Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 06/64/01. The assessment report began editorial review in August 2008 and was accepted for publication in April 2009. See the HTA programme web site for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of omalizumab for the treatment of chronic severe persistent allergic asthma, in accordance with the licensed indication, based upon the evidence submission from Novartis to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The clinical evidence comes from a randomised controlled trial comparing omalizumab as an add-on to standard therapy with placebo and standard therapy over a 28-week treatment period. For the primary outcome of the rate of clinically significant asthma exacerbations, there was no statistically significant difference between treatment groups. However, after making a post hoc adjustment for a suggested ‘clinically relevant’ imbalance between trial arms in baseline exacerbation rate, the difference became marginally statistically significant. In terms of secondary outcomes, there were statistically significant differences favouring omalizumab over placebo in total emergency visits, Asthma Quality of Life Questionnaire scores, total symptom scores and lung function. Adverse events appeared to be similar between the trial arms. Results from three other publications are included in the manufacturer’s submission as supporting evidence for the effectiveness of omalizumab, despite not meeting the inclusion criteria which adhere strictly to the licensed indication. The ERG checked and provided commentary on the manufacturer’s model using standard checklists as well as undertook one-way sensitivity analysis, scenario analysis and a probabilistic sensitivity analysis. The cost-effectiveness analysis estimates the incremental costs and consequences of omalizumab as an add-on to standard therapy. The base-case analysis of the trial’s primary intention-to-treat population estimates a cost per quality-adjusted life-year of £30,647. The ERG conducted one-way sensitivity analyses for parameters omitted from the manufacturer’s submission sensitivity analysis. The results were most sensitive to variation in the utility values for omalizumab responders, and the unit cost of omalizumab. The guidance issued by NICE in November 2007 as a result of the STA states that omalizumab is recommended as a possible treatment for adults and young people over 12 years with severe persistent allergic asthma when their asthma meets certain conditions. Omalizumab treatment should be given along with the person’s current asthma medicines. It should be prescribed by a doctor who is experienced in asthma and allergy medicine at a specialist centre. If omalizumab does not control the asthma after 16 weeks, treatment should be stopped.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS which is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG); an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA, omalizumab for severe persistent allergic asthma.
Description of the underlying health problem
Asthma is characterised by symptoms such as dyspnoea, chest tightness, wheezing and cough associated with variable airflow obstruction and airway hyper-responsiveness. The development of asthma occurs when a person comes into contact with a trigger; the bronchioles (small airways in the lungs) become inflamed, swollen and constricted and excess mucus is produced, which has an effect on the person’s airway structure and function.
Asthma attacks vary in frequency and severity. Some people who have asthma are mostly symptom-free, with only occasional episodes of shortness of breath. Other people cough and wheeze most of the time and may have severe attacks after viral infections, exercise or irritants, including cigarette smoke; however, the absence of a cough or wheeze does not mean the attack is not severe. Asthma can have an allergic component resulting in overproduction of human immunoglobulin E (IgE) in response to environmental allergens, e.g. pollen, house dust mite. IgE binds to cell membrane receptors, resulting in the release of inflammatory mediators.
There are approximately 5.2 million people with asthma in the UK (4.7 million in England and Wales). The total for the UK includes 590,000 teenagers with asthma. Approximately 5% of asthma patients have severe asthma.
Current British guidelines from the British Thoracic Society (BTS) and Scottish Intercollegiate Guidelines Network (SIGN) recommend a stepwise approach to treatment. 2 Control is maintained by stepping up treatment as necessary and stepping down when control is good.
Scope of the evidence review group report
The ERG critically evaluated the evidence submission from Novartis on the use of omalizumab for the treatment of chronic severe persistent allergic asthma.
Omalizumab has a marketing authorisation for add-on therapy to improve asthma control in adult and adolescent patients (12 years of age and above) with severe persistent allergic asthma and ALL of the following:
-
a positive skin test or in vitro reactivity to a perennial aeroallergen
-
reduced lung function (forced expiratory volume in 1 second; FEV1 < 80%), frequent daytime symptoms or night-time awakenings, multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids (ICS), plus a long-acting inhaled beta2-agonist (LABA)
-
convincing IgE-mediated asthma.
The intervention specified in the decision problem was omalizumab as an add-on therapy to standard therapy, used within its licensed indication. The comparator was treatment without omalizumab. This means standard treatment such as ICS in combination with LABA, plus other medication as necessary in accordance with the BTS/SIGN guidelines. The population was adults and adolescent patients (12 years of age and above) with severe persistent allergic asthma under the conditions specified in the marketing authorisation. The outcome measures included objective measures of lung function [e.g. FEV1, peak expiratory flow (PEF)], symptom-free days and nights, incidence of acute exacerbations (e.g. unscheduled contact with health-care professional; hospitalisation or visit to accident and emergency department), levels of ICS, use of oral corticosteroids, reduction in IgE levels, adverse effects of treatment, health-related quality of life and mortality.
Methods
The ERG report comprised a critical review of the evidence for the clinical evidence and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
The ERG checked the literature searches and applied the NICE critical appraisal checklist to the included studies and checked the quality of the manufacturer’s submission with the Centre for Reviews and Dissemination (CRD) quality assessment criteria for a systematic review. In addition, the ERG checked and provided commentary on the manufacturer’s model using standard checklists. A one-way sensitivity analysis, scenario analysis and a probabilistic sensitivity analysis were undertaken by the ERG.
Results
Summary of submitted clinical evidence
The manufacturer’s submission presents clinical evidence for omalizumab in patients with severe persistent allergic asthma based on one published multicentre international double-blind randomised controlled trial (RCT) [known as the Investigation of Omalizumab in Severe Asthma Treatment (INNOVATE) trial]. 3 (Table 1) This was the pivotal EU/UK licensing trial. The trial compares omalizumab as an add-on to standard therapy (e.g. ICS and LABA) with placebo and standard therapy over a 28-week treatment period.
| Study: Humbert et al.3 (The INNOVATE trial) | ||
|---|---|---|
| Methods | Participants | Outcomes |
|
Design: RCT Interventions: GrpA: omalizumab – 0.016 (mg/kg)/(IU/ml) per 4-week period based on the patient’s bodyweight and total serum IgE level at screening every 2 or 4 weeks for a 28-week treatment duration by subcutaneous injectionGrpB: placebo by subcutaneous injection for 28-week treatment duration by subcutaneous injection Number of centres: 108 (14 countries) |
Inclusion criteria: Positive skin prick test to ≥ 1 perennial aeroallergen and total serum IgE level of ≥ 30 – ≤ 700 IU/ml Severe persistent asthma and regular treatment with > 1000 µg/day BDP or equivalent and LABA (GINA step 4 treatment) Forced expiratory volume in 1 second (FEV1) ≥ 40 to < 80% of predicted normal value and continuing asthma symptoms FEV1 reversibility ≥ 12% from baseline within 30 minutes of inhaled (≤ 400 µg) or nebulised (≥ 5 mg) salbutamol More than two asthma exacerbations requiring systemic corticosteroids, or one severe exacerbation (PEF/FEV1 < 60% of personal best, requiring systemic corticosteroids) resulting in hospitalisation or emergency room treatment, in past 12 months Additional asthma medications taken regularly from > 4 weeks prior to randomisation permitted, including theophyllines, oral b2-agonists and anti-leukotrienes Maintenance oral corticosteroids (maximum 20 mg/day) permitted providing at least one of the exacerbations in the previous 12 months occurred whilst on this therapy Exclusion criteria: Smokers or smoking history of ≥ 10 pack-years Treatment for an exacerbation within 4 weeks of randomisation (8-week run-in could be extended if necessary) Use of methotrexate, gold salts, troleandomycin or ciclosporin within 3 months of the first visit Prior omalizumab treatment Numbers: 482 ITT; 419 (86.9%) PITT (efficacy analyses) GrpA: 209; GrpB: 210 Age: mean (SD, median, range): GrpA: 43.4 (± 13.29, 44, 12–79) GrpB: 43.3 (± 13.49, 44, 13–71) Discontinued: 44 (10.8%). GrpA –30 (12.2%); GrpB –22 (9.3) Adverse events: GrpA –11(4.5%); GrpB –4(1.7%) Lost to follow-up: GrpA –2; GrpB –6. Reasons unknown |
Primary outcomes: Rate of clinically significant asthma exacerbations Secondary outcomes: Hospitalisation, emergency visit and unscheduled doctor’s visits QoLa (weeks 0, 12 and 28) Clinical symptom score (weeks 0, 1, 2, 4, 12, 20, 24 and 28) Use of rescue medication Patients and investigators global evaluations of treatment effectiveness Use of concomitant asthma medications Pulmonary function tests (FEV1, FVC, and FEF 25–75%); weeks 0, 2, 4, 12, 20, 24 and 28 PEF am/pm and number of days with > 20% improvement in am PEF compared with personal best (diaries); weeks 0,1, 2, 4, 12, 20, 24 and 28 Other measures: Haematological assessment, urine screening and blood chemistry; weeks 0, 12, 28 and during follow-up Vital signs and physical examination Length of follow-up: 16 weeks (results not reported) |
The efficacy analyses were carried out on the ‘primary intention to treat’ (PITT) population, which excludes 13% of randomised patients (excluded due to a trial protocol amendment). With the exception of safety results, ‘true’ intention to treat (ITT) results are not reported in the main manufacturer’s submission report, or the INNOVATE journal publication. For the primary outcome of the rate of clinically significant asthma exacerbations, there was no statistically significant difference between treatment groups. However, after making a post hoc adjustment for a suggested ‘clinically relevant’ imbalance between trial arms in baseline exacerbation rate, the difference became marginally statistically significant.
In terms of secondary outcomes, there were statistically significant differences favouring omalizumab over placebo in total emergency visits, Asthma Quality of Life Questionnaire scores, total symptom scores and lung function. Adverse events appeared to be similar between the trial arms.
Results from three other publications are included in the manufacturer’s submission as supporting evidence for the effectiveness of omalizumab, despite not meeting the inclusion criteria which adhere strictly to the licensed indication. These included a 12-month open-label ‘naturalistic’ RCT, a meta-analysis of seven pharmaceutical company sponsored trials, and a Cochrane systematic review of 14 RCTs of anti-IgE treatment. The results of these publications, in differing populations of asthmatics (e.g. mild to moderate asthma), are reported to support the findings of the INNOVATE trial.
Summary of submitted cost-effectiveness evidence
The cost-effectiveness analysis (CEA) comprises a Markov state-transition model to estimate the incremental costs and consequences of omalizumab as an add-on to standard therapy. The model has been applied in a published Swedish4 and a published Canadian5 cost-effectiveness study and is reported to have been validated by asthma physicians and modelling experts.
Despite some limitations in reporting, the model is, in general, internally consistent and appropriate to severe asthma in terms of its structural assumptions. The CEA generally conforms to the NICE reference case and the scope/decision problem.
The model assumes that responders to omalizumab (those rated as ‘excellent’ or ‘good’ using the global evaluation of treatment effectiveness) at 16 weeks will continue to receive the drug for 5 years, after which they revert to standard therapy. Nonresponders to omalizumab at 16 weeks revert to standard therapy at that point. The model has a lifetime horizon.
Data from the INNOVATE trial are used to estimate the proportion of patients with clinically significant exacerbations (both severe and non-severe), the utility associated with day-to-day symptoms, and treatment costs. Utility values for clinically significant exacerbations were taken from another study. 6
The base-case analysis of the INNOVATE PITT population estimates a cost per quality-adjusted life-year (QALY) of £30,647. The base-case cost per QALY for a subgroup of ‘high risk’ patients hospitalised in the previous year was £26,509.
The base-case estimate for the INNOVATE PITT population rises as the mortality rate associated with clinically severe exacerbations decreases, with a cost per QALY of £73,177 when a 0% rate is used.
The ERG conducted one-way sensitivity analyses for parameters omitted from the manufacturer’s submission sensitivity analysis. The results were most sensitive to variation in the utility values for omalizumab responders, and the unit cost of omalizumab.
The ERG conducted scenario analyses examining the cumulative effect of varying assumptions over the asthma mortality rate, costing of omalizumab, and utilities applied to the exacerbation states and to the day-to-day symptoms state for standard care. Using a lower mortality rate than in the base case and a more realistic approach to costing omalizumab in primary care produced less favourable incremental cost effectiveness ratios (ICERs) than in the base case. ICERs were more sensitive to assumptions over the difference in utility between omalizumab responders and standard care/non-responders than to utility associated with transient changes (such as exacerbations).
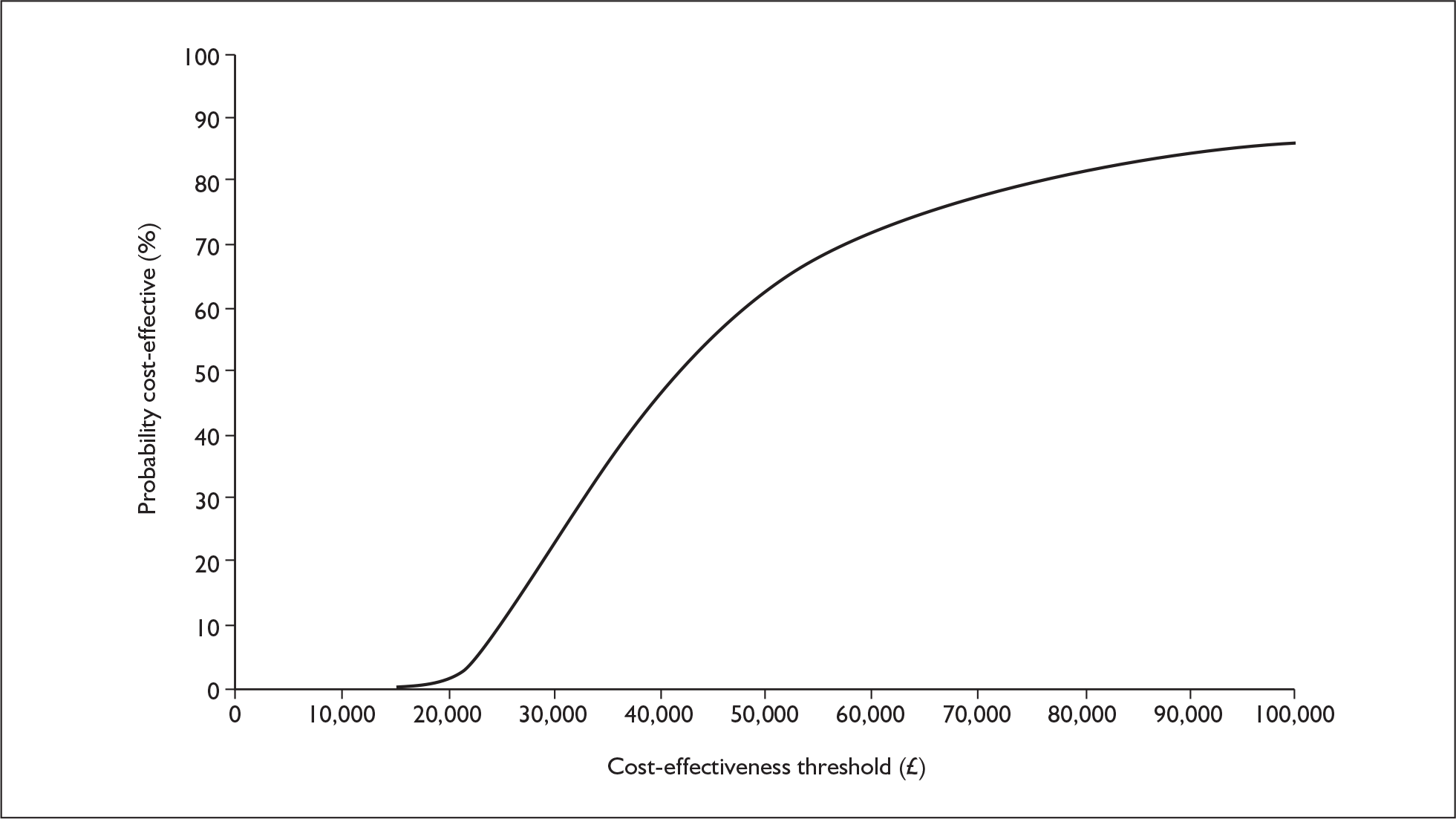
The probabilistic cost–utility analysis of the INNOVATE PITT population was £31,713 (confidence interval £23,178, £48,236) with a 50% probability of the ICER being under £32,000. A replication of the probabilistic analysis by the ERG using a lower mortality rate (2%) and omalizumab cost per vial rather than per milligram, generated a mean ICER of £38,852. At a threshold willingness to pay of £30,000 per QALY, omalizumab add-on therapy has a 23.6% probability of being cost-effective (Figure 1 and Figure 2).
FIGURE 1.
Scatter plot of the ERG probabilistic sensitivity analysis results. QALYs, quality-adjusted life-years.
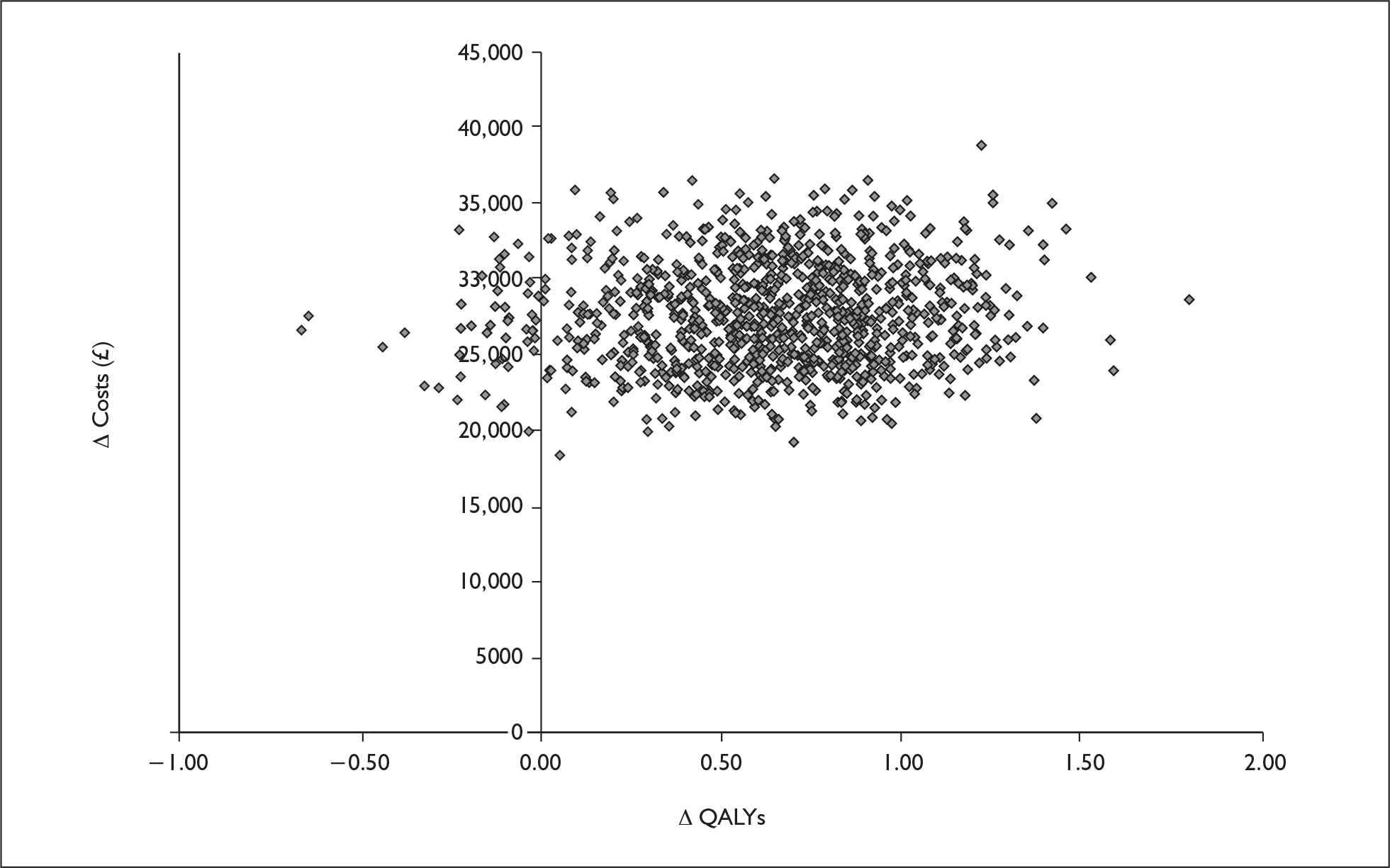
FIGURE 2.
Cost-effectiveness acceptability curve from ERG probabilistic sensitivity analysis, INNOVATE primary intention-to-treat population.
Commentary on the robustness of submitted evidence
Strengths
The manufacturer’s submission includes a systematic search for clinical effectiveness and cost-effectiveness studies of omalizumab. It appears unlikely that any additional trials would have met the inclusion criteria had the search been widened to include other databases.
The INNOVATE trial appears to be of reasonable methodological quality (with some limitations – see below) and measures a range of clinically relevant outcomes (e.g. exacerbations, day and night symptoms, health-related quality of life, emergency visits and adverse events). Taken together these outcomes accurately capture the impact of pharmacotherapy on the control of severe asthma.
The economic model appears internally consistent and structurally appropriate, and the cost-effectiveness analysis is in accordance with the NICE reference case and the scope of the appraisal.
Weaknesses
Despite a systematic search and screen of the literature, only one RCT was included. The manufacturer’s submission is therefore largely dependent upon this one trial. Although the trial has merits there are also weaknesses, notably in the statistical analysis. Further high-quality RCT evidence for the effectiveness of omalizumab in the patient group meeting the licensed indication would be beneficial.
The INNOVATE trial was subject to protocol amendments which resulted in the exclusion of 13% of randomised patients from the PITT efficacy population (although it is reported that the results of the full ITT analysis are similar to the PITT).
As acknowledged in the manufacturer’s submission, there was a strong placebo effect in the INNOVATE trial, exemplified by the relatively high physician rating of response for patients receiving placebo in addition to standard therapy. This is attributed to the optimised standard of care received by patients in the clinical trial. Consequently, the manufacturer’s submission regards the treatment effect to be an underestimate. Although an open-label RCT conducted in a setting more representative of clinical practice was presented as supporting evidence, only around half of the randomised patients in this trial met the criteria for the licensed indication.
Conclusions
Areas of uncertainty
There is uncertainty about some of the statistical methods used in the analysis of the INNOVATE trial because of post hoc adjustments to the primary outcome to correct for suggested clinically relevant imbalances in baseline exacerbation history between trial arms. The manufacturer’s submission reports that such adjustment was recommended by the Committee for Medicinal Products for Human Use. The validity of post hoc adjustments has to be viewed with caution, particularly as the difference in favour of omalizumab in the primary outcome only became statistically significant following adjustment.
The validity of including unpublished post hoc analysis for two subgroups (‘high-risk’ previously hospitalised patients, and omalizumab responders), is also questionable as both are likely to be underpowered.
Long-term published data on the effectiveness and safety of omalizumab are not yet available. The economic model extrapolates efficacy data from the 28-week INNOVATE trial over a 5-year period, and assumes full compliance. In practice, compliance is likely to vary with factors such as the standard of care, which may not be as optimal as within the context of a clinical trial.
There is no discussion in the manufacturer’s submission of possible bias introduced due to missing response data on 14 omalizumabtreated patients. There is no discussion of the characteristics of these patients and the manufacturer’s submission does not report the number of exacerbations for these patients separately.
The submission assumes that it is possible to store unused portions of vials of omalizumab and therefore costs the drug by the milligram rather than by the vial. It is unclear whether such a policy of re-use would be feasible in primary care, without incurring substantial additional costs for safe storage and managing this process.
There is substantial uncertainty over the excess mortality rate applied to severe exacerbations in the model. The rate used was derived from a Swedish observational study7 in which definitions of severe and moderate asthma exacerbations were not clearly specified, and the patient population was substantially older (62.5 years) than the mean starting age for patients in the model (40 years). The manufacturer’s submission contains no discussion or objective evidence on the extent to which the dimension that defines a clinically significant exacerbation as severe in the model (PEF or FEV1 less than 60% of personal best) is a valid predictor of risk of asthma death.
Key issues
Given that the inclusion criteria adhere strictly to the licensed indication, only one RCT was officially included in the manufacturer’s submission (the pivotal licensing trial). In this trial the primary outcome became statistically significant in favour of omalizumab only once a post hoc adjustment had been made to correct for a ‘clinically relevant’ imbalance between trial arms.
The ICER is highly sensitive to assumptions about the mortality rate associated with severe exacerbations, and to a lesser extent to whether omalizumab is costed on a per vial or per milligram basis.
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in November 2007, TA133, states that:
Omalizumab is recommended as a possible treatment for adults and young people over 12 years with severe persistent allergic asthma when all of the following circumstances apply.
-
When the person’s asthma is still severe and unstable despite best efforts to control it with other asthma medicines taken as directed by their doctor.
-
When the person has stopped smoking, if their doctor feels it is appropriate.
-
When the person has allergic asthma. This should be confirmed by checking past symptoms and skin testing for allergies.
-
When the person has had at least two asthma attacks within the past year that have needed admission to hospital, or when the person has had three or more severe asthma attacks within the past year, one of which has needed admission to hospital and the other two have needed additional treatment in an accident and emergency department.
Omalizumab treatment should be given along with the person’s current asthma medicines. It should be prescribed by a doctor who is experienced in asthma and allergy medicine at a specialist centre. If omalizumab does not control the asthma after 16 weeks, treatment should be stopped.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- NICE . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/media/8DE/74/STA_process_Guide.pdf (accessed 15 July 2009).
- British Thoracic Society, Scottish Intercollegiate GN . British Guideline on the Management of Asthma (Revised Edition November 2005) n.d. www.sign.ac.uk/guidelines/fulltext/101/index.html (accessed 15 July 2009).
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16.
- Dewilde S, Turk F, Tambour M, Sandstrom T. The economic value of anti-IgE in sever persistent, IgE-mediated (allergic) asthma patients: adaption of INNOVATE to Sweden. Curr Med Res Opin 2006;22:1765-76.
- Brown R, Turk F, Dale P, Bousquet J. Cost effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy 2007;62:149-53.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7.
- Lowhagen O, Ekstrom L, Holmberg S, Wennerblom B, Rosenfeldt M. Experience of an emergency mobile asthma trteatment programme. Resucitation 1997;35:243-47.