Cost-effectiveness outcomes Not reported |
STSC (n = 64) | SC (n = 62) | p | On EBM or any breastmilk between enrolment and discharge: | 21 (33%) | 6 (10%) | 0.0002 | Breastfeeding on discharge: | 19 (30%) | 9 (15%) | 0.004 | Clinical/health outcomes | STSC (n = 64) | SC (n = 62) | p | Developed sepsis | 2 (3%) | 1 (1.6%) | 1.0 | Developed NEC | 0 | 0 | Mean [SD] increase in head circumference (cm/week): | 1.0 [0.3] | 0.7 [0.3] | < 0.0001 | Mean [SD] head circumference at discharge (cm): | 31.5 [1.4] | 31.5 [1.6] | 0.9 | Mean [SD] weight gain (g/day): | 28.3 [11.3] | 27.5 [9.0] | 0.6 | Weight at discharge (g) median (IQR): | 1878 (160) | 1993 (452) | 0.001 | Process outcomes | STSC (n = 64) | SC (n = 62) | p | Received human milk fortifier (between enrolment and discharge): | 5 (8%) | 3 (5%) | 0.7 | Mean days postmenstrual age at discharge [SD]: | 253 [21] | 263 [16] | 1.0 | Median (IQR) days duration of hospital stay postrecruitment: | 13.5 [11.5] | 22.5 [14.0] | < 0.0001 |
128 of 225 eligible infants randomised (STSC n = 65) (SC n = 63) One infant per group died between randomisation and before start of study Data from remaining 126 infants reported |
Data were analysed using intention-to-treat model Census of study unit (2000) showed 30% breastfeeding rate among VLBW infants Authors note although infants in SC group weighed significantly more than STSC infants, they also stayed significantly longer in hospital after recruitment (p. 835) Logistic regression found only significant predictors of successful breastfeeding at discharge were infants receiving EBM at enrolment (adjusted OR: 4.1; 95% CI: 1.4–11.7; p = 0.009) or receiving EBM during intervention period (adjusted OR: 8.3; 95% CI 2.8–24.4; p < 0.0001) Authors acknowledge study limitations including differences in some characteristics at baseline, failure to obtain consent from controls and lack of blinding |
|||||||||||||||||||||||||||
| STSC (n = 64) | SC (n = 62) | p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| On EBM or any breastmilk between enrolment and discharge: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21 (33%) | 6 (10%) | 0.0002 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Breastfeeding on discharge: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 (30%) | 9 (15%) | 0.004 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical/health outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STSC (n = 64) | SC (n = 62) | p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Developed sepsis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 (3%) | 1 (1.6%) | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Developed NEC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean [SD] increase in head circumference (cm/week): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.0 [0.3] | 0.7 [0.3] | < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean [SD] head circumference at discharge (cm): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31.5 [1.4] | 31.5 [1.6] | 0.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean [SD] weight gain (g/day): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 28.3 [11.3] | 27.5 [9.0] | 0.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight at discharge (g) median (IQR): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1878 (160) | 1993 (452) | 0.001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Process outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STSC (n = 64) | SC (n = 62) | p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Received human milk fortifier (between enrolment and discharge): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 (8%) | 3 (5%) | 0.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean days postmenstrual age at discharge [SD]: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 253 [21] | 263 [16] | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median (IQR) days duration of hospital stay postrecruitment: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.5 [11.5] | 22.5 [14.0] | < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cattaneo 1998 Ethiopia (Addis Ababa) Indonesia (Yogyakarta) Mexico (Merida) Research aim To explore the effectiveness, safety, feasibility, acceptability and costs of kangaroo mother care (KMC) in low-income countries Study design Multi-centre randomised controlled trial Method of group allocation List of random numbers. In Yogyakarta, carried out in blocks of six and stratified by weight Unit of allocation Infant Unit of analysis Infant Sample size calculation Not calculated. Aimed to randomise approximately 100 infants of low birthweight per site Outcome measures Exclusive breastfeeding at discharge Predefined serious illness Hypo- and hyperthermia Weight gain Mothers’ views Adequacy and availability of structures/staff Costs |
Selection Eligible infants admitted to one major hospital in each of the three sites over an approximate 12-month period between August 1995 and September 1996 Inclusion criteria Infants Birthweight 1000–1999 g Any gestational age Not on oxygen or i.v. fluids Some ability to feed No visible major malformation Mother present and willing to collaborate Exclusion criteria Infants Multiple births not excluded unless twins randomly assigned to different groups In practice, eligible twins were enrolled only if the sibling died |
KMC; C (n = 149); (n = 136) Addis Ababa (n = 50); (n = 50); p GA; < 32 weeks; (%) 6 (32); 6 (12); 0.02 Yogyakarta (n = 52); (n = 54) Exclusive breastfeeding at enrolment (%) 45 (87); 33 (61); 0.003 Merida Primiparas (%) 27 (57); 9 (28%); 0.01 Overall Exclusive breastfeeding at enrolment (%) 100 (67%); 75 (55); 0.04 No other significant differences between KMC and C Overall KMC; C (n = 149); (n = 136) Born in same hospital 102 (68%); 85 (63) Caesarean section 45 (30%); 34 (25%) Primiparas 85 (57%); 67 (49%) Mean birthweight (g) [SD] 1622 [239]; 1638 [247] Mean gestational age (weeks) [SD] 33.7 [2.5]; 34/9 [2.2] Gestational age < 32 weeks 24 (16%); 14(10%) Exclusive breastfeeding at admission 63 (48%); 49 (40%) Median days old at enrolment (range) 10 (1–30); 8 (1–40) Mean grams weight at enrolment [SD] 1584 [223]; 1574 [251] Group comparability No differences between the groups in maternal age, reproductive history, education or social/economic conditions |
I: n = 146 Kangaroo mother care (KMC) defined as early, prolonged and continuous skin-to-skin contact between a mother and her infant, both in hospital and after discharge, until at least the 40th week of postnatal gestational age Infant stayed in 4-bed rooms with their mothers, skin-to-skin between breasts, wearing nappy and hat, and backs covered with mother’s clothes, all day and night, for an average of 20 hours/day, including when mother asleep. Occasionally replaced for a few hours by another person, usually father. For absences < 1 hour, baby left on mother’s bed, covered with a blanket. Mothers encouraged to continue KMC after discharge C: n = 133 Control, standard care Addis Ababa Infants in open cribs in a warm room (possibility of rewarming in a bulb-heated cot). All mothers stayed in hospital, in separate rooms with access for breastfeeding Yogyakarta Infants Infants in incubators. All mothers stayed in hospital, in separate rooms with access for breastfeeding Merida Infants in incubators. Mothers not allowed to stay or to visit at night, could visit any time during the day Data collection Six tools: a form to record data for each admission, a form for follow-up, two semistructured questionnaires for interviews with mothers and staff, a monthly record of observed constraints, and a monthly form for costs. Tools were tested, standardised and translated into local languages. The principal investigator in each site was in charge of monitoring the quality of data collection. Qualitative data from the two questionnaires was coded before data entering. Staff interviews were conducted during the second half of the study among 45% of the professional staff engaged in the study, proportionally distributed across the sites including 15 doctors, 31 nurses and 3 others |
Statistical techniques All data analysed in Trieste using ANOVA, chi-squared, t and Kruskal–Wallis tests, stratifying by site, gender, birthweight and socioeconomic variables Breastfeeding/breastmilk-related outcomesa Exclusive breastfeeding at discharge (%):KMCCAddis Ababa40/48 (83)40/47 (85)Yogyakarta51/52 (98)48/54 (89)Merida37/46 (80)5/32 (15)Merida, p = 0.00001; not significant for Addis Ababa or YogyakartaOverall128/146 (88)93/133 (70)p = 0.0003 (see Additional comments)Clinical/health outcomesEpisodes of severe disease (/1000 infants/day)KMCCAddis Ababa6 (12.0)6 (9.4)Yogyakarta7 (8.1)18 (15.8)Merida1 (1.6)1 (2.2)Overall14 (7.1)25 (11.2)Episodes of hypothermiab (/100 infants/day)KMCCAddis Ababa119 (23.7)158 (24.8)Yogyakarta12 (1.4)26 (2.3)Merida82 (13.5)141 (31.5)Merida, p = 0.00001; among sites, p = 0.000001Overall213 (10.8)325 (14.6)p = 0.0005 (see Additional comments)Episodes of hyperthermiac (/100 infants/day)KMCCAddis Ababa0 (0)1 (1.6)Yogyakarta1 (1.2)10 (8.8)Merida0 (0)1 (2.2)Yogyakarta, p = 0.02Overall1 (0.5)12 (5.4)p = 0.004 (see Additional comments)Mean daily weight gain (g) [SD]KMCCAddis Ababa17.9 (13.4)13.4 (10.6)Yogyakarta25.6 (11.7)21.4 (11.9)Merida20.1 (8.7)18.0 (13.9)Among sites, p = 0.00003Overall21 (11.8)17.7 (12.4)p = 0.001 (see Additional comments)Mean weight gain during study (g) [SD]KMCCAddis Ababa166 (137)154 (121)Yogyakarta367 (173)395 (262)Merida259 (200)286 (218)Among sites, p = 0.000001Overall267 (190)284 (234)Mean weight gain at discharge (g) [SD]KMCCAddis Ababa1645 (204)1615 (223)Yogyakarta2039 (140)2049 (158)Merida1852 (52)1886 (83)Merida, p = 0.04; among sites, p = 0.000001Overall1848 (220)1851 (257)Process outcomesMedian length of stay (days) after enrolment (range)KMCCAddis Ababa9 (3–31)11 (2–39)Yogyakarta13 (2–85)18 (3–60)Merida10 (2–41)12 (2–39)Among sites, p = 0.000001Overall11 (2–85)13 (2–60)p = 0.003 (see Additional comments)Mean age (days) at discharge (range)KMCCAddis Ababa17 (6–78)20 (7–65)Yogyakarta21.5 (8–97)25 (8–74)Merida27.5 (9–56)24 (3–51)Among sites, p = 0.003Overall21 (6–97)23 (3–74)Psychosocial outcomesMothers happy with assignment (%)KMCCAddis Ababa44/50 (88)23/50 (46)Yogyakarta48/52 (92)54/54 (100)Merida38/41 (93)21/22 (96)Yogyakarta, p = 0.000008; among sites, p = 0.002Overall130/143 (91)98/126 (78)p = 0.003Mothers would prefer the other assignment (%)KMCCAddis Ababa9/50 (18)34/50 (68)Yogyakarta1/52 (2)1/54 (2)Merida1/41 (2)15/22 (68)Merida, p = 0.0000001; among sites, p = 0.0000001Overall11/143 (8)50/126 (40)p = 0.0000001 (see Additional comments) Staff views 92% considered KMC as safe 90% found mothers were comfortable with KMC 69% found mothers were comfortable with standard care (control) When asked whether they would prefer KMC or CMC if they had a low birthweight infant, 100% chose KMC in Addis Ababa and Merida compared with 41% in Yogyakarta where 6% chose standard care and 47% were uncertain Cost-effectiveness outcomes Running costs (US$) KMCCSalaries11,78829,888Other items75019876 Average monthly salaries (US$) Doctors 250 Nurses 96 Other staff 55 Electricity and maintenance costs much higher in standard care. Food for mothers and laundry were more expensive for KMC than C group |
Exclusive breastfeeding at discharge (%): | KMC | C | Addis Ababa | 40/48 (83) | 40/47 (85) | Yogyakarta | 51/52 (98) | 48/54 (89) | Merida | 37/46 (80) | 5/32 (15) | Merida, p = 0.00001; not significant for Addis Ababa or Yogyakarta | Overall | 128/146 (88) | 93/133 (70) | p = 0.0003 (see Additional comments) | Clinical/health outcomes | Episodes of severe disease (/1000 infants/day) | KMC | C | Addis Ababa | 6 (12.0) | 6 (9.4) | Yogyakarta | 7 (8.1) | 18 (15.8) | Merida | 1 (1.6) | 1 (2.2) | Overall | 14 (7.1) | 25 (11.2) | Episodes of hypothermiab (/100 infants/day) | KMC | C | Addis Ababa | 119 (23.7) | 158 (24.8) | Yogyakarta | 12 (1.4) | 26 (2.3) | Merida | 82 (13.5) | 141 (31.5) | Merida, p = 0.00001; among sites, p = 0.000001 | Overall | 213 (10.8) | 325 (14.6) | p = 0.0005 (see Additional comments) | Episodes of hyperthermiac (/100 infants/day) | KMC | C | Addis Ababa | 0 (0) | 1 (1.6) | Yogyakarta | 1 (1.2) | 10 (8.8) | Merida | 0 (0) | 1 (2.2) | Yogyakarta, p = 0.02 | Overall | 1 (0.5) | 12 (5.4) | p = 0.004 (see Additional comments) | Mean daily weight gain (g) [SD] | KMC | C | Addis Ababa | 17.9 (13.4) | 13.4 (10.6) | Yogyakarta | 25.6 (11.7) | 21.4 (11.9) | Merida | 20.1 (8.7) | 18.0 (13.9) | Among sites, p = 0.00003 | Overall | 21 (11.8) | 17.7 (12.4) | p = 0.001 (see Additional comments) | Mean weight gain during study (g) [SD] | KMC | C | Addis Ababa | 166 (137) | 154 (121) | Yogyakarta | 367 (173) | 395 (262) | Merida | 259 (200) | 286 (218) | Among sites, p = 0.000001 | Overall | 267 (190) | 284 (234) | Mean weight gain at discharge (g) [SD] | KMC | C | Addis Ababa | 1645 (204) | 1615 (223) | Yogyakarta | 2039 (140) | 2049 (158) | Merida | 1852 (52) | 1886 (83) | Merida, p = 0.04; among sites, p = 0.000001 | Overall | 1848 (220) | 1851 (257) | Process outcomes | Median length of stay (days) after enrolment (range) | KMC | C | Addis Ababa | 9 (3–31) | 11 (2–39) | Yogyakarta | 13 (2–85) | 18 (3–60) | Merida | 10 (2–41) | 12 (2–39) | Among sites, p = 0.000001 | Overall | 11 (2–85) | 13 (2–60) | p = 0.003 (see Additional comments) | Mean age (days) at discharge (range) | KMC | C | Addis Ababa | 17 (6–78) | 20 (7–65) | Yogyakarta | 21.5 (8–97) | 25 (8–74) | Merida | 27.5 (9–56) | 24 (3–51) | Among sites, p = 0.003 | Overall | 21 (6–97) | 23 (3–74) | Psychosocial outcomes | Mothers happy with assignment (%) | KMC | C | Addis Ababa | 44/50 (88) | 23/50 (46) | Yogyakarta | 48/52 (92) | 54/54 (100) | Merida | 38/41 (93) | 21/22 (96) | Yogyakarta, p = 0.000008; among sites, p = 0.002 | Overall | 130/143 (91) | 98/126 (78) | p = 0.003 | Mothers would prefer the other assignment (%) | KMC | C | Addis Ababa | 9/50 (18) | 34/50 (68) | Yogyakarta | 1/52 (2) | 1/54 (2) | Merida | 1/41 (2) | 15/22 (68) | Merida, p = 0.0000001; among sites, p = 0.0000001 | Overall | 11/143 (8) | 50/126 (40) | p = 0.0000001 (see Additional comments) | KMC | C | Salaries | 11,788 | 29,888 | Other items | 7501 | 9876 |
285 of 463 eligible infants randomised (KMC n = 149; C n = 136) Six infants died after enrolment (three from each group) KMC 129/146 (88%) attended first visit 93/146 (64%) attended fourth visit C 112/133 (84%) attended first visit 82/133 (62%) attended fourth visit Authors state relative loss to follow-up was the same by group and by site |
Data were not analysed using intention-to-treat model Authors note the many differences between the sites make overall comparison between the KMC and C groups problematic. Authors warn their results should not be interpreted without continuous reference to the specific findings of each site. For example: exclusive breastfeeding significantly higher in Merida where exclusive breastfeeding at enrolment was also significantly higher among KMC group compared to C group |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusive breastfeeding at discharge (%): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 40/48 (83) | 40/47 (85) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 51/52 (98) | 48/54 (89) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 37/46 (80) | 5/32 (15) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida, p = 0.00001; not significant for Addis Ababa or Yogyakarta | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 128/146 (88) | 93/133 (70) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| p = 0.0003 (see Additional comments) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinical/health outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Episodes of severe disease (/1000 infants/day) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 6 (12.0) | 6 (9.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 7 (8.1) | 18 (15.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 1 (1.6) | 1 (2.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 14 (7.1) | 25 (11.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Episodes of hypothermiab (/100 infants/day) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 119 (23.7) | 158 (24.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 12 (1.4) | 26 (2.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 82 (13.5) | 141 (31.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida, p = 0.00001; among sites, p = 0.000001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 213 (10.8) | 325 (14.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| p = 0.0005 (see Additional comments) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Episodes of hyperthermiac (/100 infants/day) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 0 (0) | 1 (1.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 1 (1.2) | 10 (8.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 0 (0) | 1 (2.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta, p = 0.02 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 1 (0.5) | 12 (5.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| p = 0.004 (see Additional comments) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean daily weight gain (g) [SD] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 17.9 (13.4) | 13.4 (10.6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 25.6 (11.7) | 21.4 (11.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 20.1 (8.7) | 18.0 (13.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Among sites, p = 0.00003 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 21 (11.8) | 17.7 (12.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| p = 0.001 (see Additional comments) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean weight gain during study (g) [SD] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 166 (137) | 154 (121) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 367 (173) | 395 (262) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 259 (200) | 286 (218) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Among sites, p = 0.000001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 267 (190) | 284 (234) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean weight gain at discharge (g) [SD] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 1645 (204) | 1615 (223) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 2039 (140) | 2049 (158) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 1852 (52) | 1886 (83) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida, p = 0.04; among sites, p = 0.000001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 1848 (220) | 1851 (257) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Process outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median length of stay (days) after enrolment (range) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 9 (3–31) | 11 (2–39) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 13 (2–85) | 18 (3–60) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 10 (2–41) | 12 (2–39) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Among sites, p = 0.000001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 11 (2–85) | 13 (2–60) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| p = 0.003 (see Additional comments) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean age (days) at discharge (range) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 17 (6–78) | 20 (7–65) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 21.5 (8–97) | 25 (8–74) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 27.5 (9–56) | 24 (3–51) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Among sites, p = 0.003 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 21 (6–97) | 23 (3–74) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychosocial outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mothers happy with assignment (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 44/50 (88) | 23/50 (46) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 48/52 (92) | 54/54 (100) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 38/41 (93) | 21/22 (96) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta, p = 0.000008; among sites, p = 0.002 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 130/143 (91) | 98/126 (78) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| p = 0.003 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mothers would prefer the other assignment (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Addis Ababa | 9/50 (18) | 34/50 (68) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Yogyakarta | 1/52 (2) | 1/54 (2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida | 1/41 (2) | 15/22 (68) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Merida, p = 0.0000001; among sites, p = 0.0000001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overall | 11/143 (8) | 50/126 (40) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| p = 0.0000001 (see Additional comments) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Salaries | 11,788 | 29,888 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other items | 7501 | 9876 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Charpak 1997 Colombia (Bogotá) Follow-up after term to one year of corrected age is reported in Charpak 2001 Research aim To evaluate the effect of the three components of Rey-Martinez KMC (position, feeding policy and discharge policy) on a range of outcomes for LBW infants in the first year of life Study design Randomised controlled trial Method of group allocation Random numbers list (permutations of 16) Stratified block randomisation by weight Unit of allocation Product of each delivery (twins and triplets allocated to same group) Unit of analysis Infant Sample size calculation Based on previous work showing a death rate among eligible infants under traditional care of ~7%, to detect a twofold increase in the risk of dying (α = 0.05, two-tailed test, power 80%) 215 per group Outcome measures Primary: mortality, growth Secondary: length of stay, infections, any and exclusive breastfeeding |
Selection Infants with birthweight/weight at transfer ≤ 2000 g being cared for at Clínica San Pedro Claver, a tertiary care obstetric facility with 13,500–15,000 live births per year Inclusion criteria Infants eligible for KMC, i.e.: Also: Mother or relative in same household willing to care for a premature infant and comply with 1-year follow-up schedule Twins and triplets were included Exclusion criteria Referred to another institution Plans to leave Bogotá Life-threatening or major malformations Early-detected major conditions arising from perinatal problems (e.g. severe hypoxic-ischaemic encephalopathy, pulmonary hypertension) Parental/family refusal to comply with the follow-up programme For KMC group, refusal to comply with the specifics of the intervention |
Mothers I (KMC); C (controls) Mean age in years 27.3; 27.4 Stable couples 83%; 86% Education: Employment: Median per capita monthly income (Colombian pesos, 1994) Col$70,000; Col$70,000 Distribution of sociodemographic variables noted to reflect the composition and characteristics of the population served by the Social Security in Bogotá Mothers’ usual weight (kg; mean ± SD) 54 ± 6.9; 53.7 ± 7.8 Mothers’ height (cm; mean ± SD) 158 ± 7; 157 ± 6.5 Primiparas 145 (38%); 138 (38%) Multiple births 79 (21%); 57(16%) Caesarean births 264 (69%); 246 (67%) Postpartum hospital stay, median days minimum–maximum 3 (0–11); 3 (0–11) The high rate of pathological conditions such as pre-eclampsia (38%) during pregnancy and the high rate of caesarean births in both groups reflect the fact that the study population was the high-risk segment of deliveries at the study hospital Infants I (n = 382); C (n = 364) Weight at birth g (mean ± SD) 1750 ± 261; 1735 ± 256 Distribution of weight at birth, number of infants (%) ≤ 1200 g: 26 (6.8); 22(6) 1201–1500 g: 56 (14.7); 58 (16) 1501–-1800 g: 147 (38.5); 124 (34) 1801–12,000 g: 153 (40); 60 (44) Gestational age (weeks; mean ± SD) 33.6 ± 2.5; 33.9 ± 2.7 Distribution of gestational age at birth (weeks; number, % of infants) ≤ 32: 137 (36); 109 (30) 33–34: 112 (29); 97 (26) 35–36: 83 (22); 106 (29) ≥ 37: 50 (13); 52 (14) Infants never admitted to neonatal unit 132 (35%); 155 (42.5%) Infants never admitted to neonatal intensive care unit (NICU) 321 (84%); 311 (85.4%) Days on NICU for those admitted; median (range) 8 (2–44); 6 (1–34) Infants never in ventilator 330 (87%); 332 (91%) Days ventilation for those ever ventilated; median (range) 4 (1–19); 4.5 (1–11) Group comparability The only differences noted between the groups was in the number of multiple births (22 more in the KMC group, significance not reported) and number of infants never admitted to neonatal unit (greater in the control group (I: 35% vs C: 42.5%, p < 0.05) |
Prior to randomisation, some infants ≤ 2000 g were admitted to NICU and some were placed in a crib in the mother’s hospital room All were assessed for eligibility for the trial (on NICU/before mother and infant discharge, respectively) Randomisation took place once the attending physician decided the infant was eligible I: n = 382 KMC infants were discharged, regardless of weight or gestational age, to another hospital (Clínica del Nino) with their mothers immediately after randomisation, for programme of ambulatory adaptation to KMC Mothers and infants could stay at the programme as long as necessary to demonstrate appropriate adaptation Infants spent 24 hours per day in an upright position, in skin-to-skin contact, and attached to the mother’s chest Infants to remain in kangaroo position until they demonstrate discomfort by pushing out limbs, crying and fussing when mothers try to return them to the upright position Infants to be breastfed regularly Premature formula used to guarantee adequate weight gain if necessary C: n = 364 Control group infants remained in incubator care at the Clínica San Pedro Claver until able to regulate temperature and reached weight 1700 g Parents’ access to infants was severely restricted at the time of the study Post-discharge from their respective hospitals, both groups had access to the same follow-up care Data collection Interviews with mothers Clinical records |
Statistical techniques Chi-squared and Fisher exact tests; t tests or non-parametric tests; ANOVA/ANCOVA and regression Breastfeeding/breastmilk-related outcomes Exclusive breastfeeding at term I: 159/343 (46.4%) C: 145/320 (45.3%) Partial breastfeeding at term I: 177/343 (51.6%) C: 151/320 (47.2%) Only formula at term I: 7/343 (2%) C: 24/320 (7.5%) p < 0.05 Duration of any breastfeeding to 1 year Age (months)KMC (%)Control (%)pTerm98.093.30.001381.775.30.05651.648.2NS936.334.8NS1219.722.2NS More babies in KMC were breastfed to 3 months corrected age Clinical/health outcomes Deaths between eligibility and term I: 6/364 (1.6%) C: 10/345 (2.9%) Not statistically significant RR = 0.59 (95% CI: 0.22–1.6) During follow-up to 12 months: I: 11/350 deaths (3.1%) C: 19/343 deaths (5.5%) Not statistically significant Mean [SD] weight at term (g) I: 2814 [541] vs 2803 [509] Similar to term newborn infants in Bogotá (2600 m above sea level) Growth indices were almost identical in the two groups Head circumference Larger for KMC infants (as proportion of expected circumference at 12 months corrected age) than control group infants (p = 0.06 before adjustment in linear regression analysis and p = 0.014 after) Infectious episodes between eligibility and term I: 49 (14%) vs C: 44 (14%), p = 0.25 Infections not requiring readmission I: 6.7% vs C: 2.8%, p = 0.019 Readmissions for infections I: 7.6% vs C: 11% p = 0.17 Infectious episodes between term and 1 year Overall frequency of infections to 12 months in KMC and CMC groups was similar, with no differences between the groups in cumulative frequency of mild/moderate infections (requiring ambulatory use of antibiotics) or severe infections (requiring hospitalisation) Psychomotor development to 1 year No difference was found between the two groups in the proportions of infants with cerebral palsy, psychomotor delay, or visual or hearing impairment The only factor associated with an increased risk of cerebral palsy was the total number of days spent in NICU – authors note this reflects the severity of the infant’s initial condition Process outcomes CMC infants had more paediatrician visits during their (longer than KMC) hospital stay and KMC infants had more ambulatory clinic visits after their (earlier than CMC) primary discharge Number of readmissions after primary discharge was similar in the two groups Total length of hospital stay from eligibility to 12 months corrected age shorter for KMC infants, particularly for those with birthweight < 1500 g Psychosocial outcomes Not reported Cost-effectiveness outcomes After adjusting for weight at eligibility there was an average saving of 1.1 days in total hospital stay from eligibility to term in the KMC group The saving in hospital stay was related to birthweight Hospital stay from eligibility to term; mean (range) [number of infants] Birthweight ≤ 1200 g I: 8.6 (0–28) [23] C: 14.84 (3–26) [19] Birthweight 1800–2000 g I: 2.86 (0–36) [137] C: 2.77 (0–34) [139] Authors state savings in hospital stay persist up to 12 months of corrected age |
Age (months) | KMC (%) | Control (%) | p | Term | 98.0 | 93.3 | 0.001 | 3 | 81.7 | 75.3 | 0.05 | 6 | 51.6 | 48.2 | NS | 9 | 36.3 | 34.8 | NS | 12 | 19.7 | 22.2 | NS |
777 infants were randomised 31 were withdrawn (I = 14, C = 17) because evidence of severe neurological problems or intrauterine infection emerged No mothers assigned to KMC refused to participate 746 entered the study (I = 382, C = 364) Complete follow-up data for 679 (91%) at term (40–41 weeks post-conception age) |
All subjects were analysed according to allocated group, regardless of compliance with treatment or contamination of the intervention Consent was postrandomisation for the KMC group only, to avoid contamination bias from parents asking for KMC (early discharge) Ethics committee approval for not seeking consent from the control group was given because the control group received usual care Funded jointly by Instituto de Seguros Sociales de Colombia and the World Laboratory (ONG, Lausanne, Switzerland, Project Number MCD13) |
| Age (months) | KMC (%) | Control (%) | p | |||||||||||||||||||||||||||
| Term | 98.0 | 93.3 | 0.001 | |||||||||||||||||||||||||||
| 3 | 81.7 | 75.3 | 0.05 | |||||||||||||||||||||||||||
| 6 | 51.6 | 48.2 | NS | |||||||||||||||||||||||||||
| 9 | 36.3 | 34.8 | NS | |||||||||||||||||||||||||||
| 12 | 19.7 | 22.2 | NS |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Kadam 2005 India (Mumbai) Research aim What are the effects of kangaroo mother care (KMC) compared with conventional care (CC) on physiological parameters and is KMC acceptable to parents? Study design Pilot study for a randomised controlled trial Method of group allocation Sealed envelope method. No further details Unit of allocation Infant Unit of analysis Infant Mother for psychosocial outcomes Sample size calculation Not calculated Outcome measures Mean days old when started breastfeeding Deaths Episodes of sepsis, apnoea, hypothermia (36°C) and hyperthermia (38°C) Oxygen saturation Respiratory rates Length of hospital stay Mean weight at discharge Mothers’ views |
Selection Infants who met inclusion criteria in a major tertiary care centre in Mumbai between 1 November 2000 and 31 October 2001 Inclusion criteria Infants Birthweight less than 1800 g Stable cardiopulmonary status in air Apgar score of 7 at 5 minutes On breastfeeds or spoon wati feeds with expressed breastmilk Exclusion criteria Infants Sick and unstable infants Major congenital malformation Refusal of parental consent |
KMC: n = 44 CC: n = 45 Infants Mean gestational age (weeks) [SD] KMC: 33.3 [2.1] CC: 34.0 [1.7] Mean birthweight (g) [SD] KMC: 1467 [228] CC: 1461 [217] Mean length (cm) [SD] KMC: 39.9 [1.4] CC: 40.0 [2.6] Mean head circumference (cm) [SD] KMC: 28.0 [1.4] CC: 28.6 [1.7] Mothers Not reported No significant differences between the groups were found in infant characteristics |
I: n = 44 Kangaroo mother care (KMC) Infants placed between mothers’ breasts in vertical position supported by a cloth ‘dupatta’, with mothers sitting in a semireclining position. KMC for at least 1 hour each episode and continued for as long as comfortable. KMC was discontinued if baby demonstrated discomfort (crying, pushing out legs) or mother uncomfortable. Infants who developed clinical problems were transferred to CMC and after stabilisation, transferred back to KMC C: n = 45 onventional care (CC) Infants managed under radiant warmers Both groups Mothers allowed to enter and handle, change and breastfeed their infants at any hour of the day. Infants discharged following: recorded weight gain for 3+ consecutive days; maintaining temperature without warmer; feeding well; mother confident about care of infant at home Data collection Gestational age assessed by Ballard’s score within 24 hours, weighed immediately after birth, length measured at 24 hours with an infantometer, head circumference measured at 48 hours with a non-stretchable cloth tape, all by same single observer. Infants continuously monitored for oxygen saturation and heart rate by pulse oximeter. Respiratory rates counted hourly, axillary temperature taken hourly for 3 minutes (during KMC period for KMC). Interview of KMC mothers using semistructured questionnaire at end of study to assess views |
Statistical techniques Unpaired, two-tailed t test on means in KMC and CC for respiratory rate, temperature and oxygen saturation Infants: KMC (n = 44)CC (n = 45)p Breastfeeding/breastmilk-related outcomes Mean days old when started breastfeeding [SD] 4.7 [3.3]5.6 [3.9]NS Clinical/health outcomes Deaths1 (sepsis)1 (NEC)NSSepsis68NSApnoea (with sepsis)6 (3)8 (4)NSEpisodes of hypothermia1021< 0.01Episodes of hyperthermia1315NSOxygen saturation95.7 ± 1.194.8 ± 0.7< 0.01Respiratory rates36.2 ± 3.340.7 ± 2.9< 0.01 Process outcomes Mean duration of KMC contact (hours/day) [SD]: 9.8 [3.7] Transfers from KMC to CC: 15/44 (34.1%) (range 1–6 days) Reasons for transfer: Sepsis (2); apnoea (3); jaundice (6) Sepsis and apnoea (3); sepsis and jaundice (1) Mean age of enrolment (days) (range) 3.2 (1.8) (not reported by group) Mean days before discharge [SD] 8.5 [4.4]9.3 [4.5]NS Hospital stay Reported as shorter in KMC but not statistically significant (p = 0.47) Mean weight at discharge (g) [SD] 1494 [211]1462 [205]NS Psychosocial outcomes (KMC mothers) 86% happy with KMC 14% preferred CC Do you feel comfortable when giving KMC? Yes: 79% Reasons for not: pain, stress of labour Will you continue giving KMC at home? Yes: 73% Does your husband agree with KMC? Agree: 64% Cost-effectiveness outcomes Not reported |
KMC (n = 44) | CC (n = 45) | p | 4.7 [3.3] | 5.6 [3.9] | NS | Deaths | 1 (sepsis) | 1 (NEC) | NS | Sepsis | 6 | 8 | NS | Apnoea (with sepsis) | 6 (3) | 8 (4) | NS | Episodes of hypothermia | 10 | 21 | < 0.01 | Episodes of hyperthermia | 13 | 15 | NS | Oxygen saturation | 95.7 ± 1.1 | 94.8 ± 0.7 | < 0.01 | Respiratory rates | 36.2 ± 3.3 | 40.7 ± 2.9 | < 0.01 | 8.5 [4.4] | 9.3 [4.5] | NS | 1494 [211] | 1462 [205] | NS |
A total of 21/110 infants excluded on defined criteria. 89 infants randomised (KMC 44; CC 45) No withdrawals Numbers of mothers or numbers completing interview are not reported |
Data were analysed using intention-to-treat model Background breastfeeding rates not reported All infants in this study received 100% human breastmilk Researchers note their rate of transfer of KMC infants into CC (34.1%) was higher than that reported by Cattaneo 1998 (13.4%). They attribute this to their lower median age of enrolment (3.2 days) [2.8] compared with 10 days [1–74] |
||||||||||||||
| KMC (n = 44) | CC (n = 45) | p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.7 [3.3] | 5.6 [3.9] | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deaths | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 (sepsis) | 1 (NEC) | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sepsis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 8 | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Apnoea (with sepsis) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 (3) | 8 (4) | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Episodes of hypothermia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | 21 | < 0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Episodes of hyperthermia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | 15 | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxygen saturation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 95.7 ± 1.1 | 94.8 ± 0.7 | < 0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Respiratory rates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36.2 ± 3.3 | 40.7 ± 2.9 | < 0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.5 [4.4] | 9.3 [4.5] | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1494 [211] | 1462 [205] | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Roberts 2000 Australia (Darwin, Northern Territory) Research aim To compare kangaroo mother care with conventional cuddling care in premature and small for gestational age (SGA) infants Study design Randomised controlled trial Method of group allocation Randomised by means of envelopes that contained the group assignment, stratified by gender Unit of allocation Mother-infant pairs Unit of analysis Group, individual Sample size calculation Not reported Outcome measures Infant weight gain Temperature maintenance during KMC and CCC Length of hospital stay Breastfeeding duration Maternal stress and confidence |
Selection Infants in the NICU at the Royal Darwin Hospital and the nursery at the Darwin Private Hospital Inclusion criteria Parents Infants Exclusion criteria Mothers Infants |
KMC (n = 16) CCC (n = 14) Mothers Mean [SD] maternal age (years) KMC: 26 [6] CCC: 28 [6] Mean [SD] parity KMC: 1.6 [0.9] CCC:1.5 [0.9] Infants Mean weeks [SD] gestational age KMC: 31.7 [3.1] CCC: 31.2 [2.4] Mean birthweight [SD] (g) KMC: 1562 [465] CCC: 1482 [409] Mean [SD] weight at enrolment (g) KMC: 1687 [418] CCC: 1693 [212] No significant differences between the groups were found in mother or infant characteristics |
I: n = 16 Kangaroo mother care (KMC) Skin-to-skin contact during cuddling Infant dressed only in a diaper (smaller infants also wore a bonnet) Infant near-naked at or between mother’s breasts Covered with a light blanket KMC was done in private, in a comfortable chair that allowed rocking C: n = 14 Conventional cuddling care (CCC) Infants wore normal clothing and were covered with a light blanket during cuddling The only difference between the groups was that infants in the KMC group had skin-to-skin contact with the mother, whereas infants in the CCC group had contact only through clothing Data collection |
Statistical techniques Two-tailed independent t tests; one-tailed t tests, Mann–Whitney U and chi-squared; paired t tests Breastfeeding/breastmilk-related outcomes Duration of breastfeeding Breastfeeding at: KMC (n = 16)CCC (n = 14)Discharge10116 weeks963 months756 months44 Clinical/health outcomes Infant weight gain Both groups gained a mean of 23 [SD 7] g/day in hospital. No significant differences in weight gain were found between the groups at 6 weeks, 3 or 6 months KMC: 6 weeks: 52 ± 24 g/day 3 months: 39 ± 12 g/day 6 months: 30 ± 6 g/day CCC: 6 weeks: 55 ± 15 g/day 3 months: 42 ± 10 g/day 6 months: 30 ± 6 g/day Infant temperature maintenance (first 11 KMC/CCC episodes) In both groups for all 11 episodes, temperatures remained stable or rose by 0.1 to 0.2°C, with no significant difference in temperature gain found between the groups for any episode Mean temperature before KMC or CCC for both groups 36.7 ± 0.1°C After KMC or CCC for both groups 36.9 ± 0.2°C Process outcomes Mean hours cuddling per day [SD] KMC: 1.6 [0.9] CCC: 1.8 [0.9] Length of hospital stay KMC: 48 ± 28 days CCC: 46 ± 19 days Psychosocial outcomes Maternal stress The mothers expressed moderate to very stressful responses on all four subscales (nursery environment, infant appearance, relationship with the infant, staff behaviour and communication). Scores were not significantly different between the groups Maternal confidence For the whole group all items had a mean score (≥ 7.5) indicating a high level of confidence among parents in their parenting abilities. Scores were not significantly different between the groups Cost-effectiveness outcomes Not reported |
KMC (n = 16) | CCC (n = 14) | Discharge | 10 | 11 | 6 weeks | 9 | 6 | 3 months | 7 | 5 | 6 months | 4 | 4 |
30 mother-infant pairs were randomised (KMC 16, CCC 14) After the first 11 cuddling episodes, 20 mother-infant pairs remained in the study Numbers remaining in the study at other data collection points not reported Withdrawals not reported by group |
Data were not analysed using intention-to-treat model The paper mentions mothers and parents; it is not clear whether or how fathers were involved in the study Funding assistance came from the Northern Territory University and the Australian Nurse-Teachers’ Society |
|
| KMC (n = 16) | CCC (n = 14) | ||||||||||||||||||||
| Discharge | 10 | 11 | |||||||||||||||||||
| 6 weeks | 9 | 6 | |||||||||||||||||||
| 3 months | 7 | 5 | |||||||||||||||||||
| 6 months | 4 | 4 |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Rojas 2003 USA (Connecticut) Research aim To determine whether infants receiving skin-to-skin care (SSC) grew more rapidly and had a shorter duration of hospital stay than infants held in a traditional way (TH) Study design Randomised controlled trial Method of group allocation Numbered and sealed opaque envelopes previously prepared using random number table Unit of allocation Infant Unit of analysis Infant Sample size calculation 45 infants per group to provide 86% power to detect a 20% difference in major outcome scale of mother–infant interaction at an alpha level of 0.05 Outcome measures Successfully breastfed before discharge Mean discharge weight, length and head circumference Rate of head circumference growth Adverse events Frequency and length of SSC |
Selection Consecutively born preterm infants at Yale New Haven Hospital who met criteria from 31 August 1995 to 19 April 1998 Inclusion criteria Infants Gestational age of 32 weeks or less Birthweight of 1500 g or less Minimal ventilatory support (peak airway pressure < 8 cmH2O and FiO2 < 40% or extubated on nasal continuous positive airway pressure or nasal canula) Haemodynamically stable Exclusion criteria Infants Clinical evidence of perinatal asphyxia Potential transfer within first month of life Major congenital abnormality Planned adoption Grade III or IV intraventricular haemorrhage Fetal growth restriction (birthweight < 10th percentile for age) Suspected sepsis Mothers Less than 18 years History of using illicit drugs in pregnancy |
Infants SSC (n = 33); TH (n = 27); p [SD] where reported Mean birthweight (g) 906 [245]; 939 [230]; 0.3 Mean birth length (cm) 35.3 [3.0]; 35.0 [3.5]; 0.8 Mean birth head circumference (cm) 24.7 [2.0]; 24.9 [2.3]; 0.7 Mean weight at study entry (g) 1021 [268]; 1002 [219]; 0.4 Mean gestational age at birth (weeks) 26.6 [2.3]; 27.2 [2.3]; 0.1 Mean corrected age at entry (weeks) 29.4 [2.0]; 29.8 [2.3]; 0.2 Apgar ≤ 3 at 5 minutes age 2/32 (6%); 1/27 (4%); 0.6 Ventilated > 3 days 24/33 (73%); 18/27 (65%); 0.6 Female 15/33 (45%); 10/27 (37%); 0.5 Mothers Not reported No significant differences between the groups were found in infant characteristics |
I: n = 33 Skin-to-skin care (SSC) Parents were shown a video demonstrating the SSC technique. Infants held in prone, semiupright position (approx. 45°) in direct skin-to-skin contact with parent’s chest. Infants wore diaper and backs covered with a blanket. Parents not prohibited from offering TH C: n = 27 Traditional holding (TH) Parents removed infants from incubator and held them in the supine position with eye-to-eye contact. Infants wore diapers and T-shirts and wrapped in a blanket. Infants not offered SSC Both groups All infants < 1000 g wore a hat when held. Parents sat in reclining chairs and told they could hold their infants for a total of 8 hours/day (periods of up to 4 hours, twice/day). Holding time was not prescribed Data collection Continuous monitoring of heart rate, respiratory rate, core body temperature and oxygen saturation before, during and after all care. Parents asked to complete a self-assessment questionnaire after each intervention to determine duration and problems. Bedside nurse recorded adverse events Data on weight and nutritional source, caloric intake, length, head circumference measured using recognised procedures by the same research assistant until infants reached 2000 g or until hospital discharge, whichever came first Successful breastfeeding was defined as objective evidence of consistent breastfeeding with appropriate technique as judged by a lactation specialist and confirmed by retrospective review of medical records |
Statistical techniques Chi-squared or Fisher exact tests for discrete variables, Student’s t test for continuous variables. Unadjusted Kaplan–Meier survival analyses and Cox proportional hazards models for data about time from study entry to events of interest Breastfeeding/breastmilk-related outcomes Successfully breastfed before hospital discharge SSC: 18/30 (60%) TH: 9/26(35%) OR: 2.8, 95% CI: 1.0–8.3, p = 0.08 Clinical/health outcomes Surviving infantsSSC (n = 31)C (n = 26)pMean discharge weight (g) [SD]2120 [248]2012 [154]0.05Mean discharge length (cm) [SD]43 [2.2]42.6 [1.1]0.3Mean discharge head circumference (cm) [SD] (data missing from one infant in TH)32.1 [1.3]31.3 [1.0]0.001Rate of head circumference growth (cm/day) [SD]0.1 [0.03]0.08 [0.02]0.05 No significant differences found between the groups for total weight gain, total head circumference growth, total linear growth, rate of weight gain or rate of linear growth Infants SSC (n = 33)C (n = 27)pNEC1 (3%)2 (7%)0.6Sepsis5 (15%)8(30%)0.2Desaturations10(30%)15(56%)0.05 Other adverse events are reported, none with statistical significance between the groups Process outcomes Median days intervention occurred after randomisation (range)1 (0–28)1 (0–15)Occasions infants held (per week)4.0 ± 2.84.8 ± 3.5Minutes infants held (per day)79 ± 4076 ± 39Mean days from randomisation to discharge or a weight of 2000 g61 ± 2861 ± 33=Infant held by parent at least once per day from randomisation to discharge or a weight of 2000 g15 ± 1622 ± 15p = 0.03% total holding time fathers held infants31%27%p = 0.07Fathers performed their assigned intervention at least once during study period30/33 (91%)25/27 (93%)NS Authors note that compliance was low in both groups Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
Surviving infants | SSC (n = 31) | C (n = 26) | p | Mean discharge weight (g) [SD] | 2120 [248] | 2012 [154] | 0.05 | Mean discharge length (cm) [SD] | 43 [2.2] | 42.6 [1.1] | 0.3 | Mean discharge head circumference (cm) [SD] (data missing from one infant in TH) | 32.1 [1.3] | 31.3 [1.0] | 0.001 | Rate of head circumference growth (cm/day) [SD] | 0.1 [0.03] | 0.08 [0.02] | 0.05 | SSC (n = 33) | C (n = 27) | p | NEC | 1 (3%) | 2 (7%) | 0.6 | Sepsis | 5 (15%) | 8(30%) | 0.2 | Desaturations | 10(30%) | 15(56%) | 0.05 | Median days intervention occurred after randomisation (range) | 1 (0–28) | 1 (0–15) | Occasions infants held (per week) | 4.0 ± 2.8 | 4.8 ± 3.5 | Minutes infants held (per day) | 79 ± 40 | 76 ± 39 | Mean days from randomisation to discharge or a weight of 2000 g | 61 ± 28 | 61 ± 33= | Infant held by parent at least once per day from randomisation to discharge or a weight of 2000 g | 15 ± 16 | 22 ± 15 | p = 0.03 | % total holding time fathers held infants | 31% | 27% | p = 0.07 | Fathers performed their assigned intervention at least once during study period | 30/33 (91%) | 25/27 (93%) | NS |
318 infants ≤ 32 weeks’ gestation and ≤ 1500 g birthweight were born at study hospital during study period 38 died before eligibility 93 did not meet criteria 115 refused participation 12 not enrolled for other cited reasons Remaining 60 infants (19%) enrolled Three infants died after randomisation (SSC 2, TH 1) from severe respiratory failure and from NEC and sepsis respectively 31 SSC infants and 26 TH infants survived to discharge |
Data were not analysed using intention-to-treat model Background breastfeeding rates not reported Authors note study was underpowered for outcome of weight gain Authors note it was observed that parents in the SSC group would spontaneously begin to transition to TH as their infants matured and reached 1800–2000 g. Many parents expressed need to maintain eye-to-eye contact Funding supported in part by Ronald McDonald Children’s Charities of Connecticut and Western Massachusetts |
||||||||||||||||||||||||||||||||||
| Surviving infants | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SSC (n = 31) | C (n = 26) | p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean discharge weight (g) [SD] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2120 [248] | 2012 [154] | 0.05 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean discharge length (cm) [SD] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 43 [2.2] | 42.6 [1.1] | 0.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean discharge head circumference (cm) [SD] (data missing from one infant in TH) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32.1 [1.3] | 31.3 [1.0] | 0.001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rate of head circumference growth (cm/day) [SD] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0.1 [0.03] | 0.08 [0.02] | 0.05 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SSC (n = 33) | C (n = 27) | p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NEC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 (3%) | 2 (7%) | 0.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sepsis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 (15%) | 8(30%) | 0.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Desaturations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10(30%) | 15(56%) | 0.05 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median days intervention occurred after randomisation (range) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 (0–28) | 1 (0–15) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Occasions infants held (per week) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.0 ± 2.8 | 4.8 ± 3.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minutes infants held (per day) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 79 ± 40 | 76 ± 39 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean days from randomisation to discharge or a weight of 2000 g | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 61 ± 28 | 61 ± 33= | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Infant held by parent at least once per day from randomisation to discharge or a weight of 2000 g | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 ± 16 | 22 ± 15 | p = 0.03 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| % total holding time fathers held infants | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31% | 27% | p = 0.07 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fathers performed their assigned intervention at least once during study period | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30/33 (91%) | 25/27 (93%) | NS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Sloan 1994 Ecuador (Quito) The author provided additional information on breastfeeding outcomes of the study for this review Research aim To compare the effects of the Kangaroo mother method (KMM) and standard treatment on morbidity, growth and cost of care for infants weighing < 2000 g at birth Study design Randomised controlled trial Method of group allocation Random numbers table Unit of allocation Individual (infant) Unit of analysis Individual and group Sample size calculation To detect 2.5% vs 7.5% of severe disorders, given α error = 0.05, 1 – ß = 0.80, two tailed-test and potential loss to follow-up 25%, 350 infants per group Outcome measures Infant growth Morbidity Duration of hospital stay Readmissions Costs of care |
Selection Infants born at the Isidiro Ayora Maternity Hospital, Quito, Ecuador, November 1991 to December 1992 Inclusion criteria Singleton infants weighing < 2000 g Stabilised, with: Exclusion criteria Infants with serious congenital abnormalities or respiratory, metabolic or infectious disease were excluded |
Mothers Mean [SD] age at first interview (years) I (n = 123): 24.94 [6.18] C (n = 143): 24.39 [6.06] Mean [SD] years maternal education I (n = 132): 4.6 [1.8] C (n = 149): 4.4 [1.8] Married I: 64.5% C: 73.1% Living with father of child I: 74.2% C: 90.3% Mean parity [SD] I (n = 121): 2.02 [1.75] C (n = 142): 1.09 [1.95] Infants Mean [SD] birthweight (g) I (n = 130): 1704 [243] C (n = 152): 1704 [248] Male I: 57.7% C: 53.9% Mean [SD] weeks’ gestation I (n = 129): 34.6 [2.5] C (n = 152): 34.1 [2.4] Gestation < 37weeks I: 51.2% C: 61.5% Mean [SD] age at eligibility (days) I (n = 127): 12.4 [10.5] C (n = 148): 13.7 [9.9] Group comparability More than 160 variables were compared to assess the study groups’ similarity Only 5/160 showed significant differences, and 6 others suggested important differences to the authors, who note this rate (< 5%) of significant differences in baseline status between the groups is less than would be expected by chance |
I: n = 140 Kangaroo mother method (KMM) After randomisation mothers received additional instruction on how to hold the baby upright, skin to skin (diaper allowed) against the breast to avoid vomiting and provide warmth and nurture, how to breastfeed from inside the blouse and how to sleep inclined with the infant C: n = 160 Standard incubator care (control) After randomisation mothers received additional instruction about the infant’s stay in the incubator or thermal crib, scheduling visits to breastfeed, positioning of baby after feeds to avoid vomiting, how to ensure maintenance of warmth and how to arrange and maintain a crib at home following discharge Standard care All mothers were trained by nurses to care for their LBW infants including: basic hygiene and immediate notification of staff if infant turned blue or pale or showed rapid breathing or feeding problems Data collection Clinical data Cost data Equipment (incubators, heated cribs) and drugs and supplies by duration of use, professional carers’ time (number of episodes of care by doctors/nurses and the product of number and duration of instruction in child care), other daily costs of postnatal clinic visits (including transport) or hospital admission (number of visits or duration of stay, retrospectively) |
Statistical techniques Linear multiple regression analysis for infant growth and cost outcomes, Kaplan–Meier survival analysis and Cox’s proportional hazards models for infant morbidity, and other analyses by unadjusted chi-squared or t tests Breastfeeding/breastmilk-related outcomes Exclusive breastfeeding at dischargeKMCCMCn randomised (R)140160Data available (DA) (%)124 (88.6)144 (90)Exclusive bf124141(% DA, R)(100, 88.6)(97.9, 88.1)Exclusive breastfeeding at 1 month of age (KMC; control)KMCControln randomised (R)140160Data available (DA) (%)93 (66.4)111 (69.4)Exclusive bf86103% DA, R92.4, 61.492.7, 64.3Exclusive breastfeeding at 6 months of age (KMC; control)KMCControln randomised (R)140160Data available (DA) (%)56 (40.0)74 (46.3)Exclusive bf (% DA, R)6 (10.7)9 (12.16)% DA, R10.7, 4.212.2, 5.6 No significant differences between the study groups were found for breastfeeding outcomes Clinical/health outcomes Posteligibility deaths I: 11; C: 13 Infant growth No significant differences were found between the groups in growth indices during the 6-month follow-up Cumulative frequency of morbidity indices KMM (n = 131)Controls (n = 152)pSerious illness7 (5%)27 (18%)0.002Lower respiratory tract6 (5%)19 (13%)0.020Readmission4 (4%)11 (7%)0.130Signs of alarm39 (30%)61 (40%)0.080Moderate illness9 (7%)10 (7%)0.910Mild illness84 (64%)102 (67%)0.310Diarrhoea14 (11%)25 (16%)0.180 The difference in the cumulative incidence of severe illness (less in the KMM group than in the control group) was highly significant from month 2 onwards After control for pre-eligibility differences in severe morbidity (not specified in the paper) the significance of this association was p < 0.007 and recruitment to the trial was halted Process outcomes Skin-to-skin contact (KMM group only) Most infants for whom this information was available (68% at 1 months, falling to 7% at 3 months) were held against the breasts until 3 months Psychosocial outcomes Not reported Cost-effectiveness outcomes Duration of hospital stay KMM infants were 1.5 days younger at eligibility and 0.5 days older at discharge than control infants, so their length of hospital stay, from the point of eligibility, was 2.0 days greater than control infants (p < 0.05). However, more control than KMM infants were in incubators after eligibility and the cost of posteligibility, pre-discharge hospital stay was 475,000 sucres (~US$340) higher in the control group Postneonatal care for controls was 561,000 sucres (~US$401) greater for the control than KMM group at 5 months |
Exclusive breastfeeding at discharge | KMC | CMC | n randomised (R) | 140 | 160 | Data available (DA) (%) | 124 (88.6) | 144 (90) | Exclusive bf | 124 | 141 | (% DA, R) | (100, 88.6) | (97.9, 88.1) | Exclusive breastfeeding at 1 month of age (KMC; control) | KMC | Control | n randomised (R) | 140 | 160 | Data available (DA) (%) | 93 (66.4) | 111 (69.4) | Exclusive bf | 86 | 103 | % DA, R | 92.4, 61.4 | 92.7, 64.3 | Exclusive breastfeeding at 6 months of age (KMC; control) | KMC | Control | n randomised (R) | 140 | 160 | Data available (DA) (%) | 56 (40.0) | 74 (46.3) | Exclusive bf (% DA, R) | 6 (10.7) | 9 (12.16) | % DA, R | 10.7, 4.2 | 12.2, 5.6 | KMM (n = 131) | Controls (n = 152) | p | Serious illness | 7 (5%) | 27 (18%) | 0.002 | Lower respiratory tract | 6 (5%) | 19 (13%) | 0.020 | Readmission | 4 (4%) | 11 (7%) | 0.130 | Signs of alarm | 39 (30%) | 61 (40%) | 0.080 | Moderate illness | 9 (7%) | 10 (7%) | 0.910 | Mild illness | 84 (64%) | 102 (67%) | 0.310 | Diarrhoea | 14 (11%) | 25 (16%) | 0.180 |
300 infants were randomised 140 to KMM 160 to control Follow-up rate for morbidity outcomes was 94.3% (lower follow-up for other outcomes) |
Available data were reported by randomised group Of 603 babies of birthweight < 2000 g, 321 were eligible Reasons for ineligibility of 282 were: Mean birthweight (g) [SD] and range for these 282 babies (except for perinatal deaths) was: 1612 [323] 660–1985 For the 321 eligible babies it was: 1618 [317] 660–1995 Mothers of 21 eligible babies did not consent to take part in the study KMM mothers and siblings were seen more often in clinics. Cost–benefit aspects of this are discussed in the paper Funding: United States Agency for International Development Contract DPE 5966 Z 00 8083 00 and John Snow Inc |
|||||||||||||||||||||||
| Exclusive breastfeeding at discharge | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | CMC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n randomised (R) | 140 | 160 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Data available (DA) (%) | 124 (88.6) | 144 (90) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusive bf | 124 | 141 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (% DA, R) | (100, 88.6) | (97.9, 88.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusive breastfeeding at 1 month of age (KMC; control) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | Control | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n randomised (R) | 140 | 160 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Data available (DA) (%) | 93 (66.4) | 111 (69.4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusive bf | 86 | 103 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| % DA, R | 92.4, 61.4 | 92.7, 64.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusive breastfeeding at 6 months of age (KMC; control) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMC | Control | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n randomised (R) | 140 | 160 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Data available (DA) (%) | 56 (40.0) | 74 (46.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusive bf (% DA, R) | 6 (10.7) | 9 (12.16) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| % DA, R | 10.7, 4.2 | 12.2, 5.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KMM (n = 131) | Controls (n = 152) | p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Serious illness | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 (5%) | 27 (18%) | 0.002 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lower respiratory tract | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 (5%) | 19 (13%) | 0.020 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Readmission | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 (4%) | 11 (7%) | 0.130 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Signs of alarm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 39 (30%) | 61 (40%) | 0.080 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate illness | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 (7%) | 10 (7%) | 0.910 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild illness | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 84 (64%) | 102 (67%) | 0.310 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diarrhoea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 (11%) | 25 (16%) | 0.180 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Whitelaw 1998, UK (London) Research aim Does early skin-to-skin contact (SSC) for very low birthweight infants influence mothers’ confidence, infant behaviour and prolong lactation? Study design Randomised controlled trial Method of group allocation Numbered and sealed opaque envelopes previously prepared in balanced blocks of six. Eligible second twins were allocated to same group as first twin Unit of allocation Infant Unit of analysis Infant Mothers for breastfeeding outcomes Sample size calculation 36 infants per group for an 80% chance of detecting a doubling of the rate of mothers of very low birthweight infants still lactating at 6 weeks to 65% at p < 0.05 Outcome measures Duration of breastfeeding Frequency and duration of SSC Mothers’ attitudes towards infants Infants’ behaviour at discharge and 6 months |
Selection All eligible infants in Hammersmith Hospital born between August 1985 and February 1987 Inclusion criteria Infants Very low birthweight of less than 1500 g Stable breathing with no oxygen equipment At least one parent fluent in English Stable infants with congenital abnormalities such as hydronephrosis or scoliosis, intracranial lesions such as periventricular leukomalacia or ventricular dilatation Exclusion criteria Infants None |
Infants SSC (n = 35); C (n = 36) Mean birthweight (g) [SD] 1152 [220]; 1135 [263] Mean gestational age (weeks) [SD] 29.1 [2.3]; 29.5 [2.3] Mean age of infant at trial entry (days) (range) 16 (1–61); 16 (1–66) Mean Apgar score at 1 minute 5.6; 5.5 Singleton 26 (74%); 27 (75%) Female 14 (40%); 22 (61%) Mothers SSC (n=31);C (n=32) Caesarean 23; 23 White 26; 24 Afro-Caribbean 4; 4 Unsupported 2; 2 Intended to breastfeed 24; 26 Group comparability No significant differences between the groups were found in infant or maternal characteristics |
I: n = 35 Skin-to-skin contact (SSC) A nurse explained SSC to mother, with photographs if required, as a safe and enjoyable way to hold and get to know her baby. The mother was helped to position her baby inside her clothing between her breasts. Infants had a cardiac or respiration monitor attached and wore a nappy. Smallest infants also wore a hat. After two occasions, mother encouraged to hold her infant in SSC when she visited and after discharge C: n = 36 Normal handling Mother had same amount of support and encouraged to visit when she liked to take her infant(s) out of the incubator. Mother and infant remained clothed Both groups If an infant became unwell, the trial was discontinued until infant was stable Mothers SSC (n = 31); C (n = 32) Caesarean 23; 23 Primiparous 21; 19 White 26; 24 Asian 5; 8 Data collection Mothers given a questionnaire at discharge to rate themselves on a six-point scale for confidence looking after, knowing, feeling optimistic or depressed about, detached from and supported in looking after, their infant(s). A similar questionnaire was given to mothers at 6 months of corrected age. Historical data on lactation from the mother at discharge or at 6 months. At 6 months, parents asked to keep a 48-hour diary of infant’s behaviour. Home visit conducted if necessary. The questionnaires and diary were piloted |
Statistical techniques Student’s t test for data with a Gaussian distribution, Mann–Whitney U test and chi-squared test for data with a non-Gaussian distribution Breastfeeding/breastmilk-related outcomes Mothers SSC (n = 31)C (n = 32)pMean (median) duration of lactation (weeks)9.2 (7)5.1 (3.5)0.0167Lactated more than 6 weeks17 (55%)9 (28%)< 0.02 Clinical/health outcomes Not reported Process outcomes Infants SSC (n = 35)C (n = 36)Mean hours visiting/day [SD] (range)2.1 [0.8] (0.7–3.9)2.2 [0.9] (0.7–11.0)Median hours left in incubator while mother visited (range)0.1 (0–2.0)0.1 (0–5.9)Mean hours touched or cuddled with clothes on [SD] (range)1.4 [0.7] (0.2–3.4)1.8 [1.0] (0.5–5.2)Median hours SSC (range): 0.6 (0–1.5)Median days spent in study hospital (range)30 (5–83)37 (5–78) Psychosocial outcomes No significant differences between the groups of mothers was found on any of the six-point scales at discharge or at 6 months Infant behaviour at 6 months of age Infants SSC (n = 35)C (n = 36)pMean hours sleeping/day [SD]13.6 [2.3]13.4 [2.3]NSMean hours feeding/day [SD]2.5 [1.1]2.5 [0.9]NSMean hours being held/day [SD]3.0 [1.3]3.0 [1.4]NSMean hours playing/day [SD]4.5 [1.8]4.5 [1.8]NSMedian minutes crying/day (range)25 (0–100)38 (5–140)0.0422 Cost-effectiveness outcomes Not reported |
SSC (n = 31) | C (n = 32) | p | Mean (median) duration of lactation (weeks) | 9.2 (7) | 5.1 (3.5) | 0.0167 | Lactated more than 6 weeks | 17 (55%) | 9 (28%) | < 0.02 | SSC (n = 35) | C (n = 36) | Mean hours visiting/day [SD] (range) | 2.1 [0.8] (0.7–3.9) | 2.2 [0.9] (0.7–11.0) | Median hours left in incubator while mother visited (range) | 0.1 (0–2.0) | 0.1 (0–5.9) | Mean hours touched or cuddled with clothes on [SD] (range) | 1.4 [0.7] (0.2–3.4) | 1.8 [1.0] (0.5–5.2) | Median hours SSC (range): 0.6 (0–1.5) | Median days spent in study hospital (range) | 30 (5–83) | 37 (5–78) | SSC (n = 35) | C (n = 36) | p | Mean hours sleeping/day [SD] | 13.6 [2.3] | 13.4 [2.3] | NS | Mean hours feeding/day [SD] | 2.5 [1.1] | 2.5 [0.9] | NS | Mean hours being held/day [SD] | 3.0 [1.3] | 3.0 [1.4] | NS | Mean hours playing/day [SD] | 4.5 [1.8] | 4.5 [1.8] | NS | Median minutes crying/day (range) | 25 (0–100) | 38 (5–140) | 0.0422 |
Data are reported for all 71 infants (SSC = 35; C = 36) and 63 mothers (SSC = 31; C = 32) recruited Five mothers in SSC group (3 Asian, 2 white) declined SSC; their data were analysed according to allocated group Six infants per group left the trial temporarily due to apnoeas, necrotising enterocolitis or sepsis Two infants per group died between study entry and reaching 6 months of age (2 septicaemia, 1 necrotising enterocolitis, 1 sudden infant death) |
Data were analysed using intention-to-treat model Researchers had previously found 32–33% mothers of very low birthweight infants still lactating at 6 weeks Mothers’ views not reported by group |
|||||||||||||||||||
| SSC (n = 31) | C (n = 32) | p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean (median) duration of lactation (weeks) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.2 (7) | 5.1 (3.5) | 0.0167 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lactated more than 6 weeks | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 (55%) | 9 (28%) | < 0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SSC (n = 35) | C (n = 36) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean hours visiting/day [SD] (range) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 [0.8] (0.7–3.9) | 2.2 [0.9] (0.7–11.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median hours left in incubator while mother visited (range) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 0.1 (0–2.0) | 0.1 (0–5.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean hours touched or cuddled with clothes on [SD] (range) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 [0.7] (0.2–3.4) | 1.8 [1.0] (0.5–5.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median hours SSC (range): 0.6 (0–1.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median days spent in study hospital (range) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30 (5–83) | 37 (5–78) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SSC (n = 35) | C (n = 36) | p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean hours sleeping/day [SD] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.6 [2.3] | 13.4 [2.3] | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean hours feeding/day [SD] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.5 [1.1] | 2.5 [0.9] | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean hours being held/day [SD] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.0 [1.3] | 3.0 [1.4] | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean hours playing/day [SD] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.5 [1.8] | 4.5 [1.8] | NS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median minutes crying/day (range) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25 (0–100) | 38 (5–140) | 0.0422 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Wilhelm 2005 USA Research aim What are the effects of early kangaroo care (EKC) on breast skin temperatures, distress and breastmilk production in mothers of premature infants? Study design Repeated measures, crossover study Method of group allocation Assigned on postpartum day 4 using a coin toss procedure for the first participant in the study. Subsequent mothers assigned to alternate groups to achieve equal numbers Unit of allocation Mother Unit of analysis Mother Sample size calculation Based on Hurst (1997)a, estimated sample size of 30 Outcome measures Breastmilk production Breast skin temperature Change in salivary cortisol Mothers’ experiences |
Selection Mothers who gave birth to infants < 33 weeks’ gestation and/or < 2000 g, who were admitted to level III NICUs in three major medical centres in a US Midwest city Inclusion criteria: Mothers Expressing breastmilk Intending to breastfeed for at least 3 months Interested in participating in KC Non-smokers No breast surgery Able to speak and read English Infants 5-minute Apgar score of 6 or more Not expected to be ready to feed from the breast during the study period (first week of life) Early intensive care completed before postpartum day 4 Intravenous or gavage feeding 2–3 hourly Stable body temperature and vital signs for 48 hours Exclusion criteria Known thyroid or other endocrine disorder Hormonal fertility treatment or steroid medications/inhalers in third trimester Could not participate on all three days of the study Mothers of infants were unable to participate in KC during that part of the study period they were allocated to KC |
Reported for mothers included in the analyses Mothers (n = 25) Mean age in years [SD] (range) 28.76 [5.24] (19–42) Race n (%) Mean gravida (sic) [SD] (range) 1.84 [1.32] (1–6) Caesarean section n (%) 17/25 (68) Married n (%) 19/25 (76%) Private insurance n (%) 18/25 (72%) Income < $10,000/year n (%) 3/25 (12) Infants (n = 25) Mean birthweight (g) [SD] (range) 1652 [300] (820–2110) Mean gestational age (weeks) [SD] (range) 31.52 [0.92] (30–33) Weight for gestational age n (%) Group comparability No statistically significant differences between the ABB and BAA groups were found |
Intervention: Early kangaroo care (EKC) Skin-to-skin, chest-to-chest placement of the infant between the mother’s breasts beneath her clothing. The mother sat in a comfortable chair and covered herself and her infant with a blanket. Mothers spent 1 hour (9–10 am) in EKC, only on the day(s) allocated Group ABB: n = 11 Day 4 postpartum Mothers participated in EKC with their infant Days 5 and 6 postpartum Mothers did not participate in EKC. They were allowed to visit their infant at the cotside Group BAA: n = 14 Day 4 postpartum Mothers did not participate in EKC. They were allowed to visit their infant at the cotside Days 5 and 6 postpartum Mothers participated in EKC with their infant Study protocol and data collection Days 1–3 Day 4 Mothers assigned to study groups Days 4–6 Day 7 Mothers were asked to page the PI on arrival at NICU to provide breastmilk log data for day 6 postpartum Day 6,7, 8 or 9 Interviews The blanket was weighed before and after EKC if necessary to ensure any breastmilk leakage was accounted for as part of the following pumping session Interviews with mothers on their experiences using a preliminary conceptual six-staged map |
Statistical techniques Repeated measures of variance (RM-ANOVA) for a three-period, two-treatment, two-sequence crossover design. The significance of carryover effects was tested Breastfeeding/breastmilk-related outcomes Mean breastmilk production days 4–6 by group (ml) [SD] DayABB (n = 9)BAA (n = 13)4428.89 [325.46]382.23 [293.64]5557.00 [348.36]465.46 [324.73]6674.89 [417.97]474.00 [322.35] Author states that participation in EKC resulted in a decrease of 49.78 ml in milk production compared with non-participation (p < 0.05, two-tailed test) Clinical/health outcomes Mean breast skin temperature from 0900 to 1000 by group (°C) [SD] DayABB (n = 10)BAA n = (13)436.37 [0.34] EKC4 35.34 [0.59]535.94 [0.53]35.86 [0.50] EKC635.82 [0.68]35.77 [0.60] EKC Author states that participation in EKC resulted in an increase of 0.46°C over non-participation (p < 0.0001, two-tailed test) Mean salivary cortisol change between 0830 and 1030 by group (units not reported) DayABB (n = 11)BAA n = (14)4– 2.91 [7.11] EKC0.28 [5.06]5– 3.11 [4.92]– 3.83 [4.61] EKC6– 5.21 [4.40]– 2.73 [5.36] EKC Author states that there was no statistically significant change in cortisol between the two groups Process outcomes Not reported Psychosocial outcomes Mothers’ experiences (n = 18/29) Did I really have a baby? Premature delivery, very brief sight of baby in the delivery room, their own physical recovery from CS, caused some to question whether they even had a baby EKC experience Mothers who had not held their infants before KC were apprehensive. Once in KC mothers enjoyed KC and some felt it stimulated their milk supply Sensory stimulation Before KC mothers were eager to hold their infants despite their fears. Once in KC mothers could hear their infant’s heartbeat and breath sounds and some mothers said they knew their baby could hear and smell them Mean salivary cortisol change between 0830 and 1030 by group (units not reported) DayABB (n = 11)BAA n = (14)4– 2.91 [7.11] EKC0.28 [5.06]5– 3.11 [4.92]– 3.83 [4.61] EKC6– 5.21 [4.40]– 2.73 [5.36] EKC Author states that there was no statistically significant change in cortisol between the two groups Process outcomes Not reported Psychosocial outcomes Mothers’ experiences (n = 18/29) Did I really have a baby? Premature delivery, very brief sight of baby in the delivery room, their own physical recovery from CS, caused some to question whether they even had a baby EKC experience Mothers who had not held their infants before KC were apprehensive. Once in KC mothers enjoyed KC and some felt it stimulated their milk supply Sensory stimulation Before KC mothers were eager to hold their infants despite their fears. Once in KC mothers could hear their infant’s heartbeat and breath sounds and some mothers said they knew their baby could hear and smell them Intimacy Before KC many mothers did not feel connected to their infants. KC provided some ‘heart-to-heart’ privacy and intimacy Role recognition Initially mothers were unsure of their role within NICU, not knowing what to say or do or even where to stand. Early KC helped many find their niche in NICU Reality of motherhood KC helped mothers to recognise they indeed had a baby, and that the baby was theirs and did not belong to the nurses Cost-effectiveness outcomes Not reported |
Day | ABB (n = 9) | BAA (n = 13) | 4 | 428.89 [325.46] | 382.23 [293.64] | 5 | 557.00 [348.36] | 465.46 [324.73] | 6 | 674.89 [417.97] | 474.00 [322.35] | Day | ABB (n = 10) | BAA n = (13) | 4 | 36.37 [0.34] EKC | 4 35.34 [0.59] | 5 | 35.94 [0.53] | 35.86 [0.50] EKC | 6 | 35.82 [0.68] | 35.77 [0.60] EKC | Day | ABB (n = 11) | BAA n = (14) | 4 | – 2.91 [7.11] EKC | 0.28 [5.06] | 5 | – 3.11 [4.92] | – 3.83 [4.61] EKC | 6 | – 5.21 [4.40] | – 2.73 [5.36] EKC | Day | ABB (n = 11) | BAA n = (14) | 4 | – 2.91 [7.11] EKC | 0.28 [5.06] | 5 | – 3.11 [4.92] | – 3.83 [4.61] EKC | 6 | – 5.21 [4.40] | – 2.73 [5.36] EKC | Four of the 29 enrolled mothers had incomplete data for all three study outcomes and were excluded from analyses. No differences were found in maternal demographics between the excluded and included mothers. The infants of the excluded mothers weighed less at birth (p < 0.02) and were born at earlier GA (29.5 vs 31.5 weeks, p > 0.007) (p value as reported in paper) |
Standard care was said to differ between units One unit introduced KC as standard care and this was a factor in the decision to end recruitment to this study Author describes the numerous confounding variables in breastmilk production that may have contributed to the finding that participation in EKC resulted in a decrease of breastmilk production Author states that values of cortisol for mothers of premature infants were not known Abnormal fluctuations in cortisol may have been obtained during her study days |
| Day | ABB (n = 9) | BAA (n = 13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 428.89 [325.46] | 382.23 [293.64] | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 557.00 [348.36] | 465.46 [324.73] | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 674.89 [417.97] | 474.00 [322.35] | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Day | ABB (n = 10) | BAA n = (13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 36.37 [0.34] EKC | 4 35.34 [0.59] | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 35.94 [0.53] | 35.86 [0.50] EKC | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 35.82 [0.68] | 35.77 [0.60] EKC | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Day | ABB (n = 11) | BAA n = (14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | – 2.91 [7.11] EKC | 0.28 [5.06] | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | – 3.11 [4.92] | – 3.83 [4.61] EKC | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | – 5.21 [4.40] | – 2.73 [5.36] EKC | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Day | ABB (n = 11) | BAA n = (14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | – 2.91 [7.11] EKC | 0.28 [5.06] | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | – 3.11 [4.92] | – 3.83 [4.61] EKC | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | – 5.21 [4.40] | – 2.73 [5.36] EKC |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Hurst 1997 USA (Houston, Texas) Research aim To evaluate the effect of early skin-to-skin (STS) holding on lactation and duration of breastfeeding Study design Before/after (cross-sectional) Method of group allocation By date Unit of allocation Mother Unit of analysis Mother Group Sample size calculation Not reported Outcome measures Milk volumes Duration of breastfeeding |
Selection Mothers of ventilated low birthweight infants in the NICU of Texas Children’s Hospital, Houston Inclusion criteria: A: All mothers participating in STS holding between its introduction to the study NICU on 1 July 1993 and 30 September 1993 B: Mothers in the control group were taken from the 12-month period (June 1992–June 1993) before STS holding was introduced, and partially matched with the STS mothers for age, parity, twin birth, delivery type, infant birthweight, gestational age and severity of illness Exclusion criteria A: Mothers who did not begin STS holding during the 4 weeks after the birth B: Mothers of infants who began breastfeeding during the 4 weeks after the birth were excluded from the analysis |
Reported for mothers included in the analyses A (n = 8); B (n = 15) Mothers Mean ± SD age (years) 30 ± 5.7; 28 ± 6.3 Primiparas/multiparas 4/4; 7/8 Vaginal/Caesarean birth 3/5; 4/11 Previous breastfeeding experience 3; 2 Number of milk expressions per 24 hours 6; 6 Twin birth, socioeconomic status Not reported Infants Mean ± SD birthweight (g) 1129 ± 205; 1055 ± 264 Mean ± SD gestational age (weeks) 27.7 ± 1.1; 27.5 ± 1.9 Severity of illness A: STS was initiated whilst infants still receiving nasal continuous positive airway pressure B: not reported Group comparability Not reported |
A: n = 16 STS holding began when the attending neonatologist deemed the infant physiologically stable Mothers were instructed to hold their infants once a day for at least 30 minutes Details of how mothers held their infants STS are not provided in the paper During STS contact, oxygen saturation, skin temperature and frequency/duration of apnoea/ bradycardia were measured B: n = 16 No STS holding Both groups Lactation consultant contacted mothers 24–48 hours following birth to determine feeding plans Mothers planning to breastfeed were given instructions on pumping (to use a mechanical breast pump with double pumping attachment for approximately 15 minutes every 3 hours, starting within 48 hours of the birth), milk collection and storage Lactation consultant contacted mothers at least once per week to document number of pumpings per 24 hours, milk volume per pumping, non-pumping interval at night, and any problems with lactation After discharge from NICU, telephone contact continued, at least once during the first week home, then approximately fortnightly until 2 months after discharge or until breastfeeding was discontinued Data collection Clinical records |
Statistical techniques Repeated ANOVA adjusting for baseline volumes (1 week after delivery) to evaluate the difference in milk volumes between the groups Breastfeeding/breastmilk-related outcomes 24-hour milk volumes (mean ± SD) A (STS): n = 8; B (before STS): n = 15 Week 1: 499 ± 139; 218 ± 132 Week 2: 574 ± 211; 462 ± 222 Week 3: 690 ± 357; 485 ± 349 Week 4: 851± 259; 421 ± 315 The pattern of milk volumes from weeks 2 to 4 differed significantly between groups (group × time interaction p = 0.0110) At 4 weeks after the birth the adjusted mean 24-hour milk volume was: A (STS): 647 ml; B (before STS): 530 ml Breastfeeding outcomes A (STS): n = 8; B (before STS): n = 15 Exclusive breastfeeding 3 (37%); 1 (6%) Breast + formula feeding after discharge 2 (25%); 8 (50%) Quit pumping before discharge A: 3/8 (37%) reasons were returning to work (n = 2), did not intend to breastfeed (one mother of twins) 6 (37%) reason for all 6 was low milk volumes despite pumping ≥ 6 times per 24 hours Clinical/health outcomes Heart rate, respiratory rate, oxygen saturation and skin temperature measurements were within normal limits for 136/138 STS sessions In 2/138 sessions (1.4%), an infant was noted during their first STS session to have a single desaturation episode, which returned to normal limits without requiring stimulation. Both these STS sessions continued without further incident Process outcomes Infants’ age at STS initiation 8–26 days (median 15 days) Mean frequency of STS 4 sessions per week Mean duration of STS Initiation week: 30 minutes Second week: 135 minutes Psychosocial outcomes Mothers commented favourably about STS Cost-effectiveness outcomes Not reported |
A: 16 STS mothers were enrolled Eight did not begin STS holding within 4 weeks of the birth, because of extreme prematurity < 27 weeks (n = 3), sepsis (n = 4) and necrotising enterocolitis (n = 1), and were excluded Eight were included in the analysis (50%) B: 16 non-STS mothers were initially identified One began breastfeeding during the 4 weeks after the birth and was excluded 15 were included in the analysis (94%) |
Available data were analysed by group Funding not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Wahlberg 1992 Sweden (Helsingborg) Research aim What were the differences, following introduction of kangaroo care (KC), in premature infants’ length of time nursed in an incubator, weight gain, length of hospital stay and rates of breastfeeding at discharge? Study design Before/after (cross-sectional), retrospective data analysis Method of group allocation By date Unit of allocation Infant Unit of analysis Infant Mother for breastfeeding outcomes Sample size calculation Not reported Outcome measures Weight gain per week Days in incubator Days old when first out of incubator Total days in hospital Breastfeeding at discharge |
Selection Two convenience samples of eligible infants before and after implementation of KC as routine practice in a Swedish premature care unit (equivalent to US level II nurseries providing care to infants who require monitoring with less intensive technological interventions) B period: May 1984 to Nov 1985 A period: Nov 1985 to May 1987 Inclusion criteria: Healthy mother with no infections or other complications Healthy stable infant aged 1–30 days when first taken out of the incubator (no respiration problems, anomalies or other complications) Exclusion criteria None |
Reported for mothers included in the analyses Mothers A (n = 33); B (n = 33) Mean age (years) [SD] 26.5 [5.0]; 27.7 [5.7] First baby 22 (67%); 20 (61%) Caesarean 15 (45%); 19 (58%) Socioeconomic status not reported Infants A (n = 33); B (n = 33) Mean birthweight (g) [SD] 1482 [453]; 1497 [419] Mean gestational age (weeks) [SD] 31.09 [2.2]; 31.33 [2.5] Interventions prior to first contact with mother outside incubator Respirator 7 (18%); 7 (22%) Oxygen 26 (70%); 22 (69%) Continuous positive airway pressure 4 (11%); 3 (9%) Group comparability Percentages as reported in paper The two groups were matched for maternal age, parity, length of pregnancy, type of delivery, gestational age, birthweight and technological intervention prior to first contact with mother outside the incubator |
A: n = 33 Kangaroo care (KC) implemented as routine practice KC began when infants were first taken out of the incubator to be with their mothers. Infants held skin-to-skin with mothers. No other details provided, picture shows infant is unclothed and held in upright position between mother’s breasts B: n = 33 Standard prematurity care (SPC) as routine practice SPC began when infants were first taken out of the incubator to be with their mothers. Mothers held their babies dressed and with a blanket or heating pad Both groups Mothers were encouraged to hold their infants as much as they desired Data collection Hospital records for following variables: |
Statistical techniques Not reported Breastfeeding/breastmilk-related outcomes A (KC) n = 33B (SPC) n = 33pBreastfeeding at discharge n (%)27 (82)15 (45)0.005Clinical/health outcomesWeight gain per week g [SD]237.48 [96.4]195.5 [82.9]< 0.05Process outcomesDays in incubator n [SD]20.9 [13.9]30.5 [15.7]< 0.05Days old when first out of incubator n [SD]4.36 [4.7]8.0 [5.9]< 0.01Total days in hospital n [SD]41.6 [16.9]49.4 [18.9]< 0.05 Chi-squared for all five results = 7.92 (p = 0.005) Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
A (KC) n = 33 | B (SPC) n = 33 | p | Breastfeeding at discharge n (%) | 27 (82) | 15 (45) | 0.005 | Clinical/health outcomes | Weight gain per week g [SD] | 237.48 [96.4] | 195.5 [82.9] | < 0.05 | Process outcomes | Days in incubator n [SD] | 20.9 [13.9] | 30.5 [15.7] | < 0.05 | Days old when first out of incubator n [SD] | 4.36 [4.7] | 8.0 [5.9] | < 0.01 | Total days in hospital n [SD] | 41.6 [16.9] | 49.4 [18.9] | < 0.05 | Retrospective data analysis for all selected participants |
Authors explain their finding that KC-group infants were younger when first removed from the incubator as follows: standard prematurity care involved a decision about an infant’s readiness for removal from the incubator based on physical status, e.g. weight and temperature. With KC group, staff learned infants in the K position were able to maintain body temperature coming to regard the mother as a human incubator. Infants were therefore taken out of incubators to be with their mothers at an earlier age |
||||||||||||||
| A (KC) n = 33 | B (SPC) n = 33 | p | |||||||||||||||||||||||||||||||||||||||||||
| Breastfeeding at discharge n (%) | |||||||||||||||||||||||||||||||||||||||||||||
| 27 (82) | 15 (45) | 0.005 | |||||||||||||||||||||||||||||||||||||||||||
| Clinical/health outcomes | |||||||||||||||||||||||||||||||||||||||||||||
| Weight gain per week g [SD] | |||||||||||||||||||||||||||||||||||||||||||||
| 237.48 [96.4] | 195.5 [82.9] | < 0.05 | |||||||||||||||||||||||||||||||||||||||||||
| Process outcomes | |||||||||||||||||||||||||||||||||||||||||||||
| Days in incubator n [SD] | |||||||||||||||||||||||||||||||||||||||||||||
| 20.9 [13.9] | 30.5 [15.7] | < 0.05 | |||||||||||||||||||||||||||||||||||||||||||
| Days old when first out of incubator n [SD] | |||||||||||||||||||||||||||||||||||||||||||||
| 4.36 [4.7] | 8.0 [5.9] | < 0.01 | |||||||||||||||||||||||||||||||||||||||||||
| Total days in hospital n [SD] | |||||||||||||||||||||||||||||||||||||||||||||
| 41.6 [16.9] | 49.4 [18.9] | < 0.05 | |||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Collins 2004 Australia Research aim To determine the effect of artificial teats (bottle and dummies) and cups on breastfeeding in preterm infants < 34 weeks’ gestation at birth Study design RCT with four groups Method of group allocation Random number table Unit of allocation Mother (twins were assigned to the same group) Unit of analysis Infant Sample size calculation Based on breastfeeding rates of 45% (unpublished hospital data) the authors calculated a sample size of 310 to detect a 16.5% increase in the proportion full breastfeeding on discharge (α = 0.05, 80% power) between use of dummy and not irrespective of cup or bottle and between cup and bottle irrespective of use of dummy Outcome measures Breastfeeding at discharge home Breastfeeding 3 and 6 months following discharge Length of hospital stay In this study, breastfeeding was defined as mother’s milk given by direct breastfeeding or other feeding device Full breastfeeding meant no other types of milk or solids were given except vitamins and minerals |
Selection Two large tertiary hospitals in Australia, April 1996 to November 1999 Inclusion criteria Women with singleton or twin infants < 34 weeks’ gestation who wanted to breastfeed Infants requiring transfer to peripheral hospitals were included (54 peripheral hospitals participated) Exclusion criteria Infants with congenital abnormalities precluding enteral feeding |
Mothers at trial entry Randomised to cup feeding No dummy: n = 75; dummy: n = 66 Age (years) n (%): < 25:8 (11); 18(27) 25–34: 47(36); 36(55) > 35: 20(27); 12(18) Education n (%): Incomplete high school: 21 (20); 22 (35) Complete high school: 19 (26); 24 (38) Tertiary: 32(44); 17 (27) Parity n (%): 1: 34 (45); 40(61) > 1: 41(55); 26(39) Breastfed before n (%): Yes: 32/72(44); 20/64(31) No: 40/72(56); 44/64(69) Randomised to bottle feeding No dummy: n = 64; dummy: n = 73 Age (years) n (%): < 25: 10 (16); 18 (25) 25–34: 36 (58); 41 (56) > 35: 16 (26); 14 (19) Education n (%): Incomplete high school: 21 (34); 29(43) Complete high school: 26 (42); 17 (25) Tertiary:15 (24); 21 (31) Parity n (%): 1: 22/62 (35); 36/73 (49) > 1: 40/62 (65); 37/73 (51) Breastfed before n (%): Yes: 31/63 (49); 25/68(37) No: 32/63 (51); 43/68 (63) Infants at birth Randomised to cup feeding No dummy: n = 89; dummy: n = 72 Twins n (%): 28 (31); 12 (17) Mean [SD] g birthweight (range): 1325 [453] (552–2520); 1344 [488] (609–2560) Gestational age at birth (%): < 28 weeks: 25 (28); 17 (24) > 28 weeks: 64 (72); 55 (76) Mean [SD] (range): 29.2 [2.7] (24–33); 29.5 [2.7] (23–33) Randomised to bottle feeding No dummy: n = 73; dummy: n = 85 Twins n (%): 18 (25); 24 (28) Mean [SD] g birthweight (range): 1508 [463] (720–2530); 1382 [469] (500–2580) Gestational age at birth (%): < 28 weeks: 14 (19); 20 (24) > 28 weeks: 59 (81); 65 (76) Mean [SD] (range): 30.3 [2.6] (25–33); 29.6 [2.6], (24–33) Group comparability Paper states most maternal and neonatal characteristics were well balanced There were ≥ 10% differences between dummy and no dummy for primiparity and previous breastfeeding, and between cup and bottle for primiparity, and these were adjusted for in the analyses |
All participants Cup or bottle feeds, when mothers were not available to breastfeed or additional milk was required orally after breastfeeds, commenced at the discretion of the practitioner Infants in dummy group had dummy available on trial entry. A dummy was used during tube feeds and when an infant was restless Infants in no-dummy group were encouraged to use quietening behaviours (hand to mouth actions were facilitated) Recruiting hospitals received education, written instructions, literature and one-to-one support (not specified who gave this) Peripheral hospitals received written instructions, literature and telephone contact (not specified who gave this) Intervention A: Infants to be cup fed (using a small plastic medicine cup) and not given a dummy (n = 89) Intervention B: Infants to be cup fed (using a small plastic medicine cup) and given a dummy (n = 72) Intervention C: Infants to be bottle fed and not given a dummy (n = 73) Intervention D: Infants to be bottle fed and given a dummy (n = 85) Data collection Not described in the paper |
Statistical techniques Logistic regression to estimate odds ratios and 95% confidence intervals (CI), Kaplan–Meier curves, Cox’s proportional hazards models Breastfeeding/breastmilk-related outcomes Any breastfeeding at discharge home Dummy: 107/152 (70%) No dummy: 108/151 (72%) OR 0.83 (95% CI: 0.45–1.50) p = 0.53, NS Cup: 112/151 (74%) Bottle: 92/151 (61%) OR 1.37(95% CI: 0.78–2.38) p = 0.27, NS Fully breastfeeding at discharge (%) Dummy: 85/151 (56) No dummy: 79/152 (52) OR 0.84 (95% CI: 0.51–1.39) p = 0.50, NS Cup: 92/151 (61%) Bottle: 72/152 (47%) OR 1.73,(95%CI: 1.04–2.88) p = 0.03 Cup feeding significantly increased the odds of full breastfeeding at discharge home Number needed to be cupfed for one extra infant to go home fully breastfeeding = 7 (95% CI: 4–41) Any breastfeeding after discharge 3 months6 monthspDummy53/141 (38%)34/140 (24%)NSNo dummy58/142 (41%)43/141 (30%)NSCup61/144 (42%)44/142 (31%)NSBottle50/139 (36%)33/139 (24%)NS Median days hospital stay (interquartile range) Dummy: 50 (33–78) No dummy: 53 (35–74) Hazard ratio 0.98, 95% CI: 0.76–1.26, p = 0.87 NS Infants randomised to cup feeding were found to have significantly longer hospital stays than those randomised to bottle feeding Cup: 59 (37–85) Bottle: 48 (33–65) Hazard ratio 0.71, 95% CI: 0.55–0.92, p = 0.01 Clinical/health outcomes No adverse events were found to be associated with the interventions Process outcomes Non-compliance 85/161 (52.7%) infants randomised to cup feeding had a bottle introduced 1/158 (0.6%) of infants randomised to bottle feeding had a cup introduced 47/162 (31%) infants randomised to no dummy had a dummy introduced 5/157 (3.2%) infants randomised to dummy did not have a dummy introduced Compliance was higher in the hospital that had used cup feeding before the trial. Most peripheral hospitals had not used cup feeding. Compliance was higher among primiparous, tertiary educated women whose household income was from full-time work from either partner and who were mothers of singleton infants. Authors note non-compliance reduced the power of the study to identify real treatment effects Psychosocial outcomes Some staff had strong feelings against cup feeding and the withholding of dummies and some parents did not like cup feeding Reasons for introducing a bottle included infant not managing cup feeds, spilling a lot, not being satisfied, or taking too long to feed Reasons for introducing a dummy included infant unsettled and to teach the infant to suck Cost-effectiveness outcomes Not reported |
3 months | 6 months | p | Dummy | 53/141 (38%) | 34/140 (24%) | NS | No dummy | 58/142 (41%) | 43/141 (30%) | NS | Cup | 61/144 (42%) | 44/142 (31%) | NS | Bottle | 50/139 (36%) | 33/139 (24%) | NS |
278 mothers of 319 infants were randomised 303 infants (94.9%) of 265 mothers (95.3%) were included in the analyses Numbers of infant deaths and withdrawals are reported by group |
An intention-to-treat analysis was done Funded by the Mercy Hospital for Women Nurses Research fund, and the first author received a two-year midwifery fellowship from the Women’s and Children’s Hospital Foundation |
||||||||
| 3 months | 6 months | p | |||||||||||||||||||||||||||||||
| Dummy | |||||||||||||||||||||||||||||||||
| 53/141 (38%) | 34/140 (24%) | NS | |||||||||||||||||||||||||||||||
| No dummy | |||||||||||||||||||||||||||||||||
| 58/142 (41%) | 43/141 (30%) | NS | |||||||||||||||||||||||||||||||
| Cup | |||||||||||||||||||||||||||||||||
| 61/144 (42%) | 44/142 (31%) | NS | |||||||||||||||||||||||||||||||
| Bottle | |||||||||||||||||||||||||||||||||
| 50/139 (36%) | 33/139 (24%) | NS | |||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Gilks 2004 UK (Birmingham) Research aim To determine which of two methods of feeding, cup or bottle, best prepared a preterm infant for breastfeeding Study design RCT (pilot) Method of group allocation By selection of concealed cards in envelopes Stratified by gestation (< 31 and > 31 completed weeks) Unit of allocation Infant Unit of analysis Infant Sample size calculation If the rate of breastfeeding at discharge among mothers who intended to breastfeed increased from 40% to 60%, 94 babies would be needed in each arm for an 80% (ß = 20%) chance of showing a significant difference at the 5% level (p < 0.05) (α = 5%) Outcome measures Breastfeeding at discharge, term and 6 weeks post-term |
Selection Neonatal Unit Birmingham Heartlands Hospital, Birmingham, UK Inclusion criteria < 35 completed weeks’ gestation at birth > 30 weeks at time of study entry Infant absorbing full-strength full-volume nasogastric feeds Clinically well for at least 48 hours prior to entry Expected to be in NICU for at least 1 week prior to discharge Mother intended to breastfeed Exclusion criteria Infants with congenital abnormalities |
Mothers Characteristics not reported Infants Cup: n = 27; bottle: n = 27 < 31 weeks: 11; 8 Median weeks’ gestation (range): 31 (25–34);32 (26–34) Median g birthweight (range): 1560 (580–2870); 1750 (944–2980) Group comparability No significant differences found between the groups |
I: n = 27 Cup feeding (not described) Paper states cup feeding had been introduced to the unit 6 months before the trial, supported by a teaching programme and information sheet for staff. The trial started after this ‘learning curve period’ C: n = 27 Bottle feeding (not described) Paper states that on this unit, ‘historically, staff had offered bottle feeds of maternal expressed breast milk to babies when their mothers were not present, as a means of progressing from tube to breast feeds.’ Data collection Exclusive breastfeeding: all feeds in previous 24 hours were breastfeeds Any breastfeeding: any breastfeed in previous 24 hours Withdrawal: mother said she no longer wished to breastfeed or infant was too ill to breastfeed Breastfeeding failure: cessation of breastfeeding in a mother who said she still wished to breastfeed |
Statistical techniques Descriptive statistics, difference of proportions Breastfeeding/breastmilk-related outcomes Breastfeeding at discharge Cup (n = 16); bottle (n = 24) Any: 14/16; 12/24 Exclusive: 10/14; 4/12 Failed: 2/16; 4/24 Fed exclusively on breastmilk but not feeding at the breast: 0; 5/24 Exclusive breastfeeding more common in cup-feeding group Difference of proportions = 22%; 95% CI: –1 to 43% Breastfeeding at term Cup (n = 16); bottle (n = 24) Any: 11; 12 Exclusive: 7/11; 8/12 Breastfeeding 6 weeks post-term Cup (n = 16); bottle (n = 24) Any: 5; 6 Exclusive: 4/5; 3/6 Clinical/health outcomes Not reported Process outcomes Withdrawals Cup: 11/27 (41%) Bottle: 3/27 (11%) Difference of proportions 30%, 95% CI: 6–50% Post-conceptional age at withdrawal of nasogastric tube Cup: 250 days (35 weeks 5 days) Bottle: 251 days (35 weeks 6 days) Psychosocial outcomes Reasons for withdrawal Mother no longer wanted to breastfeed (8 cup, 3 bottle) Infant ill (2 cup, 1 bottle) Mother on medication contraindicating breastfeeding (1 cup) Most common reasons for stopping breastfeeding Cost-effectiveness outcomes Not reported |
54 infants randomised, 27 to each group 14/54 (26%) withdrew 40 infants included in the analysis (74%) 16 in the cup-feeding group 24 in the bottle-feeding group Data for the outcome breastfeeding at discharge appear to be missing for three infants in the bottle-feeding group |
Available data are reported by randomised group Authors note it was difficult to differentiate between withdrawal and breastfeeding failure Authors note the number of mothers who would refuse trial entry was underestimated and suggest further research on recruitment and consent for neonatal trials Funding: Not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Kliethermes 1999 USA (Kansas) Research aim To compare NGT supplementation with bottle feeding as methods for transitioning preterm infants to breastfeeding Study design Randomised controlled trial Method of group allocation Sealed envelopes physically mixed and drawn in random sequence Unit of allocation Infant Unit of analysis Group Sample size calculation Not reported Outcome measures Breastfeeding status Complications Length of stay Days using NGT |
Selection Preterm infants in the level III, 40-bed ICN of a private regional perinatal centre were enrolled over a 22-month period Inclusion criteria Infants Birthweight 1–2.5 kg < 1-week-old Mothers Providing own breastmilk Exclusion criteria Congenital and neurological abnormalities that interfered with cardiopulmonary status |
Participants Bottle (n = 46)NGT (n = 38)pMothers: mean (range) age in years25 (16–42)30 (18–44)0.003Single mothers7 (15%)7 (18%)NSWage earners36 (80%)34 (89%)0.02Mean parity (range)1.7 (1–4)2.3 (1–5)0.003Previous breastfeeding experience12 (27%)20 (56%)0.01Unsuccessful breastfeeding2 (4.4%)5 (14.3%)NSInfants: mean birthweight (range) kg1.64(1.00–2.35)1.73(1.05–2.43)NSMean weeks’ gestational age (range)32 (28–35)32 (26–35)NSSmall for gestational age10 (22%)1 (3%)0.03Twin births16 (35%)8 (21%)NS Group comparability There were significant differences between the groups. These were controlled for as appropriate in the logistic regression models |
Bottle (n = 46) | NGT (n = 38) | p | Mothers: mean (range) age in years | 25 (16–42) | 30 (18–44) | 0.003 | Single mothers | 7 (15%) | 7 (18%) | NS | Wage earners | 36 (80%) | 34 (89%) | 0.02 | Mean parity (range) | 1.7 (1–4) | 2.3 (1–5) | 0.003 | Previous breastfeeding experience | 12 (27%) | 20 (56%) | 0.01 | Unsuccessful breastfeeding | 2 (4.4%) | 5 (14.3%) | NS | Infants: mean birthweight (range) kg | 1.64(1.00–2.35) | 1.73(1.05–2.43) | NS | Mean weeks’ gestational age (range) | 32 (28–35) | 32 (26–35) | NS | Small for gestational age | 10 (22%) | 1 (3%) | 0.03 | Twin births | 16 (35%) | 8 (21%) | NS |
Bottle group: n = 52 Once oral feeds prescribed, if mother not available for breastfeeding or if additional supplemental feeds required, infant to receive oral feedings by bottle NGT removed at clinical discretion. If supplements necessary after removal of NGT, they were given via bottle NGT group: n = 47 Once oral feeds prescribed, if mother not available for breastfeeding or if additional supplemental feeds required, infant to receive feedings via indwelling 3.5-French NGT NGT removed at rooming-in. If supplements necessary after removal of NGT, they were given via cup or syringe Both groups All mothers received standardised breastfeeding education provided by two registered nurse/certified lactation consultants (CLC), including method and frequency of expressing milk;transport and storage of milk; breast care; maternal dietary and fluid intake; and instructions on how to use electric pump All mothers received an electric breast pump with double pumping capabilities Lactation consultants contacted mothers biweekly All infants were given comparable intensive care until oral feeds prescribed Mothers roomed-in with their infants 24–48 hours before discharge to establish full breastfeeding and demonstrate appropriate weight gain Data collection Clinical records and phone calls to mothers by lactation consultants at 3 days, 3 and 6 months after discharge |
Statistical techniques Ordinal logistic regression models for breastfeeding status Breastfeeding/breastmilk-related outcomes Mean (range) days old at first breastfeed was 11 (1–40) in the bottle group and 16 (9–77) in the NGT group (not statistically significant) The NGT group had higher rates of breastfeeding at all time points studied (not reported numerically in the paper) Using the ordinal logistic regression model, the method of supplementation (NGT vs bottle) was predictive of continued breastfeeding at discharge (p = 0.0001), 3 days (p = 0.0001), 3 months (p = 0.0006) and 6 months after discharge (p = 0.0016) Clinical/health outcomes Apnoea/bradycardia incidents Bottle group: mean 136 NGT group: mean 127, p = 0.0006 Apnoea/bradycardia incidents requiring stimulation Bottle group: M 32.7 NGT group: M 23.3, p = 0.0001 Process outcomes Mean length of stay was similar between the groups (34.6 vs 33 days, p = 0.68) NGT group infants had a NGT for an average of 7 days longer than bottle group infants (M 29.2 vs 21.7 days, p = 0.036) Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
99 infants were enrolled (52 to the bottle group and 47 to the NGT group) A total of 15 (15.15%) were excluded Reasons for the 6 exclusions from the bottle group were: Reasons for the 9 exclusions from the NGT group were: |
Available data were analysed by randomised group Paper states ‘to avoid artificial deflation of the standard errors because of the presence of twins, all major results of this study were confirmed with a parallel analysis in which one twin in each pair was selected at random and excluded’ Funding Medela, Inc., McHenry, Ill., donated electric breast pumps for all study mothers Neotube, Mullin Medical, Tustin, CA, donated the NGTs used in this study |
||||||||||||||||||||
| Bottle (n = 46) | NGT (n = 38) | p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mothers: mean (range) age in years | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25 (16–42) | 30 (18–44) | 0.003 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Single mothers | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 (15%) | 7 (18%) | NS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wage earners | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 (80%) | 34 (89%) | 0.02 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean parity (range) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.7 (1–4) | 2.3 (1–5) | 0.003 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Previous breastfeeding experience | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 (27%) | 20 (56%) | 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unsuccessful breastfeeding | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 (4.4%) | 5 (14.3%) | NS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Infants: mean birthweight (range) kg | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.64(1.00–2.35) | 1.73(1.05–2.43) | NS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean weeks’ gestational age (range) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 (28–35) | 32 (26–35) | NS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Small for gestational age | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 (22%) | 1 (3%) | 0.03 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Twin births | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 (35%) | 8 (21%) | NS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Mosley 2001 UK Research aim To compare the impact of two methods of supplementary feeding of preterm infants (bottle vs cup) on subsequent breastfeeding Study design Pilot RCT Method of group allocation Group allocations taken from opaque, sealed, shuffled, sequentially numbered envelopes Unit of allocation Infant Unit of analysis Infant Sample size calculation Assuming a total population of preterm babies needing supplementary feeding of 4000, a confidence level of 90%, a maximum acceptable error of 17% and an estimated percentage level of an answer as 80/20, a sample size of 15 Outcome measures Breastfeeding at discharge |
Selection Preterm infants being cared for in SCBU of a District General Hospital in the UK during a 3-month period Inclusion criteria Born at 30–37 weeks’ gestation Mothers expressed desire to breastfeed Exclusion criteria Congenital abnormality No maternal preference for cup or bottle as the method of supplementary feeding No supplementary feed via bottle or cup prior to admission |
Mothers Primparas Cup: 3/8 (37.5%) Bottle: 5/8 (62.5%) Spontaneous vaginal delivery Cup: 5/8 (62.5%) Bottle: 4/8 (50%) Assisted delivery Cup: 3/8 (37.5%) Bottle: 4/8 (50%) Age, socioeconomic status, breastfeeding experience, multiple births Not reported Infants Mean weeks gestational age Cup: 35.2 Bottle: 35.5 Gestational age < 35 weeks Cup: 3/8 (37.5%) Bottle: 2/8 (25%) Birthweight Not reported Group comparability Not reported |
All participants When the infant was considered able to progress from gastric feeding, the supplementary feed was given by bottle or cup, as allocated I: n = 8 Supplementary feeds given by cup C: n = 8 Supplementary feeds given by bottle Data collection Staff were asked to continue to fill in the feed chart after each feed, as normal, to record the type and amount of milk taken at each feed and the frequency of feeds Mothers completed a visual analogue scale about their perceptions of breastfeeding support received from staff |
Statistical techniques Fisher exact probability test Breastfeeding/breastmilk-related outcomes Exclusive breastfeeding at discharge Cup: 4/6 Bottle: 6/8 Not statistically significant Clinical/health outcomes, process outcomes Not reported by randomised group Psychosocial outcomes The sample was redivided into two groups according to whether the support mothers perceived they had received was higher or lower than the group mean Successful breastfeeding was not found to be related to perceived level of support in this sample Cost-effectiveness outcomes Not reported |
Mothers of 16 eligible infants consented to take part in the study, and characteristics of 16 infants are reported, 8 in each group 2 infants were excluded because they were given supplementary feeds (not clear when) Results reported for 14 infants (Cup group 6, Bottle group 8) |
ITT unclear Normal hospital protocol in the study unit did not include clinical guidelines for initiation of oral feeding or implementation of supplementary feeding Authors emphasise that because of the small-scale nature of their work, the data must be treated with extreme caution Funding not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Rocha 2002 Brazil (São Paulo) Research aim To examine the impact of cup feeding, compared with bottle feeding, on subsequent breastfeeding in premature infants Study design Randomised controlled trial Method of group allocation Stratified by weight and randomly assigned by drawing lots Unit of allocation Infant Unit of analysis Group and individual Sample size calculation Not reported Outcome measures Breastfeeding rates Oxygen saturations Infant weight gain |
Selection Infants born at 32–36 weeks’ gestation weighing < 1700 g who were admitted to the NICU of the University Hospital of the Faculty of Medicine of Ribeirão Preto at the University of São Paulo, Brazil, between August 1998 and February 2000 Inclusion criteria Infant whose mother wished to breastfeed, without any condition that would prevent breastfeeding Singleton infant Clinically stable Did not require intensive care Receiving a feeding quota of 150 ml/kg/day via a gastric tube Exclusion criteria Infant initially received parenteral nutrition Infant developed disease that prevented feeding for > 3 consecutive days Infant with major malformation or severe neurological problem Mother did not wish to continue providing pumped milk Parent did not follow the randomly assigned regimen |
Reported for the 78 participants included in the analyses Cup (n = 44); bottle (n = 34) Mothers Mean ± SD maternal age (years): 24.8 ± 6.9; 25.8 ± 6.3 Incomplete primary schooling: 30 (68.1%); 21 (61.7%) Annual income between US$780 and US$3000: 34 (77.3%); 24 (69.7%) Primiparas: 20 (45.5%); 11 (32.4%) Breastfed other children: 16 (36.3%); 16 (47.1%) Infants Gestational age at birth, weeks, mean ± SD: 32.7 ± 1.8; 32.5 ± 2.0 Postconceptional age at initiation of oral feeds, in weeks, mean ± SD: 37.0 ± 1.6; 37.2 ± 2.2 Birthweight (g) mean ± SD: 1276 ± 283; 1262 ± 270 Small for gestational age: 22 (50%); 17 (50%) Males: 22 (54.5%); 10 (35.3%) Group comparability No significant differences were found between the groups for maternal or infant characteristics |
All mothers were provided with instruction and support in milk expression All infants were fed by orogastric tube until they reached 1600 g, when oral feeds commenced Breastfeeding was encouraged and prioritised; when the infant was not breastfed, the appropriate volume was offered via the assigned method Infants’ supplements were mother’s expressed milk, or if this was insufficient, premature infant formula At discharge, it was suggested to mothers that they use the allocated method if supplementing up to the first clinic visit I: n = 46 Infants were fed orally by cup Infant held slightly inclined; cup touching lower lip; infant allowed to lick or sip milk from the cup; milk not to be poured into the infant’s mouth Non-nutritive sucking was provided by offering the little finger instead of a nipple or pacifier C: n = 37 Infants were fed orally by bottle Data collection Infant data verified by researchers during daily visits to the ward and at clinic visits 5–15 days and 1, 2 and 3 months post-discharge Maternal data by interview and semistructured questionnaires |
Statistical techniques Fisher exact test, Student’s t test, non-parametric Wilcoxon test Breastfeeding/breastmilk-related outcomes Cup (n = 44); bottle (n = 34) Any breastfeeding at discharge 36 (81.8%); 27 (79.4%) Any breastfeeding at 5–15 days follow-up 19 (43.2%); 15 (44.1%) Any breastfeeding third month after discharge 13 (29.5%); 5 (14.7%) Cup (n = 19); bottle (n = 15) Maintaining breastfeeding third month after discharge 13 (68.4%); 5 (33.3%); p = 0.04 Clinical/health outcomes O2 saturations Oxygen saturations 1 week after commencing oral feeding Oxygen saturation (%) before feeding, mean ± SD (range): Cup: 97.1 ± 1.7 (92–100) Bottle: 97.4 ± 1.8 (93–100) Lowest oxygen saturation (%) during feeding, mean ± SD (range): Cup: 90.8 ± 4.8 (75–99) Bottle: 87.7 ± 7.6 (68–97) Number of infants with saturation < 90% during feeding: Cup: 18 (40.9%) vs bottle 19 (55.9%) Number of infants with saturation < 85% during feeding: Cup: 6 (13.6%) vs bottle 12 (35.3%), p = 0.02 Oxygen saturation (%) after feeding, mean ± SD (range): Cup: 96.2± 2.5 (91–100) Bottle: 96.3 ± 2.4 (89–100) No differences seen in mean values of lowest saturation levels or in proportion below 90% between the groups. When values of ≤ 85% were used as the cut-off point, the bottle group had significantly more desaturation episodes than the cup group (p = 0.02) Weight gain in first week Weight gain (g/kg/day) mean ± SD (range), one week after commencing oral feeding Cup: 14.1 ± 6.1 (0.9–25.3) Bottle: 14.7 ± 5.6 (3.0–28.2) Not statistically significant Process outcomes Feeding time (minutes) mean ± SD (range) Cup: 11.8 ± 4.5 (5–25) Bottle: 13.4 ± 4.8 (6–25) Not statistically significant Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
83 infants were randomised (46 to cup feeding and 37 to bottle feeding) A total of 5 (6%) were excluded Reasons for exclusion of 2 infants from the cup-feeding group were: Reasons for exclusion of 3 infants from the bottle feeding group were: |
Data were not analysed using intention-to-treat model Before the study, the entire nursing team was trained in the proper cup-feeding technique as part of a pilot project The cup used was the protective cap of the bottle. This cup was easy to sterilise and reuse, inexpensive and readily available Milk was offered by trained personnel who took care to avoid spillage; however, spillage was not measured Funding Not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Meier 2000 USA (Chicago) Research aim Did introduction of nipple shields, by advanced nurse practitioners working to research-based protocols, increase volume of milk consumed by premature infants during breastfeeding? Study design Non-randomised crossover study with retrospective analysis of data Method of group allocation Before/ after Unit of allocation Infant/ mother pair Unit of analysis Group Sample size calculation Not reported Outcome measures Milk transfer during the feeds immediately before and after introduction of the nipple shield Findings on duration of breastfeeding are presented but not by use vs non-use of nipple shield |
Selection Preterm infants hospitalised in two large teaching hospitals during a 12-month period 1997–8 Inclusion criteria Data from 34 infants and their mothers who participated and were randomised to the intervention group in either of two clinical trials of breastfeeding interventions for preterm infants The author provided extra information confirming that of all infants in the two studies that contributed data, only and all the 34 infants whose data were included in this analysis (Meier 2000) used nipple shields Exclusion criteria None |
Mothers Maternal characteristics not reported Infants (n = 34) Mean weeks [SD] gestational age at birth (range):31.9 [3.0] (25–37) Birthweight g [SD] (range): 1702 [521] (770–2820) Small for gestational age: 6/34 (17.6%) Multiple gestation: 14/34 (41.2%) Ethnicity: Weight g [SD] at first breastfeeding (range): 1782 [403] (1080–2820) Median (range) number of breastfeeding attempts prior to introduction of nipple shield: 4 (1–10) Reasons for introduction of the nipple shield: Group comparability Not applicable |
B: n = 34 The feed immediately before use of nipple shields A: (the same 34 infants acted as their own controls) The first feed using nipple shields Intervention Small, ultra-thin, silicone nipple shields Both trials that contributed data to this retrospective study used the same research-based study protocols Under these protocols, advanced practice nurses providing breastfeeding services introduced nipple shields, for specific indications that were recorded For all infants, shields were introduced when practitioners felt use of shields would increase volume of milk consumed by preterm infants during breastfeeding Data collection Data were collected from standardised forms used in the two trials For each infant, volume of intake was compared for two consecutive breastfeedings: Volume of intake for all breastfeedings had been measured by infant test-weights |
Statistical techniques Descriptive statistics, paired t tests, Pearson correlations Breastfeeding/breastmilk-related outcomes Milk transfer during the feeds immediately before and after introduction of the nipple shield All 34 infants consumed more when the shield was used Mean [SD]RangeMilk transfer during first feed with shield (ml)8.4 [13.2]2–62Milk transfer during feed immediately before first feed with shield (ml)3.9 [7.0]0–30Increase in milk transfer with shield (ml)14.4*[9.1]2–41 *t = 9.25, p = 0.0001, paired t test |
Mean [SD] | Range | Milk transfer during first feed with shield (ml) | 8.4 [13.2] | 2–62 | Milk transfer during feed immediately before first feed with shield (ml) | 3.9 [7.0] | 0–30 | Increase in milk transfer with shield (ml) | 14.4*[9.1] | 2–41 | None |
Cut-off for prematurity unclear (range of gestational age at birth includes 37 weeks) Funding National Institutes of Health Grant NR03881 and a research grant from Medela, Inc. (McHenry, III). This company also manufactures nipple shields and the authors acknowledge this as a potential conflicting interest |
|||
| Mean [SD] | Range | |||||||||||||||||||
| Milk transfer during first feed with shield (ml) | ||||||||||||||||||||
| 8.4 [13.2] | 2–62 | |||||||||||||||||||
| Milk transfer during feed immediately before first feed with shield (ml) | ||||||||||||||||||||
| 3.9 [7.0] | 0–30 | |||||||||||||||||||
| Increase in milk transfer with shield (ml) | ||||||||||||||||||||
| 14.4*[9.1] | 2–41 | |||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Fewtrell 2001 UK Research aim To test the hypothesis that the total amount of milk expressed by mothers who used a novel manual pump (MP) would be greater than that produced by mothers who used the standard electric pump (EP) Study design RCT Method of group allocation Permuted blocks of randomised length, stratified by infant’s sex and gestation Unit of allocation Mother Unit of analysis Group (mothers and infants) Sample size calculation 76 women per group to detect a 0.5 standard deviation difference in outcome measures between groups with 80% power at the 5% significance level, allowing for 12 (15%) dropouts per group Outcome measures Maternal milk volume (MMV) Subgroup comparisons of pattern of milk flow and creamatocrit Mothers’ views of randomised pump Mode of feeding at study discharge |
Selection NICU, Rosie Hospital, Cambridge, UK, between February 1998 and January 2000 Inclusion criteria Mothers of infants born at < 35 weeks’ gestation who decided to provide milk for their infant Mothers of infants transferred into the study unit were also eligible Exclusion criteria None reported |
Mothers MP: (n = 74); EP: (n = 71) Mean age (years) 29.7; 28.4 Social class 1+2 34%; 30% ‘A’ levels or higher professional qualification 34%; 33% Living with partner 95%; 93% Number of children breastfed: none: 75%; 75% one: 18%; 23% two: 7%; 3% Used breast pump previously 15%; 16% Singleton pregnancy 61 (82%); 64 (90%) Twin pregnancy 12 (16%); 7 (10%) Triplet pregnancy 1 (1%); 0 Infants Mean weeks [SD] gestational age 29.4 [3.0]; 29.1 [3.3] Mean g birthweight [SD] 1357 [540]; 1305 [565] Males 32 (53%); 35 (55%) Group comparability No significant differences found between the groups in maternal characteristics No significant differences found between the groups in proportion of infants who required supplemental oxygen or ventilation or in the duration of support required |
MP: n = 74 Women expressed breastmilk using a novel manual pump with petals that simulate the infant’s compressive action on the areola (Philips AVENT ISIS) EP: n = 71 Women expressed breastmilk using a standard electric breast pump (Egnell Ameda Elite™); mothers were encouraged to double pump, although they decided to double or sequential pump themselves All participants were recommended to express at least six times per day, initially for 5 minutes on each breast, then increasing the length of time per breast; information and help with the pump were provided by the midwifery staff on the postnatal ward, and by the nursing staff on the neonatal unit Data collection All mothers were recruited within 3 days of the birth and were asked to provide details of method, time and volume for each milk expression until they left the study The study had five endpoints: |
Statistical techniques Student’s t test, Mann–Whitney U test for non-parametric data; chi-squared test, Fisher’s exact test to compare proportions; repeated ANOVA for subgroup analyses Breastfeeding/breastmilk-related outcomes Mode of feeding at discharge: Infants receiving > 50% of their intake as breastmilk at study completion: MP 73%, EP 76% (NS) Singletons: MP 80%, EP 71% (0.3, NS) Breastmilk expression [median (25th, 75th centiles)] MP (n = 60); EP (n = 58); p Total number of expressions: 38 (15, 69); 4 (15, 74); 0.8 Mean expressions/day: 3.7 (3.0, 4.1); 3.9 (3.2, 4.4); 0.3 Total time spent expressing (minutes): 745 (289, 1321); 515 (231, 1069); 0.06 Mean time/day spent expressing: 65 (56, 85); 51(38, 63); < 0.001 Total volume expressed (ml): 1928 (322, 5408); 2062 (728, 5485); 0.6 Mean volume/day expressed: 199 (57, 323); 218 (119, 341); 0.5 Women in the MP group spent more time expressing milk per day than the EP group. No other significant differences were reported Subgroup analyses Thirty-seven mothers (23 MP and 14 EP, p = 0.12) who attempted to breastfeed their infant while participating in the study expressed significantly greater volumes of milk and spent more time expressing with a greater total number of sessions than women who did not attempt to breastfeed. Results for breastmilk expression were not different between MP and EP groups (see above) Forty-five mothers (MP 24, EP 21) consented to participate in an extra study and expressed milk sequentially for 20 minutes (10 minutes at each breast) Mean [SD] volume expressed in 20 minutes (ml) MP 112 [69]; EP 76 [44] Difference = 36 ml (95% CI: 2–71), p = 0.04 The authors concluded that when compared on equal terms (sequential pumping), mothers who used the MP showed greater milk volume during a fixed period Creamatocrit values Among the 45 mothers, creamatocrit values at 1-minute intervals, and mean creamatocrit during a 10-minute period were not significantly different in the breastmilk of women using the two types of pumps for either breast Clinical/health outcomes Maternal health related to the use of pump: 7% of women in both groups reported sore nipples Two mothers using the EP (2%) developed mastitis Process outcomes Median days in the study MP 16, EP 14 Psychosocial outcomes Mothers who used the MP reported greater ease of use, better comfort, and that the MP was pleasant to use; the overall opinion was that the MP was better than the EP Cost-effectiveness outcomes Costs (as reported by the author) EP = ‘hundreds of pounds’ MP = ‘less than £25’ |
145 mothers recruited (MP 74, EP 71) Data from 118 mothers (81.3%) were included in the analyses for the main outcomes 27/145 mothers (18.6%) failed to fill in the daily milk collection sheets: MP 14/74 (18.9%); EP 13/71(18.3%) MP mothers had a total of 88 infants, all of whom survived EP mothers had a total of 78 infants, 4 of whom died |
Available data were analysed both by randomised group and separately by use of randomised method Supported by a grant from Canon Avent (Glemsford, Suffolk, UK), which also provided the ISIS manual pumps |
| Study details | Participant selection and inclusion/ exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Groh-Wargo 1995 USA Research aim To test the hypothesis that use of a bilateral breast pumping system increases the volume of milk expressed in mothers of premature infants Study design Randomised controlled trial Method of group allocation Not reported Unit of allocation Mother Unit of analysis Group Sample size calculation Not reported Outcome measures Milk production per week Pumping sessions per week Time spent pumping per week Serum prolactin change |
Selection Mothers of infants in a 36-cot level III NICU in a large metropolitan hospital in the US Midwest Inclusion criteria Mothers breastfeeding infants: ≤ 1500 g at birth ≤ 7 days old To be included, a mother had to have pumped for at least 4 weeks (paper does not state whether or not any mothers were excluded on this criterion) Exclusion criteria Not reported |
Mothers Single: n = 16; bilateral: n = 16 Mean age (years) 28; 28 Years education n (%): 10–12: 9 (56); 8 (50) > 12: 7 (44); 8 (50) Marital status n (%): Married: 14 (88); 13 (82) Single: 2 (12); 2 (12) Divorced: –; 1 (6) Multiparous n (%) 7 (44); 7 (44) Breastfed one other child for any length of time n (%) 5 (31); 6 (38) Infants Infant characteristics not reported Group comparability No statistically significant differences found between the groups |
I: n = 16 Mothers used Medela (McHenry, IL) bilateral electric pump Protocol: pump both breasts simultaneously for a total of 20 minutes (during data collection the time to pump instruction was changed to ‘as long as there is any flow of milk’) C: n = 16 Mothers used Medela (McHenry, IL) single electric pump Protocol: pump for 10 minutes on each breast (during data collection the time to pump instruction was changed to ‘as long as there is any flow of milk’) All participants Instructed to: Requested to: In addition, all participants: Standard care Not described Data collection Maternal questionnaire for demographics Daily milk production log kept by mothers and submitted weekly for 6 weeks Weekly State-Trait Anxiety Inventory and serum prolactin levels |
Statistical techniques Weekly means for number of pumping sessions, pumping time and milk volume Fold increase pre–post one pumping session in each subject each week, averaged for each subject and each study group (fold increase defined as doubling the original value, e.g. one fold is a 100% increase) Student’s t test, Mann–Whitney rank sum test, Pearson’s product moment correlation, multiple regression Breastfeeding/breastmilk-related outcomes Milk production (ml/week) Single: n = 16; bilateral: n = 16 2685 ± 2016; 2787 ± 1939 Not statistically significant Authors note large standard deviations show milk production varied greatly from mother to mother Clinical/health outcomes Serum prolactin change: fold increase pre-to-post-pumping Single: n = 16; bilateral: n = 16 11.4 ± 11.9; 7.7± 7.6 Not statistically significant Process outcomes Single: n = 16; bilateral: n = 16 Pumping sessions per week 28.4 ± 5.5; 28.8 ± 5.5 Not statistically significant Minutes (hours) pumped per week 664.94 ± 188.1 (11.1 ± 3.1); 58.380± 176.8 (7.6 ± 3); p = 0.003 Psychosocial outcomes State Anxiety Inventory scores obtained weekly over the 4–6 weeks of the study but results not reported in the paper Cost-effectiveness outcomes Not reported |
None reported |
It is not clear whether any randomised mothers were excluded from the analysis Data were analysed by randomised group Funding from Medela, Inc. (McHenry, IL) and from Grant RR-00080 from National Institutes of Health, General Clinical Research Center at MetroHealth Medical Center |
| Study details | Participant selection and inclusion/ exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Hill 1999b (Hill 1999b112 and Hill 2001113 report aspects of this study) USA (Chicago, IL) Research aim To compare milk production in lactating mothers of non-nursing preterm infants who use either a sequential single (Seq) or simultaneous double (Sim) breast pumping regimen To examine the influence of selected variables on adequate versus inadequate milk production Study design Randomised controlled trial Method of group allocation States randomised, does not describe how Unit of allocation Individual (mother) Unit of analysis Group (milk weight) Sample size calculation Not reported Outcome measures Mean weekly milk weight (g) Pumping frequency Other outcomes not by randomised group |
Selection Lactating mothers of non-nursing preterm infants from two tertiary care centres Inclusion criteria Mothers English or Spanish speakers Accessible by telephone Non-smokers No history of thyroid/endocrine disorder Planning to mechanically express breast milk for ≥ 6 weeks after singleton or multiple birth Infants < 32 weeks’ gestation Very low birthweight (≤ 1500g) Not expected to feed at the breast for ≥ 6 weeks Exclusion criteria Not stated |
Reported for the 39 mothers who completed the study Mothers Seq (n = 20); Sim (n = 19) Mean age (years) ± SD 28.85 ± 3.94; 30.63 ± 5.13 Income < $50,000 17 (85.0%); 5 (26.3%) Mean years of education ± SD 14.70 ± 1.81; 14.52± 1.87 White 12 (60.0%); 17 (89.5%) Previously breastfed 7 (35.0%); 2 (10.5%) Multiple births 7 sets of twins and 2 sets of triplets, not reported by mother’s randomised group Infants Mean gestational age (weeks) ± SD: Seq: 27.52 ± 2.10 Sim: 28.08 ± 1.81 Mean birthweight (g) ± SD: Seq: 1065.83 ± 261.14 Sim: 1050.04 ± 253.73 Group comparability The only statistically significant difference found between the groups was in income. More Seq group mothers reported income < $50,000 (paper reports p = 0.000) |
All participants After consent, received detailed instructions, and were tested by researchers, on how to use the Medela Lactina® (McHenry, IL) electric breast pump, milk collection, storage and transport procedures Hill 2001 adds that mothers were instructed in techniques helpful for eliciting the milk ejection reflex, such as use of warm moist breast compresses, breast massage, looking at a picture of their infant, or inhaling their infant’s scent from clothing or a blanket Protocol for both groups was to pump eight times per day Seq: sequential (single) pumping (reported for 20 mothers) Pump a minimum of 5 minutes then switch to the other side (when the milk stops spraying or dripping regularly); repeat, so that each breast is pumped 10 minutes minimum Sim: simultaneous (double) pumping (reported for 19 mothers) Pump a minimum of 10 minutes or until one breast no longer dripping Standard care Not described; however, similar amounts of kangaroo care were reported for mothers in both study groups Data collection Mothers kept a log recording day and time of each milk expression for 6 weeks Mothers brought expressed milk to the unit in labelled bags Researchers weighed milk bags Paper states ‘the weight in grams is nearly equivalent to the volume of milk in millilitres and is a more precise method of determining the amount produced’ |
Statistical techniques Descriptive statistics; repeated measures of ANOVA for milk weight; Spearman’s rho for adequate/inadequate milk production Breastfeeding/breastmilk-related outcomes No statistically significant difference found between Seq vs Sim groups in mean weekly milk weight (weeks 2–5) p = 0.164 Clinical/health outcomes Not reported Process outcomes Pumping frequency per week (weeks 2–5): Seq: 40.18 ± 8.77 Sim: 42.87 ± 9.75, p = 0.370 Psychosocial outcomes Some mothers preferred the single pumping method and others the double Cost-effectiveness outcomes Not reported Researchers conclude clinicians may encourage mothers to choose either pumping method Other results are reported but not by randomised group: |
Forty-nine mothers consented to participate and were randomised Ten withdrew (20.4%), not reported by group Reasons: |
Available data were analysed by randomised group Hill 1999b112 used ANOVA and found mothers with previous breastfeeding experience had significantly greater milk yield than those without Hill 2001113 used ANOVA and found milk weight in mothers with low frequency of pumping appeared to be positively influenced by initiating pumping soon after the birth Supported by the University of Illinois at Chicago, College of Nursing, Internal Research Support Program, Chicago, IL; National Institutes of Health, National Institute of Nursing Research, 1 R55 NR04118-01A1; and Medela, Inc. |
| Study details | Participant selection and inclusion/ exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Jones 2001 UK Research aims Study design Method of group allocation Randomised centrally into six groups (three for gestational age and two for parity) Unit of allocation Mother Unit of analysis Group Sample size calculation 39 in each arm for 80% power and 5% significance based on an improvement in milk yield of 20–50%. Interim analysis of 36 complete data sets showed highly significant results and recruitment was discontinued Outcome measures Milk weight Milk fat Mothers’ views Breastfeeding duration |
Selection Mothers of infants being cared for in the Neonatal Unit of North Staffordshire Maternity Hospital October 1997–August 1999 Inclusion criteria Mothers wishing to express breastmilk Exclusion criteria Mothers unable to express ≥ 5 times per day before the start of the study Mothers with retained products of conception Mothers living outside the study hospital’s area expecting to return to their local hospital before their infant reached term |
Mothers Age and socioeconomic status not reported I1 (Sim): n = 16; C1 (Seq): n = 17 First pregnancy 5/16; 7/17 Breastfed previously 8/16; 6/17 Multiple births (twins) 3 sets; 3 sets Infants Mean weeks gestational age 29.9; 30.2 Mean kg birthweight 1.46; 1.61 Group comparability Not reported |
Standard care Study unit had active breastfeeding policy All mothers provided with information leaflet on milk expression technique All mothers had opportunity to view a video made by the researchers covering milk expression and preterm breastfeeding All participants Egnell Ameda Electric Elite™ pump (Egnell Ameda, Taunton, Somerset, UK) with silastic shield inserts, were loaned for the length of the trial Mothers were encouraged to express × 8 per day until milk no longer entered the collection set I1: n = 25 randomised to simultaneous pumping Mothers were asked to use the study pump in simultaneous pumping mode (both breasts at once) C1: n = 27 randomised to sequential pumping Mothers were asked to use the study pump in sequential mode When milk from the first breast no longer entered the collection set, mothers were asked to switch breasts I2: Breast massage prior to pumping (all participants on two of the four study days) Gentle tactile stimulation of mammary and nipple tissue using a hand action that rolled the knuckles downwards over the breast, beginning at the ribs and working towards the areola This technique did not involve manual expression of milk C2: No breast massage prior to pumping (all participants on two of the four study days) Data collection The study took place during two 48-hour periods (total four days), starting on day 4 or when engorgement had been relieved Mothers were randomised to massage on either days 1 and 2 or days 3 and 4 of the study On study days 1 and 3, mothers familiarised themselves with breast massage and data collection Data were collected on study days 2 and 4 only Mothers logged date, time and duration of each expression, and weight of collection bottles before expression Researchers logged weight of collection bottles after expression Capillary sample from each milk sample for creamatocrit test Two questionnaires to mothers at trial completion, and mothers were asked about feeding method at 37 weeks postconception (term) |
Statistical techniques Repeated measures ANOVA on mean milk volume and mean fat content between days 2 and 4 (one day of massage and one day of non-massage for each woman) The means were calculated by the total daily volume/number of expressions that day. Both groups had a mean of 5.2 expressions per day. Results from 36 mothers with complete data Breastfeeding/breastmilk-related outcomes Fully breastfeeding or expressing milk at term (37 weeks) I1 (Sim): 1/17 did not establish breastfeeding C1 (Seq): 15/19 fully breastfeeding or expressing Weeks expressing/breastfeeding until term ranged from 5 to 13 weeks The authors state that differences in milk yield per expression were significant at the p < 0.01 level between sequential and simultaneous pumping, and between massage and non-massage. Fat concentration was similar in all groups. Total fat was significantly different between simultaneous pumping and sequential pumping (p < 0.01), but not for massage vs no-massage Mean (95% CI) breastmilk weight (g) Seq (non-massage): 51 (46–56) Seq (massage): 79 (73–85) Sim (non-massage): 88 (79–97) Sim (massage): 125 (110–140) Mean (95% CI) fat concentration (g/l) Seq (non-massage): 7.1 (5.9–8.3) Seq (massage): 6.7 (5.7–7.7) Sim (non-massage): 7.0 (5.9–8.1) Sim (massage): 6.8 (5.7–7.9) Mean (95% CI) total fat (g) Seq (non-massage): 3.1 (2.7–3.5) Seq (massage): 4.2 (3.8–4.6) Sim (non-massage): 6.0 (5.3–6.7) Sim (massage): 8.0 (6.9–9.1) Clinical/health outcomes Not reported Process outcomes 30/36 mothers began the study on postpartum day 5 and 6/36 on day 7 Simultaneous pumping took mothers 10–15 minutes compared with 25–40 minutes for sequential pumping Psychosocial outcomes Women appreciated massage and simultaneous pumping to expedite milk flow, and voiced a need for larger milk collection sets Cost-effectiveness outcomes Not reported |
52 randomised (27 Seq, 25 Sim) 16/52 lost (31%) Missing from Seq 27 – 19 = 8 Missing from Sim 25 – 17 = 8 Reasons given, but not by group: |
Available data were analysed according to randomised group for the comparison between simultaneous and sequential pumping The authors concluded that simultaneous pumping produces more milk than sequential pumping. Massage has an additive effect, improving milk production in both groups The author provided additional information about method of randomisation Funded by Baby Lifeline |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Paul 1996 India (New Delhi) Research aim To compare hand expression of breastmilk with expression using a hand-held cylindrical pump (Medela) for output of breastmilk and mothers’ views Study design Randomised crossover trial with two phases Method of group allocation Randomised (method not stated) Unit of allocation Mother Unit of analysis Milk expression session Sample size calculation Not reported Outcome measures Mean output per session Mothers’ preferences for hand or pump expression |
Selection Mothers of infants in NICU; infants unable to breastfeed and receiving expressed breastmilk by gavage tube Inclusion criteria Well and comfortable enough to come to the nursery feeding room Exclusion criteria Cracked/sore nipples |
Mothers Not reported Infants Phase 1 (22 mothers) Mean gestation 34.3 weeks (range 27–40 weeks) Phase 2 (14 mothers) Mean gestation 33.5 weeks (range 27–37 weeks) Number of infants not reported Group comparability Not applicable within each phase (crossover trials) Not reported between phase 1 and phase 2 |
Phase 1 (22 mothers) Expressed milk for 15 minutes at 10.00, 12.00 and 14.00 on postnatal days 4 and 5 Each mother alternated hand expression and pump methods Eleven mothers were randomised to begin with hand expression and 11 with pumping Mothers were advised to allocate equal time to expressing each breast at each session Method of hand expression not described The pump used (Medela) was cylindrical, made of plastic and hand-operated. By in-and-out motion with one hand, a piston mechanism produced suction at a breast cup placed around the areola. Vacuum-sucked breast milk flowed into a collecting bottle Phase 2 (14 mothers, not included in phase 1) Expressed milk for 15 minutes at 10.00, 12.00 and 14.00 on postnatal days 4 and 5 and on postnatal days 8 and 9 Each mother alternated hand expression and pump methods, following the same procedure as in phase 1 Data collection Milk volume was measured at the end of each 15-minute session to the nearest ml using a syringe Mothers were questioned about their preferences at the end of the sixth session (tool not described) |
Statistical techniques Descriptive statistics, Student’s t test Breastfeeding/breastmilk-related outcomes Phase 1 (n = 22) days 4 and 5 Expression by: HandPumpNo. sessions6666Total ml14302744Mean per session21.741.57SD10.5416.09 p < 0.001 Phase 2 (n = 14) days 3 and 4 Expression by: HandPumpNo. sessions4242Mean per session31.246.8SD15.526.3 p < 0.01 Phase 2 (n = 14) days 8 and 9 Expression by: HandPumpNo. sessions4242Mean per session38.450.4SD11.213.4 p < 0.01 Clinical/health outcomes Not reported Process outcomes Not reported Psychosocial outcomes Days 4 and 5: Phase 1 (n = 22)Phase 2 (n = 14)Prefer pump1914Prefer hand20Not sure10 Pump preferred (days 4 and 5) because it provided relief from engorgement Days 8 and 9: Phase 2 (n = 14) Prefer pump: 1 Prefer hand: 13 Hand expression preferred (days 8 and 9) because it was more gentle and convenient Cost-effectiveness outcomes Not reported |
Hand | Pump | No. sessions | 66 | 66 | Total ml | 1430 | 2744 | Mean per session | 21.7 | 41.57 | SD | 10.54 | 16.09 | Hand | Pump | No. sessions | 42 | 42 | Mean per session | 31.2 | 46.8 | SD | 15.5 | 26.3 | Hand | Pump | No. sessions | 42 | 42 | Mean per session | 38.4 | 50.4 | SD | 11.2 | 13.4 | Phase 1 (n = 22) | Phase 2 (n = 14) | Prefer pump | 19 | 14 | Prefer hand | 2 | 0 | Not sure | 1 | 0 | None reported |
Data were analysed using intention-to-treat model Numbers of sessions reported (66 × 2 in phase 1 and 42 × 4 in phase 2) were complete Author notes at the time of the study the only breast pump manufactured in India was unsuitable for milk collection and states ‘there is a genuine need for a relatively inexpensive, indigenous breast pump’ |
||||
| Hand | Pump | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. sessions | 66 | 66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total ml | 1430 | 2744 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean per session | 21.7 | 41.57 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD | 10.54 | 16.09 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hand | Pump | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. sessions | 42 | 42 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean per session | 31.2 | 46.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD | 15.5 | 26.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hand | Pump | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. sessions | 42 | 42 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean per session | 38.4 | 50.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD | 11.2 | 13.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase 1 (n = 22) | Phase 2 (n = 14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prefer pump | 19 | 14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prefer hand | 2 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not sure | 1 | 0 |
| Study details | Participant selection and inclusion/ exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Slusher 2007 Kenya and Nigeria Research aim To compare mean daily maternal milk volume (MMV) using an electric breast pump, non-electric pedal breast pump, or hand (manual) expression for mothers of premature or sick infants in special care nurseries Study design Randomised controlled trial Method of group allocation Random numbers table Unit of allocation Mother Unit of analysis Group Sample size calculation Not reported, convenience sample Outcome measures Mean daily breastmilk volumes |
Selection Special care nurseries at Jos University Teaching Hospital in Jos, Nigeria, and Tenwek Mission Hospital, in Bomet, Kenya Inclusion criteria Mothers of premature or sick infants being cared for in the study units, who were unable to breastfeed Physician expected infant to be unable to breastfeed for at least 1 week Exclusion criteria Not reported |
Mothers Maternal age (years) mean [range] A: 25 [17–38] B: 24 [16–38] C: 28 [17–40] First pregnancy A: 38% B: 38%, C: 44% Socioeconomic status, multiple births and breastfeeding experience not reported Infants Birthweight (g) mean [range] A: 1651 [907–3134] B: 1606 [850–3400] C: 1871 [1049–4600] Infant gestation (weeks) mean [range] A: 31.4 [27–38] B: 31.7 [26–40] C: 33.9 [28–40] Group comparability No significant differences found between the groups for variables reported |
Intervention A (n = 22) Women expressed breastmilk by double collection Lactina electric breast pump Mothers were instructed to use the electric pump at 2–3-hour intervals Intervention B (n = 24) Women expressed breastmilk by double collection non-electric pedal pump Control (n = 19) Women expressed breastmilk by hand expression (the clinical standard in both settings) All participants were taught: Data collection Breastmilk volumes were measured to the nearest ml and recorded at each pumping session for 10 days. Mothers contributed data for a mean of 8.7 days. All but one mother began the study within 2 days of the birth |
Statistical techniques Descriptive statistics, one-way ANOVA, Tukey HSD test Breastfeeding/breastmilk-related outcomes Mean breastmilk volume during the study: Intervention A group (electric pump): 578 ± 228 ml Intervention B (non-electric pedal pump): 463 ± 302 ml Control group (hand expression): 323 ± 199 ml The Intervention A group (electric pump) result was significantly greater than the control group result (p < 0.01) There was no significant difference between the Intervention A group and the Intervention B group results There was also no significant difference between the Intervention B group and the control group results Clinical/health outcomes Not reported Process outcomes Not reported Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
103 mothers were randomised at birth 38 withdrew (not reported by group) Infants of 26 mothers died Infants of 5 mothers were ready to feed directly from the breast within the first week of life 7 mothers withdrew (reasons not stated) 65 mothers were included in the analysis |
Available data were analysed by randomised group Funding: not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
da Silva 2001 Canada Research aim To evaluate the effectiveness of domperidone in augmenting milk production in mothers of premature infants Study design RCT Method of group allocation Random numbers table Unit of allocation Mother Unit of analysis Group Sample size calculation 20 subjects to demonstrate an increase ≥ 25% in milk production between the groups with 80% power and α = 0.05 Outcome measures Milk volume Domperidone concentrations and side effects Serum domperidone and prolactin concentrations |
Selection Mothers of infants admitted to the NICU at St Joseph’s Health Care, London, Ontario Inclusion criteria Milk production not meeting the infant’s daily oral feeding requirements Mothers using an electric breast pump with double collection kit Mothers continuing to have problems with lactation after extensive teaching by lactation consultants Exclusion criteria Mothers receiving medication known to affect serum prolactin levels Mothers with any chronic or debilitating illness |
Mothers Mean [SD] maternal age (years) I: 28.2 [5.0] C: 27.9 [6.6] SES: not reported First pregnancy I: 3/7 C: 3/9 Breastfed previously I: 1/7 C: 3/9 Multiple births I: two sets of triplets and one set of twins C: one set of multiples Mean [SD] number of days between delivery and study entry I: 31.9 [10.5] C: 33.1 [22.9] Infants Mean weeks [SD] gestational age I: 29.1 [2.0] C: 29.1 [3.7] Birthweight: not reported Group comparability No significant differences found between the groups |
Domperidone tablets were crushed and mixed with lactose. The resulting white powder was placed in clear capsules. Placebo was plain lactose powder in the same type of capsule I: n = 11 Domperidone 10 mg orally, three times per day for 7 days C: n = 9 Placebo Standard care Extensive teaching by lactation consultants for all women failing lactation (content of teaching not described) No other components of standard care are described Data collection Milk volumes for 7 days Domperidone concentrations in milk on day 5 Serum domperidone and prolactin before the initial dose and on days 5 and 10 Mothers were asked to report side effects |
Statistical techniques Chi-squared test for categorical data, Student’s t test for continuous data, Wilcoxon rank sum test for increase in milk volumes from baseline Breastfeeding/breastmilk-related outcomes Mean ml [SD] milk at baseline Domperidone (n = 7); placebo (n = 9) 112.8 [128.7]; 48.2 [63.3] Milk volume data for the 24 hours before the trial were not available for 1/7 of the intervention group and 3/9 controls Milk volume data for the 24 hours following enrolment were used in these cases Length of time between enrolment and start of the trial not reported Mean ml [SD] milk days 2–7 Domperidone (n = 7); placebo (n = 9) 162.2 [127.5]; 56.1 [48.0] Mean ml [SD] increase in milk volume between baseline and days 2–7 Domperidone (n = 7); placebo (n = 9) 49.5[29.4]; 8.0[39.5]; p = < 0.05 Proportion of infants discharged home breastfeeding not stated, but reported not to differ between the groups Clinical/health outcomes Mean [SD] serum prolactin at baseline (μg/l) I: 12.9 [7.7] vs C: 15.6 [17] p = 0.71 (NS) Mean [SD] serum prolactin on day 5 (μg/l) I: 119.3 [97.3] vs C: 18.1 [14.7] p = 0.008 Mean [SD] serum prolactin on day 10 (μg/l) (3 days after last dose) I: 12.1 [5.1] vs C: 16.5 [5.2] p = 0.11 (NS) Domperidone in milk on day 5 Milk:serum ratio 0.4 (authors state this is relatively low and much lower than metoclopramide) No side effects detected for mothers or infants Process outcomes One mother assigned to domperidone stopped taking it on day 4 because she started to breastfeed successfully Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
Withdrawals I: 4/11 withdrew from the Domperidone group Reasons: C: no withdrawals from the control group |
Available data were analysed by randomised group Compliance was monitored by capsule count Funded by a grant from the Research and Education Foundation of the Canadian Society of Hospital Pharmacists |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Feher 1989 USA Research aim To evaluate the effect of relaxation/imagery techniques on milk volume and fat content of milk expressed by mothers of hospitalised premature infants and to improve the mothers’ lactation experience Study design RCT Method of group allocation Random numbers table (open allocation) Unit of allocation Mother Unit of analysis Group Sample size calculation Authors decided a 20% increase in milk volume would be clinically significant and state they calculated the sample size accordingly. No details of the calculation are reported Outcome measures Milk volume Cream content Mothers’ views of the tape |
Selection Breastfeeding mothers of preterm infants in two units: Inclusion criteria Mothers of infants expected to be in the unit for at least 10 days Exclusion criteria Not reported |
Mothers Reported for those who completed the study I (n = 30); C (n = 25) Mean [SD] age in years 24.5 [6.2]; 26.8 [6.2] Mean [SD] years of education 13.0 [2.0]; 14.0 [1.9] Married 80%; 76% Family income < $10,000: 47%; 40% $11,000–20,000: 10%; 36% $21,000–30,000: 20%; 8% > $30,000: 23%; 16% First pregnancy 43%; 68% Breastfed previously: not reported Multiple births: not reported Infants Mean gestational age in weeks (SD not reported) 29.9; 27.6 Birthweight: not reported Eight infants in both groups were receiving mechanical ventilation at the time of milk collection Group comparability No statistically significant differences were found between the groups |
Mothers were assigned 3–5 days postpartum I: n = 38 Mothers received a 20-minute audio cassette tape Tape consisted of a progressive relaxation exercise (to alternately tense and relax muscle groups while taking deep, rhythmic breaths) followed by a guided imagery section (including descriptions of pleasant surroundings, milk flowing in the breasts, and the baby’s warm skin against the mother) Mothers were recommended to use the tape every time they wanted to express milk If necessary, a tape player was loaned to the mother C: n = 33 Routine supportive care from the nursing and medical staff, which included verbal and written instructions on how to use the electric breast pump Data collection Volume and fat content of a single expression of breastmilk obtained at the hospital 4–13 days after enrolment |
Statistical techniques Chi-squared analysis, t tests and analysis of variance Breastfeeding/breastmilk-related outcomes I (n = 30); C (n = 25) Mean ± SD milk volume (ml ) 90.1 ± 60.0; 55.4 ± 48.2; p < 0.05 Creamatocrit (%) 7.2 ± 2.9; 6.8 ± 2.4 Not statistically significant Clinical/health outcomes Not reported Process outcomes I (n = 30); C (n = 25) Mean expression time (days) 7.8; 8.1 Compliance 15/30 intervention group mothers (50%) had listened to the tape more than five times before providing the sample Authors report a dose–response relationship between milk volume and reported tape listening: Number of times mother reported listening to tape0–45–9≥ 10Number of mothers10109Mean volume expressed milk (ml)57.168.7112.7 Psychosocial outcomes Mothers’ views of the tape ‘Many positive responses and no strong negative reactions’ Examples of positive responses: Cost-effectiveness outcomes Not reported |
Number of times mother reported listening to tape | 0–4 | 5–9 | ≥ 10 | Number of mothers | 10 | 10 | 9 | Mean volume expressed milk (ml) | 57.1 | 68.7 | 112.7 |
71 mothers randomised, 38 to I group and 33 to C group Data from 55 mothers analysed: 30 in I group (79%) and 25 in C group (76%) 16 lost to follow-up, 8 from each group Reasons: |
Available data were analysed by randomised group The author provided additional information about method of randomisation Partial funding from University of New Mexico School of Medicine, General Clinical Research Center, NIH grant RR-00997-10, 11 |
||||||
| Number of times mother reported listening to tape | ||||||||||||||||||||||||
| 0–4 | 5–9 | ≥ 10 | ||||||||||||||||||||||
| Number of mothers | ||||||||||||||||||||||||
| 10 | 10 | 9 | ||||||||||||||||||||||
| Mean volume expressed milk (ml) | ||||||||||||||||||||||||
| 57.1 | 68.7 | 112.7 | ||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Fewtrell 2006 UK Research aim To test the hypothesis that oxytocin nasal spray increases early milk output in mothers expressing milk for preterm infants Study design RCT Method of group allocation Sequence drawn up by clinical trials company in permuted blocks of randomised length, stratified by parity and gestation. Identical spray containers prepared and numbered by the company with the appropriate number in the sequence Unit of allocation Mother Unit of analysis Mother and group Sample size calculation 16 in each group to detect a 5% difference with 80% power (based on previous published work) Outcome measures Milk volume Number of pumping sessions Milk volume and fat content over 20 minutes on day 5 (subgroup) Mothers’ opinions of milk expression and spray used |
Selection Mothers who gave birth at the Elizabeth Garrett Anderson Hospital, UCLH, London, between March 2003 and April 2004 Inclusion criteria Infant < 35 weeks’ gestation Exclusion criteria None stated |
Mothers I (n = 27); C ( n = 24) Mean [SD] age in years 31.5 [5.5]; 30.8 [6.7] Degree/higher professional qualification 18 (67%); 14 (58%) Married/with partner 26 (96%); 21 (87%) White/Asian 18 (67%); 16 (67%) Black 9 (33%); 8 (33%) Parity: not reported Previous breastfeeding experience 5 (19%); 5 (21%) Previous pumping experience 6 (22%); 3 (13%) Multiple birth 4 (15%); 5 (21%) Infants Mean weeks [SD] gestational age 29.9 [2.8]; 29.0 [3.7] Mean g birthweight [SD] 1380 [604]; 1315 [603] Group comparability No significant differences between the groups were found |
All participants were advised to express milk at least every 3 hours and instructed in the use of hand massage before pumping All participants used the Egnell Ameda Elite pump, generally in single mode Advice was given by staff in postnatal and neonatal wards and by a research nurse, who saw mothers daily and was available for telephone contact at all times Mothers were advised to use their spray once (100 µl) 2–5 minutes prior to expressing from each breast I: n = 27 Syntocinon nasal spray 40 IU synthetic oxytocin per ml: total 5 ml C: n = 24 Placebo spray sterile normal saline plus benzalkonium chloride Data collection Records of start and finish time and weight of milk at each expression were completed by mothers Mothers were shown how to use the scales and complete the records |
Statistical techniques Chi-squared tests, repeated ANOVA. Milk weight data were skewed therefore transformed to natural logarithms Breastfeeding/breastmilk-related outcomes Total milk production was found not to differ between the groups Oxytocin group median 667 g (25th, 75th centiles 206, 1203) vs placebo group 530 g (394, 778); p = 0.9 Significantly different patterns of milk production were found between the groups, with more milk in the oxytocin group over the first 2 days (p = 0.001), then placebo matched and by day 5 exceeded them In the physiological studies of the subgroup of mothers, no significant differences were found in milk weight or fat content Clinical/health outcomes No adverse effects were recorded Process outcomes Mean [SD] pumping sessions per day, days 1–5 Oxytocin 3.4 [0.8] Placebo 3.6 [0.9] p = 0.4 Mean minutes/day expressing milk, days 1–5 Oxytocin 84 (24) vs placebo 95 (29), p = 0.14 Psychosocial outcomes Mothers’ opinions of milk expression and spray used No significant differences in ratings found between the groups Cost-effectiveness outcomes Not reported |
51 mothers randomised, 27 to I group 24 to C group Individual data for the five study days are presented for 25/27 intervention group participants and 23/24 control group participants Complete 5-day milk recordsICTotaln = 27n = 24N = 51212142 (82%)Incomplete 5-day milk records639 (18%) Reasons: Critically ill mother I: 1 (all 5 days); C: 1 (days 4 and 5) Mother did not complete any records I: 1; C: 1 Mother did not express on one or more days I: 2; C: 1 Mother ran out of spray I: 2; C: 0 |
Complete 5-day milk records | I | C | Total | n = 27 | n = 24 | N = 51 | 21 | 21 | 42 (82%) | Incomplete 5-day milk records | 6 | 3 | 9 (18%) |
Available data were analysed by randomised group The author provided additional information about method of randomisation Funded by MRC Programme grant |
||||
| Complete 5-day milk records | ||||||||||||||||||||||||
| I | C | Total | ||||||||||||||||||||||
| n = 27 | n = 24 | N = 51 | ||||||||||||||||||||||
| 21 | 21 | 42 (82%) | ||||||||||||||||||||||
| Incomplete 5-day milk records | ||||||||||||||||||||||||
| 6 | 3 | 9 (18%) | ||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Gunn 1996 New Zealand Research aim To determine the galactopoietic response to recombinant human growth hormone (hGH) in mothers of premature infants with inadequate lactation Study design RCT Method of group allocation Medication was prerandomised and issued in sequentially numbered packets Unit of allocation Mother Unit of analysis Group Sample size calculation Not reported Outcome measures Milk production Plasma concentrations of insulin-like growth factor-1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3) and growth hormone |
Selection Mothers of infants born at 26–34 weeks’ gestation; infants otherwise healthy and being cared for in the Special Care Baby Unit (SCBU) Inclusion criteria Healthy mothers Not producing enough milk to supply their infants’ nutritional needs Receiving standard management to promote and maintain lactation Exclusion criteria Any medication Any known contraindication to hGH therapy |
Mothers Mean [SD] maternal age (years) I: 32.4 [3.6] vs C: 35.7 [4.6] SES: not stated First pregnancy: 15/18 Breastfed previously: not stated Multiple births: not stated Number [SD] days between delivery and study entry I: 39.7 [32] vs C: 31.3 [18.9] Infants Mean [SD] weeks gestational age I: 30.6 [3.2] vs C: 30.1 [3.2] Birthweight g [SD] I: 1398 [397] vs C: 1239 [552] Weight g [SD] of infants when the study began I: 2206 [455] vs C: 1576 [661] p < 0.05 Group comparability Infants in the hGH group were heavier when their mothers began the study |
Standard care Before the start of the study, all mothers had been shown how to express and were expressing from both breasts 5–6 times daily using electric pumps available in the hospital Most mothers also hired a pump to use at home I: n = 10 hGH 0.2 IU/kg/day subcutaneously to a maximum of 16 IU/day, for 7 days C: n = 10 Placebo Data collection Maternal venous blood samples on days 0 and 8 Milk volumes for days 0–1 (48 hours) and day 8 (24 hours), as the sum of milk expressed plus any suckled by the infant as determined by weighing |
Statistical techniques Paired and unpaired Student’s t tests (Bonferroni method) to compare groups Fisher’s exact test to test incidences Linear regression for relationship between milk production and hormone levels Breastfeeding/breastmilk-related outcomes Mean milk volume in ml/day [SD] Days 0–1Day 8pI: 139 [49]175 [46]< 0.01C: 93 [50]102 [69]NS Milk production increased in all hGH-treated mothers and in 4/9 placebo-treated mothers (p < 0.04) Clinical/health outcomes Plasma concentrations of both IGF-1 and IGFBP-3 rose after hGH therapy, compared with both baseline levels (p < 0.01) and with placebo levels (p < 0.01) The increases in plasma IGF-1 and IGFBP-3 were highly correlated (r = 0.8, p < 0.001) Plasma concentrations of growth hormone, measured 24 hours after the last hGH injection, did not change significantly after hGH therapy or placebo No significant correlations reported from linear regression analyses of relationships between milk production and hormone levels No adverse effects with hGH treatment were seen in mothers or infants Process outcomes Not reported Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
Days 0–1 | Day 8 | p | I: 139 [49] | 175 [46] | < 0.01 | C: 93 [50] | 102 [69] | NS | Two withdrawals:
|
Available data appear to have been analysed by randomised group No confidence intervals Study supported in part by the Health Research Council of New Zealand and Pharmacia AB, Stockholm, Sweden |
| Days 0–1 | Day 8 | p | |||||||||||||
| I: 139 [49] | 175 [46] | < 0.01 | |||||||||||||
| C: 93 [50] | 102 [69] | NS |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hansen 2005 USA (Iowa) Research aim To investigate the effect of metoclopramide on breastmilk volume and duration of breastfeeding in women delivering preterm Study design RCT Method of group allocation Randomised using computer-generated random numbers Stratified by gestation Unit of allocation Mother Unit of analysis Group Sample size calculation Based on a report240 of preterm milk volume 93 ± 19 ml before and 197 ± 32 ml after metoclopramide treatment, assuming a standard deviation [SD] of 32 ml, 30 in each group needed for the statistical test (sic), applying the Bonferroni correction, to detect a difference of at least 32 ml per day at any of the 17 days of the study period at the 0.05 significance level with 0.80 power Outcome measures Milk volumes Time mothers spent expressing Duration of breastfeeding Metoclopramide levels in milk |
Selection Mothers of infants born at 23–34 weeks’ gestation and being cared for at the University of Iowa Hospitals and Clinics Inclusion criteria Mothers planned to breastfeed Exclusion criteria Mothers using medication contraindicating breastfeeding or metoclopramide use HIV-positive mothers Infants with a congenital abnormality |
Reported for I: 31/34 (91%) of mothers randomised C: 29/35 (83%) of mothers randomised Mothers Median (25–75th percentiles) age (years) I: 28 (23–33) C: 25 (23–33) White % (n) I: 83.87 (26) C: 89.66 (26) Married I: 87.10 (27) C: 65.52 (19) Partner in white-collar occupation % (n) I: 51.85 (14) C: 30.77 (8) Preterm birth(s) prior to this one % (n) I: 25.80 (8) C: 44.82 (13) Multiple birth this time % (n) I: 12.90 (4) C: 24.14 (7) Infants Median weeks (25–75th percentiles) gestational age I: 28.1 (25.1–32.6) C: 28.0 (26.0–30.3) Birthweight: not reported Group comparability No significant differences in baseline characteristics found between the groups |
I: n = 34 10 mg metoclopramide tablet 3 times a day for 10 days C: n = 35 10 mg placebo tablet 3 times a day for 10 days Both groups Medication was started within 96 hours (4 days) of the birth A trained lactation specialist standardised all educational material (not described) given to mothers when they entered the study All study mothers were seen by a trained nurse at 3–7 days postpartum to answer questions and offer support (not described) All study mothers were provided with a Medela Classic® electric pump (Medela Inc., McHenry, IL) free of charge Standard care Not described Data collection Mothers’ journals, two verifications of milk volumes for 17 days (10 days of tablets and the 7 following days) Two milk aliquots from a subsample of mothers at days 10 ± 4 and 17 ± 5, for assessment of metoclopramide levels and fat and protein content |
Statistical techniques Fisher exact test and Wilcoxon rank-sum test for demographics Linear mixed-model analysis for repeated measures in comparison of milk volumes and between groups Breastfeeding/breastmilk-related outcomes Milk volumes Metoclopramide use was not associated with a significant increase in milk volume compared with placebo on any of the 17 days of the study No significant change in volume of milk produced in 24 hours between day 10 (last day of tablets) and day 17 was found between the groups Mean 24-hour milk volume (ml) Metoclopramide (n = 25–28)Placebo (n = 29)Day 10519 ± 60519 ± 60Day 17459 ± 63497 ± 64 Subgroup analysis by gestation (23–28 weeks and 28–34 weeks) also did not show any significant effect of metoclopramide use on milk volumes Breastfeeding duration: median weeks (IQR) I: 8.8 (3.4 to 12.0) C: 8.6 (5.6 to 16.9) Clinical/health outcomes Mean metoclopramide level found in milk from 14 I-group mothers was 44.8 ± 20.4 ng/ml, stated to be similar to levels found in studies of term subjects. Authors calculated maximum exposure to metoclopramide would be about 3% of the recommended daily dosage for children Process outcomes 3/17 milk aliquots from I-group mothers had immeasurable drug levels, suggesting that some mothers did not comply with treatment Psychosocial outcomes The nine participants who stopped breastfeeding in the first week cited a variety of reasons including ‘too little milk’, ‘too much stress’ and ‘too busy’; 4/9 (44%) were non-white, 5/9 (55%) were single, and more had had maternal complications than in the sample as a whole Cost-effectiveness outcomes Not reported |
Metoclopramide (n = 25–28) | Placebo (n = 29) | Day 10 | 519 ± 60 | 519 ± 60 | Day 17 | 459 ± 63 | 497 ± 64 |
12 of the 69 mothers randomised withdrew (17%), six from each group Reasons: I group: Neonatal death (1) Potential drug reaction (facial rash and itching) (1) Received metoclopramide from local doctor second week postpartum (1) Stopped breastfeeding in the first week of the study (3) A further three mothers stopped breastfeeding during the second week of the study; these were excluded from some analyses, but paper states they had data analysed for baseline variables and some milk volumes C group: Stopped breastfeeding in the first week of the study (6) |
The author provided additional information about method of randomisation Both sets of tablets were white. They differed slightly in size. Therefore both sets were counted and stored in darkened plastic pill vials. Contents known only to one research nurse not involved in the study Available data were analysed using intention-to-treat model Supported by grant RR00059 from the Clinical Research Center, National Center for Research Resources, National Institutes of Health, and Children’s Miracle Network grant proposal 1131 |
|
| Metoclopramide (n = 25–28) | Placebo (n = 29) | ||||||||||||||
| Day 10 | 519 ± 60 | 519 ± 60 | |||||||||||||
| Day 17 | 459 ± 63 | 497 ± 64 |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Jones 2001 UK Research aims Study design Method of group allocation Randomised centrally into 6 groups (3 for gestational age and 2 for parity) Unit of allocation Mother Unit of analysis Group Sample size calculation 39 in each arm for 80% power and 5% significance based on an improvement in milk yield of 20–50%. Interim analysis of 36 complete data sets showed highly significant results and recruitment was discontinued Outcome measures Milk weight Milk fat Mothers’ views Breastfeeding duration |
Selection Mothers of infants being cared for in the Neonatal Unit of North Staffordshire Maternity Hospital October 1997 to August 1999 Inclusion criteria Mothers wishing to express breastmilk Exclusion criteria Mothers unable to express ≥ 5 times per day before the start of the study Mothers with retained products of conception Mothers living outside the study hospital’s area expecting to return to their local hospital before their infant reached term |
Mothers Age and SES not reported I1 (Sim n = 16); C1 (Seq; n = 17) First pregnancy 5/16; 7/17 Breastfed previously 8/16; 6/17 Multiple births (twins) 3 sets; 3 sets Infants Mean weeks gestational age 29.9; 30.2 Mean kg birthweight 1.46; 1.61 Group comparability Not reported |
Standard care Study unit had active breastfeeding policy All mothers provided with information leaflet on milk expression technique All mothers had opportunity to view a video made by the researchers covering milk expression and preterm breastfeeding All participants Egnell Ameda Electric Elite pumps (Egnell Ameda, Taunton, Somerset, UK) with silastic shield inserts, were loaned for the duration of the trial Mothers were encouraged to express × 8 per day until milk no longer entered the collection set I1: n = 25 randomised to simultaneous pumping Mothers were asked to use the study pump in simultaneous pumping mode (both breasts at once) C1: n = 27 randomised to sequential pumping Mothers were asked to use the study pump in sequential mode When milk from the first breast no longer entered the collection set, mothers were asked to switch breasts I2: Breast massage prior to pumping (all participants on two of the four study days) Gentle tactile stimulation of mammary and nipple tissue using a hand action that rolled the knuckles downwards over the breast, beginning at the ribs and working towards the areola This technique did not involve manual expression of milk C2: No breast massage prior to pumping (all participants on two of the four study days) Data collection The study took place during two 48-hour periods (total 4 days), starting on day 4 or when engorgement had been relieved Mothers were randomised to massage on either days 1 and 2 or days 3 and 4 of the study On study days 1 and 3, mothers familiarised themselves with breast massage and data collection Data were collected on study days 2 and 4 only Mothers logged date, time and duration of each expression, and weight of collection bottles before expression Researchers logged weight of collection bottles after expression Capillary sample from each milk sample for creamatocrit test Two questionnaires to mothers at trial completion, and mothers were asked about feeding method at 37 weeks postconception (term) |
Statistical techniques Repeated measures ANOVA on mean milk volume and mean fat content between days 2 and 4 (1 day of massage and 1 day of non-massage for each woman) The means were calculated by the total daily volume/number of expressions that day. Both groups had a mean of 5.2 expressions per day. Results from 36 mothers with complete data Breastfeeding/breastmilk-related outcomes Fully breastfeeding or expressing milk at term (37 weeks) I1 (Sim): 1/17 did not establish breastfeeding C1 (Seq): 15/19 fully breastfeeding or expressing Weeks expressing/breastfeeding until term ranged from 5 to 13 weeks The authors state that differences in milk yield per expression were significant at the p < 0.01 level between sequential and simultaneous pumping, and between massage and non-massage. Fat concentration was similar in all groups. Total fat was significantly different between simultaneous pumping and sequential pumping (p < 0.01), but not for massage vs no massage Mean (95% CI) breastmilk weight (g): Seq (non-massage): 51 (46–56) Seq (massage): 79 (73–85) Sim (non-massage): 88 (79–97) Sim (massage): 125 (110–140) Mean (95% CI) fat concentration (g/l): Seq (non-massage): 7.1 (5.9–8.3) Seq (massage): 6.7 (5.7–7.7) Sim (non-massage): 7.0 (5.9–8.1) Sim (massage): 6.8 (5.7–7.9) Mean (95% CI) total fat (g): Seq (non-massage): 3.1 (2.7–3.5) Seq (massage): 4.2 (3.8–4.6) Sim (non-massage): 6.0 (5.3–6.7) Sim (massage): 8.0 (6.9–9.1) Clinical/health outcomes Not reported Process outcomes 30/36 mothers began the study on postpartum day 5 and 6/36 on day 7 Simultaneous pumping took mothers 10–15 minutes compared with 25–40 minutes for sequential pumping Psychosocial outcomes Women appreciated massage and simultaneous pumping to expedite milk flow, and voiced a need for larger milk collection sets Cost-effectiveness outcomes Not reported |
52 randomised (27 Seq, 25 Sim) 16/52 lost (31%) Missing from Seq 27 – 19 = 8 Missing from Sim 25 – 17 = 8 Reasons given, but not by group: |
Available data were analysed according to randomised group for the comparison between simultaneous and sequential pumping The authors concluded that simultaneous pumping produces more milk than sequential pumping. Massage has an additive effect, improving milk production in both groups The author provided additional information about method of randomisation Funded by Baby Lifeline |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mersmann 1993 USA PhD dissertation, New York University Research aim To test the hypothesis that quantity and fat content of expressed milk in mothers of non-nursing infants will be greater following therapeutic touch (TT) than following mimic therapeutic touch (MTT) or no treatment (NT) Study design Randomised crossover study Method of group allocation Treatment sequence allocated using random numbers table Unit of allocation Mother Unit of analysis Mother Sample size calculation 17 participants in a crossover design with three trials to provide a power of 0.80 to detect a medium effect size at the 0.05 level of significance Outcome measures Quantity and fat content of expressed milk |
Selection Mothers of infants in a 58-cot NICU in a major US metropolitan medical centre Inclusion criteria Mothers of non-nursing preterm infants Expressing breastmilk for at least 2 weeks Literate in English Exclusion criteria Mothers with diabetes, hypoglycaemia or an acute illness Previously received TT |
Mothers Age: Mean 31 ± 5.3 years 21–25: 4 (22%) 26–30: 5 (28%) 31–35: 6 (33%) 36–40: 2 (11%) 41: 1 (6%) Insurance Private: 16 (89%) Public assistance: 2 (11%) Marital status Married: 17 (94%) Not married: 1 (6%) Race Caucasian: 8 (44%) Hispanic: 6 (33%) Black: 4 (23%) Number of other children: 0: 11 (61%) 1: 3 (17%) 2: 3 (17%) 3: 1 (6%) Twin birth: 3/18 (17%) Breastfed previously: 6/18 (33%) Current milk expression: Days expressing milk 14–20: 9 (50%) 21–27: 2 (11%) 28–41: 3 (17%) > 42: 4 (22%) Frequency of expression (number of times/day) 2–4: 11 (61%) 5–8: 6 (33%) > 9: 1 (6%) During recruitment 16/18 reported a current problem maintaining milk supply Infants Gestational age in weeks (%) Mean ± SD, median: 31.5 ± 3.4, 32 < 30: 5 (24%) 30–34: 14 (67%) > 34: 2 (9%) Birthweight in g (%) Mean ± SD, median: 1660 ± 912, 1533 Birthweight (g) < 1000: 4 (19) 1001–1500: 6 (29) 1501–2000: 9 (43) 2001–2500: 0 (0) 2501–3000: 0 (0) > 3001: 2 (9) Group comparability Not applicable |
Intervention Therapeutic touch (TT) Followed a 6-step standard procedure: TT provided by four experienced TT nurses Length of TT was determined by the nurse’s assessment of the mother Control 1: Mimic therapeutic touch (MTT), designed as a single-blind control for TT Follows a 6-step standard procedure: MTT provided by experienced nurses who were taught MTT movements by the investigator. They had not learned TT. Length of MTT was matched with length of previous TT treatment Control 2: No treatment (NT) All study mothers The 18 mothers received TT, MTT and NT in a randomly assigned order Mothers received the treatments on three of five consecutive days, immediately before expressing breast milk with an electric breast pump Time since last expression and time of day kept constant for each mother Mothers maintained their usual milk expression routine Data collection The investigator recorded volume and performed three creamatocrits on milk expressed at each treatment |
Statistical techniques Descriptive statistics, one-way repeated measures MANOVA Breastfeeding/breastmilk-related outcomes Leaking of breastmilk during study treatments n (%) (N = 18) NoYesTT13 (72)5 (28)MTT17 (94)1 (6)NT18 (100)0 (0) More mothers experienced milk leaking after TT than MTT (p < 0.05) Volume (ml) milk expressed after study treatments (not adjusted for milk leaked) (n = 18) Mean; SD; median; range TT: 59.9; 53.9; 47; 5–200 MTT: 49.6; 49.0; 38; 4–220 NT: 47.3; 52.6; 32; 4–220 Author notes the large SDs reflect the large interparticipant variability Fat content (g/100 ml) [creamatocrit %] of a hindmilk sample expressed after study treatments (n = 18) Mean; SD; median; range TT: 6.6 [10.2]; 2.6 [3.8]; 67 [10]; 21–103 [4–16] MTT: 7.1 [10.9]; 2.8 [4.1]; 71 [11]; 23–106 [4–16] NT: 6.8 [10.5]; 3.1 [4.4]; 67 [10]; 23–142 [4–-21] Study hypothesis not supported; volume of breastmilk expressed after TT was greater than after MTT or NT (p < 0.05) but fat content was not Clinical/health outcomes Not reported Process outcomes Treatment time (minutes) Mean; SD; median; range TT: 11.0; 1.6; 11; 9–15 MTT: 10.7; 2.2; 10; 8–14 No significant difference between length of TT and MTT across mothers Expression time (minutes) TT: 19.1; 5.2; 20; 10–30 MTT: 19.8; 5.0; 20; 12–20 NT: 21.3; 4.8; 20; 14–30 No significant difference in time to express milk after TT, MTT and NT Psychosocial outcomes Mothers’ perceptions of treatment (Tx) by treatment order PerceptionTT 1stTT 2ndNo difference531st212nd24Cost-effectiveness outcomes Not reported |
No | Yes | TT | 13 (72) | 5 (28) | MTT | 17 (94) | 1 (6) | NT | 18 (100) | 0 (0) | Perception | TT 1st | TT 2nd | No difference | 5 | 3 | 1st | 2 | 1 | 2nd | 2 | 4 |
21 mothers agreed to participate Researcher reports that three were dropped Two of these were found not to meet inclusion criterion (had expressed < 2 weeks) The third was unable to complete the study because of investigator illness Data from 18 mothers of 21 infants were analysed |
Standard care on the study unit Breastfeeding pamphlets and an electric breast pump were located in a newly created breastfeeding cubicle Mothers of non-nursing preterm infants were neither encouraged to express nor discouraged from expressing milk for their infants Neither unit-based lactation consultants nor other mechanisms of support were available Lactation educators were available on request but generally assisted with infant suckling Kangaroo care was not practised Infants did not initiate suckling until they were successful at bottle feeding Possible confounders for milk production 4/18 mothers used a manual pump as well as an electric pump 2/18 mothers used a double pump accessory kit (not for the treatments) 1/18 used a heat pad 3/18 always used massage 1/18 always used imagery Funding Not reported |
|
| No | Yes | |||||||||||||||||||||||||||||
| TT | 13 (72) | 5 (28) | ||||||||||||||||||||||||||||
| MTT | 17 (94) | 1 (6) | ||||||||||||||||||||||||||||
| NT | 18 (100) | 0 (0) | ||||||||||||||||||||||||||||
| Perception | TT 1st | TT 2nd | ||||||||||||||||||||||||||||
| No difference | 5 | 3 | ||||||||||||||||||||||||||||
| 1st | 2 | 1 | ||||||||||||||||||||||||||||
| 2nd | 2 | 4 |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Amali-Adekwu 2007 Nigeria Research aim To evaluate the effect of selective hindmilk feeding on the growth of preterm low birthweight babies Study design Randomised controlled trial Method of group allocation Randomised (method of randomisation not stated) Stratified by birthweight and gestational age Unit of allocation Individual infants Units of analysis Infant groups Milk produced by mothers of the infant groups Sample size calculation None reported Outcome measures Milk volume and composition Infant weight gain Infant growth |
Selection Infants admitted to SCBU of Jos University Teaching Hospital, Nigeria Inclusion criteria Healthy preterm infants weighing between 1000 and 1499 g with a gestational age < 37weeks Exclusion criteria Infants with congenital abnormalities Infants unable to tolerate full enteral feeding by 72 hours of age Serious maternal postnatal illness Known maternal HIV infection |
Infants small for gestational age (SGA) I (n = 17); C (n = 17) Mean ± SD birthweight (g) 1278.3 ± 168.2; 1267.8 ± 149.4 Mean gestational age (weeks) 33.05 ± 2.06; 33.97 ± 1.15 Age at enrolment (days) 6.05 ± 0.80; 5.82 ± 0.70 Weight on enrolment (g) 1173.45 ± 146.57; 1191.29 ± 137.06 Infants appropriate for gestational age (AGA) I (n = 17); C (n = 17) Mean birthweight (g) I: 1380.47 ± 103.52; C: 1430.88 ± 54.42 Mean gestational age (weeks) 32.38 ± 1.68; 32.81 ± 0.81 Age at enrolment (days) 6.22 ± 0.79; 5.38 ± 0.62 Weight on enrolment (g) 1297.40 ± 144.12; 1329.76 ± 97.01 No maternal data reported Group comparability Comparable for all parameters |
All infants were fed for the first 4 days after the establishment of enteral feeding with composite milk (whole breastmilk) to ensure uniform colostrum ingestion From day 5 feeds were 2 hourly by intermittent gavage Volume of feeds was increased daily by 15 ml/kg to a maximum 200 ml/kg/day I: n = 34 (17 SGA and 17 AGA) From day 5 infants received hindmilk (defined as milk collected after the first 3 minutes of pumping; a colour difference from white to yellow was noted at the beginning of hindmilk collection) After 14 days the intervention ended and infants once more received composite milk C: n = 34 (17 SGA and 17 AGA) Infants received composite milk (whole breastmilk) throughout Data collection Breastmilk obtained via mechanical pumping Fat concentration estimated by creamatocrit Calorific values derived from creamatocrits Infants weighed naked daily on a battery-operated digital scale |
Statistical techniques Student’s t test, ANOVA, linear regression Breastfeeding/breastmilk-related outcomes Mean ± SD daily milk production (ml) I (mothers of infants fed on hindmilk): 356.33 ± 80.25 (foremilk 35.1%, hindmilk 64.9%) C (mothers of infants fed on composite milk): 218.64 ± 47 p < 0.0001 Mean daily creamatocrit (%) I: 9.23±1.89 (hindmilk) C: 5.73±1.4 (composite milk) p < 0.0001 Mean calorie content (J/ml) I: 3.73 ± 0.50 (hindmilk); 2.6 + 0.34 (foremilk, not used in the study) C: 2.8 ± 0.38 p < 0.0001 Clinical/health outcomes Infant weight gain (SGA) Mean daily weight gain g/kg/day (range) I: 12.92±10.95 (1.2 to 21.6) C: 5.01±17.37 (–15.2 to 24.2) p<0.0001 Infant weight gain (AGA) I: 12.99 ± 10.75 (– 12.2 to 28.4) C: results not reported numerically p reported as < 0.009 |
77 infants were recruited Five infants developed abdominal distension and vomiting (I = 3, C = 2) Four infants developed apnoeic attacks (I = 1, C = 3) All nine of these infants died (11.7%) 68 infants completed 14 days of feeding Results reported for these 68 |
Available data were analysed by randomised group Funding not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Hurst 2004 USA (Houston, Texas) Research aim To evaluate feeding outcomes and perceptions of mothers allocated to use test weighing, compared with those of mothers not so allocated, to manage breastfeeding of their preterm infants during the first month after hospital discharge Study design Randomised controlled trial Method of group allocation Randomised using cards previously prepared and placed in sealed envelopes Randomisation stratified by gestational age Unit of allocation Mother Unit of analysis Mothers Infants Sample size calculation Not reported Outcome measures Mothers’ perceptions of using in-home weighing Mean daily infant weight gain Attainment of breastfeeding goals Mothers’ breastfeeding concerns |
Selection Mothers of infants in Texas Children’s Hospital NICU between August 1996 and December 1997 Inclusion criteria English-speaking mothers of preterm infants 31–36 weeks’ gestation Mothers who had maintained lactation throughout the NICU stay and intended to continue breastfeeding postdischarge Exclusion criteria Infants small for gestational age Infants with any structural or functional anomalies that might interfere with feeding |
Reported for the 31 mothers who completed the study Mothers I (n = 15); C ( n = 16) Mean age [SD] (range) in years 32 [5.5] (17–40); 29 [5.7] (19–40) Caucasian, non-Hispanic 11 (73%); 9 (56%) African American 2 (13%); 4 (25%) Hispanic 1 (7%); 3 (19%) Asian 1 (7%); 0 Married 14 (93%); 12 (75%) Mean years [SD] education 15 [1.7]; 16 [2.2] Income > $30,000 3 (20%); 4 (25%) Twin birth 3 (20%); 3 (19%) First birth 9 (60%); 13 (81%) Breastfed previously 3 (20%); 3 (19%) Infants Gestational age (weeks) Mean [SD] (range) I: 33 [2] (31–36) C: 33 [2] (31–36) Birthweight (g) I: 1960 [526] (910–2725) C: 1998 [455] (1305–2885) First breastfeed (day of life) I: 11 [12] (1–24) C: 11 [17] (1–38) Hospital discharge (day of life) I: 23 [18] (2–51) C: 24 [16] (4–55) Weight at discharge (g) I: 2378 [304] (1820–2822) C: 2428 [373] (1742–3068) Group comparability The groups were found to be similar for the characteristics reported |
I: n = 24 Mothers were provided with an electronic scale for in-home use Mothers were instructed to weigh infants before and after each breastfeed to determine breastmilk intake (test-weighing) Mothers managed extra milk feedings based on prescribed volumes in discharge feeding plan and through consultation with their primary care provider C: n = 22 Mothers had no scale Mothers were instructed to determine the need for extra feedings on the basis of clinical indices (i.e. strength and duration of infant sucking, observation of swallowing, degree of breast softening during feeding, infant behaviour) and through consultation with the primary care provider Standard care NICU feeding routines included skin-to-skin contact and suckling opportunities. During transition from tube feeding to breastfeeding, oral feeds when mothers not present were mainly by bottle. Thin silicone nipple shields were used for infants needing help to sustain attachment to the breast. Test-weighing was used intermittently. A lactation consultant telephoned all mothers within a few days of discharge from NICU Data collection Demographic data from clinical records Infant weight gain recorded by research assistant at discharge and at 1, 2 and 4 weeks postdischarge Mothers maintained a record of infant feeding patterns from discharge over the first 4 weeks at home Likert-type questionnaire to rank mothers’ breastfeeding concerns at 1, 2 and 4 weeks |
Statistical techniques Univariant statistics and frequencies to describe data Chi-squared and Mann–Whitney U tests, repeated-measures ANOVA Breastfeeding/breastmilk-related outcomes Breastfeeding at discharge (infants) All infants partially breastfeeding (1–3 feeds per day) Breastfeeding at 4 weeks (mothers) I (n = 15); C (n = 16) Exclusive breastfeeding: I: 4; C: 4 Breastfeeding plus EBM: I: 3; C: 3 Breastfeeding plus EBM plus formula: I: 7;C: 8 No breastfeeding: I: 1; C: 1 Supplementary/complementary feeds (20–90% of daily feeds) were given by bottle Duration of breastfeeding I (n = 9): 5.9 months [SD 4 months] C (n = 10): 6.6 months [3 months] Clinical/health outcomes Mean daily weight gain [SD] g Week 1: 37.5 [12.4]; 35.5 [18.4] Week 2: 40.2 [15.8]; 44.7 [20.1] Week 4: 46.1 [17.1]; 48.5 [19.9] Differences not statistically significant Process outcomes Breastfeeding goals at hospital discharge (breastfeeding at 4 weeks) I (n = 15); C (n = 16) Exclusive breastfeeding: I: 3 (4); C: 2 (4) Give additional milk feeds of either EBM or formula: I: 12 (10); C: 14 (11) No breastfeeding: I: 0 (1); C: 0 (1) At 4 weeks, 19/31 mothers had met or exceeded their goals Psychosocial outcomes Breastfeeding concerns The most commonly cited concerns were the same for both groups: ‘knowing that my baby is gaining enough weight’; ‘knowing how much milk my baby takes at each feeding’; and ‘my baby taking enough milk from the breasts’ Perceptions of using in-home test-weighing Very/extremely helpful: 15 (100%) Did the scales make you nervous? Not at all: 10 (67%) Somewhat: 5 (33%) Cost-effectiveness outcomes Not reported |
46 mothers consented and were randomised (I = 24, C = 22) 15 (34.6%) did not complete the 4-week study protocol (I = 9, C = 6) Reasons: Failure to complete daily feeding records (I = 3, C = 2) Maternal decision to exclusively bottle-feed (I = 1, C = 2) Maternal illness (I = 2, C = 1) Paediatrician recommended changing to formula (I = 1) Grandmother recommended changing to formula (C = 1) Mother returned to employment (I = 2) Paper states there were no differences in maternal or infant characteristics between those who did and did not complete the study |
Available data were analysed by randomised group Numbers for infant outcomes are unclear Funding Study partly funded by Medela, Inc. (McHenry, IL) |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Griffin 2000 USA Research aims To determine the accuracy with which mothers (compared with nurses) performed creamatocrits (CRCTs) on their own mother’s milk (OMM); to elicit maternal reaction to performing CRCTs; to evaluate the cost-effectiveness of this approach; to ascertain whether income, educational and ethnic background influenced accuracy, reactions, and time spent teaching the CRCT procedure Study design Concurrent comparison Method of group allocation Convenience Unit of allocation Mother Unit of analysis Differences in CRCTs between mothers and validating nurse Sample size calculation Not stated Outcome measures Validation CRCT for mothers and nurses Mothers’ reactions to performing CRCTs Time spent teaching mothers Demographic information |
Selection Mothers expressing own mother’s milk for feedings in the NICU Inclusion criteria CRCT measures clinically indicated for management of the infant’s nutritional plan Exclusion criteria None stated |
Mothers Age not reported Income (N = 25) n (%) < $30,000: 9 (36) $30,000–75,000: 8 (32) > $75,000: 8 (32) Education (N = 26) n (%) ≤ 12 years: 8 (30.8) 13–16 years: 14 (53.8) > 16 years: 4 (15.4) Occupation (N = 26) n (%) Professional: 10 (38.5) Skilled: 10 (38.5) Homemaker: 6 (23.0) Ethnicity (N = 26) n (%) African American 9 (34.6) White: 12 (46.2) Hispanic: 5 (19.2) Parity, multiple births and breastfeeding experience not reported Infants Characteristics not reported Group comparability This group of mothers was noted to be diverse with respect to maternal age, education, occupation, income and racial/ ethnic background |
Phase 1 Mothers were taught to perform CRCTs by one of two instructional nurses (IRNs) using a standard teaching tool Mothers practised doing CRCTs over the following 72 hours Phase 2 Each mother performed two CRCTs on the same freshly expressed OMM specimen simultaneously with one of the two validating RNs (VRNs) Mothers used the same centrifuge in both phases; VRNs used a different centrifuge in an area adjacent to NICU IRNs were blind to all CRCT measures performed in Phase 2 and VRNs were blind to teaching procedure in Phase 1. For Phase 2, mothers and VRNs were blinded to each other’s CRCT measures CRCT measures by the VRNs served as the accurate standard to which mothers’ values were compared Data collection Time spent teaching mothers Demographic questionnaire Phase 2 CRCT values were recorded by the mother and nurse on separate, previously prepared index cards Four-item Likert-type questionnaire on mothers’ perceptions of learning CRCT procedure |
Statistical techniques Differences between mothers’ and nurses’ measures were compared using mean, SD, minimum and maximum differences, mean absolute difference (MAD), the percentage of differences of ≤ 0.5% and 1% CRCT, and the percentage of error. Mothers’ and nurses’ measures were compared using Pearson and Spearman correlation coefficients as appropriate and illustrated with Bland–Altman plots. Descriptive statistics for other outcomes Breastfeeding/breastmilk-related outcomes The mothers’ CRCT measurements were highly accurate compared with those of the VRN (MAD 0.69; SD 0.93; min. 0; max. 2.50; 50% of errors < 0.5%; 84/6% of errors < 1.0%, r = 0.9532) No systemic error in the differences for high, low or individual CRCT values was revealed on the Bland–Altman plot The percentage of error in mothers’ measurements (6.8%) was lower than that achieved in a pilot study by the two VRNs (10.51%) No statistically significant association was found between magnitude of maternal error and demographic variables tested Trends identified – mothers with fewer formal years of education and those who reported employment in ‘skilled’ vs ‘professional’ jobs performed CRCTs with greater accuracy Clinical/health outcomes Not reported Process outcomes Mean (range) minutes spent teaching mothers: 23.6 (10–45) Psychosocial outcomes Mothers’ reactions to performing CRCTs (n = 25) Nineteen mothers provided free text comments. Main themes of these were mothers’ feelings of involvement and control with respect to infant care, and of reassurance that their lactoengineereda OMM had the desired calories for infant feeding Cost-effectiveness outcomes For the average lactoengineering intervention, approximately 186.4 minutes of nursing time would be saved per infant if a mother performed a daily CRCT measure on her OMM |
None |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Agrasada 2005 The Philippines (Manila) Research aim To test the efficacy of home-based, postnatal peer counselling for mothers of term low-birthweight infants on breastfeeding exclusivity and duration Study design RCT Method of group allocation Random numbers table Unit of allocation Mother-infant pair Unit of analysis Mother-infant pair Sample size calculation 64 mothers per group for 80% power to detect a 30% absolute difference in exclusive breastfeeding between the two intervention groups, with α = 0.01, a two-sided test and adjustment for 20% attrition Outcome measures Exclusive breastfeeding 2 weeks to 6 months Any breastfeeding at 6 months Infant growth and health outcomes, mothers’ views |
Selection Births at Philippine General Hospital (PGH), Manila January 2001–August 2002 PGH was assessed ‘Baby-Friendly’ in 1993 Inclusion criteria First-time mother ≥ 18 years old Intended to breastfeed Vaginal delivery at term (37–42 weeks) Singleton low birthweight (LBW) infant (< 2500 g) Apgar score ≥ 8 at 5 minutes Exclusion criteria Mothers taking medications that may compromise breastfeeding Mothers not staying with their infants in the study area until the infant was 6 months old |
Mothers BC (n = 68); CC (n = 67); C (n = 69) Mean [SD] age (years) 22.7 [4.5]; 23.2 [4.4]; 23.2 [4.7] Education n (%) Primary school or none: 4 (5.9); 7 (10.4); 2 (2.9) Secondary: 41 (60.2); 36 (53.7); 33 (47.8) College: 23 (33.8); 24 (35.8); 34 (49.3) Living with partner n (%) 48 (70.6); 47 (70.1); 48 (69.6) Study/work outside home n (%) 22 (32.4); 22 (32.8); 21 (30.4) Mean [SD] income pa (US$) 1358 [126]; 1310 [100]; 1325 [126] All were first-time mothers of singleton infants Infants Mean [SD] age of gestation (weeks) BC (n = 68): 39.2 [0.5] CC (n = 67): 39.2 [0.6] C (n = 69): 39.4 [0.3] Mean [SD] birthweight (g) BC (n = 68): 2340.6 [165.6] CC (n = 67): 2368.1 [117.7] C (n = 69): 2365.4 [156.3] Median (range) birthweight (g) BC (n = 68): 2400 (1700–2490) CC (n = 67): 2400 (2000–2490) C (n = 69): 2440 (1750–2490) Group comparability Statistical significance of differences in participant characteristics between the groups not reported |
All study groups Mothers who vaginally delivered term LBW infants were sent to the rooming-in ward Term LBW infants with birthweights < 2kg were observed in the NICU for 12–24 hours While separated from their mothers, these infants received (by dropper) fresh expressed breastmilk (EBM) donated by lactating mothers on the ward As soon as infants stable, they joined their mothers on the rooming-in ward No hospital staff or volunteer was tasked to educate or assist mothers with breastfeeding in the rooming-in ward Mothers recruited and randomised during hospital stay Mothers’ breastfeeding knowledge tested at recruitment Mothers informed of group assignment as leaving hospital All study mothers were discharged on or before postnatal day 3, breastfeeding exclusively Peer counsellors 14 women health volunteers (age 22–50 years) with similar formal education to the mothers and willing to do home visits undertook 40 hours counselling training 6/14 trained by a maternal child health-care specialist became childcare counsellors (for CC) 8/14 with positive personal breastfeeding experience trained by a certified lactation specialist and became breastfeeding counsellors (for BC) Received local transport costs during training and home visits; did not receive a salary Eight home visits were scheduled (at infant age days 3–5, 7–10 and 21, then monthly up to 5.5 months) Counsellors used a semistructured home visitation guide BC: n = 68 Counsellors informed mothers of benefits of exclusive breastfeeding to 6 months Counsellors assisted mothers in preventing and managing breastfeeding problems CC: n = 67 Counsellors assisted mothers on infant care and increasing mother–infant interaction using activities such as infant massage and smile therapy C: n = 69 Clinic visits only, no input from peer counsellors Data collection Mothers were asked to come to the hospital clinic for seven infant visits (at 2 and 4 weeks then monthly until 6 months), where study measures were taken |
Statistical techniques Descriptive statistics, chi-squared tests, general estimating equation (GEE) models, survival analyses, two-sided tests of significance Breastfeeding/breastmilk-related outcomes BC (n = 68); CC (n = 67); C (n = 69) Exclusive breastfeeding from birth to 6 months 22 (32%); 2 (3%); 0 At 6 months, exclusive breastfeeding during the last 7 days 33 (44%); 5 (7%); 0 Any breastfeeding at 6 months 43 (63.2); 21 (31.3); 20 (29); p < 0.001) About 70% of CC and C mothers stopped exclusive breastfeeding at 2 weeks Half the BC mothers stopped exclusive breastfeeding at 5 weeks Using generalised estimating equation (GEE) analysis, mothers who received breastfeeding counselling were 6.3 times more likely to breastfeed exclusively than mothers of other groups (p < 0.001, 95% CI: 3.53–11.3) GEE analysis also showed the proportions of mothers in CC and C groups breastfeeding exclusively were not significantly different (p = 0.95, 95% CI: 0.50–1.91) Clinical/health outcomes Weight for age (WAZ) ± SD at 6 months BC: – 1.96 ± 0.26 to – 1.10 ± 0.83 CC: – 1.91 ± 0.18 to – 0.92 ± 0.93 C: – 1.91 ± 0.22 to – 0.92 ± 0.87 No significant differences between the groups in mean WAZ scores at birth or 6 months Death No infant in the study died Diarrhoea BC: 9/60 (15%) CC: 17/60 (28.3%) C: 18/59 (30.5%) Process outcomes Counsellors of both groups had similar caseloads and participated until the end of the study Proportions of breastfeeding outcomes of mothers with the same breastfeeding counsellor were similar Psychosocial outcomes At exit interview, mothers who had counsellors stated they were satisfied with the programme BC mothers said the counsellor was the person who had influenced their feeding decisions the most CC and C mothers said the physician had influenced their feeding decisions the most Cost-effectiveness outcomes Not reported |
204 mother-infant pairs randomised Withdrawals Overall dropout 25/204 (12.3%) |
Data were analysed using intention-to-treat model It appears that well LBW infants with birthweight 2–-2.5 kg were not separated from their mothers for 12–24 hours observation in SCBU Results for these infants are not reported separately Funding Swedish International Development Cooperation Agency (SIDA), InDevelop, the Swedish Institute, Uppsala University, The Philippine Department of Science and Technology, and the University of the Philippines, Manila |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Merewood 2006 US (Boston, MA) Research aim To examine whether peer counsellors improved the duration of breastfeeding among premature infants admitted to an urban NICU Study design RCT Method of group allocation Computer randomised (SAS) Unit of allocation Mother Unit of analysis Mother Sample size calculation Sample size based on an estimate that 10% of the infants would be breastfeeding at 12 weeks. Assuming that α = 5% and power = 80%, the sample size needed was 78 Outcome measures Any breastfeeding at 12 weeks postpartum |
Selection Mothers of infants in Level III, 15-cot NICU at Boston Medical Center (a Baby Friendly hospital) between January 2001 and September 2004 Inclusion criteria Healthy premature infant born at 26–37 weeks’ gestation Mother intended to breastfeed Mother eligible to breastfeeda Mothers spoke English or Spanish Exclusion criteria Mothers incapacitated from participation by illness or birth complications Infants with congenital abnormalities Infants with life-threatening conditions in the immediate postpartum period |
Mothers I (n = 48); C (n = 53) Insurance status n (%) Medicaid: 27 (56.3); 27 (50.9) Private/HMO: 8 (16.7); 4 (7.5) Other: 13 (27.1); 22 (41.5) Race/ethnicity n (%) African American non-Hispanic: 35 (72.9); 35 (66.0) White non-Hispanic: 3 (6.2); 2 (3.8) Hispanic: 5 (10.4); 14 (26.4) Other: 5 (10.4); 2 (3.8) US born: 12 (25.0); 17 (32.1) Non-US born: 36 (75.0); 36 (67.9) Age, parity, breastfeeding experience and multiple births not reported Infants I (n = 48); C (n = 53) Mean weeks gestational age (GA) (range): 32.6 (26.3-37); 32.7 (26.4–36.3) GA 26–32 weeks (%): 14/48 (29); 16/53 (30) GA > 32 weeks (%): 34/48 (71); 37/53 (70) Mean g birthweight (range): 1914.4 (724–3320); 1840.0 (682–3305) Group comparability No significant differences were found between the groups |
I: n = 48 Initial peer counsellor contact took place within 72 hours postpartum. Peer counsellors were women with breastfeeding experience, drawn from the local community, who were trained at a 5-day breastfeeding course. After initial contact, the peer counsellor was in contact with the mother on a weekly basis for 6 weeks. After the infant was discharged from hospital, contact was by telephone unless mother went to hospital C: n = 53 Baby Friendly standard care, which included referrals to the lactation consultant when needed, use of a breast pump, access to three breastfeeding classes per week, and staff highly trained in lactation Data collection Breastfeeding status determined using infant’s medical records and by maternal recall after discharge (breast and/or formula feeds in the previous 48 hours) |
Statistical techniques Chi-squared tests, hypothesis tests with a significance level of α = 0.05 Breastfeeding/breastmilk-related outcomes At 12 weeks women who received peer counselling were more likely to provide ‘any’b breastmilk than those who did not receive the intervention (OR = 2.81; 95% CI: 1.11–7.14; p = 0.03)c At 12 weeks, women in the intervention group were also more likely to be providing ‘mostly’d breastmilk (OR = 2.49; 95% CI: 0.97–6.40; p = 0.006) but not ‘all’ breastmilk (OR = 1.30; 95% CI: 0.30–5.65; p = 0.72) than women in the control group Subgroup analysis: When only African American women were analysed, those receiving the intervention (n = 30) had odds of providing ‘any’ breastmilk 249% greater than those without peer counselling (n = 29) (OR = 3.59; 95% CI: 1.16–11.03; p = 0.03); however, there were no significant differences between groups for ‘mostly’ or ‘all’ breastmilk Clinical/health outcomes Not reported Process outcomes Mean days hospital stay (range) I: 27.1 (2–81) C: 25.2 (1–104) Among 42/48 (90%) of the intervention group, peer counsellors discussed pumping techniques at the initial contact (100%); helped the mother pump (72.1%); accompanied the mother to NICU (72.1%); helped the mother to breastfeed, kangaroo care or both (30.2%) At 4 weeks, 37.2% of the infants remained in NICU and 81.3% of their mothers were seen in person by the peer counsellor in the NICU Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
108 randomised, 48 to the intervention group and 53 to the control group In those assigned to the intervention group, 5 did not receive intervention and 10 were lost to follow-up; 38 analysed In the control group 2 were withdrawn and 6 were lost to follow-up; 47 analysed |
Available data were analysed by randomised group Funding Study was supported by a grant from the Bureau of Maternal Child Health, in part by a grant from the National Institute of Child Health and Human Development |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Pinelli 2001 Canada (Hamilton, Ontario) Research aim To examine whether supplementary structured breastfeeding counselling for parents of very low birthweight (VLBW) infants improved the duration of breastfeeding up to 1 year of age Study design RCT Method of group allocation Random number tables Unit of allocation Parents (couple) Unit of analysis Infant Sample size calculation Sample size was based on difference between success rate (10% of the infants breastfeeding at 3 months of age) and desired success rate (30%). Assuming α = 5%, β = 2%, and a one-tailed test, the sample size needed was 116 (58 per group) Outcome measures Duration of breastfeeding |
Selection Infants in the tertiary level, 33-cot NICU at Children’s Hospital of the Hamilton Health Sciences Corporation Inclusion criteria VLBW infants (< 1500 g) Inborn or transferred with their mother within 72 hours of birth Fed mother’s milk by parental choice Exclusion criteria Multiple births Infants with severe congenital, surgical, or chromosomal abnormalities Non-English-speaking parents |
I (n = 64); C (n = 64) Mothers Mean [SD] maternal age in years: 30 [6]; 29 [7] Mean [SD] paternal age in years: 32 [6]; 33 [8] Mother living with partner: 86%; 86% Maternal education n (%) < High school: 10 (16); 10 (16) Completed high school: 17 (28); 22 (35) Postsecondary: 10 (16); 16 (26) Completed university: 25 (40); 14 (23) Social class n (%) I,II: 60 (94); 54 (85) III: 1 (2); 4 (6) IV,V: 3 (4); 6 (9) Have other children (%): 26 (41); 25 (39) Infants Mean weeks gestational age [SD]: 29 [3]; 29 [3] Mean g birthweight [SD]: 1083 [267]; 1103 [261] Group comparability No significant differences between the groups were found |
I: n = 64 Supplemented structured breastfeeding counselling (SSBC) for both parents within 72 hours of birth. This involved viewing a video on breastfeeding for preterm infants; individual counselling by the research lactation consultant (not a member of the hospital staff); weekly contact in the hospital and frequent postdischarge contact through the infants’ first year or until breastfeeding was discontinued C: n = 64 Conventional hospital breastfeeding support (CHBS). This was the standard care of breastfeeding support from staff members during the period of hospitalisation At the time of the study, there was no specialised breastfeeding clinic available to the parents, and only a small number of staff had formal education in lactation or breastfeeding support Data collection Duration of breastfeeding was determined using self-administered questionnaires |
Statistical techniques Descriptive statistics, survival analysis, chi-squared test of proportions, Cox’s regression model. Statistical significance set at p < 0.05 Breastfeeding/breastmilk-related outcomes Mean duration of breastfeeding I: 26.2 weeks (SE 2.7 weeks, 95% CI: 21.0–31.5) C: 24.2 weeks (SE 2.7 weeks, 95% CI: 19.0–29.4) Not statistically significant Exclusive breastfeeding No significant differences found between the groups at any time point (3, 6 and 12 months follow-up) Clinical/health outcomes Not reported Process outcomes In SCBU: SSBC mean (SD); CHBS mean (SD) Hours after birth pumping started: 29 (6); 26 (19) Frequency of pumping per 24 hours: 6 (2); 6 (2) Duration of pumping (minutes): 17 (6); 20 (8); p = 0.01 Milk pumped each time (ml): 72 (65); 66 (45) Day of life baby first put to breast: 25 (23); 25 (18) Breastfeeds per day: 3 (2); 4 (3) Cost of pump, Can $: 16 (8); 20 (13) Before 12 months, mothers in both groups stated that they discontinued breastfeeding because they perceived they were not producing enough breastmilk; at 12 months, the mothers stated that the infants were no longer interested in breastfeeding Psychosocial outcomes At home: > 50% of mothers in both groups experienced breastfeeding problems including sore nipples, fatigue, not enough milk, infant not gaining weight and infant not interested in breastfeeding Mothers reported using a wide range of resources for solving breastfeeding problems at home The most used resource over all time periods was the lactation consultant. This included the research lactation consultant in the intervention group and community lactation consultants in both groups Cost-effectiveness outcomes Not reported |
Unclear 128 were randomised, 64 to each group No dropouts are reported The numbers breastfeeding in each group are reported at term and at 1, 3, 6 and 12 months Other results are reported without denominators |
Available data were reported by randomised group Funding Study was supported by a grant from the National Health Research Development Program, Ottawa, Ontario |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Gonzalez 2003 USA (Norfolk, VA) Research aim To find out whether the proportion of infants given their own mother’s milk (OMM) in a children’s hospital neonatal intensive care unit changed after the introduction of a lactation support service Study design Before/after study Method of group allocation Date Unit of allocation Infant Unit of analysis Group Sample size calculation 350, based on preliminary data (not described); the authors aimed to detect a 15% point difference between study groups using a two-sided test; α = 5% and power = 80% Outcome measures Infants given OMM; factors associated with lack of OMM feedings |
Selection Infants admitted to NICU of Children’s Hospital of the King’s Daughters in Norfolk, September 1996 to March 1998 Inclusion criteria Simple random sampling of database records of all admissions The majority of infants admitted were premature (< 37 weeks’ gestation) or low birthweight (≤ 2500 g) Exclusion criteria Not reported |
Mothers Maternal age (years) Before < 20: 30 (17%) ≥ 20: 141 (81%) After < 20: 28 (16%) ≥ 20: 145 (83%) Number of mothers not reported Other maternal characteristics not reported Infants Gestational age (weeks) Before (n = 175) < 37: 105 (67%) ≥ 37: 70 (40%) After (n = 175) < 37: 117 (67%) ≥ 37: 58 (33%) Birthweight (g) Before < 2500: 99 (57%) ≥ 2500: 76 (43%) After < 2500: 111 (63%) ≥ 2500: 64 (37%) Group comparability No significant differences between groups were found |
B: n = 175 Records of infants admitted September 1996 to March 1997 Received usual support (not described) Intervention International Board Certified Lactation Consultant (IBCLC) service initiated July 1997 A: n = 175 Data from records of infants admitted September 1997 to March 1998 Infants and mothers received IBCLC support An IBCLC contacted mothers within 24 hours of their infants’ admission to NICU IBCLC counselled mothers regarding the benefits and options for providing her milk to the infant If mother chose to provide her milk, a feeding plan normally including a pumping regimen was developed IBCLC available 7 am to 6 pm to answer questions and assist with pumping Private rooms equipped with pumps were available 24/7 for mothers to express milk A telephone message service was available after hours (IBCLC would contact mothers the following morning) Data collection Infant records Note: OMM was given by breastfeeding, bottle or through nasogastric tube |
Statistical techniques Pearson’s chi-squared, Wilcoxon rank sum test, univariate and multiple logistic regression Breastfeeding/breastmilk-related outcomes During hospitalisation, 47% of infants in the intervention group received their OMM compared to 31% in the control group (p = 0.002, OR = 2.0, 95% CI: 1.3–3.0) At discharge, 37% of infants in the intervention group received their OMM compared to 23% in the control group (p = 0.004, OR = 2.0, 95% CI: 1.2–3.2) Using logistic regression analysis, factors significantly associated with giving OMM to infants in the NICU were: IBCLC support (p = 0.002) White ethnicity (p < 0.001) Male gender (p = 0.04) 5-minute Agpar score > 7 (p = 0.003) NICU stay > 7 days (p = 0.007) Clinical/health outcomes Not reported Process outcomes Length of stay in NICU (intervention; control): ≤ 7 days: I: 34%; C: 32% 8–14 days: I: 27%; C: 23% 15–30 days: I: 19%; C: 20% > 30 days: I: 19%; C: 25% Not statistically significant Discharged: Home: I: 47%; C: 51% Another hospital: I: 44%; C: 42% Died: I: 9%; C: 7% Not statistically significant Psychosocial outcomes Not reported Cost-effectiveness outcomes Total cost of NICU stay did not differ between the groups |
350 sets of records were abstracted Withdrawals unclear (results expressed as percentages) |
Data were analysed in before/after groups Intervention provided by two IBCLCs and one in training, all of whom were registered nurses Funding Not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Pereira 1984 USA (Philadelphia) Research aim To determine the incidence and duration of breastfeeding before and after the introduction of a breastfeeding counselling programme Study design Before/after Method of group allocation Before/after initiation of the intervention Unit of allocation Mother Unit of analysis Mother Sample size calculation Not reported Outcome measures In-hospital breastfeeding rate Duration of breastfeeding Mothers’ views of the programme |
Selection Mothers of infants admitted to the Infant Intensive Care Unit (ICU) of the Children’s Hospital of Philadelphia during the 6 months immediately preceding and the 6 months immediately following initiation of the intervention Inclusion criteria None specified Exclusion criteria Mothers of infants who died |
Mothers Before (n = 192) BF (%); non-BF (%); p Age < 20: 6; 17; NS 20–30: 71; 58 > 30: 23; 24 Insurance status Private: 84; 56; < 0.01 Medicaid: 13: 42 None: 3; 2 Race White: 91; 77; < 0.01 Black: 9; 23 Other: 0; 0 After (n = 210) BF (%); non-BF (%); p Age < 20: 2; 16; NS 20–30: 75 ; 64 > 30: 23; 20 Insurance status Private: 89; 77; < 0.05 Medicaid: 9; 23 None: 2; 0 Race White: 95; 74; < 0.01 Black: 5; 22 Other: 0; 4 Infant characteristics not reported Group comparability Before and after groups stated to be comparable |
Intervention At infant’s transfer to ICU, all mothers received verbal and written information about the programme Mothers interested in breastfeeding received full information from hospital social worker, who notified the programme coordinator of all mothers interested in breastfeeding Programme coordinator contacted mothers and assigned a counsellor living near them Peer counsellors Seventeen counsellors who had successfully breastfed their sick baby and were certified by the Childbirth Education Association of Greater Philadelphia were selected They received semi-annual orientation to ICU, with demonstration of the intensive care equipment and a lecture on common medical problems of the newborn Telephone counselling provided as needed Counselling included empathy and emotional support as well as advice on collection, home storage and transport to hospital of expressed breastmilk; transition from tube feeding to breastfeeding; maternal diet during lactation and medications excreted in breastmilk Data collection Questionnaires for mothers’ views, within 3 months after counselling ended |
Statistical techniques Chi-squared and independent Student’s t testing Breastfeeding/breastmilk-related outcomes Before (n = 192); after n = 210; p Number of mothers breastfeeding their infants: 32 (17%); 64 (30%); < 0.01 Number of mothers not breastfeeding their infants: 160 (83%); 146 (70%); < 0.01 Duration of breastfeeding (days) (mean ± SEM): 41.6 ± 9.4; 134 ± 12.9; < 0.001 Breastfeeding rates were significantly higher after counselling than before at 1 to 6 months inclusive. After 6 months relatively small proportions of mothers continued to breastfeed and the difference between the groups was not statistically significant 59/64 questionnaires sent to mothers were returned (93%) Programme ranking Very beneficial: 61% Somewhat beneficial: 39% Non-beneficial: 0% Ranking of four aspects of the programme Techniques of breastmilk collection (%): Very successful 90 Somewhat successful 9 Not successful 1 Nutritional information: Very successful 70 Somewhat successful 23 Not successful 7 Emotional support: Very successful 79 Somewhat successful 21 Not successful 0 Newborn care: Very successful 58 Somewhat successful 37 Not successful 5 Clinical/health outcomes Not reported Process outcomes Not reported Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
None reported | Funding not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Senn 2003 USA (Illinois) PhD thesis at Southern Illinois University at Carbondale Research aim To test the hypotheses that compared with historical controls, participation in the intervention will improve maternal perceptions about breastfeeding and breastfeeding rates, and that in the intervention group, attitude to breastfeeding will be associated with breastfeeding rates Study design Before/after study Method of group allocation Date Unit of allocation Infant Unit of analysis Infant Sample size calculation Not reported Outcome measures Breastfeeding and breastmilk feeding up to discharge Mothers’ views |
Selection Infants in NICU of Memorial Hospital of Carbondale Rural unit, serving southern 25 counties of Illinois, treating infants with birthweight ≥ 750 g not requiring surgery Inclusion criteria Preterm infants (≤ 34 weeks) who were in the study unit November 2002 to June 2003 Matched with medical records of infants in the study unit November 2001 to October 2002 Mothers of twins or triplets were invited to participate, with one infant chosen randomly for inclusion in the analyses Exclusion criteria Infant placed for adoption Mother needing intensive care postpartum Mother judged unable to provide informed consent Mother with first language other than English Mother used illicit drugs in pregnancy Mothers in the intervention group who did not attend at least one core session of the Lactation Education Breastfeeding Program |
Mothers Before (n = 25); intervention (n = 25) Mean maternal age [SD] (range) in years 27.2 [6.3] (16–38); 26.3 [4.6] (18–34) ≤ 12 years’ education n (%) 7/16 (44%); 6 (24%) ≥ 12 years’ education n (%) 9/16 (56%); 19 (76%) Caucasian n (%) 18 (72%); 25 (100%) Minority n (%) 7 (28%); 0 (0%) Married n (%) 16 (76%); 19 (76%) Not married n (%) 5 (24%); 6 (24%) Private health insurance 12 (48%); 8 (32%) Public/no health insurance 13 (52%); 17 (68%) Primiparous 10 (40%); 13 (52%) Infants Mean weeks’ gestation [SD] (range) Before: 33.5 [1.7] (29–36) Intervention: 33.0 [1.9] (29–36) Mean g birthweight [SD] (range) Before: 2023 [432] (1206–2943) Intervention: 2125 [552] (1263–3729) Group comparability The only statistically significant difference found between the groups was for race; all the intervention group were white, and seven of the historical controls belonged to minority ethnic groups (p < 0.01) |
Intervention: n = 25 Lactation Education Breastfeeding Program Two core sessions Participants given $25 Wal-Mart Gift Card for each session they attended Before: n = 25 Historical controls selected from records of preterm infants to match the intervention group Standard care Mothers were not allowed in the NICU in the mornings During the implementation of the programme, the lactation consultant began to speak with all mothers of preterm babies (not as previously only those who expressed their intention to breastfeed). As a result many mothers had an individual meeting with lactation consultant as part of standard care, and filled in the pre-intervention maternal breastfeeding questionnaire (MBQ) after their meeting with the lactation consultant Data collection MBQ; feedback forms (post-sessions); infants’ clinical and feeding records |
Statistical techniques Descriptive statistics, chi-squared test, Fisher’s exact test, ANOVA Breastfeeding/breastmilk-related outcomes Received breastmilk at least once Before (n = 25): 15 (60%) Intervention (n = 25): 20 (80%) p < 0.12 Received breastmilk within 2 days of feeding initiation Before (n = 15): 4 (27%) Intervention (n = 20): 7(35%) p = 0.6 Mean % days received breastmilk [SD] (range) Before (n = 15): 47% [25] (7–86) Intervention (n = 20): 63% [23] (6–91) p < 0.06 On days received breastmilk, mean % feedings breastmilk received [SD] (range) Before (n = 15): 32% [22] (2–71) Intervention (n = 20): 35% [22] (1–78) NS Ever fed directly from the breast (‘nursed’) Before (n = 15): 12 (80%) Intervention (n = 20): 15 (75%) p = 0.73 Mean % days nursed Before (n = 12): 20% [18] (5–71) Intervention (n = 15): 53% [17] (29–83) p < 0.01 On days nursed, mean % feedings nursed Before (n = 12): 7% [7] (2–29) Intervention (n = 15): 17% [9] (9–53) p< 0.01 Breastmilk at discharge Before (n = 15): 11 (73%) Intervention (n = 20): 16 (80%) p = 0.64 Clinical/health outcomes Not reported Process outcomes Infants up to 36 weeks gestational age were included Most of the group sessions were conducted with individual mothers Process outcomes related to the educational session are reported The lactation consultant did not know when mothers were in the Unit to breastfeed their babies for the first time, so rarely (3/25) met with mothers to assist them at the first breastfeed Mean [SD] age at discharge (range) Before (n = 25): 17.9 [11.0] (1–43) Intervention (n = 24): 19.6 [12.8] (5–17) NS Psychosocial outcomes No differences in intervention group mothers’ perceptions of breastfeeding benefits, barriers or self-efficacy before and after the intervention Responses on the MBQ were related to breastmilk feeding frequency, as well as frequency of nursing from the breast Cost-effectiveness outcomes Not reported |
25 mothers agreed to participate and were matched with 25 historical controls No withdrawals for primary outcome Guidelines were created to deal with missing feedings information |
Data were analysed by before/after group The author notes as study limitations: Funding Not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Jones 2004 UK Research aim To evaluate the effect of a specialist preterm breastfeeding programme on staff knowledge and skills, and breastfeeding rates Study design Before/after Method of group allocation Before and after training Unit of allocation Individual staff member Unit of analysis Group scores Sample size calculation Not reported Outcome measures Staff knowledge before and after training Breastfeeding rates before and after the intervention |
Selection Staff of NICU at North Staffordshire Maternity Hospital between September 2001 and February 2002 Inclusion criteria Not stated Exclusion criteria Not stated |
Professional backgrounds of staff members who completed the course (n = 34) Neonatal intensive care trained midwives (8) Neonatal intensive care trained paediatric nurses (8) Registered nurses (12) Paediatric nurses (3) Paediatric house officers (2) Paediatric registrar (1) |
Intervention A training programme of five taught modules that took a total of 10 hours to complete, plus practical assessments and tutorials Additional guidance was available in CD-ROM and video formats Teaching took place away from the clinical area to ensure an environment conducive to learning Training programme delivered by neonatal breastfeeding coordinator (midwife) Content of the five modules Data collection Pre- and post-course tests of staff knowledge Audit of medical and nursing records of infants admitted July to November 2000 (before the intervention, n = 135) and July to November 2001 (after the intervention, n = 127) |
Statistical techniques Wilcoxon signed rank test for pre- and post-test questionnaires Chi-squared test for clinical audit data before and after staff training Breastfeeding/breastmilk-related outcomes (from audit of medical and nursing notes) Mothers intending to breastfeed Before staff training (n = 135); after staff training (n = 127) Yes: B: 90 (67%); A: 76 (60%) No: B: 29 (21%); A: 47 (37%) Unclear: B: 16 (12%); A: 4 (3%) p not reported For the two cohorts of mothers intending to breastfeed: Before staff training (n = 90); after staff training (n = 76) Expressed breastmilk given B: 75/86 (86%); A: 72/74 (97%); p = 0.012 Documented problem-solving plan for milk expression B: 2/84 (2%); A: 57/66 (86%); p < 0.0001 Skin to skin contact B: 15/46 (33%); A: 63/64 (98%); p = < 0.0001 Cup feeds offered in mother’s absence B: 53/82 (65%); A: 56/66 (85%); p = 0.006 Baby put to breast B: 57/76 (75%); A: 65/69 (94%); p = 0.002 Breastfeeding on discharge B: 49/73 (67%); A: 54/68 (79%); p = 0.1 Clinical/health outcomes Not reported Process outcomes Achievable score for the pre- and post-test questionnaire was 85 Median (range) scores for the 34 staff members who completed pre- and post-test questionnaires were Pre-test: 32.5 (9–39) Post-test: 44.6 (34–60.5); p < 0.001 No participant scored fewer marks after training Two questions were problematic for all participants before training (one asked how to promote nutritive suckling in infants with both weak and irregular oro-motor responses, the other addressed the issue of sore nipples caused by poor milk expression technique). Answers to these questions improved significantly following training Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
42 staff members enrolled in the programme Eight left during the study period and were excluded from the evaluation 34 attended all five modules and completed pre-and post-test questionnaires For each question in the clinical audit, the number of positive responses is shown followed by the total number where data were available for that question. In each case this was fewer than n, because some answers were not clear from the notes and some infants died or were transferred to other hospitals |
Number of staff working on the unit is not reported The questionnaire used for pre- and post-course tests of staff knowledge was piloted for reliability and validity with five trainee neonatal nurses Three experienced specialists advised on accuracy, relevance, construction flaws and level of readability Researchers note problems consolidating some of the practical areas of the teaching programme, primarily because of the shortage of intensive care nurses Researchers note that record-keeping improved greatly following the programme, and remark that some improvements seen may have been due to this rather than to change in practice After the evaluation the training programme became mandatory for all neonatal nurses on the unit Funding Department of Health |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pineda 2006 USA Research aim To evaluate the effect on breastfeeding outcomes of a staff training intervention to deliver newly introduced individualised care plans and education and support for mothers of VLBW infants Study design Before/after study Method of group allocation Quota sampling from discharge data during study periods Unit of allocation Mother-infant pairs Unit of analysis Mother-infant pairs Sample size calculation Cohen’s criteria to detect a standard deviation change of 0.5 with a power of 80% and alpha 0.05 = 82 participants per group Outcome measures Was breastmilk ever provided in hospital? Was the infant ever breastfed while in the hospital? Was breastmilk provided at discharge? Was breastmilk provided for most of hospitalisation? |
Selection All infants admitted into NICU during study periods: B: 15 April 2004 to 7 December 2004 I: 1 March to 14 April 2005 A: 15 April 2005 to 7 December 2005 Inclusion criteria VLBW (< 1500 g) Admitted to NICU within first 3 days of life Length of stay ≥ 7 days Achieved full gastric feeds during hospital stay Hospitalised < 4 months Exclusion criteria Transferred into the study unit after third day of life Breastfeeding medically contraindicated Hospital stay crossed over from B group into intervention period |
Mothers Mean age in years B: 25.46 A: 25.62 Marital status B: Unmarried 56% A: Unmarried 57% Socioeconomic status B: Low SES 77.5% A: Low SES 70% Ethnicity Black B: 42% A: 48% Infants Birthweight mean g B: 1074 A: 1114 Gestational age at birth mean weeks B: 28.57 A: 28.70 Singletons B: 84.0% A: 83.3% Mean length of stay (days) B: 50 A: 54 Transferred to another hospital B: 43.2% A: 32.7% Group comparability Comparable for all parameters, race was dichotomised black/not black |
Standard care Not described B: n = 81 Intervention A: n = 54 Staff (n = 88) – mostly nurses Breastfeeding support education for NICU health-care professionals Self-study or attendance at in-service training Incentives for completion of training (education credits, food, prizes) Topics included: Mothers n = 54 Individualised care plans modified to require staff to document for each infant: Educational booklet issued to mothers on admission Information consistent with staff education Included space to document milk production as a basis for discussing milk supply with nurses Data collection Retrospective chart review Inter-rater agreement on chart review procedures |
Statistical techniques Pearson’s chi-squared test, non-parametric Mann–Whitney. Significance levels adjusted by ranked Bonferroni adjustment YesNoWas breastmilk ever provided in hospital?B60 (74%)21 (26%)A46 (85%)8 (15%)NS, p = 0.124 (OR: 2.013, CI: 0.818–4.95)Was the infant ever breastfed while in hospital?B21 (26%)60 (75%)A24 (44%)30 (56%)p = 0.03 (OR: 2.286, CI: 1.1–4.75)Was breastmilk provided at discharge?B29 (36%)52 (64%)A22 (41%)32 (59%)NS, p = 0.56 (OR: 1.233, C: 0.61–2.5) Breastmilk provided for most of hospitalisation B: 51% A: 57% NS, p not stated (OR: 1.219, CI: 0.61–2.4) First oral feeding at the breast occurred in 25% of mother–infant pairs in the postintervention group Process outcomes: 56/88 (63%) of health professionals participated in training programme All participating staff achieved a pass (80% score) in post-training test |
Yes | No | Was breastmilk ever provided in hospital? | B | 60 (74%) | 21 (26%) | A | 46 (85%) | 8 (15%) | NS, p = 0.124 (OR: 2.013, CI: 0.818–4.95) | Was the infant ever breastfed while in hospital? | B | 21 (26%) | 60 (75%) | A | 24 (44%) | 30 (56%) | p = 0.03 (OR: 2.286, CI: 1.1–4.75) | Was breastmilk provided at discharge? | B | 29 (36%) | 52 (64%) | A | 22 (41%) | 32 (59%) | NS, p = 0.56 (OR: 1.233, C: 0.61–2.5) |
None reported Data provided for all participants by allocated group (ITT) where detailed |
Participants in each group represented 80% of all admissions during study periods Authors acknowledge study limitations including lack of randomised sample, lack of participation in training programme by senior NICU staff, inadequate implementation of all intervention strategies by health professionals and the inability to control for other changes in NICU environment. Authors note health-care professional behaviour change and attitudes were not measured. The direct effect of the education and individualised care plans on health care cannot be assessed |
|||||||||||||
| Yes | No | ||||||||||||||||||||||||||||||||||||||||||||
| Was breastmilk ever provided in hospital? | |||||||||||||||||||||||||||||||||||||||||||||
| B | 60 (74%) | 21 (26%) | |||||||||||||||||||||||||||||||||||||||||||
| A | 46 (85%) | 8 (15%) | |||||||||||||||||||||||||||||||||||||||||||
| NS, p = 0.124 (OR: 2.013, CI: 0.818–4.95) | |||||||||||||||||||||||||||||||||||||||||||||
| Was the infant ever breastfed while in hospital? | |||||||||||||||||||||||||||||||||||||||||||||
| B | 21 (26%) | 60 (75%) | |||||||||||||||||||||||||||||||||||||||||||
| A | 24 (44%) | 30 (56%) | |||||||||||||||||||||||||||||||||||||||||||
| p = 0.03 (OR: 2.286, CI: 1.1–4.75) | |||||||||||||||||||||||||||||||||||||||||||||
| Was breastmilk provided at discharge? | |||||||||||||||||||||||||||||||||||||||||||||
| B | 29 (36%) | 52 (64%) | |||||||||||||||||||||||||||||||||||||||||||
| A | 22 (41%) | 32 (59%) | |||||||||||||||||||||||||||||||||||||||||||
| NS, p = 0.56 (OR: 1.233, C: 0.61–2.5) | |||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Gunn 2000 New Zealand (Auckland) Research aim To determine safety and effects on breastfeeding rates of earlier hospital discharge of premature infants under the supervision of nurse specialists visiting at home Study design RCT Method of group allocation Sequential list of computer-generated numbers, stratified for birthweight and multiple births Unit of allocation Infant Unit of analysis Group Sample size calculation Not reported Outcome measures Breastfeeding at discharge, 6 weeks and 6 months after discharge; weight gain; readmission rates |
Selection Preterm infants in NICU/SCBU of National Women’s Hospital, Auckland, NZ Inclusion criteria Infants < 37 weeks’ gestation In the study unit > 3 days March 1996 to August 1997 Discharged home Mother’s signed consent Exclusion criteria If the paediatrician considered an infant not ready for early discharge the infant was not randomised Infants discharged to another hospital for ongoing care Mothers whose English was insufficient to complete questionnaires Mothers who lived outside the follow-up area for the study hospital (home visiting not possible) |
Mothers Mean maternal age ± SD ED 30.5±5.7; RD29.7±5.6 Primiparous ED 63 (43%); RD 58 (36%) European ED 98 (67%); RD 105 (66%) Maori ED 20 (14%); RD 32 (20%) Pacific Islander ED 8 (5%); RD 11 (7%) Asian ED 20 (14%); RD 12 (8%) Married ED 111 (76%); RD 102 (66%) Single ED 13 (9%); RD 21 (14%) De facto ED 22 (15%); RD 32 (21%) Prenatal employment: Not employed ED 48 (33%); RD 62 (39%) Part-time ED 31 (21%); RD 31 (20%) Full-time ED 66 (46%; RD 64 (41%) Infants ED (n = 148); RD (n = 160) Mean weeks’ gestation at birth ± SD ED 33.2 ± 2.3; RD 32.9 ± 2.5; NS Mean weeks gestational age at discharge ± SD ED 36.1 ± 1.5; RD 36.4 ± 1.2; NS Mean g birthweight ± SD ED 2007 ± 503; RD 1970 ± 535; NS Mean g weight at discharge ± SD ED 2381 ± 315; RD 2460 ± 317; p = 0.05 Days of suckle feeding in hospital ED 2.5 ± 2.0; RD 4.4 ± 2.8; p < 0.0001 Twins ED 29 (20%); RD 33 (21%); NS Group comparability No significant differences were found between the groups prior to hospital discharge |
All participants Randomisation took place when the infant started to receive suckle feeds from breast or bottle, before full oral feeding was established Routine discharge (RD) control group n = 160 Preterm infants discharged from hospital when: A team of experienced Home Care Nurses contacted the family to enable visiting to occur in hospital and after discharge The Home Care Nurses made home visits or telephone contact during office hours on weekdays Visits/contacts were usually daily for the first 5 weekdays after discharge, then as necessary to support breastfeeding and other problems Early discharge (ED) intervention group n = 148 Infants met the same discharge criteria but without the need for weight gain For the first 7–10 days after discharge they were visited daily (including weekends) by a team of Visiting Nurse Specialists who were also available by telephone 24 hours/day Data collection Questionnaires to mothers in hospital; 6 weeks after discharge, Visiting Nurse Specialists interviewed mothers, weighed infants and completed questionnaire; 6 months after discharge, telephone questionnaire |
Statistical techniques Chi-squared tests, t tests Breastfeeding/breastmilk-related outcomes Any breastfeeding at discharge ED 80%; RD 83%; NS Exclusive breastfeeding at discharge ED 54.8%; RD 67.4%; NS Receiving any breastmilk 6 weeks after discharge ED 55%; RD 60%; NS Exclusive breastfeeding 6 weeks after discharge ED 31.3%; RD 40.5%; NS Receiving any breastmilk 6 months after discharge ED 36%; RD 36%; NS Exclusive breastfeeding 6 months after discharge ED 0.8%; RD 3.6%; NS Clinical/health outcomes ED (n = 148); RD (n = 160); p Mean g weight 6 weeks after discharge ± SD 4034 ± 592; 4189 ± 731; < 0.04 Weight gain (g/kg/day) 12.18 ± 2.98; 12.15 ± 3.61; NS Readmission to hospital Six weeks after discharge ED 8.8%; RD 11.9%; p = 0.37 Six months of age ED 20.2%; RD 20.3%; p = 0.96 Process outcomes Days of full oral feeds in hospital before discharge ED 2.5 ± 2.0; RD 4.4 ± 2.8; p < 0.001 Psychosocial outcomes There was one adverse comment about early discharge, from a mother of 35-week twins: ‘they were too sleepy to feed and I had to wake them round the clock. The stress and tiredness was very severe.’ All other comments on early discharge were positive, e.g. ‘transport, health and financial problems all disappeared when at home’ Cost-effectiveness outcomes Not reported |
308 infants randomised Dropout rate unclear (primary outcome reported as percentages) |
Data were analysed by randomised group Authors note a marked ‘Hawthorne effect’ with this study on timing of discharge; the average duration of hospital stay decreased even in control infants Funding Not reported |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Ortenstrand et al. 2001 and 1999 Sweden (Stockholm) Research aim To evaluate the effect of early discharge and domiciliary nursing care of preterm infants on parental anxiety, assessment of their infants’ health and breastfeeding Study design Quasi-RCT Method of group allocation Convenience allocation to one of two rooms (1:I; 2:C) in Neonatal Special Care Unit Unit of allocation Infants Unit of analysis Families for all outcomes except clinical outcomes Sample size calculation None Outcome measures Health outcomes during corresponding periods of domiciliary care and hospital stay Parental anxiety levels (personality-related ‘trait’ anxiety and situational ‘state’ anxiety) Parental experience of infant’s health Duration of any or exclusive breastfeeding Total number of visits |
Selection Infants admitted to Neonatal Special Care Unit at Sach’s Children’s Hospital between November 1992 and February 1994. Infants admitted from two referring hospitals or from NICU at Karolinska Hospital, Stockholm Inclusion criteria Gestational age < 37 weeks Clinically stable expecting special care > 1 week No apnoeic episodes Maintaining normal body temperature in open crib If required, parents able to handle oxygen equipment Staff assessment of parents’ capability of caring for infants Parents are literate and conversant with Swedish language Exclusion criteria None stated |
Mothers Mean ± SD maternal age (in years): I: 30.6 ± 4.5 C: 31.1 ± 5.5 Single parent: I: 3; C: 1 Education: Less than high school I: 5; C:3 High school I: 23; C:17 More than high school I: 12; C: 15 First pregnancy: I: 28; C: 21 Breastfed previously: not reported Pairs of twins: I: 5; C: 8 Infants Mean weeks ± SD gestational age I: 31.4 ± 2.8 C: 32.0 ± 2.3 Range: I: 24–35; C: 25–36 Birthweight (g, mean ± SD) I: 1677 ± 549 C: 1737 ± 486 Range: I: 654–2905 C: 855–2830 Weight (g, mean ± SD) at onset of study: I: 2224 ± 376 C: 2122 ± 301 Range: I: 1505–2985 C: 1160–2900 Small for gestational age at birth (diagnosed at onset of study): I: 6; C: 6 Very low birthweight (< 1500 g) (diagnosed at onset of study): I: 19; C:14 Perinatal asphyxia (diagnosed at onset of study) I: 4; C: 0 Group comparability No statistically significant differences |
I: n = 40 families Individual care plan in conjunction with parent including: Care provided by project nurse with specialist paediatric and neonatal nursing. Supported by hospital-based neonatologist, nutritionist, social worker, psychiatric team C: n = 35 families Standardised neonatal care that encouraged parents to participate in infants’ care as much as possible Data collection Infant information collected from hospital records and from domiciliary nurse records Parent postal questionnaire at approximately 1 year postdischarge Home-based structured interview based on questionnaire responses 1 week later by assistant nurse Health outcomes based on clinical judgement, not laboratory tests |
Statistical techniques t test, Mann–Whitney U test and chi-squared test Duration of any breastfeeding after domiciliary care period I: 2 not breastfeeding C: 3 not breastfeeding NS No data provided Duration of any breastfeeding at 6 months Fewer I than C mothers (χ2(1) = 3.5; p = 0.06) Mean duration of breastfeeding (months) I: 6.3 (SD: 4.1) C: 7.5 (SD: 4.0) (t = 1.2; p = 0.24) Health outcomes during period of domiciliary care or hospital stay (Infants: I: 45; C: 43) Respiratory infection I: 6/45; C: 16/43, p = 0.02 Data in favour of I group (not significant) for conjunctivitis, dermatological problems, gastrointestinal problems, jaundice requiring phototherapy, myoclonias, oxygen dependence, feeding problems, infection workup and antibiotic medications Data in favour of C group (not significant) Mean g/day weight gain (SD): I: 22.5 (2.4); C: 23.6 (9.2) p = 0.54 Process outcomes Number of scheduled/unscheduled home visits: I: 235/4 Psychosocial outcomes Satisfaction with duration of breastfeeding I: 59.5% C: 72.7% (χ2(1) = 0.8; p = 0.36) Anxiety at hospital discharge, mean (SD) Mothers’ trait anxiety: I: 32.8 (5.9) C: 33.3 (7.8) (t = 0.3; p = 0.75) Mothers’ state anxiety: I: 30.9 (6.2) C: 36.6 (8.4) (t = 3.3; p < 0.01) Fathers’ trait anxiety: I: 30.1 (5.8) C: 33.5 (7.8) (t = 2.0; p < 0.05) Fathers’ state anxiety: I: 29.5 (5.4) C: 32.8 (9.1) (t = 1.8; p = 0.08) Anxiety at end of domiciliary care programme vs comparable period: Mothers’ trait anxiety: I: 31.7 (7.1) C: 31.1 (7.8) (t = 0.3; p = 0.74) Mothers’ state anxiety: I: 27.8 (5.9) C: 30.1 (7.6) (t = 1.4; p = 0.16) Fathers’ trait anxiety: I: 29.0 (6.1) C: 32.3 (6.9) (t = 2.0; p < 0.05) Fathers’ state anxiety: I: 27.6 (6.3) C: 29.4 (5.4) (t = 1.3; p = 0.20) Experience of infants’ health during first year (0 = maximally ill; 12 = maximally good health), mean (SD) Mothers’ experience: I: 5.2 (2.7); C:4.4 (2.2) (t = 1.3; p = 0.19) Fathers’ experience: I: 5.5 (2.5); C: 4.4 (1.9) (t = 1.9; p = 0.06) Experience of infants’ strength compared with other infants in same postconceptional age: Mothers’ experience: I: 5.0 (1.8); C:4.5 (1.8) (t = 1.1; p = 0.27) Fathers’ experience: I: 5.2 (2.0); C: 4.8 (1.9) (t = 1.0; p = 0.32) Cost-effectiveness outcomes None reported |
95/225 preterm infants were eligible Reasons for non-eligibility stated and in accordance with inclusion criteria Withdrawals (infants): I: 3; C: 4 No reasons stated and not clear if postrandomisation but prior to study commencement Total study sample: 88 infants in 75 families I: 45 infants/40 families C: 43 infants/35 families Losses for 1-year follow-up: I: 4 infants/3 families C: 2 infants/2 families Reasons stated by group |
Data were analysed using available case basis Nursing teams in each Special Care Unit room were switched after 8 months to minimise effects of possible differences between nursing teams Other process outcomes are reported in Ortenstrand et al. 109 |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Bell 1995 USA (Iowa) Research aim To investigate whether breastfeeding rates in the study unit increased following a structured intervention of lactation support Study design Before/after Method of group allocation Date Unit of allocation Mother Unit of analysis Mother Sample size calculation Not reported Outcome measures Breastfeeding at discharge |
Selection Mothers of infants in the Intermediate Care Nursery, University of Iowa Hospitals and Clinics (UIHC) Inclusion criteria Mothers intended to breastfeed their preterm or ill infants Exclusion criteria Not reported |
15 mothers intended to breastfeed during the 3-month period in 1992 before the intervention The proportion of mothers who intended to breastfeed is not reported 102 mothers intended to breastfeed during the 1-year period after implementation of the intervention The proportion of mothers who intended to breastfeed was 58% No other participant characteristics are reported |
Before: n = 15 Review of charts for a 3-month period in summer 1992 Intervention A structured intervention of lactation support 1. Protocol for Breastfeeding for the Premature or Ill Infant developed (i) as a standard for the pediatric nursing division; (ii) as guidelines for orientation of residents and staff physicians; (iii) as an educational tool and guide for parents The Protocol included the following stages of breastfeeding: Initial education Nurses discuss infant feeding options with mother on her baby’s admission. Mothers who choose to breastfeed to receive written information and video on breastfeeding and instruction on pumping and storing breastmilk. Nurses ensure pumping begins within 24 hours of birth. For first several weeks, 2–3-hourly pumping recommended. All mothers provided with kit for double pumping. Lactation room with two pumps next to the unit Initiating non-nutritive time at the breast Begin when baby’s corrected gestational age is about 32 weeks; baby can swallow own secretions; baby stable outside incubator at least 10 minutes; baby tolerates kangaroo care. Aim for mother and baby to become accustomed to one another via skin-to-skin or kangaroo care. Mother housed close to the nursery if possible Nurse will help position baby at the breast. Baby not expected to suckle at this stage, will be gavage fed (via nasogastric or orogastric tube). Bottles will not be introduced Progress towards non-nutritive sucking When at the breast, baby may not latch on or suck. May swallow once or twice, may fall asleep at the breast. Continue pumping and gavage feeding, do not give bottles Progress towards nutritive sucking At this stage baby may consistently latch onto the breast, feed for about 5 minutes and show progress on the SAIB scale. Infant-led breastfeeding. No more than 5 hours between feeds. If baby suckles for less than 10 minutes, a proportion of the volume ordered by medical staff to deliver estimated need for growth will be given by gavage. Bottles not introduced until gavage supplements not needed. Continue pumping Transition to breastfeeding At this stage baby wakes for feeds and feeds well on SAIB scale; baby shows adequate hydration and weight gain without supplementation; mother is confident in her ability to breastfeed at home. Nurse will complete discharge teaching and documentation, and arrange for local breastfeeding support. 2. Assessment of staff educational needs followed by program of staff training including in-service sessions, posters and videotapes. Two certified lactation consultants employed in the hospital provided staff training. Staff received resource manual and received SAIB as a pocket reference card 3. Patient/Family Teaching Record (documents breastfeeding teaching, promotes continuity of care among the different nursing shifts) revised to include the Protocol stages of breastfeeding After: n = 102 Appears also to be a review of charts, for a 1-year period after implementation of the protocol Data collection Before and After: Numbers of mothers intending to breastfeed; successfully breastfeeding at discharge After: Proportion of mothers intending to breastfeed; number still breastfeeding; 1 week after discharge |
Statistical techniques Percentages Breastfeeding/breastmilk-related outcomes Breastfeeding at discharge Before: 40% (i.e. 6/15 mothers) After: 80% (i.e. 82/102 mothers) Breastfeeding 1 week after discharge Before: not reported After: 98% (i.e. 81/82 mothers) Clinical/health outcomes Not reported Process outcomes Not reported Psychosocial outcomes Main reason mothers gave for stopping breastfeeding Before: inadequate milk production After: not reported Cost-effectiveness outcomes Not reported |
Unclear |
Available data are presented by before/after group The paper does not define breastfeeding Funding Intervention developed by Research Utilization Committee of the Division of Pediatric Nursing (in-house) |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Bicalho-Mancini 2004 Brazil, Belo Horizonte Research aim To compare rates of exclusive breastfeeding at discharge from a neonatal intensive care unit (NICU) before and after implementation of the Baby Friendly Hospital Initiative (BFHI) Study design Before/after Method of group allocation Date Unit of allocation Infant Unit of analysis Infant Sample size calculation Not reported Outcome measures Infant feeding over the 3 days prior to discharge |
Selection All infants who appeared in the admissions, transfers, discharges and deaths register of the high-risk ward at Odete Valadares maternity hospital (OV) May 1998 to May 2000 Inclusion criteria Infants born at OV Exclusion criteria Infants admitted between December 1998 and October 1999, when the 10 steps of BFHI were being implemented Infants who died, or were transferred to another institution or to judicial care One infant with anencephaly Mothers with contraindications for breastfeeding or transferred for treatment One mother who rejected her infant until discharge and one who had no desire to breastfeed Records incomplete |
Mothers Mean age: 25.3 ± 6.7 years Education (years): 4 or less: 49.3% 5–12: 50.7% Married/stable relationship: 80.4% Single: 19.6% First pregnancy: 40.8% Second pregnancy: 24.1% ≥ Third pregnancy: 35.1% Infants Gestational age < 37 weeks: 383/494 (77.5%) > 37 weeks: 111/494 (22.5%) Small for gestational age: (SGA): 65/495 (13%) Appropriate/large for gestational age (AGA/BGA): 430/495 (87%) Birthweight < 2500 g: 384/495 (77.6%) > 2500 g: 111/495 (22.4%) Multiples: 65/495 (13%) Most common reason for admission was early respiratory difficulties Group comparability Characteristics not reported by group |
Before: n = 250 Infants admitted May–November 1998, when ‘old’ standards and routines of care were in operation (not described) Intervention OV was accredited as a Baby Friendly Hospital (BFH) in May 1999 after ‘training and modifications’ (not described) After: n = 245 Infants admitted November 1999 to May 2000, when care met BFHI standards All participants Parenteral nutrition was used for 20.2% By 10 days 67.7% were being fed with a cup or bottle and 57.9% had started suckling at the maternal breast Relactation was used in 8.7% Mean hospital stay was 23.4 ± 19.5 days Data collection From infants’ dietary charts |
Statistical techniques Chi-squared test, multiple stepwise logistic regression Breastfeeding/breastmilk-related outcomes Infant feeding over the 3 days prior to discharge BeforeAfterExclusive breastfeeding36%54.7%Mixed feeding46.8%37.1%Artificial feeding17.2%8.2% Other outcomes not reported by group Multivariate logistic regression showed independent risk factors associated with non-exclusive breastfeeding at discharge were: Use of enteral feeding (OR: 3.01, 95% CI: 1.77–5.12) < 6 antenatal consultations (OR: 2.75, 95% CI: 1.42–3.44) Relactation use (OR: 2.66, 95% CI: 1.13–6.29, p = 0.026) Birthweight < 2500 g (OR: 2.64, 95% CI: 1.55–4.50) Being born before BFHI was implemented (OR: 2.75, 95% CI: 1.55–4.50) Clinical/health outcomes Not reported Process outcomes Not reported Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
Before | After | Exclusive breastfeeding | 36% | 54.7% | Mixed feeding | 46.8% | 37.1% | Artificial feeding | 17.2% | 8.2% |
Unclear Primary outcome reported as percentages |
Data were analysed by before/after group Funding Not reported |
|
| Before | After | |||||||||||||||||
| Exclusive breastfeeding | 36% | 54.7% | ||||||||||||||||
| Mixed feeding | 46.8% | 37.1% | ||||||||||||||||
| Artificial feeding | 17.2% | 8.2% |
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Merewood 2003 USA, Boston Research aim To compare rates of breastfeeding initiation and duration in the study NICU before and after the implementation of Baby-Friendly policies Study design Before/after Method of group allocation Date Unit of allocation Infant Unit of analysis Infant Sample size calculation Not reported Outcome measures Breastfeeding initiation, breastfeeding at 2 weeks |
Selection Medical records of all infants admitted to Boston Medical Center (BMC)’s level III, 15-bed neonatal intensive care unit (NICU) in 1995 and 1999 Inclusion criteria Infant directly admitted (i.e. not transferred in) Infant survived Exclusion criteria Infant subject of adoption or custody issue Mother ineligible to breastfeed (e.g. HIV positive, substance abuse, methadone use) Other (e.g. maternal incarceration) Records missing Data missing from records |
Mothers (number not reported) BeforeAfterAge (%)< 20141120–305450> 303339Payer status (%)Medicaid5751Uninsured2928Other1320Ethnicity (%)Black6866Hispanic1915White911Other33Unknown14 Infants Before (n = 110)After (n = 117)Gestational age, weeks (%)< 304.56.030–3751.861.537 +43.632.5Birthweight, gMean ± SD2619 ± 9872506 ± 939 All the infants were receiving enteral feeds by 2 weeks of age Group comparability The only statistically significant difference found between the groups was for percentage of vaginal births (58% in 1995 and 44% in 1999, p = 0.05) |
Before | After | Age (%) | < 20 | 14 | 11 | 20–30 | 54 | 50 | > 30 | 33 | 39 | Payer status (%) | Medicaid | 57 | 51 | Uninsured | 29 | 28 | Other | 13 | 20 | Ethnicity (%) | Black | 68 | 66 | Hispanic | 19 | 15 | White | 9 | 11 | Other | 3 | 3 | Unknown | 1 | 4 | Before (n = 110) | After (n = 117) | Gestational age, weeks (%) | < 30 | 4.5 | 6.0 | 30–37 | 51.8 | 61.5 | 37 + | 43.6 | 32.5 | Birthweight, g | Mean ± SD | 2619 ± 987 | 2506 ± 939 |
Before: n = 110 In 1995, lactation support was minimal and none of the Ten Steps (Baby-Friendly policies) were in place Intervention From 1997, strategies to support breastfeeding were implemented, and BMC became a Baby-Friendly accredited hospital in December 1999 After: n = 117 In 1999, all the Ten Steps were implemented, including Step 5 ‘Show mothers how to breastfeed and how to maintain lactation, even if they should be separated from their infants’ Data collection Research assistant extracted data from medical, hospital and insurance records and from daily feed charts |
Statistical techniques Fisher’s exact test, chi-squared test Breastfeeding/breastmilk-related outcomes Infants receiving any breastmilk by any means during the first week of enteral feeds were considered to have initiated breastfeeding Breastfeeding initiation All included infants Before: 38/110 (34.6%) After: 87/117 (74.4%), p < 0.001 US-born black infants Before: 10 (34.5%) After: 16 (64%), p = 0.03 Non-US born black infants Before: 7 (27%) After: 29 (81%), p = 0.001 Breastfeeding at 2 weeks among infants still in the study unit Exclusive breastmilk Before: 4/43 (9.3%) After: 16/41 (39%) Any breastmilk Before: 12/43 (27.9%) After: 27/41 (65.9%), p < 0.001 Exclusive formula Before: 31/43 (72.1%) After: 14/41 (34.1%) Clinical/health outcomes Not reported Process outcomes Not reported Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
Data from all eligible records included in the analysis |
Data were analysed in before/after groups Boston Medical Center served a primarily impoverished population with a high number of racial minorities Funding Grant from the Centers for Disease Control and Prevention (PERT 01-008) |
||||||||||||
| Before | After | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 20 | 14 | 11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20–30 | 54 | 50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| > 30 | 33 | 39 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Payer status (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Medicaid | 57 | 51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uninsured | 29 | 28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 13 | 20 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ethnicity (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Black | 68 | 66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hispanic | 19 | 15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| White | 9 | 11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 3 | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unknown | 1 | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Before (n = 110) | After (n = 117) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gestational age, weeks (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 30 | 4.5 | 6.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30–37 | 51.8 | 61.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 37 + | 43.6 | 32.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Birthweight, g | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mean ± SD | 2619 ± 987 | 2506 ± 939 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study details | Participant selection and inclusion/exclusion criteria | Baseline characteristics of participants | Intervention details | Results | Withdrawals | Additional comments |
|---|---|---|---|---|---|---|
|
Oddy 2003 Australia (Perth) Research aim To find out whether the rate of exclusive breastfeeding on discharge from the study unit changed, after the introduction of finger feeding as part of Baby Friendly Hospital Initiative (BFHI) accreditation Study design Before/after Method of group allocation Date Unit of allocation Infant Unit of analysis Infant Sample size calculation Not reported Outcome measures Breastfeeding at discharge |
Selection Infants cared for in the Special Care Nursery (SCN) of Joondalup Private Maternity Hospital, Perth, Western Australia, during 1998 and 2001 (before and after implementation of BFHI in 1999) Inclusion criteria Infants born at the study hospital at 34–35 weeks’ gestation Exclusion criteria Not reported |
Not reported |
Standard care Routine progression to suck feeds was: Feeds were 3-hourly and took approximately 20 minutes so as not to tire the baby Before: n = 18 When suck feeds were introduced, these were breast and bottle feeds Intervention In 1999 staff were trained in the BFHI, and the hospital was accredited as a ‘Baby-Friendly Hospital’; the SCN was included in the assessment The following changes in hospital practice were undertaken: After: n = 17 When suck feeds were introduced, these were breastfeeds and finger feeds Finger feeds were used if the baby refused the breast or was too tired to breastfeed; did not latch well and therefore did not get milk well; was separated from its mother; if breastfeeding was stopped temporarily; if the mother’s nipples were so sore that she could not put the baby to the breast Staff taught parents how to use the finger lines and gave them a copy of Dr Newman’s instructionsb Data collection Paper states breastfeeding rates were measured prospectively in both groups, with available data measured and recorded by nurses in the SCN on the maternity ward |
Statistical techniques Percentages, t test for equality of means Breastfeeding/breastmilk-related outcomes Breastfeeding at discharge Before: n = 18) After: n = 17 Not breastfeeding on discharge Before: 10 After: 5 Breastfeeding on discharge Before: 8 (44%) After: 12 (71%) t test for equality of means; df = 33; 2-tailed Before: p = 0.125 After: NS Clinical/health outcomes Not reported Process outcomes Not reported Psychosocial outcomes Not reported Cost-effectiveness outcomes Not reported |
Before: 18 infants met the inclusion criteria After: 17 infants met the inclusion criteria Outcome data for all these infants are presented |
Data were analysed using intention-to-treat model Authors state the sample size was too small to demonstrate statistical power Authors note pacifier use was not documented It is not clear whether the outcome reported is exclusive or any breastfeeding Funding Not reported |
Appendix 4.2: Health economics review
Health Economics Data Extraction template
| Source |
| CRD summary |
| Type of economic evaluation |
| Study objective |
| Interventions |
| Location/setting |
| Methods |
| Results |
| Authors’ conclusions |
Appendix 5 Quality assessment tables – effectiveness review
Based on Centre for Reviews and Dissemination Report number 4,102 National Institute for Health and Clinical Excellence guidance development methodology 2005103 and Cochrane Handbook 2008. 104
| Study | Clear inclusion and exclusion criteria | Number randomised (total N) and by group (n = I/C) | A priori sample size calculation | Adequate randomisation methoda | Adequate concealment methodb | Groups comparable at baseline | Subject and investigators blind about treatment allocation | Outcome data reported appropriatelyc | Withdrawalsd n: I and C < 20%/> 20%✓ ✗ Reported by group with reason | ITT/PRE analysise | Overall quality ratingf |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blaymore Bier 1996115 | ✓ | 41 (21/20) | ✗ | ✓ | Not clear | ✓ | Not applicable | ✗ |
I: 0; C: 4 < 20% ✓ |
✗ | Moderate |
| Boo 2007141 | ✓ | 128 (65/63) | ✓ | ✓ | ✓ | ✗ Infant postmenstrual age and maternal education | Not applicable | ✓ |
None < 20% |
✓ | Moderate |
| Cattaneo 1998131 | ✓ |
285 (149/136) in three sites: (50/50) (52/54) (47/32) |
✗ | ✓ | Not clear | ✓Excl. bf at enrolment different in one site | Not applicable | ✗ |
< 20% at first visit > 20% at fourth visit ✗ |
✗ | Moderate |
| Charpak 1997,107 2001108 | ✓ |
777 (396/381) |
✓ | ✓ | ✓ | ✓ | Not applicable |
✓ Study primary outcome; ✗ % data for bf at 3-12 months |
< 20% ✗ |
✗ | Moderate |
| Kadam 2005118 | ✓ |
89 (44/45) |
✗ | Not clear | ✓ | ✓ Age at enrolment not reported; age at birth comparable | Not applicable | ✗ |
None < 20% |
✓ | Moderate |
| Roberts 2000129 | ✓ |
30 (16/14) |
✗ | Not clear | Not clear | ✓ | Not applicable | ✗ | Not stated | ✗ | Poor |
| Rojas 2003121 | ✓ |
60 (33/27) |
✗ Not for bf outcomes, not met for study | ✓ | Not clear | ✓ | Not applicable | ✓ |
I: 1; C: 0 < 20% ✗ |
✗ | Moderate |
| Sloan 1994132 | ✓ |
300 (140/160) |
✓ | ✓ | ✓ | ✓ | Not applicable | ✗ |
< 20% ✗ |
✗ | Moderate |
| Whitelaw 1988147 | ✓ |
71 (35/36) |
✓ Not met | ✓ | ✓ | ✓ | Not applicable | ✗ | None | ✓ | Moderate |
| Study | Clear inclusion and exclusion criteria | Overall sample size (n = I/C) | A priori sample size calculation | Method of group allocation | Groups comparable at baseline | Blinded outcome assessment | Outcomes measured in standard way (info on reporting outcome) | Withdrawals n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | ITT/PRE analysis | Overall quality rating |
|---|---|---|---|---|---|---|---|---|---|---|
|
Wilhelm 2005150 (crossover study) |
✓ |
25 I1: 11 I2: 14 |
✗ Interim analysis showed 100 participants needed for milk volume outcome |
Coin toss on day 4 for first mother-infant pair and alternate allocation for subsequent pairs |
Not applicable Withdrawals: significantly lower birthweight and earlier gestation age |
Not applicable | ✗ | ✗ | ✗ | Poor |
| Study | Are the groups selected from a suitable sampling frame? | Are the groups selected from the same sampling frame? | Method of allocation of participants to comparison groups | Were groups comparable at baseline? | A priori sample size calculation | Clear inclusion and exclusion criteria | What factors (other than the intervention) may affect the outcome? (state factors) | Did the authors adjust for the effects of con-founding factors? | Withdrawals n: I & C <20% / >20% ✓ ✗ Reported by group with reason | Was the analysis appropriate | Overall quality rating |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hurst 1997139 | ✓ | ✓ |
All mother-infant pairs in NICU in two time frames: B: 06/92 to 06/93 A: 01/07/93 to 30/09/93 |
✓ |
✗ Very small study: B: 15 A: 8 |
✗ |
Any other bf promotion activity during two study periods Retrospective, self-reported data collection on milk pumping and output for preceding week Any relevant factors that were not reported on/selected from case records used for retrospective before group data |
✗ | None other than legitimate losses to study |
✓ Retrospective for before group |
Poor |
| Wahlberg 1992135 | ✓ | ✓ |
Convenience sample selected by head nurse in two time frames: B: 05/84 to 11/85 A: 11/85 to 05/87 |
✓ |
✗ B: 33 A: 33 |
✓ | Any relevant factors that were not reported on/selected from case records used for retrospective data analysis for both groups |
✗ Authors acknowledge limitationof study design |
None |
✓ Retrospective for both groups |
Moderate |
| Study | Clear inclusion and exclusion criteria | Number randomised (total N) and by group (n = I/C) | A priori sample size calculation | Adequate randomisation methoda | Adequate concealment methodb | Groups comparable at baseline | Subject and investigators blind about treatment allocation | Outcome data reported appropriatelyc | Withdrawalsd n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | ITT/PRE analysise | Overall quality ratingf |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Collins 2004119 | ✓ |
278 mothers of 319 infants (161 cup, 158 bottle) Mother was unit of randomisation Infant was unit of analysis |
✓ | ✓ | ✓ | Cup and bottle groups appear similar | Not applicable | ✓ |
< 20% and ✓ at discharge < 20% at 3 and 6 months Losses reported by group but without reason |
✓ Notes high non-compliance rates but no pragmatic analysis presented (partial results of exploratory compliance analyses in the discussion) PRE unclear (when enrolled not stated) |
Good |
| Gilks 2004120 | ✓ |
54 infants (27 cup, 27 bottle) |
✓ Not met (pilot study) |
✗ (Not clear) | ✓ | Paper states no significant differences but see reported birthweights | Not applicable | ✗ |
> 20% ✓ |
ITT for any and exclusive breastfeeding at discharge No PRE analysis |
Poor (no forest plot) |
| Kliethermes 1999130 | ✓ |
99 (52 bottle, 47 tube) |
✗ | ✗ (Not clear) | ✗ (Not clear) | ✗ | Not applicable | ✗ |
< 20% ✓ |
✗ | Poor (no forest plot) |
| Mosley 2001124 | ✓ |
16 infants (8 cup, 8 bottle) |
✓ Not met (pilot study) |
✓ | ✓ | Can’t tell from very limited information in the paper | Not applicable | ✗ |
< 20% ✓ |
✓ (But babies who got a supplementary feed were legitimate PREs in this study) |
Moderate |
| Rocha 2002122 | ✓ |
83 (46 cup, 37 bottle) |
✗ | ✓ | ✗ (Not clear) | ✓ | Not applicable | ✗ |
< 20% ✓ |
✗ | Moderate |
| Study | Are the groups selected from a suitable sampling frame? | Are the groups selected from the same sampling frame? | Method of allocation of participants to comparison groups | Were groups comparable at baseline? | A priori sample size calculation | Clear inclusion and exclusion criteria | What factors (other than the intervention) may affect the outcome? (state factors) | Did the authors adjust for the effects of confounding factors? | Withdrawalsa n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | Outcome data reported appropriately, including ITT/PRE analysisb | Overall quality ratingc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meier 2000136 | ✓ | ✓ | Not applicable – crossover study | Not applicable | ✗ | ✓ | Ability to transfer milk without the shield | Yes | Not applicable (retrospective analysis of data) | Unclear | Moderate |
| Study | Clear inclusion and exclusion criteria | Number randomised (total N) and by group | A priori sample size calculation | Adequate randomisation methoda | Adequate concealment methodb | Groups comparable at baseline | Subject and investigators blind about treatment allocation | Outcome data reported appropriatelyc | Withdrawalsd n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | ITT/PRE analysise | Overall quality ratingf |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fewtrell 2001125 | ✓ |
145 (74/71) |
✓ | ✓ | ✓ | ✓ | Not applicable | ✓ |
< 20% ✓ |
ITT but not PRE | Good |
| Groh-Wargo 1995128 | ✓ |
At least 32 At least 16/at least 16) |
✗ |
Not clear ✗ |
Not clear ✗ |
✓ Mothers, no details of babies | Not applicable | Primary ✗ |
Not clear ✗ |
✓ | Moderate |
| Hill 1999112 | ✓ |
49 Not clear |
✗ |
Not clear ✗ |
Not clear ✗ |
Income differed | Not applicable | Primary ✗ |
> 20% ✗ |
✗ | Poor |
| Jones 2001114 | ✓ |
52 (27/25) |
✓ | ✓ | ✓ | Not clear | Not applicable |
Primary ✓ Secondary ✗ |
> 20% ✗ |
✗ | Moderate |
| Paul 1996: phase 1134 | ✓ | 22 | ✗ |
Not clear ✗ |
Not clear ✗ |
Not applicable | Not applicable | Primary ✗ | None reported | ✓ | Poor |
| Paul 1996: phase 2134 | ✓ | 14 | ✗ |
Not clear ✗ |
Not clear ✗ |
Not applicable | Not applicable | Primary ✗ | None reported | ✓ | Poor |
| Slusher 2007142 | ✓ |
103 Not clear |
✗ | ✓ |
Not clear ✗ |
Not clear ✗ Term babies |
Not applicable | Primary ✗ |
> 20% ✗ |
✗ | Moderate |
| Study | Clear inclusion and exclusion criteria | Number randomised (total N) and by group (n = I/C) | A priori sample size calculation | Adequate randomisation methoda | Adequate concealment methodb | Groups comparable at baseline | Subject and investigators blind about treatment allocation | Outcome data reported appropriatelyc | Withdrawalsd n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | ITT/PRE analysise | Overall quality ratingf |
|---|---|---|---|---|---|---|---|---|---|---|---|
| da Silva 2001123 | ✓ |
20 11/9 |
✓ Not met |
✓ | ✓ | ✓ | ✓ | Primary ✗ |
20% ✓ |
✗ | Moderate |
| Feher 1989146 | ✓ |
71 38/33 |
Unclear | ✓ | ✗ | ✓ | Not applicable | Primary ✗ |
> 20% ✓ |
✗ | Moderate |
| Fewtrell 2006144 | ✓ |
51 27/24 |
✓ Met |
✓ | ✓ | ✓ | ✓ | Primary ✗ |
< 20% ✓ |
✗ | Good |
| Gunn 1996132 | ✓ |
20 10/10 |
✗ | ✓ | ✓ | ✓ |
Subject ✓ Investigators ? |
Primary ✗ |
< 20% ✓ |
✓ | Moderate |
| Hansen 2005116 | ✓ |
69 34/35 |
✓ Not met |
✓ | ✓ | ✓ |
Subject ✓ Investigators ? |
Primary ✗ |
< 20% ✓ |
✗ | Moderate |
| Jones 2001114 | ✓ |
52 not applicable |
✓ | ✓ | ✓ | Crossover trial | Not applicable |
Primary ✓ Secondary ✗ |
> 20% ✗ |
✗ | Moderate |
| Mersmann 1993148 | ✓ |
19 Not applicable |
✓ Met |
✓ | Unclear | Crossover trial | Subject ✓ for 2 of 3 treatments | Primary ✗ |
< 20% ✓ |
✗ | Moderate |
| Study | Clear inclusion and exclusion criteria | Number randomised (total N) and by group (n = I/C) | A priori sample size calculation | Adequate randomisation method | Adequate concealment method | Groups comparable at baseline | Subject and investigators blind about treatment allocation | Outcome data reported appropriately | Withdrawals n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | ITT/PRE analysis | Overall quality rating |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amali-Adekwu 2007140 | ✓ |
77 (38/39)a |
✗ | ✗ | ✗ | ✓ | Not clear | ✗ |
None < 20% |
✓ | Poor |
| Hurst 2004145 | ✓ |
46 (24/22)b |
✗ | ✗ | ✓ | ✓ | Not applicable | ✗ |
I: 9; C: 6 > 20% ✓ |
✗ | Poor |
| Study | Clear inclusion and exclusion criteria | Overall sample size (n = I/C) | A priori sample size calculation | Method of group allocation | Group comparable at baseline | Blinded outcome assessment | Outcomes measured in standard way (info on reporting outcome) | Withdrawals n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | ITT/PRE analysis | Overall quality rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Griffin 200020 | ✓ | 26 milk samplesa | ✗ | ✓ | ✓ | ✓ | ✓ |
None < 20% |
✓ | Good |
| Study | Clear inclusion and exclusion criteria | Number randomised (total N) and by group (n = I/C) | A priori sample size calculation | Adequate randomisation methoda | Adequate concealment methodb | Groups comparable at baseline | Subject and investigators blind about treatment allocation | Outcome data reported appropriatelyc | Withdrawalsd n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | ITT/PRE analysise | Overall quality ratingf |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agrasada 2005117 | ✓ |
204 68, 67, 69 |
✓ | ✓ | ✓ | ✓ |
Subjects NA Investigators ✓ |
✓ |
< 20% ✓ |
✓ | Good |
| Merewood 2006143 | ✓ |
108 53, 55 |
✓ | ✓ | ✓ | ✓ |
Subjects NA Investigators ✓ |
✓ |
> 20% ✓ |
✗ | Moderate |
| Pinelli 2001128 | ✓ |
128 64, 64 |
✓ | ✓ | ✓ | ✓ |
Subjects NA Investigators unclear |
✓ |
10% dropout to 1 year except at 1 month when dropout was 24% Reported by group but no reasons |
✓ | Good |
| Study | Are the groups selected from a suitable sampling frame? | Are the groups selected from the same sampling frame? | Method of allocation of participants to comparison groupsa | Were groups comparable at baseline? | A priori sample size calculation | Clear inclusion and exclusion criteria | What factors (other than the intervention) may affect the outcome? (state factors) | Did the authors adjust for the effects of confounding factors? | Withdrawalsb n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | Outcome data reported appropriately, including ITT/PRE analysisc | Overall quality ratingd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonzalez 2003166 | ✓ | ✓ | By date | ✓ | ✓ | ✓ | Any concurrent changes in standard care | ✓ | No withdrawals | Outcomes reported as % data | Moderate |
| Pereira 1984153 | ✓ | ✓ | By date | ✓ | ✗ | ✓ | Any concurrent changes in standard care | ✗ | No withdrawals | Outcomes reported as % data | Moderate |
| Senn 2004152 | ✓ | ✓ | By date | ✗ | ✗ | ✓ | Concurrent changes in standard care (some are described) | ? | No withdrawals | ✓ | Poor |
| Study | Are the groups selected from a suitable sampling frame? | Are the groups selected from the same sampling frame? | Method of allocation of participants to comparison groups | Were groups comparable at baseline? | A priori sample size calculation | Clear inclusion and exclusion criteria | What factors (other than the intervention) may affect the outcome? (state factors) | Did the authors adjust for the effects of confounding factors? | Withdrawalsa n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | Outcome data reported appropriately, including ITT/PRE analysisb | Overall quality ratingc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jones 200481 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
Missing data from medical record review Improved documentation of data and information |
✗ | ✗ | ✗ | Moderate |
| Pineda 2006149 | ✓ | ✓ | ✓ | ✓ | ✓ Not achieved | ✓ |
Not all mothers were given the intervention booklet on admission Some mothers were not given the booklet Old versions of the individualised care plan used in the postintervention period Breastfeeding intervention may not have resulted in behavioural change in the health-care professionals |
✗ | ✓ | ✓ | Moderate |
| Study | Clear inclusion and exclusion criteria | Number randomised (total N) and by group (n = I/C) | A priori sample size calculation | Adequate randomisation methoda | Adequate concealment methodb | Groups comparable at baseline | Subject and investigators blind about treatment allocation | Outcome data reported appropriatelyc | Withdrawalsd n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | ITT / PRE analysise | Overall quality ratingf |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gunn 2000127 | ✓ | 308 (148/160) | ✗ | ✓ | Not stated |
✓ ✗ Days on full oral feeds in hospital; weight at discharge |
Not applicable |
✓ No supporting numerator or denominator data reported |
✗ Not for losses after randomisation prior to intervention ✓ Losses prior to randomisation |
Not clear: no data on losses to study and reported results as percentage data | Moderate |
| Ortenstrand 1999,109 2001110 | ✓ | 88 (45/43) | ✗ | ✗ | ✗ | ✓ | Not applicable | ✗ |
< 20% ✓ |
Not clear for breastfeeding outcomes: percentage data reported | Poor |
| Study | Are the groups selected from a suitable sampling frame? | Are the groups selected from the same sampling frame? | Method of allocation of participants to comparison groups | Were groups comparable at baseline? | A priori sample size calculation | Clear inclusion and exclusion criteria | What factors (other than the intervention) may affect the outcome? (state factors) | Did the authors adjust for the effects of confounding factors? | Withdrawals n: I and C < 20%/> 20% ✓ ✗ Reported by group with reason | Outcome data reported appropriately, including ITT/PRE analysis | Overall quality rating |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bell 1995154 |
✗ Not stated how mothers identified |
✗ Before: 3-month period After 12-month period |
Mothers of infants in Intermediate Care Nursery |
✗ No characteristics reported |
✗ | ✗ | Any other bf promotion or changes in care during unknown period between data collection | ✗ | None | ✓ | Poor |
| Bicalho-Mancini 2004151 | ✓ | ✓ | All newborn babies on high-risk ward at two time points |
✗ No data by group |
✗ | ✓ | Any other bf promotion or changes in care during 11-month period between data collection | ✗ | None | ✓ | Moderate |
| Merewood 2003137 | ✓ | ✓ | All surviving infants for one year at two time points |
✓ ✗ Significantly fewer vaginal births in After group |
✗ | ✓ | Any other bf promotion or changes in care during 3-year period between data collection | ✗ | None | ✓ | Good |
| Oddy 2003138 | ✗ | ✗ | All babies meeting retrospectively applied inclusion criteria |
✗ No characteristics reported |
✗ | ✓ | Any other bf promotion or changes in care during 2-year period between data collection | ✗ | None (assumes only babies with complete records were selected) |
✓ Excluding bf at discharge ✗ Mean % bf at discharge |
Poor |
Appendix 6 Excluded studies
Appendix 6.1: Effectiveness review
Full paper copies for 138 citations were obtained. Eighty-seven of these papers reported studies that did not meet the inclusion criteria for this review. Fourteen of the 87 papers were duplicates, and one reported an ongoing study. Reasons for exclusion of the remaining 72 papers are listed below.
| Study | Reason for exclusion | |||
|---|---|---|---|---|
| Not an evaluation of an intervention | Not special care infants | Intervention does not specifically address breastfeeding/breastmilk feeding in SCBU/NICU | Breastfeeding/breastmilk outcomes not reported | |
| Secretariat of Health of the City of Rio de Janeiro 1999222 | ✗ | |||
| Contemporary Pediatrics (commentary) 2006223 | ✗ | |||
| Alexandre 2007224 | ✗ | |||
| Anderson 1999225 | ✗ | |||
| Blaymore Bier 199724 | ✗ | |||
| Bingham 2007226 | ✗ | |||
| Blumenfeld 2006227 | ✗ | |||
| Cabral 2006228 | ✗ | |||
| Callen 2005229 | ✗ | |||
| Carfoot 2003230 | ✗ | |||
| Castrucci 2007231 | ✗ | |||
| Charpak 2007232 | ✗ | |||
| Charpak 1994233 | ✗ | |||
| Cobb 2002234 | ✗ | |||
| Cockerill 2006235 | ✗ | |||
| Conseil d’Evaluation des Technologies de la Santé du Québec (CÉTS)1997236 | ✗ | |||
| Dall’Oglio 2007237 | ✗ | |||
| Damanik 2006238 | ✗ | |||
| Ehrenkranz 1986239 | ✗ | |||
| Elliott 1998240 | ✗ | |||
| Forsythe 1998241 | ✗ | |||
| Gilks 2007242 | ✗ | |||
| Glazebrook 2007243 | ✗ | |||
| Gotsch 1991244 | ✗ | |||
| Gray 2001245 | ✗ | |||
| Gupta 1999246 | ✗ | |||
| Harding 2006247 | ✗ | |||
| Hill 2005248 | ✗ | |||
| Hurst 2007249 | ✗ | |||
| Jones 199496 | ✗ | |||
| Jones 1995250 | ✗ | |||
| Jones 2000251 | ✗ | |||
| Jones 2001252 | ✗ | |||
| Lawrence 2001253 | ✗ | |||
| Liu 200742 | ✗ | |||
| Marinelli 2001192 | ✗ | |||
| Meier 200421 | ✗ | |||
| Meier 200290 | ✗ | |||
| Meier 2007254 | ✗ | |||
| Milsom 1998255 | ✗ | |||
| Moore 200784 | ✗ | |||
| Narayanan 1988256 | ✗ | |||
| Narayanan 1991257 | ✗ | |||
| Nyqvist 1997258 | ✗ | |||
| Page-Wilson 2007259 | ✗ | |||
| Patel 2007260 | ✗ | |||
| Peterson 2002261 | ✗ | |||
| Phillips 200544 | ✗ | |||
| Pinelli 2005213 | ✗ | |||
| Premji 2002262 | ✗ | |||
| Preyde 2007263 | ✗ | |||
| Schanler 1999264 | ✗ | |||
| Senarath 2007265 | ✗ | |||
| Sheppard 2007266 | ✗ | |||
| Sisk 2006106 | ✗ | |||
| Slusher 2003195 | ✗ | |||
| Spatz 2005267 | ✗ | |||
| Symington 200685 | ✗ | |||
| Thomas 1986268 | ✗ | |||
| Toppare 1994269 | ✗ | |||
| Tosh 2006270 | ✗ | |||
| Tyson 2007271 | ✗ | |||
| VandenBerg 1999272 | ✗ | |||
| Vohr 200712 | ✗ | |||
| Wallis 2007273 | ✗ | |||
| Ward 2006274 | ✗ | |||
| Warren 2000275 | ✗ | |||
| Weimers 2007276 | ✗ | |||
| Wheeler 1999277 | ✗ | |||
| Woldt 1991278 | ✗ | |||
| Young 1994279 | ✗ | |||
| Zukowsky 2007280 | ✗ | |||
Ongoing studies
One of the 138 full paper copies reported an ongoing study. Authors we contacted informed us of another four ongoing studies. These five studies are listed below.
