Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 08/93/01. The assessment report began editorial review in May 2009 and was accepted for publication in May 2009. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of ustekinumab for the treatment of moderate to severe psoriasis based upon a review of the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The submission’s main evidence came from three randomised controlled trials (RCTs), of reasonable methodological quality and measuring a range of clinically relevant outcomes. Higher proportions of participants treated with ustekinumab (45 mg and 90 mg) than with placebo or etanercept achieved an improvement on the Psoriasis Area and Severity Index (PASI) of at least 75% (PASI 75) after 12 weeks. There were also statistically significant differences in favour of ustekinumab over placebo for PASI 50 and PASI 90 results, and for ustekinumab over etanercept for PASI 90 results. A weight-based subgroup dosing analysis for each trial was presented, but the methodology was poorly described and no statistical analysis to support the chosen weight threshold was presented. The manufacturer carried out a mixed treatment comparison (MTC); however, the appropriateness of some of the methodological aspects of the MTC is uncertain. The incidence of adverse events was similar between groups at 12 weeks and withdrawals due to adverse events were low and less frequent in the ustekinumab than in the placebo or etanercept groups; however, statistical comparisons were not reported. The manufacturer’s economic model of treatments for psoriasis compared ustekinumab with other biological therapies. The model used a reasonable approach; however, it is not clear whether the clinical effectiveness estimates from the subgroup analysis, used in the base-case analysis, were methodologically appropriate. The base-case incremental cost-effectiveness ratio for ustekinumab versus supportive care was £29,587 per quality-adjusted life-year (QALY). In one-way sensitivity analysis the model was most sensitive to the number of hospital days associated with supportive care, the cost estimate for intermittent etanercept 25 mg and the utility scores used. In the ERG’s scenario analysis the model was most sensitive to the price of ustekinumab 90 mg, the proportion of patients with baseline weight > 100 kg and the relative risk of intermittent versus continuous etanercept 25 mg. In the ERG’s probabilistic sensitivity analysis ustekinumab had the highest probability of being cost-effective at conventional NICE thresholds, assuming the same price for the 45-mg and 90-mg doses; however, doubling the price of ustekinumab 90 mg resulted in ustekinumab no longer dominating the comparators. In conclusion, the clinical effectiveness and cost-effectiveness of ustekinumab in relation to other drugs in this class is uncertain. Provisional NICE guidance issued as a result of the STA states that ustekinumab is recommended as a treatment option for adults with plaque psoriasis when a number of criteria are met. Final guidance is anticipated in September 2009.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of ustekinumab for the treatment of psoriasis.
Description of the underlying health problem
Psoriasis is a chronic systemic inflammatory skin disease. The most common form of psoriasis is chronic plaque psoriasis. This is characterised by exacerbations of thickened, erythematous, scaly patches of skin that can occur at any skin site but commonly appear on the elbows, knees, scalp and trunk. Estimates suggest that psoriasis affects approximately 2% of the population in the UK. 2 Psoriasis is associated with a significant negative impact on heath-related quality of life.
The severity of psoriasis is determined by several factors and can vary from mild, through to moderate and severe. A number of different criteria are available for determining the severity of psoriasis. One of the main accepted systems for classifying the severity of psoriasis is the Psoriasis Area and Severity Index (PASI). The limitations of this measure have been well documented3 but despite its shortcomings it is the measure used in most clinical trials. Body surface area (BSA) and the Dermatology Life Quality Index (DLQI) are also commonly used. Severe psoriasis is generally accepted as a PASI ≥ 10 when combined with a DLQI > 102 or, if taken alone, a PASI > 12. 4 Moderate psoriasis is generally defined as a PASI between 7 and 12. 4
Scope of the ERG report
The ERG critically evaluated the evidence submission from Janssen-Cilag on the use of ustekinumab for the treatment of moderate to severe plaque psoriasis.
