Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 05/16/01. The contractual start date was in September 2006. The draft report began editorial review in June 2010 and was accepted for publication in October 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
A Frost is a board member of the UK Water Treatment Association, consultant for the European Water Treatment Association and member of the Water Quality Association (USA). I Pallett was technical director and a board member of British Water until June 2009 and is now a technical consultant to British Water and also to Aqua Europa – the Federation of European Water Industry Associations.
Permissions
Copyright statement
© 2011 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
The problem of eczema
Atopic eczema (atopic dermatitis) is the most common inflammatory skin disease in childhood, with a prevalence of around 20% in England, Australia and Scandinavia. There is recent evidence of a worldwide increase in atopic eczema symptoms in primary school-aged children. 1 The term atopic eczema is synonymous with atopic dermatitis. The World Allergy Organization now suggests that the phenotype of atopic eczema should be called just eczema unless specific immunoglobulin E (IgE) antibodies are demonstrated, and we will use the term eczema throughout this report.
The burden of eczema is wide-ranging. The child’s life is affected in many ways, including the suffering of intractable itch, sleep disturbance and ostracism by other children. Family disturbance is also considerable, including sleep loss and the need to take time off work for visits to health-care professionals. 2–4 Wider economic costs are considerable. Reviews of the socioeconomic impact of eczema reveal significant burdens worldwide, including the UK,5,6 the USA7 and Australia. 8
Treatment options for childhood eczema have traditionally focused on topical medications, with topical corticosteroids being the mainstay of treatment of skin inflammation and regular use of emollients for dry skin. 9 However, many parents of children with atopic eczema worry about the side effects of conventional topical medications. 10 Although the degree of public concern about the side effects of corticosteroids, such as skin thinning and growth retardation, has not been supported by long-term studies,2 it is important to recognise these concerns and continue to look for other ways of treating atopic eczema. Options that avoid the possible side effects of conventional pharmacological treatments would be a welcome addition to the management of eczema.
Water hardness and eczema
There is evidence from ecological studies linking increasing water hardness with increasing eczema prevalence in children of primary school age. This was first demonstrated in the UK in a study of 4141 primary school children. 11 The 1-year period prevalence of eczema was 17.3% in the hardest water category and 12.0% in the lowest [odds ratio (OR) 1.54, 95% confidence interval (CI) 1.19 to 1.99 after adjustment for confounders]. Such a gradient was not seen in secondary school children in the same study. Similar results were subsequently reported in a large study in Japan of 458,284 children aged 6–12 years, in which the prevalence was 24.4% in the hardest water category and 22.9% in the lowest,12 and in a study in Spain of 3907 children aged 6–7 years, in which the lifetime prevalence was 36.5% in the hardest water category and 28.6% in the lowest. 13 There are also anecdotal reports from the patients themselves that water softeners are of benefit to eczema sufferers.
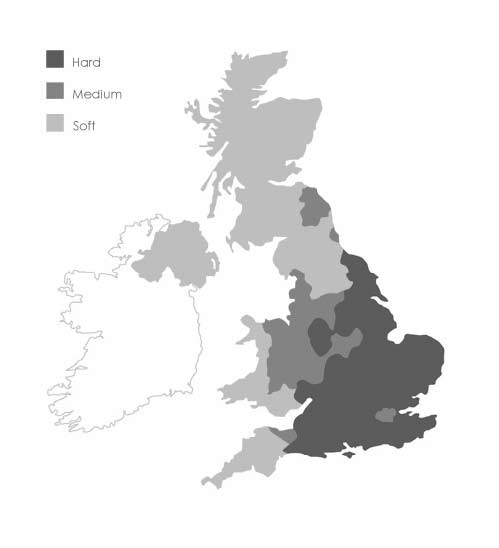
Hardness in water is due to a high mineral content, primarily calcium and magnesium ions. Calcium usually enters the water supply as calcium bicarbonate as the water passes through limestone or chalk rocks. Water hardness varies across the UK, but is generally classified as hard to very hard (> 200 mg/l calcium carbonate) throughout southern and central England (Figure 1).
FIGURE 1.
Water hardness in the UK. Soft, < 100 mg/l calcium carbonate; medium, 100–200 mg/l calcium carbonate; hard, > 200 mg/l calcium carbonate.
If the association between water hardness and eczema prevalence is a causative one, a number of possible mechanisms can be put forward to suggest why hard water could exacerbate eczema. Perhaps the most likely explanation is increased soap usage in hard water areas, the deposits of which (‘soap scum’) can cause skin irritation in eczema sufferers. 14,15 This could be from direct skin contact with soap scum during washing or from the irritant effect of residual deposits in clothes and bedding. A direct chemical irritant effect from calcium and magnesium salts is also possible, or an indirect effect of enhanced allergen penetration from skin barrier disruption16 and increased bacterial colonisation of the skin. 14
Water softeners
Ion-exchange water softening is a well-understood and widely available technology. Water softeners are mainly used in households for reducing calcium deposits in appliances. They are usually installed under the kitchen sink and plumbed into the water supply to soften water to the whole house. A typical purchase price, including installation, would be approximately £600 ($900, €700). Ion-exchange water softeners remove calcium and magnesium ions, replacing them with sodium ions (from common salt). They reduce water hardness to < 20 mg/l calcium carbonate.
To fully soften water, calcium and magnesium ions must be removed, and domestic ion-exchange water softeners are the only products specifically designed to do this. Other technologies include water conditioners (also called ‘physical water conditioners’), which reduce limescale build-up by altering the physical properties of calcium and magnesium ions, but they do not affect the chemical composition of the water and therefore do not affect its hardness. For this reason ion-exchange water softeners were installed in the Softened Water Eczema Trial (SWET), and throughout this report the term ‘water softeners’ refers to ion-exchange technology.
Despite interest from people with eczema using water softeners, a Health Technology Assessment (HTA) systematic review of eczema treatments failed to identify any trials evaluating the use of water softeners for patients with eczema. 12 The only trials of possible relevance were an inconclusive one looking at the benefits of salt baths and another that examined the use of biological versus non-biological washing powders. The search for new, relevant studies was updated in 2010 and no new evidence on the use of softened water was found (see Appendix 1 for search strategy). The HTA systematic review identified a randomised controlled trial (RCT) of water softeners as one of six urgent research priorities in eczema. As a result of this, a feasibility study was run by the University of Nottingham in 2002 involving 17 families living in Nottingham, UK. This informed the design of SWET, in which ion-exchange water softeners were compared with usual eczema care in over 300 children recruited from seven hard water areas across England.
Objectives of the trial
The Softened Water Eczema Trial (SWET) had two main objectives: (i) to assess whether installation of an ion-exchange water softener reduces the severity of eczema in children with moderate-to-severe eczema; and, if so, (ii) to establish the likely cost and cost-effectiveness of this intervention.
Chapter 2 Methods
Trial design
See also Chapter 3, Pilot study.
The Softened Water Eczema Trial (SWET) was a pragmatic, observer-blinded, parallel-group RCT of 12 weeks’ duration, followed by a 4-week observation period (Figure 2).
FIGURE 2.
Trial design.
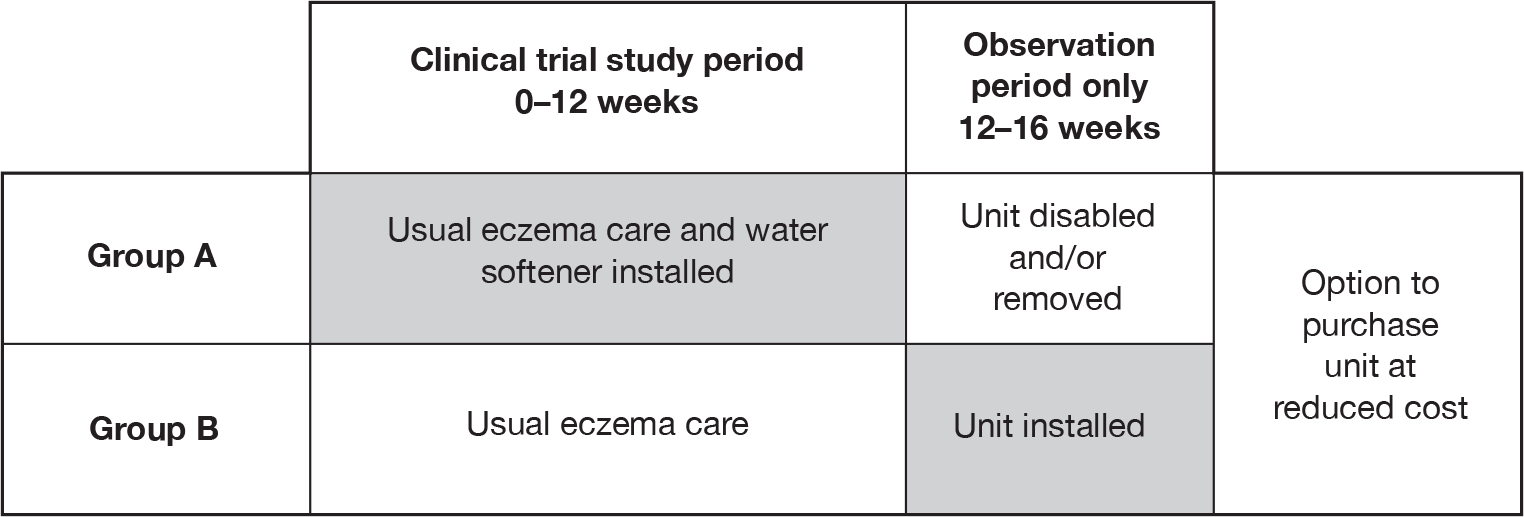
All participants were randomised to receive either immediate installation of an ion-exchange water softener, plus usual eczema care (group A), or usual eczema care, with delayed installation of a water softener after week 12 (group B). The primary outcome (eczema severity) was assessed at 12 weeks. The final 4-week period was included to provide further information on speed of onset of any effects, and to measure how quickly any benefits were lost once treatment was stopped. Feedback from the pilot study indicated that all participants would have liked to experience the intervention; hence, the inclusion of the opportunity for those not allocated to active treatment in the first 3 months to experience water softeners for the last month of the trial. In addition to helping recruitment, the provision of a water softener to group B after 12 weeks allowed a within-group comparison of speed of onset in group B if the softener was effective.
All families had the option of purchasing the water softener at reduced cost at the end of their child’s 16-week study period.
Recruiting centres
Recruitment took place at secondary and primary care referral centres in England, serving a variety of ethnic and social groups, and including both urban and periurban homes. All sites had predominantly hard water (> 200 mg/l calcium carbonate).
At the start of the trial children were recruited through four secondary care referral centres: Queen’s Medical Centre (Nottingham), Addenbrooke’s Hospital (Cambridge), Barnet and Chase Farm Hospitals NHS Trust (London) and David Hide Asthma and Allergy Research Centre, St Mary’s Hospital (Newport, Isle of Wight). As the trial progressed, a further four secondary care referral centres were opened: Leicester Royal Infirmary, United Lincolnshire Hospitals, the Royal London Hospital and St Mary’s Hospital (Portsmouth). All centres held designated paediatric clinics in which children with eczema were seen.
Participants were informed of the trial in a variety of ways. Principal investigators at each centre sent letters of invitation and information sheets to parents of children with eczema referred to these centres over the previous 12–18 months. Posters were displayed in centres, and SWET research nurses attended designated outpatient clinics, informing interested families about the trial. The National Eczema Society website included a link to the trial website. Information was included in primary school newsletters. Individual research nurses advertised the study through local radio and short articles in local newspapers. In addition, recruitment was obtained from primary care trusts local to three of the recruiting centres (Isle of Wight, Leicester and Cambridge), with letters of invitation and information sheets sent to targeted families by general practitioners (GPs) at practices within these primary care trusts.
Ethical considerations
The trial was approved by the North West Multicentre Research Ethics Committee (MREC, reference number 06/MRE08/77) and the local ethics research committee (LREC) for each participating centre prior to entering participants into the trial.
Participants
Eligibility criteria
Children were candidates for inclusion in the trial if they were aged 6 months to 16 years at recruitment visit, with moderate-to-severe eczema, and living in a property supplied by hard water. Eczema was defined by the UK refinement of the Hanifin and Rajka diagnostic criteria. 17
In order to qualify as a case of atopic eczema with the UK diagnostic criteria, the child must have:
-
an itchy skin condition in the last 12 months
-
plus three or more of:
-
onset below age 2 years (not used in children under 4 years)
-
history of flexural involvement
-
history of a generally dry skin
-
personal history of other atopic disease ( in children under 4 years, history of atopic disease in a first degree relative may be included)
-
visible flexural dermatitis as per photographic protocol.
Eczema was assessed using the Six Area, Six Sign Atopic Dermatitis (SASSAD) score. Moderate-to-severe eczema was defined as a SASSAD score of 10 or above. Children with a SASSAD score of < 10 were excluded in order to avoid floor effects, i.e. they had the potential to improve. Although children with a SASSAD score < 10 at baseline were not randomised into the trial, they were invited to contact the nurse again if their eczema worsened. The home where the child lived was assessed by a water engineer for technical suitability for the installation of an ion-exchange water softener, during a ‘home screening’ visit carried out prior to recruitment. Hard water was defined as containing ≥ 200 mg/l calcium carbonate, and was measured in the home by water engineers using the drop-count titration Hach test (counting the number of drops required to change the solution colour to determine water hardness). In order to be as inclusive as possible, approval for water softener installation was sought from local council housing departments, housing associations and private landlords.
Children were not admitted to the trial if:
-
they planned to be away from home for > 21 days during the 16-week study period, or had holidays scheduled during the 4 weeks prior to the primary outcome assessment date (to ensure adequate exposure to the intervention)
-
they had taken systemic medication (e.g. ciclosporin A, methotrexate) or ultraviolet light for their eczema within the previous 3 months (because of these treatments’ long-lasting effects)
-
they had taken oral steroids within the previous 4 weeks, or, as a result of seeing a health-care professional, had started a new treatment regimen for their eczema within the last 4 weeks
-
they lived in homes that already had a water treatment device installed, including ion-exchange softeners, polyphosphate dosing units or physical conditioners
-
they lived in a home that was unsuitable for straightforward installation of a water softener.
Screening
On expression of interest in the trial, a two-stage screening process was initiated.
Families were initially contacted by telephone by their local SWET research nurse, who then administered a telephone screen checklist (Appendix 2) to assess eligibility for the trial. This generated a study number and a request for a home screen visit by a water engineer attached to the trial. The water engineer completed a home screen checklist (Appendix 2), which was faxed to the co-ordinating centre. If the home was supplied by hard water and was technically suitable for straightforward installation of an ion-exchange water softener, an appointment was made for the child to be assessed by their local SWET research nurse for recruitment into the trial.
Informed consent
Research nurses took written consent from the child’s primary carer at the initial recruitment visit for all children aged 15 years or less. Children aged 16 years consented in their own right. Children aged 15 years or younger were invited to sign the consent form if they wanted to. Consent included permission for on-site inspection of the installed water softener by the relevant water supply company, under their duties within the statutory water fitting regulations, should this be requested.
Consent to take part in the genetic part of the study (filaggrin status) was additional to consent for the main study, i.e. it was not necessary to participate in the genetic study in order to participate in the main trial.
Interventions
Participants received either an ion-exchange water softener plus usual eczema care (group A) or usual eczema care alone with delayed installation of a water softener at 12 weeks (group B).
Ion-exchange water softeners use a synthetic styrene monomer resin to remove calcium and magnesium ions from hard household water, replacing them with sodium ions, thus removing the hardness. The resin becomes depleted of sodium and is recharged using sodium chloride (common salt). The units met all necessary regulatory standards, and were installed by trained water engineers according to the Water Regulations Advisory Scheme (WRAS) Information and Guidance Note18 and British Water’s code of practice. 19 In order to avoid favouring any one company, a generic unit was produced for the trial, which carried the SWET logo. Units were usually installed under the kitchen sink (Figure 3).
FIGURE 3.
The SWET ion-exchange water softener.

The standard procedure was to soften all water in the home, but to provide mains (hard) drinking water through an additional faucet-style tap at the side of the kitchen sink for drinking and cooking. (Occasionally, this was refused or was technically too difficult to install, in which case participants either purchased bottled water or used softened water for drinking and cooking for the duration of the study period.) Participants were therefore exposed to softened water for all washing/bathing/showering and washing of clothes, but continued to drink mains (hard) water. Participants were asked to shower/bathe and wash their clothes in their usual way. While using the water softener, participants were encouraged to reduce their soap use in line with general advice on the use of water softeners in the home (www.ukwta.org/watersofteners.php).
For those allocated to group A, a water softener unit was installed in the child’s main home as soon as possible after the baseline (recruitment) visit. Engineers were instructed to install water softeners within 10 working days, and parents were asked to be as flexible as possible when arranging suitable dates in order to achieve this. Participants allocated to group B received an active unit as soon as possible after the primary outcome had been collected at 12 weeks. Salt was supplied for all participants during the trial. Participants were reminded of the importance of replenishing the salt supply during a telephone call at 8 weeks (group A), and a weekly reminder was included in the daily symptom diary.
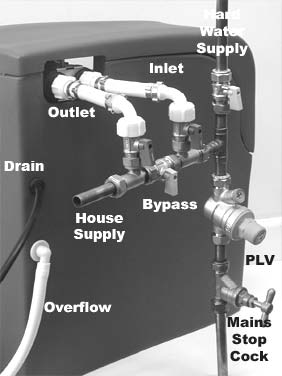
At week 12, group A participants were asked to switch their water softeners off, by turning three bypass levers to put the unit into ‘bypass mode’ (Figure 4) on the evening of the day they attended for their 12-week assessment (primary outcome), and reminded to do so with a telephone call the following day. A water engineer subsequently visited to remove the water softener and all associated pipework and fittings. However, if participants in group A indicated that they wished to purchase the water softener, the engineer ensured that the unit remained inactive for the final 4 weeks by removing the brine valve. Everything else remained in place, ready for subsequent reconnection.
FIGURE 4.
Water softener water supply and bypass levers.
Both groups received a ‘support telephone call’ from the co-ordinating centre at 8 weeks, and all participants continued with their usual eczema care for the duration of the trial. ‘Usual care’ was defined as any treatment currently being used in order to control the child’s eczema (e.g. topical corticosteroids, emollients). Newly introduced treatment regimens used during the study period were documented.
A Water Engineer’s Handbook was compiled by the trial manager in conjunction with the UK Water Treatment Association (UKWTA) giving background information about the trial and practical information about home screening and subsequent visits. The UKWTA provided engineers with SWET water softener installation instructions, based on the WRAS guidelines.
At installation, engineers gave parents a number of sampling pots, stamped addressed envelopes, and instructions for sending weekly samples taken from the hot tap in the bathroom for hardness testing. At the start of the trial (May 2007) parents were instructed to take the weekly water sample from the cold (softened) kitchen tap. Occasionally parents confused this with the new kitchen drinking faucet (mains hard water). In September 2008 a hardness alert visit revealed a home with unusual plumbing and a hard water supply to the bathroom despite a water softener installed in the kitchen. As a result parents were asked to take weekly water samples from the hot water tap in the main bathroom from October 2008 to the end of the trial. Samples were sent to Culligan UK Ltd (High Wycombe, UK) and analysed using a Palintest wavelength selection photometer (Palintest Ltd, Kingsway, Tyne & Wear, UK). Tests were carried out within 24 hours of receipt. Samples were split for analysis. The first sample was used for ‘blocking’ (setting the test unit), and the second was treated with Palintest Hardicol tablets. The test method was accurate to ± 5 mg/l calcium carbonate. If a sample contained > 20 mg/l calcium carbonate an alert was faxed through to the engineer’s co-ordinator, and copied to the co-ordinating centre. This triggered a standard procedure for dealing with the alert. If a unit was suspected to be malfunctioning, an engineer visited the home and replaced the unit. If there was an obvious reason for the hardness breakthrough (e.g. a bypass lever had been knocked out of position), this was rectified on site.
Outcome measures
Primary outcome
As this was a single-blind trial, it was important to use an objective primary outcome measure that could be assessed by blinded observers (research nurses). 20 With this in mind, the primary outcome was the mean change in eczema severity at 12 weeks compared with baseline, as measured using the SASSAD severity scale –(see Appendix 3). SASSAD is an objective severity scale that was completed by the research nurses; it did not involve input from the participant in any way. 21 SASSAD includes assessment of the severity of six signs – erythema (redness), exudation (oozing of fluid), excoriation (scratch marks), cracking, lichenification (skin thickening) and dryness – in each of six areas, the head and neck, trunk, hands, arms, legs and feet. The theoretical range of the scale is 0 to 108, although in practice scores rarely exceed 70.
Nurses were trained in the use of SASSAD during a 2-day training event at the co-ordinating centre. With the exception of one study centre (Chase Farm Hospital) all SASSAD scores for each participant were obtained by the same nurse. In July 2008 the nurse at Chase Farm Hospital went on maternity leave. Prior to her departure she trained two Medicines for Children Research Network (MCRN)-funded nurses in SASSAD scoring. The MCRN-funded nurses attended a number of joint assessment visits by SWET participants, during which the nurses scored SASSAD independently and compared their final scores. Training was deemed complete when scores were within 10% or less of each other for three consecutive assessments.
In addition to the SASSAD score, nurses scored a representative site using the Three-Item Severity (TIS) scale. This measures three clinical signs – excoriation, erythema and oedema/population – at a single representative site. 22 Its simplicity makes it a suitable tool for research studies and clinical practice, and it has been suggested that the score provides as much information about eczema severity as more complex scoring systems. 23 In SWET, this score was recorded for two reasons: (i) to compare with SASSAD for research purposes; and (ii) to assess integrity of observer (nurse) bias (information bias) using digital images of a representative site of the participant’s eczema. These digital images were intended to be scored by two independent dermatologists using the TIS scale. The location of the representative site for TIS was agreed between the nurse, parent and child and photographed using a Samsung S630 CE digital camera (Chelsey, UK).
Secondary outcomes
Night-time movement
The difference between the groups in the proportion of time spent moving during the night was included as an objective surrogate for sleep loss and itchiness (two of the defining features of eczema). Previous research has suggested that this is a suitable objective tool for assessing itch,24,25 and it has been shown to correlate with objective clinical scores in children with atopic dermatitis. 26 Movement was measured using accelerometers (Actiwatch Mini™, CamNtech Ltd, Cambridge, UK) for periods of 1 week at week 1 and for 1 week at week 12. The unit was worn by the child in the same way as a wrist watch. Data were stored on the unit and uploaded on to a laptop computer at the subsequent assessment visit. Pilot work using these units suggested that it was unusual for participants to record complete data for an entire week. As a result, the first three nights of evaluable data were used at baseline and the last three nights of evaluable data were used at week 12, in order to tie data collection as closely as possible to the date at which the participants’ eczema severity was assessed by the research nurse. Evaluable data were defined as values > 5% and < 95% of the night spent moving to remove outliers. If there were fewer than three nights of evaluable data, this variable was considered missing.
Improvement in eczema severity
The difference between the groups in the proportion of children who had the same or worse outcome (≤ 0%) or a reasonable (> 0% and ≤ 20%), good (> 20% and ≤ 50%) or excellent (> 50%) improvement in SASSAD score at 12 weeks compared with baseline.
Topical medication use
The difference between the groups in the amount of topical corticosteroid/calcineurin inhibitors used during the study period was measured. Medications were weighed at each assessment visit, using digital scales. The scales were checked for accuracy before each visit, using standardised weights. Data were split into six types of medication: mild steroids, moderate steroids, potent steroids, very potent steroids, mild calcineurin inhibitors and moderate calcineurin inhibitors.
Patient-Oriented Eczema Measure (POEM, Appendix 3)
The difference between the groups in POEM data collected at baseline and at weeks 4, 12 and 16. This scale is a well-validated tool that has been developed to capture symptoms of importance to patients. 27 Parents were asked to state the number of days in the last week that their child had been affected by a range of symptoms. These were scored as follows: no days = 0, 1–2 days = 1, 3–4 days = 2, 5–6 days = 3 and every day = 4. The POEM score was then calculated as the sum of these seven individual scores (scale 0–28).
Eczema control
The difference between the groups in the number of totally controlled weeks (TCWs) and well-controlled weeks (WCWs) was recorded. This outcome was based on a systematic review looking at ways of assessing long-term control for chronic conditions such as eczema, asthma and rheumatoid arthritis. 28 The terms TCW and WCW have been adopted for use by researchers in the field of asthma and appear to be a useful and intuitive means of capturing disease activity over time. Using this definition, a TCW is one in which symptoms are controlled throughout the week without the need to ‘step up’ treatment beyond normal maintenance care (such as emollients). A WCW is one in which symptoms and the need for ‘step-up treatment’ occurred on 2 days of the week or less. Each family was asked to keep a daily symptom diary throughout the trial. The information from this diary was used to calculate the number of TCWs and WCWs.
Dermatitis Family Impact questionnaire (Appendix 3)
The difference was measured between the groups in the mean change in the questionnaire at 12 weeks compared with baseline. This scale measures how much the child’s eczema has affected the whole family over the previous week, based on 10 questions. 29 Questions were scored as follows: not at all = 0, a little = 1, a lot = 2 and very much = 3. The Dermatitis Family Impact (DFI) score was calculated as the sum of these 10 individual scores (scale 0–30).
European Quality of Life-5 Dimensions (Appendix 3)
In order to assess whether the intervention had an impact on generic health-related quality of life, health utility was captured using the children’s version of the EQ-5D for children aged 7 years and over, or the proxy version of the European Quality of Life-5 Dimensions (EQ-5D) for children aged 3–6 years. 30,31 A utility weight was attached to the health state descriptions using the currently accepted UK adult tariff, calculated using the York A1 tariff. 32 The mean change in utility score from baseline to 12 weeks was compared for group A against group B.
Filaggrin status
The role of filaggrin gene (FLG) mutations as a predictor of treatment response was assessed. Mutations of the epidermal barrier protein filaggrin have been shown to be a predisposing factor for eczema. 16,33 Saliva samples were collected during the trial. If children were unable to spit into the container, swabs were taken from inside the cheek. Samples were shipped to the Human Genetics Unit at the University of Dundee, Dundee, UK and analysed for FLG genotyping for the common null alleles according to published protocols. 34
Assessment visits
Assessment visits were carried out in paediatric dermatology clinic rooms in one of the SWET secondary care referral centres. Occasionally the research nurse agreed to see the child in the family home for the initial recruitment visit, but parents were informed that follow-up visits would all need to take place in the local SWET referral centre, to avoid unblinding of the nurse once the child had been randomised into the trial. SWET research nurses were trained in defining eczema at an initial training session run by a dermatology nurse consultant, by attending eczema clinics run by their principal investigators and consultant colleagues, and by self-testing using the online diagnostic criteria manual (www.nottingham.ac.uk/scs/divisions/evidencebaseddermatology/methodologicalresources/diagnostictools.aspx). Assessments took place at baseline and at 4 weeks and at 12 weeks (primary outcome) and at 16 weeks (Table 1).
| Baseline | Week 4 | Week 12 | Week 16 |
|---|---|---|---|
| Eligibility criteria checked | SASSAD/TIS | SASSAD/TIS | SASSAD/TIS |
| Baseline characteristics | POEM | POEM, DFI, EQ-5D | POEM |
| SASSAD/TIS | Medications weighed | WTP questionnaire | Medications weighed |
| POEM, DFI, EQ-5D | Digital photo of index site (TIS score) | Medications weighed | Digital photo of index site (TIS score) |
| WTP questionnaire | Week 1 Actiwatch data downloaded and watch reissued | Digital photo of index site (TIS score) | |
| Medications weighed | Diary 2 issued | Week 12 Actiwatch data downloaded | |
| Digital photo of index site (TIS score) | Diary 3 issued | ||
| Saliva sample | |||
| Actiwatch issued | |||
| Diary 1 issued | |||
| Consent taken, child randomised into trial |
Data collection and monitoring
Data generated by all centres were collected on study case report forms, which were entered on to the password-protected SWET database that was created and maintained by the Nottingham Clinical Trials Unit (CTU). Data were entered by the research nurses and by staff at the co-ordinating centre. A 100% check was conducted for the primary outcome (eczema severity) and the time spent moving, and discrepancies were resolved. All other data were subject to a 10% check, which was assumed to be adequate if the maximum error rate was, < 1 in 200 (in practice it was much lower than this). Data were also checked for consistencies in range and missing data. Missing and/or ambiguous data were queried with individual research nurses and resolved wherever possible.
Randomisation
Participants were randomised using web-based randomisation, and allocated on a 1 : 1 basis according to a computer-generated code, using random permuted blocks of randomly varying size. The program was created by the Nottingham CTU in accordance with its standard operating procedure and held on a secure server. Randomisation was stratified by disease severity (baseline SASSAD score ≤ 20 or SASSAD score > 20) and recruiting centre. Access to the sequence was confined to the CTU data manager. The allocation group was indicated to the trial manager only after baseline data had been irrevocably entered into the randomisation programme by the research nurse. The sequence of treatment allocations was concealed until interventions had all been assigned, recruitment and data collection were complete, a signed-off statistical analysis plan had been received and the database locked.
Blinding/bias
Research nurses were blinded to treatment allocation throughout the trial and statisticians analysed the results based on treatment code, using an analysis plan that had been finalised prior to locking the database and prior to the blinded data analysis. The only study personnel in direct contact with study participants were the research nurses and water engineers. The trial manager and study support staff at the co-ordinating centre in Nottingham had telephone contact with parents of participants. Trial participants continued to see health-care professionals for their usual eczema care.
Participants were discouraged from discussing their treatment allocation with the research nurse and the importance of maintaining ‘blinding’ was highlighted in the participant information sheets. Records were kept of all instances when the nurses believed they had become unblinded.
Sample size
Sample size calculations, based on the results of the pilot study and previously published eczema trials, supported a target of 310 participants (155 in each group) in order to show a minimum clinically relevant difference of 20% in the change in SASSAD score between the two groups [assuming a mean baseline SASSAD score of 20 and a standard deviation (SD) in change scores of 10]. This sample size provided 90% power, assuming a significance level of 5% and dropout rate of 15%.
For the planned subgroup analysis, including children with at least one mutation in the gene coding filaggrin, a total of 90 children with the mutation was assumed to be sufficient to detect a 30% difference between the treatment groups in the primary outcome, with 80% power, 5% significance and a SD of 10.
Statistical analysis
Primary outcome
The full statistical analysis plan is included in Appendix 4. The primary efficacy end point was an intention-to-treat (ITT) analysis including all participants with evaluable data (if < 5% missing values). If > 5% of data were missing, then a general linear model was to be used to handle the missing values.
Baseline characteristics were summarised and, if any major imbalance existed, the analyses were to be adjusted to account for this, along with an adjusted analysis including the stratification variables (recruiting centre and eczema severity).
A secondary, per-protocol analysis was performed in order to establish proof of principle, and subgroup analysis was conducted, based on those with at least one mutation of the gene coding for filaggrin.
Per-protocol analysis excluded the following participants:
-
those who were randomised into the study, but who failed to receive their allocated treatment
-
those who were deemed to be major protocol violators as defined by the Protocol Violators Group [including independent members of the Trial Steering Committee (TSC)].
Criteria for protocol violators were defined prior to breaking of the code relating to treatment allocation. They were as follows:
-
missing SASSAD score at week 12
-
group A: exposed to fully softened water for < 75% of the time that their home had an active water softener in place (i.e. sleeping at home + unit fully working for < 75% of the time that their home had an installation)
-
group A: participant away from home or with partially functioning water softener for > 2 days/week for each of the 4 weeks prior to the primary outcome assessment
-
group B: participant away from home for > 2 days/week for each of the 4 weeks prior to the primary outcome assessment
-
unblinding of research nurse prior to primary outcome measurement (which could have caused observer bias)
-
participants starting new treatment prior to primary outcome assessment were examined by a dermatologist on a case-by-case basis to determine if they were violating protocol.
Sensitivity analyses
Three sensitivity analyses were planned in relation to the primary outcome: (i) including all randomised participants by replacing missing values; (ii) excluding those for whom the research nurse had become unblinded; and (iii) excluding outliers. For the analysis including all randomised participants, missing values at baseline were replaced by the maximum score from the other five areas of the SASSAD score that were completed. Missing values at week 12 were replaced by the SASSAD score at baseline or week 4, depending on which was greater. For the analysis excluding outliers, these were defined as change scores outside the range of ± 3 SD.
Secondary outcomes
Secondary end points were analysed using a complete case analysis.
In order to aid clinical interpretability, SASSAD scores were grouped into those reporting no change or worse, a reasonable reduction (> 0% and ≤ 20%), a good reduction (> 20% and ≤ 50%), or an excellent reduction (> 50%).
The average percentage of the night spent moving was calculated by taking the average of the first three nights of usable data at baseline and the last three nights of usable data at week 12. Usable data were defined as values between 5% and 95% of the night spent moving to exclude outliers.
The total amount of medication used during the 12-week study period was measured by weighing the medication at each visit. Nurses recorded how confident they were in the measurement.
The number of TCWs and WCWs were compared during the first 12 weeks of the trial. A TCW was defined as a week with zero days with an eczema bother score above 4 and zero days on which ‘stepping up’ of treatment was required. Stepping up of treatment was defined as treatment over and above that defined as ‘normal’ for an individual participant in the daily symptom diaries. Bother scores were assessed on a scale of 0–10 in answer to the following question: ‘How much bother has your child’s eczema been today?’ A WCW was defined as a week with ≤ 2 days with an eczema bother score > 4 and ≤ 2 days on which stepping up was required.
All other outcomes were scored according to the guidelines for the scale, and compared the mean change from baseline to week 12. Continuous data were analysed using a t-test and categorical data were analysed using a chi-squared test for trend.
Analyses of all secondary end points and adjusted analyses were considered to be supportive to the primary analysis, so no adjustments for multiple comparisons were made.
Analyses were performed in stata 10.1 (StataCorp LP, College Station, TX, USA) and all p-values reported are two sided, with a significance level of 5%.
Summary of changes to the protocol
A full copy of the final trial protocol and statistical analysis plan are given in Appendix 4. Changes to the protocol following MREC approval in January 2007 include minor amendments to trial documents: the inclusion of amounts of topical medications as an additional secondary outcome measure and an end of trial follow-up questionnaire. One of the secondary outcomes (patient-assessed global improvement in eczema) was replaced with broad categories as defined by the SASSAD score [the proportion of children who had a reasonable (≤ 20%), good (> 20% and ≤ 50%) or excellent (> 50%) improvement in SASSAD score], as this was felt to be more appropriate in a single-blind study. All amendments were implemented prior to breaking of the treatment allocation code and prior to finalising the analysis plan.
Trial conduct
Trial organisation
The trial was managed and co-ordinated from the Centre of Evidence Based Dermatology, University of Nottingham, Nottingham, UK. Data management was conducted through the Nottingham CTU. Statistical analysis was overseen by Professor Andrew Nunn and conducted at the Medical Research Council (MRC) CTU in London.
The Trial Management Group (TMG) was responsible for overall management of the trial. The TSC had an independent chairperson and vice chairperspon and met annually to provide overall supervision of the trial on behalf of the trial sponsor (University of Nottingham). Training sessions were held for research nurses and water engineers prior to starting the trial, and ongoing training was provided at individual sites as required.
The trial manager was responsible for day-to-day management of the trial. Details of individual participants were kept in a password-protected access database (Microsoft Corporation, Redmond, WA, USA). This included unblinded information relating to home screen outcomes, installation and removal of water softeners and hardness alerts.
As the trial involved the use of a commonly available domestic water softening unit, and did not involve the use of a medicinal product, there was no need for a Data Monitoring Committee.
Membership of the TMG and the TSC are given in Appendix 5.
Engineer co-ordination
Water engineers were subcontracted by the UKWTA. All water engineering aspects on the Isle of Wight were handled by a single subcontractor (MG Heating Ltd, Oxford, UK). Homes on the mainland were assessed by a number of local independent subcontractors co-ordinated by Lorraine Doran at European Water Care Ltd (Essex, UK, May–October 2007) and John Kyle at Kinetico UK Ltd (Hampshire, UK, October 2007 to September 2009). Fourteen subcontractors carried out water engineering aspects over the course of the trial. The majority of the work was done by the following nine subcontractors: Aquastream, Capital Softeners, Clearwater Softeners, European Water Care, Greens Water Systems, Kinetico UK, MG Heating Ltd, Silkstream and Simply Soft Water Softeners.
Consumer involvement
A consumer panel of five service users with experience of living with eczema assessed patient information sheets, symptom diaries and publicity material prior to submission for ethical approval. The panel members shared these documents with children with eczema aged 4 and 13 years. Mr David Potter acted as consumer panel representative on the TSC. Several participants from the trial assisted with trial publicity by agreeing to take part in media interviews (once their direct involvement in the trial was over). The National Eczema Society (NES) and the Nottingham Support Group for Carers of Children with Eczema (NSGCCE) helped with publicity during the recruitment phase of the trial.
Trial finances
This trial was funded by the UK National Institute for Health Research (NIHR) HTA programme. Subcontracts were established between the University of Nottingham and the MRC CTU, the consortium of water treatment companies (through the UKWTA) and the University of Portsmouth. In addition to the funding provided by the NIHR HTA programme, representatives from the water-softening industry covered the costs of the design, testing and supply of generic ion-exchange water softeners, salt supplies, hardness testing of water samples and supervision of water engineers.
Trial participants were offered a standard inconvenience allowance of £5–10 per visit in the form of gift vouchers.
Trial insurance and indemnity
The usual NHS indemnity arrangements for negligent harm applied. The University of Nottingham acted as sponsor for the trial and had third-party liability insurance in accordance with all local legal requirements, including cover for children under the age of 5 years. In addition, study engineers carried their own third-party liability insurance. The water softeners used in the study were covered by product warranty.
SWET website
The SWET website (www.swet-trial.co.uk, Figure 5) was active from May 2007 when recruitment began. The website included a password-protected researcher section where all current trial documentation was accessible for download by research nurses at individual study sites.
FIGURE 5.
Screenshot of the home page of the SWET website.
Chapter 3 Working with industry
Pilot study
A pilot study funded by Kinetico UK Ltd was carried out in 2002 by Professor Hywel Williams and his research team at the University of Nottingham. This was a randomised, double-blind, parallel-group pilot study of 12 weeks’ duration. The aims of the pilot study were (i) to test the appropriateness of the recruitment methods and trial procedures; (ii) to inform sample size calculations for the main RCT; and importantly and; (iii) to assess whether or not it was possible to blind participants to their treatment allocation (given that softened water typically produces more lather when using cleaning products).
Participants in the pilot study received either an ion-exchange water softener or a specially modified ‘placebo’ water softener, in which the internal resin beads had been replaced with inactive polypropylene. Technical difficulties meant that, for the purposes of the pilot trial, only homes with a gravity-fed boiler were eligible to take part (families with a combination boiler were excluded). Participants were instructed to continue treating their eczema according to their usual practice for the duration of the trial.
Seventeen children aged 1–10 years with moderate or severe eczema from the Nottingham area were randomised into one of two treatment groups for a period of 12 weeks.
At the end of 12 weeks, the children’s eczema was assessed, and parents/carers were asked whether they thought they had received a real or a placebo unit.
Lessons from the pilot study.
-
The pilot trial generated a lot of interest, although many families were ineligible because their homes were unsuitable for the installation of a water softener; or they had a combination boiler in the home. This led to modifications in the RCT design so that both gravity-fed and combination boiler types were eligible, and an additional home screen visit was introduced prior to randomising the participants into the trial.
-
It proved to be extremely difficult to blind participants to their treatment allocation and, as might be expected, this was particularly marked for those who received the real water softener. However, there was no evidence to suggest that the research nurse had been compromised, and so a single-blind study was recommended (with mechanisms in place to record instances when the research nurses had become aware of the treatment allocation).
-
In order to maximise exposure to the intervention, it was recommended that water softener units were installed as soon as possible after a child had been randomised into the study, and records kept of periods away from the home.
-
The number of technical difficulties experienced with the units during the 12-week study period was higher than expected. For the full study, it was recommended that engineers be employed to work exclusively for the trial, and that regular water testing be introduced.
-
Measuring chlorine content of the water proved problematic due to rapid evaporation. For the full study it was recommended that we measure water hardness only.
-
Participants randomised to receive a placebo unit expressed regret at not being able to try a ‘real’ unit for themselves. It was felt that this might impact on our ability to recruit into the main RCT, and so an additional 4-week period was introduced between weeks 12 and 16, when the control group would have a water softener installed.
Experiences from the main trial
The Softened Water Eczema Trial (SWET) was an unusual eczema clinical trial in that the intervention was not another skin cream, but altering one aspect of the child’s normal home environment (water hardness). The intervention was a piece of widely available specialised non-medical equipment, which plumbed into the mains water supply to the child’s home. This required a level of specialist knowledge and expertise that could be achieved only by close collaboration with the water-softening industry.
Water-softening industry and their trade associations
British Water is a corporate membership association covering all sectors of the water industry, and was closely involved with the pilot study and setting up the main study. Ian Pallett (Technical Director at British Water) was a co-applicant on the funding application and served as the industry representative on the TSC.
A number of meetings were held with representatives from British Water and the water-softening industry prior to setting up the main trial. These informed practical logistics, including the design of a generic water-softening unit encased in a special SWET cabinet.
Representatives from the following companies gave input to meetings prior to and during the trial: Aqua Focus Ltd (Newport, UK), Aquademic Ltd (Derby, UK), Aqua Nouveau Ltd (Basingstoke, UK), Coleman Water Ltd (Ipswich, UK), Culligan International (UK) Ltd (High Wycombe, UK), EcoWater Systems Ltd (High Wycombe, UK), Harvey Softeners Ltd (Surrey, UK), Kennet Water Components Ltd (Newbury, UK) and Kinetico (UK) Ltd (Hampshire, UK).
The UKWTA was formed in March 2006 and is a national trade organisation for companies involved in the sale and use of water treatment chemicals and equipment in the UK. The UKWTA was closely involved with delivery of the SWET trial, and Tony Frost served as the UKWTA representative on the TMG.
There was a great deal of goodwill in the industry towards the trial; an early example was the professional redrawing of a draught logo by artists working in the publicity department of Aqua Nouveau Ltd. This became the instantly recognisable SWET hippo logo, which was a great hit with children on the trial.
Engineer employment
The intention had been to employ a small number of water engineers, one for each study centre, and to pay each engineer a salary for a fixed number of days/week devoted to SWET. However, this plan was set aside in March 2006, when the UKWTA was formed. By liaising directly through the UKWTA, the trial was able to have a more flexible approach to securing water engineer expertise and cover a wider geographical area extending across south-east and central England, from the Isle of Wight to Lincolnshire. Tony Frost acted as representative on the TMG for the UKWTA, which took over responsibility for subcontracting work to a number of smaller water softener companies.
Engineer training
Over the lifetime of the study, more than 20 water engineers from over 10 companies were involved in the installation and/or removal of study units. It was felt that the advantage of increased flexibility and engineer cover outweighed the disadvantage of losing direct contact with individual engineers. A downside was the effect on engineer training. Information about the trial was passed on through engineer co-ordinators on the Isle of Wight and the mainland, rather than directly in face-to-face training sessions with members of the TMG. In response to a few instances where engineers became involved in unwarranted discussions with parents about softened water and eczema, the trial manager wrote a SWET engineer’s handbook. This was distributed to all engineers in March 2008, and included background information and a list of important ‘dos’ and ‘don’ts’.
Individual engineers’ levels of expertise and professionalism were very high. There were a few occasions when water engineers did not have the skills necessary to adequately screen homes, or to carry out installations, e.g. one engineer underestimated water hardness at home screen by not giving sufficient time for the Hach drop-count test to develop. Action to resolve the situation was swiftly implemented in all cases.
Water engineers work to tight deadlines, often driving many miles between homes and only visiting their company depot/offices as required. As a result, it was difficult for individual engineers to work to good clinical practice (GCP) standards in terms of paperwork trails. The co-ordinating centre spent many hours chasing paperwork, confirming home screens and installations/removals. In an effort to improve rapid communication between engineers and the co-ordinating centre, a dedicated telephone answering machine was introduced for engineers to leave messages whenever they had done anything for SWET.
Understanding research terminology and methodology
In order to collaborate effectively, industry colleagues needed to understand clinical methodology and terminology such as the difference between RCTs and other types of research, and the statistical interpretation of RCT blinded and unblinded outcomes. This was important both during the trial itself in terms of working to GCP standards (data protection, paperwork trails, etc.) and at the end of the trial when understanding trial results and statistical terminology. Hywel Williams, in his role of Chief Investigator, agreed to talk to a meeting of industry colleagues after the trial ended, in order to explain the results.
While the water-softening industry helped inform study design and assisted with the trial conduct by carrying out home screen visits, installing devices and monitoring water samples, it had no involvement in data collection, analysis or interpretation.
Publicity issues
The UKWTA companies involved with SWET were asked to take a responsible approach to publicity about the study on their own websites. While all additional publicity about the trial was welcomed (because it would aid recruitment) it was important that companies remained neutral and did not give any misleading information. Routine monitoring of company websites occasionally revealed problems which were rapidly resolved on our behalf by the UKWTA.
Ongoing commitment to the trial
There were numerous examples of good practice which put the needs of the trial foremost. During the first 6 months of the study the number of homes failing the ‘home screening visit’ was higher than expected, and this was addressed in meetings held within the industry, and in collaborative meetings with staff at the co-ordinating centre. As the trial progressed, the number of samples needing routine analysis for hardness levels increased, and this extra work was absorbed by Culligan UK Ltd. Kinetico UK Ltd had responsibility for building the generic SWET ion-exchange water softeners. Originally the company had been told that 100 units would be required across the 2-year recruitment period, but owing to higher than expected numbers of units being purchased by families, this number increased to 197. At an individual level, engineers attached to the SWET trial were often working in very different environments from usual. This sometimes involved installing softeners into tight or unusual spaces, and finding creative ways to solve technical problems. One engineer discovered additional pipework to a bathroom during a visit to investigate a hardness ‘alert’. As a result of this, the procedure for routine weekly water testing and home screening was changed. Other examples included staff at the co-ordinating centre contacting parents to let them know about a recent hardness alert only to be told that water engineer had already visited and rectified the problem.
Option to buy the water softener
All participants had the option of buying the water softener at a reduced price at the end of their child’s 16-week study period.
To avoid potential conflict of interest, staff at the co-ordinating centre did not get involved in payment arrangements. Standard information was included in the letter sent out after recruitment, and all requests to purchase units were directed to the UKWTA (responsible as intermediary) and Kinetico UK Ltd (responsible for invoicing and warranty).
Chapter 4 Results
Recruitment
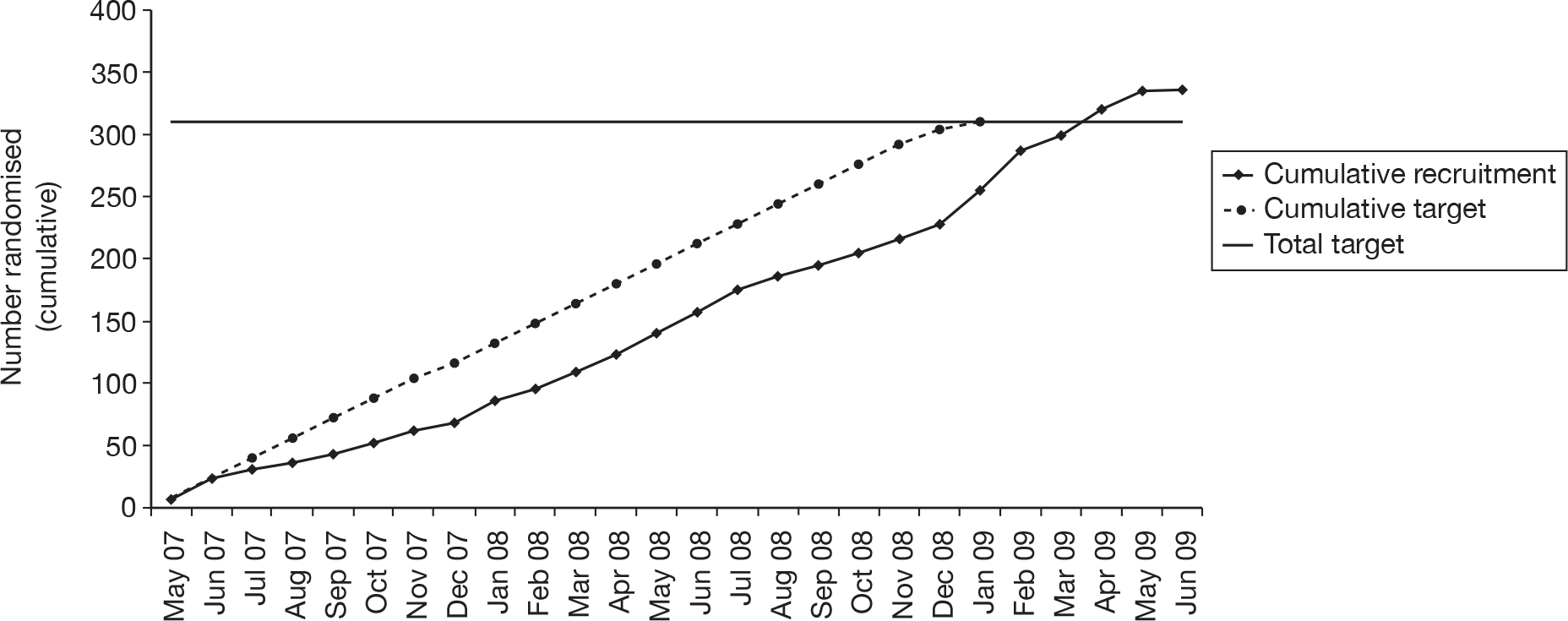
Recruitment took place between May 2007 and June 2009 (Figure 6).
FIGURE 6.
Cumulative recruitment.
Enrolment into the trial was a two-stage process (Figure 7). All those who passed the initial telephone screen were issued a study number (n = 644). Of these, 308 failed to meet the full inclusion criteria and were not randomised into the trial. The main reasons for excluding participants at this stage were that it was not possible to install a water-softening device in the child’s home or that the child’s eczema was too mild. Further details on home screening outcomes are given in Appendix 6.
FIGURE 7.
CONSORT diagram of participant flow.
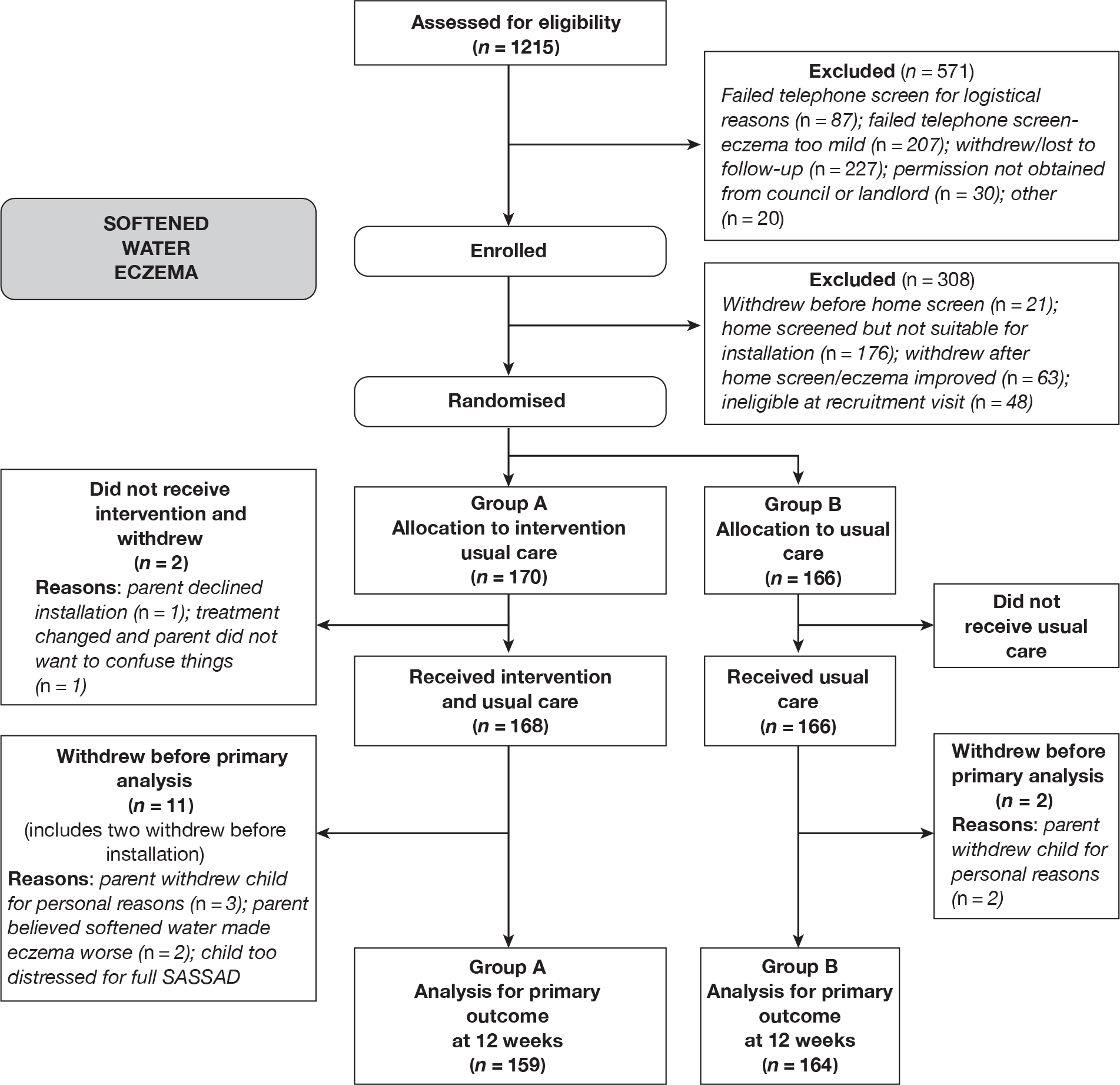
A total of 336 participants were randomised into the study (170 in group A and 166 in group B). This is higher than the original target (n = 310), as a number of families had been issued study numbers and were in the process of having home screening visits or were awaiting landlord/council decisions when the 310th participant was recruited into the trial. The ITT population consisted of the 323 participants with evaluable data (96% of all randomised participants). This included 159 in group A (water softener + usual care) and 164 in group B (usual care). Multiple imputation of missing values was not felt to be appropriate in this context owing to the very low levels of missing data.
Baseline data
The groups were generally well balanced for all baseline characteristics, although to group A (water softener + usual care) included a slightly higher proportion of older children (aged ≥ 7 years) and members of group A, were more likely to use higher potency topical therapy (potent steroids, very potent steroids or calcineurin inhibitors) and slightly more likely to use biological washing powders (Table 2). The possible impact of these differences was explored in sensitivity analysis (primary outcome).
| Baseline characteristics | Group A (water softener + usual care) | Group B (usual care) |
|---|---|---|
| Number enrolled | 170 | 166 |
| Number in ITT population | 159 | 164 |
| Age | ||
| Mean age, years (SD) | 5.8 (4.2) | 5.1 (4.0) |
| Sex, n (%) | ||
| Male | 89 (56) | 96 (59) |
| Female | 70 (44) | 68 (41) |
| Ethnicity, n (%) | ||
| White | 124 (78) | 125 (76) |
| Non-white | 34 (21) | 38 (23) |
| Not stated/unknown | 1 (1) | 1 (1) |
| Previous treatment history, n (%)a | ||
| High strength corticosteroids/calcineurin inhibitors | 91 (57) | 80 (49) |
| Low strength corticosteroids/calcineurin inhibitors | 57 (36) | 73 (45) |
| None | 11 (7) | 11 (7) |
| Filaggrin status, n (%) | ||
| Presence of mutation | 45 (28) | 47 (29) |
| Absence of mutation | 103 (65) | 109 (66) |
| Unknown | 11 (7) | 8 (5) |
| Food allergy, n (%)b | ||
| No | 97 (63) | 102 (64) |
| Yes | 58 (37) | 58 (36) |
| Baseline SASSAD score, n (%)c | ||
| Mean (SD) | 24.6 (12.7) | 25.9 (13.8) |
| Median (IQR) | 21 (15–32) | 22.5 (15.5–33.5) |
| 10–19 | 72 (45) | 68 (41) |
| > 20 | 87 (55) | 96 (59) |
| Water hardness (mg/L-1 calcium carbonate) | ||
| Mean (SD) | 309 (50) | 310 (58) |
| Median (IQR) | 308 (274–342) | 300 (270–340) |
| Washing powder, n (%)d | ||
| Biological | 20 (13) | 12 (7) |
| Fabric softener, n (%)e | ||
| Yes | 69 (44) | 81 (49) |
| Bathing frequency at home, times per weekf | ||
| Median (IQR) | 5 (3–7) | 4 (3–7) |
| Bathing frequency away from home, times per weekg | ||
| Median (IQR) | 0 (0–1) | 0 (0–0) |
| Swimming frequency, n (%)h | ||
| Never | 56 (35) | 66 (40) |
| Less than once a month | 53 (34) | 52 (32) |
| More than once a month | 49 (31) | 46 (28) |
Intervention – duration of exposure to softened water
Engineers were instructed to carry out installation of water softeners within a maximum of 2 weeks (10 working days); parents were asked to be as flexible as possible when arranging suitable dates, in order to achieve this. The average duration of exposure to softened water in group A was 10.6 weeks (range 7.6–16.4 weeks) (Table 3).
| Group | Installation status | Days (including weekends), mean ± SD |
|---|---|---|
| Group Aa (n = 168) | Time from randomisation to installation | 12.4 ± 5.5 (range 2–32) |
| Duration of installation prior to primary outcome assessment | 74 ± 7.6 (range 53–115) | |
| Group Bb (n = 156) | Time from week 12 visit to installation | 9.2 ± 6.5 (range 0–34) |
| Duration of installation prior to assessment at 16 weeks | 24.5 ± 9.0 (range 0–78) |
Primary analysis
Intention-to-treat analysis
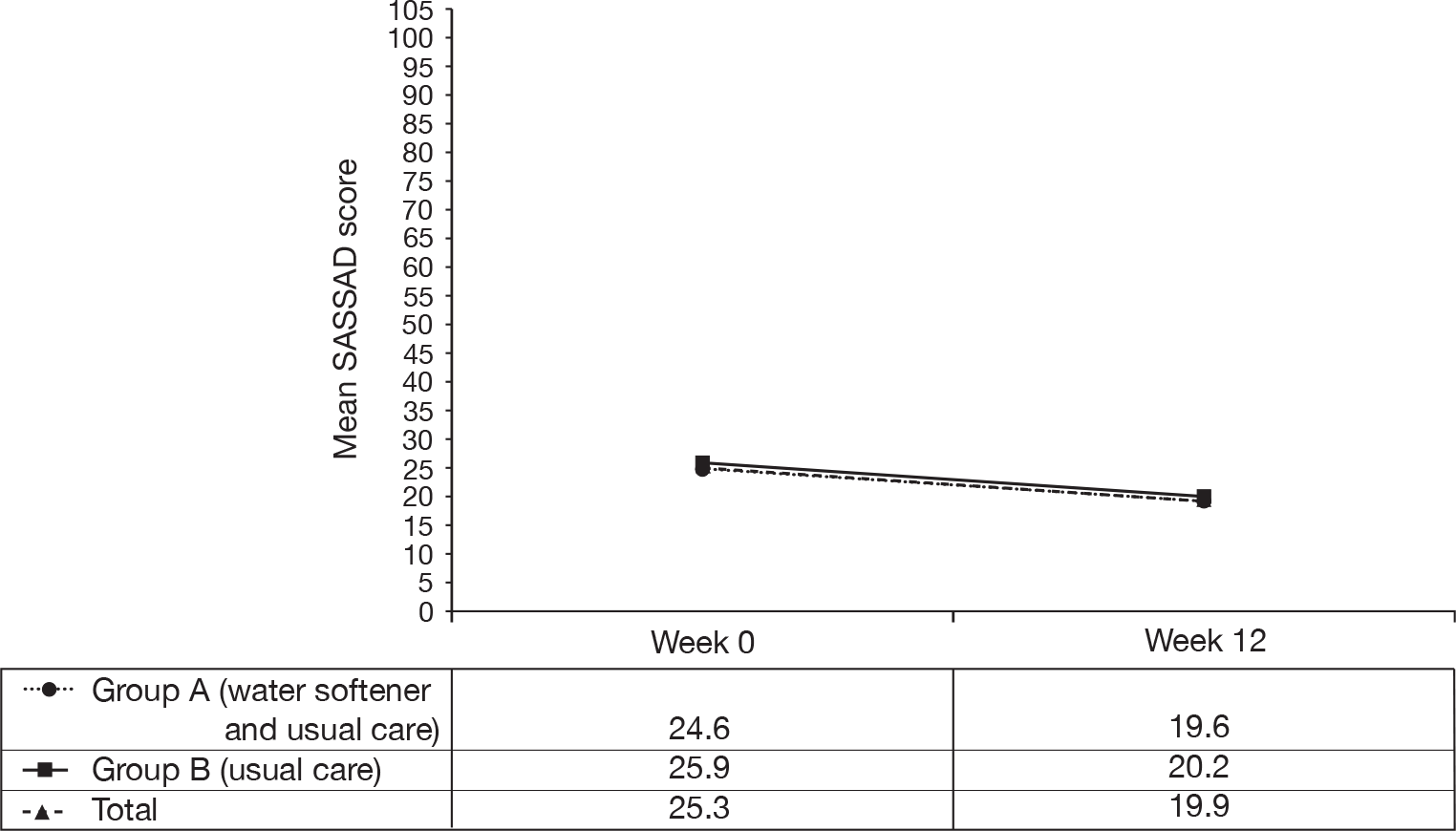
The primary end point of change in disease severity is shown in Table 4. Group A showed a mean reduction of 20% (5.0 points) in SASSAD score from an average of 24.6 at baseline to 19.6 at week 12. Group B showed a reduction of 22% (5.7 points) in SASSAD score from an average of 25.9 at baseline to 20.2 at week 12. The difference between the two groups at week 12 was 0.66 in favour of group B (95% CI –1.37 to 2.69) and was not statistically significant (p = 0.53). An additional analysis adjusting for stratification variables (baseline SASSAD score and centre) was performed, but this did not alter the conclusion. The difference between the two groups was reduced to 0.34 (95% CI –1.65 to 2.33, p = 0.74), in favour of group B.
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed a | 159 | 164 | |||
| Week 0 | Mean ± SD | 24.6 ± 12.7 | 25.9 ± 13.8 | ||
| Week 12 | Mean ± SD | 19.6 ± 12.8 | 20.2 ± 13.8 | ||
| Change | Mean ± SD | –5.0 ± 8.8 | –5.7 ± 9.8 | 0.66 (–1.37 to 2.69) | 0.53 |
These results are shown graphically in Figure 8 based on those with complete data at all time points.
As a result of the slight imbalance between the two groups at baseline in relation to age, previous treatment history and use of biological washing powder, a generalised linear model (GLM) was performed that adjusted for these baseline differences. This analysis gave similar results to the univariate t-test analysis. The difference between the two groups was 0.54 (95% CI –1.54 to 2.62, p = 0.61). (More detailed information is given in Appendix 7.)
FIGURE 8.
Six Area, Six Sign Atopic Dermatitis scores to week 12.
Per-protocol analysis
The planned per-protocol analysis supported the findings of the primary ITT analysis (Table 5). There was an 18% reduction (4.5 points) in group A and a 24% reduction (6.3 points) in group B. This represented a difference of 1.87 in favour of group B (95% CI –0.73 to 4.47), which was not statistically significant (p = 0.16).
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed a | 99 | 115 | |||
| Week 0 | Mean ± SD | 25.3 ± 13.7 | 26.3 ± 14.5 | ||
| Week 12 | Mean ± SD | 20.8 ± 13.6 | 20.0 ± 13.4 | ||
| Change | Mean ± SD | –4.5 ± 9.3 | –6.3 ± 9.9 | 1.87 (–0.73 to 4.47) | 0.16 |
Sensitivity analyses
Sensitivity analyses were performed (i) including all randomised participants by replacing missing values; (ii) excluding participants for whom the outcome assessor had been unblinded; and (iii) excluding participants with scores that were defined as being outliers (Table 6).
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N – all participants a | 170 | 166 | |||
| Week 0 | Mean ± SD | 25.5 ± 13.7 | 26.0 ± 13.9 | ||
| Week 12 | Mean ± SD | 20.7 ± 13.8 | 20.4 ± 13.9 | ||
| Change | Mean ± SD | –4.9 ± 8.7 | –5.6 ± 9.7 | 0.76 (–1.22 to 2.74) | 0.45 |
| N – excluding participants where nurse became unblinded b | 153 | 159 | |||
| Week 0 | Mean ± SD | 24.7 ± 12.8 | 26.0 ± 14.0 | ||
| Week 12 | Mean ± SD | 19.8 ± 12.9 | 19.9 ± 13.7 | ||
| Change | Mean ± SD | –4.9 ± 8.7 | –6.1 ± 9.4 | 1.26 (–0.77 to 3.28) | 0.22 |
| N – excluding outliers c | 157 | 163 | |||
| Week 0 | Mean ± SD | 24.7 ± 12.7 | 25.5 ± 12.8 | ||
| Week 12 | Mean ± SD | 19.3 ± 12.5 | 20.2 ± 13.8 | ||
| Change | Mean ± SD | –5.4 ± 8.2 | –5.3 ± 8.3 | –0.11 (–1.93 to 1.70) | 0.90 |
Results from analysis of all randomised participants supported the primary result, as the mean change in SASSAD score was 0.76 in favour of group B (95% CI –1.22 to 2.74; p = 0.45).
Results from analysis excluding unblinded participants showed a difference between the two groups of 1.26 in favour of group B (95% CI –0.77 to 3.28), which was not statistically significant (p = 0.22), and again supported the primary result.
Results from analysis excluding outliers gave a mean difference of –0.11 in favour of group A (95% CI –1.93 to 1.70) and was not statistically significant (p = 0.90).
Planned subgroup analysis – filaggrin status
The laboratory screened for the two most common mutations in the filaggrin gene (loss-of-function mutations R501X and 2282del4); variants (mutations) were either heterozygous or homozygous affected. A sample size of 90 children with at least one mutation was required for this subgroup analysis (see Chapter 2 for further details).
Of the 314 participants with test results, 94 (30%) had at least one mutation in the filaggrin gene. These were affected as follows:
-
11 wild type/heterozygous
-
71 heterozygous/heterozygous
-
12 wild type/homozygous affected.
The p-value for the interaction between the filaggrin status and the intervention was 0.87, indicating no evidence that the treatment effect varied between those with and without the mutation.
The analysis by filaggrin status is given in Table 7. The change in SASSAD score between baseline and week 12 in those in whom the mutation was absent was –5.1 in group A and –5.8 in group B. This represented a difference of 0.68 in favour of group B (95% CI –1.87 to 3.23, p = 0.60). The change in SASSAD score between baseline and week 12 in those in whom the mutation was present was –5.2 in group A and –6.3 in group B. This represented a difference of 1.05 in favour of group B (95% CI –2.36 to 4.47, p = 0.54).
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| Na – mutation absent | 103 | 109 | |||
| Week 0 | Mean ± SD | 23.2 ± 12.3 | 25.4 ± 14.2 | ||
| Week 12 | Mean ± SD | 18.1 ± 12.5 | 19.6 ± 13.9 | ||
| Change | Mean ± SD | –5.1 ± 8.0 | –5.8 ± 10.6 | 0.68 (–1.87 to 3.23) | 0.60 |
| Nb – mutation present | 45 | 47 | |||
| Week 0 | Mean ± SD | 27.2 ± 13.4 | 26.7 ± 13.4 | ||
| Week 12 | Mean ± SD | 22.0 ± 13.4 | 20.4 ± 13.9 | ||
| Change | Mean ± SD | –5.2 ± 9.5 | –6.3 ± 6.8 | 1.05 (–2.36 to 4.47) | 0.54 |
Secondary analyses
Categories of improvement in Six Area, Six Sign Atopic Dermatitis score
The SASSAD scores grouped into categories of improvement are shown in Table 8. There was no evidence of a difference between the groups (p = 0.62), which supported the primary ITT analysis.
| Level of improvement | Group A (water softener + usual care) | Group B (usual care) | Total |
|---|---|---|---|
| Number randomised | 170 | 166 | 336 |
| N analysed a | 159 | 164 | 323 |
| Same or worse (≤ 0%) | 39 (25%) | 42 (26%) | 81 (25%) |
| Reasonable (> 0% and ≤ 20%) | 37 (23%) | 30 (18%) | 67 (21%) |
| Good (> 20% and ≤ 50%) | 53 (33%) | 56 (34%) | 109 (34%) |
| Excellent (> 50%) | 30 (19%) | 36 (22%) | 66 (20%) |
Night-time movement
The percentage of the night spent moving was measured using accelerometers (Table 9). Both groups showed an increase in the percentage of the night spent moving: 3.5% in group A and 4.1% in group B. The difference between the two groups was –0.64 in favour of group A (95% CI –4.68 to 3.40) and was not statistically significant (p = 0.76). Both groups showed an increase in night-time movement during the trial. This is in contrast to the other reported outcomes, which all showed improvements over time in both groups. As a result an exploratory sensitivity analysis was conducted (see below). The correlation between the SASSAD score and the first three nights of usable data from the accelerometers at baseline was 0.11 (p = 0.06), suggesting weak evidence of a weak correlation. Comparing the change in SASSAD score from baseline to week 12 with the change in the percentage of the night spent moving from the accelerometer data gave a correlation of –0.02 (p = 0.77), suggesting no evidence of any correlation.
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed a | 114 | 121 | |||
| Week 0 | Mean ± SD | 21.2 ± 7.7 | 22.4 ± 9.7 | ||
| Week 12 | Mean ± SD | 24.7 ± 15.9 | 26.5 ± 17.9 | ||
| Change | Mean ± SD | 3.5 ± 14.5 | 4.1 ± 16.8 | –0.64 (–4.68 to 3.40) | 0.76 |
| Nb – same watch at baseline and 12 weeks | 75 | 85 | |||
| Week 0 | Mean ± SD | 20.7 ± 7.8 | 22.8 ± 10.5 | ||
| Week 12 | Mean ± SD | 26.0 ± 17.2 | 26.8 ± 18.2 | ||
| Change | Mean ± SD | 5.3 ± 16.0 | 4.0 ± 16.2 | 1.30 (–3.73 to 6.34) | 0.61 |
| Nc – participants with > 5 sleep bouts and wearing watch all night (according to diaries) | 94 | 104 | |||
| Week 0 | Mean ± SD | 21.1 ± 7.3 | 22.1 ± 9.1 | ||
| Week 12 | Mean ± SD | 23.1 ± 12.1 | 25.7 ± 17.9 | ||
| Change | Mean ± SD | 2.0 ± 11.1 | 3.6 ± 17.1 | –1.62 (–5.70 to 2.46) | 0.44 |
Sensitivity analyses
Owing to possible differences between the watches, a sensitivity analysis was performed restricted to those who wore the same watch at baseline and week 12 (n = 160). The difference between the two groups was 1.30 (95% CI –3.73 to 6.34), and was not statistically significant (p = 0.61), which supported the main analysis. Given that the direction of change for this outcome was different to that of all other reported outcomes (i.e. participants moved more rather than less during the trial), a post hoc sensitivity analysis was conducted restricted to those for whom we had the most confidence in the accuracy of the data. This was restricted to those who had five or more sleep ‘bouts’ during the analysis period (which would suggest that the units had been worn correctly), and those whose parents indicated that the watch had been worn throughout the night (information taken from diaries, n = 198). This analysis continued to show an increase in movement during the trial and the difference between the groups remained non-significant (Table 9).
Amount of medication used
Group A used on average 58.4 g (SD = 96.8 g) of medication over the 12-week period and group B used on average 67.3 g (SD = 97.3 g, Table 10). The difference between the two groups was –8.90 g (95% CI –30.50 to 12.70 g) and was not statistically significant (p = 0.42).
| Steroid strength | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed | 160 | 153 | |||
| Mild steroids (g) | Mean ± SD | 12.0 ± 29.9 | 18.2 ± 35.6 | ||
| Moderate steroids (g) | Mean ± SD | 19.7 ± 69.3 | 25.3 ± 59.1 | ||
| Potent steroids (g) | Mean ± SD | 21.5 ± 41.4 | 18.4 ± 39.7 | ||
| Very potent steroids (g) | Mean ± SD | 2.2 ± 11.7 | 1.8 ± 20.7 | ||
| Mild calcineurin inhibitors (g) | Mean ± SD | 1.9 ± 7.9 | 2.7± 12.0 | ||
| Moderate calcineurin inhibitors (g) | Mean ± SD | 1.1 ± 9.1 | 1.0 ± 7.9 | ||
| Total medications (g) | Mean ± SD | 58.4 ± 96.8 | 67.3 ± 97.3 | –8.9 (–30.50 to 12.70) | 0.42 |
Sensitivity analyses
Two further sensitivity analyses were performed based on strength of medication and confidence of the nurses in the measurements. Detailed information is given in Appendix 8. Both analyses supported the main findings.
Patient Oriented Eczema Measure scores
Group A showed a drop of 34% (5.7 points) from 16.8 at baseline to 11.1 at week 12 and group B showed a drop of 22% (3.6 points) from 16.6 at baseline to 13.0 at week 12 (Table 11). The difference between the two groups was –2.03 (95% CI –3.55 to –0.51) which was statistically significant (p < 0.01).
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed a | 161 | 162 | |||
| Week 0 | Mean ± SD | 16.8 ± 6.0 | 16.6 ± 5.6 | ||
| Week 12 | Mean ± SD | 11.1 ± 7.1 | 13.0 ± 6.7 | ||
| Change | Mean ± SD | –5.7 ± 7.2 | –3.6 ± 6.7 | –2.03 (–3.55 to –0.51) | < 0.001 |
The difference between the two groups is shown visually in Figure 9.
FIGURE 9.
Change in POEM scores.

The POEM scores at week 4 also showed a difference in favour of group A, but this was not statistically significant. Group A showed a drop of 22% (3.8 points) from 16.9 at baseline to 13.1 at week 4, and group B showed a drop of 17% (2.8 points) from 16.8 at baseline to 13.9 at week 4. The difference between the two groups was –1.0 (95% CI –2.25 to 0.30), which was not statistically significant (p = 0.13).
Totally controlled weeks and well-controlled weeks
Group A had an average of 8.3 (SD 3.8) WCWs and group B had an average of 7.3 (SD 4.1) WCWs over the 12-week study period. The difference between the groups was 0.99 (95% CI 0.04 to 1.95). This difference of just under 1 week was statistically significant (p = 0.04).
The difference between the two groups can be seen in Figure 10.
FIGURE 10.
Number of well WCWs.
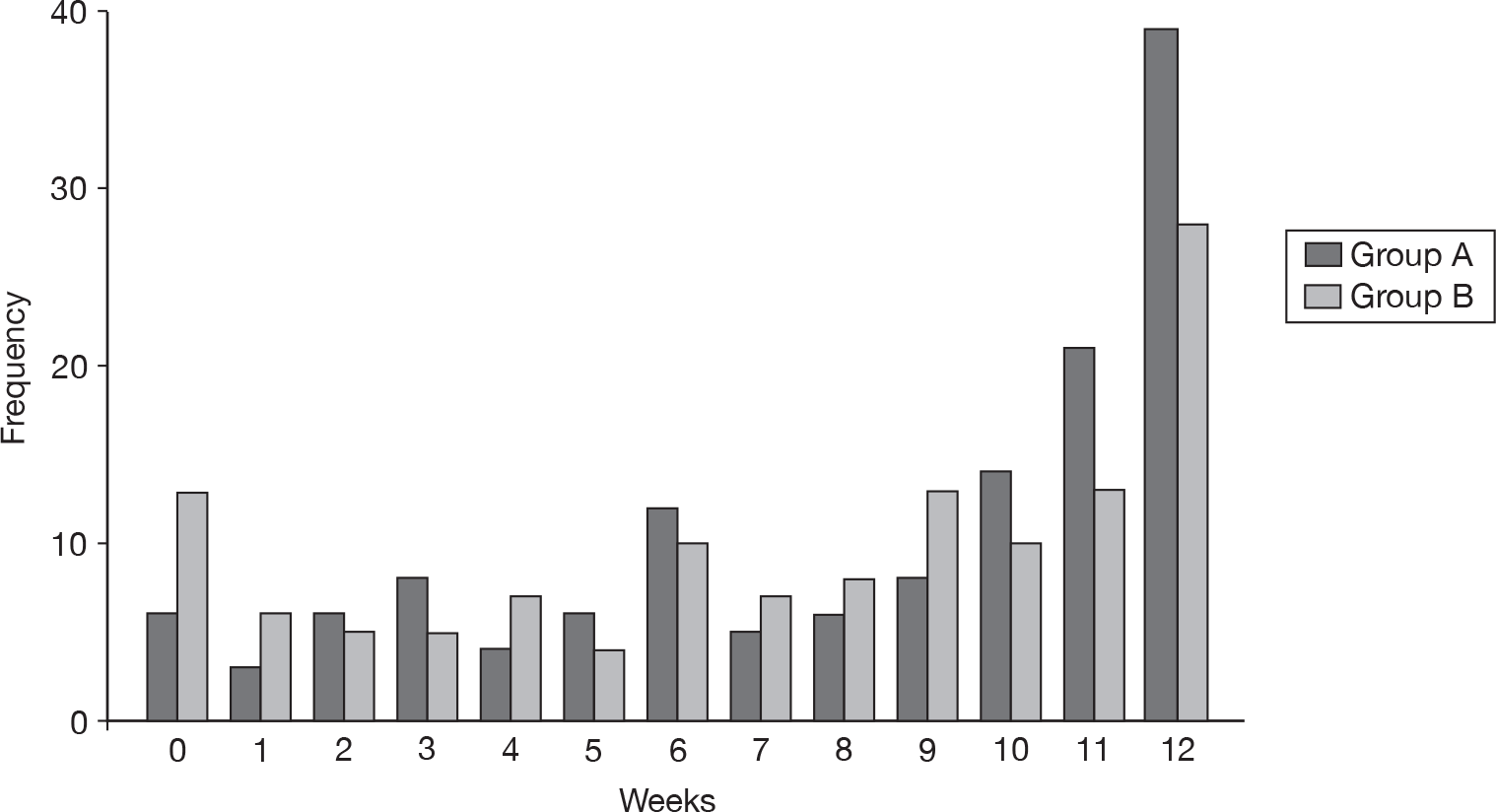
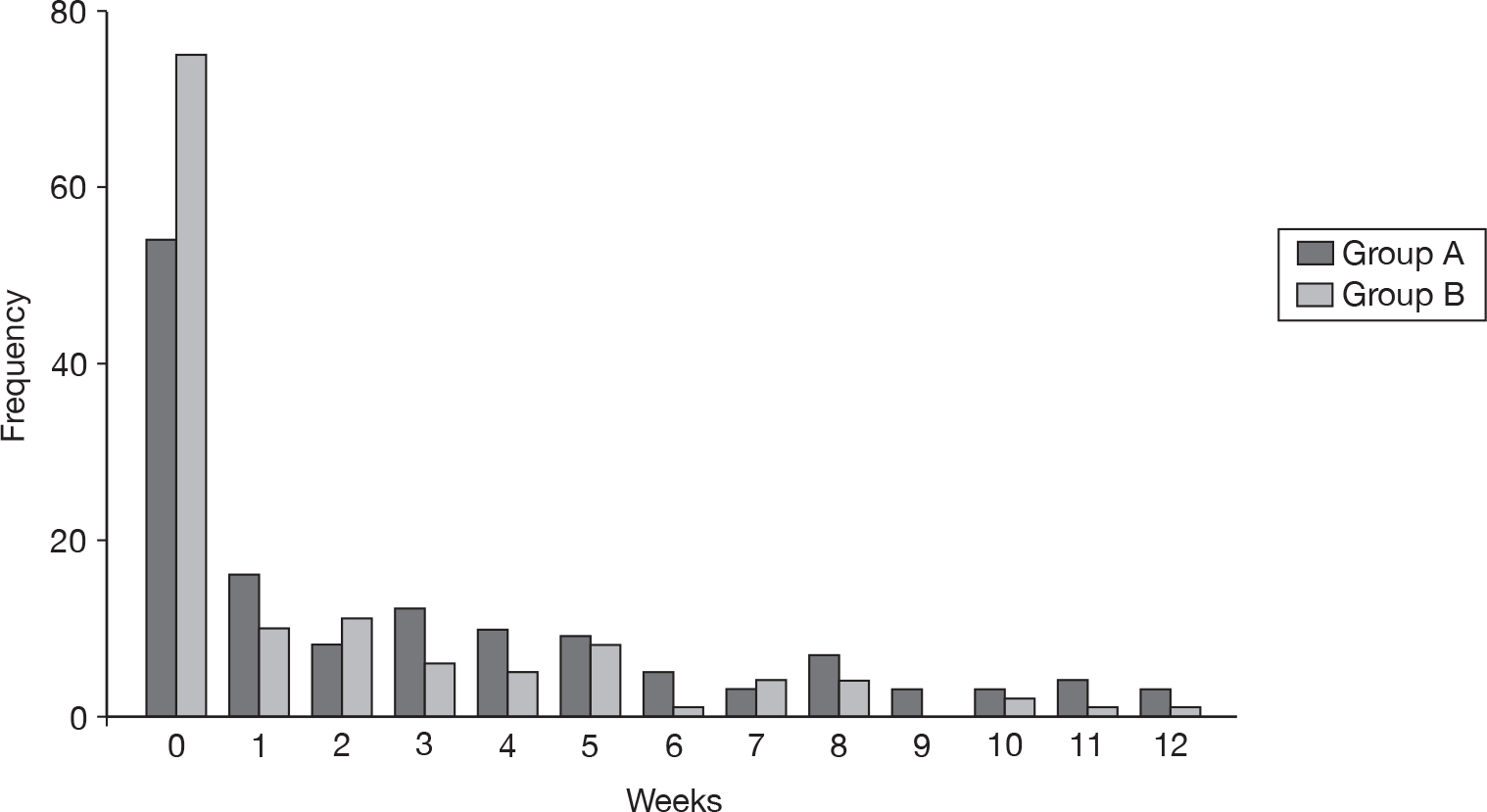
This result was also reflected in the number of TCWs. Although the majority of participants had no weeks when the eczema was totally controlled (Figure 11), group A had an average of 2.9 (SD 3.5) TCWs compared with 1.7 (SD 2.8) in group B. This represented a difference of 1.19 (95% CI 0.43 to 1.95), which was statistically significant (p < 0.01).
FIGURE 11.
Number of TCWs.
Graphs showing the number of WCWs and TCWs for the entire 16-week trial period are shown in Appendix 9.
Dermatitis Family Impact questionnaire
Both groups showed a reduction in DFI score (Table 12). Scores in group A dropped by 32% (3.2 points) and in group B by 16% (1.8 points). This represented a difference of –1.33 points in favour of group A (95% CI –2.63 to –0.03), which just achieved statistical significance (p = 0.05).
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed a | 151 | 158 | |||
| Week 0 | Mean ± SD | 10.0 ± 6.8 | 11.2 ± 7.3 | ||
| Week 12 | Mean ± SD | 6.8 ± 6.0 | 9.3 ± 7.1 | ||
| Change | Mean ± SD | –3.2 ± 6.2 | –1.8 ± 5.4 | –1.33 (–2.63 to –0.03) | 0.05 |
European Quality of Life-5 Dimensions
Both groups showed a small improvement in health-related quality of life. Scores in group A increased by 0.119 points and in group B by 0.066 points. The difference between the two groups was 0.054 (95% CI –0.015 to 0.122) and was not statistically significant (p = 0.12, Table 13).
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed a | 112 | 112 | |||
| Week 0 | Mean ± SD | 0.690 ± 0.298 | 0.693 ± 0.274 | ||
| Week 12 | Mean ± SD | 0.810 ± 0.236 | 0.759 ± 0.245 | ||
| Change | Mean ± SD | 0.119 ± 0.269 | 0.066 ± 0.250 | 0.054 (–0.015 to 0.122) | 0.12 |
Tertiary analyses
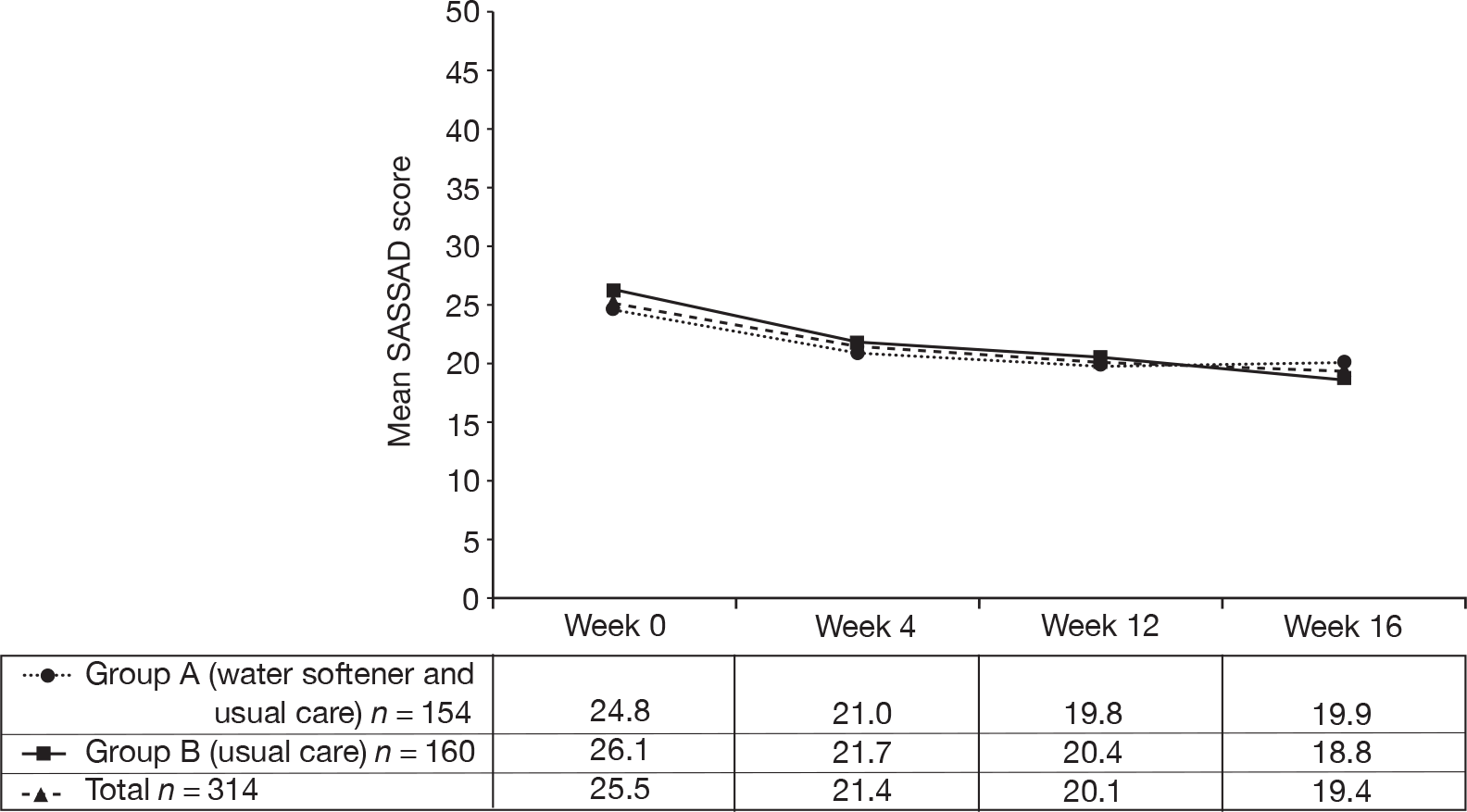
It was not appropriate to conduct analyses looking at possible duration of benefit or speed of onset of benefit, as there was no primary treatment effect. Nevertheless, the SASSAD scores collected between weeks 12 and 16 are shown for interest (when the softeners had been turned off for group A, and installed for group B) (Figure 12).
FIGURE 12.
The SASSAD scores during trial period (weeks 0–16).
Purchase of water softener
Participants had the opportunity to purchase the water softener at the end of the study period. Water softeners were purchased by 55% of participants (179 purchases from 324 installations). Purchase rates in group A (93/168 installs; 55%) were the same as purchase rates in group B (86/156 installs; 55%), even though group A had an average 10.5-week installation period prior to deciding whether to purchase, whereas group B only had an average 3.5-week installation period.
Post hoc end of trial questionnaire
Participants were sent an end of trial follow-up questionnaire once all participants had completed the study. This sought information about current eczema status, whether they had a functioning water softener and, if so, their reasons for purchase. Non-responders were followed up by telephone.
Replies were received from 290/336 participants (86% return). Summarised results (n = 290) are given in Table 14, and collation of free text comments of relevance to SWET (n = 165) in Table 15.
| Purchase status | How is child’s eczema now? (Na = 281) | |||
|---|---|---|---|---|
| Eczema clear | Eczema mild | Eczema quite bad | Eczema very bad | |
| Bought softener (n = 164) | 18 (11%) | 111 (68%) | 27 (16%) | 8 (5%) |
| Did not buy softener (n = 117) | 10 (8%) | 76 (65%) | 23 (20%) | 8 (7%) |
| Reason(s) for buying water softener given by parents who purchased the water softener unit | n |
|---|---|
| Eczema improved on SWET (though hasn’t disappeared) and believe water softener helps | 43 |
| Unsure at the time, but felt worth buying water softener in case it was beneficial in longer term | 19 |
| Eczema improved on SWET (though hasn’t disappeared) and believe water softener helps and wider benefits of having a softener | 15 |
| Wider benefits not related to child’s eczema | 11 |
| Eczema improved on SWET but have now found other factors more important than water softener e.g. avoiding certain foods; new skin care regime; avoiding stress | 9 |
| Eczema improved on SWET (and now clear or nearly gone) and believe due to water softener | 8 |
| Eczema improved on SWET but now unsure if improvement due to water softener or child growing out of it | 3 |
| Eczema improved on SWET but has relapsed and now can’t see any benefit | 3 |
| Total | 111 |
| Comments from parents who did not buy the water softener | |
| Eczema did not improve on SWET, therefore did not wish to buy | 23 |
| Could not afford to buy the water softener but would have liked to | 15 |
| Eczema improved on SWET but not enough to warrant buying a water softener | 7 |
| Eczema improved on SWET and has continued clear without a water softener | 3 |
| Could not buy for practical/technical reasons/moving home | 3 |
| Needed a longer trial period to decide whether to buy or not | 2 |
| Eczema improved on SWET but did not believe this was due to water softener | 1 |
| Total | 54 |
A fuller discussion of willingness to pay (WTP) is given in Chapter 5.
Of the 290 participants who replied, 170 purchased the water softener (59%, including three purchases of non-SWET units) and 120 did not purchase the water softener (41%).
Of the 170 purchasers, 168 gave the reason for their purchase as either ‘eczema improved on SWET’ (n = 111, 66%) or ‘because of wider benefits’ (n = 46, 27%) or both reasons (n = 11, 7%).
Three-Item Severity score and assessment of integrity of information bias
Nurses scored a representative site (target lesion) using the TIS score. Group A showed a mean reduction of 30% (1.2 points) from an average of 3.9 points at baseline to 2.7 points at week 12. Group B showed a reduction of 33% (1.3 points) from an average of 3.9 points at baseline to 2.5 points at week 12. The difference between the two groups based on the scores given by the nurses was 0.07 (95% CI –0.31 to 0.46) and was not statistically significant (p = 0.71, Table 16). This supports the primary outcome (SASSAD) data.
| Time assessed | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number enrolled | 170 | 166 | |||
| N a | 160 | 161 | |||
| Week 0 | Mean ± SD | 3.9 ± 1.8 | 3.9 ± 1.7 | ||
| Week 12 | Mean ± SD | 2.7 ± 1.9 | 2.5 ± 1.8 | ||
| Change | Mean ± SD | –1.2 ± 1.7 | –1.3 ± 1.8 | 0.07 (–0.31 to 0.46) | 0.71 |
Given the clear and consistent difference between the blinded and the non-blinded outcomes in terms of treatment effect, it is likely that blinding in the trial was maintained. In order to confirm this, attempts were made to measure information bias using the digital images. However, during the course of the study a number of practical problems emerged: (i) the quality of digital images varied widely; (ii) not all uploaded photographs were taken of the target site; and (iii) some images were missing for one or more of the assessment visits. The TMG recommended that integrity of information bias be limited to examination of baseline and 12-week images. Images were recoded and sent in the first instance to Dr Emma Veysey, Consultant Dermatologist, for scoring using the TIS scale.
Feedback from SWET research nurses indicated that digital images poorly reflected in situ skin lesions. This was confirmed by the fact that Dr Veysey was able to fully score only 376/546 images (188/273 participants), owing to variable image quality. For this reason Professor Hywel Williams also assessed a sample of 40 images, and these were compared with Dr Veysey’s scores and the in situ scores obtained by the research nurses. This showed that there was reasonable agreement in the dermatologist’s scoring of the digital images, but that the quality of the images meant that potential differences between the baseline and week 12 scores were lost. This was confirmed comparing the results of Dr Veysey’s analysis with TIS scores recorded by the nurses in situ. In light of these findings, it was agreed that the assessment of information bias would be tested through sensitivity analysis in which participants were excluded if the research nurse reported that they had become unblinded before the primary outcome assessment (n = 11).
Adverse events
This trial involved the use of a commonly available domestic water softening unit with provision for mains drinking water during the time when the water-softening unit was installed. Therefore, the TMG did not anticipate any adverse events or adverse reactions of relevance to the trial. As a result, adverse event data were not routinely collected. Events of technical relevance such as plumbing difficulties, floods or difficulties with the units were logged at the co-ordinating centre and investigated by local water engineers as a matter of urgency.
The parents of two participants believed that their child’s eczema had worsened as a direct result of installation of the water softener and asked to have the unit removed. They were instructed to switch off the unit, which was subsequently removed and their child withdrawn from the trial. The parents of a third participant expressed concern that the water softener appeared to be making their child’s eczema worse, but the child continued to take part in the trial.
Chapter 5 Health economics
Introduction
Eczema has large cost implications for society and the individual families affected. In 1995–6 the total annual UK cost of eczema in children aged ≤ 5 years was estimated to be £47M (or £79.59 per child), of which 64% was accounted for by NHS health-care costs. 35 A further UK study looking at a broader age range estimated the total annual cost to be in the order of £465M, of which £125M was incurred by the NHS, £297M by the patients and £42M by society in terms of lost working days (price year not reported, but most likely to be 1994 or 1995 prices). 6 Childhood eczema has been shown to have a similar impact on health-related quality of life as other common childhood conditions such as asthma and diabetes. 36
Current treatment consists predominantly of emollients, bath oils and topical corticosteroid creams, although some children may receive topical antibiotics, oral antibiotics, wet wraps, oral antihistamines and special dietary products. It was hypothesised at the outset of this trial that, should ion-exchange water softeners be effective, this may result in a reduction in the use of these products, and in the number of consultations, such that there might be potential cost savings for the NHS. Likewise, if effective, the costs incurred by families may also decline.
Ion-exchange water softeners are currently a private good in the UK; individual consumers are free to choose whether or not to purchase a unit out of their own disposable income. Before this trial, there was no scientific evidence about the clinical effectiveness or cost-effectiveness of ion-exchange water softeners for the treatment of eczema. As a result, the national health-care system in the UK does not currently fund this technology. One of the aims of the economic component of this trial was to assess whether the NHS should consider funding this technology.
Even though our RCT failed to find any objective evidence for the benefit of ion-exchange water softeners for improving eczema severity, an economic analysis is presented in this section because (i) it was part of the original research plan; (ii) it provides an indication of the potential costs if the intervention had been effective; (iii) while being highly prone to response bias, it is possible that the patient-generated utilities are measuring something important that physical signs alone do not capture; and (iv) the approaches and costs used may be useful for future health economic assessments of interventions for eczema or for other potential health benefits of water softeners.
Methods
Aim and perspective
The aim was to estimate the cost-effectiveness and cost–utility of ion-exchange water softeners for children with eczema, as compared with usual care. The study adopted an NHS perspective, in order to inform health policy relating to the use of ion-exchange water softeners for children with eczema.
Time frame
Costs and benefits were calculated for the 12-week study period only. As the trial has a short time frame, neither costs nor benefits were discounted.
Resource use and cost analysis
Resources collected during the trial are summarised in Table 17. The resources fall into two main categories: (i) those used to provide the intervention (consultation with a dermatologist, water softener device, installation, salt); and (ii) those that may change as a result of the intervention (NHS resource use, including number of visits to the GP, practice nurse, pharmacist, health visitor, specialist nurse, hospital admissions, hospital doctor and medication use).
| Resource use item | Typical/mean | Minimum/best case | Maximum/worst case | Source |
|---|---|---|---|---|
| Annuity factora | 9.6633 based on r = 3.5% and n = 12 years | 14.2124 based on r = 3.5% and n = 20 years | 4.5151 based on r = 3.5% and n = 5 years | Drummond et al. 200537 |
| Purchase price (£)d,e | 600 | 300 | 1800 | Industry expert opinion |
| Annuitised 12-week purchase priceb | 14.33 | 4.87 | 92.00 | |
| Installation cost (£) d,f | 230 | 175 | 380 | Industry expert opinion |
| Annuitised 12-week installation costb | 5.49 | 2.84 | 19.42 | |
| Expected lifetime years | 12 | 20 | 5 | Industry expert opinion |
| Salt, cost per box (£)d | 15.36 | – | – | Direct Salt |
| Face-to-face consultant-led follow-up attendance, paediatric dermatology | 151 | 0 (no visit assumed necessary) | 228 | NHS reference costs 2008/9 38 |
| Primary health carec | ||||
| GP (per surgery consultation) | 31 | 27 | 35 | PSSRU39 |
| Practice nurse (per consultation) | 9 | 9 | 11 | PSSRU39 |
| Health visitor (per visit home visit) | 35 | 35 | 40 | PSSRU39 |
| Pharmacist (per visit) | 42 | 34 | 85 | PSSRU39 |
| Specialist nurse (per hour) | 74 | 29 | 88 | PSSRU39 |
| Secondary health care | ||||
| Hospital outpatients visit (per follow-up attendance, paediatric dermatology) | 151 | 88 | 228 | NHS reference costs 2008/9 38 |
| Hospital admission (non-elective inpatient stay (short stay) | 493 | 329 | 588 | PSSRU39 |
| Medication and accessory costs | ||||
| Variety | – | – | – | BNF 5840 |
The dermatology consultation was included in the intervention group in the base case because it was assumed that an additional clinic visit would be required in order to prescribe a water softener device. The impact of excluding this cost is further explored in sensitivity analysis.
NHS resource use data were recorded by parents in a daily diary over the 12-week trial period. In addition, topical steroids and/or calcineurin inhibitors were weighed by trial nurses at baseline and the end of week 12. Several assumptions were required in order to cost the medication weights recorded:
-
Where the medication weights were recorded but no medication name given, the average unit cost across medications in that potency category was assumed.
-
Where no weights but some medication names were recorded it was assumed there had been no use of that medication over the 12-week study period. This applied to 25 cases (nine in group A, 16 in group B) where the nurses had rated their confidence in the assessment of weight as ‘not sure’ or ‘not at all sure’. As slightly more of the missing values were in group B, the effect of this assumption would be conservative against the intervention.
-
Where there were two medications within a single potency category it was assumed that half the weight had been used on each medication.
-
Where there were more than two medications within a single potency category, the average unit cost across medications in that potency category was used.
-
Where a medication did not have the strength or application types recorded, an assumption was made. For example, the most frequent omission was with respect to the strength of hydrocortisone. For cases where this was recorded, it was most frequently 1% strength and approximately 30 g had been used. As a result, a unit cost for those with missing information was assumed based on the cost of a 30-tube of 1% hydrocortisone.
It was assumed that all intervention households had one water softener device installed. This assumption was made despite knowing the exact number of devices installed into each home because in this study the devices were being installed into multiple homes for short periods of time. Therefore, although a minority of homes had to have replacement devices, we did not include the cost of these replacement machines because this is likely to be a consequence of the study design rather than some inherent failing of the devices, which have an expected average life span of 12 years. Salt consumption was assumed as a standard rate based on household size and the agreed amounts of salt engineers left at the time of installation (Table 18).
| Group | SWET salt supply at installation | ||
|---|---|---|---|
| Number of residents | Salt boxes (six blocks/box) | Cost of salt for 12 weeks (£) | |
| Group A (water softener + usual care) | ≤ 3 | 2 | 30.72 |
| 4–5 | 3 | 46.08 | |
| ≥ 6 | 4 | 61.44 | |
The unit costs for the resources were identified from published sources, or from expert opinion where necessary. The value and source of the proposed unit costs for each resource item are shown in Table 17. The unit cost for the actual water softener device and installation has been estimated as the appropriate proportion of the equivalent annual cost (i.e. estimated as an equivalent 12-week cost to accurately reflect the fact that the device has a typical lifespan of 12 years and depreciation has been assumed to be zero). This was done in order to express all costs on a 12-week basis. On this basis the average 12-week cost of the device and installation was £19.82. The cost analysis was undertaken using individual patient level data.
All costs are reported in UK pounds sterling for the year 2009. Mean resource use and costs per patient for group A and group B are presented along with mean difference in resource use and cost per patient and 95% CIs comparing the groups.
Outcome measurement and valuation
Two cost-effectiveness analyses are presented using (i) the proportion of participants showing a 20% reduction in SASSAD score at week 12; and (ii) the proportion of participants showing a 50% reduction in SASSAD score at week 12. This enables health policy decision-makers to compare the cost-effectiveness of water softeners for the treatment of eczema with the cost-effectiveness of other eczema treatments.
An additional, cost–utility analysis is presented using health-related quality of life (EQ-5D). Data were captured using the children’s version of the EQ-5D for children aged 7 years and over, or the proxy version of the EQ-5D for children aged 3–6 years. 30,31 A utility weight has been attached to the health state descriptions using the currently accepted UK adult tariff, calculated using the York A1 tariff. 32 Children under 36 months were excluded from this analysis, as the EQ-5D has not been designed for use in children aged < 3 years.
Quality-adjusted life-years (QALYs) for individual participants have been calculated using linear interpolation between the baseline and 12-week utility value (Figure 13). Using the area under the curve technique, the number of QALYs for the 12-week trial period was estimated for each participant as displayed in Equation 1.
FIGURE 13.
Quality-adjusted life-years.
where UB is utility at baseline, U12 is utility at 12 weeks, 0.5 reflects that we are measuring a triangle not a square and 0.230769 reflects the 12-week study period (12 weeks divided by 52 weeks).
The shaded area in Figure 13 shows an example of the QALY area being measured for each individual participant, as the area between the linear interpolation line and a line drawn horizontally from the baseline value for the 12-week study period. Figure 13 shows a gain in utility over time such that this individual would have gained a positive number of QALYs. If a loss of QALYs had occurred the diagonal line measuring utility over time would be reversed so that the line sloped downwards rather than upwards, and if there was no change in QALYs over the 12-week period then there would be no diagonal line indicating that utility had remained constant over time.
Mean QALYs per patient for group A and group B are presented along with the mean difference in QALYs per patient and 95% CIs comparing the groups. This analysis allows health policy decision-makers to compare the value of water softeners for eczema with other health technologies in other areas of health.
Cost-effectiveness analysis and presentation of results
Where non-dominance occurs (that is where the intervention is more costly and more effective or less costly and less effective) an incremental cost-effectiveness ratio is presented as an incremental cost per patient achieving a 20% improvement in SASSAD score, an incremental cost per patient achieving a 50% improvement in SASSAD score and as an incremental cost per QALY. Additionally, cost-effectiveness acceptability curves are presented for the intervention and control group,41,42 where the cost-effectiveness acceptability curve depicts the probability that an intervention is cost-effective at different levels of the cost-effectiveness threshold (that is for differing levels of WTP per QALY).
Sensitivity analysis
The base case is based on the typical, mean or most likely unit costs. As some of the unit costs are based on expert opinion, we have tested these assumptions by taking a best- and worst-case sensitivity analysis. All unit costs are varied firstly to their best case and then to the worst case to provide the range within which the true incremental cost-effectiveness ratio may lie (see Table 17). The broader this range, the greater our uncertainty in the base-case result.
For the cost–utility analysis, an additional sensitivity analysis was conducted including the children < 36 months by assuming a utility weight based on the child’s severity of eczema (SASSAD score). Those with a SASSAD score of < 20 were assigned the mean utility weight of those aged ≥ 36 months with a SASSAD score of < 20, and those with a SASSAD score of ≥ 20 were assigned the mean utility weight of those aged ≥ 36 months with a SASSAD score of ≥ 20.
Secondary analyses
The base-case analysis makes best use of the data collected in the trial. However, it took a narrow perspective and short time frame. It had originally been planned to produce a model to explore the long-term effects of the water softeners. However, given the lack of treatment effect during the trial, this was no longer felt to be appropriate.
Contingent valuation study measuring willingness to pay
This study presented a unique opportunity to compare hypothetical WTP with actual WTP for a health intervention that is not currently available from the NHS. As a result, a WTP questionnaire was included in the assessments (see Appendix 10; it was designed based on previous contingent valuation questionnaires used by researchers at Nottingham University). We provided information to parents about the likely benefits for their home of having a water softener, and the uncertainty surrounding whether or not water softeners help to improve skin conditions, before asking the WTP question. Information was also provided on the lifespan and typical cost of the device. The WTP question was administered face to face at the baseline visit by the research nurse to all parents of participant children, and again by post at 12 weeks for a subsample of parents, to elicit the maximum WTP for an ion-exchange water softener device. Note: to avoid influencing data collected in the ex-ante WTP questionnaire, the actual reduced price (£437 including VAT) was not given to parents before their child’s recruitment visit. From May 2007 to October 2008 this information was given out only after their child’s 12-week assessment visit, once they had completed the second WTP questionnaire. Feedback indicated that a number of parents were unhappy with the short time between learning the reduced price and being asked to decide if they wished to purchase the water softener. Therefore, from November 2008, parents were informed of the reduced price in the letter sent out immediately after their child’s recruitment into the trial, and the second WTP questionnaire was abandoned.
Willingness to pay prior to using the water softener
Willingness to pay elicited prior to use of the ion-exchange water softener was measured at the baseline visit. Those giving zero bids were categorised as protestors (those who bid zero for moral or political reasons) or non-protestors (those who do not value water softeners at all).
Mean WTP was estimated and the distribution of WTP bids illustrated graphically (this represents the demand curve for water softeners for the treatment of childhood eczema).
A general linear regression analysis was undertaken to estimate how WTP for softeners before the trial varied according to a number of independent variables as defined in Appendix 11.
Actual willingness to pay
The number actually willing to pay for a water softener at the discounted price (or market price if they bought a non-trial device) was estimated as a proportion of those who were hypothetically willing to pay the actual asking price at baseline. The difference in hypothetical and actual WTP is reported.
A logistic regression analysis was undertaken to see which independent variables explained a parent’s decision to purchase the water softener or not. The dependent variable was categorised into those who bought the device at the end of the trial (coded as 1) and those who did not buy the device (coded as 0). The independent variables included in the model are defined in Appendix 11.
This analysis enables a partial examination of the issue of hypothetical bias in contingent valuation in health care. However, it should be noted that as participants were offered a single price at the end of the trial, we are not able to estimate the actual maximum WTP for the water softener using this approach.
Results
Data from 323 participants (159 intervention and 164 control) were included in the base-case cost-effectiveness analysis and data from 228 participants (115 intervention and 113 control) were included in the base-case cost–utility analysis of participants aged 3 years and over. At baseline the groups were well matched (see Table 2).
Resource use and costs
Just 2.5% of weekly diaries were not completed, therefore data were not imputed for these entries. The mean total cost per patient in group A was £332 (SD £170) compared with £134 (SD £288) in group B (difference £198, 95% CI £146 to £250, p < 0.001). This significant cost difference was due to the cost of the intervention; all other resource categories (health professional visits, medications and other medical items) were not significantly different between groups (see Table 19 for resource use and Table 20 for resource costs).
| Resource use item | Group A (water softener + usual care) (n = 159) | Group B (usual care) (n = 164) | Mean difference (95% CI) |
|---|---|---|---|
| Intervention | |||
| Ion-exchange water softener | 1.00 | 0.00 | 1.00 |
| Installation | 1.00 | 0.00 | 1.00 |
| Salt blocks | 15.25 (7.42) | 0.00 (0.00) | 15.25 (14.08 to 16.41) |
| Face-to-face consultant-led follow-up attendance, paediatric dermatology | 1.00 | 0.00 | 1.00 |
| Secondary health care | |||
| Hospital outpatients visit (follow-up attendance, paediatric dermatology) | 0.26 (0.59) | 0.35 (0.90) | –0.09 (–0.26 to 0.08) |
| Hospital admission [non-elective inpatient stay (short stay)] | 0.00 (0.00) | 0.01 (0.11) | –0.01 (–0.03 to 0.01) |
| Primary and community health care (consultation/visit) | |||
| GP | 0.52 (1.00) | 0.49 (1.04) | 0.03 (–0.20 to 0.25) |
| Practice nurse | 0.04 (0.23) | 0.15 (1.19) | –0.10 (–0.29 to 0.09) |
| Health visitor | 0.01 (0.08) | 0.02 (0.19) | –0.02 (–0.05 to 0.01) |
| Pharmacist | 0.43 (1.27) | 0.41 (1.19) | 0.03 (–0.24 to 0.29) |
| Specialist nurse | 0.21 (0.85) | 0.27 (1.12) | –0.05 (–0.27 to 0.16) |
| Resource use item | Group A (water softener + usual care) (n = 159) | Group B (usual care) (n = 164) | Mean difference (95% CI) |
|---|---|---|---|
| Intervention | 215.45 (7.88) | 0.00 (0.00) | 215.45 (214.22 to 216.69) |
| Ion-exchange water softener | 14.33 | 0.00 | 14.33 |
| Installation | 5.49 | 0.00 | 5.49 |
| Salt | 44.63 (7.88) | 0.00 (0.00) | 44.63 (43.40 to 45.87) |
| Face-to-face consultant-led follow-up attendance, paediatric dermatology | 151.00 | 0.00 | 151.00 |
| Secondary health care | 38.94 (88.63) | 58.49 (167.24) | –19.56 (–48.77 to 9.65) |
| Hospital outpatients visit (follow-up attendance, paediatric dermatology) | 38.94 (88.63) | 52.48 (135.47) | –13.54 (–38.54 to 11.45) |
| Hospital admission [non-elective inpatient stay (short stay)] | 0.00 (0.00) | 6.01 (54.28) | –6.01 (–14.38 to 2.36) |
| Primary and community health care | 72.12 (116.21) | 78.68 (149.74) | –6.55 (–35.85 to 22.74) |
| GP | 16.18 (30.97) | 15.31 (32.30) | 0.87 (–6.05 to 7.80) |
| Practice nurse | 0.40 (2.11) | 1.32 (10.75) | –0.92 (–2.63 to 0.77) |
| Health visitor | 0.22 (2.78) | 0.85 (6.66) | –0.63 (–1.75 to 0.48) |
| Pharmacist | 18.23 (53.15) | 17.16 (49.85) | 1.07 (–10.22 to 12.36) |
| Specialist nurse | 15.82 (63.06) | 19.85 (82.82) | –4.03 (–20.12 to 12.06) |
| Medication and accessories | 21.28 (31.99) | 24.18 (41.15) | –2.91 (–10.97 to 5.15) |
| Total incremental cost | 331.74 (169.95) | 133.61 (288.33) | 198.13 (146.47 to 249.80) |
Cost and resource-use data for children aged ≥ 3 years (as required for the cost–utility analysis) are included in Appendix 12.
Family costs
Although the cost-effectiveness analysis adopted an NHS perspective, wider family costs were collected during the trial. Thirty-three per cent of families provided estimates of costs incurred as a result of their child having eczema over the trial period. For group A, mean family costs were £16.67 (SD £65.78, range £0–653) compared with a mean family cost of £20.82 (SD £92.29, range £0–1095) for group B, giving a difference of –£4.16 (independent samples test, p < 0.641). Items included medicinal products such as aqueous cream, E45 or supplements, travel and parking for appointments, special foods, cleaning products, clothing and bedding. It should be noted, however, that, although all participants were asked to report these data in the daily diary, it is not clear if non-reporting of these types of costs meant that they were not incurred or whether they were under-reported because in some instances it is hard to attach a monetary value. In a few cases respondents did not report a monetary value but did write a textual description of a resource item and so these items had to be excluded, for instance where respondents stated that their electricity bill or amount of clothes washing undertaken was higher. If mean costs are estimated across just those providing a cost estimate (48 participants in group A and 54 in group B), mean family costs for group A were £55.21 (SD £111.23, range £1.65–653) compared with a mean family cost of £63.24 (SD £153.17, range £1.19–1095) for group B, giving a difference of –£8.04 (independent samples test, p < 0.761). We did not collect non-monetary family costs, for instance time costs of accompanying children to visits, although the number of visits undertaken during the trial period tended to be quite small.
Cost-effectiveness analysis
The proportion of participants showing a 50% reduction in SASSAD score at week 12 compared with baseline was 18.87% (30/159 participants) in group A compared with 21.95% (36/164 participants) in group B, a difference of –3.08% (p = 0.492). The proportion of participants showing a 20% reduction in SASSAD score at week 12 compared with baseline was 48.43% (77/159 participants) in group A compared with 54.88% (90/164 participants) in group B, a difference of –6.45% (p = 0.246).
As the incremental mean cost is greater and incremental mean benefits lower for group A than for group B, it was not appropriate to estimate an incremental cost-effectiveness ratio because ion-exchange water softeners were dominated by usual care alone, i.e. they were both more expensive and no more effective than usual care, such that the NHS would not consider them a cost-effective use of NHS resources. The decision uncertainty is depicted in Figures 14 and 15. These cost-effectiveness acceptability curves show that there is around a 20% chance of the wrong decision being made if ion-exchange water softeners are not funded by the NHS.
FIGURE 14.
Decision uncertainty: plots of the cost-effectiveness acceptability curves for the proportion of participants showing a 50% reduction in eczema severity (SASSAD).
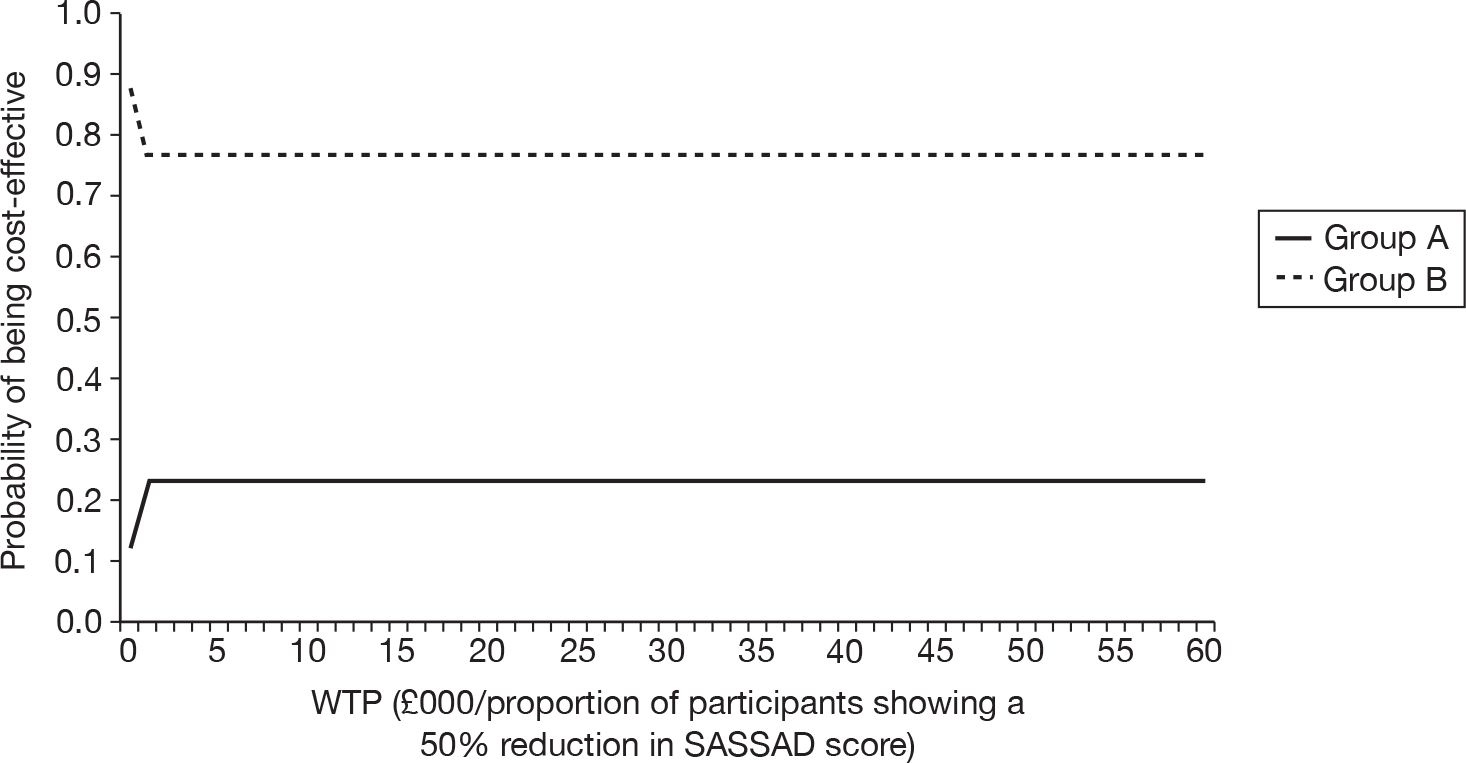
FIGURE 15.
Decision uncertainty: plots of the cost-effectiveness acceptability curves for the proportion of participants showing a 20% reduction in eczema severity (SASSAD).
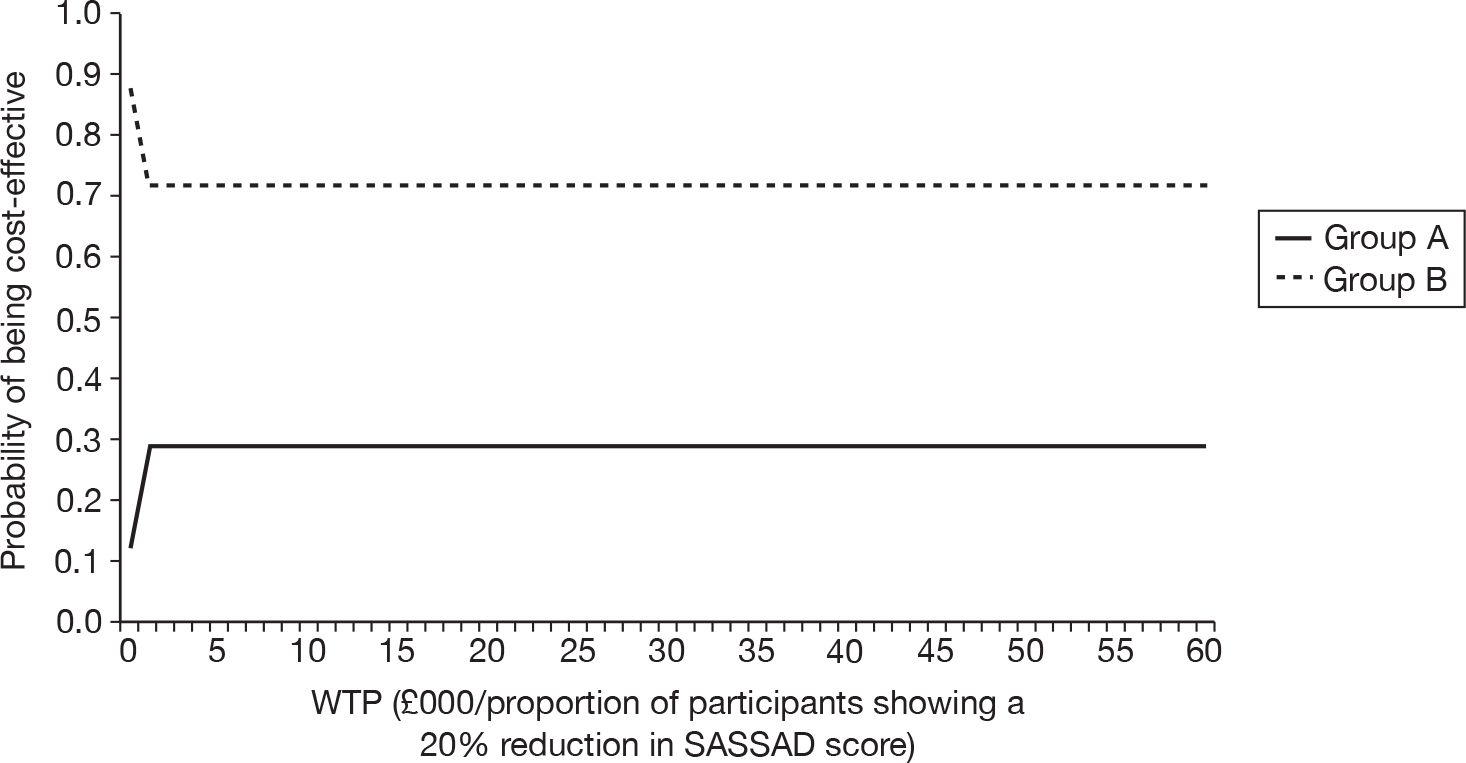
Sensitivity analysis
For the best-case analysis dermatology consultation cost was removed from the intervention costs (as this may not be necessary in practice to obtain an ion-exchange water softener). The cost-effectiveness analysis based on the proportion of participants showing either a 50% or 20% reduction in SASSAD score at week 12 supported the base-case analysis. Costs in group A were greater than those in group B [mean cost £135.50 (SD £111.25) vs £94.13 (SD £179.51), incremental cost £41.37 (95% CI £8.76 to £73.98), p = 0.013], meaning that usual care was still both cheaper and slightly more effective than the use of an ion-exchange water softener in children with eczema. The associated cost-effectiveness acceptability curves (not shown) revealed that there is around a 35% chance of the wrong decision being made if ion-exchange water softeners are not funded by the NHS.
Worst-case analysis was not deemed to be necessary given that neither the base case nor the best case suggested cost-effectiveness for the NHS.
Cost–utility analysis
Group A gained a mean of 0.119 on the EQ-5D health-related quality of life scale and group B a mean of 0.066. The mean difference in EQ-5D was 0.054 (95% CI –0.015 to 0.122) (see Table 13).
The mean total cost per patient aged ≥ 3 years in group A was £315 (SD £64) compared with £143 (SD £343) in group B (difference £172, 95% CI £101 to £243, p = 0<001). (For full details, see Appendix 12.)
Group A gained a mean of 0.014 QALYs per patient and group B a mean of 0.008 QALYs per patient. The mean difference in QALYs per patient was 0.006 (95% CI –0.002 to 0.014, p = 0.117). Using the incremental mean cost reported in Table 20, the incremental cost per QALY was estimated as £28,002. Figure 16 shows the cost-effectiveness acceptability curve for the 12-week study period. At all levels of WTP, usual care (group B) had a higher probability of being cost-effective.
FIGURE 16.
Decision uncertainty – plots of the cost-effectiveness acceptability curves for the base-case cost–utility analysis of year 3- to16- year-olds.
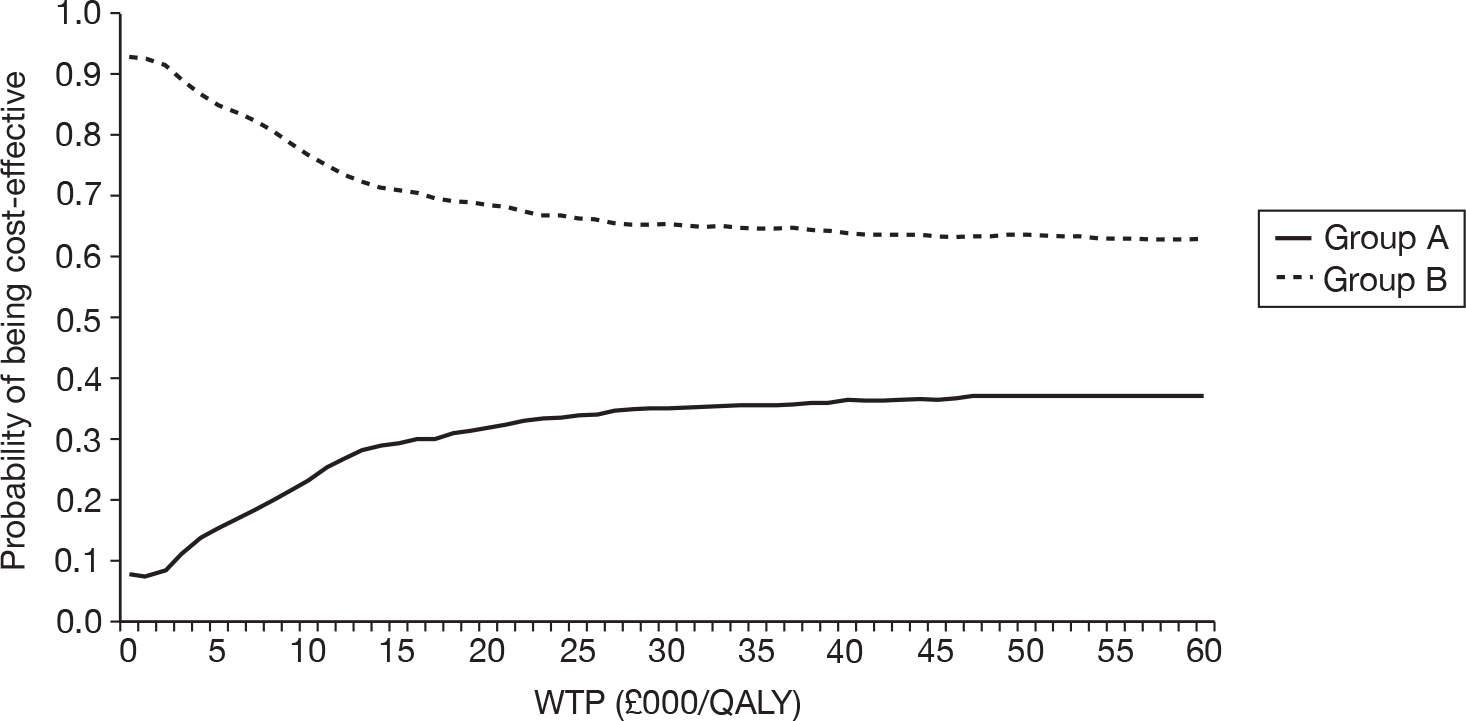
Sensitivity analysis
Sensitivity analysis including best-case costs and all participants (rather than just those aged ≥ 3 years who had EQ-5D scores) supported the main analysis. Possible incremental cost-effectiveness ratios ranged from £4548 (best case) to £53,957 (worst case).
Cost–utility sensitivity analyses for those aged ≥ 3 years are given in Appendix 13.
Secondary analyses
Modelling of long-term effects
As ion-exchange water softeners were not found to be clinically effective or cost-effective over the trial horizon, it was not felt to be appropriate to model the longer term cost-effectiveness of water softeners. However, an indication of the likely long-term cost of an ion-exchange water softener is helpful. For a typical family, the cost would be somewhere in the region of £3250. This assumes a 12-year lifespan for the device (covering initial purchase, installation and ongoing salt use) and a household of four or five people.
Contingent valuation study
Willingness to pay for water softeners before the trial
The majority of participants (333/336) provided an answer to the contingent valuation question, which asked parents to estimate the financial value of an ion-exchange water softener to them (the three not answering were all in group A). Given that water softeners are essentially private goods, it was felt that asking directly about participants’ WTP for this product would be relatively intuitive. The mean (median/SD) WTP value was £506.68 (£500/£387.73) with a range from £0 to £3000 (see Figure 17 for distribution of WTP responses). Just five (1.5%) participants (three in group A and two in group B) gave a value of zero and all were genuine zeros. Reasons given by these parents included not being willing to pay anything until proven to be of benefit for eczema, and the child’s eczema not currently causing problems.
FIGURE 17.
Willingness to pay for water softeners for children with eczema.
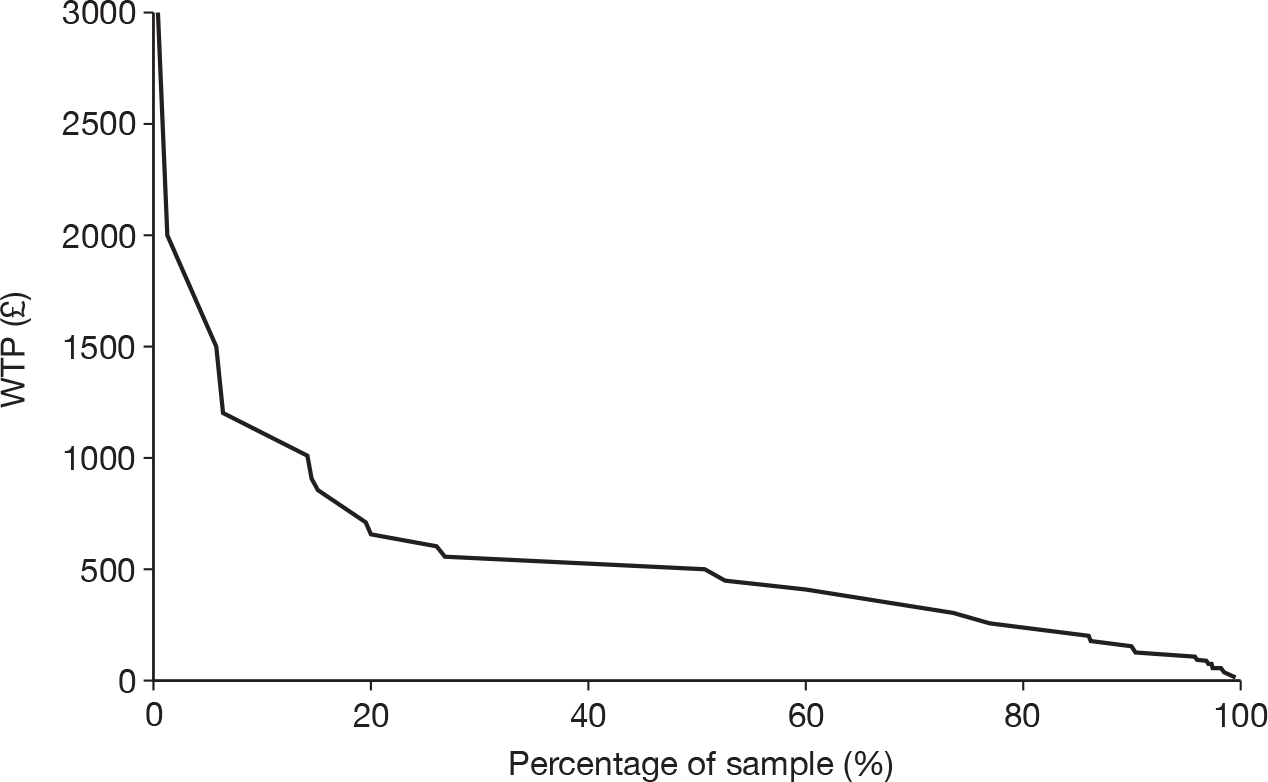
The results of the univariate general linear regression analysis in which hypothetical WTP was the dependent variable are reported in Appendix 14. The statistically significant variables associated with a positive relationship with WTP included participants stating that their value reflected the anticipated benefits of the ion-exchange water softener (such participants were willing to pay on average £126.73 more than those not stating this reason); those households with an income of ≥ £50,000 (willing to pay £173.72 more on average than those not with this income); and number of nights at home (those with more nights at home willing to pay more). Participants who found the WTP task difficult, or who stated that their WTP reflected their ability to pay, reported significantly lower values of WTP.
The typical cost of an ion-exchange water softener is £600, but could range between £300 and £1800 (based on industry opinion). Using the mean WTP prior to using the water softener as a measure of benefit for the family, it can be inferred that families perceive the benefits of an ion-exchange water softener for their family to be a mean of £506.68. As the scenario provided to families included a description of the likely non-eczema-related cost savings (resulting from less lime scale and improved efficiency of household appliances leading to less fuel consumption and soap use for instance), this estimate of WTP can be taken to mean that families would find an ion-exchange water softeners to have a positive cost–benefit ratio for the family only at the lower price end of the market (i.e. where price is < £506.68). As shown above (see Secondary analyses), there were no significant cost savings for the families associated with costs incurred by the family as a result of their child’s eczema, so these are not considered again here.
Willingness to pay for water softeners after the trial
At 12 weeks a subsample of 146 respondents (those recruited first to the study) were asked the same WTP question as at baseline to see if experience influenced valuations. Of these, only 97 (66%) provided a value (in addition two respondents stated ‘priceless’). Not all 336 participants were asked this question because during the trial it became clear that parents wanted to have some idea of the actual price of the device they could buy at the end of the study in order to save for it. Once this information was divulged this question was felt to be inappropriate.
Mean (median/SD) WTP at the end of the trial was £375 (£300/£282) with a range from £0 to £1500 (Figure 18). Values for the same 97 participants prior to the trial were £475 (£400/£346 with a range from £0 to £1500). On average, experience of using the water softeners lowered their mean WTP by approximately £100. Overall, 26% gave higher WTP values at the end of the trial, 29% gave the same value and 44% gave a lower value.
FIGURE 18.
Demand curves for water softeners before and after participation in the trial.
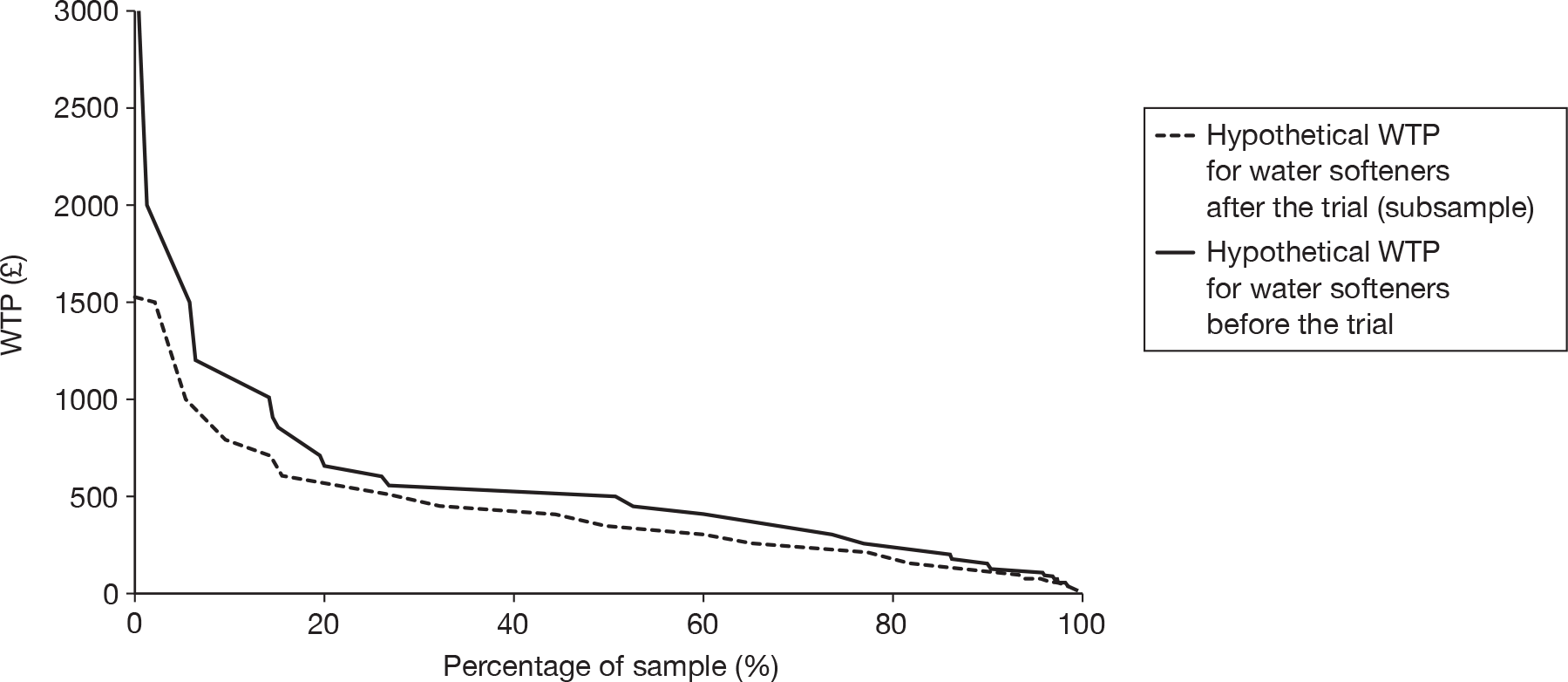
The reasons given for WTP values before and after the trial are summarised in Table 21 (respondents could give multiple responses).
| Reason given | WTP reason before the triala n (% of 336) | WTP reason after the triala n (% of 99) | |
|---|---|---|---|
| This is a reasonable or fair amount for me to pay | 136 (40) | 41 (41) | |
| This is just a guess | 109 (32) | 26 (26) | |
| This amount reflects the benefits I think my child with eczema might get from the water softener | 160 (48) | 43 (43) | |
| This amount reflects the wider benefits of installing a water softener in my home | 87 (26) | 24 (24) | |
| This is how much I think a water softener would cost | 84 (25) | 24 (24) | |
| This is how much I can afford to pay | 118 (35) | 56 (57) | |
| Other reasons | |||
| Pay more if proven to be effective but given unsure this is maximum WTP/wouldn’t buy it for other benefits | 24 | No benefits experienced/not sure it is worth this amount | 2 |
| Based WTP value on family research into price of unit | 6 | Received benefits/but cannot afford it | 2 |
| If money no object would pay more/an amount that would not cause hardship to family | 4 | Undecided about whether there is a benefit but still useful to install | 1 |
| Considered running and service costs | 2 | Amount considered paying before trial | 1 |
| All I am prepared to pay | 1 | If helped eczema be priceless | 1 |
| Already decided to buy one at the end of the trial | 1 | Not popular with the rest of the family | 1 |
| Child’s problems not so bad | 1 | Not had long enough to experience it | 1 |
| Total | 39 | Need to compare bills over same period | 1 |
| The amount I am willing to pay to see if there is any benefit | 1 | ||
| Total | 11 | ||
Hypothetical versus real willingness to pay for an ion-exchange water softener
Table 22 shows the number (percentage) of participants who stated a hypothetical WTP value that was either above or below the actual discounted price they were offered at the end of the study (rows), and whether they actually chose to purchase the ion-exchange water softener (either a study device or private device – columns). Although the percentage who indicated a hypothetical WTP above the actual asking price and bought the device and those giving a WTP below the actual asking price and not buying the device account for over 50% of the relevant participants, clearly a large number changed their preferences during the trial, some changing in favour of buying the device and others choosing not to purchase, despite initially indicating that they thought the device might be worthwhile. The two groups account for a similar percentage of respondents.
| Willingness to pay | Purchased water softener (%) | Did not purchase water softener | Total |
|---|---|---|---|
| WTP > purchase price | 106 (59.2) | 69 (43.9) | 175 (52.1) |
| WTP < purchase price | 73 (40.8) | 88 (56.1) | 161 (47.9) |
| Total | 179 (53.3) | 157 (46.7) | 336 (100) |
| Sensitivity 59% | Specificity 56% |
To try to understand what influenced the decision to purchase or not, a binary logistic regression analysis was conducted. The results are shown in Appendix 15. Only the number of medications at baseline, water hardness at baseline and household income were significant determinants. An increase in the first two explanatory factors made it more likely that an ion-exchange water softener would be purchased; however, a household income of < £30,000 per annum made it less likely that an ion-exchange water softener would be purchased.
Discussion and conclusion
Main findings
The mean costs were consistently higher for the water softener group than for those receiving normal care as a result of the intervention costs. All other costs were similar between the groups and sensitivity analysis supported these findings. It is clear that on the basis of this trial, there is no evidence to suggest that ion-exchange water softeners provide a cost-effective treatment option for the treatment of children with eczema.
The results of the cost–utility analysis supported the cost-effectiveness analysis. Despite a slight (non-significant) improvement in the EQ-5D scores for those receiving the water softener, the cost–utility analysis estimated the use of water softeners to have an incremental cost per QALY of £28,018 in the base case. Sensitivity analyses revealed that this could range from £4548 per QALY (best case) to £53,957 per QALY (worst case).
For interventions with a cost per QALY > £20,000, the latest available National Institute for Health and Clinical Excellence (NICE) methods manual43 states that decisions about whether an intervention represents a good use of NHS resources will depend on a number of factors including the uncertainty surrounding the incremental cost-effectiveness ratio and whether the assessment of health-related quality of life was adequate or unlikely to have captured benefits of an innovative technology. Although, clearly, ion-exchange water softeners are a potentially innovative technology in the health-care context and this study used NICE’s preferred measure of health-related quality of life (the EQ-5D, p. 38), this study found a large degree of uncertainty around estimates of the incremental cost per QALY, suggesting that this technology is unlikely to be viewed as offering the NHS value for money in treating the condition and age range considered.
Evidence from the contingent valuation study suggested that experience of using a water softener during the trial reduced the average WTP by approximately £100. This is possibly to be expected, given that people who believed in the value of water softeners would have been more likely to take part in the trial, and belief in the benefits of the water softeners was a significant factor in determining how much participants felt that they would be willing to pay prior to experiencing the intervention. However, the fact that many families opted to purchase the units despite little improvement in the eczema suggests that other factors were also important.
Strengths and weaknesses
This is the first economic evaluation of ion-exchange water softeners in the context of eczema care. The strengths of this economic evaluation are that it has been conducted alongside a RCT, which enabled comprehensive prospective data collection and suffered very few missing data. However, in line with all economic evaluations, it has had to employ some assumptions as detailed in Chapter 2. The uncertainty created by the most important of these was tested in sensitivity analyses, testing the extremes, but we did not include a probabilistic sensitivity analysis approach. It is a limitation of current health-related quality of life instruments as used in economic evaluations that they have not been developed or validated for very young children such that there is currently no best practice approach to valuing child health within a cost–utility framework. Although a child version of the EQ-5D has been tested in a survey of UK children aged 7–17 years,31 which is completed by parental proxies,44,45 and more recently, a revised youth version has been tested internationally,46,47 there are still a number of methodological issues in this area of valuation, including questions about the relevance of included health dimensions and who, and from what perspective, the appropriate respondents are to attach a value to children’s health states. 48,49 Consequently, we did not measure the health-related quality of life of those children aged < 3 years and these children were excluded from the cost–utility analysis. For those children aged > 3 years who had either a parental proxy or self-completed health-related quality of life score, a QALY was estimated for the trial period based on the utility weights of the York A1 tariff,32 which were derived from non-institutionalised adults. Whether this is deemed acceptable depends on whether or not one believes voters’/taxpayers’ perspectives are legitimate even when these perspective were elicited without specific reference to children’s health status. Clearly, future research and consensus in this research area is needed. Given this context, the results of the cost–utility analyses reported above should be read with some caution.
Conclusion
Ion-exchange water softeners are unlikely to be a cost-effective intervention for children with eczema from an NHS perspective because of the lack of objective evidence of benefit and because they incur a cost. We find no basis for the NHS and other government bodies to consider funding ion-exchange water softeners for childhood eczema as they do not appear to work for this condition. The contingent valuation study taking a family perspective suggests that ion-exchange water softeners may be perceived as cost beneficial to certain individual families provided the cost of the device is at the lower end of the market price range.
Chapter 6 Discussion
Main findings
The primary outcome
This study is the only RCT to date assessing whether the installation of an ion-exchange water softener can help relieve the symptoms of eczema in children with moderate-to-severe eczema. The main findings based on a blinded evaluation of the primary end point of mean change in eczema severity at 12 weeks compared with baseline (as measured using the SASSAD) showed that installing water softeners was no better than usual care in relieving the symptoms of eczema. Furthermore, there was a narrow CI around that difference, excluding small but important clinical differences.
One possible reason for the discrepancy between our null trial findings and those of previous observational studies that found that increased prevalence of eczema in children living in hard water areas is that children in the observational studies ingested the water. In other words, it is possible that ingestion of hard water or a component to water that is related to water hardness actually induces skin inflammation directly or indirectly through inflammatory gene interactions, although we are not aware of any such mechanisms in the literature to date.
It is also possible that eczema represents a heterogeneous group of distinct genetic conditions, some of which will respond to water softeners and some of which will not. Previous research has suggested an association between the presence of eczema/dry skin and mutations of the filaggrin gene. A subgroup analysis based on the presence or absence of mutations of the filaggrin gene found no evidence that the treatment effect varied between those with and without the mutation.
We wish to stress that those who participated in the study were not formally tested for atopy by means of circulating specific IgE antibodies. They did, however, represent the sort of patients with eczema typically seen in clinical hospital practice. It is possible that a subgroup analysis of atopic versus non-atopic individuals might have shown that one group benefits while the other does not. Based on our previous observation that IgE responsiveness adds little information to our predictive ability about eczema,50 added to our desire to minimise the number of subgroup analyses to avoid inappropriate post hoc findings, we chose not to measure atopic status in this pragmatic trial.
Other outcomes
In addition to our primary outcome measure, which was assessed blindly, we had a number of secondary outcomes, some of which were assessed blindly (proportion showing reasonable, good or excellent improvement, night-time movement, medication used) and some of which were assessed by parents who were not blinded (POEM, TCWs and WCWs, DFI, and EQ-5D).
For all of the blinded outcomes, there was no difference between the treatment groups. Of the unblinded secondary outcomes, all but one showed small, but statistically significant, differences in favour of the water softener group. However, the magnitude of improvement seen in these outcomes was small and unlikely to be clinically meaningful. It is also possible that our emphasis on objective outcomes meant that some important potential benefits were not captured in the primary analysis. Other factors, such as improvements in quality of life or a reduction in symptoms (e.g. dry skin), may be important drivers in determining whether or not parents perceived a benefit.
Strengths and weaknesses
The main strengths of our study was its internal validity (concealment randomisation, blinding of research nurses and investigators to allocation, minimal data attrition) and the use of an objective validated primary outcome measure. Additionally, the study recruited the required number of participants according to the initial power calculation.
The 95% CIs for the primary outcome were extremely narrow, making it unlikely that a clinically significant benefit had been excluded by chance. Indeed, performing a per-protocol analysis based on those with maximum exposure to the water softener and excluding those who had changed their usual eczema treatments during the trial did not change the overall interpretation of these results.
Although the unblinded secondary outcomes (with the exception of EQ-5D) showed small statistically significant differences in favour of the water softener group, it is most likely that these were due to observer bias.
It is also possible that treatment effects were masked by the usual eczema care that the children received. We do not consider this to be the case and, in fact, we noted that total amount of medications used was lower than expected in this patient grouping.
A potential weakness could be that the relatively short duration of the trial was insufficient to capture any treatment effect. Anecdotal reports from patients with eczema suggested that any benefit from moving to a soft water area or installing a water softener would be apparent within a few days or within 2 weeks. This led us to anticipate that, if a treatment response existed, it was likely to occur more quickly than 12 weeks. It is still possible that water softeners could have a slowly evolving and subtle benefit that would be apparent only after 1 year or more. However, both treatment groups improved in disease severity during the trial, and there was no hint that the intervention group was starting to show more improvement than the control group towards the end of the 12-week period.
The continued use of soap and soap products during the trial may have limited the observed benefits if families were using too much soap in conjunction with the water softener. However, this was a pragmatic study that aimed to capture the effects of water softeners according to standard advice. Evidence of how much soap was actually used was not collected, as we did not want to change participants’ behaviour by intensive monitoring.
Generalisability
This study has good external validity as it was designed as a pragmatic study. Participants were recruited from eight UK centres across the primary and secondary care setting, and included families of diverse socioeconomic backgrounds. Every effort was made to include participants who lived in rented accommodation as well as home owners, and participants were able to continue with their usual eczema care. Nevertheless, the results are applicable only to children with moderate-to-severe eczema. The baseline characteristics of the sample are typical of children seen in clinics with moderate-to-severe eczema. We are not able to comment on the impact of other types of water-softening devices [e.g. physical water devices; or the impact of softened water in adults and other skin conditions (including dry skin)].
Implication for health care
The results of this study are clear, and do not support the use of ion-exchange water softeners for the treatment of eczema in children. Whether or not the wider benefits of installing a water softener in the home are sufficient to justify the purchase of a softener is something for individual householders to consider on a case-by-case basis.
Implication for future research
High Priority
Assessment of other non-pharmacological therapies
This trial demonstrated overwhelming demand for non-pharmacological interventions for the treatment of eczema, and this is something that should be considered when prioritising future research in the field, especially in relation to emerging genetic subtypes. Specific non-pharmacological interventions that could be tested include specialised clothing such as silk garments51 or antibacterial garments impregnated with silver,52 or the effects of occlusive bandaging in controlling eczema flares. 53 The evaluation of educational interventions for eczema, such as nurse education, is also ripe for evaluation in a UK setting. 54
Core outcome measures
The profusion of poorly developed and validated outcome measures is also cause for concern and eczema research would benefit from harmonisation of a set of core outcomes for all clinical trials, as has been achieved by the Outcome Measures in Rheumatology (OMERACT) initiative and currently taken forward by the Core Outcome Measures in Trials (COMET) initiative. Some early work on prioritising key domains has started. 55
Medium priority
Flare factors
While this trial has shown no benefit of water softeners for the treatment of eczema, there is still a need to explore and understand the effects of the environment on the incidence and prevalence of eczema, especially a more scientific understanding of what environmental factors may be associated with flares in people with established eczema. Some research has already pointed to the possibility that multiple exposures are acting together in a complex way before a flare occurs. 56,57
Objective outcome measures
Further work is also required in the development of objective outcome measures for the assessment of eczema. The SWET trial neatly demonstrated the importance of using objective outcome measures in trials in which it is not possible to blind participants to treatment allocation. While the Actiwatches™ used in this trial showed great promise in this regard, it is concerning that data relating to the proportion of the night spent moving were poorly correlated with all of the other outcome measures. Further work is clearly required in order to understand why this might have been the case.
Low priority
Water softeners/conditioners
While we do not recommend a repeat of this study in the UK, it is always useful to see if the results can be replicated in other studies performed by different teams in other countries. Our research study evaluated only ion-exchange water softeners because they soften the water so dramatically. It is possible that other types of domestic water devices called ‘physical water conditioners’, which reduce limescale build-up by altering the physical properties of calcium and magnesium ions, could have an effect on the skin which could be explored in preliminary laboratory experiments that evaluate their effect on skin barrier parameters such as transepidermal water loss. 15 We cannot recommend either of these suggestions as priorities.
Acknowledgements
The trial would not have been possible without the valued contribution of the families who volunteered to take part.
We would like to thank our dermatology and nursing colleagues who referred patients into the trial. We are grateful for support from the following individuals and organisations: Sue Davies-Jones, Jane Grundy, Rhiannon Medhurst and Rosalind Simmonds, who were responsible for recruitment, data collection and data entry at the six main SWET study centres; David Paige and Nerys Roberts (chairperson and vice-chairperson of the SWET Steering Committee); MRC Clinical Trials Unit (Mark Sculpher provided general advice and supervision to members of the Trial Team); Nottingham Clinical Trials Unit (Daniel Simpson provided data management and IT support); David Potter, Rebecca Baker, Neroli and Chris Williams, Sally Brooks and Deena Smart contributed to the SWET Service User Panel and commented on study design and participant information sheets; Emma Veysey (Consultant Dermatologist, Singleton Hospital, Swansea) assessed digital images of target lesions; Gill Glasbey (David Hyde Asthma and Allergy Research Centre, Isle of Wight) provided additional administrative support; Mansoor Dilnawaz helped with recruitment at United Lincolnshire Hospitals Trust; research nurses who contributed to recruitment, data collection and data entry (Alison Allen, Jane Devonshire, Denise McClure and Amanda Roper); the National Eczema Society (Margaret Cox and John Fuller), the Nottingham Eczema Support Group and Lindsay Brooke (University of Nottingham) provided assistance in advertising the trial; Kinetico UK Ltd (Grant Audemard supported the original feasibility study and assisted with trial design and John Bissett and John Kyle co-ordinated the engineering aspects of the main trial); Lorraine Doran (European Water Care Ltd) co-ordinated engineering aspects in the initial stages of the trial; Robin Stevens (MG Heating) co-ordinated engineering aspects on the Isle of Wight; members of the UKWTA provided technical advice and contributed to the cost of the water softeners and salt supplies; Peter Marsh (Culligan UK) co-ordinated hardness testing of water samples; Linda Campbell (University of Dundee) conducted gene sequencing to identify filaggrin status; Mike Purday (CamNTech) provided training in the use of Actiwatches™; engineers from the following companies installed water softeners: Aquastream, Capital Softeners, Clearwater Softeners, European Water Care, Greens Water Systems, Kinetico UK, MG Heating Ltd; Silkstream and Simply Soft Water Softeners.
Contributions of authors
Kim Thomas (Associate Professor of Dermatology, non-clinical) contributed to the conception and design of the study, trial management and oversight (as Lead Investigator) interpretation of data and writing of the report.
Karin Koller (Trial Manager) contributed to trial management and oversight, data collection, analysis and interpretation of data and writing of the report.
Tara Dean (Professor of Health Sciences) contributed to the conception and design of the study, recruitment to Isle of Wight and Portsmouth study centres (as Principal Investigator), trial management and oversight, interpretation of data and writing of the report.
Caroline O’Leary (Statistician) carried out all statistical analyses and contributed to analysis and interpretation of data and writing of the report.
Tracey Sach (Senior Lecturer in Health Economics) contributed to the conception and design of the study, carried out all health economics analyses and contributed to analysis and interpretation of data and writing of the report.
Anthony Frost (Director of Aqua Focus Ltd; UKTWA representative on TMG) contributed to the conception and design of the study, trial management and oversight, and writing of the report.
Ian Pallett (British Water Technical Consultant) contributed to the conception and design of the study, trial oversight and writing of the report.
Angela M Crook (Senior Statistician) contributed to the conception and design of the study; trial management and oversight; interpretation of data and writing of the report.
Sarah Meredith (Senior Epidemiologist) contributed to the conception and design of the study, trial management and oversight, interpretation of data and writing of the report.
Andrew Nunn (Professor of Epidemiology) contributed to the conception and design of the study, trial management and oversight, interpretation of data and writing of the report.
Nigel Burrows (Consultant Dermatologist) contributed to the conception and design of the study; trial management and oversight (as Principal Investigator for Cambridge study centre); interpretation of data and writing of the report.
Ian Pollock (Consultant Paediatrician) contributed to the conception and design of the study; trial management and oversight (as Principal Investigator for North London study centre); interpretation of data and writing of the report.
Robin Graham-Brown (Consultant Dermatologist) contributed to recruitment at SWET Leicester study centre (as Principal Investigator), trial management and oversight, analysis and interpretation of data and writing of the report.
Edel O’Toole (Consultant Dermatologist) contributed to recruitment at London study centre (as Principal Investigator), trial management and oversight, interpretation of data and writing of the report.
David Potter (Retired Biochemist) contributed to the conception and design of the study; trial management and oversight and interpretation of data, and writing of the report.
Hywel Williams (Professor of Dermato-epidemiology) contributed to the conception and design of the study, trial management and oversight (as Chief Investigator and Principal Investigator of Nottingham study centre), interpretation of data and writing of the report.
Publications
-
Thomas KS, Sach TH. A multicentre randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children: protocol for the Softened Water Eczema Trial. Br J Dermatol 2008;159:561–6.
-
Devonshire J, Koller K, Davies-Jones S, Grundy J, Medhirst R, Simmonds R, et al. A trial into whether water softeners can help eczema. Dermatol Nurs 2008;7:50–1.
-
Thomas KS, Dean T, O’Leary C, Sach TH, Koller K, Frost A, et al. A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLoS Med 2011;8(2):e1000395.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. Is eczema really on the increase worldwide?. J Allergy Clin Immunol 2008;121:947-54e15.
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000;4.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics 2003;21:105-13.
- O’Connell EJ. The burden of atopy and asthma in children. Allergy 2004;78:7-11.
- Simpson CR, Newton J, Hippisley-Cox J, Sheikh A. Trends in the epidemiology and prescribing of medication for eczema in England. J R Soc Med 2009;102:108-17.
- Herd RM, Tidman MJ, Prescott RJ, Hunter JA. The cost of atopic eczema. Br J Dermatol 1996;135:20-3.
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol 2008;25:1-6.
- Su JC, Kemp AS, Varigos GA, Nolan TM. Atopic eczema: its impact on the family and financial cost. Arch Dis Child 1997;76:159-62.
- National Institute for Health and Clinical Excellence (NICE) . NICE Clinical Guideline 57: Management of Atopic Eczema in Children from Birth up to the Age of 12 Years 2007.
- Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol 2000;142:931-6.
- McNally NJ WH, Phillips DR, Smallman-Raynor M, Lewis S, Venn A, . Atopic eczema and domestic water hardness. Lancet 1998;352:527-31.
- Miyake Y, Yokoyama T, Yura A, Iki M, Shimizu T. Ecological association of water hardness with prevalence of childhood atopic dermatitis in a Japanese urban area. Environ Res 2004;94:33-7.
- Arnedo-Pena A, Bellido-Blasco J, Puig-Barbera J, Artero-Civera A, Campos-Cruanes JB, Pac-Sa MR, et al. [Domestic water hardness and prevalence of atopic eczema in Castellon (Spain) school children]. Salud Publica Mex 2007;49:295-301.
- Tanaka A, Takai M, Yoshinari Y, Matsuda H. Ultrapure soft water reduces growth of Staphylococcus aureus adopted on skins of the barrier-disrupted animal model. Allergy 2009;64.
- Tanaka A, Takai M, Yoshinari Y, Matsuda H. Rinsing Soap in Ultra-Pure Soft Water Improves Clinical Skin Conditions in Patients With Atopic Dermatitis n.d.
- van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ 2009;339.
- Williams H, Burney P, Hay R, Archer CB, Shipley MJ, Hunter JJ. The UK working party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994;131:383-96.
- Water Regulations Advisory Scheme (WRAS) . Information and Guidance Note n.d. www.wras.co.uk/wiaps/ (accessed January 2011).
- British Water . Code of Practice for the Installation of Ion Exchange Water Softeners n.d. www.britishwater.co.uk/publications/Publications_and_Technical_Guides.aspx (accessed January 2011).
- Wood L, Egger M, Gluud L, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601-5.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol 1996;135:25-30.
- Wolkerstorfer A, de Waard van der Spek FB, Glazenburg EJ, Mulder PG, Oranje AP. Scoring the severity of atopic dermatitis: three item severity score as a rough system for daily practice and as a pre-screening tool for studies. Acta Derm Venereol 1999;79:356-9.
- Charman CR, Venn AJ, Williams H. Measuring atopic eczema severity visually: which variables are most important to patients?. Arch Dermatol 2005;141:1146-51.
- Benjamin K, Waterston K, Russell M, Schofield O, Diffey B, Rees JL. The development of an objective method for measuring scratch in children with atopic dermatitis suitable for clinical use. J Am Acad Dermatol 2004;50:33-40.
- Bringhurst C, Waterston K, Schofield O, Benjamin K, Rees JL, . Measurement of itch using actigraphy in pediatric and adult populations. J Am Acad Dermatol 2004;51:893-8.
- Hon KL, Lam MC, Leung TF, Kam WY, Lee KC, Li MC, et al. Nocturnal wrist movements are correlated with objective clinical scores and plasma chemokine levels in children with atopic dermatitis. Br J Dermatol 2006;154:629-35.
- Charman C, Venn A, Williams H. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-19.
- Langan SM, Thomas KS, Williams HC. What is meant by a ‘flare’ in atopic dermatitis? A systematic review and proposal. Arch Dermatol 2006;142:1190-6.
- Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the dermatitis family impact questionnaire. Br J Dermatol 1998;138:107-13.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol 2001;144:104-10.
- Hennessy S, Kind P. Measuring Health Status in Children: Developing and Testing a Child-Friendly Version of EQ-5D n.d. www.euroqol.org (accessed August 2006).
- Dolan P. Modelling valuations for EuroQol health states. Med Care 1997;35:1095-108.
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006;38:441-6.
- Smith FJD, Irvine AD, Terron-Kwiatkowski A, Sandiland A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet 2006;38:337-42.
- Emerson R, Williams H, Allen B. What is the cost of atopic dermatitis in preschool children?. Br J Dermatol 2001;143:514-22.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 2006;60:984-92.
- Drummond M, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Department of Health . NHS Reference Costs 2008 9 n.d. www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_111607.xls#Start8 (accessed February 2010).
- Curtis L. Unit Costs of health and social care 2009. Personal Social Services Research Unit (PSSRU); n.d.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2009.
- Barton G, Briggs A, Fenwick E. Optimal cost-effective decisions: the role of the cost-effectiveness acceptability curve (CEAC), cost-effectiveness acceptability frontier (CEAF) and expected value of perfect information (EVPI). Value Health 2008;11:886-97.
- Fenwick E, Claxton K, Sculpher MJ. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal n.d. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdateJune2008.pdf (accessed January 2011).
- Badia X, Roset M, Peruler N. Validation of the EQ-5D to Be Used in Children With Persistent Asthma n.d.
- Matza L, Secnik K, Mannix S, Sallee F. Parent-proxy EQ-5D ratings of children with attention-defecit hyperactivity disorder in the US and the UK. Pharmacoeconomics 2005;23:777-90.
- Ravens-Sieberer U, Wille N, Badia X, Bonsel G, Burstrom K, Cavrini G, et al. Feasibility, reliability, and validity of the EQ-5D-Y: results from a multinational study. Qual Life Res 2010;19:887-97.
- Wille N, Badia X, Bonsel G, Burstrom K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res 2010;19:875-86.
- Griebsch I, Coast J, Brown J. Quality-adjusted life-year lack of quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics 2005;115:600-14.
- Petrou S. Methodological issues raised by prefernce-based approaches to measuring the health status of children. Health Econ 2003;12:697-702.
- Flohr C, Johansson S, Wahlgren C, Williams H. How atopic is atopic dermatitis?. J Allergy Clin Immunol 2004;114:150-8.
- Vlachou C, Thomas KS, Williams HC. A case report and critical appraisal of the literature on the use of DermaSilk in children with atopic dermatitis. Clin Exp Dermatol 2009;34:e901-3.
- Bath-Hextall F, Birnie AJ, Ravenscroft JC, Williams H. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dematol 2010;163:12-26.
- Braham S, Pugashett R, Koo J, Mailbach H. Occlusive therapy in atopic dermatitis: overview. J Dermatolog Treat 2010;21:62-7.
- Williams H. Nurse practitioners for management of childhood eczema. Br J Dermatol 2010;162:4-5.
- Schmitt J, Langan SM, Stamm T, Williams H. on behalf of the harmonizing outcome measurements in eczema (HOME) Delphi panel. Core outcome domains for controlled trials and clinical recordkeeping in eczema: International multi-perspective Delphi consensus process. J Invest Dermatol 2010.
- Langan SM, Thomas KS, Williams HC. What is meant by a ‘Flare’ in atopic dermatitis?: a systematic review and proposal. Arch Dermatol 2006;142:1190-6.
- Langan SM, Silcocks P, Williams H. What causes flares of eczema in children?. Br J Dermatol 2009;161:640-6.
Appendix 1 Information sources search strategies
Literature search strategy for RCTs:
The following databases were searched:
-
The Cochrane Skin Group Specialised Trials Register (up to 13 January 2010)
-
The Cochrane Library 2009, issue 4
-
MEDLINE (2000 to end of 2009)
-
EMBASE (2000 to end of 2009)
-
CHINAL (inception to end of 2009)
-
AHMED (inception to end of 2009).
Details of MEDLINE search
-
randomised controlled trial.pt.
-
controlled clinical trial.pt.
-
randomised.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
6 or 3 or 7 or 2 or 8 or 1 or 4 or 5
-
(animals not (human and animals)).sh.
-
9 not 10
-
Exp Dermatitis, Atopic/
-
atopic dermatitis.mp.
-
atopic eczema.mp.
-
exp NEURODERMATITIS/
-
neurodermatitis.mp.
-
infantile eczema.mp.
-
childhood eczema.mp.
-
Besniers’ Prurigo.mp.
-
Exp Eczema/or eczema.mp.
-
17 or 12 or 20 or 15 or 14 or 18 or 13 or 16 or 19
-
11 and 21
-
Limit 22 to yr = ‘2000 to 2009’
Appendix 2 Pre-recruitment screening
Telephone screen checklist carried out by research nurse
Home screening carried out by water engineer
Appendix 3 Outcome measures
Six Area, Six Sign Atopic Dermatitis score/Three-Item Severity score
Patient-Orientated Eczema Measure
Dermatitis Family Impact
European Quality of Life-5 Dimensions
Willingness-to-pay questionnaire
Appendix 4 Protocol and statistical analysis plan
Appendix 5 Committee membership
SWET Trial Management Group
Professor Hywel Williams (Chief Investigator), Professor of Dermato-epidemiology, Centre of Evidenced Based Dermatology, Nottingham University Hospitals NHS Trust, Queen’s Medical Centre, C Floor, South Block, Nottingham NG7 2UH, UK.
Dr Kim Thomas (Lead Applicant), Associate Professor in Dermatology, Centre of Evidenced Based Dermatology, University of Nottingham, King’s Meadow Campus, Lenton lane, Nottingham NG7 2NR, UK.
Dr Sarah Meredith, Senior Clinical Epidemiologist, MRC Clinical Trials Unit, 222 Euston Road, London NW1 2DA, UK.
Professor Andrew Nunn, Associate Director, MRC Clinical Trials Unit, 222 Euston Road, London NW1 2DA, UK.
Dr Angela Crook, Senior Statistician, MRC Clinical Trials Unit, 222 Euston Road, London NW1 2DA, UK.
Ms Caroline O’Leary, Statistician, MRC Clinical Trials Unit, 222 Euston Road, London NW1 2DA, UK.
Dr Ian Pollock (Principle Investigator), Consultant Paediatrician, Barnet & Chase Farm Hospital, Chase Farm Hospital, The Ridgeway, Enfield, Middlesex EN2 8JL, UK.
Dr Nigel Burrows (Principal Investigator), Consultant Dermatologist, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Hills Road, Cambridge CB2 0QQ, UK.
Professor Tara Dean (Principal Investigator), Reader in Epidemiology/Deputy Director of Asthma & Allergy Research Centre, St Mary’s Hospital, Parkhurst Road, Newport, Isle of Wight PO30 5TG, UK.
Dr Robin Graham-Brown (Principal Investigator), Consultant Dermatologist/Hon Senior Lecturer, University Hospitals of Leicester NHS Trust, Gwendolen House, Gwendolen Road, Leicester LE5 4QF, UK.
Dr Mansoor Dilnawaz (Principal Investigator), Consultant Dermatologist, Pilgrim Hospital, Boston United Lincolnshire Hospitals NHS Trust, Boston PE21 9QS.
Dr Edel O’Toole (Principal Investigator), Consultant Dermatologist, The Royal London Hospital, Whitechapel, London E1 1BB, UK.
Dr Tracey Sach, Senior Lecturer in Health Economics, University of East Anglia, Earlham Road, Norwich NR4 7TJ, UK.
Mr Grant Audemard (Water Softener Industry Rep), Kinetico UK Ltd, Bridge House, Park Gate Business Centre, Chandler’s Way, Park Gate, Hampshire SO31 1FQ, UK.
Mr Tony Frost (Water Softener Industry Rep), Aqua Focus Ltd, PO Box 47, Newport, Shropshire TF10 9WB, UK.
Dr Karin Koller (Trial Manager), Centre of Evidenced Based Dermatology, University of Nottingham, King’s meadow Campus, Lenton Lane, Nottingham NG7 2NR, UK.
Ms Jane Grundy (Research Nurse), St Mary’s Hospital, Parkhurst Road, Newport, Isle of Wight PO30 5TG, UK.
Ms Rhiannon Medhurst (Research Nurse), Barnet & Chase Farm Hospital, Chase Farm Hospital, The Ridgeway, Enfield, Middlesex EN2 8JL, UK.
Ms Rosalind Simmonds (Research Nurse), Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Hills Road, Cambridge CB2 0QQ, UK.
Ms Susan Davies-Jones (Research Nurse), Nottingham University Hospitals NHS Trust, Queen’s Medical Centre, C Floor, South Block, Nottingham NG7 2UH, UK.
Ms Amanda Roper (MCRN-funded Research Nurse), United Lincolnshire Hospital NHS Trust.
Ms Alison Allen (MCRN-funded Research Nurse), Barnet & Chase Farm Hospital, Chase Farm Hospital, The Ridgeway, Enfield, Middlesex EN2 8JL, UK.
Mr John Kyle (Engineering main contact, Mainland), Kinetico UK Ltd, Bridge House, Park Gate Business Centre, Chandler’s Way, Park Gate, Hampshire SO31 1FQ, UK.
Mr John Bisset (Engineering Services Manager), Kinetico UK Ltd, Bridge House, Park Gate Business Centre, Chandler’s Way, Park Gate, Hampshire SO31 1FQ, UK.
Mr Robin Stevens (Engineering main contact, Isle of Wight), MG Heating, 16 Little London, Newport, Isle of Wight PO30 5BS, UK.
SWET Trial Steering Committee (TSC)
Dr David Paige (TSC Independent Chair), Consultant Dermatologist, The Royal London Hospital, Whitechapel, London E1 1BB, UK.
Professor Hywel Williams (Chief Investigator), Professor of Dermato-epidemiology, Centre of Evidenced Based Dermatology, Nottingham University Hospitals NHS Trust, Queen’s Medical Centre, C Floor, South Block, Nottingham NG7 2UH, UK.
Dr Ian Pollock (Principle Investigator), Consultant Paediatrician, Barnet & Chase Farm Hospital, Chase Farm Hospital, The Ridgeway, Enfield, Middlesex EN2 8JL, UK.
Mr David Potter (Consumer representative), Research Biochemist (retired).
Professor Andrew Nunn (Associate Director), MRC Clinical Trials Unit, 222 Euston Road, London NW1 2DA, UK.
Dr Nerys Roberts (TSC Independent Deputy Chair), Consultant Dermatologist, Chelsea & Westminster Hospital, 369 Fulham Road, London SW10 9NH, UK.
Dr Ian Pallet (Technical Director), British Water, 1 Queen Anne’s Gate, London SW1H 9BT, UK.
Dr Karin Koller (Trial Manager), Centre of Evidenced Based Dermatology, University of Nottingham, King’s meadow Campus, Lenton Lane, Nottingham NG7 2NR, UK.
Appendix 6 Home screening outcomes
Home screen pass rate
Homes on the mainland were initially assessed by water engineers employed through European Water Care Ltd, but in October 2007 this company withdrew from their contract with the trial following concern about the lower than expected number of homes found suitable for installation of an ion-exchange water softener (61% of the 150 mainland homes screened from May to October 2007) and issues over quality of workmanship. The original budget was based on a home screening pass rate and subsequent installation of units in 85% of homes. From November 2007, co-ordination of water engineers on the mainland was undertaken by John Kyle at Kinetico UK Ltd and home screens (and installation of water softeners) were carried out by a number of local independent water engineering subcontractors for the remainder of the trial. There was a noticeable improvement in the rate of homes deemed suitable (79% of the 150 mainland homes screened from November 2007 to July 2008). The final per cent of all screened homes deemed suitable for straightforward installation of an ion-exchange water softener was 72%.
| Centre/area | Passed/total screened | Pass rate (%) |
|---|---|---|
| London (east)a | 4/14 | 29 |
| Nottinghamshire | 39/63 | 62 |
| Lincolnshire | 11/77 | 65 |
| London (north/north-east) | 98/144 | 68 |
| Isle of Wight | 58/85 | 68 |
| Cambridgeshire | 125/162 | 77 |
| Hampshire (Portsmouth) | 49/61 | 80 |
| Leicestershire | 66/77 | 86 |
Appendix 7 Further baseline characteristics
| Baseline characteristics | Group A (water softener + usual care) | Group B (usual care) | Total |
|---|---|---|---|
| Number randomised | 170 | 166 | 336 |
| Age, N (%) | |||
| Mean age (SD) | 5.8 (4.2) | 5.0 (4.0) | 5.4 (4.1) |
| < 3 years old | 46 (27) | 52 (31) | 98 (29) |
| 3–6 years old | 56 (33) | 67 (40) | 123 (37) |
| ≥ 7 years old | 68 (40) | 47 (28) | 115 (34) |
| Sex, N (%) | |||
| Male | 95 (56) | 98 (59) | 193 (57) |
| Female | 75 (44) | 68 (41) | 143 (43) |
| Ethnicity, N (%) | |||
| White | 133 (78) | 127 (77) | 260 (77) |
| Asian | 16 (9) | 17 (10) | 33 (10) |
| Black | 6 (4) | 4 (2) | 10 (3) |
| Mixed | 10 (6) | 9 (5) | 19 (6) |
| Other | 4 (2) | 8 (5) | 12 (4) |
| Not stated/unknown | 1 (1) | 1 (1) | 2 (1) |
| Baseline characteristics | Group A (water softener + usual care) | Group B (usual care) | Total |
|---|---|---|---|
| Number randomised | 170 | 166 | 336 |
| Previous treatment history, N (%) | |||
| High | 97 (57) | 81 (49) | 178 (53) |
| Low | 59 (35) | 74 (45) | 133 (40) |
| None | 14 (8) | 11 (7) | 25 (7) |
| Filaggrin status, N (%) | |||
| Presence of mutation | 47 (28) | 47 (28) | 94 (28) |
| Absence of mutation | 108 (64) | 110 (66) | 218 (65) |
| Unknown | 15 (9) | 9 (5) | 24 (7) |
| Food allergy, N (%)a | |||
| No | 105 (63) | 103 (64) | 208 (63) |
| Yes | 61 (37) | 59 (36) | 120 (37) |
| Baseline SASSAD, N (%)b | |||
| Mean (SD) | 25.3 (13.4) | 26.0 (13.9) | 25.6 (13.6) |
| 10–19 | 75 (44) | 68 (41) | 143 (43) |
| > 20 | 94 (56) | 98 (59) | 192 (57) |
| Baseline characteristics | Group A (water softener + usual care) | Group B (usual care) | Total |
|---|---|---|---|
| Number randomised | 170 | 166 | 336 |
| Income, N (%)a | |||
| < £10,000 | 7 (4) | 9 (5) | 16 (5) |
| £10,000–20,000 | 12 (7) | 20 (12) | 32 (10) |
| £20,000–30000 | 26 (16) | 36 (22) | 62 (19) |
| £30,000–50,000 | 54 (33) | 49 (30) | 103 (31) |
| ≥ £50,000 | 43 (26) | 38 (23) | 81 (25) |
| Do not know | 22 (13) | 12 (7) | 34 (10) |
| Home ownership, N (%)b | |||
| Do not know | 0 (0) | 1 (1) | 1 (< 0.5) |
| Owned by you outright | 16 (9) | 16 (10) | 32 (10) |
| Being brought with the help of a mortgage or loan | 118 (70) | 119 (72) | 237 (71) |
| Part-rented and part-mortgaged (shared ownership) | 4 (2) | 1 (1) | 5 (1) |
| Rented privately | 10 (6) | 12 (7) | 22 (7) |
| Rented from the council or a housing association | 19 (11) | 16 (10) | 35 (10) |
| Rent-free (e.g. in a relative or a friend’s property) | 2 (1) | 1 (1) | 3 (1) |
| Baseline characteristics | Group A (water softener + usual care) | Group B (usual care) | Total |
|---|---|---|---|
| Number randomised | 170 | 166 | 336 |
| Bathing frequency at home, times per weeka | |||
| N | 169 | 166 | 335 |
| Mean (SD) | 4.8 (2.4) | 5.0 (2.4) | 4.9 (2.4) |
| Median (IQR) | 4 (3–7) | 4 (3–7) | 4 (3–7) |
| Bathing frequency away from home, times per weekb | |||
| N | 163 | 161 | 324 |
| Mean (SD) | 0.4 (1.0) | 0.4 (1.5) | 0.4 (1.2) |
| Median (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–0) |
| Swimming frequency, N (%)c | |||
| Never | 62 (37) | 67 (40) | 129 (39) |
| Less than once a month | 56 (33) | 52 (31) | 108 (32) |
| More than once a month | 51 (30) | 47 (28) | 98 (29) |
| Baseline characteristics | Group A (water softener + usual care) | Group B (usual care) | Total |
|---|---|---|---|
| Number randomised | 170 | 166 | 336 |
| Water hardness, N (%) | |||
| Mean (SD) | 307.6 (50.2) | 309.5 (58.0) | 308.6 (54.1) |
| Median (IQR) | 306 (274–342) | 300 (270–340) | 305.5 (273.5–340.0) |
| 200–299 mg/l | 72 (42) | 66 (40) | 138 (41) |
| 300–399 mg/l | 90 (53) | 84 (51) | 174 (52) |
| 400–499 mg/l | 8 (5) | 15 (9) | 23 (7) |
| ≥ 500 mg/l | 0 (0) | 1 (1) | 1 (< 0.5) |
| Washing powder, N (%)a | |||
| Biological | 21 (13) | 12 (7) | 33 (10) |
| Non-biological | 145 (87) | 151 (92) | 296 (89) |
| Both | 1 (1) | 2 (1) | 3 (1) |
| Fabric softener, 0 (%)2 | |||
| No | 92 (55) | 84 (51) | 176 (53) |
| Yes | 75 (45) | 82 (49) | 157 (47) |
Appendix 8 Medication sensitivity analyses
In addition to examining the difference in the total amount of medication used, this was also split into low-strength and high-strength medication. Low-strength medication consisted of mild and moderate steroids, and high-strength medication consisted of potent steroids, very potent steroids and all calcineurin inhibitors.
The difference in the amount of low-strength medication used was –11.66 g (95% CI –28.92 to 5.59 g), meaning group A used on average 1 gram (g) less a week of low-strength medications than group B (Table 29). However, this difference was not statistically significant (p = 0.18). The difference in the amount of high-strength medication used over the 12-week period was 2.82 g (95% CI –8.46 to 14.11 g), with group A using slightly more high-strength medication than group B (Table 30).
| Steroid strength | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed | 160 | 154 | |||
| Mild steroids | Mean ± SD | 12.0 ± 29.9 | 18.1 ± 35.5 | ||
| Moderate steroids | Mean ± SD | 19.7 ± 69.3 | 25.3 ± 58.9 | ||
| Total low-strength medication (g) a | Mean ± SD | 31.7 ± 83.5 | 43.4 ± 71.1 | –11.66 (–28.92 to 5.59) | 0.18 |
| Steroid strength | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed | 160 | 153 | |||
| Potent steroids | Mean ± SD | 21.5 ± 41.4 | 18.4 ± 39.7 | ||
| Very potent steroids | Mean ± SD | 2.2 ± 11.7 | 1.8 ± 20.7 | ||
| Mild calcineurin inhibitors | Mean ± SD | 1.9 ± 7.9 | 2.7 ± 12.0 | ||
| Moderate calcineurin inhibitors | Mean ± SD | 1.1 ± 9.1 | 1.0 ± 7.9 | ||
| Total high-strength medication (g) | Mean ± SD | 26.6 ± 45.9 | 23.8 ± 55.3 | 2.82 (–8.46 to 14.11) | 0.62 |
Where medications were not available for weighing at the clinic visits the nurses estimated the amount of medication used. At the end of the 12-week period they recorded the total amount of medication used along with an indication of the accuracy of these figures. The above analyses were repeated using measurements that the nurse was sure or very sure were accurate, but this made no difference to the conclusions (Table 31).
| Steroid strength | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed | 115 | 113 | |||
| Mild steroids | Mean ± SD | 11.7 ± 32.7 | 16.6 ± 33.4 | ||
| Moderate steroids | Mean ± SD | 16.9 ± 53.0 | 21.5 ± 55.5 | ||
| Potent steroids | Mean ± SD | 20.7 ± 43.1 | 16.0 ± 39.0 | ||
| Very potent steroids | Mean ± SD | 1.9 ± 10.6 | 0.1 ± 1.1 | ||
| Mild calcineurin inhibitors | Mean ± SD | 2.1 ± 8.8 | 1.4 ± 5.4 | ||
|
Moderate calcineurin inhibitors |
Mean ± SD | 0.5 ± 3.3 | 0.8 ± 8.3 | ||
| Total medication (g) | Mean ± SD | 53.7 ± 91.9 | 56.5 ± 83.9 | 2.80 (–25.78 to 20.18) | 0.81 |
| Steroid strength | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed | 115 | 113 | |||
| Mild steroids | Mean ± SD | 11.7 ± 32.7 | 16.6 ± 33.4 | ||
| Moderate steroids | Mean ± SD | 16.9 ± 53.0 | 21.5 ± 55.5 | ||
| Total low-strength medication (g) | Mean ± SD | 28.6 ± 76.4 | 38.1 ± 68.3 | –9.50 (–28.41 to 9.42) | 0.32 |
| Steroid strength | Group A (water softener + usual care) | Group B (usual care) | Difference and 95% CI (A–B) | p-value | |
|---|---|---|---|---|---|
| Number randomised | 170 | 166 | |||
| N analysed | 115 | 113 | |||
| Potent steroids | Mean ± SD | 20.7 ± 43.1 | 16.0 ± 39.0 | ||
| Very potent steroids | Mean ± SD | 1.9 ± 10.6 | 0.1 ± 1.1 | ||
| Mild calcineurin inhibitors | Mean ± SD | 2.1 ± 8.8 | 1.4 ± 5.4 | ||
|
Moderate calcineurin inhibitors |
Mean ± SD | 0.5 ± 3.3 | 0.8 ± 8.3 | ||
| Total high-strength medication (g) | Mean ± SD | 25.1 ± 47.5 | 18.4 ± 42.8 | 6.70 (–5.11 to 18.51) | 0.27 |
The difference in the total amount of medication used was –2.80 g (95% CI –25.78 to 20.18 g) and was not statistically significant (p = 0.81). The difference in the amount of low-strength mediation used was –9.50 g (95% CI –28.41 to 9.42 g) and this difference was not statistically significant (p = 0.32). The difference in the amount of high-strength medication used was 6.70 g (95% CI –5.11 to 18.51 g) was not statistically significant (p = 0.27). All three analyses supported the main analysis above.
Appendix 9 Well-controlled weeks and totally-controlled weeks to 16 weeks
FIGURE 19.
Well-controlled weeks for the whole 16-week study period.
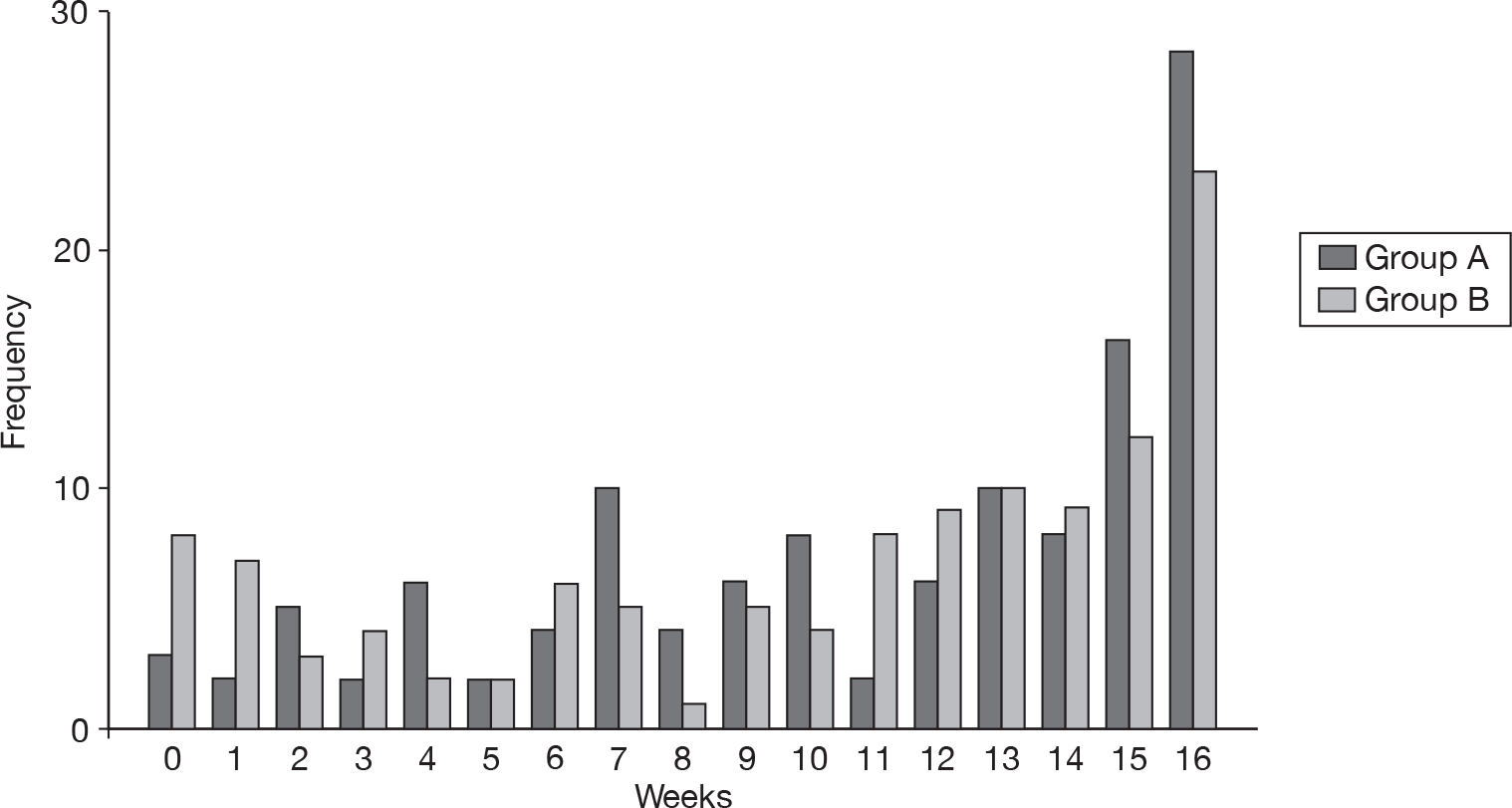
FIGURE 20.
Totally-controlled weeks for the whole 16-week study period.
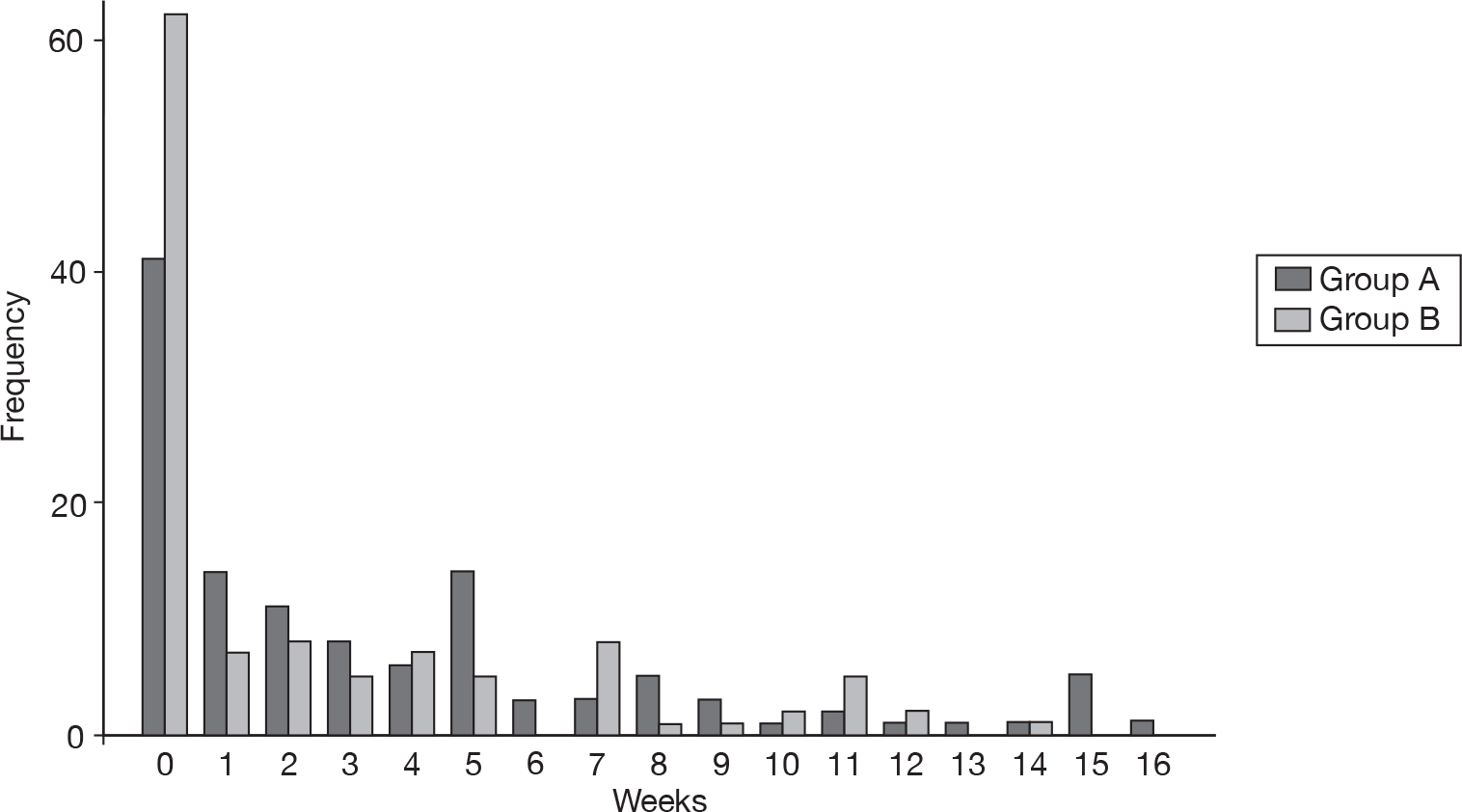
Appendix 10 Health economics questionnaire
Appendix 11 Variable definitions used in the contingent valuation study
| Variable | Definition (number in each response category) [non-responders/missing data] |
|---|---|
| WTP prior to trial | Mean £506.68 (SD £387.73; range 0–£3000), [3] |
| Purchase | 0) not purchased (157) R; 1) purchased (179), [0] |
| Child age | 1) < 3 years 98; 2) 3–7 years (123); 3) ≥ 7 years (115) R, [0] |
| Child gender | 0) female (143) R; 1) male (193), [0] |
| Child baseline SASSAD score | Mean 25.73 (SD 13.71; range 10–94), [0] |
| Child experienced a 20% reduction in SASSAD score | 0) no (146) R; 1) yes (177), [13] |
| Child filaggrin status | 0) no or unknown status (242) R; 1) positive filaggrin status 94, [0] |
| Number of nights at home | Mean 73.61 (SD 11.36; range 4–84), [2] |
| Number of medications at baseline | Mean 4.91 (SD 2.12; range 0–13), [0] |
| Household income (per annum) | 1) < £30,000 (109); 2) £30,000–50,000 (102); 3) ≤ £50,000 80 R, [45] |
| Intervention group | 0) group B (166) R; 1) group A (170), [0] |
| Water hardness at baseline | Mean 308.55 mg/l calcium carbonate (SD 54.11; range 200–540), [0] |
| Number of residents at home | Mean 4.15 (SD: 0.96, range 2–8), [12] |
| Reason given: this is a reasonable or fair amount for me to pay | 0) no (200) R; 1) yes (136), [0] |
| Reason given: this is just a guess | 0) no (227) R; 1) yes (109), [0] |
| Reason given: this amount reflects the benefits I think my child with eczema might get from the water softener | 0) no (176) R; 1) yes (160), [0] |
| Reason given: this amount reflects the wider benefits of installing a water softener in my home | 0) no (249) R; 1) yes 87, [0] |
| Reason given: this is how much I think a water softener would cost | 0) no (252) R; 1) yes 84, [0] |
| Reason given: this is how much I can afford to pay | 0) no (218) R; 1) yes (118), [0] |
| Reason given: other reasons | 0) no (297) R; 1) yes 39, [0] |
| Difficulty of WTP question | Mean 6.29 (SD: 2.52, range 0–10), [3] |
Appendix 12 Cost and resource use data for ages 3 years plus
Just 2.2% of weekly diaries were not completed; therefore data were not imputed for these entries. The mean total cost per patient in group A was £315 (SD £164) compared with £143 (SD £343) in group B [difference £172, 95% confidence interval (CI) £101 to £243, p = 0 < 001]. This significant cost difference was due to cost of the intervention all other resource categories (health professional visits, medications and other medical items) were not significantly different between groups (see Tables 34 and 35).
| Resource use item | Group A (water Softener + usual care) (n = 115) | Group B (usual care) (n = 113) | Mean difference (95% CI) |
|---|---|---|---|
| Intervention | |||
| Ion-exchange water softener | 1.00 | 0.00 | 1.00 |
| Installation | 1.00 | 0.00 | 1.00 |
| Salt (blocks) | 18.05 (2.69) | 0.00 (0.00) | 18.05 (17.55 to 18.55) |
| Face-to-face consultant-led follow-up attendance, paediatric dermatology | 1.00 | 0.00 | 1.00 |
| Secondary health care | |||
| Hospital outpatients visit (per follow-up attendance, paediatric dermatology) | 0.21 (0.55) | 0.35 (0.99) | –0.14 (–0.35 to 0.07) |
| Hospital admission (non-elective inpatient stay (short stay) | 0 (0.00) | 0.02 (0.13) | –0.02 (–0.04 to 0.01) |
| Primary and community health care (per consultation/visit) | |||
| GP | 0.46 (0.93) | 0.51 (1.13) | –0.05 (–0.32 to 0.22) |
| Practice nurse | 0.03 (0.18) | 0.19 (1.43) | –0.16 (–0.43 to 0.11) |
| Health visitor | 0.00 (0.00) | 0.01 (0.09) | –0.01 (–0.03 to 0.01) |
| Pharmacist | 0.45 (1.135) | 0.37 (1.20) | 0.08 (–0.025 to 0.41) |
| Specialist nurse | 0.17 (0.91) | 0.32 (1.32) | –0.15 (–0.45 to 0.14) |
| Resource use item | Group A (water Softener + usual care) (n = 115) | Group B (usual care) (n = 113) | Mean difference (95% CI) |
|---|---|---|---|
| Intervention | 217.03 (6.90) | 0.00 (0.00) | 217.03 (215.76 to 218.31) |
| Ion-exchange water softener | 14.33 | 0.00 | 14.33 |
| Installation | 5.49 | 0.00 | 5.49 |
| Salt | 46.21 | 0.00 | 46.21 |
| Face-to-face consultant-led follow-up attendance, paediatric dermatology | 151.00 | 0.00 | 151.00 |
| Secondary health care | 31.51 (83.66) | 60.84 (190.47) | –29.33 (–67.94 to 9.28) |
| Hospital outpatients visit (per follow-up attendance, paediatric dermatology) | 31.51 (83.66) | 52.12 (149.33) | –20.60 (–52.31 to 11.11) |
| Hospital admission (non-elective inpatient short stay) | 0.00 (0.00) | 8.73 (65.29) | –8.73 (–20.90 to 3.44) |
| Primary and community health care | 54.26 (84.47) | 58.28 (96.27) | –4.02 (–27.68 to 19.63) |
| GP | 14.29 (28.83) | 15.91 (34.93) | –1.62 (–9.99 to 6.74) |
| Practice nurse | 0.31 (1.66) | 1.75 (12.89) | –1.44 (–3.86 to 0.98) |
| Health visitor | 0.00 (0.00) |
0.31 (3.29) |
–0.31 (–0.92 to 0.30) |
| Pharmacist | 18.99 (56.80) | 15.61 (50.26) | 3.38 (–10.61 to 17.37) |
| Specialist nurse | 12.23 (67.15) | 23.58 (97.53) | –11.35 (–33.25 to 10.55) |
| Medication and accessories | 20.67 (31.44) | 25.01 (41.72) | –4.34 (–14.00 to 5.32) |
| Incremental mean cost | 315.07 (163.99) | 143.02 (343.31) | 172.05 (101.45 to 242.62) |
In both age groups it is noticeable how little health-care resource both groups used over the 12-week study period. It is also clear to observe that no resource category other than total intervention costs were significantly different between group A and group B. Thus, group A had higher costs because of receiving the intervention and there were no significant differences in any other resource item or cost. The ion-exchange water softener did not result in cost savings for the NHS as there was no reduced use of medications or other health-care resource use in group A over group B in this trial.
Appendix 13 Cost–utility sensitivity analysis for ages 3 years plus
Best case
The best-case costs for the 3 years and over age group resulted in mean costs of £130.82 (SD £110.57) for group A and £102.88 (SD £225.02) for group B [mean difference £27.94, 95% confidence interval (CI) –£18.55 to £74.44, p = 0.237]. Combining this with the incremental quality-adjusted life-years (QALYs) presented above for 3- to 16- year-olds of 0.006, the incremental cost-effectiveness ratio was estimated as £4548 in the best-case scenario. Figure 21 shows the cost-effectiveness acceptability curve (CEAC) for the 12-week study period. At a WTP of £30,000 per QALY there is a 39.0% chance of the ion-exchange water softener being cost-effective in this population.
FIGURE 21.
Decision uncertainty: plots of the CEACs for the sensitivity analysis best-case cost–utility analysis for ages ≥ 3 years.
Worst case
For worst-case costs, the mean cost for group A was £520.74 (SD £236.73) compared with £189.23 (SD £464.75) for group B, mean difference £331.51 (95% CI £234.81 to £428.21, p < 0.001). Combining the incremental cost with the incremental QALYs presented above for 3- to 16- year olds of 0.006, the incremental cost-effectiveness ratio was estimated as £53,957 in the worst-case scenario. Figure 22 shows the CEAC for the 12-week study period. Again, at all levels of WTP, normal care (group B) was more likely to be cost-effective.
FIGURE 22.
Decision uncertainty: plots of the cost-effectiveness acceptability curves for the sensitivity analysis worst-case cost–utility analysis for ages ≥ 3 years.
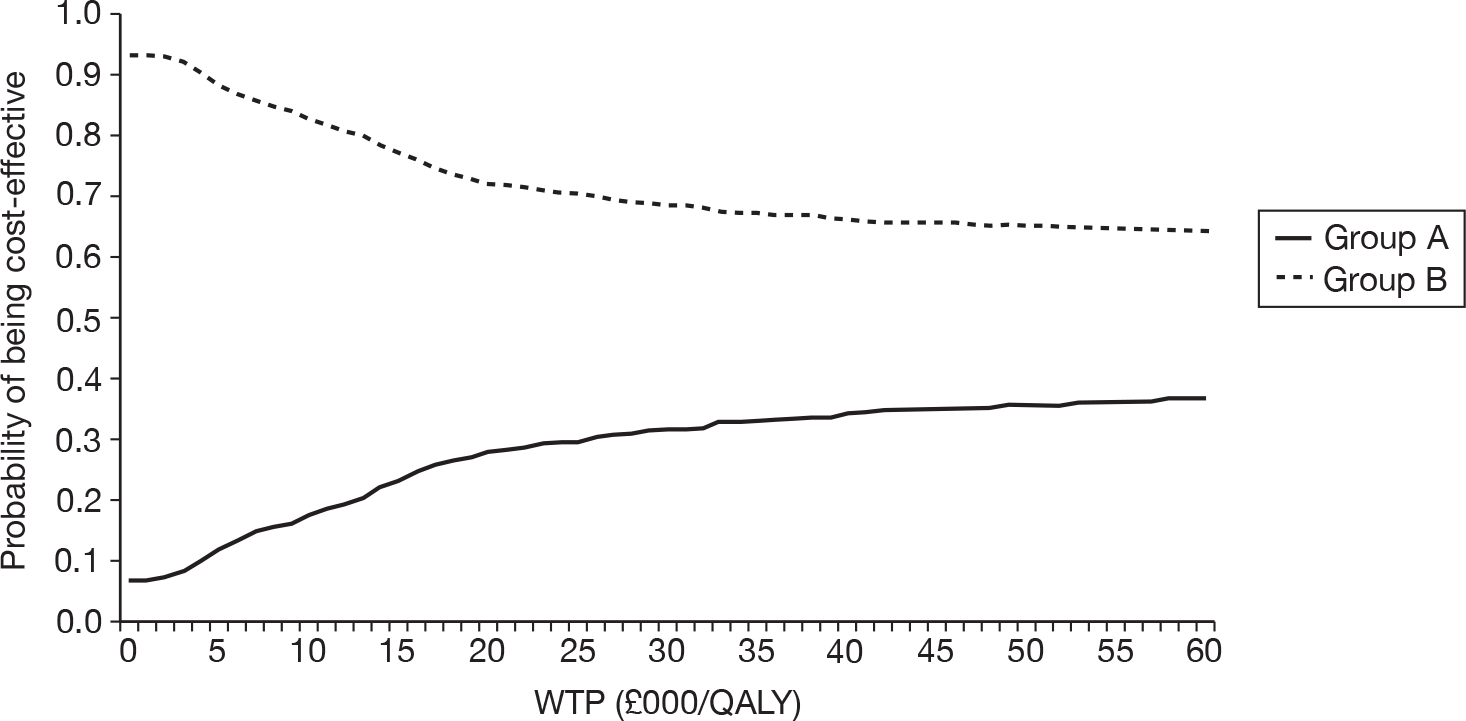
Imputing missing values
Sensitivity analysis, including all trial participants, used imputed QALY scores for children < 3 years based on their baseline SASSAD scores. Group A gained a mean of 0.014 QALYs per patient and group B a mean of 0.007 QALYs per patient. The mean difference in QALYs per patient was 0.006 (95% CI 0.001 to 0.012, p = 0.022). Using the incremental mean cost reported in Table 20, the incremental cost-effectiveness ratio for group A was estimated as £31,018.
Figure 23 shows the CEAC for the 12-week study period. Using this model, the acceptability curves crossed in favour of water softeners at a WTP of approximately £45,000 per QALY.
FIGURE 23.
Decision uncertainty: plots of the CEACs for the sensitivity analysis cost–utility analysis for all ages.
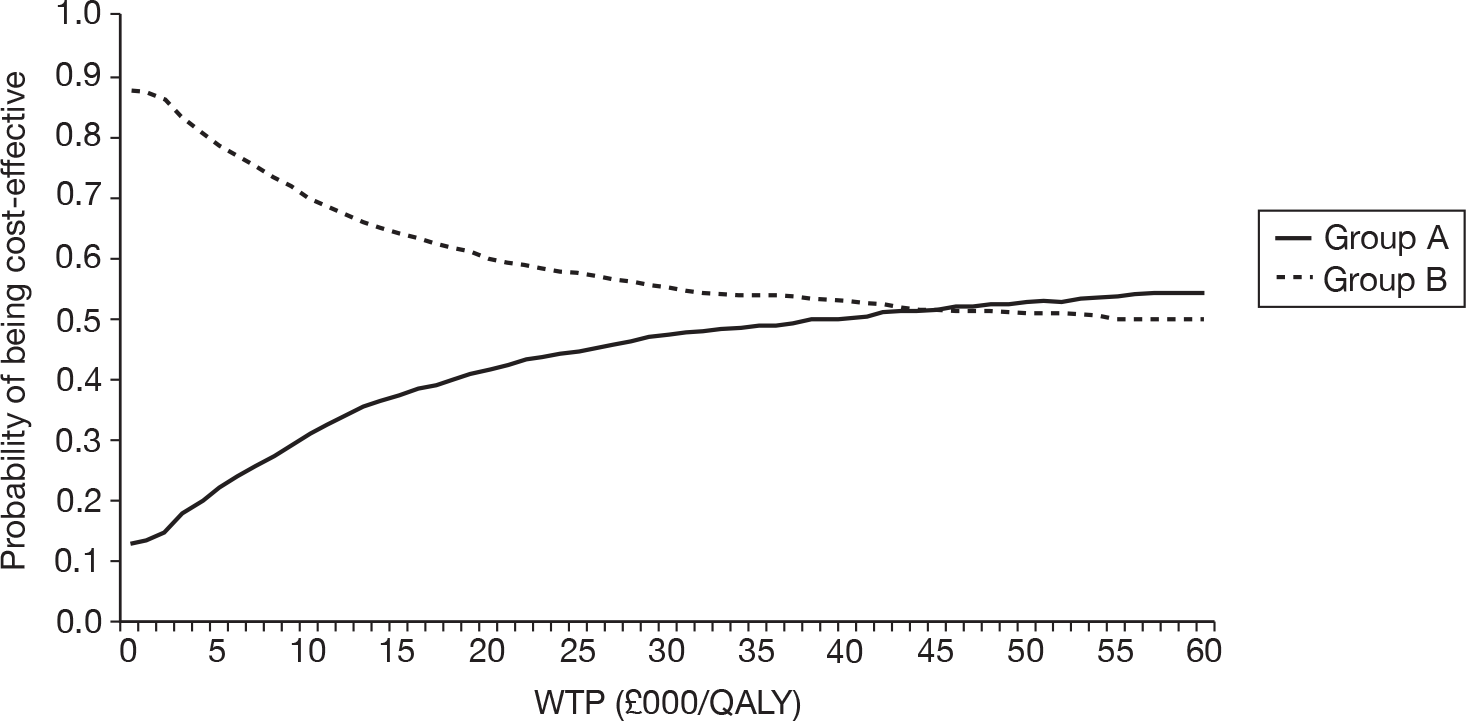
Appendix 14 Parameter estimates of the general linear regression analysis to explain variation in willingness-to-pay values prior to the trial
Parameter estimates of the general linear regression performed to explain variation in WTP values prior to the trial, data for 278a participants were included in the analysis.
| Explanatory variable | Estimate (95% CI) |
|---|---|
| Intercept | 718.226 (177.753 to 1258.699)c |
| Child age | |
| < 3 years | –57.509 (–174.794 to 59.776) |
| 3–7 years | –48.185 (–156.508 to 60.139) |
| Child gender | 30.223 (–123.057 to 62.612) |
| Child baseline SASSAD score | –0.687 (–4.188 to 2.814) |
| Child-positive filaggrin status | 92.146 (–6.162 to 190.454) |
| Number of nights at home | 4.694 (0.349 to 9.039)b |
| Number of medications at baseline | 7.148 (–16.247 to 30.543) |
| Household income (per annum) | |
| < £30,000 | –173.720 (–291.605 to –55.835)c |
| £30,000–50,000 | –155.102 (–274.014 to –36.190)b |
| Intervention group | –32.84 (–123.523 to 57.843) |
| Water hardness at baseline | –0.581 (–1.45 to 0.288) |
| Number of residents at home | –36.987 (–89.256 to 15.282) |
| Reason given: this is a reasonable or fair amount for me to pay | –103.56 (–200.008 to –7.111)b |
| Reason given: this is just a guess | –20.473 (–122.854 to 81.908) |
| Reason given: this amount reflects the benefits I think my child with eczema might get from the water softener | 126.738 (31.852 to 221.623)c |
| Reason given: this amount reflects the wider benefits of installing a water softener in my home | 20.491 (–86.644 to 127.625) |
| Reason given: this is how much I think a water softener would cost | 86.917 (–19.37 to 193.204) |
| Reason given: this is how much I can afford to pay | –102.913 (–202.548 to –3.278)b |
| Reason given: other reasons | –99.292 (–239.652 to 41.068) |
| Difficulty of WTP question | –26.354 (–44.474 to –8.235)c |
Appendix 15 Binary logistic regression analysis to explain decision to purchase
Parameter estimates of the binary logistic regression performed to explain decision to purchase an ion-exchange water softener, data for 273a participants were included in the analysis.
| Explanatory variable | OR (95% CI) |
|---|---|
| Constant | 0.705 |
| Child age | |
| < 3 years | 1.207 (0.591 to 2.465) |
| 3–7 years | 1.185 (0.618 to 2.274) |
| Child gender | 1.027 (0.584 to 1.804) |
| Child experienced a 20% reduction in SASSAD score | 1.623 (0.929 to 2.835) |
| Child-positive filaggrin status | 0.656 (0.361 to 1.192) |
| Number of nights at home | 0.987 (0.958 to 1.018) |
| Number of medications at baseline | 1.243 (1.078 to 1.434)c |
| Household income (per annum) | |
| < £30,000 | 0.345 (0.166 to 0.717)c |
| £30,000–50,000 | 0.582 (0.275 to 1.231) |
| Intervention group | 1.040 (0.602 to 1.796) |
| Water hardness at baseline | 1.007 (1.001 to 1.012)b |
| Number of residents at home | 1.026 (0.750 to 1.403) |
| Reason given: this is a reasonable or fair amount for me to pay | 1.052 (0.588 to 1.883) |
| Reason given: this is just a guess | 1.094 (0.595 to 2.012) |
| Reason given: this amount reflects the benefits I think my child with eczema might get from the water softener | 0.610 (0.341 to 1.091) |
| Reason given: this amount reflects the wider benefits of installing a water softener in my home | 1.022 (0.533 to 1.963) |
| Reason given: this is how much I think a water softener would cost | 0.847 (0.440 to 1.630) |
| Reason given: this is how much I can afford to pay | 0.912 (0.499 to 1.668) |
| Reason given: other reasons | 0.775 (0.321 to 1.874) |
| WTP prior to trial | 1.000 (1.000 to 1.001) |
| Difficulty of WTP question | 0.934 (0.834 to 1.045) |
List of abbreviations
- CI
- confidence interval
- CTU
- Clinical Trials Unit
- DFI
- Dermatitis Family Impact (questionnaire)
- eczema
- atopic eczema/atopic dermatitis
- EQ-5D
- European Quality of Life-5 Dimensions
- GCP
- good clinical practice
- GP
- general practitioner
- HTA
- Health Technology Assessment
- IgE
- immunoglobulin E
- ITT
- intention to treat
- MRC
- Medical Research Council
- MREC
- Multicentre Research Ethics Committee
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- POEM
- Patient-Oriented Eczema Measure
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SASSAD
- Six Area, Six Sign Atopic Dermatitis
- SD
- standard deviation
- SWET
- Softened Water Eczema Trial
- TCW
- totally controlled week, i.e. a week in which symptoms are controlled throughout the week without the need to ‘step up’ treatment beyond normal maintenance care (such as emollients)
- TIS
- Three-Item Severity
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UKWTA
- UK Water Treatment Association
- WCW
- well-controlled week, i.e. a week in which symptoms and the need for ‘step-up treatment’ occurred on ≤ 2 days of the week
- WRAS
- Water Regulations Advisory Scheme
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr David P Britt, Public contributor
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Public contributor
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington