Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/55/01. The contractual start date was in January 2009. The draft report began editorial review in August 2009 and was accepted for publication in January 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 General background
Basics of photodynamic therapy
Photodynamic therapy (PDT) is the use of a light-sensitive drug (a photosensitiser), in combination with light of a visible wavelength, to destroy target cells (e.g. cancerous or pre-cancerous cells). Photosensitisers can be administered systemically or topically, which targeted cells then preferentially absorb. A period of time is required to permit photosensitiser uptake (ranging from a few minutes up to several days), after which light is directed at the area to be treated. The photochemical reaction resulting from excitation of the photosensitiser produces singlet oxygen, which destroys cells (by reacting with, and damaging, cell organelles and biomolecules important to cell function).
Some of the light absorbed by photosensitisers is re-emitted at a different wavelength, a process known as fluorescence; this can be used as a means of detecting the presence and location of tumours. This technique, known as photodynamic diagnosis (PDD), may be used alongside PDT.
Development of photodynamic therapy
The photodynamic effect was discovered by chance over 100 years ago, followed shortly after by early pioneering work on PDT in Europe. 1 However, despite this early knowledge of the basic principles, it was not until the 1980s that PDT (which was then also often known as ‘photoradiation therapy’) developed to a level where it was used – to any significant extent – in both clinical research and practice. Randomised controlled trials (RCTs) of PDT in patients with malignant and pre-malignant conditions began in earnest in the 1990s. PDT has also been used to treat age-related macular degeneration, cardiovascular disease, psoriasis, acne vulgaris and viral warts. 2
Photosensitisers
Haemoglobin (which transports oxygen in the blood) and chlorophyll (an essential component of photosynthesis) molecules contain heterocyclic ring structures, known as porphyrins. Many photosensitisers are derivatives of haematoporphyrin; the first photosensitiser used clinically in PDT was haematoporphyrin derivative (HpD). Its purified fraction is known as porfimer sodium (Ps). Ps and HpD are first-generation photosensitisers. There are two major drawbacks of using Ps or HpD. One is the time taken (typically 48 hours) for tissues to accumulate sufficient levels of photosensitiser to allow the next stage of the PDT process to occur (illumination). The second is the time taken for photosensitiser concentration to fall below clinically active levels. Persistent levels will typically last for many weeks, causing photosensitivity of the skin (sunburn-like effects), unless patients avoid bright light. 3
An alternative approach to introducing the photosensitiser to the target tissue involves making use of biomolecules produced by the body. These can be exploited to naturally generate therapeutic levels of photosensitiser. An example is aminolevulinic acid (ALA), a naturally occurring intermediate in the haem biosynthetic pathway, and precursor of the photosensitising agent protoporphyrin IX (PpIX). Although ALA has no intrinsic photosensitising properties, it is metabolised to produce PpIX (the active agent in ALA–PDT). Administration of sufficient ALA results in a rapid elevation (for a few hours) of PpIX levels, meaning that illumination can take place. 4 Following this there is also rapid systemic clearance of ALA-induced PpIX, within 24 hours.
Aminolevulinic acid and its methylester, methyl aminolevulinate (MAL), are second-generation photosensitisers. Other types of photosensitiser have also been developed, including chlorins, bacteriochlorins, phthalocyanines, naphthalocyanines, pheophorbides and purpurins. 3 The mechanisms involved in the selective uptake and retention of photosensitisers by tumour cells are not yet fully understood. Table 1 provides details of the photosensitisers studied in the trials included in this systematic review.
| Photosensitiser (trade name) | Wavelength commonly used (nm) | Condition/site |
|---|---|---|
| Porfimer sodium (Photofrin®) | 630 | Barrett’s oesophagus, biliary tract cancer, brain cancer, lung cancer, nasopharyngeal carcinoma, oesophageal cancer |
| Haematoporphyrin derivative (HpD) | ∼630 | Oesophageal cancer |
| Dihaematoporphyrin ether (DHE) (Photosan®) | ∼630 | Biliary tract cancer, lung cancer, oesophageal cancer |
| Aminolevulinic acid (ALA) (Levulan®) | ∼630 | Actinic keratosis, Barrett’s oesophagus, basal cell carcinoma, Bowen’s disease, brain cancer, lung cancer |
| Methyl aminolevulinate (MAL) (Metvix®) | ∼630 | Actinic keratosis, basal cell carcinoma, Bowen’s disease |
| PsD-007/photocarcinorin | ∼630 | Oesophageal cancer |
| mTHPC/temoporfin (Foscan®) | 652 | Oesophageal cancer |
| (Radachlorin®) | 662 | Oral cancer |
| Methylene blue | 665 | Laryngeal/pharyngeal cancer |
| (Photosense®) | 670 | Oral cancer |
| Verteporfin (Visudyne®) | 688 | Basal cell carcinoma, Bowen’s disease |
Topically active agents are preferred in the treatment of dermatological cancers and pre-cancerous conditions, as most systemic photosensitisers produce prolonged generalised photosensitivity. Ideally, the photosensitiser will be evenly distributed throughout the lesion and show a high lesion–normal tissue concentration ratio. 5
Light sources
When treating skin lesions, light is directed at the treatment area by straightforward means, such as a lamp or light-emitting diode (LED) light source (which can easily illuminate large areas). Laser light is used for treating internal sites, delivered via an endoscope, or a variety of other devices, including needles, optic fibres and balloons. Lasers enable the delivery of a more precise wavelength of light than lamps.
The wavelengths and intensity of light required in PDT vary, depending on the depth of light penetration needed and on the photosensitiser being used. The greater the wavelength of light, the deeper the penetration into tissue, which has implications for the type of tumour suitable for treatment with PDT; cancers occurring deep within tissues (where adequate illumination could be problematic) generally are currently not suitable for treatment with PDT. Red light is the most commonly used in PDT, as it has the longest wavelength in the visible spectrum, although for thin lesions [such as actinic keratoses (AKs)] blue light can also be used.
Light delivery systems for PDT have improved over time. Tuneable dye lasers – which allow flexibility of wavelength – have been used in research studies but are not ideal for clinical use because of their size and limited mobility. However, the licensing of specific photosensitisers (using a specific wavelength) has led to the development of small compact lasers, such as diode lasers and LED array lasers, which are more convenient for use in clinical settings. 3
The role of photodynamic therapy
Photodynamic therapy is generally used as either as a primary treatment (usually in skin conditions) or as an adjunctive treatment alongside surgery, radiotherapy or chemotherapy, as appropriate. Trials have tended to focus on patients who have not responded to usual treatment, but more recent research is now assessing the effectiveness of PDT as a first-line intervention. Some potential advantages of PDT may include the preservation of connective tissue within the treated area and limited side effects. PDT also offers the ability to treat large areas of diseased tissue, areas not reachable by surgery, and the option of re-treatment.
Although PDT is a fairly well-accepted treatment in clinical practice for some types of skin cancer, as a treatment for other forms of cancer it has yet to be fully explored, although the National Institute for Health and Clinical Excellence (NICE) has issued a number of interventional procedure guidance documents, which make recommendations about whether the treatment is safe enough, and works well enough, for routine use. 6–14
Previous and ongoing reviews of photodynamic therapy
A number of existing reviews were identified in the initial stages of the systematic review. These were assessed by two independent reviewers using criteria developed by the Centre for Reviews and Dissemination (CRD) for the Database of Abstracts of Reviews of Effects (DARE). 15 As with the DARE database, reviews were required to meet all of the first three criteria and at least one of the last two in order to be accepted as a systematic review. As is usual practice, discrepancies were resolved by discussion, or by referral to a third reviewer when necessary. One of these reviews addressed lung cancer and PDT but only covered English-language research to 2002 and therefore did not fully answer our research questions. 16 The other reviews did not meet our criteria.
A research group from the Health Technology and Policy Unit, University of Alberta, has recently completed a scoping review of PDT for cancer in any site. 17 This review searched for English-language studies, including comparative and non-comparative designs published in the past 10 years. This scoping review was based on searches carried out in MEDLINE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane Library and Science Citation Index. Study classification and limited data extraction were carried out by only one reviewer. The project group were kind enough to supply us with draft results and the full database of references, which was cross-checked against our own searches. No further studies were identified as a result of this process.
The same research group has undertaken a full assessment of treatments for early-stage oesophageal cancer and Barrett’s oesophagus, which includes PDT as one of the interventions. The evidence is drawn from the scoping review and additional searches for other interventions (searches were limited to English language, and the last 5 years). Results were unavailable for inclusion in this report but have recently been released. 18,19
Two Cochrane reviews from the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group were identified. One intended to compare surgery versus radical endotherapies, including PDT, in the treatment of Barrett’s oesophagus, but no eligible trials were located and five retrospective studies were excluded. 20 The other review was at protocol stage at the time of writing, but has subsequently been published. 21 Having failed to locate any systematic reviews capable of answering our research questions, we therefore conducted the current review.
Chapter 2 Research questions
Introduction
In its role as part of the CRD/ Centre for Health Economics (CHE) Technology Appraisal Review team, CRD was commissioned by the Health Technology Assessment (HTA) programme, on behalf of the National Cancer Director, to undertake a systematic review looking at PDT in five areas [Barrett’s oesophagus, head and neck cancer, lung cancer, oesophageal cancer and skin cancer (including pre-cancerous conditions)]. The scope of the project was subsequently expanded to include an additional two cancer sites (bile duct, brain) at the request of the Scottish Government Health Directorates. No economic component was requested and so attention has been restricted to clinical effectiveness and safety.
Research question
What is the clinical effectiveness and safety of PDT in the treatment of Barrett’s oesophagus, pre-cancerous skin conditions and the following cancers: biliary tract, brain, head and neck, lung, oesophageal and skin?
Aims and objectives
The aim of this project was to systematically identify, evaluate and summarise the findings of all relevant studies regarding clinical effectiveness and safety. The results of the review will be used to inform decisions about the role of PDT in clinical practice and also the need for further research.
Chapter 3 Methods for reviewing clinical effectiveness
A systematic review was undertaken following the principles recommended by CRD guidance and the QUOROM statement. 22,23
Search strategy
A comprehensive search strategy was developed to ensure that all relevant sources of data were located. Searches were not restricted by language, date of publication or study design. The search strategy comprised the following main elements:
-
searching of electronic databases
-
scrutiny of bibliographies of included studies and existing reviews
-
hand searching of abstracts from recent relevant conferences
-
contact with experts in the field including manufacturers of photosensitising agents.
The following electronic databases were searched from inception to August/October 2008:
-
MEDLINE (including MEDLINE In-Process)
-
EMBASE
-
CINAHL
-
PASCAL [database of INIST (Institut de l’Information Scientifique et Téchnique)]
-
Latin American & Caribbean Health Sciences Literature (LILACS)
-
Cochrane Database of Systematic Reviews (CDSR)
-
DARE
-
NHS Economic Evaluation Database (NHS EED)
-
HTA Database
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
metaRegister of Current Controlled Trials (mRCT)
-
ISI Conference Proceedings Citation Index
-
Zetoc (British Library’s Electronic Table of Contents)
-
UK Clinical Research Network (UKCRN)
See Appendix 1 for full details of the search strategies used.
Where completed trials were identified from research registers without an associated full publication, or studies were indicated to be in progress, the principal investigator was contacted for further details and references. Reference lists from all identified reviews were checked for potentially relevant studies.
The original searches were undertaken between August and October 2008. Update searches were carried out in MEDLINE, EMBASE, CINAHL, DARE, CDSR, NHSEED, HTA and CENTRAL in May 2009.
Proceedings for two recent conferences that were not yet electronically indexed were obtained and hand searched: the 13th Congress of the European Medical Laser Association (EMLA), 23–24 August 2008 (published in Photodiagnosis and Photodynamic Therapy) and the 7th International Symposium on Photodynamic Therapy and Photodiagnosis in Clinical Practice, 7–10 October 2008.
As well as contact with the clinical advisors, the manufacturers of relevant photosensitising agents were also contacted. Where research bibliographies were kindly provided or available from websites, these were checked against the database of identified literature.
The results of all searches were imported in endnote xi bibliographic software and de-duplicated. 24
Inclusion criteria
Published and unpublished studies from any country and reported in any language were eligible for inclusion, provided that they met the following inclusion criteria.
Population
The eligible populations included people with specified pre-cancerous conditions or primary cancer in the following sites:
-
biliary tract
-
– extrahepatic cholangiocarcinoma (usually adenocarcinoma)
-
– perihilar cholangiocarcinoma
-
– distal cholangiocarcinoma
-
– gall bladder
-
– ampulla
-
-
brain
-
– gliomas (astrocytoma, ependymoma, oligodendroglioma or mixed glioma)
-
– any of the rarer brain cancer types, including invasive pituitary adenomas
-
-
head and neck
-
– laryngeal cancer
-
– hypopharyngeal cancer
-
– oropharyngeal cancer
-
– oral cavity cancer
-
-
lung
-
– small cell, non-small cell lung cancer [squamous cell carcinoma (SCC), adenocarcinoma, large cell carcinoma]
-
– tracheobronchial cancer was also classified as lung (based on advice from clinical advisors)
-
-
oesophagus
-
– Barrett’s oesophagus (a precursor to cancer)
-
– squamous cell carcinoma, adenocarcinoma or undifferentiated cancer of the oesophagus
-
-
skin
-
– pre-cancerous conditions: actinic/solar keratosis, Bowen’s disease
-
– non-melanoma skin cancers [basal cell carcinoma (BCC) (superficial and nodular), SCC, Merkel cell carcinoma, Kaposi’s sarcoma, T-cell lymphoma of skin or sarcoma].
-
We did not anticipate identifying any trials dealing with children as these cancers are extremely rare in such groups, but any data on children would have been included and considered separately where appropriate.
Where studies comprised populations covering more than one site of interest, these were included if the results were reported by diagnosis or where a minimum of 90% of patients was diagnosed with the same condition.
Intervention
Photodynamic therapy for either curative or palliative treatment The specific interventional details varied according to cancer site. There are a number of variations possible in the application of PDT, for example the type of photosensitising agent, the method of light delivery, wavelength and duration of light used. We have not restricted our review according to the details of the PDT treatment, but have extracted and reported data on agent, light source, wattage, light intensity, duration, number of treatment sessions and wavelength. Studies of prophylactic PDT alone were excluded; data relating to prophylactic PDT were not extracted when both treatment and prophylaxis were reported.
Comparators
No restrictions were placed on the inclusion criteria for comparators. The relevant comparators varied according to the cancer site. Studies comparing differing application of PDT treatments (e.g. photosensitising agents; source, duration, or wavelength of light) were also included.
Outcomes
Studies were included provided that they reported at least one relevant outcome. The primary outcomes the review focused on are listed below. These were addressed individually by site, where appropriate, due to differences in the specific outcome measures. Outcomes also reflected the curative or palliative nature of the intervention.
-
mortality
-
morbidity (symptom burden, symptom improvement, time to healing)
-
quality of life (QoL) (patient-based outcomes, such as cosmetic appearance, QoL or depression scores)
-
adverse events (AEs) (e.g. photosensitivity of skin in general, ulceration of the underlying tissues, haemoptysis, scarring, carcinogenicity, oesophageal strictures, cardiac complications, nausea, inflammation, pain, constipation)
-
resource use (e.g. length of hospital stay)
-
return to normal activities.
We also extracted data on recurrence and tumour response measures (such as tumour or lesion clearance or response), while bearing in mind the extent to which these outcomes relate to symptomatic morbidity and patient-perceived benefits.
Study designs
In the evaluation of effectiveness and safety of clinical procedures, RCTs are normally seen as providing a ‘gold standard’ of evidence that is less prone to bias. 22 However, such trials have not been conducted in large numbers for all of the conditions under investigation, so there was a need to consider other types of evidence. The particular study design inclusion criteria depended on the cancer site as shown below:
-
Skin We anticipated sufficient RCTs and therefore restricted our attention to these.
-
Barrett’s oesophagus In a change to the protocol, we restricted our attention to RCTs, as the initial screening identified a significant number of RCTs in this area.
-
All other sites Given the paucity of RCTs identified in our initial scoping searches, we considered prospective experimental studies with a control group, in addition to RCTs.
Animal models, pre-clinical and biological studies, narrative reviews, editorials, opinions and reports containing no outcome data were excluded from the reviews.
Alongside the systematic review we conducted a scoping review of studies, which met all of our inclusion criteria except the study design. The aim of this scoping review was to document the extent of the observational research in those areas in which we anticipated a paucity of controlled trials (see Chapter 3, Scoping review).
Inclusion and exclusion strategies
Based on the volume of records within the endnote library, we adopted a three-stage screening process, as shown in Figure 1. Two reviewers independently screened all titles and abstracts regardless of source at each stage. Discrepancies were resolved by discussion, or by referral to a third reviewer when necessary.
FIGURE 1.
Three-stage screening process.
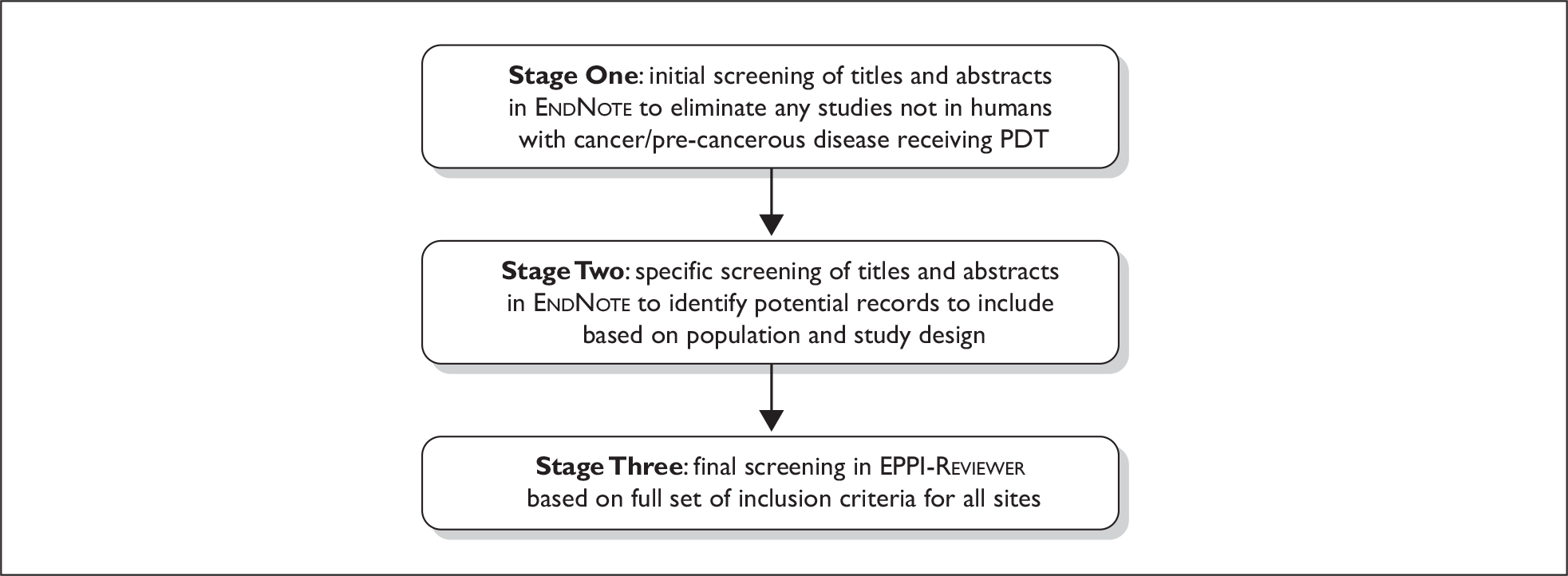
Stage One
An initial sift of the records was carried out aiming to exclude any clearly irrelevant material. Records were excluded if they met any of the following criteria, i.e. the study:
-
was not in human patients (could be animals or in vitro, cell cultures, etc. only)
-
was not of PDT (i.e. not the use of photosensitising agents in combination with a light source)
-
was of PDD without any actual therapy
-
did not include patients with the identified conditions.
Stage Two
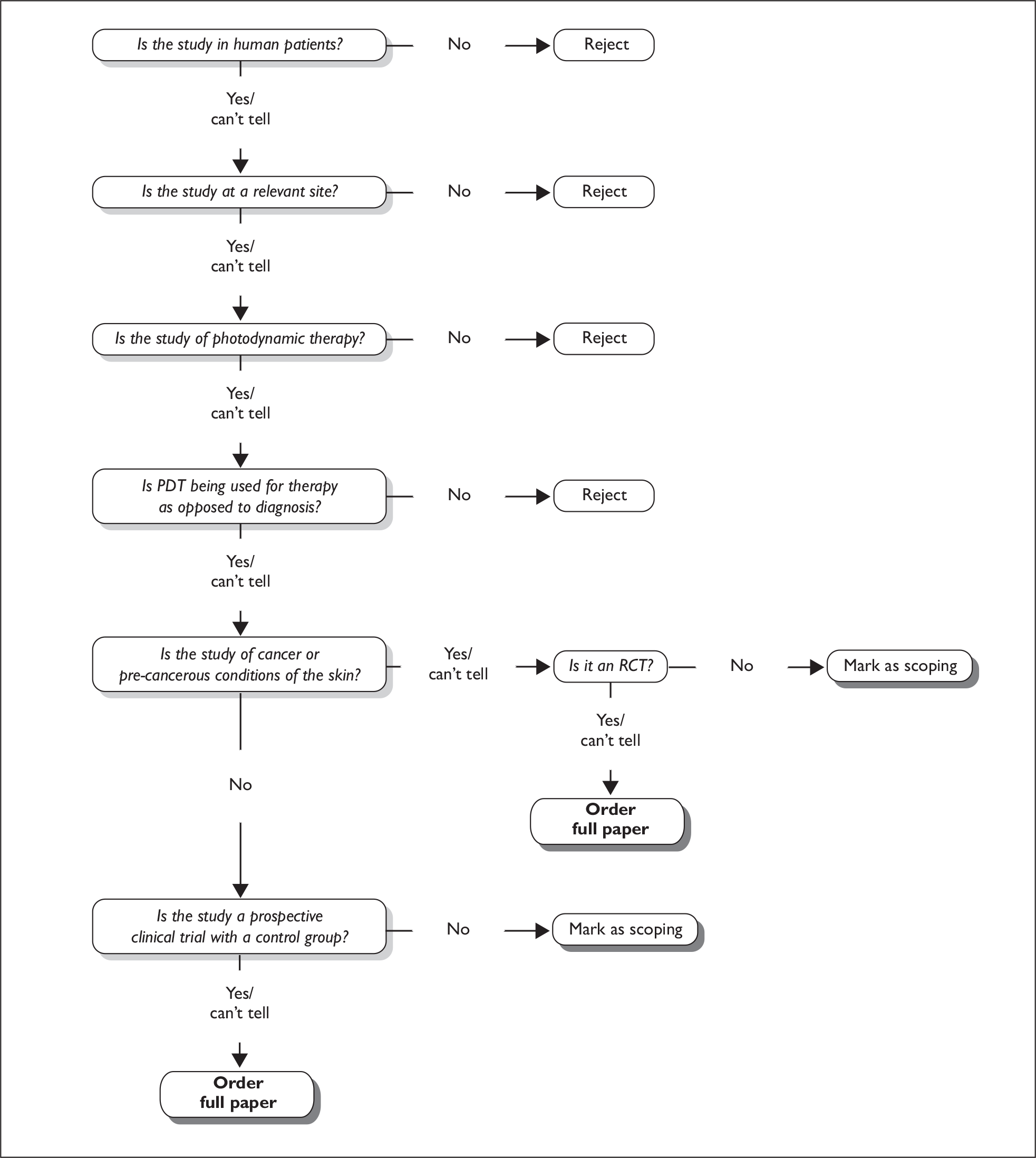
A more detailed assessment of the potentially relevant records identified in Stage One was carried out. An algorithm was used to determine which records were to be considered for inclusion (full paper obtained), which were considered for the scoping review (see Scoping review), and which should be rejected. The algorithm is described in Figure 2.
FIGURE 2.
Decision algorithm.
Stage Three
All non-rejected records from Stage Two were then imported into eppi-reviewer (systematic review software) for final assessment. 25 All references were screened and assessed, based on the complete set of inclusion criteria reported above (see Inclusion criteria). Foreign language papers were assessed by either a single reviewer who was competent in that language or an appointed external reviewer under guidance, but not checked by a second reviewer. Studies that did not fulfil all of the criteria were excluded, with documented reasons.
Data extraction and quality assessment
Data extraction and quality assessment was undertaken by one reviewer, and independently checked by a second reviewer. Any disagreements were resolved by discussion and if necessary a third reviewer was consulted. Foreign language papers were extracted by either a single reviewer who was competent in that language or an appointed external reviewer under guidance, but not checked by a second reviewer.
Data extraction
A standardised data extraction form was developed within eppi-reviewer (see Appendix 2). It was piloted by all reviewers on a selection of studies and refined to ensure consistency of data extraction between reviewers and across sites. Guidelines on its use were produced to enhance consistency.
Data from multiple publications of the same study were extracted and reported as a single study. Within the data extraction tables the term ‘linked publications’ refers to abstracts or full papers that report related information about the same patient group (see Appendices 13–21). Where publications appeared to be duplicates or linked, these were assessed independently by two reviewers.
Extraction included data on: study details (e.g. study identifier, author, year, country, setting, number of participants, and duration of follow-up), patient characteristics (e.g. age, gender, cancer site and stage), intervention (full details of photosensitising agent with dosage, light source, wavelength spectrum and method of delivery), comparator treatment (type of comparison with full details of delivery methods), and outcomes relating to effectiveness and safety as specified under Inclusion criteria, above. Attempts were made to contact authors for missing data.
Quality assessment
The quality of RCTs and non-RCTs was assessed using standard checklists, which were adapted, as necessary, to incorporate topic-specific quality issues (see Appendix 2). Quality assessment data were extracted directly into an excel spreadsheet.
Methods of analysis/synthesis
Data extracted from the studies were tabulated and discussed in a narrative synthesis. The results of the quality assessment were tabulated and graphs created, where appropriate. The influence of quality on the results of the studies and the findings of the review was discussed. Where appropriate, meta-analysis was undertaken using revman software to estimate a summary measure of effect on relevant outcomes based on intention-to-treat (ITT) populations. 26 Random effects meta-analyses were used throughout. Where ITT results were not explicitly reported, the relevant data were extracted based on data reported in the text, graphs and tables of the publications if possible. Heterogeneity was explored through consideration of the study populations, methods and interventions, by visualisation of results and, in statistical terms, by the chi-squared test for heterogeneity and the I2 statistic.
Scoping review
Given the likely paucity of RCTs in some of the cancer sites, a scoping review was undertaken alongside the screening stage of the systematic review. The aim was to document the extent of the observational research in those areas where we anticipated relatively few controlled trials. This was intended to provide as complete a picture of the evidence base as possible, while bearing in mind the inherent bias and limitations of such studies.
All records that met the population, intervention, comparator and outcome criteria reported above, but were excluded on study design, were considered in the scoping review.
For all sites, records that appeared to be uncontrolled trials, observational studies with a control group, case series or case reports were included. In addition, non-RCTs in skin and Barrett’s oesophagus were also included. These decisions were made as part of the screening process detailed above (see Figure 2). Where the decision to include in the scoping review could be made based on the title and abstract, the full paper was not ordered.
No formal data extraction or quality assessment was undertaken due to the limited time available. For four sites (biliary, brain, head and neck, lung) for which few included studies were identified in the systematic review, the scoping publications were categorised into broad study design groups as follows:
-
observational comparative studies
-
non-comparative experimental trials
-
case series (with 10 or more patients)
-
case reports (individual case reports of reports with fewer than 10 patients).
This was done by extracting the cancer site, number of patients and study design, along with study identifier, author and year of publication into an excel spreadsheet (details available on request). It should be noted that this brief categorisation might not be accurate. It was undertaken by one reviewer and based on the authors’ terminology as reported in the publication; however, many publications, often in abstract form, did not provide sufficient details to be clear.
For the remaining sites (pre-cancerous skin, skin, Barrett’s oesophagus and oesophageal) no such categorisation was undertaken and all identified references are listed together.
For further details of the findings of the scoping review, see Chapter 5.
Chapter 4 Studies included in the systematic review
The search strategies identified 12,522 references, of which 38 were located from hand searches, reference checking and contact with individuals. These were screened as described in the methods chapter, and full copies of 699 papers were obtained and assessed for inclusion in the main review. Figure 3 shows the flow of studies through the review process and the numbers excluded at each stage.
FIGURE 3.
Flow chart of study selection. *Numbers do not total as one publication reported two separate trials, and two trials included multiple distinct patient groups, thus were extracted more than once. Some publications were linked to more than one study.
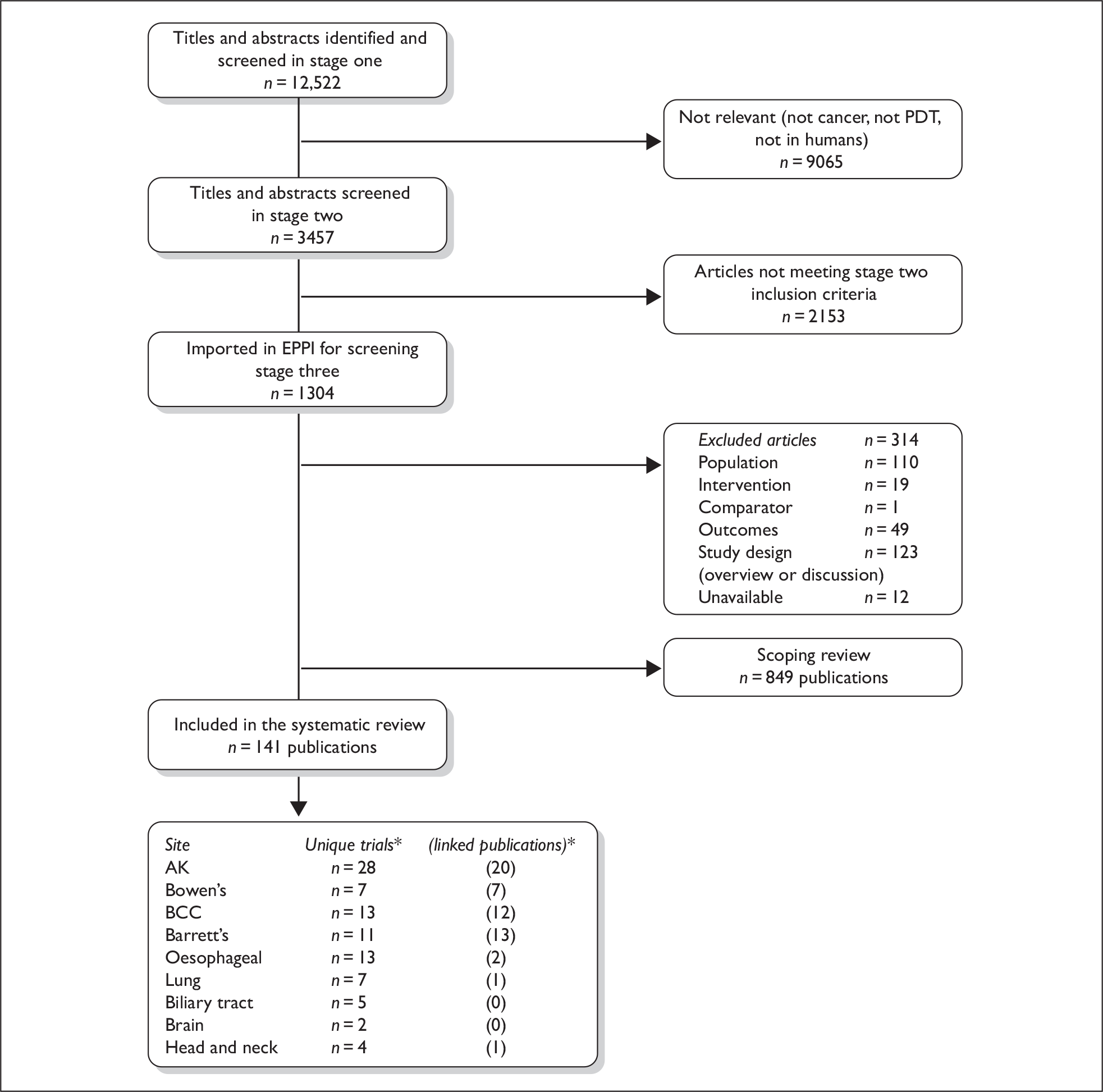
Duplicate publication of study results and multiple reports of partial data sets appeared to be common. A total of 54 papers were designated as ‘linked’ publications. Overall, we included 88 trials reported in 141 publications; one publication reported two separate trials, and two publications reported on two sites and were extracted into both relevant groups in this systematic review.
Chapter 5 Studies excluded from the systematic review
As described previously in Chapter 3, articles that met all of the agreed inclusion criteria apart from study design were included in the scoping review (Figure 4). For all sites, records that appeared to be uncontrolled trials, observational studies with a control group, case series or case reports were included. In addition, non-RCTs on skin and Barrett’s oesophagus were also included in the scoping review.
FIGURE 4.
Flow chart of scoping review. *Numbers do not total as some publications report on more than one site.
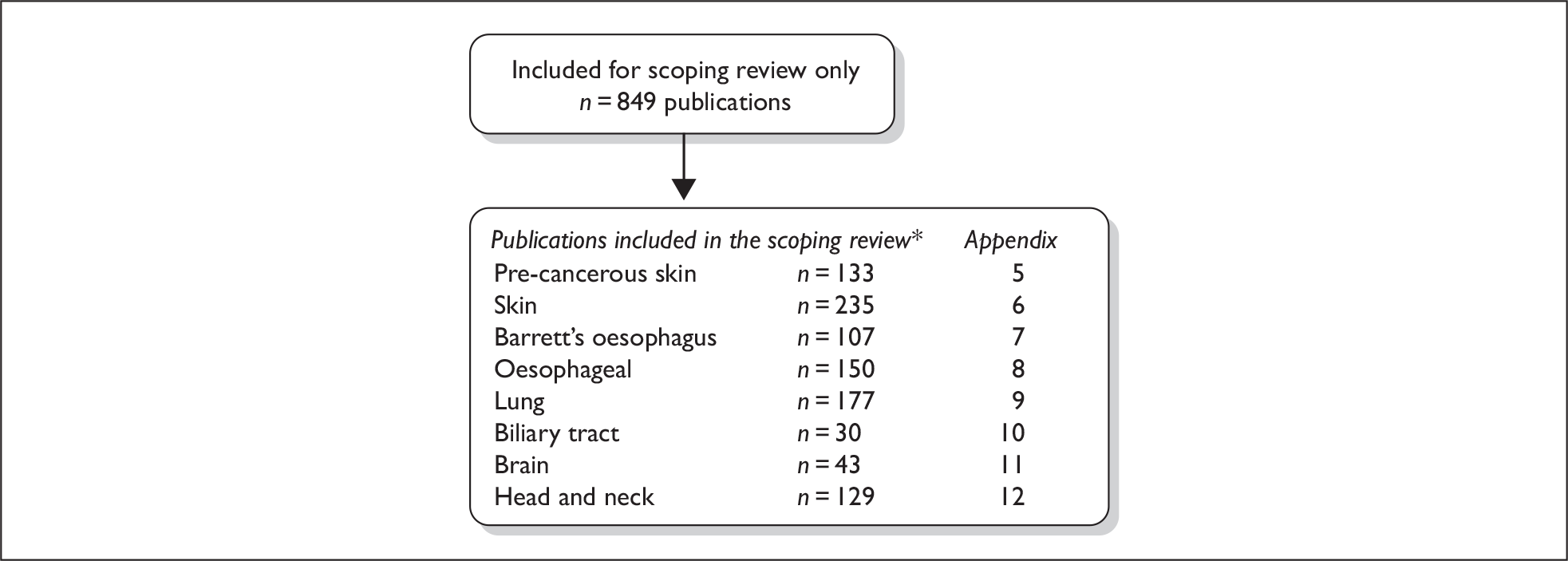
We made the decision to include non-RCTs in the scoping review rather than the systematic review for Barrett’s oesophagus because initially our screening indicated there were over 20 RCTs in this area. Subsequent examination revealed that there were only 11 unique trials due to multiple publications.
A total of 849 articles were included in the scoping review; however, this figure is likely to contain a number of linked and duplicate publications. The totals per group do not sum to 849, as some publications appeared to report data for multiple sites and have been included in each accordingly. For each of the sites we have generated a list of scoping records – please see the relevant appendices (Appendices 5–12). For four sites (biliary, brain, head and neck, lung), for which few included studies were identified in the systematic review, the records have been classified in terms of study design according to the authors’ terminology and are identified as such in the scoping lists. For all other sites an alphabetical list has been prepared.
Chapter 6 Skin cancers and pre-cancerous skin conditions
ACTINIC KERATOSIS
Background
Actinic (or solar) keratosis is a form of pre-cancerous skin lesion which, based on pathological evidence, is considered to be a precursor to SCC. AK is usually diagnosed clinically rather than histologically, with criteria including parakeratosis, epidermal atrophy or thickening, and atypia.
Actinic keratoses most commonly affect areas that are prone to chronic sun exposure (the face, scalp, backs of hands and forearms) in older populations, especially in men. Most people will have multiple AKs, although single lesions do occur. AK lesions are usually small (less than 1 cm in diameter), erythematous and scaly, and they may become enlarged or bleed. The lifetime risk of developing AK is greater than 50% in parts of Australia and the USA; this rises to 90% in people who are over 80 years old. 27,28 In England the data suggest that prevalence in people over 70 years is around 15% for men and 6% for women. 29
The exact conversion rate varies but estimates suggest that between 0.25% and 25% may progress to SCC,30 3–4% of which will metastasise;27 early treatment is therefore recommended. Evidence suggests the yearly progression of AK to invasive SCC is between 8 and 24 per 10,000 in an average-risk Australian, while around 2% of these resultant SCCs metastasise, leading to significant morbidity or death. 31 To date no studies have accurately predicted which AKs will progress to invasive SCC. Overall AK incidence is estimated as around 10 times the usual SCC rates. 30
Data suggest that there is a continuum from AK to SCC in situ (Bowen’s disease) to invasive SCC. 31 There is still some debate as to whether AKs should be classed as pre-cancerous or as early stages of actual cancer. AKs are usually graded according to Olsen’s criteria, which divide lesions according to thickness: grade I = thin, grade II = moderate and grade III = thick. 32
Treatment options for AK include destruction (e.g. cryotherapy), topical therapies [such as fluorouracil (5-FU), imiquimod, diclofenac and retinoids], resurfacing (chemical peels), excision or combinations of these. Choices may be based on location and size of the lesion, as well as cosmetic considerations. The AHRQ (Agency for Healthcare Research and Quality) report on AK states that there is a general consensus that all AKs on the lip, ear, eyelid or in immunocompromised patients should be treated because of the high rate of metastases in these areas. 31
Photosensitisers that are used to treat AK include ALA and MAL, both of which are usually applied topically as a cream, and left on the skin for an appropriate incubation period. Lesions may be prepared using abrasion or curettage (without local anaesthetic) to scrape away any excess tissue, as in the treatment of BCC. 33 An experimental photosensitiser ATX-S10(Na) has recently been developed but has not yet been trialled in humans. 34
Illumination is commonly provided by a non-coherent red light source, including an illuminant and a reflector. These can be used to treat large areas and the long wavelength light penetrates relatively deeply into the tissues. Green and blue light may be used but the depth of penetration is reduced – below 1 mm for blue and between 0.5 and 2 mm for green, compared with 1–6 mm for red – making their use suitable only for superficial lesions. 5 Lasers are also being increasingly used as light sources.
Study characteristics
Twenty-eight RCTs investigated the use of PDT for treating AK, all reporting results between 1998 and 2009 (including 10 studies in 2008) (Table 2). Seven trials were reported only as abstracts35–41 and 20 as published papers42–61 (see Table 2). In total, the studies randomised 2611 participants. One publication reported results for two different trials. 60
| Authors | No. patients | Trial treatments |
|---|---|---|
| PDT vs cryotherapy | ||
| Kaufmann et al. (2008)46 | 121 | MAL–PDT vs cryotherapy (within-participant comparison) |
| Morton et al. (2006)48 | 119 | MAL–PDT vs cryotherapy (within-participant comparison) |
| Szeimies et al. (2002)51 | 202 | MAL–PDT vs cryotherapy |
| Wennberg et al. (2008)59 | 81 | MAL–PDT vs investigator’s choice of treatment, mostly cryotherapy (organ transplant recipients, within-participant comparison) |
| PDT vs placebo PDT | ||
| Fowler and Zax (2002)44 | 243 | ALA–PDT vs placebo PDT |
| Hauschild et al. (2009),60 trial AK03 | 103 | ALA–PDT vs placebo PDT |
| Jeffes et al. (1998)41 (abstract only) | 36 | ALA–PDT vs placebo PDT (within-participant comparison) |
| Dragieva et al. (2004)42 | 17 | MAL–PDT vs placebo PDT; organ transplant recipients (within-participant comparison) |
| Pariser et al. (2008)56 | 100 | MAL–PDT vs placebo PDT |
| Pariser et al. (2003)49 | 80 | MAL–PDT vs placebo PDT |
| Szeimies et al. (2009)58 | 131 | MAL–PDT vs placebo PDT |
| PDT vs placebo PDT vs cryotherapy | ||
| Freeman et al. (2003)45 | 204 | MAL–PDT vs placebo PDT vs cryotherapy |
| Hauschild et al. (2009),60 trial AK04 | 346 | ALA–PDT vs placebo PDT vs cryotherapy |
| PDT parameter comparisons | ||
| Braathen et al. (2009)54 | 119 | MAL–PDT comparing incubation times (1 hour or 3 hours) and doses (160 mg/g or 80 mg/g) |
| Ericson et al. (2004)43 | 40 | ALA–PDT 50 mW/cm2 vs ALA–PDT 75 mW/cm2 vs ALA–PDT 30 mW/cm2 vs ALA–PDT 45 mW/cm2; total dose 100 J/cm2 (all treatments) |
| Hauschild et al. (2009)55 | 149 | Patch containing ALA (PD P 506 A) applied to lesions for 0.5, 1, 2, or 4 hours, followed by illumination with red light |
| Legat et al. (2006)36 (abstract only) | 22 | MAL–PDT with fractionated illumination vs MAL–PDT with unfractionated illumination (within-participant comparison) |
| Puizina-Ivic et al. (2008)57 | 36 | ALA–PDT with 16-hour incubation and 2 light fractions vs ALA–PDT with 5-hour incubation and a single illumination |
| Szeimies et al. (2007)38 (abstract only) | 25 | MAL–PDT with VPL vs MAL–PDT with LED light (within-participant comparison) |
| Tarstedt et al. (2005)52 | 211 | MAL–PDT single session vs MAL–PDT 2 sessions (1 week apart) |
| Touma et al. (2003)39 (abstract only) | 18 | ALA–PDT 1-hour incubation vs 2-hour incubation vs 3-hour incubation |
| Wiegell et al. (2008)53 | 30 | MAL–PDT with daylight vs MAL–PDT with red LED (within-participant comparison) |
| Wiegell et al. (2008)40 (abstract only) | 29 | PDT with 8% MAL vs PDT with 16% MAL (within-participant comparison) |
| MAL–PDT vs ALA–PDT | ||
| Moloney and Collins (2007)37 (abstract only) | 16 | MAL–PDT vs ALA–PDT |
| PDT vs 5-FU | ||
| Gupta (2004)35 (abstract only) | 50 | ALA–PDT vs 5-FU |
| Kurwa et al. (1999)47 | 17 | ALA–PDT vs 5-FU (within-participant comparison) |
| Other comparisons | ||
| Smith et al. (2003)50 | 36 | ALA–PDT with blue light vs ALA–PDT with laser light vs 5-FU |
| Sotiriou et al. (2009)61 | 30 | ALA–PDT vs imiquimod (within-participant comparison) |
Four RCTs compared PDT with cryotherapy46,48,51,59 and seven trials compared PDT with placebo PDT (cream). 41,42,44,49,56,58,60 Two three-armed trials compared PDT with both cryotherapy and with placebo PDT. 45,60 Eleven trials compared PDT using different parameters,36–40,43,52–55,57 and two compared PDT to 5-FU;35,47 one three-armed trial compared two different PDT light parameters with 5-FU. 50 One trial compared PDT with imiquimod. 61
Where studies provided participant information, it was evident that the majority of participants were male, with multiple AKs on the face or scalp. Two studies were of organ transplant recipients. 42,59
For PDT treatments, MAL and ALA were used as photosensitisers, at doses of 160 mg/g of MAL with red light, and 20% ALA with blue light, in most studies. The drug–light interval (incubation time) was around 3 hours in nearly all of the MAL populations being treated, but varied greatly in the ALA studies (between 45 minutes and 18 hours).
Study quality
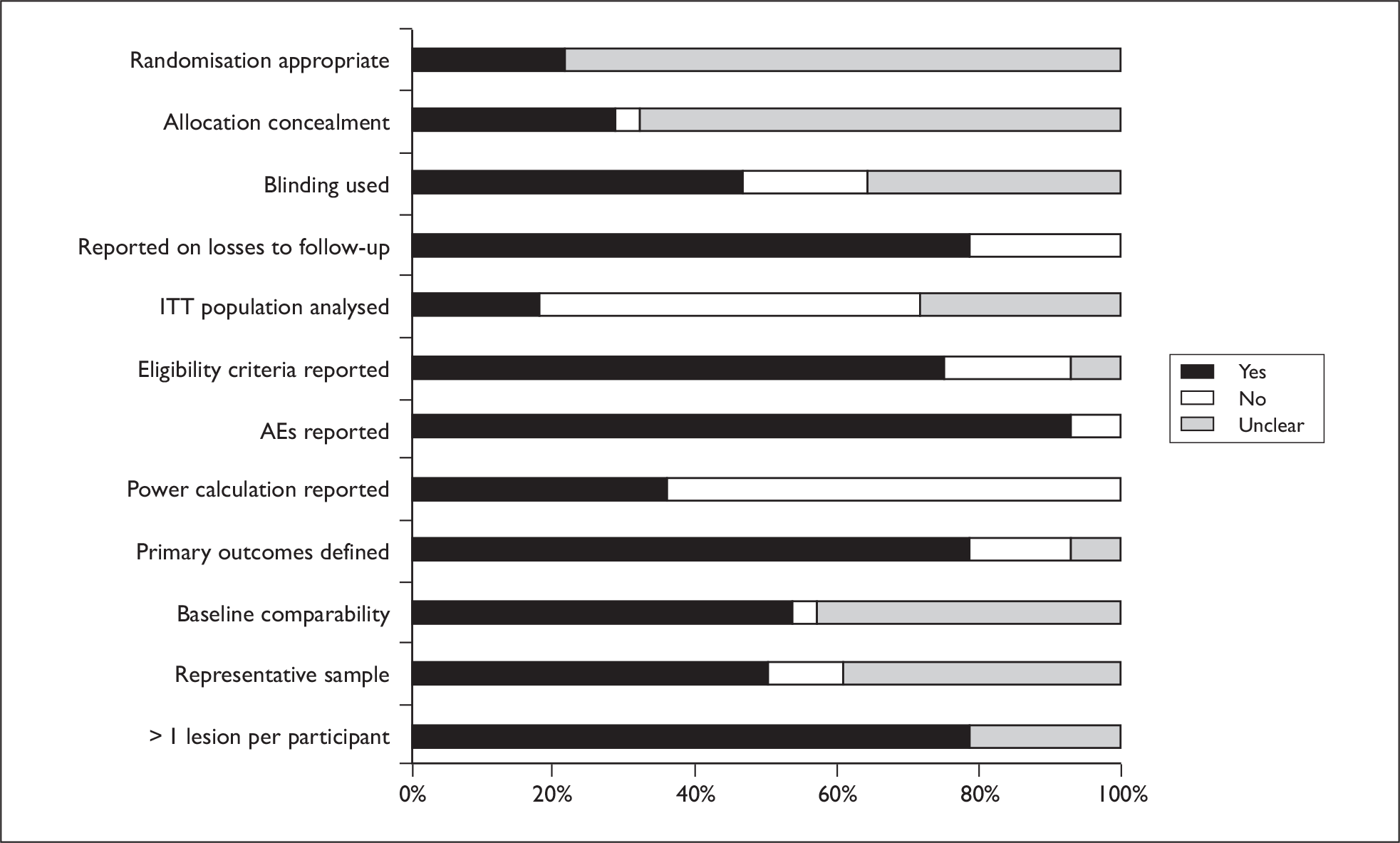
Sample sizes varied widely between 16 and 346 participants, with just over one-third of the studies reporting use of calculations to generate an appropriate sample size. Most participants had more than one lesion treated; even in studies reporting use of a power calculation it was often unclear whether the calculations had accounted for the likely existence of a correlation between lesion responses within patients. The number of independent observations per group may therefore not have been large enough for many studies. Eleven studies did however make use of a within-participant comparison design, removing the possibility of there being baseline differences between treatment groups.
Randomisation and allocation concealment procedures were generally poorly reported, although 46% of trials did report some use of blinding (generally of outcome assessors). Most trials reported incidence of AEs. A graph illustrating study quality is presented overleaf (Figure 5).
FIGURE 5.
Actinic keratosis study quality.
Results of effectiveness
The results are presented in a narrative synthesis, and for two comparisons we were able to conduct meta-analyses.
Mortality was not assessed in the AK RCTs as this outcome is less relevant for a non-invasive cancer. Resource use was not evaluated in any of the trials.
PDT vs cryotherapy
Six trials compared PDT with cryotherapy, with five using MAL45,46,48,51,59 (one of which was in organ transplant recipients59), and one using ALA. 60
Morbidity
Four RCTs of MAL–PDT versus cryotherapy, in patients (other than organ transplant recipients) with mild or moderate lesions, reported lesion complete response (CR) data for the ITT populations (two at 12 weeks45,51 and two at 24 weeks46,48). Although only one study directly reported results for the ITT population, the necessary ITT data could be extracted from the other three studies. Morton et al. 48 reported CR at both 12 and 24 weeks, with the 24-week response rates being only marginally the better; this provided the basis of our justification for pooling the 12- and 24-week data. All four studies defined a complete lesion response as complete disappearance of a lesion. The individual study results have been combined into a pooled estimate and the results presented in a forest plot (Figure 6).
FIGURE 6.
Methyl aminolevulinate–photodynamic therapy versus cryotherapy.
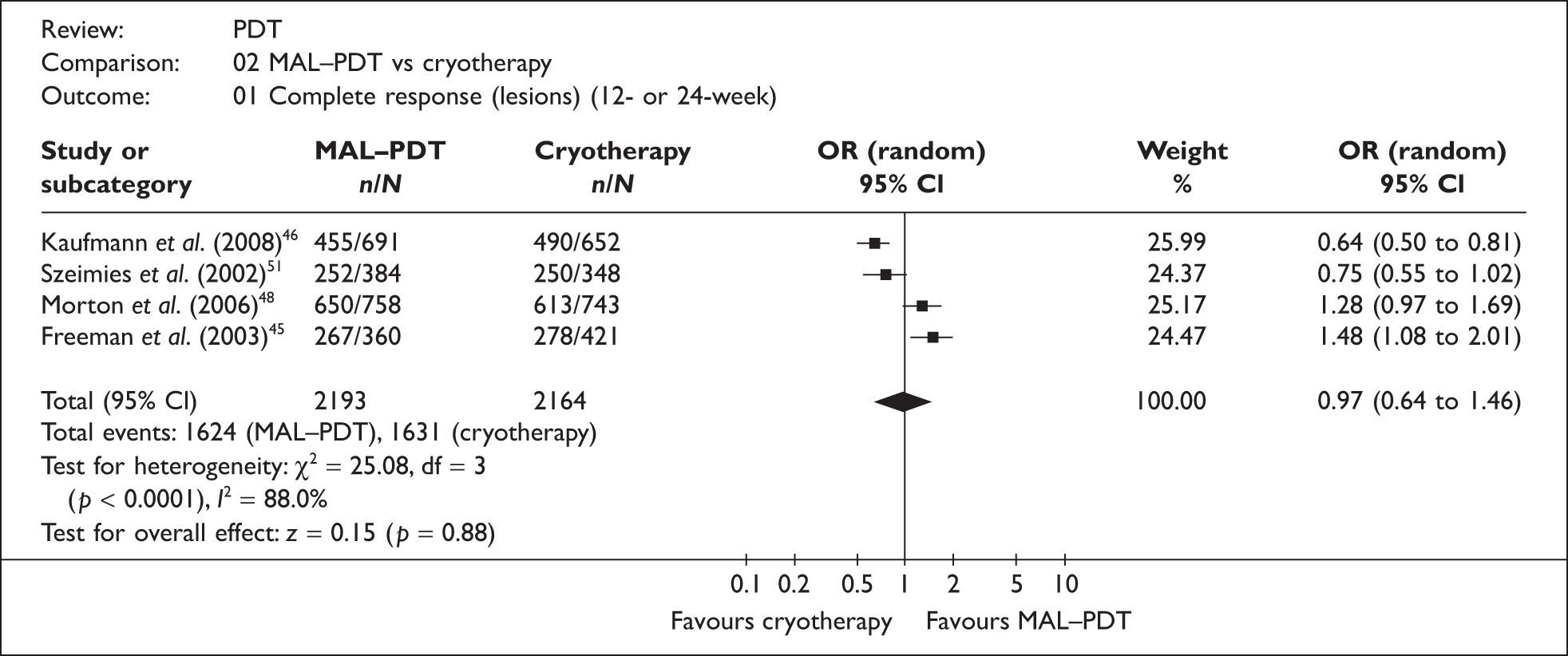
Although the pooled result [odds ratio (OR) = 0.97; 95% confidence interval (CI) 0.64 to 1.46] indicates that there is no difference in effectiveness between the treatments, the substantial statistical heterogeneity (I2 = 88%), coupled with the discernible polarity of individual study results, suggest this result may be unreliable. Two factors in particular may possibly explain the root of this variation. Firstly, the study quality of the trials was variable, with only one trial reporting the use of blinding (of outcome assessors) and reporting appropriate methods for randomisation and allocation concealment;45 the other three trials may therefore have been subject to bias. Secondly, there was variation in the cryotherapy regimens used, both within and between studies (mean freeze times ranged from 16 to 24 seconds between studies); two studies explicitly stated that individual centres could use their own preferred regimens (relevant details to clarify this information were not provided in the other two studies). 45,46 The effects of this are likely to have been exacerbated by the large numbers of recruiting sites for each trial (ranging from 9 to 25 centres). The large reported variances of freezing time means (which suggested the populations were not normally distributed) made it difficult to adequately assess the possible effect of differing freezing times (between studies). None of the studies reported median times, which could have been useful for this purpose.
Other factors may also have affected the individual study results. Although the number of lesions studied was large, the numbers of patients recruited were more modest (ranging from 119 to 202 participants), raising doubts as to whether the number of independent observations per treatment group was large enough for some trials. Additionally, trials recruiting across a large number of sites sometimes results in small numbers of participants recruited at many of the individual sites, a factor which appears to indicate reduced site performance, for example in terms of correct recruitment, or lower event rates. 62,63
The only study of PDT versus cryotherapy which used ALA as the photosensitiser utilised a standardised cryotherapy protocol (a freeze time of between 5 and 10 seconds) and reported complete clinical clearance rates at 12 weeks to be significantly better for patients treated with ALA–PDT than with cryotherapy (89% vs 77%, p = 0.007). 60 However, it should be noted that the cryotherapy freeze times used were considerably lower than for the trials comparing MAL–PDT with cryotherapy.
One RCT studied the use of MAL–PDT in organ transplant recipients. Wennberg et al. 59 evaluated MAL–PDT against investigators’ choice of other treatment, which was cryotherapy in 83% of cases. The lesion CR rate in the PDT group at 3 months was 77% compared with 74% in the control group, and recurrence rates were also similar with no statistically significant difference. The lesion response rate at 15 months was 88% in the PDT group versus 89% in the control group. Although the MAL–PDT group had more favourable outcomes for hypopigmentation, they also had more AEs, such as erythema, pain and crusting (reported by 75% of patients) compared with the control group (reported by 48% of patients).
Quality of life
Five RCTs presented cosmetic outcomes, as either investigator ratings or preferences, with all reporting PDT achieving significantly better results than cryotherapy. 45,46,48,51,60 However, in only one trial45 were the outcomes assessed by blinded investigators; the other four studies may have been subject to bias, casting doubt on the reliability of their results.
The evidence is unclear whether MAL–PDT (in five trials) or ALA–PDT (in one trial) is more effective, less effective, or equivalent to cryotherapy for treating AKs. Although PDT appears to produce a better cosmetic outcome than cryotherapy, the lack of blinding in most studies means that there is uncertainty regarding the reliability of this conclusion.
PDT vs chemotherapy creams (5-FU and imiquimod)
Three trials evaluated the use of ALA–PDT compared with 5-FU,35,47,50 although one reported just AEs as an outcome,35 and one was a three-armed trial comparing treatment with 5-FU with PDT using a blue light illuminator, and PDT using a laser. 50 Kurwa et al. 47 studied patients with a long history of AK affecting forearms and hands, and randomised (left or right) both treatments to be received in each patient. One trial was of ALA–PDT compared with imiquimod in patients with AK on the hands and forearms. 61
Morbidity
For the 5-FU studies, Kurwa et al. 47 found no statistically significant difference after 6 months in the reduction of lesional area between the treatment areas and found that no patients were completely cleared of AKs with either treatment. In a small three-armed trial, Smith et al. 50 found PDT with a laser to be somewhat less effective than PDT with blue light or 5-FU.
In the imiquimod study, at 6 months there were no statistically significant differences in overall CR (65% PDT vs 56% imiquimod), or for grade I lesions (72% for both treatments), but PDT resulted in a significantly higher rate of CR for grade II lesions (58% vs 37%, p < 0.05). 61
Quality of life
Only Smith et al. 50 evaluated QoL outcomes in the 5-FU studies, with results of skin photoageing assessments suggesting the 5-FU and PDT with blue light groups had more benefit in terms of tactile roughness, and the 5-FU and PDT laser groups had more benefit in terms of pigmentation.
Sotiriou et al. ’s imiquimod study61 reported no significant differences between treatments in investigator-assessed cosmetic outcome but did find that 69% of patients preferred PDT to imiquimod. 61
The two trials reporting effectiveness results for PDT versus 5-FU were of uncertain quality, and had small sample sizes, but they suggest there is no difference in effectiveness between the treatments. Results of the imiquimod study suggest that ALA–PDT may be superior to imiquimod for treating grade II lesions.
PDT vs PDT with placebo cream
Nine RCTs compared PDT to PDT with placebo cream, with five using MAL42,45,49,56,58 and four using ALA41,44,60 as a photosensitiser (two trials were reported in one paper). 60 One study was of organ transplant recipients. 42
Morbidity
Four RCTs of MAL–PDT versus placebo PDT, in patients (other than organ transplant recipients) with mild or moderate lesions, reported CR data (complete disappearance of the lesion) at 3 months. 45,49,56,58 Where ITT results were not explicitly reported the relevant data were extracted and the results from the individual studies have been combined and presented in a forest plot (Figure 7).
FIGURE 7.
Methyl aminolevulinate–photodynamic therapy versus placebo PDT.
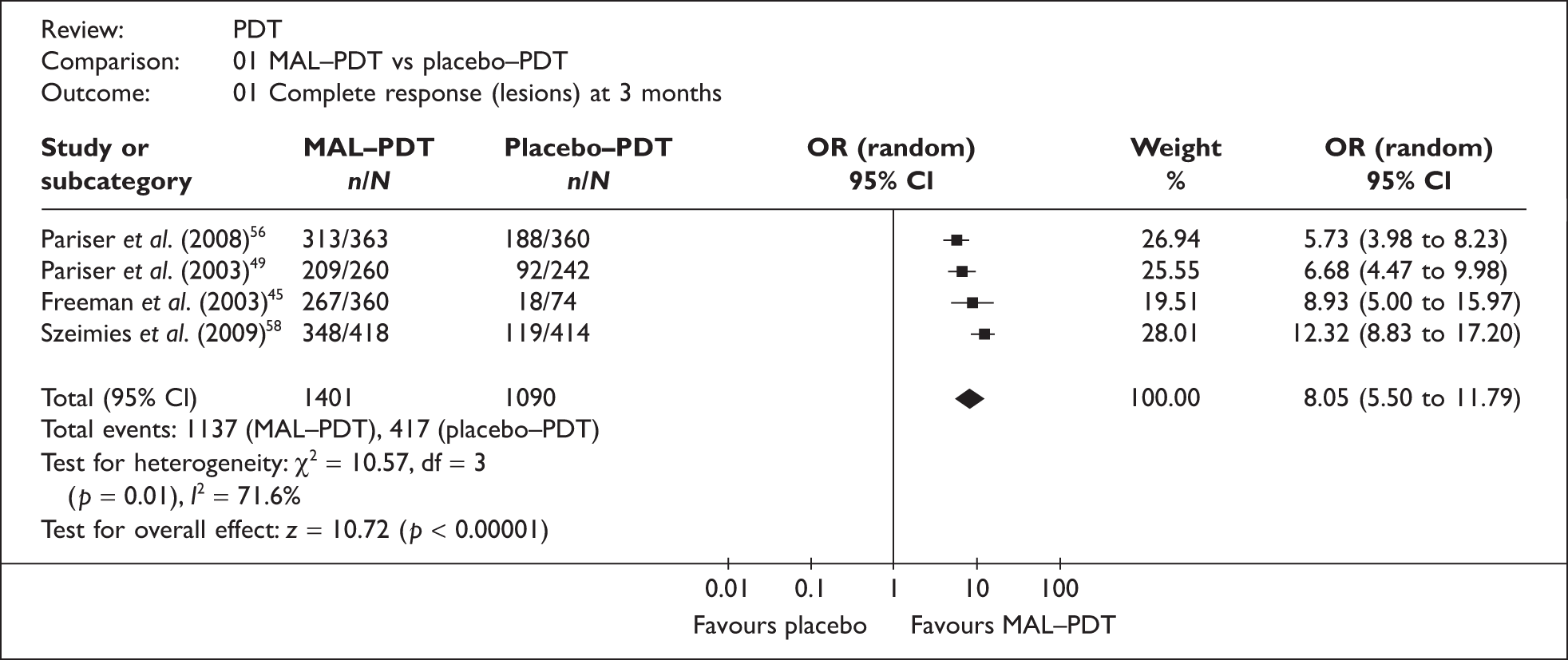
The pooled result (OR = 8.05; 95% CI 5.50 to 11.79) clearly indicates that MAL–PDT is more effective than treatment with placebo cream. However, the magnitude of effect is more uncertain as there was significant heterogeneity between studies (I2 = 72%). There appears to be no obvious explanation for this variation (all studies appeared to be generally well conducted, with study investigators blinded in all four trials), other than the fact that all were multicentre trials (with between 5 and 10 sites) with quite small numbers of participants (between 80 and 204), increasing the possibility that institutional differences (e.g. experience of clinicians), protocol deviations, and data from sites with few participants, could have affected the reliability of results.
The one other RCT comparing MAL–PDT with placebo was also generally well conducted, and was of organ transplant recipients with mild to moderate AK. 42 The authors reported overall lesion CR rates of 56/62 for the MAL–PDT group versus 0/67 in the placebo group (p = 0.0003).
Of the four RCTs comparing ALA–PDT with placebo PDT one did not report methods and results adequately. 41 Two of the other three trials were reported in one paper and used an incubation time of 4 hours; both reported significantly better lesion CR rates with ALA–PDT (89% vs 29%, and 82% vs 19%). 60 Fowler and Zax44 also reported lesion CR results strongly favouring ALA–PDT – in two trials with identical protocols – although the incubation times used were not stated.
Quality of life
None of the MAL–PDT RCTs adequately reported QoL results for both treatment groups. Pariser et al. 49 reported that investigator-assessed cosmetic outcome was ‘excellent’ or ‘good’ in 97% of patients, and that of 32 patients who had received previous types of other treatment (cryotherapy, 5-FU, operation) 73% preferred MAL–PDT.
Of the ALA–PDT trials, only Hauschild et al. 60 reported comparative QoL data, finding no significant differences in cosmetic assessment of cleared lesions.
Results from four generally well-conducted RCTs indicate that MAL–PDT is significantly more effective than placebo PDT in achieving lesion CR. The evidence for ALA–PDT, although less robust, also indicates superiority over placebo PDT. However, there remains uncertainty around which are the optimal ALA incubation times, and QoL outcomes were inadequately assessed in most trials.
PDT using different treatment parameters
Twelve RCTs evaluated the use of PDT using different treatment parameters. Five of these trials studied light sources50,53 or light doses/durations,36,38,43 and another five evaluated photosensitisers/photosensitiser doses37,40 or incubation times (duration of photosensitiser),39,55 including one which examined both doses and incubation times. 54 One compared the number of PDT sessions52 and one studied incubation times and light doses. 57
Morbidity
Five trials evaluated the effects of varying PDT light parameters, three using MAL36,38,53 and two using ALA43,50 as a photosensitiser.
Two RCTs looked at differing light sources. One trial,50 using ALA as a photosensitiser, reported cumulative clearance rates of 80% using a blue light illuminator compared with 50% using a laser. The trial using MAL as a photosensitiser53 concluded that PDT with daylight (79% decrease in lesions) was as effective as PDT with red LED light (71% decrease).
Three RCTs looked at differing light doses/durations. Ericson et al. 43 reported a significant correlation (p < 0.02) between fluence rate and treatment response (the remaining actinic area) with 30 mW/cm2 (with a narrow filter) showing the best results. Legat et al. 36 reported, in an abstract, that PDT with fractionated and unfractionated illumination were similarly effective in reducing AKs. Szeimies et al. 38 reported no significant differences in lesion scores between groups receiving LED light or variable pulsed light (VPL).
Five RCTs evaluated photosensitisers/photosensitiser doses and/or incubation times; two studied ALA,39,55 two studied MAL40,54 and one studied both photosensitisers. 37
Of the two RCTs studying photosensitiser incubation times, Hauschild et al. 55 reported that receiving an ALA patch with a 4-hour incubation showed the best response compared with 0.5-, 1- or 2-hour incubations. Touma et al. 39 evaluated ALA incubation times of 1, 2 or 3 hours; although the abstract did not report results by treatment group, the authors did report that incubation time had no effect on outcomes.
The small study by Moloney and Collins37 compared ALA–PDT with MAL–PDT and found no significant differences in efficacy (although ALA was applied for a longer incubation period). Similarly, Wiegell et al. 40 reported no differences in response rates when comparing 8% MAL with 16% MAL, with patients receiving daylight as the light source. Braathen et al. 54 compared MAL incubation times (1 or 3 hours) and doses (160 or 80 mg/g), in a study with some methodological problems (e.g. the trial protocol was often not followed), suggested that a 1-hour incubation with 160 mg/g may have potential for treating mild AK lesions.
Puizina-Ivic et al. 57 reported that a longer ALA incubation with fractionated illumination resulted in fewer patients with persistent lesions at 24 weeks than a shorter incubation with a single illumination (13% vs 75%). However, the extent of the relative contributions of the incubation time and fractionation was unclear.
Tarstedt et al. 52 concluded that a single MAL–PDT treatment was as effective as a two-treatment schedule (1 week apart) for thin AK lesions, and recommended repeat treatment for thicker or non-responding lesions.
Quality of life
For the two light sources studies, Smith et al. 50 found that, for signs of photoageing, both light treatment groups showed improvement in global response, but the signs completely resolved in two patients in the group receiving light from a blue light illuminator, compared with no patients in the laser (595 nm) group. Wiegell et al. ,53 in a within-participant comparison study, found that 62% of patients preferred treatment with daylight: 14% with LED light and 21% had no preference.
For the three light doses/durations studies, Szeimies et al. 38 found no significant differences in patient satisfaction between the LED light and variable pulsed-light treatments. However, none of the other studies reported QoL outcome.
Very few of the five trials looking at photosensitisers/photosensitiser doses and/or incubation times reported QoL outcomes, and none reported them by treatment group.
Tarstedt et al. 52 reported that cosmetic outcome was rated (by the investigator) as excellent in over 75% of lesions in both treatment groups, and that patients who had previously been treated with cryotherapy tended to prefer treatment with PDT.
The 11 trials evaluating PDT parameters were varied in their objectives, and their results suggest further research is needed to ascertain the optimum parameters; particularly since several studies provided limited details on methods/results, and/or had small sample sizes. However, two RCTs – including one good quality study – suggest that PDT using daylight (as a light source) appears to be a promising option.
Results of safety
Of the 28 RCTs, 11 assessed AEs in trials of ALA–PDT, 15 in trials of MAL–PDT, one assessed AEs for both photosensitisers, and one did not assess AEs. 57 The extent of assessment varied greatly, and was not always presented by treatment group.
MAL–PDT vs placebo
Overall, local AEs were reported by between 85% and 98% of patients receiving active PDT and in 45–60% of patients receiving placebo PDT. 49,56,58 Where an AE had been reported by patients receiving active treatment this was judged to be mild in 32–53% of cases, moderate in 42–49% and severe in 5–33%. Between 38% and 93% of patients receiving placebo reported mild AEs, 6–8% reported moderate AEs and 0–4% reported severe AEs. Dragieva et al. 42 reported that for placebo areas discomfort was judged to be mild in all cases while for active PDT discomfort was largely mild or moderate. 42
Severe local AEs (described as causing considerable interference with daily activities, may be incapacitating or life threatening) included skin-burning sensations, pain of the skin, erythema, skin exfoliation or blisters. 49,56,58 Common local AEs occurred as detailed in Table 3. 49,56,58
| Local AE | Percentage range reported (active) | Percentage range reported (placebo) |
|---|---|---|
| Burning sensations | 36–72 | 11–12 |
| Erythema | 52–77 | 5–21 |
| Crusting | 38 | 16 |
| Skin pain | 24–60 | 0–22 |
| Blisters | 15–19 | 0–5 |
| Skin oedema | 14–28 | 2–3 |
| Skin ulceration | 12 | 0 |
| Scab | 26 | 0 |
| Pruritis | 23 | 11 |
| Skin exfoliation | 11 | 4 |
| Stinging skin/discomfort | 14–23 | 2–3 |
Szeimies et al. 58 reported that most AEs started during illumination and in the case of skin pain or burning sensations these were transient, resolving within 1 day. Erythema tended to be more persistent (median 4 days’ duration) and was also reported after treatment in around 40% of cases.
ALA–PDT vs placebo
Fowler and Zax44 found the proportion of patients reporting some or all lesions being oedematous (35% vs 0%) or erythematous shortly after treatment (99% vs 79%), to be higher in the ALA–PDT group compared with the placebo group. All AEs resolved or improved by 4 weeks. PostPDT itching was reported by more patients receiving ALA–PDT than patients receiving placebo (26% vs 7%, respectively). Seven patients had a serious AE (SAE), but none was deemed to be related to treatment.
Hauschild et al. ,60 in trial AK03, deemed that transient skin discoloration in one patient was related to ALA–PDT treatment. The same trial also found that patients treated with ALA–PDT had more overall local reactions compared with patients on placebo when treatment was applied (mostly itching, 42% vs 13%, although the 13% placebo figure appeared to be pooled from the two trials). In trial AK04 the authors reported AE rates related to study treatment as being 3% in the ALA group versus 2% in the placebo group. 60
Jeffes et al. 41 reported no differences in hyperpigmentation between the treatment groups, but reported a figure only for the ALA–PDT group (11%).
MAL–PDT using different treatment parameters
One RCT (n = 25) compared LED versus VPL illumination and reported on pain scores assessed immediately after treatment using a visual analogue scale (VAS) scale. 38 Patients receiving VPL reported significantly lower levels of pain (4.3) than with LED illumination (6.4) (p < 0.001).
Legat et al. 36 (n = 22) compared fractionated and unfractionated illumination. Two patients terminated treatment due to extreme pain and six areas assigned to unfractionated illumination had to be treated with an alternative fractionated protocol after the patients complained of intense pain. PDT-induced pain was significantly less in the fractionated area according to VAS score (6.0) than in the unfractionated area (6.7) (p = 0.02).
Braathen et al. 54 (n = 119) compared different doses and incubation times of MAL–PDT across four groups. No SAEs related to the treatment were reported and most AEs were both mild and local in nature. Between 96% and 99% of patients in each group reported at least one treatment-related AE. Erythema was the most commonly reported AE by 32–50% across groups, with a median duration of 17 days. Skin pain lasted around 12 days and other AEs were transient (< 1 day).
Tarstedt et al. 52 (n = 211) compared single-session versus double-session MAL–PDT. Although more AEs were reported in the double-PDT group, there was no indication of cumulative local phototoxicity (76 events after first treatment, 46 after the second). Overall 40% of patients receiving single PDT and 50% receiving double PDT reported any AE. The majority of the AEs were mild to moderate intensity and lasted less than 1 day including pain. Median erythema duration was 5 days for single treatment and 2 days for double treatment.
One RCT comparing two doses of MAL cream with daylight as the illumination (n = 29) reported that, generally, patients had mild to moderate pain (mean 3.7 on the VAS scale). 40 Erythema and crusting were reported in both 8% and 16% groups but no further details were available.
An RCT of 30 patients compared daylight versus red LED illumination for MAL–PDT and reported that pain was significantly less for the daylight exposed areas during treatment, mean pain score 2 versus 6.7 for LED (p < 0.001). 53 These differences were no longer statistically significant 6 hours post treatment. In the LED group, 50% of patients required cold-water spray to control the pain and 25% needed mid-treatment breaks. Both treatment areas developed erythema and crusting.
ALA–PDT using different treatment parameters
Touma et al. ,39 after studying different ALA incubation times, only stated (in an abstract) that phototoxic reactions were well tolerated. Ericson et al. 43 found no correlation between fluence rates and pain scores. Hauschild et al. 55 reported that five patients had AEs that were considered to be related to study medication (patch ALA), which were: headache, moderate epistaxis and a mild increase in alanine transaminase. The study also found that local reactions during illumination appeared to be dose dependent (26% in the 0.5-hour incubation group vs 66% in the 4-hour group), and that almost all patients had local reactions after treatment. Patients with clearance experienced local reactions to a greater extent than patients without clearance.
MAL–PDT vs cryotherapy
No systemic AEs were reported by any trial. Overall levels of AE in the PDT groups ranged from 43% to 75%, and 26–72% for cryotherapy. The majority of all reported AEs were recorded as mild/moderate and were transient in nature. Only one trial46 reported any SAEs – two cases of severe cold exposure injury in the cryotherapy arm.
One trial48 reported skin discomfort after the first treatment session using a VAS scale and found no significant differences between PDT (5.2) and cryotherapy (4.9) (p = 0.24). However, data from Wennberg et al. 59 showed that 6% of patients receiving PDT discontinued treatment due to pain despite fans and cold water sprays being used, and most reports of pain were of moderate intensity.
All trials reported that common AEs included skin pain/discomfort, erythema, blistering and crusting. Szeimies et al. 51 presented percentages of these AEs by treatment group as follows: burning sensation (PDT 32%, cryotherapy 9%), skin pain (PDT 10%, cryotherapy 13%) and crusting (PDT 5%, cryotherapy 6%).
ALA–PDT vs cryotherapy
Hauschild et al. ,60 in trial AK04, reported AEs related to study treatment as being at 3% in both the ALA–PDT and cryotherapy groups.
ALA–PDT vs chemotherapy creams (5-FU and imiquimod)
Gupta,35 in an abstract, reported only that after 1 week patients receiving ALA–PDT showed few signs of irritation (e.g. erythema), but patients treated with 5-FU exhibited moderate to severe erythema. Kurwa et al. 47 found that in the first week after treatment the ALA–PDT sites were significantly more painful than the 5-FU sites, but the difference was absent in week 2, and was reversed in week 4; overall there was no significant difference between the groups. A very similar pattern of results was reported for level of erythema. One patient experienced contact sensitivity to 5-FU.
Smith et al. 50 found erythema to be the most pronounced AE, with patients receiving 5-FU having the largest average increase. Crusting was only seen in the 5-FU group.
In the imiquimod study reactions to both treatments were reported as being well-tolerated, with erythema being very common in both groups. All patients experienced burning and pain after PDT, compared to 11% (burning) and 4% (pain) after treatment with imiquimod. 61
MAL–PDT vs ALA–PDT
Moloney and Collins,37 in a split-scalp study of 16 patients, reported statistically significant greater pain at 3, 6, 12 and 16 minutes, and longer duration of discomfort post treatment, on the side treated with ALA–PDT (although ALA was applied for a longer incubation period).
MAL–PDT: No systemic or SAEs were reported in any study. Based on 16 RCTs, local skin-related AEs appear to be fairly common in patients receiving MAL–PDT. These include skin pain or discomfort, erythema, crusting, blisters and oedema of the skin. While usually transient, erythema and skin pain, in particular, may have a longer duration, and it is worth noting that despite the use of cooling fans and water sprays a small proportion of patients are unable to tolerate the pain during illumination. There is limited evidence from single small RCTs that fractionated, daylight or VPL illuminations may be less painful than standard LED illumination.
ALA–PDT: AE reporting in the ALA–PDT trials was inconsistent, but both ALA–PDT and the alternative treatments generally appear to be well tolerated.
The one study that compared ALA–PDT with MAL–PDT concluded that ALA–PDT was the more painful treatment, but the small sample size, lack of methodological details and a difference in incubation times mean that the reliability of this conclusion is uncertain.
Ongoing trials
There were eight ongoing or unpublished trials for which we could obtain no results details (Table 4).
| Investigator | Interventions | Start date | Status |
|---|---|---|---|
| Witherspoon J | ALA–PDT with pulsed-light PDT vs no treatment | August 2007 | Expected December 2008, but listed as recruiting in September 2008 |
| Hauschild A | PDT + red light vs placebo | March 2006 | December 2007 – listed as completed |
| Szeimies RM | PDT (using PD P 506 A) vs placebo or cryotherapy | March 2006 | November 2007 – listed as completed |
| Oseroff A | ALA–PDT – various exposure periods and laser doses | May 2005 | Listed as recruiting |
| Wulf H | MAL–PDT with daylight | June 2008 | Expected January 2009 |
| Roberts F | ALA–PDT – investigation for Bowen’s disease and AK, dose fractionation for BCC | February 2002 | February 2004 – listed as completed |
| Wulf H | MAL–PDT using sunlight | May 2006 | February 2007 – listed as completed |
| Pariser D | MAL–PDT vs placebo (using LED light source) | September 2007 | Expected October 2007, listed as completed |
Discussion
The placebo-controlled trials were generally well conducted and clearly illustrate that PDT is an effective treatment for AK. But the MAL–PDT-versus-cryotherapy trials produced conflicting results, probably due to methodological weaknesses, suggesting that further high-quality RCTs are required. These trials would need clearly defined protocols for administering all study treatments, longer follow-up periods, and adequate blinding of outcome assessors. Only one RCT has been conducted that compared ALA–PDT with cryotherapy, so similar uncertainties of relative efficacy also exist.
The two RCTs of PDT versus 5-FU had small sample sizes and were of uncertain quality, so further research is needed. There is also a noticeable dearth of RCTs comparing PDT with imiquimod, diclofenac and retinoids. The results of any future PDT versus cryotherapy trials should be viewed in the context of the results of trials comparing topical chemotherapy agents for AK (e.g. 5-FU vs imiquimod).
Factors such as patient preference, lesion thickness and number, availability of treatments and expertise, and whether a treatment can be given at home can all play a role in determining which therapy is used to treat AK. Having a range of options, including PDT, is therefore preferable.
The results of trials comparing different ways of delivering PDT indicate that optimum parameters have yet to been found. The suggestion that daylight could be an effective light source appears worthy of further investigation, especially as there may be additional benefits in terms of time and cost savings.
Providing enough patients can be recruited (i.e. there are enough independent comparisons), the use of within-patient comparison trials should be favoured whenever possible – ideally randomising treatment to opposite sides of the body – as this eliminates the possibility of baseline differences. Patients acting as their own controls would also mean that fewer patients would need recruiting than in conventional controlled trial designs. However, investigators would also need to be confident there would be no systemic study treatment effects, for example the possibility that PDT treatment may enhance outcomes in areas treated with cryotherapy. Systemic effects are theoretically possible as it is known that PDT can affect the immune system, although it should be noted that few systemic AEs were reported in the RCTs in this review (and no trials reported systemic photosensitisation effects). The results of 10 AK RCTs were reported in 2008, suggesting that this is still an active area of research.
BOWEN’S DISEASE
Background
Bowen’s disease is a pre-invasive form of squamous cell skin cancer, also called SCC in situ. Lesions can be located on several different parts of the body but are commonly found on the head, neck and lower limbs. 64 Bowen’s disease is most often seen in people in their 60s and 70s, and is about three times more common in women than men. 65 Whilst Bowen’s disease often occurs on chronically sun exposed sites, sun exposure does not seem to be the only explanation for its aetiology. The incidence of Bowen’s disease is about 15 per 100,000 people. 66
In Bowen’s disease the carcinoma is present within the epidermis and has not breached the basement membrane. If left untreated, the disease can invade the dermis (invasive SCC) and there is then the potential to metastasise. Approximately 3% of cases will develop into invasive disease. 67
The choice of therapy depends on patient suitability, and location and number of lesions. As lesions of Bowen’s disease are often large and multiple and commonly found on the lower legs in frail, elderly patients, treatment by destructive therapies can be associated with significant morbidity. Treatment options include surgery, cryotherapy, curettage, radiotherapy and topical therapies using 5-FU or imiquimod. Watchful waiting may be used if a patient is frail, as in Bowen’s disease only a small number of cases will become invasive.
In PDT for the treatment of Bowen’s disease a photosensitising cream is applied to the affected area, usually a few hours before treatment with the light. As with the above treatment options, it has a curative intent and can be repeated if response is incomplete. It can be used as an alternative to the options described above. It is considered to be the treatment of choice on a lower leg site. It may also be used where lesions are large or multiple or where other treatments have failed or are inappropriate. 64
Study characteristics
Seven RCTs investigated PDT for Bowen’s disease (Table 5). Six trials had a total number of 362 patients and one trial did not state participant numbers. 68 All trials were published as full papers and often as abstracts too; references in the table relate to only the full papers.
| Authors | No. of participants | Trial treatments |
|---|---|---|
| de Haas et al. (2007)69 | 40 (50 lesions) | ALA–PDT using a single illumination vs ALA–PDT with a twofold illumination |
| Morton et al. (2000)70 | 19 (70 lesions) | ALA–PDT with red light vs ALA–PDT with green light |
| Morton et al. (1996)71 | 19 (40 lesions) | ALA–PDT vs cryotherapy |
| Puizina-Ivic et al. (2008)57 | 15 | ALA–PDT with 16-hour incubation and two light fractions vs ALA–PDT with 5-hour incubation and a single illumination |
| Salim et al. (2003)72 | 40 (66 lesions) | ALA–PDT vs 5-FU |
| Lui et al. (2004)68 | Not stated (34 lesions) | PDT at 60 J/cm2 vs PDT at 120 J/cm2 vs PDT at 180 J/cm2 using intravenous verteporfin |
| Morton et al. (2006)73 | 229 (275 lesions) | MAL–PDT vs placebo PDT vs cryotherapy vs 5-FU |
Different methods of delivering PDT were explored in four trials. 57,68–70 PDT was compared with cryotherapy in two trials71,73 and with 5-FU in two trials,72,73 while one trial also had a placebo PDT treatment. 73 ALA or MAL creams or intravenous verteporfin were used as photosensitisers. The drug to light interval varied from 1 to 16 hours across the studies.
Study quality
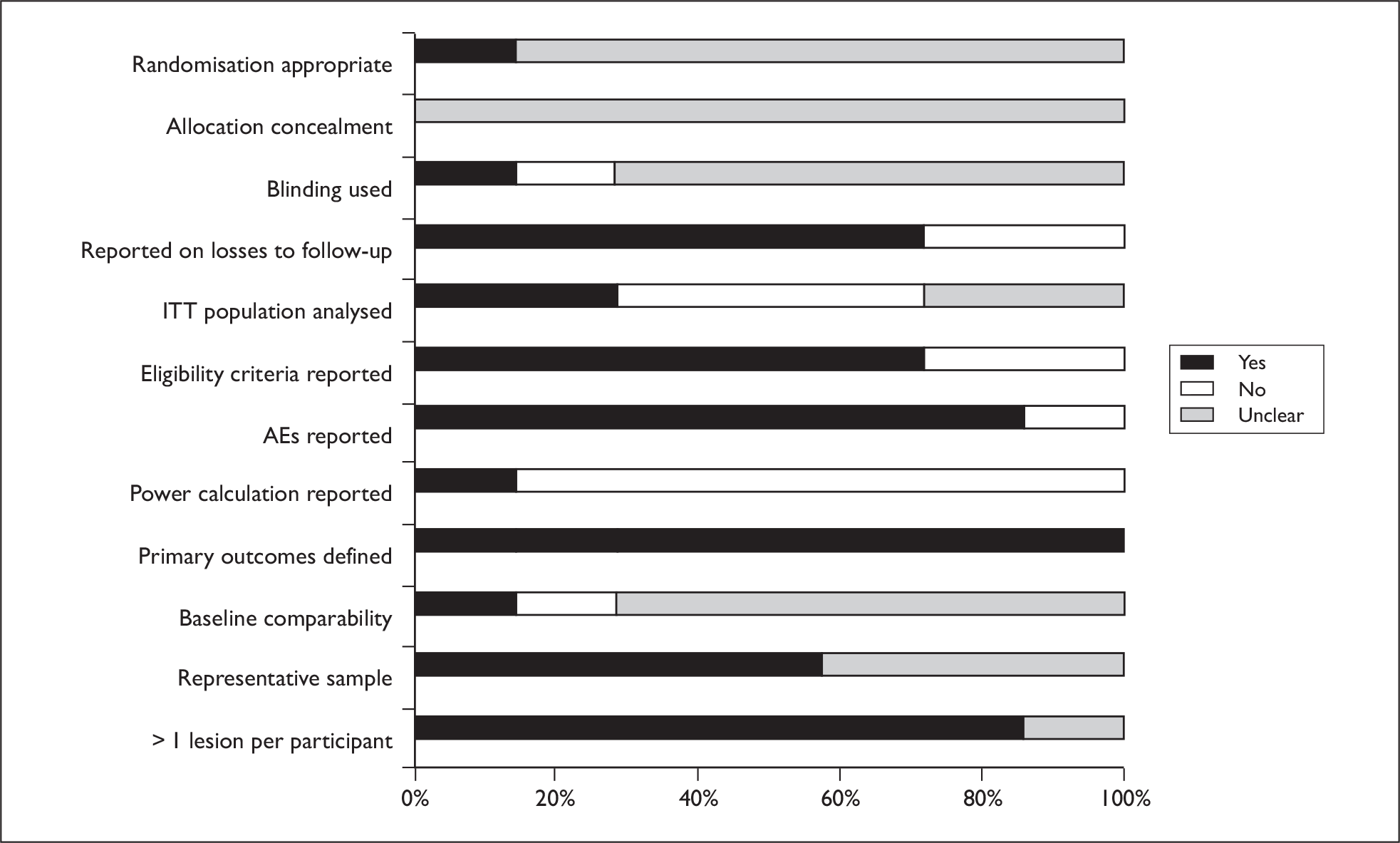
Five out of seven trials were published within the last 6 years,57,68,69,72,73 with two being considerably older. 70,71 All except one trial had fewer than 50 patients. The largest trial (229 patients) was conducted in 40 centres across 11 countries, raising the possibility of institutional differences and protocol deviations. 73 In the majority of trials, procedures for randomisation, allocation concealment and blinding of outcome assessors were unclear. It was not always clear if results presented were statistically significant. All except two trials reported AEs. 57,68 Generally reporting was limited, making the reliability of studies difficult to assess. A graph illustrating study quality is presented in Figure 8.
FIGURE 8.
Bowen’s study quality.
Results of effectiveness
Results are presented in a narrative synthesis. Meta-analysis was not possible due to heterogeneity between the trials. Mortality was not assessed in this group of studies; however, this outcome is less relevant for a non-invasive cancer. QoL outcomes were sparsely reported and resource use was not evaluated in any of the trials.
PDT vs cryotherapy
Two trials by Morton et al. 71,73 compared PDT with cryotherapy. One of the trials also compared PDT with placebo PDT and 5-FU and is further discussed below. 73 The larger trial used MAL–PDT,73 whereas the smaller one used ALA as a photosensitiser. 71
Morbidity
The larger, more recent trial by Morton et al. 73 found better CR rates and lower recurrence rates with PDT than with cryotherapy. There was a statistically significant difference between the two treatments at 12 months favouring PDT (OR = 1.77; 95% CI 1.01 to 3.12). At 24 months, sustained CR rates were similar (PDT 68%, cryotherapy 60%). The smaller, older trial by Morton et al. also found better CR rates for PDT (100%) than cryotherapy (90%) and lower recurrence rates. In this trial, taking size of lesion into account, the probability that a lesion is completely cleared at first treatment was statistically significantly better with PDT (p < 0.01). 71
Quality of life
The larger trial by Morton et al. 73 found higher rates of ‘good or excellent cosmetic outcome’ with PDT at 3 months, which was maintained at 12 and 24 months. The smaller trial reported that 12 months following clearance, four lesions had visible scarring after cryotherapy, whereas none did after PDT. 71
Based on two RCTs with some methodological limitations, PDT appears to result in better rates of CR and has a better cosmetic outcome with PDT than cryotherapy.
PDT vs 5-FU
Two trials compared PDT with 5-FU. 72,73 The larger trial by Morton et al. ,73 described above, used MAL–PDT, whereas the smaller one by Salim et al. 72 used ALA as a photosensitiser.
Morbidity
The larger, more recent trial by Morton et al. 73 found better CR rates and lower recurrence rates with PDT when compared with 5-FU. In the trial by Salim et al. ,72 after adjustment for lesion size on response, the difference in initial clearance rates was not significant. However, overall clearance at 12 months was statistically significant. The PDT group had clearance of 27 out of 33 lesions (82%), whereas the 5-FU group had clearance of 16 out of 33 lesions (48%) (OR = 4.78; 95% CI 1.56 to 14.62, p = 0.006). At 24 months sustained CR rates were similar (PDT 68%, cryotherapy 59%).
Quality of life
The trial by Morton et al. 73 found higher rates of ‘good or excellent cosmetic outcome’ with PDT at 3 months, maintained at 12 months. The trial by Salim et al. 72 did not assess this outcome.
Based on two RCTs with some methodological limitations, PDT appears to result in lower recurrence rates and hence better overall clearance than 5-FU. Cosmetic outcomes may be better but this is based on only one RCT.
PDT with different treatment parameters
Different methods of delivering PDT were explored in four trials. 57,68–70 All four trials were small. One trial had a patient population of patients with various non-melanoma skin cancers in addition to a very small number with Bowen’s disease. This trial did not present all results by diagnosis and is not discussed here. 68 One trial had a sample with either AK or Bowen’s disease, again not presenting all results by diagnosis. 57 Three trials used ALA as a sensitiser but one evaluated red versus green light,70 whereas the other two considered the relative benefits of single and twofold illumination. 57,69
Morbidity
In the trial by de Haas et al. ,69 CR rates at 12 months were not statistically significantly different between the single and twofold-illumination treatment groups. Equally, healing time was not statistically significantly different. However, the trial by Puizina-Ivic et al. 57 found fewer residual tumours at 24 months in the fractionated, longer incubation group than in the single-illumination group. In the trial by Morton et al. treatment with red light was superior to treatment with green light. Initial response rates were 94% with red light and 72% for green light (p = 0.002). There were also statistically fewer recurrences with red light (OR = 0.13; 95% CI 0.04 to 0.48).
Quality of life
Two trials did not discuss this outcome,57,69 whereas the other reported that no clinically obvious scars were evident at 1 year in either red or green light conditions. 70
This small group of trials points to the need for ongoing research into the optimum parameters for the delivery of PDT to ensure the best patient outcomes.
Results of safety
Five trials reported AEs relating to PDT and Bowen’s disease. 69–73 Results for ALA,69–72 and MAL73 are presented separately; AEs were not reported for the only trial using verteporfin. 68
ALA–PDT
In one trial comparing single with twofold illumination, no SAEs were observed in either group. 69
In the trial by Morton et al. 71 comparing PDT with cryotherapy, PDT resulted in statistically significantly less pain during treatment (p = 0.01). Six patients in this trial who received both treatments reported PDT as less painful. The trial by Salim et al. 72 found that in a comparison of intensity and duration of pain, more pain was experienced in the 5-FU group than in the PDT group (p = 0.01). However, comparison of total pain over time resulted in no statistically significant difference in the median pain scores between the two treatment groups. In the trial by de Haas et al. ,69 pain during treatment was experienced by five patients in the twofold-illumination group and by none of the patients in the single-illumination group. In the trial by Morton et al. 70 comparing red and green light, no significant difference in pain was observed between the treatment groups.
Morton et al. 71 found that there were no instances of blistering and infection in the PDT groups in a trial comparing PDT with cryotherapy. In the trial by Morton et al. 70 of red and green light, no ulceration or infection was reported in either of the red- or green-light treatment groups. In the trial by Salim et al. ,72 no patients in the PDT group experienced ulceration of the lesions and there was no clinically obvious scar formation at 12 months at any PDT treatment site.
No photosensitivity reactions were found in either group in the trial comparing red and green light,70 and in the trial comparing PDT with cryotherapy. 71
MAL–PDT
In the trial by Morton et al. ,73 most treatment-related AEs were mild (60%) or moderate (34%). Severe AEs were noted in 6% of patients treated with PDT and 12% of patients undergoing cryotherapy. SAEs were reported in four PDT patients, two placebo patients and three cryotherapy patients, of which only one event in the cryotherapy group, and none of the others, were considered to be treatment related. At 24 months, AEs were said to be of a shorter duration than with other treatments (no data provided).
Serious treatment-related AEs have not been reported for PDT in the treatment of Bowen’s disease. However, trials are generally small and rarer AEs might not, therefore, be observed. It is unclear whether PDT is more or less painful than other treatments and how altering PDT parameters might impact on pain. In the trials where this outcome was reported, photosensitivity did not emerge as a significant issue.
Ongoing trials
We are aware of two potentially ongoing/unpublished trials of PDT for Bowen’s disease but could not obtain any further information about them (Table 6).
| Investigator | Interventions | Start date | Status |
|---|---|---|---|
| Verzijl A or Krekels G | Excision vs PDT for Bowens | May 2007 | Listed as recruiting |
| Roberts F | ALA–PDT – investigation for Bowen’s and AK, dose fractionation for BCC | February 2002 | February 2004 – listed as completed |
Discussion
The majority of the trials of PDT in Bowen’s disease are small and have methodological limitations. Only three trials compared PDT to another treatment (cryotherapy and/or 5-FU). There was no investigation of imiquimod in relation to PDT. There are suggestions of better outcomes, especially of a cosmetic nature, with PDT but these would need confirmation in further, well-designed comparative trials. Such trials would also need to consider differences in AE profiles between treatments. More clarification of the optimal parameters for PDT is also needed in terms of effectiveness and safety.
BASAL CELL CARCINOMA
Background
Basal cell carcinoma is the most common form of skin cancer, and around 85% of lesions affect the head and neck areas. 74 Generally slow growing and locally invasive, BCC may take a variety of clinical appearances such as nodular, cystic, superficial, morphoeic, ulcerated or pigmented. Risk factors for BCC include fair skin phototype, tendency to freckles, and excessive exposure to ultraviolet light, male gender and smoking. Nodular BCC is the most common type in the UK, while in other countries such as the USA and Australia superficial BCC is particularly common. 75
Incidence rates for BCC vary widely across the literature, partly due to differences in latitude and sun exposure, and possibly due to incomplete registration of tumours (Table 7 provides examples). 75,76 If left untreated, BCCs can cause extensive tissue damage, particularly on the face. Superficial BCCs often occur in large multiple patches on the trunk and may not be amenable to surgery.
| Country | BCC rate per 100,000 people |
|---|---|
| Australia | 788 |
| USA | 146 |
| Western Europe | 200 |
| UK | 100 |
Treatment options for BCC include the following: surgical excision (with margins of normal tissue/excision under frozen section control/Mohs micrographic surgery in complex cases); curettage; cryosurgery; laser; radiotherapy; intralesional therapy; immunomodulation, where agents, such as imiquimod, are used to stimulate the immune system to eradicate the tumour; chemotherapy (topical 5-FU) and PDT.
Photodynamic therapy for the treatment of BCC is usually given to lesions prepared by preliminary surface curettage, although it may be given without preparation. A cream containing a photosensitising agent is applied to the area of the lesion and pre-defined margin, and a light-occlusive dressing is then applied for the incubation period. In some cases the photosensitiser may be given intravenously. Excess cream is removed and an appropriate wavelength of light used to activate the photosensitiser, resulting in tumour destruction.
The guidelines for topical PDT produced by the British Photodermatology Group emphasised that there are a number of possible light sources, and as yet disease-specific irradiance, wavelength and dose characteristics have not been agreed upon. 5 Experts suggest that the pre-illumination interval is likely to be disease dependent. Light sources for cutaneous PDT include lasers, xenon arc/discharge lamps and incandescent filament lamps; however, solid-state LEDs are now the most commonly used. The sources are usually aimed at producing the ‘red’ spectrum, around 630 nm, to maximise tissue penetration.
Early studies have indicated that photosensitiser absorption may vary between types of skin cancer, suggesting that effectiveness in one type, for example nodular, may not be a transferable finding to superficial BCC; this review therefore deals with nodular and superficial separately. 5
The key outcomes for PDT treating BCC are lesion clearance (partial or total), recurrence of lesions and cosmetic appearance. PDT may be considered where cosmetic outcomes are of a high priority and/or the lesion is too large for surgery. 12
Study characteristics
A total of 13 between-participant comparative RCTs were included, which reported on PDT for BCC (Table 8). All trials were curative in nature, and 11 were reported in full papers, the remaining two were available as single or multiple abstracts only. 77,78 Cosmetic outcomes were reported as investigator-assessed in most studies with patient-reported outcomes largely absent.
| Authors | No. of participants | Trial treatments |
|---|---|---|
| Superficial | ||
| Bassett-Seguin (2008)79 | 118 patients (219 lesions) | MAL–PDT vs cryotherapy |
| Szeimies (2008)80 | 196 patients | MAL–PDT vs excision surgery |
| Schleier (2007)81 | 24 patients (112 lesions) | ALA–PDT vs mALA–PDT |
| Soler (2000)82 | 83 patients (245 lesions) | Laser ALA–PDT vs broadband lamp ALA–PDT |
| de Haas (2006)83 | 154 patients (505 lesions) | Fractionated-illumination ALA–PDT vs single-illumination ALA–PDT |
| Nodular | ||
| Foley (2003)77 (abstract only) | 66 patients | MAL–PDT vs placebo cream PDT |
| Tope (2004)78 (abstract only) | 65 patients (80 lesions) | MAL–PDT vs placebo cream PDT |
| Kuijpers (2006)84 | 39 patients (43 lesions) | ALA–PDT vs MAL–PDT |
| Rhodes (2007)85 | 103 patients (118 lesions) | MAL–PDT vs excision surgery |
| Mosterd (2008)86 | 149 patients (173 lesions) | Fractionated-illumination ALA–PDT vs excision surgery |
| Berroeta (2007)87 | 31 patients (40 lesions) | ALA–PDT vs excision surgery |
| Superficial, nodular, BCC unspecified | ||
| Lui (2004)68 | (387 BCC lesions total) | Systemic PDT at 60 J/cm2 vs PDT at 120 J/cm2 vs PDT at 180 J/cm2 |
| Superficial and nodular reported together | ||
| Wang (2001)88 | 88 patients | ALA–PDT vs cryotherapy |
Five RCTs assessed PDT as a treatment for primary superficial BCC: one study compared different photosensitisers,81 two trials looked at different light sources,83,89 one study compared PDT with cryotherapy,79 and the final study compared MAL–PDT with surgery. 80 The following topical photosensitisers were used in these studies: 160 mg/g of methyl aminolevulinate (MAL), 10% or 20% aminolevulinic acid (ALA) cream, 10% methyl-aminolevulinic acid (mALA) cream. Photosensitising creams were applied for 3 hours prior to MAL–PDT and 3–6 hours for ALA–PDT; light dosage and sources varied.
Six RCTs reported on PDT for nodular BCC, four studies compared MAL–PDT with various other alternatives (placebo photosensitiser, ALA–PDT),77,78,84,90 and the remaining trials compared ALA–PDT with excision surgery. 87,91 In all studies the lesions were prepared with superficial curettage or debridement before application of the topical photosensitiser: 20% ALA cream or 160 mg/g of MAL cream. In some studies PDT was routinely repeated after 7 days, and in all but one trial PDT was repeated after 3 months if there was evidence of residual lesions. The drug to light interval was between 3 and 6 hours. Light was delivered at between 570 and 730 nm across the studies, most trials reported using a total light dose of 75 J/cm2, although Berroeta et al. 87 used 125 J/cm2. 87
Two further RCTs reported on mixed populations with nodular, superficial or non-specified BCCs. 68,88 One trial68 compared three different wavelengths of PDT reporting results according to BCC type; however, this older study was the only one to use intravenous verteporfin and the results were not considered to add significantly to the evidence base. The second study88 compared ALA–PDT with cryotherapy but did not report the results by BCC type, making it difficult to draw useful conclusions from the data. Full results for both studies are available in the relevant data extraction tables (Appendix 15).
Study quality
The trials on primary superficial BCC were difficult to assess as most publications did not provide detailed information on aspects of methodology that are used to assess quality, for example methods of randomisation, blinding and dropouts. One study was well conducted but questions about the implementation of the PDT treatment suggested that the results may not be reliable. 82 The trials comparing MAL–PDT with cryotherapy79 and surgery80 did not report power calculations, meaning that it is unclear if the studies were underpowered to have detected differences between treatments.
Of the six trials on nodular BCC, two were relatively small but appeared to be robust,84,87 two trials were reasonably sized, well reported and included longer-term follow-up,85,86 while the final two studies were difficult to assess based on the limited information provided. 77,78
The trial that compared different wavelengths in PDT on various types of BCC was poorly reported, little detail on the trial methods was given and the results appeared to be incomplete. 68 Wang et al. ’s trial88 was clearly reported and well conducted, suggesting that the results were likely to be reliable, although this was the only study that did not look at a particular subtype of BCC. A graph illustrating study quality is presented in Figure 9.
FIGURE 9.
Basal cell carcinoma study quality.
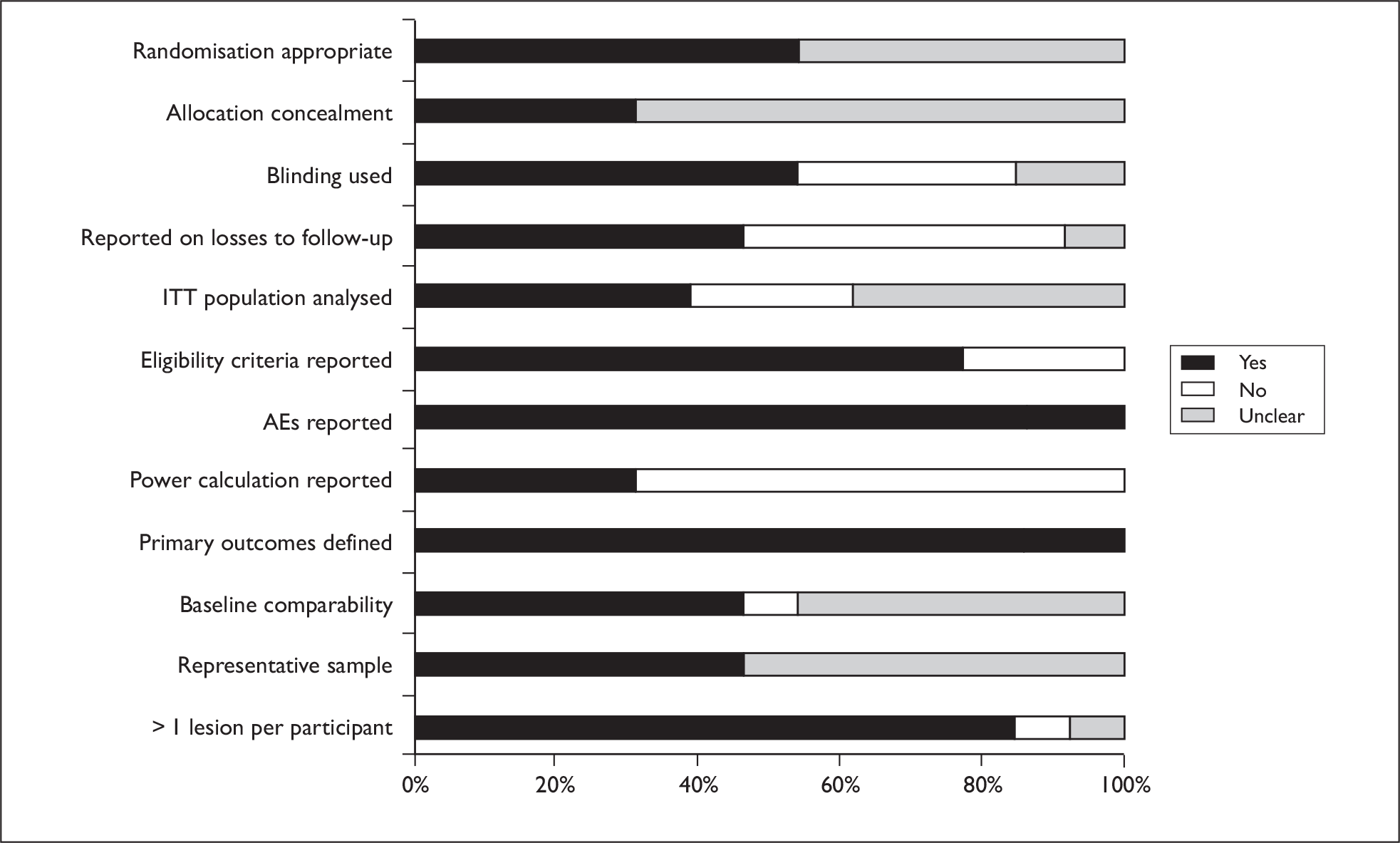
Results of effectiveness for superficial basal cell carcinoma
Results are presented in a narrative synthesis. Meta-analysis was not possible due to heterogeneity between the trials. Five RCTs (n = 575 patients, > 1000 lesions) compared types of PDT, PDT with cryotherapy or with surgery. None of the trials reported mortality or resource use data.
PDT vs cryotherapy
One RCT by Basset-Seguin et al. 79 compared MAL–PDT with double-freeze thaw cryotherapy with long-term follow-up. Data were reported at 3 months (n = 115 patients), 12 months (105 patients), 36 months (n = 107 patients) and 5 years.
Morbidity
There were no statistically significant differences in lesion recurrence or CR rates between the two treatments at any follow-up point.
Quality of life
PDT resulted in statistically better cosmetic appearance when compared with cryotherapy at both 3-month and 5-year follow-up points. The investigators reported an ‘excellent’ outcome for 30% of PDT patients at 3 months and 60% at 5 years, compared with 4% and 16% of cryotherapy patients, respectively (3-month p-value = 0.005, 5-year p-value = 0.00078).
PDT vs surgery
One RCT by Szeimies et al. 80 compared MAL–PDT with surgical excision (n = 196) and reported results from 12 months of follow-up.
Morbidity
Per-protocol analyses found similar lesion response rates for PDT and surgery, but recurrence in only the PDT arm (no statistical tests reported).
Quality of life
Cosmetic outcome was judged to be superior for patients receiving PDT by both patients and investigators, and this was reported as statistically significant (p-values not reported). Investigator assessments at 12 months were grouped such that a judgement of ‘success’ indicated cosmetic outcome across lesions was at least ‘good’. Overall, 92.8% of patients receiving PDT were considered a ‘success’ compared with 51.2% of surgery patients at 12 months (p < 0.001).
PDT with different treatment parameters
Morbidity
Schleier et al. 81 compared MAL–PDT versus ALA–PDT and recorded data for up to 6 months’ follow-up. No significant differences were found between groups for either partial success or CR in superficial BCC lesions.
De Haas et al. 83 compared fractionated ALA–PDT (laser, LED or broadband source) with single-illumination ALA–PDT. In the fractionated group, light was delivered on two occasions with 2 hours between treatments. CR rates of lesions were significantly higher in the fractionated group (p = 0.002) at 1-year follow-up; however, there were no differences between light sources noted.
Soler et al. 92 compared laser versus broadband illumination for ALA–PDT. Follow-up lasted a minimum of 6 months but was continued for patients with a CR. No significant differences were found between groups for complete, partial or non-response rates.
Quality of life
One study comparing laser versus broadband illumination PDT found no differences between the two groups;92 the other trials did not report this outcome.
These trials of PDT for superficial BCC appear to suggest that there may be few differences between light-delivery methods for ALA–PDT in terms of partial or complete lesion response rates. PDT may be no better or worse than cryotherapy or surgery at long- or short-term follow-up for lesion clearance rates; however, recurrence has not yet been fully explored. In terms of cosmesis, MAL and ALA–PDT may not differ in outcome. Cryotherapy and surgery may both result in poorer cosmetic outcomes when compared with PDT.
It is difficult to draw any definitive conclusions as the trials of cryotherapy and surgery versus PDT did not report power calculations, and it is unclear if they were suitably powered to show equivalence.
Results of effectiveness for nodular basal cell carcinoma
Results are presented in a narrative synthesis. Meta-analysis was not possible due to heterogeneity between the trials. No trials reported mortality or resource use data.
MAL–PDT vs placebo PDT
Two RCTs compared MAL–PDT versus placebo PDT (n = 131 patients). 77,78 Both trials used surface debridement prior to application of MAL or placebo creams and treatment was repeated after 7 days in both studies. Insufficient details regarding the intervention parameters were reported to establish if the treatment was similar. Follow-up appeared to last around 6 months.
Morbidity
Tope et al. 78 reported significant differences in favour of active PDT for complete clinical and histological responses. Foley et al. 77 found a significant difference in histological evaluations at 6 months; patients who received active PDT were found to have significantly fewer signs of malignancy.
Quality of life
Cosmetic outcome for the active PDT patients was reported as ‘excellent’ or ‘good’ by investigators for between 93% and 95% of patients in both trials, with one study reported similar results (90%) for the placebo group. 78
PDT vs excision surgery
Three RCTs compared PDT with excision surgery; the first87 looked at ALA–PDT versus surgery (n = 31 patients, 40 lesions); the second86 compared fractionated ALA–PDT with surgery (n = 149 patients, 173 lesions) and the third85 compared MAL–PDT versus surgery (n = 103 patients, 118 lesions). In all trials the lesion was prepared using superficial curettage before application of the photosensitiser in the PDT groups. It was not clear if patients received either one or two cycles of PDT. Each trial used illumination appropriate to the photosensitiser.
Morbidity
All three trials initially reported no significant differences between PDT and surgery at 3-month follow-up. Five-year follow-up data on MAL–PDT from one RCT reported CR rates were significantly better in the surgery group (76% for PDT vs 96% for surgery), while recurrence was more frequent in the PDT arm (14%) compared with surgery (4%) but this difference was not statistically significant. 85 Interim results from Mosterd et al. 86 at a median of 28 months’ follow-up reported significantly higher failure rates in the fractionated ALA–PDT arm (30.3%) than with surgery (2.3%) but final 5-year data are not yet available. Berroeta et al. 87 reported only 12-month follow-up data and found no significant difference in clearance rates between ALA–PDT and excision surgery in low-risk nodular BCC.
Quality of life
One trial85 reported patient-rated cosmetic outcome and found similar results for both PDT and surgery at 3 months and significant better results for PDT at 12 and 24 months. Berroeta et al. 87 reported collecting blinded cosmetic outcome data but results were not reported in the publications.
ALA–PDT vs MAL–PDT
One RCT84 compared ALA versus MAL–PDT (n = 39 patients, 43 lesions). This study used superficial curettage plus anaesthetic spray prior to application of the photosensitiser. The follow-up lasted 8 weeks.
Morbidity
Data were reported only for incomplete lesion clearance and rates did not significantly differ between treatment arms.
Quality of life
No QoL data were reported in this trial.
These trials suggest that MAL–PDT is superior to placebo PDT, but that PDT is less effective than surgical excision in terms of lesion clearance, although it may have a better cosmetic outcome. MAL–PDT may not offer any advantages over ALA–PDT; however, the trials to date have been relatively small and poorly reported. Further research to establish equivalence of treatments would require large well-designed RCTs.
Results of safety
No SAEs were reported by any trials, and pain was the most commonly reported AE in trials of superficial BCC. When comparing single illumination versus fractionated illumination, 27% of patients in the fractionated group required pain relief as opposed to 5% in the single group. 83 Comparing MAL–PDT and ALA–PDT – 8/13 and 5/11 patients, respectively – reported moderately painful sensations during treatment and two MAL–PDT patients received local anaesthetic. 81 Laser versus broadband ALA–PDT: 68% of laser and 74% of broadband lamp patients reported some degree of discomfort but this was not significantly different between the groups. 82
No serious or systemic AEs for PDT in nodular BCC were reported. The most common side effects were mild to moderate burning, stinging, erythema and pain. Where PDT was compared with a placebo, these effects occurred in both active and placebo groups. 77,78 Rhodes et al. 85 found that patients treated with PDT reported significantly more AEs than patients undergoing surgery, while Berroeta et al. 87 reported that patients treated with PDT experienced more pain than surgery patients (lidocaine was not used during treatment – confirmed by author). In one trial84 in which topical anaesthetic was administered during curettage and available after PDT treatment, there were no differences in pain ratings between MAL–PDT and ALA–PDT.
Most photosensitisers were applied topically therefore there were no problems with systemic sensitisation in these trials; however, only one trial mentioned precautions being taken subsequent to treatment (area was covered with an occlusive dressing for 1 day). In the one study that used an intravenous photosensitiser, photosensitivity was not recorded as an AE.
Overall, SAEs have not been reported and systemic photosensitisation is not a likely risk. The most commonly reported side effects were pain and discomfort during and shortly after light exposure. It was not clear in all studies if pain relieving medication was used, and in one trial the use of topical anaesthetic appeared to effectively reduce this pain.
Ongoing trials
We are aware of the following potentially ongoing/unpublished trials of PDT for the treatment of BCC but could not obtain any further information about them (Table 9). In addition we are aware of the following paper, which is in press, but not yet published when the report was written: Foley P. PDT with methyl aminolevulinate for primary nodular BCC: results of two randomized studies (accepted for publication in the International Journal of Dermatology).
| Name | Title | Start date | Completion date/status |
|---|---|---|---|
| Oseroff A | 4- to 5-hour vs 18- to 24-hour ALA–PDT (BCC and AK) | February 1997 | Recruiting |
| Kurwa H | PDT + Mohs microsurgery vs surgery alone | February 2006 | October 2007 – listed as completed |
| Foley P | MAL–PDT vs placebo | October 2000 | September 2002 – listed as completed |
| Neyndorff H | Verteporfin PDT vs placebo for multiple BCC | Terminated | |
| Tope W | MAL–PDT vs placebo | December 2000 | April 2002 – listed as completed |
| Roberts F | ALA–PDT – investigation for Bowen’s disease and AK, dose fractionation for BCC | February 2002 | February 2004 – listed as completed |
| Kelleners-Smeets N | PDT vs imiquimod vs 5-FU | March 2008 | March 2011 |
Discussion
We identified 13 unique RCTs of PDT for nodular and/or superficial BCC, which varied in sample size and methodological quality. Although not always clearly reported, it appears that in at least 10 trials multiple lesions per patient were included and analysed. As in other cancerous and pre-cancerous skin conditions, counting multiple patient lesions independently may have implications for the overall results. We did not locate any trials of PDT for morphoeic or pigmented BCC.
Of the five RCTs in superficial BCC, all used different treatment parameters and comparator arms. The limited evidence suggests that PDT may results in similar lesion response rates to surgery or cryotherapy with better cosmetic outcomes; however, these conclusions are tentative, as the trials do not appear to have been suitably powered to demonstrate equivalence. Further research is particularly needed to establish optimal treatment parameters for superficial BCC.
We identified three key RCTs comparing PDT for nodular BCC with surgery. Rhodes et al. 85 reported the longest follow-up data (5 years) for a sample of 103 patients, finding that surgery was superior to MAL–PDT for clearance rates but that PDT was significantly better for cosmetic outcomes. Interim results from a trial comparing fractionated ALA–PDT with surgery suggest that at 3 years’ treatment failure was more likely for patients receiving PDT, while a relatively small study of ALA–PDT found no difference between groups. 86,87 Overall, it appears that PDT may result in poorer long-term outcomes over 3–5 years, but cosmetic outcomes are significantly better. Therefore, there may still be scope to explore the optimal PDT regime and clarify which patients and/or lesion type will respond best, while balancing clearance or recurrence with cosmetic outcomes.
This is an active area of research, although it is interesting to note that of the ongoing or currently unpublished trials from which we were unable to obtain data, three are placebo-controlled studies, which seem less likely to add to the evidence base. The most important trials are likely to be those that compare PDT with a viable alternative such as imiquimod, 5-FU or surgery.
Chapter 7 Barrett’s oesophagus
Background
Barrett’s oesophagus is caused by the backwash of stomach acid and bile into the oesophagus, known as gastroesophageal reflux disease (GORD), which damages the normal lining. In about 10% of patients with GORD the injured lining of the oesophagus does not grow back but it is replaced by a new abnormal lining (specialised intestinal metaplasia). The Barrett’s lining begins at the bottom of the oesophagus, where the oesophagus lines the stomach and extends upwards towards the mouth. Barrett’s linings may be short (less than 3 cm) or long (3 cm or greater in length). Most people with Barrett’s oesophagus also have a hiatal hernia. However, most people who have a hiatal hernia do not have Barrett’s oesophagus. It is not known why only certain people go on to develop Barrett’s oesophagus. However, it is known that men are more at risk than women, and older age, and possibly obesity, is a risk factor. 93
Barrett’s oesophagus is confirmed in a procedure known as upper gastrointestinal endoscopy complete with biopsy. If it is confirmed, a patient will need to undergo endoscopic biopsy surveillance to determine the grade of dysplasia (abnormal changes in cells or their growth patterns). Dysplasia is normally graded from negative, indefinite, low grade up to high grade. Those with high-grade dysplasia (HGD) are at the most increased risk of developing oesophageal adenocarcinoma. Although the majority of patients with Barrett’s oesophagus do not develop cancer during long-term follow-up they are at increased risk when compared with the general population. 93
Treatment for Barrett’s oesophagus aims to control symptoms and repair oesophageal injury. Treatment with acid-suppressive treatment [e.g. proton pump inhibitors (PPIs)], antireflux surgery and lifestyle changes may be advised. Ongoing surveillance aims to detect progression to HGD and adenocarcinoma. Endoscopic therapies, such as endoscopic mucosal resection and PDT, may be offered to a patient with HGD but ongoing surveillance will still be used. Multipolar electrocoagulation (MPEC) may also be used. Radiofrequency ablation is offered in a few specialist centres. Oesophagectomy (removal of the oesophagus) is normally performed only in patients with HGD; chemotherapy and radiotherapy may be used in conjunction with this surgical procedure.
Photodynamic therapy is used as a first-line treatment for patients with HGD in Barrett’s oesophagus. 94 PDT can be used alone or in combination with a range of other therapies. The photosensitisers that have been used are Ps (Photofrin), meta-(tetrahydroxyphenyl) chlorine (mTHPC) [temoporfin (Foscan)] and 5-aminolevulinic acid (5-ALA); Photofrin and Foscan are given intravenously, and 5-ALA is given orally.
Study characteristics
Eleven RCTs investigated PDT for Barrett’s oesophagus with a total number of 594 patients (Table 10). Seven trials were published as full papers,95,97–100,102,105 and four as abstracts only. 96,101,103,104 PDT was compared with different treatments in five trials (APC in four and omeprazole alone in one). 97–101 Different methods of delivering PDT were explored in six trials,96,97,102–105 and PDT was compared with placebo in two trials. 95,96 (The numbers of trials do not add up to 11 due to some trials having three arms.) ALA was used as the photosensitiser in the majority of trials and was compared to Ps in one trial. 104
| Authors | No. of participants | Trial treatments | Dysplasia |
|---|---|---|---|
| PDT vs placebo PDT | |||
| Ackroyd et al. (2000)95 | 36 | ALA–PDT vs placebo PDT | LGD |
| Ackroyd et al. (1996)96 (abstract only) | 28 | ALA–PDT 30 mg/kg vs ALA–PDT 50 mg/kg vs placebo | Dysplasia (not specified) |
| PDT vs other treatments | |||
| Hage et al. (2004)97 | 40 | ALA- PDT with fractionated dose vs ALA–PDT single dose vs APC | Without dysplasia or with LGD |
| Kelty et al. (2004)98 | 72 | ALA–PDT vs APC | Without dysplasia |
| Overholt et al. (2007)99 | 208 | PDT with Ps and omeprazole vs omeprazole alone | HGD |
| Ragunath et al. (2005)100 | 26 | PDT with Ps vs APC | LGD or HGD |
| Zoepf et al. (2003)101 (abstract only) | 20 | ALA–PDT vs APC | Mixed |
| PDT delivery comparisons | |||
| Kelty et al. (2004)102 | 25 | ALA–PDT at 30 mg/kg or 60 mg/kg at 4- or 6-hour incubation times or with fractionated illumination | Without dysplasia |
| Mackenzie et al. (2007)103 (abstract only) | 72 | ALA–PDT with varying doses of light and comparing red or green light | HGD |
| Mackenzie et al. (2008)104 (abstract only) | 40 | ALA–PDT vs PDT with Ps | HGD |
| Mackenzie et al. (2008)105 | 27 | ALA–PDT with red light vs ALA with green light at 30 or 60 mg/kg | HGD |
Four trials specifically focused on patients with HGD,99,103–105 one trial100 included patients with LGD or HGD, one focused on LGD,95 one on low-grade or without dysplasia,97 two without dysplasia,98,102 one was mixed,101 and in one dysplasia was not specified. 96
Study quality
Eight out of the 11 RCTs were published within the last 5 years. However, the RCTs tended to be small, with the majority having fewer than 50 patients. These smaller trials are likely to have been underpowered to detect effects for all outcomes under investigation. The largest, well-conducted trial was conducted in 30 centres across four countries, raising the possibility of institutional differences and protocol deviations. 99 The majority of trials did not clearly report study methods. Procedures for randomisation, allocation concealment and blinding of outcome assessors were not always clear, making it difficult to assess the reliability of the results. The major reporting problem was that several trials were only available as abstracts, making an assessment of their quality and reliability challenging. A graph illustrating study quality is presented in Figure 10.
FIGURE 10.
Barrett’s oesophagus study quality.
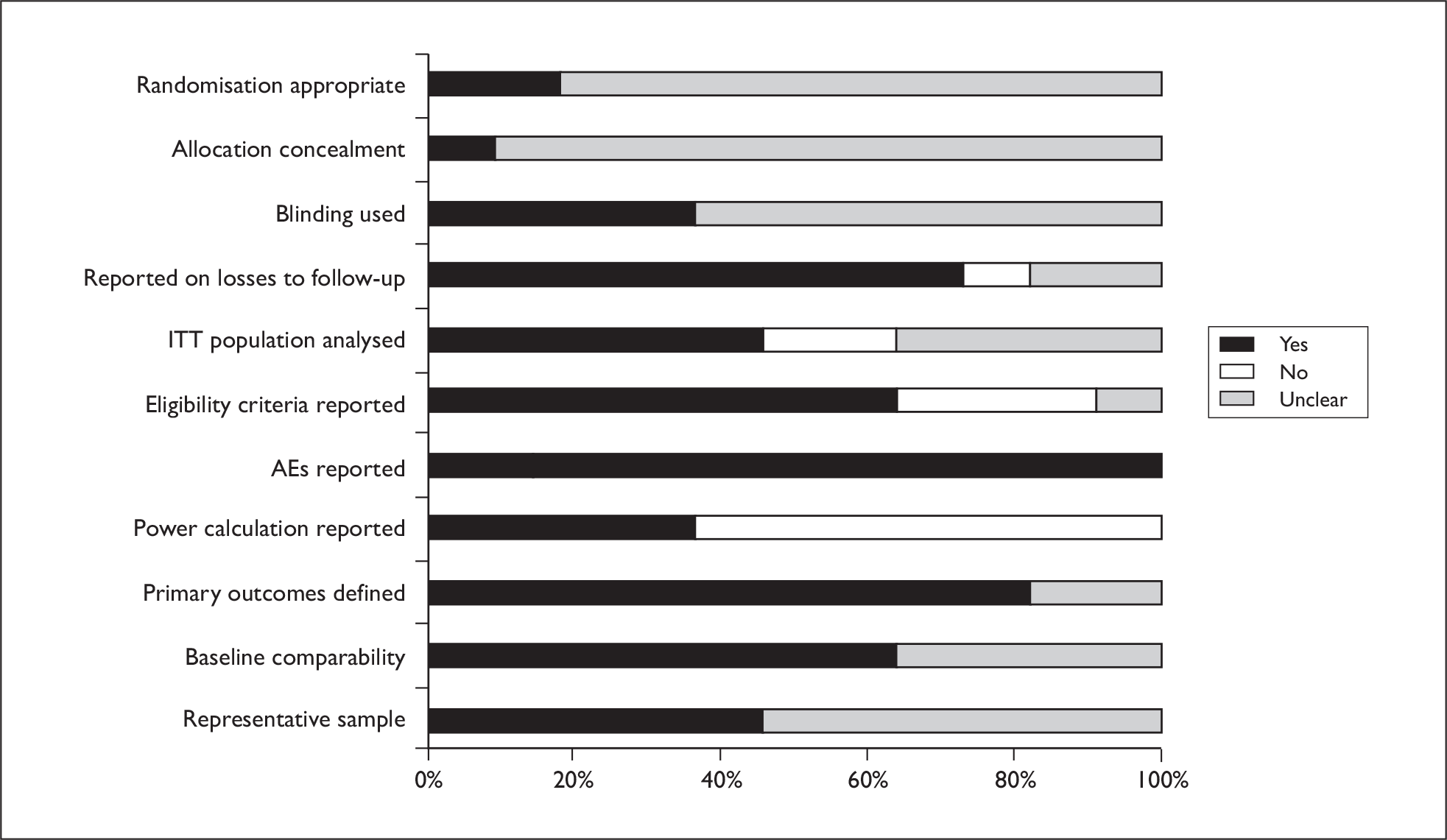
Results of effectiveness
Results are presented in a narrative synthesis. Meta-analysis was not possible due to heterogeneity between the trials. None of the trials considered QoL outcomes. One trial investigated mortality,99 and one considered resource use. 100 Outcomes are mainly related to morbidity and AEs.
PDT vs APC
Four trials compared PDT to APC. 97,98,100,101 Three trials used ALA as a photosensitiser and one used Ps. 100 One trial was of patients without dysplasia,98 one without or with LGD,97 one was LGD or HGD,100 and one was not stated. 101
Morbidity
In a trial of patients without dysplasia or with LGD, both the group receiving fractionated-dose PDT with ALA and the group receiving APC had statistically significantly better results in terms of Barrett’s oesophagus surface reduction than the group receiving single-dose PDT. 97 Differences between fractionated-dose PDT and APC were not significant. Rates of complete ablation were not significant between the groups. In a trial in patients without dysplasia, treatment led to complete reversal of the columnar segment to squamous epithelium in 50% of patients receiving ALA–PDT and 97% of patients receiving APC (p < 0.0001). 98 In a trial with patients with mixed levels of dysplasia, reported in abstract only, reduction of length was 90% for those undergoing ALA–PDT treatment and those receiving APC but fewer treatments were used for APC. 101
The final trial compared Ps to APC in a group of 26 patients with LGD or HGD. 100 Dysplasia eradication was statistically significantly better at 4 months, but not at 12 months, with PDT. This was a small trial that may have been underpowered to detect treatment effects for all outcomes.
Resource use
The small UK trial described above was accompanied by a cost-effectiveness analysis. 100 The incremental cost-effectiveness ratios (ICERs) were calculated based on differences in effects and costs between PDT with Ps and APC for Barrett’s oesophagus length eradication and dysplasia eradication. At 4 months, APC was less expensive and more effective. At 12 months, the ICER was £266; an additional £266 would be required for every percentage reduction in Barrett’s oesophagus using PDT compared with APC.
From this small body of evidence it is not possible to conclude whether PDT is superior to, equivalent to or inferior to APC. Nor is it possible to state with confidence which treatment (if any) would be most appropriate for the various levels of dysplasia.
PDT plus omeprazole vs omeprazole alone
One trial compared PDT with Ps plus omeprazole (PHOPDT) versus omeprazole alone (OM). 99 This was the largest trial for Barrett’s oesophagus (208 patients), although the 3- to 5-year follow-up phase of the trial had just 61 participants. All patients had HGD. The results of this multicentre trial were considered to be reliable but an unknown factor is the influence of any between-centre differences on the results found.
Mortality
Two patients in the PHOPDT and one in the OM group died within the first 2 years from events unrelated to Barrett’s disease. There were no additional patients who died during the 3-year follow-up period.
Morbidity
The proportion of responders (complete ablation of HGD) was significantly higher in the PHOPDT group than with OM (77% vs 39%, p < 0.0001). By the end of the 5-year follow-up period, the probability of maintaining complete ablation of HGD was 48% in PHOPDT compared with 4% in OM (p < 0.0001). The median duration of the CR was 44.8 months in the PHOPDT group and 3.2 months in the OM group. Comparison between the two groups showed that patients in the PHOPDT group had a significant delay in progression to cancer compared with patients in the OM group. After 5 years of follow-up, the rate of patients who progressed to cancer in PHOPDT was significantly lower than in OM (p = 0.027).
On the basis of one generally well-conducted trial, it appears that PDT with Ps in addition to omeprazole is more effective than omeprazole alone at producing long-term ablation of HGD and slowing/preventing progression to cancer.
PDT vs placebo
Two trials compared PDT with placebo,95,96 one of which was reported as abstract only and did not provide any effectiveness outcomes and is therefore not discussed here. 96
Morbidity
In a trial in patients with LGD, a statistically significantly larger proportion of the ALA–PDT group showed evidence of regression (89% vs 11%) and reduction in Barrett’s (30% vs 0%) than in the placebo group. 95 There was also a statistically significant reduction in prevalence of dysplasia in favour of the PDT group.
On the basis of one small trial, ALA–PDT appears to be more effective than placebo in patients with low-grade dysplasia. However, it should be noted that all patients were taking omeprazole so the evidence could be interpreted as PDT being more effective than omeprazole alone, as stated above.
ALA–PDT vs PDT with Ps
One trial compared ALA–PDT to Ps in patients with HGD but was reported in abstract only. 104 The trial reported preliminary data only, as recruitment is not yet complete.
Morbidity
Remission rates were statistically significantly superior in the ALA–PDT group than with the Ps group (100% vs 64%, p < 0.05).
The results of this ongoing trial suggest that ALA–PDT may be more effective than Ps, but conclusions cannot be drawn until all the planned patients have been treated and followed up for a longer period of time.
PDT with different treatment parameters
Five trials compared PDT of varying parameters. 96,97,102,103,105 One trial, as previously mentioned in the placebo group, was reported as abstract only and did not have any effectiveness outcomes and so is not discussed here. 96 One trial has already been discussed in the PDT vs APC section but a reminder of the findings is provided here. 97
Two of the three additional trials to be discussed in this section were reported as a full paper,102,105 while the other was reported as an abstract only. 103 Patients had HGD in the trial published as an abstract103 and in one of those reported as a full publication. 105 In the other full publication patients did not have dysplasia. 102 ALA was used as a photosensitiser in all three trials.
Morbidity
One trial found that in patients with no dysplasia the greatest reductions in Barrett’s epithelium were seen in 30-mg/kg and fractionated groups but results were not statistically significant. However, each treatment group had just five patients so is unlikely to be able to detect all treatment differences where they exist. 102 In a trial of patients without dysplasia or with LGD previously discussed in the APC section, both the group receiving fractionated dose PDT with ALA and the group receiving APC had a statistically significantly better results in terms of Barrett’s oesophagus surface reduction than the group receiving single-dose PDT. 97
One trial found that patients with HGD receiving high-dose ALA–PDT (60 mg/kg) and high-dose red light (1000 J/cm) had a significant decrease in cancer risk compared with treatment groups with lower doses of photosensitiser and/or lower light doses at 36 months (3% risk vs 24% risk). Red light was associated with lower rates of adenocarcinoma than green light (8% vs 45%, p < 0.05). 103 In the other trial, 60-mg ALA red light was more successful than 30-mg ALA red light (p = 0.03) and than 30-mg ALA green light (p = 0.005). 105
Based on the trials in this section, optimal parameters for PDT in patients without dysplasia are unknown. In HGD, according to two small trials, it appears that high-dose ALA–PDT may be more effective. Higher dose light may be more effective but this is based on one trial. The optimal parameters for PDT in HGD remain to be determined.
Results of safety
All trials of PDT and Barrett’s oesophagus reported AEs, albeit briefly. AEs are detailed separately for ALA and Ps and for the trial comparing the two. 104
ALA–PDT
Eight trials, comparing ALA with APC or placebo or ALA of various treatment parameters, provided information on AEs. 95–98,101–103,105
Serious AEs have not been reported in this group of studies. Specifically, it was reported that no major side effects in terms of perforations or strictures occurred in two trials. 98,102 No patients developed strictures in one trial comparing various ALA regimens103 and differences in stricture formation were not significant between PDT and APC groups in a further trial. 97 In a small trial comparing ALA with red or green light, there were no major complications. 105 Differences in fever and sudden death were not significant between PDT and APC groups in one trial. 97
Adverse effects were mainly short term. In one trial all patients receiving PDT experienced chest pain during treatment, which persisted for 3–5 days and was aggravated by swallowing and coughing. 95 In a further trial 23 of 26 patients receiving PDT and 5 out of 14 patients receiving APC experienced pain during treatment (p < 0.01). 97 Pain was not specifically mentioned in the other trials.
In two trials, PDT was found to result in more nausea and vomiting than APC. There were seven versus zero cases (p < 0.05) in one trial. 97 All patients in the ALA arm of a trial comparing ALA at a dose of 60 mg/kg to APC developed nausea and vomiting over a period of 4 hours after treatment, whereas there were no cases of vomiting in the APC group. 101 In a further trial comparing different modes of ALA, significant nausea and vomiting occurred in 32% of patients receiving ALA, and was more common in patients who received the higher dose of ALA. 102
Photosensitivity did not appear to be a significant problem in the trials in which this was reported. In two trials, one comparing PDT with APC and one testing various ALA treatment parameters, no patients suffered photosensitivity reactions. 101,103 In other trials small numbers experienced photosensitivity, which, where stated, tended to be mild and resolve fairly quickly. 95,96 Numbers were slightly higher in two further trials; 5 out of 25 patients in one trial102 and 5 out of 35 in another. 98
Dysphagia was not found to be a problem with PDT in the trials that specifically mentioned this. 95 In two trials, instances of dysphagia were not significantly different between the PDT and APC groups. 97,101 In one trial that specifically mentioned this, instances of odynophagia were not significantly different between PDT and APC groups. 101
Three trials suggested some disturbance in liver function tests. 96,97,105
Porfimer sodium
Two trials, one comparing Ps with APC and the other comparing PDT with Ps and omeprazole (PHOPDT) to omeprazole alone (OM) provided information on AEs. 99,100
In the omeprazole trial, events of severe intensity were similar for PHOPDT (16%) and OM (15%), with 65% of the PHOPDT group being related to the treatment compared with 2% in the OM group. 99 From years 2 to 5 in the trial there were no SAEs and, of those AEs reported, none was attributed to the treatments. The trial found that 36% of PDT patients developed oesophageal strictures, but that 94% of those with strictures were stricture free in the initial phase of the trial. 99 In the trial comparing PDT with APC, two of 13 patients in both groups developed oesophageal strictures. 100
In one trial, photosensitivity occurred in 69% of patients receiving PDT. 99 All photosensitivity events were resolved. In another trial 31% experienced photosensitivity. 100
ALA vs Ps
One trial of ALA versus Ps provided a direct comparison of the AEs of the two photosensitisers. 104 There was also a statistically significant difference in the development of strictures (6 out of 16 patients treated with Ps and 1 out of 16 treated with ALA). 104
There was a statistically significant difference in photosensitivity [7 out of 16 patients treated with Ps (one of who had to be admitted to hospital) vs no cases with ALA]. There were no other significant differences between groups regarding side effects.
In general, SAEs have not been reported for PDT in the treatment of Barrett’s oesophagus. However, trials are mainly small and rarer AEs might not, therefore, be observed. There may be differences in the rates of stricture between PDT and other treatments such as APC. However, the evidence suggests, but does not confirm, that this is more of a problem when using Ps. Pain particularly during treatment was not always reported so it is unclear if there are differences in pain between PDT and other treatments, and how this might differ when PDT is delivered using different parameters. Nausea and vomiting appeared to be problematic with ALA but may relate to dose of ALA delivered. This outcome was not evaluated for Ps. Any effects on liver function merit further investigation. Dysphagia did not appear to be more problematic for PDT than APC in the ALA trials that investigated this. Photosensitivity either did not occur or was relatively mild in the ALA trials. The two Ps trials reported higher levels of photosensitivity than the ALA trials and this finding was supported by the trial comparing the two treatments.
Ongoing trials
The following trial is ongoing and at the time of writing of this report had no detailed results to report (Table 11).
| Investigator | Interventions | Start date | Status |
|---|---|---|---|
| Lovat L | ALA–PDT vs Ps PDT to study the side effect profile and to establish measures of efficacy in the eradication of dysplasia in Barrett’s oesophagus | February 2006 | Expected end February 2009 – but authors stated that the trial was ongoing; 55 out of 66 patients were recruited by January 2009 |
We are aware of the following potentially ongoing/unpublished trials of PDT for Barrett’s disease but could not obtain any further information about them (Table 12).
| Investigator | Interventions | Start date | Status |
|---|---|---|---|
| Nava H | PDT in two light regimes for HGD and early cancer | February 2004 | Suspended, no reply to e-mail |
| Reed M | ALA–PDT (green light) vs placebo (all patients to take omeprazole) | April 1995 | Finished March 1996, no reply to e-mail |
| Wang K | Mucosal resection vs resection + PDT | September 2005 | Recruiting, no reply to e-mail |
Discussion
We concentrated on RCTs for the treatment of Barrett’s oesophagus, as a large number appeared to be available. On closer inspection, we found that many publications related to the same trials. The 11 RCTs were published in 24 publications. Additionally, the evidence presented is diverse, with variation in PDT parameters including photosensitisers, comparators and patient level of dysplasia. A further barrier to drawing firm conclusions is that the majority of the trials are small, with methodological limitations.
Nevertheless, it appears from the evidence provided that PDT might be beneficial above and beyond PPIs alone. However, its relative effectiveness is unclear compared with APC and other treatment options as yet not evaluated in trials. The relative benefits and AEs of Ps versus ALA also need further research and there is an ongoing trial in this area.
A number of trials were conducted in patient groups with no or with LGD. However, these patients are unlikely to be treated in routine practice. The priority for Barrett’s oesophagus would seem to be to determine more clearly the role of PDT and its optimal delivery to patients with HGD.
Chapter 8 Oesophageal cancer
Background
A recent review highlighted the increasing incidence of oesophageal cancer over the last 30 years in the UK. 94 It now affects around 7800 people each year in the UK. The disease is more common in men than women, and most cases are in people aged 50 years and over. 106 Depth of penetration of the tumour determines tumour stage. Tumours that are superficial or have penetrated only the submucosa are defined as early-stage cancer. The two most common types of oesophageal cancer are SCCs and adenocarcinoma, described as being strongly associated with Barrett’s oesophagus.
The prognosis for oesophageal cancer is not encouraging. Five-year survival rates for all patients diagnosed with oesophageal cancer in 2000–1 in England and Wales were 8% for both men and women. 106 Endoscopic therapies such as endoscopic mucosal resection and PDT may be offered to a patient with early cancer but ongoing surveillance will still be used. MPEC may also be used. Oesophagectomy (removal of the oesophagus) may be performed, and chemotherapy and radiotherapy may be used.
If cancer has spread or the cancer is otherwise unsuitable for surgery, palliative therapy may be offered. Options include stents to stretch the oesophagus narrowed by the tumour, ablative therapy, or possibly radiation and chemotherapy to shrink the tumour and improve symptoms, such as dysphagia, and hence maintain QoL.
Photodynamic therapy was first introduced as a palliative treatment for oesophageal cancer but it is now also used as a first-line treatment for patients with early oesophageal cancer. 94 The treatment objective in early-stage oesophageal cancer is cure, whereas in advanced cancer it continues to be a palliative option. PDT can be used alone or in combination with a range of other therapies for curative and palliative purposes.
Study characteristics
A total of thirteen studies were included, which reported on PDT in the treatment of oesophageal cancer (Table 13). 107–119 Three studies were available only as abstracts,107,110,111 the rest were published as full papers. In a small number of trials, the presented data did not appear to entirely support the authors’ conclusions; this has been indicated in the data extraction tables. 110,111,113
| Authors | Study design | No. of participants | Trial treatments |
|---|---|---|---|
| Curative | |||
| Canto et al. (2005)107 (abstract only) | Non-RCT | 80 | Ps-PDT vs Ps-PDT + EMR |
| Lecleire et al. (2008)111 (abstract only) | Non-RCT | 35 (37 tumours) | Primary Ps-PDT vs Ps-PDT following CRT |
| Grosjean et al. (1997)108 | Non-RCT | 15 (22 tumours) | Ps-PDT (630 nm vs 514 nm) |
| Scotiniotis et al. (2000)110 (abstract only) | Non-RCT | 37 | PDT vs EMR vs oesophagectomy |
| Savary et al. (1998)109 | Non-RCT | 24 (31 tumours) | HpD-PDT vs Ps-PDT vs mTHPC (various doses) |
| Palliative | |||
| Lightdale et al. (1995)112 | RCT | 236 | Ps-PDT vs Nd:YAG laser |
| Heier et al. (1995)114 | RCT | 42 | HpD-PDT vs Nd:YAG laser |
| Zhang et al. (2003)119 | RCT | 60 | HpD-PDT + radiotherapy vs radiotherapy alone |
| Maier et al. (2000)117 | Non-RCT | 119 | HpD-PDT + brachytherapy vs brachytherapy |
| Zhang et al. (2007)113 | RCT | 140 | PsD-007 PDT vs PsD-007 + PDT + 5-FU |
| Maier et al. (2000)115 | Non-RCT | 52 | HpD-PDT vs HpD-PDT + HBO |
| Maier et al. (2000)116 | Non-RCT | 75 | HpD-PDT vs HpD-PDT + HBO |
| Maier et al. (2001)118 | Non-RCT | 49 | ALA–PDT (+ HBO) vs HpD-PDT (+ HBO) |
Five non-randomised trials focused on curative PDT for early or superficial oesophageal cancers. 107–111 In some studies the patients had received prior therapies, such as radiotherapy or surgery, and the cancer types varied (adenocarcinoma, squamous, oesophagogastric). A range of photosensitisers were used across the studies: Ps, HpD, mTHPC, although one did not report this detail. Three studies compared variants of PDT,107–109 one compared primary and secondary PDT following chemoradiotherapy,111 and the final study compared PDT with two different surgical procedures. 110
Eight studies reported on palliative PDT treatments for oesophageal cancer, four RCTs112–114,119 and four non-randomised comparative trials. 115–118 Participants in six studies were diagnosed with advanced oesophageal cancer, and overall most trials included only patients who were not eligible for resection due to anatomical restrictions, poor health or refusal. Comparators in these trials included chemotherapy, radiotherapy, neodymium-doped yttrium aluminium garnet (Nd:YAG) laser or PDT. Photosensitisers used were Ps, HpD, PsD-007 and ALA.
Study quality
The non-randomised curative studies were generally poorly reported. Of the five trials only three were available as full published papers, making it difficult to assess quality based solely on a brief abstract. As is common with non-randomised studies, most of these appeared to display important baseline differences between comparison groups, making it difficult to ascribe any differences in outcome to the intervention. Sample sizes were small overall, ranging from 15 to 80, although in some patients more than one tumour was treated and counted. Most trials did not report if they were single or multicentre. All studies reported on lesion clearance rates and AEs although assessors were often not reported as blinded, and in some reports no statistical tests appeared to have been carried out.
All eight palliative trials were available as full published papers; however, the standard of reporting was variable. The four RCTs were generally better reported, though few studies used blinded outcome assessors or ITT analyses. Samples sizes ranged from 49 to 119 in the non-randomised trials and from 42 to 236 in the RCTs. At least two studies used more than one recruiting centre but this aspect was poorly reported. All trials reported on AEs, all but one trial reported mean survival time, and morbidity outcomes included stenosis length, dysphagia score and tumour length. Some trials also reported Karnofsky performance status. A graph illustrating study quality is presented in Figure 11.
FIGURE 11.
Oesophageal study quality.
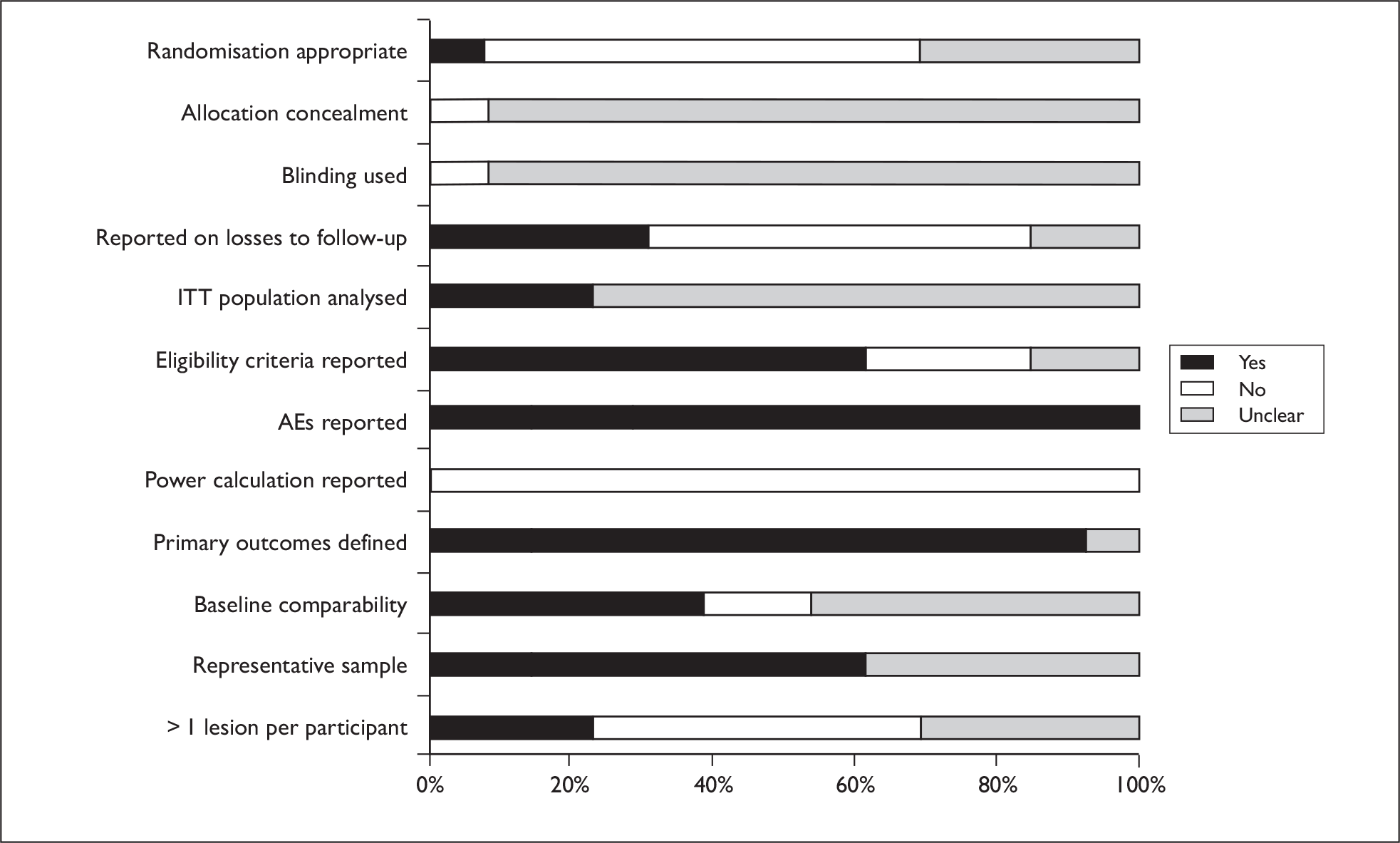
Results of effectiveness (curative intent)
Results are presented in a narrative synthesis. Meta-analysis was not possible due to heterogeneity between the trials. Outcomes reported mainly related to morbidity and AEs. Only one trial reported mortality107 and no trials reported on QoL or resource use outcomes.
PDT vs PDT (curative intent)
Two non-randomised trials have compared different kinds of PDT for early or superficial oesophageal cancer. Grosjean et al. 108 used Ps as the photosensitiser and compared two wavelengths (n = 15 patients, 22 tumours) of light. In this study, patients with both oesophageal and bronchial tumours were included; however, the majority (14/22) were oesophageal. Savary et al. 109 compared Ps, HpD and three different dosages of mTHPC using varying light doses (n = 24 patients, 31 tumours).
Morbidity
Grosjean et al. 108 reported that both 630nm (69% CR) and 514nm (67% CR) were suitable wavelengths to cure superficial oesophageal cancer. Savary et al. 109 reported overall response and failure rates according to photosensitiser: HpD CR = 89%, mTHPC CR = 86%, Ps CR = 75%.
Using 514-nm green-light illumination reduced deep tissue damage in both trials. 108,109
Two small non-randomised trials suggest that 514 nm may be the preferred wavelength for treating early or superficial oesophageal cancer when using either Ps or mTHPC. These findings are drawn from poorly reported studies, which are likely to be underpowered, and so the results should not be considered conclusive.
PDT vs CRT or EMR followed by PDT (curative intent)
Two non-randomised trials have examined primary PDT versus secondary PDT where another treatment is given initially. One trial looked at Ps-PDT versus endoscopic mucosal resection (EMR) followed by Ps-PDT (n = 80 patients),107 the second used PDT versus PDT following unsuccessful chemoradiotherapy (CRT) (n = 35 patients, 37 tumours). 111 Both of these trials were available only as abstracts and further treatment details were not reported.
Mortality
Canto et al. 107 reported that disease-specific 5-year survival was 100%.
Morbidity
No significant differences were reported between CR rates for PDT (89.7%) and EMR + PDT (91.2%) (p = 0.67). 107 There were also no significant differences in number of lesions treated successfully when PDT (73%) was compared with PDT following unsuccessful CRT (53%) (p = 0.3). 111
Two small non-randomised trials found no differences between primary PDT treatment and PDT following either EMR or unsuccessful CRT. These results are based on small samples of unclear methodological quality.
PDT vs EMR vs oesophagectomy (curative intent)
One non-randomised trial (n = 37 patients) compared PDT or EMR in poor surgical candidates with oesophagectomy in good surgical candidates in a three-armed design. 110 Only a short abstract was available and no details were reported for any of the interventions.
Morbidity
Eradication of lesions was reported for each study arm: 75% for PDT, 83% for EMR and 95% for oesophagectomy. No statistical tests were reported.
It is not possible to draw any conclusions about the relative effects of PDT, EMR or oesophagectomy from this single trial.
Results of effectiveness (palliative intent)
Results are presented in a narrative synthesis. Meta-analysis was not possible due to heterogeneity between the trials. All trials reported morbidity results, seven reported on mortality113–119 and four reported on QoL outcomes. 115–118 Only two of the studies reported resource use, both in terms of days of hospitalisation. 116,118
PDT vs Nd:YAG laser
Two relatively old RCTs compared PDT with Nd:YAG laser for oesophageal carcinoma in patients who had refused, failed or were not suitable for surgery, and who had a Karnofsky performance status of > 30%. One trial used Ps as the photosensitiser (n = 236)112 and the second used HpD (n = 42). 114 Both trials were reported as full papers, and were generally well conducted and reported.
Mortality
This outcome was only assessed by Heier et al. ,114 who reported no significant difference in mean survival between HpD-PDT (145 days) and Nd:YAG (128 days) laser treatments (p = 0.419).
Morbidity
Heier et al. 114 reported significant increases in oesophageal grade for the PDT group, while Lightdale et al. 112 found no significant differences in dysphagia grade or response rates at 1 week but did report a significant benefit in favour of PDT at 1 month.
Quality of life
Heier et al. 114 found that patients receiving PDT were judged to have significantly greater improvements in dietary performance and Karnofsky performance status. Lightdale et al. 112 did not report on this outcome.
One well-conducted, but small, RCT suggests that PDT with HpD may not be different from Nd:YAG laser in terms of mortality. PDT may also be equivalent to or better than Nd:YAG laser in terms of morbidity and QoL. One study was small and neither study reported a power calculation thus we cannot rule out the possibility that relevant differences were not detected.
PDT vs radiotherapy
One RCT and one non-randomised trial have compared HpD-PDT plus radiotherapy versus radiotherapy alone. The RCT used standard radiotherapy of 40 grays (Gy) for 4 weeks,119 while the non-randomised trial used brachytherapy and 5 Gy per session for one to four sessions. 117 In this second study, patients who were judged to be in ‘fair condition’ completed their radiotherapy via external-beam irradiation (57% of the combination group and 23% of the brachytherapy-alone group).
Mortality
The RCT by Zhang et al. 119 showed longer survival rates at 5 years for PDT plus radiotherapy over radiotherapy alone (29.9% vs 16.7%, p = 0.05), and again significantly longer for 10-year survival rates (16.7% vs 10.0%, p < 0.05).
Maier et al. 117 reported mean survival for brachytherapy as 5.6 months, for brachytherapy and external beam irradiation as 7.7 months, for PDT plus brachytherapy as 6.3 months and for PDT plus brachytherapy plus external-beam irradiation as 13.0 months.
Morbidity
Maier et al. 117 found significantly greater improvements for the PDT plus brachytherapy group versus brachytherapy alone for both dysphagia scores (p = 0.003) and mean stenosis size (p = 0.001).
Quality of life
Only Maier et al. 117 reported this outcome and found no difference in 3-month Karnofsky scores.
Two studies suggest that adding PDT to radiotherapy may be beneficial; however, the RCT was poorly reported and difficult to assess for quality, while the non-randomised study used two different forms of radiotherapy and pre-treated some patients with Nd:YAG laser. Firm conclusions cannot be drawn from these results.
PDT vs PDT plus 5-FU
One RCT compared PDT alone with PDT following 5-FU (n = 140). 113 This study was poorly reported overall, but the paper reports that after treating over 40 patients and reviewing the clinical data the randomisation was abandoned and all further patients were treated with the combination of PDT and 5-FU (no further details reported).
Mortality
Combined treatment resulted in significantly longer mean survival times for the PDT plus 5-FU group (15.1 months) than with PDT alone (8.9 months) (p < 0.01).
Morbidity
Combined treatment produced significantly greater rates of dysphagia remission (99%) than with PDT alone (87%) (p < 0.05); however, there were no significant differences in pharyngeal pain or weight loss between groups.
The evidence from this single study is difficult to assess, and so no firm conclusions can be drawn.
PDT and HBO
Two non-randomised trials have compared PDT alone versus PDT plus hyperbaric oxygen (HBO) (total n = 107)115,116 and a third non-randomised trial has compared two types of photosensitiser (HpD vs ALA), where both were given under HBO. 118 All three studies were carried out with patients who had advanced oesophageal carcinoma. The PDT versus PDT-plus-HBO studies both used HpD at 2 mg/kg and 630-nm illumination, and in both some patients received Nd:YAG and dilatation prior to PDT.
Mortality
Patients receiving PDT plus HBO were reported to have significantly longer median survival times (13.8 months and 12 months) than patients receiving PDT without HBO (8.7 months and 7 months). 115,116
Where ALA was compared with HpD, there was no significant difference in median survival (8 months vs 9 months). 118
Morbidity
Both trials evaluating the impact of HBO in addition to PDT reported significantly greater decreases in tumour length in the HBO groups (2.8 cm and 3 cm) than in the PDT without HBO group (2 cm and 2 cm). 115,116 Dysphagia scores were significantly reduced in the PDT-plus-HBO group in only one trial. 116 Stenosis length decreased overall, between 5 and 6 mm, but these changes were not significantly different between groups.
At 1-month follow-up significantly greater improvements for dysphagia, tumour stenosis and tumour length were seen in the patients receiving ALA than in the patients receiving HpD. 118
Quality of life
A semi-solid diet was possible in all groups following treatment for the two trials comparing the addition of HBO to PDT alone. 115,116 The comparison of HpD and ALA found no significant differences in Karnofsky status scores (44% vs 23%, p = 0.12). 120
Resource use
One trial evaluating the addition of HBO reported on duration of hospital stay, there was no difference between groups with an overall median of 4.9 days (range 3–9 days). 116 The duration of hospital stay in the comparison of HpD and ALA (both with HBO) ranged from 4 to 6 days in both groups. 118
Two non-randomised studies without blinded outcome assessors provide limited evidence that adding HBO to HpD-PDT can enhance the efficacy of this treatment. One small non-randomised study suggests that ALA may be more effective than HpD when used with HBO.
Results of safety
All trials of PDT and oesophageal cancer reported AEs, albeit briefly. AEs are detailed separately by treatment intention.
The AE most commonly reported in patients receiving curative PDT appears to be stricture formation. Those studies reporting this AE gave figures ranging from 10% to 50% of patients developing stricture. 107,110,111 Chest pains, fever and transient dysphagia were also reported. 108 All patients who received mTHPC109 reported a burning sensation at the injection site, and two patients who did not follow the recommended precautions developed second-degree sunburn on the face and hands.
Regarding those trials that were palliative in intention: a small RCT (n = 42)114 found very few AEs for either PDT or Nd:YAG treatments, whereas a larger RCT (n = 236) reported that patients undergoing PDT were more likely to suffer from pain, nausea and pleural effusion. 112 Patients receiving Nd:YAG, however, were significantly more likely to suffer from oesophageal perforations.
The RCT comparing PDT alone versus PDT plus 5-FU (n = 140) reported no oesophageal stenosis or perforation in either group. 113 Small numbers of patients in both groups (PDT = 7, combination = 8) reported subternal pain due to oesophageal mucosa injury and gastroesophageal reflux 1–2 days after treatment. In total, eight patients accidentally exposed themselves to sunlight and developed discoloration of the skin.
One non-randomised study of 119 patients comparing HpD-PDT with brachytherapy reported 9% of all patients experienced major complications including oesophageal perforation and severe haemorrhaging, the authors suggest that care should be taken when selecting patients for treatment to prevent serious complications. 117
The PDT plus radiotherapy RCT (n = 60) stated that all patients receiving PDT reported swelling, itchiness, pigmentation and pain on swallowing. This last AE lasted for 3–5 days in most cases, but for some patients the duration was more than 10 days and sufficient for them to discontinue treatment. 119 Deaths by group were reported but not clearly attributed to the intervention or other extraneous factors.
In the three studies using HBO, no barotrauma to the ear was reported and no major complications were recorded. 115,116,118 Common minor complications included odynophagia, fever and chest pain for up to 2 days. All patients receiving ALA reported nausea immediately after administration.
Curative: Serious AEs have not been reported in the trials to date; however, trials are mainly small and rarer AEs might not, therefore, have been observed. The most common AE when curative PDT is given appears to be stricture formation. Both curative and palliative PDT may result in chest pain and fever although these are transient.
Palliative: Pain on swallowing or pain in the oesophageal area following PDT treatment was reported variably in the included studies and appears to be a common AE although the severity is unknown.
In both curative and palliative treatment, where patients did not follow the recommended precautions in terms of light exposure, photosensitivity reactions occurred.
Serious complications were reported by one non-randomised study however some of these may be attributable to the concomitant brachytherapy treatment. From the one trial where ALA was used, all patients receiving this photosensitiser reported nausea.
Ongoing trials
We are not aware of any potentially ongoing/unpublished trials of PDT for oesophageal cancer.
Discussion
To date PDT as a curative intervention for oesophageal cancer has only been studied in non-randomised trials. The patients receiving non-surgical interventions such as PDT or CRT appear to be markedly different from those receiving surgery, and this, combined with small sample sizes and lack of randomisation, makes it difficult to draw any firm conclusions.
Randomised controlled trials have been successfully carried out for palliative PDT, and there is evidence from one older study that Nd:YAG and PDT may not differ in terms of mortality. SAEs were only reported in one trial using Nd:YAG; however, these appear to be related to the comparator rather than the PDT treatment. Stricture formation is a concern in this area; however, the two more recent trials give substantially lower rates of stricture formation and this may reduce further as research refines the appropriate dose of photosensitiser and wavelength of light required.
There is some evidence from non-randomised studies that using HBO may enhance the effectiveness of PDT, but good-quality RCTs are required to clarify the degree to which the effectiveness can be increased. In all palliative studies, there was a lack of comparability between treatment arms and a lack of detail reported on the procedures given, making it difficult to draw conclusions.
Across both curative and palliative PDT treatments, there appears to be little consensus as to the optimal regime in terms of photosensitiser, light or duration of exposure; however, where patients fail to follow the recommendations in terms of light exposure then photosensitisation may be a concern. Further research is required to clarify these parameters.
Chapter 9 Lung cancer
Background
More than 38,000 people are diagnosed with lung cancer in the UK each year. Although some people who have never smoked get lung cancer, smoking causes 90% of cases. 106 However, over 80% of patients present late with the disease, and only 15–20% are suitable for surgical resection. 106 These figures help to explain why the 5-year survival of patients with lung cancer is poor; only about 7% of patients will live for at least 5 years. 106
Surgery (removal of part, or all, of the lung), chemotherapy, radiotherapy, or a combination of these treatments, can be used, depending on the stage when the cancer is diagnosed. If the cancer is at an advanced stage, a range of options are available to help alleviate symptoms. These include brachytherapy, electrocautery, laser therapy, PDT or cryotherapy.
Photodynamic therapy for lung cancer can be delivered under general or local anaesthetic. Removal of necrotic tumour is required usually 48 hours after each treatment. It can be repeated if necessary, and can be used alongside other lung cancer treatments. PDT can be given to patients with early-stage cancer who are surgically inoperable and can have a curative intent.
When lung cancer is advanced, PDT is used palliatively, aiming to reduce symptoms, such as shortness of breath by reducing tumour bulk. A previous systematic review has concluded that ‘the palliative effect of PDT in late stage lung cancer is promising, although its effectiveness in comparison to traditional therapies requires further study’. 16
Study characteristics
Seven trials investigated PDT for lung cancer with a total number of 329 patients (Table 14). 121–127 Five trials were reported in full papers122–124,126,127 and two as abstracts only. 121,125 All trials had a palliative intent in relation to non-small cell lung cancer.
| Authors | Study design | No. of participants | Trial treatments |
|---|---|---|---|
| Baas et al. (1994)121 (abstract only) | RCT | 39 | ERT alone vs PDT with Photofrin preceding ERT vs endobronchial HDR preceding ERT |
| Diaz-Jimenez et al. (1999)122 | RCT | 31 | PDT with Photofrin vs Nd:YAG laser resection |
| Lam et al. (1991)123 | RCT | 41 | PDT with Photofrin + external radiotherapy vs external radiotherapy alone |
| Lam et al. (1987)124 | RCT | 11 | PDT with Photofrin + external radiotherapy vs radiotherapy alone |
| Leroy et al. (1998)125 (abstract only) | RCT | 141 | PDT with Photofrin vs Nd:YAG laser resection |
| Maier et al. (2002)127 | Non-RCT | 40 | ALA–PDT with HBO vs PDT with Photosan with HBO |
| Moghissi et al. (1993)126 | RCT | 26 | PDT with Photofrin or Photofrin II vs Nd:YAG laser resection |
Photodynamic radiotherapy was compared to radiotherapy [including external radiation therapy (ERT) and HDR + ERT]121,123,124 and Nd:YAG laser resection. 122,125,126 Photofrin and Photofrin II were used as photosensitisers in the six trials comparing PDT to other treatments. 121–126 Where stated, trials used a protocol of 2 mg/kg, together with light at 630 nm. The drug–light interval varied from 24 to 54 hours across the studies. However, not all intervention parameters were reported in full. ALA–PDT was compared with Photosan in one trial that also used PDT in conjunction with HBO. 127
Study quality
The most recent trial127 was published in 2002, raising issues of relevance to current practice. All trials were relatively small (the largest had 141 patients) suggesting that they may have lacked power to detect significant effects. Procedures for randomisation, allocation concealment and blinding of outcome assessors were generally unclear. All trials reported AEs (albeit briefly). However, generally reporting was poor, making the reliability of a study difficult to assess. Two trials had clear baseline differences that could have impacted on results,122,124 and only two clearly had no differences at baseline. 123,126 The trial comparing different types of PDT was not randomised and was therefore open to selection bias. 127 A graph summarising study quality is presented in Figure 12.
FIGURE 12.
Lung study quality.
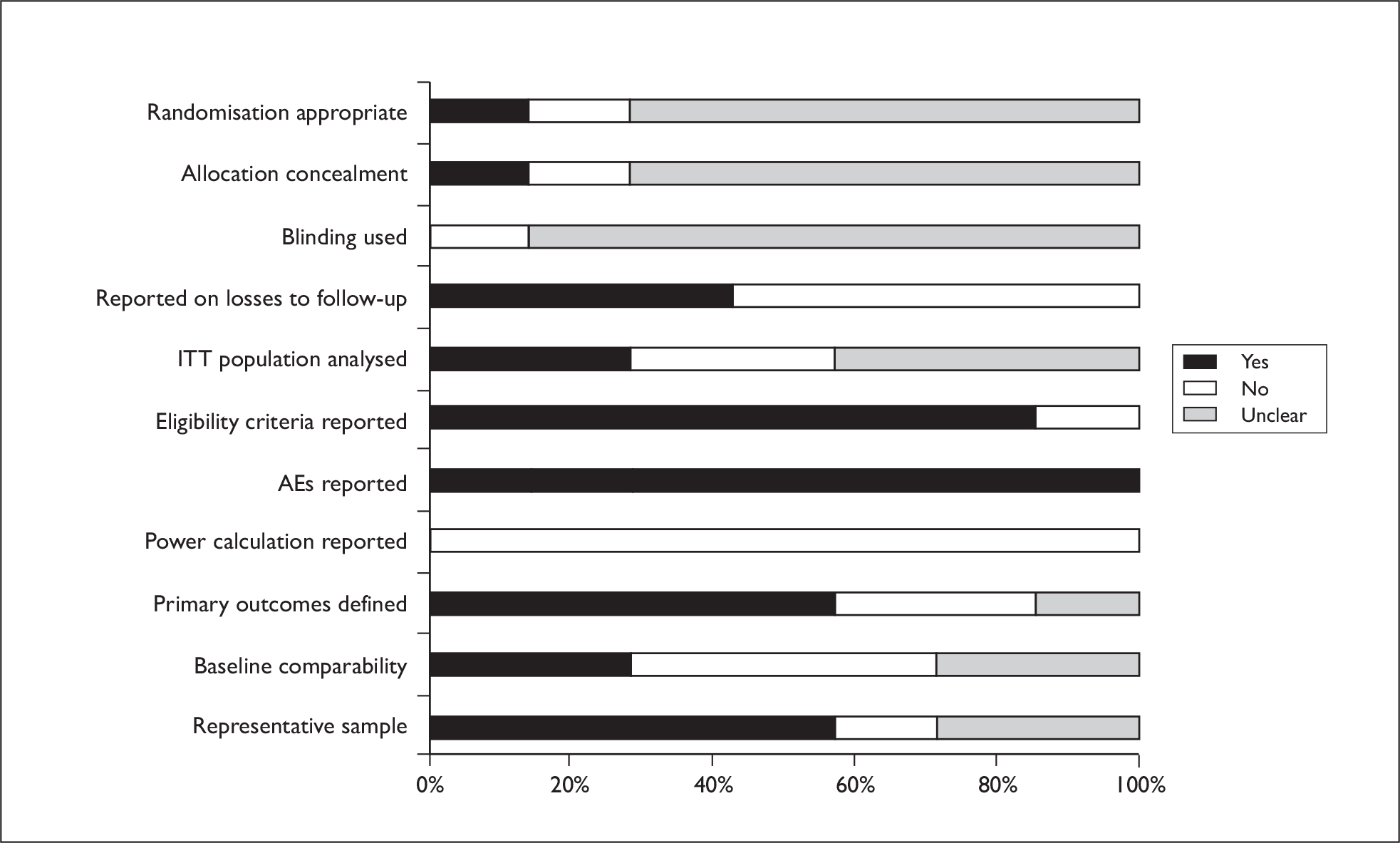
Results of effectiveness
Results are presented in a narrative synthesis. Meta-analysis was not possible due to heterogeneity between the trials. Resource use data were not presented in any of the trials of PDT and lung cancer.
PDT plus radiotherapy
Two trials (n = 52) compared PDT plus radiotherapy with radiotherapy alone,123,124 neither providing adequate methodology details, and one trial recruited only 11 participants. 124 One trial was of the effects of ERT alone versus PDT preceding ERT versus high-dose irradiation preceding ERT, but results were not presented by treatment group. 121
Mortality
Lam et al. 123 reported no differences in mortality rates or survival times (444 days PDT plus radiotherapy vs 445 days radiotherapy alone).
Morbidity
Lam et al. 123 found a greater reduction of haemoptysis (coughing up blood) and shortness of breath and cough at 1 and 3 months in the PDT-plus-radiotherapy group (p < 0.05). 123 There was also a substantial difference in both the success rate of bronchial lumen re-opening (14/20 PDT plus radiotherapy vs 2/21 radiotherapy alone) and the median interval between treatment and local recurrence (233 days’ PDT plus radiotherapy vs 107 days radiotherapy alone, p = 0.005).
Quality of life
Both trials assessed this outcome, but only the very small trial reported results, which suggested improvements from baseline in QoL and Karnofsky rating for the PDT-plus-radiotherapy group. 124
Two trials suggest that PDT plus radiotherapy may be more effective than radiotherapy alone in the palliative treatment of non-small cell lung cancer. However the small numbers involved, coupled with a lack of reporting of study methods and some outcomes, mean firmer conclusions cannot be drawn.
PDT vs Nd:YAG laser resection
Three trials (n = 198) compared PDT with Nd:YAG. 122,125,126 All had methodological limitations and did not report methods in full.
Mortality
In one trial, survival was significantly longer in the PDT group (265 days vs 95 days, p = 0.007), but the groups had important baseline differences. 122 The other two trials did not assess this outcome.
Morbidity
Similar response rates between treatment groups were found by Diaz-Jimenez et al. 122 At 1 month (but not at 1 week), Leroy et al. 125 found significant differences with a response rate of 61% for PDT versus 35% for Nd:YAG (p < 0.05). Diaz-Jimenez et al. found time elapsed to failure to be 50 days in the PDT group and 38 days in the Nd:YAG group (p = 0.03).
Leroy et al. 125 found symptomatic control to be better with PDT, although it was unclear if this result was statistically significant. Moghissi et al. 126 found that forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) improved significantly more with PDT than with Nd:YAG at 1 month after treatment. Mean difference between baseline and 1 month in FVC was 0.47 for PDT versus –0.06 for Nd:YAG (p < 0.05). The corresponding data for FEV1 was 0.35 for PDT versus 0.01 Nd:YAG (p < 0.05).
Quality of life
One trial assessed this outcome but did not report results,122 and the other two did not assess this outcome. 125,126
On the basis of three trials with methodological limitations and small numbers, it is not possible to conclude whether PDT is superior, equivalent or inferior to Nd:YAG. It is equally not possible to conclude which treatment, if any, might be most appropriate for which patients. There are suggestions of better symptomatic control with PDT but these would require further investigation. Any effects on survival would also need further investigation alongside any QoL issues that have so far not been assessed.
ALA–PDT plus HBO vs PDT plus Photosan plus HBO
One non-randomised trial, conducted as a pilot study (n = 40), compared ALA–PDT with HBO versus PDT plus Photosan plus HBO. 127
Mortality
The mean survival for the ALA groups was 9 months and the Photosan group 14 months (p = 0.020).
Morbidity
Difference in change in stenosis diameter post treatment favoured Photosan, but there was no statistically significant difference between groups on pulmonary function parameters.
Quality of life
A statistically significant difference in change in Karnofsky score was observed in favour of the Photosan group. None of the patients in the Photosan group reported a decrease in QoL due to long-lasting need for skin protection.
Although one trial suggested that Photosan was more effective than ALA, it was small and non-randomised. In addition, treatment groups had differences at baseline, and these may have impacted on results. The survival data do not solely reflect PDT treatment, as, 4 weeks after PDT, patients were allowed to receive a variety of other treatments.
Results of safety
Moghissi et al. 126 reported that there was no treatment-related mortality, but Diaz-Jimenez et al. 122 stated that one death was probably related to PDT. Lam et al. 123 noted mild skin photosensitivity in 20% of patients receiving PDT plus radiotherapy. Diaz-Jimenez et al. 122 reported photosensitisation in four of 14 PDT patients, while Leroy et al. 125 reported skin photosensitivity in 21% of the PDT patients, and Moghissi et al. 126 stated that there were no cases of photosensitivity. In the trial comparing ALA–PDT with Photosan, no major complications relating to photosensitisation were observed. 127 This same trial found only minor complications (fever and mild chest pain) in both groups, none of which required specific treatment. Diaz-Jimenez et al. 122 found bronchitis to be the most common AE (4/14 in the PDT group compared to one in the Nd:Yag group). This trial also reported that all five patients who experienced no AEs were in the Nd:YAG comparator group. Baas et al. 121 reported minor haemoptysis in 2 out of 15 combined PDT-ERT patients. Moghissi et al. 126 stated that there were no serious post-treatment complications.
Serious AEs do not appear to be common but the impact of photosensitisation is unclear. Not all trials reported the duration and seriousness of this AE, and the influence of advice and counselling is unclear. The one trial that reported such advice noted no instances of photosensitisation.
Ongoing trials
We are not aware of any potentially ongoing/unpublished trials of PDT for lung cancer.
Discussion
No trials were located in relation to early lung cancer using PDT with a curative intent. All of the trials we located related to PDT in a palliative setting where there is some uncertainty about effectiveness in relation to other treatments. However, it should be borne in mind that palliative radiotherapy, a common alternative to PDT, has significant variation in the way it is delivered across the medical community. 128 Additionally, it is not usually delivered in isolation and other agents may be used to improve symptoms. Treatment effects are measured by a variety of techniques and any improvement in symptom severity is subjective for both patient and clinician. 128 All of these present challenges in any comparison with PDT.
In any examination of the relative effectiveness of one or more treatments, it is important to identify whether there are subgroups of patients who might benefit particularly from one of the treatments. The trials identified do not enable us to identify the type of patient who might benefit from PDT. We have been advised that the small group of patients with large-airway (trachea or major bronchi) clinically invasive and inoperable tumours, which do not tend to metastasise and cause airway obstruction, might benefit most from PDT. Maintaining a patent airway in these patients is most beneficial in terms of symptoms and life expectancy (Dr JH Winter, Clinical Group Director/Respiratory Consultant, Medicine & Cardiovascular Clinical Group, Ninewells Hospital, Dundee, 24 July 2009, personal communication).
Apart from one trial that compared different photosensitisers, all of the evidence dates from the 1990s or 1980s, which may not reflect current practice. Further research is needed to determine the role of PDT in lung cancer. The scoping review identified 177 publications that did not meet the study design criteria for the review reporting on PDT in the treatment of lung cancer (see Appendix 9). Examination of these publications, including an assessment of quality, could inform the design of further trials.
Chapter 10 Biliary tract cancer
Background
Biliary tract cancer involves cancerous growths in the gall bladder and/or the bile duct. The uncontrolled epithelial cell growth occurs in the inner lining of the gall bladder and bile duct. These cancerous tumours block the flow of bile as they grow. Bile duct cancer, or cholangiocarcinoma, can occur intrahepatically or extrahepatically. Although there are some known risk factors, the majority of patients do not present with these. 129
Primary cholangiocarcinoma is relatively rare with an incidence of up to 3 per 100,000 per year,130 accounting for approximately 3% of all gastrointestinal malignancies. 33 However, incidence rates have been increasing, particularly with respect to intrahepatic cholangiocarcinoma. The 5-year relative survival rate for people diagnosed with early-stage cholangiocarcinoma is about 30%; however, only 20% of patients are diagnosed at the early stages. 131 Early cholangiocarcinoma is often asymptomatic, but as the cancer progresses and prevents bile flowing to the small intestine symptoms such as jaundice, itchy skin, abdominal discomfort, loss of appetite or weight, and fever may occur. The key efficacy outcomes for biliary tract cancers are survival, disease progression, recurrence of jaundice/stent failure and QoL.
This cancer may be treated with potentially curative surgery if detected in the early stages before vascular invasion or metastasis formation has occurred, or with a variety of palliative interventions. The survival rate at 5-years after curative resection is only 30–40%,130 and for the majority of cases surgery is not possible due to the position and extension of the tumour. Common palliative strategies include surgical bypass of the bile duct, percutaneous or endoscopic stenting and, more recently, chemotherapy, while more experimental options such as PDT, radiotherapy or brachytherapy are under investigation.
Cholangiocarcinomas have been classified in various ways according to location, extent and severity of the tumour. In general cholangiocarcinoma may be staged using the TNM system as for other cancers (see Appendix 3). The Bismuth–Corlette classification system is used for perihilar cholangiocarcinoma and is useful when decisions are being made about the possibility of successful resection:
-
tumours below the confluence of the left and right hepatic ducts (type I)
-
tumours reaching the confluence (type II)
-
tumours occluding the common hepatic duct and either the right or left hepatic duct (types IIIa and IIIb, respectively)
-
tumours that are multicentric, or that involve the confluence and both the right or left hepatic duct (type IV).
Photodynamic therapy is usually given alongside biliary stenting following endoscopic retrograde cholangiopancreatography (ERCP) rather than as a stand-alone treatment, and is a palliative option. The protocol for treatment is as follows: the photosensitising agent is injected intravenously and exposure to the light takes place around 48 hours later. The light is delivered to the target area via translucent endoscopic catheter or the light source is placed across the stricture caused by the tumour. Radiological control is used to determine correct positioning of the laser fibre. Patients are required to remain in subdued light for up to 3 days following the injection of the photosensitiser before being gradually re-adapted to bright indoor light. The treatment is repeatable. One or more percutaneous or endoscopic stents are placed to relieve biliary obstruction by facilitating drainage, reducing pruritis and therefore improving QoL. Stents may be either plastic or metal; metal mesh stents remain patent (open and unobstructed) for longer and need replacing less often but can also result in occlusion.
Study characteristics
Five controlled trials, of which two were RCTs, evaluated PDT for cholangiocarcinoma with a total number of 332 patients (Table 15). 132–136 All were available as full published papers, except one trial which was published only as an abstract. 132
| Authors | Study design | No. of patients | Trial treatments | Type of cholangiocarcinoma | Treatment intention |
|---|---|---|---|---|---|
| Dechene et al. (2007)132 (abstract only) | Non-RCT | 29 | PDT with Photosan-3 vs PDT with Photofrin II (all + plastic stents) | Not reported | Palliative |
| Kahaleh et al. (2008)133 | Non-RCT | 48 | PDT + ERCP + stenting vs ERCP + stenting alone | Perihilar – mostly Bismuth types III and IV | Palliative |
| Ortner et al. (2003)134 | RCT | 39 | Stent + PDT + ERCP + stent vs ERCP + stenting (all double stenting) | Perihilar – mostly Bismuth type IV | Palliative |
| Witzigmann et al. (2006)135 | Non-RCT | 191 (184 analysed) | PDT + double stenting vs double stenting alone vs resection | Perihilar – stenting arms mostly Bismuth stage IV | Curative and palliative arms |
| Zoepf et al. (2005)136 | RCT | 32 | PDT + stenting vs stenting alone | Perihilar, Bismuth stage IV | Palliative |
Two RCTs compared PDT plus stenting with stenting alone (n = 71);134,136 however, in Ortner et al. 134 stenting was given both before and after PDT. One non-randomised study also compared stenting and PDT plus stenting. 133 One non-randomised study compared two types of photosensitiser for palliation,132 and the final non-randomised prospective study explored curative and palliative treatments (PDT, stenting and resection). 135
All studies, apart from Witzigmann et al. ,135 included only patients with non-resectable cholangiocarcinoma. Ortner et al. ’s RCT134 was unusual in obtaining 100% histological confirmation of cholangiocarcinoma. In both trials and clinical practice, the diagnosis of cholangiocarcinoma is often not established in all patients. 137 Where other trials reported this information, histopathological confirmation was established for between 60% and 70% of all cases. In the study by Ortner et al. 134 patients were randomised only following technically successful stent placement, while in all other studies the patient group may have included those who did not achieve technically successful stenting.
These studies used a variety of photosensitisers, including Photosan-3, Photofrin II and Photofrin. The dosage of all photosensitisers was set at 2 mg/kg and light was delivered at between 630 and 635 nm across the studies where reported. The drug to light interval was 48 hours in all studies where this parameter was reported.
One trial was halted part of the way through recruitment based on established trial monitoring and stoppage rules. 134
Study quality
Although RCTs in this area were published in 2003 and 2005,134,136 more recent publications have tended to be non-randomised. 132,133,135 It is more difficult to draw firm conclusions from this kind of evidence. Overall, the two RCTs were of relatively good quality and reported their methods clearly; however, neither used large sample sizes. The non-randomised studies were generally less well reported, and only one study used groups that were comparable at baseline. All of the non-RCT studies reported on AEs, but none used power calculations or reported using ITT analysis. A graph illustrating study quality is presented in Figure 13.
FIGURE 13.
Biliary tract study quality.
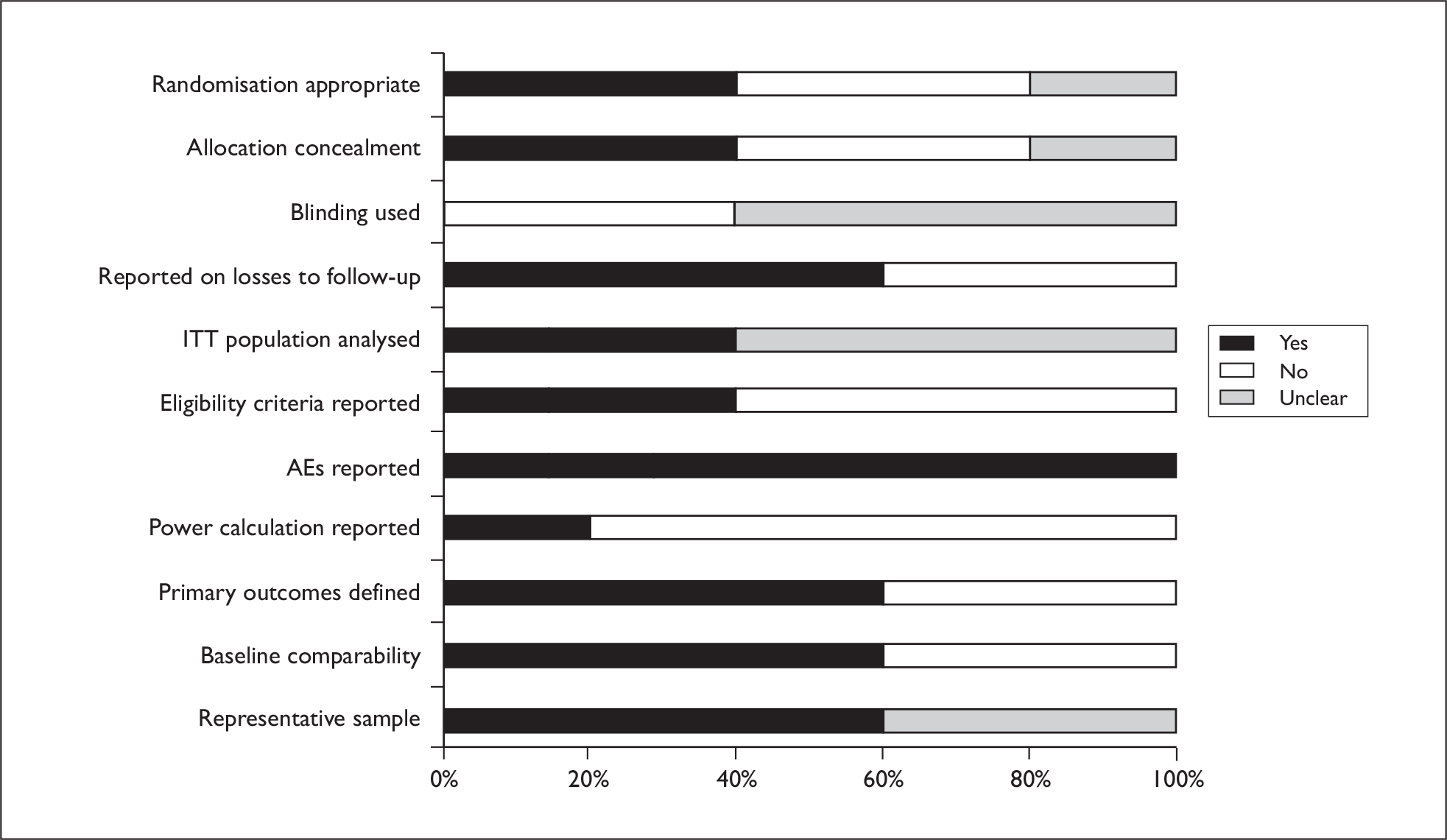
Results of effectiveness
Results are presented in a narrative synthesis. Meta-analysis was not possible due to heterogeneity between the trials.
PDT plus stenting vs stenting alone
Two RCTs (n = 71) compared PDT plus stenting with stenting alone. 134,136 Both were reported in full papers and seemed to be well conducted. One non-RCT also compared PDT plus stenting with stenting alone (n = 48), although there were important differences in baseline characteristics between the groups and definitive conclusions were difficult to draw. 133
One non-RCT (n = 191, 184 analysed) compared stenting alone with PDT plus stenting and resection for hilar cholangiocarcinoma in a prospective study over 10 years in one centre. 135 This was an unusual study, which appeared to include patients of varying cancer stages, and some patients in the resection group also received adjuvant PDT or stenting where required. Therefore, only the results of the PDT plus stenting versus stenting alone have been described here as these patients appear to be broadly comparable.
Mortality
Both RCTs found statistically significant increases in survival time in the PDT-plus-stenting groups compared with stenting alone. In the study by Ortner et al. 134 median survival in the PDT group was 493 days, and 98 days in the stenting-only group (p < 0.0001), whereas in the Zoepf et al. trial136 the PDT group had a survival time of around 21 months compared with 7 months in the stenting group (p = 0.01).
Both non-RCTs also reported statistically significant prolonged survival rates in the PDT groups. 133,135 Mortality rates were initially lower in the PDT groups but not statistically significantly different by the end of each study when the majority of patients had died (usually a result of tumour progression and complications of chronic cholangitis).
Morbidity
In the two RCTs, successful drainage and relief of bile duct stenosis was generally achieved. Zoepf et al. 136 reported the median bilirubin level after first intervention was not significantly different between the groups, while Ortner et al. 134 found that after PDT serum bilirubin reached lower levels relative to baseline and stenting alone (p < 0.01).
Kahaleh et al. 133 reported both groups having significantly decreased levels of serum bilirubin at 3 months when compared with baseline levels (p = 0.008 for PDT and p = 0.0001 for stent only), but no significant differences between the two groups in the degree of decrease (p = 0.78). In contrast, Witzigmann et al. 135 found significant reductions from baseline and significant differences in favour of the PDT treatment group (p < 0.05); successful drainage was achieved in 75% of PDT-plus-stent patients versus 39% receiving stents alone.
Quality of life
Zoepf et al. 136 found that QoL as assessed by the Karnofsky scale did not significantly change after treatment for either group, while Ortner et al. 134 reported that Karnofsky index improved after PDT, with a median 80% score, but did not improve in the stenting-only group. Ortner et al. 134 also reported that after PDT physical functioning (p < 0.01) and global QoL (p < 0.001) improved in the PDT group but not in the stenting-alone group.
Quality of life was assessed by one non-RCT study, which reported that QoL decreased in the stenting-alone patients but increased significantly in the PDT-plus-stenting group (p < 0.01). 135
Resource use
This outcome was reported for one non-RCT study. 135 Median hospital stay duration was reported as 65 days for PDT plus stenting versus 44 days for stenting alone.
Based on two relatively small RCTs and two non-randomised studies, the evidence suggests that survival is increased when PDT is given alongside stenting but it is not yet possible to draw firm conclusions regarding morbidity. The QoL results differ between trials; there is no evidence that patients’ QoL significantly declines following treatment, but there was no consistent increase across studies. This evidence base includes studies that have used different treatment protocols for stenting and those that have chosen different photosensitisers and recruited patient groups which differed in baseline health status.
These studies appear to have included perihilar cholangiocarcinoma patients only, therefore caution is advised in extending these results to other cholangiocarcinomas or to biliary obstruction due to gall bladder cancer.
Photosan-3 vs Photofrin II
One non-RCT (n = 29) compared two different photosensitisers for the treatment of non-resectable cholangiocarcinoma (further details not reported). 132 This study was available only as an abstract, therefore it was difficult to quality assess. It was a small trial and may have been underpowered to detect any possible differences between photosensitisers.
Mortality
Mortality did not differ significantly between the two groups: median survival for the Photosan-3 arm was 690 days, and 494 days for the Photofrin II arm (p = 0.87). 132
Morbidity, QoL and resource use were not reported in this study.
It is not possible to draw firm conclusions on the comparative efficacy of photosensitisers based on one small study reported only in abstract form.
Results of safety
Photosensitivity is the most common AE associated with PDT treatment, these studies reported only mild reactions occurring in between 0% and 10% of patients. 132–136 Cholangitis was reported in both the PDT-plus-stent and the stenting-alone groups, although where these rates were compared there were no significant differences. Cholangitis was usually managed with antibiotics. Other AEs included cholecystitis and stenosis (although not usually related to the intervention).
Serious AEs do not appear to be common and photosensitisation was not reported as a frequent AE. Only one study reported patients being required to remain in a darkened room following photosensitiser injection; it is unclear if this is common practice, as most trials did not report these details. Not all trials reported the duration and seriousness of this AE, and the influence of advice and counselling is unclear.
Ongoing trials
We are aware of the following trials and S Pereira has confirmed that their trial is still recruiting with no results currently available (Table 16). Efforts to contact the other trialists and obtain results have been unsuccessful.
| Name | Title | Start date | Completion date/status |
|---|---|---|---|
| Farrell J | Stent vs stent + PDT for inoperable stage III or IV cancer | Unknown | Withdrawn |
| Pereira S | PDT + stenting vs stent alone for advanced inoperable biliary tract tumours | July 2007 | July 2009 – ongoing |
| Trichereau N | Photofrin PDT vs unknown | November 2007 | November 2008 – not yet recruiting |
| Rauws E | PDT + endoscopic treatment vs endoscopic treatment alone | January 2005 | January 2008 – listed as completed |
Discussion
Overall, the evidence base for PDT in biliary tract cancers is relatively small; however, further well-designed RCTs are under way and should provide more definitive answers. The scoping review only identified 30 publications of potential relevance in this area (see Appendix 10). The majority of these have been published in the last 10 years and, compared to other sites, appear to include a larger proportion of experimental uncontrolled trials (Phase I and II and pilot studies).
It appears that PDT plus stenting may improve survival rates compared with stenting alone and SAEs are rare overall. The strongest evidence comes from an RCT by Ortner et al. 134 but it is worth noting that this was a highly selected non-resectable population; only patients who had not previously been technically successfully stented, and therefore presumably had persistent jaundice, were eligible; additionally, only those patients who were then technically successfully stented were randomised into the trial. It could be argued therefore that the results of this trial may not be generalisable to the broader population of patients. Further trials may be useful to further refine the PDT treatment procedure and identify the most suitable photosensitisers for cancers in this area.
Chapter 11 Brain cancer
Background
In the UK, there are nearly 2500 new cases of brain cancer in men, 1700 in women and 300 in children each year. 106 The growth of brain tumours causes pressure and damage to healthy brain tissue. They are categorised according to growth rate: ‘low grade’ (grades I and II) are generally regarded as being benign, and ‘high grade’ (grades III and IV) as being malignant. There are numerous different types of brain tumour, with around one-half being gliomas (of which there are three main types: astrocytoma, ependymoma and oligodendroglioma). The cause of brain tumours remains unknown. Malignant gliomas have a poor prognosis; patients who receive treatment will typically survive for only around 1 year.
Resection surgery, followed by radiotherapy and/or chemotherapy, is the treatment most commonly used for malignant brain tumours, and can be used with curative or palliative intent. Surgery rarely results in the removal of all tumour cells, hence the need for adjuvant treatment. Ultrasonic aspiration (where ultrasonic waves fragment the tumour, with fragments being removed by suction) may sometimes be an alternative to standard surgery (with a scalpel). Stereotactic radiosurgery (using highly focused X-rays) is a non-invasive procedure that can be used when invasive surgery is not appropriate, although generally it is an option only for tumours of less than 4 cm in size. Radiosurgery may also be used after conventional surgery, and is often used in combination with conventional radiotherapy.
Photodynamic therapy has been used little in the treatment of malignant brain tumours. When PDT is used it is done so by administration of photosensitiser (currently usually ALA, Photofrin or Foscan), followed up to many hours later (ranging from 3 to over 100 hours) by tumour resection, and subsequent light delivery via a special illuminating device. PDT has been used in addition to radiotherapy and/or chemotherapy, and is normally preceded by photodynamic diagnosis (PDD) to identify tumour tissue. The scope of this review does not cover studies of PDD without the use of PDT as a treatment.
Study characteristics and quality
Two trials evaluated the use of PDT for brain cancer. 138,139 Both trials recruited fewer than 30 patients, and both were reported as full papers. The treatments for the trial by Krishnamurthy et al. 138 were Ps-PDT (at 630 nm) with three different light dose ranges, in patients with recurrent or residual tumours ≤ 5 cm in diameter. This trial was non-randomised, and it was unclear whether it recruited comparable groups or used blinding. The very small sample size suggests that the study lacked power to detect significant effects.
The other trial was an RCT by Eljamel et al. 139 – for patients with glioblastoma multiforme – comparing fluorescence-guided resection followed by repetitive ALA–PDT, with standard resection (both groups also received radiotherapy). It was unclear both how many participants were randomised into the study, and what kind of randomisation and allocation processes were used, although outcome assessors were blinded to treatment allocation.
Results of effectiveness and safety
In the Ps trial, the group receiving the medium light dose (3700–4400 J), survived longer than the group receiving the highest dose (4400–5900 J) – 314 days versus 238 days. Results were not reported for the low-dose group. Five patients had postoperative permanent neurological defects (two in the medium-dose group and three in the high-dose group). 138
In the repetitive ALA–PDT trial, the PDT group survived significantly longer than the surgery group (52.8 weeks vs 24.2 weeks), and had a significantly longer time to tumour recurrence (8.6 months vs 4.8 months). Three patients had deep vein thrombosis, two of which were in the PDT group. 139
Of the two trials of PDT in brain cancer, one was of too poor quality to yield any useful evidence. The other showed interesting results for the effectiveness of fluorescence-guided resection with repetitive ALA–PDT, which need further investigation in larger trials that include safety assessment, as the incomplete and inconsistent reporting, coupled with the small sample size, limit the reliability of the authors’ conclusions.
Ongoing trials
Results from a North American RCT looking at PDT (with Ps IV) using high-light dose versus low-light dose for patients with recurrent malignant astrocytoma are due to be published in 2010. The study aimed to recruit around 120 participants. Results of a non-RCT (including around 60 participants) conducted in Belarus, of high-grade gliomas treated with surgery and Photolon (also known as Fotolon) PDT versus surgery and chemotherapy (temozolamide), were to be presented at the 2009 World Congress of the International Photodynamic Association, Seattle.
Stopped trials
Two randomised trials of Photofrin-PDT for gliomas – both by the same investigators – were not completed. 140 No peer-reviewed publication of results is available. One trial aimed to determine the effectiveness of PDT as an addition to standard therapy (surgery, radiotherapy and/or chemotherapy) in newly diagnosed patients, and the other to see whether surgery and PDT with high-light dose was superior to surgery and PDT with low-light dose, in patients with recurrent tumours. The trials were stopped prematurely after the second predetermined interim analysis showed that the statistical power would not be sufficient to show a survival advantage for PDT treatment. Differences in site recruitment and treatment techniques were also a factor (L Lilge, Associate Professor, University of Toronto, 23 July 2009, personal communication).
Discussion
The evidence-base for PDT in brain cancer is very limited, but PDD, followed by PDT, may be the way forward, as identification of the entire tumour is first needed for a curative outcome to be possible. However, although in most cancer sites the stem cells are found within the clinical target volume, and so are subject to therapy, the brain is differently organised, with the normal stem cells found only in four locations (e.g. subventricular space). This means that stem cells may be outside the clinical target volume, so any focal treatment is bound to fail if these zones are not included in the therapy (L Lilge, Associate Professor, University of Toronto, 23 July 2009, personal communication). It is therefore questionable whether PDT using current technology is a suitable treatment worthy of further study. Furthermore, the results of the scoping review (see Appendix 11) reveal there to be generally few studies of PDT in brain cancer. Of these, only two studies had comparator groups, and there have been only 10 studies published since 2004.
Chapter 12 Head and neck cancer
Background
The term ‘head and neck cancer’ encompasses, among others, cancers of the mouth, tongue, lip (oral cancers), pharynx, larynx, sinuses, salivary gland and middle ear. Head and neck cancers account for more than 5% of cancers in Western countries. Tumours commonly arise in mucosal linings and may spread locally, but most do not metastasise. The tumours may affect a patient’s ability to breathe, drink and eat. The 5-year survival rates are around 50%, but vary by type and stage. Although the cause of head and neck cancer is unknown for many patients, cancers of the mouth, larynx and pharynx are far more common in people who smoke and drink a lot of alcohol (especially spirits).
Most head and neck cancer treatment involves surgery and/or radiotherapy. Chemotherapy is sometimes used to treat certain cancers (e.g. nasopharynx). Treatment can be given with curative or palliative intent. Even for small tumours, surgery and radiotherapy can both often result in significant morbidity, disabling AEs and loss of function (swallowing, taste, speech). For advanced tumours, resection is often followed by reconstruction surgery involving free flap grafts from other parts of the body (e.g. arm, leg or hip).
Photodynamic therapy in the treatment of head and neck cancer – which is used with either curative or palliative intent – is normally a stand-alone treatment, but can be used in combination with other treatments. Laser light is normally used, with delivery via fibreoptic cables. A possible advantage of using PDT as an alternative is the prospect of preserving function with minimal toxicity, resulting in repeat treatment being an option when necessary.
Study characteristics
Four trials investigated PDT in the treatment of cancer of the head and neck with a total number of 276 participants (Table 17). 141–144 Only one trial was reported in a full paper,141 with the other three being reported as abstracts. 142–144
| Authors | Study design | No. of participants | Cancer site | Trial treatments |
|---|---|---|---|---|
| Li et al. (2006)141 | RCT | 30 | Nasopharynx | PDT-Photofrin vs chemotherapy (cisplatin and 5-FU) |
| Loukatch et al. (1995)142 (abstract only) | Non-RCT | 145 | Larynx | Surgery + PDT vs Surgery + PDT without laser vs surgery alone |
| Loukatch et al. (1996)143 (abstract only) | Non-RCT | 49 | Laryngeal part of pharynx | PDT vs PDT-laser only vs PDT-photosensitiser only |
| Vakulovskaya (2007)144 (abstract only) | Non-RCT | 52 | Mouth | PDT-Photosense vs PDT-Radachlorin |
The trials studied different cancer sites, different PDT treatments (apart from two that used methylene blue as photosensitiser142,143), and different comparator treatments. Not all treatment parameters were reported in full. Two trials were of curative intent142,143 and in two the intent was not stated. 141,144
Study quality
Trial sample sizes ranged from 30 to 145, with three trials having fewer than 60 participants. This raises questions about whether trials were sufficiently powered to detect significant effects. Use of procedures for blinding of outcome assessors (and randomisation, and allocation concealment procedures, for the one RCT) was unclear in all studies. All trials did, however, report AEs (with varying amounts of detail). Only one trial reported on whether there were losses to follow-up. 141 A graph summarising study quality is presented in Figure 14.
FIGURE 14.
Head and neck study quality.
