Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 09/59/01. The assessment report began editorial review in June 2010 and was accepted for publication in July 2010. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of gefitinib for the first-line treatment of locally advanced or metastatic non-small cell lung cancer, in accordance with the licensed indication, based upon the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal process. The submitted clinical evidence consisted of the IRESSA Pan-ASian Study (IPASS); a phase III open-label randomised controlled trial conducted in 87 centres in East Asia which compared the use of gefitinib with paclitaxel/carboplatin in 1217 chemotherapy (CTX)-naive patients with stage IIIB/IV pulmonary adenocarcinoma. The manufacturer’s submission focused on a subgroup of patients in IPASS who were epidermal growth factor receptor (EGFR) gene mutation-positive (M+) (n = 261; 21% of the total IPASS population). The primary clinical outcome was progression-free survival (PFS). Secondary outcomes included overall survival, clinically relevant improvement in quality of life and adverse events (AEs). Cost-effectiveness was measured in terms of incremental cost per quality-adjusted life-year (QALY). In the overall population, PFS was significantly longer in patients treated with gefitinib than in those treated with paclitaxel/carboplatin (hazard ratio 0.74, 95% confidence interval 0.65 to 0.85; p < 0.0001). The manufacturer reported an incremental cost-effectiveness ratio (ICER) of £20,744 per QALY gained for the target population. The probabilistic sensitivity analysis illustrated that for patients who are EGFR M+, gefitinib compared with doublet CTX was not likely to be cost-effective at what would usually be considered standard levels of willingness to pay for an additional QALY; the mean ICER for gefitinib EGFR M+ versus doublet CTX EGFR M+ was reported as £35,700 per QALY. Additional analysis by the ERG included amendments to the base-case analysis, including an alternative approach to projecting survival, inclusion of two important additional comparators, sensitivity to EGFR M+ prevalence, and AE costs and disutilities. The manufacturer’s submission provides clinical evidence to support the use of gefitinib in EGFR M+ patients with adenocarcinoma histology only. Before patients can be offered first-line treatment with gefitinib they must undergo EGFR mutation status testing which is currently not routinely available in the NHS. At the time of writing, the guidance document issued by NICE on 28 July 2010 states that ‘Gefitinib is recommended as an option for the first-line treatment of people with locally advanced or metastatic non-small-cell lung cancer (NSCLC) if they test positive for the epidermal growth factor receptor tyrosine kinase (EGFR-TK) mutation and the manufacturer provides gefitinib at the fixed price agreed under the patient access scheme’.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of the Institute. This paper presents a summary of the ERG report for the STA entitled ‘Gefitinib for the first-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC)’.
Description of the underlying health problem
Lung cancer is the leading cause of cancer death worldwide and is responsible for over 33,000 deaths a year in England and Wales. 2 NSCLC is the most common subtype, accounting for 80% of all lung cancer cases. Despite advances in early detection, most patients still present with late-stage disease.
Survival rates for lung cancer are very poor. In England, for patients diagnosed between 1993 and 1995 and followed up to 2000, 21.4% of men and 21.8% of women with lung cancer were alive 1 year after diagnosis and less than 1% of advanced NSCLC lung cancer patients were alive after 5 years. 3,4
The majority of patients with lung cancer are diagnosed, or relapse, with incurable disease and receive palliative treatment only. For otherwise fit patients with stage III/IV NSCLC, first-line treatment consists of platinum-based combination chemotherapy (CTX) followed by docetaxel CTX or erlotinib, as currently recommended in NICE clinical guidelines. 3
Scope of the evidence review group report
Gefitinib is an orally active, selective epidermal growth factor receptor (EGFR) gene tyrosine kinase inhibitor which helps to slow the growth and spread of the cancer.
Gefitinib is indicated for the treatment of adult patients with locally advanced or metastatic NSCLC with activating mutations of EGFR;5 the scope issued by NICE is for first-line treatment only.
Before patients can be offered first-line treatment with gefitinib they must undergo EGFR mutation status testing which is currently not routinely available in the NHS.
The ERG report includes an assessment of both the clinical effectiveness and cost-effectiveness evidence submitted by the manufacturer (AstraZeneca) for the use of gefitinib compared with doublet CTX for the treatment of CTX-naive patients with stage IIIB/IV pulmonary adenocarcinoma who tested positive (M+) for the EGFR mutation. Data were presented for all patients and a subgroup of patients who were EGFR M+.
The primary clinical outcome was progression-free survival (PFS). Secondary outcomes included overall survival (OS), clinically relevant improvement in quality of life (QoL) and adverse events (AEs). Cost-effectiveness was measured in terms of incremental cost per quality-adjusted life-year (QALY).
Methods
The ERG report comprised a critical review of the evidence for the clinical evidence and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
The ERG evaluated the quality of the manufacturer’s clinical effectiveness review which comprised of a systematic review, meta-analysis and mixed-treatment comparison (MTC). Searches conducted by the manufacturer were assessed for completeness and the single trial put forward as evidence of effectiveness was critically appraised using the manufacturer’s responses to specific questions in the submission template.
Cost-effectiveness evidence submitted by the manufacturer consisted of a systematic review and a de novo economic evaluation. The ERG assessed the manufacturer’s searches for completeness, critically appraised the submitted economic model using the NICE reference case checklist6 and the Drummond 10-point checklist,7 and conducted a detailed evaluation of the model and the validity of the MTC results for economic analysis of non-trial comparators.
Additional analysis by the ERG included amendments to the base-case analysis, including an alternative approach to projecting survival, inclusion of two important additional comparators, sensitivity to EGFR M+ prevalence, and AE costs and disutilities.
Results
Summary of submitted clinical evidence
Only one relevant randomised controlled trial (RCT) was identified by the manufacturer; the IRESSA Pan-ASian Study (IPASS). 8 IPASS8 is a phase III open-label RCT conducted in 87 centres in East Asia which compared the use of gefitinib with paclitaxel/carboplatin in 1217 CTX-naive patients with stage IIIB/IV pulmonary adenocarcinoma. 8 The manufacturer’s submission focused on a subgroup of patients in IPASS8 who were EGFR M+ (n = 261; 21% of the total IPASS population).
In the overall population, PFS was significantly longer in patients treated with gefitinib than in those treated with paclitaxel/carboplatin [hazard ratio (HR) 0.74, 95% confidence interval (CI) 0.65 to 0.85; p < 0.0001]. In a subgroup analysis of 261 patients who were EGFR M+, PFS was significantly longer among those who received gefitinib than among those who received paclitaxel/carboplatin (HR 0.48, 95% CI 0.36 to 0.64; p < 0.0001). In the subgroup of patients who were EGFR mutation-negative (M–) (n = 176), PFS was significantly longer among those who received paclitaxel/carboplatin (HR with gefitinib 2.85, 95% CI 2.05 to 3.98; p < 0.001).
Overall survival estimates were based on an interim analysis (37% maturity) and were similar for gefitinib and paclitaxel/carboplatin patients in the overall trial population [18.6 months for gefitinib vs 17.3 months for paclitaxel/carboplatin (HR 0.91, 95% CI 0.76 to 1.10)]. There was no significant difference in OS between gefitinib and paclitaxel/carboplatin in EGFR M+ patients groups (HR 0.78, 95% CI 0.50 to 1.20). Median OS was 12.1 months in the gefitinib EGFR M– subgroup and was 12.6 months in the paclitaxel/carboplatin EGFR M– subgroup.
Significantly more patients in the gefitinib group than in the paclitaxel/carboplatin group had a clinically relevant improvement in QoL, as assessed by scores on the Functional Assessment of Cancer Therapy – Lung questionnaire,9 [odds ratio (OR) 1.34, 95% CI 1.06 to 1.69; p = 0.01] and by scores on the Trial Outcome Index (OR 1.78, 95% CI 1.40 to 2.26; p < 0.001). Gefitinib was associated with fewer grade 3 or 4 AEs.
After late identification of interim analysis data from an ongoing RCT, the manufacturer performed a meta-analysis using data from IPASS8 and the North East Japan Gefitinib Study Group (NEJGSG). 10 Meta-analysis demonstrated significant improvement in PFS for EGFR M+ patients in the gefitinib arm compared with EGFR M+ patients in the paclitaxel/carboplatin arm (HR 0.43, 95% CI 0.34 to 0.53; p < 0.00001).
The manufacturer conducted an MTC comparing doublet CTX in CTX-naive patients with NSCLC, using paclitaxel/carboplatin evidence from IPASS8 as a baseline and including 29 RCTS. The MTC did not identify any individual doublet CTX as offering both significant clinical benefit and significantly improved tolerability over the other doublet CTX regimens.
Summary of submitted cost-effectiveness evidence
The manufacturer conducted a de novo economic evaluation. A Markov model was developed to evaluate the cost-effectiveness of gefitinib compared to four different doublet CTX regimens. The clinical data used in the economic evaluation were generated from a variety of sources. The HR for PFS for gefitinib EGFR M+ patients was derived from a meta-analysis conducted by the manufacturer and the HR for OS for gefitinib EGFR M+ patients was extrapolated from IPASS. 8 Estimates of the HRs for PFS and OS for the doublet CTX regimens were sourced indirectly from the MTC. Although the economic evaluation is primarily trial-based, there is a modelling component with regard to the extrapolation of health effects because IPASS8 is ongoing. The economic evaluation adopts a lifetime horizon for consideration of costs and benefits and the perspective is that of the UK NHS and Personal Social Services.
The manufacturer reported an incremental cost-effectiveness ratio (ICER) of £20,744 per QALY gained for the target population. In addition to the main cost-effectiveness results, ICERs for selected subgroups were presented. Univariate sensitivity analysis, scenario analyses and probabilistic sensitivity analysis (PSA) were undertaken by the manufacturer.
The PSA illustrated that for patients who are EGFR M+, gefitinib compared with doublet CTX was not likely to be cost-effective at what would usually be considered standard levels of willingness to pay for an additional QALY; the mean ICER for gefitinib EGFR M+ versus doublet CTX EGFR M+ was reported as £35,700 per QALY.
Commentary on the robustness of submitted evidence
Clinical evidence
Before patients can be offered first-line treatment with gefitinib they must undergo EGFR mutation status testing. Currently, EGFR mutation testing is not routinely available in the NHS. It is uncertain how future testing of newly diagnosed patients with NSCLC will be orchestrated within the NHS in England and Wales. In addition, patients with adenocarcinoma histology would need to be identified prior to EGFR mutation testing. This diagnostic service is not routinely available to patients in the NHS.
The ERG highlighted that the clinical validity characteristics of EGFR tests could impact on treatment outcomes with gefitinib. In particular, a positive result for EGFR mutation status does not guarantee a good outcome, as a proportion (clinical false-positives) of such patients receiving gefitinib will not experience any benefit (shorter PFS) compared with current treatment with doublet CTX and may in fact be worse off by not receiving doublet CTX (Figure 1). The implications of using EGFR mutation tests must be carefully considered for both EGFR M+ and EGFR M– patients.
FIGURE 1.
Effects of diagnostic test on treatment pathways and patient outcome. AEs, adverse events; CTX, chemotherapy; EGFR, epidermal growth factor receptor gene; M+, mutation-positive; PFS, progression-free survival; OS, overall survival; Tx, treatment.
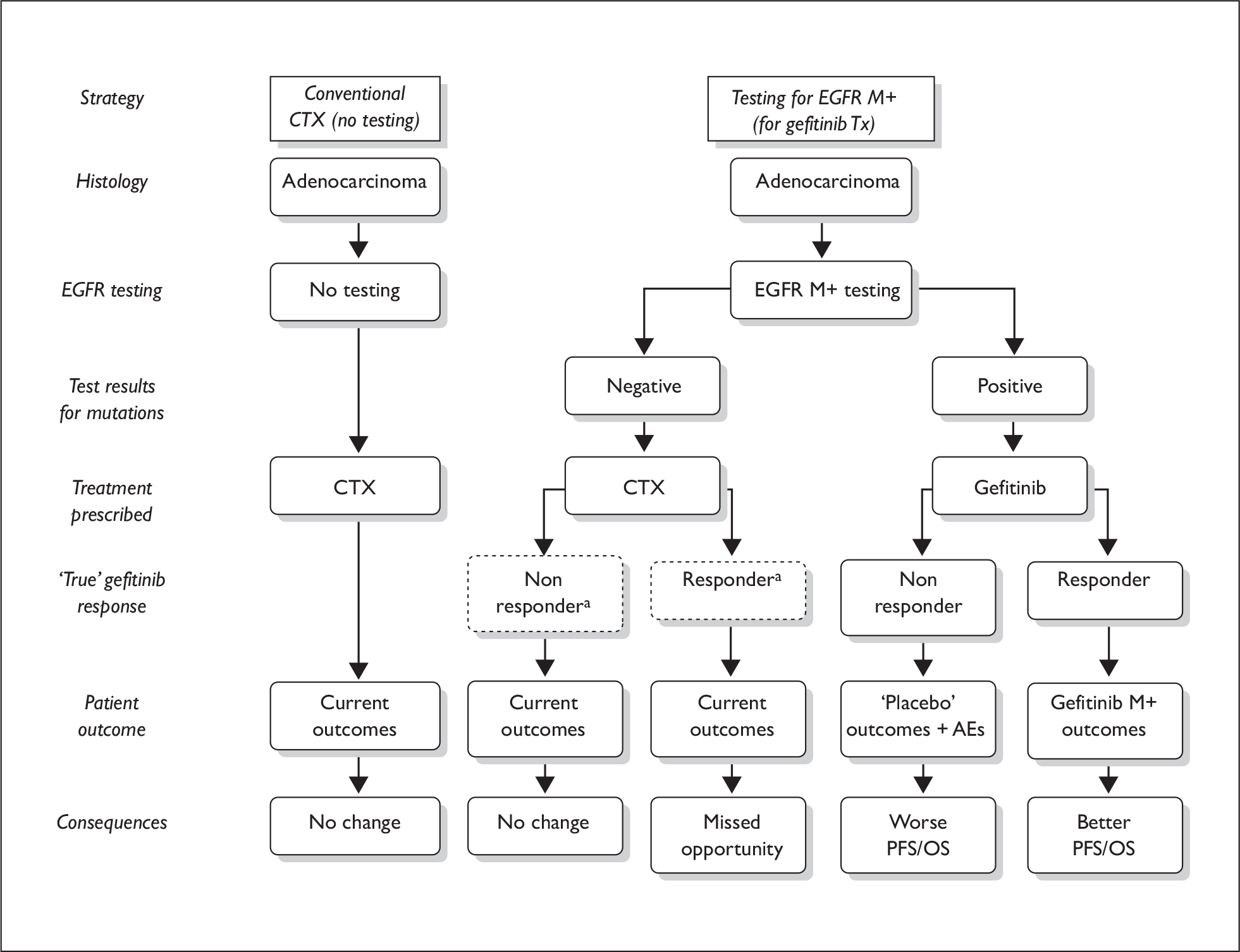
The number of patients requiring first-line treatment for NSCLC who are EGFR M+ in England and Wales is currently uncertain. A recent publication has estimated this figure to be between 5% and 10% in the Western population. 11
The clinical evidence was derived from a high quality trial in patients with NSCLC; convincing efficacy and QoL evidence were presented by the manufacturer for a specific group of patients.
The main evidence cited by the manufacturer was derived from the IPASS8 trial; this study has reached only 37% maturity for the determination of OS. The final OS estimates for patients in IPASS8 will be available in 2010. However, it may be difficult for the investigators to interpret the final OS data from IPASS8 owing to the substantial number of patients in both groups who went on to receive a variety of second-line CTX regimens.
Clinical data from two other smaller trials [the NEJGSG10 trial and the First-SIGNAL (First-line Single agent Iressa versus Gemcitabine and cisplatin trial in Never-smokers with Adenocarcinoma of the Lung)12 trial] comparing gefitinib with doublet CTX are also available.
The main focus of the manufacturer’s submission was on patients who were EGFR M+; this subgroup of patients cannot be considered to have been truly randomised in the trial as the randomisation process did not include stratification by biomarker type. In addition, the trial was not powered to perform this subgroup analysis.
The generalisability of the IPASS8 study to patients in England and Wales is limited. None of the IPASS8 centres were based in the UK; all of the patients were from East Asia. All of the IPASS8 patients had adenocarcinoma histology; in the UK patients with adenocarcinoma are estimated to make up approximately 25% of the population with NSCLC. 13 IPASS8 includes patients with performance status (PS) 2; in England and Wales, CTX is not recommended by NICE for patients with metastatic disease with PS 2 unless as part of a clinical trial. 3 The demographic characteristics of patients in IPASS8 do not match those of the relevant population in England and Wales; IPASS8 patients are predominantly female and never smokers.
In the UK, the most common first-line CTX regimen for patients with NSCLC is gemcitabine with either carboplatin or cisplatin. In IPASS,8 gefitinib is compared with paclitaxel/carboplatin; it has been estimated by the manufacturer that approximately only 5% of patients receive paclitaxel/carboplatin as a first-line treatment for NSCLC in England and Wales.
The MTC methods used by the manufacturer to compare paclitaxel/carboplatin with a range of doublet CTX regimens in unselected populations are appropriate. However, the ERG considered that the MTC was weak as it was reliant on the assumption that EGFR mutation status does not affect treatment outcomes if patients are receiving doublet CTX. The ERG believes this assumption is too strong as it is wholly reliant on the results of a subgroup analysis from a single RCT of patients with adenocarcinoma histology. The evidence base for the studies used in the comparison of gefitinib with doublet CTX may not be generalisable to the EGFR M+ population.
Economic evidence
The manufacturer’s economic evaluation did not compare gefitinib with docetaxel or pemetrexed; both of these CTX regimens are listed as relevant comparators in the final NICE scope. In response to the ERG’s clarification letter, the manufacturer provided an updated version of the MTC and included pemetrexed. The ERG considered that not including pemetrexed or docetaxel as comparators in the economic evaluation was a major weakness of the manufacturer’s submission.
The ERG identified key areas where corrections and/or adjustments to the economic model are required: CTX costs, cycles, and exposure; OS and PFS modelling; and use of discounting and continuity correction methods. Taken together, the ERG’s corrections and/or adjustments to the submitted model increased the size of the ICER for the base-case population from £20,010 to over £70,000 per QALY (Tables 1 and 2). This suggests that the cost-effectiveness of gefitinib compared to doublet CTX for CTX-naive EGFR M+ patients may be less favourable than presented by the manufacturer in the manufacturer’s submission.
| Model amendment | Gemcitabine/carboplatin | Vinorelbine/cisplatin | Gemcitabine/cisplatin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inc. Costs | Inc. QALYs | ICER (£/QALY) | Inc. Costs | Inc. QALYs | ICER (£/QALY) | Inc. Costs | Inc. QALYs | ICER (£/QALY) | |
| Submitted model | £3666 | 0.1767 | £20,744 | £8024 | 0.2229 | £35,992 | £4138 | 0.1445 | £28,633 |
| Base case with 6-year horizon | £3761 | 0.1767 | £21,284 | £8151 | 0.2229 | £36,562 | £4222 | 0.1445 | £29,217 |
| Revised MTC | £3858 | 0.1824 | £21,151 | £8149 | 0.2229 | £36,557 | £4218 | 0.1445 | £29,181 |
| Amend first-line CTX costs | £4057 | 0.1767 | £22,956 | £8447 | 0.2229 | £37,890 | £4077 | 0.1445 | £28,215 |
| Reduced cycles of CTX | £5599 | 0.1735 | £32,278 | £9547 | 0.2194 | £43,512 | £6244 | 0.1409 | £44,308 |
| Revise OS models | £1985 | 0.1174 | £16,907 | £7175 | 0.1893 | £37,905 | £2245 | 0.0788 | £28,509 |
| Revise PFS models | £5019 | 0.1630 | £30,788 | £9299 | 0.2097 | £44,356 | £5409 | 0.1313 | £41,209 |
| IPASS PFS HR (not MA) | £4450 | 0.1678 | £26,520 | £8840 | 0.2140 | £41,304 | £4911 | 0.1356 | £36,219 |
| Revise discounting method | £3674 | 0.1796 | £20,453 | £8123 | 0.2266 | £35,839 | £4146 | 0.1469 | £28,229 |
| Omit GCSF prophylaxis | £4039 | 0.1767 | £22,855 | £8429 | 0.2229 | £37,809 | £4500 | 0.1445 | £31,141 |
| Continuity correction | £3362 | 0.1767 | £19,024 | £7891 | 0.2229 | £35,398 | £3895 | 0.1445 | £26,956 |
| Correct misaligned cycles | £3762 | 0.1767 | £21,290 | £8152 | 0.2229 | £36,567 | £4223 | 0.1445 | £29,223 |
| Correct second-line CTX costs | £4380 | 0.1767 | £24,785 | £8085 | 0.2229 | £36,264 | £4657 | 0.1445 | £32,228 |
| Common CTX outcomes | £5114 | 0.1892 | £27,028 | £7043 | 0.1896 | £37,148 | £5149 | 0.1880 | £27,394 |
| CTX treatment exposure | £4543 | 0.1767 | £25,706 | £8737 | 0.2229 | £39,189 | £5067 | 0.1445 | £35,062 |
| Combined effect of all changes | £7554 | 0.1253 | £60,273 | £8842 | 0.1256 | £70,390 | £7322 | 0.1241 | £59,016 |
| Model amendment | Docetaxel/cisplatin | Pemetrexed/cisplatin | ||||
|---|---|---|---|---|---|---|
| Inc. costs | Inc. QALYs | ICER (£/QALY) | Inc. costs | Inc. QALYs | ICER (£/QALY) | |
| Submitted modela | -– | – | – | – | – | – |
| With revised MTC | £4434 | 0.1627 | £27,252 | –£134 | 0.0601 | –£2223 |
| Reduced cycles of CTXb | £6254 | 0.1593 | £39,263 | £2484 | 0.0565 | £43,984 |
| Revise OS models | £2591 | 0.1013 | £25,590 | –£3115 | –0.0379 | £82,125 |
| Revise PFS models | £5636 | 0.1494 | £37,735 | £1091 | 0.0469 | £23,271 |
| IPASS PFS HR (not MA) | £5123 | 0.1538 | £33,311 | £555 | 0.0512 | £10,838 |
| Revise discounting method | £4356 | 0.1654 | £26,340 | –£264 | 0.0610 | –£4323 |
| Omit GCSF prophylaxis | £4712 | 0.1627 | £28,961 | £144 | 0.0601 | £2402 |
| Continuity correction | £4024 | 0.1627 | £24,728 | –£600 | 0.0601 | –£9984 |
| Correct misaligned cycles | £4435 | 0.1627 | £27,257 | –£134 | 0.0601 | –£2223 |
| Correct second-line CTX costs | £4944 | 0.1627 | £30,385 | £842 | 0.0601 | £14,004 |
| CTX treatment exposure | £5200 | 0.1627 | £31,961 | £958 | 0.0601 | £15,931 |
| Combined effect of all changes | £6285 | 0.0862 | £72,908 | £1574 | –0.0560 | –£28,080 (gefitinib dominated) |
The ERG highlighted that the results of the manufacturer’s economic evaluation were predicated on the use of the EGFR mutation test (or similar) described in IPASS. 8 This means that if a different EGFR mutation test is used and/or does not demonstrate similar analytic validity, the manufacturer’s cost-effectiveness results may no longer be valid. This assessment does not relate solely to use of gefitinib, but to the specific combination of mutation testing and gefitinib treatment studied in IPASS. 8
Finally, during the clarification process the manufacturer was asked to provide individual patient data (IPD) from IPASS8 that would allow the ERG to explore a number of weaknesses identified in the economic model. The manufacturer replied that it could not share IPD, but would be willing to conduct specific analyses on behalf of the ERG. A request was made to the manufacturer to conduct these analyses. The manufacturer responded that it would not able to provide the results of the requested analyses within the timeframe of the STA process.
Conclusions
The manufacturer’s submission provides clinical evidence to support the use of gefitinib in EGFR M+ patients with adenocarcinoma histology only. Before patients can be offered first-line treatment with gefitinib they must undergo EGFR mutation status testing which is currently not routinely available in the NHS.
Major weaknesses in the clinical section of the manufacturer’s submission identified by the ERG include: (i) the clinical results of IPASS8 are not generalisable to the majority of patients with NSCLC in clinical practice in England and Wales; and (ii) to date, there are no direct clinical trial data to demonstrate that use of gefitinib as a first-line treatment by EGFR M+ patients leads to improved OS compared with the use of paclitaxel/carboplatin.
The ERG’s corrections and/or adjustments to the submitted economic model have increased the size of the ICER for the base-case population; the cost-effectiveness of gefitinib compared to doublet CTX for CTX-naive EGFR M+ patients may be less favourable than presented by the manufacturer in the manufacturer’s submission.
Summary of NICE guidance issued as a result of the STA
The guidance document issued by NICE on 28 July 2010 states that:
Gefitinib is recommended as an option for the first-line treatment of people with locally advanced or metastatic non-small-cell lung cancer (NSCLC) if:
they test positive for the epidermal growth factor receptor tyrosine kinase (EGFR-TK) mutation and
the manufacturer provides gefitinib at the fixed price agreed under the patient access scheme.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o = STAprocessguide.
- Cancer Research UK. Lung Cancer Incidence Statistics 2007. http://info.cancerresearchuk.org/cancerstats/types/lung/index.htm.
- National Institute for Health and Clinical Excellence, National Collaborating Centre for Acute Care . Clinical Guideline 24, Lung Cancer: The Diagnosis and Treatment of Lung Cancer 2009.
- National Institute for Health and Clinical Excellence . Lung Cancer: The Diagnosis and Treatment of Lung Cancer 2005.
- European Medicines Agency, Evaluation of Medicines for Human Use . Assessment Report for Iressa 2009. www.emea.europa.eu/humandocs/PDFs/EPAR/iressa/H-1016-en6.pdf.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/pdf/TAP_Methods.pdf.
- Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working. BMJ 1996;313.
- Mok TS, Wu YL, Thongsprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or Carboplatin-Paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361.
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy – Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220.
- Kobayashi K, Inoue A, Maemondo M, Sugawara S, Oizumi S, Siajo Y, et al. First-line gefitinib versus first-line chemotherapy by carboplatin (CBDCA) plus paclitaxel (TXL) in non-small cell lung cancer (NSCLC) patients with EGFR mutations: a phase III study (002) by North East Japan (NEJ) Gefitinib Study Group. J Clin Oncol 2009;27.
- Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 2007;98.
- Lee J, Park K, Kim S, Lee D, H K. A Randomized Phase III Study of Gefitinib (IRESSA) Versus Standard Chemotherapy (gemcitabine Plus Cisplatin) As a First-Line Treatment for Never Smokers With Advanced or Metastatic Adenocarcinoma of the Lung n.d.
- The Information Centre for Health and Social Care . National Lung Audit: Key Findings about the Quality of Care for People With Lung Cancer in England and Wales. Report for the Audit Period 2006 2007.