Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 07/67/01. The assessment report began editorial review in August 2008 and was accepted for publication in April 2009. See the HTA programme web site for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical and cost-effectiveness of entecavir for the treatment of chronic hepatitis B (CHB) in adults based upon a review of the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The submission’s evidence came from five randomised controlled trials (RCTs), of good methodological quality and measuring a range of clinically relevant outcomes, comparing entecavir with lamivudine. After 1 year of treatment entecavir was statistically superior to lamivudine in terms of the proportion of patients achieving hepatitis B virus (HBV) DNA suppression, alanine aminotransferase (ALT) normalisation and histological improvement, but not in terms of the proportion of patients achieving hepatitis B e antigen (HBeAg) seroconversion. The incidence of adverse or serious adverse events was similar for both treatments. The results of the manufacturer’s mixed treatment comparison (MTC) model to compare entecavir with the comparator drugs in nucleoside-naive patients were considered to be uncertain because of concerns over its conduct and reporting. For the economic evaluation the manufacturer constructed two Markov state transition models, one in HBeAg-positive and one in HBeAg-negative patients. The modelling approach was considered reasonable subject to some uncertainties and concerns over some of the structural assumptions. In HBeAg-positive patients the base-case incremental cost-effectiveness ratios (ICER) for entecavir compared with lamivudine and pegylated interferon alpha-2a were £14,329 and £8403 per quality-adjusted life-year (QALY) respectively. Entecavir was dominated by telbivudine. In HBeAg-negative patients the base-case ICERs for entecavir compared with lamivudine, pegylated interferon alpha-2a and telbivudine were £13,208, £7511 and £6907 per QALY respectively. In HBeAg-positive lamivudine-refractory patients entecavir dominated adefovir added to lamivudine. In one-way deterministic sensitivity analysis on all key input parameters for entecavir compared with lamivudine in nucleoside-naive patients, ICERs generally remained under £30,000 per QALY. In probabilistic sensitivity analysis in nucleoside-naive HBeAg-positive patients the probability of the ICER for entecavir being below £20,000 per QALY was 57%, 82% and 45% compared with lamivudine, pegylated interferon alpha-2a and telbivudine respectively. In nucleoside-naive HBeAg-negative patients the probabilities were 90%, 100% and 96% respectively. The manufacturer’s lifetime treatment scenario for HBeAg-negative patients and the ERG’s 20-year treatment scenario for HBeAg-positive patients increased the ICERs, particularly in the latter case. Amending the HBeAg-negative model so that patients with compensated cirrhosis would also receive lifetime treatment gave probabilities of entecavir being cost-effective at a willingness to pay of £20,000 and £30,000 of 4% and 40% respectively. The NICE guidance issued in August 2008 as a result of the STA states that entecavir is recommended as an option for the treatment of people with chronic HBeAg-positive or HBeAg-negative hepatitis B in whom antiviral treatment is indicated.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of entecavir for the treatment of chronic hepatitis B (CHB).
Description of the underlying health problem
Hepatitis B is an infectious disease of the liver caused by the hepatitis B virus (HBV). It is transmitted through blood-to-blood contact (e.g. through sharing of blood-contaminated needles by drug users) and sexual contact. It is also transmitted vertically from mother to infant during or soon after birth. Infected individuals develop an acute infection, which may or may not result in symptoms. The majority of those infected during adulthood make a full recovery and acquire immunity from future infection. Only about 2–10% of infected adults will develop CHB, defined as viraemia and hepatic inflammation that persists for more than 6 months after acute infection with HBV. In contrast, almost 100% of infected neonates and about 50% of infected young children will develop CHB if infected with HBV.
Active infection can be described as HBeAg positive or HBeAg negative according to whether hepatitis B ‘e’ antigen (HBeAg) is secreted. HBeAg is an indicator of viral replication, although some variant forms of the virus do not express HBeAg. The response to treatment and rates of progression differ between the two forms. People can be infected with the so-called HBeAg-negative form of the virus initially, or the viral mutation can emerge later in the course of infection in people initially infected with the HBeAg-positive form of the virus. Chronic infection with mutant strains of HBV that do not produce the ‘e’ antigen (i.e. HBeAg negative) is associated with a fluctuating course and a poor prognosis.
The Department of Health estimates that about 180,000 people in the UK have CHB. There are about 7700 new cases of CHB each year. Of these, around 300 people were infected within the UK; the remainder (mainly immigrants to the UK) were infected abroad, generally in areas of high prevalence where the virus is frequently transmitted from mother to child.
The progression to cirrhosis occurs at an annual rate of 2–5.5%, with a cumulative 5-year rate of progression of 8–20% in HBeAg-positive CHB and an annual rate of 8–10% in HBeAg-negative CHB.
Scope of the ERG report
The ERG critically evaluated the evidence submission from Bristol Myers Squibb on the use of entecavir for the treatment of CHB. Entecavir has a marketing authorisation in the UK for the treatment of chronic HBV infection in adults with compensated liver disease and evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or fibrosis.
The population considered in the scope was adults with CHB according to the licensed indication. Patient subgroups included those with HBeAg-positive and HBeAg-negative CHB and those who are treatment (nucleoside analogue) naive or refractory to lamivudine (e.g. those with persistent viraemia and/or genotypical resistance). Patients with coinfections were excluded in accordance with the scope. The intervention was entecavir alone in the treatment of CHB.
Comparators included the nucleoside analogues lamivudine and telbivudine; the nucleotide analogue adefovir dipivoxil; and the immune modifiers interferon alpha-2a and -2b and pegylated interferon alpha-2a.
Outcomes included HBeAg/HBsAg seroconversion rate, virological response (HBV DNA), histological improvement (liver inflammation and fibrosis), biochemical response (e.g. ALT levels), development of viral resistance and adverse events. Outcomes included in the scope and decision problem, but not reported in the submission include time to treatment failure, survival (unless within the context of adverse events) and health-related quality of life.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
The ERG checked the literature searches and applied the NICE critical appraisal checklist to the included studies and checked the quality of the manufacturer’s submission with the Centre for Reviews and Dissemination (CRD) quality assessment criteria for a systematic review. In addition, the ERG checked and provided commentary on the manufacturer’s model using standard checklists. A one-way sensitivity analysis, scenario analysis and a probabilistic sensitivity analysis were undertaken by the ERG.
Results
Summary of submitted clinical evidence
The manufacturer’s systematic review included five randomised controlled trials (RCTs), all of which compared entecavir with lamivudine. Three of the trials were conducted in nucleoside-naive patients (one in HBeAg-positive patients, one in HBeAg-negative patients and one in a mixed HBeAg-positive and HBeAg-negative status group). The other two were conducted in lamivudine-refractory patients (one in HBeAg-positive patients, the other in a mixed HBeAg-positive and HBeAg-negative status group). Outcome data were reported for up to 1 year of treatment, and for a subset of patients who did not achieve a complete response and who continued treatment in year 2. Cumulative proportions of all patients ever attaining a treatment response up to 2 years were also presented. Some of the patients from the RCTs have entered long-term observational extension studies, with treatment continuing for up to 5 years; however, fully published data are not yet available.
After 1 year of treatment entecavir was statistically superior to lamivudine in terms of the proportion of patients achieving HBV DNA suppression, ALT normalisation and histological improvement. There was no statistically significant difference between the treatments in the proportion of patients achieving HBeAg seroconversion (HBeAg-positive patients only, by definition). Most of the entecavir-treated patients did not have any detectable resistance-associated substitutions at 1 year of treatment. The proportions of patients with any adverse events or serious adverse events were similar for entecavir and lamivudine. The proportions of patients who withdrew during the first year because of adverse events were similar for entecavir and lamivudine except in one trial in which significantly more lamivudine patients withdrew. The number of deaths during treatment was low (< 1% in all cases).
The manufacturer also constructed a mixed treatment comparison (MTC) model to compare entecavir with the comparator drugs in nucleoside-naive patients. An MTC was not considered possible in lamivudine-refractory patients because of lack of evidence. The results of the MTC generally accord with the results of the RCTs in that, with the exception of HBeAg seroconversion, entecavir was superior to lamivudine across outcomes. The MTC suggests that entecavir is either significantly better or equivalent to the other comparators, depending on the outcome measure and the time point.
Summary of submitted cost-effectiveness evidence
The manufacturer’s economic evaluation comprised a systematic review of economic evaluations of CHB treatments and a cost–utility analysis based on a de novo economic model.
Two Markov state transition models were constructed, one in HBeAg-positive patients and one in HBeAg-negative patients. The models estimated progression to 14 health states (15 in the HBeAg-negative model) representative of progressive CHB-related liver disease (e.g. compensated and decompensated cirrhosis, hepatocellular carcinoma). The models had a lifetime horizon and a cycle length of 1 year.
In HBeAg-positive and -negative nucleoside-naive patients, the models compared entecavir with lamivudine, pegylated interferon alpha-2a and telbivudine. Treatment lasted for 2 years in HBeAg-positive patients and 5 years in HBeAg-negative patients (with the exception of pegylated interferon alpha-2a, which was given for only 1 year). In HBeAg-positive patients who were refractory to lamivudine, entecavir was compared with adefovir added to lamivudine for 2 years. Response to treatment was defined by HBeAg seroconversion and undetectable HBV DNA.
In HBeAg-positive patients the base-case incremental cost-effectiveness ratio (ICER) for entecavir compared with lamivudine was £14,329 per quality-adjusted life-year (QALY). Compared with pegylated interferon alpha-2a the ICER was £8403 per QALY. Entecavir was associated with the same number of QALYs as telbivudine, but at a slightly higher total cost and was therefore dominated. In HBeAg-negative patients the base-case ICERs for entecavir compared with lamivudine, pegylated interferon alpha-2a and telbivudine were £13,208, £7511 and £6907 per QALY respectively. In HBeAg-positive lamivudine-refractory patients entecavir dominated adefovir added to lamivudine.
One-way deterministic sensitivity analysis for entecavir compared with lamivudine on all key input parameters, and performed for nucleoside-naive patients, showed that the results were most sensitive to the baseline transition probabilities from CHB to seroconversion (spontaneous seroconversion) and active cirrhosis, the baseline transition probability from active cirrhosis to decompensated cirrhosis, baseline cirrhosis risk and treatment effects. ICERs generally remained under £30,000 per QALY.
The probabilistic sensitivity analysis in nucleoside-naive HBeAg-positive patients showed that the probability of the ICER for entecavir being below £20,000 per QALY was 57% compared with lamivudine, 82% compared with pegylated interferon alpha-2a and 45% compared with telbivudine. In nucleoside-naive HBeAg-negative patients the probabilities were 90%, 100% and 96% respectively.
The manufacturer included a lifetime treatment scenario in HBeAg-negative patients and the ERG included a scenario of up to 20 years treatment for HBeAg-positive patients. The ICERs increased as a consequence, particularly in the latter case.
The ERG updated the sensitivity analyses with utilities and drug costs varied by ± 20%. The model for HBeAg-positive patients was most sensitive to changes in response and CHB utility rates and the transition probabilities from CHB to compensated cirrhosis and CHB to seroconversion. The model for HBeAg-negative patients was most sensitive to changes in the response rates and resistance utility and the transition probabilities between compensated cirrhosis and decompensated cirrhosis and between CHB treatment and compensated cirrhosis.
The ERG conducted a probabilistic sensitivity analysis using wider uncertainty around the utilities (± 10%) and drug costs (± 20%) than presented in the manufacturer’s submission. In the HBeAg-positive model, patients with CHB were treated for 2 years with entecavir, lamivudine or telbivudine, but it was considered more appropriate for them to be treated for longer. The ERG attempted to run the HBeAg-positive model for a longer duration but the results were inconsistent with those from the deterministic scenario analyses.
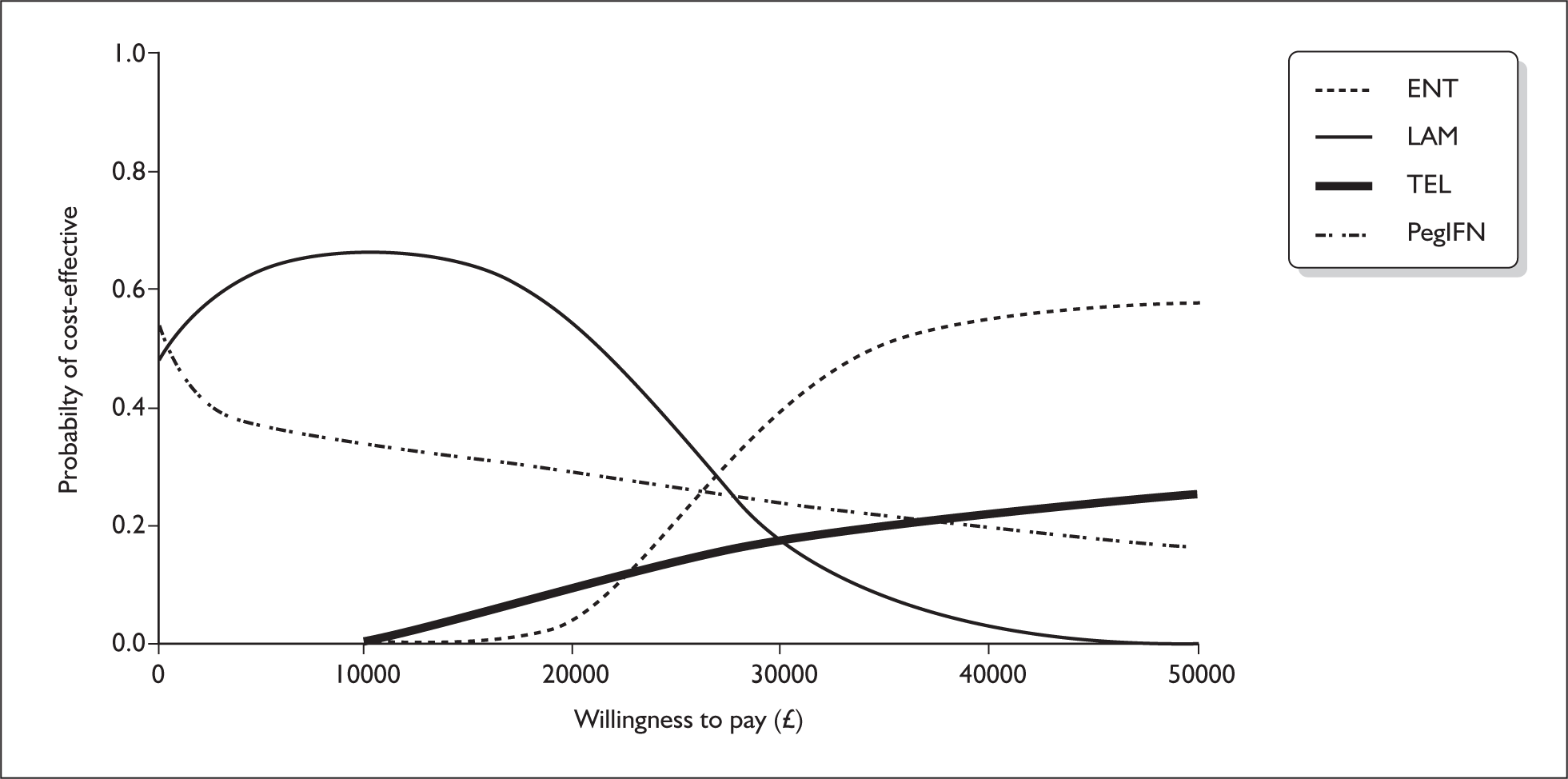
The ERG ran the HBeAg-negative model for a lifetime treatment duration. The model was amended so that patients with compensated cirrhosis would also receive treatment, lasting until they developed decompensated cirrhosis, hepatocellular carcinoma or died. As can be seen from Figure 1 the probability of entecavir being cost-effective at a willingness to pay of £20,000 and £30,000 was 4% and 40% respectively.
FIGURE 1.
Cost effectiveness acceptability curves for entecavir, lamivudine, telbivudine and pegylated interferon for the HBeAg-negative model. ENT, entecavir; LAM, lamivudine; PEG IFN, pegylated interferon alpha; TEL, telbivudine.
Commentary on the robustness of submitted evidence
Strengths
The manufacturer conducted a systematic search for clinical effectiveness and cost-effectiveness studies of entecavir. It appears unlikely that the searches missed any additional trials that would have met the inclusion criteria.
The five entecavir RCTs identified were of generally good methodological quality and measured a range of outcomes that are appropriate and clinically relevant, although health-related quality of life was not reported. Overall, the manufacturer’s submission presents an unbiased estimate of the efficacy of entecavir versus lamivudine, based on the results of the five RCTs.
Overall, the manufacturer’s economic evaluation accords with the decision problem and the NICE reference case. The approach to modelling was generally considered reasonable and the model was judged to be internally and externally consistent, subject to some uncertainties (see Conclusions).
Disease progression pathways assumed in the economic models were generally consistent with the natural history of CHB, although there were some concerns about some of the structural assumptions (see Conclusions).
Weaknesses
The MTC suffers from certain limitations in conduct and reporting, including small numbers of studies/single studies in some networks, no assessment or discussion of heterogeneity and no reporting of criteria for judging statistical significance or equivalence.
Conclusions
Areas of uncertainty
Given the concerns about the conduct and reporting of the MTC the ERG considers its results to be uncertain. This limits any conclusions that can be drawn regarding the comparative efficacy of entecavir and telbivudine and entecavir and pegylated interferon alpha-2a in nucleoside-naive patients (notwithstanding the head-to-head RCT evidence comparing entecavir with lamivudine).
There is relatively limited clinical effectiveness and cost-effectiveness evidence for entecavir in lamivudine-refractory patients. Head-to-head RCT evidence is available for entecavir versus ongoing lamivudine but only in HBeAg-positive patients. Smaller RCTs have been published comparing switching to adefovir versus adding adefovir to ongoing lamivudine, but these have not been compared in a statistical indirect comparison to entecavir. The manufacturer presented cost-effectiveness estimates only for HBeAg- positive, not HBeAg-negative, lamivudine-refractory patients.
Structural assumptions in both the HBeAg-positive and HBeAg-negative disease models precluded the patients with response from directly entering the active/compensated cirrhosis health state. The rationale for this assumption was not clear and it is not possible to estimate the impact of these structural assumptions.
Treatment of CHB in many patients will be longer than the 2 and 5 years assumed in the HBeAg-positive and HBeAg-negative disease models respectively. However, there is a paucity of published clinical effectiveness data from RCTs beyond the second year of treatment [long-term observational studies (up to 5 years) are in progress]. Increasing the treatment duration in scenario analysis resulted in higher ICERs.
No data were presented in the submission on the efficacy and safety of entecavir in combination with other licensed agents.
Contrary to the assumptions in the manufacturer’s economic evaluation, a certain proportion of CHB patients will first present with compensated cirrhosis. Moreover, it is unlikely that treatment will be terminated once patients progress to the active cirrhosis stage of disease. Changing these assumptions to reflect a more realistic scenario increased the ICER for entecavir compared with lamivudine.
Summary of NICE guidance issued as a result of the STA
The Final Appraisal Determination issued by NICE in June 2008 states that:
Entecavir, within its marketing authorisation, is recommended as an option for the treatment of people with chronic HBeAg-positive or HBeAg-negative hepatitis B in whom antiviral treatment is indicated.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.