Ustekinumab is a fully human monoclonal antibody. The licensed indication for ustekinumab for injection is for the treatment of adults with moderate to severe chronic plaque psoriasis who have had an inadequate response to or who have a contraindication to or who are intolerant to other systemic therapies.
The outcomes stated in the manufacturer’s definition of the decision problem were measures of severity of psoriasis, remission rate, relapse rate, adverse effects of treatment and health-related quality of life.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
The ERG checked the literature searches and applied the NICE critical appraisal checklist to the included studies and checked the quality of the manufacturer’s submission with the Centre for Reviews and Dissemination (CRD) quality assessment criteria for a systematic review. In addition, the ERG checked and provided commentary on the manufacturer’s model using standard checklists. One-way sensitivity analyses, scenario analyses and a probabilistic sensitivity analysis were undertaken by the ERG.
Results
Summary of submitted clinical evidence
The main evidence on efficacy in the submission came from three randomised controlled trials (RCTs), two comparing ustekinumab with placebo and one comparing ustekinumab with etanercept. One further RCT contributed to the evidence on adverse events.
Higher proportions of participants treated with ustekinumab (at both the 45-mg and 90-mg doses) than with placebo (two trials) or etanercept (one trial) achieved an improvement on the PASI of at least 75% (PASI 75) after 12 weeks. No statistical comparisons between the two ustekinumab doses were presented for any of the trials. There were also statistically significant differences in favour of ustekinumab (at both the 45-mg and 90-mg doses) over placebo for the proportion of participants achieving a PASI 50 and a PASI 90 (two trials), but again no statistical comparisons between the two ustekinumab doses were presented. In the trial comparing ustekinumab with etanercept, PASI 50 results appeared to be similar across the three treatment groups (45 mg ustekinumab, 90 mg ustekinumab and etanercept), but no statistical comparison of these data was presented. In contrast, both doses of ustekinumab led to statistically significantly higher proportions of participants achieving a PASI 90 than was observed in the etanercept group.
The manufacturer’s submission also presented PASI 75 data from a weight-based subgroup dosing analysis for each of the three included trials, but the methodological description of these analyses was limited and no statistical analysis to support the chosen weight threshold was presented.
The manufacturer’s submission did not present a narrative or quantitative synthesis of the data from the three included trials except as part of a mixed treatment comparison (MTC). The MTC was conducted using data from the ustekinumab trials in two ways, either all participants as randomised or subgroups of participants from the dose by weight analysis noted above. The result from the all participant analysis MTC for treatment with 45 mg ustekinumab was a mean probability of achieving a PASI 75 response to treatment of 69%, with a different result obtained from the weight-based ustekinumab analysis MTC. For the 90-mg ustekinumab dose the all participant analysis MTC resulted in a mean probability of achieving a PASI 75 response to treatment of 74%; again, a different result was obtained from the weight-based ustekinumab analysis MTC. For the PASI 75 MTC outcome the probability of response was greatest for infliximab, and the probability of response with ustekinumab was greater than those of the other comparators, except for infliximab.
For the reported secondary outcomes there were statistically significant differences in favour of ustekinumab over placebo and etanercept in the Physician’s Global Assessment score, and in favour of ustekinumab over placebo in the DLQI. The DLQI outcome was not reported for the ustekinumab versus etanercept trial. The incidence of adverse events appeared to be similar in the treatment and placebo arms at 12 weeks although this was not statistically tested. Withdrawals due to adverse events were low and appeared to occur less often in the ustekinumab groups than in either the placebo or the etanercept groups, although a statistical comparison was not reported in the manufacturer’s submission.
Summary of submitted cost-effectiveness evidence
The manufacturer’s economic evaluation included a review of the published economic literature on therapies used for psoriasis and a report of an economic evaluation undertaken for the NICE STA process, which included a cost-effectiveness model of treatments for psoriasis comparing ustekinumab with other biological therapies. The analysis estimated the number of individuals who responded to treatment at each time interval, the mean length of time that an individual would respond to treatment and the utility gains associated with this response. The model was based closely on the model reported in Woolacott and colleagues. 3
The model was generally internally consistent and appropriate to psoriasis in terms of structural assumptions. The cost-effectiveness analysis generally conformed to the NICE reference case, the scope and the decision problem.
The evidence-based treatment effectiveness was reported in terms of the probability of achieving a specified PASI response with each of the treatment alternatives and supportive care by the end of the trial period. Evidence was synthesised from a variety of trials for ustekinumab and the comparators using an MTC model. In the base-case analysis it was assumed that those under a weight of 100 kg (80% of patients in base case) received 45 mg ustekinumab whereas those over 100 kg (20% of patients) received 90 mg ustekinumab. The manufacturer’s submission proposed a patient access scheme (PAS) providing ustekinumab 90 mg at an equivalent cost to ustekinumab 45 mg and the model assumed these costs in the base case.
Patients who achieved improvements in PASI score were assigned an associated improvement in quality of life (a utility gain), with higher responses associated with larger improvements in quality of life. Two approaches were used to achieve this task. In the first the observed patient-level changes in DLQI were used as surrogate outcomes in the statistical modelling that related the PASI scores to utility gains assessed using the EuroQol 5 dimension (EQ-5D) questionnaire. The EQ-5D utility values derived from the DLQI were used in the base-case analysis. In the second approach the observed patient-level Short Form-36 (SF-36) scores were converted into Short Form-6D (SF-6D) utility values and aggregated according to the PASI response categories. The SF-6D utility estimates were used in the sensitivity analysis.
The base-case incremental cost-effectiveness ratio for ustekinumab compared with supportive care for patients with severe psoriasis was £29,587 per quality-adjusted life-year (QALY). The one-way sensitivity analysis reported in the manufacturer’s submission shows that the model was most sensitive to the number of hospital days associated with supportive care, the estimate of the cost of dosing for intermittent etanercept 25 mg and the use of SF-6D utility scores instead of EQ-5D utility scores (with SF-6D utility scores associated with a much higher cost-effectiveness ratio for ustekinumab in comparison to supportive care then the cost-effectiveness ratio estimated in the base-case analysis).
Scenario analyses were presented in the manufacturer’s submission that compared outcomes from the model when the efficacy estimates came from (1) the MTC subgroup data in which the ustekinumab dose regimen depends on the baseline weight and (2) the all patients according to their randomisation outcome. Scenario analysis conducted by the ERG showed that the model was most sensitive to the assumptions about the price of ustekinumab 90 mg, the proportion of patients with baseline weight > 100 kg and the relative risk of intermittent etanercept 25 mg in comparison to continuous etanercept 25 mg.
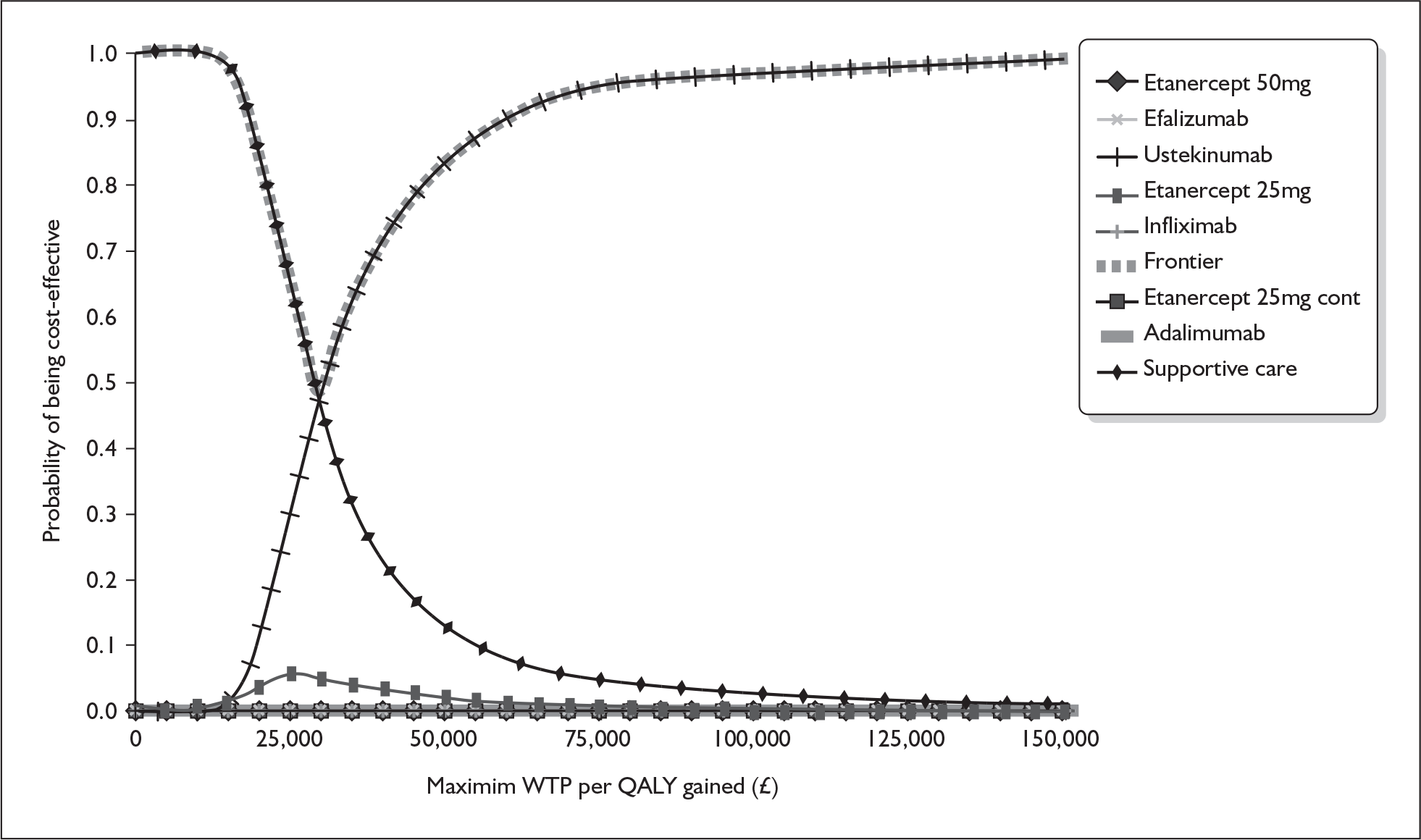
The ERG amended the manufacturer’s probabilistic sensitivity analysis to include distributions for parameters not previously included in the model. Figure 1 shows the cost-effectiveness acceptability curve assuming the same price for the 45-mg and 90-mg doses of ustekinumab. According to these results ustekinumab has the highest probability of being cost-effective at conventional NICE thresholds, whereas all other biologics have a zero probability of being cost-effective. The probability of ustekinumab being cost-effective at thresholds of £20,000 and £30,000 per QALY is 10% and 47% respectively.
FIGURE 1.
ERG analysis of the cost-effectiveness acceptability curve for biologics in the base case. QALY, quality-adjusted life-year; WTP, willingness to pay.
Commentary on the robustness of submitted evidence
Strengths
The manufacturer conducted a systematic search for clinical effectiveness and cost-effectiveness studies of ustekinumab. It appears unlikely that the searches missed any additional trials that would have met the inclusion criteria.
The three key ustekinumab trials identified and systematically reviewed were of reasonable methodological quality and measured a range of outcomes that are as appropriate and clinically relevant as possible. Overall, the manufacturer’s submission presents an unbiased estimate of treatment efficacy for ustekinumab at 12 weeks based on the results of two placebo-controlled trials and one trial comparing ustekinumab with etanercept.
The economic model presented in the manufacturer’s submission used a reasonable approach.
Weaknesses
There is a lack of information regarding the methodology used for the subgroup analysis and it was therefore difficult for the ERG to determine whether the methods used were appropriate and whether the subgroup analysis supports the weight-based categorisation presented. These clinical effectiveness estimates of the subgroup data were used in the base-case analysis of the modelled economic evaluation of ustekinumab presented in the manufacturer’s submission.
Conclusions
Areas of uncertainty
The reliability of the estimates of clinical effectiveness derived from subgroups of participants receiving differential weight-based dosing is uncertain. In addition, the impact on MTC outcomes of using a fixed-effect model rather than a random-effects model (which was used by the assessment group who developed the original MTC) is unclear.
The clinical effectiveness and cost-effectiveness of ustekinumab in relation to other drugs in the class is uncertain. A number of factors contribute to this uncertainty, including the two points above but also the assumption about the proportion of patients with baseline weight > 100 kg and the assumptions about the relative risk of intermittent etanercept 25 mg in comparison to continuous etanercept 25 mg.
It is not clear whether the estimates from the subgroup analysis, which were used in the base-case analysis in the manufacturer’s submission, were methodologically appropriate. The choice of utility estimates used for the cost-effectiveness analysis has a major impact on the estimated cost-effectiveness of ustekinumab.
Key issues
Two of the trials of ustekinumab efficacy presented by the manufacturer were placebo-controlled trials. There was also one head-to-head RCT that directly compared ustekinumab with etanercept 50 mg.
No studies were identified that directly compared ustekinumab with the other possible comparators included within the STA.
The manufacturer’s submission did not present the results of the subgroup analysis according to NICE methodological guidance and therefore the ERG was unable to determine whether the weight-based categorisation used in the cost-effectiveness analysis was justified.
Although the manufacturers carried out an MTC, the effectiveness of ustekinumab in relation to other drugs of this type remains unclear because of uncertainties about the appropriateness of some of the methodological aspects of the MTC.
All of the economic outcomes in the manufacturer’s submission were conditional on the price of ustekinumab 90 mg as indicated in the PAS. Doubling the price of ustekinumab 90 mg resulted in ustekinumab no longer dominating the comparators at a cost-effectiveness threshold of £20,000–30,000 per QALY.
Summary of NICE guidance issued as a result of the STA
The NICE guidance issued as a result of the STA states that ustekinumab is recommended as a treatment option for adults with plaque psoriasis when the following criteria are met:
-
The disease is severe, as defined by a total Psoriasis Area Severity Index (PASI) score of 10 or more and a Dermatology Life Quality Index (DLQI) score of more than 10.
-
The psoriasis has not responded to standard systemic therapies, includingciclosporin, methotrexate and PUVA (psoralen and long-wave ultraviolet radiation), or the person is intolerant of or has a contraindication to these treatments.
-
The manufacturer provides the 90 mg dose (2 × 45 mg vials) for people who weigh more than 100 kg at the same total cost as for a single 45 mg vial.
Ustekinumab treatment should be stopped in people whose psoriasis has not responded adequately by 16 weeks after starting treatment. An adequate response is defined as either:
-
a 75% reduction in the PASI score (PASI 75) from when treatment started or
-
a 50% reduction in the PASI score (PASI 50) and a 5-point reduction in the DLQI score from when treatment started.
When using the DLQI, healthcare professionals should take into account any physical, sensory or learning disabilities, or communication difficulties, that could affect the responses to the DLQI and make any adjustments they consider appropriate.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- National Institute for Health and Clinical Excellence . Etanercept and Efalizumab for the Treatment of Adults With Psoriasis 2006.
- Woolacott N, Bravo VY, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al. Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 2006;10:iii-239.
- Schmitt J, Wozel G. The Psoriasis Area and Severity Index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology 2005;210:194-9.