Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 06/41/02. The contractual start date was in July 2007. The draft report began editorial review in March 2009 and was accepted for publication in October 2009. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Paul Abrams was involved in a trial of two surgical treatments for stress urinary incontinence conducted by the Bristol Urological Institute and funded by an educational grant by American Medical Services.
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of underlying health problem
Continence mechanisms in health
Efficient mechanisms have evolved to ensure reliable urine storage and complete bladder emptying at socially convenient times. Mechanisms that prevent urine leakage involve the bladder, the urethra and the pelvic floor muscles, together with their controlling nerve pathways. The bladder is a highly compliant organ, allowing the storage of increasing quantities of urine without rise in pressure, a property underpinned by passive stretch and active relaxation of its smooth muscle (detrusor). Active central nervous control mechanisms in the pons and cerebrum inhibit detrusor contraction despite increasing sensation of bladder fullness until micturition is appropriate. Urethral mechanisms promoting continence are less well understood, but are thought to involve tonic contraction of smooth muscle in the urethral wall, together with a tight seal formed by the urethral lining (mucosa) and the highly vascular submucosal layer. Contraction of the pelvic floor striated muscles acts as an additional guarding mechanism, compressing the urethra and preventing leakage during actions that raise intra-abdominal pressure.
These features maintain continence during the storage phase of the micturition cycle by ensuring that bladder pressure is always lower than urethral closure pressure. Incontinence is therefore likely to result from deficiency of urethral closure mechanisms and/or involuntary detrusor activity, aggravated by factors that chronically increase intra-abdominal pressure. In general, maximal urethral closure pressure is higher in men and therefore incontinence is far more prevalent amongst women, who are the target population for this review. 1
Definition of urinary incontinence
The symptom of urinary incontinence is defined as the involuntary leakage of urine,2,3 and this can be subcategorised qualitatively according to the patient’s description (Box 1).
Involuntary leakage of urine associated with effort or exertion, or on sneezing or coughing
Urgency urinary incontinence (UUI)Involuntary leakage of urine accompanied, or immediately preceded, by urgency, which is a sudden compelling desire to pass urine that is difficult to defer
Mixed urinary incontinence (MUI)When complaints of both SUI and UUI coexist
This symptom categorisation2,3 is based on a detailed history and provides a useful basis for discussion of the problem with the patient, identification of patient-centred treatment goals and initiation of treatment pathways.
When the patient first reports the problem to a clinician it is usual for the clinician to define possible causative factors and commonly associated problems by further questioning, physical examination and performance of simple tests. The severity of incontinence and the degree of bother it causes the individual can be estimated by appropriate direct questioning, including pad usage, or can be quantified more objectively using validated symptom scores4 or bladder diaries.
Further categorisation of incontinence according to the underlying functional or anatomical cause requires simultaneous measurement of bladder and rectal pressure, together with observation of urine loss during bladder filling. This invasive clinical test, filling cystometry, requires catheterisation of the bladder and is therefore generally performed only when more accurate categorisation is required, for example prior to surgical treatment in women with MUI. This test will differentiate urodynamic stress incontinence (USI) due to bladder outlet weakness from detrusor overactivity (DO) incontinence due to involuntary contraction of the bladder muscle (Box 2).
Involuntary leakage of urine during increased abdominal pressure in the absence of a detrusor contraction
Detrusor overactivity (DO) incontinenceLeakage of urine due to an involuntary detrusor contraction
The test may be accompanied by radiographic visualisation of the bladder outlet to qualitatively subcategorise USI according to the degree of descent of the bladder neck on coughing (hypermobility) or loss of the sealing mechanism of the urethra (intrinsic sphincter deficiency). The diagnostic and prognostic usefulness of this additional imaging, together with related indices such as abdominal leak point pressure, is uncertain and currently not recommended for routine use by the International Continence Society. 2
Most people complaining of the symptom of SUI will have USI, which is demonstrable on cystometry, and this can be aggravated by conditions that chronically raise abdominal pressure, such as obesity and chronic obstructive pulmonary disease. In approximately 10–20% of cases, however, the symptom of SUI can result from DO provoked by coughing, for example, or from loss of bladder compliance due to fibrosis or neurological disease (low bladder compliance). It should therefore be noted that the symptom of stress incontinence does not always relate to weakness of the bladder outlet or urethral closure mechanism.
Practical definition of incontinence for outcome purposes requires a variable that can be measured before and after treatment. This presents a particular problem for evidence synthesis, as there is a lack of consensus on the most appropriate method, with a variety of variables being used to define improvement or cure (Box 3). It is also recognised that these variables may not capture outcomes of prime importance to individual women suffering SUI.
Self-report of outcome or change in symptom score
QuantifiedChange in reported episodes on bladder diaries
Weight of urine loss during exercise pad tests
Clinician definedDirect observation of urine loss
Cystometric diagnosis
Quality of life definedChange in generic ratings, such as EQ-5D
Change in condition-specific ratings, such as King’s Health Questionnaire
Epidemiology and natural history
Prevalence
Prevalence estimates vary depending on population sampled, definition of incontinence, severity threshold and survey methodology. 5 A recent longitudinal study from one county in the UK surveyed a random sample of over 15,000 community-dwelling individuals aged ≥ 40 years and found prevalences of 34% in women and 14% in men. 6 These data were in line with prevalence rates among adults living in the UK, summarised from previous studies, showing a mean (range) of 40% (2–69%) for women and 10% (2–25%) for men. 7 These ranges were consistent with findings from other developed countries, which documented rates of 10–72% for women and 3–20% for men. 7
The EPINCONT study surveyed over 34,000 community-dwelling Norwegian women and found that the prevalence of urinary incontinence increased during young adult life, reached a broad peak between the ages of 45 and 55 years, and then showed a further steady increase in the elderly (Figure 1). 8–12 This study also found that about one-quarter of women with incontinence rated it as severe, a proportion in line with previous reports. 13
FIGURE 1.
Prevalence of female urinary incontinence by age group and symptomatic type of incontinence. Compiled from data in Hannestad and colleagues (2000). 8

This study also provided differential prevalence rates for symptoms of stress, urge and mixed incontinence. 10 Overall, SUI was the most common type, experienced by 50% of incontinent women, whereas 11% reported urgency incontinence alone and 36% reported mixed symptoms (Figure 1). The pattern does vary, however, according to age group, with SUI alone being most frequently reported in women who are younger than 50–55 years, after which urgency incontinence is reported most often, either alone or in combination, possibly reflecting menopausal status. 12 This pattern was also found in a large cross-sectional European study using validated questionnaires, for which SUI was most often reported by women under 60 years old. 11 Overall prevalence rates for urinary incontinence amongst older women who are living in supported accommodation are usually much higher, reaching 40–50%. 14
This high community prevalence does not tend to lead to equivalent rates of consultation with a clinician. Studies estimate that only 15% of the women identified as suffering from SUI in cross-sectional surveys have consulted a health professional about the problem. 15,16 The reasons for this are unclear but may relate to social class, mild symptoms, lack of bother, embarrassment, disinclination towards treatment options and perceived lack of effective treatment. 17
Natural history
In comparison with the many cross-sectional prevalence surveys, fewer longitudinal studies have examined the incidence and remission of urinary incontinence symptoms. One study followed a large cohort of community-dwelling middle-aged women (mean age 46 years) for 2 years and documented an average annual incidence of new incontinence of 9%. 18 The incidence increased with age, with the majority reporting mild, non-disabling leakage (Figure 2). Subanalysis of type of incontinence showed an annual incidence of frequent or severe stress incontinence of about 2% (Figure 3). This study also documented a 7% annual remission rate among those women reporting urinary incontinence at baseline. Similar results were found from a cohort of older, postmenopausal women (mean age 64 years), followed for 2 years, and a further large cohort study of women aged > 65 years, with rates of annual incidence for SUI of 9% and 9.5%, respectively, and annual remission rates of 7% and 8%, respectively. 19,20
FIGURE 2.
Two-year incidence of urinary incontinence by severity. Based on a cohort of 33,952 American women. Any incontinence defined as leaking at least once per month; occasional incontinence defined as leaking one to three times per month; frequent incontinence defined as leaking at least once per week; severe incontinence defined as frequent leaking of quantities at least enough to wet the underwear. Adapted from Townsend and colleagues (2007). 18

FIGURE 3.
Two-year incidence of frequent (at least once weekly) urinary incontinence by incontinence type. Cases of incident frequent incontinence; missing data on incontinence type symptoms are excluded from these calculations. Adapted from Townsend and colleagues (2007). 18

A number of studies have reported questionnaire follow-up of numerically smaller cohorts of younger women (mean age 26–30 years) before and after their first vaginal delivery and reported the annual incidence of new urinary incontinence to be 5%, 1% and 4% over periods of 4, 10 and 12 years, respectively. 21–23
Factors associated with SUI
The main risk factors for female SUI are pregnancy, vaginal delivery, increasing parity, increasing age, obesity and postmenopausal status. 24–26 In older women, particularly those requiring social care, age-related changes to the lower urinary tract (such as reduced bladder capacity) and comorbidity in other organ systems (such as cardiac or cognitive impairment treated with drugs such as diuretics) can precipitate or worsen incontinence. Consideration of these multiple factors, together with the increasing preponderance of mixed urgency and stress incontinence symptoms, makes effective management of the problem in the elderly more difficult. 14
Postpartum SUI
Childbearing is the main predisposing factor that is specific for SUI, although the exact mechanism of pelvic floor injury that contributes to the development of outlet weakness during pregnancy and vaginal delivery is unclear. 26,27 Longitudinal studies have reported that two-thirds of women with SUI during their first pregnancy continue to have symptoms at a follow up of 15 years. Having antenatal SUI doubles this risk. 28 Immediately after childbirth women may expect to have higher levels of incontinence, which can often resolve spontaneously over the first 6 months. As this natural resolution might confound the effect of any intervention, data from trials in women in the immediate postpartum period were not included in the main body of the systematic review or in the cost-effectiveness analysis (results are reported in Appendix 20).
Other risk factors
In a large population-based cross-sectional study of premenopausal women, high body mass index (BMI > 30), diabetes mellitus and previous urinary incontinence surgery were identified as significant risk factors for severe SUI. 29 A history of gynaecological surgery for prolapse increased the risk of developing stress leakage over twofold, and hysterectomy and other gynaecological procedures also doubled the risk. 30,31
Significance in terms of ill health
Effect on well-being
Embarrassment associated with urinary incontinence may cause withdrawal from social situations and reduce quality of life. 32 Women with a severe or frequent problem find the leakage distressing and socially disabling. They may avoid going away from home, using public transport and sexual activity. 33 SUI does not generally lead to deterioration in physical health but can be associated with depression and other psychological morbidity. 33 The problem may also lead to withdrawal from regular physical activities, potentially harming general health. 34
Extent of problem in the UK
Assuming an overall prevalence for SUI of 15% among women aged over 20 years, it can be estimated that there are 3.3 million sufferers in the UK. 35
Cost to society
The high prevalence of urinary incontinence results in a high overall cost of treatment and containment. Precise cost is difficult to define but a recent study suggested an estimated figure for combined health care, personal and societal expenditure of £248 per person per year in the UK, which would equate to a total annual cost of £818M for SUI. 36 A further estimate, assuming that SUI accounts for 50% of cases, suggested a health care cost to the UK National Health Service (NHS) of £117M per year. 37
Description of interventions
The treatment options for SUI can be classified as non-surgical and surgical. Lifestyle changes, such as weight loss, smoking cessation, control of chronic obstructive pulmonary disease, timed voiding and oral fluid management, may reduce risk of leakage but all need continued adherence to the required adjustments in order to maintain response. Specific non-surgical interventions, such as pelvic floor muscle training and biofeedback (BF), also require long-term adherence to the taught programmes in order to produce continued benefit. However, these interventions have few or no adverse events. Surgical treatment, on the other hand, may have a higher rate of benefit but has a greater risk of complications. 38 Alternatively, the leakage can be contained using absorbent pads, an indwelling urinary catheter or, very rarely, urinary diversion.
The choice of treatment depends on patient preference and professional advice and will take into account factors, such as symptom severity, degree of interference with lifestyle, presence of related problems and degree of comorbidity. The importance of patient preference as the primary consideration in selecting a particular treatment for SUI was underlined by the findings of a recent survey which reported that most preferred less invasive treatment and management options. 39 From a health service perspective it is important to balance short- and long-term efficacy against potential adverse events and costs.
Existing guidelines
Epidemiological studies consistently demonstrate that proportionally few women who experience urinary leakage approach clinicians for advice and treatment. 15,16 It is likely that most women first seek advice from family, friends and the media and, for individually varying reasons, decide to manage the problem themselves. Those who present their problem to health-care professionals tend to have more severe symptoms, which cause interference to their social activities and they are therefore generally seeking active treatment. Most countries, such as the UK, have attempted to standardise the assessment and initial management of women with incontinence by publication of consensus documents and guidelines. 40–44 Despite this, uniformity of care remains lacking and will depend on individual clinician opinion and local service provision.
Current UK NHS care pathway
In the UK, the first port of call is likely to be the general practitioner (GP – primary care physician). An initial assessment will document the severity of the problem and the degree to which it bothers the women, and make sure that there are no more immediate health-threatening problems. Lifestyle advice, such as smoking cessation and weight loss, to modify risk factors may be offered. It is then possible that conservative therapy, in terms of bladder training (BT) or pelvic floor education and therapy, will be suggested, with referral to a practice nurse, physiotherapist or continence nurse specialist. Alternatively, or if these approaches subsequently fail, the woman will be offered referral to secondary care, to a urologist, urogynaecologist or gynaecologist, depending on local service arrangements. Such referrals will mostly result in further investigation, further conservative treatment including the use of drugs and eventually the offer of surgery to those with predominant SUI.
Lifestyle changes
Symptomatic SUI may be improved or cured by changing lifestyle factors. This can be achieved by interventions, such as weight loss, fluid restriction, reduction of caffeine or alcohol intake, limiting heavy activity, stopping smoking and treatment of constipation (Table 1). The effect of weight loss has been most intensively studied, with evidence summarised in a recent systematic review. 45 Successful weight-loss programmes require intensive therapy, involving diet, exercise and behavioural modification over a prolonged period.
| Lifestyle change | Methods | Evidence of effectiveness for SUIa |
|---|---|---|
| Weight loss | Diet | Level 1a |
| Exercise | ||
| Behavioural modification | ||
| Adjustment of fluid intake | Reduced volume | Level 2b |
| Avoidance of caffeine | ||
| Avoidance of carbonated drinks | ||
| Smoking cessation | Behavioural modification | Level 4 |
| Nicotine replacement | ||
| Exercise modification | Avoidance of provocative exercise | Level 4 |
| Regularisation of bowel habit | Interventions to prevent constipation and straining to defecate | Level 4 |
Setting
In the context of a consultation in primary care, the possible benefit of lifestyle modifications, such as weight loss, would be discussed and reinforced by a patient information leaflet, together with the offer of further therapeutic help. Intensive weight-loss programmes are not widely available at present but are most likely to be community based.
Personnel involved
Weight loss or smoking cessation therapy is most likely to be effective if it is supervised, preferably on a weekly basis, by a therapist who has undergone recognised training and obtained appropriate qualifications. Such programmes are frequently run as group sessions.
Costs
The cost of lifestyle changes will vary according to the intensity of the intervention. It might range from simple provision of information at a primary care consultation with a specialist nurse (£13.95)46 to the taking of active steps to lose weight. For example, a 6-month supervised group weight-reduction programme with the leading commercial provider in the UK, WeightWatchers®, would currently cost £152,47 with possible additional costs to the individual related to dietary changes and exercise programme.
Pelvic floor muscle training
Recommendations for the standardisation of these treatments have been published by the UK Chartered Society of Physiotherapists. 40
Basic pelvic floor muscle training (PFMT basic)
Popularised by Arnold Kegel,48 basic pelvic floor muscle training (PFMT) is generally the first-line non-surgical management for SUI. The principle behind this intervention is to condition and strengthen the striated pelvic floor muscles in order to improve the urethral sphincter closure mechanism during provocative activity (such as coughing) that raises intra-abdominal pressure. There is a variety of regimens to provide PFMT to women. The simplest involves education about pelvic floor structure and function, together with demonstration, using digital vaginal examination by the therapist or woman herself, of a correct and effective pelvic floor muscle contraction. A regular exercise programme schedule is then agreed between the woman and her therapist, with intermittent checks of progress and benefit over a 3- to 4-month period. The schedule suggested by Kegel was five contractions performed every waking hour,49 whereas that recommended by recent guidelines is a sequence of eight contractions three times daily. 43 A summary of current recommendations is given in Box 4.
-
Pelvic floor muscle awareness is taught
-
The pelvic floor is assessed and exercised in functional positions
-
The use of anticipatory pelvic floor muscle contraction immediately prior to an activity that causes urine leakage (‘The Knack’) is taught
-
A programme of pelvic floor muscle exercises is tailored to individual patients and includes exercises for both fast- and slow-twitch muscle fibres
-
Pelvic floor muscle exercises are performed several times a day until the muscle fatigues
-
Pelvic floor muscle exercises are practised for 15–20 weeks
-
Patients are initially seen weekly, but account may need to be taken of their circumstances and/or the available resources
-
Pelvic floor muscle exercises are continued on a maintenance programme
Recommendation from Laycock and colleagues (2001). 40
Augmented PFMT
The addition of BF as a teaching and performance-enhancing device, in the form of vaginal pressure recording using a perineometer or electromyographic demonstration of muscle activity, can be helpful to visually demonstrate to the patient when they are performing a correct pelvic contraction and to quantify improvement. This feedback should encourage and motivate perseverance with a regular exercise programme. Digital BF is the practice of assessing pelvic muscle strength by vaginal examination, with verbal feedback concerning correctness and strength of the contraction. Once benefit is established and the woman is discharged from the therapist’s care, a continued exercise programme is encouraged.
Biofeedback may also be used as a training device or as an aid to pelvic floor muscle exercising. Women use a pressure perineometer to monitor strength and endurance of a series of pelvic floor muscle contractions over a period of time, typically 20–30 minutes, at weekly or monthly intervals.
As an adjunct to standard pelvic floor training programmes, women can be instructed to retain graded cone-shaped weights [vaginal cones (VCs)] within the vagina to improve pelvic floor muscle strength. Starting with the lightest weight, women are advised to hold a cone in their vagina and prevent it from slipping out, while standing, moving around or coughing. It is suggested that the use of cones improves compliance with the exercise schedule, individualises the exercise regimen, gives BF and improves knowledge of the functional anatomy of the vagina and pelvic floor. Cones may also be used as a standalone treatment at home for women who do not wish to, or cannot, access a health professional. 50
A further possible adjunct to PFMT is electrical stimulation (ES). This causes the pelvic floor muscles to contract, either directly or indirectly, by excitation of the motor efferent fibres of the pudendal nerve. The electrodes can be placed on the perineal skin, within the vagina or within the anus. The vaginal route is recommended with set stimulation parameters (Box 5). It is thought that ES may be particularly useful for women who are unable to contract their pelvic floor muscles voluntarily, or to help build up muscle strength prior to a supervised PFMT programme. The reported advantages of this intervention include high patient acceptability, little or no discomfort and home-managed delivery of the treatment. 51
-
Frequency: 35 Hz
-
Pulse width: 250 microseconds (0.25 milliseconds)
-
Current type: biphasic rectangular
-
Intensity: maximum tolerated
-
Duty cycle: 5 seconds on/10 seconds off. Very weak muscles: 5 seconds on/15 seconds off
-
Treatment daily/twice daily (home treatment)
-
Treatment time: 5 minutes initially, gradually increasing to 20 minutes
Recommendation from Laycock and colleagues (2001). 40
Setting
These interventions are organised through a primary care continence or physiotherapy service, which may be located in a primary health care centre or local hospital department. The patient will typically attend weekly or fortnightly sessions over a 3- to 4-month period, depending on compliance and improvement. They will be instructed to continue the exercise programme at home, during daily activity between visits to the therapist, and to continue the programme themselves, lifelong, after discharge from the therapist’s care.
Personnel involved
These treatments will be typically supervised by a chartered physiotherapist who has undergone education to degree level and has undertaken a recognised professional training programme leading to the relevant professional registration. In some cases sessions may be delegated to trainees or assistants under supervision. Alternatively, the treatment will be administered by a continence nurse specialist who has undergone training in the provision of pelvic floor exercise programmes and has achieved appropriate competencies signified by additional qualifications.
Costs
Based on Personal and Social Services Research Unit (PSSRU) figures, the average cost for a consultation with a physiotherapist is approximately £13. 46 Additional costs for PFMT programmes would include overheads and consumables for basic intervention (£4), and additional equipment for BF (£35) and ES (£11). VCs are not provided by the UK NHS, and women would have to purchase them commercially at a cost of £20. 52 The total cost of all these interventions is determined by the number of sessions the women receive. For basic PFMT, the average cost for a 3-month cycle of treatment is £189, for BF it is £224 and for ES it is £398. These costs are similar if the programme is provided by a continence nurse specialist. 43
Bladder training
Bladder training to regain control of micturition is more predominantly used for UUI or those women with mixed symptoms. Typical programmes involve a gradually progressive voiding schedule to delay micturition, together with distraction and relaxation techniques to suppress urgency. This would generally involve an initial assessment consultation and subsequent visits or telephone follow-up over a period of 8–12 weeks. Bladder diaries are often used as additional BF and outcome tools. 53
Setting
The initial assessment would take place in a primary health care centre, with follow-up visits in the health centre, patient’s home or by telephone as appropriate.
Personnel involved
This type of therapy is generally supervised in primary care by a designated member of the district nursing team with expertise in continence promotion or by a continence nurse specialist.
Costs
UK NHS reference costs for district nursing contacts (CN301AF) average £31 (range £26–40), while continence nurse specialist contacts (CN204AF) are costed at between £48 and £114. This would give an approximate total cost, assuming fortnightly visits for 12 weeks, of £180–540. 54
Pharmacotherapy
Serotonin–noradrenaline reuptake inhibitor
Experimental studies in animals suggest that noradrenaline and serotonin (5-hydroxytryptamine) act on efferent neurons in the sacral spinal cord (Onuf’s nucleus) to encourage contraction of the periurethral striated muscle of the urethral sphincter and relaxation of the bladder wall muscle (detrusor muscle), thereby promoting urine storage and continence. Duloxetine hydrochloride, a balanced serotonin–noradrenaline reuptake inhibitor (SNRI), was tested as an antidepressant but found to have an effect on reducing stress incontinence in women. It is now licensed as a continence-promoting drug in women with SUI. 55 Although it has been shown to improve symptoms of SUI, the usefulness of duloxetine is limited by side effects, particularly nausea, which result in up to 20% of women being unable to tolerate the drug. 56 There is some evidence to suggest that this drug may be more useful as an adjunct to other conservative therapies, such as PFMT. 57 Despite poor tolerability and modest efficacy, the prescribing of this drug appears to be increasing in the UK (Figure 4). Further details on the current use of SNRI’s were obtained from a survey of members of the Association of Continence Advisors (ACA). The ACA provided a copy of their e-mail distribution list and members were surveyed during January and February 2009. Out of approximately 650 ACA members on the distribution list, 86 responded (a 13.2% response rate). Of these, 57 provided details of their SUI caseload, of which 15/57 (26.3%) advisors reported that they had patients using duloxetine for the treatment of SUI. The number being treated was 92 out of a caseload of 1234 patients (7.5%); the majority of these, however, came from the caseloads of two respondents.
FIGURE 4.
Serotonin–noradrenaline reuptake inhibitor prescriptions for stress urinary incontinence. UK NHS prescribing data obtained from NHS information centre. 58

The drug is generally prescribed in divided doses, totalling 40–80 mg per day, and the response is assessed over a 12-week period. The relatively high risk of side effects requires initial close monitoring by the specialist or GP.
The poor tolerability profile of this drug has resulted in it mainly being prescribed by specialists through hospital clinics.
The drug is predominantly initiated by hospital specialists for women with SUI, who are unsuitable for surgery or do not wish to undergo surgery.
The cost of a 56 × 40-mg tablet pack of duloxetine is £30.80. 59 This equates to an average annual cost per patient, for drugs alone, of £402. If two visits to the GP are included the total annual cost rises to £474.
Estrogen
Estrogens are thought to affect female continence through several modes of action, including maintenance of pelvic floor musculature and enhancement of urethra mucosal sealing. It was therefore considered that therapeutic estrogen supplementation in postmenopausal women would be beneficial delivered either locally or systemically. Overall, there is a high level of evidence that oral estrogen replacement therapy appears to increase incontinence symptoms amongst postmenopausal women,60–62 and its use for this indication is therefore not recommended. Recent appraisal of evidence for topical vaginal estrogen therapy suggests that it may benefit women with incontinence but not to any consistent degree,63 and it is currently only recommended for women with overactive bladder symptoms that are associated with mucosal atrophy. 43 The opinion from the expert group for the current review was in agreement with this guidance and so oral or topical estrogen were not included as a treatment option for SUI.
Adrenergic agonists
Despite a theoretical and experimental expectation that alpha-adrenergic drugs should improve urethral and bladder neck smooth muscle activity, no consistent clinical benefit was found in women with stress incontinence. 63,64 These drugs are neither licensed for this treatment indication nor recommended by current guidance,43 and were considered to be very rarely used in practice by our expert group. On this basis, the use of adrenergic agonists as a treatment option for SUI was not considered further.
Mechanical devices
Consideration of the underlying urodynamic changes that result in SUI has continued to encourage the design and testing of intraurethral or intravaginal mechanical devices to prevent leakage. The mechanism of action of intravaginal devices is either by compression of the urethra against the inferior margin of the pubis or adjunctive bladder neck support with tampons such as Contrelle™. 65 Intraurethral devices, such as NEAT Expandable Tip Continence Device™,66 and FemSoft™,67 plug the urethral lumen. The clinical effectiveness of these devices in comparison with no treatment or other conservative methods of managing stress incontinence was the subject of a recent systematic review. 68 This review included six trials with a total of 286 participants. The mechanical devices used were five intravaginal devices and five intraurethral devices. The included trials either compared a mechanical device with no treatment or with an alternative device. There were no published data comparing devices with other non-surgical interventions for the treatment of SUI. The published data were unsuited to meta-analysis and the individual trials had small sample sizes and poor methodology ratings. The review therefore concluded that there was insufficient evidence to estimate the effect of mechanical devices for the treatment of women with SUI. In summary, although these devices are conceptually attractive, they do not appear to be widely used at present. 49 Some further information was obtained from the previously described survey of ACA members. The respondents had a total caseload of 1187 women. A total of 80 women were using mechanical devices, although 35 (43.8%) of these came from the caseload of a single respondent. The vast majority of devices used were vaginal devices, used by 78/80 women (97.5%). The most popular of these was pessaries, used by 77.5% (62/80) of the women. The Contrelle Activeguard was used by 7/80 (8.8%) women, tampons by 4/80 (5%) women, the Incostress device by 2/80 (2.5%) women, and vaginal sponges by 3/80 (3.8%) women. The only non-vaginal device used was an anal plug used by 2/80 (2.5%) women.
Setting and personnel
These devices require a preliminary assessment by a trained health-care professional, who will also instruct the patient on their use. However, this could occur in primary or secondary care settings.
Costs
The Contrelle device costs £50 and can be reused, whereas the FemSoft urethral insert costs £1.50 and has to be changed for a new device after urination.
Electromagnetic stimulation
Experimental studies have shown that electromagnetic stimulation of the S3 and S4 sacral nerves increases maximum urethral closing pressure by 34% compared with baseline. 69 However, few randomised controlled trials (RCTs) have reported on the cure or improvement of SUI using electromagnetic stimulation compared with placebo,69,70 and the intervention is not in current clinical use. It will not be considered further in this review.
Periurethral injectable bulking agents
Stress urinary incontinence can be treated by periurethral injections of agents that bulk the urethral wall to encourage sealing during urine storage and hence improve continence. Conceptually, they occupy a ‘grey’ area between truly non-surgical treatments and operations. We chose to define periurethral injection therapy as surgical treatment, as this is carried out by surgically qualified clinicians, in an operating room environment, with the standard precautions and care process that this entails. This treatment modality will not therefore be included in treatment pathways, but a summary of a recently updated Cochrane review of the subject is included here for completeness. 71 This review looked at the effects of periurethral or transurethral injection therapy for the treatment of women with urinary incontinence. It identified 12 relevant trials, including 1318 participants in total, and examined eight types of injectable material (Box 6) along with one dummy injection (saline).
Macroplastique™ – silicon particles
Durasphere™ – carbon particles
Contigen™ – glutaraldehyde cross-linked bovine collagen
Coaptite™ – calcium hydroxylappatite
Uryx™ – ethylene vinyl alcohol copolymer
Permacol™ – porcine dermal collagen
Experimental useSilicon microballoons
Dextran copolymer
Alginate gels
Autologous chondrocytes
Autologous myoblasts
DiscontinuedAutologous fat
Polytef™ – polytetrafluoroethylene
Zuidex™ – dextranomer/hyaluronic acid copolymer
Full details available from Keegan and colleagues (2007). 71
One study comparing periurethral injection to dummy treatment with saline was terminated due to safety concerns, although early results had not indicated any significant difference in treatment effect prior to this. 72 There were no relevant studies comparing periurethral injection to conservative management of urinary incontinence. However, in two studies comparing periurethral injection with surgery, surgery was reported to result in significantly greater improvement as measured by clinical observation. Comparisons between different types of agents used for periurethral injection suggested a variety of new agents to be as effective as collagen, and porcine dermal implant as effective as silicone, although confidence intervals (CIs) were wide and longer-term data were required. In general, approximately 50% of patients were satisfied in the short term (less than 1 year) after injection therapy. The review concluded that methodologically robust trials were needed, particularly with longer follow-up data and when using non-surgical treatments, such as PFMT, as comparators. Currently, the lack of useful high-quality evidence makes it difficult to draw conclusions regarding the place of periurethral injection therapy in the care pathway for women with SUI and it will not be used in the care pathways described in this review.
Surgery for SUI
Since the introduction of the first surgical procedure, anterior colporrhaphy with plication of the urethra by Kelly and Dumm in 1911,73 the number of different surgical procedures used to treat stress incontinence has grown to about 100. They are covered by six published Cochrane reviews (Box 7).
Open Burch retropubic colposuspension – Lapitan and colleagues (2005)38
Laparoscopic colposuspension – Dean and colleagues (2006)74
Suburethral slings – Bezerra and colleagues (2005)75
Anterior colporrhaphy – Glazener and Cooper (2001)76
Needle urethral suspension – Glazener and Cooper (2004)77
Tension-free transvaginal tape (TVT) – Ogah and colleagues78
Tension-free transobturator vaginal tape (TVT-O) – Ogah and colleagues78
The development of a novel theory of causation of SUI by Petros and Ulmsten79 has clarified the rationale for different surgeries. Older abdominal techniques, such as Burch colposuspension and pubovaginal sling insertion, aim to reduce mobility of the bladder neck and proximal urethra by providing fixation to the pubis. Newer techniques popularised by Ulmsten aim to increase support to the mid-urethra, typically using a synthetic tape introduced through the vagina and fixed either suprapubically or in the obturator fossa without tension. 80,81 These latter two procedures – TVT and TVT-O – are currently the most popular surgical techniques for treatment of SUI in women, having the advantages of a vaginal approach, minimally invasive insertion and a favourable adverse event profile. 82 The procedures involve blind passage of a length of monofilament macroporous polypropylene tape to provide a ‘hammock’ for the mid-urethra. The tape is positioned without tension using specially designed curved needles through suprapubic (TVT) or medial groin (TVT-O) incisions, and an incision in the anterior vaginal wall beneath the mid-urethra. The procedure can be performed under local anaesthetic and sedation or, more usually, a general or regional anaesthetic, and can be accomplished within a 24-hour hospital stay. The TVT variant also requires cystoscopy to detect and correct bladder perforation. Burch colposuspension or pubovaginal sling insertion, on the other hand, are open surgical procedures requiring a formal suprapubic incision. They require a general anaesthetic and a 2- to 3-day hospital stay. The main risk of all such procedures is transient (10%) or permanent (1%) voiding difficulty requiring intermittent self-catheterisation. Surgical success rates after 1–5 years of follow-up vary between 51% and 87% for Burch colposuspension/pubovaginal sling and between 63% and 85% for TVT/TVT-O, according to definition of cure/improvement and length of follow-up. 80
Setting
All procedures require an appropriately equipped hospital or clinic environment for safe surgical conduct. This will include a preoperative area, a fully equipped operating room and facilities for postoperative recovery. The newer minimally invasive procedures, such as TVT and TVT-O, can be carried out in an ambulatory care unit that is designed for stays of up to 24 hours.
Personnel
The procedures require staff for preoperative preparation, a surgical team including an anaesthetist and appropriately trained surgeon, and postoperative recovery staff. Staff and facilities to monitor residual urine and teach intermittent self-catheterisation when required are also needed.
Cost
UK National Health service reference costs54 for TVT and TVT-O, classified as ‘lower genital tract minor procedures without complications’, are, on average (interquartile range), £1135 (£741–1357) for inpatient treatment (mean stay of 2 days) and £629 (£456–828) for day-case treatment. Colposuspension classified as an ‘open bladder neck procedure in women’ has an average (interquartile range) cost of £1396 (£1011–2013) for inpatient treatment (mean stay of 2 days). 54
Criteria for treatment
Women with incontinence who choose to seek clinician advice generally have more severe symptoms that are bothersome and therefore desire treatment to improve their continence and lessen the impact on their day-to-day life. It is the clinician’s responsibility to gather evidence of the severity, type and degree of bother suffered by the individual in order to suggest the most appropriate first-line treatment options for the patient to consider. Selection of the initial treatment is then negotiated according to patient choice, clinician opinion and local availability. Subsequent consultations will decide on the need for further trials of treatment if the initial outcome is unsatisfactory. This assessment requires the exclusion, by examination and simple testing, of complicating factors, such as relevant neurological dysfunction, impaired voiding, infection and pelvic organ prolapse. Those with modifiable lifestyle factors, such as high body mass index and smoking, should be encouraged and helped to address these either before, or concurrent with, more active treatment. The selection for active treatment then depends on the individual’s desire and motivation, the presence of comorbidity and local service availability.
For women with a predominant symptom of SUI, education about their pelvic floor anatomy and function, and supervised training to improve pelvic floor muscle function, are the most used first-line treatments in the UK. This may sometimes be augmented by transvaginal ES or BF techniques, including use of VCs. To benefit from these therapies, women require sufficient mobility, motivation and the ability to attend frequent therapy sessions. Following a trial of PFMT, the next step is likely to be referral to a specialist for consideration of surgery.
Most high-level evidence for the effectiveness of each of these treatments is based on randomised trials with set inclusion and exclusion criteria. It is uncertain how the results of these trials and meta-analysis of multiple trials relate to individual patients, particularly those who would not meet the inclusion criteria (Table 2).
| Treatment | Inclusion criteria | Exclusion criteria |
|---|---|---|
| PFMT83 | SUI | Urinary tract infection |
| USI | Incomplete voiding | |
| More than two episodes of incontinence per week | Neurological disorders | |
| 18–75 years | Cognitive impairment | |
| BT53 | Age > 35 | Previous surgery for incontinence |
| Urgency or MUI | Neurological disease | |
| DO (urodynamic diagnosis) | Urinary tract infection | |
| Predominant stress incontinence | ||
| Diabetes mellitus | ||
| Inability to reach toilet unaided | ||
| Duloxetine56 | Age 30–80 years | Pregnancy |
| SUI | Breastfeeding | |
| USI | Urinary tract infection | |
| Arrhythmias or liver disease | ||
| Advanced pelvic organ prolapse | ||
| Continence surgery within 1 year | ||
| Prior hip fracture or replacement | ||
| Prior formal PFMT | ||
| Surgery38,74–77 | SUI | Age < 21 years |
| USI | Age > 65 years | |
| Non-ambulatory | ||
| Pregnancy | ||
| Current cancer treatment | ||
| Systemic disease affecting bladder function | ||
| Urethral diverticulum | ||
| Recent pelvic surgery | ||
| Grade III incontinence | ||
| DO | ||
| Urinary tract infection | ||
| Severe medical disease |
Containment options
Methods for containment of urinary incontinence do not constitute a means of treatment and are therefore not included in the systematic review. It is acknowledged, however, that pads are widely used by women in all phases of management of their incontinence problem and that methods for urinary diversion form the ‘last resort’ for women with severe incontinence who cannot be treated, or have failed repeated attempts at curative treatment, typically following multiple surgical procedures. As such, the use of containment products is included in the economic evaluation as they form part of the care that a woman might receive over her lifetime.
Absorbent pads
The most commonly used method of incontinence management is absorbent pads. The total expenditure on such products is large and, although not altering the underlying condition, it can be a satisfactory option for women with minimal or predictable leakage, or for those who are unsuited to treatments or have failed to benefit from treatment. The products range from thin panty-liners to large nappy (diaper)-type pads. Despite their widespread use, little research has been conducted concerning patient satisfaction or effectiveness, although recent evidence summarised in a Cochrane review does suggest that specifically designed pant insert pads are superior to those designed for menstrual loss. 84,85 Containment-using pads was also the subject of a previous UK Health Technology Assessment (HTA) monograph. 86
Urinary diversion
Some women with severe intractable incontinence that has not responded to corrective methods of management may choose to undergo urinary diversion. The simplest method is transurethral or suprapubic insertion of an indwelling catheter, with continuous drainage into a collection bag. Summarised evidence regarding types of catheter and policies for their use has previously been published. 87,88 Generally, indwelling catheters are unsuited for women with long life expectancy, who are often better served with a formal surgical urinary diversion procedure. This can be performed using an ileal conduit or continent diversion. The techniques, benefits and adverse events have been described in a Cochrane review. 89
Chapter 2 Aims and objectives
The aim of this study was to estimate the effectiveness and cost-effectiveness of non-surgical treatment for women with SUI.
The objectives are to:
-
develop a series of management pathways that describe potential sequence of non-surgical and surgical treatments for SUI
-
determine the clinical effectiveness of the different individual treatments for SUI
-
determine the safety in terms of the magnitude of any risks or side effects of treatments for SUI
-
estimate the cost-effectiveness of the alternative management pathways
-
identify areas for future research.
The remainder of the report is structured as follows:
-
Chapter 3 describes the definition of the decision problems and the patient treatment pathway.
-
Chapter 4 presents the results of a survey performed on women with SUI to identify the outcomes of importance to them.
-
Chapter 5 describes the methods used for reviewing clinical effectiveness and provides information on the inclusion and exclusion criteria, search methods for identification of studies, data collection and analysis.
-
Chapter 6 describes the studies included in the systematic review of clinical effectiveness.
-
Chapter 7 assesses the clinical effectiveness of the major treatments using direct pair-wise (head-to-head) comparisons.
-
Chapter 8 assesses the clinical effectiveness of the treatment using a mixed-treatment comparison (MTC) model.
-
Chapter 9 assesses the cost-effectiveness using an economic model; this chapter describes the basics of the modelling approach and the key assumptions underpinning the estimates of cost-effectiveness results.
-
Chapter 10 discusses the results of the study.
-
Chapter 11 is the conclusion, which highlights the implications of the findings for the NHS, women and research.
Chapter 3 Definition of the decision problem
The impact of SUI on individual women with the condition, their families and carers, and on the NHS, as a whole, is huge. 43 There are many potential methods for treating SUI which might be used alone or as part of a management strategy. However, while there is some evidence of the relative effectiveness of individual treatments, there is a lack of evidence on how the various interventions might be combined into management strategies. We therefore sought to identify plausible treatment strategies comprising sequences of non-surgical and surgical interventions. The clinical pathways were based on advice from the health-care professionals and patient group representatives who were involved in the study on what interventions patients could receive and the sequencing of the interventions. Even though the focus of this study is on non-surgical treatments, surgery is considered, as it may form part of a treatment strategy. For example, it may be a viable alternative for some women seeking treatment for SUI. For other women, surgery may be resorted to if a non-surgical intervention fails to satisfactorily resolve symptoms.
The Agency for Healthcare Research and Quality (AHRQ)90 and the third International Consultation on Incontinence91 have published a comprehensive literature review and consensus opinion of treatment guidelines for therapeutic interventions for SUI (the AHRQ has declared its guidelines obsolete). It is generally accepted that there is no ‘perfect’ therapy for all patients with SUI. Many factors should be considered when determining the optimal therapy for SUI in women. These include the aetiology and type of SUI, bladder capacity, renal function, sexual function, severity of the leakage and degree of bother to the patient, the presence of associated conditions – such as vaginal prolapse – or concurrent abdominal or pelvic pathology requiring surgical correction, prior abdominal and/or pelvic surgery, and, finally, willingness to accept the costs, risks, morbidity and success (or failure) rates associated with each intervention.
For most women with SUI it is sensible to discuss first the most reversible, simplest, least invasive and least expensive service-provider interventions, such as lifestyle changes and PFMT. The clinical consensus appears to be that the initial treatments for SUI involve a variety of non-surgical interventions, including lifestyle modification, PFMT with or without BF, and other accessory teaching aids, such as electronic devices and VCs. Further strategies can be formulated, which would consist of sequences of non-surgical and surgical interventions. These strategies will take into account the mechanism by which the treatment works and will also place limits on the number of retreatments allowed, based on current concepts of the use and effectiveness of the different procedures. More invasive or expensive interventions, such as surgery, are pursued if the clinician and patient decide that the current therapy is either ineffective or otherwise undesirable.
The importance of patient preference as the primary consideration in selecting a particular treatment for SUI was underlined by the findings of a recent survey which reported that most women preferred less invasive options. 92,93 From a health service perspective it is important to balance short- and long-term effectiveness against the potential adverse events and costs. Compared with surgical treatments, non-surgical treatments, such as lifestyle changes and physical therapies (such as PFMT), are associated with limited side effects and do not preclude future changes in management. Hence, non-surgical procedures have been considered to be the choice of primary treatments for SUI. 43 In developing the care pathways we have categorised treatments as being either non-surgical or surgical procedures and then considered how these treatments might be combined into management strategies for women presenting with SUI. While it is true that the specific treatment strategy adopted will vary from woman to woman, broad exemplars of specific strategies can be defined. In the following sections, descriptions of possible management strategies of women with SUI are presented, which vary in terms of the type and sequences of treatments that might be offered.
Descriptions of the patient treatment pathways
Figure 5 describes the treatment pathways that are compared within the economic model reported in Chapter 9. Potentially there are a very large number of alternative pathways that might be considered, and the pathways selected were derived following discussion with the health-care professionals and patient representatives involved in this study. They represent plausible care pathways that women might follow and might also be used to infer the value of other potential pathways not otherwise considered.
FIGURE 5.
Description of patient care pathways

Patient treatment pathway 1
Patient treatment pathway 1, in Figure 5, details one plausible strategy of care for a woman presenting with SUI. The first line of treatment that women presenting with SUI are offered is lifestyle modification. Lifestyle modification may involve a combination of one or more elements. These are dietary factors, reduction of caffeine intake, fluid intake, smoking, weight, physical exercise, alcohol consumption and limiting heavy activity (it may be possible that there are several ways lifestyle modifications can be performed so that they can be considered as different treatments, therefore allowing someone to get more than one lifestyle modification treatment). There are three states that could arise after treatment: ‘continent’, ‘incontinent’ or ‘dead’ (from natural causes). The continent state includes women who report that they are completely cured and those that feel that their condition has improved to the extent that they require no further treatment at the present time. Women could remain continent and hence require no further treatment, but some of them may experience a return or worsening of symptoms of SUI at some point in the future and then require further treatment. The women who report that they are not cured are said to be in the incontinent state. These women could either require or not require further treatment. The women who require further treatment get the next treatment option in the pathway they are following. In the first care pathway, all of the women who need further treatment are offered the second line of treatment: physical therapy. There are different forms of physical therapies that women can receive. These include PFMT alone or in combination with an adjunct, such as BF, ES or VCs. Based on the number of sessions women receive, PFMT can be classed as ‘basic’, when a patient has a maximum of two sessions per month, or ‘PFMT with extra sessions’, when the patient receives more than two sessions per month. This pathway offers only basic PFMT without any adjunct. Women who are not cured and do not receive any further treatment may use containment products, such as pads, to manage symptoms.
If women are not cured after PFMT they progress to the third line of treatment, which is surgery. Those who are not cured and need further treatment are offered a second surgical procedure. If the second surgical procedure does not work then women use containment products until they die. It is appreciated that women may also use containment products even when they are receiving other possible interventions. Figure 6 provides a detailed description of pathway 1. Similar figures could have been developed for all of the other pathways.
FIGURE 6.
Description of alternative treatment strategies (note: women may choose not to seek further treatment at any point).

Patient treatment pathway 2
The treatment pathway option 2 is similar to that depicted in option 1. The only difference is that PFMT with extra sessions is offered as an additional non-surgical treatment between basic PFMT and surgery.
Patient treatment pathway 3
Pathway 3 extends pathway 2 by adding a drug therapy (an SNRI) between PFMT with extra sessions and surgery.
Patient treatment pathway 4
The treatment pathway 4 is substantially the same as pathway 1. The difference is that women receive PFMT with extra sessions straight away, instead of PFMT basic.
Patient treatment pathway 5
Pathway 5 extends pathway 4 by adding SNRI therapy between PFMT extra sessions and surgery.
Patient treatment pathway 6
In this pathway women would, as in all other pathways, initially receive the relevant advice or treatment aimed at modifying lifestyle. If they experienced a recurrence, had insufficient improvement or no improvement, they would proceed straight to surgery. Such a strategy might be appropriate for some subgroups of women. Alternatively, it may be popular with some women because it gives the prospect of relatively rapid relief from symptoms.
Patient treatment pathway 7
This treatment pathway is similar to pathway 1 but with VCs as a treatment option between basic PFMT and surgery.
Patient treatment pathway 8
This treatment pathway is similar to pathway 1 but with ES as a treatment option between basic PFMT and surgery.
These patient care pathways will be used in the study to inform the economic model reported in Chapter 9. These pathways will also assist in identifying outcomes of interest for the systematic review of effectiveness and identify the data required to populate the model. The key outcome of these pathways is that any need for further treatment is heavily influenced by whether the prior treatment has resulted in sufficient resolution of symptoms.
Chapter 4 Identification of outcomes of importance to women with stress urinary incontinence
Introduction
It has been recognised for many years that the embarrassment associated with urinary incontinence may cause withdrawal from social situations and reduce quality of life. 32 Many women with SUI show symptoms of depression and introverted behaviour, together with dysfunctional interpersonal relationships. 33 Furthermore, SUI may lead to withdrawal from regular physical activities and hence impair women’s general health. 34 One of the problems with the assessment of incontinence is that outcome measures frequently used in primary research may not perfectly map on to outcomes of importance to women. For example, the commonly reported outcome measure in clinical trials is ‘cure’, which is the satisfactory resolution (or near resolution) of symptoms. Using this definition usually means the patient self-report of the absence of incontinence. However, the ideal measure of how women are after treatment should reflect the ability to lead a normal social life, which may be compatible with improvements in the level of continence.
Given that much of the available literature focuses on clinical outcomes, which, as a result, may have limited relevance to the lives of women with SUI, the aim of this survey was to provide evidence on outcomes of importance to women.
The purpose of this work was to prospectively survey women with SUI to provide information on outcomes of importance to them; a secondary aim was to identify additional outcomes that ought to be collected in future primary studies and hence to define relevant outcomes for systematic reviews of the literature.
Methods
In order to identify the areas of importance to women who suffer from SUI, a questionnaire was designed. A Patient Generated Index (PGI)94 was used to allow respondents to state and evaluate the areas of their life affected by SUI. In addition to the PGI, the questionnaire included the King’s Health Questionnaire,95 the EQ-5D, and questions relating to socioeconomic and demographic information. The rationale for choosing the PGI was that as it is an individualised instrument – it would provide information on the specific concerns of women with SUI. In addition, these outcomes could then be compared with generic measures, such as the King’s Health Questionnaire and the EQ-5D.
The questionnaire
The PGI is an individualised patient-reported health instrument that allows the respondent to select, weight and rate the importance of a particular health outcome. 96 The PGI was designed with the aim of producing a valid measure of outcome that reflected areas of importance to patients’ lives. 94 The PGI involves the respondent deciding what factors are important to her. Examples of the types of factors that may be important are included to provide guidance. The aim of the PGI is therefore to capture the diverse range of concerns or priorities of respondents. Using the PGI, respondents can vary the weight they attach to these concerns or priorities, which provides researchers with an insight into each respondent’s viewpoint. An overall score for the PGI for each respondent can then be calculated by multiplying the rating for each health area by the proportion of points allocated to that particular area.
The PGI is completed in three stages. In the first stage, respondents are asked to identify up to five areas of their life that are affected by their SUI. Respondents are given a list of outcomes to act as prompts to help them think about which areas of their life might be affected by their condition. Respondents can then choose from these options or provide their own examples. In addition to the five boxes, there is a sixth box that enables respondents to rate all other areas of their life affected by their SUI. Examples of the factors to include as prompts on the PGI were drawn from three sources. The first of these was the King’s Health Questionnaire,95 which was used to generate a list of outcomes under the broad headings of: ‘role limitations’, ‘physical limitations’, ‘social limitations’ and ‘personal relationships and emotions’. These outcomes were supplemented from Cochrane reviews of non-surgical treatments. 56,75,83,97 Finally, a general literature search was also conducted, although this did not provide further additions to the 17 different outcomes identified from the King’s Health Questionnaire and the Cochrane reviews. These outcomes were then narrowed down to broad categories of those considered most relevant by members of the project team. These were ‘work’, ‘household tasks’, ‘social activities’, ‘feeling depressed/anxious’, ‘personal hygiene’ and ‘affecting sleep’. In addition to the methods used to generate the prompt list, further qualitative work could have been conducted. This could have included a focus group of women with SUI to further refine the areas to include in the prompt list. This is an area that could be considered in future use of the PGI.
In stage two of the PGI, respondents were asked to score each area listed in stage one of the PGI on a scale ranging from 0 to 6. The score given in stage two was intended to reflect how the individual was affected by their SUI in the past month. A score of 0 would signify that the effect on their life was as bad as it could possibly be and a score of 6 would correspond to an effect that was as good as it could possibly be.
Finally, in stage three, respondents were asked to ‘spend’ 10 points to indicate the relative importance of each of the areas mentioned in stage one. Respondents were requested to spend more points on areas that were the most important to them.
As noted above, in addition to the PGI, the questionnaire also contained the King’s Health Questionnaire and the EQ-5D. The King’s Health Questionnaire is a condition specific questionnaire that aims to assess the impact of urinary incontinence on an individual’s quality of life. It contains questions set in nine domains relating to: general health perception, incontinence impact, role limitations, physical limitations, social limitations, personal relationships, emotions, sleep and energy, and severity. With the exception of the final part of the questionnaire (severity measures) scores can be calculated for each domain (0–100). The higher the score the worse off an individual feels they are and the lower they perceive their quality of life to be.
The EQ-5D is a standardised instrument used to measure quality of life. The EQ-5D is applicable to a wide range of health conditions and treatments and it provides a simple descriptive profile and a single index value for health status. 98 The EQ-5D has five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) that can be converted into a utility score.
Sample
The Bladder and Bowel Foundation (formerly ‘InContact’ and the ‘Continence Foundation’) is a national charity that provides information and support to people with bladder and bowel problems, representing the interests of people with continence problems with the aim of ensuring they have access to the latest information and services available. 99 In 2006 a survey conducted by InContact was completed by 755 people affected by bladder and bowel problems. 100 Of these, 188 women with SUI gave consent for future contact about relevant research and formed the sample for the current study. In July 2007 these women were sent questionnaires for the current study by InContact. Given that this is a self-selected sample of women suffering from SUI and not a random sample of the population, it is not known how representative this sample is of the wider population.
Ethical issues
The 2006 survey in which the participants were originally identified was a service evaluation in which The Bladder and Bowel Foundation surveyed people who had previously been in touch with the charity. As such, no ethical approval was necessary. The 2006 survey materials contained an explicit assurance that confidentiality would be maintained and that identifiable data would not be passed on to third parties. Respondents were asked if they were willing to be contacted in the future for research purposes. For this study questionnaires were sent in July 2007 to 188 women with SUI who gave their consent for further contact relating to research. The questionnaires were returned directly to the charity and, after screening only anonymous data, were subsequently forwarded to the authors in accordance with the Medical Research Council’s guidance on the use of personal information in medical research and the Data Protection Act 1998. 101,102
Results
All data were analysed in spss version 17.0. Descriptive statistics and correlations of the sample were analysed and EQ-5D and PGI scores were calculated. In total, 105 out of 188 respondents (55.9%) completed and returned the questionnaire. Table 3 shows the areas of an individual’s life that they reported to be affected by their SUI, divided into four different themes. These themes were: (1) activities of daily living (work, home and social); (2) sex, hygiene and lifestyle issues; (3) emotional health; and (4) services. In total, 38 different areas were reported by respondents. Activities of daily living were the most frequently reported areas to be affected by SUI, followed by sex, hygiene and lifestyle issues and emotional health.
| Theme/specific issue | N | % |
|---|---|---|
| Activities of daily living: work, home and social | ||
| Going out/socialisinga | 58 | 13.7 |
| Sleepa | 47 | 11 |
| Shoppinga | 33 | 7.8 |
| Physical activity | 30 | 7.1 |
| Worka | 24 | 5.7 |
| Travel | 18 | 4.2 |
| Going on holiday/staying away from home | 12 | 2.8 |
| Household tasks | 10 | 2.4 |
| Family activities | 4 | 0.9 |
| Travelling on public transport | 1 | 0.2 |
| Being housebound | 1 | 0.2 |
| Inability to study/write | 1 | 0.2 |
| Activities outside the home | 1 | 0.2 |
| Total | 56.6 | |
| Sex, hygiene, lifestyle issues | ||
| Personal hygienea | 53 | 12.5 |
| Sexual relationships | 10 | 2.4 |
| Personal relationships | 7 | 1.7 |
| Sneezing/coughing/laughing | 7 | 1.7 |
| Affecting choice of clothes | 6 | 1.4 |
| Infections/skin irritations | 4 | 0.9 |
| Loss of independence | 3 | 0.7 |
| Limiting liquid intake | 2 | 0.5 |
| Continually going to toilet when not necessary | 1 | 0.2 |
| Feeling cold | 1 | 0.2 |
| Worry about leaving wet stains | 1 | 0.2 |
| Total | 22.4 | |
| Emotional health | ||
| Depressiona | 32 | 7.5 |
| Anxietya | 24 | 5.7 |
| Bladder controlling life | 2 | 0.5 |
| Embarrassment | 2 | 0.5 |
| Affecting confidence | 1 | 0.2 |
| Body image | 1 | 0.2 |
| Feeling unfeminine | 1 | 0.2 |
| It annoys me | 1 | 0.2 |
| Long-term effect it is having on me | 1 | 0.2 |
| Failure | 1 | 0.2 |
| Total | 15.6 | |
| Services | ||
| Lack of public toilets | 11 | 2.6 |
| Need to use products/pads | 8 | 1.9 |
| Time spent at doctor’s surgery/hospital | 3 | 0.7 |
| Public queues | 1 | 0.2 |
| Total | 5.4 | |
Out of 105 respondents, 73 respondents were categorised as having answered the PGI correctly. A further nine respondents made mistakes in the PGI and 23 respondents did not fully complete it (Table 4). Of the 73 respondents who correctly completed the questionnaire, 61 answered the PGI with no mistakes (all sections were completed satisfactorily). The remaining 12 respondents made a small error in completion of the PGI. This small error always occurred in section three of the PGI, where respondents had to spend 10 points. These respondents did in fact spend 10 points but they missed out spending points in area 6 (all other areas of their life affected by SUI) and totalled to 10 in box 6. This error is likely to have occurred due to the layout of the PGI. An example of the PGI used can be seen in Appendix 1.
| Responses | Frequency | Percentage | Notes |
|---|---|---|---|
| PGI answered correctly | 73 | 69.5 | PGI correct: 61 |
| PGI put total in box 6: 12 | |||
| Mistake in PGI | 9 | 8.6 | |
| PGI not fully completed | 23 | 21.9 | |
| Total | 105 | 100.0 |
Table 5 shows the demographic information of the sample, as a whole and for those individuals who correctly and incorrectly completed the PGI. The mean age for the sample as a whole was 57 years (range 28–89). As can be seen in Table 5, those respondents who correctly completed the PGI were, on average, younger than those who completed it incorrectly. In addition, those who correctly completed the PGI appear to be better educated and in higher-income groups.
| Variable | Total sample (n = 105) | Correct PGI (n = 73) | Incorrect PGI (n = 32) |
|---|---|---|---|
| Mean age of respondents (range, years) | 56.90 (28–89) | 55.16 (28–89) | 60.84 (37–87) |
| Age ranges (%) | |||
| 25–34 | 3 (2) | 3 (4) | – |
| 35–44 | 15 (14) | 11 (15) | 4 (13) |
| 45–54 | 39 (37) | 28 (38) | 11 (35) |
| 55–64 | 21 (20) | 15 (21) | 6 (19) |
| 65–74 | 8 (8) | 6 (8) | 2 (6) |
| 75+ | 19 (18) | 10 (14) | 9 (28) |
| Income (valid %) | |||
| £6000 | 10 (11) | 7 (11) | 3 (11) |
| £6001–10,000 | 16 (17) | 12 (19) | 4 (15) |
| £10,001–15,000 | 20 (22) | 11 (17) | 9 (33) |
| £15,001–20,000 | 13 (14) | 9 (14) | 4 (15) |
| £20,001–25,000 | 5 (5) | 3 (5) | 2 (7) |
| £25,001–30,000 | 10 (11) | 7 (11) | 3 (11) |
| £30,001–35,000 | 8 (9) | 6 (9) | 2 (7) |
| £35,001+ | 10 (11) | 10 (15) | – |
| Education (%) | |||
| None | 2 (2) | 1 (1) | 1 (3) |
| Secondary school | 39 (37) | 22 (30) | 17 (53) |
| College | 29 (28) | 21 (29) | 8 (25) |
| University | 35 (33) | 29 (40) | 6 (19) |
In addition to listing the outcomes of importance to women who suffer from SUI, a score of overall quality of life can also be calculated from the PGI. The score ranges from 0 to 6, with 0 reflecting a very low quality of life (‘it’s as bad as it could possibly be’) and 6 reflecting a very high quality of life (‘it’s as high as it could possibly be’). An example of the PGI and the method used to calculate the score is given in Table 6. For the respondents who successfully completed the PGI the mean score was 2.4 (SD 1.4, range 0–6). Given that a score of 6 on the PGI reflects the highest quality of life, a mean score of 2.4 in this population reflects that their quality of life falls significantly short of their hopes and expectations. In total, 101 out of 105 returned questionnaires had a fully completed EQ-5D. Scores on the EQ-5D ranged from –0.17 to 1. The mean EQ-5D score was 0.598 (SD 0.339). Correlation between the mean PGI score and the mean EQ-5D score was, as expected, positive and statistically significant.
| Part 1: list areas of life affected by urinary incontinence | Part 2: score (0–6) | Part 3: spend your 10 points | Final PGI score |
|---|---|---|---|
| 1. Interrupted sleep | 1 | 3 | 0.3 |
| 2. Affects my social life | 6 | 1 | 0.6 |
| 3. Affects my work | 3 | 2 | 0.6 |
| 4. Personal relationships | 2 | 2 | 0.4 |
| 5. It makes me feel depressed | 4 | 1 | 0.4 |
| 6. All other areas of your life affected by your urinary incontinence | 5 | 1 | 0.5 |
| Total | 2.8 |
Scores (out of 100) for each domain in the King’s Health Questionnaire can be seen in Table 7. The higher the score, the worse off an individual feels. In addition to the domains of the King’s Health Questionnaire, it also contains a section detailing the respondent’s bladder problems and how much they affect the individual’s life.
| N | Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|---|
| KHQ scores for role limitation | 101 | 0.00 | 100.00 | 53.30 | 30.64 |
| KHQ physical limitation scores | 100 | 0.00 | 100.00 | 61.83 | 30.09 |
| KHQ social limitation | 95 | 0.00 | 100.00 | 45.61 | 30.98 |
| KHQ score for personal relationships | 73 | 0.00 | 100.00 | 37.90 | 35.92 |
| KHQ score for emotions | 98 | 0.00 | 100.00 | 60.32 | 31.67 |
| Sleep energy | 100 | 0.00 | 100.00 | 60.67 | 31.02 |
| Severity measures | 98 | 6.67 | 100.00 | 68.50 | 22.55 |
Correlations of the PGI and seven domains of the King’s Health Questionnaire were also performed. Given the scoring system of the King’s Health Questionnaire we would expect to find a negative correlation between the PGI and King’s Health Questionnaire. All correlations were negative but only two were statistically significant: personal relationships (p = 0.004) and severity measures (p = 0.003).
In addition, correlations between the EQ-5D score and the domains of the King’s Health Questionnaire were also calculated. We found all seven of the King’s Health Questionnaire domains to be significantly (negatively) correlated with the EQ-5D. This result is to be expected, as many of the EQ-5D and King’s Health Questionnaire domains are similar.
Summary
The PGI has been used to quantify the effect of SUI on the quality of women’s lives for the first time. In stage one of the PGI, 38 different areas of a woman’s life affected by SUI were reported. The most frequently mentioned areas were: going out or socialising, with 14% of all respondents listing this as one of the areas of their life affected by their condition; personal hygiene (13%); and the effect their condition has on their sleep (11%). Shopping (8%), depression (8%), physical activity (6%), work (6%), anxiety (6%), travel (4%), household tasks (2%), personal (1%) and sexual relationships (2%) were all also listed as areas of their life affected by SUI.
The respondents are a self-selected sample of women who had previously been in touch with a patient support charity and who may be considered to be active help-seekers. However, there is no reason to suspect that their experience of SUI and the relative perceived impact of SUI on various aspects of their lives are different from the wider population of women affected. Nearly 70% of respondents successfully completed the questionnaire. A further 9% attempted the PGI but made mistakes in its completion, and 22% failed to fully complete the PGI (the majority of these respondents completed stage one of the PGI but failed to complete stages two or three). Those respondents who successfully completed the questionnaire were found to be younger, in higher-income groups and to have a higher level of education. For the PGI to be used as a valid and reliable measure of outcomes of importance to women with SUI and to be able to accurately quantify the effect of SUI on their lives, the response rate and successful completion of the PGI would need to be improved. Of the respondents, 31% had difficulty in completing the questionnaire and there was also a low response rate to the survey in general (56%).
In order to improve this response rate and the chance of successful completion, alterations could be made to the layout of the PGI to make it more user-friendly. The PGI, or instruments like it, have been criticised in the past. Some authors question whether they reflect the patient’s view point or, conversely, whether they are simply reflecting the views of the researchers who designed the questionnaire. 103 In this survey, while we did find a varied response in the number of outcomes listed by respondents, the majority of these did in fact come from the prompt list provided in the PGI. Of the 10 most mentioned areas, eight of these were from the prompt list. Other studies have found similar associations between the prompt list and final outcomes listed by respondents. However, it is unclear whether this association is due to the most relevant examples being selected from the prompt list, or due to respondents being unwilling or unable to think of their own examples because the prompt list is already comprehensive. 104
We correlated the mean PGI score with the mean EQ-5D scores. This correlation was, as expected, found to be positive and statistically significant. In addition to this, correlations between the King’s Health Questionnaire and the PGI were performed. Although all correlations of the PGI and King’s Health Questionnaire were as expected (negative), only two were found to be statistically significant. Given that the PGI outcomes and the domains of the King’s Health Questionnaire do not correlate well, and that many of the aspects respondents mentioned in the PGI list of outcomes do not map very well on to the dimensions of the EQ-5D, this might suggest that generic measures, like the EQ-5D, may not be a very good reflection of the preferences of people with incontinence. The PGI in this instance may therefore be capturing concerns of women who suffer from SUI which are not adequately captured by generic instruments, such as the EQ-5D. This is of particular interest in the context of health technology appraisals such as this, where EQ-5D has become the accepted standard for calculation of quality-adjusted life-year (QALY) indices for use in determination of cost-effectiveness. 105 As will be described in later chapters, health-state utilities derived from the EQ-5D have been used to estimate QALYs in the economic evaluation. Hence, in the light of findings reported in this chapter, evidence on cost-effectiveness of interventions needs careful consideration.
Conclusion
Much of the available literature on SUI focuses on doctor-selected clinical outcomes. Given the undoubted social and personal impact of SUI, these outcomes may have limited relevance to the women who suffer this condition. Thirty-eight different areas of an individual’s life affected by SUI were identified by the PGI. The PGI succeeded in capturing a diverse range of outcomes of importance to women suffering from SUI, although some respondents found the PGI difficult to complete.
The PGI was not found to correlate well with the domains of the King’s Health Questionnaire, nor to map well on to the dimensions of the EQ-5D or the King’s Health Questionnaire. This suggests that generic measures may not be a very good reflection of the preferences of people with incontinence.
Ideally, the information obtained from this survey would be used to help define outcome measures for the systematic review of clinical effectiveness. However, these outcomes were often not considered in primary studies and hence their inclusion in a systematic review was not possible. Nevertheless, it would be important to include them in future research in this area.
Chapter 5 Methods for reviewing clinical effectiveness
The next four chapters present the systematic review of clinical effectiveness. This includes the review methods (this chapter), characteristics of the included studies (Chapter 6), direct head-to-head comparisons (Chapter 7), followed by MTC models (Chapter 8).
Inclusion and exclusion criteria
Types of studies
The types of studies considered were RCTs and quasi-RCTs (alternate allocation). Trial data reported in conference abstracts, as well as full-text papers, were included. For abstracts, solely those identified from the Cochrane Incontinence Group Specialised Register of trials were used.
Types of participants
The participants were women with SUI or incontinence that was predominantly SUI (however diagnosed). Classification of diagnoses was accepted as defined by the trialists.
Owing to the small number of studies per intervention available, a pragmatic decision was made to include studies where the majority (≥ 50%) of the sample consisted of women with SUI or predominant SUI. Studies were therefore included if:
-
all women had SUI alone (type-1 population)
-
at least 50% of women had SUI alone; the remainder could have UUI or MUI (type-2 population)
-
under 50% of women had stress incontinence alone but the majority (50% or more) had MUI with stress symptoms as a predominant pattern; the remainder could have SUI, UUI or MUI (type-3 population).
Studies were excluded if the proportion of women with predominantly SUI was not reported, if the type of incontinence (stress, urge, mixed) was unknown or undiagnosed, or if predominant symptoms (stress or urgency) of women with MUI were not specified.
Women with urinary incontinence whose symptoms might be due to significant factors outside the urinary tract were excluded, for example neurological disorders, cognitive impairment, and lack of independent mobility. Studies investigating nocturnal enuresis in women were also excluded.
Incontinent women during pregnancy or in the early postpartum period were considered for inclusion. Data from these childbearing women were analysed separately on the assumption that the effect of PFMT might differ in this group due to the physiological changes of pregnancy and in the postpartum period. Studies investigating prevention of incontinence among childbearing women were excluded.
Studies that recruited mixed populations of men and women with different types of urinary incontinence were eligible for inclusion, providing that demographic and outcome data were reported separately for women with predominantly SUI.
Types of interventions
We defined non-surgical treatment as that which could be undertaken in a heath-care professional’s office or clinic and patients’ homes. Any of the following interventions, alone or in combination, were included. Treatment definitions used were as according to Cochrane Reviews where possible.
Lifestyle interventions
-
Weight loss, decreased physical exertion, fluid manipulation, decreased caffeine, nicotine or alcohol consumption, treatment of constipation and dietary interventions. 106
Physical or behavioural therapy
-
Pelvic floor muscle training, with or without biofeedback – defined as ‘a programme of repeated voluntary pelvic floor muscle contractions taught and supervised by a health care professional’. 83 This includes ‘The Knack’, the use of a timed contraction of the pelvic floor muscles before and during a cough (Miller 1998). 107 However, it excludes PFMT introduced by a leaflet only, without any contact with a health-care professional (which was classified as being equivalent to no treatment).
-
Electrical stimulation (non-implanted) – local stimulation of pelvic floor muscles. Electrical nerve stimulation (e.g. sacral nerve) was excluded.
-
Weighted vaginal cones.
-
Bladder training, either described by trialists as bladder training, bladder retraining, bladder drill or bladder re-education or an intervention ‘which included mandatory schedule or self schedule with the aim of increasing the interval between voids’. 53 Prompted or timed voiding or urge suppression techniques alone with no mention of mandatory schedule or self-schedule were not considered as BT.
Pharmacotherapy
-
SNRIs.
-
Local administration (in form of cream or pessaries) of intravaginal low-dose estrogens, given as an adjunct to other therapy (e.g. PFMT) in postmenopausal women. Hormonal treatment (estrogens) given on its own was excluded.
Trials comparing methods of delivering services (e.g. nurse-led care) were considered for inclusion if they involved one of the included interventions listed above. The use of non-surgical therapies for prevention of incontinence was excluded. Complementary therapies, such as acupuncture, hypnosis and herbal medicines, were also excluded.
Where studies reported a comparison involving a programme of interventions (e.g. PFMT plus BT), then these studies were included, provided that every participant in the intervention arm received all of the specified treatments. If, on the other hand, treatments were tailored according to participants’ diagnosis (e.g. PFMT for participants with stress incontinence alone and PFMT plus BT for participants with mixed or urge incontinence) and some participants did not receive all components available, studies were excluded, as it was not possible to distinguish effects of individual treatments for SUI.
A valid comparator was one of the included interventions or no treatment. Studies that compared non-surgical with surgical interventions were included in order to enable the performance of MTCs of non-surgical treatment. However, studies were excluded if the comparator was an excluded intervention (e.g. PFMT vs adrenergic agonists).
Types of outcome measures
The standardisation committee of the International Continence Society recommended that research investigating the effect of therapeutic interventions for urinary incontinence in adult women should incorporate outcome measurements in the following five domains: (1) the patient’s observations (symptoms); (2) quantification of symptoms (e.g. urine loss); (3) the clinician’s observations (anatomical or functional); (4) quality of life; and (5) socioeconomic measures. 108 Following this recommendation, the following measures of outcomes were sought in this review:
Primary outcomes
-
Number of women cured.
-
Number of women cured or improved (this outcome is henceforth referred to in the text as improvement).
-
Adverse events.
-
Condition-specific (and generic measures of health-related) quality of life.
Secondary outcomes
-
Quantification of symptoms:
-
– number of incontinent episodes over 24 hours
-
– number of pad changes over 24 hours
-
– mean volume or weight of urine loss on pad test
-
– number of micturitions over 24 hours.
-
-
Participant satisfaction or desire for further treatment.
-
Long-term data:
-
– number of women having incontinence surgery
-
– return of symptoms/recurrence.
-
-
Socioeconomic measures.
-
Other intermediate, explanatory or treatment-specific outcomes:
-
– measure of pelvic floor muscle function
-
– treatment adherence
-
– volume and type of fluid intake or change in body mass index.
-
The cure and improvement rate may be ascertained via women’s observation (self-report), quantification of symptoms (typically based on incontinence diaries or pad tests) or clinician’s observation. There was a considerable variability in the way these outcomes were defined by the trialists, which limited the possibilities for quantitative synthesis. We therefore chose to combine data on the cure and improvement rate from different sources. The women’s observation was given priority but for studies in which it was not reported, the rate based on diaries was used as a proxy. Where diary data were also not reported, the rate based on pad tests or any other definitions chosen by the trialists was used.
The choice of quality of life measures again varied across studies. In addition, scores were reported either as final values or changes from baseline. Final values were preferred, but where these were not reported, a change score was used. Quantitative synthesis was performed separately for final values and change scores.
Search methods for identification of studies
Literature searching was performed in two stages. First, relevant trials were identified from the Cochrane Incontinence Group Specialised Register of controlled trials of interventions for urinary incontinence (last searched 20 March 2008). When last searched for this project, the Register contained trials identified from: MEDLINE (covering January 1966 to week 4 of January 2008), the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2008), CINAHL (covering January 1982 to December 2000), and from hand searching relevant journals and conference proceedings.
Second, an extensive electronic search was carried out to identify reports of relevant published and ongoing studies, as well as grey literature and recent meeting abstracts from sources that are not currently covered for the Cochrane Incontinence Group Specialised Register of trials. A highly sensitive search strategy based upon the one developed for the Cochrane Incontinence Review Group was adopted. 109 This strategy used controlled vocabulary and text word terms that reflected the clinical condition, interventional procedures and study designs that were considered within the scope of this project. The following additional databases were searched:
-
CINAHL (January 1982 to week 1 of December 2007)
-
EMBASE (January 1980 to week 49 2007)
-
BIOSIS (January 1985 to 13 March 2008)
-
Science Citation Index and Social Science Citation Index (1970 to 2 February 2008)
-
Current Controlled Trials (searched on 29 May 2008)
-
ClinicalTrials.gov (searched on 9 June 2008)
-
UKCRN Portfolio Database (replaces UK National Research Register).
An Internet search included the websites of relevant professional organisations and manufacturers that had not been covered by the Specialised Register or the other bibliographic database searches. No language or date restrictions were applied to the searches.
Searches were run for the intervention areas listed above on each of the databases listed. The main searches were run during September to November 2007, with updates in December 2007/January–February 2008. A set of urinary incontinence terms was combined with a set of terms to cover the main interventions listed above. These terms were combined with a study design filter as appropriate for each database. InterTasc website design filters were assessed and adapted if suitable. 110 Full details of the search strategies used are provided in Appendix 2.
Data collection and analysis
Selection of studies
The titles and abstracts identified by the searches were assessed by one reviewer, having already been assessed by the Cochrane Incontinence Group Trials Search Co-ordinator. Full-text copies of all potentially relevant reports were obtained and independently assessed by two reviewers, using a form developed to determine whether the reports met the inclusion criteria (Appendix 3). Any disagreements were resolved by consensus or arbitration by a third person.
Data extraction
A data extraction form was developed to record details of study design, methods, participants, interventions and outcomes (Appendix 4). One reviewer extracted data and another reviewer checked the extracted data. Any disagreements that could not be resolved by discussion were referred to an arbiter.
For studies in which the majority of participants had stress incontinence alone (types 1 and 2 above), data were extracted for both primary and secondary outcomes. For studies where stress-predominant MUI was the majority (type 3) and studies of childbearing women, data were extracted for primary outcomes only. General background and methodological information was collected from all studies.
Assessment of risk of bias in included studies
Two reviewers independently assessed all of the studies that met selection criteria for potential risk of bias. The assessment used the adapted version of a checklist developed by the Cochrane Incontinence Group (Appendix 5). 109
Data synthesis
Data analysis was performed in two stages: (1) pairwise (head-to-head) comparison (Chapter 7), and (2) a mixed-treatments comparison (Chapter 8).
Pairwise comparisons
For trials with multiple publications, only the most up-to-date or complete data for each outcome were included. Overall, there was inconsistency in outcome measures chosen by the trialists. For this reason, quantitative synthesis was performed on primary outcomes only. A random effects model was used to derive summary estimates with 95% CI of odds ratio (OR) for dichotomous variables (cure and improvement rates) and standardised mean difference (SMD) for continuous variables (quality-of-life measures). The random effects model was chosen because of variability in the characteristics of included studies in terms of participants’ diagnoses (inclusion of women with stress, urge or mixed incontinence), variation in the treatment programmes, and the frequency and duration of treatment. Odds ratios were used because of their symmetry compared with relative risks and were therefore unaffected by outcome definitions (e.g. number of women cured or not cured). Odds ratios were also chosen to fulfil a requirement of the MTC model.
Publication bias was not formally assessed in the analysis, as the number of studies available for each comparison was very limited. Heterogeneity between studies was assessed by visual inspection of plots of the data, the chi-squared test for heterogeneity and the I2 statistic. 111 Possible reasons for heterogeneity were explored, such as differences in the populations studied, the treatment given or the way in which the outcomes were assessed. Studies were grouped and sorted by types of participants: studies solely comprising women with SUI alone (type 1), studies where the majority of participants (50% or more) had stress incontinence alone (type 2) and studies where the majority (50% or more) of participants had MUI with stress as the predominant pattern (type 3). Where a quantitative synthesis was considered to be inappropriate or not feasible, then a narrative synthesis of the results was provided. Analysis was performed in stata. 112
The duration of treatment varied between studies. No attempt was made to standardise the treatment duration. Data at the end of the prescribed treatment phase, or at the first outcome assessment, if later, were used in quantitative synthesis. This may mean that any treatment effects shown were measured when they may be considered to be showing the maximum effects. Data from further follow-ups after the end of the initial treatment phase are reported in the text but are not included in the meta-analysis.
Data from primary studies were often reported ambiguously, particularly the number of people contributing data for an outcome (i.e. there were problems caused by missing data). For example, some studies had reported percentages without reference to an actual number of participants, or where studies claimed to have used an ‘intention-to-treat’ method but this was not clearly described. Where possible, we used the number of participants with available outcome data as the denominator for the relevant time point (i.e. we did not make the assumption that all participants who dropped out ‘failed’ and were not cured). We did consider the reasons for missing data caused by withdrawal/dropout reported by the trialists and these are highlighted (Chapter 7) where these appear to be treatment related. This is particularly problematic where there is differential withdrawal between trial arms.
Further analysis was planned on the following patient subgroups:
-
nature of presentation – postpartum (within 12 months of childbirth) versus at any other time
-
nature of the incontinence – stress urinary incontinence alone versus mixed/any urinary incontinence
-
presence or absence of a co-existing anterior vaginal wall prolapse.
In the event this was not performed due to a lack of available data.
Mixed-treatment comparison
This review aimed to assess the effectiveness of several treatments for SUI. With direct, head-to-head, comparisons alone it is often not clear which treatment is the most effective. 113 Multiple treatment comparison models attempt to address this problem by analysing all of the treatments and all of the trials together in one single model. In such a model it is possible to estimate the OR of all pairs of treatments, using direct and indirect evidence.
Multiple treatment comparison models were used for key treatments and outcomes (i.e. cure and improvement rates). The models were evaluated using Bayesian methods within winbugs software. 114 A full description is given in Chapter 8.
Chapter 6 Description of studies
Results of the search
Number of studies identified
The results of the initial searches are summarised in Table 8. These results were then assessed for potential relevance to the project and to remove duplicates already present in the Specialised Register that could not be removed by reference manager duplicate checking. As described above, the numbers retrieved for CINAHL, EMBASE, BIOSIS and Science Citation Index include only the additional reports identified after excluding those identified in the Cochrane Incontinence Register. A total of 7103 titles and abstracts were identified, of which 378 were selected for full assessment.
| Database | Number of hits | Number of full text papers selected for assessment | Number of reports included in final review |
|---|---|---|---|
| Published reports | |||
| Cochrane SR | 645 (date of last search: 20 March 2008) | 322 | 175 |
| CINAHL | 1115 (date of last search: 5 February 2008) | 4 | 0 |
| EMBASE | 1031 (date of last search: 10 December 2007) | 23 | 0 |
| BIOSIS | 204 (date of last search: 13 March 2008) | 9 | 3a |
| SCI, SSCI | 3228 (date of last search: 6 February 2008) | 5 | 1b |
| Eli Lilly website | 15 (date of last search: 29 May 2008) | 15 | 0 |
| Subtotal | 6238 | 378 | 179 |
| Ongoing trial list | |||
| UKCRN Portfolio Databasec | 34 (date of last search: 9 June 2008) | NA | NA |
| CCT | 549 (date of last search: 29 May 2008) | NA | NA |
| ClinicalTrials | 282 (date of last search: 9 June 2008) | NA | NA |
| Subtotal | 865 | ||
| Total | 7013 | 378 | 179 |
Number and type of studies included
Of the papers selected for full text assessment the level of agreement between reviewers about whether a paper met the inclusion criteria was very high, and 176 papers from the search met the inclusion criteria for the review. These described 88 studies, which covered 37 distinct treatments (Table 9) and 68 treatment comparisons (Table 10). The list of included studies and associated references are listed in Appendix 6.
| Type of interventions |
|---|
| NT |
| PFMT |
| PFMT + BF |
| ES |
| VC |
| BT |
| SNRI 80 mg |
| SNRI 40 mg |
| SNRI 30 mg |
| SNRI 20 mg |
| SNRI 40 mg b.i.d., starting with 40 mg b.i.d. |
| SNRI 40 mg b.i.d., starting with 40 mg q.d. |
| SNRI 40 mg b.i.d., starting with 20 mg b.i.d. |
| PFMT + ES |
| PFMT + VC |
| PFMT + BF + BT |
| PFMT + SNRI |
| PFMT with additional sessions |
| PFMT with audiocassette |
| Strength and motor relearning PFMT |
| Motor relearning PFMT |
| PFMT in supine position |
| PFMT in supine and upright position |
| Modified pilates |
| PFMT (maximal contraction) + BF |
| PFMT (submaximal contraction) + BF |
| PFMT + urethral electrical conductance |
| PFMT + BF (vaginal) |
| PFMT + BF (vaginal and abdominal) |
| PFMT + BF + ES (faradism) |
| PFMT + BF + ES (IFT) |
| PFMT+BF + ES (maximal intensity at clinic) |
| PFMT + BF + ES (low intensity at home) |
| VC passive |
| VC active |
| PFMT + VC + BF |
| Surgery |
| Comparison | Number of trials | Participants | References |
|---|---|---|---|
| Lifestyle change | 1 | 84 | 119 |
| Comparison with no treatment | |||
| PFMT vs NT | 14 | 958 | 57, 107, 115, 118, 120–129 |
| PFMT + BF vs NT | 2 | 110 | 120, 122 |
| ES vs NT | 8 | 446 | 115, 124, 126, 130–134 |
| VC vs NT | 2 | 220 | 115, 129 |
| BT vs NT | 1 | 131 | 135 |
| SNRI 80 mg vs NT | 11 | 3891 | 57, 117, 136–144 |
| SNRI 40 mg vs NT | 2 | 342 | 144, 145 |
| SNRI 30 mg vs NT | 1 | 60 | 145 |
| SNRI 20 mg vs NT | 2 | 344 | 144, 145 |
| SNRI 40 mg b.i.d., starting with 40 mg b.i.d. vs NT | 1 | 256 | 138 |
| SNRI 40 mg b.i.d., starting with 40 mg q.d. vs NT | 1 | 247 | 138 |
| SNRI 40 mg b.i.d., starting with 20 mg b.i.d. vs NT | 1 | 253 | 138 |
| PFMT + ES vs NT | 3 | 257 | 121, 123, 126 |
| PFMT + SNRI vs NT | 1 | 99 | 57 |
| Comparison of different PFMT variants | |||
| PFMT vs PFMT + BF | 15 | 609 | 120, 122, 146–158 |
| PFMT vs PFMT with additional sessions | 4 | 178 | 116, 159–161 |
| PFMT vs PFMT with audiocassette | 2 | 157 | 162, 163 |
| Strength and motor relearning PFMT vs motor relearning PFMT | 1 | 128 | 164 |
| PFMT in supine position vs PFMT in supine and upright position | 1 | 44 | 165 |
| PFMT vs modified pilates | 1 | 11 | 166 |
| PFMT (maximal contraction) + BF vs PFMT (submaximal contraction) + BF | 1 | 37 | 167 |
| PFMT + perineometer vs PFMT + urethral electrical conductance | 1 | 34 | 168 |
| PFMT + BF (vaginal) vs PFMT + BF (vaginal and abdominal) | 1 | 38 | 169 |
| PFMT + BF vs PFMT + ES | 2 | 90 | 170, 171 |
| Comparison of different variants of ES | |||
| PFMT + BF + ES (faradism) vs PFMT + BF + ES (IFT) | 1 | 30 | 157 |
| PFMT+BF + ES (maximal intensity at clinic) vs PFMT+BF + ES (low intensity at home) | 1 | 49 | 172 |
| Comparison of different variants of VC | |||
| VC passive vs VC active | 1 | 61 | 173 |
| Comparison of different SNRI doses | |||
| SNRI 80 mg vs SNRI 40 mg | 1 | 277 | 144 |
| SNRI 80 mg vs SNRI 20 mg | 1 | 278 | 144 |
| SNRI 40 mg vs SNRI 30 mg | 1 | 59 | 145 |
| SNRI 40 mg vs SNRI 20 mg | 2 | 342 | 144, 145 |
| SNRI 30 mg vs SNRI 20 mg | 1 | 60 | 145 |
| SNRI 40 mg b.i.d., starting with 40 mg b.i.d. vs SNRI 40 mg b.i.d., starting with 40 mg q.d. | 1 | 263 | 138 |
| SNRI 40 mg b.i.d., starting with 40 mg b.i.d. vs SNRI 40 mg b.i.d., starting with 20 mg b.i.d. | 1 | 269 | 138 |
| SNRI 40 mg b.i.d., starting with 40 mg q.d. vs SNRI 40 mg b.i.d., starting with 20 mg b.i.d. | 1 | 260 | 138 |
| Comparison of different treatments (single modality) | |||
| PFMT vs ES | 7 | 222 | 115, 124, 126, 174–177 |
| PFMT vs VC | 6 | 426 | 115, 129, 152, 178–180 |
| PFMT + BF vs VC | 2 | 141 | 152, 181 |
| PFMT vs BT | 1 | 84 | 182 |
| PFMT + BF vs BT | 1 | 137 | 183 |
| PFMT vs SNRI | 1 | 102 | 57 |
| PFMT vs surgery | 2 | 105 | 184, 185 |
| ES vs VC | 4 | 191 | 115, 186–188 |
| Comparison of different treatments (dual modality) | |||
| PFMT vs PFMT + ES | 7 | 473 | 121, 123, 126, 185, 189–191 |
| PFMT + BF vs PFMT + BF + ES | 2 | 115 | 157, 172 |
| PFMT + BF vs PFMT + BF + ES (faradism)a | 1 | 30 | 157 |
| PFMT + BF vs PFMT + BF + ES (IFT)a | 1 | 30 | 157 |
| PFMT + BF vs PFMT + BF + ES (maximal intensity at clinic)a | 1 | 45 | 172 |
| PFMT + BF vs PFMT + BF + ES (low intensity at home)a | 1 | 46 | 172 |
| PFMT vs PFMT + VC | 1 | 46 | 192 |
| PFMT vs PFMT + SNRI | 1 | 102 | 57 |
| PFMT + BF vs PFMT + BF + BT | 1 | 136 | 183 |
| PFMT + ES vs ES | 1 | 22 | 126 |
| PFMT + VC vs VC | 1 | 42 | 188 |
| PFMT + BF + BT vs BT | 1 | 135 | 183 |
| PFMT + SNRI vs SNRI | 1 | 104 | 57 |
| Comparisons considered not relevant for direct head-to-head comparisonsb | |||
| PFMT vs PFMT + BF + ES (faradism) | 1 | 30 | 157 |
| PFMT vs PFMT + BF + ES (IFT) | 1 | 30 | 157 |
| PFMT + ES vs VC | 1 | 60 | 193 |
| PFMT + ES vs surgery | 1 | 54 | 185 |
| PFMT + VC vs ES | 1 | 41 | 188 |
| PFMT + BF + VC vs ES | 1 | 46 | 132 |
| PFMT + SNRI vs surgery | 1 | 197 | 194 |
| PFMT + ES + BF vs VC | 1 | 120 | 195 |
| PFMT + ES + BF vs PFMT + VC | 1 | 102 | 196 |
| Childbearing women only | |||
| PFMT, VC or both vs NT | 1 | 230 | 197 |
| PFMT vs NT | 1 | 264 | 198 |
| PFMT vs PFMT + abdominal training vs NT | 1 | 68 | 199 |
Excluded studies, with reasons for specific exclusions
A total of 199 papers were obtained but did not meet the inclusion criteria (Figure 7). Of these, 113 were excluded on the basis of study design, populations, interventions or outcomes. A further 86 papers (describing 56 studies) that did include relevant populations or interventions as part of the study were retained for further assessment and subsequently excluded through the consensus within the research team that it was impossible to attribute an effect to a particular intervention for women with SUI. Reasons for exclusions for the 86 papers excluded on further assessment are described in more detail in Appendix 7.
FIGURE 7.
Flow chart for study selection.

Ongoing studies
A list of ongoing trials is provided in Appendix 8.
Risk of bias in included studies
A summary of the assessment of risk of bias for the 88 included RCTs is presented in Table 11, and a detailed assessment for each of the included studies is reported in Appendix 9.
| Criteria | Yes (n, %) | Unclear (n, %) | No (n, %) |
|---|---|---|---|
| 1. Was the allocation sequence adequately generated? | 30 (34.1) | 53 (60.2) | 5 (5.7) |
| 2. Was allocation adequately concealed? | 16 (18.2) | 63 (71.6) | 9 (10.2) |
| 3. Were participants ‘blind’ to treatment status? | 19 (21.6) | 56 (63.6) | 13 (14.8) |
| 4. Were health-care providers ‘blind’ to treatment status? | 6 (6.8) | 63 (71.6) | 19 (21.6) |
| 5. Were outcome assessors ‘blind’ to treatment status? | 17 (19.3) | 65 (73.9) | 6 (6.8) |
| 6. Were the groups treated identically other than for the named intervention? | 86 (97.7) | 0 (0.0) | 2 (2.3) |
| 7. Was there a description of withdrawals, dropouts and those lost to follow-up? | 33 (37.5) | 33 (37.5) | 22 (25.0) |
| 8. Was the analysis on intention to treat? That is: | |||
| (a) Were trial results reported for everyone who entered the trial? | 18 (20.5) | 24 (27.3) | 46 (52.3) |
| (b) Were participants analysed in the groups they were originally allocated to? | 75 (85.2) | 13 (14.8) | 0 (0.0) |
Fourteen studies (15.9%) reported both adequate random allocation sequence generation and concealment. 57,115,117,129,137,139–141,143,144,164,181,182,199 A further two studies reported adequate allocation concealment, although the method of allocation sequence generation was not clear. 133,153 Five studies reported inadequate methods of sequence generation and allocation concealment, namely consecutive/alternate assignment,157,162,190 assignment based on hospital casenote number116 or assignment based on time of arrival at the clinic and severity of incontinence. 127 Allocation concealment was also considered to be suboptimal (sealed envelopes with no indication of third party involvement) in a further five studies. 120,147,150,172,183 The remainder (n = 47, 53.4%) did not describe the methods used in sufficient detail to assess proneness to selection bias related to random allocation sequence generation and concealment.
The majority of studies did not clearly stipulate whether participants, health-care providers or outcome assessors were ‘blinded’ to participants’ treatment status. For example, some studies would state ‘double blind’ but would not detail whether this referred to participants, health-care providers or outcome assessors. In addition, for some treatments (e.g. PFMT) it was not considered feasible to blind the health-care providers or participants to group allocation.
Nevertheless, 19 studies (21.6%) reported participant blinding. 57,117,128,130–134,136–145,189 Twelve of these involved drug therapy and used a placebo to blind participants. 57,117,136–145 In the other seven studies, participants were blinded through sham ES,130–134,189 or imitation or placebo PFMT. 57,128 Six studies (6.8%) reported that health-care providers were blinded. 57,133,140,144,183,189 However, in one of these, which was a four-arm trial comparing drug therapy with active or imitation PFMT versus PFMT with active or placebo drug,57 blinding was only possible for the drug treatment aspect of the study and not for PFMT. Blinded outcome assessment should be possible but only 17 studies (19.3%) reported that this was done. 115,121,122,127,128,130,131,133,134,147,153,163,164,166,182,189,199 Based on the available information on blinding, the majority of studies may be considered to be at modest risk of performance and detection bias.
While no study reported that groups had been treated differently in any other way apart from the named intervention, in two studies140,181 it was doubtful whether groups had been treated the same in all other ways. In one of these studies this was due to a large proportion of participants in one arm opting out of the allocated treatment (weighted VCs) and receiving the comparator treatment (PFMT). 181 In the other, participants randomised to drug therapy were permitted to reduce their dosage, suspend their treatment or augment it with other treatments. 140
Thirty-three studies (37.5%) stated numbers and reasons for withdrawals in sufficient detail,57,115–118,123,127,128,131,133,134,137–140,143,146,147,153,156,159,164,166,167,175,178,181,183,187,189,197–199 while another 33 studies (37.5%) stated the number of withdrawals but did not describe reasons for withdrawals. 107,119,121,122,129,130,132,135,141,142,144,145,150,152,154,155,162,163,165,168,172,176,179,180,184,185,188,190–193,196 The remaining 22 studies (25.0%) did not provide sufficient information about withdrawals, dropouts and those lost to follow-up.
The majority of studies (52.3%) failed to report results for everyone who entered into the trial,57,115–119,122,124,129–133,135,137–141,143–145,150,152,154,156,159,162,164,166–168,176,178,180,183,185,187–190,196–199 while in 24 studies (27.3%), it was unclear whether results accounted for all participants originally randomised. 107,120,121,134,136,142,148,153,157,158,160,161,165,169–174,179,186,193–195 Few studies explicitly stated that participants were analysed in the groups that they had been originally allocated to but no study showed clear evidence that this was not done.
Characteristics of included studies
The sample size ranged from 11 to 683, with a total of 9721 participants. 139,166 This includes 9163 non-childbearing women and 558 childbearing women (Table 12). A large proportion of the participants (n = 4197) came from 11 pharmaceutical trials comparing SNRI with placebo. Trials of physical or behavioural interventions for non-childbearing women were generally small; only four trials57,123,129,183 had 200 or more participants and this included one trial57 comparing PFMT with SNRI. Participants’ baseline characteristics are provided in Appendix 10. A brief summary of the baseline characteristics of the participants in the included studies, such as mean age, is also provided in Chapter 7, although it was not possible to summarise severity of incontinence of the participants in the included studies in any meaningful way because this was reported using diverse measures.
| Number of studies | Number of participants | |||
|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | ||
| Lifestyle | 0 | 1 | 0 | 84 |
| Physical/behavioural, ≥ 200 participants per trial | 1 | 2 | 1 | 843 |
| Physical/behavioural, 100–199 participants per trial | 6 | 4 | 1 | 1459 |
| Physical/behavioural, < 100 participants per trial | 56 | 1 | 1 | 2583 |
| Pharmaceutical (SNRI vs placebo)a | 3 | 1 | 7 | 4194 |
| Studies with childbearing women only | 1 | 2 | 0 | 558 |
| Total | 67 | 11 | 10 | 9721 |
The main characteristics of active treatments are summarised in Appendices 11–15. The majority of included studies involved PFMT as part of their interventions. The PFMT programme that was used varied in a number of ways: for example, whether a correct pelvic floor muscle contraction was confirmed prior to training, the frequency and type (e.g. strength and endurance) of contractions performed per day, the duration of training, the number of clinic sessions provided and whether training was provided individually or in groups. Advice on lifestyle change or strategies for symptoms of urge and/or frequency may also be given. However, without any structured regimen, such advice was considered as part of a broad PFMT programme and not a separate treatment.
Biofeedback may also be provided for the purpose of teaching a voluntary pelvic muscle floor contraction. A single episode of BF in the initial teaching but not thereafter was similarly considered as part of a broad PFMT programme. This excludes BF that was used repeatedly to monitor or assist PFMT, which was classified separately as an adjunct treatment (PFMT with BF). An intravaginal device is also included in the BF comparison.
While it is likely that the success or otherwise of PFMT may be contingent on the level of intensity of the training programme, characterising or categorising PFMT is difficult. We chose supervisory intensity (the frequency of clinic visits or any face-to-face contacts with health-care professionals) as a crude measure of differentiating PFMT programmes. The current guidelines43 of the National Institute for Health and Clinical Excellence (NICE) estimated from the Guidance Development Group members that four to eight sessions were typically offered for PFMT over 3 months (six visits over 3 months or two visits per month). Following this guideline, in this review a PFMT programme with up to two clinic visits per month is considered as PFMT with basic supervisory intensity (or ‘PFMT basic’), and the programme with more than two clinic visits per month is considered as PFMT with intensive supervision (or ‘PFMT extra sessions’) (Appendices 11 and 12). This is regardless of the amount or type of pelvic floor muscle contractions performed per day or the duration and precise nature of supervision; such data were poorly reported and hence could not be incorporated into our analysis.
The comparison group may be another active treatment or no treatment. No treatment in this review is equivalent to no active treatment. Leaflet-only PFMT, which was not taught by a health-care professional, is also considered as ‘no treatment’, based on the assumption that the distribution of guidelines has a small effect on behaviour200 and also that patients are not naive and can obtain knowledge of PFMT from anywhere (e.g. internet). Within the 30 studies of non-childbearing women classified as having a ‘no-treatment’ arm, participants received no treatment,107,118,120–122,124,125,135 advice on continence device or pads,115,127 sham ES,126,130–134 placebo PFMT,128 imitation PFMT with placebo drug,57 placebo drug alone,117,136–142,144,145 a booklet including the entire training programme for self-administration123 or an educational leaflet, encouragement to keep an exercise diary and an equal number of clinic visits to the comparator arm. 129 Of the three studies of childbearing women, one199 provided a relaxation massage, whereas the other two197,198 provided standard or routine care for the ‘no-treatment’ arm.
The treatment protocol for ES varied widely between studies. A common problem associated with ES is that there is no consistency in the criteria used to describe ES. 201 Treatment could be described on the basis of the type of current being used (e.g. faradic stimulation, interferential therapy), the structure being targeted (e.g. neuromuscular ES), or the current intensity, etc. No attempt was made in this review to categorise different treatment protocols.
The VCs treatment was relatively homogeneous and so was SNRI drug therapy, which was based on duloxetine. Nevertheless, one study by Kinchen and colleagues140 was a ‘naturalistic’ study, for which participants could, at any point after randomisation, choose to remain on SNRI as randomised, reduce drug doses, add other treatments to SNRI, or suspend SNRI and receive other treatments. The additional treatments used by the study participants included estrogen products, anticholinergic medications and PFMT. There was differential withdrawal due to adverse events between SNRI and placebo groups in all 10 studies with available data (see Chapter 7, Serotonin–noradrenaline reuptake inhibitors drug therapy). 117,137–145 Nine studies117,136–139,142–145 stated that they had either received sponsorship, funding or support from the manufacturer of duloxetine (Eli Lilly and Company).
Chapter 7 Assessment of clinical effectiveness
This chapter describes the results from direct pairwise (head-to-head) comparisons. Results are grouped into three sections: comparison with no treatment and variation within comparators, comparison between different treatments (single modality), and comparison between different treatments (dual modality).
A summary of the baseline characteristics of the participants and interventions in the included studies is provided at the beginning of each section. This is described in more detail in Appendix 10.
The focus is on the primary outcome data (cure, improvement, adverse events and quality of life) and, in particular, those that are relevant to the MTC analysis in the following chapter (Chapter 7). There was a great variability in the way cure and improvement was defined by the trialists. This is detailed in Appendix 16. As noted in Chapter 5 (see Types of outcome measures), we chose to substitute woman’s observation (measurement type 1) with quantified symptoms or clinician’s observation (measurement type 2), if the former was missing. The type of outcome measurement for each study is shown on the right hand side of the quantitative synthesis figure (measurement type – see Box 8). For quantitative synthesis, data at the end of the prescribed treatment phase, or at the first outcome assessment, if later, were used. Data from further follow-ups and other relevant primary outcome data are reported in the text and also in Appendix 17.
-
Women’s observation
-
Quantification of symptoms or clinician’s observation
-
All women had SUI alone
-
≥ 50% of women had SUI alone; the remainder could have UUI or MUI
-
≥ 50% of women had MUI with stress symptoms as a predominant pattern; the remainder could have SUI, UUI or MUI
Studies with different population types are analysed together. The type of population included in each trial is also shown on the right hand side of the figure (population type – Box 8). The quantitative analysis for cure and improvement rates was also performed with studies that included women diagnosed as having SUI only (population type 1); the results are presented in Appendix 18. Quantitative data syntheses for condition-specific quality of life were limited due to lack of available data; these are given in Appendix 19. Studies of childbearing women were analysed separately and provided in Appendix 20. Secondary outcome data of all relevant comparisons are reported in Appendix 21.
Comparison with no treatment and variation within comparators
Lifestyle change
No studies were found comparing lifestyle change with no treatment. The only relevant study119 was a crossover trial evaluating the effect of increasing or decreasing fluid intake on urinary symptoms in women (Table 13). Women were randomised in the order in which they increased or decreased fluids. All women were instructed to restrict caffeine. No information was available on any of the specified outcomes.
PFMT with and without BF
PFMT, with or without BF, vs no treatment
The characteristics of included studies comparing PFMT (with and without BF) and no treatment are summarised in Table 14. Fourteen studies were eligible for this comparison, in which a total of 958 participants were randomised. Studies varied widely in terms of the duration of treatment prescribed by the trialists, as well as supervisory intensity (measured in terms of the number of clinic visits or any face-to-face contacts with a health-care professional). No studies reported data on further follow-up after the end of the prescribed treatment phase. The comparison groups received no treatment,107,118,120–122,124,125 advice on continence device or pads,115,127 sham ES,126 placebo PFMT,128 imitation PFMT with placebo drug,57 a booklet including the entire behavioural training programme for self-administration123 or an educational leaflet, the equal number of clinic visits as the PFMT arm and encouragement to keep an exercise diary. 129
| Study ID | Population type | Duration (month) | Comparator | N randomised | Age | Supervisory intensitya | Notes |
|---|---|---|---|---|---|---|---|
| Aksac 2003120 | 1 | 2 | PFMT | 20 | 52.5 | Intensive | |
| PFMT + BF | 20 | 51.6 | Intensive | ||||
| NT | 10 | 54.7 | None | ||||
| Bidmead 2002121 | 1 | 3.5 | PFMT | 40b | 46.2 | Basic | |
| PFMT + sham ES | 42b | 51.5 | Basic | ||||
| NT | 20b | 47.5 | None | ||||
| Bø 1999115 | 1 | 6 | PFMT | 29 | 49.6 | Intensive | |
| NT | 32 | 51.7 | None | Advice on Continence Guard™ | |||
| Burns 1993122 | 2 | 2 | PFMT | 43c | 63.0 | Intensive | |
| PFMT + BF | 40c | 63.0 | Intensive | ||||
| NT | 40c | 63.0 | None | ||||
| Ghoniem 200557 | 1 | 3 | PFMT | 50 | 54.0 | Basic | Placebo drug |
| NT | 47 | 51.0 | Basic | Placebo drug and imitation PFMT | |||
| Goode 2003123 | 3 | 2 | PFMT | 66 | 57.7 | Basic | ‘Behavioural training’ |
| NT | 67 | 55.9 | None | Self-administered behavioural training | |||
| Henalla 1989124 | 1 | 3 | PFMT | 26 | NR | Intensive | |
| NT | 25 | NR | None | ||||
| Henalla 1990125 | 1 | 1.5 | PFMT | 8 | 54 | NR | |
| NT | 7 | NR | |||||
| Hofbauer 1990126 | 1 | 1.5 | PFMT | 11 | 51.0 | Intensive | |
| NT | 10 | 59.8 | Intensive | Sham ES | |||
| Kim 2007118 | 1 | 3 | PFMT | 35 | 76.6 | Intensive | |
| NT | 35 | 76.6 | None | ||||
| Lagro-Janssen 1991127 | 1 | 3 | PFMT | 33 | 46.1 | Basic | |
| NT | 33 | 44.6 | None | Advice on continence pads | |||
| Miller 1998107 | 1 | 0.25 | PFMT | 13 | 68.4 | Intensive | The Knack |
| NT | 14 | None | |||||
| Ramsay 1990128 | 1 | 3 | PFMT | 22 | NR | NR | |
| NT | 22 | NR | NR | Placebo PFMT | |||
| Williams 2006129 | 2 | 3 | PFMT | 79 | 55.9 | Basic | |
| NT | 79 | 56.7 | Basic | Leaflet, clinic visits and exercise diary |
Pelvic floor muscle training plus sham ES was classified as being equivalent to PFMT. Where both PFMT and PFMT plus sham ES were present in a trial,121 the dichotomous data were combined (added up).
Cure and improvement rate
Figures 8 and 9 show the number of women who were either cured or improved, respectively, in the PFMT group (with or without BF) versus the no-treatment group at the end of the prescribed treatment phase. Pooled data for cure rates showed higher cure rates for PFMT with or without BF, but with significant heterogeneity (Figure 8: PFMT vs no treatment – 23% vs 7%, OR 5.41, 95% CI 1.64 to 17.82; PFMT with BF vs no treatment – 42% vs 2%, OR 21.54, 95% CI 3.65 to 126.98). The source of the heterogeneity appeared to be the inclusion of studies with women with SUI with or without UUI symptoms (population types 2 or 3). In these studies the direction and magnitude of effect varied across studies. Of note is that the two studies reporting the lowest effect size123,129 not only included women with different types of urinary incontinence, but also had the largest sample size, less intensive supervision (fewer clinic visits) and relatively substantial provision of care for the no-treatment group (e.g. self-administered behavioural training, clinic visits), which may have contributed to the relatively small effect size.
FIGURE 8.
Cure rates for pelvic floor muscle training, with or without biofeedback, versus no training.

FIGURE 9.
Improvement rates for pelvic floor muscle training, with or without biofeedback, versus no training.
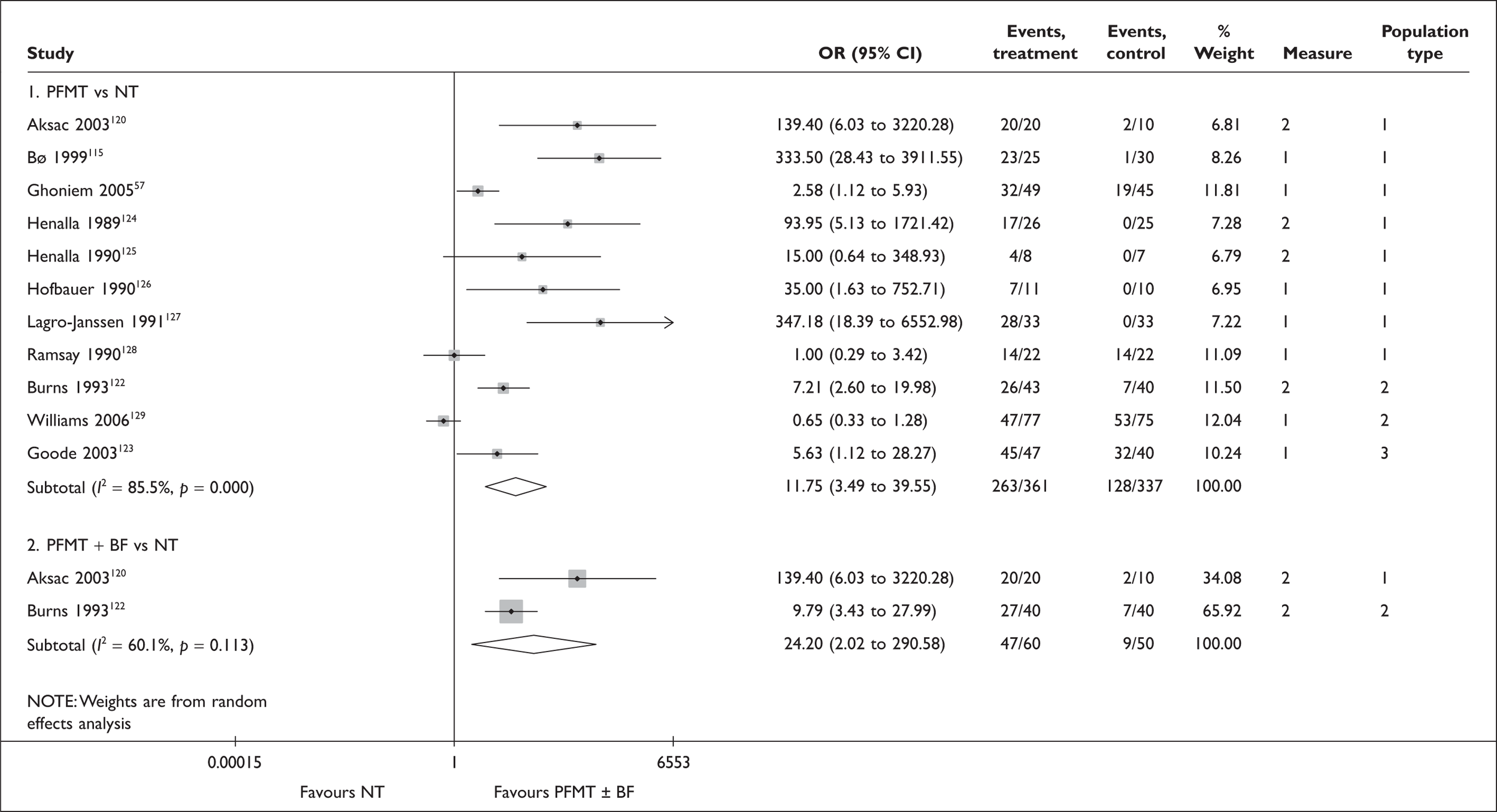
Removing these studies reduced the statistical heterogeneity but also widened the CI (as fewer data were available for meta-analysis) (Appendix 18, Comparison 01: PFMT vs no treatment – 39% vs 3%, OR 15.15, 95% CI 5.50 to 41.75; PFMT with BF vs no treatment – 80% vs 0%, OR 77.00, 95% CI 3.75 to 1581.71).
Results for improvement rates similarly favoured PFMT with or without BF compared with no treatment, though statistical heterogeneity was again evident across studies (Figure 9: no BF, OR 11.75, 95% CI 3.49 to 39.55; with BF, OR 24.20, 95% CI 2.02 to 290.58). One additional cause of heterogeneity for this outcome was the greater variability in the way ‘improvement’ was defined between studies, as the heterogeneity remained even after removing studies with mixed diagnoses (Appendix 18, Comparison 02: OR 27.07, 95 CI 4.72 to 155.35). Crucially, the smallest effects were found in those studies with placebo or imitation PFMT57,128 in the no-treatment arm and with women with mixed types of urinary incontinence. 123,129
Adverse events
Adverse events were uncommon in the PFMT group (Table 15). Nevertheless, two studies127,129 reported that up to 12% of women experienced adverse events during PFMT.
Quality of life
Condition-specific quality of life was reported using various measures, including the Social Activity Index, the Urinary Incontinence Quality of Life (I-QoL) scale, the Leicester Impact Scale, the Incontinence Impact Questionnaire (IIQ) and the Bristol Female Lower Urinary Tract Symptoms (B-FLUTS) (Appendix 19, Comparison 01). In all but two trials,123,129 results were better for the PFMT group (with or without BF). One study115 reported this outcome using two instruments (Social Activity Index and B-FLUTS), with results consistently favouring PFMT. As the outcome measures varied between studies and not all studies reported data amenable to meta-analysis, quantitative synthesis was not performed.
Two studies115,123 reported general health-related quality of life (HRQoL) using SF-36 and the Norwegian version of the Quality of Life Scale (QoLS-N). One of these studies115 reported higher quality of life for the PFMT group, whereas the other123 reported no statistically significant difference between the groups (Appendix 17, Comparison 01).
PFMT vs PFMT plus BF
Fifteen studies120,122,146–158 evaluated the effect of adding BF to PFMT (Table 16). In two studies by Glavind and colleagues150 and Wilson and colleagues,157 the women randomised to use BF had more clinic visits than the PFMT group. There was also a potential difference in supervisory intensity in a further study by Pages and colleagues,154 in which the PFMT group had group therapy for 60 minutes, five times a week, whereas the BF group had individual therapy for 15 minutes, five times a week.
In addition to devices specifically designed to provide visual and/or audio BF, the use of intravaginal resistance device,149 an endotrainer151 and an exerciser155 were also classified as BF for the purpose of this review.
| Study ID | Population type | Duration (month) | Comparator | N randomised | Age | Supervisory intensitya | Notes |
|---|---|---|---|---|---|---|---|
| Aksac 2003120 | 1 | 2 | PFMT | 20 | 52.5 | Intensive | |
| PFMT + BF | 20 | 51.6 | Intensive | ||||
| Aukee 2002146 | 1 | 3 | PFMT | 15 | 50.8 | Basic | |
| PFMT + BF | 15 | 51.8 | Basic | Home BF | |||
| Berghmans 1996147 | 1 | 1 | PFMT | 20 | 50.4 | Intensive | |
| PFMT + BF | 20 | 46.4 | Intensive | ||||
| Burns 1993122 | 2 | 2 | PFMT | 43 | 63.0 | Intensive | |
| PFMT + BF | 40 | 63.0 | Intensive | Clinic BF | |||
| Castleden 1984148 | 1 | 1 | PFMT | 19 (crossover) | 55.0 | NR | |
| PFMT + BF | 19 (crossover) | NR | Home BF | ||||
| Ferguson 1990149 | 1 | 1.5 | PFMT | 10 | 35.8 | Basic | |
| PFMT + BF | 10 | 37.1 | Basic | IVRD | |||
| Glavind 1996150 | 1 | 3b | PFMT | 20 | 45.0 | Basic | |
| PFMT + BF | 20 | Basic | Additional visits for clinic BF | ||||
| Klingler 1995151 | 1 | 3 | PFMT | 21 | 53.0 | Intensive | |
| PFMT + BF | 20 | 51.8 | Intensive | Endotrainer | |||
| Laycock 2001152 | 1 | 3 | PFMT | 20 | NR | Basic | |
| PFMT + BF | 40 | NR | Basic | Home BF | |||
| Mørkved 2002153 | 2 | 6 | PFMT | 50 | 45.4 | Intensive | |
| PFMT + BF | 53 | 47.8 | Intensive | Home + clinic BF | |||
| Pages 2001154 | 1 | 3 | PFMT | 27 | 51.1 | Intensive | Group therapy |
| PFMT + BF | 24 | Intensive | Individual therapy; clinic BF | ||||
| Shepherd 1983155 | 1 | 4.5c | PFMT | 11 | 48.4 | Basic | |
| PFMT + BF | 11 | 48.2 | Basic | Exerciser | |||
| Taylor 1986156 | 1 | 2.25 | PFMT | 13 (number in each group unclear) | NR | Intensive | |
| PFMT + BF | NR | Intensive | Clinic BF | ||||
| PFMT + BF | NR | Intensive | Clinic + home BF | ||||
| PFMT + BF | NR | Intensive | Home BF without vaginal sensor to be used as a resistive device | ||||
| Wilson 1987157 | 1 | 1.5 | PFMT | 15 | 46.8 | Basic | |
| PFMT + BF | 15 | Intensive | Clinic BF | ||||
| Wong 1997a158 | 1 | 2 | PFMT | 7 | 48.2 | Intensive | |
| PFMT + BF | 10 | Intensive |
Cure and improvement rate
Pooled data showed that at the end of the prescribed treatment phase the addition of BF to PFMT resulted in significantly higher cure and improvements than with PFMT alone (Figures 10 and 11: cure rates 34% vs 49%, OR 0.48, 95% CI 0.30 to 0.77; improvement rates 76% vs 86%, OR 0.41, 95% CI 0.18 to 0.97). Among the studies showing the largest effect size were studies by Glavind and colleagues150 and Wilson and colleagues,157 which had additional supervisory visits in the BF group relative to the PFMT group. The study by Pages and colleagues,154 which was also a trial with a potential difference in supervisory intensity, also suggested a large treatment effect in the cure data.
FIGURE 10.
Cure rates: pelvic floor muscle training (PFMT) versus PFMT plus biofeedback.

FIGURE 11.
Improvement rates: pelvic floor muscle training (PFMT) versus PFMT plus biofeedback.

Two studies150,157 conducted a further follow-up after the end of the supervised treatment in which all women were advised to continue PFMT at home without BF or any close supervision by a health-care professional. The first study157 with 6-month follow-up (4.5 months after the end of 6-week treatment) reported that women who did not use BF in the treatment phase were significantly less likely to improve than those who trained with BF (Appendix 17, Comparison 02, 4/15 vs 9/14, OR 0.20, 95% CI 0.04 to 0.98). In the second study150 with 2.5 years of follow-up, the results for cure and improvement also favoured the BF group, although the differences were not statistically significant (Appendix 17, Comparison 02, cure 0/14 vs 5/19, OR 0.09, 95% CI 0.01 to 1.80; improvement 4/14 vs 8/19, OR 0.55, 95% CI 0.13 to 2.40).
Adverse events
Adverse events were uncommon but in two studies146,153 that did report incidents, more participants (15–27%) experienced adverse events if they were using BF. Some women found the device unpleasant or painful (Table 17).
| PFMT | PFMT + BF | Notes | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Aukee 2002146 | 3/15 | 20 | 4/15 | 27 | Pain while training (of which 3/7 premenopausal) | 1 |
| Mørkved 2002153 | 3/46 | 7 | 7/48 | 15 | PFMT + BF: 7/48 found use of apparatus ‘unpleasant’; PFMT: 3/46 found PFMT itself ‘unpleasant’; ‘However, they all followed the training protocol in spite of this’ | 2 |
| PFMT | PFMT + BF | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| Social Activity Index | |||||||
| Aksac 2003120 | 20 | 7.5 (1.2) | 20 | 8.1 (0.8) | Score (median, SD) | 1 | |
| Mørkved 2002153 | 34 | 9.5 (0.74) | 36 | 9.6 (0.61) | Score (mean, SD) at 6 months | 1 | |
| Modified PRAFAB | |||||||
| aBerghmans 1996147 | 20 | 13.1 (8.6) | 20 | 11.1 (5.9) | NS | Score (mean, SD) | 1 |
| King’s Health Questionnaire | |||||||
| Laycock 2001152 | 16 | 8.13 (9.06) | 22 | 6.14 (6.20) | NS | Change in score (mean increase, SD) | 1 |
| Incontinence Impact Questionnaire | |||||||
| Wong 1997a158 | 7 | 24.5 (10.8) | 10 | 8.5 (19.9) | < 0.05 | Change in score (mean reduction, SD) | 1 |
Quality of life
Five studies120,147,152,153,158 reported condition-specific quality of life using four different measures (Table 17). In four of these studies results were similar for both groups, but in one study,158 women reported statistically significantly better quality of life in the PFMT group than with those using BF. Quantitative synthesis did not demonstrate a statistically significant difference between the groups (Appendix 19, Comparison 01, SMD for total score, –0.29, 95% CI –0.62 to 0.03; SMD for change in score, 0.49, 95% CI –0.14 to 1.12).
PFMT vs PFMT with additional sessions
The characteristics of included studies comparing PFMT with and without additional supervisory clinical sessions are summarised in Table 18.
| Study ID | Population type | Duration (month) | Comparator | N randomised | Age | Supervisory intensitya |
|---|---|---|---|---|---|---|
| Bø 1990159 | 1 | 6 | PFMT | 31 | 45.9 | Basic |
| PFMT with additional sessions | 26 | 44.9 | Intensive | |||
| Konstantinidou 2007116 | 1 | 3 | PFMT | 15 | 47.8 | Basic |
| PFMT with additional sessions | 15 | Intensive | ||||
| Wong 1997b160 | 1 | 1 | PFMT | 26 | 48.8 | Basic |
| PFMT with additional sessions | 21 | Intensive | ||||
| Zanetti 2007161 | 1 | 3 | PFMT | 21 | 54.0 | Basic |
| PFMT with additional sessions | 23 | 56.0 | Intensive |
Cure and improvement rate
Cure and improvement rates were consistently higher for women who received additional supervisory sessions (Figures 12 and 13: cure rate, 15% vs 43%, OR 0.11, 95% CI 0.03 to 0.43; improvement rate, 54% vs 97%, OR 0.05, 95% CI 0.01 to 0.28).
FIGURE 12.
Cure rates: pelvic floor muscle training (PFMT) versus PFMT with additional sessions.

FIGURE 13.
Improvement rates: pelvic floor muscle training (PFMT) versus PFMT with additional sessions.

One small study159 with 15 years of follow-up also reported that the number of women who remained continent (cured) after they were left to continue PFMT on their own was higher in the group who had additional sessions during the initial supervised treatment phase, although this difference was not statistically significant (Appendix 17, Comparison 03, 16% vs 30%, OR 0.44, 95% CI 0.11 to 1.87).
Adverse events
No studies reported any adverse events.
Quality of life
Condition-specific quality of life measures varied, but all studies consistently reported that PFMT with additional sessions was associated with better quality of life (Table 19). Two studies116,159 provided data amenable to quantitative synthesis; results showed a statistically significant difference between the groups favouring PFMT with additional sessions (Appendix 19, Comparison 02, SMD –1.07, 95% CI –1.98 to –0.15).
| PFMT | PFMT + additional sessions | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| Social Activity Index | |||||||
| Bø 1990159 | 29 | 8.2 (2.06) | 23 | 9.3 (0.73) | < 0.01 | Sum score (mean, SD) at 6 months | 1 |
| Quality-of-life index | |||||||
| aKonstantinidou 2007116 | 10 | 3.6 (1.5) | 12 | 1.7 (0.8) | 0.000 | Score (mean, SD) | 1 |
| Incontinence Quality of Life | |||||||
| Zanetti 2007161 | 21 | 79 | 23 | 89 | 0.0456 | Score (median) | 1 |
Comparisons of other PFMT variants
A further 10 studies162–171 compared other variations in the method of delivering PFMT (Table 20). Not all studies collected data for the same outcome and it was therefore not possible to combine results in a meaningful way. The detailed information for comparators is reported in Appendix 17, Comparisons 04–09. Within the data available from the six studies that reported at least one of the specified primary outcomes,164–169 there was insufficient evidence to suggest that any of these variants of PFMT were more effective than the comparator treatment shown in Table 20.
| Study ID | Population type | Duration (months) | Comparator | N randomised | Age | Supervisory intensitya |
|---|---|---|---|---|---|---|
| Borello-France 2006165 | 1 | 2.25–3 | PFMT in supine position | 22 | 51.7 | Intensive |
| PFMT in supine and upright position | 22 | 53.6 | Intensive | |||
| Edwards 2000170 | 1 | 3 | PFMT + BF | 10? | 46 | NR |
| PFMT + ES | 10? | NR | ||||
| Gallo 1997162 | 1 | 1–1.5 | PFMT | 43 | 60 | Basic |
| PFMT with audiocassette | 43 | Basic | ||||
| Hay-Smith 2003164 | 3 | 5 | Strength and motor relearning PFMT | 64 | 48.7 | Basic |
| Motor relearning PFMT | 64 | 48.9 | Basic | |||
| Johnson 2001167 | 1 | 1.5 | PFMT (maximal contraction) + BF | 37 | 49.5 | NR |
| PFMT (submaximal contraction) + BF | 51.0 | NR | ||||
| Mayne 1988168 | 1 | 4 | PFMT + perineometer | 34 | 45.0 | Basic |
| PFMT + urethral electrical conductance | 56.0 | Basic | ||||
| Nygaard 1996163 | 2 | 3 | PFMT | 71 | 53.0 | Basic |
| PFMT with audiocassette | Basic | |||||
| Pohl 2004171 | 1 | 3 | PFMT + BF | 70 | NR | NR |
| PFMT + ES | NR | NR | ||||
| Savage 2005166 | 1 | 3 | PFMT | 5 | 54.6 | Basic |
| Modified pilates | 6 | 48.2 | Basic | |||
| Wong 2001169 | 1 | 3 | PFMT + BF (vaginal) | 19 | 47.6 | Basic |
| PFMT + BF (vaginal and abdominal) | 19 | 44.4 | Basic |
Other physical and behavioural interventions
The characteristics of included studies comparing physical or behavioural interventions other than PFMT (with and without BF) are summarised in Table 21.
| Study ID | Population type | Duration (month) | Comparator | N randomised | Age | Supervisory intensitya | Notes |
|---|---|---|---|---|---|---|---|
| Bø 1999115 | 1 | 6 | ES | 32 | 47.2 | Basic | Maximum intermittent vaginal stimulation |
| VC | 29 | 49.2 | Basic | ||||
| NT | 32 | 51.7 | None | ||||
| Brubaker 1997130 | 2 | 2 | ES | 148 | 56.0 | Basic | Transvaginal stimulation |
| NT | 57.7 | None | Sham ES | ||||
| Burton 1993173 | 1 | NR | VC passive | 31 | NR | NR | VC in static position |
| VC active | 30 | NR | NR | VC while doing activities | |||
| Fantl 1991135 | 2 | 1.5 | BT | 65 | 66.0 | Intensive | |
| NT | 66 | 68.0 | None | ||||
| Henalla 1989124 | 1 | 3 | ES | 25 | NR | Intensive | IFT |
| NT | 25 | NR | None | ||||
| Hofbauer 1990126 | 1 | 1.5 | ES | 11 | 59.7 | Intensive | Faradic |
| NT | 10 | 59.8 | Intensive | Sham ES | |||
| Jeyaseelan 2000131 | 1 | 2 | ES | 14 | NR | NR | Patterned neuromuscular stimulation |
| NT | 13 | NR | NR | Sham ES | |||
| Knight 1998172 | 1 | 6 | PFMT+BF + ES (max) | 24 | NR | Intensive | ES = maximal stimulation at clinic |
| PFMT+BF + ES (low) | 25 | NR | Intensive | ES = overnight at low intensity at home | |||
| Laycock Trial 2 1993132 | 1 | 2–3 | ES | 15 | 43.7 | Intensive | IFT |
| NT | 15 | 46.2 | Intensive | Sham ES | |||
| Luber 1997133 | 1 | 3 | ES | 26 | 54.1 | Basic | |
| NT | 28 | 53.6 | Basic | Sham ES | |||
| Sand 1995134 | 1 | 3 | ES | 35 | 50.9 | Basic | |
| NT | 17 | 57.7 | Basic | Sham ES | |||
| Williams 2006129 | 2 | 3 | VC | 80 | 58.2 | Basic | |
| NT | 79 | 56.7 | Basic | Leaflet, clinic visits and exercise diary | |||
| Wilson 1987157 | 1 | 1.5 | PFMT + BF + ES (faradism) | 15 | 46.8 | Intensive | |
| PFMT + BF + ES (IFT) | 15 | Intensive |
Electrical stimulation vs no treatment
Eight studies115,124,126,130–134 compared ES with no active treatment. Five studies provided treatment by means of a device for home use, and compared it with either sham treatment130,131,133,134 or no treatment. 115 The other studies provided treatment at clinic, compared with sham treatment126,132 or no treatment. 124
Cure and improvement rate
Pooled data showed no statistically significant difference between the groups in the cure rate (6% vs 6%, OR 1.10, 95% CI 0.41 to 2.94), but ES showed a significantly higher rate for improvement (37% vs 13%, OR 3.93, 95% CI 1.43 to 10.80) compared with no active treatment, although there was some evidence of heterogeneity (Figures 14 and 15). The source of heterogeneity is unclear but it appears to be caused by the study by Bø and colleagues. 115 Removal of this study reduced statistical heterogeneity and the difference between the groups was still statistically significant (OR 2.46, 95% CI 1.14 to 5.30, figure not shown).
FIGURE 14.
Cure rates: electrical stimulation versus no treatment.
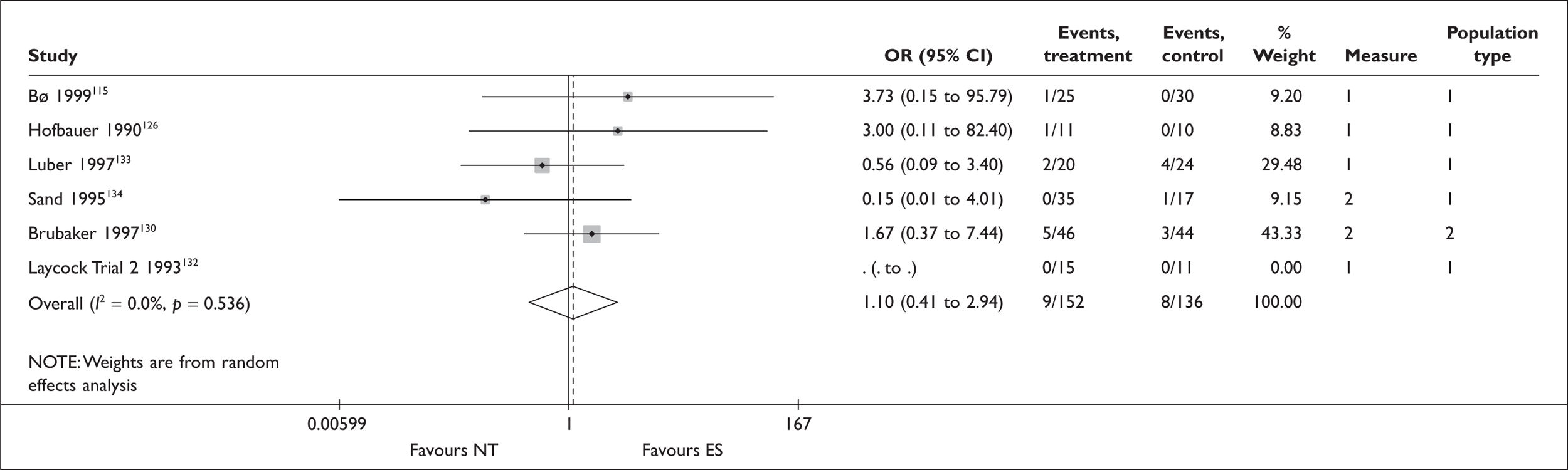
FIGURE 15.
Improvement rates: electrical stimulation versus no treatment.

Adverse events
Only two studies115,134 reported any incidence of adverse events (Table 22). All recorded cases were attributed to the treatment device, whether it was used for active or sham ES. In the study by Bø and colleagues,115 seven of the 32 participants in the ES group stopped treatment due to adverse events.
| ES | NT | Type of AEs | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| N experiencing AEs | ||||||
| Bø 1999115 | 10/32 | 31 | 0/32 | 0 | Smarting (tenderness, bleeding, discomfort), motivation problem, difficulty in using the stimulator | 1 |
| Sand 1995134 | 14/35 | 40 | 7/17 | 41 | All cases added up, although unclear if the same patient experienced more than one adverse event; vaginal irritation: ES 5/35, NT 2/17; occasional pain: ES 3/35, NT 1/17; vaginal infection: ES 4/35, NT 2/17; urinary tract infection: ES 1/35, NT 2/17 | 1 |
| ES | NT | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| Social Activity Index | |||||||
| Bø 1999115 | 25 | 0.6 (1.02) | 30 | –0.2 (1.68) | Change in score (mean, SD) | 1 | |
| Incontinence Impact Questionnaire | |||||||
| aJeyaseelan 2000131 | 12 | –4.1 (16.4) | 12 | –9.1 (17.1) | NS | Change in score (mean, SD) | 1 |
| Urogenital Distress Inventory | |||||||
| aJeyaseelan 2000131 | 12 | –11.8 (15.9) | 12 | –3.3 (8.3) | 0.01 | Change in score (mean, SD) | 1 |
Quality of life
Two studies115,131 reported condition-specific quality of life, with one of these studies131 using two instruments (questionnaires). Results were inconsistent across and within studies (Table 22 and Appendix 19, Comparison 03).
There were no statistically significant differences in general HRQoL scores (SF-36) between the groups in two studies (Appendix 17, Comparison 10). 131,134
Comparison of different variants of ES
Two studies157,172 assessed different variants of ESs. One of these studies157 compared faradism and interferential therapy (IFT), both as an adjunct treatment to PFMT with clinic-based BF, for a period of 6 weeks. After this initial supervised treatment phase, all participants (N = 30) continued with PFMT and were followed up for 6 months.
The other study172 compared maximal ES (provided at clinic) with low intensity ES (provided overnight at home), both performed in conjunction with PFMT and a home BF device. Participants (N = 49) received these treatments with supervision for 6 months and were then instructed to perform PFMT with BF for a further 6 months.
Results showed that improvement was more likely for faradism and clinic-based maximal stimulation than for interferential therapy and home-based low-intensity stimulation, respectively, both after the supervised treatment phase and at 6 months after the end of the supervised phase (Appendix 17, Comparisons 11–12). However, CIs were wide and did not rule out clinically important differences that could favour either treatment. No information was available on cure, adverse events or quality of life.
VCs vs no treatment
Cure and improvement rate
Two studies115,129 comparing VCs with no treatment reported conflicting data on improvement (Figure 16). This may stem from a range of factors such as difference in study populations (SUI with or without UUI symptoms) and sample size, duration of treatment and supervisory intensity. The intervention for the ‘no-treatment’ group also differed, with one study by Bø and colleagues115 offering instructions on a disposable vaginal device (Continence Guard™) only, whereas the other study by Williams and colleagues129 provided the same number of clinic visits as the treatment group, with leaflets giving advice on the pelvic floor muscles. Cure rates were not reported.
FIGURE 16.
Improvement rates: vaginal cones versus no treatment.

Adverse events
In one study115 62% (18/29) of the women who used weighted VCs reported abdominal pain and vaginitis, as well as difficulty in using cones and motivational problems. In the other study,129 3% (2/80) in the cones group reported urinary infection. No adverse events were reported for women receiving no treatment (Table 23).
| VC | NT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Bø 1999115 | 18/29 | 62 | 0/32 | 0 | Abdominal pain, vaginitis, bleeding, motivation problems, trouble in using the cones | 1 |
| Williams 2006129 | 2/80 | 3 | 0/79 | 0 | Urinary tract infection | 2 |
| VC | NT | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| Social Activity Index | |||||||
| Bø 1999115 | 27 | 0.1 (1.06) | 30 | –0.2 (1.68) | Change in score (mean, SD) | 1 | |
| The Leicester Impact Scale | |||||||
| aWilliams 2006129 | 79 | 2 (0.0 to 5.0) | 75 | 1.5 (0.0 to 5.0) | 0.658 | Score (0–42, median, interquartile range) | 2 |
Quality of life
There was no evidence of a difference between the groups in either study (Table 23).
Comparison of different variants of VC
One study173 of 61 women with SUI compared VCs used in a static position (‘passive cones’) with VCs used while doing activities that previously made them incontinent (‘active cones’). The results showed a slightly higher cure rate for the active cones (Appendix 17, Comparison 13, 58% vs 70%, OR 0.59, 95% CI 0.21 to 1.71).
BT vs no treatment
Only one study135 compared BT with no treatment among women with different types of urinary incontinence (SUI with or without UUI symptoms). The results favoured BT in terms of both cure and improvement, although CIs were wide (Table 24). Condition-specific quality of life similarly favoured BT. The study reported no significant difference by incontinence diagnosis (stress, mixed or urge incontinence) (Table 24).
SNRI drug therapy
The characteristics of included studies comparing SNRI drug therapy (duloxetine) with no treatment are summarised in Table 25.
| Study ID | Population type | Duration (month) | Comparator | N randomised | Age | Supervisory intensity |
|---|---|---|---|---|---|---|
| Bump 2004136 | 1 | 1 | SNRI80 | 34 | NR | NA |
| NT | 31 | NR | NA | |||
| Cardozo 2004137 | 1 | 2 | SNRI80 | 55 | 54.5 | NA |
| NT | 54 | 52.4 | NA | |||
| Castro-Diaz 2007138 | 3 | 2 | SNRI40 b.i.d., starting with 40 b.i.d. | 136 | 53.3 | NA |
| SNRI40 b.i.d., starting with 40 q.d. | 127 | 52.3 | NA | |||
| SNRI40 b.i.d., starting with 20 b.i.d. | 133 | 53.5 | NA | |||
| NT | 120 | 52.7 | NA | |||
| Dmochowski 2003139 | 3 | 3 | SNRI80 | 344 | 52.3 | NA |
| NT | 339 | 53.3 | NA | |||
| Ghoniem 200557 | 1 | 3 | SNRI | 52 | 53.0 | NA |
| NT | 47 | 51.0 | NA | |||
| Kinchen 2005140 | 3 | 3 | SNRI80 | 224 | 52.7 | NA |
| NT | 227 | 53.5 | NA | |||
| Mah 2006141 | 3 | 2 | SNRI80 | 61 | 50.7 | NA |
| NT | 60 | 48.5 | NA | |||
| Manning 2005142 | 3 | 2 | SNRI80 | 306 | NR | NA |
| NT | 311 | NR | NA | |||
| Millard 2004143 | 3 | 3 | SNRI80 | 227 | 53.7 | NA |
| NT | 231 | 52.6 | NA | |||
| Norton 2002144 | 2 | 3 | SNRI80 | 140 | 49.3 | NA |
| SNRI40 | 137 | 49.4 | NA | |||
| SNRI20 | 138 | 49.4 | NA | |||
| NT | 138 | 50.2 | NA | |||
| van Kerrebroeck 2004117 | 3 | 3 | SNRI80 | 247 | 52.0 | NA |
| NT | 247 | 54.0 | NA | |||
| Zinner 1998145 | 1 | 1.5 | SNRI40 | 33a | NR | NA |
| SNRI30 | 26a | NR | NA | |||
| SNRI20 | 34a | NR | NA | |||
| NT | 34a | NR | NA |
SNRI vs no treatment
All but one57 of the 12 trials compared SNRI (duloxetine) with placebo. The other study by Ghoniem and colleagues57 was a four-arm trial comparing duloxetine with placebo, both combined with either active or imitation PFMT. For cure and improvement as well as quality of life, data from two arms were compared (SNRI with imitation PFMT vs placebo with imitation PFMT), while for adverse events, data from all four arms were combined (SNRI with active or imitation PFMT vs placebo with active or imitation PFMT). All but three studies57,136,137 included women with SUI with UUI symptoms (population types 2 and 3).
The majority of studies used a daily dose of 80 mg, although participants in the SNRI group in the Cardozo and colleagues study137 ingested 80 mg daily for 4 weeks, escalating to 120 mg daily for another 4 weeks. Another four-arm trial by Castro-Diaz and colleagues138 varied a starting dose in the first 2 weeks (20 mg twice a day, 40 mg once a day or 40 mg twice a day), with all participants in the SNRI group taking 40 mg twice daily in the subsequent weeks. In this study, cure and improvement rates and quality of life were measured at the end of 8 weeks, whereas adverse events were reported at the end of 4 weeks when all participants had at least 2 weeks of ingesting 40 mg twice a day. In the study by Kinchen and colleagues,140 the participants were allowed to reduce or suspend study drug or use other modalities of treatment (e.g. PFMT) simultaneously with SNRI. One study by Manning and colleagues142 did not specify dosage and this was assumed to be 80 mg per day.
Cure and improvement rate
Cure and improvement rates were consistently higher across studies for SNRI than with placebo, regardless of the dosage (80, 40 or 20 mg per day) (Figures 17 and 18). Pooled data for a daily dosage of 80 mg showed that cure was reported in 11% (67/609) of the participants using SNRI compared with 8% (53/683) for placebo, and improvement was reported in 65% (1254/1939) for SNRI compared with 46% (803/1733) for placebo. The difference between the groups in improvement reached statistical significance (Figure 18: OR 2.02, 95% CI 1.67 to 2.44) but for cure this was not the case. The results also appeared to show a noticeable placebo effect. The study by Cardozo and colleagues137 showed a higher OR for improvement than other studies. The reason for this is unclear, but may partly stem from the fact that the study recruited women with SUI only and also provided a higher drug dosage.
FIGURE 17.
Cure rates: serotonin–noradrenaline reuptake inhibitors versus no treatment.

FIGURE 18.
Improvement rates: serotonin–noradrenaline reuptake inhibitors versus no treatment.

While most studies provided treatment for a period of 2–3 months, one study140 continued treatment for a total of 9 months and provided data on improvement; the results favoured the treatment group, although the difference was not statistically significant (Appendix 17, Comparison 14, 49% vs 41%, OR 1.37, 95% CI 0.93 to 2.01).
A small number of studies using a daily dosage of 40, 30 or 20 mg reported similar results. Pooled data for improvement from two studies144,145 using a daily dosage of 40 and 20 mg showed statistical heterogeneity, which may be partly explained by different sample populations, with one study145 recruiting women with stress incontinence alone and the other144 including women with different types of incontinence.
Adverse events
Table 26 shows the number of participants who experienced any treatment-related adverse events and also lists all adverse events that were defined by trialists as being significant (in terms of quantity rather than severity): the latter is not a list of all adverse events. adverse events were experienced by 52–93% of participants in the SNRI group. 137,138 Notably, 32–64% of participants in the placebo group also reported adverse events. 117,141 Nausea was the most commonly reported adverse event with SNRI. Castro-Diaz and colleagues138 reported that at 4 weeks (when all SNRI-treated participants had had at least 2 weeks’ use of 40 mg twice a day) a low starting dose of 20 mg, twice daily, significantly reduced the incidence of nausea and dizziness compared with other drug regimens.
| SNRI | NT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| N experiencing adverse events | ||||||
| SNRI 80 mg vs NT | ||||||
| Cardozo 2004137 | 43/46 | 93 | 37/52 | 71 | Adverse events that occurred in more than 10% of participants: nausea, constipation, headache, dry mouth, fatigue, dizziness, insomnia, somnolence and vomiting; serious adverse events: cardiovascular (not significantly different in both arms); increasing the dose from 80 mg to 120 mg daily did not increase efficacy or side effects | 1 |
| Ghoniem 200557 | 85/104 | 82 | 58/97 | 60 | Adverse events that were significantly more common with SNRI than with placebo (with PFMT or imitation PFMT): nausea, dizziness, dry mouth, constipation, insomnia, somnolence, aesthesia | 1 |
| Norton 2002144 | 102/140 | 73 | 84/138 | 61 | Adverse events that occurred in ≥ 5% of subjects in any treatment arm: nausea, headache, diarrhoea, constipation, dry mouth, dizziness, insomnia, sinusitis, fatigue, nasopharyngitis | 2 |
| Castro-Diaz 2007138 (40 mg b.i.d. starting dose) | 87/136 | 64 | 53/120 | 44 | Adverse events that occurred in ≥ 2 patients in first 4 weeks: nausea, dry mouth, constipation, somnolence, dizziness, insomnia, fatigue, headache, diarrhoea | 3 |
| Castro-Diaz 2007138 (40 mg q.d. starting dose) | 76/127 | 60 | 53/120 | 44 | As above | 3 |
| Castro-Diaz 2007138 (20 mg b.i.d. starting dose) | 69/133 | 52 | 53/120 | 44 | As above | 3 |
| Dmochowski 2003139 | 255/344 | 74 | 170/339 | 50 | Adverse events significantly more common with SNRI and occurring in ≥ 5% of subjects on SNRI: nausea, fatigue, insomnia, dry mouth, constipation, somnolence, dizziness, headache, diarrhoea | 3 |
| Kinchen 2005140 | 198/224 | 88 | 159/227 | 70 | Adverse events for which there are statistically significant differences between groups: nausea, fatigue, insomnia, dizziness, headache, somnolence, dry mouth, constipation, diarrhoea, vomiting, increased sweating, decreased appetite, anxiety, tremor, decreased libido, lethargy, nightmare, fungal infection | 3 |
| Mah 2006141 | 50/61 | 82 | 19/60 | 32 | Adverse events that occurred in ≥ 5% of the women randomised to the SNRI group or which occurred significantly more often with SNRI than with placebo: nausea, dizziness, anorexia, fatigue, lethargy, abdominal discomfort, somnolence, constipation, headache, dry mouth | 3 |
| Millard 2004143 | 173/227 | 76 | 137/231 | 59 | Adverse events: significantly more common with, and occurring in, ≥ 5% of subjects with SNRI: nausea, headache, insomnia, constipation, dry mouth, dizziness, fatigue, somnolence, anorexia, vomiting, increased sweating, anxiety | 3 |
| van Kerrebroeck 2004117 | 200/247 | 81 | 158/247 | 64 | Adverse events occurring in at least 5% of patients on SNRI or occurring significantly more often with SNRI than placebo: nausea, dry mouth, constipation, fatigue, insomnia, dizziness, headache, increased sweating, vomiting, somnolence, tremor | 3 |
| SNRI 40 mg vs NT | ||||||
| Norton 2002144 | 93/137 | 68 | 84/138 | 61 | Adverse events that occurred in ≥ 5% of subjects in any treatment arm: nausea, headache, diarrhoea, constipation, dry mouth, dizziness, insomnia, sinusitis, fatigue, nasopharyngitis | 2 |
| SNRI 20 mg vs NT | ||||||
| Norton 2002144 | 86/138 | 62 | 84/138 | 61 | Adverse events that occurred in ≥ 5% of subjects in any treatment arm: nausea, headache, diarrhoea, constipation, dry mouth, dizziness, insomnia, sinusitis, fatigue, nasopharyngitis | 2 |
| Discontinuation due to adverse events | ||||||
| SNRI 80 mg vs NT | ||||||
| Cardozo 2004137 | 18/55 | 33 | 3/54 | 6 | 1 | |
| Castro-Diaz 2007138 (40 mg b.i.d. starting dose) | 22/136 | 16 | 7/120 | 6 | 3 | |
| Castro-Diaz 2007138 (40 mg q.d. starting dose) | 15/127 | 12 | 7/120 | 6 | 3 | |
| Castro-Diaz 2007138 (20 mg b.i.d. starting dose) | 10/133 | 8 | 7/120 | 6 | 3 | |
| Dmochowski 2003139 | 83/344 | 24 | 14/339 | 4 | 3 | |
| Ghoniem 200557 | 28/104 | 27 | 8/97 | 8 | 1 | |
| Kinchen 2005140 | 20/224 | 9 | 5/227 | 2 | 3 | |
| Mah 2006141 | 21/61 | 34 | 5/60 | 8 | 3 | |
| Manning 2005142 | 53/306 | 17 | 9/311 | 3 | 3 | |
| Millard 2004143 | 39/227 | 17 | 4/231 | 2 | 3 | |
| Norton 2002144 | 21/140 | 15 | 7/138 | 5 | 2 | |
| van Kerrebroeck2004117 | 53/247 | 21 | 12/247 | 5 | 3 | |
| SNRI 40 mg vs NT | ||||||
| Norton 2002144 | 17/137 | 12 | 7/138 | 5 | 2 | |
| SNRI 20 mg vs NT | ||||||
| Norton 2002144 | 13/138 | 9 | 7/138 | 5 | 2 | |
It should be noted that the discontinuation rate among participants due to adverse events was high, at 8–34% in the SNRI group, compared with 2–8% in the placebo group (Table 26).
Serious adverse events were reported by three studies. One of these studies57 reported one case of rectal bleeding in the SNRI group but this was not attributed to the study drug. The second study144 reported that five subjects had adverse events that required hospitalisation (one event before randomisation, one event in the group taking SNRI 20 mg per day, two events in the group taking SNRI 40 mg per day, and one event in the group taking SNRI 80 mg per day): only one of these events (a rash) was judged by the study authors to be related to the study drug. The third study140 recorded serious adverse events (details not reported) in eight of the 224 participants (16 events) in the SNRI group and seven of the 227 participants (eight events) in the placebo group. Of these, two of the events in the placebo group but none in the SNRI group was considered by the study author to be related to the study drug (active or placebo).
Quality of life
A total of 11 studies provided information on condition-specific quality of life using various outcome measures and drug doses (Table 27). With respect to studies using a daily dosage of 80 mg, all 10 studies favoured SNRI-treated participants. This difference was reported to be statistically significant in all but two studies117,140 that performed a statistical test. One study138 using two outcome measures reported consistent results favouring duloxetine. Only three studies reported data amenable to meta-analysis, with one reporting a total score at the final evaluation,143 and the other two reporting a change in score from baseline to the final evaluation. 137,139 Pooled data from the two studies137,139 reporting a change in score found a statistically significant difference between the groups (Appendix 19, Comparison 04, OR 0.35. 95% CI 0.16 to 0.55).
| SNRI | NT | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| I-QoL | |||||||
| SNRI80 vs NT | |||||||
| Cardozo 2004137 | 52 | 10.6 (19.1) | 52 | 2.4 (9.4) | 0.003 | Change in score (mean, SD) | 1 |
| Ghoniem 200557 | 50 | 8.3 | 45 | 4.8 | Mean percentage score increase | 1 | |
| Norton 2002144 | 130 | 9.3 | 132 | 5.8 | 0.03 | Change in score (mean) | 2 |
| aCastro-Diaz 2007138 | 344 | 12.9 | 112 | 5.7 | < 0.001 | Change in score (mean). | 3 |
| Dmochowski 2003139 | 334 | 11.1 (14.8) | 332 | 6.8 (13.8) | < 0.001 | Change in score (mean, SD) | 3 |
| Kinchen 2005140 | 208 | 13 | 218 | 10.4 | 0.07 | Change in score (mean) | 3 |
| Mah 2006141 | 56 | 63.41 | 57 | 60.23 | Total score (mean) | 3 | |
| Millard 2004143 | 220 | 69.2 (23.8) | 229 | 64.7 (24.9) | Total score (mean, SD) | 3 | |
| van Kerrebroeck 2004117 | 240 | 72.2 | 245 | 68.5 | 0.127 | Total score (mean) | 3 |
| SNRI40 vs NT | |||||||
| Zinner 1998145 | 33 | 8.2 (10.8) | 34 | 2.6 (8.8) | < 0.05 | Change in score (mean, SD) | 1 |
| Norton 2002144 | 129 | 7.8 | 132 | 5.8 | 0.16 | Change in score (mean) | 2 |
| SNRI30 vs NT | |||||||
| Zinner 1998145 | 26 | 10 (6.4) | 34 | 2.6 (8.8) | < 0.05 | Change in score (mean, SD) | 1 |
| SNRI20 vs NT | |||||||
| Zinner 1998145 | 34 | 12 (16) | 34 | 2.6 (8.8) | < 0.05 | Change in score (mean, SD) | 1 |
| Norton 2002144 | 132 | 5.3 | 132 | 5.8 | 0.6 | Change in score (mean) | 2 |
| King’s Health Questionnaire | |||||||
| SNRI80 vs NT | |||||||
| bManning 2005142 | 306 | –9.2 | 311 | –2.6 | < 0.0001 | Change in score (mean) | 3 |
| ICIQ-UI SF | |||||||
| SNRI80 vs NT | |||||||
| bCastro-Diaz 2007138 | 344 | –2.8 | 112 | –1.7 | 0.004 | Change in score (mean) | 3 |
One study140 provided information for a longer treatment duration of 9 months; no significant difference between the groups was reported (Appendix 17, Comparison 14).
Two studies144,145 used a daily dose of 40, 30 and 20 mg, with one study145 favouring duloxetine and the other144 reporting no statistically significant difference between SNRI and placebo (Table 27).
Comparison of different SNRI doses
Two small studies144,145 compared different doses of SNRI. Data on cure and improvement rates are reported in Appendix 17, Comparison 15. In one study,144 slightly more participants taking a higher dose of SNRI were improved than those taking a lower dose (80 mg: 44%, 57/130; 40 mg: 37%, 48/129; 20 mg: 31%, 41/132), but no such pattern was found for cure rates (80 mg: 19%, 23/123; 40 mg: 24%, 30/123; 20 mg: 16%, 21/128). The other smaller study145 also did not find any dose dependence for improvement rates (40 mg: 45%, 15/33; 30 mg: 31%, 8/26; 20 mg: 44%, 15/34).
With respect to adverse events, the direction of effect was consistent across all comparisons, with more participants experiencing adverse events with a higher dose than with a lower dose (Appendix 17, Comparison 15).
There is no strong evidence to say whether a lower dose is associated with a better quality of life (Appendix 17, Comparison 16).
PFMT with adjunct treatment
The characteristics of included studies comparing PFMT with adjunct treatment versus no treatment are summarised in Table 28.
| Study ID | Population type | Duration (month) | Comparator | N randomised | Age | Supervisory intensitya | Notes |
|---|---|---|---|---|---|---|---|
| Bidmead 2002121 | 1 | 3.5 | PFMT + ES | 82? | 50.4 | Basic | |
| NT | 20? | 47.5 | None | ||||
| Ghoniem 200557 | 1 | 3 | PFMT + SNRI | 52 | 54.0 | Basic | |
| NT | 47 | 51.0 | Basic | Placebo drug and imitation PFMT | |||
| Goode 2003123 | 3 | 2 | PFMT + ES | 67 | 54.9 | Basic | |
| NT | 67 | 55.9 | None | Self-administered behavioural training | |||
| Hofbauer 1990126 | 1 | 1.5 | PFMT + ES | 11 | 62.9 | Intensive | |
| NT | 10 | 59.8 | Intensive | Sham ES |
PFMT plus ES vs no treatment
Cure and improvement
Three studies121,123,126 compared PFMT with adjunct ES versus no treatment (Figures 19 and 20). Pooled data from two of these studies with data123,126 showed a higher improvement rate (but not cure rate) in the intervention group, although the CI was wide (Figure 20: OR 8.69, 95% CI 1.87 to 40.32).
FIGURE 19.
Cure rates: pelvic floor muscle training and electrical stimulation versus no treatment.
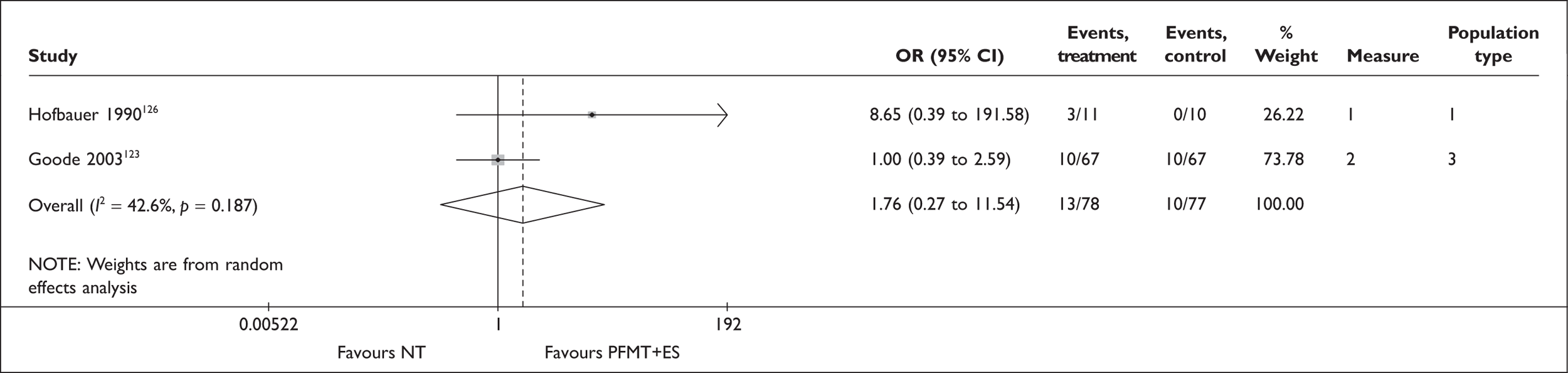
FIGURE 20.
Improvement rates: pelvic floor muscle training and electrical stimulation versus no treatment.
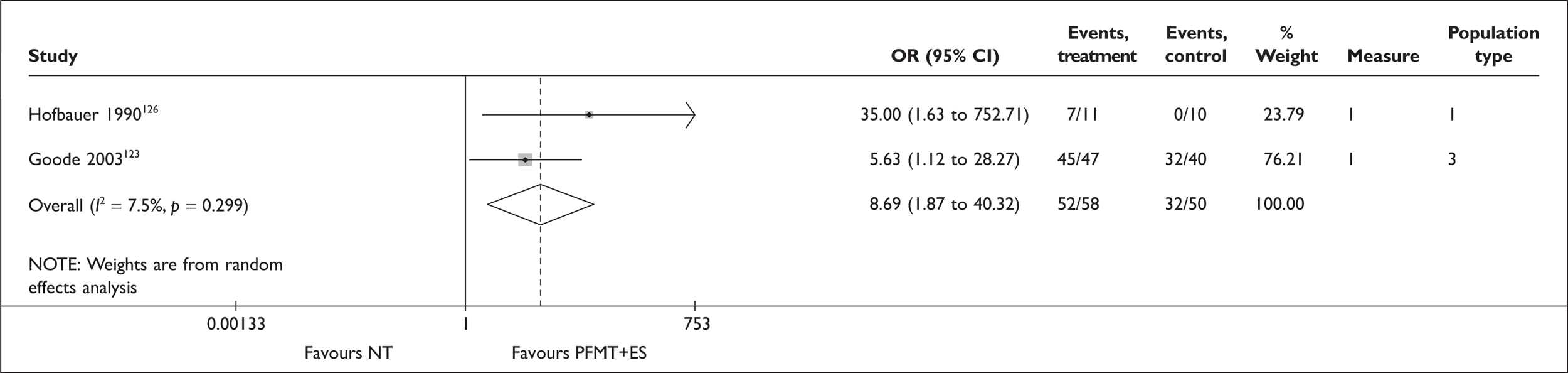
Adverse events
Only one study123 reported adverse events: 6% (4/67) of the participants using ES experienced vaginal irritation (Table 29).
| PFMT + ES | NT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Goode 2003123 | 4/67 | 6 | 0/67 | 0 | Vaginal irritation | 3 |
| PFMT + ES | NT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Incontinence Impact Questionnaire | ||||||
| Goode 2003123 | 67 | No difference | 67 | No difference | Total score | 3 |
Quality of life
The same study123 found no significant difference between the groups in condition-specific quality of life (Table 29) and general quality of life (Appendix 17, Comparison 17).
PFMT plus SNRIs vs no treatment
One four-arm trial57 reported data for a combination of PFMT plus a drug therapy (SNRI) compared with no treatment (Table 30). The ‘no-treatment’ arm received placebo drugs and also performed imitation PFMT. The PFMT-plus-SNRI group was associated with higher improvement rates and higher (better) quality of life than the no-treatment group. adverse events associated with SNRI (with PFMT or imitation PFMT) are reported separately in Table 27. No adverse events associated with PFMT were reported.
| PFMT + SNRI | NT | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Improvement rate | |||||||
| Ghoniem 200557 | 36/51 | 71 | 19/45 | 42 | 3.28 (1.41 to 7.64), p = 0.006 | 1 | 1 |
| PFMT + SNRI | NT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| I-QoL | ||||||
| Ghoniem 200557 | 51 | 13.1 | 50 | 8.3 | Change in score (mean percentage score increase) | 1 |
Comparison between different treatments: single modality
Table 31 summarises the characteristics of included studies comparing different single interventions. PFMT augmented with BF is included here as a ‘single’ intervention.
| Study ID | Population type | Duration (month) | Compa-rator | N randomised | Age (years) | Supervisory intensitya | Notes |
|---|---|---|---|---|---|---|---|
| Arvonen 2001178 | 1 | 4 | PFMT | 20 | 47.0 | Basic | |
| VC | 20 | 49.0 | Basic | ||||
| Bernardes 2000174 | 1 | 0.3 | PFMT | 7 | 44.1 | Intensive | |
| ES | 7 | 53.3 | Intensive | ||||
| Bø 1999115 | 1 | 6 | PFMT | 29 | 49.6 | Intensive | |
| ES | 32 | 47.2 | Basic | Maximum intermittent vaginal stimulation | |||
| VC | 29 | 49.2 | Basic | ||||
| Cammu 1998181 | 1 | 3 | PFMT + BF | 30 | 55.9 | Intensive | |
| VC | 30 | 56.3 | Basic | ||||
| Delneri 2000186 | 1 | 0.5 | ES | 10 | 49.5 | Intensive | Functional ES |
| 1 | VC | 10 | 41.5 | NR | |||
| Ghoniem 200557 | 1 | 3 | PFMT | 50 | 54.0 | Basic | Placebo drug |
| SNRI | 52 | 53.0 | Basic | Imitation PFMT | |||
| Hahn 1991175 | 1 | 6 | PFMT | 10 | 47.2 | Basic | IFT |
| ES | 10 | Basic | |||||
| Haken 1991179 | 1 | 2.5 | PFMT | 33 | 48.0 | Basic | |
| VC | 31 | Basic | |||||
| Henalla 1989124 | 1 | 3 | PFMT | 26 | NR | Intensive | |
| ES | 25 | NR | Intensive | IFT | |||
| Hofbauer 1990126 | 1 | 1.5 | PFMT | 11 | 51.0 | Intensive | 12 visits |
| ES | 11 | 59.7 | Intensive | 18 visits; faradism | |||
| Klarskov 1986184 | 1 | 4 | PFMT | 24 | 48.0 | Basic | |
| Surgery | 26 | NR | |||||
| Laycock 1988176 | 1 | 1–2? | PFMT | 16 | 44.0 | Intensive | Weekly visit |
| ES | 20 | Intensive | 2–3 visits per week; IFT | ||||
| Laycock 2001152 | 1 | 3 | PFMT | 20 | NR | Basic | |
| PFMT + BF | 40 | NR | Basic | ||||
| VC | 41 | NR | Basic | ||||
| Oláh 1990187 | 3 | 1 | ES | 36 | 47.9 | Intensive | 12 visits |
| VC | 33 | 43.2 | Intensive | 4 visits; IFT | |||
| Peattie 1988180 | 1 | 1 | PFMT | 22 | NR | Intensive | |
| VC | 22 | NR | Basic | ||||
| Sherburn 2007182 | 1 | 5 | PFMT | 43 | 72.0 | Intensive | |
| BT | 41 | Intensive | |||||
| Smith 1996177 | 1 | 4 | PFMT | 9 | 48.0 | Basic? | |
| ES | 9 | 53.0 | Basic? | Intravaginal neuromuscular stimulation | |||
| Tapp 1989185 | 1 | 3 | PFMT | 27 | NR | Intensive | |
| Surgery | 28 | NR | NR | ||||
| Williams 2006129 | 2 | 3 | PFMT | 79 | 55.9 | Basic | |
| VC | 80 | 58.2 | Basic | ||||
| Wise 1993188 | 1 | 3 | ES | 20 | NR | Basic | Maximal vaginal ES |
| VC | 21 | NR | Basic | ||||
| Wyman 1998183 | 2 | 3 | PFMT + BF | 69 | 62.0 | Basic | |
| BT | 68 | 60.0 | Basic |
PFMT vs ES
Seven studies provided at least one of the specified primary outcomes. 115,124,126,174–177 Of these, five studies, namely Bernardes and colleagues (2000),174 Bø and colleagues (1999),115 Henalla and colleagues (1989),124 Hofbauer and colleagues (1990)126 and Laycock and colleagues (1988),176 provided intensive supervision (more than two sessions per month) for both groups. In addition, the study by Bø and colleagues (1999)115 also provided intensive supervision for the PFMT group but basic supervision (two or fewer sessions per month) for the comparator group.
Cure and improvement
Pooled data for cure and improvement indicated that PFMT was more effective than ES in terms of both cure (Figure 21: OR 2.65, 95% CI 0.82 to 8.60) and improvement (Figure 22: OR 2.18, 95% CI 0.76 to 6.28). The direction and magnitude of effects varied across studies, particularly for the improvement rate which displayed statistical heterogeneity at the 10% level (p = 0.070). The source of heterogeneity was unclear. However, it is noteworthy that all but one study (Laycock 1988)176 that provided intensive supervision for the PFMT group showed ORs (point estimate) of greater than one (favouring PFMT on average) for both cure and improvement data.
FIGURE 21.
Cure rates: pelvic floor muscle training versus electrical stimulation.

FIGURE 22.
Improvement rates: pelvic floor muscle training versus electrical stimulation.

Adverse events and quality of life
Information on adverse events and condition-specific quality of life was provided by one small study only115 (Table 32). In this study, adverse events were experienced in 31% (10/32) of the participants using ES but none in the PFMT group. Seven participants in the ES group withdrew. Condition-specific quality-of-life scores were similar for both groups (Table 32).
| PFMT | ES | Notes | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| N experiencing AEs | ||||||
| Bø 1999115 | 0/29 | 0 | 10/32 | 31 | Smarting (tenderness, bleeding, discomfort), motivation problem, difficulty in using the stimulator | 1 |
| PFMT | ES | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Social Activity Index | ||||||
| Bø 1999115 | 25 | 0.6 (1.02) | 25 | 0.6 (1.02) | Change in score (mean, SD) | 1 |
PFMT, with or without BF, vs VC
Seven studies provided information on at least one of the specified primary outcomes. 115,129,152,178–181 Three of these studies, namely Bø and colleagues (1999),115 Cammu and colleagues (1998)181 and Peattie and colleagues (1988),180 provided intensive supervision (more than two sessions per month) for the PFMT group (with or without BF) but basic supervision (two or fewer sessions per month) for the VCs group. The other studies provided basic supervision for both groups.
Cure and improvement
There appeared to be no clear differences between PFMT, with or without BF, and VCs (Figures 23 and 24). One study by Bø and colleagues (1999)115 stands out, showing a relatively higher OR favouring PFMT. It is not clear why this is the case. One explanation may be a longer training duration and more supervisory sessions for the PFMT group relative to other studies.
FIGURE 23.
Cure rates: pelvic floor muscle training, with or without biofeedback, versus vaginal cones.

FIGURE 24.
Improvement rates: pelvic floor muscle training, with or without biofeedback, versus vaginal cones.

Adverse events
Four studies115,129,179,181 reported adverse events (Table 33). The first study115 reported that 62% (18/29) of participants in the VCs group experienced adverse events, compared with none in the PFMT group. The second study179 did not report any data but noted that PFMT was associated with difficulty remembering to use the technique, whereas participants using cones reported aesthetic dislike of the technique as well as difficulties associated with vaginal prolapse, resulting for some in discontinuation of the cone treatment. In the third study,129 there were two cases of urinary tract infection in both groups but no other side effects were reported by the participants. The fourth study181 reported that 47% (14/30) in the cones group experienced adverse events and subsequently discontinued treatment, compared with none in the PFMT-plus-BF group.
| PFMT ± BF | VC | Notes | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| PFMT vs VC | ||||||
| Bø 1999115 | 0/29 | 0 | 18/29 | 62 | Abdominal pain, vaginitis, bleeding, motivation problems, trouble in using the cones | 1 |
| Haken 1991179 | ND/33 | ND/31 | ‘Difficulty remembering to use the technique was a significant feature in the [PFMT] group which was not apparent in those using cones. Causes of withdrawal in the cones group were predominantly aesthetic dislike of the technique and difficulties associated with vaginal prolapse’; number lost to follow-up for PFMT = 3/33, for cones = 8/31 | 1 | ||
| Williams 2006129 | 2/79 | 3 | 2/80 | 3 | Urinary tract infection | 2 |
| PFMT + BF vs VC | ||||||
| Cammu 1998181 | 0/30 | 0 | 14/30 | 47 | Unpleasant feeling (n = 5), time consuming (n = 3), inability to introduce the cone when too nervous or when in a hurry (n = 2), interference with menstrual cycle (n = 2), a certain cone held in the morning could not be held any longer in the evening (muscle fatigue) (n = 2) | 1 |
| PFMT ± BF | VC | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| Social Activity Index – PFMT vs VC | |||||||
| Bø 1999115 | 25 | 0.6 (1.02) | 27 | 0.1 (1.06) | Change in score (mean, SD) | 1 | |
| King’s Health Questionnaire – PFMT vs VC | |||||||
| Laycock 2001152 | 16 | 8.13 (9.06) | 30 | 7.03 (7.74) | NS | Change in score (mean increase, SD) | 1 |
| King’s Health Questionnaire – PFMT + BF vs VC | |||||||
| Laycock 2001152 | 22 | 6.14 (6.2) | 30 | 7.03 (7.74) | NS | Change in score (mean increase, SD) | 1 |
| The Leicester Impact Scale – PFMT vs VC | |||||||
| aWilliams 2006129 | 77 | 2 (0.0 to 5.0) | 79 | 2 (0.0 to 5.0) | 0.729 | Score (median interquartile range) | 2 |
PFMT, with or without BF, vs BT
Cure and improvement
Two studies182,183 compared PFMT (with or without BF) with BT (Table 34). The first study182 recruited women with SUI only (population type 1) and reported cure rates of 48% (19/40) for PFMT and 26% (9/35) for BT immediately after the supervised treatment phase (OR 2.61, 95% CI 0.98 to 6.96). The difference was not statistically significant. The other study183 included women with other urinary incontinence symptoms (population type 2) and similarly found no clear difference between groups in cure and improvement at the end of the supervised treatment phase (Table 34: cure 13% vs 18%; improvement 76% vs 65%), and 3 months after the end of the treatment phase (Appendix 17, Comparison 18: cure 20% vs 16%; improvement 70% vs 62%). In this second study,183 a large proportion of participants from both groups appeared to have sought alternative treatments by the end of the 3-year follow-up; of those who did not receive any additional treatment, cure rates were 9% (1/11) in the PFMT group and 18% (4/22) in the BT group (Appendix 17, Comparison 18).
| PFMT ± BF | BT | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| PFMT vs BT | |||||||
| Sherburn 2007182 | 19/40 | 48 | 9/35 | 26 | 2.61 (0.98 to 6.96), p = 0.055 | 2 | 1 |
| PFMT + BF vs BT | |||||||
| Wyman 1998183 | 8/64 | 13 | 12/68 | 18 | 0.67 (0.25 to 1.76), p = 0.412 | 2 | 2 |
| Improvement | |||||||
| PFMT + BF vs BT | |||||||
| Wyman 1998183 | 48/63 | 76 | 43/66 | 65 | 1.71 (0.79 to 3.70), p = 0.171 | 1 | 2 |
| PFMT ± BF | BT | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| ICIQ-UI SF – PFMT vs BT | |||||||
| aSherburn 2007182 | 43 | 5 (4) | 41 | 8 (7) | 0.003 | Score (median interquartile range) | 1 |
| Urogenital Distress Inventory – PFMT + BF vs BT | |||||||
| aWyman 1998183 | 45 | 81.2 (39.6) | 47 | 99.2 (54.4) | Score (mean, SD) | 1 | |
| Incontinence Impact Questionnaire-Revised – PFMT + BF vs BT | |||||||
| aWyman 1998183 | 45 | 43.5 (47.4) | 47 | 68.4 (69.7) | Score (mean, SD) | 1 | |
Adverse events
No data were available on adverse events.
Quality of life
Condition-specific quality of life was better for women having PFMT with or without BF, compared with BT (Table 34). In one study182 that performed a statistical test, the difference was found to be statistically significant. General HRQoL scores [Assessment of Quality of Life (AQoL)] were similar for both groups in one study182 that reported this outcome (Appendix 17, Comparison 18).
PFMT vs SNRI (80 mg)
One study57 compared PFMT (with placebo drug) with SNRI (with imitation PFMT). The results showed greater improvement and better condition-specific quality of life in the PFMT group, although the difference was not statistically significant (Table 35).
| PFMT | SNRI | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Ghoniem 200557 | 32/49 | 65 | 27/50 | 54 | 1.60 (0.71 to 3.60), p = 0.253 | 1 | 1 |
| PFMT | SNRI | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| I-QoL | |||||||
| Ghoniem 200557 | 49 | 7.8 | 50 | 8.3 | 0.979 | Change in score (mean percentage score increase) | 1 |
PFMT vs surgery
Data on cure and improvement from two studies184,185 comparing PFMT with surgery are shown in Figures 25 and 26. One of these studies184 compared PFMT with either Burch colposuspension, vaginal repair or combined procedures, whereas the other study185 compared PFMT with Burch colposuspension. The results for both cure and improvement favoured surgery (Figures 25 and 26: OR for cure 0.08, 95% CI 0.03 to 0.23; OR for improvement 0.19, 95% CI 0.04 to 0.77). However, no information was reported on adverse events or quality of life.
FIGURE 25.
Cure rates: pelvic floor muscle training versus surgery.

FIGURE 26.
Improvement rates: pelvic floor muscle training versus surgery.

ES vs VC: cure and improvement
Figures 27 and 28 show pooled data from three studies115,187,188 reporting the number of women cured or improved after treatment with ES compared with VCs. No statistically significant difference between the groups was found (Figures 27 and 28: OR for cure 1.00, 95% CI 0.26 to 3.91, OR for improvement 1.30, 95% CI 0.59 to 2.84).
FIGURE 27.
Cure rates: electrical stimulation versus vaginal cones.

FIGURE 28.
Improvement rates: electrical stimulation versus vaginal cones.

One of these studies187 provided data at 6 months after the initial treatment phase had finished and found no statistically significant difference between the groups in terms of cure (OR 0.93, 95% CI 0.31 to 2.78) or improvement (OR 0.54, 95% CI 0.17 to 1.68) (Appendix 17, Comparison 19).
ES vs VC: adverse events
One study115 indicated that more participants experienced adverse events in the VC group (62%) than in the ES group (31%), although discontinuation due to adverse events was more common in the ES group (7/32) than in the VC group (1/29) (Table 36). Another study,187 which used cones to assess pelvic floor muscle strength in both groups, reported that nine women withdrew because of a failure to tolerate the cones during pretreatment assessment. In seven of these women, the vagina was too narrow and the cones ‘wedged’. The same study187 reported a further two adverse events (one psychiatric disorder and one death) in the cones group, which were considered by the study author to be unrelated to the study intervention.
| ES | VC | Notes | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| N experiencing AEs | ||||||
| Bø 1999115 | 10/32 | 31 | 18/29 | 62 |
ES: Smarting (tenderness, bleeding, discomfort), motivation problem, difficulty in using the stimulator VC: Abdominal pain, vaginitis, bleeding, motivation problems, trouble in using the cones |
1 |
| Oláh 1990187 | 4/36 | 11 | 5/33 | 15 | Unable to tolerate cones (used to assess pelvic floor muscle strength in both groups): the vagina was too narrow and the cones ‘wedged’; irregular uterine bleeding preventing cone use; discomfort experienced during use because of excessive scar tissue in the vagina | 3 |
| ES | VC | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Social Activity Index | ||||||
| Bø 1999115 | 25 | 0.6 (1.02) | 27 | 0.1 (1.06) | Change in score (mean, SD) | 1 |
Comparison between different treatments: dual modality
The characteristics of included studies comparing a single intervention with an intervention combined with an adjunct treatment are summarised in Table 37. Pelvic floor muscle training with sham ES was classified as being equivalent to PFMT. Where both PFMT and PFMT with sham ES were present within a trial, the dichotomous data were combined (added up). 121,190 In two three-arm trials examining the addition of ES to PFMT plus BF,157,172 the trial arms involving different types of ES (PFMT plus BF and ES) were combined for dichotomous data.
| Study ID | Population type | Duration (month) | Comparator | N randomised | Age | Supervisory intensitya | Notes |
|---|---|---|---|---|---|---|---|
| Bidmead 2002121 | 1 | 3.5 | PFMT | 40? | 46.2 | Basic | |
| PFMT + sham ES | 42? | 51.5 | Basic | ||||
| PFMT + ES | 82? | 50.4 | Basic | ||||
| Blowman 1991189 | 1 | ?1 | PFMT | 7 | 42.5 | Basic | With sham ES |
| PFMT + ES | 7 | 45.0 | Basic | ES = neurotrophic stimulation | |||
| Ghoniem 200557 | 1 | 3 | PFMT | 50 | 54.0 | Basic | |
| SNRI | 52 | 53.0 | Basic | ||||
| PFMT + SNRI | 52 | 54.0 | Basic | ||||
| Goode 2003123 | 3 | 2 | PFMT | 66 | 57.7 | Basic | |
| PFMT + ES | 67 | 54.9 | Basic | ES = biphasic pulses | |||
| Haig 1995190 | 1 | 3 | PFMT | 20 | 55.0 | Intensive | |
| PFMT + sham ES | 18 | 51.0 | Intensive | ||||
| PFMT + ES | 20 | 51.0 | Intensive | ||||
| Hofbauer 1990126 | 1 | 1.5 | PFMT | 11 | 51.0 | Intensive | |
| ES | 11 | 59.7 | Intensive | Faradic | |||
| PFMT + ES | 11 | 62.9 | Intensive | ||||
| Knight 1998172 | 1 | 6 | PFMT + BF | 21 | NR | Intensive | ES = maximal stimulation at clinic |
| PFMT + BF + ES (maximal) | 24 | NR | Intensive | ||||
| PFMT + BF + ES (low) | 25 | NR | Intensive | ES = overnight at low intensity at home | |||
| Pieber 1995192 | 1 | 3 | PFMT | 25 | 44.3 | Basic | |
| PFMT + VC | 21 | 41.7 | Basic | ||||
| Tapp 1987191 | 1 | 3 | PFMT | 15 | NR | Intensive | |
| PFMT + ES | 14 | NR | Intensive | ||||
| Tapp 1989185 | 1 | 3 | PFMT | 27 | NR | Intensive | |
| PFMT + ES | 26 | NR | Intensive | Faradic | |||
| Wilson 1987157 | 1 | 1.5 | PFMT + BF | 15 | 46.8 | Intensive | |
| PFMT + BF + ES (faradism) | 15 | Intensive | |||||
| PFMT + BF + ES (IFT) | 15 | Intensive | |||||
| Wise 1993188 | 1 | 3 | PFMT + VC | 21 | NR | Basic | |
| VC | 21 | NR | Basic | ||||
| Wyman 1998183 | 2 | 3 | PFMT + BF | 69 | 62.0 | Basic | |
| BT | 68 | 60.0 | Basic | ||||
| PFMT + BF + BT | 67 | 61.0 | Basic |
PFMT (with or without BF) vs PFMT (with or without BF) and adjunct treatment
PFMT (with or without BF) vs PFMT (with or without BF) and ES
Cure and improvement
Pooled data on cure and improvement showed that all trial estimates overlapped, and there was significant statistical heterogeneity for the cure rate at the 10% level (Figures 29 and 30: OR for cure without BF 1.02, 95% CI 0.29 to 3.55; OR for improvement without BF 0.84, 95% CI 0.34 to 2.07; OR for improvement with BF 0.86, 95% CI 0.36 to 2.08). The source of heterogeneity appears to be the study by Blowman and colleagues,189 which suggested that adding ES to PFMT was significantly better than PFMT alone. The reason for this is unclear.
FIGURE 29.
Cure rates: pelvic floor muscle training (PFMT) [± biofeedback (BF)] versus PFMT (± BF) plus electrical stimulation.

FIGURE 30.
Improvement rates: pelvic floor muscle training (PFMT) [± biofeedback (BF)] versus PFMT (± BF) plus electrical stimulation.

Two studies157,172 reported the improvement rate at 6 months after the end of the initial treatment phase (Appendix 17, Comparison 20). No differences between the treatment groups were found.
Adverse events
Only one study123 reported any adverse events (Table 38). The combined treatment group recorded four (6%) occurrences of vaginal irritation due to application of ES devices, compared with none in the PFMT group.
| PFMT | PFMT + ES | Notes | Population type | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Goode 2003123 | 0/66 | 0 | 4/67 | 6 | Vaginal irritation | 3 |
| PFMT | PFMT + ES | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Incontinence Impact Questionnaire | ||||||
| Goode 2003123 | 66 | No difference | 67 | No difference | Total score | 3 |
Quality of life
Condition-specific (Incontinence Impact Questionnaire) (Table 38) and general HRQoL (SF-36) (Appendix 17, Comparison 20) was reported by one study only. 123 The results did not differ by treatment group.
PFMT vs PFMT plus VC
One study192 reported cure and improvement rates for PFMT compared with PFMT with adjunct VCs (Table 39). The results were slightly better for the combined treatment group, but these differences were not statistically significant.
PFMT plus BF vs PFMT plus BF and BT
Cure and improvement
One study183 of 136 women with different types of incontinence reported cure and improvement at the end of the 3-month treatment phase, at 6 months (3 months after the end of treatment) and at 3.2 years (Table 40). Both cure and improvement rates were consistently higher in the combined treatment group at 3 months (Table 40: cure OR 0.32, 95% CI 0.13 to 0.79; improvement OR 0.35, 95% CI 0.13 to 0.97) and 6 months (Appendix 17, Comparison 21: cure OR 0.69, 95% CI 0.30 to 1.58; improvement OR 0.75, 95% CI 0.34 to 1.69). However, the differences were not statistically significant at follow-up at 6 months. The percentage of participants who remained cured at 3.2 years was 50% (8/16) for the combined treatment and 9% (1/11) for the comparator, although the number available for long-term assessment was very small (Appendix 17, Comparison 21).
| PFMT + BF | PFMT + BF + BT | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| Wyman 1998183 | 8/64 | 13 | 19/61 | 31 | 0.32 (0.13 to 0.79), p = 0.014 | 2 | 2 |
| Improvement rate | |||||||
| Wyman 1998183 | 48/63 | 76 | 55/61 | 90 | 0.35 (0.13 to 0.97), p = 0.044 | 1 | 2 |
| PFMT + BF | PFMT + BF + BT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Urogenital Distress Inventory | ||||||
| aWyman 1998183 | 45 | 81.2 (39.6) | 44 | 63.2 (49.2) | Score (mean, SD) | 1 |
| Incontinence Impact Questionnaire-Revised | ||||||
| aWyman 1998183 | 45 | 43.5 (47.4) | 44 | 52.3 (73.4) | Score (mean, SD) | 1 |
Quality of life
The same study183 measured condition-specific quality of life using two instruments (Table 40). The data at the end of the 3-month treatment phase were reported separately for women with SUI alone: one instrument (Urogenital Distress Inventory) favoured the group with adjunct BT, and the other (Incontinence Impact Questionnaire Revised) favoured the group without BT. The data with longer follow-up were reported combined for all participants with SUI, UUI and MUI (Appendix 17, Comparison 21). The results were again contradictory according to the different instruments.
PFMT vs PFMT plus SNRI
One study57 reported improvement and condition-specific quality of life for PFMT compared with women having a combination of PFMT plus adjunct drug therapy (Table 41). The results were slightly better for the combined treatment, but these differences were not statistically significant. adverse events associated with SNRI (with PFMT or imitation PFMT) are reported separately in Table 27.
| PFMT | PFMT + SNRI | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Ghoniem 200557 | 32/49 | 65 | 36/51 | 71 | 0.78 (0.34 to 1.82), p = 0.572 | 1 | 1 |
| PFMT | PFMT + SNRI | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| I-QoL | |||||||
| Ghoniem 200557 | 49 | 7.8 | 51 | 13.1 | 0.063 | Mean percentage score increase | 1 |
PFMT plus adjunct treatment vs the adjunct treatment
PFMT plus ES vs ES
One study126 reported the number of women cured or improved for PFMT plus ES compared with ES alone (Table 42). The ORs (point estimate) favoured the combined treatment for both cure and improvement, but CIs were wide and included one (no difference).
PFMT plus VC vs VC
One study188 compared a combination of PFMT plus VCs with VCs alone (Table 43). Between 74% and 93% of participants improved after treatment but there was no clear difference between the groups.
| PFMT + VC | VC | OR (95% CI) | Measure | Population type | ||||
|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||||
| Improvement rate | ||||||||
| Wise 1993188 | 14/15 | 93 | 14/19 | 74 | 5.00 (0.52 to 48.46), p = 0.165 | 2 | 1 | |
PFMT plus BF and BT vs BT
Cure and improvement
One study183 of 135 women with different types of urinary incontinence reported cure and improvement at the end of the 3-month treatment phase, at 6 months (3 months after the end of treatment) and 3.2 years (Table 44). The results showed higher cure and improvement rates for the combined treatment group at the end of the initial treatment phase (Table 44: cure OR 2.11, 95% CI 0.92 to 4.82; improvement OR 4.90, 95% CI 1.84 to 13.10), and at 6 months (Appendix 17, Comparison 22; cure OR 1.89, 95% CI 0.78 to 4.59; improvement OR 1.95, 95% CI 0.88 to 4.33). The difference in improvement at 3 months reached statistical significance but the CIs were wide. At 3.2 years, 50% (8/16) of the women receiving the combined treatment and 18% (4/22) receiving BT alone were continent (cured), although the number available for long-term assessment was very small (Appendix 17, Comparison 22).
| PFMT + BF + BT | BT | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| Wyman 1998183 | 19/61 | 31 | 12/68 | 18 | 2.11 (0.92 to 4.82), p = 0.076 | 2 | 2 |
| Improvement rate | |||||||
| Wyman 1998183 | 55/61 | 90 | 43/66 | 65 | 4.90 (1.84 to 13.10), p = 0.002 | 1 | 2 |
| PFMT + BF + BT | BT | Measure | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Urogenital Distress Inventory | ||||||
| aWyman 1998183 | 44 | 63.2 (49.2) | 47 | 99.2 (54.4) | Score (mean, SD) | 1 |
| Incontinence Impact Questionnaire-Revised | ||||||
| aWyman 1998183 | 44 | 52.3 (73.4) | 47 | 68.4 (69.7) | Score (mean, SD) | 1 |
Quality of life
The same study183 measured condition-specific quality of life using two instruments. The data immediately after the 3-month treatment phase were reported separately for women with stress incontinence alone and suggested higher quality of life for the combined treatment compared with BT without PFMT and BF (Table 44). The data at 6 months (3 months after the end of 3-month treatment) were reported for all participants with SUI, UUI and MUI; the direction of effect remained in favour of the combined treatment (Appendix 17, Comparison 22). Among 38 women who did not seek additional treatment before the 3.2 years’ follow-up, however, quality of life did not differ significantly between groups (Appendix 17, Comparison 22).
PFMT plus SNRI vs SNRI
One study57 compared PFMT plus drug therapy (SNRI) with drug therapy alone (Table 45). The apparent greater improvement and better quality of life in the combined therapy group was not statistically different from the group using drug therapy alone.
| PFMT + SNRI | SNRI | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Ghoniem 200557 | 36/51 | 71 | 27/50 | 54 | 2.04 (0.90 to 4.64), p = 0.087 | 1 | 1 |
| PFMT + SNRI | SNRI | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| I-QoL | ||||||
| Ghoniem 200557 | 51 | 13.1 | 50 | 8.3 | Change in score (mean percentage score increase) | 1 |
Summary
A summary of the effect sizes based on meta-analyses of cure and improvement data are given in Tables 46 and 47. Overall, it appears that most standalone interventions had, on average, higher cure and improvement rates than no (active) treatment. In particular, PFMT with or without BF was more effective than no treatment.
| Intervention 1 | Intervention 2 | Number of trials in MA (total) | Number of participants in MA | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Comparison with no treatment | ||||||
| PFMT | NT | 8 (14) | 605 | 5.41 | 1.64 to 17.82 | 0.005 |
| PFMT + BF | NT | 2 (2) | 110 | 21.54 | 3.65 to 126.98 | 0.001 |
| ES | NT | 6 (8) | 288 | 1.10 | 0.41 to 2.94 | 0.849 |
| VC | NT | 0 (2) | ||||
| SNRI80 | NT | 3 (11) | 1292 | 1.46 | 1.00 to 2.14 | 0.053 |
| SNRI40 | NT | 1 (2) | 255 | 1.81 | 0.96 to 3.39 | 0.065 |
| SNRI30 | NT | 0 (1) | ||||
| SNRI20 | NT | 1 (2) | 260 | 1.10 | 0.56 to 2.14 | 0.781 |
| BT | NT | 1 (1) | 123 | 4.03 | 0.80 to 20.23 | 0.091 |
| PFMT + ES | NT | 2 (3) | 155 | 1.76 | 0.27 to 11.54 | 0.556 |
| PFMT + SNRI | NT | 0 (1) | ||||
| Single modality | ||||||
| PFMT | ES | 5 (7) | 124 | 2.65 | 0.82 to 8.60 | 0.105 |
| PFMT | VC | 3 (6) | 245 | 0.61 | 0.09 to 3.95 | 0.606 |
| PFMT + BF | VC | 1 (2) | 46 | 0.86 | 0.25 to 2.93 | 0.806 |
| PFMT | BT | 1 (1) | 75 | 2.61 | 0.98 to 6.96 | 0.055 |
| PFMT + BF | BT | 1 (1) | 132 | 0.67 | 0.25 to 1.76 | 0.412 |
| PFMT | SNRI | 0 (1) | ||||
| PFMT | Surgery | 2 (2) | 95 | 0.08 | 0.03 to 0.23 | < 0.001 |
| ES | VC | 2 (4) | 106 | 1.00 | 0.26 to 3.91 | 0.998 |
| Dual modality | ||||||
| PFMT | PFMT + ES | 4 (7) | 133 | 1.02 | 0.29 to 3.55 | 0.976 |
| PFMT + BF | PFMT + BF + ES | 0 (2) | ||||
| PFMT | PFMT + VC | 1 (1) | 46 | 0.44 | 0.09 to 2.10 | 0.300 |
| PFMT + BF | PFMT + BF + BT | 1 (1) | 125 | 0.32 | 0.13 to 0.79 | 0.014 |
| PFMT | PFMT + SNRI | 0 (1) | ||||
| PFMT + ES | ES | 1 (1) | 22 | 3.75 | 0.33 to 43.31 | 0.290 |
| PFMT + VC | VC | 0 (1) | ||||
| PFMT + BF + BT | BT | 1 (1) | 129 | 2.11 | 0.92 to 4.82 | 0.076 |
| PFMT + SNRI80 | SNRI80 | 0 (1) | ||||
| Variation within comparators | ||||||
| Fluid increase then decrease | Fluid, decrease then increase | 0 (1) | ||||
| PFMT | PFMT + BF | 8 (15) | 370 | 0.48 | 0.30 to 0.77 | 0.002 |
| PFMT | PFMT with additional sessions | 3 (4) | 118 | 0.11 | 0.03 to 0.43 | 0.001 |
| PFMT | PFMT with audiotape | 0 (2) | ||||
| Strength and motor learning PFMT | Mortor learning PFMT alone | 1 (1) | 123 | 0.24 | 0.03 to 2.23 | 0.210 |
| PFMT (in supine) + BF | PFMT (in supine and upright) + BF | 0 (1) | ||||
| PFMT | Modified pilates | 0 (1) | ||||
| PFMT (maximal contraction) + BF | PFMT (submaximal contraction) + BF | 1 (1) | 32 | 1.80 | 0.39 to 8.22 | 0.448 |
| PFMT + perineometer | PFMT + urethral conductance | 1 (1) | 27 | 1.09 | 0.13 to 9.12 | 0.936 |
| PFMT + BF (vaginal) | PFMT + BF (vaginal and abdominal) | 0 (1) | ||||
| PFMT + BF | PFMT + ES | 0 (2) | ||||
| PFMT + BF + ES (faradism) | PFMT + BF + ES (interferential) | 0 (1) | ||||
| PFMT + BF + ES (maximal) | PFMT + BF + ES (low) | 0 (1) | ||||
| VC passive | VC active | 0 (1) | ||||
| SNRI 80 mg | SNRI 40 mg | 1 (1) | 246 | 0.71 | 0.39 to 1.32 | 0.279 |
| SNRI 80 mg | SNRI 20 mg | 1 (1) | 251 | 1.17 | 0.61 to 2.25 | 0.633 |
| SNRI 40 mg | SNRI 30 mg | 0 (1) | ||||
| SNRI 40 mg | SNRI 20 mg | 1 (2) | 251 | 1.64 | 0.88 to 3.07 | 0.118 |
| SNRI 30 mg | SNRI 20 mg | 0 (1) | ||||
| Intervention 1 | Intervention 2 | Number of trials in meta-analysis (total) | Number of participants in meta-analysis | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Comparison with no treatment | ||||||
| PFMT | NT | 11 (14) | 689 | 11.75 | 3.49 to 39.55 | < 0.001 |
| PFMT + BF | NT | 2 (2) | 110 | 24.20 | 2.02 to 290.58 | 0.012 |
| ES | NT | 7 (8) | 369 | 3.93 | 1.43 to 10.80 | 0.008 |
| VC | NT | 2 (2) | 212 | 5.43 | 0.07 to 396.77 | 0.439 |
| SNRI80 | NT | 10 (11) | 3672 | 2.02 | 1.67 to 2.44 | < 0.001 |
| SNRI40 | NT | 2 (2) | 328 | 2.43 | 0.84 to 7.07 | 0.103 |
| SNRI30 | NT | 1 (1) | 60 | 2.58 | 0.73 to 9.11 | 0.142 |
| SNRI20 | NT | 2 (2) | 332 | 2.11 | 0.56 to 7.72 | 0.258 |
| BT | NT | 1 (1) | 123 | 9.60 | 4.22 to 21.87 | < 0.001 |
| PFMT + ES | NT | 2 (3) | 108 | 8.69 | 1.87 to 40.32 | 0.006 |
| PFMT + SNRI | NT | 1 (1) | 96 | 3.28 | 1.41 to 7.64 | 0.006 |
| Single modality | ||||||
| PFMT | ES | 6 (7) | 190 | 2.18 | 0.76 to 6.28 | 0.148 |
| PFMT | VC | 5 (6) | 331 | 1.01 | 0.52 to 1.95 | 0.978 |
| PFMT + BF | VC | 1 (2) | 46 | 1.14 | 0.34 to 3.85 | 0.829 |
| PFMT | BT | 0 (1) | ||||
| PFMT + BF | BT | 1 (1) | 129 | 1.71 | 0.79 to 3.70 | 0.171 |
| PFMT | SNRI | 1 (1) | 99 | 1.60 | 0.71 to 3.60 | 0.253 |
| PFMT | Surgery | 2 (2) | 95 | 0.19 | 0.04 to 0.77 | 0.020 |
| ES | VC | 3 (4) | 141 | 1.30 | 0.59 to 2.84 | 0.514 |
| Dual modality | ||||||
| PFMT | PFMT + ES | 3 (7) | 160 | 0.84 | 0.34 to 2.07 | 0.699 |
| PFMT + BF | PFMT + BF + ES | 2 (2) | 102 | 0.86 | 0.36 to 2.08 | 0.743 |
| PFMT | PFMT + VC | 1 (1) | 46 | 0.84 | 0.26 to 2.68 | 0.767 |
| PFMT + BF | PFMT + BF + BT | 1 (1) | 124 | 0.35 | 0.13 to 0.97 | 0.044 |
| PFMT | PFMT + SNRI | 1 (1) | 100 | 0.78 | 0.34 to 1.82 | 0.572 |
| PFMT + ES | ES | 1 (1) | 22 | 4.67 | 0.77 to 28.47 | 0.095 |
| PFMT + VC | VC | 1 (1) | 34 | 5.00 | 0.52 to 48.46 | 0.165 |
| PFMT + BF + BT | BT | 1 (1) | 127 | 4.90 | 1.84 to 13.10 | 0.002 |
| PFMT + SNRI80 | SNRI80 | 1 (1) | 101 | 2.04 | 0.90 to 4.64 | 0.087 |
| Variation within comparators | ||||||
| Fluid, increase then decrease | Fluid, decrease then increase | 0 (1) | ||||
| PFMT | PFMT + BF | 7 (15) | 296 | 0.41 | 0.18 to 0.97 | 0.042 |
| PFMT | PFMT with additional sessions | 2 (4) | 74 | 0.05 | 0.01 to 0.28 | 0.001 |
| PFMT | PFMT with audiotape | 0 (2) | ||||
| Strength and motor learning PFMT | Motor learning PFMT alone | 1 (1) | 123 | 1.69 | 0.67 to 4.25 | 0.269 |
| PFMT (in supine) + BF | PFMT (in supine and upright) + BF | 0 (1) | ||||
| PFMT | Modified pilates | 0 (1) | ||||
| PFMT (maximal contraction) + BF | PFMT (submaximal contraction) + BF | 0 (1) | ||||
| PFMT + perineometer | PFMT + urethral conductance | 1 (1) | 20 | 1.17 | 0.26 to 5.29 | 0.842 |
| PFMT + BF (vaginal) | PFMT + BF (vaginal and abdominal) | 0 (1) | ||||
| PFMT + BF | PFMT + ES | 0 (2) | ||||
| PFMT + BF + ES (faradism) | PFMT + BF + ES (interferential) | 1 (1) | 30 | 1.38 | 0.29 to 6.60 | 0.691 |
| PFMT + BF + ES (maximal) | PFMT + BF + ES (low) | 1 (1) | 39 | 4.44 | 1.08 to 18.36 | 0.039 |
| VC passive | VC active | 1 (1) | 61 | 0.59 | 0.21 to 1.71 | 0.334 |
| SNRI 80 mg | SNRI 40 mg | 1 (1) | 259 | 1.32 | 0.80 to 2.17 | 0.277 |
| SNRI 80 mg | SNRI 20 mg | 1 (1) | 262 | 1.73 | 1.05 to 2.87 | 0.033 |
| SNRI 40 mg | SNRI 30 mg | 1 (1) | 59 | 1.88 | 0.64 to 5.51 | 0.253 |
| SNRI 40 mg | SNRI 20 mg | 2 (2) | 328 | 1.25 | 0.80 to 1.97 | 0.329 |
| SNRI 30 mg | SNRI 20 mg | 1 (1) | 60 | 0.56 | 0.19 to 1.65 | 0.294 |
There was insufficient evidence from direct comparisons to say whether PFMT was more effective than other modalities of treatment or which of the PFMT programmes was the most effective. Nevertheless, PFMT with additional supervisory sessions (or any face-to-face contacts with health-care professionals) appeared more effective than PFMT alone. PFMT augmented with BF was also associated with higher cure and improvement rates. With respect to PFMT combined with adjunct treatment (e.g. PFMT plus ES), it was not possible to draw firm conclusions due to the small number of studies identified from our searches.
Quality-of-life measures reported in included studies were highly variable, which made comparison across studies difficult. There were few major safety concerns for most non-surgical interventions, although some instances were reported which were related to the treatment device for BF, ES or weighted VCs. However, one notable exception was SNRI drug therapy, for which the majority of participants experienced adverse events and up to one-third of the participants discontinued treatment due to adverse events. It is unclear whether the potential mood-enhancing effect of SNRIs might or might not affect its measured effect on SUI. It is also worth mentioning the apparent placebo effects of the SNRI drug as noted above under SNRI drug therapy.
A key issue for all non-surgical interventions is their long-term performance. Data beyond the supervised treatment phase were sparse. The extent to which women continued to adhere to treatment after active supervision finished may also be an important confounding factor, although such data were poorly reported and could not be incorporated into the analysis.
Chapter 8 Mixed-treatment comparisons (direct and indirect)
Introduction
The review of effectiveness data presented in the preceding chapters has revealed that several treatments are used for SUI, with many variations and combinations of them also being used. Mixed treatment comparison (MTC) models analyse all of the treatments in one model, allowing indirect evidence to supplement direct, head-to-head evidence and thereby making comparison of treatments easier than via multiple head-to-head analyses. 113 The model produces estimates (from direct and indirect evidence) of the OR for each pair of treatments and then the cure and improvement rates of each individual treatment. Cure and improvement are defined in Chapter 5 (see Types of outcome measures). The rest of this chapter describes the data used, discusses the technical issues surrounding the application of a MTC model to these data and gives some definitions of important terms in Bayesian statistics that are used in the chapter, followed by results, discussion and summary of the work.
The data
The cured and improved outcomes were analysed in separate models. The searches identified 61 trials which have data on either or both of these outcomes. Fourteen treatments were included in the MTC analysis. The standard abbreviations for the treatments are used throughout this chapter (see List of abbreviations). Six trials (Table 48) were removed from the data set either because they compared one treatment on the list of treatments selected with one that was not, for example Klarskov and colleagues (1986),184 or because they compared two varieties of the same treatment and it was decided that separating treatments down to that level of detail was unhelpful, for example Burton (1993). 173 Some trials included some treatments that did not meet the inclusion criteria of this review, for example Tapp and colleagues (1989),185 compares PFMT, PFMT plus ES, and surgery. In this case the surgery arm was not included in the analysis.
| Trial | Reason |
|---|---|
| Burton 1993173 | Comparison of ‘passive’ VC and ‘active’ VC |
| Hay-Smith 2003164 | Comparison of ‘motor learning’ with and without PFMT |
| Johnson 2001167 | Comparison of PFMT + BF with maximal contraction and submaximal contraction |
| Klarskov 1986184 | Comparison of PFMT and surgery; surgery not included in the data set |
| Mayne 1998168 | Comparison of PFMT with perineometer and PFMT with urethral conductance |
| Zinner 1998145 | Comparison of no treatment with 20- and 40-mg doses of SNRI |
Removing these six trials resulted in 55 trials being used; of which eight reported cure results only, 17 reported improved results only and 30 reported both. Therefore, 38 trials were included in the cured analysis and 47 in the improved analysis.
The success rates in each trial were assessed by either the patient or by a clinician, with some trials reporting both. As for the direct, head-to-head meta-analyses, the patient-reported measure was used when it was available, with the clinician-reported measure being used as a proxy for it when the patient-reported measure was unavailable (see Model assumptions). The time between intervention and measurement of success varied across trials from 10 days to 6 months. Some trials also reported additional, long-term follow-up. 150,157,172,183,187 Each trial appears once in the model, using the data collected at the first time that the outcome was measured. This assumes that the measurement was taken at the point in time when the trialists believed that the treatments would have their maximal effect.
Table 49 shows which treatments are compared in each trial and the number of patients in each arm. A MTC model requires all treatments of interest to be connected to each other, which was the case for both the cured and the improved data sets (i.e. if trial 1 compares treatments A and B, trial 2 compares treatments B and C, and trial 3 compares treatments C and D, then A is connected to C through a path of trial 1, then trial 2 and A is connected to D through a path of trial 1, trial 2 and trial 3; an MTC model can only be applied if all pairs of treatments in a model have a path of trials that connect them). A total of 6608 patients were involved in the 55 trials, with 3560 providing data for cure and 6140 for improvement. It should be noted that a large proportion of these patients (36.3%, 1292/3560 for cure and 61.4%, 3772/6140 for improvement) come from the 10 trials testing SNRIs against a placebo (placebo being considered equivalent to ‘no treatment’). The cure model considered 13 treatments and the improvement model considered 14 treatments, as there were no cure data for the PFMT plus SNRI treatment.
| Trial ID | Outcome | NT | PFMT basic | PFMT extra | PFMT + BF | ES | VC | SNRI | BT | PFMT + ES | PFMT + ES + BF | PFMT + VC | PFMT + VC + BF | PFMT + BF + BT | PFMT + SNRI | Source of outcome | Number of patients in trial |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aksac 2003120 | C/I | 10 | 20 | 20 | Clinician | 50 | |||||||||||
| Arvonen 2001178 | C/I | 19 | 18 | Patient | 37 | ||||||||||||
| Berghmans 1996147 | C/I | 20 | 20 | Clinician | 40 | ||||||||||||
| Bernardes 2000174 | C | 7 | 7 | Patient | 14 | ||||||||||||
| Blowman 1991189 | C | 6 | 7 | Clinician | 13 | ||||||||||||
| Bø 1990159 | C/I | 29 | 23 | Patient | 52 | ||||||||||||
| Bø 1999115 | C/I | 30 | 25 | 25 | 27 | Patient | 107 | ||||||||||
| Bourcier 1994196 | C | 46 | 38 | Patient | 84 | ||||||||||||
| Brubaker 1997130 | C | 44 | 46 | Clinician | 90 | ||||||||||||
| I | 60 | 61 | Clinician | 121 | |||||||||||||
| Burns 1993122 | C/I | 40 | 43 | 40 | Clinician | 123 | |||||||||||
| Cammu 1998181 | C/I | 30 | 16 | Clinician | 46 | ||||||||||||
| Cardozo 2004137 | I | 52 | 51 | Patient | 103 | ||||||||||||
| Castro-Diaz 2007138 | I | 112 | 344 | Patient | 456 | ||||||||||||
| Dmochowski 2003139 | C | 322 | 286 | Clinician | 608 | ||||||||||||
| I | 332 | 334 | Patient | 666 | |||||||||||||
| Fantl 1991135 | C/I | 63 | 60 | Clinician | 123 | ||||||||||||
| Ghoniem 200557 | I | 45 | 49 | 50 | 51 | Patient | 195 | ||||||||||
| Glavind 1996150 | C | 15 | 19 | Clinician | 34 | ||||||||||||
| Goode 2003123 | C | 67 | 66 | 67 | Clinician | 200 | |||||||||||
| I | 40 | 47 | 47 | Patient | 134 | ||||||||||||
| Hahn 1991175 | C/I | 10 | 10 | Patient | 20 | ||||||||||||
| Haken 1991179 | I | 30 | 23 | Clinician | 53 | ||||||||||||
| Henalla 1989124 | I | 25 | 26 | 25 | Clinician | 76 | |||||||||||
| Henalla 1990125 | I | 7 | 8 | Clinician | 15 | ||||||||||||
| Hofbaur 1990126 | C/I | 10 | 11 | 11 | 11 | Patient | 43 | ||||||||||
| Kim 2007118 | C | 32 | 33 | Patient | 65 | ||||||||||||
| Kinchen 2005140 | I | 218 | 208 | Patient | 426 | ||||||||||||
| Klingler 1995151 | C/I | 21 | 20 | Clinician (C) /patient (I) | 41 | ||||||||||||
| Knight 1998172 | I | 18 | 39 | Patient | 57 | ||||||||||||
| Konstantinidou 2007116 | C/I | 10 | 12 | Clinician | 22 | ||||||||||||
| Lagro-Janssen 1991127 | C/I | 33 | 33 | Clinician | 66 | ||||||||||||
| Laycock 1988176 | I | 11 | 18 | Patient | 29 | ||||||||||||
| Laycock Trial 1 1993132 | C/I | 23 | 16 | Patient | 39 | ||||||||||||
| Laycock Trial 2 1993132 | C/I | 11 | 15 | Patient | 26 | ||||||||||||
| Luber 1997133 | C/I | 24 | 20 | Patient | 44 | ||||||||||||
| Mah 2006141 | I | 57 | 56 | Patient | 113 | ||||||||||||
| Manning 2005142 | I | 311 | 306 | Patient | 617 | ||||||||||||
| Millard 2004143 | C | 229 | 200 | Clinician | 429 | ||||||||||||
| I | 229 | 220 | Patient | 449 | |||||||||||||
| Mørkved 2002153 | C | 34 | 36 | Patient | 70 | ||||||||||||
| Norton 2002144 | C | 132 | 123 | Clinician | 255 | ||||||||||||
| I | 132 | 130 | Patient | 262 | |||||||||||||
| Oláh 1990187 | C/I | 30 | 24 | Patient | 54 | ||||||||||||
| Pages 2001154 | C/I | 27 | 13 | Patient | 40 | ||||||||||||
| Peattie 1988180 | I | 16 | 17 | Patient | 33 | ||||||||||||
| Pieber 1995192 | C/I | 25 | 21 | Patient | 46 | ||||||||||||
| Ramsay 1990128 | I | 22 | 22 | Patient | 44 | ||||||||||||
| Sand 1995134 | C/I | 17 | 35 | Clinician | 52 | ||||||||||||
| Seo 2004195 | I | 60 | 60 | Patient | 120 | ||||||||||||
| Shepherd 2003155 | C/I | 11 | 11 | Patient | 22 | ||||||||||||
| Sherburn 2007182 | C | 40 | 35 | Clinician | 75 | ||||||||||||
| Smith 1996177 | C/I | 9 | 9 | Clinician | 18 | ||||||||||||
| Tapp 1989185 | C/I | 21 | 23 | Clinician | 44 | ||||||||||||
| van Kerrebroeck 2004117 | I | 245 | 240 | Patient | 485 | ||||||||||||
| Williams 2006129 | C/I | 75 | 77 | 79 | Patient | 231 | |||||||||||
| Wilson 1987157 | I | 15 | 15 | 30 | Patient | 60 | |||||||||||
| Wise 1993188 | I | 16 | 19 | 15 | Clinician | 50 | |||||||||||
| Wyman 1998183 | C | 64 | 68 | 61 | Clinician | 193 | |||||||||||
| I | 63 | 66 | 61 | Patient | 190 | ||||||||||||
| Zanetti 2007161 | C | 21 | 23 | Clinician | 44 | ||||||||||||
| Totals | C | 1139 | 331 | 360 | 273 | 231 | 164 | 609 | 163 | 108 | 46 | 59 | 16 | 61 | 0 | 3560 | |
| I | 2200 | 394 | 276 | 250 | 298 | 283 | 1939 | 126 | 81 | 129 | 36 | 16 | 61 | 51 | 6140 | ||
| All | 2259 | 455 | 413 | 306 | 305 | 283 | 1939 | 163 | 108 | 175 | 74 | 16 | 61 | 51 | 6608 |
An important difference between the MTC model and the head-to-head comparisons is that PFMT has been separated into two treatments: ‘PFMT basic’ and ‘PFMT extra sessions’, where ‘PFMT extra sessions’ is defined as having more than two supervised sessions per month (see Chapter 6, Characteristics of included studies). 43 The models for each outcome were analysed twice, once with PFMT split into the two categories and once with it together as one treatment. This latter model is to facilitate comparison with the direct, head-to-head analyses. However, three trials116,159,161 are solely comparisons of the two intensities of PFMT, and as they provide no comparison with any other treatments they were removed from the data set when considering PFMT as one treatment. Hence, the former model, which considers the two intensities of PFMT separately, is taken as the base-case analysis and is presented in this chapter. The results from the models with PFMT considered as one treatment (regardless of the number of sessions) are shown in Appendix 23.
The model
The model used was developed by the Multi-parameter Evidence Synthesis (MPES) Programme at the University of Bristol. 202 The statistical theory behind the code can be found in Lu and Ades (2004),203 Caldwell and colleagues (2005),113 Lu and Ades (2006),204 and Ades and colleagues (2006). 205 Its main parameters are the log ORs of each treatment compared to a reference treatment, for which we used ‘no treatment’. A random effects model was adopted, and, as some of the trials involved had three or four arms that were relevant to the study, the multiarm version of the MTC model was used. This incorporates adjustments for the correlation between arms of the same trial. The model parameters are estimated within Bayesian methodology by the use of winbugs software. 114 The model code, currently available from the MPES website,202 is given in Appendix 22, alongside the data used from the individual trials.
Model assumptions
The data that were available required the following two key additional assumptions to be made before using the model (as previously suggested – see The data):
-
that the log OR of the success of any treatment compared to the no-treatment reference is the same when success is assessed using the clinician methods as when it is assessed by the patient
-
that the log OR of the success of any treatment compared to the no-treatment reference is independent of the time period at which the outcome was assessed.
‘No-treatment’ cure rate and improvement rate
Although the main parameters of the model are the log ORs of pairs of treatments, other statistics can be calculated from the parameters. The cure and improvement rates for each treatment can be calculated from the ORs if the success rate for one treatment is known. The distribution of the success rate of the reference treatment was estimated by applying a normal distribution to the log odds of the probability of success, with its mean and variance being estimated from a random effects model of the no-treatment arms of the studies involved (see Appendix 22).
Technical information about the running of the model
Vague prior distributions are used on the necessary parameters: the log ORs of treatment to no treatment, the individual trial baselines and the random effects standard deviation – see Appendix 22 for further details. The model was run on four different data sets; the cure and improvement data sets, with and without the PFMT treatment being split into basic and extra sessions. For the cure data set, a burn-in period of 20,000 iterations was used to ensure convergence, whereas for the improvement data set a burn-in period of 10,000 iterations was sufficient for convergence. The results were sampled for a further 100,000 iterations in both cases.
Bayesian terminology
For those who are unfamiliar with Bayesian methodology, a few terms used in the results section need to be explained. A good introduction to Bayesian methods can be found in O’Hagan and Luce’s primer for health economics. 206
-
Distribution The Bayesian method uses probability distributions to describe the uncertainty about a parameter, for example the OR between two treatments. The posterior distribution is the probability distribution gained from applying the data to the model. The posterior distribution is described through its summary statistics, such as the mean, median and percentiles. The phrase ‘the median odds ratio between …’ should be interpreted as ‘the median of the posterior distribution of the odds ratio between …’.
-
Ninety-five per cent central credible interval A 95% credible interval is one where the probability that the true value for the parameter is within that interval is 95%. The interval is central if, in addition, the probability of being below the credible interval is 2.5% and being above it is 2.5%. Bayesian analyses report credible intervals for similar reasons to the reporting of CIs in frequentist analysis (e.g. the direct, pairwise, meta-analyses in this report are performed using frequentist statistical inference), i.e. to give an indication of the uncertainty surrounding the estimate. The abbreviation CrI is used for (central) credible interval.
Results
In this section we report the ORs between treatments. The posterior distributions of the ORs are generally skewed. Therefore, we report the median OR as the point estimate. As the log ORs are roughly symmetrical, Figures 31 and 32 are plotted on the log odds scale, although labelled with the ORs.
FIGURE 31.
Cure: odds ratio of each treatment versus no treatment. Note: posterior distribution median (circle) with 95% central credible intervals. The horizontal axis is plotted on the log scale.

FIGURE 32.
Improvement: odds ratio of each treatment versus no treatment. Note: posterior distribution median (circle) with 95% central credible intervals. The horizontal axis is plotted on the log scale.

Table 50 shows the posterior distribution of the ORs for each treatment compared with no treatment for both the cure and the improvement models. These are shown graphically in Figures 31 and 32. Tables showing the distributions of the ORs for each pair of treatments are given in Appendix 23. For cure, all treatments when compared to no treatment have a median OR greater than one, indicating that on average they have higher cure rates than no treatment. PFMT extra sessions, PFMT plus BF, VCs, BT, PFMT plus ES and PFMT plus BT and BF all have their 2.5th percentile greater than one, indicating a benefit from the treatment. For improvement, the median ORs for all treatments compared with no treatment were greater than one, with all 2.5th percentiles being greater than one, except for PFMT plus VCs and BF, and PFMT plus SNRI. For both outcomes the treatment with the highest median OR is PFMT plus BT and BF. This treatment, however, appears in only one trial183 and has 61 participants in the PFMT-plus-BT-and-BF arm. Its success rate is high for both cure (19/61, 31.1%) and improvement (55/61, 90.2%). Given that the data come from only one trial, further research is needed.
| Treatment | Sample | Mean | SD | Median | 2.5th percentile | 97.5th percentile |
|---|---|---|---|---|---|---|
| Cure | ||||||
| PFMT basic | 331 | 1.4 | 0.627 | 1.28 | 0.554 | 2.92 |
| PFMT extra sessions | 360 | 12 | 5.7 | 10.7 | 5.03 | 26.2 |
| PFMT + BF | 273 | 14 | 7.38 | 12.3 | 5.35 | 32.7 |
| ES | 231 | 1.64 | 0.88 | 1.45 | 0.55 | 3.86 |
| VC | 164 | 4.18 | 2.62 | 3.55 | 1.23 | 10.9 |
| SNRI | 609 | 1.57 | 0.771 | 1.43 | 0.582 | 3.46 |
| BT | 163 | 9.34 | 7.17 | 7.53 | 2.34 | 27 |
| PFMT + ES | 108 | 3.78 | 2.84 | 3.05 | 1.09 | 10.7 |
| PFMT + ES + BF | 46 | 30.7 | 182 | 9.21 | 0.569 | 172 |
| PFMT + VC | 59 | 6.89 | 18 | 3.13 | 0.324 | 36 |
| PFMT + VC + BF | 16 | 86.4 | 2750 | 5.82 | 0.245 | 263 |
| PFMT + BT + BF | 61 | 37.8 | 55.1 | 25.2 | 4.94 | 146 |
| Improvement | ||||||
| PFMT basic | 394 | 4.97 | 2.37 | 4.47 | 2.03 | 10.9 |
| PFMT extra sessions | 276 | 29.8 | 17 | 25.7 | 10.3 | 73.1 |
| PFMT + BF | 250 | 31 | 21.8 | 25.4 | 8.68 | 86.9 |
| ES | 298 | 6.14 | 3 | 5.49 | 2.39 | 13.7 |
| VC | 283 | 7.86 | 4.65 | 6.77 | 2.6 | 19.4 |
| SNRI | 1939 | 2.29 | 0.87 | 2.14 | 1.06 | 4.4 |
| BT | 126 | 18.3 | 23.4 | 12 | 2.16 | 73.3 |
| PFMT + ES | 81 | 29.6 | 31 | 20.7 | 4.51 | 108 |
| PFMT + ES + BF | 129 | 31.2 | 35.3 | 21.6 | 4.5 | 116 |
| PFMT + VC | 36 | 21.7 | 35.6 | 12.2 | 1.83 | 99.2 |
| PFMT + VC + BF | 16 | 7.44 | 28.4 | 2.66 | 0.181 | 42 |
| PFMT + BT + BF | 61 | 160 | 401 | 69.8 | 6.59 | 852 |
| PFMT + SNRI | 51 | 7.37 | 11.8 | 4.42 | 0.646 | 31.8 |
Splitting of PFMT into basic and extra sessions
The results show that the number of sessions of PFMT does have an effect. For cure, the combined PFMT has an OR of 4.56 (95% CrI 1.95 to12.4) against no treatment, but on splitting by number of sessions, PFMT basic (two or fewer sessions per month) has an OR of 1.28 (95% CrI 0.554 to 2.92), whereas PFMT with extra sessions (more than two sessions per month) has an OR of 10.7 (95% CrI 5.03 to 26.2). The OR for PFMT with extra sessions compared with PFMT basic is 8.36 (95% CrI 3.74 to 21.7). For improvement a similar relationship appears, with combined PFMT having an OR compared with no treatment of 8.97 (95% CrI 4.4 to 20.8), whereas the model with PFMT separated into basic or with extra sessions has an OR for PFMT basic of 4.47 (95% CrI 2.03 to 10.9) and for PFMT extra sessions of 25.7 (95% CrI 10.3 to 73.1). The OR between PFMT with extra sessions and PFMT basic is 5.75 (95% CrI 2.11 to 16.2). The results from the combined PFMT models are given in Appendix 23.
Treatment success rates
The cure and improvement rates for each individual treatment are shown in Table 51, and, graphically, in Figures 33 and 34. These are calculated by combining the treatment to no-treatment ORs with the success rate for no treatment (obtained from modelling the no-treatment arms). The width of the 95% CrIs suggests that there is still considerable uncertainty surrounding the success rates.
| Cure | Improvement | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Median | 95% central CrI | N | Median | 95% central CrI | |||
| NT | 1139 | 5.8 | 3.3 | 9.9 | 2200 | 26.3 | 15.7 | 40.5 |
| PFMT basic | 331 | 7.3 | 2.8 | 17.8 | 394 | 61.5 | 36.4 | 82.7 |
| PFMT extra sessions | 360 | 39.9 | 20.2 | 65.8 | 276 | 90.2 | 74.9 | 96.9 |
| PFMT + BF | 273 | 43.3 | 21.5 | 70.2 | 250 | 90.1 | 72.0 | 97.3 |
| ES | 231 | 8.2 | 2.8 | 21.9 | 298 | 66.3 | 40.6 | 85.8 |
| VC | 164 | 18.0 | 6.1 | 43.5 | 283 | 70.8 | 43.1 | 89.3 |
| SNRI | 609 | 8.1 | 2.9 | 20.1 | 1939 | 43.3 | 22.7 | 66.7 |
| BT | 163 | 31.8 | 11.2 | 65.3 | 126 | 81.1 | 40.5 | 96.7 |
| PFMT + ES | 108 | 15.9 | 5.4 | 42.8 | 81 | 88.1 | 58.3 | 97.8 |
| PFMT + ES + BF | 46 | 36.1 | 3.2 | 91.8 | 129 | 88.5 | 58.5 | 97.9 |
| PFMT + VC | 59 | 16.1 | 1.8 | 70.3 | 36 | 81.4 | 36.7 | 97.5 |
| PFMT + VC + BF | 16 | 26.4 | 1.4 | 94.4 | 16 | 48.7 | 5.7 | 94.1 |
| PFMT + BT | 61 | 60.8 | 21.8 | 90.7 | 61 | 96.1 | 68.4 | 99.7 |
| PFMT + SNRI | 331 | 51 | 61.2 | 17.1 | 92.5 | |||
FIGURE 33.
Cure rate for each treatment. Note: posterior distribution median (circle) with 95% central credible intervals.

FIGURE 34.
Improvement rate for each treatment. Note: posterior distribution median (circle) with 95% central credible intervals.

Nevertheless, PFMT extra sessions appears to have a higher cure rate than PFMT basic and a similar rate to PFMT plus BF. In fact, PFMT extra sessions, PFMT plus BF, and PFMT plus BT and BF appear to be the best treatments in terms of cure, bearing in mind that PFMT plus BT and BF is assessed in only one trial. These three treatments also have the highest median improvement rate.
Sensitivity analysis
In the cure model, two treatments, ‘PFMT plus VCs and BF’ and ‘PFMT plus BT and BF’, appear in only one trial. PFMT plus SNRI, which appears only in the improvement model, was also only reported in one trial. As a sensitivity analysis to the inclusion of these three treatments, the cure and improvement models were run without them. This resulted in one trial132 being removed from the data set. The log ORs between the treatments and no treatment and the individual treatment success rates were satisfactorily similar to those of the original models.
Probability that each treatment is the most effective
Another measure that can be calculated from the results is the probability that each treatment is the best. In the cure model, ‘PFMT plus BT and BF’ has the highest probability (0.54) of being the best treatment, with ‘PFMT plus ES and BF’ (0.20) and ‘PFMT plus VCs and BF’ (0.17) the next highest. ‘PFMT plus BF’ (0.047) and ‘PFMT with extra sessions’ (0.025) come fourth and fifth in the ranking. For improvement, ‘PFMT plus BT and BF’ stands out as the treatment that is most likely to be the best (0.64), with ‘PFMT plus ES’ (0.092), ‘PFMT plus ES and BF’ (0.083), ‘PFMT with extra sessions’ (0.060), ‘PFMT plus VCs’ (0.052) and ‘PFMT plus BF’ (0.043) coming next.
These results need to be interpreted with caution, as ‘PFMT plus BT and BF’ appears in only one trial. In the models used in the sensitivity analysis (see Sensitivity analysis), excluding ‘PFMT plus VCs and BF’, ‘PFMT plus BT and BF’ and ‘PFMT plus SNRI’ (the interventions with only one trial) produces the following probabilities that each treatment is the most effective:
-
Cure PFMT + ES + BF (0.384), PFMT + BF (0.354), PFMT extra (0.158)
-
Improvement PFMT + ES (0.215), PFMT + ES + BF(0.212), PFMT extra (0.198), PFMT (0.186)
The results suggest that adding an adjunct (such as ES) or making PFMT more intensive in another way is likely to be the most effective. However, of the different options considered, several resulted in similar probabilities of being the most effective.
Discussion
Mixed-treatment comparison models are an effective method of handling evidence from many trials on several treatments in one analysis. Like all models, they require assumptions to be made that may or may not be reasonable. In this case, the assumptions used in this analysis indicate that the results should be interpreted with a degree of caution.
The output from the MTC model shows wide credible intervals for some treatments. For example, the intervals for the cure rates for ‘PFMT plus ES and BF’ and ‘PFMT plus VCs and BF’ are so wide (on a bounded scale) that we know very little about the effectiveness of these treatments. This is due to there being very few data about the cure rates for these treatments; the data for each treatment come from only one trial, with ‘PFMT plus ES and BF’ having 46 participants in its arm and ‘PFMT plus VCs and BF’ having 16.
There are some results that are quite clear and useful. There is clear evidence that both ‘PFMT with extra sessions’ and ‘PFMT with BF’ are better at cure than the following: no treatment; PFMT basic; ES; VCs; SNRI; and PFMT plus ES. Both ‘PFMT with extra sessions’ and ‘PFMT with BF’ are also better at improvement than: no treatment; PFMT basic; ES; VCs; and SNRI. The evidence also suggests that ‘PFMT plus BT and BF’ is better than each of the above listed treatments for cure and improvement, respectively, except for VCs, for which there is no clear evidence of a difference in improvement rates. However, conclusions about ‘PFMT plus BT and BF’ must be considered with caution, as this appears as a treatment in only one trial.
The results of the model indicate where further research might be beneficial, for example ‘PFMT plus BT and BF’ had reasonable success in the one trial that is in the data set, and its comparisons with other treatments look promising.
Comparison of the results from the model with PFMT, separated into basic and extra sessions with the combined PFMT model, shows that the median ORs between the other treatments can be different. The nature of the MTC model is such that splitting a treatment into two has an effect on the comparisons between other treatments. However, the split PFMT models do include additional trials (three for cure and two for improvement) that compare the two PFMT intensities alone, which were not used in the combined PFMT model. This appears to account for most of the discrepancies between the results of the split PFMT model compared with the combined PFMT model.
Summary
PFMT appears to be more effective when delivered with extra sessions (more than two sessions per month). Using this format of PFMT or adding BF to PFMT appears to be more effective than the other standalone treatments that have been analysed: no treatment, PFMT basic, ES, VCs, and SNRIs. Adding ES as an adjunct may also be effective.
The strengths of this approach are that all of the treatments of interest are compared in one analysis, facilitating indirect evidence to support direct evidence.
The limitations of the models lie in the assumptions taken about the equivalence of patient- and clinician-assessed success, and that the different time intervals over which the outcome is assessed within the separate trials is unimportant. The credible intervals around the estimates are often wide, indicating that there is still considerable uncertainty about the values of some parameters.
Chapter 9 Assessment of cost-effectiveness
A formal systematic review of existing economic evaluations was not attempted, as initial scoping searches failed to identify any existing prior economic evaluations that considered all comparators from the perspective of the UK NHS. Therefore, this chapter focuses on presenting the methods and results of a de novo economic model.
The subsections ‘Model framework’ and ‘Summary of key assumptions made in the economic model’ (see Methods, below) describe the basics of the modelling approach and the key assumptions underpinning the estimates of cost-effectiveness results. The remainder of the Methods section describes, in detail, how parameter estimates for the economic model were derived and how these data were used to estimate cost-effectiveness. The results from the economic model are presented in three sections. In the first section, the results of the economic model are presented in terms of number of people cured, cured or improved (referred to in the text as ‘improvement’) or incontinent in the model at different time horizons (see Results of the model presented in terms of costs and consequences). In the second section the results are based upon patients being cured of incontinence (see Cost–utility analysis based upon cure rates). In the third section (see Results based upon improvement rates), results are presented when successful treatment is defined as being either cured or improved. The final section of this chapter provides a short summary of the results.
Methods
As explained in Chapter 3, the economic model described the pathways of care for alternative management strategies of SUI. The perspective adopted for the analysis is that of the NHS (the main health service provider within the UK).
Model framework
A Markov model portraying the temporal and logical sequence of the clinical decision problem was used to provide estimates of costs, effectiveness (measured in QALYs) and cost-effectiveness. The health states within the model are considered to reflect possible outcomes of therapy, for example successful treatment (i.e. cure or improvement), failure (i.e. incontinence) and recurrence of incontinence. Graduation in the severity of incontinence was otherwise not considered in the model due to paucity of the available data on effectiveness.
In the model, women receive an initial treatment and then move into one of three states: success, failure or death. If a woman considers herself cured (or sufficiently improved so that she no longer feels the need for further treatment) then the treatment is successful. If the treatment does not result in cure (or sufficient improvement) then the woman enters the failure state. There is always a chance that over any period of time that a woman would die. If this should happen, they then move into an absorbing state (a state from which they cannot move): death. Death takes into account that patients are exposed to a very small risk of death when they are undergo surgery as well as a chance of dying from other causes at any point in time, assumed to be equivalent to all-cause mortality.
In a Markov model, people stay in a state for a minimum period of time (called the cycle length). In this model the cycle length was 3 months. This cycle length was chosen as it represents the recommended/widely used duration of PFMT before reassessment. Women who enter into the success state, stay in that state for 3 months, after which time, if they are still alive, they either continue to stay in this state or suffer a recurrence and go to the next available treatment. If a woman becomes incontinent after previously being cured, or after a treatment has failed, she proceeds to the next treatment in the strategy.
Women can continue moving through states in the model for a maximum of 40 years (equivalent to 160 cycles). This time horizon was chosen as it takes into account the average life expectancy of women who enter the model at the age of 45, which, as Figure 1 shows, is that age of peak incidence of SUI. Costs and benefits that occur in the future are discounted following standard practice. The discount rate used is the recommended rate of 3.5% for both costs and benefits. 105
It is assumed that all women are initially given advice, if appropriate, about modifying their lifestyles. The interventions included in the management strategies are thus: lifestyle changes (LS); basic PFMT (PFMT basic); PFMT with extra sessions (PFMT extra sessions); PFMT plus BF; PFMT plus ES; ES; VCs; drug treatment (SNRI), surgery [tension-free vaginal tape (TVT) or other similar self-fixing sling, e.g. transvaginal obturator tape], and the second surgery. Once all of the treatments are exhausted, it is assumed that women have to manage their symptoms using containment products. Table 52 summarises the potential strategies that are considered in the model.
| Treatment sequence | ||||||
|---|---|---|---|---|---|---|
| First treatment | Second treatment | Third treatment | Fourth treatment | Fifth treatment | Sixth treatment | |
| 1 | Lifestyle + PFMT basic | TVT/TVT-O | Second surgery | Containment | ||
| 2 | Lifestyle + PFMT basic | PFMT extra sessions | TVT/TVT-O | Second surgery | Containment | |
| 3 | Lifestyle + PFMT basic | PFMT extra sessions | SNRI | TVT/TVT-O | Second surgery | Containment |
| 4 | Lifestyle + PFMT extra sessions | TVT/TVT-O | Second surgery | Containment | ||
| 5 | Lifestyle + PFMT extra sessions | SNRI | TVT/TVT-O | Second surgery | Containment | |
| 6 | Lifestyle | TVT/TVT-O | Second surgery | Containment | ||
| 7a | Lifestyle + PFMT basic | VC | TVT/TVT-O | Second surgery | Containment | |
| 8a | Lifestyle + PFMT basic | ES | TVT/TVT-O | Second surgery | Containment | |
The difference between basic PFMT and PFMT with extra sessions was based upon the number of supervisory sessions that a woman had per month. For basic PFMT it was assumed that the woman would have six sessions in 3 months, whereas PFMT with extra sessions was defined as having 12 sessions in 3 months. 43
Pelvic floor muscle training with BF has not been explicitly included in this model, as its effectiveness is similar to the effectiveness of PFMT with extra sessions and it is plausible that the costs of the two types of therapy are similar. The impact of difference in costs for PFMT with BF is explored within a sensitivity analysis.
Figure 35 depicts an example of one of the management strategies used in the model (a more detailed description of the model is provided in Appendix 24). A woman that is diagnosed with SUI is offered PFMT. If this treatment is successful then the patient stays in a success state. When a treatment fails the woman is offered the next available treatment in that particular strategy. In this example, if PFMT fails in the next cycle the woman is offered PFMT plus an adjunct. If this fails, the woman is offered the next available treatment, drug therapy, and so on. If necessary the woman will receive each treatment until the treatment options are exhausted and their incontinence has to be managed with containment products.
FIGURE 35.
Markov model for one of the strategies. *Adjuncts include, for example, biofeedback and electrical stimulation.

Summary of key assumptions made in the economic model
Outlined below are the key assumptions that are made within the economic model. Details about how these assumptions were arrived at and their justifications are provided in the remainder of this section.
Assumptions related to the structure of the model
-
The age of the women considered in the model is 45 years. In sensitivity analysis, different starting ages were considered.
-
Cumulative costs and effectiveness were estimated for a 40-year time horizon. This would cover the average life expectancy of women aged 45 years. In sensitivity analyses the effect of shorter time horizons on costs, effects and cost-effectiveness was considered.
-
Costs and effects that occur in the future are given less weight in the analysis than costs and effects that occur in the present, i.e. they are discounted. This is recommended practice for any economic evaluation. Different discount rates were considered in a sensitivity analysis.
-
The cycle length of the model is 3 months. This cycle length determines the minimum period of time over which a woman’s continence status might change or over which treatments might change. This period was chosen as it represents the recommended/widely used duration of PFMT before reassessment.
-
Within the model, women can either be cured (or improved in the version of the model based on improvement rates) or be incontinent. Severity of disease is not otherwise considered in the model because of lack of data.
Assumptions related to the treatment strategies compared
-
The treatment strategies compared consist of a finite number of treatments. In reality women whose symptoms are not controlled to their satisfaction could continue seeking treatment until they have what they consider adequate control of their symptoms.
-
All women are initially given advice, if appropriate, about modifying their lifestyles.
-
If a woman becomes incontinent after previously been cured, or after a treatment failed, she would proceed to the next treatment in the strategy. Sensitivity analysis was performed to explore the relaxation of this assumption.
-
In the cure model analysis women were assumed not to use containment products until they had exhausted all treatments in a treatment strategy. Sensitivity analysis was performed to explore the relaxation of this assumption.
-
In the improvement model all women were assumed to use containment products. Those women who were improved used less containment products than those who were not improved. Sensitivity analysis was performed to explore the relaxation of this assumption.
-
The probability of drug therapy being successful after suffering an adverse event was assumed to be zero as we expected the woman to stop taking the drug when she suffered an adverse event.
Assumptions relating to effectiveness
-
The median values of cure and improvement rates were used in the economic model, as the data were highly skewed and the median was believed to provide a better representation of the actual difference.
-
Long-term cure rates of all interventions were based on extrapolation of available data.
-
When making an extrapolation from these data, it was assumed, for drug therapy, that at 12 months only 5.8% of those initially cured remain cured, which is equivalent to the spontaneous cure estimated for no treatment.
-
The estimates of cure and improvement rates for all treatments were assigned log-normal or normal distributions, based on the assumptions made for the mixed-treatment model in Chapter 8.
Assumptions relating to costs
-
The difference between basic PFMT and PFMT with extra sessions was based upon the number of sessions that a woman had per month.
-
The resources used in PFMT plus BF were based on the assumption that the staff providing the service was the same as those providing PFMT and that the number of sessions were the same as those for PFMT.
-
The costs of VCs were based on the assumption that the labour costs were one-third of those for PFMT (i.e. two visits with the physiotherapist).
-
The costs of exercise dairies and leaflets were considered to be negligible and it was also assumed that all patients would receive them. Therefore, their costs were not included.
-
All treatment costs were assigned log-normal distributions, as this distribution appeared to best fit the data that have skewed or symmetric ranges.
Estimation of model probabilities
The main probabilities for the model are the cure rates, improvement rates and recurrence rates of different interventions and mortality rates.
Relative differences in cure and improvement rates
The estimates of cure and improvement rates for the interventions considered in the model are based on the results of the mixed-treatment model reported in Chapter 8. Table 53 describes the median ORs for the comparison with either no treatment or PFMT. The median values were used in the economic model, as the data were highly skewed and the median was believed to provide a better representation of the actual difference.
| Intervention vs comparison | Cure rates | Improvement rates | ||
|---|---|---|---|---|
| Median | 95% CrI | Median | 95% CrI | |
| Used in base-case analysis | ||||
| PFMT basic vs NT | 1.28 | 0.55 to 2.92 | 4.47 | 2.03 to 10.9 |
| PFMT extra sessions vs NT | 10.7 | 5.03 to 26.2 | 25.7 | 10.3 to 73.1 |
| SNRI vs NT | 1.43 | 0.58 to 3.46 | 2.14 | 1.06 to 4.4 |
| Used only in sensitivity analysis | ||||
| ES vs NT | 1.45 | 0.55 to 3.86 | 5.49 | 2.39 to 13.7 |
| VC vs NT | 3.55 | 1.23 to 10.9 | 6.77 | 2.6 to 19.4 |
| ES vs PFMT basic | 1.13 | 0.39 to 3.32 | 1.23 | 0.45 to 3.25 |
| VC vs PFMTbasic | 2.77 | 0.98 to 8.51 | 1.52 | 0.58 to 3.97 |
Absolute cure and improvement rates
As mentioned in Chapter 8, while the main parameters of the model are the log ORs of the treatments, the absolute success rate for each treatment can be calculated from the relative success rates, if the absolute success rate for one treatment is known. The absolute cure and improvement rates were calculated in the model by combining the information on relative cure and improvement rates described in Table 53, above, with the absolute cure and improvement rates for no treatment which was taken to be the reference treatment. The absolute cure rate at 3 months for no treatment was median 5.8% (95% CrI: 3.3% to 9.9%) and the improvement rate at 3 months was 26.3% (95% CrI: 15.7% to 40.5%) (Chapter 8).
Transition probabilities
Recurrence rates of PFMT
Despite extensive searching, few data were identified on the long-term effectiveness (greater than 1 year) for any of the interventions. Therefore, estimates used in the model were based on extrapolations of the available data.
Only four reports159,207–209 provided long-term follow-up of women who had received PFMT. One report209 indicated that at 6 years no significant differences were found in urinary incontinence prevalence, severity or leakage episodes in the women who responded to the questionnaire between groups for PFMT with extra sessions and basic PFMT. However, this study was based on postnatal women, whom we regarded as not being representative of women with SUI. A further three reports published by other authors provided outcome data at 5 years207 and 15 years208 after receiving PFMT were identified. One report208 looked at two different intensities of pelvic floor muscle exercises over a period of 15 years. Hence, data from these three reports have been used in the model, although the sample sizes were small. Details of the values taken from these papers are described in Table 54.
| Values | Number of patients | |
|---|---|---|
| PFMT basic | PFMT extra sessions | |
| Randomised | 31 | 26 |
| At 6 months | 29 | 23 |
| At 5 years | NR | 20 |
| At 15 years | 26 | 21 |
| Surgical interventions at 5 years | 9 | 3 (3/23) |
| Surgical interventions at 15 years | 4 | 8 (8/21) |
| Pad test < 2 g (cure) at 5 years | NR | 6 (6/20) |
| No visible leakage on stress pad test at 6 months | NR | 17 (17/20) |
| No visible leakage on stress pad test at 5 years | NR | 15 (15/20) |
| Dry on severity index at 15 years | 4 (4/25) | 6 (6/20) |
Extrapolation of the long-term cure rates used in the model was generated from Kaplan–Meier survival curves, using a linear exponential distribution that manipulated by adjusting the two parameters (defined below). Using this process it is possible to estimate a hazard function using the values reported in Table 55.
| Time (years) | Probability of cure for: | |
|---|---|---|
| PFMT basic | PFMT extra sessions | |
| 0 | 1 | 1 |
| 5 | 0.67a | 0.75b |
| 15 | 0.16b | 0.30b |
The formula used to extrapolate the survival function [S(t)] is:
where:
-
S(t) is the probability of cure at any given time t
-
t is time (measured in terms of the number of cycles, where each cycle is equivalent to 12 weeks)
-
λ is the scale parameter, which describes the probability that a woman will remain/become incontinent during the next time period, given that she was continent in the current period
-
γ is the shape parameter, which, in simple terms, describes the rate of change in the probability that a woman will become incontinent over time.
Appendix 25 described how the transition probabilities (i.e. the chance or remaining cured or suffering a recurrence of symptoms) were calculated using the above formula. Figure 36 shows the shape of the linear exponential curve that was fitted to the data reported in Table 55.
Recurrence rates for other physical therapies
The recurrence rate for physical therapies was generated by multiplying the failure of PFMT by the relative effect sizes derived in the MTC reported in Chapter 8 and summarised in Table 50.
Recurrence rates for drug (SNRI) therapy
As with the PFMT, there were very few studies that had long-term follow-up of women who were using drugs to treat their incontinence. Four relevant studies were identified as part of the systematic review studies57,136–138 and an unpublished study from Eli Lilly and Company. 211 These studies had an unclear length of follow-up, a follow-up of less than 1 year or were unpublished. One study was identified that reported recurrence rates for a cohort of women at different time points after the initiation of drug treatment. 212 In making an extrapolation from these data it was assumed that at 12 months only 5.8% of those initially cured remain cured, which is equivalent to the spontaneous cure estimated for no treatment. This rate is lower than the 9% cure rate reported by Vella and colleagues. 212
Extrapolation of cure rate (disease free) was estimated from a Kaplan–Meier curve using a Weibull survival model from the data reported in Table 56. The Weibull distribution was chosen for this extrapolation as it was felt to have a suitable functional form to estimate a survival. The Weibull distribution is defined by two parameters: the scale parameter (λ) and the shape parameter (γ). The scale parameter describes the probability that the woman becomes incontinent during the next time period, given that she is continent during the current time period. The shape parameter describes the hazard function of Weibull function for the survival time. The hazard function for Weibull survival time could be increasing or decreasing with time, depending on the value of parameter γ. If the value is greater than 1, the hazard rate increases with time. If the shape parameter is less than 1, the hazard decreases with time. If the shape parameter is equal to 1, then the Weibull distribution is equivalent to an exponential distribution. Figure 37 describes the curve fitted to the data reported in Table 56. An approximate hazard function for the curve is given by:
where:
-
S(t) is the probability of cure in any given cycle
-
t is time (measured in terms of the number of cycles, where each cycle is equivalent to 12 weeks)
-
λ is the scale parameter that describes the probability that the woman becomes incontinent during the next time period, given that she is continent during the current time period
-
γ is the shape parameter, which describes the hazard function of Weibull function for the survival time.
| Time (months) | Cure rate |
|---|---|
| 0 | 1 |
| 1 | 0.31 |
| 4 | 0.12 |
| 6 | 0.1 |
| 12 | 0.09a |
FIGURE 37.
Extrapolated long-term cure rates after drug therapy. Note that each cycle is 3 months long. Based on Vella and colleagues (2008). 212
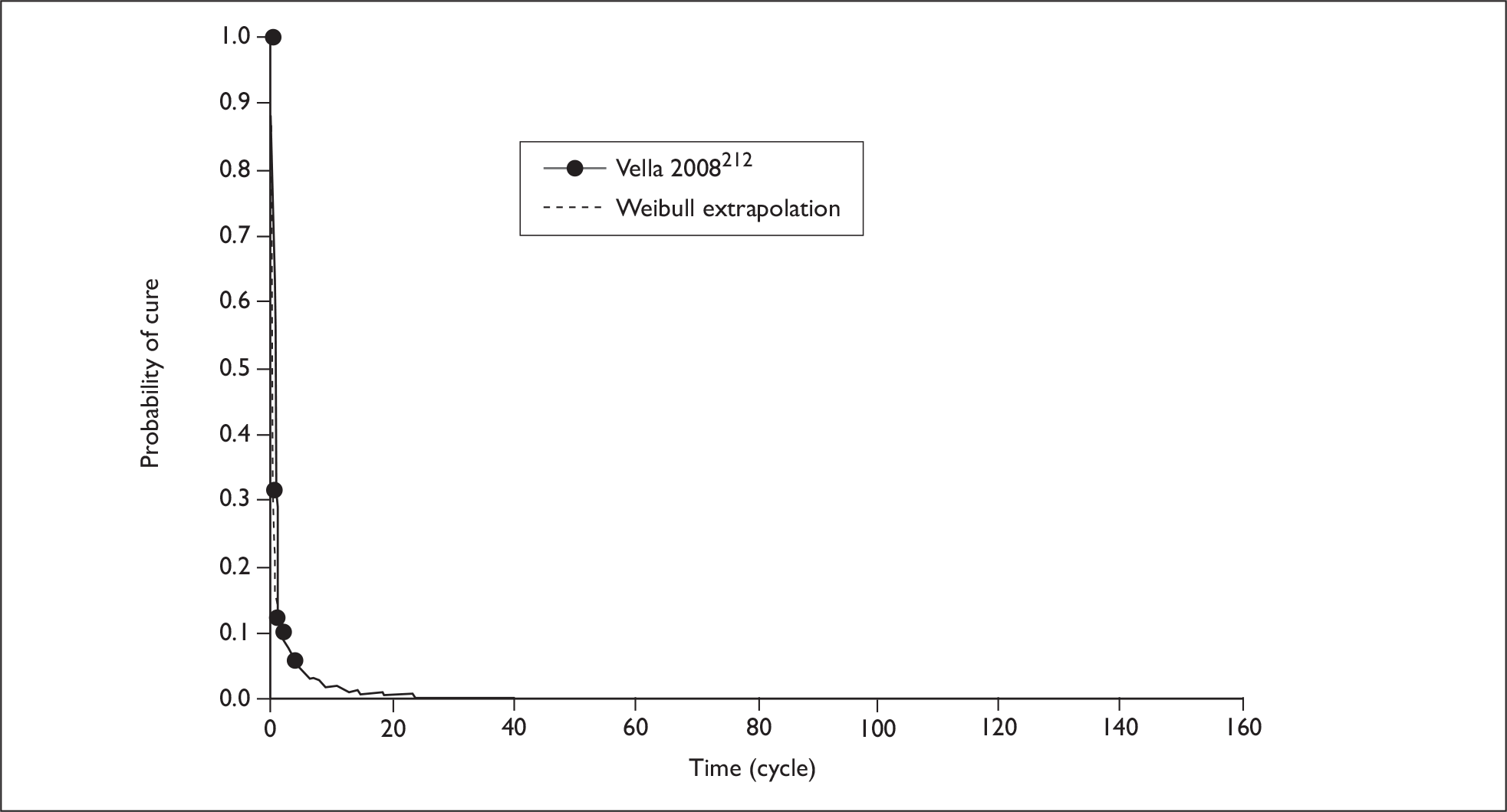
Appendix 25 describes how the above equation was used to calculate the transition probabilities required for the model (e.g. the probability of a woman who is currently cured suffering a recurrence in the next cycle and the probability of remaining cured).
Recurrence rates of TVT
The long-term recurrence rates of TVT were derived using the long-term cure rates of a recent trial conducted in the UK, comparing TVT with Burch colposuspension. 213 The data from reported cure rates for up to 5 years were used to estimate longer-term recurrence rates using a Weibull survival model (Figure 38), from the data reported in Table 57. As was the case for drug treatment, this model was chosen because it was felt to provide a reasonable representation of the estimated long-term recurrence rates.
| Time (years) | Rate of continence |
|---|---|
| 0 | 1 |
| 0.5 | 0.85 |
| 2 | 0.80 |
In this trial the cure rate at 5 years was 0.81, which is 0.01 higher than it was at 2 years, which is likely to be caused by women being lost to follow-up. Therefore, the reported data at 5 years were not used in the extrapolation, although it did help inform assumptions about what proportion of women might remain cured at 5 years.
The following survival hazard formula was defined:
where:
-
S(t) is the probability of cure
-
t is time (measured in terms of the number of cycles, where each cycle is equivalent to 12 weeks)
-
λ is the scale parameter, which describes the probability that the woman becomes incontinent during the next time period, given that she is continent during the current time period
-
γ is the shape parameter, which describes the hazard function of Weibull function for the survival time.
The transition probabilities used in the model were calculated using the formula shown in Appendix 25.
Other parameters
The other parameters (Table 58) considered in the model were:
-
The probability of adverse events (drugs only). The estimate used in the model was generated from the systematic review reported in Chapter 7 (see SNRI drug therapy).
-
The chance that women may still need to use containment products when undergoing any of the interventions. In the base-case analysis it was assumed that if women were cured then they did not need to use containment products. This assumption was tested in a sensitivity analysis. In the improvement model all women were assumed to use containment products. The cost of the containment product used by those who were improved was based on the least expensive type of containment product used (menstrual pads). 84
-
Risk of death attached to surgery. This information was taken from a previous systematic review that reported the risk of death from open colposuspension surgery. 80
| Probability | Value | Source |
|---|---|---|
| Probability of adverse event while on drug therapy | 0.45 | Estimated from systematic reviewa |
| Probability that women who failed PFMT, or for whom symptoms recurred, managed their symptoms thereafter with containment products | 0 | Assumption |
| Probability of continuing to use containment products after PFMT has failed or symptoms have recurred | 0 | Assumption |
| Probability that women who failed PFMT + adjunct, or for whom symptoms recurred, managed their symptoms thereafter with containment products | 0 | Assumption |
| Probability of continuing to use containment products after PFMT + adjunct has failed or symptoms have recurred | 0 | Assumption |
| Probability that women who failed PFMT, etc., or for whom symptoms recurred, managed their symptoms thereafter with containment products | 0 | Assumption |
| Probability of continuing to use containment management after PFMT, etc. has failed or symptoms have recurred | 0 | Assumption |
| Probability that women who failed with drugs, or for whom symptoms recurred, managed their symptoms thereafter with containment products | 0 | Assumption |
| Probability of continuing to use containment products after drugs have failed or symptoms have recurred | 0 | Assumption |
| Probability that women who failed following surgery, or for whom symptoms recurred, managed their symptoms thereafter with containment products | 0 | Assumption |
| Probability of continuing to use containment products after surgery has failed or symptoms have recurred | 0 | Assumption |
| Probability of the first surgery being successful | 0.87 | Estimated in the model |
| Probability of a second surgery being successful | 0.85 | Estimated in the model |
| Probability of drug therapy being successful after suffering an adverse event | 0 | Urinary incontinence guideline, 200643 |
| Mortality rates of surgery | 0.0005 | Cody 200380 |
All-cause mortality rates in the UK
As a woman moves through the model there will be some chance of that the woman might die. The likelihood that a woman might die was based upon the annual rates of age-specific all-cause mortality for women [based on the Office for National Statistics (ONS) interim life tables 2004–06 (database on the Internet)]. 217 Figure 39 shows the survival curve for females for the UK and Appendix 25 reports the rates of all-cause mortality used in the model.
FIGURE 39.
Age-adjusted survival Kaplan–Meier curve for females in the UK.

Resource utilisation and cost estimation
Resource use data was identified from existing studies,43 relevant literature (e.g. reports from manufacturers) and advice from experts in this field. The resources used to provide the non-surgical interventions included:
-
the number of visits to the practitioners for the sessions of therapy
-
the staff time, the appropriate grade and direct overheads associated with delivering health care, such as clerical support and administration of the sessions of therapy
-
the consumables required to provide service
-
the reusable equipment used.
The costs of exercise dairies and leaflets were considered to be negligible and it was also assumed that all patients would receive them. Therefore, their costs were not included.
Lifestyle changes
The cost of lifestyle changes were based on the cost of a single visit to the GP.
PFMT basic
As indicated in the recent NICE guidance,43 it was assumed that PFMT comprised six sessions. As acknowledged in the guidance, it is difficult to define a ‘standard’ or ‘typical’ PFMT session, and, hence, in reality, costs will vary according to the actual care provided. Within the model it was assumed that the first session of PFMT would last for 1 hour and the other five sessions would last for 30 minutes each. Each session would be conducted by a senior grade 1 women’s health physiotherapist in a hospital physiotherapy department. The consumables that would be required per session would be: gloves, KY Jelly, wipes and paper towels.
PFMT with extra sessions
The costs of PFMT with extra sessions were derived in the same way as those of basic PFMT described above, but the number of sessions was increased from six to 12.
PFMT plus BF
The resources used for PFMT plus BF were based on the assumption that the staff providing the service was the same as those providing PFMT alone, and that the number of sessions were the same as those for PFMT. Additional resources required for the BF were the equipment used, for example the hand-held single-channel EMG channel, the Neen Educator and Neen Periform vaginal probe. The educators and the probes were treated as consumables, as they were used by only one woman over all of the sessions. However, the NeuroTrac device™ was loaned to women for 3 months. Based upon information from manufacturers and from NHS users, this piece of equipment was considered to have a lifespan of 5 years, the equivalent annual cost of the equipment was calculated (using a 3.5% discount rate as recommended by the UK Treasury) and the cost per woman took into account that the equipment was used by four women per year. These data were used to help interpret the results of ‘PFMT with extra sessions’ only when it was used in the model. This is because the effectiveness of PFMT with extra sessions was similar to PFMT plus BF, and it is plausible that the costs of the two types of therapy were similar.
Vaginal cones
Although VCs are recommended as a first-line treatment, they are not routinely provided by the NHS. Cones are often bought over the counter after GP advice. The cost of VCs was based on the assumption that the labour costs were one-third of those for PFMT (i.e. two visits with the physiotherapist). Although VCs are not currently provided by the NHS, the women using VCs would still have two visits with the physiotherapist. The consumables required and consumables used were cones, gloves, paper towels and KY Jelly.
Electrical stimulation
Electrical stimulation can be provided either at home or in a clinic. The difference between the two ways of providing the therapy relates to the number of sessions that the woman receives. Home-based ES has three sessions, whereas the clinic-based therapy has 13 sessions (although this may vary in practice), with the first session lasting 1 hour and the remainder lasting 30 minutes. For the home-based ES, the woman has a 1-hour-long session in the hospital physiotherapy department to determine an appropriate programme and then two follow-up sessions. The Neen Pericalm device is loaned to women for home use for 3 months. In this analysis it was assumed that 13 sessions would be provided by a physiotherapist in a hospital department. The resources used included a Neen Periform vaginal electrode and data-reading clinical equipment, along with the same consumables that were used in the provision of PFMT (gloves, KY Jelly, couch roll, wipes and paper towels). Based upon information from manufacturers and from NHS users, the equipment was assumed to have a lifespan of 5 years and an equivalent annual cost of the equipment was calculated (using a 3.5% discount rate as recommended by the UK Treasury). A total of 200 women would be able to use it each year in a clinical setting.
Medical therapy
The cost of drug treatment was based on two consultations (an initial consultation and a review consultation) with the GP, and the drug costs for each cycle (3 months).
Surgical interventions
Surgical costs were based on the average costs of an elective minor lower urinary tract procedure without complications. 54
Containment products
The costs of containment products (such as disposable insert and menstrual pads, and washable pants with and without insert pads) were based on information reported in a systematic review of containment products. 84 The cost of other containment products, such as urethral plugs, were not included in the base analysis.
Cost estimation
As described above, costs focused on the direct health service costs that were associated with each treatment. Unit cost data were extracted from the literature or from relevant sources, such as manufacturer price lists and NHS reference costs. The year of the cost data is 2008 and the currency is pounds sterling (£). Table 59 provides a summary of the costs for each intervention.
| Intervention | Cost per cycle (£)a | Range (£) | Notes and comments |
|---|---|---|---|
| Lifestyle changes | |||
| Cost per visit | 27 | 13–40 | Range based on 1 or 3 visits to GP (Curtis 2008)46 |
| PFMT | |||
| PFMT basic | 189 | 135–243 | Range based on 4 and 8 sessions |
| PFMT + BF | 224 | 175–388 | Range based on 4 and 8 sessions |
| ES | 398 | 206–481 | Range based on 8 and 16 sessions |
| VCs | 93 | 83–103 | Assumed provided at the hospital |
| PFMT extra sessions | 351 | 243–459 | Based on 8 and 16 sessions |
| Drug therapy (SNRI) | |||
| Appointments and drugs for 12 weeks | 164 | 128–200 | Initial cost based on 2 appointments (initial and review) and the range is based on 1 or 3 visits to GP |
| Surgical therapy | |||
| TVT/TVT-O | 1135 | 741–1357 | Based on lower and upper interquartile range of reference costs (2008) for elective surgery on lower-tract minor procedures without complications |
| Colposuspension | 1396 | 1002–1618 | Range estimation based on TVT range |
| Containment products | |||
| Washable inserts | 39 | 9–75 | Initial cost based on cost per month for washable insert pants; range based on the minimum (menstrual pads) and maximum (disposable insert pants) cost of containment |
Quality of life
Summary of a structured review of reports on health-state utilities
The primary outcome for the economic analysis was QALYs. The QALY estimates within the model analysis are mainly determined by whether a woman was continent or not, as the risk of death from SUI or any of the treatment options is very low. Although there is considerable evidence about the quality of life of women with SUI, this tends to be measured using condition-specific tools, such as the I-QoL. Such data are not ideal for incorporation into an economic evaluation. As an economic evaluation seeks to inform choices about how best to allocate society’s scarce resources, it has been argued that changes in quality of life should reflect society’s valuation. One quality-of-life instrument that has a scoring system based on the preferences of the UK population is the EQ-5D. This method is the approach preferred by NICE105 in HTAs, although there may be some concerns that it is not sufficiently sensitive to changes in the symptoms of incontinence.
Estimates of the EQ-5D scores for a sample of women suffering from SUI were reported in Chapter 4. Further data were identified from the literature search that was performed for the effectiveness review and these were supplemented by information from NHS Economic Evaluation Database (EED) and the Cost-Effectiveness Analysis (CEA) Registry at Tufts Medical Center. 218 From this search, only three published studies were identified. 219–221 The first study221 was a pan-European study of women with urinary incontinence, who sought treatment, the second study219 was a clinical trial of group versus individual physiotherapy for women with SUI and the final study220 was an economic evaluation alongside an RCT, conducted in the UK, of TVT compared with Burch colposuspension.
In the first study, conducted in 14 European countries, the median EQ-5D score of women with urinary incontinence that sought treatment was 0.85. The mean score for women in the UK was 0.73 and the median 0.85. 221 Another study on the same population of women reported 0.76 as the adjusted OR for EQ-5D health-state index score. 222
Haywood and colleagues219 reported EQ-5D scores for women based on the number of incontinence episodes at baseline. Those with no episodes has a mean score of 0.85 (SD 0.24). When incontinence was suffered on a few days the mean score was 0.85 (SD 0.16). As would be anticipated, the mean score declined as severity of incontinence increased. For example, when incontinence was suffered for ‘about half the week’ the mean score was 0.81 (SD 0.20); for ‘most days’ the mean score was 0.79 (SD 0.23) and for ‘everyday’ the mean score was 0.75 (SD 0.32). 219 This study219 also reported EQ-5D scores based upon the perceived benefit from physiotherapy at 6 weeks and 5 months. At 6 weeks the mean score from those who said they had benefited was 0.85 (SD 0.23) and for those who said that they had no benefit was 0.73 (SD 0.31). At 5 months the mean scores were 0.85 (SD 0.24) and 0.74 (SD 0.38) for those who said they did and did not benefit from physiotherapy, respectively.
The third study published on women who were receiving surgery for SUI reported quality of life at baseline and at trial follow-up. 220 These data were subsequently manipulated in an HTA trial comparing different surgical treatments. 80 The EQ-5D score for women prior to surgery was 0.778 for women randomised to TVT, and 0.785 for women randomised to colposuspension. 220 Finally, as reported in Chapter 4, the survey of members of InContact previously identified as suffering from SUI reported a mean EQ-5D score of 0.598 (SD 0.339). The mean age for the women involved in this sample was 57 years (range 28–89).
Derivation of values used in the model
It was thought possible that the women involved in the survey reported in Chapter 4 might be atypical of the average woman with SUI, as they were part of a self-selected patient group. Therefore, in the economic model the utility score was based on the study by Manca and colleagues,220 and failure after treatment was based on the study by Haywood and colleagues. 219 This value was that of women who perceived that they did not benefit from physiotherapy treatment, thus indicating that it had failed. The utility assigned to successful treatment was taken as 0.85. This value was based on the women who perceived that they benefited from physiotherapy treatment at 6 months after treatment (Table 60).
Data analysis
Cost–utility analysis
As women with SUI might be either cured or ‘cured or improved’ (i.e. not requiring further treatment but possibly still having some degree of incontinence), two separate analyses were conducted. One analysis was based upon the reported cure rates (see Cost–utility analysis based upon cure rates, below) and a second analysis based upon improvement rates (see Results based upon improvement rates, below).
The estimation of the costs and consequences of the different management strategies was performed using a hypothetical cohort of 1000 women, starting at age of 45 years (the identified prevalence average age of women with SUI in Chapter 1) over a 40-year horizon. Results are presented as incremental cost per QALY. These are ratios of the differences in costs of the interventions divided by the differences in effectiveness between the different strategies. These data show the rate of return (in QALYs) to the quantity of resources used (measured in monetary terms). If for any given incremental cost-effectiveness ratio (ICER) it is judged that a treatment is efficient then it implies that society is willing to pay at least that amount to obtain an additional QALY. The value society is willing to pay for a QALY is unclear, but, typically, NICE, within the UK, recommends interventions when the incremental cost per QALY is less than £20,000–30,000. 105
Sensitivity analyses
With all parameter estimates there are elements of uncertainty owing to the lack of available information. In order to explore the importance of such uncertainties and assumptions, various sensitivity analyses were conducted by varying some of the assumptions or parameters made in the model. Two types of sensitivity analyses were performed: a probabilistic sensitivity analysis and a deterministic sensitivity analysis.
Probabilistic sensitivity analysis
One area of uncertainty within the economic model is precision of the parameter estimates used. Many of the parameters are not precisely known but the uncertainty surrounding a point estimate can be described using a statistical distribution. Probability distributions were applied to the specific ranges of the key parameters (Table 61), such as costs and samples drawn at random from these distributions to generate an empirical distribution of the cost-effectiveness ratios. All treatment costs were assigned log-normal distributions, as this distribution appeared to best fit the data that have skewed or symmetric ranges. There was no distribution to be assigned for probabilities of recurrence of all treatment (PFMT, drug and TVT surgery) due to the paucity of data. There was also no distribution to all-cause mortality rate as the number of observation used to calculate the risk was very large. The estimates of cure and improvement rates for all treatments were assigned log-normal or normal distributions based on the assumptions made in the mixed-treatment model in Chapter 8.
Deterministic sensitivity analysis
The second type of analysis undertaken to handle parameter uncertainty was to consider changes in one or more parameter value at the same time. This was used to explore structural uncertainty, extrapolations, methodological uncertainty, etc. This deterministic sensitivity analysis had been combined with probabilistic sensitivity analysis so that the joint effect of using different values (and distributions) and statistical imprecision surrounding estimates is explored. Outlined below are the details of the specific sensitivity analyses performed.
Recurrence rates of PFMT
As there were limited data relating to the long-term follow-up of PFMT, the long-term recurrence rates of PFMT in the base-case analysis were produced based on the values at 5 years210 and 15 years. 208 However, the identified data at 15 years in the study by Bø and colleagues208 was varied as in Table 55. Changes to the recurrence rates of PFMT are likely to alter cost-effectiveness of the strategies related to PFMT. Figure 40 describes the alternative long-term recurrence rates considered in the sensitivity analysis.
FIGURE 40.
Linear exponential curves with variation of recurrence rates of pelvic floor muscle training. Note that each cycle is 3 months long.
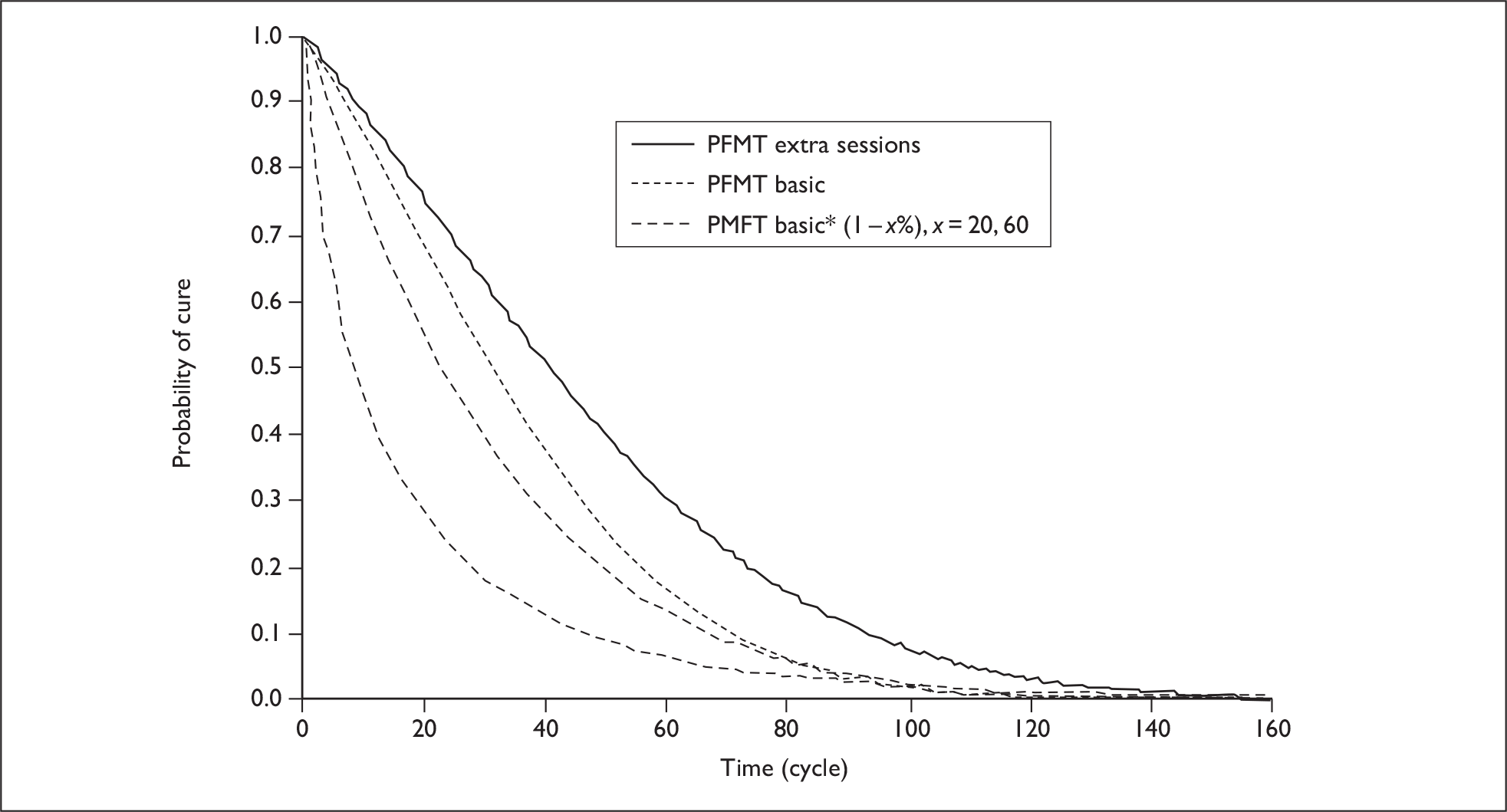
As described in the section Estimation of model probabilities, data on the long-term effectiveness of all physical interventions were not readily available. In the base-case model it was assumed that the recurrence rates for physical therapies could be derived by multiplying the recurrence of PFMT by the relative effect sizes derived in the MTC, reported in Chapter 8 and summarised in Table 50. The recurrence rates for PFMT came from Bø and colleagues. 208 Bø and colleagues208 reported that there was no difference at 15 years in the effectiveness of the two forms of PFMT compared. Therefore, in this sensitivity analysis it was assumed that the recurrence rates for all different physical treatments (PFMT plus BF, VCs and ES) had the same recurrence rate as PFMT.
Recurrence rates of TVT
As explained in Estimation of model probabilities, there was no reliable evidence related to the long-term follow-up of recurrence rate of TVT surgery. The trial by Ward and colleagues,213 which reported recurrence rates for up to 5 years’ follow-up, was used to estimate the recurrence rates of TVT surgery in the base-case analysis. This sensitivity analysis used the recurrence rates estimated in an earlier HTA that compared TVT surgery with other surgical treatments. 80 In the sensitivity analysis the long-term cure rates for TVT surgery were increased to 81% and reduced to 65% in the first year and 61% in the second year.
Starting age and time horizon
The incidence rate of SUI is likely to increase as age increases. The mean age of women with SUI in the UK is 45 years and this was taken to be the age of women in the cohort modelled. Women included in the trials included in the systematic review of effectiveness tended to be slightly older, with average ages varying from 50 to 60 years. The costs and effects in the model were estimated for a 40-year time horizon. This time horizon was chosen as it was felt to cover the expected life expectancy of women aged 45. However, few robust data are available for such a long follow-up.
In this sensitivity analysis the implications of varying the age of women at the start of treatment and the impact of adopting a shorter time horizon were explored. Therefore, the starting age of women was changed to 50, 55, and 60 years in the sensitivity analyses. The time horizons were likewise reduced to 10, 20 and 30 years.
Quality of life
As mentioned previously (see Quality of life), data from Manca and colleagues220 and Haywood and colleagues219 were used in the base-case analysis. Sensitivity analysis was performed using the EQ-5D scores derived in the survey reported in Chapter 4 to weight the utility scores taken from the study by Haywood and colleagues219 (Table 61).
The impact of the natural decline in quality of life over time was also considered in further sensitivity analysis. The values for the age-related reduction was derived based on published values for age-related quality of life. 223 The extrapolated values are illustrated in Figure 41 and Appendix 25.
FIGURE 41.
Linear extrapolation of EQ-5D score using EQ-5D adjusted by age. Note that extracted data refers to data taken from Kind and colleagues (1999). 223

Discount rate
As recommended in the NICE guidelines, an annual discount rate of 3.5% for costs and benefits was used in the base-case analyses. 105 A range of 1–6% for discount rate was considered in this sensitivity analysis.
Probability of moving to the next treatment following failure of prior treatment
Considerable efforts were made to identify the estimates for a probability that women would not seek further treatment should a treatment fail or symptoms recur, and would manage their incontinence using containment products, but few data are available. In the base-case analyses it was assumed that the women would go immediately to the next treatment after failure or recurrence. In this sensitivity analysis, the impact of allowing between 10% and 50% of women who experience a treatment failure or recurrence of symptoms not to seek further treatment was explored.
Use of containment products
Although there is anecdotal evidence that women use containment even when they are undergoing treatment, there were no data to inform what proportion of the women use containment products and what type of products they use. Therefore, in the cure and improvement model it was assumed all women used containment products, although the quantity/type used varied according to symptoms.
Mortality and success rate of TVT
Further sensitivity analyses were conducted by varying the mortality risk and success rates related to TVT in base case. In the base-case analysis a mortality risk based on the risk of undergoing open surgery (0.0005) was attached to TVT. In the sensitivity analysis this was reduced to zero. Sensitivity analysis was also performed to estimate the impact of an increase in the success rate of TVT by 5%.
Costs
As indicated earlier, it is difficult to define a ‘standard’ intervention, as practice varies greatly. The costs of interventions are dependent on the assumptions made about the number of sessions of therapy a woman could get. Maximum and minimum costs were derived by increasing and reducing the number of sessions. For example, the minimum cost of basic PFMT was derived by reducing the number of sessions to four and the maximum cost was derived by increasing the number of sessions to eight. Distributions were used in the analysis to incorporate the minimum and maximum values attached to the costs.
Results of the model presented in terms of costs and consequences
The results of the base-case deterministic analyses for 1000 women are presented in terms of summaries of the time spent cured (or improved for the improvement model), with incontinence and also the cumulative number of women who have received surgery. The results are also presented in terms of the cumulative QALYs and cost.
Analysis based on cure rates
For the analyses based on the cure rates, the strategy that used lifestyle changes and PFMT basic followed by PFMT with extra sessions followed by TVT surgery (LS–PFMT basic–PFMT extra sessions–TVT) had the best performance in terms of the highest number of successes (939 and 953) and lowest number of failures (59 and 36) at the 1- and 10-year time horizons, whereas the strategy that used lifestyle changes and PFMT basic followed by TVT surgery (LS–PMT basic–TVT) had the highest number of success at the 40-year time horizon (398). The strategy that had the least number of failures (28) was the one that used the lifestyle changes and PFMT basic followed by PFMT with extra sessions followed by SNRI followed by TVT surgery (LS–PFMT basic–PFMT extra sessions–SNRI–TVT). The strategy of lifestyle change and TVT surgery (LS–TVT) has the worst performance in terms of the lowest number of successes (726, 907 and 363) and the highest number of failures (272, 82 and 57) at all three time horizons. This strategy had the highest number of patients (998 and 989) receiving surgery at 1- and 10-year time horizons, and the strategy that used lifestyle changes and PFMT basic followed by TVT surgery (LS–PFMT basic–TVT) had the highest number (443) of patients receiving surgery at the 40-year time horizon (Table 62).
| Year | Strategy | Success performance | Receiving surgery | Population | QALYs/costs | ||
|---|---|---|---|---|---|---|---|
| Success | Failure | QALYs | Costs (£) | ||||
| 1 | LS–PFMT extra sessions–TVT | 927 | 71 | 393 | 1000 | 0.8 | 821 |
| LS–PFMT extra sessions–SNRI–TVT | 927 | 71 | 375 | 1000 | 0.79 | 855 | |
| LS–PFMT basic–PFMT extra sessions–TVT | 939 | 59 | 357 | 1000 | 0.78 | 930 | |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 936 | 62 | 333 | 1000 | 0.77 | 954 | |
| LS–PFMT basic–TVT | 845 | 153 | 926 | 1000 | 0.78 | 1261 | |
| LS–TVT | 726 | 272 | 998 | 1000 | 0.8 | 1186 | |
| 10 | LS–PFMT extra sessions–TVT | 950 | 39 | 512 | 1000 | 6.97 | 1290 |
| LS–PFMT extra sessions–SNRI–TVT | 946 | 43 | 500 | 1000 | 6.93 | 1349 | |
| LS–PFMT basic–PFMT extra sessions–TVT | 953 | 36 | 478 | 1000 | 6.90 | 1391 | |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 947 | 42 | 466 | 1000 | 6.86 | 1449 | |
| LS–PFMT basic–TVT | 932 | 57 | 937 | 1000 | 6.89 | 1676 | |
| LS–TVT | 907 | 82 | 989 | 1000 | 6.91 | 1733 | |
| 40 | LS–PFMT extra sessions–TVT | 386 | 31 | 416 | 1000 | 16.2 | 1644 |
| LS–PFMT extra sessions–SNRI–TVT | 380 | 28 | 406 | 1000 | 16.06 | 1727 | |
| LS–PFMT basic–PFMT extra sessions–TVT | 379 | 29 | 407 | 1000 | 16.02 | 1758 | |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 373 | 27 | 398 | 1000 | 15.89 | 1842 | |
| LS–PFMT basic–TVT | 398 | 45 | 443 | 1000 | 16.03 | 1886 | |
| LS–TVT | 363 | 57 | 420 | 1000 | 16.08 | 1973 | |
Analysis based on improvement rates
For the analysis based on improvement rates, the strategy that used LS–PFMT with extra sessions followed by TVT surgery had the best performance in terms of the highest number of successes (985, 951 and 410) at all the three time horizons and the lowest number of failures (13 and 22) at 1 and 10 years (Table 63). The strategy that used lifestyle changes and PFMT basic followed by PFMT with extra sessions followed by drug therapy followed by TVT surgery (LS–PFMT basic–PFMT extra sessions–SNRI–TVT) had the least number of failures (8). The strategy of lifestyle changes followed by TVT surgery (LS–TVT) had the worst performance in terms of the lowest number of successes (793, 913 and 382) and the highest number of failures (205, 60 and 40) at all three time horizons. This strategy also had the highest number of patients (998, 973 and 422) having had surgery at all three time horizons.
| Year | Strategy | Success performance | Receiving surgery | Population | QALYs/costs | ||
|---|---|---|---|---|---|---|---|
| Success | Failure | QALYs | Costs (£) | ||||
| 1 | LS–PFMT extra sessions–TVT | 985 | 13 | 23 | 1000 | 0.82 | 430 |
| LS–PFMT extra sessions–SNRI–TVT | 977 | 21 | 8 | 1000 | 0.81 | 416 | |
| LS–PFMT basic–PFMT extra sessions–TVT | 983 | 15 | 0 | 1000 | 0.81 | 252 | |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 983 | 15 | 0 | 1000 | 0.81 | 252 | |
| LS–PFMT basic–TVT | 981 | 17 | 30 | 1000 | 0.81 | 275 | |
| LS–TVT | 793 | 205 | 998 | 1000 | 0.8 | 1200 | |
| 10 | LS–PFMT extra sessions–TVT | 951 | 22 | 462 | 1000 | 7.04 | 1159 |
| LS–PFMT extra sessions–SNRI–TVT | 942 | 31 | 401 | 1000 | 7.02 | 1145 | |
| LS–PFMT basic–PFMT extra sessions–TVT | 947 | 26 | 162 | 1000 | 7.03 | 833 | |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 940 | 33 | 127 | 1000 | 7.03 | 818 | |
| LS–PFMT basic–TVT | 947 | 26 | 593 | 1000 | 7.03 | 1139 | |
| LS–TVT | 913 | 60 | 973 | 1000 | 6.96 | 1883 | |
| 40 | LS–PFMT extra sessions–TVT | 410 | 12 | 420 | 1000 | 16.37 | 1938 |
| LS–PFMT extra sessions–SNRI–TVT | 403 | 11 | 404 | 1000 | 16.27 | 1965 | |
| LS–PFMT basic–PFMT extra sessions–TVT | 406 | 9 | 410 | 1000 | 16.31 | 1795 | |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 400 | 8 | 386 | 1000 | 16.24 | 1803 | |
| LS–PFMT basic–TVT | 408 | 13 | 421 | 1000 | 16.34 | 1873 | |
| LS–TVT | 382 | 40 | 422 | 1000 | 16.2 | 2425 | |
Cost–utility analysis based upon cure rates
Deterministic results
Table 64 details the results of the mean cost and treatment effects of the model using cure rates from the mixed-treatment model in a hypothetical cohort with 1000 samples. The table reports performance of the strategies from the least to the most costly. The lower part of the table reports the ICERs when dominated and extendedly dominated strategies are omitted. The strategy that used lifestyle changes and PFMT with extra sessions followed by TVT surgery (LS–PFMT extra sessions–TVT) was the least costly (£1644) and the most effective (16.20 QALYs). The strategy that had lifestyle changes followed by TVT surgery (LS–TVT) was the most costly (£1973), and the strategy that used lifestyle changes and PFMT basic followed by PFMT with extra sessions followed by SNRI and then TVT surgery (LS–PFMT basic–PFMT extra sessions–SNRI–TVT) was the least effective (15.89 QALYs).
| Strategy | Cost (£) | Incremental cost (£) | QALYS | Incremental QALY | ICER |
|---|---|---|---|---|---|
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated |
| LS–PFMT basic–PFMT extra sessions –TVT | 1758 | 113 | 16.02 | –0.17 | Dominated |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1842 | 197 | 15.89 | –0.3 | Dominated |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated |
| Results without dominated and extendedly dominated options | |||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | |||
Probabilistic results
As the cost-effectiveness point estimates do not provide any information of uncertainty surrounding the model parameters, probabilistic sensitivity analysis using Monte Carlo simulations was also performed using these strategies. The results of the probabilistic analysis are presented in the form of cost-effectiveness acceptability curves in Figure 42. The strategy employing lifestyle changes and PFMT with extra sessions followed by TVT surgery (LS-PFMT extra sessions-TVT) has a more than 70% probability of being considered cost-effective for all threshold values for willingness to pay for a QALY presented. The other five strategies each have a probability of less than 20% of being considered cost-effective.
FIGURE 42.
Cost-effectiveness acceptability curves determined by society’s willingness to pay for a quality-adjusted life-year for the six strategies.

Sensitivity analyses
Changes to the effectiveness and cost of PFMT basic and PFMT with extra sessions
Change in the long-term recurrence rates of PFMT basic and PFMT with extra sessions
As mentioned previously (under Summary of key assumptions made in the economic model), the recurrence rates of PFMT basic and PFMT with extra sessions were estimated from the study by Bø and colleagues208 and it is possible that this value is overestimated. As long-term cure rates of PFMT basic and PFMT with extra sessions decreased, the costs of the strategies associated with long-term PFMT basic and PFMT with extra sessions are increased because the long-term recurrence estimation for PFMT basic and PFMT with extra sessions is increased. However, the outcomes associated with long-term PFMT basic and PFMT with extra sessions are decreased. The results of the sensitivity analyses of variations of long-term of recurrence rates for PFMT basic and PFMT with extra sessions are presented in Table 65. The probability that society is willing to pay for an additional QALY for the strategies associated with PFMT basic and PFMT with extra sessions generally decreases as the long-term cure rate of PFMT decreases.
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case [cure rates of PFMT basic and PFMT with extra sessions at 5 and 15 years in the Lagro-Janssen (1998)210 and Bø (2005)208 studies] | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Long-term cure rates of PFMT with extra sessions were the same as the that of PFMT basic | ||||||||||
| LS–PFMT extra sessions–TVT | 1715 | 16.17 | 66 | 65 | 64 | 63 | 63 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1802 | 88 | 16.03 | –0.14 | Dominated | 5 | 5 | 5 | 5 | 5 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1826 | 111 | 16.00 | –0.17 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–TVT | 1886 | 172 | 16.03 | –0.15 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1915 | 200 | 15.86 | –0.31 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 258 | 16.08 | –0.09 | Dominated | 24 | 26 | 27 | 27 | 27 |
| Long-term cure rate of PFMT with extra sessions and PFMT basic is reduced by 20% | ||||||||||
| LS–PFMT extra sessions–TVT | 1763 | 16.15 | 64 | 62 | 62 | 61 | 64 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1853 | 90 | 16.00 | –0.15 | Dominated | 4 | 4 | 4 | 4 | 4 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1871 | 109 | 15.98 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–TVT | 1892 | 129 | 16.02 | –0.13 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1963 | 201 | 15.84 | –0.32 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–TVT | 1973 | 210 | 16.08 | –0.07 | Dominated | 27 | 29 | 30 | 30 | 27 |
| Long-term cure rate of PFMT with extra sessions and PFMT basic is reduced by 60% | ||||||||||
| LS–PFMT extra sessions–TVT | 1879 | 16.10 | 48 | 48 | 48 | 47 | 47 | |||
| LS–PFMT basic–TVT | 1906 | 27 | 16.02 | –0.09 | Dominated | 9 | 8 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1969 | 90 | 15.94 | –0.16 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 94 | 16.08 | –0.02 | Dominated | 39 | 40 | 41 | 41 | 41 |
| LS–PFMT extra sessions–SNRI–TVT | 1974 | 95 | 15.94 | –0.16 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2065 | 186 | 15.79 | –0.32 | Dominated | 0 | 0 | 0 | 0 | 0 |
Changes to the ORs of PFMT basic and PFMT with extra sessions compared with no treatment
The results of the sensitivity analysis performed using different point estimates in clinical effectiveness for cure rates of PFMT with extra sessions and PFMT basic are reported in Table 66. When the ORs of PFMT with extra sessions were compared with no treatment decreased, the probability that the strategies associated with PFMT with extra sessions were cost-effective was also reduced. When the ORs compared with no treatment reduced to four (the value used in base case was 10.7), the likelihood that lifestyles followed by PFMT with extra sessions followed by TVT surgery (LS–PFMT extra sessions–TVT) was cost-effective fell to approximately 40%. The main strategy that gained was ‘lifestyles’, followed by ‘TVT surgery’, which has a 50% chance of being considered cost-effective over the range of values for a cost per QALY considered.
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (OR of PFMT with extra sessions compared with no treatment is 10.7) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| OR of PFMT with extra sessions compared with no treatment is 8 | ||||||||||
| LS–PFMT extra sessions–TVT | 1758 | 16.15 | 66 | 63 | 63 | 63 | 62 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1842 | 84 | 16.01 | –0.14 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1860 | 102 | 15.98 | –0.17 | Dominated | 2 | 3 | 3 | 3 | 2 |
| LS–PFMT basic–TVT | 1886 | 128 | 16.03 | –0.12 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1947 | 190 | 15.84 | –0.31 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–TVT | 1973 | 215 | 16.08 | –0.07 | Dominated | 24 | 26 | 26 | 26 | 27 |
| OR of PFMT with extra sessions compared with no treatment is 4 | ||||||||||
| LS–PFMT basic–TVT | 1886 | 16.03 | 8 | 6 | 5 | 5 | 5 | |||
| LS–PFMT extra sessions–TVT | 1926 | 39 | 16.08 | 0.05 | 769 | 39 | 40 | 41 | 40 | 41 |
| LS–TVT | 1973 | 47 | 16.08 | < 0.01 | 13,249 | 50 | 51 | 51 | 52 | 52 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2011 | 38 | 15.92 | –0.16 | Dominated | 2 | 2 | 1 | 1 | 1 |
| LS–PFMT extra sessions–SNRI–TVT | 2013 | 40 | 15.92 | –0.16 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2104 | 131 | 15.77 | –0.32 | Dominated | 1 | 1 | 1 | 1 | 1 |
When the OR of PFMT basic compared with no treatment varied (Table 67), the results were broadly similar to the best case analysis.
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (OR of PFMT basic compared with no treatment is 1.28) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| OR of PFMT basic compared with no treatment is 4 | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 75 | 72 | 71 | 71 | 71 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 1685 | 41 | 16.06 | –0.14 | Dominated | 4 | 4 | 4 | 4 | 4 |
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 5 | 5 | 5 | 5 | 5 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1767 | 122 | 15.93 | –0.26 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1790 | 145 | 16.07 | –0.13 | Dominated | 4 | 3 | 3 | 3 | 3 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 13 | 16 | 17 | 17 | 17 |
| OR of PFMT basic compared with no treatment is 8 | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1579 | 16.11 | 11 | 10 | 10 | 9 | 9 | |||
| LS–PFMT extra sessions–TVT | 1644 | 65 | 16.20 | 0.09 | 726 | 58 | 59 | 59 | 60 | 60 |
| LS–PFMT basic–TVT | 1648 | 4 | 16.13 | –0.07 | Dominated | 15 | 12 | 11 | 11 | 11 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1656 | 12 | 15.99 | –0.21 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 12 | 14 | 16 | 16 | 16 |
Changes to the cost of PFMT with extra sessions
As indicated in Resource utilisation and cost estimation, the cost of PFMT with extra sessions may be underestimated. As shown in Table 68, the probability that lifestyle–PFMT extra sessions–TVT was most cost-effective decreased and the probability that LV–TVT was cost-effective increased. However, PFMT with extra sessions would need to increase in cost by more than £400 before the base-case conclusions would substantially change.
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (cost of PFMT with extra sessions is based on 12 visits per cycle) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Cost of PFMT with extra sessions is increased by 200 | ||||||||||
| LS–PFMT extra sessions–TVT | 1844 | 16.20 | 70 | 71 | 71 | 71 | 71 | |||
| LS–PFMT basic–TVT | 1886 | 42 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT extra sessions–SNRI–TVT | 1927 | 82 | 16.06 | –0.13 | Dominated | 6 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1950 | 106 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 128 | 16.08 | –0.12 | Dominated | 19 | 19 | 19 | 19 | 19 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2034 | 190 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Cost of PFMT with extra sessions is increased by 400 | ||||||||||
| LS–PFMT basic–TVT | 1886 | 16.03 | 4 | 3 | 3 | 2 | 4 | |||
| LS–TVT | 1973 | 86 | 16.08 | 0.05 | Extendedly dominated | 24 | 22 | 22 | 22 | 24 |
| LS–PFMT extra sessions–TVT | 2044 | 72 | 16.20 | 0.17 | 1059 | 65 | 67 | 68 | 68 | 65 |
| LS–PFMT extra sessions–SNRI–TVT | 2127 | 82 | 16.06 | –0.13 | Dominated | 6 | 7 | 7 | 7 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2143 | 98 | 16.03 | –0.17 | Dominated | 2 | 1 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2227 | 182 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Cost of PFMT with extra sessions is increased by 1000 | ||||||||||
| LS–PFMT basic–TVT | 1886 | 16.03 | 10 | 4 | 3 | 3 | 2 | |||
| LS–TVT | 1973 | 86 | 16.08 | 0.05 | 1577 | 36 | 28 | 25 | 24 | 23 |
| LS–PFMT extra sessions–TVT | 2644 | 672 | 16.20 | 0.12 | 5820 | 48 | 60 | 62 | 64 | 65 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2721 | 76 | 16.02 | –0.17 | Dominated | 5 | 6 | 7 | 7 | 7 |
| LS–PFMT extra sessions–SNRI–TVT | 2727 | 82 | 16.06 | –0.13 | Dominated | 2 | 2 | 2 | 2 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2805 | 160 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
Changes to the effectiveness of surgery
Changes to the recurrence rates of TVT surgery
As indicated in Estimation of model probabilities, the long-term recurrence rates of incontinence after TVT surgery were estimated from the trial by Hilton and colleagues (2008). 214 There is some uncertainty attached to this estimate and it is probable that this value could be either an over- or underestimate. As long-term cure rates of TVT surgery increases, the costs for each strategy are decreased and QALYs increase, as women spend more time continent over the 40-year time horizon. When the probability of recurrence of TVT surgery was increased to 81%, the probability that LS–PFMT extra sessions–TVT would be considered to be cost-effective was reduced, and the probability that lifestyle changes followed by TVT surgery (LS–TVT) was cost-effective increases. When the TVT surgery cure rate was reduced to 65%, the probability that the strategy that uses lifestyle changes followed by TVT surgery (LS–TVT), was considered to be cost-effective was also reduced (Table 69).
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (Hilton 2008214) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Long-term cure rate of TVT increased to 81% | ||||||||||
| LS–PFMT extra sessions–TVT | 1526 | 16.27 | 63 | 61 | 60 | 60 | 60 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1618 | 93 | 16.12 | –0.14 | Dominated | 6 | 6 | 5 | 5 | 5 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1653 | 127 | 16.08 | –0.18 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–TVT | 1684 | 159 | 16.15 | –0.12 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1745 | 219 | 15.95 | –0.32 | Dominated | 4 | 3 | 3 | 3 | 4 |
| LS–TVT | 1778 | 252 | 16.20 | –0.07 | Dominated | 27 | 29 | 30 | 31 | 31 |
| Long-term cure rate of TVT reduced to 65% in first year and 61% in the second year | ||||||||||
| LS–PFMT extra sessions–TVT | 1826 | 16.09 | 79 | 78 | 79 | 79 | 78 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1888 | 62 | 15.97 | –0.12 | Dominated | 12 | 12 | 12 | 12 | 12 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1913 | 88 | 15.93 | –0.16 | Dominated | 8 | 8 | 8 | 8 | 8 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1983 | 158 | 15.81 | –0.28 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 2207 | 382 | 15.83 | –0.26 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–TVT | 2540 | 714 | 15.73 | –0.36 | Dominated | 1 | 1 | 1 | 2 | 2 |
Mortality and success rate of TVT surgery
These results did not change to any extent when the mortality risk of surgery was reduced to zero or the success rate of TVT surgery was increased by 5%. The results of this analysis are shown in Appendices 26–27.
Changes to the structure of the model
Probability of containment after failure or recurrence for non-surgical treatment
The likelihood that the strategies would be considered cost-effective in cure rate base case did not largely change, although the costs for the strategy associated with no surgical treatments were increased, and the outcomes (QALYs) were decreased when the proportion of women using containment products increased (Table 70).
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (the probability of containment after failure or recurrence for non-surgical treatments is 0%) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Probability of containment after failure or recurrence for non-surgical treatments is 30% | ||||||||||
| LS–PFMT extra sessions–TVT | 1917 | 15.85 | 59 | 58 | 57 | 57 | 57 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1992 | 75 | 15.72 | –0.13 | Dominated | 12 | 12 | 12 | 12 | 12 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2018 | 101 | 15.69 | –0.16 | Dominated | 14 | 14 | 14 | 14 | 15 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 2094 | 177 | 15.57 | –0.28 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 2249 | 332 | 15.67 | –0.18 | Dominated | 10 | 11 | 11 | 11 | 11 |
| LS–PFMT basic–TVT | 2263 | 346 | 15.57 | –0.28 | Dominated | 4 | 5 | 5 | 5 | 5 |
| Probability of containment after failure or recurrence for non-surgical treatments is 60% | ||||||||||
| LS–PFMT extra sessions–TVT | 2190 | 15.50 | 52 | 51 | 51 | 51 | 51 | |||
| LS–PFMT extra sessions–SNRI–TVT | 2259 | 68 | 15.39 | –0.12 | Dominated | 20 | 20 | 19 | 19 | 19 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2279 | 89 | 15.36 | –0.14 | Dominated | 16 | 16 | 17 | 17 | 17 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 2346 | 156 | 15.25 | –0.26 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 2525 | 335 | 15.27 | –0.24 | Dominated | 8 | 9 | 9 | 9 | 9 |
| LS–PFMT basic–TVT | 2639 | 449 | 15.12 | –0.39 | Dominated | 2 | 2 | 3 | 3 | 3 |
Probability of containment after failure or recurrence for first surgical treatment
For the model based on cure rates, the likelihood that the different strategies would be considered cost-effective did not change when the probability of using containment products after failure or recurrence was applied, although costs for each given strategy increased and the outcomes (QALYs) decreased when the proportion of women using containment products increased (Table 71).
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALYs (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (the probability of containment after failure or recurrence for first surgical treatments is 0%) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Probability of containment after failure or recurrence for first surgical treatments is 30% | ||||||||||
| LS–PFMT extra sessions–TVT | 1680 | 16.13 | 77 | 76 | 75 | 75 | 75 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1761 | 82 | 16.00 | –0.13 | Dominated | 9 | 9 | 9 | 9 | 9 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1794 | 114 | 15.97 | –0.17 | Dominated | 5 | 5 | 5 | 5 | 5 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1878 | 199 | 15.84 | –0.29 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1937 | 258 | 15.93 | –0.21 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 2046 | 367 | 15.94 | –0.19 | Dominated | 9 | 10 | 10 | 10 | 10 |
| Probability of containment after failure or recurrence for first surgical treatments is 60% | ||||||||||
| LS–PFMT extra sessions–TVT | 1712 | 16.07 | 74 | 74 | 73 | 73 | 73 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1791 | 79 | 15.94 | –0.13 | Dominated | 13 | 12 | 13 | 13 | 13 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1822 | 111 | 15.91 | –0.16 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1905 | 193 | 15.78 | –0.29 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–TVT | 1993 | 281 | 15.83 | –0.24 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 2127 | 415 | 15.80 | –0.27 | Dominated | 5 | 6 | 6 | 6 | 6 |
| Probability of containment after failure or recurrence for first surgical treatments is 100% | ||||||||||
| LS–PFMT extra sessions–TVT | 1755 | 15.98 | 73 | 72 | 73 | 72 | 72 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1830 | 75 | 15.86 | –0.12 | Dominated | 15 | 15 | 15 | 15 | 15 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1860 | 106 | 15.83 | –0.15 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic –PFMT extra sessions–SNRI –TVT | 1940 | 185 | 15.71 | –0.27 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–TVT | 2067 | 312 | 15.70 | –0.29 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 2234 | 479 | 15.62 | –0.37 | Dominated | 2 | 3 | 3 | 3 | 3 |
Adding strategies that use VCs and ES
As indicated in Model framework, strategies using VC and ES were to be considered in sensitivity analyses. The results of the deterministic analysis are reported in Table 72. The strategy that employed lifestyle changes and PFMT basic followed by ES followed by TVT surgery (LS–PFMT basic–ES–TVT) was dominated (more costly and less effective than another strategy). The strategy that employed lifestyle changes and PFMT basic followed by VCs followed by TVT surgery (LS–PFMT basic–VC–TVT) was dominated. The addition of strategies involving VCs and ES had a small probability of being considered cost-effective (less than 12%) and did not greatly influence the results.
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (six strategies) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Base case adding two strategies including VC and ES | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 77 | 76 | 75 | 75 | 75 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–VC–TVT | 1739 | 95 | 15.93 | –0.27 | Dominated | 1 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra session–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 13 | 15 | 15 | 16 | 16 |
| LS–PFMT basic–ES–TVT | 2159 | 514 | 15.88 | –0.32 | Dominated | 0 | 0 | 0 | 0 | 0 |
Other sensitivity analyses
Changes to starting ages, time horizon, quality of life and discount rates all had no substantial effect on the results. As the starting age was increased the costs and outcomes for each strategy were reduced because of the increase in mortality as age increased. As the time horizon reduced there was less opportunity for women who had undergone the relatively costly surgery to accrue much benefit. Reducing the discount rate meant that the sustained benefits of surgery were given more weight in the analysis as were the costs of using containment products. Therefore, the LS–TVT strategy increased in its likelihood of being cost-effective. The results of these sensitivity analyses are reported in Appendix 26.
Results based upon improvement rates
Deterministic results
In these analyses the costs were lower and the QALYs were higher than those reported in analyses based on cure rates (Table 73). The strategy that used lifestyle changes and PFMT basic followed by PFMT with extra sessions followed by TVT surgery (LS–PFMT basic–PFMT extra sessions–TVT) was the least costly (£1795) and the strategy that had lifestyle and PFMT with extra sessions followed by TVT surgery (LS–PFMT extra sessions–TVT) was the most effective (16.37 QALYs). The strategy that had lifestyle changes followed by TVT surgery (LS–TVT) was the most costly (£2425) and the least effective (16.2 QALYs).
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | £2147 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated |
| Results without dominated and extendedly dominated options | |||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | |||
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | £2147 |
Probabilistic results
Probabilistic analysis was performed comparing all these strategies. As illustrated in Figure 43, the strategy that used lifestyle and PFMT with extra sessions followed by TVT surgery (LS–PFMT extra sessions–TVT) has more than a 50% probability of being considered cost-effective when society’s willingness to pay for an additional QALY is more than £10,000. The other strategies have a less than 20% chance of being considered cost-effective, as society’s willingness to pay more for additional QALYs increases. The strategy that had the least probability of being considered cost-effective was the one that used lifestyle changes followed by TVT surgery.
FIGURE 43.
Cost-effectiveness acceptability curves determined by society’s willingness to pay for a quality-adjusted life-year for the six strategies.

Sensitivity analyses
Changes to the effectiveness and cost of PFMT basic and PFMT extra sessions
Change in the long-term recurrence rates of PFMT basic and PFMT extra sessions
When the long-term cure rates of PFMT with extra sessions were the same as PFMT basic, then the strategy that had the highest probability of being cost-effective changed from lifestyle and PFMT with extra sessions followed by TVT surgery (LS–PFMT extra sessions–TVT) to lifestyle and PFMT basic followed by TVT surgery (LS–PFMT basic–TVT) (Table 74). Similarly, reducing the cure rates of both PFMT basic and PFMT with extra sessions also meant that lifestyle and PFMT basic followed by TVT surgery (LS–PFMT basic–TVT) was most likely to be cost-effective.
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case [cure rates of PFMT basic and PFMT with extra sessions at 5 and 15 years in the Lagro–Janssen (1998)210 and Bø (2005)208 studies] | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 1973 | 294 | 16.08 | –0.23 | Dominated | 1 | 1 | 1 | 1 | 1 |
| Long-term cure rates of PFMT with extra sessions were the same as the that of PFMT basic | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1870 | 16.29 | 11 | 10 | 10 | 10 | 10 | |||
| LS–PFMT basic–TVT | 1873 | 3 | 16.34 | 0.06 | 53 | 56 | 54 | 52 | 51 | 50 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1885 | 11 | 16.2 | –0.14 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT extra sessions–TVT | 2035 | 162 | 16.34 | 0 | Dominated | 15 | 16 | 16 | 17 | 17 |
| LS–PFMT extra sessions–SNRI–TVT | 2071 | 198 | 16.23 | –0.12 | Dominated | 9 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 552 | 16.20 | –0.14 | Dominated | 2 | 3 | 5 | 5 | 6 |
| Long-term cure rate of PFMT with extra sessions and PFMT basic is reduced by 20% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1918 | 16.26 | 13 | 12 | 12 | 12 | 11 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1936 | 18 | 16.18 | –0.09 | Dominated | 7 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1939 | 21 | 16.32 | 0.06 | 376 | 54 | 50 | 49 | 48 | 47 |
| LS–PFMT extra sessions–TVT | 2101 | 162 | 16.32 | 0 | Dominated | 15 | 15 | 16 | 17 | 17 |
| LS–PFMT extra sessions–SNRI–TVT | 2142 | 203 | 16.19 | –0.13 | Dominated | 7 | 8 | 8 | 8 | 8 |
| LS–TVT | 2425 | 486 | 16.20 | –0.12 | Dominated | 5 | 8 | 9 | 10 | 10 |
| Cure rate of PFMT with extra sessions and PFMT basic is reduced by 60% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 2081 | 16.19 | 11 | 10 | 10 | 10 | 10 | |||
| LS–PFMT basic–TVT | 2098 | 17 | 16.25 | 0.06 | 278 | 55 | 50 | 47 | 46 | 45 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2112 | 14 | 16.09 | –0.17 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT extra sessions–TVT | 2260 | 162 | 16.25 | 0 | Dominated | 10 | 12 | 13 | 14 | 14 |
| LS–PFMT extra sessions–SNRI–TVT | 2313 | 215 | 16.11 | –0.14 | Dominated | 4 | 4 | 4 | 4 | 4 |
| LS–TVT | 2425 | 327 | 16.20 | –0.05 | Dominated | 17 | 21 | 23 | 23 | 24 |
Changes to the ORs of PFMT basic and PFMT extra sessions compared with no treatment
The results of the sensitivity analysis performed using different point estimates in clinical effectiveness for improvement rates of PFMT extra sessions and PFMT basic are reported in Table 75. When the OR of PFMT with extra sessions compared with no treatment falls, strategies involving this treatment are less likely to be considered cost-effective. The strategy that increased more in terms of the likelihood of being cost-effective was lifestyle changes and PFMT basic followed by TVT surgery (LS–PFMT basic–TVT).
| Strategy | Deterministic result | Probability cost–effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (OR of PFMT with extra sessions compared with no treatment is 25.4) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| OR of PFMT with extra sessions compared with no treatment is 10 | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 7 | 6 | 6 | 6 | 6 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 7 | 6 | 6 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 65 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 11 | 12 | 12 | 12 | 12 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 1 | 1 | 2 | 2 |
| OR of PFMT with extra sessions compared with no treatment is 3 | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1858 | 16.29 | 10 | 9 | 9 | 8 | 8 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1872 | 14 | 16.21 | –0.08 | Dominated | 6 | 6 | 5 | 5 | 5 |
| LS–PFMT basic–TVT | 1873 | 15 | 16.34 | 0.06 | 268 | 53 | 47 | 46 | 45 | 44 |
| LS–PFMT extra sessions–TVT | 2068 | 195 | 16.32 | –0.02 | Dominated | 24 | 29 | 30 | 32 | 32 |
| LS–PFMT extra sessions–SNRI–TVT | 2102 | 229 | 16.21 | –0.14 | Dominated | 5 | 5 | 5 | 5 | 5 |
| LS–TVT | 2425 | 552 | 16.20 | –0.14 | Dominated | 2 | 4 | 5 | 5 | 5 |
Changes to the cost of PFMT with extra sessions
As indicated in Resource utilisation and cost estimation, the cost of PFMT with extra sessions may be underestimated. When the cost of PFMT with extra sessions increases, the strategies containing this treatment became less likely to be considered cost-effective (Table 76). When the cost of PFMT increased beyond £200 there was an increasing chance that lifestyle changes and PFMT basic followed by TVT surgery (LS–PFMT basic–TVT) would be considered cost-effective.
| Strategy | Deterministic result | Probability cost–effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (cost of PFMT with extra sessions is based on 12 visits per cycle) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Cost of PFMT with extra sessions is increased by 200 | ||||||||||
| LS–PFMT basic–TVT | 1873 | 16.34 | 9 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 1939 | 66 | 16.31 | –0.04 | Dominated | 10 | 8 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1948 | 75 | 16.24 | –0.11 | Dominated | 7 | 6 | 6 | 6 | 6 |
| LS–PFMT extra sessions–TVT | 2138 | 265 | 16.37 | 0.03 | 8510 | 63 | 67 | 67 | 67 | 68 |
| LS–PFMT extra sessions–SNRI–TVT | 2165 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 11 |
| LS–TVT | 2425 | 287 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 2 |
| Cost of PFMT with extra sessions is increased by 400 | ||||||||||
| LS–PFMT basic–TVT | 1873 | 16.34 | 54 | 31 | 20 | 14 | 11 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 2084 | 211 | 16.31 | –0.04 | Dominated | 8 | 7 | 7 | 7 | 6 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2092 | 219 | 16.24 | –0.11 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT extra sessions–TVT | 2338 | 465 | 16.37 | 0.03 | 14,932 | 23 | 42 | 53 | 59 | 62 |
| LS–PFMT extra sessions–SNRI–TVT | 2365 | 27 | 16.27 | –0.10 | Dominated | 9 | 11 | 12 | 12 | 12 |
| LS–TVT | 2425 | 87 | 16.20 | –0.17 | Dominated | 1 | 3 | 3 | 3 | 3 |
| Cost of PFMT with extra sessions is increased by 1000 | ||||||||||
| LS–PFMT basic–TVT | 1873 | 16.34 | 77 | 54 | 42 | 31 | 24 | |||
| LS–TVT | 2425 | 552 | 16.20 | –0.14 | Dominated | 3 | 4 | 4 | 4 | 4 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2517 | 644 | 16.31 | –0.04 | Dominated | 4 | 5 | 6 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2526 | 652 | 16.24 | –0.11 | Dominated | 3 | 5 | 5 | 5 | 5 |
| LS–PFMT extra sessions–TVT | 2938 | 1065 | 16.37 | 0.03 | 34,199 | 8 | 23 | 34 | 43 | 50 |
| LS–PFMT extra sessions–SNRI–TVT | 2965 | 27 | 16.27 | –0.10 | Dominated | 6 | 9 | 10 | 11 | 11 |
Changes to the effectiveness of surgery
Change in the recurrence rates of TVT
As indicated in Estimation of model probabilities, the long-term recurrence rates of incontinence after TVT surgery were estimated from the trial by Ward and colleagues. 213 There is some uncertainty attached to this estimate and it is probable that this value could be either an over- or underestimate. As long-term cure rates of TVT surgery decreased, the costs associated with long-term TVT surgery for each strategy were increased because the long-term recurrence of TVT surgery was also increased leading to a decrease in long-term benefits. Table 77 describes the results of the sensitivity analyses for changes in the long-term cure rates for TVT surgery. When the probability was increased to 81%, the likelihood that lifestyle changes and PFMT with extra sessions followed by TVT (LS–PFMT extra sessions–TVT) was cost-effective fell to approximately 60%. The main gainer was the strategy of lifestyle changes followed by TVT (LS–TVT), which had a 30% chance of being considered cost-effective over the range of values considered. When the probability was reduced to 65%, the likelihood that the strategy lifestyle changes and PFMT with extra sessions followed by TVT (LS–PFMT extra sessions-TVT) was considered to be cost-effective is increased to 80% and that of lifestyle changes followed by TVT is reduced to 1% (Table 78).
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (Hilton 2008214) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Long–term cure rate of TVT increased to 81% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1767 | 16.32 | 5 | 3 | 3 | 3 | 3 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1780 | 13 | 16.25 | –0.07 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–TVT | 1808 | 41 | 16.38 | 0.06 | 698 | 14 | 10 | 9 | 8 | 8 |
| LS–PFMT extra sessions–TVT | 1884 | 76 | 16.40 | 0.02 | 3103 | 59 | 62 | 62 | 62 | 61 |
| LS–PFMT extra sessions–SNRI–TVT | 1919 | 34 | 16.30 | –0.11 | Dominated | 13 | 13 | 13 | 13 | 13 |
| LS–TVT | 2260 | 375 | 16.31 | –0.09 | Dominated | 6 | 9 | 10 | 11 | 11 |
| Long–term cure rate of TVT reduced to 65% in first year and 61% in the second year | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1831 | 16.29 | 20 | 16 | 15 | 14 | 14 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1833 | 3 | 16.22 | –0.07 | Dominated | 9 | 8 | 8 | 8 | 8 |
| LS–PFMT basic–TVT | 1963 | 132 | 16.29 | < 0.01 | Extendedly dominated | 6 | 5 | 5 | 5 | 5 |
| LS–PFMT extra sessions–TVT | 2012 | 182 | 16.33 | 0.04 | 4356 | 55 | 59 | 61 | 62 | 62 |
| LS–PFMT extra sessions–SNRI–TVT | 2029 | 17 | 16.24 | –0.10 | Dominated | 11 | 11 | 11 | 11 | 11 |
| LS–TVT | 2921 | 908 | 15.85 | –0.48 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Strategy | Deterministic result | Probability cost–effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (the probability of containment after failure or recurrence for non-surgical treatments is 0%) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Probability of containment after failure or recurrence for non-surgical treatments is 30% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1850 | 16.07 | 19 | 18 | 17 | 17 | 17 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 1855 | 5 | 16.11 | 0.04 | 113 | 40 | 38 | 38 | 37 | 37 |
| LS–PFMT basic–TVT | 2027 | 172 | 16.01 | –0.11 | Dominated | 9 | 10 | 9 | 9 | 10 |
| LS–PFMT extra sessions–TVT | 2064 | 208 | 16.08 | –0.03 | Dominated | 23 | 25 | 25 | 26 | 26 |
| LS–PFMT extra sessions–SNRI–TVT | 2074 | 218 | 16.01 | –0.11 | Dominated | 9 | 9 | 10 | 10 | 10 |
| LS–TVT | 2425 | 570 | 15.85 | –0.27 | Dominated | 0 | 0 | 1 | 1 | 1 |
| Probability of containment after failure or recurrence for non-surgical treatments is 60% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1898 | 15.90 | 37 | 36 | 35 | 35 | 35 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 1916 | 18 | 15.92 | 0.02 | 991 | 42 | 41 | 40 | 40 | 40 |
| LS–PFMT basic–TVT | 2181 | 265 | 15.67 | –0.25 | Dominated | 2 | 3 | 3 | 3 | 3 |
| LS–PFMT extra sessions–TVT | 2182 | 266 | 15.74 | –0.18 | Dominated | 14 | 15 | 16 | 16 | 16 |
| LS–PFMT extra sessions–SNRI–TVT | 2189 | 273 | 15.79 | –0.13 | Dominated | 5 | 6 | 6 | 6 | 6 |
| LS–TVT | 2425 | 509 | 15.50 | –0.42 | Dominated | 0 | 0 | 0 | 0 | 0 |
Mortality and success rate of TVT
The results did not change to any extent when the mortality risk was reduced to zero or the success rate of TVT was increased to 5%. The results of these analyses are shown in Appendix 27.
Changes to the structure of the model
Probability of containment after failure or recurrence for non-surgical treatment
The likelihood that the strategies would be considered cost-effective in the improvement model hardly changed when the probability that women might use containment products rather than progress to the next active treatment was increased to 30% and 60% (Table 78).
Probability of containment after failure or recurrence for first surgical treatment
When women might use containment products rather than resort to a second operation (if one was necessary) the likelihood of the strategy lifestyle changes and basic PFMT followed by PFMT with extra sessions followed by tension-free tape (LS-PFMT basic-PFMT extra sessions-TVT) would be considered cost-effective reduced (Table 79).
| Strategy | Deterministic result | Probability cost–effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (the probability of containment after failure or recurrence for first surgical treatments is 0%) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Probability of containment after failure or recurrence for first surgical treatments is 30% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1793 | 16.29 | 14 | 11 | 11 | 10 | 10 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1801 | 8 | 16.23 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1877 | 84 | 16.31 | 0.02 | Extendedly dominated | 11 | 9 | 8 | 8 | 8 |
| LS–PFMT extra sessions–TVT | 1940 | 147 | 16.35 | 0.05 | 2744 | 58 | 62 | 63 | 64 | 64 |
| LS–PFMT extra sessions–SNRI–TVT | 1967 | 26 | 16.25 | –0.10 | Dominated | 10 | 11 | 11 | 11 | 12 |
| LS–TVT | 2460 | 520 | 16.07 | –0.28 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Probability of containment after failure or recurrence for first surgical treatments is 60% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1792 | 16.28 | 20 | 17 | 16 | 16 | 16 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1799 | 8 | 16.21 | –0.07 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–PFMT basic–TVT | 1881 | 89 | 16.28 | 0 | Dominated | 12 | 9 | 8 | 8 | 7 |
| LS–PFMT extra sessions–TVT | 1943 | 151 | 16.32 | 0.04 | 3731 | 49 | 54 | 56 | 56 | 57 |
| LS–PFMT extra sessions–SNRI–TVT | 1968 | 25 | 16.22 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2495 | 553 | 15.94 | –0.38 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Probability of containment after failure or recurrence for first surgical treatments is 100% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1789 | 16.26 | 27 | 25 | 24 | 24 | 24 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1797 | 8 | 16.20 | –0.06 | Dominated | 13 | 11 | 11 | 11 | 11 |
| LS–PFMT basic–TVT | 1886 | 96 | 16.23 | –0.03 | Dominated | 9 | 7 | 7 | 7 | 7 |
| LS–PFMT extra sessions–TVT | 1945 | 156 | 16.28 | 0.02 | 6811 | 42 | 46 | 47 | 47 | 47 |
| LS–PFMT extra sessions–SNRI–TVT | 1970 | 24 | 16.19 | –0.09 | Dominated | 10 | 11 | 11 | 12 | 12 |
| LS–TVT | 2542 | 597 | 15.77 | –0.52 | Dominated | 0 | 0 | 0 | 0 | 0 |
Adding strategies using VCs and ES
For the sensitivity analysis the only difference was that the likelihood of the strategy involving the use of VCs (LS–PFMT basic–VC–TVT) was more likely to be considered cost-effective instead of the lifestyle changes and basic PFMT followed by TVT (LS–PFMT basic–TVT) (Table 80).
| Strategy | Deterministic result | Probability cost–effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (six strategies) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Base case adding two strategies including VC and ES | ||||||||||
| LS–PFMT basic–VC–TVT | 1615 | 16.31 | 11 | 8 | 6 | 6 | 5 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 179 | 16.31 | < 0.01 | Extendedly dominated | 1 | 1 | 1 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 188 | 16.24 | –0.07 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–ES–TVT | 1872 | 257 | 16.30 | –0.01 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–TVT | 1873 | 258 | 16.34 | 0.04 | Extendedly dominated | 9 | 6 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 323 | 16.37 | 0.07 | 4723 | 61 | 67 | 68 | 68 | 68 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
Other sensitivity analysis
Changes to starting ages, quality of life and discount rates had no substantial effect on the results. As the starting age was increased, the costs and outcomes for each strategy were reduced because of the increase in mortality as age increased. However, as the time horizon was reduced in the analysis using the improvement rates, the non-dominated strategy of lifestyle-PFMT basic-PFMT with extra sessions followed by TVT (LS–PFMT basic–PFMT extra sessions–TVT) had a slightly increased likelihood of being considered cost-effective. Reducing the discount rate meant that the sustained benefits of surgery were given more weight in the analysis as were the costs of containment products. The results of these sensitivity analyses are reported in Appendix 27.
Summary of results
The economic model presented in this chapter considered some of the management strategies that have the potential to be, or are currently being, used in managing women with SUI. These strategies included the following interventions: lifestyle changes, physical therapies, medical therapies and surgery. The effectiveness data for non-surgical treatments came from the results of mixed-treatment model. This was because the data of direct comparison were not adequate, as they were based on very few and very small studies.
There were no data on the clinical effectiveness of lifestyle modification interventions. It was therefore assumed in the model that all women have received lifestyle change advice in each strategy and then go to the next treatment. There were no reliable data on the long-term clinical effectiveness of PFMT, drug therapy and TVT surgery. Long-term recurrence rates for PFMT, drug therapy and TVT were estimated by parametric extrapolation methods of the limited evidence base. Furthermore, there was also no information associated with long-term clinical effectiveness of other non-surgical treatments.
The cost–utility analyses were conducted using EQ-5D scores to value health effects in the two base-case analyses. Broadly speaking, the results that were based on cure rates were similar to those based on improvement rates.
In the cure-rate base-case analysis there was only one non-dominated or non-extendedly dominated strategy (i.e. it provided more benefits than a less costly strategy or more benefits than could be provided should a combination of a less and more costly strategies be used):
-
lifestyle changes and PFMT with extra sessions followed by TVT (LS–PFMT extra sessions–TVT).
In the analysis based on improvement rates there were two strategies that were not dominated or extendedly dominated:
-
lifestyle changes and PFMT with extra sessions followed by TVT surgery (LS–PFMT extra sessions–TVT) and
-
lifestyle changes and PFMT basic followed by PFMT with extra sessions then TVT surgery (LS–PFMT basic–PFMT extra sessions–TVT).
For the cure-rate base-case analysis, the strategy ‘lifestyle changes and PFMT extra session followed by TVT (LS–PFMT extra sessions–TVT)’ was the least costly (£1644) and the most effective (16.20 QALYs) strategy. For the improvement-rate base-case analysis, the strategy ‘lifestyle changes and PFMT basic followed by PFMT with extra sessions followed by TVT surgery (LS–PFMT basic–PFMT extra sessions–TVT)’ was the least costly (£1795) and provided 16.31 QALYs. For both base-case analyses the strategy of ‘lifestyle changes followed by TVT surgery (LS–TVT)’ (mean cost = £1793 for cure rates and £2524 for improvement rates) was the most costly.
In the cure-rate base case, the strategy of ‘lifestyle changes and PFMT basic followed by PFMT with extra sessions followed by SNRI and then TVT surgery’ was the least effective (providing 15.89 QALYs) strategy. In the improvement-rate base-case analysis the strategy that used ‘lifestyle changes followed by TVT surgery’ was the least effective.
Although the differences between strategies in terms of costs and effects in both base-case analyses appear not to be large, the important issue is whether society is willing to pay for any additional gain. Only two strategies have a likelihood of being considered to be cost-effective of greater than 50% when the threshold is £20,000–30,000 per QALY: LS–PFMT extra session–TVT (cure rates) and LS–PFMT basic–PFMT extra sessions–TVT(improvement rates). All of the other strategies had between 0% and 20% likelihood of being considered cost-effective if society were willing to pay between £20,000 and £30,000 per QALY.
Results of probabilistic sensitivity analyses, performed to handle the uncertainty around the parameter estimates used within the model, were broadly consistent with the point estimates for both base-case analyses. The likelihood that different strategies might be considered cost-effective changed slightly in some sensitivity analyses. The results of the model were sensitive to changes in the long-term cure rates. However, these changes did not alter the conclusions.
The results of the sensitivity analyses were broadly similar to those of the base cases when different values of quality of life were used to estimate QALYs and when other assumptions made within the model were changed. For example, when the proportion of patients that used containment products after failure or recurrence for non-surgical or surgical treatments were changed.
The modelling performed in this section provided and contributed to the evidence available for evaluating the cost-effectiveness of non-surgical interventions for the treatment of SUI in women. This work also provided information on the long-term costs of managing women with SUI.
The data that were used to estimate cost-effectiveness came from the MTC model. These data represented the best available evidence to date on the relative effectiveness of the non-surgical treatments.
The lack of long-term data on the cure and recurrence rates of the interventions under consideration limits the analyses. Long-term performance was extrapolated from short-term data. Also assumptions had to be made about these parameters; for example, the recurrence rates of PFMT with extra sessions were assumed to be the same as the PFMT basic. Due to a lack of reliable data, it is unclear whether this assumption is valid or not.
There was also uncertainty around the costs estimates. It was very difficult to determine the ‘standard’ number of sessions that there were in basic PFMT. Therefore, it was assumed that basic PFMT consisted of six sessions. These assumptions were also applied to all therapies that had some form of PFMT. The other area where there was a lack of data was the quality-of-life estimates.
Chapter 10 Discussion
Stress incontinence is the most common type of urinary incontinence experienced by women. The treatment options can be classified as non-surgical and surgical. Non-surgical interventions, such as PFMT, may require long-term adherence to an exercise regimen to produce continued benefit but (apart from medical therapies) they have few or no adverse events. Surgical treatment, on the other hand, may have a higher rate of cure or improvement in symptoms but has a greater risk of complications.
The choice of treatment is influenced by patient preference, professional advice and the research evidence. It takes into account factors such as symptom severity, degree of interference with lifestyle, presence of related problems and degree of comorbidity. A woman may seek and receive several different treatments during the course of her lifetime, and, from a health service perspective, it is important to balance effectiveness, potential adverse events and costs of individual treatments and alternative care pathways. This study aimed to assess the clinical effectiveness and cost-effectiveness of alternative non-surgical treatments and treatment pathways for women with SUI.
Main results
Summary of results from the survey of factors important to women with SUI
Central to the estimation of both effectiveness and cost-effectiveness is that the outcome measures chosen reflect factors that are important to the women themselves. As reported in Chapter 4, members of The Bladder and Bowel Foundation (formerly members of InContact)99 (n = 188 women) were prospectively surveyed with the aim of gathering information on outcomes of importance to them and 105 responses were received. Areas of importance to women who suffer from SUI were assessed using a PGI. In addition, the questionnaire included the King’s Health Questionnaire and the EQ-5D. The survey identified 38 different areas of life affected by SUI. The five most frequently reported were: going out or socialising, personal hygiene, sleep, shopping and depression. Ideally, these areas would then be used to derive outcomes of relevance to women in primary studies and systematic reviews. However, these outcomes were rarely considered in primary studies. PGI scores and EQ-5D scores were positively correlated and these correlations were statistically significant. Nevertheless, the areas identified by the PGI did not map well to the EQ-5D. Correlations between the seven domains of the King’s Health Questionnaire and PGI were all negative but only two were statistically significant: personal relationships and severity measures. These data suggest that the PGI may be capturing concerns of women who suffer from SUI which are not adequately captured by generic instruments such as the EQ-5D.
Summary of results from the systematic review of effectiveness
The focus of the systematic reviews of effectiveness was on the rates of cure and cure or improvement (this latter outcome is referred to in the text as improvement), quality of life, and adverse events. The systematic review of clinical effectiveness identified 88 trials reporting data from 9721 women. The included studies covered five generic interventions (PFMT with or without BF, ES, VCs, BT and SNRI medications) with many variations and combinations of them. Data were available for 37 interventions and 68 direct head-to-head (pairwise) comparisons. The MTC included 14 treatments and took data from the 55 trials (6608 women) that reported data for these treatments. Cure data were available for 3560 women (38 trials) and improvement data were available for 6140 women (47 trials). In total, 41% and 61% of these data for cure and improvement, respectively, came from 10 trials comparing SNRI with placebo.
A summary of the treatment comparisons showing both the direct comparisons and the MTC results is given in Table 81. PFMT with or without BF appeared to be more effective than no treatment both in terms of cure and improvement. ES, SNRI, BT and PFMT plus ES had, on average, higher odds of cure and improvement compared with no treatment. VCs, and PFMT plus SNRI had higher odds of improvement compared with no treatment. The direct, head-to-head comparisons were inconclusive about whether PFMT with or without BF was better than other treatments. For example, the ORs (point estimate) for improvement favoured PFMT (with or without BF) over other standalone treatments (ES, VCs, BT and SNRI), but the CIs were wide and included one (no difference).
| Intervention | Direct comparison | MTC | |||
|---|---|---|---|---|---|
| 1 | 2 | Number of trials | Number of people | Est (95% CrI) | Est (95% CrI) |
| Cure | |||||
| PFMT | NT | 8 | 605 | 5.41 | 4.56 |
| (1.64 to 17.82) | (1.95 to 12.4) | ||||
| PFMT + BF | NT | 2 | 110 | 21.54 | 9.65 |
| (3.65 to 126.98) | (3.37 to 33.3) | ||||
| ES | NT | 6 | 288 | 1.10 | 1.63 |
| (0.41 to 2.94) | (0.506 to 5.54) | ||||
| SNRI | NT | 3 | 1292 | 1.46 | 1.42 |
| (1.00 to 2.14) | (0.377 to 5.35) | ||||
| BT | NT | 1 | 123 | 4.03 | 4.87 |
| (0.80 to 20.23) | (1.05 to 26.1) | ||||
| PFMT + ES | NT | 2 | 155 | 1.76 | 4.59 |
| (0.27 to 11.54) | (1.20 to 22.4) | ||||
| PFMT | ES | 5 | 124 | 2.65 | 2.82 |
| (0.82 to 8.60) | (0.911 to 9.3) | ||||
| PFMT | VC | 3 | 245 | 0.61 | 0.963 |
| (0.09 to 3.95) | (0.274 to 3.51) | ||||
| PFMT + BF | VC | 1 | 46 | 0.86 | 2.03 |
| (0.25 to 2.93) | (0.528 to 8.53) | ||||
| PFMT | BT | 1 | 75 | 2.61 | 0.935 |
| (0.98 to 6.96) | (0.206 to 4. 28) | ||||
| PFMT + BF | BT | 1 | 132 | 0.67 | 1.98 |
| (0.25 to 1.76) | (0.431 to 9.62) | ||||
| ES | VC | 2 | 106 | 1.00 | 0.341 |
| (0.26 to 3.91) | (0.0782 to 1.48) | ||||
| PFMT | PFMT + ES | 4 | 133 | 1.02 | 0.998 |
| (0.29 to 3.55) | (0.257 to 3.47) | ||||
| PFMT | PFMT + VC | 1 | 46 | 0.44 | 0.41 |
| (0.09 to 2.10) | (0.0246 to 6.2) | ||||
| PFMT + BF | PFMT + BF + BT | 1 | 125 | 0.32 | 0.542 |
| (0.13 to 0.79) | (0.0614 to 4.95) | ||||
| PFMT + ES | ES | 1 | 22 | 3.75 | 2.82 |
| (0.33 to 43.31) | (0.598 to 15.5) | ||||
| PFMT + BF + BT | BT | 1 | 129 | 2.11 | 3.64 |
| (0.92 to 4.82) | (0.419 to 32.7) | ||||
| PFMT | PFMT + BF | 8 | 370 | 0.48 | 0.474 |
| (0.30 to 0.77) | (0.2 to 1.07) | ||||
| PFMT basic | PFMT extra | 3 | 118 | 0.11 | 0.12 |
| (0.03 to 0.43) | (0.0462 to 0.268) | ||||
| Improvement | |||||
| PFMT | NT | 11 | 689 | 11.75 | 8.97 |
| (3.49 to 39.55) | (4.4 to 20.8) | ||||
| PFMT + BF | NT | 2 | 110 | 24.20 | 21.7 |
| (2.02 to 290.58) | (7.24 to 75.2) | ||||
| ES | NT | 7 | 369 | 3.93 | 4.75 |
| (1.43 to 10.80) | (2.02 to 11.9) | ||||
| VC | NT | 2 | 212 | 5.43 | 6.99 |
| (0.07 to 396.77) | (2.63 to 20.7) | ||||
| SNRI | NT | 10 | 3672 | 2.02 | 2.24 |
| (1.67 to 2.44) | (1.09 to 4.68) | ||||
| BT | NT | 1 | 123 | 9.60 | 11.3 |
| (4.22 to 21.87) | (1.92 to 70.1) | ||||
| PFMT + ES | NT | 2 | 108 | 8.69 | 13.1 |
| (1.87 to 40.32) | (2.91 to 67.5) | ||||
| PFMT + SNRI | NT | 1 | 96 | 3.28 | 5.63 |
| (1.41 to 7.64) | (0.784 to 43) | ||||
| PFMT | ES | 6 | 190 | 2.18 | 1.9 |
| (0.76 to 6.28) | (0.81 to 4.67) | ||||
| PFMT | VC | 5 | 331 | 1.01 | 1.29 |
| (0.52 to 1.95) | (0.527 to 3.18) | ||||
| PFMT + BF | VC | 1 | 46 | 1.14 | 3.11 |
| (0.34 to 3.85) | (0.948 to 10.7) | ||||
| PFMT + BF | BT | 1 | 129 | 1.71 | 1.92 |
| (0.79 to 3.70) | (0.336 to 12.1) | ||||
| PFMT | SNRI | 1 | 99 | 1.60 | 4.01 |
| (0.71 to 3.60) | (1.53 to 12) | ||||
| ES | VC | 3 | 141 | 1.30 | 0.675 |
| (0.59 to 2.84) | (0.235 to 1.9) | ||||
| PFMT | PFMT + ES | 3 | 160 | 0.84 | 0.686 |
| (0.34 to 2.07) | (0.153 to 3.12) | ||||
| PFMT + BF | PFMT + BF + ES | 2 | 102 | 0.86 | 0.935 |
| (0.36 to 2.08) | (0.208 to 4.29) | ||||
| PFMT | PFMT + VC | 1 | 46 | 0.84 | 0.509 |
| (0.26 to 2.68) | (0.07 to 3.34) | ||||
| PFMT + BF | PFMT + BF + BT | 1 | 124 | 0.35 | 0.352 |
| (0.13 to 0.97) | (0.0332 to 3.67) | ||||
| PFMT | PFMT + SNRI | 1 | 100 | 0.78 | 1.59 |
| (0.34 to 1.82) | (0.214 to 12.9) | ||||
| PFMT + ES | ES | 1 | 22 | 4.67 | 2.77 |
| (0.77 to 28.47) | (0.554 to 14.4) | ||||
| PFMT + VC | VC | 1 | 34 | 5.00 | 2.52 |
| (0.52 to 48.46) | (0.358 to 20.2) | ||||
| PFMT + BF + BT | BT | 1 | 127 | 4.90 | 5.5 |
| (1.84 to 13.10) | (0.551 to 60.8) | ||||
| PFMT + SNRI | SNRI | 1 | 101 | 2.04 | 2.52 |
| (0.90 to 4.64) | (0.337 to 20) | ||||
| PFMT | PFMT + BF | 7 | 296 | 0.41 | 0.414 |
| (0.18 to 0.97) | (0.143 to 1.15) | ||||
| PFMT basic | PFMT extra | 2 | 74 | 0.05 | 0.174 |
| (0.01 to 0.28) | (0.0617 to 0.473) | ||||
The MTC results provided similar results to the direct comparisons. Because this comparison was able to draw upon both the direct and indirect comparative evidence, it was able to provide more precise estimates of relative effectiveness than provided by the direct pairwise comparisons.
Pelvic floor muscle training, when supervised, with extra sessions (more than two sessions or contacts with a health-care professional per month), was better than basic PFMT in terms of both cure and improvement, as shown by both direct evidence and the MTC model (see the final row of each section of Table 81 and, for the MTC model only, see Table 82).
| Intervention | Cure, median (95% CrI) | Improvement, median (95% CrI) | |
|---|---|---|---|
| 1 | 2 | ||
| PFMT with extra sessions | |||
| PFMT extra | NT | 10.7 (5.03 to 26.2) | 25.7 (10.3 to 73.1) |
| PFMT extra | PFMT basic | 8.36 (3.74 to 21.7) | 5.75 (2.11 to 16.2) |
| PFMT extra | PFMT + BF | 0.867 (0.45 to 1.69) | 1.01 (0.32 to 3.16) |
| PFMT extra | ES | 7.43 (2.72 to 22.3) | 4.68 (1.75 to 13.2) |
| PFMT extra | VC | 3.02 (1.04 to 9.53) | 3.79 (1.32 to 11.5) |
| PFMT extra | SNRI80 | 7.44 (2.44 to 27.8) | 12 (3.92 to 42.4) |
| PFMT extra | BT | 1.41 (0.481 to 4.48) | 2.13 (0.34 to 14.5) |
| PFMT extra | PFMT + ES | 3.51 (1.12 to 10.2) | 1.24 (0.27 to 5.79) |
| PFMT extra | PFMT + ES + BF | 1.17 (0.07 to 20.5) | 1.19 (0.23 to 6.3) |
| PFMT extra | PFMT + VC | 3.45 (0.31 to 35.6) | 2.11 (0.26 to 16) |
| PFMT extra | PFMT + VC + BF | 1.87 (0.04 to 46.2) | 9.66 (0.62 to 158) |
| PFMT extra | PFMT + BT + BF | 0.422 (0.09 to 2.14) | 0.369 (0.03 to 4.4) |
| PFMT extra | PFMT + drug | – – | 5.82 (0.73 to 50.7) |
| PFMT plus BF | |||
| PFMT + BF | NT | 12.3 (5.35 to 32.7) | 25.4 (8.68 to 86.9) |
| PFMT + BF | PFMT basic | 9.63 (4.12 to 25.9) | 5.68 (1.88 to 18.3) |
| PFMT + BF | PFMT extra | 1.15 (0.59 to 2.22) | 0.99 (0.32 to 3.11) |
| PFMT + BF | ES | 8.55 (2.88 to 27.6) | 4.63 (1.37 to 16.9) |
| PFMT + BF | VC | 3.47 (1.19 to 11) | 3.75 (1.17 to 12.5) |
| PFMT + BF | SNRI80 | 8.58 (2.64 to 33.4) | 11.8 (3.37 to 48.4) |
| PFMT + BF | BT | 1.62 (0.54 to 5.18) | 2.11 (0.38 to 12.5) |
| PFMT + BF | PFMT + ES | 4.05 (1.15 to 12.7) | 1.23 (0.21 to 7.51) |
| PFMT + BF | PFMT + ES + BF | 1.34 (0.07 to 24) | 1.18 (0.27 to 5.21) |
| PFMT + BF | PFMT + VC | 3.96 (0.35 to 41.2) | 2.09 (0.25 to 16.7) |
| PFMT + BF | PFMT + VC + BF | 2.14 (0.05 to 53.7) | 9.54 (0.57 to 170) |
| PFMT + BF | PFMT + BT + BF | 0.49 (0.11 to 2.28) | 0.37 (0.04 to 3.61) |
| PFMT + BF | PFMT + drug | – – | 5.75 (0.67 to 54.2) |
Both pelvic floor muscle training with extra sessions,and PFMT with BF were also more effective in terms of both cure and improvement compared with: no treatment; ES; VCs; or SNRI (Table 82). There was considerable uncertainty about whether either is better than BT.
Evidence about PFMT (with or without BF) combined with an adjunct treatment (e.g. ES, VCs) was generally inconclusive, largely due to a lack of available data. Adding BT to PFMT with BF appears to be more effective than PFMT with BF alone or BT alone, although interpretation requires caution, as this finding is based upon data from a single trial that considered a population who had both stress and urgency incontinence symptoms. The MTC results for these pairs of treatments were also inconclusive.
All of these results need to be considered cautiously. Importantly, the longevity of any treatment effects was unclear because of the small amount of data available and limited duration of follow-up.
Summary of results from the economic model
Data from the MTCs were used to populate an economic model. The economic model presented in this report compared eight different management strategies. These were chosen because they were believed to be relevant to the NHS and they might potentially be cost-effective. The model compared cumulative costs and QALYs for a 40-year time horizon for two separate analyses; one based on cure rates and the other based on improvement rates.
In the model based on cure rates, the least costly strategy was lifestyle intervention, followed by PFMT with extra sessions (or PFMT plus BF) and then surgery if necessary, with a mean cost per woman treated of £1644. The most costly strategy was lifestyle changes followed by surgery with a mean cost per woman treated of £1973. In terms of QALYs, the least effective strategy was lifestyle changes followed by surgery (mean QALYs per woman treated = 16.1). The most effective was lifestyle changes followed by PFMT with extra sessions followed by surgery if necessary (mean QALYs per woman treated = 16.2). There were relatively modest differences between treatments in terms of both QALYs and costs. One interpretation of these results would be that any of these treatment strategies could be equally well provided by the NHS. Nevertheless, when the incremental cost-effectiveness was estimated then it was highly likely that lifestyle changes followed by PFMT with extra sessions followed by surgery if necessary would be cost-effective (there was an over 70% chance that this intervention would be considered cost-effective at a threshold value of £20,000 per QALY).
For the model based on improvement rates, the QALYs and costs for each treatment were greater than those for the model based on cure rates. This is because improvement rates were greater than cure rates but no quality-of-life data that were specific to improvement were available. Therefore, it was assumed that the utility associated with improvement was the same as the utility associated with cure. Costs were greater because it was assumed that women would incur some costs of containment products, even if their symptoms were improved. As a consequence, comparisons with the analysis made using cure rates should be interpreted cautiously.
In the model based on improvement rates, the least costly strategy was lifestyle changes, followed by PFMT basic, PFMT with extra sessions and then surgery (mean cost per woman treated = £1795), and the most costly was lifestyle changes followed by surgery with a mean cost per woman treated of £2425. In terms of QALYs, the least effective strategy was lifestyle changes followed by surgery (mean QALYs per woman treated = 16.2). The most effective was lifestyle changes followed by PFMT with extra sessions followed by surgery if necessary (mean QALYs per woman treated = 16.37). Which treatment strategy was most likely to be cost-effective depended upon society’s willingness to pay for a QALY. Below a threshold of £30,000 per QALY, lifestyle changes followed by PFMT basic and then surgery was the intervention most likely to be considered cost-effective. Above that a threshold of £30,000 per QALY, lifestyle changes followed by PFMT with extra sessions followed by surgery if necessary was most likely to be considered cost-effective. This strategy had a 55% likelihood of being considered cost-effective when society’s willingness to pay for a QALY was between £10,000 and £30,000.
The role of drug therapy appears limited, as strategies involving drug management were unlikely to be considered cost-effective. This is primarily due to the non-adherence to SNRI treatment caused by the side effects of the drugs, which limited their effectiveness. Furthermore, the strategy involving surgery without the use of non-surgical treatments was not likely to be cost-effective.
When success was defined in terms of cure or improvement, the results were insensitive to the introduction of strategies involving VCs or ES. It was also found that the interpretation of the cost-effectiveness results did not greatly change when the model was adapted to allow women to exercise preference not to seek surgery or repeat surgery, should cure or sufficient improvement not be achieved.
These data suggest that adopting an intervention such as PFMT with extra sessions (or potentially other more intensive forms of PFMT, such as PFMT with BF) would be more efficient than PFMT basic. This is important as PFMT basic is perhaps closest to the form of PFMT most common in the NHS. Therefore, although PFMT basic does appear to improve the symptoms of women with SUI, consideration should be given to whether it is practical and acceptable to both women and the NHS to provide some form of more intensive PFMT (either alone or with an adjunct, such as BF training).
The results were most sensitive to changes in the effectiveness of PFMT with extra sessions. Should the chance of PFMT with extra sessions achieving cure or improvement reduce, or should longer-term recurrence rates increase, then the likelihood that lifestyle changes followed by PFMT with extra sessions followed by surgery is cost-effective would fall. For the model based on cure rates, lifestyle changes followed by PFMT with extra sessions followed by surgery would no longer be the most cost-effective strategy when long-term cure rates were reduced by more than 60%. For the model based on improvement rates, lifestyle changes followed by PFMT with extra sessions followed by surgery would no longer the most cost-effective strategy when long-term improvement rates of PFMT with extra sessions were the same as PFMT basic. The long-term success rates of PFMT with extra sessions were based upon data from Bø and colleagues,115,159 who used a combination of group and individual sessions with women, as well as longer-term provision of training. It is unclear, however, what factors determined the outcomes they observed (e.g. was the motivation of the women atypical, was it the intensity of the intervention or some other factor?) or whether the results of this study could be replicated in routine NHS practice.
The results were also sensitive to changes in the cost of PFMT with extra sessions. Lifestyle changes followed by PFMT with extra sessions followed by surgery would no longer be cost-effective when the cost of PFMT with extra sessions was increased by nearly £1000 per woman treated in the model based on cure rates, and £400 per woman treated when it was in the model based on improvement rates.
Overall, the results of the economic evaluation suggests that further research to develop and test a more intensive PFMT intervention that is acceptable to women and feasible for the NHS is warranted.
Strengths, assumptions, limitations and uncertainties
Numerous previous studies have considered the relative effectiveness of non-surgical treatments for SUI in women. 50,56,60,64,68,71,83–85,87,88,106,201,224–228 What our study has added is the systematic review and overview of the treatments relevant to the NHS, where evidence on the relative effectiveness has been derived using more advanced methods of meta-analysis than have been used previously. These methods have allowed us to provide clearer evidence about which treatments work and how well they work than was hitherto available. The statistical approach of MTC allowed indirect evidence to supplement direct head-to-head comparisons of treatments. This made the comparison of treatments much easier than trying to interpret the data from the 68 direct head-to-head (pairwise) comparisons.
Despite extensive searching and the identification of a large number of studies, few data were available for most comparisons. Over time this may be rectified, and, indeed, an updated search conducted up to June 2009 identified additional 12 articles that appear to meet our inclusion criteria (these studies are listed in Appendix 28).
Of the studies that were identified, nearly one-half of the participants in the included trials (46%, 4554/9803) came from the 12 trials that included a SNRI as one of their trial arms. Given this, a pragmatic decision was made to include studies where women with urgency urinary incontinence symptoms also formed part of the study population (population types 2 and 3).
Generally, these studies were less likely to show large effect sizes than studies that only included women with SUI alone. The studies with mixed populations, however, tended to have a larger sample, but involved fewer supervisory sessions than studies of women with only SUI. Both of these factors may also have affected the estimated size of effect.
Even for the relatively simple clinical outcomes, such as cure or improvement, the lack of consensus on the most appropriate method for assessing incontinence presented a particular problem for evidence synthesis. Ideally, the success of a treatment should be gauged on the ability of women to lead a normal social life. Using this definition usually means that the woman herself reports a satisfactory resolution (or near resolution) of her symptoms. Unfortunately, patient-reported measures were not always available. Moreover, available data (including proxy measures based on the quantification of symptoms derived from diaries or pad tests) did not differentiate those who are sufficiently better (and do not want further treatment) from those who are better but do want further treatment.
A further challenge for evidence synthesis that is related to the lack of consistency between studies and the limited reporting of studies was that intervention protocols were complex and varied considerably across studies. For example, PFMT differed widely in terms of the precise nature of the exercises, how patients were instructed, and the frequency and duration of therapy (Appendix 12). Generally, such data were poorly reported, with the exercise protocol not consistently described using the same criteria. In an attempt to explore the impact of intensity of therapy, PFMT was defined solely by the frequency of supervisory clinic sessions or contacts with a health-care professional. Similarly, the complex nature of the intervention protocols also meant that it was difficult to decipher which aspect of the intervention actually worked. For example, the addition of BF training to PFMT appeared beneficial in enhancing the effect of PFMT. This may have occurred because women made greater use of PFMT and increased their adherence to the training programme. Introducing a BF device may also intensify the nature or the quantity of supervision provided by the health-care professional, which, in itself, may be beneficial for women. What the research does highlight is the importance of intensity of therapy on effectiveness and cost-effectiveness, and the need for the evidence-based development of more intensive regimens that can be taken forward to rigorous evaluation in adequately powered RCTs.
When assessing the effectiveness of treatment emphasis was given to using the data collected at the end of the supervised treatment phase. It was expected that these data would represent the point where the treatments would show their maximum effect. Long-term data beyond the supervised treatment phase were sparse but the evidence gathered for the economic evaluation suggests that the effectiveness was not maintained. This may be due to poor adherence to a muscle training programme or whether the effects of training can be sustained into the longer term. The results of the economic evaluation are sensitive to changes in long-term performance of non-surgical interventions. Improvements in the long-term performance will improve the cost-effectiveness of non-surgical treatments.
Although the meta-analyses have limitations, one of the strengths of this study is that it has used these rigorously assembled data within an economic evaluation. This economic evaluation compared treatment strategies relevant to the NHS and hitherto no such analysis existed. Indeed, no economic evaluations were identified which compared all the relevant treatments.
Modelling the cost-effectiveness of non-surgical treatments was challenging because of the number of potential management strategies that might be relevant and also the lack of data available. As described in Chapter 9, considerable efforts were made to identify relevant data, and extensive sensitivity analyses were used to explore the impact of uncertainties. Useful information to help guide practice has been produced but it should be remembered that, apart from drug therapy, few data were available for any of the treatments, and very few data on long-term performance were available for any therapy. Therefore, the results of this study should be treated cautiously.
All of the strategies considered within the economic model included lifestyle advice (e.g. reduce weight, restrict caffeine intake, etc.). It was assumed that, although advice would be given, it would be ineffective. A recent trial has shown that weight reduction (lifestyle change) in obese women may reduce the symptoms of incontinence and further work on lifestyle interventions may be worthwhile.
Within the economic evaluation the effectiveness of treatments were measured in QALYs, which were derived from EQ-5D values obtained from the literature (and in a sensitivity analysis from a survey conducted as part of this study). The EQ-5D has been recommended as the method of valuing health states by NICE,105 but it may not be sensitive enough to capture the concerns of women. Any failure to accurately measure the benefits of treatment may lead to erroneous conclusions about cost-effectiveness.
A further limitation of the economic model was that there were no quality-of-life data to differentiate between cure and improvement. It might be expected that the quality of life of women whose symptoms had improved but who were not cured would be less than women who were cured. Alternatively, changes in the frequency of incontinence may fail to translate into benefits of importance to women. Ideally, further research should be conducted on the quality of life of women whose symptoms are improved and who no longer seek further treatment.
Within all of the economic analyses, the preferences of women for the process of care have not been considered. Women are likely to have preferences about who provides the care, where the care is provided, and what risks and costs they face themselves. These factors are not captured by measures such as the EQ-5D. Therefore, research to elicit the preferences of women for the different outcomes and processes of treatment in a form that is suitable for incorporation into an economic evaluation is likely to be worthwhile. Such data would be complementary to existing data and could help to highlight areas in which the preferences of women for outcomes of importance to them would lead to different policy decisions.
The economic model has focused on costs to the NHS. It has been assumed that certain costs, such as those for VCs and containment products, may be incurred by the NHS. In practice, women may buy the cones and may well incur the costs of containment management themselves. Other costs that may fall on the women have not been included. These include the other costs of managing symptoms, such as laundry costs, and the time and travel costs related to accessing care. It might be expected that the more effective treatments would reduce the costs of managing symptoms borne by the women and their families. However, the more effective treatments may also require substantially more time commitment (and travel costs to access care) from the women. The net effect of these two factors is uncertain.
A further limitation with respect to costs is that the handling of facility costs and hospital management costs has not been wholly consistent. For example, for some interventions, such as the use of physiotherapy, the cost has been used upon the staff and equipment used to provide a session but has not included an element to cover the facility and management costs. However, for other interventions, for example the cost of surgery, these are based on nationally available figures that do include an element to cover facility costs and hospital management costs. The net impact of this is to make PFMT interventions more likely to be cost-effective compared with surgery. However, as the sensitivity analysis has illustrated, increases in the cost of PFMT would need to be substantial to change the overall results and it is implausible that this would actually be the case.
Nevertheless, despite the limitations of the evidence base, there is evidence from a number of trials that PFMT plus BF and PFMT with extra sessions was effective. Furthermore, strategies involving these treatments are likely to be considered cost-effective at threshold values that society might be willing to pay for a QALY.
Chapter 11 Conclusions
Implications for the NHS
-
The available data suggest that non-surgical treatments for SUI in women are effective and could potentially be cost-effective, but a judgement is required as to whether the benefits are worth the cost.
-
There is no evidence that PFMT basic (which is similar to the form of PFMT provided by the NHS)43 is any better than no treatment when success is measured in terms of cure, although it is better than no treatment when success is measured in terms of improvement.
-
There is clear evidence from a number of trials that ‘PFMT plus BF’ and ‘PFMT with extra sessions’ were effective compared with no treatment.
-
Both ‘PFMT plus BF’ and ‘PFMT with extra sessions’ are more effective (for both cure and improvement) than PFMT basic.
-
Evidence from a small number of trials suggests that other non-surgical treatments may also be effective (PFMT plus BT and BF; PFMT plus ES and BF; PFMT plus VCs and BF). There is, however, insufficient evidence to recommend their routine use by the NHS.
-
A strategy by which women can progress to surgery almost immediately is unlikely to be cost-effective, primarily because of the cost of surgery.
-
Treatment with SNRI drugs are, on average, effective (measured in terms of cure or improvement), but the frequency of side effects mean that women do not tend to use this therapy for long. Therefore, strategies involving SNRI are unlikely to be cost-effective.
-
As no treatment or treatment strategy is perfect, women should be offered support to help them articulate what it is that they hope to achieve from therapy.
-
The differences between the treatment strategies considered (measured in QALYs) were relatively modest and, although some strategies were found to be more likely to be cost-effective, the evidence base was limited.
-
The feasibility of the potentially cost-effective more intensive forms of PFMT is questionable, as there may not be sufficient trained therapists to provide this care at present. For the use of these therapies to increase, staff would need to be recruited, trained and retained. Recent surveys about the provision of care by the NHS by the Continence Foundation (Judith Wardle, formerly of the Continence Foundation, March 2009, personal communication) found that less costly therapists were being substituted for the higher-grade continence nurse specialists. Given the potential demand for care, non-specialist care providers are likely to be necessary, in addition to care provided by specialist therapists, but they will need appropriate training.
-
Opportunities for self-management by the women should be encouraged, as women can purchase VCs themselves or they could undertake PFMT without formal supervision. Therapies are most likely to be effective and cost-effective when women receive training so that they can perform the exercises correctly.
-
Continuing and ongoing support for women with SUI may be required beyond current programmes, as long-term performance is central to estimates of long-term effectiveness.
-
Conversely, providing therapies when there is no sustained follow-up may not be a good use of scarce practitioner time or resources.
Implications for women
Non-surgical treatment for SUI can provide either cure or improvement in symptoms. A non-surgical treatment might not totally resolve symptoms but may lead to sufficient improvement so that a woman considers that further treatment is not worthwhile. Although the woman may not desire further treatment, it should be noted that symptoms may still be bothersome and may require the use of containment products. The cost of using containment products and extra laundry will still fall on women and their families.
For some women, non-surgical treatments can delay or prevent the need for surgery. This may be particularly important for those women who have no desire to undergo surgery and may be prepared to accept severe incontinence rather than face surgery. The recourse to surgery early in a treatment pathway might be preferred by some women who are unable to use non-surgical treatments or who are unwilling to devote the sustained time and effort required to obtain and maintain an improvement in symptoms. However, over the longer term better outcomes might be achieved if the women try out a non-surgical treatment first.
Some of the therapies do not necessarily need involvement of the health service. Women can purchase VCs themselves, or they could undertake PFMT without formal supervision. These treatments can be effective, but whether this is worthwhile depends upon the ability of the woman to perform the therapy correctly. Therefore, women should consider whether some formal instruction by suitably trained health-care professionals would be helpful to ensure that they are performing correct contractions.
The long-term success of the treatments such as PFMT declines over time. One potential reason for why this happens is that women do not continue to perform the exercises in the long term. This requires a behaviour change that women might find difficult to initiate and maintain. Without making and maintaining this change, it is likely that symptoms will return and further treatments, including surgery, might be required.
Further research
Evidence has been provided to show that several of the non-surgical treatments for SUI can be effective, at least in the short term, and are potentially cost-effective. The evidence, however, is based upon a number of small trials, and, for many comparisons, only single trials.
-
Further definitive evidence from large well-designed studies of the most promising regimens (in terms of likely effectiveness, cost-effectiveness and feasibility within the health service) is required to provide a definitive answer.
-
Any further research, be it from a trial, on long-term outcomes, benefit assessment, or costs, should be incorporated into an updated economic evaluation, as and when it becomes available.
-
Such trials should consider the long-term effectiveness of more intensive versions of PFMT (e.g. PFMT with BF training) in typical standard health-care setting and an economic modelling exercise that will place the results of the trial into the context of other relevant research and extrapolate from the trial results.
-
The effectiveness and cost-effectiveness will depend upon the long-term effective of treatments, which, in turn, may depend upon whether any training programme is adhered to and sustained into the longer term. Research in how this might be achieved in a way that is feasible for both the NHS and women is required.
-
Further work to understand what outcomes are of importance to women and the strength of preference of women for these outcomes is required.
-
Understanding the preferences of women for different ways that care could be provided, and the trade-offs between different process and outcome measures, would be useful. Women may also have preferences about the process of care as well as the outcome of care. Ideally, such data should be suitable for incorporation into a subsequent economic evaluation.
-
The impact of costs (both in terms of out of pocket expenses and time) that fall upon women and their families should be explored further. Specifically, information is needed about the trade-off between the costs of managing symptoms (use of self-purchased containment products, laundry costs, etc.) and the time and travel costs of treatment.
-
If an effective and efficient follow-up regimen can be developed, then the incentives/disincentives faced by NHS providers may need to be reconsidered. For example, within England, performance current monitoring goals might lead to a focus on ‘first contacts’ at the expense of follow-up care.
Summary of conclusions
More intensive forms of PFMT (‘PFMT with extra sessions’ and ‘PFMT with BF’) are effective and potentially worthwhile uses of NHS resources. Nevertheless, the data came from a few small trials and further information from large well-designed studies is required to establish whether these interventions are effective, cost-effective and feasible for the NHS to provide.
Acknowledgements
We would like to thank:
-
all those researchers from around the world who provided additional information on the trials
-
the members of the Bladder and Bowel Foundation (formerly InContact) who participated in the survey of issues of importance to women and the Bladder and Bowel Foundation itself for organising the survey
-
the ACA (Association for Continence Advice) members for participating in the survey
-
the translators, including Stephen Dombrowski (German), Barbara Eberth (German), Tânia Lourenço (Spanish and Portuguese) and Nargiz Y. Tagiyeva-Milne (Azerbaijani)
-
Graham Mowatt, Senior Systematic Reviewer, Health Services Research Unit, for advice and critical comments throughout this project
-
Bronwyn Davidson, Karen McLeod, Midj Falconer, Lara Kemp, Angela May and Kathleen McIntosh for secretarial support.
The Health Services Research Unit and the Health Economics Research Unit are both core funded by the Chief Scientist Office of the Scottish Government Health Directorates.
Contribution of authors
Robert Pickard led the drafting of the background chapter and was assisted in this by Jenni Hislop. Jenni Hislop also assisted Mari Imamura in the completion of the systematic review of effectiveness. Mary Kilonzo led the drafting of the chapter outlining the decision problem and led the economic evaluation, on which she worked closely with Shihua Zhu. Laura Ternent designed, conducted and led the survey of members of the Bladder and Bowel Foundation. She was assisted in this by Brian Buckley and Cathryn Glazener. David Jenkinson led the work on mixed-treatment comparisons and drew upon the advice of Jonathan Cook and comments of Mari Imamura and Cathryn Glazener. Sheila Wallace conducted the literature searches, drafted sections of the report related to this work and provided reference management for the whole project. Paul Abrams, Christine Bain, Brian Buckley, Linda Cardozo, June Cody, Sharon Eustice, Adrian Grant, Jean Hay-Smith, Ghulam Nabi, James N’Dow, Robert Pickard and Judith Wardle all provided advice in their own areas of expertise and provided critical comments throughout the project. Luke Vale was involved in all elements of the project and, along with Mari Imamura, provided project management. All authors assisted in preparing the manuscript, reading and commenting on drafts, and reading the final draft.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Perry S, Shaw C, Assassa P, Dallosso H, Williams K, Brittain KR, et al. An epidemiological study to establish the prevalence of urinary symptoms and felt need in the community: the Leicestershire MRC Incontinence Study. Leicestershire MRC Incontinence Study Team. J Public Health Med 2000;22:427-34.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116-26.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:167-78.
- Avery KN, Bosch JL, Gotoh M, Naughton M, Jackson S, Radley SC, et al. Questionnaires to assess urinary and anal incontinence: review and recommendations. J Urol 2007;177:39-4.
- Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc 1998;46:473-80.
- McGrother CW, Donaldson MM, Shaw C, Matthews RJ, Hayward TA, Dallosso HM, et al. Storage symptoms of the bladder: prevalence, incidence and need for services in the UK. BJU Int 2004;93:763-9.
- McGrother C, Donaldson M, Wagg A, Matharu G, Williams K, Watson J, et al. Health care needs assessment: the epidemiologically based needs assessment reviews (third series). Oxford: Radcliffe Publishing Ltd; 2006.
- Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol 2000;53:1150-7.
- Hunskaar S, Arnold EP, Burgio K, Diokno AC, Herzog AR, Mallett VT. Epidemiology and natural history of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2000;11:301-19.
- Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjalmas K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology 2003;62:16-23.
- Hunskaar S, Lose G, Sykes D, Voss S. The prevalence of urinary incontinence in women in four European countries. BJU Int 2004;93:324-30.
- Hunskaar S, Burgio K, Clarke A, Lapitan MC, Nelson R, Sillen U, et al. Incontinence: 3rd International Consultation on Incontinence. Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Plymouth, UK: Health Publication Ltd; 2005.
- Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn 2000;19:137-45.
- Chang CH, Gonzalez CM, Lau DT, Sier HC. Urinary incontinence and self–reported health among the U.S. Medicare managed care beneficiaries. J Aging Health 2008;20:405-19.
- Shaw C, Tansey R, Jackson C, Hyde C, Allan R. Barriers to help seeking in people with urinary symptoms. Fam Pract 2001;18:48-52.
- Shaw C, Das GR, Williams KS, Assassa RP, McGrother C. A survey of help-seeking and treatment provision in women with stress urinary incontinence. BJU Int 2006;97:752-7.
- Huang AJ, Brown JS, Kanaya AM, Creasman JM, Ragins AI, van den Eeden SK, et al. Quality-of-life impact and treatment of urinary incontinence in ethnically diverse older women. Arch Intern Med 2006;166:2000-6.
- Townsend MK, Danforth KN, Lifford KL, Rosner B, Curhan GC, Resnick NM, et al. Incidence and remission of urinary incontinence in middle-aged women. Am J Obstet Gynecol 2007;197:167-5.
- Jackson SL, Scholes D, Boyko EJ, Abraham L, Fihn SD. Predictors of urinary incontinence in a prospective cohort of postmenopausal women. Obstet Gynecol 2006;108:855-62.
- Nygaard IE, Lemke JH. Urinary incontinence in rural older women: prevalence, incidence and remission. J Am Geriatr Soc 1996;44:1049-54.
- Yip SK, Sahota D, Chang A, Chung T. Effect of one interval vaginal delivery on the prevalence of stress urinary incontinence: a prospective cohort study. Neurourol Urodyn 2003;22:558-62.
- Altman D, Ekstrom A, Gustafsson C, Lopez A, Falconer C, Zetterstrom J. Risk of urinary incontinence after childbirth: a 10-year prospective cohort study. Obstet Gynecol 2006;108:873-8.
- Viktrup L, Rortveit G, Lose G. Does the impact of subsequent incontinence risk factors depend on continence status during the first pregnancy or the postpartum period 12 years before? A cohort study in 232 primiparous women. Am J Obstet Gynecol 2008;199:73-4.
- Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol 1997;90:983-9.
- MacArthur C, Glazener CM, Wilson PD, Lancashire RJ, Herbison GP, Grant AM. Persistent urinary incontinence and delivery mode history: a six-year longitudinal study. BJOG 2006;113:218-24.
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG 2000;107:1460-70.
- Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med 2003;348:900-7.
- Dolan LM, Hosker GL, Mallett VT, Allen RE, Smith AR. Stress incontinence and pelvic floor neurophysiology 15 years after the first delivery. BJOG 2003;110:1107-14.
- Fritel X, Ringa V, Varnoux N, Fauconnier A, Piault S, Breart G. Mode of delivery and severe stress incontinence. A cross-sectional study among 2625 perimenopausal women. BJOG 2005;112:1646-51.
- Hampel C, Artibani W, Espuna Pons M, Haab F, Jackson S, Romero J, et al. Understanding the burden of stress urinary incontinence in Europe: a qualitative review of the literature. Eur Urol 2004;46:15-27.
- Allahdin S, Harrild K, Warraich QA, Bain C. Comparison of the long-term effects of simple total abdominal hysterectomy with transcervical endometrial resection on urinary incontinence. BJOG 2008;115:199-204.
- Hunskaar S, Vinsnes A. The quality of life in women with urinary incontinence as measured by the sickness impact profile. J Am Geriatr Soc 1991;39:378-82.
- Norton PA, MacDonald LD, Sedgwick PM, Stanton SL. Distress and delay associated with urinary incontinence, frequency, and urgency in women. BMJ 1988;297:1187-9.
- Nygaard I, DeLancey JOL, Arnsdorf L, Murphy E. Exercise and incontinence. Obstet Gynecol 1990;75:848-51.
- Office for National Statistics . Office for National Statistics population estimates. Age Structure of the United Kingdom, Mid-2007: Interactive Population Pyramid n.d. www.statistics.gov.uk/populationestimates/svg_pyramid/default.htm (accessed 16 February 2009).
- Papanicolaou S, Pons ME, Hampel C, Monz B, Quail D, Schulenburg MG, et al. Medical resource utilisation and cost of care for women seeking treatment for urinary incontinence in an outpatient setting. Examples from three countries participating in the PURE study. Maturitas 2005;52:S35-47.
- Turner DA, Shaw C, McGrother CW, Dallosso HM, Cooper NJ. MRC Incontinence Team . The cost of clinically significant urinary storage symptoms for community dwelling adults in the UK. BJU Int 2004;93:1246-52.
- Lapitan MC, Cody DJ, Grant AM. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev 2005. 10.1002/14651858.CD002912.pub2.
- Robinson D, Anders K, Cardozo L, Bidmead J, Dixon A, Balmforth J, et al. What do women want?: interpretation of the concept of cure. J Pelvic Med Surg 2003;9:273-7.
- Laycock J, Standley A, Crothers E, Naylor D, Frank M, Garside S, et al. Clinical Guidelines for the Physiotherapy Management of Females Aged 16–65 Years With Stress Urinary Incontinence 2001. www.csp.org.uk (accessed 9 February 2008).
- Department of Health . Good Practice in Continence Services 2000. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4005851 (accessed 5 March 2009).
- Thuroff J, Abrams P, Andersson KE, Artibani W, Chartier-Kastler E, Hampel C, et al. Guidelines on urinary incontinence. Arnhem, the Netherlands: European Association of Urology; 2005.
- National Collaborating Centre for Women’s and Children’s Health, National Institute for Health and Clinical Excellence (NICE) . Urinary Incontinence: The Management of Urinary Incontinence in Women 2006. www.nice.org.uk/Guidance/CG40#summary (accessed 23 February 2009).
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Urinary Incontinence in Primary Care: A National Clinical Guideline 2005. www.sign.ac.uk/pdf/sign79.pdf (accessed 4 March 2009).
- Hunskaar S. A systematic review of overweight and obesity as risk factors and targets for clinical intervention for urinary incontinence in women. Neurourol Urodyn 2008;27:749-57.
- Curtis L. Unit costs of health and social care. Canterbury, UK: Personal Social Services Research Unit (PSSRU), University of Kent; 2008.
- Weight Watchers International Inc . Weight Watchers (registered Trademark) n.d. www.weightwatchers.co.uk (accessed 5 March 2009).
- Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol 1948;56:238-48.
- Anders K. Recent developments in stress urinary incontinence in women. Nurs Stand 2006;20:48-54.
- Herbison P, Dean N. Weighted vaginal cones for urinary incontinence. Cochrane Database Syst Rev 2002. 10.1002/14651858.CD002114.
- Indrekvam S, Hunskaar S. Side effects, feasibility, and adherence to treatment during home–managed electrical stimulation for urinary incontinence: a Norwegian national cohort of 3,198 women. Neurourol Urodyn 2002;21:546-52.
- Physio-Med Services Ltd . Physio-Med Services n.d. www.physio-med.com/ (accessed 5 Mar 2009).
- Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database Syst Rev 2004. 10.1002/14651858.CD001308.pub2.
- Department of Health . NHS Reference Costs 2006–07 2008. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_082571 (accessed 5 March 2009).
- Jost W, Marsalek P. Duloxetine: mechanism of action at the lower urinary tract and Onuf’s nucleus. Clin Auton Res 2004;14:220-7.
- Mariappan P, Alhasso A, Grant A, N’Dow J. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database Syst Rev 2005. 10.1002/14651858.CD004742.pub2.
- Ghoniem GM, Van Leeuwen JS, Elser DM, Freeman RM, Zhao YD, Yalcin I, et al. A randomized controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment and no active treatment in women with stress urinary incontinence. J Urol 2005;173:1647-53.
- Department of Health . Prescriptions. United Kingdom National Health Service Prescribing Data 2008. www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescriptions (accessed 5 March 2009).
- British Medical Association, Royal Pharmaceutical Society of Great Britain . British National Formulary, No. 56 2004. www.bnf.org/bnf/ (accessed 15 February 2009).
- Moehrer B, Hextall A, Jackson S. Oestrogens for urinary incontinence in women. Cochrane Database Syst Rev 2003. 10.1002/14651858.CD001405.
- Grady D, Brown JS, Vittinghoff E, Applegate W, Varner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol 2001;97:116-20.
- Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005;293:935-48.
- Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med 2008;148:459-73.
- Alhasso A, Glazener C, Pickard R, N’Dow J. Adrenergic drugs for urinary incontinence in adults. Cochrane Database Syst Rev 2005. 10.1002/14651858.CD001842.pub2.
- Thyssen H, Bidmead J, Lose G, Moller BK, Dwyer P, Cardozo L. A new intravaginal device for stress incontinence in women. BJU Int 2001;88:889-92.
- Robinson H, Schulz J, Flood C, Hansen L. A randomized controlled trial of the NEAT expandable tip continence device. Int Urogynecol J Pelvic Floor Dysfunct 2003;14:199-203.
- Dunn M, Brandt D, Nygaard I. Treatment of exercise incontinence with a urethral insert: a pilot study in women. Phys Sportsmed 2002;30:45-8.
- Shaikh S, Ong EK, Glavind K, Cook J, N’Dow JMO. Mechanical devices for urinary incontinence in women. Cochrane Database Syst Rev 2006. 10.1002/14651858.CD001756.pub4.
- Fujishiro T, Enomoto H, Ugawa Y, Takahashi S, Ueno S, Kitamura T. Magnetic stimulation of the sacral roots for the treatment of stress incontinence: an investigational study and placebo controlled trial. J Urol 2000;164:1277-9.
- But I. Conservative treatment of female urinary incontinence with functional magnetic stimulation. Urology 2003;61:558-61.
- Keegan PE, Atiemo K, Cody JD, McClinton S, Pickard R. Periurethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2007. 10.1002/14651858.CD003881.pub2.
- Lee PE, Kung RC, Drutz HP. Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J Urol 2001;165:153-8.
- Kelly HA, Dumm WM. Urinary incontinence in women, without manifest injury to the bladder. Surg Gynecol Obstet 1914;18:444-50.
- Dean NM, Ellis G, Wilson PD, Herbison GP. Laparoscopic colposuspension for urinary incontinence in women. Cochrane Database Syst Rev 2006. 10.1002/14651858.CD002239.pub2.
- Bezerra CA, Bruschini H, Cody DJ. Traditional suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev 2005. 10.1002/14651858.CD001754.pub2.
- Glazener CMA, Cooper K. Anterior vaginal repair for urinary incontinence in women. Cochrane Database Syst Rev 2001. 10.1002/14651858.CD001755.
- Glazener CM, Cooper K. Bladder neck needle suspension for urinary incontinence in women. Cochrane Database Syst Rev 2004. 10.1002/14651858.CD003636.pub2.
- Ogah J, Rogerson L, Cody J. Minimally invasive sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev 2009. 10.1002/14651858.CD006375.pub2.
- Petros PP, Ulmsten U. An anatomical classification: a new paradigm for management of female lower urinary tract dysfunction. Eur J Obstet Gynecol Reprod Biol 1998;80:87-94.
- Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al. Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence. Health Technol Assess 2003;7.
- Novara G, Ficarra V, Boscolo-Berto R, Secco S, Cavalleri S, Artibani W, et al. Tension-free midurethral slings in the treatment of female stress urinary incontinence: a systematic review and meta-analysis of randomized controlled trials of effectiveness. Eur Urol 2007;52:663-78.
- Novara G, Galfano A, Boscolo-Berto R, Secco S, Cavalleri S, Ficarra V, et al. Complication rates of tension-free midurethral slings in the treatment of female stress urinary incontinence: a systematic review and meta-analysis of randomized controlled trials comparing tension-free midurethral tapes to other surgical procedures and different devices. Eur Urol 2008;53:288-309.
- Hay-Smith E, Dumoulin C. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2006. 10.1002/14651858.CD005654.
- Fader M, Cottenden A, Getliffe K. Absorbent products for light urinary incontinence in women. Cochrane Database Syst Rev 2007. 10.1002/14651858.CD001406.pub2.
- Fader M, Cottenden AM, Getliffe K. Absorbent products for moderate–heavy urinary and/or faecal incontinence in women and men. Cochrane Database Syst Rev 2008. 10.1002/14651858.CD007408.
- Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al. Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product designs. Health Technol Assess 2008;12.
- Jahn P, Preuss M, Kernig A, Seifert-Huhmer A, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev 2007. 10.1002/14651858.CD004997.pub2.
- Niel-Weise BS, van den Broek PJ. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev 2005. 10.1002/14651858.CD004201.pub2.
- Nabi G, Cody JD, Dublin N, McClinton S, N’Dow JMO, Neal DE, et al. Urinary diversion and bladder reconstruction/replacement using intestinal segments for intractable incontinence or following cystectomy. Cochrane Database Syst Rev 2009. 10.1002/14651858.CD003306.
- Fantl JA, Newman DK, Colling J, DeLancey JOL, Keeys C, Loughery R, et al. Urinary incontinence in adults: acute and chronic management. Clinical practice guideline update. Number 2, 1996 Update. Rockville, MD: US Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research; 1996.
- Abrams P, Cardozo L, Khoury S, Wein A. 2005.
- Rovner ES, Wein AJ. Treatment options for stress urinary incontinence. Rev Urol 2004;6:S29-47.
- Berghmans LC, Hendriks HJ, de Bie RA, van Waalwijk van Doorn ES, Bø K, van Kerrebroeck PE. Conservative treatment of urge urinary incontinence in women: a systematic review of randomized clinical trials. BJU Int 2000;85:254-63.
- Ruta DA, Garratt AM, Leng M, Russell IT, MacDonald LM. A new approach to the measurement of quality of life. The Patient-Generated Index. Med Care 1994;32:1109-26.
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol 1997;104:1374-9.
- Martin F, Camfield L, Rodham K, Kliempt P, Ruta D. Twelve years’ experience with the Patient Generated Index (PGI) of quality of life: a graded structured review. Qual Life Res 2007;16:705-15.
- Brazzelli M, Shirran E, Vale L. Absorbent products for containing urinary and/or faecal incontinence in adults. Cochrane Database Syst Rev 1999. 10.1002/14651858.CD001406.
- EuroQol Group . EQ–5D n.d. www.euroqol.org/ (accessed 15 March 2008).
- The Bladder and Bowel Foundation . The Bladder and Bowel Foundation (formerly InContact and the Continence Foundation) n.d. www.bladderandbowelfoundation.org (accessed 15 March 2008).
- Buckley B, Wagg A, Winder A. Emotional well-being in faecal and urinary incontinence. Continence UK J 2007;1:66-70.
- Data Protection Act . Data Protection Act 1998 n.d. www.opsi.gov.uk/acts/acts1998/ukpga_19980029_en_1 (accessed 15 March 2008).
- Medical Research Council . Personal Information in Medical Research n.d. www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC002452 (accessed 15 March 2008).
- Tully MP, Cantrill JA. The validity of the modified patient generated index: a quantitative and qualitative approach. Qual Life Res 2000;9:509-20.
- Macduff C, Russell E. The problem of measuring change in individual health-related quality of life by postal questionnaire: use of the patient-generated index in a disabled population. Qual Life Res 1998;7:761-9.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal n.d. www.nice.org.uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides (accessed 15 March 2008).
- Nygaard IE, Bryant CM, Dowell C, Wilson D. Lifestyle interventions for the treatment of urinary incontinence in adults [protocol]. Cochrane Database Syst Rev 2002. 10.1002/14651858.CD003505.
- Miller JM, Ashton-Miller JA, DeLancey JOL. A pelvic muscle precontraction can reduce cough-related urine loss in selected women with mild stress urinary incontinence. J Am Geriatr Soc 1998;46:870-4.
- Lose G, Fantl JA, Victor A, Walter S, Wells TL, Wyman J, et al. Outcome measures for research in adult women with symptoms of lower urinary tract dysfunction. Neurourol Urodyn 1998;17:255-62.
- Grant AM, Cody DJ, Glazener CMA, Hay-Smith J, Herbison P, Lapitan MC, et al. About the Cochrane Collaboration (Cochrane Review Groups (CRGs). The Cochrane Library. Chichester: Wiley-Blackwell; 2007.
- InterTASC Information Specialists’ Sub-Group . Search Filter Resource n.d. www.york.ac.uk/inst/crd/intertasc/ (accessed 1 September 2007).
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
- StataCorp . Stata Version 10.1 [computer Program] 2008. www.stata.com/ (accessed 11 September 2008).
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS: a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Bø K, Talseth T, Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ 1999;318:487-93.
- Konstantinidou E, Apostolidis A, Kondelidis N, Tsimtsiou Z, Hatzichristou D, Ioannides E. Short-term efficacy of group pelvic floor training under intensive supervision versus unsupervised home training for female stress urinary incontinence: a randomized pilot study. Neurourol Urodyn 2007;26:486-91.
- Van Kerrebroeck P, Abrams P, Lange R, Slack M, Wyndaele JJ, Yalcin I, et al. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG 2004;111:249-57.
- Kim H, Suzuki T, Yoshida Y, Yoshida H. Effectiveness of multidimensional exercises for the treatment of stress urinary incontinence in elderly community-dwelling Japanese women: a randomized, controlled, crossover trial. J Am Geriatr Soc 2007;55:1932-9.
- Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol 2005;174:187-9.
- Aksac B, Aki S, Karan A, Yalcin O, Isikoglu M, Eskiyurt N. Biofeedback and pelvic floor exercises for the rehabilitation of urinary stress incontinence. Gynecol Obstet Invest 2003;56:23-7.
- Bidmead J, Mantle J, Cardozo L, Hextall A, Boos K. Home electrical stimulation in addition to conventional pelvic floor exercises: a useful adjunct or expensive distraction? [abstract]. Neurourol Urodyn 2002;21:372-3.
- Burns PA, Pranikoff K, Nochajski TH, Hadley EC, Levy KJ, Ory MG. A comparison of effectiveness of biofeedback and pelvic muscle exercise treatment of stress incontinence in older community-dwelling women. J Gerontol 1993;48:M167-74.
- Goode PS, Burgio KL, Locher JL, Roth DL, Umlauf MG, Richter HE, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA 2003;290:345-52.
- Henalla SM, Hutchins CJ, Robinson P, MacVicar J. Non-operative methods in the treatment of female genuine stress incontinence of urine. J Obstet Gynaecol 1989;9:222-5.
- Henalla SM, Millar DR, Wallace KJ. Surgical versus conservative management for post-menopausal genuine stress incontinence of urine [abstract]. Neurourol Urodyn 1990;9:436-7.
- Hofbauer J, Preisinger F, Nurnberger N. The value of physical therapy in genuine female stress incontinence. Z Urol Nephrol 1990;83:249-54.
- Lagro-Janssen TL, Debruyne FM, Smits AJ, van Weel C. Controlled trial of pelvic floor exercises in the treatment of urinary stress incontinence in general practice. Br J Gen Pract 1991;41:445-9.
- Ramsay IN, Thou M. A randomised, double blind, placebo controlled trial of pelvic floor exercises in the treatment of genuine stress incontinence [abstract]. Neurourol Urodyn 1990;9:398-9.
- Williams KS, Assassa RP, Gillies CL, Abrams KR, Turner DA, Shaw C, et al. A randomized controlled trial of the effectiveness of pelvic floor therapies for urodynamic stress and mixed incontinence. BJU Int 2006;98:1043-50.
- Brubaker L, Benson JT, Bent A, Clark A, Shott S. Transvaginal electrical stimulation for female urinary incontinence. Am J Obstet Gynecol 1997;177:536-40.
- Jeyaseelan SM, Haslam EJ, Winstanley J, Roe BH, Oldham JA. An evaluation of a new pattern of electrical stimulation as a treatment for urinary stress incontinence: a randomized, double-blind, controlled trial. Clin Rehabil 2000;14:631-40.
- Laycock J, Jerwood D. Does pre-modulated interferential therapy cure genuine stress incontinence?. Physiotherapy 1993;79:553-60.
- Luber KM, Wolde-Tsadik G. Efficacy of functional electrical stimulation in treating genuine stress incontinence: a randomized clinical trial. Neurourol Urodyn 1997;16:543-51.
- Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor electrical stimulation in the treatment of genuine stress incontinence: a multicenter, placebo-controlled trial. Am J Obstet Gynecol 1995;173:72-9.
- Fantl JA, Wyman JF, McClish DK, Harkins SW, Elswick RK, Taylor JR, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991;265:609-13.
- Bump R, Benson JT, Brubaker L, Brostrom S, Hampel C, Jannelli M, et al. Biomechanical and Electrophysiological Effects of Duloxetine in Women With Stress Urinary Incontinence [abstract Number 269] n.d.
- Cardozo L, Drutz HP, Baygani SK, Bump RC. Pharmacological treatment of women awaiting surgery for stress urinary incontinence. Obstet Gynecol 2004;104:511-19.
- Castro-Diaz D, Palma PC, Bouchard C, Haab F, Hampel C, Carone R, et al. Effect of dose escalation on the tolerability and efficacy of duloxetine in the treatment of women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:919-29.
- Dmochowski RR, Miklos JR, Norton PA, Zinner NR, Yalcin I, Bump RC, et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol 2003;170:1259-63.
- Kinchen KS, Obenchain R, Swindle R. Impact of duloxetine on quality of life for women with symptoms of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2005;16:337-44.
- Mah SY, Lee KS, Choo MS, Seo JT, Lee JZ, Park WH, et al. Duloxetine versus placebo for the treatment of Korean women with stress predominant urinary incontinence. Korean J Urol 2006;47:527-35.
- Manning M, Lange R, Jonas F, Meisel J, Kohoutek U, Willgerodt J, et al. Duloxetine Versus Placebo for the Treatment of German Women With Stress Urinary Incontinence (SUI) [abstract Number 211] n.d.
- Millard RJ, Moore K, Rencken R, Yalcin I, Bump RC. Duloxetine Urinary Incontinence Study Group . Duloxetine vs placebo in the treatment of stress urinary incontinence: a four-continent randomized clinical trial. BJU Int 2004;93:311-18.
- Norton PA, Zinner NR, Yalcin I, Bump RC. Duloxetine Urinary Incontinence Study Group . Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol 2002;187:40-8.
- Zinner N, Sarshik S, Yalcin I, Faries D, Riedl P, Thor KB. Efficacy and Safety of Duloxetine in Stress Urinary Incontinent Patients: Double-Blind, Placebo-Controlled Multiple Dose Study [abstract] n.d.:173-4.
- Aukee P, Immonen P, Penttinen J, Laippala P, Airaksinen O. Increase in pelvic floor muscle activity after 12 weeks’ training: a randomized prospective pilot study. Urology 2002;60:1020-3.
- Berghmans LC, Frederiks CM, de Bie RA, Weil EH, Smeets LW, van Waalwijk van Doorn ES, et al. Efficacy of biofeedback, when included with pelvic floor muscle exercise treatment, for genuine stress incontinence. Neurourol Urodyn 1996;15:37-52.
- Castleden CM, Duffin HM, Mitchell EP. The effect of physiotherapy on stress incontinence. Age Ageing 1984;13:235-7.
- Ferguson KL, McKey PL, Bishop KR, Kloen P, Verheul JB, Dougherty MC. Stress urinary incontinence: effect of pelvic muscle exercise. Obstet Gynecol 1990;75:671-5.
- Glavind K, Nohr SB, Walter S. Biofeedback and physiotherapy versus physiotherapy alone in the treatment of genuine stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1996;7:339-43.
- Klingler HC, Madersbacher S, Uher EM, Schmidbauer CP. Pelvic Floor Exercise and Endotrainer for Treatment of Female Stress Urinary Incontinence [abstract No. 122] n.d.:56-7.
- Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. Pelvic floor reeducation for stress incontinence: comparing three methods. Br J Community Nurs 2001;6:230-44.
- Morkved S, Bø K, Fjortoft T. Effect of adding biofeedback to pelvic floor muscle training to treat urodynamic stress incontinence. Obstet Gynecol 2002;100:730-9.
- Pages IH, Jahr S, Schaufele MK, Conradi E. Comparative analysis of biofeedback and physical therapy for treatment of urinary stress incontinence in women. Am J Phys Med Rehabil 2001;80:494-502.
- Shepherd A, Montgomery E, Anderson RS. A pilot study of a pelvic exerciser in women with stress urinary incontinence. J Obstet Gynaecol 1983;3:201-2.
- Taylor K, Henderson J. Effects of biofeedback and urinary stress incontinence in older women. J Gerontol Nurs 1986;12:25-30.
- Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown AD. An objective assessment of physiotherapy for female genuine stress incontinence. Br J Obstet Gynaecol 1987;94:575-82.
- Wong KS, Fung BKY, Fung ESM, Fung LCW, Tang LCH. Randomized Prospective Study of the Effectiveness of Pelvic Floor Training Using Biofeedback in the Treatment of Genuine Stress Urinary Incontinence in Chinese Population [abstract] n.d.:57-8.
- Bø K, Hagen RH, Kvarstein B, Jorgensen J, Larsen S. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: III. Effects of two different degrees of pelvic floor muscle exercises. Neurourol Urodyn 1990;9:489-502.
- Wong KS, Fung BKY, Fung LCW, Ma S. Pelvic Floor Exercises in the Treatment of Stress Urinary Incontinence in Hong Kong Chinese Women [abstract] n.d.:62-3.
- Zanetti MR, Castro RA, Rotta AL, Santos PD, Sartori M, Girao MJ. Impact of supervised physiotherapeutic pelvic floor exercises for treating female stress urinary incontinence. São Paulo Med J 2007;125:265-9.
- Gallo ML, Staskin DR. Cues to action: pelvic floor muscle exercise compliance in women with stress urinary incontinence. Neurourol Urodyn 1997;16:167-77.
- Nygaard IE, Kreder KJ, Lepic MM, Fountain KA, Rhomberg AT. Efficacy of pelvic floor muscle exercises in women with stress, urge, and mixed urinary incontinence. Am J Obstet Gynecol 1996;174:120-5.
- Hay-Smith EJC. Pelvic Floor Muscle Training for Female Stress Urinary Incontinence 2003.
- Borello-France DF, Zyczynski HM, Downey PA, Rause CR, Wister JA. Effect of pelvic-floor muscle exercise position on continence and quality-of-life outcomes in women with stress urinary incontinence. Phys Ther 2006;86:974-86.
- Savage AM. Is lumbopelvic stability training (using the pilates model) an effective treatment strategy for women with stress urinary incontinence? A review of the literature and report of a pilot study. J Assoc Chartered Physiother Womens Health 2005;97:33-48.
- Johnson VY. Effects of a submaximal exercise protocol to recondition the pelvic floor musculature. Nurs Res 2001;50:33-41.
- Mayne CJ, Hilton P. A comparison of urethral electrical conductance and perineometry during a course of pelvic floor exercises for genuine stress incontinence [abstract number 71]. Neurourol Urodyn 1988;7:264-5.
- Wong KS, Fung KY, Fung SM, Fung CW, Tang CH. Biofeedback of pelvic floor muscles in the management of genuine stress incontinence in Chinese women. Physiotherapy 2001;87:644-8.
- Edwards GJ, Wines H, Barrington JW. A comparison between pelvic floor exercises and pelvic floor exercises and electrical therapy with respect to urethral pressure profiles [abstract number IDP50]. Int Urogynecol J Pelvic Floor Dysfunct 2000;11.
- Pohl K, Jundt K, Greulich T, Drinovac V, Peschers U. Biofeedback Versus Electrostimulation in Treatment of Female Stress Urinary Incontinence [abstract Number 564] n.d.
- Knight S. Evaluation of neuromuscular electrical stimulation in the treatment of genuine stress incontinence. Physiotherapy 1998;84:61-7.
- Burton G. n.d.
- Bernardes NO, Peres FR, Souza ELBL, Souza OL. [Methods of treatment of genuine stress incontinence: a comparative study between a pelvic floor exercise program and a pelvic floor electrical stimulation]. Revista Brasileira De Gynecologia E Obstetricia 2000;22:49-54.
- Hahn I, Sommar S, Fall M. A comparative study of pelvic floor training and electrical stimulation for the treatment of genuine female stress urinary incontinence. Neurourol Urodyn 1991;10:545-54.
- Laycock J. Interferential therapy in the treatment of genuine stress incontinence [abstract]. Neurourol Urodyn 1988;7:268-9.
- Smith JJ. Intravaginal stimulation randomized trial. J Urol 1996;155:127-30.
- Arvonen T, Fianu-Jonasson A, Tyni-Lenne R. Effectiveness of two conservative modes of physical therapy in women with urinary stress incontinence. Neurourol Urodyn 2001;20:591-9.
- Haken J, Benness C, Cardozo L, Cutner A. A randomised trial of vaginal cones and pelvic floor exercises in the management of genuine stress incontinence [abstract]. Neurourol Urodyn 1991;10:393-4.
- Peattie AB, Plevnik S. Cones versus physiotherapy as conservative management of genuine stress incontinence [abstract no. 72]. Neurourol Urodyn 1988;7:265-6.
- Cammu H, van Nylen M. Pelvic floor exercises versus vaginal weight cones in genuine stress incontinence. Eur J Obstet Gynecol Reprod Biol 1998;77:89-93.
- Sherburn M, Galea M, Bø K, Bird M, Carey M. Pelvic floor muscle training or bladder training to treat stress urinary incontinence in elderly women: a single blind randomised controlled trial [abstract no. 49]. Neurourol Urodyn 2007;26:665-6.
- Wyman JF. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. Am J Obstet Gynecol 1998;179:999-1007.
- Klarskov P, Belving D, Bischoff N, Dorph S, Gerstenberg T, Okholm B, et al. Pelvic floor exercise versus surgery for female urinary stress incontinence. Urol Int 1986;41:129-32.
- Tapp AJS, Hills B, Cardozo L. Randomised study comparing pelvic floor physiotherapy with the Burch colposuspension [abstract]. Neurourol Urodyn 1989;8:356-7.
- Delneri C, Di Benedetto P. Pelvic floor rehabilitation. A comparison of two methods of treatment: vaginal cones versus functional electrical stimulation. Eura Medicophys 2000;36:45-8.
- Oláh KS, Bridges N, Denning J, Farrar DJ. The conservative management of patients with symptoms of stress incontinence: a randomized, prospective study comparing weighted vaginal cones and interferential therapy. Am J Obstet Gynecol 1990;162:87-92.
- Wise BG, Haken J, Cardozo LD, Plevnik S. A comparative study of vaginal cone therapy, cones + Kegel exercises, and maximal electrical stimulation in the treatment of female genuine stress incontinence [abstract no. 76]. Neurourol Urodyn 1993;12:436-7.
- Blowman C, Pickles C, Emery S, Creates V, Towell L, Blackburn N, et al. Prospective double blind controlled trial of intensive physiotherapy with and without stimulation of the pelvic floor in treatment of genuine stress incontinence. Physiotherapy 1991;77:661-4.
- Haig L, Mantle J, Versi E. Does Interferential Therapy (IFT) Confer Added Benefit over a Pelvic Floor Muscle Exercise Programme (PFMEP) for Genuine Stress Incontinence (GSI)? [abstract No. 111] n.d.:36-7.
- Tapp AJS, Williams S, Hills B, Cardozo LD. The role of physiotherapy in the treatment of genuine stress incontinence [abstract] n.d.:204-5.
- Pieber D, Zivkovic F, Tamussino K, Ralph G, Lippitt G, Fauland B. Pelvic floor exercise alone or with vaginal cones for the treatment of mild to moderate stress urinary incontinence in premenopausal women. Int Urogynecol J Pelvic Floor Dysfunct 1995;6:14-7.
- Terry PB, Whyte SM. n.d.:248-9.
- Karagkounis SC, Pantelis A, Parashou GC, Paplomata E, Madenis N, Chrisanthopoulos C, et al. Stress urinary incontinence: TVT OB system versus duloxetine-HCl. And the winner is? [abstract no. 5]. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:S3-4.
- Seo JT, Yoon H, Kim YH. A randomized prospective study comparing new vaginal cone and FES-Biofeedback. Yonsei Med J 2004;45:879-84.
- Bourcier A, Juras J. Randomised Study Comparing Physiotherapy and Pelvic Floor Rehabilitation [abstract] n.d.
- Wilson PD, Herbison GP. A randomized controlled trial of pelvic floor muscle exercises to treat postnatal urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1998;9:257-64.
- Woldringh C, van den WM, Albers-Heitner P, Nijeholt AA, Lagro-Janssen T. Pelvic floor muscle training is not effective in women with UI in pregnancy: a randomised controlled trial. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:383-90.
- Dumoulin C, Lemieux MC, Bourbonnais D, Gravel D, Bravo G, Morin M. Physiotherapy for persistent postnatal stress urinary incontinence: a randomized controlled trial. Obstet Gynecol 2004;104:504-10.
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8.
- Berghmans B, Bø K, Hendriks E, de Bie R, Van Kampen M. Electrical stimulation with non-implanted electrodes for urinary incontinence in adults [protocol]. Cochrane Database Syst Rev 2004. 10.1002/14651858.CD001202.pub2.
- Multi-Parameter Evidence Synthesis (MPES) Programme . Mixed Treatment Comparisons [computer Program] 2007. www.bris.ac.uk/cobm/research/mpes/mixed–treatment–comparisons.html (accessed 23 July 2008).
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24.
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006;101:447-59.
- Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1-19.
- O’Hagan A, Luce BR. A primer on bayesian statistics in health economics and outcomes research. Bethesda, MD: MEDTAP International Inc; 2003.
- Bø K, Talseth T. Long-term effect of pelvic floor muscle exercise 5 years after cessation of organized training. Obstet Gynecol 1996;87:261-5.
- Bø K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstet Gynecol 2005;105:999-1005.
- Glazener CM, Herbison GP, MacArthur C, Grant A, Wilson PD. Randomised controlled trial of conservative management of postnatal urinary and faecal incontinence: six year follow up. BMJ 2005;330:337-40.
- Lagro-Janssen T, van Weel C. Long-term effect of treatment of female incontinence in general practice. Br J Gen Pract 1998;48:1735-8.
- Eli Lilly and Company . The Safety and Effectiveness of Duloxetine Compared With Placebo and Its Long-Term Safety and Efficacy in the Treatment of Predominant Stress Urinary Incontinence n.d. www.clinicalstudyresults.org/drugdetails/?unique_id=8049%26sort=c.company_name%26page=1%26drug_id=3154 (accessed 5 June 2008).
- Vella M, Duckett J, Basu M. Duloxetine 1 year on: the long-term outcome of a cohort of women prescribed duloxetine. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:961-4.
- Ward KL, Hilton P. UK and Ireland TVT Trial Group . Tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence: 5-year follow up. BJOG 2008;115:226-33.
- Hilton P. Long-term follow-up studies in pelvic floor dysfunction: the Holy Grail or a realistic aim?. BJOG 2008;115:135-43.
- Ward K, Hilton P. behalf of the UK and Ireland TVT Trial Group . Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ 2002;325:67-73.
- Ward KL, Hilton P. UK and Ireland TVT Trial Group . A prospective multicenter randomized trial of tension-free vaginal tape and colposuspension for primary urodynamic stress incontinence: two-year follow-up. Am J Obstet Gynecol 2004;190:324-31.
- Office for National Statistics (ONS) . Interim Life Tables 2004–06 2008. www.gad.gov.uk/Demography%5FData/Life_Tables/Interim_life_tables.asp (accessed September 2008).
- The Center for the Evaluation of Value and Risk in Health, Tufts Medical Center . Cost-Effectiveness Analysis (CEA) Registry n.d. https://research.tufts–nemc.org/cear/default.aspx (accessed 11 March 2009).
- Haywood KL, Garratt AM, Lall R, Smith JF, Lamb SE. EuroQol EQ–5D and condition-specific measures of health outcome in women with urinary incontinence: reliability, validity and responsiveness. Qual Life Res 2008;17:475-83.
- Manca A, Sculpher MJ, Ward K, Hilton P. A cost–utility analysis of tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence. BJOG 2003;110:255-62.
- Monz B, Pons ME, Hampel C, Hunskaar S, Quail D, Samsioe G, et al. Patient-reported impact of urinary incontinence: results from treatment seeking women in 14 European countries. Maturitas 2005;52:S24-34.
- Monz B, Chartier-Kastler E, Hampel C, Samsioe G, Hunskaar S, Espuna-Pons M, et al. Patient characteristics associated with quality of life in European women seeking treatment for urinary incontinence: results from PURE. Eur Urol 2007;51:1073-81.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ–5D. Discussion Paper 1999. www.york.ac.uk/inst/che/publications/publicationsbyyear.htm (accessed 9 March 2009).
- Hay-Smith EJ, Bø K, Berghmans LC, Hendriks HJ, de Bie RA, van Waalwijk van Doorn ES. Pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2001. 10.1002/14651858.CD001407.
- Niel-Weise B, van den Broek P. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev 2005. 10.1002/14651858.CD004203.pub2.
- Khazali S, Jackson S, Balmforth J. Electromagnetic treatment for urinary incontinence in adults [protocol]. Cochrane Database Syst Rev 2007. 10.1002/14651858.CD006711.
- Moore K, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev 2007. 10.1002/14651858.CD006008.pub2.
- Hay-Smith J, Morkved S, Fairbrother KA, Herbison GP. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev 2008. 10.1002/14651858.CD007471.
- Macleod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al. Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews. Health Technol Assess 1998;2.
- Sandercock P, Algra A, Anderson C, Bereczki D, Berge E, Bowen A, et al. About the Cochrane Collaboration [Cochrane Review Groups (CRGs)]. The Cochrane Library. Chichester: Wiley-Blackwell; 2008.
- Royle P, Waugh N. Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system. Health Technol Assess 2003;7:1-51.
- Aksac B, Aki S, Karan A, Yalcin O, Isikoglu M, Eskiyurt N. Biofeedback and Pelvic Floor Muscle Exercises for the Rehabilitation of Stress Urinary Incontinence [abstract] n.d.
- Aukee P, Immonen P, Pettinen J, Airaksinen O. A Prospective Randomised Study Comparing FemiScan Home Trainer and Pelvic Floor Muscle Training Alone [abstract No. 205] n.d.
- Aukee P, Immonen P, Laaksonen DE, Laippala P, Penttinen J, Airaksinen O. The effect of home biofeedback training on stress incontinence. Acta Obstet Gynecol Scand 2004;83:973-7.
- Berghmans LCM, Weil EHJ, Frederiks CMA, de Bie RA, Smeets LWH, van Waalwijk van Doorn ESC, et al. Efficacy of Biofeedback for Genuine Stress Incontinence [abstract No. 115] n.d.:44-5.
- Parsons M, Mantle J, Cardozo L, Hextall A, Boos K, Bidmead J. A Single Blind, Randomised, Controlled Trial of Pelvic Floor Muscle Training With Home Electrical Stimulation in the Treatment of Urodynamic Stress Incontinence [abstract No. 296] n.d.
- Bø K. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence. Methodological studies and clinical results. Acta Obstet Gynecol Scand 1991;70:637-9.
- Bø K. Adherence to pelvic floor muscle exercise and long-term effect on stress urinary incontinence. A five-year follow-up study. Scand J Med Sci Sports 1995;5:36-9.
- Bø K, Hagen R, Jorgensen J, Kvarstein B, Larsen S. The effect of two different pelvic floor muscle exercise programs in treatment of urinary stress incontinence in women [abstract]. Neurourol Urodyn 1989;8:355-6.
- Bø K, Larsen S, Kvarstein B, Hagen RH. Classification and characterization of responders to pelvic floor muscle exercise for female urinary incontinence [abstract]. Neurourol Urodyn 1990;9:395-7.
- Bø K, Larsen S. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: classification and characterization of responders. Neurourol Urodyn 1992;11:497-50.
- Bø K, Hagen R, Kvarstein B, Larsen S. Female stress urinary incontinence and participation in different sports and social activities. Scand J Med Sci Sports 1989;11:117-21.
- Bø K, Kvarstein B, Hagen RH. The Effect of Two Different Pelvic Floor Muscle Exercise Regimens in Treatment of Female Stress Urinary Incontinence [abstract] 1991.
- Bø K. Pelvic floor muscle strength and response to pelvic floor muscle training for stress urinary incontinence. Neurourol Urodyn 2003;22:654-8.
- Bø K, Kvarstein B. 15 Year Follow-up of a Randomized Controlled Trial of Pelvic Floor Muscle Training to Treat Female Urodynamic Stress Incontinence [abstract No. 658] n.d.
- Bø K, Kvarstein B, Nygaard I. Lower urinary tract symptoms 15 years after ending a randomised controlled trial of pelvic floor muscle training for urodynamic stress incontinence [abstract no. 355]. Eur Urol Suppl 2005;4.
- Bø K, Talseth T. Single blinded randomized controlled trial on the effect of pelvic floor muscle strength training, electrical stimulation, cones or control on severe genuine stress incontinence [abstract]. Neurourol Urodyn 1998;17:421-2.
- Bø K, Talseth T. Randomized Controlled Trial on the Effect of Pelvic Floor Muscle Training on Quality of Life and Sex-Life in Genuine Stress Incontinent Women [abstract No. 175] n.d.:59-60.
- Bø K, Talseth T. Randomized controlled trial on the effect of pelvic floor muscle training on quality of life and sex-life in genuine stress incontinent women [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 1999;10.
- Bø K, Talseth T, Vinsnes A. Randomized controlled trial on the effect of pelvic floor muscle training on quality of life and sexual problems in genuine stress incontinent women. Acta Obstet Gynecol Scand 2000;79:598-603.
- Brubaker L, Benson JT, Bent A, Clark A. Transvaginal electrical stimulation is effective for treatment of detrusor overactivity [abstract]. Neurourol Urodyn 1996;15:282-3.
- Burns P, Pranikoff K, Nochajski TJ, Levy KJ. Effectiveness of biofeedback therapy for stress incontinence [abstract no. 742]. J Urol 1989;141.
- Burns PA, Pranikoff K, Reis JS, Levy KJ. Effectiveness of biofeedback therapy for stress incontinent females [abstract]. Neurourol Urodyn 1988;7:280-2.
- Burns PA, Pranikoff K, Nochajski T, Desotelle P, Harwood MK. Treatment of stress incontinence with pelvic floor exercises and biofeedback. J Am Geriatr Soc 1990;38:341-4.
- Burns PA, Nochajski TH, Pranikoff K. Factors discriminating between genuine stress and mixed incontinence. J Am Acad Nurse Pract 1992;4:15-21.
- Cammu H, van Nylen M. Pelvic Floor Exercises (PFE) Versus Vaginal Cones (VC) in the Treatment of Genuine Stress Incontinence [abstract No. 225] n.d.
- Drutz H, Cardozo L, Baygani S, Bump R. Duloxetine treatment of women with only urodynamic stress incontinence awaiting continence surgery [abstract]. Neurourol Urodyn 2003;22:523-4.
- Cardozo L, Drutz H, Baygani S, Bump R. Duloxetine response and onset of action in women with severe stress urinary incontinence (SUI) awaiting continence surgery [abstract no. 36]. Prog Urol 2004;14.
- Zinner N, Dmochowski R, Miklos J, Norton P, Yalcin I, Bump R. Duloxetine versus placebo in the treatment of stress urinary incontinence (SUI) [abstract]. Neurourol Urodyn 2002;21:383-4.
- Dumoulin C, Lemieux M, Bourbonnais D, Morin M. Conservative management of stress urinary incontinence: a single-blind, randomized controlled trial of pelvic floor rehabilitation with or without abdominal muscle rehabilitation compared to the absence of treatment [abstract]. Neurourol Urodyn 2003;22:543-4.
- Dumoulin C, Morin M, Bourbonnais D, Lemieux M, Gravel D. Effect of Adding Deep Abdominal Muscle Training to Pelvic Floor Muscle Training to Treat Stress Urinary Incontinence: A One-Year Follow up [abstract No. 662] n.d.
- Dumoulin C, Morin M, Lemieux MC, Bourbonnais D, Bravo G, Gravel D. Efficacy of deep abdominal training when combined with pelvic floor muscle training for stress urinary incontinence: a single-blind randomized controlled trial [abstract no. 44]. Prog Urol 2004;14.
- McClish DK, Fantl JA, Wyman JF, Pisani G, Bump RC. Bladder training in older women with urinary incontinence: relationship between outcome and changes in urodynamic observations. Obstet Gynecol 1991;77:281-6.
- Fantl JA, Wyman JF, Harkins SW, Taylor JR, . Bladder training in women with urinary incontinence [abstract]. Neurourol Urodyn 1988;7:276-7.
- Wyman JF, McClish DK, Ory MG, Fantl JA. Changes in quality of life following bladder training in older women with urinary incontinence [abstract]. Neurourol Urodyn 1992;11:426-7.
- Wyman JF, Fantl JA, McClish DK, Harkins SW, Uebersax JS, Ory MG. Quality of life following bladder training in older women with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1997;8:223-9.
- Schagen van Leeuwen JH, Elser D, Freeman R, Ghoniem G, Zhao Y, Yalcin I, et al. Controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment, and no treatment in women with stress urinary incontinence (SUI) [abstract]. Eur Urol Suppl 2004;3.
- Glavind K, Nohr S, Walter S. Randomized prospective trial on physiotherapy versus physiotherapy and biofeedback in treatment of genuine stress urinary incontinence [abstract]. Neurourol Urodyn 1995;14:457-9.
- Hahn I, Naucler J, Sommer S, Fall M. Urodynamic assessment of pelvic floor training. World J Urol 1991;9:162-6.
- Hay-Smith EJC, Herbison GP, Wilson PD. Pelvic floor muscle training for women with symptoms of stress urinary incontinence: A randomised trial comparing strengthening and motor relearning approaches [abstract]. Neurourol Urodyn 2002;21:371-2.
- Henalla SM, Hutchins CJ, Castleden CM. Conservative management of urethral sphincter incompetence [abstract]. Neurourol Urodyn 1987;6:191-2.
- Preisinger E, Hofbauer J, Nurnberger N, Sadil S, Schneider B. Possibilities of physiotherapy for urinary stress incontinence. Z Phys Med Balneol Med Klimatol 1990;19:75-9.
- Jeyaseelan SM, Haslam J, Roe B, Winstanley J, Oldham JA. The Evaluation of a New Pattern of Electrical Muscle Stimulation As a Treatment for Genuine Stress Incontinence: A Randomised, Double-Blind, Controlled Trial [abstract No. 522] n.d.
- Johnson VY. Effects of a Submaximal Exercise Protocol to Recondition the Circumvaginal Musculature in Women With Genuine Stress Urinary Incontinence 1997.
- Klarskov P, Vedel Jepsen P, Dorph S. Reliability of voiding colpo-cysto-urethrography in female urinary stress incontinence before and after treatment. Acta Radiol 1988;29:685-8.
- Klarskov P, Kroyer K, Kromann B, Maegaard E. Long term results of pelvic floor training and surgery for female genuine stress incontinence [abstract]. Neurourol Urodyn 1989;8:357-9.
- Klarskov P, Nielson KK, Kromann-Andersen B, Maegaard E. Long term results of pelvic floor training and surgery to female genuine stress incontinence. Int Urogynecol J 1991;2:132-5.
- Klarskov P, Belving D, Bischoff N, Dorph S, Gerstenberg T, Hald T, et al. Pelvic Floor Exercise Versus Surgery for Female Urinary Stress Incontinence: Preliminary Results [abstract] n.d.:159-61.
- Laycock J, Knight S, Naylor D. Prospective, randomised, controlled clinical trial to compare acute and chronic electrical stimulation in combination therapy for GSI [abstract]. Neurourol Urodyn 1995;14:425-6.
- Konstantinidou E, Apostolidis A, Kondelidis N, Tsimtsiou Z, Hatzichristou D, Ioannides E. Short-term efficacy of high-supervisory-intensity group pelvic floor training versus unsupervised, home training in female stress urinary incontinence: a randomised pilot study [abstract no. 678]. Eur Urol Suppl 2006;5.
- Lagro-Janssen ALM, Debruyne FMJ, Smits AJA, van Weel C. The effects of treatment of urinary incontinence in general practice. Fam Pract 1992;9:284-9.
- Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. A multi-centre, prospective, randomised, controlled, group comparative study of the efficacy of vaginal cones and PFX [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 1999;10.
- Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. A multi-centre, prospective, randomised, controlled, group comparative study of the efficacy of vaginal cones and PFX [abstract no. 47]. Neurourol Urodyn 1999;18:301-2.
- Millard R, Moore K, Yalcin I, Bump R. Duloxetine vs placebo in the treatment of stress urinary incontinence: a global phase 3 study [abstract]. Neurourol Urodyn 2003;22:482-3.
- Millard RJ, Moore K, Rencken R. Duloxetine vs placebo in the treatment of stress urinary incontinence: a global phase III study [abstract]. Aust N Z J Surg 2003;73.
- Morkved S, Fjortoft T, Bø K. Is there any effect of adding biofeedback to pelvic floor muscle training? A randomised controlled trial [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 2001;12.
- Morkved S, Bø K, Fjortoft T. Continence Status One Year After Cessation of Organised Pelvic Floor Muscle Training [abstract] n.d.:260-1.
- Morkved S, Fjortoft T, Lindland M, Bø K. Continence Status Five Years After Cessation of Organised Pelvic Floor Muscle Training [abstract No. 297] n.d.
- Bump RC, Yalcin I. for the Duloxetine UI Study Group . Pure and mixed stress urinary incontinence (UI) symptoms: comparing UI severity and treatment response [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 2001;12.
- Bump RC, Yalcin I. Mixed incontinence: duloxetine treatment response, urodynamic findings, and incontinence severity [abstract]. Obstet Gynecol 2002;99.
- Bump RC, Norton PA, Zinner NR, Yalcin I. Duloxetine Urinary Incontinence Study Group . Mixed urinary incontinence symptoms: urodynamic findings, incontinence severity, and treatment response. Obstet Gynecol 2003;102:76-83.
- Norton P, Zinner NR, Yalcin I, Bump RC. for the Duloxetine Urinary Incontinence Study Group . Duloxetine versus placebo in the treatment of stress urinary incontinence [abstract]. Neurourol Urodyn 2001;20:532-4.
- Yalcin I, Viktrup L. Comparison of physician and patient assessments of incontinence severity and improvement. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:1291-5.
- Bridges N, Denning J, Oláh KS, Farrar DJ. A prospective trial comparing interferential therapy and treatment using cones in patients with symptoms of stress incontinence [abstract]. Neurourol Urodyn 1988;7:267-8.
- Peattie AB, Taylor B, Plevnik S, Stanton SL. Cones Versus Physiotherapy for Conservative Treatment of Genuine Stress Incontinence [abstract No. 169] n.d.
- Pieber D, Zivkovic F, Tamussino K, Ralph G. Pelvic Floor Exercise Alone or With Vaginal Cones for the Treatment of Mild and Moderate Stress Incontinence in Premenopausal Women: A Randomised Trial [abstract] n.d.
- Pieber D, Zivkovic F, Tamussino K. Pelvic floor exercises without or with vaginal cones in premenopausal women with mild to moderate stress incontinence. Gynakologisch-Geburtshilfliche Rundschau 1994;34:32-3.
- Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic Floor Stimulation in the Treatment of Genuine Stress Incontinence: A Multicentrer Placebo Controlled Trial [abstract No. 27] n.d.
- Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor stimulation in the treatment of genuine stress incontinence: a multicentre placebo-controlled trial [abstract]. Neurourol Urodyn 1994;13:356-7.
- Whitmore KE, Staskin DR, Grigoriev VE, Appell RA, Sand PK, Ostergaard DR. Pelvic floor stimulation in the treatment of genuine stress incontinence: a multicenter placebo controlled trial [abstract no. 1050]. J Urol 1995;153.
- Shepherd AM, Montgomery E, Anderson RS. Treatment of genuine stress incontinence with a new perineometer: a series of graded exercises. Physiotherapy 1983;69.
- Smith JJ, Loehner D, Bingham W. Intravaginal Electrical Stimulation in the Treatment of GSUI and DI: A Controlled Study [abstract No. 25] n.d.
- Aaronson PS, Loehner D, Bingham W, Smith JJ. Intravaginal electrical stimulation in the treatment of genuine stress urinary incontinence and detrusor instability: a controlled study [abstract]. J Urol 1995;153.
- Swithinbank LV, Rogers CA, Yang Q, Shepherd AM, Abrams P. Does the amount and type of fluid intake effect urinary symptoms in women [abstract no. 104]?. Neurourol Urodyn 1999;18:371-2.
- Tapp AJS, Hills B, Cardozo L. Pelvic Floor Physiotherapy Compared With the Burch Colposuspension in the Treatment of Genuine Stress Incontinence [abstract] n.d.
- Van Kerrebroeck P, Abrams P, Lange R, Slack M, Wyndaele J, Yalcin I, et al. Duloxetine vs placebo in the treatment of stress urinary incontinence: phase 3 results from Europe and Canada [abstract]. Eur Urol Suppl 2003;2.
- Shaw C, Matthews RJ, Perry SI, Assassa RP, Williams K, McGrother C, et al. Validity and reliability of an interviewer-administered questionnaire to measure the severity of lower urinary tract symptoms of storage abnormality: the Leicester Urinary Symptom Questionnaire. BJU Int 2002;90:205-15.
- Williams KS, Assassa RP, Smith N, Rippin C, Shaw C, Mayne C, et al. Good practice in continence care: development of nurse-led service. Br J Nurs 2002;11:548-59.
- Williams K, Assassa RP, Cooper N, Turner D, Shaw C, Abrams K, et al. Randomised controlled trial of the clinical and cost effectiveness of existing continence services compared with a new nurse-led service [abstract]. Neurourol Urodyn 2003;22.
- Williams KS, Assassa RP, Cooper NJ, Turner DA, Shaw C, Abrams KR, et al. Clinical and cost-effectiveness of a new nurse-led continence service: a randomised controlled trial. Br J Gen Pract 2005;55:696-703.
- Williams K, Coleby D, Abrams K, Shaw C, Assassa P, McGrother C. Randomised controlled trial of the clinical effectiveness of services for urinary symptoms: six year follow-up [abstract no. 45]. Neurourol Urodyn 2007;26:660-1.
- Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown ADG. The Value of Physiotherapy in Female Genuine Stress Incontinence [abstract] n.d.:156-8.
- Wilson D, Herbison P, Borland M, Grant AM. A Randomised Controlled Trial of Physiotherapy Treatment of Postnatal Urinary Incontinence [abstract] n.d.
- Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC. Continence Program for Women Research Group . Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol 2002;99:281-9.
- Elser DM, Fantl JA, McClish DK. Comparison of “subjective” and “objective” measures of severity of urinary incontinence in women. Program for Women Research Group. Neurourol Urodyn 1995;14:311-16.
- Elser DM, Wyman JF, McClish DK, Robinson D, Fantl JA, Bump RC. The effect of bladder training, pelvic floor muscle training, or combination training on urodynamic parameters in women with urinary incontinence. Continence Program for Women Research Group. Neurourol Urodyn 1999;18:427-36.
- Theofrastous JP, Wyman JF, Bump RC, McClish DK, Elser DM, Bland DR, et al. Effects of pelvic floor muscle training on strength and predictors of response in the treatment of urinary incontinence. Neurourol Urodyn 2002;21:486-90.
- Wyman JF, McClish DK, Sale P, Earle B, Camp J. Long-term follow-up of behavioral interventions in incontinent women [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 1999;10.
- Yalcin I, DeBrota DJ, Thor KB. Incontinence Severity Index (ISI) in Measuring Efficacy of Duloxetine in Stress and Mixed Incontinent Patients [abstract] n.d.:171-2.
- Zinner N, Sarshik S, Yalcin I, Faries D, DeBrota D, Riedl P, et al. Evaluation of Various Efficacy Measures from 140 Stress and 146 Mixed Incontinence Patients Enrolled in a Double-Blind, Placebo-Controlled Trial of Duloxetine [abstract] n.d.:175-6.
- Alewijnse D, Mesters IEPE, Metsemakers JFM, van den Borne BHW. Program development for promoting adherence during and after exercise therapy for urinary incontinence. Patient Educ Couns 2002;48:147-60.
- Alewijnse D, Metsemakers JF, Mesters IE, Van Den BB. Effectiveness of pelvic floor muscle exercise therapy supplemented with a health education program to promote long-term adherence among women with urinary incontinence. Neurourol Urodyn 2003;22:284-95.
- Alewijnse D, Mesters I, Metsemakers J, van den Borne B. Predictors of long-term adherence to pelvic floor muscle exercise therapy among women with urinary incontinence. Health Educ Res 2003;18:511-24.
- Barroso JC, Ramos JG, Martins-Costa S, Sanches PR, Muller AF. Transvaginal electrical stimulation in the treatment of urinary incontinence. BJU Int 2004;93:319-23.
- Barroso JCV, Ramos JGL. Estimulacao eletrica transvaginal no tratamento da incontinencia urinaria. Revista Brasileira De Gynecologia E Obstetricia 2002;24.
- Bawden ME, Kartha AS, Borrie MJ, Kerr PS, Durko NA, Haslam IF, et al. Treating Women With Stress Incontinence in a Multidisciplinary Clinic: A Randomized Study [abstract No. 276] n.d.
- Borrie MJ, Bawden ME, Kartha AS, Kerr PS. A nurse/physician continence clinic triage approach for urinary incontinence: a 25 week randomized trial. Neurourol Urodyn 1992;11:364-5.
- Borrie MJ, Bawden ME, Speechley M. Continence clinic randomized controlled trial using a nurse/physician triage approach: two-year follow-up. Clin Invest Med 1995;18.
- Borrie MJ, Bawden M, Speechley M, Kloseck M. Interventions led by nurse continence advisers in the management of urinary incontinence: a randomized controlled trial. CMAJ 2002;166:1267-73.
- Kartha AS, Borrie MJ, Bawden ME, Kerr PS. The Impact of Treatment on Quality of Life in a Randomized Clinical Study of Incontinent Adults [abstract No. 287] n.d.
- Brown JS, Wing R, Barrett-Connor E, Nyberg LM, Kusek JW, Orchard TJ, et al. Lifestyle intervention is associated with lower prevalence of urinary incontinence: the Diabetes Prevention Program. Diabetes Care 2006;29:385-90.
- Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Dombrowski M, et al. Behavioral vs drug treatment for urge urinary incontinence in older women. JAMA 1998;280:1995-2000.
- Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc 2000;48:370-4.
- Burgio KL, Locher JL, Roth DL, Goode PS. Psychological improvements associated with behavioral and drug treatment of urge incontinence in older women. J Gerontol B Psychol Sci Soc Sci 2001;56:46-51.
- Goode PS, Burgio KL, Locher JL, Umlauf MG, Lloyd LK, Roth DL. Urodynamic changes associated with behavioral and drug treatment of urge incontinence in older women. J Am Geriatr Soc 2002;50:808-16.
- Burgio KL, Goode PS, Locher JL, Umlauf MG, Roth DL, Richter HE, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: a randomized controlled trial. JAMA 2002;288:2293-9.
- Burgio KL, Goode PS, Locher JL, Richter HE, Roth DL, Wright KC, et al. Predictors of outcome in the behavioral treatment of urinary incontinence in women. Obstet Gynecol 2003;102:940-7.
- De Gregorio G, Krahmann H, Bernhard A. The efficacy of pelvic floor reeducation. Randomized study. Arch Gynecol Obstet 1993;254:504-6.
- de Jong JH, Van Kampen M, Biemans B. The Effect of Whole Body Vibration Training on Women With Stress Urinary Incontinence [abstract No. 416] n.d.
- Demain S, Fereday Smith J, Hiller L, Dziedzic K. Comparison of group and individual physiotherapy for female urinary incontinence in primary care. Physiotherapy 2001;87:235-42.
- Dougherty MC, Dwyer JW, Pendergast JF, Tomlinson BU, Boyington AR, Vogel WB, et al. Community-based nursing: continence care for older rural women. Nurs Outlook 1998;46:233-44.
- Dougherty MC, Dwyer JW, Pendergast JF, Boyington AR, Tomlinson BU, Coward RT, et al. A randomized trial of behavioral management for continence with older rural women. Res Nurs Health 2002;25:3-13.
- Foote AJ, Moore KH. Qalys: an objective continence outcome measure to determine the cost effectiveness of conservative urogynaecological treatments [abstract no. 109]. Neurourol Urodyn 2000;19:518-19.
- Foote AJ, Moore KH. The cost of urogynaecological treatments: which are more cost-effective?. Aust N Z J Obstet Gynaecol 2007;47:240-6.
- Galea M, Tisseverasinghe S, Sherburn M, Phillips B. Motor Skill Training of the Pelvic Floor Muscles Using Visual Versus Tactile Feedback [abstract No. 459] n.d.
- Goode PS. Behavioral and drug therapy for urinary incontinence. Urology 2004;63:58-64.
- Gorman R. Expert system for management of urinary incontinence in women. Proc Annu Symp Comput Appl Med Care 1995:527-31.
- Hill LA, Fereday Smith J, Credgington C, Woodward AF, Knight JC, Williams AJ, et al. Bladders behaving badly: a randomized controlled trial of group versus individual interventions in the management of female urinary incontinence. J Assoc Chartered Physiother Womens Health 2007;101:30-6.
- Pepper J, Lamb SE, Fereday Smith J, Doughty G. Female Urinary Incontinence: Women’s Preferences for Group or Individual Treatment [abstract no.: Poster 31] n.d.
- Pepper J, Lamb SE, Doughty G, Fereday Smith J. Group treatment: an acceptable and effective method of physiotherapy for bladder problems?. J Assoc Chartered Physiother Womens Health 2003;93:15-8.
- Holtedahl K, Verelst M, Schiefloe A. A population based, randomized, controlled trial of conservative treatment for urinary incontinence in women. Acta Obstet Gynecol Scand 1998;77:671-7.
- Hui E, Lee PS, Woo J. Management of urinary incontinence in older women using videoconferencing versus conventional management: a randomized controlled trial. J Telemed Telecare 2006;12:343-7.
- Ishiko O, Hirai K, Sumi T, Tatsuta I, Ogita S. Hormone replacement therapy plus pelvic floor muscle exercise for postmenopausal stress incontinence. A randomized, controlled trial. J Reprod Med 2001;46:213-20.
- Janssen CCM, Lagro-Janssen ALM, Felling AJA. The effects of physiotherapy for female urinary incontinence: Individual compared with group treatment. BJU Int 2001;87:201-6.
- Jeyaseelan S, Haslam J, Oldham J. Can the Effects of Pelvic Floor Muscle Exercises Be Enhanced With a New Pattern of Electrical Stimulation in Women With Stress Incontinence? Pilot Data [abstract No. 135] n.d.:66-7.
- Johnson JL, King Baker T. Biofeedback versus verbal instruction for pelvic floor training in the treatment of urinary incontinence. J Womens Health Phys Ther 2000;24:7-13.
- Kim J. Continence efficacy intervention program for community residing women with stress urinary incontinence in Japan. Public Health Nurs 2001;18:64-72.
- Kincade JE, Dougherty MC, Carlson JR, Hunter GS, Busby-Whitehead J. Randomized clinical trial of efficacy of self-monitoring techniques to treat urinary incontinence in women. Neurourol Urodyn 2007;26:507-11.
- Kincade JE, Dougherty MC, Carlson JR, Wells EC, Hunter GS, Busby-Whitehead J. Factors related to urinary incontinence in community-dwelling women. Urol Nurs 2007;27:307-17.
- Kirschner-Hermanns R, Niehaus S, Schafer W, Jakse G. Pelvic Floor Re-Education in Female Stress-Incontinence I. and II. Follow-up Results (mean 43 Months) [abstract No. 216] n.d.:230-1.
- Lee C, Johnson C, Chiarelli P. Women’s waterworks: evaluating an early intervention for incontinence among adult women. Aust N Z Continence J 2005;11:11-6.
- Liebergall-Wischnitzer M, Hochner-Celnikier D, Lavy Y, Manor O, Arbel R, Paltiel O. Paula method of circular muscle exercises for urinary stress incontinence: a clinical trial. Int Urogynecol J Pelvic Floor Dysfunct 2005;16:345-51.
- Lin TL, Chen YC, Hu SW, Chen GD. Nursing Intervention to Enforce the Efficacy of Home Practice of Pelvic Floor Muscle Exercise in Mixed Incontinence [abstract No. 294] n.d.
- Lo SK, Naidu J, Cao Y. Additive effect of interferential therapy over pelvic poor exercise alone in the treatment of femele urinary stress and urge incontinence: a randomized controlled trial. Hong Kong Physiother J 2003;21:37-42.
- Lumley J, Small R, Brown S, Watson L, Gunn J, Mitchell C, et al. PRISM (Program of Resources, Information and Support for Mothers) Protocol for a community-randomised trial [ISRCTN03464021]. BMC Public Health 2003;3.
- Lumley J, Watson L, Small R, Brown S, Mitchell C, Gunn J. PRISM (Program of Resources, Information and Support for Mothers): a community-randomised trial to reduce depression and improve women’s physical health six months after birth [ISRCTN03464021]. BMC Public Health 2006;6.
- McFall SL, Yerkes AM, Belzer JA, Cowan LD. Urinary incontinence and quality of life in older women: a community demonstration in Oklahoma. Fam Community Health 1994;17:64-75.
- McFall SL, Yerkes AM, Cowan LD. Outcomes of a small group educational intervention for urinary incontinence: episodes of incontinence and other urinary symptoms. J Aging Health 2000;12:250-67.
- McFall SL, Yerkes AM, Cowan LD. Outcomes of a small group educational intervention for urinary incontinence: health-related quality of life. J Aging Health 2000;12:301-17.
- Moore KH, O’Sullivan RJ, Simons A, Prashar S, Anderson P, Louey M. Randomised controlled trial of nurse continence advisor therapy compared with standard urogynaecology regimen for conservative incontinence treatment: efficacy, costs and two year follow up. BJOG 2003;110:649-57.
- O’Sullivan R, Simons A, Prashar S, Anderson P, Louey M, Moore KH. Is objective cure of mild undifferentiated incontinence more readily achieved than that of moderate incontinence? Costs and 2-year outcome. Int Urogynecol J Pelvic Floor Dysfunct 2003;14:193-8.
- Mulcahy JJ, Laddu AR, Faries DE, DeBrota DJ, Kirkemo AK, Rudy DC, et al. Efficacy and safety of duloxetine in stress incontinence patients [abstract]. Neurourol Urodyn 1996;15:395-6.
- Nieto Blanco E, Moriano Bejar P, Serrano Molina L, Davila Alvarez V, Perez Llorente M. Efficiency of a nursing clinical trial on the treatment of female urinary incontinence. Actas Urol Esp 2007;31:493-501.
- O’Brien J, Austin M, Sethi P, O’Boyle P. Urinary incontinence: prevalence, need for treatment, and effectiveness of intervention by nurse. BMJ 1991;303:1308-12.
- O’Brien J, Long H. Urinary incontinence: long term effectiveness of nursing intervention in primary care. BMJ 1995;311.
- O’Brien J. Evaluating primary care interventions for incontinence. Nurs Stand 1996;10:40-3.
- Ocampo MS, Diokno AC, Ibrahim IA, Karl CR, Lajiness MJ, Hall SA. Group session teaching of behavioral modification program (BMP) for urinary incontinence (UI): a randomized, controlled trial among incontinent women [abstract no. 1348]. J Urol 2007;177.
- Dowell CJ, Bryant CM, Moore KH, Prashar S. The Efficacy and User Friendliness of the Urethral Occlusive Device [abstract] n.d.:295-6.
- Moore KH, Simons A, Dowell C, Bryant C, Prashar S. Efficacy and user acceptability of the urethral occlusive device in women with urinary incontinence. J Urol 1999;162:464-8.
- Prashar S, Moore K, Bryant C, Dowell C. The urethral occlusive device for the treatment of urinary incontinence: changes in quality of life [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 1997;8.
- O’Sullivan R, Anderson P, Louey M, Prashar S, Simons A, Bower W, et al. Long Term Results of a Randomised Controlled Trial of the Nurse Continence Advisor Versus the Urogynaecologist in Conservative Therapy [abstract No. 212] n.d.
- Prashar S, Moore K, Anderson P, Louey M, Cragg S, Simons AM, et al. A randomized controlled trial of nurse continence advisor management versus urogynaecology management of conservative continence therapy: benefits and costs [abstract]. Neurourol Urodyn 1998;17:423-4.
- Sam C, Umek W, Uorfler D, Hanzal E. Outpatient Pelvic Floor Exercises Versus Home Biofeedback (PELVEXT) [abstract No. 557] n.d.
- Sherburn M, Tisseverasinghe S, Phillips B, Galea MP. Ultrasound Visual Feedback May Be As Effective As Digital Vaginal Palpation for Pelvic Floor Muscle Training [abstract No. 613] n.d.
- Sherman RA, Davis GD, Wong MF. Behavioral treatment of exercise-induced urinary incontinence among female soldiers. Mil Med 1997;162:690-4.
- Spruijt J, Vierhout M, Verstraeten R, Janssens J, Burger C. Vaginal electrical stimulation of the pelvic floor: a randomized feasibility study in urinary incontinent elderly women. Acta Obstet Gynecol Scand 2003;82:1043-8.
- Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstet Gynecol 2002;100:72-8.
- Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol 2005;174:190-5.
- Sugaya K, Owan T, Hatano T, Nishijima S, Miyazato M, Mukouyama H, et al. Device to promote pelvic floor muscle training for stress incontinence. Int J Urol 2003;10:416-22.
- Tsai C, Engberg S. Is Biofeedback-Assisted Pelvic Floor Muscle Training (PFMT) More Effective Than Verbal Instruction in Teaching Pelvic Floor Muscle Utilization and Continence Control? A Randomised Prospective Study [abstract No. 138] n.d.:68-70.
- von der Heide S, Emons G, Hilgers R, Viereck V. Effect on Muscles of Mechanical Vibrations Produced by the Galileo 2000 in Combination With Physical Therapy in Treating Female Stress Urinary Incontinence [abstract] n.d.:192-3.
- Wagg AR, Barron D, Kirby M, Stott D, Corlett K. A randomised partially controlled trial to assess the impact of self-help vs structured help from a continence nurse specialist in women with undiagnosed urinary problems in primary care. Int J Clin Pract 2007;61:1863-73.
- Wang AC, Liang CC. Bladder Sphincter Biofeedback As Treatment of Detrusor Instability in Women Who Failed to Respond to Oxybutynin Chloride: A Preliminary Results [abstract] n.d.:162-3.
- Wells TJ, Brink CA, Diokno AC, Wolfe R, Gillis GL. Pelvic muscle exercise for stress urinary incontinence in elderly women. J Am Geriatr Soc 1991;39:785-91.
- Glazener CM, Herbison GP, Wilson PD, MacArthur C, Lang GD, Gee H, et al. Conservative management of persistent postnatal urinary and faecal incontinence: randomised controlled trial. BMJ 2001;323:593-6.
- Glazener CM, Herbison GP, Wilson PD, MacArthur C, Lang GD, Gee H, et al. Conservative management of persistent postnatal urinary and faecal incontinence: randomised controlled trial [extended electronic version]. EBMJ 2001;323:1-5.
- Rennie AM, Wilson D, Glazener C, Gee H, Lang G, MacArthur C. A Multicentre Randomised Trial of Treatment of Postnatal Incontinence [abstract] n.d.
- Wilson PD, Herbison GP, Glazener CMA, Lang G, Gee H, MacArthur C. Postnatal incontinence: a multi centre, randomised controlled trial of conservative treatment [abstract]. Neurourol Urodyn 1997;16:349-50.
- Wilson PD, Glazener C, McGee M, Herbison P, MacArthur C, Grant A. Randomised controlled trial of conservative management of postnatal urinary and faecal incontinence: long term follow-up study [abstract]. Neurourol Urodyn 2002;21.
- Yoon HS, Song HH, Ro YJ. A comparison of effectiveness of bladder training and pelvic muscle exercise on female urinary incontinence. Int J Nurs Stud 2003;40:45-50.
- Abel I. Elektrostimulation Og Lokal Ostrogenterapi Til Behandling Af Urininkontinens Hos Postmenopausale Kvinder 1997.
- Kim JS, Yoon H, Chung WS, Shim BS. The long term effect of extracorporeal magnetic innervation therapy with pelvic floor muscle exercise for stress urinary incontinence. Korean J Urol 2006;47:1334-8.
- Smidt N, te Giffel MA, Gerards-Last TM, de Vet HC. Effectiveness of myofeedback for women with stress incontinence. Ned Tijdschr Fysioter 1997;107:121-7.
- Sung MS, Hong JY, Choi YH, Baik SH, Yoon H. FES-biofeedback versus intensive pelvic floor muscle exercise for the prevention and treatment of genuine stress incontinence. J Korean Med Sci 2000;15:303-8.
- Sung MS, Choi YH, Back SH, Hong JY, Yoon H. The effect of pelvic floor muscle exercises on genuine stress incontinence among Korean women: focusing on its effects on the quality of life. Yonsei Med J 2000;41:237-51.
- Klarskov P, Hald T. Reproducibility and reliability of urinary incontinence assessment with a 60 min test. Scand J Urol Nephrol 1984;18:293-8.
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med 2005;24:2401-28.
Appendix 1 Example of the Patient Generated Index
Appendix 2 Search strategies
Search strategies for the systematic reviews of effectiveness
Cochrane Incontinence Group Specialised Register of trials
On the date that the Cochrane Incontinence Group Specialised Register was last searched for this review (20 March 2008), the Register of trials contained trials identified from:
-
MEDLINE (covering January 1966 to week 4 January 2008), searched on 31 January 2008
-
MEDLINE Extra for 30 January 2008, searched on 31 January 2008
-
the Cochrane Central Register of Controlled Trials (CENTRAL) Issue 1, 2008 (searched on 13 March 2008)
-
CINAHL (covering January 1982 to December 2000)
-
hand searching relevant journals and conference proceedings.
For more details of how the Cochrane Incontinence Group Specialised Register of trials is produced, please see the Cochrane Incontinence Group Module. 109
Search terms used (all searches were of the keyword field in reference manager version 10)
PFMT/biofeedback
{topic.urine.incon*} AND ({design.rct*} OR {design.cct*}) AND ({intvent.phys.pfe*} OR {intvent.phys.biofeed*} OR {intvent.phys.physicaltraining.} OR {intvent.phys.physiotherapy.*})
Electrical stimulation
{topic.urine.incon*} AND ({design.rct*} OR {design.cct*}) AND ({intvent.phys.electstim*})
Vaginal cones
{topic.urine.incon*} AND ({design.rct*} OR {design.cct*}) AND (intvent.phys.cones*)
SRNI
{topic.urine.incon*} AND ({design.rct*} OR {design.cct*}) AND ({intvent.chem.SNRI.snri.} OR {intvent.chem.SNRI.duloxetine*} OR {relevant.review.sri.})
Lifestyles
{topic.urine.incon*} AND ({design.rct*} OR {design.cct*}) AND ({intvent.lifestyle*} OR {intvent.chem.diet*})
Behavioural (including bladder training)
{topic.urine.incon*} AND ({design.rct*} OR {design.cct*}) AND ({intvent.psych.behavrtrain*} OR {intvent.psych.behaviouralinterventions*} OR {intvent.psych.bladdrill*} OR {intvent.psych.behaviouralinterventions*} OR {intvent.psych.behaviouraltherapy*} OR {intvent.psych.behavrtrain*} OR {intvent.psych.enhancedtoilettraining.} OR {intvent.psych.motivation*} OR {intvent.psych.psychotherapy.})
Delivery of care
{topic.urine.incon*} AND ({design.rct*} OR {design.cct*}) AND {intvent.DeliveryOfCare*} OR {intvent.nurse*} OR {intvent.ed*})
CINAHL
CINAHL on OVID (years searched: January 1982 to Week 1 December 2007). Date of last search: 5 February 2008.
-
randomized controlled trials/
-
clinical trial.pt.
-
exp clinical trials/
-
placebos/
-
placebo$.tw.
-
random$.tw.
-
research design/
-
volunteer$.tw.
-
(clin$ adj25 trial$).tw.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
-
factorial.tw.
-
crossover.tw.
-
latin square.tw.
-
(balance$ adj2 block$).tw.
-
(animals not humans).sh.
-
random assignment/
-
exp clinical trials/
-
community trials/ or factorial design/ or solomon four-group design/ or quantitative studies/
-
crossover design/ or static group comparison/
-
exp clinical research/
-
or/1-14
-
or/16-20
-
21 or 22
-
23 not 15
-
toilet$.tw.
-
(incontinen$ or continen$).tw.
-
exp urinary incontinence/
-
incontinence pads/
-
urodynamics/
-
urinary sphincter, artificial/
-
urodynamic$.tw.
-
urinary catheterization/
-
exp bladder fistula/
-
toilet training/
-
cutaneous fistula/
-
vaginal fistula/
-
vesicovaginal fistula/
-
“pelvic floor”/
-
perineomet$.tw.
-
interferential.tw.
-
cystitis, interstitial/
-
nycturia.tw.
-
((vesic$ or bladder or vagina$) adj5 (support$ or prosthes$)).tw.
-
(bladder adj5 (train$ or retrain$)).tw.
-
mmk.tw.
-
marshall marchetti krantz.tw.
-
burch.tw.
-
((bladder or neck or vesic$) adj5 suspen$).tw.
-
colposuspension$.tw.
-
guittes.tw.
-
colporrhaph$.tw.
-
pereyra.tw.
-
urethrosuspension$.tw.
-
cystoplast$.tw.
-
urethropex$.tw.
-
lyodura$.tw.
-
colpoperineoplast$.tw.
-
urethrocervicopex$.tw.
-
stamey.tw.
-
interstitial cystitis.tw.
-
(fistula$ adj5 (bladder or vesic$ or bladder-vagina$ or urin$ or vagina$ or uretero-vagina$ or ureterovagina$ or urogenital or genitourin$)).tw.
-
raz.tw.
-
((urin$ or bladder) adj5 sphincter$).tw.
-
((bladder or detrusor or vesic$) adj5 (instability or stab$ or unstable or irritab$ or hyperreflexia or dys?ynerg$ or dyskinesi$ or irritat$)).tw.
-
(void$ adj5 (prompt$ or diar$)).tw.
-
urethral syndrome.tw.
-
(urethra$ adj2 sphincter$).tw.
-
(bladder adj2 neck).tw.
-
(urin$ adj2 leak$).tw.
-
urinary fistula/
-
dribbl$.tw.
-
diaper$.tw.
-
bladder, neurogenic/
-
(bladder adj1 ulcer$).tw.
-
(hunner adj1 ulcer$).tw.
-
(vesic$ adj1 (neck$ or cervi$)).tw.
-
cystostomy.tw.
-
cystostomy/
-
vesicostom$.tw.
-
cystostom$.tw.
-
colporraph$.tw.
-
(fistula$ adj1 (urethra$ or colovesic$ or cystocol$ or cystovagina$ or vagino$)).tw.
-
(sling$ adj1 procedure$).tw.
-
(pelvi$ adj5 rehab$).tw. (55)
-
((bladder or detrusor or vesic$) adj2 (hyper$ or overactiv$)).tw.
-
(urin$ adj2 extravasat$).tw.
-
((urin$ or bladder or urethra$) adj1 (prosthes$ or endoprosthes$)).tw.
-
(detrusor adj1 sphincter$).tw.
-
(spinal adj2 bladder$).tw.
-
(bladder$ adj2 (neuropath$ or neurogen$ neurolog$)).tw.
-
bodyworn$.tw.
-
underpad$.tw.
-
(nervous adj1 pollakisur$).tw.
-
*prostate/
-
*prostatectomy/
-
*prostatic hyperplasia/
-
*prostatic neoplasm/
-
*prostatic neoplasms/
-
*bladder neoplasms/
-
*urinary tract infections/
-
*prostatitis/
-
*prostatic diseases/
-
or/94-102
-
urotherapy.tw.
-
(void$ adj2 dysfunct$).tw.
-
incontinence/ or urinary incontinence/
-
“functional incontinence (nanda)”/ or “reflex incontinence (nanda)”/ or “stress incontinence (nanda)”/ or “total incontinence (nanda)”/
-
“INCONTINENCE/ or “STRESS INCONTINENCE (NANDA)”/ or “URINARY INCONTINENCE CARE (SABA CCC)”/ or INCONTINENCE AIDS/ or “REFLEX URINARY INCONTINENCE (SABA CCC)”/ or “URGE URINARY INCONTINENCE (SABA CCC)”/ or “STRESS URINARY INCONTINENCE (SABA CCC)”/ or “URINARY INCONTINENCE CARE (IOWA NIC)”/ or URGE INCONTINENCE/ or “TOTAL URINARY INCONTINENCE (SABA CCC)”/ or “REFLEX INCONTINENCE (NANDA)”/ or URINARY INCONTINENCE/ or “URINARY INCONTINENCE AND FREQUENCY COMFORT QUESTIONNAIRE”/ or “URGE INCONTINENCE (NANDA)”/ or STRESS INCONTINENCE/ or “TOTAL INCONTINENCE (NANDA)”/
-
Continence Advisors/
-
“urinary continence: (iowa noc)”/
-
INCONTINENCE/ or “FUNCTIONAL INCONTINENCE (NANDA)”/ or “FUNCTIONAL URINARY INCONTINENCE (SABA CCC)”/
-
or/25-93
-
or/104-111
-
112 or 113
-
114 not 103
-
115 and 24
Other terms tested on CINAHL but not included as they did not add any extra relevant hits were:
-
Condition terms overactive bladder/, bladder, neurogenic/, urination disorders/, urinary retention/, bladder/, altered urinary elimination/
-
Intervention terms kegel exercises/, biofeedback/.
Key
/ = Subject heading term; $ = truncation symbol; .tw. = search in title and abstract field; adjn = word is within n words either side of this word; exp = exploded subject heading search; ? = character may or may not be present; *Subject heading = this subject heading is the major focus of the article.
CINAHL RCT filter
The only RCT filter on the INTERTASC site was from the Scottish Intercollegiate Guidelines Network (SIGN). As this filter was not sufficiently sensitive it was adapted to make it more sensitive.
CINAHL condition/intervention terms
The MEDLINE textwords from the Cochrane Incontinence Group strategy were supplemented with CINAHL Subject Headings (CINAHL’s keyword system). Duplicates were removed within OVID and further duplicates were removed on import into reference manager version 10.
EMBASE
EMBASE on OVID (years searched: January 1980 to Week 49 2007). Date of last search: 10 December 2007.
-
Randomized Controlled Trial/
-
controlled study/
-
clinical study/
-
major clinical study/
-
prospective study/
-
meta analysis/
-
exp clinical trial/
-
randomization/
-
crossover procedure/ or double blind procedure/ or parallel design/ or single blind procedure/
-
Placebo/
-
latin square design/
-
exp comparative study/
-
follow up/
-
pilot study/
-
family study/ or feasibility study/ or pilot study/ or study/
-
placebo$.tw.
-
random$.tw.
-
(clin$ adj25 trial$).tw.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
-
factorial.tw.
-
crossover.tw.
-
latin square.tw.
-
(balance$ adj2 block$).tw.
-
or/1-23
-
(nonhuman not human).sh.
-
24 not 25
-
factorial design/
-
parallel design/
-
triple blind procedure/
-
community trial/
-
intervention study/
-
experimental study/
-
prevention study/
-
quasi experimental study/
-
or/27-34
-
24 or 35
-
36 not 25
-
incontinence/ or mixed incontinence/ or stress incontinence/ or urge incontinence/ or urine incontinence/
-
continence/
-
(incontinen$ or continen$).tw.
-
or/38-40
-
*prostate/
-
*prostatectomy/
-
*prostatic hyperplasia/
-
*prostatic neoplasm/
-
*prostatic neoplasms/
-
*bladder neoplasms/
-
*urinary tract infections/
-
*prostatitis/
-
*prostatic diseases/
-
or/42-50
-
41 not 51
-
52 and 37
-
Pelvis Floor/
-
muscle training/ or pelvic floor muscle training/
-
exp feedback system/
-
muscle strength/
-
perineomet$.tw.
-
(pelvi$ adj5 rehab$).tw.
-
(behavio?rial$ adj5 therap$).tw.
-
behavior therapy/
-
biofeedback.tw.
-
kegel$.tw.
-
((pelvi$ or muscle$) adj5 (train$ or exercis$ or educat$ or reeducat$ or rehab$)).tw.
-
((behavio?r$ or cognit$ or exercis$) adj5 (therap$ or train$ or treat$ or strateg$ or interven$ or method$)).tw.
-
(urg$ adj5 suppress$).tw.
-
(frequenc$ adj5 strateg$).tw.
-
conservative treatment/
-
Electrostimulation Therapy/
-
(cone$ adj5 (weight$ or vagin$)).tw.
-
(pfmt or pfe or pfx).tw.
-
((pevi$ or muscle$) adj5 (educat$ or reeducat$)).tw.
-
((pelvi$ or muscle$) adj5 (therap$ or treat$)).tw.
-
physical therap$.tw.
-
physiotherap$.tw.
-
cone$.tw.
-
((behavio?r$ or cognit$ or exercis$) adj3 (program$ or manag$)).tw.
-
((conservative or educat$) adj5 (treat$ or therap$ or program$ or manag$)).tw.
-
(educat$ adj5 (program$ or strateg$ or interven$ or method$)).tw.
-
(bladder adj5 (train$ or retrain$ or drill or educat$ or reeducat$)).tw.
-
exercis$.tw.
-
(rehab$ adj3 therap$).tw.
-
myofeedback.tw.
-
((pelvi$ or muscle$) adj5 (retrain$ or relax$)).tw.
-
(primary adj5 interven$).tw.
-
(nurs$ adj5 interven$).tw.
-
(non adj operative).tw.
-
((pelvi$ or muscle$) adj5 retrain$).tw.
-
Electrostimulation/
-
(fluid$ adj5 (intake$ or manipulat$)).tw.
-
duloxetine/
-
116539 59 4.rn.
-
136434-34-9.rn.
-
or/54-93
-
94 and 53
Key
/ = EMTREE term; $ = truncation symbol; .tw. = search in title and abstract field; adjn = word is within n words either side of this word; exp = exploded EMTREE search; ? = character may or may not be present; *Subject heading = this EMTREE term is the major focus of the article.
RCT filter
The RCT filter was one developed for the Cochrane Incontinence Group.
Condition/intervention terms
The MEDLINE textwords from the Cochrane Incontinence Group strategy were supplemented with EMTREE terms (EMBASE’s keyword system). Duplicates were removed within OVID and further duplicates were removed on import into reference manager version 10.
BIOSIS (on ISI Web of Knowledge)
Years searched: 1 January 1985 to 10 January 2008). Date of last search: 16 January 2008.
#1 TS=(incontinen* OR continen*)
DocType=All document types; LitType=Meeting Abstract OR Meeting Address OR Meeting Paper OR Meeting Poster OR Meeting Report OR Meeting Slide OR Meeting Summary; Language=All languages; Taxa Notes=All Taxa Notes; Database=BIOSIS Previews; Timespan=1985-2008
#2 TS=(placebo*)
DocType=All document types; LitType=All literature types; Language=All languages; Taxa Notes=All Taxa Notes; Database=BIOSIS Previews; Timespan=1985-2008
#3 TS=((singl* OR doubl* OR trebl* OR tripl*) SENT (blind* OR mask*))
DocType=All document types; LitType=All literature types; Language=All languages; Taxa Notes=All Taxa Notes; Database=BIOSIS Previews; Timespan=1985-2008
#4 TS=(clin* SENT trial*)
DocType=All document types; LitType=All literature types; Language=All languages; Taxa Notes=All Taxa Notes; Database=BIOSIS Previews; Timespan=1985-2008
#5 TS=(random*)
DocType=All document types; LitType=All literature types; Language=All languages; Taxa Notes=All Taxa Notes; Database=BIOSIS Previews; Timespan=1985-2008
#6 #2 OR #3 OR #4 OR #5
DocType=All document types; LitType=Meeting Abstract OR Meeting Address OR Meeting Paper OR Meeting Poster OR Meeting Report OR Meeting Slide OR Meeting Summary; Language=All languages; Taxa Notes=All Taxa Notes; Database=BIOSIS Previews; Timespan=1985-2008
#7 #1 AND #6
DocType=All document types; LitType=Meeting Abstract OR Meeting Address OR Meeting Paper OR Meeting Poster OR Meeting Report OR Meeting Slide OR Meeting Summary; Language=All languages; Taxa Notes=All Taxa Notes; Database=BIOSIS Previews; Timespan=1985-2008
Limited literature type to: meeting abstract, meeting address, meeting paper, meeting poster, meeting report, meeting slide or meeting summary.
Key
TS = topic, searches in title, subject fields and abstracts; SENT = words must appear in the same sentence; * = truncation symbol.
RCT filter
No INTERTASC filter was available. Therefore, the approach was based on one developed for an earlier HTA systematic review229 and the Cochrane Stroke Group BIOSIS strategy. 230
Condition/intervention terms
A very general search for all incontinence was performed. The first 300 abstracts of over 1000 were assessed and it was found that new and relevant hits were related to meetings. This tied in with the findings of Royle and Waugh. 231 Therefore, the BIOSIS searches were limited to literature types: meeting abstract, meeting address, meeting paper, meeting poster, meeting report, meeting slide or meeting summary.
Science Citation Index and Social Science Citation Index (on ISI Web of Knowledge)
Years searched: 1970 to 2 February 2008). Date of last search: 6 February 2008.
#1 TS=(incontinen* OR continen*)
DocType=All document types; Language=All languages;
#2 TS=(random*)
DocType=All document types; Language=All languages;
#3 TS=(placebo*)
DocType=All document types; Language=All languages;
#4 TS=(clin* SENT trial*)
DocType=All document types; Language=All languages;
#5 TS=((singl* OR doubl* OR trebl* OR tripl*) SENT (blind* OR mask*))
DocType=All document types; Language=All languages;
#6 (#5 OR #4 OR #3 OR #2)
DocType=All document types; Language=All languages;
#7 (#6 AND #1)
DocType=All document types; Language=All languages;
Key
TS = topic, searches in title, subject fields and abstracts; SENT = words must appear in the same sentence; * = truncation symbol.
Physiotherapy Evidence Database (PEDro)
Available at: www.pedro.fhs.usyd.edu.au/
Search date: 9 May 2008
-
Problem = incontinence
-
Method = clinical trial
Overall, 277 records were retrieved. The first 40 were assessed and nothing new was found so this search was curtailed.
National Research Register/UKCRN Portfolio Database
Search date: 9 June 2008
-
Topic = renal and urogenital
-
Topic = all
-
Title/acrononym = incontinence
-
Title/acrononym = incontinent
-
Title/acrononym = continence
-
Title/acrononym = continent
Current Controlled Trials/metaRegister
All registers were searched (active and inactive) (includes clinical trials).
-
Incontinent* (searched on 22 May 2008)
-
Continen* NOT incontinent* (searched 28 May 2008)
Terms tested but not relevant:
-
GSI NOT (continen* OR incontinent*)
-
Leak* NOT (continen* OR incontinent*)
-
Urin* NOT (continen* OR incontinent*)
ClinicalTrials.gov (Available at: http://clinicaltrials.gov/ct/gui)
Search date: 9 June 2008
-
Incontinence OR incontinent
-
(Continence OR continent) NOT (incontinence OR incontinent)
Eli Lily website – Lily Clinical Trials Registry
Available at: www.lillytrials.com/
Search date: 29 May 2008
-
No search terms were used – listed all trials by therapeutic area = other
-
Looked at all those listed under Disease = stress urinary incontinence
Search strategies for the economic evaluation
NHS Economic Evaluations Database (NHS EED)
Available from: www.york.ac.uk/inst/crd/.
-
Searched on Centre for Reviews and Dissemination website, University of York.
-
Search date: June 2008
-
Search terms used: urinary incontinence, stress urinary incontinence
Search for quality of life literature
Further data were identified from the literature search performed for the effectiveness review and were supplemented by information from NHS EED and the Cost-Effectiveness Analysis (CEA) Registry at Tufts Medical Center. 218 This search was performed in June 2008.
Searching for TVT
Search to find any updates of included RCTs (search for authors ) or studies including over 1000 participants that were included in the TVT HTA monograph or population registries included in the TVT HTA monograph. 80 This search was performed in order to try to find long-term follow-up or other information important for the economic modelling.
Using last names of authors of included RCTs in TVT report
Specialised Register Cochrane Incontinence Group search date: 17 June 2008
Tried all last names: cucinella g* OR adile b* OR gugliotta f* OR lo bue a* OR grifo s* OR caputo a* OR halaska m* OR koelbl h* OR petri e* OR danes l* OR voigt r* OR otcenasek m* OR martan a* OR pohanka m* OR masata j* OR han w* OR liapis a* OR bakas p* OR creatsas g* in Author
Databases on OVID searched on 18 June 2008
-
MEDLINE (1996 to June week 1 2008) and In Process (17 June 2008)
-
EMBASE (week 1 1996 to 2008 week 24)
-
CAB Abstracts (January 1990 to May 2008)
-
CINAHL (January 1982 to June week 2 2008)
-
cucinella g$
-
adile b$
-
gugliotta f$
-
lo bue a$
-
grifo s$
-
caputo a$
-
halaska m$
-
koelbl h$
-
petri e$
-
danes l$
-
voigt r$
-
otcenasek m$
-
martan a$
-
pohanka m$
-
masata j$
-
han w$
-
liapis a$
-
bakas p$
-
creatsas g$
-
or/1-5
-
or/6-19
-
(tension or TVT).tw.
-
(21 and 22) or 20
Databases on WoS
Search date: 18 June 2008.
-
BIOSIS (January 1985 to 18 June 2008)
-
WoS (January 1970 to 18 June 2008)
-
ISI Proceedings (1970 to 18 June 2008)
-
Web Citation Index (1936 to 2005)
Put in all the surnames above combined with OR and combined with (tension OR TVT) in title.
CENTRAL Issue 2, 2008
Searched on 18 June 2008.
-
cucinella g* OR adile b* OR gugliotta f* OR lo bue a* OR grifo s* OR caputo a* OR halaska m* OR koelbl h* OR petri e* OR danes l* OR voigt r* OR otcenasek m* OR martan a* OR pohanka m* OR masata j* OR han w* OR liapis a* OR bakas p* OR creatsas g* in Author and tension OR tvt in Title, Abstract or Keywords in Cochrane Central Register of Controlled Trials”
Using last names of authors and country names of included population registries in TVT report80
Databases on OVID searched on 18 June 2008:
-
MEDLINE (1996 to June week 1 2008) and In Process (17 June 2008)
-
EMBASE (week 1 1996 to week 24 2008)
-
CAB Abstracts (January 1990 to May 2008)
-
CINAHL (January 1982 to June week 2 2008)
Databases on WoS searched on 18 June 2008:
-
BIOSIS (January 1985 to 18 June 2008)
-
WoS (January 1970 to 18 June 2008)
-
ISI Proceedings (1970 to 18 June 2008)
-
Web Citation Index (1936 to 2005)
Finnish population registry:
-
Kuuva N$
-
Nilsson c$ AND (tension or TVT)
-
Finnish AND (tension or TVT)
-
Finland AND (tension or TVT)
Austrian population registry (only the first two terms searched on WoS databases):
-
Tamussino$
-
Austrian urogynecology working group.au.
-
Hanzal e$
-
Riss p$
-
Kolle d$
-
Ralph g$
Appendix 3 Study eligibility form
Appendix 4 Data extraction form
Data extraction form: Non-surgical treatment for women with stress urinary incontinence (SUI)
Appendix 5 Risk of bias assessment form
Non-surgical treatment for women with stress urinary incontinence (SUI) Quality assessment checklist (Source: The Cochrane Incontinence Group109)
Appendix 6 References to studies included in this review
Note: * denotes the primary reference.
Aksac 2003120,232
Aksac B, Aki S, Karan A, Yalcin O, Isikoglu M, Eskiyurt N. Biofeedback and pelvic floor muscle exercises for the rehabilitation of stress urinary incontinence [abstract]. Proceedings of the International Continence Society (ICS), 32nd Annual Meeting, 28–30 August 2002, Heidelberg, Germany. p. 175.
*Aksac B, Aki S, Karan A, Yalcin O, Isikoglu M, Eskiyurt N. Biofeedback and pelvic floor exercises for the rehabilitation of urinary stress incontinence. Gynecol Obstet Invest 2003; 56(1):23–7.
Arvonen 2001178
Arvonen T, Fianu-Jonasson A, Tyni-Lenne R. Effectiveness of two conservative modes of physical therapy in women with urinary stress incontinence. Neurourol Urodyn 2001; 20(5):591–9.
Aukee 2002146,233,234
Aukee P, Immonen P, Pettinen J, Airaksinen O. A prospective randomised study comparing FemiScan home trainer and pelvic floor muscle training alone [abstract no. 205]. Proceedings of the International Continence Society (ICS), 30th Annual Meeting, 28–31 August 2000, Tampere, Finland.
*Aukee P, Immonen P, Penttinen J, Laippala P, Airaksinen O. Increase in pelvic floor muscle activity after 12 weeks’ training: a randomized prospective pilot study. Urology 2002; 60(6):1020–3.
Aukee P, Immonen P, Laaksonen DE, Laippala P, Penttinen J, Airaksinen O. The effect of home biofeedback training on stress incontinence. Acta Obstet Gynecol Scand 2004; 83(10):973–7.
Berghmans 1996147,235
Berghmans LCM, Weil EHJ, Frederiks CMA, de Bie RA, Smeets LWH, van Waalwijk van Doorn ESC, et al. Efficacy of biofeedback for genuine stress incontinence [abstract no. 115]. Proceedings of the International Continence Society (ICS), 25th Annual Meeting, 17–20 October 1995, Sydney, Australia. pp. 44–5.
*Berghmans LC, Frederiks CM, de Bie RA, Weil EH, Smeets LW, van Waalwijk van Doorn ESC, et al. Effica cy of biofeedback, when included with pelvic floor muscle exercise treatment, for genuine stress incontinence. Neurourol Urodyn 1996; 15(1):37–52.
Bernardes 2000174
Bernardes NO, Peres FR, Souza ELBL, Souza OL. [Methods of treatment of genuine stress incontinence: a comparative study between a pelvic floor exercise program and a pelvic floor electrical stimulation.] Revista Brasileira de Gynecologia e Obstetricia 2000; 22(1):49–54.
Bidmead 2002121,236
*Bidmead J, Mantle J, Cardozo L, Hextall A, Boos K. Home electrical stimulation in addition to conventional pelvic floor exercises: a useful adjunct or expensive distraction [abstract]? Neurourol Urodyn 2002; 21(4):372–3.
Parsons M, Mantle J, Cardozo L, Hextall A, Boos K, Bidmead J. A single blind, randomised, controlled trial of pelvic floor muscle training with home electrical stimulation in the treatment of urodynamic stress incontinence [abstract no. 296]. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 23–27 August 2004, Paris, France.
Blowman 1991189
Blowman C, Pickles C, Emery S, Creates V, Towell L, Blackburn N, et al. Prospective double blind controlled trial of intensive physiotherapy with and without stimulation of the pelvic floor in treatment of genuine stress incontinence. Physiotherapy 1991; 77(10):661–4.
Bø 1990159,207,208,237–246
Bø K. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: methodological studies and clinical results. Acta Obstet Gynecol Scand 1991;70:637–9.
Bø K. Adherence to pelvic floor muscle exercise and long-term effect on stress urinary incontinence. A five-year follow-up study. Scand J Med Sci Sports 1995;5(1): 36–9.
Bø K. Pelvic floor muscle strength and response to pelvic floor muscle training for stress urinary incontinence. Neurourol Urodyn 2003;22(7):654–8.
Bø K, Kvarstein B. 15-year follow-up of a randomized controlled trial of pelvic floor muscle training to treat female urodynamic stress incontinence [abstract no. 658]. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 23–27 August 2004, Paris, France.
Bø K, Larsen S. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: classification and characterization of responders. Neurourol Urodyn 1992;11(5):497–507.
Bø K, Talseth T. Long-term effect of pelvic floor muscle exercise 5 years after cessation of organized training. Obstet Gynecol 1996;87(2):261–5.
Bø K, Hagen R, Jorgensen J, Kvarstein B, Larsen S. The effect of two different pelvic floor muscle exercise programs in treatment of urinary stress incontinence in women [abstract]. Neurourol Urodyn 1989;8(4):355–6.
Bø K, Hagen R, Kvarstein B, Larsen S. Female stress urinary incontinence and participation in different sports and social activities. Scand J Sports Sci 1989;11(3):117–21.
*Bø K, Hagen RH, Kvarstein B, Jorgensen J, Larsen S. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: III. Effects of two different degrees of pelvic floor muscle exercises. Neurourol Urodyn 1990;9(5):489–502.
Bø K, Larsen S, Kvarstein B, Hagen RH. Classification and characterization of responders to pelvic floor muscle exercise for female urinary incontinence [abstract]. Neurourol Urodyn 1990;9(4):395–7.
Bø K, Kvarstein B, Hagen RH. The effect of two different pelvic floor muscle exercise regimens in treatment of female stress urinary incontinence [abstract]. Proceedings of the American Urogynecology Society, 12th Annual Meeting, 23–26 October 1991, Newport Beach, California.
Bø K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstet Gynecol 2005;105(5):999–1005.
Bø K, Kvarstein B, Nygaard I. Lower urinary tract symptoms 15 years after ending a randomised controlled trial of pelvic floor muscle training for urodynamic stress incontinence [abstract no. 355]. Eur Urol Suppl 2005;4(3):91.
Bø 1999115,247–250
Bø K, Talseth T. Single blinded randomized controlled trial on the effect of pelvic floor muscle strength training, electrical stimulation, cones or control on severe genuine stress incontinence [abstract]. Neurourol Urodyn 1998;17(4):421–2.
Bø K, Talseth T. Randomized controlled trial on the effect of pelvic floor muscle training on quality of life and sex-life in genuine stress incontinent women [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 1999;10(Suppl. 1):83.
Bø K, Talseth T. Randomized controlled trial on the effect of pelvic floor muscle training on quality of life and sex-life in genuine stress incontinent women [abstract no. 175]. Proceedings of the International Continence Society (ICS), 29th Annual Meeting, 23–26 August 1999, Denver, Colorado, 1999. pp. 59–60.
*Bø K, Talseth T, Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ 1999;318(7182):487–93.
Bø K, Talseth T, Vinsnes A. Randomized controlled trial on the effect of pelvic floor muscle training on quality of life and sexual problems in genuine stress incontinent women. Acta Obstet Gynecol Scand 2000;79(7):598–603.
Borello-France 2006165
Borello-France DF, Zyczynski HM, Downey PA, Rause CR, Wister JA. Effect of pelvic-floor muscle exercise position on continence and quality-of-life outcomes in women with stress urinary incontinence. Phys Ther 2006;86(7):974–86.
Bourcier 1994196
Bourcier A, Juras J. Randomised study comparing physiotherapy and pelvic floor rehabilitation [abstract]. Proceedings of the International Continence Society (ICS), 24th Annual Meeting, 30 August–2 September 1994, Prague, Czech Republic. p. 146.
Brubaker 1997130,251
Brubaker L, Benson JT, Bent A, Clark A. Transvaginal electrical stimulation is effective for treatment of detrusor overactivity [abstract]. Neurourol Urodyn 1996;15(4):282–3.
*Brubaker L, Benson JT, Bent A, Clark A, Shott S. Transvaginal electrical stimulation for female urinary incontinence. Am J Obstet Gynecol 1997;177(3):536–40.
Bump 2004136
Bump R, Benson JT, Brubaker L, Brostrom S, Hampel C, Jannelli M, et al. Biomechanical and electrophysiological effects of duloxetine in women with stress urinary incontinence [abstract no. 269]. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 23–27 August 2004, Paris, France.
Burns 1993122,252–255
Burns PA, Pranikoff K, Reis JS, Levy KJ. Effectiveness of biofeedback therapy for stress incontinent females [abstract]. Neurourol Urodyn 1988;7(3):280–2.
Burns P, Pranikoff K, Nochajski TJ, Levy KJ. Effectiveness of biofeedback therapy for stress incontinence [abstract no. 742]. J Urol 1989;141(4):355.
Burns PA, Pranikoff K, Nochajski T, Desotelle P, Harwood MK. Treatment of stress incontinence with pelvic floor exercises and biofeedback. J Am Geriatr Soc 1990;38(3):341–4.
Burns PA, Nochajski TH, Pranikoff K. Factors discriminating between genuine stress and mixed incontinence. J Am Acad Nurse Pract 1992;4(1):15–21.
Burns PA, Pranikoff K, Nochajski TH, Hadley EC, Levy KJ, Ory MG. A comparison of effectiveness of biofeedback and pelvic muscle exercise treatment of stress incontinence in older community-dwelling women. J Gerontol 1993;48(4):M167–74.
Burton 1993173
Burton G. Active vaginal cones therapy: a new form of treatment for genuine stress incontinence [abstract no. 134]. Proceedings of the International Continence Society (ICS), 23rd Annual Meeting, 8–11 September 1993, Rome, Italy.
Cammu 1998181,256
Cammu H, van Nylen M. Pelvic floor exercises (PFE) versus vaginal cones (VC) in the treatment of genuine stress incontinence [abstract no. 225). Proceedings of the International Continence Society (ICS), 26th Annual Meeting, 27–30 August 1996, Athens, Greece. p. 223.
*Cammu H, van Nylen M. Pelvic floor exercises versus vaginal weight cones in genuine stress incontinence. Eur J Obstet Gynecol Reprod Biol 1998;77(1):89–93.
Cardozo 2004137,257,258
Cardozo L, Drutz H, Baygani S, Bump R. Duloxetine response and onset of action in women with severe stress urinary incontinence (SUI) awaiting continence surgery [abstract no. 36). Prog Urol 2004;14(Suppl. 3):14.
*Cardozo L, Drutz HP, Baygani SK, Bump RC. Pharmacological treatment of women awaiting surgery for stress urinary incontinence. Obstet Gynecol 2004;104(3):511–19.
Drutz H, Cardozo L, Baygani S, Bump R. Duloxetine treatment of women with only urodynamic stress incontinence awaiting continence surgery [abstract]. Neurourol Urodyn 2003;22(5):523–4.
Castleden 1984148
Castleden CM, Duffin HM, Mitchell EP. The effect of physiotherapy on stress incontinence. Age Ageing 1984;13(4):235–7.
Castro-Diaz 2007138
Castro-Diaz D, Palma PC, Bouchard C, Haab F, Hampel C, Carone R, et al. Effect of dose escalation on the tolerability and efficacy of duloxetine in the treatment of women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2007;18(8):919–29.
Delneri 2000186
Delneri C, Di Benedetto P. Pelvic floor rehabilitation. A comparison of two methods of treatment: vaginal cones versus functional electrical stimulation. Eura Medicophys 2000;36(1):45–8.
Dmochowski 2003139,259
*Dmochowski RR, Miklos JR, Norton PA, Zinner NR, Yalcin I, Bump RC, et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol 2003;170(4):1259–63.
Zinner N, Dmochowski R, Miklos J, Norton P, Yalcin I, Bump R. Duloxetine versus placebo in the treatment of stress urinary incontinence (SUI) [abstract]. Neurourol Urodyn 2002;21(4):383–4.
Dumoulin 2004199,260–262
Dumoulin C, Lemieux M, Bourbonnais D, Morin M. Conservative management of stress urinary incontinence: a single-blind, randomized controlled trial of pelvic floor rehabilitation with or without abdominal muscle rehabilitation compared to the absence of treatment [abstract]. Neurourol Urodyn 2003;22(5):543–4.
*Dumoulin C, Lemieux MC, Bourbonnais D, Gravel D, Bravo G, Morin M. Physiotherapy for persistent postnatal stress urinary incontinence: a randomized controlled trial. Obstet Gynecol 2004;104(3):504–10.
Dumoulin C, Morin M, Bourbonnais D, Lemieux M, Gravel D. Effect of adding deep abdominal muscle training to pelvic floor muscle training to treat stress urinary incontinence: a one-year follow up [abstract no. 662]. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 23–27 August 2004, Paris, France.
Dumoulin C, Morin M, Lemieux MC, Bourbonnais D, Bravo G, Gravel D. Efficacy of deep abdominal training when combined with pelvic floor muscle training for stress urinary incontinence: a single-blind randomized controlled trial [abstract no. 44]. Prog Urol 2004;14(Suppl. 3):16.
Edwards 2000170
Edwards GJ, Wines H, Barrington JW. A comparison between pelvic floor exercises and pelvic floor exercises and electrical therapy with respect to urethral pressure profiles [abstract no. IDP50]. Int Urogynecol J Pelvic Floor Dysfunct 2000;11(Suppl. 1):S89.
Fantl 1991135,263–266
Fantl JA, Wyman JF, Harkins SW, Taylor JR, et al. Bladder training in women with urinary incontinence [abstract]. Neurourol Urodyn 1988;7(3):276–7.
*Fantl JA, Wyman JF, McClish DK, Harkins SW, Elswick RK, Taylor JR, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA 1991;265(5):609–13.
McClish DK, Fantl JA, Wyman JF, Pisani G, Bump RC. Bladder training in older women with urinary incontinence: relationship between outcome and changes in urodynamic observations. Obstet Gynecol 1991;77(2):281–6.
Wyman JF, McClish DK, Ory MG, Fantl JA. Changes in quality of life following bladder training in older women with urinary incontinence [abstract]. Neurourol Urodyn 1992;11(4):426–7.
Wyman JF, Fantl JA, McClish DK, Harkins SW, Uebersax JS, Ory MG. Quality of life following bladder training in older women with urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1997;8(4):223–9.
Ferguson 1990149
Ferguson KL, McKey PL, Bishop KR, Kloen P, Verheul JB, Dougherty MC. Stress urinary incontinence: effect of pelvic muscle exercise. Obstet Gynecol 1990;75(4):671–5.
Gallo 1997162
Gallo ML, Staskin DR. Cues to action: pelvic floor muscle exercise compliance in women with stress urinary incontinence. Neurourol Urodyn 1997;16(3):167–77.
Ghoniem 200557,267
*Ghoniem GM, Van Leeuwen JS, Elser DM, Freeman RM, Zhao YD, Yalcin I, et al. A randomized controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment and no active treatment in women with stress urinary incontinence. J Urol 2005;173(5):1647–53.
Schagen van Leeuwen JH, Elser D, Freeman R, Ghoniem G, Zhao Y, Yalcin I, et al. Controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment, and no treatment in women with stress urinary incontinence (SUI) [abstract]. Eur Urol Suppl 2004;3(2):52.
Glavind 1996150,268
Glavind K, Nohr S, Walter S. Randomized prospective trial on physiotherapy versus physiotherapy and biofeedback in treatment of genuine stress urinary incontinence [abstract]. Neurourol Urodyn 1995;14(5):457–9.
*Glavind K, Nohr SB, Walter S. Biofeedback and physiotherapy versus physiotherapy alone in the treatment of genuine stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1996;7:339–43.
Goode 2003123
Goode PS, Burgio KL, Locher JL, Roth DL, Umlauf MG, Richter HE, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA 2003;290(3):345–52.
Hahn 1991175,269
Hahn I, Naucler J, Sommer S, Fall M. Urodynamic assessment of pelvic floor training. World J Urol 1991;9(3):162–6.
*Hahn I, Sommar S, Fall M. A comparative study of pelvic floor training and electrical stimulation for the treatment of genuine female stress urinary incontinence. Neurourol Urodyn 1991;10(6):545–54.
Haig 1995190
Haig L, Mantle J, Versi E. Does interferential therapy (IFT) confer added benefit over a pelvic floor muscle exercise programme (PFMEP) for genuine stress incontinence (GSI)? [abstract no. 111]. Proceedings of the International Continence Society (ICS), 25th Annual Meeting 17–20 October 1995, Sydney, Australia. pp. 36–7.
Haken 1991179
Haken J, Benness C, Cardozo L, Cutner A. A randomised trial of vaginal cones and pelvic floor exercises in the management of genuine stress incontinence [abstract]. Neurourol Urodyn 1991;10(4):393–4.
Hay-Smith 2003164,270
*Hay-Smith EJC. Pelvic floor muscle training for female stress urinary incontinence. PhD thesis, University of Otago, Dunedin, New Zealand, 2003.
Hay-Smith EJC, Herbison GP, Wilson PD. Pelvic floor muscle training for women with symptoms of stress urinary incontinence: A randomised trial comparing strengthening and motor relearning approaches [abstract]. Neurourol Urodyn 2002;21(4):371–2.
Henalla 1989124,271
Henalla SM, Hutchins CJ, Castleden CM. Conservative management of urethral sphincter incompetence [abstract]. Neurourol Urodyn 1987;6(3):191–2.
*Henalla SM, Hutchins CJ, Robinson P, MacVicar J. Non-operative methods in the treatment of female genuine stress incontinence of urine. J Obstet Gynaecol 1989;9(3):222–5.
Henalla 1990125
Henalla SM, Millar DR, Wallace KJ. Surgical versus conservative management for post-menopausal genuine stress incontinence of urine [abstract]. Neurourol Urodyn 1990;9(4):436–7.
Hofbauer 1990126,272
*Hofbauer J, Preisinger F, Nurnberger N. [The value of physical therapy in genuine female stress incontinence.] [German] Z Urol Nephrol 1990;83(5):249–54.
Preisinger E, Hofbauer J, Nurnberger N, Sadil S, Schneider B. Possibilities of physiotherapy for urinary stress incontinence. Z Phys Med Balneol Med Klimatol 1990;19:75–9.
Jeyaseelan 2000131,273
Jeyaseelan SM, Haslam J, Roe B, Winstanley J, Oldham JA. The evaluation of a new pattern of electrical muscle stimulation as a treatment for genuine stress incontinence: a randomised, double-blind, controlled trial [abstract no. 522]. Proceedings of the International Continence Society (ICS), 29th Annual Meeting, 23–26 August 1999, Denver, Colorado. p. 74.
*Jeyaseelan SM, Haslam EJ, Winstanley J, Roe BH, Oldham JA. An evaluation of a new pattern of electrical stimulation as a treatment for urinary stress incontinence: a randomized, double-blind, controlled trial. Clin Rehabil 2000;14(6):631–40.
Johnson 2001167,274
Johnson VY. Effects of a submaximal exercise protocol to recondition the circumvaginal musculature in women with genuine stress urinary incontinence. PhD thesis, The University of Texas Health Science Center at San Antonio, San Antonio, Texas, 1997.
*Johnson VY. Effects of a submaximal exercise protocol to recondition the pelvic floor musculature. Nurs Res 2001;50(1):33–41.
Karagkounis 2007194
Karagkounis SC, Pantelis A, Parashou GC, Paplomata E, Madenis N, Chrisanthopoulos C, et al. Stress urinary incontinence: TVT OB system versus duloxetine-HCl. And the winner is? [abstract no. 5]. Int Urogynecol J Pelvic Floor Dysfunct 2007;18(Suppl. 1):3–4.
Kim 2007118
Kim H, Suzuki T, Yoshida Y, Yoshida H. Effectiveness of multidimensional exercises for the treatment of stress urinary incontinence in elderly community-dwelling Japanese women: A randomized, controlled, crossover trial. J Am Geriatr Soc 2007;55(12):1932–9.
Kinchen 2005140
Kinchen KS, Obenchain R, Swindle R. Impact of duloxetine on quality of life for women with symptoms of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2005;16(5):337–44.
Klarskov 1986184,275–278
Klarskov P, Belving D, Bischoff N, Dorph S, Gerstenberg T, Hald T, et al. Pelvic floor exercise versus surgery for female urinary stress incontinence: preliminary results [abstract]. Proceedings of the International Continence Society (ICS), 14th Annual Meeting, 13–15 September 1984, Innsbruck, Austria. pp. 159–61.
*Klarskov P, Belving D, Bischoff N, Dorph S, Gerstenberg T, Okholm B, et al. Pelvic floor exercise versus surgery for female urinary stress incontinence. Urol Int 1986;41(2):129–32.
Klarskov P, Vedel Jepsen P, Dorph S. Reliability of voiding colpo-cysto-urethrography in female urinary stress incontinence before and after treatment. Acta Radiol 1988;29(6):685–8.
Klarskov P, Kroyer K, Kromann B, Maegaard E. Long term results of pelvic floor training and surgery for female genuine stress incontinence [abstract]. Neurourol Urodyn 1989;8(4):357–9.
Klarskov P, Nielson KK, Kromann-Andersen B, Maegaard E. Long term results of pelvic floor training and surgery to female genuine stress incontinence. Int Urogynecol J 1991;2:132–5.
Klingler 1995151
Klingler HC, Madersbacher S, Uher EM, Schmidbauer CP. Pelvic floor exercise and endotrainer for treatment of female stress urinary incontinence [abstract no. 122]. Proceedings of the International Continence Society (ICS), 25th Annual Meeting, 17–20 October 1995, Sydney, Australia. pp. 56–7.
Knight 1998172,279
*Knight S. Evaluation of neuromuscular electrical stimulation in the treatment of genuine stress incontinence. Physiotherapy 1998;84(2):61–71.
Laycock J, Knight S, Naylor D. Prospective, randomised, controlled clinical trial to compare acute and chronic electrical stimulation in combination therapy for GSI [abstract]. Neurourol Urodyn 1995;14(5):425–6.
Konstantinidou 2007116,280
Konstantinidou E, Apostolidis A, Kondelidis N, Tsimtsiou Z, Hatzichristou D, Ioannides E. Short-term efficacy of high-supervisory-intensity group pelvic floor training versus unsupervised, home training in female stress urinary incontinence: a randomised pilot study [abstract no. 678]. Eur Urol Suppl 2006;5(2):192.
*Konstantinidou E, Apostolidis A, Kondelidis N, Tsimtsiou Z, Hatzichristou D, Ioannides E. Short-term efficacy of group pelvic floor training under intensive supervision versus unsupervised home training for female stress urinary incontinence: a randomized pilot study. Neurourol Urodyn 2007;26(4):486–91.
Lagro-Janssen 1991127,210,281
Lagro-Janssen T, van Weel C. Long-term effect of treatment of female incontinence in general practice. Br J Gen Pract 1998;48(436):1735–8.
*Lagro-Janssen TL, Debruyne FM, Smits AJ, van Weel C. Controlled trial of pelvic floor exercises in the treatment of urinary stress incontinence in general practice. Br J Gen Pract 1991;41(352):445–9.
Lagro-Janssen ALM, Debruyne FMJ, Smits AJA, van Weel C. The effects of treatment of urinary incontinence in general practice. Fam Pract 1992;9(3):284–9.
Laycock 1988176
Laycock J. Interferential therapy in the treatment of genuine stress incontinence [abstract]. Neurourol Urodyn 1988;7(3):268–9.
Laycock 2001152,282,283
Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. A multi-centre, prospective, randomised, controlled, group comparative study of the efficacy of vaginal cones and PFX [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 1999;10(Suppl. 1):49.
Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. A multi-centre, prospective, randomised, controlled, group comparative study of the efficacy of vaginal cones and PFX [abstract no. 47]. Neurourol Urodyn 1999;18(4):301–2.
*Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. Pelvic floor reeducation for stress incontinence: comparing three methods. Br J Community Nurs 2001;6(5):230–44.
Laycock Trials 1 and 2 1993132
Laycock J, Jerwood D. Does pre-modulated interferential therapy cure genuine stress incontinence? Physiotherapy 1993;79(8):553–60.
Luber 1997133
Luber KM, Wolde-Tsadik G. Efficacy of functional electrical stimulation in treating genuine stress incontinence: a randomized clinical trial. Neurourol Urodyn 1997;16(6):543–51.
Mah 2006141
Mah SY, Lee KS, Choo MS, Seo JT, Lee JZ, Park WH, et al. Duloxetine versus placebo for the treatment of Korean women with stress predominant urinary incontinence. Korean J Urol 2006;47(5):527–35.
Manning 2005142
Manning M, Lange R, Jonas F, Meisel J, Kohoutek U, Willgerodt J, et al. Duloxetine versus placebo for the treatment of German women with stress urinary incontinence (SUI) [abstract no. 211]. Proceedings of the International Continence Society (ICS), 35th Annual Meeting, 28 August–2 September 2005, Montreal, Canada.
Mayne 1988168
Mayne CJ, Hilton P. A comparison of urethral electrical conductance and perineometry during a course of pelvic floor exercises for genuine stress incontinence [abstract no. 71]. Neurourol Urodyn 1988;7(3):264–5.
Millard 2004143,284,285
Millard RJ, Moore K, Rencken R. Duloxetine vs placebo in the treatment of stress urinary incontinence: a global phase III study [abstract]. Aust NZ J Surg 2003;73:A337.
Millard R, Moore K, Yalcin I, Bump R. Duloxetine vs placebo in the treatment of stress urinary incontinence: a global phase 3 study [abstract]. Neurourol Urodyn 2003;22(5):482–3.
*Millard RJ, Moore K, Rencken R, Yalcin I, Bump RC, Duloxetine UI Study Group. Duloxetine vs placebo in the treatment of stress urinary incontinence: a four-continent randomized clinical trial. BJU Int 2004;93(3):311–18.
Miller 1998107
Miller JM, Ashton-Miller JA, DeLancey JOL. A pelvic muscle precontraction can reduce cough-related urine loss in selected women with mild stress urinary incontinence. J Am Geriatr Soc 1998;46(7):870–4.
Mørkved 2002153,286–288
Mørkved S, Fjortoft T, Bø K. Is there any effect of adding biofeedback to pelvic floor muscle training? A randomised controlled trial [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 2001;12(Suppl. 3):28.
*Mørkved S, Bø K, Fjortoft T. Effect of adding biofeedback to pelvic floor muscle training to treat urodynamic stress incontinence. Obstet Gynecol 2002;100(4):730–9.
Mørkved S, Bø K, Fjortoft T. Continence status one year after cessation of organised pelvic floor muscle training [abstract]. Proceedings of the International Continence Society (ICS), 33rd Annual Meeting, 5–9 October 2003, Florence Italy. pp. 260–1.
Mørkved S, Fjortoft T, Lindland M, Bø K. Continence status five years after cessation of organised pelvic floor muscle training [abstract no. 297]. Proceedings of the International Continence Society (ICS), 36th Annual Meeting, 27 November–1 December 2006, Christchurch, New Zealand.
Norton 2002144,289–293
Bump RC, Yalcin I, for the Duloxetine Urinary Incontinence Study Group. Pure and mixed stress urinary incontinence (UI) symptoms: comparing UI severity and treatment response [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 2001;12(Suppl. 3):2.
Bump RC, Yalcin I. Mixed incontinence: duloxetine treatment response, urodynamic findings, and incontinence severity [abstract]. Obstet Gynecol 2002;99(Suppl. 4):5.
Bump RC, Norton PA, Zinner NR, Yalcin I, Duloxetine Urinary Incontinence Study Group. Mixed urinary incontinence symptoms: urodynamic findings, incontinence severity, and treatment response. Obstet Gynecol 2003;102(1):76–83.
Norton P, Zinner NR, Yalcin I, Bump RC, for the Duloxetine UI Study Group. Duloxetine versus placebo in the treatment of stress urinary incontinence [abstract]. Neurourol Urodyn 2001;20(4):532–4.
*Norton PA, Zinner NR, Yalcin I, Bump RC, Duloxetine Urinary Incontinence Study Group. Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol 2002;187(1):40–8.
Yalcin I, Viktrup L. Comparison of physician and patient assessments of incontinence severity and improvement. Int Urogynecol J Pelvic Floor Dysfunct 2007;18(11):1291–5.
Nygaard 1996163
Nygaard IE, Kreder KJ, Lepic MM, Fountain KA, Rhomberg AT. Efficacy of pelvic floor muscle exercises in women with stress, urge, and mixed urinary incontinence. Am J Obstet Gynecol 1996;174(1):120–5.
Oláh 1990187,294
Bridges N, Denning J, Oláh KS, Farrar DJ. A prospective trial comparing interferential therapy and treatment using cones in patients with symptoms of stress incontinence [abstract]. Neurourol Urodyn 1988;7(3):267–8.
*Oláh KS, Bridges N, Denning J, Farrar DJ. The conservative management of patients with symptoms of stress incontinence: a randomized, prospective study comparing weighted vaginal cones and interferential therapy. Am J Obstet Gynecol 1990;162(1):87–92.
Pages 2001154
Pages IH, Jahr S, Schaufele MK, Conradi E. Comparative analysis of biofeedback and physical therapy for treatment of urinary stress incontinence in women. Am J Phys Med Rehabil 2001;80(7):494–502.
Peattie 1988180,295
*Peattie AB, Plevnik S. Cones versus physiotherapy as conservative management of genuine stress incontinence [abstract no. 72]. Neurourol Urodyn 1988;7(3):265–6.
Peattie AB, Taylor B, Plevnik S, Stanton SL. Cones versus physiotherapy for conservative treatment of genuine stress incontinence [abstract no. 169]. Proceedings of the Silver Jubilee British Congress of Obstetrics and Gynaecology, 4–7 July 1989, London, UK.
Pieber 1995192,296,297
Pieber D, Zivkovic F, Tamussino K. [Pelvic floor exercises without or with vaginal cones in premenopausal women with mild to moderate stress incontinence]. [German] Gynäkol Geburtshilfliche Rundsch 1994;34(1):32–3.
Pieber D, Zivkovic F, Tamussino K, Ralph G. Pelvic floor exercise alone or with vaginal cones for the treatment of mild and moderate stress incontinence in premenopausal women: a randomised trial [abstract]. Proceedings of the International Continence Society (ICS), 24th Annual Meeting, 30 August–2 September 1994, Prague, Czech Republic. p. 162.
*Pieber D, Zivkovic F, Tamussino K, Ralph G, Lippitt G, Fauland B. Pelvic floor exercise alone or with vaginal cones for the treatment of mild to moderate stress urinary incontinence in premenopausal women. Int Urogynecol J Pelvic Floor Dysfunct 1995;6:14–17.
Pohl 2004171
Pohl K, Jundt K, Greulich T, Drinovac V, Peschers U. Biofeedback versus electrostimulation in treatment of female stress urinary incontinence [abstract no. 564). Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 23–27 August 2004, Paris, France.
Ramsay 1990128
Ramsay IN, Thou M. A randomised, double blind, placebo controlled trial of pelvic floor exercises in the treatment of genuine stress incontinence [abstract]. Neurourol Urodyn 1990;9(4):398–9.
Sand 1995134,298–300
Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor stimulation in the treatment of genuine stress incontinence: a multicentre placebo-controlled trial [abstract]. Neurourol Urodyn 1994;13(4):356–7.
Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor stimulation in the treatment of genuine stress incontinence: a multicentre placebo controlled trial [abstract no. 27]. Proceedings of the American Urogynecology Society (AUGS), 15th Annual Meeting, 21–24 Sepember 1994, Toronto, Canada.
*Sand PK, Richardson DA, Staskin DR, Swift SE, Appell RA, Whitmore KE, et al. Pelvic floor electrical stimulation in the treatment of genuine stress incontinence: a multicenter, placebo-controlled trial. Am J Obstet Gynecol 1995;173(1):72–9.
Whitmore KE, Staskin DR, Grigoriev VE, Appell RA, Sand PK, Ostergaard DR. Pelvic floor stimulation in the treatment of genuine stress incontinence: a multicenter placebo controlled trial [abstract no. 1050]. J Urol 1995;153(Suppl. 4):491.
Savage 2005166
Savage AM. Is lumbopelvic stability training (using the Pilates model) an effective treatment strategy for women with stress urinary incontinence? A review of the literature and report of a pilot study. J Assoc Chartered Physiother Womens Health 2005;97:33–48.
Seo 2004195
Seo JT, Yoon H, Kim YH. A randomized prospective study comparing new vaginal cone and FES-Biofeedback. Yonsei Med J 2004;45(5):879–84.
Shepherd 1983155,301
*Shepherd A, Montgomery E, Anderson RS. A pilot study of a pelvic exerciser in women with stress urinary incontinence. J Obstet Gynaecol 1983;3(3):201–2.
Shepherd AM, Montgomery E, Anderson RS. Treatment of genuine stress incontinence with a new perineometer: a series of graded exercises. Physiotherapy 1983;69(4):113.
Sherburn 2007182
Sherburn M, Galea M, Bø K, Bird M, Carey M. Pelvic floor muscle training or bladder training to treat stress urinary incontinence in elderly women: a single blind randomised controlled trial [abstract no. 49]. Neurourol Urodyn 2007;26(5):665–6.
Smith 1996177,302,303
Aaronson PS, Loehner D, Bingham W, Smith JJ. Intravaginal electrical stimulation in the treatment of genuine stress urinary incontinence and detrusor instability: a controlled study [abstract]. J Urol 1995;153(Suppl. 4):491.
*Smith JJ, III. Intravaginal stimulation randomized trial. J Urol 1996;155(1):127–30.
Smith JJ, Loehner D, Bingham W. Intravaginal electrical stimulation in the treatment of GSUI and DI: a controlled study [abstract no. 25]. Proceedings of the American Urogynecology Society (AUGS), 15th Annual Meeting, 21–24 September 1994, Toronto, Canada.
Swithinbank 2005119,304
*Swithinbank L, Hashim H, Abrams P. The effect of fluid intake on urinary symptoms in women. J Urol 2005;174(1):187–9.
Swithinbank LV, Rogers CA, Yang Q, Shepherd AM, Abrams P. Does the amount and type of fluid intake effect urinary symptoms in women? [abstract no. 104]. Neurourol Urodyn 1999;18(4):371–2.
Tapp 1987191
Tapp AJS, Williams S, Hills B, Cardozo LD. The role of physiotherapy in the treatment of genuine stress incontinence [abstract]. Proceedings of the International Continence Society (ICS), 17th Annual Meeting, 2-5 September 1987, Bristol, UK. pp. 204–5.
Tapp 1989185,305
Tapp AJS, Hills B, Cardozo L. Pelvic floor physiotherapy compared with the Burch colposuspension in the treatment of genuine stress incontinence [abstract]. Proceedings of the Silver Jubilee British Congress of Obstetrics and Gynaecology, 4–7 July 1989, London, UK. p. 65.
*Tapp AJS, Hills B, Cardozo L. Randomised study comparing pelvic floor physiotherapy with the Burch colposuspension [abstract]. Neurourol Urodyn 1989;8(4):356–7.
Taylor 1986156
Taylor K, Henderson J. Effects of biofeedback and urinary stress incontinence in older women. J Gerontol Nurs 1986;12(9):25–30.
Terry 1996193
Terry PB, Whyte SM. Randomised trial comparing enhance with physiotherapy for the treatment of GSI [abstract]. Proceedings of the International Continence Society (ICS), 26th Annual Meeting, 27–30 August 1996, Athens, Greece. pp. 248–9.
van Kerrebroeck 2004117,306
van Kerrebroeck P, Abrams P, Lange R, Slack M, Wyndaele J, Yalcin I, et al. Duloxetine vs placebo in the treatment of stress urinary incontinence: Phase 3 results from Europe and Canada [abstract]. Eur Urol Suppl 2003;2(1):29.
*van Kerrebroeck P, Abrams P, Lange R, Slack M, Wyndaele JJ, Yalcin I, et al. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG 2004;111(3):249–57.
Williams 2006129,307–311
Shaw C, Matthews RJ, Perry SI, Assassa RP, Williams K, McGrother C, et al. Validity and reliability of an interviewer-administered questionnaire to measure the severity of lower urinary tract symptoms of storage abnormality: the Leicester Urinary Symptom Questionnaire. BJU Int 2002;90(3):205–15.
Williams KS, Assassa RP, Smith N, Rippin C, Shaw C, Mayne C, et al. Good practice in continence care: development of nurse-led service. Br J Nurs 2002;11(8):548–59.
Williams K, Assassa RP, Cooper N, Turner D, Shaw C, Abrams K, et al. Randomised controlled trial of the clinical and cost effectiveness of existing continence services compared with a new nurse-led service [abstract]. Neurourol Urodyn 2003;22(5):440.
Williams KS, Assassa RP, Cooper NJ, Turner DA, Shaw C, Abrams KR, et al. Clinical and cost-effectiveness of a new nurse-led continence service: a randomised controlled trial. Br J Gen Pract 2005;55(518):696–703.
*Williams KS, Assassa RP, Gillies CL, Abrams KR, Turner DA, Shaw C, et al. A randomized controlled trial of the effectiveness of pelvic floor therapies for urodynamic stress and mixed incontinence. BJU Int 2006;98(5):1043–50.
Williams K, Coleby D, Abrams K, Shaw C, Assassa P, McGrother C. Randomised controlled trial of the clinical effectiveness of services for urinary symptoms: six year follow-up [abstract no. 45). Neurourol Urodyn 2007;26(5):660–1.
Wilson 1987157,312
Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown ADG. The value of physiotherapy in female genuine stress incontinence [abstract]. Proceedings of the International Continence Society (ICS), 14th Annual Meeting, 13–15 September 1984, Innsbruck, Austria. pp. 156–8.
*Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown AD. An objective assessment of physiotherapy for female genuine stress incontinence. Br J Obstet Gynaecol 1987;94(6):575–82.
Wilson 1998197,313
Wilson D, Herbison P, Borland M, Grant AM. A randomised controlled trial of physiotherapy treatment of postnatal urinary incontinence [abstract]. Proceedings of the British Congress of Obstetrics and Gynaecology, 26th Meeting, 7–10 July 1992, Manchester, UK. p. 162.
*Wilson PD, Herbison GP. A randomized controlled trial of pelvic floor muscle exercises to treat postnatal urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1998;9(5):257–64.
Wise 1993188
Wise BG, Haken J, Cardozo LD, Plevnik S. A comparative study of vaginal cone therapy, cones plus Kegel exercises, and maximal electrical stimulation in the treatment of female genuine stress incontinence [abstract no. 76]. Neurourol Urodyn 1993;12(4):436–7.
Woldringh 2007198
Woldringh C, van den WM, Albers-Heitner P, Nijeholt AA, Lagro-Janssen T. Pelvic floor muscle training is not effective in women with UI in pregnancy: a randomised controlled trial. Int Urogynecol J Pelvic Floor Dysfunct 2007;18(4):383–90.
Wong 1997a158
Wong KS, Fung BKY, Fung ESM, Fung LCW, Tang LCH. Randomized prospective study of the effectiveness of pelvic floor training using biofeedback in the treatment of genuine stress urinary incontinence in Chinese population [abstract]. Proceedings of the International Continence Society (ICS), 27th Annual Meeting, 23–26 September 1997, Yokohama, Japan. pp. 57–8.
Wong 1997b160
Wong KS, Fung BKY, Fung LCW, Ma S. Pelvic floor exercises in the treatment of stress urinary incontinence in Hong Kong Chinese women [abstract]. Proceedings of the International Continence Society (ICS), 27th Annual Meeting, 23–26 September 1997, Yokohama, Japan. pp. 62–3.
Wong 2001169
Wong KS, Fung KY, Fung SM, Fung CW, Tang CH. Biofeedback of pelvic floor muscles in the management of genuine stress incontinence in Chinese women. Physiotherapy 2001;87(12):644–8.
Wyman 1998183,314–318
Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC, Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol 2002;99(2):281–9.
Elser DM, Fantl JA, McClish DK. Comparison of “subjective” and “objective” measures of severity of urinary incontinence in women. Program for Women Research Group. Neurourol Urodyn 1995;14(4):311–16.
Elser DM, Wyman JF, McClish DK, Robinson D, Fantl JA, Bump RC. The effect of bladder training, pelvic floor muscle training, or combination training on urodynamic parameters in women with urinary incontinence. Continence Program for Women Research Group. Neurourol Urodyn 1999;18(5):427–36.
Theofrastous JP, Wyman JF, Bump RC, McClish DK, Elser DM, Bland DR, et al. Effects of pelvic floor muscle training on strength and predictors of response in the treatment of urinary incontinence. Neurourol Urodyn 2002;21(5):486–90.
*Wyman JF. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. Am J Obstet Gynecol 1998;179(4):999–1007.
Wyman JF, McClish DK, Sale P, Earle B, Camp J. Long-term follow-up of behavioral interventions in incontinent women [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 1999;10(Suppl. 1):33.
Zanetti 2007161
Zanetti MR, Castro RA, Rotta AL, Santos PD, Sartori M, Girao MJ. Impact of supervised physiotherapeutic pelvic floor exercises for treating female stress urinary incontinence. Sao Paulo Med J 2007;125(5):265–9.
Zinner 1998145,319,320
Yalcin I, DeBrota DJ, Thor KB. Incontinence severity index (ISI) in measuring efficacy of duloxetine in stress and mixed incontinent patients [abstract]. Proceedings of the International Continence Society (ICS), 28th Annual Meeting, 14–17 September 1998, Jerusalem, Israel. pp. 171–2.
Zinner N, Sarshik S, Yalcin I, Faries D, DeBrota D, Riedl P, et al. Evaluation of various efficacy measures from 140 stress and 146 mixed incontinence patients enrolled in a double-blind, placebo-controlled trial of duloxetine [abstract]. Proceedings of the International Continence Society (ICS), 28th Annual Meeting, 14–17 September 1998, Jerusalem, Israel. pp. 175–6.
*Zinner N, Sarshik S, Yalcin I, Faries D, Riedl P, Thor KB. Efficacy and safety of duloxetine in stress urinary incontinent patients: double-blind, placebo-controlled multiple dose study [abstract]. Proceedings of the International Continence Society (ICS), 28th Annual Meeting, 14–17 September 1998, Jerusalem, Israel. pp. 173–4.
Appendix 7 Examples of excluded studies with reasons for exclusion
| Study ID | Number of reports | Reasons for exclusion | |
|---|---|---|---|
| 1 | Alewijnse 2003321–323 | 3 | SUI 37%, MUI 31%, UUI 9%, unspecified UI 23%. No separate data for SUI. PFMT vs PFMT plus education (three versions). N = 132 |
| 2 | Barroso 2002324,325 | 2 | MUI and UUI over 70% of the sample. No separate data for SUI. ES vs sham ES. Includes one Portuguese publication |
| 3 | Borrie 1992326–330 | 5 | Treatments tailored according to UI diagnosis. Nurse-led intervention vs NT. Includes both men and women. The related paper by Bowden (1992) reports data for women |
| 4 | Brown 2006331 | 1 | Not all women had urinary incontinence at baseline. Data from 1987 overweight women at high risk for diabetes who were enrolled in the Diabetes Prevention Program (DPP) were analysed to determine whether an intensive lifestyle intervention with improved diet and increased physical activity or metformin therapy would be associated with lower prevalence of urinary incontinence compared with a standard lifestyle intervention alone. The three interventions included an intensive lifestyle intervention, metformin at 850 mg twice daily or placebo twice daily. All participants received standard lifestyle recommendations. Urinary incontinence was determined at the end-of-trial visit using a self-administered questionnaire |
| 5 | Burgio 1998332–335 | 4 | UUI or urge-predominant MUI. Behavioural training vs oxybutynin |
| 6 | Burgio 2002336,337 | 2 | UUI or urge-predominant MUI. Behavioural training vs behavioural training with BF vs self-administered behavioural training |
| 7 | de Gregorio 1993338 | 1 | PFMT + BF + ES vs ‘standard physiotherapy’. Comparator (‘standard physiotherapy’) not clearly described and unclear if it is intervention specified in review protocol. German publication. Women with USI |
| 8 | de Jong 2006339 | 1 | PFMT vs PFMT + whole body vibration. Whole body vibration is not an intervention specified in review protocol. Women with SUI |
| 9 | Demain 2001340 | 1 | Stress or urge UI. Proportions of SUI not reported. No separate data for SUI. Individual vs group sessions for PFMT + BT |
| 10 | Dougherty 1998341,342 | 2 | SUI 18%, MUI 66%, UUI 15%. No separate data for SUI. Behavioural management (e.g. caffeine consumption, dietary change, BT and PFMT with BF) vs no treatment. Not all patients in the intervention arm received PFMT. N = 217 |
| 11 | Foote 2000343 | 1 | Treatments tailored according to diagnosis. Interventions led by nurse continence advisors (NCA) vs standard care by urogynaecologists (UG). Abstract only. Cost-effectiveness analysis. UG group = PFMT and BT, and given VC or anticholinergic SNRIs when clinically indicated. NCA group = all the treatments for the UG group, and in addition, ES and BF were also offered. N = 150. |
| 12 | Foote 2007344 | 1 | Treatments tailored according to diagnosis: given PFMT, BF, BT and ES, and also given VC or anticholinergic SNRIs when clinically indicated. Nurse-led vs standard gynaecologist-led interventions. N = 145. USI, USI + DO or DO. No separate data for SUI |
| 13 | Galea 2006345 | 1 | Type of UI unspecified. Elderly women with incontinence. PFMT with digital palpation vs PFMT + BF |
| 14 | Goode 2003346 | 1 | Urge predominant. Behavioural vs SNRI therapy |
| 15 | Gorman 1995347 | 1 | Type of UI unspecified. Comparison of PFMT not taught or supervised by a health professional (PFMT via PC software or booklet) |
| 16 | Hill 2007348–350 | 3 | SUI, MUI or UUI. Proportions of SUI not reported. No separate data for SUI. Individual vs group sessions for PFMT + BT |
| 17 | Holtedahl 1998351 | 1 | Treatment tailored according to symptoms. Estriol to all women unless they refused or were well estrogenised. All patients were instructed in PFMT. Patients with urge or mixed incontinence were also instructed in BT. For ES, patients with urge incontinence received maximal stimulation, whereas patients with stress incontinence used long-term stimulation. Immediate vs deferred treatment. SUI 57%, MUI 36%, UUI 7% |
| 18 | Hui 2006352 | 1 | USI 21%, UUI 79%. No separate data for SUI. Video conference (PFMT, fluid management, BT) vs conventional management (PFMT, fluid management, BT) |
| 19 | Ishiko 2001353 | 1 | PFMT vs PFMT + estriol tablet. Route of administration unclear. Women with SUI |
| 20 | Janssen 2001354 | 1 | Treatment tailored according to symptoms. PFMT was taught to all but BT was taught to women who frequently voided. Group vs individual sessions. USI 58–61%, MUI 32%, UUI 7–10% |
| 21 | Jeyaseelan 2002355 | 1 | The title refers to stress incontinence but unclear if predominant SUI constituted 50% or more. PFMT + BF vs ES. N = 16. N in each arm not reported. No usable data |
| 22 | Johnson 2000356 | 1 | SUI 30%, MUI 50%, UUI 20%. No separate data for SUI. PFMT + BF vs PFMT. N randomised = 20 |
| 23 | Kim 2001357 | 1 | SUI or MUI. Proportions of SUI not reported. PFMT as part of the ‘continence efficacy intervention programme’ |
| 24 | Kincade 2007358,359 | 2 | Type of UI not specified. Includes two phases. Phase 1 (N = 224): self-monitoring (‘The Knack’, caffeine consumption, fluid intake, voiding frequency, constipation) vs deferred treatment. Phase 2 (N = 301): PFMT vs PFMT + BF vs attentional control (non-incontinence-related health education) |
| 25 | Kirschner-Hermanns 1995360 | 1 | The title refers to stress incontinence but unclear if predominant SUI constituted 50% or more. N = 43. Number in each arm not reported. Data presented as complete cohort. No usable data |
| 26 | Lee 2005361 | 1 | < 50% SUI. No separate data for SUI. PFMT + BT vs deferred treatment |
| 27 | Liebergall-Wischnitzer 2005362 | 1 | Female hospital employees with SUI or MUI. Proportions of SUI not reported. No separate data for SUI. PFMT vs the Paula method |
| 28 | Lin 2004363 | 1 | MUI 100%. Dominant symptom (stress or urge) unclear. PFMT at home vs PFMT monitored by nurse |
| 29 | Lo 2003364 | 1 | Stress or urge UI. Proportions of SUI not reported. No separate data for SUI. PFMT + ES vs PFMT |
| 30 | Lumley 2006365,366 | 2 | No mention of specific UI treatment in this report. PRISM study. Primary care and community-based strategies for postnatal depression |
| 31 | McFall 2000367–369 | 3 | Majority had MUI. Dominant symptom (stress or urge) unclear. Community-based educational intervention programme (PFMT, liquid intake, BT, etc.) vs deferred treatment |
| 32 | Moore 2003370,371 | 2 | Treatment tailored according to symptoms. Nurse-led vs urogynaecologist-led interventions (PFMT, ES, BT). USI 63–67%, MUI 20–25%, DO 7–8%, sensory urgency 3–5% |
| 33 | Mulcahy 1996372 | 1 | Included one male patient. Duloxetine vs placebo |
| 34 | Nieto Blanco 2007373 | 1 | Treatment tailored according to symptoms. PFMT for SUI and MUI and BT for UUI. Systematised nursing care vs conventional care. SUI 60%, MUI 26%, UUI 14%. Spanish publication |
| 35 | O’Brien 1991374–376 | 3 | Historical control? Participants were randomised to the treatment (PFMT) or control (deferred treatment) groups, and the control group was later added to the treatment group in data analysis. For those with symptoms of urge UI, BT was also offered. The study included both men and women. Some data were reported separately for women (SUI 55%, UUI 10%, MUI 31%, and other UI 4%) |
| 36 | Ocampo 2007377 | 1 | 33% (14/44) tested positive using stress test at baseline. No separate data for SUI. Group behavioural modification programme vs NT. N = 44 |
| 37 | Prashar 1997378–380 | 3 | SUI, MUI or UUI. Proportions of SUI not reported. No separate data for SUI. PFMT + plug vs plug. Data presented as complete cohort, not by group allocation |
| 38 | Prashar 1998381,382 | 2 | USI and/or DI or sensory urgency. Proportions of USI not reported. N = 127. Treatment by nurse (PFMT, ES, anticholinergic therapy, BT) vs standard care by urogynaecologist (PFMT, anticholinergic therapy, BT). Treatments tailored based on diagnosis (e.g. DI received BT and SNRI, those with weak PF muscle received ES, etc.) |
| 39 | Sam 2004383 | 1 | SUI or MUI. Proportions of SUI not reported. No separate data for SUI. PFMT + BF vs PFMT |
| 40 | Sherburn 2005384 | 1 | Stress and/or urge UI. Proportions of SUI not reported. No separate data for SUI. PFMT vs PFMT + visual feedback from ultrasound |
| 41 | Sherman 1997385 | 1 | USI or MUI. Proportions of SUI not reported. No separate data for SUI. N = 46. PFMT + BT with or without BF |
| 42 | Spruijt 2003386 | 1 | SUI 17%, MUI 66%, UUI 17%. No separate data for SUI. Elderly women. PFMT vs ES |
| 43 | Subak 2002387 | 1 | SUI 24%, MUI 37%, UUI 37%. No separate data for SUI. PFMT, BT vs deferred treatment |
| 44 | Subak 2005388 | 1 | 53% (25/47) are urge UI alone or urge-predominant MUI. No separate data for SUI. Weight loss vs deferred treatment |
| 45 | Sugaya 2003389 | 1 | Comparison of PFMT not taught or supervised by professional health-care provider. Leaflet PFMT vs leaflet PFMT plus device that prompts women to perform PFMT with cartoon character. 61% SUI, 39% SUI with occasional UUI but did not have uninhibited bladder contractions on cystometry |
| 46 | Tsai 2002390 | 1 | SUI 31%, MUI 51%, UUI 18%. No separate data for SUI. PFMT + BF vs PFMT |
| 47 | von der Heide 2003391 | 1 | Physical therapy with entire body vibration vs physical therapy followed by entire body vibration. Entire body vibration is not treatment specified in review protocol |
| 48 | Wagg 2007392 | 1 | 1175 women with frequency, emptying, stress, urge, unspecified incontinence, strain on urination and nocturia. Proportions of SUI not reported. Structured help vs leaflet. Patients received one or more of the following: PFMT, BF, BT, VC, ES |
| 49 | Wang 1997393 | 1 | Women with urge syndrome. Unclear if RCT for relevant intervention: participants in SNRI RCT were ‘assigned’ to either PFMT or BF. PFMT vs BF |
| 50 | Wells 1991394 | 1 | PFMT vs phenylpropanolamine hydrochloride. SNRI subject to recall and will likely be banned? Not intervention specified in review protocol. USI or MUI |
| 51 | Wilson 1997209,395–399 | 6 | Treatment tailored according to symptoms. In the intervention group, PFMT for all women but with addition of BT and caffeine restriction if symptoms of frequency or urgency. Compared with standard care. Pregnant women with UI. SUI 53%, MUI 32%, UUI 16% |
| 52 | Yoon 2003400 | 1 | Type of UI not specified. BT vs PFMT + BF |
| Awaiting assessment | |||
| 53 | Abel 1997401 | 1 | Danish publication. ES vs Sham ES |
| 54 | Kim 2006402 | 1 | Korean publication. Magnetic therapy followed by PFMT vs magnetic therapy followed by no treatment. Unclear if patients were randomised and if data were reported separately for PFMT. Awaiting author’s reply |
| 55 | Smidt 1997403 | 1 | Dutch publication. Physiotherapy vs physiotherapy + BF |
| 56 | Sung 2000404,405 | 2 | Korean publication. PFMT vs ES + BF. Unclear if patients were randomised. Awaiting author’s reply |
| Total number of reports | 86 | ||
Reference list for excluded studies
Alewijnse D, Mesters IEPE, Metsemakers JFM, van den Borne BHW. Program development for promoting adherence during and after exercise therapy for urinary incontinence. Patient Educ Couns 2002;48(2):147–60.
Alewijnse D, Mesters I, Metsemakers J, van den Borne B. Predictors of long-term adherence to pelvic floor muscle exercise therapy among women with urinary incontinence. Health Educ Res 2003;18(5):511–24.
Alewijnse D, Metsemakers JF, Mesters IE, van den Borne B. Effectiveness of pelvic floor muscle exercise therapy supplemented with a health education program to promote long-term adherence among women with urinary incontinence. Neurourol Urodyn 2003;22(4):284–95.
Barroso JCV, Ramos JGL. Estimulacao eletrica transvaginal no tratamento da incontinencia urinaria. Revista Brasileira de Gynecologia e Obstetricia 2002;24(10):685.
Barroso JC, Ramos JG, Martins-Costa S, Sanches PR, Muller AF. Transvaginal electrical stimulation in the treatment of urinary incontinence. BJU Int 2004;93(3):319–23.
Bawden ME, Kartha AS, Borrie MJ, Kerr PS, Durko NA, Haslam IF, et al. Treating women with stress incontinence in a multidisciplinary clinic: a randomized study [abstract no. 276]. Proceedings of the International Continence Society (ICS), 22nd Annual Meeting, 1–4 September 1992, Halifax, UK.
Borrie MJ, Bawden ME, Kartha AS, Kerr PS. A nurse/physician continence clinic triage approach for urinary incontinence: a 25 week randomized trial. Neurourol Urodyn 1992;11(4):364–5.
Borrie MJ, Bawden ME, Speechley M. Continence clinic randomized controlled trial using a nurse/physician triage approach: Two-year follow-up. Clin Invest Med 1995; (Suppl. 4):B59.
Borrie MJ, Bawden M, Speechley M, Kloseck M. Interventions led by nurse continence advisers in the management of urinary incontinence: a randomized controlled trial. CMAJ 2002;166(10):1267–73.
Brown JS, Wing R, Barrett-Connor E, Nyberg LM, Kusek JW, Orchard TJ, et al. Lifestyle intervention is associated with lower prevalence of urinary incontinence: the Diabetes Prevention Program. Diabetes Care 2006;29(2):385–90.
Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Dombrowski M, et al. Behavioral vs SNRI treatment for urge urinary incontinence in older women. JAMA 1998;280(23):1995–2000.
Burgio KL, Locher JL, Goode PS. Combined behavioral and SNRI therapy for urge incontinence in older women. J Am Geriatr Soc 2000;48(4):370–4.
Burgio KL, Locher JL, Roth DL, Goode PS. Psychological improvements associated with behavioral and SNRI treatment of urge incontinence in older women. J Gerontol B Psychol Sci Soc Sci 2001;56(1):46–51.
Burgio KL, Goode PS, Locher JL, Umlauf MG, Roth DL, Richter HE, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: a randomized controlled trial. JAMA 2002;288(18):2293–9.
Burgio KL, Goode PS, Locher JL, Richter HE, Roth DL, Wright KC, et al. Predictors of outcome in the behavioral treatment of urinary incontinence in women. Obstet Gynecol 2003;102(5):940–7.
De Gregorio G, Krahmann H, Bernhard A. The efficacy of pelvic floor reeducation. Randomized study. Arch Gynecol Obstet 1993;254(1–4):504–6.
de Jong JH, Van Kampen M, Biemans B. The effect of whole body vibration training on women with stress urinary incontinence [abstract no. 416]. Proceedings of the International Continence Society (ICS), 36th Annual Meeting, 27 November–1 December 2006, Christchurch, New Zealand.
Demain S, Fereday Smith J, Hiller L, Dziedzic K. Comparison of group and individual physiotherapy for female urinary incontinence in primary care. Physiotherapy 2001;87(5):235–42.
Dougherty MC, Dwyer JW, Pendergast JF, Tomlinson BU, Boyington AR, Vogel WB, et al. Community-based nursing: continence care for older rural women. Nurs Outlook 1998;46(5):233–44.
Dougherty MC, Dwyer JW, Pendergast JF, Boyington AR, Tomlinson BU, Coward RT, et al. A randomized trial of behavioral management for continence with older rural women. Res Nurs Health 2002;25(1):3–13.
Dowell CJ, Bryant CM, Moore KH, Prashar S. The efficacy and user friendliness of the urethral occlusive device [abstract]. Proceedings of the International Continence Society (ICS), 27th Annual Meeting, 23–26 September 1997, Yokohama, Japan. pp. 295–6.
Foote AJ, Moore KH. Qalys: an objective continence outcome measure to determine the cost effectiveness of conservative urogynaecological treatments [abstract no. 109]. Neurourol Urodyn 2000;19(4):518–19.
Foote AJ, Moore KH. The cost of urogynaecological treatments: which are more cost-effective? Aust NZ J Obstet Gynaecol 2007;47(3):240–6.
Galea M, Tisseverasinghe S, Sherburn M, Phillips B. Motor skill training of the pelvic floor muscles using visual versus tactile feedback [abstract no. 459]. Proceedings of the International Continence Society (ICS), 36th Annual Meeting, 27 November–1 December 2006, Christchurch, New Zealand.
Glazener CM, Herbison GP, Wilson PD, MacArthur C, Lang GD, Gee H, et al. Conservative management of persistent postnatal urinary and faecal incontinence: randomised controlled trial. BMJ 2001;323(7313):593–6.
Glazener CM, Herbison GP, Wilson PD, MacArthur C, Lang GD, Gee H, et al. Conservative management of persistent postnatal urinary and faecal incontinence: randomised controlled trial [extended electronic version]. eBMJ 2001;323:1–5.
Glazener CM, Herbison GP, MacArthur C, Grant A, Wilson PD. Randomised controlled trial of conservative management of postnatal urinary and faecal incontinence: six year follow up. BMJ 2005;330(7487):337–40.
Goode PS. Behavioral and SNRI therapy for urinary incontinence. Urology 2004;63(Suppl. 3A):58–64.
Goode PS, Burgio KL, Locher JL, Umlauf MG, Lloyd LK, Roth DL. Urodynamic changes associated with behavioral and SNRI treatment of urge incontinence in older women. J Am Geriatr Soc 2002;50(5):808–16.
Gorman R. Expert system for management of urinary incontinence in women. Proc Annu Symp Comput Appl Med Care 1995;5:27–31.
Hill LA, Fereday-Smith J, Credgington C, Woodward AF, Knight JC, Williams AJ, et al. Bladders behaving badly: a randomized controlled trial of group versus individual interventions in the management of female urinary incontinence. J Assoc Chartered Physiother Womens Health 2007;101:30–6.
Holtedahl K, Verelst M, Schiefloe A. A population based, randomized, controlled trial of conservative treatment for urinary incontinence in women. Acta Obstet Gynecol Scand 1998;77(6):671–7.
Hui E, Lee PS, Woo J. Management of urinary incontinence in older women using videoconferencing versus conventional management: a randomized controlled trial. J Telemed Telecare 2006;12(7):343–7.
Ishiko O, Hirai K, Sumi T, Tatsuta I, Ogita S. Hormone replacement therapy plus pelvic floor muscle exercise for postmenopausal stress incontinence. A randomized, controlled trial. J Reprod Med 2001;46(3):213–20.
Janssen CCM, Lagro-Janssen ALM, Felling AJA. The effects of physiotherapy for female urinary incontinence: Individual compared with group treatment. BJU Int 2001;87(3):201-6.
Jeyaseelan S, Haslam J, Oldham J. Can the effects of pelvic floor muscle exercises be enhanced with a new pattern of electrical stimulation in women with stress incontinence? Pilot data [abstract no. 135]. Proceedings of the International Continence Society (ICS), 32nd Annual Meeting, 28–30 August 2002, Heidelberg, Germany. pp. 66–7.
Johnson JL, King Baker T. Biofeedback versus verbal instruction for pelvic floor training in the treatment of urinary incontinence. J Womens Health Phys Ther 2000;24(3):7–13.
Kartha AS, Borrie MJ, Bawden ME, Kerr PS. The impact of treatment on quality of life in a randomized clinical study of incontinent adults [abstract no. 287). Proceedings of the International Continence Society (ICS), 22nd Annual Meeting, 1–4 September 1992, Halifax, UK.
Kim J. Continence efficacy intervention program for community residing women with stress urinary incontinence in Japan. Public Health Nurs 2001;18(1):64–72.
Kincade JE, Dougherty MC, Carlson JR, Hunter GS, Busby-Whitehead J. Randomized clinical trial of efficacy of self-monitoring techniques to treat urinary incontinence in women. Neurourol Urodyn 2007;26(4):507–11.
Kincade JE, Dougherty MC, Carlson JR, Wells EC, Hunter GS, Busby-Whitehead J. Factors related to urinary incontinence in community-dwelling women. Urol Nurs 2007;27(4):307–17.
Kirschner-Hermanns R, Niehaus S, Schafer W, Jakse G. Pelvic floor re-education in female stress-incontinence I. and II. follow-up results (mean 43 months) [abstract no. 216]. Proceedings of the International Continence Society (ICS), 25th Annual Meeting, 17–20 October 1995, Sydney, Australia. pp. 230-1.
Lee C, Johnson C, Chiarelli P. Women’s waterworks: evaluating an early intervention for incontinence among adult women. Aust NZ Continence J 2005;11(1):11–16.
Liebergall-Wischnitzer M, Hochner-Celnikier D, Lavy Y, Manor O, Arbel R, Paltiel O. Paula method of circular muscle exercises for urinary stress incontinence: a clinical trial. Int Urogynecol J Pelvic Floor Dysfunct 2005;16(5):345–51.
Lin TL, Chen YC, Hu SW, Chen GD. Nursing intervention to enforce the efficacy of home practice of pelvic floor muscle exercise in mixed incontinence [abstract no. 294]. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 23–27 August 2004, Paris, France.
Lo SK, Naidu J, Cao Y. Additive effect of interferential therapy over pelvic poor exercise alone in the treatment of female urinary stress and urge incontinence: a randomized controlled trial. Hong Kong Physiother J 2003;21:37–42.
Lumley J, Small R, Brown S, Watson L, Gunn J, Mitchell C, et al. PRISM (Program of Resources, Information and Support for Mothers) Protocol for a community-randomised trial [ISRCTN03464021]. BMC Public Health 2003;3(1):36.
Lumley J, Watson L, Small R, Brown S, Mitchell C, Gunn J. PRISM (Program of Resources, Information and Support for Mothers): a community-randomised trial to reduce depression and improve women’s physical health six months after birth [ISRCTN03464021]. BMC Public Health 2006;6(37).
McFall SL, Yerkes AM, Belzer JA, Cowan LD. Urinary incontinence and quality of life in older women: a community demonstration in Oklahoma. Fam Community Health 1994;17(1):64–75.
McFall SL, Yerkes AM, Cowan LD. Outcomes of a small group educational intervention for urinary incontinence: episodes of incontinence and other urinary symptoms. J Aging Health 2000;12(2):250–67.
McFall SL, Yerkes AM, Cowan LD. Outcomes of a small group educational intervention for urinary incontinence: health-related quality of life. J Aging Health 2000;12(3):301–17.
Moore KH, Simons A, Dowell C, Bryant C, Prashar S. Efficacy and user acceptability of the urethral occlusive device in women with urinary incontinence. J Urol 1999;162(2):464–8.
Moore KH, O’Sullivan RJ, Simons A, Prashar S, Anderson P, Louey M. Randomised controlled trial of nurse continence advisor therapy compared with standard urogynaecology regimen for conservative incontinence treatment: efficacy, costs and two year follow up. BJOG 2003;110(7):649–57.
Mulcahy JJ, Laddu AR, Faries DE, DeBrota DJ, Kirkemo AK, Rudy DC, et al. Efficacy and safety of duloxetine in stress incontinence patients [abstract]. Neurourol Urodyn 1996;15(4):395–6.
Nieto Blanco E, Moriano Bejar P, Serrano Molina L, Davila Alvarez V, Perez Llorente M. [Efficiency of a nursing clinical trial on the treatment of female urinary incontinence.] [Spanish] Actas Urol Esp 2007;31(5):493–501.
O’Brien J, Austin M, Sethi P, O’Boyle P. Urinary incontinence: prevalence, need for treatment, and effectiveness of intervention by nurse. BMJ 1991;303(6813):1308–12.
O’Brien J, Long H. Urinary incontinence: long term effectiveness of nursing intervention in primary care. BMJ 1995;311(7014):1208.
O’Brien J. Evaluating primary care interventions for incontinence. Nurs Stand 1996;10(23):40–3.
O’Sullivan R, Anderson P, Louey M, Prashar S, Simons A, Bower W, et al. Long term results of a randomised controlled trial of the nurse continence advisor versus the urogynaecologist in conservative therapy [abstract no. 212]. Proceedings of the International Continence Society (ICS), 30th Annual Meeting, 28–31 August 2000, Tampere, Finland.
O’Sullivan R, Simons A, Prashar S, Anderson P, Louey M, Moore KH. Is objective cure of mild undifferentiated incontinence more readily achieved than that of moderate incontinence? Costs and 2-year outcome. Int Urogynecol J Pelvic Floor Dysfunct 2003;14(3):193–8.
Ocampo MS, Diokno AC, Ibrahim IA, Karl CR, Lajiness MJ, Hall SA. Group session teaching of behavioral modification program (BMP) for urinary incontinence (UI): A randomized, controlled trial among incontinent women [abstract no. 1348]. J Urol 2007;177(4 Suppl.):444.
Pepper J, Lamb SE, Doughty G, Fereday Smith J. Group treatment: an acceptable and effective method of physiotherapy for bladder problems. J Assoc Chartered Physiother Womens Health 2003;93:15–18.
Pepper J, Lamb SE, Fereday Smith J, Doughty G. Female urinary incontinence: women’s preferences for group or individual treatment [abstract no.: poster 31]. Proceedings of the Chartered Society for Physiotherapy Annual Congress, 17–19 October 2003, Birmingham, UK. p. 63.
Prashar S, Moore K, Bryant C, Dowell C. The urethral occlusive device for the treatment of urinary incontinence: changes in quality of life [abstract]. Int Urogynecol J Pelvic Floor Dysfunct 1997;8(1):S130.
Prashar S, Moore K, Anderson P, Louey M, Cragg S, Simons AM, et al. A randomized controlled trial of nurse continence advisor management versus urogynaecology management of conservative continence therapy: benefits and costs [abstract]. Neurourol Urodyn 1998;17(4):423–4.
Rennie AM, Wilson D, Glazener C, Gee H, Lang G, MacArthur C. A multicentre randomised trial of treatment of postnatal incontinence [abstract]. Proceedings of the International Confederation of Midwives, 24th Triennial Congress, 26–31 May 1996, Oslo, Norway. p. 8.
Sam C, Umek W, Uorfler D, Hanzal E. Outpatient pelvic floor exercises versus home biofeedback (PELVEXT) [abstract no. 557]. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 23–27 August 2004, Paris, France.
Sherburn M, Tisseverasinghe S, Phillips B, Galea MP. Ultrasound visual feedback may be as effective as digital vaginal palpation for pelvic floor muscle training [abstract no. 613]. Proceedings of the International Continence Society (ICS), 35th Annual Meeting, 28 August–2 September 2005, Montreal, Canada.
Sherman RA, Davis GD, Wong MF. Behavioral treatment of exercise-induced urinary incontinence among female soldiers. Mil Med 1997;162(10):690–4.
Spruijt J, Vierhout M, Verstraeten R, Janssens J, Burger C. Vaginal electrical stimulation of the pelvic floor: a randomized feasibility study in urinary incontinent elderly women. Acta Obstet Gynecol Scand 2003;82(11):1043–8.
Subak LL, Quesenberry CP, Posner SF, Cattolica E, Soghikian K. The effect of behavioral therapy on urinary incontinence: a randomized controlled trial. Obstet Gynecol 2002;100(1):72–8.
Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol 2005;174(1):190–5.
Sugaya K, Owan T, Hatano T, Nishijima S, Miyazato M, Mukouyama H, et al. Device to promote pelvic floor muscle training for stress incontinence. Int J Urol 2003;10(8):416–22.
Tsai C, Engberg S. Is biofeedback-assisted pelvic floor muscle training (PFMT) more effective than verbal instruction in teaching pelvic floor muscle utilization and continence control? … a randomised prospective study [abstract no. 138]. Proceedings of the International Continence Society (ICS), 32nd Annual Meeting, 28–30 August 2002, Heidelberg, Germany. pp. 68–70.
von der Heide S, Emons G, Hilgers R, Viereck V. Effect on muscles of mechanical vibrations produced by the Galileo 2000 in combination with physical therapy in treating female stress urinary incontinence [abstract]. Proceedings of the International Continence Society (ICS), 33rd Annual Meeting, 5–9 October 2003, Florence Italy. pp. 192–3.
Wagg AR, Barron D, Kirby M, Stott D, Corlett K. A randomised partially controlled trial to assess the impact of self-help vs structured help from a continence nurse specialist in women with undiagnosed urinary problems in primary care. Int J Clin Pract 2007;61(11):1863–73.
Wang AC, Liang CC. Bladder sphincter biofeedback as treatment of detrusor instability in women who failed to respond to oxybutynin chloride: a preliminary results [abstract]. Proceedings of the International Continence Society (ICS), 27th Annual Meeting, 23–26 September 1997, Yokohama, Japan. pp. 162–3.
Wells TJ, Brink CA, Diokno AC, Wolfe R, Gillis GL. Pelvic muscle exercise for stress urinary incontinence in elderly women. J Am Geriatr Soc 1991;39(8):785–91.
Wilson PD, Herbison GP, Glazener CMA, Lang G, Gee H, MacArthur C. Postnatal incontinence: a multi centre, randomised controlled trial of conservative treatment [abstract]. Neurourol Urodyn 1997;16(5):349–50.
Wilson PD, Glazener C, McGee M, Herbison P, MacArthur C, Grant A. Randomised controlled trial of conservative management of postnatal urinary and faecal incontinence: long term follow-up study [abstract]. Neurourol Urodyn 2002;21(4):370.
Yoon HS, Song HH, Ro YJ. A comparison of effectiveness of bladder training and pelvic muscle exercise on female urinary incontinence. Int J Nurs Stud 2003;40(1):45–50.
Appendix 8 List of identified ongoing and unpublished trials
| Project lead | Identifiers | Start date | End date | Location | Sample size | Description of intervention | Status of project |
|---|---|---|---|---|---|---|---|
| Eli Lilly | F1J-MC-SBBT | April 2003 | February 2005 | Unknown | Unclear | Duloxetine 80 mg day as 40 mg b.i.d. vs placebo | Unpublished |
| Eli Lilly | F1J-EW-SBCC | January 2003 | June 2006 | Unknown | Unclear | Duloxetine 80 mg day as 40 mg b.i.d. vs placebo | Unpublished |
| Eli Lilly | F1J-BI-SBCM | October 2005 | January 2007 | Unknown | Unclear | Duloxetine 40 mg as 20 mg b.i.d. to 80 mg taken as 40 mg b.i.d. vs placebo | Unpublished |
| Pfizer | NCT00138749 | November 2004 | August 2006 | Unknown | 402 | Reboxetine vs placebo | Unpublished |
| Pfizer | NCT00141128 | December 2005 | Unknown | Denmark | 40 | Reboxetine vs placebo | Unpublished |
| Harvey | NCT00247286 | September 2001 | October 2006 | Canada | 36 | VC vs PFMT + BF | Unpublished |
| McGrother | ISRCTN28188933 | 1999 | 2001 | UK | 432 | Topical estrogen ring vs placebo | Unpublished |
| Whelpton | ISRCTN59388318 | 2003 | 2006 | UK | 40 | PFMT + BF vs PFMT | Unpublished |
| Moran | ISRCTN37726767 | November 2003 | February 2006 | UK | 100 | PFMT + BF vs PFMT | Unpublished |
| Elton | ISRCTN15411586 | October 2005 | November 2005 | UK | 1430 | Survey with self-lead questions vs no self-lead | Unpublished |
| Bolderstone | N0128081147 | July 2000 | June 2003 | UK | 250 | PFMT + ES vs PFMT | Unpublished |
| Crothers | N0411063811 | February 2001 | January 2002 | UK | Unclear | Cueing device to enhance patient compliance to PFMT vs no cue | Unpublished |
| Watson | NCT00323245 | March 2006 | September 2008 | Canada | 48 | Conservative management of UI for osteoporosis patients | Ongoing |
| Weber/Meikle | NCT00270998 | June 2005 | December 2008 | USA | 450 | PFMT + pessary vs PFMT vs pessary | Ongoing |
| Culligan | NCT00549458 | April 2006 | March 2008 | USA | 60 | Pilates vs PFMT | Ongoing |
Appendix 9 Assessment of risk of bias in included studies
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8a | Q8b | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aksac 2003120 | U | N | U | U | U | Y | N | U | U |
| 2 | Arvonen 2001178 | U | U | N | N | N | Y | Y | N | Y |
| 3 | Aukee 2002146 | Y | U | U | U | U | Y | Y | Y | Y |
| 4 | Berghmans 1996147 | U | N | N | N | Y | Y | Y | Y | Y |
| 5 | Bernardes 2000174 | U | U | U | U | U | Y | N | U | U |
| 6 | Bidmead 002121 | U | U | U | U | Y | Y | U | U | Y |
| 7 | Blowman 1991189 | U | U | Y | Y | Y | Y | Y | N | Y |
| 8 | Bø 1990115 | U | U | U | U | U | Y | Y | N | Y |
| 9 | Bø 1999115 | Y | Y | N | N | Y | Y | Y | N | Y |
| 10 | Borello-France 2006165 | Y | U | U | N | U | Y | U | U | Y |
| 11 | Bourcier 1994196 | U | U | U | U | U | Y | U | N | Y |
| 12 | Brubaker 1997130 | Y | U | Y | N | Y | Y | U | N | Y |
| 13 | Bump 2004136 | U | U | Y | U | U | Y | N | U | U |
| 14 | Burns 1993122 | U | U | N | N | Y | Y | U | N | Y |
| 15 | Burton 1993173 | U | U | U | U | U | Y | N | U | Y |
| 16 | Cammu 1998181 | Y | Y | U | U | U | N | Y | Y | Y |
| 17 | Cardozo 2004137 | Y | Y | Y | U | U | Y | Y | N | Y |
| 18 | Castleden 1984148 | U | U | U | U | U | Y | N | U | Y |
| 19 | Castro-Diaz 2007138 | U | U | Y | U | U | Y | Y | N | Y |
| 20 | Delneri 2000186 | U | U | U | U | U | Y | N | U | Y |
| 21 | Dmochowski 2003139 | Y | Y | Y | U | U | Y | Y | N | Y |
| 22 | Dumoulin 2004199 | Y | Y | N | N | Y | Y | Y | N | Y |
| 23 | Edwards 2000170 | U | U | U | U | U | Y | N | U | U |
| 24 | Fantl 1991135 | U | U | U | U | U | Y | U | N | Y |
| 25 | Ferguson 1990149 | U | U | U | U | U | Y | N | Y | Y |
| 26 | Gallo 1997162 | N | N | U | U | U | Y | U | N | Y |
| 27 | Ghoniem 200557 | Y | Y | Y | Ya | N | Y | Y | N | Y |
| 28 | Glavind 1996150 | U | N | U | U | U | Y | U | N | Y |
| 29 | Goode 2003123 | Y | U | U | U | U | Y | Y | Y | Y |
| 30 | Hahn 1991175 | U | U | N | U | U | Y | Y | Y | Y |
| 31 | Haig 1995190 | N | N | U | U | U | Y | U | N | Y |
| 32 | Haken 1991179 | U | U | U | N | U | Y | U | U | Y |
| 33 | Hay-Smith 2003164 | Y | Y | N | N | Y | Y | Y | N | Y |
| 34 | Henalla 1989124 | U | U | U | U | U | Y | N | N | Y |
| 35 | Henalla 1990125 | U | U | U | U | U | Y | N | Y | Y |
| 36 | Hofbauer 1990126 | U | U | U | U | U | Y | N | Y | Y |
| 37 | Jeyaseelan 2000131 | Y | U | Y | U | Y | Y | Y | N | Y |
| 38 | Johnson 2001167 | Y | U | U | N | U | Y | Y | N | Y |
| 39 | Karagkounis 2007194 | U | U | U | U | U | Y | N | U | Y |
| 40 | Kim 2007118 | Y | U | U | U | U | Y | Y | N | Y |
| 41 | Kinchen 2005140 | Y | Y | Y | Y | U | N | Y | N | Y |
| 42 | Klarskov 1986184 | U | U | U | U | U | Y | U | Y | Y |
| 43 | Klingler 1995151 | U | U | U | U | U | Y | N | Y | Y |
| 44 | Knight 1998172 | Y | N | U | U | U | Y | U | U | Y |
| 45 | Konstantinidou 2007116 | N | N | U | N | N | Y | Y | N | Y |
| 46 | Lagro-Janssen 1991127 | N | N | U | U | Y | Y | Y | Y | Y |
| 47 | Laycock 1988176 | U | U | U | U | U | Y | U | N | Y |
| 48 | Laycock Trial 1 1993132 | U | U | N | N | N | Y | U | N | Y |
| 49 | Laycock Trial 2 1993132 | U | U | Y | N | N | Y | U | N | Y |
| 50 | Laycock 2001152 | Y | U | U | U | U | Y | U | N | Y |
| 51 | Luber 1997133 | U | Y | Y | Y | Y | Y | Y | N | Y |
| 52 | Mah 2006141 | Y | Y | Y | U | U | Y | U | N | Y |
| 53 | Manning 2005142 | U | U | Y | U | U | Y | U | U | Y |
| 54 | Mayne 1988168 | U | U | U | U | U | Y | U | N | Y |
| 55 | Millard 2004143 | Y | Y | Y | U | U | Y | Y | N | Y |
| 56 | Miller 1998107 | U | U | U | N | U | Y | U | U | Y |
| 57 | Mørkved 2002153 | U | Y | N | N | Y | Y | Y | U | Y |
| 58 | Norton 2002144 | Y | Y | Y | Y | U | Y | U | N | Y |
| 59 | Nygaard 1996163 | Y | U | U | U | Y | Y | U | Y | U |
| 60 | Oláh 1990187 | U | U | U | U | U | Y | Y | N | Y |
| 61 | Pages 2001154 | Y | U | U | U | U | Y | U | N | Y |
| 62 | Peattie 1988180 | U | U | U | U | U | Y | U | N | Y |
| 63 | Pieber 1995192 | Y | U | U | U | U | Y | U | Y | Y |
| 64 | Pohl 2004171 | U | U | U | U | U | Y | N | U | U |
| 65 | Ramsay 1990128 | U | U | Y | N | Y | Y | Y | Y | Y |
| 66 | Sand 1995134 | U | U | Y | U | Y | Y | Y | U | Y |
| 67 | Savage 2005166 | U | U | U | U | Y | Y | Y | N | Y |
| 68 | Seo 2004195 | U | U | U | U | U | Y | N | U | U |
| 69 | Shepherd 1983155 | U | U | U | U | U | Y | U | Y | U |
| 70 | Sherburn 2007182 | Y | Y | N | N | Y | Y | N | Y | Y |
| 71 | Smith 1996177 | U | U | U | U | U | Y | N | Y | Y |
| 72 | Swithinbank 2005119 | U | U | N | U | U | Y | U | N | U |
| 73 | Tapp 1987191 | U | U | U | U | U | Y | U | Y | Y |
| 74 | Tapp 1989185 | U | U | U | U | U | Y | U | N | Y |
| 75 | Taylor 1986156 | U | U | U | U | U | Y | Y | N | U |
| 76 | Terry 1996193 | U | U | U | U | U | Y | U | U | U |
| 77 | van Kerrebroeck 2004117 | Y | Y | Y | U | U | Y | Y | N | Y |
| 78 | Williams 2006129 | Y | Y | N | N | U | Y | U | N | Y |
| 79 | Wilson 1987157 | N | N | U | U | U | Y | N | U | Y |
| 80 | Wilson 1998197 | Y | U | U | U | U | Y | Y | N | Y |
| 81 | Wise 1993188 | U | U | U | U | U | Y | U | N | Y |
| 82 | Woldringh 2007198 | Y | U | U | U | U | Y | Y | N | Y |
| 83 | Wong 1997a158 | U | U | U | U | U | Y | N | U | Y |
| 84 | Wong 1997b160 | U | U | U | U | U | Y | N | U | U |
| 85 | Wong 2001169 | Y | U | U | U | U | Y | N | U | U |
| 86 | Wyman 1998183 | U | N | N | Y | N | Y | Y | N | Y |
| 87 | Zanetti 2007161 | Y | U | U | N | U | Y | N | U | Y |
| 88 | Zinner 1998145 | U | U | Y | U | U | Y | U | N | Y |
| 88 | 88 | 88 | 88 | 88 | 88 | 88 | 88 | 88 | ||
| Totals per category | ||||||||||
| Y | 30 | 16 | 19 | 6 | 17 | 86 | 33 | 18 | 75 | |
| U | 53 | 62 | 56 | 63 | 65 | 0 | 33 | 24 | 13 | |
| N | 5 | 10 | 13 | 19 | 6 | 2 | 22 | 46 | 0 | |
| Totals per category (%) | ||||||||||
| Y | 34.1 | 18.2 | 21.6 | 6.8 | 19.3 | 97.7 | 37.5 | 20.5 | 85.2 | |
| U | 60.2 | 70.5 | 63.6 | 71.6 | 73.9 | 0.0 | 37.5 | 27.3 | 14.8 | |
| N | 5.7 | 11.4 | 14.8 | 21.6 | 6.8 | 2.3 | 25.0 | 52.3 | 0.0 | |
Appendix 10 Characteristics of included studies: summary of efficacy and adverse events
Studies of non-pregnant women
| Study | Participant characteristics | Intervention/comparator | Objective outcomes | Subjective outcomes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Aksac 2003 120 Study design/method: 3-arm RCT, parallel design. Single centre, Turkey Duration of study: 8 weeks |
Inclusion criteria: Women with USI Exclusion criteria: Not reported N randomised: 50 N lost to follow-up: Not reported Type of incontinence: USI. Type of USI (0/1/2): I: 7/8/5, II: 4/9/7, III: 3/5/2, p > 0.05 Age (years, mean, SD): I: 52.5 (7.9), II: 51.6 (5.8), III: 54.7 (7.8), p > 0.05 Severity of incontinence: see type of SUI above Other: Body weight, parity, % postmenopausal 100% |
1. PFMT taught via digital palpation, N = 20 2. PFMT taught via BF, N = 20 3. No treatment, N = 10 PFMT + digital palpation: Voluntary pelvic floor muscle contraction (VPFMC) taught via digital palpation technique. Relaxation of abdominal and buttock muscles. Set: 10 VPFMC, with 5-second hold and 10-second rest. Progressed at 2 weeks to 10-second hold and 20-second rest. Sets per day: 3. Duration of training: 8 weeks. Supervision: Weekly clinic visits PFMT + BF: VPFMC taught via biofeedback (Myomed-932, vaginal probe); unclear if home or clinic based. Set: 20 minutes consisting of 40 VPFMC with 10 seconds hold and 20 seconds rest. Repeat 3 times per week for 8 weeks. Supervision: Weekly clinic visits with a therapist Control subjects: No exercises Additional information: All women were postmenopausal and on hormone replacement therapy (estradiol hemihydrate 2 mg/day and norethisterone acetate 1 mg/day) |
Objective Cure (≤ 1 g on 1-hour pad test): I: 15/20, II: 16/20, III: 0/10 Improvement (≥ 50% reduction in pad weight; not including ‘cure’): I: 5/20, II: 4/20, III: 2/10 Episodes of leakage (4-point ordinal scale, 1–4, median, SD): I: before 2.3 (0.7), after 3.5 (0.5), p < 0.001, II: before 2.3 (0.6), after 3.6 (0.4), p < 0.001, III: before 2.1 (0.9), after 2.4 (0.9), p > 0.05 Note: Ordinal scale. 1 = once a day, 2 = more than once a week, 3 = less than once a week, 4 = once a month 1-hour pad test (g, median, SD): I: before 19.9 (2.5), after 2.1 (0.4), p < 0.001, II: before 20.5 (1.7), after 1.2 (0.2), p < 0.001, III: before 29.1 (3.2), after 28.2 (3.7), p > 0.05 Surrogate outcomes Vaginal squeeze pressure (cmH2O, median, SD): I: before 20.3 (6.2), after 37.5 (8.7), p < 0.001, II: before 19.1 (4.8), after 50.0 (11.5), p < 0.001, III: before 18.7 (4.9), after 20.0 (3.9), p > 0.05 Digital palpation score (6-point ordinal scale, 0-5, median, SD): I: before 3.5 (0.5), after 4.8 (0.4), p < 0.001, II: before 3.3 (0.4), after 4.9 (0.2), p < 0.001, III: before 3.3 (0.4), after 3.3 (0.6), p > 0.05 |
Quality of life Social Activity Index (visual analogue scale, median, SD): I: before 4.5 (0.3), after 7.5 (1.2), p < 0.001, II: before 3.5 (0.4), after 8.1 (0.8), p < 0.001, III: before 3.6 (0.7), after 3.6 (0.6), p > 0.05; groups I and II better than group III after treatment (p < 0.001) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Arvonen 2001 178 Study design/method: 2-arm RCT. Single centre, Sweden Duration of study: 4 months |
Inclusion criteria: Women with symptoms of stress incontinence, age under 65, and an understanding of spoken Swedish Exclusion criteria: Pregnancy, cysto/rectocoele, prolapse, urinary tract infection, altered vaginal tissue, and medication affecting the functioning of the urinary tract or kidneys N randomised: 40 N lost to follow-up: I: 2/20, II: 1/20 Type of incontinence: SUI Age (years, median, range): I: 49 (32–64), II: 47 (28–65), NS Pad test (short provocation test with a standard 300-ml in bladder – g, median, range): I: 30 (2–170), II: 20 (3–80) Pelvic floor muscle strength (vaginal palpation; score range 0–5, median, range): I: 3 (1–4), II: 3 (1–3), NS Other: BMI, parity |
I. VC (balls), N = 18 II. PFMT, N = 19 (N in analysis) Vaginal balls: Starting with a ball (Vagitrim, Ipex Medical AB, Stockholm) weighing 65 g, 10 maximum VPFMC 20 seconds’ hold, 20 seconds’ rest, 2 times a day. In addition, sub-maximum VPFMC was performed by retaining the 50-g ball while moving (e.g. walking, housekeeping and doing gymnastics) once a day for 15 minutes. After 2 months the balls were replaced by ones weighing 100 g for maximum squeeze and 80 g for submaximal squeeze. Three clinic visits with physiotherapist and nurse over 4 months PFMT: Set: 10 maximum VPFMC while sitting, 5 seconds’ hold, 5 seconds’ rest, with a short break after 5 squeezes. Repeated while standing. Sets per day: 2. In addition, 15 submaximum VPFMC with 3 seconds’ hold, 3 seconds’ rest, once a day, and one static 2-minute sub-maximum PFM squeeze, once a day. Duration of training: 4 months. Supervision: 3 clinic visits with physiotherapist and nurse over 4 month Additional information: Two women who suffered from asthma encountered difficulties in using the balls because of hard coughing |
Objective No leakage (0 g on pad test) (short provocation test with a standard 300 ml in bladder): I: 9/18, II: 5/19 1- to 10-g leakage pad test (short provocation test with a standard 300-ml in bladder): I: 4/18, II: 7/19 Pad test (short provocation test with a standard 300 ml in bladder; g, median, range): I: 1 (0–100), II: 5 (0–90), p = 0.03 Surrogate outcomes Pelvic floor muscle strength (vaginal palpation; score range 0–5, 0 = no contraction, 5 = very strong pressure with a strong lift for 6–7 seconds; median, range): I: 4 (1–5), II: 3 (1–5), NS Women in the vaginal ball group ‘were not able to keep the ball inside without dropping it several times during a single set of maximum squeezes’ (p. 597) Adverse events N experiencing adverse events: I: 0/20, II: 0/20 Adverse events: I: ‘no feeling of pain while using the ball was reported’ (p. 597), II: 0/20 Discontinued treatment because of adverse events: I: 0/20, II: 0/20 |
Subjective Subjective self-rated experience of improvement (4-point scale) – ‘Good (fully recovered)’: I: 4/18, II: 0/19 Note: ‘Good (fully recovered)’ vs improved, unchanged or worse Subjective self-rated experience of improvement (4-point scale) – ‘Good (fully recovered)’ or ‘Improved’: I: 11/18, II: 11/19 Note: ‘Good (fully recovered)’ or improved vs unchanged or worse Patient satisfaction: PFMT ‘was considered satisfactory because it could be performed as part of other activities’ (p. 597) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Aukee 2002 146 Study design/method: 2-arm RCT. Single centre, Finland Duration of study: 12 weeks and 1-year follow-up |
Inclusion criteria: Women aged 21–70 with USI, without previous incontinence operations, maximal urethral closure pressure over 20 cmH2O and cough leak point pressure over 90 cmH2O Exclusion criteria: Genital protrusion beyond the vaginal hymen, inability to understand instructions for home training, pregnancy, severe diseases such as malignancies in the abdominal region, multiple sclerosis and diabetes mellitus requiring insulin N randomised: 30 for 12 week data (Aukee 2002146); 35 for 1 year data (Aukee 2004234) N lost to follow-up: 0/30 Type of incontinence: USI Age (years, mean, range): I: 15, 51.8 (35 to 61), II: 15, 50.8 (31 to 69) Leakage index score (mean, SD, range): I: 15, 45.5 ± 10.1, II: 15, 38.5 ± 11.0, p = 0.003 24-hour pad test (g, mean, SD). I: 15, 28.1 ± 29.4, II: 15, 47.1 ± 34.6, p < 0.001 Pelvic floor muscle activity while supine (μV): I: 15, 15.3 ± 4.4, II: 15, 17.8 ± 6.8 Pelvic floor muscle activity while standing (μV): I: 15, 13.5 ± 4.7, II: 15, 14.7 ± 7.2 Other: BMI, parity (vaginal deliveries), N of postmenopausal women |
I. PFMT + BF, N = 15 II. PFMT, N = 15 (N in analysis) PFMT + BF: All women were taught PFMT by the same physiotherapist, using electromyography (EMG) BF treatment in five office visits during a 12-week period. After 12 weeks, patients advised to continue training themselves. At the first visit, patients familiarised with location of levator ani muscle and pelvic anatomy. Home PFMT = verbal and written instructions to practice 20 minutes per day, five times per week over 12 weeks. BF = individual EMG-assisted home BF device (FemiScan, Mega-Electronics, Kuopio, Finland) with a vaginal prove and headphones. The device emits a voice signal if the contraction is too weak. At each session, the home-training devices were downloaded and the registered data were checked PFMT: All women were taught PFMT by the same physiotherapist in five office visits during a 12-week period. Home PFMT = verbal and written instructions to practice 20 minutes per day, five times per week over 12 weeks. After 12 weeks, patients were advised to continue training themselves |
Objective 24-hour pad test at 12 weeks (g, mean, SD): I: 15, 19.0 ± 19.7, II: 15, 22.5 ± 19.6 Note: Baseline values were significantly lower for BF group; no between-group difference after adjustment (p = 0.907) Surrogate outcomes Adherence at 12 weeks (N of training sessions recorded by BF device, mean, range): I: 68 (9–130), II: NA Adherence at 12 weeks (N of days exercised without the device, daily log written down by women, average, range) I: 47.5 (6–93), II: 56.2 (21–87) Pelvic floor muscle activity while supine at 12 weeks (μV, mean, SD): I: 25.8 (10.0), II: 20.1 (8.6) Pelvic floor muscle activity while standing at 12 weeks (μV, mean, SD): I: 21.4 (10.3), II: 20.9 (8.6) Pelvic floor muscle activity while supine at 1 year (patients not receiving surgery only; μV, mean, SD): I: 11, 23.8 ± 8.55, II: 10, 24.65 ± 9.08 Long term (1 year) N having surgery or awaiting surgery at 1 year: I: 5/16, II: 9/19 Adverse events N experiencing adverse events at 12 weeks: I: 4/15, II: 3/15 indicated pain while training. Of which 3/7 premenopausal Discontinuation due to adverse events at 12 weeks: I: 2/15, II: 0/15. The two patients in the BF group undertook PFMT only instead and were analysed on an intent-to treat basis. They were both post-menopausal and found the vaginal probe to be uncomfortable |
Quality of life Leakage Index (Bø 1994) at 12 weeks (13 types of physical exertions that trigger urinary leakage with decrease in values reflecting improvement; score, mean, SD): I: 15, 34.9 ± 10.4, II: 15, 38.1 ± 10.5 Note: Baseline values were significantly higher for BF group; no between-group difference after adjustment (p = 0.068) Satisfaction with PFMT at 1 year: % considering PFMT effective: 23/35 (67%), group breakdown not available % considering biofeedback PFMT harmful: 0/35 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Berghmans 1996 147 Study design/method: 2-arm RCT. Stratified by severity of leakage and source of referral. Two sites in the Netherlands Duration of study: 4 weeks |
Inclusion criteria: Women aged 18–70 years old, with either mild or moderate forms of stress incontinence. Some with urodynamically proven genuine stress incontinence (GSI) and others with clinical history suggestive of GSI. Able to fill out forms and willing to participate Exclusion criteria: Medication for lower urinary tract problems, pudendal nerve lesion, urinary tract infection, non-compliance in diagnostic phase, previous urological or gynaecological surgery, < 6 weeks postnatal, concomitant treatment for stress incontinence, severe stress incontinence, psychological disorders, vaginitis, pacemaker, hip prosthesis, unable to understand Dutch N randomised: 40 N lost to follow-up: 0/40 Type of incontinence: USI or SUI (mild/moderate): I: 11/9, II: 11/9 Note: Mild = grade 1: < 20 g/48 hours; moderate = grade 2: 20–100 g/48 hours (Mulder and Vierhout 1990) Age (years, mean, SD): I: 50.4 ± 10.5, II: 46.4 ± 12.1 48-hour pad test (g, mean SD) I: 29.0 ± 31.7, II: 26.6 ± 24.5 Episodes of leakage in 24 hours (mean, SD) I: 3.0 ± 3.4, II: 2.0 ± 2.1 N of pad changes in 24 hours (mean, SD): I: 2.6 (1.2), II: 2.4 (1.8) Symptom scores: I: 23.8 ± 6.9, II: 22.1 ± 4.6 Incontinence assessment: (N finding their daily activities altered:) I: 12/20, II: 12/20 N finding their social consequences altered: I: 11/20, II: 9/20; N finding self-worth altered: I: 11/20, II: 14/20 Other: Mean body weight, % prior incontinence surgery, micturitions per day |
I. PFMT, N = 20 II. PFMT + BF, N = 20 (N in analysis) PFMT: Clinic visits three times per week for 4 weeks with 25- to 35-minutes per visit. Explanation of pelvic anatomy, function of PFM and bladder. PFMT = 4 sets of 10 VPFMC (5 quick, 5 sustained, 3–30 seconds) progressed by 10 per set until 30 per set. Exercise in supine, side, standing and crawling positions. ‘The Knack’ (VPFMC with cough, stair climbing, lifting, jumping) also included. Digital palpation performed weekly by physiotherapist using PERFECT Assessment Scheme (Laycock 1992) and received home exercise programme to practice three times per day PFMT + BF: PFMT as above with addition of BF from vaginal probe (electromyographic) giving both visual and acoustic signals (Myaction 12, Uniphy BV) |
Objective Cure based on 48-hour pad test: I: 3/20, II: 5/20 Cure or improvement based on 48-hour pad test: I: 17/20, II: 19/20 Episodes of leakage in 24 hours (mean, SD): I: 1.4 ± 3.5, II: 0.8 ± 1.3 48-hour pad test (g, mean, SD): I: 12.5 ± 12.0, II: 12.2 ± 15.4 Adverse events Discontinued treatment because of adverse events: none |
Quality of life Symptom questionnaire score (a modification of the standardised PRAFAB score) (Mulder and Vierhout 1990), range 5–50 with higher scores meaning more serious problems; mean, SD): I: 13.1 ± 8.6, II: 11.1 ± 5.9 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Bernardes 2000 174 Study design/method: 2-arm RCT. Portugal – Portuguese publication Duration of study: 10 days |
Inclusion criteria: Women with USI (grade 1, 2 or 3 of pelvic muscle strength according to criteria by Oritz 1994); no surgical treatment for stress UI; no use of hormonal therapy Exclusion criteria: Pregnancy, current use of intrauterine device, use of pacemaker, urological disease, orthopaedic diseases of shoulder, stress UI grade > 3 of pelvic muscle strength, no compliance to protocol, previous physiotherapy for stress UI N randomised: 14 N lost to follow-up: Not reported Type of incontinence: USI Age (years, median, range): I: 44.1 (31–59), II: 53.3 (45–64), NS Severity of incontinence (light loss/moderate loss): I: 3 light, 4 moderate; II: 2 light, 5 moderate Pelvic floor muscle strength grade: I: grade 0, 4/7; grade 1, 2/7; grade 3, 1/7; II: grade 0, 6/7; grade 1, 1/7; grade 3. 0/7 Other: Weight, parity (% multiparous) |
I. PFMT, N = 7 II. ES, N = 7 (N in analysis) PFMT: In the first ‘experimental’ session women did six types of exercises, including two for abdominal, two for pelvic floor muscles and two for gluteus and pelvic floor muscles to see how they adapted to the programme. This was followed by 10 consecutive sessions (by the same physical therapist), involving three sets of six exercises as above, 10 times each. Daily home exercise included micturition control and perineal reinforcement. Women were evaluated at the first consultation and after the 10-day treatment ES: Dualpex (Quark Medical products) with perineal intrauterine electrode. Frequency 30 Hz, pulse 1 millisecond, sustained for 6 seconds and relaxed for 12 seconds. Contraction 10–30 mA. Treatment for 20 minutes. 10 consecutive sessions. Individualised intensity, progressing to 60 Hz. Two evaluation sessions before and after the 10-day treatment Additional information: 21 women were eligible, of whom seven did not comply with the protocol (especially urodynamic testing), as many of them were from lower social classes and could not afford treatments/tests |
Surrogate outcomes Pelvic floor muscle strength (N who had strength grade 2?): I: 4/7, II: 7/7 |
Subjective Patient perception (no symptom or no loss of urine): I: 5/7, II: 2/7 Patient perception (light loss of urine, i.e. when sneezing, strong cough or weight-lifting): I: 2/7, II: 4/7 Patient perception (moderate loss of urine, i.e. during cough, laughter or daily activity): I: 0/7, II: 1/7 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Bidmead 2002121 (abstract only) Study design/method: 4-arm RCT, parallel design. Single centre, UK Duration of study: 14 weeks |
Inclusion criteria: Women who had a new diagnosis of USI or had no treatment in previous 6 months Exclusion criteria: Not reported N randomised: 173 (recruited over 4 years) N lost to follow-up: 44/173 (I: 10, II: 15, III: 12, IV: 7); Authors reported ‘no statistical differences between women withdrawing and completing or between withdrawals across the treatment groups’ Type of incontinence: USI Age (years, mean, SD): I: 46.2 (8.5), II: 50.4 (11.5), III: 51.5 (9.7), IV: 47.5 (11.5) Pad weight (standardised pad test, g, mean, SD): I: 12.0 (3.3), II: 10.0 (1.6), III: 10.0 (2.6), IV: 8.0 (4.9) |
I. PFMT, N = 40 II. PFMT + ES, N = 82 III. PFMT + sham ES, N = 42 IV. No treatment, N = 20 (N in analysis) PFMT: ‘Conventional’ PFMT supervised by physiotherapist. Set: NR. Sets per day: NR. Duration of training: 14 weeks. Supervision: five clinic visits in 14 weeks (weeks 1, 3, 6, 10 and 14). Also given individually tailored lifestyle advice PFMT + ES: PFMT, lifestyle advice and clinic visits as above. ES = Uromax stimulator with a periform intravaginal electrode. At home PFMT + sham ES: PFMT, lifestyle advice and clinic visits as above. Sham ES = manufactured to be identical to the active device Control subjects: Deferred treatment and then crossover to PFMT + ES group with reassessment Additional information: Total N = 184, 11 more than randomised (N = 173); some of the control patients crossed over to group II? Awaiting author’s reply. Data extracted from Parsons (2004)236 |
Objective Pad weight change (fixed volume pad test with half-hour exercise programme, g, mean change, SE): I: 40, –9.62 (3.37), II: 82, –5.74 (1.91), III: 42, –2.01 (2.15), IV: 20, +3.65 (1.71) Surrogate outcomes Adherence – PFMT (excellent/good): I: 30/40, II: 65/88, III: 33/42 Adherence – ES (excellent/good): I: NA, II: 40/88, III: 19/42 Note: Excellent = performed daily; good = performed more than three times a week; poor = less often; unrecorded = not recorded or withdrawn. Based on patients’ diaries |
Quality of life King’s Health Questionnaire (mean score increase, Wilcoxon signed-rank test): Significant subjective improvement across most domains, for the PFMT + ES group and the PFMT group, but this was not mirrored by the PFMT + sham ES group. See table in paper for details |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Blowman 1991 189 Study design/method: 2-arm RCT Duration of study: ?4–6 weeks treatment + follow-up at 6 months |
Inclusion criteria: Women having USI without significant prolapse Exclusion criteria: Not reported N randomised: 14 N lost to follow-up: I: 0/7, II: 1/7, due to poor compliance judged from the electric stimulator Type of incontinence: USI Age (years, median, range): I: 45.0 (33–68), II: 42.5 (38–64) Episodes of leakage per week (median, range): I: 5 (1–14), II: 12.5 (1–31) N of micturition per week (median, range): I: 51 (36–63), II: 61.5 (53–84) Other: Height, weight, % prior incontinence surgery, parity |
I. PFMT + ES, N = 7 II. PFMT + sham ES, N = 6 (N in analysis) PFMT: Correct VPFMC taught by an obstetric physiotherapist with visual feedback using a perineometer. Set: Home exercise (1) five VPFMC, hold for five counts, increasing hold time to 10 counts. (2) 10 quick VPFMC, progressing to 20 and increasing speed and N of contractions. (3) Tighten the pelvic floor muscle in five small steps and then relax in five steps; repeat five times. Sets per day: 4 times a day. Duration of training: ?4 weeks. Self-test to be done 1 month after exercise starts (squatting, jumping, etc.). If not dry, continue exercise. Supervision: Fortnightly visits to the hospital with the same physiotherapist. ES: Neurotrophic stimulation, a method of neuromuscular electrical (NME) stimulation. Home stimulator, 60 minutes per day for 4 weeks, with the cathode placed over perineal area and buttocks. Frequency 10 Hz, 4-second hold, 4-second relax, pulse width 80 microseconds. Further therapy using a higher frequency of 35 Hz (all other parameters as before) given for 15 minutes a day over 2 weeks. Patients told to turn up the amplitude control until they just became aware of some electrical sensation. The stimulation sensation was minimal, not enough to cause a pelvic floor contraction and comfortable enough to be ignored for the duration of treatment. The stimulator had the facility to check the patients’ compliance, i.e. the N of times and the N of hours they used the stimulator. This was checked every fortnight Sham ES: Told to set the amplitude control to 3 Additional information: Apparent error in table 2 (wrong rows) |
Objective Cure at 4–6 weeks (leakage episodes reduced to zero, no accidents per week): I: 6/7, II: 1/6 Episodes of leakage per week at 4–6 weeks (1-week continence chart, median, range): I: 7, 0 (0–1), II: 6, 6 (0–21), p < 0.05 N of micturition per week at 4–6 weeks (1-week continence chart, median, range): I: 7, 41 (37–56), II: 6, 51, (44–57) Surrogate outcomes Adherence: See ‘lost to follow-up’ Maximum perineometer reading at 4-6 weeks (range 0–16, median, range): I: 7, 12 (5–16), II: 6, 9 (5–15) Maximum perineometer reading for 10 seconds hold at 4–6 weeks (scale 0–16, median, range): I: 7, 5 (2–16), II: 6, 5 (3–13) Return of symptoms at 6 months (no change since end of study/deteriorated/no reply on questionnaire): I: 4/2/1, II: 3/1/2 Adverse events N experiencing adverse events at 4–6 weeks: I: 0/7, II: 0/7. On direct questioning none of the patients reported any discomfort or side effects from the ES Discontinued treatment because of adverse events at 4–6 weeks: I: 0/7, II: 0/7 |
Subjective N requiring further treatment: I: 0/7, II: 4/6, i.e. better for group I (active ES) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Bø 1990 159 Study design/method: 2-arm RCT. Stratified by degree of leakage. Single centre, Norway Duration of study: Treatment 6 months. Follow-up 5 years (intensive PFMT group only) and at 15 years (both groups) |
Inclusion criteria: Women with USI Exclusion criteria: Detrusor instability or infection N randomised: 57 N lost to follow-up: I: 3/26, II: 2/31 Type of incontinence: USI Age (years, mean, range): I: 23, 44.9 (24–64), II: 29, 45.9 (35–63), NS 90-second pad test (g, mean, 95% CI): I: 23, 27.0 (8.8 to 45.1), II: 29, 29.5 (14.5 to 44.0) Social Activity Index (sum score, mean, 95% CI): I: 23, 7.7 (7.1 to 8.3), II: 29, 7.8 (7.2 to 8.4) Urinary Leakage Index (mean, 95% CI): I: 23, 3.0 (2.8 to 3.3), II: 29, 3.1 (2.9 to 3.3) Maximal pelvic floor muscle strength (cmH2O, mean, 95% CI, range): I: 23, 7.0 (4–10), II: 29, 7.9 (5.5–10.3), NS Other: BMI, % prior incontinence surgery, parity (mean N of children), % postmenopausal |
I. PFMT, intensive exercise, N = 23 II. PFMT, home exercise only, N = 29 (N in analysis) PFMT, intensive exercise: Education, home PFMT and monthly clinic visits as below, with addition of a special PFMT exercise course, training with instructor in groups 45 minutes once a week for 6 months. Course included sets of 8–12 VPFMC (as hard as possible) with 6- to 8-second holds in standing, sitting, lying, and kneeling with legs apart. 3–4 fast contractions added after held contraction PFMT, home exercise only: Individual instruction in pelvic anatomy and correct VPFMC with physiotherapist. Home PFMT 6 months, with monthly clinic visit for measurement of pelvic floor muscle strength using a vaginal balloon catheter connected to a pressure transducer. PFMT = 8–12 strong contractions per set, 3 times a day. Frequency of exercise was notified in a training diary |
Objective 90-second pad test at 6 months (g, mean, 95% CI): I: 23, 7.1 (0.8 to 13.4), II: 29, 23.0 (9 to 35.0); data for group II obtained from graph Surrogate outcomes Adherence at 6 months: ‘The attendance rate for both groups to the home exercise programme and for the IE (intensive exercise) group to the weekly group exercise was close to 100%’ Adherence at 5 years (PFMT at least once a week): I: 16/23, II: not reported Adherence at 15 years (PFMT at least once a week): I: 8/21, II: 5/26, p = 0.20; of those who exercised, the mean (SD) number of VPFMC per set was 12 (9.7), and the mean (SD) reported holding time was 9 (6.6) seconds Maximal pelvic floor muscle strength at 6 months (cmH2O, mean, 95% CI): I: 23, 22.5 (17.7, 27.3), II: 29, 15.3 (12.0, 18.6) Long term (15 years) N having incontinence surgery: Within the first 5 years after treatment, I: 3/21, II: 9/26; 5–15 years after treatment, I: 8/21, II: 4/26 Adverse events N experiencing adverse events: At 15 years of those that underwent surgery (I: 11/21, II: 13/26) 21% reported Adverse events, bladder emptying being the most common complaint |
Subjective Patients’ own assessment at 6 months (continent/almost continent/some improvement/unchanged/worse): I: 2/12/8/1/0, II: 0/5/14/10/0 Quality of life Social Activity Index at 6 months (sum score, mean, 95% CI): I: 23, 9.3 (9.0, 9.6), II: 29, 8.2 (7.4, 8.9) Note: Women’s perceived problems in participation in 9 different social situations registered on a 10-cm visual analogue scale (0 = impossible to take part, 10 = no problem in taking part). The nine social situations include: at work; while dancing; at the cinema, theatre, etc.; while hiking; in group exercise; other social situations (e.g. parties); in connection with sexual activity; attending educational courses; and on a bus/train, etc. Urinary Leakage Index at 6 months (mean, 95% CI): I: 23, 1.9 (1.6 to 2.2), II: 29, 2.6 (2.2 to 3.0); 95% CI obtained from graph Note: The degree of stress urinary incontinence during sneezing, coughing, laughing, walking, walking downhill, running, jumping and lifting on a 5-point scale (5 = always, 1 = never). The mean was calculated as an overall index 15 years’ follow-up (questionnaire) Severity Index (Sandvik et al. 1993; 200013) at 15 years (dry/slight/moderate/ severe/very severe): I: 6/4/9/1/0, II: 4/4/10/6/1, p = 0.34 Urinary Leakage Index at 15 years (median, range): I: 21, 1.9 (1.0, 2.6), II: 26, 1.9 (1.0, 4.7), p = 0.14 International Consultation on Incontinence Questionnaire – Urinary Incontinence Short Form (ICIQ-UI SF) at 15 years (N of women with score 0–1 taken to mean no interference on everyday life): I: 8/21, II: 14/26, p = 0.53 Note: International Consultation of Incontinence Questionnaire Urinary Incontinence Short Form; 10-point scale (0 = not at all, 10 = a great deal) Stress urinary incontinence last month during physical activity (self-report at 15 years): All respondents, I: 8/21, II: 8/26, p = 0.80, non-operated women only, I: 5/10, II: 4/13 Stress urinary incontinence last month during coughing or sneezing (self-report at 15 years): All respondents, I: 11/21, II: 13/26, p = 1.0, non-operated women only, I: 8/10, II: 7/13 Self-reported pad use at 15 years: ‘Never or only during physical activity’, I: 12/21, II: 9/26, p = 0.15; ‘always’, I: 3/21, II: 7/26, p = 0.47 Satisfaction at 15 years (‘satisfied or almost satisfied today’): all respondents, I: 17/21, II: 19/26, p = 0.21; non-operated women only, I: 8/10, II: 10/13, p-value not reported Subgroup analysis At 6 months, the ‘responders’ to intensive PFMT (group I) were significantly older, had a longer history of SUI symptoms, a higher BMI score, stronger pelvic floor muscles, and a lower resting maximum urethral pressure (MUP) before treatment than the ‘borderline responders’ Participants in group I were classified as ‘responders’ (15/23), ‘borderline responders’ (8/23) and ‘non-responders’ (0/23), using a cumulative score of the five parameters including improvement on pad test, improvement by the social activity index, etc. At 15 years, no differences were found in urinary symptoms (pad use, leakage during ‘physical activity’ and ‘coughing or sneezing’), satisfaction or training adherence between operated and non-operated women. Operated women were more likely to report severe incontinence and interference with daily life than non-operated women |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Bø 1999 115 Study design/method: 4-arm RCT, stratified by severity of leakage on pad test. Five centres, Norway Duration of study: 6 months |
Inclusion criteria: Women with USI, waiting for surgery or recruited through advertising, > 4-g leakage on pad test with standardised bladder volume Exclusion criteria: other types of incontinence, DO on urodynamics, residual urine > 50 ml, maximum uroflow < 15 ml/second, previous surgery for USI, neurological or psychiatric disease, ongoing urinary tract infection, other disease that could interfere with participation, use of concomitant treatments during trial, inability to understand instructions given in Norwegian N randomised: 122 N lost to follow-up: I: 4/29, II: 7/32, III: 2/29, IV: 2/32 Type of incontinence: USI Age (years, mean, SD): 49.6 (10.0), II: 47.2 (10.1), III: 49.2 (10.6), IV: 51.7 (8.8) Episodes of leakage in 3 days (mean, SD): I: 2.0 (1.8), II: 2.3 (2.0), III: 2.7 (2.4), IV: 2.9 (2.9) Leakage Index (mean, SD): I: 2.8 (0.6), II: 2.7 (0.5), III: 3.0 (0.6), IV: 3.0 (0.7) Social Activity Index (mean, SD): I: 8.7 (1.2), II: 8.2 (1.2), III: 8.3 (1.1), IV: 8.1 (2.3) Fixed volume stress pad test (60 seconds, g, mean, SD): I: 38.6 (34.7), II: 56.0 (53.7), III: 48.4 (51.2), IV: 51.4 (48.2) Pad test (24 hours, g, mean, SD): I: 14.5 (15.2), II: 20.9 (15.5), III: 52.3 (158.3), IV: 42.5 (116.1) The Norwegian version of the Quality of Life Scale (QoL-N, mean, SEM): I: 25, 85.3 (1.6), IV: 30, 82.3 (2.6) Other: BMI, parity, % postmenopausal |
I. PFMT, N = 25 II. ES, N = 25 III. VC, N = 27 IV. Control subjects, N = 30 (N in analysis) All participants (including controls): Explanation of anatomy, physiology, and continence mechanism by physiotherapist. Correct pelvic floor muscle contractions taught and confirmed by vaginal palpation PFMT: Correct VPFMC taught by physiotherapist and confirmed by palpation. Set: 8–12 high-intensity (close to maximal) VPFMC, with 6- to 8-second hold and 3–4 fast contractions added at the end of each hold, 6-second rest between contractions. Body position: included lying, kneeling, sitting, standing; all with legs apart. Women used preferred position. Audiotape of home training programme. A training diary was kept. Sets per day: three. Duration of training: 6 months. Supervision: Weekly 45-minute exercise class to music, with PFMT in a variety of body positions, and back, abdominal, buttock and thigh muscle exercises. Monthly clinic visit with physiotherapist ES: Maximum intermittent vaginal stimulation with MS106 Twin (Vitacon AS), 50-Hz, pulse width 0.2 ms, current 0–120 mA, 30 minutes every day. Treatment adherence electronically monitored and recorded. Monthly clinic visit with physiotherapist VC: 20 minutes per day. Mabella cones. Three cylindrical weights: 20, 40 and 70 g. Adherence noted in a training diary. Monthly clinic visit with physiotherapist Control subjects: No clinic visits. Offered instruction in use of the Continence Guard (14 accepted) Additional information: Not reported |
Objective N cured (≤ 2-g leakage on pad test with standardised bladder volume): I: 11/25, II: 7/25, III: 4/27, IV: 2/30 Episodes of leakage in 3 days (diary, mean change, 95% CI): I: –1.2 (–2.0 to –0.4), II: –0.7 (–1.5 to 1.1), III: 0.8 (–1.2 to 2.8), IV: 0.3 (–0.5 to 1.1) Stress pad test (fixed volume, 60 second) (g, mean change, 95% CI): I: –30.2 (–43.3 to 16.9), II: –7.4 (–20.9, 6.1), III: –14.7 (–27.6 to –1.8), IV: –12.7 (–27.2 to 1.8) 24-hour pad test (at home) (g, mean change, 95% CI): I: –6.6 (–12.1 to –1.1), II: –0.5 (–8.9 to 7.9), III: –22.0 (–55.7 to 11.7), IV: –7.1 (–20.2 to 6.0) Surrogate outcomes Treatment adherence (training diary or electronic record) (%, SE): I: 93 (1.5), II: 75 (2.8), III: 78 (4.4), IV: not reported Vaginal squeeze pressure (cm water, mean, 95% CI): I: 19.2 (15.3 to 23.1), II: 18.6 (13.3 to 23.9), III: 15.4 (11.1 to 19.7), IV: 16.4 (12.8 to 20.0) Note: Data for I–III from text, IV imputed from figure Adverse events Adverse events: I: 0/29, II: 10/32, III: 18/29, IV: 0/32 Adverse events: smarting (tenderness, bleeding, discomfort), abdominal pain, vaginitis, motivation problem, difficulty in using equipment Discontinued medication because of adverse events: I: 1/29, II: 7/32, III: 1/29, IV: 0/32 |
Subjective Self-rated cure/improvement: IIIIIIIVContinent2100Almost continent10251Improved1113120Unchanged271026Worse0203Total25252730 N reporting ‘unproblematic’: I: 14/25, II: 3/25, III: 2/27, IV: 1/30 Note: Recorded on a 5-point scale: unproblematic, minimal problem, moderate problem, problematic, very problematic Leakage Index (5-point scale, 5 = always, 1 = never; mean change, 95% CI): I: –0.9 (–1.1 to –0.7), II: –0.2 (–0.4 to 0.0), III: –0.3 (–0.5 to 0.1), IV: 0.1 (–0.1 to 0.3) Quality of life Social Activity Index (10-cm analogue scale, 0 = impossible to participate, 10 = no problem taking part; mean change, 95% CI): I: 25, 0.6 (0.2 to 1.0), II: 25, 0.6 (0.2 to 1.0), III: 27, 0.1 (–0.3 to 0.5), IV: 30, –0.2 (–0.8 to 0.4) Bristol Female Lower Urinary Tract Symptoms (B-FLUTS, ‘a little/somewhat/a lot’ or ‘a bit of a problem/quite a problem/a serious problem’):Note: Lifestyle questions (28–31, 33) and sex life questions (21–24) only The Norwegian version of the Quality of Life Scale (QoLS-N, mean, SEM): I: 90.1 (1.9), IV: 85.2 (2.2) Note: The higher the score, the better the Quality of life N desiring further treatment: I: 4/25, II: 19/25, III: 23/27, IV: 28/30 Note: No desire = more satisfied |
I | II | III | IV | Continent | 2 | 1 | 0 | 0 | Almost continent | 10 | 2 | 5 | 1 | Improved | 11 | 13 | 12 | 0 | Unchanged | 2 | 7 | 10 | 26 | Worse | 0 | 2 | 0 | 3 | Total | 25 | 25 | 27 | 30 | |||||||||||||||||||||
| I | II | III | IV | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Continent | 2 | 1 | 0 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Almost continent | 10 | 2 | 5 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Improved | 11 | 13 | 12 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unchanged | 2 | 7 | 10 | 26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Worse | 0 | 2 | 0 | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 25 | 25 | 27 | 30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Borello-France 2006 165 Study design/method: 2-arm RCT, USA Duration of study: 9–12 weeks (randomisation at week 3) |
Inclusion criteria: Women aged 38–70, not pregnant, ambulatory, symptoms of SUI occurring at least once a week, no symptoms of urgency or urge UI Exclusion criteria: Prior treatments for SUI (including surgery), been taught or prescribed PFMT by a health-care professional, having a pacemaker, using an intrauterine device, or having a medical history of pelvic cancer, severe endometriosis or neurological or metabolic disorders likely to impair bladder or sphincter function, any episodes of urge UI recorded on bladder diary, vaginal wall prolapse beyond the vaginal introitus, inability to demonstrate a palpable pelvic floor muscle contraction, sensory loss below the L4 dermatome, atrophic vaginitis or skin breakdown around the perineum, lumbosacral or pelvic pain or dysfunction that would interfere with PFMT, or the inability to tolerate the supine position, demonstrated urodynamic detrusor instability or an abdominal leak pressure of less than 60 cm of H2O N randomised: 44 N lost to follow-up: I: 5/22, II: 3/22 Type of incontinence (SUI/USI): I: 10/12, II: 8/14, p = 0.54 Age (years, mean, SD, range): I: 51.7 (8.9) (39–68), II: 53.6 (8.1) (39–68), p = 0.42 Episodes of leakage per week (mean, SD, range): I: 6.9 (7.0) (1.0–28.0), II: 7.2 (5.5) (1.0–21.0), p = 0.86 Pad test (g, mean, SD, range): I: 4.0 (5.2) (0.0–18.6), II: 11.7 (27.7) (0.0–120.5), p = 0.22 IIQ score (mean, SD, range): I: 54.9 (54.6) (0.0–208.0), II: 54.4 (53.8) (0.0–187.0), p = 0.99 Brink score (mean, SD, range): I: 8.6 (2.2) (5–12), II: 8.4 (2.4) (3–13), p = 0.89 Other: Parity – median 2, % postmenopausal – 57% |
I. PFMT in supine position, N = 22 II. PFMT in supine and upright position, N = 22 PFMT in supine position + BF: Education of pelvic floor muscle anatomy and physiology. Teaching of 3-second maximum contractions and 12-second contractions before randomisation at week 3. Correct VPFMC taught using a Pathway Dual-Channel Surface Biofeedback System, interfaced with Synergy Plus Software (both Prometheus Group, Dover, NH) providing EMG feedback. BF was used during each clinic visit. PFMT at home include 20 VPFMC (two sets of 10) with 3-second hold and 10 VPFMC (one set) with 12-second hold, twice a day, progressing to 60 VPFMC (three sets of 20) with 3-second hold and 30 VPFMC (three sets of 10) with 12-second hold, twice a day. Duration of training: 9–12 weeks. If subjects were continent (no episodes of urine loss recorded on the bladder diaries) for 2 consecutive weeks by session 9 (week 9) then they were scheduled for the postintervention examination. Others continued until they recorded continence for 2 consecutive weeks or completed the 12-week intervention. Supervision: clinic sessions conducted with physiotherapist at 1-week intervals. Randomisation at the third visit. After randomisation, participants continued PFMT in the supine position and were also taught ‘the stress strategies’ to contract pelvic floor muscle in anticipation of a cough, sneeze, etc. PFMT in supine and upright position + BF: PFMT (in supine position) and education as above up to randomisation at the third visit. After randomisation, participants performed one set each of the 3-second and 12-second VPFMC in the supine, sitting and standing positions. Also taught ‘the stress strategies’ to contract pelvic floor muscle in anticipation of a cough, sneeze, etc. Duration of training and supervision as above Additional information: Authors note that a large difference in baseline pad test results (although not statistically significant) ‘caused us to adjust for baseline urine loss on the pad test in subsequent bladder diary and pad test analysis’ (p. 980) |
Objective Prevalence of USI after treatment: I: 9/22, II: 9/22 Note: The prevalence of USI at baseline = I: 12/22, II: 14/22 Change in episodes of leakage per week (7-day bladder diary, mean, SD, range): I: –4.0 (4.7) (–4.0 to 18.0), II: –5.4 (4.8) (0.0 to 16.0), p = 0.30 Change in pad test [modified 1-hour pad test by ICS (Abrams 1998) with full bladder and provocative manoeuvres] (g, change in mean weight, SD, range): I: –3.9 (3.8) (–2.7 to 11.4), II: –5.1 (3.9) (–7.1 to 102.0), p = 0.29 Surrogate outcomes Adherence – all participants, including dropouts (N of visits attended, range): I: 8.4 (2.8) (2.0–12.0), II: 8.9 (3.0) (1.0–12.0), p = 0.53 Adherence – completers only, excluding dropouts (N of visits attended, range): I: 9.88 (1.11) (9.0–12.0), II: 10.16 (1.01) (9.0–12.0), p = 0.42 Means for prescribed number of VPFMC did not differ between those who completed the trial and all participants including dropouts Change in Brink score (pelvic floor muscle strength, mean, SD, range): I: 2.0 (1.7) (0.0–5.0), II: 2.2 (1.9) (0.0–6.0) p = 0.75 Note: The Brink scale considers three muscle function dimensions: muscle contraction duration, squeeze pressure felt around the examiner’s fingers, and vertical displacement of the examiner’s fingers at the pelvic floor muscles contract. The 3 subscale scores are summed to obtain a composite score ranging from 3 to 12. Increases in values indicate improvement |
Quality of life Incontinence Impact Questionnaire (change in score, mean, SD, range): I: 27.6 (32.7) (0.0–135.0), II: 24.7 (31.0) (–22.3 to 85.5), p = 0.62 Note: As the value increases, quality of life improves |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Bourcier 1994196 (abstract only) Study design/method: 2-arm RCT Duration of study: 2–3 months treatment + follow-up at 6 months |
Inclusion criteria: Women with USI Exclusion criteria: Not reported N randomised: 102 N lost to follow-up: I: 12/50, II: 6/52 Type of incontinence: USI Age (years, mean): 38 Pad test (not specified, g, average): I: 19.5, II: 27.0 Pelvic floor muscle strength and endurance (not specified, µg, average): I: 25, II: 27 |
I. PFMT + VC, N = 38 II. ES + BF (+ PFMT), N = 46 (N in analysis) PFMT + VC: 20 maximal VPFMC three times per day for 3 months. Use of unspecified cones twice daily and different exercise with instructor (‘intensive exercises’) for 30 minutes once a week. After assessment at 3 months encouraged to continue the home treatment ES + BF (+ PFMT): Treated over 6 weeks by 12 30-minute sessions, including 20 minutes of short-term maximal functional electrical stimulation (parameters unspecified) and 10 minutes of EMG/pressure biofeedback. Presumably BF of VPFMC, although this is not stated. After assessment at 3 months attended clinic weekly for 2 months Additional information: Not relevant for direct head-to-head comparisons; data were therefore extracted for primary outcomes only |
Objective Pad test (not specified) at 6 months (gram, average): I: 11.5, not significant improvement, II: 7.1, significant improvement |
Subjective ‘Continent after treatment’ at 6 months: I: 16/38, II: 31/46 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Brubaker 1997 130 Study design/method: 2-arm RCT. Four sites, USA Duration of study: 8 weeks |
Inclusion criteria: Women with USI or detrusor overactivity Exclusion criteria: Urinary incontinence other than USI, detrusor overactivity or mixed incontinence; age < 25 years, leakage episodes ≤ 3 times per week, inadequate genitourinary estrogen (minimum 3 months replacement), inadequate cognitive ability (investigator judgement), infected urine, anatomic defect that precluded use of device, postvoid residual > 100 ml, implanted electric device, genitourinary surgery ≤ 6 months previously, medication alteration ≤ 3 months previously, anticipated geographic relation during study N randomised: 148 N lost to follow-up: 27/148 Type of incontinence (SUI/MUI/DO): I: SUI 28 (46%), MUI 19 (31%), DO 14 (23%)), II: SUI 32 (53%), MUI 14 (23%), DO 14 (23%) Age (years, mean, SD): I: 56.0 (11.9), II: 57.7 (12.4) Grade of anterior vagina or cystocoele prolapse (grade 1–4): I: 1.1 (0.9), II: 1.0 (0.8) Other: Weight, % prior incontinence surgery, % genitourinary surgery, N of vaginal delivery, previous treatment for incontinence |
I. ES, N = 61 II. Sham ES, N = 60 (N in analysis) Both groups: The consent form was worded to remove expectations regarding sensory input. Telephone contact by nurse at 2nd and 6th weeks. An office visit at 4 weeks ES: Transvaginal (InCare Microgyn II), frequency 20 Hz, 2-second/4-second work–rest cycle, pulse width 0.1 microseconds. The bipolar square wave could be delivered over a range of 0–100 mA. Patients instructed to stimulate to the maximum tolerable motor response, 20 minutes twice daily for 8 weeks. Stimulator stores time and amperage during use. Telephone contact by nurse at second and sixth weeks and an office visit at 4 weeks. The consent form was worded to remove expectations regarding sensory input Sham ES: Same parameters, externally identical device but with no electrical energy supply. Telephone contact by nurse at second and sixth weeks and an office visit at 4 weeks. The consent form was worded to remove expectations regarding sensory input Additional information: Withdrawal rates varied by site (6–37%) and the difference was statistically significant (p = 0.0008). The site with the highest dropout rate serves a broad geographic area and travel inconvenience was frequently cited as a reason for dropping out Authors note that ‘interpretation of urinary diary was not possible because of large amounts of incomplete patient data’ |
Objective N cured (negative urodynamic diagnosis of stress incontinence): USI and MUI patients only, I: 5/46, II: 3/44 Episodes of leakage in 24 hours at 6 weeks (mean, SD): all patients, I: 61, 2.4 (3.1), II: 60, 2.2 (2.7), p = 0.75 N of micturition in 24 hours at 6 weeks: all patients, I: 61, 9.3 (6.8), II: 60, 9.5 (2.8), p = 0.049 Surrogate outcomes Mean subject compliance (?degree of compliance) at 8 weeks (%): all patients, I: 78.8 (20.5), II: 83.7 (14.7) |
Subjective Adequate subjective improvement (not defined): all patients, I: 21/61, II: 10/60, p = 0.027 Quality of life Condition-specific quality of life (test specific to this study, not validated, no detail): No statistically significant differences between groups |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Bump 2004136 (abstract only) Study design/method: 2-arm RCT. Multicentre (8 study sites across Europe and the US) Duration of study: 4 weeks |
Inclusion criteria: Women aged 22–73 with USI and normal bladder compliance Exclusion criteria: Detrusor overactivity N randomised: 65 N lost to follow-up: unclear Type of incontinence: USI Age (years, range): 22–73 |
I. Duloxetine 80 mg (taken as 40 mg b.i.d.), N = 34 II. Placebo, N = 31 (N randomised) Additional information: Participants self-administered duloxetine or placebo for 4 weeks and then all participants were allowed to take duloxetine in an open-label extension to the study |
Objective Change in episodes of leakage: ‘Duloxetine was significantly superior to placebo’ Decreases in N of pads used: ‘Duloxetine was significantly superior to placebo’ N of micturition (increases in the time between voids): ‘Duloxetine was significantly superior to placebo’ |
Quality of life Patient-perceived measured with a global rating scale (no further details regarding the scale): ‘Duloxetine was significantly superior to placebo’ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Burns 1993 122 Study design/method: 3-arm RCT, parallel design. Block randomisation. Method of allocation concealment not stated. Single centre, USA Duration of study: 8-week treatment, with 3- and 6-month follow-ups post intervention |
Inclusion criteria: Women aged ≥ 55 years with USI or MUI, minimum of three leakage episodes per week, demonstrates leakage with stress manoeuvres during physical examination, mentally competent (Mini-Mental Status Exam score ≥ 23), non-depressed (Center for Epidemiological Studies Depression scale) absence of glycosuria or pyuria, postvoid residual < 50 ml, maximum uroflow > 15 ml/second Exclusion criteria: Not reported N randomised: 135 (data reported for 123 who completed study) N lost to follow-up: 10/135 (no reasons given) and 2/135 excluded from analysis (no urinary diary). Group not specified Type of incontinence (USI/MUI): I: 36/4, II: 40/3, III: 36/4 Age (years, mean, SD): I: 63 (6), II: 63 (6), III: 63 (5) Episodes of leakage per week (mean, SD): I: 13 (12), II: 18 (15), III: 18 (18) Severity of incontinence (mild/moderate/severe): I: 13/9/8, II: 10/19/14, III: 14/14/12; see Outcome section for definition Vaginal electromyography (mean of five quick contractions, microvolts): I: 40, 3.5 (3.0), II: 38, 2.9 (3.2), III: 40, 3.4 (3.9) Vaginal electromyography (mean of five sustained contractions, microvolts): I: 35, 2.0 (1.5), II: 33, 1.7 (1.6), III: 34, 1.8 (1.5) Percentage cystocoele: I: 31/40, II: 28/43, III: 33/40 Other: Ethnicity 98% white, education/employment status: 57% middle class, % prior incontinence surgery, % estrogen therapy |
I. PFMT + BF, N = 40 II. PFMT, N = 43 III. No treatment, N = 40 (N in analysis) PFMT: Coached by a nurse. Set: 10 VPFMC with 3-second hold (‘quick’), and 10 VPFMC with 10-second hold (‘sustained’). Beginning with four sets of 20 (10 quick and 10 sustained) and progressed by 10 per set over 4 weeks to daily maximum of 200. Videotape (12 minutes) describing exercise protocol and booklet explaining anatomy, PFMT, and completion of exercise and urinary diaries. Sets per day: four. Duration of training: 8 weeks. Supervision: 25–35 minutes weekly clinic visits with nurse. Weekly, 3- and 6-month telephone reminder calls for appointments and weekly exercise reminder cards mailed between visits PFMT+BF: PFMT programme and supervision (including reminder phone calls and cards) as above. Visual BF from vaginal probe attached to a computerised EMG provided during clinic visits with nurse Control subjects: 8 weeks without treatment, diary or contact with study personnel Additional information: The ‘mild’ symptom group had a significant worsening of their symptoms across their follow-up period, while the ‘moderate’ and ‘severe’ groups maintained stable rates of improvements through 6 months of follow-up (treatment group not specified) |
Objective N of patients improved in episode of leakage per week at 8 weeks (by symptom severity): Improvement (%)≤ 0–4950–99100 (dry)PFMT + BF13189Mild535Moderate793Severe161PFMT17197Mild136Moderate991Severe770None3361Mild1220Moderate1211Severe930 Note: Improvement = (N of leakage episodes per week preintervention – N episodes post intervention) ÷ N episodes preintervention × 100; Symptom severity: mild = ≤ 7 leakage episodes per week, moderate = 8–21 episodes per week, severe = ≥ 22 leakage episodes per week Average % improvement at 8 weeks: I: 61%, II: 54%, III: 6% Episodes of leakage per week at 8 weeks (24-hour diary, mean, SD): I: 5 (6), II: 8 (10), III: 17 (19) Surrogate outcomes Vaginal electromyography (mean of 5 quick contractions, microvolts, SD): I: 40, 6.0 (5.1), II: 38, 3.0 (3.4), III: 40, 3.5 (4.4) Vaginal electromyography (mean of five sustained contractions, microvolts): I: 35, 4.0 (3.1), II: 33, 1.8 (2.0), III: 34, 2.0 (1.8) Significant correlations between percentage of improvement in incontinent episodes and increases in electromyography performance on both quick (r = 0.26, p < 0.005) and sustained (r = 0.22, p < 0.03) pelvic muscle contractions |
Improvement (%) | ≤ 0–49 | 50–99 | 100 (dry) | PFMT + BF | 13 | 18 | 9 | Mild | 5 | 3 | 5 | Moderate | 7 | 9 | 3 | Severe | 1 | 6 | 1 | PFMT | 17 | 19 | 7 | Mild | 1 | 3 | 6 | Moderate | 9 | 9 | 1 | Severe | 7 | 7 | 0 | None | 33 | 6 | 1 | Mild | 12 | 2 | 0 | Moderate | 12 | 1 | 1 | Severe | 9 | 3 | 0 |
Subgroup analysis Subgroup analysis by incontinence severity in Burns (1993),122 table 2 Time course of response to treatment from week 1 through week 8 to 3- and 6-month follow-ups in Burns (1993),122 figure 1 (for groups I and II) and figure 2 (by incontinence severity, treatment groups not specified) |
|||
| Improvement (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ≤ 0–49 | 50–99 | 100 (dry) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PFMT + BF | 13 | 18 | 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild | 5 | 3 | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 7 | 9 | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe | 1 | 6 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PFMT | 17 | 19 | 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild | 1 | 3 | 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 9 | 9 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe | 7 | 7 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| None | 33 | 6 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mild | 12 | 2 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Moderate | 12 | 1 | 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Severe | 9 | 3 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Burton 1993173 (abstract only) Study design/method: 2-arm RCT, Australia Duration of study: NR |
Inclusion criteria: Women with USI Exclusion criteria: Not reported N randomised: 61 N lost to follow-up: NR Type of incontinence: USI Leakage Activity Index (Bø 1991,237 mean): I: 2.2, II: 2.4 Visual Analogue Symptom Score (no further details, range 0–10, mean): I: 5.6, II: 5.0 |
I. Passive VC, N = 31 II. Active VC, N = 30 (N randomised) Passive VC (i.e. normal cone): 15 minutes twice a day in a static position. Unspecified cones Active VC: 15 minutes twice a day while doing standardised activities that previously made them incontinent. Unspecified cones |
Objective No leakage after coughing (videocystourethrography): I: 18/31, II: 21/30 40-minute pad test (ml, mean): I: 4.1, p-value for pre-post test not reported, II: 2.0, p < 0.05 (pre-post) |
Quality of life Leakage Activity Index (Bø 1991,237 5-point scales on 13 activities that could normally trigger incontinence in women with USI; mean): I: 1.0, p < 0.05 (pre-post), II: 0.5 p < 0.01 (pre-post) Note: Lower scores = better Visual Analogue Symptom Score (no further details, range 0–10, mean): I: 2.1, p < 0.05 (pre-post), II: 0.7, p < 0.01 (pre-post) Note: Lower scores = better General quality of life (Psychological Adjustment to Illness Scale, or PAIS, Derogatis et al. 1983): ‘a better result’ for active cones than for passive cones; poor print quality graph so actual scores could not be extracted |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cammu 1998 181 Study design/method: 2-arm parallel RCT. Single centre, Belgium Duration of study: 12 weeks |
Inclusion criteria: Ambulatory, mentally and physically fit white women with ‘troublesome’ USI, had a vaginal capacity permitting the use of BF or VC Exclusion criteria: postpartum, genital prolapse, pathology needing surgery, detrusor overactivity, outflow obstruction, intrinsic sphincter deficiency N randomised: 60 N lost to follow-up: I: 0/30, II: 14/30 Type of incontinence: USI Age (years, mean, SD): I: 55.9 (9.5), II: 56.3 (11.4), II-trtd: 56.4 (9.2) Episodes of leakage per week (mean, SD): I: 14.4 (10.0), II: 13.6 (12.0), II-trtd: 13.9 (18.0) N of pad changes per week (mean, SD): I: 13.4 (8.0), II: 15.8 (14.0), II-trtd: 15.1 (16.0) Perineometer squeezing capacity – fast (µV, mean, SD): I: 7.2 (4.4), II: 6.1 (3.5), II-trtd: 7.8 (4.3) Perineometer squeezing capacity – slow (µV, mean, SD): I: 7.1 (4.1), II: 6.4 (3.6), II-trtd: 8.2 (4.5) ‘Severity of incontinence’ on visual analogue scale (11-point, 0-10, no further details; mean, SD): I: 4.7 (1.7), II: 5.2 (1.7), II-trtd:, 4.0 (1.7) ‘Psychological distress’ on visual analogue scale (11-point, 0-10, no further details; mean, SD): I: 5.4 (2.4), II: 6.0 (2.1), II-trtd: 5.3 (2.5) Other: BMI/ethnicity, parity |
I. PFMT + BF, N = 30 II. VC, N = 30 (only 16/30 treated; see below) (N in analysis) Note: Data reported separately for 16/30 really treated with VC (II-trtd). See Additional information PFMT + BF: Correct VPFMC taught by vaginal palpation. Education on pelvic anatomy and purpose of treatment. PFMT = 10 brief forceful contractions, 10 sustained 10 sec contractions. Progress by increasing number of sets. ‘The patients also instructed to voluntarily contract the pelvic floor prior to a sudden intra-abdominal pressure rise’ (= The Knack?). BF = Vaginal probe with surface EMG giving visual BF, and abdominal wall electrode, at clinic visits only. A weekly 30-minute private session with physiotherapist VC: Individual training, vaginal palpation and education as in the PFMT + BF group above. VC = Femina cones, five conical weights, 20–70 g. 15 minutes twice a day at home (except during menstruation) with heaviest cone that could be retained with VPFMC in standing. Progress by increasing the passive and the active cone weight. Clinic visits fortnightly for 12 weeks. The physiotherapist sessions served mainly to assess whether the cones were correctly used. No BF used Note: The ‘passive’ cone = the heaviest weight that could be retained in place for 1 minute without VPFMC. The ‘active’ cone = the heaviest cone retained with VPFMC Additional information: After the first visit 14/30 women in the cones group withdrew and received PFMT but stayed in the VC group. Data reported separately for 16/30 really treated with VC (II-trtd). Authors reported no statistically significant difference between group I versus II and I versus II-trtd |
Objective Cure (negative stress test): I: 12/30, II: 12/30, II-trtd: 7/16 Episodes of leakage per week (1-week diary, mean, SD): I: 30, 5.6 (5.5), II: 30, 8.7 (13.0), II-trtd: 16, 8.3 (15.0) N of pad changes in 24 hours (1-week diary, mean, SD): I: 30, 6.0 (5.6), II: 30, 8.8 (13.0), II-trtd: 16, 8.6 (15.0) Surrogate outcomes Perineometer squeezing capacity – fast (µV, mean, SD): I: 30, 10.7 (5.9), II: 30, 9.0 (4.1), II-trtd: 16, 10.3 (4.1) Perineometer squeezing capacity – slow (µV, mean, SD): I: 30, 11.4 (6.2), II: 30, 9.7 (5.4), II-trtd: 16, 10.9 (4.6) Also, the women who used cones showed a 39% improvement in the passive (from a mean of 36–50g) and a 29% improvement in the active cone weight (from a mean of 48–62g) N having incontinence surgery after treatment: I: 5/30, II: 9/30, II-trtd: 4/16 Adverse events N experiencing adverse events: I: 0/30, II: 14/30 Adverse events: unpleasant feeling (N = 5), time consuming (N = 3), inability to introduce the cone when too nervous or when in a hurry (N = 2), interference with menstrual cycle (N = 2), a certain cone held in the morning could not be held any longer in the evening (muscle fatigue) (N = 2) Discontinued treatment because of adverse events: I: 0/30, II: 14/30 |
Subjective Cured or improved to a significant degree: I: 16/30, II: 17/30, II-trtd: 8/16 Quality of life ‘Severity of incontinence’ on visual analogue scale (visual analogue scale, range 0-10, no further details; mean, SD): I: 30, 2.6 (2.1), II: 30, 2.9 (2.4), II-trtd: 16, 2.3 (2.0) Note: lower score = better (less severe) ‘Psychological distress’ on visual analogue scale (visual analogue scale, range 0-10, no further details; mean, SD): I: 30, 2.1 (2.1), II: 30, 3.4 (3.3), II-trtd: 16, 2.6 (2.8) Note: lower score = better (less distress) N desiring further treatment: None of the patients wanted to use the cones after the completion of the study period of 12 months. Reasons: unpleasant feeling (N = 4), time consuming (N = 4), muscle fatigue (N = 3), positive about the use of cones but did not buy a set for continuous long-term home practice (N = 5) Subgroup analysis Non-compliance with VC significantly correlated with BMI (esp. obesity). Logistic regression, p = 0.0228, OR 1.54 (1.06–2.22). Menopause, estrogen status, age, parity, duration of symptoms, degree of incontinence, severity of symptoms and vaginal squeeze capacity were of no importance in the non-compliance with cones (p. 90) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cardozo 2004 137 Study design/method: 2-arm RCT. Multicentre (Australia, Canada, the Netherlands, UK) Duration of study: 8 weeks |
Inclusion: Women aged 18–75 years, USI, ≥ 14-week incontinence episode frequency (IEF), scheduled for continence surgery Exclusion: Not specified N randomised: 109 N lost to follow-up: I: 20/55, II: 12/54 Type of incontinence: USI (severe) Age (years, mean, SD): I: 54.5 (9.7), II: 52.4 (10.4), range 33–75 years N of leakage episodes per week (mean, SD): I: 24.7 (27.6), II: 21.2 (18.2) PGI-S (Patient Global Impression of Severity; moderate or severely abnormal urinary tract function): I: 49/55, II: 48/54 I-QoL (Incontinence Quality of Life, normalised to a scale of 0–100, with 0 representing the worst possible and 100 representing the best possible condition-specific quality of life, mean, SD): I: 53.6 (21.9), II: 53.0 (22.5) Other: BMI, ethnicity, % patients on hormone replacement therapy, % prior incontinence surgery |
I: Duloxetine, 40 mg twice daily for 4 weeks, then escalating to 60 mg twice daily for 4 weeks, N = 55 (54) II: Placebo, N = 54 (52) (N randomised; and N in ‘ITT’ analysis in brackets with women with at least one postrandomisation measure) |
Objective measures Cure or improvement (N of ‘responders’ with at least 50% decrease in leakage episodes): I: 29/46, II: 7/52 Decrease in N of leakage episodes per week (N episodes, median): I: 46, 7.1, II: 52, 2.9, p < 0.001 Decrease in N of episodes of leakage per week (median %): I: 46, 59.8%, II: 52, 26.9%, p < 0.001 (no data to extract exact numbers) Decrease in N of leakage episodes per week at 1–4 weeks (duloxetine 80 mg per day): I: 46, 54.7%, II: 52, 26.3%, p < 0.002 (no data to extract exact numbers) Decrease in N of leakage episodes per week at 5–8 weeks (duloxetine 120 mg per day): I: 46, 64%, II: 52, 28.6%, p < 0.001 (no data to extract exact numbers) Reduction in N of pad changes (median %): I: 46, 34.5%, II: 52, 4.8%, p = 0.008 Adverse events Adverse events (any): I: 43/46 (92.7%), II: 37/52 (72.2%); Increasing the dose from 80 mg to 120 mg daily neither increased efficacy nor side effects Adverse events that occurred in more than 10% of subjects: Nausea, constipation, headache, dry mouth, fatigue, dizziness, insomnia, somnolence and vomiting Serious adverse events: ‘Serious adverse events, cardiovascular events and laboratory abnormalities were rare and not significantly different with duloxetine compared with placebo’ (p. 516). Discontinued medication because of adverse events: I: 18/55 (32.7%), II: 3/54 (5.5%) |
Subjective PGI-I (‘very much better’ and ‘much better’): I: 17/51, II: 4/52 PGI-I (‘a little better’ and ‘no change’): I: 31/51, II: 42/52 PGI-I (‘a little worse’, ‘much worse’, and ‘very much worse’): I: 3/51, II: 6/52 Quality of life I-QoL (total score mean change, SD): I: 52, 10.6 (19.1), II: 52, 2.4 (9.4), p = 0.003 N desiring further treatment: 20% of women with USI were somewhat or not very much keen on continence surgery being on duloxetine compared with 0% of those in the placebo arm Subgroup analysis Intrinsic sphincter deficiency status, baseline severity of symptoms |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Castleden 1984148 Study design/method: Crossover trial in which ‘the order of treatment was randomised’. Single centre, England Duration of study: 4 weeks (each treatment period lasted 2 weeks) |
Inclusion criteria: Women with easily demonstrable SUI Exclusion criteria: Not reported N randomised: 19 N lost to follow-up: not reported Type of incontinence: SUI Age (years, mean, range): 55 (23–85) Duration of symptoms (years): 9 (0.25–32) |
I. PFMT + BF, N = 19 II. PFMT, N = 19 (N in analysis) PFMT + BF: 4–5 VPFMC per hour and midstream urine stop on every occasion. With use of portable perineometer (Kingsdown Medical, UK) with visual BF at least once per day for 2 weeks. Supervised by a physiotherapist PFMT: As above but without perineometer |
Subjective Improvement in symptom (mean change in visual analogue scale score, ranging from ‘completely dry’ to ‘wet all the time’) (mean change, range): I: 23.9 (0 to 79), II: 6.7 (–32 to 26), no significant difference between treatments Note: Greater value reflects greater improvement |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Castro-Diaz 2007 138 Study design/method: 2-arm RCT, 64 study centres in 8 countries (Brazil, Canada, France, Germany, Italy, Mexico, Puerto Rico, Spain) Duration of study: 8 weeks (+ 2-week placebo lead-in after a further 2-week screening and no-SNRI lead-in) |
Inclusion criteria: Women aged 18 with symptoms of predominant SUI (≥ 7 SUI episodes per week, and at least twice as many SUI episodes as UUI episodes). Patients also had presence of SUI confirmed within 6 months of study entry, and an average daytime voiding interval of > 2 hours, nocturnal voiding interval of ≤ 2/day and positive cough stress test result Exclusion criteria: Continence surgery within 6 months or pharmacological treatment for symptoms of overactive bladder within 14 days of visit 1, pelvic organ prolapse beyond the hymen and previous participation in a Duloxetine trial N randomised: 516 N lost to follow-up: I: 15/120, II: 33/136, III: 26/127, IV: 15/133 Type of incontinence: Predominant symptoms of SUI (MUI) Age (years, mean, SD): I: 52.7 ± 9.2, II: 53.3 ± 11.8, III: 52.3 ± 10.4, IV: 53.5 ± 10.6 Other: BMI, % postmenopausal |
I. Placebo N = 120 (112) II. Duloxetine 40mg b.i.d. (twice daily) (i.e. 80 mg/day) N = 136 (109) III. Duloxetine 40 mg q.d. (daily) for 2 weeks, escalating to 40mg b.i.d. for 6 weeks, N = 127 (112) IV. Duloxetine 20 mg b.i.d. for 2 weeks, escalating to 40 mg b.i.d. for 6 weeks, N = 133 (123) V: Pooled data for duloxetine (groups II–IV), N = 396 (344) (N randomised; and N in ‘ITT’ analysis in brackets with women with at least one postrandomisation measure) Additional information: Study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only Study contained additional discontinuation phase after 8 weeks for those randomised to SNRI arms, who were then rerandomised to placebo, 40 mg q.d. or 20 mg b.i.d. for 2 weeks before all receiving placebos for a further 2 weeks. Only data before rerandomisation are extracted |
Adverse events N experiencing any adverse events (first 4 weeks, i.e. all patients had at least 2 weeks, with escalated dose of 40 mg b.i.d.): I: 53/120, II: 87/136, III: 76/127, IV: 69/133 Adverse events (that occurred in 2 patients in first 4 weeks): nausea, dry mouth, constipation, somnolence, dizziness, insomnia, fatigue, headache, diarrhoea Discontinued treatment because of adverse events (in first 4 weeks): I: 7/120, II: 22/136, III: 15/127, IV: 10/133 |
Subjective Patient Global Impression – Improvement (PGI-I):Overall ‘Significantly more duloxetine-treated patients rated their response to treatment in one of the three “better” categories than did the placebo-treated patients’ (p < 0.01) Quality of life Mean change in total I-QoL score: I: 112, 5.7, II: 109, 15.4, p < 0.001, III: 112, 12.2, p = 0.006, IV: 123, 11.5, p = 0.004, V: 344, 12.9, p < 0.001; p-value versus placebo; no significant difference among the three duloxetine groups (II–IV) Mean change in ICIQ-SF score: I: 112, –1.7, V: 344, –2.8, p = 0.004 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Delneri 2000 186 Study design/method: 2-arm parallel RCT, Italy Duration of study: The VC treatment lasted 4 weeks and the ES lasted 16 days |
Inclusion criteria: Women with USI Exclusion criteria: Detrusor overactivity, inversion of perineal command, absent contraction of pubococcygeal (PC) muscle, neurological diseases, patients’ unwillingness to collaborate N randomised: 20 N lost to follow-up: NR. 2/10 in the VC group refused to undergo follow-up urethral pressure profile Type of incontinence: USI Age (years, mean, SD, range): I: 49.5 (14.5) (29–81), II: 41.5 (7.4) (31–54) Other: BMI, ethnicity, education, employment status, % prior incontinence surgery, Parity, % postmenopausal |
I. ES, N = 10 II. VC, N = 10 (N in analysis) ES: Functional electrical stimulation in the rehab centre. Lie in the dorsal position. Transvaginal sensors dampened before insertion. 12 consecutive sessions (excluding Saturday and Sunday), each of 30 minutes: 15 minutes at 20 Hz and the other 15 minutes at 50 Hz. Pulse duration 4 seconds, with 8-second recovery VC: Taught in the rehabilitation centre and then practised at home for 25–35 minutes per day for 4 weeks. Femcon cones, five conical weights from 20–70 g |
Objective Pad test (not defined) (g, mean): I: 9.5, II: 9.5, NS |
Quality of life Subjective rating of the overall discomfort caused by incontinence (visual analogue scale, no further detail; mean): I: 5, II: 5, NS |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Dmochowski 2003 139 Study design/method: two-arm RCT. Multicentre: 55 study centres in USA and Canada Duration of study: 12 weeks (+ 2-week no-SNRI lead-in and 2-week placebo lead-in prior to randomisation) |
Inclusion criteria: Women aged 18 with predominant SUI symptoms (i.e. ‘bothersome’ SUI persisting for ≥ 3 months), and a weekly stress incontinence episodes of ≥ 7 with micturition frequency of < 8 per day and < 3 per night; bladder capacity of ≥ 400 ml and a positive cough stress test and stress pad test Exclusion criteria: Predominant symptoms of urge incontinence, pregnancy, unable to tolerate retrograde bladder filling to 400 ml or had a first sensation of bladder filling at < 100 ml, antidepressant medication N randomised: 683 N lost to follow-up: I: 107/344, II: 44/339, p < 0.001; ‘The difference was primarily attributable to a higher rate of duloxetine early discontinuation related to side effects’ Type of incontinence: Predominant symptoms of SUI (MUI) Age (years, mean, SD): I: 52.3 ± 10.4, II: 53.3 ± 11.5, overall range (22 to 84) Episodes of leakage in 1 week (mean, SD, range): I: 18.2 ± 14.3 (14.3 to 87.0), II: 19.0 ± 14.6 (0 to 103.0), ‘extreme outliers substantially distorted the mean’ PGI-I (moderately/severe abnormal bladder function on PGI-S): I: 235/344 (68.4%), II: 226/339 (66.8%) Mean I-QOL score (mean, SD): I: 62.0 ± 20.2, II: 64.3 ± 17.7 Other: BMI, ethnicity, median stress pad test (g), % prior incontinence surgery, % with prior pelvic floor training |
I. Duloxetine 80 mg as 40 mg b.i.d. (twice daily), N = 344 II. Placebo, N = 339 (N randomised) Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only Dichotomous data calculated from percentage in paper, using the N of women included in the ‘ITT’ analysis with at least one postrandomisation measure; diary data, I = 286, II = 322; subjective data, I = 334, II = 332 Two 7-day diaries completed before randomisation. Three diaries completed after randomisation, each for the week prior to a visit at the clinic |
Objective Cure (no incontinence episodes at the last 7-day diary): I: 30/286 (10.5%), II: 19/322 (5.9%), p < 0.05 Cure or improvement (50–100% reduction in IEF/week): I: 147/286 (51.4%), II: 108/322 (33.5%), p < 0.001 Adverse events N experiencing adverse events (any): I: 255/344 (74%), II: 170/339 (50%) Adverse events (significantly more common with duloxetine and occurring in ≥ 5% of subjects on duloxetine): Nausea, fatigue, insomnia, dry mouth, constipation, somnolence, dizziness, headache, diarrhoea Discontinued treatment because of adverse events: I: 83/344 (24.1%), II: 14/339 (4.1%), due to nausea, fatigue, insomnia, somnolence, dizziness, blurred vision |
Subjective PGI-I (rating condition as improved): I: 207/334 (62.0%), II: 131/332 (39.6%) Quality of life Change in I-QoL score (mean, SD): I: 334, 11.1 (14.8), II: 332, 6.8 (13.8), p < 0.001 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Edwards 2000170 (abstract only) Study design/method: 2-arm RCT, UK Duration of study: 12 weeks |
Inclusion criteria: Premenopausal women with USI and no previous pelvic surgery Exclusion criteria: Not reported N randomised: 20? N lost to follow-up: Not reported Type of incontinence: USI Age (years): 46 (32–51) |
I. PFMT + BF, N = 10? II. PFMT + ES, N = 10? (N in analysis) PFMT + BF: No details given PFMT + ES: No details given |
Objective Objective cure (not defined): 50% (10/20), no significant difference between two groups |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fantl 1991 135 Study design/method: 2-arm RCT, USA Duration of study: 6 weeks |
Inclusion criteria: Women aged ≥ 55 years with USI or DI, living independently in the community, at least one episode of urine loss per week, mentally intact (Mini-Mental State Examination score > 23) and functionally capable of independent or assisted toileting Exclusion criteria: USI or DI, metabolic decompensation (e.g. uncontrolled diabetes), lower urinary tract infection, urinary obstruction, diverticulum, fistula, reversible cause of urinary incontinence (e.g. faecal impaction, direct SNRI effect), permanent indwelling catheter N randomised: 131 N lost to follow-up: I: 5/65, II: 3/66 Type of incontinence: I: USI 45 (75%), USI and DI 8 (13%), DI 7 (12%), II: USI 44 (70%), USI and DI 12 (19%), DI 7 (11%) Age (years, mean, SD): I: 60, 66 ± 8, II: 63, 68 ± 9 Episodes of leakage per week (mean, SD): all women I: 60, 21 ± 20, II: 63, 22 ± 20; women with USI only, I: 45, 23 ± 22, II: 43, 22 ± 20 Number of micturitions per week (diurnal): all women, I: 60, 64 ± 28, II: 63, 59 ± 26; women with USI only, I: 45, 61 ± 21, II: 43, 58 ± 20 Pad test (g, mean, SD): all women, I: 60, 37 ± 62, II: 63, 39 ± 82; women with USI only, I: 45, 24 ± 46, II: 43, 21 ± 63 Incontinence Impact Score: all women, I: 60, 0.51 ± 0.41, II: 63, 0.49 ± 0.55 Other: Ethnicity (% white), education (% > high school), income (% > US$20,000 p.a), % prior incontinence surgery, parity, % postmenopausal (% using estrogen supplementation) |
I. Bladder training, N = 60 II. No treatment, N = 63 (N in analysis) Bladder training: Weekly clinic visits of 15–20 minutes over 6 weeks. Bladder training consisted of patient education and a schedule of voluntary micturition. Patient education was audiovisual programme plus verbal and written instruction on how to adapt this to everyday lifestyle. Neurological control over the urinary tract was emphasised. Voiding schedule (for waking hours only) was self-implemented and involved micturitions scheduled for every 30–60 minutes and progressively increased by 30 minutes each week (if tolerated and a decrease in incontinence episodes had been shown), with the goal of 2.5- to 3-hour intervals between voids. Patients were instructed to ‘go to the toilet and empty your bladder as completely as you can’ regardless of desire to void. No fluid modifications were used. Each patient kept daily treatment logs Control group: No further contact and asked to return in 6 weeks |
Objective Cure (100% reduction in the number of incontinent episodes on 7-day diary): all women, I: 7/60, II: 2/63 Cured or improved (50–100% reduction in the number of incontinent episodes on 7-day diary): all women, I: 45/60, II: 15/63 Episodes of leakage per week (7-day diary, mean, SD): all women, I: 60, 9 ± 11, II: 63, 19 ± 17; women with USI only, I: 45, 10 ± 12, II: 43, 19 ± 19 N of micturition per week (7-day diary, mean, SD): all women, I: 60, 52 ± 14, II: 63, 57 ± 27; women with USI only, I: 45, 51 ± 11, II: 43, 56 ± 20 Pad test (g, mean, SD): all women, I: 60, 17 ± 36, II: 63, 47 ± 87; women with USI only, I: 45, 10 ± 21, II: 43, 29 ± 74 |
Quality of life Incontinence Impact Questionnaire Score (composite score, range 0–3 with low scores reflecting higher quality of life; mean, SD): all women, I: 39, 0.25 ± 0.29, II: 39, 0.50 ± 0.59 Additional IIQ subscale and VAS data available in Wyman (1997) alongside data (for the treatment group only) by diagnosis at 6 weeks and correlation data for both the IIQ and CES Depression Scale with pad weight and IEF/week Subgroup analysis Leakage episodes per week, pad test and N of micturitions per week also presented by age group (55–64, 65–74, 75+), and by baseline severity (1–10 episodes, 11–26 episodes, 26+ episodes) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ferguson 1990 149 Study design/method: 2-arm RCT. Single centre, USA Duration of study: 6 weeks (with follow-up between 12 and 24 months after completion of study) |
Inclusion criteria: Women with USI, stable cystometrogram and negative stress urethral pressure profile Exclusion criteria: Postmenopausal, previous urological surgery, taking medications that affect the bladder or skeletal muscle function, or complained of urgency, frequency or nocturia N randomised: 20 N lost to follow-up: Not reported Type of incontinence: USI Age (years, mean, SD): I: 37.1 ± 6.4, II: 35.8 ± 4.6 24-hour pad test (g, an, SD): I: 10, 10.6 ± 8.6, II: 10, 14.9 ± 15.9 30-minute pad test (g, mean, SD): I: 7, 4.8 ± 5.5, II: 7, 10.3 ± 8.1 Cystocoele (grade1/2/3): I: 8/2/0, II: 8/2/0 Rectocoele (mild/moderate/ severe): I: 6/4/0, II: 6/4/0 Other: Parity |
I. PFMT with IVRD (intravaginal resistance device), N = 10. II. PFMT without IVRD, N = 10 (N in analysis) PFMT with IVRD: Correct VPFMC taught by intense counselling and feedback on correct performance. Daily home exercise undertaken with intravaginal balloon in situ and with audiotape. Duration of training: 6 weeks. Supervision: clinic visits every 2 weeks, where maximal intravaginal pressure tested during five maximum VPFMC followed by three sustained VPFMC. Participants maintained a daily record of PFMT exercise and was contacted weekly by telephone to document adherence PFMT without IVRD: As above with audiotape but without intravaginal balloon in situ Additional information: Participants contacted by letter or phone 12–24 months after completing the study. Results of follow-up reported as a cohort and not by group allocation |
Objective 24-hour pad test at 6 weeks (g mean, SD): I: 10, 5.6 ± 4.7, II: 10, 5.8 ± 5.6 Change in-24 hour pad test from baseline to 6 weeks (g, mean, SD): I: 10, –5.1 ± 8.1, II: 10, –9.1 ± 13.6 30-minute pad test at 6 weeks (g, mean, SD): I: 7, 1.4 ± 1.7, II: 7, 3.4 ± 4.7 Change in 30-minute Pad Test from baseline to 6 weeks (g, mean, SD): I: 7, –3.4 ± 4.0, II: 7, –6.9 ± 7.4 Surrogate outcomes Maximum intravaginal pressure at 6 weeks (planimeter, H2O, mean, SD): I: 10, 33.4 ± 15.1, II: 10, 46.5 ± 20.7 Pelvic muscle pressure area at 6 weeks (endurance; H2O, seconds, SD, mean): I: 10, 234.4 ± 124.0, II: 10, 328.5 ± 139.7 Long term (12–24 months) Adherence (continuing PFMT) at 12–24 months after completion of the study (self-report): 9/19, data not available by group allocation N having incontinence surgery by 12–24 months after completion of the study (self-report): 3/19, data not available by group allocation Return of symptoms by 12–24 months after completion of the study (self-report): 2/19 (following discontinuation of PFMT exercises), data not available by group allocation |
Subjective Major improvement at 12–24 months post intervention (without surgery): 3/19 (of these 1/3 continuing PFMT) Moderate improvement at 12–24 months post intervention (without surgery): 2/19 (of these 1/2 continuing PFMT) Mild improvement at 12–24 months post intervention (without surgery): 6/19 (of these 5/6 continuing PFMT) Unchanged at 12–24 months post intervention (without surgery): 5/19 (of these 1/5 continuing PFMT) Improved after surgery at 12–24 months post intervention: 3/19 (of these 1/3 continuing PFMT) Data not available by group allocation |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Gallo 1997 162 Study design/method: 2-arm (quasi-) RCT. Single centre, USA. Duration of study: Approximately 4–6 weeks |
Inclusion criteria: Women aged 20–80 with a history of self-reported stress UI and the diagnosis of USI. Desire for conservative treatment, ability to complete questionnaire, willingness to participate. Note: Some patients described knowledge or performance of PFMT in the past but were not excluded Exclusion criteria: Pregnancy, psychological disorders that would make it difficult to follow PFMT instructions N randomised: 86 N lost to follow-up: I: 9/43, II: 2/43 Type of incontinence: USI Age (years, mean, range): 60 (29–80) among 75 women who completed the study ‘No significant differences’ between groups in age, ethnicity and education |
I. PFMT, no tape, N = 34 II. PFMT with audiotape, N = 41 (N in analysis) PFMT: 45-minute individual training with nurse, including education. Correct VPFMC taught using a biofeedback computer. Instruction sheet and verbal encouragement to perform PFMT at home for 10 minutes twice a day, with suggestions of potential exercise times based on the individual’s lifestyle PFMT with audiotape: PFMT and education as above with addition of audiocassette for use twice a day. Cassette contained 25 consecutive VPFMC with 10-second hold and 10-second rest counted aloud over 10 minutes. If the patient had a car, the nurse suggested use of tape on the way out of the drive way in the morning and returning home Additional information: The study aimed to assess patient compliance to PFMT. It did not correlate exercise compliance with improved muscle strength or incontinence that is assumed to have occurred |
Surrogate outcomes Adherence: Study-specific questionnaire (questions below are ‘research questions’ and may not be identical with the actual survey questions): |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ghoniem 2005 57 Study design/method: RCT, 2 × 2 design. 16 centres, in the Netherlands, UK, USA Duration of study: 12 weeks |
Inclusion criteria: Women aged 18–75 years with moderate to severe stress UI (≥ 2 stress leakage episodes per day) and either a positive cough stress test and normal voiding frequencies ( < 8 per day) at entry (N = 165) OR no detrusor overactivity within 6 months of enrolment (N = 36) Exclusion criteria: Advanced pelvic organ prolapse, active or recurrent urinary tract infections, continence surgery within 1 year, current device or pharmaceutical incontinence treatment, prior hip fracture or replacement, or any prior formal PFMT with a continence nurse or physical therapist N randomised: 201 N lost to follow-up: I: 16/52, II: 9/47, III: 19/52, IV: 9/50 Type of incontinence: USI (36/201) or SUI (165/201) Age (years, mean, range): I: 54 (31–75), II: 51 (29–68), III: 53 (34–70), IV: 54 (36–75), NS Episodes of leakage per week (median, range): I: 19.4 (10.0–70.5), II: 18.9 (10.3–299.4), III: 18.3 (6.4–78.5), IV: 22.0 (13.0–140.9) Median pad changes per week: I: 9.7 (0–53.5), II: 9.8 (0–43.1), III: 8.1 (0–44.0), VI: 8.6 (0–45.9) I-QOL score (mean, SD): I: 61.6 (22.3), II: 64.9 (17.1), III: 59.8 (20.6), VI: 61.4 (22.2) Pelvic floor muscle grade (9-point scale, mean, SD): I: 5.2 (1.7), II: not done, III: not done, IV: 5.2 (1.7) Other: BMI, ethnicity, % prior incontinence surgery (> 1 year) |
I. Duloxetine 80 mg + PFMT (‘combined treatment’), N = 52 II. Placebo SNRI + imitation PFMT (‘no active treatment’), N = 47 III. Duloxetine 80 mg + imitation PFMT (‘duloxetine only’), N = 52 IV. Placebo SNRI + PFMT (‘PFMT only’), N = 50 (N randomised) PFMT: Verbal instructions and manual feedback by qualified instructors. Correct VPFMC confirmed by the examiner during a digital pelvic examination. Written instruction to perform ‘strength training’ and ‘skill training (“The Knack”)’ for 12 weeks. Strength training = three sets of 10 long VPFMC with 6–8 seconds hold, and two sets of 10 rapid VPFMC with 1–2 seconds hold, 4 days weekly. Patients given a training log at every visit to record the N of VPFMC performed. Skill training = ‘The Knack’, as in Miller et al. 1998,107 i.e. to do VPFMC with events that cause leakage; 30 minutes’ instruction and feedback initially, and then 15-minute reinstruction and manual feedback at 4 and 8 weeks Imitation PFMT: Comprising hip abductor muscle contraction for 6–8 seconds with feet crossed at the ankles. The therapist confirmed the abductor contraction without dominant contractions of abdominal muscles. Three sets of long and two sets of rapid contractions, 4 times weekly. No recommendation for ‘skill training’ was given Additional information: The study was powered significantly to compare ‘combined treatment’ with ‘no treatment’ |
Objective N of IEF ‘responders’ (≥ 50% decrease in IEF): I: 27/44, II: 11/44, III: 26/46, IV: 12/46 Episodes of leakage per week (pooled paper diaries completed at each visit, median % decrease): I: 44, 57.4%, II: 44, 28.9%, III: 46, 56.5%, IV: 46, 34.7% N of pad changes per week (median % decrease): I: 44, 45.7%, II: 44, 10.5%, III: 46, 35.3%, IV: 46, 24.8% Surrogate outcomes Adherence (medication) at 12 weeks (% of prescribed doses taken): I: 93%, II: 92%, III: 93%, IV: 93%, no significant difference between groups Adherence (PFMT) at 12 weeks (% of prescribed contractions performed): I: 86%, II: 89%, III: 76%, IV: 88%, no significant difference between groups Pelvic floor muscle grade (9-point scale, mean increase, SD): I: 1.34 (1.28), p < 0.001, II: NA, III: NA, IV: 1.41 (1.93), p < 0.001; endpoint mean muscle grade for imitation PFMT groups II/III = 4.7 and 5.3 (groups not specified) Adverse events N experiencing adverse events that were significantly more common with duloxetine than with placebo: duloxetine: 85/104, placebo: 58/97 Adverse events that were significantly more common with duloxetine than with placebo: nausea, dizziness, dry mouth, constipation, insomnia, somnolence, aesthesia Serious adverse events: 1 patient in duloxetine group experienced rectal bleeding not attributed to the study SNRI; laboratory and vital sign data indicated no clinically relevant safety issues for duloxetine compared with placebo Discontinued treatment because of adverse events: I: 12/52, II: 4/47, III: 16/52, IV: 4/50 |
Subjective PGI-I (‘very much better’, ‘much better’ or ‘a little better’): I: 36/51, II: 19/45, III: 27/50, IV: 32/49 Quality of life I-QoL (mean score increase): I: 51, 13.1%, II: 45, 4.8%, III: 50, 8.3%, IV: 49, 7.8% |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Glavind 1996 150 Study design/method: 2-arm RCT. Single centre, Denmark Duration of study: 4 weeks treatment, plus follow-up at 3 months and questionnaire at 2–3 (median 2.5) years |
Inclusion criteria: Women with USI, with normal cystometrograms, leaking urine while coughing and jumping Exclusion criteria: Detrusor instability, previous surgery for urinary incontinence N randomised: 40 N lost to follow-up: I: 1/20, II: 5/20 Type of incontinence: USI Age (years, median, 95% CI): 45 (40-48) Pad test result (g, median, 95% CI): I: 9 (5 to 22) II:12.8 (9 to 44) |
I. PFMT + BF, N = 19 II. PFMT, N = 15 (N in analysis) PFMT + BF: Standard procedure of physiotherapy 2–3 times per patient with individual instruction from physiotherapist, receiving verbal instruction in exercises and presentation of anatomical charts of the pelvic floor muscles. Instructed to perform the same exercises at home at least three times a day, and as often as possible. In addition, four clinic visits (1 × 30-minute session per week for 4 weeks) for visual BF (the recording of the vaginal EMG) from a vaginal surface electrode (Dantec 21L20, Skovlunde, Denmark) and rectal catheter. Patient continuously observed vaginal EMG during 10 VPFMCs sustained for 5–10 seconds in supine, standing and sitting positions (i.e. 30 VPFMCs in total) PFMT: As above but without BF. 2–3 clinic visits over 4 weeks and daily home exercise programme. PFMT = at least three times a day at home Additional information: Patients in the PFMT + BF group received more instruction than the control (PFMT only) group |
Objective Cured after 3 months ( < 2 g on 1-hour pad test with a bladder volume of 3/4 of cystometric capacity): I: 11/19, II: 3/15 1-hour pad test after 1 month (with a bladder volume of 3/4 of cystometric capacity) (g, median, 95% CI): I: 2.5 (1 to 10), II: 19.0 (0 to 51) 1-hour pad test after 3 months (with a bladder volume of 3/4 of cystometric capacity) (g, median, 95% CI): I: 0.8 (0 to 4), II: 10 (2 to 27) Adherence (N doing PFMT regularly 2–3 years after treatment) I: 17/19, II: 7/14 |
Subjective Cure at 2–3 years post treatment (N considering themselves still cured): I: 5/19, II: 0/14 Improvement at 2–3 years post treatment (N considering themselves improved compared with before treatment): I: 8/19, II: 4/14 Acceptance of condition 2–3 years post treatment (those feeling they could accept their present degree of incontinence) I: 14/19, II: 7/14 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Goode 2003 123 Study design/method: 3-arm RCT. Stratified randomisation by types and severity of incontinence and race (black or white). USA Duration of study: 8 weeks |
Inclusion criteria: Community-dwelling women aged ≥ 40 years, with predominantly stress urinary incontinence demonstrated during urodynamic testing, ambulatory; average at least two incontinence episodes per week and persisting for at least 3 months Exclusion criteria: Continual leakage, post void residual urine volume greater than 150 ml, severe uterine prolapse (past the vaginal introitus), decompensated congestive heart failure, haemoglobin A1C ≥ 9, or impaired mental status N randomised: 200 N lost to follow-up: I: 12/66, II: 8/67, III: 25/67, difference in attrition rates, p = 0.001 Type of incontinence (USI/MUI with stress as the predominant pattern): I: 19/47, II: 23/44, III: 25/42 Age (years, mean, SD): I: 57.7 (10.0), II: 54.9 (9.4), III: 55.9 (10.0), p > 0.10 Episodes of leakage per week (mild (< 5)/ moderate (5–10)/severe ( > 10)]: I: 13/20/33, II: 15/17/35, III: 17/18/32, p > 0.10 Episodes of leakage per week (mean, SD): I: 66, 15.1 (13.7), II: 67, 15.6 (13.1), III: 67, 14.8 (13.9) % vaginal wall prolapse: Cyctocoele 2° or 3°: I: 23/66, II: 27/67, III: 27/67; rectocoele 2°; or 3°: I: 11/66, II: 10/67, III: 11/67; uterine prolapse: I: 5/66, II: 2/67, III: 4/67; bladder neck hypermobility: I: 32/66, II: 28/67, III: 26/67, p > 0.10 Other: Ethnicity, education (high school graduate), % prior incontinence surgery, parity |
I. Behavioural training, N = 66 II. Behavioural training + ES, N = 67 III. Self-administered behavioural programme, N = 67 (N in analysis) Behavioural training: (1) Anorectal BF to teach correct pelvic floor muscle contraction at visit 1. (2) Verbal and written instructions for three sessions of PFMT at home daily. Set: 15 VPFMC, 2–4 seconds hold, 2–4 seconds rest, progressing to maximum 10 seconds hold, 10 seconds rest. One set each lying, sitting and standing. Once daily, practice interruption or slowing of the urinary stream during voiding. Duration of training: 8 weeks. (3) Bladder control strategies, comprising ‘stress strategies’ to contract pelvic floor muscles during any activity that usually result in leakage (e.g. coughing, sneezing) and ‘urge strategies’ to contract pelvic floor muscles repeatedly to diminish urgency instead of rushing to the toilet. (4) Self-monitoring with bladder diaries. Four clinical visits at 2-week intervals. Behavioural training was implemented by female nurse practitioners who were specially trained by the behavioural psychologist (KL Burgio) and physician principal investigator (PS Goode) Behavioural training plus ES: Include all of the components of behavioural training with the addition of home ES. Four clinical visits at 2-week intervals. ES = Home unit (Hollister InCare). Vaginal probe, biphasic pulses (frequency of 20 Hz), pulse width of 1 milliseconds, and pulse train to rest period of 1:1 Current intensity adjusted to maximum tolerable level up to 100 mA. Simultaneous with each muscle contraction induced by ES, patients performed VPFMC. 15 minutes every other day. On alternate days, perform three sessions of PFMT (as in the behavioural training group Self-administered behavioural training: Given a booklet including the entire behavioural programme but completely self-administered. Given an appointment for a return visit at 8 weeks Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only |
Objective Cure (100 reduction in frequency of incontinence by 2-week bladder diary, data from figure): I: 11/66, II: 10/67, III: 10/67 Cure or improvement (≥ 50% reduction in frequency of incontinence by 2-week bladder diary, data from figure): I: 53/66, II: 57/67, III: 37/67 Adverse events N experiencing adverse events: I: 0/66, II: 4/67, III: 0/67 Adverse events: vaginal irritation Discontinued treatment because of adverse events: I: 0/66, II: 0/67, III: 0/67 |
Subjective Patient perception of treatment outcome (‘much better’ or ‘better’): I: 45/47, II: 45/47, III: 32/40 Quality of life Incontinence Impact Questionnaire (total score): Overall, improved from 93.1 to 57.6 over time. No significant group effects or group by time interactions Hopkins Symptom Checklist 90-R: No significant changes SF-36: No significant changes |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Hahn 1991 175 Study design/method: 2-arm RCT. Single centre, Sweden. Duration of study: 6 months treatment, and follow-up at 1 and 4 years |
Inclusion criteria: Women previously not operated upon with USI, consecutively referred for surgery Exclusion criteria: Neurological pathology, detrusor instability N randomised: 20 N lost to follow-up: none at 6 months Type of incontinence: USI Age (years, mean): 47.2 years (range 34–64); no difference between groups Urine loss at pad test (g, mean, SEM): I: 66.5 (14.38), II: 55.6 (21.21), no difference between groups Parity: No difference between groups |
I. PFMT, N = 10 II. ES, N = 10 (N in analysis) PFMT: Given instruction in pelvic floor anatomy and physiology Correct VPFMC taught by vaginal autopalpation. Set: (1) submaximal contraction with 2-second hold; 2) 5–10 maximum contractions with 5-second hold; also ‘The Knack’ (VPFMC with provocation such as cough); (3) submaximal contraction with 30- to 40-second hold. Exercises were performed in the supine, sitting and standing positions. Sets per day: 6–8 times. Duration of training: 6 months. Supervision: Individual training with physiotherapist weekly for 4 weeks, then monthly for 5 months ES: IFT vaginal probe, intermittent stimulation with alternating pulses at a repetition frequency of 10, 20 and 50 Hz. Home device (Contelle), 6–8 hours per night for 6 months Additional information: Patients not cured by the first treatment (after 6 months) were offered the other one (N = 13), then evaluated at 1 year and 4 years (groups combined) |
Objective N cured after treatment (essentially dry < 2 g weight increase at pad test (Sutherst et al. 1981), modified to more provocative effects): I: 1/10, II: 4/10 Pad test after treatment (Sutherst et al. 1981): I: significant improvement, p < 0.01, II: significant improvement, p < 0.05; no significant differences in the rates of improvement between the two groups, p < 0.10 Surrogate outcomes Urodynamic vaginal pressure recordings after treatment (maximal pressure during squeezing, cmH2O): I: before 15.88, after 14.59, NS, II: before 16.97, after 16.96, NS Urodynamic vaginal pressure recordings after treatment (sustained pressure response to squeezing, cmH2O): I: before 0.3, after 0.3, NS, II: before 0.2, after 0.33, NS Long term N having incontinence surgery (Burch colposuspension) at 4 years (after crossover): 5/19, four owing to inadequate primary effect of conservative treatment, one because of recurrence of symptoms Pad test at 4 years (after cross over) among 14/19 not operated upon: 4/14 further improved, 8/14 unchanged, 2/14 some degree of recurrence of SUI Adherence at 4 years (after crossover): 5/14 training regularly, 6/14 training now and then, 3/14 no training |
Subjective Subjective ratings after treatment (cured/insignificant symptoms/improved/unchanged/worse): I: 1/5/4/0/0, II: 1/4/3/2/0 Subjective ratings at 4 years (after crossover) (those who did not have surgery only): improved: 1/14, unchanged: 8/14, recurrence of symptoms: 5/14 Other: Individual patients’ data (volume of urine loss) reported in figure 2 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Haig 1995190 (abstract only) Study design/method: Quasi-RCT, consecutive allocation. Single centre, UK, pilot study Duration of study: 3 months |
Inclusion criteria: Women with USI Exclusion criteria: Not reported N randomised: 58 N lost to follow-up: I: 12/20, II: 9/20, III: 7/18 Type of incontinence: USI Age (years, mean, SD): I: 55 (7.2), II: 51 (7.5), III: 51 (7.5) N of micturition in 48 hours (mean, SD): I: 8, 18.5 (7.7), II: 11, 15.6 (4.2), III: 11, 19.4 (7.8) 48-hour pad test (g, mean, SD): I: 8, 15.7 (75.9), II: 11, 33.5 (38.9), III: 11, 87.6 (11.3) Perceived severity of leakage (visual analogue scale, not defined): I: 8, 3.3 (2.6), II: 11, 3.6 (1.9), III: 11, 4.3 (2.0) Quality of life: Perceived effect on life (visual analogue scale, not defined): I: 8, before 4.3 (2.6), after 2.0 (1.6), II: 11, before 3.2 (1.8), after 1.0 (0.7), p = 0.00078, III: 11, before 4.6 (2.4), after 2.5 (2.6), p = 0.021 Disparity of pretreatment severity of leakage between groups (see N of micturition and 48-hour pad test) |
I. PFMT, N = 8 II. PFMT + ES, N = 11 III. PFMT + Sham ES, N = 11 (N in analysis) PFMT: Correct VPFMC taught by: physiotherapist. Continence-promoting advice given (no further detail). Patients attended five times in the first month, 12 times in the second month and three times in the third month. At each visit, PFMT performed in sitting and standing position in the first and third months, and in four positions (sitting, standing, lying and kneeling) at the second month. ‘Fast’ and ‘hold’ contractions in sitting and standing at home three times daily PFMT + ES: PFMT as above. During the second attendances received 20 minutes of active interferential therapy (IFT) at 10–40 Hz using vaginal electrodes in addition to PFMT PFMT + sham ES: PFMT as above. 20 minutes of placebo IFT using vaginal electrodes during the second month’s attendances in addition to PFMT |
Objective N of micturition in 48 hours (mean, SD): I: 8, 13.1 (3.3), p = 0.033 (pre-post), II: 11, 13.3 (2.2), p = 0.006 (pre-post), III: 11, 15.5 (6.8), p = 0.0005 (pre-post) 48-hour pad test (g, mean, SD): I: 8, 12.2 (9.4), p = 0.25 (pre-post), II: 11, 10.6 (6.2), p = 0.027 (pre-post), III: 11, 38.7 (49.4), p = 0.014 (pre-post) |
Subjective Perceived severity of leakage (visual analogue scale, not defined, reduction in score reflects improvement; mean, SD): I: 8, 2.2 (2.2), p = 0.033 (pre-post), II: 11, 1.8 (1.3), p = 0.006 (pre-post), III: 11, 2.2 (1.8), p = 0.0005 (pre-post) Quality of life Perceived effect on life (visual analogue scale, not defined, mean, SD): I: 8, 2.0 (1.6), p = 0.013 (pre-post), II: 11, 1.0 (0.7), p = 0.00078 (pre-post), III: 11, 2.5 (2.6), p = 0.021 (pre-post) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Haken 1991179 (abstract only) Study design/method: 2-arm RCT. Single centre, England Duration of study: 10 weeks |
Inclusion criteria: Women with USI Exclusion criteria: Not reported N randomised: 64 N lost to follow-up: I: 3/33, II: 8/31 Type of incontinence: USI Age (years, mean): 48 years |
I. PFMT, N = 30 II. VC, N = 23 (N in analysis) PFMT: Individual training with continence advisor. Three clinic visits. Education in appropriate anatomy and physiology. PFMT = 5 VPFMC 10 times per day VC: Individual training with continence advisor. Three clinic visits. Education in appropriate anatomy and physiology. Hold cone for 15 minutes twice a day, increasing the cone weight when successful on two consecutive occasions. Femina cones [five conical weights (?)] |
Objective Improvement (on 40-minute pad test, with standardised bladder volume, ICS Proceedings 1988): I: 19/30, II: 17/23 Adverse events Adverse events: ‘Difficulty remembering to use the technique was a significant feature in the (PFMT) group which was not apparent in those using cones. Causes of withdrawal in the cones group were predominantly aesthetic dislike of the technique and difficulties associated with vaginal prolapse’ |
Subjective Subjective assessment on visual analogue scale: Significant improvement in both groups (p < 0.05) but no between-group difference Patient satisfaction: Difficulty remembering to use the technique’ apparent in the PFMT group but not in those using VC |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Hay-Smith 2003 164 Study design/method: 2-arm RCT. Single centre, New Zealand Duration of study: 20 weeks |
Inclusion criteria: Community-dwelling women with symptoms of SUI with ≥ 2 leakage episodes per week Exclusion criteria: Reversible causes of incontinence (e.g. SNRI side effects), uncontrolled metabolic conditions (e.g. diabetes), clinical history and/or uroflowmetry indicated voiding difficulty, active urinary tract infection, pelvic organ prolapse below the hymenal ring, unable to perform a correct VPFMC after instruction, use of concomitant therapies for incontinence, less than 16 years of age, inability to read, write or verbally communicate in English N randomised: 128 N lost to follow-up: I: 4/64 (2/4 had follow-up data and were included in analysis), II: 3/64 Type of incontinence: SUI or MUI with stress as predominant pattern (confirmed by author) Age (years, mean, SD): I: 48.9 (13.1), II: 48.7 (13.2) Episodes of leakage in 24 hours (mean, SD): I: 63, 1.7 (1.7), II: 62, 1.9 (2.2) Pelvic floor muscle grade (grades 1 or 2/grade 3/grades 4 or 5): I: 30/15/18, II: 28/25/6 Other: BMI, ethnicity, % prior incontinence surgery, parity, % postmenopausal |
I. Motor learning, N = 62 II. PFMT with motor learning, N = 61 (N in analysis) Motor learning: Correct VPFMC taught by: physiotherapist using vaginal palpation. Individualised, progressive, training programme. Also given a leaflet on PFMT and an insert outlining ‘The Knack’. Duration of training: 20 weeks. Supervision: four physiotherapy visits and three phone calls to progress the programme and maintain motivation PFMT with motor learning: Received all the teaching and recommendations as above. In addition, women received individualised, progressive PFMT strength training. Women were advised to complete a set of contractions, three times a day, every day (or a minimum 3 days a week) and that each contraction should be performed with maximal effort. Training progressed by one contraction, or one second per hold, each week until a set comprised 12 contractions, with each contraction held for 8 seconds Both groups: Advice on lifestyle was given where appropriate. Women voiding more than seven times during waking hours were given advice on frequency strategies. Women with urgency were advised on common urge suppression techniques. A timed voiding programme was not used Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only Author confirmed that some women had MUI but ‘only women whose predominant symptom was SUI (leakage with cough, sneeze, exercise or other exertion) were eligible for inclusion’ |
Objective Improvement (decrease by more than 4 g on 24-hour pad test): I: 18/47, II: 15/48 Adverse events N experiencing adverse events: None reported |
Subjective Self-reported change in leakage (cure/ much better/somewhat better/no change/somewhat worse/much worse): I: 4/25/19/14/0/0, II: 1/24/27/8/1/0 Quality of life King’s Health Questionnaire (mean, SD): (1) General health perception: I: 55, 18.2 (17.7), II: 60, 17.1 (19.3), p = 0.751 (2) Incontinence impact: I: 55, 38.8 (27.8), II: 60, 49.4 (24.9), p = 0.032 (3) Role limitation: I: 52, 20.5 (27.7), II: 57, 27.2 (23.7), p = 0.178 (4) Physical limitation: I: 51, 22.6 (22.8), II: 57, 31.3 (22.5), p = 0.048 (5) Social limitation: I: 51, 10.5 (21.3), II: 56, 11.8 (18.6), p = 0.728 (6) Personal relationships: I: 40, 14.6 (24.8), II: 40, 13.8 (23.2), p = 0.877 (7) Emotions: I: 51, 20.0 (24.1), II: 58, 26.1 (28.0), p = 0.236 (8) Sleep/energy: I: 51, 32.0 (19.7), II: 54, 28.4 (19.6), p = 0.346 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Henalla 1990125 (abstract only) Study design/method: 3-arm RCT, parallel design. Single centre, UK Duration of study: 6 weeks |
Inclusion criteria: Postmenopausal women with USI Exclusion criteria: No further criteria stated N randomised: 26 N lost to follow-up: None? Type of incontinence: USI Age (years): Mean 54 (range 49–64) % vaginal prolapse: See surgery Other: % postmenopausal 100% |
I. Estrogen cream, N = 11 II. PFMT, N = 8 III. No treatment, N = 7 IV. Surgery (secondary treatment), N = 22 (I: 11, II: 4, III: 7) Estrogen: Vaginal cream (Premarin) 2 g per night PFMT: No detail given Surgery: All failures (< 50% reduction from baseline pad test) were subjected to surgical repair by the abdominal or vaginal route depending on the absence or presence of associated genital prolapse. Further assessment was carried out 6 weeks following surgery |
Objective N cured or improved at 3 months (perineal pad weighing test, not defined): I: 0/11, II: 4/8, III: 0/7, IV: 21/22 Note: ‘Failure’ defined as < 50%reduction in pad weight from baseline |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Hofbauer 1990 126 Study design/method: 4-arm RCT, parallel design. Single centre, Austria. German publication Duration of study: ?6 weeks treatment and further follow-up at 6 months. Assessment immediately after treatment and at 6 months |
Inclusion criteria: Women with USI Exclusion criteria: Urge incontinence N randomised: 43 N lost to follow-up: None? Type of incontinence: USI (grade 1/2/3): I: 0/9/2, II: 3/4/4, III: 2/3/6, IV: 4/4/2 Note: Grading according to Ingelmann-Sundberg (1952) Age (years, mean): I: 62.9, II: 51.0, III: 59.7, IV: 59.8, overall mean 57.5 (SD 12), (30–88) Other: Prior incontinence surgery: 16/43 |
I. PFMT + ES, N = 11 II. PFMT, N = 11 III. ES, N = 11 IV. Sham ES, N = 10 (N in analysis) PFMT + ES: PFMT and ES as below PFMT: Exercise programme including PFMT, abdominal and hip adductor exercise, twice a week for 20 minutes with therapist, and daily home programme ES: Three times per week, 10 minutes per treatment, 6 weeks. Vaginal and perineal (active) and lumbar (inactive) electrodes. Faradic. Output increased to noticeable contraction and patient added voluntary effort Sham ES: ES as above but current so low that no effect was expected |
Objective N having incontinence surgery: Patients who were unsuccessful in treatment had surgery (number not stated) Adverse events Adverse events: None reported |
Subjective Cure (?symptom scale, at ?6weeks or ?6 months): I: 3/11, II: 6/11, III: 1/11, IV: 0/10 Cure or improvement (?symptom scale, at ?6weeks or ?6 months): I: 7/11, II: 7/11, III: 3/11, IV: 0/10 Note: Authors state that results were similar immediately after treatment and at 6 months |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Jeyaseelan 2000 131 Study design/method: 2-arm RCT. Three centres, UK Duration of study: 8 weeks |
Inclusion criteria: Women with USI, no neurological conditions Exclusion criteria: Previous ES for stress incontinence, prolapse, pregnancy, pacemakers and cardiomyopathy, abnormal urological/gynaecological findings, urinary tract/vaginal infection, recent pelvic floor surgery (within 6 months) N randomised: 27 N lost to follow-up: I: 2/14, II: 1/13 (Three withdrawals because treatment regimen too demanding) Type of incontinence: USI Episodes of leakage per week (median, range): I: 4.5 (0–9), II: 2 (0–8), p > 0.05 1-hour pad test (g, median, range): I: 11 (0–35.4), II: 4.6 (0–43) Incontinence Impact Questionnaire (score range 0–100, mean, SD): I: 32.55 (18.02), II: 39.22 (18.38) Urogenital Distress Inventory (score range 0–100, mean, SD): I: 46.27 (16.27), II: 41.58 (15.44) |
I. ES, N = 12 II. Sham ES, N = 12 (N in analysis) ES: Portable stimulator (PS1, Dynamic Medical Instruments). Patterned Neuromuscular Stimulation. Background low frequency (target slow twitch fibres) and intermediate frequency with an initial doublet (target fast twitch fibres), vaginal probe, 1 hour per day for 8 weeks (except when menstruating). Patients asked to keep stimulation diary Sham ES: One 250 microsecond/minute for 1 hour. This method of stimulation has been proven to have no physical effect on muscle Additional information Data pertaining to the frequency/volume charts (7-day) not presented (not published) due to incomplete data |
Objective Change in episodes of leakage per week (median, range): I: 0 (–5 to 4), NS, II: –2 (–4 to 0), NS Change in 1-hour pad test (g, median, range): I: 0.5 (–33 to 71), NS, II: 0.1 (–15 to 61), NS Surrogate outcomes Adherence (patient’s stimulation diary): I: 71–98%, II: 64–100% Change in pelvic floor muscle strength (perineometer, cmH2O, mean, SD): I: 3.1 (12.5), II: 1.0 (5.3), NS Change in pelvic floor muscle endurance (perineometer, cmH2O, mean, SD): I: 4.8 (13.9), II: –2.0 (5.3), NS Change in pelvic floor muscle strength (digital assessment, scale 0–15, median, range): I: 1 (–1 to 5), p < 0.01, II: 1 (–2 to 4), NS Adverse events Discontinued treatment because of adverse events: treatment regimen too demanding, I: 2/14, II: 1/13 |
Quality of life Incontinence Impact Questionnaire (score range 0–100, mean change, SD): I: –4.1 (16.4), II: –9.1 (17.1), NS Urogenital Distress Inventory (score range 0–100, 0 = not bothered by incontinence, 100 = greatly bothered by incontinence) (mean change, SD): I: –11.8 (15.9), II: –3.3 (8.3), p = 0.01 SF-36: No significant difference between groups |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Johnson 2001 167 Study design/method: 2-arm RCT. Single centre, USA Duration of study: Treatment for 6 weeks |
Inclusion criteria: Women diagnosed with USI and self-report of two or more incontinent episodes per day for the previous 3 months, English-speaking, non-pregnant, free of bladder or vaginal infection, not currently taking medications for treatment of USI, showed adequate estrogenisation of the vaginal mucosa, had no urethral collagen injection therapy, and had no history of neuromuscular disease, radical pelvic/perineal surgical intervention or other serious physical or psychological problems Exclusion criteria: N randomised: 37 (N in each arm not reported) N lost to follow-up: 5/37 Type of incontinence: USI Age (years, mean, SD): I: 16, 51.00 (10.21), II: 16, 49.50 (11.09) Leakage episodes per day (mean, SD): I: 16, 4.04 (3.32) (1.86–13.00), II: 16, 3.18 (1.85) (2.00–8.50) 10-hour weighted pad test (g, mean, SD): I: 16, 16.04 (27.26) (2.14–111.42), II: 16, 9.85 (13.98) (1.76–55.69) Pelvic floor muscle endurance (mean, SD): I: 16, 5.69 (3.42) (2.00, 12.00), II: 16, 5.94 (3.62) (2.00, 15.00) Pelvic floor muscle sustained contraction (mean, SD): I: 16, 2.35 (2.92) (0.00–9.20), II: 16, 3.60 (3.99) (0.00–10.00) Pelvic floor muscle mean maximal contraction (mean, SD): I: 16, 4.88 (6.88) (0.00–28.00), II: 16, 8.00 (6.72) (1.00–20.00) |
I. PFMT with submaximal voluntary contraction + BF, N = 16 II. PFMT with near maximal voluntary contraction + BF, N = 16 (N in analysis) PFMT with submaximal voluntary contraction + BF: InCare Contimed II (InCare Medical, Libertyville, IL) home biofeedback device was used with the vaginally inserted pressure probe tubing before daily tests of mean maximal contractions (MMC) strength and during exercise sessions. InCare Perineal Reduction System (PRS8900) was used in pre- and post-training tests for measuring MMC strength of the pelvic floor muscles and endurance testing. Participants used diagrams that indicated precalculated goal intensity, based on a daily test of MMC effort. In pretesting and post-testing, surface electrodes were placed abdominally to determine inappropriate muscle recruitment, and perianally to temporarily correlate electromyographic muscle response to pressure readings. Correct VPFMC confirmed by the investigator using biofeedback and surface EMG electrodes at first visit. Participants were given written and oral instructions to perform PFMT at specified intensity (60% of MMC force), duration (15 minutes) and repetition (three times a day for 6 weeks) using the biofeedback device. Exercise information was recorded by the devices and downloaded for analysis PFMT with near maximal voluntary contraction + BF: PFMT and devise use as above. Participants were given written and oral instructions to perform PFMT at specified intensity (90% of MMC force), duration (10 minutes) and repetition (three times a day for 6 weeks) using the biofeedback device |
Objective Cure (no episodes of urine loss on daily diary during the 8th week of the study, i.e. 1 week immediately after treatment phase): I: 4/16, II: 6/16 Episodes of leakage in 24 hours (daily diary for 8 weeks, mean, SD): I: 1.15 (2.55) (0.00–10.00), II: 0.79 (1.65) (0.00–6.57) Note: Daily diaries were recorded during the 6 weeks of treatment and 1 week before and after 10-hour pad test (g, mean, SD): I: 3.41 (4.79) (0.41–20.32), II: 3.84 (5.29) (0.12– 21.29) Surrogate outcomes Pelvic floor muscle endurance (N of contractions completed prior to fatigue, mean, SD): I: 16, 17.25 (8.80) (10.00–37.00), II: 16, 12.00 (6.12) (2.00–23.00) Pelvic floor muscle sustained contraction (seconds, mean, SD): I: 16, 9.06 (2.20) (1.40–10.30), II: 16, 9.63 (1.51) (4.70–11.30) Pelvic floor muscle mean maximal contraction (cmH2O, mean, SD): I: 16, 19.19 (10.37) (7.00–42.00), II: 16, 18.38 (14.30) (2.00–62.00) EMG amplitude (microvolts): I: 16, 4.44 (1.99) (1.00–8.00), II: 16, 3.69 (2.52) (1.00–10.00) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Karagkounis 2007194 (abstract only) Study design/method: 2-arm RCT, Greece Duration of study: > 2 weeks? (unclear) |
EMG amplitude (microvolts): I: 16, 2.00 (1.27) (1.00–5.00), II: 16, 1.94 (0.77) (1.00–3.00) Inclusion criteria: Women aged 44–68 with stress urinary incontinence (clinical and urodynamic diagnosis) Exclusion criteria: Not reported N randomised: 197 N lost to follow-up: Not reported Type of incontinence: USI Age (years, average, range): 58.7 (44–68) |
I. PFMT + SNRI, N = 98 II. Surgery, N = 99 (N randomised) PFMT + SNRI: Duloxetine–HCL 40 mg twice a day, with simultaneous PFMT (no further detail) Surgery: The TVT obturator system under local or regional anaesthesia followed by 2-day hospitalisation Additional information: Not relevant for direct head-to-head comparisons; data were therefore extracted for primary outcomes only |
Hospital length of stay: I: not reported, II: 2 days Adverse events N experiencing adverse events: I: 4/98, II: 0/99 Adverse events: Nausea, headaches, insomnia |
Subjective Cure or improvement; (based on the survey): I: 55% symptom relief in 78/98 (80%), and then 90/98 (92%) ‘in the following 2 weeks of treatment’, II: 99/99 cured Quality of life Incontinence Quality of Life: I: ‘without a lifetime cure achievement’, II: ‘total lifetime symptom relief’ (perfect score in IQoL questionnaire) Cost: I: €378/US$480 for a 6-month period (€2/US$2.67 per day), no absence from daily activities was advised, II: €910/US$1156, encouraged to return to daytime activities after 1 month |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Kim 2007 118 Study design/method: 2-arm RCT. Single centre, Japan Duration of study: 3 months (and 1-year follow-up after crossover) |
Inclusion criteria: Community-dwelling Japanese women aged 70 and older with symptoms of SUI Exclusion criteria: Urine leakage occurring less than once per month; mixed or urge UI N randomised: 70 N lost to follow-up: I: 2/35, II: 3/35. Those who attended 15 or more of the 24 exercise sessions were considered to have completed the intervention Type of incontinence: SUI Age (years, mean, SD): I: 76.6 (5.0), II: 76.6 (3.8), p = 0.96 Frequency of leakage (daily/1 every 2 days/1–2 per week/1–3 per month): I: 11/2/9/13, II: 7/4/6/18, p = 0.40 Frequency score of urine leakage: I: 3.4 (1.3), II: 3.0 (1.3), p = 0.14 Other: BMI |
I. PFMT + fitness exercise, N = 33 II. No treatment, N = 32 (N in analysis) PFMT + fitness exercise: 60-minute group sessions two times per week for 12 weeks. Taught the structure of the pelvic floor muscle. PFMT = 10 fast VPFMC with 3 seconds hold, and 10 sustained VPFMC with 6–8 seconds hold, in sitting, lying and standing. Fitness exercise = body awareness, breathing, relaxation, and strength training of the thigh, abdominal and back muscles were performed between PFMT exercise positions, with additional training including bending the knees, tilting the pelvis backwards and forwards, lifting the buttocks on the back with the knee bent, raising one leg while lying on the back, and others; also used two kinds of training balls (21 cm and 45- to 55-cm in diameter); the ball exercises included sitting on the ball, rolling the ball and pelvis forwards and backwards, moving from side to side while squeezing the thighs and others Control subjects: Instructed to lead a normal life and to refrain from special exercises aiming to increase muscle strength (not only pelvic floor muscles) or walking speed to decrease BMI, or to improve their dietary habits Additional information: The control group was crossed over to the treatment group after 3 months. After 3 months, both groups were followed for 1 year. Only data from the first 3 months were extracted The study authors judged that the 3-day diary underestimated the frequency of ‘mild’ (not ‘severe’) urine leakage and hence tended to overestimate the effect of intervention. For this reason the authors decided not to use 3-day diary data and instead use the 6-point scale |
Surrogate outcomes Adherence: In group I, 10/35 (29%) attended all 24 sessions and 23/35 (66%) attended more than 20 sessions In group I there were significant increases over time (p < 0.05) in adductor muscle strength and maximum walking speed (?or usual walking speed – table and text do not match), and also a significant decrease (p < 0.05) in body weight and BMI. No significant changes were observed in group II |
Subjective Cured of urine leakage: I: 18/33, II: 3/32 Note: In terms of urine leakage episodes based on an interview asking if woman has experienced urine leakage and, if yes, the frequency of the leakage using the 6-point scale. Frequency score of urine leakage (point, mean, SD): I: 1.5 (1.8), II: 2.4 (1.4) Note: Based on the International Consultation on Incontinence Questionnaire (ICIQ, Avery et al. 2004); 0 = no urine leakage, 1 = less than once a month, 2 = 1–3 times per month, 3 = 1–2 times per week, 4 = once every two days, 5 = every day. Subgroup analysis Factors associated with ‘cure’ at 3 months – those with a ‘decreased’ BMI (p = 0.03) and ‘increased’ (?maximum) walking speed (p = 0.04) were significantly more likely to be cured than those with ‘increased’ or ‘unchanged’ BMI or ‘decreased’ or ‘unchanged’ walking speed. No difference was found in proportion of cured subjects with improved adductor muscle strength |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Kinchen 2005 140 Study design/method: 2-arm RCT. Multicentre, 24 sites, USA Duration of study: 36 weeks (plus 1 week prerandomisation) |
Inclusion criteria: Ambulatory women aged ≥ 18, with ≥ 1 IEF/week with SUI symptoms that have been going on for ≥ 3 months Exclusion criteria: Pregnant/breastfeeding women, urinary tract infection, previous participation in a duloxetine trial, arrhythmias, poorly controlled/uncontrolled hypertension, liver disease, seizure disorders or unstable cardiac conditions N randomised: 451 N lost to follow-up: I: 62/224, II: 50/227 Type of incontinence: SUI: I: SUI 36/224 (16%), stress predominant MUI 156/224 (70%), ‘balanced’ UI 11/224 (5%), UUI 21/224 (9%), II: SUI 36/227 (16%), stress predominant MUI 155/227 (68%), ‘balanced’ UI 15/227 (7%), UUI 21/227 (9%) Age (years, mean, SD): I: 52.7 ± 13.0 , II: 53.5 ± 13.0 Frequency of stress incontinence episodes per week (> 5 stress episodes/5-13/≥ 14): I: 67/72/85, II: 68/87/72 Episodes of leakage per week (median): I: 7.5, II: 7 I-QoL (average score): I: 210, 61.5, II: 218, 60.2 Other: Ethnicity, prior incontinence surgery, PFMT use, genital prolapse on straining |
I. Duloxetine 80 mg as 40mg b.i.d. (twice daily), N = 224 (210) II. Placebo, N = 227 (218) [N randomised; and N in ‘ITT’ analysis in brackets with women with at least one postrandomisation measure (last outcome measure carried forward)] Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only This is a naturalistic study – ‘… at any point after randomization, subjects could choose to remain on study SNRI as randomized, reduce study SNRI dosing in any way, remain on study SNRI with augmentation by other treatments, or suspend study SNRI and receive other treatments. Subjects on placebo were not allowed to be switched to duloxetine, nor were duloxetine subjects ever switched to placebo … . Regardless of whether subjects continued to take the study medication, all subjects were to be followed up according to the protocol. However, subjects who chose to have surgery for urinary incontinence symptoms were discontinued from the study [N not reported]’ Women reported planned use of another intervention including weight reduction, PFMT, estrogen, anticholinergics, pseudoephedrine (phenylpropanolamine), smoking cessation, bladder retraining, ‘devices’ and biofeedback. Almost half of women reported actual use of estrogen products, and 11% reported use of anticholinergic medications. 40.4% performed PFMT inconsistently and 15.4% performed PFMT consistently |
Surrogate outcomes Adherence (N still on study SNRI at end visit): I: 85/224 (37.9%), II: 122/227 (53.7%) Adverse events N experiencing at least one adverse event: I: 198/224, II: 159/227 Adverse events (for which there are statistically significant differences between groups): nausea; fatigue; insomnia; dizziness; headache; somnolence; dry mouth; constipation; diarrhoea; vomiting; increased sweating; decreased appetite; anxiety; tremor; decreased libido; lethargy; nightmare; fungal infection Serious adverse events: I: 8/224 (16 SAEs), none of which was considered to be related to study SNRI, II: 7/227 (eight SAEs), of which two SAEs were considered to be related to study SNRI Discontinued treatment because of adverse events: I: 20/224, II: 5/227 |
Subjective PGI-I (‘better’) at 9 months: I: 103/210 (49.1%), II: 90/218 (41.5%) PGI-I (‘better’) at 3 months: I: 148/208 (71%), II: 111/218 (51%) Quality of life Change in I-QoL at 3 months: I: 208, 13.0, II: 218, 10.4, p = 0.07 Change in I-QoL at 9 months: I: 210, 13.8, II: 218, 12.1, p = 0.26 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Klarskov 1986 184 Study design/method: 2-arm RCT. Single centre, Denmark Duration of study: 4 months treatment + follow-up at 12 months and median 6 (4–8) years |
Inclusion criteria: women with USI, no previous surgery or systematic PFMT or UUI Exclusion criteria: Surgery was indicated for reasons other than urinary incontinence (e.g. prolapse operation, hysterectomy), patients expected to be unable to follow the training instructions for mental reasons N randomised: 50 N lost to follow-up: Not reported Type of incontinence: USI Age (years, median, range): 48 (31–66) Episodes of leakage in 3 days (3-day voiding and incontinence chart, median, range): I: 24, 6 (0–31), II: 26, 6 (0–39) % vaginal wall prolapse: Bladder base insufficiency: I: 13/24, II: 16/26 Posterior bladder descent: I: 9/24 , II: 7/26 Combined suspension defect: I: 2/24, II: 3/26 |
I. PFMT = 24 II. Surgery, N = 26 (N in analysis) PFMT: Weekly lessons (median 5, range 2–13) with physiotherapist to small groups of patients (2–6), including (a) pelvic floor anatomy, (b) muscle awareness training (but no objective measurement was carried out to ensure correct squeezing technique), and (c) instruction for home exercise programme of two lying, one sitting and one standing. The four exercises were done five times each and four times daily. During the 4-month treatment period practices of ‘increasing intensity’ were used, and the patients were also taught correct lifting technique Surgery: Surgery chosen on basis of cystometry, including Burch colposuspension for anterior suspension defect, vaginal repair for posterior bladder descent, or combined procedure Additional information: At the follow-up examinations (4 and 12 months), patients dissatisfied with the outcome of the treatment were given alternative treatment (PFMT→surgery, surgery→PFMT) |
Objective Cured (no leakage on 3-day chart) at 1 year (excluding those who received alternative treatment after 4 months): I: 6/10, II: 19/20 Cured (no leakage on 3-day chart) at median 6 years (excluding those who received alternative treatment after 4 months): I: 5/10, II: 11/20 Episodes of leakage in 3 days at 4 months (3-day voiding and incontinence chart, median, range): I: 24, 2 (0–20), II: 26, 0 (0–14), significantly larger reduction for surgery, p < 0.01 Note: Data by prolapse type also reported N of micturition in 3 days at 4 months (3-day voiding and incontinence chart, median, range): I: 24, 20 (11–40), II: 26, 19 (12–29) Standardised 60-minute pad test (Klarskov and Hald 1984) at 4 months: Better for the operated patients than for the PFMT patients, p < 0.0005 Note: Individual data also reported Surrogate outcomes Adherence to PFMT at 4–8 years (group not specified): At least once a week 59%, occasionally 28%, never use PFMT 14% Long term N having alternative treatment by 12 months: I: 14/24 given surgery, II: 7/26 given PFMT N having incontinence surgery at median 6 years: I: 0/24, II: NA Adverse events N experiencing adverse events at 1 year: Four following surgery (group not specified) Adverse events at 1 year: Urge incontinence, retropubic pain, persistent pelvic pain, persistent dyspareunia; no patient developed persistent bacteriuria Adverse events at 4–8 years: Three patients who had surgery (group not specified) had persistent pelvic pain and dyspareuria Discontinued treatment because of adverse events: Not reported |
Subjective Patient-perceived cure at 4 months: I: 3/24, II: 16/26 Patient-perceived cure or improvement at 4 months: I: 17/24, II: 23/26 Patient-perceived improvement at median 6 years (excluding those who received alternative treatment after 4 months): I: 1/10, II: 3/20 Patient satisfaction at 4 months: 15/24, II: 19/26 Patients remained satisfied with initial treatment at 12 months: I: 10/24 (satisfied with PFMT), II: 19/26 (satisfied with surgery) Patient perceived cure or improvement at 4 months by type of prolapse: I–1I–2I–3II–1II–2II–3Cured3001321Improved851142Other241210Total13921673 Note: I/II–1, bladder base insufficiency; I/II–2, posterior bladder descent; I/II–3, combined bladder suspension defect |
I–1 | I–2 | I–3 | II–1 | II–2 | II–3 | Cured | 3 | 0 | 0 | 13 | 2 | 1 | Improved | 8 | 5 | 1 | 1 | 4 | 2 | Other | 2 | 4 | 1 | 2 | 1 | 0 | Total | 13 | 9 | 2 | 16 | 7 | 3 | |||||||||||||||||||||
| I–1 | I–2 | I–3 | II–1 | II–2 | II–3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cured | 3 | 0 | 0 | 13 | 2 | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Improved | 8 | 5 | 1 | 1 | 4 | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other | 2 | 4 | 1 | 2 | 1 | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 13 | 9 | 2 | 16 | 7 | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Klingler 1995151 (abstract only) Study design/method: 2-arm RCT. Single centre, Australia Duration of study: 3 months |
Inclusion criteria: Women with clinical diagnosis of SUI Exclusion criteria: Not reported N randomised: 41 N lost to follow-up: None? Type of incontinence: SUI Age (years, mean): I: 51.8, II: 53 N not using continence pads: I: 3/20, II: 2/21 N of pad changes per day (?mean, range): I: 1.2 (1–4), II: 2.4 (0-7) Pad test (g, ?mean, range): I: 8.7 (1–35), II: 12 (1–70) |
I. PFMT + BF + ?IVRD (‘Endotrainer’), N = 20 II. PFMT, N = 21 (N in analysis) PFMT + BF (IVRD?): PFMT as below with addition of audiovisual biofeedback from the ‘Endotrainer’ device, which is an ‘intermittent gas-filled balloon placed in the vagina which has to be compressed by the patient’ PFMT: ‘Classic’ PFMT. In-depth instruction followed by 9-week programme. Clinic visits (30 minutes each) with physiotherapist twice a week for the first 3 weeks, then 3 weeks home programme, followed by additional 3 weeks training with physiotherapists (and presumably further 3 weeks of home programme?). Patients were evaluated at 3 months |
Objective Cure (N not using continence pads): I: 14/20, II: 15/21 N of pad changes in 24 hours (?mean, range): I: 0.1 (0–2), II: 0.6 (0–6) Pad test (not defined; g, ?mean, range): I: 1.2 (0–22), II: 2.9 (0–19) |
Subjective ‘Subjective improvement’: I: 19/20, II: 21/21 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Knight 1998 172 Study design/method: 3-arm RCT. Single centre, England Duration of study: 6 months + follow-up at 12 months |
Inclusion criteria: Women aged 16–75 with USI, sterile urine, English speaking Exclusion criteria: Urinary tract infection, unstable bladder, unable to perform VPFMC, pregnant, breastfeeding, pelvic malignancy, cardiac pacemaker, neurological condition, diabetes, hormone replacement therapy started within previous 3 months N randomised: 70 N lost to follow-up: I: 3/21, II: 6/25, III: 4/24 at 6 months; further 3 in group I, and 3 in group II by 12 months Type of incontinence: USI Age (years, range): (24–68), no difference between groups Urine loss on pad test (at 75% of the max cystometric capacity) (g, median, range): I: 18, 13.1 (2.1–75.2), II: 19, 9.8 (2.3–115.2), III: 20, 20.8 (2.0–103.4) Other: BMI, NS (no data), % smokers, % prior pelvic surgery, parity, NS (no data), % postmenopausal, NS (no data) |
I. PFMT + BF, N = 18 II. PFMT + BF + low intensity home ES, N = 19 III. PFMT+BF + maximal clinic ES, N = 20 (N in analysis) PFMT: Correct VPFMC taught by: physiotherapist with vaginal palpation. Set: individually tailored programme with progression to 10 sustained 10-second contractions, followed by 10 fast contractions. Sets per day: six. Duration of training: 6 months. Supervision: clinic visits weekly for 1 month, then fortnightly for 5 months BF: Air-filled vaginal probe (PRS900, InCare) with visual BF at clinic visits. Home BF with air-filled vaginal probe (PFX, Cardio Design) for visual BF PFMT+BF + low-intensity home ES: PFMT + BF as above. ES = overnight at low intensity at home for 6 months (not during menstruation). Vaginal electrode, 10 Hz trains with 35 Hz bursts, pulse width 200 microseconds, duty cycle 5 seconds on, 5 seconds off (DMI Ltd) PFMT+BF + acute maximal clinic ES: PFMT + BF as above. ES = 16 30-minute of maximal electrical stimulation (VSI, Neen HealthCare). Vaginal electrode, 35 Hz, pulse width 250 microseconds, duty cycle 5 seconds on, 5 seconds off. VPFMC performed with the stimulation In 7–12 months, all women were instructed to perform their final PFMT programme once a day and use home BF (pelvic floor exerciser) once a week. Any patient who had undergone pelvic surgery, become pregnant or started hormone replacement therapy was excluded from follow-up |
Objective Cured or greatly improved at 6 months (cure = dry or urine loss of < 2 g; greatly improved = 75% or more reduction in urine loss at repeat pad test): I: 13/18, II: 10/19, III: 16/20 Note: pad test at 75% of the maximal cystometric capacity Cured or greatly improved at 12 months (cure = dry or urine loss of < 2 g; greatly improved = 75% or more reduction in urine loss at repeat pad test): I: 10/14, II: 12/15, III: 17/20 Note 1: groups II and III had PFMT + BF + ES at 1–6 month but had only PFMT+BF at 7–12 months Note 2: Pad test at 75% of the maximal cystometric capacity Episodes of leakage in 24 hours at 6 months: 7-day chart incomplete and therefore not analysed Urine loss on pad test at 6 months (at 75% of the maximal cystometric capacity, g, median, range): I: 18, 0.8 (0.0–88.1), p = 0.0012 (pre-post), II: 19, 2.9 (0.0–50.9), p = 0.0062 (pre-post), III: 20, 1.5 (0.0–28.1), p = 0.0003 (pre-post) % change in urine loss on pad test at 6 months (median, range): I: 18, 90.7% (–17.1 to 100.0), II: 19, 76.5% (–580.3 to 100.0), III: 20, 91.3% (–72.4 to 100.0), no significant between-group difference % change in urine loss on pad test at 12 months (median, range): I: 15, 100% (–8.1 to 100.0), II: 16, 97.5% (–415.1, 100.0), III: 20, 100.0 (–67.5 to 100.0) Surrogate outcomes Adherence (subjective) at 6 months: I: 90% (highest among the 3 groups), II: 72.5% (lowest among the 3 groups), III: NR % change in perineometer at 6 months (maximum pressure, cmH2O, % median change, range): I: 18, 40.8 (–19.6, 132.1), II: 19, 32.7 (–8.3, 368.6), III: 20, 64.3 (–55.9, 705.7) % change in perineometer at 12 months (maximum pressure, cmH2O, median %, range): I: 15, 53.2 (–22.9 to 137.0), II: 16, 47.1 (–17.2 to 381.4), III: 20, 44.4 (–43.2 to 433.3) |
Subjective Cure or great improvement at 6 months: I: 10/18, II: 9/19, III: 16/20 Cure or great improvement at 12 months: I: 9/14, II: 7/15, III: 17/20 Note: groups II and III had PFMT + BF + ES at 1–6 months but had only PFMT+BF at 7–12 months Subgroup analysis The clinic group demonstrated a positive correlation between subjective and pad test outcome (p = 0.02) and also had a positive correlation with the duration of incontinence symptoms; women with a longer history of incontinence demonstrated greater improvement In both the home and clinic groups, subjects with initially weaker pelvic floor muscle strength demonstrated a greater improvement than those with stronger muscles |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Konstantinidou 2007 116 Study design/method: 2-arm quasi-RCT. Single centre, Greece. Pilot study Duration of study: 12 weeks |
Inclusion criteria: ‘Treatment naive’ female patients with a clinical and urodynamic diagnosis of SUI; age over 18 years, clinical diagnosis of SUI for more than 3 months, ≥ 7 incontinent episodes per week, daytime frequency of less than eight micturition episodes, nocturia of less than three episodes, positive stress test (urine leakage with coughing and with a bladder capacity of 400 ml), positive pad test, and a score of 3 or 4 in the Oxford scale (pelvic floor muscle strength, etc.) Exclusion criteria: Symptoms of urgency and urge incontinence, presence of any degree of pelvic organ prolapse, pregnancy, comorbidities from or affecting the lower urinary tract such as diabetes mellitus, neurological disease, psychiatric illness, use of medication affecting micturition, history of surgical or pharmaceutical treatment of SUI, and chronic debilitating disease, such as renal failure N randomised: 30 N lost to follow-up: I: 5/15, II: 3/15 Type of incontinence: USI Age (years, mean, SD): 47.8 (7.5) (34, 60) Episodes of leakage per week (mean, SD): I: 14.8 (6.1) (3–25), II: 12.2 (4.8) (7–21), p = 0.161 N of pad changes in 24 hours: I: 2.5 (0.9) (1–4), II: 2.0 (1.0) (1–4), p = 0.172 N of micturition in 24 hours: I: 7.6 (0.9) (6, 9), II: 7.2 (0.7) (6, 8), p = 0.201 N of women reporting underwear wetting: I: 4/15, II: 8/15 Quality-of-life index score: I: 4.9 (0.6) (4–6), II: 4.6 (1.0) (3–6), p = 0.345 The two groups were ‘comparable’ for age, height, weight, parity and birthweight |
I. PFMT, no group sessions, N = 10 II. PFMT with group sessions, N = 12 (N in analysis) PFMT, no group sessions: Participants received, in group, instructions for home PFMT, including 1-hour demonstration programme, followed by a supervised session for accurate first application of the programme. Home PFMT = three sets of fast contractions and 3–4 sets of slow contractions daily in lying, sitting and standing positions. Exercises were individualised according to the strength and endurance of the pelvic floor muscles. Supervision: followed up individually in hospital every 4 weeks. Participants were also submitted to vaginal assessment of the pelvic floor muscles on a monthly basis, and their training programme was readjusted according to their progress PFMT, with group sessions: PFMT and monthly hospital visits and assessment as above. In addition, participants attended a common weekly session in a group of five, and were given written instructions for the rest of the week |
Objective N of women reporting underwear wetting: I: 3/10, II: 0/12, p = 0.046 Cure by negative pad test (pad weight < 2 g over 24 hours; women who repeated test at 12 weeks only): I: 0/4, II: 4/6 Episodes of leakage per week (7-day diary, mean, SD): I: 12.5 (7.0), II: 2.9 (2.8), p = 0.002 N of pad changes in 24 hours (7-day diary, mean, SD): I: 2.4 (1.3), II: 0.8 (0.1), p = 0.006 N of micturitions in 24 hours (7-day diary, mean, SD): I: 7.3 (0.7), II: 6.9 (0.7), p = 0.343 Surrogate outcomes Pelvic floor muscle strength: Oxford scale (5-grade scale, mean, SD, range): I: 3.1 (0.3) (3–4), II: 3.6 (0.5) (3–4), p = 0.059 Endurance (mean, SD, range): I: 4.2 (1.6) (3–8), II: 6.3 (1.5) (4–9), p = 0.006 Repetitions (mean, SD, range): I: 4.0 (0.5) (3–7), II: 6.5 (1.2) (5–8), p = 0.001 Fast contractions (mean, SD, range): I: 8.0 (3.3) (5–15), II: 11.7 (2.6) (8–15), p = 0.004 Hold with cough, weak (N of women): I: 7/10, II: 4/12 Hold with cough, moderate (N of women): I: 2/10, II: 7/12 Hold with cough, strong (N of women): I: 1/10, II: 1/12 |
Subjective Patient Global Impression of Improvement (‘Has your condition improved over the past 4 weeks?’ – YES): I: 2/10, II: 12/12 Quality of life Quality-of life index (7-point scale, mean, SD): I: 3.6 (1.5), II: 1.7 (0.8), p = 0.000 Note: ‘How would you feel if you had to spend the rest of your life with the same urinary problem?’ 0 = delighted, 6 = disappointed. The lowest scores were reflective of a better quality of life |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Lagro-Janssen 1991 127 Study design/method: 2-arm quasi-RCT; consecutive assignment, stratified by severity of incontinence. Multicentre (13 general practitioners in the Netherlands) Duration of study: 3 months |
Inclusion criteria: Women aged 20–65 years reporting ≥ 2 leakage episodes per month Exclusion criteria: Previous incontinence surgery, neurological causes of incontinence, diabetes, urinary tract infection, temporary cause of incontinence (e.g. pregnancy) N randomised: 66 N lost to follow-up: None Type of incontinence: USI Age (years, mean, SD): I: 46.1 (10.1), II: 44.6 (8.2) Severity of symptoms (GP assessment, mild/moderate/severe): I: 4/17/12, II: 2/20/11 Episodes of leakage per week (mean, 95% CI): I: 17.3 (12.5–22.1), II: 23.1 (18.1–28.4) % vaginal wall prolapse or cystocoele: I: 11/33, II: 12/33 Other: Parity |
I. PFMT, N = 33 II. No treatment, N = 33 PFMT: Advice about incontinence pads from practice assistant. Correct VPFMC taught by GP and confirmed by palpation. Set: 10 VPFMC (as tightly as possible), with 6 seconds’ hold. Sets per day: 5–10. Duration of training: 3 months. Supervision: none Control subjects: Advice about incontinence pads only. Offered treatment after 3 months Additional information: Post-treatment evaluation for a larger sample, including USI (PFMT), urge UI (bladder training) and mixed UI (BT and PFMT) at 3 months, 12 months and 5 years, reported in separate reports |
Objective Severity of incontinence (GP assessment, dry/mild/moderate/ severe): I: 7/13/12/1, II: 0/1/21/11 Note: Based on GP assessment scores regarding frequency and amount of urine loss, use of protective pads or garments, and restrictions in daily activities owing to incontinence Episodes of leakage per week (7-day bladder chart, mean, 95% CI): I: 4.8 (2.8 to 6.8), II: 25.3 (19.9 to 30.7), p < 0.01 Surrogate outcomes Adherence (judged by patients, ‘excellent or good’/ ‘reasonable or poor’/ ‘no exercise’): I: 20/9/4, II: NA Adverse events N experiencing adverse events: I: 4/33, II: 0/33 Adverse events: pain, uncomfortable feeling during exercise Discontinued treatment because of adverse events: I: 0/33, II: 0/33 |
Subjective Subjective assessment of incontinence (‘cure’ or ‘improved’): I: 28/33, II: 0/33 Quality of life Subjective assessment of psychological impact (‘cured’ or ‘improved’): I: 23/33, II: 0/33 Subjective assessment of restrictions in activities (‘cured’ or ‘improved’): I: 24/33, II: 2/33 N desiring further treatment: ‘The majority of the patients in both groups (80%) were satisfied with treatment’ (p. 448) Further analysis: Most important factor influencing treatment outcome was compliance, and not age, parity, severity of incontinence, the duration of incontinence or the presence of cystocoele or vaginal prolapse (p. 448) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Laycock 1988176 (abstract only) Study design/method: 2-arm RCT. Single centre, England Duration of study: 4–8 weeks treatment and follow-up questionnaire at 3 months |
Inclusion criteria: Women with USI. Exclusion criteria: None stated N randomised: 36 N lost to follow-up: I: 5/16, II: 2/20 Type of incontinence: USI Age (years, range): 44 (30–74) |
I. PFMT, N = 11 II. ES, N = 18 (N in analysis) PFMT: Weekly clinic visit for 6-8 weeks and daily home exercise programme. ES: Interferential therapy (Endomed 433, Enraf Nonius), 10–50 Hz. Average 11 (range 7–13) half-hour sessions, 2–3 times a week for 4–6 weeks |
Objective Pad test (not defined) (g, mean reduction, range): I: 36.33 (7.7–72.4), p = 0.01, II: 30.55 (3.0–78.0), p = 0.01 |
Subjective Subjective improvement post-treatment (much improved/some improvement/other): I: 6/2/3, II: 9/7/2 At 3 months, most patients maintained their level of improvement |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Laycock 2001 152 Study design/method: 3-arm parallel RCT.Five sites in Australia, New Zealand, Ireland and the UK (2 sites) Duration of study: 3 months |
Inclusion criteria: Women aged 20–64 years with symptoms of stress incontinence; those without clinically significant abnormalities (except incontinence) Exclusion criteria: Pregnant or planning pregnancy; medication for bladder symptomology or medication likely to affect the lower urinary tract; hormone replacement therapy for < 3 months; neurological conditions; moderate/severe symptoms of urge incontinence; present or previous participation in research for incontinence; moderate/severe genital prolapse; urinary tract infections N randomised: 101 N lost to follow-up: I: 4/20, II: 18/40, III: 11/41; significant difference between the centres but no significant difference between the groups Type of incontinence: SUI Episodes of leakage in 24 hours (mean): I: 1.71, II: 2.04, III: 2.00 Baseline characteristics: ‘no significant difference between the groups in any of the variables’ (no further details) |
I. PFMT, N = 16 II. PFMT+BF, N = 22 III. VC, N = 30 (N in analysis) PFMT: Patients received written instruction of individual PFMT regimen, determined after digital vaginal assessment. Individually assessed number of fast and slow contractions lying, sitting and standing for 10 minutes each day. Treatment continued during menstruation. Six clinic visits PFMT + BF: BF using the PFX (Cardio Design), which is a modified Kegel Perineometer, designed for home use. Patients received written instructions on correct use of the PFX and participant’s technique was checked at each clinic visit. PFMT = Individual regimen (prescribed after digital assessment) of fast and slow contraction, lying and standing, for 10 minutes per day. Increase the number over the 3-month period. Treatment discontinued during menstruation. Six clinic visits VC: Patients received written instructions. Two Aquaflex cones (SSL-International) with unstated number of different weights. Weight added according to ability to retain cone. Treatment discontinued during menstruation. 10 minutes per day. Six clinic visits Additional information: This trial is reported in two abstracts published in 1999 (Laycock 1999) and a full-text paper published in 2001(Laycock 2001). Data reported in 1999 and 2001 do not match. The outcome data were extracted from the 1999 abstracts, as we judged them to be more complete than the 2001 paper in terms of reporting a greater number of outcomes as well as standard deviations |
Objective Reduction in episodes of leakage in 24 hours (mean, SD): I: 16, 1.13 (1.42), II: 22, 1.20 (1.29), III: 30, 1.00 (1.04), NS Reduction in N of pad changes in 24 hours (bladder diary, mean, 95% confidence bounds ±): I: 16, 1.88 (1.15), II: 22, 2.27 (1.49), III: 30, 2.9 (1.51), NS Surrogate outcomes Compliance score (estimated from exercise diary): I: 16, 81.3%, II: 22, 78.8%, III: 30, 77.0%, NS Increase in pelvic floor muscle strength (maximal muscle contraction, cmH2O, mean, 95% confidence bounds ±): I: 16, 7.13 (4.99), II: 22, 11.00 (6.28), III: 30, 9.30 (4.58), NS |
Subjective Reduction in subjective assessment score (10-cm visual analogue scale; 0 = no symptoms, 10 = the worst possible symptoms, mean, 95% confidence bounds ±): I: 16, 1.84 (0.68), II: 22, 2.35 (1.33), III: 30, 1.69 (0.71), NS Quality of life King’s Health Questionnaire (score range 0–48, mean change (‘improvement in QoL’), 95% confidence bounds ±): I: 16, 8.13 (4.44), II: 22, 6.14 (2.59), III: 30, 7.03 (2.77), NS Note: Data taken from the abstracts published in 1999. It is not clear if mean change is an increase or reduction in King’s Health Questionnaire scores relative to the baseline. In the 2001 paper all three groups show an increase in King’s Health Questionnaire scores from the baseline which is interpreted by author as improvement, even although a higher score in the King’s Health Questionnaire indicates a greater impairment of quality of life |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Laycock Trial 1 1993 132 Study design/method: 2-arm RCT, parallel design. Single centre, England Duration of study: 8 weeks (?) treatment with follow-up questionnaire at 2 years (mean 27.8 months) |
Inclusion criteria: Women with USI Exclusion criteria: Previous physiotherapy for USI, pregnant, neurological dysfunction, present or previous pelvic malignancy, cardiac pacemaker N randomised: 46 N lost to follow-up: I: 0/23, II: 6/23 Type of incontinence: USI Age (years, mean, SD): I: 41.8 (29–59), II: 39.5 (28–53) Other: parity, no difference between groups in the number of pelvic operations, urological history, parity and obesity |
I. ES, N = 23 II. PFMT + BF + VC, N = 17 (N in analysis) ES: Interferential therapy (Endomed 433, Enraf Nonius), bipolar technique (external electrodes on perineal body and inferior to symphysis pubis), 10 minutes at 1 Hz, 10 minutes at 10–40 Hz and 10 minutes at 40 Hz, maximal acceptable current intensity. Average of 10 treatment sessions (the first treatment lasted 15 minutes and subsequent treatments lasted 30 minutes). Patients agreed not to perform PFMT during study PFMT+BF+VC: Correct VPFMC taught individually by physiotherapist with vaginal palpation. Set: Patient specific regimes progressing to five maximum VPFMC of individualised duration every hour of the day. Duration of training: 8 weeks? Supervision: Weekly clinic visit for 2 weeks, then every 10 days for average 6 weeks. Treatment incorporating digital BF was given at each session. Cones supplied at second visit; use cones for 10 minutes, twice per day (except during menstruation) Additional information: Not relevant for direct head-to-head comparisons; data were therefore extracted for primary outcomes only After the treatment period, patients in both groups were instructed to practice daily PFMT as a lifelong habit |
Objective Cured (< 0.5 g increase in urine loss based on standard pad test (Sutherst et al. 1981): I: 1/23, II: 3/17 Note: Patients with urine loss < 2g pre-treatment are not included in the number cured (N = 3 for group I, N = 1 for group II) Cured (as above) or improved (> 30% decrease in urine loss based on standard pad test (Sutherst et al. 1981): I: 10/23, II: 10/17 Long term (Questionnaire at mean 27.8 months) Response rate: I: 15/23, II: 4/17; At least 30% of those ‘improved’ or ‘cured’ at end of treatment maintained their improvement. All subjects claimed to practice regular PFMT: for group I, 1 subject reported exercising daily, 6 nearly every day, and 8 once per week |
Subjective Cured: I: 1/23, II: 2/16 Cured or improved: I: 14/23, II: 7/16 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Laycock Trial 2 1993 132 Study design/method: 2-arm RCT, parallel design. Single centre, England Duration of study: 8–12 weeks (?) with follow-up questionnaire at mean 16.2 months |
Inclusion criteria: Identical to Laycock 1993, Trial 1 Exclusion criteria: Identical to Laycock 1993, Trial 1 N randomised: 30 N lost to follow-up: I: 0/15, II: 4/15 Type of incontinence: USI Age (years, mean, SD): I: 43.7 (25–62), II: 46.2 (16–66) Other: Parity, no difference between groups in parity, BMI and major pelvic surgery |
I. ES, N = 15 II. Sham ES, N = 11 (N in analysis) ES: As in Laycock 1993, Trial 1. Patients told to expect a ‘pins-and-needles’ sensation under electrodes. Patients agreed not to perform PFMT during study Sham ES: Machine (Endomed 433) modified by supplier to appear to be working but no current. Patients told to expect no sensation under electrodes. Up to 10 treatment sessions, each lasting 30 minutes. Patients agreed not to perform PFMT during study Additional information: All patients allocated to group II (sham ES) received PFMT + VC immediately after the trial and assessment period |
Objective Cured (< 0.5 g increase in urine loss based on standard pad test, Sutherst et al. 1981): I: 2/15, II: 0/11 Note: Patients with urine loss < 2g pretreatment are not included in the number cured (N = 2 for group I, N = 2 for group II) Cured (as above) or improved ( > 30% decrease in urine loss based on standard pad test, Sutherst et al. 1981): I: 11/15, II: 5/11 Note: Patients with urine loss < 2 g pretreatment are not included in the number cured or improved (N = 2 for group I, N = 2 for group II) Episodes of leakage (7-day frequency/volume chart): No significant change in either group (p > 0.05) N of micturition per day (7-day frequency/volume chart ; baseline to post-treatment; unclear if mean or median): I: 9.0 to 7.0, p = 0.0039, II: 8.9 to 7.9, p = 0.0549 Standard pad test (Sutherst et al. 1981 with modification, average % decrease): I: 56.8%, p = 0.0066, II: 21.4%, p = 0.0429, significant different between groups Surrogate outcomes Change in pelvic floor contractility (perineometer, mmHg, average increase): I: 5.4, p = 0.0166, II: 0.9, p = 0.5000 Long term (Questionnaire at mean 16.2 months) Response rate: I: 13/15; No questionnaire sent to group II. 20% of those self-classified as ‘improved’ or ‘cured’ at end of treatment sustained their improvement. Two subjects reported exercising (PFMT?) daily, five nearly every day, four once per week and two less than once per week |
Subjective Cured (subjective assessment): I: 0/15, II: 0/11 Cured or improved (subjective assessment): I: 5/15, II: 3/11 Perceived severity of incontinence (10-cm visual analogue scale, 0 = no incontinent, 10 = the most severe incontinence imaginable): I: 21.6% decrease (1 = improvement), p = 0.0219, II: 10.3% decrease, p = 0.1932 Perceived severity of incontinence (10-cm visual analogue scale; cured/ improved/no change/worse): I: 0/11/0/4, II: 0/6/1/4 Note: Any decrease from baseline classified as improvement Subjective assessment of frequency of urine loss (6-point scale, 0 = never, 5 = virtually daily): I: significant decrease, p = 0.0216), II: non-significant decrease, p = 0.0899 Subjective assessment of severity of symptom (5-point scale, 0 = no loss, 4 = sufficient to wet the floor): I: significant decrease, p = 0.0216, II: non-significant decrease, p = 0.1587 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Luber 1997 133 Study design/method: 2-arm RCT. Single centre, USA Duration of study: 3 months |
Inclusion criteria: Women with USI, had failed an attempt or chose not to pursue PFMT, the ability to adequately retain the vaginal probe and to cooperate with the study protocol Exclusion criteria: Pelvic organ prolapse of grade II or greater, detrusor instability, postvoid residual urine > 100 ml, extra urethral incontinence, history of vaginal intraepithelial neoplasia, urinary tract infection, a fixed immobile urethra, intrinsic sphincteric deficiency N randomised: 54 N lost to follow-up: I: 6/26, II: 4/28 Type of incontinence: USI Age (years, mean): I: 54.1 (N = 20), II: 53.6 (N = 24), p > 0.05 Episodes of leakage in 24 hours (?median, range): I: 20, 2.8 (1–9), II: 24, 2.7 (1–12), NS Other: Ethnicity, % prior incontinence surgery, Parity, % postmenopausal, prior PFMT: I: 18/20, II: 16/24 |
I. ES, N = 20 II. Sham ES, N = 24 (N in analysis) ES: 15 minutes twice a day for 12 weeks. Home device (Hollister, Evanson), pulse width 2 milliseconds, a work schedule of 2 milliseconds, followed by a 4-second rest, frequency 50 Hz, and an adjustable power setting ranging from 10 to 100 mA. Compliance measured by an internal memory system of the stimulator. All patients were informed that they may or may not appreciate sensation during stimulation sessions. Contacted every 2 weeks by physical therapy and nursing personnel Sham ES: Same parameter but no sensation. Patients issued vaginal probes in which the wiring from the unit to the probe was covertly discontinuous. All patients were informed that they may or may not appreciate sensation during stimulation sessions. Contacted every 2 weeks by physical therapy and nursing personnel Additional information: Statistically underpowered: ‘The study was discontinued after interim analysis revealed that after enrolment of 54 patients, no difference was observed in the outcomes between the two groups’ (Luber 1997133: 546) Placebo effect: ‘It could be that the benefit ... is the result of the probe acting much like a vaginal cone’ (Luber 1997: 548) |
Objective Cure (stress test negative): I: 3/20, II: 3/24, p = 1.000 Episodes of leakage in 24 hours (24-hour voiding diaries, ?median, range): I: 20, 2.4 (0–9), II: 24, 2.4 (0–11), NS pre-post or between groups Note: Table heading in reverse order in table 3 for no obvious reason, i.e. control on the left and treatment on the right, whereas in other tables control is on the right Surrogate outcomes Adherence: Average 82% (no details given) Adverse events N experiencing adverse events: No complications related to device use, i.e. no vaginal bleeding, vaginal erosions, urinary tract infections, worsening of urinary incontinence, electrical accidents, or discomfort that persisted after device removal Discontinued treatment because of adverse events: Discomfort: I: 3/26, II: 2/28; discouragement I: 2/26, 2/28 |
Subjective Cure (questionnaire scale 5, resolution of symptom): I: 2/20, II: 4/24, p = 1.000 Improvement (questionnaire scale 3–4, moderate improvement): I: 3/20, II: 3/24, p = 0.785 Convenience of device use (questionnaire scale 0–5, with 5 representing the most desirable outcome, ?median, range): I: 3.4 (1–5), II: 3.2 (1–5) Comfort of device use (questionnaire scale 0–5, with 5 representing the most desirable outcome, ?median, range): I: 3.9 (1–5), II: 4.1 (1–5) Subgroup analysis: ‘Those who responded to treatment (cure/improved) were found to be similar to non-respondents with regard to age, parity, prior hysterectomy, prior anti-incontinence surgery, prior PFMT and menopausal status’ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mah 2006 141 Study design/method: 2-arm RCT. Multicentred, seven centres in Korea Duration of study: 8 weeks (+ 2-week no-SNRI screening period before, and a 2-week no-SNRI period after) |
Inclusion criteria: Non-pregnant women aged ≥ 20 years, with predominant symptoms of SUI of ≥ 3 months’ duration, with one incontinent episode per day, a daytime voiding frequency of ≤ 8 voids, nocturnal frequency of ≤ 2 voids daily, and no predominant urge symptoms. Positive cough stress test with visual urine leakage Exclusion criteria: Those taking concomitant medications (including continence promoting SNRIs, antidepressants, SNRIs for obesity, including appetite suppressants and diet pills – and illicit SNRIs). Those unable to tolerate bladder filling at 100 ml/minute to 400 ml. Those experiencing first sensation of bladder filling at < 100 ml, or no sensation at all throughout filling N randomised: 121 N lost to follow-up: I: 14/60, II: 24/61 Type of incontinence: Predominant symptoms of SUI (MUI) Age (years, mean, SD): I: 48.52 ± 8.05, II: 50.67 ± 9.01 Other: BMI, % prior incontinence surgery |
I. Placebo, N = 60 II. Duloxetine 80mg (40 mg twice daily), N = 61 (N randomised) Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only Dichotomous data were calculated from percentage given in paper Denominators were assumed to be N of women with baseline and at least one postrandomisation measurement: I = 53 and II = 45 for diary data (table 2) and I = 57 and II = 56 for subjective data (table 3a) |
Objective Cure or improvement (IEF responders with ≥ 50% reduction in IEF/week based on weekly diary): I: 19/53, II: 23/45, p = 0.128 Adverse events N experiencing adverse events: I: 19/60, II: 50/61, p < 0.001 Adverse events (that occurred in 5% of the women randomised to the duloxetine group or they occurred significantly more often with duloxetine than with placebo): Nausea (p < 0.001), dizziness (p < 0.001), anorexia (p < 0.001), fatigue (p < 0.001), lethargy (p = 0.003), abdominal discomfort (p = 0.032), somnolence (NS at 0.05 level), constipation (p = 0.027), headache (p = 0.999), dry mouth (p = 0.439) Discontinued treatment because of adverse events: I: 5/60, II: 21/61 p = 0.001 |
Subjective Improvement (‘very much better’, ‘much better’ or ‘a little better' on Patient Global Impression of Improvement, or PGI-I): I: 33/57, II: 35/56 Quality of life Mean I-QoL score: I: 57, before 51.52, after 60.23, II: 56, before 48.64, after 63.41 Note: Data based on participants with diary data available Mean change in I-QoL score: I: 57, 8.71, II: 56, 14.77, p = 0.066 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Manning 2005142 (abstract only) Study design/method: 2-arm RCT, Germany Duration of study: 6 weeks |
Inclusion criteria: Women aged between 28 and 86 years with predominant SUI. SUI was diagnosed by either urodynamic studies within the previous 12 months, or using a simple question from a short 2-question instrument (the S/UIQ). ≥ 7 episodes of urinary incontinence per week, with at least twice as many being SUI episodes as UUI by S/UIQ Exclusion criteria: Not specified N randomised: 617 N lost to follow-up: Unclear Type of incontinence: Predominant symptoms of SUI (MUI) |
I. Duloxetine (unspecified dose), N = 306 II. Placebo, N = 311 (N randomised) Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only Data reported as % and N of observations not reported Denominator assumed to be the same as N randomised |
Adverse events Adverse events: Nausea was the most common adverse event and the most common symptom leading to discontinuation of duloxetine in 7.5% (23/306) of all participants Discontinued treatment because of adverse events: I: 53/306 (17.3%), II: 9/311 (2.9%), p < 0.001 |
Subjective Patient Global Impression – Improvement (PGI-I) (from graph): all ‘better’ responses, I: 196/306, II: 137/311, ‘no change’, I: 98/306, II: 159/311, all ‘worse’ responses, I: 12/306, II: 15/311 When compared with placebo, duloxetine showed significant improvements in quality of life using PGI-I instrument (p < 0.001) Quality of life Mean change in King’s Health Questionnaire score: I: 306, –9.2, II: 311, –2.6, p < 0.0001 Note: Scores for each subscale available in table 2 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mayne 1988168 (abstract only) Study design/method: 2-arm RCT, UK Duration of study: 4 months |
Inclusion criteria: Women with USI Exclusion criteria: Not specified N randomised: 34 N lost to follow-up: 7 Type of incontinence: USI Age (years, mean): I: 45 (N = 13), II: 56 (N = 14) |
I. PFMT with perineometer, N = 13 II. PFMT with urethral electrical conductance, N = 14 (N in analysis) PFMT with perineometer: Participants instructed on how to exercise their pelvic floor muscles and were seen weekly at the clinic for 1 month, then monthly for a further 3 months to check exercises are being performed correctly. At each clinic visit patients’ progress was monitored using perineometry and the results conveyed visually PFMT with urethral electrical conductance: PFMT and supervision as above. At each clinic visit, patients’ progress was monitored using a urethral electrical conductance test (Plevnik et al. 1985) and the results conveyed visually |
Objective Cured (short exercise perineal pad test; vs improved, no change, worse): I: 2/13, II: 2/14 Cured or improved (short exercise perineal pad test; vs no change or worse): I: 7/13, II: 7/14 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Millard 2004 143 Study design/method: 2-arm RCT. Multicentre; 38 centres in Poland (31%), South Africa (25%), Australia (17%), Brazil (9%), Argentina (5%), Finland (3%) and Spain (?%) Duration of study: 12 weeks, plus prerandomisation 2-week screening process, 2-week no-SNRI lead-in and 2-week placebo lead-in |
Inclusion criteria: Women aged ≥ 18 years with predominant SUI symptoms of ≥ 3 months duration, ≥ 7 incontinence episodes/week, diurnal frequency of < 9 per day, nocturnal frequency of < 3 per night. Positive cough stress and positive stress pad test (with > 2-g leakage) Exclusion criteria: Predominant symptoms of urge incontinence, unable to tolerate filling bladder to 400 ml or experienced first sensation of bladder filling at < 100 ml, or no sensation at any time during bladder filling N randomised: 458 Lost to follow-up: I: 57/227 (25%), II: 18/231 (8%) Type of incontinence: Predominant symptoms of SUI (MUI) Age (years, mean, range): I: 53.7 (29–79), II: 52.6 (27–77) Episodes of leakage per week (mean, SD): I: 18.5 ± 15.1, II: 18.3 ± 15.5 PGI-S (bladder function classed as ‘moderate’ or ‘severely’ abnormal): 165/227 (72.7%), II: 169/231 (73.1%) I-QoL score (mean, SD): I: 58.9 ± 23.5, II: 58.3 ± 22.8 Other: BMI, ethnicity (% Caucasian), % prior incontinence surgery, % currently using PFMT |
I. Duloxetine 80 mg taken as 40 mg b.i.d. (twice daily), N = 227 II. Placebo, N = 231 (N randomised) Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only Dichotomous data calculated from percentage in paper, using the N of women included in the ‘ITT’ analysis with at least one postrandomisation measure; 88% (200/227) in the duloxetine and 99% (229/231) in the placebo group completed at least one diary after randomisation, while 97% (220/227) in the duloxetine and 99% (229/231) in the placebo group completed at least one I-QoL questionnaire Diary data collected in daily diaries collected for 1 week prior to each visit (and 2 prior to randomisation – one of which was completed during the no-SNRI lead-in and the other during the placebo lead-in) High placebo response rate in this study was deemed attributable by the authors to the differences (depending on country) of previous experience/knowledge of SUI prevention methods (e.g. PFMT) |
Objective Cure (no incontinent episodes at last visit; 7-day diary): I: 14/200 (7.1%), II: 14/229 (6.1%), NS Cure or improvement (50–100% reduction in incontinence episodes; 7-day diary): I: 119/200 (59.5%), II: 99/229 (43.2%), p < 0.001 Surrogate outcomes Adherence (average % of treatment doses ingested): I: 86%, II: 94%, p < 0.001 Adverse events N experiencing adverse events: I: 173/227 (76.2%), II: 137/231 (59.3%), p < 0.001 Adverse events (significantly more common with and occurring in ≥ 5% of subjects with duloxetine): Nausea, headache, insomnia, constipation, dry mouth, dizziness, fatigue, somnolence, anorexia, vomiting, increased sweating, anxiety Note: Adverse events written in italics caused discontinuation among > 1% of participants on duloxetine Discontinued treatment because of adverse events: I: 39/227 (17.2%), II: 4/231 (1.7%), p < 0.001 |
Subjective PGI-I (‘very much better’, ‘much better’ or ‘a little better’): I: 162/220 (73.6%), II: 147/229 (64.2%) Quality of life I-QoL score (mean, SD): I: 220, 69.2 (23.8), II: 229, 64.7 (24.9) Change in I-QoL score (mean, SD): I: 220, 10.3 (16.0), II: 229, 6.4 (17.0), p = 0.007 Subgroup analysis: Change in IEF for those with severe incontinence (> 14 IEF/week at baseline) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Miller 1998 107 Study design/method: 2-arm RCT, parallel design. Single centre, USA Duration of study: 1 week |
Inclusion criteria: Community-dwelling women aged 60 years and older with self-reported symptoms of (mild-to-moderate) SUI and with leakage occurring at least weekly and up to five times per day, direct visualisation of urine loss on cough with 100 ml or more voided after stress test Exclusion criteria: Systemic neuromuscular disease, previous bladder surgery, active urinary tract infection, delayed leakage after cough (assumed DI) (N = 2), more than moderate leakage (that saturated a paper towel and/or pooled on the floor) when coughing in the standing posture (N = 2), inability to demonstrate any VPFMC despite detailed instruction during the pelvic examination (N = 3), prolapse below hymenal ring (N = 2) N randomised: 27 N lost to follow-up: None Type of incontinence: SUI Age (years, mean, SD): 68.4 (5.5) (60–84), no significant difference between groups Episodes of leakage in 24 hours (mean, SD): 1.4 (1.4) (0–5), no significant difference between groups % vaginal wall prolapse: none Other: % prior incontinence surgery – none, parity 84% parous, no significant difference between groups |
I. PFMT, N = 13 II. No treatment, N = 14 PFMT (‘The Knack’): Education on basic physiology and function of pelvic floor muscles. Correct VPFMC taught by digital palpation. At the first clinic visit taught ‘The Knack’, i.e. a single, intentionally timed VPFMC (rather than repetitive exercise) prior to hard cough maintained throughout cough until abdominal wall relaxed. Duration of training: practice at home for 1 week Additional information: Individual data (paper towel test, digital palpation score, voided volume) reported in table 2, though group not specified; within-subjects comparisons reported Control subjects cross over to treatment group at 1 month |
Objective Paper towel test, mild cough (wet area, cm2, mean, SD): I (with ‘The Knack’): 13, 0.4 (1.04), II (without ‘The Knack’): 10, 21.2 (44.8) Paper towel test, deep cough (wet area, cm2, mean, SD): I (with ‘The Knack’): 13, 5.4 (15.3), II (without ‘The Knack’): 10, 26.8 (46.7) Paper towel test, mild cough (wet area, cm2, mean, SD): I (without ‘The Knack’): 13, 23.0 (44.6) Paper towel test, deep cough (wet area, cm2, mean, SD): I (without ‘The Knack’): 13, 32.7 (33.9) Note: wet area 1 cm2 is equivalent to 0.039 ml |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mørkved 2002 153 Study design/method: 2-arm RCT, Norway Duration of study: 6 months |
Inclusion criteria: Women with symptoms of SUI and > 2 g of leakage as measured by pad test with standardised bladder volume, ability to understand Norwegian instructions Exclusion criteria: Involuntary detrusor contractions on cystometry, abnormal bladder function (residual urine > 50ml), previous surgery for stress incontinence, neurological or psychiatric disease, urinary tract infection or other diseases that might affect participation, pregnancy, use of concomitant treatments during the trial period N randomised: 103 N lost to follow-up: I: 5/53, II: 4/50 Type of incontinence: I: USI 36 (75%), MUI 12 (25%), II: USI 34 (74%), MUI 12 (26%) Age (years, mean, SD): I: 48, 47.8 ± 8.2, II: 46, 45.4 ± 8.1 Stress pad test: All women (g, mean, SD), I: 48, 25.7 ± 24.2, II: 46, 29.0 ± 34.5; women with USI only (g, mean, 95% CI), I: 36, 25.9 (17.1 to 34.8), II: 34, 27.6 (15.0 to 40.2) 48-hour pad test: All women (g, mean, SD), I: 48, 39.8 ± 36.6, II: 46, 44.6 ± 33.9; women with USI only (g, mean, 95% CI), I: 36, 41.2 (28.2 to 54.1), II: 34, 46.3 (33.6 to 59.0) Leakage index: All women (mean, SD), I: 2.8 ± 0.7, II: 46, 2.8 ± 0.5; women with USI only (mean, 95% CI), I: 36, 2.7 (2.5 to 3.0), II: 34, 2.7 (2.5 to 2.8) Social Activity Index: All women (mean, SD), I: 48, 9.1 ± 0.9, II: 46, 9.2 ± 0.6; women with USI only (mean, 95% CI), I: 9.2 (8.9 to 9.5), II: 9.3 (9.1 to 9.5) PFM strength: All women (cmH2O, mean, SD), I: 48, 13.6 ± 9.8, II: 46, 14.4 ± 7.8; women with USI only (cmH2O, mean, 95% CI), I: 36, 14.0 (10.3–17.6), II: 34, 14.5 (11.7–17.2) Other: BMI, Parity, N (%) postmenopausal, N (%) using estrogen |
I. PFMT + BF, N = 48 II. PFMT, N = 46 (N in analysis) PFMT + BF: Individual training sessions with physical therapist once per week during the first 2 months and every second week during the next 4 months. Instructed in pelvic floor anatomy. Correct VPFMC taught by physical therapist using vaginal palpation. At each clinic visit three sets of 10 VPFMC with 6–8 seconds hold, following each contraction with 3–4 fast contractions. Also encouraged to perform three sets of 10 high-intensity (close to maximum) VPFMC per day at home. BF = a home training device (BF-106, Vitacon, Norway) with a vaginal pressure probe, used in training both at home and at clinic with physiotherapist. The contractions were measured and stored in the apparatus PFMT: As above, without biofeedback Additional information: Participants were followed up for 1–5 years but data were presented as cohort and not by group allocation |
Objective Cure (< 2-g leakage) on stress pad test with standardised bladder volume at 6 months (defined by author as objective cure): All women, I: 28/48, II: 21/46; women with USI only, I: 25/36, II: 17/34 Cure on 48-hour pad test ( < 2-g leakage) at 6 months: All women, I: 31/48, 26/46; women with USI only, I: 24/36, II: 22/34 Stress pad test with standardised bladder volume at 6 months (g, mean, 95% CI): All women, I: 6.1 (3.1 to 9.1), II: 10.6 (4.7 to 16.4); women with USI only, I: 5.5 (2.1 to 9.0), II: 9.9 (2.8 to 17.0) Mean change in leakage on stress pad test with standardised bladder volume at 6 months (g, mean, 95% CI): All women, I: 19.6 (14.4 to 24.8), II: 18.5 (12.2 to 24.7); women with USI only, I: 20.4 (13.9 to 26.9), II: 17.7 (10.1 to 25.3) 48-hour pad test at 6 months (g, mean, 95% CI): All women, I: 6.5 (2.4-10.6), II: 6.0 (3.3 to 8.8); women with USI only, I: 7.0 (1.7 to 12.4), II: 3.8 (1.4 to 6.2) Mean change in leakage 48-hour pad test at 6 months (g, mean, 95% CI): All women, I: 34.1 (25.5 to 42.8), II: 38.6 (29.1 to 48.0); women with USI only, I: 33.0 (22.5 to 43.5), II: 42.5 (30.3 to 54.7) Surrogate outcomes Adherence at 6 months: I: 88.9% (43/48), II: 85.3% (39/46) were ‘training their pelvic floor muscles more than three times per week’ Pelvic floor muscle strength at 6 months (cmH2O, mean, 95% CI): All women, I: 25.9 (21.8 to 29.9), II: 25.4 (21.2 to 29.6); women with USI only, I: 26.6 (21.6 to 31.6), II: 25.9 (20.5 to 30.9) Long term N wanting surgery at end of 6-month treatment period: I: 2/48, II: 3/46 Adverse events N experiencing adverse events: I: 7/48 found use of apparatus ‘unpleasant’, II: 3/46 found PFMT itself ‘unpleasant’. ‘However, they all followed the training protocol in spite of this’ Discontinued treatment because of adverse events during 6 months: I: 1/53 (because she ‘disliked the equipment’), II: 0/50; in addition there were 4 dropouts in each group not due to adverse events but to changes in work situation, family causes, death or disease in the family or moving to other parts of the country |
Subjective Subjective assessment of severity: All women, ‘Unproblematic’ (defined by author as subjective cure), I: 19/48, II: 14/46, ‘minor problem’ I: 17/48, II: 18/46, ‘moderate problem’ I: 8/48, II: 5/46, ‘problematic’ I: 3/48, II: 6/46, ‘very problematic’ I: 1/48, II: 3/46 Women with USI only: ‘Unproblematic’ (defined by author as subjective cure) I: 16/36, II: 10/34, ‘minor problem’ I: 12/36, II: 17/34, ‘moderate problem’ I: 5/36, II: 4/34, ‘problematic’ I: 2/36, II: 2/34, ‘very problematic’ I: 1/36, II: 1/34 Quality of life Social Activity Index at 6 months (mean score, 95% CI): All women, I: 9.5 (9.3 to 9.7), II: 9.4 (9.2 to 9.7); women with USI only, I: 9.6 (9.4 to 9.8), II: 9.5 (9.3 to 9.8) Note: Social Activity Index = 10-cm visual analogue scale on 9 social settings in which women may have problems with participation; 0 = impossible to participate, 10 = no problem to participate Mean change in Social Activity Index at 6 months (mean, 95% CI): All women, I: 0.4 (–0.1 to 0.6), II: 0.3 (0.0 to 0.5); women with USI only, I: 0.4 (0.1 to 0.6), II: 0.4 (0.2 to 0.7) Leakage Index at 6 months (mean score, 95% CI): All women, I: 1.9 (1.7 to 2.1), II: 1.9 (1.7 to 2.1); women with USI only, I: 1.8 (1.6 to 2.0), II: 1.8 (1.6 to 2.0) Note: Leakage Index = 5-point scale, 1 = never, 5 = always), containing 13 types of physical activities known to trigger urinary leakage Change in Leakage Index at 6 months (mean, 95% CI): All women, I: 0.9 (0.7 to 1.0), II: 0.9 (0.7 to 1.1); women with USI only, I: 1.0 (0.7 to 1.2), II: 0.9 (0.6 to 1.1) Satisfaction with treatment at 6 months: Would recommend treatment to others, I: 100% (48/48), II: 100% (46/46), % satisfied/very satisfied with the treatment, I: 80% (38/48), II: 71% (33/46); reported improvement with the treatment, I: 97% (47/48), II: 93% (43/46); reported condition unchanged, I: 3% (1/48), II: 7% (3/46), unsatisfied with treatment, I: 0% (0/48), II: 0% (0/46) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Norton 2002 144 Study design/method: RCT (double-blind, placebo controlled). Multicentre (48 sites), USA Duration of study: 12 weeks (plus 2 weeks no-SNRI lead-in and 2-week placebo lead-in) |
Inclusion criteria: Female outpatients aged between 18 and 65 years with clinical diagnosis of predominant SUI for ≥ 3 months, with ≥ 4 incontinent episodes/week, micturition frequency of ≤7 (day) and ≤ 2 (night), positive cough stress test and stress pad test Exclusion criteria: Predominant urge symptoms, previous continence/prolapse surgery, unable to tolerate bladder infusion filling at 100 ml/minute, those with first sensation of bladder filling at < 100 ml or those with no sensation at any time during the filling N randomised: 553 N lost to follow-up: Unclear Type of incontinence: I: SUI 87/138 (63%), MUI 51/138 (37%), II: SUI 90/137 (66%), MUI 47/137 (34%), III: SUI 104/140 (74%), MUI 36/140 (26%), IV: SUI 101/138 (73%), MUI 37/138 (27%) Age (years, mean, SD): I: 49.4 ± 7.3, II: 49.4 ± 8.0, III: 49.3 ± 8.8, IV: 50.2 ± 8.9. Episodes of leakage in 24 hours (mean, SD): I: 1.6 ± 1.3, II: 1.7 ± 1.6, III: 1.9 ± 1.6, IV: 1.6 ± 1.1 N of micturition in 24 hours (mean, SD): I: 9.2 (2.6), II: 10.0 (2.6), III: 9.8 (2.5), IV: 9.4 (2.5) Other: BMI, ethnicity (% white), height (cm), weight (kg) |
I. Duloxetine 20 mg/day, N = 138 (132) II. Duloxetine 40 mg/day (taken as 20 mg b.i.d.), N = 137 (129) III. Duloxetine 80 mg/day (taken as 40 mg b.i.d.), N = 140 (130) IV. Placebo, N = 138 (132) (N randomised; and N in ‘ITT’ analysis in brackets with women with at least one postrandomisation measure) Additional information: Diary data collected daily for 1 week, in the week prior to each clinic visit |
Objective Cure rates: Based on N of incontinent episodes in 24 hours at last diary: I: 21/128, II: 30/123, III: 23/123, IV: 20/132; Based on negative cough stress test at 400 ml: I: 11/112, II: 25/112, III: 18/114, IV: 15/118 Negative stress pad test ( 2 g at 1 hour): I: 36/110, II: 48/111, III: 44/113, IV: 42/114 Reduction in episodes of leakage in 24 hours (median % reduction at last visit): I: 132, 44%, II: 129, 59%, III: 130, 58%, IV: 132, 40% Reduction in number of voids in 24 hours: I: 132, 0.8, II: 129, 1.0, III: 130, 0.8, IV: 132, 0.5 Median % change in 1-hour stress pad test: I: 132, –11% (–100 to 3240%), II: 129, –43% (–100 to 5800%), III: 130, –29% (–100 to –12333%), IV: 132, –30% (–100 to 2175%) Surrogate outcomes Adherence (ingested ≥ 80% of medication and did not miss > 4 consecutive doses): I–III: 324/415 (78%), IV: 115/138 (83%); figures calculated from % given in paper Adverse events N experiencing at least one adverse event: I: 86/138 (62%), II: 93/137 (68%), III: 102/140 (73%), IV: 84/138 (61%); figures calculated from % given in paper Adverse events (that occurred in 5% of subjects in any treatment arm): Nausea, headache, diarrhoea, constipation, dry mouth, dizziness, insomnia, sinusitis, fatigue, nasopharyngitis Also menorrhagia and somnolence listed among adverse events causing discontinuation of treatment ‘Five subjects had adverse events that required hospitalisation (one event before randomisation, one event while ingesting duloxetine 20 mg per day, two events while ingesting duloxetine 40 mg per day, and one event while ingesting duloxetine 80 mg per day); only one of these events (rash) was felt to be related to the study SNRI’ (p. 44) Discontinued treatment because of adverse events: I: 13/138 (9%), II: 17/137 (12%), III: 21/140 (15%), IV: 7/138 (5%) |
Subjective PGI-I (N rating incontinence as ‘very much better’/‘much better’): I: 41/132, II: 48/129, III: 57/130, IV: 36/132 Quality of life Mean change in I-QoL score: I: 132, 5.3, II: 129, 7.8, III: 130, 9.3, IV: 132, 5.8 Subgroup analysis I-QoL and IEF results for women with more severe SUI (baseline IEF of ≥ 14) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Nygaard 1996 163 Study design/method: 2-arm RCT. Multicentred, two sites, USA Duration of study: 12 weeks’ treatment. Follow-up at 9 months after beginning treatment |
Inclusion criteria: Non-pregnant women > 21 years old who were seen for treatment of incontinence in two tertiary gynaecological and urological incontinence clinics over a 1-year period Exclusion criteria: Genital prolapse past the vaginal introitus, parturition within the preceding 6 months, and deafness N randomised: 71 N lost to follow-up: 16/71 (6 USI, 3 DO, 7 MUI). All dropouts stated that they were unimproved at the time they discontinued the study Type of incontinence (USI/DO/MUI): 37/17/17 Age (years, mean, SD, range): 53 (13) (25–81) Cystorethrocoele: 53/71 Rectocoele: 27/71 Enterocoelic–uterine prolapse: 11/71 Other: Weight, education, % prior incontinence surgery, parity |
I. PFMT, no tape II. PFMT with audiotape (number not reported) PFMT: Visual aids to teach the location of the levator ani muscles and the pelvic anatomy. Correct VPFMC taught using vaginal palpation. PFMT for 5 minutes, twice a day at home, with VPFMC held to count of 4, progressing to count of 8. Participants were told that improvement was not anticipated for 6–8 weeks. Three clinic visits and three telephone calls over 3 months PFMT with audiotape: PFMT and education as above with the addition of audiotape. Tape contained 270 minutes of music and verbal instructions to guide women through 3 months of PFMT Additional information: Published data presented by diagnosis, not group allocation. Withdrawals included in data analysis using baseline values In all three diagnostic groups (USI, DO, MUI) the number of incontinent episodes per day decreased significantly, and muscle strength increased significantly |
Objective Improvement or cure by 3-day diary at 3 months (based on the N of incontinence episodes): All women, 50–74% improvement 31/71, 75–99% improvement 20/71, 100% improvement (cure) 9/71. Data by group allocation not reported Improvement or cure by 1-hour pad test at 3 months (based on pad weight, g): 50–74% improvement 22/71, 75–99% improvement 18/71, 100% improvement (cure) 0/71. Data by group allocation not reported Episodes of leakage in 24 hours at 3 months (3-day diary, mean, SD): Women with USI only, N = 37, Before 2.6 (1.8), after 1.7 (1.6). No between-group difference post treatment N of pad changes per day (day time) at 3 months (means, SD): Before 1.8 (1.4), after 1.5 (1.4), USI patients only, N = 37. N of micturition per day (daytime) at 3 months (means, SD): Before 6.8 (3.0), after 5.9 (2.7), USI patients only, N = 37 1-hour pad test at 3 months (g, mean, SD): Before 16.8 (37.9), after 8.6 (13.9), USI patients only, N = 37 No between-group difference post treatment Surrogate outcomes Pelvic floor muscle strength at 3 months (digital method described by Sampselle et al. 1989): Before 4.2 (1.6), after 5.5 (1.8), USI patients only, N = 37. No between-group difference post treatment N having incontinence surgery at 9 months: 10/37, USI patients only |
Quality of life Leakage index score at 3 months (stress score, mean, SD): Before 2.1 (0.6), after 1.8 (0.7). USI patients only, N = 37. No difference between groups post treatment. Note: Leakage Index modified from that described by Bø (1994). 5-point scale (0 = never, 4 = always) listing 11 activities known to trigger stress incontinence and nine activities that may trigger urge incontinence Satisfied with PFMT at 9 months: 12 of the 37 USI participants described their improvement as good and did not want any further therapy of any sort for incontinence |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Oláh 1990 187 Study design/method: 2-arm parallel design, UK Duration of study: 4 weeks treatment plus 6 months follow-up |
Inclusion criteria: Women with symptoms of incontinence (predominantly stress incontinence) referred to an outpatient department of physiotherapy Exclusion criteria: Patients who had treatment with PFMT in the last 6 months N randomised: 69 N lost to follow-up: I: 6/36 (17%) by the end treatment, further two after 6 months, II: 9/33 (27%) by the end of treatment, further five after 6 months Type of incontinence: Predominant symptoms of SUI (MUI) Age (years, mean, SD): I: 30, 47.9 (13.0), II: 24, 43.2 (8.9) Severity of symptoms (urine lost < 2 ml/2–10 ml/ 10–50 ml/> 50 ml; unclear if pad test or continence chart): I: 9/3/11/7, II: 9/5/4/6 Standard 1-hour pad test (ICS 1987) (gram, mean, SD): I: 30, 32.2 (49.1), II: 24, 27.7 (38.8) Weekly leakage (continence chart, g, mean, SD): I: 30, 19.3 (22.6), II: 24, 22.0 (31.4) Pelvic floor muscle strength (active cone weight, g, mean, SD): I: 30, 40.7 (22.9), II: 24, 47.1 (20.7) Note: Active cone weight = the weight of the heaviest cone that the patient could voluntarily retain Pelvic floor muscle strength (passive cone weight, g, mean, SD): I: 30, 30.7 (22.1), II: 24, 37.5 (22.3) Note: Passive cone weight = the weight of the heaviest cone that could be retained in the vagina for 1 minute while ambulatory without voluntary holding Other: Weight (kg), % over ideal weight by 20%, % prior incontinence surgery, parity |
I. ES (+ PFMT), N = 30 II. VC (+ PFMT), N = 24 (N in analysis) ES: Interferential therapy at a clinic three times per week for 4 weeks. Patient semirecumbent position with four vacuum electrodes, two on the abdomen and two on the inside of the thighs. Frequency 0- to 100-Hz sweeps, maximum tolerable intensity and each treatment was for 15 minutes. All participants were taught PFMT, with no further details about this VC: Clinic visit once per week for 4 weeks for supervision. Hold the heaviest cones possible for 15 minutes two times per day. Progress to the next heaviest cone when successful on two consecutive occasions. Femina cones. Nine conical weights, 20–100 g. All participants were taught PFMT, with no further details about this Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only 4/36 in the ES arm and 5/33 in the VC arm excluded from the trial because of a failure to tolerate the cone (see Adverse events) |
Objective Cure (no leakage on continence chart) after treatment: I: 10/30, II: 10/24 Note: Chart starting a week before treatment and continuing throughout the course of therapy Cure (no leakage on continence chart) after 6 months: I: 12/30, II: 12/24 Improvement in weekly urinary leakage after treatment (continence chart; including cure): I: 18/30, II: 20/24 Note: Unclear if reduction in frequency or amount of urinary leakage Improvement in weekly urinary leakage after 6 months (continence chart; including cure): I: 18/30, II: 20/24 Note: Unclear if reduction in frequency or amount of urinary leakage Improvement on standard 1-hour pad test after treatment: I: 23/30, II: 12/24 Improvement on standard 1-hour pad test after 6 months: I: 22/30, II: 14/24 Adverse events N experiencing adverse events: NR (other than below) Discontinued treatment because of failure to tolerate cones at start of trial: I: 4/36, II: 5/33 (3/36 in group I and 4/33 group II had vagina too narrow and the cone ‘wedged’; 1/33 in group II had irregular bleeding preventing cone use; 1/36 in group I had discomfort during cone use because of excessive scar tissue in vagina) Adverse events during follow-up period (after end of treatment): I: 0/36, II: 2/33 (one developed a psychiatric disorder and considered unfit for assessment, 1 died of an unrelated cause) Note: Cones were used to assess pelvic floor muscle strength in both groups |
Subjective Cured after treatment: I: 4/30, II: 4/24 Cured after 6 months: I: 12/30, II: 10/24 Improved after treatment (not including cure): I: 23/30, II: 15/24 Improved after 6 months (not including cure): I: 15/30, II: 7/24 Other: Time spent by physiotherapist with each patient (mean , SD, range): ES = 184.9 (13.4) (177–230) minutes, VS = 36.3 (12.3) (20–60) minutes |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pages 2001 154 Study design/method: 2-arm RCT. Single centre, Germany Duration of study: Approximately 3 months, including 4 weeks supervised training plus unsupervised home programme for 2 months |
Inclusion criteria: Women with mild to moderate SUI Exclusion criteria: Co-existing medical illnesses, especially neurological problems, taking medications that would influence normal bladder control and functioning N randomised: 51 N lost to follow-up: I: 0/27, II: 11/24 (excluded ‘after randomisation because they had evidence of cystitis, genital prolapse, or gynaecological haemorrhage on physical examination or they decided to withdraw after randomisation. BF is contraindicated in these conditions’.); no further dropouts Type of incontinence: SUI Age (years, average, range): 51.1 (27–80) BMI (% > 25): 23.5% |
I. PFMT, N = 27 II. PFMT + BF, N = 13 (N in analysis) PFMT with group sessions: One 90-minute introductory session for education on anatomy and incontinence. This was followed by group therapy with physical therapist (60 minutes each), five times a week, with 10 patients per group, over 4 weeks, totalling 20 sessions. Before each therapy session, each participant was instructed in finding a position that allows contraction of pelvic floor muscles without contracting adjacent muscles. Then, the patients performed PFMT in various positions and under various daily situations, such as stair climbing, singing, hiking and power walking. Home PFMT = 100 VPRMC per day during typical daily situations and specific PFMT in a supine position for 10 minutes twice a day. Additional exercises for the trunk, buttocks, abdominal muscles and respiratory exercise were taught. For aerobic conditioning and assistance in weight reduction, patients went twice a week to a warm water pool for 30 minutes. Other aerobic sports were recommended. The patients were encouraged to lose weight and to develop better eating habits. Individual counselling was undertaken when felt necessary. After 4 weeks of supervised training, patients continued PFMT at home, with 4 training units with 10 VPFMC each, five times a week. BF was used for measurement purposes only at 4 weeks and 3 months PFMT with clinic-based individual BF sessions: A general 90-minute introductory group session similar to above. One 30-minute individual session to introduce BF (Gemini 2000™, Wilest, Berlin, Germany). No additional group sessions, educational sessions or lifestyle counselling were offered afterward. Patients then received individual therapy with physical therapist for 15 minutes, five times a week for 4 weeks. Each session consisted of 4 training units with 10 VPFMC each. After 4 weeks of supervised training, patients continued PFMT without BF at home, with 4 training units with 10 VPFMC each, 5 times a week Additional information: Patient-perceived cure and improvement data (provided as %) presented in the table do not match their description in text (abstract, results, discussion). Author was contacted and confirmed that the data in text were correct and the correct data were supplied |
Objective N of micturitions in 24 hours after 4 weeks of supervised treatment (diary throughout study period, mean, SD, range): I: 5.2 ± 1.3 (3.0–8.0), II: 5.5 ± 1.0 (4.0–7.0) N of micturitions in 24 hours at 3 months (diary throughout study period, mean, SD, range): I: 5.4 ± 1.4 (3.0–8.2), II: 5.8 ± 1.1 (4.5–8.0) Surrogate outcomes Average contraction pressure at 4 weeks (measured with the BF apparatus, cmH2O, median, SD): I: 16 (10), II: 50 (14), p < 0.05 Average contraction pressure at 3 months (measured with the BF apparatus, cmH2O, median, SD): I: 17 (14), II: 43 (16) , p < 0.05 Adverse events N experiencing adverse events: I: 0/27, II: 0/13 |
Subjective Cure after 4 weeks’ treatment (no incontinence episodes and symptoms; patient questionnaire): I: 6/27 (22%), II: 9/13 (69%) Cured (as above) or improved after 4 weeks treatment (at least 50% decrease in incontinence episodes and symptoms; patient questionnaire): I: 26/27 (96%), II: 13/13 (100%) Cure at 3-month follow-up (no incontinence episodes and symptoms; patient questionnaire): I: 8/27 (28%), II: 8/13 (62%) Cured (as above) or improved at 3-month follow up (at least 50% decrease in incontinence episodes and symptoms; patient questionnaire): I: 26/27 (96%), II: 13/13 (100%) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Peattie 1988180 (abstract only) Study design/method: 2-arm parallel RCT. Single centre, England Duration of study: 4 weeks |
Inclusion criteria: Premenopausal women with USI awaiting surgery Exclusion criteria: Not reported N randomised: 37 in the abstract published in 1988 (Peattie 1988180) 44 in the Cochrane review (Hay-Smith 2001224) – see Additional information N lost to follow-up: I: 6/22, II: 5/22 Type of incontinence: USI |
I. PFMT, N = 16 II. VC, N = 17 (N in analysis) PFMT: Training with physiotherapist. Three clinic visits of 1 hour, 30 minutes and 15 minutes, respectively. Education on the anatomical relationship and means of exercising the pelvic floor muscles. Daily home exercise programme of 50 VPFMC per day for 4 weeks VC: Hold cone for 15 minutes two times per day. Weekly telephone call. Femina cones. Nine conical weights, 20–100 g Additional information: Abstract publication was of a continuing trial, with some participants awaiting assessment. More complete information was provided by the author for inclusion in the Cochrane review (Hay-Smith 2001224) |
Objective Improvement on pad test: I: 9/16, II: 12/17 Extended pad test (no detail): significant reduction in both groups, no between-group difference (at the time of reporting in 1988) Surrogate outcomes Adherence: I: 6/22 did PFMT only on alternate days or less often, 5/22 poor compliance and withdrew, II: 19/22 used cones at least once a day, 3/22 poor compliance and withdrew N having incontinence surgery after treatment (at the time of reporting in 1988): I: 4/9, II: 5/15 |
Subjective Subjective improvement: I: 10/16, II: 12/17 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pieber 1995 192 Study design/method: 2-arm parallel RCT. No attempt at blinding assessment. Single centre, Austria Duration of study: 3 months |
Inclusion criteria: Premenopausal women referred to urodynamic unit, had grade 1 or grade 2 SUI according to Ingelman-Sundberg Exclusion criteria: Grade 3 SUI, previous incontinence surgery, pelvic relaxation greater than grade 2, or detrusor instability N randomised: 46 N lost to follow-up at 6 weeks: I: 9/25 (35%), II: 8/21 (38%); further 2 from group 1 at 12 weeks Type of incontinence: USI (mild to moderate) Age (years, mean, SD): I: 44.3 (5.7), N = 16?, II: 41.7 (6.4 ), N = 13? Other: Weight, parity |
I. PFMT, N = 25 II. PFMT + VC, N = 21 (N in analysis) PFMT: Correct VPFMC taught by: physiotherapist using vaginal palpation. Perineal sonography during examination to show pelvic floor muscle contraction. Education in anatomy of pelvic floor muscle and bladder. PFMT = individualised programme with aim of 100 VPFMC during the day and ‘The Knack’ (VPFMC with increased intra-abdominal pressure and lifting). Duration of training: 12 weeks Supervision: Clinic visits at intervals of 2–4 weeks for 12 weeks PFMT + VC: PFMT and education as above with the addition of holding heaviest cone possible for 15 minutes per day. Five conical weights, 20–70 g. Instructed to use the next heaviest cone when comfortable with the last one. Clinic visits at intervals of 2–4 weeks for 12 weeks |
Surrogate outcomes Pelvic floor muscle strength: I: NR; II: Except for 2 women, who increased their cone weight, all women stayed with the cone they started N having incontinence surgery at 3 months (during study period): I: 0/25, II: 0/21 Adverse events Adverse events: None reported |
Subjective Cure (patients reported no urine loss on any occasion, and a negative pad test ) at 12 weeks: I: 3/25, II: 5/21 Cure (as above) or improvement (patients reported losing urine less often than before treatment) at 12 weeks: I: 12/25, II: 11/21 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Pohl 2004171 (abstract only) Study design/method: 2-arm RCT, Germany Duration of study: 3 months |
Inclusion criteria: Women with SUI Exclusion criteria: Not reported N randomised: 70 at the time of publication (results for 31 are shown) N lost to follow-up: Not reported Type of incontinence: SUI ‘Suffering from disease’ (visual analogue scale): I: 6.00, II: 6.70 Stress test under standardised condition (no further detail, unit of measurement unclear, average): I: 2.32, II: 2.60 Pad test under standardised condition (no further detail, unit of measurement unclear, average): I: 4.82, II: 11.4 Levator contraction (digital palpation, average): I: 1.75, II: 2.00 |
I. PFMT + ES, N = 21 II. PFMT + BF, N = 10 (N in analysis) PFMT + ES: PFMT = twice a day for 10 minutes, to perform VPFMC with 10 seconds’ hold, 10 seconds’ rest. N of contractions not reported. ES = no detail PFMT + BF: PFMT as above. BF = no detail Additional information: Ongoing trial |
Objective Stress test under standardised condition (no further detail, unit of measurement unclear, average): I: 1.50, II: 1.70 Pad test under standardised condition (no further detail, unit of measurement unclear, average): I: 6.21, II: 10.0 Surrogate outcomes Levator contraction (digital palpation, average): I: 2.55, II: 2.70 Adverse events N experiencing adverse events: I: 0/21, II: 0/10 |
Quality of life ‘Suffering from disease’ (visual analogue scale, 0-10, 0 = no suffering, 10 = maximum suffering; average score): I: 4.81, II: 5.33 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ramsay 1990128 (abstract only) Study design/method: 2-arm RCT, parallel design. Single centre, Scotland Duration of study: 3 months |
Inclusion criteria: Women whose only symptom was SUI Exclusion criteria: Not reported N randomised: 44 N lost to follow-up: None Type of incontinence: SUI (and USI?) |
I. PFMT, N = 22 II. Placebo PFMT, N = 22 PFMT: Set: Four maximum isometric VPFMC, with 4-second hold and 10-second rest. One set every waking hour. Duration of training: 3 months. Supervision: NR Placebo PFMT: As PFMT but comprising hip abductor muscle contraction with feet crossed at the ankles Additional information: It is probable that both groups received physiotherapy counselling, given that the study aimed to assess: (1) the effectiveness of physiotherapy; (2) what proportion of success can be attributed directly to PFMT as opposed to general support and counselling obtained during physiotherapy; (3) the compliance of patients undergoing home-based, taught PFMT |
Objective Pad test (not defined): I: mean reduction 2.1 g, II: mean increase 1.5 g, ‘significant’ between-group difference Surrogate outcomes Adherence: The highest number of exercise (contractions) performed in 1 week = 130 (30% of the maximum possible). The mean frequency of exercises per week = 15% of the requested level. No difference between groups. Authors note that lack of PFMT effect may be due to the very poor exercise level (poor compliance) Vaginal squeeze pressure (perineometry score): Not significantly different between groups, the mean score in each group improving |
Subjective Patient-perceived improvement (not defined): I: 14/22, II: 14/22 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Sand 1995 134 Study design/method: 2-arm RCT. Six centres, USA Duration of study: 3 months |
Inclusion criteria: Women with USI, ambulatory, community dwelling, understand questions, comply with visits, not seek other treatment, no current incontinence treatment, neurologically normal Exclusion criteria: Detrusor instability, pregnant, demand pacemaker, prior pelvic floor stimulation, pelvic implanted devices, active vaginal lesions or infections, urinary tract infection, hypermenorrhoea or menorrhagia, urinary retention > 100 ml, pelvic surgery in past 6 months, atrophic vaginitis, genital prolapse to introitus, pelvic irradiation, intrinsic sphincter indeficiency N randomised: 52 N lost to follow-up: I: 7/35, II: 1/17 Type of incontinence: USI Age (years, mean, SD): I: 50.9 (9.8), II: 57.7 (13.3), p = 0.04 Episodes of leakage in 24 hours (mean, SE): I: 28, 3.1 (0.65), II: 16, 3.0 (0.45) N of pad changes per week (weekly diary, mean, SE): I: 28, 6.2 (1.08), II: 16, 9.7 (3.37) Weight of urine lost on 20-minute pad test at a fixed bladder volume (g, mean, SE): I: 28, 45.2 (10.24), II: 16, 30.0 (10.85) Other: Height, weight, % prior incontinence surgery, parity, % postmenopausal Had tried PFMT previously: I: 14/35, II: 10/17 |
I. ES, N = 35 II. Sham ES, N = 17 (N randomised) ES: Innova (Empi, Inc.) pelvic floor stimulator. Seven office visits over 15 weeks (12 weeks’ treatment plus 2 weeks before and 1 week after treatment) with weekly telephone follow-up on non-visit weeks. A total of 70 hours of device use was planned Sham ES: No details Additional information: Denominators (N of valid observations) not provided and assumed to be N randomised ‘The investigators deliberately did not try to educate patients about pelvic muscle exercises or treatment of incontinence or modify their behaviour in any way, so that a true ‘bare bones’ assessment of the efficacy of pelvic floor stimulation could be made’ (p. 78) |
Objective Cure by diary (the absence of reported leakage episodes on 24-hour diary): I: 0/35, II: 1/17, p = 0.33 Cure or improvement by diary ( 50% in leakage episodes on 24-hour diary): I: 13/35, II: 2/17, p = 0.05 Cure by pad test (≤1 g of leakage on 20-minute fixed-volume pad test): I: 7/35, II: 2/17, p = 0.38 Cure or improvement by pad test ( 50% decrease in fluid loss on 20-minute fixed-volume pad test): I: 16/35, II: 3/17, p = 0.05 Change in episodes of leakage in 24 hours (24-hour diary, mean, SE): I: 35, –1.2 (0.5), II: 17, 0.8 (0.53), p = 0.04 Change in N of pad changes per week (weekly diary, mean change, SE): I: 35, –2.1 (0.8), II: 17, 1.5 (1.43), p = 0.07 Change in N of micturition in 24 hours (24-hour diary, mean change): I: 35, –0.2, II: 17, 0.7, NS, SE not reported Change in weight of urine lost on 20-minute pad test at a fixed bladder volume (g, mean, SE): I: 35, –29.9 (9.7), II: 17, 2.3 (5.59), p = 0.005 Surrogate outcomes Adherence (N with 80% compliance, i.e. > 50 hours of planned 70-hour device use): I: 21/35 (61%), II: 15/17 (89%) Change in vaginal muscle strength (perineometer, mmHg, mean, SE): I: 35, 4.6 (1.4), II: 17, –1.1 (1.51), p = 0.02 Adverse events N experiencing adverse events:Diarrhoea and abdominal pain thought to be unrelated to device use: I: 1/35, II: 0/17 No severe or irreversible adverse events during the study Discontinued treatment because of adverse events: I: 2/35 (persistent vaginal irritation), II: 0/17 |
Subjective Change in severity of urinary incontinence (10-point visual analogue scale, mean, SE): I: 35, –2.1 (0.5), II: 17, 0.1 (0.49), p = 0.007 Note: Decrease in values represent improvement Change in severity of stress incontinence (10-point visual analogue scale, mean, SE): I: 35, –2.3 (0.6), II: 17, –0.3 (0.60), p = 0.02 Note: Decrease in values represent improvement Quality of life SF-36: ‘No significant differences between the two groups for any changes in summary scores from baseline’. Details ‘will be reported else where’ (p. 78) Other: The ES device is ‘about US$1000’ Subgroup analysis In terms of the weight of urine lost on pad test and vaginal muscle strength, the results remained significantly better for the active ES group after controlling for age (the two groups were unbalanced at baseline) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Savage 2005 166 Study design/method: 2-arm RCT. Multicentred, two sites, UK. Pilot study Duration of study: 12 weeks |
Inclusion criteria: Women with a pure clinical history of SUI; leaks on cough, sneeze, jump or movement; frequency < 8 per day; nocturia once a day or less; occasional mild urgency only; and pelvic floor muscle strength of grade ≥ 2+ Exclusion criteria: Incontinence symptoms other than SUI (e.g. urgency, urge incontinence and faecal incontinence); dominant symptoms suggestive of prolapse; positive urinalysis; pregnancy; use of concomitant treatments (e.g. anticholinergic medication); pathology affecting ability to exercise (e.g. acute back pain, severe rheumatoid arthritis), concurrent neurological or psychiatric disease; women who had given birth or had gynaecological surgery within the previous 6 months; physiotherapy treatment for this condition within the past 2 years; women already practising lumbopelvic stability or pilates exercises; women unable to attend regular training sessions N randomised: 11 N lost to follow-up: I: 1/5, II: 0/6 Type of incontinence: SUI Age (years, mean, range): I: 54.60 (37–79), II: 48.17 (range not reported) King’s Health Questionnaire (composite score, mean, range): I: 293.02 (161.05, 522.15), II: 242.10 (122.21, 361.1) King’s Health Questionnaire (symptoms, severity, mean, range): I: 5.72 (2–10), II: 6 (4–12) Power of pelvic floor muscle (mean, range): I: 3.25 (2.5–4.0), II: 3.33 (2.5–4.0) Length (endurance) of pelvic floor muscle (mean, range): I: 7.25 (4–10), II: 6.16 (3–10) Other: BMI, parity (N of deliveries), % post- and perimenopausal |
I. PFMT, N = 4 II. Modified pilates (lumbopelvic stability training), N = 6 (N in analysis) PFMT: Before randomisation, participants received education about the anatomy and function of the pelvic floor and bladder, and were taught how to perform a correct VPFMC by therapist. Six individual physiotherapy sessions of 30–45 minutes in duration (in the outpatient physiotherapy department) over a 12-week period with the expectation that the patients would practice their allocated training at home between sessions. PFMT = maximal contractions held for 1–2 seconds (‘fast’ contractions), submaximal contractions (‘slow’ contractions) held while breathing, and staged contractions, where the pelvic floor is gradually tightened to maximum and then allowed to release slowly Contractions were taught in different positions. Encouraged to practise the PFMT sequence several times during the day. Programme tailored to individual’s ability. At each review, new goals for the coming week were identified. Also taught ‘The Knack’. Other physiotherapy modalities (e.g. electrical stimulation, vaginal cones) were not allowed. Duration of training: 12 weeks Pilates: Lumbopelvic stability training exercises were taught using the modified pilates method. Stage 1: Taught how to activate and control deep abdominal and pelvic floor muscles in a co-contraction. The importance of relearning motor control of these muscles to stabilise the pelvic region was underlined. Breathing patterns were emphasised. Stage 2: Co-contraction together with relaxed breathing were performed in different positions, progressing to antigravity positions. The physiotherapist monitored the patient by palpating the lower abdominal muscles, and by monitoring movement of the pelvis and spine. Stage 3: The exercises were developed by applying low load to the muscles through limb movement patterns. Also taught how to use a similar co-contraction with breathing during activities of daily living and in situations which cause bladder leakage. Encouraged to perform a series of exercises which they enjoyed and/or found challenging for 10–15 minutes at home at lease every other day. Six individual physiotherapy sessions of 30–45 minutes in duration over a 12-week period with the expectation that the patients would practice their allocated training at home between sessions |
Surrogate outcomes Pelvic floor muscle strength (digital vaginal assessment by palpation of the muscles and grading on the modified Oxford scale; mean, range): I: 3.5 (3–4), II: 4.41 (3–5) Pelvic floor muscle endurance (seconds, mean, range): I: 8 (6, 10), II: 9.1 (7, 10) N having incontinence surgery: I: 1/4 considering an operation (referred to consultant), II: not reported |
Quality of life King’s Health Questionnaire (composite scores regarding symptoms, severity and quality of life, mean, range): I: 256.92 (147.20– 416.64), II: 152.37 (83.82–197.20) King’s Health Questionnaire (symptom severity scores, mean, range): I: 5.5 (2–9), II: 3.5 (1–6) N desiring further treatment: I: 2/4 needed only physiotherapy follow-up, 2/4 referred for a consultant opinion, II: 0/6 needed any medical input, 2/6 required ongoing physiotherapy; individual data reported in table 8 Satisfaction, 100% (N of participants): I: 1/4, II: 1/6 Note: The authors note that this patient in group I was 100% satisfied because she now knew that PFMT would not work and felt confident that she needed an operation Satisfaction, 80–99% (N of participants): I: 1/4, II: 3/6 Note: The authors postulate over 80% satisfaction as a satisfactory clinical outcome |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Seo 2004 195 Study design/method: 2-arm parallel study. Multicentre but number of centres not stated Duration of study: 6 weeks |
Inclusion criteria: Women with SUI who required non-surgical treatment Exclusion criteria: Not reported N randomised: 120 N lost to follow-up: None? Type of incontinence: SUI Age (years, mean, SD): I: 42.7 (11.3), II: 44.5 (12.1), p = 0.63 Pad test (type of test and unit of measurement not specified): I: 5.56 (6.05), II: 6.51 (2.55), p = 0.210 Maximal vaginal pressure (mmHg): I: 17.78 (10.96), II: 23.01 (11.70), p = 0.231 Duration of pelvic floor muscle contraction (seconds): I: 4.86 (2.31), II: before 5.47 (3.48), p = 0.581 Other: Weight, parity |
I. PFMT + ES + BF, N = 60 II. VC, N = 60 (N in analysis) ES + BF (+PFMT): 20-minute sessions twice a week for 6 weeks to perform alternately functional electrical stimulation (FES) and BF (presumably BF of VPFMC but this is not stated). FES = simultaneous electrical stimulation of 35 Hz and 50 Hz for 24 seconds, and repeat this for 20 minutes ‘New’ VC: Each cone has a dumbbell shape and is 150 g. Instructed by a specially trained nurse to start lying down, and progress to sitting with the cone in place while contracting the pelvic floor muscles. VPFMC with 5-second hold, 10 seconds’ rest. Repeat this for at least 5 minutes daily for 6 weeks. Pelvic floor muscle awareness and compliance were assessed at the hospital once a week Additional information: Not relevant for direct head-to-head comparisons; data were therefore extracted for primary outcomes only |
Subjective ‘Improvement in the degree of incontinence’ (unclear how this was measured): I: 55/60, II: 53/60 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Shepherd 1983 155 Study design/method: 2-arm RCT. Single centre, England Duration of study: Length of treatment period unclear. Measurement at ‘3 months after treatment was completed’ |
Inclusion criteria: Women with USI, with stable bladders Exclusion criteria: Not reported N randomised: 22 N lost to follow-up: I: 0/11, II: 3/11 (lack of motivation suggested as possible cause of withdrawal) Type of incontinence: USI Age (years, mean, range): I: 48.2 (23–63), II: 48.4 (28–67) Duration of symptoms (years, mean, range): I: 11 (0.5–30), II: 9 (0.67–16) Number of incontinent episodes/week: I: 6.5 (1–18), II: 5.5 (3–12) Number of micturitions/day (mean, range) I: 8.6 (6–10), II: 8.0 (5–10) Other: % prior incontinence surgery, parity |
I. PFMT + IVRD + BF (‘pelvic exerciser’), N = 11 II. PFMT, N = 11 (N in analysis) PFMT + IVRD + BF: Initial measurement of pelvic floor muscle and then instructed from physiotherapist a series of graded exercises with intravaginal ‘exerciser’ connected to visual BF to perform daily at home. Weekly clinic visit PFMT: Initial measurement of pelvic floor muscle and then taught ‘conventional’ home exercises from physiotherapist. Weekly clinic visit Additional information: Paper states that ‘If there was no improvement after six attendances at weekly intervals, alternative treatment was arranged’. No further details available |
Objective Episodes of leakage per week (diary, mean, range): I: 1.1 (0–8), II: 4.1 (0–7) N of micturition in 24 hours (diary, mean, range): I: 6.1 (5–9), II: 7.8 (6–10) Pelvic squeeze (cmH2O, mean, range): I: 19.3 (10–30), II: 11.2 (5–20) |
Subjective Cure (N of patients who perceived dryness): I: 8/11 (72.7%), II: 3/11 (27.3%) Improvement (N of patients who perceived improvement; not including cure): I: 2/11 (18.2%), II: 3/11 (27.3%) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Sherburn 2007182 (abstract only) Study design/method: 2-arm RCT, parallel design. Two centres, Australia Duration of study: 20 weeks |
Inclusion criteria: Community-dwelling women over 65 years of age with USI, which is perceived by them as bothersome Exclusion criteria: > 10 cmH2O detrusor pressure rise on cystometry, incontinent due to neurological causes, PFMT intervention within the last 6 months, or unable to give informed consent N randomised: 84 N lost to follow-up: NR Type of incontinence: USI Age (years, mean, range): 72 (65–89) ‘There were no differences between groups at baseline on any measures’ |
I. PFMT, N = 43 II. BT, N = 41 (N randomised) PFMT: Weekly hour-long exercise and education classes for 20 weeks. Home programme of PFMT (no further details given) BT: Weekly hour-long exercise and education classes for 20 weeks. Home programme of BT (no further details given) |
Objective Zero leakage on the cough stress test, with no precontraction of pelvic floor muscle: I: 19/40 (47.5%), II: 9/35 (25.7%) Zero leakage on the cough stress test, with a pre-contraction of pelvic floor muscle: I: 23/40 (57.5%), II: 8/35 (22.9%) Episodes of leakage (7-day diary, median interquartile range, mean rank): I: 43, 4.5, 11, (36.47), II: 41, 8.0, 27, (47.95), p = 0.030 Stress test – cough (g, median interquartile range, mean rank): I: 43, 0.1, 1.5, (36.18), II: 41, 0.5, 2.4, (47.09), p = 0.034 Stress test – brace/cough (g, median interquartile range, mean rank): I: 43, 0.0, 0.4, (32.51), II: 41, 0.3, 0.7, (44.27), p = 0.008 |
Quality of life ICIQ-UI SF score (median interquartile range, mean rank): I: 43, 5, 4, (34.55), II: 41, 8, 7, (50.01), p = 0.003 AQoL total score (mean, SD, SEM): I: 43, 14.44 (9.14), 1.394, II: 41, 11.88 (9.27), 1.519, p = 0.217 Note: The higher the score, the poorer the QoL ‘Bothersome’ (visual analogue scale, not specified, mean, SD, SEM): I: 43, 2.26 (2.139), 0.326, II: 41, 3.68 (2.654), 0.420, p = 0.009 Participant global perception of change: the PFMT group reported a greater perception of change in symptoms, p = 0.004 Satisfaction with treatment: no significant difference between groups, p = 0.102 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Smith 1996 177 Study design/method: 4-arm RCT (only details of 2 arms given). Single centre, USA Duration of study: 16 weeks |
Inclusion criteria: Women predominantly with urodynamically proven genuine stress urinary incontinence (type 2 incontinence with Valsalva leak point of between 60 and 110 cm of water) or detrusor overactivity Exclusion criteria: Type 3 stress incontinence, pregnant, history of prolonged urinary retention, vaginal vault prolapse, diminished sensory perception, cardiac pacemaker, mixed incontinence if major/minor component (GSI or DO) not determined N randomised: predominantly GSI 18, predominantly DO 39 N lost to follow-up: predominantly GSI, I: 0/9, II: 0/9; predominantly DO, 1/39 Type of incontinence: GSI or DO as the major component of incontinence; data reported separately for women predominantly with GSI Age (years, average, range): women predominantly with GSI, I: 9, 48 (36–70), II: 9, 53 (26–72) Other: Parity; % estrogen |
I. PFMT, N = 9 II. ES, N = 9 (N in analysis) PFMT: Correct VPFMC confirmed by vaginal examination and perineal squeeze. Sixty ‘slow and quick’ VPFMC per day for 16 weeks. Clinic visits every 4–6 weeks ES: Intravaginal neuromuscular stimulation (Stimtech Products Inc). Output starting with a 5-second contraction time (range 3–15), a duty cycle of 1 to 2 (range 1–1, to 1–2), 15 minutes twice a day (at home) progressing to 60 minutes twice a day over 16 weeks. Amplitude progressed from 5–10 mA to maximum of 80 mA (range 1–100). Clinic visits every 4–6 weeks Additional information: Intervention arms I and II for women predominantly with GSI (N = 18), and III and IV for women predominantly with DO (N = 39). Details of interventions III and IV not given as comparisons not used in this review |
Objective Cured (cessation of incontinence and no longer requiring pads): women predominantly with GSI, I: 1/9, II: 2/9 Improved (≥ 50% reduction in the N of pads and episodes of urinary incontinence; not including cure): women predominantly with GSI, I: 3/9, II: 4/9 Episodes of leakage per week (voiding diaries, average, range): women predominantly with GSI, I: 2.4 (0–6), II: 1.4 (0–5) N of pad changes per week (voiding diaries, average, range): women predominantly with GSI, I: 5.4 (0–10), II: 4.0 (0–10) N having incontinence surgery (timing not reported): women predominantly with GSI, I: 3/9, II: 2/9 Adverse events N experiencing adverse events (women with GSI and DO, see Additional information section):Discontinued treatment because of adverse events: women predominantly with GSI, I: 0/9, II: 0/9 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Swithinbank 2005 119 Study design/method: Crossover trial with random allocation to the order in which participants increased or decreased decaffeinated fluids in weeks 3 or 4 Duration of study: 4 weeks |
Inclusion criteria: Women with USI or idiopathic detrusor overactivity (IDO); the USI group was naive to surgery Exclusion criteria: Urinary tract infection, hepatic, cardiac or renal disease, diabetes mellitus; those on antidepressants, anticholinergics or diuretics N randomised: Not reported N lost to follow-up: USI: 9/48, IDO: 6/36 Type of incontinence: USI 48, IDO 36; data for USI reported separately Age (years, mean, range): 54.8 (31–76) Episodes of leakage in 24 hours (median, interquartile range, women with USI only): Week 1 (baseline): 1.6 (0.6, 2.8), week 2 (decaffeinated fluids): 0.8 (0.1–1.9) N of micturition in 24 hours (median, interquartile range, women with USI only): Week 1 (baseline): 7.2 (6.2–8.4), week 2 (decaffeinated fluids): 7.0 (5.9–8.9) 24-hour pad test (g, median, interquartile range): Week 1 (baseline): 7.6 (3.3, 18.3), week 2 (decaffeinated fluids): 7.1 (2.7–12.1) Mean fluid intake per day (week 1): 1639 ml |
I. Caffeine free and increasing fluid, N = 39 II. Caffeine free and decreasing fluid, N = 39 (N in analysis, crossover, women with USI only) Treatment (crossover): During the first week, participants drank normally. In the second week all participants drank normally, but only decaffeinated fluids. After this participants were randomised to either increasing decaffeinated fluids to 3 litres (20 cups) per day for a week, followed by a week of reducing decaffeinated fluids to 750 ml (five cups) per day, or vice versa. Results from the weeks with increased and decreased fluids were compared. Urine osmolality was measured at weekly clinic visit to assess compliance Additional information: Data on patients with IDO not extracted |
Objective Episodes of leakage in 24 hours (median, interquartile range; daily diary over 4 weeks; women with USI only): I: 0.7 (0.3–3), II: 0.5 (0.2–2.1) N of micturition in 24 hours (median, interquartile range; daily diary over 4 weeks; women with USI only): I: 8.3 (7.0–10.9), II: 6.3 (5.0–7.1) 24-hour pad weight (g, median, interquartile range, women with USI only): I: 7.9 (4.0–19.7), II: 6.9 (3.1–13.9) Mean fluid intake per day (ml, all women with USI or IDO): Week 2 with decaffeinated fluids: 1630 ml; week increasing fluid: 2673 ml; week decreasing fluid: 872 ml Adverse events: Constipation, thirst |
Subjective Quality of life (Overall, how much do your urinary symptoms interfere with your life?’): Decreasing fluid intake (week 3 or 4) showed significant improvement in quality of life compared with the baseline week (p < 0.003). However, there was no difference in quality-of-life impact among any of the other weeks Shorter version of the Bristol Female Lower Urinary Tract Symptoms symptom questionnaire (all women with USI or IDO): The mean impact of urinary symptoms remained as ‘a little’ on daily life for the study period |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Tapp 1987191 (abstract only) Study design/method: 2-arm RCT Duration of study: 3 months’ treatment (plus 6 months’ follow-up but data are reported for assessment at 3 months only) |
Inclusion criteria: Women with USI with no other significant urodynamic abnormality Exclusion criteria: Previous incontinence or prolapse surgery N randomised: 29 N lost to follow-up: Not reported Type of incontinence: USI Visual analogue symptom score – stress incontinence (no detail, score %, mean, SD): I: 15, 78 (24), II: 14, 84 (20) Visual analogue symptom score – urge incontinence (no detail, score %, mean, SD): I: 15, 30 (23), II: 14, 44 (38) |
I. PFMT, N = 15 II. PFMT + ES, N = 14 (N in analysis) PFMT (‘physiotherapy’): Individual training with continence advisor. Comprehensive teaching about the mechanism of continence and the action of pelvic floor. PFMT four times per hour, every hour of the day. Weekly clinic visits for 3 months PFMT (‘physiotherapy’) +ES: PFMT (3 months) and education as above. Individual training with continence advisor. ES = faradic stimulation with vaginal probe twice a week for 1 month |
Objective Pad test (g, mean, SD): no within-group (before–after) differences or between-group differences N having incontinence surgery after treatment: I: 10/15, II: 6/14 |
Subjective Visual analogue symptom score – stress incontinence (no detail, score %, mean, SD): I: 15, 67 (29), NS (pre-post), II: 14, 65 (28), p < 0.05 (pre-post) Visual analogue symptom score – urge incontinence (no detail, score %, mean , SD): I: 15, 29 (28), NS (pre-post), II: 14, 36 (30), NS (pre-post) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Tapp 1989185 (abstract only) Study design/method: 3-arm RCT. Single centre, England Duration of study: 3 months for PFMT group, and 6 months for surgery group |
Inclusion criteria: Consecutive women with USI and no other urodynamic abnormality Exclusion criteria: A history of urological or vaginal surgery N randomised: 81 N lost to follow-up: I: 6/27, II: 3/26, III: 4/28 Type of incontinence: USI |
I. PFMT, N = 21 II. PFMT + ES, N = 23 III. Surgery, N = 24 (N in analysis) PFMT: 14 clinic visits over 3 months with continence advisor trained to teach PFMT. Patients assessed after 3-month treatment and at 6 months PFMT + ES: PFMT as above. ES = faradism. Patients assessed after 3-month treatment and at 6 months Surgery: Burch colposuspension. Patients assessed at 6 months after surgery Additional information: For PFMT and PFMT + ES, some unsatisfied patients requested surgery at the end of 3 or 6 months’ assessment This trial is published in two abstracts (Tapp 1989)185,305 but there were inconsistencies in data reporting. Authors were contacted and informed that data in Tapp (1989)185 should be used, as they are more complete |
Objective ‘Objectively cured’ (not defined) after primary treatment: I: 4/21 at 3 months, II: 3/23 at 3 months, III: 18/24 at 6 months ‘Symptomatic improvement’ (not defined) after primary treatment (not including ‘cure’): I: 9/21 at 3 months, II: 13/23 at 3 months, III: 5/24 at 6 months N having incontinence surgery as secondary treatment after 3 months: I: 11/21, II: 8/23, III: NA Objective cure or improvement at 6 months (of those who did not have surgery): IIICured at 6 months2/102/15Symptomatically better at 6 months7/108/15Symptoms relapsed and requested surgery at 6 months1/105/15 Return of symptoms at 6 months (of those who did not have surgery): see above Objectively cured at 6 months (of those who had surgery as secondary treatment): IIICured10/128/13Awaiting assessment0/123/13 |
I | II | Cured at 6 months | 2/10 | 2/15 | Symptomatically better at 6 months | 7/10 | 8/15 | Symptoms relapsed and requested surgery at 6 months | 1/10 | 5/15 | I | II | Cured | 10/12 | 8/13 | Awaiting assessment | 0/12 | 3/13 | |||||||||||||||||||||||||||||||||||||
| I | II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cured at 6 months | 2/10 | 2/15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptomatically better at 6 months | 7/10 | 8/15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms relapsed and requested surgery at 6 months | 1/10 | 5/15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| I | II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cured | 10/12 | 8/13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Awaiting assessment | 0/12 | 3/13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Taylor 1986 156 Study design/method: 4-arm RCT, pilot study. Single centre, USA Duration of study: 9 weeks |
Inclusion criteria: Women aged ≥ 55 years, currently experiencing simple urinary stress incontinence. Non-institutionalised ambulatory women who could speak English Exclusion criteria: Known neurogenic or neuromuscular disorders (e.g. diabetes, multiple sclerosis, Parkinson’s, stroke); symptoms indicating urinary tract infection; taking medications for urological disorders N randomised: 13 N lost to follow-up: 1/13 participants (group not specified) participated in the trial but results were not included in the analysis because she had missed a weekly clinic visit following a heart attack (‘Subjects had to make all nine, weekly visits to be included in the study data’) Type of incontinence: SUI ‘Demographic data concerning age, weight, parity, previous pelvic repair surgery, multiple or difficult births were collected in the health history for later analysis. |
I. PFMT II. PFMT + clinic BF and home BF III. PFMT + clinic BF and home IVRD IV. PFMT + clinic BF (N in each group unclear) PFMT: One 90-minute introductory visit at clinic where correct VPFMC was taught using an electromyographic device called the Personal Perineometer™. Participants then returned for 30-minute individual visits, once per week for 8 weeks. PFMT at home = 100 VPFMC with 10-second hold, once a day. Duration of training: 9 weeks. Instructed to repeat this exercise ‘for the rest of their lives’. Also received advice not to restrict fluid intake, and strategies to reduce frequency. Participants provided with a take-home teaching guide. Pelvic floor muscle strength was measured at clinic at entry and exit only PFMT + clinic BF + home BF: PFMT as above. In addition, clinic and home BF. Weekly BF in the clinic setting = participants measured for pelvic floor muscle strength and allotted 10 minutes of private use of the BF device. Home BF = visual BF (Personal Perineometer™) PFMT + clinic BF + home IVRD: PFMT and clinic BF as above plus vaginal probe (detached from the BF machine and so no visual BF) in situ to be used as a resistive device during PFMT PFMT + clinic BF: PFMT as above with BF used only during clinic visits. No home BF devices for daily exercises Additional information: ‘Pretest-post-test control group design with a randomly assigned, self-selected sample’ |
Subjective Cure (% of women describing themselves as continent): I: 67%, II: 100%, III: not reported, IV: not reported,; the rate obtained by groups II, III and IV as a whole was also 67% |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Terry 1996193 (abstract only) Study design/method: 2-arm parallel study. Single centre, Scotland Duration of study: 6-week treatment + follow-up at 6 months |
Inclusion criteria: Women with USI able to retain vaginal cone Exclusion criteria: Not repeated N randomised: 60 N lost to follow-up: I: 7/30, II: 19/30 Type of incontinence: USI Standardised pad test after a series of provocative exercises with a full bladder (g, mean): I: 38.2, II: 32.5 |
I. VC, N = 30 II. PFMT + ES, N = 30 (N in analysis) VC: Enhanced vaginal cones, one cylindrical weight of 75 g. No details given PFMT + ES: Supervised physiotherapy, 12 sessions over 6 weeks with a combination of interferential therapy and PFMT with no further details Additional information: Not relevant for direct head-to-head comparisons; data were therefore extracted for primary outcomes only |
Subjective Patient perception at 6 months (5-point scale, asking how the women were compared with before treatment): significant and similar improvement for both groups Patient perception at 6 months (10-cm analogue scale, asking how bad the women thought their continence was at present): significant and similar improvement for both groups Quality of life at 6 months (10-cm analogue scale, asking how badly the women’s continence affected their lifestyle): significant and similar improvement for both groups |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
van Kerrebroeck 2004 117 Study design/method: 2-arm RCT. Multicentre; 46 sites in Belgium, Canada, Denmark, France, Germany, the Netherlands, Sweden and UK Duration of study: 12 weeks (+ 2-week screening, 2-week no-SNRI, and 2-week placebo lead- in prior to randomisation) |
Inclusion criteria: Women aged ≥ 18 with clinical diagnosis of predominant and ‘bothersome’ SUI of ≥ 3 months’ duration, with seven incontinence episodes/week. Micturition frequency of < 8 per day and ≤ 2 per night; positive cough stress test and positive stress pad test Exclusion criteria: Predominant symptoms of urge incontinence, unable to tolerate bladder filling to 400 ml or with first sensation of bladder filling at ≤ 100 ml N randomised: 494 N lost to follow-up: I: 68/247 (27%), II: 21/247 (8%) Type of incontinence: Predominant symptoms of SUI (MUI) Age (years, mean, SD): I: 52 ± 11.0 , II: 54 ±10, p = 0.01 Episodes of leakage per week (mean, SD, range): I: 17.3 ± 12.9 (0–81), II: 17.2 ±12.4 (1–68), extreme outliers Episodes of leakage (N with ≥ 14 episodes per week): I: 125/247, II: 131/247 PGI-S (‘moderate’ or ‘severely’ abnormal urinary tract function): I: 166/247, II: 169/247 I-QoL score (mean, SD): I: 66.7 ± 20.1, II: 64.5 ± 21.3 Other: BMI, ethnicity, % prior incontinence surgery, % performing PFMT |
I. Duloxetine 80 mg taken as 40 mg twice daily, N = 247 II. Placebo, N = 247 (N randomised) Additional information: The study recruited ‘type 3’ population; data were therefore extracted for primary outcomes only Dichotomous data calculated from percentage in paper, using the N of women included in the ‘ITT’ analysis with at least one postrandomisation measure (last outcome measure carried forward) Diary data were completed daily during the week prior to each clinic visit. Two diaries were completed prior to randomisation and three following randomisation |
Objective Cure or improvement (50–100% reduction in incontinence episodes on daily paper diaries): I: 110/212 (51.9%), II: 81/242 (33.5%), p < 0.001 Surrogate outcomes Adherence (average % of treatment doses ingested): I: 82%, II: 94%, p < 0.001 Adverse events N experiencing adverse events: I: 200/247 (81%), II: 158/247 (64%), p < 0.001 Adverse events (occurring in at least 5% of patients on duloxetine or occurring significantly more often with duloxetine than placebo): Nausea, dry mouth, constipation, fatigue, insomnia, dizziness, headache, increased sweating, vomiting, somnolence, tremor (Note: Italic text indicates that this type of event caused withdrawal) Discontinued treatment because of adverse events: I: 53/247 (21.5%), II: 12/247 (4.9%); adverse events as above, and attention disturbance |
Subjective PGI-I (‘very much/much/a little’ better): I: 135/240 (56.2%), II: 118/245 (48.2%) Quality of life I-QoL (mean): I: 240, before 66.6, after 72.2, II: 245, before 64.4, after 68.5, p = 0.127 Note: Based on data from randomised subjects with at least one postrandomisation measure |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Williams 2006 129 Study design/method: 3-arm RCT. Leicester MRC, UK Duration of study: 3 months |
Inclusion criteria: Women with urodynamic diagnosis of USI or MUI and DO who had already had an 8-week primary care intervention (2-armed trial comparing a new nurse-led service with existing care; if, after 8 weeks’ treatment in the nursing arm of the trial, participants reported any episode of UI, day time frequency of ≥ 8, nocturia of ≥ 2 reported in a 3-day diary, 24-hour pad test weight of ≥ 8 g, or patient complaint of urgency most of the time, they were offered a urodynamic investigation) Exclusion criteria: Women who were pregnant, had urinary fistula, pelvic malignancy, severe prolapse and those currently receiving treatment for urinary symptoms (e.g. on a waiting list for continence surgery) N randomised: 238 N lost to follow-up: I: 3/79, II: 1/80, III: 3/79 Type of incontinence (USI/MUI): I: 53/26, II: 57/23, III: 56/23 Age (years, mean, SD): I: 55.9 (8.5), II: 58.2 (9.4), III: 56.7 (10.8) Episodes of leakage in 24 hours (median, interquartile range): I: 79, 1.7 (0.7–4.0), II: 80, 1.2 (0.3–3.3), III: 79, 1.0 (0.3–2.8) Leakage (several times per months or more): I: 78/79, II: 76/80, III: 72/79 Other: Parity, % postmenopausal |
I. PFMT, N = 77 II. VC, N = 79 III. ‘Standard care’, N = 75 (N in analysis) PFMT: Correct VPFMC taught by specially trained nurses. Individualised exercise regimen, including time to hold maximum contractions, the N of quick contractions and the N of sets per day (4+). Digital pelvic floor muscle assessment at 2-weekly intervals. Duration of treatment: 12 weeks. Supervision: Clinic visits at 2-weekly intervals for 6 weeks and at 12 weeks VC: Femina, Urohealth Systems Inc. At first visit, patients were taught by specially trained nurses how to use the cones and given a prescription of activity (1–lying, 2–sitting, 3–standing, 4–housework, 5–exercise), cone weight (10–60 g), length of holds (10–15 minutes), and the N of times to be used per day (2–3). Commenced using the heaviest cone that the women could hold for > 5 and < 15 minutes whilst standing, then the weight was increased until the heaviest could be held for 15 minutes twice daily whilst undertaking the most strenuous level of activity. Duration of treatment: 12 weeks. Supervision: Clinic visits at 2-weekly intervals for 6 weeks and at 12 weeks Note: Author confirmed that PFMT programme does not include standard BF treatment ‘Standard care’: Given a leaflet detailing the location of pelvic floor muscles and three steps to exercising these muscles, i.e. pelvic floor awareness (PFA). Clinic visits with nurse at 2-weekly intervals for 6 weeks and at 12 weeks Additional information: Nested within a 2-armed trial; all participants had already had an 8-week primary intervention phase, which comprised behavioural intervention, including advice on fluid intake, caffeine intake, bladder re-education, PFA and weight loss (healthy eating). PFA included teaching of ‘The Knack’ |
Objective Change in N of episodes of leakage in 24 hours (3-day diary, mean, 95% CI): I: 77, –1.03 (–1.73 to –0.32), II: 79, –0.28 (–0.87 to 0.31), III: 75, –0.59 (–1.04 to –0.13) Change in N of pad changes in 24 hours (mean, 95% CI): I: 77, 0.05 (–0.30 to 0.41), II: 79, –0.04 (–0.25 to 0.17), III: 75, –0.16 (–0.39 to 0.06) Change in N of micturition in 24 hours (mean, 95% CI): I: 77, –0.23 (–0.56 to 0.11), II: 79, 0.11 (–0.22 to 0.43), III: 75, –0.27 (–0.65 to 0.11) Change in urine loss on 1-hour pad test (g, mean, 95% CI): I: 77, –7.39 (–13.76 to –1.02), II: 79, –3.68 (–8.43 to 1.07), III: 75, –6.11 (–12.64 to 0.42) Change in urine loss on 24-hour pad test (g, mean, 95% CI): I: 77, –2.06 (–13.82 to 9.71), II: 79, –5.19 (–12.22 to 1.84), III: 75, –7.25 (–14.63 to 0.17) Surrogate outcomes Adherence (N reporting that they followed the instructions all or most of the time): I: 59/77 (76%), II: 40/79 (50%), III: 60/75 (80%) Change in perineometry (average peak/rest) pressure, mean, 95% CI): I: 77, 1.62 (–0.33 to 3.59), II: 79, 0.25 (–1.10 to 1.73), III: 75, 0.13 (–1.65 to 1.91) Change in pelvic floor function (modified Oxford scale, mean, 95% CI): I: 77, 0.56 (0.30 to 0.83), II: 79, 0.04 (–0.15 to 0.23), III: 75, 0.23 (0.04 to 0.42) Adverse events N experiencing adverse events: I: 2/79, II: 2/80, III: 0/79 Adverse events: urinary tract infection; no other side effects reported by the three groups Discontinued treatment because of adverse events: I: 0/79, II: 0/80, III: 0/79 |
Subjective N cured (no symptoms): I: 4/77, II: 7/79, III: 6/75 Mild or no problem: I: 47/77, II: 51/79, III: 53/75 Quality of life The Leicester Impact Scale (0–42, lower score = better health; median, interquartile range): I: 77, 2.0 (0.0 to 5.0), II: 79, 2.0 (0.0 to 5.0), III: 75, 1.5 (0.0 to 5.0) The Leicester Urinary Symptom Questionnaire, leakage (several times per months or more): I: 72/77, II: 69/79, III: 68/75 The Leicester Urinary Symptom Questionnaire, frequency (hourly or more): I: 27/77, II: 25/79, III: 21/75 The Leicester Urinary Symptom Questionnaire, urgency (very strong or overwhelming): I: 27/77, II: 27/79, III: 26/75 The Leicester Urinary Symptom Questionnaire, nocturia (≥ 3 per night): I: 18/77, II: 8/79, III: 7/75 N satisfied with current urinary symptoms for the rest of life: I: 30/77, II: 30/79, III: 34/75 ‘How motivated do you feel to continue with treatment?’ (A lot): I: 55/77, II: 44/79, III: 53/75 Other:Subgroup analysis: |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Wilson 1987 157 Study design/method: Quasi-RCT, consecutive assignment. Single centre, UK Duration of study: 6 weeks’ treatment, with 6 months’ follow-up |
Inclusion criteria: Women diagnosed to have USI Exclusion criteria: None stated N randomised: 60 N lost to follow-up: None at 6 weeks Type of incontinence: USI Age (years, mean, range): 46.8 (19–79) N of pad changes in 24 hours (mean, SD): I: 15, 2.0 (1.3), II: 15, 2.3 (2.0), III: 15, 3.3 (2.7), IV: 15, 3.0 (2.1) N of micturition in 24 hours (mean, SD): I: 15, 8.6 (2.7), II: 15, 8.7 (2.7), III: 15, 9.1 (3.2), IV: 12, 8.24 (2.7) Pelvic floor muscle strength (perineometer reading, mmHg, mean, SD): I: 15, 7.1 (5.4), II: 15, 5.8 (3.6), III: 15, 5.1 (4.2), IV: 14, 5.8 (4.4) Other: The four groups reported to be comparable with respect to age, weight, parity, severity of leakage and the occurrence of previous surgery for incontinence N prior incontinence surgery: 12/60 |
I. PFMT + BF, N = 15 II. PFMT + BF + ES (faradism), N = 15 III. PFMT + BF + ES (interferential therapy), N = 15 IV. PFMT, N = 15 (N in analysis) PFMT + BF: Correct VPFMC taught in the hospital physiotherapy department with a vaginal perineometer. Three series of 6 VPFMC with 5 seconds’ hold and 15 seconds’ rest, 2-minute rest between each series, performed with the perineometer in the hospital physiotherapy department for 12 sessions over 6 weeks. Patients also given an instruction leaflet for PFMT to be performed daily at home: 5 VPFMC with a few seconds hold before getting up, after getting up, then half hourly thereafter, progressing to 10 VPFMC every half hour. Duration of training: 6 weeks. Supervision: Clinic visits twice a week PFMT: One session in the hospital physiotherapy department (possibly with the help of a vaginal perineometer). Patients given an instruction sheet for PFMT to be done daily at home for 6 weeks, as in the hospital-treated group above ES (faradism): Faradism involves the use of a low frequency current to stimulate striated muscle contraction. A saddle-shaped indifferent electrode placed over the sacrum and the active buttery electrode applied to the perineum, with as strong a current as the patient could tolerate. Three sets of 12 surges with a 2-minute rest between sets performed in hospital for 12 sessions over 6 weeks ES (interferential therapy): Interferential therapy involves a low frequency stimulating current within the body while avoiding the problems of skin resistance. Four medium-sized suction electrodes (2 each on the abdomen and adductor muscles of the thighs) transmitted a 20- to 25-mA current, giving 15 pulses at a pressure peak 0.25–0.30 Pa/cm2. The first session lasted 10 minutes and then progressed to 15 minutes. Performed in hospital for 12 sessions over 6 weeks |
Objective N of pad changes in 24 hours after treatment (mean, SD): I: 15, 0.9 (1.5), II: 15, 1.3 (1.4), III: 15, 1.6 (2.3), IV: 15, 2.7 (2.5) N of micturition in 24 hours after treatment (mean, SD): I: 15, 7.6 (1.6), II: 15, 7.8 (2.0), III: 15, 8.0 (1.8), IV: 12, 8.20 (2.7) Surrogate outcomes Pelvic floor muscle strength after treatment (perineometer reading, mmHg, mean, SD): I: 15, 15.7 (10.5), II: 15, 9.0 (5.4), III: 15, 16.5 (8.3), IV: 14, 6.9 (7.7) |
Subjective Subjective improvement after treatment (‘much improved’ or ‘improved’): I: 11/15, II: 11/15, III: 10/15, IV: 4/15 Subjective improvement at 6 months (‘much improved’ or ‘improved’): I: 9/14, II: 10/15, III: 9/15, IV: 4/15 Subgroup analysis: For groups I, II and III, successes and failures were compared to identify factors contributing to treatment success |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Wise 1993188 (abstract only) Study design/method: 3-arm RCT, parallel design. Single centre, England Duration of study: 12 weeks |
Inclusion criteria: Women with USI Exclusion criteria: None stated N randomised: 61 or 62 (unclear) N lost to follow-up: I: 6/21, II: 2/21, III: 4/20 Type of incontinence: USI The groups reported to be matched for age, parity and severity of USI |
I. PFMT + VC, N = 15 II. VC, N = 19 III. ES, N = 16 (N in analysis) PFMT + VC: Correct VPFMC taught by vaginal palpation. Set: 10 VPFMC. Sets per day: 10. Duration of training: 12 weeks. Supervision: Patients seen at 2, 6 and 12 weeks. Cones used in identical manner as the VC group below VC: Not examined vaginally but just instructed to use cones for 15 minutes two times per day, progressing to heavier weight when successful on two consecutive occasions. Patients seen at 2, 6 and 12 weeks ES: Maximal vaginal electrical stimulation (CONMAX), 20 MHz, 0.75-ms pulse duration, continuous stimulation at maximum tolerable intensity between 0 and 90 mA. Home treatment 20 minutes per day for 12 weeks. Patients seen at 2, 6 and 12 weeks |
Objective Improvement (not defined) on pad test: I: 14/15, II: 14/19, III: 12/16 Reduction in weight of urine loss on pad test (40-minute test with standard bladder volume): I: p = 0.006, II: p = 0.011, III: p = 0.163. ‘… decrease in pad weight after treatment was significantly greater (p = 0.038) for the PFMT + VC group compared to the ES group, but not when comparing the PFMT + VC group and the VC group (p = 0.053)’ Surrogate outcomes Pelvic floor muscle strength: ‘In the two groups using cones, there was a significant increase in both the passive and active cone weight following treatment’. No difference between groups |
Subjective Improvement in the symptom (visual analogue symptom scores): I: p = 0.002, II: p = 0.028, III: p = 0.011. The degree of symptomatic improvement was greater in the PFMT + VC group than in both the ES and VC groups |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Wong 1997a158 (abstract only) Study design/method: RCT. Single centre, Hong Kong Duration of study: 8 weeks |
Inclusion criteria: Chinese women with USI Exclusion criteria: Previous failure to PFMT, previous incontinence surgery N randomised: Not reported. Data reported for 17 women N lost to follow-up: Not reported Type of incontinence: USI Age (years, mean, SD): 48.2 ± 7.3 ‘The two groups were comparable with respect to duration of symptoms, parity, frequency of incontinence per week and pad test result’ |
I. PFMT, N = 7 II. PFMT + BF, N = 10 (N in analysis) PFMT: ‘Standard protocol of pelvic floor re-education’. ‘8 clinic visits, two times weekly’ (two visits per week over 4 weeks? Unclear) PFMT + BF: As above with addition of BF (PRS9300) from a vaginal surface electrode and a rectal catheter to record abdominal contraction |
Objective Reduction in episodes of leakage per week (7-day diary, mean, SD): I: 9.1 ± 12.3, II: 2.0 ± 3.5, p > 0.05 Reduction in 1-hour pad test (g, mean, SD): I: 18.7 ± 24.8, II: 7.4 ± 6.1, p > 0.05 |
Subjective Change (?reduction) in Incontinence Impact questionnaire (IIQ) score (mean, SD): I: 24.5 ± 10.8, II: 8.5 ± 19.9, p < 0.05; ‘the extent of improvement is far greater for group A (= PFMT) than group B (= PFMT + BF)’ |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Wong 1997b160 (abstract only) Study design/method: 2-arm RCT. Single centre, Hong Kong Duration of study: 4 weeks |
Inclusion criteria: Chinese women in Hong Kong with USI Exclusion criteria: Not reported N randomised: 47 N lost to follow-up: Not reported Type of incontinence: USI Age (years, mean, SD): 48.8 (9.4) (33–73) 1-hour pad test (g, mean, SD): 19.9 (30.1) ‘The two groups were comparable with respect to age, BMI, results of PFMS (pelvic floor muscle strength), PFME (pelvic floor muscle endurance as holding time by perineometry), pad test and CES (Continence Efficacy Scale, measuring the subjective feeling of self-control over incontinence)’ (p. 63) |
I. PFMT, with clinic visits, N = 21 II. PFMT, home based, N = 26 (N randomised) PFMT, with clinic visits: Eight clinic visits over 4 weeks for PFMT and daily PFMT at home PFMT, home-based: Single clinic visit and daily PFMT at home for 4 weeks. Taught the same PFMT programme as the clinic-based group Additional information Cure rates presented as cohort, not by group allocation |
Objective ‘Completely continence’ (less than 2 g on 1-hour pad test): 26/47, data by group allocation not reported Episodes of leakage (7-day diary): Both groups showed ‘significant improvement’ over time but no between-group differences 1-hour pad test: Both groups showed ‘significant improvement’ over time but no between-group differences Surrogate outcomes Maximal pelvic floor muscle strength as pressure (cmH2O): Both groups showed ‘significant improvement’ over time but no between-group differences Pelvic floor muscle strength as holding time (seconds) by perineometer: Both groups showed ‘significant improvement’ over time but no between-group differences |
Quality of life Continence Efficacy Scale (subjective feeling of self-control over incontinence, 10-point visual analogue scale): Both groups showed ‘significant improvement’ over time but no between-group differences |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Wong 2001 169 Study design/method: 2-arm RCT, Single centre, Hong Kong Duration of study: 2 months? |
Inclusion criteria: Chinese women with GSI Exclusion criteria: Second- and third-degree uterine prolapse, previous failure of PFMT, previous continence surgery, neurological pathology, and pad test result of less than 2 g N randomised: 38 N lost to follow-up: Not reported Type of incontinence: USI Age (years, mean, SD): I: 47.6 (8.0), II: 44.4 (4.3), p > 0.05 Episodes of leakage per week (7-day diary, mean, SD): I: 9.1 (14.6), II: 3.5 (5.6), p > 0.05 1-hour pad test (g, mean, SD): I: 59.7 (108.5), II: 12.5 (12.7), p > 0.05 Pelvic floor muscle strength (perineometer, cmH2O): I: 12.9 (10.2), II: 11.4 (7.2), p > 0.05 Pelvic floor muscle endurance (perineometer, seconds): I: 5.8 (8.5), II: 5.0 (7.8), p > 0.05 IIQ-7 (mean): I: 28.57, II: 19.05, p > 0.05 UDI-6 (mean): I: 50.00, II: 35.70 (?possible errors in tables 2 and 3), p > 0.05 Other: Parity |
I. PFMT + BF (vaginal), N = 19 II. PFMT + BF (vaginal and abdominal), N = 19 (N randomised) PFMT + BF (vaginal): Four bi-weekly sessions with physiotherapist lasting about half an hour. Session 1 included education and teaching of VPFMC. From session 2, five sets of exercises in crook-lying, with each set including three fast and two slow VPFMC. BF = PRS9300 with a vaginal probe. Patients watched the screen for BF on their performance shown by electromyography. Patients were asked to minimise any excessive abdominal action PFMT + BF (vaginal and abdominal): As above except that patients had the surface electrode attached to the abdominal wall and a vaginal probe in addition. The rectus abdominis was identified as the prime muscle responsible for contraction of the abdominal wall. Electrodes were placed on both sides of umbilicus, recording the activity of the rectus abdominis. Patients were asked to minimise any excessive abdominal action |
Objective Episodes of leakage per week (7-day diary, mean, SD): I: 4.1 (10.7), II: 1.5 (3.0), p > 0.05 1-hour pad test (with a standardised set of exercises, g, mean, SD): I: 23.0 (69.0), II: 3.9 (3.6), p > 0.05 Note: One patient in group I had a leakage of more than 300 g in the pad test Surrogate outcomes Maximal pelvic floor muscle strength (perineometer, cmH2O): I: 21.7 (14.0), II: 16.8 (8.1) Pelvic floor muscle endurance (perineometer, seconds): I: 6.7 (3.0), II: 6.3 (2.9) |
Quality of life Incontinence Impact Questionnaire Short Form (IIO-7, Chinese translated version, mean): I: 14.29, II: 14.29, p-value for between-group difference in change from baseline = 0.037 Note: Scoring 0 = not at all, 1 = lightly, 2 = moderately, 3 = greatly. The average, which ranged from 0 to 3, was multiplied by 33.3 to transform scores into a scale of 0–100 Urogenital Inventory Short Form (UDI-6, Chinese translated version, mean): I: 16.67, II: 27.78, p-value for between-group difference in change from baseline = 0.044 Note: Scoring as for IIQ-7 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Wyman 1998 183 Study design/method: 3-arm RCT part of a multistudy (see Additional information). Block randomisation stratified by urodynamic diagnosis, baseline incontinence severity and treatment centre. Multicentre (two sites), USA Duration of study: 3 months’ treatment plus follow-up at 3 months after treatment and at mean 3.2 years (no range or SD given) |
Inclusion criteria: Women aged ≥ 45 years; independent community dwelling; at least one involuntary episode of urine loss per week; mentally intact and functionally capable of independent or assisted toileting Exclusion criteria: Uncontrolled metabolic conditions (e.g. diabetes mellitus); urinary tract infection; genitourinary fistula; reversible cause of urinary incontinence; indwelling catheter; residual urinary volume after voiding of greater than 100 ml; inability to perform VPFMC on digital examination N randomised: 204 N withdrawals/dropouts/ losses to follow-up: immediately after treatment phase: I = 0, II = 5, III = 6; 3 months after end of treatment phase: numbers unclear (numbers in tables don’t quite tally with text): I = ?6, II = ?+1, III = ?1; at long-term follow-up, I = 20, II = 17, III = 20 Type of incontinence (USI/MUI/DO): I: 48/11/9, II: 48/11/10, III: 49/8/10 Age (years, mean, SD): I = 60 (10), II = 62 (10), III = 61 (9) Episodes of leakage per week (mean, SD):Urogenital Distress Inventory (UDI) (mean, SD): Women with USI only: I: 47, 124.6 (45.9), II: 45, 114.2 (45.0), III: 44, 120.2 (48.9)Incontinence Impact Questionnaire-Revised (IIQ-R) (mean, SD): Women with USI only: I: 47, 85.7 (67.9), II: 45, 68.2 (55.7), III: 44, 90.4 (72.1)Other: BMI, ethnicity (% white), education (> high school), p = 0.035, employment status (% with income > US$20,000/year), % prior incontinence surgery, parity, % postmenopausal without hormone replacement therapy |
I. BT, N = 68 II. PFMT + BF, N = 69 III. BT + PFMT + BF, N = 67 (N randomised) BT: Trained by registered research nurses. Structured education programme (audiovisual and written). Weekly clinic visits with nurse during first 6 weeks. Patients mailed in their treatment logs weekly in weeks 7–12. Bi-weekly phone calls by nurse in weeks 7–12. Scheduled voiding: starting from baseline frequency of 30- or 60-minute voiding interval, increasing interval between voids by 30 minutes each week, aiming to get a 2.5-hour or 3-hour interval between voiding; the schedule usually remained unchanged in the last 6 weeks. Encouraged to use urge inhibition techniques such as affirmations (self-statements), distraction and relaxation techniques PFMT: Education and contacts with nurse as in the BT group. Correct VPFMC taught by registered nurses. Graded home exercise regimen with audiocassette. PFMT = five fast VPFMC with 3-second hold and 10 sustained VPFMC with 10-second hold, with 10-second relaxation between contractions twice a day. Progressed to a total of 10 fast and 40 sustained VPFMC per day. Also taught VPFMC to inhibit urge, and ‘The Knack’ (VPFMC prior to increases in intra-abdominal pressure, such as cough). BF = four weekly 30-minute sessions of visual and verbal biofeedback BT + PFMT + BF: BT in weeks 1 and 2, PFMT added in week 3. Education and clinic visits as in the BT group Additional information: This is one trial within a multicentre study including several different trials. Any women considered insufficiently ‘estrogenised’ were entered into a different trial. Any women who had a stage III or stage IV prolapse were entered into a different trial. Women diagnosed with USI only could choose between entering into this trial of behavioural therapies or could choose to enter a surgical trial |
Objective 100% reduction (cure) in episodes of leakage per week immediately after treatment (7-day diary, mean, SD): I: 12/68, II: 8/64, III: 19/61 100% reduction (cure) in episodes of leakage per week at 3 months after end of treatment (mean, SD): I: 10/62, II: 13/65, III: 16/60 N reporting no incontinence episode at mean 3.2 years (of those who did not have additional treatment): I: 4/22, II: 1/11, III: 8/16 50–100% reduction (cure or improvement) in episodes of leakage per week immediately after treatment (7-day diary, mean, SD): I: 35/68, II: 36/64, III: 43/61 50–100% reduction (cure or improvement) in episodes of leakage per week at 3 months after end of treatment (mean, SD): I: 28/62, II: 36/65, III: 35/60 N of episodes of leakage per week immediately after treatment (7-day diary, mean, SD): Women with USI only: I: 48, 12.5 (8.3), II: 46, 8.7 (0.0) [sic], III: 42, 7.2 (11.5)N of episodes of leakage per week at 3 months after end of treatment (mean, SD): All women: I: 62, 10.0 (12.0), II: 65, 9.4 (14.0), III: 60, 8.1 (12.4) N of episodes of leakage per week at mean 3.2 years: Of those who did not seek additional treatment, there were no differences among treatment groups in those who became worse in terms of incontinence episodes as compared to baseline (p = 0.80) or at 3 months after end of treatment (p = 0.97) Surrogate outcomes Adherence immediately after treatment (attended all 6 visits, denominator not specified): I = 57%, II: 53%, III = 73%, p = 0.047 Adherence to scheduled voidings (denominator not specified): immediately after treatment: I: 85%, II: NA, III: 81%; at 3 months after end of treatment: I: 44%, II: NA, III: 40%; at mean 3.2 years: 38% (group not specified) Adherence to PFMT (denominator not specified): immediately after treatment – I: NA, II: 84%, III: 78%; at 3 months after end of treatment – I: NA, II: 64%, III: 58%; at mean 3.2 years: 35% (group not specified) Balloon manometry immediately after treatment (mean sustained contraction, mmHg): I: 68, 13.1 (10.6), II: 69, 16.7 (12.9), III: NR, significant improvement from baseline for Group II Balloon manometry immediately after treatment (mean fast contraction, mmHg): I: 68, 18.3 (14.0), II: 69, 22.3 (16.2), III: NR, significant improvement from baseline for Group II The degree of improvement in continence status does not correlate directly with the degree of increase in PFM strength Long term (at mean 3.2 years) N having incontinence surgery: I: 5/48, II: 5/52, III: 8/47 N having SNRI therapy: I: 2/48, II: 10/52, III: 5/47 N having injections: I: 1/48, II: 2/52, III: 1/47 N having PFMT: I: 13/48, II: NR, III: NR N who sought any additional treatment: I: 19/48, II: 29/52, III: 18/47 Adverse events None reported |
Subjective Improvement immediately after treatment (5-point Likert scale, ‘much better’ or ‘somewhat better’): I: 43/66, II: 48/63, III: 55/61 Improvement at 3 months after end of treatment (5-point Likert scale, ‘much better’ or ‘somewhat better’): I: 37/60, II: 45/64, III: 44/58 Quality of life Urogenital Distress Inventory (UDI) immediately after treatment (Shumaker et al. 1994; mean, SD): Women with USI only: I: 47, 99.2 (54.4), II: 45, 81.2 (39.6), III: 44, 63.2 (49.2)UDI at 3 months after end of treatment (mean, SD): All women – I: 60, 91.7 (55.0), II: 64, 85.0 (52.4), III: 58, 72.8 (50.4) Incontinence Impact Questionnaire-Revised (IIQ-R) immediately after treatment (Shumaker et al. 1994; mean, SD): Women with USI only – I: 47, 68.4 (69.7), II: 45, 43.5 (47.4), III: 44, 52.3 (73.4)IIQ-R at 3 months after end of treatment (mean, SD): All women – I: 60, 65.7 (80.2), II: 64, 59.3 (67.7), III: 58, 59.8 (83.9) IIQ-R at mean 3.2 years: Of those who did not seek additional treatment, there were no differences among treatment groups in those who became worse in terms of IIQ-R scores as compared to baseline (p = 0.16) or at 3 months after end of treatment (p = 0.20) Patient satisfaction immediately after treatment (5-point Likert scale, ‘very satisfied’ or ‘slightly satisfied’): I: 48/66, II: 56/63, III: 57/61 Patient satisfaction at 3 months after end of treatment (‘very satisfied’ or ‘slightly satisfied’): I: 47/60, II: 53/64, III: 51/58 Subgroup analysis By type of incontinence (USI vs other) Change in mean incontinence episodes per week over time available: authors note that BT appears to have its greatest efficacy at 6 weeks, compared to PFMT at 11–12 weeks |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Zanetti 2007 161 Study design/method: 2-arm RCT. Single centre, Brazil Duration of study: 3 months |
Inclusion criteria: Women with USI, urinary leakage observed during physical examination; postmenopausal patients needed to have been on topical hormone replacement therapy for no less than 3 months Exclusion criteria: Any kind of disorder affecting muscle or nerve tissues, genital bleeding, pregnancy, urinary tract infection, vulvovaginitis, genital prolapse beyond the hymen, atrophic vaginitis or cardiac pacemakers N randomised: 44 N lost to follow-up: Not reported Type of incontinence: USI Age (years, median): I: 54, II: 56, p = 0.9344 N of micturitions in 24 hours (median): I: 11.0, II: 7.0, p = 0.4939 Note: table 2 provides these data as ‘micturitions per day’ but in the text this is described as ‘urine leakage episodes’ 1-hour pad test (g, median): I: 24.7, II: 20.1, p = 0.8508 Incontinence Quality of Life (median): I: 82, II: 69, p = 0.3717 Other: BMI, ethnicity, % prior incontinence surgery, parity (N of pregnancies), % postmenopausal |
I. PFMT unsupervised, N = 21 II. PFMT, supervised, N = 23 (N randomised) PFMT: All patients underwent individual physiotherapeutic evaluation to assess their pelvic floor strength by means of bidigital examination during perineal contraction without the association of gluteal and/or adductor muscles. PFMT = 10 VPFMC of 5-second hold and 5-second rest, 20 VPFMC of 2-second hold and 2-second rest, 20 VPFMC of 1-second hold and 1-second rest, and five VPFMC of 10-second hold and 10-second rest, followed by five strong contractions together with a cough, with one-minute intervals with each set. Instructed to perform the sequence daily, repeated in the orthostatic, sitting and supine positions. Monthly assessment of pelvic floor muscle strength by the same physiotherapist by means of bidigital examination, and classified from 0 to 5 in accordance with Sampselle et al. (1989). Patients were informed about their evaluation PFMT, with supervision: Individual physiotherapeutic evaluation, PFMT sequence and monthly evaluation as above. Instructed to perform the sequence daily, repeated in the orthostatic, sitting and supine positions. In addition, PFMT was performed under guidance from a physiotherapist, twice a week, for 45 minutes |
Objective Cure (pad test negative, i.e. urine leakage of no more than 2 g on 1-hour pad test): I: 2/21, II: 11/23 N of micturition in 24 hours (7-day diary, median): I: 10.0 , II: 1.0, p = 0.0002 Note: table 2 provides these data as ‘micturitions per day’ but in the text this is described as ‘urine leakage episodes’ 1-hour pad test (g, median): I: 15, II: 3.2, p = 0.0018 |
Quality of life Incontinence Quality of Life (median): I: 79, II: 89, p = 0.0456 Note: Composed of 20 questions. Total scores converted into a percentage. Higher percentage reflects better Quality of life Satisfaction (N of participants who did NOT want any other kind of treatment): I: 5/21, II: 16/23 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Zinner 1998145 (abstract only) Study design/method: 4-arm RCT. Multicentre, USA Duration of study: 6 weeks (+ 2 week placebo lead-in) |
Inclusion criteria: Women with diagnosis of SUI or MUI Exclusion criteria: Not specified N randomised: 286 N lost to follow-up: Unclear Type of incontinence: SUI: 140 (49%), MUI: 146 (51%); data reported separately for women with SUI only |
I. Duloxetine 20 mg q.d. (= once daily), N = 34–35 II. Duloxetine 30 mg q.d., N = 26-29 III. Duloxetine 40 mg (30 mg q.d. for 2 weeks, rising to 40 mg q.d. for 4 weeks), N = 33–38 I. Placebo, N = 34–35a (women with SUI only; N in analysis, depending on outcomes) Additional information: N included in analysis was unclear, as denominators were provided as a range (as above). The lowest limit of each range is used as the denominator for each arm. Data were presented for women with SUI only. Authors reported that ‘In mixed UI patients, no statistically significant changes were observed’ |
Objective Cure or improvement on N of leakage episodes per week (N of ‘responders’ who showed > 70% improvement/reduction; from graph): women with SUI only, I: 15/34, II: 8/26, III: 15/33, IV: 5/34 Cure or improvement on 1-hour stress pad test (N of ‘responders’ who showed > 70% improvement; from graph): Women with SUI only, I: 15/34, II: 7/26, III: 11/33, IV: 6/34 Cure or improvement on 24-hour pad test (N of ‘responders’ who showed > 70% improvement; from graph): Women with SUI only, I: 15/34, II: 6/26, III: 13/33, IV: 5/34 Mean reduction in N of leakage episodes per week (mean, SD): Women with SUI only, I: 34, 13.9 ± 12.8 , II: 26, 8.8 ± 6.1, III: 33, 7.6 ± 11.3 , IV: 34, 5.9 ± 7.6; duloxetine combined (I-III): 95, 10.1 ± 11.0 Mean reduction in 1-hour stress pad test (g, mean, SD): Women with SUI only, I: 34, 13.5 ± 26.2 , II: 26, 5.3 ± 16.3, III: 33, 12.2 ± 21.7, IV: 4.7 ± 15.5; Duloxetine combined (I-III): 95, 10.7 ± 22.2 Mean reduction in 24-hour pad test (g, mean, SD): Women with SUI only, I: 34, 40.8 ± 65.0, II: 26, 17.6 ± 49.2, III: 33, 19.6 ± 41.4, IV: 34, 9.4 ± 43.3; Duloxetine combined (I-III): 95, 26.7 ± 53.6 Adverse events Adverse events: Nausea, but only < 2% withdrew because of this. No serious adverse events Discontinued treatment because of adverse events: Duloxetine (I–III): 8% (8/95?), placebo (IV): 3% (1/34?); figures calculated from percentages given in paper |
Quality of life Mean change in I-QOL score: Women with SUI only, I: 34, 12.0 ± 16.0 , II: 26, 10.0 ± 6.4 , III: 33, 8.2 ± 10.8 , IV: 34, 2.6 ± 8.8; duloxetine combined (I–III): 95, 10.1 ± 12.0 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Studies of pregnant women
| Study | Participant characteristics | Intervention/comparator | Objective outcomes | Subjective outcomes |
|---|---|---|---|---|
|
Dumoulin 2004 199 Study design/method: 3-arm RCT. Single centre, Canada Duration of study: 8 weeks (plus 1-year follow-up) |
Inclusion criteria: Women younger than 45 years, premenopausal, still presenting symptoms of SUI at least once per week 3 months after their last delivery Exclusion criteria: Women who had experienced UI before pregnancy, who had had previous surgery for SUI, a neurological or psychiatric disease, or a major medical condition, or those who were taking medication that could interfere with their evaluation or treatment; current pregnancy, inability to understand French or English; those with moderate to severe urogenital prolapse; postvoid residual urine volume > 50 mg; < 5 g of leakage measured by a 20-minute pad test with fixed bladder volume; involuntary detrusor contraction on cystometry N randomised: 68 N lost to follow-up: I: 1/20, II: 0/23, III: 1/20 Type of incontinence: SUI or USI Age (years, median, 25th and 75th percentile): I: 36.00 (23.25, 39.00), II: 37.00 (34.00, 39.00), III: 35.50 (33.75, 38.25), p = 0.802 Pad test (g, median, 25th and 75th percentile): I: 12.50 (7.00, 26.75), II: 20.00 (6.00, 32.00), III: 13.00 (8.75, 42.25), p = 0.870 Other: BMI, parity |
I. PFMT (multimodal), N = 20 II. PFMT (multimodal) + abdominal muscle training, N = 23 III. Control, N = 19 (N in analysis) PFMT (+ BF + ES): Weekly sessions with an experienced physiotherapist for 8 consecutive weeks. Each session consisted of 15-minute electrical stimulation (biphasic rectangular from), followed by a 25-minute PFMT with BF, which included strengthening and motor relearning exercises, and a home exercise programme to be done 5 days per week. The UROSTIM Unit (Laborie Medical Technologies, Brossard, Quebec, Canada) was used for electrical stimulation and electromyographic BF during the whole supervised treatment PFMT (+ BF + ES) + abdominal training: Weekly sessions with an experienced physiotherapist for 8 consecutive weeks. Each session consisted of the multimodal PFMT described above plus 30 minutes of deep abdominal muscle training consisting of isolation, reeducation, and functional retraining of the transversus abdominis Control subjects: Eight weekly sessions of relaxation massage for the back and extremities performed by a physiotherapist. Women were asked not to exercise their pelvic floor muscles at home during the study but were offered the possibility of receiving a treatment at trial completion Additional information: After 8 weeks, the control group was added to the two treatment groups but it was unclear if this involved any further randomisation. Awaiting author’s reply Data extracted for outcomes at the end of 8-week treatment only |
Objective Objective cure (< 2-g urine loss on pad test): I: 14/20, II: 17/23 , III: 0/19 20-minute pad test with standardised bladder volume (g, median, 25th and 75th percentiles): I: 8.00 (4.00, 25.25), II: 19.00 (6.00, 25.00), III: 0.00 (–3.00, 9.75), p < 0.05 (I vs III), p < 0.05 (II vs III) Adverse events N experiencing adverse events: I: 0/21, II: 0/23, III: 0/20 |
Quality of life Incontinence Impact Questionnaire (total 90, change of score, median, 25th and 75th percentile): I: 13.00 (6.00, 25.00), II: 10.00 (2.00, 16.00), III: 0.50 (–6.50, 5.00); p < 0.05 (I vs III), p < 0.05 (II vs III) Urogenital Stress Inventory (total 57, change of score, median, 25th and 75th percentile): I: 7.00 (3.00, 8.00), II: 4.00 (1.00, 10.00), III: 0.00 (–2.25, 6.50); p < 0.05 (I vs III), p < 0.05 (II vs III) |
|
Wilson 1998 197 Study design/method: 4-arm RCT (women randomised to a control and intervention group, with the intervention group further randomised into three subgroups). Block randomisation, stratified by parity, N of incontinent episodes and type of delivery. Single centre, New Zealand Duration of study: 1 year after delivery and at 24–44 months after delivery |
Inclusion criteria: Women, 3 months postpartum, with urinary incontinence, within catchment area who returned postal questionnaire Exclusion criteria: Not specified N randomised: 230 N lost to follow-up: At 1 year, I: 17/38, II: 20/39, III: 22/36, IV: 26/117; at 24–44 months, I: 12/38, II: 9/39, III: 6/36, IV: 35/117 Withdrawal mainly for reasons of lack of time and inconvenience involved with the hospital-based regimen. Six people in the intervention groups (I, II and III) withdrew due to being ‘continent’ Type of incontinence: Stress 130 (57%), mixed 59 (26%), urge 35 (15%), undefined 6 (3%) Age (years, mean, 95% CI): I, II, III (combined): 29.0 (28.8 to 29.2), IV: 27.8 (27.0 to 28.7) N (%) of women with < 1 incontinent episode per day: I, II, III (combined): 101 (89%), IV: 104 (89%) Pad test (g, mean, 95% CI): I, II, III (combined): 4.0 (1.0 to 7.1), IV: 1.3 (0.9 to 1.7) Other: N (%) vaginal delivery, N (%) parity < 4, N (%) primipara, N (%) performing PFMT in previous month |
I. PFMT + VC, N = 38 II. PFMT, N = 39 III. VC, N = 36 IV. Control, N = 117 (N randomised) All women in the intervention arms (I, II and III) received instruction by one physiotherapist on four occasions in hospital at 3, 4, 6 and 9 months after delivery PFMT: VPFMC taught via a vaginal perineometer. Preparatory exercises were provided to help identify the PF muscles, followed by a basic exercise programme of 8–10 sessions/day including ‘fast’ and ‘slow’ contractions. Aim of 80-100 VPFMCs per day VC: Nine cones in each set, of increasing weight from 20–100 g. Women to retain cones of increasing weights in their vaginas for 15 minutes twice daily PFMT + VC: Combined PFMT and VC programmes as described above Control subjects: Standard postnatal PFMT as taught by physiotherapists in one antenatal group (12 women) class teaching PF anatomy and exercises, with further daily instruction on PFMT in smaller groups (of 6) from the 2nd postnatal day (if still in hospital) or via an audiotape in hospital at weekends |
Objective Home pad test (Wilson 1991) (g, mean, 95% CI): I: 12, 0.5 (0.1, 0.9), II: 18, 2.1 (–0.3, 4.5), III: 20, 0.6 (0.1, 1.1), IV: 82, 2.6 (0.1, 5.1) Long term N having incontinence surgery by 24–44 months: 9 (group not specified) Surrogate outcomes Adherence:Pelvic floor muscle function:Adverse events Discontinued treatment because of adverse events (dislike treatment): I, II, III (combined): 2, IV: 0 |
Subjective Number not cured 1 year after delivery (postal questionnaire): I: 8/14 (57%), II: 9/19 (47%), III: 10/21 (48%), IV: 69/91 (76%) (p = 0.003 between intervention groups (I–III combined) vs control group) Quality of life ‘General feeling’ at 1 year after delivery:No significant differences between the intervention groups (I–III combined) and control group regarding sexual satisfaction at 1 year after delivery Telephone questionnaire at 24–44 months after delivery Note: 168/230 were contacted. Excluding those who had either had another pregnancy or were currently pregnant (N = 72) and those who had undergone surgery (N = 9), there were 89 available for analysis (I–III: 52, IV: 37). In the 89 women, there were no significant differences between the two groups with respect to the prevalence of urinary incontinence (I–III: 58%, IV: 54%) or compliance with PFMC, which appeared to diminish significantly from 1 year postpartum (I–III: 48% at 1 year, 8% at 24–44 months) |
|
Woldringh 2007 198 Study design/method: 2-arm RCT, stratified by centre. Multicentre, the Netherlands Duration of study: The study consisted of five measurements: 22 weeks’ gestation (baseline), week 35 (after three sessions), 8 weeks postpartum (after fourth session), 6 months postpartum and 1 year postpartum |
Inclusion criteria: Women in weeks 17–20 of pregnancy, with ≥ 2 incidences of involuntary urine loss during the last month Exclusion criteria: Already receiving medical treatment for UI, suffering from comorbidity, or had insufficient knowledge of Dutch language N randomised: 264, I: 112, II: 152 N lost to follow-up: I: 47/112, II: 53/152. Withdrew due to new pregnancy/motherhood reasons: I: 9/47, II: 13/53; all other reasons for withdrawal unspecified Type of incontinence (stress/mixed/urge/NONE): i: 62/45/3/2, ii: 99/45/1/7 Age (years, mean, 95% CI): I: 31.9 (31.1 to 32.7) , II: 32.6 (32.0 to 33.3) UI severity score (score range 0-10, mean, 95% CI): I: 5.8 (5.4 to 6.2), II: 5.6 (5.2 to 5.9) Other: BMI, education, employment status, % nulliparous, % doing VPFMCs already at least once per week |
I. PFMT N = 112 II. Control N = 152 (N randomised) PFMT: Four sessions of individual therapy with physiotherapist: three sessions (with 2-week interval) between weeks 23 and 30 of pregnancy and one additional session 6 weeks after delivery. Training manual in accordance with the KNGF (Royal Dutch Society of Physiotherapists). The sessions consisted of information aimed to raise the women’s awareness of PF muscles and to encourage them to exercise these. In view of the advanced pregnancies, the physiotherapists did not perform vaginal palpation but observation and palpation of the perineal body. They also encouraged women to practice self-palpation. Additional 40-page handbook provided with information on incontinence, PF muscles and PFMT exercises Control subjects: Routine care for pregnant women. No further details, but nearly two-thirds of the control group received some instruction on PFMT |
Surrogate outcomes Adherence (following 3 sessions during pregnancy):Note: Denominator not specified |
Subjective Severity of incontinence score (composite measure, range 0–10, 0 = no UI at all) N cured (score 0) at 8 weeks postpartum (2 weeks following last intervention session): I: 31/81 (38%), II: 35/109 (32%). Difference between groups 6%, 95% CI –20 to 8%, p = 0.442 N cured (score 0) at 1 year postpartum: I: 25/60 (42%), II: 35/94 (37%); difference between groups 5%, 95%CI –21 to 11%, p = 0.610 Note: UI severity score was a combined objective and subjective score. The objective assessment was based on bladder diaries (daily during a whole week). The subjective assessment was based on the validated PRAFAB score (five questions relating to the use of protective pads or garments, the amount of UI, frequency of UI, adjustment in daily activity because of UI, and body image). The total score ranged from 0 to 10. Two dichotomous variables were constructed: (1) ‘no UI at all’ (score 0) vs ‘any UI’ (score 1-10), and (2) ‘mild UI’ (score 0–4) vs ‘moderate/severe UI’ (score 5–10). Quality of life Incontinence Impact Questionnaire Impact of incontinence on daily life at 8 weeks postpartum (N of women)Impact of incontinence on daily life at 1 year postpartum (N of women) |
Appendix 11 Characteristics of included studies: interventions and supervisory intensity
| Trial ID | N randomised | Duration (months) of prescribed treatment | N of visits per month in active treatment arm | N of clinic visits in total in active treatment arm | N of arms | Arm 1 | Arm 2 | Arm 3 | Arm 4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aksac 2003120 | 50 | 2 | 4 | 8 | 3 | PFMT | PFMT + BF | NT. No exercises | |
| 2 | Arvonen 2001178 | 40 | 4 | 0.8 | 3 | 2 | PFMT | VC | ||
| 3 | Aukee 2002146 | 30 | 3 | 1.7 | 5 | 2 | PFMT | PFMT + BF. Home BF device | ||
| 4 | Berghmans 1996147 | 40 | 1 | 12 | 12 | 2 | PFMT. Includes ‘The Knack’ | PFMT + BF | ||
| 5 | Bernardes 2000174 | 14 | 0.3 | 30.3 | 10 | 2 | PFMT. Includes micturition control and perineal reinforcement | ES | ||
| 6 | Bidmead 2002121 | 184 | 3.5 | 1.4 | 5 | 4 | PFMT | PFMT. With sham ES | PFMT + ES | NT. Deferred treatment |
| 7 | Blowman 1991189 | 14 | ?1 | 2 | 2 | 2 | PFMT + sham ES | PFMT + ES | ||
| 8 | Bø 1990159 | 57 | 6 | 1 or 5 | 6 or 30 | 2 | PFMT. Monthly clinic visit (six visits) | PFMT. Monthly clinic visits and weekly exercise class (30 visits) | ||
| 9 | Bø 1999115 | 122 | 6 | 1 or 5 | 6 or 30 | 4 | PFMT. Monthly clinic visit and weekly exercise class (30 visits) | ES. Monthly clinic visit (six visits) | VC. Monthly clinic visit (six visits) | NT. Instruction on Continence Guard. No clinic visit |
| 10 | Borello-France 2006165 | 44 | 2.25 to 3 | 4 | 9 to 12 | 2 | PFMT in supine position with clinic-based BF. Also includes stress strategies | PFMT in supine and upright positions with clinic-based BF. Also includes stress strategies | ||
| 11 | Bourcier 1994196 | 102 | 6 | 2 or 3.3 | 12 or 20 | 2 | PFMT + BF + ES. Twice a week over 6 weeks (12 visits). After assessment at 3 months, participants attended a clinic weekly for 2 months (eight visits). Cure measured at 6 months | PFMT + VC. Weekly session for 3 months (12 visits). After assessment at 3 months encouraged to continue the home treatment. Cure measured at 6 months | ||
| 12 | Brubaker 1997130 | 148 | 2 | 0.5 | 1 | 2 | ES | Sham ES | ||
| 13 | Bump 2004136 | 65 | 1 | 2 | SNRI | Placebo | ||||
| 14 | Burns 1993 122 | 123 | 2 | 4 | 8 | 3 | PFMT | PFMT + BF. BF during clinic visits | NT. No contact with study personnel | |
| 15 | Burton 1993173 | 61 | NR | NR | NR | 2 | VC. Use of cone in static position (passive) | VC. Use of cone while doing standardised activities that previously made them incontinent (active) | ||
| 16 | Cammu 1998181 | 60 | 3 | 2 or 4 | 6 or 12 | 2 | PFMT + BF. Clinic-based BF. Also instructed ‘to voluntary contract the pelvic floor prior to a sudden intra-abdominal pressure rise’. Weekly clinic visits (12 visits) | VC. Clinic visits fortnightly (six visits) | ||
| 17 | Cardozo 2004 137 | 109 | 2 | 2 | SNRI | Placebo | ||||
| 18 | Castleden 1984148 | 19 | 1 | NR | NR | 2 | PFMT (crossover) | PFMT + BF (cross over). Home BF device | ||
| 19 | Castro-Diaz 2007138 | 516 | 2 | 4 | SNRI. 2 x 40 mg daily | SNRI. 1 × 40 mg daily, then 2 × 40 mg daily | SNRI. 2 × 20 mg daily, then 2 × 40 mg daily | Placebo | ||
| 20 | Delneri 2000186 | 20 | 0.5 or 1 | 24 for ES | 12 for ES | 2 | ES. Twelve consecutive sessions (excluding Saturday and Sunday) | VC. 4 weeks. N of clinic visits not reported | ||
| 21 | Dmochowski 2003139 | 683 | 3 | 2 | SNRI | Placebo | ||||
| 22 | Dumoulin 2004 199 | 64 | 2 | 4 | 8 | 3 | PFMT (as part of multimodal rehabilitation including BF and ES). Childbearing women only | PFMT (as part of multimodal rehabilitation including BF and ES) + abdominal muscle training. Childbearing women only | NT. Relaxation massage for the back and extremities by physiotherapist. Childbearing women only | |
| 23 | Edwards 2000170 | 20 | 3 | NR | NR | 2 | PFMT + BF | PFMT + ES | ||
| 24 | Fantl 1991135 | 131 | 1.5 | 4 | 6 | 2 | BT | NT. No contacts | ||
| 25 | Ferguson 1990149 | 20 | 1.5 | 2 | 3 | 2 | PFMT. With audiotape | PFMT + BF. With audiotape. Intravaginal resistance device (IVRD) coded as BF | ||
| 26 | Gallo 1997162 | 86 | 1 to 1.5 | 0.67 to 1 | 1 | 2 | PFMT | PFMT with audiocassette | ||
| 27 | Ghoniem 200557 | 201 | 3 | 1 | 3 | 4 | PFMT. With placebo SNRI. Includes ‘skill training’ (‘The Knack’) | SNRI. With imitation PFMT | PFMT + SNRI | NT. Imitation PFMT + placebo SNRI. No instruction for ‘skill training’ |
| 28 | Glavind 1996 150 | 40 | 3 | 0.6 to 2 | 2 to 6 | 2 | PFMT. 2–3 clinic visits for 4 weeks. Cure measured at 3 months | PFMT + BF. PFMT as in arm 1 with additional four clinic visits for visual BF. Cure measured at 3 months | ||
| 29 | Goode 2003123 | 200 | 2 | 2 | 4 | 3 | PFMT. ‘Behavioural training’ including ‘stress strategies’ and ‘urge strategies’ | PFMT + ES. PFMT as in arm 1 | NT. Self-administered behavioural training using a booklet | |
| 30 | Hahn 1991175 | 20 | 6 | 1.5 | 9 | 2 | PFMT. Includes muscle contractions against resistance and during different provocative situations like coughing | ES | ||
| 31 | Haig 1995190 | 58 | 3 | 6.7 | 20 | 3 | PFMT. Five visits with physiotherapist in the first month, 12 in the second month and three in the third month | PFMT + sham ES. Sham ES in the second month | PFMT + ES. ES in the second month | |
| 32 | Haken 1991179 | 64 | 2.5 | 1.2 | 3 | 2 | PFMT | VC | ||
| 33 | Hay-Smith 2003164 | 128 | 5 | 0.8 | 4 | 2 | Strength and motor learning PFMT | Motor learning PFMT alone | ||
| 34 | Henalla 1989124 | 76 | 3 | 3.3 to 4 | 10 to 12 | 4 | PFMT. Weekly clinic visits (for 12 weeks?) | ES. 10 sessions, once per week for 10 weeks | NT. Received no treatment | Estrogen cream for 12 weeks – not assessed in review |
| 35 | Henalla 1990125 | 54 | 1.5 | NR | NR | 3 | PFMT | NT | Estrogen cream – not assessed in review | |
| 36 | Hofbauer 1990 126 | 43 | 1.5 | 8 to 12 | 12 to 18 | 4 | PFMT. Clinic visits twice a week (12 visits) | ES. At clinic, three times per week for 6 weeks (18 visits) | PFMT + ES | NT. Sham ES |
| 37 | Jeyaseelan 2000131 | 27 | 2 | NR | NR | 2 | ES | Sham ES | ||
| 38 | Johnson 2001167 | 37 | 1.5 | NR | NR | 2 | PFMT + BF. PFMT using maximal voluntary contraction | PFMT + BF. PFMT using submaximal voluntary contraction | ||
| 39 | Karagkounis 2007194 | 197 | 0.5 | NR | NR | 2 | PFMT + SNRI | Surgery. The TVT ob system under local or regional anaesthesia followed by 2-day hospitalisation | ||
| 40 | Kim 2007118 | 70 | 3 | 8 | 24 | 2 | PFMT. Includes fitness exercise | NT. Instructed to lead a normal life and to refrain from special exercises | ||
| 41 | Kinchen 2005140 | 451 | 3 | 2 | SNRI | Placebo | ||||
| 42 | Klarskov 1986184 | 50 | 4 | 1.3 | 5 | 2 | PFMT. Median 5 (range 2–13) group lessons. Also taught ‘correct lifting technique’ | Surgery. Colposuspension, vaginal repair or both. N of clinic visits unclear | ||
| 43 | Klingler 1995151 | 41 | 3 | 4 | 12 | 2 | PFMT | PFMT + BF. BF from the ‘Endotrainer’ device | ||
| 44 | Knight 1998172 | 70 | 6 | 2.33 | 14 | 3 | PFMT + BF. BF in clinic and at home | PFMT + BF + ES. ES = maximal intensity at clinic | PFMT+BF + ES. ES = overnight at low intensity at home | |
| 45 | Konstantinidou 2007116 | 30 | 3 | 1 or 5 | 3 or 15 | 2 | PFMT. Monthly hospital visit (three visits) | PFMT. Monthly hospital visit plus weekly group sessions (15 visits) | ||
| 46 | Lagro-Janssen 1991127 | 66 | 3 | 0.33 | 1 | 2 | PFMT. With advice on continent pads | NT. Advice on continent pads | ||
| 47 | Laycock 1988176 | 36 | ?1–2 | ?4 or more | ?6–11 | 2 | PFMT. Weekly clinic visits for 6–8 weeks | ES. Average 11 (range 7–13) sessions, 2–3 times a week for 4–6 weeks | ||
| 48 | Laycock 2001152 | 101 | 3 | 2 | 6 | 3 | PFMT | PFMT + BF. Home BF device | VC | |
| 49 | Laycock Trial 1 1993132 | 46 | ?2 | ?3–5 | 6–10 | 2 | PFMT + BF + VC. Weekly clinic visit for 2 weeks, then every 10 days for average 6 weeks (total six visits?) | ES. Average 10 sessions. Length of treatment period unclear | ||
| 50 | Laycock Trial 2 1993132 | 30 | ?2–3 | ?3.3–5 | 10 | 2 | ES. Average 10 sessions | NT. Sham ES | ||
| 51 | Luber 1997133 | 54 | 3 | 2 | 6 | 2 | ES | Sham ES | ||
| 52 | Mah 2006141 | 121 | 2 | 2 | SNRI | Placebo | ||||
| 53 | Manning 2005142 | 617 | 1.5 | 2 | SNRI | Placebo | ||||
| 54 | Mayne 1988168 | 34 | 4 | 1.8 | 7 | 2 | PFMT, monitored using perineometer in clinic | PFMT, monitored using urethral electrical conductance in clinic | ||
| 55 | Millard 2004143 | 458 | 3 | 2 | SNRI | Placebo | ||||
| 56 | Miller 1998107 | 27 | 0.25 | 4 | 1 | 2 | PFMT (‘The Knack’) | NT | ||
| 57 | Mørkved 2002 153 | 103 | 6 | 2.7 | 16 | 2 | PFMT | PFMT + BF. BF both in clinic and at home | ||
| 58 | Norton 2002144 | 553 | 3 | 4 | SNRI. 80 mg | SNRI. 40 mg | SNRI. 20 mg | Placebo | ||
| 59 | Nygaard 1996163 | 71 | 3 | 1 | 3 | 2 | PFMT. Three telephone calls in addition to clinic visits | PFMT with audiotape. Three telephone calls in addition to clinic visits | ||
| 60 | Oláh 1990187 | 69 | 1 | 4 or 12 | 4 or 12 | 2 | ES. Three sessions per week (12 visits) | VC. Weekly clinic visit (four visits) | ||
| 61 | Pages 2001154 | 51 | 3 | 7 | 21 | 2 | PFMT. Includes fitness exercise. Group sessions during first 4 weeks. Patients then continued PFMT at home in weeks 5–12 | PFMT + BF. Individual BF therapy at clinic during first 4 weeks. Patients then continued PFMT without BF at home in weeks 5–12 | ||
| 62 | Peattie 1988180 | 44 | 1 | 1 or 3 | 1 or 3 | 2 | PFMT. Three clinic visits | VC. Weekly telephone calls after initial clinic visit | ||
| 63 | Pieber 1995192 | 46 | 3 | ?1.3 | ?4 | 2 | PFMT. Includes ‘The Knack’ | PFMT + VC. Includes ‘The Knack’ | ||
| 64 | Pohl 2004171 | 70 | 3 | NR | NR | 2 | PFMT + BF | PFMT + ES | ||
| 65 | Ramsay 1990128 | 44 | 3 | NR | NR | 2 | PFMT | NT. Placebo PFMT | ||
| 66 | Sand 1995134 | 52 | 3 | 1.7 | 5 | 2 | ES | Sham ES | ||
| 67 | Savage 2005166 | 11 | 3 | 2 | 6 | 2 | PFMT. Includes ‘The Knack’ | Modified pilates | ||
| 68 | Seo 2004195 | 120 | 1.5 | 4 or 8 | 6 or 12 | 2 | PFMT + BF + ES. Clinic visits twice a week (12 visits) | VC. Weekly clinic visits (six visits) | ||
| 69 | Shepherd 1983155 | 22 | 4.5 | 1.3 | 6 | 2 | PFMT. Weekly clinic visits for ?6 weeks and home exercise programme. Cure rate (and other outcomes) measured at 3 months after treatment was completed (i.e. at 18 weeks) | PFMT + BF. Visual BF from ‘Exerciser’. Weekly clinic visit for ?6 weeks. Cure rate (and other outcomes) measured at 3 months after treatment was completed (i.e. at 18 weeks) | ||
| 70 | Sherburn 2007182 | 84 | 5 | 4 | 20 | 2 | PFMT | BT | ||
| 71 | Smith 1996177 | 18 | 4 | ?0.8 | ?3 | 2 | PFMT | ES | ||
| 72 | Swithinbank 2005119 | 84 | 1 | NR | NR | 2 | Caffeine free, increase fluids then decrease (crossover) | Caffeine free, decrease fluids then increase (crossover) | ||
| 73 | Tapp 1987191 | 29 | 3 | 4 or 5.3 | 12 or 16 | 2 | PFMT. Weekly visits for 12 weeks (12 visits) | PFMT + ES. PFMT as arm 1 for 12 weeks with ES twice a week for 1 month (16 visits) | ||
| 74 | Tapp 1989185 | 81 | 3 | 4.67 | 14 | 3 | PFMT. 14 visits over 3 months | PFMT + ES. 14 visits over 3 months | Surgery. Burch colposuspension. N of clinic visits unclear | |
| 75 | Taylor 1986156 | 13 | 2.25 | 4 | 9 | 4 | PFMT. With advice on ‘strategies to reduce frequency’ | PFMT + BF. Weekly BF in clinic. With advice on ‘strategies to reduce frequency’ | PFMT + BF. Weekly BF in clinic + BF device for home use. With advice on ‘strategies to reduce frequency’ | PFMT + BF. Weekly BF in clinic + home IVRD (vaginal sensors removed from the home BF machine to be used as a resistive device). With advice on ‘strategies to reduce frequency’ |
| 76 | Terry 1996193 | 60 | 1.5 | 8 | 12 | 2 | PFMT + ES. 12 visits over 6 weeks | VC. N of visits unclear | ||
| 77 | van Kerrebroeck 2004117 | 494 | 3 | 2 | SNRI. 80 mg | Placebo | ||||
| 78 | Williams 2006 129 | 238 | 3 | 1.33 | 4 | 3 | PFMT. Four clinic visits over 12 weeks | VC. Four clinic visits over 12 weeks | NT. A leaflet on the location of pelvic floor muscles and three step to exercising these muscles. Encouraged to record in an exercise diary. Four clinic visits over 12 weeks | |
| 79 | Wilson 1987157 | 60 | 1.5 | 0.7 or 8 | 1 or 12 | 4 | PFMT. One clinic visit | PFMT + BF. BF during clinic visits twice a week (12 visits) | PFMT + BF + ES. BF during clinic visits twice a week (12 visits). ES = faradism | PFMT + BF + ES. BF during clinic visits twice a week (12 visits). ES = interferential therapy |
| 80 | Wilson 1998 197 | 230 | 12? | 0.33 | 4 | 4 | PFMT, VC or both (further randomised). Childbearing women only | NT. Standard postnatal care. Childbearing women only | ||
| 81 | Wise 1993188 | 62 | 3 | 1 | 3 | 3 | ES | VC | PFMT + VC | |
| 82 | Woldringh 2007198 | 264 | 5? | 0.8? | 4 | 2 | PFMT. Childbearing women only | NT. Routine care. Childbearing women only | ||
| 83 | Wong 1997a158 | 17 | 2 | 4 | 8 | 2 | PFMT | PFMT + BF | ||
| 84 | Wong 1997b160 | 47 | 1 | 1 or 8 | 1 or 8 | 2 | PFMT. One clinic visit | PFMT. Eight clinic visits | ||
| 85 | Wong 2001169 | 38 | 2? | 2 | 4 | 2 | PFMT + BF (vaginal) | PFMT + BF (vaginal and abdominal) | ||
| 86 | Wyman 1998183 | 204 | 3 | 2 | 6 | 3 | PFMT + BF. Includes urge inhibition techniques and ‘The Knack’. Four BF sessions in clinic | BT. Includes urge-inhibition techniques. Clinic visits and contacts as in arm 1 | PFMT + BF + BT. BT in weeks 1 and 2, PFMT added in week 3. 4 BF sessions in clinic | |
| 87 | Zanetti 2007161 | 46 | 3 | 1 or 9 | 3 or 27 | 2 | PFMT. Monthly clinic visits (three visits) | PFMT. Monthly clinic visits and additional visits twice a week (27 visits) | ||
| 88 | Zinner 1998 145 | 127 | 1.5 | 4 | SNRI. 40 mg | SNRI. 30 mg | SNRI. 20 mg | Placebo |
Appendix 12 Characteristics of interventions (pelvic floor muscle training with or without biofeedback)
| Study | Duration (months) | N of clinic visits | Supervisory intensitya | Who provided supervision | N of prescribed muscle contractions at home per day | Description |
|---|---|---|---|---|---|---|
| Aksac 2003120 | 2 | 8 | Intensive | Therapist | 30 | PFMT |
| 8 | Intensive | Therapist | 17 | PFMT + BF | ||
| Arvonen 2001178 | 4 | 3 | Basic | Physiotherapist and nurse | 56 | PFMT |
| Aukee 2002146 | 3 | 5 | Basic | Physiotherapist | NR | PFMT |
| 5 | Basic | Physiotherapist | NR | PFMT + BF | ||
| Berghmans 1996147 | 1 | 12 | Intensive | Physiotherapist | NR | PFMT |
| 12 | Intensive | Physiotherapist | NR | PFMT + BF | ||
| Bernardes 2000174 | 0.3 | 10 | Intensive | Physiotherapist | 120 | PFMT |
| Bidmead 2002121 | 3.5 | 5 | Basic | Physiotherapist | NR | PFMT ± active or sham ES |
| Blowman 1991189 | 1? | 2 | Basic | Obstetric physiotherapist | 80–120 | PFMT. With sham ES |
| Bø 1990159 | 6 | 6 | Basic | Physiotherapist | 24–36 | PFMT |
| 30 | Intensive | Physiotherapist, and weekly special PFMT class by ‘instructor’ | 24–36 | PFMT with additional sessions | ||
| Bø 1999115 | 6 | 30 | Intensive | Physiotherapist | 96–180 | PFMT |
| Borello-France 2006165 | 2.25–3 | 9–12 | Intensive | Physiotherapist | 180 | PFMT in supine position with BF |
| 9–12 | Intensive | Physiotherapist | 180 | PFMT in supine and upright positions with BF | ||
| Bourcier 1994196 | 6 | 20 | Intensive | NR | NR | PFMT + BF + ES. Stated as ‘ES + BF’ but presumably BF of VPFMC |
| 12 | Basic | NR | 60 | PFMT + VC | ||
| Burns 1993122 | 2 | 8 | Intensive | Nurse trained in BF technique | 200 | PFMT |
| 8 | Intensive | Nurse trained in BF technique | 200 | PFMT + BF | ||
| Cammu 1998181 | 3 | 12 | Intensive | Physiotherapist | 20 | PFMT + BF |
| Castleden 1984148 | 1 | NR | NR | Physiotherapist | 4–5 per hour | PFMT |
| NR | NR | Physiotherapist | 4–5 per hour | PFMT + BF | ||
| Dumoulin 2004199 | 8 | 8 | Intensive | Physiotherapist | NR | PFMT (as part of multimodal rehabilitation including BF and ES) ± abdominal muscle training. Childbearing women only |
| Edwards 2000170 | 3 | NR | NR | NR | NR | PFMT + BF or PFMT + ES |
| Ferguson 1990149 | 1.5 | 3 | Basic | NR | 45? | PFMT |
| 3 | Basic | NR | 45? | PFMT + BF (intravaginal resistance device) | ||
| Gallo 1997162 | 1-1.5 | 1 | Basic | Nurse | NR | PFMT |
| 1 | Basic | Nurse | NR | PFMT with audiotape | ||
| Ghoniem 2005 57 | 3 | 3 | Basic | Qualified instructor | 29 | PFMT + active or placebo SNRI |
| Glavind 1996150 | 3 | 2 | Basic | Physiotherapist | NR | PFMT |
| 6 | Basic | Physiotherapist | NR | PFMT + BF | ||
| Goode 2003123 | 2 | 4 | Basic | Nurse practitioner specifically trained by the behavioural psychologist (KL Burgio) and physician (PS Goode) | 45 | PFMT ± ES |
| Hahn 1991175 | 6 | 9 | Basic | Physiotherapist | 42–96? | PFMT |
| Haig 1995190 | 3 | 20 | Intensive | Physiotherapist | NR | PFMT ± active or sham ES |
| Haken 1991179 | 2.5 | 3 | Basic | Continence advisor | 50 | PFMT |
| Hay-Smith 2003164 | 5 | 4 | Basic | Physiotherapist | 36 | Strength and motor relearning PFMT |
| 4 | Basic | Physiotherapist | NR | Motor relearning PFMT | ||
| Henalla 1989124 | 3 | 12 | Intensive | Physiotherapist | 5 per hour | PFMT |
| Henalla 1990125 | 1.5 | NR | NR | NR | NR | PFMT |
| Hofbauer 1990126 | 1.5 | 12 | Intensive | Therapist | NR | PFMT ± ES |
| Johnson 2001167 | 1.5 | NR | NR | Investigator (nurse?) | NR (10 minutes) | PFMT (near maximal contraction) + BF |
| NR | NR | Investigator (nurse?) | NR (15 minutes) | PFMT (sub-maximal contraction) + BF | ||
| Karagkounis 2007194 | 0.5 | NR | NR | NR | NR | PFMT + SNRI |
| Kim 2007118 | 3 | 24 | Intensive | NR | NR | PFMT |
| Klarskov 1986184 | 4 | 5 | Basic | Physiotherapist | NR | PFMT |
| Klingler 1995151 | 3 | 12 | Intensive | Physiotherapist | NR | PFMT |
| 12 | Intensive | Physiotherapist | NR | PFMT + BF (Endotrainer device) | ||
| Knight 1998172 | 6 | 14 | Intensive | Physiotherapist | 120 | PFMT + BF ± ES |
| Konstantinidou 2007116 | 3 | 3 | Basic | NR | NR | PFMT |
| 15 | Intensive | NR | NR | PFMT + additional sessions | ||
| Lagro-Janssen 1991127 | 3 | 1 | Basic | GP | 50–100 | PFMT |
| Laycock 1988176 | 1-2? | 6-8? | Intensive | NR | NR | PFMT |
| Laycock Trial 1 1993132 | 2? | 6? | Intensive | Physiotherapist | 5 per hour | PFMT + BF + VC |
| Laycock 2001152 | 3 | 6 | Basic | NR | NR | PFMT |
| 6 | Basic | NR | NR | PFMT + BF | ||
| Mayne 1998168 | 4 | 7 | Basic | NR | NR | PFMT + perineometer |
| 7 | Basic | NR | NR | PFMT + urethral electrical conductance | ||
| Miller 1998107 | 0.25 | 1 | Intensive | NR | NR | PFMT (‘The Knack’) |
| Mørkved 2002153 | 6 | 16 | Intensive | Physiotherapist | 30 | PFMT |
| 16 | Intensive | Physiotherapist | 30 | PFMT + BF | ||
| Nygaard 1996163 | 3 | 3 | Basic | NR | NR (10 minutes) | PFMT |
| 3 | Basic | NR | NR (270 minutes) | PFMT with audiotape | ||
| Pages 2001154 | 3 | 21 | Intensive | Physiotherapist | More than 100 for 4 weeks; 29 for further 2 months | PFMT (group therapy) |
| 21 | Intensive | Physiotherapist | 100 for 4 weeks; 29 for further 2 months | PFMT + BF (individual therapy) | ||
| Peattie 1988180 | 1 | 3 | Intensive | Physiotherapist | 50 | PFMT |
| Pieber 1995192 | 3 | 4? | Basic | Physiotherapist | 100 | PFMT ± VC |
| Pohl 2004171 | 3 | NR | NR | NR | NR | PFMT + BF or PFMT + ES |
| Ramsay 1990128 | 3 | NR | NR | NR | 4 per hour | PFMT |
| Savage 2005166 | 3 | 6 | Basic | Physiotherapist | NR | PFMT |
| Seo 2004195 | 1.5 | 12 | Intensive | NR | NR | PFMT + BF + ES. Stated as ES + BF but presumably BF of VPFMC |
| Shepherd 1983155 | 4.5? | 6 | Basic | Physiotherapist | NR | PFMT |
| 6 | Basic | Physiotherapist | NR | PFMT + BF (intravaginal ‘Exerciser’ connected to visual BF | ||
| Sherburn 2007182 | 5 | 20 | Intensive | NR | NR | PFMT |
| Smith 1996177 | 4 | 3? | Basic | NR | 60 | PFMT |
| Tapp 1987191 | 3 | 12 | Intensive | Continence advisor | 4 per hour | PFMT ± ES |
| Tapp 1989185 | 3 | 14 | Intensive | Continence advisor trained to teach PFMT | NR | PFMT ± ES |
| Taylor 1986156 | 2.25 | 9 | Intensive | Researcher (nurse?) | 100 | PFMT |
| 9 | Intensive | Researcher (nurse?) | 100 | PFMT + BF. BF at clinic | ||
| 9 | Intensive | Researcher (nurse?) | 100 | PFMT + BF. BF at clinic and at home | ||
| 9 | Intensive | Researcher (nurse?) | 100 | PFMT + BF. BF at clinic and the BF machine at home without vaginal sensors to be used as a resistive device | ||
| Terry 1996193 | 1.5 | 12 | Intensive | NR | NR | PFMT + ES |
| Wilson 1987157 | 1.5 | 1 | Basic | Hospital physiotherapy department | NR | PFMT |
| 12 | Intensive | Hospital physiotherapy department | NR | PFMT + BF | ||
| Wilson 1998197 | 12 | 4 | Basic | Physiotherapist | 80-100 | PFMT. Childbearing women only |
| Williams 2006129 | 3 | 4 | Basic | Specially trained nurse | NR | PFMT |
| Wise 1993188 | 3 | 3 | Basic | NR | 100 | PFMT + VC |
| Woldringh 2007198 | 5? | 4 | Basic | Physiotherapist | NR | PFMT. Childbearing women only |
| Wong 1997a158 | 2 | 8 | Intensive | NR | NR | PFMT |
| 8 | Intensive | NR | NR | PFMT + BF | ||
| Wong 1997b160 | 1 | 1 | Basic | NR | NR | PFMT |
| 8 | Intensive | NR | NR | PFMT with additional sessions | ||
| Wong 2001169 | 2? | 4 | Basic | Physiotherapist | NT | PFMT + BF (vaginal) |
| 4 | Basic | Physiotherapist | NT | PFMT + BF (vaginal and abdominal) | ||
| Wyman 1998183 | 3 | 6 | Basic | Trained registered nurse | 50 | PFMT + BF ± BT |
| Zanetti 2007161 | 3 | 3 | Basic | Physiotherapist | 180 | PFMT |
| 27 | Intensive | Physiotherapist | 180 | PFMT + additional sessions |
Appendix 13 Characteristics of interventions (electrical stimulation)
| Study | Duration (month) | Description |
|---|---|---|
| Bernardes 2000174 | 0.3 | Dualpex (Quark Medical products) with perineal intrauterine electrode. 10 consecutive clinic sessions |
| Bidmead 2002121 | 3.5 | Uromax stimulator with a periform intravaginal electrode at home |
| Blowman 1991189 | 1? | Neurotrophic stimulation, a method of neuromuscular electrical stimulation. Home stimulator, once per day |
| Bø 1999 115 | 6 | Maximum intermittent vaginal stimulation. Once per day |
| Bourcier 1994196 | 6 | Short-term maximal functional ES and EMG/pressure BF. 12 sessions |
| Brubaker 1997130 | 2 | Transvaginal electrical stimulation. Home treatment, twice daily |
| Delneri 2000186 | 0.5 | Functional electrical stimulation.12 consecutive sessions on weekdays (i.e. not weekends) |
| Edwards 2000170 | 3 | No details provided |
| Goode 2003123 | 2 | Home unit with biphasic pulses. Simultaneous with each muscle contraction induced by electrical stimulation, patients performed VPFMC (voluntary pelvic floor muscle contraction).4 clinic sessions |
| Hahn 1991175 | 6 | Interferential therapy. Home device, once daily |
| Haig 1995190 | 3 | Interferential therapy during clinic visits from second month |
| Henalla 1989124 | 3 | Interferential therapy. 10 clinic sessions |
| Hofbauer 1990126 | 1.5 | Faradism with vaginal and perineal (active) and lumbar (inactive) electrode. 3 times per week |
| Jeyaseelan 2000131 | 2 | Patterned neuromuscular electrical stimulation. Portable stimulator, once daily |
| Knight 1998172 | 6 | Overnight at low intensity at home |
| 16 × 30-minute session of maximal electrical stimulation. VPFMC performed with the stimulation | ||
| Laycock 1988176 | 1–2? | Interferential therapy, 2–3 times a week |
| Laycock Trial 1 1993132 | 2? | Interferential therapy. 10 clinic sessions |
| Laycock Trial 2 1993132 | 2–3? | Interferential therapy. 10 clinic sessions |
| Luber 1997133 | 3 | Home device, twice daily |
| Oláh 1990187 | 1 | Interferential therapy at clinic, 3 times per week |
| Pohl 2004171 | 3 | No details provided |
| Sand 1995134 | 3 | Innova (Empi Inc.) pelvic floor stimulator. 7 office visits |
| Seo 2004195 | 1.5 | Clinic sessions, twice a week, to perform alternately functional ES and BF |
| Smith 1996177 | 4 | Intravaginal neuromuscular stimulation. Twice a day at home |
| Tapp 1987191 | 3 | Faradic stimulation, twice a week |
| Tapp 1989185 | 3 | Faradism. No further details |
| Terry 1996193 | 1.5 | Interferential therapy. No further details |
| Wilson 1987157 | 1.5 | Faradism. 12 clinic sessions |
| Interferential therapy. 12 clinic sessions | ||
| Wise 1993188 | 3 | Maximal vaginal electrical stimulation. Home treatment, once daily |
Appendix 14 Characteristics of interventions (vaginal cones)
| Study | Duration (month) | Description |
|---|---|---|
| Arvonen 2001178 | 4 | Vaginal balls. The pelvic floor muscle contractions were performed by retaining the ball during movement (e.g. walking, house-keeping) |
| Bø 1999115 | 6 | Mabella cones (cylindrical), once per day |
| Bourcier 1994196 | 6 | Unspecified cones, twice daily |
| Burton 1993173 | NR | Unspecified cones, twice daily, in a static position |
| Unspecified cones, twice daily, while doing standardised activities that previously made them incontinent | ||
| Cammu 1998181 | 3 | Femina cones (conical), twice a day. Note: After the first clinic visit 14/30 women in the cones group withdrew and received PFMT but stayed in the VC group |
| Delneri 2000186 | 1 | Femcon cones (conical), once per day |
| Haken 1991179 | 2.5 | Femina cones (conical)? Twice daily |
| Laycock Trial 1 1993132 | 2? | Unspecified cones, twice daily |
| Laycock 2001152 | 3 | Aquaflex cones, once daily |
| Oláh 1990187 | 1 | Femina cones (conical), twice daily |
| Peattie 1988180 | 1 | Femina cones (conical), twice daily |
| Pieber 1995192 | 3 | Conical weights, once per day |
| Seo 2004195 | 1.5 | ‘New’ cone, which has a dumbbell shape. Use cone in place while contracting the pelvic floor muscles, once per day |
| Terry 1996193 | 1.5 | Enhance cones (cylindrical). No further details |
| Williams 2006129 | 3 | Femina cones, 2–3 times a day |
| Wilson 1998197 | 12 | Unspecified cones, twice daily. Pregnant women only |
| Wise 1993188 | 12 | Unspecified cones, twice daily |
Appendix 15 Characteristics of interventions (serotonin–noradrenaline reuptake inhibitor)
| Study | Duration (month) | Description |
|---|---|---|
| Bump 2004136 | 1 | Duloxetine 40 mg twice daily |
| Cardozo 2004137 | 2 | Duloxetine 40 mg twice daily for 4 weeks then escalating to 60 mg twice daily for another 4 weeks |
| Castro-Diaz 2007138 | 2 | Duloxetine 40 mg twice daily |
| Duloxetine 40 mg once daily for 2 weeks, escalating to 40 mg twice daily | ||
| Duloxetine 20 mg twice daily for 2 weeks, escalating to 40 mg twice daily | ||
| Dmochowski 2003139 | 3 | Duloxetine 40 mg twice daily |
| Ghoniem 200557 | 3 | Duloxetine 80 mg |
| Karagkounis 2007194 | NR | Duloxetine 40 mg twice daily |
| Kinchen 2005140 | 3 | Duloxetine 40 mg twice daily. Note: This was a ‘naturalistic’ study in which participants could, at any point after randomisation, choose to remain on duloxetine as randomised, reduce SNRI doses, add other treatments to duloxetine, or suspend duloxetine and receive other treatments |
| Mah 2006141 | 2 | Duloxetine 40 mg twice daily |
| Manning 2005142 | 1.5 | Duloxetine. Unspecified dose |
| Millard 2004143 | 3 | Duloxetine 40 mg twice daily |
| Norton 2002144 | 3 | Duloxetine 20 mg once daily |
| Duloxetine 20 mg twice daily | ||
| Duloxetine 40 mg twice daily | ||
| van Kerrebroeck 2004117 | 3 | Duloxetine 40 mg twice daily |
| Zinner 1998145 | 1.5 | Duloxetine 20 mg once daily |
| Duloxetine 30 mg once daily | ||
| Duloxetine 30 mg once daily for 2 weeks, escalating to 40 mg once daily |
Appendix 16 Cure/improvement definitions
| Author | Subjective cure | Subjective improvement | Quantified cure | Quantified improvement |
|---|---|---|---|---|
| Aksac 2003120 | ≤1 g on 1-hour pad test | Cure (≤ 1 g on 1-hour pad test) or improved (≥ 50% reduction in pad weight) | ||
| Arvonen 2001178 | ‘Good (fully recovered)’ on subjective self-rated experience of improvement (4-point scale) | ‘Good (fully recovered)’ or ‘Improved’ on subjective self-rated experience of improvement (4-point scale) | No leakage, i.e. 0 g on pad test (short provocation test with a standard 300 ml in bladder) | |
| Berghmans 1996147 | Cure based on 48-hour pad test | Cure or improvement based on 48-hour pad test | ||
| Bernardes 2000174 | Patient perception (no symptom or no loss of urine, vs light or moderate loss) | |||
| Blowman 1991189 | Leakage episodes reduced to zero (no accidents per week) | |||
| Bø 1990159 | ‘Continent’ vs ‘almost continent’, ‘improved’, ‘unchanged’ and ‘worse’ after supervised treatment | ‘Continent’, ‘almost continent’, ‘improved’ vs ‘unchanged’, ‘worse’ | ||
| Bø 1990159 | At 15 years’ follow-up, Severity Index (Sandvik et al. 1993; 200013): Dry vs slight/moderate/ severe/very severe | |||
| Bø 1999115 | ‘Continent’ vs ‘almost continent’, ‘improved’, ‘unchanged’ and ‘worse’ | ‘Continent’, ‘almost continent’, ‘improved’ vs ‘unchanged’, ‘worse’ | ≤ 2-g leakage on pad test with standardised bladder volume | |
| Bourcier 1994196 | ‘Continent after treatment’ at 6 months | |||
| Brubaker 1997130 | Adequate subjective improvement | Cure (GSI/MUI patients only) = cured of (urodynamic) stress urinary incontinence but by definition may still have DO (urge UI)? | ||
| Burns 1993122 | 100% reduction in weekly number of urine losses recorded on (24 hour?) diary | 50–100% reduction in weekly number of urine losses recorded on (24 hour?) diary; 50% defined by author as minimal level of reduction | ||
| Burton 1993173 | No leakage after coughing (videocystourethrography) | |||
| Cammu 1998181 | Cured or improved to a significant degree. 14/30 in VC group withdrew and received PFMT. Extracted data are based on 16/30 really treated with VC | Negative stress test. 14/30 in VC group withdrew and received PFMT. Extracted data are based on 16/30 really treated with VC | ||
| Cardozo 2004137 | PGI-I (‘very much better’ and ‘much better’) | Cure or improvement (N of ‘responders’ with at least 50% decrease in leakage episodes) | ||
| Castro-Diaz 2007138 | PGI-I (‘very much better’, ‘much better’ or ‘little better’). Data available to show ‘very much better’ and ‘much better’ only | |||
| Dmochowski 2003139 | PGI-I (rating condition as improved) | Cure (no incontinence episodes at the last 7-day diary) | Cure or improvement (50–100% reduction in IEF/week) | |
| Dumoulin 2004199 | < 2 g urine on pad test. Note: childbearing women only | |||
| Fantl 1991135 | Cure (100% reduction in the number of incontinent episodes on 7-day diary) | Cured or improved (50–100% reduction in the number of incontinent episodes on 7-day diary) | ||
| Ghoniem 200557 | ‘Very much better’, ‘much better’ or ‘a little better’ on PGI-I | ≥ 50% reduction in IEF (‘responders’) | ||
| Glavind 1996150 | Cure at 2–3 years post treatment (N considering themselves still cured) | Improvement at 2–3 years post treatment (N considering themselves improved compared with before treatment) | ||
| Glavind 1996150 | Cure after treatment (< 2 g on 1-hour pad test with a bladder volume of 3/4 of cystometric capacity; unclear if at 1 or 3 months) | |||
| Goode 2003123 | Much better’ or ‘better’ vs ‘about the same’ or ‘worse’ | 100% reduction in frequency of incontinence by 2-week bladder diary. Data from figure. Cure rate by urodynamic test also available | ≥ 50% reduction in frequency of incontinence by 2-week bladder diary. Data from figure | |
| Hahn 1991175 | ‘Cured’ vs ‘insignificant symptoms’, ‘improved’, ‘unchanged’, ‘worse’ | ‘Cured’, ‘insignificant symptoms’, ‘improved’ vs ‘unchanged’, ‘worse’ | Cure = by the ICS definition, i.e. essentially dry < 2-g weight increase at pad test (Sutherst 1981) – not sure what this exactly means though? | |
| Haken 1991179 | Subjective assessment on visual analogue scale; significant improvement in both groups (p < 0.05) but no between-group difference | Improved on 40-minute pad test with standardised bladder volume (ICS Proceedings 1988) | ||
| Hay-Smith 2003164 | Self-reported change in leakage (cure vs much better/somewhat better/no change/somewhat worse/much worse) | Self-reported change in leakage (cure/much better/somewhat better vs no change/somewhat worse/much worse) | Decrease by more than 4 g on 24-hour pad test | |
| Henalla 1989124 | Cure = negative following positive test; significantly improved = ≥ 50% reduction in pad weight from baseline (pad test, Sutherst et al. 1981) | |||
| Henalla 1990125 | Cure or improved vs unchanged. Cure and improvement not defined explicitly but ‘failure’ is defined as < 50% reduction in pad weight from baseline based on perineal pad weighing test (no further detail about test). Cure/improvement definitions may be similar to Henalla 1989,12 i.e. ‘cure’ = negative following positive pad test (Sutherst 1981), or ‘significantly improved’ = ≥ 50% reduction in pad weight from baseline | |||
| Hofbauer 1990126 | Cure. Symptom scale? Method of measurement unclear. Time of assessment unclear: immediately after 6-week treatment or at 6-month follow-up | Cure or improvement. Symptom scale? Method of measurement unclear. Time of assessment unclear: immediately after 6-week treatment or at 6-month follow-up | ||
| Johnson 2001167 | No episodes of urine loss on daily diary during the 8th week of the study, i.e. 1 week immediately after treatment phase | |||
| Kim 2007118 | Cured of urine leakage based on an interview asking if woman has experienced urine leakage and, if yes, the frequency of the leakage using the 6-point scale | |||
| Kinchen 2005140 | PGI-I (‘better’) | |||
| Klarskov 1986184 | ‘Cured’ vs ‘improved’, ‘unchanged’, ‘worse’ | ‘Cured’ or ‘improved’ vs ‘unchanged’ or ‘worse’ | ||
| Klingler 1995151 | ‘Subjective improvement’ | Cure (N not using continence pads) | ||
| Knight 1998172 | ‘Cure’ or ‘great improvement’ at 12 months. PFMT + BF + ES groups performed exercises with ES for the first 6 months and then without ES for the following 6 months | Cured (dry or urine loss of < 2 g) or greatly improved (75% or more reduction in urine loss at repeat pad test) at 12 months. Pad test at 75% of the maximum cystometric capacity (Janez et al. 1985). PFMT + BF + ES groups performed exercises with ES for the first 6 months and then without ES for the following 6 months | ||
| Knight 1998172 | ‘Cure’ or ‘great improvement’ at 6 months | Cured (dry or urine loss of < 2 g) or greatly improved (75% or more reduction in urine loss at repeat pad test) at 6 months. Pad test at 75% of the max cystometric capacity (Janez et al. 1985) | ||
| Konstantinidou 2007116 | Patient Global Impression of Improvement (‘Has your condition improved over the past 4 weeks?’ – yes) | N of women NOT reporting underwear wetting | ||
| Lagro-Janssen 1991127 | ‘Cured’ or ‘improved’ vs ‘unchanged’ or ‘worse’ | ‘Dry’ vs ‘mild’, ‘moderate’ or ‘severe’. Based on GP assessment scores regarding frequency and amount of urine loss, use of protective pads or garments, and restrictions in daily activities owing to incontinence | ||
| Laycock 1988176 | ‘Much improved’ or ‘some improvement’ | |||
| Laycock Trial 1 1993132 | ‘Cured’ vs ‘improved’, ‘no change’, ‘worse’ | ‘Cured’, ‘improved’ vs ‘no change’, ‘worse’ | < 0.5 g increase in urine loss based on standard pad test (Sutherst et al. 1981). Patients with urine loss < 2 g (defined as clinically insignificant) at baseline were excluded from the N cured (PV = 1, ES = 3) | Cure = < 0.5 g increase in urine loss based on standard pad test (Sutherst et al. 1981), or improved = > 30% decrease; also include patients with urine loss < 2 g (defined as clinically significant) at baseline (PV = 1, ES = 3) |
| Laycock Trial 2 1993132 | ‘Cured’ vs ‘improved’, ‘no change’, ‘worse’. Cure rates based on visual analogue scale also reported | ‘Cured’, ‘improved’ vs ‘no change’, ‘worse’. Cure and improvement rates based on visual analogue scale also reported | < 0.5 g increase in urine loss based on standard pad test (Sutherst et al. 1981). Patients with urine loss < 2 g (defined as clinically insignificant) at baseline (sham ES = 2, ES = 2) are not included in the number cured | Cure = < 0.5 g increase in urine loss based on standard pad test (Sutherst et al. 1981), or improved = > 30% decrease. Patients with urine loss < 2 g (defined as clinically insignificant) at baseline (sham ES = 2, ES = 2) are not included in the N cured or improved |
| Luber 1997133 | Resolution of symptom, scale 5 on tested but non-validated questionnaire | Cure (resolution of symptom, scale 5), or moderate improvement (scale 3–4) on tested but non-validated questionnaire | Stress test negative | |
| Mah 2006141 | PGI-I (‘very much better’, ‘much better’ or ‘a little better’) | Cure or improvement (IEF responders with ≥ 50% reduction in IEF/week based on weekly diary) | ||
| Manning 2005142 | PGI-I (all ‘better’ responses) (from graph) | |||
| Mayne 1988168 | Cured (short exercise perineal pad test, vs improved, no change, worse) | Cured or improved (short exercise perineal pad test, vs no change or worse) | ||
| Millard 2004143 | PGI-I (‘very much better’, ‘much better’ or ‘a little better’) | Cured (no incontinent episodes at last visit; 7-day diary) | Cure or improvement (50–100% reduction in incontinence episodes; 7-day diary) | |
| Mørkved 2002153 | Subjective assessment of severity, ‘unproblematic’ (defined by author as subjective cure) as opposed to ‘minor problem’, ‘moderate problem’, ‘problematic’ or ‘very problematic’ | Cure (< 2-g leakage) on stress pad test with standardised bladder volume at 6 months. Cure by 48-hour pad test also reported by stress pad test is defined by author as objective cure | ||
| Norton 2002144 | PGI-I (‘very much better’/‘much better’) | Cure based on N of incontinent episodes in 24 hours at last diary | ||
| Oláh 1990187 | Cured after 4-week treatment | Cured or improved after 4-week treatment | No leakage on continence chart after treatment. Chart starting a week before treatment and continuing throughout the course of therapy | Improvement in (the frequency of?) weekly urinary leakage after treatment (continence chart) |
| Pages 2001154 | Patient assessment at 3 months (cure vs improved/no change). Based on questionnaire responses. Cure = no incontinence episodes and symptoms. Data in table and text do not match. Data in text are used here | Patient assessment at 3 months (cure/improved vs no change). Based on questionnaire responses. Cure = no incontinence episodes and symptoms; improved = at least 50% decrease in incontinence episodes and symptoms. Data in table and text do not match. Data in text are used here | ||
| Peattie 1988180 | Improvement (not defined) – data taken from Cochrane review (Hay-Smith 2001)224 | Improvement on pad test – data taken from Cochrane review (Hay-Smith 2001)224 | ||
| Pieber 1995192 | Patients reported no urine loss on any occasion, and a negative pad test | Cure (patients reported no urine loss on any occasion, and a negative pad test ) or improvement (patients reported losing urine less often than before treatment) | ||
| Ramsay 1990128 | ‘Improvement’ vs ‘no change’ or ‘deteriorated’ | |||
| Sand 1995134 | The absence of reported leakage episodes based on 24-hour diary. Cure by pad test also reported | ≥ 50% decrease in leakage episodes on 24-hour diary. Improvement by pad test also reported | ||
| Seo 2004195 | ‘Improvement in the degree of incontinence’ (unclear how this was measured) | |||
| Shepherd 1983155 | Cure (N of patients who perceived dryness) | Improvement (N of patients who perceived improvement) | ||
| Sherburn 2007182 | Zero leakage on the cough stress test, with no precontraction of pelvic floor muscle. Test with a precontraction also reported | |||
| Smith 1996177 | Cure = cessation of incontinence and no longer requiring pads | Cure = cessation of incontinence and no longer requiring pads, or improvement = ?50% reduction in the number of pads and episodes of urinary incontinence | ||
| Tapp 1989185 | ‘Objectively cured’ (not defined) | ‘Objectively cured’ or ‘symptomatic improvement’ (not defined) | ||
| van Kerrebroeck 2004117 | PGI-I (‘very much/much/a little’ better) | Cure or improvement (50–100% reduction in incontinence episodes on daily paper diaries) | ||
| Williams 2006129 | No symptoms | Mild or no problem | ||
| Wilson 1987157 | ‘Much improved’ or ‘improved’ | |||
| Wilson 1998197 | Not incontinent (postal questionnaire). Note: childbearing women only | |||
| Wise 1993188 | Improvement on pad test (40-minute test with standard bladder volume); no further detail given | |||
| Woldringh 2007198 | ‘No UI at all’ based on a composite score ranging from 0 to 10, derived from a validated questionnaire (the PRAFAB score) and bladder diaries. Note: childbearing women only | |||
| Wyman 1998183 | ‘Much better’ or ‘somewhat better’ on 5-point Likert scale | 100% reduction in episodes of leakage per week immediately after treatment (7-day diary, mean, SD) | 50–100% reduction in episodes of leakage per week immediately after treatment (7-day diary, mean, SD) | |
| Wyman 1998183 | ‘Much better’ or ‘somewhat better’ on 5-point Likert scale at 3 months after end of 3-month treatment | 100% reduction in episodes of leakage per week immediately at 3 months after end of 3-month treatment (7-day diary, mean, SD) | 50–100% reduction in episodes of leakage per week immediately at 3 months after end of 3-month treatment (7-day diary, mean, SD) | |
| Wyman 1998183 | N reporting no incontinence episode at mean 3.2 years (of those who did not have additional treatment) | |||
| Zanetti 2007161 | Cure (pad test negative, i.e. urine leakage of no more than 2 g on 1-hour pad test) | |||
| Zinner 1998145 | N of ‘responders’ who showed > 70% improvement/reduction in leakage episodes per week; from graph. Women with SUI only |
Appendix 17 Direct pairwise comparisons: additional data tables for primary outcomes
Comparison 01 – PFMT with or without BF vs no treatment
| PFMT ± BF | NT | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| Social Activity Index | |||||||
| PFMT vs NT | |||||||
| Aksac 2003120 | 20 | 7.5 (1.2) | 10 | 3.6 (0.6) | <0.001 | Score (median, SD) | 1 |
| Bø 1999115 | 25 | 0.6 (1.02) | 30 | –0.2 (1.68) | NR | Change in score (mean, SD) | 1 |
| PFMT + BF vs NT | |||||||
| Aksac 2003120 | 20 | 8.1 (0.8) | 10 | 3.6 (0.6) | <0.001 | Score (median, SD) | 1 |
| I-QoL | |||||||
| PFMT vs NT | |||||||
| Ghoniem 200557 | 49 | 7.8 | 45 | 4.8 | NR | Mean % score increase | 1 |
| The Leicester Impact Scale | |||||||
| PFMT vs NT | |||||||
| Williams 2006a129 | 77 | 2 (0.0, 5.0) | 75 | 1.5 (0.0, 5.0) | 0.990 | Score (median, interquartile range) | 2 |
| Incontinence Impact Questionnaire | |||||||
| PFMT vs NT | |||||||
| Goode 2003123 | 66 | No difference | 67 | No difference | NR | Total score | 3 |
| PFMT n/N | NT n/N | Reported p-value | Notes | Population type | |
|---|---|---|---|---|---|
| Bristol Female Lower Urinary Tract Symptoms (B-FLUTS) | |||||
| PFMT vs NT | |||||
| Bø 1999115 | 7/25 | 10/30 | < 0.54 (sic) | 1. Problems because of avoiding places and situations (N of women) | 1 |
| Bø 1999115 | 1/25 | 12/30 | < 0.01 | 2. Problems with interference with social life (N of women) | 1 |
| Bø 1999115 | 11/25 | 24/30 | < 0.01 | 3. Problem with interference with physical activity (N of women) | 1 |
| Bø 1999115 | 14/25 | 25/30 | < 0.1 (sic) | 4. Overall interference with life (N of women) | 1 |
| Bø 1999115 | 1/25 | 11/30 | < 0.1 (sic) | 5. Unsatisfied if had to spend rest of life as now (N of women) | 1 |
| Bø 1999115 | 4/25 | 15/30 | 0.03 | 6. Sex life spoilt by urinary symptoms (N of women) | 1 |
| Bø 1999115 | 3/25 | 15/30 | 0.02 | 7. Problem with sex life being spoilt (N of women) | 1 |
| Bø 1999115 | 3/25 | 10/30 | 0.1 | 8. Problem with painful intercourse (N of women) | 1 |
| Bø 1999115 | 3/25 | 13/30 | 0.02 | 9. Urinary incontinence with intercourse (N of women) | 1 |
| PFMT | NT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| SF-36 (generic) | ||||||
| PFMT vs NT | ||||||
| Goode 2003123 | 66 | No difference | 67 | No difference | Score | 3 |
| Norwegian version of the Quality of Life Scale (QoLS-N) (generic) | ||||||
| PFMT vs NT | ||||||
| Bø 1999115 | 25 | 90.1 (9.5) | 30 | 85.2 (12.05) | Score (mean, SD) | 1 |
Comparison 02 – PFMT vs PFMT + BF
| PFMT | PFMT ± BF | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| PFMT vs PFMT + BF (≥ 1-year follow-up) | |||||||
| Glavind 1996150 | 0/14 | 0 | 5/19 | 26 | 0.09 (0.01 to 1.80), p = 0.115 | 1 | 1 |
| Improvement rate | |||||||
| PFMT vs PFMT + BF (< 1-year follow-up) | |||||||
| Wilson 1987157 | 4/15 | 27 | 9/14 | 64 | 0.20 (0.04 to 0.98), p = 0.048 | 1 | 1 |
| PFMT vs PFMT + BF (≥ 1-year follow-up) | |||||||
| Glavind 1996150 | 4/14 | 29 | 8/19 | 42 | 0.55 (0.13 to 2.40), p = 0.427 | 1 | 1 |
Comparison 03 – PFMT vs PFMT with additional sessions
| PFMT | PFMT + add | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate (≥ 1-year follow-up) | |||||||
| Bø 1990159 | 4/25 | 16 | 6/20 | 30 | 0.44 (0.11 to 1.87), p = 0.268 | 1 | 1 |
Comparison 04 – strength and motor relearning PFMT vs motor relearning PFMT alone
| S + M PFMT | M PFMT | OR (95% CI) | Measure | Population type | ||||
|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||||
| Cure rate | ||||||||
| Hay-Smith 2003164 | 1/61 | 2 | 4/62 | 6 | 0.24 (0.03 to 2.23), p = 0.210 | 1 | 3 | |
| Improvement rate | ||||||||
| Hay-Smith 2003164 | 52/61 | 85 | 48/62 | 77 | 1.69 (0.67 to 4.25), p = 0.269 | 1 | 3 | |
| King’s Health Questionnaire | ||||||||
| N | Value | N | Value | Reported p-value | Population type | |||
| aHay-Smith 2003164 | 60 | 17.1 (19.3) | 55 | 18.2 (17.7) | 0.751 | 1. General health perception (mean, SD) | 3 | |
| aHay-Smith 2003164 | 60 | 49.4 (24.9) | 55 | 38.8 (27.8) | 0.032 | 2. Incontinence impact (mean, SD) | 3 | |
| aHay-Smith 2003164 | 57 | 27.2 (23.7) | 52 | 20.5 (27.7) | 0.178 | 3. Role limitation (mean, SD) | 3 | |
| aHay-Smith 2003164 | 57 | 31.3 (22.5) | 51 | 22.6 (22.8) | 0.048 | 4. Physical limitation (mean, SD) | 3 | |
| aHay-Smith 2003164 | 56 | 11.8 (18.6) | 51 | 10.5 (21.3) | 0.728 | 5. Social limitation (mean, SD) | 3 | |
| aHay-Smith 2003164 | 40 | 13.8 (23.2) | 40 | 14.6 (24.8) | 0.877 | 6. Personal relationships (mean, SD) | 3 | |
| aHay-Smith 2003164 | 58 | 26.1 (28) | 51 | 20 (24.1) | 0.236 | 7. Emotions (mean, SD) | 3 | |
| aHay-Smith 2003164 | 54 | 28.4 (19.6) | 51 | 32 (19.7) | 0.346 | 8. Sleep/energy (mean, SD) | 3 | |
Comparison 05 – PFMT (in supine) + BF vs PFMT (in supine and upright) + BF
| PFMT supine | PFMT supine/upright | Reported p-value | Notes | Population type | |||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| Incontinence Impact Questionnaire | |||||||
| Borello-France 2006165 | 22 | 27.6 (32.7) | 22 | 24.7 (31) | 0.62 | Change in score (mean reduction, SD) | 1 |
Comparison 06 – PFMT vs PFMT via pilates
Comparison 07 – PFMT (maximal contraction) + BF vs PFMT (submaximal contraction) + BF
| PFMTmax + BF | PFMTsub + BF | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| Johnson 2001167 | 6/16 | 38 | 4/16 | 25 | 1.80 (0.39 to 8.22), p = 0.448 | 2 | 1 |
Comparison 08 – PFMT + perineometer vs PFMT + urethral conductance
Comparison 09 – PFMT + BF (vaginal) vs PFMT + BF (vaginal and abdominal)
| PFMT + BF (vag) | PFMT + BF (vag/ab) | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Incontinence Impact Questionnaire Short Form (IIQ-7) | ||||||
| aWong 2001169 | 19 | 14.29 | 19 | 14.29 | Score (mean) | 1 |
| Urogenital Inventory Short Form (UDI-6) | ||||||
| aWong 2001169 | 19 | 16.67 | 19 | 27.78 | Score (mean) | 1 |
Comparison 10 – ES vs NT
Comparison 11 – PFMT + BF + ES (faradism) vs PFMT + BF + ES (IFT)
| PFMT + BF + ES (farad) | PFMT + BF + ES (IFT) | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Improvement | |||||||
| Wilson 1987157 | 11/15 | 73 | 10/15 | 67 | 1.38 (0.29 to 6.60), p = 0.691 | 1 | 1 |
| Improvement (< 1-year follow-up) | |||||||
| Wilson (follow-up) 1987 | 10/15 | 67 | 9/15 | 60 | 1.33 (0.30 to 5.92), p = 0.705 | 1 | 1 |
Comparison 12 – PFMT + BF + ES (maximal at clinic) vs PFMT + BF + ES (low intensity at home)
| PFMT + BF + ES (max) | PFMT + BF + ES (low) | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Improvement | |||||||
| Knight 1998172 | 16/20 | 80 | 9/19 | 47 | 4.44 (1.08 to 18.36), p = 0.039 | 1 | 1 |
| Improvement (< 1-year follow-up) | |||||||
| Knight (follow-up) 1998 | 17/20 | 85 | 7/15 | 47 | 6.48 (1.32 to 31.83), p = 0.021 | 1 | 1 |
Comparison 13 – VC-passive vs VC-active
| VC-active | VC-passive | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| Burton 1993 | 18/31 | 58 | 21/30 | 70 | 0.59 (0.21 to 1.71), p = 0.334 | 2 | 1 |
Comparison 14 – SNRI vs NT
| SNRI | NT | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Improvement | |||||||
| SNRI80 vs NT (< 1-year follow-up) | |||||||
| Kinchen 2004 | 103/210 | 49 | 90/218 | 41 | 1.37 (0.93 to 2.01), p = 0.107 | 1 | 3 |
| SNRI | NT | Reported p-value | Population type | ||||
|---|---|---|---|---|---|---|---|
| N | Value | N | Value | ||||
| I-QoL | |||||||
| SNRI80 vs NT (< 1-year follow-up) | |||||||
| Kinchen 2004 | 210 | 13.8 | 218 | 12.1 | 0.26 | Change in score | 3 |
Comparison 15 – Comparison of different SNRI doses: cure, improvement and adverse events
| SNRI 1 | SNRI 2 | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| SNRI80 vs SNRI40 | |||||||
| Norton 2002144 | 23/123 | 19 | 30/123 | 24 | 0.71 (0.39 to 1.32), p = 0.279 | 2 | 2 |
| SNRI80 vs SNRI20 | |||||||
| Norton 2002144 | 23/123 | 19 | 21/128 | 16 | 1.17 (0.61 to 2.25), p = 0.633 | 2 | 2 |
| SNRI40 vs SNRI20 | |||||||
| Norton 2002144 | 30/123 | 24 | 21/128 | 16 | 1.64 (0.88 to 3.07), p = 0.118 | 2 | 2 |
| Improvement rate | |||||||
| SNRI80 vs SNRI40 | |||||||
| Norton 2002144 | 57/130 | 44 | 48/129 | 37 | 1.32 (0.80 to 2.17), p = 0.277 | 1 | 2 |
| SNRI80 vs SNRI20 | |||||||
| Norton 2002144 | 57/130 | 44 | 41/132 | 31 | 1.73 (1.05 to 2.87), p = 0.033 | 1 | 2 |
| SNRI40 vs SNRI30 | |||||||
| Zinner 1998145 | 15/33 | 45 | 8/26 | 31 | 1.88 (0.64 to 5.51), p = 0.253 | 2 | 1 |
| SNRI40 vs SNRI20 | |||||||
| Zinner 1998 | 15/33 | 45 | 15/34 | 44 | 1.06 (0.40 to 2.77) | 2 | 1 |
| Norton 2002144 | 48/129 | 37 | 41/132 | 31 | 1.32 (0.79 to 2.20) | 1 | 2 |
| Total | 63/162 | 39 | 56/166 | 34 | 1.25 (0.80 to 1.97), p = 0.329 | ||
| SNRI30 vs SNRI20 | |||||||
| Zinner 1998145 | 8/26 | 31 | 15/34 | 44 | 0.56 (0.19 to 1.65), p = 0.294 | 2 | 1 |
| N experiencing adverse events | |||||||
| n/N | % | n/N | % | Notes | Population | ||
| SNRI80 vs SNRI40 | |||||||
| Norton 2002144 | 102/140 | 73 | 93/137 | 68 | Adverse events that occurred in ≥ 5% of subjects in any treatment arm: nausea, headache, diarrhoea, constipation, dry mouth, dizziness, insomnia, sinusitis, fatigue, nasopharyngitis | 2 | |
| SNRI80 vs SNRI20 | |||||||
| Norton 2002144 | 102/140 | 73 | 86/138 | 62 | As above | 2 | |
| SNRI40 vs SNRI20 | |||||||
| Norton 2002144 | 93/137 | 68 | 86/138 | 62 | As above | 2 | |
| SNRI80, starting 40 b.i.d. vs starting 40 q.d. | |||||||
| Castro-Diaz 2007138 | 87/136 | 64 | 76/127 | 60 | Adverse events that occurred in ≥ 2 patients in first 4 weeks: nausea, dry mouth, constipation, somnolence, dizziness, insomnia, fatigue, headache, diarrhoea | 3 | |
| SNRI80, starting 40 b.i.d. vs starting 20 b.i.d. | |||||||
| Castro-Diaz 2007138 | 87/136 | 64 | 69/133 | 52 | As above | 3 | |
| SNRI80, starting 40 q.d. vs starting 20 b.i.d. | |||||||
| Castro-Diaz 2007138 | 76/127 | 60 | 69/133 | 52 | As above | 3 | |
Comparison 16 – Comparison of different SNRI doses: quality of life
| SNRI 1 | SNRI 2 | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| I-QoL | ||||||
| SNRI80 vs SNRI40 | ||||||
| Norton 2002144 | 130 | 9.3 | 129 | 7.8 | Change in score (mean) | 2 |
| SNRI80 vs SNRI20 | ||||||
| Norton 2002144 | 130 | 9.3 | 132 | 5.3 | Change in score (mean) | 2 |
| SNRI40 vs SNRI30 | ||||||
| Zinner 1998145 | 33 | 8.2 (10.8) | 26 | 10 (6.4) | Change in score (mean, SD) | 1 |
| SNRI40 vs SNRI20 | ||||||
| Zinner 1998145 | 33 | 8.2 (10.8) | 34 | 12 (16) | Change in score (mean, SD) | 1 |
| Norton 2002144 | 129 | 7.8 | 132 | 5.3 | Change in score (mean) | 2 |
| SNRI30 vs SNRI20 | ||||||
| Zinner 1998145 | 26 | 10 (6.4) | 34 | 12 (16) | Change in score (mean, SD) | 1 |
Comparison 17 – PFMT + ES vs no treatment
| PFMT + ES | NT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| SF-36 (generic) | ||||||
| Goode 2003123 | 67 | No difference | 67 | No difference | Score | 3 |
Comparison 18 – PFMT with or without BF vs BT
| PFMT ± BF | BT | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| PFMT + BF vs BT (< 1-year follow-up) | |||||||
| Wyman 1998183 | 13/65 | 20 | 10/62 | 16 | 1.30 (0.52 to 3.23), p = 0.572 | 2 | 2 |
| PFMT + BF vs BT (≥ 1-year follow-up; women who did not seek additional treatment only) | |||||||
| Wyman 1998183 | 1/11 | 9 | 4/22 | 18 | 0.45 (0.04 to 4.60), p = 0.501 | 2 | 2 |
| Improvement | |||||||
| PFMT + BF vs BT (< 1-year follow-up) | |||||||
| Wyman 1998183 | 45/64 | 70 | 37/60 | 62 | 1.47 (0.70 to 3.11), p = 0.310 | 1 | 2 |
| PFMT ± BF | BT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Urogenital Distress Inventory | ||||||
| PFMT + BF vs BT (< 1-year follow-up) | ||||||
| aWyman 1998183 | 64 | 85 (52.4) | 60 | 91.7 (55) | Score (mean, SD) | 2 |
| Incontinence Impact Questionnaire-Revised | ||||||
| PFMT + BF vs BT (< 1-year follow-up) | ||||||
| aWyman 1998183 | 64 | 59.3 (67.7) | 60 | 65.7 (80.2) | Score (mean, SD) | 2 |
| PFMT + BF vs BT (≥ 1-year follow-up) | ||||||
| Wyman 1998183 | 11 | No difference | 22 | No difference | Score (mean, SD)a | 2 |
| Assessment of quality of life (A-QoL) (generic) | ||||||
| PFMT vs BT | ||||||
| aSherburn 2007182 | 43 | 14.44 (9.14) | 41 | 11.88 (9.27) | Total score (mean, SD) | 1 |
Comparison 19 – ES vs VC
Comparison 20 – PFMT (± BF) vs PFMT (± BF) + ES
| PFMT ± BF | PFMT ± BF + ES | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Improvement rate | |||||||
| PFMT + BF vs PFMT + BF + ES (< 1-year follow-up) | |||||||
| Wilson 1987157 | 9/14 | 64 | 19/30 | 63 | 1.04 (0.28 to 3.91), p = 0.951 | 1 | 1 |
| PFMT + BF vs PFMT + BF + ES (< 1-year follow-up) | |||||||
| Knight 1998172 | 9/14 | 64 | 24/35 | 69 | 0.825 (0.224 to 3.044), p = 0.773 | 1 | 1 |
| PFMT ± BF | PFMT ± BF + ES | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| SF-36 (generic) | ||||||
| PFMT vs PFMT + ES | ||||||
| Goode 2003123 | 66 | No difference | 67 | No difference | Total score | 3 |
Comparison 21 – PFMT + BF vs PFMT + BF + BT
| PFMT + BF | PFMT + BF + BT | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| PFMT + BF vs PFMT + BF + BT (< 1-year follow-up) | |||||||
| Wyman 1998183 | 13/65 | 20 | 16/60 | 27 | 0.69 (0.30 to 1.58), p = 0.379 | 2 | 2 |
| PFMT + BF vs PFMT + BF + BT (1-year follow-up)b | |||||||
| Wyman 1998183 | 1/11 | 9 | 8/16 | 50 | 0.10 (0.01 to 0.98), p = 0.048 | 2 | 2 |
| Improvement rate | |||||||
| PFMT + BF vs PFMT + BF + BT (< 1-year follow-up) | |||||||
| Wyman 1998 | 45/64 | 70 | 44/58 | 76 | 0.75 (0.34 to 1.69), p = 0.491 | 1 | 2 |
| PFMT + BF | PFMT + BF + BT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Urogenital Distress Inventory | ||||||
| PFMT + BF vs PFMT + BF + BT (< 1-year follow-up) | ||||||
| aWyman 1998183 | 64 | 85 (52.4) | 58 | 72.8 (50.4) | Score (mean, SD) | 2 |
| Incontinence Impact Questionnaire-Revised | ||||||
| PFMT + BF vs PFMT + BF + BT (< 1-year follow-up) | ||||||
| aWyman 1998183 | 64 | 59.3 (67.7) | 58 | 59.8 (83.9) | Score (mean, SD) | 2 |
| PFMT + BF vs PFMT + BF + BT (1-year follow-up)b | ||||||
| aWyman 1998183 | 11 | No difference | 16 | No difference | Score (mean, SD) | 2 |
Comparison 22 – PFMT + BF + BT vs BT
| PFMT + BF + BT | BT | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure rate | |||||||
| PFMT + BF + BT vs BT (< 1-year follow-up) | |||||||
| Wyman 1998183 | 16/60 | 27 | 10/62 | 16 | 1.89 (0.78 to 4.59), p = 0.159 | 2 | 2 |
| PFMT + BF + BT vs BT (≥ 1-year follow-up)b | |||||||
| Wyman 1998183 | 8/16 | 50 | 4/22 | 18 | 4.50 (1.04 to 19.39), p = 0.044 | 2 | 2 |
| Improvement rate | |||||||
| PFMT + BF + BT vs BT (< 1-year follow-up) | |||||||
| Wyman 1998183 | 44/58 | 76 | 37/60 | 62 | 1.95 (0.88 to 4.33), p = 0.099 | 1 | 2 |
| PFMT + BF + BT | BT | Notes | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Urogenital Distress Inventory | ||||||
| PFMT + BF + BT vs BT (< 1-year follow-up) | ||||||
| aWyman 1998183 | 58 | 72.8 (50.4) | 60 | 91.7 (55) | Score (mean, SD) | 2 |
| Incontinence Impact Questionnaire-Revised | ||||||
| PFMT + BF + BT vs BT (< 1-year follow-up) | ||||||
| aWyman 1998183 | 58 | 59.8 (83.9) | 60 | 65.7 (80.2) | Score (mean, SD) | 2 |
| PFMT + BF + BT vs BT (≥ 1-year follow-up)b | ||||||
| Wyman 1998183 | 16 | No difference | 22 | No difference | Score (mean, SD) | 2 |
Comparison 23 – other comparisons considered not relevant to the review (cure and improvement only)
| Intervention 1 | Intervention 2 | OR (95% CI) | Measure | Population type | |||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Cure | |||||||
| PFMT + ES vs surgery | |||||||
| Tapp 1989185 | 3/23 | 13 | 18/24 | 75 | 0.05 (0.01 to 0.23), p < 0.001 | 2 | 1 |
| PFMT + BF + VC vs ES | |||||||
| Laycock Trial 1993132 | 2/16 | 13 | 1/23 | 4 | 3.14 (0.26 to 37.99), p = 0.368 | 1 | 1 |
| PFMT + ES + BF vs PFMT + VC | |||||||
| Bourcier 1994196 | 31/46 | 67 | 16/38 | 42 | 2.84 (1.17 to 6.93), p = 0.022 | 1 | 1 |
| Improvement | |||||||
| PFMT vs PFMT + BF + ES (faradism) | |||||||
| Wilson 1987157 | 4/15 | 27 | 11/15 | 73 | 0.13 (0.03 to 0.67), p = 0.014 | 1 | 1 |
| Wilson 1987157 (< 1-year follow-up) | 4/15 | 27 | 10/15 | 67 | 0.18 (0.04 to 0.87), p = 0.033 | 1 | 1 |
| PFMT vs PFMT + BF + ES (IFT) | |||||||
| Wilson 1987157 | 4/15 | 27 | 10/15 | 67 | 0.18 (0.04 to 0.87), p = 0.033 | 1 | 1 |
| Wilson 1987157 (< 1-year follow-up) | 4/15 | 27 | 9/15 | 60 | 0.24 (0.05 to 1.13), p = 0.072 | 1 | 1 |
| PFMT + ES vs surgery | |||||||
| Tapp 1989185 | 16/23 | 70 | 23/24 | 96 | 0.10 (0.01 to 0.89), p = 0.039 | 2 | 1 |
| PFMT + VC vs ES | |||||||
| Wise 1993188 | 14/15 | 93 | 12/16 | 75 | 4.67 (0.46 to 47.63), p = 0.194 | 2 | 1 |
| PFMT + BF + VC vs ES | |||||||
| Laycock Trial 1 1993132 | 7/16 | 44 | 14/23 | 61 | 0.50 (0.14 to 1.83), p = 0.294 | 1 | 1 |
| PFMT + ES + BF vs VC | |||||||
| Seo 2004195 | 55/60 | 92 | 53/60 | 88 | 1.45 (0.43 to 4.86), p = 0.545 | 1 | 1 |
Appendix 18 Direct pairwise comparisons: cure and improvement rate (population type 1 only)
COMPARISON 01 Cure rates: pelvic floor muscle training with or without biofeedback versus no treatment (population type 1 only)