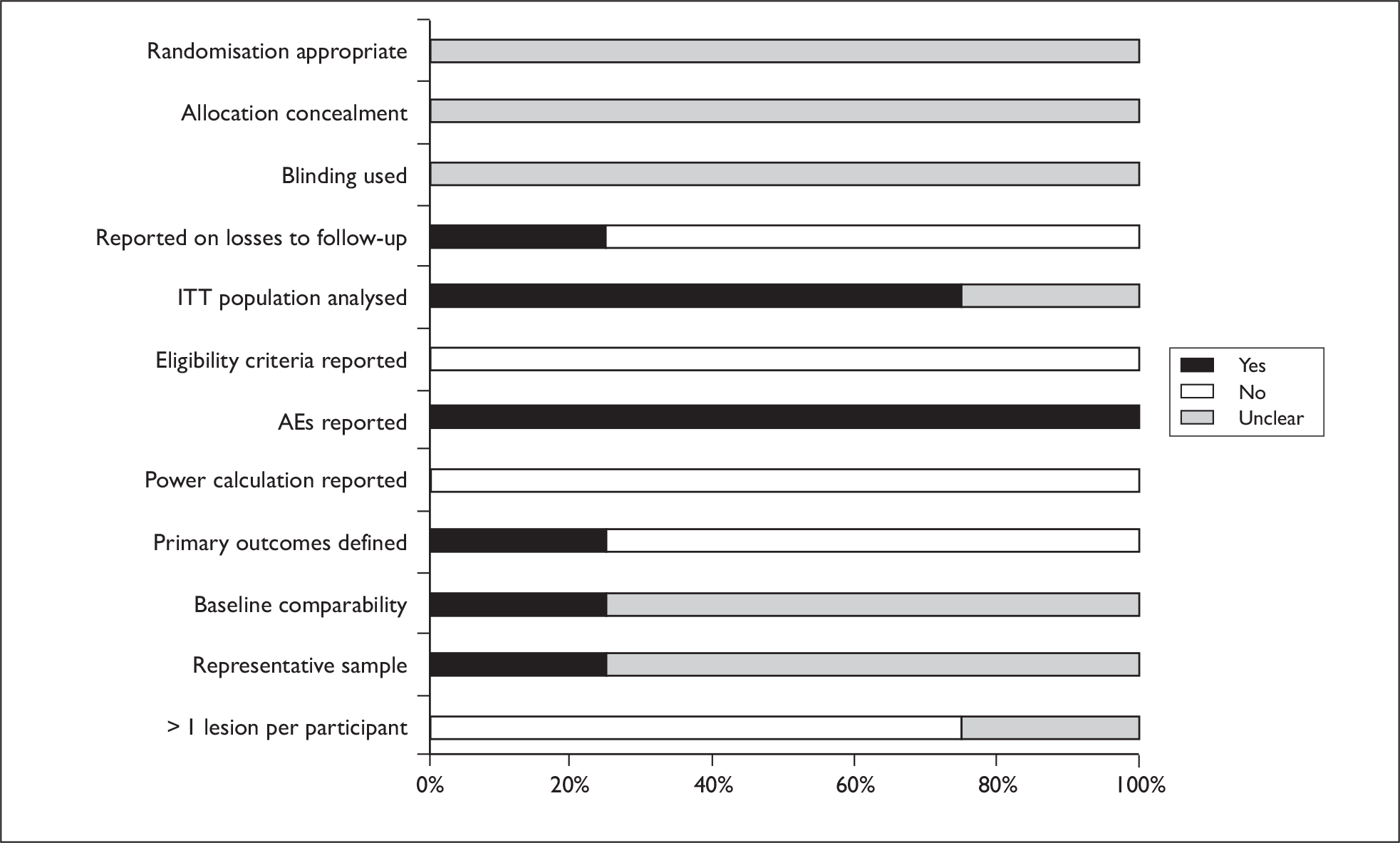
Results of effectiveness and safety
PDT vs chemotherapy
One RCT (n = 30), which compared Photofrin-PDT with chemotherapy (cisplatin and 5-FU) in patients with nasopharyngeal carcinoma, was only a small pilot study, which provided no details on randomisation procedures or use of blinding. 141 Overall, clinical response was better with PDT (p = 0.001), and there was a greater improvement in Karnofsky score (from 45 to 70 vs 40 to 50, p = 0.02). In those patients with nasal obstruction PDT was more effective at debulking of tumours (7/8 improved vs 2/8, p = 0.04).
The authors reported that the PDT related adverse reactions and side effects were generally tolerable. See Appendix 21 for fuller details.
PDT vs other treatments
The two non-RCTs studying PDT and/or surgery compared with other PDT treatments (including PDT with laser only, or photosensitiser only) had limited relevance to clinical practice. 142,143 One non-RCT compared PDT using different photosensitisers (Photosense and Radachlorin). 144 All three studies were on different cancer sites. All were also reported as abstracts, and had minimal reporting of methods and results. Results are therefore not discussed in detail here but are available in Appendix 21.
The only RCT on PDT in head and neck cancer suggested that the use of PDT to treat patients with nasopharyngeal cancer is worthy of further investigation, both with regard to effectiveness and adverse effects. Little useful evidence could be gleaned from the three non-RCTs.
Ongoing trials
We are not aware of any ongoing/unpublished comparative trials of PDT for head and neck cancer.
Discussion
Only one small RCT and three non-RCTs (all three reported only as abstracts) of PDT in head and neck cancer were identified, yielding little useful effectiveness and safety data. The true value of PDT, in relation to other treatment options, has therefore yet to be established. In light of its ability to preserve connective tissue, PDT has potential to be of great value, especially as there are few barriers to multiple re-treatments with PDT. Re-treatment by conventional means is very difficult, as there is a limit to the amount of tissue that can be surgically excised.
However, a recent editorial in a PDT journal questioned why so few people with head and neck tumours are treated with PDT, concluding it was due to a dearth of prospective clinical trials, exacerbated by a lack of advancement (in clinical PDT) in terms of photosensitisers and light sources. 145 It is also generally acknowledged that PDT cannot successfully control multiple lymphatic metastases, whereas both surgery and radiotherapy easily can, meaning that PDT is unlikely to be a primary treatment modality in the vast majority of patients with head and neck cancer.
Nevertheless, PDT may still have a key role to play, particularly in primary palliative treatment, and in the treatment of early cancers. It is, perhaps, patients with early head and neck cancer who could theoretically gain the most from PDT, as these patients are at low risk for nodal metastases. Randomised trials of PDT in patients with early-stage head and neck cancer, versus usual treatment (surgery/radiotherapy), and of palliative PDT versus re-irradiation (with or without chemotherapy) are therefore warranted. The population(s) and PDT parameters most suitable for investigation (e.g. which photosensitiser, method of light delivery, etc.) could be informed by an examination of the studies listed in the scoping section of this report (see Appendix 12). This should include an assessment of study quality.
An example of the slowed progress in PDT research is the development of the photosensitiser Foscan. It was finally licensed in the EU in 2001 but is indicated for only the palliative treatment of patients with advanced head and neck SCC who are failing prior therapies, and is unsuitable for radiotherapy, surgery or systemic chemotherapy. Foscan being licensed for use in such a minority of patients may be a reflection of its chequered history of development, rather than its true efficacy.
In 2000, the US Food and Drug Administration (FDA), shortly followed by the European Agency for the Evaluation of Medicinal Products (EMEA), rejected a licence application for Foscan as a palliative treatment for late-stage head and neck cancer. This failure to obtain licences has been attributed to a change of leadership, in 1998, in the company producing Foscan, which led to the termination of ongoing early-stage cancer research projects, and to the changing of Foscan’s modality from a treatment for early-stage cancer, to a palliative treatment for late-stage cancer, where it may not have a major advantage over other treatment modalities. 146 The licence rejections ultimately resulted in reduced investment, which consequently delayed the further development of Foscan.
Chapter 13 Discussion
Review methodology
We have conducted a rigorous systematic review of the effectiveness and safety of PDT across a range of clinical conditions. To do this, we pre-specified in a protocol inclusion and exclusion criteria for populations, intervention, comparators and outcomes. We did not place restrictions on the population, intervention or comparator. PDT, in all its variations, was eligible, as were trials that compared different PDT parameters. This comprehensive and rigorous approach also extended to the search strategy. In addition to a thorough search of a range of electronic references, we also contacted experts in the field including trialists, manufacturers and other researchers. All included trials and existing reviews were checked for eligible studies and the review was kept up to date by a further search conducted towards the end of the project. The lack of language restriction and efforts to translate foreign language studies ensured that the international literature was fully explored. Assessment of study quality is paramount in systematic reviews, to understand the weight that can be given to the evidence found. 22 In our assessment of quality we were particularly mindful of how deficiencies in study design might impact on results. The use of evidence summaries in the results narrative provides an interpretation of the findings in the context of quality. We were cautious in our approach to synthesising the data, giving careful consideration to clinical heterogeneity before deciding to pool certain study results. We were only able to conduct meta-analysis in the area of AK, and only for two comparisons. We also assessed the reliability of the pooled results of the meta-analyses conducted. In summary, we believe that the systematic review can be considered relevant, reliable and thorough.
We aimed to base the systematic review on the best evidence for each clinical area. Hence we restricted study design to RCTs where many had been conducted and to non-RCTs where not. At the same time, we were aware that in some areas PDT is an emerging field of research where few comparative trials have been conducted. Therefore, we also conducted a scoping review of the remaining, mainly observational, studies to produce a map of the complete literature. Within the time and resources available for this review, we have not been able to assess the scoping literature in any detail. However, we have categorised publications by broad study design in those areas where controlled trial evidence is lacking. Further assessment and analysis of this data may provide useful insights into informing future research.
Every systematic review has its limitations. There is the issue of unpublished studies (the file drawer problem). 147 Despite our best efforts, we did not receive replies from most trialists contacted for ongoing and unpublished studies. We do not believe that this would have altered our overall conclusions, but valuable information could have been added to the evidence base. A further problem was poor indexing on some of the electronic databases we searched. Studies may have been missed despite our rigorous search strategy. Finally, our review was limited by the quality of the underlying primary evidence.
Appraisal of the evidence base
An assessment of the quality of the primary studies allowed us to identify several shortcomings across the PDT literature. There was generally a paucity of well-conducted, adequately powered RCTs which are recognised as the gold standard of health-care research and the least prone to bias. 22 While it is acknowledged that there can be many barriers to conducting high-quality RCTs in cancer,148 there is also a need to base decisions on the best evidence available. AK had the greatest number of RCTs (28), while brain cancer had just one; the remaining sites had varying numbers and clearly RCTs present challenges in many areas. However, we identified at least one RCT in each clinical condition, suggesting that ethical and funding considerations can be overcome. We recommend that future research, where ethically possible, and where true clinical equipoise exists, should be in the form of well-designed and rigorously conducted RCTs.
Trials of adequate size to detect effects can be challenging to conduct, particularly in rarer cancers. However, we found that even in more common pre-cancerous conditions such as AK, a lack of study power appeared to be evident in several trials. A complicating factor in skin conditions was that often lesions randomised rather than patients. It was not always clear whether the analyses had taken into account the likely correlation between lesion responses within patients. Multicentre, international trials exist across the PDT literature. Potentially, they can enable larger numbers of patients to be recruited, and possibly enhance generalisability; however, statistical analyses should take centre effects in account. 149 It was not always clear from publications whether institutional differences and protocol deviations had been addressed when analysing data and interpreting results of multicentre trials.
Poor reporting of methodology is a common problem across the clinical trial literature. 150,151 The PDT literature was no exception. Randomisation and allocation concealment procedures in RCTs were generally not well reported. This means that the presence of selection bias cannot be discounted. Although blinding of patients and physicians is sometimes challenging in PDT and its comparators, even attempts to blind outcome assessors were not always reported. This is especially important where outcomes are subjective, such as cosmetic appearance. Therefore, the presence of outcome bias cannot be discounted in the majority of the clinical areas of this review. Across the review AEs were documented in the trial reports. However, improvements could be made in reporting the detail of AEs, such as duration of symptoms and need for auxiliary treatment. It should also be acknowledged that rarer AEs are unlikely to emerge in small-scale trials.
A key issue in any comparative trial is that the outcomes of effectiveness and safety relate only to the treatments being studied. Across the review, we identified trials that had clear baseline differences between groups, making it difficult to relate outcomes solely to interventions.
To provide a robust assessment of effect, trials should ideally detail any dropouts and withdrawals, and conduct an ITT analysis (where results are analysed according to allocation whether the designated treatment was received or not, or only partially completed). Such an analysis was often not conducted (or it was unclear), raising issues of attrition bias through selective dropout. Furthermore, certain trials did not always perform a test of statistical significance but merely reported differences in treatment groups in terms of raw numbers or percentages. Conclusions on improvements in outcome may not be reliable in these circumstances.
Reported outcomes mainly related to morbidity (response rates, recurrence rates and relief of symptoms), AEs and mortality where relevant to the condition. While these are obviously essential outcomes to assess, outcomes relating to QoL are also important to consider but these were reported more sparsely. Where reported, they tended to be related to cosmetic appearance, with ratings not always provided by blinded outcome assessors. Some trialists made use of the Karnofsky scale to assess QoL (see Appendix 3). Patient-reported outcomes appear to be underutilised across the PDT literature. Resource use was even more rarely assessed, and, in fact, was investigated only in the oesophageal trials and one biliary tract trial.
Often it was not possible to know whether there were problems with the design, conduct or analysis of a trial or whether there was simply poor reporting. This is a common problem in the medical literature. 152 This problem was exacerbated by the fact that several trials were reported as abstracts only. There are advantages and disadvantages in including abstracts in a systematic review. Inclusion ensures that all evidence has been found and documented. A disadvantage is that the abstract rarely presents sufficient detail for reliable critical appraisal. A further issue relating to reporting is the unclear overlap between publications. Unique studies were difficult to identify, as previous publications were not always referenced. Often an assessment of author names and participant numbers and characteristics was the only way to determine whether a publication represented a follow-up to an existing trial or even a publication reporting the same results from the same patients. Ideally, follow-up and associated publications should be clearly linked and referenced, and duplicate publication avoided in order to ensure no double counting of participants in systematic reviews and other technology assessments.
The above limitations were taken into account when assessing the weight that could be attributed to results. They are detailed here as recommendations for improvement in the conduct and reporting of research in this area.
Not all of the research located in this review was subject to the limitations detailed so far. Where trials were more robust we have been able to draw firmer conclusions. PDT is an active area of ongoing, dynamic research. Over one-half of the included trials in this review were conducted in the last 5 years. As an example, 11 RCTs of PDT for AK were reported in 2008 (out of a total of 28, altogether, in this area). The exception to this is lung cancer, for which the most recent trial was published in 2003, with all others conducted in the 1980s or 1990s.
Statement of principal findings and uncertainties
The appraisal of the evidence above helps to explain why we are unable to draw many definitive conclusions across the cancer sites and conditions investigated. What we are able to conclude, however, is that, overall, PDT appears to be a promising treatment in the majority of conditions we have reviewed. The potential place of PDT amongst the range of other treatments available for each condition is not yet clearly defined.
Skin conditions
Although 28 RCTs have been conducted on PDT for AK, the only clear evidence of effectiveness found was that both MAL–PDT and ALA–PDT appear to be superior to placebo PDT. Uncertainties still exist regarding PDT’s effectiveness relative to cryotherapy, 5-FU and other topical treatments. While optimum parameters for PDT in treating AK remain to be determined, PDT using daylight as a light source may warrant further investigation. The treatment variation found in the cryotherapy studies indicates that the optimal freezing regimens for cryotherapy also appear yet to be determined. SAEs do not appear to be common with PDT, but local skin-related AEs are fairly common. It was unclear – due to inconsistent reporting – whether ALA–PDT and MAL–PDT have different AE profiles.
A small number of trials compared PDT with either cryotherapy or 5-FU for the treatment of Bowen’s disease. There are suggestions of better outcomes with PDT, but these would need further confirmation from larger, well-designed trials. All relevant comparators should be investigated. Further research is also needed to clarify the optimal parameters for PDT in terms of effectiveness and safety.
For superficial BCC, PDT may result in similar lesion response rates to surgery or cryotherapy with better cosmetic outcomes; however, these conclusions are tentative.
The use of MAL–PDT appears superior to placebo for CR in nodular lesions. PDT has been found to be less effective than surgery for nodular BCC in terms of lesion response, but may have better cosmetic outcome. SAEs have not been reported but level of pain needs further investigation in both superficial and nodular BCC. All relevant comparators should be investigated. Further research is also needed to clarify the optimal parameters for PDT in terms of effectiveness and safety.
Barrett’s oesophagus
The 11 RCTs relating to PDT and Barrett’s oesophagus are mainly small, and there is variation between trials in levels of dysplasia, comparators and parameters of PDT, making general statements more challenging. PDT with Ps in addition to omeprazole appears to be more effective than omeprazole alone at long-term ablation of HGD and slowing/preventing progression to cancer in Barrett’s oesophagus. However, PDT’s relative effectiveness compared with other relevant treatment options is unclear. SAEs have not been reported for PDT in Barrett’s oesophagus but AE profiles need to be more clearly established. The priority for PDT research in the area of Barrett’s oesophagus is to determine more clearly the role of PDT and its optimal delivery to patients with HGD.
Oesophageal cancer
Trials have been conducted with curative and palliative intent in oesophageal cancer, but the evidence is not yet sufficiently robust to draw firm conclusions of effectiveness when compared with other treatments such as surgery, radiotherapy or 5-FU. HBO appears to enhance the efficacy of PDT in oesophageal cancer but has not yet been tested in an RCT. It is not yet clear what are the optimal parameters or preferred photosensitisers for PDT in oesophageal cancer, and this is an area of ongoing research. Questions remain around the place of PDT; for example, should PDT be offered as a primary treatment for early-stage oesophageal cancer or following endoscopic mucosal resection or unsuccessful chemoradiotherapy in a palliative setting?
The most likely AEs from the PDT treatments were stricture formation and dysphagia, which may occur in either curative or palliative treatments. Patients who did not follow recommended precautions to prevent over exposure to light were vulnerable to photosensitivity.
Lung cancer
No trials were located for early-stage lung cancer. All included trials related to PDT used with palliative intent. Additionally, with the exception of one trial, the literature dates from the 1980s and 1990s. Further research is needed to determine the role of PDT in lung cancer in relation to current comparators and to identify particular subgroups who might benefit from PDT.
Cancers of the biliary tract
Photodynamic therapy may improve survival when compared with stenting alone, and an ongoing trial should provide more definitive evidence in a more generalisable patient population. SAEs do not appear to be common. Equally, photosensitisation has not been reported as a major issue. It is unclear if there are variations in effectiveness between photosensitisers in the treatment of cholangiocarcinoma, and there do not appear to have been any controlled trials carried out in other biliary tract cancer sites.
Brain cancer
There is very limited evidence available on PDT for brain cancer and no definitive statements can currently be made. Fluorescence-guided resection with repeated ALA–PDT may possibly have some effectiveness, but whether PDT has any role in treating brain cancer, using current technologies, is a subject needing further debate.
Head and neck cancer
There is a lack of good trial evidence for head and neck cancer. The true value of PDT, in relation to other treatment options, has therefore yet to be established, as have the optimum PDT parameters. PDT’s ability to preserve connective tissue and therefore allow re-treatment makes it worthy of investigation in RCTs for both early head and neck cancers, and palliative treatment.
General uncertainties
The following uncertainties arise due to the evolving nature of PDT research and to existing gaps in the research literature.
-
As more knowledge is gained on the optimal parameters for PDT in each clinical area how will this affect the comparisons between PDT and other treatments? What effect will new developments in PDT have on effectiveness and treatment tolerability? For example, research is ongoing to investigate attaching photosensitisers to antibodies to obtain better tumour selectivity. 153 Another potential development is that of generating light chemically, so that it is not necessary to know the location of every area of cancer in order to deliver light effectively. 154
-
Patient-reported outcomes are not commonly sought across the PDT literature. We do not have patient preference data in the included trials. However, we have some anecdotal evidence of selected patient preferences for PDT (D Longman, KILLING Cancer charity, 2009, personal communication]. We also located a small number of qualitative and survey studies that aimed to assess patients’ experience of PDT155,156 but there is more work to be done in this area. In addition, it would be beneficial to examine the barriers to conducting RCTs in PDT from the perspective of the patient and the clinician.
Chapter 14 Conclusions
Implications for health care
Photodynamic therapy is currently most accepted in the treatment of malignant and pre-malignant non-melanoma skin lesions. In this review we found evidence of effectiveness for the treatment of AK and nodular BCC in relation to placebo. However, we do not yet fully know the effectiveness of PDT in relation to other treatments and optimal parameters for PDT do not appear to be firmly established. The evidence suggests that PDT might be a useful option in Barrett’s oesophagus but its effectiveness in relation to other treatments is not yet apparent.
The ideal model of delivery for the above conditions is not clear. The review did not examine cost-effectiveness and we located very little evidence on resource use. Further work would need to be done on implications for infrastructure, resourcing and development of staff to provide access to PDT in a fair and equitable way.
We did not find any clear evidence implying that PDT should definitely not be used for certain clinical conditions. Rather, there are a number of uncertainties outlined in the previous chapter that require further investigation.
Suggested research priorities
Further research is needed to clarify the uncertainties identified in this report and to continue to develop the field of PDT for cancer. Future studies should bear in mind the quality issues highlighted in the appraisal of the current evidence. The need for further research in each of the clinical areas in this review is detailed in the previous chapter; the priorities listed here apply to all the clinical areas and identify the methodologies that will allow the field of PDT to develop more robust evidence.
-
The optimal parameters of PDT need to be identified across the conditions studied.
-
Future trials need to compare PDT against all relevant comparators to establish its place in the treatment of a given condition and to identify whether subgroups of patients might respond better to PDT.
-
Future trials should study all relevant outcomes, including QoL. They need to balance an assessment of the effectiveness of PDT against the assessment of AEs, and should include a detailed assessment of any such events. Cosmetic ratings should be conducted by patients and blinded outcome assessors.
-
Research is needed on the patient experience of PDT. While QoL and cosmetic outcome data have been gathered to some extent, a deep insight into the acceptability of the treatment should be beneficial. There is no reason why this research should not be embedded in a trial. An example from the field of prostate cancer demonstrates the validity of this approach. 157
-
While the difficulties of conducting high-quality trials in rarer cancers, such as those of the brain and head and neck, are recognised, there is a need to establish where barriers are insurmountable. If RCTs cannot be conducted, other types of evidence could be considered. In some of the rarer sites further evaluation of the observational literature may be informative.
PDT is an active field of research and, as the results of ongoing trials become available, there will be a need to update this review. Research evidence exists in a number of other sites,17 and so far these have not been subject to a thorough, systematic review. Further work should focus on the cost-effectiveness of PDT in those areas where effectiveness and safety have been established.
Acknowledgements
A large number of people contributed in various ways to this project.
We would like to thank the following people for agreeing to be part of the review advisory group who commented on the protocol, answered our questions and/or reviewed relevant sections of the draft final report. Not all people who agreed to be on the advisory group were able to provide input at all stages of the review.
| Mr Roger Ackroyd | Consultant Upper Gastrointestinal Surgeon, Sheffield Teaching Hospitals NHS Trust |
| Professor Steve Bown | Professor of Laser Medicine and Surgery, Director, National Medical Laser Centre |
| Dr David DeBerker | Consultant Dermatologist, University Hospitals Bristol NHS Foundation Trust |
| Mr Sam Eljamel | Consultant Neurological Surgeon, Ninewells Hospital and Medical School, University of Dundee |
| Mr Colin Hopper | Head of the Unit of Oral and Maxillofacial Surgery, Eastman Dental Institute and Hospital; Senior Research Fellow, National Medical Laser Centre, London |
| Dr Sally Ibbotson | Clinical Senior Lecturer in Photobiology, Ninewells Hospital and Medical School, University of Dundee |
| Professor Andrew H Kaye | James Stewart Professor of Surgery, Head of Department of Surgery, The University of Melbourne |
| Professor Herwig Kostron | Department of Neurosurgery, Medical University Innsbruck |
| Dr Robert Milroy | Consultant in Respiratory Medicine, Stobhill Hospital, Glasgow |
| Dr Steve Pereira | Senior Lecturer in Hepatology & Gastroenterology, Royal Free and University College London Medical School |
| Mr Iain Tait | Consultant Surgeon and Senior Lecturer, Ninewells Hospital and Medical School, University of Dundee |
| Dr John Winter | Consultant Respiratory Physician, King’s Cross Hospital, Dundee |
We thank Dr Lothar Lilge for helpful comments and advice regarding PDT for the treatment of brain cancer. We also thank Professor Keyvan Moghissi for meeting with us and providing a range of background information and publications for consideration in the review.
We acknowledge, with thanks, the trialists who gave additional information on ongoing or completed trials. We are also grateful to researchers in the Health Technology and Policy Unit, School of Public Health, University of Alberta, for sharing their experience of systematic reviewing in this area.
We acknowledge the input of David Longman of the charity ‘KILLING Cancer’. The work of the charity is focused on promoting the patient health benefits of PDT and funding research to develop new PDT treatments. We thank him for providing information on the patient experience of PDT, in addition to background information and publications for consideration in the review.
We are grateful to Sara Suekarran and Jackie Bentinck of CRD for assistance with data extraction and production of tables, respectively. We thank Gill Forder for help with producing the final report.
We thank the individuals who helped translate studies during the study selection and data extraction process. They are Sophie Cheng, Anneliese Emmans-Dean, Yumi Nixon, Merek Grzes, Baurzhan Aituov and Huiqin Yang.
Contribution of authors
Debra Fayter contributed to all stages of the systematic review from the development of the protocol to the production of the final report, she also took day-to-day responsibility for the project. Mark Corbett contributed to all stages of the systematic review from the development of the protocol to the production of the final report. He managed the project software and devised the meta-analyses. Morag Heirs contributed to all stages of the systematic review from the development of the protocol to the production of the final report. She also took responsibility for managing the papers and the project software, and for contacting researchers and study authors. David Fox devised the search strategy, carried out the literature searches and wrote the search methodology sections of the report. Alison Eastwood contributed to all stages of the review, commented on drafts of the report and took overall responsibility for the project.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Mitton D, Ackroyd R. A brief overview of photodynamic therapy in Europe. Photodiagn Photodyn Ther 2008;5:103-11.
- Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat 2005;4:283-93.
- Leeds Centre for Photobiology and Photodynamic Therapy 2009. www.bmb.leeds.ac.uk/pdt/index.html (accessed 15 July 2009).
- Kelty CJ, Brown NJ, Reed MWR, Ackroyd R. The use of 5-aminolaevulinic acid as a photosensitiser in photodynamic therapy and photodiagnosis. Photochem Photobiol Sci 2002;1:158-68.
- Morton CA, Brown SB, Collins S, Ibbotson S, Jenkinson H, Kurwa H, et al. Guidelines for topical photodynamic therapy: report of a workshop of the British Photodermatology Group. Br J Dermatol 2002;146:552-67.
- National Institute for Health and Clinical Excellence (NICE) . Photodynamic Therapy for Early-Stage Oesophageal Cancer (Interventional Procedure Guidance: IPG200) 2006. http://guidance.nice.org.uk/IPG200.
- National Institute for Health and Clinical Excellence (NICE) . Palliative Photodynamic Therapy for Advanced Oesophageal Cancer (Interventional Procedure Guidance: IPG206) 2007. http://guidance.nice.org.uk/IPG206.
- National Institute for Health and Clinical Excellence (NICE) . Photodynamic Therapy for Advanced Bronchial Carcinoma (Interventional Procedure Guidance: IPG87) 2004. www.nice.org.uk/Guidance/IPG087.
- National Institute for Health and Clinical Excellence (NICE) . Photodynamic Therapy for High-Grade Dysplasia in Barrett’s Oesophagus (Interventional Procedure Guidance: IPG82) 2004. www.nice.org.uk/Guidance/IPG082.
- National Institute for Health and Clinical Excellence (NICE) . Photodynamic Therapy for Bile Duct Cancer (Interventional Procedure Guidance: IPG134) 2005. www.nice.org.uk/Guidance/IPG134.
- National Institute for Health and Clinical Excellence (NICE) . Photodynamic Therapy for Localised Inoperable Endobronchial Cancer (Interventional Procedure Guidance: IPG137) 2005. www.nice.org.uk/Guidance/IPG137.
- National Institute for Health and Clinical Excellence (NICE) . Photodynamic Therapy for Non-Melanoma Skin Tumours (including Premalignant and Primary Non-Metastatic Skin Lesions) (Interventional Procedure Guidance: IPG155) 2006. http://guidance.nice.org.uk/IPG155.
- National Institute for Health and Clinical Excellence (NICE) . Photodynamic Therapy for Brain Tumours (Interventional Procedure Guidance: IPG290) 2009. www.nice.org.uk/Guidance/IPG290.
- National Institute for Health and Clinical Excellence (NICE) . Interstitial Photodynamic Therapy for Malignant Parotid Tumours (Interventional Procedure Guidance: IPG259) 2008. www.nice.org.uk/Guidance/IPG259.
- Centre for Reviews and Dissemination (CRD) . CRD Website Help Page n.d. www.crd.york.ac.uk/crdweb/html/help.htm (accessed 15 July 2009).
- Maziak DE, Markman BR, MacKay JA, Evans WK. Photodynamic therapy in nonsmall cell lung cancer: a systematic review. Ann Thorac Surg 2004;77:1484-91. www.sciencedirect.com/science/article/B6T11–4C283BF-35/2/6d1bff1bcf1847ce68d8a49ded9218d8.
- Edmonton AB. Photodynamic Therapy for Cancer Summaries and Evidence Tables [unpublished Draft Report] n.d.
- Edmonton AB. Photodynamic therapy for the treatment of Barrett’s esophagus: a systematic review and economic evaluation. Health Technology & Policy Unit, School of Public Health, Department of Public Health Sciences, University of Alberta; 2009.
- Edmonton AB. Photodynamic therapy for the treatment of early esophageal cancer: a systematic review and economic evaluation. Health Technology & Policy Unit, School of Public Health, Department of Public Health Sciences, University of Alberta; 2009.
- Green S, Tawil A, Barr H, Bennett C, Bhandari P, DeCaestecker J, et al. Surgery versus radical endotherapies for early cancer and high grade dysplasia in Barrett’s oesophagus. Cochrane Database Syst Rev 2009;2. 10.1002/14651858.
- Rees J, Lao-Sireix P, Wong A, Fitzgerald R. Treatment for Barett’s oesophagus. Cochrane Database Syst Rev 2010;1.
- Centre for Reviews and Dissemination (CRD) . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare 2009.
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896-900.
- endnote . X1.0.01 Bld 2682 Edn 2009.
- eppi-reviewer 2009.
- revman 2009.
- Marks R, Jolley D, Dorevitch AP, Selwood TS. The incidence of non-melanocytic skin cancers in an Australian population: results of a five-year prospective study. Med J Aust 1989;150:475-8.
- Marks R, Selwood TS. Solar keratoses. The association with erythemal ultraviolet radiation in Australia. Cancer 1985;56:2332-6.
- Memon A, Tomenson J, Bothwell J, Freidmann P. Prevalence of solar damage and actinic keratois in a Merseyside population. Br J Dermatol 2000;142:1154-9.
- Lehmann P. Methyl aminolaevulinatephotodynamic therapy: a review of clinical trials in the treatment of actinic keratoses and nonmelanoma skin cancer. Br J Dermatol 2007;156:793-801. www.ingentaconnect.com/content/bsc/bjd/2007/00000156/00000005/art00002.
- Helfand M, Gorman AK, Mahon S, Chan BKS, Swanson N. Actinic keratoses. Portland, OR: Oregon Health & Science University, Evidence Based Practice Centre; 2001.
- Olsen EA, Abernethy L, Kulp-Shorten C, Callen JP, Glazer SD, Huntley A, et al. A double-blind vehicle controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol 1991;24:738-43.
- Hamblin MR, Mroz P. Advances in photodynamic therapy: basic, transitional and clinical. Boston: Artech House; 2008.
- Stritt A, Merk H, Braathen L, von Felbert V. Photodynamic therapy in the treatment of actinic keratosis. Photochem Photobiol 2008;84:388-98.
- Gupta AK. J Am Acad Dermatol 2004;50.
- Legat FJ, Holzer A, Wackernagel A, Hofer A, Quehenberger F, Kerl H, et al. Photodynamic therapy (PDT) with fractionated versus unfractionated illumination: a half-side comparison in patients with actinic keratoses. J Invest Dermatol 2006;126.
- Moloney FJ, Collins P. Randomised, double-blind, prospective study to compare topical 5-aminolaevulinic acid methylester with topical 5-aminolaevulinic acid photodynamic therapy for extensive scalp actinic keratosis. J Invest Dermatol 2007;127.
- Szeimies M, Babilas P, Hummel S, Koller M, Knobler R. Variable pulsed light (VPL) reduces treatment pain in patients undergoing photodynamic therapy (PDT) for actinic keratoses. J Invest Dermatol 2007;127.
- Touma D, Whitehead S, Konnikov N, Phillips T, Yaar M, Gilchrest B. Short incubation delta-ALA PDT for treatment of actinic keratoses and facial photodamage. Lasers Surg Med 2003;32.
- Wiegell SR, Haedersdal M, Wulf H. Photodynamic therapy of actinic keratoses with two different concentrations of methyl aminolevulinate and home-based daylight exposure: a randomized, controlled, double-blinded study [Abstract]. J Invest Dermatol 2008;128.
- Jeffes E, McCullough J, Weinstein G, Shull T, Kaplan R, Glazer S, et al. Photodynamic therapy of patients having multiple actinic keratoses with topical 5-aminolevulinic acid (ALA) and non-laser blue light [Abstract]. J Invest Dermatol 1998;110:680-1248.
- Dragieva G, Prinz BM, Hafner J, Dummer R, Burg G, Binswanger U, et al. A randomized controlled clinical trial of topical photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratoses in transplant recipients. Br J Dermatol 2004;151:196-200.
- Ericson MB, Sandberg C, Stenquist B, Gudmundson F, Karlsson M, Ros AM, et al. Photodynamic therapy of actinic keratosis at varying fluence rates: assessment of photobleaching, pain and primary clinical outcome. Br J Dermatol 2004;151:1204-12.
- Fowler JF, Zax RH. Aminolevulinic acid hydrochloride with photodynamic therapy: efficacy outcomes and recurrence 4 years after treatment. Cutis 2002;69:2-7.
- Freeman M, Vinciullo C, Francis D, Spelman L, Nguyen R, Fergin P, et al. A comparison of photodynamic therapy using topical methyl aminolevulinate (Metvix) with single cycle cryotherapy in patients with actinic keratosis: a prospective, randomized study. J Dermatol Treat 2003;14:99-106.
- Kaufmann R, Spelman L, Weightman W, Reifenberger J, Szeimies RM, Verhaeghe E, et al. Multicentre intraindividual randomized trial of topical methyl aminolaevulinate-photodynamic therapy vs. cryotherapy for multiple actinic keratoses on the extremities. Br J Dermatol 2008;158:994-9.
- Kurwa HA, Yong-Gee SA, Seed PT, Markey AC, Barlow RJ. A randomized paired comparison of photodynamic therapy and topical 5-fluorouracil in the treatment of actinic keratoses. J Am Acad Dermatol 1999;41:414-18.
- Morton C, Campbell S, Gupta G, Keohane S, Lear J, Zaki I, et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol 2006;155:1029-36.
- Pariser DM, Lowe NJ, Stewart DM, Jarratt MT, Lucky AW, Pariser RJ, et al. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: results of a prospective randomized multicenter trial. J Am Acad Dermatol 2003;48:227-32.
- Smith S, Piacquadio D, Morhenn V, Atkin D, Fitzpatrick R. Short incubation PDT versus 5-FU in treating actinic keratoses. J Drugs Dermatol 2003;2:629-35.
- Szeimies RM, Karrer S, Radakovic-Fijan S, Tanew A, Calzavara-Pinton PG, Zane C, et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: a prospective, randomized study. J Am Acad Dermatol 2002;47:258-62.
- Tarstedt M, Rosdahl I, Berne B, Svanberg K, Wennberg A-M. A randomized multicenter study to compare two treatment regimens of topical methyl aminolevulinate (Metvix)-PDT in actinic keratosis of the face and scalp. Acta Dermatol Venereol 2005;85:424-8.
- Wiegell SR, Haedersdal M, Philipsen PA, Eriksen P, Enk CD, Wulf HC. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br J Dermatol 2008;158:740-6.
- Braathen LR, Paredes BE, Saksela O, Fritsch C, Gardlo K, Morken T, et al. Short incubation with methyl aminolevulinate for photodynamic therapy of actinic keratoses. J Eur Acad Dermatol Venereol 2009;23:550-5.
- Hauschild A, Popp G, Stockfleth E, Meyer K-G, Imberger D, Mohr P, et al. Effective photodynamic therapy of actinic keratoses on the head and face with a novel, self-adhesive 5-aminolaevulinic acid patch. Exp Dermatol 2009;18:116-21.
- Pariser D, Loss R, Jarratt M, Abramovits W, Spencer J, Geronemus R, et al. Topical methyl-aminolevulinate photodynamic therapy using red light-emitting diode light for treatment of multiple actinic keratoses: a randomized, double-blind, placebo-controlled study. J Am Acad Dermatol 2008;59:569-76.
- Puizina-Ivic N, Zorc H, Vanjaka-Rogosic L, Miric L, Persin A. Fractionated illumination improves the outcome in the treatment of precancerous lesions with photodynamic therapy. Coll Antropol 2008;32:67-73.
- Szeimies R-M, Matheson RT, Davis SA, Bhatia AC, Frambach Y, Klovekorn W, et al. Topical methyl aminolevulinate photodynamic therapy using red light-emitting diode light for multiple actinic keratoses: a randomized study. Dermatol Surg 2009;35:586-92.
- Wennberg A-M, Stenquist B, Stockfleth E, Keohane S, Lear JT, Jemec G, et al. Photodynamic therapy with methyl aminolevulinate for prevention of new skin lesions in transplant recipients: a randomized study. Transplantation 2008;86:423-9.
- Hauschild A, Stockfleth E, Popp G, Borrosch F, Bruning H, Dominicus R, et al. Optimization of photodynamic therapy with a novel self-adhesive 5-aminolaevulinic acid patch: results of two randomized controlled phase III studies. Br J Dermatol 2009;160:1066-74.
- Sotiriou E, Apalla Z, Maliamani F, Zaparas N, Panagiotidou D, Ioannides D. Intraindividual, right-left comparison of topical 5-aminolevulinic acid photodynamic therapy vs. 5% imiquimod cream for actinic keratoses on the upper extremities. J Eur Acad Dermatol Venereol 2009;23:1061-5.
- Sylvester RJ, Pinedo HM, De Pauw M, Staquet MJ, Buyse ME, Renard J. Quality of institutional participation in multicenter clinical trials. N Engl J Med 1981;305:852-5.
- Ioannidis JP, Dixon DO, McIntosh M, Albert JM, Bozzette SA, Schnittman SM. Relationship between event rates and treatment effects in clinical site differences within multicenter trials: an example from primary. Pneumocystis Carinii 1999;20:253-66.
- Attili SK, Ibbotson SH. How we treat Bowen’s disease with topical photodynamic therapy in Dundee. Photodiagn Photodyn Ther 2009;6:41-5.
- Cancerbackup n.d. www.cancerbackup.org.uk/Home (accessed 4 June 2009).
- Chute CG, Chuang T-Y, Bergstralh EJ, Su WP. The subsequent risk of internal cancer with Bowen’s disease. A population-based study. JAMA 1991;266:816-19.
- Cox NH, Eedy DJ, Morton CA. Guidelines for management of Bowen’s disease. British Association of Dermatologists. Br J Dermatol 1999;141:633-41.
- Lui H, Hobbs L, Tope WD, Lee PK, Elmets C, Provost N, et al. Photodynamic therapy of multiple nonmelanoma skin cancers with verteporfin and red light-emitting diodes: two-year results evaluating tumor response and cosmetic outcomes. Arch Dermatol 2004;140:26-32.
- de Haas ERM, Sterenborg HJCM, Neumann HAM, Robinson DJ. Response of Bowen disease to ALA-PDT using a single and a 2-fold illumination scheme. Arch Dermatol 2007;143:264-5.
- Morton CA, Whitehurst C, Moore JV, MacKie RM. Comparison of red and green light in the treatment of Bowen’s disease by photodynamic therapy. Br J Dermatol 2000;143:767-72.
- Morton CA, Whitehurst C, Moseley H, McColl JH, Moore JV, Mackie RM. Comparison of photodynamic therapy with cryotherapy in the treatment of Bowen’s disease. Br J Dermatol 1996;135:766-71.
- Salim A, Leman JA, McColl JH, Chapman R, Morton CA. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen’s disease. Br J Dermatol 2003;148:539-43.
- Morton C, Horn M, Leman J, Tack B, Bedane C, Tjioe M, et al. Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or Fluorouracil for treatment of squamous cell carcinoma in situ: results of a multicenter randomized trial. Arch Dermatol 2006;142:729-35.
- Bath-Hextall FJ, Perkins W, Bong J, Williams HC. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev 2007;1.
- Raasch BA, Buettner PG. Multiple nonmelanoma skin cancer in an exposed Australian population. Int J Dermatol 2002;41:652-8.
- Telfer NR, Colver GB, Morton CA. British Association of Dermatologists . Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008;159:35-48.
- Foley P, Freeman M, Siller G, Gebauer K, Murrell D, Barnetson R, et al. A phase III randomized study comparing photodynamic therapy (PDT) using methyl aminolevulinate or placebo cream in nodular basal cell carcinoma (NBCC) [Abstract P9–14]. J Eur Acad Dermatol Venereol 2003;17.
- Tope WD, Menter A, El-Azhary RA, Lowe NJ. Comparison of topical methylaminolevulinate photodynamic therapy versus placebo photodynamic therapy in nodular basal cell carcinoma. J Am Acad Dermatol 2004;50.
- Basset-Seguin N, Ibbotson SH, Emtestam L, Tarstedt M, Morton C, Maroti M, et al. Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: a 5 year randomized trial. Eur J Dermatol 2008;18:547-53.
- Szeimies RM, Ibbotson S, Murrell DF, Rubel D, Frambach Y, de Berker D, et al. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8–20 mm), with a 12-month follow-up. J Eur Acad Dermatol Venereol 2008;22:1302-11.
- Schleier P, Berndt A, Kolossa S, Zenk W, Hyckel P, Schultze-Mosgau S. Comparison of aminolevulinic acid (ALA)-thermogel-PDT with methyl-ALA-thermogel-PDT in basal cell carcinoma. Photodiagn Photodyn Ther 2007;4:197-201.
- Soler AM, Angell-Petersen E, Warloe T, Tausjo J, Steen HB, Moan J, et al. Photodynamic therapy of superficial basal cell carcinoma with 5-aminolevulinic acid with dimethylsulfoxide and ethylendiaminetetraacetic acid: a comparison of two light sources. Photochem Photobiol 2000;71:724-9.
- de Haas ERM, Kruijt B, Sterenborg HJCM, Martino Neumann HA, Robinson DJ. Fractionated illumination significantly improves the response of superficial basal cell carcinoma to aminolevulinic acid photodynamic therapy. J Invest Dermatol 2006;126:2679-86.
- Kuijpers DI, Thissen MR, Thissen CA, Neumann MH. Similar effectiveness of methyl aminolevulinate and 5-aminolevulinate in topical photodynamic therapy for nodular basal cell carcinoma. J Drugs Dermatol 2006;5:642-5.
- Rhodes LE, de Rie MA, Leifsdottir R, Yu RC, Bachmann I, Goulden V, et al. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch Dermatol 2007;143:1131-6.
- Mosterd K, Thissen MRTM, Nelemans P, Kelleners-Smeets NWJ, Janssen RLLT, Broekhof KGME, et al. Fractionated 5-aminolaevulinic acid-photodynamic therapy vs. surgical excision in the treatment of nodular basal cell carcinoma: results of a randomized controlled trial. Br J Dermatol 2008;159:864-70.
- Berroeta L, Clark C, Dawe RS, Ibbotson SH, Fleming CJ. A randomized study of minimal curettage followed by topical photodynamic therapy compared with surgical excision for low-risk nodular basal cell carcinoma. Br J Dermatol 2007;157:401-3.
- Wang I, Bendsoe N, Klinteberg CA, Enejder AM, Andersson-Engels S, Svanberg S, et al. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. Br J Dermatol 2001;144:832-40.
- Soga F, Hanada K, Katoh N, Kishimoto S. Successful treatment of oral florid papillomatosis with a combination modality of photodynamic therapy and oral etretinate. Skin Res 2004;3:485-8.
- Wolfsen HC, Hemminger LL, Wallace MB, Devault KR. Clinical experience of patients undergoing photodynamic therapy for Barrett’s dysplasia or cancer. Aliment Pharmacol Ther 2004;20:1125-31.
- Mesquita A, Sa F, Issa MC, Villar E. Photodynamic therapy with methyl aminolevulinate and LED (red light) to nodular basal cell carcinoma in the leg. J Am Acad Dermatol 2007;56.
- Soler AM, Warloe T, Berner A, Giercksky KE. A follow-up study of recurrence and cosmesis in completely responding superficial and nodular basal cell carcinomas treated with methyl 5-aminolaevulinate-based photodynamic therapy alone and with prior curettage. Br J Dermatol 2001;145:467-71.
- Barrett’s Esophagus Website n.d. www.barrettsinfo.com/ (accessed 7 July 2009).
- Gray J, Fullarton G. The current role of photodynamic therapy in oesophageal dysplasia and cancer. Photodiagn Photodyn Ther 2007;4:151-9.
- Ackroyd R, Brown NJ, Davis MF, Stephenson TJ, Marcus SL, Stoddard CJ, et al. Photodynamic therapy for dysplastic Barrett’s oesophagus: a prospective, double blind, randomised, placebo controlled trial. Gut 2000;47:612-17.
- Ackroyd R, Brown N, Reed MWR. Photodynamic therapy (PDT) for Barrett’s oesophagus: establishing optimal treatment parameters [Abstract]. Eur J Surg Oncol 1996;22:551-2.
- Hage M, Siersema PD, van Dekken H, Steyerberg EW, Haringsma J, van de Vrie W, et al. 5-Aminolevulinic Acid Photodynamic Therapy Versus Argon Plasma Coagulation for Ablation of Barrett’s Oesophagus: A Randomised Trial 2004;53:785-90.
- Kelty CJ, Ackroyd R, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MWR. Endoscopic ablation of Barrett’s oesophagus: a randomized-controlled trial of photodynamic therapy vs. argon plasma coagulation. Aliment Pharmacol Ther 2004;20:1289-96.
- Overholt BF, Wang KK, Burdick JS, Lightdale CJ, Kimmey M, Nava HR, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc 2007;66:460-8.
- Ragunath K, Krasner N, Raman VS, Haqqani MT, Phillips CJ, Cheung I. Endoscopic ablation of dysplastic Barrett’s oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol 2005;40:750-8.
- Zoepf T, Alsenbesy M, Jakobs R, Apel D, Rosenbaum A, Riemann JF. Photodynamic therapy (PDT) versus argon plasma, coagulation (APC) for ablative therapy of Barrett’s esophagus. Gastrointest Endosc 2003;57.
- Kelty CJ, Ackroyd R, Brown NJ, Brown SB, Reed MWR. Comparison of high- vs low-dose 5-aminolevulinic acid for photodynamic therapy of Barrett’s esophagus. Surg Endosc 2004;18:452-8.
- Mackenzie G, Selvasekar C, Jamieson N, Mosse C, Thorpe S, Novelli M, et al. Low incidence of esophageal adenocarcinoma following optimal regimen of ALA PDT for high grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2007;65.
- Mackenzie GD, Dunn JM, Novelli MR, Mosse S, Thorpe SM, Bown SG, et al. Preliminary results of a randomised controlled trial into the safety and efficacy of ala versus photofrin photodynamic therapy for high grade dysplasia in Barrett’s oesophagus. Gut 2008;57.
- Mackenzie GD, Dunn JM, Selvasekar CR, Mosse CA, Thorpe SM, Novelli MR, et al. Optimal conditions for successful ablation of high-grade dysplasia in Barrett’s oesophagus using aminolaevulinic acid photodynamic therapy. Lasers Med Sci 2009;24:729-34.
- Cancer Research UK Website n.d. www.cancerresearchuk.org/ (accessed 2 July 2009).
- Canto MI, Gress F, Wolfsen HC. Long term outcomes of curative bare fiber porfimer sodium (Ps): Photodynamic therapy (PDT) for T1NO cancers (CA) of the esophagus and esophagogastric junction (EGJ): a multicenter prospective cohort study. Gastrointest Endosc 2005;61.
- Grosjean P, Wagnieres G, Fontolliet C, van den Bergh H, Monnier P. Clinical photodynamic therapy for superficial cancer in the oesophagus and the bronchi: 514 nm compared with 630 nm light irradiation after sensitization with Photofrin II. Br J Cancer 1998;77:1989-95.
- Savary JF, Grosjean P, Monnier P, Fontolliet C, Wagnieres G, Braichotte D, et al. Photodynamic therapy of early squamous cell carcinomas of the esophagus: a review of 31 cases. Endoscopy 1998;30:258-65.
- Scotiniotis IA, Kochman ML, Sanfilipo N, Hahn S, Rosato E, Ginsberg GG. Management of superficial esophageal cancer: outcomes of esophagectomy, photodynamic therapy, or endoscopic mucosal resection. Gastrointest Endosc 2000;51.
- Lecleire S, Di Fiore F, Antonietti M, Ben-Soussan E, Lerebours E, Michel P, et al. Photodynamic therapy in superficial esophageal cancer: a comparison between patients treated in primary intent and patients treated as a salvage therapy after local failure of chemoradiotherapy. Gastrointest Endosc 2008;67:A186-AB7.
- Lightdale CJ, Heier SK, Marcon NE, McCaughan JS, Gerdes H, Overholt BF, et al. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest Endosc 1995;42:507-12.
- Zhang NZ, Zhu Y, Pan W, Ma WQ, Shao AL. Photodynamic therapy combined with local chemotherapy for the treatment of advanced esophagocardiac carcinoma. Photodiagn Photodyn Ther 2007;4:60-4.
- Heier SK, Rothman KA, Heier LM, Rosenthal WS. Photodynamic therapy for obstructing esophageal cancer: light dosimetry and randomized comparison with Nd:YAG laser therapy. Gastroenterology 1995;109:63-72.
- Maier A, Anegg U, Fell B, Rehak P, Ratzenhofer B, Tomaselli F, et al. Hyperbaric oxygen and photodynamic therapy in the treatment of advanced carcinoma of the cardia and the esophagus. Lasers Surg Med 2000;26:308-15.
- Maier A, Tomaselli F, Anegg U, Rehak P, Fell B, Luznik S, et al. Combined photodynamic therapy and hyperbaric oxygenation in carcinoma of the esophagus and the esophago-gastric junction. Eur J Cardiothorac Surg 2000;18:649-54.
- Maier A, Tomaselli F, Gebhard F, Rehak P, Smolle J, Smolle-Juttner FM. Palliation of advanced esophageal carcinoma by photodynamic therapy and irradiation. Ann Thorac Surg 2000;69:1006-9.
- Maier A, Tomaselli F, Matzi V, Rehak P, Pinter H, Smolle-Juttner FM. Does new photosensitizer improve photodynamic therapy in advanced esophageal carcinoma?. Lasers Surg Med 2001;29:323-7.
- Zhang JW, Wang JH, Liu FW. Analysis of long term survival rate on radiotherapy combined with photodynamic therapy for advanced esophageal cancer. Zhong Guo Yi Shi Za Zhi 2003;5:1058-60.
- Maier A, Tomaselli F, Matzi V, Rehak P, Pinter H, Smolle-Juttner FM. Photosensitization with hematoporphyrin derivative compared to 5-aminolaevulinic acid for photodynamic therapy of esophageal carcinoma. Ann Thorac Surg 2001;72:1136-40.
- Baas P. Brapho Study Group . Photodynamic therapy and/or endobronchial irradiation as pretreatment in external radiation therapy for patients with inoperable non-small cell lung cancer: an interim analysis [Abstract 162]. Eur J Cancer Clin Oncol 1994;30A.
- Diaz-Jimenez JP, Martinez-Ballarin JE, Llunell A, Farrero E, Rodriguez A, Castro MJ. Efficacy and safety of photodynamic therapy versus Nd-YAG laser resection in NSCLC with airway obstruction. Eur Respir J 1999;14:800-5.
- Lam S, Grafton C, Coy P, Voss N, Fairey R. Combined photodynamic therapy (PDT) using photofrin and radiotherapy (XRT) versus radiotherapy alone in patients with inoperable obstructive nonsmall cell bronchogenic carcinoma. SPIE 1991;1616:20-8.
- Lam S, Kostashuk EC, Coy EP, Laukkanen E, LeRiche JC, Mueller HA, et al. A randomized comparative study of the safety and efficacy of photodynamic therapy using Photofrin II combined with palliative radiotherapy versus palliative radiotherapy alone in patients with inoperable obstructive non-small cell bronchogenic carcinoma. Photochem Photobiol 1987;46:893-7.
- Leroy M, Diaz-Jimenez JP, Moghissi K, Diaz-Agero P, Spinelli P, Häussiger K. A phase III randomized, comparative trial of photodynamic therapy (PDT) utilising Photofrin r versus thermal ablation therapy using the Nd:YAG laser for obstructing endobronchial lung cancer. Ann Oncol 1998;9.
- Moghissi K, Dixon K, Parsons RJ. A controlled trial of Nd-YAG laser vs photodynamic therapy for advanced malignant bronchial obstruction. Lasers Med Sci 1993;8:269-73.
- Maier A, Tomaselli F, Matzi V, Woltsche M, Anegg U, Fell B, et al. Comparison of 5-aminolaevulinic acid and porphyrin photosensitization for photodynamic therapy of malignant bronchial stenosis: a clinical pilot study. Lasers Surg Med 2002;30:12-7.
- Spin HG, Huber RM, Janes SM. Thoracic malignancies. Lausanne: European Respiratory Society; 2009.
- Alijiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg 2009;208:134-47.
- Kiesslich T, Wolkersdorfer G, Neureiter D, Salmhofer H, Berr F. Photodynamic therapy for non-resectable perihilar cholangiocarcinoma. Photochem Photobiol Sci 2009;8:23-30.
- The Cholangiocarcinoma Foundation Website n.d. www.cholangiocarcinoma.org/ (accessed 24 June 2009).
- Dechene A, Hildgard P, Maldonado-Lopez EJ, Riemann JF, Gerken G, Zoepf T. Survival difference in patients with photodynamic therapy of nonresectable bile duct cancer using different hematoporphyrins. Gastrointest Endosc 2007;65.
- Kahaleh M, Mishra R, Shami VM, Northup PG, Berg CL, Bashlor P, et al. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol 2008;6:290-7.
- Ortner MEJ, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology 2003;125:1355-63.
- Witzigmann H, Berr F, Ringel U, Caca K, Uhlmann D, Schoppmeyer K, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg 2006;244:230-9.
- Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol 2005;100:2426-30.
- Gores GJ. A spotlight on cholangiocarcinoma. Gastroenterology 2003;125:1536-8.
- Krishnamurthy S, Powers SK, Witmer P, Brown T. Optimal light dose for interstitial photodynamic therapy in treatment for malignant brain tumors. Lasers Surg Med 2000;27:224-34.
- Eljamel MS, Goodman C, Moseley H. ALA and Photofrin(R) Fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci 2008;23:361-7.
- Muller P, Wilson B, Lilge L, Yang V, Varma A, Bogaars A, et al. Clinical studies of photodynamic therapy for malignant brain tumors: Karnofsky score and neurological score in patients with recurrent gloms treated with Photofrin-PDT. SPIE 2002;4612:40-7.
- Li LB, Luo RC, Liao WJ, Zhang MJ, Luo YL, Miao JX. Clinical study of Photofrin photodynamic therapy for the treatment of relapse nasopharyngeal carcinoma. Photodiagn Photodyn Ther 2006;3:266-71.
- Loukatch E, Latyshevska G, Fekeshgazi I. Intraoperative photodynamic therapy for larynx carcinomas. SPIE 1995;2395:245-6.
- Loukatch E, Trojan V, Loukatch V. Intraoperative photodynamic therapy in laryngeal part of pharynx cancers. SPIE 1996;2924:305-8.
- Vakulovskaya E. Photodynamic therapy in oral cancer patients with second-generation photosensitizers (P276). Oral Oncol 2007;2.
- Allison RR, Sibata C, Gay H. PDT for cancers of the head and neck. Photodiagn Photodyn Ther 2009;6:1-2.
- Bouchie A. In brief: Scotia’s woes. Nat Biotechnol 2001;19.
- Sharpe D. Of apples and oranges, file drawers and garbage: why validity issues in meta-analysis will not go away. Clin Psychol Rev 1997;17:881-90.
- Fayter D, McDaid C, Eastwood A. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. J Clin Epidemiol 2007;60:990-1001.
- Takuhiro Y, Yasuo O. Investigating centre effects in a multi-centre clinical trial of superficial bladder cancer. Stat Med 1999;18:1961-71.
- Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM. Adequacy and reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ 2005;330:1057-8.
- Dumville JC, Torgerson DJ, Hewitt CE. Reporting attrition in randomised controlled trials. BMJ 2006;332:969-71.
- Huwiler-Muntener K, Juni P, Junker C, Egger M. Quality of Reporting of Randomized Trials as a Measure of Methodologic Quality. JAMA 2002;287:2801-4.
- Milgrom LR. Towards recombinant antibody-fragment targeted photodynamic therapy. Sci Prog 2008;91:241-63.
- Theodossiou T, Hothersall JS, Woods EA, Okkenhaug K, Jacobson J, MacRobert AJ. Firefly Luciferin-activated Rose Bengal: In Vitro Photodynamic Therapy by Intracellular Chemiluminescence in Transgenic NIH 3T3 Cells. Cancer Res 2003;63:1818-21.
- Goodell T. Measuring subjective side effects and symptoms in palliative photodynamic therapy. Oncol Nurs Forum 2006;33:647-50.
- Walker G, Andrew Maiden J. Understanding patients’ lived experience following photodynamic therapy for the treatment of advanced cancer. Int J Palliat Nurs 2009;15:80-5.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study * Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70.
- Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187-93.
- American Joint Committee on Cancer (AJCC) . What Is Cancer Staging? n.d. www.cancerstaging.org/mission/whatis.html (accessed 23 July 2009).
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1998;124:869-71.
- Dragieva G, Prinz B, Hafner J, Dummer R, Burg G, Binswanger U, et al. Topical photodynamic therapy with MAL in the treatment of actinic keratoses in transplant recipients. Br J Dermatol 2005;153.
- Drugs Information Online, 2006. Levulan Kerastick Official FDA Information, Side Effects and Uses n.d. www.drugs.com/pro/levulan-kerastick.html (accessed 19 June 2009).
- Piacquadio DJ, Chen DM, Farber HF, Fowler JF, Glazer SD, Goodman JJ, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol 2004;140:41-6.
- Foley P, Freeman M, Vinciullo C, Spelman L, Murrell D, Weightman W, et al. A comparison of photodynamic therapy using methyl aminolaevulinate with cryotherapy in actinic keratosis [Abstract]. Br J Dermatol 2003;149.
- Foley P, Freeman M, Vinciullo C, Spelman L, Murrell D, Weightman W, et al. Photodynamic therapy using methyl aminolaevulinate with cryotherapy in actinic keratosis: an Australian study [Abstract]. Br J Dermatol 2005;153.
- Hauschild A, Stockfleth E, Popp G, Borrosch F, Bruening H, Dominicus R, et al. Excellent cosmetic outcome after photodynamic therapy of actinic keratosis with an innovative 5-aminolaevulinic acid patch proves superior to cryosurgery. Br J Dermatol 2008;159.
- Morton C, Campbell S, Gupta G, Keohane S, Lear J, Zaki I, et al. Intra-individual comparison of MAL-PDT and cryotherapy in subjects with actinic keratoses: a multicentre, randomized, controlled study. J Invest Dermatol 2006;126.
- Morton CA, Campbell S, Gupta G, Keohane S, Lear J, Walton S, et al. Intra-individual, right-left comparison of methyl aminolaevulinate photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized, controlled study. Br J Dermatol 2006;155.
- Pariser D, Szeimies RM. Results from two randomized, multicenter, vehicle-controlled studies evaluating the efficacy and safety of methyl aminolevulinate-photodynamic therapy for the treatment of actinic keratoses. J Am Acad Dermatol 2008;58.
- Szeimies R, Babilas P, Hummel S, Koller M, Knobler R. Variable pulsed light (VPL) reduces treatment-induced pain in patients undergoing photodynamic therapy for actinic keratoses. J Invest Dermatol 2006;126.
- Szeimies R, Hummel S, Knobler R. Variable pulsed light (VPL) reduces treatment induced pain in patients undergoing photodynamic therapy for actinic keratosis. Exp Dermatol 2007;16.
- Szeimies RM, Banilas P, Hummel S, Koller M, Knobler R. Variable pulsed light (VPL) reduces treatment induced pain in patients undergoing photodynamic therapy for actinic keratosis. J Invest Dermatol 2006;126.
- Babilas P, Knobler R, Hummel S, Gottschaller C, Maisch T, Koller M, et al. Variable pulsed light less painful than light emitting diodes for topical photodynamic therapy of actinic keratoses: a prospective randomized controlled trial. Exp Dermatol 2007;16.
- Szeimies RM, Karrer S, Radakovic-Fijan S, Tanew A. Photodynamic therapy using topical methyl aminolevulinate (MAL-PDT) compared with cryotherapy for actinic keratosis: a European prospective, randomized study. J Am Acad Dermatol 2005;52.
- Szeimies RM, Karrer S, Radakovic-Fijan S, Tanew A, Calzavara-Pinton PG, Zane C, et al. Photodynamic therapy using topical methyl aminolaevulinate photodynamic therapy compared with cryotherapy for actinic keratosis: a European prospective, randomized study. Br J Dermatol 2005;153.
- Wennberg AM. Results from a 15-month update of a multicentre study of methyl aminolaevulinate photodynamic therapy in immunocompromised organ transplant recipients with nonmelanoma skin cancer [Abstract P-79]. Br J Dermatol 2006;155.
- Wennberg AM, Keohane S, Lear JT, Jemec G, Mørk C, Christensen E, et al. A multicenter study of MAL-PDT in immunocompromised organ transplant recipients with non-melanoma skin cancer [Abstract P08.77]. J Eur Acad Dermatol Venereol 2005;19.
- Lear JT, Wennberg AM, Schmook T, Keohane S, Stenquist B, Jemec G, et al. A Multicenter, Study of Photodynamic Therapy With Methyl Aminolevulinate (MAL-PDT) in Immunocompromised Organ Transplant Recipients With Non-Melanoma Skin Cancer n.d.
- Morton CA. Topical photodynamic therapy for Bowen’s disease and basal cell carcinoma: an effective therapy? [Abstract]. Br J Dermatol 1996;135.
- Morton C. Topical photodynamic therapy for Bowen’s disease. Australas J Dermatol 2005;46.
- Morton CA, Horn M, Leman J, Tack B, Bedane C, Tijioe M, et al. A placebo-controlled multicentre study comparing photodynamic therapy using methyl aminolaevulinate with cryotherapy and 5-fluorouracil in Bowen’s disease. Br J Dermatol 2004;151.
- Morton CA, Horn M, Leman J, Tack B, Bedane C, Tjioe M, et al. A randomised, placebo-controlled, European study comparing MAL-PDT with cryotherapy and 5-fluorouracil in subjects with Bowen’s disease. J Invest Dermatol 2006;126.
- Morton C, Horn M, Leman J, Tack B. A placebo-controlled European study comparing MAL-PDT with cryotherapy and 5-fluorouracil in patients with Bowen’s disease. J Am Acad Dermatol 2005;52.
- Morton C, Horn M, Leman J, Tack B, Bedane C, Tjioe M, et al. A placebo controlled European study controlling methylaminolaevulinate photodynamic therapy with cryotherapy and 5-fluorocaril in patients with Bowen’s disease. Br J Dermatol 2005;153.
- Salim A, Morton CA. Comparison of photodynamic therapy with topical 5-fluorouracil in Bowen’s disease. Br J Dermatol 2000;143.
- Basset-Seguin N, Ibbotson S, Emtestam L, Tarstedt M, Morton C, Maroti M, et al. MAL-PDT versus cryotherapy for treatment of primary superficial basal cell carcinoma: Results of a five years prospective randomized trial. J Invest Dermatol 2006;126.
- Basset-Seguin N, Ibbotson S, Emtestam L, Tarstedt M, Morton C, Maroti M, et al. Methyl aminolaevulinate photodynamic therapy vs. cryotherapy in primary superficial basal cell carcinoma: results of a 36-month follow-up. Br J Dermatol 2005;153.
- Basset-Seguin N, Ibbotson S, Emtestam L, Tarstedt M. MAL-PDT versus cryotherapy in primary superficial basal cell carcinoma. results of a 36-month follow-up. J Am Acad Dermatol 2005;52.
- Basset-Seguin N, Ibbotson S, Emtestam L, Tarstedt M, Morton C, Maroti M, et al. Photodynamic therapy using methyl aminolaevulinate is as efficacious as cryotherapy in basal cell carcinoma, with better cosmetic results. Br J Dermatol 2003;149.
- Basset-Seguin N, Ibbotson S, Emtestam L, Tarstedt M, Morton C, Maroti M, et al. Photodynamic therapy using topical methyl aminolaevulinate versus cryotherapy for treatment of primary superficial basal cell carcinoma: results of a five-year prospective randomized trial. Br J Dermatol 2006;155.
- Berroeta L, Clark C, Dawe RS, Ibbotson SH, Fleming CJ. Surgery versus debulking curettage plus topical photodynamic therapy for low-risk nodular basal cell carcinomas. Br J Dermatol 2005;153.
- Rhodes LE, De Rie M, Enstrom Y, Groves R, Morken T, Goulden V, et al. A randomized comparison of excision surgery and photodynamic therapy using methyl aminolaevulinate in nodular basal cell carcinoma. Br J Dermatol 2003;149.
- Rhodes LE, De Rie M, Enstrom Y, Groves R, Morken T, Goulden V, et al. A randomized European comparison of MAL-PDT and excision surgery in nodular basal cell carcinoma. Br J Dermatol 2005;153:28-9.
- Rhodes L, Groves R, Wolf P. A randomized European comparison of MAL-PDT and excision surgery in nodular basal cell carcinoma: a 24-month update. J Am Acad Dermatol 2005;52.
- Rhodes LE, de Rie M, Enstrom Y, Groves R, Morken T, Goulden V, et al. Photodynamic therapy using topical methyl aminolevulinate vs surgery for nodular basal cell carcinoma: results of a multicenter randomized prospective trial. Arch Dermatol 2004;140:17-23.
- Rhodes L, de Rie M, Enstrom Y, Groves R, Morken T, Goulden V, et al. A randomized European comparison of excision surgery and MAL-PDT in nodular basal cell carcinoma: results from a 36-month follow-up [Abstract P08.69]. J Eur Acad Dermatol Venereol 2005;19.
- Rhodes LE, De Rie M, Enstrom Y, Groves R, Morken T, Goulden V, et al. A randomized European comparison of MAL-PDT and excision surgery in nodular basal cell carcinoma: results from a 60 month follow-up study. J Invest Dermatol 2006;126.
- Hage M, Van Dekken H, Haringsma J, Grool TE, Steyerberg EW, Van Veen RLP, et al. 5-aminolevulinic acid-based photodynamic therapy versus argon plasma coagulation for ablation of Barrett’s esophagus: a randomized trial. Gastrointest Endosc 2003;57.
- Kelty CJ, Ackroyd R, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MWR. Endoscopic ablation of Barrett’s oesophagus: a randomised trial of photodynamic therapy (PDT) versus argon plasma coagulation (APC). Gut 2004;53.
- Kelty CJ, Ackroyd R, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MWR. Endoscopic ablation of Barrett’s esophagus: a randomized trial of photodynamic therapy (PDT) versus argon plasma coagulation (APC). Gastrointest Endosc 2004;59.
- Kelty CJ, Ackroyd R, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MWR. Endoscopic ablation of Barrett’s oesophagus: a randomised controlled trial of photodynamic therapy vs. argon plasma coagulation. Br J Surg 2004;91.
- Selvasekar CR, Novelli MR, Thorpe S, Bown SG, Lovat LB. Interim results of a randomized controlled trial (RCT) comparing green and red laser photodynamic therapy using low dose ALA for high grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2004;59.
- Mackenzie G, Selvasekar C, Clark BR, Novelli M, Thorpe S, Mosse C, et al. Randomised controlled trial of photodynamic therapy using low dose 5 aminolevulinic acid activated by red or green light for high grade dysplasia in Barrett’s esophagus. Gastroenterology 2005;128.
- Selvasekar CR, Mackenzie GD, Clark BR, Novelli MR, Thorpe SM, Mosse CA, et al. Randomised controlled trial of photodynamic therapy using low dose 5 aminolevulinic acid activated by red or green light for high grade dysplasia in Barrett’s oesophagus. Gut 2005;54.
- Mackenzie GD, Selvasekar CR, Jamieson N, Mosse CA, Thorpe SM, Novelli MR, et al. Low incidence of oesophageal adenocarcinoma following optimal regimen of aminolaevulinic acid photofrin photodynamic therapy for high grade dysplasia in Barrett’s oesophagus. Gut 2007;56.
- Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488-98.
- Overholt BF, Lightdale CJ, Wang K, Canto M, Burdick S, Barr H, et al. International, multicenter, partially blinded, randomised study of the efficacy of photodymamic therapy (PDT) using porfimer sodium (POR) for the ablation of high-grade dysplasia (HGD) in Barrett’s esophagus (BE): results of 24-month follow-up. Gastroenterology 2003;124.
- Bronner MP, Overholt BF, Taylor SL, Haggitt RC, Wang KK, Burdick JS, et al. Squamous overgrowth is not a safety concern for photodynamic therapy for Barrett’s esophagus with high-grade dysplasia. Gastroenterology 2009;136:56-64.
- Bronner M, Taylor S, Overholt B, Wang K, Burdick S, Lightdale C, et al. Squamous overgrowth in a 5-year randomized phase III trial of photodynamic therapy using porfirmer sodium in ablation of high-grade dysplasia in Barrett’s esophagus. Gastroenterology 2006;130.
- Kapoor N, Ragunath K, Raman V, Haqqani M, Krasner N. Endoscopic ablation of dysplastic Barrett’s oesophagus comparing argon plasma coagulation and photodynamic therapy: long term results of a randomised prospective trial. Gut 2005;54.
- Maier A, Anegg U, Tomaselli F, Rehak P, Sankin O, Fell B, et al. Does hyperbaric oxygen enhance the effect of photodynamic therapy in patients with advanced esophageal carcinoma? A clinical pilot study. Endoscopy 2000;32:42-8.
- Wieman TJ. Photodynamic Therapy (PDT) With Photofrin Is Effective in the Palliation of Obstructive Endobronchial Lung Cancer: Results of Two Randomized Trials [Abstract 1782] n.d.
- Vakulovskaya EG, Ungiadze GV, Tabolinovskaya TD. Photodynamic therapy of oral cancer and cancer of oropharynx with second-generation photosensitizers. Oral Oncol 2005;1.
Appendix 1 Search strategy
The core search strategy used for this review was as follows:
-
photochemotherapy/
-
photosensitizing agents/
-
((photodynamic or (photo adj dynamic)) adj2 therap$).tw.
-
PDT.tw.
-
(photosensitise$or photosensitize$or photosensiti?ing or photochemotherapy or (photo adj chemotherapy)).tw.
-
((photoradiation or (photo adj radiation)) adj2 therap$).tw.
-
PRT.tw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7
-
exp neoplasms/
-
(cancer$or neoplas$or oncolog$or tumour$or tumor$or lump or lumps).tw.
-
(sarcoma$or malignan$or carcinoma$or growth$or mass or masses or lesion$or glioma$).tw.
-
(premalig$or pre-malig$or pre malig$or cyst or cysts).tw.
-
(metastatic or metastases or metastasis or squamous cell$).tw.
-
“Barrett Esophagus”/
-
(barret$adj (oesophagus or esophagus)).tw.
-
9 or 10 or 11 or 12 or 13 or 14 or 15
-
8 and 16
-
exp Animals/not humans/
-
17 not 18
This strategy was designed for searching MEDLINE through the Ovid interface and was adapted as appropriate for all other databases searched, taking into account differences in indexing terms and search syntax for each database.
Full details of all databases searched and search strategies are provided below.
MEDLINE and MEDLINE In-Process: Ovid
http://gateway.ovid.com/athens
The MEDLINE search covered the date range 1950 to present. The search was carried out on 14 August 2008 and identified 7178 records.
-
photochemotherapy/(8857)
-
photosensitizing agents/(5949)
-
((photodynamic or (photo adj dynamic)) adj2 therap$).tw. (7397)
-
PDT.tw. (4852)
-
(photosensitise$or photosensitize$or photosensiti?ing or photochemotherapy or (photo adj chemotherapy)).tw. (8234)
-
((photoradiation or (photo adj radiation)) adj2 therap$).tw. (174)
-
PRT.tw. (651)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (17787)
-
exp neoplasms/(2011836)
-
(cancer$or neoplas$or oncolog$or tumour$or tumor$or lump or lumps).tw. (1412061)
-
(sarcoma$or malignan$or carcinoma$or growth$or mass or masses or lesion$or glioma$).tw. (2012713)
-
(premalig$or pre-malig$or pre malig$or cyst or cysts).tw. (78803)
-
(metastatic or metastases or metastasis or squamous cell$).tw. (250958)
-
“Barrett Esophagus”/(4295)
-
(barret$adj (oesophagus or esophagus)).tw. (4411)
-
9 or 10 or 11 or 12 or 13 or 14 or 15 (3478621)
-
8 and 16 (9326)
-
exp Animals/not humans/(3344509)
-
17 not 18 (7178)
The search was re-run on 28 May 2009, using the same strategy to capture recent studies, and identified 460 additional records.
EMBASE: Ovid
http://gateway.ovid.com/athens
The EMBASE search covered the date range 1980–2008 (week 32). The search was carried out on 14 August 2008 and identified 5690 records.
-
photochemotherapy/(1635)
-
photosensitizing agent/(3791)
-
((photodynamic or (photo adj dynamic)) adj2 therap$).tw. (6228)
-
PDT.tw. (3983)
-
(photosensitise$or photosensitize$or photosensiti?ing or photochemotherapy or (photo adj chemotherapy)).tw. (6580)
-
((photoradiation or (photo adj radiation)) adj2 therap$).tw. (130)
-
PRT.tw. (487)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (13196)
-
exp neoplasm/(1439539)
-
(cancer$or neoplas$or oncolog$or tumour$or tumor$or lump or lumps).tw. (1054121)
-
(sarcoma$or malignan$or carcinoma$or growth$or mass or masses or lesion$or glioma$).tw. (1531626)
-
(premalig$or pre-malig$or pre malig$or cyst or cysts).tw. (51404)
-
(metastatic or metastases or metastasis or squamous cell$).tw. (198043)
-
“Barrett Esophagus”/(5465)
-
(barret$adj (oesophagus or esophagus)).tw. (3915)
-
9 or 10 or 11 or 12 or 13 or 14 or 15 (2518901)
-
8 and 16 (7538)
-
exp animal/(18250)
-
exp animal-experiment/(1251475)
-
nonhuman/(3097648)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (2000565)
-
18 or 19 or 20 or 21 (3512709)
-
exp human/(6267311)
-
exp human-experiment/(249466)
-
23 or 24 (6268176)
-
22 and 25 (620556)
-
22 not 26 (2892153)
-
17 not 27 (5690)
The search was re-run using the same strategy on 21 May 2009 (2009, week 20) to capture recent studies and identified 357 additional records.
CINAHL: Ovid
http://gateway.ovid.com/athens
The CINAHL search covered the date range 1982–2008 (August, week 2). The search was carried out on 14 August 2008 and identified 229 records.
-
photochemotherapy/(124)
-
photosensitizing agents/(112)
-
((photodynamic or (photo adj dynamic)) adj2 therap$).tw. (213)
-
PDT.tw. (119)
-
(photosensitise$or photosensitize$or photosensiti?ing or photochemotherapy or (photo adj chemotherapy)).tw. (55)
-
((photoradiation or (photo adj radiation)) adj2 therap$).tw. (2)
-
PRT.tw. (50)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (453)
-
exp neoplasm/(88899)
-
(cancer$or neoplas$or oncolog$or tumour$or tumor$or lump or lumps).tw. (72661)
-
(sarcoma$or malignan$or carcinoma$or growth$or mass or masses or lesion$or glioma$).tw. (54848)
-
(premalig$or pre-malig$or pre malig$or cyst or cysts).tw. (1672)
-
(metastatic or metastases or metastasis or squamous cell$).tw. (6333)
-
“Barrett Esophagus”/(249)
-
(barret$adj (oesophagus or esophagus)).tw. (214)
-
9 or 10 or 11 or 12 or 13 or 14 or 15 (146875)
-
8 and 16 (235)
-
“animal studies”/(7317)
-
17 not 18 (229)
The search was re-run on 26 May 2009 (1982 to 15 May 2009) to capture recent studies, and identified 47 additional records. An amended strategy was used to search via EBSCO interface (http://web.ebscohost.com) as CINAHL was no longer available via the Ovid interface.
S20 S10 AND S19
S19 S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18
S18 TX barret* N1 esophagus
S17 TX barret* N1 oesophagus
S16 MH Barrett Esophagus
S15 TX metastatic or metastases or metastasis or squamous cell*
S14 TX premalig* or pre-malig* or pre malig* or cyst or cysts
S13 TX sarcoma* or malignan* or carcinoma* or growth* or mass or masses or
lesion* or glioma*
S12 TX cancer* or neoplas* or oncolog* or tumour* or tumor* or lump or
lumps
S11 MH neoplasms
S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9
S9 TX PRT
S8 TX photo radiation N2 therap*
S7 TX photoradiation N2 therap*
S6 TX photosensitise* or photosensitize* or photosensiti?ing or
photochemotherapy or photo
S5 TX PDT
S4 TX photo dynamic N2 therap*
S3 TX photodynamic N2 therap*
S2 MH photosensitizing agents
S1 MH photochemotherapy
Cochrane Library
Including:
-
Cochrane Database of Systematic Reviews (CDSR)
-
Health Technology Assessment Database (HTA)
-
Cochrane Central Register of Controlled Trials (CENTRAL)
-
NHS Economic Evaluation Database (NHS EED)
The search was carried out on 1 September 2008 and identified 334 records. No date limits applied.
#1 MeSH descriptor Photochemotherapy, this term only
#2 MeSH descriptor Photosensitizing Agents, this term only
#3 (photodynamic near/2 therap*):ti,ab
#4 PDT:ti,ab
#5 (photosensitise* or photosensitize* or photochemotherapy or photo chemotherapy or photo-chemotherapy):ti,ab
#6 (photoradiation near/2 therap*):ti,ab
#7 PRT:ti,ab
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
#9 MeSH descriptor Neoplasms explode all trees
#10 (cancer* or neoplas* or oncolog* or tumour* or tumor* or lump or lumps):ti,ab
#11 (sarcoma* or malignan* or carcinoma* or growth* or mass or masses or lesion* or glioma*):ti,ab
#12 (premalig* or pre-malig* or pre malig* or cyst or cysts):ti,ab
#13 (metastatic or metastases or metastasis or squamous cell*):ti,ab
#14 MeSH descriptor Barrett Esophagus, this term only
#15 (barret* near/1 (oesophagus or esophagus)):ti,ab
#16 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15)
#17 (#8 AND #16)
The search was re-run using the same strategy on 26 May 2009 to capture recent studies, and identified 5 additional records.
Database of Abstracts of Reviews of Effects (DARE)
The search was carried out on 7 September 2008, using the internal CRD administration system, and identified 122 records. No date limits applied.
RESTRICT MH Photochemotherapy
photochemotherapy:OK
photochemotherapy:CK
RESTRICT MH Photosensitizing Agents
Photosensitizing Agents:OK
Photosensitizing Agents:CK
photodynamic*
photo dynamic
PDT
photosensiti*
photochemotherapy
photoradiation
PRT
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
or #9 or #10 or #11 or #12 or #13
RESTRICT MH Neoplasms
Neoplasms:OK
Neoplasms:CK
cancer$or neoplas$or oncolog$or tumour$or
tumor$or lump or lumps
sarcoma* or malignan* or carcinoma* or growth*
or mass or masses or lesion* or glioma*
premalig* or pre-malig* or pre malig* or cyst or
cysts
metastatic or metastases or metastasis or squamous
cell*
RESTRICT MH Barrett Esophagus
Barrett Esophagus:OK
Barrett Esophagus:CK
barret* esophagus
barret* oesophagus
#15 or #16 or #17 or #18 or #19 or #20 or #21
or #22 or #23 or #24 or #25 or #26
#14 and #27
The search was re-run using the same strategy on 20 May 2009 to capture recent studies, and identified 19 additional records.
LILACS (Latin American and Caribbean Health Sciences Literature)
The search was carried out on 1 September 2008 and identified 80 records. No date limits applied.
-
photochemotherapy or photosensiti$or photodynamic therap$or PDT or photoradiation therap$(in WORDS)
AND
cancer$or neoplas$or oncolog$or tumour$or tumor$or lump or lumps or sarcoma$or malignan$or carcinoma$or mass or masses or lesion$or glioma$or premalig$or pre-malig$or pre malig$or cyst or cysts or metastatic or metastases or metastasis or squamous cell$or barret$oesophagus or barret$esophagus (in WORDS)
PASCAL [database of INIST (Institut de l’Information Scientifique et Téchnique)]
The search was carried out on 12 September 2008 and identified 2278 records. No date limits applied.
b 144
s (photodynamic or photo(w)dynamic)(2n)(therapy or therapies)
s (photoradiation or photo(w)radiation)(2n)(therapy or therapies)
s PDT or PRT
s photosensitis? or photosensitiz? or
photochemotherapy or photo(w)chemotherapy
s s1:s4
s cancer? or neoplas? or oncolog? or tumour?
or tumor? or lump or lumps or sarcoma? or
malignan? or carcinoma? or growth? or mass or
masses or lesion? or glioma? or premalig? or pre(w)
malig? or cyst or cysts or metastatic or metastases
or metastasis or squamous(w)cell?
s barret?(w)(oesophagus or esophagus)
s s6:s7
s s5 and s8
Current Controlled Trials
The search was carried out on 12 September 2008 and identified 204 records. No date limits applied.
photochemotherapy OR photosensitiser OR
photosensitizer OR “photodynamic therapy”
or “photodynamic therapies” or PDT or
“photoradiation therapy” or PRT
ISI Conference Proceedings Citation Index- Science (CPCI-S)
The search was carried out on 20 October 2008 and identified 958 records. No date limits applied.
Basic search (restricted to CPCI-S);
photochemotherapy OR photosensiti*
OR photodynamic therap* OR PDT OR
photoradiation therap* OR PRT
AND
cancer* OR neoplas* OR oncolog* OR tumour* OR tumor* OR lump OR lumps OR sarcoma* OR malignan* OR carcinoma* OR mass OR masses OR lesion* OR glioma* OR premalig* OR pre-malig* OR pre malig* OR cyst OR cysts OR metastatic OR metastases OR metastasis OR squamous cell* OR barret* oesophagus OR barret* esophagus OR Bowen* OR keratos*
Zetoc (British Library’s Electronic Table of Contents)
The search was carried out on 22 October 2008 and identified 754 records. No date limits applied. Searches were run separately, and results combined and de-duplicated.
conference: photodynamic neoplas* (22)
conference: photochemotherapy neoplas* (16)
conference: PDT neoplas* (3)
conference: photosensiti* neoplas* (2)
conference: photoradiation neoplas* (nil)
conference: PRT neoplas* (nil)
conference: photodynamic cancer* (426)
conference: photochemotherapy cancer* (52)
conference: PDT cancer* (86)
conference: photosensiti* cancer* (98)
conference: photoradiation cancer* (1)
conference: PRT cancer* (nil)
conference: photodynamic barret* (26)
conference: photochemotherapy barret* (2)
conference: PDT barret* (4)
conference: photosensiti* barret* (1)
conference: photoradiation barret* (nil)
conference: PRT barret* (nil)
conference: photodynamic bowen* (14)
conference: photochemotherapy bowen* (1)
conference: PDT bowen* (nil)
conference: photosensiti* bowen* (nil)
conference: photoradiation bowen* (nil)
conference: PRT bowen* (nil)
Appendix 2 Data extraction and quality assessment
Guidelines for data extraction
Population details
Type of cancer and histology
Tick box for type then provide numbers on histology in text box.
Patient characteristics
Use ‘overall population’ figures where available, or where they can be calculated (e.g. 56% male, even though results were given by treatment group), and only break down figures by treatment group when the overall figure cannot be calculated.
Characteristics to look out for, and extract
-
Percentage male: (just state number, no % after).
-
Age range.
-
Mean age.
-
Cancer stage: state numbers if provided, stage II, 2; stage III, 4; stage IV: 7.
-
Number with recurrent tumour.
If this section is lengthy say that ‘Further patient characteristics were reported’.
Eligibility criteria: check for
Age, Karnofsky status, time since last chemotherapy/radiotherapy, inoperability, previous treatment allowed? Other details such as ‘non-pregnant women’ may be extracted.
If this section is lengthy say that ‘Further eligibility criteria were reported’.
Treatment details
Describing PDT (use ‘Not stated’ when necessary)
Check for the following:
-
photosensitiser used (including mode of application)
-
dose of photosensitiser
-
duration of photosensitiser (drug to light interval)
-
light source and duration
-
wavelength of light source
-
power density (mW/cm)
-
total light dose (J/cm)
-
maximum number of sessions/doses allowed
-
postoperative care/advice
-
baseline bronchoscopy (only state if this is absent, using the Study appraisal field)
-
postoperative care/advice.
Rather than stating ‘Dose, duration, etc., were not reported’ state ‘Further PDT parameters were not reported’.
Describing comparators
-
Type and mode of delivery.
-
Dose and duration.
-
Maximum number of sessions/doses allowed.
-
Postoperative care/advice.
Results
Note: Only extract outcome data that are broken down by treatment group.
Morbidity
-
Recurrence and tumour response measures.
-
Symptom burden.
-
Symptom improvement.
-
Time to healing.
Quality of life
-
Quality of life scores.
-
Depression scores.
-
Cosmetic appearance.
Resource use
-
Length of hospital stay.
Adverse events
List all, especially detailing photosensitisation (mention if this is not reported). Treatment-related mortality is detailed here, not in ‘mortality’, which focuses on survival.
Interpretation
Brief study appraisal
In addition to study quality, highlight issues such as ‘were all the assessed outcomes reported?’.
Quality assessment – additional notes
Distinction between ‘no’ and ‘unclear’
Only enter ‘no’ if the paper is explicit.
Losses to follow-up reported?
That is, did the authors explicitly report on whether or not there were any losses to follow-up, or did they say nothing on this issue? If the authors said that there were no losses to follow-up, then answer ‘yes’.
| Section A: Study details | |
|---|---|
| A.1 Authors, year and master endnote number | A.1.1 Specify |
| A.2 Linked endnote numbers | A.2.1 Specify |
| A.3 Data source | A.3.1 Full published paper |
| A.3.2 Abstract | |
| A.3.3 Other (specify) | |
| A.4 Country | |
| A.5 Language | |
| A.6 Study design | A.6.1 RCT |
| A.6.2 Non-RCT | |
| A.7 No. of participants | A.7.1 Total |
| A.7.2 Intervention | |
| A.7.3 Comparator | |
| A.7.4 2nd comparator | |
| A.7.5 3rd comparator | |
| A.7.6 4th comparator | |
| A.8 No. of recruiting centres | A.8.1 Specify |
| A.8.2 Not stated | |
| A.9 Follow-up period and frequency | A.9.1 Specify |
| Section B: Population details | |
| B.1 Treatment intention | B.1.1 Curative |
| B.1.2 Palliative | |
| B.1.3 Not stated | |
| B.2 Type(s) of cancer and histology | B.2.1 Specify |
| B.2.2 Not stated | |
| B.3 Main eligibility criteria | B.3.1 Specify |
| B.3.2 Not stated | |
| B.4 Patient characteristics | B.4.1 Specify |
| B.4.2 Not stated | |
| B.5 Concomitant treatment | B.5.1 Specify |
| B.5.2 Not stated | |
| B.5.3 None | |
| Section C: Treatment details | |
| C.1 Trial treatments | C.1.1 Specify |
| C.2 Intervention | C.2.1 Specify |
| C.3 Comparator | C.3.1 Specify |
| C.4 2nd comparator | C.4.1 Specify |
| C.5 3rd comparator | C.5.1 Specify |
| C.6 4th comparator | C.6.1 Specify |
| Section D: Results | |
| D.1 Mortality | D.1.1 Specify |
| D.1.2 Not assessed | |
| D.1.3 Assessed but not reported | |
| D.2 Morbidity | D.2.1 Specify |
| D.2.2 Not assessed | |
| D.2.3 Assessed but not reported | |
| D.3 QoL and return to normal activity | D.3.1 Specify |
| D.3.2 Not assessed | |
| D.3.3 Assessed but not reported | |
| D.4 Adverse events | D.4.1 Specify |
| D.4.2 Not assessed | |
| D.4.3 Assessed but not reported | |
| D.5 Resource use | D.5.1 Specify |
| D.5.2 Not assessed | |
| D.5.3 Assessed but not reported | |
| Section E: Interpretation | |
| E.1 Authors’ conclusions | E.1.1 Specify |
| E.2 Brief study appraisal | E.2.1 Specify |
Quality assessment tool
| Was randomisation used appropriately? | Yes/no/unclear |
| Was allocation concealment used appropriately? | Yes/no/unclear |
| Was blinding used appropriately? | Yes/no/unclear |
| Were any losses to FU reported? | Yes/no/unclear |
| Was an ITT analysis conducted? | Yes/no/unclear |
| Were trial eligibility criteria reported? | Yes/no/unclear |
| Were AEs reported? | Yes/no |
| Was a power calculation reported? | Yes/no |
| Were primary outcomes defined? | Yes/no/unclear |
| Were groups comparable at baseline? | Yes/no/unclear |
| Did the trial have a representative sample of patients? | Yes/no/unclear |
| Was there more than 1 lesion per patient (skin sites only)? | Yes/no/unclear |
Appendix 3 Karnofsky performance status
Quality of life may be proxied by performance status scores such as the Karnofsky Performance status. 158 The Karnofsky score runs from 100 to 0, where 100 is ‘perfect’ health and 0 is death. Although the score has been described with intervals of 10, a practitioner may choose decimals if he or she feels that a patient’s situation holds somewhere between two marks. It is named after Dr David A Karnofsky, who described the scale with Dr Joseph H Burchenal in 1949.
| 100% – normal, no complaints, no signs of disease |
| 90% – capable of normal activity, few symptoms or signs of disease |
| 80% – normal activity with some difficulty, some symptoms or signs |
| 70% – caring for self, not capable of normal activity or work |
| 60% – requiring some help, can take care of most personal requirements |
| 50% – requires help often, requires frequent medical care |
| 40% – disabled, requires special care and help |
| 30% – severely disabled, hospital admission indicated but no risk of death |
| 20% – very ill, urgently requiring admission, requires supportive measures or treatment |
| 10% – moribund, rapidly progressive fatal disease processes |
| 0% – death |
Appendix 4 Classification systems
There are a number of different staging systems to classify cancer; one of the most common is the TNM classification but numerical systems are also used.
TNM stages
The TNM system159 provides a framework for classifying solid tumours according to the site of the primary tumour, the histological type and degree to which the cancer has spread.
-
‘T’ refers to the primary tumour:
-
– T0 – no evidence of primary tumour
-
– Tis – carcinoma in situ
-
– T1, T2, T3, T4 – size/extent of the primary tumour
-
– TX – primary tumour cannot be evaluated.
-
-
‘N’ refers to regional lymph node involvement:
-
– N0 – no regional lymph node involvement
-
– N1, N2, N3 – involvement of regional lymph nodes (number/extent of spread)
-
– NX – regional lymph nodes cannot be evaluated.
-
-
‘M’ refers to metastasis:
-
– M0 – no metastasis (cancer has not spread to other parts of the body)
-
– M1 – metastasis (cancer has spread to other parts of the body)
-
– MX – metastasis cannot be evaluated.
-
Number staging systems
The number system159 uses numerical values (often written using Roman numerals) to distinguish stages:
-
Stage 0 = carcinoma in situ.
-
Stage I cancers are localized to one part of the body.
-
Stage II cancers are locally advanced.
-
Stage III cancers are also locally advanced. Whether a cancer is designated as Stage II or Stage III can depend on the specific type of cancer. The specific criteria for Stages II and III therefore differ according to diagnosis.
-
Stage IV cancers have often metastasised (spread to other organs or throughout the body).
The stages can be further subdivided using the letters a, b, c, etc. (e.g. Stage IIIb).
The TNM combinations often correspond to one of these numbered stages, although the criteria for this differ for different types of cancer.
Fitzpatrick skin type
Within dermatology, generally, patient skin type may be described in terms of Fitzpatrick score. 160 This is a numerical schema that classifies skin according to how it reacts to UV light. The overall score includes genetic disposition, reaction to sun exposure and tanning habits.
-
Type 1 (scores 0–7) White; very fair; red or blond hair; blue eyes; freckles. Always burns, never tans.
-
Type II (scores 8–16) White; fair; red or blond hair; blue, hazel or green eyes. Usually burns, tans with difficulty.
-
Type III (scores 17–25) Cream white; fair with any eye or hair color; very common. Sometimes mild burn, gradually tans.
-
Type IV (scores 25–30) Dark brown; typical Mediterranean Caucasian skin. Rarely burns, tans with ease.
-
Type V (scores over 30) Dark brown; Middle Eastern skin types. Very rarely burns, tans very easily.
-
Type VI Black; never burns, tans very easily.
Appendix 5 Pre-cancerous skin scoping
One hundred and thirty-three publications, with study designs that did not meet the inclusion criteria for the review, reported on patients with pre-cancerous skin conditions being treated with PDT. The references are listed below, in alphabetical order; they have not been categorised and may still contain a number of duplicate publications.
References
-
Alexiades-Armenakas MR, Bernstein LJ, Chen J, Jacobson L, Geronemus R. Laser-assisted photodynamic therapy of actinic keratoses: long-term follow-up. Lasers Surg Med 2003;15(Suppl.):45.
-
Alexiades-Armenakas MR, Geronemus RG. Laser-mediated photodynamic therapy of actinic keratoses. Arch Dermatol 2003;139:1313–20.
-
Ammann R, Hunziker T. Photodynamic therapy for mycosis fungoides after topical photosensitization with 5-aminolevulinic acid. J Am Acad Dermatol 1995;33:541.
-
Antoniou C, Katsambas A, Rigopoulos D, Palaskas E, Tsikrikas GN. Aminolevulinic acid: topical photodynamic therapy in skin cancers and solar keratoses. Skin Cancer 1996;11:81–4.
-
Attili S, Cochrane A, McNeill A, Camacho-Lopez M, Moseley H, Ibbotson S, et al. An open pilot study of ambulatory photodynamic therapy using a wearable, low-irradiance, organic LED light source in the treatment of nonmelanoma skin cancer. Br J Dermatol 2008;159:130–1.
-
Bakos RM, Bakos L, Ferlin E, Cestari T, Orlandini T, Rezende R, et al. [Photodynamic therapy with delta-aminolevulinic acid for superficial keratinocytic neoplasms.] An Bras Dermatol 2003;78:197–207.
-
Baptista J, Martinez C, Leite L, Cochito M. Our PDT experience in the treatment of non-melanoma skin cancer over the last 7 years. J Eur Acad Dermatol Venereol 2006;20:693–7.
-
Baptista J, Paris F, Serrao V, Cochito M. Topical photodynamic therapy and imiquimod cream in the treatment of actinic keratoses: a case report. Skin Cancer 2006;21:49–53.
-
Baron E, Domingo DS, Hsia A, Colussi V, Oleinick N, Foster T, et al. Silicon phthalocyanine photodynamic therapy for treatment of cutaneous neoplasms. J Invest Dermatol 2008;128:395.
-
Berking C, Herzinger T, Flaig MJ, Brenner M, Borelli C, Degitz K. The efficacy of photodynamic therapy in actinic cheilitis of the lower lip: a prospective study of 15 patients. Dermatol Surg 2007;33:825–30.
-
Berroeta L, Lewis-Jones MS, Evans AT, Ibbotson SH. Woringer-Kolopp (localized pagetoid reticulosis) treated with topical photodynamic therapy (PDT). Clin Exp Dermatol 2005;30:446–7.
-
Breuninger H, Bonnekoh B, Gollnick H. Photodynamic therapy with methylaminooxopentanoate (MetvixR) and a broad band light source (PhotoDyn 501): Experiences in complicated patients with actinic keratoses and basal cell carcinomas [Multiple letters]. JDDG (Journal of the German Society of Dermatology) 2005;3:397.
-
Britton JER, Goulden V, Stables G, Stringer M, Sheehan-Dare R. Investigation of the use of the pulsed dye laser in the treatment of Bowen’s disease using 5-aminolaevulinic acid phototherapy. Br J Dermatol 2005;153:780–4.
-
Brookes PT, Jhawar S, Hinton CP, Murdoch S, Usman T. Bowen’s disease of the nipple: a new method of treatment. Breast (Edinburgh, Scotland) 2005;14:65–7.
-
Buchanan RB, Carruth JA, McKenzie AL, Williams SR. Photodynamic therapy in the treatment of malignant tumours of the skin and head and neck. Eur J Surg Oncol 1989;15:400–6.
-
Cairnduff F, Stringer MR, Hudson EJ, Ash DV, Brown SB. Superficial photodynamic therapy with topical 5-aminolaevulinic acid for superficial primary and secondary skin cancer. Br J Cancer 1994;69:605–8.
-
Calzavara-Pinton PG. Repetitive photodynamic therapy with topical delta-aminolaevulinic acid as an appropriate approach to the routine treatment of superficial non-melanoma skin tumours. J Photochem Photobiol B 1995;29:53–7.
-
Calzavara-Pinton PG, Venturini M, Sala R, Capezzera R, Parrinello G, Specchia C, et al. Methylaminolaevulinate-based photodynamic therapy of Bowen’s disease and squamous cell carcinoma. Br J Dermatol 2008;159:137–44.
-
Calzavara-Pinton PG, Zane C, Facchetti F, Carlino A, Blanzuoli L, Marocolo D, et al. [Photodynamic therapy of non-melanoma skin tumours with topical delta-aminolevulinic acid.] G Ital Dermatol Venereol 1997;132:15–21.
-
Carruth JAS, Barrett JM, Barnes DWH, Buchanan RB, Sansom JM. A trial of photodynamic therapy for the treatment of tumors of the skin and head and neck, and the results of an experimental-study to determine the Interrelationships between the tissue effects of ionizing-radiation and photodynamic therapy. SPIE Proceedings Series 1993;1616:14–19.
-
Cavicchini S, Moretti D, Tanzi C, Tourlaki A. MAL-PDT in “difficult to treat’’ Bowen’s disease. J Invest Dermatol 2006;126:S36.
-
Ceylan C, Erboz S, Ozdemir F, Alper S. Topical photodynamic therapy for intraepidermal epithelioma. J Eur Acad Dermatol Venereol 2002;16:292–4.
-
Ceylan C, Erboz S, Ozdemir F, Kazandi A. [The effectiveness of topical photodynamic therapy in actinic.] Deri Hast Frengi Ars 2001;35:213–18.
-
Cochito M, Campos Lopes JM, Leite L. Topical photodynamic therapy in a case of Bowen’s disease of the face. Skin Cancer 1996;11:215–18.
-
Counters J, Zelickson B, Coles C, Selim M. A comparison of the V-beam and IPL in photodynamic therapy for reduction of actinic keratosis. Lasers Surg Med 2004;34:42.
-
de Haas ER, Sterenborg HJ, Neumann HA, Robinson DJ. The influence of light fractionation on the response of superficial skin cancer to aminolevulinic-acid photodynamic therapy. J Invest Dermatol 2006;126:S73.
-
de Haas ERM, de Vijlder HC, Sterenborg HJCM, Neumann HAM, Robinson DJ. Fractionated aminolevulinic acid-photodynamic therapy provides additional evidence for the use of PDT for non-melanoma skin cancer. J Eur Acad Dermatol Venereol 2008;22:426–30.
-
Dijkstra AT, Majoie IM, van Dongen JW, van Weelden H, van Vloten WA. Photodynamic therapy with violet light and topical 6-aminolaevulinic acid in the treatment of actinic keratosis, Bowen’s disease and basal cell carcinoma. J Eur Acad Dermatol Venereol 2001;15:550–4.
-
Dragieva G, Hafner J, Dummer R, Schmid-Grendelmeier P, Roos M, Prinz BM, et al. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen’s disease in transplant recipients. Transplantation 2004;77:115–21.
-
Erboz S, Ceylan C, Ozdemir F, Kazandi A, Ozol A. [Photodynamic treatment in solar keratosis.] Deri Hast Frengi Ars 1999;33:75–8.
-
Fernandez-Guarino M, Harto A, Perez-Garcia B, Martin-Gonzalez M, Urrutia S, Jaen P. Photodynamic therapy in disseminated superficial actinic porokeratosis. J Eur Acad Dermatol Venereol 2009;23:176–7.
-
Fijan S, Honigsmann H, Ortel B. Photodynamic therapy of epithelial skin tumours using delta-aminolaevulinic acid and desferrioxamine. Br J Dermatol 1995;133:282–8.
-
Fink-Puches R, Hofer A, Smolle J, Kerl H, Wolf P. Primary clinical response and long-term follow-up of solar keratoses treated with topically applied 5-aminolevulinic acid and irradiation by different wave bands of light. J Photochem Photobiol B 1997;41:145–51.
-
Fowler JF, Jr, Zax RH. Aminolevulinic acid hydrochloride with photodynamic therapy: efficacy outcomes and recurrence 4 years after treatment. Cutis 2002;69:2–7.
-
Fritsch C, Stege H, Saalmann G, Goerz G, Ruzicka T, Krutmann J. Green light is effective and less painful than red light in photodynamic therapy of facial solar keratoses. Photodermatol Photoimmunol Photomed 1997;13:181–5.
-
Gniazdowska B, Rueff F, Hillemanns P, Przybilla B. Allergic contact dermatitis from delta-aminolevulinic acid used for photodynamic therapy. Contact Dermatitis 1998;38:348–9.
-
Gold MH. Treatment of actinic cheilitis using photodynamic therapy with methylaminolevulinate: report of three cases [Commentary]. Dermatol Surg 2005;31:1348.
-
Goldman M, Atkin D. ALA/PDT in the treatment of actinic keratosis: spot versus confluent therapy. J Cosmetic Laser Ther 2003;5:107–10.
-
Gonzalez-Perez R, Garcia JG, Badiola IB, Calleja JMV, Sanchez SA, Diaz-Perez JL. [Topical photodynamic therapy with 5-amino levulinic acid: Experience at the Hospital of Cruces.] Actas Dermosifiliogr 1997;88:561–5.
-
Guarneri C, Vaccaro M. Erosive pustular dermatosis of the scalp following topical methylaminolaevulinate photodynamic therapy. J Am Acad Dermatol 2009;60:521–2.
-
Gupta G, Morton CA, Whitehurst C, Moore JV, MacKie RM. Photodynamic therapy with meso-tetra(hydroxyphenyl) chlorin in the topical treatment of Bowen’s disease and basal cell carcinoma. Br J Dermatol 1999;141:385–6.
-
Ha XW, Sun XM, Xie JG, Fan XJ, Zhang YH, Mei QC, et al. Clinical use of hematoporphyrin derivative in malignant tumors. Chin Med J 1983;96:754–8.
-
Hauschild A, Lischner S, Lange-Asschenfeldt B, Egberts F. Treatment of actinic cheilitis using photodynamic therapy with methyl aminolevulinate: report of three cases. Dermatol Surg 2005;31:1344–7.
-
Hegyi J, Frey T, Arenberger P. The treatment of unilesional mycosis fungoides with methyl aminolevulinate-photodynamic therapy. J Eur Acad Dermatol Venereol 2008;22:1134–5.
-
Hyung SK, Jong YY, Kwang HC, Oh SK, Sang EM, Vinciullo C. Topical photodynamic therapy using intense pulsed light for treatment of actinic keratosis: clinical and histopathologic evaluation [Commentary]. Dermatol Surg 2005;31:33–7.
-
Itoh Y, Ninomiya Y, Henta T, Tajima S, Ishibashi A. Topical delta-aminolevulinic acid-based photodynamic therapy for Japanese actinic keratoses. J Dermatol 2000;27:513–18.
-
Jeffes EW, McCullough JL, Weinstein GD, Fergin PE, Nelson JS, Shull TF, et al. Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid. A pilot dose-ranging study. Arch Dermatol 1997;133:727–32.
-
Jiraskova M, Jirasek L, Stork J, Vosmik F, Jirsa M. [Photodynamic diagnosis and therapy in dermatology. Experience with use of TPPS4 in skin diseases.] Cas Lek Cesk 2003;142:493–9.
-
Jiraskova M, Vosmik F, Krajsova I, Lapes M, Jirsa M. [Experience with photodynamic therapy in some skin affections.] Cesk Dermatol 1999;74:161–7.
-
Jiraskova M, Vosmik F, Lapes M, Jirsa M, Stadnik B. Experiences with local photodynamic therapy with TPPS4 and non-coherent light. Biomed Tech 1997;42(Suppl.):447–8.
-
Jones CM, Mang T, Cooper M, Wilson BD, Stoll HL, Jr. Photodynamic therapy in the treatment of Bowen’s disease. J Am Acad Dermatol 1992;27:979–82.
-
Jungersted JM, Dam TN, Bryld LE, Agner T. Allergic reactions to Metvix (ALA-ME). Contact Dermatitis 2008;58:184–6.
-
Kaae J, Philipsen PA, Haedersdal M, Wulf HC. Immediate whealing urticaria in red light exposed areas during photodynamic therapy. Acta Dermatol Venereol 2008;88:480–3.
-
Kacerovska D, Pizinger K, Majer F, Smid F. Photodynamic therapy of nonmelanoma skin cancer with topical hypericum perforatum extract: a pilot study. Photochem Photobiol 2008;84:779–85.
-
Kacerovska D, Pizinger K, Resl V, Cetkovska P, Jirsa M, Smid F. [Comparison of efficacy between the two photosensitizers in the photodynamic therapy of cutaneous tumours.] Cesko Dermatol 2006;81:148–52.
-
Karrer S, Baumler W, Abels C, Hohenleutner U, Landthaler M, Szeimies RM. Long-pulse dye laser for photodynamic therapy: investigations in vitro and in vivo. Lasers Surg Med 1999;25:51–9.
-
Karrer S, Szeimies RM, Landthaler M. Topical photodynamic therapy with 5-ala in the treatment of arsenic-induced skin tumors. SPIE Proceedings Series 1995;2371:222–5.
-
Karrer S, Szeimies RM, Sauerwald A, Landthaler M. Topical photodynamic therapy with 5-aminolevulinic acid in the treatment of actinic keratoses: a first clinical study. SPIE Proceedings Series 1996;2625:48–50.
-
Kasche A, Luderschmidt S, Ring J, Hein R. Photodynamic therapy induces less pain in patients treated with methyl aminolevulinate compared to aminolevulinic acid. J Drugs Dermatol 2006;5:353–6.
-
Kawczyk-Krupka A, Sieron A, Suwata-Jurczyk B, Adamek M. [Photodynamic therapy (PDT) using topically applied delta-aminolevulinic acid (ALA) for the treatment of malignant skin tumours.] Przegl Dermatol 2000;87:235–40.
-
Kerr AC, Ferguson J, Ibbotson SH. Acute phototoxicity with urticarial features during topical 5-aminolaevulinic acid photodynamic therapy. Clin Exp Dermatol 2007;32:201–2.
-
Kim EH, Kang HY, Lee E-S, Kim YC. Mycosis fungoides showing inCR to topical 5-aminolaevulinic acid phototherapy. Eur J Dermatol 2007;17:343–5.
-
Kim HS, Song KH, Kim KH. [Photodynamic therapy of actinic keratoses using 585nm dye laser and variable lights.] Korean J Dermatol 2005;43:53–9.
-
Kim HS, Yoo JY, Cho KH, Kwon OS, Moon SE. Topical photodynamic therapy using intense pulsed light for treatment of actinic keratosis: clinical and histopathologic evaluation. Dermatol Surg 2005;31:33–6; discussion 6–7.
-
Kodama M, Watanabe D, Akita Y, Tamada Y, Matsumoto Y. Photodynamic therapy for the treatment of actinic cheilitis. Photodermatol Photoimmunol Photomed 2007;23:209–10.
-
Kreutzer K, Bonnekoh B, Franke I, Gollnick H. [Photodynamic therapy with methylaminooxopentanoate (Metvix) and a broad band light source (PhotoDyn 501): practical experiences in problem-patients with actinic keratoses and basal cell carcinomas.] JDDG (Journal of the German Society of Dermatology) 2004;2:992–9.
-
Lang S, Baumgartner R, Struck R, Leunig A, Gutmann R, Feyh J. [Photodynamic diagnosis and therapy of neoplasms of the facial skin after topical administration of 5-aminolevulinic acid.] Laryngorhinootologie 1995;74:85–9.
-
Lee JS, Kim YJ, Kang HY, Lee ES, Oh CH, Kim YC. [Topical photodynamic therapy for treatment of actinic keratosis using light-emitting diode (LED) device.] Korean J Dermatol 2005;43:469–74.
-
Loncaster JA, Moore JV, Allan D, Allan E. An ultrasound analysis of the response of Gorlin syndrome-related and sporadic basal cell carcinomas to aminolaevulinic acid photodynamic therapy. Photodiag Photodyn Ther 2005;2:149–55.
-
Marcus L. Photodynamic therapy for actinic keratosis followed by 5-fluorouracil reaction. Dermatol Surg 2003;29:1061–4; discussion 4–5.
-
Markham T, Sheahan K, Collins P. Topical 5-aminolaevulinic acid photodynamic therapy for tumour-stage mycosis fungoides. Br J Dermatol 2001;144:1262–3.
-
Maydan E, Nootheti PK, Goldman MP. Development of a keratoacanthoma after topical photodynamic therapy with 5-aminolevulinic acid. J Drugs Dermatol 2006;5:804–6.
-
McCaughan JS, Jr, Guy JT, Hicks W, Laufman L, Nims TA, Walker J. Photodynamic therapy for cutaneous and subcutaneous malignant neoplasms. Arch Surg 1989;124:211–16.
-
Mehta RK, Langmack K, Sarkany R, Norris PG. Light emitting diode lamp as a novel light source for photodynamic therapy. Br J Dermatol 1999;141:114–18.
-
Meijnders PJN, Star WM, De Bruijn RS, Treurniet-Donker AD, Van Mierlo MJM, Wijthoff SJM, et al. Clinical results of photodynamic therapy for superficial skin malignancies or actinic keratosis using topical 5-aminolaevulinic acid. Lasers Med Sci 1996;11:123–31.
-
Morton CA, Whitehurst C, McColl JH, Moore JV, MacKie RM. Photodynamic therapy for large or multiple patches of Bowen disease and basal cell carcinoma. Arch Dermatol 2001;137:319–24.
-
Morton CA, Whitehurst C, Moseley H, Moore JV, Mackie RM. Development of an alternative light source to lasers for photodynamic therapy: 3. Clinical evaluation in the treatment of pre-malignant non-melanoma skin cancer. Lasers Med Sci 1995;10:165–71.
-
Moseley H, Allen JW, Ibbotson S, Lesar A, McNeill A, Camacho-Lopez MA, et al. Ambulatory photodynamic therapy: a new concept in delivering photodynamic therapy. Br J Dermatol 2006;154:747–50.
-
Moseley H, Ibbotson S, Woods J, Brancaleon L, Lesar A, Goodman C, et al. Clinical and research applications of photodynamic therapy in dermatology: experience of the Scottish PDT Centre. Lasers Surg Med 2006;38:403–16.
-
Nagano T, Yamada Y, Ikeda T, Kamo T, Nishioka E, Nishigori C. [Successful treatment of superficial cutaneous malignant neoplasms with photodynamic therapy (PDT) using light-emitting diode (LED) light source.] Skin Res 2005;4:188–93.
-
Nayeemuddin FA, Wong M, Yell J, Rhodes LE. Topical photodynamic therapy in disseminated superficial actinic porokeratosis. Clin Exp Dermatol 2002;27:703–6.
-
Paoli J, Halldin C, Ericson MB, Wennberg AM. Nerve blocks provide effective pain relief during topical photodynamic therapy for extensive facial actinic keratoses. Clin Exp Dermatol 2008;33:559–64.
-
Park SY, Kim KT, Yoon TJ. [A case of actinic keratosis treated by topical photodynamic therapy with low intensity dye laser.] Korean J Dermatol 2006;44:636–8.
-
Parlette EC. Red light laser photodynamic therapy of Bowen’s disease. J Drugs Dermatol 2004;3:S22–4.
-
Pérez W J, González S, Goset K, Sánchez B, Zelaya G. [Photodynamic therapy in actinic keratoses and Bowen’s disease: preliminary report.] Rev Chil Dermatol 2003;19:256–60.
-
Perrett CM, McGregor J, Proby C, Harwood C. A comparative study of topical 5-fluorouracil and topical photodynamic therapy using methylaminolevulinate for actinic keratosis and Bowen’s disease in organ transplant recipients. J Am Acad Dermatol 2006;54:A7.
-
Philipp CM, Muller U, Urban P, Berlien HP. Potential of systemic photosensitizers for PDT of skin malignancies. SPIE Proceedings Series 2008;6991:19.
-
Piaserico S, Belloni Fortina A, Rigotti P, Rossi B, Baldan N, Alaibac M, et al. Topical photodynamic therapy of actinic keratosis in renal transplant recipients. Transplant Proc 2007;39:1847–50.
-
Pinzi C, Campolmi P, Moretti S, Guasti A, Rossi R, Cappugi P. [Photodynamic therapy of primary and secondary (non-melanoma) skin tumors with topical delta-aminolevulinic acid.] G Ital Dermatol Venereol 2000;135:427–31.
-
Polak-Pacholczyk I, Lassota-Falczewska M, Bartkowiak R, Kaszuba A. [Photodynamic therapy of the precancerous states and non-melanoma skin cancers using the methyl-derivative of aminolevulinic acid (MAL-PDT).] Przegl Dermatol 2007;94:599–605.
-
Pons P, Pittau R, Boetto N, Garzon R, Aoki A. [Photodynamic therapy of neoplastic skin lesions: a two year experience.] Revista Argent Dermatol 2001;82:208–15.
-
Pons P, Pittau RF, Boetto NA, Garzon R, Aoki A. [Prototype of light source for photodynamic therapy in Centre of Electron Microscopy.] Rev Fac Cien Med Cordoba 2000;57:31–6.
-
Robinson PJ, Carruth JA, Fairris GM. Photodynamic therapy: a better treatment for widespread Bowen’s disease. Br J Dermatol 1988;119:59–61.
-
Rossi R, Mavilia L, Ghersetich I, Lotti T. Photodynamic therapy of actinic keratoses with methyl-aminolevulinate (METVIX). G Ital Dermatol Venereol 2005;140:381–7.
-
Runfola MA, Weber TK, Rodriguez-Bigas MA, Dougherty TJ, Petrelli NJ. Photodynamic therapy for residual neoplasms of the perianal skin. Dis Colon Rectum 2000;43:499–502.
-
Sandberg C, Stenquist B, Rosdahl I, Ros A-M, Synnerstad I, Karlsson M, et al. Important factors for pain during photodynamic therapy for actinic keratosis. Acta Dermatol Venereol 2006;86:404–8.
-
Sega GM, Keohane SG. MAL-PDT in the treatment of ‘field changes’ in patients with previous nonmelanoma skin cancer. Br J Dermatol 2005;153:96.
-
Shim SD, Kim YC, Chung PS, Rhee CK. [A case of actinic keratosis treated with topical photodynamic therapy with a 632 nm diode laser.] Korean J Dermatol 2004;42:1221–4.
-
Sieron A, Kawczyk-Krupka A, Wojciech Cebula MA, Szygula M, Zieleznik W, Gruk M, et al. Photodynamic therapy (PDT) using topically applied delta-aminolevulinic acid (ALA) for the treatment of malignant skin tumors. Photodiag Photodyn Ther 2004;1:311–17.
-
Song KH, Lee CW, Kim KH. [Photodynamic treatment for precancerous disease.] Korean J Dermatol 2003;41:609–16.
-
Souza CS, Felicio LBA, Bentley MV, Tedesco AC, Ferreira J, Kurachi C, et al. Topical photodynamic therapy for Bowen’s disease of the digit in epidermolysis bullosa. Br J Dermatol 2005;153:672–4.
-
Stables GI, Stringer MR, Robinson DJ, Ash DV. Large patches of Bowen’s disease treated by topical aminolaevulinic acid photodynamic therapy. Br J Dermatol 1997;136:957–60.
-
Stables GI, Stringer MR, Robinson DJ, Ash DV. The treatment of Bowen’s disease by topical aminolaevulinic acid photodynamic therapy. Br J Dermatol 1998;139:74.
-
Stender IM, Wulf HC. Photodynamic therapy with 5-aminolevulinic acid in the treatment of actinic cheilitis. Br J Dermatol 1996;135:454–6.
-
Svanberg K, Andersson T, Killander D, Wang I, Stenram U, Andersson-Engels S, et al. Photodynamic therapy of non-melanoma malignant tumours of the skin using topical delta-amino levulinic acid sensitization and laser irradiation. Br J Dermatol 1994;130:743–51.
-
Szeimies RM, Karrer S, Sauerwald A, Landthaler M. Photodynamic therapy with topical application of 5-aminolevulinic acid in the treatment of actinic keratoses: an initial clinical study. Dermatology 1996;192:246–51.
-
Szeimies RM, Landthaler M. Treatment of Bowen’s disease with topical photodynamic therapy. J Dermatolog Treat 1993;4:207–9.
-
Szeimies RM, Landthaler M. [Topical photodynamic therapy in treatment of superficial skin tumors.] Hautarzt 1995;46:315–18.
-
Tan B, Sinclair R, Foley P. Photodynamic therapy for subungual Bowen’s disease. Australas J Dermatol 2004;45:172–4.
-
Toll A, Parera Ma E, Velez M, Pujol RM. Photodynamic therapy with methyl aminolevulinate induces phototoxic reactions on areas of the nose adjacent to basal cell carcinomas and actinic keratoses. Dermatol Surg 2008;34:1145–7.
-
Toll A, Parera ME, Velez M, Pujol RM. Letter: photodynamic therapy with methyl aminolevulinate induces phototoxic reactions on areas of the nose adjacent to basal cell carcinomas and actinic keratoses. Dermatol Surg 2008;34:1145–7; discussion 7–8.
-
Tosca AD, Balas CJ, Stefanidou MP, Katsantonis JC, Georgiou S, Tzardi MN, et al. Prediction of ALA-PDT efficacy through remote color inspection and post therapy sequential histologic observations of skin malignancies. SPIE Proceedings Series 1997;3191:221–30.
-
Touma D, Yaar M, Whitehead S, Konnikov N, Gilchrest BA. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol 2004;140:33–40.
-
Tschen E, Pariser D, Wong DS, Dunlap FE. Photodynamic therapy using aminolevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: long-term histopathologic results of a phase IV multicenter clinical trial. J Am Acad Dermatol 2005;52:P164.
-
Tschen EH, Wong DS, Pariser DM, Dunlap FE, Houlihan A, Ferdon MB, et al. Photodynamic therapy using aminolaevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: phase IV multicentre clinical trial with 12-month follow-up. Br J Dermatol 2006;155:1262–9.
-
Usmani N, Stables GI, Telfer NR, Stringer MR. Subungual Bowen’s disease treated by topical aminolevulinic acid-photodynamic therapy. J Am Acad Dermatol 2005;53:S273–6.
-
Usmani N, Telfer N, Stringer M, Stables G. Subungual Bowen’s disease treated by topical photodynamic therapy. J Am Acad Dermatol 2005;52:P164.
-
Vaicova M, Ettler K. [Our clinical experience with the use of photodynamic therapy in patients with the basal cell carcinoma and morbus Bowen (comparison of efficacy of two photosensitizers).] Cesk Dermatol 2004;79:200–4.
-
Varma S, Anstey A, Wilson H, Kurwa HA. Photodynamic therapy for the treatment of Bowen’s disease, solar keratoses, and superficial basal cell carcinomas: 12 months experience with a novel light source. Br J Dermatol 1998;139:19.
-
Varma S, Wilson H, Kurwa HA, Charman C. One year relapse rates for Bowen’s disease, basal cell carcinomas and solar keratoses treated by photodynamic therapy: analysis of 189 lesions. Br J Dermatol 1999;141:114–18.
-
Varma S, Wilson H, Kurwa HA, Gambles B, Charman C, Pearse AD, et al. Bowen’s disease, solar keratoses and superficial basal cell carcinomas treated by photodynamic therapy using a large-field incoherent light source. Br J Dermatol 2001;144:567–74.
-
Wang J, Gao M, Wen S, Wang M. Photodynamic therapy for 50 patients with skin cancers or precancerous lesions. Chin Med Sci J 1991;6:163–5.
-
Wang JB, Gao ML, Wen SJ, Wang MJ. Study of photodynamic therapy in skin cancers and precancerous lesions. SPIE Proceedings Series 1993;1616:139–42.
-
Wang XL, Wang HW, Guo MX, Xu SZ. Treatment of skin cancer and pre-cancer using topical ALA-PDT: a single hospital experience. Photodiag Photodyn Ther 2008;5:127–33.
-
Weisser H, Meyer-Rogge D, Meyer-Rogge E. [First experiences in medical practice with the new topical photosensitizer MAOP for actinic keratosis and basal cell carcinoma.] Aktuelle Dermatologie 2004;30:306–11.
-
Wennberg AM, Lindholm LE, Alpsten M, Larko O. Treatment of superficial basal cell carcinomas using topically applied delta-aminolaevulinic acid and a filtered xenon lamp. Arch Dermatol Res 1996;288:561–4.
-
Wolf P, Fink-Puches R, Cerroni L, Kerl H. Photodynamic therapy for mycosis fungoides after topical photosensitization with 5-aminolevulinic acid. J Am Acad Dermatol 1994;31:678–80.
-
Wolf P, Fink-Puches R, Reimann-Weber A, Kerl H. Development of malignant melanoma after repeated topical photodynamic therapy with 5-aminolevulinic acid at the exposed site. Dermatology 1997;194:53–4.
-
Wolf P, Rieger E, Kerl H. Topical photodynamic therapy with endogenous porphyrins after application of 5-aminolevulinic acid. An alternative treatment modality for solar keratoses, superficial squamous cell carcinomas, and basal cell carcinomas? [Erratum published in J Am Acad Dermatol 1993;29(1):41.] J Am Acad Dermatol 1993;28:17–21.
-
Wolfe CM, Hatfield K, Cognetta AB, Jr. Cellulitis as a postprocedural complication of topical 5-aminolevulinic acid photodynamic therapy in the treatment of actinic keratosis. J Drugs Dermatol 2007;6:544–8.
-
Wong TW, Sheu HM, Lee JY, Fletcher RJ. Photodynamic therapy for Bowen’s disease (squamous cell carcinoma in situ) of the digit. Dermatol Surg 2001;27:452–6.
-
Xu S, Wang X, Xu W. [Evaluation of photodynamic therapy of skin cancers with delta-aminolevulinic acid.] Med J Wuhan Univ 2003;24:86–9.
-
Zelickson B, Coles C. Treatment of actinic keratosis with PDT and chemical light patch. Lasers Surg Med 2003;15(Suppl.):167.
Appendix 6 Skin cancer scoping
Two hundred and thirty-five publications, with study designs that did not meet the inclusion criteria for the review, reported on patients with skin cancer being treated with PDT. The references are listed below in alphabetical order; they have not been categorised and may still contain a number of duplicate publications.
References
-
Abels C, Karrer S, Baumler W, Goetz AE, Landthaler M, Szeimies RM. Indocyanine green and laser light for the treatment of AIDS-associated cutaneous Kaposi’s sarcoma. Br J Cancer 1998;77:1021–4.
-
Alecu M, Ursaciuc C, Halalau F, Coman G, Merlevede W, Waelkens E, et al. Photodynamic treatment of basal cell carcinoma and squamous cell carcinoma with hypericin. Anticancer Res 1998;18:4651–4.
-
Allison RR, Mang TS, Wilson BD, Vongtama V. Tin ethyl etiopurpurin-induced photodynamic therapy for the treatment of human immunodeficiency virus-associated Kaposi’s sarcoma. Curr Ther Res Clin Exp 1998;59:23–7.
-
Alvanopoulos K, Antoniou C, Melpo P, Vareltzidis A, Katsambas A. Photodynamic therapy of superficial basal cell carcinomas using exogenous 5-aminolevulinic acid and 514-nm light. J Eur Acad Dermatol Venereol 1997;9:134–6.
-
Antoniou C, Katsambas A, Rigopoulos D, Palaskas E, Tsikrikas GN. Aminolevulinic acid: topical photodynamic therapy in skin cancers and solar keratoses. Skin Cancer 1996;11:81–4.
-
Attili S, Cochrane A, McNeill A, Camacho-Lopez M, Moseley H, Ibbotson S, et al. An open pilot study of ambulatory photodynamic therapy using a wearable, low-irradiance, organic LED light source in the treatment of nonmelanoma skin cancer. Br J Dermatol 2008;159:130–1.
-
Baas P, Saarnak AE, Oppelaar H, Neering H, Stewart FA. Photodynamic therapy with meta-tetrahydroxyphenylchlorin for basal cell carcinoma: a phase I/II study. Br J Dermatol 2001;145:75–8.
-
Bagnato VS, Kurachi C, Ferreira J, Marcassa LG, Sibata CH, Allison RR. PDT experience in Brazil: a regional profile. Photodiag Photodyn Ther 2005;2:107–18.
-
Bakos RM, Bakos L, Ferlin E, Cestari T, Orlandini T, Rezende R, et al. [Photodynamic therapy with delta-aminolevulinic acid for superficial keratinocytic neoplasms.] An Bras Dermatol 2003;78:197–207.
-
Bandieramonte G, Marchesini R, Melloni E, Andreoli C, di Pietro S, Spinelli P, et al. Laser phototherapy following HpD administration in superficial neoplastic lesions. Tumori 1984;70:327–34.
-
Baptista J, Martinez C, Leite L, Cochito M. Our PDT experience in the treatment of non-melanoma skin cancer over the last 7 years. J Eur Acad Dermatol Venereol 2006;20:693–7.
-
Baron E, Domingo DS, Hsia A, Colussi V, Oleinick N, Foster T, et al. Silicon phthalocyanine photodynamic therapy for treatment of cutaneous neoplasms. J Invest Dermatol 2008;128:395.
-
Baron ED, Hanneman K, Scull HM, Hsia A, McCormick T, Oleinick NL, et al. Silicon phthalocyanine (Pc 4) photodynamic therapy for the treatment of pre-malignant and malignant skin conditions: an update. J Invest Dermatol 2005;125:942.
-
Bendsoe N, Persson L, Johansson A, Axelsson J, Svensson J, Grafe S, et al. Fluorescence monitoring of a topically applied liposomal Temoporfin formulation and photodynamic therapy of nonpigmented skin malignancies. J Environ Pathol Tox 2007;26:117–26.
-
Bendsoe N, Persson L, Johansson A, Axelsson J, Svensson J, Grafe S, et al. Fluorescence monitoring of a topically applied liposomal temoporfin formulation and photodynamic therapy of nonpigmented skin malignancies. J Environ Pathol Tox 2007;26:117–26.
-
Bernstein ZP, Wilson BD, Oseroff AR, Jones CM, Dozier SE, Brooks JS, et al. Photofrin photodynamic therapy for treatment of AIDS-related cutaneous Kaposi’s sarcoma. AIDS 1999;13:1697–704.
-
Betz CS, Rauschning W, Stranadko EP, Riabov MV, Albrecht V, Nifantiev NE, et al. Optimization of treatment parameters for Foscan-PDT of basal cell carcinomas. Lasers Surg Med 2008;40:300–11.
-
Biel MA. Photodynamic therapy and the treatment of malignancies of the head and neck. SPIE Proceedings Series 1995;2392:55–62.
-
Bieniek A, Fraczek M, Cislo M, Maj J, Szybejko-Machaj G, Barancewicz-Losek M, et al. [Cancer of the skin of the nose. Epidemiology and treatment.] Dermatol Klin 2007;9:165–9.
-
Bloznelyte L, Cepulis V, Ponomarev I, Dougherty TJ. Intra-arterial PDT and ordinary PDT in head and neck cancer. SPIE Proceedings Series 1996;2675:76–9.
-
Bloznelyte L, Garlaite D, Felinskaite E. Differences of Photodamage in various malignant tissues which appear after application of Photodynamic therapy, using different laser systems. SPIE Proceedings Series 1995;2392:106–10.
-
Bogelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Dermatol Venereol 2007;87:330–4.
-
Breuninger H, Bonnekoh B, Gollnick H. [Photodynamic therapy with methylaminooxopentanoate (MetvixR) and a broad band light source (PhotoDyn 501): Experiences in complicated patients with actinic keratoses and basal cell carcinomas (multiple letters).] JDDG (Journal of the German Society of Dermatology) 2005;3:397.
-
Buchanan RB, Carruth JA, McKenzie AL, Williams SR. Photodynamic therapy in the treatment of malignant tumours of the skin and head and neck. Eur J Surg Oncol 1989;15:400–6.
-
Cairnduff F, Stringer MR, Hudson EJ, Ash DV, Brown SB. Superficial photodynamic therapy with topical 5-aminolaevulinic acid for superficial primary and secondary skin cancer. Br J Cancer 1994;69:605–8.
-
Calista D, Coccia L. Photodynamic therapy for the treatment of in situ squamous cell carcinoma of the left eyelid. Int J Dermatol 2008;47:1319–21.
-
Calzavara F, Tomio L. Photodynamic therapy: clinical experience at the Department of Radiotherapy at Padova General Hospital. J Photochem Photobiol B 1991;11:91–5.
-
Calzavara-Pinton PG. Repetitive photodynamic therapy with topical delta-aminolaevulinic acid as an appropriate approach to the routine treatment of superficial non-melanoma skin tumours. J Photochem Photobiol B 1995;29:53–7.
-
Calzavara-Pinton PG, Venturini M, Sala R, Capezzera R, Parrinello G, Specchia C, et al. Methylaminolaevulinate-based photodynamic therapy of Bowen’s disease and squamous cell carcinoma. Br J Dermatol 2008;159:137–44.
-
Calzavara-Pinton PG, Zane C, Capezzera R, Venturini M, Sala R. MAL-PDT of in situ, microinvasive and invasive squamous cell carcinoma. J Invest Dermatol 2007;127:S44.
-
Calzavara-Pinton PG, Zane C, Facchetti F, Carlino A, Blanzuoli L, Marocolo D, et al. [Photodynamic therapy of non-melanoma skin tumours with topical delta-aminolevulinic acid.] G Ital Dermatol Venereol 1997;132:15–21.
-
Campbell SM, Morton CA, Alyahya R, Horton S, Pye A, Curnow A. Clinical investigation of the novel iron-chelating agent, CP94, to enhance topical photodynamic therapy of nodular basal cell carcinoma. Br J Dermatol 2008;159:387–93.
-
Cappugi P, Massi D, Salvini MD. Nodular basal cell carcinoma of the nose treated by photodynamic therapy (PDT). Photodynamic Therapy and Photodiagnosis in Clinical Practice, Brixen/Bressanone, 7–11 October, 2008. Poster session – CD12.
-
Cappugi P, Mavilia L, Campolmi P, Reali EF, Mori M, Rossi R. New proposal for the treatment of nodular basal cell carcinoma with intralesional 5-aminolevulinic acid. J Chemother 2004;16:491–3.
-
Carruth JAS, Barrett JM, Barnes DWH, Buchanan RB, Sansom JM. A trial of photodynamic therapy for the treatment of tumors of the skin and head and neck, and the results of an experimental-study to determine the interrelationships between the tissue effects of ionizing-radiation and photodynamic therapy. SPIE Proceedings Series 1993;1616:14–19.
-
Castro García J, Rincón Duran N, Gordon Parra M, Marcano Olaizola A, Aranguren L. Terapia fotodinámica: en cáncer de la piel. Rev Venez Oncol 2007;19:3–19.
-
Ceylan C, Ozdemir F, Erboz S, Kazandi A, Ozol A. [Topical photodynamic treatment in basal cell carcinoma.] Deri Hast Frengi Ars 1999;33:153–7.
-
Chapas A, Zeltser R, Geronemus R, Gilchrest B. Intralesional photodynamic therapy of nonmelanoma skin cancer. Lasers Surg Med 2006;38:27.
-
Chapas AM, Gilchrest BA. Broad area photodynamic therapy for treatment of multiple basal cell carcinomas in a patient with nevoid basal cell carcinoma syndrome. J Drugs Dermatol 2006;5:3–5.
-
Christensen E, Skogvoll E, Viset T, Warloe T, Sundstrom S. Photodynamic therapy with 5-aminolaevulinic acid, dimethylsulfoxide and curettage in basal cell carcinoma: a 6-year clinical and histological follow-up. J Eur Acad Dermatol Venereol 2009;23:58–66.
-
Clark C, Bryden A, Dawe R, Moseley H, Ferguson J, Ibbotson SH. Topical 5-aminolaevulinic acid photodynamic therapy for cutaneous lesions: outcome and comparison of light sources. Photodermatol Photoimmunol Photomed 2003;19:134–41.
-
de Haas ERM, de Vijlder HC, Sterenborg HJCM, Neumann HAM, Robinson DJ. Fractionated aminolevulinic acid-photodynamic therapy provides additional evidence for the use of PDT for non-melanoma skin cancer. J Eur Acad Dermatol Venereol 2008;22:426–30.
-
Devirgiliis V, Panasiti V, Curzio M, Gobbi S, Rossi M, Roberti V, et al. Complete remission of nodular basal cell carcinoma after combined treatment with photodynamic therapy and imiquimod 5% cream. Dermatol Online J 2008;14.
-
Dijkstra AT, Majoie IM, van Dongen JW, van Weelden H, van Vloten WA. Photodynamic therapy with violet light and topical 6-aminolaevulinic acid in the treatment of actinic keratosis, Bowen’s disease and basal cell carcinoma. J Eur Acad Dermatol Venereol 2001;15:550–4.
-
Domaniecki J, Stanowski E, Graczyk A, Kalczak M, Struzyna J, Kwasny M, et al. Photodynamic method used for the treatment of malignant melanoma and Merkel cell carcinoma. SPIE Proceedings Series 1997;3188:122–7.
-
Dougherty TJ, Cooper MT, Mang TS. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg Med 1990;10:485–8.
-
Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res 1978;38:2628–35.
-
Edstrom DW, Hedblad MA. Long-term follow-up of photodynamic therapy for mycosis fungoides. Acta Dermatol Venereol 2008;88:288–90.
-
Edstrom DW, Porwit A, Ros AM. Photodynamic therapy with topical 5-aminolevulinic acid for mycosis fungoides: clinical and histological response. Acta Dermatol Venereol 2001;81:184–8.
-
Eibenschutz L, Marenda S, Mariani G, Ferrari A, Silipo V, Catricala C. MAL-PDT for the treatment of large basal cell carcinomas. J Invest Dermatol 2006;126:S16.
-
El-Far M, Setate A, El-Maddawy M. First initial clinical application of photodynamic therapy (PDT) in Egypt: two case reports. Laser Life Sci 1998;8:27–35.
-
Elliott S, Keller G, Razum N, Parks J, White R, Seiler A. Photodynamic therapy of nonmelanoma skin-cancer using a ktp-pumped dye-laser. SPIE Proceedings Series 1993;1881:2–9.
-
Elliott SL. The incidence and etiology of nonmelanoma skin cancer and selected management with photodynamic therapy. Laser Nursing 1992;6:83–91.
-
Evtushenko VA, Soldatov AN, Vusik MV, Reimer IV. Treatment of basal-cellular skin cancer and heavy concomitant diseases by a photodynamic therapeutic method with a dye laser LITT-PDT. SPIE Proceedings Series 2007;6938:32.
-
Fai D. MAL-PDT for the treatment of multiple basal cell carcinomas in a patient with Gorlin-Goltz syndrome. J Invest Dermatol 2006;126:S34-S.
-
Fijan S, Honigsmann H, Ortel B. Photodynamic therapy of epithelial skin tumours using delta-aminolaevulinic acid and desferrioxamine. Br J Dermatol 1995;133:282–8.
-
Filonenko E, Sokolov V, Sukhin D. Fluorescent diagnostics and photodynamic therapy of malignant cutaneous tumours. Photodynamic Therapy and Photodiagnosis in Clinical Practice, Brixen/Bressanone, 7–11 October, 2008. Poster session – SPO5.
-
Fink-Puches R, Soyer HP, Hofer A, Kerl H, Wolf P. Long-term follow-up and histological changes of superficial nonmelanoma skin cancers treated with topical delta-aminolevulinic acid photodynamic therapy. Arch Dermatol 1998;134:821–6.
-
Fink-Puches R, Wolf P, Kerl H. Photodynamic therapy of superficial basal cell carcinoma by instillation of aminolevulinic acid and irradiation with visible light. Arch Dermatol 1997;133:1494–5.
-
Gaal M, Gyulai R, Baltas E, Kui R, Olah J, Kemeny L. [Photodynamic therapy in dermatooncology.] Orv Hetil 2007;148:2227–33.
-
Gayl Schweitzer V. Photofrin-mediated photodynamic therapy for treatment of aggressive head and neck nonmelanomatous skin tumors in elderly patients. Laryngoscope 2001;111:1091–8.
-
Ghaffar SA, Clements SE, Lear JT. Epidermoid cysts mimicking recurrence of superficial basal cell carcinoma following photodynamic therapy. Clin Exp Dermatol 2007;32:223–4.
-
Gonzalez-Perez R, Garcia JG, Badiola IB, Calleja JMV, Sanchez SA, Diaz-Perez JL. [Topical photodynamic therapy with 5-amino levulinic acid: Experience at the Hospital of Cruces.] Actas Dermosifiliogr 1997;88:561–5.
-
Gregory GF, Hopper C, Fan K, Grant WE, Bown SG, Speight PM. Photodynamic therapy and lip vermilion dysplasia: a pilot study. Eur J Cancer 1995;5:346–7.
-
Guillen C, Sanmartin O, Escudero A, Botella-Estrada R, Sevila A, Castejon P. Photodynamic therapy for in situ squamous cell carcinoma on chronic radiation dermatitis after photosensitization with 5-aminolaevulinic acid. J Eur Acad Dermatol Venereol 2000;14:298–300.
-
Gupta G, Morton CA, Whitehurst C, Moore JV, MacKie RM. Photodynamic therapy with meso-tetra(hydroxyphenyl) chlorin in the topical treatment of Bowen’s disease and basal cell carcinoma. Br J Dermatol 1999;141:385–6.
-
Ha XW, Sun XM, Xie JG, Fan XJ, Zhang YH, Mei QC, et al. Clinical use of hematoporphyrin derivative in malignant tumors. Chin Med J 1983;96:754–8.
-
Haller JC, Cairnduff F, Slack G, Schofield J, Whitehurst C, Tunstall R, et al. Routine double treatments of superficial basal cell carcinomas using aminolaevulinic acid-based photodynamic therapy. Br J Dermatol 2000;143:1270–5.
-
Hanneken S, Sterzinger AA, Schulte KW, Reifenberger J. [Photodynamic therapy for a nevoid basal cell carcinoma syndrome.] Hautarzt 2005;56:363–4.
-
Harth Y, Bergman R, Gotfried V, Kimel S, Friedman-Birnbaum R. A case of basal cell carcinoma treated with photodynamic therapy: changes in histological features and bcl-2 expression. J Eur Acad Dermatol Venereol 1996;7:163–6.
-
Harth Y, Hirshovitz B. [Topical photodynamic therapy in basal and squamous cell carcinoma and penile Bowen’s disease with 20% aminolevulinic acid, and exposure to red light and infrared light.] Harefuah 1998;134:602–5.
-
Harth Y, Hirshowitz B, Kaplan B. Modified topical photodynamic therapy of superficial skin tumors, utilizing aminolevulinic acid, penetration enhancers, red light, and hyperthermia. Dermatol Surg 1998;24:723–6.
-
Hebeda KM, Huizing MT, Brouwer PA, van der Meulen FW, Hulsebosch HJ, Reiss P, et al. Photodynamic therapy in AIDS-related cutaneous Kaposi’s sarcoma. J Acquir Immune Defic Syndr Hum Retrovirol 1995;10:61–70.
-
Heinritz H, Benzel W, Sroka R, Iro H. Photodynamic therapy of superficial skin tumors following local application of delta-aminolaevulinic acid. Adv Otorhinolaryngol 1995;49:48–52.
-
Hintschich C, Feyh J, Beyer-Machule C, Riedel K, Ludwig K. Photodynamic laser therapy of basal-cell carcinoma of the lid. Ger J Ophthalmol 1993;2:212–17.
-
Hoerauf H, Huttmann G, Diddens H, Thiele B, Laqua H. [Photodynamic therapy of eyelid basalioma after topical administration of delta-aminolevulinic acid.] Ophthalmologe 1994;91:824–9.
-
Horn M, Wolf P, Wulf HC, Warloe T, Fritsch C, Rhodes LE, et al. Topical methyl aminolaevulinate photodynamic therapy in patients with basal cell carcinoma prone to complications and poor cosmetic outcome with conventional treatment. Br J Dermatol 2003;149:1242–9.
-
Hunzelmann VVN, Kubler AC. [Systemical photodynamic therapy for the treatment of nodular basalioma (case-report).] Z Hautkrankheiten 2000;75:712–14.
-
Hurlimann AF, Hanggi G, Panizzon RG. Photodynamic therapy of superficial basal cell carcinomas using topical 5-aminolevulinic acid in a nanocolloid lotion. Dermatology 1998;197:248–54.
-
Isanov T, Graschew G, Shopova M, Mitrov G, Mitev U, Jori G. Photodynamic therapy for the treatment of skin cancer. A case report. Radiobiol Radiother 1987;28:5–7.
-
Itkin A, Gilchrest BA. Delta-aminolevulinic acid and blue light photodynamic therapy for treatment of multiple basal cell carcinomas in two patients with nevoid basal cell carcinoma syndrome. Dermatol Surg 2004;30:1054–61.
-
Itoh Y, Henta T, Ninomiya Y, Tajima S, Ishibashi A. Repeated 5-aminolevulinic acid-based photodynamic therapy following electro-curettage for pigmented basal cell carcinoma. J Dermatol 2000;27:10–15.
-
Jiraskova M, Jirasek L, Stork J, Vosmik F, Jirsa M. [Photodynamic diagnosis and therapy in dermatology. Experience with use of TPPS4 in skin diseases.] Cas Lek Cesk 2003;142:493–9.
-
Jiraskova M, Vosmik F, Krajsova I, Lapes M, Jirsa M. [Experience with photodynamic therapy in some skin affections.] Cesk Dermatol 1999;74:161–7.
-
Jiraskova M, Vosmik F, Lapes M, Jirsa M, Stadnik B. Experiences with local photodynamic therapy with TPPS4 and non-coherent light. Biomed Tech 1997;42(Suppl.):447–8.
-
Jungersted JM, Dam TN, Bryld LE, Agner T. Allergic reactions to Metvix (ALA-ME). Contact Dermatitis 2008;58:184–6.
-
Kaae J, Philipsen PA, Haedersdal M, Wulf HC. Immediate whealing urticaria in red light exposed areas during photodynamic therapy. Acta Dermatol Venereol 2008;88:480–3.
-
Kacerovska D, Pizinger K, Majer F, Smid F. Photodynamic therapy of nonmelanoma skin cancer with topical hypericum perforatum extract: a pilot study. Photochem Photobiol 2008;84:779–85.
-
Kacerovska D, Pizinger K, Resl V, Cetkovska P, Jirsa M, Smid F. [Comparison of efficacy between the two photosensitizers in the photodynamic therapy of cutaneous tumours.] Cesk Dermatol 2006;81:148–52.
-
Kapinus VN, Kaplan MA. Photodynamic therapy of locally invasive cutaneous carcinoma. In: Laser Helsinki 2008, 13th International Congress of EMLA (European Medical Laser Association) in conjunction with EMLA Finland and MAL (Medical Acupuncture and Laser) in cooperation with ASLMS (American Society for Laser Medicine and Surgery); 2008; Helsinki. 2008.
-
Karagas MR, Stukel TA, Umland V, Tsoukas MM, Mott LA, Sorensen HT, et al. Reported use of photosensitizing medications and basal cell and squamous cell carcinoma of the skin: results of a population-based case-control study. J Invest Dermatol 2007;127:2901–3.
-
Karrer S, Szeimies RM, Hohenleutner U, Heine A, Landthaler M. Unilateral localized basaliomatosis: treatment with topical photodynamic therapy after application of 5-aminolevulinic acid. Dermatology 1995;190:218–22.
-
Karrer S, Szeimies RM, Landthaler M. Topical photodynamic therapy with 5-ala in the treatment of arsenic-induced skin tumors. SPIE Proceedings Series 1995;2371:222–5.
-
Kaviani A, Ataie-Fashtami L, Fateh M, Sheikhbahaee N, Ghodsi M, Zand N, et al. Photodynamic therapy of head and neck basal cell carcinoma according to different clinicopathologic features. Lasers Surg Med 2005;36:377–82.
-
Kawczyk-Krupka A, Sieron A, Suwata-Jurczyk B, Adamek M. [Photodynamic therapy (PDT) using topically applied delta-aminolevulinic acid (ALA) for the treatment of malignant skin tumours.] Przegl Dermatol 2000;87:235–40.
-
Keller GS, Razum NJ, Doiron DR. Photodynamic therapy for nonmelanoma skin cancer. Facial Plast Surg 1989;6:180–4.
-
Kennedy J. HPD photoradiation therapy for cancer at Kingston and Hamilton. Adv Exp Med Biol 1983;160:53–62.
-
Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B 1990;6:143–8.
-
Keohane S. Successful clearance of residual superficial basal cell carcinoma with MAL-PDT following Mohs surgery. J Am Acad Dermatol 2005;52:P164-P.
-
Kerr AC, Ferguson J, Ibbotson SH. Acute phototoxicity with urticarial features during topical 5-aminolaevulinic acid photodynamic therapy. Clin Exp Dermatol 2007;32:201–2.
-
Konig K, Boehncke WH, Ruck A, Kaufmann R, Steiner R, Sterry W. Photodynamic effects on T-Cells and skin-lesions of a patient with mycosis fungoides using porphyrin photosensitizers. SPIE Proceedings Series 1994;2086:268–76.
-
Kopera D, Cerroni L, Fink-Puches R, Kerl H. Different treatment modalities for the management of a patient with the nevoid basal cell carcinoma syndrome. J Am Acad Dermatol 1996;34:937–9.
-
Kreutzer K, Bonnekoh B, Franke I, Gollnick H. [Photodynamic therapy with methylaminooxopentanoate (Metvix) and a broad band light source (PhotoDyn 501): practical experiences in problem-patients with actinic keratoses and basal cell carcinomas.] JDDG (Journal of the German Society of Dermatology) 2004;2:992–9.
-
Kubler AC, de Carpentier J, Hopper C, Leonard AG, Putnam G. Treatment of squamous cell carcinoma of the lip using Foscan-mediated photodynamic therapy. Int J Oral Maxillofac Surg 2001;30:504–9.
-
Kubler AC, Haase T, Staff C, Kahle B, Rheinwald M, Muhling J. Photodynamic therapy of primary nonmelanomatous skin tumours of the head and neck. Lasers Surg Med 1999;25:60–8.
-
Kuijpers DIM, Smeets NWJ, Krekels GAM, Thissen MRTM. Photodynamic therapy as adjuvant treatment of extensive basal cell carcinoma treated with Mohs micrographic surgery. Dermatol Surg 2004;30:794–8.
-
Lane JE, Allen JH, Lane TN, Lesher JL, Jr. Unilateral Basal cell carcinomas: an unusual entity treated with photodynamic therapy. J Cutan Med Surg 2005;9:336–40.
-
Lang S, Baumgartner R, Struck R, Leunig A, Gutmann R, Feyh J. [Photodynamic diagnosis and therapy of neoplasms of the facial skin after topical administration of 5-aminolevulinic acid.] Laryngorhinootologie 1995;74:85–9.
-
Langeland J. Successful treatment of superficial basal cell carcinoma with MAL-PDT: two case reports. J Am Acad Dermatol 2005;52:P165-P.
-
Leman JA, Dick DC, Morton CA. Topical 5-ALA photodynamic therapy for the treatment of cutaneous T-cell lymphoma. Clin Exp Dermatol 2002;27:516–18.
-
Leman JA, Morton CA. Topical photodynamic therapy for basal cell carcinoma: optimizing response. Br J Dermatol 2003;149:47.
-
Li JH, Guo ZH, Jin ML, Zhao FY, Cai WM, Gao ML, et al. Photodynamic therapy in the treatment of malignant tumours: an analysis of 540 cases. J Photochem Photobiol B 1990;6:149–55.
-
Lui H, Salasche S, Kollias N, Wimberly J, Flotte T, McLean D, et al. Photodynamic therapy of nonmelanoma skin cancer with topical aminolevulinic acid: a clinical and histologic study. Arch Dermatol 1995;131:737–8.
-
Martinez C, Ferreira A, Cochito M, Campos Lopes JM, Ferreira MF, Bajanca R, et al. Topical photodynamic therapy in a case of basal-cell carcinoma of the nose. Skin Cancer 1999;14:49–52.
-
McCaughan JS, Jr. Photoradiation of malignant tumors presensitized with hematoporphyrin derivative. Prog Clin Biol Res 1984;170:805–27.
-
McCaughan JS, Jr. Overview of experiences with photodynamic therapy for malignancy in 192 patients. Photochem Photobiol 1987;46:903–9.
-
McCaughan JS, Jr. Photodynamic therapy of malignant tumors. Prog Clin Biol Res 1988;278:163–9.
-
McCaughan JS, Jr. Photodynamic therapy of skin and esophageal cancers. Cancer Invest 1990;8:407–16.
-
McCaughan JS, Jr, Guy JT, Hawley P, Hicks W, Inglis W, Laufman L, et al. Hematoporphyrin-derivative and photoradiation therapy of malignant tumors. Lasers Surg Med 1983;3:199–209.
-
McCaughan JS, Jr, Guy JT, Hicks W, Laufman L, Nims TA, Walker J. Photodynamic therapy for cutaneous and subcutaneous malignant neoplasms. Arch Surg 1989;124:211–16.
-
Mehta RK, Langmack K, Sarkany R, Norris PG. Light emitting diode lamp as a novel light source for photodynamic therapy. Br J Dermatol 1999;141:114–18.
-
Meijnders PJN, Star WM, De Bruijn RS, Treurniet-Donker AD, Van Mierlo MJM, Wijthoff SJM, et al. Clinical results of photodynamic therapy for superficial skin malignancies or actinic keratosis using topical 5-aminolaevulinic acid. Lasers Med Sci 1996;11:123–31.
-
Mesquita A, Sa F, Issa MC, Villar E. Photodynamic therapy with methyl aminolevulinate and LED (red light) to nodular basal cell carcinoma in the leg. J Am Acad Dermatol 2007;56:A165.
-
Miller JD, Nancy O, Scull HM, Hsia A, Cooper KD, Baron ED. Phase I clinical trial using topical silicon phthalocyanine Pc 4-photodynamic therapy for the treatment of malignant and pre-malignant skin conditions: an update. J Invest Dermatol 2006;126:272.
-
Mitropoulos P, Norman R. Nevoid basal cell carcinoma syndrome (Gorlin syndrome): updated review of minimally invasive treatments. Cutis 2008;81:53–60.
-
Mori M, Campolmi P, Mavilia L, Rossi R, Cappugi P, Pimpinelli N. Topical photodynamic therapy for primary cutaneous B-cell lymphoma: a pilot study. J Am Acad Dermatol 2006;54:524–6.
-
Mori M, Mavilia L, Campolmi P, Rossi R, Cappugi P, Pimpinelli N. [Topical photodynamic therapy in cutaneous lymphomas.] G Ital Dermatol Venereol 2005;140:123–7.
-
Morton CA, Burden AD. Treatment of multiple scalp basal cell carcinomas by photodynamic therapy. Clin Exp Dermatol 2001;26:33–6.
-
Morton CA, Fraser C, Leman JA, Smith B. Effects of protocol variation on response rate of superficial basal cell carcinoma to topical photodynamic therapy. Br J Dermatol 2005;153:25.
-
Morton CA, MacKie RM, Whitehurst C, Moore JV, McColl JH. Photodynamic therapy for basal cell carcinoma: effect of tumor thickness and duration of photosensitizer application on response. Arch Dermatol 1998;134:248–9.
-
Morton CA, Whitehurst C, McColl JH, Moore JV, MacKie RM. Photodynamic therapy for large or multiple patches of Bowen disease and basal cell carcinoma. Arch Dermatol 2001;137:319–24.
-
Moseley H, Ibbotson S, Woods J, Brancaleon L, Lesar A, Goodman C, et al. Clinical and research applications of photodynamic therapy in dermatology: experience of the Scottish PDT Centre. Lasers Surg Med 2006;38:403–16.
-
Nagano T, Yamada Y, Ikeda T, Kamo T, Nishioka E, Nishigori C. [Successful treatment of superficial cutaneous malignant neoplasms with photodynamic therapy (PDT) using light-emitting diode (LED) light source.] Skin Research 2005;4:188–93.
-
Naidenov N, Dencheva R, Tsankov N. Recurrence rate of basal cell carcinoma after topical aminolevulinic acid-based photodynamic therapy. Acta Dermatovenerol Croat 2004;12:157–61.
-
Nikkels AF, Pierard-Franchimont C, Nikkels-Tassoudji N, Bourguignon R, Pierard GE. Photodynamic therapy and imiquimod immunotherapy for basal cell carcinomas. Acta Clin Belg 2005;60:227–34.
-
Nikkels AF, Thirion L, Quatresooz P, Pierard GE. Photodynamic therapy for cutaneous verrucous carcinoma. J Am Acad Dermatol 2007;57:516–19.
-
Oakford M, Keohane SG. Photodynamic therapy to manage residual superficial basal cell carcinomas following Mohs’ surgery. Br J Dermatol 2004;151:96.
-
O’Connell M, Walker M, Scott D, Layton AM. A combined photodynamic therapy, imiquimod, curettage and cautery approach in the treatment of large recalcitrant basal cell carcinomas. Br J Dermatol 2007;157:P-104.
-
Orenstein A, Haik J, Tamir J, Winkler E, Trau H, Malik Z, et al. Photodynamic therapy of cutaneous lymphoma using 5-aminolevulinic acid topical application. Dermatol Surg 2000;26:765–9; discussion 9–70.
-
Orenstein A, Kostenich G, Goldan O, Weisman O, Winkler E, Haik J, et al. Clinical experience in controlled photodynamic therapy of skin lesions using topical 5-aminolevulinic acid. Photodynamic Therapy and Photodiagnosis in Clinical Practice, Brixen/Bressanone, 7–11 October 2008. Session 9–2.
-
Orenstein A, Kostenich G, Roitman L, Tsur H, Katanick D, Kopolovic J, et al. Photodynamic therapy of malignant lesions of the skin mediated by topical application of 5-aminolevulinic acid in combination with DMSO and EDTA. Laser Life Sci 1996;7:49–57.
-
Orenstein A, Kostenich G, Tsur H, Roitman L. Photodynamic therapy of human skin tumors using topical application of 5-aminolevulinic acid, dimethylsulfoxide (DMSO), and edetic acid disodium salt (EDTA). SPIE Proceedings Series 1994;2325:100–5.
-
Oseroff AR, Blumenson LR, Wilson BD, Mang TS, Bellnier DA, Parsons JC, et al. A dose ranging study of photodynamic therapy with porfimer sodium (Photofrin) for treatment of basal cell carcinoma. Lasers Surg Med 2006;38:417–26.
-
Oseroff AR, Shieh S, Frawley NP, Cheney R, Blumenson LE, Pivnick EK, et al. Treatment of diffuse basal cell carcinomas and basaloid follicular hamartomas in nevoid basal cell carcinoma syndrome by wide-area 5-aminolevulinic acid photodynamic therapy. Arch Dermatol 2005;141:60–7.
-
Paech V, Lorenzen T, Stoehr A, Lange K, Merz H, Meigel WN, et al. Remission of a cutaneous Mycosis fungoides after topical 5-ALA sensitisation and photodynamic therapy in a patient with advanced HIV-infection. Eur J Med Res 2002;7:477–9.
-
Panjehpour M, Julius CE, Hartman DL, Dougherty TJ. Photodynamic therapy for skin cancer. SPIE Proceedings Series 1996;2675:29–31.
-
Pennington DG, Waner M, Knox A. Photodynamic therapy for multiple skin cancers. Plast Reconstr Surg 1988;82:1067–71.
-
Peris K, Surrenti T, Bianchi L, Papoutsaki M. MAL-PDT in the treatment of basal cell carcinoma. J Am Acad Dermatol 2005;52:P161.
-
Perrett CM, Tan SK, Cerio R, Goldsmith PC, McGregor JM, Proby CM, et al. Treatment of basal cell carcinoma with topical methylaminolaevulinate photodynamic therapy in an organ-transplant recipient. Clin Exp Dermatol 2006;31:146–7.
-
Peter S, Witold Z, Peter H, Alexander B. Comparison between mALA- and ALA-PDT in the treatment of basal cell carcinomas. SPIE Proceedings Series 2006;6139:279–86.
-
Philipp CM, Muller U, Urban P, Berlien HP. Potential of systemic photosensitizers for PDT of skin malignancies. SPIE Proceedings Series 2008;6991:19.
-
Pinzi C, Campolmi P, Moretti S, Guasti A, Rossi R, Cappugi P. [Photodynamic therapy of primary and secondary (non-melanoma) skin tumors with topical delta-aminolevulinic acid.] G Ital Dermatol Venereol 2000;135:427–31.
-
Polak-Pacholczyk I, Lassota-Falczewska M, Bartkowiak R, Kaszuba A. [Photodynamic therapy of the precancerous states and non-melanoma skin cancers using the methyl-derivative of aminolevulinic acid (MAL-PDT).] Przegl Dermatol 2007;94:599–605.
-
Pons P, Pittau R, Boetto N, Garzon R, Aoki A. [Photodynamic therapy of neoplastic skin lesions: a two year experience.] Rev Arg Dermatol 2001;82:208–15.
-
Privalov VA, Lappa AV, Seliverstov OV, Faizrakhmanov AB, Yarovoy NN, Kochneva EV, et al. Clinical trials of a new chlorin photosensitizer for photodynamic therapy of malignant tumors. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XI. SPIE Proceedings 2002;4612:178–89.
-
Pye A, Campbell S, Curnow A. Enhancement of methyl-aminolevulinate photodynamic therapy by iron chelation with CP94: an in vitro investigation and clinical dose-escalating safety study for the treatment of nodular basal cell carcinoma. J Cancer Res Clin Oncol 2008;134:841–9.
-
Recio ED, Zambrano B, Alonso ML, de Eusebio E, Martin M, Cuevas J, et al. Topical 5-aminolevulinic acid photodynamic therapy for the treatment of unilesional mycosis fungoides: a report of two cases and review of the literature. Int J Dermatol 2008;47:410–13.
-
Reshetnikov AV. Summary on Radachlorin/Radachlorin Research [unpublished.] In. Moscow: RADA-PHARMA Co. Ltd; 2005.
-
Rossi R, Puccioni M, Mavilia L, Campolmi P, Mori M, Cappuccini A, et al. Squamous cell carcinoma of the eyelid treated with photodynamic therapy. J Chemother 2004;16:306–9.
-
Runfola MA, Weber TK, Rodriguez-Bigas MA, Dougherty TJ, Petrelli NJ. Photodynamic therapy for residual neoplasms of the perianal skin. Dis Colon Rectum 2000;43:499–502.
-
Sacchini V, Melloni E, Marchesini R, Luini A, Bandieramonte G, Spinelli P, et al. Preliminary clinical studies with PDT by topical TPPS administration in neoplastic skin lesions. Lasers Surg Med 1987;7:6–11.
-
Sacchini V, Melloni E, Santoro O, Marchesini R, Cascinelli N, Bandieramonte G. Photodynamic therapy by TPPS topical application and dye-laser in basal-cell carcinoma. SPIE Proceedings Series 1989;1066:173–6.
-
Salgado A, Fujimura M, Secco L. Case report: Basal cell carcinoma treated with photodynamic therapy and yellow light. J Am Acad Dermatol 2007;56:A206.
-
Salvini C, Massi D, Cappugi P. Recurrent basal cell carcinoma of the nose successfully treated by photodynamic therapy. J Eur Acad Dermatol Venereol 2009;23:73–5.
-
Santoro O, Bandieramonte G, Melloni E, Marchesini R, Zunino F, Lepera P, et al. Photodynamic therapy by topical meso-tetraphenylporphinesulfonate tetrasodium salt administration in superficial basal cell carcinomas. Cancer Res 1990;50:4501–3.
-
Schleier P, Berndt A, Kolossa S, Wood M. Comparison between mALA- and ALA-PDT in the treatment of basal cell carcinomas. Oral Oncol 2005;1(Suppl.):67–8.
-
Schleier P, Hyckel P, Berndt A, Bode H-P, Albrecht V, Hindermann W, et al. Photodynamic therapy of virus-associated epithelial tumours of the face in organ transplant recipients. J Cancer Res Clin Oncol 2004;130:279–84.
-
Schweitzer VG, Visscher D. Photodynamic therapy for treatment of AIDS-related oral Kaposi’s sarcoma. Otolaryngol Head Neck Surg 1990;102:639–49.
-
Sieron A, Kawczyk-Krupka A, Wojciech Cebula MA, Szygula M, Zieleznik W, Gruk M, et al. Photodynamic therapy (PDT) using topically applied delta-aminolevulinic acid (ALA) for the treatment of malignant skin tumors. Photodiag Photodyn Ther 2004;1:311–17.
-
Smucler R. Surgical excision versus ALA-PDT-PDL versus laser coagulation with diode laser in treatment of basal cell carcinoma (2 years study). Lasers Surg Med 2005;36:S17.
-
Smucler R, Vlk M. Combination of PDT and Er:YAG laser in treatment of nodular basal cell carcinoma. Lasers Surg Med 2007;39:S19.
-
Smucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med 2008;40:153–8.
-
Soler AM, Warloe T, Berner A, Giercksky KE. A follow-up study of recurrence and cosmesis in completely responding superficial and nodular basal cell carcinomas treated with methyl 5-aminolaevulinate-based photodynamic therapy alone and with prior curettage. Br J Dermatol 2001;145:467–71.
-
Soler AM, Warloe T, Tausjo J, Berner A. Photodynamic therapy by topical aminolevulinic acid, dimethylsulphoxide and curettage in nodular basal cell carcinoma: a one-year follow-up study. Acta Dermatol Venereol 1999;79:204–6.
-
Soler AM, Warloe T, Tausjo J, Giercksky KE. Photodynamic therapy of residual or recurrent basal cell carcinoma after radiotherapy using topical 5-aminolevulinic acid or methylester aminolevulinic acid. Acta Oncol 2000;39:605–9.
-
Souza CS, Neves ABS, Felicio LAB, Ferreira J, Kurachi C, Bagnato VS. Optimized photodynamic therapy with systemic photosensitizer following debulking technique for nonmelanoma skin cancers. [Erratum published in Dermatol Surg 2007;33:523.] Dermatol Surg 2007;33:194–8.
-
Stables GI, Stringer MR, Hudson EJ, Ash DV. Topical 5-aminolaevulinic acid photodynamic therapy for Bowen’s disease and basal cell carcinoma. Br J Dermatol 1994;131:23.
-
Star WM, van’t Veen AJ, Robinson DJ, Munte K, de Haas ERM, Sterenborg HJCM. Topical 5-aminolevulinic acid mediated photodynamic therapy of superficial basal cell carcinoma using two light fractions with a two-hour interval: long-term follow-up. Acta Dermatol Venereol 2006;86:412–17.
-
Stolz W, Landthaler M, Braun-Falco O. [Photodynamic therapy of cutaneous T-cell lymphoma in special locations: two case reports.] Hautarzt 1999;50:109–14.
-
Stranadko E, Purtskhvanidze V, Radaev A. Photodynamic therapy for skin cancer with minimized light doses and minimized doses of chlorin derivatives photosensitizers. Photodynamic Therapy and Photodiagnosis in Clinical Practice, Brixen/Bressanone, 7–11 October 2008. Poster session – CD08.
-
Stranadko E, Skobelkin O. Experience of PDT of cancer with two Russian-produced photosensitizers. SPIE Proceedings Series 1995;2392:93–105.
-
Stranadko EF, Purtskhvanidze VA, Radaev AA. Photodynamic therapy for skin cancer with chlorin derivatives under the outpatient conditions. Laser Helsinki 2008, 13th International Congress of EMLA (European Medical Laser Association) in conjunction with EMLA Finland and MAL (Medical Acupuncture and Laser) in cooperation with ASLMS (American Society for Laser Medicine and Surgery), Helsinki, 2008.
-
Stranadko EF, Skobelkin OK, Litvin GD, Astrakhankina TA. Photodynamic therapy of human malignant tumours: a comparative study between photohem and tetrasulfonated aluminium phthalocyanine. SPIE Proceedings Series 1996;2625:440–8.
-
Stranadko EF, Skobelkin OK, Makeev YM, Markichev NA, Riabov MV, Armitchev AV, et al. Laser treatment of skin cancer: dissection, photodestruction, photodynamic therapy. SPIE Proceedings Series 1996;2887:63–5.
-
Stranadko EF, Skobelkin OK, Vorozhtsov GN, Beshleul SE, Markitchev NA, Riabov MV. Photodynamic therapy of cancer, five-year clinical experience. SPIE Proceedings Series 1997;3191:253–62.
-
Stranadko EP, Ponomarev GV, Mechkov VM, Riabov MV, Ivanov AV, Reshetnikov AV, et al. The first experience of photodithazine clinical application for photodynamic therapy of malignant tumors. SPIE Proceedings Series 2000;3909:138–44.
-
Surrenti T, De Angelis L, Di Cesare A, Fargnoli MC, Peris K. Efficacy of photodynamic therapy with methyl aminolevulinate in the treatment of superficial and nodular basal cell carcinoma: an open-label trial. Eur J Dermatol 2007;17:412–15.
-
Surrenti T, De Angelis L, Fargnoli MC, Peris K. Efficacy, tolerability, and cosmetic outcome of MAL-PDT in the treatment of basal cell carcinoma. J Am Acad Dermatol 2006;54:A187.
-
Svanberg K, Andersson T, Killander D, Wang I, Stenram U, Andersson-Engels S, et al. Photodynamic therapy of non-melanoma malignant tumours of the skin using topical delta-amino levulinic acid sensitization and laser irradiation. Br J Dermatol 1994;130:743–51.
-
Szeimies RM, Landthaler M. [Topical photodynamic therapy in treatment of superficial skin tumors.] Hautarzt 1995;46:315–18.
-
Taber SW, Fingar VH, Coots CT, Wieman TJ. Photodynamic therapy using mono-L-aspartyl chlorin e6 (Npe6) for the treatment of cutaneous disease: a Phase I clinical study. Clin Cancer Res 1998;4:2741–6.
-
Tardivo JP, Del Giglio A, Paschoal LH, Baptista MS. New photodynamic therapy protocol to treat AIDS-related Kaposi’s sarcoma. Photomed Laser Surg 2006;24:528–31.
-
Thiele B, Grotmann P, Huttmann G, Diddens H, Horauf H. [Topical photodynamic therapy of basal cell carcinomas (BCCS): clinical, histological and experiment results (first report).] Z Hautkrankheiten 1994;69:161–4.
-
Thissen MR, Schroeter CA, Neumann HA. Photodynamic therapy with delta-aminolaevulinic acid for nodular basal cell carcinomas using a prior debulking technique. Br J Dermatol 2000;142:338–9.
-
Thissen MRTM, Kuijpers DIM, De Groot AC. [Photodynamic therapy of superficial basal cell carcinomas: 5 years experience.] Nederland Tijdschr Dermatol Venereol 2004;14:307–13.
-
Thompson MS, Andersson-Engels S, Svanberg S, Johansson T, Palsson S, Bendsoe N, et al. Photodynamic therapy of nodular basal cell carcinoma with multifiber contact light delivery. J Environ Pathol Tox 2006;25:411–24.
-
Tindholdt TT, Tonseth KA, Bjaerke TK, Abyholm FE. [Photodynamic therapy of facial basal cell carcinoma.] Tidsskr Nor Laegeforen 2007;127:1928–30.
-
Tobola J, Witkowska A, Ostrowski J, Kucharczyk Z. [Topical treatment of superficial basal cell carcinomas with 5% 5-fluorouracil ointment.] Przegl Dermatol 2003;90:357–62.
-
Toll A, Parera Ma E, Velez M, Pujol RM. Photodynamic therapy with methyl aminolevulinate induces phototoxic reactions on areas of the nose adjacent to basal cell carcinomas and actinic keratoses. Dermatol Surg 2008;34:1145–7.
-
Toll A, Parera ME, Velez M, Pujol RM. Letter: photodynamic therapy with methyl aminolevulinate induces phototoxic reactions on areas of the nose adjacent to basal cell carcinomas and actinic keratoses. Dermatol Surg 2008;34:1145–7; discussion 7–8.
-
Tomio L, Corti L, Polico C, Maluta S, Calzavara F, Norberto L, et al. [Laser-photo-radiotherapy in the treatment malignant tumors.] Radiol Med 1987;73:313–16.
-
Tope WD, Ross EV, Kollias N, Martin A, Gillies R, Anderson RR. Protoporphyrin IX fluorescence induced in basal cell carcinoma by oral delta-aminolevulinic acid. Photochem Photobiol 1998;67:249–55.
-
Tosca AD, Balas CJ, Stefanidou MP, Katsantonis JC, Georgiou S, Tzardi MN, et al. Prediction of ALA-PDT efficacy through remote color inspection and post therapy sequential histologic observations of skin malignancies. SPIE Proceedings Series 1997;3191:221–30.
-
Tse DT, Kersten RC, Anderson RL. Hematoporphyrin derivative photoradiation therapy in managing nevoid basal-cell carcinoma syndrome. A preliminary report. Arch Ophthalmol 1984;102:990–4.
-
Umegaki N, Moritsugu R, Katoh S, Harada K, Nakano H, Tamai K, et al. Photodynamic therapy may be useful in debulking cutaneous lymphoma prior to radiotherapy. Clin Exp Dermatol 2004;29:42–5.
-
Usmani N, Telfer NR, Stables GI. Subungual Bowen’s disease treated by topical photodynamic therapy. Br J Dermatol 2004;151:96.
-
Vaicova M, Ettler K. [Our clinical experience with the use of photodynamic therapy in patients with the basal cell carcinoma and morbus Bowen (comparison of efficacy of two photosensitizers).] Cesk Dermatol 2004;79:200–4.
-
Vakoulovskaia E, Chental V, Kuvshinov Y, Poddubny B, Ivanov AV, Kazaryan MA. New approaches to photodynamic therapy of tumors with Al phthalocyanine. SPIE Proceedings Series 2000;4059:32–8.
-
Vakulovskaya EG, Kemov YV, Zalevsky ID, Reshetnikov AV, Umnova LV, Vorozhcsov GN. Photodynamic therapy and fluorescent diagnostics of skin cancer with radochlorine and photosense, comparing efficacy and toxicity. SPIE Proceedings Series 2004;5315:148–51.
-
Van de Mierop F, Lightdale CJ. Use of atropine during endoscopy: benefit during photodynamic therapy. Gastrointest Endosc 1996;44:512.
-
Van Oosten EJ, Kuijpers DIM, Thissen MRTM. Different pain sensations in photodynamic therapy of nodular basal cell carcinoma: results from a prospective trial and a review of the literature. Photodiag Photodyn Ther 2006;3:61–8.
-
Varma S, Anstey A, Wilson H, Kurwa HA. Photodynamic therapy for the treatment of Bowen’s disease, solar keratoses, and superficial basal cell carcinomas: 12 months experience with a novel light source. Br J Dermatol 1998;139:19.
-
Varma S, Wilson H, Kurwa HA, Charman C. One year relapse rates for Bowen’s disease, basal cell carcinomas and solar keratoses treated by photodynamic therapy: analysis of 189 lesions. Br J Dermatol 1999;141:114–18.
-
Varma S, Wilson H, Kurwa HA, Gambles B, Charman C, Pearse AD, et al. Bowen’s disease, solar keratoses and superficial basal cell carcinomas treated by photodynamic therapy using a large-field incoherent light source. Br J Dermatol 2001;144:567–74.
-
Vinciullo C, Elliott T, Francis D. MAL-PDT in “difficult-to-treat” basal cell carcinoma, an Australian study: 48 month follow-up data. J Invest Dermatol 2006;126:S34.
-
Vinciullo C, Elliott T, Francis D, Gebauer K, Spelman L, Nguyen R, et al. Photodynamic therapy with topical methyl aminolaevulinate for ‘difficult-to-treat’ basal cell carcinoma. Br J Dermatol 2005;152:765–72.
-
Viniciullo C, Elliott T, Gebauer K, Spelman L. Photodynamic therapy using methyl aminolevulinate in patients with basal cell carcinoma. J Am Acad Dermatol 2004;50:P478.
-
Waldow SM, Lobraico RV, Kohler IK, Wallk S, Fritts HT. Photodynamic therapy for treatment of malignant cutaneous lesions. Lasers Surg Med 1987;7:451–6.
-
Wang J, Gao M, Wen S, Wang M. Photodynamic therapy for 50 patients with skin cancers or precancerous lesions. Chin Med Sci J 1991;6:163–5.
-
Wang JB, Gao ML, Wen SJ, Wang MJ. Study of photodynamic therapy in skin cancers and precancerous lesions. SPIE Proceedings Series 1993;1616:139–42.
-
Wang XL, Wang HW, Guo MX, Xu SZ. Treatment of skin cancer and pre-cancer using topical ALA-PDT: a single hospital experience. Photodiag Photodyn Ther 2008;5:127–33.
-
Warloe T, Peng Q, Heyerdahl H, Moan J, Steen HB, Giercksky KE. Photodynamic therapy with 5-aminolevulinic acid induced porphyrins and DMSO/EDTA for basal cell carcinoma. SPIE Proceedings Series 1995;2371:226–35.
-
Weisser H, Meyer-Rogge D, Meyer-Rogge E. [First experiences in medical practice with the new topical photosensitizer MAOP for actinic keratosis and basal cell carcinoma.] Aktuelle Dermatol 2004;30:306–11.
-
Wennberg AM, Lindholm LE, Alpsten M, Larko O. Treatment of superficial basal cell carcinomas using topically applied delta-aminolaevulinic acid and a filtered xenon lamp. Arch Dermatol Res 1996;288:561–4.
-
Whitaker IS, Shokrollahi K, James W, Mishra A, Lohana P, Murison MC. Combined CO(2) laser with photodynamic therapy for the treatment of nodular basal cell carcinomas. Ann Plast Surg 2007;59:484–8.
-
Wilson BD, Mang TS, Cooper M, Stoll H. Use of photodynamic therapy for the treatment of extensive basal cell carcinomas. Facial Plast Surg 1989;6:185–9.
-
Wilson BD, Mang TS, Stoll H, Jones C, Cooper M, Dougherty TJ. Photodynamic therapy for the treatment of basal cell carcinoma. Arch Dermatol 1992;128:1597–601.
-
Wolf P, Fink-Puches R, Reimann-Weber A, Kerl H. Development of malignant melanoma after repeated topical photodynamic therapy with 5-aminolevulinic acid at the exposed site. Dermatology 1997;194:53–4.
-
Wolf P, Rhodes L, Fritsch C. MAL-PDT in the treatment of difficult-to-treat basal cell carcinoma: 36-month update. J Am Acad Dermatol 2005;52:P162.
-
Wolf P, Rieger E, Kerl H. Topical photodynamic therapy with endogenous porphyrins after application of 5-aminolevulinic acid. An alternative treatment modality for solar keratoses, superficial squamous cell carcinomas, and basal cell carcinomas? [Erratum published in J Am Acad Dermatol 1993;29:41.] J Am Acad Dermatol 1993;28:17–21.
-
Xu S, Wang X, Xu W. [Evaluation of photodynamic therapy of skin cancers with delta-aminolevulinic acid.] Med J Wuhan Univ 2003;24:86–9.
-
Xu S, Wang X, Xu W, Xia Y, Zhang C. Evaluation of photodynamic therapy of skin cancers with partial differential alpha-aminolevulinic acid. Chin Med J 2002;115:1141–5.
-
Yokoyama S, Nakano H, Nishizawa A, Kaneko T, Harada K, Hanada K. A case of photocontact urticaria induced by photodynamic therapy with topical 5-aminolaevulinic acid. J Dermatol 2005;32:843–7.
-
Zane C, Venturini M, Sala R, Calzavara-Pinton P. Photodynamic therapy with methylaminolevulinate as a valuable treatment option for unilesional cutaneous T-cell lymphoma. Photodermatol Photoimmunol Photomed 2006;22:254–8.
-
Zeng CY, Yang D, Wang KH, Cao QQ. Interstitial photodynamic therapy for cancers of cavum oris, skin and cervix. SPIE Proceedings Series 1993;1616:102–7.
Appendix 7 Barrett’s oesophagus scoping
Seven non-RCTs reported in 11 publications were identified, which reported on patients with Barrett’s oesophagus being treated with PDT. 1–11 These were originally to be included in the main systematic review; however, as 24 publications reporting 11 RCTs were identified, these less robust non-randomised designs were subsequently excluded from the review.
A further 96 publications with study designs that did not meet the inclusion criteria for the review were identified. 12–107 The references are listed below in alphabetical order, they have not been categorised and may still contain a number of duplicate publications.
References
Non-randomised controlled trials
-
1. Gross SA, Gill KR, Wolfsen HC. Comparative outcomes of photodynamic therapy and radiofrequency ablation for the treatment of Barrett’s esophagus with high grade dysplasia. Gastrointest Endosc 2008;67:A179.
-
2. Jamieson N, Mosse A, Thorpe S, Novelli M, Bown S, Lovat L. High-grade dysplasia in Barrett’s esophagus: successful ablation by photodynamic therapy with ALA requires intensive therapy. Gastroenterology 2003;124:A298.
-
3. Jamieson NF, Thorpe S, Mosse A, Bown SG, Lovat LB. High grade dysplasia in Barrett’s oesophagus: successful ablation by photodynamic therapy with ALA requires intensive therapy. Gut 2003;52:73.
-
4. Mackenzie G, Clark BR, Selvasekar C, Jamieson N, Novelli M, Thorpe S, et al. Photodynamic therapy with 5 aminolevulinic acid for high grade dysplasia in Barrett’s esophagus: longterm follow-up of 51 patients. Gastroenterology 2005;128:A238.
-
5. Mackenzie GD, Clark B, Selvasekar CR, Jamieson NF, Novelli MR, Thorpe SM, et al. Photodynamic therapy with 5 aminolevulinic acid for high grade dysplasia in Barrett’s oesophagus: long term follow-up of 51 patients. Gut 2005;54:53.
-
6. Mackenzie GD, Jamieson NF, Novelli MR, Mosse CA, Clark BR, Thorpe SM, et al. How light dosimetry influences the efficacy of photodynamic therapy with 5-aminolaevulinic acid for ablation of high-grade dysplasia in Barrett’s esophagus. Lasers Med Sci 2008;23:203–10.
-
7. May A, Gossner L, Pech O, Fritz A, Gunter E, Mayer G, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14:1085–91.
-
8. Mellidez JC, Mackenzie G, Selvasekar C, Novelli M, Thorpe S, Mosse C, et al. Reversal of Barrett’s esophagus following photodynamic therapy using high dose 5 aminolevulinic acid activated by red or green laser light. Gastroenterology 2005;128:A239.
-
9. Panjehpour M, Overholt BF, Phan MN, Haydek JM. Optimization of light dosimetry for photodynamic therapy of Barrett’s esophagus: efficacy vs. incidence of stricture after treatment. Gastrointest Endosc 2005;61:13–18.
-
10. Panjehpour M, Phan MN, Overholt BF, Haydek JM. Optimization of light dosimetry for photodynamic therapy of Barrett’s esophagus. SPIE Proceedings Series 2004;5315:100–6.
-
11. Phan MN, Panjehpour M, Overholt BF. Optimization of photodynamic therapy for Barrett’s esophagus: treatment efficacy and stricture incidence as a function of light dose. Gastroenterology 2004;126:A178.
Other scoping publications
-
12. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. [Erratum published in Gastrointest Endosc 2005;62:488–498.] Gastrointest Endosc 2006;63:359.
-
13. Ackroyd R, Brown NJ, Davis MF, Stephenson TJ, Stoddard CJ, Reed MW. Aminolevulinic acid-induced photodynamic therapy: safe and effective ablation of dysplasia in Barrett’s esophagus. Dis Esophagus 2000;13:18–22.
-
14. Ackroyd R, Brown NJ, Davis MF, Stephenson TJ, Stoddard CJ, Reed MWR. Aminolaevulinic acid-induced photodynamic therapy in the treatment of dysplastic Barrett’s oesophagus and adenocarcinoma. Lasers Med Sci 1999;14:278–85.
-
15. Ackroyd R, Kelty CJ, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MWR. Eradication of dysplastic Barrett’s oesophagus using photodynamic therapy: long-term follow-up. Endoscopy 2003;35:496–501.
-
16. Attila T, Kortan P, Kandel GT, Marcon N. Photodynamic therapy (PDT) for Barrett’s esophagus with high grade dysplasia (BE-HGD). Gastrointest Endosc 2005;61:A127.
-
17. Ban S, Mino M, Nishioka NS, Puricelli W, Zukerberg LR, Shimizu M, et al. Histopathologic aspects of photodynamic therapy for dysplasia and early adenocarcinoma arising in Barrett’s esophagus. Am J Surg Pathol 2004;28:1466–73.
-
18. Barr H, Shepherd NA, Dix A, Roberts DJ, Tan WC, Krasner N. Eradication of high-grade dysplasia in columnar-lined (Barrett’s) oesophagus by photodynamic therapy with endogenously generated protoporphyrin IX. Lancet 1996;348:584–5.
-
19. Barr H, Tan WC, Shepherd NA, Krasner N. Photodynamic therapy (PDT) using 5-aminolaevulinic acid (5ALA) for oesophageal adenocarcinoma associated with Barrett’s metaplasia. Lasers Med Sci 1997;12:291–2.
-
20. Behrens A, May A, Gossner L, Gunter E, Pech O, Vieth M, et al. Curative treatment for high-grade intraepithelial neoplasia in Barrett’s esophagus. Endoscopy 2005;37:999–1005.
-
21. Buttar NS, Wang KK, Lutzke LS, Krishnadath KK, Anderson MA. Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett’s esophagus. Gastrointest Endosc 2001;54:682–8.
-
22. Chen WR, Huang Z, Korbelik M, Nordquist RE, Liu H. Photoimmunotherapy for cancer treatment. J Environ Pathol Tox 2006;25:281–91.
-
23. Etienne J, Canard JM. [Photodynamic therapy in liberal practice.] Acta Endoscopica 2007;37:397–8.
-
24. Etienne J, Dorme N, Bourg-Heckly G, Raimbert P, Fekete F. [Local curative treatment of superficial adenocarcinoma in Barrett’s esophagus. First results of photodynamic therapy with a new photosensitizer.] Bull Acad Natl Med 2000;184:1731–44; discussion 44–47.
-
25. Etienne J, Dorme N, Bourg-Heckly G, Raimbert P, Fekete F, Mercadier Mc, et al. Local curative treatment of superficial adenocarcinoma in Barrett’s esophagus. First results of photodynamic therapy with a new photosensitizer. Bull Acad Natl Med 2000;184:1731–47.
-
26. Etienne J, Dorme N, Bourg-Heckly G, Raimbert P, Flejou JF. Photodynamic therapy with green light and m-tetrahydroxyphenyl chlorin for intramucosal adenocarcinoma and high-grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2004;59:880–9.
-
27. Foroulis CN, Thorpe JAC. Photodynamic therapy (PDT) in Barrett’s esophagus with dysplasia or early cancer. Eur J Cardiothorac Surg 2006;29:30–4.
-
28. Globe J, Kelty CJ, Smythe A, Ackroyd R. The effect of photodynamic therapy (PDT) on oesophageal motility in Barrett’s oesophagus. Gut 2004;53:118.
-
29. Globe J, Smythe A, Kelty CJ, Reed MWR, Brown NJ, Ackroyd R. The effect of photodynamic therapy (PDT) on oesophageal motility and acid clearance in patients with Barrett’s oesophagus. J Photochem Photobiol B Biol 2006;85:17–22.
-
30. Go JT, Dumot JA, Lopez R, Vargo J, Zuccaro G, Falk G, et al. Photodynamic therapy in Barrett’s esophagus with high grade dysplasia and intra-mucosal carcinoma. Am J Gastroenterol 2006;101:66.
-
31. Gossner L, Stolte M, Sroka R, Rick K, May A, Hahn EG, et al. Photodynamic ablation of high-grade dysplasia and early cancer in Barrett’s esophagus by means of 5-aminolevulinic acid. Gastroenterology 1998;114:448–55.
-
32. Heller SJ, Van Dam J, Barr H. Eradication of high-grade dysplasia using 5-ALA and acid suppression: a (photo)dynamic duo. Gastroenterology 1997;112:2156–8.
-
33. Hemminger LL, Wolfsen HC. Photodynamic therapy for Barrett’s esophagus and high grade dysplasia: results of a patient satisfaction survey. Gastroenterol Nurs 2002;25:139–41.
-
34. Hinnen P, de Rooij FWM, Hop WCJ, Edixhoven A, van Dekken H, Wilson JHP, et al. Timing of 5-aminolaevulinic acid-induced photodynamic therapy for the treatment of patients with Barrett’s oesophagus. J Photochem Photobiol B Biol 2002;68:8–14.
-
35. Hur C, Wittenberg E, Nishioka NS, Gazelle GS. Quality of life in patients with various Barrett’s esophagus associated health states. Health Qual Life Outcomes 2006;4:45.
-
36. Ikeguchi M, Lightdale CJ, Choi YA, Bukowski K. A new light delivery system for photodynamic therapy with aminolevulinic acid (ALA) for treatment of high-grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2005;61:A233.
-
37. Jamieson N, Thorpe S, Mosse A, Novelli M, Lovat L, Bown S. Photodynamic therapy using mTHPC for early Barrett’s-associated esophageal cancer. Gastroenterology 2003;124:A298.
-
38. Jamieson NF, Thorpe S, Masse A, Bown SG, Lovat LB. Photodynamic therapy using mTHPC for early Barrett’s-associated oesophageal cancers. Gut 2003;52:170.
-
39. Javaid B, Watt P, Krasner N. Photodynamic therapy (PDT) for oesophageal dysplasia and early carcinoma with mTHPC (m-tetrahydroxyphenyl chlorin): a preliminary study. Lasers Med Sci 2002;17:51–6.
-
40. Javaid B, Watt P, Krasner N, Brouwer PA. Oesophageal photodynamic therapy with mTHPC (m-tetra hydroxyphenyl chlorin) using a laser and a non-laser light source: a pilot study. SPIE Proceedings Series 2001;4156:262–5.
-
41. Jenkins JT, Charles A, Mitchell KG, Fullarton GM. Photodynamic therapy for Barretts’ adenocarcinoma associated with an Angelchik device. Photodiag Photodyn Ther 2005;2:197–200.
-
42. Kashtan H, Umansky M, Birkenfeld S, Scherubl H, Haddad R, Greenberg R, et al. Photodynamic therapy of Barrett’s esophagus with dysplasia using systemic aminolevulinic acid and a non-laser light source. A phase I/II study. Gastrointest Oncol 2002;4:153–7.
-
43. Kashtan H, Umansky M, Birkenfeld S, Scherubl H, Haddad R, Greenberg R, et al. Photodynamic therapy of Barrett’s esophagus with dysplasia using systemic aminolevulinic acid and a non-laser light source. A phase I/II study. Gastrointest Oncol 2002;4:153–8.
-
44. Keeley SB, Luketich JD, Landreneau RJ, Christie NA, Rivera MA, McGrath K. Using photodynamic therapy to cure esophageal cancer and Barrett’s high-grade dysplasia. Ann Surg Oncol 2006;13:85.
-
45. Keeley SB, Pennathur A, Gooding W, Landreneau RJ, Christie NA, Luketich J. Photodynamic therapy with curative intent for Barrett’s esophagus with high grade dysplasia and superficial esophageal cancer. Ann Surg Oncol 2007;14:2406–10.
-
46. Krishna M, Nakhleh RE, Hemminger LL, Wolfsen HC. Histologic characteristics and follow-up of 37 cases of Barrett’s esophagus with high grade dysplasia and intramucosal adenocarcinoma treated with photodynamic therapy. Lab Invest 2006;86:504.
-
47. Krishna M, Nakhleh RE, Hemminger LL, Wolfsen HC. Histologic characteristics and follow-up of 37 cases of Barrett’s esophagus with high grade dysplasia and intramucosal adenocarcinoma treated with photodynamic therapy. Mod Pathol 2006;19:504.
-
48. Krishnadath KK, Wang KK, Taniguchi K, Sebo TJ, Buttar NS, Anderson MA, et al. Persistent genetic abnormalities in Barrett’s esophagus after photodynamic therapy. Gastroenterology 2000;119:624–30.
-
49. Laukka MA, Wang KK. Initial results using low-dose photodynamic therapy in the treatment of Barrett’s esophagus. Gastrointest Endosc 1995;42:59–63.
-
50. Lovat LB, Jamieson NF, Novelli MR, Mosse CA, Selvasekar C, Mackenzie GD, et al. Photodynamic therapy with m-tetrahydroxyphenyl chlorin for high-grade dysplasia and early cancer in Barrett’s columnar lined esophagus. Gastrointest Endosc 2005;62:617–23.
-
51. Luketich JD, Fernando HC, Christie NA, Litle VR, Ferson PF, Buenaventura PO, et al. Photodynamic therapy in thoracic oncology: a single institution experience. SPIE Proceedings Series 2001;4248:28–33.
-
52. Macrae FA, Rajesekaram R, Thomas R, Bhathal PS. Photodynamic therapy in high grade dysplasia in Barrett’s oesophagus using 5 amino-laevulinic acid sensitization. Gastrointest Endosc 2004;59:A252.
-
53. Mino-Kenudosn M, Ban S, Deshpande V, Shimizu M, Lauwers GY. Buried dysplasia and early adenocarcinoma arising in Barrett esophagus (BE) after photodynamic therapy (PDT): not uncommon findings. Mod Pathol 2006;19:516.
-
54. Mino-Kenudson M, Ban S, Deshpande V, Shimizu M, Lauwers GY. Buried dysplasia and early adenocarcinoma arising in Barrett Esophagus (BE) after photodynamic therapy (PDT): not uncommon findings. Lab Invest 2006;86:114A.
-
55. Mino-Kenudson M, Ban S, Ohana M, Puricelli W, Deshpande V, Shimizu M, et al. Buried dysplasia and early adenocarcinoma arising in Barrett esophagus after porfimer-photodynamic therapy. Am J Surg Pathol 2007;31:403–9.
-
56. Mino-Kenudson M, Lauwers GY. Pathology of Barrett esophagus after photodynamic therapy. Mod Pathol 2007;20:550.
-
57. Moghissi K, Dixon K. The role of photodynamic therapy (PDT) in pre-malignant and malignant oesophageal lesions: can PDT compete with surgery? Laser Helsinki 2008, 13th International Congress of EMLA (European Medical Laser Association) in conjunction with EMLA Finland and MAL (Medical Acupuncture and Laser) in cooperation with ASLMS (American Society for Laser Medicine and Surgery), Helsinki, 2008.
-
58. Moghissi K, Dixon K, Thorpe JAC, Stringer MR. The role of photodynamic therapy (PDT) in oesophageal pre-neoplastic and malignant neoplastic lesions of the oesophagus: a review study. Photodynamic Therapy and Photodiagnosis in Clinical Practice, Brixen/Bressanone. 7–11 October 2008. Session 6 – invited lecture.
-
59. Ohana M, Mino-Kenudson M, Ban S, Puricelli W, Nishioka N. Frequent persistence of dysplasia by systematic pathological evaluation after photodynamic therapy for Barrett’s esophagus with high-grade dysplasia or early adenocarcinoma. Gastroenterology 2005;129:1109.
-
60. Ohana M, Mino-Kenudson M, Ban S, Puricelli W, Nishoka N. Frequent persistence of dysplasia by systematic pathological evaluation after photodynamic therapy for Barrett’s Esophagus with high-grade dysplasia or early adenocarcinoma. Gastrointest Endosc 2005;61:A94.
-
61. Ohana M, Mino-Kenudson M, Ban SC, Puricelli W, Nishioka N, Lauwers G. Frequent persistence of dysplasia by systematic pathological evaluation after photodynamic therapy for Barrett’s Esophagus with high-grade dysplasia or early adenocarcinoma. Gastroenterology 2005;128:A72.
-
62. Ortner MA, Zumbusch K, Liebetruth J, Ebert B, Fleige B, Dietel M, et al. Is topical delta-aminolevulinic acid adequate for photodynamic therapy in Barrett’s esophagus? A pilot study. Endoscopy 2002;34:611–16.
-
63. Overholt B, Panjehpour M, Tefftellar E, Rose M. Photodynamic therapy for treatment of early adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc 1993;39:73–6.
-
64. Overholt BF, Panjehpour M. Photodynamic therapy in Barrett’s esophagus: reduction of specialized mucosa, ablation of dysplasia, and treatment of superficial esophageal cancer. Semin Surg Oncol 1995;11:372–6.
-
65. Overholt BF, Panjehpour M. Barrett’s esophagus: photodynamic therapy for ablation of dysplasia, reduction of specialized mucosa, and treatment of superficial esophageal cancer. Gastrointest Endosc 1995;42:64–70.
-
66. Overholt BF, Panjehpour M. Barretts esophagus – photodynamic therapy for ablation of dysplasia, reduction of specialized mucosa and treatment of superficial esophageal cancer. SPIE Proceedings Series 1995;2371:375–84.
-
67. Overholt BF, Panjehpour M. Photodynamic therapy in Barrett’s esophagus. J Clin Laser Med Surg 1996;14:245–9.
-
68. Overholt BF, Panjehpour M. Photodynamic therapy for Barrett’s esophagus: clinical update. Am J Gastroenterol 1996;91:1719–23.
-
69. Overholt BF, Panjehpour M. Photodynamic therapy for Barrett’s esophagus. Gastrointest Endosc Clin N Am 1997;7:207–20.
-
70. Overholt BF, Panjehpour M, Ayres M. Photodynamic therapy for Barrett’s esophagus: cardiac effects. Lasers Surg Med 1997;21:317–20.
-
71. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc 2003;58:183–8.
-
72. Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc 2003;58:183–8.
-
73. Overholt BF, Panjehpour M, Haydek JM. Photodynamic therapy for Barrett’s esophagus: follow-up in 100 patients. Gastrointest Endosc 1999;49:1–7.
-
74. Pacifico RJ, Wang KK, Wongkeesong L-M, Buttar NS, Lutzke LS. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett’s esophagus. Clin Gastroenterol Hepatol 2003;1:252–7.
-
75. Panjehpour M, Overholt BF, Dougherty TJ. Photodynamic therapy in Barrett’s esophagus: review of current results. SPIE Proceedings Series 1996;2675:20–8.
-
76. Panjehpour M, Overholt BF, Dougherty TJ. Photodynamic therapy of dysplasia in Barrett’s esophagus: an update. SPIE Proceedings Series 1997;2972:27–36.
-
77. Panjehpour M, Overholt BF, Haydek JM, Dougherty TJ. Photodynamic therapy with photofrin followed by thermal ablation for elimination of dysplasia and early cancer in Barrett’s esophagus: follow-up in 100 patients. SPIE Proceedings Series 1998;3247:14–24.
-
78. Panjehpour M, Overholt BF, Phan MN, Haydek JM, Robinson AF. Photodynamic therapy for Barrett’s esophagus using a 20-mm diameter light-delivery balloon (invited paper). SPIE Proceedings Series 2002;4612:13–20.
-
79. Panjehpour M, Phan MN, Overholt BF. Relationship between acute mucosal injury, treatment efficacy and esophageal stricture following photodynamic therapy of Barrett’s esophagus. Gastrointest Endosc 2004;59:A251.
-
80. Pech O, Gossner L, May A, Rabenstein T, Vieth M, Stolte M, et al. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett’s cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc 2005;62:24–30.
-
81. Pech O, Gossner L, May AD, Stolte M, Ell C. Long term results of PDT for early neoplasia in Barrett’s esophagus. Gastrointest Endosc 2004;59:A257.
-
82. Peters F, Kara M, Rosmolen W, Aalders M, Ten Kate F, Krishnadath K, et al. Poor results of 5-aminolevulinic acid-photodynamic therapy for residual high-grade dysplasia and early cancer in barrett esophagus after endoscopic resection. Endoscopy 2005;37:418–24.
-
83. Peters F, Kara M, Rosmolen W, ten Kate F, Bultje B, Krishnadath S, et al. Endoscopic resection combined with photodynamic therapy for high grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc 2004;59:A251.
-
84. Peters FP, Kara MA, Rosmolen WD, Aalders MCG, Ten Kate FJW, Bultje BC, et al. Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett’s esophagus. Gastrointest Endosc 2005;61:506–14.
-
85. Prasad GA, Wang KK, Buttar NS, Wongkeesong L-M, Krishnadath KK, Nichols FC, III, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology 2007;132:1226–33.
-
86. Prasad GA, Wang KK, Buttar NS, Wongkeesong L-M, Lutzke LS, Borkenhagen LS. Predictors of stricture formation after photodynamic therapy for high-grade dysplasia in Barrett’s esophagus. Gastrointest Endosc 2007;65:60–6.
-
87. Rahmani EY, Rex DK, Ciaccia D, Turpin CS. Photodynamic therapy for ablation of esophageal high grade dysplasia and superficial cancer. Gastrointest Endosc 2000;51:3539.
-
88. Rahmani EY, Turpin C, Arney K. Photodynamic therapy for dysplastic Barrett’s esophagus and early esophageal adenocarcinoma should be first line therapy! Gastrointest Endosc 2004;59:A250.
-
89. Schembre DB, Huang JL, Lin OS, Cantone N, Low DE. Treatment of Barrett’s esophagus with early neoplasia: a comparison of endoscopic therapy and esophagectomy. Gastrointest Endosc 2008;67:595–601.
-
90. Schweitzer VG. Photodynamic therapy: 1994 treatment of benign and malignant upper aerodigestive tract disease. SPIE Proceedings Series 1995;2371:403–17.
-
91. Shah AK, Wolfsen HC, Hemminger LL, Shah AA, DeVault KR. Changes in esophageal motility after porfimer sodium photodynamic therapy for Barrett’s dysplasia and mucosal carcinoma. Dis Esophagus 2006;19:335–9.
-
92. Sylantiev C, Schoenfeld N, Mamet R, Groozman GB, Drory VE. Acute neuropathy mimicking porphyria induced by aminolevulinic acid during photodynamic therapy. Muscle Nerve 2005;31:390–3.
-
93. Triadafilopoulos G. Photodynamic therapy for the treatment of patients with Barrett’s high-grade dysplasia and mucosal adenocarcinoma. Nat Clin Pract Gastroenterol Hepatol 2005;2:136–7.
-
94. van Hillegersberg R, Haringsma J, ten Kate FJW, Tytgat GNJ, van Lanschot JJB. Invasive carcinoma after endoscopic ablative therapy for high-grade dysplasia in Barrett’s oesophagus. Dig Surg 2003;20:440–4.
-
95. Wang KK, Buttar NS, Lutzke LS, Anderson MA, Krisnadath K. Long-term results of photodynamic therapy for Barrett’s esophagus. Gastrointest Endosc 2000;51:4915.
-
96. Wang KK, Gutta K, Laukka MA. Phototherapy of Barrett’s esophagus. SPIE Proceedings Series 1994;2133:33–8.
-
97. Wang KK, Song L, Buttar N, Papenfuss S, Lutzke L. Barretts esophagus after photodynamic therapy: Risk of cancer development during long term follow-up. Gastroenterology 2004;126:A50.
-
98. Weiss A, Wiesinger H, Owen D. Photodynamic therapy of high grade dysplasia in Barrett’s esophagus: results from treatment of 17 patients. Gastrointest Endosc 2005;61:A145.
-
99. Weiss AA, Wiesinger HAR, Owen D. Photodynamic therapy in Barrett’s esophagus: results of treatment of 17 patients. Can J Gastroenterol 2006;20:261–4.
-
100. Wolfsen HC, Hemminger LI. Photodynamic therapy for dysplastic Barrett’s esophagus and mucosal adenocarcinoma. Gastrointest Endosc 2004;59:A251.
-
101. Wolfsen HC, Hemminger LL, Raimondo M, Woodward TA. Photodynamic therapy and endoscopic mucosal resection for Barrett’s dysplasia and early esophageal adenocarcinoma. South Med J 2004;97:827–30.
-
102. Wolfsen HC, Hemminger LL, Wallace MB, Devault KR. Clinical experience of patients undergoing photodynamic therapy for Barrett’s dysplasia or cancer. Aliment Pharmacol Ther 2004;20:1125–31.
-
103. Wolfsen HC, Ng CS. Cutaneous consequences of photodynamic therapy. Cutis 2002;69:140–2.
-
104. Wolfsen HC, Woodward TA. Photofrin PDT for Barrett’s esophagus and early esophageal cancer. Recent Advances in Diseases of the Esophagus, 8th World Congress of the International Society for Diseases of the Esophagus, Sao Paulo, Brazil, 2001. pp. 149–53.
-
105. Wolfsen HC, Woodward TA, Raimondo M. Photodynamic therapy for dysplastic Barrett esophagus and early esophageal adenocarcinoma. Mayo Clin Proc 2002;77:1176–81.
-
106. Yachimski P, Puricelli WP, Nishioka NS. Patient predictors of esophageal stricture development after photodynamic therapy. Clin Gastroenterol Hepatol 2008;6:302–8.
-
107. Zopf T, Rosenbaum A, Apel D, Jakobs R, Arnold JC, Riemann JF. [Photodynamic therapy of dysplasias and early carcinomas in Barrett esophagus with a diode laser system: a pilot study.] Med Klin 2001;96:212–16.
Appendix 8 Oesophageal cancer scoping
One hundred and fifty publications, with study designs that did not meet the inclusion criteria for the review, reported on patients with oesophageal cancer being treated with PDT. The references are listed below in alphabetical order; they have not been categorised and may still contain a number of duplicate publications.
References
-
Anonymous. Photodynamic therapy effective for swallowing problems. Oncology 1999;13:937.
-
Baconnier M, Phelip JM, Germain E, Durand A, Balosso J, Bichard P, et al. [External radiotherapy and photodynamic therapy for esophageal carcinoma: a dangerous association?] Gastroenterol Clin Biol 2008;32:221–3.
-
Bai XM, Shen GR, Chen WG, Guo T. Observation of curative effect photodynamic therapy for 42 cases of moderate or late stage in esophagus cancer. SPIE Proceedings Series 1998;3344:114–16.
-
Ban S, Mino M, Nishioka NS, Puricelli W, Zukerberg LR, Shimizu M, et al. Histopathologic aspects of photodynamic therapy for dysplasia and early adenocarcinoma arising in Barrett’s esophagus. Am J Surg Pathol 2004;28:1466–73.
-
Calzavara F, Tomio L. Photodynamic therapy: clinical experience at the Department of Radiotherapy at Padova General Hospital. J Photochem Photobiol B 1991;11:91–5.
-
Calzavara F, Tomio L, Corti L, Zorat PL, Barone I, Peracchia A, et al. Oesophageal cancer treated by photodynamic therapy alone or followed by radiation therapy. J Photochem Photobiol B 1990;6:167–74.
-
Calzavara F, Tomio L, Norberto L, Peracchia A, Corti L, Zorat PL, et al. Photodynamic therapy in the treatment of malignant tumours of the upper aerodigestive tract. Lasers Med Sci 1989;4:279–84.
-
Chissov VI, Sokolov VV, Trakhtenberg AK, Mamontov AS. Photodynamic therapy of early stage cancer of lung, esophagus, and stomach with two different photosensitizers. SPIE Proceedings Series 1995;2625:466–75.
-
Corti L, Noverto L, Belfontali S, Schaffer M. Long survival-rate in patients with esophagus cancer treated with PDT. In Waidelich W, Waidelich R, Hofstetter A, editors. Lasers in medicine. Munich, Germany: Springer-Verlag; 1993. pp. 268–71.
-
Corti L, Skarlatos J, Boso C, Cardin F, Kosma L, Koukourakis MI, et al. Outcome of patients receiving photodynamic therapy for early esophageal cancer. Int J Radiat Oncol Biol Phys 2000;47:419–24.
-
Craig C, Gray J, Macpherson M, Hodgson H, Zammit M, Fullarton G. Porfimer sodium photodynamic therapy in the treatment of early oesophageal carcinoma. Photodiag Photodyn Ther 2007;4:244–8.
-
Craig C, Macpherson M, Hodgson H, Gray J, Zammit M, Fullarton G. Photodynamic therapy of early oesophageal carcinoma. Gut 2006;55:76.
-
Craig CF, MacPherson M, Hodgson H, Gray J, Zammit M, Fullarton G. Photodynamic therapy is of benefit in treatment of early oesophageal carcinoma. Gastroenterology 2006;130:A414.
-
Dal Fante M, Lombardo L, Conio M, Mancini A, Spinelli P. [Photodynamic therapy of the superficial esophageal carcinoma in Barrett’s esophagus.] Giorn Ital Endosc Digest 1995;18:171–6.
-
Eickhoff A, Jakobs R, Weickert U, Hartmann D, Schilling D, Alsenbesy M, et al. Long-segment early squamous cell carcinoma of the proximal esophagus: curative treatment and long-term follow-up after 5-aminolevulinic acid (5-ALA)-photodynamic therapy. Endoscopy 2006;38:641–3.
-
Etienne J, Canard JM. [Photodynamic therapy in liberal practice.] Acta Endoscopica 2007;37:397–8.
-
Everette SS, Sethi A, DeCosta GB, Zfass AM. A 10 year, single center experience with PDT in esophageal lesions. Am J Gastroenterol 2006;101:94.
-
Fujimaki M, Nakayama K. Endoscopic laser treatment of superficial esophageal cancer. Semin Surg Oncol 1986;2:248–56.
-
Go JT, Dumot JA, Lopez R, Vargo J, Zuccaro G, Falk G, et al. Photodynamic therapy in Barrett’s esophagus with high grade dysplasia and intra-mucosal carcinoma. Am J Gastroenterol 2006;101:66.
-
Goodell TT, Jacques SL, Gregory KW, Dougherty TJ. Evaluating clinical outcomes of PDT. SPIE Proceedings Series 2001;4248:1–9.
-
Gossner L, May A, Sroka R, Stolte M, Hahn EG, Ell C. Photodynamic destruction of high grade dysplasia and early carcinoma of the esophagus after the oral administration of 5-aminolevulinic acid. Cancer 1999;86:1921–8.
-
Gossner L, Stolte M, Sroka R, May A, Hahn EG, Ell C. [Photodynamic therapy of early squamous epithelial carcinomas and severe squamous epithelial dysplasias of the esophagus with 5-aminolevulinic acid.] Z Gastroenterologie 1998;36:19–26.
-
Grosjean P, Savary JF, Mizeret J, Wagnieres G, Woodtli A, Theumann JF, et al. Photodynamic therapy for cancer of the upper aerodigestive tract using tetra(m-hydroxyphenyl)chlorin. J Clin Laser Med Surg 1996;14:281–7.
-
Grosjean P, Savary JF, Wagnieres G, Mizeret J, Woodtli A, Fontolliet C, et al. [Phototherapy of pharyngeal, oesophageal and bronchial early squamous cell carcinomas after sensitisation by tetra (m-hydroxyphenyl) chlorin (mTHPC).] Med Chir Digest 1996;25:411–13.
-
Grosjean P, Savary JF, Wagnieres G, Mizeret J, Woodtli A, Theumann JF, et al. Tetra(m-hydroxyphenyl)chlorin clinical photodynamic therapy of early bronchial and oesophageal cancers. Lasers Med Sci 1996;11:227–35.
-
Ha BW, Kim JI, Hwang EM, Oh YK, Cheung DY, Park SH, et al. [A case of photodynamic therapy for early esophageal cancer recurred after esophagectomy.] Taehan Sohwagi Hakhoe chi) 2007;49:331–5.
-
Ha XW, Sun XM, Xie JG, Fan XJ, Zhang YH, Mei QC, et al. Clinical use of hematoporphyrin derivative in malignant tumors. Chin Med J 1983;96:754–8.
-
Hayata Y, Kato H, Furuse K, Kusunoki Y, Suzuki S, Mimura S. Photodynamic therapy of 168 early stage cancers of the lung and oesophagus: a Japanese multi-centre study. Lasers Med Sci 1996;11:255–9.
-
Hayata Y, Kato H, Konaka C, Okitsu H, Suga S, Sayami P. [Laser endoscopy in photodynamic therapy.] Chirurgie 1988;59:81–9.
-
Hochain P, Ducrotte P, Paillot B, Touchais JY, Thorel JM, Petit A, et al. [Photodynamic therapy by coloring laser. Can it represent a therapeutic alternative for small epidermoid cancers of the esophagus?] Gastroenterol Clin Biol 1992;16:552–7.
-
Hochain P, Ducrotte P, Touchais JY, Paillot B, Hecketsweller P. Extended necrosis of the oesophageal wall after photodynamic therapy: report of two cases. Lasers Med Sci 1993;8:147–50.
-
Jamieson N, Thorpe S, Mosse A, Novelli M, Lovat L, Bown S. Photodynamic therapy using mTHPC for early Barretts-associated esophageal cancer. Gastroenterology 2003;124:A298.
-
Jamieson NF, Thorpe S, Masse A, Bown SG, Lovat LB. Photodynamic therapy using mTHPC for early Barrett’s-associated oesophageal cancers. Gut 2003;52:170.
-
Javaid B, Watt P, Krasner N. Photodynamic therapy (PDT) for oesophageal dysplasia and early carcinoma with mTHPC (m-tetrahydroxyphenyl chlorin): a preliminary study. Lasers Med Sci 2002;17:51–6.
-
Javaid B, Watt P, Krasner N, Brouwer PA. Oesophageal photodynamic therapy with mTHPC (m-tetra hydroxyphenyl chlorin) using a laser and a non-laser light source: a pilot study. SPIE Proceedings Series 2001;4156:262–5.
-
Karanov S, Kostadinov D, Shopova M, Kurtev P. Photodynamic therapy in lung and gastrointestinal cancers. J Photochem Photobiol B1990;6:175–81.
-
Karanov S, Shopova M, Getov H. Photodynamic therapy in gastrointestinal cancer. Lasers Surg Med 1991;11:395–8.
-
Karasic DS, Pearson VE. Urticaria and respiratory distress due to porfimer sodium. Ann Pharmacother 2000;34:1208–9.
-
Kashtan H, Konikoff F, Haddad R, Skornick Y. Photodynamic therapy of cancer of the esophagus using systemic aminolevulinic acid and a non laser light source: a phase I/II study. Gastrointest Endosc 1999;49:760–4.
-
Kashtan H, Konikoff F, Haddad R, Umansky M, Skornick Y, Halpern Z. [Photodynamic therapy for dysphagia due to esophageal carcinoma.] Harefuah 1999;137:441–4.
-
Kato H, Kito T, Furuse K, Sakai E, Ito K, Mimura S, et al. [Photodynamic therapy in the early treatment of cancer.] Gan To Kagaku Ryoho 1990;17:1833–8.
-
Keeley SB, Luketich JD, Landreneau RJ, Christie NA, Rivera MA, McGrath K. Using photodynamic therapy to cure esophageal cancer and Barrett’s high-grade dysplasia. Ann Surg Oncol 2006;13:85.
-
Keeley SB, Pennathur A, Gooding W, Landreneau RJ, Christie NA, Luketich J. Photodynamic therapy with curative intent for Barrett’s esophagus with high grade dysplasia and superficial esophageal cancer. Ann Surg Oncol 2007;14:2406–10.
-
Kouzu T, Konno H, Sakuma Y, Hishikawa E, Onoda S, Isono K. The present condition and the future of PDT for esophageal carcinoma in Japan. Recent Advances in Bronchoesophagology: Proceedings of the 6th World Congress of Bronchoesophagology, Tokyo, Japan, 1990. Elsevier Science Publishers. pp. 41–8.
-
Lecleire S, Di Fiore F, Antonietti M, Ben-Soussan E, Hochain P, Lerebours E, et al. Nonoperable patients with superficial esophageal cancer treated by photodynamic therapy after chemoradiotherapy have more severe complications than patients treated in primary intent. Am J Gastroenterol 2008;103:2215–19.
-
Li JH, Guo ZH, Jin ML, Zhao FY, Cai WM, Gao ML, et al. Photodynamic therapy in the treatment of malignant tumours: an analysis of 540 cases. J Photochem Photobiol B1990;6:149–55.
-
Likier HM, Levine JG, Lightdale CJ. Photodynamic therapy for completely obstructing esophageal carcinoma. Gastrointest Endosc 1991;37:75–8.
-
Litle VR, Luketich JD, Christie NA, Buenaventura PO, Alvelo-Rivera M, McCaughan JS, et al. Photodynamic therapy as palliation for esophageal cancer: experience in 215 patients. Ann Thorac Surg 2003;76:1687–92; discussion 92–93.
-
Lovat LB, Jamieson NF, Novelli MR, Mosse CA, Selvasekar C, Mackenzie GD, et al. Photodynamic therapy with m-tetrahydroxyphenyl chlorin for high-grade dysplasia and early cancer in Barrett’s columnar lined esophagus. Gastrointest Endosc 2005;62:617–23.
-
Luketich JD, Christie NA, Buenaventura PO, Weigel TL, Keenan RJ, Nguyen NT. Endoscopic photodynamic therapy for obstructing esophageal cancer: 77 cases over a 2-year period. Surg Endosc 2000;14:653–7.
-
Luketich JD, Fernando HC, Christie NA, Litle VR, Ferson PF, Buenaventura PO, et al. Photodynamic therapy in thoracic oncology: a single institution experience. SPIE Proceedings Series 2001;4248:28–33.
-
Luketich JD, Nguyen NT, Weigel TL, Keenan RJ, Ferson PF, Belani CP. Photodynamic therapy for treatment of malignant dysphagia. Surg Laparosc Endosc Percutan Tech 1999;9:171–5.
-
Maier A, Woltsche M, Fell B, Prettenhofer U, Domej W, Roger GM, et al. Local and systemic treatment in small cell carcinoma of the esophagus. Oncology Reports 2000;7:187–92.
-
Malhi-Chowla N, Wolfsen HC, DeVault KR. Esophageal dysmotility in patients undergoing photodynamic therapy. Mayo Clin Proc 2001;76:987–9.
-
Mall JW, Zuckermann-Becker H, Pollmann C, Opitz I, Rogalla P, Walter M. Esophageal necrosis and perforation of the left main bronchus following photodynamic therapy of esophageal carcinoma. Thorac Cardiovasc Surg 2002;50:111–13.
-
Marcon NE. Photodynamic therapy and cancer of the esophagus. Semin Oncol 1994;21:20–3.
-
Marcon NE. Photodynamic therapy and cancer of the esophagus. Semin Oncol 1994;21:20–3.
-
Maunoury V, Mordon S, Bulois P, Mirabel X, Hecquet B, Mariette C. Photodynamic therapy for early oesophageal cancer. Dig Liver Dis 2005;37:491–5.
-
McCaughan JS, Jr. Photoradiation of malignant tumors presensitized with hematoporphyrin derivative. Prog Clin Biol Res 1984;170:805–27.
-
McCaughan JS, Jr. Overview of experiences with photodynamic therapy for malignancy in 192 patients. Photochem Photobiol 1987;46:903–9.
-
McCaughan JS, Jr. Photodynamic therapy of malignant tumors. Prog Clin Biol Res 1988;278:163–9.
-
McCaughan JS, Jr. Photodynamic therapy of skin and esophageal cancers. Cancer Invest 1990;8:407–16.
-
McCaughan JS. Photodynamic therapy versus Nd-Yag laser treatment of endobronchial or esophageal malignancies. Photodyn Ther Biomed Lasers 1992;1011:23–36.
-
McCaughan JS, Jr. Photodynamic therapy of endobronchial and esophageal tumors: an overview. J Clin Laser Med Surg 1996;14:223–33.
-
McCaughan JS, Jr. Photodynamic therapy for obstructive esophageal malignancies. Diagn Ther Endosc 1999;5:167–74.
-
McCaughan JS, Jr, Ellison EC, Guy JT, Hicks WJ, Jones JJ, Laufman LR, et al. Photodynamic therapy for esophageal malignancy: a prospective twelve-year study. Ann Thorac Surg 1996;62:1005–9; discussion 9–10.
-
McCaughan JS, Jr, Ellison EC, Guy JT, Hicks WJ, Jones JJ, Laufman LR, et al. Photodynamic therapy for esophageal malignancy: a prospective twelve-year study [Discussion]. Ann Thorac Surg 1996;62:1005–10.
-
McCaughan JS, Jr, Guy JT, Hawley P, Hicks W, Inglis W, Laufman L, et al. Hematoporphyrin-derivative and photoradiation therapy of malignant tumors. Lasers Surg Med 1983;3:199–209.
-
McCaughan JS, Jr, Hicks W, Laufman L, May E, Roach R. Palliation of esophageal malignancy with photoradiation therapy. Cancer 1984;54:2905–10.
-
McCaughan JS, Jr, Nims TA, Guy JT, Hicks WJ, Williams TE, Jr, Laufman LR. Photodynamic therapy for esophageal tumors. Arch Surg 1989;124:74–80.
-
McCaughan JS, Jr, Williams TE, Jr, Bethel BH. Palliation of esophageal malignancy with photodynamic therapy. Ann Thorac Surg 1985;40:113–20.
-
Messmann H, Holstege A, Szeimies RM, Lock G, Bown SG, Scholmerich J. Photodynamic therapy: a safe and effective treatment for tumor overgrowth in patients with oesophageal cancer and metal stents. Endoscopy 1995;27:629.
-
Messmann H, Szeimies RM, Baumler W, Knuchel R, Zirngibl H, Scholmerich J, et al. Enhanced effectiveness of photodynamic therapy with laser light fractionation in patients with esophageal cancer. Endoscopy 1997;29:275–80.
-
Mimura S, Ichii M, Imanishi K, Otani T, Okuda S. [Indications for and limitations of HpD photodynamic therapy for esophageal cancer and gastric cancer.] Gan To Kagaku Ryoho 1988;15:1440–4.
-
Mimura S, Narahara H, Ishihara R, Iishi H. Long-term survival after photodynamic therapy with Photofrin for superficial esophageal cancer and early gastric cancer. 14th World Congress of the International Society for Laser Surgery and Medicine, Chennai, India, 2001. pp. 41–5.
-
Mimura S, Otani T, Okuda S. Photodynamic therapy for superficial esophageal cancer using an excimer dye laser. Diagn Ther Endosc 1994;1:99–105.
-
Mlkvy P. [Photodynamic therapy of gastrointestinal tumours – a pilot study.] Lekarsky Obzor 2003;52:171–5.
-
Moghissi K. Comparison of endoscopic PDT with surgical resection for early oesophageal cancer. Photodiag Photodyn Ther 2008;5:A O-12.
-
Moghissi K, Dixon K. Photodynamic therapy (PDT) in esophageal cancer: a surgical view of its indications based on 14 years experience. Technol Cancer Res Treat 2003;2:319–26.
-
Moghissi K, Dixon K. The role of photodynamic therapy (PDT) in pre-malignant and malignant oesophageal lesions: can PDT compete with surgery? Laser Helsinki 2008, 13th International Congress of EMLA (European Medical Laser Association) in conjunction with EMLA Finland and MAL (Medical Acupuncture and Laser) in cooperation with ASLMS (American Society for Laser Medicine and Surgery); 2008; Helsinki. 2008.
-
Moghissi K, Dixon K, Hudson E, Stringer M. Photodynamic therapy of oesophageal cancer. Lasers Med Sci 1995;10:67–71.
-
Moghissi K, Dixon K, Thorpe JA, Stringer M, Moore PJ. The role of photodynamic therapy (PDT) in inoperable oesophageal cancer. Eur J Cardiothorac Surg 2000;17:95–100.
-
Moghissi K, Dixon K, Thorpe JAC, Stringer MR. The role of photodynamic therapy (PDT) in oesophageal pre-neoplastic and malignant neoplastic lesions of the oesophagus: a review study. Photodynamic Therapy and Photodiagnosis in Clinical Practice, Brixen/Bressanone, 7–11 October 2008. Session 6 – invited lecture.
-
Monnier P, Fontolliet C, Wagnieres G, Braichotte D, Vandenbergh H. Further appraisal of PDI and PDT of early squamous-cell carcinomas of the pharynx, esophagus and bronchi. Photodyn Ther Biomed Lasers 1992;1011:7–14.
-
Monnier P, Savary M, Fontolliet C, Wagnieres Chatelain GA, Cornaz P, Depeursinge C, et al. Photodetection and photodynamic therapy of ‘early’ squamous cell carcinomas of the pharynx, oesophagus and tracheo-bronchial tree. Lasers Med Sci 1990;5:149–68.
-
Monnier P, Savary M, Fontolliet C, Wagnieres G, Vandenbergh H, Chatelain A. Photodynamic therapy (PDT) of 25 early pharyngoesophageal carcinomas: results and complications. Dis Esophagus 1990;1:359–71.
-
Muto M, Yano T, Katada C, Mera K, Doi T, Ishikura S, et al. Salvage photodynamic therapy (PDT) for locoregional failure after definitive chemoradiotherapy (CRT) for esophageal cancer (EC). J Clin Oncol 2004;22:4171.
-
Nakamura T, Fukui H, Shirakawa K, Fujii Y, Fujimori T, Terano A. Photodynamic therapy of superficial esophageal cancer with a transparent hood. Gastrointest Endosc 2004;60:120–4.
-
Norberto L, Segalin A, Ruol A, Baessato M, Cusumano A, Bardini R, et al. Results after ND:YAG laser therapy and photo dynamic therapy (PDT) of inoperable esophageal and cardial cancers. Recent Advances in Bronchoesophagology, Proceedings of the 6th World Congress of Bronchoesophagology, 1990, pp. 191–7.
-
Okunaka T, Kato H, Conaka C, Yamamoto H, Bonaminio A, Eckhauser ML. Photodynamic therapy of esophageal carcinoma. Surg Endosc 1990;4:150–3.
-
Okushima N, Muto Y, Kaburagi Y, Muroi M, Ide H, Suzuki S, et al. [Photodynamic therapy: its application for the treatment of esophageal cancer.] Nippon Geka Gakkai Zasshi 1989;90:1594–7.
-
Okushima N, Yoshida M, Muroi M. [Endoscopic photoradiation therapy for esophageal cancer.] Gastroenterol Endosc 1985;27:177–83.
-
Orth K, Ruck A, Beck G, Stanescu A, Beger HG. [Photodynamic therapy of small adenocarcinomas with methylene blue.] Chirurgie 1995;66:1254–7.
-
Orth K, Ruck A, Stanescu A, Beger HG. Intraluminal treatment of inoperable oesophageal tumours by intralesional photodynamic therapy with methylene blue. Lancet 1995;345:519–20.
-
Orth K, Stanescu A, Ruck A, Russ D, Beger HG. [Photodynamic ablation and argon-plasma coagulation of premalignant and early-stage malignant lesions of the oesophagus: an alternative to surgery?] Chirurgie 1999;70:431–8.
-
Overholt BF, Panjehpour M. Photodynamic therapy in Barrett’s esophagus: reduction of specialized mucosa, ablation of dysplasia, and treatment of superficial esophageal cancer. Semin Surg Oncol 1995;11:372–6.
-
Overholt BF, Panjehpour M. Barretts esophagus: photodynamic therapy for ablation of dysplasia, reduction of specialized mucosa and treatment of superficial esophageal cancer. SPIE Proceedings Series 1995;2371:375–84.
-
Overholt BF, Panjehpour M. Photodynamic therapy in Barrett’s esophagus. J Clin Laser Med Surg 1996;14:245–9.
-
Overholt BF, Panjehpour M. Photodynamic therapy for Barrett’s esophagus: clinical update. Am J Gastroenterol 1996;91:1719–23.
-
Overholt BF, Panjehpour M, Ayres M. Photodynamic therapy for Barrett’s esophagus: cardiac effects. Lasers Surg Med 1997;21:317–20.
-
Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett’s esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc 2003;58:183–8.
-
Overholt BF, Panjehpour M, Haydek JM. Photodynamic therapy for Barrett’s esophagus: follow-up in 100 patients. Gastrointest Endosc 1999;49:1–7.
-
Overholt BF, Panjehpour M, Teffetellar E, Rose M. Photodynamic therapy (PDT) utilizing photofrin for treatment of early esophageal cancer. SPIE Proceedings Series 1993;1881:35–40.
-
Pan LN, Zhang ZY, Li M, Su RL. [Effect of photodynamic therapy for esophageal carcinoma in near future.] World Chin J Digestol 2006;14:530–2.
-
Panjehpour M, Overholt BF, Haydek JM, Dougherty TJ. Photodynamic therapy with photofrin followed by thermal ablation for elimination of dysplasia and early cancer in Barrett’s esophagus: follow-up in 100 patients. SPIE Proceedings Series 1998;3247:14–24.
-
Patrice T, Foultier MT, Yactayo S, Adam F, Galmiche JP, Douet MC, et al. Endoscopic photodynamic therapy with hematoporphyrin derivative for primary treatment of gastrointestinal neoplasms in inoperable patients. Digestive Diseases & Sciences 1990;35:545–52.
-
Patrice T, Foultier MT, Yactayo S, Douet MC, Maloisel F, Le Bodic L. Endoscopic photodynamic therapy with haematoporphyrin derivative in gastroenterology. J Photochem Photobiol B 1990;6:157–65.
-
Pazurek M, Malecka-Panas E. Photodynamic therapy in the palliative treatment of esophageal cancer. Photodiag Photodyn Ther 2005;2:73–7.
-
Peters F, Kara M, Rosmolen W, Aalders M, Ten Kate F, Krishnadath K, et al. Poor results of 5-aminolevulinic acid-photodynamic therapy for residual high-grade dysplasia and early cancer in barrett esophagus after endoscopic resection. Endoscopy 2005;37:418–24.
-
Privalov VA, Lappa AV, Seliverstov OV, Faizrakhmanov AB, Yarovoy NN, Kochneva EV, et al. Clinical trials of a new chlorin photosensitizer for photodynamic therapy of malignant tumors. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XI 2002;4612:178–89.
-
Radu A, Grosjean P, Fontolliet C, Wagnieres G, Woodtli A, Bergh HV, et al. Photodynamic therapy for 101 early cancers of the upper aerodigestive tract, the esophagus, and the bronchi: a single-institution experience. Diagn Ther Endosc 1999;5:145–54.
-
Rahmani EY, Rex DK, Ciaccia D, Turpin CS. Photodynamic therapy for ablation of esophageal high grade dysplasia and superficial cancer. Gastrointest Endosc 2000;51:3539.
-
Regula J, MacRobert AJ, Gorchein A, Buonaccorsi GA, Thorpe SM, Spencer GM, et al. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX: a pilot study. Gut 1995;36:67–75.
-
Sanfilippo NJ, Hsi A, DeNittis AS, Ginsberg GG, Kochman ML, Friedberg JS, et al. Toxicity of photodynamic therapy after combined external beam radiotherapy and intraluminal brachytherapy for carcinoma of the upper aerodigestive tract. Lasers Surg Med 2001;28:278–81.
-
Savary JF, Monnier P, Fontolliet C, Mizeret J, Wagnieres G, Braichotte D, et al. Photodynamic therapy for early squamous cell carcinomas of the esophagus, bronchi, and mouth with m-tetra (hydroxyphenyl) chlorin. Arch Otolaryngol Head Neck Surg 1997;123:162–8.
-
Savary JF, Monnier P, Wagnieres G, Braichotte D, Fontolliet C, Vandenbergh H. Preliminary clinical-studies of photodynamic therapy with meso-tetrahydroxyphenyl chlorin (M-Thpc) as a photosensitizing agent for the treatment of early pharyngeal, esophageal and bronchial carcinomas. SPIE Proceedings Series 1994;2078:330–40.
-
Scheider DM, Siemens M, Cirocco M, Haber GB, Kandel G, Kortan P, et al. Photodynamic therapy for the treatment of tumor ingrowth in expandable esophageal stents. Endoscopy 1997;29:271–4.
-
Schweitzer VG. Photodynamic therapy – 1994 treatment of benign and malignant upper aerodigestive tract disease. SPIE Proceedings Series 1995;2371:403–17.
-
Schweitzer VG, Bologna S, Batra SK. Photodynamic therapy for treatment of esophageal cancer: a preliminary report. Laryngoscope 1993;103:699–703.
-
Schweitzer VG, Bologna S, Batra SK, Cummings C. Photodynamic therapy for treatment of esophageal cancer: a preliminary report [Discussion]. Laryngoscope 1993;103:699–703.
-
Segalin A, Little AG, Ruol A, Ferguson MK, Bardini R, Norberto L, et al. Surgical and endoscopic palliation of esophageal carcinoma. Ann Thorac Surg 1989;48:267–71.
-
Shah AK, Wolfsen HC, Hemminger LL, Shah AA, DeVault KR. Changes in esophageal motility after porfimer sodium photodynamic therapy for Barrett’s dysplasia and mucosal carcinoma. Dis Esophagus 2006;19:335–9.
-
Shim CS, Cheon YK, Jung IS, Ko BM, Cho JY, Lee JS, et al. Photodynamic therapy for early esophageal cancer. Gastrointest Endosc 2007;65:A149.
-
Sibille A, Lambert R, Souquet JC, Sabben G, Descos F. Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology 1995;108:337–44.
-
Smucler R. Surgical excision versus ALA-PDT-PDL versus laser coagulation with diode laser in treatment of basal cell carcinoma (2 years study). Lasers Surg Med 2005;36:S17.
-
Spinelli P, Dal FM. Photodynamic therapy of solid tumors. Semin Hematol 1992;29:142–54.
-
Spinelli P, Dalfante M, Mancini A, Massetti M. Endoscopic photodynamic therapy of early cancer and severe dysplasia of the esophagus. Photodyn Ther Biomed Lasers 1992;1011:262–5.
-
Spinelli P, Mancini A, Dal Fante M. Endoscopic treatment of gastrointestinal tumors: indications and results of laser photocoagulation and photodynamic therapy. Semin Surg Oncol 1995;11:307–18.
-
Stranadko EP, Ponomarev GV, Mechkov VM, Riabov MV, Ivanov AV, Reshetnikov AV, et al. The first experience of photodithazine clinical application for photodynamic therapy of malignant tumors. SPIE Proceedings Series 2000;3909:138–44.
-
Sun Y, Guan SC. A study of the clinical-application of PDT to the treatment of alimentary-tract cancer and precancerous gastric diseases. SPIE Proceedings Series 1993;1616:122–6.
-
Tajiri H, Oguro Y. Laser endoscopic treatment for upper gastrointestinal cancers. J Laparoendosc Surg 1991;1:71–8.
-
Tan WC, Fulljames C, Stone N, Dix AJ, Shepherd N, Roberts DJ, et al. Photodynamic therapy using 5-aminolaevulinic acid for oesophageal adenocarcinoma associated with Barrett’s metaplasia. J Photochem Photobiol B 1999;53:75–80.
-
Tanaka T, Sueyoshi S, Fujita H, Toh U, Fujii T, Kubota M, et al. Photodynamic therapy for superficial esophageal carcinoma. Recent Advances in Diseases of the Esophagus, 8th World Congress of the International Society for Diseases of the Esophagus, Sao Paulo, Brazil, 2001. pp. 593–7.
-
Thomas RJ, Abbott M, Bhathal PS, St John DJ, Morstyn G. High-dose photoirradiation of esophageal cancer. Ann Surg 1987;206:193–9.
-
Tian ME, Qui SL, Ji Q. Preliminary results of hematoporphyrin derivative-laser treatment for 13 cases of early esophageal carcinoma. Adv Exp Med Biol 1985;193:21–5.
-
Tomio L, Corti L, Polico C, Maluta S, Calzavara F, Norberto L, et al. [Laser-photo-radiotherapy in the treatment malignant tumors]. Radiol Med 1987;73:313–16.
-
Vandenbergh H. Photodynamic therapy and photodetection of early cancer in the upper aerodigestive tract, the tracheobronchial tree, the oesophagus and the urinary bladder. Hadron Ther Oncol 1994;1077:577–621.
-
Wolfsen HC, Hemminger LL, Raimondo M, Woodward TA. Photodynamic therapy and endoscopic mucosal resection for Barrett’s dysplasia and early esophageal adenocarcinoma. South Med J 2004;97:827–30.
-
Wolfsen HC, Hemminger LL, Wallace MB, Devault KR. Clinical experience of patients undergoing photodynamic therapy for Barrett’s dysplasia or cancer. Aliment Pharmacol Ther 2004;20:1125–31.
-
Wolfsen HC, Woodward TA. Photofrin PDT for Barrett’s esophagus and early esophageal cancer. Recent Advances in Diseases of the Esophagus, 8th World Congress of the International Society for Diseases of the Esophagus, Sao Paulo, Brazil. International Society for Diseases of the Esophagus; 2001. pp. 149–53.
-
Wolfsen HC, Woodward TA, Raimondo M. Photodynamic therapy for dysplastic Barrett esophagus and early esophageal adenocarcinoma. Mayo Clin Proc 2002;77:1176–81.
-
Woodward TA, Wolfsen HC, Anderson RR, Bartels KE, Bass LS, Garrett CG, et al. Photodynamic therapy (PDT) with endoscopic ultrasound for the treatment of esophageal cancer. SPIE Proceedings Series 2000;3907:422–5.
-
Xu K, Niu L, Guo Z, Zuo J. Endoscopic photodynamic therapy for symptomatic palliation in advanced esophageal cancer. Ann Oncol 2006;17:91.
-
Yachimski P, Puricelli WP, Nishioka NS. Patient predictors of esophageal stricture development after photodynamic therapy. Clin Gastroenterol Hepatol 2008;6:302–8.
-
Yano T, Muto M, Minashi K, Hattori S, Ohtsu A, Yoshida S. Endoscopic mucosal resection (EMR) and photodynamic therapy (PDT) as curative salvage treatments for local failure after definitive chemoradiotherapy (CRT) for esophageal Cancer(EC). Gastrointest Endosc 2007;65:A143.
-
Yano T, Muto M, Minashi K, Ohtsu A, Yoshida S. Photodynamic therapy as salvage treatment for local failures after definitive chemoradiotherapy for esophageal cancer. Gastrointest Endosc 2005;62:31–6.
-
Yoshida K, Suzuki S, Mimura S, Ichii M, Sakai H, Shimao H, et al. [Photodynamic therapy for superficial esophageal cancer: a phase III study using PHE and excimer dye laser]. Gan To Kagaku Ryoho 1993;20:2063–6.
-
Yoshida K, Suzuki S, Mimura S, Narahara H, Tanimura H, Nagai Y, et al. A clinical study of photodynamic therapy for superficial esophageal carcinoma by YAG-OPO laser. Diagn Ther Endosc 1998;4:173–6.
-
Zammit M, Fullarton G. Treatment of early upper gastrointestinal tumors using photodynamic therapy. Gastrointest Endosc 2004;59:A258.
-
Zopf T, Rosenbaum A, Apel D, Jakobs R, Arnold JC, Riemann JF. [Photodynamic therapy of dysplasias and early carcinomas in Barrett esophagus with a diode laser system – a pilot study]. Med Klin 2001;96:212–16.
Appendix 9 Lung cancer scoping
One hundred and seventy-seven publications, with study designs that did not meet the inclusion criteria for the review, reported on patients with lung cancer being treated with PDT. Details are lacking for many of these publications, so the categorisations may not be reliable.
There are 10 observational comparative studies, with sample sizes ranging from 29 to 687, although the sample size is unclear for one-half of them. 1–10 For some of these studies, all of the patients receive PDT, for others it is series of patients undergoing treatment for lung cancer, only a proportion of which receive PDT.
Eleven publications are described as trials but without a comparator group (Phase I/II/pilot studies), with sample sizes ranging from 9 to 54. 11–21
One hundred and nineteen publications report case series;22–140 19 have over 100 patients, eight publications report on case series with between 50 and 100 patients, and 71 report less than 50 patients. For 21 publications it is not clear what the sample size is. Many of these publications are by the same authors and appear to be updated series of patients or duplicate reports published in different journals. Therefore, these publications may double count patients to a certain degree.
Thirty publications report less than 10 patients,141–170 16 of which are single case reports. 142,143,146,147,149,151,152,155–157,161,163,164,166,167,169
A further seven publications remain uncategorised (e.g. the paper is unavailable, it is unclear what is being reported, or possible duplicate publication). 171–177
References
Observational comparative studies
-
1. Furukawa K, Okunaka T, Yamamoto H, Tsuchida T, Usuda J, Kumasaka H, et al. Effectiveness of photodynamic therapy and Nd-YAG laser treatment for obstructed tracheobronchial malignancies. Diagn Ther Endosc 1999;5:161–6.
-
2. Kato H, Okunaka T, Konaka C. Photodynamic therapy for bronchogenic carcinoma. Nippon Geka Gakkai Zasshi 1997;98:36–40.
-
3. McCaughan JS. Photodynamic therapy versus Nd-Yag laser treatment of endobronchial or esophageal malignancies. Photodyn Ther Biomed Lasers 1992;1011:23–36.
-
4. McCaughan JS. PDT of endobronchial tumors on the same day as injection of the photosensitizer DHE photodynamic therapy. Clin Laser Mon 1993;11:57–8.
-
5. McCaughan JS, Barabash R, Hawley P. Stage-III endobronchial squamous-cell cancer: survival after Nd-Yag laser combined with photodynamic therapy vs Nd-Yag laser or photodynamic therapy alone. SPIE 1991;1426:279–86.
-
6. McCaughan JS, Jr, Barabash RD, Hatch DR, McMahon DC. Gold vapor laser versus tunable argon-dye laser for endobronchial photodynamic therapy. Lasers Surg Med 1996;19:347–50.
-
7. McCaughan JS, Jr, Dougherty TJ. Photodynamic therapy repeated without reinjection of Photofrin (R) (porfimer sodium). SPIE 1998;3247:31–4.
-
8. McCaughan JS, Jr, Hawley PC, Walker J. Management of endobronchial tumors: a comparative study. Semin Surg Oncol 1989;5:38–47.
-
9. Moghissi K. Lasers in broncho-pulmonary cancer. Radiol Oncol 1994;28:359–64.
-
10. van Boxem AJ, Westerga J, Venmans BJ, Postmus PE, Sutedja G. Photodynamic therapy, Nd-YAG laser and electrocautery for treating early-stage intraluminal cancer: which to choose? Lung Cancer 2001;31:31–6.
Experimental non-comparative studies
-
11. Calzavara F, Tomio L, Norberto L, Peracchia A, Corti L, Zorat PL, et al. Photodynamic therapy in the treatment of malignant tumours of the upper aerodigestive tract. Lasers Med Sci 1989;4:279–84.
-
12. Friedberg JS, Metz JM, Stevenson J, Zhu T, Mick R, Smith D, et al. Increased survival and local control is observed for patients with stage 3B non-small cell lung cancer (NSCLC), secondary to malignant pleural effusions and gross pleural carcinomatosis, in a phase II trial combining surgery and photodynamic therapy. Chest 2002;122:166S.
-
13. Friedberg JS, Mick R, Stevenson JP, Zhu T, Busch TM, Shin D, et al. Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J Clin Oncol 2004;22:2192–201.
-
14. Furuse K, Fukuoka M, Horai T, Hitomi S, Kato H, Hayata Y. Phase-II study of photodynamic therapy (PDT) with photofrin-II for hilar type early lung-cancer. Photodyn Ther Biomed Lasers 1992;1011:614–17.
-
15. Furuse K, Fukuoka M, Kato H, Horai T, Kubota K, Kodama N, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol 1993;11:1852–7.
-
16. Kato H, Furukawa K, Sato M, Okunaka T, Kusunoki Y, Kawahara M, et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003;42:103–11.
-
17. Moghissi K, Dixon K, Hudson E, Stringer M, Brown S. Endoscopic laser therapy in malignant tracheobronchial obstruction using sequential Nd YAG laser and photodynamic therapy. Thorax 1997;52:281–3.
-
18. Oakford M, Keohane SG. Photodynamic therapy to manage residual superficial basal cell carcinomas following Mohs’ surgery. Br J Dermatol 2004;151:96.
-
19. Okunaka T, Kato H, Tsutsui H, Ishizumi T, Ichinose S, Kuroiwa Y. Photodynamic therapy for peripheral lung cancer. Lung Cancer 2004;43:77–82.
-
20. Sutedja T, Baas P, Stewart F, van Zandwijk N. A pilot study of photodynamic therapy in patients with inoperable non-small cell lung cancer. Eur J Cancer 1992;28A:1370–3.
-
21. Tomaselli F, Maier A, Pinter H, Stranzl H, Smolle-Juttner FM. Photodynamic therapy enhanced by hyperbaric oxygen in acute endoluminal palliation of malignant bronchial stenosis (clinical pilot study in 40 patients). Eur J Cardiothorac Surg 2001;19:549–54.
Case series (10 or more patients)
-
22. Ablitsov UA, Kuzin MI, Loginov LE, Uspensky LV. Photodynamic therapy of lung cancer. SPIE Proceedings Series 1995;2625:451–2.
-
23. Balchum OJ, Doiron DR, Huth GC. Photoradiation therapy of endobronchial lung cancers employing the photodynamic action of hematoporphyrin derivative. Lasers Surg Med 1984;4:13–30.
-
24. Balchum OJ, Doiron DR, Huth GC. HpD photodynamic therapy for obstructing lung cancer. Prog Clin Biol Res 1984;170:727–45.
-
25. Chida M, Handa M, Sato M, Matsumura Y, Okada Y, Shimada K, et al. [Re-evaluation of bronchoplasty for central-type lung cancer.] Nippon Geka Gakkai Zasshi 2001;102:530–4.
-
26. Chissov VI, Sokolov VV, Trakhtenberg AK, Mamontov AS. Photodynamic therapy of early stage cancer of lung, esophagus, and stomach with two different photosensitizers. SPIE Proceedings Series 1995;2625:466–75.
-
27. Cicenas S, Jackevicius A, Aleknavicius E, Bloznelyte L. Photodynamic therapy (PDT) in combined non small cell lung cancer (NSCLC) treatment. Radiother Oncol 1996;40:606.
-
28. Cortese DA, Edell ES, Kinsey JH. Photodynamic therapy for early stage squamous cell carcinoma of the lung. Mayo Clin Proc 1997;72:595–602.
-
29. Cortese DA, Kinsey JH. Endoscopic management of lung cancer with hematoporphyrin derivative phototherapy. Mayo Clin Proc 1982;57:543–7.
-
30. Cortese DA, Kinsey JH. Bronchoscopic phototherapy using hematoporphyrin derivative. Surg Clin North Am 1984;64:941–6.
-
31. Corti L, Toniolo L, Boso C, Colaut F, Fiore D, Muzzio P-C, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 2007;39:394–402.
-
32. Edell ES, Cortese DA. Bronchoscopic phototherapy with hematoporphyrin derivative for treatment of localized bronchogenic carcinoma: a 5-year experience. Mayo Clin Proc 1987;62:8–14.
-
33. Edell ES, Cortese DA. Photodynamic therapy: Treatment early stage lung cancer results and follow-up. Recent advances in bronchoesophagology. Proceedings of the 6th World Congress of Bronchoesophagology, Tokyo, Japan. Elsevier Science Publishers BV; 1990. pp. 205–10.
-
34. Edell ES, Cortese DA. Photodynamic therapy in the management of early superficial squamous cell carcinoma as an alternative to surgical resection. Chest 1992;102:1319–22.
-
35. Ernst A, Freitag L, Feller-Kopman D, LoCicero J, Ost D. Photodynamic therapy for endobronchial obstruction is safely performed with flexible bronchoscopy. J Bronchol 2003;10:260–3.
-
36. Freitag L, Ernst A, Thomas M, Prenzel R, Wahlers B, Macha HN. Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax 2004;59:790–3.
-
37. Freitag L, Korupp A, Itzigehl I, Dankwart F, Tekolf E, Reichle G, et al. [Experiences with fluorescence diagnosis and photodynamic therapy in a multimodality therapy concept of operated, recurrent bronchial carcinoma.] Pneumologie 1996;50:693–9.
-
38. Fujimura S, Sakurada A, Sagawa M, Saito Y, Takahashi H, Tanita T, et al. A therapeutic approach to roentgenographically occult squamous cell carcinoma of the lung. Cancer 2000;89:2445–8.
-
39. Furukawa K, Kato H, Konaka C, Okunaka T, Usuda J, Ebihara Y. Locally recurrent central-type early stage lung cancer < 1.0 cm in diameter after complete remission by photodynamic therapy. Chest 2005;128:3269–75.
-
40. Furuse K, Okunaka T, Sakai H, Konaka C, Kato H, Aoki M, et al. [Photodynamic therapy (PDT) in roentgenographically occult lung cancer by photofrin II and excimer dye laser.] Gan To Kagaku Ryoho 1993;20:1369–74.
-
41. Grosjean P, Savary JF, Wagnieres G, Mizeret J, Woodtli A, Fontolliet C, et al. [Phototherapy of pharyngeal, oesophageal and bronchial early squamous cell carcinomas after sensitisation by tetra (m-hydroxyphenyl) chlorin (mTHPC).] Med Chir Digest 1996;25:411–13.
-
42. Grosjean P, Savary JF, Wagnieres G, Mizeret J, Woodtli A, Theumann JF, et al. Tetra(m-hydroxyphenyl)chlorin clinical photodynamic therapy of early bronchial and oesophageal cancers. Lasers Med Sci 1996;11:227–35.
-
43. Harada M. [Recent advances in photodynamic therapy; an expanding role in non-small cell lung cancer.] Jpn J Lung Cancer 2005;45:687–92.
-
44. Hayata Y. Applications of laser photoradiation in the diagnosis and treatment of lung cancer. [Japanese]. Jpn Ann Thorac Surg 1983;3:203–10.
-
45. Hayata Y, Kato H, Furuse K, Fukuoka M. Photodynamic therapy in early stage lung cancer. In: Motta G, editor. Lung cancer: frontiers in science and treatment. Genoa, Italy: Rapallo; 1993. pp. 247–54.
-
46. Hayata Y, Kato H, Furuse K, Kusunoki Y, Suzuki S, Mimura S. Photodynamic therapy of 168 early stage cancers of the lung and oesophagus: a Japanese multi-centre study. Lasers Med Sci 1996;11:255–9.
-
47. Hayata Y, Kato H, Konaka C, Amemiya R, Ono J, Ogawa I, et al. Photoradiation therapy with hematoporphyrin derivative in early and stage 1 lung cancer. Chest 1984;86:169–77.
-
48. Hayata Y, Kato H, Konaka C, Okitsu H, Suga S, Sayami P. [Laser endoscopy in photodynamic therapy.] Chirurgie 1988;59:81–9.
-
49. Hayata Y, Kato H, Konaka C, Okunaka T. Photodynamic therapy (PDT) in early stage lung cancer. Lung Cancer 1993;9:287–93.
-
50. Hayata Y, Kato H, Konaka C, Ono J, Amemiya R, Kinoshita K, et al. Hematoporphyrin derivative and photoradiation therapy in early stage lung cancer. Lasers Surg Med 1984;4:39–47.
-
51. Hayata Y, Kato H, Konaka C, Ono J, Takizawa N. Hematoporphyrin derivative and laser photoradiation in the treatment of lung cancer. Chest 1982;81:269–77.
-
52. Hayata Y, Yamamoto H, Kato H, Furukawa K. [Photodynamic therapy in early stage lung cancer using an excimer dye laser.] Nippon Rinsho 1990;48:201–7.
-
53. Hill HC, Nwogu CE, Loewen G, Pelow J, Dougherty TJ, Anderson TM. Off-label management of primary and metastatic endobronchial tumors with photodynamic therapy. Clin Pulmon Med 2004;11:107–11.
-
54. Hugh-Jones P, Gardner WN. Laser photodynamic therapy for inoperable bronchogenic squamous carcinoma. Q J Med 1987;64:565–81.
-
55. Imamura S, Kusunoki Y, Takifuji N, Kudo S, Matsui K, Masuda N, et al. Photodynamic therapy and/or external beam radiation therapy for roentgenologically occult lung cancer. Cancer 1994;73:1608–14.
-
56. Jones BU, Helmy M, Brenner M, Serna DL, Williams J, Chen JC, et al. Photodynamic therapy for patients with advanced non-small-cell carcinoma of the lung. Clin Lung Cancer 2001;3:37–41; discussion 2.
-
57. Karanov S, Kostadinov D, Shopova M, Kurtev P. Photodynamic therapy in lung and gastrointestinal cancers. J Photochem Photobiol B 1990;6:175–81.
-
58. Kato H, Harada M, Ichinose S, Usuda J, Tsuchida T, Okunaka T. Photodynamic therapy (PDT) of lung cancer: experience of the Tokyo Medical University. Photodiag Photodyn Ther 2004;1:49–55.
-
59. Kato H, Kawate N, Kinoshita K, Yamamoto H, Furukawa K, Hayata Y. Photodynamic therapy of early-stage lung cancer. Ciba Found Symp 1989;146:183–94; discussion 95–97.
-
60. Kato H, Kito T, Furuse K, Sakai E, Ito K, Mimura S, et al. [Photodynamic therapy in the early treatment of cancer.] Gan To Kagaku Ryoho 1990;17:1833–8.
-
61. Kato H, Konaka C, Kawate N, Yoneyama K, Saito M, Shinohara H, et al. [Qualitative improvement of the surgical treatment of cancer using laser equipment: surgical technic after photodynamic therapy.] Gan To Kagaku Ryoho 1987;14:1468–76.
-
62. Kato H, Konaka C, Kinoshita K, Kito T, Otomo S, Okunaka T, et al. [Photoradiation therapy (PRT). 7. Clinical study of photoradiation therapy: clinical application and efficacy. b. Lung neoplasms and bronchial neoplasms.] Nippon Rinsho 1987;45:806–11.
-
63. Kato H, Konaka C, Kinoshita K, Taira O, Okunaka T, Yamamoto H, et al. Laser endoscopy with photodynamic therapy in the respiratory tract. Gann Monogr Cancer Res 1990;37:139–52.
-
64. Kato H, Konaka C, Nishimiya K, Takahashi H, Kitoh T, Aizawa K, et al. [Photodynamic therapy in lung cancer.] Gan No Rinsho (Japan Journal of Cancer Clinics) 1985;31:690–6.
-
65. Kato H, Konaka C, Ono J, Kawate N, Nishimiya K, Shinohara H, et al. Preoperative laser photodynamic therapy in combination with operation in lung cancer. J Thorac Cardiovasc Surg 1985;90:420–9.
-
66. Kato H, Konaka C, Ono J, Matsushima Y, Nishimiya K, Lay J, et al. Effectiveness of HPD and radiation therapy in lung cancer. Adv Exp Med Biol 1983;160:23–39.
-
67. Kato H, Konaka C, Saito M, Nishimiya K, Kawate N, Aizawa K, et al. [Laser photodynamic therapy with hematoporphyrin derivative in lung cancer.] Nippon Geka Gakkai Zasshi 1985;86:1059–63.
-
68. Kato H, Okunaka T, Konaka C, Furuse K, Kusunoki Y, Horai T, et al. Photodynamic therapy with YAG-OPO laser for early stage lung cancer. Diagn Ther Endosc 1997;4:75–81.
-
69. Kato H, Okunaka T, Shimatani H. Photodynamic therapy for early stage bronchogenic carcinoma. J Clin Laser Med Surg 1996;14:235–8.
-
70. Kato H, Usuda J, Okunaka T, Furukawa K, Honda H, Sakaniwa N, et al. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg Med 2006;38:371–5.
-
71. Kawaguchi T, Furuse K, Kawahara M, Yamamoto S, Sutedja TG. Histological examination of bronchial mucosa after photodynamic therapy showing no selectivity of effect between tumour and normal mucosa. Lasers Med Sci 1998;13:265–70.
-
72. Kawaguchi T, Yamamoto S, Naka N, Okishio K, Atagi S, Ogawara M, et al. Immunohistochemical analysis of Bcl-2 protein in early squamous cell carcinoma of the bronchus treated with photodynamic therapy. Br J Cancer 2000;82:418–23.
-
73. Kawahara M, Naka N, Kodama N, Ogawara M. Survival and occurrence of second primary tumor of patients with roentgenologically occult lung cancer treated with photodynamic therapy. 33rd Annual Meeting of the American Society of Clinical Oncology, Denver, CO. American Society of Clinical Oncology; 1997. pp. 1613.
-
74. Konaka C, Okunaka T, Furukawa K, Sakai H, Kato H. [Photodynamic therapy for multiple primary lung cancer.] Gan To Kagaku Ryoho 1996;23:31–5.
-
75. Konaka C, Okunaka T, Furukawa K, Sakai H, Shimatani H, Shibuya H, et al. [Laser photodynamic therapy for central-type lung cancer.] Nihon Kyōbu Shikkan Gakkai Zasshi 1996;34(Suppl.):107–10.
-
76. Konaka C, Okunaka T, Sakai H, Furukawa K, Hayata Y, Kato H. Photodynamic therapy for multiple primary lung cancer. Photodyn Ther Biomed Lasers 1992;1011:618–21.
-
77. Konaka C, Usuda J, Kato H. [Preoperative photodynamic therapy for lung cancer.] Nippon Geka Gakkai Zasshi 2000;101:486–9.
-
78. Kostadinov D, Benov E, Mitchev K, Vlasov V, Jory G. Our 3-years experience in application of PDT in the treatment of lung cancer. SPIE Proceedings Series 1993;1616:70–4.
-
79. Kubota K. Long-term follow-up of patients with roentgenographically occult lung cancer treated with photodynamic therapy (PDT). Nippon Gan Chiryo Gakkai Shi 1994;30:143.
-
80. Kubota K, Furuse K, Kawahara M, Kodama N, Yamamoto M, Ogawara M, et al. [Photodynamic therapy of roentgenographically occult lung cancer.] Kyobu Geka 1992;45:80–3.
-
81. Lam S, Muller NL, Miller RR, Kostashuk EC, Szasz IJ, LeRiche JC, et al. Predicting the response of obstructive endobronchial tumors to photodynamic therapy. Cancer 1986;58:2298–306.
-
82. Li JH, Chen YP, Zhao SD, Zhang LT, Song SZ. Application of hematoporphyrin derivative and laser-induced photodynamical reaction in the treatment of lung cancer: a preliminary report on 21 cases. Lasers Surg Med 1984;4:31–7.
-
83. Li JH, Guo ZH, Jin ML, Zhao FY, Cai WM, Gao ML, et al. Photodynamic therapy in the treatment of malignant tumours: an analysis of 540 cases. J Photochem Photobiol B 1990;6:149–55.
-
84. Liang YM, Tu QX, Liu GY, Zhang XS. [Short term result of lung cancer treated by photodynamic therapy (PDT).] Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology] 1987;9:50–2.
-
85. LoCicero J, Metzdorff M, Almgren C. Photodynamic therapy in the palliation of late stage obstructing non-small cell lung cancer. Chest 1990;98:97–100.
-
86. Luketich JD, Fernando HC, Christie NA, Litle VR, Ferson PF, Buenaventura PO, et al. Photodynamic therapy in thoracic oncology: a single institution experience. SPIE Proceedings Series 2001;4248:28–33.
-
87. McCaughan FM, Janes SM, Read CA, Mosse CA, Bown S, George PJ. Photodynamic therapy in the treatment of early stage lung cancer [unpublished abstract of internal UCH report to Use of Medicines Committee.] In. London: University College Hospital.
-
88. McCaughan JS, Jr. Overview of experiences with photodynamic therapy for malignancy in 192 patients. Photochem Photobiol 1987;46:903–9.
-
89. McCaughan JS, Jr. Photodynamic therapy of malignant tumors. Prog Clin Biol Res 1988;278:163–9.
-
90. McCaughan JS, Jr. Photodynamic therapy of endobronchial and esophageal tumors: an overview. J Clin Laser Med Surg 1996;14:223–33.
-
91. McCaughan JS, Jr, Hawley PC, Bethel BH, Walker J. Photodynamic therapy of endobronchial malignancies. Cancer 1988;62:691–701.
-
92. McCaughan JS, Jr, Williams TE. Photodynamic therapy for endobronchial malignant disease: a prospective fourteen-year study. J Thorac Cardiovasc Surg 1997;114:940–6; discussion 6–7.
-
93. McCaughan JS, Jr, Williams TE, Jr, Bethel BH. Photodynamic therapy of endobronchial tumors. Lasers Surg Med 1986;6:336–45.
-
94. Miyazu Y, Miyazawa T, Kurimoto N, Iwamoto Y, Kanoh K, Kohno N. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am J Respir Crit Care Med 2002;165:832–7.
-
95. Moghissi K, Dixon K. Photodynamic therapy (PDT) of central type lung cancer: current indications based on 15 years experience and literature review. Lasers Surg Med 2005;36:S17.
-
96. Moghissi K, Dixon K, Stringer M, Brown S, Altshuler GB, Chiesa F, et al. Photodynamic therapy (PDT) in advanced inoperable bronchial carcinoma. SPIE Proceedings Series 1996;2922:295–301.
-
97. Moghissi K, Dixon K, Stringer M, Freeman T, Thorpe A, Brown S. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg 1999;15:1–6.
-
98. Moghissi K, Dixon K, Thorpe JAC, Oxtoby C, Stringer MR. Photodynamic therapy (PDT) for lung cancer: The Yorkshire Laser Centre experience. Photodiag Photodyn Ther 2004;1:253–62.
-
99. Moghissi K, Dixon K, Thorpe JAC, Stringer M, Oxtoby C. Photodynamic therapy (PDT) in early central lung cancer: a treatment option for patients ineligible for surgical resection. Thorax 2007;62:391–5.
-
100. Moghissi K, Dixon K, Thorpe JAC, Stringer MR, Oxtoby C. Photodynamic therapy (PDT) in lung cancer with disease limited to endobronchial lesions. Thorax 2004;59:53.
-
101. Monnier P, Fontolliet C, Wagnieres G, Braichotte D, Vandenbergh H. Further appraisal of PDI and PDT of early squamous-cell carcinomas of the pharynx, esophagus and bronchi. Photodyn Ther Biomed Lasers 1992;1011:7–14.
-
102. Monnier P, Savary M, Fontolliet C, Wagnieres Chatelain GA, Cornaz P, Depeursinge C, et al. Photodetection and photodynamic therapy of ‘early’ squamous cell carcinomas of the pharynx, oesophagus and tracheo-bronchial tree. Lasers Med Sci 1990;5:149–68.
-
103. Okunaka T. Diagnosis and treatment of early stage central type lung cancer photodynamic therapy for early and advanced stage bronchogenic carcinomas [symposium.] Jpn Soc Bronchol 2000;22:636–9.
-
104. Okunaka T, Furukawa K, Tsutsui H, Usuda J, Nitadori J, Kuroiwa Y, et al. The role of PDT for bronchogenic carcinoma. Bronchol Bronchoesophageal State of the Art 2001;1217:131–3.
-
105. Okunaka T, Hiyoshi T, Furukawa K, Yamamoto H, Tsuchida T, Usuda J, et al. Lung cancers treated with photodynamic therapy and surgery. Diagn Ther Endosc 1999;5:155–60.
-
106. Okunaka T, Kato H. [Laser bronchoscopic therapy of lung cancer.] Gan To Kagaku Ryoho 1995;22:179–84.
-
107. Okunaka T, Kato H. Photodynamic therapy for central type lung cancer. 1st International Congress of Thorax Surgery. Athens; 1997. pp. 581–6.
-
108. Okunaka T, Kato H. Photodynamic therapy for lung cancer: state of the art and expanded indications. Nippon Geka Gakkai Zasshi 2002;103:258–62.
-
109. Okunaka T, Kato H, Konaka C, Kawate N, Bonaminio A, Yamamoto H, et al. Photodynamic therapy for multiple primary bronchogenic carcinoma. Cancer 1991;68:253–8.
-
110. Ono R, Egawa S, Ikeda S. [Combined treatment of endoscopic laser irradiation and radiotherapy in lung cancer.] Gan To Kagaku Ryoho 1989;16:1418–24.
-
111. Ono R, Ikeda S. [Evaluation of the combined procedure of Hp-D (hematoporphyrin derivative) phototherapy and radiation therapy in advanced lung cancer.] Nippon Gan Chiryo Gakkai Shi 1986;21:2189–95.
-
112. Ono R, Ikeda S. [Indication and evaluation of effectiveness of the HpD (hematoporphyrin-derivative) phototherapy in lung cancer.] Nippon Ika Daigaku Zasshi 1986;46:919–25.
-
113. Ono R, Ikeda S. [The hematoporphyrin derivative (HpD) phototherapy in roentgenologically occult cancer in the trachea and bronchus.] Nippon Gan Chiryo Gakkai Shi 1986;21:60–7.
-
114. Ono R, Ikeda S, Suemasu K. Hematoporphyrin derivative photodynamic therapy in roentgenographically occult carcinoma of the tracheobronchial tree. Cancer 1992;69:1696–701.
-
115. Pass HI, Delaney T, Smith PD, Bonner R, Russo A. Bronchoscopic phototherapy at comparable dose rates: early results. Ann Thorac Surg 1989;47:693–9.
-
116. Patelli M, Lazzari Agli L, Poletti V, Falcone F. Photodynamic laser therapy for the treatment of early-stage bronchogenic carcinoma. Monaldi Arch Chest Dis 1999;54:315–18.
-
117. Radu A, Grosjean P, Fontolliet C, Wagnieres G, Woodtli A, Bergh HV, et al. Photodynamic therapy for 101 early cancers of the upper aerodigestive tract, the esophagus, and the bronchi: a single-institution experience. Diagn Ther Endosc 1999;5:145–54.
-
118. Ross P, Jr, Grecula J, Bekaii-Saab T, Villalona-Calero M, Otterson G, Magro C. Incorporation of photodynamic therapy as an induction modality in non-small cell lung cancer. Lasers Surg Med 2006;38:881–9.
-
119. Sakai H, Okunaka T, Konaka C, Kato H. [Photodynamic therapy for early stage lung cancer.] Nippon Rinsho 1996;54:1332–6.
-
120. Santos RS, Raftopoulos Y, Keenan RJ, Halal A, Maley RH, Landreneau RJ. Bronchoscopic palliation of primary lung cancer: single or multimodality therapy? Surg Endosc 2004;18:931–6.
-
121. Savary JF, Monnier P, Fontolliet C, Mizeret J, Wagnieres G, Braichotte D, et al. Photodynamic therapy for early squamous cell carcinomas of the esophagus, bronchi, and mouth with m-tetra (hydroxyphenyl) chlorin. Arch Otolaryngol Head Neck Surg 1997;123:162–8.
-
122. Savary JF, Monnier P, Wagnieres G, Braichotte D, Fontolliet C, Vandenbergh H. Preliminary clinical-studies of photodynamic therapy with meso-tetrahydroxyphenyl chlorin (M-Thpc) as a photosensitizing agent for the treatment of early pharyngeal, esophageal and bronchial carcinomas. SPIE Proceedings Series 1994;2078:330–40.
-
123. Spinelli P, Dal Fante M. Endoscopic photodynamic therapy in lung cancer. Lasers Med Sci 1990;5:181–3.
-
124. Stranadko E, Skobelkin O. Experience of PDT of cancer with two Russian-produced photosensitizers. SPIE Proceedings Series 1995;2392:93–105.
-
125. Stranadko EF, Skobelkin OK, Litvin GD, Astrakhankina TA. Photodynamic therapy of human malignant tumours: a comparative study between photohem and tetrasulfonated aluminium phthalocyanine. SPIE Proceedings Series 1996;2625:440–8.
-
126. Stranadko EP, Ponomarev GV, Mechkov VM, Riabov MV, Ivanov AV, Reshetnikov AV, et al. The first experience of photodithazine clinical application for photodynamic therapy of malignant tumors. SPIE Proceedings Series 2000;3909:138–44.
-
127. Sutedja TG, Schreurs AJ, Vanderschueren RG, Kwa B, van der Werf TS, Postmus PE. Bronchoscopic therapy in patients with intraluminal typical bronchial carcinoid. Chest 1995;107:556–8.
-
128. Taber SW, Buschemeyer WC, III, Fingar VH, Wieman TJ. The treatment of malignant endobronchial obstruction with laser ablation. Surgery 1999;126:730–3; discussion 3–5.
-
129. Takahashi H, Gi H, Tamachi Y, Tsuchida T, Taira O, Kato H. [Targeting therapy for lung cancer.] Gan To Kagaku Ryoho 1994;21:749–54.
-
130. Tomaselli F, Maier A, Sankin O, Anegg U, Stranzl U, Pinter H, et al. [Bronchoscopic PDT combined with hyperbaric oxygen in palliation of malignant bronchogenic stenosis.] Zeitschrift fur Herz-, Thorax- und Gefaesschirurgie 2000;14:245–51.
-
131. Tomaselli F, Maier A, Sankin O, Anegg U, Stranzl U, Pinter H, et al. Acute effects of combined photodynamic therapy and hyperbaric oxygenation in lung cancer – a clinical pilot study. Lasers Surg Med 2001;28:399–403.
-
132. Tomio L, Corti L, Polico C, Maluta S, Calzavara F, Norberto L, et al. [Laser-photo-radiotherapy in the treatment malignant tumors.] Radiol Med 1987;73:313–16.
-
133. Usuda J, Kato H, Okunaka T, Furukawa K, Honda H, Suga Y, et al. Photodynamic therapy using Laserphyrin for centrally located early stage lung cancer. J Clin Oncol 2006;24:7229.
-
134. Usuda J, Tsutsui H, Honda H, Ichinose S, Ishizumi T, Hirata T, et al. Photodynamic therapy for lung cancers based on novel photodynamic diagnosis using talaporfin sodium (NPe6) and autofluorescence bronchoscopy. Lung Cancer 2007;58:317–23.
-
135. Vakoulovskaia E, Chental V, Kuvshinov Y, Poddubny B, Ivanov AV, Kazaryan MA. New approaches to photodynamic therapy of tumors with Al phtalocyanine. SPIE Proceedings Series 2000;4059:32–8.
-
136. Vandenbergh H. Photodynamic therapy and photodetection of early cancer in the upper aerodigestive tract, the tracheobronchial tree, the oesophagus and the urinary bladder. Hadron Ther Oncol 1994;1077:577–621.
-
137. Vincent RG, Dougherty TJ, Rao U, Boyle DG, Potter WR. Photoradiation therapy in advanced carcinoma of the trachea and bronchus. Chest 1984;85:29–33.
-
138. Wile AG, Coffey J, Nahabedian MY, Baghdassarian R, Mason GR, Berns MW. Laser photoradiation therapy of cancer: an update of the experience at the University of California, Irvine. Lasers Surg Med 1984;4:5–12.
-
139. Yamamoto H, Kato H, Okunaka T, Eckhauser ML, Bonaminio A, Konaka C, et al. Photodynamic therapy with the excimer dye laser in the treatment of respiratory tract malignancies. Laser Life Sci 1991;4:125–33.
-
140. Yoon SH, Han KT, Kim GN, Lee SI. [Effect of photodynamic therapy in lung cancer.] Chin J Tuberc Respir Dis 2004;57:358–63.
Case reports (single case or fewer than 10 patients)
-
141. Andouin H, Patrice T, Foultier MT, Courtin V, Dabouis G. HpD-PDT for cancer treatment in bronchology. Chest 1987;92:767–8.
-
142. DeArmond DT, Mahtabifard A, Fuller CB, McKenna RJ, Jr. Photodynamic therapy followed by thoracoscopic sleeve lobectomy for locally advanced lung cancer. Ann Thorac Surg 2008;85:e24–6.
-
143. Downie GH, Cuenca RE, Allison RR, McIlroy BW. A comparison of interstitial and superficial light delivery for photodynamic therapy of intraluminal neoplasms. J Bronchol 2002;9:193–6.
-
144. Downie GH, Qureshi A, Loewen G, Cuenca R, Allison R, Sibata C. Endobronchial ablation of typical carcinoid tumor with photodynamic therapy. J Bronchol 2007;14:10–14.
-
145. Feintrenie X, Lignon D, Arboit F, Menard O, Jonveaux E, Martinet Y, et al. [Experience of photodynamic therapy in inoperable bronchogenic cancer in the city of Nancy. Apropos of 3 cases.] Rev Pneumol Clin 1992;48:129–30.
-
146. Gamarra F, Baumgartner R, Stepp H, Rick K, Leberig A, Huber RM. 5-aminolaevulinic acid for fluorescence diagnosis and photodynamic therapy of bronchial cancer – a case report. SPIE 1995;2371:398–402.
-
147. Haussinger K, Huber RM, Krug M, Baumgartner R, Stepp H, Unsold E, et al. Bronchoscopic photodynamic therapy of bronchial carcinoma. Endoscopy 1989;21:285–8.
-
148. Haussinger K, Huber RM, Krug M, Breyer G, Baumgartner R, Beyer W, et al. [Photodynamic therapy of bronchial cancer.] Pneumologie 1990;44:687–93.
-
149. Horai T, Nakamura SI, Nishio H, Sakuma T, Ikegami H, Matsuda M. A five-year disease-free survivor of multiple unresectable lung cancer treated by photoradiation therapy with haematoporphyrin derivative. Lasers Med Sci 1989;4:1–5.
-
150. Jang TW, Kim HK, Oak CH, Jung MH. Photodynamic therapy in early lung cancer: a report of two cases. Korean J Intern Med 2006;21:178–82.
-
151. Kato H, Konaka C, Kawate N, Shinohara H, Kinoshita K, Noguchi M, et al. Five-year disease-free survival of a lung cancer patient treated only by photodynamic therapy. Chest 1986;90:768–70.
-
152. Kato H, Konaka C, Okunaka T, Nakamura H, Taguchi M, Masada S, et al. Ten year disease-free survival of a lung cancer patient treated only by photodynamic therapy (PDT). Laser Life Sci 1994;6:9–14.
-
153. Kim YK, Lee YM, Kim KU, Uh ST, Kim YH, Park CS. [Clinical experience of photodynamic therapy in five patients with advanced lung cancer.] Tuberc Respir Dis 2004;57:72–7.
-
154. Konaka C, Kato H, Hayata Y. Lung cancer treated by photodynamic therapy alone: survival for more than three years. Lasers Med Sci 1987;2:17–19.
-
155. Kondo K, Miyoshi T, Takizawa H, Kenzaki K, Sakiyama S, Tangoku A. Photodynamic therapy for submucosal tumor of the central bronchus. J Med Invest 2005;52:208–11.
-
156. Kseibi SA, Childs CJ, Allison RR, Cuenca RE, Downie GH. Interventional endoscopic modalities for primary tracheal malignancy: Photodynamic therapy and temporary stenting. Chest 2003;124:291S.
-
157. Kubota K, Furuse K, Kawaguchi T, Kawahara M, Ogawara M, Yamamoto S. A case of long-term survival with stage IV small cell lung cancer and early-stage central-type squamous cell lung cancer treated by photodynamic therapy. Jpn J Clin Oncol 1999;29:45–8.
-
158. McCaughan FM, Janes SM, Read CA, Mosse CA, Bown S, George PJ. Photodynamic therapy in the treatment of early stage lung cancer: internal report to the Use of Medicines Committee [unpublished]. London: University College Hospital; 2009.
-
159. McCaughan JS, Jr. Photoradiation of malignant tumors presensitized with hematoporphyrin derivative. Prog Clin Biol Res 1984;170:805–27.
-
160. McCaughan JS, Jr, Guy JT, Hawley P, Hicks W, Inglis W, Laufman L, et al. Hematoporphyrin-derivative and photoradiation therapy of malignant tumors. Lasers Surg Med 1983;3:199–209.
-
161. Moghissi K, Dixon K. Photodynamic therapy for synchronous occult bronchial cancer 17 years after pneumonectomy. Interact Cardiovasc Thorac Surg 2005;4:327–8.
-
162. Moghissi K, Parsons RJ, Dixon K. Photodynamic therapy (PDT) for bronchial carcinoma with the use of rigid bronchoscope. Lasers Med Sci 1992;7:381–5.
-
163. Mortman KD, Frankel KM. Pulmonary resection after successful downstaging with photodynamic therapy. Ann Thorac Surg 2006;82:722–4.
-
164. Nelson JS, Fairshter RD, Berns MW. Long-term survival of a lung cancer patient treated with photodynamic therapy. Lasers Surg Med 1990;10:208–10.
-
165. Okunaka T, Kato H, Konaka C, Furukawa K, Harada M, Yamamoto Y. Photodynamic therapy of lung cancer with bronchial artery infusion of photofrin. Diagn Ther Endosc 1996;2:203–6.
-
166. Saito M, Kato H, Konaka C, Okunaka T, Furukawa K, Sakai H, et al. Synchronous quadruple lung cancer treated curatively by photodynamic therapy. Diagn Ther Endosc 1996;3:115–19.
-
167. Saito M, Wei BR, Furukawa K, Konaka C, Kato H, Ebihara Y. [A case of squamous cell carcinoma of the lung treated by right sleeve upper lobectomy after photodynamic therapy.] Nippon Kyobu Geka Gakkai 1992;40:1788–91.
-
168. Shimatani H, Okunaka T, Shibuya H, Furukawa T, Ikeda N, Konaka C, et al. [Preoperative PDT for early stage lung cancer accompanied with infiltration to the central airway.] Kyobu Geka 2001;54:957–61.
-
169. Sutedja T, Kwa B, van Kamp H, van Zandwijk N. Photodynamic therapy as an alternative treatment for surgery in a patient with lung cancer undergoing bone marrow transplantation. Chest 1993;103:1908–9.
-
170. Takehara N, Ohsaki Y, Fujiuchi S, Yamaguchi S, Akiba Y, Nakano H, et al. [Effects of photodynamic therapy in inoperable early stage lung cancer patients.] Jpn J Lung Cancer 1995;35:127–32.
Uncategorised
-
171. Downie G, Qureshi A, Childs C, Ron A, Claudio S, Rosa C, et al. Endobronchial ablation of typical carcinoid tumor with photodynamic therapy. Lung Cancer 2005;49:S155-S6.
-
172. Isaev VM, Zenger VG, Musatenko LI, Nasedkin AN, Petlev AA. [Updated laser technologies in otorhinolaryngology.] Vestn Otorinolaringol 2001;5:33–5.
-
173. Li PS. [Photoradiation treatment of broncho-pulmonary carcinoma with hematoporphyrin derivative and microwaves through broncho-fiberscopy.] Chung Hua Chieh Ho Ho Hu Hsi 1986;9:98–9.
-
174. Loewen GM, Pandey R, Bellnier D, Henderson B, Dougherty T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg Med 2006;38:364–70.
-
175. Mantareva V, Kassabov K, Shopova M, Mueller S. Influence of photodynamic therapy on the delay of metastasis development in Lewis lung carcinoma. SPIE 1994;2325:355–63.
-
176. Sanfilippo NJ, Hsi A, DeNittis AS, Ginsberg GG, Kochman ML, Friedberg JS, et al. Toxicity of photodynamic therapy after combined external beam radiotherapy and intraluminal brachytherapy for carcinoma of the upper aerodigestive tract. Lasers Surg Med 2001;28:278–81.
-
177. Sutedja G, Risse R, Van Mourik JC, Postmus PE. Photodynamic therapy for treatment of bronchial carcinomas. Thorax 1994;49:289–90.
Appendix 10 Biliary tract cancer scoping
Thirty publications, with study designs that did not meet the inclusion criteria for the review reported on patients with biliary tract cancer being treated with PDT. Details are lacking for many of these publications, so the categorisations may not be reliable.
There is one comparative study, which is published in Korean;1 the information is taken from the English abstract, which provides no methodological details. This study, of patients with advanced hilar cholangiocarcinoma, is a retrospective analysis of 27 patients who were treated with PDT under percutaneous cholangioscopy plus additional percutaneous biliary drainage compared with 20 patients who were treated with endoscopic biliary drainage alone.
Twelve publications are described as trials but without a comparator group (Phase I/II/pilot studies), with sample sizes ranging from 1 to 44. 2–13 One of these, a Phase II trial of 24 patients with Bismuth III/IV cholangiocarcioma treated with PDT after sensitization with Ps, also reports a retrospective comparison with a historical control group of 20 patients who fulfilled the inclusion criteria for the prospective study. 4
Seven publications report case series with at least 10 patients but all of them have less than 50 patients. 14–20 Ten publications report fewer than 10 patients,21–30 three of which are single case reports. 21–23
References
Observational comparative studies
-
1. Cheon YK, Cho YD, Baek SH, Cha SW, Moon JH, Kim YS, et al. [Comparison of survival of advanced hilar cholangiocarcinoma after biliary drainage alone versus photodynamic therapy with external drainage][see comment.]Korean J Gastroenterol 2004;44:280–7.
Experimental non-comparative studies
-
2. Berr F, Tannapfel A, Lamesch P, Wiedmann M. Endoscopic palliation of bile duct cancer with photodynamic therapy. Hepatology 1997;26:200.
-
3. Berr F, Wiedmann M, Tannapfel A, Halm U, Kohlhaw KR, Schmidt F, et al. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology 2000;31:291–8.
-
4. Dumoulin FL, Gerhardt T, Fuchs S, Scheurlen C, Neubrand M, Layer G, et al. Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma. Gastrointest Endosc 2003;57:860–7.
-
5. Harewood GC, Baron TH, Rumalla A, Wang KK, Gores GJ, Stadheim LM, et al. Pilot study to assess patient outcomes following endoscopic application of photodynamic therapy for advanced cholangiocarcinoma. J Gastroenterol Hepatol 2005;20:415–20.
-
6. Kaya M, de Groen PC, Angulo P, Nagorney DM, Gunderson LL, Gores GJ, et al. Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience. Am J Gastroenterol 2001;96:1164–9.
-
7. Ortner MA, Liebetruth J, Mansmann U, Voderholzer W, Michetti P, Schachschal G, et al. Photodynamic therapy of malignant bile duct tumors. Gastroenterology 2003;124:A565.
-
8. Pereira SP, Aithal G, Devlin J, Meadows HM. Photostent 1: a phase II trial of porfimer sodium photodynamic therapy in locally advanced biliary tract carcinoma. Gut 2006;55:A116.
-
9. Pereira SP, Ayaru L, Rogowska A, Mosse A, Hatfield ARW, Bown SG. Photodynamic therapy of malignant biliary strictures using meso-tetrahydroxyphenylchlorin. Eur J Gastroenterol Hepatol 2007;19:479–85.
-
10. Pereira SP, Ragunath K, Devlin J, Meadows HM. Preliminary results of a phase II trial to examine the safety and efficacy of porfimer sodium photodynamic therapy (PDT) in locally advanced biliary tract carcinoma (BTC). J Clin Oncol 2005;23:A4180.
-
11. Wiedmann M, Berr F, Schiefke I, Witzigmann H, Kohlhaw K, Mossner J, et al. Photodynamic therapy in patients with non-resectable hilar cholangiocarcinoma: 5-year follow-up of a prospective phase II study. Gastrointest Endosc 2004;60:68–75.
-
12. Wiedmann M, Caca K, Berr F, Schiefke I, Tannapfel A, Wittekind C, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer 2003;97:2783–90.
-
13. Wolkersdorfer G, Emmanuel K, Denzer U, Puespoek A, Neureiter D, Kiesslich T, et al. P-150 Temoporfin improves tumoricidal efficacy of photodynamic therapy (PDT) for bile duct cancer. International Liver Cancer Association Annual Conference, Chicago, 2008. p. 60.
Case series (10 or more patients)
-
14. Abulafi AM, Allardice JT, Williams NS, van Someren N, Swain CP, Ainley C. Photodynamic therapy for malignant tumours of the ampulla of Vater. Gut 1995;36:853–6.
-
15. Itoi T, Sofuni A, Itokawa F, Shinohara Y, Takeda K, Nakamura K, et al. Salvage therapy in patients with unresectable hilar cholangiocarcinoma. Digestive Endoscopy 2006;18:232–8.
-
16. Maunoury V, Mordon S, Boyer J, Quentin V, Barthet M, Laugier R, et al. Results of a multicenter open study of photodynamic therapy (Photofrin) in 49 patients with cholangiocarcinoma. Photodynamic Therapy and Photodiagnosis in Clinical Practice, Brixen/Bressanone, 7–11 October 2008.
-
17. Ortner M. Photodynamic therapy for cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2001;8:137–9.
-
18. Prasad GA, Wang KK, Baron TH, Buttar NS, Wongkeesong L-M, Roberts LR, et al. Factors associated with increased survival after photodynamic therapy for cholangiocarcinoma. Clin Gastroenterol Hepatol 2007;5:743–8.
-
19. Shim CS, Cheon YK, Cha SW, Bhandari S, Kim YS, Cho YD, et al. Percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and role of intraductal ultrasonography (IDUS) in the follow-up and response assessment. Gastrointest Endosc 2003;57:A199.
-
20. Shim CS, Cheon YK, Cha SW, Bhandari S, Moon JH, Cho YD, et al. Prospective study of the effectiveness of percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment. Endoscopy 2005;37:425–33.
Case reports (single case or fewer than 10 patients)
-
21. Berr F, Tannapfel A, Lamesch P, Pahernik S, Wiedmann M, Halm U, et al. Neoadjuvant photodynamic therapy before curative resection of proximal bile duct carcinoma. J Hepatol 2000;32:352–7.
-
22. Burdick JS, Magee D, Miller G, Wright KB. Biliary photodynamic therapy alternative delivery technique. Endoscopy 2000;32:S63.
-
23. McCaughan JS, Jr, Mertens BF, Cho C, Barabash RD, Payton HW. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report. Arch Surg 1991;126:111–13.
-
24. Nanashima A, Yamaguchi H, Shibasaki S, Ide N, Sawai T, Tsuji T, et al. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: a preliminary study. J Gastroenterol 2004;39:1095–101.
-
25. Ortner MA, Liebetruth J, Schreiber S, Hanft M, Wruck U, Fusco V, et al. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology 1998;114:536–42.
-
26. Rumalla A, Baron TH, Wang KK, Gores GJ, Stadheim LM, de Groen PC. Endoscopic application of photodynamic therapy for cholangiocarcinoma. Gastrointest Endosc 2001;53:500–4.
-
27. Suzuki S, Inaba K, Yokoi Y, Ohata K, Ota S, Azuma M, et al. Photodynamic therapy for malignant biliary obstruction: a case series. Endoscopy 2004;36:83–7.
-
28. Zoepf T, Jakobs R, Arnold JC, Apel D, Rosenbaum A, Riemann JF. Photodynamic therapy for palliation of nonresectable bile duct cancer – preliminary results with a new diode laser system. Am J Gastroenterol 2001;96:2093–7.
-
29. Zoepf T, Jakobs R, Rosenbaum A, Apel D, Arnold JC, Riemann JF. Photodynamic therapy with 5-aminolevulinic acid is not effective in bile duct cancer. Gastrointest Endosc 2001;54:763–6.
-
30. Zoepf T, Jakobs R, Arnold JC, Apel D. Photodynamic therapy (PDT) for palliation of non resectable bile duct cancer: first results with a new diode laser system. Gastroenterology 1999;116:G2357.
Appendix 11 Brain cancer scoping
Forty-three publications, with study designs that did not meet the inclusion criteria for the review, reported on patients with brain cancer being treated with PDT. Details are lacking for many of these publications, so the categorisations may not be reliable.
There are two studies with some form of comparison group, but both studies are poorly reported and lack methodological details. 1–2
One comparative study reports the results of a Phase II trial of PDD/PDT in 26 patients with WHO grade IV recurrent glioblastoma who were sensitised with mTHPC (FOSCAN) prior to fluorescent guided resection and intraoperative PDT after 4 days. 2 This group of patients was compared with a control group of matched patients, but no details of how they were matched are provided.
The other comparative study reported on 30 patients [27 glioma (nine of which were recurrences), two malignant meningioma, two metastatic brain cancer] treated with high-dose PDT (haematoporphyrin derivative and pumped dye laser) in addition to craniotomy with a radical or partial excision of the tumour. 1 The authors state that 30 comparable patients who were treated with surgery alone were selected at random as control subjects, but no details are provided so it is not clear how these patients were selected.
Thirteen publications are described as being trials but without a comparator group, the number of patients ranging from 3 to 186. 3–15 Some of the publications appear to be related, either potential duplicate publications or reporting different outcomes for the same patients.
Twenty-two publications report case series with at least 10 patients16–37 and six publications report less than 10 patients,38–43 two of which are single case reports. 38–43 Of the 22 publications, four had between 50 and 100 patients,26,27,29,30 and only three had more than 100 patients (one of which included various treatments and so not all patients will have undergone PDT). 32,33,35
Many of the publications appear to be from the same clinical groups (14 are authored by Muller et al. and six by Kostron et al. ) and there may well be significant overlap in the results presented (updating as more patients have been treated). Muller et al. began two RCTs that were not completed (see Chapter 11, Stopped trials) and some of the publications authored by them appear to report characteristics of the cohort of trial patients, but not comparative results.
References
Observational comparative studies
-
1. Chen ZQ, Wu S, Zhu SG. Adjuvant photodynamic therapy in surgical management of cerebral tumors. SPIE 1993;1616:94–7.
-
2. Kostron H, Fiegele T, Akatuna E. Combination of FOSCAN mediated fluorescence guided resection and photodynamic treatment as new therapeutic concept for malignant brain tumors. Med Laser Appl 2006;21:285–90.
Experimental non-comparative studies
-
3. Fedulov AS, Sakovich II, Sliakhtsin SV, Trukhachova TV. PDT of high-grade gliomas with Fotolon. Results of an open-label randomized clinical trial. Photodiagn Photodyn Ther 2008;5(Suppl. 1):S7.
-
4. Kostron H, Fritsch E, Grunert V. Photodynamic therapy of malignant brain tumours: a phase I/II trial. Br J Neurosurg 1988;2:241–8.
-
5. Laws ER, Jr, Cortese DA, Kinsey JH, Eagan RT, Anderson RE. Photoradiation therapy in the treatment of malignant brain tumors: a phase I (feasibility) study. Neurosurgery 1981;9:672–8.
-
6. Marks PV, Belchetz PE, Saxena A, Igbaseimokumo U, Thomson S, Nelson M, et al. Effect of photodynamic therapy on recurrent pituitary adenomas: clinical phase I/II trial: an early report. Br J Neurosurg 2000;14:317–25.
-
7. Muller P, Wilson B, Dougherty TJ. Photodynamic therapy of supratentorial gliomas. SPIE 1997;2972:14–26.
-
8. Muller P, Wilson B, Lilge L, Hitchcock M, Hertzel F, Chen Q, et al. Photodynamic therapy of malignant brain tumors: results from a Phase II trial and demographics from a Phase III trial [Abstract.] Can J Neurol Sci 1999;26:S31.
-
9. Muller P, Wilson B, Lilge L, Varma S, Bogaards A, Fullagar, et al. Clinical studies of photodynamic therapy for malignant brain tumors: facial nerve palsy after temporal fossa photoillumination. SPIE 2003;4952:97–103.
-
10. Muller P, Wilson B, Lilge L, Yang V, Hetzel, Chen Q, et al. Photofrin: Photodynamic therapy for malignant brain tumors. SPIE 2001;4248:34–45.
-
11. Muller P, Wilson B, Lilge L, Yang V, Hitchcock M, Hetzel F, et al. Clinical trials of photodynamic therapy of malignant brain tumors. SPIE 2000;3909:10–19.
-
12. Muller P, Wilson B, Lilge L, Yang V, Varma A, Bogaars A, et al. Clinical studies of photodynamic therapy for malignant brain tumors: Karnofsky score and neurological score in patients with recurrent gloms treated with Photofrin-PDT. SPIE 2002;4612:40–7.
-
13. Origitano TC, Reichman OH. Photodynamic therapy for intracranial neoplasms: development of an image-based computer-assisted protocol for photodynamic therapy of intracranial neoplasms. Neurosurgery 1993;32:587–95, discussion 95–96.
-
14. Rosenthal MA, Kavar B, Uren S, Kaye AH. Promising survival in patients with high-grade gliomas following therapy with a novel boronated porphyrin. J Clin Neurosci 2003;10:425–7.
-
15. Varma AK, Muller PJ. Cranial neuropathies after intracranial Photofrin-photodynamic therapy for malignant supratentorial gliomas: a report on 3 cases. Surg Neurol 2008;70:190–3.
Case series (10 or more patients)
-
16. Beck TJ, Kreth FW, Beyer W, Mehrkens JH, Obermeier A, Stepp H, et al. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg Med 2007;39:386–93.
-
17. Chen L-f, Ke Y-q, Yang Z-l, Wang S-q, Xu R-x. [Effect of photodynamic therapy combined with interstitial chemotherapy for gliomas.] Di Yi Jun Yi Da Xue Xue Bao 2005;25:116–18.
-
18. Kaneko S. Clinical results of stereotactic intratumoral photodynamic therapy for malignant brain tumors. Clin Lasers Diagn 2000;1:222–30.
-
19. Kaye AH, Morstyn G, Brownbill D. Adjuvant high-dose photoradiation therapy in the treatment of cerebral glioma: a phase 1–2 study. J Neurosurg 1987;67:500–5.
-
20. Kostron H, Grunert V. [Photodynamic therapy of malignant brain tumors.] Wien Klin Wochenschr 1987;99:389–92.
-
21. Kostron H, Obwegeser A, Jakober R, Zimmermann A, Rueck A, Dougherty TJ. Experimental and clinical results of mTHPC (Foscan R)-mediated photodynamic therapy for malignant brain tumors. SPIE 1998;3247:40–5.
-
22. Kostron H, Plangger C, Fritsch E, Maier H. Photodynamic treatment of malignant brain tumors. Wien Klin Wochenschr 1990;102:531–5.
-
23. Kostron H, Weiser G, Fritsch E, Grunert V. Photodynamic therapy of malignant brain tumors: clinical and neuropathological results. Photochem Photobiol 1987;46:937–43.
-
24. McCulloch GA, Forbes IJ, See KL, Cowled PA, Jacka FJ, Ward AD. Phototherapy in malignant brain tumors. Prog Clin Biol Res 1984;170:709–17.
-
25. Mehrkens J, Stummer W, Beck T, Tonn J, Kreth F. Interstitial photodynamic therapy of recurrent malignant gliomas using 5-aminolevulinic acid (5-ALA). Neuro Oncol 2005;7:110.
-
26. Muller P, Wilson B. Photodynamic therapy of malignant brain-tumors – supplementary postoperative light delivery by implanted optical fibers: field fractionation. SPIE 1991;1426:254–65.
-
27. Muller P, Wilson B, Dougherty TJ. Photodynamic therapy of supratentorial gliomas. SPIE 1998;3247:2–13.
-
28. Muller PJ, Wilson BC. Photodynamic therapy of malignant primary brain tumours: clinical effects, post-operative ICP, and light penetration of the brain. Photochem Photobiol 1987;46:929–35.
-
29. Muller PJ, Wilson BC. Photodynamic therapy of malignant brain tumours. Can J Neurol Sci 1990;17:193–8.
-
30. Muller PJ, Wilson BC. Photodynamic therapy for recurrent supratentorial gliomas. Semin Surg Oncol 1995;11:346–54.
-
31. Muller PJ, Wilson BC. Photodynamic therapy for malignant newly diagnosed supratentorial gliomas. J Clin Laser Med Surg 1996;14:263–70.
-
32. Muller PJ, Wilson BC. Photodynamic therapy of brain tumors: a work in progress. Lasers Surg Med 2006;38:384–9.
-
33. Obwegeser A, Ortler M, Seiwald M, Ulmer H, Kostron H. Therapy of glioblastoma multiforme: a cumulative experience of 10 years. Acta Neurochir 1995;137:29–33.
-
34. Schmidt MH, Meyer GA, Reichert KW, Cheng J, Krouwer HG, Ozker K, et al. Evaluation of photodynamic therapy near functional brain tissue in patients with recurrent brain tumors. J Neurooncol 2004;67:201–7.
-
35. Stylli SS, Kaye AH, MacGregor L, Howes M, Rajendra P. Photodynamic therapy of high grade gliom: long term survival. J Clin Neurosci 2005;12:389–98.
-
36. Zhu SG. [Application of adjuvant photodynamic therapy in the treatment of malignant brain tumors.] Chin J Clin Oncol 1993;20:894–6.
-
37. Zhu SG, Wu S, Chen ZQ, Sun W. Photodynamic therapy of recurrent cerebral glioma. SPIE 1993;1616:115–18.
Case reports (single case or fewer than 10 patients)
-
38. Benzer A, Putensen C, Kostron H. Photodynamic therapy. Lancet 1989;2:382–3.
-
39. Kaneko S. [Photoradiation therapy (PRT). 7. Clinical study of photoradiation therapy: clinical application and efficacy. d. Brain neoplasms.] Nippon Rinsho 1987;45:817–25.
-
40. Muller PJ, Wilson BC. Photodynamic therapy: cavitary photoillumination of malignant cerebral tumours using a laser coupled inflatable balloon. Can J Neurol Sci 1985;12:371–3.
-
41. Perria C, Carai M, Falzoi A, Orunesu G, Rocca A, Massarelli G, et al. Photodynamic therapy of malignant brain tumors: clinical results of, difficulties with, questions about, and future prospects for the neurosurgical applications. Neurosurgery 1988;23:557–63.
-
42. Powers SK, Cush SS, Walstad DL, Kwock L. Stereotactic intratumoral photodynamic therapy for recurrent malignant brain tumors. Neurosurgery 1991;29:688–95; discussion 95–96.
-
43. Stummer W, Beck T, Beyer W, Mehrkens JH, Obermeier A, Etminan N, et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: case report. J Neurooncol 2008;87:103–9.
Appendix 12 Head and neck cancer scoping
One hundred and twenty-nine publications, with study designs that did not meet the inclusion criteria for the review, reported on patients with head and neck cancer being treated with PDT. Details are lacking for many of these publications, so the categorisations may not be reliable.
There are three studies that appear to be comparative, although the precise design is not clear for any of them. 1–3
Fifteen publications are described as trials but without a comparator group (Phase I/II pilot studies), with sample sizes ranging from 5 to 121. 4–18
Eighty-seven publications report case series;19–105 eight have over 100 patients,26–28,33,61,87,94,105 but not all of these contain patients with only head and neck cancer; some studies report multiple cancer site series. Eleven publications report on case series, with between 50 and 100 patients, and 56 report less than 50 patients. For 12 publications it is not clear what the sample size is. 19,39,40,48,56,57,60,64,68,82,85,99 Many of these publications are by the same authors and appear to be updated series of patients, or duplicate reports published in different journals. Therefore, these publications may double count patients to a certain degree.
Nineteen publications report fewer than 10 patients,106–124 seven of which are single case reports. 108,110,112,115,120,121,124
A further five publications remain uncategorised. 125–129
References
Observational comparative studies
-
1. Inoue H. [Voice evaluation after photodynamic therapy for laryngeal cancer.]Otolaryngol Head Neck Surg 1997;69:407–10.
-
2. Lofgren LA, Hallgren S, Nilsson E, Westerborn A, Nilsson C, Reizenstein J. Photodynamic therapy for recurrent nasopharyngeal cancer. Arch Otolaryngol Head Neck Surg 1995;121:997–1002.
-
3. Smucler R, Vlk P. Combination of laser induced interstitial thermotherapy and photodynamic therapy in the treatment of neck cancer. Lasers Surg Med 2006;39:S18.
Experimental non-comparative studies
-
4. Biel MA. Photodynamic therapy as an adjuvant intraoperative treatment of recurrent head and neck carcinomas. Arch Otolaryngol Head Neck Surg 1996;122:1261–5.
-
5. Buchanan RB, Carruth JA, McKenzie AL, Williams SR. Photodynamic therapy in the treatment of malignant tumours of the skin and head and neck. Eur J Surg Oncol 1989;15:400–6.
-
6. Calzavara F, Tomio L, Norberto L, Peracchia A, Corti L, Zorat PL, et al. Photodynamic therapy in the treatment of malignant tumours of the upper aerodigestive tract. Lasers Med Sci 1989;4:279–84.
-
7. Carruth JA, McKenzie AL. Preliminary report of a pilot study of photoradiation therapy for the treatment of superficial malignancies of the skin, head and neck. Eur J Surg Oncol 1985;11:47–50.
-
8. Carruth JAS, Barrett JM, Barnes DWH, Buchanan RB, Sansom JM. A trial of photodynamic therapy for the treatment of tumors of the skin and head and neck, and the results of an experimental-study to determine the interrelationships between the tissue effects of ionizing radiation and photodynamic therapy. SPIE 1993;1616:14–19.
-
9. Feyh J. Photodynamic therapy of head and neck tumors. Adv Otorhinolaryngol 1995;49:53–7.
-
10. Feyh J. Photodynamic treatment for cancers of the head and neck. J Photochem Photobiol B 1996;36:175–7.
-
11. Grossweiner LI, Hill JH, Lobraico RV. Photodynamic therapy of head and neck squamous cell carcinoma: optical dosimetry and clinical trial. Photochem Photobiol 1987;46:911–17.
-
12. Hopper C, Kubler A, Lewis H, Tan IB, Putnam G. mTHPC-mediated photodynamic therapy for early oral squamous cell carcinoma. Int J Cancer 2004;111:138–46.
-
13. Lou PJ, Jager HR, Jones L, Theodossy T, Bown SG, Hopper C. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br J Cancer 2004;91:441–6.
-
14. Rauschning W, Tan IB, Dolivet G. Photodynamic therapy (PDT) with mTHPC in the palliation of advanced head and neck cancer in patients who have failed prior therapies and are unsuitable for radiotherapy, surgery or systemic chemotherapy. J Clin Oncol 2004;22:5596.
-
15. Savary JF, Monnier P, Fontolliet C, Mizeret J, Wagnieres G, Braichotte D, et al. Photodynamic therapy for early squamous cell carcinomas of the esophagus, bronchi, and mouth with m-tetra (hydroxyphenyl) chlorin. Arch Otolaryngol Head Neck Surg 1997;123:162–8.
-
16. Schweitzer VG. Photodynamic therapy: 1994 treatment of benign and malignant upper aerodigestive tract disease. SPIE 1995;2371:403–17.
-
17. Schweitzer VG, Dougherty TJ. Hematoporphyrin-mediated photodynamic therapy for treatment of head and neck cancer: clinical update 1996. SPIE 1996;2675:47–66.
-
18. Suhr MA, Hopper C, MacRobert AJ, Speight PM, Kubler AC, Kunz L. [Clinical pilot study of interstitial photodynamic therapy for treatment of advanced head and neck tumors.] Mund Kiefer Gesichtschir 2001;5:277–82.
Case series (10 or more patients)
-
19. Bagnato VS, Kurachi C, Ferreira J, Marcassa LG, Sibata CH, Allison RR. PDT experience in Brazil: a regional profile. Photodiagn Photodyn Ther 2005;2:107–18.
-
20. Biel MA. Photodynamic therapy and the treatment of neoplastic diseases of the larynx, oral cavity, pharynx and tracheobronchial tree. SPIE 1993;1881:10–19.
-
21. Biel MA. Photodynamic therapy and the treatment of neoplastic diseases of the larynx. Laryngoscope 1994;104:399–403.
-
22. Biel MA. Photodynamic therapy of head and neck cancers. Semin Surg Oncol 1995;11:355–9.
-
23. Biel MA. Photodynamic therapy and the treatment of neoplastic diseases of the larynx. SPIE 1995;2395:239–44.
-
24. Biel MA. Photodynamic therapy and the treatment of malignancies of the head and neck. SPIE 1995;2392:55–62.
-
25. Biel MA. Photodynamic therapy and the treatment of head and neck cancers. J Clin Laser Med Surg 1996;14:239–44.
-
26. Biel MA. Photodynamic therapy and the treatment of head and neck neoplasia. Laryngoscope 1998;108:1259–68.
-
27. Biel MA, Dougherty TJ. Photodynamic therapy using photofrin and foscan and the treatment of malignancies of the head and neck. SPIE 1998;3247:25–30.
-
28. Bloznelyte L, Cepulis V, Ponomarev I, Dougherty TJ. Intra-arterial PDT and ordinary PDT in head and neck cancer. SPIE 1996;2675:76–9.
-
29. Cai WM. [Result and indication of photodynamic therapy in the treatment of malignant tumors: analysis of 165 patients.] Zhonghua Zhong Liu Za Zhi 1990;12:69–71.
-
30. Copper MP, Tan IB, Oppelaar H, Ruevekamp MC, Stewart FA. Meta-tetra(hydroxyphenyl)chlorin photodynamic therapy in early-stage squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 2003;129:709–11.
-
31. Copper MP, Triesscheijn M, Tan IB, Ruevekamp MC, Stewart FA. Photodynamic therapy in the treatment of multiple primary tumours in the head and neck, located to the oral cavity and oropharynx. Clin Otolaryngol 2007;32:185–9.
-
32. Cuenca RE, Sibata CH, Downie GH, Brodish B, Camnitz P, Allison RR. Photodynamic therapy for head and neck neoplasia using Photofrinin. Ann Surg Oncol 2006;13:16.
-
33. D’Cruz AK, Robinson MH, Biel MA. mTHPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study of 128 patients. Head Neck 2004;26:232–40.
-
34. de Haas ER, Sterenborg HJ, Neumann HA, Robinson DJ. The influence of light fractionation on the response of superficial skin cancer to aminolevulinic-acid photodynamic therapy. J Invest Dermatol 2006;126:S73.
-
35. Decorbiere S, Ouayoun M, Sequert C, Freche C, Chabolle F. Use of photodynamic therapy in the treatment of vocal cord carcinoma retrospective study 1986–1992: on 41 cases. Photodyn Ther Biomed Lasers 1992;1011:656–61.
-
36. Delbove H, de Corbiere S, Fugain C, Freche C, Chabolle F. [Photochemotherapy in the treatment of carcinoma of the vocal cords of early stage (Tis, T1).] Ann Otolaryngol Chir Cervicofac 1996;113:155–61.
-
37. Dilkes M, Bapat U. Foscan photodynamic therapy for early head and neck cancer. Otolaryngol Head Neck Surg 2004;131:74–5.
-
38. Dilkes MG, Benjamin E, Ovaisi S, Banerjee AS. Treatment of primary mucosal head and neck squamous cell carcinoma using photodynamic therapy: results after 25 treated cases. J Laryngol Otol 2003;117:713–17.
-
39. Dilkes MG, DeJode ML, Gardiner Q, Kenyon GS, McKelvie P. Treatment of head and neck cancer with photodynamic therapy: results after one year. J Laryngol Otol 1995;109:1072–6.
-
40. Dilkes MG, DeJode ML, Rowntree-Taylor A, McGilligan JA, Kenyon GS, McKelvie P. m-THPC photodynamic therapy for head and neck cancer. Lasers Med Sci 1996;11:23–9.
-
41. Fan KF, Hopper C, Speight PM, Buonaccorsi G, MacRobert AJ, Bown SG. Photodynamic therapy using 5-aminolevulinic acid for premalignant and malignant lesions of the oral cavity. Cancer 1996;78:1374–83.
-
42. Fan KF, Hopper C, Speight PM, Buonaccorsi GA, Bown SG. Photodynamic therapy using mTHPC for malignant disease in the oral cavity. Int J Cancer 1997;73:25–32.
-
43. Feyh J, Bujia J, Kastenbauer E. [Photodynamic therapy with hematoporphyrin derivatives in cervico-facial basaliomas and epidermoid carcinoma in stage I and II.] Acta Otorrinolaringol Esp 1993;44:121–4.
-
44. Feyh J, Goetz A, Muller W, Konigsberger R, Kastenbauer E. Photodynamic therapy in head and neck surgery. J Photochem Photobiol B 1990;7:353–8.
-
45. Freche C, De Corbiere S. Use of photodynamic therapy in the treatment of vocal cord carcinoma. J Photochem Photobiol B 1990;6:291–6.
-
46. Frisch C, Vocks E, Herzog M, Vogt HJ, Borelli S. [Persistent photosensitivity after systemic photodynamic therapy.] Aktuelle Dermatol 1996;22:98–103.
-
47. Gluckman JL. Hematoporphyrin photodynamic therapy: is there truly a future in head and neck oncology? Reflections on a 5-year experience. Laryngoscope 1991;101:36–42.
-
48. Gluckman JL, Waner M, Shumrick K, Peerless S. Photodynamic therapy. A viable alternative to conventional therapy for early lesion of the upper aerodigestive tract? Arch Otolaryngol Head Neck Surg 1986;112:949–52.
-
49. Grant WE, Hopper C, Speight PM, Macrobert AJ, Bown SG. Photodynamic therapy of malignant and premalignant lesions in patients with ‘field cancerization’ of the oral cavity. J Laryngol Otol 1993;107:1140–5.
-
50. Ha XW, Sun XM, Xie JG, Fan XJ, Zhang YH, Mei QC, et al. Clinical use of hematoporphyrin derivative in malignant tumors. Chin Med J 1983;96:754–8.
-
51. Herzog M. [Photodynamic laser therapy and fluorescence diagnosis in squamous epithelial cancer of the oral cavity.] Fortschr Kiefer Gesichtschir 1993;38:139–43.
-
52. Herzog M, Fellbaum C, Horch HH. [Initial clinical experiences with the photodynamic therapy (PDT) of oral cavity carcinomas.] Dtsch Zahn Mund Kieferheilkd Zentralbl 1992;80:141–3.
-
53. Herzog M, Fellbaum C, Wagnermanslau C, Horch HH. Clinical, MRI and histological results after photodynamic therapy (PDT) of oral-cancer. SPIE 1992;1645:52–5.
-
54. Hopper C, Fan K, Grant WE, Speight PM, Bown SG. Photodynamic therapy using 5-aminolaevulinic acid in oral malignancy and premalignancy. SPIE 1995;2371:254–5.
-
55. Jiang X. The application of laser and PDT to treatment recurrent cancer in the paranasal sinuses. SPIE 1993;1616:127–8.
-
56. Keller GS, Doiron DR, Fisher GU. Photodynamic therapy in otolaryngology: head and neck surgery. Arch Otolaryngol Head Neck Surg 1985;111:758–61.
-
57. Kulapaditharom B, Boonkitticharoen V. Photodynamic therapy in the treatment of head and neck cancers: a two-year experience. J Med Assoc Thai 1996;79:229–35.
-
58. Kulapaditharom B, Boonkitticharoen V. Photodynamic therapy for residual or recurrent cancer of the nasopharynx. J Med Assoc Thai 1999;82:1111–17.
-
59. Kulapaditharom B, Boonkitticharoen V. Photodynamic therapy in management of head and neck cancers and precancerous lesions. J Med Assoc Thai 2000;83:249–58.
-
60. Kurachi C, Cestari G, Bagnato VS. Photodynamic therapy in the treatment of oral lesions. J Dent Res 2003;82:217.
-
61. Li JH, Guo ZH, Jin ML, Zhao FY, Cai WM, Gao ML, et al. Photodynamic therapy in the treatment of malignant tumours: an analysis of 540 cases. J Photochem Photobiol B 1990;6:149–55.
-
62. Li LB, Luo RC, Liao WJ, Zhang MJ, Liu XJ, Luo YL, et al. [PHOTOFRIN photodynamic therapy for treating laryngeal carcinoma and improving voice function.] Chin J Clin Rehab 2005;9:160–1.
-
63. Lorenz KJ, Maier H. [Squamous cell carcinoma of the head and neck. Photodynamic therapy with Foscan.] HNO 2008;56:402–9.
-
64. Malczewski M, Birecki J, Symonowicz K, Bronowicz A, Ziolkowski P, Rabczynski J, et al. [Clinical applications of photodynamic therapy in the treatment of laryngeal lesions.] Otolaryngol Pol 1999;53:671–5.
-
65. Marchiori C, Zorat PL, Canaviglia G. [Photodynamic therapy with hematoporphyrin and red light for the treatment of head and neck cancer.] Acta Otorhinolaryngol Ital 1985;35:289–94.
-
66. Monnier P, Savary M, Fontolliet C, Wagnieres Chatelain GA, Cornaz P, Depeursinge C, et al. Photodetection and photodynamic therapy of ‘early’ squamous cell carcinomas of the pharynx, oesophagus and tracheo-bronchial tree. Lasers Med Sci 1990;5:149–68.
-
67. Monnier P, Savary M, Fontolliet C, Wagnieres G, Vandenbergh H, Chatelain A. Photodynamic therapy (PDT) of 25 early pharyngoesophageal carcinomas: results and complications. Dis Esophagus 1990;1:359–71.
-
68. Radu A, Grosjean P, Fontolliet C, Wagnieres G, Woodtli A, Bergh HV, et al. Photodynamic therapy for 101 early cancers of the upper aerodigestive tract, the esophagus, and the bronchi: a single-institution experience. Diagn Ther Endosc 1999;5:145–54.
-
69. Savary JF, Monnier P, Wagnieres G, Braichotte D, Fontolliet C, Vandenbergh H. Preliminary clinical-studies of photodynamic therapy with meso-tetrahydroxyphenyl chlorin (M-Thpc) as a photosensitizing agent for the treatment of early pharyngeal, esophageal and bronchial carcinomas. SPIE 1994;2078:330–40.
-
70. Schuller DE, McCaughan JS, Jr, Rock RP. Photodynamic therapy in head and neck cancer. Arch Otolaryngol Head Neck Surg 1985;111:351–5.
-
71. Schweitzer VG. Photodynamic therapy for treatment of head and neck cancer. Otolaryngol Head Neck Surg 1990;102:225–32.
-
72. Schweitzer VG. PHOTOFRIN-mediated photodynamic therapy for treatment of early stage oral cavity and laryngeal malignancies. Lasers Surg Med 2001;29:305–13.
-
73. Shubina AM, Kaplan MA, Kapinus VN, Spichenkova IS, Polkin VV. The photodynamic therapy of malignant neoplasms of oral cavity and lips. Laser Helsinki 2008, 13th International Congress of EMLA (European Medical Laser Association) in conjunction with EMLA Finland and MAL (Medical Acupuncture and Laser) in cooperation with ASLMS (American Society for Laser Medicine and Surgery), Helsinki, 2008.
-
74. Sieron A, Namyslowski G, Misiolek M, Adamek M, Kawczyk-Krupka A. Photodynamic therapy of premalignant lesions and local recurrence of laryngeal and hypopharyngeal cancers. Eur Arch Otorhinolaryngol 2001;258:349–52.
-
75. Sieron A, Namyslowski G, Misiolek M, Adamek M, Kawczyk-Krupka A. [Clinical response to photodynamic therapy in premalignant lesions and advanced head and neck carcinomas.] Otolaryngol Pol 2003;57:501–4.
-
76. Stranadko E, Skobelkin O. Experience of PDT of cancer with two Russian-produced photosensitizers. SPIE 1995;2392:93–105.
-
77. Stranadko EF, Garbusov MI, Markitchev NA, Riabov MV. Photodynamic therapy of recurrent tumours of oropharyngeal area as an out-patient procedure. SPIE 1997;3191:237–42.
-
78. Stranadko EF, Garbuzov MI, Zenger VG, Nasedkin AN, Markichev NA, Riabov MV, et al. [Photodynamic therapy of recurrent and residual oropharyngeal and laryngeal tumors.] Vestn Otorinolaringol 2001:36–9.
-
79. Stranadko EF, Skobelkin OK, Litvin GD, Astrakhankina TA. Photodynamic therapy of human malignant tumours: a comparative study between photohem and tetrasulfonated aluminium phthalocyanine. SPIE 1996;2625:440–8.
-
80. Stranadko EF, Skobelkin OK, Markichev NA, Riabov MV. Photodynamic therapy of recurrent cancer of oral cavity, an alternative to conventional treatment. SPIE 1996;2924:292–7.
-
81. Stranadko EF, Skobelkin OK, Markichev NA, Riabov MV, Khorana BM, Junheng L, et al. Photodynamic therapy of recurrent cancer of oral cavity. SPIE 1996;2887:233–5.
-
82. Stranadko EF, Skobelkin OK, Vorozhtsov GN, Beshleul SE, Markitchev NA, Riabov MV. Photodynamic therapy of cancer, five-year clinical experience. SPIE 1997;3191:253–62.
-
83. Stranadko EF, Titova VA, Rjabov MV, Petrovsky VY. Photodynamic therapy of squamous-cellular cancer of head and neck. Laser Helsinki 2008, 13th International Congress of EMLA (European Medical Laser Association) in conjunction with EMLA Finland and MAL (Medical Acupuncture and Laser) in cooperation with ASLMS (American Society for Laser Medicine and Surgery), Helsinki, 2008.
-
84. Stranadko EP, Garbusov MI, Markitchev NA, Riabov MV, Ivanov AV, Kazaryan MA. Photodynamic therapy for recurrent and residual malignant tumors of oropharyngeal area. SPIE 2000;4059:39–45.
-
85. Stranadko EP, Ponomarev GV, Mechkov VM, Riabov MV, Ivanov AV, Reshetnikov AV, et al. The first experience of photodithazine clinical application for photodynamic therapy of malignant tumors. SPIE 2000;3909:138–44.
-
86. Sun ZQ. [Hematoporphyrin derivative (HPD) plus laser photodynamic therapy for nasopharyngeal carcinoma: analysis of 57 cases.] Zhonghua Zhong Liu Za Zhi 1990;12:120–2.
-
87. Sun ZQ. [Photodynamic therapy of nasopharyngeal carcinoma by argon or dye laser: an analysis of 137 cases.] Zhonghua Zhong Liu Za Zhi 1992;14:290–2.
-
88. Tan I, Dolivet G, Ceruse P, Vander Poorten V, Mahy P, Roest G, et al. Temoporfin-mediated photodynamic therapy in advanced, incurable head and neck squamous cell carcinoma: a prospective multicenter study [abstract from 2008 ASCO annual meeting]. J Clin Oncol 2008;26:17001.
-
89. Titova VA, Kharchenko NV, Stranadko EF, Tishenko VA, Petrovsky VY, Kreynina JM. Photodynamic therapy in multimodality treatment of locally advanced and recurrent oral cancer. Laser Helsinki 2008, 13th International Congress of EMLA (European Medical Laser Association) in conjunction with EMLA Finland and MAL (Medical Acupuncture and Laser) in cooperation with ASLMS (American Society for Laser Medicine and Surgery), Helsinki, 2008.
-
90. Titova VA, Kharchenko V, Dykhno Y, Petrovsky Y, Stranadko F, Kreinina M, et al. Photodynamic therapy and lazer intensed thermotherapy in locally advanced and recurrent oropharyngeal cancer multimodal treatment. Radiother Oncol 2007;82 (Suppl.1).
-
91. Tong MC, van Hasselt CA, Woo JK. Preliminary results of photodynamic therapy for recurrent nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol 1996;253:189–92.
-
92. Troillet FX, Dumonceau JM, Margalith D, Dorta G, Ortner MA. [New techniques in diagnostic and interventional endoscopy.] Med Hyg 2004;62:218–22.
-
93. Vakouloskaia EG, Chental VV, Abdoullin NA, Kuvshinov YP, Tabolinovskaia TD, Edinak NJ, et al. Photodynamic therapy of head and neck tumors. SPIE 1996;2924:309–13.
-
94. Vakoulovskaia E, Chental V, Kuvshinov Y, Poddubny B, Ivanov AV, Kazaryan MA. New approaches to photodynamic therapy of tumors with Al phtalocyanine. SPIE 2000;4059:32–8.
-
95. Vakoulovskaia EG, Chental VV, Abdoullin NA, Kuvshinov YP, Tabolinovskaia TD, Edinak NJ, et al. Photodynamic therapy of head and neck cancer with different sensitizers. SPIE 1997;3191:232–6.
-
96. Vakulovskaya EG, Ungiadze GV, Tabolinovskaya TD. Photodynamic therapy of oral cancer and cancer of oropharynx with second-generation photosensitizers. Oral Oncol 2005;1(Suppl.):68.
-
97. Wang KH. [Interstitial photodynamic therapy of oral squamous cancers.] Zhonghua Zhong Liu Za Zhi 1988;10:473–5.
-
98. Wenig BL, Kurtzman DM, Grossweiner LI, Mafee MF, Harris DM, Lobraico RV, et al. Photodynamic therapy in the treatment of squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 1990;116:1267–70.
-
99. Wile AG, Dahlman A, Burns RG, Berns MW. Laser photoradiation therapy of cancer following hematoporphyrin sensitization. Lasers Surg Med 1982;2:163–8.
-
100. Wile AG, Novotny J, Mason GR, Passy V, Berns MW. Photoradiation therapy of head and neck cancer. Prog Clin Biol Res 1984;170:681–91.
-
101. Yoshida T. Clinical research of photodynamic therapy in the head and neck cancer. Nippon Gan Chiryo Gakkai Shi 1994;30:247.
-
102. Yoshida T, Kato H, Okunaka T, Saeki T, Ohashi S, Okudaira T, et al. Photodynamic therapy for head and neck cancer. Diagn Ther Endosc 1996;3:41–51.
-
103. Zeng CY, Yang D, Wang KH, Cao QQ. Interstitial photodynamic therapy for cancers of cavum oris, skin and cervix. SPIE 1993;1616:102–7.
-
104. Zhao FY, Zhang KH, Jiang F, Wu MJ. Photodynamic therapy for treatment of cancers in oral and maxillofacial regions: a long-term follow-up study in 72 complete remission cases. Lasers Med Sci 1991;6:201–4.
-
105. Zhao FY, Zhang KH, Ma DQ, He ZQ, Chen S, Ni XM, et al. Treatment of 570 cases of oral squamous cell carcinoma. Ann Acad Med Singapore 1989;18:533–6.
Case reports (single case or fewer than 10 patients)
-
106. Biel MA. Photodynamic therapy as an adjuvant intraoperative treatment of recurrent head and neck carcinomas. SPIE 1994;2133:53–7.
-
107. Biel MA. Photodynamic therapy as an adjuvant intraoperative treatment of recurrent head and neck carcinomas. SPIE 1995;2395:235–8.
-
108. Chen H-M, Chen C-T, Yang H, Lee M-I, Kuo MY-P, Kuo Y-S, et al. Successful treatment of an extensive verrucous carcinoma with topical 5-aminolevulinic acid-mediated photodynamic therapy. J Oral Pathol Med 2005;34:253–6.
-
109. Chen SZ, Cao JY. [Hemodynamic and imageological analysis of oral and maxillofacial tumor with photodynamic therapy.] J Clin Rehabil Tissue Eng Res 2007;11:923–5.
-
110. Feyh J, Goetz A, Martin F, Lumper W, Muller W, Brendel W, et al. [Photodynamic laser tumor therapy with a hematoporphyrin derivative in spinocellular carcinoma of the external ear.] Laryngorhinootologie 1989;68:563–5.
-
111. Grant WE, Hopper C, MacRobert AJ, Speight PM, Bown SG. Photodynamic therapy of oral cancer: photosensitisation with systemic aminolaevulinic acid. Lancet 1993;342:147–8.
-
112. Mang TS, Sullivan M, Cooper M, Loree T, Rigual N. The use of photodynamic therapy using 630 nm laser light and porfimer sodium for the treatment of oral squamous cell carcinoma. Photodiagn Photodyn Ther 2006;3:272–5.
-
113. McCaughan JS, Jr, Guy JT, Hawley P, Hicks W, Inglis W, Laufman L, et al. Hematoporphyrin-derivative and photoradiation therapy of malignant tumors. Lasers Surg Med 1983;3:199–209.
-
114. Ofner JG, Bartl B, Konig S, Thumfart WF. Photodynamic therapy in selected cases at the ENT Clinic, Innsbruck: case reports. J Photochem Photobiol B 1996; 36:185–7.
-
115. Poate TW, Dilkes MG, Kenyon GS. Use of photodynamic therapy for the treatment of squamous cell carcinoma of the soft palate. Br J Oral Maxillofac Surg 1996;34:66–8.
-
116. Prabhakar J, Fan K, Hopper C, Bown SG. Interstitial photodynamic therapy in head and neck tumours. Eur J Surg Oncol 1998;24:348.
-
117. Schweitzer VG. Photodynamic therapy improves Kaposi’s sarcoma in AIDS patients. Am Fam Physician 1988;38:260.
-
118. Schweitzer VG. Photodynamic therapy for treatment of aids-related mucocutaneous Kaposi’s sarcoma. Photodyn Ther Biomed Lasers 1992;1011:49–64.
-
119. Schweitzer VG. Photodynamic therapy for treatment of AIDS-related mucocutaneous Kaposi’s sarcoma. SPIE 1992;1645:10–32.
-
120. Soudant J, Castro DJ, Fougeront B, Saxton RE. The use of photodynamic therapy and photochemotherapy with lasers for palliative treatment of head and neck carcinomas: an initial human case report. J Clin Laser Med Surg 1992;10:367–71.
-
121. Takebayashi S. [Photodynamic therapy for laryngeal cancer.] Otolaryngol Head Neck Surg 2004;76:502–3.
-
122. Tanaka H, Hashimoto K, Yamada I, Masumoto K, Ohsawa T, Murai M, et al. Interstitial photodynamic therapy with rotating and reciprocating optical fibers. Cancer 2001;91:1791–6.
-
123. Tomio L, Corti L, Polico C, Maluta S, Calzavara F, Norberto L, et al. [Laser-photo-radiotherapy in the treatment malignant tumors.] Radiol Med 1987;73:313–16.
-
124. Wang C-P, Chang Y-L, Chen C-T, Yang T-H, Lou P-J. Photodynamic therapy with topical 5-aminolevulinic acid as a post-operative adjuvant therapy for an incompletely resected primary nasopharyngeal papillary adenocarcinoma: a case report. Lasers Surg Med 2006;38:435–8.
Uncategorised
-
125. Biel MA. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochem Photobiol 2007;83:1063–8.
-
126. Bing Tan I, Dolivet G, Ceruse P, Vander Poorten V, Roest GJ, Rauschning W. Foscan–mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study [unpublished]. Dublin: Biolitec Pharma Ltd.
-
127. Jager HR, Taylor MN, Theodossy T, Hopper C. MR imaging-guided interstitial photodynamic laser therapy for advanced head and neck tumors. AJNR Am J Neuroradiol 2005;26:1193–200.
-
128. Luo GY, Sun ZQ, Wang HT. The study on photodynamic therapy of nasopharyngeal carcinoma by argon or dye laser (treatment of 108 cases). Chin J Cancer 1995;14:368–70.
-
129. Vandenbergh H. Photodynamic therapy and photodetection of early cancer in the upper aerodigestive tract, the tracheobronchial tree, the oesophagus and the urinary bladder. Hadron Ther Oncol 1994;1077:577–621.
Appendix 13 Actinic keratosis data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Braathen et al. (2008)54 Data source Full published paper Country Not stated, ‘Europe’ Language English Study design RCT No. of participants Total: 119 randomised, 112 treated (384 lesions) Intervention: 28 (1 hr; 160 mg/g) Comparator: 30 (3 hr; 160 mg/g) 2nd Comparator: 25 (1 hr; 80 mg/g) 3rd Comparator: 29 (3 hr; 80 mg/g) No. of recruiting centres Eight Follow-up period and frequency FU at wk 1 and 2, then at 2, 3, 6 and 12 mth |
Treatment intention Curative Type(s) of lesion and histology Primary AK Main eligibility criteria Patients of 18 yr or older with Fitzpatrick skin type I, II or II with primary previously untreated, non-pigmented and non-infiltrating AK lesions (up to four lesions). Extensive exclusion criteria were reported Patient characteristics % Male: 56, age not reported. The majority of patients had three or fewer lesions; most were located on the face/scalp and were thin or moderately thick Concomitant treatment Not stated |
Trial treatments MAL–PDT comparing incubation time (1 hr or 3 hr) and dose (160 mg/g or 80 mg/g) Intervention MAL–PDT (1 hr + 160 mg/g). Most lesions were prepared using dermal curette to remove scales and crusts. 1-mm-thick layer of MAL cream applied to each lesion and to 5 mm of surrounding area. Lesions covered with occlusive dressing for specified incubation period then wiped off with saline. Lesions were illuminated with red lamp light (wavelength 570–670 nm, intensity 70 – 190 mW/cm2) to provide total light dose of 75 J/cm2. The mean illumination time was around 9.5 min. Any lesions without CR at 2 or 3 mth were given 2nd PDT treatment. Any non-CR at 6 mth offered alternative treatment Comparator MAL–PDT (3 hr + 160 mg/g) as above 2nd comparator MAL–PDT (1 hr + 80 mg/g) as above 3rd comparator MAL–PDT (3 hr + 80 mg/g) as above |
Morbidity Lesion response (based on 110 patients with 380 lesions, two patients with four lesions excluded due to wrong diagnosis). Overall lesion response (not clear if CR) 85% 3 hr + 160 mg/g 76% 1 hr + 160 mg/g 74% 3 hr + 80 mg/g 77% 1 hr + 80 mg/g. Note: About one-third of lesions were not debrided as per protocol. For the 3 hr + 160 mg/g group CR was higher for debrided lesions (89%) than non-debrided (78%) Lesion recurrence (evaluated in 97 patients with 299 lesions that had responded completely): The lowest recurrence rates occurred in the 3 hr + 160 mg/g group (11%) compared with between 26% and 45% for the other groups Patients who received two PDT sessions: Lesion recurrence was lower for the 1-hr and 3 hr + 160 mg/g groups (19% and 17%) than for the 80 mg/g groups (44–45%). For debrided lesions in the 3 hr + 160 mg/g recurrence was 10% vs 14% for non-debrided lesions QoL and return to normal activity Cosmetic outcome (assessed by VAS score at 12 mth) was rated at between 8.4 cm and 9.3 cm by both investigator and patients indicating an excellent cosmetic outcome AEs Most AEs were mild intensity and the majority were local. The most commonly reported AE was erythema with a median duration of 17 d. Other AEs included skin pain, pruritis, burning sensation on skin, oedema and suppuration. Four patients experienced SAEs but these were not considered to be treatment related Treatment-related AE %: 1 hr + 160 mg/g, 98% 3 hr + 160 mg/g, 99% 1 hr + 80m/g, 98% 3 hr + 80 mg/g, 96% |
Authors’ conclusions PDT using a 1-hr incubation with 160 mg/g MAL cream may have potential for treating relatively mild AK lesions and offers practical advantages, but regular substitution is not recommended Brief study appraisal As the authors acknowledge, there were some important flaws in the design of this study, including the lack of blinding of outcome assessors, and having response rate judgements made clinically rather than histologically. Although the mean incubation and illumination times were consistent according to the protocol, it was clear that a large proportion of patients received only 1 PDT session (when 2 are recommended) and around one-third of lesions had not been debrided. Given that the protocol does not appear to have been followed closely, or has not utilised PDT optimally, these results should be considered with caution. No p-values or formal tests of statistical significance appear to have been carried out, making it difficult to assess the real differences between treatment groups |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Dragieva et al. (2004)42 Linked publications161 Data source Full published paper Country Switzerland Language English Study design RCT No. of participants Total: 17 (34 lesional areas with 129 AK; two lesional areas per patient were randomised for treatment) Intervention: 17 lesional areas (62 AK) Comparator: 17 lesional areas (67 AK) No. of recruiting Centres One Follow-up period and frequency FU 1, 4, 8 and 16 wk after 2nd treatment. AEs also recorded at these times and before and after illumination |
Treatment intention Curative Type(s) of lesion and histology Mild to moderate AK Main eligibility criteria Male and female organ transplant recipients (18 or over) with mild to moderate AK (confirmed by 4-mm punch biopsy from thickest region). Patients with porphyria or known allergy to compounds or excipients of the cream were excluded Patient characteristics % Male: 76 Age range: 44–76 yr Mean age: 61 yr Most lesions were located on the face or scalp. Lesions were either untreated or had received previous ineffective treatment over 1 mth ago Concomitant treatment 1 g of oral paracetamol 1 hr before illumination and a fan was used on the affected area |
Trial treatments MAL–PDT vs PDT with placebo cream (within-participant comparison) Intervention MAL–PDT: Two consecutive treatments 1 wk apart, performed on areas of maximum size 4 x 4 cm. Superficial curettage followed by application of 1-mm-thick MAL cream then covered with an occlusive dressing for 3 hr. After removal of dressing and cleaning of area using saline solution, PDT was delivered at 80 mW/cm2 (75 J/cm2) by a non-coherent light source (emission spectrum 600–730 nm) Comparator PDT with placebo cream: As for MAL but with placebo cream |
Morbidity Week 16: For the MAL–PDT group there was CR of 13/17 lesional areas (95% CI 9 to 16) and PR in 3/17. There was no reduction in size or number of AK in 1/17 MAL–PDT treated area and in all placebo-treated areas. Overall lesion CR rate for MAL–PDT was 56/62 and for placebo 0/67 (p = 0.0003) QoL and return to normal activity Not assessed AEs For the MAL–PDT group discomfort (using the VAS scale) was mild in 11/17 and moderate in 6/17 (1st illumination) – and mild in 6/17, moderate in 9/17 and severe in 2/17 (2nd illumination). Mild to moderate intensity AEs for the MAL–PDT group included erthyema, oedema and crusting. For placebo treated areas discomfort was mild in all cases |
Authors’ conclusions PDT with methyl aminolevulinate is safe and effective for AK in transplant recipients. It may also reduce the risk of transformation of AKs to invasive, and potentially fatal, SCC Brief study appraisal This study was generally of high quality in methods and reporting. As the population recruited were immunosuppressed transplant recipients the results may not be generalisable to other populations |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Ericson et al. (2004)43 Data source Full published paper Country Sweden Language English Study design RCT No. of participants Total: 40 (37 analysed) Intervention: Nine (broad filter, 50 mW/cm2) Comparator: 10 (broad filter, 75 mW/cm2) 2nd Comparator: Nine (narrow filter, 30 mW/cm2) 3rd Comparator: Nine (narrow filter, 45 mW/cm2) No. of recruiting centres Multicentre Follow-up period and Frequency 7 wk |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Clinically typical AK; either one AK lesion, minimum diameter of 20 mm, or three lesions within area exceeding 25 cm2 Patient characteristics % Male: 80 Mean age: 71 yr Lesions were located on the face, scalp, neck and upper chest Concomitant treatment Not stated |
Trial treatments ALA–PDT 50 mW/cm2 (broad filter) vs ALA–PDT 75 mW/cm2 (broad filter) vs ALA–PDT 30 mW/cm2 (narrow filter) vs ALA–PDT 45 mW/cm2 (narrow filter). Total dose 100 J/cm2 (all treatments) Intervention ALA–PDT: Crusts and scales were removed then 20% ALA cream was applied using an occlusive bandage and removed after 3 hr. The Photo Demarcation System 1, Prototype 5, was used to deliver 50 mW/cm2, total dose 100 J/cm2. Fluorescence imaging recordings (365 and 405 nm, 0.5 mW/cm2) took place before treatment, during treatment (after 5, 10, 20 and 40 J/cm2) and after finishing treatment (100 J/cm2) Comparator ALA–PDT with 75 mW/cm2 (broad filter); other treatment details as before 2nd comparator ALA–PDT with 30 mW/cm2 (narrow filter); other treatment details as before 3rd comparator ALA–PDT with 45 mW/cm2 (narrow filter); other treatment details as before |
Morbidity There was a significant correlation between fluence rate and treatment outcome (p < 0.02); the highest number of patients with complete remission was in the 30 mW/cm2 (narrow filter) group (8/9 patients). There was a non-significant trend towards a smaller proportion of remaining AK for the narrow filter (p = 0.07). No significant difference was found between 45 mW/cm2 (narrow) and 50mW (broad) groups implying preferable treatment outcome was attributable to fluence rate not spectral emission QoL and return to normal activity Not assessed AEs There was no significant correlation between fluence rate and VAS score. The VAS value increased up to a peak after a cumulative light dose of 20 J/cm2 |
Authors’ conclusions Photobleaching rate and primary treatment outcomes are dependent on fluence rate. A low fluence rate (30 mW/cm2) seems preferable when performing PDT of AK using non-coherent light sources Brief study appraisal The details of this small trial were poorly reported therefore the reliability of the conclusions is unclear; however, the authors acknowledge that a larger RCT is required |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Fowler and Zax (2002)44 Data source Full published paper. A summary of results of 2 trials with identical treatment protocols. Results for most outcomes were only available as combined data. Further data were obtained from drugs.com162 Country Not stated Language English Study design RCT No. of participants Total: Reported as being 243, across two trials – 116 in trial ALA-018, and 125 in trial ALA-019 (which totals 241) Intervention: ALA-018: 87 ALA-019: 93 Comparator: ALA-018: 29 ALA-019: 32 No. of recruiting centres Multicentre Follow-up period and frequency 1, 4, 8, and 12 wk, then FU at intervals of approximately 3 to 6 mth, up to 48 mth |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Patients with 4–15 grade I or II AKs on the face or scalp. Excluded patients had a history of cutaneous photosensitisation, porphyria, hypersensitivity to porphyrins, photodermatosis or inherited or acquired coagulation defects Patient characteristics % Male: 90 Age range: 34–89 yr The number of treated lesions per patient ranged from 4 to 15 Treated areas were the face or scalp, but not both in the same patient Concomitant treatment Not stated |
Trial treatments ALA–PDT vs PDT with placebo cream Intervention ALA–PDT: Topical 20% ALA and 10 J/cm2 of visible blue light (from the BLU-U illuminator, for 1000 s) were applied. Lesions that had not cleared were re-treated at 8 wk and all patients were re-evaluated at 12 wk. After 12-wk long-term lesion recurrence was based on complete chart review (including lesion photographs and treatment during most recent patient visits at wk 36–48) Comparator Not described |
Morbidity At wk 8, CR rate (100%), by no. of patients: ALA-018: 60/87 (69%) ALA–PDT vs 4/29 (14%) placebo ALA-019: 59/93 (63%) ALA–PDT vs 4/32 (13%) placebo At wk 8, > 75% clearance rate, by no. of patients: ALA-018: 68/87 (78%) ALA–PDT vs 6/29 (21%) placebo ALA-019: 71/93(76%) ALA–PDT vs 8/32 (25%) placebo When results for the two trials were pooled, the CR rate, for number of lesions, was: Lesion grade I: 666/756 (88%) ALA–PDT vs 122/302 (40%) placebo Lesion grade II: 495/632 (78%) ALA–PDT vs 52/199 (26%) placebo 34% of patients were re-treated at 8 wk At wk 12 CR rate was 129/180 (72%) in the ALA–PDT group vs 7/61(11%) in placebo treated patients. A response rate of least 75% was reported in 158/180 (88%) of ALA–PDT patients vs 12/61(20%) placebo-treated patients. The clearance rate was higher for facial lesions than scalp lesions At 4 yr after PDT, of 32 lesions in four patients (PDT group), 69% (22) remained cleared, 9% (3) were ‘recurrent’ and 22% (7) were ‘uncertain’. Further results were reported QoL and return to normal activity For ALA–PDT cosmetic response was rated as ‘good’ to ‘excellent’ by investigators in 92% of lesions; patients rated cosmetic response as ‘good’ to ‘excellent’ in 94% AK lesions; 85% of patients previously treated with 5-FU or cryotherapy indicated a preference for ALA–PDT for future management AEs Severe stinging and/or burning was reported by at least 50% of PDT patients; less than 3% stopped treatment. In 99% of PDT patients, some or all lesions were erythematous shortly after treatment vs 79% in the placebo group. In 35% of PDT patients some or all lesions were oedematous vs 0% in the placebo group. Both types of AE resolved or improved by 4 wk. More ALA patients also reported postPDT itching (26% vs 7%) Seven patients had an SAE – all were deemed remotely, or not related to, treatment |
Authors’ conclusions The excellent short- and long-term cosmetic results, low recurrence rate and high rate of patient and physician satisfaction associated with ALA–PDT indicate definite advantages over other existing treatment modalities for AK Brief study appraisal Few methodological details were reported, and details of the study population were unclear. The sources of information appeared contradictory in reporting that FU both ceased at 12 mth, and also continued for 4 yr. The 4-yr results were presented for just four patients and this, coupled with the lack of a clinically relevant comparison treatment, further questions the reliability of the authors’ conclusions. (Attempts were made to contact authors for further details.) |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Freeman (2003)45 Data source Full published paper Country Australia Language English Study design RCT No. of participants Total: 204 Intervention: 88 (360 AKs) (active PDT) Comparator: 23 (74 AKs) (placebo PDT) 2nd Comparator: 89 (421 AKs) (cryotherapy) No. of recruiting centres Nine Follow-up period and frequency FU of 3 mth (after a run-in period of up to 2 wk) |
Treatment intention Curative Type(s) of lesion and histology Mild to moderate non-pigmented AK Main eligibility criteria Patients with mild to moderate non-pigmented AK of the face or scalp suitable for cryotherapy (with the largest diameter of each lesion at least 5 mm) Patient characteristics % Male: 56 active PDT; 70 placebo PDT; 61 cryotherapy Mean age: 64 Age range: 33–89 yr Cancer stage: Grade I: 209 active PDT; 35 placebo PDT; 232 cryotherapy Grade II: 151 active PDT; 39 placebo PDT; 45 cryotherapy. All patients were Caucasian, most had Fitzpatrick skin type I or II Concomitant treatment Not stated |
Trial treatments MAL–PDT vs PDT with placebo cream vs conventional cryotherapy Intervention MAL–PDT: Scales and crusts were removed and lesion surface roughened with a curette. MAL cream (160 mg/g) was applied (1 mm thickness) under occlusion for 3 hr then PDT with red light (570–670 nm), intensities of 50–250 mW/cm2, total dose 75 J/cm2. 2 identical lamps (Curelight, Photocure ASA, Oslo) illuminated fields with maximum 5.5cm diameter, mean exposure time 10 min with a maximum of six treatment sites (mapped with acetate sheets and AKs marked) per patient. Anatomical landmarks and polaroid photography also used. This PDT procedure was repeated after 7 d Comparator PDT with placebo: As for MAL–PDT but with colour-matched cream base instead of MAL 2nd comparator Cryotherapy: AKs were outlined and lesions were frozen uniformly with a 1- to 2-mm rim. The locally accepted regimen was used, i.e. the protocol specified a single-timed freeze–thaw cycle with no exact freeze time (the time from formation of an ice ball to commencement of thawing). Lesions with a mean diameter less than 10 mm had a mean (SD) freeze time of 0.12 s (0.13); 10- to 20-mm lesions 0.16 s (0.15) and more than 20-mm lesions 0.26 s (0.11) |
Morbidity Lesion response rate was significantly higher (91%, 267/295) in the MAL–PDT group, than the placebo-PDT group (30%, 18/61) and the cryotherapy group (68%, 278/407), p < 0.001 for both. Response rates were higher in the thin lesions than the moderately thick lesions with MAL–PDT; in the cryotherapy group, response rates were higher for thicker lesions QoL and return to normal activity A significantly higher proportion of MAL–PDT patients graded overall cosmetic outcome as excellent than with cryotherapy patients (83% vs 51% as assessed by investigator, p < 0.001; 76% vs 56% as assessed by the patient, p = 0.013). Hypopigmentation was present in 5% MAL–PDT treated sites vs 29% cryotherapy sites. Hyperpigmentation, scar formation or tissue defects were present in less than 6% of total lesion sites. MAL–PDT patient satisfaction was rated better than previous treatment in 61%, equal in 24% and worse in 15%. Placebo-PDT patient satisfaction was rated better than previous treatment (cryotherapy, surgery or 5-FU) in 21%, equal in 14% and worse in 64% AEs No systemic AEs were reported. The most common AEs were local reactions (74%). 73% patients experienced at least 1 local AE after the 1st PDT session and 66% after the 2nd PDT session; 35% after cryotherapy; 30% after the 1st and 27% after the 2nd placebo PDT. Most of the AEs in the MAL–PDT group were mild (48%) or moderate (40%) intensity. Other reported AEs common with MAL–PDT were: burning sensation, stinging, painful skin (46%), erythema (23.9%), oedema (8.5%), skin peeling (5.1%), blisters (3.4%), itching (5.1%) and crusting (2.3%). Median duration was 1 wk or less (all events). 1 MAL–PDT patient discontinued due to the burning sensation |
Authors’ conclusions PDT with MAL–PDT is an excellent treatment option, particularly for patients with widespread damage or AK lesions in cosmetically sensitive areas Brief study appraisal Generally a well-conducted and reported study; although the conclusions appear likely to be reliable, it should be noted that cryotherapy was delivered using ‘locally accepted regimens’ allowing clinical variation between the nine centres |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Gupta (2004)35 Data source Abstract Country Canada Language English Study design RCT No. of participants Total: 50 Intervention: 25 Comparator: 25 No. of recruiting centres One Follow-up period and frequency FU at wk 4, 8, 12 and 26 |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Participants with 5–20 lesions of moderate to severe AKs were eligible Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments ALA–PDT vs 5-FU Intervention ALA–PDT: Incubation with ALA for 45–60 min before illumination with a Blue Light PDT Illuminator (400–450 nm). Treatment repeated at wk 8 if there was less than a 75% reduction in AKs. No further details reported Comparator 5-FU: Application of 5-FU to the face or scalp twice daily for 2–4 wk as tolerated |
Morbidity Not assessed QoL and return to normal activity Not assessed AEs At 1 wk FU ALA–PDT patients showed few signs of irritation (erythema, scaling and crusting); 5-FU patients exhibited moderate to severe erythema |
Authors’ conclusions If short-duration ALA–PDT is shown to be as effective as 5% 5-FU at wk 12 and 26 then it may be a suitable treatment alternative for subjects with multiple moderate to severe AKs Brief study appraisal As there were few details available in the abstract, the efficacy of the treatments was not described and the study is not yet complete, the reliability of the author’s conclusion is unclear. (Attempts were made to contact the study author.) |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Hauschild et al. (2008),60 Trial AK03 Linked publications166 Data source Full published paper Country Germany Language English Study design RCT No. of participants Total: 103 (587 lesions) Intervention: 69 Comparator: 34 No. of recruiting centres 29 (unclear whether this was for each trial) Follow-up period and frequency FU after 12 wk |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Caucasian males and females (at least 18 yr old) with skin types I–IV were eligible for inclusion. AK lesions had to be mild to moderate (Cockrell definition) with a maximum diameter of 1.8 cm and interlesional distance of at least 1 cm. The following were excluded: women of child-bearing potential, non-responders to previous PDT, patients with particular dermatological conditions, porphyria, dementia or clinically relevant immunosuppression, topical treatment that may affect response 4 wk before and during the study (various criteria), treatment with cytostatics or radiation 3 mth prior to/during study, and intolerance to ingredients of ALA Patient characteristics % Male: 82 Mean Age: ALA 70.4; Placebo 71.4 Age Range: 51–89 yr Skin Type: I 9%; II 83%; III 1% Concomitant treatment Not stated |
Trial treatments ALA–PDT patch vs placebo PDT patch Intervention ALA–PDT: 3–6 patches (4 cm2) containing 8 mg of 5-ALA was applied to lesions without preparation. One patch per lesion. After 4 h, illumination with red light (37 J/cm2, 630 ± 3nm) with an LED source. Patients were instructed to protect lesions from light for 48 h after therapy Comparator Placebo-PDT: As for ALA–PDT but with placebo on the patches |
Morbidity CCCR (lesions) was 82% (316/384) for ALA–PDT vs 19% (34/179) for placebo, p < 0.0001. Corresponding clearance rates on a patient basis were 62% (41/66) vs 6% (2/33), p < 0.0001 QoL and return to normal activity There was no difference between PDT or placebo in the cosmetic assessment of ‘cleared lesions’ (patient assessment p = 0.35; investigator assessment 0.54). Pigmentation status classed as ‘normal’ in the ALA–PDT groups were 91% vs 12%. There was no statistical difference from those treated with placebo-PDT (p = 0.95). 95% of ALA–PDT patients were very satisfied or satisfied with the overall cosmetic outcome vs 44% with placebo-PDT. Patient satisfaction with overall outcome was greater with PDT (p < 0.0001) AEs One AE was described as relating to therapy (transient discoloration of the skin with ALA) Transient skin discoloration in one patient was related to ALA treatment. ALA patients had more overall local reactions when treatment was applied (mostly itching, 42% vs 13%; the 13% placebo figure appears to be pooled from the 2 trials) |
Authors’ conclusions ALA–PDT is an easy to handle one-step procedure for therapy of isolated mild to moderate AK lesions. Compared with current PDT procedures, pre-treatment (e.g. curettage) is not needed and handling is considerably facilitated. ALA–PDT leads to efficacy rates superior to placebo Brief study appraisal This study appeared to be generally well conducted; however, it was unclear how many centres were used and the reporting of results was sometimes unclear |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Hauschild et al. (2008),60 Trial AK04 Linked publications166 Data source Full published paper Country Germany Language English Study design RCT No. of participants Total: 349 (1950 lesions) Intervention: 148 Comparator: 149 (cryosurgery) 2nd Comparator: 49 (placebo) No. of recruiting centres 29 (unclear whether this was for each trial) Follow-up period and frequency FU after 12 wk |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Caucasian males and females (at least 18 yr old) with skin types I–IV were eligible for inclusion. AK lesions had to be mild to moderate (Cockrell definition) with a maximum diameter of 1.8 cm and interlesional distance of at least 1 cm. The following were excluded: women of child-bearing potential, non-responders to previous PDT, patients with particular dermatological conditions, porphyria, dementia or clinically relevant immunosuppression, topical treatment that may affect response 4 wk before and during the study (various criteria), treatment with cytostatics or radiation 3 mth prior to/during study and intolerance to ingredients of placebo or known reactions to cryotherapy Patient characteristics % Male: 72 Mean age: PDT 70, cryosurgery 71, placebo 72 Age range: 41–94 yr Skin type: I 18%, II 66%, III 15%, IV 1% Concomitant treatment Not stated |
Trial treatments ALA–PDT patch vs cryosurgery vs Placebo PDT patch Intervention ALA–PDT patch: 4–8 patches (4 cm2) containing 8 mg of 5-ALA were applied to lesions without preparation, one patch per lesion. After 4 hr, illumination with red light (37 J/cm2, 630 ± 3 nm) with Omnilux (11 centres). Patients were instructed to protect lesions from light for 48 hr after therapy Comparator cryosurgery: A standardised protocol was used. Open spraying procedure with liquid nitrogen in 1 cycle was used (with size C nozzles) and freeze time (of 5–10 s) started after ice ball formation 2nd comparator Placebo PDT patch: As for ALA–PDT but with placebo on the patches |
Morbidity The complete clinical clearance rates (lesions) were 89% (664/750) for ALA–PDT, 77% (530/692) for cryosurgery and 29% (75/259) for placebo. PDT was significantly better than placebo PDT (p < 0.001) and cryosurgery (p = 0.007). Clearance rates (patients) were ALA–PDT 67% (86/129), cryosurgery 52% (66/126) and placebo 12% (5/43). PDT was significantly better than placebo and cryosurgery (p < 0.001 for both) QoL and return to normal activity Patients’ and investigators’ assessment of cosmetic outcome of cleared lesions was significantly better for ALA–PDT than cryosurgery (p < 0.001). 95% of ALA–PDT patients were very satisfied or satisfied with the overall cosmetic outcome vs 82% with cryosurgery. It appears to be reported that ALA patients were significantly more satisfied than placebo and cryosurgery (p < 0.0001). Pigmentation status classed as ‘normal’ in the ALA–PDT groups was 88%. Hypopigmentation was seen in 33% of lesions with cryosurgery. Hyperpigmentation was seen in 9% PDT patients vs 4% placebo. Pigmentation status was significantly different between ALA–PDT and cryosurgery (p < 0.001) but the difference between ALA–PDT and placebo was not significant (p = 0.87) AEs Three per cent in the ALA and cryosurgery arms and 2% placebo reported an AE related to therapy. 99% of ALA patients experienced an adverse reaction at some stage of treatment (placebo data was pooled with trial AK03) |
Authors’ conclusions ALA–PDT is an easy to handle one-step procedure for therapy of isolated mild to moderate AK lesions. Compared with current PDT procedures, pre-treatment is not needed and handling is considerably facilitated. A single PDT treatment results in efficacy rates being statistically significantly superior to placebo and cryosurgery Brief study appraisal The study appears to be generally well conducted but reporting of some of the methodology and results was unclear |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Hauschild et al. (2008)55 Data source Full published paper Country Germany Language English Study design RCT No. of participants Total: 149: 146 patients completed study, of which 140 (520 lesions) were in the per protocol population Intervention: 34 (128 lesions) 0.5-hr incubation Comparator: 38 (138 lesions) 1-hr incubation 2nd Comparator: 34 (124 lesions) 2-hr incubation 3rd Comparator: 34 (130 lesions) 4-hr incubation No. of recruiting centres 12 Follow-up period and frequency 4 and 8 wk after treatment |
Treatment intention Curative Type(s) of lesion and histology AK (mild to moderate) Main eligibility criteria Caucasian males or females aged at least 18 yr with histologically confirmed mild to moderate AK. Lesions were required to have maximum diameter of 1.8 cm and interlesional distance of at least 1 cm. Patients with dermatological conditions likely to impact on results were excluded. Further eligibility criteria were reported Patient characteristics % Male: 74 Age range: 39–91 yr across groups Mean age: 72 yr, 72 yr, 70 yr, 70 yr, respectively, for the four treatment groups Most lesions were located on the scalp or forehead in all groups, severity was fairly evenly split between mild and moderate. Mean lesion diameter was similar across groups at around 8–9 cm Concomitant treatment Any other topical treatment able to affect AK not permitted 4 wk prior to and during study. No urea and salicylic acid-containing preparations permitted 2 wk prior and during study |
Trial treatments Patch containing ALA (PD P 506 A) applied to lesions for 0.5, 1, 2 or 4 hr followed by illumination with red light Intervention PDT patch + 0.5-hr incubation: Between three and four lesions per patient were treated using a PDT patch (one patch per lesion) containing 8 mg of ALA. Patch is lightproof therefore provides occlusive protection. After patch was removed, lesion illuminated with red light (dose 37 J/cm2, wavelength around 630 nm). Further PDT parameters were not reported Comparator PDT patch + 1-hr incubation: see above 2nd comparator PDT patch + 2-hr incubation: see above 3rd comparator PDT patch + 4-hr incubation: see above |
Morbidity The majority of lesions showed clearance 8 wk after PDT and the 4-hr incubation group showed the best response – estimated 86% clearance rate and this was ‘statistically selected as the best treatment’. All but 1 of the 12 centres found similar results (details not reported). In some patients, lesions that appeared ‘cleared’ at wk 4 then worsened by wk 8 – this effect was most apparent in the 0.5-hr group, and was not present in the 4-hr group QoL and return to normal activity Not assessed AEs Five patients reported AE considered to be related to the study treatment: headache, moderate epistaxis and mild increase of alanine transaminase. Local reactions during application and incubation included burning, pruritis and erythema – all but one case was rated as mild or moderate. Local reactions during illumination appeared to be dose dependent and ranged from 26% in the 0.5-hr group to 66% in the 4-hr group. Most frequent reactions were pain, burning and pruritis. One patient’s treatment was interrupted due to severe pain. Almost all patients had local reactions after treatment, with erythema, scabbing, desquamation, burning and pruritis being common. Patients with clearance experienced local reactions to a greater extent than patients without clearance |
Authors’ conclusions PD P 506 A-PDT patches are suitable for the treatment of up to eight AK lesions of mild to moderate intensity on the head and face, and 4-hr application results in excellent outcomes. Further Phase III trials are required to confirm these outcomes Brief study appraisal This study was relatively poorly reported making it difficult to assess the reliability of the methodology or results. The use of multiple centres in a small trial raises the possibility of centre-effects on treatment and outcomes. Patients were only followed up for 8 wk. No statistical test results were reported; therefore it is difficult to be confident in the conclusions of efficacy |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Jeffes et al. (1998)41 Data source Abstract Country USA Language English Study design RCT No. of participants Total: 36 Intervention: 36 Comparator: 36 No. of recruiting centres Not stated multicentre Follow-up period and frequency 8 and 16 wk |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Patients with four AKs on the face and scalp Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments ALA–PDT vs PDT with placebo (within-participant comparison) Intervention ALA–PDT: Two AKs were treated with 20% ALA solution (Levulan) and after 14- to 18-hr exposed to blue (non-laser) light at doses of 2, 5 and 10 J/cm2. Patients were re-treated at 8 wk if necessary. Further PDT parameters were not reported Comparator Placebo PDT: As above except placebo control was used in place of ALA solution |
Morbidity At 8 wk, 66% of AKs treated with ALA had a CR vs 17% with placebo (p < 0.001). At 16 wk. CR was seen in 56/66 (85%) of ALA–PDT patients (no results reported for placebo). AKs treated with ALA–PDT at 5 or 10 J/cm2 of light resulted in a significantly better response than the corresponding placebo group, although there was no significant difference between the groups at 2 J/cm2 QoL and return to normal activity Not assessed AEs The authors reported that hyperpigmentation was seen in 11% of AKs treated with ALA–PDT, which was not different from the placebo group. The treatment was well tolerated and no patients withdrew due to an AE |
Authors’ conclusions The authors did not report any conclusions Brief study appraisal Little useful evidence could be retrieved from this abstract which provided very few methodological or result details, and involved a fairly small sample followed up for 16 wk |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Kaufmann et al. (2008)46 Data source Full published paper Countries Australia, Belgium, Germany, UK Language English Study design RCT No. of participants Total: 121 (1343 lesions) Intervention: 121 (691 lesions) Comparator: 121 (652 lesions) No. of recruiting centres 24 Follow-up period and frequency FU at wk 12 and 24. Additional telephone calls were made at wk 1 and 13 when patients were re-treated |
Treatment intention Curative Type(s) of lesion and histology Non-hyperkeratotic AK Main eligibility criteria Males and non-pregnant women aged 18 or over, with a clinical diagnosis of non-hyperkeratotic AK, of mild or moderate thickness, on locations other than the face or scalp, were eligible for inclusion. Patients had to have at least four comparable symmetrical AKs, of similar severity and total number on both sides of the body. Further eligibility criteria were reported Patient characteristics % Male: 65 Age range: 38–89 yr Mean age: 68.9 yr Cancer stage: Grade I, 687; grade II, 656 Patients had (a mean of) six lesions per side. Further patient characteristics were reported Concomitant treatment Not stated |
Trial treatments MAL–PDT vs cryotherapy (within-participant comparison) Intervention MAL–PDT: After scraping of lesions, a 1-mm layer of 160-mg/g MAL cream was applied to each lesion (including 5 mm of surrounding tissue) for 3 hr (under occlusion). After saline cleansing, a standard LED lamp illuminated lesions with narrow band red light (average 630 nm, dose 37 J/cm2, mean time 8 min 36 s). Lesions with a non-CR were re-treated after 12 wk Comparator Cryotherapy: Double freeze–thaw cryotherapy using liquid nitrogen spray applied with a 1- to 2-mm frozen rim outside the lesion outline. Timing of freeze–thaw application was as per usual practice of each centre (mean time 20 s ± 14 s) |
Morbidity At wk 24 the mean reduction in lesion count from baseline was 78% for MAL–PDT and 88% for cryotherapy (per-protocol population) (p = 0.002), 95% CI of the bilateral difference (MAL–PDT/cryotherapy) was between –16.6% and 3.9%. ITT (last observation carried forward) analysis confirmed this (75% reduction with MAL–PDT vs 87% with cryotherapy, p < 0.001). 76% (455) of lesions were cured with MAL–PDT vs 88% (490) with cryotherapy. The difference was similar for mild- and moderate-thickness lesions QoL and return to normal activity Investigator-assessed cosmetic outcome was significantly better for MAL–PDT than cryotherapy (p < 0.001). In the MAL–PDT group, 79% of lesions had an excellent cosmetic outcome, 19% good, 3% fair and 0% poor (compared with 56% excellent, 36% good, 8% fair and 0.9% poor with cryotherapy). After 24 wk, 50% of patients preferred MAL–PDT in terms of cosmetic outcome compared with 22% for cryotherapy (p < 0.001). 28% had no preference (ITT analysis). Patients preferred MAL–PDT to cryotherapy for all questions in the patient questionnaire (between 12% and 58% of difference). The differences were marked apart from effectiveness of treatment (39% favoured MAL–PDT vs 26% cryotherapy, not significant). Patients preferred MAL–PDT in terms of comfort (60% vs 10%, p < 0.001), procedure (49% vs 28%, p = 0.05) and healing (64% vs 6%, p < 0.001). Overall patient satisfaction favoured MAL–PDT (49% vs 20%, p < 0.001). If re-treatment was required 59% would prefer MAL–PDT over cryotherapy (25%, p < 0.001) AEs There were 63% patients with 99 AEs with cryotherapy vs 45% patients with 67 AEs with MAL–PDT. Most were dermatological and related to treatment. The most commonly reported AE for MAL–PDT was photosensitivity reaction (43% of patients with 63 AEs) and cold exposure injury for cryotherapy (62% patients with 95 AEs). Most were of mild intensity. Two patients in the cryotherapy group reported severe cold exposure injury |
Authors’ conclusions MAL–PDT showed inferior efficacy for treatment of non-face/scalp AK compared with cryotherapy. However, both treatments showed high efficacy, and MAL–PDT conveyed the advantages of better cosmesis and higher patient preference Brief study appraisal The study was quite well conducted, but it was open in design and therefore there was potential for investigator/patient bias. The possibility of institutional differences and/or protocol deviation (24 centres in four countries) affecting the reliability of results was illustrated by the wide variation of freeze–thaw timings used for cryotherapy |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Kurwa et al. (1999)47 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 17 Intervention: 17 Comparator: 17 No. of recruiting centres Not stated Follow-up period and frequency 1 wk, 4 wk and 6 mth |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria It appeared that patients with a long history of AKs affecting the forearms and hands were eligible for inclusion Patient characteristics % Male: 47 Age range: 53–79 yr Concomitant treatment Not stated |
Trial treatments ALA–PDT vs 5-FU (within-participant comparison) Intervention ALA–PDT: Thick surface scale was removed if present then 20% 5-ALA was applied topically and covered in a light-impermeable dressing for 4 hr. PDT was administered with a halogen lamp (580–740 nm, 150 J/cm2, mean fluence rate 80 mW/cm2) Comparator 5-FU cream (5%) was applied topically twice a day to one hand for 3 wk by thorough massage |
Morbidity The mean lesional area before treatment for PDT was 1322 mm2 and 6 mth after treatment was 291 mm2 (a reduction of 73%, 95% CI 61% to 84%). For 5-FU, mean lesional area was 1390 mm2 before treatment and 297mm2 after (a reduction of 70%, 95% CI 61% to 80%). There was no statistically significant difference in reduction of lesional area between PDT and 5-FU at 6 mth (p = 0.72). No patients were completely cleared of AKs with either treatment QoL and return to normal activity Not assessed AEs All patients experienced mild to moderate pain at PDT sites. In wk 1, PDT sites were significantly more painful than 5-FU sites, but this difference was absent in wk 2 and reversed in wk 4. There was no significant difference between treatments overall over the 4-wk period. Daily symptom diaries were completed by 11 patients. In wk 1, PDT sites were significantly more erythematous than 5-FU sites but this difference was absent in wk 2 and reversed in wk 3 and 4. There was no significant difference between treatments overall over the 4-wk period. There was no blistering, ulceration, scarring or photosensitivity reaction after either treatment method. One patient experienced contact sensitivity to 5-FU |
Authors’ conclusions One treatment with PDT using topical 5-ALA appears to be as effective and well tolerated as 3 wk of twice-daily topical 5-FU, a cheap and widely available alternative Appraisal This study appeared to be too small to detect significant treatment effects. The methods and results were not clearly reported and measures to reduce bias (e.g. randomisation, blinding and allocation concealment) were not reported at all so the reliability of the conclusions is questionable |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Legat et al. (2006)36 Data source Abstract Country Austria Language English Study design RCT No. of participants Total: 22 (mean number AKs 47, range 17–89) Intervention: 22 (no. of AK not reported) Comparator: 22 (no. of AK not reported) 2nd Comparator: six (no. of AK not reported) No. of recruiting centres Not stated Follow-up period and frequency FU at 4, 12 and 24 wk |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Not stated Patient characteristics % Male: 100 Age range: 59–84 yr Median age: 75 yr Patients had multiple AKs Concomitant treatment Not stated |
Trial treatments PDT with fractionated illumination vs PDT with unfractionated illumination. Within-participant comparison, but six patients had PDT with fractionated illumination vs alternative fractionated illumination, due to severe pain after 1st fractionated dose Intervention PDT with unfractionated illumination: Following application of MAL cream for 3 hr, red light illumination (peak emission 635 nm) of a single dose of 37 J/cm2 was applied Comparator PDT with fractionated illumination: As for PDT with unfractionated illumination except PDT was given in two doses of 18.5 J/cm2 divided by a dark interval of 15 min 2nd comparator PDT with alternative fractionated illumination: As for PDT with unfractionated illumination except with three doses of 12.3 J/cm2, with two dark intervals of 5 min |
Morbidity The mean number of AK at wk 4, 12 and 24 was reduced by 65, 60 and 48% with unfractionated PDT and 56, 53, and 50% with fractionated PDT respectively (difference not significant, n = 14). Similar results seen for alternative fractionated PDT group QoL and return to normal activity Not assessed AEs PDT induced pain (VAS score) was 6.7 (SE 0.5) for unfractionated PDT and 6.0 (0.5) for fractionated PDT (n = 14, p = 0.02). There was no significant difference in pain between fractionated and alternative fractionated patients [8.0 (0.7) vs 8.2 (0.3) respectively] |
Authors’ conclusions PDT with fractionated and unfractionated illumination were similarly effective in reducing AKs. However, pain sensation during PDT was significantly less intense with standard fractionated than unfractionated illumination Brief study appraisal Few methodological details were provided in the abstract and the results of this small study may not be generalisable |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Moloney et al. (2007)37 Data source Abstract Country Not stated Language English Study design RCT No. of participants Total: 16 Intervention: 16 Comparator: 16 No. of recruiting centres Not stated Follow-up period and frequency 1 mth |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Not stated Patient characteristics % Male: 100 Age range: 59–87 yr All patients had AKs on the scalp Concomitant treatment Not stated |
Trial treatments MAL–PDT vs ALA–PDT Intervention MAL–PDT: MAL cream was applied for 3 hr. Further PDT parameters were not reported Comparator ALA–PDT: 20% ALA cream was applied for 5 hr. Further PDT parameters were not reported |
Morbidity AK counts reduced by 5.6 ± 3.2 (MAL) vs 6.2 ± 1.9 (ALA) (p = 0.588) (n = 15) QoL and return to normal activity Not assessed AEs All patients (n = 15) experienced pain, which was of greater intensity on the ALA treated side at all time points: 3 min, p = 0.151; 6 min, p = 0.085; 12 min, p = 0.012; 16 min, p = 0.029. There was also longer duration of discomfort post treatment with ALA (p = 0.044) |
Authors’ conclusions Both ALA and MAL–PDT result in significant reduction in scalp AKs. There is no significant difference in efficacy. However, ALA is more painful than MAL–PDT in the treatment of extensive scalp AKs Brief study appraisal The abstract provided few methodological details and involved a small sample, followed up for only 1 mth, so the reliability of the conclusions is unclear |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Morton et al. (2006)48 Data source Full published paper Countries Ireland, UK Language English Study design RCT No. of participants Total: 119 (1501 lesions) Intervention: 119 (758 lesions) Comparator: 119 (743 lesions) No. of recruiting centres 25 Follow-up period and frequency FU at wk 12 and 24. Additional telephone FU was performed at wk 1 and 13 |
Treatment intention Curative Type(s) of lesion and histology Non-hyperkeratotic AK Main eligibility criteria Males and females over 18 yr (16 yr in Scotland) with clinical diagnosis of (at least three) non-hyperkeratotic AK on the face and/or scalp (of similar severity and number on both sides) were eligible for inclusion. Patients that received topical treatment within the previous 3 mth, regular UV therapy, patients with thick or pigmented lesions or porphyria were excluded Patient characteristics % Male: 91 Age range: 54–93 yr Mean age: 75 yr Mean no. of lesions per side: six Median lesion diameter: 7 mm Further patient characteristics were reported Concomitant treatment Not stated |
Trial treatments MAL–PDT vs cryotherapy (within-participant comparison) Intervention MAL–PDT: After scraping of lesions, a 1-mm layer of MAL 160 mg/g cream was applied (including 5 mm of surrounding tissue) for 3 hr (under occlusion). Following saline cleansing, lesions were illuminated with narrowband light (approximately 630 nm, dose 37 J/cm2) using a standard LED light source. Mean illumination time was 8 min 41 s. Lesions with a non-CR were re-treated at 12 wk Comparator Double freeze–thaw cryotherapy: Liquid nitrogen spray applied to achieve a 1- to 2-mm frozen rim around the marked outline of the lesion. Mean freezing time was 16 s (± 7 s) |
Morbidity At wk 12 lesion reduction with MAL–PDT was 87% vs 76% (p < 0.001). Reduction in lesion count at wk 24 was 89% with MAL–PDT vs 86% with cryotherapy (p = 0.2, n = 108). At wk 12 CR was 83% with MAL–PDT vs 72% with cryotherapy. At wk 24 it was 86% (650/758) with MAL–PDT vs 83% (613/743) with cryotherapy. 21% of lesions with CR at wk 24 had needed re-treatment with cryotherapy at wk 12 vs 10% with MAL–PDT, results were independent of initial severity grade or location (ITT population) QoL and return to normal activity Overall participant preference (i.e. cosmetic outcome, efficacy, and skin discomfort) was 49% for MAL–PDT vs 21% for cryotherapy (p < 0.001, ITT analysis). Results reported for per-protocol population were 45% vs 10%, p < 0.001. Investigator preference for cosmetic outcome was 43% for MAL–PDT vs 12% for cryotherapy, p < 0.001, and for overall preference was 52% vs 16%, p < 0.001. Most subjects were ‘satisfied’ to ‘very satisfied’ with MAL–PDT (compared with cryotherapy and previous other AK treatments) for 7/11 questions of a satisfaction questionnaire. More than 90% were satisfied with MAL–PDT in terms of effectiveness of treatment, scarring, skin colour and appearance at 1- and 3-mth FU. Cryotherapy was preferred for time taken over treatment (92%) compared to 78% for MAL–PDT. 65% would prefer to be re-treated with MAL–PDT vs 32% with cryotherapy (n = 108) AEs Skin discomfort VAS scores (mean) were 5.2 with MAL–PDT vs 4.9 with cryotherapy (p = 0.24) after 1st treatment. For re-treated lesions, mean VAS scores were 3.7 vs 4.4. Patients preferred cryotherapy in terms of skin discomfort after 1st treatment (45% vs 33%, no preference 22%, p = 0.07), but there was no difference for re-treated lesions. Skin-related AEs were reported by 62% MAL–PDT patients vs 72% for cryotherapy. There was one discontinuation with MAL–PDT due to a local reaction. Most skin-related AEs were mild to moderate and transient |
Authors’ conclusions When treated with both MAL–PDT and cryotherapy, patients significantly prefer MAL–PDT treatment for AK. MAL–PDT is an attractive treatment option for AK, with comparable efficacy and superior cosmetic outcomes compared with double freeze–thaw cryotherapy Brief study appraisal This study was generally well conducted and reported; however, it was an open-label trial, which can lead to bias in favour of a particular treatment. The possibility of institutional differences and/or protocol deviation (25 centres in two countries) affecting the reliability of results was illustrated by the variation of freezing times used for cryotherapy |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Pariser et al. (2003)49 Data source Full published paper Country USA Language English Study design RCT No. of participants Total: 80 (502 lesions) Intervention 42 (260 lesion) Comparator: 38 (242 lesions) No. of recruiting centres Five Follow-up period and frequency FU was at 3 mth. AEs were assessed during, immediately after PDT, at wk 2 and 3 mth after the 2nd PDT treatment |
Treatment intention Curative Type(s) of lesion and histology Mild and moderate AK Main eligibility criteria Males and females over 18 yr with 4–10 previously untreated mild to moderate non-pigmented AK on the face and scalp (at least 3 mm diameter) were eligible for inclusion. Further eligibility criteria were reported Patient characteristics % Male: 88 Age range: 31–84 yr Mean age: MAL–PDT 64; placebo PDT 67 The majority of lesions were mild and located on the face. Most patients were Fitzpatrick skin type I or II Concomitant treatment Not stated |
Trial treatments MAL–PDT vs PDT with placebo cream Intervention MAL–PDT: Scales and crusts were removed using a curette MAL cream (160 mg/g) was applied (1-mm thickness and 5 mm around lesion) for mean 3 hr under occlusion. Cream was washed off using a 0.9% saline solution, then a non-coherent red light was applied: 570–670 nm, dose 75 J/cm2, mean intensity 155 mW/cm2 (range 50–200 mW/cm2). Treatment was repeated after 1 wk Comparator PDT with placebo cream: As for MAL–PDT but with placebo cream |
Morbidity For the MAL–PDT group, patient response was 32/39 (82%) vs 8/38(21%) in placebo, treatment difference –61% (p = 0.001) For the MAL–PDT group lesion response rate was 209/236 (89%) vs 92/241 (38%) for placebo. Response rate was similar for mild and moderate lesions in the MAL group (90% and 84%, respectively); placebo response was higher in the mild lesions (44% and 25%) QoL and return to normal activity Investigator assessed cosmetic outcome in the MAL–PDT group was ‘excellent’ or ‘good’ in 31/32 (97%) patients and when assessed by patients this was 29/32 (91%). The outcome was not rated ‘poor’ by either investigator or patient. 73% of 32 patients preferred MAL–PDT to previous treatments (5-FU, cryotherapy, surgery) AEs 38 (90%) MAL–PDT patients had an AE vs 22 (58%) in placebo. One MAL–PDT patient discontinued due to AE. Common local AEs were: burning sensation of the skin (27 MAL patients vs 4); erythema (22 vs 8); crusting (16 vs 6); pain on the skin (10 vs 0); blisters (8 vs 2); skin oedema (6 vs 1); stinging skin (6 vs 1) and skin ulceration (5 vs 0). More details in paper |
Authors’ conclusions PDT using topical MAL was a safe and effective treatment for AK with excellent cosmetic outcome. It is a promising treatment that could benefit from further study Brief study appraisal This study appeared to be generally well conducted |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Pariser et al. (2008)56 Linked publications169 Data source Full published paper Country USA Language English Study design RCT No. of participants Total: 100 (723 lesions) (four patients were treated as ‘training’ population) Intervention: 49 (363 lesions) Comparator: 47 (360 lesions) No. of recruiting centres Eight Follow-up period and frequency FU 3 mth after last treatment |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Previously untreated males and non-pregnant/non-lactating females with adequate contraception during and 1 mth after treatment that were at least 18 yr old with 4–10 non-pigmented, non-hyperkeratotic grade I or II lesions on the face and scalp (at least 3-mm diameter) were eligible for inclusion. Exclusion criteria were extensive but included: immunosuppression, porphyria, allergy to MAL or similar, allergy to nut products or protein antigens, regular UV therapy or treatment of face and scalp with local therapy in previous 30 d, topical therapy in previous 3 mth Patient characteristics % Male: 82 Mean age: MAL–PDT 66.1; Placebo PDT 66.7 Age range: 43–89 yr Skin type: I 23%; II 50%; III/IV 27% Grade of lesions: Grade I 73%; grade II 27% The majority of lesions were thin (grade I) and located on the face. About 50% of patients had between 8 and 10 lesions in total Concomitant treatment Not stated |
Trial treatments MAL–PDT vs Placebo PDT Intervention MAL–PDT: Preparation of lesions by removal of scales and crusts by dermal curette, then application of 1-mm-thick MAL cream to lesion and surrounding 5 mm skin. Occlusive dressing applied for at least 3 hr (permitted range 2.5–4 hr) and avoidance of exposure to sunlight, bright indoor light and extreme cold. Area then illuminated (average duration, 8 min) with red LED (total dose 37 J/cm2, 5–8 cm from skin). This procedure was repeated after 1 wk Comparator Placebo PDT: As for MAL–PDT but with placebo cream |
Morbidity Lesion CR rate was 86% for MAL–PDT vs 52% for placebo, OR 6.9 (95% CI 4.7 to 10.3, p < 0.0001). Patient CR rate was 59% (29/49) for MAL–PDT vs 15% (7/47) for placebo, OR 13.2 (95% CI 4.1 to 43.1, p < 0.0001). 3 mth after treatment 31% (15/49) MAL–PDT patients had 42 new lesions vs 26% (12/47) placebo-PDT patients with 34 new lesions. Four MAL–PDT patients had at least five new lesions vs two placebo. In the MAL–PDT group 67% of new lesions were on the face vs 50% placebo (p = 0.16), no difference between location of new lesions (p = 0.16) QoL and return to normal activity Not assessed AEs Any AE/any local AE was reported by 98% (52/53) in the MAL–PDT group. 45% (22/47) reported any AE in placebo group, 45% (21/47) reported any local AE. In the MAL group 32% were mild (vs 38%), 49% were moderate (vs 6%) and 17% were severe (vs 0%). Commonly reported local AEs for the MAL–PDT (n = 53) (vs placebo, n = 47) patients were: erythema (77 vs 15%), skin burning sensation (72 vs 11%), pain of skin (60 vs 21%), pruritis (23 vs 11%), skin oedema (28 vs 2%), scab (26 vs 0%), skin discomfort (23 vs 2%), blister (15 vs 0%) and skin exfoliation (11 vs 4%) |
Authors’ conclusions Given that MAL–PDT has proved excellent cosmetic outcomes, superior to conventional therapy, this suggests a role for MAL–PDT using a red LED light source in patients with multiple (up to eight) AK lesions Brief study appraisal This study appeared to be generally well conducted and explored centre-effects on the results, although further details of the PDT light treatment would have been useful |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Puizina-Ivic et al. (2008)57 Data source Full published paper Country Croatia Language English Study design RCT No. of participants Total: 36 Intervention: Fractionated illumination: 16 Comparator: Single illumination: 20 No. of recruiting centres One Follow-up period and frequency 24 wk |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Previous histologically confirmed diagnosis of AK Patient characteristics Not stated |
Trial treatments ALA–PDT with 16-hr incubation and two light fractions vs ALA–PDT with 5-hr incubation and a single illumination Intervention ALA–PDT with 16-hr incubation and two light fractions: Thick crusts were 1st removed with ointments (and wet dressings). After cleaning the area with a saline solution, the 20% ALA cream was applied to a thickness of approximately 1 mm, covering the treated area, and 1 cm of the surrounding skin. The area was covered by occlusive dressing. Aluminium foil was placed on top in order to protect skin from ambient light. There was then a 16-hr incubation period before red light (635 nm) was applied. The total of 100 J/cm2 was delivered in 2 doses of 50 J/cm2 with a fluence intensity of 30 mW/cm2. There was a 2-hr break between illuminations. Spraying of water and cooling with fan was carried out to minimise pain sensations. After the treatments sunblock ointments were recommended for the next few days in addition to sun protection measures. Biopsies were performed 24 wk after illumination in patients with fluorescence detected after 4 hr Comparator PDT with 5-hr incubation with ALA cream and single light fraction: Preparation for illumination was as for the intervention group. There was then a 5-hr incubation period before red light (635 nm) was applied. 100 J/cm2 was delivered in 1 dose, with a fluence intensity of 30 mW/cm2. Spraying of water and cooling with fans, and sun protection measures were as for the intervention group. Biopsies were performed 24 wk after illumination in patients whom fluorescence after 3-hr incubation with ALA was detected |
Morbidity At 24 wk, residual tumour was found in 15 of 20 (75%) biopsied patients in the single illumination, shorter-incubation group. Treatment was repeated. At 24 wk, there was persistence of tumour in 2 of 16 (13%) biopsied patients in the fractionated, longer-incubation group QoL and return to normal activity Not assessed AEs Not assessed |
Authors’ conclusions PDT delivered as fractionated illumination with 16 hr of incubation separated by a 2-hr dark interval significantly improves therapeutic outcome in tumour eradication Brief study appraisal This trial had only a small number of patients. Procedures of randomisation and blinding of outcome assessors were unclear. No patient details were provided. Although the group receiving fractionated illumination had better outcomes, the relative contribution of the longer incubation time and the fractionated delivery are unclear. It is also unclear if there were any AEs |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Sotiriou et al. (2009)61 Data source Full published paper Country Greece Language English Study design RCT No. of participants Total 30 (256 lesions) Intervention 30 (133 lesions) Comparator 30 (123 lesions) No. of recruiting centres Not stated Follow-up period and frequency Followed up at 1 and 6 mth after treatment; if given a 2nd treatment then cycle final FU was at 6 mth after last treatment |
Treatment intention Curative Type(s) of cancer and histology Non-hyperkeratotic AK Main eligibility criteria Clinical diagnosis of non-hyperkeratotic grade I (mild) and grade II (moderate) AK on dorsa of hands and forearms. Each patient required to have at least three lesions of comparable severity on each side of body (total of six lesions minimum). Exclusion criteria were any other dermatological diseases or conditions in treatment area or within 3 cm, topical treatments for AK within previous 2 mth, and any invasive tumours in the treatment area Patient characteristics % Male: 83 Mean age: 64 yr Age range: 49–79 yr Severity of lesions: Grade I: 54% (PDT), 54% (Imiquimod); grade II: 46% (PDT), 46% (Imiquimod) Concomitant treatment None |
Trial treatments ALA–PDT vs Imiquimod 5% intraindividual right/left comparison Intervention ALA–PDT: lesions prepared by removing crusts and curettage, where lesions were not prepared these were all grade I. 20% 5-ALA cream applied to lesions and 5-mm surrounding skin and left for 4 hr. Illumination immediately following removal of dressing using non-coherent red light source, light dose 75 J/cm2, and fluence rate of 75 mW/cm2. Patients given two PDT sessions on same day, 1st session targeting the hand and lower forearm, 2nd treating upper two-thirds of the forearm. Two complete PDT treatments were delivered to all areas 15 d apart. Pain management during treatment included use of a fan and/or cooling sprays Comparator Imiquimod: treatment based on approved dosage regime for the head. Patients applied 500 mg of imiquimod 5% cream daily for 3 d/wk prior to sleep, cream left on skin for at least 8 hr. This treatment continued for 4 wk (course 1). Following a 4-wk post-treatment period for observation, any patients with lesions remaining repeated the process for a further 4 wk (course 2) |
Morbidity 1 mth: PDT CR rates were significantly better overall and for grade I and grade II lesions. CR for PDT 70% (87/124) and 18% (21/115) for imiquimod (p < 0.05). Grade I lesion CR was 75% for PDT (50/67) and 34% (21/61) for imiquimod (p < 0.05). Grade II CR for PDT was 65% (37/57), no grade II lesions achieved CR with imiquimod (0/54). 6 mth: The CR was 65% (81/124) for PDT and 56% (64/115) for imiquimod, but the difference was not statistically significant (p > 0.05). There was also no significant difference for grade I lesions, 72% (48/67, PDT) vs 72% (44/61, imiquimod). CR were significantly higher for grade II lesions in the PDT treatment areas (58%, 33/57) than with imiquimod (37%, 20/54, p < 0.05) QoL and return to normal activity Cosmetic outcome was assessed by the investigators 6 mth after treatment according to scarring, atrophy, erythema and pigment change. Outcomes were graded as excellent, good, fair or poor. No significant differences between the groups were observed; excellent outcomes were 85% in PDT and 75% for imiquimod. A patient-completed questionnaire at 6 mth assessed treatment preferences. The PDT procedure was preferred by 69% of patients, 55% favoured PDT in terms of efficacy and 70% would prefer PDT for future treatments AEs No unexpected safety issues were recorded. PDT reactions during treatment: stinging (83%), burning (100%), pain (100%), moderate erythema (100%), oedema (67%), blistering (27%). Reactions were well tolerated, no further treatment was required and all resolved within 7–15 d. Imiquimod reactions were most commonly application site related: itching (21%), burning (11%), pain (4%). Local skin reactions in the treatment area were mostly mild to moderate and well tolerated, and more intense during course 1 than course 2: erythema (93%), crusting (11%), scaling (11%), erosions/ulcerations (7%), oedema (7%) |
Authors’ conclusions ALA–PDT and imiquimod 5% cream are comparable treatments for upper extremity AK. ALA–PDT should be considered as a 1st-line therapy for both grade I and grade II AK of the extremities Brief study appraisal Although this trial used an intraindividual randomisation design for treatment, it failed to report on aspects of methods (including randomisation) and did not use an ITT analysis Outcome assessors were not blinded and only investigator-assessed cosmetic outcomes were reported. The results suggest there may be little difference between PDT and imiquimod for grade I lesions, but PDT may be better for grade II These results can be considered moderately reliable, but uncertainties do exist about the choice of statistical techniques used, the inconsistent reporting of some results, and the sample size (as the authors assumed that lesions within patients were independent) |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Smith et al. (2003)50 Data source Full published paper Country USA Language English Study design RCT No. of participants Total: 36 Intervention 12 (ALA with blue light) Comparator: 12 (ALA with laser light) 2nd Comparator:12 (5-FU) No. of recruiting centres Not stated Follow-up period and frequency FU at end of treatment, 2 wk and 4 wk |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria It appeared that Caucasian patients with a minimum of four non-hyperkeratotic AK of the face or scalp were eligible for inclusion Patient characteristics % Male: 81 Mean age: ALA with blue light, 58.9; ALA with laser light 61.0; 5-FU 64.3 Mean no. of lesions: 6–7 per patient Concomitant treatment Patients with more dramatic cutaneous reactivity (6/11 5-FU and 1/12 PDT-laser patients) were treated with dilute acetic acid soaks and topical low potency corticosteroid (2–3 times daily) |
Trial treatments Broad area ALA–PDT with blue light vs broad area ALA–PDT with laser light vs 5-FU Intervention ALA–PDT (blue light): Following topical application of ALA for 1 hr to a broad area, illumination with blue-light PDT for 1000 s. Two treatments 30 d apart were applied Comparator ALA–PDT (with laser): Following broad area topical ALA application for 1 hr, pulsed dye laser was administered (595 nm, 75 J/cm2 with 10-ms pulse duration using a 10-mm spot size, 10% overlap of each laser impact and two passes across treatment area). Patients received two treatments 30 d apart 2nd comparator 5-FU: 5% fluorouracil cream applied once or twice daily for 4 wk |
Morbidity 75% or more lesions were cleared in 9/12 PDT-blue light patients vs 5/12 PDT-laser vs 9/11 5-FU. 100% of lesions were cleared in 6/12 PDT-blue light patients vs 1/12 PDT-laser vs 6/11 5-FU. The cumulative clearance rate (or individual AK lesion rate) was 80% (PDT-blue light), 50% (PDT-laser) and 79% (5-FU) QoL and return to normal activity All three treatments showed improvement in global response, tactile roughness and mottled hyperpigmentation; the 5-FU and PDT-blue light groups tended towards more benefit for tactile roughness, whereas the 5-FU and PDT-laser groups favoured pigmentation. PDT-blue light was the only group in which the signs of photoageing completely resolved based on the global response score (two patients). None of the treatments worsened signs of photoageing AEs Four PDT-blue light and 3 PDT-laser patients reported mild or moderate stinging directly after therapy but not at subsequent FU. Erythema was the most pronounced AE; 5-FU patients had the greatest average increase and showed residual erythema at 4 wk. Crusting and erosions were only seen with 5-FU. There was one discontinuation in the 5-FU group due to a severe confluent erythematous reaction |
Authors’ conclusions Broad area PDT treatment with ALA plus activation with blue light appears to be as effective as 5-FU in the treatment of AK. ALA plus laser light is somewhat less effective than the above therapies Brief study appraisal This was a small study with poorly reported methodology. Additional treatment was given to patients that had severe reactions, which may have been a confounding factor. The reliability of the results is therefore uncertain |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Szeimies et al. (2007)38 Data source Abstract Country Not stated Language English Study design RCT No. of participants Total: 25 (238 lesions) Intervention: Not stated Comparator: Not stated No. of recruiting centres Not stated Follow-up period and frequency FU at 2 wk and 3 mth |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Not stated Patient characteristics % Male: 68 Mean age: 73 yr Concomitant treatment Not stated |
Trial treatments MAL–PDT with VPL vs MAL–PDT with LED light (within-participant comparison) Intervention PDT-VPL: MAL cream was applied to target area for 3 hr. One side received VPL at 80 J/cm2 (double pulsed at 40 J/cm2) with a pulse train of 15 impulses, each of 5-ms duration, using a 610–950 nm filtered hand piece. The opposite side received LED light (37 J/cm2 for 12 min) Comparator PDT-LED: See above |
Morbidity Infiltration and keratoses score: No significant difference between LED 0.86 (0.71) and VPL 1.05 (0.74), p = 0.292 QoL and return to normal activity No significant differences in patient satisfaction between treatments (p = 0.425) AEs Pain assessment (VAS) immediately after PDT showed significantly lower pain levels for the VPL side (4.3 vs 6.4) |
Authors’ conclusions The use of VPL is an efficient and useful alternative in the photodynamic treatment of AK, where otherwise pain development can be a limiting factor for the performance of PDT Brief study appraisal Minimal reporting of both methods and results means little can be deduced from this conference abstract |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Szeimies et al. (2002)51 Data source Full published paper Countries Austria, Germany, Italy, Switzerland, the Netherlands Language English Study design RCT No. of participants Total: 202 (732 lesions) Intervention: 102 (384 lesions) Comparator: 100 (348 lesions) No. of recruiting centres 13 Follow-up period and frequency At 2 wk and 3 mth |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Patients > 18 yr old, with up to 10 AK lesions suitable for cryotherapy and no treatment within the last 4 wk when eligible. Diagnosis was based on clinical assessment (and histology where needed). Patients receiving regular UV therapy and patients with pigmented lesions or porphyria were excluded Patient characteristics % Male: 61 Age range: 42–89 yr Mean age: 71 yr (PDT), 72 yr (cryotherapy) 58% of patients had 1–3 lesions, 33% had 4–7 lesions, and 9% had 8–10 lesions. Lesions were mostly of thin (40%) or moderate (52%) grade, and most were located on the face (63%) or scalp (28%) Concomitant treatment Not stated |
Trial treatments MAL–PDT vs Cryotherapy Intervention MAL–PDT: Loose crusts were removed using a curette and the surface gently roughened. MAL cream (160 mg/g) was applied as a 1-mm-thick layer and to 5 mm of surrounding normal tissue. The area was covered with an occlusive dressing for 3 hr, after which the cream was washed off with a saline solution, followed by illumination with non-coherent red light (570–670 nm) with a total light dose of 75 J/cm2 and a light intensity of 70–200 mW/cm2. The mean illumination time was 11 min. Up to 10 lesions were treated at the same session. The procedure was repeated after 1 wk in lesions not on the face or scalp (8% of patients) Comparator Cryotherapy: preparation with superficial curettage, followed by cryotherapy with liquid nitrogen spray to achieve a 1- to 2-mm frozen rim outside the marked lesion outline. The mean total freezing time was 24 s. The freezing procedure was performed in two cycles during the single treatment session |
Morbidity The overall CR rate was 69% (252/367) for PDT vs 75% (250/332) for cryotherapy. Higher response rates were observed in grade I (thin) lesions than in thicker lesions. Grade I facial lesions showed the best response regardless of intervention arm QoL and return to normal activity Cosmetic outcome was significantly better in PDT group (p = 0.035), where 96% of investigators and 98% of patients graded outcome as excellent or good (vs 81% and 91%, respectively, for cryotherapy group). In the PDT group, of the 43 previously treated patients (various treatments such as cryotherapy and 5-FU) 32 rated PDT as better, 10 as equal, and one worse than the previous treatment AEs Local AEs were reported by 44 (43%) of PDT patients vs 26 (26%) of cryotherapy patients. The commonest were burning sensation (PDT 32% vs cryotherapy 9%), skin pain (10% vs 13%) and crusting (5% vs 6%). Three patients stopped treatment due to local reactions – one PDT (burning) and two cryotherapy (pain) |
Authors’ conclusions PDT for treating AK has a similar response rate to cryotherapy, but with superior cosmetic results and high patient satisfaction Brief study appraisal In relation to CR, the authors’ conclusions appeared only to relate to thin facial lesions, with uncertainty surrounding other results (which frequently lacked p-values). Cosmetic outcome and satisfaction results were based on smaller patient numbers. The study was not blinded which coupled with the real possibility of institutional differences and protocol deviation (13 centres in five countries) casts further doubt on the reliability of the results |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Szeimies et al. (2009)58 Linked publications169 Data source Full published paper Countries Germany, USA Language English Study design RCT No. of participants Total: 131 Intervention: 57 (plus 16 not randomised – included only in AE analysis) Comparator: 58 No. of recruiting centres 10 Follow-up period and frequency FU 3 mth after last treatment |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Males and non-pregnant, non-lactating women aged over 18 yr with 4–10 previously untreated, non-pigmented, non-hyperkeratotic grade I or II lesions on the face and scalp (at least 3-mm diameter) were eligible for inclusion. Exclusion criteria were extensive and included: immunosuppression, porphyria, allergy to MAL or similar, hypersensitivity to nut products or other protein antigens, regular UV treatment of face or scalp in previous 30 d, topical therapy in previous 3 mth Patient characteristics % Male: 79 Mean age: MAL–PDT 69.5; Placebo PDT 67.0 Age range: 41–90 yr skin type: I 19%; II 44%; III/IV 27%; IV 10% lesions: grade I (thin) 41%; grade II (moderate) 59% The majority of lesions were located on the face or scalp, patients had a median of seven lesions each and the median maximum diameter was 9 mm Concomitant treatment 22 patients (14 MAL, 8 placebo) received oral analgesic treatment or fentanyl patches |
Trial treatments MAL–PDT vs Placebo PDT Intervention MAL–PDT: After debridement of lesions, 1-mm-thick MAL cream (160 mg/g) was applied to each lesion and 5-mm surrounding skin for 3 hr (permitted range 2.5–4 hr) under occlusion. Lesions were cleaned with a saline solution, then illuminated with non-coherent LED red light (average duration 9 min, mean dose 37 J/cm2, mean intensity 74 mW/cm2, range 56–83 mW/cm2) with the light source kept at 5–8 cm from skin. Breaks in illumination were allowed provided that treatment was completed within 4 h of occlusion. This process was repeated after 1 wk Comparator Placebo PDT: As for MAL–PDT but with placebo cream |
Morbidity Lesion response rate was 83% (348/418) with MAL vs 29% (119/414) for placebo, OR 13.8 (9.5 to 19.9, p < 0.001). Patient CR rate with MAL was 68% (39/57) vs 7% (4/59) for placebo, OR 39.5 (10.5 to 149.2, p < 0.001). At 3 mth after treatment, development of new lesions was 18% (10/56) in MAL patients vs 34% (20/58) for placebo, p = 0.04. 73% were on the scalp in the MAL group vs 59%. Response rates were higher for small lesions (3–10 mm) on the face but better for large lesions (> 20 mm) on the scalp QoL and return to normal activity Not assessed AEs 85% (62/73) MAL patients reported AEs vs 60% (35/58) and most were associated with treatment site (312/368 and 56/89, respectively). The most commonly reported local AEs with MAL were pain of the skin 55% (40/73) vs 22% (13/58), erythema 52% (38/73) vs 5% (3/58), and skin burning sensation 36% (26/73) vs 12% (21/58). In the placebo group all but six local AEs were mild; most MAL AEs were mild to moderate. Nineteen MAL patients had severe local AEs considered treatment related: pain of the skin 13, erythema six, skin burning sensation five, skin exfoliation four, scab one, skin swelling one and face swelling one. There were also six non-local AEs: dizziness and increased perspiration, eyelid oedema (three reports) and headache (one report). Two MAL patients discontinued due to severe skin pain |
Authors’ conclusions Topical MAL–PDT using an LED is an effective treatment for multiple AKs Brief study appraisal Generally a well-conducted trial that considered centre effects on the results; however, a subgroup analysis of patients that received additional painkillers may have been useful |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Tarstedt et al. (2005)52 Data source Full published paper Country Sweden Language English Study design RCT No. of participants Total: 211 (413 lesions) Intervention: 105 (198 lesions) Comparator: 106 (215 lesions) No. of recruiting centres 21 Follow-up period and frequency At 3 mth |
Treatment intention Curative Type(s) of lesion and histology AK (grade I or II) Main eligibility criteria Patients aged at least 18 yr with up to 10 clinically diagnosed AK lesions on the face and/or scalp, which were mild (grade I) or moderate (grade II) and non-pigmented, were eligible Patient characteristics % Male: 39 Mean age: 69 yr single-session group, 68 yr 2-session group Around one-half of the patients had received prior treatment for AK. Mean lesion diameter was around 10 mm. The majority of patients (76–83% in both groups) had 1 or 2 lesions, and 90% of lesions were on the face, with the rest being on the scalp. Further characteristics were reported Concomitant treatment Local anaesthetic if needed during treatment |
Trial treatments MAL–PDT single session vs MAL–PDT 2 sessions (1 wk apart) Intervention MAL–PDT single session: Any lesion crust was removed using a curette or scalpel (without anaesthetic) and a 1-mm-thick layer of MAL (Metvix, 160 mg/g) was applied to each lesion and 5 mm of surrounding tissue and covered with an occlusive dressing for mean of 3 hr. The dressing was removed and the cream washed off with 0.9% saline solution immediately before illumination, for mean of 8 min, with red LED light (peak wavelength 634 ± 3 nm, light dose 37 J/cm2, irradiance 50 mW/cm2 at 50 mm from skin). Re-treatment if there was a non-CR after 3 mth Comparator MAL–PDT two sessions: See above, but two treatment sessions, 1 wk apart |
Morbidity Analyses based on 400 lesions (198 single session; 202 double session MAL–PDT). The lesion CR rates were similar (81% single treatment vs 87% 2 treatments). A further 22 lesions showed a CR after re-treatment (increasing the CR for the single treatment group to 92%). Single and two-treatment schedules had similar CRs for thin lesions (93% vs 89%), but not for moderately thick lesions (70% vs 84%), although again this improved after re-treatment (88%) QoL and return to normal activity Cosmetic outcome was rated as excellent for each of four parameters in > 75% of lesions in each group. 66% of single treatment patients, who had previously been treated with cryotherapy, preferred PDT to cryotherapy vs 58% in the two-treatment group AEs AEs were reported in 42 single-treatment patients and in 53 two-treatment patients. Most local AEs were of mild to moderate intensity and of relatively short duration. Burning of skin occurred in 15% of single treatment patients vs 19% of the treatment group, whereas pain occurred in 9% and 18% of patients, respectively. One patient randomised to two sessions discontinued due to moderate erythema (which resolved completely) |
Authors’ conclusions Single MAL–PDT treatment is as effective as a two-treatment schedule for thin AK lesions. Repeated treatment is recommended for thicker or non-responding lesions Brief study appraisal Although this study appears to have been quite well conducted, it was unclear whether the outcome assessors were blinded to treatment allocation. 21 centres recruited the 211 patients (around five participants per centre on average) increasing the possibility that protocol deviation and institutional differences would affect results. p-values were not reported |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Touma et al. (2003)39 Data source Abstract Country USA Language English Study design RCT No. of participants Total: 18 Intervention: Not stated Comparator: Not stated 2nd Comparator: Not stated No. of recruiting centres Not stated Follow-up period and frequency Day 1, then at 1 wk, and 1 and 5 mth |
Treatment intention Curative Type(s) of lesion and histology Non-hypertrophic AK Main eligibility criteria Patients with at least four non-hypertrophic AK and diffuse photodamage Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments ALA–PDT, 1-hr incubation vs 2-hr incubation vs 3-hr incubation Intervention 1-hr incubation: Pre-treatment with 40% urea cream (penetration enhancer) and xylocaine HCL 3% (pain control) followed by 20% ALA–PDT with 417-nm blue light. Further PDT parameters were not reported Comparator 2 hr incubation: See above 2nd comparator 3-hr incubation: See above |
Morbidity Results not broken down by treatment group, but it was reported that there was no effect of ALA incubation time or urea cream on any of the measured outcomes. At 1 mth, 90% of AKs had cleared (analysis on 17 patients) QoL and return to normal activity Patient satisfaction with cosmetic results was reported as moderately high, and all patients commented on improved skin texture AEs Phototoxic reactions were well tolerated |
Authors’ conclusions Short incubation (1–3 hr) broad area ALA–PDT appears as effective in eradicating AKs as the long incubation application, with tolerable phototoxicity Brief study appraisal Little information on methods and very limited results presented for this very small study. The conclusions appear inappropriate if they were based on the study results, as short incubation appears to be defined as 1–3 hr, yet no group received long incubation treatment |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Wennberg et al. (2008)59 Data source Full published paper Country Not stated, ‘Europe’ Language English Study design RCT No. of participants Total: 81 (889 lesions, 90% AK) Intervention: 476 lesions Comparator: 413 lesions No. of recruiting centres 11 Follow-up period and frequency 3, 9, 15, 21 and 27 mth after initial treatment |
Treatment intention Curative. This trial aimed to treat existing lesions and also prevent recurrence. Only response rates and cosmetic outcomes have been reported here Type(s) of lesion and histology AK (some BCC and SCC in situ included but data not reported) Main eligibility criteria Organ transplant recipients who had received immunosuppressive therapy for more than 3 yr and had between two and 10 lesions in two symmetrical 50-cm2 contralateral areas on the face, scalp, neck, trunk or extremities. All patients were required to have received at least one previous treatment for the lesions. Exclusion criteria were reported Patient characteristics % Male: 68 Age range: 30–78 yr Median age: 57 yr Most patients had Fitzpatrick skin types I–III, organ transplantation occurred between 3 and 34 yr previously (median 16 yr) with the majority more than 10 yr prior to treatment. Selected treatment areas were mostly on the face, scalp or extremities. AKs were mostly grade I or II and almost all were ≤ 10 mm in diameter Concomitant treatment Not stated |
Trial treatments MAL–PDT vs investigator’s choice of treatment (within-participant comparison) Intervention MAL–PDT: 2 treatments were given, 1 wk apart (baseline and 1 wk after randomisation). Additional single treatments were given at 3, 9 and 15 mth for a total of five treatments. Any visible lesions at 21 and 27 mth were treated at the investigators’ discretion. Lesions were prepared using a small curette to debride the area. MAL 160 mg/g cream applied in 1-mm layer to 50-cm2 treatment area and covered for 3 hr with an occlusive dressing. Excess cream was removed with saline and the area illuminated with non-coherent red light from a lamp (630 nm, light dose 37 J/cm2). Patients’ eyes were protected during treatment. Fans and cold water spraying were used for all patients to minimise pain Comparator Control area was treated at the investigator’s discretion utilising any suitable therapy in accordance with normal clinical practice EXCEPT 5-FU cream or imiquimod cream. Treatment was carried out at baseline and 3, 9 and 15 mth later. Any visible lesions at 21 or 27 mth were treated according to the investigator’s preference. Control treatments utilised: cryotherapy (83%) curettage and cautery (4%) laser therapy (2%) surgery (1%) |
Morbidity This trial aimed to treat existing lesions and also prevent recurrence. Only response rates and cosmetic outcomes have been reported here. Lesion CR rate at 3 mth: MAL–PDT 77% and control 74% (no p-value reported). Lesion response rate at 15 mth: MAL–PDT 88% and control 89% Recurrence rates for lesions that were present at baseline and rated as CR by 3 mth were similar in both groups (PDT 24% and 20% control) with no significant difference QoL and return to normal activity Cosmetic outcomes were rated by the investigator on a 3-point scale. Overall MAL–PDT resulted in more favourable outcomes than the control treatments for hypopigmentation (p < 0.001). At 15 mth, more hypopigmentation was reported in the control group and obvious hypopigmentation was 0% (MAL–PDT) vs 25% (control). No significant difference was found between groups for scar formation AEs Local AEs associated with MAL–PDT were reported by 75% of patients although most were transient and resolved within 1 wk (erythema, pain and crusting). 6% of patients discontinued MAL–PDT due to pain; pain was judged as severe in 17 out of 420 treatment sessions but most reported were of moderate pain. 48% of patients reported AEs in the control area including pain, erythema, crusting or blistering of mild to moderate intensity |
Authors’ conclusions Treating field cancerisation in organ transplant recipients with topical MAL–PDT is efficacious and generally well tolerated (further conclusions relating to prevention of AK reported) Brief study appraisal This study was primarily intended to evaluate prophylactic PDT in immunosuppressed patients and this should be borne in mind. Few details were provided about concealment of allocation, and blinding was not used. The control treatment was not predefined and intercentre differences are likely to have impacted on the results (of which few figures were provided). As a comparative study, the results should be considered with caution and may not be reliable |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Wiegell et al. (2008)53 Data source Full published paper Country Denmark Language English Study design RCT No. of participants Total: 30 (29 treated) Intervention: 30 (29 treated) Comparator: 30 (29 treated) No. of recruiting centres One Follow-up period and frequency FU at 1–3 d (AEs) and 3 mth (CR) |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Patients with AKs symmetrically distributed on the face or scalp. Pregnant or lactating women were excluded Patient characteristics % Male: 79 Age range: 63–90 yr Mean age: 78 yr Most lesions were grade I, with around a third grade II and very small number were grade III Concomitant treatment Sunscreen for exposed areas not covered by treatment |
Trial treatments MAL–PDT with daylight vs MAL–PDT with red LED (within-participant comparison) Intervention PDT with daylight: Before treatment, lesions were counted, graded, mapped, and photographed. AK lesions of face or scalp were marked into two symmetrical treatment areas (∼80 cm2 each) and scales and hyperkeratoses were removed using a curette. Around 1 g of MAL cream was applied to each area and covered with a dressing and light-impermeable lead rubber. Following 30 min indoors, the daylight area had the dressing removed and patients spent 2.5 hr outside in daylight (mean effective total dose 43 J/cm2, mean effective red light dose 1.9 J/cm2, patients treated in period between July and September), before returning to have MAL cream from both treatment areas removed. The area randomised to red LED light was treated with a light dose of 37 J/cm2 (effective red light dose 1.2 J/cm2, peak irradiance at 632 nm) after covering the daylight treatment area with light-impermeable rubber Comparator PDT with red LED: See above |
Morbidity At 3 mth the absolute decrease in the mean number of lesions compared to baseline was 8.0 (71%) in the LED areas vs 8.4 (79%) in the daylight areas (p = 0.13 for percentage, p = 0.50 for mean number) QoL and return to normal activity Eighteen patients (62%) preferred the daylight treatment, four (14%) LED treatment, and six (21%) had no preference AEs Analyses based on 24 patients: The daylight areas were significantly less painful on a 10-point VAS scale than the LED areas (mean maximal pain score, 2.0 for daylight vs 6.7 for LED, p < 0.0001). In the LED light group, 15 patients needed cold water spray to make pain tolerable, and one-half of these patients needed one or two breaks during illumination. In two patients this was not enough, so treatments were stopped after one-third of illumination time. Pain scores 6 hr after LED treatment were not significantly different (mean maximal score of 1 for daylight vs 1.3 for LED, p = 0.14). Both areas developed erythema and crusting after treatment. These AEs were most severe in the daylight area in 10 patients (42%), in the LED area in five patients (21%) and there was no difference between the areas in nine patients (38%) |
Authors’ conclusions Continuous activation PDT using daylight exposure was as effective as, and better tolerated than, conventional PDT Brief study appraisal This was a generally well conducted but small study and the results appear likely to be reliable. It should be noted though that six patients had previously received PDT in the treated areas (although more than 1 yr before the study), and that the LED illumination time was not clearly stated |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Wiegell et al. (2008)40 Data source Abstract Country Denmark Language English Study design RCT No. of participants Total: 29 Intervention: 29 Comparator: 29 No. of recruiting centres Not stated Follow-up period and frequency FU at 3 mth |
Treatment intention Curative Type(s) of lesion and histology AK Main eligibility criteria Patients with AK of the face and scalp Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments PDT with 8% MAL vs PDT with 16% MAL (within-participant comparison) Intervention PDT 8% MAL: Patients were given both treatments, randomised to two symmetric areas, one area was given 8% MAL cream and the other 16% MAL cream. Patients were sent home and instructed to spend as much time as possible outside, in daylight. Patients spent an average of 210 min outdoors (range 62–372 min). Light exposure was measured using an electronic dosimeter watch Comparator PDT 16% MAL: See above |
Morbidity At 3 mth, there was no significant difference in CR rate (77% in 16% area vs 80% in 8% area), p = 0.37 QoL and return to normal activity Not assessed AEs Erythema and crusting occurred in both treatments (and were similar to inflammation seen after conventional PDT). Pain diaries were used but not reported by intervention arm |
Authors’ conclusions PDT using daylight activation will make AK treatment more time and cost-effective, and more convenient for the patient Brief study appraisal The authors compared overall pain scores, and AEs in a small sample, with those seen in conventional PDT, but using a comparator treatment of conventional PDT in this study would have been much more informative. This abstract also featured minimal reporting of methods and results |
Appendix 14 Bowen’s disease data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors de Haas et al. (2007)69 Data source Full published paper Country The Netherlands Language English Study design RCT No. of participants Total: 40 (50 Bowen’s disease patches) Intervention: 25 lesions (participant no. not stated) Comparator: 25 lesions (participant no. not stated) No. of recruiting centres One Follow-up period and frequency 4 wk, then at 3 mth intervals up to 28 mth |
Type(s) of lesion and histology Bowen’s disease Main eligibility criteria Not stated Patient characteristics % Male: 43 Age range: 49–91 yr Mean age: 74 yr Mean lesion diameter: 14.5 mm (range 5–40 mm) Locations: Trunk 12, lower leg 11, hand 8, ear 7, upper leg 4, cheek and/or nose 3, eyelid 2, arm 1, frontal and/or temporal area 1, scalp 1 The sample included seven organ recipients Concomitant treatment Lidocaine, 2% without adrenaline was used if patients required it |
Trial treatments ALA–PDT using a single illumination vs ALA–PDT with a twofold illumination Intervention Single Illumination: Surface scale or crusts were removed. 20% ALA, locally produced, was applied topically and left in place for 4 hr with a margin of 1 cm. A diode laser and light emitting diode provided illumination at a wavelength of 630 nm, 4 hr after ALA application at a dose of 75 J/cm2 Further PDT parameters were not reported Comparator Twofold illumination: As for single illumination except patches were light treated 4 and 6 hr after ALA application at doses of 20 and 80 J/cm2, respectively, separated by a 2-hr dark interval. Each illumination was delivered at 50 mW/cm2 |
Morbidity In the single illumination group, CR was seen in 22 patches (80%) at 12 mth. In the twofold-illumination group CR was seen in 22 patches (88%) (NS). Patients reported a 3-wk maximum healing time, which was not different between treatments QoL and return to normal activity Not assessed AEs All patients in both groups experienced some discomfort during treatment but all finished therapy. No SAEs were seen in either group. In the single-illumination group none of the patients complained of pain during treatment. In the twofold-illumination group five patients complained about pain in the treatment of six patches. Lidocaine without adrenaline was used in four patches |
Authors’ conclusions ALA–PDT may offer the best treatment option for Bowen’s disease. This study shows the potential of light fractionation for enhancing the response of the disease to ALA–PDT and illustrates the need for a larger, suitably powered trial to determine if the effect is statistically significant Brief study appraisal This was a small trial with unclear methods of randomisation and blinding. Treatment methods were reported but not all outcomes were detailed. This study shows the potential of PDT and its enhancement through light fractionation but would need confirmation, as the authors state, in an adequately powered trial |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Lui et al. (2004)68 Data source Full published paper Countries Canada, USA Language English Study design RCT No. of participants Total: Not stated by diagnosis (34 lesions) Intervention: Not stated by diagnosis (27 lesions) Comparator: Not stated by diagnosis (one lesion) 2nd comparator: Not stated by diagnosis (six lesions) No. of recruiting centres Four Follow-up period and frequency 6 wk, and 3, 6, 12, 18 and 24 mth |
Type(s) of lesion and histology Superficial BCC, 277 lesions (66%); nBCC, 93 lesions (22%); Bowen’s disease, 34 lesions (8%); BCC unspecified 17 lesions (4%) Main eligibility criteria Patients with at least two biopsy-proven superficial or nBCC or Bowen’s lesions Patient characteristics Not stated for Bowen’s group Concomitant treatment Oral analgesics for pain |
Trial treatments PDT at 60 J/cm2 vs PDT at 120 J/cm2 vs PDT at 180 J/cm2 Intervention PDT at 60 J/cm2: 10 min intravenous infusion of 14 mg/m2 verteporfin followed 1–3 hr later by exposure to 60 J/cm2 of red light (688 ± 10 nm) from a non-thermal LED panel. The exposed area had a margin of 3–4 mm around the lesion. The irradiance delivered was 200 ± 40 mW/cm2. Tumours re-treated at 3 mth if necessary (with dose increased to 18 mg/m2) Comparator PDT at 120 J/cm2: See above 2nd comparator PDT at 180 J/cm2: See above |
Morbidity At 6 mth, the histopathological response (i.e. no residual tumour) was 85% at 60 J/cm2, 100% at 120 J/cm2, and 50% at 180 J/cm2 QoL and return to normal activity Assessed but not reported for Bowen’s group AEs Assessed but not reported for Bowen’s group |
Authors’ conclusions A single course of verteporfin PDT showed treatment benefit for patients with multiple non-melanoma skin cancers Brief study appraisal There was a lack of information on issues such as blinding and allocation concealment, and the authors did not present many results and population details by diagnosis. It is therefore difficult to make any reliable conclusions about the efficacy of verteporfin in patients with Bowen’s disease, particularly as they formed a small proportion of the overall number of lesions |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Morton et al. (2000)70 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 19 (70 lesions) randomised 16 (61 lesions) followed up Intervention: 32 lesions (no of patients not specified) Comparator: 29 lesions (no of patients not specified) No. of recruiting centres Not stated Follow-up period and frequency After clearance, all patients were reviewed at monthly intervals, up to 12 mth |
Type(s) of lesion and histology Bowen’s disease Main eligibility criteria Biopsy-proven disease with individual lesions of ≤ 21 mm in diameter. No lesion had been previously treated Patient characteristics Age range: 50–87 yr Mean age: 73 yr No. of lesions per patient varied between one and six (median three) Concomitant treatment Local anaesthetic (1% plain lidocaine by intradermal injection) was offered during PDT treatment |
Trial treatments ALA–PDT with red light vs ALA–PDT with green light Intervention Red light: Surface crusts were removed and the surface was gently abraded. Topical ALA in an oil-in-water emulsion was applied to the lesions 4 h pre-illumination. Approximately 50 mg/cm2 of cream was applied to cover the entire field of illumination, including a clinically disease-free margin of at least 4 mm. The cream was kept in place under an occlusive dressing A ‘Paterson’ lamp with 300W xenon short arc plasma discharge was adjusted using appropriate filters to 630 ± 15 nm for red light. At a fluence rate of 86 mW/cm2 lesions received 125 J/cm2 of red light A repeat treatment was given after 2 mth if necessary Comparator As for red light except a wavelength of 540 ± 15 nm was used to deliver 62.5 J/cm2 of light |
Morbidity In the red light group 24 lesions cleared following one treatment and a further six after a repeat treatment giving an initial response rate of 94%. Eighteen lesions treated using green light cleared after one treatment with a further three clearing on repeat giving an initial response of 72%. Difference in response was statistically significant (p = 0.002). A high recurrence rate was observed in the green light group with seven recurrences in comparison with two lesions relapsing after PDT with red light. OR for recurrence = 0.13 (95% CI 0.04 to 0.48) QoL and return to normal activity No clinically obvious scars were evident at 1yr in either group AEs No ulceration or infection complicating therapy and no photosensitivity reactions were documented after PDT treatment in either group. No significant difference in pain was seen between the treatment groups. No red light patients needed anaesthesia whereas two lesions (one patient) treated in the green light group needed anaesthesia Twelve patients received both types of light. Four stated that green light was the most painful, six that red light was the most painful, while two patients could not distinguish between the light used |
Authors’ conclusions Green light is less effective than red light in the treatment of Bowen’s disease by ALA–PDT Brief study appraisal A small study, with unclear methods of randomisation, allocation concealment and blinding of outcome assessors |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Morton et al. (1996)71 Linked publications179 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 19 (40 lesions) Intervention: 20 lesions, participant no. not stated Comparator: 20 lesions, participant no. not stated No. of recruiting centres Not stated Follow-up period and frequency 2 d and 10 d for AEs. 2-monthly intervals for clinical response. Following clearance, 2-monthly intervals for 12 mth for recurrence and late AEs |
Main eligibility criteria Histological confirmation of Bowen’s with individual lesions ≤ 21 mm in diameter. No lesion had been previously treated Patient characteristics % Male: 16 Age range: 62–88 yr Mean age: 76 yr Location and no. of lesions: legs 33, face five, hand two Concomitant treatment Patients were offered local anaesthetic (1% plain lidocaine by intradermal injection) during treatment |
Trial treatments PDT with ALA vs Cryotherapy Intervention PDT: Surface crusts were removed and the surface gently abraded prior to application with topical 5-ALA in an oil-in-water emulsion 20%. Approximately 50 mg/cm2 of cream was applied to cover the entire irradiation field including the clinically disease-free margin. The cream was kept in place under an occlusive dressing Four hours later lesions were illuminated with a prototype lamp. The lamp incorporated a 300 W xenon short arc plasma discharge producing a continuous wave broadband flat spectral output across the entire visible spectrum. Using filters, the spectral output of the lamp was adjusted to a 30-nm bandwidth, about 630 nm. To broaden the treatment field and to produce uniform irradiation of the lesions, a 25-mm collimating lens was attached to the 5-mm fibre bundle. Allowing at least a 10% margin around lesions in the field of irradiation permitted the treatment of lesions ≤ 21 mm in diameter, at a fluence rate of 70 mW/cm2 and a treatment time of 30 min, lesions received 125 J/cm2. Following therapy, 3-mm punch biopsies were performed in lesions where there was doubt over clinical clearance or recurrence. Repeat treatments were administered if lesions persisted Comparator Cryotherapy: Liquid nitrogen was applied to lesions via a hand-held Cry-Ac spray. After initial ice field formation, the freeze was maintained for 20 s. A single freeze–thaw cycle technique was used with a 2–3 mm rim of clinically healthy tissue included in the treatment field. Following therapy, 3-mm punch biopsies were performed in lesions where there was doubt over clinical clearance or recurrence. Repeat treatments were administered if lesions persisted |
Morbidity Clearance after one treatment: PDT, 15 of 20 lesions; cryotherapy, 10 of 20 lesions Clearance after two treatments: PDT, five remaining lesions; cryotherapy, six lesions The remaining four lesions in the cryotherapy group required a third treatment. There was no significant difference between the two treatments in clearance rates. However, by chance lesions treated by PDT were overall larger than those in the cryotherapy group. In a linear regression model taking size of lesion into account, the probability that a lesion of any size is completely cleared at the 1st treatment was significantly greater with PDT than with cryotherapy (p < 0.01). Recurrence Rate during 12-mth following clinical clearance: PDT zero; cryotherapy two (6 mth and 8 mth). CR rate was, therefore, 100% for PDT and 90% for cryotherapy QoL and return to normal activity Visible scarring 12 mth following clearance (no. of lesions): PDT zero, cryotherapy four AEs Pain during treatment (no. of lesions): PDT, six mild and five moderate; cryotherapy, 12 mild and seven moderate (p = 0.01). Free from pain 10 d following treatment (no. of lesions): PDT 15; cryotherapy 10 All six patients who received both treatments, due to having multiple lesions, reported PDT as less painful. Blistering (no. of lesions): PDT zero; cryotherapy seven. Ulceration (no. of lesions): PDT zero; cryotherapy five. Secondary infection (no. of lesions): PDT zero; cryotherapy two. No photosensitivity reactions occurred after PDT |
Authors’ conclusions PDT is at least as effective as cryotherapy in the treatment of Bowen’s disease and was associated in this study with fewer AEs and a lower recurrence rate Brief study appraisal This was a small trial using a prototype lamp as a light source. Results were reported by lesion rather than by patient. By chance lesions were significantly larger in the PDT group but this was taken into account when assessing clearance rates. The results of the trial are promising but would require confirmation in larger trials |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Morton et al. (2006)73 Data source Full published paper Countries Not stated, 11 European countries Language English Study design RCT No. of participants Total: 229 randomised, 225 treated (275 lesions) Intervention: 96 (124 lesions) Comparator: 17 (24 lesions) 2nd comparator: 82 (91 lesions) 3rd Comparator: 30 (36 lesions) No. of recruiting centres 40 Follow-up period and frequency 3, 12 and 24 mth after last treatment |
Type(s) of lesion and histology Bowen’s disease Main eligibility criteria Inclusion criteria: Patients 18 yr or older with histologically confirmed diagnosis of SCC in situ from a biopsy specimen taken within the last 5 mth and with no evidence of any change in appearance suggestive of lesion progression Lesions that had been treated within the previous 3 mth or that were strongly pigmented, less than 6 mm or more than 40 mm in diameter or located on the genitalia were excluded Patient characteristics For the treated patients: % Male: 39 Age range: 39–99 yr Mean age: 73 yr Location of lesion (no. of lesions): face (scalp) 68, neck, trunk 34, extremities 173 Concomitant treatment Not stated |
Trial treatments MAL–PDT vs Placebo PDT vs Cryotherapy vs 5-FU Intervention MAL–PDT: Lesions were prepared by gentle surface debridement with a curette. 160 mg/g of topical MAL cream was applied to the lesions. It remained on the skin for 3 hr then was washed off with 0.9% saline solution before illumination with non-coherent red light. Wavelength was 570–670 nm, light dose was 75 J/cm2. Mean illumination time was 10 min 37 s. Treatment was repeated once after 1 wk for a complete treatment cycle. Lesions with a PR at 12 wk were re-treated Comparator Placebo cream: As for MAL–PDT. Lesions with a PR at 12 wk were re-treated 2nd comparator Cryotherapy: A handheld liquid nitrogen spray was used in a single freeze–thaw cycle. After an initial ice field formation with a 2-mm rim of clinically healthy tissue, the ice field was maintained for a minimum of 20 s. Mean total freezing time was 25 s Lesions with a PR at 12 wk were re-treated 3rd comparator 5-FU: Topical 5% 5-FU cream was applied for 4 wk, once daily during the 1st week, and twice daily thereafter. Mean number of applications was 42 and 45 in the 1st and 2nd treatments, respectively. Lesions with a PR at 12 wk were re-treated |
Morbidity CR rate at 3 mth: PDT, 103/111 (93%); Placebo, 4/19 (21%); Cryotherapy, 73/85 (86%), and 5-FU 24/29, (83%). 12-mth recurrence rate: PDT, 15%; Placebo, not stated; Cryotherapy, 24% and 5-FU, 21%. Estimated sustained CR rate at 12 mth: PDT, 80%; Placebo, not stated; cryotherapy, 67%; 5-FU, 69%. There was a statistically significant difference between MAL–PDT and combined standard therapy (OR = 1.73; 95% CI 1.03 to 2.93). MAL–PDT was significantly different from cryotherapy (OR = 1.77 to 1.01, 3.12). Estimated sustained CR rate at 24 mth: PDT, 68%; placebo, 11%; cryotherapy, 60%; 5-FU, 59% QoL and return to normal activity Good or excellent cosmetic outcome at 3 mth: PDT, 77/82 (94%); cryotherapy, 43/65 (66%); 5-FU, 16/21 (76%). This was maintained at 12 mth AEs SAEs: PDT, 6%, cryotherapy, 12%. SAEs (including four deaths) were reported. PDT, four patients; placebo cream, two patients; cryotherapy, three patients |
Authors’ conclusions MAL–PDT is an effective treatment option for Bowen’s disease with excellent cosmetic outcome Brief study appraisal The authors’ conclusions appear appropriate, although mitigating factors include the fact that 11% of treated lesions were excluded from the per-protocol population, the lack of reporting on methods of randomisation and allocation concealment, and the possibility of institutional differences and protocol deviation (40 centres in 11 countries) affecting results |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Puizina-Ivic et al. (2008)57 Data source Full published paper Country Croatia Language English Study design RCT No. of participants Total: 15 Intervention: Nine Comparator: Six No. of recruiting centres One Follow-up period and frequency 24 wk |
Treatment intention Curative Type(s) of lesion and histology Bowen’s disease Main eligibility criteria Previous histologically confirmed diagnosis of Bowen’s disease Patient characteristics Not stated |
Trial treatments ALA–PDT with 16-hr incubation and two light fractions vs ALA–PDT with 5-hr incubation and a single illumination Intervention ALA–PDT with 16-hr incubation and two light fractions: Thick crusts were 1st removed with ointments (and wet dressings). After cleaning the area with a saline solution, the 20% ALA cream was applied to a thickness of approximately 1 mm, covering the treated area and 1 cm of the surrounding skin. The area was covered by occlusive dressing. Aluminium foil was placed on top in order to protect skin from ambient light. There was then a 16-hr incubation period before red light (635 nm) was applied. The total of 100 J/cm2 was delivered in two doses of 50 J/cm2 with a fluence intensity of 30 mW/cm2. There was a 2-hr break between illuminations. Spraying of water and cooling with fan was done to minimise pain sensations. After the treatments sunblock ointments were recommended for the next few days in addition to sun protection measures. Biopsies were performed 24 wk after illumination in patients with fluorescence detected after 4 hr Comparator PDT with 5 hr incubation with ALA cream and single light fraction: Preparation for illumination was as for the intervention group. There was then a 5-hr incubation period before red light (635 nm) was applied. 100 J/cm2 was delivered in one dose, with a fluence intensity of 30 mW/cm2. Spraying of water and cooling with fans, and sun protection measures were as for the intervention group. Biopsies were performed 24 wk after illumination in patients whom fluorescence after 3-hr incubation with ALA was detected |
Morbidity At 24 wk, residual tumour was found in four of six (67%) biopsied patients in the single-illumination, shorter-incubation group. Treatment was repeated. At 24 wk, there was persistence of tumour in two of nine (22%) biopsied patients in the fractionated, longer incubation group QoL and return to normal activity Not assessed AEs Not assessed |
Authors’ conclusions PDT delivered as fractionated illumination with 16 hr of incubation separated by a 2-hr dark interval significantly improves therapeutic outcome in tumour eradication Brief study appraisal This trial had only a small number of patients with Bowen’s disease. Procedures of randomisation and blinding of outcome assessors were unclear. No patient details were provided. Although the group receiving fractionated illumination had better outcomes, the relative contribution of the longer incubation time and the fractionated delivery are unclear. It is also unclear if there were any AEs |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Salim et al. (2003)72 Linked Publications185 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 40 Intervention: 20 (33 lesions) Comparator: 20 (33 lesions) No. of recruiting centres Two Follow-up period and frequency 12 mth |
Type(s) of lesion and histology Bowen’s disease Main eligibility criteria Patients with 1–3 lesions of previously untreated, histologically proven Bowen’s disease measuring 0.5–4 cm Patient characteristics % Male: 20 Age range: 65–88 yr Mean age: 76 yr Lesion site: Legs 58, arms 4, face 4 Patients had between one and three lesions and were of skin types I–III Concomitant treatment Local anaesthetic (1% plain lidocaine by intradermal injection) was offered to patients experiencing pain during PDT treatment |
Trial treatments PDT vs 5-FU Intervention 20% ALA in an oil-in-water emulsion was applied to lesions including a 5-mm margin of clinically normal skin 4-hr before PDT. Illumination was with a 300-W xenon lamp at a dose of 100 J/cm squared with a density of 50–90 mW/cm squared narrowband red light (630 ± 15 nm). The time of illumination was dependent on lesion size and ranged from 12 to 40 min. All patients were reviewed at 6 wk and PDT was repeated if required. Further PDT parameters were not reported Comparator 5-FU (Efudix) was applied thinly to the lesions initially once daily during wk 1 and then twice daily (wk 2–4). All patients were reviewed at 6wk and 5-FU was repeated if required |
Morbidity 29 of 33 lesions (88%) showed initial complete clinical clearance with PDT with a PR in the four remaining lesions. 22 of 33 lesions (67%) had complete clinical clearance after 5-FU and six had PR. Five lesions were withdrawn prior to completion of a single cycle of 5-FU. After adjustment for the influence of lesion size on response, the difference in clearance rates was NS. At 12 mth FU there were two recurrences in the PDT group (at 6 and 7 mth). There were six recurrences in the 5-FU group (at 5, 7, 8, 11 and 2 at 12 mth). Overall clearance (at 12 mth) in the PDT group was 27 of 33 lesions (82%) vs 16 of 33 lesions in the 5-FU group (48%). OR = 4.78 (95% CI 1.56 to 14.62, p = 0.006) QoL and return to normal activity Not assessed AEs Three patients with five lesions from the 5-FU group developed widespread dermatitic reactions over the treated limbs and were withdrawn from the study. Another patient with two lesions from the 5-FU group had widespread dermatitic reactions but elected to continue the treatment. In the 5-FU group, three lesions ulcerated and two developed painful erosions on completion of the treatment cycle. The ulcerated lesions healed leaving prominent scarring. There were no reactions in the PDT group and no clinically obvious scar formation at 12 mth at any PDT treatment site. 10 of 15 patients in the 5-FU group and 14 of 19 in the PDT group reported pain during the treatment cycle. During PDT pain was rated ‘mild’ by six patients, ‘moderate’ by six and ‘severe’ by two. Pain settled following illumination in 4 patients and persisted to 24 hr in four. Mild discomfort was reported by the remaining six patients lasting 7–42 d (mean 14). In the 5-FU group pain was rated ‘mild’ by six patients, ‘moderate’ by two and ‘severe’ by two. Discomfort persisted in the 5-FU group for 7–42 d (mean 21). In assessment of intensity and duration of pain, more pain was found in the 5-FU group (p = 0.01). Comparison of total pain over time revealed no statistically significant difference in the median pain scores between the two groups |
Authors’ conclusions Topical ALA–PDT is more effective than topical 5-FU in the treatment of Bowen’s disease with fewer AEs. ALA should be considered one of the 1st-line therapeutic options for Bowen’s disease Brief study appraisal Methods of randomisation, allocation concealment and blinding of outcome assessors were not described. This study, although small, highlights the potential of PDT for Bowen’s disease but results should be confirmed in further trials |
Appendix 15 Basal cell carcinoma data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Bassett-Seguin et al. (2008)79 Data source Full published paper Country Not stated Language English Study design RCT (between-participant comparison) No. of participants Total: 120 randomised, 118 treated (219 lesions) Intervention: 60, 58 treated and analysed (103 lesions) Comparator: 58, 57 treated and analysed (98 lesions) No. of recruiting centres 13 across seven European countries Follow-up period and frequency 3 mth, then at 1, 2, 3, 4 and 5 yr |
Treatment intention Curative Type(s) of cancer and histology Primary superficial BCC Main eligibility criteria Patients aged 18 yr or older with up to 10 previously untreated primary superficial BCC lesions suitable for cryotherapy. Diagnosis confirmed using punch biopsy. Lesions had to have diameter > 6 mm but < 15 mm on face/scalp, < 20 mm on neck/extremities, or < 30 mm on trunk. Further eligibility criteria were reported Patient characteristics Not stated Concomitant treatment Concomitant treatment with immunosuppressive medication was prohibited |
Trial treatments MAL–PDT vs Cryotherapy Intervention MAL–PDT: A single treatment was initially given. Lesions were prepared by surface debridement. MAL cream, 160 mg/g, was applied in a layer of 1 mm to the lesion and 5 mm of surrounding tissue for 3 hr. The cream was washed off using a saline solution and the treated area was then illuminated with non-coherent red light (wavelength 570–670 nm) using a light dose of 75 J/cm. In patients with incomplete CR at 3-mth treatment was repeated (two consecutive MAL–PDT sessions 7 d apart) Comparator Cryotherapy: Cryotherapy was applied in two freeze–thaw cycles using liquid nitrogen spray applied to the lesion and a 3-mm surrounding area of healthy tissue. Procedure was repeated after a thaw period of two to three times the freeze duration. In patients with an incomplete response at 3-mth treatment was repeated (double freeze–thaw cryotherapy) |
Morbidity 3 mth (115 patients): Lesions with inCR after 3 mth were 32% in the PDT group and 30% in the cryotherapy group. CR rates did not differ between the groups (PDT: 97% vs cryotherapy: 95%, p = 0.49) 12 mth (105 patients): Fewer lesions recurred with MAL–PDT than with cryotherapy (8% vs 16%) More patients had an ‘excellent/good’ cosmetic outcome with MAL–PDT than with cryotherapy at 3 and 12 mth 36 mth (107 patients): Proportion of lesions in CR was 66% for MAL–PDT and 67% for cryotherapy (NS). 74% estimated CR rate in both groups according to per-protocol population. The lesion recurrence rates in lesions with CR 3 mth after the last treatment were 23% for MAL–PDT and 20% for cryotherapy The overall cosmetic outcome was rated by physicians as ‘excellent’ or ‘good’ for 89% of the MAL–PDT patients and 63% of the cryotherapy patients 5 yr: CR rates did not differ between the groups (PDT: 75% vs cryotherapy: 74%, p = 0.90). Cumulative recurrence rate after 5 yr was PDT: 22% and cryotherapy: 20%, p = 0.86 QoL and return to normal activity Cosmetic outcome was better with PDT at both 3 mth and 5 yr 3 mth: 30% of PDT patients had an ‘excellent’ outcome compared with 4% for cryotherapy (p = 0.0005) 5 yr: 60% of PDT patients had an ‘excellent’ outcome compared with 16% for cryotherapy (p = 0.00078) All cosmetic outcomes rated by investigators using a 4-point scale AEs AEs were reported by 73% (44/60) PDT patients and 79% (46/58) cryotherapy patients. Most AEs were local and transient, no patients discontinued as a result of treatment-related AEs Pain: 37% PDT, 33% cryotherapy Crusting: 35% PDT, 47% cryotherapy Erythema: 30% PDT, 21% cryotherapy Mild: 80% PDT 73% cryotherapy Moderate: 13% PDT, 25% cryotherapy Severe: 5% PDT, 1% cryotherapy |
Authors’ conclusions This study demonstrated that lesion recurrence rate with MAL–PDT treatment was comparable to double freeze thaw cryotherapy for treatment of superficial BCC and provided a better cosmetic outcome Brief study appraisal Overall this trial was well conducted clearly reported. The lack of a power calculation means it is unclear if there was no difference between the treatments, or if the study was underpowered to detect such a difference. This trial did include a long term FU of 5 yr as well as examination of safety, efficacy and cosmetic outcomes |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Berroeta et al. (2007)87 Linked publications191 Data source Full published paper (letter) Country UK Language English Study design RCT (between-participant comparison) No. of participants Total: 31 (40 lesions) Intervention: 18 (21 lesions) Comparator: 13 (19 lesions) No. of recruiting centres One Follow-up period and frequency FU to assess response at 3, 6 and 12 mth. FU to assess pain at 3, 6, 24 and 48 hr and also at 1 wk |
Treatment intention Curative Type(s) of cancer and histology Nodular BCC Main eligibility criteria Non-pregnant adults (18 or over) with well-defined BCCs ≤ 2cm on anatomically non-critical sites were eligible Patients with recurrent BCCs, or BCCs at high-risk sites or patients with immunodeficiency or photosensitivity were excluded % Male: 100 Concomitant treatment Not stated |
Trial treatments ALA–PDT (following superficial curettage) vs Excision surgery Intervention Initial 4-mm punch biopsy to assess tumour depth, followed by superficial curettage (without anaesthetic) and PDT with 630-nm laser after 20% ALA applied for 6 hr (under occlusion). Irradiance was 12 mW/cm2 and total dose 125 J/cm2. PDT repeated at 3 mth if residual BCC was clinically evident Comparator Excision with surgical margins as recommended by the British Association of Dermatologists. Scalpel surgery was performed under infiltrative lidocaine anaesthesia but surgical re-excisions were not conducted |
Morbidity For the PDT group 13/21 lesions (62%) were clear at 1 yr compared to 15/19 lesions (79%) in the surgery group, p = 0.24 There were five persistent BCCs in the PDT group but none in the surgery group (ns) QoL and return to normal activity There was no difference in mean scar severity (on a 1–4 scale) between the groups when judged independently by 10 non-medical men (1.9 for PDT vs 2.1 for surgery, p = 0.42) or 10 non-medical women (2.2 for PDT vs 2.5 for surgery, p = 0.23) AEs Median pain scores for the 1st BCC treated (scored out of 10) both during treatment, and immediately after treatment, were five for the PDT group, and 0 for surgery group (p = 0.001, and p = 0.004, respectively). Both groups had a score of zero at later assessments |
Authors’ conclusions There was no suggestion that PDT was, in general, better than surgery. PDT appears more painful than surgery for low-risk nBCCs. Surgery remains the 1st treatment choice for nBCCs Brief study appraisal This small pilot study was generally of high quality in its methods and reporting. The absence of anaesthetic for the PDT group before curettage may explain the differences in pain scores |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors de Haas et al. (2006)83 Data source Full published paper Country The Netherlands Language English Study design RCT (between-participant comparison) No. of participants Total: 154 (505 lesions) Intervention: 55 (262 lesions) Comparator: 100 (243 lesions) No. of recruiting centres One Follow-up period and frequency FU four times a year in 1st year, then twice yearly. Patients tending to develop more lesions were seen more frequently. Minimum FU period was 1 yr, maximum FU period was 5 yr |
Treatment intention Curative Type(s) of cancer and histology Primary superficial BCC Main eligibility criteria Not stated Patient characteristics Age range: 31–83 yr Mean age: 57 yr All participants were Caucasians Concomitant treatment Paracetamol, lidocaine (without adrenaline) or bupivacaine if required |
Trial treatments Fractionated illumination PDT vs single-illumination PDT Intervention Fractionated illumination PDT: Crusts and scaling were gently removed using a disposable curette before ALA application. Illumination using doses of 20 and 80 J/cm2 (at 50 mW/cm2) delivered 4 and 6 hr after administration of 20% ALA ointment (containing 2% lidocaine) with a 1-cm margin. One of three different light sources were used on each lesion (a 630-nm diode laser, coupled into a 600-µm optical fibre and using a combination of lenses for uniform fluence rate; a light-emitting diode 633 nm with a bandwidth of 20 nm; or a 2nd broadband source with an output of between 590 and 650 nm), with a margin of at least 5 mm. A light-protective bandage (including aluminium foil) was used to provide the 2-hr dark interval between fractions. Participants were instructed to stay out of the cold Comparator PDT with placebo cream: Patients received two cycles (1 wk apart) of placebo cream PDT. There was surface debridement and slight lesion debulking prior to PDT. BCC with partial clinical response at 3 mth were re-treated. Further parameters were not reported |
Morbidity CR of lesions was significantly greater using fractionated illumination compared with single illumination (at 1 yr, 97% vs 89%, p = 0.002). The results were very similar when analysis was undertaken on a subgroup of histologically proven BCCs. 10/262 (4%) lesions failed to respond, or recurred, in the fractionated-illumination group compared with 32/243 (13%) in the single-illumination group (p = 0.0002). There were no significant differences in response rates, within each illumination group, for the different light sources used QoL and return to normal activity Assessed but not reported AEs 5/100 (5%) patients required pain relief in the single illumination group compared to 15/55 (27%) patients in the fractionated illumination group |
Authors’ conclusions There is a significant increase in the CR rate of PDT using two-light fraction illumination scheme compared with a single-illumination scheme Brief study appraisal Although treatment methods were very well described, study design details on issues such as randomisation, blinding, and dropouts (were 154 or 155 patients treated?) were not provided, making it difficult to assess the reliability of the results |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Foley et al. (2003)77 Data source Abstract Country Australia Language English Study design RCT (between-participant comparison) No. of participants Total: 66 Intervention: 33 Comparator: 33 No. of recruiting centres Not stated Follow-up period and frequency Not clear, but appeared to be at least 6 mth |
Treatment intention Curative patients with histologically confirmed nBCC Type(s) of cancer and histology nBCC Main eligibility criteria Patients with histologically confirmed nBCC Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments PDT (methyl aminolevulinate) vs PDT (placebo cream) Intervention Lesions 1st prepared by debridement/debulking. PDT with 160 mg/g of methyl aminolevulinate cream and 3 hr of red light (570–670 nm) with a total light dose of 75 J/cm2. Treatment repeated after 7 d. Lesions with PR at 3 mth were re-treated. Further PDT parameters were not reported Comparator As for active PDT group, but using placebo cream |
Morbidity At 6 mth, histological evaluation there were no signs of malignancy in 73% of the active PDT group vs 21% in the placebo PDT group (p < 0.001) QoL and return to normal activity Cosmetic outcome rated as excellent or good in 95% of the active PDT patients AEs There were no treatment-related serious or systemic AEs. Burning, stinging, pain, and erythema were transient, and graded as mild or moderate |
Authors’ conclusions PDT is a good alternative to existing therapies, particularly in areas where an excellent cosmetic outcome is crucial Brief study appraisal Limited reporting of methods and results makes meaningful interpretation difficult. No details were reported on who assessed cosmetic outcomes, and whether outcome assessors were blinded. Outcomes not always reported for both groups, or broken down by group |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Kuijpers et al. (2006)84 Data source Full published paper Country The Netherlands Language English Study design RCT (between-participant comparison) No. of participants Total: 43 BCCs in 39 patients Intervention: 22 BCCs Comparator: 21 BCCs No. of recruiting centres One Follow-up period and frequency 8 wk |
Treatment intention Curative Type(s) of cancer and histology Nodular primary BCC Main eligibility criteria Patients with nodular primary BCC located anywhere on skin except periocular area and hairy scalp, with a clinical diameter smaller than 20 mm. Pigmented BCCs and patients with more than five BCCs were excluded, as were patients with porphyria, contraindications to surgery, or hypersensitivity to daylight or to either of the creams Patient characteristics % Male: 62 Age range: 39–87 yr Mean age: 68 yr Most tumours were less than 10 mm in diameter Concomitant treatment Topical emollient for pain |
Trial treatments PDT with 5-aminolevulinate (ALA–PDT) vs PDT with methyl aminolevulinate (MAL–PDT) Intervention ALA–PDT: All tumour tissue above skin level was removed by curettage (with ethyl chloride spray anaesthetic). The visible tumour, plus 5-mm margin, was covered in a layer of 20% ALA cream (around 2 mm thick) and polyurethane and opaque dressings were applied. After 3 hr the area was cleaned and illuminated with light of 600–730 nm (from metal halogen source) with an intensity of 100 mW/cm2 giving a total dose of 75 J/cm2. After illumination the area was covered with a light protective dressing for 1 d Procedure repeated after 7 d (but without debulking) Comparator MAL–PDT: Same methods as for PDT with 5-aminolevulinate group, except 16% methyl aminolevulinate cream was used instead of 20% ALA |
Morbidity There was no statistically significant difference in incomplete clearance rates [6/22 (27%) ALA vs 6/21 (29%) MAL, p = 0.92] QoL and return to normal activity Not assessed AEs Average intensity of pain did not differ significantly between groups (1st treatment: VAS = 4.4 for ALA vs 2.8 for MAL, p = 0.09, 2nd treatment: VAS = 4.8 for ALA vs 3.9 for MAL, p = 0.4), nor did character of pain. Most pain was described as being burning or stinging |
Authors’ conclusions The study found no difference in short-term efficacy between ALA–PDT and MAL–PDT, so both can be equally recommended as photosensitisers Brief study appraisal This pilot study blinded both patients and outcome assessors. However, there was no mention of a power calculation and the sample size was very small; a larger study would have been more informative, particularly on differences in pain scores |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Lui et al. (2004)68 Data source Full published paper Countries Canada, USA Language English Study design RCT (between-participant comparison) No. of participants Total: Not stated by diagnosis (387 lesions classified as BCC) Intervention: Superficial BCC 120 lesions; nBCC 47 lesions; BCC (not specified) zero Comparator: Superficial BCC 77 lesions; nBCC 30 lesions; BCC (not specified) nine 2nd comparator: Superficial BCC 80 lesions; nBCC 16 lesions, BCC (not specified) eight No. of recruiting centres Four Follow-up period and frequency FU at 6 wk, and 3, 6, 12, 18 and 24 mth (optional beyond 6 mth) |
Treatment intention Curative Type(s) of cancer and histology Superficial BCC, 277 lesions (66%); nBCC, 93 lesions (22%); Bowen’s disease, 34 lesions (8%); BCC unspecified 17 lesions (4%) Main eligibility criteria Patients with at least two biopsy-proven superficial or nBCC or Bowen’s lesions Patient characteristics Not stated for BCC only (study also included SCC) but overall: Average tumours treated per patient = eight Age range: 22–79 yr Mean age: 55 yr Most patients had Fitzpatrick skin type II or III Concomitant treatment Oral analgesic drugs for pain |
Trial treatments PDT at 60 J/cm2 vs PDT at 120 J/cm2 vs PDT at 180 J/cm2 Intervention PDT at 60 J/cm2: 10 min intravenous infusion of 14 mg/m2 verteporfin, followed 1–3 hr later by exposure to 60 J/cm2 of red light (688 ± 10 nm) from a non-thermal LED panel. The exposed area included a margin of 3–4 mm around the lesion. The irradiance delivered was 200 ± 40 mW/cm2. Tumours re-treated at 3 mth if necessary (with dose increased to 18 mg/m2) Comparator PDT at 120 J/cm2: See above 2nd comparator PDT at 180 J/cm2: See above |
Morbidity At 6 mth, the histopathological response (i.e. no residual tumour) was: nBCC: 76% at 60 J/cm2, 82% at 120 J/cm2, and 100% at 180 J/cm2; superficial BCC: 63%, 80%, and 97%; BCC not specified: 0%, 56% and 75% There was a trend indicating a better response with a higher light dose (p = 0.06) QoL and return to normal activity Reported by light dose rather than tumour type AEs Reported by light dose, rather than by tumour type |
Authors’ conclusions A single course of verteporfin PDT showed treatment benefit for patients with multiple non-melanoma skin cancers Brief study appraisal No clinically relevant comparator treatment was used, there was a lack of information on issues such as blinding and allocation concealment, and the authors did not present many results and population details by diagnosis. It is therefore difficult to make any reliable conclusions about the efficacy of verteporfin in patients with BCC |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Mosterd et al. (2008)86 Data source Full published paper Country The Netherlands Language English Study design RCT (between-participant comparison) No. of participants Total: 149 (173 lesions randomised, 171 treated) Intervention: 85 lesions Comparator: 88 lesions No. of recruiting centres One Follow-up period and frequency 1–2 wk for surgery, then 3, 6, 12 and 18 mth; 2, 3, 4 and 5 yr |
Treatment intention Curative Type(s) of cancer and histology nBCC Main eligibility criteria Previously untreated primary nBCC with maximum diameter of 20 mm in patients of 18 yr or older. Pregnancy, life expectancy of less than 5 yr and use of photosensitive drugs were exclusion criteria. Tumours were excluded if recurrent, pigmented or located on hairy or concave areas. Further details were reported Patient characteristics % Male: 50 Mean age: 65 yr Most tumours were located on the forehead/temple, back or nose area. Maximum mean tumour diameter 9.1 mm Concomitant treatment Not stated |
Trial treatments ALA–PDT vs Surgical excision Intervention ALA–PDT: Partial tumour debulking performed under local anaesthetic 3 wk prior to PDT treatment. 20% ALA cream applied to lesion including 5-mm surrounding area and covered with occlusive dressing for 4 hr. Lesion then illuminated using a broadband metal-halogen light source for 15 min with intensity of 100 mWcm2 and dose of 75 J/cm2. Area was then covered and re-illuminated after 60 min. This produced a fractionated treatment on the same day with a total light dose of 150 J/cm2. Any incomplete responses or recurrent tumours were re-treated surgically Comparator Surgical Excision: Local anaesthetic using lidocaine (1%) with adrenaline followed by excision of the tumour and a 3-mm surrounding margin. Closure was by sutures or transposition/transplantation depending on lesion location. Sections of the lateral and deep margins were histologically examined; if residual tumour was found then this was regarded as a treatment failure and re-excisions were performed until margins were free from tumour |
Morbidity 3 mth: 78/83 (94%) CR in PDT and 86/88 (98%) in SE patients, p = 0.27 Failure rates: Cumulative incidence of failure probability at 3 yr was 2.3% for SE and 30.3% for PDT (p < 0.001) QoL and return to normal activity Not assessed AEs No serious complications were observed |
Authors’ conclusions Treatment of nBCC with SE is significantly more effective than treatment with ALA–PDT after debulking. PDT should not therefore be used as a standard treatment for nBCC Brief study appraisal This was a well-conducted and generally well-reported study, which draws appropriate conclusions and can be considered to be reliable. As the authors suggest further studies are required to explore possible variations in PDT treatment |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Rhodes et al. (2007)85 Data source Full published paper Countries Not stated ‘European hospitals’ Language English Study design RCT (between-participant comparison) No. of participants Total: 103 randomised (101 treated) Intervention: 53 (60 lesions) Comparator: 50 (58 lesions) No. of recruiting centres 13 Follow-up period and frequency 3 mth, then at 1, 2, 3, 4 and 5 yr |
Treatment intention Curative Type(s) of cancer and histology Primary nBCC Main eligibility criteria Histologically confirmed nBCC, previously untreated in patients ≥ 18 yr. Patients with more than 10 lesions, porphyria, or Gorlin syndrome were excluded. Further exclusion criteria were reported Patient characteristics % Male: 60 Age range: 38–95 yr Mean age: 69 yr (PDT group), 67 yr (surgery group) Around 90% of patients had only one lesion, and around three-quarters of lesions were between 5 and 14 mm in diameter. The majority of patients were classified as Fitzpatrick skin type II or III, and lesions were mostly on the face/scalp/trunk and neck |
Trial treatments MAL–PDT vs excision surgery Intervention Surface scale removed using scalpel or curette (without anaesthesia). Then one or two PDT cycles with methyl aminolevulinate (160 mg/g), each comprising of two sessions (1 wk apart). Cream was applied 1 mm thick and to 5 mm of surrounding tissue, then covered with an occlusive dressing for 3 hr. Cream then washed off with 0.9% saline solution immediately before illumination with non-coherent red light (570–670 nm, total fluence 75 J/cm2, fluence rate of 50 to 200 mW/cm2), mean light density of 127 mW/cm2 from a standard light source If not CR by 3mth, 2nd treatment cycle administered. 76% of lesions treated with one cycle only Comparator Simple elliptical excision surgery with at least 5-mm margins. Local anaesthesia |
Mortality Four patients in each group died during FU (all considered to be unrelated to treatment) Morbidity At 3 mth: 48 lesions (91%, 50 patients) in the PDT group, and 51 lesions (98%, 47 patients) in the surgery group, showed CR, mean diff = 4.8%, ns. At 1 yr: 44/53 PDT lesions (83%) had CR vs 50/52 (96%) surgery lesions (p = 0.15). Recurrence was 4% in PDT group vs 0% in surgery group. At 2 yr: 32/53 (60%) PDT lesions had CR vs 44/52 (85%) surgery lesions. By this stage, 11 (21%) of PDT lesions were lost to FU vs six (11%) surgery lesions. Recurrence was 9% in PDT group vs 2% in surgery group. At 36 mth: CR 79% in PDT group vs 96% in surgery group. Recurrence was 10% in PDT group vs 2% in surgery group. At 5 yr: CR was 76% in the PDT group and 96% in the surgery group (per-protocol population, p = 0.01). There was recurrence in 14% of lesions in PDT group and 4% in the surgery group (p = 0.09). Only one lesion (in the surgery group) recurred within the 3- to 5-yr FU period. In the PDT group, there was no evidence that the recurrence rate was higher in larger lesions QoL and return to normal activity Cosmetic outcome was rated by investigator as being: At 3mth, excellent or good in 36/44 patients (82%) having PDT vs 15/45 patients (33%) having surgery (p < 0.001). At 1 yr, excellent or good in 33 of 42 (79%) PDT patients vs 17 of 45 (38%) surgery patients (p < 0.001). At 2 yr, excellent or good in 24/29 (83%) PDT patients vs 16 of 39 (41%) surgery patients (p < 0.001). At 36 mth, excellent or good in 83% of PDT patients vs 37% surgery patients. At 5yr, excellent or good in 27 of 31 (87%) PDT patients vs 19 of 35 (54%) surgery patients, p = 0.007). Patients also rated global cosmetic outcome on a 4-point scale at 3, 12 and 24 mth. No significant difference at 3 mth, at 12 mth excellent or good in 41/42 (98%) for PDT patients vs 36/43 (84%) for surgery, p = 0.03. At 24 mth, PDT patients reported 28/29 (97%) vs 27/36 (75%) for surgery, p = 0.04 AEs More PDT patients reported AEs [27/52 (52%) vs 14/49(29%), p = 0.03]. Most AEs were transient local reactions such as burning sensations, skin pain, or erythema. One PDT patient had to stop treatment due to a severe burning sensation; three surgery patients had skin infections |
Authors’ conclusions Long-term FU indicates superior efficacy of surgery to PDT. However, PDT is also an effective treatment and exhibits a more favourable cosmetic outcome Brief study appraisal This generally well-conducted trial, which had a long FU period, was reported in two papers and four abstracts. The results are likely to be reliable, although the 3-mth CR results did vary slightly between reports |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Schleier et al. (2007)81 Data source Full published paper Country Germany Language English Study design RCT (between-participant comparison) No. of participants Total: 24 (112 lesions) Intervention: 13 Comparator: 11 No. of recruiting centres One Follow-up period and frequency 2, 4 and 12 wk and 6 mth after primary treatment |
Treatment intention Curative Type(s) of cancer and histology Superficial BCC Main eligibility criteria Histologically verified BCC of the skin, histologically proven superficial BCC with no deep infiltration (< 2 mm), no morpheic and pigmented BCC and good patient compliance. Exclusion criteria were: Unclear histology, clinically nBCC, expected poor compliance of the patient, untreated diabetes mellitus and pregnancy Patient characteristics % Male: 54 Age range: 42–96 yr Mean age 74 yr The vast majority of the tumours were located in the head and neck area. The average diameter of the lesions was 7 mm (range 3–12 mm). Three patients with GGS were included in the study Concomitant treatment Not stated |
Trial treatments ALA-based PDT vs mALA-based PDT Intervention ALA and mALA gels were prepared less than 1hr before treatment by dissolving in a cold (approx 4°C) thermo gel (Lutrol F-127) up to a concentration of 10% of ALA (mALA)/ml (w/v). The gel was applied 3 mm beyond the visible margin of the tumour and was approximately 5 mm thick. The area was covered with plaster and protected from light. 3 hr later, residues were removed and tumour areas circled with a blue skin marker. The lesion was then illuminated with a diode laser equipped with a microlens fibre. The power density was 0.1 W/cm2 and the energy density was 120 J/cm2. A diameter of the irradiated area of approximately 10 mm was selected and distance laser-diffuser-skin corresponded to 15 mm. The procedure was performed with or without local anaesthesia according to the pain management needs of the patient. In cases where treatment was only partially successful, the therapy was repeated after the final examination (12th wk). Further PDT parameters were not reported Comparator See ‘Intervention’ for details |
Morbidity ALA group: 44 of 72 BCC (61%) showed a CR 12 wk after the 1st treatment vs mALA group: 23 of 40 BCC (58%) NS There was no statistically significant difference in partial successes (reduction of the diameter of the BCC of at least 50% of the initial tumour size) between the groups. Three tumours (4%) in the ALA group and one BCC (3%) did not respond to treatment and showed no reduction in tumour size. These patients were given surgical treatment. Eight BCCs in the ALA group and five in the mALA group developed a recurrence during the 6-mth period. After a second PDT, seven lesions in the ALA group and seven in the mALA group were treated successfully QoL and return to normal activity Not assessed AEs During illumination, eight ALA patients and five mALA patients experienced moderately painful sensations in the treated are (1–4 on the pain scale). Two patients in the mALA group had stabbing pain sensations (level 6–7 on the pain scale) during the laser application and had to be treated with local anaesthetic. Five patients in ALA group and two in mALA group felt moderate pain sensations up to the 3rd day post illumination (1–3 on the pain scale) |
Authors’ conclusions The therapeutic outcome of this pilot study showed no difference between PDT with ALA and mALA. This preliminary result will require confirmation in further research Brief study appraisal This was a pilot study in preparation for a larger clinical trial. As such, it is likely to have been underpowered to detect statistically significant differences for at least some of the outcomes investigated. Treatment methods were well described but study methods, such as methods of randomisation, concealment of allocation and blinding, were not reported in detail |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Soler et al. (2000)82 Data source Full published paper Country Norway Language English Study design RCT (between-participant comparison) No. of participants Total: 83 (245 lesions) Intervention: 41 (111 lesions) Comparator: 42 (134 lesions) No. of recruiting centres One Follow-up period and frequency FU at 1 wk, and 3 and 6 mth. Some participants also followed up after 1 and 2 yr |
Treatment intention Curative Type(s) of cancer and histology Superficial BCC Main eligibility criteria Patients with histologically/cytologically confirmed superficial BCC with thickness < 1 mm, and diameter < 3 cm. Patients with fewer than six lesions Patient characteristics % Male: 47 Mean age: 62 yr All Caucasian Concomitant treatment Not stated |
Trial treatments PDT-laser vs PDT-broadband lamp Intervention PDT-laser: Pre-treatment with dressing soaked with 99% dimethylsulphoxide for 15 min followed by 20% ALA cream and covering with occlusive dressing for 3h. Cream was washed off before exposure to light of 630 nm from a copper vapour laser pumping a dye laser. Irradiance of 120–150 mW/cm2, and a light dose of 100–150 J/cm2 (median dose 100 J/cm2) Comparator PDT-broadband lamp: As for PDT-laser except light source was a 150 W halogen bulb broadband lamp, giving filtered light of between 570 and 740 nm. Irradiance was 100–180 mW/cm2 and total light dose ranged from 150–200 J/cm2 with median light dose of 200 J/cm2. Total irradiance including infra-red was 135–240 mW/cm2 |
Morbidity Overall, there were no statistically significant differences (p = 0.49) in response rates (complete, partial, or none) between the groups [CR was 95/111 lesions (86%) for laser vs 110/134 (82%) for lamp]. Patients with CR were followed up beyond the protocol 6-mth period. Data were presented, but not statistically analysed – recurrence after 2 yrs occurred in four lesions for the laser group, and five lesions for the lamp group QoL and return to normal activity Overall there were no statistically significant differences in cosmetic results (p = 0.075). Results were scored as being excellent or good in 80 lesions (84%) in the laser group vs 102 lesions (92%) in the lamp group AEs During the 1st wk after treatment 68% of the laser group and 74% of the lamp group patients reported some degree of discomfort (e.g. stinging, itching, pain). There were no statistically significant differences between the groups for AEs either during or after treatment. No SAEs were reported during 6-mth FU period |
Authors’ conclusions Topical ALA–PDT with a broadband halogen light source gives cure rates and cosmetic outcome similar to those obtained with a laser source Brief study appraisal Although this was quite a well-conducted study, there were still issues which question the reliability of its results: the authors acknowledged that the optimum wavelength for ALA–PDT is 635 nm, but 630 nm was used for the laser group, for reasons of practicality; light doses varied between patients within a treatment group; and the M/F ratio differed substantially between the treatment groups |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Szeimies et al. (2008)80 Data source Full published paper Countries Australia, Germany, Switzerland, UK Language English Study design RCT (between-participant comparison) No. of participants Total: 196 (182 analysed) Intervention: 100 Comparator: 96 No. of recruiting centres 27: 10 in UK, 10 in Germany, two in Switzerland, five in Australia Follow-up period and frequency 3, 6 and 12 mth |
Treatment intention Curative Type(s) of cancer and histology Superficial BCC Main eligibility criteria Patients over 18 yr with histologically confirmed primary sBCC suitable for simple excision surgery. Patients with more than five lesions, lesions in the mid-face area, lesions smaller than 8 mm or larger than 20 mm were excluded (other criteria reported) Patient characteristics % Male: 67 Age range: 31–92 yr Mean age: 64 yr All patients were Caucasian, and the mean no. of lesions per patient was 1.4 (range 1–5). Most lesions were located on the trunk or neck Concomitant treatment concurrent treatment on the lesion areas was not permitted |
Trial treatments MAL–PDT vs Surgery Intervention MAL–PDT: Patients received two treatment sessions, 7 d apart. Lesions were prepared prior to each session if deemed necessary by removing crusts and roughening the surface. 160 mg/g of MAL cream was applied 1 mm thick to the lesion and surround 5–10 mm of skin and covered with an occlusive dressing for 3 hr. Cream was washed off using saline solution and the area exposed to red light from a large-field LED source for between 7 and 10 min, total light dose 37 J/cm2. Mini-desk fans were provided to cool the irradiation sites during light exposure Comparator Surgery: One simple elliptical excision surgery was performed according to the investigators routine practice with an estimated 3-mm margin from estimated edge of the lesion |
Morbidity (all per protocol analyses) 3-mth complete lesion response: 118/128 (92%) for PDT vs 117/118 (99%) in surgery 12-mth lesion recurrence: 11/118 (9%) for PDT vs 0.117 (0%) for surgery QoL and return to normal activity Cosmetic outcome assessed by patient and investigator, in both cases PDT treatment was judged to be superior 12-mth investigator rated assessment: 77/83 (93%) for PDT and 44/86 (51%) for surgery were considered as a ‘success’, p < 0.001 AEs Treatment-related AE were higher in the PDT (37%) than surgery (15%) group. Most related AEs were of mild to moderate severity and were most commonly photosensitivity (31%) reaction for PDT and wound infection (5%) for surgery patients 11% of PDT patients with related AEs required treatment while 57% of surgery patients with related AEs needed treatment No SAEs were recorded that were considered to be related to either treatment |
Authors’ conclusions MAL–PDT has high levels of efficacy and excellent cosmetic outcomes when treating sBCC and should be considered as an alternative to surgery Brief study appraisal This was a well-reported and conducted trial; however, longer-term FU would provide useful outcome data on the recurrence rates. It was not clear if the study was adequately powered to show equivalence of treatments. Given that included patients were restricted to those eligible for surgery, as the authors have highlighted it seems plausible that PDT may be more effective than shown here |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Tope et al. (2004)78 Data source Abstract Country USA Language English Study design RCT (between-participant comparison) No. of participants Total: 65 (80 lesions) Intervention: 33 patients (41 lesions) Comparator: 32 patients (39 lesions) No. of recruiting centres Not stated but described as being multicentre Follow-up period and frequency Not clear, but appeared to be at least 6 mth |
Treatment intention Curative Type(s) of cancer and histology nBCC Main eligibility criteria Not stated Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments MAL–PDT (methyl aminolevulinate) vs PDT (placebo cream) Intervention MAL–PDT: Patients received two cycles (1 wk apart) of methyl aminolevulinate PDT. There was surface debridement and slight lesion debulking prior to PDT. BCC with partial clinical response at 3 mth were re-treated. Further PDT parameters were not reported Comparator As for MAL–PDT using placebo cream |
Morbidity Complete clinical response was 80% (33/41 lesions) for active PDT vs 51% (20/39 lesions) for placebo PDT. Complete histological response was 78% (32/41 lesions) vs 33% (13/39 lesions), both appeared to be at p < 0.001 QoL and return to normal activity For sites showing complete clinical response, investigator-assessed cosmetic outcome was excellent or good in 93% of active PDT vs 90% of placebo PDT Patient satisfaction with PDT compared with previous treatment was better in 60% of MAL–PDT patients, and 52% in placebo PDT patients AEs There were no systemic AEs in either group. Local AEs: 91% in the active group vs 75% in the placebo group. Mild to moderate erythema, burning, stinging, and pain found in both groups. Pain occurred for a median of 2 d in active PDT group vs 3–6 d in placebo PDT group. All SAEs in both groups were not related to treatment |
Authors’ conclusions PDT was clinically and histologically superior to placebo PDT in treating nBCC Brief study appraisal Very little information was available in this abstract. It was not always clear whether results were for lesions or individual patients |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Wang et al. (2001)88 Data source Full published paper Country Sweden Language English Study design RCT (between-participant comparison) No. of participants Total: 88 Intervention: 47 Comparator: 41 No. of recruiting centres One Follow-up period and frequency FU at 1, 4, 8 and 12 wk, and at 1 yr |
Treatment intention Curative Type(s) of cancer and histology Non-morphoeic BCCs (superficial and nodular) Main eligibility criteria Patients aged 20–90, with histopathologically verified BCCs suitable for both PDT and cryosurgery, were eligible. Exclusion criteria (e.g. pregnancy) were also reported Patient characteristics % Male: 50 Age range: 42–88 yr There were 39 patients with superficial BCCs and 49 with nBCCs. 54% were on the trunk, 28% on head and neck, 11% on legs, and 7% on arms Concomitant treatment Local anaesthetic available during procedures. Use of analgesic drugs was permitted for pain relief during the week following procedures |
Trial treatments ALA–PDT vs Cryotherapy Intervention Lesions were 1st prepared (removal of stratum corneum material using scalpel/96% alcohol/isotonic saline). 20% ALA was then applied to lesion with 1-cm margin, and covered with a thin occlusive dressing. 6 hr after ALA, 635-nm light through a 600-µm optical fibre (with a clear-cut polished end) from a Nd:YAG laser was applied. The single light dose was 60 J/cm2, and the mean fluence rate 80 mW/cm2. Larger lesions had to be illuminated with more than one light source. Patients with pain during light exposure received water spray at 15–20°C. Additional treatment given if there was evidence of residual tumour growth at the 4, 8 or 12 wk examinations Comparator Treatment with a liquid nitrogen unit using a spray technique. Two freeze–thaw cycles were given, and the area frozen for 25–30 s each time, with a thawing period of 2–4 min in between. Additional treatment given if there was evidence of residual tumour growth at the 4-, 8- or 12-wk examinations |
Mortality One patient died in each group after the 3-mth FU. Both deaths were unrelated to BCC and its treatment Morbidity More participants in the PDT group had to be re-treated (13/44, 30%) compared with the cryosurgery group (1/39, 3%). The recurrence rate at 1 yr was higher in the PDT group (11/44, 25% vs 6/39, 15%), though not statistically significant (and the PDT group had fewer clinically obvious recurrences). After 1 wk, the PDT group had a significantly shorter healing time in terms of leakage and oedema (p < 0.001), but not erythema. There was also a significant difference in leakage at 1 mth, favouring the PDT group QoL and return to normal activity The cosmetic outcome was significantly better at 1 yr in the PDT group for hypopigmentation, scar formation, tissue defects (all p < 0.001), and hyperpigmentation (p < 0.05) AEs There was no statistically significant difference in mean pain VAS scores during treatment (PDT 43 vs cryosurgery 32). One PDT patient required local anaesthetic. One cryosurgery patient developed a bacterial infection at the treatment site. During the 1st week post treatment eight PDT patients and two cryosurgery patients used analgesic medication (p < 0.05) |
Authors’ conclusions ALA–PDT is comparable with cryosurgery as a treatment modality for BCCs. Retreatments are more common with PDT, but this can easily be performed due to shorter healing times, less scarring, and better cosmetic outcome, which follows ALA–PDT Brief study appraisal This study was generally quite well conducted and the results are likely to be reliable. However, more information on losses to FU and any sample size calculation used, would have been useful |
Appendix 16 Barrett’s oesophagus data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Ackroyd et al. (2000)95 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 36 Intervention: 18 Comparator: 18 No. of recruiting centres Not stated Follow-up period and frequency FU at 1, 6, 12 and 24 mth |
Treatment intention Curative Type(s) of cancer and histology BO – LGD Main eligibility criteria Patients with LGD in circumferential BO of at least 3 cm in length, who were receiving omeprazole were eligible. However, histological re-examination after biopsy had to confirm the diagnosis Patient characteristics % Male: 83 Age range: 30–71 yr Median age: 56 yr Range of pre-treatment lengths of Barrett’s: 3–15 cm Concomitant treatment Patients were given analgesic and antiemetic drugs as required following treatment. Patients were also supplied with antacids to take as needed. Throughout the treatment and FU period patients were maintained on 20 mg omeprazole daily |
Trial treatments ALA–PDT vs Placebo-PDT Intervention Patients drank 30 mg/kg ALA (dissolved in 50 ml of orange juice) followed 4 hr later by laser endoscopy (under intravenous sedation and analgesia) when the extent of Barrett’s area was recorded. A copper vapour laser delivered by a fibre with a diffuser tip was used to deliver green light (514 nm) at a power density of 120 mW/cm2 for 500 s per 3-cm length. All patients had two separate treatments (distal, then proximal, total treatment time 1000 s, energy density 60 J/cm2) so that 6 cm of oesophagus was treated (upper 6 cm of Barrett’s mucosa). This represented complete treatment of Barrett’s epithelium in one-half of the patients. Patients remained in hospital until dark, and were advised to avoid bright light for 24 hr Comparator As for above, except orange juice alone was used as placebo |
Mortality Not assessed Morbidity In the PDT group 16/18 (89%) showed macroscopic evidence of regression at FU endoscopy, compared with 2/18 (11%) in the placebo group; the corresponding median reduction in areas were 30% for PDT vs 0% for placebo (both p < 0.001). All regression cases displayed normal squamous mucosa when biopsied. There was a reduction in prevalence of dysplasia in favour of the PDT group (0/18 vs 12/18, p < 0.001). Although it was unclear as to which FU point these results relate to, the authors did state that the effects of treatment were maintained for up to 24 mth AEs All PDT patients experienced chest pain during treatment that persisted for 3–5 d, and was aggravated by swallowing or coughing. One patient developed a mild skin rash on exposure to sunlight (resolved within 48 hr). No patients complained of dysphagia. No results appeared to have been reported for the placebo group Resource use Not assessed |
Authors’ conclusions ALA–PDT can provide safe and effective ablation of low-grade dysplastic epithelium Brief study appraisal This small study was generally well conducted, and the results appear reliable. However, it should be noted that no results appear to have been reported on AEs in the placebo group, and it was unclear to which FU the main study results relate |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Ackroyd et al. (1996)96 Data source Abstract Country UK Language English Study design RCT No. of participants Total: 28 Intervention: Not stated Comparator: Not stated No. of recruiting centres Not stated Follow-up period and frequency Not stated |
Treatment intention Curative (dosing study) Type(s) of cancer and histology Dysplastic BO Main eligibility criteria Not stated Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments ALA–PDT 30 mg/kg vs ALA–PDT 50 mg/kg vs placebo Intervention Oral ALA at 30 or 50 mg/kg, or placebo, was followed 4 hr later by light administration. No further parameters were reported Comparator See ‘Intervention’ 2nd comparator See ‘Intervention’ |
Mortality Not assessed Morbidity Not assessed AEs In the 30-mg/kg group, one patient had mild photosensitivity, but no other AEs were seen. In the 50-mg/kg group, oesophageal discomfort, hair loss, and transient disturbance of liver function test results were observed Resource use Not assessed |
Authors’ conclusions ALA–PDT at 30 mg/kg should provide optimal treatment conditions in BO Brief study appraisal The absence of important methodological, population, and result details in this abstract of a small study, means it is difficult to assess the reliability of its results |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Hage et al. (2004)97 Linked publications198 Data source Full published paper Country The Netherlands Language English Study design RCT No. of participants Total: 40 Intervention: PDT 20 + 100: 13 Comparator: PDT100: 13 2nd comparator: APC: 14 No. of recruiting centres Not stated Follow-up period and frequency 6 wk, 6, 12, 18 and 24 mth |
Treatment intention Curative Type(s) of cancer and histology Patients with BO without dysplasia or with LGD Main eligibility criteria Patients 18 yr or over with BO without dysplasia or with LGD on histological examination were eligible. Patients had to have a BO length of 2–5 cm and specialised intestinal metaplasia. All patients were taking PPIs for at least 6 mth before treatment. Exclusion criteria were intolerance to (repeated) endoscopy, pregnancy, acute porphyria and intercurrent diseases precluding survival during the study period Patient characteristics % Male: 78 Median age: 59 yr Age range: 41–72 yr Mean BO length: 3 cm (range 2–5 cm) Dysplasia: None 32; LGD eight Concomitant treatment If complete elimination of BO was not achieved by the designated treatment at 6 wk, the remaining BO was ablated by additional APC with a maximum of two sessions at 4-wk intervals. Patients were treated with a daily dose of at least 40 mg of omeprazole for the duration of the study. Mean dose 47.5 mg (range 40–80mg) |
Trial treatments PDT with fractionated dose (20 + 100) ALA vs PDT with single-dose ALA vs APC Intervention Fractionated PDT: 60 mg/kg ALA was dissolved in 20 ml of orange juice. All patients were kept in a darkened room for 36 hr. A KTP/532 dye laser module was used to deliver light at a wavelength of 630 nm. PDT was performed with a fluence of 20 J/cm2 at 1 hr and 100 J/cm2 at 4 hr after ALA administration. Light delivery was performed using an inflatable balloon with an inflated diameter of 2.5 cm. Calculated total fluence rate was 100 mW/cm2 Comparator Single-dose ALA–PDT: As for fractionated PDT with the exception that there was a single illumination of 100 J/cm2 at 4 hr after ALA administration 2nd comparator APC: an Argon Beamer 2 device, APC 300 was used with a gas flow rate of 2 l/min at a power setting of 65 W. The aim was to ablate two-thirds of the oesophageal circumference of BO during the 1st session and in the following session to ablate the remainder. APC involved a maximum of two treatment sessions per patient at 4-wk intervals |
Mortality Not assessed Morbidity At 6 wk, mean endoscopic BO surface reduction was 51% (range 20–100%) in the single dose PDT group, 86% (range 0–100%) in the fractionated PDT group and 93% (range 40–100%) in the APC group. This was statistically significant for the comparison between single-dose PDT and fractionated dose PDT and single-dose PDT and APC. Differences between fractionated dose PDT and APC were not significant. Rates of complete ablation were ns between the groups. 6-, 12- and 18-mth data not extracted as patients were then eligible to receive APC AEs 23 of 26 patients across the two PDT groups and five of 14 in the APC group experienced pain during treatment (p < 0.01). There were more cases of nausea and vomiting with PDT (7 vs 0 in APC, p < 0.05) and patients had more elevated liver enzyme results in tests (20 vs 0, p < 0.01). Differences in odynophagia, fever, sudden death and stricture formation were not significant Resource use Not assessed |
Authors’ conclusions APC alone or ALA–PDT in combination with APC can lead to complete reversal of Barrett’s epithelium in at least two-thirds of patients when administered in multiple treatment sessions. The authors did not recommend use of these techniques for prophylactic ablation of BO Brief study appraisal This was a small trial and the low numbers of patients across the three groups may have meant there was insufficient power to detect treatment differences where they existed. A further problem is that all patients who did not respond adequately were given APC, so the long-term effect of PDT alone is unclear |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Kelty et al. (2004)102 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 25 Intervention: Five Comparator: Five 2nd comparator: Five 3rd Comparator: Five 4th Comparator: Five No. of recruiting centres One Follow-up period and frequency 4 wk |
Treatment intention Curative Type(s) of cancer and histology Non-dysplastic BO Main eligibility criteria Patients already participating in a large cohort study on BO with biopsy proven Barrett’s epithelium Patient characteristics % Male: 80 Median age: 63 yr Age range: 31–81 yr Median length of Barrett’s epithelium was 4 cm (range 2–15 cm), no patients had high or LGD on biopsy Concomitant treatment 40 mg esomeprazole daily |
Trial treatments ALA–PDT at various doses (30 mg/kg or 60 mg/kg) at 4- or 6-hr incubation times or with fractionated illumination Intervention 30 mg/kg ALA–PDT, light delivered by endoscopy after 4-hr PDT protocol: ALA dissolved in 50 ml of orange juice and taken orally. Patients kept in dimly lit room prior to treatment. At appropriate time patients underwent endoscopy with intravenous sedation, analgesia and an antiemetic drug. Balloon applicator was placed over a guidewire in the oesophagus and position confirmed endoscopically. Balloon was inflated to around 20 mmHg and light delivered by a 5-cm cylindrical diffuser fibre. Red light (635 nm, 2W diode laser) was used at fluence rate of 68 mW/cm2 for a total dose of 85 J/cm2. Patients recovered in a dimly lit room, discharged with oral analgesia and advice to avoid bright lights for 24 hr Comparator 30 mg/kg ALA–PDT, light delivered by endoscopy after 6 hr 2nd comparator 30 mg/kg ALA–PDT repeated at 2 hr, light delivered by endoscopy after 4 hr 3rd comparator 60 mg/kg ALA–PDT, light delivered by endoscopy after 4 hr 4th comparator 60 mg/kg ALA–PDT, light delivered by endoscopy after 6 hr |
Mortality Not assessed Morbidity At 1 mth FU all patients showed a reduction in the area of Barrett’s epithelium in the treated area, median reduction for all 25 patients was 60%. Median reduction in area varied between 30% and 60% for each treatment group. The greatest reductions were seen in the fractionated and 30 mg/kg groups (all 60%), but this difference was not statistically significant AEs No major AEs – no perforations or strictures. Significant N&V occurred in 32% of patients who required further anti-emetic treatment. N&V was more common in patients who received the higher dose of ALA. Five patients had a documented photosensitivity reaction – all were mild cases Resource use Not assessed |
Authors’ conclusions Low dose ALA–PDT appears to be a safe protocol for the ablation of BO. The authors recommend that ALA should be given orally as 30 mg/kg 4- to 6-hr before activation and could be taken at home Brief study appraisal This was a small study that aimed to establish optimum dosage regimes. Although patients were randomised to treatment, no information on blinding or allocation concealment was provided. The sample size appears to have been too small to view the authors’ conclusions as being reliable |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Kelty et al. (2004)98 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 72 Intervention: 35 (PDT) Comparator: 37 (APC) No. of recruiting centres One Follow-up period and frequency 1 day, 4 wk then 6, 12 and 24 mth after successful treatment or five sessions |
Treatment intention Curative Type(s) of cancer and histology BO Main eligibility criteria Patients were invited to take part from an endoscopic screening programme (over 150 were approached). No further details reported Patient characteristics % Male: 81 Age range: 28–83yr Median age: 61 yr All patients had biopsy proven Barrett’s epithelium (median length of 4 cm, range 2–15cm). No patients had high or LGD Concomitant treatment 40 mg esomeprazole daily, with oral analgesia as required |
Trial treatments ALA–PDT vs APC Intervention ALA–PDT: 30 mg/kg of ALA dissolved in 50 ml of orange juice taken orally, patient kept in a dim room prior to treatment (46 hr later). Endoscopy was carried out (with intravenous sedation, analgesia and an antiemetic) and a balloon applicator was placed over a guidewire (position confirmed endoscopically) and inflated to approximately 20 mmHg. Light was delivered using a cylindrical diffuser fibre – red laser light (635 nm 3W) at a fluence rate of 68 mW/cm2 and total light dose of 85 J/cm2. Patients were discharged and advised to avoid bright lights for 24 hr. Follow-up at 4 wk – if residual Barrett’s epithelium patient was re-treated until re-epithelisation was complete or to a maximum of five treatments Comparator APC: endoscopy as per PDT protocol. APC generator set with gas flow of 2 l/min and power setting of 65 W. APC probe passed down biopsy channel and positioned with tip of probe 1 cm distal to the end of the scope. APC performed in a linear fashion coagulating strips of tissue approximately 2 mm wide at each pass. One-half of the affected circumference was treated at any one sitting on the rationale of reducing chances of stricture. Repeat treatment as per PDT but using APC |
Mortality Not assessed Morbidity Significantly fewer treatments were performed in the APC group (median 3) than in the PDT group (median 5), p = 0.016. The median number of treatments required for successful ablation was two in the PDT group and three in the APC group, p = 0.189 Complete macroscopic reversal of the columnar segment to squamous epithelium was achieved in 50% of PDT patients and 97% of APC patients, p < 0.0001 AEs Major side effects for PDT were minimal with no strictures or perforations. Significant N&V occurred in 32% of patients who required further antiemetic treatment. Five patients reported cutaneous photosensitivity (mild erythema and pain). All APC patients reported discomfort and 91% reported transient dysphagia and odynophagia. All were resolved with oral analgesia over 3 d. No oesophageal perforations occurred, one patient developed dysphagia to solids and required four dilatations Resource use Not assessed |
Authors’ conclusions PDT and APC are both effective for ablating BO. APC appears more effective but larger studies should assess impact on carcinoma development Brief study appraisal This was a relatively robust comparative trial (despite the sample size), which would also have benefited from the use of blinded outcome assessors. However, this study, like its related dosing study102 was of BO patients without dysplasia; such patients are often not treated at all, so the results appear to be of limited use in relation to clinical practice |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Mackenzie et al. (2008)105 Data source Full paper Country UK Language English Study design RCT No. of participants Total: 27 Intervention (ALA with red light): 14 Comparator: (ALA with green light): 13 No. of recruiting centres One Follow-up period and frequency 4 wk then every 3 mth for the 1st year then every 6 mth for the 2nd year, then yearly |
Treatment intention Curative Type(s) of cancer and histology BO with HGD Main eligibility criteria Patients with BO with HGD. Patients were not allowed to receive chemotherapy or radiotherapy within 1 mth prior to PDT. Other exclusion criteria were provided Patient characteristics Not stated Concomitant treatment All patients received PPIs. Intravenous fluids and antiemetics were given pre-operatively |
Trial treatments ALA–PDT with red light vs ALA–PDT with green light Intervention Phase 1 (eight patients) ALA with red light: at 635 nm delivering a dose of 200 J/cm2. Laser treatment was applied 4 hr after oral ALA administration (30 mg/kg). Patients received up to three treatments with PDT 1 mth apart Phase 2 (six patients) As above but with 60 mg/kg ALA Comparator Phase 1 (eight patients) ALA with green light at 512 nm otherwise as for intervention Phase 2 (five patients) As above but with 60 mg/kg ALA |
Mortality Not assessed Morbidity Phase 1 4 of 16 patients (25%) had HGD eradicated (three red light, one green light) The trial was paused following interim analysis. It then proceeded to Phase 2 Phase 2 Six of six patients in the 60-mg red light group had successful treatment, whereas one of five was successful in the 60-mg green light group (p = 0.01) 60-mg ALA red light was also more successful than 30-mg ALA red light (p = 0.03) and than 30 mg ALA green light (p = 0.005) AEs AEs were not all reported by group. All patients receiving 60 mg PDT showed minor, self-limiting abnormalities in the results of their liver function tests Resource use Not assessed |
Authors’ conclusions PDT with ALA at 30 mg/kg with green or red laser is ineffective for eradication of HGD in BO. ALA at 60 mg/kg activated by 1000 J/cm red laser light has high efficacy for HGD in BO Brief study appraisal This trial, although small, was able to suggest a greater effectiveness with 60 mg red light. Such findings would need to be confirmed in larger trials and any AEs documented |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Mackenzie et al. (2008)104 Data source Abstract Country UK Language English Study design RCT No. of participants Total: 40 recruited of a planned 66. 32 were treated Intervention: ALA–PDT: 16 Comparator: PDT with Photofrin: 16 No. of recruiting centres Not stated Follow-up period and frequency 6 wk, 4 mth and 1 yr post therapy |
Treatment intention Not stated Type(s) of cancer and histology BO with HGD Main eligibility criteria Patients with BO with HGD confirmed by two independent pathologists were eligible for the trial. Any visible nodules of HGD were removed and patients only treated if residual HGD was still present Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments ALA–PDT vs PDT with Photofrin Intervention 60 mg/kg ALA activated by 1178 J/cm of red laser light Comparator Photofrin PDT with the standard protocol or as previously shown to be the most effective (no further details given) |
Mortality Not assessed Morbidity Five patients are undergoing repeat therapy (three Photofrin, two ALA). Remission rates are 14 of 14 (100%) in the ALA–PDT group and nine of 14 (64%) in the Photofrin group (p < 0.05) AEs Strictures developed in six of 16 patients treated with Photofrin and one of 16 treated with ALA (probably not related to treatment), p < 0.05. Skin photosensitivity developed in seven of 16 patients treated with Photofrin, one of whom had to be briefly admitted to hospital. No instances of photosensitisation were found with ALA (p < 0.05). There were no other significant differences between groups regarding side effects Resource use Not assessed |
Authors’ conclusions The preliminary data suggest that ALA–PDT is both safer and potentially more effective than PDT with Photofrin but FU is short and not all patients in the trial have been treated as yet Brief study appraisal This trial was reported in abstract form only so full details of the methods are not available. The data presented are promising but would need confirmation in longer FU and with all the planned patients treated |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Mackenzie et al. (2007)103 Linked publications205 Data source Abstract Country UK Language English Study design RCT No. of participants Total: 24 appeared to have been randomised to either red or green light and were part of a larger study of 72 patients Intervention: High-dose ALA (60 mg/kg) with high-dose red or green light (1000 J/cm) – not stated Comparator: High-dose ALA (60 mg/kg) with low-dose red light (500–700 J/cm) – not stated 2nd comparator: Low-dose ALA (30 mg/kg) with high-dose red or green light (1000 J/cm) – not stated No. of recruiting centres Not stated Follow-up period and frequency 36 mth |
Treatment intention Not stated Type(s) of cancer and histology BO with HGD Main eligibility criteria Not stated Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments High-dose ALA (60 mg/kg) with High-dose red or green light (1000 J/cm) vs High-dose ALA (60 mg/kg) with low-dose red light (500–700 J/cm) vs Low-dose ALA (30 mg/kg) with high-dose red or green light (1000 J/cm) Intervention High-dose ALA (60 mg/kg) with high-dose red or green light (1000 J/cm) Comparator High-dose ALA (60 mg/kg) with low-dose red light (500–700 J/cm) 2nd comparator Low-dose ALA (30 mg/kg) with high-dose red or green light (1000 J/cm) |
Mortality Not assessed Morbidity Patients in the group receiving High-dose ALA–PDT and High-dose red light had a significant decrease in cancer risk when compared with the other treatment groups at 36 mth (24% risk vs 3%). The difference in adenocarcinoma rates were significant when red light was compared with green (8% vs 45%, p < 0.05) AEs No patients suffered photosensitivity reactions or developed oesophageal strictures Resource use Not assessed |
Authors’ conclusions The data from this trial support the use of the optimal regimen of ALA in a RCT of ALA vs Photofrin PDT Brief study appraisal This small study is reported in abstract form only and no further publication is available. It is, therefore, difficult to assess the quality of the trial and the reliability of the findings |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Overholt et al. (2007)99 Data source Full published paper Country Not stated Language English Study design RCT No. of participants Total: 208 (61 in long-term phase group) Intervention: PHOPDT: 138 (48 in long-term phase group) Comparator: OM: 70 (13 in long term phase group) No. of recruiting centres 30 (in four unnamed countries) Follow-up period and frequency Every 3 mth until four consecutive biopsy results were negative for HGD, then biannually until 5 yr |
Treatment intention Curative Type(s) of cancer and histology BO with HGD Main eligibility criteria Patients were eligible if they were diagnosed with BO with HGD proven by biopsy and be ≥ 18yr. Exclusion criteria were: cancer other than non-melanoma skin cancer within the last 5yr; prior PDT to the oesophagus, oesophageal strictures unresponsive to dilatation and further criteria detailed in full in the paper Patient characteristics % Male: 85 Mean age: 67 yr Further patient characteristics were reported Concomitant treatment 9% of patients in the PHOPDT group underwent an oesophagectomy or other endoscopic ablation (3%). 19% of the patients in the OM group had PHOPDT treatment, 10% underwent an oesophagectomy and 2.9% had another endoscopic ablation technique |
Trial treatments PDT with PHOPDT vs OM alone Intervention PHOPDT: Patients in this arm received a maximum of three courses of PDT over 5 yr separated by at least 3 mth. One course of PDT consisted of a 2-mg/kg PHO injection followed by one laser light session (630 nm) applied to the oesophageal segment with HGD 40–50 hr after injection. The light dose was 130 J/cm of diffuser length with a centring balloon. A 2nd light application of 50 J/cm without the cantering balloon could be given 96–120hr after PHO injection but only for areas with insufficient mucosal response after the 1st light application. A maximum of 7 cm of BO was treated during one course of PDT. It was required that the entire length of Barrett’s mucosa be treated. Patients also received 20 mg of OM twice daily. Patients had to avoid exposure of eyes and skin to direct sunlight and high intensity light for at least 30 d. They were told to wear dark sunglasses for a 30-d period when outdoors Comparator OM: Patients received 20 mg OM twice daily |
Mortality Two patients in the PHOPDT and one patient in the OM group died within the 1st 2 yr from events unrelated to Barrett’s disease. No deaths were related to the treatment. There were no additional patients who died over the course of the additional 3 yr of FU Morbidity The proportion of responders (complete ablation of HGD) was significantly higher in PHOPDT than with OM (77% vs 39%, p < 0.0001). Of the omeprazole alone responders there were 26% of the PHOPDT and 52% of the OM patients who terminated the trial with either HGD or cancer. Analysis of responders for both treatment groups at 10 specific time points showed a proportion of responders almost twice as large in PHOPDT compared with OM at all assessment periods (data not shown). There was a significant difference between the median time to CR in the 2 groups: PHOPDT, 113 d and OM, 551 d, p < 0.0001. Over the trial period 10% of PHOPDT patients had HGD compared with 31% of the OM patients By the end of the 5-year FU period, the probability of maintaining complete ablation of HGD was 48% in PHOPDT compared with 4% in OM, p < 0.0001. The median duration of the CR was 44.8 mth in the PHOPDT group and 3.2 mth in the OM group. A 2-yr responder in the PHOPDT group had a 90% chance of maintaining the response for 5 yr compared with 30% for a 2-yr responder in the OM group. Comparison between the 2 groups showed that patients in the PHOPDT group had a significant delay in progression to cancer compared with patients in the OM group. In the PHOPDT group, 21 (15%) patients progressed to cancer from d48 to 1793. In the OM group, 20 (29%) patients progressed to cancer from d63 to 1092. After 5yr of FU, the rate of patients who progressed to cancer in PHOPDT was significantly lower than in OM (p = 0.027). There was no significant difference in squamous overgrowth between groups when compared per patient or per biopsy or when the average no. of biopsies with squamous overgrowth were compared per patient. Squamous overgrowth did not obscure the most advanced neoplasia in any patient AEs In the initial phase of the trial, the most common AEs were photosensitivity (69%) and oesophageal strictures (36%). All photosensitivity events were resolved and 94% of patients with strictures were stricture free during the course of the initial phase. Events of severe intensity were similar for PHOPDT (16%) and OM (15%) with 65% of the PHOPDT group being related to the treatment compared with 2% in the OM group. From years 2 to 5, there were no SAEs and of those AEs reported, none was attributed to the treatments. There were no photosensitivity AEs occurring during the long term phase. Full details of all AES, both related and unrelated to treatment are available in the paper Resource use Not assessed |
Authors’ conclusions This trial shows that PDT with Photofrin is a clinically and statistically effective therapy in producing long-term ablation of HGD and reducing the potential impact of cancer compared with OM Brief study appraisal This was a RCT with procedures for blinding of outcome assessors. Outcomes were defined and appropriately assessed and AEs noted. Longer-term FU was used to trace the development of dysplasia and progression to cancer. The results of this trial appear to be reliable with the caveat being the large number of recruiting centres (and very small numbers of patients at some sites) which may have resulted in between-site differences, such as the delivery of the intervention (e.g. varying expertise in delivering PDT), which may have affected the overall results. The number recruited at individual sites ranged from one to 51 participants |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Ragunath et al. (2005)100 Linked publications210 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 26 Intervention: PDT: 13 Comparator: APC: 13 No. of recruiting centres Not stated Follow-up period and frequency 4 mth and 12 mth |
Treatment intention Curative Type(s) of cancer and histology BO with LGD or HGD Main eligibility criteria Patients with BO ≥ 3 cm and LGD or HGD with histological diagnosis confirmed on biopsy no more than 3 mth before study entry were eligible. The following were excluded: patients with oesophageal malignancy of any form, previous oesophageal resection, previous mucosal ablative therapy or endoscopic mucosal resection, patients with predominantly tongues rather than circumferential Barrett’s oesophagus, patients with porphyria or patients intolerant to endoscopy. Patients pregnant, trying to get pregnant or not using contraception were also excluded Patient characteristics % Male: 81 Median age: 60 yr Age range: 35–86 yr Median BO length: 4 cm Dysplasia: HGD, 3(12%), 23(88%) Concomitant treatment After the procedures, patients received a high-dose PPI, lansoprazole 60 mg/d, during the treatment period and were then maintained on 30 mg/d. All patients also received two tablets of co-codamol, to be taken every 6 hr as pain relief for 24–48 hr after the treatment. A few patients also received 1 g of sucralfate every 6 hr for retrosternal discomfort and transient dysphagia |
Trial treatments PDT with Photofrin vs APC Intervention 2 mg/kg of Photofrin was injected intravenously 48 hr before illumination with laser. PDT was performed using 630-nm red laser light with a power output of 840 mW delivering 200 J/cm through an endoscopically inserted PDT balloon. Endoscopy was performed under intravenous sedation with midazolam 5–15 mg and fentanyl 50–100 µg. Intravenous buscopan was used as an antimotility agent. A 3-cm window PDT balloon was inserted over a guidewire and inflated after positioning, adjacent to the Barrett’s segment. The laser fibre was inserted into the balloon and positioned to allow uniform laser light distribution. The procedure was repeated for every additional 3 cm of the Barrett’s segment. Further treatment parameters were described. All patients were admitted to the gastroenterology ward and nursed in a semi-dark room. The ward nursing staff and the patients were given instructions and an information leaflet about avoiding direct sunlight and bright indoor lights for 4–8 wk Comparator APC: Endoscopy was performed under intravenous sedation with midazolam 5–15 mg. After assessment of the Barrett’s segment, APC was performed using the ERBE ICC 200 Argon Beamer. APC was applied until a white coagulum appeared at a power setting of 65 W and argon gas flow of 1.8 l/min. Depending on the length of the Barrett’s segment and patient tolerability, APC was carried out in one or more sessions with an interval of 2–4 wk between the sessions. The treatment goal was complete ablation of BO and dysplasia or a maximum of six sessions when complete ablation was not achieved. Further details were provided in the paper |
Mortality Not assessed Morbidity Median length of BO eradicated at 4-mth FU: PDT 57% (3 cm); APC 65% (3 cm) Median length of BO eradicated at 12-mth FU: PDT 60% (3 cm); APC 56% (2.5 cm) Dysplasia eradication at 4 mth: PDT 77%; APC 62% (p = 0.03) Dysplasia eradication at 12 mth: PDT 77%; APC 67% NS Development of malignancy at 12 mth: PDT one; APC zero AEs Severe AEs: PDT: four of 13 (31%), photosensitivity two; oesophageal stricture two APC: three of 13 (23%), Oesophageal stricture, two; severe chest pain, odynophagia and fever requiring hospital admission, one Resource use A cost-effectiveness analysis was conducted from the perspective of the UK NHS. The cost of PDT per patient was calculated at £2804 and that of APC £1341. The ICERs were calculated based on differences in cost and effects between the two procedures for BO length eradication and dysplasia eradication at 4 and 12 mth. At 4 mth APC was the dominant strategy being less expensive and more effective. At 12 mth the incremental cost ratio was £266, i.e. it would cost an additional £266 for every percentage reduction in Barrett’s using PDT. Full details of the cost-effectiveness analysis are provided in the paper |
Authors’ conclusions PDT and APC are equally effective in eradicating Barrett’s mucosa. However, PDT is more effective in eradicating dysplasia. Long-term FU is needed to assess cancer prevention and the durability of the neosquamous epithelium. These interventions cannot be recommended as yet for routine practice Brief study appraisal This was a small trial that will likely have been underpowered to detect treatment differences for all outcomes. This would also have impacted on the cost effectiveness analysis, which accompanied this trial. Treatment protocols are well described but study methods such as procedures for randomisation and blinding are less well described. The authors advise a larger trial and also highlight the need for longer-term FU |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Zoepf et al. (2003)101 Data source Abstract Country Germany Language English Study design RCT No. of participants Total: 20 Intervention: PDT 10 Comparator: APC 10 No. of recruiting centres Not stated Follow-up period and frequency PDT: Median 27 mth, range 12–42 mth APC: Median 24 mth, range 4–46 mth |
Treatment intention Curative Type(s) of cancer and histology Long-segment BO Main eligibility criteria Not stated Patient characteristics % Male: 65 Median age: 68 yr Age range: 44–77 yr PDT: 4/10 LGD, 6/10 HGD APC: 5/10 LGD, 5/10 No dysplasia Concomitant treatment Not stated |
Trial treatments PDT vs APC Intervention PDT: 60 mg/kg bw of ALA. Cylindrical diffuser fibre in the centre of a balloon applicator and illuminated using a diode laser system with 150 J/cm2. Number of treatment sessions was two (1–5). Further PDT parameters were not reported Comparator APC: APC was applied with a power of 70 W. The number of treatment sessions was four (2–9) |
Mortality Not assessed Morbidity Reduction of length was 90% for PDT (range 0–100%) and 90% for APC (range 50–100%) AEs All patients with PDT developed nausea and vomiting over a period of 4 hr after treatment. 4/10 PDT patients showed transient dysphagia. No skin phototoxity was found after PDT. There was no vomiting in the APC group but 3/10 patients developed transient dysphagia and one mediastinal emphysema and treatment had to be interrupted Resource use Not assessed |
Authors’ conclusions Both APC and PDT can ablate BO but for APC only half as many treatment sessions needed Brief study appraisal This small trial was reported in abstract only and no further full publication was located. Many of the study details and methods were unclear from the abstract and the quality of the trial was therefore difficult to assess. Treatment groups do not appear comparable in terms of dysplasia |
Appendix 17 Oesophageal cancer data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Canto et al. (2005)107 Data source Abstract Country Not stated Language English Study design Non-RCT No. of participants Total: 80 Intervention: 58 Comparator: 22 No. of recruiting centres Not stated, multicentre Follow-up period and frequency FU at 4–6 wk, every 3 mth (year 1), every 3–6 mth (year 2) then every 6–12 mth. EUS and CT scans taken every 6 mth (year 1) then every 6–12 mth. Mean FU 31.2 mth (6–96) |
Treatment intention Curative Type(s) of cancer and histology T1 oesophageal cancer Main eligibility criteria Patients with oesophageal SCC, adenocarcinoma of the oesophagus, EGJ staged as T1 N0 M0 by EUS or CT and that refused, or were unfit for oesophagectomy, or declined radiation therapy, or declined chemoradiation therapy were eligible for inclusion Patient characteristics % Male: 70 Mean age: 73 yr Age range: 43–91 yr; 74 Barrett’s oesophageal carcinomas, two oesophageal squamous cell cancers, four oesophagogastric junction adenocarcinomas Concomitant treatment Not stated |
Trial treatments Bare fibre Ps PDT alone vs PDT plus EMR Intervention PDT alone: Ps infusion (2 mg/kg), then EGD plus PDT (dose 175–300 J/cm fibre, with a 1.0, 2.5, 5 or 7cm diffuser fibre without a balloon centring device) Comparator PDT with EMR: Lesions were staged and removed by EMR before PDT from 2001 to 2004 |
Mortality Overall and disease specific 5-yr survival was 88% and 100%, respectively Morbidity The CR rate was 89.7% for PDT alone vs 91.2% for PDT + EMR (p = 0.67). Nine patients had a 2nd course for ablation of HGD or cancer. Four patients with new HGD in residual Barrett’s oesophageal cancers of 9–14 cm were treated successfully with PDT. Five (6.2%) subsquamous lesions diagnosed at FU with HGD/cancer were treated successfully with PDT (2), chemoradiation (1) or surgery (2) QoL and return to normal activity Not assessed AEs Six patients (7.5%) required hospitalisation for nausea, vomiting, dehydration, transient dysphagia, bleeding or pain); nine (11.2%) developed PDT-related strictures. There were no treatment-related deaths Resource use Not assessed |
Authors’ conclusions Ps-PDT without a balloon centring device, with or without EMR, is a safe and highly effective curative treatment for early cancer of the oesophagus/EGJ Brief study appraisal This study was available only as a short abstract, the methodology was not clearly reported and it was unclear which results were applicable to each treatment group. As a non-randomised study, the authors’ conclusions may be overly strong and should be regarded with caution |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Grosjean et al. (1998)108 Data source Full published paper Country Switzerland Language English Study design Non-RCT No. of participants Total: 15 (22 tumours) Intervention: 13 tumours (630 nm PDT) Comparator: Nine tumours (514 nm PDT) No. of recruiting centres Not stated Follow-up period and frequency FU at 7–10 d, then 3 mth after treatment and twice per year thereafter |
Treatment intention Curative Type(s) of cancer and histology Superficial oesophageal and bronchial cancers Main eligibility criteria It appeared that men and women with one or several biopsy-proven superficial SCC of the bronchi or oesophagus Patient characteristics % Male: 80 Age range: 46–79 yr Mean Age: 59.8 yr All patients had previously received radiotherapy and/or surgery for primary invasive cancer of the head and neck Concomitant treatment Not stated |
Trial treatments PDT at 630 nm with Photofrin II vs PDT at 514 nm with Photofrin II Intervention PDT with Photofrin (630 nm): After injection with Photofrin II (1 or 2 mg/kg) irradiation with 630 nm, 100 mW/cm2 (total dose 100 J/cm2) argon ion pumped-dye laser under general anaesthetic. Microlens and/or cylindrical light distributors were used in the bronchi and 180 or 240° windowed cylindrical light distributors in the oesophagus. Ten tumours had a drug–light interval of 72 hr, three tumours had a drug–light interval of 1 hr. If there was less than CR at 3-mth endoscopy, PDT was repeated. Patients were advised to avoid direct sunlight for 4–6 wk after drug administration Comparator PDT at 514 nm: As for PDT at 630 nm but using 514 nm and five tumours had a drug–light interval of 72 h, four tumours had a drug–light interval of 1 hr |
Mortality Not assessed Morbidity CR was seen in 9/13 of the superficial tumours (69%) with 630-nm PDT vs 6/9 tumours (67%) with 514-nm PDT. In the 630-nm PDT group three tumours showed a PR (vs three in 524-nm group) and one tumour only minimally reduced in size. In the oesophagus, both wavelengths were effective in eradicating in situ and intramucosal cancer but did both cure more than half of the submucosal tumours. 2/10 tumours treated at a drug–light interval of 1 hr achieved a CR QoL and return to normal activity Not assessed AEs No major complications were observed in either treatment group. Three 630-nm PDT patients reported chest pains with associated high-grade fever for 10 d after PDT (two with pleural effusion, the 3rd with endoscopic evidence of oedema and erythema on the posterior wall of the trachea at the level of the oesophageal cancer). All three patients recovered with antimicrobial therapy Resource use Not assessed |
Authors’ conclusions PDT with 514-nm light has the potential to cure superficial cancer in the oesophagus and bronchi with the same probability as 630-nm PDT. In the oesophagus, green light prevents deep tissue damage, thus reducing the risk of perforation Brief study appraisal The numbers included in this study were small and the methods were not clearly reported – particularly in terms of comparability of the two groups. The conclusions may not therefore be reliable. Note: The majority of patients in this trial had oesophageal tumours (14/22), therefore the results have been included in the oesophageal cancer group |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Heier et al. (1995)114 Data source Full published paper Country USA Language English Study design RCT No. of participants Total: 42 Intervention: 22 Comparator: 20 No. of recruiting centres Not stated Follow-up period and frequency FU at 1 wk, then once per month. CT every 3 mth |
Treatment intention Palliative Type(s) of cancer and histology Oesophageal cancer Main eligibility criteria Patients with dysphagia caused by biopsy-proven oesophageal malignancy that were not suitable for, had refused or failed surgery, radiotherapy and chemotherapy were eligible for inclusion. Prior therapy had to have ended at least 1 mth before enrolment. Exclusion criteria were: tracheal involvement by bronchoscopy and Karnofsky performance status < 30 Patient characteristics % Male: 62 Age range: 42–87 yr Mean age: 70 yr (PDT) 73yr Nd:YAG Mean Karnofsky status for both groups was around 73–74 Overall, 60% of tumours were squamous and 40% were adenocarcinoma. Most patients had some kind of prior therapy Concomitant treatment Not stated |
Trial treatments PDT with DHE vs Nd:YAG laser Intervention PDT: IV DHE was given (2 mg/kg bw), then illumination with an argon pumped-dye laser. 630 ± 2 nm (300 J/cm) red light was delivered by cylinder-diffusing fibres, and tumour segments were treated sequentially in a retrograde fashion. Power density was 400 mW/cm fibre tip. Tissue dose was calculated from light dose delivered and surface area exposed, estimated from segmental luminal diameter. A 2nd dose could be given if necessary (13 patients). Patients advised to restrict sun exposure for at least 30 d post injection. If there was a recurrence of tumour obstruction another course of PDT was given if 1 mth had elapsed since DHE injection Comparator Nd:YAG: Standard technique was used at 90 W in a retrograde fashion. Laser pulses were delivered through quartz fibres (bare or coaxial air flow). Therapy was delivered every 2–4 d until luminal patency was achieved. Most patients required two sessions |
Mortality Mean survival was not significantly different between PDT and Nd:YAG (145 vs 128 d, p = 0.419) Morbidity At 1 mth PDT was associated with a greater increase in dietary performance (p = 0.006), Karnofsky performance status (p < 0.001) and oesophageal grade (p = 0.002) compared with Nd:YAG. Mean duration of response with PDT was 84 d vs 53 for Nd:YAG, p = 0.008. The difference in dietary levels at 1 wk and weight change between treatment groups was ns. CR was seen in two PDT patients vs one QoL and return to normal activity Not assessed AEs In the randomised trial patients complications were relatively few: fistula (one PDT patient vs two), stricture (zero vs two), skin photoreaction (four vs zero), fever (five vs one) and luminal plugging (five vs five) Resource use Not assessed |
Authors’ conclusions PDT can relieve oesophageal obstruction from squamous cell and adenocarcinoma and is an alternative to Nd:YAG thermal necrosis with a longer duration of response. However, PDT requires patient precautions to minimise skin photoreactions Brief study appraisal This was a small but well-conducted and reported study despite the apparent lack of blinding of outcome assessors. The analyses adjusted for confounding variables, and the significant benefits in favour of PDT could be considered as promising |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Lecleire et al. (2008)111 Data source Abstract Country Not stated Language English Study design Non-RCT No. of participants Total: 35 (37 lesions) Intervention: 21 (22 lesions) (primary intent PDT) Comparator: 14 (15 lesions) (PDT indicated after local failure of CRT) No. of recruiting centres Not stated Follow-up period and frequency Median FU 15 mth |
Treatment intention Curative Type(s) of cancer and histology Early stage oesophageal cancer Main eligibility criteria Not stated Patient characteristics Not reported in detail, paper states no significant differences between groups. Median tumour length 2cm Concomitant treatment Not stated |
Trial treatments PDT in patients treated in primary intent vs PDT in patients treated with PDT after local failure of definitive CRT Intervention Primary intent PDT: After IV Photofrin (2 mg/kg), illumination with a dye laser between 48h. The mean number of PDT sessions was 1.18. Control endoscopies with routine biopsies were planned 6–8 wk after PDT. No further details were reported Comparator PDT after failed CRT: As for primary intent PDT except mean number of PDT sessions was 1.33 |
Mortality Not assessed Morbidity 16/22 lesions (73%) were successfully treated in the primary intent group vs 8/15 (53%) after failed CRT, p = 0.3. There was no difference in recurrence rate between groups (9 vs 14%, ns) QoL and return to normal activity Not assessed AEs Severe complications were 10% in the primary intent PDT group vs 50% for failed CRT (p = 0.015) (two strictures requiring endoscopic dilation vs two perforations and five strictures requiring dilation respectively). Death rate directly related to PDT was 0% in primary intent patients vs 14% for failed CRT (ns) Resource use Not assessed |
Authors’ conclusions PDT when indicated as a salvage therapy in patients with local failure after CRT for oesophageal cancer tended to be less effective than when indicated as a 1st-line treatment. Moreover, severe complications, including death-related procedures were significantly more frequent in patients treated after prior CRT Brief study appraisal Few methodological and patient details were reported in this abstract of a small study so the reliability of the conclusions is unclear. The authors appear to have drawn conclusions at odds with the statistical test results The authors’ conclusions do not follow from the results reported |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Lightdale et al. (1995)112 Data source Full published paper Country USA Language English Study design RCT No. of participants Total: 236 (218 treated) Intervention: 118 (110 treated) Comparator: 118 (108 treated) No. of recruiting centres 24 Follow-up period and frequency FU at 1 wk, then 1, 2, 3 and 6 mth |
Treatment intention Palliative Type(s) of cancer and histology Oesophageal carcinoma Main eligibility criteria Patients with a biopsy-proven oesophageal malignancy and that were too debilitated for, refused, failed to respond to or had a recurrence following chemotherapy, radiation therapy or surgery were eligible for inclusion. Also patients with SCC or adenocarcinoma, and that were symptomatic, had malignancy-caused dysphagia to solid foods and a Karnofsky status of at least 30% were eligible. Prior therapy was required to be terminated at least 4 wk before randomisation. Bronchoscopy was required for tumours at or above the level of the carina. Patients with involvement of the tracheobronchial tree and those that had prior treatment for oesophageal carcinoma with PDT or Nd:YAG laser were excluded. Concurrent radiation and chemotherapy were not permitted Patient characteristics % Male: 72 Median age: PDT 68 yr; Nd:YAG 72 yr Median Karnofsky performance status: 80% Prior therapy: 45% Median tumour length: PDT 6 cm; Nd:YAG 5 cm Adenocarcinoma 51%, the rest had SCC Concomitant treatment Not stated |
Trial treatments PDT vs Nd:YAG laser Intervention PDT: 40–50 hr after single intravenous injection with Ps (2 mg/kg bw), illumination by red light (630 nm) provided by a continuous wave argon pumped-dye laser and delivered via an optical quartz fibre with a cylindrical diffusing tip (400 mW/cm, total dose 300 J/cm). After 2–3 d patients were re-endoscoped to debride the necrotic tumour and residual tumour could be treated with a 2nd application of laser light (same dose). A maximum of three courses at 1-mth intervals was permitted Comparator Nd:YAG laser therapy: A laser power setting of 15–90 W and pulse duration of 0.5–4.0 s was used (delivered with either contact or non-contact technique via quartz fibres). Each session ended when the endoscopist thought maximum achievable benefit or maximum patient tolerance was reached. Repeat sessions could be given if initial response was deemed insufficient (the course was deemed complete when the investigator believed dysphagia had been palliated or further therapy would be futile) |
Mortality Not assessed Morbidity Dysphagia grades significantly improved from baseline at 1 wk (PDT –0.73 vs Nd:YAG –0.90) and 1 mth (PDT –0.75 vs Nd:YAG –0.68) with both treatments (ns difference between treatment groups). Objective tumour response (responders: patients with CR or PR) was 44% PDT vs 48% Nd:YAG (ns) at 1 wk and 32% PDT vs 20% Nd:YAG at 1 mth (p < 0.05). Data was obtained from 80% patients at 1 wk and 60% at 1 mth. Subgroup analyses showed ns differences between groups but there was a trend in favour of PDT for objective tumour response rates in the upper and lower third of the oesophagus, tumours > 10 cm and in patients that received prior therapy. There was no difference between groups for patients with squamous cell cancer and adenocarcinoma QoL and return to normal activity Not assessed AEs Significantly more PDT patients had an AE (92% vs 82%, p < 0.05). Sunburn (19% PDT vs 0%), nausea (8% vs 2%), fever (16% vs 5%) and pleural effusion (10% vs 2%) were significantly greater for PDT and oesophageal perforation greater for Nd:YAG (1% vs 7%) p < 0.05). All PDT patients were photosensitive for 1–2 mth, none severe. Treatment-related respiratory insufficiency occurred in 3% PDT (vs 1%). Severe AEs were equal overall for both treatments Resource use Not assessed |
Authors’ conclusions PDT with porfimer solution has overall equal efficacy to Nd:YAG laser thermal ablation for palliation of dysphagia in oesophageal cancer, and equal or better objective tumour response rate. Temporary photosensitivity is a limitation, but PDT is carried out with greater ease and is associated with fewer acute perforations than Nd:YAG laser therapy Brief study appraisal The methods were generally well-described though a few features were not reported. It may have been useful to gauge patient/investigator opinion of ease of treatment and importance of photosensitivity to QoL to inform the conclusions |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Maier et al. (2000)115 Data source Full published paper Country Austria Language English Study design Non-RCT No. of participants Total: 52 Intervention: 23 (PDT) Comparator: 29 (PDT under HBO) No. of recruiting centres Not stated Follow-up period and frequency FU at 1 mth, then every 3 mth |
Treatment intention Palliative Type(s) of cancer and histology Advanced cancer of the upper gastrointestinal tract Main eligibility criteria It appeared that patients with advanced cancer of the upper gastrointestinal tract who were not eligible for resection treatment due to poor performance status, functional and/or anatomic inoperability, and/or anatomic inoperability, and/or refusing surgery were eligible for inclusion Patient characteristics % Male: 81 Age range: 46–87 yr Mean age: 67.3 yr Most patients’ cancers were judged to be stage III, dysphagia scores varied across levels 2, 3 and 4 and no significant differences at baseline were found. Tumours were SCC in 25 cases, adenocarcinoma in 25 cases. Further patient characteristics were reported Concomitant treatment Before PDT, 12 (seven in PDT, five in PDT/HBO) patients underwent dilatation and retrograde Nd:YAG laser disobliteration |
Trial treatments PDT vs PDT under HBO Intervention PDT: Photosan-3 (2 mg/kg) was administered intravenously. After 48 hr, PDT, using a fibre with a 1-cm tip radial light-diffusing cylinder, was inserted using an endoscope. Illumination dose was 300 J/cm of fibre, 630 nm applied by a KTP-Nd:YAG laser. Treatment was under short-term intravenous anaesthesia. 2–3 d after PDT, endoscopy was repeated, and necrotic tissue removed mechanically if necessary. Treatment could be repeated after 3 mth if necessary but most patients received one session Comparator PDT under HBO (PDT/HBO): As for PDT but under HBO at a level of 2 ATA in a hyperbaric chamber. Oxygen was administered with the Scuba valve, transcutaneous Po2 levels were 500–750 mmHg. Before HBO, patients had an ear, nose and throat check-up |
Mortality Median survival after PDT was 8.7 mth vs 13.8 mth for PDT/HBO (p = 0.021) Morbidity At 3 mth, dysphagia score had decreased in both groups but there was no significant difference between treatments (p = 0.43). At 3 mth, mean decrease in stenosis was 5.6 mm in the PDT group vs 6.3 mm PDT/HBO, p = 0.065. Mean decrease in tumour length was 2 cm PDT vs 2.8 cm PDT/HBO, p = 0.002 QoL and return to normal activity A semi-solid diet was possible in all patients after PDT or PDT/HBO AEs No major complications related to PDT, HBO and photosensitisation, and no barotrauma of the ear or lung or sunburn was observed. Minor complications included: postinterventional odynophagia (eight PDT vs nine PDT/HBO), fever up to 39° (five vs nine) and chest pain for 1 or 2 d (five vs nine). Six oesophagotracheal fistulas were found in two cases (PDT at 5 and 17 mth) and four cases (PDT/HBO at 4, 7, 14 and 24 mth). Stenting with coated, self-expandable stents was performed in one PDT patient at 16 mth and two PDT/HBO patients at 14 and 17 mth. One patient had haemorrhage of the tumour 18 mth after PDT/HBO Resource use Not assessed |
Authors’ conclusions Combined PDT/HBO represents a new approach in the treatment of oesophageal and cardial cancer, which appears to have enhanced the efficacy of PDT Brief study appraisal This was a small pilot study that, despite not being randomised, does seem to have achieved reasonable comparison groups. The authors comment that randomisation was not possible due to the variable availability of the oxygen chamber. The conclusions are reasonable but the study would have benefited from blinded outcome assessors |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Maier et al. (2000)116 Linked publications211 Data source Full published paper Country Austria Language English Study design Non-RCT No. of participants Total: 75 Intervention: 31 Comparator: 44 No. of recruiting centres One Follow-up period and frequency FU after 1 mth, then every 3 mth |
Treatment intention Not stated Type(s) of cancer and histology Advanced oesophageal carcinoma Main eligibility criteria Patients that were not eligible for resection treatment due to significant comorbidity were included Patient characteristics % Male: 80 Mean age: PDT alone, 67 yr; PDT/HBO 67.5 Age range: 46–87 yr Cancer stage: III, 59; IV, 16 Dysphagia score: level 2, 23; level 3, 37; level 4, 15. 40 SCC, 35 adenocarcinoma Further patient characteristics were reported Concomitant treatment Not stated |
Trial treatments PDT vs PDT/HBO Intervention PDT: HpD given intravenously (2 mg/kg) and camouflage skin protection used for 2 wk, then sunblock for 10 wk. PDT given 48 hr after sensitisation with a fibre (1-cm tip, radial light diffusing cylinder) inserted through the biopsy channel of the endoscope (several placements were necessary). Light dose was 300 J/cm, 630 nm applied with a KTP-Nd:YAG laser with DYE box. Treatment given under short-term anaesthesia. Endoscopy was repeated 2–3 d after PDT and necrotic tissue removed mechanically if necessary. Prior to PDT, dilatation and retrograde Nd:YAG was necessary in 15 cases Comparator PDT/HBO: As for PDT except patients had ear, nose and throat check-up, then PDT given under HBO (2 atmospheres) in a walk-in hyperbaric chamber |
Mortality Median overall survival with PDT was 7 mth (vs 12 mth in PDT/HBO group), p = 0.0098. 12-mth survival with PDT was 25% vs 52% with PDT/HBO Morbidity At 3 mth, stenosis decreased in both groups by 6 mm In the PDT group median tumour length decrease was 2 cm vs 3 cm in the PDT/HBO group, p = 0.0002 At 3-mth FU (or last FU in case of death)in the PDT group dysphagia score could be lowered by one level in eight cases (vs nine) and two levels in 23 cases (vs 33) (and in the PDT/HBO group it could be lowered by three levels in two cases); this significantly favoured PDT/HBO (p = 0.0064). No recurrent dysphagia was observed at 3-mth FU for either group QoL and return to normal activity At least a semi-solid diet was possible in all patients after either treatment AEs There were no major postinterventional complications or skin photosensitisation related to either treatment. No barotrauma was observed. Minor complications included: odynophagia (PDT group 6 vs PDT/HBO 8); fever up to 39° in the afternoon of the interventional day, one in PDT vs three in PDT/HBO); chest pain for 1 or 2 d (four in PDT vs seven in PDT/HBO). 30-day mortality was 0%. Six oesophagotracheal fistulas in two patients were found (PDT, two cases; PDT/HBO four cases) Resource use Hospitalisation in both groups was 3–9 d (median 4.9 d) |
Authors’ conclusions Combined PDT/HBO represents a new approach in the treatment of oesophageal and cardial cancer which appears to have enhanced the efficiency of PDT Brief study appraisal This study was not randomised (and so may have been subject to bias) and included a fairly small number of patients. The authors did though acknowledge that definitive conclusions could not be drawn based on these results. This publication appears to be the same study as a report of a pilot study211, although the authors were not explicit about this |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Maier et al. (2000)117 Data source Full published paper Country Austria Language English Study design Non-RCT No. of participants Total: 119 Intervention: 44 (PDT and brachyradiotherapy) Comparator: 75 (brachyradiotherapy) No. of recruiting centres Not stated Follow-up period and frequency FU at 1 mth, then every 3 mth |
Treatment intention Palliative Type(s) of cancer and histology Advanced oesophageal carcinoma Main eligibility criteria Patients who were not eligible for resection due to tumour involvement of the adjacent tissue, poor performance status plus inoperable status as a result of comorbidity, refusal of surgical intervention, or a combination of these, were included Patient characteristics % Male: 81 Mean Age: Men, 67 yr; women 65 yr Age Range: 27–93 yr Cancer stage: III, 80; IV, 39. 68 SCC and 51 adenocarcinoma Further patient characteristics were reported Concomitant treatment Prior to therapy 21 patients required initial dilatation and tumour obliteration with Nd:YAG |
Trial treatments PDT and brachytherapy vs brachytherapy alone Intervention PDT and brachytherapy: Intravenous haematoporphyrin was administered (mean 1.5 injections/patient)(2 mg/kg), after 48 hr PDT treatment using a fibre with 2-cm radial light-diffusing cylinder inserted through biopsy channel of an endoscope (dose 300 J/cm fibre, 630 nm applied by a KTP-Nd:YAG laser with DYE-box). 2–3 d after PDT, endoscopy repeated and necrotic tissue removed. Endoscopy was performed after 1 mth, then every 3 mth. PDT was not repeated within 3 mth. Iridium-192 brachyradiotherapy was given by insertion of the afterloading catheter. 5 Gy per session was given, patients received one to four sessions in total depending on endoscopic findings and dysphagia, with 3–7 d between sessions. This process was carried out under short-term intravenous anaesthesia, combined with topical anaesthesia with supported breathing. In 25 patients with Karnofsky score of > 80 (fair condition) treatment was completed by external beam irradiation using the multiple field technique to deliver mean dose of 44 Gy Comparator Brachytherapy: Iridium-192 brachyradiotherapy was given by insertion of the afterloading catheter. 5 Gy per session was given, patients received one to four sessions in total depending on endoscopic findings and dysphagia, with 3–7 d between sessions. This process was done under short-term intravenous anaesthesia, combined with topical anaesthesia with supported breathing. In 17 patients with Karnofsky score of > 80 (fair condition) treatment was completed by external beam irradiation using the multiple field technique to deliver mean dose of 44 Gy |
Mortality Mean survival was 5.6 mth for brachytherapy; 7.7 mth brachytherapy and external beam irradiation; 6.3 mth PDT brachytherapy; 13 mth PDT, brachytherapy and external beam irradiation. There was a significant difference with PDT* (p = 0.0129) and external beam irradiation* (p = 0.0001). This was biased, as groups without external beam irradiation contained patients that died before entering the irradiation regimen. (Authors’ comment.) *It was unclear which patient groups these results referred to Morbidity Dysphagia score improved in all patients by one to three levels and was significantly greater in the PDT group (p = 0.0003). The mean increase in opening diameter of tumour-related stenosis was significantly greater in the PDT group with or without external beam irradiation than in either of the brachytherapy conditions QoL and return to normal activity There were no significant differences between the two groups in terms of Karnofsky performance status scores at 3 mth, p = 0.28 AEs Major complications occurred in 9% (11/119) of patients, including oesophageal perforation after brachytherapy, severe haemorrhaging 3 d after PDT, spontaneous perforation of distal oesophagus with oesophagomediastinopleural fistula and concurrent pleural emphysema 5 mth after PDT, tracheo-oesophageal or tracheobronchial fistula. Due to strictures, dilation was necessary after PDT (four patients) and Nd:YAG (45 patients). After PDT 28 patients reported odynophagia for 2–5 d Resource use Not assessed |
Authors’ conclusions PDT has been shown to be an effective palliative treatment of advanced oesophageal cancer. However, proper patient selection is necessary to prevent serious complications Brief study appraisal A sizeable proportion of patients in each group were pre-treated with Nd:YAG, which did not appear to be accounted for in the analysis. This trial appears to have contained four separate arms, although this was poorly described. Patients in good/fair condition received a slightly different treatment protocol. These limitations make it difficult to evaluate reliability of the authors’ conclusions |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Maier et al. (2001)118 Linked publications120 Data source Full published paper Country Austria Language English Study design Non-RCT No. of participants Total: 49 Intervention: 22 (ALA–PDT) Comparator: 27 (Photosan-PDT) No. of recruiting centres Not stated Follow-up period and frequency FU at 1 mth, then every 3 mth |
Treatment intention Palliative Type(s) of cancer and histology Advanced oesophageal cancer Main eligibility criteria Patients that were not eligible for resection treatment due to poor performance status, functional and/or anatomical inoperability, and/or refusing surgery were included Patient characteristics % Male: 78 Age range: 46–88 yr Mean age: ALA, 69 yr; Photosan 68 yr Cancer stage: III, 17; IV 32. Dysphagia score varied with most in level 2 or 3 but no significant differences Overall there were 13 SCC, 14 adenocarcinoma Further patient characteristics were reported Concomitant treatment Not stated |
Trial treatments ALA- PDT vs PDT with Photosan (HpD). Both performed with additional hyperbaric oxygenation Intervention ALA–PDT: Diagnostic work-up was performed using barium oesophagogram, oesophagogastroscopy, bronchoscopy and CT scans. Oral administration of ALA (60 mg/kg) then skin protection by camouflage for 24 hr. 6–8 hr after ALA, PDT was carried out using a fibre with 2-cm tip radial light-diffusing cylinder, inserted through the biopsy channel of the endoscope. Light dose was 300 J/cm fibre and 630-nm light was applied by KTP-Nd: YAG laser having a DYE module. Additional hyperbaric oxygenation was applied (after an ear, nose and throat check-up) at level 2 ATA using a Scuba valve system. Treatment was performed under short-term intravenous anaesthesia. Endoscopy was performed 2–3 d after PDT and necrotic tissue removed. Endoscopy was then performed after 1 mth, then every 3 mth. Increased tumour length and dysphagia at FU indicated further PDT treatment. No treatment was repeated within 3 mth after the 1st PDT session Comparator Photosan-PDT: As for ALA–PDT except intravenous administration of Photosan (2 mg/kg), 48 hr before PDT |
Mortality Median survival for ALA group was 8 mth vs 9mth, p = 0.44 (Kaplan–Meier survival curve in paper) Morbidity At 1 mth, there was significantly more improvement in the ALA group than the Photosan group for the following outcomes: dysphagia (p = 0.02), tumour stenosis (p = 0.00000) and tumour length (p = 0.000014) QoL and return to normal activity Karnovsky Performance status improved by 23% for ALA vs 44% for Photosan, not significant (p = 0.12) AEs No barotrauma of the ear was observed. No sunburn occurred in either group. There were no major AEs. 30-day mortality was 0%. Minor complications were: postinterventional odynophagia (nine in ALA group vs 13); fever up to 39° in the afternoon of the interventional day (five vs eight); chest pain for 1 or 2 d (nine vs 13). After ALA administration all patients experienced nausea Resource use Hospitalisation was 4–6 d in both treatment groups |
Authors’ conclusions Despite the limitations of a non-randomised study, photosensitisation with Photosan seems to be more effective in PDT of advanced oesophageal carcinoma compared with ALA Brief study appraisal The conclusions that could be drawn were limited as this was a small, non-randomised study. Baseline characteristics were largely similar apart from M stage and the authors’ cautious conclusions appear reliable. This study appears to have been published twice (see ref. 120) with Photosan being described as HpD. The patients, treatments and results appear to be identical, therefore only one study has been data extracted |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Savary et al. (1998)109 Data source Full published paper Country Switzerland Language English Study design Non-RCT No. of participants Total: 24 (31 tumours) Intervention: Nine tumours (HpD-PDT) Comparator: Eight tumours (Photofrin II-PDT) 2nd comparator: Two tumours (mTHPC-PDT, 0.15 mg/kg with 652 nm) 3rd Comparator: One tumour (mTHPC-PDT, 0.3 mg/kg with 514 nm) 4th Comparator: 11 tumours (mTHPC-PDT, 0.15 mg/kg with 514 nm) No. of recruiting centres Not stated Follow-up period and frequency FU at 10 d, 3 mth, then 6-mth intervals |
Treatment intention Curative Type(s) of cancer and histology Early SCC of the oesophagus Main eligibility criteria Biopsy confirmed early SCC. Patients with porphyria were excluded Patient characteristics % Male: 92 Age range: 42–79 yr Mean age: 56 yr Twenty-two patients had a history of primary invasive head and neck cancer, two had no such history. Two patients had synchronous tumours, five patients developed metachronous early cancers Concomitant treatment None |
Trial treatments HpD PDT vs Photofrin II PDT vs mTHPC 0.15 mg/kg (652 nm) PDT vs mTHPC 0.3 mg/kg (514 nm) PDT vs mTHPC 0.15 mg/kg (514 nm) PDT Intervention HpD PDT: Intravenous HpD was injected (3 mg/kg), then PDT given with an argon ion pumped-dye laser after 72 hr (630 nm, 100 J/cm2, 80 mW/cm2) for 21 min. Surface irradiation using 180 or 240° windowed cylindrical light distributors (15 mm diameter) was used Comparator Photofrin II PDT: intravenous Photofrin II was injected (1 or 2 mg/kg), then PDT given with an argon ion pumped-dye laser after 72 hr [630 nm (most patients) or 514nm, mean light dose 100 J/cm2, 90 mW/cm2] for 19 min. Surface irradiation using 180° or 240° windowed cylindrical light distributors (15-mm diameter) was used 2nd comparator mTHPC 0.15 mg/kg (652 nm) PDT: Intravenous mTHPC was injected (0.15 mg/kg), then PDT given with an argon ion pumped-dye laser after 20 hr (652 nm, 6 or 8 J/cm2, 40 mW/cm2) for 3 min. Surface irradiation using 180 or 240° windowed cylindrical light distributors (15-mm diameter) were used 3rd comparator mTHPC 0.3 mg/kg (514 nm) PDT: intravenous mTHPC was injected (0.3 mg/kg), then PDT given with a argon ion pumped-dye laser after 20 hr (514 nm, 30 J/cm2, 50 mW/cm2) for 10 min. Surface irradiation using 180 or 240 windowed cylindrical light distributors (15-mm diameter) was used 4th comparator mTHPC 0.15 mg/kg (514 nm) PDT: intravenous mTHPC was injected (0.15 mg/kg), then PDT given with an argon ion pumped-dye laser after 20 or 96 hr (514 nm, 75 J/cm2, 90 mW/cm2) for 14 min. Surface irradiation using 180 or 240° windowed cylindrical light distributors (15-mm diameter) was used |
Mortality Not assessed Morbidity No truly selective necrosis was seen with HpD, Photofrin II or mTHPC when irradiation was at 20 hr. With mTHPC (irradiation at 96 h) some patients had necroses that were selective. CR rates: HpD = 89%, mTHPC = 86%, Photofrin II = 75%. Failures of treatment according to sensitiser used were 1/9 (11%) in the HpD group; 2/8 (25%) Photofrin II group; 2/14 (14%) mTHPC group. Failures of treatment according to wavelength used were 2/15 (13%)for 630 or 652 nm; 3/16 (19%) for 514 nm QoL and return to normal activity Not assessed AEs All patients reported burning sensation during the injection of mTHPC. Major complications were: Stenoses (two), oesophagotracheal fistulas in PDT patients (630 or 652 nm) (two, one of which complicated by oesophageal stenosis). Three patients that did not follow prescribed precautions (not in methods) developed 2nd-degree sunburn on the face and hands (one HpD patient at 2 mth; 2 0.15 mg/kg mTHPC patients at 6 d) Resource use Not assessed |
Authors’ conclusions PDT eradicates early SCCs (T1a and T1b) of the oesophagus efficiently. Transmural necroses leading to fistulas can be avoided using a low-penetrating wavelength of laser light (green light at 514.5 nm instead of red light at 630 or 652 nm). Stenoses always result from circumferential irradiation of the oesophageal wall, and this can be avoided by using a 180° or 240° windowed cylindrical light distributor Brief study appraisal This was a relatively small trial with multiple comparator arms, the methods were not clearly reported and as the authors themselves comment – the small samples preclude any firm conclusions, so the results may not be reliable |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Scotiniotis et al. (2000)110 Data source Abstract Country USA Language English Study design Non-RCT No. of participants Total: 37 Intervention: 12 (PDT) Comparator: Six EMR 2nd comparator: 19 (oesophagectomy) No. of recruiting centres Not stated Follow-up period and frequency Mean FU 15 mth (range 2–28 mth). PDT and EMR patient FU 4–6 wk after treatment, then every 3–6 mth; oesophagectomy patient FU dictated by symptoms |
Treatment intention Curative Type(s) of cancer and histology Superficial oesophageal cancer Main eligibility criteria Superficial oesophageal cancer determined by EUS and CT including HGD, carcinoma in situ or intramucosal carcinoma Patient characteristics Mean age: PDT, 76; EMR, 73; oesophagectomy, 65 Thirty-six adenocarcinomas, one SCC Concomitant treatment Not stated |
Trial treatments PDT vs EMR vs Oesophagectomy Intervention PDT: No details reported Comparator EMR: No details reported 2nd comparator Oesophagectomy: No details reported |
Mortality Not assessed Morbidity Eradication of lesions was achieved in 9/12 (75%) PDT, 5/6 (83%) EMR and 18/19 (95%) oesophagectomy patients QoL and return to normal activity Not assessed AEs Stricture occurred in 6/12 (50%) PDT, 0/6 EMR and 10/19 (53%) oesophagectomy patients. ≥ 3 dilatations occurred in 4/12 (33%) PDT, 0/6 EMR and 7/19 (37%) oesophagectomy patients. Other complications were reported for small numbers of patients Resource use Not assessed |
Authors’ conclusions In poor surgical candidates with superficial oesophageal carcinoma PDT and EMR achieved outcomes comparable to oesophagectomy in good surgical candidates. PDT and EMR are reasonable alternatives to oesophagectomy for selected patients Brief study appraisal This small study was available only as an abstract and few methodological details were reported. The study populations for the different interventions did not appear to be comparable at baseline with PDT/EMR patients chosen if suboptimal for surgery. In addition no statistical tests were carried out to verify the findings, the results of this study may therefore not be reliable The authors’ conclusions do not follow from the results reported |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Zhang et al. (2003)119 Data source Full published paper Country China Language Chinese Study design RCT No. of participants Total: 60 Intervention: 30 Comparator: 30 No. of recruiting centres One hospital (outpatients) Follow-up period and frequency FU at 5 and 10 yr |
Treatment intention Palliative Type(s) of cancer and histology Advanced oesophageal cancer Main eligibility criteria Diagnosed with advanced oesophageal cancer, suitable for treatment Patient characteristics Age: under 70 yr Cancer length: 5–10 cm. No metastases Concomitant treatment Not stated |
Trial treatments PDT with radiotherapy vs radiotherapy alone Intervention PDT with radiotherapy: Radiotherapy for 4 wk (40 Gy). Then intravenous haematoporphyrin derivative (5 mg/kg bw) before illumination with 630-nm red light (400–500 W/cm2 at each part of the tumour for 15 min) at 48 and 72 hr Comparator Radiotherapy: 40 Gy for 4 wk |
Mortality The 5-yr survival rate was 29.9% in the PDT group compared with 16.7% (p = 0.05). The 10-yr survival rate was 16.7% in the PDT group vs 10.0% (p < 0.05) Morbidity Not assessed QoL and return to normal activity Not assessed AEs All PDT patients experienced pigmentation, and swelling and itchiness. All PDT patients also experienced pain when swallowing for 3–5 d (some patients had pain for > 10 d and discontinued treatment). 23 died in the PDT group: loss to FU two; uncontrolled localisation 13 including one due to blockage of oesophagus; metastases eight; other disease one; unknown cause one. 26 died in the radiotherapy group: loss to FU one; uncontrolled localisation 18, including two due to blockage of oesophagus; metastases four; other disease two; unknown cause two Resource use Not assessed |
Authors’ conclusions Radiotherapy combined with PDT could obviously enhance the long-term survival rate of patients with advanced oesophageal cancer Brief study appraisal This was a brief report and some methodological aspects were not clearly reported. The p-values were not reported consistently between the abstract and the text making it difficult to clarify the significant differences between groups |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Zhang et al. (2007)113 Data source Full published paper Country China Language English Study design RCT No. of participants Total: 140 Intervention: 42 Comparator: 98 No. of recruiting centres Not stated; appears to be two centres in China Follow-up period and frequency FU at 1 mth. Over 70% patients were followed up for 12–36 mth |
Treatment intention Palliative Type(s) of cancer and histology Advanced oesophagocardiac carcinoma Main eligibility criteria Biopsy proven advanced oesophageal carcinoma Patient characteristics % Male: 79 Age range: 40–81 yr Median age: PDT 58; PDT with 5-FU 62 Cancer stage: Stage II 84; stage III 53; stage IV 3 Concomitant treatment Not stated |
Trial treatments PDT vs PDT with 5-FU Intervention PDT: After intravenous injection of the photosensitiser PSD-007 (photocarcinorin) (3–5 mg/kg bw) and patients were kept in the dark. Irradiation was performed at 24 and 48 hr with either 630-nm copper vapour pumped-dye laser or 632.8 nm high power He-Ne laser (total dose 200–400 J/cm fibre length, median 300 J/cm). This was delivered by cylindrical diffusers under endoscope assistance. Irradiation was carried out in one to four segments (with slight overlap between each segment) depending on lesion length and diffuser. Treatment could be repeated after 1 mth unless evaluation showed effectiveness or symptoms were remitted. Patients were advised to avoid sunlight exposure for over 1 mth Comparator PDT with 5-FU: As for PDT but in addition, before irradiation, 200–500 mg 5-FU was locally injected into tumour tissue under endoscopic guidance. Before injection the extent of the lesions was confirmed. Most patients received four to eight injections per tumour |
Mortality Mean survival time was 8.9 mth with PDT alone compared with 15.1 mth for PDT and 5-FU (p < 0.01) Morbidity The rate of dysphagia remission was 87% for PDT compared with 99% with PDT and 5-FU (p < 0.05). Differences in pharyngeal pain and weight loss were not significant. With PDT alone one patient achieved complete remission (vs five with combined therapy, p < 0.05), eight significant remission (vs 36) and five no remission (vs nine). With combined therapy 48 patients also achieved minor remission QoL and return to normal activity Not assessed AEs Subternal pain due to oesophageal mucosa injury and gastroesophageal reflux 1–2 d after treatment was reported by seven patients in PDT only and eight patients in combination treatment Eight patients in total accidentally exposed themselves to sunlight and developed discoloration of the skin No oesophageal stenosis or perforation reported in either group Resource use Not assessed |
Authors’ conclusions PDT is safe and effective for advanced oesophagocardiac cancer. Its therapeutic effect can be further improved when combined with local chemotherapy Brief study appraisal This study was poorly reported (e.g. method of randomisation, whether ITT analysis was used) but most importantly it appears that after over 40 patients had been treated, interim analysis was carried out and all subsequent patients were treated with combined PDT and 5-FU. These analyses were not further reported. Given this shift from an RCT to experimental without a comparator it is difficult to determine the reliability of the study results overall The authors’ conclusions do not follow from the results reported |
Appendix 18 Lung cancer data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Baas et al. (1994)121 Data source Abstract Country The Netherlands Language English Study design RCT No. of participants Total: 39 Intervention: 15 Comparator: 12 2nd comparator: 12 No. of recruiting centres Not stated Follow-up period and frequency Not stated |
Treatment intention Palliative Type of Lung Cancer and Histology Non-small cell; no further details given Main eligibility criteria Histologically proven inoperable locoregional NSCLC, weight loss < 10% and a PS (sic) > 70% Patient characteristics % Male: 88 Concomitant treatment Not stated |
Trial treatments ERT alone vs PDT preceding ERT vs HDR preceding ERT Intervention Photofrin 2 mg/kg, 200 J/cm, 630 nm given 2 wk before ERT. Other PDT parameters not stated Comparator ERT alone: 14 x 2.5 + 8 x 2.5 Gy to the tumour area in 4 wk 2nd comparator HDR + ERT: As above preceded by HDR: 15 Gy at 1-cm distance along the tumour 2 wk before ERT |
Mortality Assessed but not reported per group Morbidity Not assessed AEs Minor haemoptysis in two PDT-ERT patients. Skin photosensitivity was acceptable in patients treated with PDT (no data provided). Other AEs reported but not broken down by group |
Authors’ conclusions No conclusions specific to PDT Brief study appraisal This small study was an interim analysis of a RCT presented in abstract form. Although it indicated that updated results would be presented, no further information could be located. Most of the study’s methodological details were not available from the abstract and the majority of the results were not broken down by treatment group |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Diaz-Jimenez et al. (1999)122 Data source Full published paper Country Spain Language English Study design RCT No. of participants Total: 31 Intervention: 14 Comparator: 17 No. of recruiting centres Not stated Follow-up period and frequency FU at 1, 2, 3, 6 and 12 mth (and 18 mth if possible) |
Treatment intention Palliative Types of Lung Cancer and Histology Non-small cell 25 SCC, three adenocarcinoma, three undifferentiated carcinoma Main eligibility criteria Biopsy-proven inoperable cancer with totally or partially obstructive endobronchial lesions with or without extrabronchial tumour. Patients > 18 yr, non-pregnant, infertile or postmenopausal. Karnofsky status ≥ 40%, ≥ 4 wk from last chemotherapy cycle and ≥ 3 wk from last radiation dose. Patients who had previous PDT or Nd:YAG were excluded. Further eligibility criteria were reported Patient characteristics % Male: 100 Age range: Not stated Mean age: 65 yr Cancer stage: Stage I, four patients; stage II, one; stage IIIA, six; stage IIIB, 10; stage IV, seven No. with recurrent tumour: three Concomitant treatment Not stated |
Trial treatments PDT vs Nd:YAG laser resection Intervention Intravenous DHE at dose of 2 mg/kg with 630-nm argon dye laser, 40–50 hr after injection. Maximum of three doses (six photoradiations). Other parameters not reported Comparator Nd:YAG resection using 15- to 80-W pulses of 0.5–1.5 s. Procedure repeated every 2–4 d as necessary |
Mortality Survival significantly longer in PDT group (265 vs 95 d, p = 0.007). 4/14 (PDT) and 4/17 (Nd:YAG) still alive at end of study Morbidity Similar response in both groups: 38.5% PDT vs 23.5% Nd:YAG at 1 mth (p = ns). PR at 1 mth in three PDT and four Nd:YAG patients. CR at 1 mth in one PDT patient. Time elapsed until treatment failure: 50 d (PDT) vs 38 d (Nd:YAG) (p = 0.03) QoL and return to normal activity Assessed but not reported AEs Bronchitis was the most common (four cases in PDT group, one in Nd:YAG group) Photosensitisation in four PDT patients. Five patients had no AEs, all in Nd:YAG group. One death probably related to PDT |
Authors’ conclusions PDT is a valid method of palliation in partially or totally obstructing NSCLC Brief study appraisal Difficult to evaluate results due to important baseline differences (presence of cough, and stage of cancer) between groups. Karnofsky performance and FU after 1 mth assessed but not reported. No details on blinding |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Lam et al. , (1991)123 Data source Full published paper Country Canada Language English Study design RCT No. of participants Total: 41 Intervention: 20 Comparator: 21 No. of recruiting centres Not stated Follow-up period and frequency FU at 1, 2, 3, 6, 12, 18 and 24 mth |
Treatment intention Palliative Types of Lung Cancer and Histology Non-small cell 34 squamous cell, three adenocarcinoma, four large cell Main eligibility criteria Biopsy-proven stage III non-small cell bronchogenic carcinoma with obstructing or partially obstructing endobronchial lesion. Karnofsky rating ≥ 40 and ability to tolerate multiple bronchoscopies. Patients who had previous PDT or radiotherapy, or concurrent chemotherapy or Nd:YAG laser therapy were excluded, as were patients whose tumours were invasive to major blood vessels on CT scan Patient characteristics % Male: 76 Mean age: Around 67 yr Tumour location: 78% in main stem bronchus, 22% in lobar bronchus Concomitant treatment See ‘Eligibility criteria’ |
Trial treatments PDT + radiotherapy vs Radiotherapy alone Intervention Intravenous Photofrin at 2 mg/kg followed with 40–50 hr of red (630nm) light from argon-dye laser delivered by a single-step index quartz fibre inserted into the biopsy channel of a flexible fibreoptic bronchoscope (a cylindrical diffuser tip was inserted 1–2 cm into tumour). Power density was 400 mW/cm, total light dose was 200 J/cm. Residual tumour treated by a repeat light exposure The duration of light was not stated. Radiotherapy – see below Comparator Radiation at 3000 cGy in 10 fractions with a 4-MeV linear accelerator over 2 wk, using a parallel pair technique. Field size defined by the 50% isodose line with 2-cm margin of normal tissue |
Mortality 16 patients died in radiotherapy-alone group compared with 14 in PDT + radiotherapy group, in both groups eight deaths were due to metastases. Three patients in the PDT + radiotherapy group died from massive haemoptysis (67, 187 and 567 d, respectively, after treatment), compared with none in the Radiotherapy-alone group. No difference between groups in median survival times (444 d in PDT + radiotherapy vs 445 d in Radiotherapy-alone group) Morbidity Significantly greater reduction of haemoptysis and shortness of breath, and cough at 1 and 3 mth, in the PDT + radiotherapy group (p < 0.05) 14/20 PDT + radiotherapy and 2/21 radiotherapy alone achieved complete re-opening of bronchial lumen. Four patients in Radiotherapy-alone group failed to respond to treatment, none failed in PDT + radiotherapy group Median interval between treatment and local recurrence was significantly longer in PDT + radiotherapy group (233 d vs 107 d, p = 0.005) QoL and return to normal activity Assessed but not reported (Karnofsky performance) AEs Photosensitivity with mild erythema, which resolved without treatment, was seen in four of the PDT + radiotherapy group |
Authors’ conclusions The addition of PDT prior to radiotherapy provides significantly better and longer-lasting local control than radiotherapy alone Brief study appraisal No details of methods of randomisation, allocation concealment or blinding were reported for this small study (raising reliability issues). Some outcomes assessed but not reported |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Lam et al. (1987)124 Data source Full published paper Country Canada Language English Study design RCT No. of participants Total: 11 Intervention: Five Comparator: Six No. of recruiting centres Not stated Follow-up period and frequency FU at 4 and 12 wk, then quarterly thereafter (unless progression of tumour occurs) |
Treatment intention Palliative Types of Lung Cancer and Histology Non-small cell: nine squamous cell, two large cell Main eligibility criteria Patients with inoperable non-small cell bronchogenic carcinoma, partially or completely obstructing a central airway, who had received no prior treatment (e.g. chemotherapy or radiotherapy). Patients with evidence of metastatic disease were excluded Patient characteristics % Male: 82 Mean age: 66 yr All patients had tumours at more than one site Concomitant treatment Not stated |
Trial treatments PDT + radiotherapy vs Radiotherapy alone Intervention Intravenous Photofrin II 24–48 hr prior to red light (630 nm) from a continuous argon pumped-dye laser, via single-step index quartz fibres inserted into channel of a double lumen flexible fibreoptic bronchoscope (or a single channel instrument). A cylindrical diffuser tip (0.5, 1.0 or 1.5 cm) was inserted into the tumour. Power density was 400 mW/cm and total light dose 300 J/cm. Clean-up bronchoscopy followed 2 d later, with further light at 300 J/cm (but no more Photofrin II) for any residual tumours. PDT was given 1st, with radiotherapy starting within 1 wk of PDT Tumours that could not be inserted due to small size, or hardness, received 200 J/cm2 at power density of 200 mW/cm2 using microlens fibre The dose of Photofrin and duration of light were not stated. For radiotherapy, see below Comparator All patients had 3000 cGy in 10 fractions over 2 wk using a parallel-pair technique from a 4 MeV linear accelerator. Field size defined by the 50% isodose line with 2-cm margin of normal tissue |
Mortality One patient died in the PDT + radiotherapy group vs three in the Radiotherapy-alone group Morbidity Both groups had significantly improved respiratory symptoms at 4 wk (with mean scores falling from 7 to 1 in the PDT + radiotherapy group, and 7 to 4 in the Radiotherapy-alone group, p < 0.05). The mean score for the Radiotherapy-alone group was back up to 7 at 12 wk, but the PDT + radiotherapy group had a mean score of 2, which was significantly different from baseline (p < 0.05) At 4 wk, the PDT + radiotherapy group had a significant reduction in% airway obstruction (99 vs 21) and improvement in arterial oxygen (63 vs 80, both p < 0.05) when compared to baseline, with percentage airway obstruction still significantly improved at 12 wk (99 vs 25, p < 0.05). There were no significant reductions in the Radiotherapy-alone group QoL and return to normal activity At 4 wk, the PDT + radiotherapy patients had significant improvements (p < 0.05) in both Karnofsky rating (78 vs 93) and QoL (56 vs 39), compared to baseline scores. There were no significant differences in the Radiotherapy-alone group AEs One patient receiving PDT remained photosensitive for 8 wk |
Authors’ conclusions The addition of PDT prior to radiotherapy provides significantly better and longer-lasting palliation, than radiotherapy alone, for patients with obstructive endobronchial tumours. The combined treatment may also improve survival Brief study appraisal Very small sample size coupled with poorly reported methods make it difficult to draw any robust conclusions |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Leroy et al. (1998)125 Linked publications212 Data source Abstract Country Not stated Language English Study design RCT No. of participants Total: 141 Intervention: Not stated Comparator: Not stated No. of recruiting centres Not stated Follow-up period and frequency FU at 1 wk and 1 mth |
Treatment intention Palliative Type of Lung Cancer and Histology Non-small cell Main eligibility criteria Not stated Patient characteristics Not stated Concomitant treatment Not stated |
Trial treatments PDT vs Nd:YAG laser Intervention 2 mg/kg of Photofrin followed 48 hr later by light activation Light source and duration, wavelength of light, power density, total light dose, maximum no. of sessions allowed, and postoperative advice not stated Comparator No details provided |
Mortality Not assessed Morbidity CR + PR comparable between groups at wk 1 (PDT 65% vs Nd:YAG 61%), but significantly different at 1 mth (PDT 61% vs Nd:YAG 35%, p < 0.05). At 1 mth PDT also improved symptoms of dyspnoea (28% vs 13%), cough (33% vs 11%), haemoptysis (33% vs 19%), and sputum production (22% vs 14%), p-values not stated QoL and return to normal activity Not assessed AEs Mild-to-moderate skin photosensitivity in 21% of PDT patients. No further details |
Authors’ conclusions Photofrin is at least similar to or better than Nd:YAG thermal ablation in re-establishing the patency of the obstructed lumen and palliating symptoms at wk 1 and mth 1 following treatment Brief study appraisal The very limited information provided, particularly on study methods and basic results (e.g. no numbers on randomisation by treatment group) makes assessment of reliability difficult |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Maier et al. (2002)127 Data source Full published paper Country Austria Language English Study design Non-RCT No. of participants Total: 40 Intervention: 16 Comparator: 24 No. of recruiting centres One Follow-up period and frequency 1 wk and 4 wk |
Treatment intention Palliative Type of Lung Cancer and Histology Non-small cell Main eligibility criteria Patients with malignant tracheobronchial stenosis, not eligible for resection treatment because of poor performance status, functional and/or anatomical inoperability, and/or refusing surgery Patient characteristics % Male: 75 Mean age: ALA, 64 yr; Photosan, 66 yr Cancer stage: Stage IIb, seven; Stage IIIa, 13; Stage IIIb, six; Stage IV, 14 Squamous cell 27, Adenocarcinoma 10, Large cell carcinoma three Stenosis mean (range): ALA, 79% (50–90%); Photosan, 50% (20–95%) 22 patients with radiological and clinical signs of poststenotic pneumonia Karnofsky status mean (range): ALA, 78 (60–90); Photosan, 70 (60–80) Further patient characteristics were reported Concomitant treatment At least 4 wk after combined PDT/HBO, all patients were considered for further treatment, including high-dose rate brachyradiotherapy, external beam irradiation and/or chemotherapy |
Trial treatments PDT with 5-ALA and HBO vs PDT with Photosan and HBO Intervention ALA was orally administered at a dose of 60 mg/kg, 6–8 hr prior to PDT In cases of severe tumour stenosis, PDT was carried out by using a fibre with a 2-cm tip radial light-diffusing cylinder, which was inserted through the biopsy channel of the endoscope. In cases of moderate tumour stenosis a 2-cm balloon applicator system was used for homogeneous light distribution. During treatment the radial light diffusing cylinder and/or balloon applicator system was closely applied to the surface of the tumour. The light dose was 100 J/cm2. Light at 630 nm was applied by a KTP-Nd: YAG laser with a DYE module. In both groups additional hyperbaric oxygenation at a level of 2 ATA in a walk-in hyperbaric chamber was undertaken. Oxygen was applied using a Scuba valve system. Each treatment was performed under short-term intravenous anaesthesia with endotracheal intubation and spontaneous breathing. Skin protection was managed by use of a camouflage (Covermark, Milan, Italy) for 24 hr after photosensitisation Comparator Photosan-3 was administered intravenously at a dosage of 2 mg/kg, 48 hr prior to PDT. See intervention for details of PDT delivery. Skin protection was by using a commercially available sun blocker for 12 wk |
Mortality The mean survival for the ALA group was 9 mth and the Photosan group 14 mth (p = 0.020) Morbidity 4 wk FU In the ALA group, stenosis diameter dropped from a mean value of 79% to 63%. In the Photosan group the mean value dropped from 50% to 19%, p = 0.00073, in favour of Photosan. Dyspnoea was improved in 10/16 ALA patients and 19/24 Photosan patients. Haemoptysis subsided in 13/16 ALA patients and 20/24 Photosan patients. Radiological and clinical signs of poststenotic pneumonia subsided in 5/9 ALA patients and 9/13 Photosan patients. There was no statistically significant difference between groups on pulmonary function parameters QoL and return to normal activity Mean Karnofsky value changed from 78 to 79 in the ALA group, and from 70 to 78 in the Photosan group, showing a significant difference in favour of the Photosan group (p = 0.00015). One patient had an improvement of 10% in the ALA group, whereas 11 patients in the Photosan group improved by 10% and five improved by 20%. None of the patients in the Photosan group reported a decrease in QoL due to long-lasting need for skin protection AEs No sunburn occurred in either group. No major complications relating to photosensitisation, PDT or HBO were observed. Minor complications were: fever in the afternoon after PDT (12 in ALA group, 18 in Photosan group) and mild chest pain for 1 or 2 d (6 in the ALA group and 13 in the Photosan group). None of the AEs required specific treatment |
Authors’ conclusions Photosan seems to be more effective than ALA in PDT of malignant tracheobronchial stenosis. However, these results would need confirming in a randomised, blinded trial Brief study appraisal This small pilot study was non-randomised and the groups had differences at baseline which may have impacted on results. The survival data do not solely reflect the effectiveness of the PDT treatment, as 4 wk after PDT patients were eligible to receive a variety of other treatments. There are also doubts as to whether the ALA dosage was optimal |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Moghissi et al. (1993)126 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 26 Intervention: 15 Comparator: 11 No. of recruiting centres Not stated Follow-up period and frequency At 1, 2 and 3 mth, then at 3-monthly intervals |
Treatment intention Palliative Type of Lung Cancer and Histology Non-small cell Main eligibility criteria Stage III inoperable NSCLC with > 50% intraluminal bronchial obstruction Patient characteristics % Male: 81 Age range: 43–76 yr Mean age: Intervention 60, comparator 66 (ns) Concomitant treatment Some patients had additional modalities of treatment after 1 mth |
Trial treatments PDT vs Nd:YAG laser Intervention Intravenous Photofrin or Photofrin II at dose of 2 mg/kg followed 48–54 hr later by red light (630 nm) from a copper vapour pumped-dye laser delivered through a 600-nm quartz fibre with a terminal cylindrical diffuser. Dose of 200 J/cm at 400 mW/cm. Duration of light dose was 500 s. Further sessions provided if needed. Thorough debridement and physiotherapy after treatment. Careful counselling on photosensitivity Comparator Nd:YAG laser (Fibrelase 100, Pilkington) with a 400-µm diameter delivery fibre using 40–50 W pulses of 3–5 s. Dose dependent on extent of tumour. Further sessions provided if needed |
Mortality No treatment-related mortality. Longer-term mortality not assessed Morbidity At 1-mth, luminal diameter, as percentage of normal diameter, was significantly greater in PDT group (83%) than the Nd:YAG group (61%), p < 0.0006. Both FVC and FEV1 improved significantly more with PDT 1 mth after treatment when compared to pre-treatment measurements: mean difference in FVC, 0.47 PDT vs –0.06 Nd:YAG, p < 0.05, mean difference in FEV1, 0.35 PDT vs 0.01 Nd:YAG, p < 0.05 All patients had a PR at 1 mth AEs No serious post-treatment complications. Mild fever in two Nd:YAG patients. No photosensitivity in PDT patients |
Authors’ conclusions PDT is more effective than Nd:YAG in patients with extensive obstructive lung cancer Brief study appraisal A small study with no details of methods of randomisation, allocation concealment or blinding (raising reliability issues) |
Appendix 19 Biliary tract cancer data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Dechene et al. (2007)132 Data source Abstract Country Not stated Language English Study design Non-RCT No. of participants Total: 29 Intervention: 16 Comparator: 13 No. of recruiting centres Not stated Follow-up period and frequency Not stated |
Treatment intention Palliative Type(s) of cancer and histology Non-resectable bile duct cancer Main eligibility criteria Advanced bile duct cancer (no further details given) Patient characteristics % Male: 76 Median age: 67–70 Histological confirmation was not reported Concomitant treatment Peri-interventional antibiotic prophylaxis |
Trial treatments PDT with Photosan-3 vs PDT with Photofrin II Intervention 2 mg/kg Photosan-3 administered 48 hr before radiation. A 4-cm quartz fibre and a diode laser system (635 nm; 1, 1 W, 220 J/cm). Light protection was advised for 4–6 wk. Further PDT parameters were not reported Comparator 2 mg/kg Photofrin II administered 48 hr before radiation. A 4-cm quartz fibre and a diode laser system (635 nm; 1, 1 W, 220 J/cm). Light protection was advised for 4–6 wk. Further PDT parameters were not reported |
Mortality Median survival in the PS-3 group was 690 d (95% CI 448 to 931) and in the PF2 group it was 494 d (95% CI 84 to 903), p = NS Morbidity Not assessed QoL and return to normal activity Not assessed AEs There was no substantial skin reaction observed (no data provided). 23% of patients in the PF2 group and 26% of patients in the PS3 group developed ‘considerable’ cholangitis Resource use Not assessed |
Authors’ conclusions PDT has the potential to considerably prolong survival in non-resectable bile duct cancer. The effect is not dependent on the type of haematoporphyrin photosensitiser Brief study appraisal This study was reported in abstract form only and did not provide details of methodology such as randomisation, blinding and allocation concealment. This is a small trial which may be underpowered to detect a difference between the photosensitisers |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Kahaleh et al. (2008)133 Data source Full published paper Country USA Study design Non-RCT No. of participants Total: 48 Intervention: 19 Comparator: 29 No. of recruiting centres One Follow-up period and frequency FU at 1 mth and every 3 mth thereafter (or earlier if there were complications) |
Treatment intention Palliative Type(s) of cancer and histology Non-resectable cholangiocarcinoma Main eligibility criteria Unclear, PDT offered after December 2004 to all patients with non-resectable cholangiocarcinoma or resectable lesions deemed inoperable Patient characteristics % Male: 50 Age range: 26–94 yr Mean age: 66.6 yr Tumour extension: Bismuth I, 6%; Bismuth II, 19%; Bismuth III, 35%; Bismuth IV, 40% Further patient characteristics were reported Pathological diagnosis was confirmed in 69% of cases Concomitant treatment Twenty-two patients had chemotherapy and 19 had radiotherapy. All patients received periprocedure antibiotic prophylaxis |
Trial treatments ERCP with PDT and stent vs ERCP with Stent alone Intervention Selective decompression of all opacified, dilated segments was attempted with bougie and balloon dilatation to assist in the placement of polyethylene stents Intravenous Photofrin at 2 mg/kg 48 hr prior to 633-nm (± 3-nm) light from a 2000-mW diode laser, delivered through a 3-m length fibre having a 2.5-cm-long cylindrical diffuser at distal end (diffuser was inserted into a 10F sheath of a plastic stent) Photoactivation performed at 633 nm* with a light dose of 180 J/cm2, fluence of 0.25 W/cm2 and duration of 750 s. One or two segments treated at discretion of endoscopist. PDT repeated at 3-mth intervals when all stents were replaced (this was done earlier if premature occlusion or migration occurred) *Although reported as 620 nm in the paper, based on the type of laser used this appears to have been a typographic error Comparator Selective decompression of all opacified, dilated segments was attempted with bougie and balloon dilatation to assist in the placement of polyethylene stents (7F, 8.5F and 10F in diameter). Repeated if indicated until patient refusal or death |
Mortality At end of study 10 patients were still alive, eight being from the PDT group There was statistically significant prolonged survival in the PDT group (mean 16.2 mth, SD 2.4) compared with the Stent-alone group (mean 7.4 mth, SD 1.6), p < 0.003. Mortality rates were significantly lower in the PDT group at 3 mth (0% vs 28%, p = 0.01), and 6mth (16% vs 52%, p = 0.01), but not at 12 mth (56% vs 82%, p = 0.08) vs Stent-alone group Morbidity Both groups had significantly decreased levels of serum bilirubin at 3 mth when compared to baseline levels (p = 0.008 for PDT and p = 0.0001 for stent only), although there was no significant difference between the two groups in the degree of decrease (p = 0.78) QoL and return to normal activity Not assessed AEs Stent-alone group: 10 patients developed cholangitis (with two patients dying as a consequence). Four patients developed pancreatitis and one had duodenal perforation. Further results reported. PDT group: three patients experienced skin phototoxicity. Seven patients developed cholangitis, two developed cholecystitis, and two haemobilia. Further results reported Resource use Not assessed |
Authors’ conclusions ERCP with PDT seems to increase survival in patients with unresectable cholangiocarcinoma when compared with ERCP alone, although it remains to be proved whether this is due to PDT or the number or ERCP sessions Brief study appraisal The aims of this study at its inception are uncertain as the study began in 2001, but PDT only became available for use in 2004. From this point on, PDT was offered to all patients, making it difficult to recruit groups with similar baseline characteristics. However, the authors acknowledged that the study design prevented definitive conclusions from being drawn |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Ortner et al. (2003)134 Data source Full published paper Country Germany Language English Study design RCT No. of participants Total: 39 Intervention: 20 Comparator: 19 No. of recruiting centres Two Follow-up period and frequency 14 d, 3 mth, 6 mth after the intervention. Survivors then followed up at 6-mth intervals |
Treatment intention Palliative Type(s) of cancer and histology NCC Main eligibility criteria Inclusion criteria were patients at least 18 yr of age with a proximal malignant tumour of the bile ducts (Bismuth types II–IV, TNM stages III and IV). They had a large (> 3 cm in diameter), imaging-confirmed, non-resectable tumour (assessed by two independent surgeons), positive histology and no evidence of cancer of another organ. Exclusion criteria were porphyria, previous chemotherapy or radiotherapy, previous technically successful stenting (details in paper), insertion of a metal stent, partial resection of cholangiocarcinoma, diagnostic ERCP more than 1 mth previously and a Karnofsky index of < 30%. Further detail is presented in the paper Patient characteristics % Male: Not stated Age range: 53–85 Median age: Intervention 64; control 68 (NS) Cancer stage: Stage III, seven; stage IVa, 19; stage IVb 13 Bismuth type: II, two; III, six; IV, 31 100% of all cases were histologically confirmed Further patient characteristics were reported Concomitant treatment Oral ciprofloxacin therapy, 250 mg twice daily, was started before ERCP and continued for 14 d |
Trial treatments PDT + Double stenting vs Stenting alone Intervention See comparator for details of stenting. PDT patients received Photofrin at a dosage of 2 mg/kg body wt intravenously 48 hr before laser activation. Endoprostheses were removed and an endoscopic Huibregtse Cotton set catheter was introduced proximally above the strictures. Intraluminal photoactivation was performed with a laser quartz fibre with a cylindrical diffuser tip, length 40 mm, core diameter 400 µm. Photoactivation was performed at 630 nm using a light dose of 180 J/cm2, fluence of 0.241 W/cm2 and irradiation time of 750 s under a continuous saline perfusion. A new set of endoprostheses was inserted after completion of PDT. Further technical details of the procedure are available in the paper. Patients remained in a darkened room for 3–4 d after injection and thereafter patients were gradually adapted to light. If any FU examination showed evidence of tumour in the bile duct, PDT was repeated. Mean number of treatments was 2.4 (minimum 1, maximum 5), the mean no of illuminations per patient was 5.3 and the median treatment time was 79 min (minimum 40; maximum 180). All patients received oxygen via a nasal catheter to optimise the PDT effect Comparator Endoscopic double-stenting followed diagnostic ERCP (all patients received oral ciprofloxacin therapy 250 mg twice daily before ERCP and continued for 14 d). At least two 10F endoprostheses had to be placed above the main strictures in every patient. Endoscopic plastic endoprostheses or percutaneous plastic prostheses were used. Successful drainage after technically successful stenting (definition given in paper) was defined as a decrease in bilirubin level > 50% within 7 d after stenting. When the 1st procedure did not lead to technically successful stenting, a 2nd procedure was performed. When the 2nd procedure was not satisfactory, percutaneous stenting was performed. Patients were randomised only after technically successful stenting. Stent exchanges were performed every 3 mth. Eight patients received extra interventions (chemotherapy, four; PDT, three; immunotherapy, one) as a last resort treatment |
Mortality Median survival in the PDT group was 493 d (95% CI 276 to 710) and 98 d in the Stenting-only group (95% CI 87 to 107) p < 0.0001. RR = 0.21 (95% CI 0.12 to 0.35). Two patients in the PDT group were still alive at the time of evaluation. the study was terminated early due to the superiority of PDT Morbidity Successful drainage was achieved in 21% of patients in both groups. After PDT serum bilirubin reached lower levels relative to baseline and stenting (p < 0.01). Successful drainage was obtained in 72% Mean number of stent exchanges: PDT group = 3.8, stenting alone = 3.7 QoL and return to normal activity The Karnofsky index improved after PDT with a median 80% score (minimum 50%, maximum 100%); mean change from baseline 3.00 but did not improve in the Stenting-alone group. The difference in change from baseline between the PDT + Stenting group and the Stenting-alone group was 11.43 (95% CI 2.92 to 19.95, p < 0.01). After PDT physical functioning (p < 0.01) and global QoL (p < 0.001) improved in the PDT group but not in the Stenting-alone group. The results of individual factors relating to QoL measures are listed in the paper AEs Burden of treatment was lower in PDT vs Stenting alone (p < 0.001). Photosensitivity was reported by two (10%) of PDT patients; all reactions were mild and resolved completely. Any cholangitis occurring during FU was considered an AE. There were three cases in the PDT group and seven in the Stenting-alone group. Stenosis probably related to therapy was reported by two in the PDT group and zero in the Stenting-alone group. Fatal cholangitis, sepsis possibly related to therapy: PDT group two of 18, Stenting-alone group six of 19 The following were causes of death probably not related to therapy: Pulmonary embolism: PDT group one of 18, Stenting alone three of 19 Cachexia: PDT group one of 18, Stenting one of 19 Cardiac failure: one of 18, one of 19, respectively Metastases: 12 of 18, eight of 19, respectively Chronic renal failure: one of 18, zero of 19, respectively Resource use Not assessed |
Authors’ conclusions PDT added to best supportive care improves survival and QoL in patients with NCC. Prolonged survival time was not associated with a high rate of AEs Brief study appraisal This was a well-conducted and reported trial, which was halted early due to the superiority of the PDT treatment |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Witzigmann et al. (2006)135 Data source Full published paper Country Germany Language English Study design Non-RCT No. of participants Total: 184 (191 if including seven patients not analysed due to missing FU data) Intervention: 68 Comparator: 56 2nd comparator: 60 No. of recruiting centres Not stated Follow-up period and frequency Unclear – patients were recruited over 10 yr, which appears to be the FU period |
Treatment intention Curative palliative Type(s) of cancer and histology Hilar cholangiocarcinoma Main eligibility criteria Not stated Patient characteristics % Male: 52 Age range: 22–91 yr Tumour stage: Patients from all stages, but mostly IB and IIA Tumour extent: Patients from all Bismuth types, but mostly type IV. According to both Bismuth–Corlette and UICC classifications there were more advanced tumours in the palliative treatment groups than in the resection group (p < 0.05). Further patient characteristics were reported The number of cases confirmed histologically was not clearly reported Concomitant treatment Chemotherapy, radiation therapy, chemoradiation and iridium implants were occasionally used |
Trial treatments PDT + stenting vs Stenting alone vs Resection Intervention PDT + stenting: Intravenous Photofrin at 2 mg/kg with photoactivation after 1–4 d. Repeated when there was evidence of tumour progression. Further PDT parameters were not reported. See below for stenting Comparator Stenting: At least two 9F or 11.5F plastic endoprostheses were placed above the main strictures and exchanged every 3 mth 2nd comparator Resection: One of right-sided hemihepatectomy, right trisegmentectomy, left hemihepatectomy, hilar resection alone or liver transplantation, with additional types of surgery when required. Neoadjuvant PDT and biliary drainage also given if required |
Mortality PDT + stenting vs Stenting alone:1- and 2-yr survival rates were 51% and 16% vs 23% and 10%, respectively; median survival time 12 mth vs 6.4 mth (p < 0.01); 63(93%) vs 51(91%) patients had died by the end of the study (the main causes were tumour progression and complications of chronic cholangitis). Resection: The 30- and 60-day death rates were 8.3% and 11.7%, respectively. Multiple organ failure from infective complications was the most common cause of death. Overall 1-, 3- and 5-yr survival rates including post op deaths were 73%, 40% and 27%, respectively, with a median survival of 23 mth. Neoadjuvant PDT before resection resulted in 1-, 3- and 5-yr survival rates of 88%, 42% and 42% compared with 66%, 28% and 19% after surgery alone (ns). There was no significant difference in median survival time between the (R1 and R2) resection group and the PDT + Stenting group Morbidity PDT + stenting and Stenting alone: At 3 mth, in the PDT + stenting group, there was a significant difference in mean bilirubin levels relative to baseline (p < 0.001). PDT + stenting group had significantly lower levels of bilirubin than the Stenting-alone group (4.1 mg/dl vs 7.3 mg/dl, p < 0.05). Successful drainage achieved in 75% of patients receiving PDT + stenting compared with 39% receiving stenting alone. Resection: Recurrence in 27 patients QoL and return to normal activity At 3 mth, median pre-treatment Karnofsky performance status increased by 2% for PDT + Stenting and decreased by 8% in Stenting-alone group (p < 0.01) AEs PDT + stenting/Stenting alone: There were no procedure-related deaths. Eight PDT patients had skin toxicity (grades I and II). Bacterial cholangitis seen in 38 PDT + Stenting patients and 32 Stenting-alone patients (ns). Resection: major complications reported in 52% of patients (37% required relaparotomy). The most common complication was bile leakage (eight patients) Resource use Median hospital stays were 65 d for PDT + stenting, 44 d for stenting alone, and 48 d for resection |
Authors’ conclusions Only complete tumour resection, including hepatic resection, enables long-term survival for patients with hilar cholangiocarcinoma. Palliative PDT + Stenting resulted in longer survival than stenting alone and has a similar survival time compared with incomplete R1 and R2 resection Brief study appraisal No firm conclusions can be drawn from the results of this study for several reasons. There was heterogeneity of treatments within the three groups (e.g. some patients in the resection group also received neoadjuvant PDT). The groups were also clinically heterogeneous (significantly different at baseline for several parameters), and there was wide variation in length of FU between groups and patients |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Zoepf et al. (2005)136 Data source Full published paper Country Germany Language English Study design RCT No. of participants Total: 32 Intervention: 16 Comparator: 16 No. of recruiting centres Not stated Follow-up period and frequency 1, 3, 6, 9, 12 and every 3 mth. Earlier endoscopic interventions were performed when needed in case of clotting or dislocation |
Treatment intention Palliative Main eligibility criteria Advanced, non-resectable BDC Patient characteristics % Male: 63 Median age: 68 yr (range 52–80 yr) Cancer stage: Stage II + lymph nodes, one; stage IV, 31 Initial median Karnofsky performance status: 90% (70–100) Overall 63% of cases were confirmed histologically Concomitant treatment All patients received prophylactic antibiotic treatment before the procedure with 1 g of Ceftriaxon, given intravenously 30 min before intervention. Ongoing antibiotic oral treatment with chinolone was given for a total of 14 d. The 1st eight PDT patients had intravenous Cefriaxon over 3 d but did not receive continuing oral antibiotic treatment |
Trial treatments PDT + endoscopic drainage vs Endoscopic drainage alone Intervention Photosan-3 was given intravenously at a dose of 2 mg/kg bw 48 hr before laser irradiation. A flexible cylindrical diffuser probe was used. The probe was mounted on a 400-µm quartz fibre with an active length of 4cm and a radiopaque marker at the distal fibre tip. A diode laser system with a maximum power output of 2 W and a wavelength of 633 ± 3 nm was used as light source. Irradiation time was calculated for a light energy density of 200 J/cm2. In most cases this was around 550 s. the power density was 450–500 mW/cm and energy dose was 250–275 J/cm of diffuser length. For transpapillary PDT the light applicator was inserted through the working channel of a duodenoscope. For percutaneous PDT the quartz fibre was guided in four patients through the partially removed percutaneous catheter and in one patient through the working channel of a cholangioscope. The 1st PDT session was performed at a median of 4.5 mth (1–9) after diagnosis of BDC. Nine patients received a 2nd PDT session after 3–9 mth and one patient a 3rd session 6 mth after the 2nd session. Further PDT parameters were not reported. All patients were provided with plastic endoprostheses immediately after the PDT treatment Comparator The aim of the endoprosthetic treatment was bilateral hilar drainage. Endoprostheses were regularly changed every 3 mth or earlier in case of clotting or dislocation |
Mortality The PDT group had a longer survival time compared to the endoprosthesis group (21 mth vs 7 mth, p = 0.01). In the PDT group, 30-d mortality was 0% and 6% in the endoprosthesis group. At the time of evaluation, three patients in the PDT group and one in the endoprosthesis group were still alive. The PDT patients were deemed to be in good clinical condition without significant cholestasis. No details were provided on the survivor in the endoprosthesis group. In the PDT group 12 patients died of tumour-related causes, and one patient of a perforated gastric ulcer. Causes of death were not reported for the endoprosthesis group Morbidity 4 wk after initial PDT, most PDT patients showed an almost complete elimination of bile duct stenosis in the treated area as shown with cholangiography (data not provided). Median bilirubin level after 1st intervention was not significantly different between the groups QoL and return to normal activity QoL as assessed by the Karnofsky scale did not significantly improve after PDT AEs Three patients developed an infected bilioma with prolonged cholangitis after PDT treatment, which could be managed by antibiotic treatment. Another patient developed cholecystitis 2 mth after a 2nd treatment session, which led to surgical laparoscopic cholecystectomy. There was no skin phototoxicity observed in any of the three patients. One patient developed severe bacterial cholangitis during endoprosthetic therapy, which was successfully treated by standard antibiotic therapy Resource use Not assessed |
Authors’ conclusions PDT is minimally invasive but there is a considerable risk of cholangitis after the procedure. PDT was found to result in a substantial prolongation of survival time but this would need confirmation in further patient series. PDT has the potential to result in a changeover of current palliative treatment of BDC Brief study appraisal A small trial with clear reporting of procedures. The results appear to be reliable but would need confirmation in a larger trial |
Appendix 20 Brain cancer data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Eljamel et al. (2008)139 Data source Full published paper Country UK Language English Study design RCT No. of participants Total: 27 analysed (42 randomised?) Intervention: 13 Comparator: 14 No. of recruiting centres One Follow-up period and frequency 3-monthly, until death |
Treatment intention Curative Type(s) of cancer and histology GBM Main Eligibility Criteria Patients over 17 yr, with a new MRI diagnosis of GBM and a Karnofsky score ≥ 60 Patient characteristics % Male: 67 Mean age: 59.8 yr Mean Karnofsky performance score: 70 Concomitant treatment Some study patients received additional treatments such as chemotherapy and further surgery. However, there were no statistically significant differences between the groups in patients receiving additional treatments |
Trial treatments Fluorescence-guided resection and repetitive PDT and radiotherapy vs standard resection and radiotherapy Intervention PDT: Patients were given 2 mg/kg Photofrin intravenously 48 h before surgery, and 20 mg/kg ALA orally 3 h before surgery. After removal of bulk of tumour violet-blue light (375–440 nm) with a 440-nm observation filter was used to illuminate the cavity with fluorescence detected by a high-quality photodiagnosis camera, and detected tumour was removed until no further fluorescence was detected. A laser-based (405 nm) protoporphyrin-IX spectroscopy detection system was used to detect any remaining tumour cells at the margins, which were removed. A size-10 balloon catheter was inflated to fit the cavity with 0.8% intralipid solution. After the patient awoke from surgery the 1st PDT treatment, using 630-nm diode laser (600 mW), was given in theatre recovery at 100 J/cm2; more PDT was given at 72, 96, 120 and 144 hr. Patients also received standard radiotherapy. Advice was given on sun protection measures Comparator Tumour removal using the same neuronavigation and surgical microscope as the PDT group. Patients also received standard radiotherapy |
Mortality Mean survival in the PDT group was 52.8 wk vs 24.2 wk in the surgery group (p < 0.001) Morbidity There was no residual tumour on discharge scan in 10/13 PDT patients vs 4/14 surgery patients. Mean time to tumour progression was 8.6 mth in the PDT group vs 4.8 mth in the surgery group (p < 0.01) QoL and return to normal activity The Karnofsky score at 6 mth had improved from 70 (at baseline) to 80 in the PDT group, although the authors reported an improvement of 20 points. The scores remained the same for the surgery group (at 70) AEs Three patients had deep vein thrombosis, two of which were in the PDT group. No infections or seizures occurred Resource use There was no difference between the groups in length of hospital stay (both had a mean stay of 7 d) |
Authors’ conclusions ALA and Photofrin fluorescence-guided resection with repetitive PDT offer a worthwhile survival advantage, without added risk, to patients with GBM Brief study appraisal The Karnofsky results were reported inconsistently within the paper, making interpretation difficult. It was unclear how many patients had actually been randomised and treated, as 14 patients with negative biopsy results were subsequently excluded from analyses. The analysed population does not therefore appear to reflect the population presenting clinically (patients with an MRI diagnosis). Although the study made use of blinding to assess outcomes, it was nevertheless unclear whether suitable methods had been used to randomise and allocate participants to treatments. No results were reported on possible photosensitisation effects. The authors did though acknowledge the need for a much larger study |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Krishnamurthy et al. (2000)138 Data source Full published paper Country USA Language English Study design Non-RCT No. of participants Total: 18 Intervention: Six Comparator: Six 2nd comparator: Six No. of recruiting centres One Follow-up period and frequency Days 1 and 2, at discharge from hospital, at 1, 4 and 6 wk, then at 3-mth intervals |
Treatment intention Curative Palliative Type(s) of cancer and histology 12 glioblastoma, five anaplastic astrocytoma, one malignant ependymoma Main eligibility criteria Patients aged 18–75 yr, with supratentorial primary malignant brain tumours ≤ 5 cm in diameter, a Karnofsky rating ≥ 60, and who had recurrent or residual tumours were eligible. Patients had to have received radiation therapy > 3 mth prior to PDT treatment. Further eligibility criteria were reported Patient characteristics Age range: 32–70 yr Median Karnofsky score: 90 Tumour locations also reported. All patients had initial surgery, radiation therapy and chemotherapy before recurrence Concomitant treatment Steroid therapy if required |
Trial treatments PDT 1500–3700 J vs PDT 3700–4400 J vs PDT 4400–5900 J Intervention PDT 1500–3700 J (group 1): Intravenous Photofrin at 2 mg/kg, followed 24 hr later by anaesthetic and CT or MRI scan which locates tumour using stereotactic arc system. Six optical diffusion tip fibres (1.6 mm) and central fibre inserted through drill holes into skull. Red light (630 nm) from an argon pumped-dye laser was delivered through optical fibres and beam splitters to individual diffusion tip fibres. Tumours were biopsied (and if no malignancy was found the patient was excluded from the study). Patients were advised about sunlight protection, and about avoiding direct sunlight and bright artificial light for 6 wk Comparator PDT 3700–4400 J (group 2): See above 2nd comparator PDT 4400–5900 J (group 3): See above |
Mortality Mean survival time was 314 d (sd = 106) in group 2 vs 238 d in group 3 (sd = 61). Group 1 not stated Morbidity 16 patients had recurrence after PDT (four in group 1, six in group 2 and six in group 3), and two did not. Time to tumour recurrence was a mean of 150 d in group 2 vs 131d in group 3. Group 1 not stated QoL and return to normal activity Median Karnofsky rating changed from 90 (pre-PDT) to 85 (post PDT) AEs Five patients had postoperative permanent neurological defects (zero in group 1, two in group 2, and three in group 3) Resource use Not assessed |
Authors’ conclusions Increasing the light dose increases the odds of having permanent neurological deficit, but does not increase survival time, or time to tumour progression. There was no difference in recurrence with increasing light dose Brief study appraisal This non-randomised study appeared to have far too small a sample size to provide clinically meaningful results. Results were not always provided for all three groups |
Appendix 21 Head and neck cancer data extraction
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Li et al. (2006)141 Data source Full published paper Country China Language English Study design RCT No. of participants Total: 30 Intervention: 15 Comparator: 15 No. of recruiting centres Not stated Follow-up period and frequency FU at 1, 3 and 6 mth |
Treatment intention Not stated, appears palliative Type(s) of cancer and histology Nasopharyngeal carcinoma Main eligibility criteria Patients who had relapsed and who had failed radiotherapy Patient characteristics % Male: 80 Age range: 28–72 Mean age: 54 yr Cancer stage: All stage IV and had local relapse. All had prior (failed) radiotherapy; some also had prior chemotherapy, 6–12 mth before trial treatment Concomitant treatment Anti-vomiting treatment for chemotherapy group. Chinese herbs (unspecified) |
Trial treatments PDT vs Chemotherapy (cisplatin and 5-FU) Intervention Intravenous Photofrin of 2 mg/kg. Local anaesthetic (lidocaine) before light (630 nm) at 200–300 J/cm from a diode laser through a cylindrical diffuser (1–5 cm) 48 hr after injection. Light was applied to one to three overlapping segments for 12 min per segment. Segments had at least a 0.5-cm margin beyond the lesion. After 48-hr necrotic tissue removed by biopsy forceps and newly exposed lesions were re-treated after cleaning. Cleaning was repeated when necessary. Patients asked to avoid sunlight for 4–6 wk after treatment. Maximum number of sessions was not stated Comparator Cisplatin 80 mg/m2 and 5-FU 500 mg/m2, both divided into five. Each cycle lasted 4 wk. Two cycles were given |
Mortality Not assessed Morbidity Overall clinical response was statistically significantly better with PDT than chemotherapy (p = 0.001). No patients achieved CR. The PDT group had more patients with a significant response (i.e. 50% reduction for 1 mth, 12 vs 2). In those patients with nasal obstructions PDT produced more effective debulking (p = 0.04) (subgroup of 16 patients, 7/8 improved vs 2/8) QoL and return to normal activity PDT group had a statistically significant greater improvement in Karnofsky score (p = 0.02). PDT group increased from 45 to 70 vs chemotherapy group increased from 40 to 50 AEs All PDT related adverse effects and reactions were tolerable. Treatment to the laryngopharynx area resulted in slight pain and increased nasal cavity secretion – resolved in 3–5 d One case of severe laryngopharynx swelling and pain. Resolved with treatment 1 wk later, may be related to light exposure One case of photosensitivity dermatitis after accidental exposure to daylight. Resolved after treatment, 1 wk later One case of skin pigmentation, no treatment required |
Authors’ conclusions PDT is effective and safe for the treatment of advanced nasal pharyngeal cancer and the management of nasal obstruction Brief study appraisal This small pilot study provided no details on randomisation, blinding and other study quality parameters raising questions about the validity of the results. Treatment details were well described. It does not appear to have led on to a larger study, though the authors rightly stated that further investigation was needed |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Loukatch et al. (1995)142 Data source Abstract Country Ukraine Language English Study design Non-RCT No. of participants Total: 145 Intervention: 42 Comparator: 51 2nd comparator: 52 No. of recruiting centres Not stated Follow-up period and frequency 3 yr |
Treatment intention Curative Type(s) of cancer and histology Planocellular cancer of larynx Main eligibility criteria Not stated Patient characteristics Cancer stage: Stage Ib, 79; stage IIa, 66. All cancers were of middle localisation. No further characteristics were reported Concomitant treatment Not stated |
Trial treatments Surgery/intraoperative PDT vs Surgery/intraoperative PDT without laser vs Surgery alone Intervention Cordectomy with local anaesthetic, followed by administration of 0.4% solution of methylene blue photosensitiser. A He-Ne laser of wavelength 633 nm emitting at 25–30 mW/cm2 for 5 min was used. Further PDT parameters were not reported Comparator Cordectomy with local anaesthetic followed by laser only (same parameters as PDT group) 2nd comparator Cordectomy with local anaesthetic only |
Morbidity After 3 yr there was recurrence in one patient (2%) and no cases of metastasis in the PDT group, four cases of recurrence (8%) in the laser group and one case of metastasis, and five cases of recurrence (10%) and two cases of metastasis in the Surgery-alone group QoL and return to normal activity Not assessed AEs PDT group: Some patients had oedema of laryngeal mucous membrane and one patient had a small haemorrhage. Surgery-alone group: One patient had a small haemorrhage. Both haemorrhages were managed by conservative treatment |
Authors’ conclusions Intraoperative PDT in patients with stages I and II laryngeal cancer could be effective for preventing recurrence and metastasis of tumours Brief study appraisal This study used comparator treatments such that the results offer little insight to how PDT compares to other treatments adjunctive to surgery. The study also examined few outcomes and there was sparse reporting of methods, patient characteristics and results |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Loukatch et al. (1996)143 Data source Abstract Country Ukraine Language English Study design Non-RCT No. of participants Total: 49 Intervention: 19 Comparator: 15 2nd comparator: 15 No. of recruiting centres Not stated Follow-up period and frequency 3 yr |
Treatment intention Curative Type(s) of cancer and histology SCC in laryngeal part of pharynx Main eligibility criteria Not stated Patient characteristics Cancer stage: Stage II, 25; stages III and IV, 24 No further characteristics were reported Concomitant treatment Pharyngotomy for stage II patients and hemilaryngopharyngotomy for stage III–IV patients with general anaesthetic. Postoperative cobalt therapy (45-Gy dose). Neck dissection operation for patients developing metastases |
Trial treatments PDT vs PDT with Laser only vs PDT with Photosensitiser only Intervention PDT with methylene blue (injected locally) as photosensitiser, followed by 633-nm light for 5 min from a He-Ne laser at 25–30 mW/cm2. Further PDT parameters were not reported Comparator As for PDT group but without photosensitiser 2nd comparator As for PDT group but without laser |
Mortality 2-yr survival PDT group, 100% (stage II), 89% (stages III and IV); Laser-only group: 88% (stage II), 86% (stages III and IV); Photosensitiser-only group: 71% (stage II), 75% (stages III and IV) 3-yr survival PDT group, 100% (stage II), 67% (stages III and IV); Laser-only group: 43% (stages III and IV); Photosensitiser-only group: 38% (stages III and IV). Stage II results not available for last two groups Morbidity After 1 year, none of the stage II patients had recurrence or metastasis. For stage III and stage IV patients, there was recurrence in one patient (11%) in the PDT group, two patients (29%) in the Laser-only group and two patients (25%) in the Photosensitiser-only group After 2 yr, in the PDT group: recurrence in 10% (stage II) and 11% (stages III and IV); in the Laser-only group: recurrence in 25% (stage II), and 43% (stages III and IV); in the Photosensitiser-only group: recurrence in 43% (stage II), and 50% (stages III and IV) During the 3-yr period there were regional metastases in 0% of PDT group (stage II), 11% in PDT (stages III and IV), 13% of Laser-only group (stage II), 14% Laser-only (stages III and IV), 14% Photosensitiser-only (stage II), 25% Photosensitiser-only (stages III and IV) QoL and return to normal activity Not assessed AEs No complications postoperatively, except some patients had minor oedema of the pharyngeal mucous membrane (resolved within 5 d) |
Authors’ conclusions PDT appears effective in treating tumours of the pharynx, but larger studies with longer FU are needed Brief study appraisal This study used comparator treatments such that the results offer little insight to how PDT compares with other treatments adjunctive to surgery. There was sparse reporting of methods and patient characteristics, and results were sometimes incomplete |
| Study details | Population details | Treatment details | Results | Interpretation |
|---|---|---|---|---|
|
Authors Vakulovskaya (2007)144 Linked publications213 Data source Abstract Country Russia Language English Study design Non-RCT No. of participants Total: 52 Intervention: Not stated Comparator: Not stated No. of recruiting centres Not stated Follow-up period and frequency Not stated (although survival monitored up to 3 yr) |
Treatment intention Not stated Type(s) of cancer and histology Oral cancer (SCC) Main eligibility criteria Not stated Patient characteristics Patients had tumours in the oral cavity, oropharynx or lower lip. Further patient characteristics were not reported Concomitant treatment Not stated |
Trial treatments PDT with Photosense vs PDT with Radachlorin Intervention Intravenous Photosense (0.4–0.8 mg/kg) with semiconductive lasers (Milon 660, Biospec 672) at a total light dose of 400–600 J/cm2. Further PDT parameters were not reported Comparator Intravenous Radachlorin (1.2–2.4 mg/kg) with semiconductive lasers (Milon 660, Biospec 672) at a total light dose of 200–300 J/cm2. Further PDT parameters were not reported |
Mortality Not broken down by treatment group Morbidity For Photosense a CR was seen in 57% of patients and a PR in 38%. For Radachlorin a CR was seen in 20% and a PR in 50% QoL and return to normal activity Not assessed AEs Main side effect with Photosense was increased skin sensitivity to direct sunlight, with Radachlorin skin sensitivity is short term. No further details were given |
Authors’ conclusions Our experience showed pronounced efficacy of PDT with high functional and cosmetic effects for oral cancer of different localisations Brief study appraisal Minimal reporting of both methods and results means little can be deduced from this conference abstract |
Glossary
- Actinic/solar keratosis
- A form of pre-cancerous skin lesion, which, based on pathological evidence, is considered to be a precursor to squamous cell carcinoma.
- Adverse event
- Any untoward medical occurrence in a patient who is administered a treatment, and which does not necessarily have a causal relationship with the treatment.
- Allocation concealment
- When neither patients nor health-care professionals are aware of the randomisation schedule or allocation of treatment to any one individual.
- Barrett’s oesophagus
- A condition in which the injured lining of the oesophagus is replaced by a new abnormal lining (specialised intestinal metaplasia).
- Basal cell carcinoma
- A skin cancer that may take a variety of clinical appearances, such as nodular, cystic, superficial, morphoeic, ulcerated or pigmented.
- Bowen’s disease
- A pre-invasive form of squamous cell skin cancer, also called squamous cell carcinoma (SCC) in situ.
- Brachytherapy
- Form of radiotherapy where a radioactive source is placed inside or next to the area requiring treatment.
- Chi-squared (χ2) test
- A statistical test used to assess heterogeneity by testing the null hypothesis that the true treatment effects are the same in each study.
- Cholangiocarcinoma
- Cancer of the bile ducts.
- Confidence interval
- The range of uncertainty about an estimate of a treatment effect. It is the range of values above and below the point estimate that is likely to include the true value of the treatment effect. A 95% confidence interval (CI) indicates that there is a 95% probability that the CI calculated from a particular study includes the true value of a treatment effect.
- Cryotherapy
- The application of extreme cold to destroy abnormal or diseased tissue.
- DYE laser
- A laser that uses an organic dye to obtain a desired wavelength.
- External validity
- The extent to which the effects observed in a study can be expected to apply in routine clinical practice, i.e. to people who did not participate in the study.
- Fixed effect model
- A statistical model that assumes only within-study variation as influencing the uncertainty of results [as reflected in the confidence interval (CI)] of a meta-analysis. Variation between the estimates of effect from each study (heterogeneity) does not affect the CI in a fixed effect model.
- Forest plot
- A graphical display designed to illustrate the relative strength of treatment effects in multiple quantitative studies addressing the same question.
- Fluorouracil (5-FU)
- A chemotherapy agent.
- Hazard ratio
- The degree of increased or decreased risk of death or other clinical outcome over a period of time.
- Heterogeneity
- Heterogeneity can be used as a statistical term: the differences/variability between the individual studies in the estimates of effects, or in terms of clinical variation between participants, interventions or settings.
- Homogeneity
- The degree to which the results of studies are similar.
- I2 statistic
- A measure to estimate how much of the total variation between the treatment estimates can be attributed to statistical heterogeneity rather than chance. It gives the proportion of the total variation that is due to heterogeneity between study results.
- Incidence
- The number of new cases of a specific condition occurring during a certain period in a specified population.
- Incubation time
- The period of time from drug application to light administration.
- Intention-to-treat analysis
- An analysis based on the initial treatment intent, not on the treatment eventually administered.
- Internal validity
- The degree to which a result (of a measurement or study) is likely to be true and free of bias.
- Karnofsky score
- An attempt to categorise a patient’s general well-being. It runs from 100 to 0, where 100 is ‘perfect’ health, 50% is ‘requires help often’, and 0 is ‘death’ (see Appendix 3).
- KTP laser
- A laser that is directed through a potassium titanyl phosphate (KTP) crystal to produce a beam in the green visible spectrum.
- Meta-analysis
- A method of combining studies to produce an overall summary of the treatment effect across studies (see also Fixed effect model and Random effects model).
- Odds ratio
- A way of comparing whether the odds, or likelihood of a certain event is the same for two groups; the odds refers to the ratio of the number of people having an event to the number not having an event.
- Per-protocol analysis
- An analysis based on patients who complete/adhere to the course of treatment.
- Prevalence
- The proportion of people in a population who have a given disease or attribute at a given point in time.
- Proton pump inhibitor
- A drug used for treatment of erosion and ulceration of the oesophagus, caused by gastroesophageal reflux disease.
- Quality of life(health-related quality of life)
- A concept incorporating all of the factors that might impact on an individual’s life, including factors such as the absence of disease or infirmity, as well as other factors that might affect their physical, mental and social well-being.
- Random effects model
- A statistical model, sometimes used in meta-analysis, in which both within-study sampling error (variance) and between-study variation are included in the assessment of the uncertainty (confidence interval) of the results of a meta-analysis.
- Serious adverse event
- Any untoward medical occurrence that results in death, is life threatening, requires hospitalisation, or results in significant disability or incapacity.
- Stricture
- An abnormal contraction of any passage or duct of the body (e.g. due to scar tissue).
List of abbreviations
- 5-FU
- fluorouracil
- AE
- adverse event
- AK
- actinic keratosis
- ALA
- aminolevulinic acid
- APC
- argon plasma coagulation
- BCC
- basal cell carcinoma
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHE
- Centre for Health Economics
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CR
- complete response
- CRD
- Centre for Reviews and Dissemination
- CRT
- chemoradiotherapy
- CT
- computerised tomography
- DARE
- Database of Abstracts of Reviews of Effects
- DHE
- dihaematoporphyrin ether
- MR
- endoscopic mucosal resection
- ERCP
- endoscopic retrograde cholangiopancreatography
- FEV1
- forced expiratory volume in one second
- FU
- follow-up
- FVC
- forced vital capacity
- GORD
- gastroesophageal reflux disease
- Gy
- gray
- HGD
- high-grade dysplasia
- HpD
- haematoporphyrin derivative
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LED
- light-emitting diode
- LGD
- low-grade dysplasia
- MAL
- methyl aminolevulinate
- MeSH
- medical subject headings in the MEDLINE thesaurus
- mTHPC
- meta-(tetrahydroxyphenyl)chlorine
- Nd:YAG
- neodymium-doped yttrium aluminium garnet (laser)
- NHS
- National Health Service
- NICE
- National Institute for Health and Clinical Excellence
- NSCLC
- non-small cell lung carcinoma
- OR
- odds ratio
- PDD
- photodynamic diagnosis
- PDT
- photodynamic therapy
- PPI
- proton pump inhibitor
- PpIX
- protoporphyrin IX
- PR
- partial response
- Ps
- porfimer sodium
- PsD-007
- photocarcinorin
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SCC
- squamous cell carcinoma
- VAS
- visual analogue scale
- VPL
- variable pulsed light
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Riemsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost-effectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.
By Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al.
-
Gemcitabine for the treatment of metastatic breast cancer.
By Jones J, Takeda A, Tan SC, Cooper K, Loveman E, Clegg A.
-
Varenicline in the management of smoking cessation: a single technology appraisal.
By Hind D, Tappenden P, Peters J, Kenjegalieva K.
-
Alteplase for the treatment of acute ischaemic stroke: a single technology appraisal.
By Lloyd Jones M, Holmes M.
-
Rituximab for the treatment of rheumatoid arthritis.
By Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, et al.
-
Omalizumab for the treatment of severe persistent allergic asthma.
By Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al.
-
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma.
By Boland A, Bagust A, Hockenhull J, Davis H, Chu P, Dickson R.
-
Adalimumab for the treatment of psoriasis.
By Turner D, Picot J, Cooper K, Loveman E.
-
Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a single technology appraisal.
By Holmes M, C Carroll C, Papaioannou D.
-
Romiplostim for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a single technology appraisal.
By Mowatt G, Boachie C, Crowther M, Fraser C, Hernández R, Jia X, et al.
-
Sunitinib for the treatment of gastrointestinal stromal tumours: a critique of the submission from Pfizer.
By Bond M, Hoyle M, Moxham T, Napier M, Anderson R.
-
Vitamin K to prevent fractures in older women: systematic review and economic evaluation.
By Stevenson M, Lloyd-Jones M, Papaioannou D.
-
The effects of biofeedback for the treatment of essential hypertension: a systematic review.
By Greenhalgh J, Dickson R, Dundar Y.
-
A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study.
By Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al.
-
Lapatinib for the treatment of HER2-overexpressing breast cancer.
By Jones J, Takeda A, Picot J, von Keyserlingk C, Clegg A.
-
Infliximab for the treatment of ulcerative colitis.
By Hyde C, Bryan S, Juarez-Garcia A, Andronis L, Fry-Smith A.
-
Rimonabant for the treatment of overweight and obese people.
By Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, et al.
-
Telbivudine for the treatment of chronic hepatitis B infection.
By Hartwell D, Jones J, Harris P, Cooper K.
-
Entecavir for the treatment of chronic hepatitis B infection.
By Shepherd J, Gospodarevskaya E, Frampton G, Cooper, K.
-
Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal.
By Stevenson M, Pandor A.
-
Rivaroxaban for the prevention of venous thromboembolism: a single technology appraisal.
By Stevenson M, Scope A, Holmes M, Rees A, Kaltenthaler E.
-
Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck.
By Greenhalgh J, Bagust A, Boland A, Fleeman N, McLeod C, Dundar Y, et al.
-
Mifamurtide for the treatment of osteosarcoma: a single technology appraisal.
By Pandor A, Fitzgerald P, Stevenson M, Papaioannou D.
-
Ustekinumab for the treatment of moderate to severe psoriasis.
By Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A.
-
Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model.
By Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al.
-
Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation.
By Chen Y-F, Jowett S, Barton P, Malottki K, Hyde C, Gibbs JSR, et al.
-
Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY) – a pharmacoepidemiological and qualitative study.
By Wong ICK, Asherson P, Bilbow A, Clifford S, Coghill D, R DeSoysa R, et al.
-
ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening.
By Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al.
-
The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation.
By Black C, Clar C, Henderson R, MacEachern C, McNamee P, Quayyum Z, et al.
-
Randomised preference trial of medical versus surgical termination of pregnancy less than 14 weeks’ gestation (TOPS).
By Robson SC, Kelly T, Howel D, Deverill M, Hewison J, Lie MLS, et al.
-
Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes.
By Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al.
-
VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers.
By Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al.
-
A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial
By Michaels JA, Campbell WB, King BM, MacIntyre J, Palfreyman SJ, Shackley P, et al.
-
Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice.
By Kai J, Ulph F, Cullinan T, Qureshi N.
-
Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation.
By Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al.
-
Development of a toolkit and glossary to aid in the adaptation of health technology assessment (HTA) reports for use in different contexts.
By Chase D, Rosten C, Turner S, Hicks N, Milne R.
-
Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation.
By Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, et al.
-
Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report.
By Bond M, Wyatt K, Lloyd J, Welch K, Taylor R.
-
Are adverse effects incorporated in economic models? An initial review of current practice.
By Craig D, McDaid C, Fonseca T, Stock C, Duffy S, Woolacott N.
-
Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE).
By Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al.
-
Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation.
By Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al.
-
The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation.
By Fleeman N, McLeod C, Bagust A, Beale S, Boland A, Dundar Y, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer.
By Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TRL, et al.
-
Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study).
By Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al.
-
A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation for chronic obstructive pulmonary disease followed by telephone or conventional follow-up.
By Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA.
-
The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation.
By Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al.
-
Dissemination and publication of research findings: an updated review of related biases.
By Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al.
-
The effectiveness and cost-effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision model.
By Hemingway H, Henriksson M, Chen R, Damant J, Fitzpatrick N, Abrams K, et al.
-
Comparison of case note review methods for evaluating quality and safety in health care.
By Hutchinson A, Coster JE, Cooper KL, McIntosh A, Walters SJ, Bath PA, et al.
-
Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation.
By Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al.
-
Self-monitoring of blood glucose in type 2 diabetes: systematic review.
By Clar C, Barnard K, Cummins E, Royle P, Waugh N.
-
North of England and Scotland Study of Tonsillectomy and Adeno-tonsillectomy in Children (NESSTAC): a pragmatic randomised controlled trial with a parallel non-randomised preference study.
By Lock C, Wilson J, Steen N, Eccles M, Mason H, Carrie S, et al.
-
Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial.
By Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al.
-
A randomised controlled multicentre trial of treatments for adolescent anorexia nervosa including assessment of cost-effectiveness and patient acceptability – the TOuCAN trial.
By Gowers SG, Clark AF, Roberts C, Byford S, Barrett B, Griffiths A, et al.
-
Randomised controlled trials for policy interventions: a review of reviews and meta-regression.
By Oliver S, Bagnall AM, Thomas J, Shepherd J, Sowden A, White I, et al.
-
Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review.
By McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N.
-
A systematic review of outcome measures used in forensic mental health research with consensus panel opinion.
By Fitzpatrick R, Chambers J, Burns T, Doll H, Fazel S, Jenkinson C, et al.
-
The clinical effectiveness and cost-effectiveness of topotecan for small cell lung cancer: a systematic review and economic evaluation.
By Loveman E, Jones J, Hartwell D, Bird A, Harris P, Welch K, et al.
-
Antenatal screening for haemoglobinopathies in primary care: a cohort study and cluster randomised trial to inform a simulation model. The Screening for Haemoglobinopathies in First Trimester (SHIFT) trial.
By Dormandy E, Bryan S, Gulliford MC, Roberts T, Ades T, Calnan M, et al.
-
Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis.
By Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, et al.
-
A randomised controlled trial of cognitive behaviour therapy and motivational interviewing for people with Type 1 diabetes mellitus with persistent sub-optimal glycaemic control: A Diabetes and Psychological Therapies (ADaPT) study.
By Ismail K, Maissi E, Thomas S, Chalder T, Schmidt U, Bartlett J, et al.
-
A randomised controlled equivalence trial to determine the effectiveness and cost–utility of manual chest physiotherapy techniques in the management of exacerbations of chronic obstructive pulmonary disease (MATREX).
By Cross J, Elender F, Barton G, Clark A, Shepstone L, Blyth A, et al.
-
A systematic review and economic evaluation of the clinical effectiveness and cost-effectiveness of aldosterone antagonists for postmyocardial infarction heart failure.
By McKenna C, Burch J, Suekarran S, Walker S, Bakhai A, Witte K, et al.
-
Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review.
By Chilcott JB, Tappenden P, Rawdin A, Johnson M, Kaltenthaler E, Paisley S, et al.
-
BoTULS: a multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A.
By Shaw L, Rodgers H, Price C, van Wijck F, Shackley P, Steen N, et al. , on behalf of the BoTULS investigators.
-
Weighting and valuing quality-adjusted life-years using stated preference methods: preliminary results from the Social Value of a QALY Project.
By Baker R, Bateman I, Donaldson C, Jones-Lee M, Lancsar E, Loomes G, et al.
-
Cetuximab for the first-line treatment of metastatic colorectal cancer.
By Meads C, Round J, Tubeuf S, Moore D, Pennant M and Bayliss S.
-
Infliximab for the treatment of acute exacerbations of ulcerative colitis.
By Bryan S, Andronis L, Hyde C, Connock M, Fry-Smith A and Wang D.
-
Sorafenib for the treatment of advanced hepatocellular carcinoma.
By Connock M, Round J, Bayliss S, Tubeuf S, Greenheld W and Moore D.
-
Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B infection.
By Jones J, Colquitt J, Shepherd J, Harris P and Cooper K.
-
Prasugrel for the treatment of acute coronary artery syndromes with percutaneous coronary intervention.
By Greenhalgh J, Bagust A, Boland A, Saborido CM, Fleeman N, McLeod C, et al.
-
Alitretinoin for the treatment of severe chronic hand eczema.
By Paulden M, Rodgers M, Griffin S, Slack R, Duffy S, Ingram JR, et al.
-
Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer.
By Fleeman N, Bagust A, McLeod C, Greenhalgh J, Boland A, Dundar Y, et al.
-
Topotecan for the treatment of recurrent and stage IVB carcinoma of the cervix.
By Paton F, Paulden M, Saramago P, Manca A, Misso K, Palmer S, et al.
-
Trabectedin for the treatment of advanced metastatic soft tissue sarcoma.
By Simpson EL, Rafia R, Stevenson MD, Papaioannou D.
-
Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia.
By Edlin R, Connock M, Tubeuf S, Round J, Fry-Smith A, Hyde C, et al.
-
The safety and effectiveness of different methods of earwax removal: a systematic review and economic evaluation.
By Clegg AJ, Loveman E, Gospodarevskaya E, Harris P, Bird A, Bryant J, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital chlamydia infection in women and men.
By Hislop J, Quayyum Z, Flett G, Boachie C, Fraser C, Mowatt G.
-
School-linked sexual health services for young people (SSHYP): a survey and systematic review concerning current models, effectiveness, cost-effectiveness and research opportunities.
By Owen J, Carroll C, Cooke J, Formby E, Hayter M, Hirst J, et al.
-
Systematic review and cost-effectiveness evaluation of ‘pill-in-the-pocket’ strategy for paroxysmal atrial fibrillation compared to episodic in-hospital treatment or continuous antiarrhythmic drug therapy.
By Martin Saborido C, Hockenhull J, Bagust A, Boland A, Dickson R, Todd D.
-
Chemoprevention of colorectal cancer: systematic review and economic evaluation.
By Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, et al.
-
Cross-trimester repeated measures testing for Down’s syndrome screening: an assessment.
By Wright D, Bradbury I, Malone F, D’Alton M, Summers A, Huang T, et al.
-
Exploring the needs, concerns and behaviours of people with existing respiratory conditions in relation to the H1N1 ‘swine influenza’ pandemic: a multicentre survey and qualitative study.
By Caress A-L, Duxbury P, Woodcock A, Luker KA, Ward D, Campbell M, et al.
-
Influenza A/H1N1v in pregnancy: an investigation of the characteristics and management of affected women and the relationship to pregnancy outcomes for mother and infant.
By Yates L, Pierce M, Stephens S, Mill AC, Spark P, Kurinczuk JJ, et al.
-
The impact of communications about swine flu (influenza A H1N1v) on public responses to the outbreak: results from 36 national telephone surveys in the UK.
By Rubin GJ, Potts HWW, Michie S.
-
The impact of illness and the impact of school closure on social contact patterns
By Eames KTD, Tilston NL, White PJ, Adams E, Edmunds WJ.
-
Vaccine effectiveness in pandemic influenza – primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine.
By Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses: a Cochrane review.
By Jefferson T, Del Mar C , Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al.
-
Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR).
By Peek GJ, Elbourne D, Mugford M, Tiruvoipati R, Wilson A, Allen E, et al.
-
Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation.
By Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, et al.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Consultant Adviser, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of NETSCC External Relations
-
Ms Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Warwick Clinical Trials Unit
-
Director, Nottingham Clinical Trials Unit
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Ms Kay Pattison, NHS R&D Programme/DH, Leeds
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Mr A S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital
-
Dr Dianne Baralle, Consultant & Senior Lecturer in Clinical Genetics, Human Genetics Division & Wessex Clinical Genetics Service, Southampton, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Service User Representative
-
Professor Anthony Robert Kendrick, Professor of Primary Medical Care, University of Southampton
-
Dr Susanne M Ludgate, Director, Medical Devices Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr David Mathew Service User Representative
-
Dr Michael Millar, Lead Consultant in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, University College London
-
Mrs Una Rennard, Service User Representative
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr W Stuart A Smellie, Consultant, Bishop Auckland General Hospital
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Alan J Williams, Consultant in General Medicine, Department of Thoracic Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist Commissioning Advisory Group (NSCAG), Department of Health
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook Clinical Programmes Director, Bazian Ltd, London
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr Colin Greaves Senior Research Fellow, Peninsular Medical School (Primary Care)
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Miss Nicky Mullany, Service User Representative
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Service User Representative
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust, Bristol
-
Reader in Wound Healing and Director of Research, University of Leeds, Leeds
-
Professor Bipin Bhakta Charterhouse Professor in Rehabilitation Medicine, University of Leeds, Leeds
-
Mrs Penny Calder Service User Representative
-
Professor Paul Carding, Professor of Voice Pathology, Newcastle Hospital NHS Trust, Newcastle
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry, London
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol, Bristol
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and the London School of Medicine and Dentistry, London
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester, Manchester
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham, Nottingham
-
Dr Kate Radford, Division of Rehabilitation and Ageing, School of Community Health Sciences. University of Nottingham, Nottingham
-
Mr Jim Reece, Service User Representative
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton, Southampton
-
Dr Pippa Tyrrell, Stroke Medicine, Senior Lecturer/Consultant Stroke Physician, Salford Royal Foundation Hospitals’ Trust, Salford
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford, Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University, Cardiff
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health , London
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Dr Ursula Wells PRP, DH, London
Interventional Procedures Panel
-
Consultant Surgeon & Honorary Clinical Lecturer, University of Sheffield
-
Mr David P Britt, Service User Representative, Cheshire
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Mr Michael Thomas, Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Mrs Isabel Boyer, Service User Representative, London
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Professor Robin Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Service User Representative, Gloucestershire
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician & Cardiologist, County Durham & Darlington Foundation Trust
-
Mr John Needham, Service User, Buckingmashire
-
Ms Mary Nettle, Mental Health User Consultant, Gloucestershire
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Howard Ring, Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry & Warwickshire Partnership Trust
-
Dr Alastair Sutcliffe, Senior Lecturer, University College London
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Professor Tom Walley, HTA Programme Director, Liverpool
-
Dr Ursula Wells, Policy Research Programme, DH, London
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington