Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 06/87/01. The assessment report began editorial review in September 2008 and was accepted for publication in April 2009. See the HTA programme web site for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group report into the clinical effectiveness and cost-effectiveness of rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma (NHL), in accordance with the licensed indication, based upon the evidence submission from Roche Products Ltd to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The submitted clinical evidence included two randomised controlled trials [European Organisation for Research and Treatment of Cancer (EORTC) and German Low Grade Lymphoma Study Group – Fludarabine, Cyclophosphamide and Mitoxantrone and (GLSG-FCM)] comparing the clinical effects of chemotherapy with or without rituximab in the induction of remission at first or second relapse and the clinical benefits of rituximab maintenance therapy versus the NHS’s current clinical practice of observation for follicular lymphoma (FL) patients. Both trials showed that in patients with relapsed FL the addition of rituximab to chemotherapy induction treatment increased overall response rates. Furthermore, rituximab maintenance therapy increased the median length of remission when compared with observation only. Safety data from the two trials showed that while the majority of patients reported some adverse events, the number of patients withdrawing from treatment in the EORTC trial was low, with rates not being reported for the GLSG-FCM trial. The most commonly reported adverse events were blood/bone marrow toxicity, skin rashes and allergies. The ERG reran the manufacturer’s economic model after altering several of the assumptions and parameter values in order to recalculate the cost–utility ratios, quality-adjusted life-years (QALYs) and estimates of benefits. The manufacturer reported that maintenance therapy with rituximab was cost-effective compared with observation against commonly applied thresholds, with an incremental cost-effectiveness ratio of £7721 per QALY gained. The greatest clinical effectiveness is achieved by R-CHOP followed by rituximab maintenance (R-CHOP > R) and this treatment strategy had the greatest probability of being cost-effective for a QALY of approximately £18,000 or greater. The guidance issued by NICE as a result of the STA states that in people with relapsed stage III or IV follicular NHL, rituximab is now an option in combination with chemotherapy to induce remission or alone as maintenance therapy during remission. Rituximab monotherapy is also an option for people with relapsed or refractory disease when all alternative treatment options have been exhausted.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG); an external organisation independent of the Institute. This paper presents a summary of the ERG report for the STA entitled ‘Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma’. 2
Description of the underlying health problem
Non-Hodgkin’s lymphoma (NHL) represents about 3% of all cancers diagnosed in the UK. In 2002 there were 9443 people diagnosed with NHL in the UK3 with an incidence of 16 per 100,000 in England and 15.6 per 100,000 in Wales. The overall rate is increasing at 3–4% per year, which is greater than would be expected from simply a combination of the effects of an ageing population plus improved diagnostic techniques. 4 Follicular lymphoma (FL) is the second most common type of NHL (22%) with a UK incidence of approximately 4 per 100,0005 and a prevalence of about 40 per 100,000. 4,5
Low-grade or indolent disease is differentiated from high-grade or aggressive disease by histology. Histological grading of the disease is determined by the World Health Organization classification grades I, II or IIIa or IIIb. 5 The grade is determined by the number and size of abnormal cells taken from lymph node biopsies. There is a growing consensus that histological grade III and, in particular, grade IIIb disease should be classified as aggressive and treated as such rather than treated as indolent disease.
Survival for patients with FL is prolonged. Different figures for median survival have been reported, but 8–10 years from diagnosis is typical. 6,7 However, these are likely to be underestimates as there is good evidence from recent large population-based8 and single institution studies9,10 that survival is improving. 11 This is probably as a consequence of improved treatment, especially the introduction of rituximab, which is the first drug treatment for this disease to demonstrate an ability to improve overall survival in randomised controlled trials.
Scope of the evidence review group report
The ERG report presents the results of the assessment of the manufacturer’s/sponsor’s evidence submission regarding the use of rituximab for the treatment of relapsed/refractory FL. The ERG report includes an assessment of both the clinical and the cost-effectiveness evidence submitted by Roche Products Limited. The manufacturer’s submission (MS) considers two ways of using rituximab: firstly, in conjunction with cytotoxic chemotherapy in order to induce remission in relapsed FL; and secondly, as maintenance therapy after successful induction of remission, regardless of the chemotherapy used to induce remission. The manufacturer presents clinical evidence to support the use of (1) rituximab plus chemotherapy (e.g. R-CHOP and R-FCM) in the induction phase and (2) rituximab versus observation in the maintenance phase of treatment for FL patients. Only clinical evidence from the CHOP comparisons is used in the cost-effectiveness analyses. The MS claims that there is no new evidence for the use of rituximab in adult patients with stage III–IV FL who are chemoresistant or are in their second or subsequent relapse after chemotherapy. Therefore the MS presents no new case for the use of rituximab in this patient population.
Methods
The ERG report comprised a critical review of the clinical and cost-effectiveness evidence presented in the MS to NICE as part of the STA process. The ERG assessed the quality of the clinical effectiveness review using a checklist, and attempted to replicate relevant clinical effectiveness and cost-effectiveness literature searches. The ERG re-ran the manufacturer’s economic model after altering several of the assumptions and parameter values in order to recalculate the cost–utility ratios, quality-adjusted life-years (QALYs) and estimates of benefits.
Results
Summary of submitted clinical evidence
The MS provides clinical evidence from two randomized controlled trials (EORTC and GLSGFCM). Both trials were included in the clinical systematic review and compare the clinical effects of chemotherapy with or without rituximab in the induction of remission at first or second relapse, and the clinical benefits of rituximab maintenance therapy versus the NHS’s current clinical practice of observation for FL patients. Both trials had two points of randomisation. The induction phases included 465 and 147 patients with relapsed FL in EORTC and GLSG-FCM trials respectively. The maintenance phases included 395 and 176 patients who had responded to induction therapy in EORTC and GLSG-FCM trials respectively. Only 113 patients in the GLSG-FCM trial who received maintenance therapy or observation were FL patients. Both trials showed that in patients with relapsed FL the addition of rituximab to chemotherapy induction treatment increased overall response rates; 72.3% (CHOP) versus 85.1% (R-CHOP) in the EORTC trial and 70% (FCM) versus 94% (R-FCM) in the GLSG-FCM trial. Furthermore, rituximab maintenance therapy increased the median length of remission when compared with observation only. In the EORTC trial, median progression-free survival (PFS) was 14.9 months for those on observation compared with 51.5 months for those receiving rituximab. In the GLSG-FCM trial for FL patients who received R-FCM, median PFS in the observation group was 26 months, and for those receiving rituximab median PFS was not reached.
Safety data from the two trials showed that while the majority of patients reported some adverse events, the number of patients withdrawing from treatment in the EORTC trial was low: 3% in each group at induction and 4% in the rituximab group at maintenance (rates were not reported for the GLSG-FCM trial). The most commonly reported adverse events were blood/bone marrow toxicity, skin rashes and allergies.
Summary of submitted cost-effectiveness evidence
The MS presents the results of two sets of economic evaluations. The first compares the use of rituximab maintenance (following response to an induction therapy) with observation only (no treatment until relapse). This is referred to as the maintenance two-arm model. A three-state transition model (progression free, progressive disease and death) is used to capture the costs and benefits of relapsed/refractory FL.
The second model compares the use of rituximab maintenance therapy with observation only for patients responding to chemotherapy with or without rituximab, and tests whether the use of rituximab as an induction therapy in addition to maintenance therapy is cost-effective. This is referred to as the induction plus maintenance four-arm model. A five-state transition model (progression free in the induction setting, progression free in the maintenance setting, progression free but not in the induction or maintenance setting, progressive disease and death) captures the costs and benefits of relapsed/refractory FL.
Evidence from the EORTC trial is the principal source of clinical data used in the economic evaluations. A half-cycle correction is applied in both models. Patients in the economic evaluation are followed through the health states in monthly cycles over a period of 30 years in order to capture the entire lifetime costs and effects of the population. Patients only exit the model due to death.
In the MS, the two-arm model is used to demonstrate that maintenance therapy with rituximab when compared with observation is cost-effective against commonly applied thresholds. The manufacturer reports an incremental cost-effectiveness ratio (ICER) of £7721 per QALY gained for this comparison. In the MS, when subject to extensive univariate and probabilistic sensitivity analysis (PSA), this ICER is shown to be robust (Table 1). In the MS, the four-arm economic model illustrates that the greatest clinical effectiveness is achieved by R-CHOP followed by rituximab maintenance (R-CHOP > R). The MS concludes that R-CHOP > R is cost-effective when compared with the second most clinically effective intervention of CHOP induction followed by rituximab maintenance therapy (CHOP > R); the estimated ICER is £16,749 per QALY gained. Again, in the MS this ICER is shown to be robust (Table 2).
| Treatment group | Total costs | QALYs gained | Incremental cost per QALY gained |
|---|---|---|---|
| Rituximab | £21,608 | 4.2250 | |
| ‘Observation’ | £14,722 | 3.3331 | |
| Incremental | £6886 | 0.8919 | £7721 |
| Treatment and comparator groups | Costs | QALYs gained | Incremental cost per QALY gained |
|---|---|---|---|
| R-CHOP>R | £28,585 | 4.0906 | |
| CHOP>R | £22,389 | 3.7207 | |
| Incremental | £6196 | 0.3699 | £16,749 |
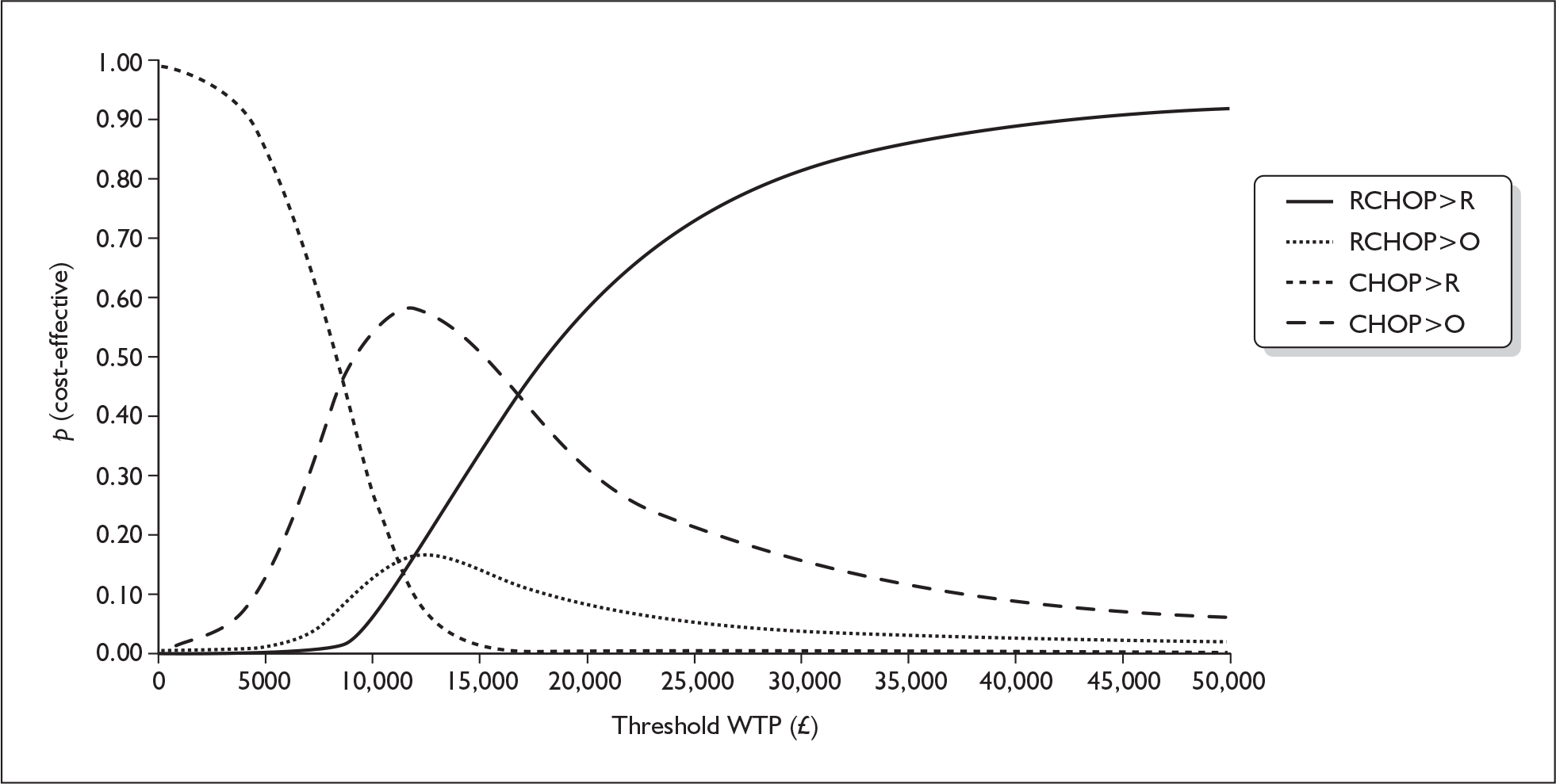
For the PSA, scatter plots and cost-effectiveness acceptability curves were calculated. For the four-arm model, the manufacturer presents a scatter plot to illustrate the considerable overlap of costs and QALYs across the four treatment groups (Figure 1). The cost-effectiveness acceptability curve shows that at a willingness to pay (WTP) for a QALY of approximately £18,000 or greater, the R-CHOP > R treatment strategy had the greatest probability of being cost-effective (Figure 2).
FIGURE 1.
Scatter plot showing incremental cost and effect of maintenance therapy over CHOP>observation across 2000 simulations of the economic model.
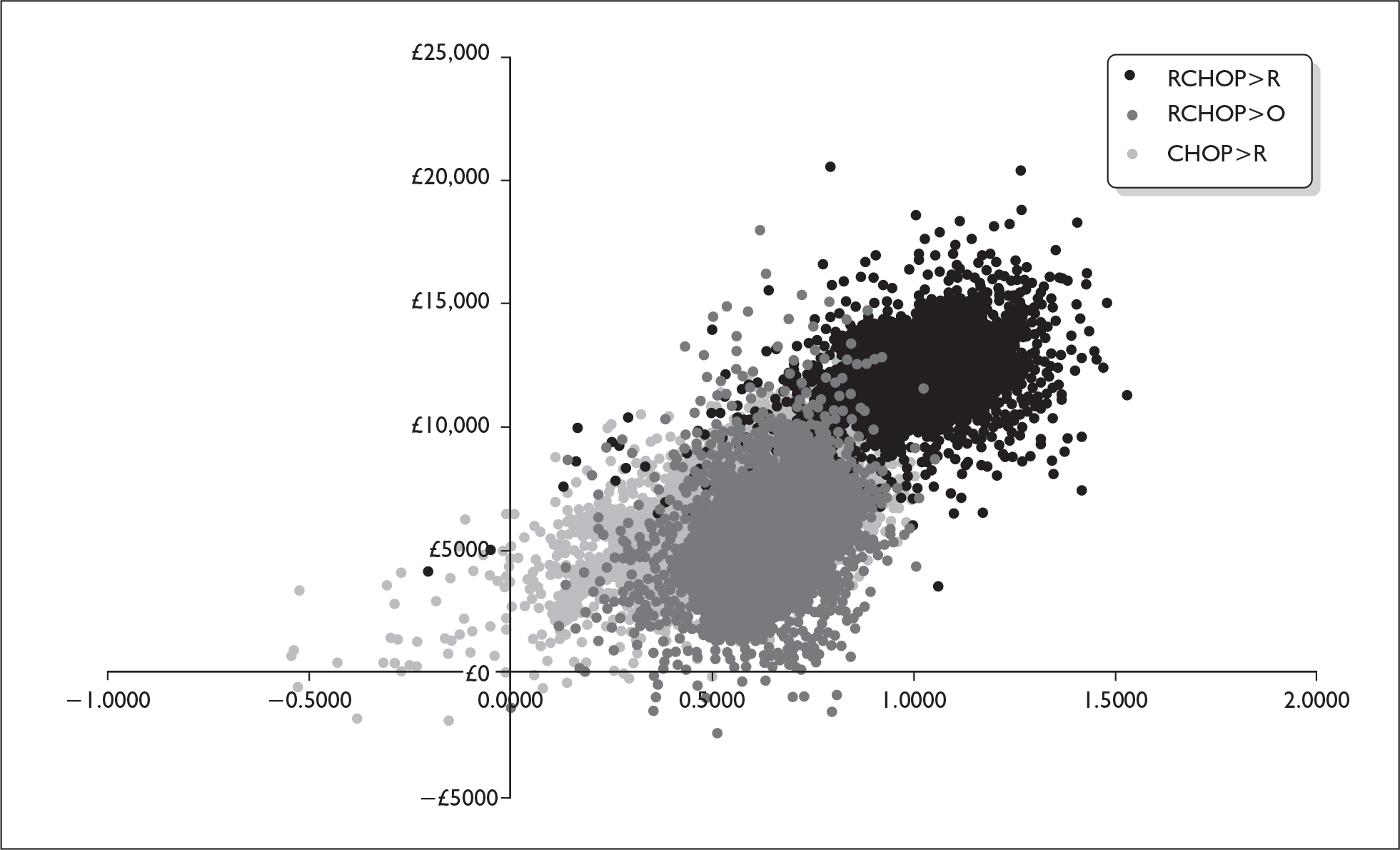
FIGURE 2.
Cost-effectiveness acceptability curve – probability that each treatment practice is cost-effective at a given willingness-to-pay (WTP) threshold.
Commentary on the robustness of submitted evidence
Strengths
The MS includes supporting clinical data from two randomised controlled trials, both of which closed early due to interim analyses showing a significant clinical benefit for rituximab treatment as induction and/or maintenance therapy before enrolment was complete.
The two economic models submitted by the manufacturer are implemented to a generally high standard, clearly presented and with a large amount of source information included to aid traceability. The layouts of the various elements of the models are generally logical, and the formulae employed are straightforward.
Weakness
The systematic review (SR) reported in the MS does not clearly specify the inclusion and exclusion criteria employed, which results in ambiguity regarding reasons for the exclusion of some trials. In addition, the MS fails to describe adequately the existing clinical evidence for the use of rituximab monotherapy in the treatment of relapsed FL.
The GLSG-FCM trial includes FL, mantle cell and lymphocytoid lymphoma patients. Evidence to support the use of rituximab as maintenance from the GLSG-FCM trial is inconclusive due to missing clinical data for FL patients only.
From the available clinical evidence, the ERG concludes that the maintenance two-arm economic model is too simplistic and therefore the ERG concentrates on the results generated by the induction plus maintenance four-arm model.
Uncertainty
The clinical effectiveness of R-CHOP induction in patients previously treated with rituximab cannot be assessed from this STA as patients in the EORTC trial are rituximab naive at entry. In 2006, R-CVP was approved by NICE12 as a first-line treatment for patients with FL. It is therefore unlikely that future patients with relapsed FL in the NHS in England and Wales will be rituximab naive.
The ERG raised some concerns about the modelling of the survival data. The ERG was unable to overcome such concerns (e.g. by conducting PSA) as the manufacturer did not provide the requested additional information on the disposition of patients in the EORTC trial and the mean time spent in each segment of the treatment pathway.
Conclusions
The ERG acknowledges that the economic models submitted by the manufacturer are implemented to a generally high standard, clearly presented and with a large amount of source information included to aid traceability. The layouts of the various elements of the models are generally logical, and the formulae employed are straightforward.
On detailed examination of the models, the ERG identified a minor anomaly in the model coding that affected estimates of both costs and outcomes. Correction of this anomaly favoured the rituximab patients. In terms of costs, the ERG made two adjustments which increased the R-CHOP > R versus CHOP > R ICER. Firstly, the outpatient cost (£86) is replaced by a chemotherapy administration cost (£504) in order to reflect that demanding chemotherapy regimens are typically given within a day-case setting and the ICER increases from £16,749 to £18,204. Secondly, the calculation of alternative postprogression treatment costs by the ERG also increases the ICER from £16,749 to £22,688.
In terms of utilities, changing the postprogression utility values does not have a major impact on the ICERs. However, the preferred approach to survival modelling does impact on the size of the ICER for every possible combination in the four-arm model. The ERG identifies four areas of concern regarding the manufacturer’s estimation of lifetime benefits from use of rituximab. In order to overcome such concerns, the ERG requested additional information from the manufacturer about the disposition of patients and the mean time spent in each segment of the treatment pathway. The manufacturer declined to provide this information. Consequently, the ERG used the observed and reported evidence on PFS and overall survival (OS) from the EORTC trial rather than the manufacturer’s projections. In doing so, the ICERs for the six possible combinations now range from £13,895 to £73,140.
The ERG calculated the cumulative effect of all of the changes on the ICERs (Table 3). It is clear that the single-use strategies are the most cost-effective options, i.e. use of rituximab for induction of remission (£13,122 per QALY gained) or for maintenance of remission (£16,488 per QALY gained). Dual-use strategies compared with single-use strategies are the least cost-effective options at around £42,000 per QALY gained. A comparison of dual use of rituximab with no use of rituximab also appears to be moderately cost-effective (£26,000 per QALY gained). However, in order to fully inform decision-making about the preferred use of rituximab for FL, a comprehensive PSA in the form of a cost-effectiveness acceptability plot is required. However, as all of the necessary data were not available to the ERG, it was not possible to carry out this assessment.
| Comparison | Model projections | ERG modifications but using original outcome projections | ERG modifications including K–M outcome estimates | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IC | IQ | ICER | IC | IQ | ICER | IC | IQ | ICER | |
| R-CHOP>R vs CHOP>R | £6196 | 0.3699 | £16,749 | £8849 | 0.3705 | £23,882 | £8660 | 0.2015 | £42,982 |
| R-CHOP>R vs R-CHOP>0 | £5531 | 0.4646 | £11,904 | £7686 | 0.4656 | £16,509 | £7289 | 0.1728 | £42,192 |
| R-CHOP>R vs CHOP>0 | £11,927 | 1.0014 | £11,910 | £12,149 | 1.0034 | £12,108 | £12,157 | 0.4680 | £25,978 |
| CHOP>R vs R-CHOP>0 | –£665 | 0.0947 | Dominant | –£1,163 | 0.0951 | –£12,232 | –£1371 | –0.0287 | £47,734 |
| CHOP>R vs CHOP>0 | £5731 | 0.6315 | £9076 | £3300 | 0.6329 | £5,214 | £3497 | 0.2665 | £13,122 |
| R-CHOP>0 vs CHOP>0 | £6396 | 0.5368 | £11,916 | £4463 | 0.5378 | £8,298 | £4867 | 0.2952 | £16,488 |
In summary, the ERG agrees that the use of rituximab for the treatment of FL is probably cost-effective, but cannot confidently recommend either or both single-use strategies over the dual-use strategy, based on the available data.
Summary of NICE guidance issued February 2008 as a result of the STA
In people with relapsed stage III or IV follicular NHL, rituximab is now an option in combination with chemotherapy to induce remission or alone as maintenance therapy during remission. Rituximab monotherapy is also an option for people with relapsed or refractory disease when all alternative treatment options have been exhausted.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal. 2004. www.nice.org.uk/pdf/TAP_Methods.pdf (accessed June 2007).
- Bagust A, Boland A, Hockenhull J, Davis H, Chu P, Dickson R. ERG Report: Rituximab for the Treatment of Relapsed or Refractory Stage III or IV Follicular Non-Hodgkin’s Lymphoma 2007.
- Cancer Research UK . Cancer Facts and Figures. 2006. www.cancerresearchuk.org (accessed 27 June 2007).
- National Institute for Clinical Excellence . Guidance on the Use of Rituximab for Recurrent or Refractory Stage III or IV Follicular Non-Hodgkin’s Lymphoma 2002.
- British Committee for Standards in Haematology . BCSH Guidelines on Nodal Non-Hodgkin’s Lymphoma 2002. www.bcshguidelines.com/pdf/NHL_100903.pdf (accessed June 2007).
- Horning S, Rosenberg S. Natural history of initially untreated low-grade non-Hodgkin’s lymphoma. New Engl J Med 1984;311:1471-5.
- Lister T. The management of follicular lymphoma. Annals Oncol 1991;2:131-5.
- Swenson WT, Wooldridge JE, Lynch CF, FormanHoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol 2005;23:5019-26.
- Dillman R, Chico S. Improved survival of lymphoma patients after introduction of rituximab. Blood 2005;106.
- Liu Q, Fayad L, Cabanillas F, Hagemeister F, Ayers G, Hess M, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at the University of Texas. J Clin Oncol 2006;24:1582-9.
- Fisher R, LeBlanc M, Press O, Maloney D, Unger J, Miller T. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol 2005;23:8447-52.
- National Institute for Health and Clinical Excellence . Rituximab for the Treatment of Follicular Lymphoma: NICE Technology Appraisal Guidance 10 2006.