Paper published since our update search
The following paper published after our update search meets the inclusion criteria for this review. It is summarised in Appendix 10: Hake-Brooks SJ, Cranston Anderson G. Kangaroo care and breastfeeding of mother-preterm infant dyads 0–18 months, a randomised controlled trial. Neonatal Network 2008;27:1–9.
Appendix 6.2: Health economics review
Quality assessment
The quality assessment planned to use two methods; firstly, a modified version of the 35-point checklist developed for the authors of economic evaluations submitted to the British Medical Journal. The modification took the form of an additional item (no. 36) intended to ascertain whether the authors had assessed the generalisability of the results. Each checklist item could be given one of four outcomes: (a) yes, (b) no, (c) not clear or (d) not applicable. The checklists were to be completed by two health economists and any discrepancies discussed until final consensus was reached.
Secondly, for each study that met the inclusion criteria, a critical textual summary was planned. This included an appraisal of the choice of comparator(s), the validity of the clinical effectiveness results, the validity of the measure of benefit, the validity of the cost estimates, an assessment of the methodology used, and a variety of other important issues. The data extraction form developed is shown in Appendix 4.2.
| Author | Title | Reason for rejection |
|---|---|---|
| Barton 2001285 | Clinical and economic outcomes of infants receiving breastmilk in the NICU | The aim of the study was to evaluate breastmilk compared with formula. No interventions related to the promotion or encouragement of breastfeeding were considered |
| Drane 1997286 | Breastfeeding and formula feeding: a preliminary economic analysis | The aim of the study was to evaluate breastmilk compared with formula in terms of cases of illness episodes. This was a preliminary analysis and did not meet the criteria of a full economic analysis |
| Cattaneo 1998130 | Kangaroo mother care for low birth weight infants: a randomised controlled trial in different settings | The study compared kangaroo mother care to conventional treatment. Only limited aggregate cost difference data were provided; therefore the analysis did not meet the criteria of a full economic analysis |
| Daga 1985287 | Impact of breast milk on the cost-effectiveness of the special care unit for the newborn | The study evaluated the impact of introducing sleeping facilities adjacent to the SCBU for mothers whose infants were in the unit. However, due to the setting and age of the study it did not meet the inclusion criteria specified |
Appendix 7 Data extraction tables of five systematic reviews used to identify studies for the effectiveness review
| First author, year, design | Review/Research question | Included studies | Main results | Confounders/comments | Quality (mark ✓ or ✗) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Collins 2003160 Cochrane SR |
Review question What are the effects of a policy of early discharge of stable preterm infants with home support of gavage (tube) feeding compared with a policy of discharge of such infants when they have reached full sucking feeds? Data sources The authors state that they used the standard search strategy of Cochrane Neonatal Group with additional searches including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1966–April 2003), CINAHL (1982 to April 2003), and EMBASE (1980 to 2003 week 15) Free text and MeSH terms specified No language restrictions Inclusion criteria Study types Randomised or quasi-randomised trials Participants Infants born at less than 37 weeks’ gestation and not requiring intravenous supplementation at discharge (infants receiving supplemental oxygen were not excluded) Interventions Early discharge home with gavage feeds and health-care support, vs later discharge home when full sucking feeds attained (studies without home support for gavage feeding were excluded) Outcomes Primary: feeding and growth, hospital readmission, adverse events Secondary: satisfaction and anxiety, cost, health service use Quality assessment The reviewers state that they used standard methods of the Neonatal Review Group |
One quasi-randomised trial was included in the review: Örtenstrand 1999 Sample: 88 physiologically stable infants < 37 weeks at birth with need for special care for at least 1 week Singletons and twins included Intervention: early discharge with home visits, gavage feeding at home by parents Control: infants discharged home when clinically well, on full breast and/or bottle feeds Study quality: Allocation concealment – no; the nursery in the trial had two separately staffed rooms. The rooms were randomly designated as experimental or control. The infants were allocated according to bed availability Blinding of treatment – not possible Blinding of outcome assessment – yes Follow-up – 7% attrition for primary outcomes All data were analysed according to the treatment group allocated |
Breastfeeding/breastmilk-related outcomes There were no significant differences between the groups for duration of any or exclusive breastfeeding at any time point: Home gavageControlRR (fixed) [95% CI]Women who had stopped fully breastfeedingDischarge13/4110/411.30[0.64–2.62]3 months8/416/411.33[0.51–3.50]6 months24/4124/411.00[0.69–1.44]Women who had stopped any breastfeedingDischarge2/414/410.50 0.10–2.58]3 months8/415/411.60 [0.57–4.48]6 months20/4112/411.67 [0.94–2.95] Clinical/health outcomes Weight: No significant difference between weight gain from trial entry to discharge from home gavage programme (experimental group) and weight gain from trial entry to hospital discharge (control group) was detected [Weighted mean difference (WMD) –1.10 g/day (– 3.94 to 1.74)] Infection: Infants in the home gavage programme had a lower risk of infection during the home gavage period than the control group during the corresponding period in hospital (RR 0.35 [0.17, 0.69]), p = 0.003 Mortality: No significant difference seen between the groups in death within the first 12 months postdischarge Process outcomes Length of hospital stay: Mean stay 9.3 days shorter for gavage group [WMD –9.30 (–18.49 to –0.11)] Infants in the gavage group spent a mean of 19.6 [SD 9.2] days on the home gavage programme Psychosocial outcomes Parental confidence in handling their infant, parental feelings of preparedness to take responsibility for the care of their infant and parental anxiety were measured at time of discharge from home gavage programme (intervention group) or discharge from hospital (control group). No significant differences were found either for mothers or for fathers Cost-effectiveness outcomes Not reported Were outcomes reported for different gestational ages of the baby and/or ability to co-ordinate sucking and swallowing? No Planned subgroup analyses were not undertaken due to lack of data |
Home gavage | Control | RR (fixed) [95% CI] | Women who had stopped fully breastfeeding | Discharge | 13/41 | 10/41 | 1.30[0.64–2.62] | 3 months | 8/41 | 6/41 | 1.33[0.51–3.50] | 6 months | 24/41 | 24/41 | 1.00[0.69–1.44] | Women who had stopped any breastfeeding | Discharge | 2/41 | 4/41 | 0.50 0.10–2.58] | 3 months | 8/41 | 5/41 | 1.60 [0.57–4.48] | 6 months | 20/41 | 12/41 | 1.67 [0.94–2.95] |
The study included in this review was conducted in Sweden The mean age of the infants has not been reported; a potential confounding factor No economic data were presented to compare the costs of the 19.6 days on the home programme with the costs saved on the 9.3 days |
The review was well conducted; however, the one study included in the review has methodological limitations Inclusion/exclusion criteria reported: ✓ Description of SR methodology: ✓ Rigorous literature search: ✓ Quality of studies assessed and taken into account: ✓ Sufficient details about individual studies: ✓ Studies appropriately combined: only one study |
|||||||
| Home gavage | Control | RR (fixed) [95% CI] | |||||||||||||||||||||||||||||||||||||||
| Women who had stopped fully breastfeeding | |||||||||||||||||||||||||||||||||||||||||
| Discharge | 13/41 | 10/41 | 1.30[0.64–2.62] | ||||||||||||||||||||||||||||||||||||||
| 3 months | 8/41 | 6/41 | 1.33[0.51–3.50] | ||||||||||||||||||||||||||||||||||||||
| 6 months | 24/41 | 24/41 | 1.00[0.69–1.44] | ||||||||||||||||||||||||||||||||||||||
| Women who had stopped any breastfeeding | |||||||||||||||||||||||||||||||||||||||||
| Discharge | 2/41 | 4/41 | 0.50 0.10–2.58] | ||||||||||||||||||||||||||||||||||||||
| 3 months | 8/41 | 5/41 | 1.60 [0.57–4.48] | ||||||||||||||||||||||||||||||||||||||
| 6 months | 20/41 | 12/41 | 1.67 [0.94–2.95] | ||||||||||||||||||||||||||||||||||||||
|
Conde-Agudelo 2003164 Cochrane SR |
Review question Is there evidence to support the use of kangaroo mother care (KMC) in low birthweight (LBW) infants as an alternative to conventional care, after the initial common period of stabilisation with conventional care? Data sources The authors followed Cochrane Neonatal Group methods, which included searches of MEDLINE, EMBASE, LILACS, POPLINE and CINAHL databases and the Cochrane Controlled Trials Register (January 1982 to December 2002) using key word terms ‘kangaroo mother care’ or ‘kangaroo care’ or ‘kangaroo mother method’ or ‘skin-to-skin contact’ and ‘infants’ or ‘low birth weight infants’; relevant trials were sought from the Neonatal Review Group’s Specialised Register; cross-referencing, conference proceedings and journal hand searching (journals unspecified) No language restrictions were imposed Inclusion criteria Study types Randomised controlled trials Participants LBW infants (birthweight less than 2500 irrespective of gestational age) Interventions KMC versus standard neonatal care in LBW infants Outcomes reported Primary: mortality Secondary: infection/illness, failure to establish breastfeeding, readmission to hospital, growth, psychomotor development, parental dissatisfaction, mother’s attachment behaviour Quality assessment The authors assessed each paper on the following criteria: allocation concealment, completeness of follow-up and blinding of assessment outcome (they also described methods of randomisation) |
Three trials were included in the review: Sloan 1994 (RCT) Intervention: KMC (kept in upright position, in skin-to-skin contact, diapers allowed) against the mother’s breasts and had frequent breastfeeding (n = 140) Control: Incubator or thermal crib and were breastfed at scheduled times (n = 160) Charpak 1997 (RCT) Intervention: KMC (24 hours/day in a strict upright position, in skin-to-skin contact firmly attached to the mother’s chest) (n = 396) Control: Infants kept in an incubator until they were able to regulate temp. or were thriving (n = 381) Cattaneo 1998 (RCT) Intervention: KMC (close and continuous skin-to-skin between mother’s breasts, diaper only) Control: skin-to-skin contact between mothers and infants not allowed. Infants kept in incubators (two hospitals) and in a warm room with open cribs with the possibility of rewarming in a bulb-heated cot at the third (unknown number of infants initially randomised to each group) Conde-Agudelo 2003 state the included trials were of moderate to poor quality: No trial described procedures of allocation concealment or reported blinding No trial provided complete outcome data |
Reviewer states intention-to-treat analysis was impossible because of incomplete outcome data, but does not specify whether available data were or were not analysed according to treatment group All results except those for breastfeeding at 1 month are based on data from one trial Breastfeeding/breastmilk-related outcomes Not exclusively breastfeeding at discharge: Significantly fewer KMC infants ‘not exclusively’ breastfeeding (i.e. more exclusively breastfeeding) at discharge (RR 0.41 [95% CI 0.25, 0.68], p = 0.0005) (Cattaneo 1998) No differences seen in exclusive breastfeeding at 41 weeks corrected gestational age, 1 or 6 months or 12 months corrected age Clinical/health outcomes Infant mortality: No differences seen in infant mortality assessed from eligibility to any of the review time points Infection/illness Nosocomial infection at 41 weeks corrected gestational age: KMC associated with lower risk (RR 0.49 [95% CI 0.25, 0.93], p = 0.03) Severe illness at 6-month follow-up: KMC associated with lower risk (RR 0.30 [95% CI 0.14, 0.67], p = 0.003) Lower respiratory tract disease at 6-month follow-up: KMC associated with lower risk (RR 0.37 [95% CI 0.15, 0.89], p = 0.03) No differences seen in severe infection at 41 weeks corrected gestational age or at 12 months corrected age, diarrhoea, or mild or moderate illness at 6 months follow-up Growth Daily weight gain at discharge (g/day): KMC infants gained more (WMD 3.6 g/day [95% CI 0.8 to 6.4], p = 0.01) No differences seen in weight, length or head circumference at 41 weeks corrected gestational age, at discharge or at 12 months corrected age Process outcomes Readmission to hospital: No differences seen at 41 weeks corrected gestational age or at 6 months follow-up Psychosocial outcomes Maternal dissatisfaction with method of care: KMC associated with lower risk (RR 0.41 [95% CI 0.22 to 0.75], p = 0.004) Cost-effectiveness outcomes Costs stated to be less for KMC in two studies, but incompletely reported Were outcomes reported for different gestational ages of the baby and/or ability to co-ordinate sucking and swallowing? No – planned subgroup analyses were not undertaken due to lack of data |
Sloan 1994: Ecuador Charpak 1997: Colombia Cattaneo 1998: Ethiopia, Indonesia and Mexico; conventional care was not defined and Conde-Agudelo 2003 states insufficient detail about breastfeeding promotion and maternal involvement in care of the newborn was provided for control groups This review focused on low birthweight infants and may not be applicable to all premature infants Conde-Agudelo 2003 states that respiratory, thermal and feeding stabilisation are crucial for the success of this intervention, but ‘stabilisation’ was not defined. Gestational age and weight were stated to be the main variables associated with respiratory, thermal and feeding functions |
Inclusion/exclusion criteria reported: ✓ Description of SR methodology: ✓ Rigorous literature search: ✓ Quality of studies assessed and taken into account: ✓ Sufficient details about individual studies: ✓ Studies appropriately combined: not enough data to combine |
||||||||||||||||||||||||||||||||||||
|
Edmond 2006155 SR (World Health Organization Technical Review) |
Review question What is the evidence on feeding low birthweight (LBW) infants? How should LBW infants in developing countries be fed in the first 6 months of life? Data sources Search terms: LBW, preterm, premature, SGA, intrauterine growth restriction/retardation (IUGR), mortality, breastfeeding, human milk Electronic databases: Cochrane database of SRs of RCTs, Cochrane controlled trials register, DARE, Cochrane Neonatal collaborative review group specialised register, MEDLINE (1966–2005), EMBASE (1966–2005) Reference lists of articles, personal communications, technical reports, conference proceedings, review articles, books, dissertations and experts in the field were also accessed Key journals (unspecified) were hand searched Non-English language articles and abstracts actively sought Inclusion criteria Study types: systematic and non-systematic reviews, randomised and quasi-randomised trials, cohort and case–control studies related to feeding of LBW infants Participants Infants with birthweight (BW) < 2500 g or gestational age (GA) at birth < 37 weeks Interventions All nutritional exposures or interventions to improve feeding of LBW infants in the first 6 months of life Outcomes reported Mortality Severe morbidity Neurodevelopment Growth Other outcomes (including rates of any and exclusive breastfeeding (bf)) Quality assessment Authors comment on quality of evidence (Annex 3), but quality assessment criteria are not described |
80 studies listed in summary tables Other studies referred to in the text Number of studies included in the review not stated Exposures/interventions stratified and reported under six headings: 1. Nutrition This section did not report bf/ breastmilk-related outcomes 2. Feeding methods Two studies evaluated the effects of cup feeding compared with bottle-feeding on bf patterns: Collins 2004 (RCT) (LII)a After bf or when mother unable to be present, infants (GA < 34 weeks) to be fed by cup (n = 151) or bottle (n = 152) Rocha 2002 (RCT) (LII) Infants (GA 32–36 weeks) to be fed by cup (n = 44) or bottle (n = 34) 3. Feeding schedules (oral and intragastric feeding) This section did not report bf/breastmilk-related outcomes 4. Support Four studies evaluated the effects of kangaroo mother care (KMC) compared with conventional care on bf patterns: Cattaneo 1998 (RCT) (LII) KMC (n = 146) compared with conventional care (n = 133); stable infants BW 1000–2000 g Charpak 1997 (RCT) (LII) KMC (n = 343) compared with conventional care (n = 320); stable infants BW < 2000 g Charpak 2001 (RCT) (LII) KMC (n = 320) compared with conventional care (n = 305); stable infants BW < 2000 g Sloan 1994 (RCT) (LII) KMC (n = 93) compared with conventional care (n = 111); stable infants BW < 2000 g Three studies evaluated the effects of breastfeeding counselling on bf patterns: Pinelli 2001 (RCT) (LII) Bf counselling package (n = 64) compared with standard package (n = 64); parents of infants BW < 1500 g who intended to bf Bhandari 2003 (cluster RCT) (LII) Subgroup of intervention group (community promotion of exclusive bf for 6 months, n = 159) compared with control group (n = 124); mothers of infants BW < 2500 g Agrasada 2005 (RCT) (LII) Home-based bf counselling (n = 60) compared with home-based counselling in general child care (n = 59) and no counselling at home (n = 71); mothers of term infants BW < 2500 g who were admitted to hospital Three studies evaluated the effects of metoclopramide or domperidone therapy on daily breastmilk volume in women who gave birth at < 34 weeks’ gestation: Ehrenkranz 1986 (cohort study) (LIII-3 or above) Metoclopramide 10 mg three times per day for 7 days de Silva 2001 (randomised study) (LIII-3 or above) Women having difficulty maintaining milk production by expression randomised to domperidone or placebo for 7 days Hansen 2005 (randomised study) (LIII-3 or above) Metoclopramide 10 mg or placebo three times per day for 7 days 5. Monitoring This section did not report bf/breastmilk-related outcomes 6. Feeding in exceptionally difficult circumstances (HIV) This section did not report bf/breastmilk-related outcomes |
For the interventions that report bf/breastmilk-related outcomes, the authors report their findings as follows: Cup feeding compared with bottle feeding Cup feeding led to higher rates of exclusive/predominant bf at hospital discharge than bottle feeding Cup feeding was associated with lower risk of bradycardia or desaturation than bottle feeding No evidence of increased risk of aspiration when cup feeding correctly done (infant upright and milk not poured into the mouth; ‘lapping milk’) Most of the evidence came from studies conducted in developed countries No data for term LBW infants KMC compared with conventional care In clinically stable preterm infants with BW < 2000 g, there is evidence that KMC is at least as effective as conventional care in reducing mortality KMC may improve exclusive BF rates and weight gain and may reduce infections Insufficient data on effects of KMC in infants with BW < 1500 g. Many of these infants were excluded from the available studies because they were not clinically stable Preliminary evidence from resource-poor settings that KMC may be effective even in clinically unstable LBW infants including those with BW < 1500 g Most of the evidence came from studies conducted in developing countries No data for term LBW infants Breastfeeding counselling Among preterm infants 32–36 weeks’ gestation and term LBW infants, bf counselling improves the rates of exclusive bf at 3 months No disadvantage in growth rates or malnutrition prevalence apparent The evidence came from developing and developed countries Few data on infants < 32 weeks’ gestation Metoclopramide or domperidone therapy The available evidence suggests that metoclopramide or domperidone increases breastmilk volume in mothers of infants of < 32 weeks’ gestation, particularly those who were having difficulty in maintaining milk production No efficacy data for mothers of infants 32–36 weeks’ gestation or of term LBW infants Cost-effectiveness outcomes Not reported Were outcomes reported for different gestational ages of the baby and/or ability to co-ordinate sucking and swallowing? Infants classified by GA and (where GA not available) on BW as follows: Authors note it was not possible to present the findings of most studies separately for preterm infants whose size was appropriate for gestational age (AGA) from those who were small for gestational age (SGA). Some studies only reported BW, not GA |
Applicability to the UK The aim of this technical review was to develop guidelines for feeding LBW infants in developing countries. Due to the paucity of data from developing countries, most of the evidence came from studies conducted in developed countries. The authors state that care was taken in extrapolating information from developed countries to developing country settings. Similarly, care should be taken when applying conclusions intended to guide practice in developing countries to the UK and other developed country settings Additional information The Background to the Results section presents: Gaps in evidence on interventions relevant to the current review Breastfeeding supplementer The authors note they found two small case series that described the impact of the breastfeeding supplementer on exclusive breastfeeding rates. However, they judged these studies were likely to suffer from selection and observer bias so did not draw conclusions from them Non-nutritive sucking Executive summary states that encouraging the infant to suck on the ‘emptied’ breast, after expression of breastmilk, may result in improved breastfeeding rates at discharge and follow-up. Text on p. 85 states this evidence came from a small study in 32 babies with an average gestation of 33 weeks, but this study is unreferenced Demand feeding Executive summary states demand feeding may be feasible for some infants of 32–36 weeks’ gestation and may reduce length of hospitalisation. Text on p. 78 lists limitations of this evidence. What and how the babies were fed is not mentioned. The authors note that starting demand feeding as early as possible may be advantageous because of risks and costs of hospitalisation. They also note demand feeding initially requires more monitoring and training |
Inclusion/exclusion criteria reported: inclusion ✓ exclusion û Description of SR methodology: ✓ This technical review considered consensus statements, expert committee reports and advice from experts in the field as well as evidence from the studies Rigorous literature search: ✓ Some papers found after the searches had been done were included in the review Quality of studies assessed and taken into account: ✓ Authors state they assessed limitations, internal and external validity, and wider implications of each study but do not say how Some details of individual studies not clearly or consistently reported: ✓ e.g. country not specified, date appears only in the reference list, not all studies appear in summary tables and details of these may be missing (as with the studies on metoclopramide or domperidone therapy) Studies appropriately combined: ✓ (narrative synthesis) |
||||||||||||||||||||||||||||||||||||
| First author, year, design quality | Review/research question | Included studies | Main results | Confounders/comments | Quality (mark ✓ or ✗) |
|---|---|---|---|---|---|
|
Flint 2007163 Cochrane SR |
Review question What are the effects of cup feeding vs other forms of supplemental enteral feeding on weight gain and achievement of successful breastfeeding in newborn infants who are unable to fully breastfeed?a Data sources The authors state that they used the Cochrane Neonatal Group methods including the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2006), CINAHL (1987–April 2006), MEDLINE (1966–April 2006). Electronic searches were based on MeSH terms ‘Infant, Newborn’ OR ‘Nurseries, Hospital’ OR ‘Intensive Care Units, Neonatal’ AND the textword ‘cup’ Previous reviews, abstracts, and conference proceedings were searched as well as expert informants English-language journals were hand searched (journals unspecified) No other language restrictions applied Inclusion criteria Study types Randomised and quasi-randomised trials (crossover studies were excluded) Participants Newborn infants, up to 44 weeks postmenstrual age or 28 days postnatal age, unable to fully breastfeed Interventions Oral feeding of either expressed breastmilk or a combination of expressed breastmilk and artificial formula via a cup (or a container of similar design that allows the infant to ‘lap’ the milk) versus other forms of supplemental enteral feeding such as tube or bottle feeds Outcomes reported Primary: weight gain, proportion not breastfeeding/not fully breastfeeding at hospital discharge and at ages 3 and 6 months Secondary: average time per feed, length of hospital stay Quality assessment The reviewers state that they used standard methods of the Neonatal Review Group |
Four trials were included in the review: Rocha 2002 (RCT) Intervention: Supplemental feeds via cup (n = 46) Control: bottle feeds (n = 37) Mean gestational age = 32.5–32.7 weeks Collins 2004 (RCT) Intervention: Supplemental feeds via cup (n = 161; mean gestational age = 29.3 weeks) Control: bottle feeds (n = 158; 30.0 weeks) Gilks 2004 (RCT) Intervention: Supplemental feeds via cup in addition to tube feeds and breastfeeding (n = 27; mean gestational age = 31.0 weeks) Control: Bottle feeds (n = 27; 32.0 weeks) Mosley 2001 (pilot RCT) Intervention: Supplemental feeds via cup (n = 8) Control: Bottle feeds (n = 158) Mean gestational age = 35.2–35.5 weeks For all studies, Flint 2007 states that allocation concealment was adequate (with the exception of Rocha 2002, where allocation concealment was unclear), blinding could not be achieved because of the nature of the intervention, and follow-up appeared to be complete at hospital discharge but decreased over time. Data were analysed according to the treatment group allocated |
Breastfeeding/breastmilk-related outcomes Not breastfeeding at hospital discharge: No significant difference between the groups (meta-analysis of three trials, I2 0%) RR 0.82 [95% CI 0.62 to 1.09] Not breastfeeding at 3 months: No significant difference between the groups (meta-analysis of two trials, I2 0%) RR 0.88 [95% CI 0.76 to 1.03] Not breastfeeding at 6 months: No significant difference between the groups (one study reported this outcome) RR 0.90 [95% CI 0.78 to 1.05] Not fully breastfeeding at hospital discharge: Significantly more of the infants allocated to cup feeding were fully breastfed at hospital discharge (meta-analysis of three trials, I2 0% – Rocha 2002 did not report this outcome); RR 0.75 [95% CI 0.61 to 0.92], p = 0.007, number needed to treat 7.3 Not fully breastfeeding at 3 months: No significant difference between the groups (one study reported this outcome); RR 1.18 [95% CI 0.88 to 1.58] Not fully breastfeeding at 6 months: No significant difference between the groups (one study reported this outcome); RR 1.31 [95% CI 0.89 to 1.92] Clinical/health outcomes Weight gain: No significant difference between the groups (one study reported this outcome) Mean difference – 0.60 g/kg/day [95% CI – 3.21 to 2.01] Process outcomes Average time per feed: No significant difference between the groups (one study reported this outcome) Mean difference – 1.60 minutes [95% CI – 3.69 to 0.49] Length of hospital stay: Significantly increased length of stay among infants allocated to cup feeding (only one study, Collins 2004, reported this outcome – see comments). Mean difference 10.1 days [95% CI 3.9 to 16.3] Psychosocial outcomes Not reported by group In Collins 2004, some of the mothers of infants allocated to cup feeding introduced a bottle. Some of the mothers who made this decision reported they did not like/had problems with cup feeding, including: infant not managing cup feeds, spilling a lot, not being satisfied or taking too long to feed. Mothers also reported that staff refused to cup feed their infant Cost-effectiveness outcomes Not reported Were outcomes reported for different gestational ages of the baby and/or ability to co-ordinate sucking and swallowing? No |
Applicability to the UK: Rocha 2002: conducted in Brazil – single centre trial Collins 2004: conducted in Australia – involved two tertiary hospitals and 54 peripheral hospitals that received babies from the tertiary hospitals Gilks 2004: conducted in the UK – single-centre trial Mosley 2001: conducted in the UK – single-centre trial Flint 2007 states Collins 2004 reports high levels of non-compliance to the experimental intervention, with 85/151 of infants allocated to cup feeding (65%) having a bottle introduced. Collins 2004 contributes 76.3% of the weight to the finding of significantly more infants allocated to cup feeding being fully breastfed at discharge Flint 2007 states that in Collins 2004 (the only study that reported length of stay), infants were not permitted to go home cup feeding (thus confounding the results). Also, this may not be the case in UK hospitals that are not referring babies to peripheral hospitals over a large geographical area The authors conclude that cup feeding may not be recommended over bottle feeding. However, it appears that there is a lack of good-quality evidence to make these conclusions. Also, there are many confounding factors (i.e. training of hospital staff, time provided to staff) that would likely affect the results in a direction towards bottle feeding. Although the authors suggest that more research would be futile, firm recommendations can’t be made until this is done |
Inclusion/exclusion criteria reported: ✓ Description of SR methodology: ✓ Rigorous literature search: ✓ Quality of studies assessed and taken into account: partially; no info provided on randomisation methods. Sufficient details about individual studies: ✓ Studies appropriately combined: a meta-analysis was conducted |
| First author, year, design quality | Review/research question | Included studies | Main results | Confounders/comments | Quality (mark ✓ or ✗) |
|---|---|---|---|---|---|
|
McInnes 2006161 NHS Health Scotland SR |
Review question What interventions support breastfeeding in neonatal units? Data sources The authors searched CDSR, DARE, AMED, British Nursing Index, CINAHL, EMBASE, MEDLINE, PsycINFO for studies published in English from 1990 to June 2005. In addition, MIDIRS was searched. Bibliographies of included studies were also checked for additional relevant studies. A list of specific search terms was not provided Inclusion criteria Study types Experimental studies were included in the review (the actual studies included were RCTs and CTs, with one cohort study and one case–control study Participants Preterm, low birthweight infants or their parents or neonatal unit staff Interventions The authors did not specify interventions of interest in their inclusion criteria Outcomes reported To be included in the review, studies had to examine breastfeeding or the provision of breastmilk as an outcome Quality assessment The reviewers included a lengthy list of criteria to assess quality (not referenced) |
36 studies were included in the review: Four studies evaluated expression of breast milk: Fewtrell 2001 (RCT) (UK, quality score 82%) Standard electric pump vs hand pump in mothers with preterm infants (< 35 weeks) (n = 145) Jones 2001 (RCT) (UK, quality score 66%) Sequential vs simultaneous breastmilk expression in mothers with preterm infants (mean gestation = 30 weeks) (n = 36) Paul 1996 (quasi-experimental) (India, quality score 63%) Manual expression (hand) vs pump in mothers of infants in NICU (size/age of infants not specified) (n = 22) Groh-Wargo 1995 (RCT) (USA, quality score 53%) Sequential vs simultaneous breastmilk expression in mothers with infants ≤ 1500 g and ≤ 7 days old (n = 32) Two studies evaluated support for parents: Gonzalez 2003 (case–control) (USA, quality score 83%) Lactation counselling service for women of preterm (< 37 weeks) or LBW (< 2.5 kg) infants (n = 175) Pinelli 2001 (RCT) (Canada, quality score 61%) Breastfeeding counselling for both parents of VLBW healthy babies (< 1.5 g) (n = 128) Nine studies evaluated cup feeding, bottles, teats or dummies (seven that evaluated breastfeeding duration as an outcome are listed here): Collins 2004 (RCT) (Australia, quality score 86%) Cup feeding vs bottle feeding in preterm infants (< 34 weeks) (n = 303) Mosley 2001(RCT) (UK, quality score 75%) Cup feeding vs bottle feeding in preterm infants (30–37 weeks) (n = 16) Rocha 2002 (RCT) (Brazil, quality score 71%) Cup feeding vs bottle feeding in preterm (32–36 weeks) or LBW (< 1700 g) infants (n = 78) Kliethermes 1999 (RCT) (USA, quality score 53%) NG vs bottle supplementation in infants weighing 1–2.5 kg (n = 84) Gilks 2004 (RCT) (UK, quality score 25%) Cup feeding vs bottle feeding in preterm infants (< 35 weeks at birth and > 30 weeks at start of trial) (n = 54) Meier 2000 (quasi-experimental) (USA, quality score 46%) Use of nipple shields in preterm infants (25–37 weeks) (n = 34) Oddy 2003 (quasi-experimental) (Australia, quality score 7%) Finger feeding in preterm infants (< 37 weeks) Two studies evaluated early discharge: Gunn 2000 (RCT) (New Zealand, quality score 78%) Early discharge with home support (n = 308) Örtenstrand 2001 (quasi-experimental) (Sweden, quality score 71%) Early discharge with home support (n = 75) One study evaluated neonatal staff education: Siddell 2003 (cohort) (USA, quality score 57%) BF education (n = 51) Eleven studies evaluated kangaroo mother care (KMC) and skin-to-skin contact (SSC): Rojas 2003 (RCT) (USA, quality score 94%) SSC vs traditional holding in preterm infants (≤ 32 weeks) or LBW (≤ 1500 g) (n = 60) Charpak 1997 (RCT) (Colombia, quality score 86%) KMC in LBW infants (≤ 2000 g) (n = 746) Charpak 2001 (RCT) (Colombia, quality score 78%) KMC in LBW infants (≤ 2000 g) (n = 693) Cattaneo 1998 (RCT) (Ethiopia, Mexico and Indonesia, quality score 76%) KMC in LBW infants (1000–1999 g) (n = 285) Kadam 2005 (RCT) (India, quality score 73%) KMC in LBW infants (≤ 1800 g) (n = 89) Blaymore Bier 1996 (RCT) (USA, quality score 72%) SSC vs standard care (n = 41 mothers, 50 infants) Charpak 1994 (RCT) (Colombia, quality score 71%) KMC in LBW infants (≤ 2000 g) (n = 332) Wahlberg 1992 (CT) (Sweden, quality score 57%) KMC (n = 66) Hurst 1997 (quasi-experimental) (USA, quality score 47%) SSC (n = 23) Sloan 1994 (RCT) (Ecuador, quality score 44%) KMC in LBW infants (≤ 2000 g) (n = 275) Roberts 2001 (‘randomised trial’) (Australia, quality score 21%) KMC vs cuddling care (n = 30) One study evaluated breastmilk fortification (but will not be addressed here) Three studies evaluated galactagogues: DaSilva 2001 (RCT) (Canada, quality score 100%) Domperidone on milk production (n = 20) Hansen 2005 (RCT) (USA, quality score 87%) Metoclopramide on milk production (n = 57) Gunn 1996 (RCT) (New Zealand, quality score 65%) Growth hormone on milk production (n = 20) One study evaluated The Baby Friendly Initiative: Merewood 2003 (quasi-experimental) (USA, quality score 72%) (n = 227) Two studies evaluated test weighing (but they did not evaluate breastfeeding) |
The authors of the SR summarised their main findings as follows: Expression of breastmilk Simultaneous pumping with an electric pump was quicker than sequential pumping with an electric pump; however, an appropriate hand pump may be more effective; breast massage assisted breastmilk expression; mothers preferred simultaneous electric pumping compared to sequential electric pumping, although a hand pump was preferred over an electric pump; none of the mothers in the UK studies expressed milk as many times a day as recommended Support for parents (counselling) Counselling and support services for both parents did not affect breastfeeding duration or exclusivity in an advantaged and motivated population; provision of clinical support and individualised care planning may increase the number of mothers willing to express their own milk for their infants The use of bottles, teats, cups and dummies The impact of cup feeding on breastfeeding is inconclusive; however, there is no evidence that cup feeding has a negative impact There is a lack of studies on the use of dummies, but their use in preterm infants is not currently associated with any adverse effects in terms of breastfeeding duration The impact of nipple shields on breastfeeding has not been adequately assessed Early discharge from the neonatal unit Breastfeeding rates were not significantly affected by early discharge with home support; early discharge with support was acceptable to both parents Neonatal staff education There is a lack of studies that evaluate the impact of educational interventions on knowledge or attitudes of neonatal staff or on breastfeeding/expression rates in the neonatal unit Kangaroo mother care and skin-to-skin contact KMC or SSC may increase breastfeeding amongst LBW infants, particularly in countries where breastfeeding is less prevalent Large trials that the examine the short- and long-term effectiveness of KMC and SSC on breastfeeding duration have not been conducted in westernised countries Galactagogues Short-term use of domperidone was associated with increased milk volumes; growth hormone achieved modest increases in milk volumes; metoclopramide did not demonstrate an effect There is no evidence that the use of galactagogues increases breastfeeding duration in mothers of premature infants; however, they do not decrease breastfeeding duration The Baby Friendly Initiative There is limited evidence of an impact of BFI in neonatal units where breastfeeding rates have traditionally been low; further research is needed Cost-effectiveness outcomes Not reported Were outcomes reported for different gestational ages of the baby and/or ability to co-ordinate sucking and swallowing? No |
Applicability to the UK: The authors of the SR noted that only five of the studies were UK based, thus the findings from other studies may be less applicable to the UK The authors also noted that due to the heterogeneity of the studies, it was difficult to draw any firm conclusions about effective practice Many of the studies had small sample size |
Inclusion/exclusion criteria reported: partially Description of SR methodology: ✓ Rigorous literature search: ✓ (search terms not provided) Quality of studies assessed and taken into account: partially; no info provided on randomisation methods Sufficient details about individual studies: ✓ Studies appropriately combined: ✓ (narrative synthesis) |
Appendix 8 Probabilistic sensitivity analysis distributions – health economics review
| Description | Parameters/info |
|---|---|
| Odds ratio of NEC given own mother’s milk vs some mothers’ milk plus donor milk | Log-normal, u (mean of logs) = – 0.1223, sigma (SD of logs) = 0.692; expected value: 1.124267823 |
| Odds ratio of NEC given some mothers’ milk plus donor milk vs some mothers’ milk plus formula | Log-normal, u (mean of logs) = – 0.7663, sigma (SD of logs) = 0.656; expected value: 0.576297073 |
| Odds ratio of NEC given formula vs some mothers’ milk plus formula | Log-normal, u (mean of logs) = 1.1007, sigma (SD of logs) = 0.396; expected value: 3.251472595 |
| Odds ratio of sepsis given own mother’s milk vs some mothers’ milk plus donor | Log-normal, u (mean of logs) = – 0.3446, sigma (SD of logs) = 0.378; expected value: 0.760972544 |
| Odds ratio of sepsis given some mothers’ milk plus donor milk vs some mothers’ milk plus formula | Log-normal, u (mean of logs) = – 0.0028, sigma (SD of logs) = 0.341; expected value: 1.056900428 |
| Odds ratio of sepsis given formula vs some mothers’ milk plus formula | Log-normal, u (mean of logs) = – 0.2189, sigma (SD of logs) = 0.149; expected value: 0.812369901 |
| Odds ratio of mortality given Gram-positive infection | Log-normal, u (mean of logs) = 0.476, sigma (SD of logs) = 0.119; expected value: 1.621060395 |
| Odds ratio of mortality given Gram-negative infection | Log-normal, u (mean of logs) = 1.983, sigma (SD of logs) = 0.141; expected value: 7.337076747 |
| Odds ratio of mortality given fungal infection | Log-normal, u (mean of logs) = 1.787, sigma (SD of logs) = 0.183; expected value: 6.072342834 |
| Odds ratio of mortality given medical NEC | Log-normal, u (mean of logs) = 0.720, sigma (SD of logs) = 0.141; expected value: 2.074957144 |
| Odds ratio of mortality given surgical NEC | Log-normal, u (mean of logs) = 1.139, sigma (SD of logs) = 0.123; expected value: 3.147361554 |
| Odds ratio of NDI given sepsis | Log-normal, u (mean of logs) = 0.825, sigma (SD of logs) = 0.069; expected value: 2.287319253 |
| Odds ratio of NDI given medical NEC | Log-normal, u (mean of logs) = 0.172, sigma (SD of logs) = 0.194; expected value: 1.210239169 |
| Odds ratio of NDI given surgical NEC | Log-normal, u (mean of logs) = 0.686, sigma (SD of logs) = 0.188; expected value: 2.0211608 |
| Odds ratio of normal vs enhanced staff contact | Log-normal, u (mean of logs) = -0.6931, sigma (SD of logs) = 0.2018; expected value: 0.510309242 |
| Level one cost | Gamma, alpha = 9.16, lambda = 0.009758; expected value: 938.716950195 |
| Level two cost | Gamma, alpha = 14.15, lambda = 0.021088; expected value: 670.997723824 |
| SCBU cost | Gamma, alpha = 16.47, lambda = 0.040662; expected value: 405.046480744 |
| Disutility for no disability | Gamma, alpha = 0.25, lambda = 4.167; expected value: 0.0599952 |
| Disutility for mild disability | Gamma, alpha = 2.11, lambda = 14.048; expected value: 0.150199317 |
| Disutility for moderate disability | Gamma, alpha = 9.53, lambda = 26.843; expected value: 0.355027381 |
| Disutility for severe disability | Gamma, alpha = 4.49, lambda = 8.480; expected value: 0.529481132 |
| Probability of intention to breastfeed | Beta, integer parameters only, n = 8210, r = 5911; expected value: 0.719975639 |
| Major cost | Gamma, alpha = 3.26, lambda = 0.002155; expected value: 1512.761020882 |
| Probability of mothers’ milk given ITB | Dirichlet, Alphas list = List(78;32;5); expected value: 0.67826087; 0.27826087; 0.043478261 |
| Probability of some mothers’ milk given ITB | Dirichlet, Alphas list = List(32;78;5); expected value: 0.27826087; 0.67826087; 0.043478261 |
| Probability of mothers’ milk given NITB | Dirichlet, Alphas list = List(13;41;27); expected value: 0.160493827; 0.50617284; 0.333333333 |
| Probability of some mothers’ milk given NITB | Dirichlet, Alphas list = List(41;13;27); expected value: 0.50617284; 0.160493827; 0.333333333 |
| Probability of formula only given ITB | Dirichlet, Alphas list = List(5;78;32); expected value: 0.043478261; 0.67826087; 0.27826087 |
| Distribution information for the 500–999 g population | |
| Baseline death no disease | Beta, integer parameters only, n = 4401, r = 905; expected value: 0.205635083 |
| Baseline probability of NDI given no disease | Beta, integer parameters only, n = 402, r = 195; expected value: 0.485074627 |
| Probability of sepsis given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(185;24;22;449); expected value: 0.272058824; 0.035294118; 0.032352941; 0.660294118 |
| Probability of medical NEC given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(24;185;22;449); expected value: 0.035294118; 0.272058824; 0.032352941; 0.660294118 |
| Probability of surgical NEC given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(22;185;24;449); expected value: 0.032352941; 0.272058824; 0.035294118; 0.660294118 |
| Probability of no disease given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(449;185;24;22); expected value: 0.660294118; 0.272058824; 0.035294118; 0.032352941 |
| Probability of mild disability | Dirichlet, Alphas list = List(105;58;32); expected value: 0.538461538; 0.297435897; 0.164102564 |
| Probability of moderate disability | Dirichlet, Alphas list = List(58;105;32); expected value: 0.297435897; 0.538461538; 0.164102564 |
| Distribution information for the 1000–1749 g population | |
| Baseline death no disease | Beta, integer parameters only, n = 4401, r = 352; expected value: 0.079981822 |
| Baseline probability of NDI given no disease | Beta, integer parameters only, n = 431, r = 178; expected value: 0.412993039 |
| Probability of sepsis given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(17;60;17;2776); expected value: 0.005923345; 0.020905923; 0.005923345; 0.967247387 |
| Probability of medical NEC given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(60;17;17;2776); expected value: 0.020905923; 0.005923345; 0.005923345; 0.967247387 |
| Probability of surgical NEC given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(17;17;60;2776); expected value: 0.005923345; 0.005923345; 0.020905923; 0.967247387 |
| Probability of no disease given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(2776;17;60;17); expected value: 0.967247387; 0.005923345; 0.020905923; 0.005923345 |
| Probability of mild disability | Dirichlet, Alphas list = List(122;42;14); expected value: 0.685393258; 0.235955056; 0.078651685 |
| Probability of moderate disability | Dirichlet, Alphas list = List(42;122;14); expected value: 0.235955056; 0.685393258; 0.078651685 |
| Distribution information for the 1750–2500 g population | |
| Baseline death no disease | Beta, integer parameters only, n = 4401, r = 220; expected value: 0.049988639 |
| Baseline probability of NDI given no disease | Beta, integer parameters only, n = 767, r = 263; expected value: 0.342894394 |
| Probability of sepsis given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(19;96;19;19155); expected value: 0.000985017; 0.00497693; 0.000985017; 0.993053035 |
| Probability of medical NEC given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(24;185;22;449); expected value: 0.035294118; 0.272058824; 0.032352941; 0.660294118 |
| Probability of surgical NEC given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(22;185;24;449); expected value: 0.032352941; 0.272058824; 0.035294118; 0.660294118 |
| Probability of no disease given some mothers’ milk plus supplement | Dirichlet, Alphas list = List(449;185;24;22); expected value: 0.660294118; 0.272058824; 0.035294118; 0.032352941 |
| Probability of mild disability | Dirichlet, Alphas list = List(171;55;37); expected value: 0.650190114; 0.209125475; 0.140684411 |
| Probability of moderate disability | Dirichlet, Alphas list = List(55;171;37); expected value: 0.209125475; 0.650190114; 0.140684411 |
Appendix 9 Sensitivity analyses – health economics review
Sensitivity analyses incremental values for enhanced staff support compared to normal support
| 500 to 999 g | 1000 to 1749 g | 1750 to 2500 g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ∆ Cost | ∆ Effect | ∆ C/E (ICER) | ∆ Cost | ∆ Effect | ∆ C/E (ICER) | ∆ Cost | ∆ Effect | ∆ C/E (ICER) | ||
| (£) | (QALY) | (£/QALY) | (£) | (QALY) | (£/QALY) | (£) | (QALY) | (£/QALY) | ||
| 1 | Base case | – 586.02 | 0.251 | – 293.04 | 0.056 | – 66.08 | 0.009 | |||
| 2 | Gonzalez effect only | – 571.18 | 0.246 | – 284.25 | 0.054 | – 62.12 | 0.009 | |||
| 3 | 0.6 ITB + 0.4 NITB | – 516.57 | 0.217 | – 246.80 | 0.049 | – 45.21 | 0.008 | |||
| 4 | 0.4 ITB + 0.6 NITB | – 705.38 | 0.304 | – 369.28 | 0.066 | – 100.50 | 0.010 | |||
| 5 | Midwife | – 514.42 | 0.251 | – 221.43 | 0.056 | 5.77 | 0.009 | 663.22 | ||
| 6 | Half length of stay | – 286.71 | 0.251 | – 131.74 | 0.056 | 14.26 | 0.009 | 1,639.08 | ||
| 7 | Donor, no formula | – 78.18 | 0.290 | 225.25 | 0.064 | 3530.56 | 328.11 | 0.009 | 34,905.32 | |
| 8 | Formula | – 709.39 | 0.284 | – 375.30 | 0.060 | – 101.53 | 0.009 | |||
| 9 | Expression kits | – 332.59 | 0.251 | – 100.27 | 0.056 | 48.64 | 0.009 | 5,590.80 | ||
| 10 | Formula + Express | – 388.72 | 0.251 | – 182.54 | 0.060 | 13.19 | 0.009 | 1,516.09 | ||
| 11 | Donor + Formula | – 149.69 | 0.290 | 161.58 | 0.064 | 2532.60 | 283.06 | 0.009 | 30,112.77 | |
| 12 | Donor + Formula + Express | 103.00 | 0.290 | 354.68 | 354.11 | 0.064 | 5550.31 | 397.63 | 0.009 | 42,301.06 |
| 13 | Reduced donor costs | – 458.71 | 0.290 | – 115.36 | 0.064 | 89.30 | 0.009 | 9,500.00 | ||
| 14 | Red donor + F | – 530.22 | 0.290 | – 179.03 | 0.064 | 44.09 | 0.009 | 4,690.43 | ||
| 15 | N Ireland ITB 56% | – 569.27 | 0.231 | – 277.72 | 0.053 | – 61.77 | 0.008 | |||
| 16 | ITB 50% | – 563.00 | 0.223 | – 271.97 | 0.052 | – 60.15 | 0.008 | |||
| 17 | ITB 90% | – 604.87 | 0.274 | – 310.27 | 0.059 | – 70.93 | 0.009 | |||
| 18 | Lower disability 1750 to 2500 g | – 56.06 | 0.008 | |||||||
| 19 | Vohr formula effect | 81.82 | 0.080 | Intervention dominated | 152.38 | – 0.005 | Intervention dominated | 134.96 | – 0.001 | Intervention dominated |
Sensitivity analyses for enhanced staff support compared to normal support
| 1 | Base case | Base-case assumptions |
| 2 | Gonzalez effect only | The effectiveness estimate of enhanced staff support was based on Gonzalez et al. alone |
| 3 | 0.6 ITB + 0.4 NITB | The effectiveness of enhanced support was higher for mothers who intended to breastfeed |
| 4 | 0.4 ITB + 0.6 NITB | The effectiveness of enhanced support was higher for mothers who did not intend to breastfeed |
| 5 | Midwife | A midwife rather than a registered nurse was assumed to provide the support at higher cost |
| 6 | Half length of stay | All length of stay assumptions were halved |
| 7 | Donor, no formula | The cost of donor milk was added for mothers who expressed milk but could not supply all the infant’s milk intake |
| 8 | Formula | The cost of formula milk was added for all milk intake other than mother’s milk |
| 9 | Expression kits | The costs of expression kits were added |
| 10 | Formula + Express | The costs of formula milk and expression kits were added |
| 11 | Donor + Formula | The costs of donor milk and formula milk were added |
| 12 | Donor + Formula + Express | The costs of donor milk, formula milk and expression kits were all added |
| 13 | Reduced donor costs | The cost of producing a litre of donor milk was reduced from £289.12 to £119.89 |
| 14 | Red donor + F | The lower costs of donor milk and formula milk were added to the base case |
| 15 | N Ireland ITB 56% | The intention to breastfeed rate was lowered from 72% to the N Ireland rate of 56% |
| 16 | ITB 50% | The intention to breastfeed rate was reduced to 50% |
| 17 | ITB 90% | The intention to breastfeed rate was increased to 90% |
| 18 | Lower disability 1750 to 2500 g | The rate of severe disability was reduced from 14.1% to 5% for the 1750 to 2500-g subgroup |
| 19 | Vohr formula effect | The odds ratio of getting confirmed NEC was reduced from 3.01 to 1.48 |
Appendix 10 Mother and baby contact interventions – additional study
Arecent RCT evaluating the kangaroo method of skin-to-skin contact was identified,288 which was published outside the search dates for this review. As this study met the remaining inclusion criteria for this review, a brief analysis is provided to be considered in conjunction with the existing evidence base.
The intervention comprised a ‘medium’-level kangaroo method of skin-to-skin contact (mean of 4.47 hours per day) among healthy, English-speaking mothers of singleton infants with a birthweight of 1300–3000 g and gestational age of 32–36 weeks. This trial excluded infants with a condition that could prevent KMC or a severe congenital abnormality, or who required CPAP or mechanical ventilation by 48 hours postbirth. All mothers included in the analysis for this paper intended to breastfeed. The authors also report that the nurse researchers assisted mothers with initial breastfeeding experiences and recognition of subtle infant feeding cues and encouraged self-regulatory feedings in response to these cues. Infants were brought to mothers remaining on the labour and delivery unit due to a medical complication. Standard care includes access to lactation consultants although the authors note that coverage is likely to be incomplete.
This study reported that KC dyads breastfed significantly longer than control dyads (5.08 ± 5.48 months vs 2.05 ± 2.15 months; p = 0.003; t = – 2.86) and more KC dyads than control dyads breastfed exclusively at discharge (I: 72%; C: 60%) and at 1.5 (I: 33%; C: 17%), 3 (I: 19%; C: 3%) and 6 (I: 8%; C: 0%) months. Exclusive breastfeeding is defined as Index of Breastfeeding Status levels 1 and 2, namely ‘exclusive’ (only human milk) and almost exclusive (allows vitamins, small amounts of water or juice or ritual feedings).
A rapid appraisal of study quality suggests this RCT is of moderate quality overall having employed an adequate randomisation method and incurring small losses, reported by group with reason. An ITT analysis was not conducted, breastfeeding rates are self-reported, and it is not clear if adequate concealment of allocation was achieved. Acceptability of the intervention was not reported although losses to study following commencement of the intervention were small.
Findings from this trial suggest that the combination of ‘medium’-level kangaroo skin-to-skin contact and personal education and support from a skilled nurse is feasible among women in the USA and will increase duration of any and exclusive breastfeeding up to 6 months among mothers who intend to breastfeed.
Appendix 11 UNICEF UK Baby Friendly Initiative Best Practice Standards
Appendix 11.1: UNICEF UK Baby Friendly Initiative Best Practice Standards for establishing and maintaining lactation and breastfeeding in neonatal units
1. Have a written (neonatal unit) breastfeeding policy which is routinely communicated to all staff
The neonatal unit should have a written breastfeeding policy that addresses all these standards and protects breastfeeding. The policy should be formulated in conjunction with the maternity and community services (where relevant) in order to ensure a seamless delivery of care. It should identify clearly the professional groups which will act as the point of first referral to support mothers to breastfeed.
A summary of the policy should be prominently displayed in the unit. The full policy and any supporting guidance should be available on request. The policy and summary should be translated into other languages where appropriate. All neonatal unit managers should be able to locate easily a copy of the policy and be able to describe the process of staff orientation to the policy.
Compliance with the policy should be audited annually and the results of this audit used to ensure continuing full implementation of all standards.
Breastmilk feeding and breastfeeding rates on discharge from the unit should be recorded and progress reported to all staff.
All policies and procedures should support breastmilk feeding and the establishment of breastfeeding in line with these standards.
2. Educate all health-care staff in the skills necessary to implement the policy
All health-care staff should receive orientation to both the breastfeeding policy and any supporting guidance as soon as their employment on the neonatal unit begins.
Education programmes that cover all of the standards will be provided for each professional group and area of responsibility.* Clear curricula or course outlines for each group should be developed. A training schedule for new employees should exist.
All staff caring for mothers and their babies should have received breastfeeding training appropriate to their role or, if new, have received orientation on arrival and be scheduled to receive training within 6 months.
*It is recommended that the training for staff who have primary responsibility for supporting mothers to initiate and maintain lactation have at least 18 hours breastfeeding education including a minimum of 3 hours supervised clinical practice relating to teaching a mother how to breastfeed and how to hand express breastmilk.
3. Inform all parents of the benefits of breastmilk and breastfeeding for babies in the neonatal unit
All parents whose baby is admitted, or is likely to be admitted, to the neonatal unit should have a one-to-one discussion with a suitably qualified* health professional about the crucial importance of breastmilk to the preterm and ill infant.** This discussion along with the parents’ decision should be documented in the baby’s records.
Written materials provided to parents on the benefits of breastmilk and breastfeeding should be accurate and effective.
*Suitably qualified health professionals would include paediatricians, infant feeding specialists, midwives and nurses who have been appropriately educated in breastfeeding and lactation management.
**The discussion on the importance of breastfeeding should emphasise the particular importance of breastmilk to the preterm and ill infant and will need to include information about the importance of breastmilk in relation to the prevention of necrotising enterocolitis and improvement of neurological development. The longer term benefits of breastfeeding to babies and mothers should also be explained.
4. Facilitate skin-to-skin contact (kangaroo care) between mother and baby
The benefits of skin-to-skin contact should be discussed with all parents at an appropriate time to allow informed decision-making. Skin-to-skin contact between mother and baby should be initiated in an unhurried environment as soon as the baby’s condition allows.
Skin-to-skin contact should continue to be offered as often as possible (at least on a daily basis) or whenever the mother is available and the baby’s condition allows.
5. Support mothers to initiate and maintain lactation through expression of breastmilk
All mothers with a baby on the neonatal unit should be encouraged to initiate lactation as soon after delivery as the mother’s condition allows. All mothers whose babies cannot breastfeed or take full feeds from the breast should be taught how to express their milk by hand and by pump. Expression of breastmilk should be encouraged at least 6–8 times in 24 hours, including at night. Emphasis should be on frequent expressing and the avoidance of long intervals between expressions.
Well-maintained equipment for the safe expression of breastmilk should be available at all times.
Facilities should be available to allow mothers to express breastmilk in comfort either near their baby or in private if preferred.
Local policies on the safe handling, storage and transportation of breastmilk should be developed in line with nationally agreed guidelines.
A system for the provision of breast pumps for home use should also be in place.
6. Support mothers to establish and maintain breastfeeding
All breastfeeding mothers should be offered help with a first breastfeed as soon their baby’s condition permits. Breastfeeding mothers should receive information, help and support to achieve correct positioning and attachment.*
When the baby is not yet able to take a full feed from the breast, mothers should be encouraged to practise positioning techniques.
Parents should be given information on the importance of baby-led feeding (as soon as appropriate) for the continuation of breastfeeding. They should be taught to recognise feeding cues and be encouraged to use all available opportunities to initiate breastfeeds.
The unit should have a policy of open visiting for parents. Facilities for rooming-in should be available and where possible mothers and babies should be enabled to room-in together.**
All written materials on infant feeding provided for parents should be accurate and effective.
*It is recognised that some mothers may not wish to breastfeed, but may decide to continue expressing breastmilk. In this circumstance the mothers should be supported to continue providing breastmilk and given an informed choice regarding the short- and long-term benefits to baby of feeding directly from the breast.
**It is recognised that rooming-in facilities may not be available within the neonatal unit. However, new mothers should at least be cared for in the same hospital as their baby. Where facilities are available, breastfeeding mothers should be encouraged to room-in with their baby in the neonatal unit.
7. Encourage exclusive breastmilk feeding
No food or drink other than breastmilk should be given to a baby who is being breastfed or receiving breastmilk unless this is clinically indicated or the result of a fully informed parental decision.
A mother’s own breastmilk is the first choice for infant feeding. Where mother’s own milk is not available the use of donor milk should be considered and where possible obtained.
When mothers are separated from their babies, mechanisms should exist to enable the regular transportation of the mother’s milk to the facility caring for the baby.
All written guidelines and protocols should support exclusive breastfeeding.*
No promotion for breastmilk substitutes, feeding bottles, teats or dummies should be displayed or distributed to parents or staff in the facility.
*Protocols for conditions such as hypoglycaemia, jaundice requiring phototherapy or slow weight gain should protect exclusive breastfeeding. If breastmilk fortifiers are used, protocols should be developed to ensure use is limited to clear clinical indication, for example for very low birthweight (< 1500-g) babies when a biochemical assessment indicates a need.
8. Avoid the use of teats or dummies for breastfed babies unless clinically indicated
Babies who are unable to feed directly from the breast should be fed breastmilk by a method appropriate to the baby’s developmental ability. Parents wishing to breastfeed who request that their baby be fed by teat must have the potential risks discussed and alternatives offered.
Dummy use should be limited to when there is a clear clinical indication or fully informed parental choice.
Skin-to-skin contact and breastfeeding should be promoted for comforting babies and relieving pain during minor procedures such as heel pricks. Feeding and comforting methods appropriate to the baby’s condition, and with reference to the presence or absence of the parents at any given time, should be discussed with the parents. The discussion should be evidence based and include all potential benefits, risks and alternatives to allow informed decision-making. The discussion and the parents’ choice should be recorded in the baby’s notes or care plan.
9. Promote breastfeeding support through local and national networks
All mothers should be provided with the contact details of midwives, health visitors, community neonatal nurses (where these exist), breastfeeding support networks and organisations that support parents of ill and premature babies for help with breastfeeding on admission to a neonatal unit and on discharge of the baby from hospital.
A formal mechanism should exist to ensure that information on breastfeeding progress is passed on during handover of care from the neonatal unit to the community health-care team.
Appendix 11.2: UNICEF UK Baby Friendly Best Practice Standards in neonatal units – interventions examined in this review
Baby Friendly accreditation of maternity services has been demonstrated to be a highly effective framework, and guidance for promotion of initiation of breastfeeding in maternity services generally80,204–206 (UNICEF UK BFI 2000, Bartington et al. 204 in Dyson et al. 200659). This is reflected in national guidance recommending increased implementation in the UK. 3,59 Neonatal units are included to a limited extent within the Baby Friendly best practice standards for maternity units. Maternity units seeking Baby Friendly accreditation are expected to ensure that all staff working on the neonatal unit are trained to support breastfeeding adequately. The information and support given to mothers who are separated from their baby to express their breastmilk is also assessed.
The UNICEF UK Baby Friendly Initiative standards for neonatal units were created in recognition of the need for clear guidance on what constitutes best practice for breastfeeding when babies are preterm or ill and separated from their mothers. They provide guidance on the specific standards required in order to allow neonatal unit staff to fully promote, protect and support breastfeeding within their clinical area (see Appendix 11a for the BFI standards for neonatal units). At present, UNICEF UK does not provide an accreditation programme for neonatal units.
To assist in the analysis of the review findings, and in the implementation of best practice, we have mapped the findings of the effectiveness of interventions from this review onto the Best Practice standards for neonatal units (Table 88). The findings from this mapping process can assist policy makers and practitioners in two key areas:
-
To identify effective interventions to inform the successful implementation of specific Baby Friendly standards within each unit.
-
To highlight the integrated nature of the Baby Friendly standards and the likelihood of one intervention having a positive effect on several standards simultaneously.
There is currently no accreditation programme for these standards, therefore no evaluations of the effectiveness of Baby Friendly accreditation for neonatal units have yet been undertaken. Studies evaluating the effectiveness of Baby Friendly accreditation for maternity hospitals in improving breastfeeding outcomes in neonatal units have been conducted. These, and studies evaluating any interventions that are consistent with one or more of the Baby Friendly neonatal care standards, are included in the matrix. Results from poor-quality studies are excluded from this matrix due to potential for misinterpretation. Use of ITT analysis to generate findings of effectiveness for good and moderate quality studies is also reported for each study. This is based on ITT analysis conducted by the authors of this review, including adjustment for postrandomisation exclusions as appropriate. Details of all studies are provided in Chapter 4.
| Baby Friendly Best Practice standard for neonatal units | Study | Intervention | Study design | Targeted population group/country | Quality rating of study | Breastfeeding outcome(s) | Effect of intervention |
|---|---|---|---|---|---|---|---|
| 1. Written neonatal unit breastfeeding policy | See Related evidence below: ‘BFI accreditation of maternity hospital comprising neonatal units’ | ||||||
| 2. Education of health-care staff on skills to implement policy | Jones 200481 | Evidence-based staff education programme taught over 10 hours in five modules by neonatal breastfeeding coordinator | Before/after |
Range of health professionals Mothers who planned to breastfeed UK |
Moderate Not ITT analysis |
Infants receiving expressed breastmilk, cup feeds offered, put to the breast | Positive for all three transitional outcomes |
| Any breastfeeding at discharge | Positive | ||||||
| Pineda 2006149 | Comprehensive educational programme, use of individualised pathway of care plan and education and support to mothers | Before/after |
VLBW infants USA |
Moderate ITT analysis |
Mothers ever providing breastmilk | No effect | |
| Mothers who ever breastfed | Positive | ||||||
| Provision of breastmilk in hospital and at discharge | No effect | ||||||
| 3. Inform parents of benefits of breastmilk and breastfeeding | See related standard on ‘Education of health-care staff on skills to implement policy’ above | ||||||
| 4. Facilitate skin-to-skin contact (kangaroo care) between mother and baby | Charpak 1997,107 2001108 | Kangaroo skin-to-skin, early discharge and regular bf | RCT |
LBW/VLBW Colombia |
Moderate ITT |
Any breastfeeding at 40–41 weeks corrected age | No effect |
| Exclusive breastfeeding at 40–41 weeks corrected age | No effect | ||||||
| Cattaneo 1998131 | Kangaroo skin-to-skin | RCT |
LBW/VLBW Ethiopia/Mexico/Indonesia |
Moderate ITT |
Exclusive breastfeeding at discharge | No effect | |
| Sloan 1994132 | Kangaroo skin-to-skin | RCT |
LBW/VLBW Ecuador |
Moderate ITT |
Exclusive breastfeeding at discharge | No effect | |
| Kadam 2005118 | Kangaroo skin-to-skin | RCT |
LBW/VLBW India |
Moderate ITT |
Timing of initiation | No effect | |
| Rojas 2003121 | Kangaroo skin-to-skin | RCT |
VLBW USA |
Moderate ITT |
Any breastfeeding before discharge | No effect | |
| Boo 2007141 | Short kangaroo skin-to-skin | RCT |
VLBW Malaysia |
Moderate ITT |
Any breastfeeding before discharge | Positive | |
| Any breastfeeding at discharge | Positive | ||||||
| Blaymore Bier 1996115 | Kangaroo skin-to-skin | RCT |
VLBW USA |
Moderate ITT |
Any breastfeeding at discharge | No effect | |
| Any breastfeeding one month after discharge | Positive | ||||||
| Whitelaw 1988147 | Kangaroo skin-to-skin | RCT |
VLBW UK |
Moderate ITT |
Any breastfeeding for more than 6 weeks | Positive | |
| Wahlberg 1992135 | Kangaroo skin-to-skin | Before/after |
LBW Sweden |
Moderate ITT |
Any breastfeeding at discharge | Positive | |
| 5. Support mothers to express breastmilk | Fewtrell 2001125 | Hand-powered sequential pumping vs electric sequential or simultaneous pumping | RCT |
Mothers expressing for infants of < 35 weeks’ gestation UK |
Good Not ITT |
Breastmilk output by volume | No effect |
| Any breastmilk feedings at discharge or transfer | No effect | ||||||
| > 50% intake as breastmilk at discharge or transfer | No effect | ||||||
| Groh-Wargo 1995128 | Electric simultaneous pumping vs electric sequential pumping | RCT |
Mothers expressing for infants of VLBW USA |
Moderate ITT |
Breastmilk output by volume | No effect | |
| Jones 2001114 | Electric simultaneous pumping vs electrical sequential pumping | RCT |
Mothers expressing for infants of VLBW UK |
Moderate Not ITT |
Breastmilk output by weight | Positive | |
| Slusher 2007142 | Electrical simultaneous pumping vs hand expression | RCT |
Mothers expressing for infants of all birthweights and gestational age Kenya and Nigeria |
Moderate Not ITT |
Breastmilk output by volume | Positive | |
| Griffin 200020 | Teaching mothers CRCT technique to measure fat content of expressed breastmilk | Concurrent comparison |
Mothers expressing (no infant characteristics) USA |
Good ITT |
Accuracy of mothers’ measurements vs registered nurses | Positive | |
| Fewtrell 2006144 | Five-day course of oxytocin nasal spray plus daily support from research nurse | RCT |
Mothers who had recently given birth to premature infants UK |
Good ITT |
Total milk weight over days 1–5 | No effect | |
| Fat content of expressed milk | No effect | ||||||
| Hansen 2005116 | Ten-day course of oral metoclopramide 10 mg three times a day | Randomised crossover |
Mothers who had recently given birth to premature infants USA |
Moderate Not ITT |
Milk volume on day 10 and 1 week after | No effect | |
| Median weeks duration of breastfeeding | No effect | ||||||
| da Silva 2001123 | Seven-day course of oral domperidone 10 mg three times a day | RCT |
Mothers who had been expressing for at least a month and whose milk production was not meeting their infants’ needs Canada |
Moderate Not ITT Very small sample: I: 7; C: 9 |
Mean milk volume at baseline and over study days 2–7 | Positive | |
| Gunn 1996133 | Seven-day course of recombinant human growth hormone 0.2 IU/kg/day subcutaneously to a maximum of 16 IU/day | RCT |
Mothers who had been expressing for at least a month and whose milk production was not meeting their infants’ needs New Zealand |
Moderate Not ITT |
Mean daily milk volume over study days 0–1 compared to mean milk volume on day 8 for each group | Positive (significant for I group but not C) | |
| Feher 1989146 | 20-minute audio-cassette of relaxation imagery/techniques recommended for use when mothers wanted to express | RCT | USA |
Moderate Not ITT |
Mean milk volume at a single expression during second postnatal week | Positive | |
| Fat content of a single sample using a creamatocrit test | No effect | ||||||
| Jones 2001114 | Breast massage by mother vs no breast massage prior to pumping (in addition to simultaneous pumping vs sequential pumping detailed above | Randomised crossover | UK |
Moderate Not ITT |
Milk yield per expression |
Positive Breast massage improved milk production for both groups conducting simultaneous or sequential pumping |
|
| Mersmann 1993148 | Therapeutic touch (TT) vs mimic therapeutic touch vs no treatment | Randomised crossover |
Mothers who had been expressing for 2 weeks USA |
Moderate Not ITT |
Milk volume | Positive after TT vs mimic TT and no treatment | |
| 6. Support mothers to establish and maintain breastfeeding | Pinelli 2001126 | Individual counselling by lactation consultant with video and regular (weekly) contact in hospital and at home while breastfeeding | RCT |
No details Canada |
Good ITT |
Intake from breastmilk/breastfeeding at term | No effect |
| Any breastfeeding at 12 and 24 weeks | No effect | ||||||
| Gonzalez 2003166 | Individual support service to mothers within the NICU by an International Board Certified Lactation Consultant | Before/after | USA |
Moderate Not ITT |
Ever receiving mother’s own milk | Positive | |
| Receiving mother’s own milk at hospital discharge | Positive | ||||||
| Gunn 2000127 | Early discharge with home support for suckle feeding infants without demonstrated sustained weight gain | RCT |
Mothers of European and Maori descent who mainly intended to breastfeed their preterm, LBW or VLBW infant(s) New Zealand |
Moderate Not clear if ITT |
Any breastfeeding at discharge or at 6 weeks and 6 months after discharge | No effect | |
| 7. Encourage exclusive breastmilk feeding | See related standard ‘Support mothers to establish and maintain breastfeeding’ above | ||||||
| 8. Avoid use of teats or dummies for breastfed babies unless clinically indicated | Collins 2004119 | Cup vs bottle feeding | RCT |
< 34 weeks’ gestation Australia |
Good but low compliance ITT |
Any breastfeeding at discharge | No effect |
| Exclusive breastfeeding at discharge | Positive effect | ||||||
| Any breastfeeding at 3 or 6 months | No effect | ||||||
| Collins 2004119 | Pacifier vs no pacifier | RCT |
< 34 weeks’ gestation Australia |
Good but low compliance ITT |
Any breastfeeding at discharge and 3 or 6 months after discharge | No effect | |
| Exclusive breastfeeding at discharge | No effect | ||||||
| Mosley 2001124 | Cup vs bottle feeding | RCT |
30–37 weeks’ gestation UK |
Moderate ITT |
Exclusive breastfeeding at discharge | No effect | |
| Rocha 2002122 | Cup and finger feeding vs bottle feeding and pacifier | RCT |
32–36 weeks’ gestation LBW/VLBW Brazil |
Moderate ITT |
Any breastfeeding at discharge | No effect | |
| Any breastfeeding at 5–15 days or 3 months | No effect | ||||||
| Meier 2000136 | Ultra-thin silicone nipple shields vs no shield for mothers with breastfeeding problems | Retrospective crossover |
Preterm LBW USA |
Moderate Unclear if ITT |
Milk transfer to infant | Positive | |
| 9. Promote breastfeeding support through local and national networks (see standard 6 for related evidence base) | Agrasada 2005117 | Community-based breastfeeding peer counselling vs community-based child care peer counselling vs no peer counselling | RCT |
Mothers exclusively breastfeeding their term LBW infants Philippines |
Good ITT |
Any breastfeeding at 12 and 24 weeks | Positive for breastfeeding peer counselling group only |
| Exclusive breastfeeding from birth to 6 months | Positive for breastfeeding peer counselling group only | ||||||
| Merewood 2006143 | (Trained) Peer counsellor support within 73 hours of birth and for six weeks (BFI accredited care for both groups) | RCT |
Mothers of preterm, healthy infants USA |
Moderate Not ITT |
Any breastfeeding at 12 weeks | Positive | |
| Pereira 1984153 | (Trained) peer counsellor support by community-based volunteer counsellors | Before/after |
Mothers of infants in NICU who intended to breastfeed USA |
Moderate Not ITT |
Duration of any breastfeeding prior to hospital discharge | Positive | |
| Mean duration of breastfeeding | Positive | ||||||
| Related evidence from this review | |||||||
| BFI accreditation of maternity hospital comprising neonatal unit | Merewood 2003137 | BFI accreditation | Before/after |
Preterm infants of mainly black American or Hispanic mothers USA |
Good ITT |
Receiving any breastmilk during first week of enteral feeds | Positive |
| Exclusive breastfeeding at 2 weeks (in hospital) | Positive | ||||||
| Any breastfeeding at 2 weeks (in hospital) | Positive | ||||||
| Bicalho-Mancini 2004151 | BFI accreditation | Before/after |
Mothers who wished to breastfeed their preterm infants of LBW/VLBW Brazil |
Moderate ITT |
Any breastfeeding at discharge | Positive | |
Baby Friendly Best Practice Standards in neonatal units: summary
Evidence from this review has identified three types of intervention to directly inform the successful implementation of four Baby Friendly standards in UK neonatal units:
-
training and education of health-care staff – Standards 2 and 6
-
kangaroo method of skin-to-skin contact – Standards 4 and 6
-
community-led peer support interventions – Standards 9 and 6.
The evidence base to inform implementation of Standards 2 and 4 comprises studies conducted in the UK, USA and Malaysia (Kuala Lumpar) among clinically stable, very low and low birthweight infants. The effective promotion of breastfeeding support through local and national networks (Standard 9) has been most clearly demonstrated by community-led peer counselling programmes delivered in both the hospital and home settings. These studies include one RCT and one before/after study conducted in the USA and one RCT in the Philippines. Community-led peer counselling programmes,115,141,151 education of health-care staff (Pineda 2006,149 Jones 200481) and the kangaroo method of skin-to-skin contact24,133,139,145 have also been demonstrated to support mothers to establish and/or maintain breastfeeding (Standard 6). Details of the characteristics of these interventions and mother-infant dyads for whom these interventions have been found to be effective are provided in Chapter 4.
Several of these interventions also included an educational component to inform parents of the benefits of breastmilk and breastfeeding (Standard 3). Such information may promote breastfeeding among this vulnerable population although it is more likely that the combination of information with practical and emotional support, delivered through peer counselling programmes or as a result of appropriate staff training, will improve breastfeeding outcomes.
Findings from this evidence review are promising, although not conclusive, in relation to some interventions to support mothers to express breastmilk (Standand 5). Relaxation techniques,144 breast massage113 and therapeutic touch146 have been found to increase milk volume among mothers of preterm or low birthweight infants. Evidence from one moderate-quality RCT conducted in the UK shows that electrical simultaneous pumping compared to electrical sequential pumping had a significant effect on breastmilk output by weight. 113 However, in a study conducted in the USA126 a similar intervention did not report a positive effect on breastmilk output by volume. The positive finding reported in the UK study may be due to the lack of time restrictions placed on pumping and/or a lack of intention-to-treat analysis. One moderate-quality trial conducted in Kenya and Nigeria found that electrical pumps significantly increased breastmilk output by volume compared to hand pumps. 140 These findings suggest that strategies to support relaxation and other therapeutic approaches may warrant consideration at the local level and/or strategies to facilitate and support electrical pumping, particularly unrestricted double pumping, in neonatal units to increase volumes of expressed breastmilk. Limited evidence suggests that use of domperidone121 and recombinant human growth hormone131 may have a positive effect on milk volumes among women who had been expressing for at least a month and whose milk production was not meeting their infant’s needs.
The findings of effectiveness for Baby Friendly accreditation for the maternity service on breastfeeding outcomes in neonatal settings lend support to the potential extension of principles, and findings, from Baby Friendly accreditation in term, healthy infants to preterm and/or low birthweight infants in neonatal units. Two large before/after studies of good and moderate quality demonstrated significant increases in the number of infants being fed breastmilk during their first week of enteral feeds, any breastfeeding at discharge and any and exclusive breastfeeding at 2 weeks during the hospital stay. These studies were conducted among preterm infants, mostly of low birthweight, suggesting that replication of the benefits of Baby Friendly accreditation can be extended from the clinically stable, term population to clinically stable infants of low birthweight with a gestational age of 30 weeks or more.
One study included mothers who wished to breastfeed and the other was conducted among a population with typically low breastfeeding rates. These findings suggest that Baby Friendly accreditation can be effective among relatively lower risk infants of mothers with different feeding intentions. Generalisability of these findings to the UK may be problematic, however, as one study was conducted in Brazil and the other among mainly black American or Hispanic mothers. White Americans represented approximately 20% of the total sample. A pattern of effectiveness for Baby Friendly accreditation across a diverse range of populations and country settings does appear to be emerging, however.
Findings from this evidence review are inconclusive in relation to breastfeeding outcomes for Standard 8 (Avoid use of teats or dummies), although there are also important safety considerations in relation to this standard. One retrospective study in the USA demonstrated a significant increase in milk transfer to preterm, low birthweight infants as a result of ultra-thin silicone nipple shields for mothers with breastfeeding problems.
A lack of evidence was available to inform the effectiveness of interventions to encourage exclusive breastmilk feeding among infants in neonatal care (Standard 7). No studies were identified that evaluated the effect of a written neonatal unit breastfeeding policy (Standard 1).
Baby Friendly standards for neonatal units: conclusion
Implementation of the kangaroo method of skin-to-skin contact and training of health professionals in the neonatal unit have been shown to be effective at increasing breastfeeding outcomes among mainly preterm infants of very low birthweight. Community-led peer support interventions in the hospital and home settings have been shown to be effective among mainly lower risk, preterm infants of low birthweight. Increased implementation of these interventions would progress neonatal units towards complying with Best Practice Standards 2, 4, 6, 9 and standard 8 (in relation to safety outcome). Neonatal units should also encourage and support the achievement of Baby Friendly accreditation status for the maternity hospital to achieve significant increases in breastfeeding rates.
Glossary
- Acceptability curves
- Cost-effectiveness acceptability curves show the probability that an intervention is more cost-effective than its comparator at different cost-effectiveness thresholds.
- Any or partial breastfeeding
- Some breastfeeding plus water-based fluids, solids, milks or gruels.
- Appropriate for gestational age
- An infant’s birthweight that lies between the 10th and 90th centiles for gestational age at birth.
- Baby Friendly accreditation
- The Baby Friendly Initiative accredits maternity and community health-care facilities and higher education institutions that have implemented best practice for breastfeeding and have passed an external assessment.
- Baby Friendly Initiative
- A worldwide programme of the World Health Organization and UNICEF to encourage maternity hospitals to implement the Ten Steps to Successful Breastfeeding and to practise in accordance with the International Code of Marketing of Breastmilk Substitutes.
- Base-case model
- A model that normally includes the best assumptions and data estimates in the analysis.
- Bolus feeding
- A calculated amount of fluid, given intermittently depending on weight and gestational age.
- Breastfeeding counsellors
- Women who have breastfed and completed an accredited training. In the UK these are run by volunteer organisations: the National Childbirth Trust; La Leche League; the Breastfeeding Network; and the Association of Breastfeeding Mothers.
- Breastmilk subsitute
- Any fluid or food other than breastmilk that is used to feed infants. It may be used instead of, or as well as, breastmilk.
- Catch-up growth
- A growth trajectory that crosses centile lines upwards or an improvement in the standard deviation score indicating reversion to the genetically determined body size.
- Chronological age
- The age of the infant in weeks from the date of birth without correcting for prematurity.
- Comparator
- The treatment with which the intervention in question is compared.
- Complementary food
- Any food, whether manufactured or locally prepared, that is suitable as a complement to breastmilk or infant formula to satisfy the nutritional requirements of the infant.
- Composite milk
- A combination of the fore- and hindmilk produced by lactating mothers.
- Corrected age
- The age of the infant in weeks from the date of birth minus the number of weeks of prematurity.
- Cup feeding
- A method of feeding in which the infant licks or sips breastmilk from a specially designed cup.
- Duration of breastfeeding
- The period beyond the first nutritive breastfeed for which a baby continues to feed at the breast.
- Enhanced staff contact
- The provision of specially trained staff to advise and support mothers about milk expression and breastfeeding.
- Enteral feeding
- The administration of any feed into the gastrointestinal tract.
- EQ-5D data
- The preference measure of health states produced by the EuroQol Group, which contains five dimensions of health, where 1 is perfect health and 0 is death.
- Exclusive breastfeeding
- Breastfeeding with no supplemental liquid or solid foods other than medications or vitamins.
- Finger feeding
- A method of feeding in which a tube filled with, or attached to a container of, expressed breastmilk is attached to the caregiver’s finger and inserted into the infant’s mouth to enable ingestion of breastmilk.
- Foremilk
- The low-fat, higher volume breastmilk obtained at the beginning of a breastfeed.
- Formula
- Cow’s or soy milk modified in line with Codex Alimentarius standards to provide the nutritional requirements of infants.
- Fortified feeds, fortifiers
- Milk protein, vitamins and minerals that are added to breastmilk with the aim of meeting preterm infants’ specific nutritional needs.
- Gavage feeds
- The introduction of food into the stomach by means of a tube inserted through the mouth (orogastric) or the nose (nasogastric).
- Gestational age
- The age in weeks and days of the fetus counted from the first day of the mother’s last menstrual period.
- Galactagogue
- Any substance (e.g. food, medicine) that aims to increase breastmilk production.
- Growth restricted
- Describing infants who have experienced intrauterine growth restriction.
- Hand expression
- The expression of milk from the breast by hand.
- Hindmilk
- The high-fat breastmilk produced after the foremilk.
- Industrialised setting
- A country or region whose economy is based on industry; generally located in the northern and western hemispheres (Natural Resources Defense Council). This level of economic development usually translates into a high income per capita and a high Human Development Index (HDI) for populations within that country or region.
- Initiation of breastfeeding
- In the context of neonatal care settings, initiation of breastfeeding is defined as the mother putting the baby to the breast and the baby demonstrating nutritive sucking.
- Initiation of feeding with breastmilk
- In the absence of agreed definitions, we suggest: ‘In the context of neonatal care settings, initiation of feeding with breastmilk for the baby is defined as the baby receiving mother’s or donor breastmilk by any method. For the mother, it is defined as any attempt to express breastmilk by any method.’
- Intention to treat
- All participants are analysed by original allocated group including those who were lost to the study.
- International Code of Marketing of Breast Milk Substitutes
- A code ratified by the World Health Assembly in 1981, and amended by its subsequent resolutions. It sets out the conditions under which breastmilk substitutes may be marketed to the public and health professionals. It has been adopted in whole or in part into the laws of several countries.
- Interquartile range
- Shows the spread of the central 50% of a distribution.
- Kangaroo skin-to-skin contact
- Ongoing skin-to-skin contact with the infant held between the mother’s breasts in an upright position.
- Lactation consultant
- An International Board Certified Lactation Consultant (IBCLC) is a health-care professional who specialises in the clinical management of breastfeeding. IBCLCs are certified by the International Board of Lactation Consultant Examiners.
- Low birthweight infant
- An infant with a birthweight of less than 2500 g.
- Medicaid
- A scheme that provides medical benefits to groups of low-income people with no or inadequate medical insurance in the USA.
- Milk banking
- A service that collects, screens, processes, stores and distributes breastmilk.
- Multiples
- Infants born as a result of the multiple birth of more than two infants (i.e. triplets, quadruplets and more).
- Nasogastric
- Describing the administration of feeds via gavage tube in the nose.
- Non-nutritive sucking/suckling
- Sucking using a pacifier or other object, or at the breast without ingestion of breastmilk.
- Oral feeding
- The administration of any feed into the oral cavity.
- Orogastric feeding
- The administration of feeds via gavage tube in the mouth.
- Oxygen saturation
- The percentage of circulating haemoglobin that is oxygenated.
- Parenteral feeding
- The partial or total intravenous provision of fluid and nutrients when infants are unable to accept these by the gastrointestinal route.
- Peer support
- Support offered by women, usually trained, who have breastfed and are from a similar socioeconomic, ethnic or cultural background to the client.
- Postconceptional age
- The age in weeks and days of the infant from conception.
- Postrandomisation exclusions
- Losses from the study after the point of randomised allocation.
- Post-term birth
- A birth occurring after 42 completed weeks of gestational age.
- Preterm birth
- A birth occurring before 37 completed weeks of gestational age.
- Preterm formula
- Cow’s or soy milk modified in line with Codex Alimentarius standards to provide the specific nutritional needs of preterm infants, principally those born before 32 weeks’ gestation or weighing under 1500 g at birth.
- Preterm infant
- An infant born before 37 weeks’ completed gestation from the first day of the mother’s last menstrual period.
- Respiratory support
- Facilitation of the infant’s gas exchange by continuous positive airways pressure or ventilation delivered through an endotracheal tube, face mask or nasal device.
- Small for gestational age
- Describing an infant whose birthweight is less than the 10th centile for gestational age at birth.
- Spoon feeding
- Feeding from a spoon.
- Stable infant
- An infant whose vital functions, respiration and heart rate are not subject to rapid and unexpected worsening, nor dependent on continuous medical monitoring and support.
- Term birth
- A birth occurring after 37 completed weeks and before 42 completed weeks of gestational age.
- Test weighing
- Weighing of infants before and after intake of breastmilk.
- US Department of Agriculture’s Special Supplemental Nutrition Program for Women, Infants, and Children (WIC)
- Health sector initiatives delivered at the local level as part of the national WIC Program targeting women of low incomes in the USA.
- Very low birthweight
- Describing an infant with a birthweight of less than 1500 g.
List of abbreviations
- AGA
- appropriate for gestational age
- B/A
- before/after study
- BF (bf)
- breastfeeding
- BFI
- Baby Friendly Initiative
- BW
- bodyweight
- C
- control
- CA
- chronological age
- CEMACH
- Confidential Enquiry into Maternal and Child Health
- CHBS
- Conventional Hospital Breastfeeding Support
- CI
- confidence interval
- CPAP
- continuous positive airway pressure
- EBM
- expressed breastmilk
- FDA
- Food and Drug Administration
- GA
- gestational age
- HDI
- Human Development Index
- HEED
- Health Economic Evaluations Database
- I
- intervention
- IBCLC
- International Board Certified Lactation Consultant
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITB
- intention to breastfeed
- ITT
- intention-to-treat
- KMC
- kangaroo mother care
- LBW
- low birthweight
- MTT
- mimic therapeutic touch
- NDI
- neurodevelopmental impairment
- NEC
- necrotising enterocolitis
- NGT
- nasogastric tube
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Clinical Excellence
- NICU
- Neonatal Intensive Care Unit
- NIDCAP
- Newborn Individualised Developmental Care and Assessment Programme
- NIPPV
- nasal intermittent positive pressure ventilation
- NITB
- no intention to breastfeed
- non-RCT
- non-randomised controlled trial
- OMM
- own mother’s milk
- OR
- odds ratio
- PES
- Parental Expectations Survey
- PRE
- postrandomisation exclusion
- PSA
- probabilistic sensitivity analysis
- PSS-NICU
- Parental Stressor Scale – NICU
- QALY
- quality-adjusted life year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAIB
- Systematic Assessment of the Infant at the Breast (scale)
- SCBU
- Special Care Baby Unit
- SD
- standard deviation
- SEM
- standard error of the mean
- SES
- socioeconomic status
- SGA
- small for gestational age
- SSBC
- Supplemented Structured Breastfeeding Counselling
- STS
- skin-to-skin contact
- TT
- therapeutic touch
- UN
- United Nations
- VLBW
- very low birthweight
- WHO
- World Health Organization
- WIC
- (Program for) Women, Infants, and Children (US Department of Agriculture’s Special Supplemental Nutrition Program)
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and costeffectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebocontrolled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of Science Support, NETSCC, HTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington