Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 06/16/01. The assessment report began editorial review in July 2007 and was accepted for publication in November 2008. See the HTA programme web site for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the evidence for the clinical effectiveness and cost-effectiveness of gemcitabine with paclitaxel for the first-line treatment of metastatic breast cancer (MBC) in patients who have already received chemotherapy treatment with an anthracycline, compared with current standard of care, based upon the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The clinical evidence for gemcitabine as a treatment for MBC comes from the unpublished JHQG trial (some data commercial-in-confidence): overall survival was 3 months longer for the gemcitabine/paclitaxel arm (18.5 months) than for the paclitaxel arm (15.8 months) (p = 0.0489); gemcitabine/paclitaxel also improved tumour response and time to documented progression of disease compared with paclitaxel monotherapy, but haematological serious adverse events were more common. In the absence of any formal methods of indirect comparison there is insufficient robust evidence to compare the relative effectiveness of gemcitabine/paclitaxel with docetaxel monotherapy or docetaxel/capecitabine combination therapy. The manufacturers used a Markov state transition model to estimate the effect of treatment with five different chemotherapy regimes, adopting a 3-year time horizon with docetaxel monotherapy as the comparator. Health state utilities for different stages of disease progression and for patients experiencing treatment-related toxicity are used to derive quality-adjusted life expectancy with each treatment. The base-case cost-effectiveness estimate for gemcitabine/paclitaxel versus docetaxel is £17,168 per quality-adjusted life-year (QALY). When longer survival with docetaxel is assumed in a sensitivity analysis, the incremental cost-effectiveness ratio (ICER) is £30,000 per QALY. Probabilistic sensitivity analysis estimates a 70% probability of gemcitabine/paclitaxel being cost-effective relative to docetaxel at a willingness-to-pay threshold of £35,000. There is considerable uncertainty over the results because of the lack of formal quality assessment or assessment of the comparability of the 15 trials included in the input data, and the questionable validity of the indirect comparison method adopted. An illustrative analysis using a different method for indirect comparison carried out by the ERG produces an ICER of £45,811 per QALY for gemcitabine/paclitaxel versus docetaxel. The guidance issued by NICE in November 2006 as a result of the STA states that gemcitabine in combination with paclitaxel, within its licensed indication, is recommended as an option for the treatment of MBC only when docetaxel monotherapy or docetaxel plus capecitabine is also considered appropriate.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of gemcitabine for advanced metastatic breast cancer. 2
Description of the underlying health problem
Breast cancer is classified into four clinical stages. Stages I and II are known as primary or early breast cancer, and stages III and IV represent advanced breast cancer. Stage IV is metastatic disease, characterised by the spread of secondary tumours to distant sites. A small proportion of incident breast cancers present as stage IV, i.e. they have overt metastases at the time of diagnosis. Approximately 40% of patients treated for early breast cancer will relapse and develop metastatic breast cancer (MBC). Patients who present with stage IV disease at first diagnosis are described by the manufacturer as being unsuitable for treatment with gemcitabine as they will not have received prior anthracycline therapy.
Scope of the ERG report
The submission’s scope is the use of gemcitabine with paclitaxel for the first-line treatment of MBC in patients who have already received chemotherapy treatment with an anthracycline, compared with current standard of care. This reflects the licensed indication, and is an appropriate question for the NHS within the context of the available evidence.
Methods
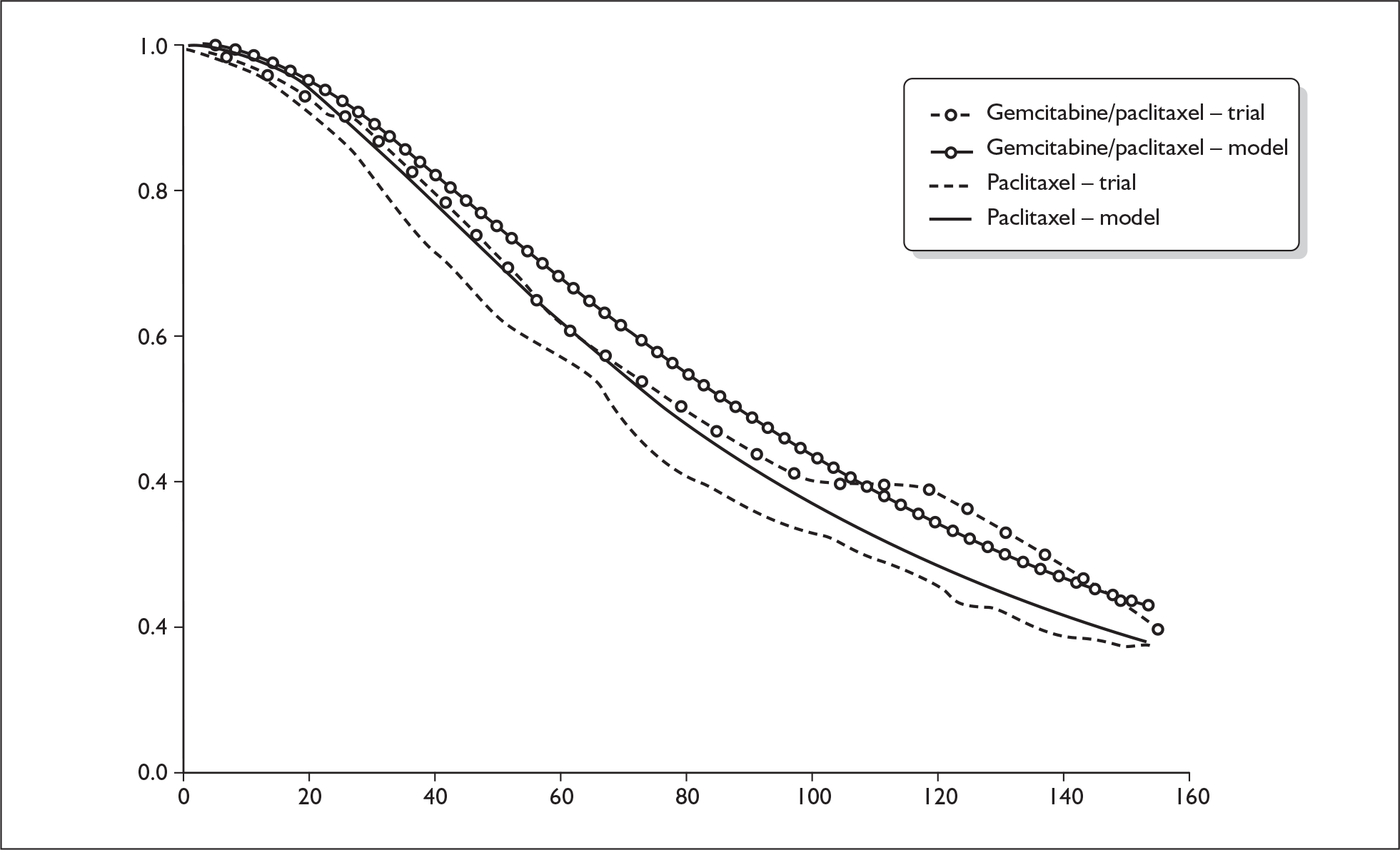
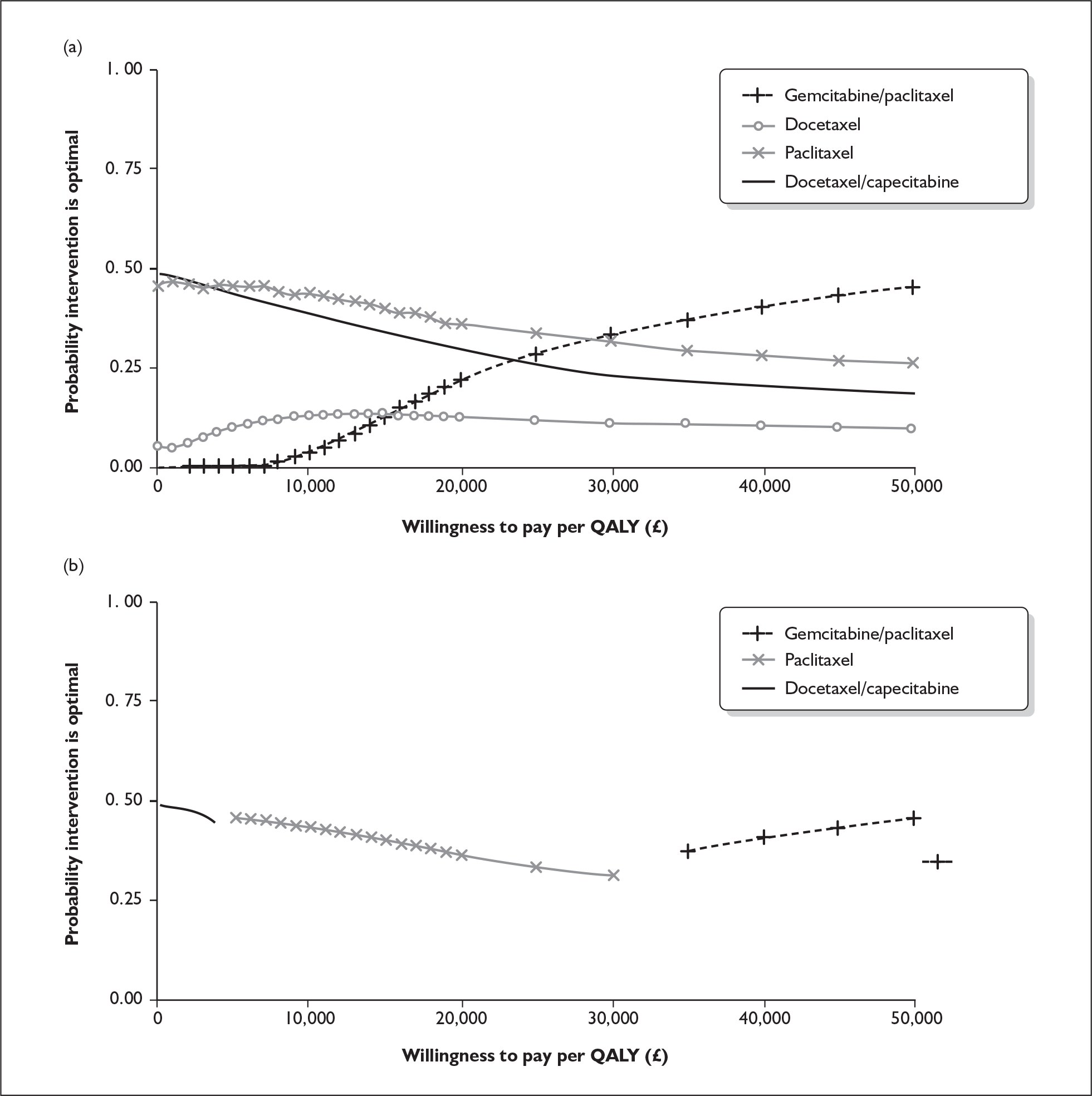
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process. It also included a critical assessment of the company’s submitted economic model. The ERG examined the excel model submitted by the manufacturer for accuracy and consistency and evaluated structural assumptions. In addition, the ERG estimated the survival probabilities and risk of disease progression for patients in the paclitaxel arm of the trial from survival plots reported in the conference presentation by Albain and colleagues,3 and fitted a parametric survival function to these data using the outputs from an ordinary least squares regression on a log-cumulative hazard. 4 The ERG estimated the external validity of the manufacturer’s model by running it with survival estimates from the JHQG trial, and with median survival times for gemcitabine/paclitaxel and paclitaxel as shown in the JHQG trial (Figure 1). In addition, one-way sensitivity analyses for key model parameters were carried out (Table 1), and key input data were replaced with pooled estimates from plausible alternative sources (e.g. the estimates observed in the JHQG trial). A scenario analysis was conducted using effectiveness data from the JHQG trial for both gemcitabine/paclitaxel and paclitaxel, and the pooled estimates from trials including anthracycline-pretreated patients for other chemotherapy regimes. To determine whether the results of the company’s probabilistic sensitivity analysis are sensitive to the choice of included trials, the ERG reran the company’s probabilistic sensitivity analysis using the pooled estimates for overall survival, time to disease progression and overall response rate for paclitaxel monotherapy with values from the JHQG trial (Figure 2). The ERG constructed cost-effectiveness acceptability curves comparing each of four taxane-based chemotherapy regimes against each other (Figure 3).
FIGURE 1.
Estimated survival for gemcitabine/paclitaxel and paclitaxel predicted by the model compared with Kaplan–Meier curves from the JHQG trial.
| Variable | Base case | Inputs | CE ratios (£) | Range (£) | ||
|---|---|---|---|---|---|---|
| Lower | Upper | Lower input | Upper input | |||
| Response rates (%) | 46 | 39.0 | 52.9 | 17,199 | 17,052 | 147 |
| Time to progression (weeks) | 26 | 21.5 | 30.5 | 16,601 | 17,406 | 805 |
| Overall survival (weeks) | 80.60 | CIC | CIC | 30,446 | 12,310 | 18,136 |
| AE discontinuation rate (%) | 6.7 | 3.7 | 9.7 | 16,335 | 17,994 | 1659 |
| Health state utilities: | ||||||
| Stable | 0.80 | 0.65 | 0.92 | 23,656 | 13,546 | 10,110 |
| Response | 0.72 | 0.60 | 0.83 | |||
| Progression | 0.46 | 0.29 | 0.63 | |||
| Adverse event, e.g. stable neuropathy, utility rates | 0.70 | 0.55 | 0.83 | 17,396 | 16,972 | 424 |
| Non-drug costs | ||||||
| Post–patient paclitaxel cost reduction (cost/course, £): | –25% | +25% | 17,988 | 16,348 | 1640 | |
| Gemcitabine/paclitaxel | 2442 | 1862 | 2442 | 5872 | 17,168 | 11,296 |
| Paclitaxel | 1462 | 862 | 1462 | |||
FIGURE 2.
Cost-effectiveness acceptability curves for gemcitabine/paclitaxel versus paclitaxel using (a) pooled estimates used in the basecase analysis and (b) values from the JHQG trial. QALY, quality-adjusted life-year.
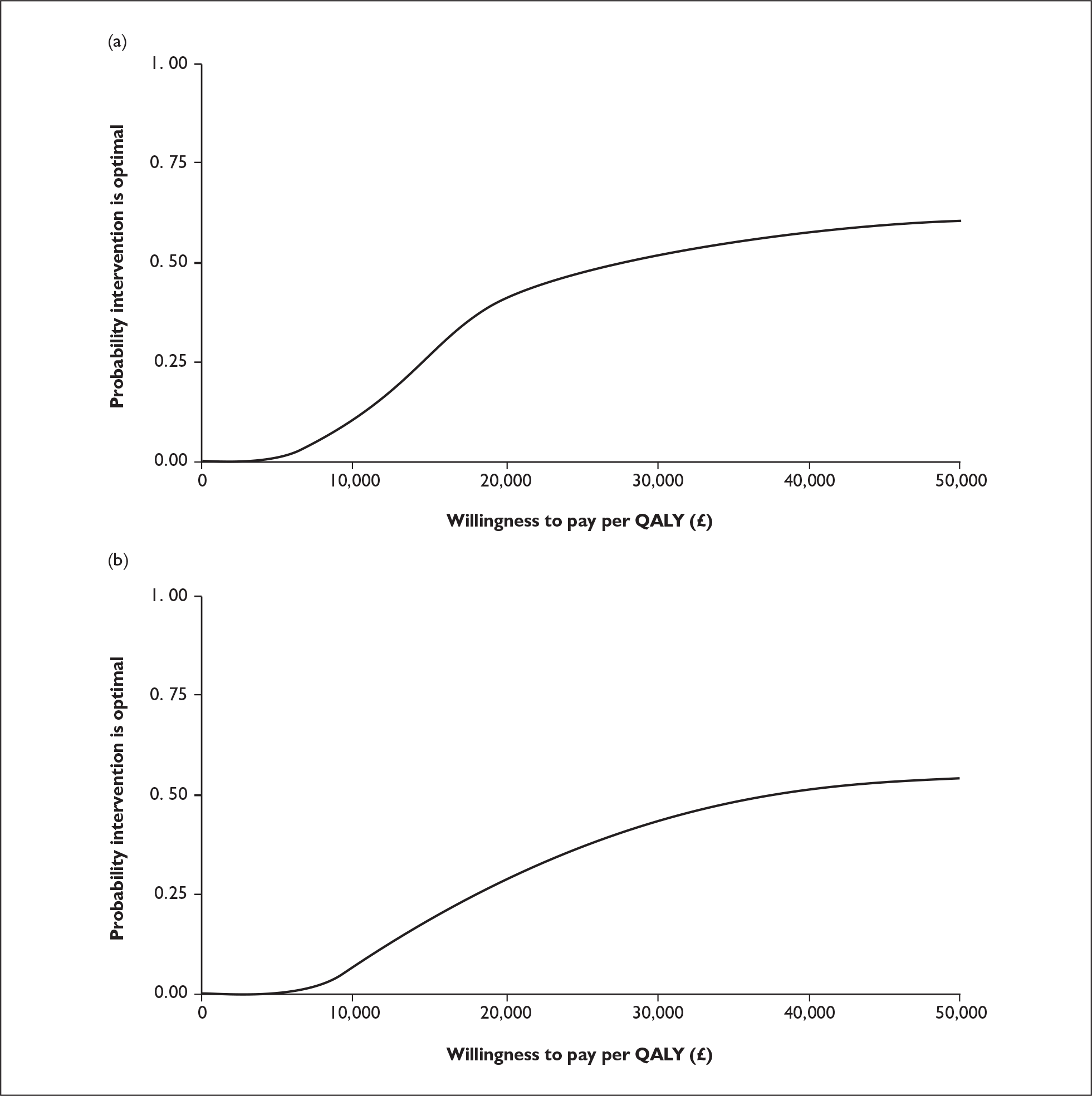
FIGURE 3.
(a) Multiple cost-effectiveness acceptability curves (CEAC) comparing four taxane-based chemotherapy regimes. (b) CEA frontier. QALY, quality-adjusted life-year.
Results
Summary of submitted clinical evidence
The clinical evidence for gemcitabine with paclitaxel compared with paclitaxel monotherapy as a treatment for MBC comes from the JHQG trial, which was published in conference abstracts5–7 in 2003–4, but has not yet been fully published. The data in the industry submission come from the unpublished trial and so are mostly marked as commercial-in-confidence. Results from two other published trials are included in the submission to provide a comparison with docetaxel monotherapy8 and docetaxel/capecitabine combined therapy. 9 The JHQG trial compared gemcitabine/paclitaxel (GT) with paclitaxel (T) in patients with MBC. The trial by Jones and colleagues8 compared docetaxel monotherapy with paclitaxel, and the trial by O’Shaughnessy and colleagues9 compared docetaxel monotherapy with docetaxel/capecitabine combination therapy.
Overall survival, the primary outcome measure for the JHQG trial, was approximately 3 months longer for the gemcitabine/paclitaxel arm (18.5 months in Albain et al. abstract,5 18.6 months in manufacturer’s submission) than for the paclitaxel arm (15.8 months). 7 This difference is of borderline statistical significance (p = 0.0489), but represents a clinically significant difference to patients. Results from the JHQG trial suggest that gemcitabine added to paclitaxel also improves tumour response and time to documented progression of disease compared with paclitaxel monotherapy. Haematological serious adverse events were more common in the gemcitabine/paclitaxel arm than in the paclitaxel monotherapy arm.
In the absence of any formal methods of indirect comparison, there is insufficient robust evidence to compare the relative effectiveness of gemcitabine/paclitaxel with docetaxel monotherapy or docetaxel/capecitabine combination therapy.
Summary of submitted cost-effectiveness evidence
The cost-effectiveness analysis in the manufacturer’s submission uses a Markov state transition model to estimate the effect of treatment with five different chemotherapy regimes, adopting a 3-year time horizon. Base-case results are presented, with docetaxel monotherapy as the comparator for all interventions (assuming that docetaxel is the standard of care for UK practice). Additional scenario analyses are presented using alternative comparators and for a price reduction for paclitaxel once the patent expires. Treatment effects in the model are derived from pooling data from 15 clinical trials – only three of these are discussed in the clinical effectiveness section of the submission. No formal assessment of trial comparability or any quality assessment was presented. Health state utilities for different stages of disease progression and for patients experiencing treatment-related toxicity are used in the model to derive quality-adjusted life expectancy with each treatment. The base-case cost-effectiveness estimate for gemcitabine/paclitaxel relative to docetaxel is £17,168 per quality-adjusted life-year (QALY). When longer survival with docetaxel is assumed in a sensitivity analysis, the incremental cost-effectiveness ratio (ICER) increases to approximately £30,000 per QALY. Probabilistic sensitivity analysis estimates a 70% probability of gemcitabine/paclitaxel being cost-effective relative to docetaxel at an arbitrary threshold willingness to pay of £35,000.
The lack of formal quality assessment or assessment of the comparability of trials included in the input data, and the questionable validity of the indirect comparison method adopted, leads to considerable uncertainty over the cost-effectiveness of gemcitabine/paclitaxel. An illustrative analysis using a different method for indirect comparison presented in this report produces an ICER of £45,811 per QALY for gemcitabine/paclitaxel relative to docetaxel.
Commentary on the robustness of submitted evidence
Strengths
The structure of the manufacturer’s economic model is appropriate for the stated decision problem and reflects accepted methodology.
Weaknesses
The manufacturer performed a systematic review, which identified two abstracts (and missed a third) reporting interim results of the JHQG trial. However, commercial-in-confidence data were presented as ‘confidential – not to be cited’ in the manufacturer’s submission; they are due to be published later this year.
Although a systematic review was carried out, there is contradiction and a lack of methodological rigour regarding a number of the references included for the economic evaluation. The ERG therefore considers that, although the model’s structure is appropriate, selection bias could potentially have affected the data inputs for the economic model.
The attempted indirect comparison in the clinical effectiveness section simply tabulates data from the JHQG trial and the two comparator trials. It might have been possible to perform a formal statistical indirect comparison of the JHQG trial with that by Jones and colleagues8 (docetaxel monotherapy versus paclitaxel) as they have a common comparator arm. However, differences in the patient characteristics between the trials may have invalidated such an approach.
Conclusions
In the absence of a randomised controlled trial (RCT) directly comparing gemcitabine with docetaxel there does not appear to be sufficient evidence to compare the relative effectiveness of these treatments. The evidence for gemcitabine’s clinical effectiveness comes from an RCT comparing gemcitabine/paclitaxel with paclitaxel. However, the economic evaluation uses docetaxel as the comparator in the reference case.
The manufacturer suggests that gemcitabine should be considered as one option for first-line therapy for MBC in some patients, but does not appear to advocate that it should replace any of the current taxane treatments.
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in November 2006 states that:
Gemcitabine in combination with paclitaxel, within its licensed indication, is recommended as an option for the treatment of metastatic breast cancer only when docetaxel monotherapy or docetaxel plus capecitabine is also considered appropriate.
Acknowledgement
We thank members of the Resource and Information Service at the Wessex Institute for Health Research and Development, and J. Bryant of SHTAC for acting as internal editor. We would also like to thank Dr N Murray, Consultant in Oncology, Southampton University Hospitals Trust for acting as a clinical advisor for this review.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process n.d. www.nice.org.uk/page.aspx?o=STAprocessguide (accessed 19 September 2006).
- Jones J, Takeda A, Tan S, Cooper K, Loveman E, Clegg A, et al. Gemcitabine for Metastatic Breast Cancer 2006.
- Albain KS, Nag S, Calderillo-Ruiz G, Jordaan JP, Llombart A, Pluzanska A, et al. Global Phase III Study of Gemcitabine Plus Paclitaxel (GT) Vs. Paclitaxel (T) As Frontline Therapy for Metastatic Breast Cancer (MBC): First Report of Overall Survival n.d. www.asco.org/portal/site/ASCO/menuitem.34d60f5624ba07fd506fe310ee37a01d/?vgnextoid=76f8201eb61a7010VgnVCM100000ed730ad1RCRD%26vmview=abst_detail_view%26confID=26%26abstractID=2708 (accessed 1 April 2006).
- Collett D. Parametric proportional hazards models. Modelling survival data in medical research. Boca Raton, FL: Chapman & Hall/CRC; 2003.
- Albain KS, Nag S, Calderillo-Ruiz G, Jordaan JP, Llombart A, Pluzanska A, et al. Global phase III study of gemcitabine plus paclitaxel (GT) vs. paclitaxel (T) as frontline therapy for metastatic breast cancer (MBC): first report of overall survival. J Clin Oncol 2004;22.
- Manpour C, Wu J, Donaldson G, Liepa A, Melemed A, O’Shaughnessy J, et al. Gemcitabine plus paclitaxe (GT) versus paclitaxel (T) as first-line treatment for anthracycline pre-treated metastatic breast cancer (MBC): Quality of Life (QoL) and pair palliation results from the global phase III study. J Clin Oncol 2004;22.
- O’Shaughnessy J, Nags S, Calderillo-Ruiz G, Jordan J, Llombart A, Pluzanska A, et al. Gencitibine plus paclitaxel (GT) vs. paclitaxel (T) as first-line treatment for anthrycline pre-treated metastatic breast cancer (MBC): interim results of a global phase III study. Proc Am Soc Clin Oncol 2003;22.
- Jones SE, Erban J, Overmoyer B, Budd G, Hutchins L, Lower E, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 2005;23:5542-51.
- O’Shaughnessy J, Miles D, Vukelja S, Moiseyenko V, Ayoub JP, Cervantes G, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 2002;12:2812-23.