Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 07/10/01. The assessment report began editorial review in May 2008 and was accepted for publication in May 2009. See the HTA programme web site for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of lapatinib for the treatment of advanced or metastatic HER2overexpressing breast cancer based upon a review of the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The scope included women with advanced, metastatic or recurrent HER2-overexpressing breast cancer who have had previous therapy that includes trastuzumab. Outcomes were time to progression, progression-free survival, response rates, overall survival, health-related quality of life and adverse effects. The submission’s evidence came from one randomised controlled trial (RCT) of reasonable methodological quality, although it was not powered to detect a statistically significant difference in mean overall survival. Median time to progression was longer in the lapatinib plus capecitabine arm than in the capecitabine monotherapy arm {27.1 [95% confidence interval (CI) 17.4 to 49.4] versus 18.6 [95% CI 9.1 to 36.9] weeks; hazard ratio 0.57 [95% CI 0.43 to 0.77; p = 0.00013]}. Median overall survival was very similar between the groups [67.7 (95% CI 58.9 to 91.6) versus 66.6 (95% CI 49.1 to 75.0) weeks; hazard ratio 0.78 (95% CI 0.55 to 1.12; p = 0.177)]. Median progression-free survival was statistically significantly longer in the lapatinib plus capecitabine group than in the capecitabine monotherapy group [27.1 (95% CI 24.1 to 36.9) versus 17.6 (95% CI 13.3 to 20.1) weeks; hazard ratio 0.55 (95% CI 0.41 to 0.74); p = 0.000033]. The manufacturer’s economic model to estimate progression-free and overall survival for patients with HER2-positive advanced/metastatic breast cancer who had relapsed following treatment with an anthracycline, a taxane and trastuzumab was appropriate for the disease area. The base-case incremental cost-effectiveness ratios (ICERs) for lapatinib plus capecitabine compared with capecitabine monotherapy or vinorelbine monotherapy were higher than would conventionally be considered cost-effective. When compared with trastuzumab-containing regimes, lapatinib plus capecitabine dominated. In sensitivity analyses the ICER for lapatinib plus capecitabine compared with capecitabine monotherapy or vinorelbine monotherapy was robust to variation in assumptions. In all sensitivity analyses the ICERs remained higher than would conventionally be considered cost-effective. ICERs for trastuzumab-containing regimes were particularly sensitive to assumptions over the frequency of treatment, which had a large effect on the cost-effectiveness of lapatinib plus capecitabine. In conclusion, there was a general lack of evidence on the effectiveness of comparators included in the model and on key parameters such as dose adjustments and the model outputs need to be interpreted in the light of this uncertainty. At the time of writing, NICE were still considering the available evidence for this appraisal.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of the clinical effectiveness and cost-effectiveness of lapatinib for the treatment of metastatic breast cancer.
Description of the underlying health problem
Breast cancer is the most common cancer in the UK, accounting for one-third of all cancers in women. 2 Increasing age is the strongest risk factor for breast cancer, and the disease is rare in women under the age of 40.
In 2004 there were 36,939 new cases of breast cancer in women in England, which represents a crude rate of 144.6 per 100,000 women. 3 In 2005 there were 2364 new registrations in Wales, giving a rate of 155.4 per 100,000 women. These figures equate to age-standardised rates per 100,000 population of 120.7 [95% confidence interval (CI) 119.5 to 121.9] for England and 120.8 (95% CI 115.9 to 125.7) for Wales. 4 A recent review by the Office for National Statistics5 found a 20-year survival rate of 64% for women diagnosed with breast cancer between the ages of 50 and 69 years.
Breast cancer is classified on a clinical basis according to the internationally recognised tumour, node, metastases (TNM) staging system. 6 The TNM system is based on three sets of codes relating to the primary tumour, involvement of lymph nodes and evidence of distant metastases. Four clinical stages are defined by particular combinations of these codes. Stage IV is metastatic disease, regardless of lymph node assessment or size of primary tumour. Approximately 25–30% of people with metastatic breast cancer have HER2-positive disease, that is, their tumours overexpress the HER2 gene. 7
Scope of the ERG report
The ERG critically evaluated the evidence submission from GlaxoSmithKline UK for the use of lapatinib for the treatment of advanced or metastatic ErbB2 (HER2: human epidermal growth factor receptor 2)-overexpressing breast cancer, in accordance with the predicted licensed indication. Lapatinib is a dual kinase inhibitor of epidermal growth factor receptor (ErbB1) and HER2 (ErbB2). It works intracellularly and, unlike monoclonal antibodies, it can block signalling through receptors that have lost or mutated their extracellular domains. Lapatinib is administered orally, in conjunction with capecitabine.
At the time of writing, lapatinib had not yet received its marketing authorisation. The final scope issued by NICE stated that the population should be women with advanced, metastatic or recurrent breast cancer that overexpresses the HER2 receptor who have had previous therapy that includes trastuzumab. The outcomes stated in the manufacturer’s definition of the decision problem were time to progression (primary end point), progression-free survival, response rates, overall survival, health-related quality of life and adverse effects.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
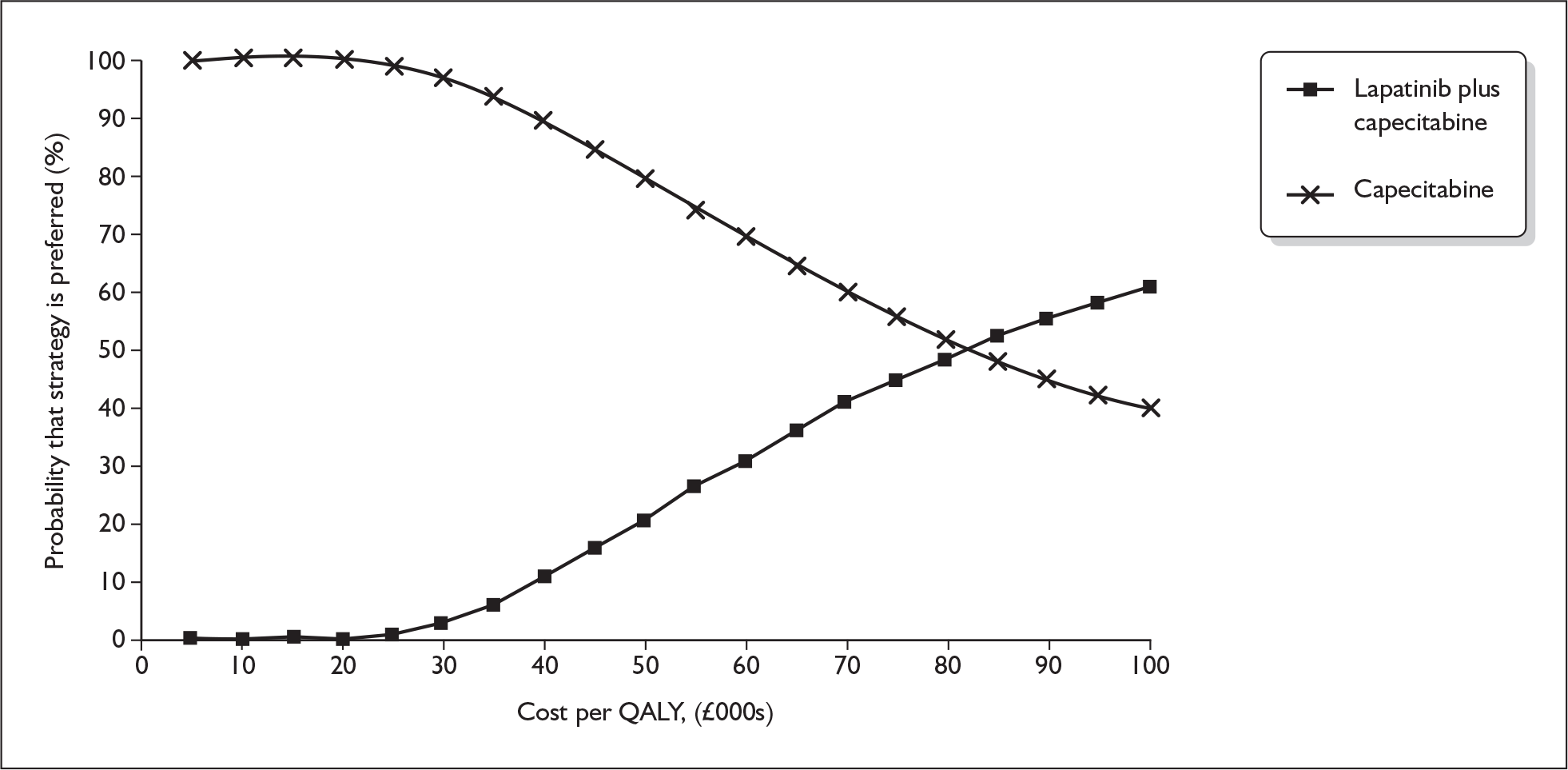
The ERG checked the literature searches and applied the NICE critical appraisal checklist to the included studies and checked the quality of the manufacturer’s submission with the Centre for Reviews and Dissemination (CRD) quality assessment criteria for a systematic review. In addition, the ERG checked and provided commentary on the manufacturer’s model using standard checklists. A one-way sensitivity analysis, scenario analysis and a probabilistic sensitivity analysis (PSA) were undertaken by the ERG. The cost-effectiveness acceptability curves for capecitabine monotherapy and lapatinib plus capecitabine from the ERG’s PSA are shown in Figure 1.
FIGURE 1.
Cost-effectiveness acceptability curves for capecitabine monotherapy and lapatinib plus capecitabine from the ERG’s probabilistic sensitivity analysis. QALY, quality-adjusted life-year.
Results
Summary of submitted clinical evidence
The main evidence in the submission came from one multicentre, multinational, open-label randomised controlled trial (RCT), EGF100151. Interim analyses from the trial were published in 2006, but the evidence in the report was from a later time point. These later data were expected to be published in June 2007,8 but had not been published when the ERG report and this summary were written.
Median time to progression was longer in the lapatinib plus capecitabine arm than in the capecitabine monotherapy arm [27.1 weeks (95% CI 17.4 to 49.4) versus 18.6 weeks (95% CI 9.1 to 36.9)], although the CIs overlapped. The hazard ratio reported in the manufacturer’s submission was 0.57 (95% CI 0.43 to 0.77; p = 0.00013).
Median overall survival was very similar between the two groups [67.7 weeks (95% CI 58.9 to 91.6) versus 66.6 weeks (95% CI 49.1 to 75.0) for lapatinib plus capecitabine versus capecitabine monotherapy respectively)]. The hazard ratio was 0.78 (95% CI 0.55 to 1.12; p = 0.177).
Median progression-free survival was statistically significantly longer in the lapatinib plus capecitabine group than in the capecitabine monotherapy group [27.1 weeks (95% CI 24.1 to 36.9) versus 17.6 weeks (95% CI 13.3 to 20.1); hazard ratio 0.55 (95% CI 0.41 to 0.74); p = 0.000033].
Summary of submitted cost-effectiveness evidence
The cost-effectiveness analysis used survival modelling methodology to estimate progression-free and overall survival for patients with HER2-positive advanced/metastatic breast cancer who had relapsed following treatment with an anthracycline, a taxane and trastuzumab. The incremental costs and consequences of treatment with lapatinib plus capecitabine were estimated relative to each of five different comparator regimes. Comparators were capecitabine monotherapy, vinorelbine monotherapy, trastuzumab monotherapy, trastuzumab plus capecitabine and trastuzumab plus vinorelbine.
The model was generally internally consistent and appropriate to metastatic breast cancer in terms of structural assumptions, although it used a different approach from previous economic evaluations of treatments for metastatic breast cancer. 9–13 The cost-effectiveness analysis generally conformed to the NICE reference case and the scope/decision problem.
Treatment effects for lapatinib plus capecitabine and capecitabine monotherapy were derived from direct clinical trial evidence. In the absence of data on the effectiveness of vinorelbine monotherapy, it was assumed to be identical to that of capecitabine monotherapy. The effectiveness of trastuzumab-containing regimes was based on pooling of data on time to disease progression, which was used in an unadjusted indirect comparison.
Utilities for preprogression survival were based on responses to the EuroQol 5 dimensions (EQ-5D) questionnaire in the EGF100151 trial. There were substantial missing data for the quality of life assessment in the trial. The utility reduction following disease progression was based on a published study,14 which reported general population valuations of disease progression and the impact of treatment-related adverse events.
The base-case incremental cost-effectiveness ratios (ICERs) for lapatinib plus capecitabine compared with capecitabine monotherapy or vinorelbine monotherapy were higher than would conventionally be considered cost-effective. When compared with trastuzumab-containing regimes, lapatinib plus capecitabine dominated (i.e. gave improved outcome at lower cost).
Sensitivity analyses reported in the manufacturer’s submission and undertaken by the ERG showed that the ICER for lapatinib plus capecitabine compared with capecitabine monotherapy or vinorelbine monotherapy was robust to variation in assumptions. In all sensitivity analyses the ICERs remained higher than would conventionally be considered cost-effective. ICERs for trastuzumab-containing regimes were highly sensitive to assumptions over the frequency of treatment (weekly or three-weekly), the distribution of weight and body surface area of patients receiving treatment, and wastage for infusional regimes.
Commentary on the robustness of submitted evidence
Strengths
The manufacturer’s submission was well written and presented a clear description of the evidence base. The manufacturer conducted a systematic review for this appraisal and searched all relevant databases using appropriate search strategies.
The identified RCT EGF100151 appeared to be of reasonable methodological quality, although enrolment was terminated before the required sample size had been met.
The economic model presented with the manufacturer’s submission used an appropriate approach for the disease area and given the available data.
Weaknesses
There was some deviation from the scope issued by NICE in terms of the timing of previous lines of therapy, and of comparator treatments.
Only one relevant RCT was identified by the manufacturer’s systematic review and the evidence base for lapatinib plus capecitabine in the manufacturer’s submission was largely based on this one trial. Early termination of enrolment meant that there was insufficient power to detect a statistically significant difference in mean overall survival.
The trastuzumab studies pooled for an indirect comparison contained a variety of treatment regimens. None of the studies contained a capecitabine monotherapy arm and so it was not possible for the manufacturer to perform an adjusted indirect comparison. 15 The manufacturer therefore used a methodologically weaker unadjusted indirect comparison. The resulting pooled mean of median time to progression values for trastuzumab may not be a reliable estimate and should therefore be treated with caution.
There was no evidence in the manufacturer’s submission of a systematic search for model parameters, in particular cost inputs and utilities.
Conclusions
Areas of uncertainty
Trastuzumab monotherapy was included as a comparator. Consultation with clinical advisors suggested that trastuzumab is used beyond progression in combination with chemotherapy agents in some primary care trusts, but not others. Clinical advisors indicated that trastuzumab monotherapy is unlikely to be continued beyond disease progression.
The manufacturer’s submission included a post hoc subgroup analysis of patients with brain metastases. It is likely that this is underpowered and so it should be treated with caution.
There was a lack of robust and reliable evidence on the effectiveness of the majority of comparators included in the economic model (vinorelbine monotherapy and all of the trastuzumab-containing regimes).
There was uncertainty over the pattern of treatment with trastuzumab if it is continued beyond disease progression, in particular whether treatment is weekly or three-weekly. This had a large effect on the cost-effectiveness of lapatinib plus capecitabine.
Key issues
The included trial was not powered to detect a statistically significant difference in overall survival between lapatinib plus capecitabine and capecitabine monotherapy.
There was a general lack of evidence on the effectiveness of comparators included in the economic model. A lack of evidence on other key parameters (such as dose adjustments) meant that there was a great deal of uncertainty and model outputs need to be interpreted in the light of that uncertainty.
Summary of NICE guidance issued as a result of the STA
At the time of writing, NICE were still considering the available evidence for this appraisal.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- Office for National Statistics . Breast Cancer: Incidence Rises While Deaths Continue to Fall 2005. www.statistics.gov.uk/cci/nugget_print.asp?ID=575 (accessed 1 December 2005).
- Office for National Statistics . Cancer Statistics Registrations: Registrations of Cancer Diagnosed in 2004, England 2007. www.statistics.gov.uk/statbase/Product.asp?vlnk=8843 (accessed 29 August 2007).
- Welsh Cancer Intelligence and Surveillance Unit . Cancer Incidence in Wales 2007. www.wales.nhs.uk/sites3/page.cfm?orgid=242%26pid=21936.
- Rachet B, Coleman M, Cooper N, Quinn M, Wood H. Breast Cancer Survival, England and Wales, 1991– 2003. Predicted Trends in Long-Term Survival from Breast Cancer in Women: Age; And Government Office Region in England and Wales 2006. www.statistics.gov.uk/statbase/ssdataset.asp?vlnk=9132%26More=Y (accessed 1 December 2005).
- Harris JR, Lippman ME, Veronesi U, Willett W. Breast cancer (2). N Engl J Med 1992;327:390-8.
- Penault-Llorca F, Vincent-Salomon A, Mathieu M, Trillet-Lenoir V, Khayat D, Marty M, et al. Incidence and implications of HER2 and hormonal receptor overexpression in newly diagnosed metastatic breast cancer (MBC). J Clin Oncol 2005;23.
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer [see comment]. N Engl J Med 2006;355:2733-43.
- Brown RE, Hutton J. Cost–utility model comparing docetaxel and paclitaxel in advanced breast cancer patients. Anticancer Drugs 1998;9:899-907.
- Brown RE, Hutton J, Burrell A. Cost effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics 2001;19:1091-102.
- Cooper NJ, Abrams KR, Sutton AJ, Turner D, Lambert PC. A Bayesian approach to Markov modelling in cost-effectiveness analyses: application to taxane use in advanced breast cancer. J R Stat Soc Sers A Stat Soc 2003;166:389-405.
- Hutton J, Brown R, Borowitz M, Abrams K, Rothman M, Shakespeare A. A new decision model for cost–utility comparisons of chemotherapy in recurrent metastatic breast cancer. Pharmacoeconomics 1996;9:8-22.
- Launois R, Reboul-Marty J, Henry B, Bonneterre J. A cost–utility analysis of second-line chemotherapy in metastatic breast cancer. Docetaxel versus paclitaxel versus vinorelbine. Pharmacoeconomics 1996;10:504-21.
- Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer 2006;95:683-90.
- Glenny A, Altman D, Song F, Sakarovitch C, Deeks J, D’Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9.