COMPARISON 02 Improvement rates: pelvic floor muscle training with or without biofeedback versus no treatment (population type 1 only)

COMPARISON 03 Cure rates: pelvic floor muscle training versus pelvic floor muscle training + biofeedback (population type 1 only)

COMPARISON 04 Cure rates: pelvic floor muscle training versus pelvic floor muscle training + biofeedback (population type 1 only)

COMPARISON 05 Cure rates: electrical stimulation versus no treatment (population type 1 only)

COMPARISON 06 Improvement rates: electrical stimulation versus no treatment (population type 1 only)

COMPARISON 07 Improvement rates: serotonin–noradrenaline reuptake inhibitor versus no treatment (population type 1 only)

COMPARISON 08 Cure rates: pelvic floor muscle training with or without biofeedback versus vaginal cones (population type 1 only)

COMPARISON 09 Improvement rates: pelvic floor muscle training with or without biofeedback versus vaginal cones (population type 1 only)

COMPARISON 10 Improvement rates: electrical stimulation versus vaginal cones (population type 1 only)

COMPARISON 11 Cure rates: pelvic floor muscle training (± biofeedback) versus pelvic floor muscle training (biofeedback) + electrical stimulation (population type 1 only)

COMPARISON 12 Improvement rates: pelvic floor muscle training (± biofeedback) versus pelvic floor muscle training (biofeedback) + electrical stimulation (population type 1 only)
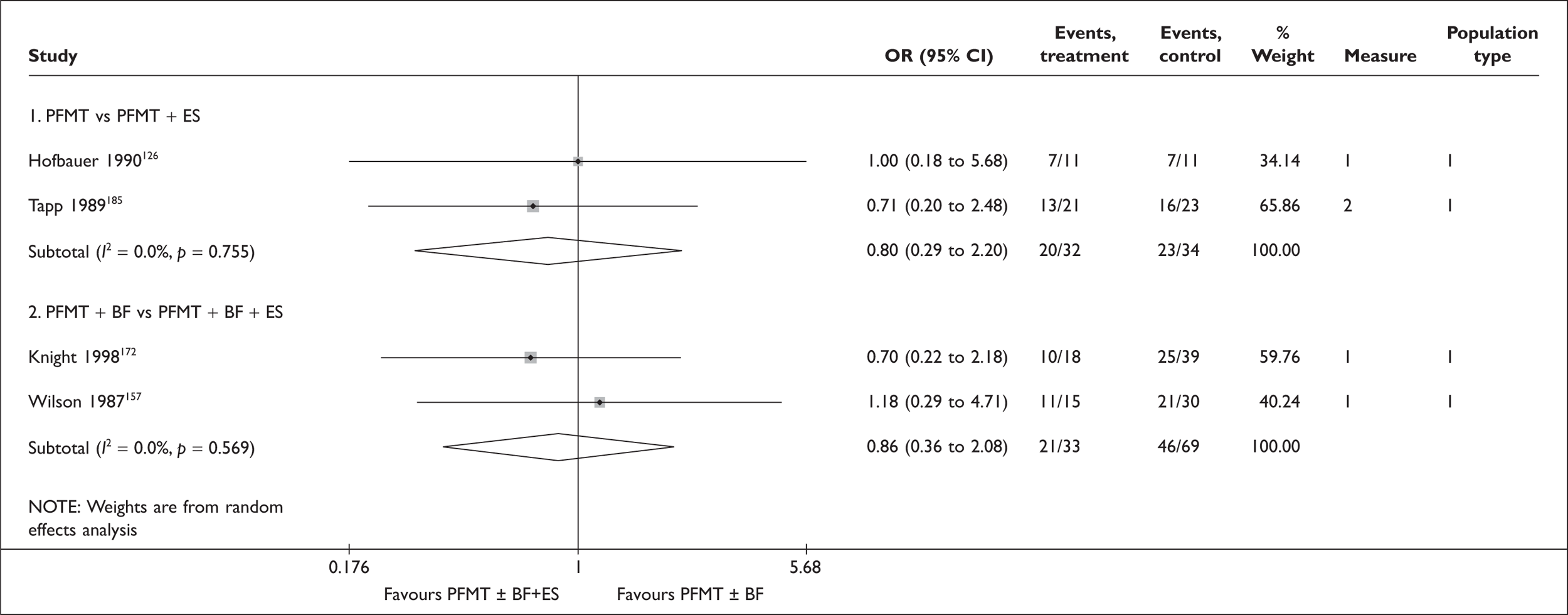
Appendix 19 Direct pairwise comparisons: quality of life
COMPARISON 01 Condition-specific quality of life: pelvic floor muscle training versus pelvic floor muscle training with biofeedback

COMPARISON 02 Condition-specific quality of life: pelvic floor muscle training versus pelvic floor muscle training with additional sessions

COMPARISON 03 Condition-specific quality of life: electrical stimulation versus no treatment
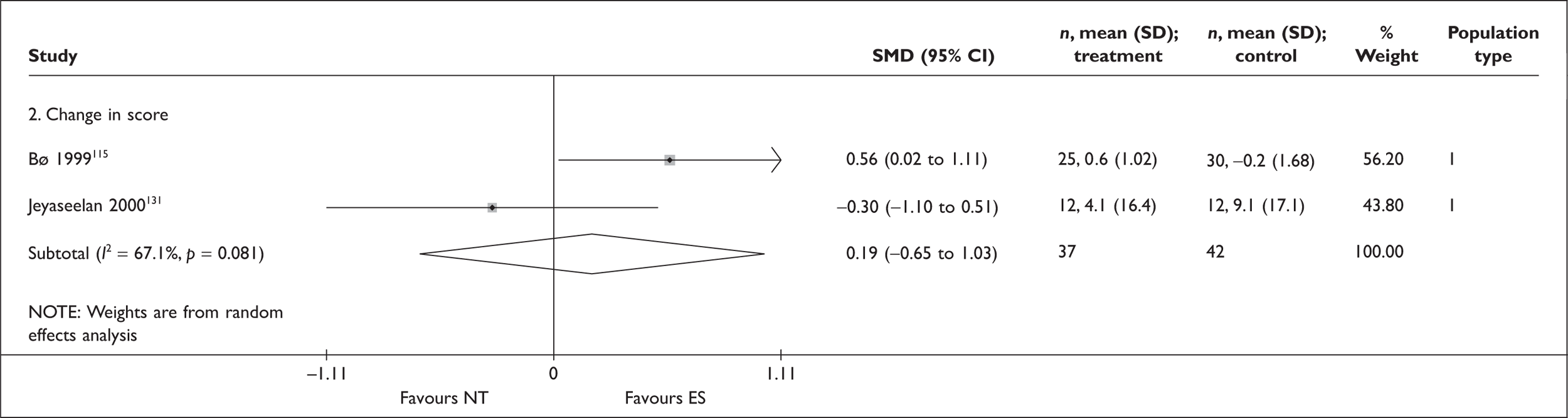
COMPARISON 04 Condition-specific quality of life: serotonin–noradrenaline reuptake inhibitor 80 mg versus no treatment

COMPARISON 05 Condition-specific quality of life: pelvic floor muscle training versus vaginal cones
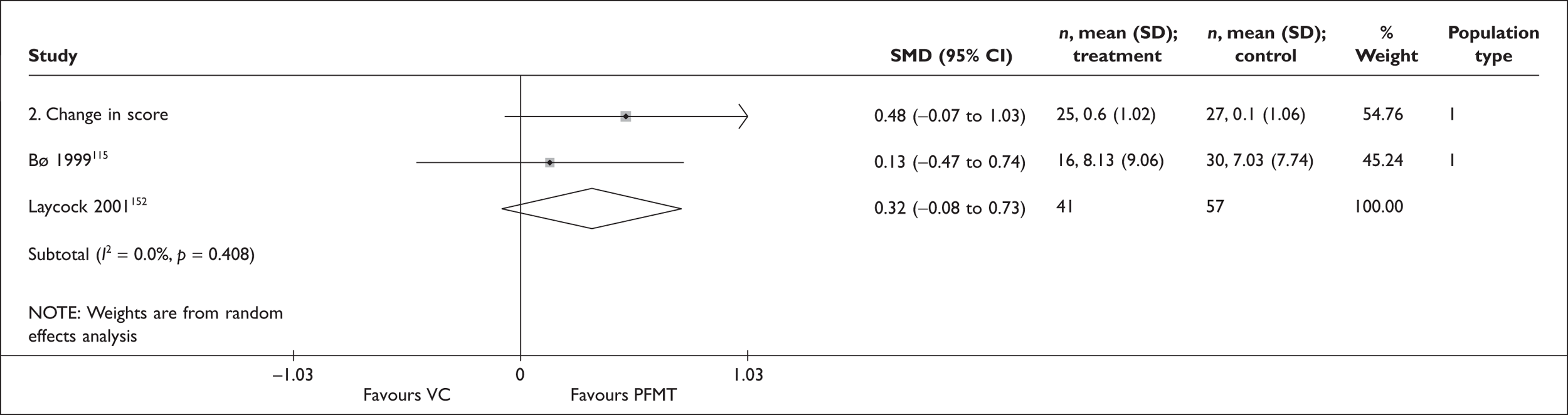
Appendix 20 Trials of treatment for stress urinary incontinence in childbearing women
Three randomised trials197–199 have tested the efficacy of treatments for SUI for women after childbirth and their baseline characteristics are summarised in Table 83.
| Study ID | Population type | Duration (month) | Comparator | N randomised | Age | Supervisory intensitya | Note |
|---|---|---|---|---|---|---|---|
| Dumoulin 2004199 | 1 | 2 | PFMT | 21 | 36.0 | Intensive | PFMT as part of multimodal rehabilitation with BF and ES |
| PFMT + abdominal muscle training | 23 | 37.0 | Intensive | PFMT as part of multimodal rehabilitation with BF and ES | |||
| NT | 20 | 35.5 | Intensive | Relaxation massage for the back and extremities; women asked not to perform PFMT | |||
| Wilson 1998197 | 2 | 12 | PFMT | 39 | 29.0 | Average | |
| VC | 36 | 29.0 | Average | ||||
| PFMT + VC | 38 | 29.0 | Average | ||||
| NT (routine care) | 117 | 27.8 | Average | Routine care with some instructions on PFMT | |||
| Woldringh 2007198 | 2 | 8 weeks postpartum | PFMT | 112 | 31.9 | Average | |
| NT | 152 | 32.6 | Average | Routine care with some instructions on PFMT |
| Arm 1 | Arm 2 | OR (95% CI) | Measure | Population type | ||||
|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||||
| Cure rate | ||||||||
| PFMT vs NT | ||||||||
| Dumoulin 2004199 | 14/20 | 70 | 0/19 | 0 | 87.00 (4.53 to 1671.63), p = 0.03 | 2 | 1 | |
| Wilson 1998197 | 10/19 | 53 | 22/91 | 24 | 3.48 (1.26 to 9.67), p = 0.016 | 1 | 2 | |
| Woldringh 2007198 | 31/81 | 38 | 35/109 | 32 | 1.31 (0.72 to 2.39), p = 0.378 | 1 and 2 | 2 | |
| PFMT vs NT (< 1-year follow-up) | ||||||||
| Woldringh 2007 | 25/60 | 42 | 35/94 | 37 | 1.20 (0.62 to 2.33), p = 0.582 | 1 and 2 | 2 | |
| PFMT + abdominal training vs NT | ||||||||
| Dumoulin 2004199 | 17/23 | 74 | 0/19 | 0 | 105.00 (5.51 to 2002.02), p = 0.02 | 2 | 1 | |
| PFMT vs PFMT + abdominal training | ||||||||
| Dumoulin 2004199 | 14/20 | 70 | 17/23 | 74 | 0.82 (0.22 to 3.13), p = 0.776 | 2 | 1 | |
| PFMT + VC vs NT | ||||||||
| Wilson 1998197 | 6/14 | 43 | 22/91 | 24 | 2.35 (0.74 to 7.52), p = 0.149 | 1 | 2 | |
| VC vs NT | ||||||||
| Wilson 1998197 | 11/21 | 52 | 22/91 | 24 | 3.45 (1.29 to 9.21), p = 0.013 | 1 | 2 | |
| PFMT vs VC | ||||||||
| Wilson 1998197 | 10/19 | 53 | 11/21 | 52 | 1.01 (0.29 to 3.50), p = 0.987 | 1 | 2 | |
| PFMT vs PFMT + VC | ||||||||
| Wilson 1998197 | 10/19 | 53 | 6/14 | 43 | 1.48 (0.37 to 5.95), p = 0.579 | 1 | 2 | |
| PFMT + VC vs VC | ||||||||
| Wilson 1998197 | 6/14 | 43 | 11/21 | 52 | 0.68 (0.17 to 2.66), p = 0.581 | 1 | 2 | |
| Incontinence Impact Quality of Life | ||||||||
| N | Value | N | Value | Reported p-value | ||||
| PFMT vs NT | ||||||||
| Dumoulin 2004199 | 20 | 13.00 (6.00 to 25.00) | 19 | 0.50 (–6.50 to 5.00) | < 0.05 | Change in score (median, 25th and 75th percentile) | 1 | |
| PFMT + abdominal training vs NT | ||||||||
| Dumoulin 2004199 | 23 | 10.00 (2.00 to 16.00) | 19 | 0.50 (–6.50 to 5.00) | < 0.05 | Change in score (median, 25th and 75th percentile) | 1 | |
| PFMT vs PFMT + abdominal training | ||||||||
| Dumoulin 2004199 | 20 | 13.00 (6.00 to 25.00) | 23 | 10.00 (2.00 to 16.00) | p > 0.05 | Change in score (median, 25th and 75th percentile) | 1 | |
The first study199 recruited women with persistent SUI symptoms (and urodynamic stress incontinence) 3 months or more after last delivery and randomised them to three groups: PFMT, PFMT plus abdominal muscle training, and control (relaxation massage). PFMT was provided as part of multimodal pelvic floor rehabilitation (including BF and ES). More than 70% of the women in both treatment groups (70% in the PFMT group and 74% in the PFMT-plus-abdominal-training group) were cured, compared with none in the control group. The results showed a greater change (improvement) in scores on condition-specific quality of life in both treatment groups than the control group but no statistically significant difference was found between the two treatment groups.
The second study197 recruited women complaining of stress urinary incontinence 3 months postpartum and randomised them to a control group treated with basic PFMT and an intervention group split between intensive PFMT alone, VCs alone or both intensive PFMT and VCs. The results showed a statistically significantly higher cure rate in the intervention group than in the control groups (50% against 24%, p = 0.0003), although there was no difference in cure rate between the three intervention arms. No information regarding improvement rates or changes in quality of life were reported.
The third study198 randomised women who complained of SUI, during weeks 17–20 of pregnancy, between intensive PFMT consisting of three physiotherapy sessions between weeks 23 and 30 of pregnancy together with a fourth session 6 weeks after delivery, and routine care that included brief instruction on pelvic floor muscle exercises. The results showed no difference in cure rates between the intervention and control arms at 8 weeks (38% against 32%, p = NS) and 12 months (42% against 37%, p = NS) after delivery nor in change to condition-specific quality of life. Improvement rates were not reported.
Appendix 21 Data on secondary outcomes (population types 1 and 2 only)
Number of incontinence episodes
| Intervention 1 | Intervention 2 | Outcome definition | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Comparison with no treatment | ||||||
| PFMT vs NT | ||||||
| Aksac 2003120 | 20 | 3.5 (0.5) | 10 | 2.4 (0.9) | N of episodes (4-point ordinal scale, median, SD); 1 = once a day, 4 = once a month | 1 |
| Bø 1999115 | 25 | –1.2 (2.04) | 30 | 0.3 (2.24) | Change in N of episodes in 3 days (diary, mean change, SD) | 1 |
| Ghoniem 200557 | 46 | –34.7 | 44 | –28.9 | Change in N of episodes per week (pooled paper diaries completed at each visit, median % decrease) | 1 |
| Lagro-Janssen 1991127 | 33 | 4.8 (5.86) | 33 | 25.3 (15.83) | N of episodes per week (mean, SD); 7-day bladder chart | 1 |
| Burns 1993122 | 43 | 8 (10) | 40 | 17 (19) | N of episodes per week (24-hour diary, mean, SD) | 2 |
| Williams 2006129 | 77 | –1.03 (3.16) | 75 | –0.59 (2.01) | Change in N of episodes in 24 hours (3-day diary, mean, SD) | 2 |
| PFMT + BF vs NT | ||||||
| Aksac 2003120 | 20 | 3.6 (0.4) | 10 | 2.4 (0.9) | N of episodes (4-point ordinal scale, median, SD); 1 = once a day, 4 = once a month | 1 |
| Burns 1993122 | 40 | 5 (6) | 40 | 17 (19) | N of episodes (24-hour diary, mean, SD) | 2 |
| VC vs NT | ||||||
| Bø 1999115 | 27 | 0.8 (5.3) | 30 | 0.3 (2.24) | Change in N of episodes in 3 days (diary, mean change, SD) | 1 |
| Williams 2006129 | 79 | –0.28 (2.68) | 75 | –0.59 (2.01) | Change in N of episodes in 24 hours (3-day diary, mean, SD) | 2 |
| BT vs NT | ||||||
| Fantl 1991135 | 45 | 10 (12) | 43 | 19 (19) | N of episodes (7-day diary, mean, SD) | 1 |
| SNRI 80 mg vs NT | ||||||
| Bump 2004136 | 34 | No data | 31 | No data | Change in N of episodes: ‘Duloxetine was significantly superior to placebo’ | 1 |
| Cardozo 2004137 | 46 | –7.1 | 52 | –2.9 | Change in N of episodes per week (median decrease) | 1 |
| Cardozo 2004137 | 46 | –59.8 | 52 | –26.9 | Change in N of episodes per week (median % decrease) | 1 |
| Ghoniem 200557 | 46 | –56.5 | 44 | –28.9 | Change in N of episodes per week (pooled paper diaries completed at each visit, median % decrease) | 1 |
| Norton 2002144 | 130 | –58 | 132 | –40 | Change in N of episodes in 24 hours (median % reduction at last visit) | 2 |
| SNRI 40 mg vs NT | ||||||
| Zinner 1998145 | 33 | –7.6 (11.3) | 34 | –5.9 (7.6) | Change in N of episodes per week (mean reduction, SD) | 1 |
| Zinner (40/30/20 mg groups combined) 1998145 | 95 | –10.1 (11) | 34 | –5.9 (7.6) | Change in N of episodes per week (mean reduction, SD) | 1 |
| Norton 2002144 | 129 | –59 | 132 | –40 | Change in N of episodes in 24 hours (median % reduction at last visit) | 2 |
| SNRI 30 mg vs NT | ||||||
| Zinner 1998145 | 26 | –8.8 (6.1) | 34 | –5.9 (7.6) | Change in N of episodes per week (mean reduction, SD) | 1 |
| SNRI 20 mg vs NT | ||||||
| Zinner 1998145 | 34 | –13.9 (12.8) | 34 | –5.9 (7.6) | Change in N of episodes per week (mean reduction, SD) | 1 |
| Norton 2002144 | 132 | –44 | 132 | –40 | Change in N of episodes in 24 hours (median % reduction at last visit) | 2 |
| PFMT + SNRI vs NT | ||||||
| Ghoniem 200557 | 44 | –57.4 | 44 | –28.9 | Change in N of episodes per week (pooled paper diaries completed at each visit, median % decrease) | 1 |
| Comparison of different variants of PFMT | ||||||
| PFMT vs PFMT + BF | ||||||
| Aksac 2003120 | 20 | 3.5 (0.5) | 20 | 3.6 (0.4) | N of episodes of leakage (4-point ordinal scale, median, SD). 1 = once a day, 4 = once a month | 1 |
| Berghmans 1996147 | 20 | 1.4 (3.5) | 20 | 0.8 (1.3) | N of episodes in 24 hours (mean, SD) | 1 |
| Laycock 2001152 | 16 | –1.13 (1.42) | 22 | –1.2 (1.29) | Change in N of episodes in 24 hours (mean, SD) | 1 |
| Shepherd 1983155 | 11 | 4.1 (0 to 7) | 11 | 1.1 (0 to 8) | N of episodes per week (diary, mean, range) | 1 |
| Wong 1997a158 | 7 | –9.1 (12.3) | 10 | –2 (3.5) | Change in N of episodes per week (7-day diary, mean reduction, SD) | 1 |
| Burns 1993122 | 43 | 8 (10) | 40 | 5 (6) | N of episodes per week at 8 weeks (24-hour diary, mean, SD) | 2 |
| PFMT vs PFMT with additional sessions | ||||||
| Konstantinidou 2007116 | 10 | 12.5 (7) | 12 | 2.9 (2.8) | N of episodes of per week (7-day diary, mean, SD) | 1 |
| Wong 1997b160 | 26 | No data | 21 | No data | N of episodes of (7-day diary). Both groups showed ‘significant improvement’ over time but no between-group differences | 1 |
| PMFT (in supine position) + BF vs PFMT (in supine and upright position) + BF | ||||||
| Borello-France 2006165 | 22 | –4 (4.7) | 22 | –5.4 (4.8) | Change in N of episodes per week (7-day bladder diary, mean, SD) | 1 |
| PFMT (maximal contraction) + BF vs PFMT (submaximal contraction) + BF | ||||||
| Johnson 2001167 | 16 | 0.79 (1.65) | 16 | 1.15 (2.55) | N of episodes of in 24 hours (daily diary for 8 weeks, mean, SD) | 1 |
| PFMT + BF (vaginal) vs PFMT + BF (vaginal and abdominal) | ||||||
| Wong 2001169 | 19 | 4.1 (10.7) | 19 | 1.5 (3.0) | N of episodes per week (7-day diary, mean, SD), p > 0.05 | 1 |
| Comparison of different SNRI doses | ||||||
| SNRI 80 mg vs SNRI 40 mg | ||||||
| Norton 2002144 | 130 | –58 | 129 | –59 | Change in N of episodes in 24 hours (median % reduction at last visit) | 2 |
| SNRI 80 mg vs SNRI 20 mg | ||||||
| Norton 2002144 | 130 | –58 | 132 | –44 | Change in N of episodes in 24 hours (median % reduction at last visit) | 2 |
| SNRI 40 mg vs SNRI 30 mg | ||||||
| Zinner 1998145 | 33 | –7.6 (11.3) | 26 | –8.8 (6.1) | Change in N of episodes per week (mean reduction, SD) | 1 |
| SNRI 40 mg vs SNRI 20 mg | ||||||
| Zinner 1998145 | 33 | –7.6 (11.3) | 34 | –13.9 (12.8) | Change in N of episodes per week (mean reduction, SD) | 1 |
| Norton 2002144 | 129 | –59 | 132 | –44 | Change in N of episodes in 24 hours (median % reduction at last visit) | 2 |
| SNRI 30 mg vs SNRI 20 mg | ||||||
| Zinner 1998145 | 26 | –8.8 (6.1) | 34 | –13.9 (12.8) | Change in N of episodes per week (mean reduction, SD) | 1 |
| Comparison of different treatments (single modality) | ||||||
| PFMT vs ES | ||||||
| Bø 1999115 | 25 | –1.2 (2.04) | 25 | –0.7 (3.32) | Change in N of episodes in 3 days (diary, mean, SD) | 1 |
| PFMT vs VC | ||||||
| Bø 1999115 | 25 | –1.2 (2.04) | 27 | 0.8 (5.3) | Change in N of episodes in 3 days (diary, mean change, SD) | 1 |
| Laycock 2001152 | 16 | –1.13 (1.42) | 30 | –1 (1.04) | Change in N of episodes in 24 hours (mean reduction, SD) | 1 |
| Williams 2006129 | 77 | –1.03 (3.16) | 79 | –0.28 (2.68) | Change in N of episodes in 24 hours (3-day diary, mean, SD) | 2 |
| PFMT + BF vs VC | ||||||
| Cammu 1998181 | 30 | 5.6 (5.5) | 16 | 8.3 (15) | N of episodes per week (1-week diary, mean, SD) | 1 |
| Laycock 2001152 | 22 | –1.2 (1.29) | 30 | –1 (1.04) | Change in N of episodes in 24 hours (mean reduction, SD) | 1 |
| PFMT vs BT | ||||||
| Sherburn 2007182 | 43 | 4.5 (11) | 41 | 8 (27) | N of episodes (7-day diary, median interquartile range) | 1 |
| PFMT + BF vs BT | ||||||
| Wyman 1998183 | 46 | 8.7 (0) | 48 | 12.5 (8.3) | N of episodes per week at 3 months (7-day diary, mean, SD). Women with USI only | 1 |
| Wyman (follow-up) 1998183 | 65 | 9.4 (14) | 62 | 10 (12) | N of episodes of per week at 6 months (7-day diary, mean, SD). 3 months after the 3-month treatment period. Women with USI/MUI/DO | 2 |
| PFMT vs SNRI 80 mg | ||||||
| Ghoniem 200557 | 46 | –34.7 | 46 | –56.5 | Change in N of episodes per week (pooled paper diaries completed at each visit, median % decrease) | 1 |
| PFMT vs surgery | ||||||
| Klarskov 1986184 | 24 | 2 (0 to 20) | 26 | 0 (0 to 14) | N of episodes in 3 days at 4 months (3-day voiding and incontinence chart, median, range) | 1 |
| Comparison of different treatments (dual modality) | ||||||
| PFMT vs PFMT + ES | ||||||
| Blowman 1991189 | 6 | 6 (0 to 21) | 7 | 0 (0 to 1) | N of episodes per week (1-week continence chart, median, range). p < 0.05 | 1 |
| PFMT vs PFMT + SNRI 80 mg | ||||||
| Ghoniem 200557 | 46 | –34.7 | 44 | –57.4 | Change in episodes of leakage per week (pooled paper diaries completed at each visit, median % decrease) | 1 |
| PFMT + BF vs PFMT + BF + BT | ||||||
| Wyman 1998183 | 46 | 8.7 (0) | 42 | 7.2 (11.5) | N of episodes per week at 3 months (7-day diary, mean, SD). Women with USI only | 1 |
| Wyman (follow-up) 1998183 | 65 | 9.4 (14) | 60 | 8.1 (12.4) | N of episodes per week at 6 months (7-day diary, mean, SD); 3 months after the 3-month treatment period. Women with USI/MUI/DO | 2 |
| PFMT + BF + BT vs BT | ||||||
| Wyman 1998183 | 42 | 7.2 (11.5) | 48 | 12.5 (8.3) | N of episodes per week at 3 months (7-day diary, mean, SD). Women with USI only | 1 |
| Wyman (follow-up) 1998183 | 60 | 8.1 (12.4) | 62 | 10 (12) | N of episodes per week at 6 months (7-day diary, mean, SD); 3 months after the 3-month treatment period. Women with USI/MUI/DO | 2 |
| PFMT + SNRI vs SNRI | ||||||
| Ghoniem 200557 | 44 | –57.4 | 46 | –56.5 | Change in N of episodes per week (pooled paper diaries completed at each visit, median % decrease) | 1 |
Pad test (urine loss)
| Intervention 1 | Intervention 2 | Outcome definition | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Comparison with no treatment | ||||||
| PFMT vs NT | ||||||
| Aksac 2003120 | 20 | 2.1 (0.4) | 10 | 28.2 (3.7) | 1-hour pad test (g, median, SD) | 1 |
| Bidmead 2002121 | 40 | –9.62 (21.31) | 20 | 3.65 (7.65) | Change in pad weight (fixed volume pad test with half-hour exercise programme) (g, mean change, SD) | 1 |
| Bø 1999115 | 25 | –30.2 (33.67) | 30 | –12.7 (40.52) | Change in stress pad test (fixed volume, 60 second) (g, mean change, SD) | 1 |
| Bø 1999115 | 25 | –6.6 (14.03) | 30 | –7.1 (36.61) | Change in 24-hour pad test (g, mean change, SD) | 1 |
| Henalla 1989124 | 26 | 6 | 25 | 12 | Pad test at 3 months (Sutherst et al. 1981) (g, from graph) | 1 |
| Miller 1998107 | 13 | 0.4 (1.04) | 10 | 21.2 (44.8) | Paper towel test, mild cough (wet area, cm2, mean, SD). Cough with ‘The Knack’ for PFMT group, cough without ‘The Knack’ for control group. Wet area 1 cm2 is equivalent to 0.039 ml | 1 |
| Miller 1998107 | 13 | 5.4 (15.3) | 10 | 26.8 (46.7) | Paper towel test, deep cough (wet area, cm2, mean, SD). Cough with ‘The Knack’ for PFMT group, cough without ‘The Knack’ for control group. Wet area 1 cm2 is equivalent to 0.039 ml | 1 |
| Ramsay 1990128 | 22 | –2.1 | 22 | 1.5 | Change in pad test (not defined, g, mean change). ‘Significant’ between-group difference | 1 |
| Williams 2006129 | 77 | –7.39 (28.52) | 75 | –6.11 (28.85) | Change in urine loss on 1-hour pad test (g, mean, SD) | 2 |
| Williams 2006129 | 77 | –2.06 (52.67) | 75 | –7.25 (32.7) | Change in urine loss on 24-hour pad test (g, mean, SD) | 2 |
| PFMT (+ sham ES) vs NT | ||||||
| Bidmead 2002121 | 42 | –2.01 (13.93) | 20 | 3.65 (7.65) | Change in pad weight (fixed volume pad test with half hour exercise programme) (g, mean change, SD) | 1 |
| PFMT + BF vs NT | ||||||
| Aksac 2003120 | 20 | 1.2 (0.2) | 10 | 28.2 (3.7) | 1-hour pad test (g, median, SD) | 1 |
| ES vs NT | ||||||
| Laycock Trial 2 1993132 | 15 | –56.8 | 11 | –21.4 | Percentage change in standard pad test (Sutherst et al. 1981 with modification, average % decrease). Significant different between groups | 1 |
| Jeyaseelan 2000131 | 12 | 0.5 (–33 to 71) | 12 | 0.1 (–15 to 61) | Change in 1-hour pad test (g, median, range) | 1 |
| Sand 1995134 | 35 | –29.9 (57.39) | 17 | 2.3 (23.05) | Change in weight of urine lost on 20-minute pad test at a fixed bladder volume (g, mean, SD) | 1 |
| Henalla 1989124 | 25 | 9.5 | 25 | 12 | Pad test at 3 months (Sutherst et al. 1981) (g, from graph) | 1 |
| Bø 1999115 | 25 | –7.4 (34.44) | 30 | –12.7 (40.52) | Change in stress pad test (fixed volume, 60-second) (g, mean change, SD) | 1 |
| Bø 1999115 | 25 | –0.5 (21.43) | 30 | –7.1 (36.61) | Change in 24-hour pad test (at home) (g, mean change, SD) | 1 |
| VC vs NT | ||||||
| Bø 1999115 | 27 | –14.7 (34.2) | 30 | –12.7 (40.52) | Change in stress pad test (fixed volume, 60 second) (g, mean change, SD) | 1 |
| Bø 1999115 | 27 | –22 (89.34) | 30 | –7.1 (36.61) | Change in 24-hour pad test (at home) (g, mean change, SD) | 1 |
| Williams 2006129 | 79 | –3.68 (21.54) | 75 | –6.11 (28.85) | Change in urine loss on 1-hour pad test (g, mean, SD) | 2 |
| Williams 2006129 | 79 | –5.19 (31.88) | 75 | –7.25 (32.7) | Change in urine loss on 24-hour pad test (g, mean, SD) | 2 |
| BT vs NT | ||||||
| Fantl 1991135 | 45 | 10 (21) | 43 | 29 (74) | Pad test (g, mean, SD) | 1 |
| SNRI 80 mg vs NT | ||||||
| Norton 2002144 | 130 | –29 (–100 to 12,333) | 132 | –30 (–100 to 2175) | Median % change in 1-hour stress pad test | 2 |
| SNRI 40 mg vs NT | ||||||
| Zinner 1998145 | 33 | 12.2 (21.7) | 34 | 4.7 (15.5) | Mean reduction in 1-hour stress pad test (g, mean, SD) | 1 |
| Zinner 1998145 | 33 | 19.6 (41.4) | 34 | 9.4 (43.3) | Mean reduction in 24-hour pad test (g, mean, SD) | 1 |
| Zinner (40-, 30- and 20-mg groups combined) 1998145 | 95 | 10.7 (22.2) | 34 | 4.7 (15.5) | Mean reduction in 1-hour stress pad test (g, mean, SD) | 1 |
| Zinner (40-, 30- and 20-mg groups combined) 1998145 | 95 | 26.7 (53.6) | 34 | 9.4 (43.3) | Mean reduction in 24-hour pad test (g, mean, SD) | 1 |
| Norton 2002144 | 129 | –43 (–100 to 5800) | 132 | –30 (–100 to 2175) | Median % change in 1-hour stress pad test | 2 |
| SNRI 20 mg vs NT | ||||||
| Zinner 1998145 | 34 | 13.5 (26.2) | 34 | 4.7 (15.5) | Mean reduction in 1-hour stress pad test (g, mean, SD) | 1 |
| Zinner 1998145 | 34 | 40.8 (65) | 34 | 9.4 (43.3) | Mean reduction in 24-hour pad test (g, mean, SD) | 1 |
| Norton 2002144 | 132 | –11 (–100 to 3240) | 132 | –30 (–100 to +2175) | Median % change in 1-hour stress pad test | 2 |
| PFMT + ES vs NT | ||||||
| Bidmead 2002121 | 82 | –5.74 (17.3) | 20 | 3.65 (7.65) | Change in pad weight (fixed volume pad test with half-hour exercise programme) (g, mean change, SD) | 1 |
| Comparison of different variants of PFMT | ||||||
| PFMT vs PFMT + BF | ||||||
| Aksac 2003120 | 20 | 2.1 (0.4) | 20 | 1.2 (0.2) | 1-hour pad test (g, median, SD) | 1 |
| Aukee 2002146 | 15 | 22.5 (19.6) | 15 | 19 (19.7) | 24-hour pad test at 12 weeks (g, mean, SD). Note: baseline values were significantly lower for BF group; no between-group difference after adjustment (p = 0.907) | 1 |
| Berghmans 1996147 | 20 | 12.5 (12) | 20 | 12.2 (15.4) | 48-hour pad test (g, mean, SD) | 1 |
| Ferguson 1990149 | 7 | 3.4 (4.7) | 7 | 1.4 (1.7) | 30-minute pad test at 6 weeks (g, mean, SD, range) | 1 |
| Ferguson 1990149 | 7 | –6.9 (7.4) | 7 | –3.4 (4) | Change in 30-minute pad test from baseline to 6 weeks (g, mean, SD) | 1 |
| Ferguson 1990149 | 10 | 5.8 (5.6) | 10 | 5.6 (4.7) | 24-hour pad test at 6 weeks (g, mean, SD) | 1 |
| Ferguson 1990149 | 10 | –9.1 (13.6) | 10 | –5.1 (8.1) | Change in-24 hour pad test from baseline to 6 weeks (g, mean, SD) | 1 |
| Glavind 1996150 | 15 | 19 [0, 51] | 19 | 2.5 (1 to 10) | 1-hour pad test after 1 month (with a bladder volume of 3/4 of cystometric capacity) (g, median, 95% CI) | 1 |
| Glavind 1996150 | 15 | 10 [2, 27] | 19 | 0.8 (0 to 4) | 1-hour pad test after 3 months (with a bladder volume of 3/4 of cystometric capacity) (g, median, 95% CI) | 1 |
| Klingler 1995151 | 21 | 2.9 [0, 19] | 20 | 1.2 (0 to 22) | Pad test (not defined; g, ?mean, range) | 1 |
| Mørkved 2002153 | 34 | 9.9 (21.12) | 36 | 5.5 (10.56) | Stress pad test with standardised bladder volume at 6 months (g, mean, SD). | 1 |
| Mørkved 2002153 | 34 | 17.7 (22.61) | 36 | 20.4 (19.9) | Mean change in leakage on stress pad test with standardised bladder volume at 6 months (g, mean, SD) | 1 |
| Mørkved 2002153 | 34 | 3.8 (7.14) | 36 | 7 (16.38) | 48-hour pad test at 6 months (g, mean, SD) | 1 |
| Mørkved 2002153 | 34 | 42.5 (36.29) | 36 | 33 (32.14) | Mean change in leakage 48-hour pad test at 6 months (g, mean, SD). | 1 |
| Wong 1997a158 | 7 | 18.7 (24.8) | 10 | 7.4 (6.1) | Reduction in 1-hour pad test (g, mean, SD) | 1 |
| PFMT vs PFMT + additional sessions | ||||||
| Bø 1990159 | 29 | 23 (35.72) | 23 | 7.1 (15.42) | 90-second pad test at 6 months (g, mean, SD). Data for PFMT with additional sessions obtained from graph | 1 |
| Wong 1997b160 | 26 | No data | 21 | No data | 1-hour pad test: both groups showed ‘significant improvement’ over time but no between-group differences | 1 |
| Zanetti 2007161 | 21 | 15 | 23 | 3.2 | 1-hour pad test (g, median) | 1 |
| PFMT (in supine position) + BF vs PFMT (in supine and upright position) + BF | ||||||
| Borello-France 2006165 | 22 | –3.9 (3.8) | 22 | –5.1 (3.9) | Change in pad test [modified 1-hour pad test by ICS (Abrams 1998) with full bladder and provocative manoeuvres] (g, mean, SD) | 1 |
| PFMT (maximal contraction) + BF vs PFMT (submaximal contraction) + BF | ||||||
| Johnson 2001167 | 16 | 3.84 (5.29) | 16 | 3.41 (4.79) | 10-hour pad test (g, mean, SD) | 1 |
| PFMT + BF (vaginal) vs PFMT + BF (vaginal and abdominal) | ||||||
| Wong 2001169 | 19 | 23 (69.0) | 19 | 3.9 (3.6) | 1-hour pad test (with a standardised set of exercises, g, mean, SD), p > 0.05. Note: One patient in the vaginal BF group had a leakage of more than 300 g in the pad test | 1 |
| PFMT + BF vs PFMT + ES | ||||||
| Pohl 2004171 | 10 | 1.7 | 21 | 1.5 | Stress test under standardised condition (no further detail, unit of measurement unclear, average) | 1 |
| Pohl 2004171 | 10 | 10 | 21 | 6.21 | Pad test under standardised condition (no further detail; unit of measurement unclear) | 1 |
| Comparison of different variants of ES | ||||||
| PFMT + BF + ES (maximal intensity at clinic) vs PFMT + BF + ES (low intensity at home) | ||||||
| Knight 1998172 | 20 | 1.5 (0.0 to 28.1) | 19 | 2.9 (0.0 to 50.9) | Urine loss on pad test at 6 months [at 75% of the max cystometric capacity (Janez et al. 1985)] (g, median, range) | 1 |
| Knight 1998172 | 20 | 91.3 (–72.4 to 100.0) | 19 | 76.5 (–580.3 to 100.0) | % change in urine loss on pad test at 6 months (median, range). No significant between-group difference | 1 |
| Knight 1998172 | 20 | 100 (–67.5 to 100.0) | 16 | 97.5 (–415.1 to 100.0) | % change in urine loss on pad test at 12 months (median, range) | 1 |
| Comparison of different variants of VC | ||||||
| VC passive vs VC active | ||||||
| Burton 1993 | 31 | 4.1 | 30 | 2 | 40-minute pad test (ml, mean) | 1 |
| Comparison of different SNRI doses | ||||||
| SNRI 80 mg vs SNRI 40 mg | ||||||
| Norton 2002144 | 130 | –29 (–100 to 12,333) | 129 | –43 (–100 to 5800) | Median % change in 1-hour stress pad test | 2 |
| SNRI 80 mg vs SNRI 20 mg | ||||||
| Norton 2002144 | 130 | –29 (–100 to 12,333) | 132 | –11 [–100 to 3240] | Median % change in 1-hour stress pad test | 2 |
| SNRI 40 mg vs SNRI 30 mg | ||||||
| Zinner 1998145 | 33 | 12.2 (21.7) | 26 | 5.3 (16.3) | Change in 1-hour stress pad test (g, mean reduction, SD) | 1 |
| Zinner 1998145 | 33 | 19.6 (41.4) | 26 | 17.6 (49.2) | Change in 24-hour pad test (g, mean reduction, SD) | 1 |
| SNRI 40 mg vs SNRI 20 mg | ||||||
| Zinner 1998145 | 33 | 12.2 (21.7) | 34 | 13.5 (26.2) | Change in 1-hour stress pad test (g, mean reduction, SD) | 1 |
| Zinner 1998145 | 33 | 19.6 (41.4) | 34 | 40.8 (65) | Change in 24-hour pad test (g, mean reduction, SD) | 1 |
| Norton 2002144 | 129 | –43 (–100 to 5800) | 132 | –11 (–100 to 3240) | Median % change in 1-hour stress pad test | 2 |
| SNRI 30 mg vs SNRI 20 mg | ||||||
| Zinner 1998145 | 26 | 5.3 (16.3) | 34 | 13.5 (26.2) | Change in 1-hour stress pad test (g, mean reduction, SD) | 1 |
| Zinner 1998145 | 26 | 17.6 (49.2) | 34 | 40.8 (65) | Change in 24-hour pad test (g, mean reduction, SD) | 1 |
| Comparison of different treatments (single modality) | ||||||
| PFMT vs ES | ||||||
| Laycock 1988176 | 11 | 36.33 (7.7 to 72.4) | 18 | 30.55 (3.0 to 78.0) | Change in pad test (not defined) (g, mean reduction, range) | 1 |
| Henalla 1989124 | 26 | 6 | 25 | 9.5 | Pad test at 3 months (Sutherst et al. 1981) (g, from graph) | 1 |
| Bø 1999115 | 25 | –30.2 (33.67) | 25 | –7.4 (34.44) | Change in stress pad test (fixed volume, 60 second) (g, mean change, SD) | 1 |
| Bø 1999115 | 25 | –6.6 (14.03) | 25 | –0.5 (21.43) | Change in 24-hour pad test (g, mean change, SD) | 1 |
| PFMT vs VC | ||||||
| Arvonen 2001178 | 19 | 5 [0, 90] | 18 | 1 (0 to 100) | Pad test (short provocation test with a standard 300 ml in bladder) (g, median, range) | 1 |
| Bø 1999115 | 25 | –30.2 (33.67) | 27 | –14.7 (34.2) | Change in stress pad test (fixed volume, 60 second) (g, mean change, SD) | 1 |
| Bø 1999115 | 25 | –6.6 (14.03) | 27 | –22 (89.34) | Change in 24-hour pad test (g, mean change, SD) | 1 |
| Peattie 1988180 | 16 | No data | 17 | No data | Extended pad test (no detail): significant reduction in both groups, no between-group difference | 1 |
| Williams 2006129 | 77 | –7.39 (28.52) | 79 | -3.68 (21.54) | Change in urine loss on 1-hour pad test (g, mean, SD) | 2 |
| Williams 2006129 | 77 | –2.06 (52.67) | 79 | –5.19 (31.88) | Change in urine loss on 24-hour pad test (g, mean, SD) | 2 |
| PFMT vs BT | ||||||
| Sherburn 2007182 | 43 | 0.1 (1.5) | 41 | 0.5 (2.4) | Stress test – cough (g, median interquartile range), p = 0.034 | 1 |
| Sherburn 2007182 | 43 | 0 (0.4) | 41 | 0.3 (0.7) | Stress test – brace/cough (g, median interquartile range), p = 0.008 | 1 |
| PFMT vs surgery | ||||||
| Klarskov 1986184 | 24 | No data | 26 | No data | Standardised 60-minute pad test (Klarskov and Hald 1984)406 at 4 months. Better for the operated patients than for the PFMT patients, p < 0.0005 | 1 |
| ES vs VC | ||||||
| Bø 1999115 | 25 | –7.4 (34.44) | 27 | –14.7 (34.2) | Change in stress pad test (fixed volume, 60 second) (g, mean change, SD) | 1 |
| Bø 1999115 | 25 | –0.5 (21.43) | 27 | –22 (89.34) | Change in 24-hour pad test (g, mean change, SD) | 1 |
| Delneri 2000186 | 10 | 9.5 | 10 | 9.5 | Pad test (not defined) (g, mean). No between-group difference | 1 |
| Comparison of different treatments (dual modality) | ||||||
| PFMT vs PFMT + ES | ||||||
| Bidmead 2002121 | 40 | –9.62 (21.31) | 82 | –5.74 (17.3) | Change in pad weight (fixed-volume pad test with half-hour exercise programme) (g, mean change, SD) | 1 |
| Haig 1995190 | 8 | 12.2 (9.4) | 11 | 10.6 (6.2) | 48-hour pad test (g, mean, SD) | 1 |
| Tapp 1987191 | 15 | No data | 14 | No data | Pad test. No within-group (before/after) differences or between-group differences | 1 |
| PFMT (+ sham ES) vs PFMT + ES | ||||||
| Bidmead 2002121 | 42 | –2.01 (13.934 | 82 | –5.74 (17.296 | Change in pad weight (fixed-volume pad test with half-hour exercise programme) (g, mean change, SD) | 1 |
| Haig 1995190 | 11 | 38.7 (49.4) | 11 | 10.6 (6.2 | 48-hour pad test (g, mean, SD) | 1 |
| PFMT + BF vs PFMT + BF + ES (maximal intensity at clinic) | ||||||
| Knight 1998172 | 18 | 0.8 (0.0 to 88.1) | 20 | 1.5 (0.0 to 28.1) | Urine loss on pad test at 6 months [at 75% of the max cystometric capacity (Janez et al. 1985)] (g, median, range) | 1 |
| Knight 1998172 | 18 | 90.7 (–17.1 to 100.0) | 20 | 91.3 (–72.4 to 100.0) | % change in urine loss on pad test at 6 months (median, range). No significant between-group difference | 1 |
| Knight 1998172 | 15 | 100 (–8.1 to 100.0) | 20 | 100 (–67.5 to 100.0) | % change in urine loss on pad test at 12 months (median, range) | 1 |
| PFMT + BF vs PFMT + BF + ES (low intensity at home) | ||||||
| Knight 1998172 | 18 | 0.8 (0.0 to 88.1) | 19 | 2.9 (0.0 to 50.9) | Urine loss on pad test at 6 months [at 75% of the maximal cystometric capacity (Janez et al. 1985)] (g, median, range) | 1 |
| Knight 1998172 | 18 | 90.7 (–17.1 to 100.0) | 19 | 76.5 (–580.3 to 100.0) | % change in urine loss on pad test at 6 months (median, range). No significant between-group difference | 1 |
| Knight 1998172 | 15 | 100 (–8.1 to 100.0) | 16 | 97.5 (–415.1 to 100.0) | % change in urine loss on pad test at 12 months (median, range) | 1 |
| PFMT + VC vs VC | ||||||
| Wise 1993188 | 15 | no data | 19 | no data | Reduction in weight of urine loss on pad test (40-minute test with standard bladder volume). No significant difference between groups (p = 0.053) | 1 |
Number of pad changes
| Intervention 1 | Intervention 2 | Outcome definition | Population type | |||
|---|---|---|---|---|---|---|
| N | Value | N | Value | |||
| Comparison with no treatment | ||||||
| PFMT vs NT | ||||||
| Ghoniem 200557 | 46 | –24.8 | 44 | –10.5 | Change N of pad changes per week (median % decrease) | 1 |
| Williams 2006129 | 77 | 0.05 (1.59) | 75 | –0.16 (0.99) | Change in N of pad changes in 24 hours (mean, SD) | 2 |
| ES vs NT | ||||||
| Sand 1995134 | 35 | –2.1 (4.73) | 17 | 1.5 (5.9) | Change in N of pad changes per week (weekly diary, mean, SD) | 1 |
| VC vs NT | ||||||
| Williams 2006129 | 79 | –0.04 (0.95) | 75 | –0.16 (0.99) | Change in N of pad changes in 24 hours (mean, SD) | 2 |
| SNRI 80 mg vs NT | ||||||
| Bump 2004136 | 34 | No data | 31 | No data | Decreases in N of pads used. ‘Duloxetine was significantly superior to placebo’ | 1 |
| Cardozo 2004137 | 46 | –34.5 | 52 | –4.8 | Change in N of pad changes (median % reduction) | 1 |
| Ghoniem 200557 | 46 | –35.3 | 44 | –10.5 | Change in N of pad changes per week (median % decrease) | 1 |
| PFMT + SNRI vs NT | ||||||
| Ghoniem 200557 | 44 | –45.7 | 44 | –10.5 | Change in N of pad changes per week (median % decrease) | 1 |
| Comparison of different variants of PFMT | ||||||
| PFMT vs PFMT + BF | ||||||
| Klingler 1995151 | 21 | 0.6 (0 to 6) | 20 | 0.1 (0 to 2) | N of pad changes in 24 hours (?mean, range) | 1 |
| Laycock 2001152 | 16 | –1.88 (2.35) | 22 | –2.27 (3.57) | Change in N of pad changes in 24 hours (bladder diary, mean reduction, SD) | 1 |
| Wilson 1987157 | 15 | 2.7 (2.5) | 15 | 0.9 (1.5) | N of pad changes in 24 hours after treatment (mean, SD) | 1 |
| PFMT vs PFMT + additional sessions | ||||||
| Bø 1990159 | 26 | 9 | 21 | 12 | Self-reported pad use at 15 years (N of women). ‘Never or only during physical activity’. p = 0.15 | 1 |
| Bø 1990159 | 26 | 7 | 21 | 3 | Self-reported pad use at 15 years (N of women). ‘Always’. p = 0.47 | 1 |
| Konstantinidou 2007116 | 10 | 2.4 (1.3) | 12 | 0.8 (0.1) | N of pad changes in 24 hours (7-day diary, mean, SD) | 1 |
| Comparison of different variants of ES | ||||||
| PFMT + BF + ES (faradism) vs PFMT + BF + ES (IFT) | ||||||
| Wilson 1987157 | 15 | 1.3 (1.4) | 15 | 1.6 (2.3) | N of pad changes in 24 hours after treatment (mean, SD) | 1 |
| Comparison of different treatments (single modality) | ||||||
| PFMT vs VC | ||||||
| Laycock 2001152 | 16 | –1.88 (2.35) | 30 | –2.9 (4.22) | Change in N of pad changes in 24 hours (bladder diary, mean reduction, SD) | 1 |
| Williams 2006129 | 77 | 0.05 (1.59) | 79 | –0.04 (0.95) | Change in N of pad changes in 24 hours (mean, SD) | 2 |
| PFMT + BF vs VC | ||||||
| Cammu 1998181 | 30 | 6 (5.6) | 16 | 8.6 (15) | N of pad changes in 24 hours (1-week diary, mean, SD) | 1 |
| Laycock 2001152 | 22 | –2.27 (3.56) | 30 | –2.9 (4.22) | Change in N of pad changes in 24 hours (bladder diary, mean reduction, SD) | 1 |
| PFMT vs SNRI | ||||||
| Ghoniem 200557 | 46 | –24.8 | 46 | –35.3 | Change in N of pad changes per week (median % decrease) | 1 |
| Comparison of different treatments (dual modality) | ||||||
| PFMT + BF vs PFMT + BF + ES (faradism) | ||||||
| Wilson 1987157 | 15 | 0.9 (1.5) | 15 | 1.3 (1.4) | N of pad changes in 24 hours after treatment (mean, SD) | 1 |
| PFMT + BF vs PFMT + BF + ES (IFT) | ||||||
| Wilson 1987157 | 15 | 0.9 (1.5) | 15 | 1.6 (2.3) | N of pad changes in 24 hours after treatment (mean, SD) | 1 |
| PFMT vs PFMT + SNRI | ||||||
| Ghoniem 200557 | 46 | –24.8 | 44 | –45.7 | Change in N of pad changes per week (median % decrease) | 1 |
| PFMT + SNRI vs SNRI | ||||||
| Ghoniem 200557 | 44 | –45.7 | 46 | –35.3 | Change in N of pad changes per week (median % decrease) | 1 |
Other secondary outcomes
Data on the following secondary outcomes are reported in Appendix 10.
| Type of outcome and study ID | Type of interventions |
|---|---|
| Number of micturitions | |
| Blowman 1991189 | PFMT vs PFMT + ES |
| Brubaker 1997130 | ES vs NT |
| Bump 2004136 | SNRI vs placebo |
| Fantl 1991135 | BT vs NT |
| Haig 1995190 | PFMT vs PFMT + sham ES vs PFMT + ES |
| Klarskov 1986184 | PFMT vs surgery |
| Konstantinidou 2007116 | PFMT vs PFMT with additional sessions |
| Laycock Trial 2 1993132 | ES vs NT |
| Norton 2002144 | SNRI 80 mg vs 40 mg vs 20 mg vs placebo |
| Pages 2001154 | PFMT vs PFMT + BF |
| Sand 1995134 | ES vs NT |
| Shepherd 1983155 | PFMT vs PFMT + BF |
| Williams 2006129 | PFMT vs VC vs NT |
| Wilson 1987157 | PFMT vs PFMT + BF vs PFMT + BF + ES (faradism) vs PFMT + BF + ES (IFT) |
| Zanetti 2007161 | PFMT vs PFMT with additional sessions |
| Participants satisfaction or desire for further treatment | |
| Arvonen 2001178 | PFMT vs VC |
| Blowman 1991189 | PFMT vs PFMT + ES |
| Bø 1990159 | PFMT vs PFMT with additional sessions |
| Bø 1999115 | PFMT vs ES vs VC vs NT |
| Cammu 1998181 | PFMT + BF vs VC |
| Glavind 1996150 | PFMT vs PFMT + BF |
| Haken 1991179 | PFMT vs VC |
| Klarskov 1986184 | PFMT vs surgery |
| Lagro-Janssen 1991127 | PFMT vs NT |
| Luber 1997133 | ES vs NT |
| Mørkved 2002153 | PFMT vs PFMT + BF |
| Savage 2005166 | PFMT vs modified pilates |
| Sherburn 2007182 | PFMT vs BT |
| Williams 2006129 | PFMT vs VC vs NT |
| Wyman 1998183 | PFMT + BF vs BT vs PFMT + BF + BT |
| Zanetti 2007161 | PFMT vs PFMT with additional sessions |
| Number of women having incontinence surgery | |
| Aukee 2002146 | PFMT vs PFMT + BF |
| Bø 1990159 | PFMT vs PFMT with additional sessions |
| Cammu 1998181 | PFMT + BF vs VC |
| Klarskov 1986184 | PFMT vs surgery |
| Mørkved 2002153 | PFMT vs PFMT + BF |
| Peattie 1988180 | PFMT vs VC |
| Pieber 1995192 | PFMT vs PFMT + VC |
| Savage 2005166 | PFMT vs modified pilates |
| Tapp 1987191 | PFMT vs PFMT + ES |
| Tapp 1989185 | PFMT vs PFMT + ES vs surgery |
| Wyman 1998183 | PFMT + BF vs BT vs PFMT + BF + BT |
| Recurrence | |
| Blowman 1991189 | PFMT vs PFMT + ES |
| Henalla 1989124 | PFMT vs ES vs NT |
| Tapp 1989185 | PFMT vs PFMT + ES vs surgery |
| Measures of pelvic floor muscle functions | |
| Aksac 2003120 | PFMT vs PFMT + BF vs NT |
| Arvonen 2001178 | PFMT vs VC |
| Aukee 2002146 | PFMT vs PFMT + BF |
| Bernardes 2000174 | PFMT vs ES |
| Blowman 1991189 | PFMT vs PFMT + ES |
| Bø 1990159 | PFMT vs PFMT with additional sessions |
| Bø 1999115 | PFMT vs ES vs VC vs NT |
| Borello-France 2006165 | PFMT in supine position + BF vs PFMT in supine and upright positions + BF |
| Burns 1993122 | PFMT vs PFMT + BF vs NT |
| Cammu 1998181 | PFMT + BF vs VC |
| Ferguson 1990149 | PFMT vs PFMT + BF |
| Ghoniem 200557 | PFMT vs SNRI vs PFMT + SNRI vs NT |
| Hahn 1991175 | PFMT vs ES |
| Henalla 1989124 | PFMT vs ES vs NT |
| Jeyaseelan 2000131 | ES vs NT |
| Johnson 2001167 | PFMT (maximal contraction) + BF vs PFMT (submaximal contraction) + BF |
| Knight 1998172 | PFMT + BF vs PFMT + BF + ES (maximal intensity) vs PFMT+BF + ES (low intensity) |
| Konstantinidou 2007116 | PFMT vs PFMT with additional sessions |
| Laycock 2001152 | PFMT vs PFMT + BF vs VC |
| Laycock Trial 2 1993132 | ES vs NT |
| Mørkved 2002153 | PFMT vs PFMT + BF |
| Pages 2001154 | PFMT vs PFMT + BF |
| Pohl 2004171 | PFMT + BF vs PFMT + ES |
| Ramsay 1990128 | PFMT vs NT |
| Sand 1995134 | ES vs NT |
| Savage 2005166 | PFMT vs modified pilates |
| Shepherd 1983155 | PFMT vs PFMT + BF |
| Williams 2006129 | PFMT vs VC vs NT |
| Wilson 1987157 | PFMT vs PFMT + BF vs PFMT + BF + ES (faradism) vs PFMT + BF + ES (IFT) |
| Wise 1993188 | ES vs VC vs PFMT + VC |
| Wong 1997b160 | PFMT vs PFMT with additional sessions |
| Wong 2001169 | PFMT + BF (vaginal) vs PFMT + BF (vaginal and abdominal) |
| Wyman 1998183 | PFMT + BF vs BT vs PFMT + BF + BT |
| Treatment adherence | |
| Aukee 2002146 | PFMT vs PFMT + BF |
| Bidmead 2002121 | PFMT vs PFMT + sham ES vs PFMT + ES vs NT |
| Blowman 1991189 | PFMT vs PFMT + ES |
| Bø 1990159 | PFMT vs PFMT with additional sessions |
| Bø 1999115 | PFMT vs ES vs VC vs NT |
| Borello-France 2006165 | PFMT in supine position + BF vs PFMT in supine and upright positions + BF |
| Brubaker 1997130 | ES vs NT |
| Gallo 1997162 | PFMT vs PFMT with audiocassette |
| Ghoniem 200557 | PFMT vs SNRI vs PFMT + SNRI vs NT |
| Glavind 1996150 | PFMT vs PFMT + BF |
| Jeyaseelan 2000131 | ES vs NT |
| Kim 2007118 | PFMT vs NT |
| Knight 1998172 | PFMT + BF vs PFMT + BF + ES (maximal intensity) vs PFMT + BF + ES (low intensity) |
| Lagro-Janssen 1991127 | PFMT vs NT |
| Laycock 2001152 | PFMT vs PFMT + BF vs VC |
| Mørkved 2002153 | PFMT vs PFMT + BF |
| Norton 2002144 | SNRI 80 mg vs 40 mg vs 20 mg vs placebo |
| Peattie 1988180 | PFMT vs VC |
| Ramsay 1990128 | PFMT vs NT |
| Sand 1995134 | ES vs NT |
| Williams 2006129 | PFMT vs VC vs NT |
| Wyman 1998183 | PFMT vs VC vs NT |
Appendix 22 Mixed-treatment comparison model
The winbugs code for the multiarm random effects mixed-treatment comparison model is shown below. It is taken from the website of the Multi-parameter Evidence Synthesis Research Group from the Department of Community Based Medicine at the University of Bristol. 114,202 The code has been adapted to allow the calculation of the log odds ratios and the odds ratios in both directions.
Choice of prior distributions
Three sets of parameters: the log odds ratios of treatment to no treatment, the individual trial baselines and the random effects standard deviation require a prior distribution to be applied to them. The prior distributions shown before are those given in the code from the MPES programme website. 202 These are vague priors, i.e. they are designed to express little information about the parameter. The MPES programme provides advise to users to be considered over the choice of prior for the random effects standard deviation parameter. Several alternatives for its prior distributions were explored, based on suggestions in Lambert and colleagues407 on both the cure and improvement models (PFMT split by number of sessions only). The difference between the estimates from these alternative priors to those from the prior reported above were small enough to be considered irrelevant.
Data for cure, with PFMT split
Data for cure, with PFMT combined
Data for improvement, with PFMT split
Data for improvement, with PFMT combined
Model of ‘no-treatment’ arms
The winbugs model used to estimate the distribution of the absolute cure rate for no treatment is given below. It uses the no-treatment trial arms and applies a random effects model. The posterior mean and standard deviation of the ‘logitP’ term were used as the values of the parameters of the normal distribution applied to the ‘mA’ term in the main model.
Appendix 23 Mixed treatment comparisons: tables of odds ratios
These tables show the posterior distribution of the odds ratios between each pair of treatments, displaying the mean, SD, median and the 2.5th and 97.5th percentiles. As stated in Chapter 8 (see Results), the median should be used as the point estimate, in preference to the mean. Intervention 1 is being compared to intervention 2, so an odds ratio greater than one implies that intervention 1 is better than intervention 2.
| Intervention 1 | Intervention 2 | Mean | SD | Median | 2.5% | 97.5% |
|---|---|---|---|---|---|---|
| PFMT basic | NT | 1.4 | 0.627 | 1.28 | 0.554 | 2.92 |
| PFMT extra sessions | NT | 12 | 5.7 | 10.7 | 5.03 | 26.2 |
| PFMT + BF | NT | 14 | 7.38 | 12.3 | 5.35 | 32.7 |
| ES | NT | 1.64 | 0.88 | 1.45 | 0.55 | 3.86 |
| VC | NT | 4.18 | 2.62 | 3.55 | 1.23 | 10.9 |
| SNRI | NT | 1.57 | 0.771 | 1.43 | 0.582 | 3.46 |
| BT | NT | 9.34 | 7.17 | 7.53 | 2.34 | 27 |
| PFMT + ES | NT | 3.78 | 2.84 | 3.05 | 1.09 | 10.7 |
| PFMT + ES + BF | NT | 30.7 | 182 | 9.21 | 0.569 | 172 |
| PFMT + VC | NT | 6.89 | 18 | 3.13 | 0.324 | 36 |
| PFMT + VC + BF | NT | 86.4 | 2750 | 5.82 | 0.245 | 263 |
| PFMT + BT + BF | NT | 37.8 | 55.1 | 25.2 | 4.94 | 146 |
| NT | PFMT basic | 0.855 | 0.387 | 0.782 | 0.342 | 1.81 |
| PFMT extra sessions | PFMT basic | 9.47 | 4.82 | 8.36 | 3.74 | 21.7 |
| PFMT + BF | PFMT basic | 11 | 5.89 | 9.63 | 4.12 | 25.9 |
| ES | PFMT basic | 1.32 | 0.793 | 1.13 | 0.393 | 3.32 |
| VC | PFMT basic | 3.26 | 2.07 | 2.77 | 0.982 | 8.51 |
| SNRI | PFMT basic | 1.35 | 1.02 | 1.11 | 0.333 | 3.8 |
| BT | PFMT basic | 7.49 | 6.31 | 5.9 | 1.7 | 23.1 |
| PFMT + ES | PFMT basic | 2.95 | 2.21 | 2.37 | 0.859 | 8.49 |
| PFMT + ES + BF | PFMT basic | 22.1 | 151 | 7.16 | 0.502 | 121 |
| PFMT + VC | PFMT basic | 4.91 | 11.1 | 2.43 | 0.3 | 24.4 |
| PFMT + VC + BF | PFMT basic | 71.2 | 2300 | 4.55 | 0.19 | 213 |
| PFMT + BT + BF | PFMT basic | 30.2 | 49.8 | 19.6 | 3.79 | 121 |
| NT | PFMT extra sessions | 0.0997 | 0.0417 | 0.0933 | 0.0381 | 0.199 |
| PFMT basic | PFMT extra sessions | 0.129 | 0.0577 | 0.12 | 0.0462 | 0.268 |
| PFMT + BF | PFMT extra sessions | 1.22 | 0.421 | 1.15 | 0.592 | 2.22 |
| ES | PFMT extra sessions | 0.153 | 0.0863 | 0.135 | 0.0448 | 0.368 |
| VC | PFMT extra sessions | 0.383 | 0.228 | 0.332 | 0.105 | 0.959 |
| SNRI | PFMT extra sessions | 0.156 | 0.103 | 0.134 | 0.036 | 0.41 |
| BT | PFMT extra sessions | 0.819 | 0.517 | 0.709 | 0.223 | 2.08 |
| PFMT + ES | PFMT extra sessions | 0.339 | 0.227 | 0.285 | 0.0984 | 0.895 |
| PFMT + ES + BF | PFMT extra sessions | 2.72 | 16.3 | 0.858 | 0.0489 | 15.3 |
| PFMT + VC | PFMT extra sessions | 0.621 | 1.42 | 0.29 | 0.0281 | 3.24 |
| PFMT + VC + BF | PFMT extra sessions | 7.5 | 214 | 0.535 | 0.0217 | 24.1 |
| PFMT + BT + BF | PFMT extra sessions | 3.25 | 3.75 | 2.37 | 0.468 | 11.4 |
| NT | PFMT + BF | 0.0882 | 0.041 | 0.0813 | 0.0306 | 0.187 |
| PFMT basic | PFMT + BF | 0.113 | 0.0538 | 0.104 | 0.0386 | 0.243 |
| PFMT extra sessions | PFMT + BF | 0.92 | 0.324 | 0.867 | 0.45 | 1.69 |
| ES | PFMT + BF | 0.136 | 0.0843 | 0.117 | 0.0362 | 0.348 |
| VC | PFMT + BF | 0.333 | 0.201 | 0.288 | 0.0913 | 0.841 |
| SNRI | PFMT + BF | 0.138 | 0.0979 | 0.117 | 0.03 | 0.379 |
| BT | PFMT + BF | 0.715 | 0.458 | 0.617 | 0.193 | 1.86 |
| PFMT + ES | PFMT + BF | 0.304 | 0.228 | 0.247 | 0.0789 | 0.868 |
| PFMT + ES + BF | PFMT + BF | 2.47 | 18.2 | 0.745 | 0.0417 | 13.5 |
| PFMT + VC | PFMT + BF | 0.549 | 1.31 | 0.252 | 0.0243 | 2.87 |
| PFMT + VC + BF | PFMT + BF | 7.13 | 253 | 0.467 | 0.0186 | 21.4 |
| PFMT + BT + BF | PFMT + BF | 2.75 | 3 | 2.06 | 0.439 | 9.33 |
| NT | ES | 0.777 | 0.413 | 0.689 | 0.259 | 1.82 |
| PFMT basic | ES | 1.02 | 0.599 | 0.884 | 0.301 | 2.55 |
| PFMT extra sessions | ES | 8.71 | 5.37 | 7.43 | 2.72 | 22.3 |
| PFMT + BF | ES | 10.2 | 6.83 | 8.55 | 2.88 | 27.6 |
| VC | ES | 2.96 | 2.05 | 2.47 | 0.759 | 8.2 |
| SNRI | ES | 1.22 | 0.938 | 0.986 | 0.26 | 3.61 |
| BT | ES | 6.9 | 6.32 | 5.22 | 1.27 | 22.6 |
| PFMT + ES | ES | 2.76 | 2.44 | 2.1 | 0.624 | 8.8 |
| PFMT + ES + BF | ES | 22.6 | 171 | 6.35 | 0.36 | 127 |
| PFMT + VC | ES | 4.98 | 13.6 | 2.16 | 0.207 | 27.1 |
| PFMT + VC + BF | ES | 52.2 | 1750 | 4.01 | 0.2 | 162 |
| PFMT + BT + BF | ES | 27.8 | 45.8 | 17.4 | 2.89 | 114 |
| NT | VC | 0.324 | 0.196 | 0.281 | 0.0922 | 0.811 |
| PFMT basic | VC | 0.413 | 0.243 | 0.361 | 0.118 | 1.02 |
| PFMT extra sessions | VC | 3.58 | 2.44 | 3.02 | 1.04 | 9.53 |
| PFMT + BF | VC | 4.12 | 2.72 | 3.47 | 1.19 | 11 |
| ES | VC | 0.486 | 0.334 | 0.406 | 0.122 | 1.32 |
| SNRI | VC | 0.51 | 0.439 | 0.402 | 0.0962 | 1.57 |
| BT | VC | 2.82 | 2.78 | 2.12 | 0.507 | 9.32 |
| PFMT + ES | VC | 1.15 | 1.19 | 0.856 | 0.233 | 3.8 |
| PFMT + ES + BF | VC | 9.36 | 89.2 | 2.57 | 0.143 | 51.5 |
| PFMT + VC | VC | 2.04 | 5.84 | 0.87 | 0.0817 | 11 |
| PFMT + VC + BF | VC | 27 | 930 | 1.63 | 0.064 | 77.5 |
| PFMT + BT + BF | VC | 11.3 | 21.4 | 7.11 | 1.18 | 45.9 |
| NT | SNRI | 0.775 | 0.391 | 0.699 | 0.29 | 1.72 |
| PFMT basic | SNRI | 1.08 | 0.783 | 0.901 | 0.263 | 3.01 |
| PFMT extra sessions | SNRI | 9.35 | 7.74 | 7.44 | 2.44 | 27.8 |
| PFMT + BF | SNRI | 10.9 | 9.34 | 8.58 | 2.64 | 33.4 |
| ES | SNRI | 1.28 | 1.08 | 1.01 | 0.277 | 3.85 |
| VC | SNRI | 3.25 | 3.07 | 2.49 | 0.636 | 10.4 |
| BT | SNRI | 7.28 | 8.04 | 5.27 | 1.23 | 25.4 |
| PFMT + ES | SNRI | 2.98 | 3.94 | 2.1 | 0.591 | 10.4 |
| PFMT + ES + BF | SNRI | 24.6 | 174 | 6.44 | 0.344 | 140 |
| PFMT + VC | SNRI | 5.41 | 15.8 | 2.19 | 0.197 | 29.9 |
| PFMT + VC + BF | SNRI | 74 | 3220 | 4.09 | 0.154 | 201 |
| PFMT + BT + BF | SNRI | 29.8 | 62 | 17.6 | 2.79 | 131 |
| NT | BT | 0.158 | 0.108 | 0.133 | 0.0371 | 0.428 |
| PFMT basic | BT | 0.207 | 0.153 | 0.17 | 0.0433 | 0.589 |
| PFMT extra sessions | BT | 1.68 | 1.13 | 1.41 | 0.481 | 4.48 |
| PFMT + BF | BT | 1.94 | 1.32 | 1.62 | 0.539 | 5.18 |
| ES | BT | 0.247 | 0.213 | 0.191 | 0.0443 | 0.788 |
| VC | BT | 0.614 | 0.564 | 0.471 | 0.107 | 1.97 |
| SNRI | BT | 0.248 | 0.238 | 0.19 | 0.0394 | 0.811 |
| PFMT + ES | BT | 0.562 | 0.675 | 0.401 | 0.0968 | 1.98 |
| PFMT + ES + BF | BT | 4.75 | 47.4 | 1.21 | 0.0601 | 26.1 |
| PFMT + VC | BT | 1.02 | 3.27 | 0.414 | 0.0327 | 5.57 |
| PFMT + VC + BF | BT | 13 | 482 | 0.768 | 0.0262 | 39.4 |
| PFMT + BT + BF | BT | 4.54 | 5.41 | 3.35 | 0.74 | 15.5 |
| NT | PFMT + ES | 0.373 | 0.219 | 0.328 | 0.0932 | 0.92 |
| PFMT basic | PFMT + ES | 0.477 | 0.277 | 0.422 | 0.118 | 1.16 |
| PFMT extra sessions | PFMT + ES | 4.05 | 2.43 | 3.51 | 1.12 | 10.2 |
| PFMT + BF | PFMT + ES | 4.78 | 3.16 | 4.05 | 1.15 | 12.7 |
| ES | PFMT + ES | 0.573 | 0.408 | 0.476 | 0.114 | 1.6 |
| VC | PFMT + ES | 1.45 | 1.13 | 1.17 | 0.263 | 4.29 |
| SNRI | PFMT + ES | 0.582 | 0.461 | 0.475 | 0.096 | 1.69 |
| BT | PFMT + ES | 3.22 | 2.86 | 2.49 | 0.504 | 10.3 |
| PFMT + ES + BF | PFMT + ES | 9.64 | 45.9 | 3.03 | 0.148 | 55 |
| PFMT + VC | PFMT + ES | 2.26 | 5.56 | 1.02 | 0.0835 | 11.9 |
| PFMT + VC + BF | PFMT + ES | 24.6 | 552 | 1.87 | 0.0656 | 91.2 |
| PFMT + BT + BF | PFMT + ES | 12.7 | 17.4 | 8.37 | 1.16 | 50.2 |
| NT | PFMT + ES + BF | 0.336 | 4.37 | 0.109 | 0.00583 | 1.76 |
| PFMT basic | PFMT + ES + BF | 0.393 | 6.51 | 0.14 | 0.00829 | 1.99 |
| PFMT extra sessions | PFMT + ES + BF | 4.09 | 130 | 1.17 | 0.0656 | 20.5 |
| PFMT + BF | PFMT + ES + BF | 4.98 | 204 | 1.34 | 0.0739 | 24 |
| ES | PFMT + ES + BF | 0.53 | 10.3 | 0.158 | 0.0079 | 2.78 |
| VC | PFMT + ES + BF | 1.33 | 22.8 | 0.389 | 0.0194 | 7.01 |
| SNRI | PFMT + ES + BF | 0.54 | 9.06 | 0.155 | 0.00715 | 2.91 |
| BT | PFMT + ES + BF | 2.97 | 27.1 | 0.827 | 0.0384 | 16.7 |
| PFMT + ES | PFMT + ES + BF | 1.43 | 66.5 | 0.33 | 0.0182 | 6.78 |
| PFMT + VC | PFMT + ES + BF | 0.487 | 0.668 | 0.341 | 0.0647 | 1.77 |
| PFMT + VC + BF | PFMT + ES + BF | 33.4 | 2240 | 0.633 | 0.00919 | 69 |
| PFMT + BT + BF | PFMT + ES + BF | 12.9 | 283 | 2.75 | 0.107 | 70 |
| NT | PFMT + VC | 0.634 | 1.59 | 0.32 | 0.0278 | 3.09 |
| PFMT basic | PFMT + VC | 0.735 | 1.73 | 0.412 | 0.041 | 3.34 |
| PFMT extra sessions | PFMT + VC | 7.16 | 26.9 | 3.45 | 0.309 | 35.6 |
| PFMT + BF | PFMT + VC | 8.41 | 40.1 | 3.96 | 0.349 | 41.2 |
| ES | PFMT + VC | 0.973 | 2.79 | 0.464 | 0.0369 | 4.84 |
| VC | PFMT + VC | 2.42 | 6.67 | 1.15 | 0.0912 | 12.2 |
| SNRI | PFMT + VC | 1.01 | 3.26 | 0.457 | 0.0334 | 5.08 |
| BT | PFMT + VC | 5.64 | 16.5 | 2.42 | 0.179 | 30.6 |
| PFMT + ES | PFMT + VC | 2.28 | 12.6 | 0.982 | 0.0838 | 12 |
| PFMT + ES + BF | PFMT + VC | 4.21 | 6.97 | 2.93 | 0.565 | 15.5 |
| PFMT + VC + BF | PFMT + VC | 67.7 | 4860 | 1.86 | 0.0369 | 150 |
| PFMT + BT + BF | PFMT + VC | 23.4 | 94.7 | 8.04 | 0.486 | 129 |
| NT | PFMT + VC + BF | 0.661 | 3.6 | 0.172 | 0.0038 | 4.09 |
| PFMT basic | PFMT + VC + BF | 0.865 | 4.51 | 0.22 | 0.00469 | 5.27 |
| PFMT extra sessions | PFMT + VC + BF | 7.72 | 80.9 | 1.87 | 0.0415 | 46.2 |
| PFMT + BF | PFMT + VC + BF | 9.31 | 173 | 2.14 | 0.0467 | 53.7 |
| ES | PFMT + VC + BF | 0.904 | 15.5 | 0.249 | 0.00619 | 5.01 |
| VC | PFMT + VC + BF | 2.58 | 20.3 | 0.615 | 0.0129 | 15.6 |
| SNRI | PFMT + VC + BF | 1.08 | 12.6 | 0.244 | 0.00497 | 6.49 |
| BT | PFMT + VC + BF | 6.07 | 47.7 | 1.3 | 0.0254 | 38.2 |
| PFMT + ES | PFMT + VC + BF | 2.47 | 16.8 | 0.534 | 0.011 | 15.3 |
| PFMT + ES + BF | PFMT + VC + BF | 19.5 | 324 | 1.58 | 0.0145 | 109 |
| PFMT + VC | PFMT + VC + BF | 4.37 | 37.5 | 0.539 | 0.00667 | 27.1 |
| PFMT + BT + BF | PFMT + VC + BF | 25.2 | 255 | 4.36 | 0.0721 | 154 |
| NT | PFMT + BT + BF | 0.0558 | 0.0672 | 0.0397 | 0.00685 | 0.203 |
| PFMT basic | PFMT + BT + BF | 0.0724 | 0.091 | 0.051 | 0.00825 | 0.264 |
| PFMT extra sessions | PFMT + BT + BF | 0.594 | 0.763 | 0.422 | 0.0879 | 2.14 |
| PFMT + BF | PFMT + BT + BF | 0.665 | 0.825 | 0.487 | 0.107 | 2.28 |
| ES | PFMT + BT + BF | 0.0874 | 0.141 | 0.0574 | 0.00877 | 0.347 |
| VC | PFMT + BT + BF | 0.215 | 0.307 | 0.141 | 0.0218 | 0.851 |
| SNRI | PFMT + BT + BF | 0.0886 | 0.147 | 0.0568 | 0.00765 | 0.359 |
| BT | PFMT + BT + BF | 0.401 | 0.425 | 0.299 | 0.0646 | 1.35 |
| PFMT + ES | PFMT + BT + BF | 0.202 | 0.425 | 0.12 | 0.0199 | 0.862 |
| PFMT + ES + BF | PFMT + BT + BF | 1.69 | 18.8 | 0.363 | 0.0143 | 9.36 |
| PFMT + VC | PFMT + BT + BF | 0.362 | 1.58 | 0.124 | 0.00773 | 2.06 |
| PFMT + VC + BF | PFMT + BT + BF | 5.83 | 317 | 0.23 | 0.00651 | 13.9 |
| Intervention 1 | Intervention 2 | Mean | SD | Median | 2.5% | 97.5% |
|---|---|---|---|---|---|---|
| PFMT basic | NT | 4.97 | 2.37 | 4.47 | 2.03 | 10.9 |
| PFMT extra sessions | NT | 29.8 | 17 | 25.7 | 10.3 | 73.1 |
| PFMT + BF | NT | 31 | 21.8 | 25.4 | 8.68 | 86.9 |
| ES | NT | 6.14 | 3 | 5.49 | 2.39 | 13.7 |
| VC | NT | 7.86 | 4.65 | 6.77 | 2.6 | 19.4 |
| SNRI | NT | 2.29 | 0.87 | 2.14 | 1.06 | 4.4 |
| BT | NT | 18.3 | 23.4 | 12 | 2.16 | 73.3 |
| PFMT + ES | NT | 29.6 | 31 | 20.7 | 4.51 | 108 |
| PFMT + ES + BF | NT | 31.2 | 35.3 | 21.6 | 4.5 | 116 |
| PFMT + VC | NT | 21.7 | 35.6 | 12.2 | 1.83 | 99.2 |
| PFMT + VC + BF | NT | 7.44 | 28.4 | 2.66 | 0.181 | 42 |
| PFMT + BT + BF | NT | 160 | 401 | 69.8 | 6.59 | 852 |
| PFMT + SNRI | NT | 7.37 | 11.8 | 4.42 | 0.646 | 31.8 |
| NT | PFMT basic | 0.241 | 0.104 | 0.224 | 0.0916 | 0.493 |
| PFMT extra sessions | PFMT basic | 6.62 | 3.8 | 5.75 | 2.11 | 16.2 |
| PFMT + BF | PFMT basic | 6.78 | 4.5 | 5.68 | 1.88 | 18.3 |
| ES | PFMT basic | 1.39 | 0.748 | 1.23 | 0.454 | 3.25 |
| VC | PFMT basic | 1.71 | 0.904 | 1.52 | 0.581 | 3.97 |
| SNRI | PFMT basic | 0.546 | 0.309 | 0.48 | 0.159 | 1.32 |
| BT | PFMT basic | 4.18 | 5.37 | 2.7 | 0.416 | 17 |
| PFMT + ES | PFMT basic | 6.62 | 7.05 | 4.62 | 0.915 | 24.4 |
| PFMT + ES + BF | PFMT basic | 6.65 | 6.52 | 4.86 | 1.02 | 23.3 |
| PFMT + VC | PFMT basic | 4.47 | 6.34 | 2.74 | 0.437 | 18.9 |
| PFMT + VC + BF | PFMT basic | 1.66 | 9.93 | 0.595 | 0.0375 | 9.26 |
| PFMT + BT + BF | PFMT basic | 35.3 | 91.3 | 15.6 | 1.36 | 186 |
| PFMT + SNRI | PFMT basic | 1.64 | 2.4 | 0.988 | 0.13 | 7.17 |
| NT | PFMT extra sessions | 0.0431 | 0.022 | 0.0389 | 0.0137 | 0.097 |
| PFMT basic | PFMT extra sessions | 0.197 | 0.108 | 0.174 | 0.0617 | 0.473 |
| PFMT + BF | PFMT extra sessions | 1.17 | 0.769 | 0.988 | 0.316 | 3.11 |
| ES | PFMT extra sessions | 0.241 | 0.132 | 0.214 | 0.0758 | 0.571 |
| VC | PFMT extra sessions | 0.304 | 0.181 | 0.264 | 0.0873 | 0.761 |
| SNRI | PFMT extra sessions | 0.0978 | 0.0629 | 0.0835 | 0.0236 | 0.255 |
| BT | PFMT extra sessions | 0.73 | 0.923 | 0.469 | 0.069 | 2.99 |
| PFMT + ES | PFMT extra sessions | 1.09 | 1.02 | 0.806 | 0.173 | 3.67 |
| PFMT + ES + BF | PFMT extra sessions | 1.19 | 1.31 | 0.842 | 0.159 | 4.34 |
| PFMT + VC | PFMT extra sessions | 0.832 | 1.34 | 0.475 | 0.0627 | 3.82 |
| PFMT + VC + BF | PFMT extra sessions | 0.282 | 0.936 | 0.104 | 0.00632 | 1.61 |
| PFMT + BT + BF | PFMT extra sessions | 6.1 | 14.6 | 2.71 | 0.227 | 32.5 |
| PFMT + SNRI | PFMT extra sessions | 0.303 | 0.49 | 0.172 | 0.0197 | 1.37 |
| NT | PFMT + BF | 0.0455 | 0.0277 | 0.0394 | 0.0115 | 0.115 |
| PFMT basic | PFMT + BF | 0.206 | 0.13 | 0.176 | 0.0548 | 0.531 |
| PFMT extra sessions | PFMT + BF | 1.2 | 0.775 | 1.01 | 0.322 | 3.16 |
| ES | PFMT + BF | 0.261 | 0.183 | 0.216 | 0.0592 | 0.731 |
| VC | PFMT + BF | 0.317 | 0.211 | 0.266 | 0.0798 | 0.855 |
| SNRI | PFMT + BF | 0.103 | 0.0762 | 0.0845 | 0.0207 | 0.297 |
| BT | PFMT + BF | 0.695 | 0.799 | 0.475 | 0.0798 | 2.63 |
| PFMT + ES | PFMT + BF | 1.23 | 1.47 | 0.814 | 0.133 | 4.82 |
| PFMT + ES + BF | PFMT + BF | 1.12 | 1.02 | 0.849 | 0.192 | 3.68 |
| PFMT + VC | PFMT + BF | 0.874 | 1.45 | 0.479 | 0.0599 | 4.04 |
| PFMT + VC + BF | PFMT + BF | 0.305 | 1.13 | 0.105 | 0.0059 | 1.75 |
| PFMT + BT + BF | PFMT + BF | 5.46 | 10.9 | 2.73 | 0.277 | 26.9 |
| PFMT + SNRI | PFMT + BF | 0.319 | 0.556 | 0.174 | 0.0185 | 1.5 |
| NT | ES | 0.199 | 0.0909 | 0.182 | 0.0731 | 0.418 |
| PFMT basic | ES | 0.926 | 0.511 | 0.815 | 0.308 | 2.2 |
| PFMT extra sessions | ES | 5.4 | 3.08 | 4.68 | 1.75 | 13.2 |
| PFMT + BF | ES | 5.74 | 4.34 | 4.63 | 1.37 | 16.9 |
| VC | ES | 1.42 | 0.833 | 1.23 | 0.441 | 3.51 |
| SNRI | ES | 0.452 | 0.276 | 0.39 | 0.125 | 1.15 |
| BT | ES | 3.5 | 4.72 | 2.19 | 0.329 | 14.6 |
| PFMT + ES | ES | 5.37 | 5.72 | 3.78 | 0.752 | 19.7 |
| PFMT + ES + BF | ES | 5.72 | 6.4 | 3.94 | 0.745 | 21.5 |
| PFMT + VC | ES | 3.84 | 6.34 | 2.23 | 0.326 | 16.9 |
| PFMT + VC + BF | ES | 1.17 | 3.72 | 0.483 | 0.0375 | 6.27 |
| PFMT + BT + BF | ES | 29.9 | 78.5 | 12.6 | 1.06 | 159 |
| PFMT + SNRI | ES | 1.42 | 2.48 | 0.806 | 0.0964 | 6.41 |
| NT | VC | 0.166 | 0.088 | 0.148 | 0.0515 | 0.384 |
| PFMT basic | VC | 0.743 | 0.392 | 0.66 | 0.252 | 1.72 |
| PFMT extra sessions | VC | 4.45 | 2.77 | 3.79 | 1.32 | 11.5 |
| PFMT + BF | VC | 4.54 | 3.18 | 3.75 | 1.17 | 12.5 |
| ES | VC | 0.928 | 0.528 | 0.811 | 0.285 | 2.27 |
| SNRI | VC | 0.376 | 0.252 | 0.317 | 0.0896 | 1.01 |
| BT | VC | 2.83 | 3.81 | 1.78 | 0.263 | 11.8 |
| PFMT + ES | VC | 4.52 | 5.2 | 3.05 | 0.553 | 17.3 |
| PFMT + ES + BF | VC | 4.38 | 4.32 | 3.19 | 0.682 | 15.1 |
| PFMT + VC | VC | 3.07 | 4.73 | 1.8 | 0.264 | 13.6 |
| PFMT + VC + BF | VC | 1.09 | 4.23 | 0.393 | 0.0244 | 6.12 |
| PFMT + BT + BF | VC | 23.7 | 58.6 | 10.3 | 0.862 | 126 |
| PFMT + SNRI | VC | 1.16 | 2.01 | 0.652 | 0.0761 | 5.28 |
| NT | SNRI | 0.497 | 0.186 | 0.467 | 0.227 | 0.943 |
| PFMT basic | SNRI | 2.45 | 1.56 | 2.09 | 0.76 | 6.29 |
| PFMT extra sessions | SNRI | 14.8 | 10.8 | 12 | 3.92 | 42.4 |
| PFMT + BF | SNRI | 15.4 | 13.3 | 11.8 | 3.37 | 48.4 |
| ES | SNRI | 3.04 | 1.99 | 2.57 | 0.871 | 8 |
| VC | SNRI | 3.89 | 2.93 | 3.16 | 0.987 | 11.2 |
| BT | SNRI | 9.09 | 12.9 | 5.59 | 0.887 | 38.7 |
| PFMT + ES | SNRI | 14.7 | 19.3 | 9.66 | 1.82 | 58.2 |
| PFMT + ES + BF | SNRI | 15.5 | 20 | 10.1 | 1.84 | 61.8 |
| PFMT + VC | SNRI | 10.7 | 19.4 | 5.69 | 0.754 | 51.2 |
| PFMT + VC + BF | SNRI | 3.69 | 14.5 | 1.24 | 0.0781 | 21.1 |
| PFMT + BT + BF | SNRI | 79.4 | 214 | 32.5 | 2.79 | 438 |
| PFMT + SNRI | SNRI | 3.49 | 5.8 | 2.06 | 0.289 | 15.3 |
| NT | BT | 0.121 | 0.14 | 0.0833 | 0.0137 | 0.464 |
| PFMT basic | BT | 0.583 | 0.803 | 0.371 | 0.059 | 2.41 |
| PFMT extra sessions | BT | 3.45 | 5.07 | 2.13 | 0.335 | 14.5 |
| PFMT + BF | BT | 3.19 | 3.96 | 2.11 | 0.38 | 12.5 |
| ES | BT | 0.726 | 0.97 | 0.456 | 0.0683 | 3.04 |
| VC | BT | 0.908 | 1.35 | 0.562 | 0.085 | 3.81 |
| SNRI | BT | 0.277 | 0.361 | 0.179 | 0.0258 | 1.13 |
| PFMT + ES | BT | 3.49 | 7.74 | 1.72 | 0.177 | 17.4 |
| PFMT + ES + BF | BT | 3.41 | 6.52 | 1.8 | 0.203 | 16.1 |
| PFMT + VC | BT | 2.54 | 7.02 | 1.01 | 0.0827 | 14.3 |
| PFMT + VC + BF | BT | 0.89 | 4.94 | 0.22 | 0.00909 | 5.52 |
| PFMT + BT + BF | BT | 11.9 | 28.8 | 5.77 | 0.602 | 59.6 |
| PFMT + SNRI | BT | 0.896 | 2.64 | 0.366 | 0.027 | 4.83 |
| NT | PFMT + ES | 0.0653 | 0.061 | 0.0484 | 0.00926 | 0.222 |
| PFMT basic | PFMT + ES | 0.303 | 0.309 | 0.217 | 0.0411 | 1.09 |
| PFMT extra sessions | PFMT + ES | 1.69 | 1.62 | 1.24 | 0.273 | 5.79 |
| PFMT + BF | PFMT + ES | 1.88 | 2.3 | 1.23 | 0.207 | 7.51 |
| ES | PFMT + ES | 0.369 | 0.369 | 0.265 | 0.0507 | 1.33 |
| VC | PFMT + ES | 0.478 | 0.54 | 0.328 | 0.0579 | 1.81 |
| SNRI | PFMT + ES | 0.149 | 0.163 | 0.104 | 0.0172 | 0.549 |
| BT | PFMT + ES | 1.14 | 2.04 | 0.582 | 0.0574 | 5.65 |
| PFMT + ES + BF | PFMT + ES | 1.9 | 3.48 | 1.05 | 0.123 | 8.78 |
| PFMT + VC | PFMT + ES | 1.31 | 3.22 | 0.59 | 0.0544 | 6.88 |
| PFMT + VC + BF | PFMT + ES | 0.44 | 1.96 | 0.127 | 0.00606 | 2.67 |
| PFMT + BT + BF | PFMT + ES | 9.77 | 30.7 | 3.36 | 0.2 | 56.5 |
| PFMT + SNRI | PFMT + ES | 0.467 | 1.04 | 0.214 | 0.0173 | 2.45 |
| NT | PFMT + ES + BF | 0.0635 | 0.063 | 0.0462 | 0.00866 | 0.222 |
| PFMT basic | PFMT + ES + BF | 0.283 | 0.28 | 0.206 | 0.0429 | 0.98 |
| PFMT extra sessions | PFMT + ES + BF | 1.71 | 1.94 | 1.19 | 0.231 | 6.3 |
| PFMT + BF | PFMT + ES + BF | 1.58 | 1.53 | 1.18 | 0.272 | 5.21 |
| ES | PFMT + ES + BF | 0.364 | 0.406 | 0.254 | 0.0466 | 1.34 |
| VC | PFMT + ES + BF | 0.428 | 0.428 | 0.314 | 0.0662 | 1.47 |
| SNRI | PFMT + ES + BF | 0.144 | 0.161 | 0.0994 | 0.0162 | 0.544 |
| BT | PFMT + ES + BF | 1.04 | 1.81 | 0.557 | 0.062 | 4.92 |
| PFMT + ES | PFMT + ES + BF | 1.75 | 2.87 | 0.957 | 0.114 | 8.13 |
| PFMT + VC | PFMT + ES + BF | 1.21 | 2.83 | 0.565 | 0.0546 | 6.27 |
| PFMT + VC + BF | PFMT + ES + BF | 0.435 | 2.15 | 0.123 | 0.00567 | 2.63 |
| PFMT + BT + BF | PFMT + ES + BF | 8.4 | 23.4 | 3.21 | 0.222 | 47.1 |
| PFMT + SNRI | PFMT + ES + BF | 0.449 | 1.03 | 0.205 | 0.0167 | 2.33 |
| NT | PFMT + VC | 0.131 | 0.168 | 0.0819 | 0.0101 | 0.547 |
| PFMT basic | PFMT + VC | 0.567 | 0.681 | 0.364 | 0.0529 | 2.29 |
| PFMT extra sessions | PFMT + VC | 3.56 | 5 | 2.11 | 0.262 | 16 |
| PFMT + BF | PFMT + VC | 3.68 | 5.75 | 2.09 | 0.247 | 16.7 |
| ES | PFMT + VC | 0.727 | 0.959 | 0.449 | 0.0591 | 3.07 |
| VC | PFMT + VC | 0.892 | 1.16 | 0.556 | 0.0734 | 3.79 |
| SNRI | PFMT + VC | 0.298 | 0.429 | 0.176 | 0.0195 | 1.33 |
| BT | PFMT + VC | 2.29 | 5.84 | 0.987 | 0.0701 | 12.1 |
| PFMT + ES | PFMT + VC | 3.58 | 7.01 | 1.7 | 0.146 | 18.4 |
| PFMT + ES + BF | PFMT + VC | 3.62 | 7.1 | 1.77 | 0.159 | 18.3 |
| PFMT + VC + BF | PFMT + VC | 0.857 | 4.64 | 0.216 | 0.00808 | 5.42 |
| PFMT + BT + BF | PFMT + VC | 19.4 | 95.4 | 5.71 | 0.262 | 114 |
| PFMT + SNRI | PFMT + VC | 0.91 | 2.36 | 0.362 | 0.0227 | 5.15 |
| NT | PFMT + VC + BF | 0.978 | 2.89 | 0.376 | 0.0238 | 5.53 |
| PFMT basic | PFMT + VC + BF | 4.64 | 14 | 1.68 | 0.108 | 26.7 |
| PFMT extra sessions | PFMT + VC + BF | 27.6 | 92.2 | 9.66 | 0.62 | 158 |
| PFMT + BF | PFMT + VC + BF | 29.3 | 102 | 9.54 | 0.572 | 170 |
| ES | PFMT + VC + BF | 4.99 | 13.7 | 2.07 | 0.16 | 26.7 |
| VC | PFMT + VC + BF | 7.18 | 23.6 | 2.54 | 0.163 | 41 |
| SNRI | PFMT + VC + BF | 2.25 | 7.39 | 0.805 | 0.0475 | 12.8 |
| BT | PFMT + VC + BF | 17.9 | 103 | 4.55 | 0.181 | 110 |
| PFMT + ES | PFMT + VC + BF | 27.4 | 126 | 7.87 | 0.375 | 165 |
| PFMT + ES + BF | PFMT + VC + BF | 29 | 118 | 8.13 | 0.38 | 177 |
| PFMT + VC | PFMT + VC + BF | 20.4 | 161 | 4.63 | 0.184 | 124 |
| PFMT + BT + BF | PFMT + VC + BF | 156 | 1010 | 26.2 | 0.748 | 987 |
| PFMT + SNRI | PFMT + VC + BF | 7.24 | 39.1 | 1.66 | 0.0587 | 44.5 |
| NT | PFMT + BT + BF | 0.0302 | 0.0725 | 0.0143 | 0.00117 | 0.152 |
| PFMT basic | PFMT + BT + BF | 0.143 | 0.348 | 0.0641 | 0.00537 | 0.738 |
| PFMT extra sessions | PFMT + BT + BF | 0.848 | 2.67 | 0.369 | 0.0308 | 4.4 |
| PFMT + BF | PFMT + BT + BF | 0.737 | 1.71 | 0.366 | 0.0372 | 3.61 |
| ES | PFMT + BT + BF | 0.179 | 0.48 | 0.0791 | 0.00628 | 0.946 |
| VC | PFMT + BT + BF | 0.223 | 0.667 | 0.0971 | 0.00793 | 1.16 |
| SNRI | PFMT + BT + BF | 0.0687 | 0.17 | 0.0308 | 0.00228 | 0.359 |
| BT | PFMT + BT + BF | 0.34 | 0.659 | 0.174 | 0.0168 | 1.66 |
| PFMT + ES | PFMT + BT + BF | 0.868 | 3.5 | 0.298 | 0.0177 | 5.01 |
| PFMT + ES + BF | PFMT + BT + BF | 0.822 | 3.97 | 0.312 | 0.0213 | 4.51 |
| PFMT + VC | PFMT + BT + BF | 0.656 | 5 | 0.175 | 0.00875 | 3.82 |
| PFMT + VC + BF | PFMT + BT + BF | 0.219 | 1.58 | 0.0382 | 0.00101 | 1.34 |
| PFMT + SNRI | PFMT + BT + BF | 0.222 | 1.46 | 0.0634 | 0.00281 | 1.32 |
| NT | PFMT + SNRI | 0.366 | 0.517 | 0.226 | 0.0315 | 1.55 |
| PFMT basic | PFMT + SNRI | 1.73 | 2.71 | 1.01 | 0.14 | 7.67 |
| PFMT extra sessions | PFMT + SNRI | 10.8 | 19.1 | 5.82 | 0.729 | 50.7 |
| PFMT + BF | PFMT + SNRI | 11.3 | 24.8 | 5.75 | 0.668 | 54.2 |
| ES | PFMT + SNRI | 2.23 | 3.8 | 1.24 | 0.156 | 10.4 |
| VC | PFMT + SNRI | 2.82 | 5.08 | 1.53 | 0.189 | 13.1 |
| SNRI | PFMT + SNRI | 0.805 | 1.21 | 0.484 | 0.0653 | 3.46 |
| BT | PFMT + SNRI | 6.72 | 18.9 | 2.73 | 0.207 | 37.1 |
| PFMT + ES | PFMT + SNRI | 10.7 | 26.1 | 4.68 | 0.409 | 57.7 |
| PFMT + ES + BF | PFMT + SNRI | 11.3 | 28.9 | 4.89 | 0.43 | 60 |
| PFMT + VC | PFMT + SNRI | 7.89 | 45.7 | 2.76 | 0.194 | 44.1 |
| PFMT + VC + BF | PFMT + SNRI | 2.65 | 12.7 | 0.602 | 0.0225 | 17 |
| PFMT + BT + BF | PFMT + SNRI | 60.5 | 329 | 15.8 | 0.759 | 357 |
| Intervention 1 | Intervention 2 | Mean | SD | Median | 2.5% | 97.5% |
|---|---|---|---|---|---|---|
| PFMT | NT | 5.22 | 2.85 | 4.56 | 1.95 | 12.4 |
| PFMT + BF | NT | 11.8 | 8.56 | 9.65 | 3.37 | 33.3 |
| ES | NT | 1.98 | 1.42 | 1.63 | 0.506 | 5.54 |
| VC | NT | 6.25 | 5.58 | 4.75 | 1.24 | 20.2 |
| SNRI | NT | 1.77 | 1.47 | 1.42 | 0.377 | 5.35 |
| BT | NT | 7.06 | 8.43 | 4.87 | 1.05 | 26.1 |
| PFMT + ES | NT | 6.36 | 6.62 | 4.59 | 1.2 | 22.4 |
| PFMT + ES + BF | NT | 378 | 19,000 | 32.7 | 0.842 | 1540 |
| PFMT + VC | NT | 40.3 | 281 | 11.2 | 0.671 | 227 |
| PFMT + VC + BF | NT | 2610 | 372,000 | 6.61 | 0.175 | 453 |
| PFMT + BT + BF | NT | 40 | 125 | 17.7 | 1.83 | 206 |
| NT | PFMT | 0.239 | 0.113 | 0.219 | 0.0805 | 0.512 |
| PFMT + BF | PFMT | 2.32 | 1.07 | 2.11 | 0.936 | 4.99 |
| ES | PFMT | 0.418 | 0.268 | 0.355 | 0.108 | 1.1 |
| VC | PFMT | 1.27 | 0.953 | 1.04 | 0.285 | 3.65 |
| SNRI | PFMT | 0.42 | 0.422 | 0.313 | 0.0571 | 1.45 |
| BT | PFMT | 1.44 | 1.59 | 1.07 | 0.234 | 4.85 |
| PFMT + ES | PFMT | 1.27 | 1.09 | 1 | 0.288 | 3.9 |
| PFMT + ES + BF | PFMT | 71.7 | 6490 | 7.15 | 0.197 | 275 |
| PFMT + VC | PFMT | 7.3 | 41.5 | 2.44 | 0.161 | 40.7 |
| PFMT + VC + BF | PFMT | 351 | 44,500 | 1.43 | 0.0367 | 94.4 |
| PFMT + BT + BF | PFMT | 7.72 | 17.6 | 3.9 | 0.41 | 37.6 |
| NT | PFMT + BF | 0.119 | 0.0719 | 0.104 | 0.0301 | 0.297 |
| PFMT | PFMT + BF | 0.514 | 0.227 | 0.474 | 0.2 | 1.07 |
| ES | PFMT + BF | 0.211 | 0.166 | 0.168 | 0.0409 | 0.633 |
| VC | PFMT + BF | 0.624 | 0.52 | 0.493 | 0.117 | 1.89 |
| SNRI | PFMT + BF | 0.209 | 0.231 | 0.148 | 0.0232 | 0.763 |
| BT | PFMT + BF | 0.685 | 0.715 | 0.505 | 0.104 | 2.32 |
| PFMT + ES | PFMT + BF | 0.647 | 0.668 | 0.475 | 0.109 | 2.23 |
| PFMT + ES + BF | PFMT + BF | 33.2 | 2090 | 3.4 | 0.08 | 141 |
| PFMT + VC | PFMT + BF | 3.75 | 19.3 | 1.16 | 0.0667 | 20.8 |
| PFMT + VC + BF | PFMT + BF | 237 | 36,000 | 0.674 | 0.016 | 46.9 |
| PFMT + BT + BF | PFMT + BF | 3.45 | 7.06 | 1.85 | 0.202 | 16.3 |
| NT | ES | 0.731 | 0.489 | 0.615 | 0.181 | 1.98 |
| PFMT | ES | 3.38 | 2.29 | 2.82 | 0.911 | 9.3 |
| PFMT + BF | ES | 7.72 | 6.63 | 5.95 | 1.58 | 24.4 |
| VC | ES | 3.89 | 3.61 | 2.93 | 0.678 | 12.8 |
| SNRI | ES | 1.3 | 1.64 | 0.872 | 0.14 | 5 |
| BT | ES | 4.75 | 6.65 | 3.01 | 0.491 | 19.7 |
| PFMT + ES | ES | 4.14 | 5.12 | 2.82 | 0.598 | 15.5 |
| PFMT + ES + BF | ES | 206 | 7050 | 20.2 | 0.481 | 956 |
| PFMT + VC | ES | 25 | 133 | 6.89 | 0.373 | 144 |
| PFMT + VC + BF | ES | 810 | 104,000 | 4 | 0.126 | 232 |
| PFMT + BT + BF | ES | 25.9 | 79.8 | 10.9 | 0.954 | 138 |
| NT | VC | 0.266 | 0.219 | 0.211 | 0.0496 | 0.807 |
| PFMT | VC | 1.19 | 0.927 | 0.963 | 0.274 | 3.51 |
| PFMT + BF | VC | 2.65 | 2.4 | 2.03 | 0.528 | 8.53 |
| ES | VC | 0.451 | 0.415 | 0.341 | 0.0782 | 1.48 |
| SNRI | VC | 0.472 | 0.7 | 0.299 | 0.041 | 1.93 |
| BT | VC | 1.68 | 3.1 | 1.03 | 0.154 | 7.13 |
| PFMT + ES | VC | 1.5 | 2.04 | 0.966 | 0.177 | 6.03 |
| PFMT + ES + BF | VC | 116 | 15,500 | 6.86 | 0.154 | 341 |
| PFMT + VC | VC | 8.97 | 74.3 | 2.35 | 0.12 | 52.4 |
| PFMT + VC + BF | VC | 668 | 109,000 | 1.38 | 0.0323 | 102 |
| PFMT + BT + BF | VC | 9.06 | 29.8 | 3.75 | 0.304 | 48 |
| NT | SNRI | 0.883 | 0.727 | 0.706 | 0.187 | 2.66 |
| PFMT | SNRI | 4.68 | 5.73 | 3.2 | 0.69 | 17.5 |
| PFMT + BF | SNRI | 10.6 | 15.1 | 6.77 | 1.31 | 43.1 |
| ES | SNRI | 1.78 | 2.52 | 1.15 | 0.2 | 7.15 |
| VC | SNRI | 5.63 | 9.12 | 3.35 | 0.52 | 24.4 |
| BT | SNRI | 6.36 | 12.5 | 3.44 | 0.466 | 30.3 |
| PFMT + ES | SNRI | 5.81 | 10.7 | 3.21 | 0.514 | 26.8 |
| PFMT + ES + BF | SNRI | 357 | 17,700 | 23.1 | 0.469 | 1360 |
| PFMT + VC | SNRI | 37.8 | 308 | 7.9 | 0.355 | 215 |
| PFMT + VC + BF | SNRI | 2600 | 373,000 | 4.64 | 0.0988 | 395 |
| PFMT + BT + BF | SNRI | 37.3 | 159 | 12.5 | 0.922 | 206 |
| NT | BT | 0.278 | 0.275 | 0.205 | 0.0383 | 0.956 |
| PFMT | BT | 1.26 | 1.29 | 0.935 | 0.206 | 4.28 |
| PFMT + BF | BT | 2.73 | 2.91 | 1.98 | 0.431 | 9.62 |
| ES | BT | 0.512 | 0.689 | 0.332 | 0.0509 | 2.04 |
| VC | BT | 1.56 | 2.39 | 0.975 | 0.14 | 6.51 |
| SNRI | BT | 0.493 | 0.798 | 0.291 | 0.033 | 2.15 |
| PFMT + ES | BT | 1.6 | 2.67 | 0.935 | 0.143 | 7.07 |
| PFMT + ES + BF | BT | 85.2 | 4000 | 6.74 | 0.134 | 355 |
| PFMT + VC | BT | 9.58 | 63.4 | 2.3 | 0.1 | 56.1 |
| PFMT + VC + BF | BT | 1110 | 175,000 | 1.36 | 0.0253 | 110 |
| PFMT + BT + BF | BT | 7.01 | 17.8 | 3.64 | 0.419 | 32.7 |
| NT | PFMT + ES | 0.274 | 0.226 | 0.218 | 0.0446 | 0.836 |
| PFMT | PFMT + ES | 1.22 | 0.901 | 0.998 | 0.257 | 3.47 |
| PFMT + BF | PFMT + ES | 2.79 | 2.68 | 2.11 | 0.448 | 9.21 |
| ES | PFMT + ES | 0.483 | 0.483 | 0.355 | 0.0645 | 1.67 |
| VC | PFMT + ES | 1.51 | 1.73 | 1.04 | 0.166 | 5.67 |
| SNRI | PFMT + ES | 0.481 | 0.665 | 0.311 | 0.0373 | 1.95 |
| BT | PFMT + ES | 1.71 | 2.51 | 1.07 | 0.142 | 6.99 |
| PFMT + ES + BF | PFMT + ES | 70.3 | 3310 | 7.15 | 0.146 | 323 |
| PFMT + VC | PFMT + ES | 8.62 | 47.8 | 2.44 | 0.113 | 49.5 |
| PFMT + VC + BF | PFMT + ES | 452 | 68,700 | 1.43 | 0.0293 | 102 |
| PFMT + BT + BF | PFMT + ES | 9.19 | 29.5 | 3.91 | 0.279 | 48.1 |
| NT | PFMT + ES + BF | 0.2 | 1.63 | 0.0306 | 0.000648 | 1.19 |
| PFMT | PFMT + ES + BF | 0.878 | 7.8 | 0.14 | 0.00364 | 5.07 |
| PFMT + BF | PFMT + ES + BF | 2.13 | 20.9 | 0.294 | 0.00712 | 12.5 |
| ES | PFMT + ES + BF | 0.347 | 2.4 | 0.0496 | 0.00105 | 2.08 |
| VC | PFMT + ES + BF | 1.12 | 10.3 | 0.146 | 0.00294 | 6.51 |
| SNRI | PFMT + ES + BF | 0.357 | 2.97 | 0.0434 | 0.000739 | 2.13 |
| BT | PFMT + ES + BF | 1.32 | 16.2 | 0.148 | 0.00282 | 7.45 |
| PFMT + ES | PFMT + ES + BF | 1.16 | 12.7 | 0.14 | 0.0031 | 6.88 |
| PFMT + VC | PFMT + ES + BF | 0.732 | 2.36 | 0.342 | 0.0319 | 3.63 |
| PFMT + VC + BF | PFMT + ES + BF | 51 | 5330 | 0.205 | 0.00111 | 47.9 |
| PFMT + BT + BF | PFMT + ES + BF | 8.1 | 137 | 0.544 | 0.00759 | 37.4 |
| NT | PFMT + VC | 0.261 | 0.877 | 0.0892 | 0.0044 | 1.49 |
| PFMT | PFMT + VC | 1.11 | 3.69 | 0.41 | 0.0246 | 6.2 |
| PFMT + BF | PFMT + VC | 2.64 | 9.59 | 0.861 | 0.048 | 15 |
| ES | PFMT + VC | 0.467 | 1.85 | 0.145 | 0.00693 | 2.68 |
| VC | PFMT + VC | 1.42 | 5.52 | 0.426 | 0.0191 | 8.37 |
| SNRI | PFMT + VC | 0.478 | 2.48 | 0.127 | 0.00466 | 2.81 |
| BT | PFMT + VC | 1.63 | 7.55 | 0.435 | 0.0178 | 9.99 |
| PFMT + ES | PFMT + VC | 1.51 | 7.78 | 0.41 | 0.0202 | 8.89 |
| PFMT + ES + BF | PFMT + VC | 6.25 | 17.9 | 2.92 | 0.276 | 31.4 |
| PFMT + VC + BF | PFMT + VC | 114 | 14,700 | 0.595 | 0.00561 | 85.4 |
| PFMT + BT + BF | PFMT + VC | 9.37 | 77.1 | 1.59 | 0.0454 | 55.5 |
| NT | PFMT + VC + BF | 0.931 | 6.55 | 0.151 | 0.00221 | 5.71 |
| PFMT | PFMT + VC + BF | 4.51 | 36.7 | 0.701 | 0.0106 | 27.2 |
| PFMT + BF | PFMT + VC + BF | 10.5 | 100 | 1.49 | 0.0213 | 62.7 |
| ES | PFMT + VC + BF | 1.3 | 12.8 | 0.25 | 0.0043 | 7.94 |
| VC | PFMT + VC + BF | 5.4 | 66.9 | 0.726 | 0.0098 | 30.9 |
| SNRI | PFMT + VC + BF | 1.67 | 17.4 | 0.215 | 0.00254 | 10.1 |
| BT | PFMT + VC + BF | 6.96 | 197 | 0.736 | 0.00913 | 39.5 |
| PFMT + ES | PFMT + VC + BF | 5.91 | 87.3 | 0.698 | 0.00978 | 34.1 |
| PFMT + ES + BF | PFMT + VC + BF | 462 | 50,000 | 4.87 | 0.0209 | 903 |
| PFMT + VC | PFMT + VC + BF | 35.7 | 617 | 1.68 | 0.0117 | 178 |
| PFMT + BT + BF | PFMT + VC + BF | 38.2 | 1290 | 2.67 | 0.0243 | 206 |
| NT | PFMT + BT + BF | 0.112 | 0.271 | 0.0565 | 0.00486 | 0.547 |
| PFMT | PFMT + BT + BF | 0.501 | 1.08 | 0.257 | 0.0266 | 2.44 |
| PFMT + BF | PFMT + BT + BF | 1.03 | 2.39 | 0.542 | 0.0614 | 4.95 |
| ES | PFMT + BT + BF | 0.206 | 0.626 | 0.0916 | 0.00727 | 1.05 |
| VC | PFMT + BT + BF | 0.623 | 2.02 | 0.267 | 0.0209 | 3.29 |
| SNRI | PFMT + BT + BF | 0.203 | 0.722 | 0.0802 | 0.00485 | 1.09 |
| BT | PFMT + BT + BF | 0.51 | 1.1 | 0.275 | 0.0306 | 2.39 |
| PFMT + ES | PFMT + BT + BF | 0.665 | 2.64 | 0.256 | 0.0208 | 3.59 |
| PFMT + ES + BF | PFMT + BT + BF | 57.4 | 8750 | 1.84 | 0.0268 | 132 |
| PFMT + VC | PFMT + BT + BF | 4.08 | 64.7 | 0.629 | 0.018 | 22 |
| PFMT + VC + BF | PFMT + BT + BF | 149 | 16,600 | 0.374 | 0.00485 | 41.2 |
| Intervention 1 | Intervention 2 | Mean | SD | Median | 2.5% | 97.5% |
|---|---|---|---|---|---|---|
| PFMT | NT | 9.9 | 4.35 | 8.97 | 4.4 | 20.8 |
| PFMT + BF | NT | 26.6 | 18.9 | 21.7 | 7.24 | 75.2 |
| ES | NT | 5.31 | 2.66 | 4.75 | 2.02 | 11.9 |
| VC | NT | 8.18 | 4.98 | 6.99 | 2.63 | 20.7 |
| SNRI | NT | 2.4 | 0.932 | 2.24 | 1.09 | 4.68 |
| BT | NT | 17.5 | 23.6 | 11.3 | 1.92 | 70.1 |
| PFMT + ES | NT | 18.6 | 20.1 | 13.1 | 2.91 | 67.5 |
| PFMT + ES + BF | NT | 34.2 | 40.4 | 23.2 | 4.71 | 130 |
| PFMT + VC | NT | 32.6 | 62.3 | 17.7 | 2.55 | 153 |
| PFMT + VC + BF | NT | 6.52 | 21.5 | 2.28 | 0.147 | 37.3 |
| PFMT + BT + BF | NT | 151 | 467 | 62.2 | 5.39 | 818 |
| PFMT + SNRI | NT | 9.7 | 15.7 | 5.63 | 0.784 | 43 |
| NT | PFMT | 0.118 | 0.0462 | 0.111 | 0.0481 | 0.227 |
| PFMT + BF | PFMT | 2.8 | 1.64 | 2.42 | 0.869 | 6.98 |
| ES | PFMT | 0.577 | 0.268 | 0.526 | 0.214 | 1.23 |
| VC | PFMT | 0.861 | 0.419 | 0.778 | 0.315 | 1.9 |
| SNRI | PFMT | 0.28 | 0.15 | 0.25 | 0.0835 | 0.655 |
| BT | PFMT | 1.92 | 2.5 | 1.26 | 0.191 | 7.68 |
| PFMT + ES | PFMT | 1.96 | 1.83 | 1.46 | 0.321 | 6.53 |
| PFMT + ES + BF | PFMT | 3.54 | 3.47 | 2.58 | 0.555 | 12.2 |
| PFMT + VC | PFMT | 3.3 | 5.37 | 1.97 | 0.3 | 14.3 |
| PFMT + VC + BF | PFMT | 0.687 | 2.02 | 0.256 | 0.0156 | 3.91 |
| PFMT + BT + BF | PFMT | 15.8 | 40.7 | 6.94 | 0.572 | 85 |
| PFMT + SNRI | PFMT | 1.07 | 1.65 | 0.63 | 0.0776 | 4.68 |
| NT | PFMT + BF | 0.0536 | 0.0334 | 0.046 | 0.0133 | 0.138 |
| PFMT | PFMT + BF | 0.474 | 0.269 | 0.414 | 0.143 | 1.15 |
| ES | PFMT + BF | 0.266 | 0.192 | 0.218 | 0.0585 | 0.755 |
| VC | PFMT + BF | 0.386 | 0.264 | 0.322 | 0.0935 | 1.06 |
| SNRI | PFMT + BF | 0.127 | 0.096 | 0.103 | 0.0251 | 0.372 |
| BT | PFMT + BF | 0.772 | 0.915 | 0.52 | 0.0828 | 2.98 |
| PFMT + ES | PFMT + BF | 0.914 | 1.12 | 0.601 | 0.0978 | 3.57 |
| PFMT + ES + BF | PFMT + BF | 1.43 | 1.31 | 1.07 | 0.233 | 4.8 |
| PFMT + VC | PFMT + BF | 1.54 | 3.01 | 0.811 | 0.0971 | 7.33 |
| PFMT + VC + BF | PFMT + BF | 0.316 | 1.19 | 0.106 | 0.00549 | 1.83 |
| PFMT + BT + BF | PFMT + BF | 5.98 | 13.3 | 2.84 | 0.272 | 30.1 |
| PFMT + SNRI | PFMT + BF | 0.492 | 0.901 | 0.261 | 0.0259 | 2.37 |
| NT | ES | 0.231 | 0.108 | 0.211 | 0.0841 | 0.496 |
| PFMT | ES | 2.11 | 1.02 | 1.9 | 0.81 | 4.67 |
| PFMT + BF | ES | 5.75 | 4.45 | 4.59 | 1.33 | 17.1 |
| VC | ES | 1.71 | 1.01 | 1.48 | 0.525 | 4.26 |
| SNRI | ES | 0.552 | 0.343 | 0.473 | 0.147 | 1.43 |
| BT | ES | 3.88 | 5.64 | 2.39 | 0.337 | 16.5 |
| PFMT + ES | ES | 3.95 | 4.26 | 2.77 | 0.554 | 14.4 |
| PFMT + ES + BF | ES | 7.29 | 8.88 | 4.93 | 0.907 | 27.7 |
| PFMT + VC | ES | 6.67 | 11.9 | 3.74 | 0.531 | 30.5 |
| PFMT + VC + BF | ES | 1.2 | 3.29 | 0.484 | 0.0345 | 6.59 |
| PFMT + BT + BF | ES | 32.6 | 97.6 | 13.2 | 1 | 181 |
| PFMT + SNRI | ES | 2.16 | 3.7 | 1.2 | 0.139 | 9.97 |
| NT | VC | 0.161 | 0.0882 | 0.143 | 0.0484 | 0.38 |
| PFMT | VC | 1.43 | 0.701 | 1.29 | 0.527 | 3.18 |
| PFMT + BF | VC | 3.78 | 2.69 | 3.11 | 0.948 | 10.7 |
| ES | VC | 0.775 | 0.446 | 0.675 | 0.235 | 1.9 |
| SNRI | VC | 0.384 | 0.264 | 0.321 | 0.0883 | 1.05 |
| BT | VC | 2.62 | 3.79 | 1.61 | 0.226 | 11.1 |
| PFMT + ES | VC | 2.76 | 3.2 | 1.87 | 0.338 | 10.5 |
| PFMT + ES + BF | VC | 4.64 | 4.78 | 3.32 | 0.689 | 16.4 |
| PFMT + VC | VC | 4.48 | 7.91 | 2.52 | 0.358 | 20.2 |
| PFMT + VC + BF | VC | 0.938 | 3.37 | 0.329 | 0.0189 | 5.31 |
| PFMT + BT + BF | VC | 21.6 | 62.8 | 8.89 | 0.69 | 118 |
| PFMT + SNRI | VC | 1.49 | 2.71 | 0.814 | 0.0872 | 6.98 |
| NT | SNRI | 0.477 | 0.184 | 0.447 | 0.214 | 0.921 |
| PFMT | SNRI | 4.69 | 2.84 | 4.01 | 1.53 | 12 |
| PFMT + BF | SNRI | 12.6 | 10.9 | 9.72 | 2.69 | 39.9 |
| ES | SNRI | 2.52 | 1.69 | 2.11 | 0.7 | 6.79 |
| VC | SNRI | 3.88 | 2.96 | 3.12 | 0.953 | 11.3 |
| BT | SNRI | 8.34 | 13.2 | 5.02 | 0.75 | 35.6 |
| PFMT + ES | SNRI | 8.82 | 10.7 | 5.84 | 1.11 | 34.6 |
| PFMT + ES + BF | SNRI | 16.2 | 21.5 | 10.4 | 1.84 | 65.8 |
| PFMT + VC | SNRI | 15.5 | 32.6 | 7.93 | 1.01 | 74.7 |
| PFMT + VC + BF | SNRI | 3.14 | 12.7 | 1.02 | 0.0599 | 18.1 |
| PFMT + BT + BF | SNRI | 71.8 | 232 | 27.7 | 2.17 | 404 |
| PFMT + SNRI | SNRI | 4.4 | 7.12 | 2.52 | 0.337 | 20 |
| NT | BT | 0.133 | 0.158 | 0.0886 | 0.0143 | 0.521 |
| PFMT | BT | 1.26 | 1.7 | 0.796 | 0.13 | 5.24 |
| PFMT + BF | BT | 3 | 4.07 | 1.92 | 0.336 | 12.1 |
| ES | BT | 0.69 | 1.01 | 0.419 | 0.0607 | 2.97 |
| VC | BT | 1.03 | 1.5 | 0.62 | 0.0899 | 4.43 |
| SNRI | BT | 0.319 | 0.44 | 0.199 | 0.0281 | 1.33 |
| PFMT + ES | BT | 2.42 | 5.24 | 1.16 | 0.116 | 12.3 |
| PFMT + ES + BF | BT | 4.1 | 8.34 | 2.07 | 0.227 | 20 |
| PFMT + VC | BT | 4.24 | 17.2 | 1.57 | 0.121 | 23.1 |
| PFMT + VC + BF | BT | 0.868 | 5.16 | 0.202 | 0.00793 | 5.26 |
| PFMT + BT + BF | BT | 11.8 | 31.5 | 5.5 | 0.551 | 60.8 |
| PFMT + SNRI | BT | 1.3 | 6.41 | 0.502 | 0.0346 | 7.22 |
| NT | PFMT + ES | 0.103 | 0.095 | 0.0764 | 0.0148 | 0.344 |
| PFMT | PFMT + ES | 0.923 | 0.849 | 0.686 | 0.153 | 3.12 |
| PFMT + BF | PFMT + ES | 2.55 | 3.07 | 1.66 | 0.28 | 10.2 |
| ES | PFMT + ES | 0.506 | 0.516 | 0.362 | 0.0693 | 1.81 |
| VC | PFMT + ES | 0.781 | 0.882 | 0.535 | 0.0949 | 2.96 |
| SNRI | PFMT + ES | 0.245 | 0.259 | 0.171 | 0.0289 | 0.901 |
| BT | PFMT + ES | 1.73 | 3.53 | 0.864 | 0.0814 | 8.63 |
| PFMT + ES + BF | PFMT + ES | 3.26 | 5.52 | 1.77 | 0.21 | 15.2 |
| PFMT + VC | PFMT + ES | 3.02 | 6.62 | 1.36 | 0.126 | 16.2 |
| PFMT + VC + BF | PFMT + ES | 0.609 | 2.35 | 0.175 | 0.00789 | 3.7 |
| PFMT + BT + BF | PFMT + ES | 14.6 | 82.5 | 4.77 | 0.268 | 86.3 |
| PFMT + SNRI | PFMT + ES | 0.963 | 2.35 | 0.434 | 0.034 | 5.02 |
| NT | PFMT + ES + BF | 0.0599 | 0.0608 | 0.043 | 0.00772 | 0.212 |
| PFMT | PFMT + ES + BF | 0.529 | 0.525 | 0.388 | 0.0819 | 1.8 |
| PFMT + BF | PFMT + ES + BF | 1.26 | 1.21 | 0.935 | 0.208 | 4.29 |
| ES | PFMT + ES + BF | 0.295 | 0.324 | 0.203 | 0.0361 | 1.1 |
| VC | PFMT + ES + BF | 0.415 | 0.417 | 0.301 | 0.0611 | 1.45 |
| SNRI | PFMT + ES + BF | 0.143 | 0.165 | 0.0966 | 0.0152 | 0.545 |
| BT | PFMT + ES + BF | 0.93 | 1.84 | 0.484 | 0.0499 | 4.4 |
| PFMT + ES | PFMT + ES + BF | 1.03 | 1.81 | 0.564 | 0.066 | 4.75 |
| PFMT + VC | PFMT + ES + BF | 1.75 | 4.57 | 0.754 | 0.0701 | 9.4 |
| PFMT + VC + BF | PFMT + ES + BF | 0.358 | 1.75 | 0.0991 | 0.00405 | 2.18 |
| PFMT + BT + BF | PFMT + ES + BF | 7.44 | 23 | 2.67 | 0.169 | 42.8 |
| PFMT + SNRI | PFMT + ES + BF | 0.559 | 1.64 | 0.244 | 0.0183 | 2.94 |
| NT | PFMT + VC | 0.0919 | 0.119 | 0.0565 | 0.00654 | 0.392 |
| PFMT | PFMT + VC | 0.807 | 1.04 | 0.509 | 0.07 | 3.34 |
| PFMT + BF | PFMT + VC | 2.21 | 3.44 | 1.23 | 0.137 | 10.3 |
| ES | PFMT + VC | 0.439 | 0.601 | 0.267 | 0.0328 | 1.88 |
| VC | PFMT + VC | 0.651 | 0.874 | 0.397 | 0.0494 | 2.79 |
| SNRI | PFMT + VC | 0.219 | 0.313 | 0.126 | 0.0134 | 0.992 |
| BT | PFMT + VC | 1.52 | 3.73 | 0.638 | 0.0432 | 8.29 |
| PFMT + ES | PFMT + VC | 1.56 | 3.16 | 0.738 | 0.0619 | 7.96 |
| PFMT + ES + BF | PFMT + VC | 2.78 | 5.63 | 1.33 | 0.106 | 14.3 |
| PFMT + VC + BF | PFMT + VC | 0.539 | 2.63 | 0.128 | 0.00438 | 3.34 |
| PFMT + BT + BF | PFMT + VC | 12.7 | 56.9 | 3.5 | 0.146 | 78.1 |
| PFMT + SNRI | PFMT + VC | 0.859 | 2.6 | 0.318 | 0.0177 | 4.84 |
| NT | PFMT + VC + BF | 1.2 | 3.69 | 0.438 | 0.0268 | 6.8 |
| PFMT | PFMT + VC + BF | 11.3 | 38 | 3.91 | 0.256 | 64.3 |
| PFMT + BF | PFMT + VC + BF | 31 | 119 | 9.47 | 0.546 | 182 |
| ES | PFMT + VC + BF | 5.28 | 16.4 | 2.07 | 0.152 | 29 |
| VC | PFMT + VC + BF | 9.25 | 37.6 | 3.04 | 0.188 | 53 |
| SNRI | PFMT + VC + BF | 2.9 | 9.99 | 0.981 | 0.0551 | 16.7 |
| BT | PFMT + VC + BF | 21 | 114 | 4.95 | 0.19 | 126 |
| PFMT + ES | PFMT + VC + BF | 21.6 | 122 | 5.73 | 0.271 | 127 |
| PFMT + ES + BF | PFMT + VC + BF | 39.6 | 197 | 10.1 | 0.46 | 247 |
| PFMT + VC | PFMT + VC + BF | 46.4 | 2720 | 7.8 | 0.299 | 228 |
| PFMT + BT + BF | PFMT + VC + BF | 190 | 2870 | 27.1 | 0.709 | 1130 |
| PFMT + SNRI | PFMT + VC + BF | 12.2 | 106 | 2.47 | 0.0827 | 74 |
| NT | PFMT + BT + BF | 0.0354 | 0.0836 | 0.0161 | 0.00122 | 0.186 |
| PFMT | PFMT + BT + BF | 0.33 | 0.822 | 0.144 | 0.0118 | 1.75 |
| PFMT + BF | PFMT + BT + BF | 0.729 | 1.62 | 0.352 | 0.0332 | 3.67 |
| ES | PFMT + BT + BF | 0.182 | 0.489 | 0.0758 | 0.00553 | 0.996 |
| VC | PFMT + BT + BF | 0.269 | 0.739 | 0.113 | 0.00851 | 1.45 |
| SNRI | PFMT + BT + BF | 0.0849 | 0.232 | 0.0361 | 0.00248 | 0.46 |
| BT | PFMT + BT + BF | 0.37 | 0.969 | 0.182 | 0.0165 | 1.82 |
| PFMT + ES | PFMT + BT + BF | 0.631 | 1.94 | 0.21 | 0.0116 | 3.74 |
| PFMT + ES + BF | PFMT + BT + BF | 1.04 | 3.73 | 0.374 | 0.0234 | 5.92 |
| PFMT + VC | PFMT + BT + BF | 1.12 | 5.89 | 0.286 | 0.0128 | 6.85 |
| PFMT + VC + BF | PFMT + BT + BF | 0.229 | 1.83 | 0.0369 | 0.000886 | 1.41 |
| PFMT + SNRI | PFMT + BT + BF | 0.349 | 2.01 | 0.091 | 0.00359 | 2.09 |
| NT | PFMT + SNRI | 0.296 | 0.464 | 0.178 | 0.0233 | 1.28 |
| PFMT | PFMT + SNRI | 2.82 | 4.97 | 1.59 | 0.214 | 12.9 |
| PFMT + BF | PFMT + SNRI | 7.75 | 16.7 | 3.84 | 0.422 | 38.6 |
| ES | PFMT + SNRI | 1.55 | 2.85 | 0.836 | 0.1 | 7.21 |
| VC | PFMT + SNRI | 2.38 | 5.02 | 1.23 | 0.143 | 11.5 |
| SNRI | PFMT + SNRI | 0.677 | 1.11 | 0.396 | 0.0499 | 2.97 |
| BT | PFMT + SNRI | 5.36 | 32.1 | 1.99 | 0.139 | 28.9 |
| PFMT + ES | PFMT + SNRI | 5.47 | 14.9 | 2.31 | 0.2 | 29.5 |
| PFMT + ES + BF | PFMT + SNRI | 9.99 | 27.4 | 4.1 | 0.34 | 54.7 |
| PFMT + VC | PFMT + SNRI | 9.62 | 35.3 | 3.14 | 0.207 | 56.7 |
| PFMT + VC + BF | PFMT + SNRI | 1.95 | 11.7 | 0.404 | 0.0135 | 12.1 |
| PFMT + BT + BF | PFMT + SNRI | 45.9 | 245 | 11 | 0.478 | 279 |
Appendix 24 Cost-effectiveness: model structure
FIGURE 44.
Diagram of Markov model for non-surgical treatment.

FIGURE 45.
Diagram of Markov model for surgical treatment following non-surgical treatment.

Appendix 25 Cost-effectiveness: estimation of transition probabilities
| Cycle | Probability | Cycle | Probability |
|---|---|---|---|
| 0 | 0 | 36 | 0.032883 |
| 1 | 0.014915 | 37 | 0.033392 |
| 2 | 0.015433 | 38 | 0.0339 |
| 3 | 0.015951 | 39 | 0.034408 |
| 4 | 0.016468 | 40 | 0.034916 |
| 5 | 0.016985 | 41 | 0.035423 |
| 6 | 0.017502 | 42 | 0.03593 |
| 7 | 0.018019 | 43 | 0.036437 |
| 8 | 0.018535 | 44 | 0.036944 |
| 9 | 0.019051 | 45 | 0.03745 |
| 10 | 0.019567 | 46 | 0.037956 |
| 11 | 0.020082 | 47 | 0.038462 |
| 12 | 0.020598 | 48 | 0.038968 |
| 13 | 0.021113 | 49 | 0.039473 |
| 14 | 0.021627 | 50 | 0.039978 |
| 15 | 0.022142 | 51 | 0.040483 |
| 16 | 0.022656 | 52 | 0.040987 |
| 17 | 0.02317 | 53 | 0.041492 |
| 18 | 0.023684 | 54 | 0.041996 |
| 19 | 0.024197 | 55 | 0.042499 |
| 20 | 0.02471 | 56 | 0.043003 |
| 21 | 0.025223 | 57 | 0.043506 |
| 22 | 0.025735 | 58 | 0.044009 |
| 23 | 0.026248 | 59 | 0.044512 |
| 24 | 0.02676 | 60 | 0.045014 |
| 25 | 0.027271 | 61 | 0.045516 |
| 26 | 0.027783 | 62 | 0.046018 |
| 27 | 0.028294 | 63 | 0.04652 |
| 28 | 0.028805 | 64 | 0.047021 |
| 29 | 0.029316 | 65 | 0.047522 |
| 30 | 0.029826 | 66 | 0.048023 |
| 31 | 0.030336 | 67 | 0.048524 |
| 32 | 0.030846 | 68 | 0.049024 |
| 33 | 0.031356 | 69 | 0.049524 |
| 34 | 0.031865 | 70 | 0.050024 |
| 35 | 0.032374 | ||
| 71 | 0.050523 | 116 | 0.072732 |
| 72 | 0.051023 | 117 | 0.073219 |
| 73 | 0.051522 | 118 | 0.073707 |
| 74 | 0.05202 | 119 | 0.074194 |
| 75 | 0.052519 | 120 | 0.074681 |
| 76 | 0.053017 | 121 | 0.075167 |
| 77 | 0.053515 | 122 | 0.075653 |
| 78 | 0.054013 | 123 | 0.076139 |
| 79 | 0.05451 | 124 | 0.076625 |
| 80 | 0.055007 | 125 | 0.077111 |
| 81 | 0.055504 | 126 | 0.077596 |
| 82 | 0.056001 | 127 | 0.078081 |
| 83 | 0.056497 | 128 | 0.078566 |
| 84 | 0.056993 | 129 | 0.07905 |
| 85 | 0.057489 | 130 | 0.079535 |
| 86 | 0.057985 | 131 | 0.080019 |
| 87 | 0.05848 | 132 | 0.080502 |
| 88 | 0.058975 | 133 | 0.080986 |
| 89 | 0.05947 | 134 | 0.081469 |
| 90 | 0.059964 | 135 | 0.081952 |
| 91 | 0.060459 | 136 | 0.082435 |
| 92 | 0.060953 | 137 | 0.082917 |
| 93 | 0.061446 | 138 | 0.083399 |
| 94 | 0.06194 | 139 | 0.083881 |
| 95 | 0.062433 | 140 | 0.084363 |
| 96 | 0.062926 | 141 | 0.084845 |
| 97 | 0.063419 | 142 | 0.085326 |
| 98 | 0.063911 | 143 | 0.085807 |
| 99 | 0.064404 | 144 | 0.086287 |
| 100 | 0.064896 | 145 | 0.086768 |
| 101 | 0.065387 | 146 | 0.087248 |
| 102 | 0.065879 | 147 | 0.087728 |
| 103 | 0.06637 | 148 | 0.088208 |
| 104 | 0.066861 | 149 | 0.088687 |
| 105 | 0.067351 | 150 | 0.089166 |
| 106 | 0.067842 | 151 | 0.089645 |
| 107 | 0.068332 | 152 | 0.090124 |
| 108 | 0.068822 | 153 | 0.090602 |
| 109 | 0.069312 | 154 | 0.091081 |
| 110 | 0.069801 | 155 | 0.091558 |
| 111 | 0.07029 | 156 | 0.092036 |
| 112 | 0.070779 | 157 | 0.092514 |
| 113 | 0.071267 | 158 | 0.092991 |
| 114 | 0.071756 | 159 | 0.093468 |
| 115 | 0.072244 | 160 | 0.093944 |
| Cycle | Probability | Cycle | Probability |
|---|---|---|---|
| 0 | 0 | 44 | 0.023618 |
| 1 | 0.011617 | 45 | 0.023896 |
| 2 | 0.011898 | 46 | 0.024173 |
| 3 | 0.012179 | 47 | 0.02445 |
| 4 | 0.012459 | 48 | 0.024727 |
| 5 | 0.01274 | 49 | 0.025004 |
| 6 | 0.01302 | 50 | 0.025281 |
| 7 | 0.0133 | 51 | 0.025558 |
| 8 | 0.013581 | 52 | 0.025835 |
| 9 | 0.013861 | 53 | 0.026112 |
| 10 | 0.014141 | 54 | 0.026388 |
| 11 | 0.014421 | 55 | 0.026665 |
| 12 | 0.014701 | 56 | 0.026941 |
| 13 | 0.014981 | 57 | 0.027218 |
| 14 | 0.015261 | 58 | 0.027494 |
| 15 | 0.015541 | 59 | 0.02777 |
| 16 | 0.01582 | 60 | 0.028047 |
| 17 | 0.0161 | 61 | 0.028323 |
| 18 | 0.016379 | 62 | 0.028599 |
| 19 | 0.016659 | 63 | 0.028875 |
| 20 | 0.016938 | 64 | 0.02915 |
| 21 | 0.017217 | 65 | 0.029426 |
| 22 | 0.017496 | 66 | 0.029702 |
| 23 | 0.017776 | 67 | 0.029978 |
| 24 | 0.018055 | 68 | 0.030253 |
| 25 | 0.018333 | 69 | 0.030529 |
| 26 | 0.018612 | 70 | 0.030804 |
| 27 | 0.018891 | 71 | 0.031079 |
| 28 | 0.01917 | 72 | 0.031355 |
| 29 | 0.019448 | 73 | 0.03163 |
| 30 | 0.019727 | 74 | 0.031905 |
| 31 | 0.020005 | 75 | 0.03218 |
| 32 | 0.020284 | 76 | 0.032455 |
| 33 | 0.020562 | 77 | 0.03273 |
| 34 | 0.02084 | 78 | 0.033004 |
| 35 | 0.021119 | 79 | 0.033279 |
| 36 | 0.021397 | 80 | 0.033554 |
| 37 | 0.021675 | 81 | 0.033828 |
| 38 | 0.021952 | 82 | 0.034103 |
| 39 | 0.02223 | 83 | 0.034377 |
| 40 | 0.022508 | 84 | 0.034651 |
| 41 | 0.022786 | 85 | 0.034925 |
| 42 | 0.023063 | 86 | 0.0352 |
| 43 | 0.023341 | 87 | 0.035474 |
| 88 | 0.035748 | 125 | 0.045831 |
| 89 | 0.036022 | 126 | 0.046102 |
| 90 | 0.036295 | 127 | 0.046373 |
| 91 | 0.036569 | 128 | 0.046644 |
| 92 | 0.036843 | 129 | 0.046914 |
| 93 | 0.037116 | 130 | 0.047185 |
| 94 | 0.03739 | 131 | 0.047456 |
| 95 | 0.037663 | 132 | 0.047726 |
| 96 | 0.037937 | 133 | 0.047997 |
| 97 | 0.03821 | 134 | 0.048267 |
| 98 | 0.038483 | 135 | 0.048538 |
| 99 | 0.038756 | 136 | 0.048808 |
| 100 | 0.039029 | 137 | 0.049078 |
| 101 | 0.039302 | 138 | 0.049348 |
| 102 | 0.039575 | 139 | 0.049618 |
| 103 | 0.039848 | 140 | 0.049888 |
| 104 | 0.040121 | 141 | 0.050158 |
| 105 | 0.040394 | 142 | 0.050428 |
| 106 | 0.040666 | 143 | 0.050698 |
| 107 | 0.040939 | 144 | 0.050967 |
| 108 | 0.041211 | 145 | 0.051237 |
| 109 | 0.041483 | 146 | 0.051507 |
| 110 | 0.041756 | 147 | 0.051776 |
| 111 | 0.042028 | 148 | 0.052045 |
| 112 | 0.0423 | 149 | 0.052315 |
| 113 | 0.042572 | 150 | 0.052584 |
| 114 | 0.042844 | 151 | 0.052853 |
| 115 | 0.043116 | 152 | 0.053122 |
| 116 | 0.043388 | 153 | 0.053391 |
| 117 | 0.04366 | 154 | 0.05366 |
| 118 | 0.043931 | 155 | 0.053929 |
| 119 | 0.044203 | 156 | 0.054197 |
| 120 | 0.044474 | 157 | 0.054466 |
| 121 | 0.044746 | 158 | 0.054735 |
| 122 | 0.045017 | 159 | 0.055003 |
| 123 | 0.045288 | 160 | 0.055272 |
| 124 | 0.04556 |
| Cycle | Probability | Cycle | Probability |
|---|---|---|---|
| 0 | 0 | 44 | 0.053443 |
| 1 | 0.823528 | 45 | 0.052689 |
| 2 | 0.386011 | 46 | 0.051962 |
| 3 | 0.292955 | 47 | 0.05126 |
| 4 | 0.242917 | 48 | 0.050581 |
| 5 | 0.210574 | 49 | 0.049926 |
| 6 | 0.187546 | 50 | 0.049292 |
| 7 | 0.170126 | 51 | 0.048678 |
| 8 | 0.156382 | 52 | 0.048083 |
| 9 | 0.145201 | 53 | 0.047507 |
| 10 | 0.135886 | 54 | 0.046948 |
| 11 | 0.12798 | 55 | 0.046406 |
| 12 | 0.121166 | 56 | 0.04588 |
| 13 | 0.11522 | 57 | 0.045368 |
| 14 | 0.109974 | 58 | 0.044871 |
| 15 | 0.105306 | 59 | 0.044388 |
| 16 | 0.101118 | 60 | 0.043918 |
| 17 | 0.097335 | 61 | 0.043461 |
| 18 | 0.093898 | 62 | 0.043015 |
| 19 | 0.090757 | 63 | 0.042581 |
| 20 | 0.087875 | 64 | 0.042158 |
| 21 | 0.085217 | 65 | 0.041746 |
| 22 | 0.082757 | 66 | 0.041344 |
| 23 | 0.080473 | 67 | 0.040952 |
| 24 | 0.078345 | 68 | 0.040569 |
| 25 | 0.076355 | 69 | 0.040195 |
| 26 | 0.074491 | 70 | 0.03983 |
| 27 | 0.07274 | 71 | 0.039474 |
| 28 | 0.071092 | 72 | 0.039125 |
| 29 | 0.069536 | 73 | 0.038784 |
| 30 | 0.068065 | 74 | 0.038451 |
| 31 | 0.066672 | 75 | 0.038125 |
| 32 | 0.06535 | 76 | 0.037806 |
| 33 | 0.064093 | 77 | 0.037494 |
| 34 | 0.062897 | 78 | 0.037188 |
| 35 | 0.061756 | 79 | 0.036889 |
| 36 | 0.060667 | 80 | 0.036596 |
| 37 | 0.059627 | 81 | 0.036308 |
| 38 | 0.058631 | 82 | 0.036027 |
| 39 | 0.057677 | 83 | 0.03575 |
| 40 | 0.056761 | 84 | 0.03548 |
| 41 | 0.055882 | 85 | 0.035214 |
| 42 | 0.055038 | 86 | 0.034954 |
| 43 | 0.054225 | ||
| 87 | 0.034698 | 124 | 0.027701 |
| 88 | 0.034447 | 125 | 0.02756 |
| 89 | 0.034201 | 126 | 0.027421 |
| 90 | 0.033959 | 127 | 0.027283 |
| 91 | 0.033722 | 128 | 0.027148 |
| 92 | 0.033489 | 129 | 0.027013 |
| 93 | 0.033259 | 130 | 0.026881 |
| 94 | 0.033034 | 131 | 0.02675 |
| 95 | 0.032813 | 132 | 0.026621 |
| 96 | 0.032595 | 133 | 0.026494 |
| 97 | 0.032382 | 134 | 0.026368 |
| 98 | 0.032171 | 135 | 0.026243 |
| 99 | 0.031964 | 136 | 0.02612 |
| 100 | 0.031761 | 137 | 0.025999 |
| 101 | 0.031561 | 138 | 0.025879 |
| 102 | 0.031364 | 139 | 0.02576 |
| 103 | 0.03117 | 140 | 0.025643 |
| 104 | 0.030979 | 141 | 0.025527 |
| 105 | 0.030791 | 142 | 0.025412 |
| 106 | 0.030606 | 143 | 0.025299 |
| 107 | 0.030424 | 144 | 0.025187 |
| 108 | 0.030245 | 145 | 0.025076 |
| 109 | 0.030068 | 146 | 0.024967 |
| 110 | 0.029894 | 147 | 0.024859 |
| 111 | 0.029723 | 148 | 0.024752 |
| 112 | 0.029554 | 149 | 0.024646 |
| 113 | 0.029387 | 150 | 0.024541 |
| 114 | 0.029223 | 151 | 0.024437 |
| 115 | 0.029061 | 152 | 0.024335 |
| 116 | 0.028902 | 153 | 0.024234 |
| 117 | 0.028744 | 154 | 0.024133 |
| 118 | 0.028589 | 155 | 0.024034 |
| 119 | 0.028436 | 156 | 0.023936 |
| 120 | 0.028285 | 157 | 0.023839 |
| 121 | 0.028136 | 158 | 0.023742 |
| 122 | 0.027989 | 159 | 0.023647 |
| 123 | 0.027844 | 160 | 0.023553 |
| Cycle | Probability | Cycle | Probability |
|---|---|---|---|
| 0 | 0 | 44 | 0.001726 |
| 1 | 0.129507 | 45 | 0.001696 |
| 2 | 0.023541 | 46 | 0.001668 |
| 3 | 0.015666 | 47 | 0.00164 |
| 4 | 0.012052 | 48 | 0.001613 |
| 5 | 0.00992 | 49 | 0.001588 |
| 6 | 0.008496 | 50 | 0.001563 |
| 7 | 0.007469 | 51 | 0.001539 |
| 8 | 0.006689 | 52 | 0.001516 |
| 9 | 0.006074 | 53 | 0.001493 |
| 10 | 0.005575 | 54 | 0.001472 |
| 11 | 0.005161 | 55 | 0.001451 |
| 12 | 0.004812 | 56 | 0.001431 |
| 13 | 0.004512 | 57 | 0.001411 |
| 14 | 0.004253 | 58 | 0.001392 |
| 15 | 0.004025 | 59 | 0.001374 |
| 16 | 0.003823 | 60 | 0.001356 |
| 17 | 0.003644 | 61 | 0.001339 |
| 18 | 0.003482 | 62 | 0.001322 |
| 19 | 0.003336 | 63 | 0.001306 |
| 20 | 0.003204 | 64 | 0.00129 |
| 21 | 0.003083 | 65 | 0.001274 |
| 22 | 0.002971 | 66 | 0.001259 |
| 23 | 0.002869 | 67 | 0.001245 |
| 24 | 0.002775 | 68 | 0.00123 |
| 25 | 0.002687 | 69 | 0.001217 |
| 26 | 0.002605 | 70 | 0.001203 |
| 27 | 0.002529 | 71 | 0.00119 |
| 28 | 0.002458 | 72 | 0.001177 |
| 29 | 0.002391 | 73 | 0.001164 |
| 30 | 0.002329 | 74 | 0.001152 |
| 31 | 0.00227 | 75 | 0.00114 |
| 32 | 0.002214 | 76 | 0.001129 |
| 33 | 0.002161 | 77 | 0.001117 |
| 34 | 0.002111 | 78 | 0.001106 |
| 35 | 0.002064 | 79 | 0.001095 |
| 36 | 0.002019 | 80 | 0.001085 |
| 37 | 0.001976 | 81 | 0.001074 |
| 38 | 0.001936 | 82 | 0.001064 |
| 39 | 0.001897 | 83 | 0.001054 |
| 40 | 0.00186 | 84 | 0.001044 |
| 41 | 0.001824 | 85 | 0.001035 |
| 42 | 0.00179 | 86 | 0.001025 |
| 43 | 0.001758 | ||
| 87 | 0.001016 | 124 | 0.000772 |
| 88 | 0.001007 | 125 | 0.000768 |
| 89 | 0.000999 | 126 | 0.000763 |
| 90 | 0.00099 | 127 | 0.000758 |
| 91 | 0.000981 | 128 | 0.000754 |
| 92 | 0.000973 | 129 | 0.000749 |
| 93 | 0.000965 | 130 | 0.000745 |
| 94 | 0.000957 | 131 | 0.00074 |
| 95 | 0.000949 | 132 | 0.000736 |
| 96 | 0.000942 | 133 | 0.000732 |
| 97 | 0.000934 | 134 | 0.000727 |
| 98 | 0.000927 | 135 | 0.000723 |
| 99 | 0.000919 | 136 | 0.000719 |
| 100 | 0.000912 | 137 | 0.000715 |
| 101 | 0.000905 | 138 | 0.000711 |
| 102 | 0.000898 | 139 | 0.000707 |
| 103 | 0.000892 | 140 | 0.000703 |
| 104 | 0.000885 | 141 | 0.000699 |
| 105 | 0.000878 | 142 | 0.000695 |
| 106 | 0.000872 | 143 | 0.000692 |
| 107 | 0.000866 | 144 | 0.000688 |
| 108 | 0.00086 | 145 | 0.000684 |
| 109 | 0.000853 | 146 | 0.000681 |
| 110 | 0.000847 | 147 | 0.000677 |
| 111 | 0.000841 | 148 | 0.000673 |
| 112 | 0.000836 | 149 | 0.00067 |
| 113 | 0.00083 | 150 | 0.000667 |
| 114 | 0.000824 | 151 | 0.000663 |
| 115 | 0.000819 | 152 | 0.00066 |
| 116 | 0.000813 | 153 | 0.000656 |
| 117 | 0.000808 | 154 | 0.000653 |
| 118 | 0.000803 | 155 | 0.00065 |
| 119 | 0.000797 | 156 | 0.000647 |
| 120 | 0.000792 | 157 | 0.000643 |
| 121 | 0.000787 | 158 | 0.00064 |
| 122 | 0.000782 | 159 | 0.000637 |
| 123 | 0.000777 | 160 | 0.000634 |
| Age | Cycle | Probability | Age | Cycle | Probability |
|---|---|---|---|---|---|
| 45 | 0 | 0.0003865 | 56 | 44 | 0.00107025 |
| 45.25 | 1 | 0.000398813 | 56.25 | 45 | 0.001092875 |
| 45.5 | 2 | 0.000411125 | 56.5 | 46 | 0.0011155 |
| 45.75 | 3 | 0.000423438 | 56.75 | 47 | 0.001138125 |
| 46 | 4 | 0.00043575 | 57 | 48 | 0.00116075 |
| 46.25 | 5 | 0.00045075 | 57.25 | 49 | 0.001186188 |
| 46.5 | 6 | 0.00046575 | 57.5 | 50 | 0.001211625 |
| 46.75 | 7 | 0.00048075 | 57.75 | 51 | 0.001237063 |
| 47 | 8 | 0.00049575 | 58 | 52 | 0.0012625 |
| 47.25 | 9 | 0.00050875 | 58.25 | 53 | 0.001299313 |
| 47.5 | 10 | 0.00052175 | 58.5 | 54 | 0.001336125 |
| 47.75 | 11 | 0.00053475 | 58.75 | 55 | 0.001372938 |
| 48 | 12 | 0.00054775 | 59 | 56 | 0.00140975 |
| 48.25 | 13 | 0.0005555 | 59.25 | 57 | 0.001442313 |
| 48.5 | 14 | 0.00056325 | 59.5 | 58 | 0.001474875 |
| 48.75 | 15 | 0.000571 | 59.75 | 59 | 0.001507438 |
| 49 | 16 | 0.00057875 | 60 | 60 | 0.00154 |
| 49.25 | 17 | 0.000598938 | 60.25 | 61 | 0.001580438 |
| 49.5 | 18 | 0.000619125 | 60.5 | 62 | 0.001620875 |
| 49.75 | 19 | 0.000639313 | 60.75 | 63 | 0.001661313 |
| 50 | 20 | 0.0006595 | 61 | 64 | 0.00170175 |
| 50.25 | 21 | 0.000670125 | 61.25 | 65 | 0.0017415 |
| 50.5 | 22 | 0.00068075 | 61.5 | 66 | 0.00178125 |
| 50.75 | 23 | 0.000691375 | 61.75 | 67 | 0.001821 |
| 51 | 24 | 0.000702 | 62 | 68 | 0.00186075 |
| 51.25 | 25 | 0.000716313 | 62.25 | 69 | 0.001902813 |
| 51.5 | 26 | 0.000730625 | 62.5 | 70 | 0.001944875 |
| 51.75 | 27 | 0.000744938 | 62.75 | 71 | 0.001986938 |
| 52 | 28 | 0.00075925 | 63 | 72 | 0.002029 |
| 52.25 | 29 | 0.000775 | 63.25 | 73 | 0.00209375 |
| 52.5 | 30 | 0.00079075 | 63.5 | 74 | 0.0021585 |
| 52.75 | 31 | 0.0008065 | 63.75 | 75 | 0.00222325 |
| 53 | 32 | 0.00082225 | 64 | 76 | 0.002288 |
| 53.25 | 33 | 0.000838125 | 64.25 | 77 | 0.002343563 |
| 53.5 | 34 | 0.000854 | 64.5 | 78 | 0.002399125 |
| 53.75 | 35 | 0.000869875 | 64.75 | 79 | 0.002454688 |
| 54 | 36 | 0.00088575 | 65 | 80 | 0.00251025 |
| 54.25 | 37 | 0.000908 | 65.25 | 81 | 0.002577313 |
| 54.5 | 38 | 0.00093025 | 65.5 | 82 | 0.002644375 |
| 54.75 | 39 | 0.0009525 | 65.75 | 83 | 0.002711438 |
| 55 | 40 | 0.00097475 | 66 | 84 | 0.0027785 |
| 55.25 | 41 | 0.000998625 | 66.25 | 85 | 0.002844688 |
| 55.5 | 42 | 0.0010225 | 66.5 | 86 | 0.002910875 |
| 55.75 | 43 | 0.001046375 | |||
| 66.75 | 87 | 0.002977063 | 76 | 124 | 0.008308 |
| 67 | 88 | 0.00304325 | 76.25 | 125 | 0.008546375 |
| 67.25 | 89 | 0.003121813 | 76.5 | 126 | 0.00878475 |
| 67.5 | 90 | 0.003200375 | 76.75 | 127 | 0.009023125 |
| 67.75 | 91 | 0.003278938 | 77 | 128 | 0.0092615 |
| 68 | 92 | 0.0033575 | 77.25 | 129 | 0.009546063 |
| 68.25 | 93 | 0.003448938 | 77.5 | 130 | 0.009830625 |
| 68.5 | 94 | 0.003540375 | 77.75 | 131 | 0.010115188 |
| 68.75 | 95 | 0.003631813 | 78 | 132 | 0.01039975 |
| 69 | 96 | 0.00372325 | 78.25 | 133 | 0.010697563 |
| 69.25 | 97 | 0.003801063 | 78.5 | 134 | 0.010995375 |
| 69.5 | 98 | 0.003878875 | 78.75 | 135 | 0.011293188 |
| 69.75 | 99 | 0.003956688 | 79 | 136 | 0.011591 |
| 70 | 100 | 0.0040345 | 79.25 | 137 | 0.011940688 |
| 70.25 | 101 | 0.004159938 | 79.5 | 138 | 0.012290375 |
| 70.5 | 102 | 0.004285375 | 79.75 | 139 | 0.012640063 |
| 70.75 | 103 | 0.004410813 | 80 | 140 | 0.01298975 |
| 71 | 104 | 0.00453625 | 80.25 | 141 | 0.013396375 |
| 71.25 | 105 | 0.00469825 | 80.5 | 142 | 0.013803 |
| 71.5 | 106 | 0.00486025 | 80.75 | 143 | 0.014209625 |
| 71.75 | 107 | 0.00502225 | 81 | 144 | 0.01461625 |
| 72 | 108 | 0.00518425 | 81.25 | 145 | 0.015069063 |
| 72.25 | 109 | 0.0053295 | 81.5 | 146 | 0.015521875 |
| 72.5 | 110 | 0.00547475 | 81.75 | 147 | 0.015974688 |
| 72.75 | 111 | 0.00562 | 82 | 148 | 0.0164275 |
| 73 | 112 | 0.00576525 | 82.25 | 149 | 0.016904313 |
| 73.25 | 113 | 0.0059625 | 82.5 | 150 | 0.017381125 |
| 73.5 | 114 | 0.00615975 | 82.75 | 151 | 0.017857938 |
| 73.75 | 115 | 0.006357 | 83 | 152 | 0.01833475 |
| 74 | 116 | 0.00655425 | 83.25 | 153 | 0.01876875 |
| 74.25 | 117 | 0.006769438 | 83.5 | 154 | 0.01920275 |
| 74.5 | 118 | 0.006984625 | 83.75 | 155 | 0.01963675 |
| 74.75 | 119 | 0.007199813 | 84 | 156 | 0.02007075 |
| 75 | 120 | 0.007415 | 84.25 | 157 | 0.020737063 |
| 75.25 | 121 | 0.00763825 | 84.5 | 158 | 0.021403375 |
| 75.5 | 122 | 0.0078615 | 84.75 | 159 | 0.022069688 |
| 75.75 | 123 | 0.00808475 | 85 | 160 | 0.022736 |
| Age | Cycle | Values | Age | Cycle | Values |
|---|---|---|---|---|---|
| 45 | 0 | 0.85 | 56 | 44 | 0.8005 |
| 45.25 | 1 | 0.848875 | 56.25 | 45 | 0.799375 |
| 45.5 | 2 | 0.84775 | 56.5 | 46 | 0.79825 |
| 45.75 | 3 | 0.846625 | 56.75 | 47 | 0.797125 |
| 46 | 4 | 0.8455 | 57 | 48 | 0.796 |
| 46.25 | 5 | 0.844375 | 57.25 | 49 | 0.794875 |
| 46.5 | 6 | 0.84325 | 57.5 | 50 | 0.79375 |
| 46.75 | 7 | 0.842125 | 57.75 | 51 | 0.792625 |
| 47 | 8 | 0.841 | 58 | 52 | 0.7915 |
| 47.25 | 9 | 0.839875 | 58.25 | 53 | 0.790375 |
| 47.5 | 10 | 0.83875 | 58.5 | 54 | 0.78925 |
| 47.75 | 11 | 0.837625 | 58.75 | 55 | 0.788125 |
| 48 | 12 | 0.8365 | 59 | 56 | 0.787 |
| 48.25 | 13 | 0.835375 | 59.25 | 57 | 0.785875 |
| 48.5 | 14 | 0.83425 | 59.5 | 58 | 0.78475 |
| 48.75 | 15 | 0.833125 | 59.75 | 59 | 0.783625 |
| 49 | 16 | 0.832 | 60 | 60 | 0.7825 |
| 49.25 | 17 | 0.830875 | 60.25 | 61 | 0.781375 |
| 49.5 | 18 | 0.82975 | 60.5 | 62 | 0.78025 |
| 49.75 | 19 | 0.828625 | 60.75 | 63 | 0.779125 |
| 50 | 20 | 0.8275 | 61 | 64 | 0.778 |
| 50.25 | 21 | 0.826375 | 61.25 | 65 | 0.776875 |
| 50.5 | 22 | 0.82525 | 61.5 | 66 | 0.77575 |
| 50.75 | 23 | 0.824125 | 61.75 | 67 | 0.774625 |
| 51 | 24 | 0.823 | 62 | 68 | 0.7735 |
| 51.25 | 25 | 0.821875 | 62.25 | 69 | 0.772375 |
| 51.5 | 26 | 0.82075 | 62.5 | 70 | 0.77125 |
| 51.75 | 27 | 0.819625 | 62.75 | 71 | 0.770125 |
| 52 | 28 | 0.8185 | 63 | 72 | 0.769 |
| 52.25 | 29 | 0.817375 | 63.25 | 73 | 0.767875 |
| 52.5 | 30 | 0.81625 | 63.5 | 74 | 0.76675 |
| 52.75 | 31 | 0.815125 | 63.75 | 75 | 0.765625 |
| 53 | 32 | 0.814 | 64 | 76 | 0.7645 |
| 53.25 | 33 | 0.812875 | 64.25 | 77 | 0.763375 |
| 53.5 | 34 | 0.81175 | 64.5 | 78 | 0.76225 |
| 53.75 | 35 | 0.810625 | 64.75 | 79 | 0.761125 |
| 54 | 36 | 0.8095 | 65 | 80 | 0.76 |
| 54.25 | 37 | 0.808375 | 65.25 | 81 | 0.758875 |
| 54.5 | 38 | 0.80725 | 65.5 | 82 | 0.75775 |
| 54.75 | 39 | 0.806125 | 65.75 | 83 | 0.756625 |
| 55 | 40 | 0.805 | 66 | 84 | 0.7555 |
| 55.25 | 41 | 0.803875 | 66.25 | 85 | 0.754375 |
| 55.5 | 42 | 0.80275 | 66.5 | 86 | 0.75325 |
| 55.75 | 43 | 0.801625 | |||
| 66.75 | 87 | 0.752125 | 76 | 124 | 0.7105 |
| 67 | 88 | 0.751 | 76.25 | 125 | 0.709375 |
| 67.25 | 89 | 0.749875 | 76.5 | 126 | 0.70825 |
| 67.5 | 90 | 0.74875 | 76.75 | 127 | 0.707125 |
| 67.75 | 91 | 0.747625 | 77 | 128 | 0.706 |
| 68 | 92 | 0.7465 | 77.25 | 129 | 0.704875 |
| 68.25 | 93 | 0.745375 | 77.5 | 130 | 0.70375 |
| 68.5 | 94 | 0.74425 | 77.75 | 131 | 0.702625 |
| 68.75 | 95 | 0.743125 | 78 | 132 | 0.7015 |
| 69 | 96 | 0.742 | 78.25 | 133 | 0.700375 |
| 69.25 | 97 | 0.740875 | 78.5 | 134 | 0.69925 |
| 69.5 | 98 | 0.73975 | 78.75 | 135 | 0.698125 |
| 69.75 | 99 | 0.738625 | 79 | 136 | 0.697 |
| 70 | 100 | 0.7375 | 79.25 | 137 | 0.695875 |
| 70.25 | 101 | 0.736375 | 79.5 | 138 | 0.69475 |
| 70.5 | 102 | 0.73525 | 79.75 | 139 | 0.693625 |
| 70.75 | 103 | 0.734125 | 80 | 140 | 0.6925 |
| 71 | 104 | 0.733 | 80.25 | 141 | 0.691375 |
| 71.25 | 105 | 0.731875 | 80.5 | 142 | 0.69025 |
| 71.5 | 106 | 0.73075 | 80.75 | 143 | 0.689125 |
| 71.75 | 107 | 0.729625 | 81 | 144 | 0.688 |
| 72 | 108 | 0.7285 | 81.25 | 145 | 0.686875 |
| 72.25 | 109 | 0.727375 | 81.5 | 146 | 0.68575 |
| 72.5 | 110 | 0.72625 | 81.75 | 147 | 0.684625 |
| 72.75 | 111 | 0.725125 | 82 | 148 | 0.6835 |
| 73 | 112 | 0.724 | 82.25 | 149 | 0.682375 |
| 73.25 | 113 | 0.722875 | 82.5 | 150 | 0.68125 |
| 73.5 | 114 | 0.72175 | 82.75 | 151 | 0.680125 |
| 73.75 | 115 | 0.720625 | 83 | 152 | 0.679 |
| 74 | 116 | 0.7195 | 83.25 | 153 | 0.677875 |
| 74.25 | 117 | 0.718375 | 83.5 | 154 | 0.67675 |
| 74.5 | 118 | 0.71725 | 83.75 | 155 | 0.675625 |
| 74.75 | 119 | 0.716125 | 84 | 156 | 0.6745 |
| 75 | 120 | 0.715 | 84.25 | 157 | 0.673375 |
| 75.25 | 121 | 0.713875 | 84.5 | 158 | 0.67225 |
| 75.5 | 122 | 0.71275 | 84.75 | 159 | 0.671125 |
| 75.75 | 123 | 0.711625 | 85 | 160 | 0.67 |
Appendix 26 Cost-effectiveness: sensitivity analyses based on cure rates
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (base case mortality rate of TVT is 0.0005, success rate of TVT is based on Ward study213,215,216) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Success rate of TVT is increased by 5% | ||||||||||
| LS–PFMT extra sessions–TVT | 1559 | 16.25 | 73 | 70 | 70 | 70 | 69 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1644 | 85 | 16.11 | –0.14 | Dominated | 4 | 5 | 5 | 5 | 5 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1676 | 117 | 16.07 | –0.17 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1763 | 203 | 15.94 | –0.31 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–TVT | 1774 | 215 | 16.10 | –0.15 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–TVT | 1855 | 296 | 16.16 | –0.09 | Dominated | 20 | 23 | 23 | 24 | 24 |
| Mortality rate of TVT is 0 | ||||||||||
| LS–PFMT extra sessions–TVT | 1645 | 16.20 | 76 | 74 | 73 | 73 | 73 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.07 | –0.13 | Dominated | 6 | 6 | 5 | 5 | 5 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.90 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1887 | 242 | 16.04 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 329 | 16.09 | –0.11 | Dominated | 15 | 18 | 18 | 18 | 19 |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (starting age = 45years) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Starting age = 50 years | ||||||||||
| LS–PFMT extra sessions–TVT | 1618 | 15.27 | 69 | 67 | 67 | 66 | 66 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1698 | 81 | 15.13 | –0.14 | Dominated | 8 | 8 | 8 | 8 | 8 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1730 | 112 | 15.09 | –0.18 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1812 | 195 | 14.96 | –0.32 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1864 | 247 | 15.10 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1946 | 329 | 15.16 | –0.11 | Dominated | 19 | 21 | 21 | 21 | 22 |
| Starting age = 60 years | ||||||||||
| LS–PFMT extra sessions–TVT | 1530 | 12.58 | 68 | 65 | 64 | 64 | 64 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1606 | 75 | 12.45 | –0.13 | Dominated | 8 | 8 | 8 | 8 | 8 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1639 | 109 | 12.40 | –0.18 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1715 | 185 | 12.27 | –0.31 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1800 | 270 | 12.41 | –0.17 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–TVT | 1872 | 342 | 12.48 | –0.10 | Dominated | 19 | 22 | 22 | 22 | 23 |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (time horizon = 40 years) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Time horizon = 30 years | ||||||||||
| LS–PFMT extra sessions–TVT | 1614 | 14.67 | 78 | 77 | 75 | 75 | 75 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1697 | 82 | 14.57 | –0.11 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1728 | 114 | 14.53 | –0.14 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1812 | 198 | 14.43 | –0.25 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1854 | 240 | 14.52 | –0.15 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1931 | 317 | 14.56 | –0.11 | Dominated | 12 | 14 | 15 | 15 | 16 |
| Time horizon = 20 years | ||||||||||
| LS–PFMT extra sessions–TVT | 1525 | 11.69 | 84 | 83 | 82 | 82 | 82 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1603 | 78 | 11.62 | –0.07 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1637 | 112 | 11.58 | –0.11 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1715 | 190 | 11.51 | –0.18 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1791 | 266 | 11.56 | –0.13 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1853 | 328 | 11.59 | –0.10 | Dominated | 9 | 9 | 10 | 10 | 10 |
| Time horizon = 10 years | ||||||||||
| LS–PFMT extra sessions–TVT | 1290 | 6.97 | 88 | 87 | 87 | 86 | 87 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1349 | 59 | 6.93 | –0.04 | Dominated | 7 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1391 | 101 | 6.90 | –0.07 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1449 | 159 | 6.86 | –0.11 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1676 | 387 | 6.89 | –0.09 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–TVT | 1733 | 443 | 6.91 | –0.06 | Dominated | 4 | 6 | 6 | 6 | 6 |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (Manca220 and Haywood219 studies) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Quality of life adjusted by age | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 14.95 | 77 | 76 | 75 | 75 | 75 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 14.82 | –0.13 | Dominated | 5 | 5 | 5 | 5 | 5 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 14.78 | –0.18 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 14.65 | –0.31 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 14.78 | –0.17 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 14.85 | –0.11 | Dominated | 16 | 17 | 18 | 18 | 18 |
| Quality of life from Chapter 4 | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 12.45 | 79 | 77 | 76 | 75 | 75 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 12.35 | –0.10 | Dominated | 5 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 12.32 | –0.13 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 12.22 | –0.23 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 12.32 | –0.13 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 12.36 | –0.09 | Dominated | 13 | 15 | 16 | 16 | 16 |
| Base case (Manca220 and Haywood219 studies) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Quality of life of success after treatment is increased by 5% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.96 | 77 | 76 | 75 | 75 | 75 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.81 | –0.15 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.77 | –0.19 | Dominated | 3 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 16.63 | –0.33 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.76 | –0.20 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 16.80 | –0.16 | Dominated | 13 | 14 | 14 | 14 | 14 |
| Quality of life of success after treatment is increased by 10% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 17.72 | 79 | 79 | 79 | 79 | 79 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 17.56 | –0.16 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 17.52 | –0.20 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 17.36 | –0.35 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 17.49 | –0.23 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 17.52 | –0.20 | Dominated | 11 | 11 | 11 | 12 | 12 |
| Base case (Manca220 and Haywood219 studies) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Quality of life of success after treatment is increased by 15% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 18.48 | 78 | 78 | 77 | 77 | 77 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 18.31 | –0.17 | Dominated | 9 | 9 | 9 | 9 | 9 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 18.26 | –0.21 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 18.10 | –0.38 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 18.22 | –0.26 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 18.24 | –0.24 | Dominated | 9 | 10 | 10 | 10 | 10 |
| Quality of life of failure after treatment is decreased by 5% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.16 | 79 | 78 | 78 | 78 | 78 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.02 | –0.14 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 15.98 | –0.18 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.84 | –0.31 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 15.97 | –0.19 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 16.01 | –0.15 | Dominated | 11 | 12 | 13 | 13 | 13 |
| Base case (Manca220 and Haywood219 studies) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Quality of life of failure after treatment is decreased by 10% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.12 | 78 | 77 | 77 | 77 | 77 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 15.97 | –0.14 | Dominated | 9 | 9 | 9 | 9 | 9 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 15.93 | –0.18 | Dominated | 2 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.79 | –0.32 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 15.90 | –0.21 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 15.93 | –0.19 | Dominated | 9 | 10 | 11 | 11 | 11 |
| Quality of life of failure after treatment is decreased by 15% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.08 | 80 | 79 | 79 | 79 | 79 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 15.93 | –0.15 | Dominated | 9 | 9 | 9 | 9 | 9 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 15.89 | –0.19 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.74 | –0.33 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 15.84 | –0.23 | Dominated | 1 | 1 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 15.85 | –0.22 | Dominated | 7 | 8 | 8 | 8 | 8 |
| Quality of life of success after treatment is increased by 5% and quality of life of failure after treatment is decreased by 5% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.92 | 79 | 79 | 78 | 78 | 78 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.77 | –0.15 | Dominated | 7 | 7 | 7 | 6 | 6 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.73 | –0.19 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 16.58 | –0.34 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.70 | –0.22 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 16.72 | –0.19 | Dominated | 10 | 11 | 11 | 11 | 11 |
| Quality of life of success after treatment is increased by 10% and quality of life of failure after treatment is decreased by 10% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 17.64 | 80 | 79 | 79 | 79 | 79 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 17.47 | –0.17 | Dominated | 8 | 8 | 8 | 8 | 8 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 17.43 | –0.21 | Dominated | 4 | 4 | 4 | 4 | 4 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 17.26 | –0.38 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 17.37 | –0.27 | Dominated | 1 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 17.37 | –0.27 | Dominated | 7 | 7 | 8 | 8 | 8 |
| Quality of life of success after treatment is increased by 15% and quality of life of failure after treatment is decreased by 15% in base case | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 18.36 | 80 | 80 | 80 | 80 | 80 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 18.18 | –0.18 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 18.13 | –0.23 | Dominated | 4 | 4 | 4 | 4 | 4 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 17.95 | –0.41 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 18.03 | –0.32 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 1973 | 328 | 18.01 | –0.35 | Dominated | 5 | 5 | 5 | 5 | 5 |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (discount rates of cost and benefits was 3.5%) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| Discount rates of cost and benefits was 1% | ||||||||||
| LS–PFMT extra sessions–TVT | 1934 | 23.87 | 55 | 54 | 54 | 54 | 54 | |||
| LS–PFMT extra sessions–SNRI–TVT | 2044 | 109 | 23.75 | –0.12 | Dominated | 12 | 13 | 12 | 12 | 12 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2067 | 133 | 23.73 | –0.14 | Dominated | 9 | 9 | 9 | 9 | 9 |
| LS–PFMT basic–TVT | 2100 | 165 | 23.70 | –0.16 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2181 | 247 | 23.61 | –0.26 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–TVT | 2204 | 270 | 23.71 | –0.16 | Dominated | 17 | 18 | 18 | 18 | 18 |
| Discount rates of cost and benefits was 6% | ||||||||||
| LS–PFMT extra sessions–TVT | 1459 | 11.80 | 85 | 84 | 83 | 82 | 82 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1522 | 63 | 11.67 | –0.13 | Dominated | 3 | 3 | 3 | 3 | 3 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1557 | 98 | 11.62 | –0.18 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1620 | 161 | 11.49 | –0.31 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1751 | 292 | 11.63 | –0.17 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–TVT | 1833 | 375 | 11.70 | –0.09 | Dominated | 12 | 13 | 13 | 14 | 15 |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (the probabilities of containment after failure or recurrence for non–surgical treatments are 0%) | ||||||||||
| LS–PFMT extra sessions–TVT | 1644 | 16.20 | 74 | 72 | 71 | 71 | 71 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1727 | 82 | 16.06 | –0.13 | Dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT basic–PFMT extra sessions–TVT | 1758 | 113 | 16.02 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1842 | 197 | 15.89 | –0.30 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT basic–TVT | 1886 | 242 | 16.03 | –0.17 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 1973 | 328 | 16.08 | –0.12 | Dominated | 15 | 18 | 18 | 19 | 19 |
| The probabilities of containment after failure or recurrence for non-surgical treatments are 30% | ||||||||||
| LS–PFMT extra sessions–TVT | 1917 | 15.85 | 59 | 58 | 57 | 57 | 57 | |||
| LS–PFMT extra sessions–SNRI–TVT | 1992 | 75 | 15.72 | –0.13 | Dominated | 12 | 12 | 12 | 12 | 12 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2018 | 101 | 15.69 | –0.16 | Dominated | 14 | 14 | 14 | 14 | 15 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2094 | 177 | 15.57 | –0.28 | Dominated | 1 | 1 | 1 | 1 | 1 |
| LS–TVT | 2249 | 332 | 15.67 | –0.18 | Dominated | 10 | 11 | 11 | 11 | 11 |
| LS–PFMT basic–TVT | 2263 | 346 | 15.57 | –0.28 | Dominated | 4 | 5 | 5 | 5 | 5 |
| The probabilities of containment after failure or recurrence for non-surgical treatments are 60% | ||||||||||
| LS–PFMT extra sessions–TVT | 2190 | 15.50 | 52 | 51 | 51 | 51 | 51 | |||
| LS–PFMT extra sessions–SNRI–TVT | 2259 | 68 | 15.39 | –0.12 | Dominated | 20 | 20 | 19 | 19 | 19 |
| LS–PFMT basic–PFMT extra sessions–TVT | 2279 | 89 | 15.36 | –0.14 | Dominated | 16 | 16 | 17 | 17 | 17 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2346 | 156 | 15.25 | –0.26 | Dominated | 2 | 2 | 2 | 2 | 2 |
| LS–TVT | 2525 | 335 | 15.27 | –0.24 | Dominated | 8 | 9 | 9 | 9 | 9 |
| LS–PFMT basic–TVT | 2639 | 449 | 15.12 | –0.39 | Dominated | 2 | 2 | 3 | 3 | 3 |
Appendix 27 Cost-effectiveness: sensitivity analyses based on improvement rates
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost | QALYs | Incremental QALYs | Incremental cost per QALY | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (base case mortality rate of TVT is 0) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Success rate of TVT is increased by 5% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1756 | 16.33 | 5 | 4 | 3 | 3 | 3 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1769 | 13 | 16.26 | –0.07 | Dominated | 4 | 4 | 3 | 3 | 3 |
| LS–PFMT basic–TVT | 1815 | 58 | 16.38 | 0.05 | 1128 | 11 | 6 | 5 | 5 | 5 |
| LS–PFMT extra sessions–TVT | 1886 | 71 | 16.41 | 0.03 | 2706 | 68 | 73 | 73 | 73 | 73 |
| LS–PFMT extra sessions–SNRI–TVT | 1917 | 32 | 16.30 | –0.11 | Dominated | 11 | 12 | 12 | 12 | 12 |
| LS–TVT | 2335 | 449 | 16.27 | –0.14 | Dominated | 1 | 2 | 3 | 4 | 4 |
| Mortality rate of TVT is 0 | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 6 | 6 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 6 | 5 | 5 | 5 | 4 |
| LS–PFMT basic–TVT | 1873 | 79 | 16.35 | 0.04 | 2059 | 11 | 7 | 7 | 7 | 7 |
| LS–PFMT extra sessions–TVT | 1938 | 65 | 16.38 | 0.03 | 2138 | 66 | 71 | 72 | 72 | 72 |
| LS–PFMT extra sessions–SNRI–TVT | 1966 | 27 | 16.28 | –0.10 | Dominated | 9 | 9 | 9 | 9 | 9 |
| LS–TVT | 2426 | 487 | 16.21 | –0.17 | Dominated | 1 | 1 | 1 | 2 | 2 |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost | QALYs | Incremental QALYs | Incremental cost per QALY | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (starting age = 45 years) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Starting age = 50 years | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1720 | 15.38 | 8 | 7 | 6 | 6 | 6 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1724 | 4 | 15.31 | –0.07 | Dominated | 7 | 6 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1814 | 94 | 15.41 | 0.04 | Extendedly dominated | 13 | 9 | 9 | 8 | 8 |
| LS–PFMT extra sessions–TVT | 1875 | 156 | 15.45 | 0.07 | 2249 | 59 | 62 | 63 | 63 | 64 |
| LS–PFMT extra sessions–SNRI–TVT | 1899 | 23 | 15.34 | –0.11 | Dominated | 13 | 13 | 13 | 13 | 13 |
| LS–TVT | 2370 | 494 | 15.28 | –0.17 | Dominated | 2 | 2 | 3 | 3 | 3 |
| Starting age = 60 years | ||||||||||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1472 | 12.63 | 11 | 10 | 9 | 9 | 9 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 1479 | 7 | 12.68 | 0.06 | 126 | 10 | 8 | 7 | 7 | 7 |
| LS–PFMT basic–TVT | 1628 | 149 | 12.71 | 0.03 | Extendedly dominated | 9 | 8 | 7 | 7 | 7 |
| LS–PFMT extra sessions–TVT | 1677 | 197 | 12.74 | 0.06 | 3344 | 51 | 53 | 53 | 54 | 53 |
| LS–PFMT extra sessions–SNRI–TVT | 1689 | 12 | 12.65 | –0.09 | Dominated | 18 | 19 | 19 | 19 | 19 |
| LS–TVT | 2209 | 533 | 12.58 | –0.16 | Dominated | 2 | 3 | 4 | 4 | 5 |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost | QALYs | Incremental QALYs | Incremental cost per QALY | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (time horizon = 40 years) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Time horizon = 30 years | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1700 | 14.78 | 10 | 8 | 6 | 6 | 6 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1701 | 1 | 14.73 | –0.05 | Dominated | 7 | 5 | 5 | 5 | 5 |
| LS–PFMT basic–TVT | 1794 | 94 | 14.80 | 0.02 | Extendedly dominated | 8 | 5 | 5 | 4 | 4 |
| LS–PFMT extra sessions–TVT | 1853 | 153 | 14.82 | 0.04 | 3471 | 65 | 72 | 73 | 73 | 73 |
| LS–PFMT extra sessions–SNRI–TVT | 1877 | 24 | 14.75 | –0.08 | Dominated | 9 | 10 | 10 | 10 | 10 |
| LS–TVT | 2335 | 482 | 14.66 | –0.16 | Dominated | 0 | 1 | 1 | 1 | 1 |
| Time horizon = 20 years | ||||||||||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1403 | 11.76 | 8 | 5 | 4 | 4 | 4 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 1419 | 16 | 11.78 | 0.02 | 731 | 27 | 14 | 11 | 10 | 10 |
| LS–PFMT basic–TVT | 1613 | 195 | 11.78 | < 0.01 | Extendedly dominated | 3 | 1 | 1 | 1 | 1 |
| LS–PFMT extra sessions–TVT | 1641 | 222 | 11.81 | 0.02 | 10,058 | 54 | 69 | 73 | 74 | 75 |
| LS–PFMT extra sessions–SNRI–TVT | 1650 | 9 | 11.76 | –0.05 | Dominated | 9 | 10 | 10 | 10 | 10 |
| LS–TVT | 2161 | 520 | 11.67 | –0.13 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Time horizon = 10 years | ||||||||||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 818 | 7.03 | 21 | 10 | 7 | 5 | 5 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 833 | 15 | 7.03 | < 0.01 | 3722 | 68 | 62 | 49 | 39 | 33 |
| LS–PFMT basic–TVT | 1139 | 306 | 7.03 | 0 | Dominated | 0 | 0 | 0 | 0 | 0 |
| LS–PFMT extra sessions–SNRI–TVT | 1145 | 312 | 7.02 | –0.01 | Dominated | 1 | 4 | 5 | 5 | 5 |
| LS–PFMT extra sessions–TVT | 1159 | 326 | 7.04 | 0.01 | 62,613 | 10 | 24 | 40 | 51 | 58 |
| LS–TVT | 1883 | 724 | 6.96 | –0.08 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (Manca220 and Haywood219 studies) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Quality of life adjusted by age | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 15.06 | 13 | 9 | 9 | 8 | 8 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 15.00 | –0.07 | Dominated | 13 | 12 | 12 | 11 | 11 |
| LS–PFMT basic–TVT | 1873 | 78 | 15.10 | 0.03 | Extendedly dominated | 7 | 7 | 7 | 7 | 7 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 15.13 | 0.06 | 2207 | 58 | 61 | 62 | 62 | 62 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 15.03 | –0.10 | Dominated | 10 | 10 | 11 | 11 | 11 |
| LS–TVT | 2425 | 487 | 14.96 | –0.17 | Dominated | 0 | 1 | 1 | 1 | 1 |
| Quality of life weighed by obtained in Chapter 4 | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 12.53 | 8 | 6 | 6 | 6 | 6 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 12.48 | –0.05 | Dominated | 5 | 4 | 4 | 4 | 4 |
| LS–PFMT basic–TVT | 1873 | 78 | 12.56 | 0.03 | Extendedly dominated | 10 | 7 | 7 | 6 | 6 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 12.59 | 0.05 | 2793 | 68 | 72 | 73 | 73 | 73 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 12.51 | –0.08 | Dominated | 9 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 12.45 | –0.13 | Dominated | 0 | 1 | 1 | 1 | 1 |
| Base case (Manca220 and Haywood219 studies) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Quality of life of success after treatment is increased by 5% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 17.10 | 10 | 7 | 6 | 6 | 6 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 17.02 | –0.08 | Dominated | 8 | 7 | 7 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 17.13 | 0.04 | Extendedly dominated | 5 | 5 | 5 | 5 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 17.17 | 0.07 | 2013 | 65 | 69 | 70 | 71 | 71 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 17.06 | –0.11 | Dominated | 10 | 11 | 11 | 11 | 11 |
| LS–TVT | 2425 | 487 | 16.95 | –0.22 | Dominated | 1 | 1 | 1 | 1 | 1 |
| Quality of life of success after treatment is increased by 10% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 17.89 | 9 | 6 | 6 | 5 | 5 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 17.80 | –0.08 | Dominated | 8 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 17.92 | 0.04 | Extendedly dominated | 5 | 5 | 5 | 4 | 4 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 17.96 | 0.08 | 1895 | 67 | 71 | 72 | 72 | 72 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 17.84 | –0.12 | Dominated | 11 | 11 | 12 | 12 | 12 |
| LS–TVT | 2425 | 487 | 17.70 | –0.26 | Dominated | 0 | 0 | 1 | 1 | 1 |
| Base case (Manca220 and Haywood219 studies) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Quality of life of success after treatment is increased by 15% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 18.68 | 10 | 8 | 7 | 7 | ||||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 18.59 | –0.09 | Dominated | 10 | 9 | 9 | 9 | |
| LS–PFMT basic–TVT | 1873 | 78 | 18.71 | 0.04 | Extendedly dominated | 5 | 5 | 4 | 4 | |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 18.76 | 0.08 | 1789 | 65 | 69 | 69 | 70 | |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 18.63 | –0.13 | Dominated | 9 | 10 | 10 | 10 | |
| LS–TVT | 2425 | 487 | 18.45 | –0.30 | Dominated | 1 | 1 | 1 | 1 | |
| Quality of life of failure after treatment is decreased by 5% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.29 | 10 | 7 | 6 | 5 | 5 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.22 | –0.07 | Dominated | 10 | 9 | 8 | 8 | 7 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.33 | 0.03 | Extendedly dominated | 5 | 5 | 5 | 5 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.36 | 0.07 | 2112 | 60 | 64 | 65 | 66 | 66 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.25 | –0.11 | Dominated | 15 | 16 | 16 | 16 | 16 |
| LS–TVT | 2425 | 487 | 16.15 | –0.21 | Dominated | 0 | 1 | 1 | 1 | 1 |
| Base case (Manca220 and Haywood219 studies) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Quality of life of failure after treatment is decreased by 10% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.28 | 10 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.20 | –0.08 | Dominated | 10 | 8 | 8 | 8 | 8 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.31 | 0.03 | Extendedly dominated | 6 | 5 | 5 | 5 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.34 | 0.07 | 2078 | 65 | 69 | 69 | 69 | 70 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.23 | –0.11 | Dominated | 10 | 11 | 11 | 11 | 11 |
| LS–TVT | 2425 | 487 | 16.10 | –0.24 | Dominated | 0 | 1 | 1 | 1 | 1 |
| Quality of life of failure after treatment is decreased by 15% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.26 | 10 | 7 | 6 | 6 | 6 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.18 | –0.08 | Dominated | 11 | 10 | 9 | 9 | 9 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.29 | 0.03 | Extendedly dominated | 5 | 4 | 4 | 4 | 4 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.33 | 0.07 | 2045 | 65 | 69 | 70 | 70 | 70 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.21 | –0.12 | Dominated | 9 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.05 | –0.28 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Quality of life of success after treatment is increased by 5% and quality of life of failure after treatment is decreased by 5% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 17.08 | 9 | 8 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 17.00 | –0.08 | Dominated | 10 | 8 | 8 | 7 | 7 |
| LS–PFMT basic–TVT | 1873 | 78 | 17.12 | 0.04 | Extendedly dominated | 6 | 6 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 17.15 | 0.07 | 1982 | 66 | 69 | 70 | 70 | 71 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 17.04 | –0.12 | Dominated | 9 | 9 | 9 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.90 | –0.25 | Dominated | 0 | 0 | 0 | 1 | 1 |
| Quality of life of success after treatment is increased by 10% and quality of life of failure after treatment is decreased by 10% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 17.86 | 9 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 17.76 | –0.09 | Dominated | 10 | 9 | 8 | 8 | 8 |
| LS–PFMT basic–TVT | 1873 | 78 | 17.89 | 0.03 | Extendedly dominated | 6 | 4 | 4 | 4 | 4 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 17.93 | 0.08 | 1841 | 66 | 70 | 70 | 70 | 70 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 17.80 | –0.13 | Dominated | 9 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 17.60 | –0.33 | Dominated | 0 | 0 | 0 | 0 | 0 |
| Quality of life of success after treatment is increased by 15% and quality of life of failure after treatment is decreased by 15% in base case | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 18.63 | Dominated | 7 | 6 | 6 | 6 | 6 | ||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 18.53 | –0.10 | Extendedly dominated | 12 | 10 | 10 | 10 | 10 |
| LS–PFMT basic–TVT | 1873 | 78 | 18.66 | 0.03 | 1718 | 4 | 4 | 4 | 4 | 4 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 18.71 | 0.08 | Dominated | 69 | 71 | 71 | 72 | 72 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 18.57 | –0.14 | Dominated | 8 | 8 | 8 | 9 | 9 |
| LS–TVT | 2425 | 487 | 18.30 | –0.41 | 0 | 0 | 0 | 0 | 0 | |
| Strategy | Deterministic result | Probability cost-effective for different threshold values for society’s willingness to pay for a QALY (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | 10,000 | 20,000 | 30,000 | 40,000 | 50,000 | |
| Base case (discount rates of cost and benefits was 3.5%) | ||||||||||
| LS–PFMT basic–PFMT extra sessions–TVT | 1795 | 16.31 | 8 | 7 | 7 | 7 | 7 | |||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1803 | 8 | 16.24 | –0.07 | Dominated | 7 | 7 | 6 | 6 | 6 |
| LS–PFMT basic–TVT | 1873 | 78 | 16.34 | 0.04 | Extendedly dominated | 10 | 7 | 6 | 6 | 5 |
| LS–PFMT extra sessions–TVT | 1938 | 143 | 16.37 | 0.07 | 2147 | 64 | 68 | 69 | 69 | 69 |
| LS–PFMT extra sessions–SNRI–TVT | 1965 | 27 | 16.27 | –0.10 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–TVT | 2425 | 487 | 16.20 | –0.17 | Dominated | 1 | 2 | 2 | 2 | 3 |
| Discount rates of cost and benefits was 1% | ||||||||||
| LS–PFMT basic–TVT | 2462 | 24.03 | 16 | 11 | 10 | 10 | 9 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 2512 | 50 | 24.00 | –0.04 | Dominated | 9 | 8 | 8 | 8 | 8 |
| LS–PFMT extra sessions–TVT | 2548 | 85 | 24.06 | 0.03 | 2928 | 40 | 43 | 43 | 44 | 44 |
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 2551 | 3 | 23.91 | –0.15 | Dominated | 10 | 10 | 10 | 10 | 10 |
| LS–PFMT extra sessions–SNRI–TVT | 2612 | 64 | 23.96 | –0.10 | Dominated | 19 | 19 | 19 | 19 | 19 |
| LS–TVT | 2899 | 351 | 23.86 | –0.20 | Dominated | 6 | 8 | 9 | 9 | 9 |
| Discount rates of cost and benefits was 6% | ||||||||||
| LS–PFMT basic–PFMT extra sessions–SNRI–TVT | 1360 | 11.85 | 2 | 2 | 2 | 2 | 2 | |||
| LS–PFMT basic–PFMT extra sessions–TVT | 1364 | 4 | 11.91 | 0.05 | 80 | 7 | 5 | 4 | 3 | 3 |
| LS–PFMT basic–TVT | 1504 | 140 | 11.93 | 0.03 | Extendedly dominated | 8 | 5 | 4 | 4 | 4 |
| LS–PFMT extra sessions–TVT | 1565 | 200 | 11.96 | 0.06 | 3554 | 77 | 83 | 83 | 84 | 84 |
| LS–PFMT extra sessions–SNRI–TVT | 1571 | 7 | 11.87 | –0.09 | Dominated | 6 | 6 | 6 | 6 | 6 |
| LS–TVT | 2146 | 581 | 11.80 | –0.16 | Dominated | 0 | 0 | 0 | 0 | 0 |
Appendix 28 Additional trials identified by an update search (29 June 2009)
| Reference | Sample size | Intervention | |
|---|---|---|---|
| 1 | Borello-France DF, Downey PA, Zyczynski H, Rause CR. Continence and quality-of-life outcomes 6 months following an intensive pelvic-floor muscle exercise programme for female stress urinary incontinence: a randomized trial comparing low- and high-frequency maintenance exercise. Phys Ther 2008;88(12):1545–53. | N = 36, SUI | Basic vs intensive PFMT |
| 2 | Castro RA, Arruda RM, Zanetti MR, Santos PD, Sartori MG, Girao MJ. Single-blind, randomized, controlled trial of pelvic floor muscle training, electrical stimulation, vaginal cones, and no active treatment in the management of stress urinary incontinence. Clinics (São Paulo, Brazil) 2008;5(4):465–72. | N = 118, USI | PFMT vs ES vs VC vs NT |
| 3 | Demirturk F, Akbayrak T, Karakaya IC, Yuksel I, Kirdi N, Demirturk F, et al. Interferential current versus biofeedback results in urinary stress incontinence. Swiss Med Wkly 2008;138(21–2):317–21. | N = 41, USI | PFMT + BF vs ES (interferential current) |
| 4 | Harvey MA, Johnston SL. A randomized, single-blind prospective trial comparing pelvic floor physiotherapy with biofeedback versus weighted vaginal cones in the treatment of female genuine stress urinary incontinence: a pilot study [abstract no. 318]. Int Urogynecol J 2006;17(Suppl. 2):235–36. | N = 44, SUI or USI | PFMT + BF vs VC |
| 5 | Wells T, Mayer R, Brink C, Brown R. Pelvic muscle exercise: a controlled clinical trial. 1999. | N = 286, baseline UI 173/242 (71%), MUI 48/242 (20%), UUI 21/242 (9%) | PFMT vs PFMT with resistive device vs attention control (no treatment with clinic visits) vs control (no treatment with no visits); unpublished manuscript |
| 6 | Wells TJ. ‘Curiouser and curiouser …’. J Wound Ostomy Continence Nurs 2003;30(6):300–4. | Related to Wells (1999), above; inaugural lecture script, with one paragraph on the above trial | |
| 7 | Erdinc A, Gurates B, Celik H, Polat A, Kumru S, Simsek M. The efficacy of venlafaxine in the treatment of women with stress urinary incontinence. Arch Gynecol Obstet 2009;279(3):343–8. | N = 40, SUI | Venlafaxine (5-HT and NA reuptake inhibitor) vs placebo |
| 8 | Klarskov N, Scholfield D, Soma K, Darekar A, Mills I, Lose G. Measurement of urethral closure function in women with stress urinary incontinence. J Urol 2009;181(6):2628–33; discussion 2633. | N = 17 (crossover), SUI or SUI-predominant MUI | Esreboxetine (highly SNRI) vs placebo |
| 9 | Lin AT, Sun MJ, Tai HL, Chuang YC, Huang ST, Wang N, et al. Duloxetine versus placebo for the treatment of women with stress predominant urinary incontinence in Taiwan: a double-blind, randomized, placebo-controlled trial. BMC Urol 2008;8:2. | N = 121, predominant symptom of SUI | Duloxetine vs placebo |
| 10 | Schagen van%%Leeuwen JH, Lange RR, Jonasson AF, Chen WJ, Viktrup L. Efficacy and safety of duloxetine in elderly women with stress urinary incontinence or stress-predominant mixed urinary incontinence. Maturitas 2008;60(2):138–47. | N = 265, SUI or SUI-predominant MUI | Duloxetine vs placebo |
| 11 | Cardozo L, Drutz H, Baygani S, Bump R. Duloxetine response and onset of action in women with severe stress urinary incontinence (SUI) awaiting continence surgery [abstract number 210]. Int Urogynecol J 2003;14(Suppl. 1):63–4. | N = 109, USI (severe) | Duloxetine vs placebo; related to Cardozo 2004,137 already included the review |
| 12 | Sherburn M, Bø K, Galea M. Investigation of 2D real-time ultrasound as a measurement tool in a randomised controlled trial of pelvic floor muscle training in older women [abstract no. 91]. Neurourol Urodyn 2008;27(7):676–8. | N = 76, older women | PFMT vs BT; related to Sherburn 2007,182 already included in the review |
List of abbreviations
- BF
- biofeedback
- b.i.d.
- twice daily
- BT
- bladder training
- CI
- confidence interval
- CrI
- credible interval
- DO
- detrusor overactivity
- EMG
- electromyography
- ES
- electrical stimulation
- GP
- general practitioner
- GSI
- genuine stress incontinence
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICIQ-UI SF
- International Consultation on Incontinence Questionnaire – Urinary Incontinence Short Form
- IDO
- idiopathic detrusor overactivity
- IEF
- incontinence episode frequency
- IFT
- interferential therapy
- I-QoL
- Urinary Incontinence Quality of Life Scale
- LS
- lifestyle advice
- MTC
- mixed-treatment comparison
- MUI
- mixed urinary incontinence
- NA
- not applicable
- NHS
- National Health Service
- NICE
- National Institute for Health and Clinical Excellence
- NR
- not reported
- NS
- not statistically significant
- NT
- no treatment
- OR
- odds ratio
- PFMT
- pelvic floor muscle training
- PFMT basic
- PFMT, delivered with up to two sessions or contacts with a health-care professional per month
- PFMT extra sessions
- PFMT, delivered with more than two sessions or contacts with a health-care professional per month
- PGI
- Patient Generated Index
- QALY
- quality-adjusted life-year
- q.d.
- once daily
- RCT
- randomised controlled trial
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMD
- standardised mean difference
- SNRI
- serotonin–noradrenaline reuptake inhibitor
- SUI
- stress urinary incontinence
- TVT
- tension-free vaginal tape
- TVT-O
- tension-free transobturator vaginal tape
- USI
- urodynamic stress incontinence
- UUI
- urgency urinary incontinence
- VC
- vaginal cone
- VPFMC
- voluntary pelvic floor muscle contraction
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Riemsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA, Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant LD, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost-effectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.
By Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al.
-
Gemcitabine for the treatment of metastatic breast cancer.
By Jones J, Takeda A, Tan SC, Cooper K, Loveman E, Clegg A.
-
Varenicline in the management of smoking cessation: a single technology appraisal.
By Hind D, Tappenden P, Peters J, Kenjegalieva K.
-
Alteplase for the treatment of acute ischaemic stroke: a single technology appraisal.
By Lloyd Jones M, Holmes M.
-
Rituximab for the treatment of rheumatoid arthritis.
By Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, et al.
-
Omalizumab for the treatment of severe persistent allergic asthma.
By Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al.
-
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma.
By Boland A, Bagust A, Hockenhull J, Davis H, Chu P, Dickson R.
-
Adalimumab for the treatment of psoriasis.
By Turner D, Picot J, Cooper K, Loveman E.
-
Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a single technology appraisal.
By Holmes M, C Carroll C, Papaioannou D.
-
Romiplostim for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a single technology appraisal.
By Mowatt G, Boachie C, Crowther M, Fraser C, Hernández R, Jia X, et al.
-
Sunitinib for the treatment of gastrointestinal stromal tumours: a critique of the submission from Pfizer.
By Bond M, Hoyle M, Moxham T, Napier M, Anderson R.
-
Vitamin K to prevent fractures in older women: systematic review and economic evaluation.
By Stevenson M, Lloyd-Jones M, Papaioannou D.
-
The effects of biofeedback for the treatment of essential hypertension: a systematic review.
By Greenhalgh J, Dickson R, Dundar Y.
-
A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study.
By Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al.
-
Lapatinib for the treatment of HER2-overexpressing breast cancer.
By Jones J, Takeda A, Picot J, von Keyserlingk C, Clegg A.
-
Infliximab for the treatment of ulcerative colitis.
By Hyde C, Bryan S, Juarez-Garcia A, Andronis L, Fry-Smith A.
-
Rimonabant for the treatment of overweight and obese people.
By Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, et al.
-
Telbivudine for the treatment of chronic hepatitis B infection.
By Hartwell D, Jones J, Harris P, Cooper K.
-
Entecavir for the treatment of chronic hepatitis B infection.
By Shepherd J, Gospodarevskaya E, Frampton G, Cooper K.
-
Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal.
By Stevenson M, Pandor A.
-
Rivaroxaban for the prevention of venous thromboembolism: a single technology appraisal.
By Stevenson M, Scope A, Holmes M, Rees A, Kaltenthaler E.
-
Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck.
By Greenhalgh J, Bagust A, Boland A, Fleeman N, McLeod C, Dundar Y, et al.
-
Mifamurtide for the treatment of osteosarcoma: a single technology appraisal.
By Pandor A, Fitzgerald P, Stevenson M, Papaioannou D.
-
Ustekinumab for the treatment of moderate to severe psoriasis.
By Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A.
-
Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model.
By Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al.
-
Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation.
By Chen Y-F, Jowett S, Barton P, Malottki K, Hyde C, Gibbs JSR, et al.
-
Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY) – a pharmacoepidemiological and qualitative study.
By Wong ICK, Asherson P, Bilbow A, Clifford S, Coghill D, R DeSoysa R, et al.
-
ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening.
By Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al.
-
The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation.
By Black C, Clar C, Henderson R, MacEachern C, McNamee P, Quayyum Z, et al.
-
Randomised preference trial of medical versus surgical termination of pregnancy less than 14 weeks’ gestation (TOPS).
By Robson SC, Kelly T, Howel D, Deverill M, Hewison J, Lie MLS, et al.
-
Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes.
By Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al.
-
VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers.
By Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al.
-
A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial.
By Michaels JA, Campbell WB, King BM, MacIntyre J, Palfreyman SJ, Shackley P, et al.
-
Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice.
By Kai J, Ulph F, Cullinan T, Qureshi N.
-
Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation.
By Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al.
-
Development of a toolkit and glossary to aid in the adaptation of health technology assessment (HTA) reports for use in different contexts.
By Chase D, Rosten C, Turner S, Hicks N, Milne R.
-
Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation.
By Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, et al.
-
Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report.
By Bond M, Wyatt K, Lloyd J, Welch K, Taylor R.
-
Are adverse effects incorporated in economic models? An initial review of current practice.
By Craig D, McDaid C, Fonseca T, Stock C, Duffy S, Woolacott N.
-
Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE).
By Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al.
-
Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation.
By Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al.
-
The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation.
By Fleeman N, McLeod C, Bagust A, Beale S, Boland A, Dundar Y, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer.
By Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TRL, et al.
-
Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study).
By Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al.
-
A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation for chronic obstructive pulmonary disease followed by telephone or conventional follow-up.
By Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA.
-
The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation.
By Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al.
-
Dissemination and publication of research findings: an updated review of related biases.
By Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al.
-
The effectiveness and cost-effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision model.
By Hemingway H, Henriksson M, Chen R, Damant J, Fitzpatrick N, Abrams K, et al.
-
Comparison of case note review methods for evaluating quality and safety in health care.
By Hutchinson A, Coster JE, Cooper KL, McIntosh A, Walters SJ, Bath PA, et al.
-
Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation.
By Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al.
-
Self-monitoring of blood glucose in type 2 diabetes: systematic review.
By Clar C, Barnard K, Cummins E, Royle P, Waugh N.
-
North of England and Scotland Study of Tonsillectomy and Adeno-tonsillectomy in Children (NESSTAC): a pragmatic randomised controlled trial with a parallel non-randomised preference study.
By Lock C, Wilson J, Steen N, Eccles M, Mason H, Carrie S, et al.
-
Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial.
By Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al.
-
A randomised controlled multicentre trial of treatments for adolescent anorexia nervosa including assessment of cost-effectiveness and patient acceptability – the TOuCAN trial.
By Gowers SG, Clark AF, Roberts C, Byford S, Barrett B, Griffiths A, et al.
-
Randomised controlled trials for policy interventions: a review of reviews and meta-regression.
By Oliver S, Bagnall AM, Thomas J, Shepherd J, Sowden A, White I, et al.
-
Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review.
By McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N.
-
A systematic review of outcome measures used in forensic mental health research with consensus panel opinion.
By Fitzpatrick R, Chambers J, Burns T, Doll H, Fazel S, Jenkinson C, et al.
-
The clinical effectiveness and cost-effectiveness of topotecan for small cell lung cancer: a systematic review and economic evaluation.
By Loveman E, Jones J, Hartwell D, Bird A, Harris P, Welch K, et al.
-
Antenatal screening for haemoglobinopathies in primary care: a cohort study and cluster randomised trial to inform a simulation model. The Screening for Haemoglobinopathies in First Trimester (SHIFT) trial.
By Dormandy E, Bryan S, Gulliford MC, Roberts T, Ades T, Calnan M, et al.
-
Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis.
By Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, et al.
-
A randomised controlled trial of cognitive behaviour therapy and motivational interviewing for people with Type 1 diabetes mellitus with persistent sub-optimal glycaemic control: A Diabetes and Psychological Therapies (ADaPT) study.
By Ismail K, Maissi E, Thomas S, Chalder T, Schmidt U, Bartlett J, et al.
-
A randomised controlled equivalence trial to determine the effectiveness and cost–utility of manual chest physiotherapy techniques in the management of exacerbations of chronic obstructive pulmonary disease (MATREX).
By Cross J, Elender F, Barton G, Clark A, Shepstone L, Blyth A, et al.
-
A systematic review and economic evaluation of the clinical effectiveness and cost-effectiveness of aldosterone antagonists for postmyocardial infarction heart failure.
By McKenna C, Burch J, Suekarran S, Walker S, Bakhai A, Witte K, et al.
-
Avoiding and identifying errors in health technology assessment models: qualitative study and methodological review.
By Chilcott JB, Tappenden P, Rawdin A, Johnson M, Kaltenthaler E, Paisley S, et al.
-
BoTULS: a multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A.
By Shaw L, Rodgers H, Price C, van Wijck F, Shackley P, Steen N, et al. , on behalf of the BoTULS investigators.
-
Weighting and valuing quality-adjusted life-years using stated preference methods: preliminary results from the Social Value of a QALY Project.
By Baker R, Bateman I, Donaldson C, Jones-Lee M, Lancsar E, Loomes G, et al.
-
Cetuximab for the first-line treatment of metastatic colorectal cancer.
By Meads C, Round J, Tubeuf S, Moore D, Pennant M, Bayliss S.
-
Infliximab for the treatment of acute exacerbations of ulcerative colitis.
By Bryan S, Andronis L, Hyde C, Connock M, Fry-Smith A, Wang D.
-
Sorafenib for the treatment of advanced hepatocellular carcinoma.
By Connock M, Round J, Bayliss S, Tubeuf S, Greenheld W, Moore D.
-
Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B infection.
By Jones J, Colquitt J, Shepherd J, Harris P, Cooper K.
-
Prasugrel for the treatment of acute coronary artery syndromes with percutaneous coronary intervention.
By Greenhalgh J, Bagust A, Boland A, Saborido CM, Fleeman N, McLeod C, et al.
-
Alitretinoin for the treatment of severe chronic hand eczema.
By Paulden M, Rodgers M, Griffin S, Slack R, Duffy S, Ingram JR, et al.
-
Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer.
By Fleeman N, Bagust A, McLeod C, Greenhalgh J, Boland A, Dundar Y, et al.
-
Topotecan for the treatment of recurrent and stage IVB carcinoma of the cervix.
By Paton F, Paulden M, Saramago P, Manca A, Misso K, Palmer S, et al.
-
Trabectedin for the treatment of advanced metastatic soft tissue sarcoma.
By Simpson EL, Rafia R, Stevenson MD, Papaioannou D.
-
Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia.
By Edlin R, Connock M, Tubeuf S, Round J, Fry-Smith A, Hyde C, et al.
-
The safety and effectiveness of different methods of earwax removal: a systematic review and economic evaluation.
By Clegg AJ, Loveman E, Gospodarevskaya E, Harris P, Bird A, Bryant J, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of rapid point-of-care tests for the detection of genital chlamydia infection in women and men.
By Hislop J, Quayyum Z, Flett G, Boachie C, Fraser C, Mowatt G.
-
School-linked sexual health services for young people (SSHYP): a survey and systematic review concerning current models, effectiveness, cost-effectiveness and research opportunities.
By Owen J, Carroll C, Cooke J, Formby E, Hayter M, Hirst J, et al.
-
Systematic review and cost-effectiveness evaluation of ‘pill-in-the-pocket’ strategy for paroxysmal atrial fibrillation compared to episodic in-hospital treatment or continuous antiarrhythmic drug therapy.
By Martin Saborido C, Hockenhull J, Bagust A, Boland A, Dickson R, Todd D.
-
Chemoprevention of colorectal cancer: systematic review and economic evaluation.
By Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, et al.
-
Cross-trimester repeated measures testing for Down’s syndrome screening: an assessment.
By Wright D, Bradbury I, Malone F, D’Alton M, Summers A, Huang T, et al.
-
Exploring the needs, concerns and behaviours of people with existing respiratory conditions in relation to the H1N1 ‘swine influenza’ pandemic: a multicentre survey and qualitative study.
By Caress A-L, Duxbury P, Woodcock A, Luker KA, Ward D, Campbell M, et al.
-
Influenza A/H1N1v in pregnancy: an investigation of the characteristics and management of affected women and the relationship to pregnancy outcomes for mother and infant.
By Yates L, Pierce M, Stephens S, Mill AC, Spark P, Kurinczuk JJ, et al.
-
The impact of communications about swine flu (influenza A H1N1v) on public responses to the outbreak: results from 36 national telephone surveys in the UK.
By Rubin GJ, Potts HWW, Michie S.
-
The impact of illness and the impact of school closure on social contact patterns.
By Eames KTD, Tilston NL, White PJ, Adams E, Edmunds WJ.
-
Vaccine effectiveness in pandemic influenza – primary care reporting (VIPER): an observational study to assess the effectiveness of the pandemic influenza A (H1N1)v vaccine.
By Simpson CR, Ritchie LD, Robertson C, Sheikh A, McMenamin J.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses: a Cochrane review.
By Jefferson T, Del Mar C, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al.
-
Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR).
By Peek GJ, Elbourne D, Mugford M, Tiruvoipati R, Wilson A, Allen E, et al.
-
Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation.
By Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, et al.
-
Barrett’s oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin.
By Fayter D, Corbett M, Heirs M, Fox D, Eastwood A.
-
Towards single embryo transfer? Modelling clinical outcomes of potential treatment choices using multiple data sources: predictive models and patient perspectives.
By Roberts SA, McGowan L, Hirst WM, Brison DR, Vail A, Lieberman BA.
-
Sugammadex for the reversal of muscle relaxation in general anaesthesia: a systematic review and economic assessment.
By Chambers D, Paulden M, Paton F, Heirs M, Duffy S, Craig D, et al.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Consultant Adviser, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of NETSCC External Relations
-
Ms Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Warwick Clinical Trials Unit
-
Director, Nottingham Clinical Trials Unit
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Ms Kay Pattison, NHS R&D Programme/DH, Leeds
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Mr A S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital
-
Dr Dianne Baralle, Consultant & Senior Lecturer in Clinical Genetics, Human Genetics Division & Wessex Clinical Genetics Service, Southampton, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Service User Representative
-
Professor Anthony Robert Kendrick, Professor of Primary Medical Care, University of Southampton
-
Dr Susanne M Ludgate, Director, Medical Devices Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr David Mathew Service User Representative
-
Dr Michael Millar, Lead Consultant in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, University College London
-
Mrs Una Rennard, Service User Representative
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr W Stuart A Smellie, Consultant, Bishop Auckland General Hospital
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Alan J Williams, Consultant in General Medicine, Department of Thoracic Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist Commissioning Advisory Group (NSCAG), Department of Health
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook Clinical Programmes Director, Bazian Ltd, London
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr Colin Greaves Senior Research Fellow, Peninsular Medical School (Primary Care)
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Miss Nicky Mullany, Service User Representative
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Service User Representative
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust, Bristol
-
Reader in Wound Healing and Director of Research, University of Leeds, Leeds
-
Professor Bipin Bhakta Charterhouse Professor in Rehabilitation Medicine, University of Leeds, Leeds
-
Mrs Penny Calder Service User Representative
-
Professor Paul Carding, Professor of Voice Pathology, Newcastle Hospital NHS Trust, Newcastle
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry, London
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol, Bristol
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and the London School of Medicine and Dentistry, London
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester, Manchester
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham, Nottingham
-
Dr Kate Radford, Division of Rehabilitation and Ageing, School of Community Health Sciences. University of Nottingham, Nottingham
-
Mr Jim Reece, Service User Representative
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton, Southampton
-
Dr Pippa Tyrrell, Stroke Medicine, Senior Lecturer/Consultant Stroke Physician, Salford Royal Foundation Hospitals’ Trust, Salford
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford, Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University, Cardiff
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health , London
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Dr Ursula Wells PRP, DH, London
Interventional Procedures Panel
-
Consultant Surgeon & Honorary Clinical Lecturer, University of Sheffield
-
Mr David P Britt, Service User Representative, Cheshire
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Mr Michael Thomas, Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Mrs Isabel Boyer, Service User Representative, London
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Professor Robin Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Service User Representative, Gloucestershire
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician & Cardiologist, County Durham & Darlington Foundation Trust
-
Mr John Needham, Service User, Buckingmashire
-
Ms Mary Nettle, Mental Health User Consultant, Gloucestershire
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Howard Ring, Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry & Warwickshire Partnership Trust
-
Dr Alastair Sutcliffe, Senior Lecturer, University College London
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Ms Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Professor Tom Walley, HTA Programme Director, Liverpool
-
Dr Ursula Wells, Policy Research Programme, DH, London
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington

