Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 05/40/04. The contractual start date was in November 2006. The draft report began editorial review in August 2008 and was accepted for publication in March 2009. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Biomarkers
There is intense interest in the measurement and evaluation of biomarkers in order to better target clinical care for many diseases. 1,2 The hope is that biomarkers will provide new information about the patient and his or her disease condition, which will help optimise the type, amount or timing of subsequent intervention. The development, evaluation and use of biomarkers represents a major technology in health care, with growing investment from large companies, including Dade Behring, Roche Diagnostics, Abbott Diagnostics, Acon International and Beckman Instruments. Within the last decade there have been rapid increases in the number of reports of individual biomarkers and their incorporation into prognostic risk scores. 3 This interest has in part been stimulated by the high cost and long timescales involved in the development of new therapeutic drugs and devices. The concept of ‘personalised medicine’ seeks to exploit information from biomarkers in order to maximise the probability of benefit and minimise harms for a given treatment.
A biomarker has been defined as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.’4 Thus the term ‘biomarker’ encompasses a range of measures; biomarkers that are circulating – that is, assessed from a blood sample – have been the focus of most interest in prognosis research. Compared with imaging biomarkers (e.g. computed tomography or magnetic resonance imaging), circulating biomarkers have the advantages of being relatively low cost, low burden (patients expect to have blood taken) and no risk (compared with the radiation exposure of computed tomography). Clinicians are used to interpreting and acting on a single numerical value from a blood test (e.g. low haemoglobin defines anaemia), and are increasingly using numerical values derived from scores. In the setting of coronary artery disease, more than 100 measures (beyond those widely made in routine clinical practice) have been related to the risk of subsequent death or heart attack in one or more study. 1
Prognosis, outcomes and NHS quality initiatives
Clinicians are increasingly invited to scrutinise the outcomes of their care, in an effort to improve quality. Cardiac surgery in children,5 and subsequently in adults, has been the subject of high profile inquiries into the performance of individual units and clinicians. Under the Darzi review,6 from April 2010 all health-care providers working for the NHS will be legally obliged to publish ‘quality accounts’ on safety, patients’ experience and clinical outcomes, in the same way that they publish financial accounts. Indeed, the 2007 White Paper Trust, Assurance and Safety7 states that ‘recertification will be supported by information that shows how clinically effective each doctor’s treatment of his or her patients has been’ requiring ‘analysis of the outcomes of their treatment’.
Coronary artery bypass grafting and the NCEPOD Report
Coronary artery bypass grafting (CABG) remains the standard of care for patients with three-vessel or left main coronary artery disease, because the use of CABG, as compared with percutaneous coronary intervention (PCI), resulted in lower rates of the combined end point of major adverse cardiac or cerebrovascular events. 8 The 2008 National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report on death after CABG9 found that ‘in the opinion of the advisors for 57/821 (7%) of cases there was a delay from referral to the first cardiothoracic review and in 33 of these patients outcome was adversely affected.’ One of the principal recommendations was to ‘use protocols for referrals. These protocols should be standardised nationally for patients who require coronary artery bypass graft surgery. The degree of urgency of referral should be emphasised within these protocols.’
Thus, cost-effective means of improving institutional performance are of considerable interest. There is an established culture of using risk prediction scores (the euroSCORE; European System for Cardiac Operative Risk Evaluation)10 for operative mortality after CABG, for the purposes of risk-adjusting comparisons between institutions and individual surgeons. This score does not use novel biomarkers and is not designed to assess event rates on the waiting list.
Stable angina
In women and men, coronary heart disease (CHD) is the most common cause of premature death in the UK and most Western countries,11 and is predicted to remain so at least until the next decade. Coronary disease commonly presents as angina pectoris, which is characterised by chest pain or discomfort typically on exertion and relieved by rest, in association with atherosclerotic narrowing of the coronary arteries (assessed at angiography). Between 1991 and 2003, while the incidence of heart attack declined rapidly (about 8% per year), the prevalence of angina pectoris diagnosed by a doctor based on five waves of Health Survey for England data showed no evidence of decline,12 suggesting that the relative importance of chronic symptomatic coronary disease may be increasing. The prevalence of angina in the UK is about 3–5% for women and men,13 suggesting that approximately 1.3 million people have symptoms (www.heartstats.org/datapage). The economic burden of angina is high, estimated at 1.3% of the NHS budget in the UK14 and costing $75 billion in 2000 in the USA. 15
Angina prognosis
The public health impact of angina comes from its immediate impact on health functioning and disability, as well as the elevated risk of future acute vascular events including myocardial infarction, other acute coronary syndromes, sudden death and stroke. Overall, coronary mortality is estimated at about 1–2% per annum among people with angina in primary care, and approximately 3% of annual all-cause mortality (ACM). 16 An important feature of angina is the wide variation in risk; while some patients die within the first 3 months of diagnosis, others live a normal life expectancy. Recently, efforts have been made to develop risk scores to discriminate between such very high and very low risk groups of patients. For example, the ACTION (A Coronary Disease Trial Investigating Outcome with Nifedipine GITS) trial data were re-analysed to generate a risk score for which those in the bottom 10th of the risk score distribution had a 5-year risk of death, non-fatal myocardial infarction or disabling stroke of 4%, compared with a 35% risk for patients in the top decile. 17 Efforts to improve the precision of such risk prediction scores have focused on the use of emerging blood-based markers. However, these efforts have been hampered by a lack of precise, unbiased estimates of the independent strength of effect for each biomarker.
Current use of circulating biomarkers in coronary disease
Several types of blood measurement are widely used in the management of coronary disease. These include markers of myocardial necrosis – evolving from aspartate transaminase in the 1950s, creatinine kinase (CK) in the 1960s, CK-MB in the 1970s, and troponins in the 1980s – which are used primarily as diagnostic tests with high negative and positive predictive value. This is one of the clearest examples in clinical medicine where marker measurement and urgent clinical decision-making are closely related. However, a range of other blood markers are routinely ‘taken’ among patients with coronary disease, but their use, if any, in clinical decision-making is less clear. For example, a measure of kidney function, the serum creatinine, has been estimated among people with suspected coronary disease for decades, but only in the last decade has its potential prognostic value been considered.
Proposed use of circulating biomarkers in stable angina
Circulating biomarkers have been recommended as potentially useful measures in the management of patients with stable angina. For example, the Centers for Disease Control/American Heart Association statement for health-care professionals recommended that, among patients with stable coronary disease, one biomarker, C-reactive protein (CRP), ‘may be useful as an independent prognostic marker’. 18 The costs and other characteristics of selected biomarkers are shown in Table 1. The European Society of Cardiology angina guidelines recommend (class IIb, strength of evidence B) measurement of CRP, lipoprotein a [Lp(a)], apoA-I (apolipoprotein A-I), apoB (apolipoprotein B), homocysteine, N-terminal brain natriuretic peptide (NT-proBNP), fibrinogen and interleukin 6 (IL-6). 19 Although there are no surveys on the variability between clinicians and centres in which biomarkers are measured, anecdotal evidence suggests that in NHS practice in 2008 most, if not all, of these eight biomarkers are not routinely evaluated among patients with angina. The biological mechanisms by which these markers may influence prognosis are varied, and span molecules with differing function, including markers of inflammation, lipids, hormones and vitamins. For the purposes of the evaluation of biomarkers, their biological functions are of secondary importance; the question is the extent to which they predict risk, not how.
| Biomarker | What is the molecule? | Why might it cause cardiovascular events? | Usual range in healthy populationsa | Higher levels associated with | What can be done to improve levels? | Cost of test in researchb |
|---|---|---|---|---|---|---|
| Widely performed in routine care | ||||||
| eGFR estimated from creatinine | Breakdown product of creatinine phosphate in muscle | Hypertension, dyslipidaemia, inflammation, calcification | > 60 ml/min | Increased risk: threshold? | Avoid causes of renal disease | Routine |
| Haemoglobin | Protein carrying oxygen in blood | Carries oxygen to heart muscle and all organs | > 13 g/dl | Decreased risk: threshold? | Avoid causes of anaemia | Routine |
| Fasting glucose | Sugar, a small molecule | Dyslipidaemia, hyperinsulinaemia | < 7 mmol/l | Increased risk: threshold? | Avoid causes of diabetes | Routine |
| Total cholesterol | Fat | Atherosclerosis | < 5.2 mmol/l | Increased risk | Statins lower levels and risk | Routine |
| LDL cholesterol | Lipoprotein | Atherosclerosis | < 3.3 mmol/l | Increased risk | Statins lower levels and risk | Routine |
| Novel: often not measured in routine care | ||||||
| CRP | Inflammatory protein | Degree of inflammatory activity may be related to plaque instability | < 1 mg/l | Increased risk | No specific agents but statins and aspirin may non-specifically lower CRP | £6 |
| Fibrinogen | Clotting protein | Formation of clot; inflammatory activity | 2–4 g/l | Increased risk | No specific agents but fibrates lower fibrinogen | £10 |
| Lp(a) | Lipoprotein | Reduces fibrinolysis; stimulates thrombogenesis; atherosclerosis (because of LDL cholesterol content) | 130 mg/l | Increased risk | No specific agents, but niacin and aspirin may reduce levels | £8 |
| ApoA-1 | Lipoprotein | The major protein component of HDL cholesterol | 1.4 g/l | Decreased risk | Cholesterol ester transfer protein inhibitors raise HDL, but concerns over safety | £10 |
| ApoB | Lipoprotein | The major protein component of LDL cholesterol | 1 g/l | Increased risk | RCTs of statins show risk lowering | £10 |
| Homocysteine | Amino acid | Oxidises low-density lipoprotein; pro-thrombotic | 1.6 mg/l | Increased risk | RCTs of B vitamins decrease levels, but do not improve events | £25 |
| BNP | Peptide | Released from ventricular myocardium as a response to ventricular dilatation and pressure overload in patients with heart failure and acute coronary syndrome | < 80 ng/l | Increased risk | No specific agents but ACE inhibitors beneficial in heart failure | £35 |
| IL-6 | Cytokine (signalling protein)/glycoprotein | Inflammation | < 5 ng/l | Increased risk | No specific agents | £36 |
Biomarkers and specific clinical decisions
Existing biomarker measurement recommendations, remarkably, do not specify which clinical decisions might be influenced in the light of the biomarker information. It is implicitly assumed that more information might lead to better clinical decision-making in general. Specifically, there are no professional body or government recommendations for the measurement of biomarkers in the invasive management of angina pectoris.
Revascularisation for angina
The goals of treatment for angina are to reduce mortality, lower the risk of major non-fatal events (heart attack and stroke) and to improve symptoms and quality of life. In both women and men, the diagnosis of angina is associated with markedly increased death rates from coronary disease compared with the general population: five-fold excesses among patients aged 45–55 years, and three-fold excesses among patients aged 65–75 years. 16 Coronary angiography – one of the most widely performed procedures in clinical medicine (annual numbers estimated at around 1 million in the USA, and 100,000 in the UK) – is the invasive X-ray used to diagnose coronary artery disease; without this test revascularisation cannot be considered. Among patients with angiographic luminal narrowings, coronary revascularisation is effective at relieving symptoms and improving quality of life, compared with medical management. Revascularisation with PCI with balloon angioplasty, with or without stenting, was initially proposed for patients with single- or double-vessel disease. CABG is a major surgical procedure carried out under general anaesthetic, in which the narrowings in the coronary arteries are bridged using vessels from the patient – leg veins (saphenous) or an artery from the inside of the chest wall (internal mammary). CABG is a higher cost procedure, which is associated with improvements in survival20 (unlike PCI), and tends to be associated with longer waiting times.
NHS waiting time initiatives for coronary artery bypass grafting
Coronary artery bypass grafting is carried out after an interval of days or weeks from the date the decision for surgery is made. In the 2003 report from the National Adult Cardiac Database there were about 25,000 CABG procedures carried out annually between 1997 and 2003,21 with no evidence of a decline in this number of procedures. There have been dramatic falls in the waiting time – defined as starting from the date of angiography to CABG – since the median waiting times of 214 days in 1994/5. 22 The implementation of the National Service Framework for CHD in 2000 led to declines in waiting time, and since March 2005 no NHS patient has waited longer than 3 months for CABG. 7 The most recent figures (August 2008) from the Department of Health suggest that about half the patients waiting for CABG have been waiting for between 1 and 3 months, and about half for up to 1 month. Previous policy was based on waiting from the time of angiography – which represents only one segment of patients’ waiting experience. The most recent policy focuses on the whole wait, from time of initial referral to receipt of definitive treatment, in this case CABG, with a ceiling of 18 weeks.
International comparisons in waiting times
Internationally and across different systems of health-care provision, waiting times for CABG have been the subject of targets set by politicians and by professional bodies. 23 Waiting times continue to vary within and between countries, with published comparisons between the USA, Sweden and Netherlands24 and other countries. 25 Recent Canadian guidelines state: ‘The target for bypass surgery in those with high-risk anatomy is 14 days; for all others, the target is six weeks … there is an ongoing need to continually reassess current risk stratification methods to limit adverse events in patients on waiting lists and assist clinicians in triaging patients for invasive therapies.’26
Events on waiting list
People with stable coronary disease are at increased risk of death or heart attack,16 compared with the general population. Being on a waiting list for CABG per se probably has no measurable impact on these event rates. 27 Patients awaiting CABG experience continued symptoms, and some, but not all, studies suggest longer waits are associated with more anxiety and disutility. 28 There is no evidence of any benefit in deferring surgery among patients with stable coronary disease without acute myocardial infarction history. Among patients in whom there is a history of recent acute myocardial infarction, the possible increased risk of early surgery may be balanced against the potential for improved remodelling, improved quality of life and decreased hospital stay costs. 29
Need for prioritising waiting lists
Irrespective of whether target waiting times for CABG are 14 days, 6 weeks or 3 months from the date of angiography, clinicians (and the administrative systems in which they work) are faced with deciding whether an individual patient merits listing for surgery sooner. That is, does a strategy of ordering the waiting interval, according to formal scores, improve clinical outcomes and, if so, is this strategy cost-effective? However the cost-effectiveness of any strategy may be hypothesised to be lower in countries with lower median waiting times.
Usual practice
Clinicians informally prioritise waiting lists. Without recourse to formal scores, published evidence suggests that time to invasive management of coronary disease is not random but, on average, is ordered at least according to urgent, semi-urgent and non-urgent categories. 30,31 But the rules for deciding which combination of simple clinical information would place a patient in one group or another are not explicit. Although enough information is routinely collected that would allow calculation of a formal urgency or risk score (including information on some circulating biomarkers), these formal scores are seldom derived in NHS practice.
Different formal prioritisation strategies
The dominant technology, which has been proposed as a means of improving on such implicit means of prioritising waiting lists, has been the use of ‘urgency’ or ‘acuity’ scores. These scores have been developed, and to some extent implemented,31 in Canada32 and New Zealand. 33 These urgency scores apply weightings to clinical covariates based on anatomical disease severity and symptom severity, both of which are predictors of mortality. 34 The principle is that higher risk patients should undergo an operation sooner. Biomarkers are not included in these urgency scores. Scores that predict the long-term risk of events among people with stable coronary disease have been developed,17 but are not widely used and were not developed among patients awaiting CABG.
Framework for evaluation
Conventionally, the effectiveness of different health-care technologies is rigorously evaluated in randomised controlled trials, in order to address confounding. There are no randomised trials comparing different prioritisation strategies for CABG in stable coronary disease, and these are unlikely ever to be performed. Thus, observational data with decision modelling has been demonstrated as a robust, evidence-based method of evaluation that can inform policy-making and clinical decision-making. Thus, observational studies are likely to be the main basis for estimating the effects of biomarkers in the context of prioritising patients on waiting lists. It is increasingly recognised that, to inform decisions about the effectiveness and cost-effectiveness of new technologies and health-care programmes, decision-analytic models provide a valuable framework. 35 These methods are now central to the National Institute for Health and Clinical Excellence’s (NICE’s) technology appraisal programme. 36 Decision analysis is a framework for supporting decisions rather than a source of data as provided by randomised trials and observational methods. To inform decisions, these methods facilitate the synthesis of available evidence and explicit assumptions and judgements about, for example, the duration of treatment effects. Importantly, decision analysis provides a means of quantifying the uncertainty in existing evidence and hence prioritising future research.
Scientific uncertainties addressed in this monograph
The following is not known:
-
The quality of individual studies reporting biomarkers in the prognosis of stable coronary disease, and the potential for biasing meta-analytic estimates of the effects of biomarkers.
-
The strength of effect (relative risks) and precision of these estimates [95% confidence intervals (CIs)] of five routinely assessed and eight novel biomarkers in the prediction of CHD, cardiovascular disease (CVD) and ACM events among people with stable coronary disease.
-
The most appropriate structure and input parameters for a decision-analytic model to evaluate alternative prioritisation strategies.
-
Are circulating biomarkers cost-effective at prioritising the clinical acuity (urgency) of patients awaiting CABG?
-
What is the incremental cost-effectiveness ratio (ICER) of adding one novel biomarker, one routinely assessed biomarker or both to a risk score to prioritise patients awaiting CABG?
Chapter 2 Aims, objectives and overview of decision problem
We sought to address the absence of previous meta-analyses of the prognostic value of circulating biomarkers in stable coronary disease, and the absence of previous decision models to establish the cost-effectiveness of such biomarkers. We carried out systematic reviews and meta-analyses of biomarkers (currently measured and novel) to structure and populate a decision-analytic model as a framework for addressing the policy question regarding the value of differences in long-term costs and quality-adjusted survival duration predicted between alternative prioritisation strategies.
Aim
To determine the clinical effectiveness and cost-effectiveness of a range of formal strategies based on conventional clinical information and novel circulating biomarkers (singly or in combination) for prioritising patients with stable angina awaiting CABG.
Objectives
-
To estimate the prognostic value of circulating biomarkers in predicting events among patients with stable coronary disease. The prognostic value was determined using systematic review and meta-analytic approaches in order to estimate summary relative risks of effects on prognosis for biomarkers for which measurement in angina patients in NHS practice is widespread [e.g. estimated glomerular filtration rate (eGFR)], and is recommended but not routine (e.g. highly sensitive CRP).
-
To explore sources of uncertainty in the estimates of effect of the biomarkers which may influence the clinical effectiveness and cost-effectiveness estimates, specifically to assess the precision of estimates, the quality of individual studies, publication bias and other sources of heterogeneity.
-
To develop a decision-analytic model to compare alternative approaches to prioritisation in terms of cost-effectiveness using lifetime costs and quality adjusted life-years (QALYs). The strategies of interest in relation to circulating biomarkers were:
-
– single routine biomarker (e.g. eGFR)
-
– single novel biomarker (e.g. CRP)
-
– combination of routine and novel biomarkers (e.g. eGFR + CRP),
-
-
added to a risk equation, as compared with other alternative approaches which are relevant comparators. These alternatives include both current practice and the more formal use of alternative methods or prioritisation based on conventional clinical information and urgency scores (e.g. New Zealand and Ontario algorithms).
-
To estimate the contemporary rates of events (death, non-fatal myocardial infarction and non-fatal stroke) among patients with chronic stable angina on a waiting list for CABG, and the extent to which conventional clinical information predicts these event rates, and thereby offer a means of prioritisation.
-
To estimate the costs of gathering prognostic information, the costs of alternative management interventions, the long-term outcomes (fatal, non-fatal and health-related quality of life), and the efficacy of revascularisation with respect to patients’ baseline risks and over time.
-
To populate the decision-analytic model using robust estimates of the full range of relevant inputs required to estimate mean lifetime costs, QALYs and overall cost-effectiveness of the alternative strategies in the context of a representative cohort of patients on a waiting list for CABG.
-
To undertake sensitivity analysis to examine the robustness of the results of the decision-analytic model to alternative input values and assumptions in relation to potentially important drivers of cost-effectiveness.
Overview of decision problem
This section summarises the key elements of the decision problem considered.
-
Patient population Patients with stable coronary artery disease who have been placed on the waiting list for CABG. It is assumed that all patients will undergo CABG within 3 months of being placed on the waiting list but, within this period, prioritisation between patients is possible. Alternative scenarios representing shorter target waiting times for CABG of 15 and 40 days are also considered.
-
Technology of interest Standard clinical information together with circulating biomarkers singly and in combination as a basis of prioritisation. This is represented in the form of a risk score combining information relating to clinical parameters and circulating biomarkers.
-
Comparators Alternative forms of prioritisation: no formal prioritisation (routine clinical practice), urgency scores (New Zealand and Ontario algorithms), a formal risk score based on standard clinical information (without biomarkers).
-
Basis of evaluation To establish which approach to prioritisation is the most cost-effective. Cost-effectiveness is determined based on a comparison of the expected (mean) estimates of costs and QALYs for the alternative strategies considered. The alternative strategies are then compared by estimating the differential costs and outcomes between successively more expensive (or more effective) strategies, expressed in terms of an ICER representing the incremental cost per additional QALY gained. The ICER can then be compared with external thresholds used to establish whether or not this represents potential value for money to the NHS. The threshold applied here is in the region of £20,000–30,000 per additional QALY, based on decisions made by NICE.
Overview of analytical approach
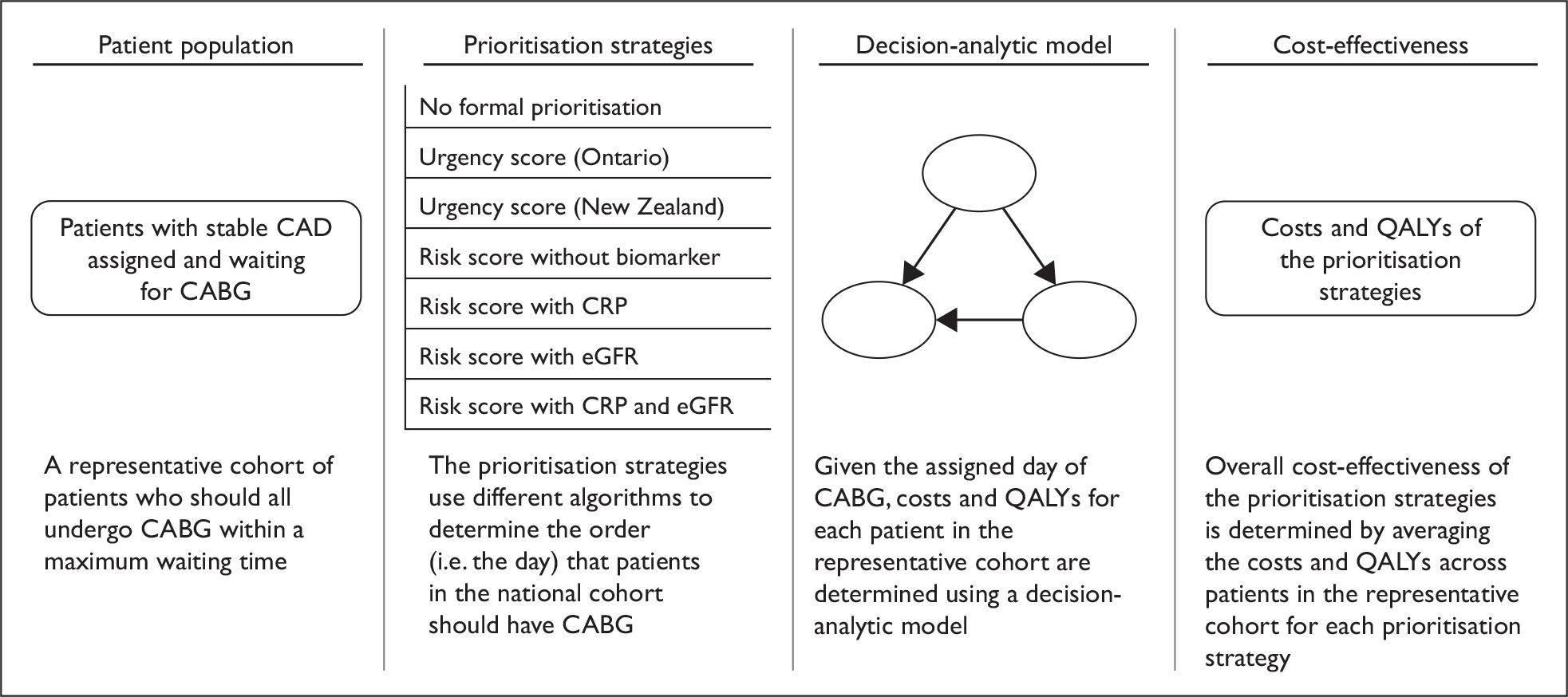
In order to determine the cost-effectiveness of alternative strategies (including the use of biomarkers) for prioritising patients on the waiting list for CABG, several analytical steps are required. These are outlined schematically in Figure 1. These steps are broken down into four inter-related elements, comprising:
-
Defining the baseline characteristics of the representative cohort This represents variation in baseline characteristics and risk factors among patients on a waiting list for CABG. In the base-case analysis, all patients in the cohort are assumed to have the procedure within 3 months, but the order in which they undergo the procedure is then determined by the alternative methods of prioritisation under investigation. A different ordering of patients may result in different costs and health outcomes, as these are determined by the risk of cardiovascular events while awaiting CABG, the risk of the procedure itself and the risk of cardiovascular events after CABG. These costs and health outcomes will vary according to the baseline characteristics and risk factors of the representative cohort. It should be noted that we modelled a waiting list based on a fixed cohort of patients. The reason we did not model a more complex, dynamic situation (in which patients enter and leave the cohort over time) is because once patients are given a date for their operation it is rarely appropriate to change this.
-
Establishing the clinical effectiveness of the alternative strategies for prioritisation Systematic review and appropriate evidence synthesis approaches are required to generate measures of clinical effectiveness (in terms of relative risks) for the alternative strategies considered, reflecting the effects on prognosis for biomarkers. Ultimately, what is of interest is the extent to which utilising such information actually changes the order in which patients are prioritised for CABG and the subsequent costs and outcomes of such an approach.
-
Developing and populating a decision-analytic model in order to evaluate the lifetime costs and health outcomes of the alternative strategies The decision-analytic model provides an explicit analytical framework to combine data on a range of effectiveness, resource use and value parameters necessary to provide guidance on optimal reimbursement decisions. The decision-analytic model is used to structure the decision problem to identify the relevant parameters, and the amount and quality of available evidence can then be reflected in the inputs assigned to these parameters. The model is structured around the patient population of interest, characterising the potential events that may occur within both the short-term (e.g. events on a waiting list and procedural risk) and the longer term (e.g. subsequent prognosis after CABG) in terms of overall quality-adjusted life expectancy and costs and also reflecting the specific effect of the alternative strategies on these elements. This process inevitably involves methods for synthesising evidence for a range of parameters beyond simply the effectiveness of an intervention, including generating baseline event rates (e.g. to represent current practice), quality of life estimates and costs.
-
Estimation of the mean lifetime cost and health outcomes of the different prioritisation strategies in order to determine overall cost-effectiveness Propagation of the full range of input parameters into the decision-analytic model enables the expected lifetime costs and health outcomes for patients with different characteristics and risk factors to be established, conditional on the assigned day of CABG. For example, a particular patient in the representative cohort could be assigned CABG at day 10 with one prioritisation strategy and day 20 with an alternative prioritisation strategy. In this case it is necessary to estimate the costs and health outcomes associated with undergoing CABG at day 10 and day 20 for this particular patient. Overall cost-effectiveness of the prioritisation strategies can then determined by averaging the costs and health outcomes across patients in the representative cohort for each prioritisation strategy and comparing the subsequent ICER estimates with external thresholds representing value for money to the NHS. 36
FIGURE 1.
Overview of the analytical approach. CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; QALY, quality-adjusted life-year.
Major data inputs
Several data sources and analytical approaches are required in order to carry out the analyses outlined above. The two major elements required to populate the decision model are: (1) systematic review and synthesis of existing evidence related to the clinical effectiveness of biomarkers themselves and (2) other evidence necessary to populate the decision-analytic model and the range of alternative strategies including the risk of cardiovascular events (on the waiting list, procedural and after CABG) as well as the effect on costs and health-related quality of life. The methods and results of the systematic review and synthesis of existing evidence related to clinical effectiveness of biomarkers are described in detail in Chapters 3 and 4. The results from this review provide one of the major inputs required for the overall decision model. The methods, range of data sources and results of the decision model are reported in Chapters 5 and 6. The key data source for a number of separate elements of the decision model, including the characteristics and risk factors of the representative cohort, was the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). 37 This registry includes consecutive patients without exclusion criteria in all 30 centres in Sweden, and covers a total of 201,000 angiographies. Furthermore, the decision on further management after angiography is also available in SCAAR, making it possible to identify a representative cohort of patients with a decision to perform CABG as well as longer term prognosis. The SCAAR registry, alongside other published sources used to populate the decision model, is presented in detail in Chapter 5.
Chapter 3 Methods of systematic review of circulating biomarkers
We carried out systematic reviews and meta-analyses of literature-based estimates of the effects of circulating biomarkers on the prognosis of stable coronary disease. We carried out the systematic reviews and meta-analyses in accordance with standards for reporting set out by the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. 38 The MOOSE standards are focused on healthy population studies; there are no standards for the design and reporting of meta-analyses for prognostic studies (i.e. among patients with established disease).
Inclusion criteria
An eligible publication had to meet five criteria:
-
prospective study design
-
patients with stable coronary disease
-
one (or more) eligible biomarker
-
eligible outcome
-
relative risk and 95% CI reported.
Appendix 3 shows the proforma used to assess articles for eligibility.
Eligible study designs
We included any prospective study (observational cohort studies, prospective nested case–control studies, randomised controlled trials) that assessed biomarkers at one time point, and patients were followed up for outcomes at a later time point. Cross-sectional studies were not eligible.
Eligible patient populations
We included patient populations that had stable coronary disease, defined as clinically diagnosed chronic stable angina pectoris or coronary artery disease defined by luminal narrowing at coronary angiography, or a history of previous acute coronary syndrome at least 2 weeks prior to biomarker measurement. We excluded studies in which the biomarker was measured during an admission with an acute coronary syndrome. We also excluded studies in which the biomarker was measured after a coronary procedure had been performed but before discharge from hospital. The ideal population (most relevant to the decision model) was defined as patients who had undergone an angiogram and were waiting for CABG.
Eligible biomarkers
We defined five routinely measured circulating biomarkers as eligible for systematic review: serum creatinine, eGFR, fasting glucose, haemoglobin, total cholesterol, and low density lipoprotein (LDL) cholesterol.
We defined eight novel circulating biomarkers as eligible for systematic review: CRP, brain natriuretic peptide (BNP), IL-6, fibrinogen, ApoA-I, ApoB, Lp(a) and homocysteine. These were chosen because of policy relevance and inclusion in the 2006 guidelines for the management of angina published by the European Society of Cardiology. 19 They represent a range of costs (see Table 1).
Eligible outcomes
Eligible outcomes were defined as CHD events (including coronary mortality, sudden cardiac death, acute non-fatal myocardial infarction, unplanned emergency admissions with unstable angina, acute coronary revascularisation, or the development of severe, worsening or rest pain), CVD events (where acute coronary events were reported in combination with other non-coronary events including heart failure, stroke and peripheral arterial disease) and ACM. For the decision model, the most important outcomes were those for which risk might be reduced by CABG – coronary death and acute coronary events. We therefore defined a hierarchy where biomarker effects on specifically coronary causes and death were given the highest preference, and non-fatal events and ACM were used in their absence. As many papers reported two or more different end points, we present end points according to the hierarchy:
| Death | Non-fatal events | Abbreviation used in tables |
|---|---|---|
| 1. Coronary | None | } CHD |
| 2. Coronary | + Coronary | |
| 3. Cardiovascular | None | } CVD |
| 4. Cardiovascular | + Cardiovascular | |
| 5. All cause | + Cardiovascular | } ACM |
| 6. All cause | None | |
| 7. None | + Cardiovascular | Morbidity |
Exclusion criteria
We did not exclude any studies based on methodological standards, sample size, duration of follow-up, publication year or language of publication.
Search strategies
We searched MEDLINE (PubMed) and EMBASE databases between 1966 and 30 November 2008 using a strategy developed with an expert librarian (who has a Postgraduate Diploma in Information and Library Science and 10 years experience as a medical librarian) based on terms for coronary disease (Cochrane Library of Systematic Reviews and Protocols), prognostic studies39 and biomarker. The final search combined these three searches with the connector word ‘AND’. Details of the search terms are shown in Appendix 2.
Reference management
Titles and abstracts were downloaded to reference manager (version 10.0) into separate databases for the MEDLINE and EMBASE results, which were then merged and checked for duplicates. Unique study identifiers were assigned to each article based on the reference manager reference identifier (Ref ID). The duplicated references were then eliminated.
Reviewing titles and abstracts for eligibility
Three reviewers (NF, JD and KM) reviewed article titles and abstracts for eligibility and obtained full text articles where eligibility was definite or unclear. Multiple publications from one study data set were eligible where they reported results from two or more different biomarkers.
Data extraction
Two reviewers (NF and JD) independently abstracted data from eligible articles using a pre-defined coding protocol (see Appendix 4). Non-English articles were translated. Individual item disagreement between the two reviewers was resolved by consensus or, rarely, adjudication by a third reviewer (HH). The main details extracted were the year of publication; the number of patients at baseline that were included in the analysis, their mean age and the percentage of women; the baseline coronary morbidity (proportion with symptoms of angina, angiographic disease or previous myocardial infarction); mean [standard deviation (SD)] levels of biomarker at baseline [or median (interquartile range)]; type of assay; follow-up duration; the number and type (CHD, CVD, or ACM) of outcome events; the crude annual risk of these events calculated; whether or not the multivariate adjustment models included terms for age, sex, smoking status, obesity (nearly always body mass index), diabetes and one or more lipid variable [from total cholesterol, LDL cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides]; and the comparisons (grouped or continuous).
Selection of relative risks
Within one study, two or more relative risks were commonly reported, based on different combinations of outcomes (e.g. reporting effects for coronary death and all-cause death separately) or different combinations of adjustment factors. We identified and extracted the relative risk and 95% CIs for the most specific cardiac end point combination according to the seven-level hierarchy defined above. Where two or more relative risks were reported for the most specific end point combination, we selected the most highly adjusted relative risk (i.e. with the largest number of adjustment variables). Where men and women were reported separately, these were taken as two separate study populations. Where separate effects were reported in active treatment and placebo arms of randomised trials, we selected the effect from the placebo arm.
Data extraction for study quality
There are no widely used, validated methods of scoring the overall quality of individual reports from studies of prognostic biomarkers. We based our definitions of quality items on those in the guidelines for reporting tumour biomarkers (REMARK)40 and those discussed in Hayden. 41 Such approaches assess the quality of the reporting of the study, rather than the quality of the study per se. However, the quality of reporting is related to the quality of the research in some, but not all, studies.
We summarised the quality of individual studies according to whether there was a clear statement or description or evidence of each item. A clear description is indicated in the table with a ‘•’ in the study row, and absence of adjustment for a factor or lack of a clear description was indicated with a ‘○’. The actual content of the descriptions among those with a ‘•’ are not summarised in this monograph (details are available from the authors on request), because they bear no simple relation with quality. For example, whether a data set is drawn from a randomised trial or a registry does not have a necessary relation to study quality.
The following quality items were systematically extracted from each paper included in the meta-analysis:
-
Pre-specified research question: a bibliographic reference stating that studying the relation of the biomarker on coronary events was part of the rationale for collecting the patient sample at the outset of the study.
Population
-
Setting: the clinic or hospital circumstances in which the cohort was recruited (e.g. primary care, at the time of angiography, or combinations thereof).
-
Duration of CHD: the average length of time since the first symptomatic clinical presentation of CHD (years).
-
Flow diagram of patient inclusion: illustrating the reasons for exclusion and numbers of patients.
-
N eligible patients: the total number of patients who were invited to participate in the study, i.e. before exclusion criteria or missing data on covariates reduces the number of patients available for analysis.
-
Exclusion criteria: relating to other conditions which, for example, might influence inflammatory markers.
-
Consent: written informed patient consent.
Biomarker measurement
-
Fasting status: the fasting status of the patients when blood tests were drawn.
-
Storage: fresh sample or if the blood is stored, at what the temperature (fresh/temperature/no statement/not applicable).
-
Manufacturer: the name of the company that makes the assay for the biomarker.
-
Assay: the type of assay used to measure the biomarker (e.g. turbidimetric, ELISA, nephelometry).
Outcomes
-
Masking: if clinical details were masked during the appraisal of outcome events.
-
Validation: outcome events were cross-checked by independent sources.
-
Primary outcome: a single disease end point, or a single combination of end points, for the analysis. The report must use the word ‘primary’.
-
Pre-specified primary outcome: the primary outcome was pre-specified in the study protocol.
Confounders
-
Confounder measurement: were the following potential confounders measured: age, sex, smoking status, LDLs and triglycerides, body mass index and diabetes? However, these confounders do not necessarily have to be included in the multivariate analysis to be included.
-
Rationale for including adjustment variable: states the method by which factors were selected to be in the multivariate adjustment models (response categories: a priori, stepwise procedures, univariate p-values).
Analytical decisions
-
Missing values: how were patients without valid information on the biomarker or confounders were dealt with in the analysis (response categories: complete case analysis, multiple imputation)?
-
Cut-point rationale: how were the cut-points for the biomarker determined for the estimation of relative risks (response categories: a priori, quantiles)?
-
Power: statistical sample size or power calculation (yes/not stated).
-
Multiple publications: other publications using the same study population report other relative risks for the same biomarker.
Statistical analysis
Different categorical and continuous scales of effect
Studies reported relative risk on continuous and categorical scales, and within each a wide variety of approaches was used (Table 2). Thus, for continuous scales some studies reported per SD of untransformed data, while others reported per unit, or per unit on the log scale. These relative risks are not directly comparable. For categorical scales, some studies reported for two, three, four or five equally sized groups, and for others the group size was not clear. Clearly, assuming a linear relation between biomarker and events, the effects for the top versus the bottom fifth of the distribution will, for example, be more extreme than for the portion above and below the median.
| RR comparison reported in the paper | Number of studies | Method | Example | ||
|---|---|---|---|---|---|
| Study reference | Reported RR (95% CI) | Scaled RR Top vs bottom (95% CI) | |||
| Continuous | |||||
| Per SD | 7 | Scaling factor is 2.18 (the difference in means between T3 and T1) | Blankenberg 200642 | 1.10 (0.99 to 1.23) | 1.23 (0.98 to 1.57) |
| Per tertile | 4 | Scaling factor is 2.00 | Anderson 200043 | 1.42 (1.12 to 1.80) | 2.02 (1.25 to 3.24) |
| Per quartile | 3 | Scaling factor is 2.32 | Chan 200344 | 1.32 (1.12 to 1.56) | 2.04 (1.34 to 3.14) |
| Per standard unit (e.g. mg/l for CRP) | 8 | First convert to SD using study-specific SD | Inaguma 200745 | 1.05 (1.02 to 1.08) | 1.40 (1.14 to 1.69) |
| Per log 10 (biomarker) | 14 | First convert to SD, and then to natural log scale | Aguilar 200646 | 1.70 (1.39 to 2.08) | 1.76 (1.42 to 2.19) |
| Equal size groups: top vs bottom | |||||
| Two groups | 10 | Scaling factor is 1.37 (2.18/1.59; difference in means is between top 50% vs bottom 50% | Huang 200847 | 1.66 (1.04 to 2.64) | 2.00 (1.06 to 3.78) |
| Three groups (tertiles) | 9 | Reported (no scaling required) | Fathi 200548 | 1.85 (1.13 to 3.03) | 1.85 (1.13 to 3.03) |
| Four groups (quartiles) | 11 | Scaling factor 0.858 (2.18/2.54; difference in means between T4 and T1) | Chew 2001a49 | 3.68 (1.51 to 8.99) | 3.06 (1.42 to 6.58) |
| Unequal size groups: top vs bottom | |||||
| Two groups | 35 | Calculated the means in the groups, assuming the underlying distribution to be normal and use these differences as the scaling factor | Crea 200250 | 2.51 (1.30 to 4.8) | 3.27 (1.40 to 7.53) |
| Three groups | 2 | Calculated the means in three groups, assuming the underlying distribution to be normal and use these differences as the scaling factor | Morrow 200651 | 3.90 (1.8 to 5.6) | 2.93 (1.59 to 3.91) |
Conversion of literature estimates of relative risk to a common scale (tertiles)
We therefore needed to convert continuous and categorical relative risks on to a common scale of effect. We chose thirds of the biomarker distribution (tertiles) because this has been the approach in the large-scale meta-analyses of these biomarkers in aetiological (healthy population) studies,52 and because the number of events in, for example, fifths of the distribution would become very small given the average size of study.
Converting continuous scales
For a normally distributed variable, the difference between the means of the top (T3) and bottom (T1) third of the distribution is 2.18 SD units. Thus to estimate the relative risk for T3 versus T1, relative risks reported per SD of log CRP were raised to the power of a scaling factor of 2.18, as previously reported. 53 This method assumes that CRP is log normally distributed and that the association with disease risk is log linear; both these assumptions have empirical support in healthy population studies of CRP. 54 Similarly, for relative risks expressed as either top 50% versus bottom 50% or by quartiles, scaling factors equal to 1.37 (2.18/1.59) and 0.86 (2.18/2.54) respectively, were used. 1.59 is the difference, in SD units, between the means of the top and bottom (T1) halves of the distribution; 2.54 is the difference, in SD units, between the top (T4) and bottom quarters of the distribution. Where two groups were of unequal size, we calculated the means in the groups, assuming the underlying distribution to be normal and used these differences as the scaling factor.
However, for a log normally distributed variable, the difference in means between T3 and T1 depends upon both the mean and SD of the untransformed distribution. The implication of this is that for relative risks reported per unit or SD of CRP (untransformed), we needed to obtain study-specific scaling factors, based on the means and SD of the untransformed data. Simulating one million observations from a log normal distribution with specified mean and SD allowed us to compute the difference in means between T3 and T1 for any combination of mean and SD. In addition, as there is an exact relationship between the means and SD of the normal and log normal distributions, the T3 versus T1 differences could be computed whenever means and SD were specified for either CRP or log CRP. In the absence of reported estimates of the mean and SD, these were estimated for the log CRP distribution from the interquartile range (IQl, IQu) as: mean = (IQl + IQu)/2, SD = (IQu – IQl)/1.349, where 1.349 is the distance in SD units between the 25th and 75th centile of the normal distribution.
Some studies reported the mean and SD within subgroups, rather than of the overall sample. In this situation where we knew the sufficient statistics (N, mean, SD) within groups, we calculated the mean and SD of the overall sample from these values.
Relative risks reported per mg/l were converted first to an SD change, using the study-specific SD, and thence to tertiles as above. For those studies that provided regression-based estimates per tertile, i.e. assuming a log linear relationship for CRP, the relative risk for the comparison of the highest third with the lowest third of the CRP distribution was obtained by using a scaling factor of 2. The middle tertile estimate was obtained by taking the square root of the T3 versus T1 estimate from the summary estimate of the meta-analysis.
Conversion of literature estimates of relative risk to a common cut-point
For eGFR, fasting glucose and haemoglobin, a single value is used to define chronic kidney disease, diabetes and anaemia respectively. However, reports used different single cut-points, and two or more cut-points. Therefore, in order to obtain an estimate for the presence versus absence of these diseases, we illustrate the method with chronic kidney disease, as defined by an eGFR lower than 60, versus its absence (eGFR ≥ 60). eGFR differs further from the situation with CRP, because eGFR is normally distributed and the relation between eGFR and CHD risk is not linear, as demonstrated in a previous meta-analysis in healthy population studies. 55
Where the relative risk was reported on a continuous scale, we used the mean and SD to estimate the proportion of the sample lying above and below the cut-point of 60 ml/min. Then we calculated the difference in means, expressed on the standard normal deviate scale, between these two groups and multiplied this by the reported eGFR SD to obtain the scaling factor.
Where the relative risk was reported in categories with the reference group as greater than or equal to 60 ml/min, we combined the two (or three) risk groups by weighting the relative risk estimates by the inverse of their variance. When the reference group was greater than or equal to 90 or to 75, we used that as reference group.
Meta-analysis
For each study, the relative risk estimate and its corresponding standard error were transformed to their natural logarithms to stabilise the variance and normalise the distributions. Summary relative risk estimates and their 95% CIs were estimated from a random effects model that considers both within- and between-study variation. 56 Statistical heterogeneity among studies was evaluated using the Cochran’s Q and I2 statistics. 57 Small study bias, consistent with publication bias was assessed with funnel plot (i.e. a plot of study results against precision), by Begg’s adjusted rank correlation test, and by Egger’s regression asymmetry test. 58 To explore other potential sources of study heterogeneity, such as age, sex, annual risk rate, CRP levels, sample size, degree of covariates adjustment, duration of follow-up, study start year, events number and type of adjustment, we employed a meta-regression model that included these variables as covariates in categorical forms. We also performed meta-regression for continuous covariates, but due to the fact that these were often aggregated individual-level covariates, the results were interpreted with caution because of possible ecological bias. All analyses were conducted using stata, version 8.0 (StataCorp, College Station, TX, USA). All statistical tests were two-sided.
Chapter 4 Results of systematic review of circulating biomarkers
Results of searches
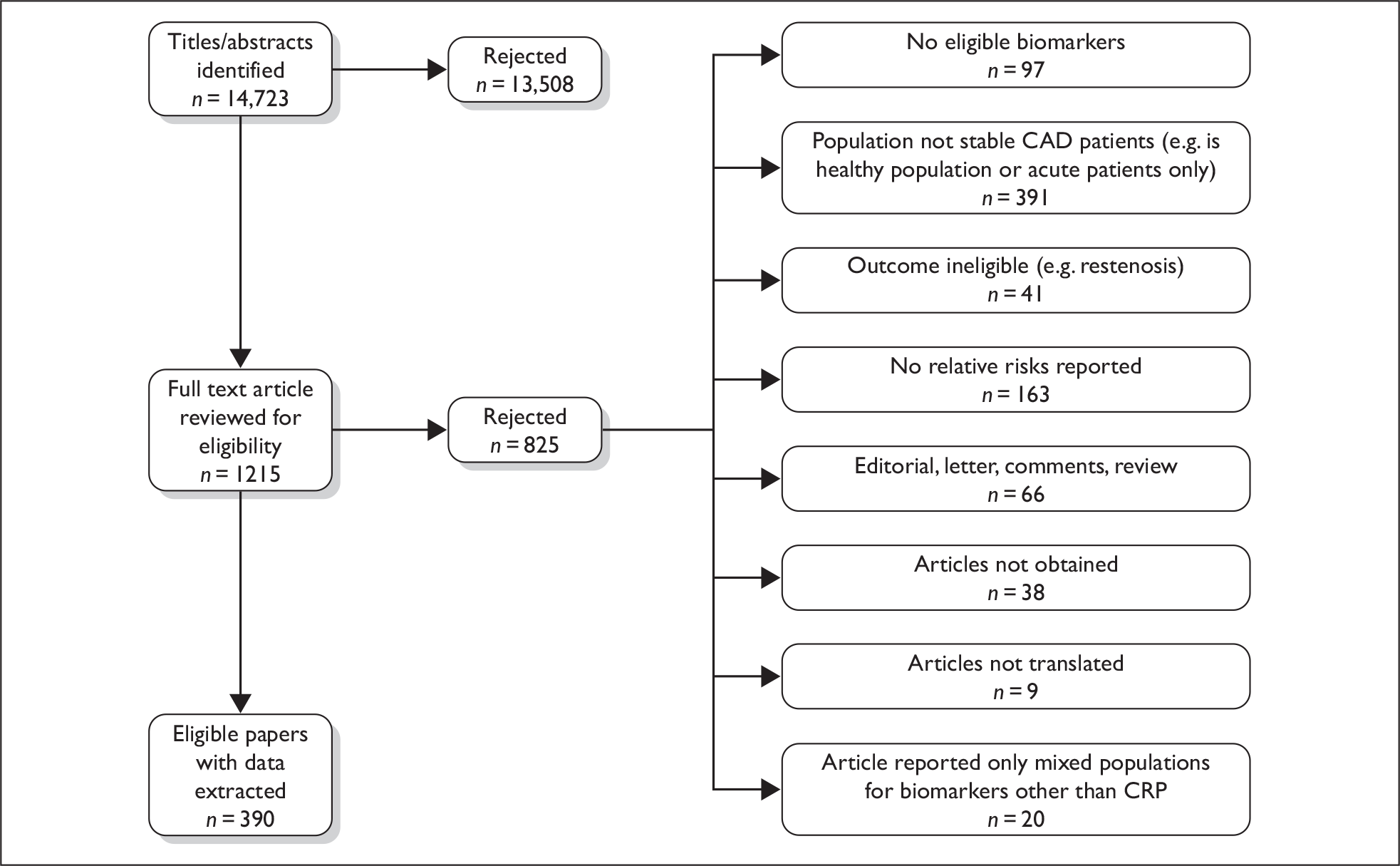
We reviewed a total of 14,723 unique titles and abstracts for eligibility and included 390 reports (see Figure 2 and Appendix 2). We translated studies from French, German, Italian, Portuguese, Spanish, Russian, Farsi, Japanese, Mandarin and Czech. A list of full text articles reviewed and rejected is given in the appendices.
FIGURE 2.
Flow chart of search results and selection of eligible studies.
We identified no previous meta-analyses, or systematic reviews, of any circulating biomarker in the prognosis of stable coronary disease. We identified no eligible studies in the ‘ideal’ population – among patients on the waiting list for CABG – in relation to novel circulating biomarkers.
Quality of individual studies
We included 390 reports of biomarker effects in our review. The number of events per study, and the demographic and clinical characteristics of patients were similar across biomarkers. The quality of individual study reports was similar across different biomarkers, and is summarised for the CRP studies in Table 3. Given that one data set commonly reported relative risks for the effects of more than one biomarker, and given that the quality of studies did not vary substantially between biomarkers, we show the 109 studies that reported results for CRP, the biomarker with the most eligible studies. No (0%) studies reported a clear statement of pre-specified research question.
| Author/publication year (study name) | Pre-specified research question | Population | Biomarker measurement | Confounders | Outcomes | Analytical decisions | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multiple publications | Setting | Duration of CHD | Number of eligible patients | Flow diagram for patient inclusion | Exclusion criteria | Informed consent | Fasting status | Storage temperature | Manufacturer | Assay | Confounders measured | Rationale for adjustments | Masking of clinical details | Validation | A primary outcome | Pre-specification of primary outcome | Missing values | Cut-point rationale | Power | ||
| Aguilar 200646 (WIZARD) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ● | ○ | ● | ● | ○ | ○ | ● | ● | ● | ○ | ○ | ● | ○ |
| Anderson 200043 (–) | ○ | ○ | ● | ○ | ○ | ○ | ○ | ● | ● | ● | ● | ● | ○ | ● | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Arroyo-Espliguero 200484 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Arroyo-Espliguero 200894 (–) | ○ | ○ | ● | ● | ● | ○ | ● | ● | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Artieda 200790 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ○ | ○ | ● | ○ |
| Aytekin 2003130 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | ● | ○ | ● | ○ | ○ | ○ | ○ |
| Biancari 2003135 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ● | ● | ○ |
| Bickel 200273 (Atherogene) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ |
| Blankenberg 2001a82 (Atherogene) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Blankenberg 2001b113 (Atherogene) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Blankenberg 200268 (Atherogene) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Blankenberg 200367 (Atherogene) | ○ | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Blankenberg 200642 (HOPE) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ● | ○ |
| Bogaty 200177 (–) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Bogaty 200888 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ○ | ○ | ● | ● | ● | ● | ● | ○ | ● | ● | ○ | ○ | ● | ● |
| Brilakis 200575 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Chan 200344 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Chew 2001b225 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Chirinos 2005a78 (–) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Chirinos 2005b76 (–) | ○ | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ● | ● | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ○ |
| Crea 200250 (4S Study) | ○ | ● | ● | ○ | ● | ○ | ● | ○ | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ● | ○ |
| Dai 200786 (–) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ |
| Dai 200887 (–) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ |
| de Winter 2002226 (–) | ○ | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| de Winter 2003227 (–) | ○ | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| de Winter 2004228 (–) | ○ | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ |
| Dibra 2003111 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ○ | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ○ |
| Espinola-Klein 200798 (–) | ○ | ○ | ● | ○ | ● | ○ | ○ | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Fathi 200548 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ○ | ○ | ○ | ○ | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Falcone 200661 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Fang 2007101 (–) | ○ | ○ | ● | ○ | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Gach 2007121 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Garcia-Moll 200081 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Gaspardone 1998136 (–) | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Grander 2004148 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ○ | ○ | ● | ○ |
| Haim 200789 (BIP) | ○ | ○ | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ● | ○ | ○ | ● | ○ |
| Harb 2002152 (THROMBO) | ○ | ○ | ● | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ |
| Haverkate 199764 (ECAT) | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ○ | ○ | ● | ○ |
| Hoffmeister 2005153(–) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Horne 200066 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Huang 2006138 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Huang 200847 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Ijsselmuiden 2003115 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ | ○ | ○ | ● |
| Ikonomidis 2005124 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● |
| Inaguma 200745 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Inoue 200799 (–) | ○ | ○ | ● | ● | ● | ○ | ● | ● | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Inoue 200896 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Janoskuti 2005118 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Kangasniemi 2006108 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Karha 2006125 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Khor 2004102 (Intermountain Heart Collaborative Study) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Kinjo 2003126 (OACIS) | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | ● | ○ |
| Kinjo 2005133 (OACIS) | ○ | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ● | ○ |
| Kip 2005151 (WISE) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ | ○ | ● | ● |
| Krzewina 2003157 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ● | ○ |
| Kubica 2005146 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Kwaijtaal 2005127 (EXIT) | ○ | ● | ● | ○ | ● | ● | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Lee 2006149 (–) | ○ | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Leu 2004122 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Liu 2003110 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | ● | ○ | ● | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Low 2004120 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Lu 2003134 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Lubos 200662 (Atherogene) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Marcinkowski 2007100 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Milazzo 1999139 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ |
| Minoretti 200683 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ○ | ● | ● | ○ | ● | ● | ○ | ○ | ● | ○ |
| Morrow 200651 (AtoZ) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ○ | ● | ● | ○ | ● | ○ | ○ | ● | ○ |
| Muhlestein 2000109 (Intermoutain Heart Collaborative Study) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Muhlestein 2004141 (Intermountain Heart Collaborative Study) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Ndrepepa 2006a71 (–) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ● | ○ |
| Ndrepepa 2006b71 (–) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Niccoli 2007112 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Otsuka 200272 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Palazzuoli 2006140 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ○ | ● | ● | ○ | ● | ○ | ○ | ● | ○ |
| Palmerini 2005132 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Palmerini 200792 (Bologna Registry) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ● | ○ | ● | ● | ● | ● | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Papa 200895 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ● | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Park 2007147 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ● | ● | ○ | ● | ● |
| Patti 200249 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Qi 2003a79 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Qi 2003b80 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Rahel 2003144 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Retterstol 2002154 (–) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Rothenbacher 2006150 (–) | ○ | ○ | ● | ○ | ● | ○ | ○ | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Sabatine 2007142 (PEACE) | ○ | ○ | ● | ○ | ● | ○ | ○ | ○ | ○ | ● | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ● | ○ |
| Saleh 2005143 (–) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Saleh 200674 (–) | ○ | ● | ● | ○ | ○ | ○ | ○ | ● | ● | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Sargento 2002131 (–) | ○ | ○ | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Schaan 200769 (–) | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Schnabel 2005a107 (Atherogene) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ● | ● | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Schnabel 2005a107 (Atherogene) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Schnabel 2005b155 (Atherogene) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ○ | ● | ● | ○ |
| Shlipak 200897 (Heart and Soul) | ○ | ● | ● | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Sinning 200659 (Atherogene) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Soeki 199965 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ○ | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Spiedl 2002145 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Susen 200585 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Thompson 199560 (–) | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ | ● | ○ | ○ | ● | ○ |
| van der Harst 2006137 (QUO VADIS) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ● | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Veselka 2004229 (–) | ○ | ○ | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ | ○ | ○ |
| West 200891 (LIPID) | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ● | ○ | ● | ○ | ○ | ● | ○ |
| Wolk 200463 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ○ | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Wu 2005117 (–) | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ | ○ | ● | ○ |
| Zairis 2002129 (GENERATION) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ○ | ● | ○ | ○ | ● | ● | ○ | ● | ○ |
| Zairis 2004128 (GENERATION) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Zebrack 2002103 (–) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Zebrack 2002b105 (–) | ○ | ● | ● | ○ | ● | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Zebrack 2003104 (–) | ○ | ● | ● | ○ | ○ | ○ | ● | ● | ○ | ○ | ● | ● | ○ | ● | ○ | ○ | ● | ○ | ○ | ● | ○ |
| Zhu 200170 (–) | ○ | ○ | ● | ○ | ○ | ○ | ○ | ● | ○ | ● | ● | ● | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ● | ○ |
| Number of ‘●’ from total of 109 reports | 0 | 43 | 109 | 5 | 55 | 3 | 93 | 84 | 42 | 69 | 95 | 92 | 39 | 56 | 17 | 20 | 38 | 3 | 4 | 91a | 5 |
| (%) | 0 | 39 | 100 | 5 | 50 | 3 | 85 | 77 | 39 | 63 | 87 | 84 | 36 | 50 | 16 | 18 | 35 | 3 | 4 | 83a | 5 |
Quality of reports of study populations
The clinical setting of the studies was clearly described, but there were concerns about how the final study population available for analysis was derived. Thus, only five (5%) studies reported the duration of history of coronary disease and 3% reported a flow diagram of patient inclusion. However, 85% reported the number of eligible patients as distinct from the number of patients included in the final analysis.
Quality of reports of biomarker measurement
Minimal standards for reporting biomarker measurement methods were not universally applied. Thus, 39% of studies reported a clear description of the fasting status of the patient and 63% gave details of sample storage.
Quality of reports of confounders
There was not always clarity about which confounders were measured and, among those that were, what rationale directed their inclusion in multivariate models. Thus, 50% of studies reported any rationale guiding the inclusion of confounders in multivariate models.
Quality of reports of outcomes (end points)
Given that nearly all studies used a combination of disease processes as an outcome event (e.g. combining different types of fatal and non-fatal events, with different combinations of non-fatal events) it was of particular concern that 35% of studies defined a primary outcome, but in only 3% of studies was this primary outcome pre-specified. Likewise, validation of outcome events, or masking of the event ascertainment or classification to clinical details, was seldom reported.
For these reasons, few studies reported on precisely the end point combination that we implement in the decision model (ACM + non-fatal myocardial infarction + non-fatal hospitalised stroke).
Quality of reporting of analytical decisions
No study reported pre-specified hypotheses or analytical plans. Four per cent of studies commented on missing values of biomarkers. Most studies that reported relative risks for cut-points of biomarkers gave a rationale for the choice of cut-point. However, few studies gave a rationale as to prior decision of whether to analyse the biomarker as a continuous or a categorical exposure. Five per cent of studies reported a sample size or power calculation.
C-reactive protein systematic review
We identified a total of 109 reports among patients with stable coronary disease where CRP was related to the risk of subsequent events (Table 4). Current angina symptoms were present in median 70.5 (range 9–100%) among the 45 studies providing data. Previous myocardial infarction was present in median 39 (range 8–100%) of patients in the 65 studies providing data. The mean age of patients across studies was a median of 62 years, and only one study had a mean age above 70 years. The median proportion of women in studies was 24.1%, and only three studies reported separate estimates among women. Eight thousand three hundred and sixty-nine outcome events were reported, with a median number of events per study of 51 (range 4–825).
| Author/publication year (study name) | Number of patients | Age (years) | % Women | Baseline coronary morbidity (%) | CRP mean (mg/l) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk | Adjustments | Comparison group | RR | 95% CI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | ||||||||||||||||
| Blankenberg 200643 (HOPE) | 3199 | 65.4 | 23.2 | – | 100 | – | 2.7 | N | 4.5 | CVD | 501 | 3.48 | ● | ● | ○ | ● | ○ | ● | Continuous (per SD) | 1.1 | 0.99 to 1.23 | |||
| Sinning 200659 (Atherogene) | 1806 | 61.7 | 21.3 | 100 | 100 | 47.5 | 2.8 | LPE | 3.5 | CVD | 131 | 2.07 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.13 | 1.0 to 1.26 | |||
| Thompson 199560 (ECAT) | 2806 | 53.8 | 14.8 | 37.0 | 75.8 | 44.3 | 1.6 | N | 2 | CHD | 106 | 1.89 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.24 | 1.00 to 1.55 | |||
| Falcone 200661 (–) | 1014 | 64.6 | 27.2 | 82.9 | 100 | 44.9 | 0.6 | – | 2.7 | CVD | 105 | 3.84 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per SD) | 1.50 | 1.21 to 1.69 | |||
| Lubos 200662 (Atherogene) | 1945 | 61.2 | 21.1 | – | 100 | 37.5 | 3.2 | LPE | 2.6 | CVD | 75 | 1.48 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.80 | 154 to 2.2 | |||
| Wolk 200463 (–) | 382 | 62.0 | 30.0 | – | 100 | 20 | 1.1 | LPE | 4 | CVD | 44 | 2.88 | Crude | Continuous (per SD) | 1.39 | 0.84 to 1.05 | ||||||||
| Haverkate 199764 (ECAT) | 743 | 56 | 14.1 | 100 | – | 42 | 1.7 | MEIA | 2 | CHD | 29 | 1.95 | ● | ○ | ○ | ○ | ○ | ○ | Continuous (per SD) | 1.5 | 1.01 to 2.18 | |||
| Soeki 199965 (–) | 106 | 62.3 | 25.5 | – | – | 35.8 | 2.4 | LXAG | 4.17 | CHD | 11 | 2.49 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per SD) | 1.55 | 1.08 to 2.23 | |||
| Anderson 200043 (–) | 1002 | 64.9 | 22.7 | – | 100 | – | 23.4 | FP | 3.0 | ACM | 118 | 3.93 | ● | ○ | ○ | ● | ○ | ● | Continuous (per tertile) | 1.42 | 0.80 to 1.12 | |||
| Horne 200066 (–) | 172 | 63 | 29 | 45 | 100 | 23 | 22 | FP | 3 | ACM | – | – | – | – | – | – | – | – | Continuous (per tertile) | 0.97 | – | |||
| Chan 200344 (–) | 937 | 69.5 | 31.1 | – | 100 | 28.4 | 4.0 | – | 1 | ACM | 149 | 15.9 | ○ | ● | ● | ○ | ○ | ● | Continuous (per quartile) | 1.32 | 1.12 to 1.56 | |||
| Blankenberg 200367 (Atherogene) | 771 | 61.7 | 23.3 | 70.5 | 100 | 48.7 | – | LPE | 4.1 | CVD | 97 | 3.07 | ● | ● | ● | ● | ● | ● | Continuous (per quartile) | 0.8 | 0.6 to 1.1 | |||
| Blankenberg 200268 (Atherogene) | 1229 | 61.8 | 25.5 | 65.8 | 100 | 47.0 | 4.0 | LPE | 3.9 | CVD | 95 | 1.88 | ● | ○ | ○ | ● | ○ | ● | Continuous (per quartile) | 0.94 | 0.70 to 1.25 | |||
| Inaguma 200745 (–) | 790 | 67.7 | 27.1 | – | – | 64.1 | 3.2 | – | 2.31 | CVD | 110 | 6.03 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per mg/dl) | 1.05 | 1.02 to 1.08 | |||
| Schaan 200769 (–) | 123 | 58.2 | 48.9 | – | 37.8 | 100 | 5.8 | N | 2.27 | CHD | – | – | ● | ○ | ○ | ○ | ○ | ○ | Continuous (per mg/dl) | 1.059 | 1.00 to 1.12 | |||
| Zhu 200170 (–) | 890 | 65.3 | 22.9 | – | 100 | – | 23.4 | FP | 3 | ACM | 167 | 6.25 | ● | ● | ● | ● | ○ | ● | Continuous (per mg/dl) | 1.08 | 1.03 to 1.14 | |||
| Ndrepepa 200671 (–) | 507 | 69.1 | 33.9 | – | 100 | 45.6 | 7.8 | T | 4 | ACM | 103 | 5.08 | ● | ○ | ○ | ○ | ○ | ○ | Continuous (per 5-mg/l increase) | 1.04 | 1.00 to 1.08 | |||
| Otsuka 200272 (–) | 363 | 65.3 | 29.5 | – | 100 | 27.5 | 3.9 | LXAG | 0.54 | CVD | 89 | 45.4 | – | – | – | – | – | – | Continuous (per mg/dl) | 1.14 | 0.82 to 1.58 | |||
| Bickel 200273 (Atherogene) | 791 | 61.9 | 24.7 | – | 100 | 49.1 | 14 | LPE | 2.9 | CHD | 88 | 3.84 | ● | ● | ● | ● | ● | ● | Continuous (per mg/dl) | 1.8 | 1.14 to 2.83 | |||
| Saleh 200574 (–) | 891 | 65 | 32 | 58 | 100 | 39 | 2.3 | N | 2.6 | ACM | 75 | 3.23 | ● | ○ | ○ | ○ | ○ | ● | Continuous (per mg/l) | 1.04 | 0.99 to 1.09 | |||
| Brilakis 200575(–) | 466 | 60.1 | 38 | – | 75.8 | 15 | 2.9 | T | 4 | ACM | 61 | 3.27 | ● | ● | ● | ● | ○ | ○ | Continuous (per 1.32mg/dl) | 1.34 | 1.05 to 1.72 | |||
| Chirinos 2005a76 (–) | 160 | 62.1 | 0 | – | 81.9 | – | – | AUTO | 4.4 | ACM | 37 | 5.26 | Crude | Continuous (per mg/dl) | 1.15 | 1.00 to 1.33 | ||||||||
| Bogaty 200177 (–) | 100 | 57.6 | 10 | – | 100 | 50 | 4.4 | N | 4 | Morbidity | 23 | 5.75 | ○ | ○ | ○ | ● | ○ | ○ | Continuous (per mg/dl) | 5.4 | 1.9 to 17.2 | |||
| Chirinos 200578 (–) | 122 | 63.9 | 0 | – | 100 | 39 | 0.7 | N | 3 | ACM | – | – | ● | ● | ○ | ○ | ○ | ○ | Continuous (per mg/dl) | 1.26 | 1.02 to 1.55 | |||
| Qi 2003a79 (–) | 134 | 64.1 | 19.4 | 48.5 | 100 | 34.4 | 3.3 | EIA | 1 | CHD | 32 | 23.9 | – | – | – | – | – | – | Continuous (per unit increase) | 2.03 | 1.13 to 2.05 | |||
| Qi 2003b80 (–) | 121 | 64.1 | 21.5 | 43.8 | 100 | 30.6 | 3.4 | EIA | 0.08 | ACM | 16 | 165.3 | – | – | – | – | – | – | Continuous (per unit increase) | 1.06 | 0.80 to 1.18 | |||
| Aguilar 200646 (WIZARD) | 3319 | 62 | 18.3 | – | – | 100 | 2.6 | N | 3.08 | ACM | 825 | 8.07 | ● | ● | ● | ● | ● | ● | Continuous (log10 mg/l) | 1.52 | 1.30 to 1.76 | |||
| Garcia-Moll 200081 (–) | 911 | 63.1 | 35.9 | 100 | 23.9 | 31.7 | 4.01 | – | 1.6 | CVD | 89 | 6.11 | ● | ● | ○ | ○ | ○ | ○ | Continuous (log10) | 1.68 | 1.04 to 2.72 | |||
| Blankenberg 200182 (Atherogene) | 1240 | 61.9 | 24.7 | – | 100 | 49.1 | 5.0 | LPE | 2.7 | CHD | 88 | 2.63 | – | – | – | – | – | – | Continuous (log mg/dl) | 1.34 | 1.09 to 1.9 | |||
| Minoretti 200683 (–) | 799 | 64.9 | 25.6 | 100 | 100 | 46.3 | 0.5 | – | 2.7 | CVD | 69 | 3.20 | ● | ● | ● | ● | ● | ● | Continuous (log transformed mg/dl) | 1.42 | 1.12 to 1.81 | |||
| Arroyo-Espliguero 200484 (–) | 700 | 63 | 25.0 | 100 | – | 39 | 2.3 | – | 1.0 | CHD | 68 | 9.71 | ● | ● | ○ | ○ | ○ | ● | Continuous (log mg/l) | 1.9 | 1.1 to 3.5 | |||
| Susen 200585 (–) | 488 | 61.0 | 22.0 | 69.0 | 100 | 19.0 | 2.6 | N | 1.24 | CHD | 44 | 7.27 | ● | ● | ● | ● | ● | ○ | Continuous (per unit by log transformation) | 2.05 | 1.21 to 3.47 | |||
| Dai 200786 (–) | 568 | 62.5 | 33.8 | 100 | 100 | – | 2.0 | N | 1.85 | CVD | 61 | 5.8 | ● | ● | ● | ● | ○ | ○ | Continuous (log transformed) | 1.51 | 1.28 to 1.77 | |||
| Dai 200887 (–) | 345 | 64.6 | 26.7 | – | 100 | 15 | 4.2 | N | 3 | CHD | 56 | 5.41 | ● | ● | ● | ● | ○ | ● | Continuous (–) | 1.99 | 1.11 to 3.56 | |||
| Bogaty 200888 (–) | 1210 | 62 | 25 | 37 | – | 28 | 4.97 | N | 1 | ACM | 142 | 11.74 | ● | ○ | ○ | ○ | ○ | ● | Continuous (log transformed) | 1.12 | 0.93 to 1.34 | |||
| Haim 200789 (BIP) | 2979 | 60 | 8.6 | 57.3 | – | 78 | 4.96 | CL | 6.2 | CHD | – | – | ● | ● | ● | ● | ● | ● | Continuous (per natural log unit) | 1.28 | 1.04 to 1.59 | |||
| Artieda 200790 (–) | 132 | 55.2 | 0 | 100 | 72.7 | 0 | 0.5 | N | 3.98 | CVD | 33 | 6.28 | ● | ○ | ● | ● | ○ | ● | Continuous (log mg/dl) | 2.17 | 0.87 to 5.43 | |||
| West 200891 (LIPID) | 500 | 63 | 15 | – | 100 | – | – | LPE | 2.5 | CVD | 250 | 20 | ● | ○ | ○ | ○ | ○ | ● | – | – | 0.9 | 0.40 to 1.50 | ||
| Palmerini 200792 (Bologna Registry) | 108 | 69.1 | 23 | 28.7 | 100 | 32.5 | – | N | 0.75 | ACM | 11 | 13.58 | ● | ○ | ○ | ○ | ○ | ● | < 1.22 | ≥ 1.22 | 5.87 | 1.67 to 20.62 | ||
| Niccoli 200793 (–) | 40 | 61 | 15 | 35 | – | – | 2.7 | N | 0.5 | ACM | 14 | 70 | ○ | ○ | ○ | ○ | ○ | ○ | ≤ 3 | > 3 | 10.9 | 1.0 to 119 | ||
| Arroyo-Espliguero 200894 (–) | 790 | 63.1 | 29.5 | 100 | 100 | 31 | – | LPE | 1 | CHD | 71 | 8.99 | ● | ○ | ○ | ○ | ○ | ○ | < Median (median not specified) | > Median (median not specified) | 1.9 | 1.1 to 3.2 | ||
| Papa 200895 (–) | 422 | 64 | 19.9 | – | 100 | – | – | FP | 3 | CHD | 13 | 1.03 | – | – | – | – | – | – | ≤ 0.8 | > 0.8 | 10.15 | 1.26 to 81.79 | ||
| Inoue 200896 (–) | 158 | 63 | 28 | 53 | 82.9 | 29.7 | 0.6 | N | 7 | CVD | 56 | 5.06 | – | – | – | – | – | – | – | – | 1.45 | 0.88 to 2.77 | ||
| Shlipak 200897 (Heart and Soul) | 979 | 67 | 18 | – | 100 | 53.7 | – | T | 3.7 | CHD | 142 | 3.92 | ● | ● | ● | ○ | ● | ● | ≤ 4.93 | > 4.93 | 1.82 | 1.24 to 2.67 | ||
| Espinola-Klein 200798 (–) | 694 | 62.4 | 27.4 | – | 92.1 | 43.3 | 4.8 | LPE | 6.5 | CVD | 75 | 1.66 | ● | ● | ● | ● | ● | ● | < 4.8 | ≥ 4.8 | 1.2 | 0.8 to 2.2 | ||
| Huang 200847 (–) | 205 | 68 | 11.5 | – | 62.9 | 0 | 2.3 | N | 4 | Morbidity | 84 | 10.24 | ● | ○ | ● | ○ | ● | ● | < 1.1 | ≥ 1.1 | 1.66 | 1.04 to 2.64 | ||
| Inoue 200799 (–) | 149 | 63 | 29 | 53.7 | 83.2 | 29.5 | 2.1 | LPE | 7 | CVD | 58 | 5.56 | ○ | ○ | ● | ○ | ○ | ○ | – | – | 2.28 | 0.92 to 6.81 | ||
| Marcinkowski 2007100 (–) | 100 | 58.3 | 22.4 | – | – | 0 | 4.4 | N | 1.48 | CHD | 15 | 10.14 | Crude | ≤ 1.83 | > 1.83 | 14.39 | 1.94 to 106.7 | |||||||
| Fang 2007101 (–) | 258 | 58.7 | 36.8 | 26.7 | 100 | – | 1.9 | – | 1.01 | CHD | 102 | 39.14 | – | – | – | – | – | – | < 2.64 | > 2.64 | 2 | 0.9 to 6.7 | ||
| Khor 2004102 (Intermountain Heart Study) | 2254 | 66 | 23.8 | – | 100 | 20 | 13.4 | FP | 3.1 | ACM | 570 | 8.16 | ● | ○ | ● | ● | ● | ● | < 1.2 | ≥ 1.2 | 1.6 | 1.3 to 1.9 | ||
| Zebrack 2002a103 (Intermountain) | 1848 | 65.5 | 22.6 | – | 100 | – | 12.2 | FP | 2.1 | CHD | 235 | 6.01 | ● | ● | ● | ● | ○ | ● | < 1.0 | ≥ 1.0 | 1.9 | 1.3 to 2.8 | ||
| Zebrack 2003104 (Intermountain) | 1484 | 64.0 | 33.0 | 72.0 | 76.0 | – | 13 | FP | 3 | ACM | 205 | 4.6 | – | – | – | – | – | – | < 1.0 | ≥ 1.0 | 1.9 | 1.2 to 2.8 | ||
| Crea 200250 (4S) | 258 | 61.9 | 10.9 | – | – | – | – | N | 5 | ACM | 129 | 10.00 | ○ | ○ | ● | ● | ○ | ○ | < 4.1 | ≥ 4.1 | 2.51 | 1.3 to 4.8 | ||
| Zebrack 2002b105 (Intermountain) | 285 | 66.3 | 23.0 | 100 | 100 | – | 13.1 | FP | 2.8 | ACM | 117 | 14.7 | ● | ● | ● | ● | ○ | ● | < 1.15 | ≥ 1.15 | 2.3 | 1.1 to 4.6 | ||
| Schnabel 2005106 (Atherogene) | 1872 | 61 | 20.9 | – | 100 | – | 3.14 | LPE | 2.6 | CVD | 114 | 2.34 | ● | ● | ● | ● | ● | ● | ≤ 5.44 | > 5.44 | 1.51 | 0.98 to 2.2 | ||
| Schnabel 2005a107 (Atherogene) | 639 | 61.7 | 27.8 | 79.3 | 86.8 | – | 3.8 | LPE | 7.1 | CVD | 112 | 2.47 | ● | ● | ● | ○ | ○ | ● | < 10.1 | ≥ 10.1 | 1.27 | 0.80 to 2.01 | ||
| Kangasniemi 2006108 (–) | 843 | 60.6 | 21.2 | – | – | 9.5 | – | T | 12 | CHD | 119 | 1.18 | ● | ○ | ○ | ○ | ○ | ● | < 1.0 | ≥ 1.0 | 1.65 | 0.95 to 2.88 | ||
| Muhlestein 2000109 (Intermountain) | 985 | 65.8 | 23.0 | 44.4 | 100 | 23.0 | 23.0 | FP | 2.7 | ACM | 110 | 4.14 | ● | ● | ● | ● | ○ | ● | < 1.2 | < 1.2 | 2.40 | 1.4 to 4.1 | ||
| Liu 2003110 (–) | 247 | 62 | 27 | – | 100 | 20 | 37.0 | – | 1.6 | CHD | 87 | 22.01 | Crude | ≤ 7.5 | > 7.5 | 1.8 | 1.2 to 3.8 | |||||||
| Dibra 2003111 (–) | 1152 | 66.1 | 26.6 | 100 | 100 | 31.5 | – | – | 1 | ACM | 86 | 7.47 | ● | ○ | ● | ● | ○ | ● | ≤ 5 | > 5 | 1.8 | 1.1 to 2.9 | ||
| Ndrepepa 2006b112 (–) | 989 | 66.3 | 21.0 | – | 100 | 39.9 | 1.2 | T | 3.6 | ACM | 85 | 2.39 | ● | ● | ● | ● | ● | ● | < 1.2 | ≥ 1.2 | 2.3 | 1.40 to 3.78 | ||
| Blankenberg 2001a113 (Atherogene) | 983 | 62.2 | 26.4 | 78.4 | 100 | 51.8 | – | LPE | 3.1 | CHD | 70 | 2.30 | ● | ● | ● | ● | ● | ● | < 9.6 | ≥ 9.6 | 3.10 | 1.2 to 8.1 | ||
| de Winter 2002114 (–) | 501 | 61.8 | 26.1 | 14.2 | 100 | – | 3.48 | N | 1.16 | CHD | 69 | 11.87 | – | – | – | – | – | – | ≤ 3 | > 3 | 2.54 | 1.44 to 4.47 | ||
| Ijsselmuiden 2003115 (–) | 400 | 60.7 | 19 | 67.5 | 100 | 38.0 | – | – | 0.5 | CVD | 64 | 32.0 | – | – | – | – | – | – | ≤ 10.0 | > 10.0 | 1.94 | 1.0 to 3.7 | ||
| de Winter 2003116 (–) | 1458 | 61.5 | 27.6 | – | 100 | – | 6.6 | N | 1.16 | CHD | 55 | 3.25 | ● | ● | ● | ○ | ○ | ● | ≤ 3 | > 3 | 3.60 | 1.8 to 7.2 | ||
| Wu 2005117 (–) | 150 | 67.8 | 9.3 | 100 | 100 | 19.7 | – | – | 1.5 | CHD | 48 | 21.3 | ○ | ○ | ○ | ○ | ○ | ○ | < 1.0 | ≥ 1.0 | 1.91 | 0.98 to 3.74 | ||
| Janoskuti 2005118 (–) | 387 | 59 | 26.9 | – | – | 48.1 | 3.89 | N | 5.06 | ACM | 41 | 2.09 | ● | ● | ● | ○ | ● | ○ | < 6.24 | ≥ 6.24 | 5.21 | 1.76 to 5.43 | ||
| Veselka 2005119 (–) | 300 | 63.5 | 31.0 | 100 | 99.5 | 57.0 | – | N | 0.5 | ACM | 40 | 26.7 | ● | ○ | ● | ● | ○ | ● | ≤ 3.0 | > 3.0 | 1.00 | 0.51 to 1.95 | ||
| Low 2004120 (–) | 347 | 58 | 34.6 | 69.2 | – | 16.5 | – | IPA | 2.5 | CVD | 37 | 4.27 | ○ | ○ | ○ | ○ | ○ | ○ | < 1.0 | ≥ 1.0 | 3.47 | 1.76 to 6.84 | ||
| Gach 2007121 (–) | 89 | 60.2 | 24.7 | 100 | 100 | 25.8 | 3.4 | N | 6.6 | CHD | 36 | 6.13 | – | – | – | – | – | – | < 3.0 | ≥ 3.0 | 1.05 | 0.09 to 1.02 | ||
| Leu 2004122 (–) | 75 | 68.1 | 12 | 100 | 100 | 25.3 | 1.02 | ELISA | 3.33 | CVD | 33 | 13.2 | ● | ● | ● | ● | ○ | ○ | ≤ 1.0 | > 1.0 | 2.78 | 1.21 to 6.41 | ||
| de Winter 2004123 (–) | 1172 | 62.0 | 33.0 | 78.0 | 100 | 46.5 | 6.4 | N | 1.16 | ACM | 32 | 2.35 | – | – | – | – | – | – | ≤ 3 | > 3 | 2.02 | 0.91 to 4.48 | ||
| Ikonomidis 2005124 (–) | 100 | 54 | 16 | 100 | 100 | 52 | – | N | 6 | CHD | 31 | 5.17 | ● | ○ | ● | ○ | ○ | ○ | < 2.4 | ≥ 2.5 | 6.24 | 1.74 to 22.42 | ||
| Karha 2006125 (–) | 652 | 65.4 | 32.4 | – | – | – | 3.3 | T | 1 | ACM | 31 | 4.75 | ● | ● | ○ | ○ | ○ | ● | < 3.3 | ≥ 3.3 | 6.5 | 2.2 to 19.3 | ||
| Kinjo 2003126 (OACIS) | 1307 | 63.4 | 22.0 | – | – | 12.5 | 1.3 | N | 1.4 | CVD | 29 | 1.58 | ● | ● | ● | ● | ● | ● | < 0.38 | ≥ 0.38 | 9.58 | 1.17 to 78.4 | ||
| Kwaijtaal 2005127 (EXIT) | 213 | 53.6 | 21.8 | 9.9 | 100 | 26.8 | 3.7 | ELISA | 2 | CHD | 25 | 5.34 | ● | ● | ● | ○ | ● | ● | ≤ 3.0 | > 3.0 | 2.50 | 1.1 to 5.7 | ||
| Zairis 2004128 (Generation) | 474 | 59.3 | 18.1 | 22.6 | 100 | 8.6 | 7.0 | N | 3 | CHD | 25 | 1.76 | – | – | – | – | – | – | < 0.68 | ≥ 0.68 | 4.0 | 1.8 to 8.9 | ||
| Zairis 2002a129 (Generation) | 483 | 59.3 | 18.0 | 22.2 | 100 | 8.7 | 5.8 | T | 3 | CHD | 20 | 1.38 | – | – | – | – | – | – | < 0.68 | ≥ 0.68 | 3.16 | 1.25 to 7.98 | ||
| Aytekin 2003130 (–) | 116 | 56.5 | 22.4 | 34.5 | 100 | 14.7 | – | – | 0.5 | CHD | 19 | 32.8 | Crude | ≤ 0.5 | > 0.5 | – | – | |||||||
| Sargento 2002131(–) | 64 | 58 | 7.8 | – | – | 100 | – | – | 1.67 | ACM | 19 | 17.8 | – | – | – | – | – | – | < 97% | ≥ 97% | 9 | – | ||
| Palmerini 2005132 (–) | 83 | 72.0 | 40.0 | 25.0 | 100 | 33.0 | – | N | 0.75 | ACM | 18 | 28.9 | ● | ○ | ○ | ○ | ○ | ● | < 10.36 | ≥ 10.36 | 11.5 | 2.5 to 52 | ||
| Kinjo 2005133 (OACIS) | 1191 | 62.4 | 25.9 | – | – | 15.4 | 9.2 | N | 1 | ACM | 14 | 1.18 | ● | ● | ● | ● | ● | ● | < 2.9 | ≥ 2.9 | 1.28 | 0.21 to 7.23 | ||
| Lu 2003134 (–) | 153 | 71.0 | 13.1 | 100 | 100 | 30.1 | – | N | 1.33 | CVD | 14 | 6.88 | Crude | ≤ 0.5 | > 0.5 | 0.77 | 0.17 to 3.47 | |||||||
| Biancari 2003135 (–) | 764 | 64 | 24.9 | – | 100 | 43.7 | – | – | 0.014a | ACM | 13 | 121.5 | – | – | – | – | – | – | < 1.00 | ≥ 1.00 | 6.97 | 1.45 to 33.42 | ||
| Gaspardone 1998136 (–) | 76 | 58.7 | 14.5 | 100 | 100 | – | 2.3 | T | 1 | CHD | 13 | 17.11 | Crude | ≤ 0.5 | > 0.5 | – | – | |||||||
| van der Harst 2006137 (QUO VADIS) | 87 | 62.9 | 14.9 | 7.4 | 100 | 41.4 | 1.9 | ELISA | 7.6 | CVD | 11 | 1.66 | ● | ● | ● | ● | ● | ● | ≤ 1.9 | > 1.9 | 4.3 | 0.75 to 24.55 | ||
| Huang 2006138 (–) | 185 | 69.4 | 47 | – | 100 | – | – | ELISA | 3 | CVD | 10 | 1.80 | ○ | ○ | ● | ● | ● | ○ | ≤ 3.0 | > 3.0 | 4.6 | 2.51 to 6.47 | ||
| Milazzo 1999139 (–) | 86 | 64.7 | 14.3 | 17.4 | 100 | 47.7 | – | N | 3.2 | ACM | 4 | 1.45 | Crude | < 3.0 | ≥ 3.0 | – | – | |||||||
| Palazzuoli 2006140 (–) | 208 | 70.6 | 33.7 | 21.2 | – | 31.7 | – | N | 1 | Morbidity | – | – | ● | ● | ● | ● | ● | ● | ≤ 5.0 | > 5.0 | 1.4 | 1.14 to 2.08 | ||
| Fathi 200548 (–) | 4522 | 65 | 29.1 | – | 100 | 42.4 | – | T | 1.7 | ACM | 332 | 4.32 | ● | ● | ● | ○ | ● | ● | < 1.0 | 1.0–3.0 | > 3.0 | 1.85 | 1.13 to 3.03 | |
| Muhlestein2004141 (Intermountain Heart Study) | 2924 | 65.0 | 24.0 | 43.0 | 100 | 26.0 | – | – | 2.4 | ACM | 277 | 3.95 | – | – | – | – | – | – | < 1.2 | 1.2–1.7 | > 1.7 | 2.30 | 1.6 to 3.2 | |
| Sabatine 2007142 (PEACE) | 3771 | 63.7 | 18.9 | – | – | 56.1 | 1.7 | T | 4.8 | CVD | 131 | 0.724 | ● | ● | ● | ● | ● | ● | < 1.0 | 1.0–3.0 | > 3.0 | 1.67 | 1.00 to 2.78 | |
| Saleh 2005143 (–) | 891 | 63.6 | 27.0 | 68 | 100 | 43 | 2.25 | N | 2.6 | ACM | 76 | 3.28 | ● | ● | ○ | ○ | ○ | ● | ≤ 1.0 | 1.1–3.1 | > 3.2 | 1.41 | 0.77 to 2.60 | |
| Rahel 2003144 (–) | 600 | 61.6 | 31.3 | – | 100 | – | 4.5 | ELISA | 0.67 | ACM | 54 | 13.4 | ● | ○ | ● | ○ | ○ | ○ | – | – | – | 1.39 | 0.62 to 3.10 | |
| Speidl 2002145 (–) | 119 | 39.3 | 23.5 | – | 100 | 78.2 | – | – | 4.5 | CHD | 30 | 5.60 | ● | ● | ● | ● | ● | ● | < 1.59 | 1.69–5.51 | > 5.58 | 2.7 | 0.94 to 7.75 | |
| Kubica 2005146 (–) | 80 | 56.0 | 27.5 | 87.5 | 100 | 50 | 1.2 | N | 1 | CHD | 28 | 35.0 | – | – | – | – | – | – | < 0.85 | 0.85–2.0 | > 2.00 | 4.17 | 1.27 to 13.65 | |
| Park 2007147 (–) | 1650 | 60.3 | 28.7 | 53.7 | 100 | 8.0 | – | LPE | 1 | CHD | 23 | 1.39 | ● | ○ | ● | ○ | ○ | ○ | < 1.2 | 1.2–3.1 | > 3.1 | 9.94 | 1.28 to 77.14 | |
| Grander 2004148 (–) | 81 | 61.6 | 30.9 | – | 100 | – | 4.3 | – | 0.57 | CVD | 17 | 36.82 | ○ | ○ | ○ | ○ | ○ | ○ | 0.7–4.8 | 0.23–0.69 | ≤ 0.22 | 0.045 | 0.004 to 0.522 | |
| Morrow 200651 (AtoZ) | 3817 | 60.7 | 24.1 | – | – | 39 | 2.4 | T | 2 | CVD | – | – | ● | ● | ● | ● | ○ | ● | < 1.0 | 1.0–3.0 | > 3.0 | 3.9 | 1.8 to 5.6 | |
| Lee 2006149 (–) | 1050 | 60.8 | 27.1 | – | – | – | – | CL | 8.5 | ACM | 231 | 2.59 | ● | ● | ● | ● | ● | ● | ≤ 0.88 | 0.88–1.97 | 1.97–5.16 | ≥ 5.16 | 2.12 | 1.38 to 3.27 |
| Rothenbacher 2006150 (–) | 1051 | 58.5 | 15.1 | – | 100 | 58.2 | – | LPE | 4.1 | CVD | 95 | 2.20 | ● | ● | ● | ● | ○ | ● | ≤ 1.24 | 1.25–3.51 | 3.52–8.61 | > 8.61 | 1.6 | 0.91 to 2.83 |
| Kip 2005151 (WISE) | 580 | 58 | 100 | – | 61 | – | 2.9 | – | 4.7 | CVD | 92 | 3.37 | ● | ○ | ● | ○ | ○ | ● | < 0.17 | 0.17–0.36 | 0.37–0.83 | ≥ 0.84 | 1.92 | 1.04 to 3.54 |
| Chew 2001b149 (–) | 727 | 65.9 | 28.6 | – | 100 | 30.9 | 5.0 | – | 0.082 | ACM | 71 | 119.1 | – | – | – | – | – | – | < 0.16 | 0.16–0.40 | 0.41–1.10 | > 1.10 | 3.68 | 1.51 to 8.99 |
| Harb 2002152 (THROMBO) | 957 | – | 24.6 | 32.2 | – | 100 | – | – | 2.17 | CHD | 69 | 3.32 | ○ | ● | ○ | ○ | ○ | ● | ≤ 0.09 | 0.10–0.23 | 0.24–0.58 | > 0.59 | 1.22 | 0.58 to 2.55 |
| Hoffmeister 2005153 (–) | 300 | 57.9 | 14.4 | – | 100 | 61.3 | – | N | 3.2 | CVD | 60 | 6.25 | ● | ● | ● | ● | ● | ● | < 0.69 | 0.70–1.27 | 1.28–2.84 | > 2.85 | 1.3 | 0.6 to 2.8 |
| Retterstol 2002154 (–) | 247 | 52.7 | 21.9 | – | – | 100 | 2.4 | LPE | 10 | CHD | 36 | 1.46 | ● | ○ | ● | ● | ○ | ○ | ≤ 1.19 | 1.20–2.36 | 2.37–4.19 | ≥ 4.20 | 4.09 | 1.20 to 3.93 |
| Schnabel 2005b155 (Atherogene) | 570 | – | – | 100 | 100 | – | – | LPE | 2 | CVD | 31 | 2.72 | ● | ● | ● | ● | ● | ● | – | – | – | > 6.1 | 2.40 | 1.1 to 4.6 |
| Patti 2002a156 (–) | 73 | 6.0 | 15.0 | 51.0 | 100 | 55.0 | 2.5 | N | 1.5 | CHD | 12 | 11.0 | Crude | – | – | – | – | 5.28 | 0.68 to 40.92 | |||||
| Krzewina 2003157 (–) | 154 | 57.1 | 25.3 | 89.6 | – | 41 | – | N | 1 | CHD | 31 | 20.1 | – | – | – | – | – | – | Unclear | 2.10 | – | |||
C-reactive protein meta-analysis
The 109 study reports came from 77 unique studies. The pooled relative risk from the random effects model of top versus bottom third of CRP based on 77 unique studies was 1.96 (95% CI 1.76 to 2.17). There was marked heterogeneity, with an I-squared value of 79.1%. Evidence of small study bias is seen with smaller studies showing more extreme (positive) results. The funnel plot (not shown) was asymmetrical and the Egger test was significant (p < 0.001). This overall effect was weaker among those with more adjustment variables and earlier studies, but study characteristics did not account for the substantial heterogeneity between studies. Effects did not differ according to morbidity at baseline or among studies which reported CHD, CVD or ACM outcome events (data not shown).
Estimated glomerular filtration rate systematic review and meta-analysis
The systematic review of eGFR is shown in Table 5 and serum creatinine in Table 6. Given that eGFR is a more accurate reflection of renal function, taking account of age and sex, we did not meta-analyse the serum creatinine results. For routinely measured biomarkers, relative risks were 2.00 (95% CI 1.65 to 2.42) for eGFR below versus above 60 ml/min (based on 12 studies, 31,839 patients, 1639 outcome events).
| Author/publication year (study name) | Number of patients | Age (years) | % Women | Baseline coronary morbidity (%) | Creatinine mean (ml/min) | Measurement type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison group | RR | 95% CI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | ||||||||||||||||
| Inaguma 200745 (–) | 790 | 67.7 | 27.1 | – | – | 64.1 | 66.1 | eGFR-MD | 2.31 | CVD | 110 | 6.03 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per ml/min) | 0.97 | 0.96 to 0.98 | |||
| Gibson 2007158 (–) | 1938 | 65 | 23 | – | – | 49 | 63.9 | Autoanalyser | 3.6 | CHD | – | – | ● | ● | ● | ○ | ○ | ● | Continuous (per ml/min) | 0.97 | - | |||
| Deckers 2006159 (EUROPA) | 12,218 | 60 | 15 | – | 25 | 65 | 85 | eGFR-CG | 4.1 | CHD | 1091 | 2.18 | ● | ● | ● | ● | ○ | ● | Continuous (per ml/min) | 1.01 | 1.01 to 1.02 | |||
| Lipsic 2005160 (Intervention Cardiology Risk Stratification Study) | 143 | 61.5 | 28.7 | 100 | 100 | 32.9 | 79.51 | – | 3.7 | CVD | 19 | 3.59 | ○ | ○ | ○ | ○ | ○ | ○ | Continuous (per ml/min) | 1.0 | 0.98 to 1.02 | |||
| Reinecke 2003161 (–) | 689 | 62.6 | 0 | – | 100 | 30.2 | 79.48 | eGFR-CG | 2 | ACM | 62 | 4.50 | ○ | ○ | ○ | ○ | ○ | ○ | Continuous (per mg/dl) | 1.76 | 1.40 to 2.21 | |||
| Hillis 2006162 (–) | 2067 | 66 | 23 | 100 | 100 | 50 | 64.1 | eGFR-MD | 2.3 | ACM | 158 | 3.32 | ● | ● | ○ | ○ | ○ | ● | Continuous (per ml/min per 1.73 m3) | 0.98 | 0.97 to 0.99 | |||
| Reddan 2003163 (–) | 4584 | 63 | 33.5 | – | 100 | 50.8 | – | eGFR-CG | 5 | ACM | – | – | ● | ○ | ○ | ○ | ○ | ● | Continuous (per 10 ml/min decline) | 1.14 | 1.09 to 1.20 | |||
| Tang 2007164 (–) | 1472 | 61.6 | 28.2 | 26 | 100 | 33.3 | – | – | 1 | ACM | 141 | 9.58 | ● | ● | ● | ○ | ● | ● | Continuous | 0.01 | 0.99 to 1.00 | |||
| Vittinghoff 2003165 (HERS) | 2763 | 66.6 | 100 | 26.4 | 100 | – | – | eGFR-CG | 4.1 | CHD | 361 | 3.19 | ● | ○ | ● | ● | ● | ● | > 40 | ≤ 40 | 1.56 | 1.16 to 2.11 | ||
| Zebrack 2003104 (–) | 1484 | 64 | 33 | 72 | 76 | – | 73 | eGFR-MD | 3 | ACM | 159 | 3.57 | ○ | ○ | ○ | ○ | ○ | ○ | ≥ 60 | < 60 | 3 | 2.1 to 4.2 | ||
| de Winter 2004123 (–) | 1172 | 62 | 32.9 | 78 | 100 | 46.5 | – | eGFR-CG | 1.16 | ACM | 32 | 2.35 | ○ | ○ | ○ | ○ | ○ | ○ | > 51 | ≤ 51 | 3.06 | 1.22 to 7.64 | ||
| Zakeri 2005166 (–) | 4403 | 63.4 | 21 | – | 100 | 50.6 | – | eGFR-CG | 2.4 | ACM | – | – | – | – | – | – | – | – | ≥ 60 | < 60 | 1.56 | 1.14 to 2.13 | ||
| Shlipak 2001167 (HERS) | 2763 | 66.5 | 100 | – | 100 | 51.9 | – | eGFR-CG | 4.1 | CHD | 127 | 1.12 | ● | ● | ● | ● | ● | ● | > 60 | 40–60 | < 40 | 2.56 | 1.5 to 4.3 | |
| Rothenbacher 2006150 (–) | 1051 | 58.5 | 15.1 | – | 100 | 58.3 | – | eGFR-CG | 4.1 | CVD | 95 | 2.20 | ● | ● | ● | ● | ○ | ● | ≥ 90 | 60–90 | < 60 | 1.39 | 0.59 to 3.23 | |
| Almquist 2006168 (APSIS) | 808 | 59.4 | 31 | 100 | – | 16 | 77.6 | eGFR-CG | 3.4 | CVD | 69 | 2.51 | ● | ● | ○ | ○ | ○ | ● | ≥ 90 | 60–89 | < 60 | 1.99 | 0.87 to 4.56 | |
| Shlipak 2004169 (HERS II) | 2266 | 71.0 | 100 | – | 100 | – | – | eGFR-MD | 6.8 | CHD | – | – | ● | ● | ○ | ● | ○ | ● | ≥ 60 | 40–60 | < 40 | 2.97 | 1.49 to 5.93 | |
| Solomon 2006170 (PEACE/Placebo) | 4127 | 63.8 | 18 | – | 70.5 | 54.9 | 77.6 | eGFR-MD | 4.8 | CVD | 352 | 1.78 | ● | ● | ○ | ○ | ○ | ● | > 75 | 60–74.9 | 45–59.9 | < 45.0 | 2.8 | 1.70 to 4.60 |
| Fathi 2005148 (–) | 4522 | 65 | 29.1 | – | 100 | 42.4 | 77 | eGFR-MD | 1.7 | ACM | 332 | 4.32 | ● | ● | ● | ○ | ● | ● | ≥ 90 | 60–89 | 30–59 | ≤ 29 | 3.65 | 2.24 to 5.94 |
| Chen 2006171 (ACRE) | 1144 | 60.7 | 0 | – | 80.5 | – | – | eGFR-MD | 7 | ACM | 280 | 3.50 | ● | ● | ● | ○ | ● | ● | ≥ 60 | 45–59.9 | 30.0–44.9 | < 30 | 4.77 | 2.95 to 7.70 |
| Chen 2006171 (ACRE) | 465 | 60.7 | 100 | – | 55.1 | – | – | eGFR-MD | 7 | ACM | 102 | 3.13 | ● | ● | ● | ○ | ● | ● | ≥ 60 | 45–59.9 | 30.0–44.9 | < 30 | 10.4 | 3.97 to 27.4 |
| Dai 200887 (–) | 345 | 64.6 | 26.7 | – | 100 | 15 | 70.9 | eGFR-MD | 3 | CHD | 56 | 5.41 | ● | ● | ● | ● | ○ | ● | – | 0.99 | 0.98 to 1.01 | |||
| Author/publication year (study name) | Number of patients | Age (years) | % Women | Baseline coronary morbidity (%) | Creatinine mean (µmol/l) | Measurement type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison group | RR | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | |||||||||||||||||
| Dai 200887 (–) | 345 | 64.6 | 26.7 | – | 100 | 15 | 1.3 mg/dl | SCr | 3 | CHD | 56 | 5.41 | Crude | – | 1.17 | 1.03 to 1.32 | |||||||||
| Assmus 2007172 (TOPCARE-CHD trial) | 121 | 62 | 13 | – | 100 | 100 | 1.17 mg/dl | SCr | 1.58 | ACM | 14 | 7.32 | ● | ○ | ○ | ○ | ○ | ● | – | 4.7 | 1.8 to 12.1 | ||||
| Falcone 200661 (–) | 1014 | 64.6 | 27.2 | 82.9 | 100 | 44.9 | 79.6 | SCr | 2.7 | CVD | 105 | 3.84 | ○ | ● | ● | ○ | ○ | ○ | Continuous (per SD) | 1.04 | 0.99 to 1.85 | ||||
| Shah 2008173 (–) | 2886 | 66.2 | – | – | 100 | 31.8 | 1.3 mg/dl | – | 5.025 | ACM | 961 | 6.63 | ● | ○ | ○ | ○ | ● | ○ | Continuous (per mg/dl) | 0.841 | – | ||||
| Ruilope 2007174 (ACTION) | 7665 | 63.4 | 20.5 | 100 | – | 51 | 1.09 mg/dl | – | 4.94 | CVD | 784 | 2.07 | ● | ● | ● | ○ | ○ | ● | Continuous (per mg/dl) | 1.75 | 1.27 to 2.40 | ||||
| Clayton 200517 (ACTION) | 7311 | 63.5 | 20.6 | 100 | 70 | 50.8 | 96.4 | SCr | 3 | ACM | 569 | 2.59 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per mg/dl) | 1.09 | 1.04 to 1.14 | ||||
| Exaire 2006175 (REPLACE-2) | 6002 | 62 | 25.6 | – | 100 | 8.2 | – | SCr | 1 | ACM | 128 | 2.13 | – | – | – | – | – | – | Continuous (per mg/dl) | 1.66 | 1.23 to 2.24 | ||||
| Minoretti 200683 (–) | 799 | 64.9 | 25.6 | 100 | 100 | 46.3 | 79.6 | SCr | 2.7 | CVD | 69 | 3.20 | ● | ● | ● | ● | ● | ● | Continuous (per mg/dl) | 1.01 | 0.98 to 1.31 | ||||
| Matts 1993176 (POSCH) | 416 | 50.6 | 7.7 | – | – | 100 | 98.1 | SCr | 7 | CHD | 32 | 1.10 | – | – | – | – | – | – | Continuous (per mg/dl) | 1.59 | – | ||||
| Chirinos 200578 (–) | 122 | 63.9 | 0 | – | 100 | 39 | 123.8 | SCr | 3 | ACM | – | – | ○ | ○ | ○ | ○ | ○ | ○ | Continuous (per mg/dl) | 1.37 | 0.94 to 1.99 | ||||
| Hu 2006177 (DESIRE) | 1280 | 59.8 | 24.5 | – | 100 | 9.4 | 132.6 | SCr | 2.27 | ACM | 158 | 5.34 | ● | ● | ○ | ● | ○ | ○ | Continuous (per mg/l) | 1.32 | 0.90 to 1.67 | ||||
| Stassano178 2006 (–) | 175 | 62.7 | 22.9 | – | 100 | 16.6 | 106.1 | SCr | 2 | ACM | 11 | 3.14 | – | – | – | – | – | – | Continuous (per unit increase by log transformation) | 1.48 | 1.03 to 2.11 | ||||
| DiMauro 2007179 (–) | 1884 | 64.5 | 16.9 | – | 100 | 46.6 | 1.1 mg/dl | SCr | 7.5 | ACM | 117 | 0.83 | – | – | – | – | – | – | < 2.0 | ≥ 2.0 | 4.1 | 2.6 to 6.4 | |||
| McKechnie 2004180 (–)b | 45,165 | 63.1 | 33.9 | – | 100 | 34.2 | 106.1 | SCr | 0.014a | ACM | 641 | 101.3 | ● | ○ | ○ | ○ | ○ | ● | ≤ 132.6 | > 132.6 | 2.14 | 1.75 to 2.63 | |||
| Duffy 2006181 (–)b | 1046 | 62.3 | 29.3 | – | 100 | 23.6 | – | – | 2.58 | ACM | 144 | 5.34 | ● | ○ | ○ | ○ | ○ | ○ | ≤ 132.6 | > 132.6 | 1.26 | 1.16 to 1.37 | |||
| Elisheva 2000182 (ISCAB)b | 4738 | 64.7 | 21.3 | – | 100 | – | – | SCr | 0.083 | ACM | 147 | 3.74 | ● | ● | ○ | ○ | ○ | ● | < 123.8 | ≥ 123.8 | 2.28 | – | |||
| Schnabel 2005106 (Atherogene)b | 1872 | 61 | 20.9 | – | 100 | – | 83.1 | – | 2.6 | CVD | 114 | 2.34 | ● | ● | ● | ● | ● | ● | < 90.2 | ≥ 90.2 | 1.8 | 1.1 to 2.5 | |||
| Cesena 2004183 (–)b | 574 | 61 | 27.5 | 97.4 | 100 | 65.9 | 106.1 | SCr | 0.47 | CHD | 107 | 3.97 | ○ | ○ | ○ | ○ | ○ | ○ | < 132.6 | ≥ 132.6 | 1.60 | 1.0 to 2.8 | |||
| Van Domburg 2002184 (–) | 832 | 62.8 | 24 | 32 | 100 | 26 | – | – | 5.2 | ACM | 92 | 2.13 | ● | ● | ○ | ● | ○ | ● | ≤ 150 | > 150 | 2.8 | 1.4 to 5.5 | |||
| Szczech 2002185 (BARI)b | 3608 | 61.5 | 26.0 | 71.2 | 100 | 52.0 | – | SCr | 7 | CHD | – | – | ● | ● | ● | ○ | ● | ● | ≤ 132.6 | > 132.6 | 3.00 | 1.87 to 4.82 | |||
| Yamamuro 2000186 (–)b | 739 | 74.0 | 20.8 | – | 100 | 58.6 | 118.5Ψ | SCr | 4.25 | ACM | – | – | ○ | ○ | ○ | ○ | ○ | ○ | ≤ 141.4 | > 141.4 | 1.73 | 1.14 to 2.61 | |||
| Zakeri 2005166 (–) | 4403 | 63.4 | 21 | – | 100 | 50.6 | – | – | 2.4 | ACM | – | – | – | – | – | – | – | – | < 130 | ≥ 130 | 1.65 | 1.25 to 2.18 | |||
| Weerasinghe 2001187 (–) | 1197 | – | 16.2 | – | 100 | 56.6 | 98.0 | SCr | 0.025 | ACM | 45 | 150.4 | ● | ● | ● | ● | ○ | ● | < 130 | 130–149 | ≥ 150 | 7 | to 1 | ||
| Wattanakit 2005188 (ARIC)b | 766 | 57.1 | 24.7 | 0 | – | 86.0 | – | SCr | 8.7 | CVD | 313 | 4.70 | ● | ● | ● | ● | ○ | ● | < 88.4 | 88.4–97.2 | 106.1–114.9 | ≥ 123.8 | 1.66 | 1.1 to 2.6 | |
| Schnabel 2005A107 (Atherogene)b | 639 | 61.7 | 27.8 | 86.8 | – | 27.8 | 97.2 | SCr | 7.1 | CVD | 112 | 2.47 | ● | ● | ● | ● | ○ | ● | < 83.1 | 83.1–91.9 | 91.9–105.2 | > 105.2 | 2.48 | 1.22 to 5.02 | |
| Nygard 1997189 (–) | 578 | 62 | 18.6 | – | 100 | 57.4 | – | SCr | 4.6 | ACM | 64 | 2.41 | ● | ● | ● | ● | ○ | ● | < 80 | 80–119 | 120–149 | ≥ 150 | 2.55 | 0.82 to 7.92 | |
| Kaplan 2002190 (–) | 2677 | 63.8 | 37.9 | 40.1 | – | 100 | 110.6 | SCr | 3.4 | CHD | 445 | 4.89 | ● | ● | ● | ● | ● | ● | 94.4–104.3 | < 84 | 84–94.3 | 104.4–120.5 | > 120.5 | 1.77 | 1.31 to 2.38 |
| Matts 1993176 (POSCH)b | 416 | 50.6 | 7.7 | – | – | 100 | 98.1 | SCr | 7 | CHD | 32 | 1.10 | – | – | – | – | – | – | ≤ 0.9 | 88.4 | 97.2 | 106.1 | ≥ 114.9 | 1.54 | – |
| Reinecke 2003161 (–) | 689 | 62.6 | 0 | – | 100 | 30.2 | 79.48 (5 SDs) (23.4) | eGFR-CG | 2 | ACM | 62 | 4.50 | ○ | ○ | ○ | ○ | ○ | ○ | Continuous (per mg/dl) | 1.76 | 1.40–2.21 | ||||
Summary of systematic review for five routinely measured and eight novel biomarkers
We included 390 reports of biomarker effects in our review (Table 7), and Appendix 1 contains the results of the systematic reviews of each of the biomarkers. The number of events per study, and age, sex and baseline morbidity characteristics were similar across all the biomarkers. The quality issues identified for the CRP studies were likewise found for the other biomarkers (data not shown). Routinely assessed biomarkers contributed fewer studies than did novel biomarkers. Thus, CRP had 109 reports in the systematic review and 100 in the meta-analysis, by contrast with haemoglobin (15 and 4 respectively).
| Number of studies in systematic review | Number of studies in meta- analysisa | Number of patients | Number of eventsb | Comparison | Relative risk (95% CI) | |
|---|---|---|---|---|---|---|
| Widely performed in routine clinical care | ||||||
| eGFR | 15 | 12 | 31,839 | 1639 | Chronic kidney disease < 60 ml/min | 2.00 (1.65 to 2.42) |
| Fasting glucose | 28 | 11 | 63,957 | 14716 | Diabetes > 7 mmol/l | 1.74 (1.15 to 2.63) |
| Haemoglobin | 15 | 4 | 52,113 | 1741 | Anaemia (haemoglobin < 13g/dl) | 2.92 (0.40 to 21.1) |
| Total cholesterol | 47 | 31 | 53,129 | 4441 | T3 vs T1 | 1.30 (1.16 to 1.45) |
| LDL cholesterol | 39 | 29 | 33,817 | 5874 | T3 vs T1 | 1.33 (1.16 to 1.52) |
| Novel biomarkers | ||||||
| hs-CRPc | 109 | 77 | 56,496 | 5798 | T3 vs T1 | 1.96 (1.76 to 2.17) |
| Fibrinogen | 40 | 31 | 36,739 | 3692 | T3 vs T1 | 1.59 (1.39 to 1.82) |
| Lp(a)c | 20 | 17 | 17,602 | 2322 | T3 vs T1 | 1.24 (1.12 to 1.38) |
| Apolipoprotein A-Ic | 14 | 11 | 15,044 | 1398 | T3 vs T1 | 0.81 (0.71 to 0.92) |
| Apolipoprotein Bc | 13 | 12 | 16,706 | 1645 | T3 vs T1 | 1.39 (1.07 to 1.79) |
| Homocysteinec | 16 | 12 | 6100 | 817 | T3 vs T1 | 2.06 (1.69 to 2.50) |
| NT-BNPc | 20 | 14 | 18,326 | 1620 | T3 vs T1 | 2.93 (2.03 to 4.23) |
| Il-6 | 14 | 9 | 8200 | 1148 | T3 vs T1 | 1.63 (1.09 to 2.43) |
The estimated summary relative risks were 1.74 for fasting glucose higher than 7 mmol/l, 2.92 for haemoglobin lower than 13 g/dl, and 1.30 and 1.33 for total and LDL cholesterol (top versus bottom tertile) respectively.
For novel circulating biomarkers, relative risks comparing the top and bottom third were: 1.96 (95% CI 1.76 to 2.17) for CRP (based on 77 studies, 56,496 patients, 5798 outcome events) and, based on a smaller literature, 2.93 for BNP, 2.06 for homocysteine, 1.63 for IL-6, 1.59 for fibrinogen, 1.39 for ApoB, 1.24 for Lp(a) and 0.81 for ApoA-I. The quality of individual study reports was variable with many studies lacking clear description of population selection, and variable adjustment for simple clinical information – age, sex, smoking, diabetes, obesity and lipids.
Chapter 5 Methods of decision model
Introduction
As outlined in Chapter 2, several analytical steps are required in order to determine the cost-effectiveness of alternative strategies for prioritising patients on the waiting list for CABG. While the results of the systematic review provide the most appropriate basis for estimating the clinical effectiveness of single and combination biomarkers (both routine and novel) in terms of their prognostic value in predicting events among patients with stable coronary disease, this addresses only one element of the overall decision problem. These results do not directly consider the effect of employing biomarkers in terms of their effect on final health outcomes expressed in generic terms (e.g. QALYs gained), subsequent health-care resource utilisation and costs; neither do these results provide a comparison against a range of alternative approaches to prioritisation which may be considered relevant comparators. Hence, in order to address the overall decision problem outlined in Chapter 2, a number of additional steps are subsequently required to determine the cost-effectiveness of alternative prioritisation strategies.
The additional steps comprise the methods and analytical approaches of the decision-analytic model itself, as well as the additional approaches required to integrate the results from the systematic review of circulating biomarkers within this framework. This chapter provides details of the methods, analytical approaches and sources of input data into the decision-analytic model. The chapter also outlines the approaches required to incorporate the results from Chapter 4 within the broader evaluation of cost-effectiveness. The results of the separate analyses, alongside the final estimates of cost-effectiveness, are reported in detail in Chapter 6.
Cost-effectiveness analysis
The cost-effectiveness analysis was undertaken from a UK health service perspective and costs were expressed in UK pounds sterling (GBP) at 2006/7 prices. A lifetime time horizon was employed and health outcomes were estimated in terms of QALYs. Costs and QALYs were discounted by 3.5% per annum. 191
Prioritisation strategies
There are several ways of formally prioritising patients waiting for revascularisation, of which circulating biomarkers represent one potential approach. It is not possible to establish the cost-effectiveness of using biomarkers without an explicit comparison against other formal approaches to prioritisation that are considered relevant and feasible options which could also be implemented in the NHS. Similarly, the use of any formal approach to prioritising waiting lists for CABG also needs to be evaluated in the context of current NHS practice. That is, any additional costs that may be imposed on the NHS because of the use of novel biomarkers (or any formal approach to prioritising waiting lists) need to be considered in relation both to the additional gains in health outcomes that may subsequently be achieved as well as to other ways in which these resources might be productively used elsewhere within the NHS. It is only through such an explicit comparison that the cost-effectiveness of alternative prioritisation strategies can subsequently be determined.
We identified four general approaches to the prioritisation of patients on a waiting list for CABG. These approaches comprised: (1) no formal prioritisation (routine clinical practice); (2) urgency scores; (3) risk score without the use of biomarkers; and (4) risk score with biomarkers. Within several of the general approaches there also exists a number of alternative approaches that could be considered (e.g. different approaches to evaluating urgency scores, different single and combination biomarkers comprising both novel and routine biomarkers, etc.). Each of these general approaches (and variants therein) represents potentially relevant and separate strategies that should be considered as part of an overall evaluation of cost-effectiveness.
While it remains desirable to evaluate all plausible strategies in the context of the decision problem, this also needs to be balanced against the analytical feasibility and data requirements required to provide robust inputs into such a comprehensive evaluation that would then provide a robust basis for informing NHS policy. This issue is particularly pertinent to the evaluation of alternative strategies that could be considered within the general approach of using risk score approaches with biomarkers. The five routine and eight novel biomarkers considered in Chapter 4 could feasibly be used either singly or in combination with any one or more of the remaining biomarkers. Consequently, there exists a large number of potentially relevant strategies – even with the eight novel biomarkers there are over 40,000 possible combinations that could be considered, and when combined with the five routine biomarkers this increases to 16 factorial. Ultimately, any attempt to comprehensively evaluate all of these strategies is likely to be a relatively futile exercise, the problems of which will also be compounded by the lack of robust clinical data on the majority of these strategies and the potential difficulties that may subsequently be encountered in terms of linking this evidence to the broader decision model itself.
Hence, rather than attempting to be comprehensive in terms of the strategies considered, with particular reference to the biomarker strategies, the decision model evaluates a more restrictive range of strategies. The strategies that were finally selected were chosen on the basis that these were considered to be particularly important questions relevant to existing NHS decision-making in terms of routine biomarkers that are already widespread (e.g. eGFR) and those whose use remains variable (e.g. CRP). A total of seven separate strategies, within the four general approaches, were thus evaluated in detail as part of the decision model. These separate strategies are summarised below.
Strategy 1: No formal prioritisation (routine clinical practice) This strategy reflects how prioritisation is currently undertaken in routine clinical practice. Routine clinical practice will inevitably reflect the variability that exists in different centres in terms of the approaches employed to prioritising patients on a waiting list. This variability will reflect differences in the formal and informal approaches to ordering waiting lists that are currently being applied. This provides an appropriate baseline with which to assess the alternative prioritisation strategies that are based on a more formal and systematic approach to prioritisation.
Strategies 2–3: Urgency scores Within this general approach, formal prioritisation is guided by the use of urgency scores. Explicit urgency scores, where those with the most disabling symptoms and worst prognosis should be prioritised first, have been proposed as a formal approach to the prioritisation of waiting lists. The two particular algorithms considered here are based on the Ontario31 and New Zealand168 scoring systems. These algorithms are subsequently evaluated as two separate strategies within the decision model.
Strategy 4: Risk score without biomarkers An alternative to the use of urgency scores based primarily on symptom measures is to consider formal prioritisation approaches guided entirely by the predicted risk of cardiovascular events. Such an approach could be implemented by employing a risk equation based on routinely measured and/or observable risk factors (excluding information based on biomarkers) which can be demonstrated to be predictive of the potential risk of experiencing a cardiovascular event. The use of such an approach would mean that individuals within a cohort of subjects on a waiting list could be stratified according to their individual risk score, with patients predicted to be at a higher absolute risk of experiencing a cardiovascular event on the waiting list subsequently prioritised above individuals predicted to be at a lower absolute risk.
Strategies 5–7: Risk score with biomarkers This general approach is similar to that outlined for Strategy 4. However, in addition to the routinely available information considered within Strategy 4, the risk score is refined by including additional prognostic information provided by the biomarkers themselves. A total of three separate strategies are considered based on a risk prediction equation that also incorporates the additional prognostic information generated by biomarkers. The three strategies considered were: (1) adding a single, routinely available biomarker to the risk prediction equation (eGFR); (2) adding a single, novel biomarker (CRP); and (3) adding a combination of biomarkers (both CRP and eGFR). Hence, the strategies reflect the use of a single routine or a novel biomarker as well as employing a combination of biomarkers.
Choice of biomarkers
We focused on two biomarkers: CRP, because our systematic reviews demonstrated that it has been investigated in many more studies than any of the other eight biomarkers assessed, and thus the available evidence is likely to be more reliable. eGFR was chosen because renal function is currently routinely assessed and used by surgeons to assess operative risk (e.g. euroSCORE). Thus, if cost-effective, extension of its use in prioritisation is likely to be feasible.
In order to evaluate the separate strategies, obtaining contemporary data representative of current clinical practice is critical. This provides an appropriate baseline which can then be used to evaluate potential changes in health outcomes and costs related to the application of more formal approaches to the prioritisation of waiting lists. Ultimately, the value of the formal prioritisation strategies will be determined by three main issues: (1) the degree to which the additional prognostic information they provide alters the subsequent ordering of individual subjects in terms of their position on a waiting list from that based on current practice; (2) the degree to which a different ordering results in meaningful improvements in terms of subsequent long-term health outcomes and costs; and (3) the costs of generating and applying this prognostic information.
The primary analysis considered here (hereafter referred to as the ‘base-case’ analysis) evaluates the impact of alternative prioritisation approaches within the context of a maximum waiting time of 3 months (90 days). However, separate scenarios are also presented that consider the value of prioritisation approaches for shorter proposed maximum waiting times (2 weeks and 6 weeks). Consequently, the results are generalisable to different settings with longer and shorter maximum waiting times.
Details of how the prioritisation strategies were implemented and evaluated in the present analysis are provided in the following sections. Given that the decision-analytic model provides the overall analytical framework for the cost-effectiveness analysis, a detailed description of the structure of the model is provided initially.
Model structure
The decision-analytic model reflects both the overall structure of the decision problem as well as the analytical framework necessary to combine the various inputs required to evaluate expected lifetime costs and QALYs for patients on a waiting list for CABG. Given that the overall objective is to assess the impact of alternative prioritisation approaches in terms of the effect they have on the ordering of a waiting list (and hence in terms of the overall time an individual experiences on a waiting list prior to the procedure), the model needs to evaluate the expected lifetime costs and QALYs based on a CABG procedure undertaken anytime between day 1 and day 90, representing the minimum and maximum waiting times possible in the context of the base-case analysis. To assess the cost-effectiveness of the different prioritisation strategies, the decision model also needs to evaluate the potential impact each prioritisation strategy has on the proposed timing of the procedure and how this subsequently affects the expected lifetime estimates of costs and QALYs.
Central to this is the structure of the decision-analytic model itself. The model developed here has a Markov structure,35 employing a similar structure to previously developed decision-analytic models in the cardiovascular field. 193,194 In a Markov structure, hypothetical individuals reside in one out of a set of mutually exclusive health states at particular points in time. During discrete time intervals of equal length (normally referred to as Markov cycles), individuals can either remain in a particular health state or move to a separate health state (e.g. because of a patient experiencing a particular clinical event). The movements between states represent the potential clinical pathways that a patient may follow at different time points and over his or her remaining lifetime. The likelihood that an individual remains in a particular health state, or moves to a separate state, is estimated in terms of transition probabilities. Defining and subsequently estimating these transition probabilities represent both key structural and analytical elements of the decision model.
In addition to defining the potential health states and estimating the transition probabilities, the costs and the quality of life effect of the states themselves also need to be evaluated. For the purpose of cost-effectiveness analysis, it is essential that quality of life is assessed in terms of a generic measure. Decisions concerning resource allocation typically need to be taken across specialties and disease areas. If these decisions are to be informed by cost-effectiveness analysis then it is crucial that the outcome measure adopted is generic, i.e. that it has meaning outside the clinical area within which it is used. The use of QALYs as the primary outcome of the model allows the cost-effectiveness of the different strategies to be compared with other potential uses of these resources within the NHS. In order to estimate QALYs, it is necessary to quality adjust the period of time the average patient is alive within the model using an appropriate utility or preference score. The utility scores represent the quality of life of the separate states in the model. The costs and health outcomes from each Markov cycle are then accumulated and summarised for the cohort of hypothetical individuals at the termination of the analysis. These estimates then provide the basis for the cost-effectiveness estimates.
The Markov structure is shown in detail in Figure 3. The health states comprising the structure of the model are illustrated by ovals in the figure. The boxes indicate events occurring during a Markov cycle. For instance, the box named ‘CABG day 1–90’ illustrates that revascularisation has occurred during a cycle. Similarly, the boxes named ‘Stroke/MI/death’ are used to illustrate that a patient has experienced a composite clinical event. However, these events do not represent health states as such, instead they simply provide the mechanism by which the specific health state (e.g. stroke, myocardial infarction or death) in which a patient resides at the end of a cycle is estimated. The arrows represent the possible movements between health states in any given cycle.
FIGURE 3.
Model structure showing key transitions before and after CABG. 1. Rates of cardiovascular death, non-fatal myocardial infarction or non-fatal stroke on the waiting list for CABG. 2. Procedural risk of death, non-fatal myocardial infarction or non-fatal stroke. 3. Rates of cardiovascular death, non-fatal myocardial infarction or non-fatal stroke after successful CABG. 4. Rates of subsequent cardiovascular events while on the waiting list for CABG. 5. Rates of subsequent cardiovascular events after successful CABG. 6. Conditional probability of a composite event being non-fatal myocardial infarction, non-fatal stroke or death. Note, at any point in time, all patients (regardless of their health state) are also at risk of dying from non-cardiovascular causes. This risk is assumed to be independent of particular health states and so these transitions are not illustrated separately in the schematic. MI, myocardial infarction.
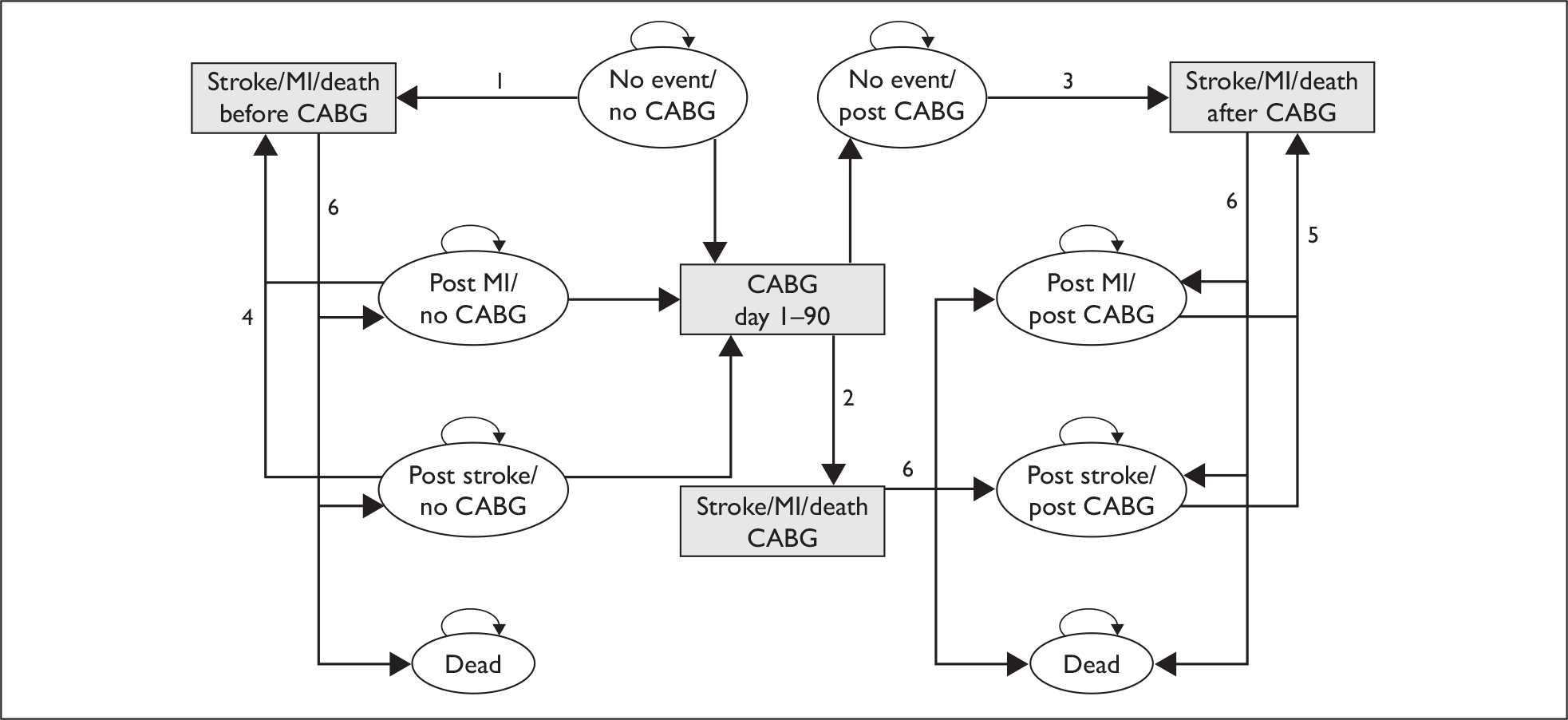
For the first 90 days of the model, representing the total period in which all patients are assumed to undergo CABG in the base-case analysis, daily cycles are applied. After 90 days, annual cycles are applied. All patients in the representative cohort start in the ‘no event/no CABG’ state. Patients face a risk of a composite end point (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) while awaiting CABG (denoted transition 1 in Figure 3). This transition is implemented in the model as a daily probability of the composite end point. This probability is applied in the model before patients receive CABG. When CABG is performed (note that CABG can be performed any day between day 1 and 90 as determined by the prioritisation strategies), patients face a procedural risk (denoted transition 2 in Figure 3). This risk is applied as an instant ‘one-off’ risk in the cycle where CABG is performed, although this actually represents the probability of an event up to 30 days after the procedure has been performed. Patients who have a successful CABG (i.e. without a procedural event) make a transition to the ‘no event/post CABG’ state. In this state, patients still face an ongoing risk of experiencing the composite end point (denoted transition 3 in Figure 3), although this risk is now lower than the risk for those on the waiting list as the protective effect of revascularisation is incorporated into this estimated risk. This transition is implemented in the model as a daily probability between the day of CABG and 90 days, and as an annual probability thereafter.
Patients suffering a non-fatal stroke or a non-fatal myocardial infarction anywhere in the model make a transition to the post-stroke and post-myocardial infarction states respectively. In these states, patients are at risk of a further composite end point (denoted transitions 4 and 5 in Figure 3). As for patients without an event, this risk is different depending on whether or not CABG has been performed. Patients suffering a non-fatal myocardial infarction or stroke before CABG are assumed to undergo CABG as planned. If a composite end point occurs at any time in the model, a further calculation determines whether this event is fatal, a non-fatal stroke or a non-fatal myocardial infarction (denoted transition 6 in Figure 3). At any point of time, patients are also at risk of mortality from other than cardiovascular causes (transitions not shown in Figure 3).
Data sources – Swedish Coronary Angiography and Angioplasty Registry
Transition probabilities
In addition to the systematic review of circulating biomarkers presented previously, the key data source for estimating transition probabilities was a registry of coronary angiography. The ideal registry in which to develop our decision-analytic model has several characteristics. It should: identify large numbers of patients at the time of angiography and record details of the intention to perform CABG and baseline clinical information including biomarkers; be multicentre or national; reflect contemporary practice; and have follow-up for fatal and non-fatal events. No such registry exists in the UK. One of the few registries in the world that meets these criteria is SCAAR. 37
The Swedish Coronary Angiography and Angioplasty Registry is a national registry that includes all angiographies performed in Sweden. The registry covers high volume dedicated research centres as well as low volume centres. Data for this analysis were available from 2000 to 2005. The registry covers a total of 201,000 angiographies. In 2005, a total of 9500 angiographies in patients with stable coronary artery disease were reported. In SCAAR the decision on further management after angiography is also available, making it possible to identify patients with a decision to undergo CABG, which comprises the patient population of interest in the present analysis. Follow-up for death, non-fatal myocardial infarction and non-fatal stroke was carried out through linkage to national hospitalisation registers. As the SCAAR registry is contemporary, has good coverage, and reflects current clinical practice, this data source was considered to represent the best available evidence to inform the decision model, despite not being from a UK population. Furthermore, we had access to comprehensive individual-patient data from SCAAR, which is required in order to estimate event risks with sufficient detail to be useful in the decision-analytic model.
Data from the SCAAR registry were employed to develop risk equations which were used to define several of the prioritisation strategies (discussed further below) and also to estimate transition probabilities in terms of the risk of cardiovascular events on the waiting list for CABG, procedural risk and the risk after CABG to be applied within the decision-analytic model. Furthermore, SCAAR was also used to define the cohort representing the characteristics and baseline risk factors of a representative cohort on a waiting list for CABG patients. We used the full SCAAR sample to generate the risk equations in order to obtain reliable estimates for the risk factor coefficients. Prioritisation strategies were applied to a cohort of patients in SCAAR with complete data including time to CABG (required to implement a strategy of no formal prioritisation) and eGFR (added to the data set in 2005). In terms of age, sex and coronary anatomy, this cohort (n = 338) was representative of the earlier sample (data not shown).
The baseline characteristics of patients in SCAAR with stable coronary artery disease and a decision to undergo CABG after angiography between 2000 and 2005 are shown in Table 8.
| Variable | Total number of patients | Number of patients with characterisation | Mean |
|---|---|---|---|
| Age | 10,129 | Continuous | 66.02 |
| Gender (male) | 10,130 | 8025 | 0.79 |
| Smoker (previous or current) | 1117 | 623 | 0.56 |
| Hypertension treatment | 1149 | 688 | 0.60 |
| Lipid lowering treatment | 1148 | 842 | 0.73 |
| Diabetes | 10,130 | 1461 | 0.14 |
| Body mass index | 783 | Continuous | 27.28 |
| Angina symptoms Canadian class (3 or 4) | 5097 | 2261 | 0.44 |
| Left main vessel or three-vessel disease | 9936 | 7801 | 0.79 |
| Previous myocardial infarction | 10,130 | 3008 | 0.30 |
| Previous PCI | 6254 | 694 | 0.11 |
| Previous CABG | 6254 | 211 | 0.03 |
| Previous stroke | 10,130 | 617 | 0.06 |
| Heart failure | 10,130 | 839 | 0.08 |
| Peripheral vascular disease | 10,130 | 446 | 0.04 |
| S-creatinine (µmol/l) | 857 | Continuous | 88.95 |
| Renal failure | 10,130 | 96 | 0.01 |
| Chronic obstructive lung disease | 10,130 | 326 | 0.03 |
| Cancer diagnosis | 10,130 | 210 | 0.02 |
Data from SCAAR were subsequently used to estimate the separate transition probabilities illustrated in Figure 4. The approaches employed to estimation are described in detail below. The results themselves are reported in Chapter 6.
FIGURE 4.
Implementation of the Ontario algorithm into SCAAR. LAD, left anterior descending.
Transition 1: Rates of cardiovascular events while on the waiting list for CABG
All patients in SCAAR between 2000 and 2005 with stable coronary artery disease and who were assigned a primary decision of CABG after angiography were included in this analysis. In order to facilitate the incorporation of the estimated risks as transition probabilities in the decision-analytic model, a parametric time-to-event model with an exponential distribution was estimated. 195 The event considered in this analysis was the composite end point of death, myocardial infarction or stroke. Patients not reaching the composite end point were censored at date of CABG, date of PCI or the date 90 days after the decision to undergo CABG. The candidate covariates for the time-to-event model are shown in Table 8. The choice of covariates to be included in the final model was based on availability and statistical significance. Some covariates had to be dropped on the basis that they were not reported for a large enough number of patients. The general rule was to keep covariates that were statistically significant at the 5% level. The transition probabilities needed to populate the Markov structure were derived from the results of the final exponential time-to-event model. Furthermore, the additional prognostic information provided by biomarkers was added to the predicted risk of the composite end point (see Implementing prioritisation strategies and Adjustment factors for details).
Transition 2: Procedural risk of cardiovascular events
For this analysis, the patients assigned and actually undergoing CABG were included. The same composite end point of death, myocardial infarction or stroke was used in this analysis. For the purpose of this analysis, events occurring within 30 days of CABG were defined as procedural. A standard logistic regression was applied in order to estimate procedural risk for patients with different characteristics and risk factors. This analysis included the same covariates as those included in the time-to-event model estimated for the risk of a composite event while on the waiting risk.
The logistic regression estimates the odds of particular events. It should be noted that the odds of an event is the ratio of two complementary probabilities, and therefore does not represent a probability required to populate the cost-effectiveness model. To obtain the relevant probabilities (p) from the logistic regression, the inverse logistic transformation was used,196 given by:
for the covariates, X, and the estimated coefficients on the log scale, β.
Transition 3: Rates of cardiovascular events after successful CABG
In estimating this risk, we used the patients assigned and undergoing CABG and not experiencing a composite procedural event. Hence, the starting date for this analysis was the date of CABG plus 30 days. A parametric time-to-event model employing an exponential distribution was estimated using the same end point and risk factors as in the statistical models used for the other transitions. Patients not experiencing the composite end point were censored at 31 December 2005. In a similar manner to the equation estimating transition 1, the additional prognostic information provided by biomarkers was added to the predicted risk of the composite end point. Transition probabilities were derived from this time-to-event model employing the same formulas outlined for transition 1.
Transition 4: Rates of subsequent cardiovascular events while on the waiting list for CABG
Patients suffering a non-fatal event in the model are at risk of further cardiovascular events (transition 4) in the model. We did not have sufficient data to estimate this risk for patients on the waiting list for CABG. Instead, the time-to-event model estimated for transition 1 was used, updating the covariates of previous myocardial infarction and previous stroke to provide an estimate of this risk.
Transition 5: Rates of subsequent cardiovascular events after successful CABG
Similar to patients on the waiting list for CABG, patients with a non-fatal event post CABG are at risk of further cardiovascular events (transition 5). As for transition 4, there were not sufficient data for estimating this risk. Similar to transition 4, the time-to-event model estimated for transition 3 was used, updating the covariates of previous myocardial infarction and previous stroke to provide an estimate of this risk.
Transition 6: Conditional probability of a composite event being non-fatal myocardial infarction, non-fatal stroke or death
In order to determine whether a composite event was fatal, a non-fatal myocardial infarction or a non-fatal stroke, the proportions of the observed events in the estimated equations were used.
Death from non-cardiovascular causes
The probability of death from non-cardiovascular causes was also included. This was assumed to be independent of the (non-fatal) health states considered in the overall model. Hence, the same probability of non-CVD mortality was assigned to each health state in the model. The respective probability of non-CVD mortality was estimated using UK sex- and age-specific life tables adjusted to exclude cardiovascular mortality. 197,198
Costs
The estimated costs for different states in the model are reported in Table 9, together with the cost of the procedure itself. The cost of CABG is derived from NHS reference costs,199 and the estimated costs associated with the health states in the model are based on previous detailed costing work undertaken using the Nottingham Heart Attack Registry. 200 The cost of the CRP was estimated to be £6 (see Table 1). No additional cost was assigned to eGFR on the basis that this is already routinely collected and hence the opportunity cost of using this for the purposes of prioritising a waiting list for CABG would be negligible.
| Cost item | Mean value |
|---|---|
| Annual cost of ischaemic heart disease without an event | £483 |
| Annual cost of the first year after a myocardial infarction | £2201 |
| Annual cost of the second and subsequent years after a myocardial infarction | £774 |
| Annual cost of the first year after a stroke | £9845 |
| Annual cost of the second and subsequent year after a stroke | £2597 |
| Cost of CABG | £8203 |
Health-related quality of life
The estimated utilities, or quality adjustment weights, representing the health-related quality of life of the separate health states in the model are shown in Table 10. These estimates are based on previous work201 which employed systematic approaches to identify appropriate utility estimates to apply to patients with ischaemic heart disease, myocardial infarction and stroke, representing the major health states of the model.
| Health state | Mean utility |
|---|---|
| Ischaemic heart disease (no event state) | 0.718 |
| First year after myocardial infarction | 0.683 |
| Second and subsequent years after myocardial infarction | 0.718 |
| Post stroke (combining disabling and non-disabling stroke)a | 0.612 |
Defining the representative cohort
In order to determine costs and health outcomes of the prioritisation strategies, a cohort of patients to be prioritised is required. As previously described, a representative cohort is an important element of the model and is used to characterise the variation in the baseline characteristics and risk factors of a representative group of patients on a waiting list for CABG. Ultimately, it is this cohort that provides the basis for estimating the order in which individuals are assumed to receive the CABG procedure for each of the alternative methods of prioritisation under investigation. Similarly, the baseline covariates of this cohort determine the subsequent effect of the separate risk equations in terms of health outcomes and costs. These costs and health outcomes will vary according to the baseline characteristics and risk factors of the representative cohort as well as to the different ordering of this cohort predicted by the alternative strategies.
A total of 338 patients from the SCAAR registry who underwent CABG were used to define the representative cohort. As noted previously, these patients did not differ in terms of baseline covariates from the larger sample. A further 22 patients were sampled randomly and reintroduced as ‘duplicate’ patients to make a total of 360 patients and an even number of procedure ‘slots’ per day over 90 days (i.e. four operations per day) to simplify the subsequent analytical implementation of the model. The 338 patients from SCAAR were those with complete covariate data (i.e. ensuring that the complete set of risk equations could be applied for these patients), and their times to CABG from angiography were available (i.e. making it possible to implement a strategy of clinical practice in the representative cohort). For example, creatinine was only routinely reported in SCAAR from 2005, thus substantially reducing the number of patients eligible for the representative cohort as creatinine was required to implement the prioritisation strategy using a risk score with eGFR. Finally, the presence of complete covariates was also necessary because of the additional analytical steps that were needed to impute CRP levels for the SCAAR patients. This imputation was required as this biomarker was not actually collected as part of the SCAAR registry. Further details of the imputation approaches are reported in later sections. There were 680 patients with complete covariates in SCAAR. Of these 680 patients, 338 also had a time to CABG registered and were thus included in the representative cohort.
Implementing prioritisation strategies
This following section outlines how the prioritisation strategies were implemented in relation to the representative cohort. For several of the proposed strategies, a number of additional assumptions and analytical approaches were required to generate the appropriate estimates necessary to implement the strategies within the decision model.
No formal prioritisation
For all patients in the representative cohort, the time to CABG is actually reported in the SCAAR registry (i.e. the time from decision to when the actual procedure was performed). Hence, the strategy of no formal prioritisation simply reflects the implicit ordering of patients based on these reported waiting times. While this approach does not comprise an explicit approach to formally ordering the waiting list, it reflects the reality of existing practice and the subsequent ordering that this implies. Hence, patients in the representative cohort with a shorter reported time to CABG are prioritised first when the strategy of no formal prioritisation is modelled.
Urgency scores
The complete set of variables needed to implement either the Ontario or the New Zealand urgency scoring systems was not available in the SCAAR data set. However, given the importance of including a range of alternative formal approaches to prioritisation of the waiting list, separate mapping exercises were undertaken between the variables reported in the SCAAR registry and those included in both the Ontario and the New Zealand urgency scoring algorithms.
The algorithm for mapping Ontario into the SCAAR patients is shown in Figure 4. The results of the mapping were used to estimate the urgency scores for each of the individual patients within the representative cohort. The ordering of patients in terms of the Ontario score was thus determined by the estimated scores of the individual patients within this cohort, with a lower Ontario score indicating a higher prioritisation and hence an earlier position on the scheduling of CABG within the 3 months considered.
The algorithm for mapping New Zealand into the SCAAR patients is shown in Figure 5. A similar approach was employed to that used for the strategy based on Ontario urgency scores, except that patients with higher New Zealand scores are prioritised first within this strategy.
FIGURE 5.
Implementation of the New Zealand algorithm into SCAAR. CAD, coronary artery disease; LAD, left anterior descending; VD, vessel disease.
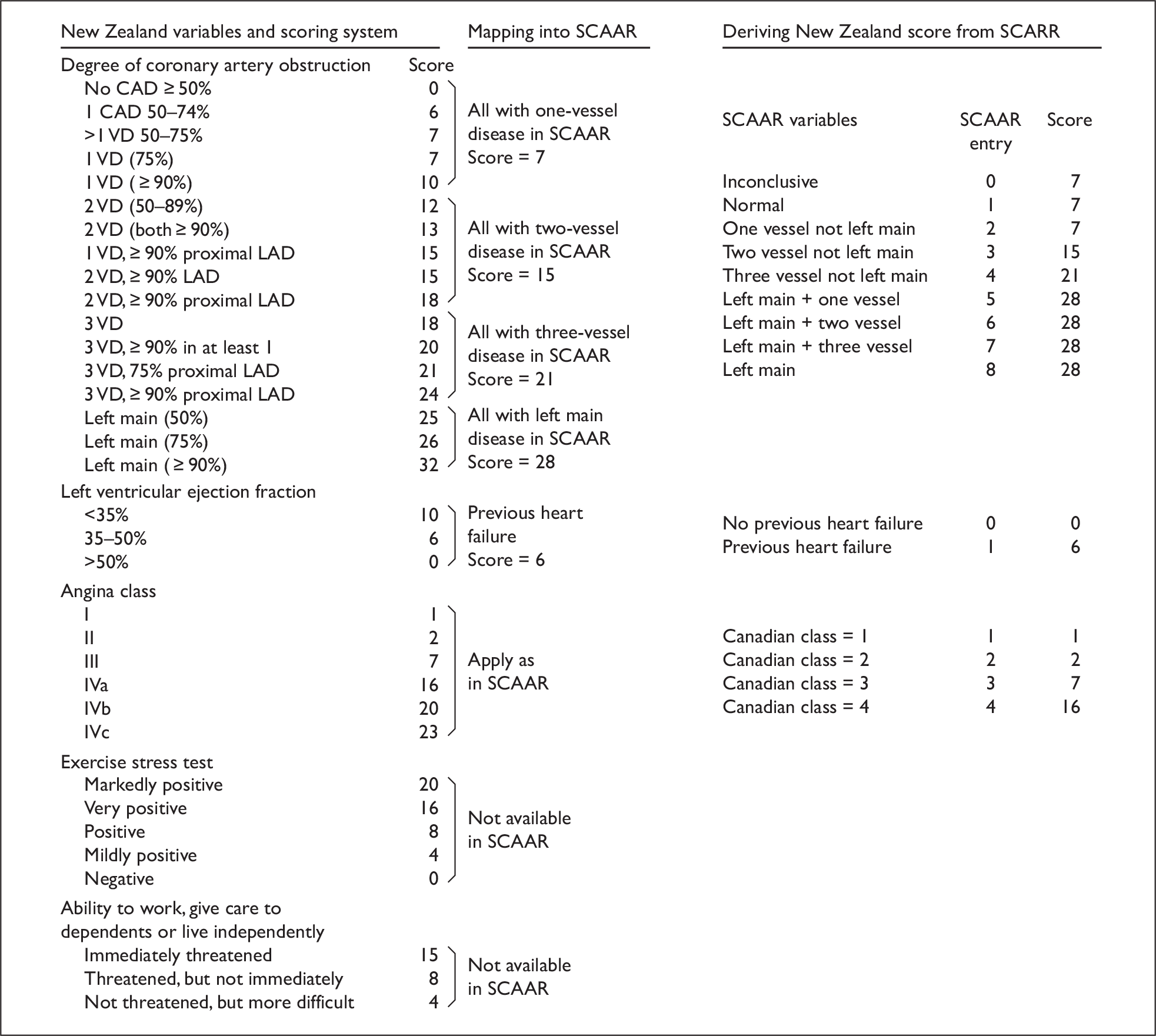
Risk score without biomarkers
With this strategy, patients were prioritised according to their predicted risk of cardiovascular events while on the waiting list for CABG. To implement this strategy in the representative cohort, the risk estimated by the time-to-event model used to derive the probability of transition 1 was used. A daily rate of the composite end point was derived for each patient in the representative cohort. With this strategy the information from biomarkers was not included in the equation. Patients within the representative cohort with the highest estimated risk of the composite end point were prioritised first within this strategy.
Risk score with biomarkers
This prioritisation approach is implemented in a similar way to the use of a risk score without biomarkers. The only difference with this set of strategies is that the additional prognostic information provided by the biomarkers is included in the risk equation for estimating transition 1. As stated earlier in this section, the three strategies considered within this general approach were: (1) adding a single, routinely available biomarker to the risk prediction equation (eGFR); (2) adding a single, novel biomarker (CRP); and (3) adding both CRP and eGFR to the risk equation. As with the approach to prioritisation without biomarker information, patients with the highest predicted risk (including the additional prognostic information provided by the biomarkers) of a composite event were prioritised first.
The additional prognostic value of the separate biomarker strategies was derived from the estimates of relative risk presented in Chapter 4. However, several additional steps were subsequently required in order to implement these results within the decision model itself. These additional steps were required to address two specific issues that prevented these results being incorporated directly into the proposed decision model framework and into the proposed risk equations.
The first issue represents the need to consider the prognostic effect of the biomarkers, represented by the other covariates within the risk equation. Hence, a set of adjustment factors was required to account for the changes to the regression coefficients of the other covariates included in the risk equations when the biomarker covariates were added. The second issue concerns the absence of CRP data within the SCAAR registry. Hence, in order to implement a prioritisation strategy incorporating this biomarker, it was necessary to impute CRP levels for patients in the representative cohort based on the covariate data that were available within the SCAAR registry. Both of these issues required access to an additional data set to address these issues and to provide appropriate estimates to populate the final model.
Methods of incorporating biomarker information within the decision model
An additional data set (St George’s Hospital, London, Principal Investigator Kaski) was obtained in order to generate the adjustment factors and to impute CRP values in the representative cohort derived from the SCAAR registry. St George’s data set consists of 643 patients with chronic stable angina undergoing coronary angiography at St George’s Hospital, London, in whom CRP was measured along with the same covariates as are available in the SCAAR registry. Patients in this data set were followed up for mortality over 7 years. Details of the St George’s data set have been previously published. 203 The St George’s data did not include waiting list information or date of CABG receipt and thus were not suitable for running the decision model.
Adjustment factors
Further to the risk equations estimated from SCAAR data, the impact of biomarker information on the risk of the composite end point was added. These estimates were obtained from the systematic review outlined in Chapter 3. Integrating the literature-based estimates of the impact of biomarker information on the composite end point with the time-to-event model developed from the SCAAR data may potentially influence the parameter estimates in the time-to-event model estimated from SCAAR data. To account for this, adjustment factors were employed. 204,205
Using the St George’s data set, adjustment factors were calculated by fitting parametric proportional hazards regression models using a constant baseline hazard function, i.e. assuming that time to an event followed an exponential distribution, with and without one or more novel biomarkers included as a three-level categorical variable, with levels representing tertiles, with the exception of eGFR which was included as a binary covariate using a cut-off of 60 ml/min. Thus, equation [1] estimates the hazard ratios, i.e. exp(β1), …, exp(βp) associated with p covariates, while equation [2] estimates the hazard ratios, i.e. exp(β1*), …, exp(βp*) associated with the same p covariates when a biomarker, BM1 is included in the model.
The adjustment factors, α0,…, αp, for the p covariates and the baseline hazard, are then calculated by equation [3].
The hazard ratios obtained from fitting the corresponding model in SCAAR (without a biomarker) were then adjusted, when the relative risks from the meta-analysis in Chapter 3 were applied, by exp(α0), …, exp(αp).
Imputation of C-reactive protein in SCAAR
As CRP was not available in the SCAAR registry, the level of CRP of patients in the representative cohort had to be imputed. The St George’s data set was used to develop a prediction model using covariates common to both St George’s and the SCAAR registry. An ordinal logistic model was used to estimate the tertiles of CRP. 206 Applying this model, the following cumulative odds can be defined:
which can then be modelled in terms of q covariates as in equation [4].
Hence, after estimating the parameters of equation [4], the cumulative probabilities of a specific patient being in either the first or the first or second tertile are given by:
Thus, the probabilities of a specific patient having a CRP value within each tertile are given by:
The meta-analysis outlined in Chapter 3 estimates the relative risk of an event for a patient being in the second tertile compared with the first, RR1,2, and the relative risk of an event for a patient being in the third tertile compared with the first, RR1,3, using meta-analysis techniques.
Using equations [4], [5] and [6], the probability that each individual patient in the SCAAR registry is in each CRP tertile is estimated. Following this, the baseline risk for each individual patient of an event in each of the three tertiles is estimated, adjusted for the fact that CRP is now included in the model, thus:
Having estimated the hazard of an event (using CRP and clinical information) using equation [7] for a patient being in each of the tertiles, this hazard is then averaged according to the probability of a patient being in each tertile, to yield λ*, i.e. λ*=π1λ1+π2λ2+π3λ3. The averaged estimate is then used to rank patients in the representative cohort for the strategy using a risk score with CRP alone and the strategy using a risk score with both eGFR and CRP.
The transition probabilities needed to populate the Markov structure were derived from the results of the final exponential time-to-event model including the integrated biomarker information. The survivor function of the exponential distribution is given by S(t)=e−λt and thus the transition probability of a composite end point in Markov cycle t, tp(t), is given by tp(t)=1−exp(λ(t−1)−λt) [8]. When evaluating the decision model, transition probabilities were derived for each tertile of CRP, thus providing three estimates of costs and QALYs for each patient. These estimates were then averaged according to the probability of a patient being in each tertile to generate a cost and QALY estimate for a particular patient (operated on a particular day within the maximum waiting time).
Analysis of decision model
Several analytical steps are required in order to estimate the cost-effectiveness of the different prioritisation strategies according to the methods outlined above. In the first step, the statistical risk prediction equations are populated based on the SCAAR registry data, with the results of the systematic review incorporated using the adjustment factors. The final risk equations represent our best prediction of clinical events based on the various data sources, and provide the basis for the subsequent transition probabilities applied in the model itself. It is important to note that the transition probabilities for each strategy are actually populated using the same risk equations representing: (1) the risk on the waiting list for CABG; (2) the procedural risk; and (3) the risk after the CABG procedure. The differences between strategies will actually be reflected in the different ordering of patients on the waiting list. This ordering determines the predicted time at which a given individual will ‘switch’ between the separate equations in the model for each separate strategy. The ordering of patients on the waiting list and how this varies by strategy comprises the next step.
In the second step of the analysis the prioritisation strategies are implemented in the representative cohort in order to assign each patient in the cohort a day of CABG with the alternative prioritisation strategies. The different approaches to prioritisation will imply a different ordering of the waiting list and hence a potentially different day on which the procedure would be undertaken. In the base-case analysis it is assumed that all patients in the representative cohort should have their procedure within 90 days (i.e. four procedures per day), representing the maximum waiting time being considered. The importance of the maximum waiting time itself on overall results of cost-effectiveness was subsequently investigated in alternative scenarios by decreasing the number of maximum days on the waiting list to 40 (i.e. nine procedures per day) and 15 days (i.e. 24 procedures per day). Regardless of which time period is chosen to represent the maximum waiting time, the ordering of the representative cohort will remain the same in each strategy.
In the third step of the analysis, costs and health outcomes are determined for each patient given the assigned day of CABG with the different prioritisation strategies. Hence, if a particular patient is assigned to undergo CABG at day 20 by a specific means of prioritisation, the costs and health outcomes for this particular patient are determined by running the decision-analytic model with the day of CABG set to 20 and applying the covariate pattern of this particular patient. This procedure is then repeated for all patients in the representative cohort and for all prioritisation strategies. It should be noted that alternative prioritisation strategies will differ in terms of the different assigned days of CABG and thus different estimates of the subsequent costs and health outcomes for the same patient. However, given that the same risk equations are employed for each strategy, if the timing of receipt of CABG for a given individual is the same for particular strategies, then the subsequent estimates of lifetime costs and QALYs will be identical. The overall cost-effectiveness of the prioritisation strategies is subsequently determined by averaging the costs and health outcomes across patients in the representative cohort for each prioritisation strategy and evaluating the associated ICERs.
Chapter 6 Results of decision model
The results of the decision model are presented as follows: (1) the final risk equations and the associated set of transition probabilities; (2) the impact of the different prioritisation strategies in terms of the actual ordering of the representative cohort within the waiting list for CABG; and (3) the overall costs and health outcomes of the alternative prioritisation strategies and their relative cost-effectiveness.
