Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 08/91/01. The assessment report began editorial review in June 2009 and was accepted for publication in July 2009. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of topotecan in combination with cisplatin for the treatment of recurrent and stage IVB carcinoma of the cervix, in accordance with the licensed indication, based upon the evidence submission from the manufacturer to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The outcomes measured were overall survival, progression-free survival, response rates, adverse effects of treatment, health-related quality of life (HRQoL) and quality-adjusted life-years (QALYs) gained. The manufacturer stated that topotecan plus cisplatin is the only combination regimen to date to have demonstrated a statistically significant survival advantage compared to cisplatin monotherapy in the licensed population. The clinical evidence came from three clinical trials comparing topotecan plus cisplatin with cisplatin monotherapy (GOG-0179), topotecan plus cisplatin with paclitaxel plus cisplatin (GOG-0169), and four cisplatin-based combination therapies: topotecan plus cisplatin, paclitaxel plus cisplatin, gemcitabine plus cisplatin, and vinorelbine plus cisplatin (GOG-0204). Results from GOG-0179 showed greater median overall survival with topotecan plus cisplatin than with cisplatin monotherapy: 9.4 months versus 6.5 months. Similar results were also reported for median progression-free survival. Response rates also showed an advantage with topotecan plus cisplatin compared with cisplatin monotherapy. The response rates in patients receiving cisplatin monotherapy were very low, but the potential reasons for this were not discussed in the manufacturer’s submission. Patients receiving topotecan plus cisplatin experienced a greater number of adverse events and the ERG was concerned with some of the assumptions related to HRQoL. In the base-case direct comparison, the incremental cost-effectiveness ratio (ICER) of topotecan plus cisplatin versus cisplatin monotherapy was £17,974 per QALY in the main licensed population, £10,928 per QALY in the cisplatin-naive population (including stage IVB patients) and £32,463 per QALY in sustained cisplatin-free interval patients. In response to the point for clarification raised by the ERG, the manufacturer submitted a revised indirect comparison incorporating HRQoL and a longer time horizon. Where the hazard ratio derived from GOG-0169 was employed, paclitaxel plus cisplatin was dominated by topotecan plus cisplatin, but, where the hazard ratio from GOG-0204 was adopted, paclitaxel plus cisplatin was found to have an ICER of £13,260 per QALY versus topotecan plus cisplatin. At present there is a paucity of evidence available on the clinical effects of topotecan plus cisplatin and the effects of palliative treatment in general for women with advanced and recurrent carcinoma of the cervix. Further trials, or the implementation of registries, are required to establish the efficacy and safety of topotecan plus cisplatin. The guidance issued by NICE in September 2009 as a result of the STA states that topotecan in combination with cisplatin is recommended as a treatment option for women with recurrent or stage IVB cervical cancer, only if they have not previously received cisplatin. Women who have previously received cisplatin and are currently being treated with topotecan in combination with cisplatin for the treatment of cervical cancer should have the option to continue therapy until they and their clinicians consider it appropriate to stop.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG); an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA entitled ‘Topotecan for the treatment of recurrent and stage IVB carcinoma of the cervix’. 2
Description of the underlying health problem
Cervical cancer is the second most common malignant neoplastic disease among women worldwide, with a standardised incidence rate of 8.4 per 100,000 females in the UK.
Most patients in the UK are diagnosed with early disease and surgery may be curative. In more advanced non-metastatic disease, radiotherapy may be administered as a potentially curative treatment. For recurrent or metastatic disease, treatment is, in most cases, palliative. Stage IVB cervical cancer is the most advanced form of the disease, in which the cancer has spread to more distant organs. 3 The median survival for stage IVB cervical cancer is very low, at approximately 9–10 months, with 30% survival at 1 year and 2–5% survival at 2 years (P Symonds, GlaxoSmithKline, 2009, personal communication).
Cisplatin has long been considered the most effective platinum-based chemotherapy for the treatment of recurrent or advanced cervical cancer,4–8 either alone or in combination with other chemotherapies. Although the use of combination therapies, particularly paclitaxel in combination with either cisplatin or carboplatin or topotecan in combination with cisplatin, has increased, only topotecan in combination with cisplatin has been explicitly licensed for this indication; recommended for restricted use within NHS Scotland and NHS Wales for the treatment of cisplatin-naive patients only.
Scope of the evidence review group report
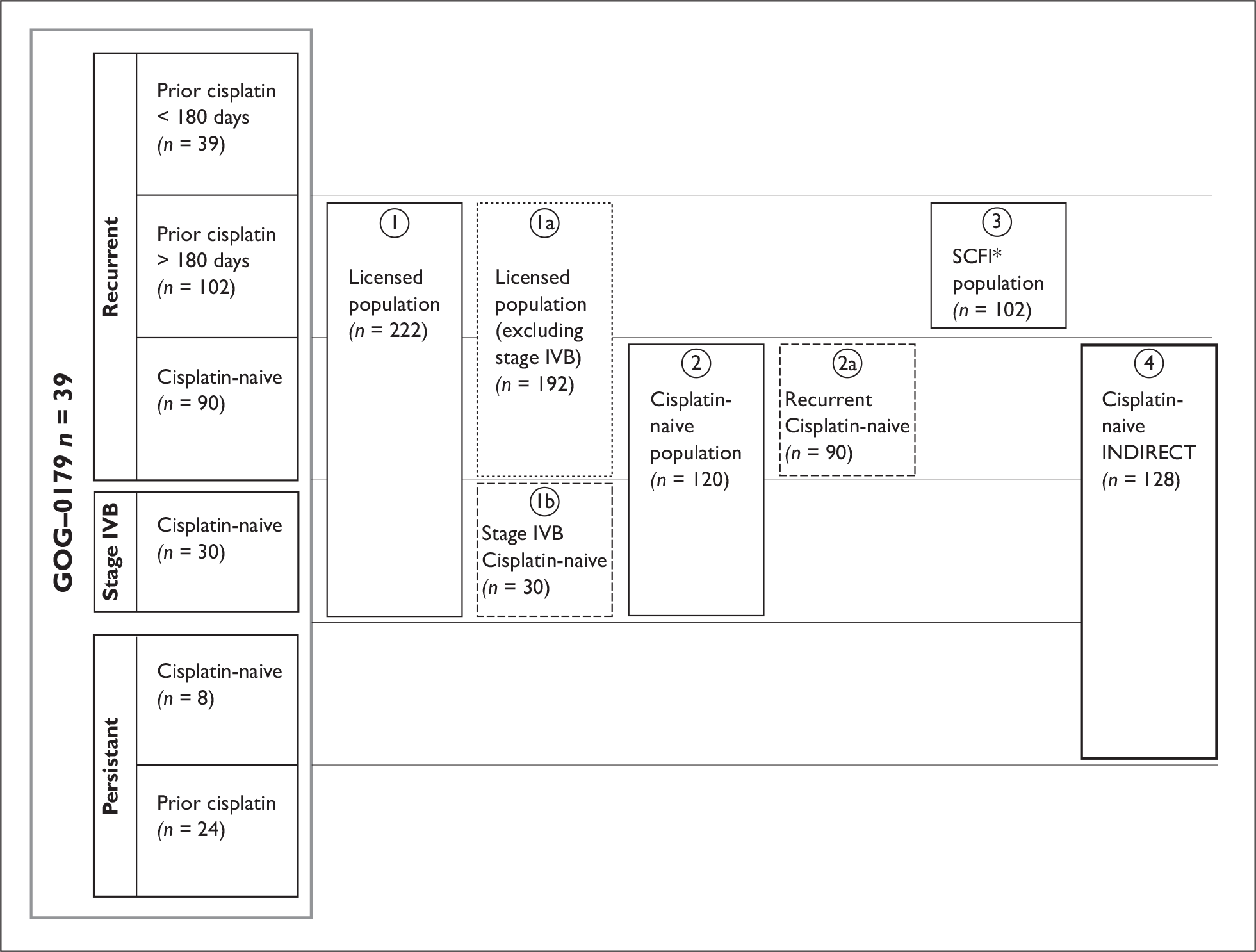
The ERG report appraised the clinical effectiveness and cost-effectiveness of topotecan in combination with cisplatin (within its licensed indications – see Figure 1) for the treatment of recurrent and stage IVB carcinoma of the cervix. The outcomes measured were overall survival, progression-free survival, response rates, adverse effects of treatment, health-related quality of life (HRQoL) and quality-adjusted life-years (QALYs). The manufacturer stated that topotecan plus cisplatin is the only combination regimen to date to have demonstrated a statistically significant survival advantage compared to cisplatin monotherapy in the licensed population.
FIGURE 1.
Schematic of study population and subgroups analysed in the manufacturer’s submission 1. Licensed population, consisting of: 1a. licensed population excluding IVB patients; 1b. stage IVB patients (by definition cisplatin-naive, as they are newly presenting). 2. Cisplatin-naive population, consisting of: 2a. cisplatin-naive recurrent population excluding stage IVB patients; b. stage IVB patients. 3. Patients with a sustained cisplatin-free interval (SCFI; prior cisplatin > 180 days). 4. A further subgroup was analysed specifically for an indirect comparison of topotecan plus cisplatin versus paclitaxel plus cisplatin. The cisplatin-naive (for indirect analysis) population contains all cisplatin-naive patients in GOG-0179 for comparison with patients in a second study (GOG-0169).
The manufacturer recommended that topotecan is administered in combination with cisplatin; 0.75 mg/m2 per day of topotecan, administered as 30-minute intravenous infusion on days 1, 2 and 3, with one dose of 50 mg/m2 per day of cisplatin administered on day 1 following topotecan. Treatment is repeated every 21 days for six cycles or until disease progression.
The manufacturer’s submission focused on direct evidence from a phase III randomised controlled clinical trial (GOG-0179) comparing topotecan plus cisplatin with cisplatin monotherapy, and indirect clinical evidence from a phase III trial (GOG-0169) comparing topotecan plus cisplatin with paclitaxel plus cisplatin. A second direct comparison trial (GOG-0204) was mentioned, which compared four cisplatin-based combination therapies: topotecan plus cisplatin, paclitaxel plus cisplatin, gemcitabine plus cisplatin, and vinorelbine plus cisplatin. GOG-0179 included patients outside the licensed population, and the manufacturer undertook subgroup analyses to reflect the different subgroups within the licensed population, namely: licensed population including or excluding stage IVB patients, cisplatin-naive patients, and patients with sustained cisplatin-free interval (SCFI) longer than 180 days.
The manufacturer submitted two separate cost-effectiveness comparisons: a trial-based direct comparison between topotecan plus cisplatin and cisplatin monotherapy based on patient-level data from GOG-0179 and evaluated using the statistical package sas®, considered by the manufacturer to be the primary analysis within their submission; and a Microsoft excel model-based indirect comparison between topotecan plus cisplatin and paclitaxel plus cisplatin, considered to be a secondary analysis.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s submission to NICE as part of the STA process.
The ERG replicated the manufacturer’s amended search strategy, and attempted to reproduce its patient-level analysis. The ERG was unable to comprehensively validate the patient-level analysis because of the manufacturer’s failure to provide a fully executable sas®-based model, and instead focused on the excel-based analysis. The ERG made a number of revisions to the manufacturer’s model, including altering the assumptions related to utility values, the costs of administering treatment, and the number of vials of topotecan utilised.
Results
Summary of submitted clinical evidence
The GOG-0179 trial reported greater median overall survival with topotecan plus cisplatin than with cisplatin monotherapy: 9.4 months versus 6.5 months. The unadjusted hazard ratio (HR) of 0.76 [95% confidence interval (CI) 0.59 to 0.98, p = 0.033] translates into a 24% reduction in mortality with combination therapy. Similar results were also reported for median progression-free survival in GOG-0179: 4.6 months (topotecan plus cisplatin) versus 2.9 months (cisplatin), HR 0.76 (95% CI 0.60 to 0.97, p = 0.027).
Response rates also showed an advantage with topotecan plus cisplatin (24%) compared with cisplatin monotherapy (12%) (p = 0.0073). The response rates in patients receiving cisplatin monotherapy were very low, but the potential reasons for this were not discussed in the manufacturer’s submission.
The safety profile of topotecan plus cisplatin was reported to be predictable and manageable, and there was reportedly no evidence to suggest that HRQoL was significantly reduced in patients receiving combination therapy. However, patients receiving topotecan plus cisplatin experienced a greater number of adverse events and the ERG is concerned with some of the assumptions related to HRQoL.
Subgroup analyses were undertaken and showed favourable results towards topotecan plus cisplatin (Table 1), but the results should be interpreted with caution as the number of patients in quite a few of the subgroups was small and some of the analyses were performed post hoc.
| Licence population | Cisplatin-naive population | Sustained cisplatin-free interval population | Cisplatin-naive (for indirect analysis) population | |||||
|---|---|---|---|---|---|---|---|---|
| Cisplatin (n = 115) | Topotecan plus cisplatin (n = 107) | Cisplatin (n = 62) | Topotecan plus cisplatin (n = 58) | Cisplatin (n = 53) | Topotecan plus cisplatin (n = 49) | Cisplatin (n = 64) | Topotecan plus cisplatin (n = 64) | |
| Overall survival time (months) | ||||||||
| Mean | 9.9 | 12.9 | 11.1 | 15.1 | 7.9 | 9.5 | 11.1 | 14.4 |
| Median | 7.3 | 11.9 | 8.5 | 14.5 | 6.3 | 9.9 | 8.5 | 12.5 |
| 95% CI for median survival time | 6.0 to 9.5 | 9.4 to 13.7 | 6.4 to 11.1 | 11.5 to 17.5 | 4.9 to 9.5 | 7.0 to 12.6 | 6.5 to 11.3 | 9.2 to 17.4 |
| Log rank p-value | 0.0041 | 0.0098 | 0.1912 | 0.0206 | ||||
| Hazard ratio (95% CI) | 0.652 (0.485 to 0.875) | 0.587 (0.389 to 0.884) | 0.75 (0.492 to 1.155) | 0.633 (0.428 to 0.935) | ||||
| Minimum | 0.3 | 0.4 | 1.3 | 0.4 | 0.3 | 0.6 | 1.3 | 0.4 |
| Maximum | 39.0 | 34.4 | 34.0 | 31.0 | 17.2 | 27.1 | 38.9 | 34.4 |
| Observed events | 100 (87.0%) | 81 (75.7%) | 55 (89.0%) | 40 (69.0%) | 45 (84.9%) | 41 (83.7%) | 57(89.1%) | 46 (71.9%) |
| Censored events | 15 (13.0%) | 26 (24.3%) | 7 (11.0%) | 18 (31.0%) | 8 (15.1%) | 8 (16.3%) | 7 (10.9%) | 18 (28.1%) |
For overall survival, the indirect comparison between GOG-0179 and GOG-0169 showed non-significant results in favour of topotecan plus cisplatin compared with paclitaxel plus cisplatin: HR 0.72 (95% CI 0.46 to 1.15).
The GOG-0204 trial was closed early as all experimental arms were unlikely to demonstrate a significant advantage compared with paclitaxel plus cisplatin. In response to a point for clarification raised by the ERG, the manufacturer conducted direct and indirect comparisons including data from GOG-0204. The direct comparison favoured paclitaxel plus cisplatin (HR 1.27, 95% CI 0.96 to 1.69), while the pooled data using direct and indirect evidence from GOG-0169, GOG-0179 and GOG-0204 favoured topotecan plus cisplatin (HR 0.98, 95% CI 0.73 to 1.23), but neither result was statistically significant.
Summary of submitted cost-effectiveness evidence
In the base-case direct comparison, the incremental cost-effectiveness ratio (ICER) of topotecan plus cisplatin versus cisplatin monotherapy was £17,974 per QALY in the main licensed population, £10,928 per QALY in the cisplatin-naive population (including stage IVB patients) and £32,463 per QALY in SCFI patients.
Results for the indirect comparison were presented only for a cisplatin-naive population, and outcomes were expressed in terms of life-years gained only. In the base-case indirect comparison, paclitaxel plus cisplatin was dominated by topotecan plus cisplatin, which in turn had a cost per life-year gained of £19,964 versus cisplatin monotherapy; where the HR used to calculate overall survival with paclitaxel plus cisplatin was taken from GOG-0204 (rather than derived from GOG-0169, as in the base case), paclitaxel plus cisplatin was found to have a cost per life-year gained of £982 versus topotecan plus cisplatin.
In response to the point for clarification raised by the ERG, the manufacturer submitted a revised indirect comparison incorporating HRQoL and a longer time horizon. Similar to the previous analysis, where the HR derived from GOG-0169 was employed, paclitaxel plus cisplatin was dominated by topotecan plus cisplatin, but, where the HR from GOG-0204 was adopted, paclitaxel plus cisplatin was found to have an ICER of £13,260 per QALY versus topotecan plus cisplatin.
The ERG made a number of revisions to this model to explore alternative assumptions to those employed by the manufacturer. Where the number of vials used was assumed to be minimised (or maximised) because of alternative assumptions about possible wastage, the ERG found topotecan plus cisplatin to have an ICER versus cisplatin monotherapy of £26,778 (£34,327) per QALY in the cisplatin-naive patient population and £58,872 (£73,833) per QALY in the full licensed population from GOG-0179. These ICERs were considered to be potentially conservative as no account was taken of the potential impact of dose reductions because of adverse events on the acquisition costs of the interventions. In order to consider the potential impact of dose reduction, the ERG employed a ‘hybrid’ approach combining estimates from the manufacturer’s patient level and the ERG’s revised model analyses. Where wastage of vials was assumed to be minimised, the ICER of topotecan plus cisplatin versus cisplatin monotherapy fell to £19,815 in the cisplatin-naive population and £53,868 in the licensed population. While assuming maximum wastage of topotecan, the ICER of topotecan plus cisplatin versus cisplatin monotherapy rose to £27,362 in the cisplatin-naive population and £68,826 in the licensed population.
Topotecan plus cisplatin, paclitaxel plus cisplatin, and cisplatin monotherapy were compared in a fully incremental analysis; topotecan plus cisplatin was found to extendedly dominate paclitaxel plus cisplatin in most scenarios where the GOG-0169 HR was adopted, but was dominated by paclitaxel plus cisplatin in all scenarios where the GOG-0204 HR was adopted.
Commentary on the robustness of submitted evidence
The main strength of the direct comparison was the potential for the results to have a very high internal validity due to the use of patient-level data from a recent, relevant and seemingly well-conducted trial (GOG-0179). This was considered to be a potential strength only because the manufacturer did not provide in a timely manner the necessary code and data sets for the ERG to validate fully the programming of this comparison.
A further strength of the direct comparison was the presentation of results for the main licensed population and a series of subgroups within that, highlighting the population gaining most benefit from treatment, and allowing variability in the cost-effectiveness estimates to be considered. However, the limitations of subgroup analyses should be borne in mind.
The main strengths of the indirect comparison were the relatively high degree of transparency within the submitted excel model and the high degree of consistency between the electronic model and the submitted report.
The lack of transparency regarding the literature search and rationale for exclusion of potentially relevant trials was a limitation, and this was not satisfactorily addressed in the manufacturer’s response document.
For the direct comparison, the results from GOG-0204 were not formally included in the submission. For the indirect comparison, it was not clear that a comprehensive network of evidence was investigated. Potentially relevant studies were excluded by the manufacturer on the basis that the comparators were not licensed for use in this population; however, the comparator selected for the indirect comparison (i.e. paclitaxel plus cisplatin) was not licensed – this contradiction was not satisfactorily explained.
The analyses submitted for the cost-effectiveness evidence were incomplete and required considerable clarification. The lack of transparency regarding the programming of the direct comparison was a significant weakness; the coding was incompletely submitted in a non-executable form and with evidence of errors. There were also concerns surrounding the methods used, which may potentially overestimate the incremental QALY gains associated with topotecan plus cisplatin. The primary analysis based on GOG-0179 suffers from a lack of external validity as it makes no comparison between topotecan plus cisplatin and other relevant treatment comparators other than cisplatin monotherapy.
The indirect comparison initially submitted neglected to consider HRQoL, reporting life-years gained instead of QALYs, although this was rectified following a request from the ERG. The results were only presented for a single population (cisplatin-naive patients, including patients with persistent disease) and the model was not probabilistic, so that uncertainty surrounding the cost-effectiveness results could not be appropriately quantified.
Both comparisons also failed to properly justify a number of assumptions over costs, including the cost of administering treatments, the number of vials of topotecan needed per cycle and the costs of adverse events, all of these were considered for revision by the ERG.
Conclusions
At present there is a paucity of evidence available on the clinical effects of topotecan plus cisplatin and the effects of palliative treatment in general (including various off-license drugs regularly used in UK clinical practice) for women with advanced and recurrent carcinoma of the cervix.
Further trials, or the implementation of registries, are required to establish the efficacy and safety of topotecan plus cisplatin. Such research should assess all aspects of quality of life, including the impact of treatment toxicities, scheduling and convenience to the patient. It is also important to untangle further which patients will benefit the most from treatments and the factors that potentially moderate these benefits. Further research to provide appropriate utility values for patients with cervical cancer, reflecting both the stage and course of disease (e.g. impact of disease progression) as well as the specific impact of individual therapies, would be beneficial.
Key issues
For the direct comparison submitted by the manufacturer, there was a paucity of clinical effectiveness evidence available, and the manufacturer made limited use of the results from GOG-0204. The ERG questioned the handling and reporting of quality of life data and whether the results were representative of the whole patient experience. For the economic evaluation, key issues relate to the appropriateness of the mapped utility values adopted, the reasonableness of the costing assumptions, the external validity of an analysis with only a single comparator, and (perhaps most importantly) the validity and transparency of the sas analysis.
In terms of the indirect comparison, a potentially relevant network of indirect evidence has not been fully explored, although the ERG acknowledges that the quality of such evidence would be limited. The inclusion of direct evidence from GOG-0204 (further results will shortly be available) and evidence from a forthcoming Cochrane Review would increase the network of evidence and enable further assessment of the clinical effectiveness and cost-effectiveness of treatments used in current UK practice.
Key issues in relation to the indirect comparison were the appropriateness of the utility values, the reasonableness of the costing assumptions, and the appropriate source of the HR used to estimate survival for paclitaxel plus cisplatin – deriving this HR from GOG-0169 favours topotecan plus cisplatin, while deriving it from GOG-0204 favours paclitaxel plus cisplatin.
Areas of uncertainty
There is uncertainty surrounding the population(s) that will benefit most from treatment with topotecan plus cisplatin. The number of patients who have received chemoradiation is likely to increase in the future, thus the number of cisplatin-naive patients will diminish. This raises the question of the applicability of the results to current and future clinical practice.
The economic submissions are subject to significant uncertainty over the utility values and cost assumptions adopted by the manufacturer, and this uncertainty feeds into the results of the subsequent analyses.
Summary of NICE guidance issued as a result of the STA
At the time of writing, the final appraisal consultation document issued by NICE on 9 June 2009 states that:
Topotecan in combination with cisplatin, is recommended as a treatment option for women with recurrent or stage IVB cervical cancer only if they have not previously received cisplatin.
Women who have previously received cisplatin and are currently being treated with topotecan in combination with cisplatin for the treatment of cervical cancer should have the option to continue therapy until they and their clinicians consider it appropriate to stop.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- NICE . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- Paton F, Paulden M, Saramago P, Manca A, Misso K, Palmer S, et al. Topotecan for the treatment of recurrent and stage IVB carcinoma of the cervix: A single technology appraisal. York: Centre for Reviews and Dissemination; 2009.
- Monk BJ, Huang HO, Cella D, Long HJ. Quality of life outcomes from a randomised phase III trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: a Gynecologic Oncology Group Study. J Clin Oncol 2005;23:4617-25.
- Scottish Intercollegiate Guidelines Network . Management of Cervical Cancer. A National Clinical Guideline. No. 99 n.d. www.sign.ac.uk/.
- GlaxoSmithKline UK Ltd . Hycamtin Summary of Product Characteristics 2008.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2009.
- Hirte HW, Strychowsky JE, Oliver T, Fung-Kee-Fung M, Elit L, Oza A. Chemotherapy for recurrent, metastatic or persistent cervical cancer: a systematic review. Int J Gynecol Cancer 2007;17:1194-204.
- Long HJ, Bundy BN, Grendys EC, Benda JA, McMeekin JS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol 2005;23:4626-33.