Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 06/25/01. The assessment report began editorial review in July 2007 and was accepted for publication in December 2008. See the HTA programme website (www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical and cost-effectiveness of cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck (LA SCCHN) considered inappropriate for chemoradiotherapy but appropriate for radiotherapy, based upon the evidence submission from Merck Pharmaceuticals to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The manufacturer’s submission was generally of good quality and was an accurate representation of the original reference data. One good-quality randomised controlled trial comparing radiotherapy plus cetuximab with radiotherapy alone in patients with stage III or IV non-metastatic LA SCCHN was included, demonstrating that the duration of locoregional control was significantly longer with radiotherapy plus cetuximab than with radiotherapy alone; also, overall and progression-free survival were significantly longer and the overall response rate was significantly better with the combination therapy. Cetuximab did not exacerbate the common toxic effects associated with radiotherapy of the head and neck. No supporting evidence for these findings are available. The patient population in the trial included a high proportion of patients who would be expected to be suitable for chemoradiotherapy and therefore does not match the population described in the submission’s decision problem. Also, the radiotherapy regimens used in the trial are not typical of current UK practice. The ERG considered the manufacturer’s economic evaluation to comprise the only relevant evidence to consider for the purposes of this STA. The economic model was considered appropriate for the decision problem. The results suggested that cetuximab plus radiotherapy was cost-effective compared with radiotherapy alone under a broad range of different assumptions on the basis of a cost-effectiveness threshold of £20,000. In the base case the incremental cost-effectiveness ratio of cetuximab plus radiotherapy compared with radiotherapy alone in the treatment of patients with LA SCCHN was £6390 per additional QALY. Simple sensitivity analyses to examine the robustness of the results were undertaken, suggesting that areas of uncertainty that emerged in the modelling are unlikely to have a material effect on the conclusions. The guidance issued by NICE in May 2007 as a result of the STA states that cetuximab in combination with radiotherapy is not recommended for patients with LA SCCHN.
Introduction
The National Institute of Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). 2
Description of the underlying health problem
Head and neck cancer is a broad term for any cancer from the base of the neck upwards,3 excluding tumours of the brain and related tissues and malignant melanomas. 4,5 The most common histological type of head and neck cancer is a squamous cell carcinoma, particularly affecting the oral cavity and larynx, although patients may present with more than one primary cancer. 3,5,6 In 2003 there were over 5000 new cases of cancer of the oral cavity, oropharynx, hypopharynx and larynx in England. Male prevalence dominates (70%), possibly because of lifestyle factors (smoking, drinking), as does increasing age (median 60–64 years). Only 1965 of the above new cases related specifically to cancer of the oropharynx, hypopharynx and larynx. 7 A recent audit of head and neck cancer treatment, specifically that of the oral cavity and larynx, indicated that 51% of all patients present with early-stage disease, although these figures may be skewed by the fact that laryngeal cancer is often detected early because of patients presenting with voice alteration. 5
Prognosis is dependent on many factors, not least the origin of the cancer and stage at diagnosis. 3 There is considerable variation in the severity of the cancer at diagnosis or presentation. Laryngeal cancers have higher 5-year survival rates than oral cancers because an obvious symptom of the cancer is voice alteration, which often prompts patients to consult a doctor earlier than do patients with oral cancers, which may only manifest as painless ulcers. Ultimately, patients with cancer diagnosed and treated at an earlier stage have a much better prognosis. 3 Treatment usually consists of a combination of surgery and radiotherapy and may include chemotherapy. 3
Scope of the ERG report
The ERG report critically evaluated the evidence submission from Merck Pharmaceuticals on the clinical and cost-effectiveness of cetuximab (Erbitux®) in combination with radiotherapy relative to radiotherapy alone in patients with LA SCCHN who are considered inappropriate for chemoradiotherapy but appropriate for radiotherapy.
Cetuximab in combination with radiotherapy is specifically licensed only for the treatment of LA SCCHN. 8
Methods
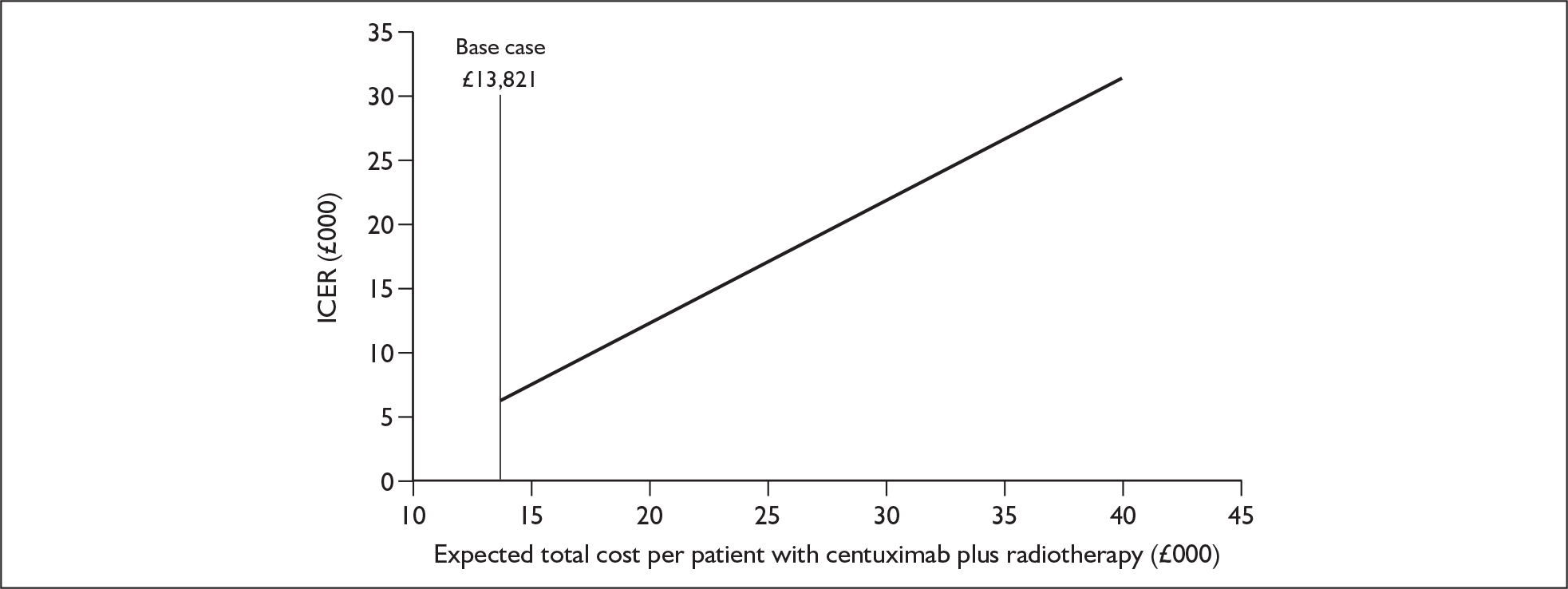
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process. The report identified the strengths and weaknesses of the manufacturer’s submission and presented additional work to address issues and uncertainties identified during the structured critique of the manufacturer’s submission. Simple sensitivity analyses to examine the robustness of the results were undertaken by (1) examining what change in the average utility value for patients in the cetuximab plus radiotherapy arm would be required to increase the incremental cost per quality-adjusted life-year (QALY) gained of cetuximab plus radiotherapy to levels that may not be considered cost-effective; the base-case average utility in the two groups was identified (ignoring discounting) by dividing the estimated QALYs in each group by the estimated life-years (Figure 1); and (2) examining what change in total average costs for the cetuximab plus radiotherapy arm would be required, ceteris paribus, for cetuximab plus radiotherapy not to be considered cost-effective (Figure 2).
FIGURE 1.
Average utility with cetuximab plus radiotherapy and its impact on the incremental cost per quality-adjusted life-year gained for the combination therapy. The average utility with radiotherapy alone remains at 0.69.
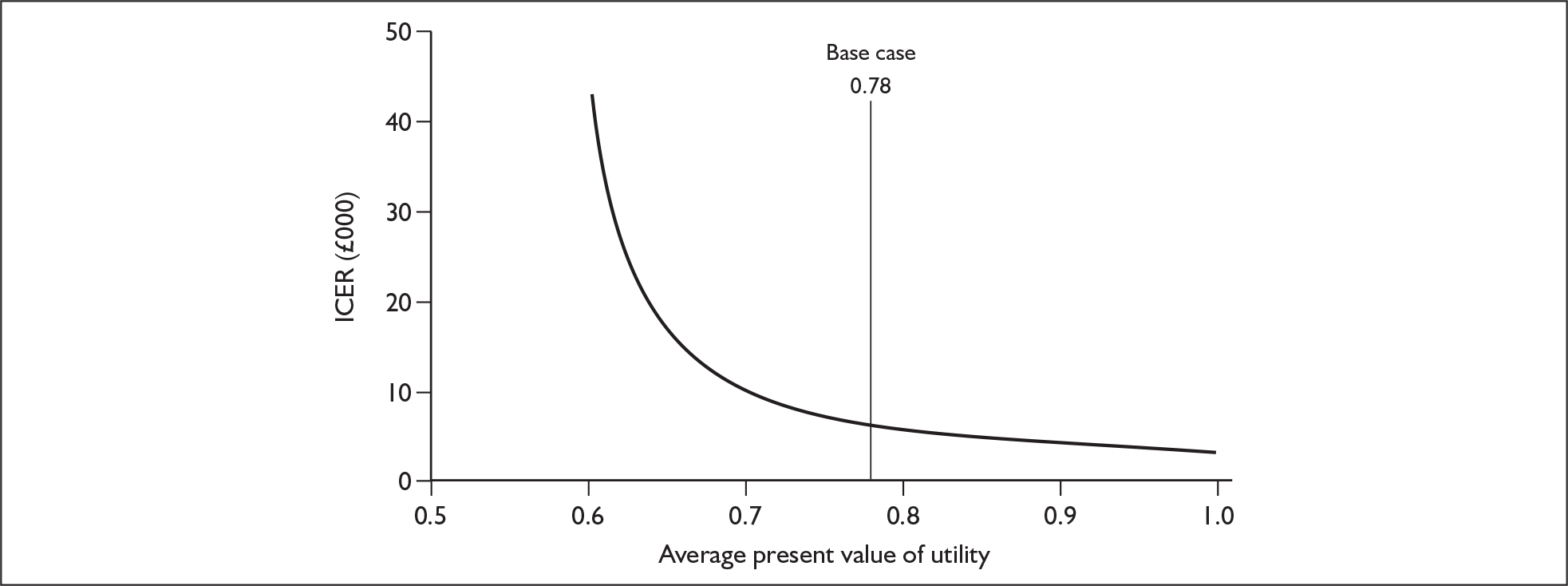
FIGURE 2.
Average total cost with cetuximab plus radiotherapy and its impact on the incremental cost per quality-adjusted life-year gained for the combination therapy. The average cost with radiotherapy alone remains at £7195. ICER, incremental cost-effectiveness ratio.
Results
Summary of submitted clinical evidence
One study was included in the submission. 9 This study was a fully published and well-designed and -conducted randomised controlled trial that compared radiotherapy plus cetuximab with radiotherapy alone in patients with stage III or IV non-metastatic squamous cell carcinoma of the oropharynx, hypopharynx or larynx. Efficacy was evaluated on an intention to treat basis and included all randomised patients. Safety was evaluated in all patients who received treatment. The trial demonstrated that the duration of locoregional control (the primary end point) was significantly longer with radiotherapy plus cetuximab than with radiotherapy alone. With respect to secondary end points both overall and progression-free survival were significantly longer and the overall response rate was significantly better with the combination therapy than with radiotherapy alone (Table 1). Cetuximab did not exacerbate the common toxic effects associated with radiotherapy of the head and neck. Severe (grade 3–5) acneiform rash and infusion reaction occurred more frequently with radiotherapy plus cetuximab than with radiotherapy alone, whereas the converse applied to severe anaemia.
| Variable | Radiotherapy alone (ITT population n = 213) |
Radiotherapy plus cetuximab (ITT population n = 211) |
|---|---|---|
| Locoregional control, median duration in months | 14.9 | 24.4 |
| Progression-free survival, median duration in months | 12.4 | 17.1 |
| Overall survival, median duration in months | 29.3 | 49.0 |
| Response rate (complete response + partial response) total number (%) | 137 (64%) | 155 (74%) |
Summary of submitted cost-effectiveness evidence
No previous studies were identified by the manufacturer or by the ERG that would help inform this STA. Therefore, the manufacturer’s economic evaluation is considered by the ERG to comprise the only relevant evidence to consider for the purposes of this STA.
The manufacturer’s submission included a de novo economic evaluation to estimate the cost-effectiveness of treatment with (1) cetuximab plus radiotherapy and (2) radiotherapy alone. The economic model (including the comparator) was considered appropriate for the decision problem. The results suggested that cetuximab plus radiotherapy was cost-effective compared with radiotherapy alone under a broad range of different assumptions on the basis of a cost-effectiveness threshold of £20,000. In the base case the incremental cost-effectiveness ratio of cetuximab plus radiotherapy compared with radiotherapy alone in the treatment of patients with LA SCCHN was £6390 per additional QALY (Table 2).
| Incremental cost | Incremental QALYs | Incremental cost per QALY | |
|---|---|---|---|
| Cetuximab plus radiotherapy vs radiotherapy alone | £6626 | 1.26 | £6390 |
Commentary on the robustness of submitted evidence
The ERG felt that the manufacturer’s submission was generally of good quality. There were no major errors or omissions and the majority of the data quoted within the submission were a fair and accurate representation of the original reference data.
The main weakness of the submission was that the evidence for the clinical effectiveness of cetuximab plus radiotherapy is based on a single clinical trial. Therefore, no supporting evidence for the findings is available.
The ERG felt that there were two major areas of uncertainty:
-
The patient population in the pivotal trial by Bonner et al. 9 included a high proportion of patients who would be expected to be suitable for chemoradiotherapy and therefore it does not match the population that is the focus of the submission’s decision problem, i.e. patients who are considered inappropriate for chemoradiotherapy. No data are available regarding the number of patients in the trial who would be considered inappropriate for radiotherapy and hence no subgroup analysis on the population specified in the decision problem has been carried out. Therefore, the trial results may not be directly applicable to the target population. However, the clinical experts consulted by the ERG were of the opinion that the Bonner et al. trial is a good source for the comparison of radiotherapy plus cetuximab with radiotherapy alone and use of the whole trial population is appropriate because the factors that would lead to chemotherapy being inappropriate are highly variable.
-
The radiotherapy regimens used in the trial are not typical of current UK practice. Once-daily radiotherapy is the regimen most representative of current UK practice (used in about 80% of patients according to a survey by the Royal College of Radiologists). In the Bonner et al. trial, however, altered fractionation regimens (twice daily and concomitant boost) were selected for 18% and 56% of patients respectively (74% in total).
Another possible area of uncertainty was whether there are subgroups of patients who may derive more benefit than others from cetuximab with radiotherapy. The Bonner et al. trial was not powered to detect treatment-related differences for subgroups, such as patients who received once-daily radiotherapy or those with laryngeal or hypopharyngeal cancer,10 but some results for subgroups are presented in the published paper, although with no confidence intervals or p-values. In view of the lack of power of the trial, caution needs to be exercised in drawing conclusions; however, the results presented raise questions as to whether there are subgroups of patients who may derive more benefit than others from the combination therapy. In patients with oropharyngeal cancer, locoregional control and overall survival durations appeared to be longer than those in patients with laryngeal or hypopharyngeal cancer. Furthermore, the once-daily radiotherapy regimen may have been less effective in terms of overall survival than the two altered fractionation regimens, and overall survival appeared to be longer with radiotherapy plus cetuximab than with radiotherapy alone in patients who received the concomitant boost regimen. Further clinical trials are needed to resolve these issues. Details of these subgroup analyses are included in the structured critical appraisal of the Bonner et al. trial presented in Appendix 3 of the full ERG report. 2
Conclusions
A number of areas of uncertainty emerged in the manufacturer’s cost-effectiveness modelling. These relate mainly to the extrapolation methods and the assumptions used to derive the utility and cost estimates. However, based on the sensitivity analyses undertaken by the manufacturer and some additional ERG analyses, these areas of uncertainty are unlikely to have a material effect on the conclusions of the cost-effectiveness analysis.
Future research into establishing which patients are likely to derive most benefit from cetuximab in conjunction with radiotherapy would be useful, as would further research on the clinical effectiveness of cetuximab plus radiotherapy in those patients with locally advanced SCCHN who are considered inappropriate for chemoradiotherapy. Setting up a patient register to collect post-treatment observational data of patients treated with cetuximab may be useful.
Summary of NICE guidance issued as a result of the STA
The following guidance was issued by NICE in May 2007:
This guidance on the use of cetixumab in combination with radiotherapy, for patients with locally advanced squamous cell cancer of the head and neck, is based on evidence submitted by the manufacturer. The evidence submitted was insufficient to enable a recommendation to be made on the use of cetuximab in combination with radiotherapy, as an alternative in patients for whom chemoradiotherapy is inappropriate. Cetuximab in combination with radiotherapy is not recommended for patients with locally advanced squamous cell cancer of the head and neck. People currently receiving cetuximab should have the option to continue therapy until they and their clinicians consider it appropriate to stop.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al. Cetuximab Plus Radiotherapy for the Treatment of Locally Advanced Squamous Cell Carcinoma of the Head and Neck 2006.
- National Institute for Health and Clinical Excellence (NICE) . Guidance on Cancer Services: Improving Outcomes in Head and Neck Cancers: The Manual 2004.
- L. Epidemiology of head and neck cancer: magnitude of the problem. Cancer Metastasis Rev 2005;24:9-17.
- NHS Health and Social Care Information Centre . DAHNO (Data for Head and Neck Oncology) First Annual Report: Key Findings from the National Head and Neck Cancer Audit, January 2004–November 2005 2006. www.ic.nhs.uk/services/national-clinical-audit-support-programme-ncasp/audit-reports/head-and-neck-cancer.
- Worrall SF. Oral Cancer – an Overview 2001. www.boams.org.uk/info/cancer/oral.pdf (accessed 28 September 2006).
- Office for National Statistics . Cancer Statistics – Registrations, England, 2003 2006.
- Merck Pharmaceuticals . Erbitux 2 Mg Ml Solution for Infusion: Summary of Product Characteristics 2006. http://emc.medicines.org.uk (accessed 31 July 2006).
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78.
- Bonner JA, Spencer SA, Sharon A, Ravinsky EK, . Cetuximab plus radiotherapy for head and neck cancer. The authors and a colleague reply. N Engl J Med 2006;354.