Final risk equations
Risk on the waiting list
The observed events and estimated hazard ratios while on the waiting list for CABG are shown in Table 11. Age, heart failure, diabetes and previous stroke were all associated with a statistically significant elevated risk of the composite end point. Previous myocardial infarction and left main vessel disease and/or three-vessel disease were very close to statistical significance at the 5% level and were retained in the time-to-event model. The hazard ratios of biomarker information (CRP and eGFR), estimated from the systematic review and meta-analysis, are also shown in Table 11. In the last column of the table, the adjusted hazard ratios are presented.
| Eventsa | Number of events/total number of patients | Hazard ratio | 95% CI | Adjusted hazard ratio |
|---|---|---|---|---|
| Dead | 83/9935 | |||
| Myocardial infarction | 84/9935 | |||
| Stroke | 30/9935 | |||
| Dead, myocardial infarction or stroke | 184/9935 | |||
| Variables in survival model | Number of patients with characteristic/total number of patientsb | |||
| Age (per year) | 66.03 | 1.05 | 1.03 to 1.06 | 1.04 |
| Heart failure | 816/9935 | 2.43 | 1.69 to 3.50 | 2.45 |
| Previous myocardial infarction | 2947/9935 | 1.32 | 0.97 to 1.80 | 1.29 |
| Diabetes | 1432/9935 | 1.57 | 1.11 to 2.23 | 1.56 |
| Previous stroke | 598/9935 | 1.85 | 1.21 to 2.83 | 1.89 |
| Left main or three-vessel disease | 7801/9935 | 1.51 | 0.99 to 2.31 | 1.51 |
| CRP 2nd tertile | 1.40 | 1.33 to 1.47 | 1.40 | |
| CRP 3rd tertile | 1.96 | 1.76 to 2.17 | 1.96 | |
| eGFR | 2.00 | 1.65 to 2.42 | 2.00 |
This is the final equation applied when determining transition probabilities for the decision-analytic model. The equation also provides the basis for the ordering of patients using the prioritisation strategy based on absolute clinical risk with biomarkers. The unadjusted equation in the first column, without the biomarker coefficients, was used when ordering patients for the prioritisation strategy based on absolute risk without biomarkers.
It should be noted that the assumption of a constant hazard within the exponential distribution was tested by employing an alternative distribution (Weibull) for this time-to-event model in order to investigate whether there was indication of a changing hazard from time of the decision to perform CABG to the censoring date. The separate analysis employing a Weibull distribution did not support a time-dependent hazard function, thus providing additional justification for the distributional assumption made.
Procedural risk
The number of procedural events and estimated procedural risk are shown in Table 12. The covariates included in the ‘waiting list model’ described above were also significant in the logistic model estimated to predict procedural risk associated with CABG. It should be noted that the results in Table 12 are based on all patients included in the ‘waiting list model’ who went on to have CABG regardless of the time from the decision to CABG to the actual procedure. Of all procedures included in the analysis, 77% were performed within 90 days of the decision to perform CABG. The proportion of patients in this group suffering a composite end point was 5.1% compared with 6.1% for those patients having the procedure more than 90 days after the decision to perform CABG. The overall estimate based on the events reported in Table 12 was 5.4%.
| Eventsa | Number of events/total number of patients | Odds ratio | 95% CI |
|---|---|---|---|
| Dead | 90/7375 | ||
| Myocardial infarction | 224/7375 | ||
| Stroke | 106/7375 | ||
| Dead, myocardial infarction or stroke | 395/7375 | ||
| Variables in logistic model | Number of patients with characteristic/total number of patientsb | ||
| Age (per year) | 65.71 | 1.04 | 1.02 to 1.05 |
| Heart failure | 554/7375 | 1.82 | 1.35 to 2.44 |
| Previous myocardial infarction | 2124/7375 | 1.52 | 1.22 to 1.89 |
| Diabetes | 1015/7375 | 2.00 | 1.56 to 2.56 |
| Previous stroke | 422/7375 | 2.14 | 1.55 to 2.95 |
| Left main or three-vessel disease | 5768/7375 | 1.62 | 1.20 to 2.18 |
Risk after coronary artery bypass grafting
The observed events and estimated hazard ratios after the CABG procedure are shown in Table 13. All covariates included in the ‘waiting list model’ were statistically significant at the 5% level, with the exception of previous myocardial infarction. However, previous myocardial infarction was very close to statistical significance and was retained in the time-to-event model. As for the ‘waiting list model’, the hazard ratios of biomarker information (CRP and eGFR), as estimated from the meta-analysis, were integrated in this model. In the last column of the table, the adjusted hazard ratios are presented. This is the final equation applied when determining transition probabilities for the decision-analytic model.
| Eventsa | Number of events/total number of patients | Hazard ratio | 95% CI | Adjusted hazard ratio |
|---|---|---|---|---|
| Dead | 478/6980 | |||
| Myocardial infarction | 137/6980 | |||
| Stroke | 161/6980 | |||
| Dead, myocardial infarction or stroke | 680/6980 | |||
| Variables in survival analysis | Number of patients with characteristic/total number of patientsb | |||
| Age (per year) | 65.55 | 1.05 | 1.04 to 1.06 | 1.05 |
| Heart failure | 485/6980 | 2.23 | 1.81 to 2.75 | 2.25 |
| Previous myocardial infarction | 1957/6980 | 1.15 | 0.98 to 1.36 | 1.13 |
| Diabetes | 912/6980 | 1.68 | 1.39 to 2.03 | 1.67 |
| Previous stroke | 372/6980 | 2.07 | 1.63 to 2.62 | 2.11 |
| Left main or three-vessel disease | 5426/6980 | 1.22 | 1.00 to 1.49 | 1.22 |
| CRP 2nd tertile | 1.40 | 1.33 to 1.47 | 1.40 | |
| CRP 3rd tertile | 1.96 | 1.76 to 2.17 | 1.96 | |
| eGFR | 2.00 | 1.65 to 2.42 | 2.00 |
A separate analysis was undertaken, employing a Weibull distribution in order to investigate whether the assumption of a constant hazard, from the starting time of the analysis to the censoring date, was appropriate. The analysis indicated that the assumption of a constant hazard was appropriate.
Transition probabilities
Transition probabilities to be applied in the decision-analytic model were derived from the equations presented in Chapter 5. These were estimated for each individual in the representative cohort. For illustrative purposes, the estimated transition probabilities for patients with selected baseline characteristics and risk factors are shown in Table 14.
| Patient characteristics | Waiting list before CABGa | CABGb | After CABGc | ||||
|---|---|---|---|---|---|---|---|
| No event | Post MI | Post stroke | No event | Post MI | Post stroke | ||
| 65 years, male | 0.00008 | 0.00010 | 0.00015 | 0.0229 | 0.009 | 0.010 | 0.019 |
| 55 years, male, heart failure, previous MI | 0.00017 | 0.00021 | 0.00031 | 0.0439 | 0.014 | 0.016 | 0.029 |
| 65 years, male, heart failure, previous MI | 0.00025 | 0.00033 | 0.00048 | 0.061 | 0.023 | 0.026 | 0.048 |
| 55 years, male, diabetes, heart failure, previous MI, main-vessel and/or three-vessel disease | 0.00039 | 0.00050 | 0.00074 | 0.129 | 0.028 | 0.032 | 0.058 |
| 65 years, male, diabetes, heart failure, previous MI, main-vessel and/or three-vessel disease | 0.00060 | 0.00078 | 0.00114 | 0.173 | 0.046 | 0.053 | 0.095 |
In Table 15, the number of composite end points being fatal, non-fatal myocardial infarction and non-fatal stroke are shown together with the probabilities applied for this transition in the decision-analytic model.
| Waiting list | CABG procedure | After CABG | ||||
|---|---|---|---|---|---|---|
| Number of events | Probability | Number of events | Probability | Number of events | Probability | |
| Non-fatal stroke | 26 | 0.14 | 100 | 0.25 | 152 | 0.22 |
| Non-fatal myocardial infarction | 83 | 0.45 | 222 | 0.56 | 130 | 0.19 |
| Death | 75 | 0.41 | 73 | 0.19 | 398 | 0.59 |
| Total | 184 | 1 | 395 | 1 | 680 | 1 |
Implementation of strategies and impact on the ordering of the waiting list
As previously described, the value of the formal prioritisation strategies will be determined by the degree to which they alter the subsequent ordering of individual subjects in terms of their position on a waiting list based on no formal prioritisation and, in turn, whether the different ordering results in meaningful improvements in terms of subsequent long-term health outcomes and costs.
The derived scores by the alternative means of prioritisation and assigned day of CABG for 12 selected patients in the representative cohort are shown in Table 16. The derived scores are the result of implementing the prioritisation strategies on the patients in the cohort using the approaches outlined in Chapter 5. The scores are then used to rank patients from highest to lowest risk, where the patients with higher risk are prioritised to receive CABG at an earlier time than lower risk patients. The position on the waiting list and hence the timing of the procedure are illustrated by the assigned day of CABG reported in the table. In the event that a prioritisation strategy produced the same score for patients in the representative cohort, then the subsequent ranking within these clusters of patients was assigned randomly. Hence, randomness is more pertinent in the somewhat crudely implemented Ontario and New Zealand urgency scores (as indicated by the scores in columns three and four of the table). It should be noted that for the strategy of no formal prioritisation, the derived ‘score’ is simply the actual number of days on the waiting list that particular patient actually experienced in the representative cohort.
| Patient | Derived scores for prioritisation | Assigned day of CABG | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clin pract | Urg Ont | Urg NZ | Risk no biom | Risk CRP | Risk eGFR | Risk eGFR + CRP | Clin pract | Urg Ont | Urg NZ | Risk no biom | Risk CRP | Risk eGFR | Risk eGFR + CRP | |
| 1 | 10 | 6.15 | 23 | 0.000508 | 0.000426 | 0.000301 | 0.000426 | 6 | 63 | 58 | 7 | 11 | 15 | 18 |
| 2 | 46 | 5.40 | 30 | 0.000197 | 0.000188 | 0.000122 | 0.000188 | 55 | 25 | 23 | 57 | 56 | 59 | 55 |
| 3 | 29 | 6.15 | 23 | 0.000121 | 0.000119 | 0.000076 | 0.000119 | 34 | 61 | 63 | 80 | 78 | 81 | 78 |
| 4 | 22 | 6.15 | 23 | 0.000165 | 0.000162 | 0.000103 | 0.000162 | 22 | 52 | 59 | 67 | 65 | 67 | 65 |
| 5 | 42 | 6.15 | 23 | 0.000216 | 0.000224 | 0.000267 | 0.000447 | 53 | 51 | 70 | 49 | 43 | 18 | 16 |
| 6 | 24 | 6.15 | 23 | 0.000260 | 0.000233 | 0.000161 | 0.000233 | 26 | 49 | 69 | 38 | 40 | 39 | 43 |
| 7 | 34 | 6.80 | 17 | 0.000109 | 0.000099 | 0.000068 | 0.000099 | 43 | 85 | 86 | 84 | 83 | 84 | 84 |
| 8 | 60 | 6.00 | 28 | 0.000258 | 0.000266 | 0.000317 | 0.000532 | 65 | 45 | 42 | 40 | 33 | 13 | 12 |
| 9 | 42 | 6.15 | 23 | 0.000197 | 0.000188 | 0.000245 | 0.000376 | 52 | 59 | 70 | 56 | 55 | 21 | 23 |
| 10 | 70 | 6.15 | 23 | 0.000236 | 0.000189 | 0.000145 | 0.000189 | 69 | 69 | 64 | 44 | 54 | 46 | 55 |
| 11 | 35 | 6.15 | 23 | 0.000151 | 0.000130 | 0.000094 | 0.000130 | 46 | 65 | 54 | 71 | 75 | 72 | 75 |
| 12 | 27 | 6.15 | 23 | 0.000270 | 0.000289 | 0.000165 | 0.000289 | 30 | 53 | 55 | 35 | 28 | 37 | 33 |
The decision model is then used to estimate the resulting differences in costs and QALYs obtained through the different orderings of the waiting list for each individual strategy.
Cost-effectiveness
To estimate lifetime costs and QALYs, the model is run for a period of 60 cycles (equivalent to 60 years), after which the vast majority of patients will have died in the model. Therefore, the mean QALYs per patient can be calculated for each strategy, as well as the mean lifetime costs. With the assigned day of CABG (as illustrated in Table 16), the cost and health outcomes with each prioritisation strategy for each individual patient in the representative cohort were estimated. The costs and health outcomes across the individual patients in the cohort for each prioritisation strategy were then averaged to obtain a mean (per patient) estimate of costs and QALYs, and the relative cost-effectiveness of the different strategies was then estimated.
The results of the cost-effectiveness analysis are presented in two ways. Firstly, mean costs and QALYs for the various comparators are presented. Secondly, the cost-effectiveness of the different strategies is compared using standard decision rules, estimating ICERs as appropriate. 207 The ICER examines the additional costs that one strategy incurs over another and compares this with the additional benefits. The ICER estimate reports the additional cost required to generate one additional unit of health outcome (QALY). When more than two strategies are being compared, the ICERs are calculated using the following process:
-
The strategies are ranked in terms of mean QALYs (from the least effective to the most effective).
-
If a strategy is more expensive and less effective than any previous strategy, then this strategy is said to be dominated and is excluded from the calculation of the ICERs.
-
The ICERs are calculated for each successive alternative, from the least effective to the most effective. If the ICER for a given strategy is higher than that of any more effective strategy, then this strategy is ruled out on the basis of extended dominance.
-
Finally, the ICERs are recalculated, excluding any strategies that are ruled out by principles of dominance or extended dominance.
The resulting ICERs then provide the basis for establishing which strategy appears optimal based on cost-effectiveness considerations. That is, which strategy (or strategies) appears to provide good value for money to the NHS. Guidance from NICE suggests that an incremental cost per additional QALY of around £20,000–30,000 is considered to represent an appropriate threshold to establish value for money to the NHS.
The model was run several times, once for the main base-case analysis and then for a number of alternative scenarios to consider alternative assumptions related to key aspects of the base-case approach.
Base-case analysis
Table 17 reports the results of the mean lifetime costs, life-years, QALYs and ICERs for the base-case analysis using a 3-month (90-day) maximum waiting time for CABG. Mean estimates of the lifetime costs and QALYs are reported in detail for each strategy, together with the associated ICER estimates for non-dominated strategies.
| Strategy | 90-day maximum waiting time | |||
|---|---|---|---|---|
| Cost (£) | Life-year | QALY | ICER (£)a | |
| No formal prioritisation | 16099.77 | 11.6611 | 8.2796 | |
| Ontario urgency score | 16100.00 | 11.6646 | 8.2822 | 88 |
| New Zealand urgency score | 16100.87 | 11.6663 | 8.2835 | ED |
| Risk score without biomarker | 16101.98 | 11.6713 | 8.2871 | ED |
| Risk score with CRP | 16107.99 | 11.6714 | 8.2872 | D |
| Risk score with eGFR | 16102.22 | 11.6721 | 8.2877 | 405 |
| Risk score with CRP + eGFR | 16108.19 | 11.6723 | 8.2878 | 57,842 |
Each of the alternative formal prioritisation strategies appears more costly and more effective than no formal prioritisation. Therefore, the comparison of ICERs is an important consideration. Applying the decision rules outlined in the previous section, one prioritisation strategy is ruled out based on dominance considerations and hence excluded from the final ICER estimates. The strategy of using a risk score with CRP is dominated by the risk score employing eGFR. This means that the single novel biomarker strategy is associated with additional costs (principally the additional £6 cost of the biomarker itself) and the prognostic information based on this strategy appears less informative than that based on a strategy incorporating the routine biomarker. Furthermore, the prioritisation strategies using a risk score without biomarker information and New Zealand urgency score are extendedly dominated by the strategy based on a risk score with eGFR.
Hence, of the seven initial strategies considered, four remain after dominance considerations. These four strategies provide the basis for the final ICER estimates in the base-case analysis. No formal prioritisation is associated with the lowest mean cost and QALY estimates. Given that this is the least effective (and non-dominated) strategy considered, this provides the initial reference point for the subsequent ICER estimates. Compared with no formal prioritisation, the strategy with Ontario urgency score is more effective and more costly. The ICER of a strategy based on Ontario urgency score compared with no formal prioritisation is £88 per additional QALY. As this is below the threshold used to establish cost-effectiveness, the next consideration is whether the additional costs and health outcomes generated by the next non-dominated strategy are cost-effective. This comparison is now made against the Ontario urgency score. That is, as it has been established that a risk equation with Ontario urgency score is potentially cost-effective, the relevant question becomes whether the remaining strategy is cost-effective with reference to this strategy? The ICER of a risk score with eGFR compared with Ontario urgency score is £405 per additional QALY. Given that this is also below the threshold used to establish cost-effectiveness, it has to be considered whether the remaining strategy of a risk score with CRP and eGFR is cost-effective compared with a risk score with eGFR alone. The ICER for this comparison is £57,842 per additional QALY.
Applying a threshold of between £20,000 and £30,000 per QALY, a strategy employing a risk score with both CRP and eGFR cannot be considered cost-effective. In contrast, the most effective prioritisation strategy with an ICER below the threshold is a risk score based on the routinely collected biomarker eGFR. This indicates that a risk score with eGFR is the most cost-effective prioritisation strategy.
The increasing ICER estimates between the single and combination biomarker strategies clearly demonstrate that while additional predictive information is informative in terms of deriving more precise estimation of a patient’s individual risk, the actual value of this increased precision will ultimately be determined by the difference this information then makes to the ordering of a waiting list (and hence to the resulting estimates of costs and QALYs). This is most evident in the comparison between the strategies based on a risk score with eGFR alone and a risk score with both eGFR and CRP. While the latter risk score provides a more precise estimate of an individual’s predicted risk, the resulting difference in outcomes between the strategies is 0.0001 QALYs. Putting this into context, this is equivalent to a mean, per patient, gain of 0.04 days of perfect health over a patient’s lifetime. Hence, although the additional cost imposed by the use of a novel biomarker appears relatively minor (£6), the subsequent impact in terms of the ordering of the waiting list and on longer-term costs and QALYs appears marginal. Consequently, when the additional costs of such a strategy are compared with the additional predicted gain in QALYs, the resulting ratio of costs to benefits leads to an ICER of approximately £58,000 per QALY.
Separate analyses were also undertaken to examine the impact of the uncertainty in the relative effectiveness estimates for biomarkers reported in Chapter 4, as well as variation that may exist in relation to the cost of the biomarkers themselves.
Sensitivity analyses
Cost-effectiveness results comparing alternative maximum waiting times
Table 18 compares the results of the mean lifetime costs, life-years, QALYs and ICERs for the base-case analysis using a 3-month (90-day) maximum waiting time for CABG, with similar estimates based on maximum waiting times of 40 days and 15 days. In each of these analyses, the same strategies are ruled out on the grounds of dominance and extended dominance. Interestingly, the ICER for a strategy of using a risk score with eGFR remains remarkably stable and well below the conventional threshold of value for money in the NHS.
| Strategy | 90-day maximum waiting time | 40-day maximum waiting time | 15-day maximum waiting time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Life-years | QALY | ICERa | Cost (£) | Life-years | QALY | ICERa | Cost (£) | Life-years | QALY | ICERa | |
| No formal prioritisation | 16099.77 | 11.6611 | 8.2796 | 16095.47 | 11.6845 | 8.2973 | 16093.22 | 11.6963 | 8.3062 | |||
| Ontario urgency score | 16100.00 | 11.6646 | 8.2822 | 88 | 16095.53 | 11.6861 | 8.2984 | 55 | 16093.24 | 11.6969 | 8.3066 | 31 |
| New Zealand urgency score | 16100.87 | 11.6663 | 8.2835 | ED | 16095.91 | 11.6868 | 8.2990 | ED | 16093.38 | 11.6972 | 8.3068 | ED |
| Risk score without biomarker | 16101.98 | 11.6713 | 8.2871 | ED | 16096.37 | 11.6891 | 8.3006 | ED | 16093.53 | 11.6980 | 8.3074 | ED |
| Risk score with CRP | 16107.99 | 11.6714 | 8.2872 | D | 16102.37 | 11.6891 | 8.3007 | D | 16099.54 | 11.6980 | 8.3074 | D |
| Risk score with eGFR | 16102.22 | 11.6721 | 8.2877 | 405 | 16096.47 | 11.6894 | 8.3009 | 380 | 16093.57 | 11.6981 | 8.3075 | 362 |
| Risk score with CRP + eGFR | 16108.19 | 11.6723 | 8.2878 | 57,842 | 16102.46 | 11.6895 | 8.3009 | 133,287 | 16099.57 | 11.6982 | 8.3075 | 374,371 |
Cost-effectiveness results comparing alternative relative risk estimates for biomarkers
The results of the systematic review of the clinical effectiveness of circulating biomarkers demonstrated a relatively high level of uncertainty surrounding the estimated hazard ratios representing the prognostic importance of the various biomarkers. The results from the base-case analysis are based on the mean estimates of the hazard ratios. In order to examine the robustness of the cost-effectiveness results to this source of uncertainty, additional scenarios were considered based on the lower and upper bounds of the 95% CIs for the estimates of the hazard ratio associated with the biomarker information. The results of these analyses are shown in Tables 19 and 20 respectively, presented in the context of maximum waiting times of 90, 40 and 15 days.
| Strategy | 90-day maximum waiting time | 40-day maximum waiting time | 15-day maximum waiting time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALY | ICERa | Cost (£) | QALY | ICERa | Cost (£) | QALY | ICERa | |
| No formal prioritisation | 16125.44 | 8.4148 | 16120.43 | 8.4309 | D | 16117.85 | 8.4390 | D | |
| Ontario urgency score | 16125.44 | 8.4172 | 0.4 | 16120.40 | 8.4320 | 16117.83 | 8.4394 | ||
| New Zealand urgency score | 16126.08 | 8.4183 | ED | 16120.67 | 8.4325 | ED | 16117.93 | 8.4396 | ED |
| Risk score without biomarker | 16126.71 | 8.4213 | ED | 16120.93 | 8.4338 | ED | 16118.02 | 8.4401 | ED |
| Risk score with CRP | 16132.71 | 8.4214 | D | 16126.93 | 8.4339 | D | 16124.02 | 8.4401 | D |
| Risk score with eGFR | 16126.80 | 8.4216 | 306 | 16120.97 | 8.4340 | 285 | 16118.03 | 8.4402 | 268 |
| Risk score with CRP + eGFR | 16132.78 | 8.4217 | 78,915 | 16126.96 | 8.4340 | 165,707 | 16124.03 | 8.4402 | 552,300 |
| Strategy | 90-day maximum waiting time | 40-day maximum waiting time | 15-day maximum waiting time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALY | ICERa | Cost (£) | QALY | ICERa | Cost (£) | QALY | ICERa | |
| No formal prioritisation | 16064.57 | 8.1424 | 16061.23 | 8.1616 | 16059.44 | 8.1713 | |||
| Ontario urgency score | 16065.09 | 8.1452 | 182 | 16061.41 | 8.1629 | 141 | 16059.49 | 8.1718 | 111 |
| New Zealand urgency score | 16066.22 | 8.1466 | ED | 16061.90 | 8.1635 | ED | 16059.67 | 8.1720 | ED |
| Risk score without biomarker | 16067.96 | 8.1508 | ED | 16062.63 | 8.1654 | ED | 16059.93 | 8.1727 | ED |
| Risk score with CRP | 16073.98 | 8.1510 | D | 16068.63 | 8.1655 | D | 16065.93 | 8.1728 | D |
| Risk score with eGFR | 16068.44 | 8.1518 | 511 | 16062.83 | 8.1658 | 480 | 16060.00 | 8.1729 | 460 |
| Risk score with CRP + eGFR | 16074.42 | 8.1519 | 39,111 | 16068.83 | 8.1659 | 81,830 | 16066.00 | 8.1729 | 229,083 |
Table 19 shows the results when the biomarker coefficients in the risk equations are set to the lower 95% CI value (i.e. biomarkers carrying less information). This approach results in a decrease in the absolute risk of clinical events for all patients regardless of the prioritisation strategy employed, relative to those applied in the base-case analysis. Furthermore, a strategy of no formal prioritisation is now dominated by the strategy of Ontario urgency score. Hence, Ontario urgency score is now the least effective (and non-dominated) strategy considered, and provides the initial reference point for the subsequent ICER estimates. The ICER comparing a risk score using eGFR with the Ontario urgency is £306 per additional QALY. The ICER of a risk score with CRP and eGFR compared with a risk score using eGFR alone was well above the threshold of value for money in the NHS (£79,000–552,000 for different maximum waiting times). Hence, even when eGFR carries less information than in the base-case scenario, this prioritisation strategy was associated with the highest ICER below the NHS threshold, and is still the most cost-effective strategy. It should be noted that the meta-analysis showed evidence of small study (publication) bias and incomplete adjustment for simple clinical information such as age, sex, smoking, diabetes, and obesity, suggesting that the estimated coefficients for biomarkers may be lower than those reported in the base-case meta-analysis. Hence, the lower bound of 95% CI is more likely to be closer to the true biomarker effect than the point estimate used in the base-case estimates; our conclusions regarding cost-effectiveness are unlikely to change with such a scenario.
In Table 20, the results of setting all biomarker coefficients in the risk equation to their upper 95% CI value are presented. This scenario results in an associated increase in the absolute risk of clinical events for all patients regardless of the prioritisation strategy employed, resulting in a reduction in the mean QALY estimates for all strategies compared with the base-case analysis. With this scenario, the results are similar to the results of the base-case scenario. The estimates of the ICER for the risk score with CRP and eGFR, compared with the risk score with eGFR alone, are more favourable in this scenario. However, using a 90-day maximum waiting time the ICER is £39,000, which is still above the threshold level of £20,000–30,000 per QALY. Thus, a prioritisation strategy with a risk score using both CRP and eGFR is unlikely to be cost-effective.
In Figure 6, the difference in the assigned day of CABG between non-dominated prioritisation strategies is plotted for the three different maximum waiting times. Hence, the figure illustrates the degree to which a prioritisation strategy actually alters, when compared with a relevant comparator, the subsequent ordering of individual subjects in terms of their position on a waiting list. Furthermore, the ICERs for the relevant comparisons are given, illustrating the link between different ordering of the waiting list and cost-effectiveness. In Figure 6 it is demonstrated that adding the novel circulating biomarker CRP to the routinely measured eGFR has little scope for improved effectiveness (changing day of CABG) with 90-day maximum waiting times, and almost none for shorter maximum waiting times.
FIGURE 6.
Difference in assigned day of CABG and ICERs for non-dominated prioritisation strategies. Each dot represents a patient in the representative cohort and shows the change in assigned day of CABG for the particular comparison. Comparing Ontario urgency score with no formal prioritisation employing a 90-day maximum waiting time (top left panel) resulted in 48.6% of patients undergoing operation on average 28.2 days later and 50.0% of patients undergoing operation on average 27.4 days sooner. Comparing a risk score with eGFR and CRP with a risk score using eGFR alone employing a 15-day maximum waiting time (bottom right panel) resulted in 23.3% of patients undergoing operation on average 1.2 days later and 24.2% of patients undergoing operation on average 1.1 days sooner.
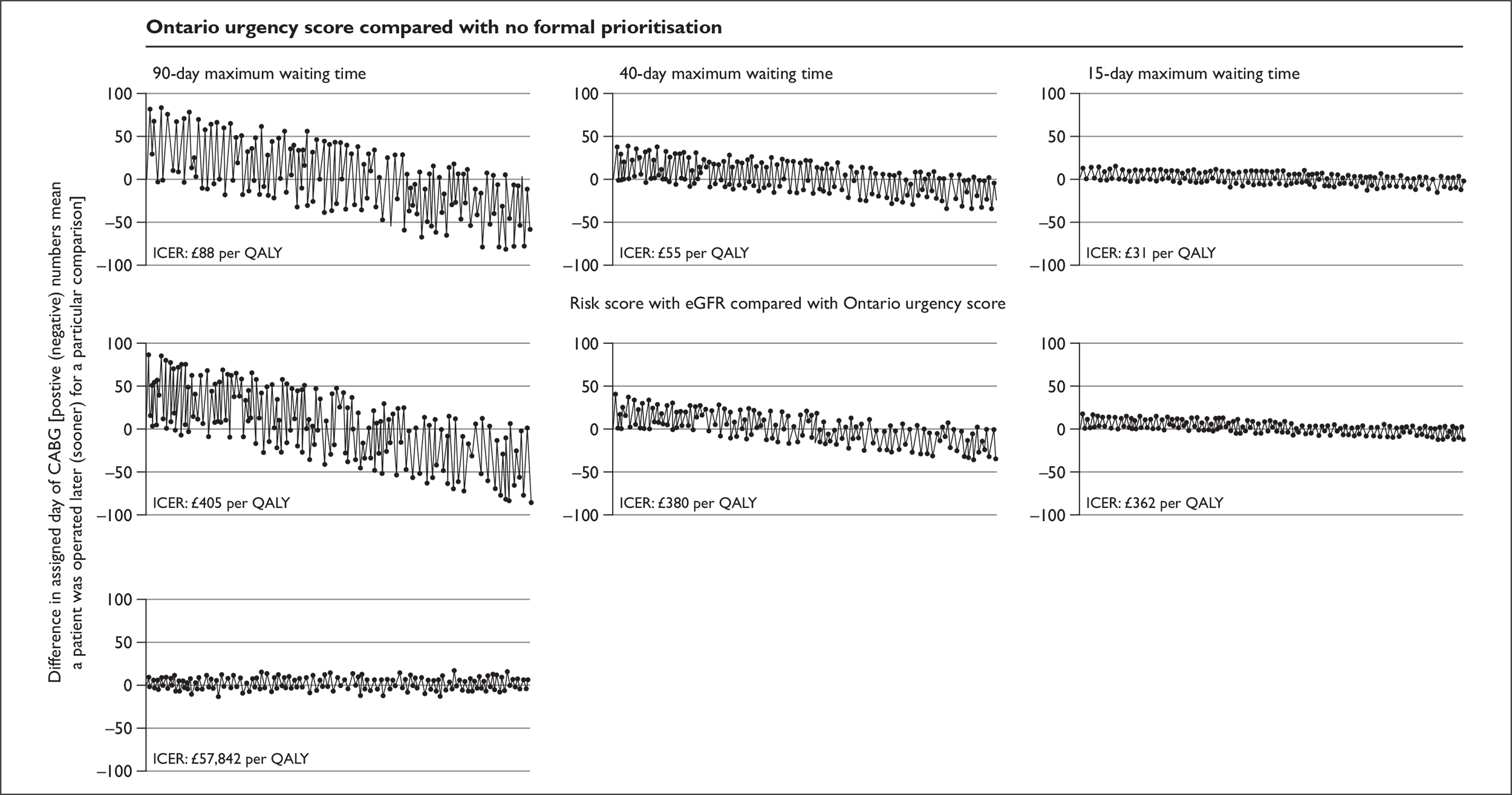
Cost-effectiveness results comparing alternative prices
The base-case results are based on a cost of £6 for CRP derived from the costs of the test in a research setting. However, this cost may vary depending upon the setting and may also differ if novel biomarkers become more widely used in the NHS. The potential robustness of the cost-effectiveness results to this issue is an important consideration. Clearly, if the costs of CRP are lower than £6, then the subsequent cost-effectiveness of a prioritisation approach employing a risk score with eGFR and CRP will become more favourable. Considering a 90-day waiting time, lowering the cost of CRP to £3 (£6 in the base-case analysis) reduces the ICER for the comparison of the risk score with eGFR and CRP with the risk score based on CRP alone to approximately £29,000 per QALY. At a cost of £2 for CRP, the subsequent ICER reduces to below the lower bound of the NICE threshold (approximately £19,000 per QALY). The cost-effectiveness of employing a risk score with eGFR and CRP is therefore somewhat sensitive to the cost of the test itself if the maximum waiting time is 90 days. Employing shorter waiting times, the cost of CRP has to be less than £1.30 for a strategy with eGFR and CRP to be cost-effective with a 40-day maximum waiting time (ICER approximately £22,000 with a CRP cost of £1) and below £0.5 with a 15-day maximum waiting time (ICER approximately £21,500 with a CRP cost of £0.35).
Organisation and training issues
A related issue also concerns the potential organisational and training implications (including costs) of using any formalised approach to prioritisation that has not been formally quantified here and would need to be considered against expected health gains. Given that the risk equations (including and excluding eGFR) are derived from routinely collected data, we have assumed that the opportunity cost of acquiring this information for the purposes of risk stratification of a waiting list would be zero. However, the subsequent application of this information within a formal risk scoring system could incur additional costs, as could the aforementioned organisational and training implications that would arise from a more systematic approach to prioritising waiting lists more generally. Equally, it should be recognised that while such changes may impose additional ‘up front’ costs to the NHS, at the level of an individual patient these costs may be negligible as such costs would ultimately be shared among the large number of patients who would then benefit from this approach. However, to more formally consider this issue and to explore the robustness of the base-case results to it, additional analyses were undertaken to identify the threshold cost at which the application of a formal prioritisation strategy would cease to become cost-effective compared with current practice. This analysis demonstrated that the per patient cost of implementing a risk equation incorporating eGFR would have to exceed £190 for the ICER estimate to exceed £30,000 per QALY. Hence, the results appear likely to be robust to this issue.
Other novel biomarkers not formally considered in decision model: the example of brain natriuretic peptide
Our results provide a provisional basis to consider the potential cost-effectiveness of the other (n = 7) novel biomarkers that were included in the systematic review, but were not formally considered in the model. The evaluation of the risk equations employing eGFR and one based on a combination of eGFR and CRP provides a suitable reference point to consider the necessary requirements that need to hold for an alternative novel biomarker to be cost-effective. The first requirement is that an alternative novel biomarker is both cheaper and at least as effective as CRP. However, as CRP is the cheapest of the novel biomarkers considered (see Table 1), this requirement is not met by any of the current alternatives to CRP. Thus, the second requirement is a more important consideration; an alternative and more expensive biomarker must provide comparatively greater gains in QALYs than those obtained using CRP to justify value for money. The summary results presented in Table 7 suggest that only BNP appears to meet this requirement as it may provide additional prognostic value compared with CRP (it has a higher point estimate of relative risk – 2.93). However, the cost of BNP is also markedly higher at £35. Consequently, the additional health gains that would be required to justify this additional cost compared with CRP will inevitably need to be markedly higher than those achieved using CRP. The results from the current model suggest that the gains in QALYs using BNP would need to be approximately 5.5 times greater than those obtained using CRP, to demonstrate value for money. Although it is not possible to directly link the relative risk estimates reported in Table 1 and subsequent QALY gains without formally modelling this strategy, the size of the relative risk estimates suggest that it is unlikely that BNP would be a cost-effective alternative to CRP.
Chapter 7 Discussion
Objective addressed
We developed a novel framework for evaluating the cost-effectiveness of formally incorporating biomarkers – routine, novel or both – into clinical decision-making. This framework evaluates methods of prioritising patients with respect to long-term costs and health outcomes. We found that a prioritisation strategy employing a single, routinely available biomarker (eGFR) appears cost-effective and robust to alternative assumptions, including variation in the maximum waiting list times. However, the additional costs and value of more precise information obtained from employing multiple biomarkers (e.g. eGFR and CRP) appears less clear and is unlikely to be cost-effective in the context of shorter waiting list times.
Systematic review of pooled relative risks
We report the first meta-analysis of any circulating biomarker in the prognosis of stable coronary disease, offering a synopsis of the field. Analysing 390 biomarker prognosis relative risks, we present a comparison of the strength of reported effects across a wide range of routinely recorded and novel biomarkers. The pooled relatives risks estimated from random effects models ranged from 2.00 (eGFR) to 1.96 (CRP) to 2.93 (BNP). However, these estimates are subject to a range of biases.
Systematic review of publication bias and missing studies
We found evidence that small studies were likely to report stronger effects, consistent with publication bias. Selective reporting within publications may also operate: those CRP results not mentioned in the abstract were less likely to be positive. More extreme effects were also observed among recently started studies, opposite to the widely observed situation where early literature tends to report inflated relative risks. 208 Subgroups of literature-based meta-analyses are unreliable means to explain such findings, and they should be seen as hypothesis generating.
Systematic review of the relative lack of evidence on routinely assessed biomarkers
Novel biomarkers contributed more studies than did routinely assessed markers. Thus, in the meta-analysis there were 77 studies available for CRP and four for haemoglobin. Biases may operate from funders of research, researchers and journal editors favouring enquiry into new markers at the expense of more robustly understanding the prognostic importance of widely performed measures. Further, the effects of novel markers are commonly not adjusted for routinely available biomarkers. Other routine markers fell outside our review, e.g. serum potassium, albumin, urate.
Systematic review of the relevance of the literature identified
We identified no studies in which a novel biomarker was related to prognosis among patients on the waiting list for CABG. Many studies reported only that patients had anatomical evidence of coronary artery disease but make no statement about the proportion of them with symptoms of angina or the severity of these symptoms. This reflects the lack of internationally agreed case definitions for what constitutes stable angina pectoris. This is of particular importance when considering the generalisability of findings to symptomatic patients awaiting CABG. Very few studies (with the possible exception of the European Concerted Action on Thrombosis and Disabilities study)209 were designed with the purpose of assessing the incremental prognostic value of biomarkers. No studies were identified that reported the incremental prognostic value of biomarkers in relation to a clearly defined clinical decision. Instead, the focus of many of the reports was assessing biological hypotheses such as plaque stability. Notwithstanding these caveats, the estimates lie within plausible ranges, consistent with those observed in healthy population (aetiologic) studies, which reported an effect of 1.5 for CRP41 and 1.41 for eGFR. 210
Systematic review of the quality of individual studies
Of those studies that were included, we identified concerns about the quality of reports of individual studies with potential for bias at each stage of population selection, biomarker measurement, rationale for inclusion of confounders, lack of primary outcome specification and post hoc analytical decisions over the choice of statistical model. Such potential biases have been identified in systematic reviews of cancer prognostic markers. 211,212 The extent and direction of these potential biases is not always clear but previous meta-analyses of observational studies of novel risk factors have suggested that they are likely to inflate estimates. Thus, more precise, less biased estimates of the relative risk would be hypothesised to be weaker than those observed.
Systematic review of the incremental prognostic value
A goal of novel biomarker estimation is to contribute information beyond that already provided by routinely measured markers. We found that the quantity and quality of adjustments for potential confounding factors in the systematic review was highly variable, and as few studies systematically adjusted for the routinely recorded factors known to relate to both CRP and outcome (including smoking, diabetes, obesity and lipids), residual confounding of the relative risks cannot be excluded. Thus, better use of information already obtained in clinical practice (i.e. at zero marginal cost) might contribute at least part of the prognostic information reported for CRP. No study assessed whether CRP might add discrimination to standard clinical factors in the prognostic risk scores for patients with angina developed in the ACTION17 and EuroAngina213 studies.
Angiography registry: SCAAR
The ideal registry in which to develop our decision-analytic model has several characteristics. It should identify large numbers of patients at the time of angiography and record details of the intention to perform CABG and baseline clinical information including biomarkers; be multicentre or national; reflect contemporary practice; and have follow-up for fatal and non-fatal events. No such registry exists in the UK. One of the few registries in the world that meets these criteria is SCAAR. 36 The Swedish angiography patients observed in SCAAR are likely to be generalisable to the UK because patient characteristics are similar to those reported in UK series. 214 Sweden has a similar health-care system (free at the point of use), and broadly comparable rates of coronary heart disease and rates of angiography and CABG. Sweden does differ from the UK in not having comparable ethnic minority populations; 14% of patients appropriate for CABG in the UK are reported as being of South Asian ethnicity. 215 Such ethnic differences are unlikely to significantly alter our conclusions because ethnicity is not associated with event rates nor with waiting time up to 90 days. 215 In Sweden, as in the UK, routine clinical practice involves informal, implicit prioritisation in which patients are given a qualitative order (urgent, semi-urgent and routine) after a joint clinical meeting between cardiologists and surgeons. However, there is a lack of national comparative research into the processes of current practice.
Smoking was not included in our risk model, despite its association with risk among patients with coronary disease,216 because it would serve to bring smokers forward in the queue. The contrary position – that smokers should be put to the back of the queue – which has attracted interest from media and ethicists is not addressed by our model. But our score could be used to investigate whether smokers are being given different priority and (according to viewpoint) discriminated against.
Cost-effectiveness: general methods
In order to assess the value of circulating biomarkers in prioritising CABG waiting lists, it is necessary to estimate their effect on health system costs and patients’ quality-adjusted life expectancies compared with other forms of prioritisation. It is then necessary to assess how any improvement in patients’ health compares with the health decrement associated with removing or reducing the use of other interventions or programmes elsewhere in the NHS to fund the use of biomarkers in this way (i.e. opportunity costs). The choice of all the relevant options for prioritising CABG waiting lists is critical in understanding the value of the specific strategies associated with biomarkers. The need to include routine clinical practice as an option is clear; although no formal prioritisation strategy may be used, decisions are inevitably taken about the order in which patients receive their procedure.
Urgency scores incorporate symptom severity which is an example of a (non-circulating) biomarker, which is routinely assessed. We also evaluated a strategy of ordering the CABG waiting list based on a risk prediction equation without biomarkers. Although formal use of risk scores is likely to be rare in routine practice, they essentially make explicit what clinicians would be expected to do routinely – assess the chance of a patient experiencing a fatal or severe non-fatal event while on the CABG waiting list. There are no risk prediction scores that are in widespread use in any aspect of the management of stable angina in general. Recently, two prognostic risk scores have been developed among patients with stable angina,17,217 but neither were suitable for use in the decision model because they were not developed in populations with severe coronary artery disease awaiting CABG; the scores did not assess 90-day risk (they assessed long-term risk over 1–5 years); and they used clinical covariates that are not routinely available (e.g. exercise electrocardiographic findings, left ventricular ejection fraction).
Changing the day of CABG is a surrogate of biomarker effectiveness
To assess the cost-effectiveness of circulating biomarkers, it is then necessary to establish their value, over and above routinely collected information incorporated into the risk equation, in predicting serious events.
To be able to demonstrate their cost-effectiveness in informing the prioritisation of patients on the CABG waiting list, it is necessary to show that information on biomarkers (as part of a risk prediction equation) will achieve a change in the order in which patients receive their procedure. In effect, ‘order’ becomes a surrogate of the effectiveness of biomarker information in this context. As with any surrogate, it is necessary to assess its link with final changes in patients’ health and with costs. The decision-analytic model quantifies this link by estimating the rate of prognostic events (death, non-fatal myocardial infarction and non-fatal stroke) for patients with different baseline characteristics while on the waiting list, during the procedure and beyond the procedure. Each event affects health service costs and patients’ quality-adjusted life expectancy. Hence, the key to biomarkers showing value is the extent to which they affect the order in which CABG is given and this change in order affects event rates.
Cost-effectiveness: base-case results
The base-case results of the cost-effectiveness analysis suggest that the choice of prioritisation strategy is between no formal prioritisation, Ontario urgency score, a risk score with eGFR and a risk score with both eGFR and CRP, with the other options being subject to dominance or extended dominance. Given the cost-effectiveness thresholds used by NICE (£20,000–30,000 per QALY),36 the base-case results suggest that incorporating eGFR into the risk model would be cost-effective, while using both eGFR and CRP is unlikely to be cost-effective.
It should be emphasised that the differences in mean costs and QALYs between current practice and the full range of formal prioritisation strategies are relatively small. Hence, although the ICER estimates indicate that the use of a formal prioritisation approach based on a risk score with eGFR appears to represent good value for money to the NHS, the predicted difference in health outcomes at the level of an individual needs to be considered in terms of whether this is clinically meaningful. Clearly, the biggest difference in outcomes between strategies is that observed based on current clinical practice and the most precise strategy based on a risk prediction equation including information generated by a combination of biomarkers. For this comparison, the resulting difference in mean QALYs is approximately 0.008 (equivalent to an additional 2.9 days of good health over a patient’s lifetime).
The relatively minor differences in terms of health outcomes estimated between the different strategies may not be entirely surprising given the nature of the decision problem under investigation. Ultimately, the different prioritisation strategies can result in a different ordering of patients only within the waiting list itself, and all patients (except those experiencing a fatal event on the waiting list) will eventually receive a CABG within 90 days. Equally, it should be noted that although the differences in quality-adjusted survival are small between strategies, formally employing more information in the prioritisation of patients appears to result in improved health outcomes.
Cost-effectiveness: alternative scenarios
Given that all surviving patients will eventually receive CABG, the maximum waiting time represents an important consideration with respect to the additional value that formal prioritisation approaches will have in the context of prioritising waiting lists. While the base-case analysis has been undertaken within the context of a maximum waiting list time, the value of alternative approaches to prioritisation within shorter waiting list times is an important consideration. To explore the robustness of the base-case results to this aspect, additional scenarios were considered based on a reduction in the maximum waiting list time (see Table 18). The results suggest that the use of a risk score with eGFR is cost-effective (subject to a £20,000–30,000 cost-effectiveness threshold) for maximum waiting list timing ranging from 15 days to 90 days. The use of the risk score with both eGFR and CRP would, however, have an ICER above NICE thresholds for maximum waiting times of 40 days and 15 days.
As noted above, less-biased estimates of the relative risk of biomarkers would be hypothesised to be weaker than those observed. The sensitivity scenario applying the lower 95% confidence limit value gives an indication of how these biases may influence the cost-effectiveness results. With biomarkers carrying less information, the risk score with eGFR and CRP appears even less cost-effective. However, the strategy employing a risk score with eGFR is still cost-effective employing the 95% lower limit value. It is difficult to estimate the magnitude of the bias on the biomarker estimates. A weaker estimate for CRP will clearly make the conclusions of this study stronger, as a risk score including CRP will look even less cost-effective. It is less clear how this bias will influence the cost-effectiveness of a risk score with eGFR. Clearly, the bias needs to have a large effect on the estimated coefficient to make the strategy employing a risk score with eGFR alone cost-ineffective.
Cost-effectiveness: limitations
It is important to be aware of limitations in the cost-effectiveness analysis. All parameters in the model are estimated with uncertainty – some of these are estimated with considerable imprecision (e.g. the predictive effects of biomarkers). Ideally, the model would have been subject to probabilistic sensitivity analysis in which the uncertainty in all parameters is systematically propagated through the model using simulation to show the consequent uncertainty in cost-effectiveness results. Given the complexity of the model – in effect the model is run for each patient in the notional cohort – probabilistic sensitivity analysis using the existing modelling platform would have taken large periods of time to compute. The authors have been funded by the National Institute for Health Research to establish a large new patient cohort of patients with angina, and assess a range of biomarkers in relation to prognosis and use of probabilistic sensitivity analysis in decision-analytic models. In the absence of probabilistic sensitivity analysis, a series of scenario analyses are presented including a sensitivity analysis on the value of the parameters relating to the relative risk with biomarkers.
We imputed values of CRP in SCAAR and cannot exclude the possibility that this diluted its effect. However, our conclusions are likely to be robust to this possibility because the literature-based estimates are likely to be inflated because of publication bias and inadequate adjustment for the routinely recorded factors known to relate to both CRP and outcome (including smoking, diabetes, obesity and lipids). Furthermore, even when using the upper 95% confidence limit for the effect, CRP had an ICER exceeding £40,000 per QALY, and is thus unlikely to be considered cost-effective. However, the manner in which we imputed the effect of CRP on risk, i.e. by averaging over tertiles, will capture some of the uncertainty associated with having to use imputation.
A further limitation is that we have simplified the process of prioritisation. In the modelling, it is assumed that prioritisation is undertaken on a single cohort of patients who join the list simultaneously and who receive their CABG in the order determined by the model. In reality, there is a dynamic process to a waiting list, in that new patients are being added to existing patients over time. In principle, this means that a formal prioritisation strategy would have to be run every time someone leaves or joins the list. A number of decision rules could be used in this more complex situation which may involve a patient’s anticipated day for surgery changing a number of times, or there may be constraints on how many changes are permitted. Revising the date of operation with changes in the pool of people waiting has its own information and scheduling costs, and the feasibility of such an approach as well as its acceptability to the patient are likely to be limited.
Further research is needed to include these more complex prioritisation algorithms into our modelling framework.
Furthermore, the model has not compared all the feasible strategies that could be used to prioritise patients on the waiting list for CABG. Specifically, as discussed in Chapter 4, there is a very large number of potential strategies involving different biomarkers individually or jointly. We focused on a circulating biomarker that is either routinely available (eGFR) or is beginning to be used in some centres to inform the care of stable angina patients (CRP). The model reported here provides a framework that can be adapted to look at other prioritisation strategies. Importantly, it provides a general approach to evaluating the cost-effectiveness of biomarkers to stratify patients by risk in a number of contexts which might include, for example, revascularisation versus best medical management and choice of revascularisation.
Implications for policy-makers
Notwithstanding these caveats, we expect our results to inform changes in clinical practice. The widespread practice of using only implicit or informal means of clinically ordering the waiting list may be harmful and we hope would be replaced with formal prioritisation approaches. The recently published Syntax trial compared CABG and PCI in the management of severe coronary artery disease and reported lower primary end point rates in those randomised to CABG. 8 It is possible that this positive trial will increase the number of patients referred for surgery, increase pressure on waiting time, and further emphasise the importance of our findings.
In our decision model we found that incorporation of a routinely available biomarker (eGFR) to a risk score was associated with changes in the day of assigned CABG, leading to higher QALYs at modest additional cost. This explicit strategy of formally prioritising the waiting list was cost-effective and robust to alternative assumptions, including maximum waiting list times of only 14 days. Although the QALY gains averaged across patients are small, our findings suggest that implementing the eGFR strategy would offer worthwhile gains in health – 780 QALYs per 100,000 patients.
This gain in health needs to be set against the organisational and training implications (including costs) of using any formalised approach to prioritisation, which we did not quantify. Several lines of evidence suggest that cost and organisational barriers to implementation of formal prioritisation scores, while real, may not be large. First, use of routinely collected data for scores for calculating operative mortality risk (e.g. euroSCORE24) is already widespread, suggesting that the information technology infrastructure and clinical culture for implementing scores already exist. Second, ‘formal protocols’ for prioritisation have recently been recommended. 25
This estimated change in health related to the cheapest biomarker compared with routine clinical practice. In moving from eGFR to potentially more effective novel biomarkers in terms of risk prediction, the incremental gains in health are likely to be quite small and their scope to be cost-effective given an additional acquisition cost may be quite limited.
Brain natriuretic peptide and other biomarkers not formally included in decision model
Our results provide a provisional basis to consider the potential cost-effectiveness of the other biomarkers that were included in the systematic review but were not formally considered in the model. Haemoglobin was the routine biomarker with the highest relative risk (2.9) and given its (zero) cost, further research that reduced the uncertainty around this estimate would be worthwhile. Given that all the other novel biomarkers are more expensive than CRP, an alternative and more expensive biomarker must provide comparatively greater gains in QALYs than those obtained using CRP to justify value for money. The summary results presented in Table 7 suggest that only BNP appears to have a stronger relative risk (2.93 for BNP versus 1.96 for CRP). However, the cost of BNP is also markedly higher at £35. Consequently, the additional health gains that would be required to justify this additional cost compared with CRP will inevitably need to be markedly higher (more than five-fold) than those achieved using CRP. Our model suggests that it is unlikely that BNP would be a cost-effective alternative to CRP. The predictive ability of multiple biomarkers has not been widely assessed and, to date, findings are conflicting. 218,219 Findings from our model suggest that combinations of costly biomarkers are unlikely to be cost-effective. Whether common genetic polymorphisms might contribute prognostic information is also not known, but the marginal cost of adding multiple genetic variants to a testing panel is low.
The issue of the maximum waiting time is important to consider for two reasons. The first is that the maximum waiting time will influence the cost-effectiveness of different prioritisation strategies (see Table 19) – in general, the shorter the maximum wait the smaller the scope for re-allocation of time slots with a prioritisation strategy with or without biomarkers, and hence the smaller the potential health gains to set against any cost of the prioritisation. The second reason for the importance of the maximum waiting time is that it may be a better use of NHS resources to reduce this time further rather than to invest in prioritisation strategies with a fixed maximum waiting time. This is because reducing the maximum wait will itself improve health outcomes – for example, Table 18 shows that a move from a maximum wait of 90 days to a maximum of 40 days, with patients’ order determined by routine practice, would increase QALYs by 0.03 for the average patient, i.e. a relatively large effect. This gain would have to be set against the cost of reducing waiting times. Figures released in August 2008 from the Department of Health suggest that about half the patients waiting for CABG have been waiting for between 1 and 3 months, and about half for up to 1 month.
Recommendations for further research
-
To establish and develop a national register of coronary angiography in the UK, which would provide a platform for health technology appraisal and other outcomes-based research. Such a register should include details of angiographic findings, clinical details required for basic risk equation, routinely estimated circulating biomarker information (eGFR) and follow-up for events and revascularisation (electronic patient record, Connecting for Health).
-
To develop initiatives for improving the quality of biomarker prognosis research,230 for example by developing standards for reporting which have been influential in other types of research and to foster collaborations of individual participant data sets. The potential shortfalls in the design, conduct, analysis and reporting of the studies highlighted in this review are consistent with those reported in meta-analyses of biomarkers in the prognosis of cancer. 220,221 Reporting standards, when adopted by journal editors, have been instrumental in reporting the quality of randomised trials (CONSORT) and although standards for observational aetiological studies (STROBE)222 exist, there is no counterpart for prognosis research. 223 A promising approach has been applied by Hayden41 and REMARK guidelines. 40 Ultimately, registration of prognosis research studies may prove as important as it has been for trials,224 especially because the rationale for many of the patient collections in this review was unclear.
-
To develop the decision-analytic framework by incorporating estimates of parameter uncertainty with probabilistic sensitivity analysis.
-
To use ‘value of information’ analysis to target where better research is needed. For example, to overcome problems of imputed data and parameters estimated from ‘non-optimal’ sources.
Conclusions
Formally employing more information in the prioritisation of patients awaiting CABG appears to be a cost-effective approach and may result in improved health outcomes. The most robust results relate to a strategy employing risk stratification using conventional clinical information together with a single biomarker (eGFR). The additional prognostic information conferred by collecting a novel circulating biomarker (CRP) or multiple biomarkers in terms of waiting list prioritisation is likely to be a cost-effective approach only in those countries with particularly long waiting lists.
Acknowledgements
Contributions of authors
Harry Hemingway (Professor of Clinical Epidemiology) was principal investigator on the grant, led the systematic reviews and co-ordinated the first and final draft of the manuscript; he also acts as guarantor of the study. Martin Henriksson (Research Fellow) designed, implemented and analysed the decision model and wrote drafts of Chapters 2, 5 and 6. Ruoling Chen (Statistician) carried out the meta-analyses of the biomarkers. Jacqueline Damant (Research Officer) is a systematic reviewer who carried out the searches, reviewed titles and abstracts for eligibility and extracted study data. Natalie Fitzpatrick (Research Programme Co-ordinator) is a systematic reviewer and project co-ordinator who reviewed titles and abstracts for eligibility and extracted study data. Keith Abrams (Professor of Medical Statistics) supervised the statistical aspects of the meta-analyses and the decision-analytic modelling. Aroon Hingorani (Professor of Genetic Epidemiology) advised on the scope of the biomarkers to include and on the interpretation of the biomarker results in biological and clinical context. Magnus Janzon (Cardiologist) was instrumental in obtaining the necessary ethical clearance for the use of SCAAR data and its linkage to the Swedish death and hospital admission registries. Martin Shipley (Statistician) derived study-specific scaling factors for the meta-analysis. Gene Feder (GP) was a co-applicant on the grant and advised on clinical aspects. Sir Bruce Keogh (Professor of Cardiac Surgery, University College London; NHS Medical Director) stimulated initial interest in the project and contributed the perspective of a cardiothoracic surgeon as well as, now, a policy-maker. Ulf Stenestrand (Cardiologist), as principal investigator on the SCAAR registry, obtained permission to use these data for this project, and helped in their appropriate interpretation. Kate McAllister (Research Assistant) was involved at inception and at study end in compiling and checking the report and study references, and updating the systematic review. Juan-Carlos Kaski (Professor of Cardiovascular Science) made the St George’s angina data set available for sharing, which allowed imputation of CRP in SCAAR and the calculation of adjustment factors. Adam Timmis (Professor of Clinical Cardiology) contributed clinical insights into the design and analysis of the meta-analyses and the decision-analytic modelling. Stephen Palmer (Senior Research Fellow of Health Economics) contributed to the overall design and implementation of the decision model and wrote much of the discussion. Mark Sculpher (Professor of Health Economics) led the overall design of the decision-analytic and cost-effectiveness models.
Special mention
We acknowledge the help of Heather Bailey (student, University College London) in the systematic review.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 2006;113:2335-62.
- Morrow DA. Cardiovascular biomarkers: pathophysiology and disease management. Totowa, NJ: Humana Press; 2006.
- Grunkemeier GL, Jin R. Receiver operating characteristic curve analysis of clinical risk models. Ann Thorac Surg 2001;72:323-6.
- Biomarkers Definitions Working Group . Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89-95.
- Aylin P, Alves B, Best N, Cook A, Elliott P, Evans SJ, et al. Comparison of UK paediatric cardiac surgical performance by analysis of routinely collected data 1984–96: was Bristol an outlier?. Lancet 2001;358:181-7.
- Darzi A. High Quality Care for All: NHS Next Stage Review Final Report 2008.
- Department of Health . Trust, Assurance and Safety: The Regulation of Health Professionals in the 21st Century. 2007.
- Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass9grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72.
- NCEPOD . Death Following a First Time, Isolated Coronary Artery Bypass Graft. The Heart of the Matter. A Report of the National Confidential Enquiry into Patient Outcome and Death 2008.
- Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9-13.
- Allender S, Scarborough P, Peto V, Rayner M, Leal J, Luengo-Fernandez R, et al. European cardiovascular disease statistics 2008. Eur J Cardiovasc Prev Rehabil 2009;16:S43-47.
- Aitsi-Selmi A, Shipley M, Hemingway H. Stable angina pectoris: comparison of trends in symptoms and diagnosed cases of angina from 1991 to 2003 with trends in MI mortality. EuroPrevent, Paris, 2008 n.d.
- Hemingway H, Langenberg C, Damant J, Frost C, Pyorala K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation 2008;117:1526-36.
- Stewart S, Murphy N, Walker A, McGuire A, McMurray JJ. The current cost of angina pectoris to the National Health Service in the UK. Heart 2003;89:848-53.
- Javitz HS, Ward MM, Watson JB, Jaana M. Cost of illness of chronic angina. Am J Manag Care 2004;10:S358-69.
- Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimäki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA 2006;295:1404-11.
- Clayton TC, Lubsen J, Pocock SJ, Voko Z, Kirwan BA, Fox KA, et al. Risk score for predicting death, myocardial infarction, and stroke in patients with stable angina, based on a large randomised trial cohort of patients. BMJ 2005;331.
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499-511.
- Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, et al. Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 2006;27:1341-81.
- Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration*. Lancet 1994;344:563-70.
- The Society of Cardiothoracic Surgeons of Great Britain and Ireland . Fifth National Adult Cardiac Surgical Database Report 2003. Improving Outcomes for Patients. 2004.
- Black N, Langham S, Coshall C, Parker J. Impact of the 1991 NHS reforms on the availability and use of coronary revascularisation in the UK (1987–1995). Heart 1996;76:1-31.
- Willcox S, Seddon M, Dunn S, Edwards RT, Pearse J, Tu JV. Measuring and reducing waiting times: a cross-national comparison of strategies. Health Aff (Millwood) 2007;26:1078-87.
- Bernstein SJ, Rigter H, Brorsson B, Hilborne LH, Leape LL, Meijler AP, et al. Waiting for coronary revascularization: a comparison between New York State, The Netherlands and Sweden. Health Policy 1997;42:15-27.
- Carroll RJ, Horn SD, Soderfeldt B, James BC, Malmberg L. International comparison of waiting times for selected cardiovascular procedures. JACC 1995;25:557-63.
- Graham MM, Norris CM, Galbraith PD, Knudtson ML, Ghali WA. Quality of life after coronary revascularization in the elderly. Eur Heart J 2006;27:1690-8.
- Legare JF, MacLean A, Buth KJ, Sullivan JA. Assessing the risk of waiting for coronary artery bypass graft surgery among patients with stenosis of the left main coronary artery. CMAJ 2005;173:371-5.
- Sampalis J, Boukas S, Liberman M, Reid T, Dupuis G. Impact of waiting time on the quality of life of patients awaiting coronary artery bypass grafting. CMAJ 2001;165:429-33.
- Raghavan R, Benzaquen BS, Rudski L. Timing of bypass surgery in stable patients after acute myocardial infarction. Can J Cardiol 2007;23:976-82.
- Hemingway H, Crook AM, Feder G, Dawson JR, Timmis A. Waiting for coronary angiography: is there a clinically ordered queue?. Lancet 2000;355:985-6.
- Naylor CD, Morgan CD, Levinton CM, Wheeler S, Hunter L, Klymciw K, et al. Waiting for coronary revascularization in Toronto: 2 years’ experience with a regional referral office. CMAJ 1993;149:955-62.
- Naylor CD, Baigrie RS, Goldman BS, Basinski A. Assessment of priority for coronary revascularisation procedures. Revascularisation Panel and Consensus Methods Group. Lancet 1990;335:1070-3.
- Hadorn DC, Holmes AC. The New Zealand priority criteria project. Part 2: Coronary artery bypass graft surgery. BMJ 1997;314:135-8.
- Hemingway H, Fitzpatrick NK, Gnani S, Feder G, Walker N, Crook AM, et al. Prospective validity of measuring angina severity with Canadian Cardiovascular Society class: The ACRE study. Can J Cardiol 2004;20:305-9.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- NICE . Guide to the Methods of Technology Appraisal 2008.
- Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med 2007;356:1009-19.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12.
- Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005;97:1180-4.
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37.
- Blankenberg S, McQueen MJ, Smieja M, Pogue J, Balion C, Lonn E, et al. Comparative impact of multiple biomarkers and N-Terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2006;114:201-8.
- Anderson JL, Muhlestein JB, Horne BD, Carlquist JF, Bair TL, Madsen TE, et al. Plasma homocysteine predicts mortality independently of traditional risk factors and C-reactive protein in patients with angiographically defined coronary artery disease. Circulation 2000;102:1227-32.
- Chan AW, Bhatt DL, Chew DP, Reginelli J, Schneider JP, Topol EJ, et al. Relation of inflammation and benefit of statins after percutaneous coronary interventions. Circulation 2003;107:1750-6.
- Inaguma D, Tatematsu M, Shinjo H, Suzuki S, Mishima T, Inaba S, et al. Relationship between renal function at the time of percutaneous coronary intervention and prognosis in ischemic heart disease patients. Clin Exp Nephrol 2007;11:56-60.
- Aguilar D, Fisher MR, O’Connor CM, Dunne MW, Muhlestein JB, Yao L, et al. Metabolic syndrome, C-reactive protein, and prognosis in patients with established coronary artery disease. Am Heart J 2006;152:298-304.
- Huang PH, Lu TM, Wu TC, Lin FY, Chen YH, . Usefulness of combined high-sensitive C-reactive protein and N-terminal-probrain natriuretic peptide for predicting cardiovascular events in patients with suspected coronary artery disease. Coron Artery Dis 2008;19:187-93.
- Fathi RB, Gurm HS, Chew DP, Gupta R, Bhatt DL, Ellis SG. The interaction of vascular inflammation and chronic kidney disease for the prediction of long-term death after percutaneous coronary intervention. Am Heart J 2005;150:1190-7.
- Chew DP, Bhatt DL, Robbins MA, Penn MS, Schneider JP, Lauer MS, et al. Incremental prognostic value of elevated baseline C-reactive protein among established markers of risk in percutaneous coronary intervention. Circulation 2001;104:992-7.
- Crea F, Monaco C, Lanza GA, Maggi E, Ginnetti F, Cianflone D, et al. Inflammatory predictors of mortality in the Scandinavian Simvastatin Survival Study. Clin Cardiol 2002;25:461-6.
- Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, et al. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation 2006;114:281-8.
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387-97.
- Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998;279:1477-82.
- Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol 2009;38:217-31.
- Di AE, Danesh J, Eiriksdottir G, Gudnason V. Renal function and risk of coronary heart disease in general populations: new prospective study and systematic review. PLoS Med 2007;4.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Egger M, Davey Smith G, Altman DG. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group; 2001.
- Sinning JM, Bickel C, Messow CM, Schnabel R, Lubos E, Rupprecht HJ, et al. Impact of C-reactive protein and fibrinogen on cardiovascular prognosis in patients with stable angina pectoris: the AtheroGene study. Eur Heart J 2006;27:2962-8.
- Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med 1995;332:635-41.
- Falcone C, Minoretti P, D’Angelo A, Buzzi MP, Coen E, Emanuele E, et al. Markers of eosinophilic inflammation and risk prediction in patients with coronary artery disease. Eur J Clin Invest 2006;36:211-17.
- Lubos E, Schnabel R, Rupprecht HJ, Bickel C, Messow CM, Prigge S, et al. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: results from the AtheroGene study. Eur Heart J 2006;27:150-6.
- Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol 2004;44:1819-24.
- Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet 1997;349:462-6.
- Soeki T, Tamura Y, Shinohara H, Tanaka H, Bando K, Yui Y, et al. Fibrinolytic factors, serum lipid and C-reactive protein predicting cardiac events in Japanese patients with coronary atherosclerotic lesions. Jpn Circ J 1999;63:976-80.
- Horne BD, Muhlestein JB, Carlquist JF, Bair TL, Madsen TE, Hart NI, et al. Statin therapy, lipid levels, C-reactive protein and the survival of patients with angiographically severe coronary artery disease. J Am Coll Cardiol 2000;36:1774-80.
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003;107:1579-85.
- Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 2002;106:24-30.
- Schaan BD, Quadros AS, Sarmento-Leite R, De LG J, Bender A, . Correction: Serum transforming growth factor beta-1 (TGF-beta-1) levels in diabetic patients are not associated with pre-existent coronary artery disease. Cardiovasc Diabetol 2007;6.
- Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation 2001;103:45-51.
- Ndrepepa G, Kastrati A, Braun S, Koch W, Kolling K, Mehilli J, et al. A prospective cohort study of predictive value of homocysteine in patients with type 2 diabetes and coronary artery disease. Clin Chim Acta 2006;373:70-6.
- Otsuka M, Hayashi Y, Ueda H, Imazu M, Kohno N. Predictive value of preprocedural fibrinogen concerning coronary stenting. Atherosclerosis 2002;164:371-8.
- Bickel C, Rupprecht HJ, Blankenberg S, Espiniola-Klein C, Schlitt A, Rippin G, et al. Relation of markers of inflammation (C-reactive protein, fibrinogen, von Willebrand factor, and leukocyte count) and statin therapy to long-term mortality in patients with angiographically proven coronary artery disease. Am J Cardiol 2002;89:901-8.
- Saleh N, Braunschweig F, Jensen J, Tornvall P. Usefulness of preprocedural serum N-terminal pro-brain natriuretic peptide levels to predict long-term outcome after percutaneous coronary intervention in patients with normal troponin T levels. Am J Cardiol 2006;97:830-4.
- Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J 2005;26:137-44.
- Chirinos JA, Zambrano JP, Chakko S, Schob A, Goldberg RB, Perez G, et al. Ability of serum to decrease cellular acylCoA:cholesterol acyl transferase activity predicts cardiovascular outcomes. Circulation 2005;112:2446-53.
- Bogaty P, Poirier P, Simard S, Boyer L, Solymoss S, Dagenais GR. Biological profiles in subjects with recurrent acute coronary events compared with subjects with long-standing stable angina. Circulation 2001;103:3062-8.
- Chirinos JA, Zambrano JP, Chakko S, Schob A, Veerani A, Perez GO, et al. Usefulness of C-reactive protein as an independent predictor of death in patients with ischemic cardiomyopathy. Am J Cardiol 2005;95:88-90.
- Qi X, Li S, Li J. The prognostic value of IL-8 for cardiac events and restenosis in patients with coronary heart diseases after percutaneous coronary intervention. Jpn Heart J 2003;44:623-32.
- Qi X, Li J, Gu J, Li S, Dang Y, Wang T. Plasma levels of IL-8 predict early complications in patients with coronary heart disease after percutaneous coronary intervention. Jpn Heart J 2003;44:451-61.
- Garcia-Moll X, Zouridakis E, Cole D, Kaski JC. C-reactive protein in patients with chronic stable angina: differences in baseline serum concentration between women and men. Eur Heart J 2000;21:1598-606.
- Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001;104:1336-42.
- Minoretti P, Falcone C, Calcagnino M, Emanuele E, Buzzi MP, Coen E, et al. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J 2006;27:802-7.
- Arroyo ER. C-reactive protein elevation and disease activity in patients with coronary artery disease. Eur Heart J 2004;25:401-8.
- Susen S, Sautiere K, Mouquet F, Cuilleret F, Chmait A, McFadden EP, et al. Serum hepatocyte growth factor levels predict long-term clinical outcome after percutaneous coronary revascularization. Eur Heart J 2005;26:2387-95.
- Dai DF, Lin JW, Kao JH, Hsu CN, Chiang FT, . The effects of metabolic syndrome versus infectious burden on inflammation, severity of coronary atherosclerosis, and major adverse cardiovascular events. J Clin Endocrinol Metab 2007;92:2532-7.
- Dai DF, Hwang JJ, Lin JL, Lin JW, Hsu CN, Lin CM, et al. Joint effects of N-terminal pro-B-typenatriuretic peptide and C-reactive protein vs angiographic severity in predicting major adverse cardiovascular events and clinical restenosis after coronary angioplasty in patients with stable coronary artery disease. Circ J 2008;72:1316-23.
- Bogaty P, Boyer L, Simard S, Dauwe F, Dupuis R, . Clinical utility of C-reactive protein measured at admission, hospital discharge, and 1 month later to predict outcome in patients with acute coronary disease. The RISCA (recurrence and inflammation in the acute coronary syndromes) study. J Am Coll Cardiol 2008;51:2339-46.
- Haim M, Tanne D, Goldbourt U, Doolman R, Boyko V, Brunner D, et al. Serum homocysteine and long-term risk of myocardial infarction and sudden death in patients with coronary heart disease. Cardiology 2007;107:52-6.
- Artieda M, Cenarro A, Ganan A, Lukic A, Moreno E, Puzo J, et al. Serum chitotriosidase activity, a marker of activated macrophages, predicts new cardiovascular events independently of C-reactive protein. Cardiology 2007;108:297-306.
- West MJ, Nestel PJ, Kirby AC, Schnabel R, Sullivan D, Simes RJ, et al. The value of N-terminal fragment of brain natriuretic peptide and tissue inhibitor of metalloproteinase-1 levels as predictors of cardiovascular outcome in the LIPID study. Eur Heart J 2008;29:923-31.
- Palmerini T, Marzocchi A, Marrozzini C, Reggiani LB, Savini C, Marinelli G, et al. Preoperative C-reactive protein levels predict 9-month mortality after coronary artery bypass grafting surgery for the treatment of left main coronary artery stenosis. Eur J Cardiothorac Surg 2007;31:685-90.
- Niccoli G, Ferrante G, Mongiardo R, Perfetti M, Belloni F, Burzotta F, et al. Cardiovasc Revasc Med 2007;8:156-60.
- Arroyo-Espliguero R, Avanzas P, Quiles J, Kaski JC. Predictive value of coronary artery stenoses and C-reactive protein levels in patients with stable coronary artery disease. Atherosclerosis 2009;204:239-43.
- Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophillymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta 2008;395:27-31.
- Inoue T, Komoda H, Nonaka M, Kameda M, Uchida T, Node K. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease. Int J Cardiol 2008;124:319-25.
- Shlipak MG, Ix JH, Bibbins-Domingo K, Lin F, Whooley MA. Biomarkers to predict recurrent cardiovascular disease: the Heart and Soul Study. Am J Med 2008;121:50-7.
- Espinola-Klein C, Rupprecht HJ, Bickel C, Lackner K, Schnabel R, Munzel T, et al. Inflammation, atherosclerotic burden and cardiovascular prognosis. Atherosclerosis 2007;195:e126-34.
- Inoue T, Kotooka N, Morooka T, Komoda H, Uchida T, Aso Y, et al. High molecular weight adiponectin as a predictor of long-term clinical outcome in patients with coronary artery disease. Am J Cardiol 2007;100:569-74.
- Marcinkowski M, Czarnecka D, Jastrzebski M, Fedak D, Kawecka-Jaszcz K. Inflammatory markers 10 weeks after myocardial infarction predict future cardiovascular events. Cardiol J 2007;14:50-8.
- Fang Y, Huang L, Li A, Song Y, Geng Z, . Significance of the ratio of circulating endothelial cell expressing endothelial lipase and supersensitivity C-reactive protein in prognosis of patients with coronary arterty disease. Chin Crit Care Med 2007;19:644-6.
- Khor LL, Muhlestein JB, Carlquist JF, Horne BD, Bair TL, Maycock CA, et al. Sex- and age-related differences in the prognostic value of C-reactive protein in patients with angiographic coronary artery disease. Am J Med 2004;117:657-64.
- Zebrack JS, Muhlestein JB, Horne BD, Anderson JL. C-reactive protein and angiographic coronary artery disease: independent and additive predictors of risk in subjects with angina. J Am Coll Cardiol 2002;39:632-7.
- Zebrack JS, Anderson JL, Beddhu S, Horne BD, Bair TL, Cheung C. Do associations with C-reactive protein and extent of coronary artery disease account for the increased cardiovascular risk of renal insufficiency?. J Am Coll Cardiol 2003;42:57-63.
- Zebrack JS, Anderson JL, Maycock CA, Horne BD, Bair TL, Muhlestein JB. Usefulness of high-sensitivity C-reactive protein in predicting long-term risk of death or acute myocardial infarction in patients with unstable or stable angina pectoris or acute myocardial infarction. Am J Cardiol 2002;89:145-9.
- Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res 2005;97:e53-9.
- Schnabel R, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Torzewski M, Lubos E, et al. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene study. J Am Coll Cardiol 2005;45:1631-7.
- Kangasniemi OP, Biancari F, Luukkonen J, Vuorisalo S, Satta J, Pokela R, et al. Preoperative C-reactive protein is predictive of long-term outcome after coronary artery bypass surgery. Eur J Cardiothorac Surg 2006;29:983-5.
- Muhlestein JB, Horne BD, Carlquist JF, Madsen TE, Bair TL, Pearson RR, et al. Cytomegalovirus seropositivity and C-reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation 2000;102:1917-23.
- Liu PY, Li YH, Tsai WC, Chao TH, Tsai LM, Wu HL, et al. Prognostic value and the changes of plasma levels of secretory type II phospholipase A2 in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur Heart J 2003;24:1824-32.
- Dibra A, Mehilli J, Braun S, Hadamitzky M, Baum H, Dirschinger J, et al. Association between C-reactive protein levels and subsequent cardiac events among patients with stable angina treated with coronary artery stenting. Am J Med 2003;114:715-22.
- Ndrepepa G, Kastrati A, Braun S, Mehilli J, Niemoller K, von Beckerath N, et al. N-terminal probrain natriuretic peptide and C-reactive protein in stable coronary heart disease. Am J Med 2006;119:355-8.
- Blankenberg S, Rupprecht HJ, Bickel C, Espinola-Klein C, Rippin G, Hafner G, et al. Cytomegalovirus infection with interleukin-6 response predicts cardiac mortality in patients with coronary artery disease. Circulation 2001;103:2915-21.
- de Winter RJ, Heyde GS, Koch KT, Fischer J, van Straalen JP, Bax M, et al. The prognostic value of pre-procedural plasma C-reactive protein in patients undergoing elective coronary angioplasty. Eur Heart J 2002;23:960-6.
- Ijsselmuiden AJJ, Serruys PW, Scholte J, Kleimeneij F, Slagboom T, Van der Weiken LR, et al. Direct coronary stent implantation does not reduce the incidence of in-stent restenosis or major adverse cardiac events: Six month results of a randomized trial. Eur Heart J 2003;24:421-9.
- de Winter RJ, Koch KT, van Straalen JP, Heyde G, Bax M, Schotborgh CE, et al. C-reactive protein and coronary events following percutaneous coronary angioplasty. Am J Med 2003;115:85-90.
- Wu TC, Leu HB, Lin WT, Lin CP, Lin SJ, Chen JW. Plasma matrix metalloproteinase-3 level is an independent prognostic factor in stable coronary artery disease. Eur J Clin Invest 2005;35:537-45.
- Janoskuti L, Forhecz Z, Hosszufalusi N, Kleiber M, Walentin S, Balint O, et al. High levels of C-reactive protein with low total cholesterol concentrations additively predict all-cause mortality in patients with coronary artery disease. Eur J Clin Invest 2005;35:104-11.
- Veselka J, Prochazkova S, Duchonova R, Homolova I, Tesar D. Relationship of C-reactive protein to adverse cardiovascular events in patients treated by percutaneous coronary intervention for stable angina pectoris. Int Heart J 2005;46:195-204.
- Low AF, Seow SC, Yeoh KG, Lim YT, Tan HC, Yeo TC. High-sensitivity C-reactive protein is predictive of medium-term cardiac outcome in high-risk Asian patients presenting with chest pain syndrome without myocardial infarction. Ann Acad Med Singapore 2004;33:407-12.
- Gach O, Legrand V, Biessaux Y, Chapelle JP, Vanbelle S, Pierard LA. Long-term prognostic significance of high-sensitivity C-reactive protein before and after coronary angioplasty in patients with stable angina pectoris. Am J Cardiol 2007;99:31-5.
- Leu HB, Lin CP, Lin WT, Wu TC, Chen JW. Risk stratification and prognostic implication of plasma biomarkers in nondiabetic patients with stable coronary artery disease: the role of high-sensitivity C-reactive protein. Chest 2004;126:1032-9.
- de Winter RJ, Stroobants A, Koch KT, Bax M, Schotborgh CE, Mulder KJ, et al. Plasma N-terminal pro-B-type natriuretic peptide for prediction of death or nonfatal myocardial infarction following percutaneous coronary intervention. Am J Cardiol 2004;94:1481-5.
- Ikonomidis I, Lekakis J, Revela I, Andreotti F, Nihoyannopoulos P. Increased circulating C-reactive protein and macrophage-colony stimulating factor are complementary predictors of long-term outcome in patients with chronic coronary artery disease. Eur Heart J 2005;26:1618-24.
- Karha J, Bavry AA, Rajagopal V, Henderson MR, Ellis SG, Brener SJ. Relation of C-reactive protein level and long-term risk of death or myocardial infarction following percutaneous coronary intervention with a sirolimus-eluting stent. Am J Cardiol 2006;98:616-18.
- Kinjo K, Sato H, Ohnishi Y, Hishida E, Nakatani D, Mizuno H, et al. Impact of high-sensitivity C-reactive protein on predicting long-term mortality of acute myocardial infarction. Am J Cardiol 2003;91:931-5.
- Kwaijtaal M, van Diest R, Bar FW, van der Ven AJ, Bruggeman CA, de Baets MH, et al. Inflammatory markers predict late cardiac events in patients who are exhausted after percutaneous coronary intervention. Atherosclerosis 2005;182:341-8.
- Zairis MN, Ambrose JA, Lyras AG, Thoma MA, Psarogianni PK, Psaltiras PG, et al. C-reactive protein, moderate alcohol consumption, and long term prognosis after successful coronary stenting: four year results from the GENERATION study. Heart 2004;90:419-24.
- Zairis MN, Ambrose JA, Manousakis SJ, Stefanidis AS, Papadaki OA, Bilianou HI, et al. The impact of plasma levels of C-reactive protein, lipoprotein (a) and homocysteine on the long-term prognosis after successful coronary stenting: The Global Evaluation of New Events and Restenosis After Stent Implantation Study. J Am Coll Cardiol 2002;40:1375-82.
- Aytekin S, Catakoglu AB, Aytekin, Kocazeybek B, Demiroglu C, Demiroglu C. C-reactive protein as a pre-procedural predictor of early and late outcomes of percutaneous coronary interventions. Int J Angiol 2003;12:229-33.
- Sargento L, Do Rosario HS, Perdigao C, Monteiro J, Saldanha C, Silva JM. Biohemorheologic factors and cardiovascular events curve in survivors of transmural acute myocardial infarct-–24-month follow-up. Rev Port Cardiol 2002;21:165-71.
- Palmerini T, Marzocchi A, Marrozzini C, Ortolani P, Saia F, Bacchi-Reggiani L, et al. Preprocedural levels of C-reactive protein and leukocyte counts predict 9-month mortality after coronary angioplasty for the treatment of unprotected left main coronary artery stenosis. Circulation 2005;112:2332-8.
- Kinjo K, Sato H, Sakata Y, Nakatani D, Mizuno H, Shimizu M, et al. Relation of C-reactive protein and one-year survival after acute myocardial infarction with versus without statin therapy. Am J Cardiol 2005;96:617-21.
- Lu TM, Ding YA, Lin SJ, Lee WS, Tai HC. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J 2003;24:191-9.
- Biancari F, Lahtinen J, Lepojarvi S, Rainio P, Salmela E, Pokela R, et al. Preoperative C-reactive protein and outcome after coronary artery bypass surgery. Ann Thorac Surg 2003;76:2007-12.
- Gaspardone A, Crea F, Versaci F, Tomai F, Pellegrino A, Chiariello L, et al. Predictive value of C-reactive protein after successful coronary-artery stenting in patients with stable angina. Am J Cardiol 1998;82:515-18.
- van der HP, Voors AA, Volbeda M, Buikema H, van Veldhuisen DJ, van Gilst WH. Usefulness of preoperative C-reactive protein and soluble intercellular adhesion molecule-1 level for predicting future cardiovascular events after coronary artery bypass grafting. Am J Cardiol 2006;97:1697-701.
- Huang W, Chen QW, Lei H, Deng W, Ke DZ. Predictive value of fibrinogen and high-sensitivity C-reaction protein for cardiovascular events in patients with stable coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34:718-21.
- Milazzo D, Biasucci LM, Luciani N, Martinelli L, Canosa C, Schiavello R, et al. Elevated levels of C-reactive protein before coronary artery bypass grafting predict recurrence of ischemic events. Am J Cardiol 1999;84:459-61.
- Palazzuoli A, Dekkers J, Calabrò A, Campagna MS, Nuti R, Pastorelli M, et al. Brain natriuretic peptide and other risk markers for outcome assessment in patients with non-st-elevation coronary syndromes and preserved systolic function. Am J Cardiol 2006;15:1322-8.
- Muhlestein JB, Anderson JL, Horne BD, Carlquist JF, Bair TL, Bunch TJ, et al. Early effects of statins in patients with coronary artery disease and high C-reactive protein. Am J Cardiol 2004;94:1107-12.
- Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 2007;115:1528-36.
- Saleh N, Svane B, Hansson LO, Jensen J, Nilsson T, Danielsson O, et al. Response of serum C-reactive protein to percutaneous coronary intervention has prognostic value. Clin Chem 2005;51:2124-30.
- Rahel BM, Visseren FL, Suttorp MJ, Plokker TH, Kelder JC, de Jongh BM, et al. Preprocedural serum levels of acute-phase reactants and prognosis after percutaneous coronary intervention. Cardiovasc Res 2003;60:136-40.
- Speidl WS, Graf S, Hornykewycz S, Nikfardjam M, Niessner A, Zorn G, et al. High-sensitivity C-reactive protein in the prediction of coronary events in patients with premature coronary artery disease. Am Heart J 2002;144:449-55.
- Kubica J, Kozinski M, Krzewina-Kowalska A, Zbikowska-Gotz M, Dymek G, Sukiennik A, et al. Combined periprocedural evaluation of CRP and TNF-alpha enhances the prediction of clinical restenosis and major adverse cardiac events in patients undergoing percutaneous coronary interventions. Int J Mol Med 2005;16:173-80.
- Park DW, Lee CW, Yun SC, Kim YH, Hong MK, Kim JJ, et al. Prognostic impact of preprocedural C-reactive protein levels on six-month angiographic and one-year clinical outcomes after drug-eluting stent implantation. Heart 2007;93:1087-92.
- Grander W, Dichtl W, Prokop W, Roithinger FX, Moes N, Friedrich G, et al. C-reactive protein plasma levels but not factor VII activity predict clinical outcome in patients undergoing elective coronary intervention. Clin Cardiol 2004;27:211-16.
- Lee KW, Hill JS, Walley KR, Frohlich JJ. Relative value of multiple plasma biomarkers as risk factors for coronary artery disease and death in an angiography cohort. CMAJ 2006;174:461-6.
- Rothenbacher D, Koenig W, Brenner H. Comparison of N-terminal pro-B-natriuretic peptide, C-reactive protein, and creatinine clearance for prognosis in patients with known coronary heart disease. Arch Intern Med 2006;166:2455-60.
- Kip KE, Marroquin OC, Shaw LJ, Arant CB, Wessel TR, Olson MB, et al. Global inflammation predicts cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Am Heart J 2005;150:900-6.
- Harb TS, Zareba W, Moss AJ, Ridker PM, Marder VJ, Rifai N, et al. Association of C-reactive protein and serum amyloid A with recurrent coronary events in stable patients after healing of acute myocardial infarction. Am J Cardiol 2002;89:216-21.
- Hoffmeister Rothenbacker D, Kunze M, Brenner H, Koenig W. Prognostic value of inflammatory markers alone and in combination with blood lipids in patients with stable coronary artery disease. Eur J Intern Med 2005:16-62.
- Retterstol L, Eikvar L, Bohn M, Bakken A, Erikssen J, Berg K. C-reactive protein predicts death in patients with previous premature myocardial infarction – a 10 year follow-up study. Atherosclerosis 2002;160:433-40.
- Schnabel R, Rupprecht HJ, Lackner KJ, Lubos E, Bickel C, Meyer J, et al. Analysis of N-terminal-pro-brain natriuretic peptide and C-reactive protein for risk stratification in stable and unstable coronary artery disease: results from the AtheroGene study. Eur Heart J 2005;26:241-9.
- Patti G, Di SG, D’Ambrosio A, Dicuonzo G, Abbate A, Dobrina A. Prognostic value of interleukin-1 receptor antagonist in patients undergoing percutaneous coronary intervention. Am J Cardiol 2002;89:372-6.
- Krzewina-Kowalska A, Kubica J, Kozinski M, Piasecki R, Berent B, . Selected acute phase proteins in patients undergoing coronary angioplasty. Folia Cadiol 2003;10:733-42.
- Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J 2007;154:995-1002.
- Deckers JW, Goedhart DM, Boersma E, Briggs A, Bertrand M, Ferrari R, et al. Treatment benefit by perindopril in patients with stable coronary artery disease at different levels of risk. Eur Heart J 2006;27:796-801.
- Lipsic E, Asselbergs FW, Van-Der-Meer. Anaemia predicts cardiovascular events in patients with stable coronary artery disease. Neth Heart J 2005;13:254-8.
- Reinecke H, Trey T, Wellmann J, Heidrich J, Fobker M, Wichter T, et al. Haemoglobin-related mortality in patients undergoing percutaneous coronary interventions. Eur Heart J 2003;24:2142-50.
- Hillis GS, Croal BL, Buchan KG, El Shafei H, Gibson G, Jeffrey RR, et al. Renal function and outcome from coronary artery bypass grafting: impact on mortality after a 2.3-year follow-up. Circulation 2006;113:1056-62.
- Reddan DN, Szczech LA, Tuttle RH, Shaw LK, Jones RH, Schwab SJ, et al. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol 2003;14:2373-80.
- Tang WH, Steinhubl SR, Van Lente F, Brennan D, McErlean E, Maroo A, et al. Risk stratification for patients undergoing nonurgent percutaneous coronary intervention using N-terminal pro-B-type natriuretic peptide: a Clopidogrel for the Reduction of Events During Observation (CREDO) substudy. Am Heart J 2007;153:36-41.
- Vittinghoff E, Shlipak MG, Varosy PD, Furberg CD, Ireland CC, Khan SS, et al. Risk factors and secondary prevention in women with heart disease: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2003;138:81-9.
- Zakeri R, Freemantle N, Barnett V, Lipkin GW, Bonser RS, Graham TR, et al. Relation between mild renal dysfunction and outcomes after coronary artery bypass grafting. Circulation 2005;112:I270-5.
- Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD. Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol 2001;38:705-11.
- Almquist T, Forslund L, Rehnqvist N, Hjemdahl P. Prognostic implications of renal dysfunction in patients with stable angina pectoris. J Intern Med 2006;260:537-44.
- Shlipak MG, Stehman-Breen C, Vittinghoff E, Lin F, Varosy PD, Wenger NK, et al. Creatinine levels and cardiovascular events in women with heart disease: do small changes matter?. Am J Kidney Dis 2004;43:37-44.
- Solomon SD, Rice MM, Jablonski A, Jose P, Domanski M, Sabatine M, et al. Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation 2006;114:26-31.
- Chen R, Kumar S, Timmis A, Feder G, Yaqoob MM, Hemingway H. Comparison of the relation between renal impairment, angiographic coronary artery disease, and long-term mortality in women versus men. Am J Cardiol 2006;97:630-2.
- Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res 2007;100:1234-41.
- Shah B, Kumar N, Garg P, Kang E, Grossi E, Lorin JD, et al. Metabolic syndrome does not impact survival in patients treated for coronary artery disease. Coron Artery Dis 2008;19:71-7.
- Ruilope LM, Kirwan BA, de Brouwer S, Danchin N, Fox KA, Wagener G, et al. Uric acid and other renal function parameters in patients with stable angina pectoris participating in the ACTION trial: impact of nifedipine GITS (gastro-intestinal therapeutic system) and relation to outcome. J Hypertens 2007;25:1711-18.
- Exaire JE, Butman SM, Ebrahimi G, Kleimen NS, Harrington RA, Schweiger MJ. Provisional glycoprotein IIb/IIIa blockade in a randomized investigation of bivalirudin versus heparin plus planned glycoprotein IIb/IIIa inhibition during percutaneous coronary intervention: Predictors and outcome in the Randomized Evaluation in Percutaneous coronary intervention Linking Angiomax to Reduced Clinical Events (REPLACE)–2 trial. Am Heart J 2006;152:157-63.
- Matts JP, Karnegis JN, Campos CT, Fitch LL, Johnson JW, Buchwald H. Serum creatinine as an independent predictor of coronary heart disease mortality in normotensive survivors of myocardial infarction. POSCH Group. J Fam Pract 1993;36:497-503.
- Hu R, Ma CS, Nie SP, Lu Q, Kang JP, Du X, et al. Effect of metabolic syndrome on prognosis and clinical characteristics of revascularization in patients with coronary artery disease. Chin Med J (Engl) 2006;119:1871-6.
- Stassano P, Di Tommaso L, Vitale DF, Monaco M, Iannelli G, Mottola M, et al. Aortic valve replacement and coronary artery surgery: determinants affecting early and long-term results. Thorac Cardiovasc Surg 2006;54:521-7.
- DiMauro M, Gagliardi M, Iaco AL, Contini M, Bivona A, Bosco P, et al. Does off-pump coronary surgery reduce postoperative acute renal failure? The importance of preoperative renal function. Ann Thorac Surg 2007;84:1496-502.
- McKechnie RS, Smith D, Montoye C, Kline-Rogers E, O’Donnell MJ, DeFranco AC, et al. Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation 2004;110:271-7.
- Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol 2006;97:993-6.
- Elisheva S, Noya G, Simchen E, Galai N, Zitser-Gurevich Y, Braun D, et al. Sequential logistic models for 30 days mortality after CABG: Pre-operative, intra-operative and post-operative experience – The Israeli CABG study (ISCAB). Eur J Epidemiol 2000;16:543-55.
- Cesena FH, Favarato D, Cesar LA, de Oliveira SA, Da Luz PL. Cardiac complications during waiting for elective coronary artery bypass graft surgery: incidence, temporal distribution and predictive factors. Eur J Cardiothorac Surg 2004;25:196-202.
- van Domburg RT, Takkenberg JJ, van Herwerden LA, Venema AC, Bogers AJ. Short-term and 5-year outcome after primary isolated coronary artery bypass graft surgery: results of risk stratification in a bilocation center. Eur J Cardiothorac Surg 2002;21:733-40.
- Szczech LA, Best PJ, Crowley E, Brooks MM, Berger PB, Bittner V, et al. Outcomes of patients with chronic renal insufficiency in the bypass angioplasty revascularization investigation. Circulation 2002;105:2253-8.
- Yamamuro M, Lytle BW, Sapp SK, Cosgrove DM, Loop FD, McCarthy PM. Risk factors and outcomes after coronary reoperation in 739 elderly patients. Ann Thorac Surg 2000;69:464-74.
- Weerasinghe A, Hornick P, Smith P, Taylor K, Ratnatunga C. Coronary artery bypass grafting in non-dialysis-dependent mild-to-moderate renal dysfunction. J Thorac Cardiovasc Surg 2001;121:1083-9.
- Wattanakit K, Folsom AR, Chambless LE, Nieto FJ. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2005;149:606-12.
- Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997;337:230-6.
- Kaplan RC, Heckbert SR, Furberg CD, Psaty BM. Predictors of subsequent coronary events, stroke, and death among survivors of first hospitalized myocardial infarction. J Clin Epidemiol 2002;55:654-64.
- HM Treasury . The Green Book. Appraisal and Evaluation in Central Government 2003.
- Jackson NW, Doogue MP, Elliott JM. Priority points and cardiac events while waiting for coronary bypass surgery. Heart 1999;81:367-73.
- Henriksson M, Epstein DM, Palmer SJ, Sculpher MJ, Clayton TC, Pocock SJ, et al. The cost-effectiveness of an early interventional strategy in non-ST-elevation acute coronary syndrome based on the RITA 3 trial. Heart 2008;94:717-23.
- Palmer S, Sculpher M, Philips Z, Robinson M, Ginnelly L, Bakhai A, et al. Management of non-ST-elevation acute coronary syndromes: how cost-effective are glycoprotein IIb/IIIA antagonists in the UK National Health Service?. Int J Cardiol 2005;100:229-40.
- Collett D. Modelling survival data in medical research. London: Chapman and Hall/CRC; 1994.
- Greene W. Econometric analysis. New York: Prentice Hall; 2003.
- Bray H. 2006-based national population projections for the UK and constituent countries. Population trends. Government Actuary Department; 2006.
- National Statistics . Review of the Registrat General on Dealths by Cause, Sex and Age, in England and Wales 2003.
- Department of Health . NHS Reference Costs 2005–06 2006.
- Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al. Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling. Health Technol Assess 2005;9:iii-xi.
- Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al. Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation. Health Technol Assess 2004;8:iii-196.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996;143:1-13.
- Cosin-Sales J, Kaski JC, Christiansen M, Kaminski P, Oxvig C, Overgaard MT, et al. Relationship among pregnancy associated plasma protein-A levels, clinical characteristics, and coronary artery disease extent in patients with chronic stable angina pectoris. Eur Heart J 2005;26:2093-8.
- Steyerberg EW, Eijkemans MJ, Van Houwelingen JC, Lee KL, Habbema JD. Prognostic models based on literature and individual patient data in logistic regression analysis. Stat Med 2000;19:141-60.
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1-30.
- McCullagh P, Nelder JA. Generalized linear models. London: Chapman and Hall; 1989.
- Drummond MF, Sculpher M.J., Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Ioannidis JP, Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG. Genetic associations in large versus small studies: an empirical assessment. Lancet 2003;361:567-71.
- Thompson SG, Duckert F, Haverkate F, Thomson JM. The measurement of haemostatic factors in 16 European laboratories: quality assessment for the Multicentre ECAT Angina Pectoris Study. Report from the European Concerted Action on Thrombosis and Disabilities (ECAT). Thromb Haemost 1989;61:301-6.
- Ye Z, Song H, Higgins JP, Pharoah P, Danesh J. Five glutathione S-transferase gene variants in 23,452 cases of lung cancer and 30,397 controls: meta-analysis of 130 studies. PLoS Med 2006;3.
- Kyzas PA, Loizou KT, Ioannidis JP. Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst 2005;97:1043-55.
- Kyzas PA, axa-Kyza D, Ioannidis JP. Almost all articles on cancer prognostic markers report statistically significant results. Eur J Cancer 2007;43:2559-79.
- Daly CA, Clemens F, Lopez Sendon JL, Tavazzi L, BOERSMA E, Danchin N, et al. The initial management of stable angina in Europe, from the Euro Heart Survey: a description of pharmacological management and revascularization strategies initiated within the first month of presentation to a cardiologist in the Euro Heart Survey of Stable Angina. Eur Heart J 2005;26:1011-22.
- Hemingway H, Crook AM, Feder G, Banerjee S, Dawson JR, Magee P, et al. Underuse of coronary revascularization procedures in patients considered appropriate candidates for revascularization. N Engl J Med 2001;344:645-54.
- Feder G, Crook AM, Magee P, Banerjee S, Timmis AD, Hemingway H. Ethnic differences in invasive management of coronary disease: prospective cohort study of patients undergoing angiography. BMJ 2002;324:511-16.
- Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290:86-97.
- Daly CA, De SB, Sendon JL, Tavazzi L, Boersma E, Clemens F, et al. Predicting prognosis in stable angina--results from the Euro heart survey of stable angina: prospective observational study. BMJ 2006;332:262-7.
- Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008;358:2107-16.
- Schnabel R, Blankenberg S. Oxidative stress in cardiovascular disease: successful translation from bench to bedside?. Circulation 2007;116:1338-40.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Jones DR, Heney D, et al. Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer 2003;88:1191-8.
- Kavvoura FK, Liberopoulos G, Ioannidis JP. Selection in reported epidemiological risks: an empirical assessment. PLoS Med 2007;4.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7.
- Hemingway H. Prognosis research: why is Dr Lydgate still waiting?. J Clin Epidemiol 2006;59:1229-38.
- Krleza-Jeric K, Chan AW, Dickersin K, Sim I, Grimshaw J, Gluud C. Principles for international registration of protocol information and results from human trials of health related interventions: Ottawa statement (part 1). BMJ 2005;330:956-8.
- Chew DP, Bhatt DL, Robbins MA, Penn MS, Schneider JP, Lauer MS, et al. Incremental prognostic value of elevated baseline C-reactive protein among established markers of risk in percutaneous coronary intervention. Circulation 2001;104:992-7.
- de Winter RJ, Heyde GS, Koch KT, Fischer J, van Straalen JP, Bax M, et al. The prognostic value of pre-procedural plasma C-reactive protein in patients undergoing elective coronary angioplasty. Eur Heart J 2002;23:960-6.
- de Winter RJ, Koch KT, van Straalen JP, Heyde GH, Bax M, Schotborgh CE, et al. C-reactive protein and coronary events following percutaneous coronary angioplasty. Am J Med 2003;115:85-90.
- de Winter RJ, Stroobants A, Koch KT, Bax M, Schotborgh CE, Mulder KJ, et al. Plasma N-terminal pro-B-type natriuretic peptide for prediction of death or nonfatal myocardial infarction following percutaneous coronary intervention. Am J Cardiol 2004;94:1481-5.
- Veselka J, Prochazkova S, Duchonova R, Homolova I, Tesar D. Relationship of C-reactive protein to adverse cardiovascular events in patients treated by percutaneous coronary intervention for stable angina pectoris. Int Heart J 2005;46:195-204.
- Hemingway H, Riley RD, Altman DG. Ten steps towards improving prognosis research. BMJ 2009;339. 10.1136/bmjb4184.
Appendix 1 A systematic review of four routine and seven novel biomarkers
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | Hb (g/dl) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison groups | RR | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | |||||||||||||||||
| Burr 1992(1) (DART) | 1755 | 60.3 | 0 | – | – | 100 | 15 | – | 1.5 | ACM | 92 | 3.49 | ● | ○ | ● | ○ | ○ | ○ | Continuous (per SD) | 0.72 | – | ||||
| Arant 2004(2) (WISE) | 864 | 58.4 | 100 | – | 63.9 | – | – | – | 3.3 | ACM | 155 | 5.44 | ● | ○ | ○ | ● | ○ | ● | Continuous (per g/dl) | 1.20 | – | ||||
| Duffy 2006(3) (–) | 1046 | 62.3 | 29.3 | – | 100 | 23.6 | 13.2 | – | 2.58 | ACM | 144 | 5.34 | ● | ○ | ○ | ○ | ○ | ○ | Continuous (per g/dl) | 0.82 | 0.74 to 0.9 | ||||
| Exaire 2006(4) (REPLACE-2) | 6002 | 62 | 25.6 | – | 100 | 8.2 | – | – | 1 | ACM | 128 | 2.13 | Adjustment variables unclear | Continuous (per mg/dl) | 0.76 | 0.68 to 0.86 | |||||||||
| Rajagopal 2004(5) (EPIC/EPISTENT/EPILOG)b | 2982 | 60.2 | 28.9 | 84.4 | 100 | 57.7 | – | – | 3 | ACM | 219 | 2.45 | ● | ● | ● | ○ | ● | ○ | Continuous (per unit increase) | 0.90 | 0.81 to 0.98 | ||||
| Fathi 2005(6) (–) | 4522 | 65 | 29.1 | – | 100 | 42.4 | 13.6 | – | 1.7 | ACM | 332 | 4.32 | ● | ● | ● | ○ | ● | ● | Continuous (per g/dl by log transformation) | 0.74 | 0.69 to 0.79 | ||||
| daSilveira 2008 (7) (–) | 310 | 63 | 38.7 | – | – | 51.5 | 13.4 | – | 3.67 | CVD | 43 | 3.78 | ● | ● | ● | ● | ● | ● | ≥ 12 women; ≥ 13 men | < 12 women, < 13 men | 3.28 | 1.66 to 6.50 | |||
| Elisheva 2000(8) (ISCAB)o | 4644 | 64.7 | 21.3 | – | – | – | – | – | 0.083 | ACM | 115 | 29.8 | ○ | ○ | ○ | ○ | ○ | ○ | > 12.9 | ≤ 12.9 | 6.85 | – | |||
| Lipsic 2005(9) (Intervention Cardiology Risk Stratification Study)o | 143 | 61.5 | 28.7 | – | 100 | 32.9 | 14.02 | – | 3.7 | CVD | 19 | 3.59 | ● | ● | ● | ● | ○ | ● | > 13.0 | ≤ 13.0 | 5.74 | 1.49 to 22.13 | |||
| McKechnie 2004(10) (–) | 45,165 | 65.4 | 33.9 | – | 100 | 34.2 | 13.6 | – | 0.014a | ACM | 1553 | 245.6 | ● | ○ | ○ | ○ | ○ | ● | > 13.0 | ≤ 13.0 | 1.2 | 1.05 to 1.34 | |||
| Muzzarelli 2006(11) (TIME) | 253 | 79.1 | 43 | 100 | 89.3 | 50 | 13.3 | – | 4 | CHD | 51 | 5.04 | > 13.0 | ≤ 13.0 | 1.76 | – | |||||||||
| Lee 2004(12) (–) | 6116 | 65.4 | 31.6 | 31.1 | 100 | 27.6 | – | – | 0.083 | ACM | 107 | 21.1 | ● | ● | ○ | ○ | ○ | ● | > 12 | 10–12 | < 10 | 1.9 | 1.2 to 6.0 | ||
| Skinner 1999(13) (–)b | 353 | 57.2 | 15.9 | 98 | 100 | 61 | – | EDTA | 5 | CHD | 16 | 0.91 | Crude | – | – | – | 0.20 | 0.04 to 0.95 | |||||||
| Martin 1991(14) (–)c | 1716 | – | 0 | – | – | 100 | 15.0 | – | 2 | CHD | 126 | 3.67 | ● | ○ | ○ | ○ | ○ | ○ | – | – | – | – | 0.5 | – | |
| Reinecke 2003(15) (–) | 689 | 62.6 | 0 | – | 30.2 | 100 | – | – | 2 | ACM | 62 | 4.5 | ● | ○ | ● | ○ | ○ | ● | ≤ 12.9 | 13.0–13.8 | 13.9–14.5 | 14.6–15.2k | ≥ 15.3 | 4.09p | 1.52 to 11.05 |
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | Fasting glucose (mmol/l) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison groups | RR | 95% CI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | ||||||||||||||||||
| Shah 2008(16) (–) | 2886 | 66.2 | – | – | 100 | 31.8 | 7.48 | – | 5.025 | ACM | 961 | 6.63 | ● | ○ | ○ | ○ | ● | ○ | Continuous (per mg/dl) | 0.999 | – | |||||
| Munoz 2007(17) (ICAR) | 983 | 63.9 | 25.5 | – | – | – | 5.7 | – | 3 | CVD | 32 | 1.09 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per mmol/l) | 1.01 | 1.00 to 1.02 | |||||
| Vaidya 2007(18) (WAVE) | 400 | 65.1 | 100 | – | 100 | 42.5 | 5.73 | Glucose oxidase method | 2.6 | ACM +nf MI | 26 | 2.5 | ● | ○ | ○ | ○ | ○ | ○ | Continuous (per 10 mg/dl) | 1.11 | 1.06 to 1.16 | |||||
| Karlson 2000(19) (–) | 624 | 63 | 33 | 49 | – | 100 | 4.8 | – | 10 | ACM | 41 | 0.66 | ● | ● | ● | ○ | ○ | ● | Continuous (per quartile) | 1.07 | 1.02 to 1.12 | |||||
| Zindrou 2001(20) (–) | 878 | 61.7 | 17.7 | 38.2 | 100 | 6.9 | 5.3 | Colorimetric oxidase | 0.082 | ACM | 30 | 41.2 | Crude | Continuous (per quartile) | 1.39 | 1.15 to 1.67 | ||||||||||
| Dankner 2003(21) (BIP)m | 14,539 | 60.3 | 18.9 | – | 100 | 72.2 | 6.34 | – | 5.2 | CHD | 1055 | 1.39 | ● | ● | ● | ● | ● | ● | Continuous (per 40 mg/dl) | 1.21 | 1.16 to 1.26 | |||||
| Wong 1989(22) (Framingham)m | 344 | 62.2 | 31.7 | – | – | 100 | 5.33 | – | 32 | CHD | 126 | 1.14 | ● | ○ | ○ | ○ | ○ | ○ | Continuous (per 25 mg/dl) | 1.11 | 1.03 to 1.20 | |||||
| Shah 2005(23) (–)m | 1746 | 65.3 | 0.8 | – | 100 | 20.9 | 6.99 | – | – | ACM | – | – | ● | ○ | ○ | ○ | ● | ● | Continuous (per 10 mg/dl) | 1.03 | 1.02 to 1.05 | |||||
| de Lorgeril 1999(24) (Lyon Diet Heart Study)m | 423 | – | – | – | – | 100 | – | – | 3.8 | CVD | 58 | 3.61 | Crude | Continuous (per mmol/l) | 1.11 | 0.89 to 1.36 | ||||||||||
| Stewart 2008(25) (LIPID) | 8733 | 61 | 16.6 | – | – | 72 | – | – | 7.8 | CVD | – | – | ● | ● | ○ | ○ | ○ | ○ | ≤ 7.0 | > 7.0 | 1.59 | 1.33 to 1.90 | ||||
| Leander 2007(26) (Stockholm Heart Epidemiology Program) | 1105 | 58.6 | 0 | – | – | 0 | 5.3 | – | 7.2 | CHD | 320 | 4.02 | ○ | ○ | ● | ● | ● | ● | < 6.0 | ≥ 6.0 | 1.2 | 0.7 to 1.8 | ||||
| Leander 2007(26) (Stockholm Heart Epidemiology Program) | 538 | 62.1 | 100 | – | – | 0 | 5.2 | – | 7.2 | CHD | 141 | 3.64 | ○ | ○ | ● | ● | ● | ● | < 5.8 | ≥ 5.8 | 2.00 | 0.9 to 4.7 | ||||
| Corsetti 2007(27) (THROMBO) | 173 | 60.3 | 32.9 | – | – | 24.3 | 8.69 | – | 2.17 | CHD | 51 | 13.59 | Crude | ≤ 10.55 | > 10.55 | 1.99 | 1.13 to 3.52 | |||||||||
| Nigam 2006(28) (CASS)m | 24,958 | 52.9 | 24.4 | – | 72.3 | – | 5.79 | – | 12.6 | ACM | 10,302 | 3.28 | ● | ● | ● | ● | ○ | ● | < 6.1 | ≥ 6.1 | 1.33 | 1.25 to 1.41 | ||||
| Arcavi 2004(29) (BIP)m | 3122 | 60.1 | 8.6 | 57 | – | 100 | 5.58 | – | 6.2 | Morbidity | 847 | 4.38 | ● | ● | ● | ● | ● | ● | < 7.0 | ≥ 7.0 | 1.4 | 1.18 to 1.77 | ||||
| The Coronary Drug Project Research Group 1975(30) (–)m | 2789 | 47 | 0 | – | – | 100 | 5.61 | – | 5 | ACM | 584 | 4.19 | Crude | < 5.6 | ≥ 5.6 | – | – | |||||||||
| Schlant 1982(31) (Coronary Drug Project) | 2789 | 52.4 | 0 | – | – | 100 | – | – | 5 | CHD | 461 | 3.31 | Crude | < 5.6 | ≥ 5.6 | – | – | |||||||||
| Dibra 2005(32) (–)m | 990 | 65.4 | 20.3 | 100 | 100 | 39.8 | – | Dehydrogenase method | 1 | ACM | 54 | 5.45 | ● | ● | ● | ● | ● | ● | < 5.6 | 5.6–6.05 | 2.3 | 1.29 to 4.06 | ||||
| Held 2005(33) (APSIS) | 740 | 59.2 | 30.9 | 100 | 6.4 | 22.8 | 5.2 | Autoanalyser | 3.4 | CVD | 55 | 2.19 | ● | ○ | ● | ○ | ○ | ● | < 6.1 | ≥ 6.1 | 2.79 | 1.97 to 3.84 | ||||
| Yun 2006(34) (–)m | 98 | 59.8 | 35.7 | 29.6 | – | 5.6 | 5.72 | – | 3 | ACM | 6 | 2.04 | Adjustment variables unclear | < 6.1 | ≥ 6.1 | 4.58 | 0.28 to 84.0 | |||||||||
| Hu 2006(35) (DESIRE)m | 1280 | 59.8 | 24.5 | – | 100 | 9.4 | 6.21 | – | 2.27 | ACM | 158 | 5.43 | ● | ● | ○ | ● | ○ | ○ | < 5.6 | ≥ 5.6 | 1.43 | 1.04 to 1.87 | ||||
| Anderson 2004(36) (Intermountain Heart Collaborative study)m | 2035 | 65 | 24 | 1 | 100 | 17 | – | – | 2.8 | ACM | 345 | 6.05 | ● | ● | ● | ● | ● | ● | < 6.1 | 6.1–6.9 | ≥ 6.9 | 1.68 | 1.48 to 1.90 | |||
| Wilhelmsen 2001(37) (4S) | 1468 | 58.7 | 0 | – | 100 | 100 | – | – | 5.4 | CHD | 431 | 5.44 | Crude | < 5.0 | 5.0–5.9 | ≥ 6.0 | 1.34 | 1.05 to 1.70 | ||||||||
| Rubins 2002(38) (VA-HIT)m | 1260 | 64.4 | 0 | – | – | 61.1 | 6.42 | – | 5.1 | CHD | 328 | 5.10 | ● | ● | ● | ● | ● | ● | < 6.1 | 6.1–6.9 | ≥ 7.0 | 1.72 | 1.01 to 2.68 | |||
| Van de Veire 2006(39) (–)m | 160 | 65.3 | 0 | – | 100 | 73 | 5.72 | – | 2.7 | ACM | 25 | 5.79 | Crude | < 6.1 | 6.1–6.9 | ≥ 7.0 | – | – | ||||||||
| Canner 2005(40) (Coronary Drug Project)m | 3906 | – | 0 | – | – | 100 | – | – | 6.2 | CHD | 1194 | 6.22 | Crude | < 5.3 | 5.3–5.8 | 5.8–6.9 | ≥ 7.0 | – | – | |||||||
| Kanaya 2005(41) (HERS)m | 2763 | 67.2 | 100 | – | 100 | 17.2 | 6.05 | Hexokinase enzymaticmethod | 6.8 | CHD | 254 | 1.35 | ● | ● | ● | ○ | ● | ● | < 5.6 | 5.6–6.0 | 6.1–6.9 | ≥ 7.0 | 2.11 | 1.55 to 2.88 | ||
| Fisman 2004(42) (BIP)m | 14670 | 59.8 | 18.9 | – | – | 71.5 | 6.13 | Autoanalyser | 8 | CHD | 1470 | 1.25 | ● | ● | ● | ● | ● | ● | ≤ 3.8 | 3.9–4.4 | 4.4–6.0k | 6.1–6.9 | 7.0–7.7 | ≥ 7.7 | 2.27 | 2.00 to 2.57 |
| The Coronary Drug Project Research Group 1977(43) (–)m | 2770 | 47 | 0 | – | – | 100 | 5.65 | Autoanalyser | 5 | CHD | 449 | 3.24 | ● | ● | ● | ● | ○ | ● | < 5.0 | 5.0–5.5 | 5.6–6.0 | 6.1–6.6 | 6.7–7.7 | ≥ 7.8 | – | – |
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | TC (mmol/l) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk(%) | Adjustments | Comparison groups | RR | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC/LDL/HDL/TG) | Obesity | Diabetes | |||||||||||||||||
| Asztalos 2008(44) (VA-HIT) | 754 | 64 | – | – | – | – | 4.32 | Standard enzymatic methods | 5.1 | CHD | 168 | 4.37 | ● | ○ | ● | ● | ● | ● | Continuous (per SD) | 1.13 | 0.95 to 1.33 | ||||
| Inaguma 2007(45) (–) | 790 | 67.7 | 27.1 | – | – | 64.1 | 4.61 | – | 2.31 | CVD | 110 | 6.03 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per mg/dl) | 0.99 | 0.99 to 1.00 | ||||
| Munoz 2007(17) (ICAR) | 983 | 63.9 | 25.5 | – | – | – | 5.4 | – | 3 | CVD | 32 | 1.09 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per mmol/l) | 1.0 | 0.99 to 1.01 | ||||
| Inoue 2007(46) (–) | 149 | 63 | 29 | 53.7 | 83.2 | 29.5 | 4.77 | – | 7 | CVD | 58 | 5.56 | Crude | Method unclear | 1.5 | 0.5 to 2.8 | |||||||||
| Bolibar 2000(47) (ECAT) | 2806 | 55.8 | 15.9 | 36.0 | – | 45.0 | 6.57 | – | 2 | CHD | 106 | 1.89 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.17 | 0.97 to 1.42 | ||||
| Falcone 2006(48) (–) | 1014 | 64.6 | 27.2 | 82.9 | 100.0 | 44.9 | 5.08 | Autoanalyser | 2.7 | CVD | 105 | 3.84 | ● | ● | ○ | ● | ○ | ○ | Continuous (per SD) | 1.31 | 1.09 to 1.58 | ||||
| Soeki 1999(49) (–)n | 106 | 62.3 | 25.5 | – | – | 35.8 | 5.26 | EIA | 4.2 | CHD | 11 | 2.47 | Crude | Continuous (per SD) | 0.92 | 0.51 to 1.66 | |||||||||
| Retterstol 2002(50) (–) | 247 | 52.7 | 21.9 | – | – | 100.0 | 6.9 | EIA | 10 | CHD | 36 | 1.46 | ● | ○ | ● | ● | ○ | ○ | Continuous (per quartile) | 1.07 | 0.78 to 1.45 | ||||
| Retterstol 2001(51) (–) | 247 | 52.7 | 21.9 | 44.5 | – | 100.0 | 6.9 | EIA | 10 | CHD | 35 | 1.42 | ● | ○ | ● | ● | ○ | ○ | Continuous (per quartile) | 1.10 | 0.8 to 1.6 | ||||
| Deckers 2006(52) (EUROPA) | 12,218 | 60.0 | 15 | – | – | 65.0 | 5.4 | – | 4.1 | CHD | 1091 | 2.18 | ● | ● | ● | ● | ● | ● | Continuous (per mmol/l) | 1.15 | 1.09 to 1.21 | ||||
| Minoretti 2006(53) (–) | 799 | 64.9 | 25.6 | 100.0 | 100.0 | 46.3 | 5.1 | Autoanalyser | 2.7 | ACM | 69 | 3.20 | ● | ● | ● | ● | ● | ● | Continuous (per mmol/l log increase) | 1.21 | 0.96 to 1.54 | ||||
| Brilakis 2005(54) (–) | 466 | 60.1 | 38 | – | 75.8 | 15 | 5.4 | EIA | 4 | ACM | 61 | 3.27 | ● | ● | ● | ● | ○ | ○ | Continuous (per 1.17 mmol/l) | 0.75 | 0.55 to 1.04 | ||||
| de Lorgeril 1999(24) (Lyon Diet Heart Study) | 423 | – | – | – | – | 100.0 | 6.16 | – | 3.8 | CVD | 58 | 3.61 | Crude | Continuous (per mmol/l) | 1.31 | 1.05 to 1.65 | |||||||||
| Tervahauta 1995(55) (Seven Countries Study) | 171 | 72.2 | 0.0 | – | 100.0 | – | 6.2 | EIA | 5 | CHD | 42 | 4.91 | ● | ● | ● | ● | ● | ○ | Continuous (per mmol/l) | 1.30 | 1.0 to 1.7 | ||||
| Bittner 2002(56) (BARI) | 1514 | 61.2 | 27 | 96.0 | 100.0 | 53.0 | 5.57 | – | 5.4 | Morbidity | – | – | ● | ● | ● | ● | ● | ● | Continuous (per 0.26 mmol/l) | 1.04 | 1.00 to 1.09 | ||||
| Simes 2002(57) (LIPID) | 4502 | 53.0 | – | – | – | – | 5.65 | – | 5 | CHD | – | – | ● | ● | ● | ● | ○ | ● | Continuous (per mmol/l) | 1.24 | 1.08 to 1.44 | ||||
| Qi 2003a(58) (–) | 134 | 64.1 | 19.4 | 48.5 | 100.0 | 34.4 | 3.83 | – | 1 | ACM | 32 | 23.88 | Crude | Continuous (method unclear) | 0.98 | 0.93 to 1.06 | |||||||||
| Qi 2003b(59) (–) | 121 | 64.1 | 21.5 | 43.8 | 100.0 | 30.6 | 3.87 | – | 0.077 | ACM | 16 | 171.73 | Crude | Continuous (method unclear) | 1.03 | 0.96 to 1.05 | |||||||||
| Hu 2006(35) (DESIRE) | 1280 | 59.8 | 24.5 | – | 100 | 9.4 | 4.80 | – | 2.27 | ACM | 158 | 5.43 | ● | ● | ○ | ● | ○ | ○ | Continuous (per mg/l) | 1.00 | 0.99 to 1.01 | ||||
| Dankner 2003(21) (BIP)n | 14,539 | 60.3 | 18.9 | – | 100.0 | 72.2 | 5.80 | – | 5.2 | CHD | 1055 | 1.40 | ● | ● | ● | ● | ● | ● | Continuous (per 40 mg/dl) | 1.09 | 1.02 to 1.15 | ||||
| Wong 1989(22) (Framingham)n | 344 | 62.2 | 31.7 | – | – | 100.0 | 6.31 | – | 32 | CHD | 126 | 1.14 | ● | ● | ○ | ● | ○ | ● | Continuous (per 50 mg/dl) | 1.34 | 1.12 to 1.59 | ||||
| Susen 2005(60) (–)n | 488 | 61.0 | 22 | 69.0 | 100.0 | 19.0 | 5.23 | – | 1.24 | ACM | 44 | 7.27 | Crude | Continuous (per mg/dl log increase) | 1.00 | 0.99 to 1.00 | |||||||||
| Dibra 2003(61) (–)n | 1152 | 66.1 | 26.6 | 100.0 | 100.0 | 31.5 | 5.26 | – | 1 | ACM | 86 | 7.46 | ● | ● | ○ | ● | ○ | ● | Continuous (per 50 mg/dl) | 1.10 | 0.90 to 1.40 | ||||
| Buchwald 2001(62) (POSCH)b | 838 | – | – | – | – | 100.0 | – | – | 9.7 | CHD | 119 | 1.46 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per unit increase) | 1.00 | – | ||||
| Lundstam 2002(63) (–) | 963 | 59.0 | 23 | 93.9 | 100.0 | 51.0 | – | EIA | 11.7 | ACM | 363 | 3.22 | Adjustment variables unclear | Continuous (ln) | 0.50 | 0.3 to 0.8 | |||||||||
| Arcavi 2004(29) (BIP)n | 3122 | 60.1 | 8.6 | 57.0 | – | 100.0 | 5.50 | – | 6.2 | Morbidity | 847 | 4.38 | ● | ● | ● | ● | ● | ● | Continuous (–) | 1.01 | 1.00 to 1.01 | ||||
| van Lennep 2000(64) (–) | 838 | 64.8 | 20.4 | – | 100.0 | 47.2 | 7.1 | EIA | 2.99 | ACM | 101 | 4.03 | ● | ● | ● | ● | ○ | ● | Continuous (–) | 0.73 | 0.38 to 1.38 | ||||
| Bosevski 2005(65) (–) | 90 | 62.3 | 27 | – | 100.0 | 54.4 | 5.64 | – | 3 | ACM | – | – | ● | ● | ● | ● | ○ | ● | Continuous (–) | 0.05 | – | ||||
| Schlant 1982(31) (Coronary Drug Project)n | 2789 | 52.4 | 0.0 | 57.8 | 100.0 | – | – | – | 5 | ACM | 591 | 4.24 | Crude | < 6.48 | ≥ 6.48 | – | – | ||||||||
| The Coronary Drug Project Research Group 1975(30)n | 2789 | 47.0 | 0 | – | – | 100.0 | 6.45 | – | 5 | ACM | 583 | 4.18 | Crude | < 6.48 | ≥6.48 | – | – | ||||||||
| Behar 1997(66) (BIP)n | 11,563 | 59.8 | 21.7 | 28.9 | – | 70.6 | 5.85 | EIA | 3.3 | CHD | 535 | 1.40 | ● | ● | ● | ● | ○ | ● | ≤ 4.14 | > 4.14 | 1.09 | 0.76 to 1.56 | |||
| Zhukovskii 1982(67) (–)n | 475 | 49.5 | 0.0 | 28.0 | – | 27.6 | – | – | 3.8 | CHD | 186 | 10.30 | Adjustment variables unclear | < 7.0 | ≥ 7.0 | – | – | ||||||||
| Glader 2002(68) (–) | 1196 | 59.4 | 18.2 | 100.0 | 100.0 | 53.0 | 6.9 | EIA | 6.7 | CVD | 152 | 1.90 | Crude | < 6.5 | ≥ 6.5 | 0.90 | 0.7 to 1.3 | ||||||||
| Frank 1973(69) (–)n | 745 | 44.0 | 0.0 | 36.9 | – | 63.0 | – | – | 4.5 | CHD | 105 | 3.13 | Crude | ≤ 7.0 | > 7.0 | – | |||||||||
| Berge 1982(70) (The Coronary Drug Project)n | 354 | 47.0 | 0 | – | – | 100.0 | – | Autoanalyser | 5 | ACM | 80 | 4.52 | Crude | < 6.48 | ≥ 6.48 | – | – | ||||||||
| Takahashi 1997(71) (–)n | 312 | 60.0 | 24.8 | – | 100.0 | 48.0 | 4.98 | EIA | 4 | CVD | 53 | 4.25 | ● | ○ | ○ | ○ | ○ | ○ | < 5.70 | ≥ 5.70 | 2.30 | 1.2 to 4.2 | |||
| Wu 2005(72) (–)n | 150 | 67.8 | 9.3 | 100.0 | 100.0 | 19.7 | 4.70 | ELISA | 1.48 | CHD | 48 | 21.62 | Crude | < 5.18 | ≥ 5.18 | 1.42 | 0.80 to 2.56 | ||||||||
| Fukushima 2004(73) (–)n | 120 | 65.6 | 37.5 | – | 100.0 | – | 5.23 | – | 1.7 | CHD | 44 | 21.57 | Crude | ≤ 5.70 | > 5.70 | 1.20 | 0.6 to 2.3 | ||||||||
| Chikamori 2000(74) (–)n | 392 | 60.6 | 25.3 | – | 100.0 | – | 5.26 | EIA | 1.4 | CHD | 43 | 7.84 | Adjustment variables unclear | ≤ 5.18 | > 5.18 | 1.50 | 0.80 to 2.70 | ||||||||
| Janoskuti 2005(75) (–) | 387 | 59.0 | 26.9 | – | – | 48.1 | 5.9 | EIA | 5.1 | ACM | 41 | 2.08 | ● | ● | ● | ● | ● | ○ | ≥ 5.2 | < 5.2 | 2.90 | 1.02 to 8.42 | |||
| Lipsic 2005(9) (Intervention Cardiology Risk Stratification Study) | 143 | 61.5 | 28.7 | – | 100.0 | 32.9 | – | – | 3.7 | CVD | 19 | 3.59 | ● | ● | ● | ● | ○ | ● | ≤ 6.5 | > 6.5 | 3.74 | 1.26 to 11.04 | |||
| Cesena 2004(76) (–)n | 574 | 61.0 | 27.5 | 97.4 | 100.0 | 65.9 | 5.57 | – | 0.47 | CHD | 12 | 4.45 | Crude | < 6.22 | ≥ 6.22 | 3.68 | – | ||||||||
| Zotz 2000(77) (–)n | 251 | 64.5 | 19.5 | – | 100.0 | 64.0 | 6.37 | – | 1 | ACM | 10 | 3.98 | Crude | ≤ 5.18 | > 5.18 | – | – | ||||||||
| Wilhelmsen 2001(37) (4S) | 1490 | – | 0.0 | – | 100.0 | – | – | EIA | 5.4 | CHD | 431 | 5.36 | Crude | ≤ 6.40 | 6.41–7.00 | ≥ 6.5 | 1.22 | 1.00 to 1.49 | |||||||
| Hoffmann 1980(78) (–)n | 1414 | 65.6 | 0.0 | – | 100.0 | 65.5 | 6.86 | Autoanalyser | 5 | ACM | 175 | 2.48 | Crude | < 6.48 | 6.48–9.07 | > 9.07 | – | – | |||||||
| Nygard 1997(79) (–) | 574 | 62.0 | 18.6 | – | 100.0 | 57.4 | – | Chemical assay | 4.6 | ACM | 64 | 2.42 | ● | ● | ● | ● | ○ | ● | < 5.50 | 5.50–6.99 | 7.00–8.99 | ≥ 9.0 | 1.59 | 0.50 to 5.07 | |
| Ulvenstam 1984(80) (–) | 1204 | 53.2 | 0.0 | – | – | 100.0 | 7.01 | EIA | 11 | ACM | 254 | 1.92 | Crude | ≤ 5.99 | 6.0–6.69 | 6.7–7.29 | 7.3–8.03 | ≥ 8.04 | – | – | |||||
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | LDL (mmol/l) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison groups | RR | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | |||||||||||||||||
| Vittinghoff 2003(81) (HERS) | 2763 | 66.6 | 100 | 26.4 | 100 | – | 3.8 | – | 4.1 | CHD | 361 | 3.19 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.10 | 1.00 to 1.22 | ||||
| Bolibar 2000(47) (ECAT) | 2806 | 55.8 | 15.9 | 36 | – | 45 | 4.7 | – | 2 | CHD | 106 | 1.89 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.23 | 0.99 to 1.53 | ||||
| Falcone 2006(48) (–) | 1014 | 64.6 | 27.2 | 82.9 | 100 | 44.9 | 3.46 | Autoanalyser | 2.7 | CVD | 105 | 3.84 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.28 | 1.09 to 1.52 | ||||
| Asztalos 2008(44) (VA-HIT) | 754 | 64 | – | – | – | – | 2.89 | Friedwald formula | 5.1 | CHD | 168 | 4.37 | ● | ○ | ● | ● | ● | ● | Continuous (per SD) | 1.08 | 0.93 to 1.26 | ||||
| Munoz 2007(17) (ICAR) | 983 | 63.9 | 25.5 | – | – | – | 2.68 | – | 3 | CVD | 32 | 1.09 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per mmol/l) | 1.0 | 0.99 to 1.02 | ||||
| vonEnyatten 2008(82) (–) | 1051 | 59 | 15.1 | – | 95.2 | 58.2 | 2.59 | Friedwald formula | 4.7 | CVD | 95 | 1.92 | ● | ● | ● | ○ | ● | ● | Continuous (per mmol/l) | 1.4 | 1.07 to 1.84 | ||||
| Volzke 2007(83) (–) | 988 | 61.2 | 21.7 | – | 100 | 67 | 3.9 | Friedwald formula | 8 | ACM | 535 | 6.77 | Crude | Continuous (per mmol/l) | 1.12 | 1.05 to 1.20 | |||||||||
| Glader 2002(68) (–) | 1046 | 59.4 | 18.2 | 100 | 100 | 53 | 4.8 | Friedwald formula | 6.7 | CVD | 152 | 2.19 | Crude | Continuous (per mmol/l) | 0.98 | 0.84 to 1.14 | |||||||||
| Bittner 2002(56) (BARI) | 1514 | 61.2 | 27 | 96 | 100 | 53 | 3.66 | Friedwald formula | 5.4 | Morbidity | – | – | ● | ● | ● | ● | ● | ● | Continuous (per mmol/l) | 1.033 | 0.98 to 1.09 | ||||
| Simes 2002(57) (LIPID) | 4502 | 53 | – | – | – | – | 3.88 | Friedwald formula | 5 | CHD | – | – | ● | ● | ● | ● | ○ | ● | Continuous (per mmol/l) | 1.28 | 1.10 to 1.46 | ||||
| LaRosa 2007(84) (TNT) | 9769 | 61 | 19 | 81.5 | – | 58.4 | 2.53 | – | 4.9 | CHD | – | – | ● | ● | ● | ○ | ● | ● | Continuous (per mg/dl) | 1.15 | 1.1 to 1.2 | ||||
| Aronow 2002A(85) (–)n | 1410 | 80.5 | 65.4 | – | – | 100 | 3.97 | – | 3 | CHD | 838 | 19.8 | ● | ○ | ● | ● | ○ | ● | Continuous (per mg/dl) | 1.01 | 1.007 to 1.012 | ||||
| Kastelein 2008(86) (TNT/IDEAL) | 18,018 | 61.4 | 18.9 | – | – | – | 2.32 | Friedwald formula | 4.8 | CHD | 1783 | 2.06 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per 27.4 mg/dl) | 1.15 | 1.10 to 1.20 | ||||
| Sacks 1998(87) (CARE)n | 4159 | 59.0 | 14.0 | – | – | 100 | 3.60 | – | 2 | CHD | 486 | 5.84 | ● | ● | ● | ● | ○ | ● | Continuous (per 25 mg/dl) | 1.21 | 1.11 to 1.31 | ||||
| Aronow 2002(88) (–)n | 529 | 79 | 67.7 | – | – | 100 | 4.01 | – | 2.42 | CHD | 405 | 34.2 | ● | ○ | ● | ● | ○ | ○ | Continuous (per mg/dl) | 1.01 | 1.005 to 1.011 | ||||
| Olsson 2005(89) (MIRACL) | 2739 | 65 | 33.3 | – | – | 54 | – | Friedwald formula | 0.31 | ACM | 121 | 14.3 | – | – | – | – | – | – | Continuous (mg/dl) | 1.001 | 0.996 to 1.007 | ||||
| Yanase 2004(90) (–)n | 102 | 61.3 | 16.7 | – | 100 | 76.5 | 3.34 | – | 4 | ACM | 23 | 5.64 | Crude | Continuous (per mg/dl) | 0.99 | 0.98 to 1.01 | |||||||||
| Buchwald 2001(62) (POSCH)n | 838 | – | – | – | – | 100 | – | – | 9.7 | CHD | 119 | 1.46 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per unit increase) | 1.01 | – | ||||
| Linden 1998(91) (–) | 964 | 60.8 | 23 | – | – | 50.6 | 4.57 | Friedwald formula | 5.2 | ACM | 168 | 3.35 | Crude | Continuous (per unit increase by log transformation) | 0.84 | 0.72 to 0.97 | |||||||||
| Minoretti 2006(53) (–) | 799 | 64.9 | 25.6 | 100 | 100 | 46.3 | 3.47 | Friedwald formula | 2.7 | CVD | 69 | 3.20 | ● | ● | ● | ● | ● | ● | Continuous (per unit increase by log transformation) | 1.21 | 0.81 to 1.56 | ||||
| Susen 2005(60) (–)n | 488 | 61.0 | 22.0 | 69 | 100 | 19 | 3.42 | – | 1.24 | ACM | 44 | 7.27 | Crude | Continuous (per unit increase by log transformation) | 1.00 | 0.99 to 1.00 | |||||||||
| Guang-da 2004(92) (–) | 131 | 65.0 | 49.6 | – | 100 | – | 3.04 | EIA | 7 | CHD | 39 | 4.25 | ● | ○ | ○ | ● | ○ | ○ | Continuous (per unit increase by log transformation) | 1.25 | 0.98 to 1.58 | ||||
| Lundstam 2002(63) (–) | 908 | 59 | 23 | 100 | – | 51 | – | Friedwald formula | 11.7 | ACM | 363 | 3.42 | – | – | – | – | – | – | Continuous (ln) | 0.5 | 0.3 to 0.7 | ||||
| Qi 2003a(58) (–) | 134 | 64.1 | 19.4 | 48.5 | 100 | 34.4 | 1.69 | – | 1 | ACM | 32 | 23.9 | Crude | Continuous | 0.46 | 0.31 to 1.12 | |||||||||
| Qi 2003b(59) (–) | 121 | 64.1 | 21.5 | 43.8 | 67 | 30.6 | 1.66 | – | 0.077 | ACM | 16 | 171.73 | Crude | Continuous | 0.88 | 0.12 to 1.16 | |||||||||
| Benchimol 2000(93) (–) | 319 | 57 | 14.1 | 56.4 | 100 | 43.6 | 4.5 | Precipitation | 2 | CHD | 12 | 1.89 | ○ | ○ | ○ | ○ | ○ | ○ | Continuous | 1.8 | 1.1 to 3.0 | ||||
| Bosevski 2005(65) (–) | 90 | 62.3 | 27 | – | – | 100 | 3.52 | – | 3 | ACM | – | – | ● | ● | ● | ● | ○ | ● | Continuous | 0.25 | – | ||||
| Aronow 2001(94) (–)n | 613 | 79.1 | 67.7 | – | – | 100 | – | – | 2.42 | CHD | 460 | 31.0 | ● | ● | ● | ● | ● | ○ | < 3.24 | ≥ 3.24 | 1.42 | 1.15 to 1.75 | |||
| Fukushima 2004(73) (–)n | 120 | 65.6 | 37.5 | – | 100 | – | 3.32 | – | 1.7 | CHD | 44 | 21.57 | Crude | ≤ 3.57 | > 3.57 | 1.0 | 0.6 to 1.8 | ||||||||
| Janoskuti 2005(75) (–) | 387 | 59 | 26.9 | –1 | 100 | 44.9 | 3.7 | Friedwald formula | 5.1 | ACM | 41 | 2.08 | ● | ● | ● | ● | ● | ○ | ≥ 0.92 | < 0.92 | 0.63 | 0.25 to 1.55 | |||
| Leu 2004(95) (–)n | 75 | 68.1 | 12 | 100 | 100 | 25.3 | 2.97 | Friedwald formula | 3.33 | CVD | 33 | 13.2 | Crude | ≤ 4.14 | > 4.14 | 2.92 | 1.19 to 7.13 | ||||||||
| Zotz 2000(77) (–)n | 251 | 64.5 | 19.5 | – | 100 | 64 | 4.10 | – | 1 | CVD | 10 | 3.98 | Crude | ≤ 3.89 | > 3.89 | – | – | ||||||||
| Wilhelmsen 2001(37) (4S) | 1490 | 58.7 | 0 | – | 100 | – | – | Friedwald formula | 5.4 | CHD | 431 | 5.36 | Crude | ≤ 4.5 | 4.51–5.15 | ≥ 5.16 | 1.16 | 0.95 to 1.42 | |||||||
| van Lennep 2000(64) (–) | 848 | 64.8 | 20.4 | – | 100 | 47.2 | 4.9 | Friedwald formula | 2.99 | ACM | 101 | 3.98 | ● | ○ | ○ | ○ | ○ | ○ | – | – | Median | 1.16 | 0.80 to 1.67 | ||
| Wattanakit 2005(96) (ARIC)n | 766 | 57.1 | 24.7 | – | 100 | 81 | – | – | 8.7 | CVD | 313 | 4.70 | ● | ● | ○ | ○ | ○ | ○ | < 3.2 | 3.2–3.8 | 3.9–4.4 | ≥ 4.4 | 1.84 | 1.2 to 2.7 | |
| Schlitt 2005(97) (–)n | 1294 | 61.8 | 25.8 | – | 100 | – | 3.65 | EIA | 3.9 | CVD | 158 | 3.13 | ● | ● | ● | ● | ● | ● | ≤ 3.0 | 3.0 –3.6 | 3.6–4.3 | > 4.3 | 1.66 | 0.67 to 4.1 | |
| Sacks 2000(98) (CARE)n | 788 | 60.0 | 13.0 | – | – | – | 3.6 | EIA | 5 | CHD | 418 | 10.6 | ● | ○ | ● | ● | ○ | ○ | – | – | – | – | – | 1.73 | 1.1 to 2.7 |
| Inoue 2007 (46) (–) | 149 | 63 | 29 | 53.7 | 83.2 | 29.5 | 3.13 | – | 7 | CVD | 58 | 5.56 | Crude | – | 1.5 | 0.8 to 2.45 | |||||||||
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | Fibrinogen mean (mg/dl) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison groups | RR | 95% CI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids | Obesity | Diabetes | ||||||||||||||||
| Blankenberg 2006(99) (HOPE) | 3199 | 65.4 | 23.2 | – | 100 | – | 353 | von Claus method | 4.5 | CVD | 501 | 3.48 | ● | ● | ○ | ● | ○ | ● | Continuous (per SD) | 1.15 | 1.01 to 1.25 | |||
| Sinning 2006(100) (Atherogene)c | 1806 | 61.7 | 21.3 | 100 | 100 | 47.5 | 314 | Derived method | 3.5 | CVD | 131 | 2.07 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.26 | 1.09 to 1.46 | |||
| Thompson 1995(101) (ECAT)c | 2806 | 53.8 | 14.8 | 37.0 | 75.8 | 44.3 | 301 | – | 2 | CHD | 106 | 1.89 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.31 | 1.07 to 1.61 | |||
| Falcone 2006(48) (–) | 1014 | 64.6 | 27.2 | 82.9 | 100 | 44.9 | 341 | – | 2.7 | CVD | 105 | 3.84 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per SD) | 1.87 | 0.32 to 4.21 | |||
| Morange 2006(102) (Atherogene)b | 1057 | 61.5 | 23.2 | 69.8 | 100 | 48.5 | – | Derived method | 6.6 | CVD | 135 | 1.94 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 1.27 | 1.04 to 1.55 | |||
| Soeki 1999(49) (–)j | 106 | 62.3 | 25.5 | – | – | 35.8 | – | Claus method | 4.17 | CHD | 11 | 2.47 | Crude | Continuous (per SD) | 1.07 | 0.61 to 1.87 | ||||||||
| Burr 1992(1) (DART)c | 1706 | 60.3 | 0 | – | – | 100 | 450 | N | 1.5 | ACM | 85 | 3.32 | ● | ○ | ● | ○ | ○ | ○ | Continuous (per SD) | 1.34 | – | |||
| Thompson 1996(102) (–)c | 209 | 53 | 20 | 100 | – | 47 | 320 | Von Claus method | 9 | ACM | 45 | 2.39 | ● | ● | ● | ○ | ○ | ○ | Continuous (per SD) | 1.29 | 0.96 to 1.73 | |||
| Cooper 1991(104) (PARIS I)h | 70 | 52 | – | – | – | 100 | – | – | 4 | ACM | 20 | 7.14 | ● | ● | ● | ○ | ○ | ○ | Continuous (per SD) | 1.69 | 0.96 to 2.97 | |||
| Soeki 1999(49) (–)j | 106 | 62.3 | 25.5 | – | – | 35.8 | – | Claus method | 4.17 | CHD | 11 | 2.47 | Crude | Continuous (per SD) | 1.07 | 0.61 to 1.87 | ||||||||
| Hartmann 2006(105) (–) | 60 | 58 | 17 | 100 | 100 | 35 | 278 | N | 1.5 | Morbidity | 19 | 21.1 | ● | ● | ● | ● | ○ | ● | Continuous (per SD) | 1.01 | 1.00 to 1.03 | |||
| Wolk 2004(106) (–) | 382 | 62.0 | 30.0 | – | 100 | 20 | 467 | – | 4 | CVD | 44 | 2.88 | Crude | Continuous (per SD) | 1.39 | 1.10 to 1.75 | ||||||||
| Retterstol 2002(50) (–)c | 247 | 52.7 | 21.9 | – | – | 100 | 340 | – | 10 | CHD | 36 | 1.45 | ● | ○ | ● | ● | ○ | ○ | Continuous (per quartile) | 1.03 | 0.72 to 1.47 | |||
| Glader 2002(68) (–)c | 1150 | 59.4 | 18.2 | 100 | 100 | 53 | 340 | Thrombin reaction rate method | 6.7 | CVD | 152 | 1.97 | ● | ● | ● | ● | ● | ● | Continuous (per g/l) | 1.21 | 0.98 to 1.48 | |||
| Benchimol 2000(93) (–)c | 319 | 57 | 14.1 | 56.4 | 100 | 43.6 | 350 | Von Claus method | 9 | CHD | 13 | 0.45 | ○ | ○ | ○ | ○ | ● | ○ | Continuous (per g/l) | 2.0 | 1.15 to 3.46 | |||
| Otsuka 2002(107) (–) | 363 | 65.3 | 29.5 | – | 100 | 27.5 | 313.5 | Von Claus method | 0.54 | CVD | 89 | 45.4 | Adjustment variables unclear | Continuous (per 100 mg/dl) | 1.82 | 1.35 to 2.46 | ||||||||
| Blankenberg 2001(108) (Atherogene) | 1240 | 61.9 | 24.7 | – | 88.4 | 49.1 | 334 | Derived method | 2.7 | CHD | 88 | 2.63 | ● | ● | ○ | ○ | ● | ○ | Continuous (log transformed) | 0.6 | 0.6 to 7.8 | |||
| Minoretti 2006(53) (–) | 799 | 64.9 | 25.6 | 100 | 100 | 46.3 | 341 | – | 2.7 | CVD | 69 | 3.20 | ● | ● | ● | ● | ● | ● | Continuous (log transformed) | 1.01 | 0.98 to 1.10 | |||
| Bosevski 2005(65) (–)c | 90 | 62.3 | 27 | – | 100 | 54.4 | 429 | Von Claus method | 3 | ACM | 8 | 2.96 | ● | ● | ● | ● | ○ | ● | Continuous | 2.78 | – | |||
| Palmerini 2007(109) (Bologna Registry) | 108 | 69.1 | 23 | 28.7 | 100 | 32.5 | – | Von Claus method | 0.75 | ACM | 11 | 13.58 | Crude | < 439 | ≥ 439 | 5.24 | 1.39 to 19.77 | |||||||
| Shlipak 2008(110) (Heart and Soul) | 979 | 67 | 18 | – | 100 | 53.7 | – | Von Claus method | 3.7 | CHD | 142 | 3.92 | ● | ● | ● | ○ | ● | ● | ≤ 443 | > 443 | 1.15 | 0.78 to 1.69 | ||
| Espinola-Klein 2007(111) (–) | 694 | 62.4 | 27.4 | – | 92.1 | 43.3 | 335.6 | LPE | 6.5 | CVD | 75 | 1.66 | ● | ● | ● | ● | ● | ● | < 332 | ≥ 332 | 2.1 | 1.2 to 3.7 | ||
| Sjoland 2007(112) (–) | 589 | 63.4 | 18.9 | 99 | 100 | 57.3 | 360 | Von Claus method | 10 | ACM | – | – | ● | ● | ● | ● | ● | ● | ≤ 360 | > 360 | 1.39 | 0.99 to 1.96 | ||
| Marchioli 2001(113) (GISSI-Prevenzione) | 9601 | 56.1 | 0 | – | – | 100 | – | – | 4 | ACM | 904 | 2.35 | ● | ● | ● | ● | ○ | ● | < 400 | ≥ 400 | 1.62 | 1.41 to 1.86 | ||
| Marchioli 2001(113) (GISSI-Prevenzione) | 1647 | 63.9 | 100 | – | – | 100 | – | – | 4 | ACM | 167 | 2.53 | ● | ● | ● | ● | ○ | ● | < 400 | ≥ 400 | 1.12 | 0.81 to 1.53 | ||
| Bickel 2002(114) (Athrerogene) | 1240 | 61.9 | 24.7 | – | 100 | 49.1 | 360 | Derived method | 2.9 | CHD | 88 | 2.45 | ● | ● | ● | ● | ● | ● | < 410.3 | ≥410.3 | 1.7 | 1.01 to 2.8 | ||
| Retterstol 2001(51) (–)c | 247 | 52.7 | 21.9 | 44.5 | – | 100 | – | Semi-automatically | 10 | CHD | 35 | 1.42 | ● | ○ | ● | ● | ○ | ○ | ≤ 390 | ≥ 400 | 2.2 | 1.1 to 4.4 | ||
| Volzke 2003(115) (–)c | 220 | 63.9 | 21.7 | – | 100 | – | 350 | Von Claus method | 2 | ACM | 20 | 4.55 | Adjustment variables unclear | < 350 | ≥ 350 | 1.59 | 1.08 to 2.33 | |||||||
| Huang 2006(116) (–)c | 185 | 69.4 | 47 | – | 100 | – | 380 | EIA | 3 | CVD | 10 | 1.80 | ○ | ○ | ● | ● | ● | ○ | ≤ 400 | 4 00 | 2.98 | 1.22 to 3.78 | ||
| Liem 2003(117) (–)c | 593 | 65.2 | 22.1 | – | 100 | 56 | 399 | EIA | 2 | ACM | – | – | ○ | ● | ○ | ○ | ○ | ● | < 500 | ≥ 500 | 2.1 | 1.23 to 3.56 | ||
| Held 2000(118) (APSIS) | 714 | 59 | 31 | 100 | 5 | 16 | 391 | Modified thrombin time | 3.3 | CVD | 60 | 2.54 | ○ | ○ | ● | ○ | ○ | ● | ≤ 345 | 3.46–425 | > 425 | 2.61 | 0.99 to 6.86 | |
| Behar 1999(119) (BIP) | 3011 | 59.5 | – | – | – | 70 | 364 | Kinetic method | 6.25 | CHD | 173 | 0.91 | ● | ○ | ● | ● | ○ | ○ | < 314 | 315–373 | > 373 | 1.44 | 0.99 to 2.09 | |
| Benderly 1996(120) (–) | 3092 | 59 | 0 | – | – | 76 | 346 | Kinetic method | 3.2 | CHD | 111 | 1.12 | ● | ○ | ● | ○ | ○ | ● | < 308 | 308–368 | > 368 | 1.75 | 1.06 to 2.88 | |
| Haim 2007(121) (BIP)e | 138 | 62.1 | 6 | 57.2 | – | 75.4 | 479.59 | Liquid chromatography | 6.2 | CHD | 69 | 8.06 | ○ | ○ | ● | ○ | ○ | ● | < 408.2 | 408.2–530.6 | > 530.6 | 2.3 | 0.87 to 5.64 | |
| Rahel 2003(122) (–)c | 600 | 61.6 | 31.3 | – | 100 | – | 313 | ELISA | 0.67 | ACM | 54 | 13.4 | ● | ○ | ● | ○ | ○ | ○ | – | – | – | 3.74 | 1.08 to 12.95 | |
| Haim 2002(123) (BIP) | 272 | 61.5 | 5 | 61.4 | – | 80.9 | 356.5 | – | 6.2 | CHD | 136 | 8.06 | Crude | – | – | – | – | 1.4 | 0.73 to 2.75 | |||||
| Martin 1991(14) (–)c | 1716 | – | 0 | – | – | 100 | 446 | N | 2 | CHD | 126 | 3.67 | ● | ○ | ○ | ○ | ○ | ○ | – | – | – | – | 2.5 | – |
| Redondo 2001(124) (–)c | 194 | 57.4 | 12.4 | – | 97 | – | 280 | Von Claus method | 2 | CHD | 37 | 9.53 | ● | ● | ● | ● | ● | ● | – | – | – | – | 1.3 | 0.47 to 3.70 |
| Wattanakit 2005(96) (ARIC) | 766 | 57.1 | 24.7 | – | 100 | 81 | – | – | 8.7 | CVD | 313 | 4.70 | ● | ● | ● | ● | ● | ● | < 277 | 277–310 | 311–357 | ≥ 358 | 1.13 | 0.8 to 1.7 |
| Papa 2008(125) (–) | 422 | 64 | 19.9 | – | 100 | – | 344 | – | 3 | CHD | 13 | 1.03 | Crude | – | 3.77 | 1.04 to 13.73 | ||||||||
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | Lp(a) (mg/dl) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison groups | RR | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | |||||||||||||||||
| Bolibar 2000(47) (ECAT)c | 2806 | 55.8 | 15.9 | 36 | – | 45 | 105 | – | 2 | CHD | 106 | 1.89 | ● | ● | ● | ○ | ● | ● | Continuous (per SD) | 1.02 | 0.79 to 1.31 | ||||
| Falcone 2006(48) (–) | 1014 | 64.6 | 27.2 | 82.9 | 100 | 44.9 | 21.0 | EIA | 2.7 | CVD | 105 | 3.84 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per SD) | 1.8 | 1.26 to 2.56 | ||||
| Soeki 1999(49) (–) | 106 | 62.3 | 25.5 | – | – | 35.8 | 29.7 | T | 4.17 | CHD | 11 | 2.47 | Crude | Continuous (per SD) | 0.98 | 0.48 to 1.70 | |||||||||
| Saely 2006(126) (–) | 451 | 62.3 | 30.2 | – | – | – | 16.0 | T | 3.9 | CVD | 81 | 4.61 | ● | ● | ● | ● | ● | ○ | Continuous (per SD) | 1.41 | 1.12 to 1.90 | ||||
| Corsetti 2008(127) (THROMBO) | 215 | 58.9 | 24.7 | – | – | 23 | 0.79q | EIA | 2.17 | CHD | 42 | 9.00 | – | – | – | – | – | – | Continuous (per µmol/l) | 1.67 | 1.26 to 2.22 | ||||
| Minoretti 2006(53) (–) | 799 | 64.9 | 25.6 | 100 | 100 | 46.3 | 18.0 | EIA | 2.7 | CVD | 69 | 3.20 | ● | ● | ● | ● | ● | ● | Continuous (per unit increase by log transformation) | 1.2 | 0.98 to 1.47 | ||||
| Guang–Da 2004(92) (–)b | 131 | 65 | 49.6 | – | 100 | – | 31.64 | ELISA | 7 | CHD | 39 | 4.25 | ● | ○ | ○ | ● | ○ | ○ | Continuous (per unit increase by log transformation) | 1.56 | 1.14 to 2.12 | ||||
| Hartmann 2006(105) (–) | 60 | 58 | 17 | 100 | 100 | 35 | 25.0 | N | 1.5 | Morbidity | 19 | 21.1 | ● | ● | ● | ● | ○ | ● | Continuous (per unit increase by log transformation) | 10.2 | 2.36 to 44.13 | ||||
| Lundstam 2002(63) (–)b | 964 | 59 | 23 | 93.9 | 100 | 51 | – | ELISA | 11.7 | ACM | 363 | 3.22 | Adjustment variables unclear | ≤ 3 | > 3 | 1.04 | 0.8 to 1.3 | ||||||||
| Vittinghoff 2003(81) (HERS) | 2763 | 66.6 | 100 | 26.3 | 100 | – | 25.3 | – | 4.1 | CHD | 361 | 3.19 | ● | ● | ● | ● | ● | ● | ≤ 25.3 | > 25.3 | 1.44 | 1.06 to 1.96 | |||
| Glader 2002(68) (–)b | 1216 | 59.4 | 18.2 | 100 | 100 | 53 | 25.1 | EIA | 6.7 | CVD | 152 | 1.87 | ● | ● | ● | ● | ● | ● | < 300 | ≥ 300 | 1.40 | 1.0 to 2.0 | |||
| Zairis 2002(128) (GENERATION) | 483 | 59.3 | 18 | 22.2 | 100 | 8.7 | 19 | N | 3 | CHD | 20 | 1.38 | Adjustment variables unclear | < 25 | ≥ 25 | 1.27 | 0.48 to 3.34 | ||||||||
| Maher 1995(129) (FATS) | 146 | – | 0 | – | 100 | – | 30.8 | ELISA | 2.5 | ACM | 15 | 4.11 | Adjustment variables unclear | < 46.2 | ≥ 46.2 | – | – | ||||||||
| Wehinger 1999(130) (–) | 2223 | 62.9 | 23.3 | – | 100 | – | 14.4 | N | 1 | CHD | – | – | ● | ● | ○ | ○ | ○ | ○ | ≤ 56 | > 56 | 1.04 | 0.85 to 1.28 | |||
| Wilhelmsen 2001(37) (4S) | 1490 | – | 0 | – | 100 | – | – | ELISA | 5.4 | CHD | 431 | 5.36 | ● | ○ | ● | ● | ● | ● | ≤ 120 | 121 to 400 | ≥ 181 | 1.09 | 0.89 to 1.32 | ||
| Rahel 2003(131) (–)b | 600 | 61.6 | 31.3 | – | 100 | – | 19.99 | – | 0.67 | ACM | 54 | 13.4 | ● | ○ | ● | ○ | ○ | ○ | – | – | – | 1.66 | 0.73 to 3.76 | ||
| Skinner 1997(132) (–)b | 347 | 57.2 | 16 | 98 | 100 | 61 | – | ELISA | 5 | CHD | 16 | 0.92 | Adjustment variables unclear | < 12.3 | 12.3–33.1 | > 33.1 | 0.4 | 0.08 to 2.06 | |||||||
| Wattanakit 2005(96) (ARIC)i | 766 | 57.1 | 24.7 | – | 100 | 86 | – | – | 8.7 | CVD | 313 | 4.7 | ● | ● | ● | ● | ○ | ● | < 2.6 | 2.6–7.1 | 7.2 to 18.0 | ≥ 18.1 | 1.41 | 1.0 to 2.1 | |
| Shlipak 2000(110) (HERS) | 1383 | 66.7 | 100 | – | – | – | 25.3 | EIA | 4.1 | CHD | 182 | 3.21 | ○ | ● | ● | ● | ● | ● | ≤ 7.0 | 7.1–25.3 | 25.4 to 54.9 | 55.0–236.0 | 1.54 | 1.0 to 2.4 | |
| Lloyd 2001(133) (FLARE)c | 823 | 60.8 | 17 | – | 100 | 32.8 | 13.0 | ELISA | 0.82 | ACM | 190 | 25.2 | Adjustment variables unclear | ≤ 4 | 5–10 | 11–19 | 20–52 | > 53 | – | – | |||||
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | ApoA (g/l) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison groups | RR | 95% CI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | ||||||||||||||||||
| Asztalos 2008(44) (VA-HIT) | 754 | 64 | – | – | – | – | 1.096 | T | 5.1 | CHD | 168 | 4.37 | ● | ○ | ● | ● | ● | ● | Continuous (per SD) | 0.93 | 0.76 to 1.14 | |||||
| Bolibar 2000(47) (ECAT) | 2806 | 55.8 | 15.9 | 36 | – | 45 | 1.28 | – | 2 | CHD | 106 | 1.89 | ● | ● | ● | ○ | ● | ● | Continuous (per SD) | 0.66 | 0.54 to 0.81 | |||||
| Soeki 1999(49) (–)f | 106 | 62.3 | 25.5 | – | – | 35.8 | 1.138 | T | 4.2 | CHD | 11 | 2.47 | Crude | Continuous (per SD) | 0.54 | 0.23 to 1.22 | ||||||||||
| Simes 2002(57) (LIPID) | 4502 | 53 | – | – | – | – | 1.3 | N | 5 | CHD | – | – | ● | ● | ● | ○ | ○ | ● | Continuous (g/l) | 0.41 | 0.24 to 0.69 | |||||
| van Lennep 2000(64) (–) | 848 | 64.8 | 20.4 | – | 100 | 47.2 | 1.4 | N | 2.99 | ACM | 101 | 3.98 | Adjustment variables unclear | Continuous (g/l) | 0.29 | 0.09 to 0.97 | ||||||||||
| Lundstam 2002(63) (–) | 871 | 59 | 23 | 93.9 | 100 | 51 | – | N | 11.7 | ACM | 363 | 3.56 | Adjustment variables unclear | Continuous (ln) | 0.3 | 0.2 to 0.5 | ||||||||||
| Harb 2002(134) (THROMBO)b | 957 | – | 24.6 | 32.2 | – | 100 | – | – | 2.17 | CHD | 69 | 3.32 | ○ | ● | ○ | ○ | ○ | ● | – | – | 2.14 | 1.24 to 3.69 | ||||
| Skinner 1999(13) (–)b | 353 | 57.2 | 15.9 | 98 | 100 | 61 | – | N | 5 | CHD | 16 | 0.91 | Crude | – | – | – | 0.13 | 0.02 to 1.01 | ||||||||
| Leander 2007(26) (Stockholm Heart Epidemiology Program) | 1105 | 58.6 | 0 | – | – | 0 | 1.5 | – | 7.2 | CHD | 320 | 4.02 | ○ | ○ | ● | ● | ● | ● | > 1.3 | ≤ 1.3 | 0.9 | 0.6 to 1.3 | ||||
| Leander 2007(26) (Stockholm Heart Epidemiology Program) | 538 | 62.1 | 100 | – | – | 0 | 1.5 | – | 7.2 | CHD | 141 | 3.64 | ○ | ○ | ● | ● | ● | ● | > 1.3 | ≤ 1.3 | 2.3 | 1.1 to 5.0 | ||||
| Wilhelmsen 2001(37) (4S)d | 1476 | – | 0 | – | 100 | – | – | T | 5.4 | CHD | 426 | 5.34 | ● | ○ | ● | ● | ● | ● | ≤ 1.3 mmol/l | 1.1–1.2 mmol/l | ≤ 1.0 mmol/l | 1.13 | 0.90 to 1.43 | |||
| Held 1997(118) (APSIS) | 786 | 60 | 31 | 100 | 6 | 16 | 1.35 | N | 3.3 | CVD | 67 | 2.58 | ● | ● | ● | ○ | ○ | ● | > 1.54 | 1.34–1.54 | < 1.34 | 1.6 | 0.82 to 3.09 | |||
| Schlitt 2005(97) (–) | 1294 | 61.8 | 25.8 | – | 100 | – | 1.33 | T | 3.9 | CVD | 158 | 3.13 | ● | ● | ● | ○ | ● | ● | ≤ 1.16 | 1.16–1.31 | 1.31–1.47 | > 1.47 | 0.41 | 0.22 to 0.78 | ||
| Moss 1999(135) (–)f | 1045 | – | 24 | – | – | 100 | 1.19 | Immunochemical | 2.2 | CHD | 81 | 3.52 | ○ | ● | ○ | ○ | ○ | ● | – | – | – | < 1.01 | 1.73 | 0.86 to 3.46 | ||
| van der Steeg 2008(136) (IDEAL) | 8564 | 61.7 | 19.1 | – | – | 100 | 1.39 | N | 4.8 | CVD | 679 | 1.65 | ● | ● | ● | ● | ● | ○ | < 1.25 | 1.25–1.45 | 1.45–1.65 | 1.65–1.80 | 1.80–1.95 | > 1.95 | 0.71 | 0.29 to 1.75 |
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | ApoB (g/l) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison groups | RR | 95% CI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | ||||||||||||||||
| Bolibar 2000(47) (ECAT) | 2806 | 55.8 | 15.9 | 36 | – | 45 | 1.28 | – | 2 | CHD | 106 | 1.89 | ● | ● | ● | ○ | ● | ● | Continuous (per SD) | 1.27 | 1.03 to 1.56 | |||
| Soeki 1999(49) (–)f | 106 | 62.3 | 25.5 | – | – | 35.8 | 1.142 | T | 4.2 | CHD | 11 | 2.47 | Crude | Continuous (per SD) | 1.59 | 0.97 to 2.58 | ||||||||
| Lee 2006(137) (–) | 1050 | 60.8 | 27.1 | – | – | – | 0.97 | – | 8.5 | CHD | 95 | 1.06 | ● | ● | ● | ● | ● | ● | Continuous (per 0.2 g/l) | 1.36 | 1.17 to 1.59 | |||
| Simes 2002(57) (LIPID) | 4502 | 53 | – | – | – | – | 1.32 | N | 5 | CHD | – | – | ● | ● | ● | ○ | ○ | ● | Continuous (g/l) | 2.07 | 1.32 to 3.22 | |||
| Lundstam 2002(63) (–) | 872 | 59 | 23 | 93.9 | 100 | 51 | – | N | 11.7 | ACM | 363 | 3.56 | Adjustment variables unclear | Continuous (ln) | 0.6l | 0.3 to 1.0 | ||||||||
| Linden 1998(91) (–) | 964 | 60.8 | 23 | – | – | 50.6 | 1.48 | N | 5.2 | ACM | 168 | 3.35 | ○ | ● | ● | ● | ○ | ● | Continuous (g/l) | 0.41 | 0.20 to 0.83 | |||
| van Lennep 2000(64) (–) | 848 | 64.8 | 20.4 | – | 100 | 47.2 | 1.5 | N | 2.99 | ACM | 101 | 3.98 | Adjustment variables unclear | Continuous (g/l) | 7.94 | 1.09 to 57.72 | ||||||||
| Kastelein 2008(138) (TNT/IDEAL) | 18,018 | 61.4 | 18.9 | – | – | – | 0.989 | N | 4.8 | CHD | 1783 | 2.06 | ● | ● | ○ | ○ | ○ | ○ | Continuous (per 27.2 mg/dl) | 1.19 | 1.14 to 1.24 | |||
| Harb 2002(134) (THROMBO)b | 957 | – | 24.6 | 32.2 | – | 100 | – | – | 2.17 | CHD | 69 | 3.32 | ○ | ● | ○ | ○ | ○ | ● | – | – | 1.9 | 1.11 to 3.25 | ||
| Wilhelmsen 2001(37) (4S)d | 1476 | – | 0 | – | 100 | – | – | T | 5.4 | CHD | 426 | 5.34 | ● | ○ | ● | ● | ● | ● | ≤ 1.0 | 1.1–1.2 | ≥ 1.3 | 1.23 | 0.99 to 1.53 | |
| Held 1997(118) (APSIS) | 786 | 60 | 31 | 100 | 6 | 16 | 1.35 | N | 3.3 | CVD | 67 | 2.58 | ● | ● | ● | ○ | ○ | ● | < 1.31 | 1.31–1.60 | > 1.60 | 1.2 | 0.67 to 2.16 | |
| Schlitt 2005(97) (–) | 1294 | 61.8 | 25.8 | – | 100 | – | 1.2 | T | 3.9 | CVD | 158 | 3.13 | ● | ● | ● | ○ | ● | ● | ≤ 1.01 | 1.01–1.17 | 1.17–1.37 | > 1.37 | 0.78 | 0.42 to 1.46 |
| Moss 1999(135) (–)f | 1045 | – | 24 | – | – | 100 | 1.23 | EIA | 2.2 | CHD | 81 | 3.52 | ○ | ● | ○ | ○ | ○ | ● | – | – | – | > 1.4 | 1.93 | 1.03 to 3.62 |
| Author publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | Homocysteine mean (µmol/l) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison group | RR | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | |||||||||||||||||
| Lee 2006(137) (–) | 1050 | 60.8 | 27.1 | – | – | – | 14.2 | CLIMA | 8.5 | CHD | 95 | 1.06 | ● | ● | ● | ● | ● | ● | Continuous (µmol/l) | 1.05 | 1.02 to 1.08 | ||||
| Lu 2003(139) (–) | 153 | 71.0 | 13.1 | 100 | 100 | 34 | 13.1 | EIA | 1.33 | CVD | 51 | 25.1 | Crude | Continuous (µmol/l) | 1.01 | 0.93 to 1.09 | |||||||||
| Ndrepepa 2006(140) (–) | 507 | 69.1 | 33.9 | – | 100 | 45.6 | 12.9 | FP | 4 | CVD | 62 | 30.6 | ● | ○ | ○ | ○ | ○ | ○ | Continuous (per 5 µmol/l) | 1.04 | 0.80 to 1.36 | ||||
| Retterstol 2003(141) (–) | 247 | 52.7 | 21.9 | – | – | 100 | – | LC | 10 | CHD | 36 | 1.46 | ○ | ○ | ● | ● | ○ | ○ | Continuous (per quartile) | 1.40 | 1.03 to 1.90 | ||||
| Schnyder 2002(142) (–) | 549 | 62 | 19.7 | – | 100 | 54.6 | 10.1 | LC | 1.12 | CHD | 6 | 0.956 | Crude | Continuous (per µmol/l) | 1.2 | 1.08 to 1.33 | |||||||||
| Zebrack 2003(143) (Intermountain) | 1128 | 64.0 | 33.0 | 72.0 | 76.0 | – | 6.8 | – | 3 | ACM | 208 | 6.15 | ● | ○ | ● | ● | ○ | ● | ≤ 15 | > 15 | 1.3 | – | |||
| Anderson 2000(144) (–) | 1002 | 64.9 | 22.7 | – | 100 | – | 15.5 | FP | 3.0 | ACM | 118 | 2.78 | ● | ○ | ○ | ● | ○ | ● | < 11.83 | 11.83–16.1 | ≥ 16.2 | 1.64 | 1.13 to 2.38 | ||
| Leu 2004(95) (–) | 75 | 68.1 | 12 | 100 | 100 | 25.3 | 12.61 | ELISA | 3.33 | CVD | 33 | 13.2 | Crude | ≤ 12 | > 12 | 2.83 | 1.21 to 6.62 | ||||||||
| Palma Reis 2000(145) (–) | 110 | 48.1 | 8 | – | – | 100 | – | – | 7 | CVD | 28 | 3.64 | Crude | < 10.10 | ≥ 10.10 | – | – | ||||||||
| Liem 2003(146) (–) | 593 | 65.2 | 22.1 | – | 100 | 56 | 12.1 | FP | 2 | ACM | – | – | ○ | ● | ○ | ○ | ○ | ● | < 13.1 | ≥ 13.1 | 1.96 | 1.15 to 3.33 | |||
| Schnyder 2002a(147) (–) | 205 | 61 | 23.5 | – | 100 | 54.6 | 9.9 | – | 0.38 | CHD | 47 | 60.3 | Crude | < 9.0 | ≥ 9.0 | – | – | ||||||||
| Rossi 2006(148) (GENICA) | 262 | 66.3 | 100 | – | 54.6 | – | 11.6 | LC | 3.6 | CVD | 15 | 1.59 | Adjustment variables unclear | ≤ 8.7 | 8.8–12.4 | ≥ 12.5 | – | – | |||||||
| Nygard 1997(149) (–) | 587 | 62 | 18.6 | – | 100 | 57.4 | 11.2 | LC | 4.6 | ACM | 64 | 2.37 | ● | ● | ● | ● | ○ | ● | < 9.0 | 9.0–14.9 | 15.0–19.9 | ≥ 20.0 | 4.51 | 1.22 to 16.6 | |
| Schnabel 2005a(150) (Atherogene) | 639 | 61.7 | 27.8 | 79.3 | 86.8 | – | 14.2 | LC | 7.1 | CVD | 112 | 2.47 | ● | ● | ● | ● | ○ | ● | < 11.3 | 11.3–13.7 | 13.7–16.8 | > 16.8 | 3.00 | 1.35 to 6.66 | |
| Knekt 2001a(151) (Mobile Clinic Health Examination Survey)s | 477 | 55.7 | 0 | – | – | – | 0.154 | LC | 12 | CHD | 166 | 2.9 | ○ | ● | ● | ● | ● | ● | < 7.9 | 7.9–9.1 | 9.2–10.4 | 10.5–12.3 | ≥ 12.4 | 2.23 | 1.03 to 4.85 |
| Knekt 2001b(152) (Mobile Clinic Health Examination Survey) | 221 | 58.8 | 100 | – | – | – | 11.7 | LC | 12 | CHD | 74 | 2.79 | ● | ● | ● | ● | ● | ● | < 8.1 | 8.1–9.8 | 9.9–11.3 | 11.4–13.4 | > 13.5 | 3.32 | 1.05 to 10.5 |
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | NT-proBNP (pg/ml) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison group | RR | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart Failure | Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | ||||||||||||||||
| Lubos 2006(153) (Atherogene) | 1945 | 61.2 | 21.1 | – | – | 100 | 37.5 | 50.9 | FP | 2.6 | CVD | 75 | 1.48 | ● | ● | ● | ● | ● | ● | Continuous (per SD) | 2.7 | 2.0 to 3.9 | |||
| Bibbins-Domingo 2007(154) (Heart and Soul) | 987 | 67 | 18.5 | 17.5 | – | – | 53.4 | 174.8 | EIA | 3.7 | CHD | 34 | 0.93 | ● | ● | ● | ● | ○ | ● | Continuous (per SD) | 2.6 | 1.3 to 5.0 | |||
| Tang 2007(155) (CREDO) | 1472 | 61.6 | 28.2 | 8.4 | 26 | 100 | 33.3 | 131 | Electrocheminescence | 1 | ACM | 141 | 9.58 | ● | ● | ● | ○ | ● | ● | Continuous (per unit increase by log transformation) | 1.25 | 1.07 to 1.46 | |||
| Dai 2008(156) (–) | 345 | 64.6 | 26.7 | – | – | 100 | 15 | 212.2 | ELISA | 3 | CHD | 56 | 5.41 | ● | ● | ● | ● | ○ | ● | Continuous (per unit increase by log transformation) | 2.68 | 1.74 to 4.13 | |||
| Schnabel 2005(157) (Atherogene) | 1872 | 61 | 20.9 | – | – | 100 | – | 52.55 | FP | 2.6 | CVD | 114 | 2.34 | ● | ● | ● | ● | ● | ● | ≤ 102.31 | > 102.31 | 1.96 | 1.28 to 3.0 | ||
| Shlipak 2008(110) (Heart and Soul) | 979 | 67 | 18 | 17.6 | – | 100 | 53.7 | – | CL | 3.7 | CHD | 142 | 3.92 | ● | ● | ● | ○ | ● | ● | ≤ 459 | > 459 | 2.13 | 1.43 to 3.18 | ||
| Ndrepepa 2006b(158) (–)g | 989 | 66.3 | 21.0 | 100 | – | 100 | 39.9 | 279.9 | Autoanalyser | 3.6 | ACM | 85 | 2.39 | ● | ● | ● | ● | ● | ● | < 279.9 | ≥ 279.9 | 2.0 | 1.05 to 3.58 | ||
| Richards 2006(237) (ANZ Heart Failure trial and Christchurch Cardioendocrine post-myocardial infarction cohort) | 1049 | 63.4 | 21.0 | 27.8 | – | – | 97 | 819.8 | ELISA | 1 | ACM | 79 | 7.53 | ● | ● | ○ | ○ | ○ | ● | Below median | Above median | 2.08 | 1.12 to 3.89 | ||
| Saleh 2006(160) (–) | 891 | 65 | 32 | 21 | 58 | 100 | 39 | 179 | SEISA | 2.6 | ACM | 75 | 3.23 | ● | ○ | ○ | ○ | ○ | ● | ≤ 490 | > 490 | 3.72 | 1.44 to 9.65 | ||
| Ndrepepa 2005(161) (–)g | 1059 | 66.6 | 21.1 | 100 | 100 | 100 | 40.6 | 369.4 | Autoanalyser | 3.6 | CVD | 70 | 1.84 | ● | ● | ○ | ● | ● | ● | < 120.6 | – | – | ≥ 808.4 | 5.98 | 1.55 to 23.13 |
| de Winter 2004(162) (–) | 1172 | 62.0 | 33.0 | 46.1 | 78.0 | 100 | 46.5 | 321.5 | – | 1.16 | ACM | 32 | 2.35 | Crude | ≤ 456 | > 456 | 7.06 | 3.30 to 15.08 | |||||||
| Lindahl 2005(163) (FRISC-II)g | 1189 | 68 | 29 | 63.0 | – | – | 29 | 343 | EIA | 2 | ACM | – | – | ● | ● | ○ | ○ | ○ | ● | Reference group | Double reference group | 1.46 | 1.06 to 2.03 | ||
| Blankenberg 2006(99) (HOPE) | 3199 | 65.4 | 23.2 | – | – | 100 | – | 160.2 | SEISA | 4.5 | CVD | 501 | 3.48 | ● | ● | ○ | ● | ○ | ● | – | – | – | 2.25 | 1.74 to 2.89 | |
| März 2007(164) (LURIC) | 1640 | 61.1 | 30.7 | – | – | 100 | 32.2 | – | CL | 5.45 | CVD | 129 | 1.44 | ● | ● | ● | ● | ● | ● | 5–81 | 82–194 | 195–521 | 522–35000 | 3.92 | 1.76 to 8.74 |
| West 2008(165) (LIPID) | 500 | 63 | 15 | – | – | 100 | – | 689 | SEISA | 2.5 | CVD | 250 | 20 | ● | ○ | ○ | ○ | ○ | ● | < 117 | 117–268 | 268–646 | > 646 | 2.22 | 1.15 to 4.29 |
| Omland 2007(166) (PEACE) | 3761 | 63.6 | 19 | – | – | 100 | 56.2 | 139.3 | CL | 4.8 | CHD | 241 | 1.33 | ● | ● | ● | ● | ● | ● | Men 5–66; women 5–105 | Men 66–127; women 105–196 | Men 127–253; women 196–372 | Men 253–5590; women 372–4593 | 1.19 | 0.77 to 1.83 |
| Kragelund 2005(167) (–) | 1034 | 58.7 | 22.3 | 100 | 89.6 | 80.1 | 531 | 169 | EIA | 9.2 | ACM | 288 | 3.03 | ● | ● | ● | ● | ● | ● | < 63 | 63–169 | 170–456 | > 456 | 2.4 | 1.5 to 4.0 |
| Rothenbacher 2006(168) (–)g | 1051 | 58.5 | 15.1 | 20.4 | – | 100 | 58.2 | 568.4 | EIA | 4.1 | CVD | 95 | 2.20 | ● | ● | ● | ● | ○ | ● | ≤ 278.3 | 278.3–564.7 | 564.7–1097 | > 1097 | 2.35 | 1.14 to 4.88 |
| Schnabel 2005b(169) (Atherogene) | 417 | – | – | – | 100 | 100 | – | – | EIA | 2 | CVD | 31 | 2.72 | ● | ● | ● | ● | ● | ● | < 86.7 | 86.7–192.0 | 192.0–487.9 | > 487.9 | 3.96 | 1.13 to 13.9 |
| Assmus 2007(170) (TOPCARE-CHD) | 121 | 62 | 13 | – | – | 100 | 100 | – | SEISA | 1.58 | ACM | 14 | 7.32 | ● | ○ | ○ | ○ | ○ | ● | – | 7.2 | 2.4 to 22.2 | |||
| Author/publication year (study name) | Number of patients | Age | % Women | Baseline coronary morbidity (%) | IL-6 mean (pg/ml) | Assay type | Follow-up (years) | Event combination | Number of events | Crude annual risk (%) | Adjustments | Comparison groups | RR | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Angiographic CAD | Prior MI | Age | Sex | Smoking | Lipids (TC, LDL, HDL, TG) | Obesity | Diabetes | |||||||||||||||||
| Blankenberg 2006(99) (HOPE) | 3199 | 65.4 | 23.2 | – | 100 | – | 3.27 | EIA | 4.5 | CVD | 501 | 3.48 | ● | ● | ○ | ● | ○ | ● | Continuous (per SD) | 0.9 | 0.8 to 1.1 | ||||
| Blankenberg 2003(171) (Atherogene)b | 771 | – | – | 70.5 | 100 | 48.7 | – | LPE | 4.1 | CVD | 97 | 3.07 | ● | ● | ● | ● | ● | ● | Continuous (per quartile) | 1.10 | 0.80 to 1.45 | ||||
| Blankenberg 2002(172) (Atherogene) | 1229 | 61.2 | 25.5 | 65.8 | 100 | 46.9 | 8.4 | ELISA | 3.9 | CVD | 95 | 1.98 | ● | ○ | ○ | ● | ○ | ● | Continuous (per quartile) | 1.15 | 0.90 to 1.49 | ||||
| Lee 2006(137) (–)g | 1050 | 60.8 | 27.1 | – | – | – | 2.3 | EIA | 8.5 | CHD | 95 | 1.06 | ● | ● | ● | ● | ● | ● | Continuous (ng/l) | 1.08 | 1.01 to 1.16 | ||||
| Ikonomidis 2008(173) (–) | 106 | 62 | 14 | 100 | 100 | 53 | 2.27 | Highly sensitive immunoassay | 5.3 | CHD | 36 | 6.41 | ● | ● | ● | ● | ○ | ○ | Continuous (per unit increase) | 1.17 | 0.94 to 1.45 | ||||
| Shlipak 2008(110) (Heart and Soul) | 979 | 67 | 18 | – | 100 | 53.7 | – | CL | 3.7 | CHD | 142 | 3.92 | ● | ● | ● | ○ | ● | ● | ≤ 4.2 | > 4.2 | 1.76 | 1.22 to 2.53 | |||
| Espinola–Klein 2007(111) (–) | 694 | 62.4 | 27.4 | – | 92.1 | 43.3 | 11.6 | LPE | 6.5 | CVD | 75 | 1.66 | ● | ● | ● | ● | ● | ● | < 11.3 | ≥ 11.3 | 1.3 | 0.8 to 2.1 | |||
| Kwaijtaal 2005(174) (Exhaustion Intervention trial) | 213 | 53.6 | 21.6 | 9.9 | 100 | 26.8 | 2.01 | ELISA | 2 | CHD | 25 | 5.87 | ● | ● | ● | ○ | ● | ● | – | – | 3.9 | 1.70 to 9.0 | |||
| Inoue 2008(175) (–) | 158 | 63 | 28 | 53 | 82.9 | 29.7 | 9.25 | SEISA | 7 | CVD | 56 | 5.06 | – | – | – | – | – | – | – | – | 0.92 | 0.27 to 3.57 | |||
| West 2008(165) (LIPID) | 500 | 63 | 15 | – | 100 | – | 25.5 (unit not specified) | ELISA | 2.5 | CVD | 250 | 20 | ● | ○ | ○ | ○ | ○ | ● | Lower quartile (not specified) | Upper quartile (not specified) | 1.0 | 0.50 to 1.80 | |||
| Rahel 2003(122) (–) | 600 | 61.6 | 31.3 | – | 100 | – | 2.88 | ELISA | 0.67 | ACM | 54 | 13.4 | ● | ○ | ● | ○ | ○ | ○ | – | – | – | 0.75 | 0.35 to 1.62 | ||
| Kip 2005(176) (WISE) | 580 | 58 | 100 | – | 61 | – | – | EIA | 4.7 | CVD | 92 | 3.37 | ● | ● | ● | ○ | ○ | ● | < 1.68 | 1.68–2.92 | 2.93–5.27 | ≥ 5.28 | 2.31 | 1.20 to 4.44 | |
| Hoffmeister 2005(177) (–) | 300 | 57.9 | 14.3 | – | 100 | 61.3 | 2.6 | ELISA | 3.2 | CVD | 60 | 6.25 | ● | ● | ● | ● | ● | ● | < 1.50 | 1.51–2.09 | 2.10–3.58 | > 3.59 | 1.8 | 0.90 to 3.60 | |
| Fisman 2006(178) (BIP) | 258 | 61 | 4.7 | 59.7 | 100 | 79 | 3.53 | CLIMA | 6.3 | CHD | 129 | 7.94 | ○ | ○ | ○ | ○ | ● | ○ | < 1.05 | 1.05–1.61 | 1.61–2.33 | 2.33–3.67 | ≥ 3.67 | 3.33 | 1.47 to 8.13 |
Key to tables
-
Estimated average length of stay for coronary event admission = 5 days.
-
Original units mg/l.
-
Original units g/l.
-
Original units mmol/l – unchanged on this table.
-
(Fibrinogen) converted from µmol/l to mg/dl by dividing by 0.0294.
-
Original units mg/dl.
-
Original units ng/l.
-
Original units mg/ml.
-
Original units µg/ml.
-
Omitted from table, as quoted as a percentage.
-
Reference group.
-
Men 0.4 (0.2 to 0.7); women 2.1 (0.6 to 6.3).
-
(Fasting glucose) converted from mg/dl to mmol/l by multiplying by 0.0555.
-
(LDL/total cholesterol) converted from mg/dl to mmol/l by multiplying by 0.0259.
-
(Hb) converted from mmol/l to g/dl by dividing by 0.6206.
-
Relative risk first vs fourth quartile (low vs high).
-
Original units pmol/l – unchanged.
-
(Creatinine) converted from mg/dl to µmol/l by multiplying by 88.4.
-
(Homocysteine) converted from mg/dl to µmol/l by multiplying by 73.97.
Reference list – papers included in biomarker systematic review
(One paper may contribute reports on more than one biomarker)
- Burr ML, Holliday RM, Fehily AM, Whitehead PJ. Haematological prognostic indices after myocardial infarction: evidence from the diet and reinfarction trial (DART). Eur Heart J 1992;13:166-70.
- Arant CB, Wessel TR, Olson MB, Bairey Merz CN, Sopko G, Rogers WJ, et al. Hemoglobin level is an independent predictor for adverse cardiovascular outcomes in women undergoing evaluation for chest pain: results from the National Heart, Lung, and Blood Institute Women’s Ischemia Syndrome Evaluation Study. J Am Coll Cardiol 2004;43:2009-14.
- Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol 2006;97:993-6.
- Exaire JE, Fathi RB, Brener SJ, Karha J, Ellis SG, Bhatt DL. Impaired myocardial perfusion score and inflammatory markers in patients undergoing primary angioplasty for acute myocardial infarction. Arch Cardiol Mex 2006;76:376-82.
- Rajagopal V, Gurm HS, Bhatt DL, Lincoff AM, Tcheng JE, Kereiakes DJ, et al. Relation of an elevated white blood cell count after percutaneous coronary intervention to long-term mortality. Am J Cardiol 2004;94:190-2.
- Fathi RB, Gurm HS, Chew DP, Gupta R, Bhatt DL, Ellis SG. The interaction of vascular inflammation and chronic kidney disease for the prediction of long-term death after percutaneous coronary intervention. Am Heart J 2005;150:1190-7.
- da Silveira AD, Ribeiro RA, Rossini AP, Stella SF, Ritta HA, Stein R, et al. Association of anemia with clinical outcomes in stable coronary artery disease. Coron Artery Dis 2008;19:21-6.
- Elisheva S, Noya G, Simchen E, Galai N, Zitser-Gurevich Y, Braun D, et al. Sequential logistic models for 30 days mortality after CABG: pre-operative, intra-operative and post-operative experience – The Israeli CABG study (ISCAB). Eur J Epidemiol 2000;16:543-55.
- Lipsic E, Asselbergs FW, Van der Meer P. Anaemia predicts cardiovascular events in patients with stable coronary artery disease. Neth Heart J 2005;13:254-8.
- McKechnie RS, Smith D, Montoye C, Kline-Rogers E, O’Donnell MJ, DeFranco AC, et al. Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation 2004;110:271-7.
- Muzzarelli S, Pfisterer M. Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J 2006;152:991-6.
- Lee CH, de Feyter P, Serruys PW, Saia F, Lemos PA, Goedhart D, et al. Beneficial effects of fluvastatin following percutaneous coronary intervention in patients with unstable and stable angina: results from the Lescol intervention prevention study (LIPS). Heart 2004;90:1156-61.
- Skinner JS, Farrer M, Albers CJ, Neil HA, Adams PC. High apolipoprotein AI concentrations are associated with lower mortality and myocardial infarction five years after coronary artery bypass graft surgery. Heart 1999;81:488-94.
- Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet 1991;338:1409-11.
- Reinecke H, Trey T, Wellmann J, Heidrich J, Fobker M, Wichter T, et al. Haemoglobin-related mortality in patients undergoing percutaneous coronary interventions. Eur Heart J 2003;24:2142-50.
- Shah B, Kumar N, Garg P, Kang E, Grossi E, Lorin JD, et al. Metabolic syndrome does not impact survival in patients treated for coronary artery disease. Coron Artery Dis 2008;19:71-7.
- Munoz MA, Vila J, Cabanero M, Rebato C, Subirana I, Sala J, et al. Efficacy of an intensive prevention program in coronary patients in primary care, a randomised clinical trial. Int J Cardiol 2007;118:312-20.
- Vaidya D, Kelemen MD, Bittner V, Tardif JC, Thompson P, Ouyang P. Fasting plasma glucose predicts survival and angiographic progression in high-risk postmenopausal women with coronary artery disease. J Womens Health (Larchmt) 2007;16:228-34.
- Karlson BW, Wiklund O, Hallgren P, Sjolin M, Lindqvist J, Herlitz J. Ten-year mortality amongst patients with a very small or unconfirmed acute myocardial infarction in relation to clinical history, metabolic screening and signs of myocardial ischaemia. J Intern Med 2000;247:449-56.
- Zindrou D, Taylor KM, Bagger JP. Admission plasma glucose: an independent risk factor in nondiabetic women after coronary artery bypass grafting. Diabetes Care 2001;24:1634-9.
- Dankner R, Goldbourt U, Boyko V, Reicher-Reiss H. Predictors of cardiac and noncardiac mortality among 14,697 patients with coronary heart disease. Am J Cardiol 2003;91:121-7.
- Wong ND, Cupples LA, Ostfeld AM, Levy D, Kannel WB. Risk factors for long-term coronary prognosis after initial myocardial infarction: the Framingham Study. Am J Epidemiol 1989;130:469-80.
- Shah B, Liou M, Grossi E, Mass H, Lorin JD, Danoff A, et al. Relation of elevated periprocedural blood glucose to long-term survival after percutaneous coronary intervention. Am J Cardiol 2005;96:543-6.
- de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999;99:779-85.
- Stewart RA, North FM, Sharples KJ, Simes RJ, Tonkin AM, White HD. Differences in cardiovascular mortality between Australia and New Zealand according to socioeconomic status: findings from the Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study. N Z Med J 2008;121:11-23.
- Leander K, Wiman B, Hallqvist J, Andersson T, Ahlbom A, de FU. Primary risk factors influence risk of recurrent myocardial infarction/death from coronary heart disease: results from the Stockholm Heart Epidemiology Program (SHEEP). Eur J Cardiovasc Prev Rehabil 2007;14:532-7.
- Corsetti JP, Ryan D, Moss AJ, Rainwater DL, Zareba W, Sparks CE. Glycoprotein Ib-alpha polymorphism T145M, elevated lipoprotein-associated phospholipase A2, and hypertriglyceridemia predict risk for recurrent coronary events in diabetic postinfarction patients. Diabetes 2007;56:1429-35.
- Nigam A, Bourassa MG, Fortier A, Guertin MC, Tardif JC. The metabolic syndrome and its components and the long-term risk of death in patients with coronary heart disease. Am Heart J 2006;151:514-21.
- Arcavi L, Behar S, Caspi A, Reshef N, Boyko V, Knobler H. High fasting glucose levels as a predictor of worse clinical outcome in patients with coronary artery disease: results from the Bezafibrate Infarction Prevention (BIP) study. Am Heart J 2004;147:239-45.
- Clofibrate and niacin in coronary heart disease. JAMA 1975;231:360-81.
- Schlant RC, Forman S, Stamler J, Canner PL. The natural history of coronary heart disease: prognostic factors after recovery from myocardial infarction in 2789 men. The 5-year findings of the coronary drug project. Circulation 1982;66:401-14.
- Dibra A, Ndrepepa G, Mehilli J, Pineck S, Mayer S, Bollwein H, et al. Prognostic value of impaired fasting glucose for outcomes of patients with stable angina pectoris treated with percutaneous coronary interventions. Am J Cardiol 2005;96:1113-15.
- Held C, Bjorkander I, Forslund L, Rehnqvist N, Hjemdahl P. The impact of diabetes or elevated fasting blood glucose on cardiovascular prognosis in patients with stable angina pectoris. Diabet Med 2005;22:1326-33.
- Yun KH, Jeong MH, Kim KH, Hong YJ, Park HW, Kim JH, et al. The effect of insulin resistance on prognosis of non-diabetic patients who underwent percutaneous coronary intervention. J Korean Med Sci 2006;21:212-16.
- Hu R, Ma CS, Nie SP, Lu Q, Kang JP, Du X, et al. Effect of metabolic syndrome on prognosis and clinical characteristics of revascularization in patients with coronary artery disease. Chin Med J (Engl) 2006;119:1871-6.
- Anderson JL, Horne BD, Jones HU, Reyna SP, Carlquist JF, Bair TL, et al. Which features of the metabolic syndrome predict the prevalence and clinical outcomes of angiographic coronary artery disease?. Cardiology 2004;101:185-93.
- Wilhelmsen L, Pyorala K, Wedel H, Cook T, Pedersen T, Kjekshus J. Risk factors for a major coronary event after myocardial infarction in the Scandinavian Simvastatin Survival Study (4S). Impact of predicted risk on the benefit of cholesterol-lowering treatment. Eur Heart J 2001;22:1119-27.
- Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT). Arch Intern Med 2002;162:2597-604.
- Van de Veire NR, de Winter O, Gillebert TC, De Sutter J. Diabetes and impaired fasting glucose as predictors of morbidity and mortality in male coronary artery disease patients with reduced left ventricular function. Acta Cardiol 2006;61:137-43.
- Canner PL, Furberg CD, Terrin ML, McGovern ME. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol 2005;95:254-7.
- Kanaya AM, Herrington D, Vittinghoff E, Lin F, Bittner V, Cauley JA, et al. Impaired fasting glucose and cardiovascular outcomes in postmenopausal women with coronary artery disease. Ann Intern Med 2005;142:813-20.
- Fisman EZ, Motro M, Tenenbaum A, Leor J, Boyko V, Mandelzweig L, et al. Is hypoglycaemia a marker for increased long-term mortality risk in patients with coronary artery disease? An 8-year follow-up. Eur J Cardiovasc Prev Rehabil 2004;11:135-43.
- [No authors listed] . The prognostic importance of plasma glucose levels and of the use of oral hypoglycemic drugs after myocardial infarction in men. Diabetes 1977;26:453-65.
- Asztalos BF, Collins D, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Relation of gemfibrozil treatment and high-density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Metabolism 2008;57:77-83.
- Inaguma D, Tatematsu M, Shinjo H, Suzuki S, Mishima T, Inaba S, et al. Relationship between renal function at the time of percutaneous coronary intervention and prognosis in ischemic heart disease patients. Clin Exp Nephrol 2007;11:56-60.
- Inoue T, Kotooka N, Morooka T, Komoda H, Uchida T, Aso Y, et al. High molecular weight adiponectin as a predictor of long-term clinical outcome in patients with coronary artery disease. Am J Cardiol 2007;100:569-74.
- Bolibar I, von Eckardstein A, Assmann G, Thompson S. Short-term prognostic value of lipid measurements in patients with angina pectoris. The ECAT Angina Pectoris Study Group: European Concerted Action on Thrombosis and Disabilities. Thromb Haemost 2000;84:955-60.
- Falcone C, Minoretti P, D’Angelo A, Buzzi MP, Coen E, Emanuele E, et al. Markers of eosinophilic inflammation and risk prediction in patients with coronary artery disease. Eur J Clin Invest 2006;36:211-17.
- Soeki T, Tamura Y, Shinohara H, Tanaka H, Bando K, Yui Y, et al. Fibrinolytic factors, serum lipid and C-reactive protein predicting cardiac events in Japanese patients with coronary atherosclerotic lesions. Jpn Circ J 1999;63:976-80.
- Retterstol L, Eikvar L, Bohn M, Bakken A, Erikssen J, Berg K. C-reactive protein predicts death in patients with previous premature myocardial infarction--a 10 year follow-up study. Atherosclerosis 2002;160:433-40.
- Retterstol L, Kierulf P, Pedersen JC, Bohn M, Bakken A, Erikssen J, et al. Plasma fibrinogen level and long-term prognosis in Norwegian middle-aged patients with previous myocardial infarction. A 10 year follow-up study. J Intern Med 2001;249:511-18.
- Deckers JW, Goedhart DM, Boersma E, Briggs A, Bertrand M, Ferrari R, et al. Treatment benefit by perindopril in patients with stable coronary artery disease at different levels of risk. Eur Heart J 2006;27:796-801.
- Minoretti P, Falcone C, Calcagnino M, Emanuele E, Buzzi MP, Coen E, et al. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J 2006;27:802-7.
- Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J 2005;26:137-44.
- Tervahauta M, Pekkanen J, Nissinen A. Risk factors of coronary heart disease and total mortality among elderly men with and without preexisting coronary heart disease. Finnish cohorts of the Seven Countries Study. J Am Coll Cardiol 1995;26:1623-9.
- Bittner V, Hardison R, Kelsey SF, Weiner BH, Jacobs AK, Sopko G. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI). Circulation 2002;106:2537-42.
- Simes RJ, Marschner IC, Hunt D, Colquhoun D, Sullivan D, Stewart RA, et al. Relationship between lipid levels and clinical outcomes in the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Trial: to what extent is the reduction in coronary events with pravastatin explained by on-study lipid levels?. Circulation 2002;105:1162-9.
- Qi X, Li S, Li J. The prognostic value of IL-8 for cardiac events and restenosis in patients with coronary heart diseases after percutaneous coronary intervention. Jpn Heart J 2003;44:623-32.
- Qi X, Li J, Gu J, Li S, Dang Y, Wang T. Plasma levels of IL-8 predict early complications in patients with coronary heart disease after percutaneous coronary intervention. Jpn Heart J 2003;44:451-61.
- Susen S, Sautiere K, Mouquet F, Cuilleret F, Chmait A, McFadden EP, et al. Serum hepatocyte growth factor levels predict long-term clinical outcome after percutaneous coronary revascularization. Eur Heart J 2005;26:2387-95.
- Dibra A, Mehilli J, Braun S, Hadamitzky M, Baum H, Dirschinger J, et al. Association between C-reactive protein levels and subsequent cardiac events among patients with stable angina treated with coronary artery stenting. Am J Med 2003;114:715-22.
- Buchwald H, Boen JR, Nguyen PA, Williams SE, Matts JP. Plasma lipids and cardiovascular risk: a POSCH report. Program on the Surgical Control of the Hyperlipidemias. Atherosclerosis 2001;154:221-7.
- Lundstam U, Herlitz J, Karlsson T, Linden T, Wiklund O. Serum lipids, lipoprotein(a) level, and apolipoprotein(a) isoforms as prognostic markers in patients with coronary heart disease. J Intern Med 2002;251:111-18.
- van Lennep JE, Westerveld HT, van Lennep HW, Zwinderman AH, Erkelens DW, van der Wall EE. Apolipoprotein concentrations during treatment and recurrent coronary artery disease events. Arterioscler Thromb Vasc Biol 2000;20:2408-13.
- Bosevski M, Kostoska S, Tosev S, Borozanov V. Prognostic importance of haemostatic parameters in polyarterial disease. Prilozi 2005;26:81-92.
- Behar S, Graff E, Reicher-Reiss H, Boyko V, Benderly M, Shotan A, et al. Low total cholesterol is associated with high total mortality in patients with coronary heart disease. The Bezafibrate Infarction Prevention (BIP) Study Group. Eur Heart J 1997;18:52-9.
- Zhukovskii GS. Ischemic heart disease mortality of men 40 to 59 years old and its basic risk factors (based on prospective study data). Kardiologiia 1982;22:76-81.
- Glader CA, Birgander LS, Stenlund H, Dahlen GH. Is lipoprotein(a) a predictor for survival in patients with established coronary artery disease? Results from a prospective patient cohort study in northern Sweden. J Intern Med 2002;252:27-35.
- Frank CW, Weinblatt E, Shapiro S. Angina pectoris in men. Prognostic significance of selected medical factors. Circulation 1973;47:509-17.
- Berge KG, Canner PL, Hainline A. High-density lipoprotein cholesterol and prognosis after myocardial infarction. Circulation 1982;66:1176-8.
- Takahashi T, Chikamori T, Yonezawa Y, Sugimoto K, Yamada M, Takata J, et al. Prognostic value of serum cholesterol level in Japanese patients with coronary artery disease. Jpn Circ J 1997;61:139-44.
- Wu TC, Leu HB, Lin WT, Lin CP, Lin SJ, Chen JW. Plasma matrix metalloproteinase-3 level is an independent prognostic factor in stable coronary artery disease. Eur J Clin Invest 2005;35:537-45.
- Fukushima H, Sugiyama S, Honda O, Koide S, Nakamura S, Sakamoto T, et al. Prognostic value of remnant-like lipoprotein particle levels in patients with coronary artery disease and type II diabetes mellitus. J Am Coll Cardiol 2004;43:2219-24.
- Chikamori T, Sugimoto K, Hamada T, Kitaoka H, Furuno T, Seo H, et al. Efficacy of cholesterol-lowering treatment in Japanese elderly patients with coronary artery disease and normal cholesterol level using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. J Cardiol 2000;35:95-101.
- Janoskuti L, Forhecz Z, Hosszufalusi N, Kleiber M, Walentin S, Balint O, et al. High levels of C-reactive protein with low total cholesterol concentrations additively predict all-cause mortality in patients with coronary artery disease. Eur J Clin Invest 2005;35:104-11.
- Cesena FH, Favarato D, Cesar LA, de Oliveira SA, Da Luz PL. Cardiac complications during waiting for elective coronary artery bypass graft surgery: incidence, temporal distribution and predictive factors. Eur J Cardiothorac Surg 2004;25:196-202.
- Zotz RB. Prospective analysis after coronary-artery bypass grafting: Platelet GP IIIa polymorphism (HPA-1b/Pl(A2)) is a risk factor for bypass occlusion, myocardial infarction, and death. Thromb Haemost 2000;83:404-7.
- Hoffmann RG, Blumlein SL, Anderson AJ, Barboriak JJ, Walker JA, Rimm AA. The probability of surviving coronary bypass surgery. Five-year results from 1,718 patients. JAMA 1980;243:1341-4.
- Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997;337:230-6.
- Ulvenstam G, Bergstrand R, Johansson S, Vedin A, Wilhelmsson C, Wedel H, et al. Prognostic importance of cholesterol levels after myocardial infarction. Prev Med 1984;13:355-66.
- Vittinghoff E, Shlipak MG, Varosy PD, Furberg CD, Ireland CC, Khan SS, et al. Risk factors and secondary prevention in women with heart disease: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2003;138:81-9.
- von Eynatten M, Hamann A, Twardella D, Nawroth PP, Brenner H, Rothenbacher D. Atherogenic dyslipidaemia but not total- and high-molecular weight adiponectin are associated with the prognostic outcome in patients with coronary heart disease. Eur Heart J 2008;29:1307-15.
- Volzke H, Henzler J, Menzel D, Robinson DM, Hoffmann W, Vogelgesang D, et al. Outcome after coronary artery bypass graft surgery, coronary angioplasty and stenting. Int J Cardiol 2007;116:46-52.
- LaRosa JC, Grundy SM, Kastelein JJ, Kostis JB, Greten H. Safety and efficacy of Atorvastatin-induced very low-density lipoprotein cholesterol levels in Patients with coronary heart disease (a post hoc analysis of the treating to new targets [TNT] study). Am J Cardiol 2007;100:747-52.
- Aronow WS, Ahn C. Incidence of new coronary events in older persons with prior myocardial infarction and serum low-density lipoprotein cholesterol > or = 125 mg/dl treated with statins versus no lipid-lowering drug. Am J Cardiol 2002;89:67-9.
- Kastelein JJ, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008;117:3002-9.
- Sacks FM, Moye LA, Davis BR, Cole TG, Rouleau JL, Nash DT, et al. Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the Cholesterol and Recurrent Events trial. Circulation 1998;97:1446-52.
- Aronow WS, Ahn C, Gutstein H. Reduction of new coronary events and new atherothrombotic brain infarction in older persons with diabetes mellitus, prior myocardial infarction, and serum low-density lipoprotein cholesterol >/=125 mg/dl treated with statins. J Gerontol A Biol Sci Med Sci 2002;57:M747-50.
- Olsson AG, Schwartz GG, Szarek M, Sasiela WJ, Ezekowitz MD, Ganz P, et al. High-density lipoprotein, but not low-density lipoprotein cholesterol levels influence short-term prognosis after acute coronary syndrome: results from the MIRACL trial. Eur Heart J 2005;26:890-6.
- Yanase M, Takatsu F, Tagawa T, Kato T, Arai K, Koyasu M, et al. Insulin resistance and fasting hyperinsulinemia are risk factors for new cardiovascular events in patients with prior coronary artery disease and normal glucose tolerance. Circ J 2004;68:47-52.
- Linden T, Taddei-Peters W, Wilhelmsen L, Herlitz J, Karlsson T, Ullstrom C, et al. Serum lipids, lipoprotein(a) and apo(a) isoforms in patients with established coronary artery disease and their relation to disease and prognosis after coronary by-pass surgery. Atherosclerosis 1998;137:175-86.
- Guang-da X, You-ying L, Zhi-Song C, Yu-Sheng H, Xiang-Jiu Y. Apolipoprotein e4 allele is predictor of coronary artery disease death in elderly patients with type 2 diabetes mellitus. Atherosclerosis 2004;175:77-81.
- Benchimol D, Dubroca B, Bernard V, Lavie J, Paviot B, Benchimol H, et al. Short- and long-term risk factors for sudden death in patients with stable angina. Int J Cardiol 2000;76:147-56.
- Aronow WS, Ahn C. Effect of beta blockers on incidence of new coronary events in older persons with prior myocardial infarction and diabetes mellitus. Am J Cardiol 2001;87:780-1.
- Leu HB, Lin CP, Lin WT, Wu TC, Chen JW. Risk stratification and prognostic implication of plasma biomarkers in nondiabetic patients with stable coronary artery disease: the role of high-sensitivity C-reactive protein. Chest 2004;126:1032-9.
- Wattanakit K, Folsom AR, Chambless LE, Nieto FJ. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2005;149:606-12.
- Schlitt A, Blankenberg S, Bickel C, Meyer J, Hafner G, Jiang XC, et al. Prognostic value of lipoproteins and their relation to inflammatory markers among patients with coronary artery disease. Int J Cardiol 2005;102:477-85.
- Sacks FM, Tonkin AM, Shepherd J, Braunwald E, Cobbe S, Hawkins CM, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation 2000;102:1893-900.
- Blankenberg S, McQueen MJ, Smieja M, Pogue J, Balion C, Lonn E, et al. Comparative impact of multiple biomarkers and N-Terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2006;114:201-8.
- Sinning JM, Bickel C, Messow CM, Schnabel R, Lubos E, Rupprecht HJ, et al. Impact of C-reactive protein and fibrinogen on cardiovascular prognosis in patients with stable angina pectoris: the AtheroGene study. Eur Heart J 2006;27:2962-8.
- Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med 1995;332:635-41.
- Morange PE, Bickel C, Nicaud V, Schnabel R, Rupprecht HJ, Peetz D, et al. Haemostatic factors and the risk of cardiovascular death in patients with coronary artery disease: the AtheroGene study. Arterioscler Thromb Vasc Biol 2006;26:2793-9.
- Thompson SG, Fechtrup C, Squire E, Heyse U, Breithardt G, van de Loo JC, et al. Antithrombin III and fibrinogen as predictors of cardiac events in patients with angina pectoris. Arterioscler Thromb Vasc Biol 1996;16:357-62.
- Cooper J. Fibrinogen level as a predictor of mortality in survivors of myocardial infarction. Fibrinolysis 1991;5:108-15.
- Hartmann M, von Birgelen C, Mintz GS, Stoel MG, Eggebrecht H, Wieneke H, et al. Relation between lipoprotein(a) and fibrinogen and serial intravascular ultrasound plaque progression in left main coronary arteries. J Am Coll Cardiol 2006;48:446-52.
- Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol 2004;44:1819-24.
- Otsuka M, Hayashi Y, Ueda H, Imazu M, Kohno N. Predictive value of preprocedural fibrinogen concerning coronary stenting. Atherosclerosis 2002;164:371-8.
- Blankenberg S, Rupprecht HJ, Bickel C, Hafner G, Meyer J. The role of inflammation and infection in acute coronary syndrome. Herz 2001;26:9-18.
- Palmerini T, Marzocchi A, Marrozzini C, Reggiani LB, Savini C, Marinelli G, et al. Preoperative C-reactive protein levels predict 9-month mortality after coronary artery bypass grafting surgery for the treatment of left main coronary artery stenosis. Eur J Cardiothorac Surg 2007;31:685-90.
- Shlipak MG, Ix JH, Bibbins-Domingo K, Lin F, Whooley MA. Biomarkers to predict recurrent cardiovascular disease: the Heart and Soul Study. Am J Med 2008;121:50-7.
- Espinola-Klein C, Rupprecht HJ, Bickel C, Lackner K, Schnabel R, Munzel T, et al. Inflammation, atherosclerotic burden and cardiovascular prognosis. Atherosclerosis 2007;195:e126-34.
- Sjoland H, Tengborn L, Stensdotter L, Herlitz J. Lack of very strong association between pre-treatment fibrinogen and PAI-1 with long-term mortality after coronary bypass surgery. Cardiology 2007;108:82-9.
- Marchioli R, Avanzini F, Barzi F, Chieffo C, Di Castelnuovo A, Franzosi MG, et al. Assessment of absolute risk of death after myocardial infarction by use of multiple-risk-factor assessment equations: GISSI-Prevenzione mortality risk chart. Eur Heart J 2001;22:2085-103.
- Bickel C, Rupprecht HJ, Blankenberg S, Espiniola-Klein C, Schlitt A, Rippin G, et al. Relation of markers of inflammation (C-reactive protein, fibrinogen, von Willebrand factor, and leukocyte count) and statin therapy to long-term mortality in patients with angiographically proven coronary artery disease. Am J Cardiol 2002;89:901-8.
- Volzke H, Robinson DM, Kleine V, Hertwig S, Schwahn C, Grimm R, et al. Preoperative plasma fibrinogen levels predict mortality after coronary artery bypass grafting. Thromb Haemost 2003;89:885-91.
- Huang W, Chen QW, Lei H, Deng W, Ke DZ. Predictive value of fibrinogen and high-sensitivity C-reaction protein for cardiovascular events in patients with stable coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34:718-21.
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: effects on clinical outcomes. J Am Coll Cardiol 2003;41:2105-13.
- Held C, Hjemdahl P, Hakan WN, Bjorkander I, Forslund L, Wiman B, et al. Inflammatory and hemostatic markers in relation to cardiovascular prognosis in patients with stable angina pectoris. Results from the APSIS study. The Angina Prognosis Study in Stockholm. Atherosclerosis 2000;148:179-88.
- Behar S. Lowering fibrinogen levels: clinical update. BIP Study Group. Bezafibrate Infarction Prevention. Blood Coagul Fibrinolysis 1999;10:S41-3.
- Benderly M, Graff E, Reicher-Reiss H, Behar S, Brunner D, Goldbourt U. Fibrinogen is a predictor of mortality in coronary heart disease patients. The Bezafibrate Infarction Prevention (BIP) Study Group. Arterioscler Thromb Vasc Biol 1996;16:351-6.
- Haim M, Tanne D, Goldbourt U, Doolman R, Boyko V, Brunner D, et al. Serum homocysteine and long-term risk of myocardial infarction and sudden death in patients with coronary heart disease. Cardiology 2007;107:52-6.
- Rahel BM, Visseren FL, Suttorp MJ, Plokker TH, Kelder JC, de Jongh BM, et al. Preprocedural serum levels of acute-phase reactants and prognosis after percutaneous coronary intervention. Cardiovasc Res 2003;60:136-40.
- Haim M, Tanne D, Boyko V, Reshef T, Goldbourt U, Leor J, et al. Soluble intercellular adhesion molecule-1 and long-term risk of acute coronary events in patients with chronic coronary heart disease. Data from the Bezafibrate Infarction Prevention (BIP) Study. J Am Coll Cardiol 2002;39:1133-8.
- Redondo M, Carroll VA, Mauron T, Biasiutti FD, Binder BR, Lammle B, et al. Hemostatic and fibrinolytic parameters in survivors of myocardial infarction: a low plasma level of plasmin–alpha2-antiplasmin complex is an independent predictor of coronary re-events. Blood Coagul Fibrinolysis 2001;12:17-24.
- Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta 2008;395:27-31.
- Saely CH, Koch L, Schmid F, Marte T, Aczel S, Langer P, et al. Lipoprotein(a), type 2 diabetes and vascular risk in coronary patients. Eur J Clin Invest 2006;36:91-7.
- Corsetti JP, Ryan D, Rainwater DL, Moss AJ, Zareba W, Block RC, et al. Lp(a) and risk of recurrent cardiac events in obese postinfarction patients. Obesity (Silver Spring) 2008;16:2217-22.
- Zairis MN, Ambrose JA, Manousakis SJ, Stefanidis AS, Papadaki OA, Bilianou HI, et al. The impact of plasma levels of C-reactive protein, lipoprotein (a) and homocysteine on the long-term prognosis after successful coronary stenting: The Global Evaluation of New Events and Restenosis After Stent Implantation Study. J Am Coll Cardiol 2002;40:1375-82.
- Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a). JAMA 1995;274:1771-4.
- Wehinger A, Kastrati A, Elezi S, Baum H, Braun S, Neumann FJ, et al. Lipoprotein(a) and coronary thrombosis and restenosis after stent placement. J Am Coll Cardiol 1999;33:1005-12.
- Rahel BM, Visseren FL, Suttorp MJ, Plokker TH, Kelder JC, de Jongh BM, et al. Preprocedural serum levels of acute-phase reactants and prognosis after percutaneous coronary intervention. Cardiovasc Res 2003;60:136-40.
- Skinner JS, Farrer M, Albers CJ, Piper K, Neil HA, Adams PC. Serum Lp(a) lipoprotein concentration is not associated with clinical and angiographic outcome five years after coronary artery bypass graft surgery. Heart 1997;78:131-5.
- Lloyd GW, Jackson G, Foley DP, Boersma E, Shepherd J, Serruys PW. The influence of plasma lipoprotein (a) on angiographic restenosis and coronary events in patients undergoing planned coronary balloon angioplasty. Ancillary analysis of the Fluvastatin Angioplasty Restenosis (FLARE) trial. Atherosclerosis 2001;158:445-54.
- Harb TS, Zareba W, Moss AJ, Ridker PM, Marder VJ, Rifai N, et al. Association of C-reactive protein and serum amyloid A with recurrent coronary events in stable patients after healing of acute myocardial infarction. Am J Cardiol 2002;89:216-21.
- Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H, et al. Thrombogenic factors and recurrent coronary events. Circulation 1999;99:2517-22.
- van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ES, et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol 2008;51:634-42.
- Lee KW, Hill JS, Walley KR, Frohlich JJ. Relative value of multiple plasma biomarkers as risk factors for coronary artery disease and death in an angiography cohort. CMAJ 2006;174:461-6.
- Kastelein JJ, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, et al. Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation 2008;117:3002-9.
- Lu TM, Ding YA, Lin SJ, Lee WS, Tai HC. Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur Heart J 2003;24:1912-19.
- Ndrepepa G, Kastrati A, Braun S, Koch W, Kolling K, Mehilli J, et al. A prospective cohort study of predictive value of homocysteine in patients with type 2 diabetes and coronary artery disease. Clin Chim Acta 2006;373:70-6.
- Retterstol L, Paus B, Bohn M, Bakken A, Erikssen J, Malinow MR, et al. Plasma total homocysteine levels and prognosis in patients with previous premature myocardial infarction: a 10-year follow-up study. J Intern Med 2003;253:284-92.
- Schnyder G, Flammer Y, Roffi M, Pin R, Hess OM. Plasma homocysteine levels and late outcome after coronary angioplasty. J Am Coll Cardiol 2002;40:1769-76.
- Zebrack JS, Anderson JL, Beddhu S, Horne BD, Bair TL, Cheung A, et al. Do associations with C-reactive protein and extent of coronary artery disease account for the increased cardiovascular risk of renal insufficiency?. J Am Coll Cardiol 2003;42:57-63.
- Anderson JL, Muhlestein JB, Horne BD, Carlquist JF, Bair TL, Madsen TE, et al. Plasma homocysteine predicts mortality independently of traditional risk factors and C-reactive protein in patients with angiographically defined coronary artery disease. Circulation 2000;102:1227-32.
- Reis RP, Azinheira J, Reis HP, Bordalo e Sa, Tavares J, Adao M, . Prognosis significance of blood homocysteine after myocardial infarction. Rev Port Cardiol 2000;19:581-5.
- Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: effects on clinical outcomes. J Am Coll Cardiol 2003;41:2105-13.
- Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA 2002;288:973-9.
- Rossi GP, Maiolino G, Seccia TM, Burlina A, Zavattiero S, Cesari M, et al. Hyperhomocysteinemia predicts total and cardiovascular mortality in high-risk women. J Hypertens 2006;24:851-9.
- Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997;337:230-6.
- Schnabel R, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Torzewski M, Lubos E, et al. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene study. J Am Coll Cardiol 2005;45:1631-7.
- Knekt P, Reunanen A, Alfthan G, Heliovaara M, Rissanen H, Marniemi J, et al. Hyperhomocystinemia: a risk factor or a consequence of coronary heart disease?. Arch Intern Med 2001;161:1589-94.
- Knekt P, Alfthan G, Aromaa A, Heliovaara M, Marniemi J, Rissanen H, et al. Homocysteine and major coronary events: a prospective population study amongst women. J Intern Med 2001;249:461-5.
- Lubos E, Schnabel R, Rupprecht HJ, Bickel C, Messow CM, Prigge S, et al. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: results from the AtheroGene study. Eur Heart J 2006;27:150-6.
- Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA 2007;297:169-76.
- Tang WH, Steinhubl SR, Van Lente F, Brennan D, McErlean E, Maroo A, et al. Risk stratification for patients undergoing nonurgent percutaneous coronary intervention using N-terminal pro-B-type natriuretic peptide: a Clopidogrel for the Reduction of Events During Observation (CREDO) substudy. Am Heart J 2007;153:36-41.
- Dai DF, Hwang JJ, Lin JL, Lin JW, Hsu CN, Lin CM, et al. Joint effects of N-terminal pro-B-type-natriuretic peptide and C-reactive protein vs angiographic severity in predicting major adverse cardiovascular events and clinical restenosis after coronary angioplasty in patients with stable coronary artery disease. Circ J 2008;72:1316-23.
- Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res 2005;97:e53-9.
- Ndrepepa G, Kastrati A, Braun S, Mehilli J, Niemoller K, von Beckerath N, et al. N-terminal probrain natriuretic peptide and C-reactive protein in stable coronary heart disease. Am J Med 2006;119:355-8.
- Richards M, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, et al. Comparison of B-type natriuretic peptides for assessment of cardiac function and prognosis in stable ischemic heart disease. J Am Coll Cardiol 2006;47:52-60.
- Saleh N, Braunschweig F, Jensen J, Tornvall P. Usefulness of preprocedural serum N-terminal pro-brain natriuretic peptide levels to predict long-term outcome after percutaneous coronary intervention in patients with normal troponin T levels. Am J Cardiol 2006;97:830-4.
- Ndrepepa G, Braun S, Niemoller K, Mehilli J, von Beckerath N, von Beckerath O, et al. Prognostic value of N-terminal pro-brain natriuretic peptide in patients with chronic stable angina. Circulation 2005;112:2102-7.
- de Winter RJ, Stroobants A, Koch KT, Bax M, Schotborgh CE, Mulder KJ, et al. Plasma N-terminal pro-B-type natriuretic peptide for prediction of death or nonfatal myocardial infarction following percutaneous coronary intervention. Am J Cardiol 2004;94:1481-5.
- Lindahl B, Lindback J, Jernberg T, Johnston N, Stridsberg M, Venge P, et al. Serial analyses of N-terminal pro-B-type natriuretic peptide in patients with non-ST-segment elevation acute coronary syndromes: a Fragmin and fast Revascularisation during In Stability in Coronary artery disease (FRISC)-II substudy. J Am Coll Cardiol 2005;45:533-41.
- Marz W, Tiran B, Seelhorst U, Wellnitz B, Bauersachs J, Winkelmann BR, et al. N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem 2007;53:1075-83.
- West MJ, Nestel PJ, Kirby AC, Schnabel R, Sullivan D, Simes RJ, et al. The value of N-terminal fragment of brain natriuretic peptide and tissue inhibitor of metalloproteinase-1 levels as predictors of cardiovascular outcome in the LIPID study. Eur Heart J 2008;29:923-31.
- Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R, et al. Prognostic value of B-Type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol 2007;50:205-14.
- Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med 2005;352:666-75.
- Rothenbacher D, Koenig W, Brenner H. Comparison of N-terminal pro-B-natriuretic peptide, C-reactive protein, and creatinine clearance for prognosis in patients with known coronary heart disease. Arch Intern Med 2006;166:2455-60.
- Schnabel R, Rupprecht HJ, Lackner KJ, Lubos E, Bickel C, Meyer J, et al. Analysis of N-terminal-pro-brain natriuretic peptide and C-reactive protein for risk stratification in stable and unstable coronary artery disease: results from the AtheroGene study. Eur Heart J 2005;26:241-9.
- Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res 2007;100:1234-41.
- Blankenberg S, Rupprecht HJ, Bickel C, Jiang XC, Poirier O, Lackner KJ, et al. Common genetic variation of the cholesteryl ester transfer protein gene strongly predicts future cardiovascular death in patients with coronary artery disease. J Am Coll Cardiol 2003;41:1983-9.
- Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 2002;106:24-30.
- Ikonomidis I, Athanassopoulos G, Stamatelopoulos K, Lekakis J, Revela I, Venetsanou K, et al. Additive prognostic value of interleukin-6 at peak phase of dobutamine stress echocardiography in patients with coronary artery disease. A 6-year follow-up study. Am Heart J 2008;156:269-76.
- Kwaijtaal M, van Diest R, Bar FW, van der Ven AJ, Bruggeman CA, de Baets MH, et al. Inflammatory markers predict late cardiac events in patients who are exhausted after percutaneous coronary intervention. Atherosclerosis 2005;182:341-8.
- Inoue T, Komoda H, Nonaka M, Kameda M, Uchida T, Node K. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease. Int J Cardiol 2008;124:319-25.
- Kip KE, Marroquin OC, Shaw LJ, Arant CB, Wessel TR, Olson MB, et al. Global inflammation predicts cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Am Heart J 2005;150:900-6.
- Hoffmeister A, Rothenbacher D, Kunze M, Brenner H, Koenig W. Prognostic value of inflammatory markers alone and in combination with blood lipids in patients with stable coronary artery disease. Eur J Intern Med 2005;16:47-52.
- Fisman EZ, Benderly M, Esper RJ, Behar S, Boyko V, Adler Y, et al. Interleukin-6 and the risk of future cardiovascular events in patients with angina pectoris and/or healed myocardial infarction. Am J Cardiol 2006;98:14-8.
Appendix 2 Search strategy for disease, study design and biomarkers in MEDLINE and EMBASE
Our original search was performed on all the literature until April 2007. At the invitation of the reviewers, we updated this in November 2008. The updated search was performed using the date parameters 1 January 2007 to 31December 2008 in order to (1) identify any papers published January–April 2007 that were not stored on databases at that time and (2) identify any papers in the public domain that had dates after the search date (e.g. advanced publications online).
Search terms as performed in April 2007
| PubMed | Results | EMBASE | Results |
|---|---|---|---|
| SEARCH 1: ACS OR ANGINA | |||
| “Angina, Unstable”[MeSH] | 295,088 hits | Exp “unstable-angina-pectoris”[SU] | 196,727 hits |
| OR unstable angina*[tw] | |||
| OR “Myocardial Ischemia”[MeSH] | Exp “Heart-muscle-ischemia”[SU] | ||
| OR coronary disease[tw] | |||
| OR coronary syndrome[tw] | |||
| OR myocardial infarct*[tw] | |||
| OR myocardial ischemi*[tw] | Myocardial isch?emi*[tw] | ||
| OR myocardial ischaemi*[tw] | |||
| OR “Coronary Thrombosis”[MeSH] | Exp “Coronary-Artery-Thrombosis”[SU] | ||
| OR coronary thrombos*[tw] | |||
| OR non q-wave[tw] | Non?q?wave | ||
| OR non q wave[tw] | |||
| OR nstemi[tw] | |||
| OR stemi[tw] | |||
| OR heart infarct*[tw] | |||
| OR coronary arteriosclerosis[tw] | |||
| OR acute coronary[tw] | |||
| OR “Angina Pectoris”[MeSH] | Exp “angina-pectoris”[SU] | ||
| OR “Angina Pectoris, variant”[MeSH] | Exp “variant-angina-pectoris”[SU] | ||
| OR angina*[tw] | |||
| SEARCH 2: STUDY DESIGN | |||
| “Prognosis”[MeSH] | 1,559,421 hits | Exp “Prognosis”[SU] | 642,517 hits |
| OR Diagnosed[tw] | |||
| OR “Cohort Studies”[MeSH] | Exp “Cohort-analysis”[SU] | ||
| OR predictor*[tw] | |||
| OR death[tw] | |||
| OR “Models, statistical”[MeSH] | Exp “statistical-model”[SU] | ||
| SEARCH 3: CRP | |||
| “C-reactive protein”[MeSH] | 20,582 hits | Exp “C reactive protein”[SU] | 20,027 hits |
| OR C reactive protein[tw] | OR C?reactive?protein | ||
| OR Hs?c?reactive protein | |||
| OR High?sensitivity?c?reactive?protein | |||
| OR Hs?CRP | |||
| OR High?sensitivity?CRP | |||
| SEARCH 3: BIOMARKERS | |||
| “C-reactive protein”[MeSH] | 626,118 hits | Exp “C reactive protein”[SU] | 446,082 hits |
| OR C reactive protein[tw] | OR C?reactive?protein | ||
| OR Hs?c?reactive protein | |||
| OR High?sensitivity?c?reactive?protein | |||
| OR Hs?CRP | |||
| OR High?sensitivity?CRP | |||
| OR “Serum albumin”[MeSH] | OR Exp “albumin”[SU] | ||
| OR Albumin[tw] | |||
| OR “Apolipoproteins A”[MeSH] | OR Exp “Apolipoprotein A”[SU] | ||
| OR Apolipoprotein* A[tw] | Apolipoprotein?A | ||
| OR ApoA[tw] | |||
| OR “Apolipoproteins B”[MeSH] | OR “Apolipoprotein B”[SU] | ||
| OR Apolipoprotein* B[tw] | Apolipoprotein?B | ||
| OR ApoB[tw] | |||
| OR “Creatinine”[MeSH] | Exp “Creatinine”[SU] | ||
| OR Creatinine[tw] | |||
| Or “Blood Glucose”[MeSH] | Exp “Glucose-blood-level”[SU] | ||
| OR Fasting glucose[tw] | |||
| OR 2 hour glucose[tw] | n/a | ||
| OR Fasting blood sugar[tw] | |||
| OR 2-hour postprandial blood sugar[tw] | n/a | ||
| OR “Fibrinogen”[MeSH] | Exp “Fibrinogen”[SU] | ||
| OR Fibrinogen[tw] | |||
| OR “Hemoglobins”[MeSH] | Exp “Hemoglobin”[SU] | ||
| OR Haemoglobin[tw] | H?emoglobin[tw] | ||
| OR Hemoglobin[tw] | – | ||
| OR Hgb[tw] | |||
| OR “Hemoglobin A, glycosylated”[MeSH] | Exp “Hemoglobin-A1c”[SU] | ||
| OR Glycated hemoglobin[tw] | Glycated h?emoglobin | ||
| OR Glycated haemoglobin[tw] | – | ||
| OR Hemoglobin A1c[tw] | H?emoglobin A1c | ||
| OR Haemoglobin A1c[tw] | – | ||
| OR Glycohaemoglobin[tw] | Glycoh?emoglobin[tw] | ||
| OR Glycohemoglobin[tw] | – | ||
| OR HbA1c[tw] | |||
| OR Glycosylated hemoglobin[tw] | Glycosylated h?emoglobin[tw] | ||
| OR Glycosylated haemoglobin[tw] | – | ||
| OR “Lipoproteins, HDL”[MeSH] | Exp “high-density-lipoprotein”[SU] | ||
| OR High density lipoprotein[tw] | OR High?density?lipoprotein[tw] | ||
| OR HDL[tw] | |||
| OR “Lipoproteins, LDL”[MeSH] | Exp “low-density-lipoprotein”[SU] | ||
| OR Low density lipoprotein[tw] | OR Low?density?lipoprotein[tw] | ||
| OR LDL[tw] | |||
| OR “Homocysteine”[MeSH] | Exp “homocysteine”[SU] | ||
| OR Homocysteine[tw] | |||
| tHcy[tw] | |||
| OR “Interleukin-6”[MeSH] | Exp “Interleukin-6”[SU] | ||
| OR Interleukin-6[tw] | Interleukin?6[tw] | ||
| OR IL-6[tw] | IL?6[tw] | ||
| OR IL 6[tw] | – | ||
| OR “Lipoprotein(a)”[MeSH] | Exp “lipoprotein-A”[SU] | ||
| OR “lipoprotein(a)”[tw] | OR lipoprotein?(a)[tw] | ||
| OR lipoprotein a[tw] | OR Lp?(a)[tw] | ||
| OR “Lp(a)”[tw] | OR lipoprotein?a[tw] | ||
| OR “Lp a”[tw] | OR Lp?a[tw] | ||
| OR Pro-brain natriuretic peptide (1–76)[Substance name] | |||
| OR N-terminal pro-BNP[tw] | |||
| OR NT-BNP[tw] | |||
| OR NTproBNP[tw] | NT?proBNP | ||
| OR NT-proBNP[tw] | – | ||
| OR Amino-terminal pro-brain natriuretic peptide[tw] | |||
| OR “Cholesterol”[MeSH] | Exp “Cholesterol-blood-level”[SU] | ||
| OR total cholesterol[tw] | |||
| OR “Triglycerides”[MeSH] | Exp “Triacyglycerol”[SU] | ||
| Triacyglycerol[tw] | |||
| OR triglycerides[tw] | |||
| OR “Leukocyte count”[MeSH] | Exp “Leukocyte count”[SU] | ||
| OR leukocyte count[tw] | |||
| OR white blood cell count[tw] | |||
| OR WBC[tw] | |||
Search terms as performed in November 2008 (with date parameters set 1 January 2007 to 31 December 2008)
EMBASE standard search terms had changed in the intervening period so the initial search strategy could not be replicated exactly.
| PubMed | Results | EMBASE | Results |
|---|---|---|---|
| SEARCH 1: ACS OR ANGINA | |||
| “Angina, Unstable”[MeSH] | 21,594 hits | exp Unstable Angina Pectoris/ | 8501 hits |
| OR unstable angina*[tw] | unstable angina pectoris.mp | ||
| OR “Myocardial Ischemia”[MeSH] | exp Heart Muscle Ischemia/ | ||
| OR coronary disease[tw] | Heart Muscle Ischemia.mp | ||
| OR coronary syndrome[tw] | |||
| OR myocardial infarct*[tw] | |||
| OR myocardial ischemi*[tw] | |||
| OR myocardial ischaemi*[tw] | myocardial isch?emi?.mp | ||
| OR “Coronary Thrombosis”[MeSH] | exp Coronary Artery Thrombosis/ | ||
| OR coronary thrombos*[tw] | Coronary Artery Thrombosis.mp | ||
| OR non q-wave[tw] | non q-wave.mp | ||
| OR non q wave[tw] | non q wave.mp | ||
| OR nstemi[tw] | non-q-wave.mp | ||
| OR stemi[tw] | non-q wave.mp | ||
| OR heart infarct*[tw] | exp Angina Pectoris/ | ||
| OR coronary arteriosclerosis[tw] | angina pectoris.mp | ||
| OR acute coronary[tw] | exp Variant Angina Pectoris/ | ||
| OR “Angina Pectoris”[MeSH] | Variant Angina Pectoris.mp | ||
| OR “Angina Pectoris, variant”[MeSH] | |||
| OR angina*[tw] | |||
| SEARCH 2: STUDY DESIGN | |||
| “Prognosis”[MeSH] | 227,140 hits | exp Prognosis/ | 47,362 hits |
| OR Diagnosed[tw] | prognosis.mp | ||
| OR “Cohort Studies”[MeSH] | exp Cohort Analysis/ | ||
| OR predictor*[tw] | cohort analysis.mp | ||
| OR death[tw] | exp Statistical Model/ | ||
| OR “Models, statistical”[MeSH] | statistical model.mp | ||
| SEARCH 3: CRP | |||
| “C-reactive protein”[MeSH] | 5980 hits | exp C Reactive Protein/ | 7914 hits |
| OR C reactive protein[tw] | C?Reactive?Protein.mp | ||
| c reactive protein.mp | |||
| CRP.mp | |||
| Hs?c?reactive protein.mp | |||
| high?sensitivity?c?reactive?protein.mp | |||
| Hs?CRP.mp | |||
| high?sensitivity?CRP.mp | |||
| SEARCH 3: BIOMARKERS | |||
| “C-reactive protein”[MeSH] | 53,951 hits | exp C Reactive Protein/ | 82,067 hits |
| OR C reactive protein[tw] | C?Reactive?Protein.mp | ||
| c reactive protein.mp | |||
| CRP.mp | |||
| Hs?c?reactive protein.mp | |||
| high?sensitivity?c?reactive?protein.mp | |||
| OR “Serum albumin”[MeSH] | Hs?CRP.mp | ||
| OR Albumin[tw] | high?sensitivity?CRP.mp | ||
| OR “Apolipoproteins A”[MeSH] | exp Albumin/ | ||
| OR Apolipoprotein* A[tw] | albumin.mp | ||
| OR ApoA[tw] | serum albumin.mp | ||
| OR “Apolipoproteins B”[MeSH] | exp Serum Albumin/ | ||
| OR Apolipoprotein* B[tw] | exp Albumin Blood Level/ | ||
| OR ApoB[tw] | albumin blood level.mp | ||
| OR “Creatinine”[MeSH] | apolipoprotein a.mp | ||
| OR Creatinine[tw] | Apolipoprotein A/ | ||
| Or “Blood Glucose”[MeSH] | Apolipoprotein A1/ | ||
| OR Fasting glucose[tw] | apolipoprotein a1.mp | ||
| OR 2 hour glucose[tw] | Apolioprotein B/ | ||
| OR Fasting blood sugar[tw] | apolipoprotein b.mp | ||
| OR 2-hour postprandial blood sugar[tw] | Creatinin Blood Level/or Creatinine/ | ||
| OR “Fibrinogen”[MeSH] | Creatinine.mp | ||
| OR Fibrinogen[tw] | creatinine blood level.mp | ||
| OR “Hemoglobins”[MeSH] | Glucose/ | ||
| OR Haemoglobin[tw] | Glucose Blood Level/ | ||
| OR Hemoglobin[tw] | Glucose.mp | ||
| OR Hgb[tw] | Glucose Blood Level.mp | ||
| OR “Hemoglobin A, glycosylated”[MeSH] | Fibrinogen Blood Level/or Fibrinogen/ | ||
| OR Glycated hemoglobin[tw] | |||
| OR Glycated haemoglobin[tw] | Glycosylated Hemoglobin/of Hemoglobin/ | ||
| OR Hemoglobin A1c[tw] | h?emoglobin.mp | ||
| OR Haemoglobin A1c[tw] | glycated h?emoglobin.mp | ||
| OR Glycohaemoglobin[tw] | glycoh?emoglobin.mp | ||
| OR Glycohemoglobin[tw] | Hemoglobin Blood Level/ | ||
| OR HbA1c[tw] | h?emoglobin blood level.mp | ||
| OR Glycosylated hemoglobin[tw] | High Density Lipoprotein/ | ||
| OR Glycosylated haemoglobin[tw] | high density lipoprotein.mp | ||
| OR “Lipoproteins, HDL”[MeSH] | Low Density Lipoprotein/ | ||
| OR High density lipoprotein[tw] | low density lipoprotein.mp | ||
| OR HDL[tw] | hdl.mp | ||
| OR “Lipoproteins, LDL”[MeSH] | ldl.mp | ||
| OR Low density lipoprotein[tw] | homopcysteine.mp | ||
| OR LDL[tw] | Homocysteine/ | ||
| OR “Homocysteine”[MeSH] | tHcy.mp | ||
| OR Homocysteine[tw] | Interleukin 6/ | ||
| Interleukin 6.mp | |||
| OR “Interleukin-6”[MeSH] | il?6.mp | ||
| OR Interleukin-6[tw] | interleukin?6.mp | ||
| OR IL-6[tw] | Lipoprotein A/ | ||
| OR IL 6[tw] | lipoprotein a.mp | ||
| OR “Lipoprotein(a)”[MeSH] | lipoprotein a?mp | ||
| OR “lipoprotein(a)”[tw] | lipoprotein?a?.mp | ||
| OR lipoprotein a[tw] | Lp a.mp | ||
| OR “Lp(a)”[tw] | Lp?a?.mp | ||
| OR “Lp a”[tw] | Amino Terminal Pro Brain Natriuretic Peptide/or Brain Natriuretic Peptide/ | ||
| OR Pro-brain natriuretic peptide (1–76)[Substance name] | Brain Natriuretic Peptide.mp | ||
| OR N-terminal pro-BNP[tw] | Amino Terminal Pro Brain Natriuretic Peptide.mp | ||
| OR NT-BNP[tw] | NT?pro?BNP.mp | ||
| OR NTproBNP[tw] | BNP.mp | ||
| OR NT-proBNP[tw] | Cholesterol Blood Level/ | ||
| OR Amino-terminal pro-brain natriuretic peptide[tw] | Cholesterol/ | ||
| OR “Cholesterol”[MeSH] | cholesterol.mp | ||
| OR total cholesterol[tw] | cholesterol blood level.mp | ||
| OR “Triglycerides”[MeSH] | Triacylglycerol/ | ||
| Triacylglycerol.mp | |||
| OR triglycerides[tw] | Leukocyte Count/ | ||
| OR “Leukocyte count”[MeSH] | leukocyte count.mp | ||
| OR leukocyte count[tw] | |||
| OR white blood cell count[tw] | |||
| OR WBC[tw] | |||
| MeSH, (MEDLINE) Medical Subject Heading; TW, text word | |||
Appendix 3 Eligibility criteria for biomarker studies in systematic review
| Criterion | Definition | Example of ineligible articles |
|---|---|---|
| Prospective design | Any prospective study including observational cohort studies, prospective nested case–control, randomised controlled trials | Cross-sectional studies, meta-analyses, editorials, reviews, comments |
| Patients with stable coronary disease | Studies which include patients described as having stable coronary disease, chronic stable angina, or a history of acute coronary syndromes for at least 2 weeks prior to biomarker measurement | Patients with: unstable angina, acute coronary syndrome, acute myocardial infarction, emergency revascularisation, undiagnosed coronary disease at biomarker measurement (i.e. healthy population) |
| One or more eligible biomarker | The article must discuss one or more of the following biomarkers: total cholesterol, low density lipoprotein, high density lipoprotein, triglycerides, fasting glucose, haemoglobin, white cell count, creatinine (serum creatinine, creatinine clearance or glomerular filtration rate), apolipoprotein A, apolipoprotein B, lipoprotein (a), fibrinogen, homocysteine, C-reactive protein, N-terminal B natriuretic protein, interleukin 6 | Studies measuring other circulating biomarkers |
| Eligible outcomes | Any prospective study which measured the following outcomes: coronary death, non-fatal coronary event, coronary revascularisation, cardiovascular death, all-cause mortality (these can be in combination with non-fatal vascular events) | Studies which measure ONLY non-fatal vascular events (e.g. stroke, heart failure, peripheral arterial disease) |
| Relative risks and 95% confidence intervals | Any prospective study which reported a hazard/odds ratio OR studies which provide the number of events and number of patients in two or more biomarker comparison groups |
Appendix 4 Coding protocol for extraction of eligible studies
| Variable name | Definition | Coding | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||
| RefmanID | reference manager ID for the article | |||||||||||
| Study number | Unique number given to the article by the reviewers | |||||||||||
| Author | Last name of the first author of the article | |||||||||||
| Pubyear | Publication year of the article | |||||||||||
| Studyname | Name of study discussed in the article | |||||||||||
| Startyear | Year the study began (i.e. when first patients were recruited) | When the start year is not stated, give an estimate of 5 years before publication date | ||||||||||
| Country | Country where study was conducted | |||||||||||
| Population/design | ||||||||||||
| Prespec | Whether the research question was pre-specified in a peer-reviewed, dated protocol or grant |
1 = Yes –1 = Not stated |
||||||||||
| Designcom | The sources of data collection reported in the article. From the list opposite, indicate the sources of data collection reported in the article. Each source has a three-digit number; <designcom> is written as a string of one or several three-digit numbers |
101 = Randomised controlled trial 102 = Observational: bespoke prospective baseline collection, assessing more variables than routine clinical care 103 = Extraction of clinical records 104 = Extraction from routinely collected electronic data 105 = Ongoing coronary disease registry 106 = Retrospective 108 = Nested case–control 109 = Cross-sectional |
||||||||||
| Multiplepub | Whether the biomarker of interest is analysed for the same data set in a separate article |
1 = Yes –1 = Not stated |
||||||||||
| Sample | The method by which the study sample of the article was selected | |||||||||||
| Multicentre | Indicates the number of patient centres in the study (e.g. hospital units, clinics) |
1 = 1 2 = 2 3 = 3 or more –1 = Not stated |
||||||||||
| Negligible | The total number of patients who were invited to participate in the study, i.e. the number before exclusion criteria or missing data reduced the number of analysed patients in the article | |||||||||||
| Npatients | Number of patients included in the analysis of the biomarker | |||||||||||
| Nsampleclear | Whether there is a clear description of how the number analysed was derived from the number eligible |
1 = Yes –1 = Not stated |
||||||||||
| Excritcom |
The combination of exclusion criteria reported in the article. Each reason for exclusion is a three-digit number; <excritcom> is written as a string of one or several three-digit numbers. The codes for the exclusion criteria were categorised as follows: Acute coronary or cardiovascular events: codes 101–129 Treatment: 201–236 Infections: 301–305 Inflammatory disorder: 401–408 Malignancy: 501 Other: 601–627 |
|||||||||||
| Ptresponse | The percentage of <N eligible> patients who agreed to be part of the study | |||||||||||
| Consent | Was written informed patient consent obtained? |
1 = Stated –1 = Not stated |
||||||||||
| Age | Average age of <N patients> | |||||||||||
| Ageavetype | How was <Age> estimated |
1 = Mean, as stated in the article 2 = Median, as stated in the article 3 = Calculated weighted mean 4 = Calculated weighted median 5 = Mid-point of stated age range –1 = Not stated |
||||||||||
| Pctwomen | Percentage of <N patients> who were women | |||||||||||
| Settcom | From the list opposite, indicate the setting in which the patients were recruited and the study was carried out |
101 = Patients presenting to and diagnosed in primary care 201 = Patients undergoing an exercise electrocardiogram or other non-invasive ischaemic test 202 = Patients presenting to chest pain clinics 203 = Patients referred to other hospital outpatient departments 204 = Patients undergoing coronary angiography 205 = Patients undergoing revascularisation: PCI, CABG 301 = Patients recruited AFTER coronary event 401 = Randomised controlled trial –1 = Not stated |
||||||||||
| Ptcom |
The combination of types of patients analysed in the article Articles with patients with 300 or 400 codes ONLY were considered ineligible |
From the list below, indicate the combination of characteristics that make up the patient population of the article. Each characteristic is a three-digit number; <ptcom> is written as a string of one or several three-digit numbers, e.g. the string 100301105 indicates stable and unstable angina patients admitted for an elective PCI Stable/chronic diseaseHistory of previous coronary heart diseaseAcute or unstable disease at time of biomarker measurementOther vascular diseaseSubgroup identification |
Stable/chronic disease | History of previous coronary heart disease | Acute or unstable disease at time of biomarker measurement | Other vascular disease | Subgroup identification | |||||
| Stable/chronic disease | History of previous coronary heart disease | Acute or unstable disease at time of biomarker measurement | Other vascular disease | Subgroup identification | ||||||||
| AveHx | Time since the first presentation of coronary disease (in average years) | |||||||||||
| MinHx | The minimum known time of patients having coronary disease (in years) | |||||||||||
| PctMI | Percentage of <N patients> who have suffered a previous myocardial infarction | |||||||||||
| PctCAD | Percentage of <N patients> with angiographically confirmed coronary artery disease | 100 if cohort admitted for revascularisation | ||||||||||
| Pctangina | Percentage of <N patients> with stable angina at the time of recruitment | |||||||||||
| Pctstatin | Percentage of <N patients> on statins at time of recruitment | |||||||||||
| FU | Follow-up time, in years | |||||||||||
| FUtype | Type of measure for follow-up time |
1 = Mean 2 = Median 3 = Maximum follow-up time stated in the analysis 4 = Average length of hospital stay for coronary event (5 days) –1 = Not stated |
||||||||||
| PctlostFU | Percentage of patients lost to follow-up |
Percentage stated in the article –1 = Not stated |
||||||||||
| CharaclostFU | Whether the article states the characteristics of patients followed up compared with those lost to follow-up |
1 = Stated –1 = Not stated |
||||||||||
| Biomarker measurement | ||||||||||||
| Biomarker identification number | Identification number of biomarker reported in article. If there is more than one biomarker analysed in the article, begin a new row of data entry |
1 = Total cholesterol 2 = Low density lipoprotein 3 = High density lipoprotein 4 = Triglycerides 5 = Fasting glucose 6 = Haemoglobin 7 = White blood cell count 8 = Creatinine (including serum creatinine, glomerular filtration rate, creatinine clearance) 9 = Apolipoprotein A-1 10 = Apolipoprotein B 11 = Lipoprotein (a) 12 = Fibrinogen 13 = Homocysteine 14 = C-reactive protein 15 = N-terminal pro-B type natriuretic peptide 16 = IL-6 |
||||||||||
| BMmeascom | From the list opposite, indicate which biomarkers have been measured in the article. Each biomarker has a three-digit number; <BMmeascom> is written as a string of one or several three-digit numbers. For example, when both C-reactive protein and triglycerides are measured in the blood analysis, the entry is 114104 |
101 = Total cholesterol 102 = Low density lipoprotein 103 = High density lipoprotein 104 = Triglycerides 105 = Fasting glucose 106 = Haemoglobin 107 = White blood cell count 108 = Creatinine, glomerular filtration rate, creatinine clearance 109 = Apolipoprotein A 110 = Apolipoprotein B 111 = Lipoprotein (a) 112 = Fibrinogen 113 = Homocysteine 114 = C-reactive protein 115 = N-terminal pro-B natriuretic peptide 116 = Interleukin-6 |
||||||||||
| Fasting | Whether the blood sample was taken while patients were fasting |
1 = Fasting 2 = Casual –1 = Not stated |
||||||||||
| Tempstor | The temperature at which the blood samples were stored |
1 = Fresh (i.e. sample assayed immediately and not frozen) 2 = Sample frozen at –80°C 3 = Sample frozen at –79°C to –60°C 4 = Sample frozen at–59°C to –40°C 5 = Sample frozen at –39°C to –1°C 6 = Refrigerated at 0°C to 10°C |
||||||||||
| Manufacturer | The reagent manufacturer biomarker measurement | Write in free text the name of the company source (e.g. Dade Behring, Abbott Diagnostics) or in house | ||||||||||
| Assay | The method of assay of biomarker measurement |
1 = Turbidimetric 2 = Nephelometric 3 = EIA (Enzyme immunoassay) 4 = ELISA (Enzyme linked immunoradiometric assay) 5 = Fluorescence polarisation 6 = Chemiluminescent 7 = Latex particle enhanced 8 = Autoanalyser 9 = HPLC (high pressure liquid chromatography) 10 = Latex agglutination 11 = von Claus method 12 = Precipitation with sodium/magnesium/phosphates 13 = Infrared particle immunoassay 14 = Sandwich enzyme linked immunosorbent assay 15 = MEIA (microparticle capture enzyme immunoassay) |
||||||||||
| BMrepmeas | The number of times the biomarker was measured |
1 = Biomarker was measured one time only 2 = Biomarker was measured at two different time points 3 = etc. –1 = Not stated |
||||||||||
| BMave | The average value of the biomarker at baseline irrespective of subsequent outcome events status | |||||||||||
| BMavetype | How was <BMave> obtained? |
1 = Mean, as stated in the article 2 = Median, as stated in the article 3 = Calculated weighted mean 4 = Calculated weighted median 5 = Geometric mean 6 = Weighted average of geometric mean 7 = Average of geometric mean –1 = Not stated –2 = Not applicable |
||||||||||
| BMSD | Standard deviation of the biomarker | |||||||||||
| BMunit | Measurement unit of the biomarker used in the article |
1 = mg/l 2 = g/l 3 = µg/l 4 = ng/l 5 = mg/dl 6 = g/dl 7 = mg/ml 8 = µg/ml 9 = ng/ml 10 = pg/ml 11 = mmol/l 12 = µmol/l 13 = pmol/l 14 = ml/min 15 = ml/min/m2 |
||||||||||
| BMavewithev | Average value of the biomarker at baseline for patients who had subsequent outcome event | |||||||||||
| BMavetypewithevt | How was <BMavewithev> obtained for patients who had an outcome event |
1 = Mean 2 = Median 3 = Weighted average of means 4 = Weighted average of medians –1 = Not stated |
||||||||||
| BMevtSD | Standard deviation for the biomarker at baseline for patients who did not experience an event over the follow-up period | |||||||||||
| BMavenoevt | The average value of the biomarker for patients without an event | |||||||||||
| BMavetypenoevt | How was this <BMavenoevt> obtained for patients who had an outcome event |
1 = Mean 2 = Median 3 = Weighted average of means 4 = Weighted average of medians –1 = Not stated |
||||||||||
| BMnoevtSD | Standard deviation for biomarker for patients without an outcome event | |||||||||||
| Blindisease | Whether the measurement of biomarker measurements was blinded to disease status |
1 = Stated –1 = Not stated |
||||||||||
| hsCRP | Whether CRP was measured by high-sensitivity method |
1 = CRP 2 = high-sensitivity CRP –1 = Not stated –2 = Not applicable (i.e. if biomarker other than CRP is analysed, e.g. total cholesterol) |
||||||||||
| Outcomes | ||||||||||||
| Evcom | The combination of events (i.e. outcomes/end points) analysed in the article. Articles which reported outcomes with 300 codes ONLY were considered ineligible |
From the list below, indicate the combination of outcomes events analysed in the article. Each event is a three-digit number; <evcom> is written as a string of one or several three-digit numbers, e.g. when analysed events are cardiovascular death and myocardial infarction, the entry is 105200 DeathNon-fatal coronary outcomesOther non-fatal vascular outcomes |
Death | Non-fatal coronary outcomes | Other non-fatal vascular outcomes | |||||||
| Death | Non-fatal coronary outcomes | Other non-fatal vascular outcomes | ||||||||||
| EvN | Number of patients experiencing an event, used in the analysis | |||||||||||
| Masking | Whether the assessment of outcomes/events was blinded to biomarker results and other clinical details |
1 = Stated –1 = Not stated |
||||||||||
| Power | Whether there was evidence of power, or a statistical sample size calculation |
1 = Stated –1 = Not stated |
||||||||||
| Validation | Whether the outcomes/events were validated by two independent assessors |
1 = Stated –1 = Not stated |
||||||||||
| Primary outcome | Whether a single disease end point, or a single combination of end points, for the analysis. The report must use the word ‘primary’ |
1 = Stated –1 = Not stated |
||||||||||
| Pre-specified primary outcome | Whether the primary outcome was pre-specified in the study protocol |
1 = Stated –1 = Not stated |
||||||||||
| Adjustments | ||||||||||||
| Adjcom |
Combination of adjustment variables reported in the analysis. Each adjustment variable is a three-digit number; <adjcom> is written as a string of one or several three-digit numbers. The codes were categorised as follows: Patient history: codes 100–111 Comorbidity: 200–225 Physical examination: 300–310 Routine blood tests: 400–423 Non-invasive ischaemic testing: 500–509 Invasive imaging: 600–611 Treatment: 700–731 Novel biomarkers: 800–832 |
|||||||||||
| Confounder measurement | Were the following potential confounders measured: age, sex, body mass index, smoking status, diabetes, low density lipoproteins and triglycerides? However, these confounders do not necessarily have to be included in the multivariate analysis |
1 = Yes –1 = Not stated |
||||||||||
| Adjrational | Rationale given for the inclusion of the adjustment variables |
1 = A priori 2 = Stepwise selection 3 = Univariate p-value –1 = Not stated (i.e. no rationale provided) –2 = Not applicable (e.g. if the analysis was univariate) |
||||||||||
| Analytical | ||||||||||||
| Rowno |
Number given to the row for each relative risk reported in the article A new row of data is created for: each biomarker each outcome event men and women subgroups placebo and treatment subgroups each analysis (i.e. if the biomarker is analysed both continuously and categorically) |
|||||||||||
| RowID | A unique identifier for each row was created by merging the study number (integer) and the corresponding row numbers (decimal) | e.g. Study number 23 has five rows. The respective rows IDs are: 23.1, 23.2, 23.3, 23.4, 23.5 | ||||||||||
| RRtype | Indicate the type of relative risk reported in the article |
1 = Odds ratio 2 = Hazard ratio 3 = Relative risk |
||||||||||
| MissBMan | Method used to deal with missing biomarker values in the analysis |
1 = Imputation 2 = Complete case analysis –1 = Not stated |
||||||||||
| Missadjan | Method used to deal with missing values of confounders in the analysis |
1 = Imputation 2 = Complete case analysis –1 = Not stated |
||||||||||
| Nriskgr | Number of risk groups reported in the article |
1 = Continuous (per SD or incremental unit increase) 2 = With one cut-off point, median 3 = Tertiles, two cut-off points 4 = Quartiles, etc. |
||||||||||
| Quant | Whether the article reports risk groups in ‘quan’tiles (e.g. tertiles, quartiles, quintiles, etc.) |
0 = No: derived cut-points, continuous analysis 1 = Yes (risk groups are equal numbers: ‘quan’tiles) –1 = Not stated/unclear |
||||||||||
| Cut-point rationale | How were the cut-points for the biomarker determined for the estimation of relative risks (response categories: a priori, quantiles) |
1 = A priori: using estimates from other general population study or review (e.g. cites reference, World Health Organization, etc.) 2 = Median (50th centile) 3 = Quantile 4 = Value of top quantile vs combined lower quantiles (e.g. Q4 vs Q1–3) –1 = Not stated –2 = Continuous |
||||||||||
| Refdef | Range of biomarker values for the reference group of the analysis |
e.g. < 1.0 (mg/dl) If continuous: per SD, log transformation, per unit increase |
||||||||||
| Refn | Number of events in the reference group | |||||||||||
| Refn_1 | Number of patients in the reference group | |||||||||||
| R1def | Range of biomarker values for group 1 | e.g. 1.0–3.0 (mg/dl); or ≥ 1.0 (mg/dl) | ||||||||||
| R1n | Number of events in risk group 1 | |||||||||||
| R1n_1 | Number of patients in risk group 1 | |||||||||||
| R1RR | Relative risk reported for risk group 1 compared with reference group | |||||||||||
| R195_CI | 95% confidence interval reported for relative risk for group 1 versus reference group | |||||||||||
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost-effectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.
By Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al.
-
Gemcitabine for the treatment of metastatic breast cancer.
By Jones J, Takeda A, Tan SC, Cooper K, Loveman E, Clegg A.
-
Varenicline in the management of smoking cessation: a single technology appraisal.
By Hind D, Tappenden P, Peters J, Kenjegalieva K.
-
Alteplase for the treatment of acute ischaemic stroke: a single technology appraisal.
By Lloyd Jones M, Holmes M.
-
Rituximab for the treatment of rheumatoid arthritis.
By Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, et al.
-
Omalizumab for the treatment of severe persistent allergic asthma.
By Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al.
-
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma.
By Boland A, Bagust A, Hockenhull J, Davis H, Chu P, Dickson R.
-
Adalimumab for the treatment of psoriasis.
By Turner D, Picot J, Cooper K, Loveman E.
-
Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a single technology appraisal.
By Holmes M, C Carroll C, Papaioannou D.
-
Romiplostim for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a single technology appraisal.
By Mowatt G, Boachie C, Crowther M, Fraser C, Hernández R, Jia X, et al.
-
Sunitinib for the treatment of gastrointestinal stromal tumours: a critique of the submission from Pfizer.
By Bond M, Hoyle M, Moxham T, Napier M, Anderson R.
-
Vitamin K to prevent fractures in older women: systematic review and economic evaluation.
By Stevenson M, Lloyd-Jones M, Papaioannou D.
-
The effects of biofeedback for the treatment of essential hypertension: a systematic review.
By Greenhalgh J, Dickson R, Dundar Y.
-
A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study.
By Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al.
-
Lapatinib for the treatment of HER2-overexpressing breast cancer.
By Jones J, Takeda A, Picot J, von Keyserlingk C, Clegg A.
-
Infliximab for the treatment of ulcerative colitis.
By Hyde C, Bryan S, Juarez-Garcia A, Andronis L, Fry-Smith A.
-
Rimonabant for the treatment of overweight and obese people.
By Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, et al.
-
Telbivudine for the treatment of chronic hepatitis B infection.
By Hartwell D, Jones J, Harris P, Cooper K.
-
Entecavir for the treatment of chronic hepatitis B infection.
By Shepherd J, Gospodarevskaya E, Frampton G, Cooper, K.
-
Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal.
By Stevenson M, Pandor A.
-
Rivaroxaban for the prevention of venous thromboembolism: a single technology appraisal.
By Stevenson M, Scope A, Holmes M, Rees A, Kaltenthaler E.
-
Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck.
By Greenhalgh J, Bagust A, Boland A, Fleeman N, McLeod C, Dundar Y, et al.
-
Mifamurtide for the treatment of osteosarcoma: a single technology appraisal.
By Pandor A, Fitzgerald P, Stevenson M, Papaioannou D.
-
Ustekinumab for the treatment of moderate to severe psoriasis.
By Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A.
-
Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model.
By Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al.
-
Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation.
By Chen Y-F, Jowett S, Barton P, Malottki K, Hyde C, Gibbs JSR, et al.
-
Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY) – a pharmacoepidemiological and qualitative study.
By Wong ICK, Asherson P, Bilbow A, Clifford S, Coghill D, R DeSoysa R, et al.
-
ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening.
By Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al.
-
The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation.
By Black C, Clar C, Henderson R, MacEachern C, McNamee P, Quayyum Z, et al.
-
Randomised preference trial of medical versus surgical termination of pregnancy less than 14 weeks’ gestation (TOPS).
By Robson SC, Kelly T, Howel D, Deverill M, Hewison J, Lie MLS, et al.
-
Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes.
By Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al.
-
VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers.
By Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al.
-
A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial
By Michaels JA, Campbell WB, King BM, MacIntyre J, Palfreyman SJ, Shackley P, et al.
-
Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice.
By Kai J, Ulph F, Cullinan T, Qureshi N.
-
Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation.
By Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al.
-
Development of a toolkit and glossary to aid in the adaptation of health technology assessment (HTA) reports for use in different contexts.
By Chase D, Rosten C, Turner S, Hicks N, Milne R.
-
Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation.
By Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, et al.
-
Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report.
By Bond M, Wyatt K, Lloyd J, Welch K, Taylor R.
-
Are adverse effects incorporated in economic models? An initial review of current practice.
By Craig D, McDaid C, Fonseca T, Stock C, Duffy S, Woolacott N.
-
Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE).
By Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al.
-
Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation.
By Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al.
-
The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation.
By Fleeman N, McLeod C, Bagust A, Beale S, Boland A, Dundar Y, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer.
By Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TRL, et al.
-
Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study).
By Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al.
-
A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation, followed by telephone or conventional follow-up.
By Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA.
-
The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation.
By Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al.
-
Dissemination and publication of research findings: an updated review of related biases.
By Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of Science Support, NETSCC, HTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington
List of abbreviations
- ACM
- all-cause mortality
- apoA-I
- apolipoprotein A-I
- apoB
- apolipoprotein B
- BNP
- brain natriuretic peptide
- CABG
- coronary artery bypass graft
- CI
- confidence interval
- CHD
- coronary heart disease
- CRP
- C-reactive protein
- CVD
- cardiovascular disease
- eGFR
- estimated glomerular filtration rate
- HDL
- high density lipoprotein
- ICER
- incremental cost-effectiveness ratio
- IL-6
- interleukin 6
- LDL
- low density lipoprotein
- Lp(a)
- lipoprotein a
- NICE
- National Institute for Health and Clinical Excellence
- NT-proBNP
- N-terminal brain natriuretic peptide
- PCI
- percutaneous coronary intervention
- QALY
- quality-adjusted life-year
- RR
- relative risk
- SCAAR
- Swedish Coronary Angiography and Angioplasty Register
- SD
- standard deviation
- TC
- total cholesterol
- TG
- triglycerides
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.