Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 07/13/01. The assessment report began editorial review in December 2009 and was accepted for publication in January 2010. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of certolizumab pegol (CZP) for adults with active rheumatoid arthritis (RA) that have not responded adequately to treatment with conventional disease modifying anti-rheumatic drugs (DMARDs) including methotrexate (MTX), in accordance with the licensed indication, based upon the evidence submission from the manufacturer to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The outcome measures included American College of Rheumatology (ACR) 20, 50 and 70 response rates and quality of life measures after 3 months and 6 months of treatment. The ERG examined the submission’s search strategies and considered they appeared comprehensive and that it was unlikely that relevant studies would have been missed. Only English language studies were considered in the submission and non-English language studies relevant to the decision problem may possibly have been ignored. The ERG analysed the first submitted economic model so as to itemise in detail clarification points that were brought to the attention of the manufacturer. In response the manufacturer submitted a modified cost-effectiveness analysis. The ERG undertook further analysis of this second model and other additional submitted evidence. The clinical evidence was derived from two multicentre blinded randomised controlled trials (RCTs) comparing CZP + MTX to placebo + MTX (the RAPID 1 and RAPID 2 trials). RAPID 1 lasted 52 weeks with 982 patients and RAPID 2 24 weeks with 619 patients. Evidence for clinical effectiveness of CZP in mono-therapy came from the 24-week FAST4WARD trial with 220 patients that compared CZP (400 mg every 4 weeks) versus placebo. The three key RCTs demonstrated statistically significant superiority of CZP + MTX versus placebo + MTX and of CZP versus placebo with respect to a variety of outcomes including ACR 20, ACR 50 and ACR 70 measures and quality of life measures at 3 and 6 months. On the basis of results from the indirect comparison meta-analyses, the manufacturer suggested that CZP may be at least as effective as other ‘biological’ DMARD (bDMARD) comparators and, in a few ACR measures at 3 and 6 months, more effective. CZP is an effective therapy for adult RA patients whose disease has failed to respond adequately to cDMARDs including MTX or who are intolerant of MTX. The cost-effectiveness of CZP relative to other bDMARDs is unclear because the economic modelling undertaken may have ignored relevant effectiveness data and potential differences between trial populations, and so may have included effectiveness results that were biased in favour of CZP; underestimated uncertainty in the relative effectiveness of compared DMARDs; and ignored the potential influence of differences between bDMARDs with regard to adverse events and their related costs and health impacts. The NICE guidance issued in October 2009 states that: the Committee is minded not to recommend certolizumab pegol as a treatment option for people with RA; and the Committee recommends that NICE asks the manufacturer of CZP for more information on the clinical effectiveness and cost-effectiveness of CZP for the treatment of people with RA. On receipt of this information and details of a patient access scheme NICE issued final guidance recommending CZP, under certain criteria, as a treatment option for people with RA.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of the Institute.
This paper presents a summary of the ERG report for the STA submission that considered the clinical effectiveness and cost-effectiveness of certolizumab pegol (CZP) for adults with active rheumatoid arthritis (RA) that has not responded adequately to treatment with conventional disease modifying anti-rheumatic drugs (cDMARDs) including methotrexate (MTX). 2 CZP is a ‘biological’ DMARD (bDMARD) whose effectiveness could be compared to cDMARDs or to other bDMARDs administered within their licensed indications.
Description of the underlying health problem
This section is taken from the NICE scope for this STA.
Rheumatoid arthritis is a chronic, disabling autoimmune disease characterised by inflammation of the synovial tissue of the peripheral joints, which causes swelling, stiffness, pain and progressive joint destruction. For a small proportion of people, inflammatory disease outside the joints (e.g. eye and lung disease, vasculitis) can also pose a significant problem. RA is heterogeneous, it is usually a chronic relapsing condition which has a pattern of flare-ups followed by periods of lower disease activity, but in a minority of cases the disease is constantly progressive. Most patients with RA develop damage to affected joints, with the amount of damage ranging from mild to severe. RA has a severe impact on quality of life and it is estimated that 40% of people with RA will stop working within 5 years of diagnosis.
Rheumatoid arthritis is three times more prevalent in women than in men. It can develop at any age, but usually starts between 40 and 60 years of age. RA affects 1% of the population, or approximately 400,000 people in England and Wales. Of these, approximately 15% have severe disease.
People with RA are usually treated in an outpatient setting rather than in primary care. There is no cure, and treatment aims to improve quality of life and to prevent or reduce joint damage. Treatment for RA usually includes: non-steroidal anti-inflammatory agents (NSAIDs) which reduce pain, fever and joint swelling/inflammation; and DMARDS which slow the disease process and reduce joint damage. Corticosteroids may also be used to control inflammation. DMARDs are usually started soon after diagnosis. MTX and sulfasalazine are two commonly used DMARDs. NICE guidance recommends the use of a TNF (tumour necrosis factor)-α inhibitor (adalimumab, etanercept and infliximab; types of bDMARD) after the failure of two cDMARDs such as MTX and sulfasalazine. NICE guidance recommends the use of rituximab (a bDMARD that depletes B cells) after the failure of a TNF inhibitor, but does not recommend the use of abatacept after the failure of a TNF inhibitor.
Scope of the evidence review group report
The scope for this STA was to address the clinical effectiveness and cost-effectiveness of CZP relative to cDMARDs and to bDMARDs for the treatment of adults with active RA whose disease had not responded adequately to cDMARDs including MTX. The STA was initiated prior to the granting of formal marketing authorisation. The anticipated marketing authorisation for CZP specified a dose regimen of 400 mg administered subcutaneously on weeks 0, 2 and 4, followed by 200 mg every other week. CZP is indicated for use in ‘combination’ therapy with MTX or as ‘monotherapy’ (without MTX) for patients intolerant of MTX. The acquisition cost of CZP is £357.50 per 200-mg syringe, excluding VAT (value added tax).
The key sources of evidence on clinical effectiveness of CZP in combination therapy came from two multicentre blinded randomised controlled trials (RCTs) comparing CZP + MTX to placebo + MTX [the RA Prevention of Structural Damage (RAPID) 13 and RAPID 24 trials]. RAPID 1 lasted 52 weeks with 982 patients and RAPID 2 24 weeks with 619 patients. Evidence for clinical effectiveness of CZP in mono-therapy came from the 24-week FAST4WARD trial5 with 220 patients that compared CZP (400 mg every 4 weeks) versus placebo. There were no head-to-head trials that compared the effectiveness of CZP to the other bDMARDs. To estimate the relative clinical effectiveness between bDMARDs the manufacturer undertook indirect comparison meta-analyses (ICMs)6 using the results from various placebo-controlled trials of bDMARDs.
The manufacturer submitted a de novo economic model that was used to estimate the cost per quality-adjusted life-year (QALY) gained from CZP in comparison with anti-TNF agents (adalimumab, etanercept and infliximab) or with rituximab. Model inputs for clinical effectiveness of the different bDMARDs were derived from results from ICMs and based on the American College of Rheumatology (ACR) 20, 50 and 70 response rates7 after 3 months and 6 months of treatment. The estimated ACR response rates in the absence of bDMARD treatment were single point values (no associated uncertainty) and were obtained by simple aggregation of the rates reported across the control arms of the included trials.
Health-related quality of life (HRQoL) utilities for the first 6 months of treatment were obtained by regression analysis of the relationship between ACR response and European Quality of Life-5 Dimensions (EQ-5D) scores observed for European patients participating in CZP trials. Utilities while continuing on treatment and utility after cessation of treatment were obtained by converting Health Assessment Questionnaire measures using a published algorithm proposed by Brennan et al. 8 Costs were mainly obtained from standard sources.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
Owing to the central importance of the RAPID 1, RAPID 2 and FAST4WARD studies, these were formally fully appraised by the ERG, taking advantage of responses to requests for clarification from the manufacturer.
The ERG examined the submission’s search strategies and considered they appeared comprehensive and that it was unlikely that relevant studies would have been missed. Only English language studies were considered in the submission and non-English language studies relevant to the decision problem may possibly have been ignored.
The ERG critically appraised the submitted ICM with focus on the validity of selection of studies for inclusion, the reproducibility of results and the exploration of heterogeneity. The ERG considered the relative merits of alternative approaches to the ICM submitted.
The ERG analysed the first submitted economic model so as to itemise in detail clarification points that were brought to the attention of the manufacturer. In response the manufacturer submitted a modified cost-effectiveness analysis. The ERG undertook further analysis of this second model and other additional submitted evidence.
Results
Summary of submitted clinical evidence
The three key RCTs demonstrated statistically significant superiority of CZP + MTX versus placebo + MTX and of CZP versus placebo with respect to a variety of outcomes including ACR 20, ACR 50 and ACR 70 measures and quality of life measures at 3 and 6 months.
On the basis of results from the ICMs, the manufacturer suggested that CZP may be at least as effective as other bDMARD comparators and, in a few ACR measures at 3 and 6 months, more effective. These ICM estimates were associated with considerable uncertainty. Some evidence was presented that CZP inhibits progression of structural damage to joints.
Summary of submitted cost-effectiveness evidence
The inputs for the first model were modified in the second model submitted. Some modifications were introduced in response to NICE’s requests for clarification, others depended on new results obtained from unprompted reanalyses of trial data undertaken by the manufacturer. The main changes made were exclusion of adverse events, exact calculation of discontinuation rates and modified annual utility decrement upon cessation of bDMARD treatment. The main results from the second model are shown in Table 1. The submission also included an economic analysis encompassing a proposed patient access scheme. At the time, this scheme was not approved by the Department of Health (DoH) and as such it was not considered in the first appraisal meeting.
| Mean cost (£) | Mean QALYs | ICER | |
|---|---|---|---|
| Combination therapy | |||
| CZP + MTX | 89,158 | 6.654 | – |
| Etanercept + MTX | 86,165 | 6.589 | 46,192 |
| Adalimumab + MTX | 86,034 | 6.412 | 12,937 |
| Rituximab + MTX | 82,940 | 6.362 | 21,345 |
| Infliximab + MTX | 95,599 | 6.196 | CZP dominates |
| Monotherapy | |||
| CZP | 85,424 | 6.305 | – |
| Etanercept | 85,941 | 6.435 | (3991)a |
| Adalimumab | 84,201 | 6.09 | 5687 |
Commentary on the robustness of submitted evidence
In the three CZP trials there were large numbers of early patient withdrawals from the control arms that were imposed for lack of a rapidly established clinical effectiveness response.
In the RAPID 1 trial, of 199 patients receiving placebo + MTX, 63% had withdrawn by week 16 and 78% by the end of the trial; this compared to 21% and 35%, respectively, of patients receiving CZP. In RAPID 2, 87% of patients in the placebo + MTX arm had withdrawn by the end of the trial (week 24). In the FAST4WARD mono-therapy trial, 54% of control arm patients had withdrawn by week 12 and 74% by the end of the trial at 24 weeks.
The high withdrawal rates at early phases of the CZP trials, especially seen in the control arms, necessitated that estimates of effectiveness at later time points required many ‘last observations’ to be carried forward, and somewhat compromised the robustness of these estimates.
Owing to a lack of head-to-head trials of different bDMARDs, the manufacturer undertook random effects ICMs to gain an estimate of their relative clinical effectiveness.
The effectiveness of CZP relative to other bDMARDs was based on ACR 20, ACR 50 and ACR 70 outcomes measured at 12 and 24 weeks in various trials analysed by ICM. The robustness of these comparisons was potentially compromised by the high withdrawal rates in the CZP trials relative to those observed in the other included trials.
For combination therapy ACR responses at 24 weeks, the ICM included 10 trials [two with CZP (RAPID 1 and 2), three with adalimumab, two with infliximab and one each with etanercept, rituximab and tocilizumab]. Seven trials were used for responses at 12 weeks [two with CZP (RAPID trials), two with etanercept and one each with adalimumab, infliximab and tocilizumab]. For monotherapy ACR responses at 12 and 24 weeks, the ICM included four trials (two with adalimumab and one each with CZP and etanercept).
The reported results from the ICMs (odds ratios) were associated with considerable uncertainty and there were some errors in the reported values for ACR 70. Of the 85 indirect comparisons made between pairs of bDMARDs, only four reached statistical significance. Two of these were for superiority of CZP at 24 weeks in the ACR 20 outcome.
Several aspects of the ICMs reported by the manufacturer were a cause of concern:
-
(a) The inclusion and exclusion of studies for the ICM did not appear to be systematic.
-
(b) The inclusion of data from the included studies lacked some consistency.
-
(c) There was a possibility that relevant information from several excluded studies, including an unpublished industry sponsored randomised trial of CZP + MTX versus MTX + placebo (study C87014), could have been used in the ICM.
-
(d) There was insufficient consideration and exploration of underlying heterogeneity amongst the studies included for ICM.
-
(e) The development of effectiveness input for the economic analysis included data for a bDMARD comparator omitted from the subsequent economic analysis, raising the issue of whether data for other omitted bDMARDs should also have been included.
-
(f) The development of clinical effectiveness input for the economic analysis used a point estimate derived by aggregation across trial control arms and sacrificed some of the strengths of randomisation and underestimated associated uncertainty.
The validity of ICM rests on an assumption of exchangeability between trials such that the placebo arms of the trials are interchangeable. The submission lacked an assessment or discussion of clinical or statistical heterogeneity amongst the trials used for ICM and did not comment on whether baseline characteristics of participants were similar across these RCTs. As such there was no consideration of potential sources of non-comparability of the placebo-controlled arms of the trials.
The ERG undertook an analysis of the heterogeneity amongst the control arms of the studies used in the estimation of effectiveness for the 24-week ACR 20 outcome for combination therapy. This choice was made because it involved the largest number of studies and the largest number of events. The results are shown in Figure 1. Data for four study level variables (chosen by the ERG) are also included in the figure.
FIGURE 1.
Risk of ACR 20 in placebo plus MTX arms of trials used for ICM at 6 months. ACR, American College of Rheumatology; CI, confidence interval; DMARDs, disease modifying anti-rheumatic drugs; MTX, methotrexate.
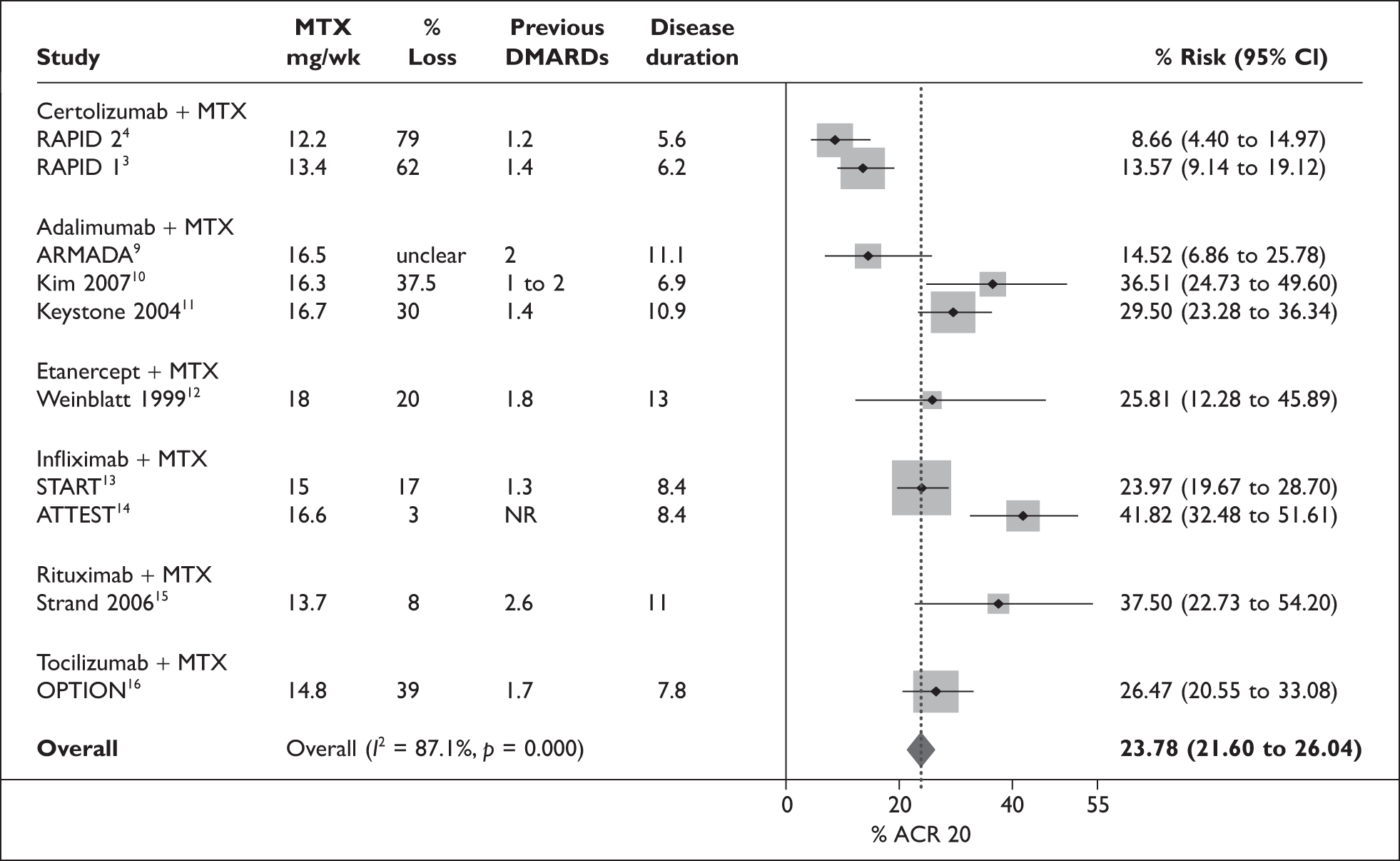
The control rate in the two CZP RCTs was the lowest amongst the 10 trials, and the I2 statistic indicated considerable heterogeneity. When the two CZP studies were omitted from the analysis, the I2 statistic was reduced to 70% and the pooled estimate increased to 28%.
The four study level variables that were looked at as potential contributors to the observed heterogeneity were: entry level MTX dose as a potential indicator of treatment intensity and population differences; percentage withdrawals for the ACR 20 outcome as indicator of completeness of data; duration of RA; and number of previous DMARDs trialed as indicators of possible population differences. For each of these variables the two CZP RCTs were at the extreme of the distributions. The brief examination of heterogeneity amongst the studies used for ICMs indicated that an indirect comparison or mixed-treatment analysis with methods that allow for differences in control rate or baseline risk (similar to the Bayesian analyses undertaken by Nixon et al. 17) probably represents the preferred choice of methodology for the decision problem.
Regarding the economic model, the robustness of quality of life and health-utility inputs was difficult to determine through lack of detail of how many patients were given HRQoL questionnaires and what response rates were elicited. It was not clear how this uncertainty might affect the estimates of cost-effectiveness generated by the model.
Adverse event costs as well as their related health outcomes were not included in the revised model although they were included in the original submission. There was a lack of information to justify this revision, so it was unclear what sources of data were used in this exercise. An assumption of no difference in adverse effects between drugs (CZP, infliximab, adalimumab, etanercept, rituximab) may on average be shown to be reasonable, but on the basis of the submitted information the assumption cannot be considered to be evidence based.
Conclusions
Certolizumab pegol is an effective therapy for adult RA patients whose disease has failed to respond adequately to cDMARDs including MTX or who are intolerant of MTX.
A reasonable interpretation of the results is that there is little convincing evidence that CZP is more or less effective than the comparators examined.
Patients with RA may respond differently to different bDMARDs and effectiveness of a bDMARD for a specific patient is currently unpredictable; an increase in the variety of available bDMARDs might potentially increase the overall proportion of patients responsive to these drugs.
The cost-effectiveness of CZP relative to other bDMARD is unclear because the economic modelling undertaken may have ignored relevant effectiveness data and potential differences between trial populations, and so may have included effectiveness results that were biased in favour of CZP; underestimated uncertainty in the relative effectiveness of compared DMARDs; and ignored the potential influence of differences between bDMARDs with regard to adverse events and their related costs and health impacts.
Summary of NICE guidance issued as a result of the STA
At the time of drafting this report, the guidance appraisal consultation document issued by NICE in October 2009 states that:
1.1 The Committee is minded not to recommend certolizumab pegol as a treatment option for people with rheumatoid arthritis (RA).
1.2 The Committee recommends that NICE asks the manufacturer of certolizumab pegol for more information on the clinical effectiveness and cost-effectiveness of certolizumab pegol for the treatment of people with RA. This information should be made available for the second Appraisal Committee meeting, and should cover the following issues:
Estimation of the clinical effectiveness of certolizumab pegol relative to other TNF-α inhibitors for the treatment of RA, including consideration of uncertainty around the estimate. In order to clarify this issue the Committee requests:
-
provision of a mixed-treatment comparison (MTC) analysis, rather than an indirect comparison meta-analysis
-
details of potentially relevant studies, including study C87014, that were excluded from the analysis
-
provision of data from the C87014 trial and an assessment of the impact on the incremental cost-effectiveness ratios (ICERs) when the American College of Rheumatology (ACR) response for certolizumab pegol in combination with methotrexate is calculated using data from the C87014 plus the RAPID 1 and 2 trials.
Clarification of how the original economic model was revised:
-
further justification of why a utility decrease of 0.037 per year after assessment of clinical response at 6 months was assumed in the original model, but a utility increase of 0.0402 per year was assumed in the revised model
-
further details of how the assumed utility decrease of 0.0025 per year when treatment is discontinued was derived
-
clarification and a full breakdown of the direct and indirect costs included in the model, including an explanation of why the mean cost for the intervention and comparators differed between the original and revised models, and an explanation of how these changes relate to costs associated with adverse events
-
clarification of how incorporating an estimated relationship between ACR 20, 50 and 70 would affect cost effectiveness in the revised model.
Provision of an incremental cost-effectiveness analysis:
-
comparing certolizumab pegol with other TNF-α inhibitors (that is, not including rituximab)
-
including univariate sensitivity analysis exploring the effect of lowering the estimate for the cost of administering infliximab in line with the range of costs used in previous appraisals
-
including a comparison of all treatments with full reporting of results of probabilistic sensitivity analysis, including but not limited to presentation of cost-effectiveness acceptability curves, with all treatments plotted and a scatter plot of all treatments on the same cost-effectiveness plane.
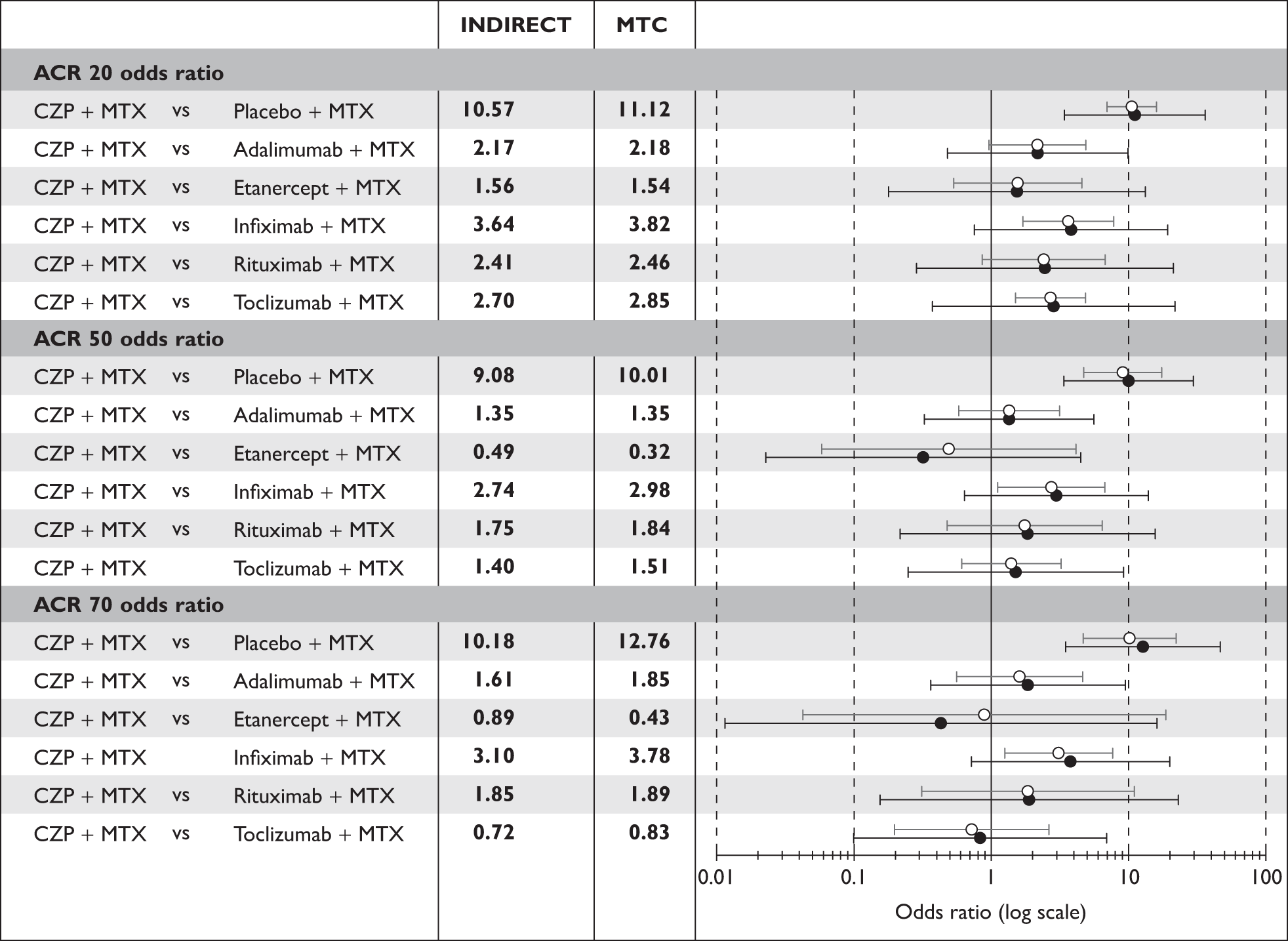
The manufacturer responded to the ACD with a new submission that incorporated a MTC that included the industry sponsored CZP study C87014 and two additional studies previously excluded from the ICMs. The major change to the economic analysis was the introduction of a DOH-approved patient access scheme (PAS) that considerably reduced the initial cost of CZP treatment by making early treatment syringes free of charge.
The MTC more faithfully reflected the inherent uncertainty in the estimates of relative effectiveness of the compared DMARDs (Figure 2) and the PAS improved the cost-effectiveness of CZP treatment. The main new cost-effectiveness results submitted are summarised in Table 2.
FIGURE 2.
Manufacturer’s results for ICM (hollow symbol) and MTC (solid symbol) with associated uncertainty: ACR 20, 50, 70 outcomes. ACR, American College of Rheumatology; CZP, certolizumab pegol; MTC, mixed-treatment comparison; MTX, methotrexate.
| Mean cost (£) | Mean QALYs | ICER | |
|---|---|---|---|
| Combination therapy | |||
| CZP + MTX | 85,583 | 6.654 | – |
| Etanercept + MTX | 86,165 | 6.589 | CZP dominates |
| Adalimumab + MTX | 86,034 | 6.412 | CZP dominates |
| Rituximab + MTX | 82,940 | 6.362 | 9072 |
| Infliximab + MTX | 95,599 | 6.196 | CZP dominates |
| Monotherapy | |||
| CZP | 81,849 | 6.305 | – |
| Etanercept | 85,941 | 6.435 | (31,582)a |
| Adalimumab | 84,201 | 6.09 | CZP dominates |
The final appraisal document for this technology was issued by NICE shortly before this article was sent to press. The appraisal document states:
Cetolizumab pegol is recommended as an option for the treatment of people with rheumatoid arthritis only if:
-
certolizumab pegol is used as described for other tumour necrosis factor (TNF) inhibitor treatments in ‘Adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis (NICE technology appraisal guidance 130) and
-
the manufacturer provides the first 12 weeks of certolizumab pegol (10 pre-loaded 200-mg syringes) free of charge to all patients starting treatment.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- NICE . Guide to Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- Connock MJ, Tubeuf S, Malottki K, Uthman A, Round J, Bayliss S, et al. Certolizumab Pegol (CIMZIA®) for the Treatment of Rheumatoid Arthritis n.d. www.nice.org.uk/guidance/index.jsp?action=download%26o=45795.
- Keystone E, Heijde DV, Mason D, Landewe R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: Findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319-29.
- Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797-804.
- Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis 2009;68:805-11.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727-35.
- Brennan A, Bansback N, Nixon R. Modelling the Cost Effectiveness of TNF-a Inhibitors in the Management of Rheumatoid Arthritis: Results from the British Society for Rheumatology Biologics Registry 2006.
- Weinblatt M, Keystone E, Furst D, Moreland L, Weisman M, Birbara C, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA Trial. Arthritis Rheum 2003;48:35-4.
- Kim H-Y. A randomized, double-blind, placebo-controlled, phase III study of the human anti-tumor necrosis factor antibody adalimumab administered as subcutaneous injections in Korean rheumatoid arthritis patients treated with methotrexate. APLAR Journal of Rheumatology 2007;10:9-16.
- Keystone E, Kavanaugh A, Sharp J, Tannenbaum H, Hua Y, Teoh L, et al. Radiographic, Clinical, and Functional Outcomes of Treatment with Adalimumab (a human anti-tumour necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled 52-week trial. Arthritis Rheum 2004;50:1400-11.
- Weinblatt M, Kremer J, Bankhurst A, Bulpitt K, Fleischmann R, Fox R, et al. A Trial of Etanercept, a Recombinant Tumor Necrosis Factor Receptor: Fc fusion protein, in patients with Rheumatoid Arthritis receiving Methotrexate. N Engl J Med 1999;340:253-9.
- Westhovens R, Yocum D, Han J. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum 2006;54:1075-86.
- Schiff M, Keiserman M. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 2008;67:1096-103.
- Strand V, Balbir-Gurman A, Pavelka K. Sustained benefit in rheumatoid arthritis following one course of rituximab: improvements in physical function over 2 years. Rheumatology (Oxford) 2006;45:1505-13.
- Smolen JSB. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987-97.
- Nixon RM, Bansback N, Brennan A. Using mixed treatment comparisons and meta-regression to perform indirect comparisons to estimate the efficacy of biologic treatments in rheumatoid arthritis. Stat Med 2007;26:1237-54.