Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 08/239/01. The assessment report began editorial review March 2010 and was accepted for publication in May 2010. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
This is a summary of the evidence review group (ERG) report on the clinical effectiveness and cost-effectiveness of adjuvant imatinib post resection of KIT-positive gastrointestinal stromal tumours (GISTs) compared with resection only in patients at significant risk of relapse. The ERG report is based on the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The bulk of the clinical evidence submitted was in the form of one randomised controlled trial (RCT), the Z9001 trial, funded by the manufacturer, which compared resection + adjuvant imatinib for 1 year to resection only. Results were immature, with median recurrence-free survival (RFS) not yet having been reached at the time of analysis. The trial did provide evidence of a delay in disease recurrence [1-year RFS rate of 98% in the imatinib arm vs 83% in the placebo arm [hazard ratio (HR) 0.35, 95% confidence interval (CI) 0.22 to 0.53, p < 0.0001)] but no evidence of an overall survival benefit. There was no long-term evidence around the rate of imatinib resistance over time with different treatment strategies (± adjuvant treatment). The relevant patient group for this appraisal is those at significant risk of relapse. These form a subgroup of the Z9001 trial, and all information regarding this group was designated ‘Commercial-in-Confidence’ (CIC). Median observation time for RFS was also CIC. The manufacturer constructed a Markov model comprising 10 health states designed to estimate costs and effects of treatment over a lifetime time horizon. The manufacturer’s estimate of the base-case incremental cost-effectiveness ratio (ICER) was £22,937/quality-adjusted life-year (subsequently amended by the manufacturer to £23,601). While the structure of the model reasonably reflected the natural history of the disease, the ERG had numerous concerns regarding the selection of, and assumptions around, input parameters (utilities, monthly probabilities of recurrence and death). Furthermore, the model was set up in such a way that any delay in recurrence translated directly into a survival benefit, an assumption that has no evidence base. A further assumption not supported by evidence was that any treatment benefit gained in the first year is carried on for a further 2 years at the same rate. Appropriate probabilistic sensitivity analysis was undertaken on the base case only, but not on scenario analyses, or choice of model used to estimate long-term survival data. The model was not amenable to changes in input values, thus limiting any additional analyses by the ERG to test assumptions. Due to the large number of uncertainties and assumptions, the estimated ICERs should be regarded as highly uncertain. The guidance issued by NICE in June 2010 as a result of the STA does not recommend imatinib as adjuvant treatment after resection of gastrointestinal stromal tumours, although individuals currently receiving adjuvant imatinib should have the option to continue treatment until they and their clinician consider it appropriate to stop.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the UK NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process1 is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor (here, Novartis Pharmaceuticals UK Ltd). Typically, it is used for new pharmaceutical products that are close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of the Institute. This paper presents a summary of the ERG report for the STA entitled ‘Imatinib as adjuvant treatment following resection of KIT-positive gastrointestinal stromal tumours (GISTs)’. 2
Description of the underlying health problem
Patients eligible for adjuvant imatinib according to the UK licence are those who have had a resection of KIT (CD117)-positive GIST and are deemed to be at significant risk of relapse. ‘Significant’ risk is not defined in the licence. In the industry submission it includes those patients in the moderate- and high-risk groups as defined by the Miettinen and Lasota criteria,3 which take into account tumour size, location and mitotic count. Risk of relapse and choice of treatment also depend on the specific type of KIT exon gene mutations.
Based on the findings of studies in different countries, GIST has an annual incidence of between 6.8 and 14.5 per million; around two-thirds of patients with GIST are thought to be resectable. Of the resected patients, around one-half may have a significant risk of relapse. 4
Survival after resection ranged from 48% to 80% at 5 years for low-risk GIST before the introduction of imatinib; the 5-year survival rate (approximately 95%) is similar to that of the general population, while for high-risk GISTs the 5-year survival rate ranged from 0% to 30% before the introduction of imatinib. 5 As imatinib is a relatively recent treatment for GIST, there are fewer long-term survival estimates. In a trial of imatinib for advanced GIST, with a reported follow-up of up to 71 months, median overall survival increased from 18 months to 60 months. 6
Most patients eventually show resistance to imatinib due to secondary mutations in the KIT and/or PDGFRA (alpha-type platelet-derived growth factor receptor) kinase domains. One study found that secondary or acquired resistance develops after a median of about 2 years of treatment. 7
Current guidelines state that imatinib increases recurrence-free survival (RFS) and suggest that it may be an effective treatment to prevent recurrence following primary surgery in those patients with a high risk of recurrence; these patients should be considered for inclusion in clinical trials of neoadjuvant and adjuvant therapy with imatinib. 8 Optimal treatment duration with adjuvant imatinib is not yet established, nor whether adjuvant treatment was a clinically effective or cost-effective option.
Scope of the ERG report
The research question was the clinical effectiveness and cost-effectiveness of adjuvant imatinib following resection compared with resection only in patients with KIT-positive GISTs who are at significant risk of relapse. This is consistent with the licence indication.
The clinical effectiveness data was primarily based on one ongoing randomised controlled trial (RCT), the Z9001 trial4 (n = 713), which compared resection + adjuvant imatinib for 1 year with resection alone.
Data on those patients who were at significant risk of relapse and who formed a subgroup of the trial (n = 302) were supplied as ‘Commercial in Confidence’ (CIC) data. Classification of patients according to risk was retrospective and was performed for only 78% of patients, making the results susceptible to bias. It is likely that patients in this trial are similar to patients in the UK who would be eligible for treatment with adjuvant imatinib, although there is a possibility of differing thresholds for what constitutes ‘significant’ risk.
Outcome measures in the Z9001 trial were RFS, overall survival (OS) and adverse events. Quality-of-life outcomes were not collected. Median follow-up time for OS was 19.7 months (data is CIC for RFS and for both RFS and OS in the significant-risk subgroup). It should be noted that on disease progression, all patients received treatment with imatinib or other treatment options (e.g. sunitinib) as appropriate, regardless of the treatment arm to which they were allocated; it is, in effect, different treatment strategies (one commencing with adjuvant imatinib) that are being compared long term.
The manufacturer submitted an economic model to assess the cost per quality-adjusted life-year (QALY) of resection with 3 years’ adjuvant imatinib (note: this differs from the trial where imatinib is given for 1 year) compared with resection only. Recurrence results were taken from the trial and extrapolated for longer time periods. Mortality results for patients in various health states were obtained from a variety of literature sources. Utility estimates were not available from the trial and were therefore taken from other literature sources or estimated by the manufacturer.
Methods
The ERG report comprised a critical review of the evidence for the clinical evidence and cost-effectiveness of the technology based on the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
Additional searches to confirm the completeness of published data on effectiveness and cost-effectiveness were undertaken. The ERG independently assessed the validity of the Z9001 trial and analysed CIC results for the significant-risk subgroup, which were provided separately by the manufacturer.
The model provided by the manufacturer was complex and not amenable to changes in parameter values, particularly with regard to running alternative probabilistic sensitivity analyses (PSAs). This limited the scope for the ERG to fully validate the model, and thus reduced the ERG’s confidence in the results of the model. There was also a lack of information around uncertainty estimates for certain parameters, particularly utility values, again restricting any additional sensitivity analyses.
Results
Summary of submitted clinical evidence
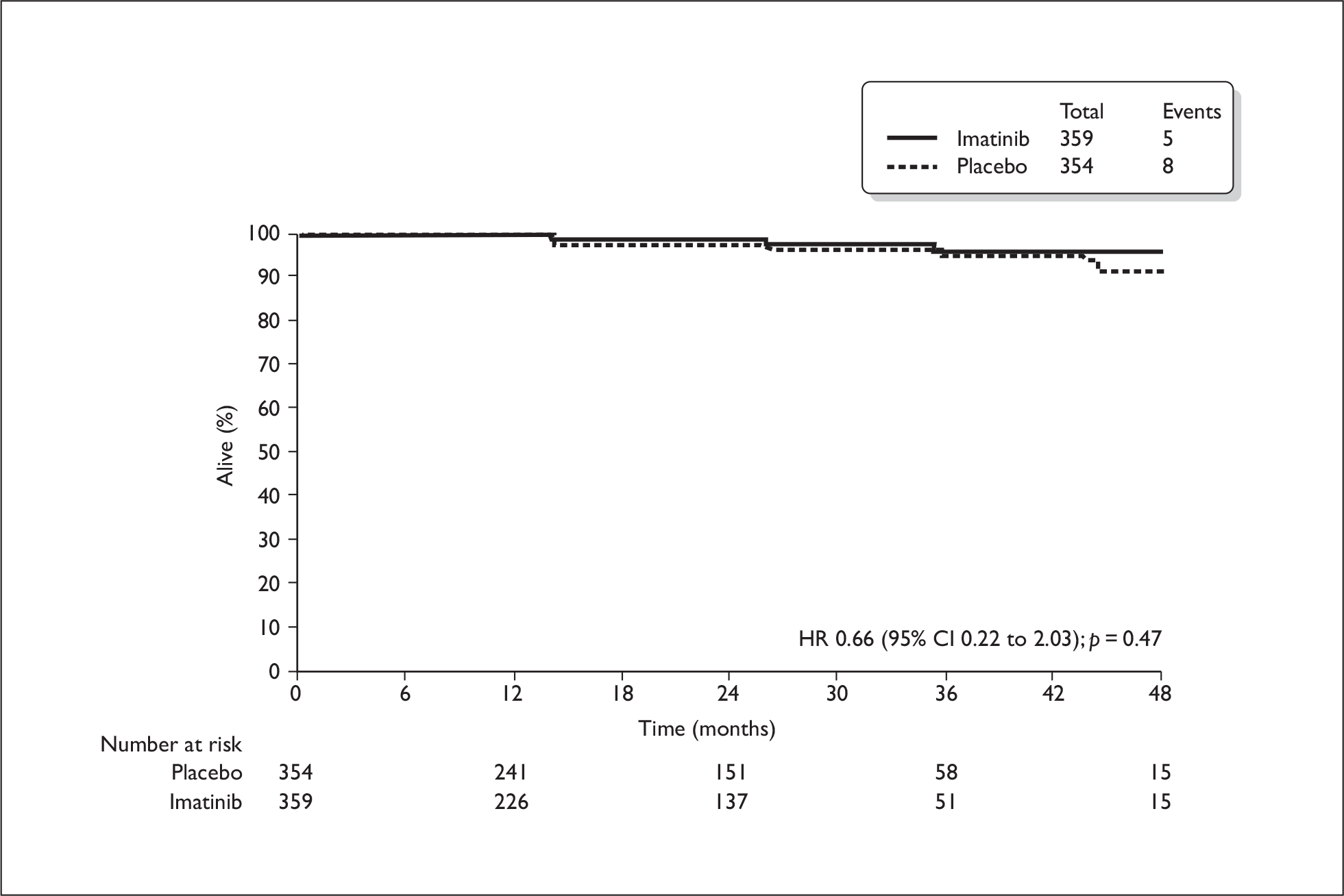
All information relating to the relevant subgroup of patients (those at significant risk of relapse) was CIC and therefore cannot be reported here. The total trial population included patients at low risk of relapse, who would not be eligible for adjuvant treatment in the UK. For this total population, the estimated 1-year RFS rate was 98% in the imatinib arm and 83% in the placebo arm (HR 0.35, 95% CI 0.22 to 0.53, p < 0.0001), therefore a delay in recurrence was evident (Figure 1). Median RFS had not yet been reached at the time of analysis and few patients were evaluable at later time points. The OS rates for the total population were similar and most patients were still alive at the time of data analysis (Figure 2). Results from the Z9001 trial on subsequent use of imatinib in patients who have previously had adjuvant imatinib were also CIC. The ERG identified no additional results on imatinib resistance rates with subsequent use in the long term.
FIGURE 1.
Recurrence-free survival in total population (from manufacturer’s submission).
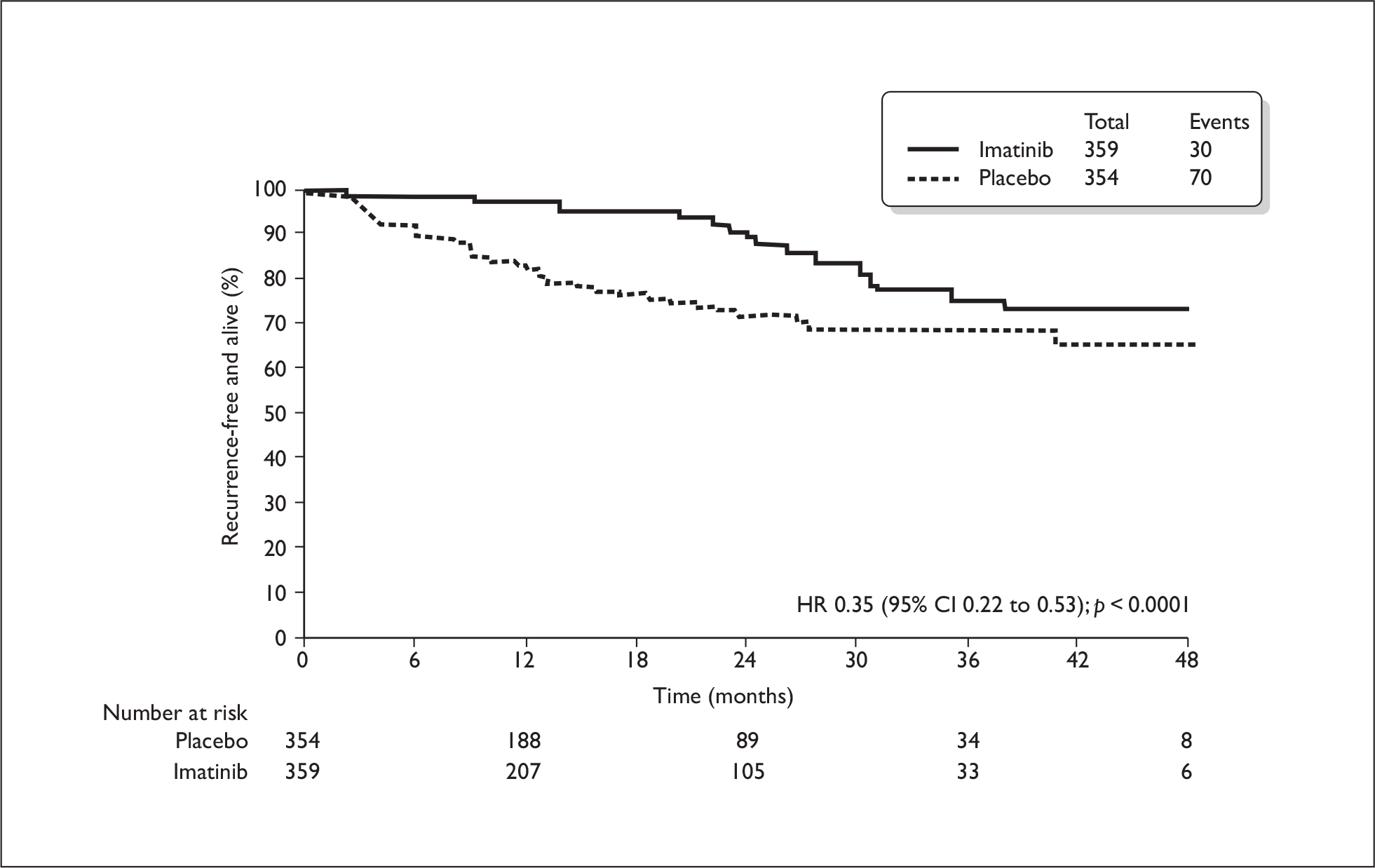
FIGURE 2.
Overall survival in total population (from manufacturer’s submission).
Summary of submitted cost-effectiveness evidence
The manufacturer’s estimate of the base-case incremental cost-effectiveness ratio (ICER) was £22,937/QALY (subsequently amended by the manufacturer to £23,601). This estimate relies on patients receiving adjuvant imatinib for 3 years, for which there is no evidence from the Z9001 trial, which used 1 year of adjuvant treatment. The manufacturer’s base-case analysis suggested that there was an approximately 60% chance that imatinib was cost-effective at willingness-to-pay thresholds of between £20,000 and £30,000 per QALY. Four additional analyses were submitted: (1) significant-risk patients, receiving imatinib for 1 year; (2) the overall at-risk population (no treatment time specified); (3) the high-risk only population, receiving 1 year of imatinib; and (4) the high-risk only population, receiving 3 years of imatinib. ICERs were £13,550, £32,981, £6109 and £19,813, respectively.
Commentary on the robustness of submitted evidence
Clinical effectiveness
The population relevant to this appraisal was a subgroup of patients with significant risk of recurrence. Assignment of risk level was retrospective, and only 78% of patients were categorised according to risk. There is therefore a possibility of imbalances at baseline and risk of bias. Baseline characteristics of the significant risk population in the two trial arms are CIC.
There was some uncertainty around the handling of missing data and which definition of ‘recurrence’ was used for the analyses in the submission; the ERG was unable to gauge the potential impact on results. The results from the trial were immature, as follow-up times were short, and results at later time points were based on few patients at risk. There is no evidence to show that adjuvant imatinib given for 1 year prolongs overall survival. Median overall survival estimates were not reached in either treatment arm (total trial population). There is no good long-term evidence on recurrence rates (resistance) when imatinib is given repeatedly. Quality of life was not measured as part of the Z9001 trial.
Cost-effectiveness
The model provided by the manufacturer contained no programming errors and the structure of the model reasonably reflected the natural history of the disease. However, the ERG was unable to conduct more than a limited range of alternative analyses to test assumptions made by the manufacturers, as the model was not amenable to changes in input values. Furthermore, the ERG had a number of concerns relating to the monthly probabilities of death in various health states and their application in the model. The manufacturer assumed that all monthly probabilities post health states A, B and D (Figure 3) were the same in both treatment arms, i.e. the probability of recurrence or death did not depend on whether a patient received adjuvant treatment or resection only. This seems implausible to the ERG. The result of this is that any differences in a delay in progression translate directly into a survival gain of the same length.
FIGURE 3.
Transition pathways in manufacturer’s model.
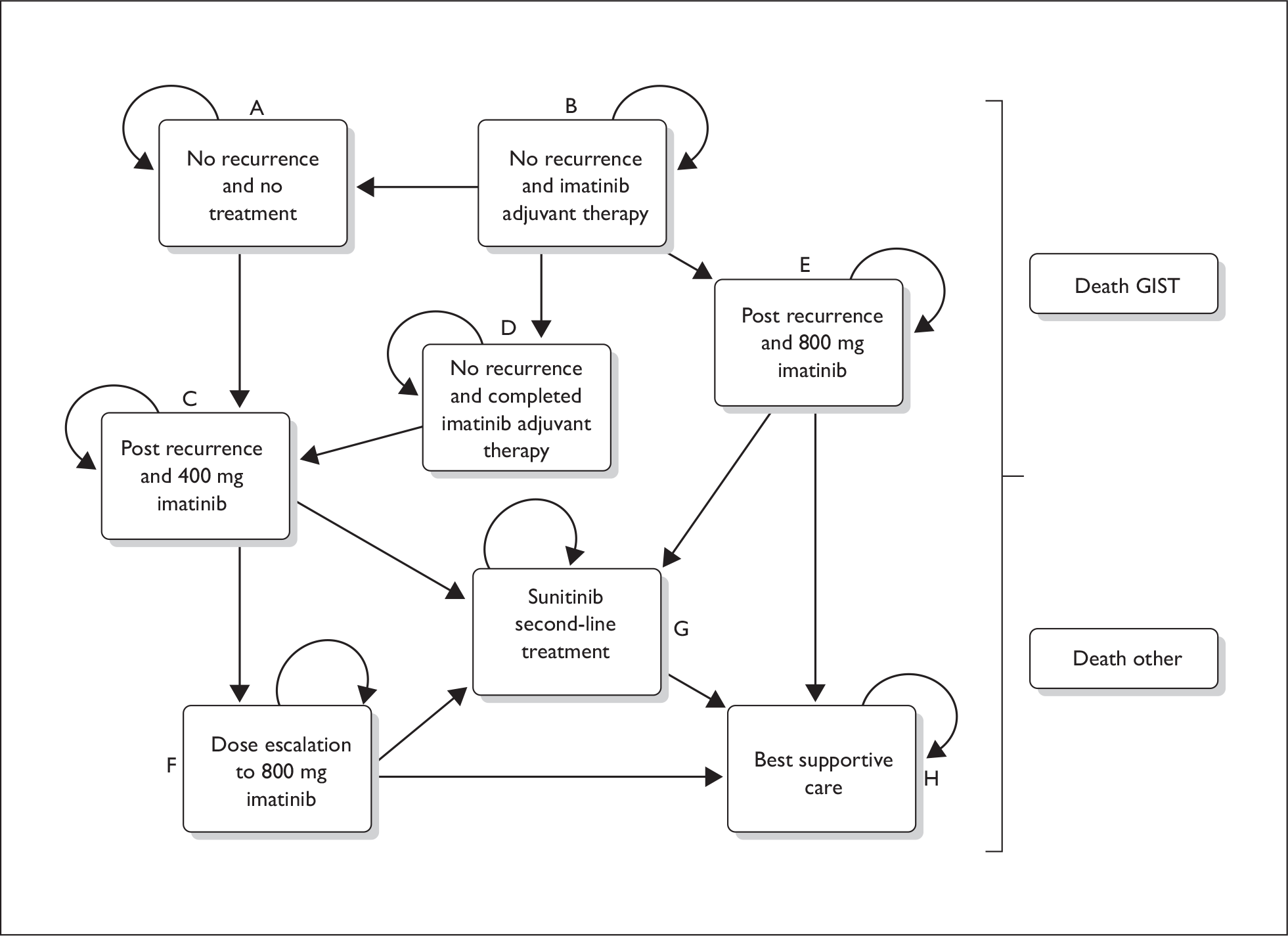
The manufacturer also provided no justification for the selection of studies from which the input parameters were derived, no details on how the death and recurrence rates were calculated, and there appeared to be some errors and inconsistencies. The impact on the ICER is unlikely to be large – because of the model structure, patients in both treatment arms received the same inputs post health states A, B and D – but this does not impart confidence in the modelling process.
The assumption of sustained benefit from treatment for 2 years beyond the evidence base is a generous one and systematically favours imatinib, resulting in a reduced ICER. Because of this way of extrapolating the treatment benefit, and because of the model structure, the logic of the model is that it is not sensible to stop adjuvant imatinib at any time point but to continue indefinitely. The ERG suggests that there is no proven benefit for this treatment strategy.
A further concern is that appropriate PSA was undertaken on the base case only, and not on the other scenario analyses. In particular, PSA was not undertaken on the subgroup analyses. One-way sensitivity analyses were conducted as part of the clarification process, but no scenario analyses were undertaken on choice of model used to estimate long-term survival data. The absence of PSA for the subgroup analyses provided further exacerbates the paucity of the evidence on the uncertainty around the cost-effectiveness estimates provided in the submission.
The utility values used relied heavily on one study (Chabot et al. 9) based on treatment with sunitinib (after imatinib failure). The authors of this study advised caution in the interpretation of their results due to large uncertainty. The ERG also identified flaws in how health-state utilities were modelled, for example relating to age adjustment, and the use of a mean utility value only (rather than a range).
Table 1 shows ERG estimates of the likely impact on the ICER of a number of parameter assumptions/changes.
| Parameter | Effect on ICER (↑ = increase, ↓ = decrease) |
|---|---|
| Decrease in utility value for RFS to 0.95 and 0.9 (manufacturer assumed a utility value of 1) | ↑ (small) |
| No estimate of uncertainty associated with recurrence-free health state in model (benefit for patients likely to have been overestimated) | ↑ |
| No utilities < 0 included (should have been included as within range of possible utilities) | ↑ |
| No disutility associated with adverse events of adjuvant treatment | ↑ |
| Gradual increase in recurrence rates after year 1 with adjuvant treatment (rather than sustained benefit over 3 years, which seems implausible) | ↑ |
| Correction of potential double-counting of utility loss (for health state and age) | ↑ |
| Error identified by manufacturer relating to recurrence rates | ↑ (also wider 95% CI) |
| Increased resistance to imatinib over time (manufacturer’s sensitivity analysis found a reduced ICER – this seems implausible to the ERG and no adequate explanation was given) | ↑ |
| Reduction in survival benefit with adjuvant treatment (manufacturer’s sensitivity analysis found a reduced ICER – this seems implausible as this means a net benefit from patients dying earlier) | ↑ |
| Reduction in length of time of imatinib use (1 year only) | ↓ |
| Use of adjuvant imatinib in high-risk population only | ↓ |
Conclusions
A survival benefit with adjuvant imatinib has to date not been shown. There is a lack of good long-term evidence around the rate of imatinib resistance over time with different treatment strategies (± adjuvant imatinib, for 1 year or 3 years), and the effect on overall survival. There are serious concerns around the validity and application in the manufacturer’s model of a number of input parameters, such as utilities and monthly probabilities of death. The model also makes a basic assumption that any benefit in delay of recurrence translates directly into an increase in survival over the long term; this assumption is not supported by any evidence and does not take into account the possibility of differing rates of imatinib resistance between the two treatment arms. Due to the large number of uncertainties and assumptions, the estimated ICERs should be regarded as highly uncertain. It is possible that results from ongoing trials will inform this issue. The EORTEC 62024 trial10 in particular has as an end point time to imatinib resistance, which may be a more useful proxy for overall survival. Should adjuvant imatinib treatment be shown to be beneficial in the future, further research would also be required into the type of patient most likely to benefit from adjuvant treatment based on mutational analysis.
Summary of NICE guidance issued as a result of the STA
NICE guidance issued in June 2010 does not recommend imatinib as adjuvant treatment after resection of gastrointestinal stromal tumours, although individuals currently receiving adjuvant imatinib should have the option to continue treatment until they and their clinician consider it appropriate to stop.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence (NICE) . Guide to Single Technology (STA) Process n.d. www.nice.org.uk/media/42D/B3/STAGuideLrFinal.pdf (accessed 24 September 2010).
- Dretzke J, Round J, Connock M, Tubeuf S, Pennant M, Fry-Smith A, et al. Imatinib As Adjuvant Treatment Following Resection of KIT-Positive Gastrointestinal Stromal Tumours 2009. http:www.nice.org.uk/nicemedia/live/12178/48128/48128.pdf.
- Miettinen M, Lasota J. Gastrointestinal stromal tumours: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83.
- DeMatteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PWT, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104.
- Park CK, Lee EJ, Kim M, Lim HY, Choi DI, Noh JH, et al. Prognostic stratification of high-risk gastrointestinal tumours in the era of targeted therapy. Ann Surg 2008;247:1011-18.
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, et al. Long-term results from a randomised phase II trial of standard versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumours expressing KIT. J Clin Oncol 2008;26:620-5.
- Demetri GD, van Oosterom AT, Garrett CR. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38.
- Reid R, Bulusu R, Buckels JEA. Guidelines for the Management of Gastrointestinal Stromal Tumours (GISTs) 2009. www.mccn.nhs.uk/userfiles/documents/04%20GIST_Mngmnt_Gdlns.pdf.
- Chabot I, LeLorier J, Blackstein ME. The challenge of conducting pharmacoeconomic evaluations in oncology using crossover trials: the example of sunitinib for gastrointestinal stromal tumours. Eur J Cancer 2008;44:972-7.
- US National Institutes of Health . Imatinib Mesylate or Observation Only in Treating Patients Who Have Undergone Surgery for Localised Gastrointestinal Stromal Tumor 2010. www.clinicaltrials.gov/ct2/show/NCT00103168?term=62024%26rank=1.