Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/49/01. The contractual start date was in July 2009. The draft report began editorial review in February 2010 and was accepted for publication in June 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2011 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health. A complex relationship between genetic,1 biochemical, neural and psychological factors, and environmental aspects2 can come into play in the development of overweight and obesity. Studies have shown that age, gender and ethnicity are key risk factors for weight gain. 3–5 Without intervention, reversal of overweight and obesity is uncommon. 4
The most commonly used measure for classifying overweight and obesity is the body mass index (BMI). This is a simple ratio that is defined as the weight in kilograms divided by the square of the height in metres (kg/m2). Overweight in adults is most commonly defined as a BMI of 25 kg/m2 or over, and obesity as a BMI of 30 kg/m2 or over (Table 1).
| Classification | BMI (kg/m2) |
|---|---|
| Underweight | < 18.50 |
| Normal range | 18.50–24.99 |
| Overweight | ≥ 25.00 |
| Pre-obese | 25.00–29.99 |
| Obese | ≥ 30.00 |
| Obese class I | 30.00–34.99 |
| Obese class II | 35.00–39.99 |
| Obese class III (morbid obesity) | ≥ 40.00 |
Description of the underlying health problem
It has been estimated that globally, in 2005, approximately 1.6 billion adults (aged 15 years and over) were overweight and at least 400 million adults were obese. 7 A recent systematic review estimated that across European countries the prevalence of obesity (BMI > 30 kg/m2) ranged from 4% to 28.3% in men, and 6.2% to 36.5% in women. 8 A 2009 NHS Information Centre report,9 drawing on data from the Health survey for England 2007 (HSE)10 states that in England in 2007, 65% of men and 56% of women were overweight or obese. In Wales in 2007, 57% of adults were classified as overweight or obese, including 21% obese. 11 In 2008, 68.5% of men and 61.8% of women aged 16 years or over in Scotland were overweight or obese. 12 Data from Northern Ireland in 2005/6 show that 59% of adults were either overweight (35%) or obese (24%), and rates were similar across genders. 13
Prevalence of obesity varies by age as well as by gender. In the 2009 NHS Information Centre report9 prevalence of overweight and obesity varied by age, with high rates in older age groups (Table 2). In the Northern Ireland health and social wellbeing survey 2005/06, obesity (BMI > 30 kg/m2) was most prominent among those aged between 35 and 64 years. 13
| Overweight or obese | Age (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 16–24 years | 25–34 years | 35–44 years | 45–54 years | 55–64 years | 65–74 years | 75 + years | All ages | |
| All adults | 33 | 49 | 65 | 68 | 74 | 73 | 69 | 61 |
| Men | 33 | 54 | 71 | 75 | 79 | 77 | 71 | 65 |
| Women | 32 | 44 | 58 | 62 | 68 | 69 | 67 | 56 |
Overweight or obesity in women is more common in lower income households (defined using equivalised household income which takes account of the number of people in the household) than in women in the highest income households. 9 In men this pattern is not seen. Other demographic characteristics potentially associated with overweight and obesity include urbanisation, marital status and ethnicity. Available data for rates of obesity from the HSE 2004 report showed that men from Bangladeshi and Chinese minority ethnic groups had the lowest prevalence (both 6%) while Black Caribbean and Irish men had the highest prevalence (both 25%). 9 There are also regional differences in England in the prevalence of overweight and obesity, with generally higher levels in the North. 14 Estimates from survey data also suggest these geographical differences are widening in women but not in males (based on Health Survey data from 1993 and 2004). 14
The prevalence of obesity (BMI > 30 kg/m2) among adults is increasing. Estimates from England in 2007 reported obesity prevalence was 24% for both men and women. This shows a clear increase from the figures shown in 1993, which were 13% for men and 16% for women. 9 In Wales there has also been an upward trend in obesity over time among people aged over 16 years. 10 Data from Scotland also show that there has been a steady upward trend (data is for overweight and obesity) in adults since 1995 (55.6% of men and 47.2% of women aged 16–64 years in 1995 compared with 66.3% and 59.6%, respectively in 2008). 12
The prevalence of obesity is predicted to rise in the future. The World Health Organization (WHO) has projected that by 2015 more than 700 million adults worldwide will be obese. A projection of the UK prevalence of obesity (BMI > 30 kg/m2) extrapolating data from 1993 to 2004 onto 2012, showed that the prevalence would be 32.1% in men and 31.0% in women, suggesting nearly 13 million people will be obese by 2012. 15 The projected prevalence for adults was higher for those in manual social classes (48%) than non-manual social classes (35%). Another estimate of obesity prevalence, modelled in the UK’s Foresight project ‘Tackling obesities: future choices’, showed that if current trends persist, 36% of men and 28% of women aged 21–60 years will be obese in 2015. 16
Health consequences of overweight or obesity
Obesity can cause a variety of adverse health consequences. An increased risk of health problems starts when someone is only very slightly overweight; this risk increases as someone becomes more and more overweight. 7,17 The predominant serious health consequences associated with overweight and obesity include Type 2 diabetes, cardiovascular disease, musculoskeletal disorders such as osteoarthritis, and many cancers (Table 3).
| Greatly increased risk (relative risk > 3)a | Moderately increased risk (relative risk 2–3)a | Slightly increased risk (relative risk 1–2)a |
|---|---|---|
| Type 2 diabetes | Coronary heart disease | Cancer (breast cancer in postmenopausal women, endometrial cancer, colon cancer) |
| Gallbladder disease | Hypertension | Reproductive hormone abnormalities |
| Dyslipidaemia | Osteoarthritis (knees) | Polycystic ovary syndrome |
| Insulin resistance | Hyperuricaemia and gout | Impaired fertility |
| Breathlessness | Low back pain due to obesity | |
| Sleep apnoea | Increased risk of anaesthesia complications | |
| Fetal defects associated with maternal obesity |
A recent systematic review and meta-analysis of 89 studies reporting incidence of comorbidities related to obesity and overweight found statistically significant associations for multiple comorbidities. 18 Being overweight (BMI 25.00–29.99 kg/m2) was associated with the increased incidence of Type 2 diabetes, most cancers (except oesophageal in females, pancreatic and prostate cancer), all cardiovascular diseases, asthma, gallbladder disease, osteoarthritis and chronic back pain. Being obese (BMI ≥ 30 kg/m2) was associated with an increased incidence of Type 2 diabetes, all cancers (except oesophageal and prostate), all cardiovascular diseases, asthma, gallbladder disease, osteoarthritis and chronic back pain. The associations were most strongly observed for the incidence in Type 2 diabetes in females, for those overweight and those obese.
A recent meta-analysis of prospective observational studies19 assessed the association between increases in BMI and the incidence of common adult cancers. Positive and strong associations with a 5 kg/m2 increase in BMI in men were identified for cancer of the oesophagus [relative risk (RR) 1.52], thyroid cancer (RR 1.33), colon cancer (RR 1.24) and renal cancer (RR 1.24). In women, positive and strong associations with a 5 kg/m2 increase in BMI were seen for endometrial cancer (RR 1.59), gallbladder cancer (RR 1.59), oesophageal cancer (RR 1.51) and renal cancer (RR 1.34). Weaker positive associations were identified for rectal cancer and malignant melanoma in men, postmenopausal breast cancer, pancreatic, thyroid, and colon cancers in women, and leukaemia, multiple myeloma and non-Hodgkin’s lymphoma in both sexes. These findings are similarly reflected in a recently published report on the relationship between food, nutrition, and physical activity and the prevention of cancer by the World Cancer Research Fund. 20 Based on a series of systematic reviews this report shows evidence of a convincing risk of oesophageal, pancreatic, colorectal, breast, endometrial and kidney cancers with increased body fatness. The association with oesophageal cancer noted in these latter studies is in contrast to that of the systematic review noted above and may reflect differences in the analysis.
The relationship between BMI and mortality has also recently been investigated in an analysis of 57 prospective studies. 17 Deaths of known cause during a mean of 8 years of follow-up (adjusted for age, gender, smoking status and study) showed that in both sexes and at all ages, mortality was lowest in people with a BMI of about 22.5–25 kg/m2. A progressive excess mortality above this range was shown, with each 5 kg/m2 higher BMI being associated with approximately 30% higher overall mortality {hazard ratio [HR] per 5 kg/m2 1.29 [95% confidence interval (CI) 1.27 to 1.32]}. For vascular mortality, diabetic, renal and hepatic mortality the HRs were 1.41 (95% CI 1.37 to 1.45), 2.16 (95% CI 1.89 to 2.46), 1.59 (95% CI 1.27 to 1.99) and 1.82 (95% CI 1.59 to 2.09), respectively (ranging from 40% to 120% higher mortality). There were no specific causes of death that were inversely associated with a BMI above the range 22.5–25 kg/m2. This study showed that mortality from cancer was 10% higher with each 5 kg/m2 higher BMI [HR 1.10 (95% CI 1.06 to 1.15)]. 17 In this study there was a greater proportional increase in the risk of mortality at younger ages (35–59 years) with each 5 kg/m2 increase in BMI, but the trend for higher overall mortality per increment in BMI was still seen in the older age groups. This is different to the findings of an earlier systematic review21 of elevated BMI in those aged 65 years or over. The conclusions of this latter study suggested that a BMI in the overweight range was not associated with a significantly increased risk of mortality. 21
Psychosocial consequences of overweight and obesity
Alongside the health consequences of overweight and obesity a number of self-perceived problems such as low self-esteem and disturbance of body image can also affect an individual. Socially, obese people often encounter discrimination and prejudice at work and in public. 22 This can lead to negative economic and social consequences such as low educational attainment and lower income. 23,24 In general, quality of life (QoL), whether physical, psychological or social, is lower in those who are overweight or obese. 23 There is growing evidence to show that there is a strong negative correlation between degree of overweight and QoL; greater impairments in QoL are associated with greater degrees of weight increase. 22,23 One recent analysis based on surveillance data25 found that being obese was associated with a significant deterioration in health-related quality of life (HRQoL) but being overweight was not. Reduced physical health and QoL associated with obesity can contribute to impaired mental well being. 25 This may also be influenced by gender as excess weight among women has been shown to be associated with an increased risk of depression, suicidal thoughts and suicide attempts, whereas this is less common in males. 24 A recent large population study (n = 43,534)26 investigated the relationship between depression and BMI. This study noted that there was a significant U-shaped association between BMI categories and depression such that being obese and being underweight were associated with depression.
Benefits of weight loss
Overweight and obesity together are the second leading cause of preventable death, primarily through effects on cardiovascular disease risk factors (hypertension, dyslipidaemia and Type 2 diabetes). 27 Weight loss improves these risk factors, and some evidence suggests that benefits can persist as long as weight loss is maintained. 28,29 A systematic review30 found that weight loss from various interventions was associated with decreased risk of development of diabetes, and a reduction in low-density lipoprotein cholesterol, total cholesterol and blood pressure (BP) in the long term. The results of weight loss on mortality are however less certain. One systematic review31 undertaken in 2007 suggests that weight loss has long-term benefits on all-cause mortality, particularly for women and for those with diabetes. However, a recent (2009) meta-analysis32 showed that weight loss had a neutral effect on all-cause mortality. This study did find evidence of benefit in terms of mortality in those classified as ‘unhealthy and obese’. A recent evidence synthesis of surgery for obesity compared with non-surgical options found that weight loss in the longer term reduced the incidence of risk factors such as metabolic syndrome, hypertriglyceridaemia and hyperuricaemia, and increased the proportion of people with remission from Type 2 diabetes. 33
After weight loss, HRQoL has also been shown to be improved, even when weight loss has been small to moderate. 23,24 Participants in one cohort study showed improved HRQoL after a 1-year weight loss programme, and a strong relationship between the amount of weight lost and the change in QoL was also seen. This suggests that greater weight loss leads to greater improvement in HRQoL. 34
In addition to health benefits resulting from weight loss there are cost implications to the health service. As many of the factors making up this cost are attributable to comorbidities [such as stroke, coronary heart disease (CHD), hypertension and diabetes mellitus], any reduction in the incidence of these conditions as a consequence of weight loss is likely to produce a cost saving to the health service.
However, for many people the lifestyle factors that have contributed to weight gain are difficult to change in order to lose weight. Although attempts at weight loss are often successful in the short term, sustaining the weight loss can be difficult.
Current service provision
Management of disease
Overweight and obesity are initially managed within the general practice setting. The recommendation within the clinical section of the National Institute for Health and Clinical Excellence (NICE) obesity guideline35 is that the level of intervention discussed with patients should be based on patient BMI, waist circumference measurement, and presence of comorbidities (Table 4).
| BMI classification | Waist circumference | Comorbidities present | ||
|---|---|---|---|---|
| Low | High | Very high | ||
| Overweight (BMI 25.00–29.99 kg/m2) | General advice on healthy weight and lifestyle | Diet and physical activitya | Diet and physical activity; consider drugs | |
| Obesity I (BMI 30.00–34.99 kg/m2) | ||||
| Obesity II (BMI 35.00–39.99 kg/m2) | Diet and physical activity; consider drugs | |||
| Obesity III (BMI 40 kg/m2 and above) | Diet and physical activity; consider drugs; consider surgery | |||
Non-surgical interventions based on a combination of diet and physical activity, accompanied by strategies to support behavioural (lifestyle) changes, form the cornerstone of overweight and obesity treatment. Recommendations regarding lifestyle interventions (LIs) for overweight and obesity have been provided within the clinical section of recommendations within the NICE guideline. 35 These recommendations state that ‘Multicomponent interventions are the treatment of choice. Weight management programmes should include behaviour change strategies to increase people’s physical activity levels or decrease inactivity, improve eating behaviour and the quality of the person’s diet and reduce energy intake’. 35 It is also recommended that treatments should be individualised, both in terms of the type of treatment suggested and the level of support provided.
The guidelines give some indication of the information or content within a multicomponent intervention that should be provided for the weight loss, behavioural and physical activity aspects. For weight loss, adults should be informed that a realistic maximum weekly weight loss target is 0.5–1 kg (1–2 lb), and the overall aim should be to lose 5–10% of original weight. Diets that have a 600 kcal/day deficit (that is, they contain 600 kcal fewer than the person needs to stay the same weight) or that reduce calories by lowering the fat content (low-fat diets), in combination with expert support and intensive follow-up, are recommended for sustainable weight loss. The guidance states that low-calorie diets (1000–1600 kcal/day) may be considered, but are less likely to be nutritionally complete. Very-low-calorie diets (VLCD) (< 1000 kcal/day) may be used for a maximum of 12 weeks continuously, or intermittently with a low-calorie diet (for example, for 2–4 days per week), by people who are obese and have reached a plateau in weight loss. There should be clinical supervision of any diet of < 600 kcal/day.
Behavioural therapy interventions should be delivered with the support of an appropriately trained professional. The strategies included in behavioural therapy interventions need to be appropriate for the person, and can include:
-
self-monitoring of behaviour and progress
-
stimulus control
-
goal-setting
-
slowing rate of eating
-
ensuring social support
-
problem solving
-
assertiveness
-
cognitive restructuring (modifying thoughts)
-
reinforcement of changes
-
relapse prevention
-
strategies for dealing with weight regain.
The physical activity recommendation is for at least 30 minutes of moderate-intensity physical activity on 5 days or more a week. To prevent obesity, people may need to do 45–60 minutes of moderate-intensity activity a day, particularly if they do not reduce their energy intake.
The NICE guideline35 also states that the distinction between losing weight and maintaining weight loss should be explained and stresses the importance of developing skills for both phases. The change from losing weight to maintenance typically happens after 6–9 months. In the longer term, people should move towards eating a balanced diet, consistent with other healthy eating advice and continue with regular physical activity to avoid regaining weight.
In addition to the clinical recommendations the NICE guideline35 also provides recommendations for the wider public health setting. In this section the guideline states that weight loss programmes (including commercial or self-help groups, slimming books or websites) are recommended only if they are based on a balanced healthy diet, encourage regular physical activity, and expect people to lose no more than 0.5–1 kg (1–2 lb) a week. The guidance indicates that programmes that do not meet these criteria are unlikely to help people maintain a healthy weight in the long term and that people with certain medical conditions – such as Type 2 diabetes, heart failure or uncontrolled hypertension or angina – should check with their general practitioner (GP) or hospital specialist before starting a weight loss programme.
In some people who are overweight or obese, in particular those with other comorbidities or class I obesity, a prescription for weight control drugs can be considered. Surgery is usually only considered as a last resort if a number of criteria are fulfilled (e.g. BMI ≥ 40 kg/m2 or between 35 kg/m2 and 40 kg/m2 with other significant disease and all appropriate non-surgical measures have been tried previously).
Current service cost
Estimating the costs of overweight and obesity has been approached in different ways. The impact of BMI on prescribing costs in the UK has been recently reported. 36 This study found that the attributable cost of overweight and obesity accounted for 23% of spending on all drugs, with 16% attributable to obesity. The minimum annual cost of any drug prescriptions at BMI 20 kg/m2 rose from £50.71 for men and £62.59 for women by £5.27 and £4.20, respectively, for each unit increase in BMI to a BMI of 25 kg/m2. Increases for each BMI unit were greater to BMI 30 kg/m2, and greater still, £8.27 (men) and £4.95 (women) to BMI 40 kg/m2, giving total annual prescribing costs at this level of obesity of £63.59 (men) and £27.16 (women).
A House of Commons Health Committee report37 included an update of the National Audit Office estimate of obesity costs for England in 1998 using cost data for 2002 (Table 5). This update estimated that the direct treatment costs of obesity for 2002 were between £46M and £49M. The costs included in calculating this estimate were those for GP consultations, ordinary admissions, day cases, outpatient attendances and prescriptions. The costs of treating the consequences of obesity (comorbidities) lay between £945M and £1075M. Combining the total costs of treating obesity with the total costs of treating the consequences of obesity results in total direct costs of £990–1225M (2.3%–2.6% of net NHS expenditure in 2001–2). When indirect costs are also included the costs of obesity rise further.
| Included costs | 2002 (£M) |
|---|---|
| GP consultations | 12–15 |
| Ordinary admissions | 1.9 |
| Day cases | 0.1 |
| Outpatient attendances | 0.5–0.7 |
| Prescriptions | 31.3 |
| Total cost of treating obesity | 45.8–49.0 |
| Consequences of obesity | |
| GP consultations | 90–105 |
| Ordinary admissions | 210–250 |
| Day cases | 10–15 |
| Outpatient attendances | 60–90 |
| Prescriptions | 575–625 |
| Total cost of treating the consequences of obesity | 945–1075 |
| Lost earnings due to attributable mortality | 1050–1150 |
| Lost earnings due to attributable sickness | 1300–1450 |
| Total indirect costs | 2350–2600 |
| Total cost of obesity | 3340–3724 |
These figures were based on people with a BMI of 25 kg/m2 and over and the Health Committee report stresses that these figures are likely to underestimate the true cost of treating obesity and its consequences. A more recent study, which included a wider range of costs, estimated the direct cost of overweight and obesity to the UK NHS at £3.2B per year. 38 The majority of the costs attributable to overweight and obesity were from treating stroke, CHD, hypertensive disease and diabetes mellitus. The cost estimate from this study may be higher than those of other published sources because cost estimates are sensitive to the chosen cut-off point for BMI. In this case people with a BMI of 22 kg/m2 and above were included, whereas some other studies have taken a BMI cut-off of 25 kg/m2.
The UK’s Foresight project ‘Tackling obesities: future choices’16 estimated that in 2007, overweight and obesity would account for £4.2B of the overall £17.4B estimated total annual cost to the NHS of diseases for which elevated BMI is a risk factor. The only factor considered in the model used to provide these estimates was BMI.
It has not been possible to determine the costs of adult weight management schemes within a primary care trust (PCT). There are a number of reasons for this but chiefly a lack of any centralised reporting of such information. When cost information for individual PCTs is found, for example on PCT websites or within press releases, the costs quoted are very variable. This variation in costs is because of factors that include:
-
Differences between PCTs in the proportion of adult weight management schemes provided within different settings such as leisure centres, primary care, pharmacies and schemes delivered in partnership with commercial weight loss organisations.
-
Costs for adult weight management services may be reported within an overall value that includes adult, child, and family-centred weight management services.
-
Differences in the target adult population: some schemes are only available to those who are obese, others are available to the overweight and the obese.
In general it appears that these weight management schemes are provided by PCTs to eligible adults who are not charged for using the service.
Variation in services
The large numbers of people in the population who are overweight and/or obese place a high demand on services from primary care. However, at the same time many practices have limited capacity to manage these people, and few evidence-based interventions to choose from. This has led to variation across the UK in the service offered to overweight and obese people. In 2001 a National Audit Office report39 concluded that approaches to weight management were inconsistent and that effective strategies for weight management were needed. This view was similarly echoed in a report of the House of Commons Health Committee in 2004. 37 More recently a survey by the Dr Foster organisation published in 200540 showed that primary care organisations employ a number of innovative approaches to the management of obesity; however, there is considerable national and regional variation in the service provided. The survey also showed that while more organisations had established weight management clinics than in their previous survey in 2003 (up by 5%), in the majority of general practices (69%) there was still no organised weight-management clinic.
Relevant national guidelines
The most comprehensive guideline for the prevention and management of obesity in adults in England and Wales is the NICE guideline. 35 This guideline, referred to in detail above, covers both primary and secondary care. Other guidelines which are relevant to the prevention and management of obesity in adult populations in the UK have been produced by the National Obesity Forum41 and the Northern Ireland Clinical Resource Efficiency Support Team. 42 They are consistent with the NICE guideline35 but focus on either primary care41 or secondary care. 42 Several UK guidelines are also available on the prevention and management of obesity in children and young people [e.g. the Scottish Intercollegiate Guidelines Network guideline43 (currently being updated; spring 2010)], and on the management of obesity using pharmacological and surgical approaches. However, these populations and interventions are outside the scope of the current assessment (see Chapter 2).
There are currently no National Service Frameworks (NSFs) that specifically focus on overweight or obesity. Guidance on avoiding obesity, with a focus on healthy diet and exercise, is included in the current NSF for CHD. 44
Existing systematic reviews
Weight management interventions have been the focus of a number of systematic reviews in recent years. Ten systematic reviews, published between 1997 and 2009, have included studies with weight management or weight maintenance interventions that comprised diet, exercise and/or behavioural components and reported weight outcomes for adults, and are summarised in Table 6. 30,45–53 Seven of these systematic reviews were restricted to randomised controlled trials (RCTs)30,45–50 and three focused on RCTs but also permitted inclusion of other study designs. 51–53 The minimum follow-up for weight management outcomes required for studies to be included in the systematic reviews was 12 months in the majority (n = 7), 12 weeks (n = 1), 24 months (n = 1) or was not specified (n = 1) (Table 6).
| Authors and date | Goal | Principal inclusion criteria | ||
|---|---|---|---|---|
| Design and intervention | Population | Follow-up | ||
| Glenny et al. 199751 | Determine effectiveness of interventions for obesity prevention and treatment, weight loss and weight maintenance | RCTs (other study designs accepted for prevention goal) | Overweight and obese adults and/or children | ≥ 12 months |
| McLean et al. 200345 | Evaluate family involvement in weight control or weight loss | RCTs with at least one family-based intervention | Adults and/or children | ≥ 12 months |
| McTigue et al. 200347 | Determine effectiveness of adult obesity screening and treatment | RCTs of fair or good quality | BMI ≥ 25 kg/m2 | ≥ 12 months |
| Avenell et al. 200446 | Systematically review effectiveness of exercise ± diet ± and behaviour therapy for weight loss and other outcomes | RCTs; specific details of interventions required; weight change an explicit outcome | Adults with minimum, mean or median BMI 28 kg/m2 | ≥ 12 months from randomisation |
| Avenell et al. 200430 | Systematically review obesity treatments in adults to identify therapies that achieve weight reduction, risk factor modification or improved clinical outcomes | RCTs; sufficient details of interventions required | Adults with minimum, mean or median BMI 28 kg/m2 | ≥ 12 months from randomisation |
| Franz 200448 | Not clearly stated but focus on weight management interventions targeting women | RCTs of weight loss and maintenance | Women, BMI > 25 kg/m2 | ≥ 12 months |
| Tsai et al. 200553 | Describe major commercial and self-help weight loss programmes in the USA that provide structured in-person or online counselling | RCTs (other designs permitted); ≥ 10 participants; assessed intervention under same conditions as offered to public | Adults | ≥ 12 weeks |
| Söderlund et al. 200949 | Determine effectiveness of exercise ± diet ± behaviour therapy in overweight and obese healthy adults | RCTs; minimum n = 15 per intervention | Overweight or obese otherwise healthy adults | ≥ 12 months (or intervention ≥ 12 months) |
| Turk et al. 200950 | Summarise findings of RCTs that tested strategies for weight loss | RCTs with weight maintenance intervention after initial weight loss | Adults | Follow-up not stated |
| Brown et al. 200952 | Determine the effectiveness of long-term lifestyle interventions for the prevention of adult weight gain and morbidity | RCTs and controlled before–after studies | Adults, BMI < 35 kg/m2 | Weight reported ≥ 24 months after randomisation |
Eligible interventions varied between these systematic reviews. Their inclusion criteria required interventions to comprise diet, exercise and/or behavioural components, but not necessarily all three together. None of the systematic reviews precisely matched the ‘multicomponent’ approach supported in the NICE guidelines. The goal of the systematic review by Söderlund and colleagues49 appears the most similar, but this review did not formally require interventions to include diet or behavioural components. The remaining nine systematic reviews also included some interventions with diet, exercise and/or behavioural components but the reviews all had different goals (Table 6) from assessing multicomponent approaches per se. For example, the systematic review by Avenell and colleagues46 focused on interventions that included exercise, behaviour therapy and/or drugs and was not limited to those that also contained a diet component. In addition, many of their included studies provided limited details of their interventions and many were of relatively short-term follow-up.
The conclusions of the 10 systematic reviews were mixed, which in part reflects their differing objectives and inclusion criteria. Some reviews (including Söderlund and colleagues49) suggested that the most effective weight loss interventions are those that combine diet, exercise and behaviour components to achieve weight loss. 30,49,51 There was also some support for interventions that contained only one or two of these three elements. 30,48,52 However, it is difficult to draw any firm conclusions regarding the long-term benefits of multicomponent weight management approaches from these systematic reviews due to their different objectives and inclusion criteria.
There is therefore a need to systematically synthesise all relevant evidence from good-quality studies that report long-term results, in order to compare the effectiveness and cost-effectiveness of different weight management programmes in delivering sustained weight loss.
Description of technology under assessment
Weight management programmes aim to improve the eating behaviour and the quality of a person’s diet, to reduce energy intake, and to also increase people’s physical activity levels or decrease inactivity. 35 A multicomponent intervention is defined as one which combines more than one mutually reinforcing strategy to achieve a common outcome. It is thought that addressing multiple influences on overweight and obesity will be an effective way to reduce and maintain healthy weight. The components may be distinguished from each other in terms of setting, location, provider, format, media and content. As previously stated, good practice guidelines promote weight management programmes that are multicomponent interventions that usually involve a combination of diet, exercise and behavioural therapy with ongoing support. In this sense the components differ primarily in terms of content (i.e. focus on diet or physical activity), but may also differ, to varying extents, in terms of setting (e.g. clinics, leisure centres), provider (e.g. dietitians, behavioural therapists) and other attributes. They work towards the common goal of encouraging weight loss and maintenance.
Dietary approaches incorporated in weight management programmes used in the UK may involve strategies such as calorie restriction, VLCDs, low fat, low carbohydrate, high fibre, meal replacement, food combining, or low glycaemic index foods. As described above (see Current service provision), dietary goals, in terms of the number of kilocalories per day permitted, may be set according to the type of diet followed. Nutritionists, dietitians or trained nurses may deliver dietary interventions. Broader lifestyle approaches may also accompany dietary goals, including education around food labelling, cookery skills and identifying where healthier foods can be purchased.
Physical activity elements of weight management programmes include exercise training and endurance exercises (e.g. running, swimming) and resistance training (e.g. use of weights). Physiotherapists, specially trained staff or fitness coaches may deliver physical activity sessions to individuals or groups with differing degrees of supervision in settings such as leisure centres or community centres. Physical activity may also be self-supervised, with participants given exercise goals to be reached through activities of their own choosing, for example daily living activities, walking or cycling.
Behavioural therapy may include a number of specific techniques including: self-monitoring (e.g. systematically observing and recording one’s own behaviour); problem analysis (e.g. dealing with situations that might interfere with reaching dietary or exercise goals); alteration of cognitive patterns such as cognitive reframing and coping imagery; cognitive strategies for replacing negative thinking with positive statements (e.g. modifying self-defeating thoughts about dieting, constructive self-statements); relapse prevention (e.g. identifying when lapses might occur in diet or physical activity and how to deal with high-risk situations); goal-setting; menu planning; stimulus control (e.g. avoiding stimuli that might encourage eating, such as reducing the visibility of food in the home, slowing the pace of eating, avoiding fast food restaurants); and may be delivered by psychologists, trained interventionists and counsellors. Ongoing support may take the form of personal contact, meetings, telephone calls and internet technology, and be individual, group or family based. Therapy may be theoretically based, for example drawing on the stages of change (SOC) approach of the Transtheoretical Model, or Social Cognitive Theory. 54–56 However, it is recognised that definitions of behavioural techniques are not always standardised, or explicitly linked to a theoretical model of behaviour change. 57
Multicomponent interventions may also include surgery and prescribed or over-the-counter (OTC) weight loss drugs, although this is more often as a second-line treatment if weight loss goals have not been attained through diet, physical activity and behavioural therapy strategies. There has been recent interest in the use of OTC drugs for obesity and their place within multicomponent approaches to weight management. In 2009 the European Union approved the use of 60 mg orlistat per day (Alli™, GlaxoSmithKline) for purchase following consultation with a pharmacist for people with a BMI over 28 kg/m2. It is possible that people participating in multicomponent interventions may use OTC orlistat as an additional weight loss strategy, though it has been suggested that some may use it in place of diet and exercise. Research is needed to establish how it might be best used in practice. 58
Weight management programmes may be delivered in the primary care setting, by GPs supported by specialists as mentioned above, and tutors trained specifically to deliver such programmes, or through professionals in specialist hospital clinics. For example, the Counterweight Programme54 is a primary care-based programme in the UK that aims to help obese people to achieve weight loss (e.g. 5%–10% over 3–6 months) and weight maintenance through changes in diet and exercise. In addition to NHS-based programmes, commercial and self-help programmes are available such as Weight Watchers®, Slimming World, Rosemary Conley™, LighterLife™ and others. LIs in overweight working populations are also available. 59
Weight management programmes vary in terms of their duration. Generally there will be an initial period where the aim is to achieve a desired weight loss goal (e.g. 6–12 months). Thereafter there may be a weight maintenance phase where the aim is to sustain weight lost in the first stage. There is some evidence to suggest that weight is lost rapidly at first, and the point of greatest loss occurs 6 months after beginning treatment; weight is then slowly regained and often reaches near the original level. 60 For example, approximately a third of lost weight may be regained in the first year after treatment and often continues with the average loss of about 1.8 kg remaining at 4 years after treatment. 60 Therefore, it is important that any weight management scheme facilitates weight maintenance after the target weight has been reached. Resulting changes to lifestyle must become part of everyday life for long-term health benefits to be realised. 35 It is important to acknowledge, however, that weight gain in the general population may occur throughout life and therefore people who have participated in a weight loss intervention may still be at lower weight than if they have not participated.
Well-managed, multicomponent weight management schemes are not thought to lead to any specific adverse events for participants;35 however, there are some suggestion that negative outcomes for some participants may occur from weight management programmes. This appears to be largely based on ‘dieting’ in general, where evidence suggests that for some people dieting can lead to eating disorders, negative psychological and emotional effects and increased social stigma. 61
Overall aims of this assessment
The aim of this review was to assess the long-term clinical effectiveness and cost-effectiveness of multicomponent weight management schemes for adults in terms of weight loss and maintenance of weight loss.
Chapter 2 Methods
The a priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness are described in the research protocol (see Appendix 1), which underwent comment by our expert advisory group. Although helpful comments were received relating to the general content of the research protocol, there were none that identified specific problems with the methods of the review. The methods outlined in the protocol are briefly summarised below.
Search strategy
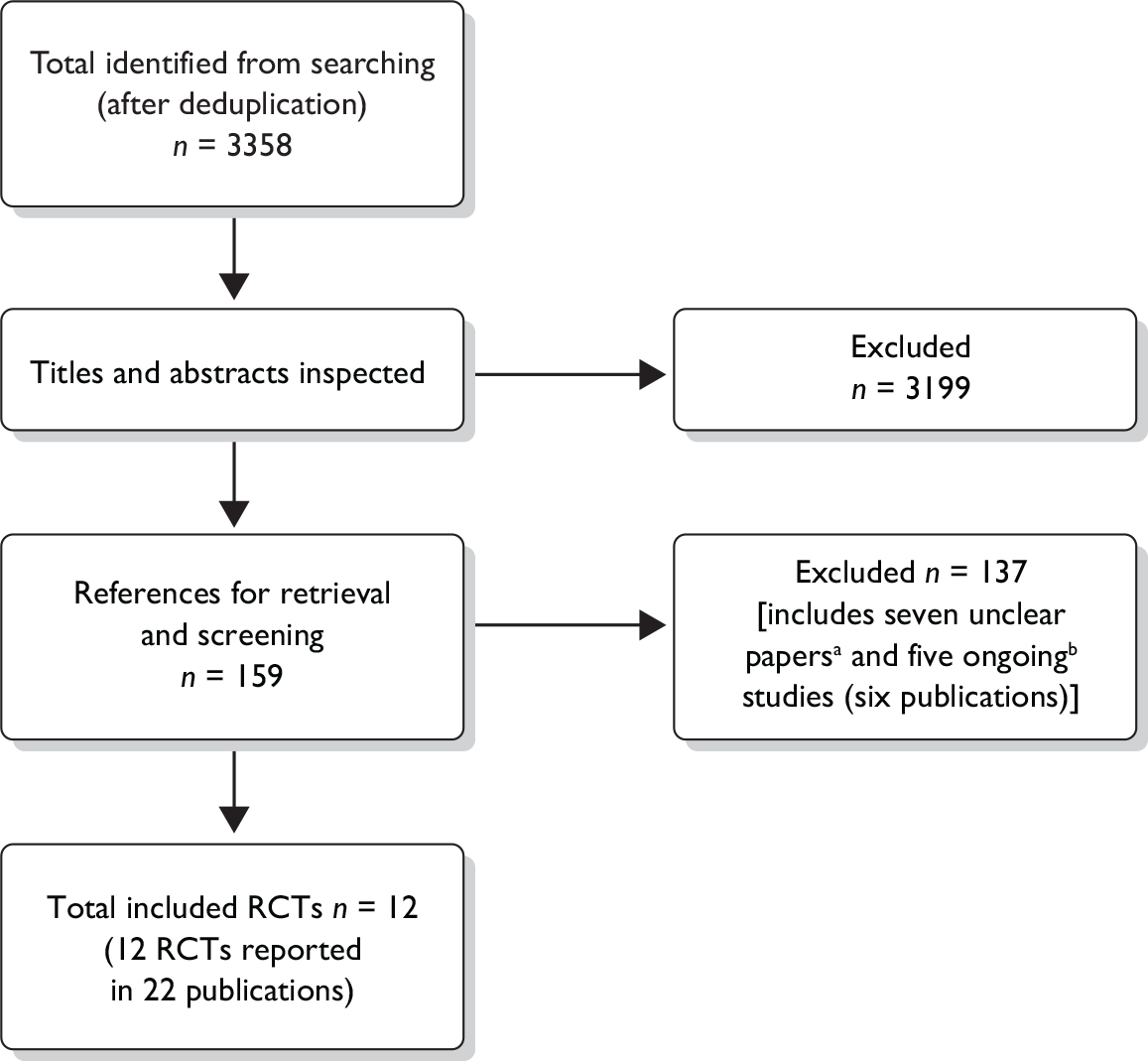
The search strategy was developed, tested and refined by an experienced information scientist. Separate searches were conducted to identify studies of clinical effectiveness, cost-effectiveness and epidemiology. Sources of information and search terms are provided in Appendix 2 and a flow chart of identification of studies can be seen in Figure 1.
FIGURE 1.
Flow chart of identification of studies for inclusion in the review. (a) Details of the interventions in six RCTs (seven publications) nearly met our threshold of containing enough detail (see Methods). (b) Five studies (six publications) were assessed as ongoing and are described as such in Assessment of effectiveness of multicomponent interventions versus non-active intervention comparators.
Searches for clinical effectiveness and cost-effectiveness literature were undertaken from database inception to December 2009. Electronic databases searched included: MEDLINE; EMBASE; MEDLINE In-Process & Other Non-Indexed Citations; The Cochrane Library including the Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database and HTA databases; Web of Science; PsycINFO; BIOSIS; and databases listing ongoing clinical trials.
Searches were restricted to the English language. Bibliographies of related papers were screened for relevant studies, the expert advisory group were also contacted for advice and peer review and to identify additional published and unpublished references.
Inclusion process
Titles and abstracts identified by the search strategy for the clinical effectiveness section of the review were assessed for possible eligibility by two reviewers independently. The full texts of relevant papers were then obtained and inclusion criteria were applied by one reviewer and checked by a second reviewer using a previously piloted inclusion flow chart (see Appendix 3). Any disagreements over eligibility were resolved by discussion or by recourse to a third reviewer.
Titles and abstracts identified by the search strategy for the systematic review of cost-effectiveness were assessed for potential eligibility by two reviewers independently. Economic evaluations were considered for inclusion if they reported both health service costs and effectiveness of multicomponent adult weight management programmes, or presented a systematic review of such evaluations. Two reviewers formally assessed full papers independently, with respect to their potential relevance to the research question. Any differences in judgement were resolved through discussion.
Inclusion criteria
Population
-
Adults (≥ 18 years) classified as overweight or obese, i.e. people with a BMI of ≥ 25 kg/m2 and ≥ 30 kg/m2, respectively.
-
Studies in children and people with eating disorders were not included, nor were studies specifically in people with a pre-existing medical condition such as diabetes, heart failure, uncontrolled hypertension or angina.
Intervention
-
Structured, sustained multicomponent weight management programmes (i.e. the intervention had to be a combination of diet and physical activity with a behaviour change strategy to influence lifestyle).
-
Components of the programme had to be clearly specified (i.e. details provided of the diet, behavioural definition, and exercise components; see below).
-
Programmes that included a long-term follow-up of more than 18 months.
-
The programme was delivered by the health sector, in the community or commercially.
-
Multicomponent programmes that involved the use of OTC medicines that are licensed in the UK for overweight or obesity were also included. Programmes that involved non-OTC drug therapies or surgery for obesity were not included.
-
Interventions incorporating other lifestyle changes such as efforts at smoking cessation or reduction of alcohol intake were not included.
Comparators
-
Normal practice (as defined by the study).
-
Single-component weight management strategies.
-
Other structured multicomponent weight management programmes.
Outcomes
-
Studies were required to include a measure of weight loss.
-
Data on the following outcomes were also eligible for extraction where reported in the included studies: study-defined success rates at more than 18 months, attrition rates at more than 18 months, barriers and facilitators of weight loss and maintenance of weight loss.
-
Outcomes of cost-effectiveness studies were costs, benefits in terms of weight loss and cost-effectiveness.
Types of studies
-
For the systematic review of clinical effectiveness RCTs were included.
-
For the systematic review of cost-effectiveness eligible study types were full cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses and cost–consequence analyses.
-
Studies published as abstracts or conference presentations were only included if sufficient details were presented to allow an appraisal of the methodology and the assessment of results to be undertaken.
-
Case series, case studies, cohort studies, narrative reviews, feasibility studies, editorials and opinions were not included.
-
Systematic reviews were used as a source of references.
-
Non-English language studies were excluded.
As stated above, all three components of the intervention had to be clearly specified for a trial to meet the inclusion criteria. This was firstly to ensure that interventions were ‘multicomponent’ (providing evidence of the three components rather than simply reporting that an intervention had a diet, exercise and behavioural component) and secondly to ensure that included interventions were, as far as possible, reproducible. It has been proposed that interventions that include a behaviour change element should clearly describe the content, the characteristics of the providers, the setting, the mode of delivery, the intensity, the duration and adherence to protocols. 62 There have also been calls for greater standardisation of specific behavioural techniques used, including explicit links to guiding theory. 57 Where details of these attributes of the included interventions were available these have been reported.
In many cases discussion as regards study selection were straightforward as one or more of the components were absent or no details whatsoever were provided for one or more of the components. In other cases a decision had to be made whether the level of detail about the component was sufficient. In these cases the following criteria were looked for in each of the three components:
-
diet
-
– type of diet
-
– calories
-
– proportion of diet (e.g. proportion of diet made up of fats, protein, carbohydrate)
-
monitoring
-
-
exercise
-
– mode
-
– type
-
– frequency/length sessions
-
– delivered by
-
– level of supervision
-
– monitoring
-
-
behaviour modification
-
– mode
-
– type
-
– content
-
– frequency/length sessions
-
– delivered by.
-
Using these criteria any studies that provided detail on only one aspect of one (or more) of the three main components were excluded (e.g. only the type of diet, but no detail of calorie goals, proportions of diets, or how the diet was monitored), and studies which referred to a secondary source for the description of their interventions were marked as unclear and authors were contacted for further information.
Data extraction and quality assessment strategy
Data were extracted by one reviewer using a standardised and pre-piloted data extraction form and checked by a second reviewer. Any disagreements were resolved by discussion, if necessary involving a third reviewer.
Within the clinical effectiveness review the quality of included studies was assessed using criteria based on those recommended by the Centre for Reviews and Dissemination (CRD)63 (see Appendix 4). Each RCT was assessed by one reviewer and checked by a second reviewer. Any disagreements were resolved by discussion, if necessary involving a third reviewer. The reviewers assessed the adequacy of reporting: randomisation; allocation concealment; population baseline characteristics; blinding of assessors, care providers and participants; imbalances in attrition; outcomes; intention-to-treat (ITT) analyses; and analyses of missing data. For ease of presentation and interpretation of the quality assessment of RCTs the responses were then translated into judgements of ‘high’, ‘low’ or ‘uncertain’ risk of bias based on the Cochrane risk of bias criteria. 64 For example, where a positive response to a question indicated an adequate procedure to minimise bias (e.g. ‘was the allocation adequately concealed?’) this was translated into a ‘low’ risk of bias. Similarly, where a positive response to a question indicated an inadequate procedure to minimise bias (e.g. ‘is there any evidence to suggest that the authors measured more outcomes than they reported?’) this was translated into a ‘high’ risk of bias.
For the systematic review of cost-effectiveness, the methodological quality of the cost-effectiveness studies was assessed using a critical appraisal checklist based on that by Philips and colleagues,65 Drummond and Jefferson66 and the NICE reference case requirements. 67 These checklists were combined to include the key elements that were relevant to internal and external validity, and the generalisability of the studies to the UK NHS.
Data synthesis
Data were synthesised through a narrative review with tabulation of results of all included studies. Full data extraction forms for the clinical effectiveness review are presented in Appendix 5. Within the clinical effectiveness section studies using similar interventions were grouped together to aid interpretation. Studies were categorised according to which of the intervention components (diet, exercise, and/or behaviour modification) were of primary interest and how they differed from the comparator intervention. This led to four groups: studies in which the comparator was a non-intervention group; studies in which the intervention focused on the diet aspect versus another active comparison; studies in which the intervention focused on the exercise aspect versus another active comparison; and studies where other variables were the focus of the study.
Where trials reported several interventions, decisions were made by consensus among the review group as to which was the ‘key’ intervention of relevance to the scope of this review (as noted above, these are multicomponent interventions involving a combination of diet, exercise and behavioural therapy) and which comparators were relevant. For some trials there was little discernible difference between the interventions tested and as such the focus of our report is limited to those which appeared to be the most relevant to our research protocol, with complete details of all interventions reported (see Appendix 5).
Our pre-defined inclusion criteria excluded studies with < 18 months’ follow-up from randomisation, in order to focus the review on long-term weight loss and maintenance of weight loss given that weight regain in the long term is an intractable problem. Therefore, where included studies report interim data before 18 months’ follow-up these have not been included. However, we do provide comment in the text where these data are available in the primary studies.
It was considered inappropriate to combine the results of the studies in a meta-analysis due to differences in the interventions, comparators and populations in the included studies.
Chapter 3 Clinical effectiveness
Quantity and quality of research available
Searching identified 3358 references after deduplication. After initial screening of titles and abstracts, 159 references were retrieved for further inspection. The total number of published papers included at each stage of the systematic review is shown in the flow chart in Figure 1. References to the studies retrieved for further inspection but subsequently excluded can be seen in Appendix 6. The most common reason for exclusion was the inadequate length of follow-up (54 studies); lack of, or only minimal, detail reported on one or more of the components of the intervention (see Chapter 2) led to 20 studies being excluded; another 21 were not deemed to be multicomponent interventions, and 19 were not RCTs. In addition, one study did not have an appropriate comparator; two studies did not report weight loss outcomes and the participants in seven studies did not meet the inclusion criteria. The level of agreement between reviewers assessing study eligibility was generally good although this was not formally measured.
Six RCTs (seven publications) appeared to meet all of the inclusion criteria except that the details of the multicomponent interventions were judged to be below our threshold (see Chapter 2). Authors of these six studies were contacted for further detail. A response from one author was received; however, the information provided did not clarify any further whether the interventions did indeed meet our criteria. These studies were undertaken between 2 and 15 years ago, which may explain the poor response from the authors. As such there were six studies that we were unable to identify whether they met the inclusion criteria of the review. These studies can be seen in Appendix 7.
Twelve RCTs met all of the inclusion criteria,68–79 of which five had two intervention arms, one had three arms, four had four arms and two had five arms (Table 7). In many of these RCTs data were reported in multiple publications; for ease of presentation the primary reference is used here, with full details of all secondary publications given in Appendix 5. Not all of the intervention arms were considered relevant to the scope of the current review; this is outlined in the subsequent sections where this is the case. The key methodological attributes of these trials are summarised in Table 8, with full details of the interventions, outcomes and quality assessments provided in Appendix 5. All included trials were individual-level interventions; no community-level interventions met the inclusion criteria.
| Study details | Study armsa | Target population and selected baseline characteristics |
|---|---|---|
|
Burke et al. 200871 Country: USA Design: RCT Follow-up: 18 months |
Preference for standard diet (pref STD-D), n = 48 Preference for lacto-ovo-vegetarian diet (pref LOV-D), n = 35 No preference for standard diet (no pref STD-D), n = 48 No preference for lacto-ovo-vegetarian diet (no pref LOV-D), n = 45 Total n = 176b |
Target population: overweight men and women aged 18–55 years Mean age (years): pref STD-D 43.2; pref LOV-D 44.3; no pref STD-D 43.2; no pref LOV-D 43.2 Sex (% M : F): pref STD-D 12.5 : 87.5; pref LOV-D 20 : 80; no pref STD-D 12.5 : 87.5; no pref LOV-D 9 : 91 Mean BMI (kg/m2): pref STD-D 34.5; pref LOV-D 34.1; no pref STD-D 32.9; no pref LOV-D 33.7 Mean weight (kg): pref STD-D 97.2; pref LOV-D 96.7; no pref STD-D 92.4; no pref LOV-D 91.8 |
|
Tate et al. 200777 Country: USA Design: RCT Follow-up: 30 months |
High physical activity (HPA), n = 109 Standard behavioural treatment (SBT), n = 93 Total n = 202 |
Target population: overweight men and women aged 25–50 years Mean age (years): overall 42.2 Sex (% M : F): overall 42 : 58 Mean BMI (kg/m2): overall 31.7 Mean weight (kg): approximately 90.5 for both interventions |
|
Logue et al. 200572 Country: USA Design: RCT Follow-up: 24 months |
Transtheoretical model and chronic disease paradigm (TM-CD), n = 329 Augmented usual care (AUC), n = 336 Total n = 665 |
Target population: overweight men and women aged 40–69 years Age (years): mean not reported. In both arms ≥ 80% aged 40–59 years Sex (% M : F): TM-CD 29 : 71; AUC 33 : 67 BMI (kg/m2): not reported Weight: not reported |
|
Stevens et al. 200170 Country: USA Design: RCT Follow-up: 36 months |
Weight loss (WL), n = 595 Usual-care control group (C), n = 596 Total n = 1191 |
Target population: overweight men and women with non-medicated diastolic blood pressure (BP) of 83–89 mmHg and systolic BP < 140 mmHg and aged 30–54 years Mean age (years): WL 43.4; C 43.2 Sex (% M : F): WL 63 : 37; C 68 : 32 Mean BMI (kg/m2): men: WL 31.0; C 31.0; women: WL 31.0; C 30.8 Mean weight (kg): WL 93.4; C 93.6 |
|
Jeffery et al. 199876 Country: USA Design: RCT Follow-up: 18 months |
Standard behavioural therapy (SBT), n = 40 SBT + supervised exercise (SBTE), n = 41 SBT + trainer (SBTT), n = 42 SBT + incentive (SBTI), n = 37 SBT + trainer + incentive (SBTTI), n = 36 Total n = 196 |
Target population: overweight men and women aged 25–55 years Mean age (years): SBT 40.0; SBTE 41.5; SBTT 41.0; SBTI 42.6; SBTTI 40.7 Sex (% M : F): SBT 18 : 82; SBTE 17 : 83; SBTT 21 : 79; SBTI 14 : 86; SBTTI 14 : 86 Mean BMI (kg/m2): SBT 31.4; SBTE 31.5; SBTT 31.4; SBTI 31.5; SBTTI 30.6 Mean weight (kg): SBT 85.6; SBTE 87.1; SBTT 84.7; SBTI 87.7; SBTTI 85.7 |
|
Simkin-Silverman et al. 199873 Country: USA Design: RCT Follow-up: 54 months |
Lifestyle intervention (LI), n = 260 Assessment-only control group (C), n = 275 Total n = 535 |
Target population: perimenopausal women, aged 44–50 years, of whom a subgroup were overweight Mean age (years): LI 47; C 47 Sex (% M : F): LI 0 : 100; C 0 : 100 Mean BMI (kg/m2): LI 25; C 25 Mean weight, lb (kg)c: LI 148.0 (67.1); C 147.6 (67.0) |
|
Weinstock et al. 199878 Country: USA Design: RCT Follow-up: 96 weeks |
Diet plus combined strength and aerobic training (DSA), n = 29d Diet plus strength training (DS), n = 31d Diet plus aerobic training (DA), n = 31d Diet alone (D), n = 29d Total n = 120d |
Target population: overweight women Mean age (years): DSA 42.8; DS 40.0; DA 40.8; D 41.0 Sex (% M : F): 0 : 100 in all arms Mean BMI (kg/m2): DSA 35.3; DS 36.5; DA 37.3; D 36.4 Mean weight (kg): DSA 92.4; DS 96.8; DA 98.7; D 96.3 |
|
Skender et al. 199679 Country: USA Design: RCT Follow-up: 2 years |
Diet + exercise (D + E), n = 42 Diet (D), n = 42 Exercise (E), n = 43 Waiting list (n = 38; data not reported) Total n = 127 (165) |
Target population: overweight men and women aged 25–45 years Age (years): not reported Sex (% M : F): D + E 50 : 50; D 52 : 48; E 53 : 47 BMI (kg/m2): not reported Mean weight (kg): D + E 97.60; D 97.65; E: 93.92 |
|
Jeffery and Wing 199575 Country: USA Design: RCT Follow-up: 30 months |
Standard behavioural treatment (SBT), n = 40 SBT + food provision (SBT + FP), n = 40 SBT + incentives (SBT + I), n = 41 Combined intervention (SBT + FP + I), n = 41 Control group (no intervention) (C), n = 40 Total n = 202 |
Target population: overweight men and women aged 25–45 years Mean age (years): SBT 37.5; SBT + FP 38.5; SBT + I 38.1; SBT + FP + I 37.6; C 35.7 Sex (% M : F): Not reported by intervention group BMIe (kg/m2): SBT 30.9; SBT + FP 30.8; SBT + I 31.1; SBT + FP + I 31.1; C 31.1 Weighte (kg): SBT 89.4; SBT + FP 88.1; SBT + I 92.3; SBT + FP + I 91.1; C 88.2 |
|
Stevens et al. 199374 Country: USA Design: RCT Follow-up: 18 months |
Weight loss (WL), n = 308 Usual-care control group (C), n = 256 Total n = 564 |
Target population: overweight men and women, average diastolic BP of 80–89 mmHg and aged 30–54 years Mean age (years): WL 43.1; C 42.4 Sex (% M : F): WL 73 : 27; C 63 : 37 Mean BMI (kg/m2): WL 29.5; C 29.5 Mean weight (kg): WL 90.2; C 89.3 |
|
Wadden et al. 198869 Country: USA Design: RCT Follow-up: 3 years |
Behaviour therapy (BT), n = 18 Combined treatment (BT + VLCD), n = 23 Very-low-calorie diet (VLCD), n = 18 Total n = 59 |
Target population: overweight men and women Mean age (years): BT 44.3; BT + VLCD 43.6: VLCD 44.3 Sex (% M : F): BT 19 : 81; BT + VLCD 11 : 89; VLCD 13 : 87 BMI (kg/m2): not reported Weightf (kg): BT 112.2; BT + VLCD 108.0; VLCD 106.4 |
|
Dubbert and Wilson 198468 Country: USA Design: RCT Follow-up: 30 months after end of intervention (circa 34 months after randomisation) |
Individual treatment with weekly (distal) goalsf Individual treatment with daily (proximal) goalsf Couples’ treatment with weekly (distal) goalsf Couples’ treatment with daily (proximal) goalsf Total n = 62 |
Target population: overweight married men and women currently living with spouse; spouse willing to attend sessions Age (years): not reported Sex (M : F): overall 23 : 77 BMI (kg/m2): not reported Weightf lb (kg)c: individual, distal 207.7 (94.2); individual, proximal 208.9 (94.8); couples, distal 190.4 (86.4); couples, proximal 195.0 (88.5) |
| Study | Randomisation sequence | Allocation concealment | Similarity of groups | Blinding of assessors | Blinding of care providers | Participant blinding | Dropout imbalance | Missing outcomes | ITT analysis | Missing data explained |
|---|---|---|---|---|---|---|---|---|---|---|
| Burke et al. 200871 | ? | ? | High | ? | ? | ? | Low | High | High | High |
| Tate et al. 200777 | ? | ? | Low | ? | ? | ? | ? | Low | ? | ? |
| Logue et al. 200572 | Low | ? | Low | ? | ? | ? | Low | High | Low | Low |
| Stevens et al. 200170 | Low | Low | Low | Low | ? | ? | Low | Low | High | ? |
| Jeffery et al. 199876 | ? | ? | Low | ? | ? | ? | ? | Low | High | High |
| Simkin-Silverman et al. 199873 | Low | Low | Low | Low | ? | ? | Low | Low | Low | High |
| Weinstock et al. 199878 | ? | ? | Low | ? | ? | ? | Low | Low | High | High |
| Skender et al. 199679 | Low | ? | ? | ? | ? | ? | High | Low | ? | High |
| Jeffery and Wing 199575 | ? | ? | Low | ? | ? | ? | ? | High | ? | ? |
| Stevens et al. 199374 | ? | Low | Low | ? | ? | ? | ? | Low | ? | ? |
| Wadden et al. 198869 | ? | ? | ? | ? | ? | ? | Low | Low | ? | High |
| Dubbert and Wilson 198468 | ? | ? | Low | ? | ? | ? | ? | Low | ? | High |
All RCTs that met the inclusion criteria were conducted in the USA. Most (83%) of these were published between 1993 and 2008, with two older RCTs, published in 198468 and 1988. 69 The total number of participants randomised ranged from 5969 to 1191,70 while the number of participants per intervention group ranged from 1869 to 596. 70 Only four of the 12 RCTs had sample sizes > 100 participants per intervention group. 70,72–74 The earliest of the RCTs68 did not report the number of participants randomised. Power analyses to calculate the necessary sample sizes were reported in only five trials. 70–74
In all but one73 of the 12 RCTs the target population was stated as being overweight. The RCT by Simkin-Silverman and colleagues73 enrolled middle-aged women and aimed to prevent the rise in weight and low-density lipoprotein (LDL) cholesterol observed during the menopause. The baseline weight, however, indicated that participants were overweight (mean BMIs > 25 kg/m2). The two trials reported by Stevens and colleagues70,74 were conducted as part of a larger study of risk factors for hypertension [Trials of Hypertension Prevention (TOHP)] and included overweight participants.
Nine of the 12 RCTs reported the starting BMI of their participants. 70–78 According to the international classification of overweight or obesity,6 participants would be considered overweight (pre-obese) in two RCTs,73,74 class I obese in five RCTs,70,71,75–77 and class II obese in one RCT. 78 The remaining RCT72 contained a mixture of pre-obese (18%–22%), class I obese (32%–36%), class II obese (21%–24%) and class III obese (22%–24%) participants (see Appendix 5). Seven of the 12 RCTs specified that their participants, aside from being overweight, were in good health. 68,70,73–77 One RCT, by Weinstock and colleagues,78 excluded women with bulimia but not those with binge eating disorder. The remaining four RCTs69,71,72,79 did not include health status as an inclusion criterion.
Three studies did not mention age among their inclusion criteria (however participants were all adults). 68,69,78 The upper age limit specified for inclusion of participants was 45 years in two RCTs,75,79 50 years in two RCTs,73,77 54 years in two RCTs,70,74 55 years in two RCTs,71,76 and 69 years in the remaining RCT. 72 None of the trials specifically included elderly populations.
Descriptions of the baseline characteristics of the populations included in the RCTs varied considerably in their detail (see Table 7). In eight (67%) of the RCTs the mean age of participants within each intervention group was in the range 35.7–47.0 years. 69–71,73–76,78 Two RCTs did not report the age by intervention group but stated that the overall mean age was 42.2 years77 or that most participants (≥ 80%) were aged 40–59 years. 72 The remaining two RCTs did not report the age of their participants. 68,79 In two of the RCTs the participants were all female,73,78 in five RCTs they were mostly (67%–91%) female,68,69,71,72,76 in two RCTs they were mostly (63%–73%) male,70,74 and in two RCTs the gender balance was nearly equal (42%–53% male). 77,79 One RCT75 did not report the gender balance of its participants.
Weight change from baseline was reported as a primary outcome in 11 RCTs68–77,79 and as a secondary outcome in one RCT. 78 Two RCTs also reported changes in BMI, either as a primary outcome75 or as a secondary outcome. 71 The proportion of participants maintaining weight loss was also a primary outcome in one RCT69 (see Table 7).
In one study the setting of the intervention was reported to be in primary care. 72 Six studies70,71,73,74,76,78 reported that their study was clinic based; in some cases these were clinical practice-based research units within universities. Five studies68,69,75,77,79 did not report the setting of their intervention.
The duration of follow-up (post randomisation) ranged from 18 to 54 months. Eight68,69,71,72,74,76,78,79 of the 12 RCTs provided weight outcome follow-up data for a single time point after randomisation, three RCTs70,75,77 provided follow-up data for two time points, and one RCT70 provided follow-up data for four time points. Six68–70,73,75,77 of the RCTs included follow-up beyond 2 years (see Table 7). The duration of interventions is discussed more fully in the following three sections.
The methodological detail reported varied considerably among the RCTs. As an indication of methodological rigour, the risk of bias was assessed for each of the 10 methodological criteria (see Table 8). Quality assessment of each individual trial can be seen in Appendix 5.
Trials that do not properly randomise their participants or conceal the allocation of interventions could be at risk of selection bias. Trials were assessed according to whether they used an adequate method to generate random allocations and to conceal their intervention allocations. Four trials provided information on their randomisation sequence70,72,73,79 and three described their allocation concealment,70,73,74 and all of these were judged to have a low risk of selection bias (see Table 8). For the remaining trials there is an uncertain risk of bias as there was either inadequate or no information reported from which this could be assessed.
Blinding of participants, care providers and outcome assessors can help to reduce the risk that participants and trial personnel become aware of intervention assignment, so potentially influencing their performance in the trial (detection bias). None of the trials clearly reported blinding of their participants or care providers, so it is unknown whether blinding occurred. It seems unlikely that care providers and participants would have been blinded as it would have been difficult to conceal the identity of the weight loss interventions without substantial spatial and/or temporal separation of the care providers and participant groups that were allocated to different interventions. Blinding of outcome assessors, however, is more feasible but was only reported in two trials. 70,73 In both of these the method of blinding was judged as adequate, with a low risk of bias (see Table 8).
Sources of measurement bias in clinical trials include losses of participants to follow-up, unequal dropout rates between interventions, selective reporting of outcomes (missing outcomes) and failure to explain why participants are missing (e.g. whether they are missing at random). Six RCTs were judged to have low risk of bias from dropout (no dropout imbalance);69,73,78 five, however, provided inadequate or no information to assess risk of bias from dropout,68,74–77 and one had unequal dropout between interventions, indicating a high risk of bias. 79 All 12 trials provided sufficient information to permit an evaluation of reporting bias,68–79 of which three had a high risk of bias (some outcomes not reported)71,72,75 and nine had a low risk of bias (all outcomes were adequately reported). 68–70,73,74,76–78,79 Four trials were judged to have a high risk of bias because they did not report an ITT analysis,70,71,76,78 and seven trials were judged to have a high risk of bias because their analysis and interpretation did not account for missing data68,69,71,73,76,78,79 (see Table 8).
Given the lack of methodological information reported it was not possible to rank the RCTs according to their methodological rigour or risk of bias. The extent to which methodological criteria were reported did not appear to show any patterns in relation to the publication date, suggesting there has not been an obvious improvement or deterioration in rigour through time. Where appropriate, the implications of risk of bias are considered in more detail for specific trials in the following three sections of this report.
The 12 RCTs varied considerably in the structure and content of their interventions and comparators and were consequently grouped into four categories (see Chapter 2). The four categories are: trials in which the comparator was a non-active intervention group, for example described as usual care (five trials);70,72–75 trials where the focus of the study was predominantly on diet (two trials);69,71 trials where the focus of the study was predominantly on exercise (four trials);76–79 trials where the focus was on other factors (one trial). 68 Studies are reported in the following sections grouped in these four categories. In these subsequent sections of the report we describe the key aspects of the interventions and results of the studies. As described above there were differences between studies in terms of quality, follow-up, participant characteristics and sample sizes that are referred to where relevant in the subsequent sections. Studies were statistically powered to detect a difference between groups unless stated.
Assessment of effectiveness of multicomponent interventions versus non-active intervention comparators
Five trials70,72–75 compared multicomponent interventions against a non-active intervention comparator group, and can be seen in Table 9. The nature of the comparator differed slightly between these studies. In two of the RCTs, reported by Stevens and colleagues,70,74 the comparator was described only as a usual-care control group, with no further details provided. In one RCT, reported by Jeffery and Wing,75 the comparator was a control group whose participants received no instruction or guidance and were free to act independently to achieve weight loss. The remaining two RCTs, reported by Logue and colleagues72 and Simkin-Silverman and colleagues,73 provided participants in their comparator group with limited general guidance on improving diet72 or reducing cardiovascular risk factors73 (see Table 9). The trial by Jeffery and Wing75 differed in that it had five arms (four active interventions and one non-active comparator), with only 40 or 41 participants randomised per arm. The four active intervention arms reported by Jeffery and Wing75 were standard behavioural therapy/treatment (SBT) alone, or SBT in combination with food provision (FP) and/or incentives (see Table 7). For the purpose of this review we consider SBT as the multicomponent intervention of most relevance. Details of the other interventions can be found in Appendix 5.
| Logue et al. 200572 | |
|---|---|
| Length of intervention: 24 months, follow-up immediate | |
| Transtheoretical model applied to chronic disease | Augmented usual care |
|
Diet: Calorie goal: reduce calories Proportions diet: increase fruit and vegetables, and reduce fat Exercise: Energy expenditure goal: increase activity and exercise but no details provided Type: not reported Behaviour modification: Mode: not reported explicitly but assume individual Frequency: face-to-face counselling once every 6 months. Telephone counselling every month Content – see Table 10 Delivered by: Registered dietitian and weight loss advisor Ongoing support: Written materials mailed on request |
Diet: Calorie goal: reduce calories Proportions diet: increase fruit and vegetables, and reduce fat Exercise: Energy expenditure goal: not reported Type: not reported Behaviour modification: Mode: not reported Frequency: counselling once every 6 months Delivered by: Registered dietitian Ongoing support: None reported |
| Stevens et al. 200170 | |
| Length of intervention: intensive phase – 14 weeks; transitional phase – 16–18 months; follow-up 36 months | |
| Weight loss intervention | Usual-care control |
|
Diet: Calorie goal: men: < 1500 kcal/day. Women: < 1200 kcal/day Proportions diet: decreasing consumption of excess fat, sugar and alcohol Exercise: Energy expenditure goal: approximately 40%–55% of heart rate reserve. From initially 10–15 minutes at least 3 days per week to 30–45 minutes per day, 4–5 days per week Type: primarily brisk walking (moderate intensity) Behaviour modification: Mode: individual then groups of 11–34 Frequency: weekly for 14 weeks (intensive phase) then six biweekly meetings and then monthly meetings for additional 3–4 months (transitional phase) Content – see Table 10 Delivered by: Dietitians and health educators and some psychologists Ongoing support: Variety of options including refreshing or redelivering the intervention content. Biweekly contacts for three to six sessions, offered six times a year (participants expected to attend at least three) |
No description provided |
| Simkin-Silverman et al. 199873 | |
| Length of intervention: phase 1 – 20 weeks; phase 2 – 6–54 months; follow-up 54 months | |
| Lifestyle intervention | Control |
|
Phase 1: Diet: Calorie goal: 1300–1500 kcal/day meal plan in the first month. When weight goal met, intake increased gradually until weight stabilised Proportions diet: for the first month dietary fat reduced to 25%, saturated fat to 7% of daily calories, and cholesterol to 100 mg/day Exercise: Energy expenditure goal: expend 1000 kcal per week from week 3. Already active women to expend 1500 kcal per week, those already expending 1500 kcal per week encouraged to maintain this Behaviour modification: Mode: group, size ≈ 20 Frequency: weekly for 10 weeks, biweekly for 10 weeks Content – see Table 10 Delivered by: Behavioural psychologists and nutritionists Ongoing support Phase 2: Meetings in months 6, 7, 8, 10, 12 and 14. After month 14 participants offered refresher programmes to help with maintenance of behaviour change |
Assessment only (received a health education pamphlet on reducing cardiovascular risk factors and for those who were smokers, advice to quit) |
| Jeffery and Wing 199575 | |
| Length of intervention: 18 months; follow-up 30 months | |
| Standard behavioural treatment | Control |
|
Diet: Calorie goal: 1000 or 1500 kcal per day on the basis of baseline body weight to produce an estimated weight loss of about 1 kg per week Proportions diet: not stated Exercise: Energy expenditure goal: 50 kcal per day for 5 days a week to increase to a final goal of 1000 kcal per week Type: Walking or cycling Behaviour modification: Mode: group, size ≈ 20 Frequency: weekly for first 20 weeks and then once a month Content – see Table 10 Delivered by: Trained interventionists with advanced degrees in nutrition or behavioural science Ongoing support: Not specifically mentioned but content of monthly group meetings noted above may fill this role |
No intervention. Participants could do whatever they wished to lose weight on their own |
| Stevens et al. 199374 | |
| Length of intervention: phase 1 – 6 months; phase 2 – 12 months; follow-up 18 months | |
| Weight loss intervention | Usual-care control |
|
Phase 1: Diet: Calorie goal: not to fall below 1200 kcal/day, no upper limit reported Proportions diet: not stated (optional method of counting fat intake noted) Exercise: Energy expenditure goal: 4–5 days per week with between 30 and 45 minutes of exercise per session, at an intensity of 40%–55% of heart rate reserve Type: Principally walking Behaviour modification: Mode: group 7–20, plus occasional friends or family members Frequency: 14 weekly meetings Content – see Table 10 Delivered by: Dietitian and a psychologist or exercise physiologist Phase 2: Ongoing support: Continued monitoring of weight and exercise encouraged. Goal of at least one contact per month for the remainder of the trial. The type and exact number of contacts varied monthly according to individual needs |
No description provided |
One trial did not explicitly report an aim. 72 The trial reported by Simkin-Silverman and colleagues73 was restricted to premenopausal women and aimed to prevent the natural gain in weight and adverse changes in lipid profiles that occur during the menopause. The remaining three trials stated that they aimed to achieve specific weight loss goals, which were to lose 4.5 kg during the first 6 months,70,74 or to achieve participants’ self-selected weight loss goals of 14, 18, or 23 kg together with an exercise energy expenditure goal of 1000 kcal/week by the final week. 75 Three of the RCTs also included weight maintenance in their aim (see below). 70,73,74
Only Logue and colleagues72 explicitly stated that their intervention was based on a theoretical model (the Transtheoretical Model). Their intervention was from the Reasonable Eating and Activity to Change Health (REACH) trial (not included in this systematic review) and the diet component was based on the US Department of Agriculture (USDA) Food Guide Pyramid Dietary Guidelines for Americans. The intervention for perimenopausal women reported by Simkin-Silverman and colleagues73 was described as an LI (the Women’s Healthy Lifestyle Project). Jeffery and Wing75 reported interventions that they described as standard behaviour treatment. The two remaining interventions by Stevens and colleagues70,74 were referred to as weight loss interventions (see Table 9) which were from the TOHP study.
The RCTs ranged in duration from 18 to 54 months. In the three trials that specifically referred to weight maintenance, the weight maintenance phase was assisted with ongoing support (details are given below). The RCTs reported by Stevens and colleagues70,74 consisted of a 6-month weight loss phase followed by a weight maintenance phase of 12 months74 or 36 months (up to 48 months for some data). 70 The trial reported by Simkin-Silverman and colleagues73 consisted of a 20-week initial phase of weight stabilisation followed by a maintenance phase up to 54 months.
Four of the RCTs specified quantitative calorie goals in the diet component of their interventions. 70,73–75 These were daily limits of: < 1500 kcal for men or < 1200 kcal for women;70 1000 or 1500 kcal according to body weight;75 ≥ 1200 kcal (no upper limit specified);74 or 1300–1500 kcal, gradually increased until weight stabilised. 73 Three RCTs specified dietary composition,70,72,73 but only one, by Simkin-Silverman and colleagues,73 mentioned quantities (dietary fat reduced to 25%, saturated fat to 7% and cholesterol to 100 mg/day). The remaining two trials specified an increase in fruit and vegetables, and reduction in fat,72 or decreased consumption of excess fat, sugar and alcohol70 (see Table 9).
The same four RCTs that specified quantitative calorie goals also provided quantitative exercise goals. 70,73–75 In two of these, exercise intensity was expressed as approximately 40%–55% of the heart rate reserve, achieved principally by increasing the frequency and duration of walking. 70,74 In one trial, exercise energy expenditure was specified as 1000 or 1500 kcal week, depending on participants’ initial activity levels, with maintenance of 1500 kcal/week encouraged, but the mode of exercise was not specified. 73 The remaining trial specified an exercise energy expenditure goal of 50 kcal/day for 5 days/week, increasing to 1000 kcal/week, achieved by walking or cycling. 75 Two of the RCTs72,73 did not specify the mode of exercise (such as walking or cycling) (see Table 9).
Four RCTs reported that the mode of behavioural therapy involved group delivery of the intervention. 70,73–75 Group sizes were 7–20,74 approximately 20,73,75 or 11–34 participants. 70 The frequency of group meetings was initially weekly for the first 10 weeks,73 14 weeks,70,74 or 20 weeks. 75 Subsequent meetings were then fortnightly for 10 weeks,73 fortnightly for 12 weeks, then monthly for 3–4 months,70 or monthly. 75 The behavioural therapy intervention reported by Logue and colleagues72 was not described in detail but appeared to involve an individual mode of delivery, as telephone counselling was provided every month (see Table 9).
The personnel who delivered the behavioural therapy interventions were described only briefly. Behaviour therapy in the trial by Logue and colleagues72 was delivered by a registered dietitian and a weight loss advisor, but it is unclear how many people delivered these two roles. None of the other RCTs specified the number of people who delivered the intervention. In four RCTs the behavioural therapy interventions were delivered partly by dietitians,70,72,74 or nutritionists. 73 These RCTs also included delivery by a weight loss advisor,72 a health educator and psychologist,70 behavioural psychologists,73 or a psychologist or exercise physiologist. 72 The remaining trial used trained interventionists with advanced degrees in nutrition or behavioural science to deliver the intervention75 (see Table 9). None of the studies reported where the behaviour therapy was undertaken.
At least 10 different components of behaviour therapy were identifiable among descriptions provided in the RCTs and are summarised in Table 10. As mentioned above, Logue and colleagues72 did not specify which components they included, but instead reported the behavioural model they used. The model employed is widely documented and accessible, and five individual target behaviours for change were specified for the intervention. Among the remaining four RCTs, the behavioural components represented most commonly were goal-setting or action plans (four studies),70,73–75 relapse prevention (four studies), 70,73–75 problem solving (three studies),73–75 self-monitoring (three studies),70,73,74 and social support (three studies). 70,74,75 Two RCTs included cognitive restructuring,73,75 motivation enhancing, and stimulus control, whereas only one trial included behaviour modelling and none explicitly included self-reinforcement or skill development (see Table 10).
| Cognitive restructuring | Goal-setting/action plans | Modelling | Motivation enhancing | Problem solving | Relapse prevention | Self-monitoring | Self-reinforcement | Skill development | Social assertion (SA)/social support (SS) | Stimulus control | Theoretical model | Others (n) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Logue et al. 200572 | ✓ | ||||||||||||
| Stevens et al. 200170 | ✓ | ✓ | ✓ | SS | 1 | ||||||||
| Simkin-Silverman et al. 199873 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | SA | ✓ | 1 | |||
| Jeffery and Wing 199575 | ✓ | ✓ | ✓ | ✓ | ✓ | SA and SS | ✓ | ||||||
| Stevens et al. 199374 | ✓ | ✓ | ✓ | ✓ | SS |
Ongoing support for participants in the intervention group was reported in four RCTs and varied in its type and frequency. 70,72–74 Logue and colleagues72 provided written materials, which were mailed to participants on request. Stevens and colleagues70,74 provided a variety of options, including refreshing or redelivering the intervention content and encouraging a number of participant contacts, the type and number of which varied according to the needs of individual participants. Simkin-Silverman and colleagues73 provided five meetings for participants during months 7–14, after which they were offered refresher programmes to assist maintenance of behaviour change (see Table 9).
The population characteristics of these RCTs limit the generalisability of their findings. The RCT by Simkin-Silverman and colleagues73 is relevant only to perimenopausal women and based on a subgroup of the total population who were mildly overweight (total population mean BMI 20–34 kg/m2; aged 44–50 years). The remaining RCTs were mostly limited to people with an upper BMI of approximately 34–37 kg/m2, with an upper age limit of 45–54 years. This precludes generalising the findings to very obese and/or older people. In contrast, the trial by Logue and colleagues72 represented overweight participants whose BMI was ≥ 40 kg/m2 and included people aged up to 69 years. The trials, which were all conducted in the USA, recruited predominantly white volunteers who responded to media advertisements and, who apart from being overweight, were free from serious disease. Such people are unlikely to be representative of the UK overweight population. Where reported, participants were relatively well educated and employed. Three of the trials used incentives to enhance participation, which could also influence their generalisability. 72,73,79
The starting weight and BMI of the trial participants were reported for four of the five trials (excluding Logue and colleagues72) (see Table 7). The participants would be classed as pre-obese in two RCTs73,74 and class I obese in two RCTs. 70,75
The appraisal of methodological quality (see Quantity and quality of research available) does not enable any of these five RCTs to be considered ‘best’ or ‘worst’ in terms of risk of bias. This mainly reflects the lack of, or unclear, reporting of the methodological variables. However, for three70,72,73 of these studies the overall risk of bias is generally low, with many key attributes required to reduce potential bias present, while for the remaining two studies74,75 the risk of bias remains uncertain.
Clinical effectiveness
Weight loss
Logue and colleagues72 used three statistical analysis approaches which indicated that at 24 months’ follow-up the Transtheoretical Model-based intervention yielded weight losses of 0.21–0.23 kg relative to the augmented usual-care comparator group (whose participants had received limited general guidance on improving diet). This difference was not statistically significant (NS) (p = 0.50, reported only for an unadjusted analysis) (Table 11). In this study,72 attrition ranged from 31% to 46% depending upon the study arm and time of follow-up. Attrition was 9% higher in the usual-care control arm than in the intervention arm at 18 months’ follow-up, and 7% higher in the control arm at 30 months’ follow-up. The analysis was undertaken using an ITT analysis however.
| Study | Treatment arms | Difference (95% CI); p-value between groups | |
|---|---|---|---|
| Logue et al. 200572 | Transtheoretical model (n = 271) | Augmented usual care (n = 266) | |
| Mean ± SE weight change, kg (95% CI) 0–24 months | |||
| Unadjusted | –0.39 ± 0.38 (–1.1 to 0.4) | –0.16 ± 0.42 (–1.0 to 0.7) | 0.23 (–1.4 to 0.9); p = 0.50 (NS) |
| Adjusted for baseline weight and other covariates | Not reported | Not reported | 0.22 (CI and p-value not reported) |
| Adjusted for participants with missing final weight | Not reported | Not reported | 0.21 (CI and p-value not reported) |
| Stevens et al. 200170 | Weight loss intervention (1) n = 545; (2) n = 547 | Usual care (1) n = 551; (2) n = 554 | |
| Mean ± SD weight change, kg (95% CI) | |||
| 0–18 months | –2.0 ± 5.8 (–2.5 to –1.5) | 0.7 ± 4.2 (0.4 to 1.6) | –2.7 ± 0.3 (–3.3 to –2.1); p < 0.001 |
| 0–36 months | –0.2 ± 5.9 (–0.7 to 0.3) | 1.8 ± 5.3 (1.3 to 2.2) | –2.0 ± 0.2 (–2.6 to –1.3); p < 0.001 |
| Simkin-Silverman et al. 199873 | Lifestyle intervention (n = 260) | Control (n = 275) | |
| Mean ± SD weight change, kg (% of initial weight lost) | |||
| Subgroup 1: overweight at baseline (BMI: 25–29.9 kg/m2) | Subgroup 1 (n = 95): | Subgroup 1 (n = 95): | Subgroup 1: |
| 18 months | –3.5 ± 5.8 (–4.6) | 0.1 ± 4.0 (0.07) | p < 0.001 |
| 30 months | –2.7 ± 5.4 (–3.5) | 0.3 ± 5.1 (0.41) | p < 0.001 |
| 42 months | –1.4 ± 5.7 (–1.7) | 1.3 ± 5.5 (1.9) | p < 0.001 |
| 54 months | 0.1 ± 6.1 (0.31) | 1.5 ± 5.2 (2.2) | Stated NS (no p-value) |
| Subgroup 2: obese at baseline (BMI ≥ 30 kg/m2) | Subgroup 2 (n = 22): | Subgroup 2 (n = 36): | Subgroup 2: |
| 18 months | –6.6 ± 8.4 (–7.7) | –0.5 ± 4.5 (–0.36) | p < 0.01 |
| 30 months | –4.3 ± 6.7 (–5.0) | 2.9 ± 5.4 (3.5) | p < 0.01 |
| 42 months | –2.0 ± 6.4 (–2.3) | 1.9 ± 5.7 (2.5) | p < 0.05 |
| 54 months | –0.2 ± 6.9 (–0.17) | 3.1 ± 7.7 (3.7) | Stated NS (no p-value) |
| Jeffery and Wing 199575 | Standard behavioural therapy (n = 40) | Control (n = 40) | |
| Mean ± SD a weight change, kg | |||
| 0–18 months | –4.6 (n = 26)b | 0.0 (s = 34)b | Reported for any differences between groups only (see Appendix 5) |
| 0–30 months | –1.4 ± 7.2 (n = 40) | 0.6 ± 5.3 (n = 40) | |
| Stevens et al. 199374 | Weight loss intervention (n = 308)c | Control (n = 256)c | |
| Mean weight change, kg | |||
| 0–18 months |
Men –4.7c Women –1.6c |
Men: stated no change (no data reported) Women 0.2c |
p < 0.001c |
Stevens and colleagues’70 weight loss intervention yielded a weight loss relative to the control group of 2.7 kg at 18 months’ follow-up and 2.0 kg at 36 months’ follow-up. These intervention–comparator differences were both statistically significant (p < 0.001). The attrition rates in this study were reported to be similar between groups at approximately 7%–9%. 70 The earlier RCT by Stevens and colleagues,74 which also compared a weight loss intervention against a control group, also reported a statistically significant intervention–comparator difference in weight loss (p < 0.001). However, Stevens and colleagues74 did not specify the amount of weight lost in kg for the intervention and comparator groups as a whole group (see Table 11). In this study the attrition rate was unclear. 74 It was also not reported for either RCT70,74 whether their statistical analyses were powered for testing differences in weight change (tests were powered for the primary outcome, BP).
Simkin-Silverman and colleagues73 demonstrated that their LI for perimenopausal women resulted in large initial weight losses relative to their control group (which had received limited general guidance on reducing cardiovascular risk factors). However, their results were presented for posthoc subgroups of participants who were overweight or obese at baseline. It is unclear whether the study was powered for this analysis and the results should therefore be interpreted cautiously. At 18 months’ follow-up, weight losses were 3.6 kg more in the intervention overweight subgroup than in the comparator overweight subgroup, and 6.1 kg more in the intervention obese subgroup than in the comparator obese subgroup. For the overweight subgroups, weight-change differences in the intervention group at 30, 42 and 54 months exceeded those in the comparator group by 3 kg, 2.7 kg and 1.4 kg, respectively. For the obese subgroups, the corresponding differences at these follow-up times were 7.2 kg, 3.9 kg and 3.3 kg. For each subgroup, the differences at 18, 30 and 42 months’ follow-up were reported to be statistically significant, but at 54 months’ follow-up the differences were stated to be not statistically significant. Participant attrition was 4%–6% in both intervention groups. 73 Full details are given in Table 11.
Jeffery and Wing75 reported large initial weight losses for their standard behaviour treatment interventions compared with the control group. At 18 months’ follow-up, the standard behaviour treatment alone resulted in 4.6 kg weight loss compared with no weight change in the control group. At 30 months’ follow-up, weight loss in the standard behaviour treatment group was 2.0 kg more than in the control group. For the results of the other interventions in this trial see Appendix 5. Pair-wise statistical comparisons of differences relative to the control group at 30 months were not reported for individual interventions. Instead, statistical tests were conducted to detect any differences between the groups. These differences were not statistically significant (overall test for differences among groups p > 0.45; post hoc test for differences between any interventions and comparator p < 0.08). Statistical power calculations for this analysis were not reported (for full details see Appendix 5). Attrition rates were not reported by study arm but were reported to be 12% overall.
Although it is possible that their starting weight and BMI may have influenced participants’ desire or likelihood of achieving weight loss, none of the studies stratified populations by weight or BMI at entry, and the results show no consistent pattern with regard to whether pre-obese or class I obese participants lost more weight.
All of the active interventions resulted in weight change relative to baseline, and in all cases where follow-up was assessed at multiple time points, the amount of weight lost relative to baseline decreased with increasing time after randomisation (see Table 11). While not directly tested, three studies offer data that suggest that long-term weight regain occurred following the end of the active interventions. 70,73,75 Stevens and colleagues70 show that the difference between the weight loss group and control group at the end of the 18-month intervention was similar to the difference at the 36-month end point (2.7 kg and 2.0 kg, respectively). However, comparison of weight loss from baseline to 18 months and from baseline to 36 months (see Table 11) suggests that weight was gained in both the intervention and control arms during 18–36 months, despite participants in the weight loss arm receiving ongoing support for weight maintenance. This finding is difficult to interpret in detail as Stevens and colleagues70 did not explicitly report weight maintenance or weight regain for any time periods of their study. The study by Jeffery and Wing75 appears to show a similar pattern, based on observation of differences between weight changes from baseline to 18 months and from baseline to 30 months, although their study differed from that by Stevens and colleagues70 in that it did not provide ongoing support for the participants after 18 months (note that weight maintenance or regain were not explicitly reported as outcomes; not all randomised participants were followed up to 30 months; and no statistical analyses were presented for this comparison). Similar patterns were seen in the study by Simkin-Silverman and colleagues73 in that despite ongoing maintenance participants appeared to regain weight over the duration of the study. Similar caveats apply as per the discussion of the two studies above, but also because these results are based on subgroups of the overall study population.
Barriers and facilitators
Only one of the five RCTs, by Jeffery and Wing,75 considered participants’ perceived barriers to behaviour change. These were assessed by self-report using a 15-item questionnaire with items scored on a 1–5 scale. The barriers were not reported as outcomes, although the authors stated that at the 30-month follow-up there were no significant differences between the groups in perceived barriers.
Other outcomes reported in the trials (not evaluated here)
The most frequently-reported outcomes other than weight change that were reported in the RCTs were: physical activity;70,72–75 dietary intake;70,72–75 BP;70,72–74 and blood lipids. 72,73 The number of other outcomes reported in addition to weight change ranged from two (BP and attendance)74 to 10 (including various psychosocial, mental and physical health assessments). 72 Adherence to the interventions was assessed and reported in three of the five RCTs, by Stevens and colleagues, 70 Simkin-Silverman and colleagues,73 and Jeffery and Wing. 75
Summary of effectiveness
Five RCTs compared multicomponent interventions to non-active intervention groups. 70,72–75 One of these RCTs provided evidence that longer term (up to 42 months) weight change in the intervention group was significantly different from that of the control group, although much of the weight lost had been regained. 73 However, these long-term benefits are relevant only to perimenopausal women and the analyses were based on posthoc subgroups which may not have been statistically powered. One trial with a 24 month duration found no statistically significant differences in weight loss between the two study arms although it should be noted that attrition was relatively high in this study and appeared to differ between the study arms. 72 One RCT did not directly test for statistical significance between the intervention and the control, although data were available for 30 months’ follow-up and did appear to show greater weight loss in the intervention group than in the control group. 75 Two trials, by Stevens and colleagues, showed statistically significant difference in weight loss (using similar weight loss interventions) at 18 months74 or 36 months. 70 The results of the Stevens and colleagues70 study provide the opportunity to assess weight change 18 months after the intervention had finished. This trial was also among those that would be judged overall to have the lowest risk of bias. Issues over differences in the interventions and differences in the duration of follow-up, and issues around the generalisability of the studies and their risk of bias make comparisons across these studies difficult. However, the weight loss interventions used in the Stevens and colleagues70,74 studies may offer a model that could be tested with relevant UK overweight populations. These trials are from the TOHP study group.
Assessment of effectiveness for multicomponent interventions with a focus on diet
Two69,71 included studies compared a multicomponent intervention which had a focus on the dietary component compared with another active comparator. The two studies are briefly described below and the main components are summarised in Table 12.
| Burke et al. 200871 | |||
|---|---|---|---|
| Length of intervention: 12 months; follow-up 18 months | |||
| STD-D (no preference group) | LOV-D (no preference group) | ||
|
Diet: Calorie goal: reduce maximum daily calorie intake to 1200 kcal (women) 1500 kcal (men) for those weighing < 90.5 kg at baseline; 1500 kcal (women) 1800 kcal (men) for those weighing > 90.5 kg at baseline. Minimum daily intake was 1000 kcal Proportions diet: reduce fat intake to 25% of total kilocalorie intake, but < 10% fat Exercise: Energy expenditure goal: participants encouraged to walk at least 50 minutes per week initially, gradually increasing to at least 150 minutes per week by week 6 Type: mostly walking Behaviour modification: Mode: group, size 10–20 participants Frequency: 32 treatment sessions (lasting 60 minutes) over 12 months. Sessions held in the evening weekly for first 6 months, then every 2 weeks for months 7–9 and monthly for months 10–12 Content – see Table 13 Delivered by: Dietitian with masters degree, exercise physiologist, or nurse behavioural scientist Ongoing support: None. After 12 months the maintenance phase began and no further contact was made with participants until the final 18 month assessment |
Diet: Calorie goal: reduce maximum daily calorie intake to 1200 kcal (women) 1500 kcal (men) for those weighing < 90.5 kg at baseline; 1500 kcal (women) 1800 kcal (men) for those weighing > 90.5 kg at baseline. Minimum daily intake was 1000 kcal Proportions diet: reduce fat intake to 25% of total kilocalorie intake, but < 10% fat. The diet was lacto-ovo-vegetarian (meat, poultry and fish eliminated) Exercise: Energy expenditure goal: participants encouraged to walk at least 50 minutes per week initially, gradually increasing to at least 150 minutes per week by week 6 Type: mostly walking Behaviour modification: Mode: group, size 10–20 participants Frequency: 32 treatment sessions (lasting 60 minutes) over 12 months. Sessions held in the evening weekly for first 6 months, then every 2 weeks for months 7–9 and monthly for months 10–12 Content – see Table 13 Delivered by: Dietitian with masters degree, exercise physiologist, or nurse behavioural scientist. Also vegetarian nutritionist Ongoing support: None. After 12 months the maintenance phase began and no further contact was made with participants until the final 18 month assessment |
||
| Wadden et al. 198869 | |||
| Length of intervention: 4 months (VLCD arm); 6 months (SBT and SBT and VLCD arm); follow-up 3 years | |||
| SBT | SBT and VLCD | VLCD only | |
|
Diet: Calorie goal: 1000–1200 kcal/day Proportions diet: ‘balanced’ diet of participants choosing Exercise: Energy expenditure goal: not reported Type: involved walking and using stairs Behaviour modification: Mode: group, size 4–7 participants Frequency: weekly 90-minute sessions Content – see Table 13 Delivered by: Doctoral-level clinical psychologists Ongoing support: 11 scheduled follow-up meetings: fortnightly for 2 months post-treatment, then monthly for 4 months then at 2-month intervals for 6 months. No other details reported |
Diet: Calorie goal (kcal/day): month 1, 1000–1200; months 2–3, 400–500; months 4–6, 1000–1200 Proportions diet: month 1: ‘balanced’ diet; months 2–3: meat, fish, fowl, plus daily supplements of 3 g each of potassium and sodium chloride and 800 mg of calcium; month 4: conventional food, with introduction of fruits, vegetables, breads, cereals, and fats; months 5–6: chosen by participants Exercise: Energy expenditure goal: not reported Type: involved walking and using stairs Behaviour modification: Mode: group, size 4–7 participants Frequency: weekly 90-min sessions Content – see Table 13 Delivered by: Doctoral-level clinical psychologists Ongoing support: 11 scheduled follow-up meetings: fortnightly for 2 months post-treatment, then monthly for 4 months then at 2-month intervals for 6 months. No other details reported |
Diet: Calorie goal (kcal/day): month 1, 1000–1200; months 2–3, 400–500; month 4, 1000–1200 Proportions diet: month 1: ‘balanced’ diet; months 2–3: meat, fish, fowl, plus daily supplements of 3 g each of potassium and sodium chloride and 800 mg of calcium; month 4: conventional food, with introduction of fruits, vegetables, breads, cereals, and fats Exercise: Energy expenditure goal: not reported Type: not reported Behaviour modification: No behaviour modification. Delivered by: Doctoral-level clinical psychologists Ongoing support: Six scheduled follow-up meetings at 1, 2, 3, 6, 9, 12 months post-treatment. No other details reported |
|
Burke and colleagues71 hypothesised that choice of either a standard calorie and fat-restricted diet (standard diet, STD-D) or a calorie- and fat- restricted vegetarian diet (lacto-ovo-vegetarian diet, LOV-D) would result in greater weight loss than having one of these diets randomly assigned. The authors’ secondary hypothesis was that LOV-D would result in greater weight loss than STD-D. There were four arms to the trial but for the purpose of this review we consider just two of these arms to be the most relevant, those where participants were randomised to either the intervention with STD-D or LOV-D and therefore had no choice regarding the type of diet they received. Wadden and colleagues69 compared three active interventions. For our review we consider the SBT the intervention of relevance to the scope. The other two groups were SBT and VLCD, and VLCD alone.
In the Burke and colleagues71 study the interventions only differed in the type of diet participants were asked to adhere to. For the duration of the 12-month interventions participants in both groups who weighed < 90.5 kg at baseline aimed to reduce their maximum daily calorie intake to 1200 kcal (women) or 1500 kcal (men). Those who weighed over 90.5 kg at baseline had goals of 1500 kcal (women) and 1800 kcal (men). Participants were advised to maintain a minimum daily intake of at least 1000 kcal. All participants also aimed to reduce their fat intake to 25% of total kilocalorie intake, but were advised not to consume < 10% of total kilocalorie intake as fat. The only difference between the two groups was that the LOV-D group eliminated meat, poultry and fish first from breakfast, then lunch, and then dinner so that by the sixth week they had eliminated these foods from their diet. In the Wadden and colleagues study69 participants in the SBT group consumed a 1000–1200 kcal/day ‘balanced’ diet of their choosing for the 6-month intervention period. The mean [standard deviation, (SD)] baseline weight of participants in this study was 112.2 kg (21.5kg) in the SBT group, 108.0 kg (21.5kg) in the SBT and VCLD group, and 106.4 kg (18.4kg) in the VLCD alone group. So for participants over 90.5 kg the weekly calorie goal was lower than in the Burke and colleagues study. 71 Participants in the SBT and VLCD, and VLCD only groups consumed a 1000–1200 kcal/day ‘balanced’ diet of their choosing for the first month of intervention, then they began a 2-month VLCD protein-sparing modified fast of only 400–500 kcal/day. This diet consisted of lean meat, fish and fowl plus potassium, sodium chloride and calcium supplements. In the fourth month fruits and vegetables, breads and cereals, and finally fats were reintroduced to the diet, in that order, to return to a more conventional diet. The VLCD intervention ended at this point (4 months), whereas the SBT and VLCD group continued at 1000–1200 kcal/day diet for a further 2 months (months 5 and 6).
All participants engaged in physical activity for the duration of the Burke and colleagues71 study. Participants were encouraged to walk at least 50 minutes per week initially, gradually increasing this to at least 150 minutes per week, by week 6. In the Wadden and colleagues study69 only two of the three groups had an exercise component. The SBT and SBT and VLCD groups each received the same instruction on increasing lifestyle activity by walking and using the stairs. However, unlike the study by Burke and colleagues,71 it does not appear that there was a specific activity goal.
For the first 6 months of the Burke and colleagues71 study participants attended weekly 60-minute sessions in groups of about 10–20 participants. Sessions were held in the evenings biweekly for months 7–9 and then monthly for the final 3 months of the intervention period. Dietitians, exercise physiologists and nurse behavioural scientists were involved in delivering the sessions, with the LOV-D group also receiving advice from a vegetarian nutritionist for four sessions on how to adopt the eating plan. The behavioural aspect of the intervention was based on several models of motivation and behavioural change such as Social Cognitive Theory. The range of features and strategies included in the behavioural component of the interventions are summarised in Table 13. It is not reported in the study where the behaviour therapy was undertaken. At the end of the 12-month intervention participants had no further contact with the interventionists until the final 18-month assessment. In the Wadden and colleagues study69 the intervention periods of 4 months for VLCD alone, and 6 months for the SBT and SBT and VLCD groups were all shorter than for the Burke and colleagues71 study. All participants were treated weekly for 90 minutes during the intervention period in groups of four to seven participants, a smaller group number than in the Burke and colleagues71 study. A doctoral-level clinical psychologist led the groups. Only two groups, SBT and SBT and VLCD, were taught traditional behavioural methods of weight control following a published manual, although it was not clear whether this was based on a particular behavioural theory. The range of features and strategies included in the behavioural component of the intervention can be seen in Table 13. The setting for the behaviour therapy was not reported. The SBT and VLCD group received additional instruction in the final 3 months of the intervention on maintenance of the weight loss achieved with the VLCD, including relapse prevention training and strategies for handling weight regain. The VLCD alone group did not receive any formal instruction about modifying their eating and exercise habits, and did not receive any behaviour therapy. Following the intervention period participants in the SBT group and SBT and VLCD group attended 11 follow-up meetings in the year after treatment. The timing of these meetings was not reported. Participants in the VLCD only group attended six follow-up meetings at 1, 2, 3, 6, 9 and 12 months post treatment. There was then no further contact with participants in any of the groups until the 3-year follow-up.
| Cognitive restructuring | Goal-setting/action plans | Modelling | Motivation enhancing | Problem solving | Relapse prevention | Self-monitoring | Self-reinforcement | Skill development | Social assertion (SA)/social support (SS) | Stimulus control | Theoretical model | Others (n) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burke et al. 200871 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | SA | ✓ | ✓ | 1 | |
| Wadden et al. 198869 | ✓ | ✓ | ✓ | SS | ✓ | 1 |
The Burke and colleagues71 study enrolled a much higher proportion of women than men, and the majority of participants (about 70%) were white with average socioeconomic status. The majority of the participants recruited by Wadden and colleagues69 were women, but the ethnicity of participants was not reported. The generalisability of the results to men and people of different ethnicities is therefore unknown. The mean ages of the participants in both trials were similar, at approximately 43 years. Wadden and colleagues69 charged a fee to cover medical tests and all other expenses, and in addition participants had to pay a deposit which was refundable after the first year of follow-up. This may have affected the type of people willing to be recruited into the trial.
The starting weight of the trial participants was reported by both trials69,71 and Burke and colleagues71 also reported starting BMI. In the Burke and colleagues study71 starting weight was slightly over 90 kg in both groups (Table 7) and participants would be categorised as having class I obesity according to their starting BMIs. Participants in the Wadden and colleagues study69 were heavier than those in the Burke and colleagues study with baseline weights over 100 kg in each group. Although BMI was not reported, if height is assumed to be 170 cm, then these participants would be categorised as having class II obesity.
Details of the methods of the Burke and colleagues71 trial either were not reported or were unclear (see Quantity and quality of research available). In the Wadden and colleagues69 study the majority of items contributing to the assessment of study quality were not reported. This may be a reflection of the age of the study, which was undertaken over 20 years ago. Therefore, when interpreting the results of these studies the likelihood that they may be associated with a high risk of bias should be borne in mind (see Table 8).
Clinical effectiveness
Weight loss
Table 14 shows the results of the two included studies on measures of weight loss. 69,71 In the Burke and colleagues71 study, by the 18-month assessment attrition from the STD-D group was 29% (14 of 48 participants) and from the LOV-D group was 24% (11 of 45 participants). It is therefore important that the outcome analyses study were conducted on an ITT basis (although nine participants who were randomised between all four arms of the study were excluded because they were found to be ineligible to take part). The primary outcome of the Burke and colleagues71 study was percentage change in body weight from baseline to 18 months. Participants in the STD-D group had a mean (± SD) loss of 8.0% (± 7.8%) of their baseline body weight, which was not statistically different from the mean loss reported for the LOV-D group (7.9% ± 8.1%, p = 0.30). The percentage BMI change from baseline to 18 months also appears similar between the groups although a p-value for the between group comparison was not reported [mean (SD)% BMI change STD-D –7.8 (7.9) vs LOV-D –7.9 (8.2)]. Burke and colleagues71 provide data on weight change at 6-, 12-, and 18-month follow-ups. Weight loss occurred during the 12-month intervention period (mean 9.7 kg loss in STD-D and LOV-D groups) but between the end of the intervention and the 18-month follow-up participants had already begun to regain weight (mean weight regain between 12 and 18 months of 2.4 kg in the STD-D group and 1 kg in the LOV-D group). As this study did not report beyond 18 months of follow-up it was not clear whether weight regain continued at the same rate over time.
Wadden and colleagues69 followed participants at 3 years but at the 3-year assessment attrition, which was only reported for the whole study population, was 24% (14 of 59 participants). Therefore results were only available for 45 of the 59 participants who had been randomised between the three groups. Furthermore, in the period since the end of the intervention some participants had received additional weight loss therapy. Therefore Wadden and colleagues69 included a correction for this (see Table 14) and presented the results of both the uncorrected and corrected analyses. There were no statistically significant differences between the three groups in mean (kg) weight loss at 3 years’ follow-up [corrected analysis: SBT 3.54 (SD 6.26); SBT and VLCD 5.11 (SD 8.28); VLCD 2.20 (SD 8.50), stated not significant, p-value not reported] and less than a fifth of participants in each group had maintained their weight loss to within 2 kg of their end-of-treatment weight (SBT 19%; SBT and VLCD 13%; VLCD 7%; no p-value reported). There were no statistically significant differences between the groups in the proportion of participants who equalled or exceeded their pre-treatment weight (SBT 38%; SBT and VLCD 47%; VLCD 43%; no p-value reported). Less than half the participants followed up at 3 years had maintained a 5 kg or greater weight loss; proportions for the three groups ranged from 29% to 44% with no significant differences between the groups (p-value not reported). The proportion maintaining a ≥ 10 kg weight loss was less than a third, ranging from 7% to 31%. Again there were no significant differences between the groups (p-values not reported). No analysis of the statistical power to detect a difference between groups was reported for this study.
| Study | Treatment arms | p-value between groups | ||
|---|---|---|---|---|
| Burke et al. 200871 | STD-D (n = 48) | LOV-D (n = 45) | ||
| % weight change, baseline to 18 months (mean SD) | –8.0 (7.8) | –7.9 (8.1) | 0.30 | |
| Weight change, baseline to 12 months, mean kga | –9.7 | –9.7 | ||
| Weight change, 12–18 months maintenance phase, mean kga | 2.4 | 1 | ||
| % BMI (kg/m2) change, baseline to 18 months, mean (SD) | –7.8 (7.9) | –7.9 (8.2) | ||
| Wadden et al. 199869 | SBT (n = 14) | SBT and VLCD (n = 16) | VLCD (n = 15) | |
| Weight loss at 3 years, mean ± SD (kg) | ||||
| Uncorrected analysisb | 4.76 ± 6.56 | 6.53 ± 9.50 | 3.76 ± 8.85 | Stated NS; no p-values reported |
| Corrected analysisa | 3.54 ± 6.26 | 5.11 ± 8.28 | 2.20 ± 8.50 | |
| Mean proportion (%) of participants who equalled or exceeded their pre-treatment weightc,d | 38 | 47 | 43 | Stated NS; no p-values reported |
| Mean proportion (%) of participants who maintained weight loss within 2 kg of their end-of-treatment weightd | 19 | 13 | 7 | Not reported |
| Mean proportion (%) of participants who maintained weight lossc,d | ||||
| 5 kg or greater | 44 | 33 | 29 | Stated NS; no p-values reported |
| 10 kg or greater | 31 | 27 | 7 | |
As the follow-up periods differed between the studies it was not possible to determine whether participants in the Burke and colleagues71 trial who had class I obesity lost more or less weight than the participants in the Wadden and colleagues69 trial who were heavier (and likely to have class II obesity).
Barriers and facilitators
Neither study reported on potential barriers or facilitators.
Other outcomes reported in the trials (not evaluated here)
Burke and colleagues71 also presented results for changes in cholesterol, glucose levels, insulin levels, kilocalorie consumption, fat consumption, carbohydrate consumption, animal protein consumption, vegetable protein consumption, and fibre consumption. Wadden and colleagues69 reported on outcomes at 1 year in an earlier publication but due to the short follow-up this did not meet the inclusion criteria of this review. Wadden and colleagues69 did not report whether they measured participants’ attendance at sessions, whereas Burke and colleagues71 did measure this.
Summary of effectiveness
Two studies69,71 assessed multicomponent interventions that differed only in terms of the dietary component. One of these studies69 also had a third arm to investigate the dietary component VLCD alone. Participants in both studies lost weight but there were no statistically significant differences in weight loss between study groups. After completing the intervention participants from both studies regained weight over time. The two studies were not directly comparable owing to different interventions, duration of the interventions, and duration of follow-up. The generalisability of these findings to participants in the UK is not clear.
Assessment of effectiveness for multicomponent interventions with a focus on exercise
Four76–79 of the included studies compared a multicomponent intervention to an active comparator in which the focus of the study in general was on exercise. The four studies are briefly described below and the main components are summarised in Tables 15 and 16.
| Tate et al. 200777 | |||
|---|---|---|---|
| Length of intervention: 18 months; follow-up 30 months | |||
| SBT | HPA | ||
|
Diet: Calorie goal: to reduce daily energy intake to 1000–1500 kcal depending on initial body weight. Proportions diet: consume < 20% of energy as fat Exercise: Energy expenditure goal: to build up from energy expenditure of 250 kcal/week, increasing by 250 kcal/week, to energy expenditure of 1000 kcal/week (roughly equivalent to walking for 30 minutes/day) Type: Not reported Behaviour modification: Mode: group, size 10–20 Frequency: weekly meetings for first 6 months, biweekly from 6 to 12 months, then monthly from 12 to 18 months Content – see Table 16 Delivered by: Trained interventionists (nutritionists, exercise physiologists, or psychologists) with expertise in both content areas (i.e. physical activity and nutrition) and behavioural therapy Ongoing support: None |
Diet: Calorie goal: to reduce daily energy intake to 1000–1500 kcal depending on initial body weight. Proportions diet: consume < 20% of energy as fat Exercise: Energy expenditure goal: to build up to an energy expenditure of 2500 kcal/week by the end of the first 6 months of the intervention (roughly equivalent to walking < 75 minutes/day) Type: Not reported Behaviour modification: Mode: group, size 10–20 Frequency: weekly meetings for first 6 months, biweekly from 6 to 12 months, then monthly from 12 to 18 months Content – see Table 16 Delivered by: Trained interventionists (nutritionists, exercise physiologists, or psychologists) with expertise in both content areas (i.e. physical activity and nutrition) and behavioural therapy Ongoing support: None |
||
| Jeffery et al. 199876 | |||
| Length of intervention: 18 months; follow-up 18 months | |||
| SBT | SBTE | ||
|
Diet: Calorie goal: 1000 kcal/day if weight was < 91 kg and 1500 kcal/day if weight was ≥ 91 kg Proportions diet: restrict fat to 20% or less of calories (22 g/day for 1000 kcal and 33 g/day for 1500 kcal) Exercise: Energy expenditure goal: to exercise to the equivalent of 250 kcal/week and to gradually increase to a minimum of 1000 kcal/week Type: Primarily walking or cycling Behaviour modification: Mode: group, size ~ 20 Frequency: weekly for 24 weeks and once per month thereafter Content – see Table 16 Delivered by: Trained interventionists with advanced degrees in nutrition or behavioural sciences Ongoing support: Not stated except for as part of the programme described above (monthly meetings after first 24 weeks) |
Diet: Calorie goal: 1000 kcal/day if weight was < 91 kg and 1500 kcal/day if weight was ≥ 91 kg Proportions diet: restrict fat to 20% or less of calories (22 g/day for 1000 kcal and 33 g/day for 1500 kcal) Exercise: Energy expenditure goal: to exercise to at least 1000 kcal/week. Regular attendance at supervised sessions would produce approximately 750 kcal/week Type: Primarily walking or cycling Behaviour modification: Mode: group, size ~ 20 Frequency: weekly for 24 weeks and once per month thereafter Content – see Table 16 Delivered by: Trained interventionists with advanced degrees in nutrition or behavioural sciences Ongoing support: Not stated except for as part of the programme described above (monthly meetings after first 24 weeks) |
||
| Skender et al. 199679 | |||
| Length of intervention: 1 year; follow-up 2 years | |||
| Combination intervention (diet and exercise) | Exercise intervention | Diet intervention | |
|
Diet: Calorie goal: adjust caloric intake so weight loss not more than 1 kg/week Proportions diet: 30% of calories as fat, 50% as carbohydrate and 20% as protein Exercise: Energy expenditure goal: based on heart rate, breathing difficulty, and perceived effort to be ‘vigorous’ but never ‘strenuous’. Goal was three to five sessions per week of 45 minutes or more per session Type: Brisk walking Behaviour modification: Mode: group, size ≈ 15 Frequency: 12 weekly 60 minute group instructional sessions, then three biweekly sessions, then eight monthly maintenance sessions (total 1 year) Content – see Table 16 Delivered by: By registered dietitians who were trained and experienced in behaviour modification Ongoing support: Only as part of maintenance sessions reported above. No ongoing support following the end of the intervention |
Diet: Calorie goal: not reported. Participants were asked to maintain their current eating habits and nutrition was not discussed Proportions diet: not applicable Exercise: Energy expenditure goal: based on heart rate, breathing difficulty, and perceived effort to be ‘vigorous’ but never ‘strenuous’. Goal was three to five sessions per week of 45 minutes or more per session Type: Brisk walking Behaviour modification: Mode: group, size ≈ 15 Frequency: 12 weekly 60 minute group instructional sessions, then three biweekly sessions, then eight monthly maintenance sessions (total 1 year) Content – as reported for ‘combination’ intervention Delivered by: By registered dietitians who were trained and experienced in behaviour modification Ongoing support: Only as part of maintenance sessions reported above. No ongoing support following the end of the intervention |
Diet: Calorie goal and proportions diet: as reported for the combination intervention Exercise: Energy expenditure goal: none Type: Normal physical activity only (no new exercise programmes) Behaviour modification: Mode: group, size ≈ 15 Frequency: 12 weekly 60 minute group instructional sessions, then three biweekly sessions, then eight monthly maintenance sessions (total 1 year) Content – as reported for ‘combination’ intervention Delivered by: By registered dietitians who were trained and experienced in behaviour modification Ongoing support: Only as part of maintenance sessions reported above. No ongoing support following the end of the intervention |
|
| Weinstock et al. 199878 | |||
| Length of intervention: 48 weeks; follow-up 96 weeks | |||
| Diet and aerobic training | Diet alone | ||
|
Diet: Calorie goal: 900–925 kcal/day (weeks 2–17). Increasing to 1250 kcal/day (week 18–20); 1500 kcal/day (weeks 22–48) Proportions diet: 150 kcal, 15 g protein, 11.2 g carbohydrate, 5 g fat (per serving of a liquid meal replacement four times per day, weeks 2–17); 280–300 kcal, 20 g protein, 35–40 g carbohydrate, 7 g fat (per dinner entrée, weeks 2–17); 12–15% calories from protein, 55–60% from carbohydrate, and 15–30% from fat (weeks 22–48) Exercise: Energy expenditure goal: three sessions per week for 28 weeks, two per week during weeks 29–48. 12 minutes at week 1, additional 2 minutes to routine each week, by week 14 performed 40 minutes of stepping Type: Step aerobics Behaviour modification: Mode: group, size 7–10 participants Frequency: 28 weekly 90 minute sessions, followed by biweekly maintenance programme sessions (weeks 29 to 48) Content – see Table 16 Delivered by: Clinical psychologists, dietitian and graduate students in exercise physiology Ongoing support: Participants attended group sessions once every 3 months in the year following treatment. Between weeks 48 and 96 the women were encouraged to continue exercising unsupervised |
Diet: Calorie goal: 900–925 kcal/day (weeks 2–17). Increasing to 1250 kcal/day (week 18–20); 1500 kcal/day (weeks 22–48) Proportions diet: 150 kcal, 15 g protein, 11.2 g carbohydrate, 5 g fat (per serving of a liquid meal replacement four times per day, weeks 2–17); 280–300 kcal, 20 g protein, 35–40 g carbohydrate, 7 g fat (per dinner entrée, weeks 2–17); 12–15% calories from protein, 55–60% from carbohydrate, and 15–30% from fat (weeks 22–48) Exercise: None. Participants agreed not to engage during the study in any programme of regular activity that resembled the aerobic or strength training conditions (but they were allowed to maintain lifestyle activities such as occasionally playing tennis, bowling or lunchtime walks) Behaviour modification: Mode: group, size 7–10 participants Frequency: 28 weekly 90 minute sessions, followed by biweekly maintenance programme sessions (weeks 29–48) Content – see Table 16 Delivered by: Clinical psychologists, dietitian and graduate students in exercise physiology Ongoing support: Mentions only that participants attended group sessions once every 3 months in the year following treatment |
||
| Cognitive restructuring | Goal-setting/action plans | Modelling | Motivation enhancing | Problem solving | Relapse prevention | Self-monitoring | Self-reinforcement | Skill development | Social assertion (SA)/social support (SS) | Stimulus control | Theoretical model | Others (n) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tate et al. 200777,a | ✓ | ✓ | ✓ | ✓ | ? | SS | ✓ | 0 | |||||
| Jeffery et al. 199876 | ✓ | ✓ | ✓ | ✓ | ✓ | SA and SS | ✓ | 0 | |||||
| Skender et al. 199679 | ✓ | ✓ | ✓ | ✓ | ?b | 3 | |||||||
| Weinstock et al. 199878,c | ✓ | ?b |
Of the four studies, those by Tate and colleagues77 and Jeffery and colleagues76 were the most similar in terms of intervention characteristics and design, having been conducted by some of the same investigators (these are therefore discussed first in this subsection, with the characteristics of the remaining two studies presented afterwards). Both studies evaluated an 18-month-long ‘SBT intervention’ comprising similar behavioural change techniques, alongside exercise and diet components. The most intensive phase in both studies appeared to be the first 6 months with weekly meetings to encourage weight loss, followed by biweekly or monthly meetings to promote weight maintenance. Final outcomes were measured at the end of the 18-month period in the study by Jeffery and colleagues study,76 but in the Tate and colleagues77 study they were measured 1 year following the end of the intervention (i.e. at 30 months).
The Tate and colleagues study77 compared SBT with a ‘high physical activity (HPA)’ arm, identical to the SBT group but with higher weekly energy expenditure goals and a greater level of supervision and social support (see Appendix 5). The aim of the study was to determine whether higher levels of energy expenditure (2500 kcal/week) would result in greater weight loss compared with SBT (1000 kcal/week). In contrast, Jeffery and colleagues76 compared SBT with four other arms including SBT plus supervised exercise (SBTE), SBT and supervised exercise plus a personal trainer (SBTT), SBT and supervised exercise plus incentives to exercise (SBTI), and SBT and supervised exercise plus both personal trainer and incentives to exercise (SBTTI). The hypothesis was that a greater number of exercise sessions would be attended by those receiving support from a personal trainer or financial incentives than SBT alone, and that a combination of trainer and incentives would result in greater exercise adherence and therefore greater energy expenditure in exercise and greater weight loss.
For both studies we considered the SBT arm as being the multicomponent intervention of most relevance to the scope of this review. In the Jeffery and colleagues76 study we have compared SBT with SBTE only, hence the characteristics and results of the other three arms are not reported here (for further details of these see Appendix 5).
The diet component, which was the same in both arms in each study, was similar between the two studies, with an emphasis on calorie restriction to between 1000 and 1500 kcal per day, with ≤ 20% of calories from fat. Both studies encouraged a gradual increase in exercise (e.g. walking, cycling) to build up energy expenditure to at least 1000 kcal per week (or, as mentioned, to 2500 kcal in the HPA arm of the Tate and colleagues study77). Exercise activities were conducted either individually or as part of group sessions.
Likewise, the behavioural component, which was the same in both arms in each study, was similar between the two studies, with therapy delivered in small groups (10–20 participants) on a weekly basis for the first 6 months, then biweekly or monthly thereafter until the end of the 18-month intervention. The sessions were led by interventionists trained in exercise, nutrition or the behavioural sciences and incorporated motivation enhancement, problem solving, stimulus control (e.g. reducing the visibility of food in the home), and relapse prevention techniques (e.g. recognising precursors and consequences of dietary lapses) (see Table 16).
The third study in this subsection was a trial of a cognitive behavioural weight control intervention by Skender and colleagues79 The trial compared a multicomponent ‘combination’ intervention comprising diet, exercise and behaviour therapy with an intervention focused on exercise, and with another intervention focused on diet (a waiting list control group was also included, but no outcome data were reported for this group so it is not mentioned any further in this subsection). All three interventions included the same behavioural component. The aim was to assess whether the addition of exercise to diet would lead to greater weight loss. For the purpose of this review the ‘combination’ intervention was considered to be most relevant to the scope.
The interventions took place over 1 year with weekly 1-hour meetings over a 12-week period, then three biweekly sessions followed by the maintenance phase comprising eight monthly meetings. Final outcomes were measured at 2 years from baseline (i.e. a year after the end of the intervention). The dietary component featured in the combination intervention and the diet-focused intervention encouraged a caloric intake to attain weight loss of up to 1 kg per week, with 30% of calories to come from fat, 50% from carbohydrate and 20% from protein. Participants were instructed to plan their meals based on the Help Your Heart Eating Plan (HYHEP) low-cholesterol diet. In the exercise focus group participants were asked to maintain their current eating habits and nutrition was not discussed.
The exercise component featured in the combination intervention and the exercise-focused intervention. Information was provided on the benefits of exercise and instruction was given on correct methods of walking. Participants were instructed to self-regulate the intensity of brisk walking based on heart rate, breathing difficulty, and perceived effort. They were instructed to exercise at a level that felt ‘vigorous’ but never ‘strenuous’, and were encouraged to exercise for three to five sessions per week for 45 minutes or more per session. Those in the diet-focused intervention were asked to maintain their relatively sedentary lifestyles and not to begin any new exercise programme.
The behavioural therapy component (common to all three interventions) involved the use of self-monitoring contracts to reward behaviour change (contingency contracting), stress management, stimulus control, goal-setting and maintenance techniques (see Table 16). The behavioural component followed the principles outlined in the LEARN® programme for weight control. This is a 12-week educational lifestyle weight management programme (‘Lifestyle, Exercise, Attitudes, Relationships and Nutrition’). It aims to enable people to lose between 1 and 2 lb per week through a decrease in dietary caloric intake and an increase in exercise to burn stored calories. The interventions were delivered by registered dietitians who were trained and experienced in behaviour modification.
The fourth study, a 48-week trial by Weinstock and colleagues,78 randomised participants to either a diet only intervention or one of three exercise interventions, diet with aerobic training, diet with strength training and diet with aerobic and strength training. In the exercise interventions the diet and behaviour components were the same across the arms. For the purpose of this review we considered that the diet and aerobic training intervention was the multicomponent intervention of most relevance to the scope. In this intervention the exercise type was ‘step’ aerobics. Details of the other exercise interventions can be seen in Appendix 5. An increasing daily kilocalorie goal was used through the 48-week intervention, increasing to 1500 kcal by week 22 which remained then until week 48. In the first 17 weeks a 900–925 kcal/day goal was used and this was as a liquid meal replacement. For the rest of the duration of the study participants were advised on the proportions of their diet to be made up from protein, carbohydrate and fat (see Table 15). Participants maintained weekly diet diaries.
Participants met weekly in groups of 7–10 people until week 28 for their behavioural therapy and biweekly thereafter. The components of the behavioural aspect of the intervention were poorly described (see Table 15). The features and strategies covered appeared to be centred on skill development. The intervention was delivered by clinical psychologists, a dietitian and graduate students in exercise physiology. For the first 28 weeks participants exercised with members of their behavioural treatment groups by step aerobics. They were required to participate in three sessions per week for 28 weeks and then two sessions per week during weeks 29–48. The time spent undertaking the step aerobics increased from 12 minutes at week 1 to 40 minutes by week 14. In the diet alone arm no prescribed exercise was undertaken, although the participants were allowed to maintain any lifestyle activities as normal. After the 48-week intervention participants were followed up at week 96. In the interim, group sessions occurred every 3 months as a form of ongoing support.
The multicomponent interventions included in the four studies appear to be similar in terms of content and structure. All studies encouraged participants to choose low-calorie diets and to reduce the proportion of calories from fat. They were also supported to take regular exercise, primarily through walking and cycling, according to set weekly goals. Behavioural techniques such as stimulus control, self-monitoring and goal-setting were also common aspects of the interventions, typically led by trained interventionists in small groups of < 20 participants. None of the studies reported where the behaviour therapy was undertaken. The level of detail in which the interventions were reported precludes a full assessment of generalisability. However, all studies were conducted in the USA with mostly white, educated populations who were paid monetary incentives to participate. The mean ages of participants were between 40 and 42 years for three studies,76–78 but in one study were not reported. 79 With the exception of Tate and colleagues,77 the interventions were delivered in the early to mid-1990s. It is not clear whether the LEARN® programme or the HYHEP diet are necessarily representative of current UK practice.
The value of the Weinstock and colleagues78 study to this systematic review is limited for a number of reasons. The trial predominantly reported changes in body composition and resting energy expenditure (REE), appetite and mood, and the long-term weight loss outcomes reported in the study were from a subgroup of the overall trial population. In addition, the number of participants randomised to each intervention was not explicitly reported (but has been estimated by our reviewers to be between 29 and 31 per intervention group) and the results presented were for three of the groups combined rather than for all randomised interventions.
In terms of starting weight, two of the studies included participants classified as BMI obesity I,76,77 and in a third study78 the participants were classified as BMI obesity II. The fourth study79 did not report baseline BMI; however, it did report weight in terms of kg that appeared to be reasonably similar to the other studies.
Poor reporting of methodological details of the studies hampers an informed judgement regarding potential risks of bias (see Quantity and quality of research available) and therefore these risks are generally regarded as uncertain, which needs to be considered when interpreting results presented below.
Clinical effectiveness
Weight loss
Table 17 reports the results of the four studies in terms of weight loss. 76–79 More weight in kilograms was lost by participants in the HPA intervention than by participants in the SBT group in the study by Tate and colleagues77 at both 18 months (6.7 ± 8.1 vs 4.1 ± 8.3, respectively, p = 0.04) and 30 months (2.86 ± 8.6 vs 0.9 ± 8.9, respectively, p = 0.16), though the difference was only statistically significant at the end of the 18-month intervention. In contrast, more weight was lost by those receiving SBT than those undergoing SBT plus supervised exercise at 18 months in the study by Jeffery and colleagues76 [–7.6 kg (standard error, SE 1.1) vs –3.8 kg (SE 1.3), respectively, p = 0.03]. Statistical power calculations were not reported for either of these two studies. 76,77 By the 30-month follow-up between 21% and 23% of participants across the study groups had dropped out of the study by Tate and colleagues77 An ITT analysis was conducted, and participants with missing data at any time point were assumed not to have lost any weight and their baseline weight was carried forward. In the study by Jeffery and colleagues76 by 18 months 22% of participants had dropped out. This figure was for the study population in general, so it is not known whether the rate of dropout varied between study groups. It was not reported whether an ITT analysis had been conducted and hence whether dropouts had been included in the analysis, so caution is advised in the interpretation of the results.
| Study | Treatment arms | p-value between groups | |||
|---|---|---|---|---|---|
| Tate et al. 200777 | SBT (n = 109) | HPA (n = 93) | |||
| Weight change kg, baseline to 18 months | –4.1 ± 8.3 (n = 80)a | –6.7 ± 8.1 (n = 87)a | p = 0.04 | ||
| Weight change kg, baseline to 30 monthsb | –0.9 ± 8.9 (n = 74)a | –2.86 ± 8.6 (n = 84)a | p = 0.16 | ||
| Weight loss (%) of initial body weight, baseline to 30 monthsb | 1 | 3 | States no significant difference, p-value not reported | ||
| Success at 30 months |
Total weight loss ≥ 5% achieved by 26% Total weight loss ≥ 10% achieved by 12% |
States no significant difference, p-value not reported | |||
| Jeffery et al. 199876 | SBT (n = 40)c | SBTE (n = 41)c | |||
| Weight change in kg at 18 months’ follow-up, mean (SE) | –7.6 (1.1) | –3.8 (1.3) | p = 0.03d | ||
| Skender et al. 199679 | Combination intervention (n = 21) | Diet-focused intervention (n = 15) | Exercise-focused intervention (n = 25) | ||
| Mean ± SD weight change (kg) from baseline (0–2 years)e | –2.2 ± 6.7 | + 0.9 ± 7.7 | –2.7 ± 9.2 | p = 0.36 | |
| Number (%) of participants with clinical success (weight loss > 4.5 kg) | 8 (38%) | 2 (13%) | 6 (24%) | p = 0.36 | |
| Number (%) of participants with no weight change (within ± 4.5 kg)f | 10 (48%) | 9 (60%) | 18 (72%) | Not reported | |
| Weinstock et al. 199878 | Diet and aerobic exercise (n = 29) | Diet only (n = 29) | |||
| Maintenance of weight loss at week 96 | Not reported | Not reported | |||
In the study by Skender and colleagues,79 the greatest amount of weight was lost by the exercise-focused intervention (–2.7 kg ± 9.2), followed by the combination intervention (–2.2 kg ± 6.7) over the 2-year follow-up period. During this time the mean weight in the diet-focused intervention increased to +0.9 kg (± 7.7). The differences between the three groups were not statistically significant (p = 0.36). However, the proportion of participants classified as achieving clinical success (defined as weight loss > 4.5 kg) was highest in the combination group (38%), followed by the exercise-focused group (24%) and the diet-focused group (13%). Again, this difference was not statistically significant (p = 0.36). The proportion of participants classified as having no weight change was lowest in the combination group (48%), followed by the diet-focused group (60%) and then the exercise-focused group (72%), (p-value not reported). Caution is advised in the interpretation of these results as outcomes at the 2-year follow-up were based only on those participants who returned for assessment [61 (48%) of the 127 randomised to the three active interventions]. Also, no statistical power analysis was reported for this study.
Weinstock and colleagues78 did not report outcomes for the 96-week evaluation between intervention arms; rather they reported outcomes as the change from baseline for more than one group combined. Data presented were also from a subgroup of the original population. Therefore there is little in the way of useful data from this study (see Appendix 5 for full details).
No clear pattern was evident from the results in the trials in terms of the degree of weight lost according to the starting weight (e.g. whether those who were more obese at baseline lost more weight).
Weight regain
Table 18 reports data on weight regain or gain from the two studies that report this outcome. In the study by Tate and colleagues77 mean weight regain in each group between the end of the intervention at the 18- and the 30-month follow-up appeared generally similar (around 5–6 kg), with a non-statistically significant difference between groups (p-value not reported). Skender and colleagues79 reported the proportion of participants who gained in excess of 4.5 kg of weight from baseline to the 2-year follow-up. This category was distinct from the mutually exclusive categories of participants whose weight did not change and those with clinical success (both of which are reported in Table 17). Less than a third of participants came under this category, with the highest proportion in the diet-focused intervention (27%), followed by the combination intervention (14%) and the exercise-focused intervention (4%). No statistical tests were reported for this outcome. As mentioned above, caution is required when interpreting results of the Skender and colleagues79 study as they are based on less than half of those originally randomised.
| Study | Treatment arms | p-value between groups | |||
|---|---|---|---|---|---|
| Tate et al. 200777 | SBT (n = 109) | HPA (n = 93) | |||
| Weight regain kg, 18–30 monthsa | 5.3 ± 7.0 (n not reported)b | 5.9 ± 5.9 (n not reported)b | States no significant difference, p-value not reported | ||
| Skender et al. 199679 | Combination intervention (n = 21) | Diet-focused intervention (n = 15) | Exercise-focused intervention (n = 25) | ||
| Number (%) of participants with weight gain > 4.5 kgc | 3 (14) | 4 (27) | 1 (4) | Not reported | |
Data from these two studies can be used to assess weight regain following the end of the active interventions. In the Tate and colleagues77 study results suggest a lack of long-term success after the end of the intervention (at 18 months). In the Skender and colleagues79 study, interim data were presented for weight loss at the end of the 12-month intervention (Note: 12-month data do not meet the inclusion criteria for our review as the inclusion criteria state that studies had to report follow-up data at 18 months or longer – see Chapter 2, Data synthesis). These data appear to show a similar pattern, with the greatest weight loss at the end of the intervention and then a gradual increase in weight again prior to the 24-month follow-up.
Barriers and facilitators
Only one of the studies mentioned measuring barriers and facilitators. 76 Jeffery and colleagues76 used a 15-item questionnaire to assess participants’ perceptions of practical, social and interpersonal barriers to successful behaviour change. However, no results were reported.
Other outcomes reported in the trials (not evaluated here)
Physical activity was a primary outcome measure in the study by Jeffery and colleagues76 A variety of secondary outcome measures were reported including physical activity or exercise,76,77,79 dietary intake,76,77 attitudes to diet and exercise,79 cardiorespiratory fitness,79 percentage body fat,79 depression,76 binge eating76 and adverse effects. 77 Weinstock and colleagues78 also reported body composition, REE, appetite, mood, insulin resistance, glucose tolerance and BP as outcome measures. The four studies measured adherence to the intervention, but in varying ways. 76–79 Skender and colleagues79 measured self-reported adherence to diet and exercise which participants recorded in questionnaires, whilst Jeffery and colleagues76 and Weinstock and colleagues78 measured attendance at the group sessions. Tate and colleagues77 report outcomes for a selected ‘high-adherence’ exercise group.
Summary of effectiveness
Four studies in which a multicomponent intervention was compared with another active comparator (in which the focus of the study was generally about physical activity) have been presented in terms of changes in weight up to 30 months. Most of the interventions were associated with weight loss at the 18-month to 2-year follow-up time points, with losses of up to 7.6 kg. However, mean weight loss had diminished at 30 months in the one study that reported outcomes at this time point, suggesting a lack of longer-term weight maintenance. There was no consistent pattern in terms of comparative effectiveness between the SBT intervention and the SBTE or HPA variants. In one study the HPA participants lost significantly more weight than the SBT participants (but by 30 months the difference was no longer statistically significant). Conversely, in another study SBT participants lost significantly more weight than participants who received SBTE. There were no statistically significant differences when comparing the combination of diet and physical activity with these two components separately (all of which included a behavioural therapy component). 79 The results of this study are difficult to interpret given the high loss to follow-up rates. Lack of details in the publications precludes full judgements of risk of bias and generalisability.
Assessment of effectiveness for multicomponent interventions where the focus was on other variables
One included study by Dubbert and Wilson68 did not appear to fit into the categories of active intervention versus no-active comparator, focusing on the dietary component, or focusing on the exercise component. This study is therefore considered separately in this section. The trial tested two variables – goal-setting (daily or weekly), and spouse involvement (or not) – in their multicomponent approach to weight loss. The intervention is briefly described here and in Table 19. BMI at baseline was not reported in this study (see Table 7); however, the participants were required to be at least 15 lb (approximately 7 kg) overweight and participants in both of these groups had mean weights around 208 lb (∼ 94 kg). If height is assumed to be 170 cm, then these participants would be categorised as having class I obesity.
| Dubbert and Wilson 198468 | |
|---|---|
| Length of intervention: 19 weeks; follow-up 34 months | |
| Treatment with daily goals | Treatment with weekly goals |
|
Diet: Calorie goal: from week 5 recommended 1215 kcal/day for women or 1525 kcal/day for men. Participants were encouraged to set daily calorie goals and to divide these into subgoals for portions of the day Proportions diet: not reported Exercise: Energy expenditure goal: states individual-based goal-setting to meet a daily goal. From week 5 minimal caloric-expenditure goals were recommended, starting at 145 cal/day above initial baseline. Goals were increased each week by 25 cal/day if previous week’s goals were met on at least 4 days Type: Aerobic exercise walking programme Behaviour modification: Mode: group, size not reported Frequency: weeks 1–4: 2-hour lectures and small group discussion meetings; week 5: individual sessions of 15–20 minutes duration commenced (one per week during weeks 5–7); fortnightly thereafter Content – see Table 20 Delivered by: Clinical psychology graduate student therapists (one experienced in behavioural weight-control treatment) Ongoing support: None reported |
Diet: Calorie goal: participants were encouraged to set weekly calorie goals of 8500 kcal for women or 10,675 kcal for men Proportions diet: not reported Exercise: Energy expenditure goal: states individual-based goal-setting to meet a weekly goal Type: Aerobic exercise walking programme Behaviour modification: Mode: group, size not reported Frequency: weeks 1–4: 2-hour lectures and small group discussion meetings; week 5: individual sessions of 15–20 minutes duration commenced (one per week during weeks 5–7); fortnightly thereafter Content – see Table 20 Delivered by: Clinical psychology graduate student therapists (one experienced in behavioural weight-control treatment) Ongoing support: None reported |
In the trial68 the interventions lasted for 19 weeks, with follow-up 30 months later. Participants were randomised to essentially the same multicomponent intervention undertaken either as an individual or with a spouse, and with the calorie and exercise goals set either daily or weekly. For the purpose of this review we consider the interventions with individuals and with daily goals as the multicomponent intervention of most relevance to the scope. The individual treatment with weekly goals is treated as the comparator intervention. The details of the couples’ interventions can be seen in Appendix 5. Multicomponent interventions often involve family members; however, in this study the spouse monitored adherence and progress with the weight loss programme and was involved in the problem solving and goal-setting.
In the daily goal-setting treatment group participants were recommended to set a goal of 1215 kcal per day (females) or 1525 kcal per day (males) from week 5. Participants were encouraged to divide the daily goals into subgoals for portions of the day. No detail was given for the proportions of the diet but participants were encouraged to record their weight daily as a form of monitoring. The exercise component of this intervention also set daily goals for energy expenditure from an aerobic exercise and walking programme. Participants started at an initial 145 kcal per day (equivalent to a 1.5-mile walk or 1.5 hours of active housework) above their initial baseline of activity and this increased by 25 kcal/day if the previous week’s goals had been met on at least 4 days. In addition, participants were instructed to walk for at least 30 minutes on 5 days each week and to monitor their heart rate to ensure that they were exercising within a 70%–80% range of their age-predicted heart rate (see Appendix 5). The participants recorded daily records of activities. The behavioural modification element of the interventions was undertaken in groups (size not reported) and included lectures and small group discussions in weeks 1–4; weekly 15- to 20-minute individual sessions in weeks 5–7; and then fortnightly sessions thereafter. The setting for the behaviour therapy was not reported. Table 20 describes the range of features and strategies covered in the behavioural treatment component of the study. These appeared to centre on goal-setting, cognitive restructuring and problem-solving skills. Clinical psychology graduate students delivered the sessions. The intervention with weekly goal-setting was very similar to the daily goal-setting intervention, with a few exceptions. The participants were encouraged to set weekly calorie goals of 8500 kcal for women and 10,675 kcal for men and the exercise component was to meet a weekly exercise goal equivalent to the sum of the same number of days for those in the daily goal-setting intervention (1000 kcal in the first week) (see Appendix 5). Participants were instructed to weigh themselves only once per week.
| Cognitive restructuring | Goal-setting/action plans | Modelling | Motivation enhancing | Problem solving | Relapse prevention | Self-monitoring | Self-reinforcement | Skill development | Social assertion (SA)/social support (SS) | Stimulus control | Theoretical model | Others (n) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aDubbert and Wilson 198468 | ✓ | ✓ | ✓ | ✓ | SA | ? |
There are questions over the generalisability of the findings of this study to UK practice. Participants in this study had answered a media advertisement and paid a refundable fee to take part in the study, which may have affected the types of people taking part. The number of participants allocated to each treatment arm was not explicitly reported in the study report, and there was no report of results of any statistical significance testing of the data. The mean age of the participants in the study was not reported. Another factor that needs to be considered when interpreting results of this study is that the daily goal-setting for the diet component was likely to be more detailed than common practice. Also, the exercise component used a daily goal. The study was also undertaken some 25 years ago, before the advent of standards for reporting of RCTs, and this is reflected in the assessment of the trial quality that suggests an uncertain risk of bias (see Table 8).
Clinical effectiveness
Weight loss
Dubbert and Wilson68 reported weight change as their primary outcome. At 34 months (30 months after the end of treatment) weight was similar in the daily goal-setting group (194 lb) and the weekly goal-setting group (200 lb) (see Table 21). No statistical significance testing was reported, there was no information that the analysis was statistically powered and no data on drop-out rates within the individual study arms were provided. Mean weight change from baseline was –14.9 lb in the daily goal-setting group and –7.7 lb in the weekly goal-setting group, again with no test for statistical significance reported. Participants were likely to be class I obese (estimated from mean weight at baseline).
| Study | Treatment arms | p-value, 95% CI between groups | |
|---|---|---|---|
| Dubbert and Wilson, 198468 | Individual; daily goals (n not reported) | Individual; weekly goals (n not reported) | |
| Weight, lb (kg)b at 30 monthsa | 194 (88) | 200 (91) | Not reported |
| Weight change from baseline lb (kg)b at 30 monthsa | –14.9 (6.7) | –7.7 (3.5) | Not reported |
In the Dubbert and Wilson study68 interim data were presented for weight loss at the end of the 19-week intervention (note: the 19-week data do not meet the inclusion criteria for our review as the inclusion criteria state that studies had to report follow-up data at 18 months or longer – see Chapter 2, Inclusion criteria). Although not formally assessed, these data show that for those in the daily goal group at 19 weeks there was a loss of 20.2 lb (9.1 kg) compared with a loss of 16.6 lb (7.5 kg) in the weekly goal group. Over time it would therefore appear (when informally comparing 19-week weight change with 30-month weight change) that there was weight regain over the longer term.
Barriers and facilitators
Dubbert and Wilson68 did not report potential barriers or facilitators.
Other outcomes reported in the trials (not evaluated here)
Dubbert and Wilson68 also reported cardiovascular fitness, BP, marital satisfaction, spouse weight, spouse co-operation, body image, satisfaction, depression, aerobic fitness, and binge eating and measured attendance at sessions.
Summary of effectiveness
One multicomponent approach to weight loss that did not fit the other categories used in the present review was included. This study tested both goal-setting and spouse involvement. 68 The study suffered from considerable methodological shortcomings. Participants in the two goal-setting arms lost weight and it appeared that weight loss was greatest in those given daily goals, but there were no statistical analyses presented to support this observation.
Overall comments on clinical effectiveness of multicomponent weight management interventions
As noted in the methods section, and demonstrated in the above sections, studies using similar goals were grouped together to aid interpretation of their results. To attempt to summarise the results of these studies across the four categories would therefore be unproductive. However, in order to attempt to inform future policy as to what might be the most effective intervention we have considered all of the included studies in terms of their length of follow-up (≥ 2 years), sample size (≥ 100 participants per arm), any methodological limitations, reproducibility, generalisability and overall effect size. While not a formal analysis (therefore caution is required) this has enabled us to make suggestions as to which studies may be the most relevant to informing policy. We focused our assessment on those studies that tested against a control/usual care arm (see Appendix 8). From this, speculatively, the Stevens and colleagues70 study appears to be the most robust, although it should be stressed that the US setting (different health system and population) places a restriction on its generalisability. This study is discussed more fully in the Discussion.
Ongoing studies
The following studies, which may meet the review eligibility criteria, were identified in searches and are currently ongoing:
Ross and colleagues80 Prevention and Reduction of Obesity through Active Living (PROACTIVE). This is a Canadian RCT of 491 adults with BMI 25–39.9 kg/m2. The goal of the study is to reduce abdominal adiposity, increase physical activity, and maintain and improve diet quality. Participants are randomised to the intervention group or a usual-care control group. Trial outcomes were unpublished as of December 2009.
Stolley and colleagues81 Obesity Reduction Black Intervention Trial (ORBIT). This is a US RCT of a culturally proficient weight loss intervention compared with a general health control group in 213 obese black women. Short-term (6-month) outcomes were published in January 2009.
van Weir and colleagues82 ALIFE@work trial aims to evaluate a lifestyle intervention among a Dutch overweight working population. Participants (BMI > 25 kg/m2) (n = 1386) were randomised to either the LI via the telephone, the internet or to a control group. Trial outcomes were unpublished as of December 2009.
Williams and colleagues83 Work, Weight, and Wellness: The 3W program. This is a worksite obesity prevention and intervention trial being conducted on 11,559 employees on the island of Oahu in Hawaii, USA. Work sites are randomised to either a minimal intervention or an intensive intervention. Trial outcomes were unpublished as of December 2009.
Østbye and colleagues84,85 Active Mothers Postpartum. This is a trial being undertaken in the USA of women with a BMI > 25 kg/m2 post partum. A total of 450 women were randomly assigned to either an intervention group (healthy eating classes, physical activity classes, telephone counselling) or a control group. Short-term (12-month) outcomes were published in 2009.
Modifying Obesogenic Homes: Impact on Weight Maintenance, is an RCT which aims to deliver 18 months of SBT or 18 months of SBT plus direct modifications to the home environment (SBT + Home) to people who are BMI > 25. The study of approximately 200 participants is currently recorded as ongoing, but no longer recruiting participants and is funded by the National Heart, Lung and Blood Institute in the USA.
Internet Assisted Obesity Treatment (iReach) is an RCT comparing three weight loss interventions: internet alone, internet and periodic in-person support, and in-person support alone. A US study this is funded by the National Institute of Diabetes and Digestive and Kidney Diseases. The study aims to follow-up participants with a BMI > 25 kg/m2 at baseline for up to 18 months. This study is recorded as ongoing but no longer recruiting participants.
The Take HEED (Healthy Eating and Exercise Decisions) RCT is a US study which aims to examine the effect of a culturally-adapted weight loss programme in African American females aged 40–65 years. Take HEED is a combination of two active interventions, Therapeutic Lifestyle Changes diet and the ‘change’ exercise programme. The study is ongoing but no longer recruiting and is funded by Kaiser Permanente and the Garfield Memorial Foundation.
Maintenance-Tailored Obesity Treatment is an RCT comparing state-of-the-art, SBT for weight loss with a maintenance tailored treatment and will follow participants until 30 months. Participants are required to be BMI between 30.0 and 37.0 kg/m2 to be included in this study which is ongoing but no longer recruiting participants. The study is a US study funded by the National Institute of Diabetes and Digestive and Kidney Diseases.
Participants in an 18-month study funded by the National Heart, Lung and Blood Institute will be randomly assigned to either a standard behavioral weight loss programme or a stepped-care weight loss programme. The study is currently recruiting participants with a BMI between 25.0 and 39.9 kg/m2 to the ‘Step-up Study’ which is expected to complete in January 2012.
Another ongoing study is comparing a wellness-centred intervention with a weight-centred intervention (LEARN® programme) in people with a BMI between 30 and 45 kg/m2. The interventions are delivered over 6 months with follow-up at 18 months. The study is active but no longer recruiting participants and is funded by The Reading Hospital and Medical Center and The Edna G. Kynett Memorial Foundation in the USA.
Chapter 4 Cost-effectiveness
The aim of this section is to assess the cost-effectiveness of multicomponent adult weight management programmes compared with standard care or other weight management programmes through a systematic review of the literature. The methods used for the search strategy are described in Chapter 2, and inclusion criteria are shown in Chapter 2, Inclusion criteria. Data from the studies were extracted and evaluated for their quality and generalisability to the UK, using a critical appraisal checklist. 65 The studies are then described in more detail, together with a discussion of the key issues arising from each of the studies, and an exploratory sensitivity analysis around the programme costs presented. The full data extraction forms for both of the studies are shown in Appendix 9.
Quantity and quality of published research
A total of 419 potentially relevant references were identified in the cost-effectiveness searches. Of these, the full texts of 15 papers were retrieved but no studies met all of the a priori inclusion criteria. A summary of the selection process and the reasons for exclusion are presented in Figure 2 and a list of excluded studies in Appendix 6. Two studies86,87 met the core criteria of evaluating diet, exercise and behaviour interventions in overweight or obese populations using a lifetime economic model. However, one of these included the use of prescription antiobesity drugs in a small number of participants87 and the length of follow-up in the other study was < 18 months (12 months). In the absence of any other evidence a pragmatic decision was taken to describe these two studies in the review of cost-effectiveness, as these studies were considered relevant and useful to the research question. However, caution is required in their interpretation. Characteristics of the studies are shown in Table 22.
FIGURE 2.
Flow chart of identification of studies for inclusion in the review of cost-effectiveness. a, The abstracts provided insufficient details of methods and results to allow inclusion in the systematic review; b, a pragmatic decision was taken to describe these studies despite them not fully meeting the a priori inclusion criteria.
| Characteristics | Author | |
|---|---|---|
| Counterweight Programme study87 | Roux et al.86 | |
| Publication year | 2010 | 2006 |
| Country | UK | USA |
| Funding source | Roche Products Ltd | Not stated |
| Study type | Cost–utility analysis | Cost–utility analysis |
| Perspective | UK NHS | Societal |
| Study population | Obese adults (BMI > 30 kg/m2) or adults with BMI > 28 kg/m2 with comorbidities | Overweight and obese women (BMI > 24.9 kg/m2) free from known CHD |
| Intervention(s) | Diet, exercise and behaviour intervention vs no treatment. About 10%–20% of participants received pharmacotherapy |
Routine care Diet Diet and exercise Diet and pharmacotherapy Diet, exercise and behaviour modification |
| Intervention effect |
Mean weight change at 12 months, for those still attending, was –3.0 kg (95% CI –3.5 to –2.4) and at 24 months was –2.3 kg (95% CI –3.2 to –1.4) Mean change in BMI kg/m2 at 12 months: –1.1 (95% CI –1.3 to –0.89) |
Change in BMI kg/m2: Routine care 0.26 Diet –1.98 Diet and exercise –2.55 Diet and pharmacotherapy –4.55 Diet, exercise and behaviour modification –3.11 |
| Intervention cost | £60 per participant | Direct medical costs per participant were US$3040 for diet, exercise and behavioural modification |
| Currency base | UK£ (2001–3) | US$ (2001) |
| Model type, health states | Patient level simulation model, with health states for CHD, diabetes and colon cancer | Monte Carlo simulation with health states for CHD |
| Time horizon | Lifetime | Lifetime |
| Baseline cohort | UK adult population | Hypothetical cohort of 10,000 healthy, non-pregnant 35-year-old overweight and obese women with original BMI > 24.9 kg/m2 and free from known CHD |
| Base case results | For no active intervention, lifetime quality-adjusted life-years (QALYs) are 28.32, for baseline intervention lifetime QALYs are 28.38. The lifetime QALY gain is 0.056 with a cost saving of £27. The incremental cost-effectiveness ratio (ICER) is –£473 per QALY gained (cost saving) |
Diet, exercise and behaviour modification was the dominant strategy The lifetime QALY gain is 0.24 compared with routine care at an extra cost of US$3080. The ICER is US$60,390 (£36,000) per life-year gained (LYG) and US$12,640 (£7600) per QALY when compared with routine care |
Critical appraisal of economic evaluations
The cost-effectiveness studies were assessed against a critical appraisal checklist (Table 23). This checklist assessed the quality of the studies and their generalisability to the UK and was adapted by the review authors from checklists by Philips and colleagues,65 Drummond and colleagues66 and the NICE reference case requirements. 67 More details of the studies are given in Chapter 4, Description and results of the published economic evaluations.
| Item | Counterweight Programme study 201087 | Roux et al. 200686 | |
|---|---|---|---|
| 1 | Is there a clear statement of the decision problem? | Y | Y |
| 2 | Is the comparator routinely used in UK NHS? | Y | Y |
| 3 | Is the participant group in the study similar to those of interest in UK NHS? | Y | Y |
| 4 | Is the health care system or setting comparable to UK? | Y | ? |
| 5 | Is the perspective of the model clearly stated? | Y | Y |
| 6 | Is the study type and modelling methodology reasonable? | Y | Y |
| 7 | Is the model structure described and does it reflect the disease process? | Y | Y |
| 8 | Are assumptions about model structure listed and justified? | Y | Y |
| 9 | Are the data inputs for the model described and justified? | ? | ? |
| 10 | Is the effectiveness of the intervention established based on a systematic review? | N | ? |
| 11 | Are health benefits measured in QALYs? | Y | Y |
| 12 | Are health benefits measured using a standardised and validated generic instrument? | Y | ? |
| 13 | Are the resource costs described and justified? | Y | ? |
| 14 | Have the costs and outcomes been discounted? | Y | Y |
| 15 | Has uncertainty been assessed? | ? | Y |
| 16 | Has the model been validated? | N | ? |
Both economic evaluations involved complex models. Although descriptions of the model structures are given and these reflect the natural history and disease progression associated with obesity, omissions in reporting details of the modelling methodology and data inputs reduce transparency and make it difficult to draw conclusions about the results.
Neither study described the transition probabilities in sufficient detail although, some parameters are listed and sources for values given. In one study87 the effectiveness of the intervention was not based on a systematic review of the literature. In the other study86 a ‘comprehensive’ review was undertaken but no details are provided on the studies identified and then selected as evidence of effectiveness. In spite of these limitations the effectiveness data used in the models seem consistent with the effect size in the studies in our systematic review of clinical effectiveness (see Chapter 3, Quantity and quality of research available).
The quality of the methodology used for estimating quality-adjusted life-years (QALYs) was mixed. One study86 used utilities that were rescaled from data from a longitudinal study; but it was not reported how this was done. The other study87 used appropriate methodology to derive utility estimates stratified by BMI, age and gender.
Methods for estimating costs were also variable across the studies. The Counterweight Programme study,87 while describing and justifying the resource costs, appears to have miscalculations and may not include all relevant costs needed for an accurate estimation of total programme costs. The other study86 reports cost per participant in the weight loss programme, but details of all the elements that contribute to this are not provided so it is difficult to comment on costs. Both studies applied discounting to costs and benefits.
One study assessed uncertainty through sensitivity analyses86 while the other study only conducted five alternative scenario analyses. 87 Finally, no details have been given on validation of the models in either studies.
In summary, the cost-effectiveness studies lack detail on some aspects of methodology so it was not possible to assess individual components and interpretation of the results was difficult. However, the studies appear credible and the results are consistent between them. The Counterweight Programme study87 is of most relevance to the UK as the health-care system in the study was the NHS and the study was conducted from the perspective of the UK NHS.
Description and results of the published economic evaluations
The Counterweight Programme
The Counterweight Programme is a weight management programme in primary care in the UK. 87 An economic evaluation was undertaken using an existing model developed for the NICE clinical guidelines on obesity. 35 The evaluation examines the impact of weight loss interventions on health outcomes and costs. The model incorporates the relationship between weight gain and the increased risk of developing a range of associated conditions. The analysis compared the costs and outcomes of the Counterweight Programme with no active intervention. The cost per patient of the Counterweight Programme was derived from an analysis of the budgetary impact of implementing the programme. 54 The analysis was conducted from the perspective of the UK NHS.
Modelling approach
A patient-level simulation model was used which had previously been developed to provide input to the UK national guidance on obesity. 35 The model works by randomly selecting an individual whose characteristics are based on those of the UK adult population (for example, BMI, age and gender) and following their health-care costs and outcomes until death. The model assesses people over 6-month cycles. During each cycle, individuals may experience a change in BMI (increase or decrease) or no change. Based on their characteristics, individuals can develop diabetes, CHD, or colon cancer depending on the prevalence of each disease at the BMI they are currently experiencing. There is a utility score associated with each health state. Patients are at increased risk of death if they experience one of these conditions. To determine whether the individual is experiencing each particular health state at any one time, the incidence of the chronic health states was calculated, based upon the prevalence of the condition for that individual’s characteristics. The model was run for a cohort of 10,000 people.
Assumptions
In the base-case analysis, patients are assumed to regain all the weight lost in 2 years following removal of the programme while those obese adults not on the programme are assumed to gain a background rate of 1 kg per year (Figure 3). 87 Those patients who were not attending at 12 months were assumed to gain weight at the same rate as those not on the programme.
FIGURE 3.
Assumptions of weight change for the economic evaluation of the Counterweight Programme.
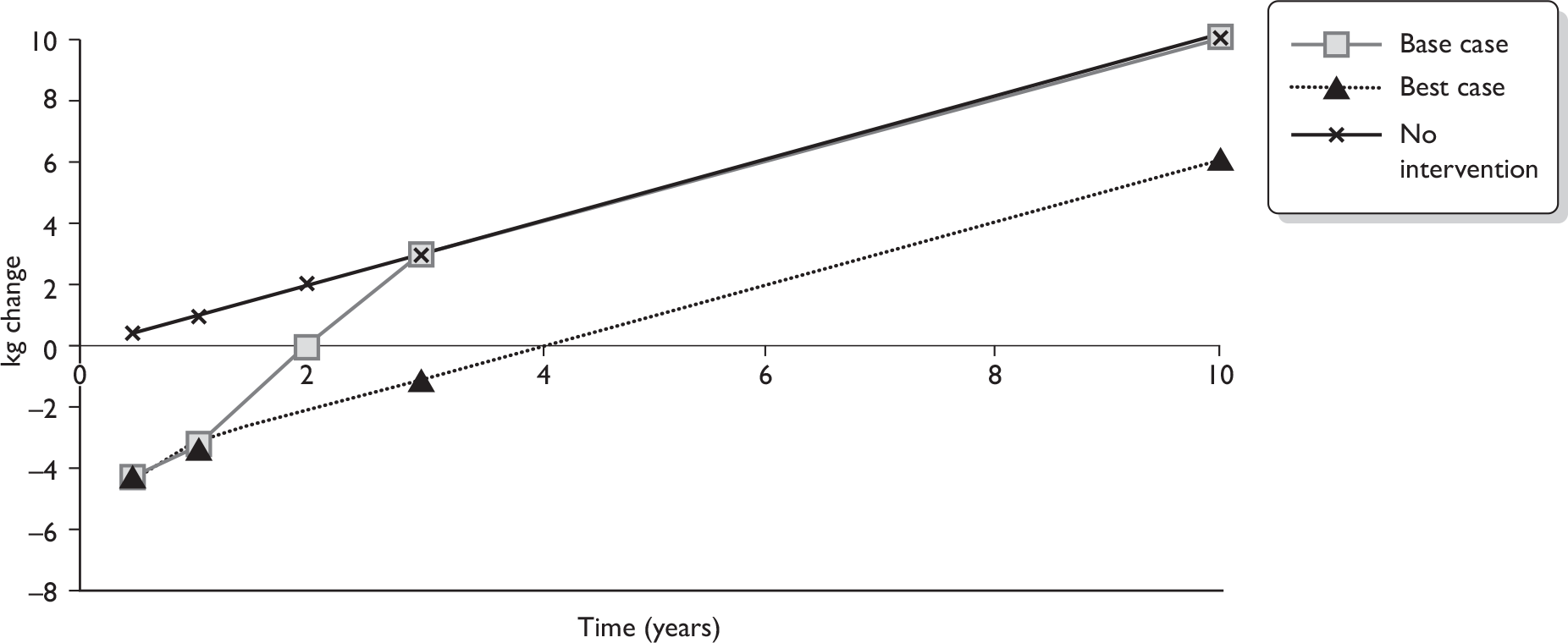
Effectiveness of intervention
The estimates of the effectiveness of the Counterweight Programme were based on a prospective evaluation of 1906 obese adults in 65 UK general practices. 54 These patients had a mean age of 49.4 years, a mean BMI of 37.1 kg/m2 and were mostly female (77%). All patients received the intervention, as the study was not a controlled trial, and the results are compared with weight gain in observational studies of obese adults. The intervention consisted of diet, exercise and behaviour modification components. First-line interventions were a prescribed eating plan, using a goal-setting approach, or a group intervention. These were all aimed at achieving an energy deficit of more than 500 kcal/day. Patients were asked to commit to nine appointments in 12 months, including six individual appointments (10–30 minutes) or six group sessions (1 hour each) over a 3-month period and then follow-up appointments at 6, 9, 12 and 24 months. Mean weight change at 12 months (n = 642) was –3.0 kg (95% CI –3.5 to –2.4 kg) and at 24 months (n = 357) was –2.3 kg (95% CI –3.2 to –1.4 kg) compared with original weight. Mean change in BMI was –1.1 kg/m2 (95% CI –1.3 to –0.9 kg/m2) at 12 months. Untreated patients are assumed to gain 1 kg weight per year. There were a large number of dropouts from the programme. Of 1419 attendees, 54% of patients (761) provided data at or beyond 12 months. These dropouts are likely to have incurred additional costs to the programme.
Caution is required in the interpretation of the evidence of effectiveness from the Counterweight Programme study. The effect of the intervention is not based on evidence from an RCT or a study with a concurrent control group and as such there is a higher risk of bias than there would be with a study using a more rigorous design.
Estimation of QALYs
The Counterweight Programme economic evaluation uses QoL utility values stratified for BMI levels, age and gender based on a discussion paper by Macran88 which estimated HRQoL based on European Quality of Life-5 Dimensions (EQ-5D) scores in the HSE, 1996 (Table 24). The utility values were for the general population that included individuals with CHD, diabetes and cancer. These values were adjusted to derive utility values for the general population without these chronic health states for different BMI scores. For a person with a relevant comorbidity, multipliers were applied to these utility scores to obtain their individual utility estimate. The multipliers for diabetes and CHD (0.8661 and 0.867, respectively) were from a manufacturer submission to NICE for the cost-effectiveness of sibutramine (Meridia®, Abbott Laboratories), published in NICE guidance for obesity. 89 Individuals with colon cancer had their utility score reduced by 5%. The utility values for the different health states do not appear to have been derived from a systematic search.
| BMI (kg/m2) | Male | Female |
|---|---|---|
| < 21 | 0.86 | 0.85 |
| 21–25 | 0.87 | 0.87 |
| 26–30 | 0.86 | 0.82 |
| 31–39 | 0.82 | 0.78 |
| > 39 | 0.88 | 0.75 |
Estimation of costs
The cost of the Counterweight Programme was based on participants from the evaluation of the weight management programme and scaled up to the national population. The costs used in the economic evaluation were the actual costs observed and recorded, and they were limited to the services actually provided. Costs included remuneration for all clinicians’ time required for the intervention – training, GPs’ time for clinical and motivational assessment, practice nurse time for assessment, motivation, delivery of advice and review. Costs for the Counterweight Programme project team, training resources and patient information materials were also included in the analysis. Costs for the intervention were based on the optimal attendance rate of at least six visits in 12 months. In addition, the costs of weight management medications prescribed according to protocol were incorporated. Around 20% of patients followed up at 12 months or around 9% of the total intervention group were prescribed weight management drugs. The scaled-up national costs, assuming that the programme was available in all practices in the UK and would be taken up by 20% of eligible obese patients, included the following: (i) initial staff recruitment and training (£2M); (ii) weight management advisor team and support staff (£8.5M/year); and (iii) resources and practice staff time for meetings, GP discussion, intervention, equipment, medication, exercise referral and secondary care referral (£16M/year). After the initial year, each cohort was assumed to require 50% of year 1 costs. The total 5-year UK costs were about £200M for 3.3 million patients, which equates to about £60 per patient.
This is a very low intervention cost. Looking at the costs in more detail, they appear to have been calculated incorrectly. The cost for the intervention is £5707 per one GP practice for 92 patients for the first year. The authors assume the follow-up for the second year is one-half of the rate in the first year (e.g. three individual contacts instead of six). Management costs for the scheme are about £15.25 per person. Thus the cost per patient would be about £108.30. This is still a low estimate for the programme, as there does not appear to be any time allocated for administration by the practice nurse or the GP and the time in practice to run the programme may be considerably more than allocated. In the study, patients attended GP appointments for other clinical matters and as part of this the GP suggested practice nurse appointments to discuss weight management. In this way there is no cost allocated for this GP appointment. There is also no time allocated to specialist advisors such as dietitians, cognitive behavioural therapy counsellors or health trainers, except as part of the initial nurse training. There are also no costs included for the fitness programme, such as leisure centre costs. We suggest that the NHS or other government departments pay for part or all of exercise referral schemes. We suggest that the resource costs are more likely to be similar to those used by a recent NICE report for bariatric surgery. 33 This suggested that for a diet-only programme there would be four contacts with a GP per year and two additional contacts per year with a community dietitian, practice nurse and district nurse at a total cost of £282 per year.
As mentioned above, the practice nurse is expected to run almost the entire Counterweight Programme. The practice nurse only receives one full day of training before the start of the course but is expected to gain extensive competencies, in order to run diet, exercise and behavioural components. This training was provided by registered dietitians, who also provided subsequent peer support for about 6 months. It is likely to be the case that GP practices require more than one practice nurse to run the scheme and that they need more support from specialist health professionals and hence the cost incurred will be substantially higher.
The costs of the chronic conditions were based upon previous studies and converted to 2005 prices. Yearly costs included medical conditions such as CHD (£1637), diabetes (£653) and colon cancer (£7320).
Follow-up data were available for only 642 of 1419 participants at 12 months, with the remainder not reaching these data collection points. The costs and effect size have been estimated for these 642 participants. It is unclear what the costs and effect size are for these dropouts, and the weight loss may be lower for these individuals while they will still have incurred the intervention cost.
Cost-effectiveness results
The model considers the impact of the 12-month observed outcomes of the Counterweight Programme. For the non-active intervention, lifetime QALYs are 28.32. For the baseline intervention, lifetime QALYs are 28.38, i.e. a QALY gain of 0.056 for the intervention. The lifetime cost to the NHS of the non-active intervention is £1884 and for the baseline intervention is £1857. The incremental cost-effectiveness ratio (ICER) is –£473 per QALY gained, i.e. it is cost saving. No sensitivity analyses were conducted for structural, methodological or parameter uncertainty. Scenario analyses were conducted for background weight change for the untreated population. For background weight change of 0.5 kg/year and 0.3 kg/year the ICER was £2017 and £2651, respectively.
Summary of key issues
-
The study has evaluated a diet, exercise and behavioural programme for obese people in the UK, using the actual costs observed in the Counterweight Programme.
-
There appear to be some miscalculations in the intervention costs used and the costs recorded are lower than reported in other studies.
-
The intervention effects are based upon a single observational study, rather than a controlled trial or systematic review.
-
There are high dropout rates in this study that will have an added cost.
-
The methodology of the model and the derivation of the QoL utility values seem reasonable.
Roux and colleagues86 conducted an economic evaluation of outpatient weight loss strategies in overweight and obese women in North America. Each weight loss intervention consisted of a 6-month intervention followed by a 6-month maintenance programme. The strategies were: routine care; diet only; diet and exercise; diet and pharmacotherapy; and diet, exercise and behavioural modification. In all strategies women who successfully completed the 6-month weight loss intervention were modelled to enter the 6-month maintenance phase. Women who were unable to lose weight or maintain weight loss were assumed to remain at their age-adjusted original BMI. The base case simulated the natural history of a hypothetical cohort of healthy, non-pregnant 35-year-old overweight and obese women with original BMI > 25 kg/m2 and free from known CHD. The model was used to project lifetime costs and gains in life-years and QALYs, and the analysis was from a societal perspective. Costs were in US$ and the price year was 2001. The study used benefits from published RCTs and costs were derived from published data, Medicare reimbursement rates and primary data for indirect costs.
Modelling approach
A first-order Monte Carlo simulation model was developed to simulate the natural history of a hypothetical cohort of otherwise healthy, non-pregnant 35-year-old overweight and obese women. The model uses a state-transition framework, where the natural history of obesity in the hypothetical cohort is characterised as a sequence of annual transitions from one health state to another. Women enter the model aged 35 years, free from known CHD, and their BMI is randomly chosen from a uniform distribution of BMIs > 24.9 kg/m2. Each year a woman’s BMI is adjusted for age-related increases and predicts the risk of developing hypertension, Type 2 diabetes or hypercholesterolaemia that in turn predicts her risk of CHD and CHD death. Each woman’s clinical course was tracked until death, when summary statistics such as quality-adjusted survival and total lifetime costs were recorded. The sample size was 10,000 women, for whom average life expectancy, QALYs and costs were calculated. Significant intervention-attributable weight loss was defined as a 10% BMI reduction after completion of the 6-month weight loss intervention. Short-term success was defined as maintenance of a reduced BMI post intervention for at least 6 months. Long-term success was defined as maintenance of a reduced BMI for at least 5 years after the weight loss intervention.
Assumptions
It was assumed that the probability of long-term weight maintenance was 20% with lifestyle modification programmes and 10% without lifestyle modifications based on published literature. Women who successfully maintained their weight loss for at least 5 years were assumed to remain at their postintervention BMI for the remainder of their lifetime. Figure 4 shows the assumptions used in the model for weight change for the intervention versus routine care (no intervention), although the study did not clearly describe how weight changed over time in the routine care group. Age- and BMI-specific obesity-related disease complications were assumed to be lifelong. It was assumed that a woman who achieves only short-term success accrues the clinical benefit associated with a lower BMI until she regains weight. Women who do not lose weight or who lose weight but do not maintain the weight loss are subject to age-adjusted cardiovascular disease risks based on their original BMI.
FIGURE 4.
Assumptions of weight change for the economic evaluation by Roux and colleagues86 Weight changederived by assuming average height in cohort of women of 1.63m and those who do not maintain weight loss return tooriginal weight.
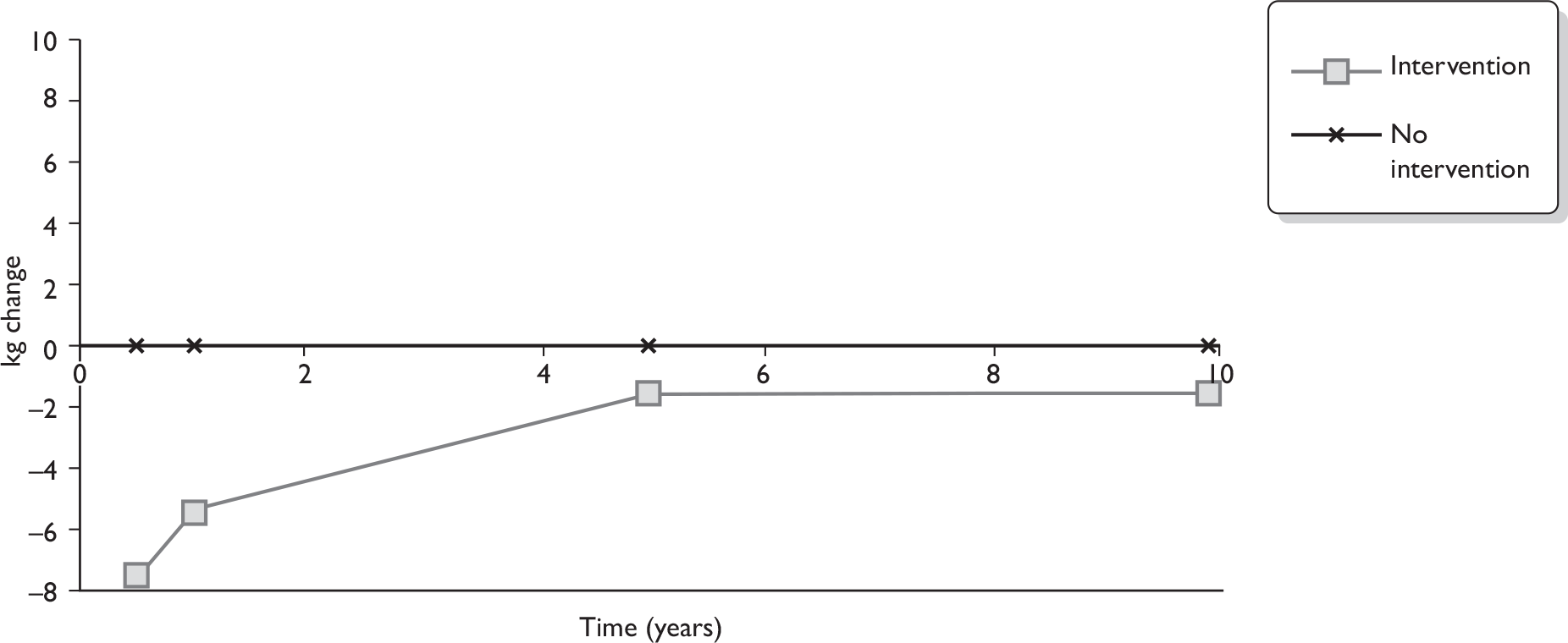
Effectiveness of intervention
The dietary component in all modelled strategies, except routine care, was defined as the reduction in caloric intake necessary to achieve a 10% weight loss under the supervision of a dietitian, in accordance with the American Heart Association guidelines. For strategies incorporating exercise, the exercise protocol consisted of three 45-minute structured exercise sessions per week of moderate intensity, led by a certified instructor, and two sessions per month to review clinical progress with an exercise therapist. The strategy that incorporated pharmacotherapy was modelled to consist of 120 mg orlistat three times a day for 6 months, then half this dose per day during the 6-month maintenance phase. The behavioural modification strategy was modelled to consist of a 1-hour cognitive therapy counselling session led by a psychologist every other week.
The estimates of the effectiveness of the weight loss interventions were based on a ‘comprehensive review’ of the published literature from which four studies were selected on the basis of their methodological quality. However, no details are given and the study references are reported to be in an online appendix that is not available. The probability of weight loss for each strategy was estimated using bootstrapping methods by sampling random numbers to predict the change in BMI for 1000 representative women. Thus, the size of the treatment effect used in the evaluation was a change in BMI post intervention of 0.26 kg/m2 for routine care, –1.98 kg/m2 for diet only, –2.55 kg/m2 for diet and exercise, –4.55 kg/m2 for diet and pharmacotherapy, and –3.11 kg/m2 for diet, exercise and behavioural modification. The estimates of weight loss for the diet, exercise and behavioural modification strategy are higher than seen in our systematic review (see Chapter 3, Quantity and quality of research available).
Estimation of QALYs
General age-specific quality weights were derived from the Beaver Dam Health Outcomes Study90 (referenced but no details given). These were applied to women more than 45 years of age and adjusted to reflect weight loss and comorbid diseases using a multiplicative function. The quality weights for weight loss were obtained using primary data from a survey of 100 weight management programme participants. The average per cent reduction in life expectancy that participants were willing to give up to achieve weight loss was elicited, through a hypothetical treatment (a single pill, free of charge and side effects but which would not prevent or cure health problems or incur survival benefit). This was scaled to give utility scores for a sustained BMI reduction to the average weight for their height (0.87) and for a 10% reduction in BMI (0.93). A disease-specific quality weight of 0.75 (age-adjusted) was used for CHD and Type 2 diabetes.
Temporary decrements in utility attributable to the interventions were found from primary data analysis to be related to the intensity of effort required to participate in a particular programme. For routine care that required minimal effort, there was no utility decrement, whereas for the diet, exercise and behavioural modification programme there was a decrement, of 0.09 for a 6-month period. Other programmes of intermediate intensity were assigned decrement values between 0 and 0.09. Thus, for this 6-month period, the utility decrement cancelled out any gain in utility for a reduction in BMI. The derivation of this utility decrement has not been described and it is unclear why such a short period has been chosen.
Estimation of costs
Micro costing techniques were used to estimate resource use associated with each weight loss intervention. Direct medical costs were based on published data and valued using average Medicare reimbursement rates. Drug costs were obtained from the 2001 pharmaceutical pricing index, based on average wholesale prices. Direct medical costs associated with obesity-related morbidity and mortality included annual age- and sex-specific treatment-related costs for hypertension, Type 2 diabetes and cardiovascular disease. The source of costs was reported but individual elements were not noted and so their unit costs and the quantities used were not reported. The costs were reported as the average cost per patient. Direct medical costs per patient were US$700 for routine care, US$2150 for diet only, US$2750 for diet and exercise, US$2820 for diet and pharmacotherapy and US$3040 for diet, exercise and behavioural modification. The programme costs appear to be based upon those from a weight management programme at the Brigham and Women’s Hospital in Boston, MA, USA, but the numbers of consultations and other resources used for the programme have not been described. However, the strategies appear to include consultations with a physician, nutritionist, exercise therapist and psychologist and included other investigations such as laboratory tests, X-ray, electrocardiogram and exercise stress test.
Direct non-medical costs (e.g. fitness attire, travel costs, diet-related costs) were estimated using primary self-reported cost and quantity data derived from the community sample of 100 participants. The study was from a societal perspective. As such, productivity costs are included, which are not included in studies taking an NHS perspective. Time–cost was estimated directly from the sample of 100 women by applying wage rates specific to their occupation. The analysis was repeated using 2001 US national-level average wage rate data to value time lost from work or leisure activity. Time lost from performing household duties was valued using wage rates for domestic child care and light duty cleaning services. The costs and quantities were not reported separately.
Cost-effectiveness results
The most cost-effective and efficient strategy was the three component intervention of diet, exercise and behavioural modification with a discounted quality-adjusted life expectancy of 18.43 years and discounted lifetime costs of US$124,200. The lifetime QALY gain is 0.24 compared with routine care, at an extra cost of $3080. This had a cost per QALY gained of US$12,640 compared with routine care or a cost per life-year saved of US$60,390. The diet-only strategy was less effective and more costly than routine care (strongly dominated), the diet and pharmacotherapy and diet and exercise strategies were less effective and less costly and had a higher ICER versus routine care (weakly dominated) than with the diet, exercise and behaviour modification strategy.
Sensitivity analyses showed that the results were most influenced by the QoL utility values for obesity and the probabilities of weight loss maintenance. No probabilistic sensitivity analyses were included.
Summary of key issues
-
It is unclear how generalisable the model parameters and results are to the UK as the study is conducted in North America and from a societal perspective. In particular the health-care costs are much higher than the UK, non-medical costs are included, and it is unclear what the results would be if the study were adapted for the UK.
-
The study was conducted for overweight and obese women.
-
Adequate details are provided on the model structure and assumptions and the methodology used and results obtained seem credible.
-
There are some methodological limitations and uncertainties such as no details about the source of clinical effectiveness data, an intervention effect which may be optimistic in terms of change in BMI, extrapolation of long-term maintenance of weight loss based on authors’ assumptions and use of aggregated costs.
Comparison of cost-effectiveness analyses
The intervention effect varied between the evaluations. The mean weight change was –3.0 kg at 12 months (–1.1 BMI kg/m2 at 12 months) and –2.3 kg at 24 months for the Counterweight Programme. 87 Roux and colleagues86 reported weight change of –3.11 kg/m2 change after 6 months intervention and thereafter the probability of maintaining a 10% weight loss was 67% at 1 year and 20% at 5 years. The weight loss from the Counterweight Programme87 seems consistent with that seen in the trials in our clinical effectiveness systematic review (see Chapter 3), while the weight loss from Roux and colleagues86 appears optimistic.
There was a range of assumptions used to extrapolate weight change beyond the follow-up period of the intervention. These are shown graphically in Figure 5. Roux and colleagues86 assumed that after 5 years, 20% of women on the diet, exercise and behaviour programme would maintain weight loss and that they would remain at their postintervention weight or maintain their weight loss post intervention for the remainder of their lifetime. In the base case, the Counterweight Programme87 assumed that those on the programme return to the same weight as the control population within 2 years of finishing the programme. In a ‘best-case’ scenario, they assumed that individuals remain at a lower weight than the control population lifelong. From the evidence presented in our systematic review of clinical effectiveness, we suggest that the control group are likely to gain weight but at less than 1 kg/year, perhaps in the region of 0.3–0.5 kg/year. Individuals finishing the weight loss programme would be likely to regain weight within 2–4 years after the end of the intervention, and thereafter gain weight at a similar rate to the control group. Figure 5 shows the weight loss of the intervention group compared with the control groups in the two economic analyses and one of the studies from the systematic review of clinical effectiveness that seemed the most robust (Stevens and colleagues70). It is likely that although people may regain lost weight eventually, because the population gains weight throughout life, people may still be at a lower weight than those who never received a weight loss intervention. Compared with the Stevens and colleagues study,70 the assumptions used to extrapolate beyond the follow-up period of the intervention seem reasonable for Roux and colleagues,86 but for the Counterweight Programme study87 seem pessimistic, as they do not assume any long-term difference between the intervention and control groups. On the other hand, in the best-case scenario of the Counterweight Programme87 the long-term weight difference between the intervention and control group seems optimistic.
FIGURE 5.
Assumptions of weight loss difference between the intervention group and control group for the economic evaluations. Weight change derived for Roux and colleagues86 by assuming average height in cohort of women of 1.63 metres and those who do not maintain weight loss return to original weight.
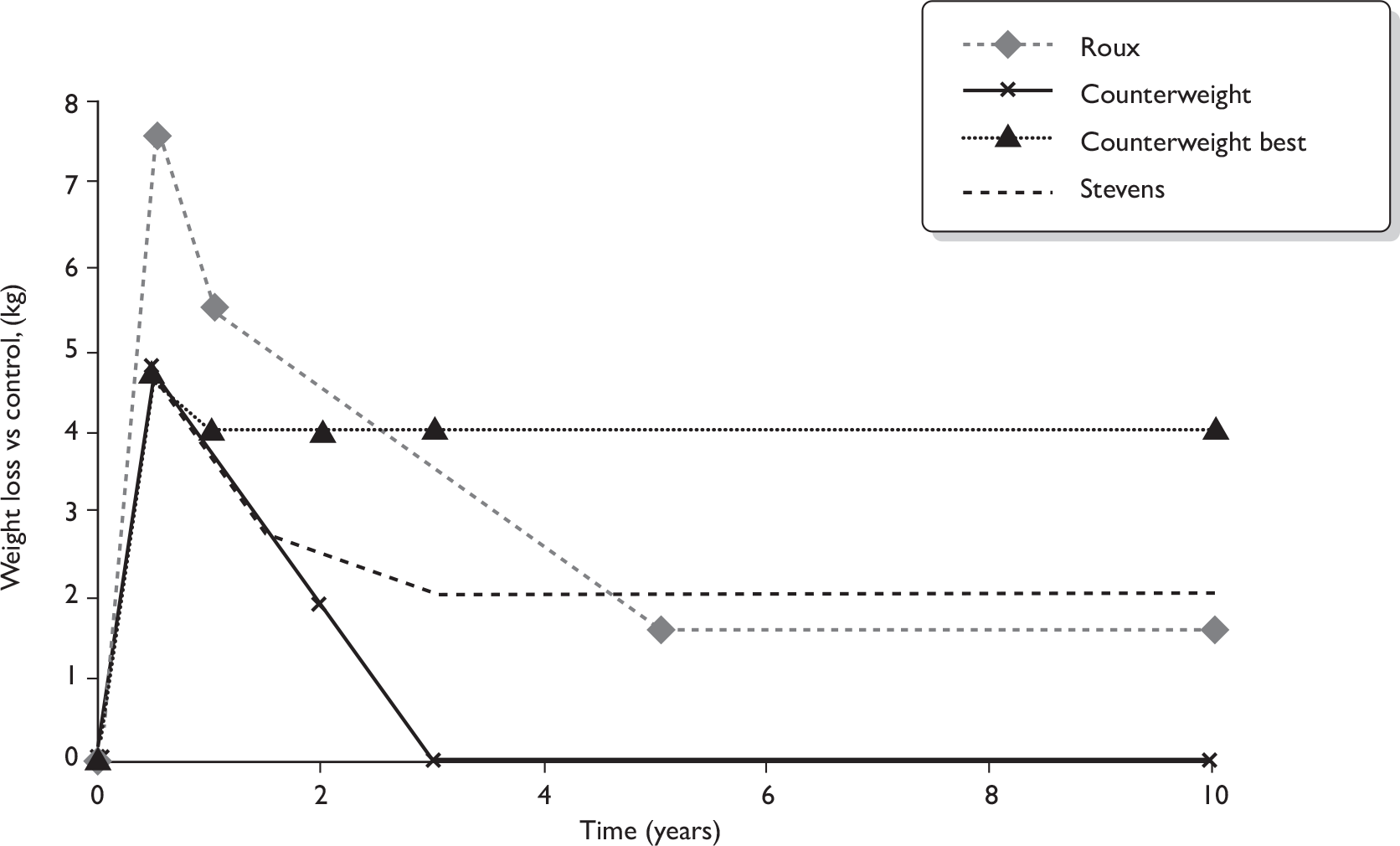
The gain in QALY compared with routine care varies between 0.06 and 0.243 for the two studies. These differences are mainly due to the differences in the assumptions for long-term weight loss maintenance and the utility values chosen for weight change. Neither study provided a clear explanation of the derivation of HRQoL values or was based upon a review of QoL literature. The studies adopt very different estimates for the effect on QoL of a reduction in weight. Roux and colleagues86 use a HRQoL value of 0.93 for individuals with obesity and 0.87 for those who achieved a 10% weight loss, i.e. a change in BMI of roughly 3 kg/m2. The Counterweight Programme study87 used values shown in Table 24, for example females have a utility of 0.82 and 0.78 for BMI between 26 and 30 and between 31 and 39 kg/m2, respectively. The change in utility values associated with a unit change in BMI varied between about 0.006 for the Counterweight Programme87 and 0.02 for Roux and colleagues. 86
The cost of the intervention varies between around £60 per patient in the Counterweight Programme study87 and US$3040 (£1820) for the diet, exercise and behaviour programme in the Roux and colleagues study. 86 Roux and colleagues86 itemised the costs included in their programme and costs are included that would not be included in UK economic evaluations, for example non-medical and time per patient costs. There is also a large difference between the costs due to the personnel used to deliver the programmes. For example, Roux and colleagues86 state that the medical cost of the programme includes consultation costs for physician, nutritionist, exercise therapist and psychologist while the Counterweight Programme used six 20-minute consultations with a practice nurse to cover diet, exercise and behavioural components (total cost £43). Furthermore, the Roux and colleagues86 programme included other investigations such as laboratory tests, X-ray, electrocardiogram and exercise stress test which were not included in the Counterweight Programme. 87
Sensitivity analysis
The Counterweight Programme study did not include sensitivity analyses and Roux and colleagues86 did not include a sensitivity analysis relevant to the likely UK programme costs. We conducted a simple sensitivity analysis using both of the studies to show the effect of changes to the results using different programme costs. This is an exploratory analysis based on the published estimates.
Based on a previous review of the literature,35 we estimate that the cost of diet, exercise and behavioural modification programme may be in the range £500–1000 per participant. Using a programme cost of £750 per participant, the ICER of the Counterweight Programme study87 changes from –£473 to £11,600 per QALY gained and the ICER for the study by Roux and colleagues86 changes from £7600 to £3200 per QALY gained. These exploratory analyses demonstrate that the results are sensitive to the programme costs and that the diet, exercise and behavioural modification programme remains cost-effective, at commonly used thresholds of £20,000–30,000 per QALY gained, for both models if the cost of the intervention is £750 per participant.
Summary of cost-effectiveness studies
-
Two cost-effectiveness studies were described in our review of economic evaluations.
-
Each study used a lifetime chronic disease model to evaluate the effect of changes in individuals’ weight. The models included the costs and benefits from avoiding chronic illnesses such as CHD and diabetes.
-
Omissions in reporting details of the modelling methodology and data inputs reduced transparency and make it difficult to draw conclusions about the results; however, the results and methodology of the studies seemed reasonable.
-
The Counterweight Programme study87 is of most relevance to the UK as the health-care system in the study was the NHS and the study was conducted from the perspective of the UK NHS.
-
There were limitations to each study. For the Roux and colleagues North American study,86 the costs were much higher than for the UK NHS perspective as non-medical costs have been included. For the UK study,87 the intervention effect is not based upon an RCT, and there is a likely underestimate of the actual costs for the intervention. Neither study has fully explored the uncertainty around the results.
-
Both studies found the interventions to be cost-effective, with estimates varying between –£473 and £7200 ($12,640) per QALY gained.
-
One study compared86 a diet, exercise and behavioural modification strategy with strategies for routine care, diet only, diet and exercise, and diet and pharmacotherapy, and found diet, exercise and behavioural modification to be the most effective and efficient strategy.
-
Sensitivity analyses conducted in one of the studies86 showed that the results were most influenced by the probability of weight loss maintenance and utility values for obesity.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
Twelve RCTs68–79 that compared multicomponent interventions with another weight loss intervention or control and which reported results on weight loss in their overweight or obese populations were included. In order to inform practice we attempted to minimise potential differences between studies given the complexity of these types of interventions. A prerequisite for including trials in this review was a minimum length of follow-up of 18 months. This was considered important to enable an assessment to be made of the impact of interventions on sustained weight loss, as weight regain is a known problem associated with weight loss treatment. Another requirement was that studies included in the systematic review needed to have reported their interventions in sufficient detail for them to be reproducible and hence useful to practice. This criterion was also included to allow identification of which, if any, aspects of the components appeared to relate to differences in outcome. However, in practice there were few similarities between the included studies; consequently, the number of meaningful conclusions that can be drawn from the range of studies included is limited (discussed in more detail below).
Five RCTs70,72–75 compared multicomponent interventions with non-active comparator groups. In general, weight loss did appear to be greater in the intervention groups than in the comparator groups, although weight losses were relatively small and their clinical meaningfulness is unclear. Where studies measured outcomes at a time point after the active intervention phase, it was seen that the intervention groups began to regain the weight they had lost. Despite this a statistically significant difference in weight loss in favour of the intervention group was maintained in two studies up to 36 months of follow-up.
Two RCTs69,71 compared multicomponent interventions that we classed as having the diet component as their focus. Participants in both studies lost weight, but there were no statistically significant differences in weight loss between study groups. After completing the intervention participants from both studies regained weight over time.
Four RCTs76–79 compared multicomponent interventions in which the focus was on the physical activity component. In one study77 participants assigned to the HPA intervention lost more weight at 18 months than those assigned to a SBT intervention, which also included physical activity but with a lower caloric expenditure goal. By 30 months the difference was not statistically significant (the trend remained however). In another study76 weight loss was greater in those in a SBT group than in those in a SBTE group. In the third study79 weight loss was similar between those allocated to a diet and physical activity combined group and those allocated to a diet alone or exercise alone group. In these physical activity focused studies any weight lost was generally small. Data were presented for a subgroup of participants only in the fourth study,78 limiting its value to this review.
One RCT68 compared multicomponent weight loss interventions but the study focus was not on diet or exercise but on other variables. It appeared that weight loss was greatest in participants given daily dietary and exercise goals compared with those given weekly goals. However, no statistical analyses were presented to support this observation. This study suffered from additional methodological limitations.
No studies were identified which used OTC weight loss medications.
In general, weight changes across the included studies were small. The degree of weight loss achieved, however, may be meaningful depending on what threshold is considered a marker of success and from whose perspective it is taken. People who are overweight or obese, and the health professionals involved in their care, may differ in the degree of weight loss they see as ‘significant’. There does not appear to be a consensus as to what would constitute a clinically meaningful weight loss. It is difficult to establish clinical significance because trends in the weight of the general population, the starting weight of individual participants, and the time over which the weight loss is measured would all need to be taken into account. In the studies we included there was a range in the starting weight and BMI of participants (discussed within each section). This might have led to variation in: the ease with which participants lost weight; their perceptions of the importance of losing weight; and their perceptions of meaningful weight loss. If we assume that a 5-kg threshold for the degree of weight loss is meaningful then participants in six68,69,71,73,76,77 of the 12 studies would be considered to have received clinical benefit from their weight loss. An alternative assumption could be that for participants to benefit meaningfully from an intervention any weight loss at the end of the intervention followed by longer-term weight stability would be acceptable. Based on this criterion none of the five studies that presented weight change results at more than one time point would be considered to have demonstrated acceptable weight loss. As there is a natural tendency for weight gain over time in the general population, a further, alternative, assumption could be that no weight regain beyond baseline may be of importance. In this instance all four included studies presenting outcomes over more than one time period would be considered to have shown clinical benefit. 70,73,75,77
Cost-effectiveness
Two cost-effectiveness studies that used lifetime chronic disease models to evaluate the diet, exercise and behavioural interventions for overweight and obese people were described. 86,87 The models included the costs and benefits, in terms of HRQoL, from avoiding chronic illnesses such as CHD and diabetes. One study was conducted from the perspective of the UK NHS, the Counterweight Programme study,87 and the other was conducted for a societal perspective for a North America health setting. 86 There were limitations to both studies and there were omissions in reporting details of the modelling methodology and data inputs which made it more difficult to draw conclusions about the results. The UK study was not based upon an RCT or a systematic review, and so caution is advised in the interpretation of the effectiveness of the intervention. In addition, the costs of the intervention appeared to be underestimated. The Roux and colleagues86 North American study was conducted for overweight and obese women and the intervention costs were much higher than would be expected in the UK. Furthermore, non-medical costs, such as patient time, have been included. Despite the limitations of the studies, the results and methodology of the studies seemed reasonable. Both studies found the interventions to be cost-effective compared with a commonly used threshold of £20,000–30,000 per QALY gained, with estimates varying between –£473 and £7200 ($12,640) per QALY gained.
General discussion
Even though studies in the review of clinical effectiveness were grouped to try to keep the most similar studies together, the studies were still rarely comparable. Differences in the types and durations of interventions, and any subsequent weight maintenance strategies, the length of follow-up, issues around generalisability to the UK and the risk of bias of the studies mean that it is difficult to draw robust conclusions as to the effectiveness of multicomponent weight management programmes. It is also difficult to establish what the core components of such programmes may need to be to maximise and sustain weight loss. With such complex interventions it is difficult to establish with any precision what the ‘active ingredient(s)’ causing any demonstrated effect is. It may be that there are necessary elements to successful weight loss, but with so few data, and so few similarities between interventions, it is difficult to draw any conclusions on this.
As noted earlier we informally assessed all of the studies included in this evaluation in terms of their length of follow-up, sample size, risk of bias, degree of reproducibility of components of the interventions, generalisability and overall effect size (see Appendix 8). Speculatively, the weight loss interventions evaluated by the Stevens and colleagues70,74 studies may offer a useful model for long-term weight management. In particular the Stevens and colleagues70 TOHP-II study, which has a large sample size, reported a statistically significant effect on weight loss at least 24 months after the active intervention, and the intervention appears to be reproducible. In terms of overall methodological rigour, the study was judged to have a generally low risk of bias. It is unclear whether the study was statistically powered to detect an effect of weight change (because the study was powered for reduction in BP); however, the large sample size provides no reason to suspect that the weight change outcome would not have been adequately powered. Its generalisability to the UK is uncertain. Although this study, based on the Trials of Hypertension, had some shortcomings it appears on balance to be best placed among those included in this systematic review to provide a model for further exploration and testing with overweight populations in the UK.
This study had two main phases: an intensive phase (14 weeks) and a maintenance phase (16–18 months), with some maintenance continuing until follow-up at 36 months. The participants’ goals were to lose at least 4.5 kg during the first 6 months of the intervention and to maintain that weight loss for the remainder of the trial (participants did regain weight over time but their weight at 36 months remained just lower than at baseline). Key features of the intervention were a calorie-controlled diet with a focus on decreasing consumption of excess fat, sugar and alcohol, a gradual increase in walking, monitoring through self-report (diaries) and at group sessions, and a relatively simple behavioural therapy intervention. This comprised goal-setting or action plans, relapse prevention, self-monitoring and social support. These components appear consistent with recent NICE obesity guideline advice. The extended support and relatively straightforward goals for diet and exercise may be contributing factors to the weight change results shown in the intervention group compared with the usual-care control group but we are unable to test this. However, the resource requirements to deliver an intervention such as this are unclear at the present time as few details were reported in the study publication.
In the studies included in our review the interventions varied in terms of their length, their components, the personnel involved and the ongoing maintenance/support mechanisms involved. For example, if we look at the number of contacts with participants, personnel involved in the interventions saw or had contact with participants weekly for at least 14 weeks in most of the included studies. It is unclear whether this is realistic in terms of the likely resource availability or as an expectation of the participants. In the intervention that we feel may have the most potential for testing in a UK setting70 the intervention was delivered by dietitians and health educators with some input from psychologists. It included 14 weekly sessions, then six biweekly sessions and then three to four monthly sessions during the 18-month intervention and then at least three contacts during the 18-month ongoing support phase. Weight change was generally positive and dropout rates were around 10% in both the intervention and a usual-care control group.
Throughout our report we have commented on issues of generalisability of the populations where appropriate. For example, where the populations are of a certain age or gender, have answered media advertisements for entry into the studies and/or paid deposits to do so and the fact that none of the studies were carried out in a UK setting and so may have different health systems and populations. A potential example of a more generalisable intervention is the Counterweight Programme. 87 This is currently being rolled out in primary care in many areas of the UK. However, the evaluation of the Counterweight Programme87 intervention was based on a non-randomised study and thus did not meet the inclusion criteria for our systematic review of clinical effectiveness. We are therefore unable to ascertain whether the intervention is clinically effective. The economic evaluation of the Counterweight Programme87 was summarised in our systematic review of cost-effectiveness studies and appeared to be cost saving. However, caution in interpretation is required as the study did not meet the inclusion criteria for this review and there were uncertainties around estimates of the costs of the programme and around assumptions about the extrapolation of effectiveness estimates over time.
Comparison to existing systematic reviews
We have identified 10 existing systematic reviews that focused on weight management interventions for adults (see Table 6). All of these systematic reviews had included studies of interventions comprising diet, exercise and/or behavioural components, but none specifically included only studies with all three components as recommended in the NICE obesity guideline. There appeared to be a consensus from these systematic reviews that the most effective weight loss interventions were those that were multicomponent. Only one of these existing systematic reviews looked specifically at long-term evidence of the interventions, but its aims and inclusion criteria were different to those of our systematic review, precluding comparisons.
Other issues and methodological concerns
The scope of this review was to focus on studies that would be of most use to policy and practice. We therefore sought studies with long-term follow-up and in which the interventions were clearly described (to enable replicability). Despite this, we identified evaluations of a range of different multicomponent interventions, making it difficult to compare results and to make meaningful conclusions. There are a number of other factors that should also be considered in the interpretation of the studies.
Weight regain was common among participants in the included interventions, even in studies with extended ongoing support. Unfortunately, the data available do not allow us the opportunity to offer any inference as to what might be causing this and it is unclear whether this is clinically meaningful in some way.
Incentives to recruit people to participate were used in three of the included studies. 72,73,79 Although not directly tested it does not appear that there was any relationship between using incentives and the likelihood of weight change.
Our review aimed to comment on any particular barriers to or facilitators of weight loss that may help to establish what the key components of these types of interventions should be, but the evidence we reviewed offers no insights into this. From the available data it is not possible to determine whether the weight loss interventions had any negative effects on participants.
Weight loss success may depend on whether trial participants have previously attempted weight loss or how long they have experienced overweight or obesity. Data on these factors could potentially be used to target interventions to certain populations. We attempted to capture these data; however, treatment history was only reported in three72,75,76 of the included studies and the duration that participants had been overweight or obese was not reported in any study. As such there are not enough data for us to reflect on any patterns in the results seen.
Even in those studies with a non-active comparator (e.g. usual care) it was difficult to establish the true estimate of the effects of an intervention as very few studies adequately described their non-active comparator. It seems likely that participants assigned to control arms would still have received some contact with health-care professionals, which may have the potential to underestimate the treatment effect.
The methodological quality of the included studies varied. None of the studies reported all the details necessary to assess all 10 of the quality assessment criteria that we used. In most studies fewer than half of the criteria could be adequately assessed. Similarly, between the included studies there were no individual quality assessment criteria that were reported by all of the studies. A recent systematic review91 of 63 RCTs of any intervention for weight loss found that while reporting seemed to have improved since the publication of the revised consolidated standards of reporting trials (CONSORT) statement in 2001, reporting of some key aspects was still poor. In the review, 60% of the overall CONSORT criteria were satisfied by the RCTs, but the reporting of criteria relating to the methods varied, with just 19% of studies satisfying the reporting criteria relating to treatment allocation. The authors of that systematic review suggest that there is considerable room for improvement in the adherence to the CONSORT reporting criteria.
Many studies included in our evaluation were undertaken more than 10 years ago. It is likely that this would have had a bearing on the reporting of methods used in the studies and the interpretation of results. For example, the general population today is more exposed (e.g. by mass media) to the issue of overweight and obesity and, as a result, likely to be more aware of healthy eating and physical activity messages. It is therefore possible that the effect of interventions conducted a decade or more ago might not be so large in the present day. Over time the roles of health professionals may also have changed, so the types of people delivering weight management interventions today may be different from those who would have delivered such interventions in the past. Practice may also vary geographically, including within countries. The studies in our review provided little detail on the process of training providers.
In the two studies86,87 that were described in our cost-effectiveness review the costs of the multicomponent weight loss programme varied between £60 and US$3040 (£1820) per participant. The costs reported in the other reviews for weight programmes appear also to be within this range. A NICE guidance report35 for the management of obesity included a review of diet, exercise and behavioural treatment and the resource costs for these components. In the NICE report, physical activity interventions cost between £532 and £737; diet interventions cost between £103 and £621; and a diet and behavioural intervention cost £672 per patient. One study included in the NICE review of a pharmacological trial for sibutramine included diet and exercise advice as the control arm with a cost of £243.48 per person. 92 Avenell and colleagues30 reviewed treatment for obesity and constructed an economic model for a diet and exercise intervention for individuals with impaired glucose tolerance. The total estimated cost for the LI was £324 per person in the first year and £178 per person for subsequent years. The resource costs reported for other weight loss interventions seem to concur with our conclusion that the costs in the Counterweight Programme study87 are too low, and those from the Roux and colleagues North American study86 do not reflect UK costs.
The gain in QALY compared with routine care varies considerably between the two studies86,87 due to the differences in the assumptions for long-term weight loss maintenance and the utility values chosen for weight change. The change in utility values associated with a unit change in BMI varied between about 0.006 for the Counterweight Programme87 and 0.02 for Roux and colleagues. 86 Neither study based these utility estimates upon a review of the QoL literature. A recent Health Technology Assessment report33 on the clinical effectiveness and cost-effectiveness of bariatric surgery for obesity conducted a targeted search to identify published utility estimates for BMI values relevant to obese adults. The authors of this study stated that the values from the study by Hakim and colleagues93 represented the most methodologically sound estimates derived. Hakim and colleagues93 found that a one-unit decrease in BMI in obese individuals without diabetes was associated with a gain of 0.017, which was independent of age or gender. The utilities values from this study are consistent with those chosen by Roux and colleagues,86 but much higher than those used by the Counterweight Programme study, suggesting that the Counterweight Programme study may be underestimating some of the health gain associated with weight loss. However, although the QALY gains are small in these studies, the interventions are still cost-effective by generally accepted thresholds, because the interventions have such low costs.
One of the studies discussed in our review (Roux and colleagues86) included a utility decrement associated with the effort involved with participating in a weight management scheme. This resulted in the situation where participants of a diet-only strategy had lower lifetime QALY than those having routine care. As the primary data analysis to derive these data was not reported, it is unclear how these data were derived and whether the assumptions are valid. Removing this assumption would result in a more favourable cost-effectiveness estimate.
The cost-effectiveness results in the two studies described in our cost-effectiveness review are consistent with other cost-effectiveness studies for diet and exercise interventions (but without a behavioural component). Gallani and colleagues94,95 described a Markov model for a diet and exercise intervention in Switzerland in overweight and obese people. In their analysis the diet and exercise intervention was cost-effective for all subgroups with an ICER < £5000 per QALY. Bemelmans and colleagues96 described a cost–utility study in the Netherlands in overweight and obese people. The ICER for the diet and exercise intervention was €7400 (£6700) per QALY saved. Furthermore, a NICE review35 for diet, exercise and behavioural treatments concluded that ‘the cost per QALY in the best-performing non-pharmacological studies ranges from £174 to £9971’. This suggests that even though there were limitations to each of the studies in our cost-effectiveness review, the results appear to be consistent with other studies.
Strengths and limitations
This review has the following strengths:
-
It is independent of any vested interest.
-
It has been undertaken following the principles for conducting a systematic review. The methods were set out in a research protocol (see Appendix 1), which defined the research question, inclusion criteria, quality criteria, data extraction process and methods to be employed at different stages of the review.
-
An advisory group has informed the review from its initiation. The research protocol was informed by comments received from the advisory group and the advisory group has reviewed and commented on the final report.
-
The review brings together the evidence for the clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults who are overweight or obese. This evidence has been critically appraised and presented in a consistent and transparent manner.
In contrast, this review also has certain limitations:
-
Synthesis of the included studies was through narrative review. Although 12 studies were included in the review of clinical effectiveness, differences in the interventions meant that meta-analysis was inappropriate.
-
No cost effectiveness or cost-effectiveness studies were found which met our full inclusion criteria and we therefore took a pragmatic approach to discuss two studies that met many of the attributes our review was looking for. Caution is therefore required in the interpretation of our results in terms of cost-effectiveness.
-
Searches were limited to the English language. Therefore, we may have omitted non-English language, but otherwise includable, studies from our review.
-
Many studies were excluded from our review because they either did not include a behavioural therapy element or they reported that there was a behavioural element but did not provide any details of how this was implemented. Although we attempted to contact authors of a number of studies to try to ascertain further details, this was unproductive. It may be that there are other studies with a long-term view of a weight management intervention that we were therefore unable to include. However, it is also apparent that the term ‘behavioural’ appears to be used in many situations to mean that a study aimed to change behaviours, but that this was as a result of the diet and/or exercise intervention components rather than of a specific behavioural therapy. It is unclear whether this is the case in any of these situations.
Need for further research
There have been a number of RCTs of multicomponent weight management interventions, and despite a number of differences between the many studies, there does appear to be some degree of success in general. It is uncertain whether any one particular intervention is best; however, the results of our review suggest that the TOHP intervention70,74 may be useful for testing further in a UK population. If any future RCT takes place, then replicating the intervention used in the TOHP study should be considered.
As most interventions succeeded in short-term weight loss, more research into the most appropriate long-term support to improve long-term maintenance of weight loss is required. Researchers should consider the Medical Research Council framework for developing evaluations of complex interventions97 and the National Obesity Observatory standard evaluation framework for weight management interventions98 when they design their studies.
There is a need for information on barriers to and facilitators of weight change in weight management interventions to be reported and, if possible, evaluated in clinical trials. As well as providing useful evidence on individual factors associated with greater or lesser weight change, such information could assist understanding of which of its components a multicomponent intervention should focus on.
There is a need for better reporting of behaviour therapy interventions, in terms of clear details of the techniques used, theoretical model, the format, setting and provider. 62 Authors of studies should more critically consider the reproducibility of their interventions. A taxonomy of a variety of behaviour change techniques has been devised and tested, to facilitate a common classification by intervention providers and researchers. 57 It would be advantageous for future evaluations to employ such a system in the reporting of interventions to facilitate a greater understanding of the specific components of interventions associated with effective health-related behaviour change.
An economic model for weight management interventions is needed for the UK NHS that includes a complete explanation of model structure, assumptions and data inputs. This model should be based upon evidence of the intervention effect, from an RCT or systematic review, with UK-relevant data inputs, particularly with regard to costs.
Chapter 6 Conclusions
Weight management interventions were generally shown to promote weight loss in overweight or obese adults. Weight changes were small, however, and weight regain was common in those studies that measured it longer term. There were few similarities between the included studies that mean that interpretation of the results is difficult to make overall. Weight management interventions appear to be a cost-effective option, although caution is required as there were a number of limitations to the two cost-evaluation studies described here, not least that neither study met the full inclusion criteria of our review in terms of length of follow-up. There were no UK-based RCTs included in the review and as such there is a research need to evaluate the effects of long-term multicomponent weight management interventions in a UK setting.
Acknowledgements
We would like to thank members of our advisory group who provided expert advice and comments on the protocol and/or a draft of this report:
-
Dr A Avenell, Senior Lecturer, University of Aberdeen
-
Dr Ian Brown, Centre for Health and Social Care Research, Sheffield Hallam University
-
Dr David Haslam, Chair, National Obesity Forum
-
Professor Paul Little, General Practitioner and Professor of Primary Care Research, University of Southampton
-
Professor Barrie Margetts, Professor of Public Health Nutrition, Institute of Human Nutrition, University of Southampton
-
Professor Jane Ogden, Department of Psychology, University of Surrey
-
Professor Carolyn Summerbell, Professor of Human Nutrition, School of Medicine and Health, Durham University.
We are also grateful to Andrea Takeda, Senior Research Fellow, Southampton Health Technology Asssessments Centre (SHTAC), for reviewing a draft of this report and Angela Vincent, Research Programme Administrator, for retrieval of references.
Contributions of authors
E Loveman (Senior Research Fellow) developed the research protocol, drafted the background section, assisted in the development of the search strategy, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted and edited the final report, and project managed the study; G Frampton (Research Fellow) drafted the background section, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted the final report; J Shepherd (Principal Research Fellow) drafted the background section, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted the final report; J Picot (Research Fellow) drafted the background section, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted the final report; K Cooper (Senior Research Fellow), assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted the report; J Bryant (Principal Research Fellow) developed the research protocol, assessed studies for inclusion, extracted data from and quality assessed included studies, synthesised evidence, drafted the report; K Welch developed the search strategy, undertook literature searches and edited the final report; and A Clegg (Professor/Director of SHTAC) developed the research protocol, drafted and edited the final report and acted as guarantor.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Walley AJ, Blakemore AIF, Froguel P. Genetics of obesity and the prediction of risk for health. Hum Mol Genet 2006;15:R124-R130.
- Wilding JPH. Pathophysiology and aetiology of obesity. Medicine 2006;34:501-5.
- Rissanen A, Heliovaara M, Aromaa A. Overweight and anthropometric changes in adulthood: a prospective study of 17,000 Finns. Int J Obes 1988;12:391-40.
- McTigue KM, Garrett JM, Popkin BM. The natural history of the development of obesity in a cohort of young U.S. adults between 1981 and 1998. Ann Intern Med 2002;136:857-64.
- Kahn HS, Cheng YJ. Longitudinal changes in BMI and in an index estimating excess lipids among white and black adults in the United States. Int J Obes 2008;32:136-43.
- Obesity: preventing and managing the global epidemic. World Health Organ Tech Rep Ser; 2000.
- World Health Organization . Overweight and Obesity 2006.
- Berghofer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008;8.
- The NHS. Statistics on obesity, physical activity and diet: England, February 2009. Leeds: The Health and Social Care Information Centre; 2009.
- The Information Centre . Health Survey for England 2007 n.d. www.ic.nhs.uk/pubs/hse07healthylifestyles (accessed 21 October 2010).
- Issued by Statistical Directorate Welsh Assembly Government . Welsh Health Survey 2007 2008.
- Corbett J, Given L, Gray L, Leyland A, MacGregor A, Marryat A, et al. The Scottish health survey 2008. Edinburgh: The Scottish Government; 2009.
- Northern Ireland Statistics and Research Agency . Northern Ireland Health and Social Wellbeing Survey 2005 06 2007.
- Scarborough P, Allender S. The North-South gap in overweight and obesity in England. Br J Nutr 2008;100:677-84.
- Zaninotto P, Head J, Stamatakis E, Wardle H, Mindell J. Trends in obesity among adults in England from 1993 to 2004 by age and social class and projections of prevalence to 2012. J Epidemiol Community Health 2009;63:140-6.
- McPherson K, Marsh T, Brown M. Tackling obesities: future choices – modelling future trends in obesity and the impact on health. London: The Foresight Programme; 2007.
- Prospective Studies Collaboration . Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96.
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of comorbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78.
- World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective 2007.
- Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev 2007;8:41-59.
- Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev 2001;2:219-29.
- Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: intervention and 24 month follow-up effects. Health Qual Life Outcomes 2006;4.
- Kushner RF, Foster GD. Obesity and quality of life. Nutrition 2000;16:947-52.
- Hassan MK, Joshi AV, Madhavan SS, Amonkar MM. Obesity and health-related quality of life: a cross-sectional analysis of the US population. Int J Obes 2003;27:1227-32.
- de Wit LM, van Straten A, van Herten M, Penninx BWJH, Cuijpers P. Depression and body mass index, a u-shaped association. BMC Public Health 2009;9.
- Flegal K, Graubard B, Williamson D, Gail M. Excess deaths associated with underweight, overweight and obesity. JAMA 2005;293:1861-7.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-40.
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50.
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8.
- Poobalan AS. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev 2007;8:503-13.
- Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev 2009;22:93-108.
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical-effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13.
- Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res 2001;9:564-71.
- National Institute for Health and Clinical Excellence . Obesity – Guidance on the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children 2006.
- Counterweight Project Team . Influence of body mass index on prescribing costs and potential cost savings of a weight management programme in primary care. J Health Serv Res Policy 2008;13:158-66.
- House of Commons Health Committee . Obesity: Third Report of Session 2003–04 2004.
- Allender S, Rayner M. The burden of overweight and obesity-related ill health in the UK. Obes Rev 2007;8:467-73.
- National Audit Office . Tackling Obesity in England: Report by the Comtroller and Auditor General 2001.
- Dr Foster . Primary Care Management of Adult Obesity 2005.
- National Obesity Forum . Guidelines on Management of Adult Obesity and Overweight in Primary Care 2009.
- Clinical Resource Efficiency Support Team (CREST) . Guidelines for the Management of Obesity in Secondary Care 2005.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Obesity in Children and Young People 2003.
- Department of Health . Coronary Heart Disease: National Service Framework for Coronary Heart Disease – Modern Standards and Service Models 2000.
- McLean N, Griffin S, Toney K, Hardeman W. Family involvement in weight control, weight maintenance and weight-loss interventions: a systematic review of randomised trials. Int J Obes 2003;27:987-1005.
- Avenell A, Brown TJ, Mcgee MA, Campbell MK, Grant AM, Broom J, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J Hum Nutri Diet 2004;17:293-316.
- McTigue KM, Harris R, Hemphill B, Lux L, Sutton S, Bunton AJ, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. preventive services task force. Ann Intern Med 2003;139:933-49.
- Franz MJ. Effectiveness of weight loss and maintenance interventions in women. Curr Diab Rep 2004;4:387-93.
- Söderlund A, Fisher A, Johansson T. Physical activity, diet and behaviour modification in the treatment of overweight and obese adults: a systematic review. Perspect Public Health 2009;129:132-42.
- Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance a review. J Cardiovasc Nurs 2009;24:58-80.
- Glenny AM, O’Meara S, Melville A, Sheldon T, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. Int J Obes 1997;21:715-37.
- Brown T, Avenell A, Edmunds LD, Moore H, Whittaker V, Avery L, et al. Systematic review of long-term lifestyle interventions to prevent weight gain and morbidity in adults. Obes Rev 2009;10:627-38.
- Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Inten Med 2005;142:56-6.
- Counterweight Project Team . Evaluation of the Counterweight Programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract 2008;58:548-54.
- Prochaska JO, Velicer WF, Rossi JS, Goldstein MG, Marcus BH, Rakowski W, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol 1994;13:39-46.
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot 1997;12:38-4.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87.
- Over-the-counter medicines: in whose best interests?. Lancet 2009;373.
- Gabel JR, Whitmore H, Pickreign J, Ferguson CC, Jain A, Shova KC, et al. Obesity and the workplace: current programs and attitudes among employers and employees. Health Aff 2009;28:46-5.
- Jeffery R, Epstein L, Wilson GT, Drewnawski A, Stunkard AJ, Wing RR. Long-term maintenance of weight loss: current status. Health Psychol 2000;19:5-16.
- Lowe MR, Timko CA. Dieting: really harmful, merely ineffective or actually helpful?. Br J Nutr 2004;92:S19-S22.
- Davidson KW, Goldstein M, Kaplan RM, Kaufmann PG, Knatterud GL, Orleans CT, et al. Evidence-based behavioral medicine: what is it and how do we achieve it?. Ann Behav Med 2010;26:161-71.
- Centre for Reviews and Dissemination . Systematic Reviews. CRD’s Guidance for Conducting Reviews in Healthcare 2009.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration 2009. www.cochrane-handbook.org (accessed 21 October 2010).
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ economic evaluation working party. BMJ 1996;313:275-83.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008.
- Dubbert PM, Wilson GT. Goal-setting and spouse involvement in the treatment of obesity. Behav Res Ther 1984;22:227-42.
- Wadden TA, Stunkard AJ, Liebschutz J. Three-year follow-up of the treatment of obesity by very low calorie diet, behavior therapy, and their combination. J Consult Clin Psychol 1988;56:925-8.
- Stevens V, Obarzanek E, Cook N, Lee IM, Appel LJ, Smith West D, et al. Long term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Intern Med 2001;134:1-11.
- Burke LE, Warziski M, Styn MA, Music E, Hudson AG, Sereika SM. A randomized clinical trial of a standard versus vegetarian diet for weight loss: the impact of treatment preference. Int J Obes 2008;32:166-76.
- Logue E, Sutton K, Jarjoura D, Smucker W, Baughman K, Capers C. Transtheoretical model-chronic disease care for obesity in primary care: a randomized trial. Obes Res 2005;13:917-27.
- Simkin-Silverman LR, Wing RR, Boraz MA, Meilahn EN, Kuller LH. Maintenance of cardiovascular risk factor changes among middle-aged women in a lifestyle intervention trial. J Womens Health 1998;4:255-71.
- Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med 1993;153:849-58.
- Jeffery RW, Wing RR. Long-term effects of interventions for weight loss using food provision and monetary incentives. J Consult Clin Psychol 1995;63:793-6.
- Jeffery RW, Wing RR, Thorson C, Burton LR. Use of personal trainers and financial incentives to increase exercise in a behavioral weight-loss program. J Consult Clin Psychol 1998;66:777-83.
- Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain?. Am J Clinl Nutr 2007;85:954-9.
- Weinstock RS, Dai H, Wadden TA. Diet and exercise in the treatment of obesity: effects of 3 interventions on insulin resistance. Arch Intern Med 1998;158:2477-83.
- Skender ML, Goodrick GK, Del Junco DJ, Reeves RS, Darnell L, Gotto AM, et al. Comparison of 2-year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. J Am Diet Assoc 1996;96:342-6.
- Ross R, Blair SN, Godwin M, Hotz S, Katzmarzyk PT, Lam M, et al. Prevention and reduction of obesity through active living (PROACTIVE): rationale, design and methods. Br J Sports Med 2009;43:57-63.
- Stolley MR, Fitzgibbon ML, Schiffer L, Sharp LK, Singh V, Van HL, et al. Obesity reduction black intervention trial (ORBIT): six-month results. Obesity 2009;17:100-6.
- van Wier MF, Ariens GA, Dekkers JC, Hendriksen IJ, Pronk NP, Smid T, et al. ALIFE@Work: a randomised controlled trial of a distance counselling lifestyle programme for weight control among an overweight working population. BMC Public Health 2006;6.
- Williams AE, Vogt TM, Stevens VJ, Albright CA, Nigg CR, Meenan RT, et al. Work, weight, and wellness: the 3W program: a worksite obesity prevention and intervention trial. Obesity 2007;15:16S-26S.
- Ostbye T, Krause KM, Lovelady CA, Morey MC, Bastian LA, Peterson BL, et al. Active mothers postpartum. A randomised controlled weight-loss intervention trial. Am J Prev Med 2009;37:173-80.
- Ostbye T, Krause KM, Brouwer RJ, Lovelady CA, Morey MC, Bastian LA, et al. Active Mothers Postpartum (AMP): rationale, design, and baseline characteristics. J Womens Health 2008;17:1567-75.
- Roux L, Kuntz KM, Donaldson C, Goldie SJ. Economic evaluation of weight loss interventions in overweight and obese women. Obesity 2006;14:1093-106.
- Trueman P, Haynes SM, Felicity LG, Louise ME, McQuigg MS, Mongia S, et al. Long-term cost-effectiveness of weight management in primary care. Int J Clin Pract 2010;64:828-9.
- Macran S. The Relationship Between Body Mass Index and Health-Related Quality of Life n.d. www.york.ac.uk/inst/che/pdf/DP190.pdf (accessed 21 October 2010).
- National Institute for Health and Clinical Excellence . Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children 2006.
- Fryback DG, Dasbach EJ, Klein JD, Klein BE, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making 2010;13:89-102.
- Thabane L, Chu R, Cuddy K, Douketis J. What is the quality of reporting in weight loss intervention studies? A systematic review of randomized controlled trials. Int J Obes 2007;31:1554-9.
- Warren E, Brennan A, Akehurst R. Cost-effectiveness of sibutramine in the treatment of obesity. Med Decis Making 2004;24:9-19.
- Hakim Z, Wolf A, Garrison LP. Estimating the effect of changes in body mass index on health state preferences. Pharmacoeconomics 2008;20:393-404.
- Galani C, Schneider H, Rutten FFH. Modelling the lifetime costs and health effects of lifestyle intervention in the prevention and treatment of obesity in Switzerland. Int J Public Health 2007;52:372-82.
- Galani C, Al M, Schneider H, Rutten FFH. Uncertainty in decision-making: value of additional information in the cost-effectiveness of lifestyle intervention in overweight and obese people. Value Health 2008;11:424-34.
- Bemelmans W, van Ball P, Wendel-Vos W, Schuit J, Feskens E, Ament A, et al. The costs, effects and cost-effectiveness of counteracting overweight on a population level. A scientific base for policy targets for the Dutch national plan for action. Preventive Medicine 2008;46:127-32.
- Medical Research Council . Developing and Evaluating Complex Interventions: New Guidance n.d. www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d%20=%20MRC004871 (accessed 25 January 2010).
- National Obesity Observatory . Standard Evaluation Framework for Weight Management Interventions n.d. www.noo.org.uk/uploads/doc/vid_3534_NOOSEFreportJuly09.pdf (accessed 29 January 2010).
- Centre for Reviews and Disseminaiton . Undertaking Systematic Reviews of Research on Effectiveness 2001.
- Centre for Reviews and Dissemination . NHS Economic Evaluation Database Handbook 2007. www.york.ac.uk/inst/crd/pdf/nhseed-handb07.pdf (accessed 20 August 2008).
- Griffin S, Bojke L, Main C, Palmer S. Incorporating direct and indirect evidence using Bayesian methods: an applied case study in ovarian cancer. Value Health 2006;9:123-32.
- Nixon RM, Bansback N, Brennan A. Using mixed treatment comparisons and meta-regression to perform indirect comparisons to estimate the efficacy of biologic treatments in rheumatoid arthritis. Stat Med 2007;26:1237-54.
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900.
- Burke LE, Choo J, Music E, Warziski M, Styn MA, Kim Y, et al. PREFER study: a randomized clinical trial testing treatment preference and two dietary options in behavioral weight management--rationale, design and baseline characteristics. Contemp Clin Trials 2006;27:34-48.
- Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women’s healthy lifestyle project: a randomized clinical trial: results at 54 months. Circulation 2001;103:32-7.
- Jeffery RW, Wing RR, Thorson C, Burton LR, Raether C, Harvey J, et al. Strengthening behavioral interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol 1993;61:1038-45.
- Simkin-Silverman L, Wing RR, Hansen DH, Klem ML, Pasagian-Macaulay AP, Meilahn EN, et al. Prevention of cardiovascular risk factor elevations in healthy premenopausal women. Prev Med 1995;24:509-17.
- Foreyt JP, Goodrick GK, Reeves RS, Raynaud AS, Darnell L, Brown AH, et al. Response of free-living adults to behavioral treatment of obesity: attrition and compliance to exercise. Behav Ther 1993;24:659-69.
- Whelton PK, Hebert PR, Cutler J, Applegate WB, Eberlein KA, Klag MJ, et al. Baseline characteristics of participants in phase I of the trials of hypertension prevention 1992;2:295-310.
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000;35:544-9.
- Whelton PK, Kumanyika S, Cook NR, Cutler JA, Borhani NO, Hennekens CH, et al. Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: results from phase 1 of the trials of hypertension prevention. Am J Clin Nutr 1997;65:652S-660S.
- Satterfield S, Cutler JA, Langford HG, Applegate WB, Borhani NO, Brittain E, et al. Trials of hypertension prevention. Phase I design. Ann Epidemiol 1991;1:455-71.
- TOHP Collaborative Research Group . Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The trials of hypertension prevention, phase II. Arch Int Med 1997;157:657-67.
- Appel LJ, Hebert PR, Cohen JD, Obarzanek E, Yamamoto M, Buring J, et al. Baseline characteristics of participants in phase II of the trials of hypertension pevention (TOHP II). Ann Epidemiol 1995;5:149-55.
- Lasser VI, Raczynski JM, Stevens VJ, Mattfeldt-Beman MK, Kumanyika S, Evans M, et al. Trials of Hypertension Prevention, Phase II. Structure and content of the weight loss and dietary sodium reduction interventions. Ann Epidemiol 1995;5:156-64.
- Hebert PR, Bolt RJ, Borhani NO, Cook NR, Cohen JD, Cutler JA, et al. Design of a multicenter trial to evaluate long-term life-style intervention in adults with high-normal blood pressure levels. Trials of Hypertension (phase II). Ann Epidemiol 1995;5:130-9.
- Hollis JF, Satterfield S, Smith F, Fouad M, Allender PS, Borhani N, et al. Recruitment for phase II of the trials of hypertension prevention. Effective stratagies and predictors of randomisation. Ann Epidemiol 1995;5:140-8.
- Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. Am J Clin Nutr 1991;53:1631S-1638S.
- Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome?. Am J Clin Nutr 2003;78:684-9.
- Raynor HA, Jeffery RW, Tate DF, Wing RR. Relationship between changes in food group variety, dietary intake, and weight during obesity treatment. Int J Obes 2004;28:813-20.
- Wadden TA, Stunkard AJ. Controlled trial of very low calorie diet, behavior therapy, and their combination in the treatment of obesity. J Consul Clin Psychol 1986;54:482-8.
- Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, et al. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consul Clin Psychol 1997;65:269-77.
- Borg P, Kukkonen-Harjula K, Fogelholm M, Pasanen M. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomised trial. Int J Obes 2002;26:676-83.
- Hakala P. Weight reduction programmes at a rehabilitation centre and a health centre based on group counselling and individual support: short- and long-term follow-up study. Int J Obes 1994;18:483-9.
- Hakala P, Karvetti RL, Ronnemaa T. Group vs. individual weight reduction programmes in the treatment of severe obesity – a five year follow-up study. Int J Obes 1993;17:97-102.
- Linde JA, Erickson DJ, Jeffery RW, Pronk NP, Boyle RG. The relationship between prevalence and duration of weight loss strategies and weight loss among overweight managed care organization members enrolled in a weight loss trial. Int J Behav Nutr Phys Act 2006;3.
- Ashley JM, Sachiko T, Perumean-Chaney SE, Schrage J, Bovee V. Meal replacements in weight intervention. Obes Res 2001;9:312S-320S.
- Svetkey L, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomised controlled trial. JAMA 2008;299:1139-48.
- Lewis JD, Brown A, Localio AR, Schwartz JS. Initial evaluation of rectal bleeding in young persons: a cost-effectiveness analysis. Ann Intern Med 2002;136:99-110.
- Gregg EW, Cadwell BL, Cheng YJ, Cowie CC, Williams DE, Geiss L, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care 2004;27:2806-12.
- Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995;122:327-34.
Appendix 1 Methods from the research protocol and commissioning brief (scope) for project
Report methods for the synthesis of clinical- and cost-effectiveness evidence
A review of the evidence for clinical effectiveness and cost-effectiveness will be undertaken systematically following the general principles outlined in CRD Report Number 4 (2nd Edition) Undertaking Systematic Reviews of Research on Effectiveness. 99
Search strategy
The search strategies will be devised and tested by an experienced information scientist. The strategies will be designed to identify (i) clinical effectiveness studies reporting on comparisons between different adult weight management schemes, and comparisons between adult weight management schemes and normal practice; and (ii) studies reporting on the costs and cost-effectiveness of different adult weight management schemes.
The following electronic databases will be searched: MEDLINE; EMBASE; PREMEDLINE In-Process & Other Non-Indexed Citations; the Cochrane Library including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, DARE, NHS EED and HTA databases; Web of Knowledge Science Citation Index; Web of Knowledge ISI Proceedings; PsycINFO; CRD; BIOSIS.
Searches will be carried from database inception to current date and will be limited to the English language. All searches will be updated when the draft report is under review, prior to submission of the final report.
Bibliographies of related papers will be assessed for relevant studies where possible.
Members of an expert advisory group will be asked to review the adequacy of the searches and to indicate whether they are aware of any additional published or unpublished evidence.
Inclusion and exclusion criteria
Population
-
Adults (≥ 18 years) classified as overweight or obese, i.e. people with a BMI of ≥ 25 kg/m2 and ≥ 30 kg/m2, respectively.
-
Studies in children and people with eating disorders will not be included, nor will studies specifically in people with a pre-existing medical condition such as diabetes, heart failure, uncontrolled hypertension or angina.
Intervention
-
Structured, sustained multicomponent weight management programmes (i.e. the intervention must be a combination of diet and physical activity with a behaviour change strategy to influence lifestyle).
-
Components of the programme have to be clearly specified (i.e. details of the diet, behavioural definition, exercise component details).
-
Programmes include a long-term follow up of more than 18 months.
-
The programme may be delivered by the health sector, in the community or commercially.
-
Multicomponent programmes that involve the use of OTC medicines that are licensed in the UK for overweight or obesity will be included. Programmes that involve non-OTC drug therapies or surgery for obesity will not be included.
-
Interventions incorporating other lifestyle changes such as efforts at smoking cessation or reduction of alcohol intake will not be included.
Comparators
-
Normal practice (defined).
-
Single component weight management strategies.
-
Other structured multicomponent weight management programme.
Outcomes
-
The main outcome of included studies must be explicitly stated as a measure of weight loss. Initial weight loss and maintenance of weight loss will be expressed as weight change (e.g. kilograms, BMI kg/m2) either reported or calculated from reported baseline weight.
-
Studies must report more than 18 months’ follow-up to be included.
-
Data will also be extracted on the following outcomes where reported in the included studies: success rates at more than 18 months’ attrition rates at more than 18 months, barriers and facilitators of weight loss and maintenance of weight loss.
-
Outcomes of cost-effectiveness studies will be costs, benefits in terms of weight loss and cost-effectiveness.
Types of studies
-
For the systematic review of clinical effectiveness RCTs will be included.
-
For the systematic review of cost-effectiveness study types will include full cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses and cost–consequence analyses.
-
Studies published as abstracts or conference presentations will only be included if sufficient details are presented to allow an appraisal of the methodology and the assessment of results to be undertaken.
-
Case series, case studies, cohort studies, narrative reviews, feasibility studies, editorials and opinions will not be included.
-
Systematic reviews will be used as a source of references.
-
Non-English language studies will be excluded.
Reference screening, data extraction, and quality assessment process
Reference screening strategy
The titles and abstracts of studies identified by the search strategy will be assessed for potential eligibility using the inclusion/exclusion criteria detailed above. This will be performed by two reviewers. Full papers of studies that appear potentially relevant will be requested for further assessment. These will be screened by two reviewers and a final decision regarding inclusion will be agreed. At each stage, any disagreements will be resolved by discussion, with involvement of a third reviewer where necessary.
Data extraction strategy
Data will be extracted by one reviewer using a standardised data extraction form. A second reviewer will independently check extracted data. Discrepancies will be resolved by discussion, with involvement of another reviewer when necessary.
Quality assessment strategy
The quality of the clinical effectiveness studies will be assessed according to criteria based on CRD (University of York) criteria. 99 Economic evaluations will be assessed using criteria recommended by Drummond and colleagues,66 and/or the format recommended and applied in the CRD NHS Economic Evaluation Database (using principles outlined in the NHS EED Handbook100). For any studies based on decision models the checklist for assessing good practice in decision analytic modelling will be used (Philips and colleagues65).
The quality of the individual studies will be assessed by one reviewer, and independently checked by a second reviewer. Any disagreements will be resolved by consensus, and if necessary a third reviewer will be consulted.
Methods of data analysis/synthesis
Studies will be synthesised through a narrative review with tabulation of results of included studies. Where possible the results from individual studies will be synthesised through meta-analysis, with causes of heterogeneity of results examined. The specific methods for meta-analysis and for the detection and investigation of heterogeneity will depend upon the summary measure selected. When head-to-head randomised evidence is limited or unavailable, mixed-treatment comparison (MTC) is increasingly being used to inform clinical decision making. 101,102 MTC is an extension of traditional, pair-wise, meta-analysis. 103 A statistical analysis of the network of trial evidence is used to produce comparable estimates of effectiveness for a range of treatments. Where limited or no direct evidence of the relative effectiveness of the interventions is identified, a MTC will be considered. This will consider RCTs of weight management programmes that include a common comparator. Data on weight loss will be extracted from the clinical trials and a network of evidence constructed. Heterogeneity and consistency between included trials will be assessed before conducting the analysis. Fixed and random treatment effects will be considered. Sensitivity and subgroup analyses will be undertaken where appropriate.
Information on barriers and facilitators to weight loss will be summarised through narrative overview only.
Commissioning brief: the effectiveness and cost-effectiveness of weight management schemes for adults
Research question:
-
What is the comparative effectiveness and cost-effectiveness of different weight management schemes for adults?
-
– Technology: weight management schemes that combine dietary and other approaches (whether delivered by the health sector or commercially).
-
– Population group: overweight or obese adults.
-
– Setting: OECD countries or settings with relevance to the UK.
-
– Control or comparator treatment: normal practice.
-
– Design: evidence synthesis, to include: (1) systematic review of the effectiveness of individual programmes’ approaches to losing weight; (2) systematic review of direct comparisons of their effectiveness with statistical modelling for indirect comparisons; (3) researchers should explore the effects of barriers and facilitators to weight management (e.g. determinants of compliance); and (4) researchers should identify the key recommendations for future primary evaluation research. The HTA programme is also interested in longer-term QoL and health outcomes and would welcome ideas on exploring the feasibility of modelling these.
-
– Outcomes: both weight loss and maintenance of weight loss are of interest. Other outcomes to consider include duration of follow-up; attrition/success rates at 1 year or longer; cost of the intervention per unit of weight lost.
-
Obesity affects at least 300 million people worldwide. In the UK, levels of obesity have trebled in the past 20 years to 25% of adults. Projections suggest that unless urgent and effective action is taken, by 2010 almost 30% of UK adults will be obese.
A huge amount of time and effort is spent on a variety of programmes to help people lose weight and maintain weight loss. Evidence about the effectiveness and cost-effectiveness of the approaches underpinning such programmes is lacking.
The Department of Health has an interest and has requested this piece of work from the HTA programme. Research is therefore required, in the form of evidence synthesis, comparing different weight management approaches to identify the most effective way of losing weight and maintaining weight loss. (The place of drugs in helping people lose weight is not part of this vignette.)
Appendix 2 Sources of information and search terms
A list of all databases searched for the systematic review, as well as searches for ongoing trials is presented below.
| Database searched | Clinical effectiveness searches | Cost-effectiveness searches |
|---|---|---|
| BIOSIS (via Web of Science) | All available years | All available years |
| Cochrane | Searched 8 December 2009 | Searched 22 December 2009 |
| Centre for Reviews and Dissemination | Searched 8 December 2009 | Searched 22 December 2009 |
| EMBASE | All available years | All available years |
| MEDLINE | 1950–2009 | 1950–2009 |
| MEDLINE In-Process & Other Non-Indexed Citations | Searched 8 December 2009 | Searched 22 December 2009 |
| NHS Economic Evaluation Database | N/A | Searched 22 December 2009 |
| PsychINFO | All available years | N/A |
| Web of Science: Conference Proceedings Citation Index | 1990–2009 | 1970–2009 |
| Web of Science: Science Citation Index | 1990–2009 | 1970–2009 |
Searched for ongoing trials
UK Clinical Research Network portfolio; controlled-trials multiple register (includes the International Standard Randomised Controlled Trial Number Register, National Institutes of Health, ClinicalTrials.gov, Action Medical Research, Medical Research Council, NHS Health Technology Assessment, Wellcome Trust, UK Clinical Trials Gateway); World Health Organization, International Clinical Trials Registry Platform; Australian New Zealand Clinical Trials Registry; GSK Clinical Trial Register.
Clinical effectiveness
The MEDLINE search strategy for the clinical effectiveness section was adjusted as necessary for cost-effectiveness searches and other electronic database searches. Search strategies for the systematic review are available from the authors on request.
MEDLINE search strategy
-
exp Obesity/or exp Obesity, Morbid/ (90,428)
-
exp weight gain/ (15,851)
-
Overweight/ (3101)
-
(overweight or over weight or overeat* or over eat* or overfeed* or over feed*).ti,ab. (19924)
-
(weight adj1 gain*).ti,ab. (31,017)
-
obes*.ti,ab. (96,808)
-
or/1-6 (162,369)
-
(modific* or therap* or intervention* or strateg* or program* or management or scheme* or group* or pathway*).ti,ab. (3,985,663)
-
(weight adj1 los*).ti,ab. (36,353)
-
(weight adj1 reduc*).ti,ab. (6674)
-
exp weight loss/ (18,198)
-
8 and (9 or 10 or 11) (24,208)
-
Obesity/dh, pc, th [Diet Therapy, Prevention & Control, Therapy] (17,839)
-
Obesity, Morbid/pc, dh, th [Prevention & Control, Diet Therapy, Therapy] (630)
-
8 and (13 or 14) (8782)
-
Diet Therapy/ (8676)
-
Diet, Fat-Restricted/ (1984)
-
Diet, Reducing/ (7805)
-
Dietetics/ed, mt (1228)
-
(diet or diets or dieting).ti,ab. (170,996)
-
(low calorie or hypocaloric or calorie control*).ti,ab. (2549)
-
(health* adj1 eating).ti,ab. (1335)
-
(diet* adj2 (modific* or therapy or intervention* or strateg* or program* or management or scheme*)).ti,ab. (10,919)
-
(nutrition adj2 (modific* or therapy or intervention* or strateg* or program* or management or scheme*)).ti,ab. (3939)
-
Weight Watchers.ti,ab. (46)
-
slimming world.ti,ab. (2)
-
“lighterlife”.ti,ab. (0)
-
or/16-27 (191,002)
-
8 and 28 (88,245)
-
exp exercise/ (46,280)
-
exercise therapy/ (18,044)
-
(exercise and (therapy or activity or class* or program* or group* or session* or scheme*)).ti,ab. (62,959)
-
(Gym and (trainer* or therap* or activit* or class* or program* or group* or session* or scheme* or club*)).ti,ab. (157)
-
(walk* or step* or jog* or run*).ti,ab. (391,392)
-
(aerobic* or physical therap* or physical activit*).ti,ab. (74,788)
-
(fitness adj (class or regime* or program* or group* or session* or scheme*)).ti,ab. (522)
-
(reduc* adj2 sedentary behavio?r).ti,ab. (33)
-
(dance and (therap* or activit* or class* or program* or group* or session* or scheme*)).ti,ab. (615)
-
personal trainer*.ti,ab. (27)
-
gym.mp. or gyms.ti,ab. [mp = title, original title, abstract, name of substance word, subject heading word] (321)
-
or/30-40 (533,389)
-
8 and (30 or 31 or 34 or 35) (199,653)
-
32 or 33 or 36 or 37 or 38 or 39 or 40 or 42 (238,474)
-
cognitive therapy/ (8971)
-
Counseling/ (22,190)
-
behavior therapy/ (19,855)
-
cognitive therapy/ (8971)
-
behavio?ral intervention*.ti,ab. (2688)
-
(change* adj2 lifestyle*).ti,ab. (3214)
-
(changing adj2 lifestyle*).ti,ab. (169)
-
(lifestyle adj2 modif*).ti,ab. (1993)
-
Hypnosis/ (7452)
-
Counseling/ (22,190)
-
(counseling or counselling).ti,ab. (11,969)
-
or/44-54 (72,017)
-
Randomised Controlled Trials as Topic/ (59,060)
-
randomised controlled trial.pt. (266,956)
-
controlled clinical trial.pt. (78,767)
-
Controlled Clinical Trial/ (78,767)
-
placebos/ (27,688)
-
random allocation/ (63,466)
-
Double-Blind Method/ (100,248)
-
Single-Blind Method/ (12,666)
-
(random* adj2 allocat*).tw. (14,022)
-
placebo*.tw. (114,670)
-
((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. (97,304)
-
Research Design/ (55,162)
-
((random* or control*) adj5 (trial* or stud*)).tw. (340,615)
-
Clinical Trials as Topic/ (141,960)
-
randomly.ab. (128,769)
-
(randomised or randomised).ab. (211,527)
-
Evaluation studies as topic/ (118,080)
-
comparative study/ (1,422,290)
-
(matched communities or matched populations).mp. (105)
-
(control* adj (trial* or stud* or evaluation*)).mp. (474,265)
-
(comparison group* or control* group*).mp. (192,124)
-
Matched-Pair Analysis/ (3158)
-
matched pair*.ti,ab. (3780)
-
Meta-Analysis/ (20,524)
-
meta analy*.ti,ab. (23,386)
-
“Outcome Assessment (Health Care)”/ (31,921)
-
outcome stud*.ti,ab. (4109)
-
intervention studies/ (3959)
-
follow up studies/ (377,220)
-
(systematic* adj (review* or methodolog* or research* or search*)).ti,ab. (20,137)
-
((hand or manual or computer or electronic or database) and search*).ti,ab. (27,863)
-
(hand adj search*).ti,ab. (2016)
-
(medline or embase or Cochrane or cinahl or psychlit or psychinfo or scisearch or pubmed).ab. (34,073)
-
Health technology assessment*.ab,in. (801)
-
(pooled adj analys*).ti,ab. (1661)
-
(electronic* adj search*).ti,ab. (1108)
-
(synthes* adj5 (literature* or research* or studies or data)).ti,ab. (18,568)
-
or/56-92 (2,647,287)
-
12 or 15 (28,964)
-
7 and 93 and 94 (6560)
-
7 and 28 and 93 (9785)
-
7 and 29 and 93 (6480)
-
7 and 41 and 93 (5762)
-
7 and 43 and 93 (4440)
-
7 and 55 and 93 (1554)
-
96 or 98 or 100 (14,089)
-
97 or 99 or 100 (9844)
-
96 and 98 and 100 (395)
-
96 and 98 (2040)
-
96 and 100 (677)
-
98 and 100 (690)
-
104 or 105 or 106 (2617)
-
97 and 99 (1728)
-
97 and 100 (628)
-
99 and 100 (654)
-
108 or 109 or 110 (2250)
-
103 or 107 or 111 (2617)
-
Anti-Obesity Agents/ (1742)
-
(sibutramine or orlistat or rimonabant).ti,ab,nm. (2771)
-
exp Bariatric Surgery/ (7820)
-
exp obesity/su (5586)
-
113 or 114 or 115 or 116 (13,194)
-
112 not 117 (2410)
-
limit 118 to (english language and humans) (2137)
-
limit 119 to (“all infant (birth to 23 months)” or “newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)”) (360)
-
119 not 120 (1777)
-
(editorial or comment or letter).pt. (936,062)
-
121 not 122 (1766)
-
from 123 keep 1-1000 (1000)
-
from 123 keep 1001-1766 (766)
Reference lists
Reference lists of retrieved articles and reviews were hand searched for additional references.
Other searches
Where possible, authors and relevant experts were contacted in order to obtain information about further references, missing data and any ongoing trials.
Appendix 3 Inclusion criteria worksheet for full papers
| First author and reference ID: | Comments/flags | |||
|---|---|---|---|---|
| Participants | ||||
|
Participants classified as overweight or obese (BMI ≥ 25 or ≥ 30 kg/m2)? Note: exclude eating disorders, focus on pre-existing medical condition |
Yes ↓ Next question |
Unclear ↓ Next question |
No → EXCLUDE |
|
|
Participants described as ‘adults’ Note: exclude those < 18 years |
Yes ↓ Next question |
Unclear ↓ Next question |
No → EXCLUDE |
Note if mixture children/adults |
| Design | ||||
|
RCT or systematic review/meta-analysis? If abstract only needs to have sufficient information |
Yes ↓ Next question |
Unclear ↓ Next question |
No → EXCLUDE |
Abstract? Any cost-effectiveness studies? Systematic review? |
| Outcomes | ||||
|
Report one or more of primary outcomes: weight loss, weight change, BMI change from reported baseline weight? Note: other outcomes will also be included if primary outcomes reported |
Yes ↓ Next question |
Unclear ↓ Next question |
No → EXCLUDE |
Any cost outcomes? |
| Intervention | ||||
|
Multicomponent programme? Note: must contain a diet, physical activity, or behaviour change strategy. a Over the counter drug therapy acceptable. Other drug treatments, surgery, or other lifestyle changes exclude |
Yes ↓ Next question |
Unclear ↓ Next question |
No → EXCLUDE |
|
| Comparator of normal practice (defined), single component programme or other structural multicomponent programme? |
Yes ↓ Next question |
Unclear ↓ Next question |
No → EXCLUDE |
|
| Components clearly specified? | ||||
| Diet |
Yes ↓ Next question |
Unclear ↓ Next question |
No | Any linked references with likely detail of intervention: |
| Exercise |
Yes ↓ Next question |
Unclear ↓ Next question |
No | |
| Behavioural |
Yes ↓ Next question |
Unclear ↓ Next question |
No | |
| Follow-up for > 18 months from point of randomisation? |
Yes ↓ Next question |
Unclear ↓ Next question |
No → EXCLUDE |
Length of follow-up? |
| Final decision | INCLUDE |
UNCLEAR (Discuss) |
EXCLUDE | Results of discussion: |
Appendix 4 Quality assessment criteria
| 1. Was the method used to generate random allocations adequate? |
| 2. Was the allocation adequately concealed? |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? |
| 4. Were outcome assessors blinded to the treatment allocation? |
| 5. Was the care provider blinded? |
| 6. Was the participant blinded? |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? |
| (ii) If so, were they explained or adjusted for? |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? |
| 9. (i) Did the analysis include an intention to treat analysis? |
| (ii) If so, was this defined? |
| 10. (i) Did the analysis account for missing data? |
| (ii) If so, were the methods appropriate? |
Appendix 5 Data from included studies
Studies are listed alphabetically.
Burke et al.
| Study details | Participants | Outcome measures | |||
|---|---|---|---|---|---|
|
Author: Burke et al. 71 (Burke et al. 104) Year: 2008 Country: USA Study design: RCT Number of centres: one Funding: National Institutes of Health, Center for Research in Chronic Disorders, Obesity and Nutrition Research Center, Heinz Nutrition Laboratory, General Clinical Research Center, University of Pittsburgh Recruitment dates: three cohorts recruited between September 2002 and May 2004 Setting: university Length of follow-up: 18 months |
Number of participants randomised: 200 Intervention 1 – preference for standard diet (pref STD-D) (n = 48) Intervention 2 – preference for lacto-ovo-vegetarian diet (pref LOV-D) (n = 35) Intervention 3 – no preference for standard diet (no pref STD-D) (n = 48) Intervention 4 – no preference for lacto-ovo-vegetarian diet (no pref LOV-D) (n = 45) Sample attrition/dropout: 44 were lost to follow-up or dropped out of the groups as follows: pref STD-D – 12 pref LOV-D – 7 no pref STD-D – 14 no pref LOV-D – 11 Plus 15 ‘discarded’ from intervention one, and nine excluded after becoming ineligible following randomisation. Total attrition rate was 68 (34%) Attendance at sessions measured: yes Other measures of adherence: yes Sample crossovers: none Inclusion criteria for study entry: Age 18–55 years, BMI of 27–43 kg/m2, willingness to be randomised to one of two treatment preference conditions and one of two dietary conditions, successful completion of a 5-day food diary, willingness and ability to provide informed consent Exclusion criteria for study entry: current medical condition requiring physician supervision of diet or physical activity, physical limitation restricting exercise ability, pregnancy or intention to become pregnant during the study, current treatment with a medication that might affect weight, alcohol intake > 4 drinks/day, participation in a weight loss programme or use of weight loss medication in past 6 months, abstention from eating meat, poultry or fish in the past month Characteristics of participants: Gender, M : F, n (%): pref STD-D – 6 (12.5) : 42 (87.5); pref LOV-D – 7 (20) : 28 (80); no pref STD-D – 6 (12.5) : 42 (87.5); no pref LOV-D – 4 (9) : 41 (91) Age (years), mean (SD): pref STD-D – 43.2 (9.4); pref LOV-D – 44.3 (8.4); no pref STD-D – 43.2 (8.4); no pref LOV-D – 43.2 (8.6) Ethnicity – white : non-white, n (%): pref STD-D – 34 (71) : 14 (29); pref LOV-D – 25 (71) : 10 (29); no pref STD-D – 34 (71) : 14 (29); no pref LOV-D – 31 (69) : 14 (31) Paffenbarger Activity Questionnaire (kilocalories expended/week), mean (SD) 1942.20 (2291.78)a BMI kg/m2, mean (SD): pref STD-D – 34.5 (3.9); pref LOV-D – 34.1 (3.5); no pref STD-D – 32.9 (4.1); no pref LOV-D – 33.7 (4.3) Weight (kg), mean (SD): pref STD-D – 97.2 (12.9); pref LOV-D – 96.7 (12.1); no pref STD-D – 92.4 (16.1); no pref LOV-D – 91.8 (15.4) Baseline data are provided for the following factors, but have not been extracted here: low- and high-density lipoprotein cholesterol, triglycerides, glucose levels, insulin levels, BP, waist circumference, Beck Depression Inventory-II scores, physical and psychological function scores, hunger satiety, weight efficacy lifestyle scores, employment status, educational attainment, marital status, total energy, total fat, carbohydrates, animal protein, vegetable protein and fibre Comorbid conditions, n (%):b coronary heart disease – 2 (1.0); hypertension – 48 (26.4); elevated cholesterol – 35 (19.2); history of emotional/psychological problems – 11 (6.0) % weight lost before starting: not reported |
Primary outcomes: change in body weight from baseline to 18 months Secondary outcomes: BMI, high- and low-density lipoprotein cholesterol, triglycerides, glucose levels, insulin levels, BP, waist circumference Facilitators and barriers: Barriers to Healthy Eating Scale (22-item questionnaire), Correlates of Maintenance to a Low-Fat Diet (25-item scale), Hunger Satiety Scale (6-item), Self-Efficacy in Weight Management (measures of adherence) Methods of assessing outcomes: weight measured on the Tanita Digital Scale. Height measured on a wall mounted stadiometer |
|||
| The PREFER study | |||||
|
Aim or goal: weight loss phase (up to 12 months) based on standard weight loss treatment goal of 1–2 lb per week. Weight maintenance phase (months 13–18) Study hypothesis is that choice of either a standard calorie and fat-restricted diet (STD-D) or a calorie- and fat-restricted (LOV-D) would result in greater weight loss compared with having one of these diets randomly assigned. Secondary hypothesis is LOV-D results in greater weight loss than STD-D |
|||||
| Intervention details | |||||
| Randomised group 1 – dietary preference | Randomised group 2 – no dietary preference | ||||
| Participants choose between STD-D and LOV-D | Participants randomised to STD-D and LOV-D | ||||
| No pref STD-D (n = 48) |
No pref LOV-D (n = 45) |
STD-D (n = 48) |
LOV-D (n = 35) |
||
|
Diet: Details, type of diet: calorie and fat restriction Calories: reduce maximum daily calorie intake to 1200 kcal (women) 1500 kcal (men) for those weighing < 90.5 kg at baseline; 1500 kcal (women) 1800 kcal (men) for those weighing > 90.5 kg at baseline. Minimum daily intake was 1000 kcal Proportions of diet: Reduce fat intake to 25% of total kilocalorie intake, but not less than 10% fat Monitoring: participants recorded their calorie and fat content of foods eaten in a weekly diary. At each session a new diary was provided and completed diaries were collected and returned at the next session after interventionists reviewed and annotated the diaries Exercise: Mode: instruction to exercise given during group meetings, with the actual exercise to be done individually Type: mostly walking Frequency and length of each session and total number sessions: participants encouraged to walk at least 50 minutes per week initially, gradually increasing to at least 150 minutes per week by week 6 Delivered: exercise physiologist Level of supervision: not reported Monitoring: daily recording of exercise in diaries, as above under ‘diet’ Behaviour modification: Mode: group, 10–20 participants Type: standard cognitive behaviour therapy. Based on several models of motivation and behavioural change, such as Social Cognitive Theory Content: environmental modification, problem solving, modelling, relapse prevention, goal-setting, self-monitoring, self-reinforcement, cognitive restructuring, stimulus control, social assertion, and skill development. A cooking class and shopping tour was also given Frequency and length of each session and total number sessions: 32 treatment sessions (lasting 60 minutes) over 12 months. Sessions held in the evening weekly for first 6 months, then every 2 weeks for months 7–9 and monthly for months 10–12 Delivered: master’s prepared dietitian, exercise physiologist, or nurse behavioural scientist. Intervention manuals provided to ensure integrity of protocol Ongoing support: None. After 12 months the maintenance phase began and no further contact was made with participants until the final 18 month assessment Other details: Participants received Cooking Light magazine as an incentive |
Diet: Details, type of diet: calorie and fat restriction, and elimination of meat, poultry and fish consumption by the sixth week. Participants were instructed to eliminate these foods at breakfast, then lunch, then dinner and to record in their diaries when they ate meals containing these foods. Four sessions by a vegetarian nutritionist who advised participants on how to adopt the eating plan as well as including family members. Otherwise the content and behavioural strategies taught were the same as intervention 1 Calories: as intervention 1 Proportions of diet: as intervention 1 Monitoring: as intervention 1 Exercise: As intervention 1 Behaviour modification: As intervention 1 Ongoing support: As intervention 1 Other details: Participants received Vegetarian Times magazine as an incentive |
Diet: As intervention 1 Exercise: As intervention 1 Behaviour modification: As intervention 1 Ongoing support: As intervention 1 |
Diet: As intervention 2 Exercise: As intervention 1 Behaviour modification: As intervention 1 Ongoing support: As intervention 1 |
||
Results
| Outcomes | Pref STD-D (n = 48) | Pref LOV-D (n = 35) | No pref STD-D (n = 48) | No pref LOV-D (n = 45) | p-value, 95% CI |
|---|---|---|---|---|---|
| % weight change (baseline to 18 months), mean (SD) | –3.9 (6.1) | –5.3 (6.2) | –8.0 (7.8) | –7.9 (8.1) | 0.30 |
| Maintenance of weight loss | |||||
| Weight change (baseline to 12 months), kga | –7.6 | –7.9 | –9.7 | –9.7 | |
| Weight change (12–18 months maintenance phase), kga | + 2.9 | + 3.3 | + 2.4 | + 1 | |
| % change in BMI kg/m2, mean (SD) | –3.9 (5.9) | –4.5 (7.4) | –7.8 (7.9) | –7.9 (8.2) | Not reported for between group comparisons |
| Other intermediate outcomes | Between months 6 and 18 there was a significant difference in weight regain between preference groups. Participants who chose their diet (i.e. pref STD-D or pref LOV-D) regained 4.5% (95% CI –5.8 to –3.2), while those with assigned diets (i.e. no pref STD-D or no pref LOV-D) regained 2.1% (95% CI –3.4 to –0.8), p< 0.001 | ||||
| Over time there was no preference × diet interaction, p = 0.34 | |||||
| Comments: results also presented for changes in cholesterol, glucose levels, insulin levels, kilocalorie consumption, fat consumption, carbohydrate consumption, animal protein consumption, vegetable protein consumption, and fibre consumption but not extracted here | |||||
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Unclear |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | No |
| 4. Were outcome assessors blinded to the treatment allocation? | Unclear |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | No |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Yes |
| 9. (i) Did the analysis include an intention to treat analysis? | No |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | No |
| (ii) If so, were the methods appropriate? |
Dubbert and Wilson
| Study details | Participants | Outcome measures | ||||||
|---|---|---|---|---|---|---|---|---|
|
Author: Dubbert and Wilson68 Year: 1984 Country: USA Study design: RCT Number of centres: not reported Funding: not reported. The research was based on the first author’s doctoral dissertation Recruitment dates: not reported Setting: not reported Length of follow-up: 30 months after end of treatment (~ 34 months after randomisation) |
Number of participants: Individual treatment with weekly (distal) goals: number not reported Individual treatment with daily (proximal) goals: number not reported Couples’ treatment with weekly (distal) goals: number not reported Couples’ treatment with daily (proximal) goals: number not reported Total randomised: 62 Sample attrition/dropout: Not reported separately by intervention but stated that attrition was spread evenly across the interventions Completed treatment: overall 47 (75.8%) Completed 30-month follow-up: overall 45 (72.6%) Attendance at sessions measured: yes Other measures of adherence: yes Sample crossovers: none reported. However, the authors observed from questionnaire responses that some participants assigned daily goals reported that they were actually setting weekly goals, and vice versa (sample sizes not reported) Inclusion/exclusion criteria for study entry: Inclusion: responders to newspaper advertisements and public service announcements on local radio; married and currently living with spouse; at least 15 lb overweight (Metropolitan Life Insurance norms, US Department of Health, Education and Welfare); not more than 100% overweight; no medical problems other than obesity; not taking medications affecting appetite or weight; spouse willing to attend at least eight sessions, including four group sessions; physician consultation indicating diet, exercise and step testing were not contraindicated Exclusion: failure to meet the above inclusion criteria; schedule conflicts (assumed to mean participants unable to attend sessions as scheduled because of other commitments); failure to complete the application |
Primary outcomes: weight; percentage of participants overweight; percentage of body fat Secondary outcomes: reported, but not stated explicitly that these were secondary outcomes; data not extracted Cardiovascular fitness; BP; marital satisfaction; spouse weight; spouse co-operation; body image; satisfaction; depression; aerobic fitness; binge eating Facilitators and barriers: none reported Methods of assessing outcomes: weight measured using a balance beam scale. Per cent overweight calculated from Metropolitan Life Insurance Company norms for medium frame men or women of the participants’ height (reference cited). Per cent body fat estimated from skinfold sums (reference cited) Subgroup analyses: none reported |
||||||
| Characteristics of participants (sample sizes, parameter and variance estimates not reported): | ||||||||
| Risk factors noted: none reported | ||||||||
| 1. Individual/distal | 2. Individual/proximal | 3. Group/distal | 4. Group/proximal | |||||
| Weight (units not stated, assumed lb): | 207.7a | 208.9a | 190.4a | 195.0a | ||||
| Per cent overweight: | 53.6a | 51.4a | 47.9a | 39.6a | ||||
| Estimated per cent body fat: | 41.2a | 42.7a | 44.0a | 41.3a | ||||
| Age (years), mean ± SD: | Not reported | Not reported | Not reported | Not reported | ||||
| Gender, M : F (% M : F): | Overall 14 : 48 (23 : 77) (not reported separately by intervention) | |||||||
| BMI kg/m2, n (%): | Not reported | Not reported | Not reported | Not reported | ||||
| % weight lost before starting: | Not reported | Not reported | Not reported | Not reported | ||||
| Duration of overweight/obesity: | Not reported | Not reported | Not reported | Not reported | ||||
| Previous weight loss attempts: | Not reported | Not reported | Not reported | Not reported | ||||
| Physical activity level: | Not reported | Not reported | Not reported | Not reported | ||||
| Ethnicity: | Not reported | Not reported | Not reported | Not reported | ||||
| Socioeconomic position: | Not reported | Not reported | Not reported | Not reported | ||||
| Intervention details | ||||||||
| 1. Individual treatment with weekly (distal) goals: n not reported | 2. Individual treatment with daily (proximal) goals: n not reported | 3. Couples’ treatment with weekly (distal) goals: n not reported | 4. Couples’ treatment with daily (proximal) goals: n not reported | |||||
|
Aim or goal: participants to lose 1 lb weight per week and change selected eating and exercise behaviours (individually set) Diet: Type of diet: calorie restriction in which participants monitored, modelled and reinforced improved eating habits, adherence to self-monitoring and adherence to calorie restriction Frequency and length of each session and total number sessions: as reported below for behaviour modification. Specific weekly (distal) calorie-counting prescriptions given to participants at first individual session (week 5) Level of supervision: small groups supervised by 1–4 therapists but numbers of participants and therapists/group not stated Calories: as for intervention 3 Proportions of diet: not reported Monitoring: as for intervention 3 Exercise: Mode: small groups (size not reported). Individual-based goal-setting Type: as for intervention 3 Frequency and length of each session and total number sessions: as reported below for behaviour modification. Prescription for exercise programme given in week 5 Delivered: as reported below Level of supervision: as reported above for diet Monitoring: weekly records of activities including type, duration and exercise heart rate Behaviour modification: Mode: small groups (size not reported) Type: individual-based problem solving and goal-setting for weight management. No specific instructions for spouse co-operative behaviours were provided (participants attended intervention sessions alone; spouses were involved only in assessment sessions) |
Aim or goal: same as intervention 1 Diet: Type of diet: same as intervention 1 Frequency and length of each session and total number sessions: as reported below for behaviour modification. Specific daily (proximal) calorie-counting prescriptions given to participants at first individual session (week 5) Level of supervision: same as intervention 1 Calories: as for intervention 4 Proportions of diet: not reported Monitoring: as for intervention 4 Exercise: Mode: as for intervention 1 Type: as for intervention 4 Frequency and length of each session and total number sessions: as for intervention 1 Delivered: as for intervention 1 Level of supervision: as for intervention 1 Monitoring: daily records of activities including type, duration and exercise heart rate Behaviour modification: Mode: same as intervention 1 |
Aim or goal: same as intervention 1 but goals set by participant and their spouse Diet: Type of diet: same as intervention 4 Frequency and length of each session and total number sessions: as reported below for behaviour modification. Specific weekly (distal) calorie-counting prescriptions given to participants at first couple session (week 5) Level of supervision: same as intervention 1 Calories: participants were encouraged to set weekly calorie goals, equivalent to the weekly sums of the same number of days’ calories for the proximal goal (intervention 4) (i.e. 8500 kcal for women or 10,675 kcal for men) Proportions of diet: not reported Monitoring: participants recorded their weight weekly; more frequent weighing was discouraged Exercise: Mode: As for intervention 4 Type: as for intervention 4 except that the flexibility of arranging activities to meet a weekly goal was emphasised instead of a daily expenditure goal Frequency and length of each session and total number sessions: as for intervention 1 Delivered: as for intervention 1 Level of supervision: as for intervention 1 |
Aim or goal: same as intervention 3 Diet: Type of diet: calorie restriction in which spouses monitored, modelled and reinforced improved eating habits, adherence to self-monitoring and adherence to calorie restriction Frequency and length of each session and total number sessions: as reported below for behaviour modification. Specific daily (proximal) calorie-counting prescriptions given to participants at first couple session (week 5) Level of supervision: same as intervention 1 Calories: from week 5 recommended 1215 kcal/day for women or 1525 kcal/day for men. Participants were encouraged to set daily calorie goals and to divide these into subgoals for portions of the day (calorie recording forms were designed to assist this) Proportions of diet: not reported Monitoring: participants recorded their weight daily Exercise: Mode: small groups. Exercise goals were set by the participant and their spouse Type: aerobic exercise walking programme with calorie monitoring in which participants monitored, modelled and reinforced improved adherence to exercise. From week 5, daily minimal caloric-expenditure goals were recommended, starting at 145 kcal/day above initial baseline (equivalent to 1.5 mile walk or 1.5 hours active housework). Goals were increased each week by 25 kcal/day (equivalent to walking 5–10 extra minutes), but only if participants met the previous week’s goals on at least 4 days. In addition to calorie expenditure goals, participants were instructed to walk for at least 30 minutes on 5 days/week and to monitor their heart rate so they could exercise in the recommended range for improving fitness (70%–80% of their age-predicted heart rate) (reference cited). Adherence to the walking programme enabled participants to meet ≥ 50% of their daily caloric expenditure requirements |
|||||
|
Content: facts about weight reduction; basic nutrition; techniques for controlling eating; safely increasing exercise; coping with negative emotions and self-defeating cognitions; asserting oneself to obtain necessary support from significant others; importance of keeping records and setting goals. Some of eating behaviour change suggestions based on existing manuals (references cited) Weeks 1–4: education and goal-setting. Participants instructed to identify 1–2 eating and exercise behaviours they wanted to change, and to set goals to accomplish this. Also advised to reduce calorie intake while increasing daily activity Weeks 5–16: implementation of diet, exercise and behaviour goal prescriptions. Participants received an individualised computer printout showing their progress at weeks 5, 10 and post-treatment Frequency and length of each session and total number sessions: Weeks 1–4: 2-hour lectures and small-group discussion meetings Week 5: individual sessions of 15–20 minutes duration commenced, with one/week during weeks 5–7 then fortnightly thereafter. In alternate weeks participants weighed in and their calorie records were collected Delivered: by four (two male, two female) clinical psychology graduate student therapists (one experienced in behavioural weight-control treatment; three had been or were overweight) Monitoring: not reported specifically for behaviour modification component All sessions comprised weigh-in by research assistant, review of previous week’s records, new information or treatment prescriptions, problem-solving discussion concerning actual or anticipated difficulties with meeting calorie intake or expenditure goals, and distribution of new self-monitoring forms Ongoing support: None reported |
Type: individual-based problem solving and goal-setting for weight management with limited spouse support. Same as intervention 1, except that participants were told to solicit support from their spouses and other significant others. With the exception of a brief discussion of assertiveness, no explicit directions were given as to how to get spouse support (participants attended intervention sessions alone; spouses were involved only in assessment sessions) Content: same as intervention 1 Frequency and length of each session and total number sessions: same as intervention 1 Delivered: as for intervention 1 Monitoring: same as intervention 1 Ongoing support: None reported |
Monitoring: same as intervention 1 Behaviour modification: Mode: same as intervention 1 Type: same as intervention 4 Content: same as intervention 1 Frequency and length of each session and total number sessions: same as intervention 1 Delivered: as for intervention 1 Monitoring: same as intervention 4 Ongoing support: None reported |
Frequency and length of each session and total number sessions: same as intervention 1 Delivered: as for intervention 1 Level of supervision: as for intervention 1 Monitoring: same as intervention 2 Behaviour modification: Mode: same as intervention 1 Type: spouse-assisted problem solving and goal-setting for weight management. Participants and their spouses were encouraged to be inventive in applying the techniques discussed during the educational phase to meet their goals. Spouses were instructed to praise their weight-reducing partner for goal attainment and day-to-day adherence to the calorie plan and expenditure. In the presence of the participant, therapists instructed spouses to try to follow the same eating and exercise habit changes prescribed for their partner; educational materials were also provided to spouses. In the first 4 weeks spouses were asked to keep records of their own and of their weight-reducing partner’s adherence. From week 5 onwards couples were asked to identify specific spouse behaviour changes which would assist the weight-reducing partner’s effort Content: same as intervention 1 Frequency and length of each session and total number sessions: same as intervention 1 Delivered: as for intervention 1 Monitoring: as for intervention 1. In addition a simple contract form was provided and spouses were encouraged to make a written as well as verbal commitment to the specified behaviour changes Ongoing support: None reported |
|||||
| Other details | ||||||||
|
Financial deposits/fees/incentives: non-refundable US$15 registration fee. Refundable US$50 deposit with partial or full refunds contingent on the number of sessions and assessments attended Training/supervision of trainers: therapists received 2 hours of training in behavioural weight-control techniques, including role playing interactions with participants and spouses. Throughout the programme they had regular meetings with clinical psychology faculty supervisors (timing not stated). Therapist sessions did not deviate from the treatment protocol (checked by audio-taping sessions) |
||||||||
Results
| Outcomes (n = 47) | 1. Individual; distal goals (n not reported) | 2. Individual; proximal goals (n not reported) | 3. Couples; distal goals (n not reported) | 4. Couples; proximal goals (n not reported) | p-value, 95% CI |
|---|---|---|---|---|---|
| Weight (lb) at 30 monthsa | 200 | 194 | 176 | 185 | Not reported |
| Weight (lb) change from baselinea | –7.7 | –14.9 | –14.4 | –10 | Not reported |
| Facilitators | None reported | None reported | None reported | None reported | None reported |
| Barriers | None reported | None reported | None reported | None reported | None reported |
| Other intermediate outcomes: not reported for 30 months | Attendance at sessions: after excluding dropouts, individual-treatment participants attended on average 16.5 (97%) of the sessions while couples-treatment participants attended 15.5 (91%) of the sessions (t-test, p < 0.05). Spouses in the couples’ treatment attended on average 11.9 sessions (70%) | ||||
| Other measures of adherence: food and exercise calorie self-monitoring records (including records for dropouts) were not reported separately by intervention but indicated | |||||
| Adherence to record keeping was best during week 2 then declined and stabilised for several weeks and then declined rapidly after about week 9. Seventy-five per cent of participants in both spouse-involvement conditions reported adherence to aerobic exercise programmes during the first week but adherence declined thereafter. At 6-month follow-up only half of participants reported at least three exercise sessions/week and at 12-month follow-up exercise adherence was only slightly above pre-treatment level | |||||
| On average calorie records were kept for 10.5 of the 16 weeks | |||||
| 29% of participants followed instructions to record their heart rate during exercise | |||||
| 57.1% of spouses were willing to make written behaviour change contracts (i.e. a notable proportion failed to adhere formally to the goal-setting and contracting components of the couples treatment package) | |||||
| The total numbers of days for which participants recorded calorie intake and output were each significantly associated with weight change during treatment (p< 0.05) | |||||
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Unclear |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | Unclear |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | Not reported |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | No |
| (ii) If so, were the methods appropriate? |
Jeffery et al.
| Study details | Participants | Outcome measures | ||
|---|---|---|---|---|
|
Author: Jeffery et al. 76 Year: 1998 Country: USA Study design: RCT Number of centres: two Funding: National Heart, Lung and Blood Institute Recruitment dates: not given Setting: clinic, possibly university Length of follow-up: 18 months |
Number of participants: abstract states 193, but n’s per group = 196 and description of participants states 196 Standard behavioural therapy (SBT): n = 40 SBT + supervised exercise (SBTE): n = 41 SBT + trainer (SBTT): n = 42 SBT + incentive (SBTI): n = 37 SBT + trainer + incentive (SBTTI): n = 36 Sample attrition/dropout: states that 78% completed the 18-month evaluation, no details of dropout by intervention group Attendance at sessions measured: yes Other measures of adherence: no Sample crossovers: none reported Inclusion/exclusion criteria for study entry: Between 14 and 32 kg overweight according to 1983 insurance industry standards, 25–55 years of age, free of serious diseases, able to walk for exercise and willing to be randomised Characteristics of participants: Any risk factors noted: none reported Gender (M : F), n (%): SBT 7 : 33 (18 : 82); SBTE 7 : 34 (17 : 83); SBTT 9 : 33 (21 : 79); SBTI 5 : 32 (14 : 86); SBTTI 5 : 31(14/86) Age (years), mean (SE): SBT 40.0 (1.3); SBTE 41.5 (1.3); SBTT 41.0 (1.3); SBTI 42.6 (1.4); SBTTI 40.7 (1.4) BMI, kg/m2, mean (SE): SBT 31.4 (0.3); SBTE 31.5 (0.3); SBTT 31.4 (0.3); SBTI 31.5 (0.4); SBTTI 30.6 (0.4) Body weight (kg), mean (SE): SBT 85.6 (1.7); SBTE 87.1 (1.6); SBTT 84.7 (1.6); SBTI 87.7 (1.7); SBTTI 85.7 (1.7) Ever in a weight programme (%): SBT 45; SBTE 71; SBTT 62; SBTI 68; SBTTI 58 Exercise (kcal/week) mean (SE): SBT 681 (103); SBTE 725 (113); SBTT 699 (108); SBTI 768 (128); SBTTI 628 (99) Ethnicity (% white): SBT 82; SBTE 71; SBTT 88; SBTI 73; SBTTI 86 % weight lost before starting: not reported Duration of overweight/obesity: not reported Also reports baseline education status, marital status, energy intake, fat intake, Beck Depression Inventory score, Gormally Binge Eating Questionnaire eating score and perceived barriers to adherence |
Primary outcomes: exercise behaviours (Paffenbarger Physical Activity Questionnaire not extracted here), weight Secondary outcomes: also attendance at walks (where relevant by intervention); habitual energy and fat intake (by Block Food Frequency questionnaire), depression (by Beck Depression Inventory), and binge eating (by Gormally Binge Eating Questionnaire) but these were not data extracted here Facilitators and barriers: perceived barriers to adherence Methods of assessing outcomes: Weight measured on a balance beam scale with participant wearing light clothing without shoes Barriers to adherence were assessed by a 15-item questionnaire devised to assess participants’ perceptions of practical, social, and interpersonal barriers to successful behaviour change. Reference to authors’ own work, unclear if validated in any way |
||
| Intervention details | ||||
| 1. SBT (n = 40) | 2. SBTE (n = 41) | 3. SBTT (n = 42) | 4. SBTI (n = 37) | 5. SBTTI (n = 36) |
|
Aim or goal: not stated Diet: Details: type of diet: not defined Calories: 1000 kcal/day if weight was < 91kg and 1500 kcal/day if weight was ≥ 91 kg Proportions of diet: restrict fat to 20% or less of calories (22 g/day for 1000 kcal and 33g/day for 1500 kcal). Given menus for five breakfasts and dinners per week along with grocery shopping lists Monitoring: recorded calorie and fat intake every day for the first 24 weeks and 1 week per month thereafter Exercise: Mode: assume individual Type: primarily walking or cycling Frequency and length of each session and total number sessions: to exercise to the equivalent of 250 kcal/week and to gradually increase to a minimum of 1000 kcal/week Delivered: by same group leaders as noted below Level of supervision: none specifically Monitoring: recorded distances walked in the daily food record Behaviour modification: Mode: group (approximately 20) Type: not stated Content: stimulus control techniques, problem solving strategies, social assertion, short-term goal-setting and techniques for enhancing motivation, cognitive strategies for altering self-defeating thoughts, relapse prevention, and social support Frequency and length of each session and total number sessions: weekly for 24 weeks and once per month thereafter Delivered: led by trained interventionists with advanced degrees in nutrition or behavioural sciences Ongoing support: Not stated except for as part of the programme described above (monthly meetings after first 24 weeks) |
Aim or goal: not stated Diet: As SBT intervention Exercise: Mode: group and individual Type: primarily walking or cycling. Supervised walking (see below) initially 0.5 miles (0.8 km), increased over first 3 months to 2.5 miles (4.0 km) Frequency and length of each session and total number sessions: to exercise to at least 1000 kcal/week. Regular attendance at supervised sessions would produce approximately 750 kcal/week Delivered: by same group leaders as noted below Level of supervision: mixture of supervised and unsupervised – three supervised walking sessions per week. One at same time and day as group session, two on other days but at the same time of day and location Monitoring: assume as per SBT Behaviour modification: As SBT intervention Ongoing support: As per SBT |
Aim or goal: not stated Diet: As SBT intervention Exercise Mode: group and individual Type: primarily walking or cycling. Supervised walking as per SBTE Frequency and length of each session and total number sessions: assume as per SBTE Delivered: personal trainer (student or staff assistant) assigned to work with three or four participants Level of supervision: The personal trainer walked with the participants, made reminder telephone calls, and scheduled walking sessions to accommodate participants’ own schedules Monitoring: assume as per SBT Behaviour modification: As SBT intervention Ongoing support: As per SBT |
Aim or goal: not stated Diet: As SBT intervention Exercise Mode: group and individual Type: primarily walking or cycling. Supervised walking as per SBTE Frequency and length of each session and total number sessions: assume as per SBTE Delivered: assume as per SBTE Level of supervision: assume as per SBTE. Also financial award based on the number of walks attended at the end of each month. These were modest and increased in value with increments in cumulative attendance. Participants were paid per walk: US$1 for their first 25 walks, US$1.50 for the next 50 walks, US$2 for the next 50 walks, and US$3 for any remaining walks Monitoring: assume as per SBTE Behaviour modification: As SBT intervention Ongoing support: As per SBT |
Aim or goal: not stated Diet: As SBT intervention Exercise Mode: group and individual Type: primarily walking or cycling. Supervised walking as per SBTE Frequency and length of each session and total number sessions: assume as per SBTE Delivered: personal trainer as per SBTT Level of supervision: as per SBTT and SBTI Monitoring: assume as per SBTE Behaviour modification: As SBT intervention Ongoing support: As per SBT |
Results
| Outcomes | SBT (n = 40 at baseline) | SBTE (n = 41 at baseline) | SBTT (n = 42 at baseline) | SBTI (n = 37 at baseline) | SBTTI (n = 36 at baseline) | p-value |
|---|---|---|---|---|---|---|
| Weight change in kg, mean (SE) | –7.6 (1.1) | –3.8 (1.3) | –2.9 (1.1) | –4.5 (1.2) | –5.1 (1.3) | p = 0.03a |
| Perceived barriers | Data not reported | Data not reported | Data not reported | Data not reported | Data not reported | Not significantb |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Unclear |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | Unclear |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | No |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | No |
| (ii) If so, were the methods appropriate? |
Jeffery and Wing
| Study details | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|
|
Author: Jeffery and Wing;75 Jeffery et al. 106 Country: USA Study design: RCT Number of centres: two Funding: National Institutes of Health grants HL41332 and HL41330 Recruitment dates: not reported Setting: not reported Length of follow-up: 30 months |
Number of participants: 202 (equal numbers of men and women), randomised to five groups: Control (C): n = 40 Standard behavioural treatment (SBT): n = 40 SBT + food provision (SBT + FP): n = 40 SBT + incentives (SBT + I): n = 41 SBT + FP + I: n = 41 Sample attrition/dropout: 177 (88%) completed the 30-month follow-up evaluation. No differences among treatment groups, centres, or sex in the per cent of participants lost to follow-up. Number by treatment group not reported. 85% (172, calculated by reviewer) completed the 18-month follow-up. Overall attrition at 6 and 12 months also reported Attendance at sessions measured: yes Other measures of adherence: yes Sample crossovers: not reported Inclusion/exclusion criteria for study entry: between 14 kg and 32 kg overweight according to 1983 insurance industry standards, 25–45 years of age, non-smokers, drank fewer than three alcoholic drinks per day, not on a special diet or allergic to any foods, able to exercise, free of current serious disease, not taking prescription medications including oral contraceptives, and agreeable to conditions of participation Characteristics of participants: Any risk factors noted: no Paper does not indicate if data reported are means, and no measures of variance reported Gender (M : F), n (%): not reported by group Age (years): C 35.7; SBT 37.5; SBT + FP 38.5; SBT + I 38.1; SBT + FP + I 37.6 BMI (kg/m2) C 31.1; SBT 30.9; SBT + FP 30.8; SBT + I 31.1; SBT + FP + I 31.1 Mean weight (kg): C 88.2; SBT 89.4; SBT + FP 88.1; SBT + I 92.3; SBT + FP + I 91.1 % weight lost before starting: not reported Duration of overweight/obesity: not reported Previous weight loss attempts (%): C 50.0; SBT 57.5; SBT + FP 62.5; SBT + I 63.4; SBT + FP + I 58.5 Physical activity level (kcal/week): C 1032.4; SBT 1445; SBT + FP 820; SBT + I 1,103; SBT + FP + I 1039 Ethnicity white (%): C 92.5; SBT 87.5; SBT + FP 97.5; SBT + I 90.2; SBT + FP + I 92.7 Socioeconomic position: not reported Pre-existing medical condition: not reported p-values reported, all not statistically significant Baseline data for each group also reported on: non-college graduate; married, weight, nutrient intake (kcal/day; calories from fat); total barriers to adherence; eating behaviours inventory; knowledge (15-item test; calorie estimates) |
Primary outcomes: change in obesity (weight and BMI) Secondary outcomes: nutrient intake (total energy intake, % of energy from fat); exercise – not data extracted Facilitators and barriers: process measures (potential mediators of weight change) were assessed – attendance at treatment sessions and weigh-ins; adherence; perceived barriers to adherence; adherence to behavioural weight control strategies; nutritional knowledge Methods of assessing outcomes: Adherence: calculated from completion of the 7-day food diaries that were requested at each group treatment session. The number of completed days was divided by the number of assigned days. No indication that this measure was validated in any way Perceived barriers to adherence: derived from a 15-item questionnaire designed specifically for this study. Covered practical and motivational barriers rated on a 5-point scale from ‘not at all a problem for me (1)’ to ‘a very important problem for me (5)’ Adherence to behavioural weight control strategies: the 26-item eating behaviours inventory of weight control practices (reference provided) Nutritional knowledge: a 15-item multiple-choice true–false test, and a test to estimate the caloric content of 22 food items |
||||
| Intervention details: study reports a weight management (weight loss) intervention (duration of intervention 18 months) with participants followed up for a further year after the end of the intervention (to determine how well weight loss maintained, but no intervention or contact with study staff in this period) | ||||||
| Intervention details | ||||||
| SBT (n = 40) | Control (n = 40) | SBT + FP + I (n = 41) | SBT + FP (n = 40) | SBT + I (n = 41) | ||
|
Aim or goal: subjects selected a weight loss goal (14, 18 or 23 kg) to try to achieve during the programme. Exercise goals increased to a final goal of 1000 calories a week Diet: Details, type of diet: emphasised the importance of remaining below caloric goals, but restriction of fat and increasing consumption of complex carbohydrates also stressed Calories: either 1000 or 1500 calories per day on the basis of baseline body weight. Goal derived by multiplying baseline body weight by 12, subtracting 1000 calories per day, and rounding to the closest caloric goal to produce an estimated weight loss of about 1 kg per week Proportions of diet: not stated Monitoring: recorded caloric intake in daily food records for the first 20 weeks and for 1 week each month thereafter Subjects who reached their weight loss goal during treatment had their caloric goals adjusted upward to a level estimated to maintain this body weight Exercise: Mode: individual (not explicitly stated) Type: based on walking or cycling Frequency and length of each session and total number sessions: an amount equivalent to 50 calories per day for 5 days a week Delivered: self-directed (not explicitly stated) Level of supervision: none (not explicitly stated) Monitoring: recorded distances walked or duration of bicycling Behaviour modification: Mode: group of about 20 Type: not stated Content: included stimulus control techniques, problem solving strategies, social assertion, short-term goal-setting and reinforcement techniques for enhancing motivation, cognitive strategies for replacing negative thinking, relapse prevention, social support Frequency and length of each session and total number sessions: met weekly for first 20 weeks and then once a month Delivered: trained interventionists with advanced degrees in nutrition or the behavioural sciences Ongoing support: Not specifically mentioned but content of group meetings seem to fill this role Mode: group of about 20 Type: behavioural counselling. Included a weigh-in, presentations of information by the interventionist, group discussion and a review of progress. During the period of monthly group sessions, participants were also encouraged to attend weekly weigh-in session to monitor progress Frequency and length of each session and total number sessions: met weekly for first 20 weeks and then once a month Other details None |
No intervention. Participants could do whatever they wished to lose weight on their own |
Aim or goal: as described for SBT group Diet: as described for SBT group Exercise: as described for SBT group Behaviour modification: as described for SBT group Ongoing support: as described for SBT group Other details Food provision: food provided for five breakfasts and five dinners a week. Pre-packaged breakfasts consisted of cereal, milk, juice, and fruit. Dinners typically consisted of lean meat, potato or rice, and vegetable. For 1 or 2 days a week, a frozen dinner, such as a Weight Watchers® or Lean Cuisine meal, was provided. A meal plan outlined what foods were to be eaten for which meals. Recipes were provided to guide food preparation. Recommendations for lunches were provided Incentives: cash payments received based on the amount of weight lost each week in relation to the weight loss goal. Minimum payment of US$2.50 if participants did not gain weight; US$12.50 if weight loss was 50% of goal. Maximum of US$25 if goal reached and maintained. Incentives paid weekly by cheque at time of weigh-in |
Aim or goal: unclear if meeting same goals as other group. Diet: as described for SBT group Exercise: as described for SBT group Behaviour modification: as described for SBT group Ongoing support: as described for SBT group Other details: Food provision: as described for SBT + FP + I group |
Aim or goal: weight loss goals as SBT + FP + I. Unclear if had the same exercise goal Diet: identical to SBT but without any FP Exercise: as described for SBT group Behaviour modification: as described for SBT group Ongoing support: as described for SBT group Other details Incentives: as described for SBT + FP + I group |
||
Results
| Outcomes | SBT + FP + I (n = 41) | SBT+I (n = 41) | SBT + FP (n = 40) | SBT (n = 40) |
Control (n = 40) | p-value, 95% CI |
|---|---|---|---|---|---|---|
|
Weight change, baseline to 18 months, mean kg, n contributing data (data estimated from figure and n contributing data calculated by reviewer) |
–6.4, n = 34 | –4.0, n = 35 | –6.6, n = 36 | –4.6, n = 26 | 0.0, n = 28 | Not reported |
| Weight loss, baseline to 30 months, meana kg (SD) | 1.6 (6.3) | 1.6 (5.5) | 2.2 (6.6) | 1.4 (7.2) | Gain 0.6 (5.3) | No overall difference between treatment groups ANOVA p > 0.45 |
| Loss of ≥ 9 kg from baseline to 30 months, % of participants |
Ranged from 8% to 17% in the active treatment groups No detail by group reported. |
0% | Not tested | |||
|
BMI kg/m2 – baseline, n 18 months, n (calculated by reviewer) |
31.26, n = 41 28.95, n = 34 |
30.77, n = 41 29.28, n = 35 |
30.66, n = 40 28.17, n = 36 |
30.85, n = 40 29.10, n = 26 |
30.88, n = 40 30.67, n = 28 |
Not reported |
| Maintenance of weight loss | The proportion maintaining some weight loss ranged from 51% to 73% | 53% | Not tested | |||
| Other intermediate outcomes: | The post hoc planned contrast analyses indicated an effect, comparing all treatment groups with the no-treatment group, which approached conventional levels of statistical significance, (F1, 157 = 3.14, p < 0.08). No adjustment of p-value for significance due to multiple comparisons however. Mean weight losses of the SBT groups (all SBT groups) were 4.1 kg at 18 months; in the groups provided with food, mean weight losses increased to 6.4 kg at 18 months. For 18 months, data are based on the analysis restricted to subjects who attended all assessment sessions. The percentage of participants who completed all three follow-ups to provide 18 month data differed by treatment group (p = 0.03) | |||||
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Not reported |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | Not reported |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Yes – some outcomes reported at 18 months, not reported on at 30 months |
| 9. (i) Did the analysis include an intention to treat analysis? | Not reported |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | Not reported |
| (ii) If so, were the methods appropriate? |
Logue et al.
| Study details | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|
|
Author: Logue et al. 72 Year: 2005 Country: USA Study design: RCT Number of centres: 15 primary-care practices Funding: study supported by Agency for Healthcare Research and Quality and the National Institute of Diabetes, Digestive, and Kidney Diseases grants and by Nutrition and Exercise grants from the Summa Health System Foundation Recruitment dates: not reported; study conducted July 1998 to December 2002 Setting: primary care Length of follow-up: 18 and 24 months after randomisation |
Number of participants: Transtheoretical Model and Chronic Disease Paradigm (TM-CD): 329 Augmented usual care (AUC): 336 Total randomised: 665 Sample attrition/dropout: Attrition, n (%) 18 months: TM-CD: 123 (37); AUC: 155 (46) Attrition, n (%) 24 months: TM-CD: 103 (31); AUC: 127 (38) Attendance at sessions measured: not reported Other measures of adherence: not reported Sample crossovers: none reported Inclusion/exclusion criteria for study entry: Inclusion: Participants of one of the primary-care practices affiliated with the study; had to provide written informed consent; men and women, 40–69 years of age; elevated BMI (> 27 kg/m2) or elevated waist-to-hip ratio (> 0.95 for men or > 0.8 for women) Exclusion: No access to a telephone; difficulty understanding eight-grade spoken or written English; pregnancy, lactation, or < 6 months post partum; use of a wheelchair for mobility; high-risk participants with severe heart or lung disease |
Primary outcome: Change in body weight Secondary outcomes (data not extracted): Waist girth; blood lipids; BP; behavioural and cognitive-based estimates of daily energy intake and total energy expenditure; psychosocial assessments including self-efficacy, social support, decisional balance for healthy eating and exercise; general physical and mental health; social desirability; anxiety, depression; binge-eating disorder; stages of change. Facilitators and barriers: none explicitly assessed Methods of assessing outcomes: Weight measured using a standardised calibrated scale Subgroup analyses: none |
||||
| Characteristics of participants: | ||||||
| Risk factors noted: none | ||||||
| TM-CD (n = 329) | AUC (n = 336) | 95% CI of difference | ||||
| Gender (M : F) (%): | 97 : 232 (29 : 71)a | 110 : 226 (33 : 67) | –3.8 to 10 (for number of men) | |||
| Age group (years), n (%) | ||||||
| 40–49: | 139 (42) | 129 (38) | –11 to 3.6 | |||
| 50–59: | 138 (42) | 141 (42) | –7.5 to 7.5 | |||
| 60–69: | 52 (16) | 66 (20) | –2.0 to 9.6 | |||
| Weight (kg), mean ± SD | Not reported | Not reported | Not reported | |||
| Total number of minutes exercised | Not reported | Not reported | Not reported | |||
| Energy expenditure | Not reported | Not reported | Not reported | |||
| BMI kg/m2, n (%) | ||||||
| 25–29.9 | 59 (18) | 73 (22) | –2.3 to 9.8 | |||
| 30–34.9 | 119 (36)a | 107 (32) | –12 to 2.9 | |||
| 35–39.9 | 69 (21) | 82 (24) | –2.9 to 9.8 | |||
| 40.0 + | 79 (24) | 74 (22) | –8.4 to 4.4 | |||
| % weight lost before starting | Not reported | Not reported | Not reported | |||
| Duration of overweight/obesity | Not reported | Not reported | Not reported | |||
| Previous weight loss attempts n (%) | 306 (93)a | 303 (90)a | –7.0 to 1.4 | |||
| Previous commercial weight loss programmes, n (%) | 147 (45)a | 155 (46)a | –6.1 to 9.0 | |||
| Physician said to lose weight, n (%) | 246 (75)a | 262 (78)a | –3.3 to 9.7 | |||
| Ethnicity: n (%) African American | 88 (27)a | 87 (26)a | –7.5 to 5.8 | |||
| Socioeconomic position | Not reported | Not reported | Not reported | |||
| Prior/current psychotropic medicine, n (%) | 85 (26) | 79 (24) | –8.9 to 4.2 | |||
| Hypertension, n (%) | 138 (42)a | 151 (45)a | –4.5 to 11 | |||
| Elevated blood cholesterol, n (%) | 107 (33)a | 115 (34)a | –5.5 to 8.9 | |||
| Diabetes, n (%) | 41 (12)a | 51 (15)a | –2.5 to 8.0 | |||
| Intervention details | ||||||
| 1. TM-CD (n = 329) |
2. AUC: (n = 336) |
|||||
|
Aim or goal: not explicitly reported Diet: Type of diet: based on either the United States Department of Agriculture (USDA) Food Guide Pyramid (Dietary Guidelines for Americans) or a standard prescription to reduce calories, increase fruit and vegetables, and reduce fat Frequency and length of each session and total number sessions: 10 minutes of in-person counselling (not stated whether group or individual) once every 6 months and mean of 15 minutes telephone counselling every month Level of supervision: no further details reported Calories: not reported (consult the materials referred to above) Proportions of diet: not reported (references given; see above) Monitoring: participants were asked to provide dietary data every 6 months and other information as reported below for behaviour modification Exercise: Mode: not reported whether individual or group contact Type: included a standard prescription to increase activity and exercise but no details provided Frequency and length of each session and total number sessions: as reported above for diet Delivered: by registered dietician (RD) and weight loss advisor (WLA). The RD prepared written exercise prescriptions based on the information from dietary recalls. The WLA provided phone counselling Level of supervision: no further details reported Monitoring: participants were asked to provide exercise data every 6 months and other information as reported below for behaviour modification Behaviour modification: Mode: not reported, however, assume from description of telephone calls that is individual Type: behavioural techniques based on TM-CD were taught consistent with Prochaska’s description of the relationship between the processes of change and the stages of change (SOC) for increasing five target behaviours (exercise, usual activity, dietary portion control, dietary fat control, fruit and vegetable intake) Content: participants were mailed stage- and behaviour-matched workbooks that corresponded to their most recent SOC profile as identified by monitoring. Content of WLA telephone contacts not reported |
Aim or goal: not explicitly reported Diet: Type of diet: based on either the USDA Food Guide Pyramid (Dietary Guidelines for Americans) or a Soul Food Guide Pyramid. Included a standard prescription to reduce calories, increase fruit and vegetables, and reduce fat Frequency and length of each session and total number sessions: 10 minutes of counselling once every 6 months Level of supervision: no further details reported Calories: not reported (references given) Proportions of diet: not reported (references given) Noted that participants were advised to discuss their lipid and BP values with their primary care physician, but not stated whether or how the results of such discussions influenced the diet Monitoring: participants were asked to provide anthropometric and dietary data every 6 months Exercise: Mode: not reported whether individual or group contact Type: not reported Frequency and length of each session and total number sessions: as reported above for diet Delivered: by a registered dietitian who prepared written exercise prescriptions based on the information from the exercise recalls Level of supervision: no further details reported Monitoring: participants were asked to provide exercise data every 6 months Behaviour modification: Mode: not reported whether individual or group contact Type: not reported Content: counselling based on 6-monthly review of diet, exercise and anthropometric monitoring, consistent with behavioural self-monitoring principles |
|||||
|
Frequency and length of each session and total number sessions: as reported above for diet Delivered: by a RD and a WLA trained to apply the processes of change that corresponded to a participant’s SOC profile. The RD prepared written dietary prescriptions based on the information from dietary recalls. The primary-care physicians were expected to counsel participants on obesity issues but only when issues were raised by participants or at infrequent (one to three times/year) chronic disease visits (diabetes check-ups). Overall, physicians had little involvement (6% of participants) in dietary, exercise or weight issues Monitoring: formal evaluation for anxiety, depression and binge eating disorder every 6 months. A SOC assessment for five behaviours was completed every 2 months (references cited). Self-monitoring by participants was recommended but self-monitoring records were not reviewed by the physician or the WLA Ongoing support: Upon request, the WLA mailed participants with public domain handouts and other materials (menu suggestions, mall walking impacts, descriptions of local walking trails) Other details: Financial incentives: participants were paid US$25 for completing each postbaseline assessment (6, 12, 18 and 24 months) Training/supervision of trainers: a part-time pharmaceutical representative was trained to provide academic detailing to physicians on the use of the SOC profiles, the processes of change, and how to use a SOC flip chart when counselling participants in the examination room. The project psychologist (KS) monitored implementation of the WLA telephone protocol and periodically debriefed the WLAs and advised WLAs how to interact with problematic participants |
Frequency and length of each session and total number sessions: as reported above for diet Delivered: as reported above for exercise Monitoring: as reported for diet and exercise. (No evaluation was carried out for anxiety, depression and binge eating disorder as it was considered unethical to do this without informing the primary care physicians) Ongoing support: None reported Other details Financial incentives: participants were paid US$25 for completing each postbaseline assessment (6, 12, 18 and 24 months) |
|||||
Results
| Outcomes | 1. TM-CD: n = 226 unless stated | 2. AUC: n = 209 unless stated | Difference (TM-CD – AUC) 95% CI, p-value |
|---|---|---|---|
| Mean (SE), 95% CI weight change (kg) from baseline to 24 monthsa |
–0.39 (0.38), –1.1 to 0.4 For adjusted differenceb n = 271 |
–0.16 (0.42), –1.0 to 0.7 For adjusted differenceb n = 266 |
Unadjusted difference 0.23 kg –1.4 to 0.9, p = 0.50 (NS) Adjusted differencec 0.22 kg (CI and p-value not reported) Adjusted differenceb 0.21 kg (CI and p-value not reported) |
| Facilitators | None explicitly reported | None explicitly reported | |
| Barriers | None explicitly reported | None explicitly reported |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Yes |
| 2. Was the allocation adequately concealed? | Unclear |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Was the care provider blinded? | Unclear |
| 6. Was the participant blinded? | Unclear |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | No |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | Yes |
| 9. (i) Did the analysis include an intention to treat analysis? | Yes |
| (ii) If so, was this defined? | Yes |
| 10. (i) Did the analysis account for missing data? | Yes |
| (ii) If so, were the methods appropriate? | Yes |
Simkin-Silverman et al.
| Study details | Participants | Outcome measures |
|---|---|---|
|
Author: Simkin-Silverman et al. 73,100,105,107 Year: 1998 Country: USA Study design: RCT Number of centres: one Funding: National Heart, Lung and Blood Institute Recruitment dates: August 1992 to March 1994 Setting: clinic (unclear whether university clinic) Length of follow-up: 54 months |
Number of participants: 535 randomised. Lifestyle intervention (LI) n = 260, control (C) n = 275. Only results for the subgroups classified as overweight or obese at baseline are reported here (LI n = 117; C n = 131) Sample attrition/dropout: 509 attended 54-month visit and were analysed. Fourteen participants missing from the LI, and 12 from the C (reasons given) Attendance at sessions measured: yes Other measures of adherence: yes Sample crossovers: not reported Inclusion/exclusion criteria for study entry: aged 44–50 years, < 3 months amenorrhea in 6 months prior to initial interview; not taking hormone replacement therapy; no surgically induced menopause (hysterectomy or bilateral oophorectomy); diastolic BP < 95 mmHg; BMI between 20 and 34 kg/m2; fasting glucose < 140 mg/dl; not taking any lipid-lowering agents, insulin, thyroid, antihypertensive, psychotropic medications; not treated for cancer in the past 5 years; not having participated in a weight reduction programme within the past 4 months Characteristics of participants: Any risk factors noted: none, other than baseline values for high- and low-density lipoprotein cholesterol, triglycerides, BP, menopausal status during follow-up Gender (M : F), n (%): 100% female Age (years), mean (SD): LI = 47 years (SD = 2); C = 47 (SD = 2) Mean BMI, kg/m2: LI = 25 (SD = 3); C = 25 (SD = 3) 53.6% (287/535) were normal weight (BMI ≤ 24.9 kg/m2) (LI = 143; C = 144) 35.5% (190/535) were overweight (BMI = 25–29.9 kg/m2) (LI = 95; C = 95) 10.8% (58/535) were obese (BMI ≥ 30 kg/m2). (LI = 22; C = 36) Weight lb, mean (SD): LI 148.0 (21.3); C 147.6 (21.9) Weight kg, mean (calculated by reviewer): LI 67.1; C 67.0 % weight lost before starting: not reported Duration of overweight/obesity: not reported Previous weight loss attempts: not reported Physical activity level (kcal/wk): LI = 1248 (SD = 1064); C = 1412 (SD = 1386) Ethnicity: white LI = 92.1%; C = 91.8% Socioeconomic position: educated beyond high school (LI = 83.2%; C = 86.2%), employed for wages (LI = 86.2%; C = 86.1%) |
Primary outcomes: weight, body fat distribution, and body composition. Measured in terms of BMI, waist-to-hip ratio and changes in fat-free mass (FFM) (Note: not consistently reported which outcomes were primary) Secondary outcomes: Physical activity, nutrient intake Lipids, BP, glucose levels, cigarette smoking, alcohol intake, menopausal status (not data extracted) Facilitators and barriers: not reported Methods of assessing outcomes: Weight measured with balanced beam scale Height measured by a stationary vertical height board |
| Intervention details | ||
| 1. Intervention 1 (n = 260): LI (Women’s Healthy Lifestyle Project) | 2. Intervention 2 (n = 275): | |
|
Aim or goal: to prevent naturally occurring weight gain and sustain baseline lipid profiles during the perimenopausal to postmenopausal transition. Two phases – phase 1 (weeks 1–20) focus on modest weight loss, described as the intensive phase. Phase 2 (months 6–54) continued focus on weight loss, but also then weight stabilisation and maintenance Phase 1 included 10 weekly group meetings followed by biweekly meetings for the remaining 10 weeks (in total there were 15 group meetings with approximately 20 women per group). Phase 2 – following the initial 5 months group meetings occurred at months 6, 7, 8, 10, 12 and 14. Participants attended refresher programmes on nutrition, weight control and physical activity between months 14 and 54 (no detail on frequency of these sessions) Diet: Details, type of diet: reduced fat and calorie diet (also lipid-lowering dietary strategies). Weight loss goals were tailored to baseline BMI. Women with BMI of 25 to 26 kg/m2 were given 10 lb and women with a BMI or ≥ 27 kg/m2 were given 15 lb weight loss goals. Women with normal weight (BMI ≤ 24 kg/m2) were asked to lose 5 lb Calories: participants were given a 1300–1500 kcal meal plan (for first month). As participants met their weight goal their caloric intake was gradually increased until weight stabilised Proportions of diet: lowering of dietary fat to 25% of daily calories, saturated fat to 7%, and dietary cholesterol to 100 mg/day (for first month) Monitoring: self-monitoring daily using 7-day pocket diaries for 6 months Exercise: Mode: group meetings Type: recommended activities: walking, aerobic dance, cycling, swimming, strength training Frequency and length of each session and total number sessions: phase 1 (10 weekly group meetings followed by 10 biweekly meetings). At the third week participants instructed to increase physical activity in step-wise manner to expend 1000 kcal per week. Women already active but expending < 1500 kcal per week were encouraged to gradually increase activity to 1500 kcal. Women already expending 1500 kcal encouraged to maintain this level. During phase 2 there were refresher meetings which covered physical activity, among other things Delivered: behavioural psychologists and nutritionists Level of supervision: appears that participants supervised themselves largely, but there were regular group meetings in phase 1 and in phase 2 there was regular mail and telephone contact Monitoring: self-monitoring on a daily basis for first 6 months Behaviour modification: Mode: group (approximately 20 women per group) Type: mentions that it is an empirically-based cognitive behavioural approach to weight control, citing two references, one of which is the NIH Clinical Guidelines on obesity in adults, the other being a chapter in a handbook of obesity Content: included the following: stimulus control, goal-setting, self-monitoring, modelling, problem solving, assertiveness training, relapse prevention, and cognitive and motivational techniques. For instance, participants were taught to identify cues in their environment to promote healthy eating and activity. They were instructed on how to set realistic goals and extensive time was spent on problem solving within the group. The coping strategies taught were based on the relapse prevention model Frequency and length of each session and total number sessions: not explicit whether each of the weekly/biweekly sessions included behavioural approaches, but presume that most of the psychological techniques were taught during phase 1. Additional behavioural skills, support and motivation was provided in phase 2, where sessions focused on adherence, emotions and eating Delivered: behavioural psychologists and nutritionists Ongoing support: After month 14 (in phase 2) participants were offered 6-week refresher programmes, specifically to help with maintenance of behaviour change Mode: presume group Type: individual or small group consultation was provided to those who experienced a rise in weight gain during phase 2. Mail and telephone contact (newsletter, self-monitoring diaries) also used Frequency and length of each session and total number sessions: not stated Other details: Incentives and lotteries were used periodically for healthy lifestyle prizes to enhance attendance at group programmes and to encourage return of self-monitoring diaries Other features of the intervention included: cooking demonstrations, and low-fat taste panels Calcium supplementation (1200 mg/day) was given to offset any decreases in calcium during weight loss |
Assessment only control group (received a health education pamphlet on reducing cardiovascular risk factors and for those who were smokers, advice to quit) | |
Results
| Outcomes | |||
|---|---|---|---|
| Subset of participants overweight at baseline (BMI = 25–29.9 kg/m2) | LI (n = 95) | C (n = 95) | p-value between groups |
| Weight change, mean kg (SD) % of initial weight lost 18 months | –3.5 (5.8) –4.6a | 0.1 (4.0) 0.07 | p < 0.001 |
| Weight change, mean kg (SD) % of initial weight lost 30 months | –2.7 (5.4) –3.5 | 0.3 (5.1) 0.41 | p < 0.001 |
| Weight change, mean kg (SD) % of initial weight lost 42 months | –1.4 (5.7) –1.7 | 1.3 (5.5) 1.9 | p < 0.001 |
| Weight change, mean kg (SD) % of initial weight lost 54 months | 0.1 (6.1) 0.31 | 1.5 (5.2) 2.2 | Not significant |
| Subset of participants obese at baseline (BMI ≥ 30 kg/m2) | LI (n = 22) | C (n = 36) | p-value between groups |
| Weight change, mean kg (SD) % of initial weight lost 18 months | –6.6 (8.4) –7.7 | –0.5 (4.5) –0.36 | p < 0.01 |
| Weight change, mean kg (SD) % of initial weight lost 30 months | –4.3 (6.7) –5.0 | 2.9 (5.4) 3.5 | p < 0.01 |
| Weight change, mean kg (SD) % of initial weight lost 42 months | –2.0 (6.4) –2.3 | 1.9 (5.7) 2.5 | p < 0.05 |
| Weight change, mean kg (SD) % of initial weight lost 54 months | –0.2 (6.9) –0.17 | 3.1 (7.7) 3.7 | Not significant |
| Percentage of participants at or below baseline weight at 54 months – (subset overweight at baseline) | 57.3% (51/89) | Not reported | p = 0.352b |
| Percentage of participants at or below baseline weight at 54 months – (subset obese at baseline) | 40% (8/20) | Not reported | |
| Facilitators | Not reported | Not reported | Not reported |
| Barriers | Not reported | Not reported | Not reported |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Yes |
| 2. Was the allocation adequately concealed? | Yes |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Yes |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | No |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | Yes |
| (ii) If so, was this defined? | Yes |
| 10. (i) Did the analysis account for missing data? | No |
| (ii) If so, were the methods appropriate? |
Skender et al.
| Study details | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|
|
Author: Skender et al. 79 (Baseline population characteristics reported by Foreyt et al. 108) Year: 1996 Country: USA Study design: RCT Number of centres: not reported Funding: study supported in part by research grant DK30921 from the National Institutes of Health, Bethesda, MD Recruitment dates: not reported Setting: not reported. Participants were recruited from an urban area of Houston, TX Length of follow-up: 2 years after randomisation (1 year after end of treatment) |
Number of participants: Diet and exercise (D + E): n = 42 Exercise only (E only): n = 43 Diet only (D only): n = 42 Waiting list control group (no data reported for these): n = 38 Total randomised: n = 165 Sample attrition/dropout: Numbers reported but not reasons Completed 1-year treatment: n = 86/127 (68%) Completed 2-year follow-up: Statistically significant differences between groups (overall p = 0.03; difference between diet and exercise groups p = 0.014) D + E: 21 (50%) D only: 15 (35.7%) E only: 25 (58%) Attendance at sessions measured: not reported Other measures of adherence: yes Sample crossovers: none reported Inclusion/exclusion criteria for study entry: men and women aged 25–45 years; volunteers recruited through (unspecified) media announcements; at least 14 kg overweight (Metropolitan Insurance height-weight tables); not engaged in regular exercise. No exclusion criteria specified |
Primary outcomes: (Reported, but not stated explicitly that this was a primary outcome): changes in body weight Secondary outcomes: (Reported, but not stated explicitly that these were secondary outcomes; not data extracted): attitudes to diet and exercise; adherence to diet and exercise; physical activity (1 year); % body fat (1 year); cardiorespiratory fitness (1 year) Facilitators and barriers: none explicitly assessed Methods of assessing outcomes: Weight measured using a balance beam scale Subgroup analyses: none. Note that attrition was reported separately by gender |
||||
|
Characteristics of participants: Risk factors noted: none Gender, M : F (%): 1. D + E: baseline: 21 : 21 (50 : 50) n = 42; 2-year follow-up: 10 : 11 (48 : 52) n = 21 2. D only: baseline: 22 : 20 (52 : 48) n = 42; 2-year follow-up: 9 : 6 (60 : 40) n = 15 3. E only: baseline: 23 : 20 (53 : 47) n = 43; 2-year follow-up: 13 : 12 (52 : 48) n = 25 Baseline characteristics Reported by Foreyt et al. 108 for 86 participants who completed treatment Weight (kg), mean ± SD: 1. (D + E): 97.60 ± 25.48 (n = 27); 2. (D only): 97.65 ± 21.96 (n = 29); 3. (E only): 93.92 ± 20.83 (n = 30) (stated NS in text) Reported by Skender et al. 79 for 61 participants who completed 2-year follow-up (not reported for all randomised participants): Weight (kg), mean ± SD: 1. (D + E): 100.1 ± 27.4 (n = 21); 2. (D only): 98.5 ± 25.9 (n = 15); 3. (E only): 93.7 ± 21.1 (n = 25) (p = 0.66; NS) Baseline characteristics for all participants not reported. Foreyt et al. 108 reported only for unspecified cohorts of the population (sample sizes variable but unexplained; data not extracted): % body fat, mean ± SD: reported for unspecified cohort only (D + E: n = 24; D: n = 22; E: n = 27) Waist circumference (cm), mean ± SD: reported for unspecified cohort only (D + E: n = 24; D: n = 23; E: n = 27) Total number of minutes exercised: reported for unspecified cohort only (D + E: n = 15; D: n = 18; E: n = 17) Energy expenditure: reported for unspecified cohort only (D + E: n = 15; D: n = 17; E: n = 16) Age: not reported BMI, kg/m2: not reported % weight lost before starting: not reported Duration of overweight/obesity: not reported Previous weight loss attempts: not reported Physical activity level: not reported Ethnicity: not reported Socioeconomic position: not reported |
||||||
| Intervention details | ||||||
| 1. D+E (n = 42) |
2. E only (n = 43) |
3. D only (n = 42) |
||||
|
Aim or goal: not explicitly reported Diet: Type of diet: participants were instructed to plan their daily meals and snacks from the foods recommended in the HYHEP, a nutritionally adequate, well-balanced low-cholesterol diet (reference cited). A table listing the calorie content of popular foods was provided. Instructors advised participants to adjust their caloric intake so that weight loss would not exceed 1 kg/week. Class instructors reviewed the food records weekly and returned them to participants at the next class Frequency and length of each session and total number sessions: 12 weekly group instructional sessions followed by three biweekly sessions then eight monthly maintenance sessions (total 1 year). (Note discrepancy in text.) The group sessions were 60 minutes long, delivered as reported below for exercise Calories: not reported Proportions of diet: to provide 30% of calories as fat, 50% as carbohydrate, and 20% as protein, based on HYHEP Monitoring: daily food intake was monitored by participants recording the food eaten and calorie content of each portion in food diaries and (separately?) completing a self-monitoring questionnaire about diet (no details reported) Exercise: Mode: groups of approximately 15 participants Type: lecture and discussion focused on the physical and psychological benefits of exercise. Proper methods of walking were taught on an indoor track during two supervised sessions. The walking regimen was adapted from a very gradual plan designed for the treatment of depression (reference cited) to maximize adherence. Participants were instructed to self-regulate the intensity of brisk walking based on heart rate, breathing difficulty, and perceived effort. They were instructed to exercise at a level that felt ‘vigorous’ but never ‘strenuous’ Frequency and length of each session and total number sessions: as reported above for diet. The duration of beginning exercise sessions was as short as 5 minutes. The goal was three to five sessions per week of 45 minutes or more per session Delivered: by registered dietitians who were trained and experienced in behaviour modification (exercise qualifications and competencies not reported) Level of supervision: supervision only of intervention groups reported Monitoring: self-monitoring questionnaire which included an hedonic five-point rating scale for exercise (no further details provided) Behaviour modification: Mode: groups of approximately 15 participants Type: followed the principles outlined in the LEARN programme for weight control (reference cited) Content: both diet behaviour modification and exercise behaviour modification involved the teaching or use of self-monitoring contracts to reward behaviour change (contingency contracting), stress management, stimulus control, goal-setting and maintenance techniques (references cited) Frequency and length of each session and total number sessions: as reported above for diet Delivered: as reported above for exercise Ongoing support:: None reported other than ‘maintenance’ sessions noted above Other details Financial deposits: at the start of the study participants deposited US$100 in an account which was refunded in increments according to the number of sessions attended Financial incentives: participants were offered US$35 for fulfilling the 2-year follow-up requirements |
Aim or goal: not explicitly reported Diet: Type of diet: participants were asked to maintain their current eating habits and nutrition was not discussed Frequency and length of each session and total number sessions: not reported (participants continued their current eating habits) Calories: not reported Proportions of diet: not reported (participants continued their current eating habits) Monitoring: none reported (participants continued their current eating habits) Exercise: Mode: as reported for D + E Type: as reported for D + E Frequency and length of each session and total number sessions: as reported for D + E Delivered: as reported for D + E Level of supervision: as reported for D + E Monitoring: as reported for D + E Behaviour modification: Mode: as reported for D + E Type: as reported for D + E Content: as reported for D + E Frequency and length of each session and total number sessions: as reported for D + E Delivered: as reported for D + E Ongoing support:: None reported Other details Financial deposits and incentives: as reported for D + E |
Aim or goal: to produce 1kg/week loss of weight Diet: Type of diet: as reported for D + E Frequency and length of each session and total number sessions: as reported for D + E Calories: not reported Proportions of diet: as reported for D + E Monitoring: as reported for D + E Exercise: Mode: as reported for D + E Type: normal physical activity only: participants were asked to maintain their relatively sedentary lifestyles and not to begin any new exercise programme Frequency and length of each session and total number sessions: no exercise Delivered: as reported for D + E Level of supervision: as reported for D + E Monitoring: none reported (participants continued their current physical activity) Behaviour modification: Mode: as reported for D + E Type: as reported for D + E Content: as reported for D + E Frequency and length of each session and total number sessions: as reported for D + E Delivered: as reported for D + E Ongoing support:: None reported Other details Financial deposits and incentives: as reported for D + E |
||||
Results
| Outcomes | D + E (n = 21) | D only (n = 15) | E only (n = 25) | p-value |
|---|---|---|---|---|
| Mean ± SD weight change (kg) from baseline (0–2 years)b,c | –2.2 ± 6.7 | 0.9 ± 7.7 | –2.7 ± 9.2 | p = 0.36 (NS)a |
| Number (%)d of participants with weight gain (> 4.5 kg) |
3 (14%) (Variance not reported) |
4 (27%) (Variance not reported) |
1 (4%) (Variance not reported) |
Not reported |
| Number (%)d of participants with no weight change (within ± 4.5 kg) |
10 (48%) (Variance not reported) |
9 (60%) (Variance not reported) |
18 (72%) (Variance not reported) |
Not reported |
| Number (%) of participants with clinical success (weight loss > 4.5 kg) |
8 (38%) (Variance not reported) |
2 (13%) (Variance not reported) |
6 (24%) (Variance not reported) |
p = 0.36 (NS)e |
| Facilitators | None reported | None reported | None reported | |
| Barriers | None reported | None reported | None reported |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Yes |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Unclear |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | Yes |
| (ii) If so, were they explained or adjusted for? | No |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | Not reported |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | No |
| (ii) If so, were the methods appropriate? |
Stevens et al. and Whelton et al.
| Study details | Participants | Outcome measures |
|---|---|---|
|
Author: Stevens et al. 74 and Whelton et al. 109 Linked to He et al. 110 (one participating centre only. Outcome data not extracted) Year: 1993 Country: USA Study design: RCT Number of centres: six (although 10 for entire TOHP study) Funding: National Heart, Lung and Blood institute Recruitment dates: September 1987 to October 1988 Setting: not explicitly stated, appears to be hospital clinics Length of follow-up: 18 months |
Number of participants: total n = 564, weight loss intervention n = 308, usual-care control n = 256 [as part of a bigger study looking at non-pharmacological interventions to lower BP. See also Whelton et al. 111 which compares three active treatments (combined) with controls so is therefore not relevant] Sample attrition/dropout: only adherence/attendance reported. Unclear how many participants may have dropped out completely Attendance at sessions measured: yes Other measures of adherence: no Sample crossovers: not applicable Inclusion/exclusion criteria for study entry: aged between 30 and 54 years, between approximately 115% and 165% of desirable body weight, BMI of 26.1–36.1 kg/m2 for men, 24.3–36.1 kg/m2 for women, average BP (DBP) of 80–89 mmHg. Exclusion criteria: history of cardiovascular disease, diabetes mellitus, gastrointestinal tract disease, chronic renal failure, malignant neoplasm, current pregnancy or intent to become pregnant during the study, recent history of psychiatric disorders, or unwillingness to accept randomisation into any study group Characteristics of participants: overall (data also reported separately for men and women in each group) Any risk factors noted: yes, but for high BP (all high-normal BP) Gender (M : F), n (%): weight loss 224 : 84 (72.7 : 27.3); control 161 : 95 (62.9 : 37.1) (n’s and % calculated by reviewer) Age (years), mean (SD): weight loss: 43.1 ± 6.0; control 42.4 ± 6.2 BMI kg/m2 mean (SD): weight loss: 29.5 ± 2.9; control 29.5 ± 2.8 Weight kg mean (SD): weight loss: 90.2 ± 13.3; control 89.3 ± 13.0 % weight lost before starting: not reported Duration of overweight/obesity: not reported Previous weight loss attempts: not reported Physical activity level, vigorous exercise (resulting in perspiration) times/week mean (SD): weight loss: 2.0 ± 2.2; control 2.1 ± 2.3 Ethnicity %: weight loss: white 81.8, black 16.6; control: white 76.6, black 21.1 Socioeconomic position: not reported If a mixed group of participants with pre-existing medical condition report n (%)’s with the condition: not applicable Baseline information reported but not data extracted on % college graduates, % employed full-time, % married, health status [systolic BP (SBP), DBP, heart rate, height, cigarette smoking, alcohol intake, urinary sodium excretion), and energy intake (overall, and % energy from fat, % energy from saturated fat) |
Primary outcomes: weight loss Secondary outcomes: change in BP (SBP and DBP). Attendance (not data extracted) Facilitators and barriers: not reported Methods of assessing outcomes: Weights were taken without shoes but including light, indoor clothing. Weights recorded for all participants during official clinic visits 3, 6, 12, and 18 months after study entry. Weights also recorded throughout the weight loss intervention Methods of BP assessment not data extracted Any self-reported outcomes? Yes – food diaries and exercise recorded (outcomes not reported) Any subgroup analysis: weight loss by gender reported, and within gender by white ethnicity |
| Intervention details: This study was part of phase 1 of the TOHP study. The comparison is one of three LIs that were tested in people with high to normal BP to study the efficacy and safety of non-pharmacologic therapy for the prevention of hypertension | ||
|
Weight loss intervention ( n = 308) Aim or goal: to achieve a weight loss of at least 4.5 kg during the first 6 months of intervention and to maintain this weight loss throughout the remaining 12 months of trial Study is not described as a weight maintenance study. However there were two phases. Firstly an intensive phase of an individual counselling session followed by 14 weekly group meetings (for the intensive phase it is generally unclear from study description what aspects of the intervention were provided during the individual sessions, and which in the weekly group meetings). After the intensive phase participants asked to attend monthly meetings for the duration of follow-up (18 months). This phase is described as ‘Extended Intervention’ and details are noted under ‘Ongoing support’ below |
Usual-care control ( n = 256) No description provided |
|
|
Diet: Details, type of diet: focus on reducing total energy consumption by reducing fat, sugar and alcohol intake. Nutrition topics discussed included guidelines for healthy eating, reducing energy intake, identifying sources of dietary fat and methods for reducing fat intake, recipe modification, restaurant eating, social eating, menu planning, label reading, and shopping strategies. The importance of nutritional balance was discussed at group meetings and incorporated into comments on the food records. Goal of achieving gradual weight loss not to exceed 0.9 kg per week. After reaching goal weight, participants adjusted their energy intake gradually to maintain the new weight level Calories: average energy intake not to fall below 1200 kcal, no upper limit stated Proportions of diet: not explicitly stated. Counting energy intake from fat and the percentage of daily energy intake from fat suggested as an optional method for focussing on major sources of energy intake Monitoring: participants encouraged to make series of small progressive steps to reduce energy intake. To help this, participants expected to keep food diaries for the first 14 weeks of the intervention, recording food intake for 3 of 7 days initially, increasing to 5 or more days per week by the fourth week of intervention. Entries included food description, estimate of amount eaten, and estimate of its energy value. Participants also asked to maintain graph of weight change from baseline Exercise: Mode: individual Type: principally walking. Participants were given general exercise guidelines including warm-up and cool-down exercises, and appropriate application of such exercising as walking, cycling, circuit training, and selected recreational activities. Participants encouraged to become more aware of their normal daily routines and to incorporate more physical activities, such as using stairs rather than elevators, to enhance daily energy expenditure Frequency and length of each session and total number sessions: initially to walk at least 3 days per week for a minimum of 20 minutes per session. As the intervention progressed exercise goal was 4–5 days per week with between 30 and 45 minutes of exercise per session, at an intensity of 40% to 55% of heart rate reserve (heart rate reserve had been determined empirically before intervention start) Delivered: mainly self-directed Level of supervision: mainly unsupervised, except for exercise demonstrations presented during meetings. Several meetings included supervised exercise periods in which the group leaders helped participants adjust their intensity of exercise to be consistent with protocol guidelines Monitoring: participants asked to record daily exercise time as a bar graph, superimposed on the weight graph Behaviour modification: Mode: group 7–20 participants (plus occasional friends or family members there to provide support). At some point during each intervention session, the group was divided into smaller discussion groups for intensive review of each person’s progress and plans for the next week Type: behavioural self-management techniques (two references provided). Relapse prevention was also addressed (reference provided) Content: strategies included setting reasonable short-term goals, formulating specific plans of action to achieve these goals, developing reinforcement and social support for each major element of the plan, keeping records to assess progress (monitoring of diet and exercise as noted above), and regularly evaluating and modifying action plans by using these records. During the smaller discussion groups, participants displayed graphs and discussed self-management efforts for the past week. Leaders facilitated discussion so that individuals worked on problem solving and developing specific and detailed goals and action plans for the next week. Relapse prevention included: introducing the concept of high-risk situations; identifying high-risk situations in which relapse was likely to occur; developing alternative coping strategies; teaching participants strategies for minimising the occurrence of high-risk situations. Walking opportunities were often made available Frequency and length of each session and total number sessions: 14 weekly meetings, each of 90 minutes Delivered: by a registered dietitian and a psychologist or exercise physiologist Ongoing support: After the intensive phase, intervention leaders attempted to make at least one intervention contact per month for the remainder of the trial. The type and exact number of contacts varied monthly according to individual needs Mode: attendance options included any one or combination of the following: (1) monthly extended intervention group sessions, (2) group weigh-in sessions, (3) individual weigh-in sessions, and (4) individual counselling sessions Type: extended follow-up groups were formed by combining several initial intervention groups. The format of the extended intervention meetings was similar to that of the initial intervention meetings. They featured formal presentations and group discussions on selected nutrition, exercise, and behavioural change topics as well as time for general discussion and problem solving, and for demonstrations/participation in exercise opportunities. A series of extended intervention session outlines were developed on the basis of the perceived needs of the participants. Each centre could adapt the sequence and content of session to meet the ethnic and situational needs of the participants Frequency and length of each session and total number sessions: monthly meetings, length and number not explicitly stated Diet and Exercise: during the extended intervention phase, subjects were encouraged to continue monitoring their weight and exercise. If a graph was not maintained an individual monitoring system of some type, such as recording the information on a calendar on in an appointment book, was encouraged |
||
|
Other details: If a meeting was missed an intervention leader scheduled a make-up visit as soon as possible. When participants were unable or unwilling to attend make-up visits, attempts were made to maintain contact through telephone calls and mailings For the extended intervention frequent conference calls helped timely sharing and review of meeting experiences. All sessions were evaluated for effectiveness and archived for easy access Attendance (at extended intervention meetings) was encouraged by the addition of occasional special events such as cooking demonstrations and guest speakers A brief, informal meeting with a weigh-in was also offered between the monthly-extended sessions for those who missed the scheduled intervention meetings or who desired more frequent contact. Current weight and amount of exercise since last contact was obtained and individual strategies were discussed with participants during these weigh-ins. Walking opportunities were often made available in conjunction with the weigh-ins Interventionists collaborated in the preparation of a detailed, session by session protocol and tested its feasibility at each centre with volunteer pilot subjects. This pilot was used to prepare the final version of the protocol. Ongoing quality control activities included biweekly conference calls, two all-centre staff training meetings, and a site visit to each centre |
||
Results
| Outcomes | Weight loss (n = 308) | Control (n = 256) | p-value, 95% CI |
|---|---|---|---|
| Weight loss at 18 months, mean kg | Men 4.7, women 1.6 | Men unchanged (no value provided), women + 0.2 | p < 0.001. |
| Difference in weight loss at 18 months between intervention and control groups, mean ± SEMa |
Overall: 3.9 ± 0.4 Men: 4.7 ± 0.5 Women: 1.8 ± 0.8 |
p < 0.01 p < 0.01 |
|
| Success at 18 monthsb | Men 45%, women 26% | Men 12%, women 18% | |
| Success at more than 24 or 30 months? | Phase II study included separately | ||
| Facilitators | Not reported | ||
| Barriers | Not reported | ||
| Other intermediate outcomes | Not reported |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Not reported |
| 2. Was the allocation adequately concealed? | Yes |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Unclear |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | Not reported |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | Not reported |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | Not reported |
| (ii) If so, were the methods appropriate? |
Stevens et al. and TOHP collaborative research group
| Study details | Participants | Outcome measures |
|---|---|---|
|
Author: Stevens et al. 70 and TOHP collaborative research group113 Data also reported in studies114–118 Year: 2001 Country: USA Study design: RCT Number of centres: nine Funding: numerous grants from National Heart, Lung and Blood Institute Recruitment dates: December 1990 to March 1992 Setting: clinics Length of follow-up: minimum of 36 months; additional data at 42 (n = 1458, 61%) and 48 (n = 464, 19%) months depending on randomisation date |
Number of participants: n = 1191 Weight loss (WL) group, n = 595 Usual-care (UC) group, n = 596 Part of an RCT of four groups: weight loss only, sodium reduction only, weight loss and sodium reduction, usual-care controls (n = 2382) Sample attrition/dropout: at 18 months 50 were not included in the analysis in the WL group and 45 in the UC group; at 36 months these rates were 48 and 42, respectively Attendance at sessions measured: yes, although rates could differ depending on delay before first group session Other measures of adherence: yes Sample crossovers: none reported Inclusion/exclusion criteria for study entry: overweight adults with non-medicated DBP of 83–89 mmHg and SBP < 140 mmHg, aged 30–54 years, BMI of 26.1–37.4 kg/m2 for men and 24.4–37.4 kg/m2 for women (approximately 110%–165% of ideal weight based on 1983 Metropolitan life tables) Exclusion criteria: current hypertension or treatment with medications that might affect BP, clinical or laboratory evidence of cardiovascular disease, diabetes mellitus, renal insufficiency [serum creatinine concentration ≥ 150 μmol/l (≥ 1.7 mg/dl) for men and ≥ 132 μmol/l (≥ 1.5 mg/dl) for women] or other serious illness, current or planned pregnancy, non-fasting serum glucose concentration of ≥ 200 mg/dl, alcohol intake of ≥ 21 drinks per week, residing more than 50 miles from the centre, evidence of unwillingness to adhere to the trial intervention or data collection procedures Characteristics of participants: Any risk factors noted: all participants had high–normal BP Gender (M : F), n (%): WL 375 : 220 (63.0 : 37.0); UC 407 : 189 (68.3 : 31.7) Age (years), mean (SD): WL 43.4 (6.1); UC 43.2 (6.1) BMI kg/m2, mean (SD), M : F: WL 31.0 (2.9) : 31.0 (3.6); UC 31.0 (2.9) : 30.8 (3.5) Weight, kg, mean (SD), M : F: WL 98.9 (12.3) : 84.1 (11.9); UC 98.54 (11.7) : 82.9 (10.9) Overall weight kg, mean (SE): WL 93.4 (14.1); UC 93.6 (13.5) Vigorous exercise, times per week: WL 2.0 (4.0); UC 1.8 (1.9) Ethnicity % white : black: WL 78 : 17.8; UC 79.5 : 17.3 |
Primary outcomes: BP (not extracted), weight loss Secondary outcomes: dietary intake, physical activity, medication use – not extracted Facilitators and barriers: none Methods of assessing outcomes: Weight measured to the nearest 0.2 kg (0.5 lb) by using a calibrated balance beam scale; participants wore indoor clothing without shoes Subgroup analyses by gender and ethnicity (not extracted) |
| Intervention details | ||
| WL (n = 595) |
UC (n = 596) |
|
|
Aim or goal: to lose at least 4.5 kg (10 lb) during the first 6 months of the intervention and to maintain the weight loss for the remainder of the trial. The intervention has a pre-intensive phase (while clinics accrued enough participants for the group intervention), an intensive phase, a transitional phase and then an extended phase. The transitional phase was designed to prevent relapse and to ease transition from weekly to less frequent contacts. The goal of the final extended phase was to maintain participants’ behaviour changes Diet: Details, type of diet: focus on reducing caloric intake but weight loss of > 0.9 kg (2 lb) per week was discouraged Calories: suggested that men not consume < 1500 kcal/day and women < 1200 kcal/day but with experience participants determined the caloric intake that produced moderate weight loss for them Proportions of diet: states decreasing consumption of excess fat, sugar and alcohol Monitoring: self-monitoring with daily food diaries, known as ‘scorekeepers’. Asked to record intake for at least 6 days a week during the intensive phase, after this time the frequency was individualised. Progress also monitored at group meetings and by frequent measurement of weight Exercise: Mode: group discussion of goals but individual exercise Type: primarily brisk walking, states moderate intensity exercise of approximately 40%–55% of heart rate reserve Frequency and length of each session and total number sessions: to gradually increase activity from 10–15 minutes at least 3 days per week to 30–45 minutes per day, 4–5 days per week at an intensity of 40%–55% of heart rate reserve Delivered: group discussion of exercise by dietitians and health educators, otherwise exercise was undertaken individually (four of the 14 sessions were specifically designated for engaging in physical activity) Level of supervision: discussed at group interventions, otherwise assume self-supervised Monitoring: graphs of physical activity per day used and recorded activity in the ‘scorekeepers’. Progress reviewed at the group meetings Behaviour modification: Mode: individual counselling session until groups could be formed (at least one) and some group meetings or by telephone during the ‘preintensive’ phase, followed by group sessions thereafter. Groups of 11–34 participants Type: states based on behaviour change principles, but no further details Content: focus on self-directed behaviour change (behavioural self-management), nutrition education, information on physical activity, and social support for making and maintaining behaviour changes. Specifically included self-monitoring, short-term goal-setting, developing specific action plans to achieve objectives, developing alternative strategies for situations which trigger problem eating Frequency and length of each session and total number sessions: monthly contact (pre-intensive phase) weekly for 14 weeks (intensive phase) then six biweekly meetings and then monthly meetings for additional 3–4 months (although one study publication suggests for 18 months) (transitional phase) Delivered: led by dietitians and health educators (and some psychologists) who were centrally trained and had experience of conducting weight loss interventions Ongoing support: In the extended phase of the study participants were given options to keep them informed, including individual counselling sessions, special refresher group sessions (mini-modules) and biweekly meetings Mode: could be group/individual/telephone/postcards/faxes Type: refreshing or redelivering the intervention content, especially for those who had not lost weight initially or relapsed. Modules included a wide range of topics and activities that were determined by a combination of participant and interventionist interest, as well as by centre and local area resources, season of the year and current events Frequency and length of each session and total number sessions: biweekly contacts for three to six sessions in each mini-module, offered six times a year (participants expected to attend at least three) Other details: Family members were invited to group meetings when the participant felt it helpful |
No details reported | |
Results
| Outcomes | WL group (n = 595) | UC group (n = 596) | Difference (SE), 95% CI, p-value |
|---|---|---|---|
| Weight change from baseline at 18 months, kg (SD), 95% CI, n | –2.0 (5.8), –2.5 to –1.5, n = 545 | 0.7 (4.2), 0.4 to 1.6, n = 551 | –2.7 (0.3), –3.3 to –2.1, p < 0.001 |
| Weight change from baseline at 36 months, kg (SD), 95% CI, n | –0.2 (5.9), –0.7 to 0.3, n = 547 | 1.8 (5.3), 1.3 to 2.2, n = 554 | –2.0 (0.2), –2.6 to –1.3, p < 0.001 |
| Facilitators | Not reported | Not reported | |
| Barriers | Not reported | Not reported | |
| Other intermediate outcomes | None | None |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Yes |
| 2. Was the allocation adequately concealed? | Yes |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Yes |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | No |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | No |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | Not reported |
| (ii) If so, were the methods appropriate? |
Tate et al.
| Study details | Participants | Outcome measures | |
|---|---|---|---|
|
Author: Tate et al. 77 (Linked to Jeffery et al. 119 and Raynor et al. 120) Year: 2007 Country: USA Study design: RCT (two arms) Number of centres: not reported (recruitment was from two centres) Funding: National Institutes of Health grants HL41330 and HL41332 Recruitment dates: not reported Setting: not reported but recruitment by public advertisement Length of follow-up: 30 months |
Number of participants: Standard behavioural treatment (SBT) n = 93 High physical activity (HPA) treatment group n = 109 Total number randomised: N = 202 Sample attrition/dropout: HPA retention: 94% at 6 months, 79% at 12 months, 80% at 18 months, 77% (84/109) at 30 months SBT retention: 90% at 6 months, 82% at 12 months, 87% at 18 months, 79% (74/93) at 30 months Total number of drop outs at 30 months: n = 44 Attendance at sessions measured: not reported Other measures of adherence: none reported Sample crossovers: none Inclusion/exclusion criteria for study entry: age 25–50 years; overweight of 14–32 kg according to actuarial norms; free from serious concurrent medical or psychological problems thought to interfere with treatment Characteristics of participants: most demographic and baseline data not reported separately for each group. States there were no significant differences between treatment groups for age, gender, % of college graduates, white ethnicity, and mean BMI Any risk factors noted: no Gender (M : F), n (%): 85 : 117 (42% : 58%) (9n:n calculated by reviewer) Age (years), mean (SD): 42.2 (6.4) BMI kg/m2, n (%): mean (SD): BMI 31.7 (2.6) kg/m2, range 26–44 Weight kg, mean: approximately 90.5 for both interventions (data extracted from graph by reviewer) % weight lost before starting: not reported Duration of overweight/obesity: not reported Previous weight loss attempts: not reported Physical activity level (assessed by self-report with Paffenbarger Physical Activity Questionnaire): baseline weekly energy expenditure (kcal/week), mean (SD): HPA 1278.0 (1369), n = 109. SBT 1286.0 (1258.0), n = 93 Ethnicity: 80% white Socioeconomic position: not reported Pre-existing medical conditions: not reported |
Primary outcomes: change in body weight Secondary outcomes: physical activity (energy expenditure); Dietary intake (energy intake kcal/day; protein g/day; fat g/day; carbohydrate g/day); Adverse effects of exercise programme – not extracted Facilitators and barriers: none reported Methods of assessing outcomes: Body weight: measured in clinic using a calibrated scale while the subject wore light street clothes and no shoes Height (used for BMI calculation): wall-mounted stadiometer Subgroup analyses: Tate et al. paper77 reports weight changes for subgroups of consistently high exercises versus all others |
|
| Intervention details | |||
| SBT group (n = 93) |
HPA group (n = 109) |
||
|
Aim or goal: encourage increasing physical activity to reach standard 1000 kcal physical activity/week prescription during a behavioural weight loss programme Diet: Type of diet: calorie restriction, low fat Frequency and length of each session and number of sessions: not reported explicitly for diet; probably as reported below for behaviour modification as this included nutritionists Calories: goal to reduce daily energy intake to 1000–1500 kcal depending on initial body weight (no further details provided) Proportions of diet: consume < 20% of energy as fat Monitoring: participants asked to keep complete diet records daily for the first 6 months and for 1 week/month thereafter during the 18-month intervention phase Exercise: Mode: not stated but appears to be individual Type: not stated, but goal was to build up from energy expenditure of 250 kcal/week, increasing by 250 kcal/week, to energy expenditure of 1000 kcal/week (roughly equivalent to walking for 30 minutes/day) Frequency and length of each session and total number sessions: not stated, but goal was to initiate a regular physical activity programme Delivered: not stated, but appears to be no set class, instead self-directed by participant Level of supervision: not stated, but appears to be unsupervised Monitoring: participants were asked to keep complete physical activity records daily for the first 6 months and for 1 week/month thereafter during the 18-month intervention phase Behaviour modification: Mode: small groups, e.g. 10–20 participants Type: no theoretical basis or definition of behaviour modification component reported Content: didactic presentations of material needed to develop obesity management skills, group discussions, and problem solving. Session content adapted from prior research (referenced by Jeffery et al. 119) included diet, physical activity, stimulus control, problem solving, goal-setting, social support, motivation, and relapse prevention topics Frequency and length of each session and total number sessions: weekly meetings for first 6 months, biweekly from 6 to 12 months, then monthly from 12 to 18 months. No treatment contact after the 18-month programme until participants were re-contacted at 30 months for the final assessment Delivered: led by trained interventionists (nutritionists, exercise physiologists, or psychologists) with expertise in both content area (i.e. physical activity and nutrition) and behavioural therapy Other details: Participants were not encouraged to recruit friends or family members Financial incentives: none |
Aim or goal: encourage increasing physical activity to reach 2500 kcal physical activity/week during a behavioural weight loss programme Study reports a weight management (weight loss) intervention (duration of intervention 18 months) with participants followed up for a further year after the end of the intervention (to determine how well weight loss maintained, but no intervention in this period) Diet: identical goals as the SBT group Exercise: Mode: not explicitly stated but appears to be a mix of individual and group (with support partners, n = 1–3; see ‘Other’ details below) Type: not stated, but goal was to build up to an energy expenditure of 2500 kcal/week by the end of the first 6 months of the intervention (roughly equivalent to walking < 75 minutes/day) Frequency and length of each session and number of sessions: not reported explicitly for diet; probably as reported below for behaviour modification as this included exercise physiologists Appears also to be participant determined Delivered: by exercise coaches (also referred to as exercise physiologists) skilled in exercise science and prescription Level of supervision: the exercise coaches met with small groups of study participants before or after each group session. They reviewed exercise progress with each participant individually and provided encouragement, support, and problem-solving strategies for participants who were having difficulty reaching their physical activity goals Monitoring: same as the SBT group Behaviour modification: identical to that of the SBT group Ongoing support: Other than contact as described above during the intervention, there was no contact between the end of the intervention at 18 months and the final follow-up at 30 months Other details: Participants were strongly encouraged to recruit friends or family members to participate in the study with them due to prior research suggesting benefits of social support for exercise and maintenance of weight loss. Participants were encouraged to recruit one to three partners, overall 54% of this group recruited one or more support partners. Entry criteria for support partners were wider than for trial participants but they went through the same screening, received the same intervention and participated in same outcome assessments Financial incentives: incentives of US$3 for each week that participants achieved or exceed the energy expenditure goal of 2500 kcal/week during the last 6 months of active intervention (months 12–18). Participants were paid US$50 for completing the 30-month assessment |
||
Results
| Outcomes: | 1. HPA | 2. SBT | p-value |
|---|---|---|---|
| Weight loss kg, baseline to 18 months |
6.7 ± 8.1 (n = 87) (variance estimate not defined)a |
4.1 ± 8.3 (n = 80) (variance estimate not defined)a |
p = 0.04 |
| Weight loss kg, baseline to 30 months (unclear whether this is an ITT analysis) |
2.86 8.6 (n = 84) (variance estimate not defined) |
0.9 ± 8.9 (n = 74) (variance estimate not defined) |
States no significant difference, p = 0.16 |
| Weight loss % of initial body weight, baseline to 30 months (unclear whether this is an ITT analysis) |
3 (variance estimate not reported) |
1 (variance estimate not reported) |
States no significant difference, p-value not reported |
| Weight regain from 18 to 30 months, kg (unclear whether this is an ITT analysis) |
5.9 5.9 (n not reported) (variance estimate not defined) |
5.3 ± 7.0 (n not reported) (variance estimate not defined) |
States no significant difference, p-value not reported |
| Success at 30 months (ITT, assuming no weight loss for missing data) |
Total weight loss ≥ 5% achieved by 26% Total weight loss ≥ 10% achieved by 12% |
States no significant difference, p-value not reported | |
| Facilitators | Not reported | Not reported | Not reported |
| Barriers | Not reported | Not reported | Not reported |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Not reported |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | Unclear |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | Yes |
| (ii) If so, was this defined? | No |
| 10. (i) Did the analysis account for missing data? | Yes |
| (ii) If so, were the methods appropriate? | Unclear |
Wadden et al.
| Study details | Participants | Outcome measures | ||||
|---|---|---|---|---|---|---|
|
Years: 1986, 1988 Country: USA Study design: RCT Number of centres: not reported Funding: researchers supported by three grants from: National Institute of Mental Health; National Institute of Child Health and Human Development; MacArthur Foundation’s Network on Health Promoting and Disease Preventing Behaviors Recruitment dates: not reported Setting: not reported; VLCD intervention aimed to simulate physician’s outpatient practice Length of follow-up: 3 years |
Number of participants: Standard behaviour therapy (SBT) [referred in publication as Behavioural Therapy (BT)]: n = 18 Combined treatment (VLCD + SBT): n = 23 Very-low-calorie diet (VLCD): n = 18 Total: N = 59 Sample attrition/dropout: Not reported separately by intervention but stated that attrition was spread evenly across the interventions At end of treatment (4 or 6 months): overall 15.3% (nine participants) At 1-year follow-up: overall 18.6% (11 participants) At 3-year follow-up: overall 23.7% (14 participants) Attendance at sessions measured: not reported Other measures of adherence: none reported Sample crossovers: none reported Inclusion/exclusion criteria for study entry: Inclusion: written approval from participant’s physician; responders to newspaper advertisements; at least 25 kg overweight (based on height-weight tables of Metropolitan Insurance Company) Exclusion: recent cardiac abnormality including myocardial infarction; history of cerebrovascular, kidney, or liver disease; cancer; type I diabetes; severe psychiatric illness |
Primary outcomes: (reported, but not stated explicitly that these were primary outcomes): weight loss; percentage of participants maintaining weight loss at 1 and 3 years’ follow-up Secondary outcomes: (reported, but not stated explicitly that these were secondary outcomes; not data extracted): depression; psychological and physical consequences of regaining weight (not validated); BP Facilitators and barriers: none reported Methods of assessing outcomes: Weight measured using a balance beam scale Subgroup analyses: Interventions each stratified into three groups based on degree overweight Post hoc comparison of participants who after 1-year follow-up did (n = 19) or did not (n = 26) receive additional therapy from external weight loss programmes |
||||
| Characteristics of participants: | ||||||
| Risk factors noted: 14 participants (23.7%) were taking antihypertensive treatment | ||||||
| Reported only for 50 participants who completed treatment (VLCD = 15; SBT = 16; VLCD + SBT = 19): | ||||||
| Gender (M : F), n (%): | VLCD: 2 : 13 (13 : 87) | SBT: 3 : 13 (19 : 81) | VLCD + SBT: 2 : 17 (11 : 89) | |||
| Age (years), mean ± SD: | VLCD: 44.3 ± 8.7 | SBT: 44.3 ± 8.6 | VLCD + SBT: 43.6 ± 7.8 | |||
| Height (cm), mean ± SD: | VLCD: 162.1 ± 7.0 | SBT: 166.5 ± 10.3 | VLCD + SBT: 165.6 ± 7.3 | |||
| Weight (kg), mean ± SD: | VLCD: 106.4 ± 18.4 | SBT: 112.2 ± 21.5 | VLCD + SBT: 108.0 ± 21.5 | |||
| Degree overweight (%), mean ± SD: | VLCD: 85.4 ± 27.4 | SBT: 91.8 ± 32.2 | VLCD + SBT: 90.7 ± 37.4 | |||
| BMI, kg/m2, n (%): | Not reported | Not reported | Not reported | |||
| % weight lost before starting: | Not reported | Not reported | Not reported | |||
| Duration of overweight/obesity: | Not reported | Not reported | Not reported | |||
| Previous weight loss attempts: | Not reported | Not reported | Not reported | |||
| Physical activity level: | Not reported | Not reported | Not reported | |||
| Ethnicity: | Not reported | Not reported | Not reported | |||
| Socioeconomic position: | Not reported | Not reported | Not reported | |||
| Intervention details | ||||||
| Three weight loss interventions of duration 4 months (VLCD), 6 months (SBT) or 6 months (SBT + VLCD), each with follow-up at 1 year (reported in Wadden et al.121). Weight loss or maintenance then assessed after 3 years (reported in Wadden et al.69). During years 1–3, some participants (19%) received additional weight therapy from unspecified external sources while the remainder (81%) did not receive any additional weight therapy | ||||||
| Intervention details | ||||||
| SBT (n = 18) |
VLCD + SBT (n = 23) |
VLCD (n = 18) |
||||
|
Aim or goal: not reported Diet: Type of Diet: 6-month duration, 1000–1200 kcal/day balanced diet of participants’ choosing Frequency and length of each session and total number sessions: weekly 90-minute sessions of groups of 4–7 participants led by two doctoral-level clinical psychologists, following procedures described in detailed treatment manuals which differed for each intervention (no details reported) Calories: Months 1–6: 1000–1200 kcal/day Proportions of Diet: months 1–6: chosen by participants. No further details reported Monitoring: none reported Exercise: Mode: three groups of 4–7 people Type: involved walking and using stairs. No other details reported Frequency and length of each session and total number sessions: as reported above for diet Delivered: by two doctoral-level clinical psychologists Level of supervision: not reported (assumed as for VLCD) Monitoring: none reported Behaviour modification: Mode: three groups of 4–7 people Type: training in skills needed for weight loss maintenance Content: traditional behavioural methods of weight control taught (based on cited references). Included: recording eating behaviour; controlling stimuli associated with eating; slowing rate of consumption; modifying self-defeating thoughts and emotions associated with dieting; social support; and reinforcing changes in behaviour Frequency and length of each session and total number sessions: as reported for diet Delivered: by two doctoral-level clinical psychologists Ongoing support: Scheduled follow-up meetings (no other details reported) Type: group Frequency and length of each session and total number sessions: 11 scheduled follow-up meetings: fortnightly for 2 months post-treatment, then monthly for 4 months then at 2-month intervals for 6 months (no other details reported) Other details: Subjects paid US$10 per visit plus US$40 which was refunded at the 1-year follow-up |
Aim or goal: not reported Diet: Type of Diet: 6-month duration including a 2-month VLCD comprising a protein-sparing modified fast (months 1–4 same as VLCD; months 5–6 same as SBT): Month 1: 1000–1200 kcal/day balanced diet of participants’ choosing Months 2–3: VLCD comprising a protein-sparing modified fast Month 4: return to conventional food Months 5–6: 1000–1200 kcal/day balanced diet of participants’ choosing Frequency and length of each session and total number sessions: same as SBT Calories: months 1–4 same as VLCD; months 5–6 same as SBT: Month 1: 1000–1200 kcal/day Months 2–3: 400–500 kcal/day Month 4: return to 1000–1200 kcal/day (managed refeeding) Months 5–6: 1000–1200 kcal/day Proportions of Diet: Month 1: ‘balanced calorie diet’ (no details reported) Months 2–3: VLCD comprising meat, fish, fowl, plus daily supplements of 3 g each of potassium and sodium chloride and 800 mg of calcium Month 4: conventional food, with introduction of (in order) fruits and vegetables, breads and cereals, and fats Months 5–6: chosen by participants Monitoring: not reported (assumed the same as VLCD) Exercise: Mode: three groups of 4–7 people Type: involved walking and using stairs. No other details reported Frequency and length of each session and total number sessions: as reported for diet Delivered: by the same people as SBT Level of supervision: not reported (assumed as for VLCD) Monitoring: none reported Behaviour modification: Mode: as reported for SBT Type: as reported for SBT Content: as reported for SBT Frequency and length of each session and total number sessions: as reported for SBT Delivered: as reported for SBT Ongoing support: Scheduled follow-up meetings (no other details reported) Mode: group; size not reported Type: group Frequency and length of each session and total number sessions: as reported for SBT Other details: As reported for SBT |
Aim or goal: to simulate treatment as delivered in a physician’s outpatient practice (no quantitative goal specified) Diet: Type of Diet: 4-month duration including a 2-month VLCD comprising a protein-sparing modified fast Months 1–4: same as VLCD + SBT Frequency and length of each session and total number sessions: same as SBT Calories: Months 1–4: same as VLCD + SBT Proportions of Diet: Months 1–4: same as VLCD + SBT Monitoring: participants were encouraged to record their food intake Exercise: Mode: three groups of 4–7 people Type: no formal instruction in modifying exercise habits Frequency and length of each session and total number sessions: as reported for diet Delivered: by the same people as SBT Level of supervision: supervision only of discussion groups Monitoring: participants were encouraged to record their exercise Behaviour modification: No behaviour modification. At weekly group meetings participants discussed their reactions to the diet but received no formal instruction in modifying their eating and exercise habits Ongoing support: Scheduled follow-up meetings (no other details reported) Mode: group; size not reported Type: group Frequency and length of each session and total number sessions: six scheduled follow-up meetings at 1, 2, 3, 6, 9, and 12 months post-treatment (no other details reported) Other details: As reported for SBT |
||||
Results
| Outcomes | VLCD + SBT (n = 16) | VLCD (n = 15) | SBT (n = 14) | p-value, 95% CI |
|---|---|---|---|---|
| Mean ± SD weight loss (kg) | ||||
| (a) uncorrected analysisa | (a) 6.53 ± 9.50 | (a) 3.76 ± 8.85 | (a) 4.76 ± 6.56 | Stated NS; no p-values reported |
| (b) corrected analysisa |
(b) 5.11 ± 8.28 (variance not reported) |
(b) 2.20 ± 8.50 (Variance not reported) |
(b) 3.54 ± 6.26 (Variance not reported) |
|
|
Mean proportion (%) of participants who equalled or exceeded their pre-treatment weight (stated that the % are approximate) (based on corrected analysisa) |
38 (Variance not reported) |
43 (Variance not reported) |
47 (Variance not reported) |
Stated NS; no p-values reported |
|
Mean proportion (%) of participants who maintained weight loss within 2 kg of their end-of-treatment weight (based on corrected analysisa) |
19 (Variance not reported) |
13 (Variance not reported) |
7 (Variance not reported) |
Not reported |
| Mean proportion (%) of participants who maintained weight loss: | ||||
| (a) 5 kg or greater | (a) 44 | (a) 33 | (a) 29 | Stated NS; no p-values reported |
|
(b) 10 kg or greater (based on corrected analysisa) Stated that the percentages are approximate |
(b) 31 (Variance not reported) |
(b) 27 (Variance not reported) |
(c) 7 (Variance not reported) |
|
| Stated that the percentages are approximate | ||||
| Facilitators | None reported | None reported | None reported | |
| Barriers | None reported | None reported | None reported | |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Not reported |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Uncleara |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | No |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | Not reported |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | No |
| (ii) If so, were the methods appropriate? |
Weinstock et al.
| Study details | Participants | Outcome measures | ||
|---|---|---|---|---|
|
Author: Weinstock et al. 78,122 Year: 1998 Country: USA Study design: RCT Number of centres: two Funding: National Institute of Mental Health/National Institutes of Health Recruitment dates: not stated Setting: university (Syracuse and Pennsylvania) Length of follow-up: 96 weeks |
Number of participants: 128 women randomised to four groupsa: Intervention 1: diet plus aerobic training (DA) (n = 31) Intervention 2: diet alone (D) (n = 29) Intervention 3: diet plus strength training (DS) (n = 31) Intervention 4: diet plus combined strength and aerobic training (DSA) (n = 29) Sample attrition/dropout: At week 48, 29 participants had discontinued treatment. Numbers discontinuing in each study group not given, though it is reported that there were no differences in attrition between interventions At week 96 it is stated that 22 participants returned for follow-up visit, though this is based on a subgroup analysis of a total of 45 women in intervention groups 2, 3 and 4 Attendance at sessions measured: yes Other measures of adherence: adherence to diet was measured at weeks 5, 9, 13 and 17 based on weekly diet diaries Sample crossovers: none Inclusion/exclusion criteria for study entry: women only; overweight (BMI 39.5 ± 0.9 kg/m2). Excluded if bulimia nervosa; significant depression; major psychiatric disturbance (but not binge eating disorder); recent myocardial infarction; cerebrovascular, kidney, or liver disease; cancer; type I diabetes; pregnancy; use of medications known to affect weight and energy expenditure Characteristics of participants: Any risk factors noted: none Gender (M : F) – all female Age (years): mean (SD): DSA: 42.8 (8.3); DS: 40.0 (9.1); DA: 40.8 (7.9); D: 41.0 (8.8) BMI kg/m2: mean (SD): DSA: 35.3 (4.4); DS: 36.5 (6.0); DA: 37.3 (5.1); D: 36.4 (5.5) Weight kg, mean (SD): DSA: 92.4 (14.8); DS: 96.8 (14.2); DA: 98.7 (12.5); D: 96.3 (8.8) Age of onset of overweight/obesity, years (SD): DSA: 20.9 (11.3); DS: 20.0 (10.6); DA: 20.1 (9.5); D: 19.5 (8.8) Ethnicity: n = 99: Caucasian, 28 African American, one Hispanic Baseline data on weight (kg), height, fat, % fat, fat free mass (FFM) and REE not data extracted |
Primary outcomes: body composition, in terms of fat-free mass (FFM) and REE (as mentioned in study hypothesis) Secondary outcomes: weight, appetite, mood, insulin resistance, glucose tolerance, BP Facilitators and barriers: none Methods of assessing outcomes: weight measured using a balance-beam scale. Only weight outcomes are extracted here |
||
| Intervention details | ||||
| Aim or goal: preservation of FFM and REE at weeks 24 and 48, resulting in superior maintenance of weight loss at week 48 (for participants taking part in the three exercise conditions compared with those who received diet alone). Women who received strength training, whether alone or in combination with aerobic activity, were expected to achieve best maintenance of FFM | ||||
|
1. DA Diet: Details, type of diet: meal replacement plus dinner entrée (weeks 2–17), refeeding (weeks 17–26), self-selected diet (weeks 22–48) Calories: 900–925 kcal/day (weeks 2–17). Increasing to 1250 kcal/day (weeks 18–20); 1500 kcal/day (weeks 22–48) Proportions of diet: 150 kcal, 15 g protein, 11.2 g carbohydrate, 5 g fat (per serving of the liquid meal replacement four times per day, weeks 2–17) 280–300 kcal, 20 g protein, 35–40 g carbohydrate, 7 g fat (per dinner entrée, weeks 2–17) 12–15% calories from protein, 55–60% from carbohydrate, and 15–30% from fat (weeks 22–48) Monitoring: participants kept weekly diet diaries. As part of the behavioural treatment component it is stated that participants were instructed in traditional behavioural methods that included recording food intake (amounts, calories, etc.). The refeeding protocol was supervised by a registered dietitian who coled group sessions from weeks 17–26 Exercise: Mode: participants exercised with members of their behavioural treatment groups (up to week 28) Type: step aerobics Frequency and length of each session and total number sessions: Three sessions per week (non-consecutive days) for first 28 weeks, two per week during weeks 29–48. 12 minutes at week 1, additional 2 minutes to routine each week, by week 14 performed 40 minutes of stepping During weeks 29–48 they were assisted in developing at home exercise to replace the third exercise session deleted from their supervised training Delivered: graduate students in exercise physiology who followed structured protocols Level of supervision: all sessions were supervised (no further detail given) Monitoring: Borg Rating of perceived Exertion Scale (to assess intensity of exercise). The aim was to exercise at moderate intensity Behaviour modification: Mode: group sessions (7–10 members each) Type: described as cognitive behavioural weight loss programme, based on the OPTIFAST programme Content: practicing skills to maintain weight loss (first 28 weeks only). Participants were given manuals summarising materials for the first 28 weeks, and weeks 29–48 Frequency and length of each session and total number sessions: 28 weekly 90 minute sessions, followed by biweekly maintenance programme sessions (weeks 29–48) Delivered: by clinical psychologists and groups co-led by a dietitian (weeks 17–26) In addition the exercisers took approximately 5–10 minutes each week to discuss adherence to their exercise programme Ongoing support: Participants attended group sessions once every 3 months in the year following treatment. Between weeks 48–96 the women were encouraged to continue exercising unsupervised |
2. D Diet: As intervention DA Exercise: None. Participants agreed not to engage during the study in any programme of regular activity that resembled the aerobic or strength training conditions (but they were allowed to maintain lifestyle activities such as occasionally playing tennis, bowling or lunchtime walks). This was recorded in their activity logs Behaviour modification: As DA except there was no discussion of adherence Ongoing support: Mentions only that participants attended group sessions once every 3 months in the year following treatment |
3. DS Diet: As intervention DA Exercise: Mode: participants exercised with members of their behavioural treatment groups (up to week 28) Type: strength training, using gym equipment such as the bench press, latissimus pull down, shoulder press (targeting large muscle groups). Exercises were performed with a resistance that allowed them to do ≥ 10 repetitions but not > 14 Frequency and length of each session and total number sessions: Three sessions per week (non-consecutive days) for first 28 weeks, two per week during weeks 29–48. Initial workouts lasted approx. 20 minutes, weeks 3–14 an extra set of exercises were added, participants eventually did two sets of each exercise at each session. By end of week 14 (until week 48) weight training lasted approx. 40 minutes per session. Resistance increased whenever participants were able to perform > 14 repetitions for two consecutive sets During weeks 29–48 they were assisted in developing a personal programme of strength training to replace the third session deleted from the supervised practice (e.g. joining a health club) Delivered: graduate students in exercise physiology who followed structured protocols Level of supervision: all sessions were supervised (no further detail given) Monitoring: not reported Behaviour modification: As DA Ongoing support: Participants attended group sessions once every 3 months in the year following treatment. Between weeks 48–96 the women were encouraged to continue exercising unsupervised |
4. DSA Diet: As intervention DA Exercise: Mode: participants exercised with members of their behavioural treatment groups (up to week 28) Type: 60% strength training, 40% aerobic activity. Women in the Syracuse cohort did step aerobics, women in the Pennsylvania cohort did treadmill walking and stationary bicycling (due to space constraints) Frequency and length of each session and total number sessions: three sessions per week (non-consecutive days) for first 28 weeks, two per week during weeks 29–48. Women in this intervention progressed through the sequence of training on approximately the same schedule as those in interventions 2 and 3 Delivered: graduate students in exercise physiology who followed structured protocols Level of supervision: all sessions were supervised (no further detail given) Monitoring: Borg Rating of perceived Exertion Scale (to assess intensity of exercise) Behaviour modification: AS DA except there was no discussion of adherence Ongoing support: Mentions only that participants attended group sessions once every 3 months in the year following treatment |
|
Results
| Outcomes | D/DS/SA (groups 2, 3 and 4) combined | p-value, 95% CI |
|---|---|---|
| Maintenance of weight loss at week 96a, mean (SE) kg | 87.6 (2.8) kg. A 9.9 kg net weight loss from baseline | Reports no significant differences between the diet and exercise groups at week 96 |
| Weight regain (weeks 44 to 96)a | 76% of participants gained weight, and 14 (64%) of 22 gained more than 5 kg | |
| BMI kg/m2, mean (SE) | Dropped to 32.7 (1.2) from 36.4 (1.4) at baseline (loss of 3.7) |
Methodological comments/notes
|
| General comments |
|---|
|
Quality criteria for assessment
| 1. Was the method used to generate random allocations adequate? | Not reported |
| 2. Was the allocation adequately concealed? | Not reported |
| 3. Were the groups similar at the outset of the study in terms of prognostic factors, e.g. severity of disease? | Yes |
| 4. Were outcome assessors blinded to the treatment allocation? | Not reported |
| 5. Was the care provider blinded? | Not reported |
| 6. Was the participant blinded? | Not reported |
| 7. (i) Were there any unexpected imbalances in dropouts between groups? | No |
| (ii) If so, were they explained or adjusted for? | |
| 8. Is there any evidence to suggest that the authors measured more outcomes than they reported? | No |
| 9. (i) Did the analysis include an intention to treat analysis? | No |
| (ii) If so, was this defined? | |
| 10. (i) Did the analysis account for missing data? | No |
| (ii) If so, were the methods appropriate? |
Appendix 6 Excluded studies
Participants
-
Leermakers EA, Anglin K, Wing RR. Reducing postpartum weight retention through a correspondence intervention. Int J Obes 1998;22:1103–9.
-
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. a
-
Calfas KJ, Sallis JF, Zabinski MF, Wilfley DE, Rupp J, Prochaska JJ, et al. Preliminary evaluation of a multicomponent program for nutrition and physical activity change in primary care: PACE + for adults. Prev Med 2002;34:153–61.
-
Dzator JA, Hendrie D, Burke V, Gianguilio N, Gillam HF, Beilin LJ, et al. A randomized trial of interactive group sessions achieved greater improvements in nutrition and physical activity at a tiny increase in cost. J Clin Epidemiol 2004;57:610–19.
-
Eriksson KM, Westborg CJ, Eliasson MC. A randomized trial of lifestyle intervention in primary healthcare for the modification of cardiovascular risk factors. Scand J Public Health 2006;34:453–61.
-
Anderson LM, Quinn TA, Glanz K, Ramirez G, Kahwati LC, Johnson DB, et al. The effectiveness of worksite nutrition and physical activity interventions for controlling employee overweight and obesity: a systematic review. Am J Prev Med 2009;37:340–57.
-
Lombard CB, Deeks AA, Teede HJ. A systematic review of interventions aimed at the prevention of weight gain in adults. Public Health Nutr 2009;12:2236–46.
Design
-
Abrams DB, Follick MJ. Behavioral weight loss intervention at the worksite: feasibility and maintenance. J Consult Clin Psychol 1983;51:226–33.
-
Andersson K, Karlstrom B, Freden S, Petersson H, Ohrvall M, Zethelius B. A two-year clinical lifestyle intervention program for weight loss in obesity. Food Nutr Res 2008;52.
-
Clifford PA, Tan SY, Gorsuch RL. Efficacy of a self-directed behavioral health change program: weight, body composition, cardiovascular fitness, blood pressure, health risk, and psychosocial mediating variables. J Behav Med 1991;14:303–23.
-
Daggy B, Schwartz SM, Foster GD, Addrizzo J, Neher K. Demographics, weight loss and satisfaction results for consumers enrolled in the online behavioral support program for non-prescription 60 mg Orlistat. Int J Obes 2008;32:S172.
-
Dornelas EA, Wylie-Rosett J, Swencionis C. The DIET study: long-term outcomes of a cognitive-behavioral weight-control intervention in independent-living elders. Dietary intervention: evaluation of technology. J Am Diet Assoc 1998;98:1276–81.
-
Eichler K, Zoller M, Steurer J, Bachmann LM. Cognitive-behavioural treatment for weight loss in primary care: a prospective study. Swiss Med Wkly 2007;137:489–95.
-
Franz MJ. Effectiveness of weight loss and maintenance interventions in women. Curr Diab Rep 2004;4:387–93.
-
Graham LE, Taylor CB, Hovell MF, Siegel W. Five-year follow-up to a behavioral weight-loss program. J Consult Clin Psychol 1983;51:322–3.
-
Jakicic JM, Otto AD. Motivating change: modifying eating and exercise behaviors for weight management. ACSMs Health & Fitness Journal 2005;9:6–12.
-
Jerome GJ, Young DR, Brantley PJ, Coughlin JW, Erlinger TP, Cooper LS, et al. Effects of lifestyle modification interventions on social support: results from the PREMIER trial. Circulation 2006;113:e370.
-
McLean N, Griffin S, Toney K, Hardeman W. Family involvement in weight control, weight maintenance and weight loss interventions: a systematic review of randomised trials. Int J Obes 2003;27(9):987–1005.
-
Miller PM, Sims KL. Evaluation and component analysis of a comprehensive weight control program. Int J Obes 1981;5:57–65.
-
Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes 1997;21:941–7.
-
O’Meara S, Glenny AM, Sheldon T, Melville A, Wilson C. Systematic review of the effectiveness of interventions used in the management of obesity. J Hum Nutr Diet 1998;11:203–6.
-
Padiyar A. Nonpharmacologic management of hypertension in the elderly. Clin Geriatr Med 2009;25:213–19.
-
Stevens VJ, Funk KL, Brantley PJ, Erlinger TP, Myers VH, Champagne CM, et al. Design and implementation of an interactive website to support long-term maintenance of weight loss. J Med Internet Res 2008;10:e1.
-
Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005;142:56–66.
-
Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance. A review. J Cardiovasc Nurs 2009;24:58–80.
-
Wong ML, Koh D, Lee MH, Fong YT. Two-year follow-up of a behavioural weight control programme for adolescents in Singapore: predictors of long-term weight loss. Ann Acad Med Singapore 1997;26:147–53.
Intervention
-
Dale KS, McAuley KA, Taylor RW, Williams SM, Farmer VL, Hansen P, et al. Determining optimal approaches for weight maintenance: a randomized controlled trial. CMAJ 2009;180:E39–E46.
-
Dale KS, Mann JI, McAuley KA, Williams SM, Farmer VL. Sustainability of lifestyle changes following an intensive lifestyle intervention in insulin resistant adults: follow-up at 2-years. Asia Pac J Clin Nutr 2009;18:114–20.
-
Elder C, Ritenbaugh C, Mist S, Aickin M, Schneider J, Zwickey H, et al. Randomized trial of two mind-body interventions for weight-loss maintenance. J Altern Complement Med 2007;13:67–78.
-
Eriksson J, Lindstrom J, Valle T, Aunola S, Hamalainen H, Ilanne-Parikka P, et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999;42:793–801.
-
Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160–7.
-
Hill JO, Hauptman J, Anderson JW, Fujioka K, O’Neil PM, Smith DK, et al. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1-y study. Am J Clin Nutr 1999;69:1108–16.
-
Jones SE, Owens HM, Bennett GA. Does behaviour therapy work for dietitians? An experimental evaluation of the effects of three procedures in a weight reduction clinic. Hum Nutr Appl Nutr 1986;40:272–81.
-
Kalodner CR, DeLucia JL. The individual and combined effects of cognitive therapy and nutrition education as additions to a behavior modification program for weight loss. Addict Behav 1991;16:255–63.
-
Karvetti RL, Hakala P. A seven-year follow-up of a weight reduction programme in Finnish primary health care. Eur J Clin Nutr 1992;46:743–52.
-
Kumanyika SK, Brancato J, Brewer A, Carnaghi M, Doroshenko L, Rosen R, et al. Interventions in the trial of nonpharmacologic interventions in the elderly: an effective approach to weight and sodium reduction among older adults. Circulation 1996;94:I690.
-
Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:89. a
-
Miura J, Arai K, Tsukahara S, Ohno M, Ikeda Y. The long term effectiveness of combined therapy by behavior modification and very low calorie diet: 2 years follow-up. Int J Obes 1989;13(Suppl. 2):73–7.
-
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97. a
-
Rossner S, Sjostrom L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res 2000;8:49–61.
-
Ryttig KR, Flaten H, Rossner S. Long-term effects of a very low calorie diet (Nutrilett) in obesity treatment. A prospective, randomized, comparison between VLCD and a hypocaloric diet + behavior modification and their combination. Int J Obes 1997;21:574–9.
-
Seo DC, Sa J. A meta-analysis of psycho-behavioral obesity interventions among US multiethnic and minority adults. Prev Med 2008;47:573–82.
-
Tinker LF, Bonds DE, Margolis KL, Manson JE, Howard BV, Larson J, et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women. The women’s health initiative randomised controlled dietary modification trial. Arch Int Med 2008;168:1500–11. a
-
Waleekhachonloet O-A, Limwattananon C, Limwattananon S, Gross CR. Group behavior therapy versus individual behavior therapy for healthy dieting and weight control management in overweight and obese women living in rural community. Obes Res Clin Pract 2007;1:223–32.
-
Wein P, Beischer N, Harris C, Permezel M. A trial of simple versus intensified dietary modification for prevention of progression to diabetes mellitus in women with impaired glucose tolerance. Aust N Z J Obstet Gynaecol 1999;39:162–66. a
-
Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev 2009;10:313–23.
-
Yancy J, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med 2004;140:769–77.
Comparator
-
Epstein LH, Wing RR, Koeske R, Andrasik F, Ossip DJ. Child and parent weight loss in family-based behavior modification programs. J Consult Clin Psychol 1981;49:674–85.
Outcomes
-
Stahre L, Hallstrom T. A short-term cognitive group treatment program gives substantial weight reduction up to 18 months from the end of treatment. A randomized controlled trial. EWD 2005;10:51–8.
-
van Wier MF, Ariens GA, Dekkers JC, Hendriksen IJ, Pronk NP, Smid T, et al. ALIFE@Work: a randomised controlled trial of a distance counselling lifestyle programme for weight control among an overweight working population. BMC Public Health 2006;6:140.
Details intervention
-
Black DR, Lantz CE. Spouse involvement and a possible long-term follow-up trap in weight loss. Behav Res Ther 1984;22:557–62.
-
Carels RA, Darby L, Cacciapaglia HM, Douglass OM, Harper J, Kaplar ME, et al. Applying a stepped-care approach to the treatment of obesity. J Psychosom Res 2005;59:375–83.
-
Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA 2003;289:1792–8.
-
Johnson SS, Paiva AL, Cummins CO, Johnson JL, Dyment SJ, Wright JA, et al. Transtheoretical model-based multiple behavior intervention for weight management: effectiveness on a population basis. Prev Med 2008;46:238–46.
-
Kostis JB, Wilson AC, Shindler DM, Cosgrove NM, Lacy CR. Non-drug therapy for hypertension: do effects on weight and sodium intake persist after discontinuation of intervention? Am J Med 2000;109:734–6.
-
Kumanyika SK, Shults J, Fassbender J, Whitt MC, Brake V, Kallan MJ, et al. Outpatient weight management in African-Americans: the Healthy Eating and Lifestyle Program (HELP) study. [erratum appears in Prev Med 2006;42:397]. Prev Med 2005;41:488–502.
-
Leermakers EA, Perri MG, Shigaki CL, Fuller PR. Effects of exercise-focused versus weight-focused maintenance programs on the management of obesity. Addict Behav 1999;24:219–27.
-
Melin I, Karlstrom B, Lappalainen R, Berglund L, Mohsen R, Vessby B. A programme of behaviour modification and nutrition counselling in the treatment of obesity: a randomised 2-y clinical trial. Int J Obes 2003;27:1127–35.
-
Murphy JK, Williamson D, Buxton A, Moody SC, Absher N, Warner M. The long term effects of spouse involvement upon weight loss and maintenance. Behav Ther 1982;13:681–93.
-
Perri MG, McAdoo WG, McAllister DA, Lauer JB, Yancey DZ. Enhancing the efficacy of behavior therapy for obesity: effects of aerobic exercise and a multicomponent maintenance program. J Consult Clin Psychol 1986;54:670–5.
-
Perri MG, McAllister DA, Gange JJ, Jordan RC, McAdoo G, Nezu AM. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol 1988;56:529–34.
-
Perri MG, McAdoo WG, Spevak PA, Newlin DB. Effect of a multicomponent maintenance program on long-term weight loss. J Consult Clin Psychol 1984;52:480–1.
-
Rapoport L, Clark M, Wardle J. Evaluation of a modified cognitive-behavioural programme for weight management. Int J Obes 2000;24:1726–37.
-
Rosenthal B, Allen GJ, Winter C. Husband involvement in the behavioural treatment of overweight women: initial effects and long-term follow-up. Int J Obes 1980;4:165–73.
-
Sikand G, Kondo A, Foreyt JP, Jones PH, Gotto AM, Jr. Two-year follow-up of patients treated with a very-low-calorie diet and exercise training. J Am Diet Assoc 1988;88:487–8.
-
Stahre L, Tarnell B, Hakanson CE, Hallstrom T. A randomized controlled trial of two weight-reducing short-term group treatment programs for obesity with an 18-month follow-up. Int J Behav Med 2007;14:48–55.
-
Torgerson JS, Lissner L, Lindroos AK, Kruijer H, Sjostrom L. VLCD plus dietary and behavioural support versus support alone in the treatment of severe obesity. A randomised two-year clinical trial. Int J Obes 1997;21:987–94.
-
Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH, Kostis JB, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons. JAMA 1998;279:839–46.
-
Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes 1996;20:56–62.
-
Yeh MC, Rodriguez E, Nawaz H, Gonzalez M, Nakamoto D, Katz DL. Technical skills for weight loss: 2-y follow-up results of a randomised trial. Int J Obes 2003;27:1500–6.
Length of follow-up
-
Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA 2003;289:2083–93.
-
Adachi Y, Sato C, Yamatsu K, Ito S, Adachi K, Yamagami T. A randomized controlled trial on the long-term effects of a 1-month behavioral weight control program assisted by computer tailored advice. Behav Res Ther 2007;45:459–70.
-
Ash S, Reeves M, Bauer J, Dover T, Vivanti A, Leong C, et al. A randomised control trial comparing lifestyle groups, individual counselling and written information in the management of weight and health outcomes over 12 months. Int J Obes 2006;30:1557–64.
-
Ashby WA, Wilson GT. Behavior therapy for obesity: booster sessions and long-term maintenance of weight loss. Behav Res Ther 1977;15:451–63.
-
Befort CA, Nollen N, Ellerbeck EF, Sullivan DK, Thomas JL, Ahluwalia JS. Motivational interviewing fails to improve outcomes of a behavioral weight loss program for obese African American women: a pilot randomized trial. J Behav Med 2008;31:367–77.
-
Black DR, Coe WC, Friesen JG, Wurzmann AG. Minimal interventions for weight control: a cost-effective alternative. Addict Behav 1984;9:279–85.
-
Burnett KF, Taylor CB, Agras WS. Ambulatory computer-assisted therapy for obesity: a new frontier for behavior therapy. J Consult Clin Psychol 1985;53:698–703.
-
Carels RA, Darby L, Cacciapaglia HM, Konrad K, Coit C, Harper J, et al. Using motivational interviewing as a supplement to obesity treatment: a stepped-care approach. Health Psychol 2007;26:369–74.
-
Carels RA, Konrad K, Young KM, Darby LA, Coit C, Clayton AM, et al. Taking control of your personal eating and exercise environment: a weight maintenance program. Eat Behav 2008;9:228–37.
-
Cussler EC, Teixeira PJ, Going SB, Houtkooper LB, Metcalfe LL, Blew RM, et al. Maintenance of weight loss in overweight middle-aged women through the Internet. Obesity 2008;16:1052–60.
-
Dechamps A, Gatta B, Bourdel-Marchasson I, Tabarin A, Roger P. Pilot study of a 10-week multidisciplinary Tai Chi intervention in sedentary obese women. Clin J Sport Med 2009;19:49–53.
-
Donahoe CP, Jr, Lin DH, Kirschenbaum DS, Keesey RE. Metabolic consequences of dieting and exercise in the treatment of obesity. J Consult Clin Psychol 1984;52:827–36.
-
Donnelly JE, Smith BK, Dunn L, Mayo MM, Jacobsen DJ, Stewart EE, et al. Comparison of a phone vs clinic approach to achieve 10% weight loss. Int J Obes 2007;31:1270–6.
-
Ely AC, Banitt A, Befort C, Hou Q, Rhode PC, Grund C, et al. Kansas primary care weighs in: a pilot randomized trial of a chronic care model program for obesity in 3 rural Kansas primary care practices. J Rural Health 2008;24:125–32.
-
Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc 2008;40:1213–19.
-
Fuller PR, Perri MG, Leermakers EA, Guyer LK. Effects of a personalized system of skill acquisition and an educational program in the treatment of obesity. Addic Behav 1998;23:97–100.
-
Gold BC, Burke S, Pintauro S, Buzzell P, Harvey-Berino J. Weight loss on the web: a pilot study comparing a structured behavioral intervention to a commercial program. Obesity 2007;15:155–64.
-
Gormally J, Rardin D. Weight loss and maintenance and changes in diet and exercise for behavioral counseling and nutrition education. J Couns Psychol 1981;28:295–304.
-
Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns 2008;70:31–9.
-
Harvey-Berino J. Changing health behavior via telecommunications technology: using interactive television to treat obesity. Behav Ther 1998;29:505–19.
-
Hill JO, Schlundt DG, Sbrocco T, Sharp T, Pope-Cordle J, Stetson B, et al. Evaluation of an alternating-calorie diet with and without exercise in the treatment of obesity. Am J Clin Nutr 1989;50:248–54.
-
Jeffery RW, Linde JA, Finch EA, Rothman AJ, King CM. A satisfaction enhancement intervention for long-term weight loss. Obesity 2006;14:863–9.
-
Johnson WG, Stalonas PM, Christ MA, Pock SR. The development and evaluation of a behavioral weight-reduction program. Int J Obes 1979;3:229–38.
-
Katz DL, Chan W, Gonzalez M, Larson D, Nawaz H, Abdulrahman M, et al. Technical skills for weight loss: preliminary data from a randomized trial. Prev Med 2002;34:608–15.
-
Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in WHO class II-III obese men losing weight with very-low-energy diet and behaviour modification: a randomised clinical trial. Int J Obes 2002;26:487–95.
-
Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res 2003;11:689–94.
-
Lafortuna CL, Resnik M, Galvani C, Sartorio A. Effects of non-specific vs individualized exercise training protocols on aerobic, anaerobic and strength performance in severely obese subjects during a short-term body mass reduction program. J Endocrinol Invest 2003;26:197–205.
-
Lutes LD, Winett RA, Barger SD, Wojcik JR, Herbert WG, Nickols-Richardson SM, et al. Small changes in nutrition and physical activity promote weight loss and maintenance: 3-month evidence from the ASPIRE randomized trial. Ann Behav Med 2008;35:351–7.
-
Meyers AW, Graves TJ, Whelan JP, Barclay DR. An evaluation of a television-delivered behavioral weight loss program: are the ratings acceptable? J Consult Clin Psychol 1996;64:172–8.
-
Micco N, Gold B, Buzzell P, Leonard H, Pintauro S, Harvey-Berino J. Minimal in-person support as an adjunct to internet obesity treatment. Ann Behav Med 2007;33:49–56.
-
Munsch S, Biedert E, Keller U. Evaluation of a lifestyle change programme for the treatment of obesity in general practice. Swiss Med Wkly 2003;133:148–54.
-
Narayan KM, Hoskin M, Kozak D, Kriska AM, Hanson RL, Pettitt DJ, et al. Randomized clinical trial of lifestyle interventions in Pima Indians: a pilot study. Diabet Med 1998;15:66–72.
-
Oh EG, Hyun SS, Kim SH, Bang SY, Chu SH, Jeon JY, et al. A randomized controlled trial of therapeutic lifestyle modification in rural women with metabolic syndrome: a pilot study. Metabolism 2008;57:255–61.
-
Perri MG, Shapiro R M, Ludwig WW, Twentyman CT, McAdoo WG. Maintenance strategies for the treatment of obesity: an evaluation of relapse prevention training and posttreatment contact by mail and telephone. J Consult Clin Psychol 1984;52:404–13.
-
Perri MG, Nezu AM, Patti ET, McCann KL. Effect of length of treatment on weight loss. J Consult Clin Psychol 1989;57:450–2.
-
Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol 2001;69:722–6.
-
Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med 2008;168:2347–54.
-
Pettman TL, Misan GMH, Owen K, Warren K, Coates AM, Buckley JD, et al. Self-management for obesity and cardio-metabolic fitness: description and evaluation of the lifestyle modification program of a randomised controlled trial. Int J Behav Nutr Phys Act 2008;5:53.
-
Polzien KM, Jakicic JM, Tate DF, Otto AD. The efficacy of a technology-based system in a short-term behavioral weight loss intervention. Obesity 2007;15:825–30.
-
Ramirez EM, Rosen JC. A comparison of weight control and weight control plus body image therapy for obese men and women. J Consult Clin Psychol 2001;69:440–6.
-
Rippe JM, Price JM, Hess SA, Kline G, DeMers KA, Damitz S, et al. Improved psychological well-being, quality of life, and health practices in moderately overweight women participating in a 12-week structured weight loss program. Obes Res 1998;6:208–18.
-
Rolland C, Hession M, Murray S, Wise A, Broom I. Randomized clinical trial of standard dietary treatment versus a low-carbohydrate/high-protein diet or the LighterLife Programme in the management of obesity. J Diabetes 2009;1:207–17.
-
Rothert K, Strecher VJ, Doyle LA, Caplan WM, Joyce JS, Jimison HB, et al. Web-based weight management programs in an integrated health care setting: a randomized, controlled trial. Obesity 2006;14:266–72.
-
Sbrocco T, Nedegaard RC, Stone JM, Lewis EL. Behavioral choice treatment promotes continuing weight loss: preliminary results of a cognitive-behavioral decision-based treatment for obesity. J Consult Clin Psychol 1999;67:260–6.
-
St GA, Bauman A, Johnston A, Farrell G, Chey T, George J. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol 2009;24:399–407.
-
Stalonas PM, Jr., Johnson WG, Christ M. Behavior modification for obesity: the evaluation of exercise, contingency management, and program adherence. J Consult Clin Psychol 1978;46:463–9.
-
Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA 2001;285:1172–7.
-
Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA 2003;289:1833–6.
-
Truby H, Baic S, deLooy A, Fox KR, Livingstone MB, Logan CM, et al. Randomised controlled trial of four commercial weight loss programmes in the UK: initial findings from the BBC ‘diet trials’ [erratum appears in BMJ 2006;332:1418]. BMJ 2006;332:1309–14.
-
Tuomilehto HP, Seppa JM, Partinen MM, Peltonen M, Gylling H, Tuomilehto JO, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009;179:320–7.
-
Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med 2006;166:860–6.
-
Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol 1994;62:165–71.
-
Wing RR, Epstein LH, Shapira B. The effect of increasing initial weight loss with the Scarsdale Diet on subsequent weight loss in a behavioral treatment program. J Consult Clin Psychol 1982;50:446–7.
-
Wylie-Rosett J, Swencionis C, Ginsberg M, Cimino C, Wassertheil-Smoller S, Caban A, et al. Computerized weight loss intervention optimizes staff time: the clinical and cost results of a controlled clinical trial conducted in a managed care setting. J Am Diet Assoc 2001;101:1155–62.
aDiabetes prevention studies
A number of studies listed above were diabetes prevention studies. The diabetes prevention studies are aimed at a group with an existing risk factor to try to prevent the onset of disease. The interventions are therefore focused on the disease and not general weight loss. This differs from the hypertension prevention studies that are aimed at a group of overweight people without known disease to prevent them getting a risk factor for a disease, not the disease itself. The interventions are therefore focused on general weight loss. As a general rule the diabetes prevention studies would have been excluded from the present review on this basis. However, in the listing above it can be seen that individual diabetes prevention studies were excluded on other grounds, such as the intervention not being a multicomponent approach.
Cost-effectiveness review
Abstracts (insufficient information)
-
Forster M. Cost-effectiveness analysis of interventions to reduce overweight and obesity in Australia. 7th World Congress on Health Economics. 2009. Beijing
-
Gustafson A, Samuel-Hodge C, Khavjou O, Keyserling T, Lindsley S, Garcia B, et al. Cost-effectiveness of a WISEWOMAN behavioral weight loss intervention for low-income women: the Weight-Wise Program. Obesity 2008;16:S158.
-
Rasu R, Hunter C, Maruska H, Peterson A, Foreyt J. Economic evaluation of an internet based weight management program. Value Health 2009;12:A133.
Participants
-
Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2001;8(21).
-
National Institute for Health and Clinical Excellence. Guidance on the prevention, identification, assessment and management of overweight and obesity in adults and children. London: National Institute for Health and Clinical Excellence; 2006.
Intervention
-
Bemelmans W, van BP, Wendel-Vos W, Schuit J, Feskens E, Ament A, et al. The costs, effects and cost-effectiveness of counteracting overweight on a population level. A scientific base for policy targets for the Dutch national plan for action. Prev Med 2008;46:127–32.
-
Galani C, Schneider H, Rutten FFH. Modelling the lifetime costs and health effects of lifestyle intervention in the prevention and treatment of obesity in Switzerland. Int J Public Health 2007;52:372–82.
-
Galani C, Al M, Schneider H, Rutten FFH. Uncertainty in decision-making: value of additional information in the cost-effectiveness of lifestyle intervention in overweight and obese people. Value Health 2008;11:424–34.
-
Gusi N, Reyes MC, Gonzalez-Guerrero JL, Herrera E, Garcia JM. Cost-utility of a walking programme for moderately depressed, obese, or overweight elderly women in primary care: a randomised controlled trial. BMC Public Health 2008;8:231.
-
Jensen C, Flum DR. The costs of nonsurgical and surgical weight loss interventions: Is an ounce of prevention really worth a pound of cure? Surg Obes Relat Dis 2005;1:353–7.
-
Sherwood NE, Jeffery RW, Pronk NP, Boucher JL, Hanson A, Boyle R, et al. Mail and phone interventions for weight loss in a managed-care setting: weigh-to-be 2-year outcomes. Int J Obes 2006;30:1565–73.
Study design
-
Fineberg HV. An economic analysis of eating and physical activity behaviors: exploring effective strategies to combat obesity. Am J Prev Med 2004;27:172–4.
-
Meenan RT, Stevens VJ, Funk K, Bauck A, Jerome GJ, Lien LF, et al. Development and implementation cost analysis of telephone- and Internet-based interventions for the maintenance of weight loss. Int J Technol Assess Health Care 2009;25:400–10.
Appendix 7 Unclear studies
It was not clear from the description of the interventions in the following studies whether the multicomponent intervention met the criteria of the current review and therefore trial authors were contacted for further advice. At the point of writing no further details had been received.
| Study author | Length of follow-up | Details diet | Details exercise | Details behaviour |
|---|---|---|---|---|
| a Borg et al. 2002123 | 29 months | Yes | Yes | Unclear |
| Hakala et al. 1994124 | 5 years | Unclear | Unclear | Unclear |
| Hakala et al. 1993125 | 5 years | Unclear | Unclear | No |
| Linde et al. 2006126 | 24 months | Unclear | Unclear | Unclear |
| Ashley et al. 2001127 | 24 months | Yes | Yes | Unclear |
| Svetkey et al. 2008128 | 30 months | Unclear | Unclear | Unclear |
Appendix 8 Informal assessment of key attributes of included studies
| Study | Length of follow-up ≥ 2 years | Sample size per arm ≥ 100 | Risk of bias low | Statistically significant effect ≥ 2 years | Generalisability | Intervention reproduciblea |
|---|---|---|---|---|---|---|
| Logue et al. 200572 | Yes | Yes | Yes | No | Unclear | No |
| Stevens et al. 200170 | Yes | Yes | Yes | Yes | Unclear | Yes |
| Simkin-Silverman et al. 199873 | Yes | Yes | Yes | Yes | No | Yes |
| Jeffery and Wing 199575 | Yes | No | No | Unclear | Unclear | Yes |
| Stevens et al. 199374 | No | Yes | No | N/A | Unclear | Approximately |
Appendix 9 Data extractions for full papers for included studies in cost-effectiveness review
Reference
The Counterweight Programme, 201087
Research question
What are the stated objectives of the evaluation?
To estimate the economic effectiveness of the Counterweight Programme
Funding source
Roche Products Ltd
Study population
What definition was used for (condition)?
Obese and overweight adults
What are the characteristics of the baseline cohort for the evaluation?
1906 adult patients (aged 18–75) in 65 UK general practitioner (GP) practices with BMI ≥ 30 kg/m2 or ≥ 28 kg/m2 with obesity-related comorbidities. Mean age 49.4 years, 77% female. Mean BMI 37.1 kg/m2. Diabetes present 13.5%. Cardiovascular disease 8%
Interventions and comparators
What interventions/strategies were included?
Diet, exercise and behaviour intervention
Was a no treatment/supportive care strategy included?
Compared with no active intervention. Note: economic evaluation was based on a cohort study, rather than a randomised controlled trial (RCT)
Describe interventions/strategies
First line interventions were a prescribed-eating plan, a goal-setting approach, or a group intervention. These were all aimed at achieving an energy deficit of ≥ 500 kcal/day. Patients were asked to commit to nine appointments in 12 months, including six individual appointments (10–30 minutes) and six group sessions (I hour each) over a 3-month period and then follow-up at 6, 9, 12 and 24 months. In addition to dietary component, physical activity and behaviour management components were also included. The physical activity component consisted of encouraging patients to take more than 30 minutes moderate physical activities on most days by incorporating activity into daily living, for example through walking more, and referral to existing exercise schemes. Patients who did not achieve more than 5% weight loss at 3 months were eligible for pharmacotherapy. Antiobesity medication was prescribed to approximately 8% of patients during the first 12 months
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third party payer, societal (i.e. including costs borne by individuals and lost productivity)?
UK NHS
Study type
Cost-effectiveness/cost–utility/cost–benefit analysis?
Cost–utility
Institutional setting
Where is/are the intervention(s) being evaluated usually provided?
Primary care
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
UK £. 2001–3 for intervention and 2005 for model
Effectiveness
Were the effectiveness data derived from: a single study, a review/synthesis of previous studies or expert opinion?
A single study (the Counterweight Programme)
Give the definition of treatment effect used in the evaluation
Mean weight change
Give the size of the treatment effect used in the evaluation
Mean weight change (kg) at 12 months (n = 642) was –3.0 (95% CI –3.5 to –2.4) and at 24 months (n = 357) was –2.3 kg (95% CI –3.2 to –1.4). Untreated patients are assumed to gain 1 kg weight per year
Intervention costs
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion?
A single study (the Counterweight Programme)
Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Costs of the intervention are described in another article (J Health Serv Res Policy 2008)36
List the direct intervention costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used
The cost was estimated for all practices in the UK having access to it over a 5-year period. Costs included remuneration for all clinicians time required for the intervention – training, GPs time for clinical and motivational assessment, practice nurse time for assessment, motivation, delivery of advice and review. Costs for the Counterweight Programme team, training resources and patient information materials were also included in the analysis. Costs for the intervention were based on the optimal attendance rate of at least six visits in 12 months. In addition the costs of weight management medications prescribed according to protocol were incorporated. Around 20% of patients followed up at 12 months or around 9% of the total intervention group were prescribed weight management drugs
Costs shown in Appendices 3 and 4 of prescribing article. However there are some discrepancies between the appendices
Summary of costs:
In year 0 there is a one-off cost of £1.9M to recruit and train the Weight Management Advisors (WMAs). In year 1 the costs are as follows:
National co-ordination costs £120,000
WMA teams cost £7.8M
Meetings with local staff cost £368,000
Costs to practices of the first wave of audit and training are £1.4M
Costs of the intervention in the first-wave practices are £23M
The total cost in year 1 is £33M, two-thirds of which is the practice nurse time and resources for the intervention
Costs in year 2 are similar but it is assumed that first wave practices recruit a further cohort of patients, so the total cost is higher at £45M. Similarly in year 3 first- and second-wave practices recruit further cohorts of patients so total costs are £56M, with costs in years 4 and 5 being £68M and £80M, respectively
The total cost over this period (including set-up) is £196M. In this time 2400 practices will have recruited five cohorts of 92 patients, 2400 practices will have recruited four cohorts, 2400 will have recruited three cohorts, and so on. In total 3.3 million people will have been recruited. The average cost per person recruited is thus £56.60 per patient
Indicate the source for individual cost values (if appropriate)
Other direct costs (costs incurred directly in treating patients)
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion?
Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Taken from published studies
List the costs used in the evaluation – if quantities of resource use are reported separately from cost values, show sources for the resource estimates as well as sources for unit costs used
Yearly costs per year included for medical conditions such as CHD (£1637), diabetes (£653) and colon cancer (£7320), based on (Ara and Brennan, 2005) and (O’leary, 2004)
Indicate the source for individual cost values (if appropriate)
Indirect costs (costs due to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
Not included
Describe how indirect costs were estimated (e.g. how days of lost productivity were estimated and how those days were valued)
N/A
Indicate the source for individual cost values (if appropriate)
Health state valuations/utilities (if study uses quality of life adjustments to outcomes)
Were the utility data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Several studies
List the utility values used in the evaluation
Utility values from the general population (Macranand colleagues88) were adjusted to obtain QALYs for individuals with any of the comorbidities, such as diabetes and CHD. The multipliers for diabetes and CHD were provided by Ara and Brennan (0.8661 and 0.8670, respectively). Individuals with colon cancer had a 5% lower QALY based on Lewis and colleagues129
| BMI (kg/m2) | Male | Female |
|---|---|---|
| < 21 | 0.86 | 0.85 |
| 21–25 | 0.87 | 0.87 |
| 26–30 | 0.86 | 0.82 |
| 31–39 | 0.82 | 0.78 |
| > 39 | 0.88 | 0.75 |
As such, a man with a BMI of 31–39 who also has CHD, diabetes and colon cancer would have a utility value of 0.58 compared with the 0.82 reported in Table 24 for an individual with no comorbidities
Indicate the source for individual cost values (if appropriate)
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original
A patient-level simulation model was used which was originally developed to provide input to the UK national guidance on obesity. (NICE, 2006)
What was the purpose of the model (i.e. why was a model required in this evaluation)?
To estimate lifetime outcome of a model cohort reflective of UK adult population
What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported? (List them if reported)
The model works by randomly selecting an individual whose characteristics are based on those of the population (for example, BMI, age and gender) and following their healthcare costs and outcomes until death. The model assesses people over 6-month cycles. Each cycle each individual will experience a change in BMI (increase, decrease or no change). The individual can develop diabetes, CHD, or colon cancer depending on the prevalence of each disease at the BMI they are currently experiencing. There is a QALY associated with each health state. Patients are at increased risk of death if they experience one of these conditions
Patients are assumed to regain all 4 kg weight difference effect in two years following removal of the programme
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text)
Prevalence of diabetes based on BMI level from Gregg and colleagues. 130 Prevalence of CHD based on Framingham equation as set out by Brindle and colleagues depending on age, smoking, blood pressure (BP), cholesterol, diabetes. Prevalence of colon cancer was derived from a study by Giovannucci and colleagues131
What is the model time horizon?
Lifetime
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
3.5%
Results/analysis
What measure(s) of benefit were reported in the evaluation?
QALY gain
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
For no active intervention, lifetime QALYs are 28.32, for baseline intervention lifetime QALYs are 28.38. QALY gain is 0.056
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
The lifetime cost to the NHS of no active intervention is £1884 and for the baseline intervention is £1857
Synthesis of costs and benefits – are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
The ICER is –£473 per QALY gained, i.e. cost saving
Give results of any statistical analysis of the results of the evaluation
N/A
Was any sensitivity analysis performed – if yes, what type(s) [i.e. deterministic (one-way, two-way etc.) or probabilistic]
One-way sensitivity analysis performed
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
No sensitivity analyses for structural, methodological or assumptions
Give a summary of the results of the sensitivity analysis – did they differ substantially from the base case analysis? If so, what were the suggested causes?
Sensitivity analyses were conducted for background weight change for the untreated population. For background weight change of 0.5 kg/year and 0.3 kg/year the ICER was £2017 and £2651 respectively
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis
Even based on very limited estimates of the costs of obesity, the Counterweight Programme is highly cost-effective and will provide cost savings in the medium to long term
What are the implications of the evaluation for practice?
Counterweight represents a highly efficient use of health-care resources
Reference
Roux and colleagues 200686
Research question
What are the stated objectives of the evaluation?
To conduct a clinical and economic evaluation of outpatient weight loss strategies in overweight and obese adult US women
Funding source
Not stated
Study population
What definition was used for (condition)?
Overweight and obesity defined as BMI > 24.9 kg/m2
What are the characteristics of the baseline cohort for the evaluation?
Hypothetical cohort of 10,000 healthy, non-pregnant 35-year-old overweight and obese women with original BMI > 24.9 kg/m2 and free from known CHD
Interventions and comparators
What interventions/strategies were included?
Each strategy consisted of a 6-month weight loss intervention followed by a 6-month maintenance programme
Diet only
Diet and pharmacotherapy
Diet and exercise
Diet, exercise and behavioural modification
Women unable to lose weight or maintain successful weight loss were assumed to remain at their age-adjusted original BMI
Was a no treatment/supportive care strategy included?
Routine care (not defined)
Describe interventions/strategies
Diet: reduction in caloric intake necessary to achieve a 10% weight loss under the supervision of a dietitian, in accordance with the American Heart Association guidelines
Pharmacotherapy: 120 mg orlistat three times per day for 6 months, then half this dose per day for 6 months maintenance phase
Exercise: three 45-minute structured exercise sessions per week of moderate intensity, led by a certified instructor, and two sessions per month to review clinical progress with an exercise therapist
Behavioural modification: 1 hour cognitive therapy counselling session led by a psychologist every other week
Analytical perspective
What is the perspective adopted for the evaluation (health service, health and personal social services, third party payer, societal (i.e. including costs borne by individuals and lost productivity)?
Societal
Study type
Cost-effectiveness/cost–utility/cost–benefit analysis?
Cost-effectiveness and cost–utility
Institutional setting
Where is/are the intervention(s) being evaluated usually provided?
Outpatient setting? Single urban setting (p. 1103)
Country/currency
Has a country setting been provided for the evaluation? What currency are costs expressed in and does the publication give the base year to which those costs relate?
Canada. Currency US$. Base year 2001
Effectiveness
Were the effectiveness data derived from: a single study, a review/synthesis of previous studies or expert opinion?
Review (methods not clear) of published literature to identify RCTs from which four studies were selected for estimating efficacy (only referenced in online appendix, not accessible)
Give the definition of treatment effect used in the evaluation
10% BMI reduction after 6-month weight loss programme
Short-term success defined as maintenance of reduced BMI postintervention for at least 6 months
Long-term success defined as maintenance of reduced BMI for at least 5 years after intervention
Give the size of the treatment effect used in the evaluation
Change in BMI postintervention:
Routine care 0.26
Diet only –1.98
Diet and exercise –2.55
Diet and pharmacotherapy –4.55
Diet, exercise and behavioural modification –3.11
Intervention costs
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion?
Not clear
Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Not enough information
List the direct intervention costs used in the evaluation – include resource estimates (and sources for these estimates, if appropriate) as well as sources for unit costs used
| Routine care | Diet only | Diet and exercise | Diet and pharmacotherapy | Diet, exercise, behavioural modification | |
|---|---|---|---|---|---|
| Direct non-medical costs and time-related per participant programme costs (6-month, US$) | 0 | 120 | 630 | 120 | 630 |
| Direct medical per participant programme costs (6 month) US$ (referenced overall but not individually, Table 1) | 700 | 2150 | 2750 | 2820 | 3040 |
Indicate the source for individual cost values (if appropriate)
Other direct costs (costs incurred directly in treating patients)
Were the cost data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion?
Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
Micro-costing techniques used to estimate resource use
Direct costs based on published data and valued using Medicare reimbursement rates
(Includes consultations, laboratory tests, chest X-rays, electrocardiogram and exercise stress test, and educational materials)
Unit costs and quantities not reported separately
Obesity-related morbidity and mortality, such as diabetes and CHD, used annual age and sex-specific treatment related costs (referenced)
Age-specific costs associated with CHD for women represented a published weighted average of the expected management costs of a non-fatal myocardial infarction, cardiac arrest and angina pectoris (referenced)
Annual age-specific direct health-care costs not specific to obesity-related morbidity were included (referenced)
List the costs used in the evaluation – if quantities of resource use are reported separately from cost values, show sources for the resource estimates as well as sources for unit costs used
| Costs | |
|---|---|
| Cost of drug per pill (US$) (published data) | 1.32 |
| 6-month maintenance per-participant programme costs (US$) | Programme incorporating any combination of diet, exercise and behaviour modification: 150 |
| Programme incorporating pharmacotherapy: 360 | |
| Routine care: 0 | |
| Annual comorbidity treatment cost (US$) | Hypertension: 616.62 |
| Hypercholesterolemia: 176.34 | |
| Type 2 diabetes age-specific | |
| CHD first year: 10,850 | |
| CHD subsequent years: 1710 | |
| CHD fatal: 3665 |
Indicate the source for individual cost values (if appropriate)
Indirect costs (costs due to lost productivity, unpaid inputs to patient care)
Were indirect costs included?
Yes (dietary changes, exercise equipment, fitness monitoring devices, fitness apparel, and transportation and time costs)
Describe how indirect costs were estimated (e.g. how days of lost productivity were estimated and how those days were valued)
Primary data collected from a survey of a community sample (n = 100) of female weight management programme participants. The survey elicited demographic information and cost information using modified version of a previously described generic UK cost and use survey (referenced). Direct non-medical resources were valued using self-reported items prices. Self-reported mileage travelled to and from classes and associated physician visits to estimate travel costs. Total annual distance travelled in miles was valued per participant, based on published estimates. These per-person annual travel costs were totalled and averaged across all participants. Time costs estimated using survey data; time was valued by applying wage rates specific to their occupation. Time lost from work was valued using US national average wage rate. Wage rates for domestic child care and light cleaning services were used to wage time lost from performing household duties
Indicate the source for individual cost values (if appropriate)
Health state valuations/utilities (if study uses quality of life adjustments to outcomes)
Were the utility data derived from: a single (observational) study, a review/synthesis of previous studies expert opinion. Were the methods for deriving these data adequately described (give sources if using data from other published studies)?
General age-specific quality weights were derived from the Beaver Dam Health Outcomes study91 and applied for women > 45 years of age. This was a longitudinal cohort study of health status and health-related quality of life for a random sample of adults (mean 64.1, range 45–89 years) in a community population. Four measures were used: Short-Form questionnaire-36 items, Quality of Well-being index, self-reported health status on a five point scale from ‘excellent’ to ‘poor’ and evaluation of current health using time trade-offs. These results were adjusted to reflect weight loss using quality weights derived from the study sample and comorbid diseases using a multiplicative function (and an additive function in sensitivity analyses). No further details given
Quality of life for the reduction in weight loss estimated from community sample with average per cent reductions in life expectancy that subjects were willing to give up through treatment with a single pill, free of charge and side effects but which would not prevent or cure health problems or incur survival benefit, to achieve sustained BMI reduction to the average weight for their height (0.87) and for 10% reduction in BMI (0.93). Quality weights rescaled to economic uses between death (0) and perfect health (1) using previously described methods (referenced)
Temporary decrements in QoL attributable to the interventions were assumed to be related to the intensity of effort required to participate in a particular programme from primary data analysis and were assigned for a 6-month period: QoL = 1 for routine care; QoL = 0.91 for diet, exercise, behavioural modification programme. Other programs of intermediate intensity were assigned values between 0.91 and 1
List the utility values used in the evaluation
Disease-specific quality weights:
Obesity = 0.87
10% reduction weight loss = 0.93
CHD = 0.75 (age-adjusted)
Type 2 diabetes = 0.75 (age-adjusted)
Indicate the source for individual cost values (if appropriate)
Modelling
If a model was used, describe the type of model used (e.g. Markov state transition model, discrete event simulation). Was this a newly developed model or was it adapted from a previously reported model? If an adaptation, give the source of the original
First-order Monte Carlo simulation
What was the purpose of the model (i.e. why was a model required in this evaluation)?
Decision analytic techniques can be used to estimate effectiveness and cost-effectiveness of a number of alternative strategies to reduce BMI in overweight and obese women, taking into account best available data and future uncertainties in costs and benefits
What are the main components of the model (e.g. health states within a Markov model)? Are sources for assumptions over model structure (e.g. allowable transitions) reported? (List them if reported)
The model uses a state-transition framework with the natural history of obesity in a cohort of hypothetical women characterised as a sequence of annual transitions from one health state to another
Women enter the model aged 35 years free from known CHD. Each year a woman’s BMI predicts the risk of developing hypertension, Type 2 diabetes or hypercholesterolemia, which predicts her risk of CHD and CHD death (diagram given)
Extract transition probabilities for (natural history/disease progression) model and show sources (or refer to table in text)
| From Table 186 | Routine care | Diet only | Diet and exercise | Diet and pharmacotherapy | Diet, exercise, behavioural modification |
|---|---|---|---|---|---|
| Probability of programme compliance | 1 | 0.84 | 0.86 | 0.69 | 0.90 |
| Probability 10% weight loss at 6 months | 0.05 | 0.26 | 0.68 | 0.96 | 0.95 |
| Probability of weight loss maintenance at 1 year | 0.5 | 0.15 | 0.55 | 0.37 | 0.67 |
| Probability of weight loss maintenance at 5 years | Programmes without lifestyle modification: 0.1 | 0.2 | |||
What is the model time horizon?
Lifetime horizon
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
3% discount rate for costs and benefits
Results/analysis
What measure(s) of benefit were reported in the evaluation?
Life years and QALYs
Provide a summary of the clinical outcome/benefits estimated for each intervention/strategy assessed in the evaluation
| From Table 2 86 | Routine care | Diet only | Diet and exercise | Diet and pharmacotherapy | Diet, exercise, behavioural modification |
|---|---|---|---|---|---|
| Discounted life expectancy (weeks) | 24.119 | 24.120 | 24.129 | 24.128 | 24.170 |
| Discounted QALY? (months) | 18.183 | 18.169 | 18.255 | 18.248 | 18.426 |
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
| From Table 2 | Routine care | Diet only | Diet and exercise | Diet and pharmacotherapy | Diet, exercise, behavioural modification |
|---|---|---|---|---|---|
| Discounted lifetime costs US$ | 121,120 | 122,440 | 123,240 | 122,660 | 124,200 |
Synthesis of costs and benefits – are the costs and outcomes reported together (e.g. as cost-effectiveness ratios)? If so, provide a summary of the results
Diet, exercise and behavioural modification was the dominant strategy. The ICER was US$60,390 per life-year gained and US$12,640 per QALY when compared with routine care
Give results of any statistical analysis of the results of the evaluation
N/A
Was any sensitivity analysis performed – if yes, what type(s) [i.e. deterministic (one-way, two-way etc.) or probabilistic]
Deterministic (one-way) sensitivity analysis performed
What scenarios were tested in the sensitivity analysis? How do these relate to structural uncertainty (testing assumptions over model structure such as relationships between health states), methodological uncertainty (such as choices of discount rate or inclusion of indirect costs) or parameter uncertainty (assumptions over values of parameters in the model, such as costs, quality of life or disease progression rates)?
Discount rate (methodological)
6-month programme costs (parameter)
Probability of compliance with 6-month intervention (parameter)
Mortality rates for CHD (parameter)
Comorbidity QoL (parameter)
Drug costs (parameter)
Give a summary of the results of the sensitivity analysis – did they differ substantially from the base case analysis? If so, what were the suggested causes?
Results were most sensitive to variation in the obesity-related effects on QoL and the likelihood of long-term weight loss maintenance. (Hard to tell which parameters had most effect on results as axis on the graph not labelled properly)
Conclusions/implications
Give a brief summary of the author’s conclusions from their analysis
Authors concluded that a multidisciplinary weight management programme of diet, exercise and behavioural modification for overweight and obese women may represent good value for money
What are the implications of the evaluation for practice?
Authors concluded also that although a three-component strategy appears to provide reasonable return on resources invested, the ‘worthwhileness’ of such a programme would depend on the resources displaced to fund it
Authors recommend that future research should aim to confirm the impacts of such combined programmes on QoL and the likelihood of long-term weight loss maintenance. Investments that improve long-term maintenance, even if costly, may provide good return in terms of population health gain for resources invested
List of abbreviations
- BMI
- body mass index (kg/m2)
- BP
- blood pressure
- CRD
- Centre for Reviews and Dissemination
- CHD
- coronary heart disease
- CI
- confidence interval
- EQ-5D
- European Quality of Life-5 Dimensions
- FP
- food provision
- GP
- general practitioner
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HSE
- Health Survey for England
- HYHEP
- Help Your Heart Eating Plan
- HPA
- high physical activity
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LDL
- low-density lipoprotein
- LI
- lifestyle intervention
- LOV-D
- lacto-ovo-vegetarian diet
- LYG
- life-year gained
- NICE
- National Institute for Health and Clinical Excellence
- NS
- not statistically significant
- NSF
- National Service Framework
- OTC
- over-the-counter
- PCT
- primary care trust
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RD
- registered dietitian
- REE
- resting energy expenditure
- RR
- relative risk
- SBT
- standard behavioural therapy/treatment
- SBTE
- standard behavioural therapy and exercise
- SBTT
- standard behavioural therapy and trainer
- SBTI
- standard behavioural therapy and incentive
- SBTTI
- standard behavioural therapy and trainer and incentive
- SD
- standard deviation
- SE
- standard error
- SOC
- stages of change
- SS
- social support
- STD-D
- standard diet
- TOHP
- Trials of Hypertension Prevention
- UC
- usual care
- USDA
- US Department of Agriculture
- VLCD
- very-low-calorie diet
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Service User Representative
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Service User Representative
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Service User Representative
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Service User Representative
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Service User Representative
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Service User Representative
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Service User Representative
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Service User Representative
-
Mr David P Britt, Service User Representative
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Service User Representative
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Service User Representative
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Service User Representative
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Service User Representative
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Service User Representative
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington