Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 05/55/01. The assessment report began editorial review in July 2007 and was accepted for publication in November 2008. See the HTA programme website (www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical and cost-effectiveness of rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma (FNHL) based upon the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The manufacturer’s scope restricts the intervention to rituximab in combination with CVP (cyclophosphamide, vincristine and prednisolone) (R-CVP); the only comparator used was CVP alone. The evidence from the one included randomised controlled trial (RCT) suggests that the addition of rituximab to a CVP chemotherapy regimen has a positive effect on the outcomes of time to treatment failure, disease progression, overall tumour response, duration of response and time to new lymphoma treatment in patients with stage III/IV FNHL compared with CVP alone. Adverse events were comparable between the two arms. This study was confirmed as the only relevant RCT. The economic analyses provided by the manufacturer were modelled using a three-state Markov model with with the health states being defined as progression-free survival (PFS), progressed (in which patients have relapsed) and death (which is an absorbing state). The model generated results for a cohort of patients with an initial age of 53 and makes no distinction between men and women. The model is basic in design, with several serious design flaws and key parameter values that are probably incompatible. Attempting to rectify the identified errors and limitations of the model did not increase the incremental cost-effectiveness ratio (ICER) above £30,000. Although the cost-effectiveness results obtained appear to be compelling in support of R-CVP compared with CVP for the trial population the results may not be so convincing for a more representative population. The results of the ERG analysis on the impact of age suggest that ICERs increase steadily with age, as the proportion of PFS that can be converted to overall survival (OS) is diminished by rising mortality rates in the general population. For the most extreme scenario (no OS gain) the ICER appears to remain below £30,000 per QALY gained. On balance the evidence indicates that R-CVP is more cost-effective than CVP. The guidance issued by NICE in July 2006 as a result of the STA states that rituximab within its licensed indication (in combination with cyclophosphamide, vincristine and prednisolone) is recommended as an option for the treatment of symptomatic stage III/IV follicular non-Hodgkin’s lymphoma in previously untreated patients.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma (FNHL).
Description of the underlying health problem
In the UK non-Hodgkin’s lymphoma (NHL) represents about 3% of all diagnosed cancers. In 2002 the incidence of NHL was 16 per 100,000 population and 15.6 per 100,000 population in England and Wales respectively. The overall rate of NHL is increasing by 3–4% annually. This is greater than expected when considering the ageing population and improvements in diagnosis. Follicular lymphoma (FL) is the second most common form of NHL in the UK with an incidence of approximately 4 per 100,000 population. It is considered incurable and the aim of treatment is to induce periods of remission, to lengthen remission and to improve survival and quality of life. There is no single accepted therapy for first-line treatment of stage III/IV FNHL, with current treatment options falling into four main categories: alkylator-, anthracycline-, fludarabine- and R-CVP-(rituximab plus cyclophosphamide, vincristine and prednisolone)-based therapies. Guidelines from the British Committee for Standards in Haematology (BCSH)2 recommend CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone), an anthracycline-based therapy, for treatment of grade III FNHL, although no guidance for the treatment of grade IV FNHL is given. There is currently no consensus as to whether combination therapy provides additional treatment benefits over monotherapy. However, recent published clinical guidelines3 suggest that trials have shown advantages for combination therapy or extended chemotherapy with more frequent and longer lasting remissions and improvements to quality of life.
Scope of the ERG report
The ERG report presented the results of the assessment of the manufacturer’s report regarding the use of rituximab (within the context of the licensed indication) in combination with CVP for the first-line treatment of stage III/IV FNHL. 4 The scope of the appraisal is presented in Table 1. The report included an assessment of both the clinical and cost-effectiveness evidence submitted by the manufacturer (Roche) of rituximab (MabThera®), indicated for the treatment of previously untreated patients with stage III/IV FNHL in combination with CVP chemotherapy.
| Clinical effectiveness | Cost-effectiveness | |
|---|---|---|
| Population | Adults with stage III/IV non-Hodgkin’s lymphoma who have not received any previous treatment | |
| Intervention | Rituximab in combination with CVP (cyclophosphamide, vincristine and prednisolone) | |
| Comparators | CVP | |
| CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) | ||
| CNOP (cyclophosphamide, mitoxantrone, vincristine and prednisolone) | ||
| MCP (mitoxantrone, chlorambucil and prednisolone) | ||
| Chlorambucil | ||
| Outcomes | Time to treatment failure | Incremental cost per quality-adjusted life-year |
| Tumour response (complete response, unconfirmed complete response, partial response, progressive disease) | From the draft scope: Details of the time horizon for the economic evaluation based on the time period over which costs and benefits can reasonably be expected given the progression of the disease | |
| Duration of response | ||
| Overall survival | ||
| Disease-free survival | ||
| Adverse effects of treatment | ||
| Health-related quality of life | ||
| Study design | Randomised controlled trial | Economic analysis |
| Inclusion criteria | Main focus of follicular lymphoma | Main focus of follicular lymphoma |
| Clinical trial data publications | Full economic evaluation | |
| Exclusion criteria | Clinical trials in previously treated patients | No attempt to synthesis costs and benefits |
| Reviews | Letters, editorials, commentaries or methodological papers | |
| Animal studies or in vitro research work | ||
Methods
The ERG report comprised a critical review of the evidence for the clinical and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
As part of their critical review the ERG repeated the searches for studies of clinical and cost-effectiveness. An accepted tool5 relating to rigour of the review process and clarity of reporting was used to assess the methodological quality of the manufacturer’s literature review. The ERG assessed whether each paper reported in the manufacturer’s submission met the inclusion criteria according to: publication date; language; type of study (whether a full economic evaluation was included); intervention; and subjects. They conducted a detailed critique of the single efficacy trial included in the manufacturer’s submission. They critiqued the manufacturer’s economic model and the model was rerun after correcting for errors relating to costs and life-years gained; a Weibull survival curve was used to estimate survival.
In addition, because the submitted model is based on a cohort of patients with an unrealistically low average age (53 years), the ERG explored this further. It was observed that it was possible that at higher ages the apparently promising cost-effectiveness ratios may not be so attractive and could become unacceptable. It proved to be impossible to modify the model to allow accurate adjustment of age because of inherent structural problems and inherent inconsistencies in the model structures and, therefore, the ERG attempted a supplementary analysis. These results are necessarily imprecise approximations and should not be viewed as more than a general indication of the types of variations that may be expected if the ERG’s assumptions prove to be valid.
Results
Summary of submitted clinical evidence
The submitted clinical evidence includes one randomised controlled trial (RCT), M30921, comparing CVP chemotherapy alone with CVP in combination with rituximab and involving a total study population of 322 patients with stage III or IV FNHL. The evidence from this trial suggests that the addition of rituximab to a CVP chemotherapy regimen has a positive effect on the primary outcome of time to treatment failure; it is reported to increase from 6.6 months in patients receiving CVP to 27 months in patients in the R-CVP arm with a risk reduction of 66% (95% CI 55%–74%). Other positive outcomes were measured for disease progression, overall tumour response, duration of response and time to new lymphoma treatment. Overall survival (OS) was not estimable at 42 months and the 38% risk reduction had not reached statistical significance. Adverse events were comparable between the two arms for the proportion of patients experiencing at least one adverse event, although the proportion experiencing an adverse event in the first 24 hours was greater for the R-CVP arm (71% versus 51%). These are primarily represented by infusion-related events. Similar proportions of patients in each arm experienced grade 3–4 haematological toxicity and infection except for neutropenia (14.5% CVP versus 24.1% R-CVP).
Summary of submitted cost-effectiveness evidence
The submitted review of economic studies included 15 studies, only eight of which actually met the inclusion criteria established for the review. None of these studies, however, compared R-CVP with CVP. The data extraction of the economic literature undertaken by the manufacturer was lacking in depth and no quality assessment of the included studies was provided. However, given the fact that these studies do not compare the same health-care technologies as the manufacturer’s own economic evaluation, this is of limited importance.
The economic model included in the manufacturer’s submission is a three-state Markov model, with the health states being defined as:
-
progression-free survival (PFS)
-
progressed (in which patients have relapsed)
-
death (which is an absorbing state).
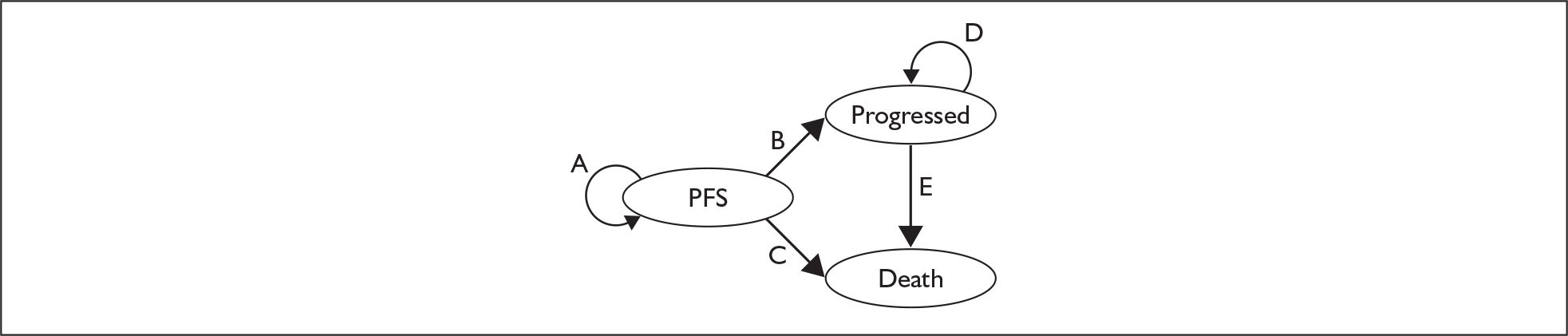
Patients begin in the PFS state and at the end of each cycle (cycle length 1 month) can either stay within this health state or move to the progressed health state or death state. Once in the progressed health state patients either move to the death state or continue in the progressed health state; once in the progressed health state they cannot return to PFS (Figure 1). However, the progressed state has been adjusted (in terms of utility) to account for periods of PFS. Movement between health states is governed by transition probabilities. The probabilities applied to the PFS health state vary over time but are generally similar between the two arms. The probabilities applied to the progressed health state are constant and do not differ between the two arms. The submitted model generates results for a cohort of patients with an initial age of 53 and makes no distinction between men and women.
FIGURE 1.
Structure of the Markov model (adapted from the manufacturer’s submission). PFS, progression-free survival.
Commentary on the robustness of submitted evidence
The single study included in the manufacturer’s submission was confirmed as the only relevant RCT. In the manufacturer’s submission the only comparator used was CVP alone. A wide range of treatment options are used in the UK for the treatment of FNHL, but currently there is no consensus on the most effective treatment. These include alkylator-based regimens (e.g. CVP, chlorambucil) or anthracycline-based regimens (e.g. CHOP, CNOP, MCP) used either alone or in combination with rituximab. Clinical guidelines, however, note a lack of data directly comparing outcomes with alternative therapeutic strategies. There is mention in the manufacturer’s submission of other studies using a variety of treatments; however, no analyses were carried out comparing the results with R-CVP. Preliminary findings of a meta-analysis, available only as a conference abstract, are discussed descriptively.
There is an issue relating to the rationale for the outcomes used, including an explanation of the reasons for using time to treatment failure as the primary outcome instead of OS as is usual for oncology clinical trials. However, although OS is a preferred outcome measure, in the case of FNHL the submission presents a persuasive rationale for the use of time to treatment failure.
The model submitted in support of the manufacturer’s submission is basic in design. It suffers from several serious design flaws and key parameter values are probably incompatible. The ERG attempted to rectify the identified errors and limitations of the model, none of which increased the incremental cost-effectiveness ratio (ICER) above the conventional threshold of £30,000. However, because of design flaws within the model as outlined in the report it was impossible for the ERG to simultaneously correct all of the errors and limitations within it. Although the cost-effectiveness results obtained appear to be compelling in support of R-CVP compared with CVP for the trial population, it could be argued that the results would not be so convincing for a more representative population.
The results of the ERG analysis on the impact of age (Table 2) suggest that ICERs increase steadily with age, as the proportion of PFS that can be converted to OS is diminished by rising mortality rates in the general population. For the most extreme scenario (no OS gain) the ICER appears to remain below £30,000 per quality-adjusted life-year gained.
Conclusions
On balance the evidence indicates that R-CVP is more cost-effective than CVP.
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in July 2006 states that:
Rituximab within its licensed indication (that is in combination with cyclophosphamide, vincristine and prednisolone) is recommended as an option for the treatment of symptomatic stage III and IV follicular lymphoma in previously untreated patients.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology Appraisal (STA) Process 2006. https://www.nice.org.uk/page.aspx?o=STAprocessguide (accessed June 2007).
- British Committee for Standards in Haematology . BCSH Guidelines on Nodal Non-Hodgkin’s Lymphoma 2002. www.bcshguidelines.com/pdf/NHL_100903.pdf.
- North West Haematology Guidelines 2005. www.nwhaems.co.uk/secure/newhaems/newdocs/GUIDELINESv18.pdf.
- Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al. Rituximab for the First-Line Treatment of Stage III–IV Follicular Non-Hodgkin’s Lymphoma 2006.
- The University of Sheffield School of Health and Related Research . Critical Appraisal of Secondary Research 2006. www.shef.ac.uk/scharr/ir/mschi/unit5/3appraising.htm#casr.