Notes
Article history
The research reported in this issue of the journal was commissioned by the National Coordinating Centre for Research Methodology (NCCRM), and was formally transferred to the HTA programme in April 2007 under the newly established NIHR Methodology Panel. The HTA programme project number is 05/516/08. The contractual start date was in December 2007. The draft report began editorial review in June 2010 and was accepted for publication in September 2010. The commissioning brief was devised by the NCCRM who specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2011 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
The number of randomised controlled trials (RCTs) of medicines for children is increasing as initiatives are put in place to improve access to medicines licensed for use in children. 1–3 Significant resources are invested in the design and set-up of these trials, yet experience shows that many children’s trials struggle to enrol and retain sufficient participants. This results in trials being delayed or abandoned4,5 or publishing with very small sample sizes. 6 Numerous studies have reported interventions to improve recruitment to adult trials. 7–9 However, ‘children are not small adults’10 and their recruitment involves greater complexity, such as gaining proxy consent from a parent or guardian. Therefore, we cannot extrapolate directly from research in adults. While difficulties in enrolling sufficient participants to clinical trials of medicines for children need to be addressed if improvements in treatment are to be realised, it is also important to ensure that recruitment is conducted in a way that is appropriate to families and practitioners and is ethically sound. Concern to ensure adequate accrual therefore needs to be balanced with the concern to ensure good conduct in recruitment to clinical trials and that prospective participants feel able to make informed and voluntary decisions. 11
Recruitment to trials of medicines for children involves three key groups: the young people themselves, their parents and the practitioners who are responsible for the trial. This chapter gives an overview of the existing research on the recruitment process as relevant to each of the groups before presenting the rationale and objectives of the current study.
Parents
Recruitment, information and understanding
By far the largest body of evidence on recruitment to children’s trials has been gathered from parents, and largely from paediatric oncology (specifically leukaemia) and neonatology. There is little work beyond these specialties. Research on parents is dominated by quantitative investigations of the recruitment process in terms of the information families receive and how much of that information they understand and recall. 12–15
Information can affect parents’ satisfaction and willingness to participate in a trial. 16–18 However, what constitutes the ‘right’ level and type of information is probably impossible to define,19 not least because information can simultaneously serve different functions20 and parents can vary in their preferences for trial information. 21,22 More information is not necessarily better: Miller and colleagues23 reported a trend for more information to be associated with greater parental anxiety and less control. While a baseline level of information may be necessary for informed consent and informed decline,24 research suggests that parents benefit from an approach in which practitioners select and tailor information according to individual need and preference. 23,25
Researchers consistently report that parents’ understanding of trials is poor. 26–28 Families whose first language is different from that used by practitioners, minority group members and those from disadvantaged backgrounds may experience particular difficulties. 27 It can also be a struggle for patients and families to separate discussions about treatment options and management from those about trial participation,29–31 particularly when faced with serious illness. 24,32 Based on a series of investigations of paediatric oncology trial discussions, followed by assessments of parents’ understanding, Simon and colleagues33 emphasised the need for a clearer distinction between trial-related issues and standard treatment issues, along with clearer explanations of randomisation, confirming findings from previous studies. 12
Interventions to improve understanding
When asked for feedback on how to improve informed consent discussions, parents of children with leukaemia who were approached about trial entry advocated a sequenced or staged approach to informed consent,21,34 including scheduling discussion of the trial after patients have achieved a basic understanding of the disease and treatment. An intervention based on this feedback, and on studies stressing the importance of encouraging parental participation,12,23,33 was used to train practitioners in communication techniques, such as simplifying information, prompting parents’ interactions and encouraging them to ask questions. 35 The training was evaluated using researcher-assessed measures of parental understanding. Improvements were found in parents’ understanding of the voluntary nature of the trial but not in their understanding of randomisation. Parents found nurse–family research education sessions helpful to prepare them for the trial discussion with their doctor, and felt that the sessions helped them ask questions and understand the information provided by the doctor. 36
Qualitative work on the recruitment of adults into trials has also indicated that patients find trials hard to understand; concepts such as voluntariness, equipoise and randomisation can be particularly difficult for patients to grasp. 37–39 These studies further indicated that a failure to understand these concepts can result in patients becoming distrustful of the trial or the doctor, and simple interventions to provide more or ‘better’ information may not always be sufficient to ameliorate these difficulties. 37–39 Adaptations to the practitioners’ communication, such as changing the way in which the trial arms were presented to emphasise their equivalence, increased the perceived acceptability of a trial to patients39 and its randomisation rate. 7 Wade and colleagues40 reported that styles of consultation that were participant led rather than practitioner led, and which involved practitioners using open questions, pauses and ‘ceding the floor’, enabled participants to express their concerns more easily.
The context and experience of trial recruitment
While these adaptations to practitioner presentation and communication about trials may be transferable to the paediatric context, parents considering trial entry for their child have a very different role from the adult considering trial entry for him or herself. 41 Parents are responsible for their child and are entrusted to act in the child’s best interests, a difficult and complex position when trials, by their nature, cannot promise to act in the best interests of the individual child. 42–44 Consent can often be sought soon after diagnosis, at birth or when the child is very ill and the parents are distressed and vulnerable. 30,45,46 Despite such challenges, parents have reported that they, rather than doctors or nurses, should be the ones who make the decision about whether a child should enter a trial,15,32,43,47,48 although parents value doctors’ advice. 49 Making a decision for one’s child is further complicated by the need to make the ‘right decision’ and the anticipation that one might later regret a decision. 31,50,51
The generally high rates of recruitment to trials in neonatology52 and childhood cancer53 suggest that the threat of a child’s illness and parents’ consequent need for hope could be important influences on how parents view trials. Parents of chronically ill children may, in general, be prepared to take greater risks in treatment in the hope of helping their child42 than the parents of healthy children considering participation in vaccine research, who have been found to believe that children should take part in research only when the medical benefits outweigh any potential risk. 54
Influences on parents’ decision to enter their child into a trial
Most studies on the reasons why parents choose whether or not to enter their child in a trial have used questionnaire and survey methods. Particularly common worries for parents when considering a trial are that their child might be randomised to the treatment arm that they perceive to be less effective50,55 and the responsibility that they would feel if the child later deteriorated. 56 Nevertheless, parents do perceive several benefits of trial participation for their own child, such as receiving extra medical attention. 57–60 Such benefits can be influential in their decision. However, evidence indicates that parents’ primary concern when considering a trial is their child’s safety61 and to protect their child from harm. 43 Altruism, although frequently cited by parents as a motivation for participation in trials,49,62,63 may be a secondary consideration in their decision. 26,45,64
The seriousness of the child’s condition and the urgency surrounding trial entry and parents’ resulting sense of vulnerability have also been found to be important influences on how parents experience recruitment to trials. 31,32,55,65 However, the relationship between anxiety, vulnerability and trial decisions may be mediated or moderated by factors such as trust in medical research66 and the parent–practitioner relationship. 23,56,67–70 The practicalities of trial participation are less commonly mentioned;71 this may reflect the fact that most studies have been conducted in neonatology and oncology, when the child is usually in hospital at the time of the trial approach.
Summary
A relatively large body of work has been conducted on parents and trial recruitment within oncology and neonatology; however, other areas have been largely overlooked, including trials in other specialties and studies investigating the experiences of parents who decline trials. The quantitative nature of much of the existing literature reflects the priorities of researchers and presupposes what is important to parents when they are approached about a trial. 72 In adult clinical trials, the information content of the trial discussion and the patient ratings of the interpersonal aspects of communication, such as a belief that the doctor listened to them, has been found to influence the trial decision and the patient’s confidence in that decision. 73 With a few notable exceptions, little research has addressed how the trial approach is experienced by parents in a way that allows them to define what they consider meaningful and important during this process. 21,30,55,74 Finally, no studies have compared parents’ experience of trial recruitment with the actual content of the trial discussion.
Young people
Young people’s experiences of trial recruitment cannot be considered in isolation from their parents’; it is usually necessary to obtain parental consent, which ultimately guarantees that the parent will be involved in the discussion and decision. However, although parents give consent on behalf of their children, when young people are old enough to have an opinion then their assent to trial entry should be sought. 75,76 This means that when a parent’s and young person’s wishes differ, they must discuss the decision. While interventions to improve parental understanding of trials have been reported,35,36 we are not aware of any studies investigating interventions to promote young people’s understanding of research.
Young people’s understanding of research
Like their parents, young people may struggle to understand trials. 77–79 The voluntary nature of research and the right to withdraw were understood by young people in some studies78,80 but not in others. 81 In their study of young people with cancer or human immunodeficiency virus (HIV), Chappuy and colleagues81 link this difference to the young people’s lack of information and understanding of their life-threatening condition and proximity to diagnosis. Like parents, young people may find it difficult to distinguish between their treatment and research,82 and this may be particularly true in the cancer setting, where the two are particularly entwined. One study reported that young people with diabetes were much better able to differentiate between the research they were involved in and their treatment regimen than those with cancer,82 whereas one-half of the young people participating in one oncology research project did not know that their treatment was part of a clinical research study. 83
Young people’s reasons for participation in research
Like the available studies in parents, most studies on the reasons why young people choose whether or not to participate in research has been conducted using questionnaire and survey methods. Personal benefit is the most popular reason cited by young people consenting to an actual or hypothetical trial; the desire to help others is significantly less influential in young people’s decisions than perception of personal benefit,84–86 as it also is for their parents,87 although occasionally young people cite benefit to others as more important than personal benefit. 88,89 Young people give a number of reasons for declining a trial, including worries about blood or urine samples or the doctor’s examination,89 extra clinic visits,90 belief that the trial will take up too much time,88 belief that the ‘research methods are too involved or too burdensome’ and lack of interest in the research topic. 91 Refusal rates have been found to be higher in studies that include blood sampling, and higher in boys than in girls. 91
Young people’s role in the trial discussion
Discussions about young people’s participation in a trial entail more complex dynamics than is usually typical of adult trial recruitment. 92,93 Compared with adult trials, such dynamics are likely to present additional challenge to paediatric trial recruiters in managing the consultation and balancing the involvement of each party. 94 However, studies of health-care consultations, in general, report that young people often play little active part. 95,96 While few studies have investigated their role in discussions about trial entry, Olechnowicz and colleagues94 reported that young people recently diagnosed with cancer said very little in the trial discussion. There was considerable variability in the extent to which practitioners directed the discussion towards the patient. Older patients spoke more than younger ones, largely because they asked more questions, but few of their questions were about the trial.
Young people’s role in the trial decision
The role of young people in the decision about trial entry is complex. Some researchers have argued that while young people’s dissent should be respected, they are not in a position to make a decision on trial entry without significant guidance from their parents,97,98 and that, while it is important that they are consulted, they should be protected from decisions that they are unwilling, or unable, to make. 99 A key complexity in considering research in this area is that much of it has investigated young people’s decision-making about entry into hypothetical trials or non-therapeutic clinical research,87,100–105 rather than focusing on young people who have been approached about a ‘real’ clinical trial. Moreover, some research in this area has been conducted with samples of healthy children, rather than children who have recent experience of illness. Because of the uncertainties in interpreting the relevance of such research, we have limited our discussion of it in this section; we draw on it only where other evidence is unavailable.
Young people approached for assent to clinical anaesthesia and surgery research80 or to clinical trials of treatments for cancer or HIV81 are strongly influenced by their parents when making a decision on trial entry. This probably stems from young people’s reliance on their parents to guide and protect them, and from both parties’ concern to make the ‘right’ decision. 92 Sometimes, parents encourage or persuade children to take part in research if they think it is in their child’s best interests. 57,85
The potential for tension between young people and parents regarding decisions about research participation, and the difficulty for practitioners in balancing their needs, is highlighted by several studies. A survey of 7- to 14-year-olds who were enrolled in clinical research or receiving clinical care for cancer or asthma reported that approximately 90% of young people felt that they should be involved in decisions, whereas only approximately 60% of parents agreed. 84 Just over 40% of parents were against or unsure about whether children should have decisional authority over participation in non-therapeutic clinical research,100 while nearly 70% of parents responding to a hypothetical research scenario said that they would impose their own views on research participation regardless of their child’s wishes. 102
Particularly where the child has a life-threatening illness, some parents report excluding the child from the decisions about research altogether in order to protect them from distressing information, or including their child in discussions but making the trial decision themselves. 94,106 In a departure from much of the decision-making literature, which has largely focused on young people in hypothetical research scenarios, Unguru83 presented the views of young people in relation to the cancer research in which they were participating. Many young people in Unguru’s study felt minimally involved in decision-making. They had wanted to be involved but did not want to make the decision on their own – rather they wanted to decide jointly with their parents and doctors. By contrast, some healthy young people102,104 and those with a chronic condition (asthma, diabetes or epilepsy) responding to hypothetical research scenarios want the ultimate decision to be theirs, although other research has indicated that some young people feel that their parents ‘know best’ and take the view that if their parents did not want them to participate then they would not do so. 105
Among the challenges for triallists when considering the young person’s role in decision-making are the conflicting legislative frameworks governing consent for research versus those governing consent for treatment. While legislation governing consent to treatment recognises young people’s agency and autonomy,107 Medicines for Human Use (Clinical Trials) Regulations give power of legal consent to parents. The wishes of under-16-year-olds are ‘considered’ but they do not have legal force in the context of clinical trials of medicines. 76 Interestingly, evidence provides some support for this legislation. Young people are more willing to take part in above-minimal-risk hypothetical studies than their parents,87 and adolescents are particularly more likely to ‘consent’ than parents. 87 If they are less risk averse than their parents in actual clinical trials, parental consent may serve a valuable function in helping to protect young people from accepting inappropriate research risks. 105 Where family decisions are initially discordant about hypothetical research scenarios, the final decision usually reflects the parents’ opinions, although the young people are less happy with the final family decision as a result. 101
Summary
Considered alongside the legislative frameworks governing consent for children’s trials,108,109 data on hypothetical scenarios and non-therapeutic clinical research can alert us to some important considerations. However, as we outline above, the applicability of these findings to families of seriously ill children approached about a real clinical trial is questionable. Moreover, the survey nature of much research on young people means that, like the literature on parents, it assumes rather than explores what is important to young people when they are invited to join a clinical trial.
Practitioners
Research involving practitioners has focused in two key areas: their role as information givers in trial discussions and their views on barriers to trial recruitment. Rarely have studies asked practitioners about their own priorities for, and experiences of, discussing trials with families.
Practitioners’ communication in the trial discussion
Clinical trials have a long and successful history in paediatric oncology. It is no surprise then that almost all research on practitioners’ communication about clinical trials has been conducted in this area. In an early survey of clinicians’ practice and parents’ opinion, practitioners noted the need for a flexible and adaptable approach to accommodate families’ individual needs, but they also sometimes noted a conflict between the need to start treatment and the ‘deliberate pace necessary for an optimal consent’. 46 However, practitioners’ concerns about informed consent were not shared by parents, who were generally more satisfied with the consent process than practitioners, 50% of whom felt that families were given too much information. 46 These disparities between parents and practitioners are important. However, the brief survey data gathered from this and similar studies limit interpretation. In response to an open-ended question in another survey, one-quarter of practitioners’ suggestions for improving the informed consent process related to simplifying information for parents, while suggestions for improving the timing and staging of the discussions were a close second. 110
Barriers to recruitment
A relatively large body of work has investigated so-called barriers to recruiting adults and children to clinical research. Evidence from adult trial recruitment suggests that, when offered a trial, most patients accept,73 and some paediatric practitioners described selectively approaching those families who they thought most likely to consent. 111 Non-invitation of eligible participants is therefore likely to contribute to trial recruitment difficulties in trials in all specialties. In paediatric trials, where the pool of eligible participants may already be relatively small,111 this source of recruitment difficulty is an important concern.
Many studies in adult care settings cite practitioners’ time constraints as one of the most common barriers to recruitment or involvement in research at all. 112–115 However, based on a study of general practitioners (GPs) who declined to facilitate an adult trial, Salmon and colleagues112 pointed out that such justifications may be markers of the low priority that practitioners give to research and the perception that research has limited relevance to their clinical work. Other evidence on practitioners’ reasons for not approaching patients about research has indicated that practitioners do not recruit to studies because they see their patients as vulnerable,113 in need of protection;116 have concerns about the impact of research on the doctor–patient relationship,113,117 which may be a particular difficulty in community-based trials;52 or have strongly held preferences for one treatment over another118 and perceive other ethical difficulties. 115
Practitioners also cite time constraints as a barrier to recruitment in paediatric trials. 119 They are also concerned about the impact of research on the doctor–patient relationship,119,120 the use of placebo and randomisation,111,119 the practical burden of participation for families,111 potential harms to participants and unpleasant procedures, such as venepuncture. 111,119 Some practitioners describe an allegiance to the patient, which they regard as being in conflict with the uncertainty inherent in trials,120 and believe that families are liable to be confused by, or mistrust, trials. 119
Particular barriers exist in paediatric research in critical care settings where parents may be unavailable for consent or be so emotionally distraught as to make an approach about research untenable. 121 In a personal account of avoiding approaching vulnerable families, Walterspiel122 described the ‘emotional burden’ of obtaining informed consent under such circumstances. Particularly in critical situations, as is often the case in neonatal trials, practitioners express concern about parents’ competence to give informed consent because of their vulnerable position and emotional state, as well as their lack of knowledge and time to decide. 32
Practitioners may also have concerns over their own skill and confidence in approaching patients. 113 While some practitioners regard trials more positively, for example as a chance to offer their patients potentially effective therapies at an early stage,115 a prominent feature of much of the research on practitioners is the negative way in which they construct trials and oppose the interests of trials and the interests of the doctor–patient relationship. One study123 reported that a significant proportion of clinicians in adult cancer care rated seeking consent to clinical trials as their main communication problem – ranking it as more difficult than breaking news of serious illness to patients. In one focus group study, some paediatricians described how they disliked approaching parents and felt ‘rejected’ if parents said ‘no’ to a trial, while others described discomfort at expressing uncertainty because they were concerned it might damage parents’ trust in their expertise. 119
Summary
Recruiting to children’s trials brings additional complexities compared with recruiting adults to trials, which probably affects the practitioner’s experience of recruitment. Paediatric trials also confer specific potential risks – such as patient discomfort, pain and fear of separation from parents or familiar surroundings – that are either absent in adult trials or harder to assess in paediatric settings. 124 Much of the research on practitioners in both adult and paediatric settings indicates that they construct trials in rather negative ways, but evidence directly comparing practitioners’ and parents’ constructions of the same trials is limited.
Rationale and objectives
While there are strands of evidence relevant to the three different stakeholder groups – young people, parents and practitioners – involved in recruitment to trials, much of this has been collected in a way that assumes rather than explores what is important to the groups. None of the evidence has been collected in a way that directly compares the perspectives of the three groups, which means that it is impossible to identify where their perspectives converge and diverge and to use this to inform clinical trial recruitment. Also missing from the existing literature are studies that have linked the experience of trial recruitment to the actual conduct of trial discussions. This is an important omission because we cannot assume that the ‘look’ of trial recruitment consultations, from the viewpoint of third-party researchers, is the same as the ‘feel’ of these consultations from the viewpoint of those involved. Finally, much of the existing evidence comes from neonatology and paediatric oncology trials, or from hypothetical trials or non-therapeutic clinical studies. It is important to extend the evidence base to families who have been approached about ‘real’ clinical trials across a range of different specialties and to include the perspectives of those who decline as well as those who consent. Existing interventions to improve recruitment and its conduct in children’s trials have generated some promising findings,35,36 although these are in the early stages of development. This study is therefore important to provide a comprehensive investigation of the processes of recruitment, with the aim of identifying strategies to improve recruitment and its conduct that can be applied across the spectrum of trials of medicines for children.
As stated in the original protocol, the data for our study were (1) trial recruitment discussions between families and trial recruiters; (2) follow-up semi-structured interviews with families (young people and parents); and (3) follow-up semi-structured interviews with practitioners. Our specific objectives were to describe:
-
how recruitment consultations (trial discussions) between families and trial recruiters (practitioners) are conducted and how information about trials is exchanged during these encounters
-
from the perspective of families, the experience of trial recruitment and the communication needs and other priorities that are served or thwarted by recruitment consultations
-
from the perspective of trial recruiters, the goals of recruitment consultations, the functions of these goals, how they interface with the conduct of the consultation, and families’ communication needs and other priorities.
In the next chapter we describe the study methods, and, in keeping with recommendations in qualitative research,125 how these evolved over the course of the study.
Chapter 2 Methods
Sampling of trials and sites
RECRUIT [processes in recruitment to randomised controlled trials of medicines for children] ran alongside four placebo-controlled, double-blind RCTs of medicines for children. All trials were National Institute for Health Research (NIHR) – Medicines for Children Research Network (MCRN) portfolio trials and were selected to represent different conditions, disease status and trial design to maximise the transferability of findings. The trials also differed in the timing and circumstances of the approach for recruitment and in the relationship between family and the practitioners responsible for recruitment. Where circumstances allowed, we selected sites in the north-west of England in preference to other regions for logistical reasons, but where north-west sites were unavailable we included sites in other regions. Two or three teams from each trial facilitated RECRUIT. MCRN local research networks (LRNs) helped to facilitate the research and development (R&D) process and assisted in liaison with the trial teams. Brief details of the four participating trials are given in Table 1 and further outlined below.
| Trial title | Age range | Disease status | Medicine delivery |
|---|---|---|---|
| MASCOT | 6–15 years | Chronic | Inhaled and oral |
| MENDS | 3–15 years | Chronic | Oral |
| POP | 4–18 years | Chronic | Oral |
| TIPIT | Infants born < 28 weeks’ gestation | Acute | i.v. |
MASCOT – Management of Asthma in School-aged Children On Therapy
-
This was an RCT comparing the add-on treatments salmeterol (long-acting β2-agonist) and montelukast (leukotriene receptor antagonist) for young people whose asthma was poorly controlled with fluticasone (low-dose inhaled corticosteroid).
-
The initial approach about the trial was usually via a letter from the GP (interested families returned a slip to the trial team) or a personal approach when the child was attending a secondary care centre. Interested families received participant information leaflets (PILs) and a telephone call from a research nurse before attending an appointment with a respiratory consultant and research nurse, specifically arranged to discuss trial entry.
-
The parent PIL was 12 pages long (approximately 5500 words), excluding consent forms.
-
Prior to randomisation, there was a 4-week run-in period, during which families were provided with information about asthma and its management. All young people were prescribed the same low-dose inhaled corticosteroid (fluticasone), which was continued throughout the trial period. Families also completed an asthma diary throughout the trial period. Young people whose asthma control had not improved after the run-in phase were randomised to one of the three treatment arms. There was a 48-week treatment period. Including the screening and baseline assessments, families made five clinic visits and received one telephone call. There was a genetic substudy for which consent was taken separately after the run-in phase.
-
At the time this report was prepared, 772 patients had been assessed for eligibility, 151 had registered for the trial, 54 had declined the trial, and 567 were ineligible or excluded for other reasons.
MENDS – the use of MElatonin in children with Neurodevelopmental Disorders and impaired Sleep
-
This was an RCT of melatonin in young people with neurodevelopmental disorders and impaired sleep.
-
The initial approach about the trial was usually made by community paediatricians at a routine clinic visit. Interested parents received PILs from their community paediatrician or by post, and had a telephone conversation with a research nurse before attending an appointment with a consultant neurologist and research nurse, specifically arranged to discuss trial entry.
-
The parent PIL was 11 pages long (approximately 5500 words), excluding consent forms. Melatonin was not licensed in the UK for children at the time the trial was conducted, and this was stated in the parent PIL.
-
Prior to randomisation there was a 4-week behavioural intervention using established techniques to reduce the child’s sleeping problems. During the trial parents completed a sleep diary every day and the child wore an ActiGraph (Ambulatory Monitoring Inc., Ardsley, NY, USA; www.ambulatory-monitoring.com) watch to record movement. Saliva samples were collected before randomisation and towards the end of the treatment phase to measure melatonin levels. There was a 12-week treatment period. Families made three additional hospital visits, and received four home visits by the research nurse and three telephone calls. There was a genetic substudy for which separate consent was taken.
-
At the time this report was prepared, 241 patients had registered for the trial. A further 147 had not proceeded to registration because of an inappropriate referral, or ineligibility at screening or because the family was not contactable; 170 had refused screening.
POP – Prevention and treatment of steroid-induced OsteoPenia in children and adolescents with rheumatic diseases
-
This was an RCT to establish if the bisphosphonate risedronate or the vitamin D analogue 1-alphahydroxycholecalciferol was better than placebo in preventing/reducing bone loss in young people (aged 4–18 years) with rheumatic diseases who were treated with corticosteroids.
-
Usually the trial was briefly introduced to families by a doctor from the rheumatology clinical team. The trial design allowed considerable flexibility so practitioners could select an appropriate time to approach the family. After the initial introduction, a more formal discussion would be arranged to coincide with a routine hospital appointment with the rheumatology team. Research nurses and doctors had trial discussions with families, although only doctors took consent.
-
The parent PIL was 6.5 pages long (approximately 3500 words), excluding the consent forms.
-
There was no run-in phase and the treatment phase lasted for 1 year. All groups received calcium and vitamin D supplementation daily. Young people were seen seven times over the course of the year. However, this was timed to coincide with routine clinic visits where possible. Blood samples were also taken, but at the same time as routine visits and tests. Young people gave regular urine samples and had three DEXA (dual-emission X-ray absorptiometry) scans and two bone radiographs taken as part of the trial. There was a genetic substudy that was discussed at the time of consent; young people could participate in the main trial without consenting to the storage of their genetic material.
-
At the time this report was prepared, 318 patients had been screened for the study, 132 had been recruited, 63 had declined the trial, 60 were potential future participants, 42 were ineligible and 41 did not take part for another or unstated reason.
TIPIT – Thyroxine In Preterm Infants Trial
An RCT of thyroxine in preterm infants under 28 weeks’ gestation.
-
As the trial name indicates, this was a trial of thyroid hormone supplementation in babies born before 28 weeks’ gestation. The primary outcome related to brain growth.
-
Necessarily, the initial trial discussion was often conducted either on the neonatal unit or at the mother’s bedside and with a practitioner unknown to the parents. A research nurse was not usually present during these discussions.
-
The PIL was three pages long (approximately 1700 words), excluding the consent forms.
-
Trial medication commenced within 5 days of birth and was given every day, through intravenous or feeding tube, until the baby was 32 weeks old, with follow-up until the baby went home. The mother had a single blood test and bloods were taken at several points from the baby alongside those for routine care (no bloods were taken from the baby specifically for the trial). There was a brain magnetic resonance imaging (MRI) study for which there was a separate consent, taken at a later date.
-
The trial closed to recruitment in 2009 when target accrual was reached. In total, 153 babies took part in the trial (20 twin pairs) and the parents of 210 babies were approached for the trial.
Procedure for recorded trial discussions
Practitioners facilitating RECRUIT sought permission to audio-record the trial recruitment discussions from families whom they approached for their trials. Families often had more than one discussion about the trial but only one was recorded. If permission was declined, the audio-recorder was not activated and the trial discussion progressed as normal. If permission was given, the practitioner activated a digital recorder. The practitioner briefly described RECRUIT, gave parents and young people the RECRUIT PIL and asked the family for permission to pass their contact details to the RECRUIT team. A member of the RECRUIT team subsequently discussed the study in full with the family and sought their consent to participate, explaining RECRUIT’s independence from the trial and clinical team. Audio-recordings of the trial discussions were released to the RECRUIT team only after the written consent of participants had been obtained. If the family declined RECRUIT, the recorded trial discussion was erased. Where families were approached without a recorded trial discussion, the practitioner described the RECRUIT study and asked for permission to pass the family’s contact details to the RECRUIT team.
Procedure for interviews
Parents
We asked parents to describe the trial discussions from their perspective, how they felt about the encounter, the recruiter, the written and verbal information exchanged, whether there was anything that was unclear or surprising about this information, and whether anything might have been handled differently. Each interview was informed by the interviewers’ detailed knowledge of the features of the particular trial concerned. The interviewers used this knowledge to develop specific prompts about, for example, what the trial involved for families such as hospital visits, or about the trial run-in period.
Parents were also prompted about (1) other (unrecorded) experiences of family–practitioner discussions about the trial; (2) their broader views on clinical research involving children; (3) their prior knowledge and experience of such research; (4) how they saw trial participation in the context of the child’s illness and future well-being and their relationships with health professionals; and (5) how trial decision-making was negotiated within the family. We consulted with the RECRUIT steering group, which included two parent representatives, on the content of the parent and practitioner prompt guides. We also consulted with the study steering group and MCRN young persons’ advisory group on the prompt guides for young people.
Young people
Where possible we interviewed parents and young people separately. This was to avoid the difficulties of interpreting individual experiences from data collected at joint interviews, which may be particularly difficult in the case of young people interviewed in the presence of their parents.
Individual interviews with young people followed the same topics as those with their parents, although the number and complexity of prompts was greatly reduced, particularly for younger participants (7–10 years). We explored young people’s feelings about particular aspects of the trial and the extent to which they felt involved and able to influence trial decision-making.
Prior to the interviews, we usually consulted with parents about how best to approach interviewing their child and to select a time for the interview that would be convenient for the young person. Where possible we met with the young person some days before the interview so that we were not complete strangers to them by the time the interviews took place. On the day of the interviews we spent some time building rapport with the young person before starting the interview and we used various activities to conduct the interviews in a developmentally appropriate and child-friendly way. With younger children we sometimes used a ‘spidergram’ to find out about their likes and dislikes prior to starting the interview. Here the young person is shown a picture of a ‘friendly’ spider’s body and together with the interviewer they suggest four likes and four dislikes, making up the spider’s legs. To demystify and allay any potential fears that younger participants might have had about the audio-recording we encouraged them to listen to a short sample recording that was made during the warm-up questions if they wanted to, and help to switch on and off the digital recorder.
Interviews with older children and adolescents were very similar in style to the parent interviews and, where appropriate, followed the same prompt guide or one that was slightly simplified. However, with younger members, if they chose to, we introduced picture cards so as to vary the question-and-answer format; this also provided a shared reference point for the young person and the interviewer and avoided the young person potentially feeling ‘in the spotlight’ by an exclusive focus on him or herself. Each card had a familiar picture on one side, and a question on the other. These were spread on the floor and the interviewer asked the young person to find the card with a certain picture on it. Depending on the individual’s preference and reading ability, the young person or the researcher would ask the question out loud. The researcher then used additional prompts to explore the topic further. When the topic was finished, the young person could choose a sticker to place on the card to mark it as complete.
Irrespective of their age or developmental level, for all of the young people we took particular care to emphasise that:
-
their responses to the questions would not get anyone into trouble
-
there were no right or wrong answers and we were interested in their thoughts and feelings.
Practitioners
Interviews with practitioners followed a similar course to the parent interviews, but we steered these using a separate topic guide. We prompted practitioners to (1) contrast their experiences of this trial discussion with their experiences of other trial discussions [although, as we describe later (see Other clarifications and changes to the methodology), this was often not possible]; (2) describe their experiences of families who declined and those who agreed to trials; (3) describe what information children and parents required to inform their decision-making, and their experiences of managing the involvement of each party; (4) describe how they responded to the needs and requirements of families; (5) describe their experiences of deciding which families to approach, including their accounts of deciding not to approach eligible families; and, finally, (6) compare their role in approaching families about trials with other aspects of their role as clinical practitioners or researchers.
General interview and transcription procedure
The pace, sequencing and duration of all the interviews were shaped by the participants. All participants were given the opportunity to ask questions before and after their interviews. We audio-recorded and then transcribed all of the interviews, reviewed the transcripts for accuracy and pseudoanonymised them. Transcription was verbatim, recording all words spoken, as well as hesitation and overlapping speech; for the recorded trial discussions we also recorded markers of disfluency. We continually reviewed the transcripts from trial discussions and interviews, as the study was ongoing, to develop the prompt guides and thereby ground and inform subsequent interviews. VS and ES conducted all of the interviews and kept reflexive notes to record systematically the contextual details of the interviews and to inform the analysis.
Analysis
Analysis was interpretative, followed the general principles of the constant comparative method126,127 and was informed by several procedural steps to ensure its quality. 128–131 VS led a process of ‘cycling’ between the developing analysis and new data in consultation with BY. The complete team developed and tested the analysis by periodic discussion, based on detailed reports of the analysis containing extensive data extracts.
Initially, a member of the team read each transcript several times before developing open codes to describe each relevant unit of meaning. VS usually did this, although ES and BY also contributed. Broadly, interview transcripts were analysed for evidence of the families’ experiences, needs and priorities when approached about a trial, with reference to their different trajectories in relation to the trials; for practitioners we analysed for evidence about their goals in discussing the trials with families and how they responded to families’ cues. Through comparison within and across the whole transcripts, VS, ES and BY developed the open codes into theoretical categories and subcategories to reflect and test the developing analysis. VS organised the categories into a framework to code and index the transcripts using nvivo software (QSR International, Doncaster, VIC, Australia), and VS, ES and BY continually checked and modified the framework categories to ensure an adequate ‘fit’ with the data in relation to the interview as a whole, as well as the proximal content, while also accounting for deviant cases. A second member of the project team checked the categories and the assignment of data to them. A record was kept of the analysis process, including definitions of the categories and their application.
We initially focused on participants’ expressed accounts whether in trial discussions or interviews. However, as the study progressed, we interpreted the accounts to theorise beyond what participants made explicit, for example we considered participants’ overall stance in their interviews and what they chose not to focus on in their responses as well as what they did focus upon.
We linked transcripts from trial discussions and interviews to compare our observations of exchanges during trial discussions, with families’ interpretations and experiences of these exchanges. Thereby we compared the ‘look’ and ‘feel’ of the trial discussions. In this way, we also identified discrepancies between interpretations and observations to inform our analysis of how communication could go awry and lead to misunderstandings. We produced summaries of the verbal interactions during the trial discussion to capture the ‘look’ of these in terms of parents’ and young people’s spoken interactivity. We calculated the proportion of speech and number of questions asked by parents and young people as indicators of their ‘observed’ level of interactivity, and recorded the frequency of practitioners’ use of open and closed questions. We particularly focused on practitioners’ invitations to elicit parents’ thoughts and questions about the trial and explore their understanding.
In the following results chapters, extracts from parents’ interviews and trial discussions are followed by identification codes beginning ‘F’, those from practitioners beginning ‘P’. Where extracts from trial discussions are included this is indicated in the text and the parent’s identification code beginning ‘F’ is used. When the extract is from a young person, the codes identify their age as 8–10 years, 11–14 years or 15–16 years. For all extracts, square brackets containing three dots […] indicate short sections of omitted speech; square brackets containing text indicate explanation added during transcribing or analysis, usually to replace a name; double parentheses signify expressions such as laughter. While original transcription recorded hesitation, overlapping speech and disfluency, for ease of reading we edited out most of these markers from the excerpts that we present in Chapters 3 and 4.
Sampling of trial discussions, families and practitioners and participant characteristics
Trial discussions
In order to link trial discussions and interviews, the sample of families interviewed largely comprised those for whom recorded trial discussions were available. However, during the course of the study we also included families for whom there was no recorded trial discussion but who had potentially informative experiences of recruitment. The recruitment of families is summarised in Figure 1, which shows the numbers of families with and without recorded trial discussions.
FIGURE 1.
(a) Recruitment of families with recorded trial discussion to RECRUIT, showing families’ trajectory in relation to the trials. a, Five families with recorded discussions from TIPIT were not approached for interview. Two had transferred to another hospital before they could be approached. Three babies had died; the RECRUIT steering group advised the team not to approach bereaved parents. (b) Recruitment of families without recorded trial discussion to RECRUIT, showing families’ trajectory in relation to the trials.
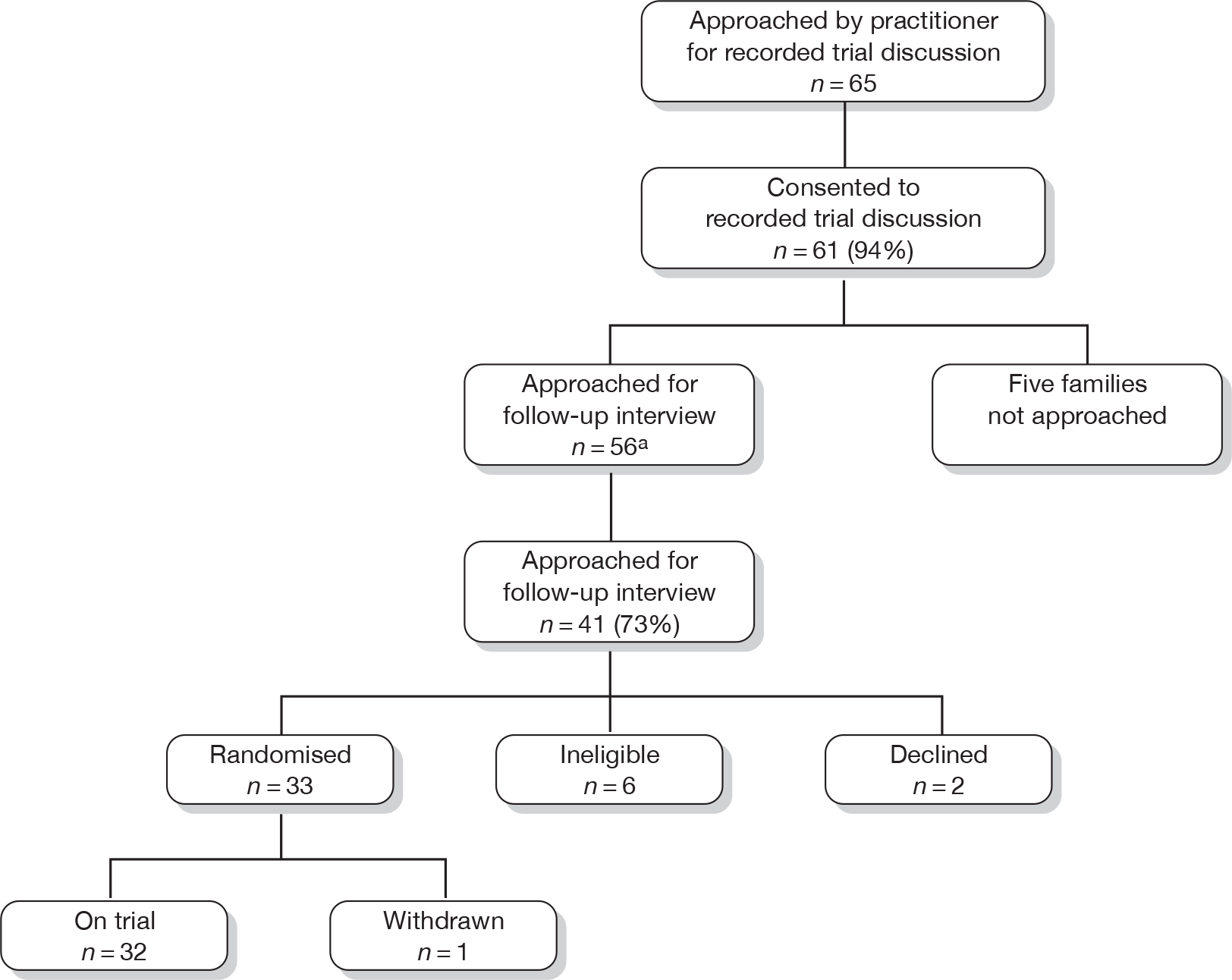

Families
We interviewed 84 members of 60 families: 58 mothers, 4 fathers and 22 young people. Figure 1 illustrates the recruitment process, recruitment rates and families’ trajectories in relation to the trials, i.e. those who consented to the trial, declined, were ineligible or withdrew. Table 2 shows family demographics and trial participation status for each of the four trials. Each trial had an initial target to enrol 15 families in RECRUIT. The MENDS trial reached this target in January 2009 and from that point we purposively sampled only those families who declined MENDS, as decliners were under-represented in the wider RECRUIT sample. Families who declined MENDS did so before attending a clinic appointment and, as such, no recorded trial discussions were available for them.
| MASCOT | MENDS | POP | TIPIT | |
|---|---|---|---|---|
| n (% of total 60) | 10 (17) | 22 (37) | 16 (26) | 12 (20) |
| n | ||||
| Randomised | 5 | 9 | 13 | 11 |
| Declined | 1 | 8 | 0 | 1 |
| Ineligible at consent | 1 | 1 | 0 | 0 |
| Ineligible after run-in | 3 | 3 | NA | NA |
| Withdrawn | 0 | 1 | 2 | 0 |
| Median (range) age of child (years) | 10 (8–14) | 7 (4–13) | 13 (4–16) | – |
| n (%) not self-identified as white British | 0 | 2 (9) | 1 (6) | 6 (50) |
| n (%) Index of Multiple Deprivation132 quintile | ||||
| Lowest | 5 (50) | 15 (68) | 2 (13) | 9 (75) |
| Highest | 2 (20) | 1 (5) | 2 (13) | 0 |
| Doctorsa | 2 | 4 | 3 | 5 |
| Research nurses | 4 | 3 | 4 | 1 |
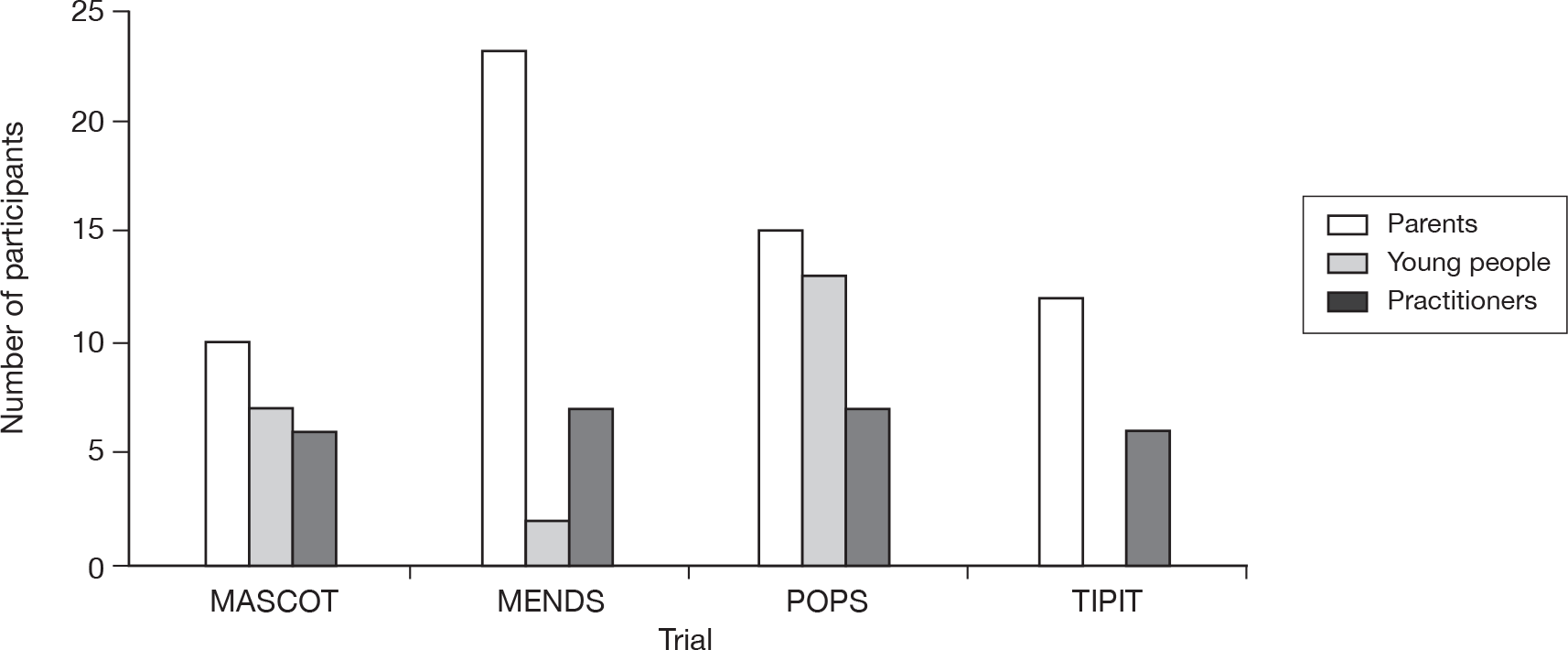
In total, 22 young people were interviewed, with the permission of their parents. [Through the remaining chapters of this report, we usually use the terms ‘young person/people’ in preference to ‘child/children’ unless the term child/children is used by the parent or patient themselves or is necessary for accuracy (e.g. when referring to the child of a parent).] Our protocol stated that, in the case of children < 7 years old, only the parents would be interviewed, as very young children are more likely to experience difficulties in articulating their experiences in interviews. Of the 22 young people interviewed, two were from MENDS, both of whom were aged 9 years, seven were from MASCOT, with a median age of 10 years (range 8–14 years), and 13 from POP, median age 13 years (range 8–16 years). We did not interview any 7-year-olds. Figure 2 shows the number of parents and young people interviewed by trial.
FIGURE 2.
Number of parents, young people and practitioners by trial. We interviewed 84 members of 60 families (58 mothers, 4 fathers and 22 young people). In one MENDS family and two POP families, both parents were interviewed. In one POP family, the parent declined to be interviewed but the young person was. Five doctors were also interviewed who regularly recruited to trials but were not recruiting to the four participating trials.
Interviews with families took place a median of 42 days after the recorded trial discussion (range 14–126 days). Table 3 shows the time lag between the recorded discussion and the RECRUIT family interviews by trial. We interviewed 48 of the 60 families in their homes, nine at the trial site and three by telephone interview. Interviews with parents lasted approximately 45–60 minutes and those with young people lasted approximately 15–30 minutes. Of the 22 young people interviewed, eight chose to be interviewed with one of their parents (sometimes for pragmatic reasons if the interviews were being conducted in the hospital). An interview with one young person was excluded from analysis as it contained no content relating to the trial.
| MASCOT | MENDS | POP | TIPIT | |
|---|---|---|---|---|
| Median (range) days between recorded discussion and interview |
35 (30–69) n = 6 |
38 (14–69) n = 15 |
42 (14–119) n = 8 |
52 (20–126) n = 12 |
Practitioners
We interviewed 31 practitioners: 12 were research nurses who were part of the trial teams for one of the four trials and 19 were doctors (14 of whom were also members of the trial teams). Twenty practitioners were directly involved in approaching the families who participated in RECRUIT; of these, 11 had audio-recorded trial discussions included in the study; nine were directly involved in approaching families within the study but were not present during or did not lead the recorded trial discussion. A further six were directly involved in recruiting families to the participating trials but had not been involved in the recruitment of specific families within this study. The remaining five practitioners did not recruit families to any of the participating trials, but we interviewed them because they had considerable experience of recruiting families to other trials. These were practitioners who responded to a request at one of the participating centres inviting those with extensive experience of recruitment to children’s trials to be interviewed. Table 2 and Figure 2 show the numbers of practitioners by trial, alongside those for families. The majority of interviews with practitioners were conducted at the trial site and lasted approximately 45–60 minutes.
Changes to protocol
The North West 5 Research Ethics Committee (REC) approved the study on 2 March 2007 (ref. 07/MRE08/6). The study was subsequently given R&D approval to run in 10 Trusts (seven hospital, three primary care). Two amendments were made to the protocol during the study, both of which aimed to increase access to data or participants.
The REC approved a substantial amendment to the protocol on 28 October 2008:
-
Under the original protocol, families gave verbal permission for the practitioner to audio-record the trial discussion. Full written consent for the release of this recording to the RECRUIT team was sought at the same time as consent to be interviewed for the RECRUIT study. The original version of the protocol required these recordings to be destroyed if the family declined the RECRUIT interview. The substantial amendment allowed us to seek separate written consent from families who declined RECRUIT interviews to include their recorded discussions in the analyses, hence avoiding the unnecessary destruction of potentially valuable data.
The REC approved a second substantial amendment to the protocol on 24 February 2009:
-
The amendment allowed us to post an invitation letter and information sheet to families in certain circumstances, rather than relying exclusively on a personal approach by a practitioner. Invitation letters contained a reply slip and postage-paid envelope, which interested families returned directly to the RECRUIT team. Recruiters to the trials could therefore identify and post invitation letters to eligible families.
This approach was to be used if, for example, RECRUIT had not been discussed with the family when they were approached about a trial, where the trial discussion was held prior to the RECRUIT study opening at a particular centre. We were particularly keen to explore if this method would allow us to access more decliners. For example, in the MASCOT trial the approach about the trial was itself made by a postal invitation letter. We sent a RECRUIT mailshot to 100 families from three general practices who did not respond to the original MASCOT mailshot and hence had declined the trial. However, we abandoned this strategy when we received no responses from families who had declined MASCOT and wished to participate in RECRUIT.
Other clarifications and changes to the methodology
-
Compared with other qualitative studies, RECRUIT was a large and complex study. It had 156 data collection points and required numerous additional visits and discussions with trial teams. The data consisted of approximately 111 hours of audio-recording, which produced an estimated 2200 pages of transcript. Simultaneously co-ordinating the study across four trials and 11 trial teams, obtaining R&D approval and conducting the data collection and analysis was a challenging task. At RECRUIT’s outset it took some time to win the confidence of the trial teams. Because RECRUIT was to run contemporaneously alongside the trials rather than retrospectively, it also took several months to clarify with the National Research Ethics Service and the Trust R&D departments the nature of the relationship between the trials and RECRUIT. It was eventually established that RECRUIT was a separate entity to the trials, rather than an add-on or nested study. This clarification was fundamental to RECRUIT’s progress. Owing to the slow recruitment and delayed starts to some of the trials (e.g. one trial was delayed by > 1 year), and delays in obtaining R&D approvals, we were not able to concentrate sampling at particular sites in time-limited blocks as planned. Because of these delays we were unable to use maximum variation sampling and we pragmatically used a mix of purposive and consecutive sampling instead. Delays to the trials also resulted in uneven recruitment between trials.
-
The protocol stated that most RECRUIT interviews would be conducted within a few days of the recorded trial discussion and within an outside limit of 2 weeks. This element of the protocol was not acceptable to three of the four trial teams. In MENDS and MASCOT there was a run-in period, meaning that trial discussions were recorded 4 weeks before randomisation. To avoid any actual or perceived impact on recruitment to the main trial, it was necessary to delay interviewing families until after the randomisation appointment. In TIPIT, discussions were recorded within days of the birth of a critically ill premature baby. The mother was often unwell herself. Mothers were not approached by the RECRUIT team until the TIPIT teams were happy that mother and baby were stable. In many cases this entailed a lag of several weeks between the trial discussion and RECRUIT interview.
-
For each trial our goal was to enrol approximately equal numbers of families who consented to the trial and families who declined. This proved unachievable within the original design of the study, which relied on practitioners introducing RECRUIT to families during the face-to-face trial discussion. We found that for all trials participating in RECRUIT except TIPIT, families who attended a face-to-face trial discussion almost always consented to the trial. Families who declined often did so prior to this discussion in a number of ways: by not responding to written invitation, by telephone or by not attending an appointment. Throughout the study we sought every opportunity to recruit families who declined, and this necessitated several changes to our methodology, some of which required ethical approval. These in turn brought delays which further hindered our efforts to recruit declining families. In the case of TIPIT, the REC amendment (see Changes to Protocol) was sought to access declining families where RECRUIT had not been discussed with the family when they were approached about the trial because the trial discussion was held prior to RECRUIT opening at a particular centre. However, practitioners felt unable to send letters to families who were no longer in their care, and this route for accessing TIPIT decliners was therefore impossible to implement. We explored alternative ways of enrolling participants who declined the trials without a face-to-face discussion. These involved approaching families who (1) declined during a telephone call with the research nurse prior to the main trial discussion; (2) declined to be referred to the trial team when the study was initially mentioned to them by the community paediatrician; and (3) did not respond to an invitation letter from their GP regarding the MASCOT trial. Of these three strategies, only the first was successful, yielding 11 trial decliners who expressed an interest in RECRUIT, seven of whom entered the study.
-
We initially intended to link practitioners’ interviews to the specific trial discussions that they recorded with families participating in RECRUIT. This proved to be impractical as some practitioners recorded several of their trial discussions, while others were unable to draw to mind a particular discussion. Instead, we adapted the interview topic guide. For example, the interviewers prompted practitioners to describe specific trial discussions that they felt exemplified the ‘easier’ and the ‘more difficult’ trial discussions.
Chapter 3 Analysis strand 1: communication about trials as observed and experienced
Summary of objectives
This chapter is about the way in which families were approached to consider a clinical trial. Using data from audio-recorded trial discussions and interviews with parents, young people and practitioners, we compare the experience of trial recruitment for these groups with the conduct of the discussion itself.
As well as the linked trial discussion and interview data, the analysis also includes interview data from the 20 parents (of 19 children) and nine young people for whom we had no recorded trial discussions but whose insights add to our understanding of the experience of being approached to take part in a trial. These included families who declined or withdrew from the trial and the young people for whom we had no recorded trial discussion.
This chapter is organised into several sections. First, we discuss the features of communication, specifically the percentage of speech contributed by the parents and young people and the number and type of questions each party asked during the trial discussion. We also examine parents’ misunderstandings of the trial discussions and trial methodology. In the second part of the chapter we bring in data from interviews with parents and young people (including those for whom we had no recorded trial discussion) to discuss the trial approach ‘as experienced’ by all parties, the function and value of the PIL, and the impact of different types of relationship with the trial team on the recruitment experience. Finally, we present some suggestions from parents and young people on ways to improve the trial approach.
Communication as observed
Parents’ interactivity in the trial discussion
A striking finding from the trial discussions was that parents’ observed level of interactivity was generally low – the median percentage of speech by parents was 16% and ranged from just 1% to 49%. We categorised the discussions into three groups according to the amount of speech contributed by the parents: those discussions where parents contributed ≤ 10% of the speech (n = 12), those where parents contributed 11%–24% (n = 16) and those where parents contributed 25%–50% of the speech (n = 13) (Table 4).
| Group | Low interactivity | Medium interactivity | High interactivity |
|---|---|---|---|
| n | 12 | 16 | 13 |
| Median percentage speech by parent (range) | 5 (1–7) | 16 (11–21) | 29 (25–49) |
| Median number of questions (range) | 1 (0–4) | 2 (0–6) | 1 (0–7) |
| Mean duration of discussion (SD and range) | 8.5 minutes (3.48, 5–16) | 13 minutes (6.21, 5–23) | 13 minutes (5.46, 6–19.5) |
| No. in which more than one practitioner present | 3 | 9 | 11 |
| No. in which more than one parent/adult present | 1 | 3 | 3 |
| No. in which young person presentb | 6 | 10 | 12 |
Typically, a ‘low-interactivity’ discussion was the first time that a family had heard about the trial in any great detail – sometimes it was the first time the trial had been mentioned to them – and its purpose was to impart information. Often families had not received the information sheet in advance. In three cases consent was taken after one of these discussions. These discussions, which were usually very brief, typically began with an explanation of the rationale for the trial and moved on to cover the key procedural and methodological aspects of the trial. Practitioners presented trial information in a systematic way and did not actively try to involve the parent. A frequent goal of these discussions was to set the scene for future discussion, and invariably they concluded with the practitioner encouraging the parent to read the PIL and discuss it with his/her family.
Practitioners rarely asked families any questions other than to gain required information, such as the date of next appointments or discharge, although occasionally they asked questions pertinent to the trial which might have allowed parents to influence the direction of the discussion, for example ‘do you know much about thyroid at all?’ (F10). It was relatively unusual for practitioners in these discussions to elicit parents’ thoughts or questions about the trial, although 6 out of 12 discussions had at least one such instance, for example ‘That’s a lot of information so far. Have you any questions you want to ask me?’ (F42). (The 41 recorded trial discussions were made by 16 practitioners. The 12 discussions in which parents contributed ≤ 10% of the speech were recorded by five different practitioners.) Practitioners in this group often imparted a ‘chunk’ of trial information and then loosely checked back with the parents for confirmation that they were happy with what they had been told, often in one word such as ‘alright?’ or ‘OK?’. These questions generally elicited one-word responses from parents, such as ‘OK’, ‘right’ or ‘yeah’. For some parents, these responses formed the main content of their turn taking in the discussion. Practitioners made few enquiries to explore whether the parent had understood the information, this being the case in only 3 out of 12 discussions, for example ‘Obviously there’s a one in two chance he may get the dummy medicine. Um, do you understand that part?’ (F29).
For ‘high-interactivity’ discussions, parents had typically received the PIL prior to the discussion and had taken part in at least one brief conversation about the trial prior to the recorded discussion. Where the parent had received the PIL in advance, practitioners often used it as a starting point for the discussion and to offer parents the opportunity to ask questions at an early stage (n = 6), for example ‘You’ve had the information sheets […] about the study? And what did you think of that when you, you read through it?’ (F18). In five of the six discussions that began in this way, the practitioner also asked questions later in the discussion, for example ‘so, reading over it, were there any concerns you had?’ (F20).
When the parent had not read the PIL in advance, the discussion was initially much closer in style to those of the low-interactivity group, but, over time, it gradually became more interactive as the focus shifted to the child’s condition. In all four discussions, the practitioner asked questions that appeared to be aimed at exploring the parents’ understanding, but because the discussion had not been preceded by a written information sheet, the questions were necessarily more closed, for example ‘In a nutshell, does that make sense?’ (F3), ‘Are you with me so far?’ (F2).
In all 13 of these high-interactivity discussions, the practitioners asked parents lots of questions. These were almost all related to the child’s condition and seemed aimed at establishing eligibility rather than eliciting parents’ thoughts or questions about the trial. Although the agenda was always set by the practitioner, the parents in this group were more able to influence the direction of the discussion because they had valuable input about their child’s condition to contribute. Conversation directly related to the trial was dominated by practitioners.
Irrespective of their level of interactivity in the trial discussion, parents asked few questions. Ten families asked no questions at all and a further 13 asked just one. Questions asked by families were most often concerned with the practical and procedural requirements of the study (n = 37) although questions relating to safety and potential side effects (n = 16) and to clarify aspects of methodology and rationale (n = 17) were also common. Parents rarely asked questions relating to potential benefits to their child (n = 4). Examples of the types of questions parents asked are shown in Box 1.
‘Is there not a chance that it could go wrong and something could harm, harm him or something like that?’ (F41)
Practical and procedural
‘Does it stay on all day, or is it just on the evenings?’ (F15)
‘And would it be mouth form or injection?’ (F25)
Methodology and rationale
‘[At] the end of the study? Do we get told whether she had the dummy?’ (F17)
‘What is it meaning by here, to be randomised?’ (F22)
Benefit to child
‘What my question is, if they say he’s gonna take the placebo, […], the dummy one what is he going to benefit from the study?’ (F32)
Practitioners always gave reassurances on safety and absence of procedures that might be distressing to a child, irrespective of the level of a parent’s interactivity in the discussion, for example:
All the treatments that we are using in the study are licensed in children of [your child’s] age […] They’re all fully available so there’s nothing new or unusual about them.
(F48)
But if she wasn’t having bloods normally she wouldn’t give bloods for the study.
(F37)
It is therefore possible that in some cases practitioners’ reassurances pre-empted parents’ questions and contributed to their relatively low number.
Mismatches and misunderstandings
In this section we present several cases in which parents appeared to have misunderstood some aspect of the trial. We identified potential misunderstandings by reading the parent interview transcripts several times to search for descriptions or explanations that were not consistent with the trial rationale or methodology. We then cross-referenced these with the transcripts from the recorded trial discussions to examine whether the source of the misunderstanding could be potentially linked to the trial discussion. In most cases no direct link could be identified; however, in a few cases it was conceivable that a misunderstanding could have had its origins in the trial discussion, although other sources cannot be excluded. These misunderstanding cases are intended to indicate topics that might warrant particular attention from practitioners when recruiting families. An important aspect of our interviews was that they were led by parents and their beliefs. We explored their beliefs or misunderstandings sensitively as our aim was to describe parents’ experiences in a way that avoided influencing, ‘testing’ or altering their beliefs about the trials.
Parents often made general statements about having experienced a sense of misunderstanding and confusion that they linked to their emotional situation at the time of the trial approach (e.g. F12 and F25). Three parents in the neonatal trial told us that they were comfortable with the limitations of their understanding because they felt that their baby was in safe hands (F41, F45 and F46). However, we have identified several possible cases in which parents had specific misunderstandings had the potential to influence families’ decisions on trial entry.
A number of parents, particularly from the TIPIT and MENDS trials, had some degree of misunderstanding linked to equipoise and/or the rationale for the trial. In TIPIT particularly, a number of parents did not seem to recognise that the trial had been designed to test whether or not the trial medication was beneficial to that group of babies; rather they saw the trial as an opportunity to receive a medication that would be of benefit to their child, and which was otherwise unavailable to them.
Her only real way of getting it is to go and take part in the trial because then, I’ve got sort of a 50/50 chance of either she gets the drug or she gets the placebo. But she wouldn’t be getting it otherwise.
(F21)
These ‘therapeutic misconceptions’ represent a misunderstanding of the rationale of the trial but do not represent a direct misunderstanding of what parents had been told; practitioners often began the trial discussions by mentioning previous positive research. Therefore, the framework for parents’ understanding was that they had a 50 : 50 chance of ‘getting the right medication’ (F31). Indeed, the practitioner’s use of the term ‘I’m afraid’ in one trial discussion might have indicated to the parent that the practitioner was not in equipoise.
So we are running this study for the last year now, where I’m afraid half the [babies] get the supplement and half the [babies] doesn’t get it.
(F29)
In the MENDS trial, parents most common area of ‘misunderstanding’ also comprised a general lack of equipoise. Additionally, parents were often under the impression that the trial medication was not available outside the trial. One family told us:
We’d already made our mind up that we were going to. Before we’d even got the information […] we just weren’t getting sleep […] it’s like, we have to do something.
(F22)
Like a number of other parents, they entered the trial as a means, albeit randomised, of accessing the trial medication ‘in order to get that tablet he has to participate in the trial’ (F32). In fact, the trial medication was regularly prescribed outside the trial; in the trial discussion with one of these families the practitioner explained:
We’ve been using it for […] ten years or so […] and the whole idea of this study is to do it properly and to get the proof that it works so that we can use it more widely and for many more doctors to prescribe it, if it, if it is successful.
(F22)
This potentially sent mixed messages about the efficacy and availability of medication to parents who, in some cases, had asked for help and felt that trial participation was the only option open to them.
A few parents were confused about randomisation. Here, a practitioner explains randomisation to a parent during the trial discussion:
We will randomise [your son] into the study […] which means there’s a lottery as to whether he gets the trial medication […] or a thing called placebo dummy […] and they look exactly the same […] and no one knows which one contains [trial medication] and which one contains placebo.
(F1)
But in her interview the parent wondered whether the allocation of medicine might be more individually tailored:
You do kind of think ‘I wonder who does actually make the decision, who goes on what and who doesn’t’. I mean, do they actually look at all the information before they decide who goes on what?
(F1)
A second parent seemed to grasp the concept of randomisation in the trial discussion:
And by randomised, what we mean is that we just randomly pick one. So it’s almost like a sort of, it’s usually a computer program that just sort of.
A lucky dip?
Yeah it is exactly like that. Like a lucky dip […] so it’s almost like just, you know, saying well we just picked this out of a hat and [your son]’s got this treatment.
However, this parent’s comments in her interview indicate that she had misunderstood the explanation to mean that the drugs to be studied would be selected at random:
A bit wary, […] because I thought, ‘Oh what’s, just going to give him some random tablets or anything’.
(F48)
For one parent (see Case 1, below), a relatively straightforward misunderstanding of the procedures involved in the trial led her to decline to participate in the trial because she felt it would cause distress to her child. While the mother did not say that she would be put off research in the future, this type of misunderstanding has the potential to leave parents with negative views about research as well as having an immediate adverse impact on trial enrolment.
[Doctor] told me, I think, they’re going to put like kind of a small tube inside him, so […] I remember after that, I just hated this idea of the tubes were going to go in! So I don’t remember why they was putting the tube. I think they were studying something, but I just, I just didn’t like the idea from the beginning so I didn’t give it more attention. (F34)
The trial medication was to be given through lines already in use to feed the baby, but this was perhaps insufficiently explicit in the trial discussion:
The hormone is given through err … an infusion. It’s called err a syringe pump basically, which is, you know, puts medicine to the veins. Um and we will give it once a day.
A further layer of complexity in this case is that in the trial discussion the parent gave no verbal indication to the practitioner that she was unclear or unhappy with what had been said, she simply responded ‘OK’ to the practitioner’s statement. Therefore, her misunderstanding was not explored or corrected. The parent later declined the trial citing her strong feelings about the ‘tube’ as the reason.
Interestingly, the practitioner clarified later in the discussion:
All these procedures won’t cause distress to your baby […] if your baby is not getting fully milk feeds, then we will use the lines. Once a baby is going on to full or milk feeds, then we will give drops and things […] so it won’t cause any distress.
However, the practitioner gave this clarification some time after the initial discussion of the trial medication. As the mother explained in her interview, she simply ‘switched off’ after the practitioner had first mentioned ‘the tube’ so she probably did not hear this clarification.
Other examples of ‘misunderstandings’ could perhaps be better thought of as ‘positive projections’ whereby the parent had constructed the trial around what they ‘wanted’ or hoped from it, rather than what the trial had been designed to investigate. This is illustrated in Case 2, below, where the parent believed that her child’s steroids would be reduced as part of participation in the POP trial. The parent’s perception of the trial was some distance from its actual purpose. Such examples can be distinguished from situations in which parents believed that the trial medication would benefit their child. While strictly speaking this is also a misunderstanding of the trial’s rationale, such a misunderstanding is arguably not so far removed from the purpose of the trial, which although designed to assess the efficacy of the trial medication, would not be conducted unless there was reason to believe that it might offer benefit above standard treatment.
This mother (F20) believed that participation in the POP study meant that her child’s steroids would be reduced, whereas the trial’s rationale was to compare two means of preventing and treating steroid-induced osteopenia. Steroid reduction was not part of the trial (although this could happen as part of normal clinical care).
And I just thought, I asked her [daughter], did she want to try something like that. She said ‘Will it mean coming off steroids?’ and I said ‘Yeah’. But they won’t take her off them like straightaway; they reduced the amount and all that.
In this particular case there is a section in the trial discussion that may be linked to the parent’s misunderstanding:
Um did you understand how long it was going on for, [young person’s name]? Did you see how long the trial is for?
It’s 12 months isn’t it?
For, 12 months, that’s right.
Yeah.
Now that doesn’t mean you’ll be on steroids for 12 months, OK ((laughs)).
((laughs)).
Big sigh of relief, OK. What it does mean is that we think you’ll be on steroids for at least three months, OK? And that’s because even if we were to carry on reducing your steroids, depending on how you are, we’d have to do it slowly over time, OK. But the effects of steroids on your bones are very long term.
In the interview this mother had stated that her child was unhappy about taking steroids and wanted to stop them. From the section above it is possible to see how the misunderstanding linking the trial and the steroid reduction might have arisen if that was what a parent very much wanted to hear. This confusion may have been avoided if the discussion had begun with clarification of what would constitute normal clinical care in this situation, regardless of the option of trial participation.
The importance of practitioners’ continued careful exploration of parents’ understanding is highlighted in Case 3 (F28). We have no recorded trial discussion for this family (F28) so it is not possible to trace the particular source of the parent’s misunderstanding, and the trial discussion had been some months before the interview. However, the excerpts from the parent’s interview show an important misunderstanding of the trial methodology: the mother describes how pleased she is that her child is ‘just on a placebo thing’ and not taking any more medication even though the trial was blinded.
She just explained that he’d be on some meds and it’d last for it was only for a year. And these meds what he’s on for it […] he’s just on a placebo thing.
She explained that there’s like a proper meds […] I think there’s four different ones and two of them are a placebo […] anyway, he ended up being on one of the placebo ones. So it weren’t, it weren’t too bad.
[The nurse] just said like all forms had been done and that and they’d been sorted. They’d pluck out what’d be best for [my son] to go on.
I felt better, because he weren’t having to take any more tablets. Because it just seemed like, for somebody so young to be on so much medication, it’s like no youngster should have to go through it.
In a second similar case, the mother appeared to be unaware not only of the rationale of the trial, but also of the fact that her baby was involved in research at all – a misunderstanding that was compounded by language difficulties (Case 4).
This case involved a mother (F39) whose first language was not English (which also made it difficult to interview her). The mother seemed very unclear that her baby was actually involved in a trial. While she was very clear that she had been given a choice and asked to make a decision (‘I can say yes and I can say no. The decision is mine’) from her interview it appeared that she thought that she had decided to have the treatment that the doctor had recommended ‘he explained there are many children have it before it’s very, very good […] they just want to seek my consent before’ rather than enter a study where there was a 50% likelihood that her baby would have received a placebo. She seemed very unclear about research as a concept, as the following excerpt from her interview highlights:
Was the term ‘research’ mentioned at all?
I’ve not heard about that.
No. Did he say it was research, trying to find out things?
He might have said. Maybe I forgot. You know, is it a medical term you’re using now?
The mother hardly spoke in the trial discussion so the practitioner had little evidence regarding her understanding. At several points during the trial discussion, which lasted < 6 minutes, the practitioner paused and asked ‘OK?’ to which the mother responded ‘OK’ or ‘uh-huh’. However, the practitioner did not ask any open questions or enquire if the mother had any questions of her own. The practitioner generally focused on a previously published trial with positive results, before leading into a description of the current trial, which he or she described in less detail. The mother’s language problems may have made it difficult for her to separate the current trial from the previous one.
Young people’s interactivity in the trial discussion
Of the 41 recorded trial discussions, 12 were from the neonatal trial and one was conducted between the parent and research nurse by telephone. Young people were present in the remaining 28 discussions; 12 of these young people took part in interviews for this study. A further nine interviews with young people are included where there was no trial discussion.
Young people said very little in the 12 recorded trial discussions. Two said nothing at all and a further three said just one, two or four words. The remaining seven uttered between 1% and 4.5% of the total words spoken (calculated in the same way as their parents’ speech had been). One young person who did not speak in the recorded discussion observed ‘I don’t think we really said anything. I think it was just [the nurse]’ (F35, 15–16 years).
Few young people asked any questions during the trial discussion. Parents’ accounts suggested that when young people had questions they tended to direct them to their parents after the consultation. However, two young people did ask one question each. Both were considering entry to the POP trial. Their questions were ‘Are they big tablets?’ (F60, 11–14 years) and ‘Even though one of them might not work for the bones and things will it do some good for me?’ (F49, 11–14 years).
Interestingly, practitioners did invite young people’s questions; 8 of the 12 young people were asked if they had any questions. Practitioners also asked other questions of the young people, although, as with the parents, these were often closed questions, such as ‘Does all that make sense?’ (F54, 8–10 years), which prompted one-word responses from young people. Two young people were asked open questions:
Do you know what an evidence base means?
Yeah.
What does it mean?
Err, proof that they actually work.
Exactly.
(F42, 15–16 years)
Can I ask, what do you understand by randomised? […] it’s a funny word isn’t it? What, what do you think it means?
(F20, 11–14 years)
(*For labelling of young person excerpts with more than one speaker, ‘YP’ is used for the young person.)
One way in which practitioners sought to involve young people in the discussion was to direct questions about their condition (which were necessary to establish eligibility) towards the young people themselves, rather than the parents. These attempts were not always successful, however. In two of the recorded trial discussions the practitioner attempted to specifically direct questions to the child by name but the parent answered for them.
Even when a young person was unlikely to contribute much to the discussion, owing to his or her age or developmental level, a number of practitioners placed the child at the centre of the discussion by speaking directly to him or her at the beginning of the consultation and explaining what was going to happen:
My name is [doctor], I’m one of the consultants here. And do you know why you’re here today?
Yeah, because I can’t go, go to sleep.
That’s right. And there’s a, there’s a new type of medicine, which we’ve been using for a few years, but we’re doing a study to see how well it works and whether it works for everybody or not. So that’s what I’m going to talk to your mum about.
OK.
Yeah, is that alright?
(F18, 8–10 years)
Similarly, in two discussions during which neither the young person nor parent spoke a great deal, the practitioner’s comments are directed towards the young person, for example ‘your’ appointments ‘your’ condition (F42, F60). However, in 2/12 discussions no attempt was made to involve the young person at all. Both of these were the first formal discussions that the family had had regarding the trial and both were ‘low-interactivity’ discussions for the parents also.
Research nurses often had an important role in talking to young people about the trial, going through the information leaflet with them while the parent was in discussion with the doctor. In two of the trial discussions in this study, it was the nurse who enquired whether the young person had read the information leaflet and whether he or she had any questions.
In some cases young people were absent for a considerable amount of the trial discussion because they were being weighed and measured by nurses as part of the trial while the parent was in discussion with the doctor. This constrained the potential for practitioners to involve young people, as well as for the young person to contribute.
Practitioners asked five of the young people if they were happy to proceed with the trial. As with the other questions, these tended to be closed questions to check that the young person accepted participation rather than open invitations for his or her thoughts, for example ‘And are you OK with this?’ (F18), ‘Happy to move forward?’ (F42). Although 10 out of 12 discussions recorded were consent discussions, the actual consent and assent forms were not always completed while the recorder was switched on, and it is possible that the other young people were asked at the time of their assent.
Communication as experienced
Parents’ experiences of the trial approach
Parents’ interactivity in the trial discussions was often low, but they did not tell us that they felt inhibited or constrained in any way. On the contrary, parents often described positive experiences of the verbal information that they had received, and emphasised their sense of ease in asking questions despite their low interactivity.
Some explained that they had not asked questions during the trial discussion because they were happy with the information that they had received, ‘It was explained the way it was meant to be explained. Anybody could have understood it’ (F2), and because the trial team were highly accessible and they felt comfortable in contacting the team with any questions as these occurred to them, ‘most doctors wouldn’t say, ‘Here’s my mobile. Any worries, just phone me there and then’ (F48) or ‘ring her anytime’ (F36). Indeed, parents described practitioners as ‘friendly’ (F56), ‘approachable’ (F13), ‘open and honest’ (F51), ‘relaxed’ (F60) and ‘comfortable to talk to’ (F38).
No parents commented on their lack of interactivity or involvement in these trial discussions. Rather, parents emphasised their overall experience of the discussion as a social encounter and their experiences of the practitioners.
I thought it was perfect and I don’t think there is any room for improvement, to be honest. [the nurse] was totally friendly and, and thoughtful and she just, she approached us and asked us whether we would like to participate in this study. Really I, I don’t see where she could have done it in a better way.
(F3)
Parents spoke of their sense of comfort during the discussion, their confidence in, and liking for, the practitioner, and their sense that the trial was safe and that their child’s health (rather than the trial) was the practitioners’ over-riding concern. Flexibility, consideration, kindness and effort on the part of the trial team were also appreciated. Parents praised practitioners for being confident, knowledgeable and enthusiastic about the trial, and spoke of how this helped them to also feel confident and enthusiastic, and fostered a sense that the trial was worthwhile. While parents valued practitioners for conveying a sense that the trial was important, they also spoke of how their child’s and family’s well-being, rather than the trial and the need to recruit participants, was the primary concern of practitioners. Therefore, a practitioner’s focus on the young person and the difficulties of the family’s situation helped to convey a sense of confidence and safety to parents. Excerpts from parents’ accounts of the trial approach are given in Box 2.
‘I mean, as I say they were lovely people, they were really, really nice and made us feel really welcome and really comfortable and they did explain, you know, things really, really well.’ (F1)
‘It weren’t like a job to do it, she actually seemed interested in it. So I was happy with that […] she kind of cared, if you know what I mean, so.’ (F9)
‘You could see he was passionate about the, about the research and the trial, so, and that just, just aids things. It makes it more comfortable.’ (F51)
‘It was the way that they treated K3 that influenced me a lot, that […] you know, his,his opinions did matter.’ (F13)
‘I don’t understand a lot of the medical terms and things but I know it’s not harming him or anything so for me, you know, I was like “Go ahead with it 100%”.’ (F41)
‘She knew we had other things on our minds […] And she said everything I’ve said is, is in here, so don’t worry if you can’t remember what I’m saying to you, have a read of that. She was brilliant, really was brilliant.’ (F37)
Parents who declined (n = 10) were also positive about their experiences of the trial, and the majority were indistinguishable from those who consented. It was not unusual for these families to also describe the practitioner as ‘really friendly’ (F36) or ‘absolutely lovely’ (F23). Nor did these families report feeling pressurised by the trial practitioner to take part: ‘we knew we wouldn’t be pushed into something’ (F24), ‘everything in terms of mannerisms, conduct, behaviour was absolutely spot on’ (F52). However, in two cases, the parents voiced criticisms of the approach:
He was fine […] he just explained the thing. (F34) Later in the interview the same parent said No I didn’t feel pushed, but I think the place was wrong. (F34) (parent was approached on a busy ward)
He was alright. It was just the fact that I got annoyed with all the waiting and when I tried to explain to him about the [medication] he wasn’t listening at first.
(F2)
In both cases the parent had strong reasons for declining; one as a result of a misunderstanding of the practitioner’s explanation of a trial procedure and the potential for distress to her child (see Case 1), while the other believed that her child had an adverse drug reaction to the trial medication in the past.
Parents’ experiences of the timing of the trial approach
In two of the trials parents were sometimes approached when they were fearful for their child’s survival or well-being, and it was not uncommon for these parents to acknowledge that they had found it difficult to concentrate during the trial discussion. As one mother commented, ‘it went sort of like in one ear and out the other […] she was so small and so poorly’ (F46). However, when asked if the trial discussion could have been better handled this mother echoed other parents in remarking:
No, I don’t think so. The doctor was really nice, he was clear and asked if we needed, you know, if had any questions either that day or later on then, you know, just arrange to speak to them.
(F46)
Parents acknowledged the difficulty of discussing clinical trials in the circumstances they faced. However, they were generally accepting of the need for research and the need for the approach to be made.
I didn’t really have a problem with it, her coming to see me so soon […] the sooner really you get in there, it’s the better isn’t it?
(F9)
I knew they needed to start it straight away and I think even the day after wouldn’t have been no good […] The day I was coming home wouldn’t have been any better or […] I don’t think there is a perfect time for stuff like that.
(F45)
I think the doctors definitely [should] feel like they should be able to approach the parents. I definitely think that because without approaching them obviously they won’t be able to do the trials.
(F40)
Parents are sometimes asked to consider entry to more than one trial. One mother’s (F29) experience highlights the importance of paying attention to the parent as an individual and the timing of the approach, as she described very different responses to being approached about two trials when her baby was in a critical condition. The mother and father consented to both trials having evaluated the information given to them, but their experiences of each trial’s recruitment contrasted sharply (Case 5).
The approach for trial 1 (non-RECRUIT trial) had been made during labour by a number of different practitioners:
Doctors coming in every fifteen minutes, ‘Have you heard about this? Have you heard about this? Have you heard about this?’ And it was sort of going to my husband, ‘Get them out ((laughs)). I don’t want to know right now. Get them out’. (lines 155–7)
The approach for the second trial (RECRUIT trial) was made after the baby was born ‘but by that point I’d seen [my baby], I was settled […] I felt safe’. (lines 101–2)
I felt safer knowing and talking about it than what it had been with the other trial, which was sort of, ‘Here, here, here. Pamphlet, pamphlet, pamphlet […] do this, do that’. (lines 249–54)
Her recollection of the practitioners who approached her about the first trial was negative:
In the [hospital name] there is some of them doctors who all they can think about is the amount of ticks they can get for what they’ve done. (lines 811–13)
While her recollection of the practitioner who approached her for the second trial was very positive, despite the fact that she hardly spoke in the trial discussion (5% interactivity):
She was really good in how she explained things and how she handled things […] and, you know, she said, ‘If you want me to go at any time because you’re tired of things, just let know and I’ll, I’ll leave you to it’. She was really nice. The approach was a lot more well presented compared to the other trial. (lines 409–14)
I can’t fault […] the way. I mean every time we seen her she’d ask how I was, she was, it wasn’t just about, about the child, it was about the family […] which really helped. It’s not all clinical […] it’s making you know you are there, you are important […] and it helps. (lines 734–740)
Two parents of children with a chronic condition felt that the timing of the trial approach could have been better: ‘the timing of when they asked as opposed to how they explained it […] if they’d given us even an hour for the diagnosis to sink in’ (F25), ‘It wasn’t just that, it was everything else […] it was her first admission […] I was worried about that’ (F12). However, this contrasted with one parent who viewed being approached when her child was unwell and an inpatient as appropriate because ‘there were a lot more people on hand’ (F13) to ask questions. What mattered for this mother was the manner of the approach rather than its timing: ‘I think if they do it in the right way then it’s OK to approach’ (F13).
Interestingly, several parents described being ‘excited’ (e.g. F1, F10 and F21) at being asked if their child would participate and remarked on how ‘passionate’ (F51) and ‘enthusiastic’ (F10) practitioners were about research. This seemed to inspire parents’ confidence in practitioners’ expertise and commitment. A few parents described how they would have felt disappointed if they had not been invited:
You don’t want to think […] there’s some sort of a trial that could improve your child’s [condition] and your child hasn’t been offered that […] I would like to be asked and be given the opportunity to say no.
(F50)
Parents also emphasised how making a decision about their child at such a difficult time gave a sense that they were involved in their child’s care when there was little else they could do for their child.
A parent needs that little bit of control, just so that they know there is still something that they’re doing for their child, because other than that there is nothing.
(F29)
Without exception, and irrespective of their level of interactivity in the trial discussion, parents felt that the decision on trial entry was theirs, they felt happy with the decision that they had made and they were clear that they did not feel pressurised by practitioners to consent to the trial.
Responding to the young person’s needs
Parents’ impressions of the trial discussion and the practitioners themselves were also influenced by efforts made by the trial staff to address the needs and preferences of their child. While the actual steps or gestures that practitioners took to do this varied widely, it was the effort and appropriateness of the action in the light of their child’s needs that parents valued. For example, parents of children who were very young, or who had behavioural or developmental difficulties, often found it hard to include their child in the trial discussion in a meaningful way. For many of these parents it was the efforts made by the trial team to engage the child in play or conversation so that the parent was able to concentrate on his or her conversation with the practitioner that they valued.
Yeah, he was kept occupied while I was getting, you know, they were asking me questions and, you know, he just loved it. He took to [the nurse] right away.
(F6)
The nurse, she’s, like she actually even asked on the phone as well, before we went through, ‘What kind of interests has [your son]?’ as well. So I thought it was really nice because as soon as we went in she came up and she introduced herself to[my son] […] So it made it easier because that way [my son]’s not like panicking, like ‘Who’s this person?’
(F15)
By contrast, for parents of older children, it was the efforts practitioners made to involve their child in the discussion and ask for their thoughts and views that parents valued.
They didn’t approach us, they actually asked [my daughter], which I thought was brilliant. And I think she was treated like a person instead of a, a thing […] she saw that as well […] you know, ‘they’re talking to me, they’re not talking to you two. They’re asking me’, […] that’s important, I think.
(F37)
Young people’s experiences of the trial approach
The sample of young people varied considerably in age and developmental level and, as a result, so did the depth of understanding and involvement that they had in the recruitment process. Although we encouraged young people to be interviewed individually, eight of the families preferred (sometimes for pragmatic reasons if the interviews were being conducted in the hospital) to hold the interview jointly between the RECRUIT interviewer, parent and young person. In these family interviews the parents’ views often dominated. Many of the young people said little about the process of considering trial entry. It is difficult to interpret what this means but it could indicate that these young people had not spent much time thinking about the trial or had found it difficult to engage with the subject of trial recruitment.
All of the young people included in this analysis were aware that they were taking part in a trial, knew the name of the trial and had some understanding of what it involved for them, although particularly for younger children, their understanding was often quite limited ‘researching about asthma […] and see how you catch it and stop it’ (F54, 8–10 years). Specific discussions with practitioners were less salient for them than for parents, particularly where a familiar practitioner was the first person to discuss the trial with them. In these situations the distinction between normal clinical care and trial participation may have been difficult for young people to discern, indeed this could be challenging for parents. Sometimes young people were confused about elements of the trial. Where a family was taking part in more than one piece of research, some of the young people’s comments, which might be interpreted as misunderstandings, could in fact be accurate descriptions of another study. Many of the young people in one trial were also taking part in a mainly questionnaire-based study to gather information about their medical conditions, and it is possible that there may have been some blurring between the two: ‘I just thought it’ll help people later on […] or find out what they’ve got’ (F13, 15–16 years).
Those young people who did have a clear recollection of the discussion were more likely to reflect on the content of what they were told rather than the social interaction. These were often delivered as rather factual descriptions of the study:
Got to take another tablet like you don’t know which one it is yet, only like the computer knows and it’s to see in a year’s time if it’s helped you get better and see if other people can take it or not.
(F12, 11–14 years)
They basically said that some people would get the real thing and others needed to get the placebo.
(F14, 11–14 years)
They’re trying to make a new drug about asthma and they don’t know if it works or until they’ve tried it on people […] so they’re like testing it.
(F48, 8–10 years)
Young people were often able to give very accurate descriptions of the practical requirements of the trial, although they sometimes viewed their participation in the trial as serving their individual needs rather than the need for research evidence.
To help me get to sleep
(F17, 8–10 years*)
(*Children in this trial had some degree of developmental delay, which may affect their understanding.)
What they’re trying to do they’re giving you inhalers that they’ve already given to other people […] and they’re giving you them to see if it’s OK for you.
(F57, 11–14 years)
Some young people did not tell us anything about their experience of the trial discussion; several others described the trial discussion as a ‘normal’ conversation (F13, F14 and F60). However, two young people described how they had concerns prior to the discussion which the discussions with the trial team had allayed.
I was quite frightened at first because I thoughts I’d have to do tests.
(F57, 11–14 years)
[and] I felt comfortable because they [the trial team] were understanding.
(F57, 11–14 years)
Apprehensive really […] because it was like un-treaded waters, you know, you don’t actually know what’s gonna happen, but the way that [the trial team] dealt with it, I thought was really good actually […] And I felt with the people that were running that they were really like, they were nice, and like at any point I could just ask them whatever or just walk out if I didn’t agree with something which I thought was really good.
(F58, 11–14 years)
Generally, the young people felt that they had been included and spoken to during the trial discussions. One boy remarked on how this differed from his usual experience of doctors’ appointments and another commented on how this was something that he valued:
Normally like when I go to the doctor’s it’s all, they always make my mum talk and not me […] but this time I talked and not my mum.
(F48, 8–10 years)
Very important for me, that I felt […] like I was important to what was going on.
(F58, 11–14 years)
Two girls (F14 and F55) explained how, while they felt involved and able to take part in the discussion, they preferred to be quiet because they felt awkward talking to people.
I usually just sit there quietly and just listen to them. If they ask me anything then I just talk I suppose and then I go back to being very quiet again.
(F14, 11–14 years)
Young people told us that they felt able to ask questions but, like their parents, often did not feel the need to:
I was able to ask questions but I didn’t ask any because they were explaining it already […] so I didn’t need to ask any questions.
(F51, 8–10 years)
Sometimes the young people gave slightly contradictory accounts that made it unclear whether or not they were happy with the way that they had been approached, for example: ‘The doctors were talking to my mum and dad and about every five minutes they’d just go, is that OK with you? So I was, I was kind, I was a bit bored eventually’, but later on in the interview this young person said ‘The people who were doing it were really nice and they explained it well’ (F57, 11–14 years).
One young person simply replied ‘no’ when asked if people had spoken to her during the discussion but also stated ‘I weren’t worried and I was able to ask questions so that made me less worried as well’ (F26, 8–10 years).
Therefore, it was sometimes hard to gauge how the young people felt about the trial discussion and whether they were as involved as they would have liked to be. However, no one told us that they felt excluded from the conversation or unable to contribute and ask questions.
Only one young person discussed the timing of the trial approach, describing how the trial discussion was held at the end of a consultation during which he had received a worse than expected diagnosis. Reflecting the views of some parents in similar situations, the young man commented ‘that was the least, the last thing on our mind’. Later in the interview he reiterated ‘maybe on another day would have been better […] it was a bit too much to take in at the time’ (F35, 15–16 years). Another described how she had found the first trial discussion ‘a bit overwhelming at first’ but that follow-up conversations had made things ‘more clear’ (F42, 15–16 years).
Practitioners’ perspectives on trial recruitment
Like young people, practitioners spoke more about the content of trial discussions and less about their experience of the process as a social encounter than parents. They were particularly concerned about ensuring that parents understood the trial and described how the amount and complexity of information, particularly written information, they had to give parents could undermine this objective and could lead to a situation where ‘we bamboozle […] families with information’ (P7).
It’s taken a number of years before people are getting towards striking a right balance between providing crucial information, but not overwhelming […] the parents, patients with information, so they can’t sometimes see the wood for the trees.
(P15)
Acknowledging the near infinite amount of information required for ‘informed consent’, one consultant questioned whether, even if parents did read the leaflets, this would be ‘enough’.
To really give informed consent just how much would you have to do to be properly informed? Would you have to go to medical school and learn all about these treatments and to make informed decisions? Or is just reading the information leaflet enough?
(P18)
Some practitioners spoke of their concern when families were eager to agree to the trial, as they felt it their duty to ensure that families achieved a certain level of understanding. They remarked how they felt ‘happier’ (P6) when families had ‘got questions because you feel like they’re wanting to be fully informed themselves’ (P6). They also commented on how in the absence of such feedback ‘it was hard to know whether or not he truly understood what he was consenting to’ (P28).
The families I worry about […] are the families that just say, ‘Yeah don’t … yeah that’s fine […] I don’t need to read the information sheet. I’m happy, whatever you say’ […] and just really don’t want to question you about it. And, um, those are the ones where I might […] go over it a little bit more than perhaps they want me to but I’m just trying to check they have absolutely […] understood.
(P5)
Occasionally, practitioners remarked on discussions where they had annoyed or deterred a parent by continuing to explain the trial after the parent had said they were happy for their child to enter the trial: ‘the mere fact that you insist on talking about things will, will put them off […] the fact that they’ve said “yes I want to be part of the study” and you somehow want to argue with them’ (P1), ‘he seemed to be getting more, you know, more annoyed with me the more I was talking’ (P28), a view reinforced by one of the parents who told us ‘and I actually cut her short. I said “please don’t go on about it. Just do it. Just do it quickly” ’ (F57). This posed a dilemma for practitioners in ensuring parents understood what they are consenting to, while also ensuring that the parent felt listened to: ‘sometimes […] they’re not bothered about all that, but I feel we have to tell them’ (P26).
Other practitioners emphasised how parents’ understanding was something that was achieved over time and might require several discussions. Some questioned whether informed consent was achievable, particularly when the child’s condition was critical. They referred to how some parents could make a decision that they (the parents) were comfortable with based on their general disposition towards research and on only the most essential information about the trial. They regarded such families’ decisions not to seek detailed information about the trial as an appropriate form of autonomy in a stressful situation.
If a family wants to exercise its autonomy by saying yes this is a good idea, don’t really mind about the details just let me sign it […] then I’m quite happy with that.
(P1)
This concept about patient has to understand, make a rational decision. That’s true but at the same time there are patients who don’t want to go through all these things. ‘I’m perfectly happy with my decision – get on with it. I don’t want all this paper’. So we have to respect that view as well.
(P14)
Based on an understanding that the role of ethics committees and regulatory agencies is to protect the public from harmful medical research, and therefore the safety of families is protected whatever their level of understanding, some practitioners were very comfortable with parents determining how much information they wished to receive.
There are some that are very obviously want to know very little, and they’ll say, ‘Yes doc, go ahead, let’s do it.’ And others will ask questions. And if they ask questions I allow the time to answer them. But I don’t think it’s the same for every patient at all. I don’t tailor; I go by what those families want.
(P30)
A number of practitioners made a clear distinction between the ‘required’ content of the trial discussion conveyed in programmes such as Good Clinical Practice (GCP) training and their own particular approach.
Oh GCP, but that […] doesn’t teach you how to talk to people. It just tells you what the rules are really. And knowing what the rules are doesn’t mean that you follow them and it doesn’t mean that you are any good at explaining to people what the study’s about.
(P28)
These practitioners emphasised the need to be adaptable to the needs of individual parents.
Implies that there’s a right and a wrong way of doing it and I’m not sure that there is, except to be sensitive to different people having different needs at different times and to listen, which is part of communication anyway.
(P11)
Practitioners’ comfort and discomfort with approaching families
Practitioners described different levels and sources of difficulty in approaching families about trials. The majority did not describe approaching families as something that they found personally difficult, but many nevertheless believed that being approached about a trial could exacerbate the emotional impact of the child’s illness: ‘these are very, very sick kids […] you’re going up to them and this is yet another consideration for them; whether they want their child to go onto a research project’ (P2). Such concerns were particularly prominent in the rheumatology and neonatal trial, where the children were often severely or critically ill. Other practitioners expressed a strong sense of personal disquiet or anxiety about approaching families about trials: ‘each parent is different and causes me great anxiety’ (P16), ‘I will go and approach them but I feel, I feel very uncomfortable doing it every single time’ (P18). The primary source of these practitioners’ discomfort was the intensity of families’ fears and distress. This led some practitioners to ask searching questions about the morality of approaching such families: ‘this family’s at a terrible time and really is it right to be asking them to do this?’ (P19). Several research nurses expressed a lack of confidence when working on trials outside of their specialty, and some practitioners spoke in strong terms of a range of other difficulties, such as the pressure to reach recruitment targets (P26), feeling ‘like a salesman’ (P19), discomfort with children’s medication being selected at random (P31) and concerns that families were vulnerable and therefore liable to be unduly influenced by practitioners (P18). A few practitioners described how their belief in the importance of research helped them to overcome their own discomfort.
Much more stressful for the family and much more stressful for you […] it’s only because you believe that intervention is critically important to investigate that you feel that you can kind of carry on.
(P12)
Occasionally, however, practitioners echoed the sentiment of the parent interviews saying that, on the whole, parents did not object to being approached about research:
The more you recruit people […] you feel less apprehensive yourself about asking them and […] you realise that actually asking them and them saying no, you haven’t upset them. You actually […] haven’t changed anything.
(P28)
Although the trial approach was personally difficult for some practitioners, none commented that this prevented them from approaching families. Indeed, a number of practitioners, whether they felt personal discomfort or not, described a strong sense of duty to the research and a sense that all families had the right to be informed about relevant studies: ‘every child I feel that has an illness should have the option to opt into research […] and we owe it to them and the future generations to do that’ (P24). ‘And it’s taking that choice away from them if it’s not mentioned to them’ (P6). ‘Gate-keeping’, which prevented an approach being made, was sometimes viewed as unhelpful, particularly when coming from professionals outside of the trial team: ‘frustrating if the other people don’t understand what the trial’s about but they’re just doing it from sort of general protectiveness’ (P1).
When practitioners told us about their decisions not to approach families who were eligible for a study, they described their reasons in terms of the families’ difficulties with comprehension or literacy: ‘you’re obliged ethically to make a judgement as to whether […] they can make an informed decision themselves’ (P15); understanding English; compliance; and their social situation such as marital problems (P8) or the involvement of social services (P12). Hence, practitioners emphasised how practical hurdles were the reason that they sometimes avoided approaching some families, rather than their own discomfort.
The design of the POP trial in particular allowed practitioners to select a time to conduct the approach in a way that was not possible in the other trials. As a result, rather than choosing not to approach a family at all, practitioners in this trial could titrate the approach: ‘I mightn’t necessarily give them an information sheet then, but I would just put the seed in their head that there is something going on’ (P20).
Participant information leaflets
Parents
Most parents were mildly positive, equivocal or neutral about the PILs, particularly when talking about them in general or global terms. Their comments, or in some cases lack of comment, implied that while the leaflets were ‘alright’, ‘OK’, ‘fine’, ‘useful’, ‘good’, they viewed them as rather unremarkable documents and had not given them much thought after the consent meeting.
I must have thought it was alright because I don’t remember having any thoughts about it.
(F11)
There is a lot of information on it. Yeah. But it was well worth reading.
(F18)
Several others commented positively on the format of the PILs – the question-and-answer format was particularly appreciated in allowing parents to scan the leaflets and select the most relevant sections to focus upon. The thoroughness of the leaflets and the presentation of the advantages and disadvantages of trial participation and the use of plain English were also commented upon favourably.
It seemed to answer questions for you, while you were going through it as well. So it was good the way that it, the way that the parents one was written out.
(F15)
However, parents remarked that the PILs were ‘a lot to read’, ‘wordy’, ‘intense’ and ‘overwhelming’. One parent commented: ‘It looks like half a forest’ (F54). Fourteen out of 59 parents specifically stated that they personally found the PIL too long or complicated, and a further four commented that while they personally were happy with the PIL, they thought it might be too much for others.
There is a lot of written information to go through […] so it’s a bit overwhelming, to try to take everything all in.
(F4)
It was a big document to read. I mean I can’t remember how many pages it was but I can remember thinking ‘oh my goodness’.
(F8)
One parent who suggested ‘don’t blind them with science’ told us that what she wanted from the PIL was to know that there was no risk, whether the trial medication would be beneficial, how long the trial was going to take and that it was voluntary: ‘I don’t want to know that there’s a β-agonist’ (F54).
Nevertheless, a few parents pointed to information that was absent from the PILs that they believed to be important, such as the licensing of the trial medication (F33), how to take trial medication (F16), urine samples (F25), use of non-trial medication (F59) and eligibility criteria (F5).
Despite these problems, parents emphasised how valuable the PILs had been in enabling them to reflect on the trial in their own time and space. Parents valued the opportunity to go over the details of the trial in the calm and quiet of their own home, particularly when their child’s condition was serious or if he or she had been noisy or demanding during the trial discussion.
I went home and I read it through […] that’s what I like to do. Come home, then read through properly and, you know. Because, like, I’m better here in my own house where I can relax and, you know, not worry.
(F6)
They described how leaflets allowed them to ‘sit down and read’, ‘relax’, ‘go over everything properly’, ‘read over it again’ and ‘go back to’.
If it wasn’t for […] the information sheets […] I’d probably forgotten a lot of what was said, you know. So it was good to have them to refer back to.
(F12)
Several mentioned reading the leaflet and then making their decision about the trial, or cited a detail from the leaflet as being important in their decision. In this way, we theorise that the PILs were important in providing parents with a sense that it was their own decision. Parents also described PILs as helping them to discuss the trial and share the decision with family members and friends who did not attend the consultation. The severity of the child’s condition and the parent’s emotional state may increase the importance of this function.
I spoke to [my family] about it and they’ve read the, the leaflet and me dad’s like me. ‘Well, why not try it?’ You know if, as long as it doesn’t cause her any harm, then there’s no, no problem.
(F16)
Almost without exception, parents placed greater value on the face-to-face discussion with practitioners than they did on PILs and would not consider participating in a trial without it.
You can only get so much information from a sheet, […] whereas, you know, the nurse […] you can ask […] any questions that you’ve got.
(F12)
If that just came through the door and I didn’t feel it’d got […] any personal contact, I’d just think, ‘no, I’m not doing it’.
(F11)
Parents’ suggestions for improving PILs centred on reducing the content and avoiding medical jargon because they thought ‘that would probably scare a lot of families off’ (F15). A website was suggested as a useful alternative to long PILs ‘so that they could go on to and get more information if they want to’ (F31). The emphasis was to provide sufficient information for everybody in a basic document while providing further information for those who wanted it.
More bullet points more pictures and a number to call rather than lots of information […] you just need a one sheeter really I would have thought.
(F5)
Young people
Like the parents, most young people had little to say about the PILs they were given and their comments were neutral or slightly positive, ‘the leaflet was helpful’ (F12, 11–14 years), ‘I thought it was fine’ (F17, 8–10 years), or mildly critical, ‘it was alright […] just less writing […] there were a fair lot of pages on there […] because really you don’t wanna sit there and read through a full booklet’ (F13, 15–16 years), ‘shorten it down I think’ (F60, 11–14 years). Only one young person specifically said that he felt the PIL was inappropriate for his age, ‘I felt more connected to the adult one […] I think it was a bit like aimed at younger children’ (F58, 11–14 years).
Young people also saw the face-to-face conversation as more important because ‘just talking it through with people makes it easier’ (F60, 11–14 years):
Myself, I always prefer the talking because then if you think about it, you can’t ask the leaflet a question.
(F42, 15–16 years)
Personally I thought the chat was more important […] the information, it kind of took second place really.
(F58, 11–14 years)
Separate PILs for young people may serve a valuable social function, in addition to their informational one, in that they recognise the young person as an individual who deserves to be informed and involved. As such, young people may appreciate having their own PIL, even if they do not necessarily read them. As one young person explained:
I liked the idea that you got your own because it makes you feel like a big person […] and it makes you feel more involved, it makes you feel, it doesn’t make you feel as though it’s your mum’s response, it makes you think that you’re able to make the decision yourself.
While at the same time stating:
I don’t read them but my mum does and, and she goes through them.
(F42, 15–16 years)
Parents also valued how young people were given their own leaflet, but recognised that often they would not read them. Parents attributed this to young people’s preference for verbal rather than written explanations (which young people themselves sometimes viewed as ‘work’) and that they trusted their parents to pass on essential information. Some parents acknowledged the difficulty of pitching the PILs appropriately, and they described rephrasing the leaflet for their child ‘to try and put it for [my son]’s language, so it didn’t scare [him]’ (F15, child aged ≤ 6 years).
Young people were clear that they wanted colour and pictures in their PILs, and PILs with such features received positive comment. Young people regarded PILs that did not have these features unfavourably:
It could have been more colourful, there’s nothing that would attract them […] they’d probably want to read it more if there was like more colours and stuff on it.
(F47, 15–16 years POP study, no pictures)
Good presentation […] because it’s got loads of pictures and it’s like in order.
(F51, 8–10 years, MASCOT study, pictures)
Two interviews indicated the potential for leaflets to cause distress to young people. One young person (F14, 11–14 years), prompted by her mother, agreed that she had found elements of the leaflet embarrassing, although the incident is described much more strongly by the mother in her own interview:
Is it the period bit? […] that’s a bit personal I suppose.
Do you remember your reaction the first time, you went, ‘What? I’m never going to answer that’ […]
I suppose everyone would mainly have the same reaction I would have.
You had the sheet and you weren’t feeling very good and you were going ‘why do they want to know that? That’s just embarrassing’.
One young person highlighted the unintentional impact that medical words can have in children’s leaflets: ‘It’s got like medication pills and inhaler and what is the medicinal device or procedure being tested and that word “tested” gave me a few worries’ (F57, 11–14 years).
The same young person suggested that to avoid additional distress to children when they attend hospital visits, information leaflets might carry a photograph of the investigator because ‘the children could worry about like “who is it mummy?” ’ (F57, 11–14 years). A number of parents, particularly in the MENDS trial, have identified that their children do not like to meet new people. This suggestion may go some way to alleviating that difficulty.
Practitioners
Practitioners commented that PILs were ‘not straightforward’ (P12), ‘too long’ (P2), ‘too detailed, too comprehensive, too busy’ (P3) and ‘too complex’ (P9). In total, 24/31 thought that the leaflets were too long and complex, with a further three stating that, although they were happy with the PIL for the trial participating in RECRUIT, they thought that PILs in general were too complicated.
Some practitioners regarded PILs as primarily serving the requirements of ethics committees and legal regulations for PILs to be comprehensive, rather than the needs of families for simple, accessible documents.
We try and produce pretty simple information sheets and in general the contribution by ethics committees is to make them more complicated. And I don’t think it’s helpful.
(P11)
A particular concern for practitioners was that families would find PILs containing scientific details overwhelming and threatening and may be deterred from participating in a trial.
If you do it according to the book then it is an intimidating document which doesn’t enhance patients’ autonomy and does occasionally turn them off being involved in the trial.
(P1)
It undermines them, it undermines their confidence and […] rather than empowering them it could actually make them feel very anxious and […] threatened. So I think it’s a difficult balance and I’m not overly sure we’ve got it completely right.
(P8)
In contrast with those who saw families at risk of being overwhelmed by information, one consultant commented that many of the details provided in PILs were ‘obvious’ and therefore superfluous.
It’s not necessarily too much information […] it’s making the assumption that everything has to be explained when actually a lot of it, I think, is pretty obvious anyway […] people understand the research process, I think, better than maybe we give them credit for.
(P9)
Some spoke of how leaflets and the information they provided were of value in principle, but in practice often failed to strike the right balance between providing meaningful information and being accessible for families, particularly those with learning or literacy difficulties. Practitioners sometimes implied that the PILs often went unread. Like the parents, practitioners placed greater importance on the personal discussion they had with families than on the PILs.
The face-to-face discussion is the time to make sure they really understand what’s, what’s going on […] because it’s only on that face to face interaction you can look at people and you know whether they’re getting it or not.
(P5)
Some suggested that, without discussion, families would not be willing to participate in trials or would find it more difficult to make up their minds.
Practitioners did acknowledge that PILs had a useful, though somewhat limited, role to play in the context of trial discussions with families. Some practitioners remarked on how leaflets were useful for families to prepare for discussions and support their participation in the discussion. They could be used to scaffold discussions, to emphasise certain points and to make abstract concepts more concrete. Practitioners also saw PILs as helping families remember the trial discussions or as a resource to which families could refer back, use to supplement their understanding and use to explain the trial to other members of the family.
It certainly lets them go back to it time and time and again sort of, you know, over a few days or whatever as a reference point really.
(P6)
Practitioners made suggestions for improving the PILs, which, like the parents’ suggestions, mostly focused on reducing their length and content. Practitioners were concerned about the readability of the document in general and some were also concerned that the parents they knew would find them difficult to understand. However, in making PILs more accessible, a balance had to be struck to ensure sufficient information was provided and to meet with ethical requirements.
I would prefer a simple, you know, say A4 sheet of this is a trial, this is what we’re doing, contact this person […] And then probably a more wordy document and especially for those people who ask for it.
(P14)
It is quite lengthy but then, you know it’s I suppose it’s a balance of the two isn’t it? If you, if you make it shorter, then you withdraw information.
(P4)
Impact of relationships on the trial approach
Parents
Parents who knew the practitioner prior to the trial approach tended to value the relationship as one that provided reassurances of safety and good care and continuity for their child. When the trial was introduced by a trusted health-care professional and then ‘handed over’ to an unknown researcher, these reassurances seemed to be extended to the new practitioner by association. Almost all of the families who had an existing relationship with the trial team were from the POP trial.
For the majority of the other families in our study, the trial team were completely unknown to them. For parents in the MENDS trial this practice was not unusual to the families because their child’s ongoing health care regularly required them to meet new professionals: ‘I deal with that many different people […] I’m not really fussed.’ (F16).
For parents in the TIPIT trial, approached within a few days of the birth of their very sick preterm baby, all of the practitioners responsible for the baby’s care were virtually unknown to the parents, so having a stranger approach them about research was not regarded as unusual: ‘I’d just meet them once, like, and then I’d see someone else and I didn’t really have a specific person to speak to anyway’ (F10). In their accounts, these parents often stressed the importance of the practitioner’s manner and their confidence in his or her expertise.
Parents were comfortable about speaking to someone whom they did not know if the approach was made in the right way: ‘I just clicked with him straight away and that was it’ (F43). In this sense, families did not show a consistent preference for being approached by someone they knew rather than by a practitioner they had only just met through the trial. Rather, they were consistently happy with whichever ‘model’ they had encountered (Box 3). Whatever the length of relationship that they had with the trial practitioner, none of the parents spontaneously told us they would have preferred something other than they had experienced in the circumstance. Only one parent acknowledged the potentially negative implication of an existing relationship with the practitioner:
In a way I think it would have been easier if I hadn’t have met her because I think I might have felt a bit more pressured into […] doing it.
(F36, declined trial on telephone, had never met practitioner face to face)
Where relationships existed, they were valued:
‘I felt that having known her, known what she could do for [my daughter] with her expertise and all of that and the fact that she’s doing the study […] that would have given me more of an interest in terms of than if someone was coming in to be cold that I hadn’t known […] I wouldn’t have been as warm to have participated.’ (F60)
And trust was extended to trial practitioners if the study was introduced by a known practitioner:
‘You feel safe, I mean in their hands, so when somebody that is working with them, you kind of […] trust them, because they are a part of the same team, so to speak.’ (F35)*
Parents were happy to be approached by an unfamiliar practitioner but emphasised the importance of the practitioner’s expertise and manner:
‘I don’t think it would make a difference whether it was a doctor, a nurse or a stranger off the street really. As long as they know what they’re talking about.’ (F45)
‘They were all very nice and very friendly and very reassuring so you sort of felt like you might have met them before.’ (F56)
(*Interestingly, this parent’s son thought that the separation from the clinical team was important.)
Young people
Not all young people talked about the practitioner with whom they had discussed the trial. In many cases they either did not know the practitioner or could not remember who it was. One young person when asked if he knew the practitioner said: ‘No. I know her well now though’ (F18, 8–10 years). This continuity once a young person had entered the trial and the developing relationship seemed to be more important to them than knowing the practitioner before they were approached about the trial.
We developed a very strong relationship with [the nurse] […] it’s better than like seeing different people every time and then you might, you’d probably feel more uncomfortable.
(F58, 11–14 years)
This young person described how the manner of the trial team overcame any potential discomfort at not knowing them prior to the trial discussion:
They were such nice people to be honest that it was easy really.
(F58, 11–14 years)
This contrasted with situations where there was not the same level of continuity:
You have to keep meeting new people and my, some like, some people, they might feel scared every time they go because they don’t know whether they’re meeting another person or not.
(F51, 8–10 years)
Where young people did give an opinion on whether it was important to know the practitioner before the trial approach, their views mirrored the pattern seen in the parents’ accounts. That is, they preferred whatever ‘model’ they had encountered:
If I didn’t know the doctor I would have been a bit more worrying, you know worry about it a bit more because I wouldn’t have known the person.
(F42, 15–16 years)
I think it helps if you are talking to someone you know.
(F47, 15–16 years)
Both of these young people knew the practitioner who discussed the trial with them and valued that relationship. By contrast, one young person that had not met the practitioner before the recorded trial discussion suggested that ‘Maybe it was better, maybe someone separate […] in case they might object to us not, um, taking part in the study’ (F35, 15–16 years). However, once the trial was under way, like others in the study, this young person valued the developing relationship: ‘It’s good to have someone specific that we know’ (F35, 15–16 years).
Practitioners
Perhaps not surprisingly, we found a similar pattern in the practitioners whom we interviewed. Those who had an existing relationship with a family were very happy to approach those families; those who were generally meeting families face to face for the first time found this perfectly acceptable also. Where practitioners did not know the family in advance they stressed the importance of making a good impression:
I always try and make a very good impression with the patient, particularly, but also with the parents, right from the beginning, so I think, once you’ve done that and established a good relationship, everything else that’s good leads on from that.
(P15)
Practitioners who knew the families well often felt that the relationship was important and, in particular, they stressed how this helped families trust to have confidence in them:
They know about their child, they know about their disease and they can explain the study to them in the context of their own child and, and make it meaningful as opposed to ‘would you like your child to be part of a study?’
(P21)
Indeed, some research nurses who did not know the families would introduce themselves as working in association with the clinical team in order to make the families feel comfortable: ‘they have very, very good relationships with the consultants […] that’s why we felt it was important for the consultants to approach the patient first of all to introduce it (P2)’*.
(*In these situations, although the research nurses discussed the trial with the family, consent was always taken by the consultant so the relationship to the medical team remained important.)
However, a number of practitioners felt that consent for research should be taken by someone not involved in the child’s clinical care and were concerned that families would feel obliged to take part as a result of the existing clinical relationship:
You don’t have to do it, just because he’s done this for you.
(P17)
You wouldn’t want to do it just because they trust me if they knew me […] it may be a bit of a conflict of interests kind of thing.
(P23)
I think, it is probably better for the patient, not for the studies […] you probably will get more consent if the clinician asks.
(P16)
These concerns were more commonly articulated by research nurses and neonatologists. Some practitioners spoke of the dilemma that this posed for best practice:
A good thing and a bad thing really […] I’m sure it would be much nicer for families if they, they knew me better because then they would […] perhaps be able to […] have more trust in what I say about the study […] Whereas if they don’t know you then they can make up their mind on the merits of the study […] they’re not […] putting themselves in a position where they feel they might upset a future therapeutic relationship with the consultants.
(P5)
Parents’ and young people’s suggestions for improving the trial approach
We found parents and young people to be largely very positive about the way they were approached to take part in the trial. Nevertheless, they did have some suggestions for ways to improve the process. Two POP parents suggested that it would be helpful if the young people being approached were able to talk to other young people:
They could say ‘[child’s name] can you talk to that girl because we’re, she’s thinking about it but she’s got questions’, because she would be the best one to give the answers then.
(F42)
It would be helpful for certain children, if they wanted to, to be able to approach other children, or other parents. Just to be able to maybe share the experience and see what they think of things.
(F30)
Parents who were approached about the neonatal trial felt strongly that it was important that they were given advance notice that they were going to be approached for research, perhaps by one of the nurses caring for their baby. The importance of such preparation in this context cannot be underestimated as one parent described: ‘I was really scared initially […] I automatically thought that […] there was a problem with the baby’ (F21). Some parents also suggested that where possible the conversation should take place in a quiet room, where they would be better able to concentrate.
For families in the MENDS trial the suggestions were often of a practical nature, such as warning the family how long the consultations would last, to enable them to make arrangements to bring another family member to look after their child. Similarly, parents suggested that the appointments be arranged so that the elements that required their child to be present could be dealt with at the start of the appointment and then a family member could take the child out. One parent suggested that involving families in the planning and design of trials might help to overcome such barriers:
Giving parents some say in how the study is conducted […] there may be circumstances in which they could have adapted certain aspects which would have helped at least some proportion of the parents who would otherwise said ‘no’ to have said ‘yes’.
(F52)
Often the first information that parents received about MASCOT was posted to them. One parent who declined the trial suggested:
Maybe, you know, if you weren’t given all that information and maybe if I’d had an initial meeting with somebody where it could have been explained […] maybe if I’d had a face to face with somebody it might have been a little bit different, my decision.
(F55)
As discussed in Impact of relationships on the trial approach, one mother and her daughter who were approached about MASCOT stressed the importance of seeing the same practitioners in the early stages of the recruitment process (F51).
Young people also made several suggestions to improve the trial approach. These were quite often practical rather than to do with the conduct or content of the actual discussion. In some cases, the older young people made suggestions as to how to improve the approach for younger children. These included ‘a play room to keep them occupied’ (F57) and colouring books for younger children and Nintendo DS (Nintendo Co., Ltd, Redmond, WA, USA) for older children (F57) or book tokens to collect as a way of keeping children involved (F14).
Some young people gave considerable thought to their answers and offered suggestions that were not necessarily criticisms of how they themselves were approached but were focused on making young people feel comfortable and not scared or intimidated (Box 4).
‘Try and not scare anyone […] Try and be as friendly as possible and not like push it, but just by trying to get to know them.’ (F14)
‘I wouldn’t do it in like a quiet room and just sit us down because it might make them feel like scared to do it […] but if they had like something, they were doing something fun […] and then just ask them questions while they were doing their activity.’ (F49)
‘Come to the house of the participant and like get to know them a bit quite informally first […] I think especially with younger children that would be better.’ (F58)
‘Well if they were young children I’d say make it a bit more fun don’t be a bit, don’t be too dull and don’t be as a doctor per se.’ (F42)
‘Depending on how confident the child is, speak to the child separate to the parents. I think I had that choice and I chose not to, but I think it’s still nice to have that choice.’ (F58)
Summary
In this chapter we have described the way in which families are approached to consider a trial, in terms of the data from recorded trial discussions and experiential evidence from the interviews with parents, young people and practitioners. Our analysis has highlighted a number of important considerations.
Parents and young people generally said very little in the trial discussions and asked few questions. Although research suggests that such features are important to good communication, the families in this study were unconcerned about their lack of interaction in the trial discussion and spoke instead of a sense of safety and comfort and a knowledge that they could ask questions if they wanted to. Even in extremely difficult situations parents were understanding and accepting of the approach, and many were positive about the opportunity to take part in a trial. The family’s experience of the trial approach did not seem to be associated with whether or not they knew the practitioner beforehand, although some practitioners were conscious that families might be unduly influenced if they were approached by a member of the clinical team that was responsible for a child’s care.
While parents described positive experiences of the trial approach, our analysis did identify several areas of misunderstanding. The cases described in this chapter raise important topics for discussion, such as at what point the process of consent should be considered complete; the need to describe the treatment arms in a neutral fashion and stress that the purpose of the trial is to assess the efficacy of the trial medication; and the difficulty in avoiding parents constructing the trial in terms that make sense to them but are inconsistent with the trial design.
Practitioners themselves were often very concerned about overburdening families with information and described a difficult balance between providing sufficient information that families might make a decision that was ‘informed enough’ while avoiding overwhelming families with information. For some practitioners, concerns about overburdening families were particularly salient if the child was seriously or critically ill and the family already distressed. This concern provoked personal discomfort and anxiety and made it difficult for these practitioners to approach families. This is a serious concern, as it is not only at odds with the positive experiences that parents described, but also has several negative consequences for the practitioners themselves, and for trials and potential participants. These will be discussed in Chapter 5.
The PILs, which are an integral part of the trial approach, indicated convergence and divergence between the views of parents and practitioners. Both groups valued the face-to-face discussion more highly than the PILs and as a result both groups felt that the PILs could be shorter and less complex. However, while practitioners tended to be critical of leaflets, often viewing them as intimidating and off-putting, if read at all, parents told us that they valued the PILs for the opportunity to reflect on the trial in their own time and space and to share the information with their family. For young people, the separate PIL seemed to serve a social function as well as an informational one, in that it signalled respect for young people as individuals with their own views.
Chapter 4 Analysis strand 2: what influenced decision-making?
Summary of objectives
This chapter is about families’ decision on whether to enter a child into a trial and what influences this decision. We have included interview data from 60 families and 31 practitioners in the analysis that underpins this chapter. Extracts from interviews have identifying code numbers as explained in Chapter 2(Methods).
This chapter is organised into two main parts. In the first part, we examine what factors parents took into account when they were considering entering their child in a trial, and their views on non-participation. We discuss whether parents felt pressure for their child to take part, or uncomfortable about declining. We also examine their experiences of ineligibility. In the remainder of this section we consider parents’ experiences of their own and practitioners’ roles in the trial decision and parents’ views on medicine and research, as a whole, as influence on their decisions. The factors that practitioners thought influenced parents’ decisions about trials showed considerable overlap with the factors that parents themselves identified. Therefore, we have kept the descriptions of practitioners’ views brief, except where they contrasted with parents’ or provided additional insights.
In the second part of the chapter we discuss what factors young people thought to be important in considering trial entry. We also examine their role in decision-making and the importance that they and their parents placed on their involvement in this decision.
What is important to parents when being approached about trials?
Prioritising the child’s safety and well-being
Almost without exception, parents described how important it was for them to feel confident that the trial was safe and would not harm their child. Similarly, practitioners recognised the importance to parents of safety when considering entering their child in a trial. Parents particularly wanted reassurance that (1) the risks of side effects from the trial medication were minimal; and (2) the trial would cause no or minimal distress to their child and, notably, that it would not involve procedures that children find distressing (e.g. venepuncture):
My partner was like, ‘Is it going to harm him? Is it going to, you know, is it going to put him through pain or anything?’ You know, that was […] the only question.
(F41)
Some parents favourably contrasted the safety of the trial that they had been approached about with other types of research. Parents, particularly in MASCOT, spoke of how they were reassured by explanations from practitioners that the trial medications were routinely prescribed: ‘There wasn’t any sort of risk because it wasn’t trialling a new drug or anything’ (F53). In MENDS, parents derived similar reassurance from the fact that ‘Melatonin is something we do naturally anyway so it’s not as if […] I was giving her some sort of strong chemical’ (F5). Occasionally, parents contrasted the safety of the trials in this study with those trials that were testing new medicines and particularly those trials that had received extensive negative press coverage, notably the Northwick Park trial in 2006. More than one practitioner recognised the potential impact of media coverage and how this could raise safety as a concern for parents: ‘How safe is it? Because there’s always the sort of connotation […] are they sort of trying […] something not tried and, you know, trusted and tested’ (P13).
Some parents were very clear that they would not have entered their child in the trial if they doubted the safety of the trial medication. As this mother implied, the knowledge not only that a medicine had previously been tested in humans, but also that it had already been widely used with children, was important in parents’ decisions to take part:
I don’t think children should be used as guinea pigs where safety, their sort of health safety is an issue. But […] quite a lot of children already have these tablets […] but I would never have done it if it was testing something for the first, like it was a test, test of something for the first time. I would never have done that. I wouldn’t have risked it going wrong.
(F56)
Her perception that the trial is safe was critical in her decision. However, her comments suggest that she was unaware that a drug’s regular use in the population did not necessarily mean it was licensed to be used (for the particular indication in the particular age group). Similarly, she seemed unaware that having such a licence did not necessarily remove the need for further research.
Parents prioritised their child’s safety and well-being irrespective of whether the child went on to participate in the trial. For several parents who declined or withdrew from a trial, it was the perception of possible harm that drove their decision:
My main concern was actually taking it, he’s so stable at the moment, apart from a few hiccups which we can control. And I was really concerned that taking him off his medication would mess his asthma up and that was the main reason for not taking part.
(F55)
(*This comment suggests that the parent may have misunderstood the MASCOT trial design. On this trial children’s medication was changed, but it was not ceased altogether.)
One mother’s discovery, via an internet search done by the child’s father, that the trial drug was unlicensed for children had left her concerned about the safety of the trial and she subsequently withdrew her child from MENDS (although the licensing status of the trial medication was given in the PIL).
It just scared me when it said not to be given to children under 20 […] I didn’t understand they weren’t licensed for children. […] And that’s what I thought it was, just to see if it worked, not to actually like so then it could be licensed.
(F33)
Benefits to the child, the family and others
Most parents spoke of the potential benefits that participating in a trial might hold for their own child. For some, participation was seen as a way of gaining access to medication that they thought was otherwise unavailable. For others, participation offered hope that their child might receive a treatment during the trial that would prove to be effective:
I didn’t see why I […] could say ‘no’ to it. Because I thought, well it’s, you know, a 50/50 chance of her getting […] this additional help which she might need.
(F21)
A number of parents also told us that they perceived very real benefits to participation even if their child did not receive active trial treatment in the sense that they would get ‘additional attention’ (F60). Parents saw additional monitoring, vitamin and mineral supplements (specific to the POP trial) and appointments with a specialist as positive aspects of participation (e.g. F14 and F56).
For 4 out of 10 parents who declined a trial, benefit to their child was an important consideration, but it was the lack of definite and immediate benefit of the trial for their child that they emphasised as being significant in their decision. Some practitioners acknowledged this as potentially problematic for recruitment, ‘It’s just that parents want the best treatment’ (P15).
Parents also discussed the importance of benefit to other children and families. Most recognised that their family might not benefit personally from the trial, but for some this possible drawback was offset by the knowledge that even if their child did not benefit personally, his or her participation could make a valuable contribution:
We might make a bit of a difference somewhere along the line and even if it doesn’t help [my son] it might help someone else […] It’s just nice to think you’re, you know, doing something useful.
(F56)
You kind of want to, to help in any way, you almost feel it’s kind of your obligation as a human being to help […] not in a pressured sort of way but, but well you want to do what you can.
(F35)
For some parents, participation was an opportunity to ‘give something back somewhere along the line’ (F51). Those parents who had had preterm babies in the past or who themselves had asthma were particularly conscious that the treatment their child was receiving was a direct result of previous generations of families choosing to take part in research. These parents indicated that they wanted to reciprocate:
Agreeing to it wasn’t really a problem because, at the end of the day, the medicines that my lad’s had I know have been tested at some time or other many years ago […] he wouldn’t be around today if they hadn’t been.
(F45)
Parents’ accounts of the benefits to others were often expressed with a considerable sense of conviction, though the benefits they identified could be quite general or idealised. For example, families in the POP trial had often experienced lengthy, painful and frightening periods of illness before their child was diagnosed and treated. Some saw participation in the trial as offering a way to prevent future children suffering in the way that their child had, even though the trial itself could not specifically contribute evidence to speed the diagnosis of the condition. Parents saw their contribution to the trial as helping towards the general aim of improving medical knowledge about their child’s condition:
It was the most horrible thing I’ve ever […] lived through in my life, to be honest. So to help other people […] For somebody else to suffer that, I’d rather, as long as it wasn’t going to hurt [my son] I would do anything to stop that.
(F26)
While it was common for parents to stress that they thought participation would ‘help other kids’ (F54), their priorities lay with their own child, and benefits to others were a secondary consideration.
Firstly to, to get his asthma better and secondly because I think it was a good thing to do to try and get sort of the different varieties of the medication, the different things more researched and better used for children.
(F59)
I’d hate it to be in vain from our point of view. Yes it might help the study as a whole but from a selfish point of view I’d prefer [my son] to get something out of it.
(F56)
Indeed, parents described a combination of several factors as important in their decision. In general order of priority, this typically included securing their own child’s safety, benefit to child/family and benefit to others. However, for many parents there was also some kind of trade-off between these factors when considering trial entry:
Maybe he might get [the trial drug], maybe he mightn’t. Maybe, if he does get it, it might help him in some way and if he doesn’t get it then, you know, at least I tried to help [you] with the study.
(F9)
If it’s going to hurt them, I wouldn’t let him do it. If it’s not going to hurt him, there’s no harm in them doing it. If it helps somebody else then he does it.
(F2)
Most practitioners recognised that parents wanted to help other families and their doctors, and that this desire informed their decisions. Trials such as TIPIT, which involve extremely premature babies, present an intensely stressful and emotional environment for parents to consider trial entry, particularly given the high mortality and morbidity rate in this group. One practitioner spoke of how, even in the most intensely distressing situations, some parents might take comfort from their child’s participation in a trial: ‘I think they also found it a little bit helpful that maybe the babies were going to die but maybe all of that wasn’t in, in vain if they were helping push things forward with one aspect of care’ (P1). However, reflecting our observation from parents’ accounts that their foremost concern was their own child, another practitioner noted: ‘Their first question is “My child.” But I think when they’ve made up their mind they also say, “Yeah, and it’s going to help other people.” […] So it’s not necessarily an immediate response’ (P25).
Practicalities of participation
Many families discussed in considerable detail the simple practical difficulties and inconveniences of participating in a trial, such as school absences or the need to travel to hospital for additional appointments. Six families who declined the trial cited practical reasons, such as frequent hospital visits, although none gave practical reasons as the only reason for non-participation:
[My husband] said, ‘How many times are you going to, you know, how many times are we going to have to go to the hospital?’ And different things about school and him having time off.
(F19)
Decliners’ comments about practicalities were often tempered in ways that suggested that a practicality would be weighed differently if the trial’s benefit to the child and family was stronger. In this sense, placebo-controlled trials could fail, in parents’ minds, to outweigh the inconveniences of the trial: ‘it just seemed like a very long drawn-out process for something that wasn’t guaranteed that she was going to get the medication’ (F44).*
(*This parent declined MENDS. This trial required a 4-week run-in period during which the parent was required to keep sleep diaries and follow sleep hygiene guidelines prior to the 12-week trial period.)
Similarly, several consenting families commented that had participation placed more demands on them, they would have said ‘no’. This indicates that, like the decliners, these families had weighed practical considerations against the potential benefits of participation but, for them, the balance had fallen in favour of the trial:
If it had meant that we had to go extra times to [the hospital] again […] I, I wouldn’t, I wouldn’t, I wouldn’t have had any problems saying ‘no’. I would have apologised but I would have said no.
(F35)
It really wasn’t a big decision. It sounded very much the right thing to do […] and the inconvenience was absolutely minimal […] so it definitely was an easy decision.
(F60)
The same parent noted, ‘Had it been additional time required, you know, it certainly would have been a factor in my decision’ (F60).
Practicalities of participation were not a concern for parents considering the neonatal trial, as trial procedures were conducted alongside the baby’s routine treatment in the neonatal unit.
The majority of practitioners acknowledged that practical considerations were important to families. However, they sometimes seemed to downplay or dismiss the significance of these factors in parents’ decisions about the trial: ‘that’s probably a partial reason but it just demonstrates their reluctance to be part of the study rather than being, you know, an actual reason’ (P5).
Saying ‘no’ to a trial
Parents described how practitioners had emphasised that the trial was voluntary and that families were free to withdraw from it if they wished. Practitioners’ efforts to emphasise these points were highly valued by parents. Indeed, several parents commented that they were reassured to know that they could change their minds if their subsequent first-hand experience of the trial left them feeling that it was not appropriate for their child or themselves. Knowing that there would be no implications for their child’s care whatever decision a family reached was particularly important:
It was made clear that it wouldn’t be a problem, you know, and it wouldn’t affect her treatment, you know, if we decided not to so.
(F12)
I think it was quite an easy decision because I knew, I knew I could pull out of it at any time.
(F59)
All parents told us that they were aware that they could decline and none told us that he or she felt pressure from the trial team to consent to the trial. However, some parents who consented described reasons why they personally would have difficulty in saying ‘no’ (Box 5).
‘I discussed it with [the doctor] and, you know, did I want to do the study? “No”, is my honest answer. But I suppose I felt like, if I could, I should, but I don’t know why. Well, I suppose because I think if nobody does it then nothing moves on.’ (F11)
Obligation to hospital and practitioners
‘I would have felt awful to say “no”, and it’s purely because of everything they’ve done for [my son] […] there’s nothing you can do for them like they’ve done for us, for [my son]. And you just think, you send them thank you cards, but you can’t really express how grateful you are for what they’ve done. So you think by doing this, you’re sort of like, you are giving something back.’ (F8)
Personal commitment
‘By the time you’ve been for your first appointment and going back for your second one, […] I really don’t feel like I could say I’d pull out even if, but I don’t find it easy to do things like that at the best of times, so that’s a me thing […] there’s nothing they could do to make that any easier […] I don’t like to let people down when I’ve said I’ll do something.’ (F56)
Anticipated regret
Two parents from the neonatal trial described how, while they had felt able to decline, they would have regretted making that decision when ‘upset and emotional’ (F41). One mother thought that if she had said ‘no’, she might regret hindering the progress of neonatal medicine and ‘might have lived with that guilt’ (F41); the second felt she would regret a decision which might deny her child potentially beneficial medication (F21).
Five of the parents who declined MENDS (F23, F36, F38, F44 and F52) specifically described feeling ‘guilty’ at their decision not to take part. All traced their unease to a recognition that the trial was important and a wish to help – ‘if I could have helped then I would have done’ (F36) – but they also explained how they had been unable to reconcile participation with other considerations, such as the practical inconveniences of the trial, a desire to begin medication immediately and the stress that participating might cause their child:
It was difficult to say ‘no’ because I just thought, you know, I’m hindering a study which could potentially help other parents with similar difficulties. So you do feel, well I felt a certain sense of guilt in saying that ‘no’. Not because I was made to feel guilty but just out of your own conscience you feel that you’re letting them down.
(F52)
Several parents who declined the trial told us that they were pleased to be interviewed for RECRUIT as a way of making an alternative contribution, which perhaps lends credibility to the accounts of those parents who described feeling guilty about declining the trial. However, it is worth reiterating that none of the parents, including those who described feeling ‘guilty’ or uncomfortable at saying ‘no’, said that he or she had experienced any pressure from the trial team. Rather, their feelings were linked to concerns that by declining the trial they were delaying important research and a sense that they ‘didn’t want to let anybody down’ (F44). Moreover, all were able to rationalise these feelings by citing their belief that, ultimately, they had acted appropriately for their child and family.
A number of practitioners were conscious of the potential difficulty for parents in declining a trial. Several research nurses described how parents might feel uneasy about disappointing the research team because ‘they’re not […] helping with the research’ (P6), or because they recognised the value of the research but ‘they don’t want their own child to be in it’ (P25). Another nurse spoke of the ‘guilt’ that a parent might feel if their decision to decline meant that their child would not have access to a potentially beneficial medicine: ‘there must be nothing worse than have to accept that you’ve been offered this lifeline or a chance for your child and for whatever reason you just can’t do it. And the guilt that may go with that’ (P26). Sensing the difficulty that some parents might have in voicing their reluctance, some nurses described how they gave parents ‘permission’ to say ‘no’:
You could see the body language and I said ‘you can say no if you want to’. And she said ‘no, I don’t want to’ then.
(P17)
I sometimes just sort of say ‘listen if it’s not right for you, that’s fine’. And it’s almost like instead of them having to say ‘it’s not right for me’, they just have to say ‘yes it’s not right for me’. So it’s taking […] that embarrassment off them a little bit.
(P26)
Being ineligible for a trial
An often overlooked aspect of being invited to participate in a trial concerns the experiences of those families who are happy to consent but then, sometimes after a run-in period, they do not fulfil eligibility criteria. We explored this with the eight families who had been ineligible for a trial. One mother described her conflicting emotions over ineligibility:
It was one of these no-win situations because I was quite disappointed that they didn’t want to see him any more and then thinking ‘oh that’s really good, you know, he’s at a level now, he doesn’t need to go’. So, you know, it was sad and it was happy at the same time […] even [my son] was a bit, Oh so I don’t have to come again. He was quite upset.
(F48)
This family’s sense of disappointment seemed to be linked to the supportive relationship that they had formed with the trial team and the realisation that ineligibility meant this relationship would end. This mother was also dissatisfied with the routine care her child received outside the trial and the prospect of returning to this care. Two other parents from the same trial expressed similar concerns about returning to the ‘pre-trial’ health care. One family had also been ineligible after the run-in period (F53), while a second had been randomised but was already concerned about their child’s care at the end of the trial period (F58). It is important to note that all of the ineligible families who we interviewed had been approached about MENDS or MASCOT. In both trials, one child was ineligible prior to the run-in period, and three post run-in. Our finding that it was only parents in MASCOT who expressed disappointment at ineligibility further suggests that concern about returning to ‘pre-trial’ health care was the source of parents’ disappointment, although ineligibility at this stage meant that their child’s asthma was better controlled. One parent who was ineligible for MENDS before the run-in expressed disappointment, but this was as much because she was not screened out prior to attending an appointment as it was about her child’s ineligibility for the trial.
The potential for families to be disappointed because their child was ineligible was something that several practitioners recognised: ‘disappointment. Almost like they’d failed a test’ (P5). Others spoke of the rigid eligibility criteria for trials, which one practitioner commented are sometimes set ‘without any particularly logical reason behind it’ (P8). One research nurse described the need to ‘let them down gently’ and the importance of choosing words carefully so that a family did not feel rejected or as if they had failed in some way: ‘the study won’t be suitable for your child’ rather than ‘your child’s not suitable for the study’ (P31).
The role of practitioners in parents’ decisions
Without exception, parents were clear that the decision on trial entry should rest with them and, where appropriate, their child, rather than the practitioner. For some parents, the decision on trial entry was theirs by right, reflecting their status as parents:
It’s not for the doctors. They’re not the ones that brought the child into the world. The mother and father did. So, they make the decision.
(F2)*
[*One young person also spoke of the decision in terms of her ‘human rights’ (F42, 15–16 years).]
Other parents framed the decision as one which, rather than owning by right, should be theirs because of their knowledge of, and relationship with, their child. Parents were better placed than doctors to make a decision that was in keeping with their child’s interests and preferences (e.g. F8, F24 and F48). Several parents indicated they had found the decision difficult, particularly when ‘getting my head around’ (F60) the child’s condition. Despite this, even parents in the neonatal trial, arguably the most ‘vulnerable’ group in our study, told us how important it was for them to be the ones to decide about the trial:
As much as everyone else’s opinions counted and I took that into consideration, it was gonna be my decision no matter what at the end of it.
(F9)
These parents spoke of the decision on trial entry as offering ‘choice’ (F43) and ‘control’ (F29 and F45), that ‘there is still something that they’re doing for their child’ (F29). The difficult environment of the neonatal unit meant that parents often felt detached from their child’s care, and in this context being asked to consider trial entry could be a positive experience:
The doctors make enough decisions while your baby’s in this situation and which are out of your hands, so I think having some input in something is really, you know, good for the parents.
(F41)
Several practitioners acknowledged this view: ‘it actually empowers the patient and their family a little bit because it allows them to be part of a learning process […] part of improving care […] we underestimate how positive that can be to families’ (P24).
By contrast, several parents whose children had chronic conditions framed their decision on trial entry within the more general context of their child’s health care. The decision was not an isolated event, but one of a series of decisions they had to make regarding their child’s care. As such, some parents remarked on how the trial decision was not a particularly significant one:
It didn’t bother me because I’ve had to make that many decisions about [my son] anyway about his health.
(F38)
It’s just all that part of her life. It’s just all hospital […] sort of stuff.
(F14)
When we talk about [my son]’s [condition] I feel like that’s more serious than the [trial] so I feel like I’m probably more relaxed.
(F8)
No practitioners spoke of how families’ experience with chronic illness could leave them feeling ‘more relaxed’ (F8) about a trial decision.
While parents were clear that the decision on trial entry was the prerogative of families, they still valued the support and expert advice of practitioners in their decision-making: ‘they’re the experts’ (F53). Some contrasted this expertise with their own limited knowledge: ‘I don’t know if it should 100% be the parents’ decision. I mean what do I know at the end of the day […] compared to what my doctor knows?’ (F50). Another parent remarked that she would be happy to take advice from her doctor despite ‘always want[ing] control over what happens with your child’ because her ‘judgement might be clouded’ (F27) while the doctor would be able to offer an informed and more impartial opinion.
However, some practitioners were concerned about how their status as trusted professionals and their relationship with a family might make it difficult for families to say ‘no’ to a trial, ‘which perhaps isn’t fair’ (P28). Practitioners were also concerned that a family might think that consenting to a trial ‘must be the right thing’ (P19) simply because their practitioner was the person asking them (P19). One practitioner spoke of the potential to inadvertently influence families: ‘am I really trying to force them down whichever route I want them to go?’ (P18). Another explained that there was no way to avoid such influence. For this practitioner it was impossible to avoid families thinking that the doctor would be ‘annoyed’ if the family declined or ‘impressed if I take part’; the only remedy was to be clear to parents that they could withdraw at any time (P14). Other practitioners resolved this conflict by invoking the ‘duty to do research’ (P1). As such, providing a trial was sound and had been properly reviewed, ‘it is a good thing for the family to become involved with’ (P1).
Contrary to some practitioners’ concerns that they might inadvertently but unduly influence a family’s decision, all parents were clear that the decision had been theirs and their family’s and that it had been voluntary. One parent even suggested that it would be appropriate for practitioners to use their influence more – ‘a gentle nudge from someone in that position to show the benefits that there can be you know can help them to get on board’ (F60).
Trust in medical research
Parents’ decisions about the four trials were influenced by their perceptions of a trial’s safety, benefits and practical inconvenience, as we have described. But it is also likely that their personal beliefs and prior experiences, including their attitudes towards medical research and medicine in general, shaped how they weighed these factors. Many parents who chose to take part in the trial described a strong sense of ‘trust’ and even ‘faith’ in doctors and some linked this to doctors’ expertise. However, this trust also extended beyond the specific practitioner or team who approached them about the trial to encompass the institutions involved in the trial and, indeed, to medicine as a whole. In this way, parents’ confidence in the trial appeared to stem from an assumption that health care in general, and research practice in particular, was well governed. Some parents also expected that research involving children was subject to special, tighter regulation (Box 6).
Some parents commented on their trust in doctors and medicine as a whole:
‘He’s not just somebody because he’s a medical doctor.’ (F39)
‘You trust a doctor, don’t you, and you trust them to sort of do what’s right by your baby.’ (F21)
‘I put a lot of trust in the doctors and I always believe everything they say and I always have done.’ (F1)
‘I am a person who I’ll put a lot of faith in them […] they are the powers that be and they know what’s what […] I trust in what they know really […] I put that in my doctor’s hands.’ (F50)
Parents believed that research was well governed and therefore safe:
‘I probably don’t know what is in place but I’m sure it’s definitely being monitored properly by someone.’ (F31)
‘Those sort of trials, you just feel a bit more secure with them, I think, because it’s under the hospital.’ (F53)
Sometimes parents suggested that research with children was particularly tightly controlled:
‘Obviously they’re not gonna test really, really, really strong tablets on children, so you know that’s safe.’ (F4)
Most parents who consented to the trial were positively inclined towards medical research: ‘brilliant idea […] help better us, as humans’ (F40). For some, this inclination came from a sense that they or their child had personally benefited from research. However, while most parents who consented were positive about research, a few used descriptions that suggested that they viewed research as a necessity, rather than an appealing endeavour – ‘otherwise we’d all be living in the dark ages, we wouldn’t have got anywhere’ (F58) – and as ‘something that needs to be done’ (F53).
None of the parents who declined or withdrew from the trial made comments that suggested that he or she mistrusted individual practitioners, institutions, research or medicine as a whole. Indeed, 3 (F24, F52 and F55) of the 10 parents who declined expressed strongly positive views of research in general:
It’s invaluable. I mean when I think of things, things are progressing all the time […] we kind of think without the research where would we be?
(F24)
However, overt expressions of ‘trust’ or positivity about research were less prominent in the accounts of the other decliners and those parents who withdrew. Those parents were particularly inclined to describe research as a necessity rather than in positive terms, and spoke of research as ‘bound to be safe’ (F30) and the trial medication as ‘not harmful’ (F44), rather than as potentially beneficial.
Changing views of research
Occasionally, consenting parents described how their discussions with practitioners about the trial and experience of participation had altered their initial stereotypical and negative perceptions of research:
You’re just thinking like American TV things where you’re all in little rooms and everyone’s watching you, there’s loads of white coats […] but it’s not like that at all.
(F4)
I’ve always thought it was like really clinical, like white suits basically, everybody clinical. But it’s not been that at all actually, it’s been more relaxed as well than I thought it would have been.
(F15)
One consenting parent expressed strongly negative views of research in general (which she attributed to media reports on animal experimentation) and a consequent urge to immediately decline the trial:
So the word research to me automatically is pain, needles, horrible. No, that’s it straightaway, your first reaction is ‘no’. So that’s why, but once obviously I got it explained to me, it’s not that way.
(F9)
This parent highly valued the consideration and kindness with which she was treated by the practitioner, whose approach possibly helped her to overcome her fears and misunderstandings about research:
She was one of them people you could just like straightaway and trust […] she made me feel at ease, so yeah I felt fine about it.
(F9)
Several parents commented on the storage of genetic materials as part of the trial. The vast majority were unconcerned. Indeed, for some the only concern was the practical difficulty of securing their child’s co-operation with the sample collection process. However, a few parents spoke of concerns connected to publicity about recent lapses in data protection. One mother described her apprehension at having her child’s DNA (deoxyribonucleic acid) on record, which she linked to television reports about DNA databases and people being wrongly identified or information being used in a way that had not been intended (F58). Another expressed initial concerns over the use of the sample: ‘Someone’s gonna have my child’s DNA out there […] it could be used for anything, we don’t know, do we really?’ (F4). In all cases parents were reassured by the trial team.
Reflecting parents’ accounts of their views about research, many practitioners spoke of the need for public education about the value and conduct of research, and this was explicitly validated by one parent:
It’s about public education about what research is and you still occasionally get the view ‘oh I’m not going to be a guinea pig, I don’t want my child to be a guinea pig’. Well, you know, that’s difficult and that’s partly why we know as little as we know about the way that drugs work in children […] You know, have people on the Archers entered into drug trials. Have things about it on, say, other, you know, television soaps, and so on. But it’s about changing the whole attitude of people to experimentation.
(P11)
Because I’m not going to go out and ask for it, but if asked to do it I will, you know. So maybe, maybe we should all openly be asked to be involved a bit more if, because I’m sure they need us.
(F50)
Young people
What is important to young people when considering a trial?
Young people reported many of the same concerns and considerations as parents in deciding about the trials. Many had a clear appreciation that a trial’s purpose was to benefit future patients. Some expressed a passionate desire to help other young people or their doctors and explained that this was an important influence on their decision to take part:
I just wanted to help, just the cause, and just to see if other people could get better quicker.
(F35, 15–16 years)
I will give it a go because, because I might be helping other people right now […] I felt like, like a life saver.
(F48, 8–10 years)
Many young people, particularly in the POP trial, were unwell for long periods and some remarked that they hoped their participation would make life easier for other children in the same position:
It would benefit me and other children in the future for like if they have the same thing they can get medicine and not have to do the study but like get it straight away because I helped.
(F49, 11–14 years)
One practitioner commented on how, compared with parents, young people were motivated to enter a trial by the contribution their participation could make to the broader population: ‘they have a very good altruistic view of sort of wanting to make everything better not just themselves. Parents are often much more focused on their own child’ (P8). Another noted, ‘children generally, as long as it’s not going to hurt them more than necessary are very, very happy to take part […] it’s not like a big block for them usually’ (P12).
Some young people from the POP trial were concerned with the amount of medication that they were already taking and described how they were reluctant to increase this (e.g. F14, F12 and F20). Like parents, a number of the young people expressed concerns about the safety and side effects of medication and whether the trial would require extra injections or blood being taken. In particular, a number described the importance of reassurance that the trial did not involve ‘new medications that they’d never used before’ (F35, 15–16 years):
You have all the thoughts of like, like will there be side effects or whatever then you realise that actually there won’t be because it’s not that kind of trial is it, it’s not that kind of drug.
(F57, 11–14 years)
Some young people, many of whom already had numerous hospital appointments, mentioned the practicalities of participation. For these young people, extra visits to the hospital could deter them from participating:
Just because I would have to go to hospital more and I wouldn’t have wanted to have done that so I wouldn’t have done it.
(F13, 15–16 years)
If it would have impacted on you know, having to, say, either go to the hospital or go swimming, or go out with your friends […] then I would have maybe said it’s not worth, you know, giving up all my extra time, but it hasn’t impacted on it that was, so it’s good.
(F42, 15–16 years)
Like parents, the potential for personal benefit was important for some young people, and they also described their decisions in ways that suggested they weighed up the different aspects of the trial against each other:
I was thinking, this could be really good for me but what if it’s the placebo then, um, it’s like I’m doing it for nothing basically.
(F14, 11–14 years)
It was all new to me, the whole thing, […] at first I thought ‘oh no, I’m not going to do this’ but then I realised that it had benefits for me as well.
(F35, 15–16 years)
You just think like ‘oh […] what are we gonna have to do’ like, ‘what medicines are we gonna take’ and then obviously […] ‘what will happen as a consequence of the medicine’.
(F57, 11–14 years)
Young people’s part in decision-making
Young people’s views on their involvement in the decision varied considerably. Most described how they had been involved in the joint decision to take part in the trial ‘with the doctors and my mum and dad’ (F28, 11–14 years). These young people were content for their parents to guide them and valued their support. They also described how they were happy with the decision and their part in making it:
The nurse went out and I talked to my mum a bit and I just went with her decision and it was good so I just stuck with that.
(F48, 8–10 years)
I wanted my mum, because I like my mum being in on it so that if there’s anything I’m concerned about I can say to my mum.
(F42, 15–16 years)
It was not unusual for young people to describe how their parents ‘sort of influenced me to go ahead with it’ (F60, 11–14 years) or that the decision had really been their parents’, but the young person ‘didn’t have any objection to it’ (F35, 15–16 years).
Occasionally, they described how they themselves had initially been more positive than their parents, and emphasised the importance of reaching a decision that all parties were content with and could support:
I think at first mum was quite negative, not negative, she had loads of questions because she, I think she was worried about side effects and things and later in the interview, well I was really joint with my mum about it really, because I wasn’t going to do it if she wasn’t happy.
(F57, 11–14 years)
I wanted to do it as soon as I found out about it but, and so did daddy but mummy had her concerns […] I didn’t want to do it unless mummy was comfortable doing it.
(F49, 11–14 years)
If I said ‘yes’ but my mum and dad didn’t approve well, well I just probably would say, say ‘can we talk about it?’ because if they don’t approve I won’t be, feel confident about it will I? […] because your family and support is more or less what people need to do things.
(F53, 11–14 years)
These descriptions imply that these young people often had their own ideas about the trial but saw the decision as shared within the context of a supportive family. Indeed, of the 21 young people in this study, only one described making the decision independently. (This young person chose to participate in the RECRUIT study even though her mother declined to be interviewed.)
It was my decision […] because your mum’s not really taking part in it, is she?
(F47, 15–16 years)
The great majority of the young people we interviewed were happy with the level of involvement they had in the decision on trial entry and the decision that was made, but there were some exceptions. Two cases (Cases 6 and 7) warrant particular discussion because they highlight the complex difficulties that a few young people experienced in decision-making about the trial.
One young person described in detail her personal difficulty with making a decision about POP. In the interview she also described finding some elements of trial participation embarrassing and unpleasant. She discussed how she was often uncomfortable talking to people and making eye contact with them, which she was concerned would be perceived as ‘rude’. She seemed to be having great difficulty dealing with her illness generally, and the trial was an additional consideration:
I don’t like saying ‘no’ because I feel like I’m disappointing people and everything so I had to end up very indecisive and having to say ‘yes’ when I don’t really want to say ‘yes’.
She described difficulties relating to decision-making as common in her interactions with health-care professionals in general, and not solely relating to the trial decision:
Sometimes when they say ‘it’s your choice’ it’s like it’s not your choice but they sometimes do just make your choice up for you […] and you feel kind of ‘why do you say it’s my choice when it basically isn’t my choice?’
While this was not a joint interview, the young person’s mother intervened at one point to put her daughter’s comments in context, as if to defend the trial team:
I did sort of feel a bit under pressure and certainly stressed at times. And um sometimes I feel that way, that I’m being pushed into some other things.
I think I ought to explain that you feel that about a lot of things in your life.
yeah I do, I do.
In their joint interview, another young person and her mother discussed what happened when the daughter withdrew from POP. During the interview it gradually became clear that the parents had originally encouraged their daughter to enter the trial. However, for the daughter, the immediate inconveniences of the trial outweighed any potential long-term benefit and she decided to withdraw:
I didn’t think I really wanted to go on at the start but mum and dad persuaded me to. And so, […] when I was getting really fed up I just said ‘No I don’t want to’, because I didn’t like the taste [of the medicine].
Her mother acknowledged that she and her husband had strongly influenced their daughter’s decision but justified this in terms of her future health, adding that she and her husband had reluctantly accepted their daughter’s decision to withdraw from the trial:
Unfortunately it was us really that wanted her to do it more than anything, I’ve got to be honest there. And we did persuade [her], and as I say, we didn’t manage to persuade her for too long because then she backed out.
And when [she] did stop the study we were disappointed […] We didn’t particularly want [her] to have any more medication, but if we thought that it was going to help prevent anything, any bone thinning later on, then we thought […] it’s got to be a good thing.
She knew it was optional because she’d been told that in the first place. Well if she hadn’t been told it was optional we might have managed to keep her on!
Parents’ and practitioners’ views on young people’s part in decision-making
Some parents and a few practitioners described young people’s involvement in the decision as empowering for them whichever direction the decision took and allowing young people to exercise some autonomy or ‘control’. Young people themselves never spoke of the trial decision in these terms.
Perhaps it empowers them a bit, that they are somehow, you know, taking control of their treatment by being part of the study.
(P21)
We’ve had two teenagers refuse the trial […] and that may be a sort of control issue […] ‘I can say no, I’m not going into the study’.
(P7)
I think she probably feels that she’s not had a lot of control since she became ill, […] And that’s why I think it’s so important to allow her to have control over these kind of decisions […] because there’s so much of her life that she can’t control.
(F14)
And [my daughter] was kept fully on board and communicated to directly, along with myself […] I think that was very good as well because [she] felt she had ownership in terms of whether or not to participate.
(F60)
However, one parent emphasised how her 10-year-old son was not old enough ‘to decide things like that’ and described how the team had placed too much importance on involving him in the decision:
It’s a good thing that obviously the child’s opinion is asked […] but it, it was sort of, it could have become a bit of a power thing with the, with him, with the children, […] I thought they sort of, they went on a bit much about it.
(F56)
This highlights the difficulties for practitioners in balancing the interests of both parties when seeking assent and proxy consent, particularly as the precise balance needed will undoubtedly vary from family to family. Most parents felt that it was important for their child to be involved in the decision in a way that was appropriate for them. For very young children and those with learning difficulties it was often the case that parents had made the decision, but sought their child’s assent themselves:
We explained to [my son] as well, if he doesn’t want to do it any more he can just say and it can stop at any time as well, so he understands he’s not being forced to do anything and, and he asked why he was doing it, so we explained it can help other little boys and girls […] so he thinks that’s good.
(F15)
For older children there was a clear recognition from parents that it was important for their child to be involved in the decision, and in some cases parents implied that their child should have decisional authority:
We always sort of discuss everything with her fully, because it’s not happening to me. It’s happening to her.
(F14)
I can say ‘yes’ a hundred times; if you say ‘no’, that’s the end of it […] it’s her body, it’s her life. I’m just the taxi driver.
(F42)
Other parents spoke of guiding their child’s decision – ‘we would have also persuaded her to do it’ (F60) – if they felt that the child was not too keen on participation because ‘I think it’s important’ (F51).
I think they rely on you, as an adult, to say, you know, ‘Is everything, you know, OK? You know, is there anything I really, really need to know?’
(F13)
Summary
In this chapter, we have described the factors which parents and young people felt to be important when considering entry to a trial. Almost without exception, parents prioritised safety over other considerations. However, the benefit to their child and family as well as the potential benefit to others were also important factors in their decision. Practitioners recognised the importance to parents of these considerations, and these same factors were also reflected in the accounts of the young people. Many parents stressed the importance of practical considerations when considering trial entry, and some parents who declined the trial cited practical reasons in explaining their decisions. The importance of minimising a trial’s inconvenience was recognised by practitioners, but some may underestimate the significance of these considerations for parents’ decisions.
Parents’ accounts also indicated that the notion of research as voluntary is more complex than simply being told that they do not have to take part. While all parents told us that they felt no pressure from the trial team and were aware that they could say ‘no’ to the trial, several described personal beliefs and values that would make them reluctant to decline the trial. Similarly, several of those parents who did decline described feeling some degree of discomfort at making this decision.
Many of the parents taking part in this study had positive views of research in general and described a sense of trust in doctors and medicine as a whole. As such, parents valued the advice and opinions of practitioners but were very clear that the decision on trial entry should be theirs, and that it was a decision they valued highly. Parents did not describe the decision as burdensome. Most felt that it was very important to involve their child in the decision and this was something that the young people also emphasised strongly. Young people mostly felt the decision was shared with their parents, while some parents spoke of how they steered their child’s decision. Occasionally, a young person’s emerging autonomy seemed difficult for parents to accept, particularly if parents felt their child was making an ‘unwise’ choice. Some parents described how their views on research had been changed through discussion with trial practitioners. The need to raise public awareness and understanding of research was recognised by both practitioners and parents.
Chapter 5 Discussion
Summary of main findings
This study provides evidence on how to improve recruitment and its conduct in clinical trials of medicines for children. Few previous studies have reported qualitative evidence on the recruitment process from the perspectives of different stakeholders. In this study we interviewed parents, young people and practitioners to identify convergences and divergences in their perspectives and compared these with audio-recordings of family–practitioner discussions of the trials.
Parents’ and practitioners’ experiences of the trial approach
One of the study’s most striking findings was the marked divergence between parents and practitioners in how they regarded the trial approach. While many practitioners viewed an approach as a burden for parents, even in the most difficult situations parents did not mind being asked about trials and they did not describe the approach as burdensome. Indeed, some viewed the trial approach as a positive or exciting opportunity.
Our findings therefore challenge the protective stance towards families that imbues some thinking in this field. Nevertheless, practitioners’ accounts illustrate vividly the complexities they experienced in recruiting to children’s trials, particularly in situations in which parents were already distressed and anxious about their child’s health. Practitioners in all specialties were concerned to avoid overburdening parents with information and strove to find a balance between providing sufficient information, while not overwhelming them. The accounts of some practitioners suggested that they found approaching families about trials to be aversive. (In this chapter we use the term ‘parent’ or ‘young people’ when referring to a specific group and ‘families’ when the comment can be more generally applied to both groups.)
Parents did not express a consistent opinion on whether it was better to be approached by the child’s regular practitioner or one who was not responsible for their child’s clinical care and so was unknown to them. Rather, they tended to emphasise the benefit of whichever ‘model’ they had encountered. However, some young people seemed to prefer interacting with practitioners whom they knew, particularly once they were participating in the trial. Parents felt it important to involve their child in the decision-making and young people concurred, but parents sometimes described trying to steer their child’s decision if they felt their child might choose unwisely. All parties valued the face-to-face discussion more highly than the PILs, and wanted shorter and less complex written information. Some parents expressed disappointment when their children were ineligible to enter a trial, which seemed to be linked to their concerns about returning to ‘pre-trial’ health care.
The ‘look’ and ‘feel’ of trial recruitment
The study’s novel design allowed us to take account of a fundamentally important, but often overlooked, complexity in research on communication: meaning arises in how people experience what is said133 and cannot simply be ‘observed’ in their dialogue. 134 That is, communication can ‘look’ and ‘feel’ very different. Previous studies that have used observational or qualitative interview data to study recruitment cannot take account of this difference. We did so in this study by simultaneously collecting and comparing observational and interview data. Previous research on the ‘look’ of trial communication has been critical of how practitioners communicate about trials and encouraged them to facilitate interactivity. 12,33,35,40,135–137 We found that parents and young people said very little in trial discussions and asked few questions. Evidence suggests that low family interactivity is common to many types of practitioner–family consultation, not just trial discussions. 96,138–140 However, the proportion of family speech relative to practitioners’ was particularly low in our study and may reflect the information-giving function of the trial discussion and the requirement for practitioners to explain the trials. This is further supported by our finding that the trial discussions in which parents and children spoke most tended to be those that required their input to establish eligibility, thereby dictating a more even balance of family versus practitioner speech.
We found that while parents and young people took little part in the trial discussions, they nevertheless felt involved and were highly satisfied about how they had been approached. Judged solely via families’ accounts of the ‘feel’ of the approach, practitioners were communicating well. However, our interviews with parents also identified several cases in which parents had important misunderstandings of the trial. Some of these could stem from the ways in which practitioners communicated and particularly their tendency to use closed rather than open questions in discussing the trial with parents. This often meant that parents’ views about the trial were not explored before practitioners sought their decisions about trial entry.
Families who consent, withdraw or decline
RECRUIT is also one of the few qualitative studies to have compared the views of families who consented to the trial versus those who declined or withdrew. Regardless of whether or not they consented, the factors that influenced parents’ decisions on trial entry were the child’s safety and well-being; potential benefits to the child and family; potential benefits to others; and the practicality of participation. Of these, parents’ paramount consideration was their perception of the trial’s safety. However, the interpretation of all these considerations differed between those who consented and those who declined; for example, while the majority of parents evaluated the trials as ‘safe’, two parents who declined and one who withdrew did so for reasons of ‘safety’. How these factors are evaluated by parents depends not just on the trial itself and how it is presented, but also on individual parents’ previous experiences and beliefs. Parents were clear that they did not feel pressurised by the trial team to participate, but a number described how their personal values made them reluctant to decline, and several parents who did decline described a passing sense of discomfort at the time they made their decision.
These findings advance our understanding of how parents and young people experience trial recruitment and the factors that influence their decisions. Later in this chapter we will discuss the significance of these findings with regard to their implications for enhancing recruitment and its conduct. However, first we will consider the study’s strengths and limitations.
Strengths and limitations
Transferability of findings
Besides its novel multiperspective methods, one of RECRUIT’s key strengths was its scale and scope. We interviewed 60 families who had been approached about one of four trials, from four different paediatric specialties. Eleven different trial teams across seven hospitals recruited the families and took part in interviews themselves. The diversity of the research participants, the children’s medical situations and the trials means our findings have transferability to a broad range of trials that recruit children. The selection of four different trials in contrasting specialties has enabled us to identify how certain types of trials give rise to particular challenges. For example, the complexities for practitioners who recruit to trials of treatments for patients with recently diagnosed serious/critical illness versus those recruiting to trials of treatments for patients with chronic illness, or how trials that use letters as the first step of the approach are likely to have recruitment problems linked to the value parents place on the face-to-face approach. Despite the diversity of the trials included in RECRUIT, it is important to note that all were placebo-controlled trials; were perceived to be ‘low risk’; were largely conducted in hospital rather than community settings; and investigated the efficacy of treatments that were add-ons to the child’s standard clinical care rather than trialling protocols that constituted the core of the child’s treatment.
Study procedures and implementation
Procedurally the study worked well though it was not free of challenges, complexities and delays, as we describe earlier (see Chapter 2, Methods). One aspect of the study’s procedure warrants particular discussion. Trial practitioners sought verbal consent to record the trial discussion and to pass on families’ details to the RECRUIT team. The RECRUIT team later sought informed consent, and this consent was a prerequisite for the trial teams to release the audio-recordings to the RECRUIT team. Despite our initial concerns about the acceptability to families of the audio-recordings and this consent process, most (94%) gave permission for the trial discussions to be recorded. While it could be argued that families might have found it difficult to decline the recordings, 30% of those with recorded trial discussions later declined RECRUIT, indicating that families were able to exercise their autonomy in deciding whether or not to participate. When we asked parents for their opinions about recording and consent procedure for the trial discussions, without exception all reported that they had found these acceptable. Previous studies in the UK have reported difficulty in obtaining such audio-recordings141–143 and we noted reluctance among some practitioners to record trial discussions. This is a potential source of bias in the sampling of trial discussions, as some families may have been perceived as ‘easier’ to approach about the audio-recordings than others.
Sampling of participants
Despite trying several different routes to reach our quota of families who declined a trial (described in Chapter 2, Methods: see Changes to protocol and Other clarifications and changes to the methodology, item 3), we recruited fewer decliners than anticipated and most of those we did recruit were from the MENDS trial (this reflects the efforts of the trial team to assist us in sampling such families and is not necessarily a reflection of the recruitment rate to the trial itself). We acknowledge that this is a limitation of the study. However, as we will suggest in Recommendations for research, below, we believe the recruitment of such families to be a unique methodological challenge, worthy of research in its own right. Nevertheless, as well as the 10 decliners, we also accessed two groups that we did not anticipate sampling at the study outset: families who had withdrawn from a trial and those who were ineligible. The experiences of these groups have previously been little investigated and their accounts provided important insights into the different trajectories families experience after the trial approach. If trial conduct is to be improved from the perspective of all approached families, it is important to investigate their experiences regardless of their trajectory through the trial. Given the divergences that we found between parents and practitioners and the under-representation of decliners in our sample, an important question is how far families’ positive views of the trial approach and the trial itself were a reflection of the limitations of our sampling? This is difficult to resolve. However, it is worth noting that we identified few differences between those who joined and remained in the trials and those who did not. A further question is whether parents felt reticent about directly criticising a practitioner for fear that the relationship or their child’s clinical care may be affected. However, we were careful to reassure families of our independence from the trial teams, and a reluctance to criticise might predict neutral or mildly positive accounts rather than the highly positive ones that parents often gave. Mirroring other research on children’s health, there were few fathers in our sample.
Of the young people we interviewed, most (60%) came from the POP trial. Relatively few spoke in detail about their experiences of being approached about the trials. Although we tried to interview young people separately from their parents, 8 out of 21 opted to be interviewed jointly with their parent. Parents often interjected on behalf of the young people; sometimes this was because young people looked to them to do so. It is conceivable that the young people’s interviews might have taken a different direction had they been interviewed alone. However, it is difficult to see how this could be resolved: some young people may be reticent about speaking to a researcher who was a stranger to them. Without the option to be interviewed in the presence of their parent, they may not have participated at all.
Comparison with existing evidence: what does this study add?
Parents
Interactivity is not synonymous with involvement but clarity is important
Parents’ positive experience of the trial approach did not depend on actively participating in the discussion but it did depend on them feeling valued, cared for and comfortable to interject in the discussion if they wanted. Parents did not explicitly use the word involvement, but their descriptions of the trial approach indicated that, despite their low interactivity, they experienced a sense of involvement shaped by their impressions of the trial approach as a social encounter and their relationship with the practitioner. These findings sit comfortably with research from other clinical contexts in suggesting that interactivity is not synonymous with involvement, and that it is the opportunity, not the obligation, to be involved that is important,144–147 alongside the sense of being free to accept or decline the practitioner’s invitation. 134 Our findings also echo the experience of adult diabetic patients who described associating a sense of ‘involvement’ with the ‘friendly and welcoming’ ‘feel’ of consultations and the ‘interested and respectful’ manner of practitioners. 148 However, previous research on communication about trials has suggested that face-to-face trial discussions have an important role in improving participants’ understanding,149 and that efforts by practitioners to facilitate patient interactivity may be important in helping patients to understand trials. 35,40 In our study, practitioners rarely asked open questions to explore parents’ views or understanding of the trial. We also found that several parents misunderstood aspects of the trials. Such misunderstandings could possibly have been avoided or clarified if practitioners had asked open questions to explore parents’ views on the trial before seeking their decisions about trial entry, as previous research has suggested. 12 Other work has investigated various multimedia interventions to improve informed consent with mixed results,149 although a recent study comparing conventional paper-based methods of delivering information with multicomponent computer-based intervention, comprising visual aids, diagrams and video clips, reported improvements in the understanding of adult patients with diabetes. 150
Discussing trials with parents
In considering how to facilitate a parent’s understanding of a trial, particular attention should be given to the overwhelming priority that he or she gives to doing their best for his or her child and the implications that this has for how practitioners present the trial to parents. Of course, it is important that practitioners present the trial in a way that does not unduly influence a parent’s decision about entry, and, as previous research has identified, that practitioners describe the trial arms in a neutral fashion. 7 Parents often enter a trial in the hope of better treatment. 41 As such they are inclined, as our findings demonstrate, to see the trial medication as offering guaranteed benefits. In view of the insight into parents’ mind-set that our study has provided, it is important that practitioners present the trial as a choice that arises from the need to test the efficacy of the trial medication, rather than as providing access to something that will benefit children if they are allocated to the ‘right’ treatment.
We also found that some parents constructed their understanding of the trial rationale around what they hoped or wanted from it rather than the trial’s actual purpose. To some extent this is beyond the control of the practitioner. However, as others have suggested,151 a process of continual consent throughout the length of trial participation may help to ensure that a parent’s understanding remains adequate. Similarly, trial discussions may benefit from greater clarity about what constitutes routine care and what is additional because it is part of the trial. 33
Nevertheless, our findings point to the complexity of a practitioner’s task in approaching parents about trials, especially in balancing the need to ensure that parents understand the trial while also attending the social norms of the clinical encounter. 152 Parents’ emphasis on the significance of the social dynamics of the trial approach indicates how important it is to avoid disrupting the conversational, welcoming and caring nature of the trial discussion. Their accounts serve to caution against turning the approach into a test or interrogation of parents’ understanding of the trial. Those practitioners in this study who spoke of the importance of listening to parents and not ‘annoying’ them, by continuing to explain the trial after parents had signalled they had heard enough, clearly recognised the need to balance the social and informational functions of the trial discussion. Decision aids are thought to be valuable to adult patients when making difficult treatment or screening decisions. The value of these aids in helping adult patients make decisions about clinical trials has also been reported153 and further studies are under way in adult trials. 154 The value of decision aids for parents considering a trial for their child has not yet been investigated. Future research might consider whether these aids could help maintain the balance between the informational and social functions of the consultation.
Parents of children who were ineligible for one of the trials told us they were disappointed that their child could not enter the trial and expressed concerns about returning to ‘pre-trial’ health care, while parents of participating children spoke of how they valued the extra monitoring and appointments with a specialist that trial entry brought. We are not aware of any previous research on the disappointment associated with ineligibility, and evidence from this study is limited owing to the small number of families concerned. Some practitioners were aware that ineligibility could lead to disappointment and sought to ameliorate it; nevertheless, this issue may warrant further consideration by trial teams.
Prioritising safety
Almost without exception, and as reported in other studies,42,43,51,61 when asked what had influenced their decision on trial entry parents prioritised safety and avoiding harm (which included the temporary distress that procedures such as venepuncture might cause a child). It was clear that parents would not have entered their child if they had doubted the safety of the trial medication. Knowing that a medicine had previously been tested in humans and that it was already in widespread use with children provided parents with a ‘marker’ of a trial medication’s safety and this information was often important in their decisions.
While parents seemed reluctant for their child to be involved in research on ‘new’ medicines, they rarely mentioned the licensing of medicines for children. The use of unlicensed or ‘off-label’ drugs is commonplace in paediatrics. 155,156 Children need access to medication but the necessary clinical trials have often not been conducted, leaving paediatric practitioners with no option but to prescribe based on evidence from adults. Lenk and colleagues157 point out that ‘off-label’ prescribing in paediatrics is ‘so commonplace that it is often probably not specifically mentioned when a drug is administered to a patient’. Not surprisingly therefore, licensing was discussed in only 3 out of the 41 trial discussions that we recorded (two of these were MASCOT trial discussions and both involved practitioners emphasising how all the trial medications were licensed in children – MASCOT is the only trial in RECRUIT in which the trial medications were licensed for the particular indication in the particular age group). The parents we interviewed seemed unaware of the complexities surrounding licensing children’s medicines. Given the emphasis they placed upon safety, it is conceivable that knowing that trial medication was unlicensed for children could have made parents reluctant to consent. Indeed, one parent withdrew her child from the trial after discovering, via the internet, that the trial medication was unlicensed for children. In a quantitative survey comparing parents of healthy children and those of chronically ill children, Lenk and colleagues157 reported that only 28% and 35% of the respective parent groups were aware that children are sometimes prescribed unlicensed medicines, and 20% and 9% of these groups, respectively, said that they would refuse such treatments. Future qualitative research is necessary to investigate parents’ understanding and views about the use of unlicensed medicines in children and the role of trials in the licensing process.
Benefits and practicalities
Having established that the trial was safe, parents emphasised the trial’s benefits and practicalities as important influences on their decisions. They spoke of the benefits that the trial might provide to the family as a whole, as well as to their child specifically. In describing their decisions, parents also cited altruistic considerations, such as the benefits of the trial to future generations of children, and to society and medicine. The practicalities involved in participating in a trial were also important to parents. However, these appeared to be the factors that swung parents’ decisions towards trial entry or decline after they had first considered the trial’s safety and benefits, rather than being the factors that parents considered first.
Conditional altruism
The accounts of many parents in this study suggested that altruistic considerations were important in their trial entry decisions. Similarly, studies have found that parents frequently endorse altruism as a reason for consenting to trials. 60,62,71 The role of altruism in trial decision-making is difficult to unpack, particularly as participants were retrospectively explaining their decisions. It is important not to underestimate the role of altruism for families in their overall experience of trial participation; however, at what stage it becomes important is less clear. Many families, arguably more so when children are very ill, may draw comfort from the knowledge that their participation may contribute to medical knowledge and the well-being of future children. Jollye51 presents the pathway described by parents considering a neonatal trial, having first to determine that the trial would not harm the baby before they could proceed to describe ‘altruistic views’ influencing their decisions. Parents who perceived harm at this stage said ‘no’ to the research. Some went on to describe feeling guilty, such feelings perhaps representing the opposite of the ‘feel-good factor’ that partners a sense of acting for the benefit of others. We found that while parents mentioned altruism frequently, and with some conviction, at the same time these accounts were generalised or idealised. Parents therefore talked about ‘making things better’, ‘helping other people’ or ‘moving things on’ rather than citing specific objectives of the trial and what their participation would achieve. In indicating that parents had perhaps given less in-depth consideration to the altruistic aspects of trial participation relative to safety, personal benefits and practicalities, our findings broadly support previous findings from adult trial participants that the motivating effect of altruism is conditional upon believing that a trial is safe and potentially offers an opportunity for personal benefit – ‘helping others’ was not seen as a sufficient reason for taking part. 158 Altruism is a complex and contested concept and its role in the decision-making process when considering a trial for oneself remains difficult to specify with precision. Moreover, respondents’ constructions of their decision-making process are potentially vulnerable to social desirability. 159 The role of altruism when deciding on behalf of another individual, be it one’s child or an adult who does not have capacity, is further complicated. The extent to which one can act altruistically on behalf of another is a bioethical debate beyond the remit of this report. However, given the children’s vulnerability, the special character of the parent–child relationship and parents’ role in protecting children’s well-being, it is conceivable that altruistic considerations play a relatively smaller role, and safety and personal benefits play relatively greater roles in parents’ deliberations about children’s trials compared with patients’ deliberations about adults’ trials. Future research comparing trials that recruit both adult and child participants would be necessary to investigate this.
Prioritising influences on trial entry
While safety, benefits and practicalities have previously been identified as important in families’ decision-making14,49,50,57,59,71 this study adds two important dimensions. First, the factors identified in this study were voiced by the parents themselves, whereas much of the previous literature has asked parents to rate the importance of factors that researchers have predetermined. Our study therefore provides more insight into the relative importance to parents of the different influences on their decisions. It also avoids the likelihood that parents respond positively to questions about ‘altruism’ simply because they tick a box corresponding to the most socially desirable response. This study provides new evidence that parents’, young people’s and practitioners’ views on what is important when considering a trial are broadly convergent, with the exception of practicalities. This convergence is important because it suggests that the practitioners in our study were well placed to structure their explanations according to families’ needs and priorities. The positive accounts that parents gave about how practitioners conducted the trial approach confirm this, including parents’ remarks about how well practitioners anticipated their questions. Previous research suggesting that practitioners and parents differ in their prioritisation of the benefits of research160 was not confirmed by our study. However, the groups in our study differed in how they prioritised practicalities. Parents in our study placed considerable importance on trial practicalities, yet practitioners sometimes gave relatively little emphasis to such considerations in the trial discussions and in their interviews. This finding could help practitioners to structure the trial approach in a way that better reflects families’ priorities.
Young people
Eliciting questions
A number of studies have investigated young people’s views on their role in decision-making or communication about their health. 95,161 Few previous studies have investigated young people’s views of trials82,83 and none has contrasted young people’s views of trials with data from the trial discussions with practitioners. In this study, young people told us that they felt involved and were highly satisfied with how they had been approached, yet in the trial discussions they said very little – significantly less than their parents. Despite most practitioners inviting their questions, only two young people asked a question. Because formulating questions in response to a direct invitation can be challenging, even for adults, as our findings on parents testify, it is perhaps not surprising that most young people did not ask questions even when invited. An invitation such as ‘do you want to ask any questions?’ may be too general and direct for many young people. To elicit their questions, practitioners may need to pace, scaffold and break down their invitations into more manageable chunks such as, ‘is there anything you would like to ask me about the tablets?’. 162–164 Such techniques may also be helpful in eliciting parents’ questions.
Not silencing young people, not sidelining parents
Most young people described a joint decision-making process with their parents, as reported elsewhere,94,102,104 but the relative influence of parents and young people in this process was complex. Young people felt that their role in the decision was important, but they often looked to their parents for guidance and were sometimes happy for their parent to steer the decision. The majority of young people told us that they were also happy with their level of involvement in the decision, but the two specific cases discussed in Chapter 4 (see Young People’s part in decision-making) raise two considerations. First, young people may find it difficult to engage with unfamiliar adults, particularly those in a position of authority. For younger children especially, it may be helpful for practitioners to seek parents’ advice on how best to approach young people about trials, so as to gain an initial insight into their preferences regarding who should explain the trial. Second, practitioners should, nevertheless, consider the influence that parents can have on their children’s decisions. For some families the young person’s emerging autonomy may be difficult to accept, particularly if they are seen to be making choices that parents consider to be unwise. Practitioners may therefore consider offering young people the chance to discuss the trial separately from their parents, with the agreement of all parties. We found that when parents and young people were interviewed together, parents could dominate the discussion and it is possible that practitioners may be better able to identify a young person’s reticence about the trial when his or her parents are not present. While considerable emphasis is placed on the number of participants recruited to trials, retention and compliance are also key to a trial’s success. Again, practitioners must maintain a difficult balance between not sidelining parents, whose opinions and advice are valued by the young people and important in their own right, while at the same time recognising that young people may find it hard to interject in a conversation between adults and may need additional support.
We noted that when young people spoke of the trial approach, they often responded as if they were talking about the preferences or concerns of other young people, rather than directly voicing these concerns as their own. For example, young people spoke of what ‘younger children’ or other ‘kids’ would think rather than what ‘I’ thought. This tendency was prominent when young people were speaking of how they might respond emotionally to the trial approach and the importance of practitioners not ‘scaring people’. In their interviews, young people may have spoken in this way in an attempt to distance themselves from their concerns and thereby save face, because they did not want to upset anyone, or because of concerns that their comments might result in the trial staff being reprimanded. It is common practice to use puppets or cartoons when asking young children to talk about things which they may find difficult, allowing them to express their views in the third person. 165 We can only speculate on whether the young people in our study were themselves intuitively using this ‘third-person’ technique to distance themselves from their views. Whatever the reason, young people need support in expressing their views when approached about trials. Practitioners need to be alert to the possibility that young people might express their views in rather indirect ways. Indeed, practitioners could perhaps explore with a young patient what other young people might think about trials, as a prelude to asking more direct questions regarding the young person’s own views about the trial.
Practitioners
Helping parents understand without overwhelming them
Practitioners were often concerned about overburdening parents with information, both written and verbal, as has previously been reported. 46 They particularly described the difficulty in ensuring that a parent had sufficient understanding to make a decision but not overwhelming them. 46,110 However, some practitioners accepted that parents might make a decision on trial entry with a rudimentary understanding; they saw families’ preferences to constrain trial information, or to seek a practitioner’s advice about a trial, as an appropriate response in a stressful situation. This parallels Kukla’s166 postulations that autonomy is not synonymous with self-determination, and that when patients are faced with a decision that they feel unable, or have insufficient expertise, to make, deciding to trust a practitioner’s judgement can be a meaningful form of autonomy.
Parents as intermediaries
Practitioners voiced concerns about the amount and level of information they had to provide parents rather more often than they voiced such concerns in relation to young people. We speculate that practitioners in this study primarily focused on making sure that parents understood the trials, and that they accorded less importance to ensuring young people’s understanding. In this way, practitioners acted in accordance with parents’ ‘executive role’,95 whereby parents operate as intermediaries for their children, for example in interpreting the trial information for them. Practitioners’ accounts here also reflect the legal framework governing children’s trials, as it is parents, not children, who provide informed consent for children to enter trials. This intermediary parental role echoes previous research92,102 and seemed to fit with how parents in this study themselves described their role, for example in going through the children’s PIL with their child to rephrase its content in line with their child’s needs. It also corresponded with some parents’ descriptions of how their children tended to ask them questions after the consultation rather than ask the practitioner. This seemed to be a role that parents expected to fulfil. Indeed, it was arguably one that all parties co-operatively engineered. Given parents’ unique knowledge of their child, and young people’s reticence in interacting with unfamiliar practitioners, parents have an important role in explaining the trial to young people in a way that is understandable and non-threatening. Of course, this assumes that parents understand the trial themselves.
Supporting practitioners in approaching families
Many practitioners commented that approaching a family to consider a trial could be an additional burden for parents. This was particularly prominent in the accounts of those practitioners recruiting to trials where the child was seriously or critically ill, some of whom described the approach in ways that suggested that they found this aspect of their work aversive. These experiences are at odds with the accounts of parents who, as already discussed, were accepting of the trial approach. Practitioners’ accounts point to potential negative consequences for their morale and future involvement in clinical trials. None of the practitioners in this study reported that his or her personal discomfort stopped him or her approaching families, but previous studies have made such a link. 111,122 If practitioners are deterred from approaching families for fear of overburdening them, it may deny potential participants the opportunity to make their own choice on research, which the parents in our study valued. Our study shows that the dilemmas inherent in research may be of more concern for the practitioner than for the parent. Parents in this study were on the whole predisposed to help with research as long as they perceived no harm to their child, often perceiving participation as acting for the general good. This could be invaluable knowledge for practitioners to bear in mind as it may help them to overcome the discomfort some experience in approaching families, particularly under difficult circumstances.
Implications
The findings of this study have a number of important implications for practitioner training and the conduct and design of trials.
Mentoring for practitioners
Some practitioners described the trial approach in ways that suggested that they found it aversive, while many were concerned that the approach was burdensome for families. Ongoing ‘moral support’ or mentoring for recruiting practitioners, alongside training in the recruitment process and informed consent, would allow practitioners to reflect openly on the emotional and ethical aspects of recruitment. Having opportunities for practitioners to informally discuss their concerns and get advice and feedback on practice may be particularly beneficial for less experienced practitioners and those working in specialties in which families are under considerable emotional strain. This support could potentially be built into existing mentoring arrangements, or developed with the help of LRNs. Dissemination of the RECRUIT study findings will have an important part to play in this process, for example reflection on the finding that parents were generally predisposed to help with research if they felt able to may ease the discomfort that some practitioners experience in approaching families.
Practitioner training
Practitioner training should reflect how trial recruitment is a social process, as well as a procedural and legal one. While previous research has addressed clarity in trial discussions, the importance of the experience or ‘feel’ of the trial approach to the different parties has largely been overlooked. Our findings indicate the social and emotional complexity of the trial approach and how practitioners have to negotiate several tensions including (1) giving ‘enough’ information without overwhelming families; (2) attending to the social norms of the encounter while ensuring clarity and understanding of the trial; and (3) balancing the needs of parent and young person. Given the considerable importance that families in this study placed on the ‘feel’ of the trial discussion relative to its informational content, it is important to consider the social and emotional dynamics of the trial discussion in designing recruitment training, and not focus exclusively on the procedural and informational aspects of informed consent.
Participant information leaflets
Both practitioners and parents were dissatisfied with the current PILs, viewing them as too lengthy and complicated. Practitioners attributed these flaws to the need to follow what they perceived as stringent guidelines and directions from RECs on the design and content of PILs. These findings add to the growing body of literature suggesting that regulatory guidelines may be at odds with the requirements of families and practitioners, and hinder rather than facilitate families’ understanding of trials. 167,168 A review of the current guidelines on PILs,169 taking into account parents’, young people’s and practitioners’ perspectives, may enhance the value and usefulness of these documents for all parties.
Clarity in the trial discussion
In the trial discussions, practitioners rarely asked parents open questions. We identified parental misunderstandings, some of which may have been avoided had practitioners used open questions to elicit families’ views and understanding of the trial before seeking their trial entry decisions. However, as we note below, to reflect the social dynamics of family–practitioner interactions, such questioning needs to be conversational and conducted in a way with which families and practitioners are comfortable.
Establishing what constitutes normal clinical care at the start of the discussion may also help parents to understand what procedures and treatments are part of the trial and thereby help them to distinguish between routine clinical care and care that is given as part of a trial. A policy of continuous consent may help to maintain this distinction. Many parents choose to enter a trial in the hope that it will benefit their child. Our analysis showed that some parents saw the trial medication as offering a guaranteed benefit. For clarity, it is important that practitioners describe the treatment arms in a neutral fashion and stress that the purpose of the trial is to assess the efficacy of the trial medication.
To help young people in the trial discussion, practitioners may consider asking parents’ advice on how best to approach them. This could involve offering young people the chance to discuss the trial separately from their parents, if this is acceptable to all parties. Practitioners should also be aware that young people might find it difficult to express their views directly and may need additional support.
Parents’ discomfort in saying ‘no’ to research
Several parents who consented to the trial expressed discomfort in saying ‘no’ to research. Many of the reasons parents gave suggested a personal sense of ‘moral duty’. For example, they described an obligation to medicine, to the hospital and to the practitioners. They felt that non-participation would let down the trial team or hinder the advancement of medical knowledge. Five of the parents who declined described a passing sense of ‘guilt’ at their decision not to take part. All traced their unease to recognition that the trial was important and a wish to help.
Mirroring parents, several practitioners were conscious of the potential difficulty for parents in declining a trial. While parents were clear that they did not feel pressurised to consent by practitioners, these data raise the question of how far practitioners should go to facilitate parents in saying ‘no’ to research. For some families saying ‘no’ to research may be difficult without ‘permission’ from the practitioner. However, such facilitation could inadvertently send the message that the trial is not important. Moreover, how far can practitioners be responsible for parents’ personal beliefs and values that are the source of such discomfort and which, in this study at least, were transient for those who did decline?
Such tensions indicate caution in prescribing how practitioners should manage these situations and point to the value of enabling practitioners to respond according to their judgement of each family’s individual situation. When parents do decline, discomfort could perhaps be ameliorated if practitioners endorsed parents’ decisions, for example by acknowledging that, above all else, it is important that families feel able to make a decision that they are comfortable with.
Trial designs that make participation easier
Parents and some young people in this study described the importance of the practical requirements of the trial in terms of the disruption associated with extra trips to hospital and absences from school or employment. Some parents cited such practicalities as the reason why they had declined the trial, while others stated that if the trial placed greater demands upon them, they would have declined. The practical requirements of a trial could also potentially affect retention (although not investigated in this study). Practicalities are therefore important to consider at the trial design stage, in patient and public involvement consultation with families. Working with families at the trial design stage to arrange the schedule of visits to the trial site, providing staffing for home or school visits, and designing trials with built-in flexibility, in terms of both out-of-hours appointments and the time-frame in which appointments must happen, could allow more parents to say ‘yes’. It is also important to discuss practicalities with families at the time of the trial approach: practitioners in this study often spent more time explaining the trial design and rationale than discussing its practicalities, which could inadvertently send a message to families that such factors are an afterthought.
Aiding families’ understanding
Although the majority of families in this study had a working understanding of issues such as randomisation and placebos, the use of simple educational aids embedded in the trial discussion, such as mock trial medications and family-friendly flow charts, might help families to visualise the different trial stages and arms of the trials. The use of such aids may help practitioners craft explanations that help avoid misunderstandings and may be particularly helpful when explaining trials to children and young people.
Public education about research
Most parents in this study were positively orientated towards medical research in general. Nonetheless, several expressed initial reservations about medical research, which they linked to the negative connotations that medical research had for them, for example referring to ‘white coats’ and animal experimentation. Such stereotyped views suggest a need for greater public awareness and education to help transform beliefs about medical research in general. Public education about clinical trials would also be helpful so that when families are approached about a particular trial they already have some basic research literacy. This would reduce the amount of effort involved in deciding on trial entry at what is sometimes a very difficult point in families’ lives. Such education might also help to create a culture in which people expect to be asked to participate in research. If the concept of research and trials was less alien to families, this would also ease the burden of explanation that currently falls to trial practitioners, allowing them to focus on the specifics of the trial at hand.
Recommendations for research
As described above, the findings of this study have led directly to a number of implications for practitioner training, current practice and trial design. Implementation of several of these could be informed by further research:
-
Research with practitioners to identify (1) the specific forms of support that practitioners need and how to deliver it in an acceptable, effective and efficient way, particularly for those practitioners who experience difficulties in approaching or recruiting participants, and (2) how to make clinical trial work more attractive to paediatric practitioners, perhaps through research with practitioners who choose not to be involved in clinical trial work, or who discontinue their involvement in trials at an early stage in their careers.
-
The findings of the current study should be expanded by research to explore the views of more parents and young people who decide not to take part in a trial. This should include those who do not have a positive disposition towards medical research to provide insights into the difficulties practitioners encounter when they approach such families and thereby inform practitioner support and training. Such research would also inform strategies for public education about research. This study encountered considerable difficulty accessing families who declined trials. The question of how best to recruit these families is a research question in its own right. People decline trials at a number of different stages, and possibly for different reasons that are as yet poorly understood. Fathers’ views about children’s participation in trials should also be investigated.
-
Some families who declined a trial expressed guilt at saying ‘no’. Some who consented described how they personally would feel uncomfortable declining. This requires further investigation and clarification, perhaps with particular attention to how declining is perceived to affect the doctor–patient relationship.
-
Qualitative research to more fully investigate parents’ knowledge and views about the use of unlicensed medicines in children and how they might be influenced by information about the role of trials in the licensing process is required.
RECRUIT has also shed light on several topics, which, although less central to the main findings and implications, are potential areas for further investigation:
-
Who should approach families about trials? Evidence from this study does not indicate that parents consistently prefer an approach by a known practitioner over one who is not responsible for their child’s treatment and whom they have not met before. Nor did parents report being pressurised by practitioners. However, it is possible that parents are influenced to consent to a trial, arising from their relationship with the practitioner, in ways that are difficult for them to verbalise. Other types of evidence, combined with ethical analyses and theory, are required to fully address this complex question.
-
Research is needed into family, practitioner and public perspectives on trials with designs that differ from those considered in this study, including trials with ‘controversial’ consent processes such as those in emergency medicine.
-
Differences between trial recruitment in adult and child care settings should be investigated to establish the different support needs in approaching child versus adult patients about clinical trials.
-
The value of decision aids has not yet been investigated in trials of medicines for children. Work is needed to evaluate the role, effectiveness and acceptability of such aids, particularly when families are making decisions about complex trials or when the family is already under considerable strain. Similarly, investigation of the role and effectiveness of simple educational aids embedded in the trial discussion would be illuminating.
As highlighted in Chapter 1, there is a large but disparate literature on recruitment to trials of medicines for children. With the increase in clinical trial activity in this area, the number of publications on families’ and practitioners’ perspectives is likely to increase. A systematic review of this literature, with particular emphasis on ways to improve trial design to make it more acceptable to families, would inform future trials and further improve recruitment and its conduct.
Acknowledgements
We thank all of the participants, the staff at the LRNs and the RECRUIT steering group members for their generous help with the study. We thank the trial teams that recruited families to the study:
-
MASCOT University Hospital of North Staffordshire, Stoke-on-Trent, UK; Royal Manchester Children’s Hospital, Manchester, UK; Alder Hey Children’s NHS Foundation Trust, Liverpool, UK
-
MENDS Alder Hey Children’s NHS Foundation Trust, Liverpool, UK; Royal Manchester Children’s Hospital, Manchester, UK
-
POP Musgrave Park Hospital, Belfast, UK; Alder Hey Children’s NHS Foundation Trust, Liverpool, UK; Bristol Royal Hospital for Children, Bristol, UK
-
TIPIT Liverpool Women’s Hospital, Liverpool, UK; St Mary’s Hospital, Manchester, UK; Royal Preston Hospital, Preston, UK.
During the final preparation of this report, VS was employed by the Child Health Group, Peninsula Medical School, University of Exeter, Exeter, which we thank for its support.
We would like to thank the three anonymous reviewers for their positive, thoughtful and constructive comments.
Contribution of authors
Valerie Shilling co-ordinated the study, recruitment, interviewing and data analysis and led the drafting of the report. Paula Williamson conceived the initial idea for the study, which Bridget Young developed and designed in consultation with Paula Williamson, Rosalind Smyth and Helen Hickey. Paula Williamson, Rosalind Smyth and Helen Hickey were grant co-applicants. They contributed to the management of the study, reviewed detailed reports of the analysis and contributed to the drafting of the report. Emma Sowden contributed to the recruitment and interviewing of participants and to data analysis. Bridget Young was principal investigator and grant holder. She supervised all aspects of the research and contributed to the data analysis and drafting of the report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Klasser TP, Hartling L, Hamm M, van der Lee JH, Ursum J, Offringa M. StaR Child Health: an initiative for RCTs in children. Lancet 2009;374:1310-12.
- Smyth RL. Making a difference: the clinical research programme for children. Arch Dis Child 2007;92:835-7.
- Smyth RL, Edwards AD. A major new initiative to improve treatment for children. Arch Dis Child 2006;91:212-13.
- Brown JJ, Zacharin MR. Attempted randomized controlled trial of pamidronate versus calcium and calcitriol supplements for management of steroid-induced osteoporosis in children and adolescents. J Paediatr Child Health 2005;41:580-2.
- Sinha SK, Lacaze-Masmonteil T, Valls i Soler A, Wiswell TE, Gadzinowski J, Hajdu J, et al. A multicenter, randomized, controlled trial of lucinactant versus poractant alfa among very premature infants at high risk for respiratory distress syndrome. Pediatrics 2005;115:1030-8.
- Sammons HM, Choonara I. Clinical trials of medication in children, 1996–2002. Eur J Clin Pharmacol 2005;61:165-7.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70.
- Jenkins V, Fallowfield L, Solis-Trapala I, Langridge C, Farewell V. Discussing randomised clinical trials of cancer therapy: evaluation of a Cancer Research UK training programme. BMJ 2005;330.
- Brown RF, Butow PN, Boyle F, Tattersall MH. Seeking informed consent to cancer clinical trials; evaluating the efficacy of doctor communication skills training. Psychooncology 2007;16:507-16.
- Ungar D, Joffe S, Kodish E. Children are not small adults: documentation of assent for research involving children. J Pediatr 2006;149:31-3.
- Steinbrook R. Testing medications in children. N Engl J Med 2002;347:1462-70.
- Kodish E, Eder M, Noll RB, Ruccione K, Lange B, Angiolillo A, et al. Communication of randomization in childhood leukemia trials. JAMA 2004;291:470-5.
- Campbell FA, Goldman BD, Boccia ML, Skinner M. The effect of format modifications and reading comprehension on recall of informed consent information by low-income parents: a comparison of print, video, and computer-based presentations. Patient Educ Couns 2004;53:205-16.
- Ballard HO, Shook LA, Desai NS, Anand KJ. Neonatal research and the validity of informed consent obtained in the perinatal period. J Perinatol 2004;24:409-15.
- Burgess E, Singhal N, Amin H, McMillan DD, Devrome H. Consent for clinical research in the neonatal intensive care unit: a retrospective survey and a prospective study. Arch Dis Child Fetal Neonatal Ed 2003;88:F280-5.
- Ruccione K, Kramer RF, Moore IK, Perin G. Informed consent for treatment of childhood cancer: factors affecting parents’ decision making. J Pediatr Oncol Nurs 1991;8:112-21.
- Paediatric European Network for Treatment of AIDS (PENTA) . Parents’ attitudes to their HIV-infected children being enrolled into a placebo-controlled trial: the PENTA 1 trial. Paediatric European Network for Treatment of AIDS. HIV Med 1999;1:25-31.
- Tait AR, Voepel-Lewis T, Malviya S. Factors that influence parents’ assessments of the risks and benefits of research involving their children. Pediatrics 2004;113:727-32.
- Masty J, Fisher CB. A goodness-of-fit approach to informed consent for pediatric intervention research. Ethics Behav 2008;18:139-60.
- Quirk M, Mazor K, Haley HL, Philbin M, Fischer M, Sullivan K, et al. How patients perceive a doctor’s caring attitude. Patient Educ Couns 2008;72:359-66.
- Eder ML, Yamokoski AD, Wittmann PW, Kodish ED. Improving informed consent: suggestions from parents of children with leukemia. Pediatrics 2007;119:e849-59.
- Postlethwaite RJ, Reynolds JM, Wood AJ, Eminson DM. Recruiting patients to clinical trials: lessons from studies of growth hormone treatment in renal failure. Arch Dis Child 1996;74.
- Miller VA, Drotar D, Burant C, Kodish E. Clinician–parent communication during informed consent for pediatric leukemia trials. J Pediatr Psychol 2005;30:219-29.
- Snowdon C, Elbourne D, Garcia J. Declining enrolment in a clinical trial and injurious misconceptions: is there a flipside to the therapeutic misconception?. Clin Ethics 2007;2:193-200.
- Fisher CB. Commentary: SES, ethnicity and goodness-of-fit in clinician-parent communication during pediatric cancer trials. J Pediatr Psychol 2005;30:231-4.
- Wiley FM, Ruccione K, Moore IM, McGuire-Cullen P, Fergusson J, Waskerwitz MJ, et al. Parents’ perceptions of randomization in pediatric clinical trials. Children Cancer Group. Cancer Pract 1999;7:248-56.
- Simon C, Zyzanski SJ, Eder M, Raiz P, Kodish ED, Siminoff LA. Groups potentially at risk for making poorly informed decisions about entry into clinical trials for childhood cancer. J Clin Oncol 2003;21:2173-8.
- Stenson BJ, Becher JC, McIntosh N. Neonatal research: the parental perspective. Arch Dis Child Fetal Neonatal Ed 2004;89:F321-3.
- Glannon W. Phase I oncology trials: why the therapeutic misconception will not go away. J Med Ethics 2006;32:252-5.
- Levi RB, Marsick R, Drotar D, Kodish ED. Diagnosis, disclosure, and informed consent: learning from parents of children with cancer. J Pediatr Hematol Oncol 2000;22:3-12.
- Stevens PE, Pletsch PK. Ethical issues of informed consent: mothers’ experiences enrolling their children in bone marrow transplantation research. Cancer Nurs 2002;25:81-7.
- Mason SA, Allmark PJ. Obtaining informed consent to neonatal randomised controlled trials: interviews with parents and clinicians in the Euricon study. Lancet 2000;356:2045-51.
- Simon CM, Siminoff LA, Kodish ED, Burant C. Comparison of the informed consent process for randomized clinical trials in pediatric and adult oncology. J Clin Oncol 2004;22:2708-17.
- Angiolillo AL, Simon C, Kodish E, Lange B, Noll RB, Ruccione K, et al. Staged informed consent for a randomized clinical trial in childhood leukemia: impact on the consent process. Pediatr Blood Cancer 2004;42:433-7.
- Yap TY, Yamokoski A, Noll R, Drotar D, Zyzanski S, Kodish ED. A physician-directed intervention: teaching and measuring better informed consent. Acad Med 2009;84:1036-42.
- Yamokoski AD, Hazen RA, Kodish ED. Anticipatory guidance to improve informed consent: a new application of the concept. J Pediatr Oncol Nurs 2008;25:34-43.
- Featherstone K, Donovan JL. ‘Why don’t they just tell me straight, why allocate it?’ The struggle to make sense of participating in a randomised controlled trial. Soc Sci Med 2002;55:709-19.
- Featherstone K, Donovan JL. Random allocation or allocation at random? Patients’ perspectives of participation in a randomised controlled trial. BMJ 1998;317:1177-80.
- Mills N, Donovan JL, Smith M, Jacoby A, Neal DE, Hamdy FC. Perceptions of equipoise are crucial to trial participation: a qualitative study of men in the ProtecT study. Control Clin Trials 2003;24:272-82.
- Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28.
- Shilling V, Young B. How do parents experience being asked to enter a child in a randomised controlled trial?. BMC Med Ethics 2009;10.
- Caldwell PH, Butow PN, Craig JC. Parents’ attitudes to children’s participation in randomized controlled trials. J Pediatr 2003;142:554-9.
- Singhal N, Oberle K, Burgess E, Huber-Okrainec J. Parents’ perceptions of research with newborns. J Perinatol 2002;22:57-63.
- Kumar A, Soares H, Wells R, Clarke M, Hozo I, Bleyer A, et al. Are experimental treatments for cancer in children superior to established treatments? Observational study of randomised controlled trials by the Children’s Oncology Group. BMJ 2005;331.
- Liaschenko J, Underwood SM. Children in research: fathers in cancer research: meanings and reasons for participation. J Fam Nurs 2001;7:71-9.
- Kodish ED, Pentz RD, Noll RB, Ruccione K, Buckley J, Lange BJ. Informed consent in the Childrens Cancer Group: results of preliminary research. Cancer 1998;82:2467-81.
- Snowdon C, Elbourne D, Garcia J. Zelen randomization: attitudes of parents participating in a neonatal clinical trial. Control Clin Trials 1999;20:149-71.
- Morley CJ, Lau R, Davis PG, Morse C. What do parents think about enrolling their premature babies in several research studies?. Arch Dis Child Fetal Neonatal Ed 2005;90:F225-8.
- Zupancic JA, Gillie P, Streiner DL, Watts JL, Schmidt B. Determinants of parental authorization for involvement of newborn infants in clinical trials. Pediatrics 1997;99.
- Eiser C, Davies H, Jenney M, Glaser A. Mothers’ attitudes to the randomized controlled trial (RCT): the case of acute lymphoblastic leukaemia (ALL) in children. Child Care Health Dev 2005;31:517-23.
- Jollye S. An exploratory study to determine how parents decide whether to enrol their infants into neonatal clinical trials. J Neonatal Nurs 2009;15:18-24.
- Campbell H, Surry SA, Royle EM. A review of randomised controlled trials published in Archives of Disease in Childhood from 1982-96. Arch Dis Child 1998;79:192-7.
- Ablett S, Pinkerton CR. Recruiting children into cancer trials: role of the United Kingdom Children’s Cancer Study Group (UKCCSG). Br J Cancer 2003;88:1661-5.
- Chantler TE, Lees A, Moxon ER, Mant D, Pollard AJ, Fiztpatrick R. The role familiarity with science and medicine plays in parents’ decision making about enrolling a child in vaccine research. Qual Health Res 2007;17:311-22.
- Kupst MJ, Patenaude AF, Walco GA, Sterling C. Clinical trials in pediatric cancer: parental perspectives on informed consent. J Pediatr Hematol Oncol 2003;25:787-90.
- Chappuy H, Doz F, Blanche S, Gentet JC, Pons G, Treluyer JM. Parental consent in paediatric clinical research. Arch Dis Child 2006;91:112-16.
- Pletsch PK, Stevens PE. Inclusion of children in clinical research: lessons learned from mothers of diabetic children. Clin Nurs Res 2001;10:140-62.
- Yeo GS, Barnwell AJ, Barkovich AJ, Partridge JC. Parents’ decisions to enroll asphyxiated infants in a research study: understanding and satisfaction. Invest Ophthalmol Vis Sci 1996;37.
- Gammelgaard A, Knudsen LE, Bisgaard H. Perceptions of parents on the participation of their infants in clinical research. Arch Dis Child 2006;91:977-80.
- van Stuijvenberg M, Suur MH, de Vos S, Tjiang GC, Steyerberg EW, Derksen-Lubsen G, et al. Informed consent, parental awareness, and reasons for participating in a randomised controlled study. Arch Dis Child 1998;79:120-5.
- Tait AR, Voepel-Lewis T, Siewert M, Malviya S. Factors that influence parents’ decisions to consent to their child’s participation in clinical anesthesia research. Anesth Analg 1998;86:50-3.
- Sammons HM, Atkinson M, Choonara I, Stephenson T. What motivates British parents to consent for research? A questionnaire study. BMC Pediatr 2007;7.
- Hoehn KS, Wernovsky G, Rychik J, Gaynor JW, Spray TL, Feudtner C, et al. What factors are important to parents making decisions about neonatal research?. Arch Dis Child Fetal Neonatal Ed 2005;90:F267-9.
- Reynolds WW, Nelson RM. Risk perception and decision processes underlying informed consent to research participation. Soc Sci Med 2007;65:2105-15.
- Pletsch PK, Stevens PE. Children in research: informed consent and critical factors affecting mothers. J Fam Nurs 2001;7:50-7.
- Verheggen FW, Jonkers R, Kok G. Patients’ perceptions on informed consent and the quality of information disclosure in clinical trials. Patient Educ Couns 1996;29:137-53.
- Leask J, Chapman S, Hawe P, Burgess M. What maintains parental support for vaccination when challenged by anti-vaccination messages? A qualitative study. Vaccine 2006;24:7238-45.
- Hoffman TM, Taeed R, Niles JP, McMillin MA, Perkins LA, Feltes TF. Parental factors impacting the enrollment of children in cardiac critical care clinical trials. Pediatr Cardiol 2007;28:167-71.
- Harth SC, Thong YH. Parental perceptions and attitudes about informed consent in clinical research involving children. Soc Sci Med 1995;41:1647-51.
- Pritchard-Jones K, Dixon-Woods M, Naafs-Wilstra M, Valsecchi MG. Improving recruitment to clinical trials for cancer in childhood. Lancet Oncol 2008;9:392-9.
- Hayman RM, Taylor BJ, Peart NS, Galland BC, Sayers RM. Participation in research: informed consent, motivation and influence. J Paediatr Child Health 2001;37:51-4.
- Dixon-Woods M, Ashcroft RE, Jackson CJ, Tobin MD, Kivits J, Burton PR, et al. Beyond ‘misunderstanding’: written information and decisions about taking part in a genetic epidemiology study. Soc Sci Med 2007;65:2212-22.
- Albrecht TL, Eggly SS, Gleason ME, Harper FW, Foster TS, Peterson AM, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol 2008;26:2666-73.
- Hoehn KS, Nathan A, White LE, Ittenbach RF, Reynolds WW, Gaynor JW, et al. Parental perception of time and decision-making in neonatal research. J Perinatol 2009:29-11.
- Slater AE. The European Unions’ Clinical Trials Directive. J R Soc Med 2001;94:557-8.
- Medicines and Human Use (Clinical Trials) Regulations 2004. www.opsi.gov.uk/si/si2004/20041031 (accessed 15 March 2011).
- Susman EJ, Dorn LD, Fletcher JC. Participation in biomedical research: the consent process as viewed by children, adolescents, young adults, and physicians. J Pediatr 1992;121:547-52.
- Koelch M, Singer H, Prestel A, Burkert J, Schulze U, Fegert JM. ‘. because I am something special’ or ‘I think I will be something like a guinea pig’: information and assent of legal minors in clinical trials – assessment of understanding, appreciation and reasoning. Child Adolesc Psychiatry Ment Health 2009;3.
- Johnson SB, Baughcum AE, Rafkin-Mervis LE, Schatz DA. Participant and parent experiences in the oral insulin study of the Diabetes Prevention Trial for Type 1 Diabetes. Pediatr Diabetes 2009;10:177-83.
- Tait AR, Voepel-Lewis T, Malviya S. Do they understand? (Part II): assent of children participating in clinical anesthesia and surgery research. Anesthesiology 2003;98:609-14.
- Chappuy H, Doz F, Blanche S, Gentet JC, Treluyer JM. Children’s views on their involvement in clinical research. Pediatr Blood Cancer 2008;50:1043-6.
- Broome ME, Richards DJ, Hall JM. Children in research: the experience of ill children and adolescents. J Family Nurs 2001;7:32-49.
- Unguru Y. Children’s views on their involvement in clinical research. Pediatr Blood Cancer 2008;51:716-17.
- Varma S, Jenkins T, Wendler D. How do children and parents make decisions about pediatric clinical research?. J Pediatr Hematol Oncol 2008;30:823-8.
- Cain M, McGuinness C. Patient recruitment in paediatric clinical trials. Practical Diabetes Int 2005;22:328-32.
- Fogas BS, Oesterheld JR, Shader RI. A retrospective study of children’s perceptions of participation as clinical research subjects in a minimal risk study. J Dev Behav Pediatr 2001;22:211-16.
- Brody JL, Annett RD, Scherer DG, Perryman ML, Cofrin KM. Comparisons of adolescent and parent willingness to participate in minimal and above-minimal risk pediatric asthma research protocols. J Adolesc Health 2005;37:229-35.
- Read K, Fernandez CV, Gao J, Strahlendorf C, Moghrabi A, Pentz RD, et al. Decision-making by adolescents and parents of children with cancer regarding health research participation. Pediatrics 2009;124:959-65.
- Wolthers OD. A questionnaire on factors influencing children’s assent and dissent to non-therapeutic research. J Med Ethics 2006;32:292-7.
- Tercyak KP, Johnson SB, Kirkpatrick KA, Silverstein JH. Offering a randomized trial of intensive therapy for IDDM to adolescents. Reasons for refusal, patient characteristics, and recruiter effects. Diabetes Care 1998;21:213-15.
- Gattuso J, Hinds P, Tong X, Srivastava K. Monitoring child and parent refusals to enrol in clinical research protocols. J Adv Nurs 2006;53:319-26.
- Broome ME, Richards DJ. The influence of relationships on children’s and adolescents’ participation in research. Nurs Res 2003;52:191-7.
- Gabe J, Olumide G, Bury M. ‘It takes three to tango’: a framework for understanding patient partnership in paediatric clinics. Soc Sci Med 2004;59:1071-9.
- Olechnowicz JQ, Eder M, Simon C, Zyzanski S, Kodish E. Assent observed: children’s involvement in leukemia treatment and research discussions. Pediatrics 2002;109:806-14.
- Young B, Dixon-Woods M, Windridge KC, Heney D. Managing communication with young people who have a potentially life threatening chronic illness: qualitative study of patients and parents. BMJ 2003;326.
- Tates K, Meeuwesen L. Doctor–parent–child communication. A (re)view of the literature. Soc Sci Med 2001;52:839-51.
- John T, Hope T, Savulescu J, Stein A, Pollard AJ. Children’s consent and paediatric research: is it appropriate for healthy children to be the decision-makers in clinical research?. Arch Dis Child 2008;93:379-83.
- Wendler D, Shah S. Should children decide whether they are enrolled in nonbeneficial research?. Am J Bioeth 2003;3:1-7.
- Joffe S, Fernandez CV, Pentz RD, Ungar DR, Mathew NA, Turner CW, et al. Involving children with cancer in decision-making about research participation. J Pediatr 2006;149:862-8.
- Swartling U, Helgesson G, Hansson MG, Ludvigsson J. Split views among parents regarding children’s right to decide about participation in research: a questionnaire survey. J Med Ethics 2009;35:450-5.
- Brody JL, Annett RD, Scherer DG, Turner C, Dalen J. Enrolling adolescents in asthma research: adolescent, parent, and physician influence in the decision-making process. J Asthma 2009;46:492-7.
- Miller VA, Reynolds WW, Nelson RM. Parent–child roles in decision making about medical research. Ethics Behav 2008;18:161-81.
- Brody JL, Scherer DG, Annett RD, Turner C, Dalen J. Family and physician influence on asthma research participation decisions for adolescents: the effects of adolescent gender and research risk. Pediatrics 2006;118:e356-62.
- Geller G, Tambor ES, Bernhardt BA, Fraser G, Wissow LS. Informed consent for enrolling minors in genetic susceptibility research: a qualitative study of at-risk children’s and parents’ views about children’s role in decision-making. J Adolesc Health 2003;32:260-71.
- Rossi WC, Reynolds W, Nelson RM. Child assent and parental permission in pediatric research. Theor Med Bioeth 2003;24:131-48.
- Snethen JA, Broome ME, Knafl K, Deatrick JA, Angst DB. Family patterns of decision-making in pediatric clinical trials. Res Nurs Health 2006;29:223-32.
- General Medical Council (GMC) . 0–18 Years: Guidance for All Doctors 2007.
- European Union . Ethical considerations for clinical trials on medicinal products conducted with the paediatric population. Eur J Health Law 2008;15:223-50.
- European Union Clinical Trials Directive. Bull Med Ethics 2001;169:13-24.
- Simon C, Eder M, Raiz P, Zyzanski S, Pentz R, Kodish ED. Informed consent for pediatric leukemia research: clinician perspectives. Cancer 2001;92:691-700.
- Amiel P, Moreau D, Vincent-Genod C, Alberti C, Hankard R, Ravaud P, et al. Noninvitation of eligible individuals to participate in pediatric studies: a qualitative study. Arch Pediatr Adolesc Med 2007;161:446-50.
- Salmon P, Peters S, Rogers A, Gask L, Clifford R, Iredale W, et al. Peering through the barriers in GPs’ explanations for declining to participate in research: the role of professional autonomy and the economy of time. Fam Pract 2007;24:269-75.
- Mason VL, Shaw A, Wiles NJ, Mulligan J, Peters TJ, Sharp D, et al. GPs’ experiences of primary care mental health research: a qualitative study of the barriers to recruitment. Fam Pract 2007;24:518-25.
- Spaar A, Frey M, Turk A, Karrer W, Puhan MA. Recruitment barriers in a randomized controlled trial from the physicians’ perspective: a postal survey. BMC Med Res Methodol 2009;9.
- Hales G, Beveridge A, Smith D. The conflicting roles of clinicians versus invetigators in HIV randomised clinical trials. Cult Health Sex 2001;3:67-79.
- White C, Gilshenan K, Hardy J. A survey of the views of palliative care healthcare professionals towards referring cancer patients to participate in randomized controlled trials in palliative care. Support Care Cancer 2008;16:1397-405.
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess 1999;3.
- Kaas R, Hart AA, Rutgers EJ. The impact of the physician on the accrual to randomized clinical trials in patients with primary operable breast cancer. Breast 2005;14:310-16.
- Caldwell PH, Butow PN, Craig JC. Pediatricians’ attitudes toward randomized controlled trials involving children. J Pediatr 2002;141:798-803.
- Caldwell PH, Craig JC, Butow PN. Barriers to Australian physicians’ and paediatricians’ involvement in randomised controlled trials. Med J Aust 2005;182:59-65.
- Chamberlain JM, Lillis K, Vance C, Brown KM, Fawumi O, Nichols S, et al. Perceived challenges to obtaining informed consent for a time-sensitive emergency department study of pediatric status epilepticus: results of two focus groups. Acad Emerg Med 2009;16:763-70.
- Walterspiel JN. Informed consent: influence on patient selection among critically ill premature infants. Pediatrics 1990;85:119-21.
- Fallowfield L, Saul J, Gilligan B. Teaching senior nurses how to teach communication skills in oncology. Cancer Nurs 2001;24:185-91.
- Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 2004;364:803-11.
- Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 2000;284:357-62.
- Glaser B, Strauss A. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
- Strauss A, Corbin J. The basics of qualitative research: techniques and procedures for developing grounded theory. Thousand Oaks, CA: Sage; 1998.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Spencer L, Ritchie J, Lewis J, Dillon L. Quality in qualitative evaluation: a framework for assessing research evidence (monograph online). London: Cabinet Office; 2003.
- Seale C. The quality of qualitative research. London: Sage; 1988.
- Yardley L. Dilemmas in qualitative health research. Psych Health 2000;15:215-28.
- Office for National Statistics . 2001 Census: Digitised Boundary Data (England and Wales) 2001.
- Goldsmith DJ, Fitch K. The normative context of advice as social support. Hum Commun Res 1997;23:454-76.
- Mendick N, Young B, Holcombe C, Salmon P. The ethics of responsibility and ownership in decision-making about treatment for breast cancer: triangulation of consultation with patient and surgeon perspectives. Soc Sci Med 2010;70:1904-11.
- Siminoff LA. Why learning to communicate with our patients is so important: using communication to enhance accrual to cancer clinical trials. J Clin Oncol 2008;26:2614-15.
- Brown RF, Butow PN, Ellis P, Boyle F, Tattersall MH. Seeking informed consent to cancer clinical trials: describing current practice. Soc Sci Med 2004;58:2445-57.
- Simon CM, Kodish ED. Step into my zapatos, doc: understanding and reducing communication disparities in the multicultural informed consent setting. Perspect Biol Med 2005;48:123-38.
- Cahill P, Papageorgiou A. Triadic communication in the primary care paediatric consultation: a review of the literature. Br J Gen Pract 2007;57:904-11.
- Cahill P, Papageorgiou A. Video analysis of communication in paediatric consultations in primary care. Br J Gen Pract 2007;57:866-71.
- Nova C, Vegni E, Moja EA. The physician-patient-parent communication: a qualitative perspective on the child’s contribution. Patient Educ Couns 2005;58:327-33.
- Snowdon C, Elbourne D, Garcia J. ‘It was a snap decision’: parental and professional perspectives on the speed of decisions about participation in perinatal randomised controlled trials. Soc Sci Med 2006;62:2279-90.
- de Salis I, Tomlin Z, Toerien M, Donovan J. Using qualitative research methods to improve recruitment to randomized controlled trials: the Quartet study. J Health Serv Res Policy 2008;13:92-6.
- Canvin K, Jacoby A. Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials 2006;7.
- Thompson AG. The meaning of patient involvement and participation in health care consultations: a taxonomy. Soc Sci Med 2007;64:1297-310.
- Mead N, Bower P, Hann M. The impact of general practitioners’ patient-centredness on patients’ post-consultation satisfaction and enablement. Soc Sci Med 2002;55:283-99.
- Saba GW, Wong ST, Schillinger D, Fernandez A, Somkin CP, Wilson CC, et al. Shared decision making and the experience of partnership in primary care. Ann Fam Med 2006;4:54-62.
- Madsen SM, Holm S, Riis P. Participating in a cancer clinical trial? The balancing of options in the loneliness of autonomy: a grounded theory interview study. Acta Oncol 2007;46:49-5.
- Entwistle V, Prior M, Skea ZC, Francis JJ. Involvement in treatment decision-making: its meaning to people with diabetes and implications for conceptualisation. Soc Sci Med 2008;66:362-75.
- Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA 2004;292:1593-601.
- Karunaratne AS, Korenman SG, Thomas SL, Myles PS, Komesaroff PA. Improving communication when seeking informed consent: a randomised controlled study of a computer-based method for providing information to prospective clinical trial participants. Med J Aust n.d.;192:388-92.
- Allmark P, Mason S. Improving the quality of consent to randomised controlled trials by using continuous consent and clinician training in the consent process. J Med Ethics 2006;32:439-43.
- Manson NC, O’Neill O. Rethinking informed consent in bioethics. Cambridge: Cambridge University Press; 2007.
- Juraskova I, Butow P, Lopez A, Seccombe M, Coates A, Boyle F, et al. Improving informed consent: pilot of a decision aid for women invited to participate in a breast cancer prevention trial (IBIS-II DCIS). Health Expect 2008;11:252-62.
- Brehaut JC, Lott A, Fergusson DA, Shojania KG, Kimmelman J, Saginur R. Can patient decision aids help people make good decisions about participating in clinical trials? A study protocol. Implement Sci 2008;3.
- Conroy S, McIntyre J, Choonara I. Unlicensed and off label drug use in neonates. Arch Dis Child Fetal Neonatal Ed 1999;80:F142-4.
- McIntyre J, Conroy S, Avery A, Corns H, Choonara I. Unlicensed and off label prescribing of drugs in general practice. Arch Dis Child 2000;83:498-501.
- Lenk C, Koch P, Zappel H, Wiesemann C. Off-label, off-limits? Parental awareness and attitudes towards off-label use in paediatrics. Eur J Pediatr 2009;168:1473-8.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11.
- Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer 2000;82:1783-8.
- Tait AR, Voepel-Lewis T, Robinson A, Malviya S. Priorities for disclosure of the elements of informed consent for research: a comparison between parents and investigators. Paediatr Anaesth 2002;12:332-6.
- Stegenga K, Ward-Smith P. The adolescent perspective on participation in treatment decision making: a pilot study. J Pediatr Oncol Nurs 2008;25:112-17.
- Ashcroft R, Goodenough T, Williamson E, Kent J. Children’s consent to research participation: social context and personal experience invalidate fixed cutoff rules. Am J Bioeth 2003;3:16-8.
- Coyne I, Hayes E, Gallagher P. Research with hospitalised children: ethical, methodological and organisational challenges. Childhood 2009;16:413-29.
- Backett K, Alexander H. Talking to young children about health: methods and findings. Health Educ J 1991;50:34-8.
- Walker CL. Stress and coping in siblings of childhood cancer patients. Nurs Res 1988;37:208-11.
- Kukla R. Conscientious autonomy: displacing decisions in health care. Hastings Cent Rep 2005;35:34-4.
- Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening?. J Clin Oncol 2007;25:e13-14.
- Freer Y, McIntosh N, Teunisse S, Anand KJ, Boyle EM. More information, less understanding: a randomized study on consent issues in neonatal research. Pediatrics 2009;123:1301-5.
- NHS, National Patient Safety Agency, National Research Ethics Service . Consent Guidance 2011. www.nres.npsa.nhs.uk/applications/guidance/consent-guidance-and-forms/.
Appendix 1 Final study protocol (version 3, 30 January 2009)
Research protocol
Processes in recruitment to randomised controlled trials (RCTs) of medicines for children (RECRUIT study).
Background and project justification
Forthcoming policy developments will increase the number of children’s RCTs initiated, but children’s triallists face considerable challenges in recruiting to RCTs. Despite these difficulties, most research on recruitment has concentrated on adult trials, leaving those working on children’s trials with little evidence to inform their recruitment strategies. Many RCTs recruit too few child participants, which can mean that the answers provided by the trials are unclear or misleading. There are also distinctive ethical, regulatory, pragmatic and psychosocial considerations surrounding children’s trials. The recruitment of children to trials therefore merits study in its own right. By informing strategies to improve recruitment and its conduct, this study will produce evidence to underpin the new policy developments for children’s medicines. These policies will significantly increase the resource invested in paediatric RCTs. By assisting children’s triallists with one of the most difficult aspects of their work, this study will help to ensure these long-awaited resources for children’s health are used to best effect.
Planned investigation
Introduction
To improve the treatments available to children, it is now widely recognised that more RCTs of medicines for children are needed [Smyth 2001, Royal College of Paediatrics and Child Health (RCPCH) 2004]. The Health Technology Assessment (HTA) Medicines for Children Open Call is itself a response to this need, and it is expected that the European Union (EU) and the UK will soon adopt further policies to increase the number of children’s RCTs initiated [Medical Research Council (MRC) 2004]. These are likely to be similar to measures that have produced dramatic rises in the numbers of children’s RCTs initiated in the USA. But the experience in the USA demonstrates that increasing the number of child RCTs initiated is not enough: attention to the adequacy of recruitment and its appropriate conduct is also vital (Steinbrook 2002). However, children’s triallists face considerable challenges in achieving these goals because recruiting children to RCTs is a particularly complex task (Smyth and Weindling 1999). Despite this complexity, most research on recruitment has concentrated on adult trials, so children’s triallists lack a suitable evidence base to inform their recruitment strategies. To ensure optimal recruitment rates and good conduct in recruitment, research is needed which takes account of the special configuration of ethical, regulatory, pragmatic and psychosocial issues raised by children’s trials, as these are likely to shape recruitment processes in distinctive ways. Children’s trials therefore merit study in their own right. The imminent legislative and policy changes, which will significantly increase the resource invested in children’s RCTs, lend particular urgency to the need for evidence on the recruitment of children, if these resources are to be used effectively.
Aims and objectives
Our qualitative study will describe processes in recruitment to four ‘case study’ trials of medicines in children with the aim of identifying strategies to improve recruitment and its conduct. We will sample children and parents (families) who have been approached to participate in a trial and will include families with different recruitment experiences and responses, that is those who have declined participation as well as those who have accepted. We will also sample practitioners who are responsible for recruiting families to trials (trial recruiters) and include those who have avoided approaching a family about a trial, even though the child was eligible. In order to examine processes in recruitment, we will directly access family–recruiter encounters (recruitment consultations) and conduct follow-up interviews with the various parties involved. Our specific objectives are to:
-
describe how recruitment consultations between families and trial recruiters are conducted and how information about trials is exchanged during these encounters
-
describe, from the perspective of families, the experience of trial recruitment and the communication needs and other priorities that are served or thwarted by recruitment consultations
-
describe, from the perspective of trial recruiters, the goals of recruitment consultations, the functions of these goals, how they interface with the conduct of the consultation and families’ communication needs and other priorities.
The scale of recruitment difficulties in children’s RCTs
Numerous individual reports testify to how children’s clinical studies have been compromised, delayed or abandoned because of recruitment difficulties (e.g. Chadwick 2005, Sinhaet al. 2005, Brown and Zacharin 2005, Caultonet al. 2004, Nicklin and Spencer 2004, Lenney and Child 2002, Cadeet al. 2000, Davidsonet al. 1998). More systematic evidence on recruitment difficulties in children’s trials is quite sparse. One review of trials published in a single paediatric journal points to considerable variability in recruitment, with RCTs in research intense and critical illness settings, such as neonatology and childhood cancer, achieving very high consent rates, but just over half of children’s community-based RCTs reporting high consent rates (Campbellet al. 1998). The picture provided by this review is clouded by trial reporting difficulties: only half the reviewed studies included consent rates, and some of these rates may have been inflated by inadequate counting methods. Other evidence is provided by an investigation of a meta-analysis of eight acute otis media trials, which estimated the recruitment rate across these studies to be lower than 44% (Bain 2001).
Published accounts of trials are likely underestimate recruitment difficulties, as many abandoned studies simply pass unreported into obscurity (Prescottet al. 1999). A useful indication of the scale of recruitment problems in unpublished children’s RCTs can be found in media reports about ongoing trials in the USA. Not long after the introduction of special legislative and policy measures to increase the number of children’s RCTs, pharmaceutical company reports indicated that over half of the recently implemented trials were ‘in crisis’ owing to severe recruitment difficulties (Zimmerman 2002, Steinbrook 2002). It is important to note that these difficulties arose in the USA where it is customary to provide financial incentives to families in order to boost recruitment. In countries such as the UK, where such incentives are prohibited on ethical grounds, recruitment difficulties are likely to be more pronounced.
Examination of the sample sizes in children’s RCTs provides further evidence of the challenges facing paediatric triallists, with one review estimating that the median number of participants recruited to children’s trials is only 50 (Sammons and Choonara 2005) and another indicating that more than 70% of trials in cystic fibrosis recruited 30 children or fewer (Chenget al. 2000). Inadequate recruitment is a serious threat to improving the evidence base on medicines for children. It means that many children’s trials lack the statistical power to detect even moderate treatment effects and are at risk of compromised validity. Severe recruitment problems may also lead to trial abandonment and to inefficiencies in the use of limited resources.
Previous research on factors in recruitment to children’s RCTs
Despite these difficulties, relatively few studies have investigated processes in recruitment to children’s RCTs. Two previous reviews which consider trial recruitment do not offer any specific comment or recommendations for triallists who work with children (Prescottet al. 1999, McDonaldet al. 2006). Little is therefore known about what children’s and parents’ priorities are when approached to participate in a trial and what factors influence their decision-making. Still less is known about the processes determining which families are even approached by practitioners (Caldwellet al. 2002). Most research that has specifically focused on recruitment processes in children’s trials has concentrated on RCTs for childhood cancer and neonates (Snowdonet al. 2004 a,b, Kodishet al. 2004, Mason and Allmark 2000, Snowdonet al. 1999, Zupancicet al. 1997, Snowdonet al. 1997), but there are serious questions about the validity of generalising from the emotionally fraught and uniquely research intense settings of neonatology and childhood cancer to other paediatric settings, where the challenges in recruiting children to trials are likely to be very different.
The small body of work to include a focus on trial recruitment outside childhood cancer and neonatology has largely involved retrospective surveys of parents (van Stuijvenberget al. 1998). However, retrospective investigations are subject to particular biases, while surveys reveal little of the intricate communication processes involved in trial recruitment, and children’s perspectives on trials may be quite distinct from those of their parents (Olechnowiczet al. 2002). Therefore, prospective work, which directly accesses the communication processes in different trial contexts, and from the perspectives of all parties involved, is needed. In order to understand the influences on the decision-making of each party, it is also important that this work investigates the views of children, parents and recruiters with different recruitment responses and experiences.
Differences between children’s and adult RCTs
Recommendations derived from studies of recruitment to adult trials may have some application to children’s trials, but this is likely to be limited: recruiting children is very different to recruiting competent adults. Children are considered to be more vulnerable than adults and there is clear expectation that they are entitled to special protection (RCPCH 2000, MRC 2004). This means that children’s triallists operate in a distinct ethical, regulatory, pragmatic and psychosocial context to adults’ triallists. Firstly, children’s RCTs are subject to more complex and tighter ethical and regulatory requirements (Smyth and Weindling 1999, RCPCH 2004). For example, there are distinctive regulations governing who can provide consent, which turn upon judgements about the competence of the child (RCPCH 2000). The acceptable risk–benefit ratio to individual child participants versus those to science and society also differs (Sugarman 2004, Wendleret al. 2005). Secondly, the psychosocial and pragmatic context of children’s RCTs is different (Punch 2002). Where children are too young to be consulted, their parents are responsible for deciding whether they should participate in a trial, but making a decision on behalf of a vulnerable third party is fundamentally different to making a decision for oneself (Caldwellet al. 2003, 2004). The special nature of the parent–child relationship with its powerful protective urges adds to the difficulty of parental decision-making (Younget al. 2002a, 2003), while children themselves are likely to have important and distinctive concerns about participating in clinical trials. In the situation of older children, recruitment consultations will involve the child, parent(s) and recruiter, and therefore entail more complex dynamics than the dyadic consultations typical of adult recruitment consultations (Broome and Richards 2003, Gabeet al. 2004). This presents serious challenges to trial recruiters in managing the consultation and balancing the involvement of each party (Olechnowiczet al. 2002).
Inadequate recruitment to trials, whether of adults or children, is widely recognised as one of the principal reasons for trial failure (MRC 2003). Work on recruitment processes in adult trials has provided valuable strategies for those designing and recruiting to adult RCTs (Donovanet al. 2002). However, these processes have not been studied in children’s RCTs. The wide-ranging contextual differences between children’s and adults’ trials indicate that recruitment processes in children’s trials merit study in their own right.
Ethical considerations
Any research on trial recruitment is permeated by tensions between facilitating informed consent and improving recruitment. Such research has the potential to lead to changes in the ways in which trial information is framed and exchanged, which may in turn exert considerable influence on the decision-making of prospective participants (Postlethwaiteet al. 1995). Donovan’s study of recruitment of adult trial participants illustrates how apparently small adjustments to the content and presentation of trial information were associated with dramatic increases in rates of recruitment (Donovanet al. 2002). Such techniques inevitably raise fears about potential manipulation or coercion of participants (Little 2002). Because children have special entitlements to protection, we consider these tensions to be especially pronounced in this study (RCPCH 2000). This has shaped our dual focus on improving the conduct of recruitment to trials and increasing the number of children recruited. We describe how we will manage the challenges that this presents below (see Research methods/Research governance: project steering group).
Research methods
Overview
This qualitative study will investigate recruitment processes in four ‘case-study’ RCTs of medicines for children chosen to represent features that are common to many children’s trials and that are likely to influence recruitment and its conduct. The data will be (1) trial recruitment consultations between families and trial recruiters; (2) follow-up semi-structured interviews with families (children and parents); and (3) follow-up semi-structured interviews with recruiters. With participants’ permission these will be audio-recorded and transcribed verbatim.
The inclusion of data from recruitment consultations is a key feature of our study. Their use is warranted by theory and empirical observations of communication in a variety of settings, including trial recruitment, which demonstrates that what people hear is often different to what is said (Roter and Hall 1992, Kodishet al. 2004, Salmon and Young 2005). Methods that completely rely on what participants say about trial recruitment, such as surveys and interviews, are therefore unsuited to studying trial recruitment processes, as these only access participants’ interpretations (Murphyet al. 1998). By audio-recording consultations our study avoids this difficulty. Indeed, our methodology is further strengthened by permitting us to compare our observations of exchanges during recruitment consultations with participants’ interpretations of these exchanges obtained during interviews. This will allow us to pin-point how families’ communication needs and priorities are served or negated. For example:
-
comparing consultation and interview data within families will identify gaps or discrepancies between the two data sets
-
systematic analysis of the patterning of these across families will highlight the circumstances, topics or phrases that are associated with communication or other difficulties
-
the exposition of these patterns will provide the foundation to identify strategies to improve recruitment and its conduct in children’s RCTs.
Recordings of consultations have demonstrated their worth in investigations of communication processes in a range of clinical settings (Byrne and Long 1976, Silverman 1981, Makoulet al. 1995, Rogers and Todd 2002). Individuals appear to rapidly habituate to recording devices when used routinely, and evidence suggests they have little influence on the communication behaviour of practitioners or patients (Coleman 2000). Comparing consultation and interview data therefore provides a powerful method for understanding communication processes in clinical settings (Burkitt Wrightet al. 2004, Barryet al. 2000), as further evidenced by its successful application in an adult RCT to investigate recruitment processes, and additional work using the same technique is ongoing (Donovanet al. 2002, Donovan, QUARTET study).
Sampling and heterogeneity
There are numerous potential sources of heterogeneity in trial recruitment processes and no single study can address all of these if it is to achieve a robust analysis. To permit a robust analysis of heterogeneity arising from our four case study trials, and from individuals’ recruitment experiences and responses, we have decided, as far as possible, to constrain heterogeneity arising from (1) trial sites by selecting broadly typical sites within trials and (2) the organisational infrastructure of trials by focusing upon UK Medicines for Children Research Network (UKMCRN) trials. There are compelling reasons for understanding recruitment processes within networked trials: high-quality children’s trials are increasingly likely to be conducted within these networks, indeed it is within these that the most pressing scientific questions about children’s health are investigated. Considerable public resource is also at stake if such trials encounter recruitment difficulties. Understanding the perspectives of individuals with different recruitment experiences and responses (that is, families and recruiters) is crucial in identifying appropriate strategies to improve recruitment and its conduct.
Sampling of trials and sites
We will sample four ‘case study’ RCTs from the candidate trials identified in Table 1. These cover a range of features that are common in children’s trials. In order to maximise the transferability of findings, our final selection will include trials that allow us to study the influence on recruitment processes of the following: (1) disease status – acute and chronic illnesses; (2) trial design – placebo controlled and two-way active comparison trials; (3) method of medicine delivery; and (4) banking of biological material for use in future pharmacogenetics studies. These features are likely to influence trial acceptability and recruitment, so representing them in our sample is important. To select broadly typical sites within trials, we will take account of the nature and number of sites for each trial. Where circumstances allow, sites in the north west will be selected in preference to other regions for pragmatic reasons, but where typical, north-west sites are unavailable we will sample from sites in other regions. All trials are funded or going forward for funding and the principal investigators (PIs) have agreed to collaborate with our study.
| Trial title | Lead investigator(s) | Age range | Disease status | Trial type | Medicine delivery | Bank of material | Agreement |
|---|---|---|---|---|---|---|---|
| (MENDs) The use of melatonin in children with neurodevelopmental disorders and impaired sleep | Dr Richard Appleton, Dr Paul Gringras | 5–18 years | Chronic | Randomised, double-blind, placebo-controlled, parallel | Oral | Yes | CI agreed |
| (TIPIT) A randomised controlled trial of thyroxine in pre-term infants under 28 weeks’ gestation | Professor A Michael Weindling | Infants born at < 28 weeks’ gestation | Acute | Randomised, double-blind | i.v. | No | |
| (MASCOT) How should asthma in school-aged children be managed when not controlled with low-dose inhaled corticosteroids? | Dr Warren Lenney | 7–15 years | Chronic | Randomised, double-blind, placebo-controlled, parallel | Inhaled | Yes | |
| (POPs) Prevention and treatment of steroid-induced osteopaenia in children and adolescents with rheumatic diseases | Dr Madeleine Rooney | 4–18 years | Randomised, double-blind, placebo-controlled | Oral |
Sampling of families (particularly those who decline trial participation) and recruiters
A small number of previous studies in the USA and Europe have studied recruitment processes to children’s trials and demonstrate the broad feasibility of our methods. Parental response rates to a survey on decisions about participation in a trial for neonates were 83% for those who agreed to the trial compared with 86% for those who declined (Zupancicet al. 1997). A study that was nested in a leukaemia trial in the USA used audio-recording of recruitment consultations and follow-up family interviews to investigate the quality of informed consent. Eighty-five per cent of parents agreed to the informed consent study, including parents who later declined the trial (Kodishet al. 2004). In the UK, a similar study of recruitment to perinatal trials involved some parents who declined trial participation (Snowdenet al. 2006). Beyond the highly specialised contexts of childhood cancer and neonatology, no previous studies have used similar techniques to investigate children’s recruitment. However, these findings provide some indication that families who decline trials are, nevertheless, willing to participate in studies of their experiences of trial recruitment.
For each trial, approximately equal numbers of families will be sampled, to include families who agree for the child to participate in a trial and those who decline. In order to link consultations and interviews, the sample of families will comprise those for whom audio-recordings of consultations are available. However, as analysis proceeds we may also include a limited number of families with potentially informative experiences of recruitment (e.g. families who initially agree to trials but decline shortly afterwards), even if audio-recordings of their recruitment consultations are unavailable. Within these constraints, maximum variation sampling will be used to represent family socioeconomic status and child age. All sites involved will keep records to allow sampling in line with these requirements, and the research associates (RAs) and UKMCRN Senior Trials Manager will liaise closely with trial sites to ensure that adequate records are maintained. Previous studies of recruitment have mostly neglected the experiences of children (Olechnowiczet al. 2002). The inclusion of their perspectives in this study reflects the growing emphasis on the value of listening to children (MRC 2004). Additionally, children’s perspectives as trial participants are likely to be crucial in improving how trial recruitment is conducted. Children of 7 years upwards and their parents will be interviewed, but where children are less than 7 years old only their parents will be interviewed, as very young children are more likely to experience difficulties in articulating their experiences in interviews.
To mirror the pairing of families and recruitment consultations, we will also aim to link recruiters and recruitment consultations. It follows that sampling of trial recruiters will largely be tied to the sampling of families, and that recruiters will mostly be those directly involved in approaching the families within this study and for whom audio-recordings of consultations are available. However, as analysis proceeds we may also include recruiters with potentially informative views on the research questions, arising from their experiences of recruitment and their key roles in the trials, even if they have not provided audio-recordings of recruitment consultations. This will assist in sampling recruiters who have avoided approaching families of eligible children. Trial recruitment records will allow identification of these individuals. Within the above constraints, sampling for maximum variation will be used to represent professional background (that is, doctors, nurses, researchers).
Proposed sample size
The sample will comprise families of 60 children (parents and their 7- to 16-year-old children) to include 30 families who decline trial participation and 30 who agree, and 30 trial recruiters (clinicians, nurses, researchers, etc.) across four ‘case study’ RCTs. Our sampling strategy will be periodically reviewed in the light of the ongoing data analysis and modified if necessary to develop and test our emerging findings. For qualitative studies, sampling is ideally judged to be complete when theoretical saturation has been reached (Mason 2002). In practice it can be difficult to demonstrate this point and it cannot be precisely specified in advance as it depends on the variability in the sample and the properties of the data. However, based on previous studies, we anticipate that our sample will balance the need to (1) include RCTs with contrasting features; (2) include families and practitioners with different recruitment responses and experiences; and (3) conduct a rigorous data analysis that allows us to compare data from consultations and interviews and triangulate our analysis on the study objectives.
Data collection: time frame, procedure and ethical considerations
The time frame for beginning this study is dictated by the trials. These are due to start recruitment between spring and summer 2007. To allow the trails to become established and avoid the initial ‘teething’ phase that most trials experience, our data collection will not begin until each of the trials has been recruiting for approximately 4 months. Data collection will continue over the course of each of the trials until autumn 2008, with the most active phase of data collection occurring between autumn 2007 and summer 2008. Where possible during this phase, sampling to our study will roll from site to site (within trials) in blocks of up to 3 months’ duration; sampling may also be temporarily suspended from time to time if accrual to our study is progressing well. Concentrating sampling at particular sites in time-limited blocks, with the possibility of planned suspensions, will minimise the impact of the study on the RCTs and the risk of overburdening particular sites. It will also facilitate liaison with the sites and assist recruiters in routine audio-recording of consultations. Sampling from summer 2008 until autumn 2008 will address any shortfalls in our sampling targets.
Recruiters will routinely seek the permission to audio-record trial recruitment consultations from the families whom they approach for the trials. If permission is declined the audio-recorder will not be activated and the recruitment consultation will progress as normal. If permission is given the recruiter will activate a recording device. At the end of the trial recruitment consultation he or she will discuss the current study with the family and seek their permission to pass their details to one of the RAs on the study. If permission is given, an RA with proven skills in investigating sensitive issues and in qualitative interviewing will meet with selected families to explain the study and seek their consent to participate. He or she will explain his/her independence from the trial and clinical team, emphasise that participation is voluntary, and that children and parents have a right to withdraw at any time.
Should a family who gave permission for their consultation to be recorded later decide that they do not wish to be interviewed as part of the RECRUIT study, they will be asked for their consent for the recording of their consultation to be included in the study analyses. Recordings from families who decline both to be interviewed for RECRUIT, and for their recorded consultations to be included in the analyses, will be erased as soon as practicable. All families who express an interest but are not selected for follow-up interview will be contacted by letter to thank them and inform them that their recordings have been erased. It is anticipated that conducting sampling at sites in time-limited blocks, with planned suspensions of sampling if accrual to the current study allows, will help to minimise the numbers of families who are approached but not selected.
In some circumstances it may be necessary to approach families by way of an invitation letter and information sheet rather than a personal approach by the practitioner. Examples of such situations would be if the RECRUIT study was not discussed with the family when they were approached about the main trial, if the trial discussion was held prior to the RECRUIT study opening at a centre or if the approach by the main trial was itself made by a postal invitation letter to which the family did not respond hence declining the main trial (specific to the MASCOT trial). In these circumstances, eligible families will be identified and approached by letters from recruiters to the main trial. Invitation letters will contain a reply slip and postage-paid envelope that interested families will return directly to the RECRUIT team. The RECRUIT team will not have access to families’ contact details unless they return the reply slip.
For ethical reasons, audio-recordings of the recruitment consultations will be released to the researchers on the current study only after the consent of participants has been obtained. The feasibility of routine audio-recording of trial recruitment consultations has been demonstrated in a previous study of recruitment processes in an adult trial (Donovanet al. 2002), and the PIs of all the candidate trials are aware of this requirement.
Interviews
The RAs will conduct the semi-structured interviews with families and recruiters. These will usually be carried out within days of the recruitment consultation, with an upper limit of 2 weeks. Where possible we will interview parents and children separately. This is to avoid the difficulties of interpreting individual experiences from data collected at joint interviews, whereby it would be necessary to interpret the accounts of each party in the context of how it is shaped by the presence of the other party. It is crucial that all interviews are conducted so as to minimise the risk of obtaining generalised or idealised accounts. Therefore, transcripts from consultations and interviews will be continually reviewed and used to ground and inform subsequent interviews. However, RAs will also ensure that interviews are conversational in tone and their pace, sequencing and duration are shaped by the participants. Reflexive notes will be maintained by the interviewer to record systematically the contextual details of the interviews.
Interviews with families will take place in a private room at the trial site or in their homes, as they choose. A topic guide with two intersecting components, each based on our previous published experience of using interviews to explore perspectives in the context of clinical communication, will be used as a steer (Dixon-Woodset al. 2001, Younget al. 2002a, 2003). First, we will need to frame the interview and focus on what parents and children expected of the recruitment consultation and how they reacted to these consultations. To do this, the RA will ask children and parents to describe the recruitment consultation from their perspective. He or she will prompt them about how they felt about the encounter, the recruiter, the written and verbal information exchanged, whether there was anything that was unclear or surprising about this information, and whether anything might have been handled differently. Importantly, each interview will be informed by detailed knowledge of the features of the particular trial concerned in order to develop specific prompts: for example ‘When the doctor said “the computer will decide whether your daughter gets the placebo or the medicine” what went through your mind?’.
Second, families will also be prompted about (1) other (unrecorded) experiences of family–recruiter discussions and exchanges; (2) their broader views on clinical research involving children; (3) their prior knowledge and experience of such research; (4) how they see trial participation in the context of the child’s illness and future well-being and their relationships with health professionals; and (5) how trial decision-making was negotiated within the family. For children, we will explore their feelings about particular aspects of the trial and the extent to which they felt involved and able to influence trial decision-making. For parents, we will explore any tensions between ensuring their child’s best interests and trial participation. The content of this aspect of the interview will evolve to reflect and test the developing analysis; however, we will take care to avoid our questions becoming too directive – it is important that we avoid asking participants to do the analytical work for us. Interview guides will be adapted so that they are appropriate for parents and children, and in line with the families’ recruitment responses and experiences.
Interviews with trial recruiters will follow a similar course to the family interviews, but will be steered by a separate topic guide. For recruiters for whom audio-recorded consultations are available, interviews will be informed by a detailed review of a recording from a recent consultation for that recruiter to develop specific interview prompts. Interviews will therefore initially focus on a particular consultation to explore the goals of the recruiter in this consultation and how well it proceeded in relation to these goals. The RA will go on to prompt recruiters to (1) contrast their experiences of this recruitment consultation with their experiences of other recorded and unrecorded consultations; (2) describe their experiences of families who decline and agree to trials; (3) describe what information children and parents require to inform their decision-making, and their experiences of managing the involvement of each party; (4) describe how they respond to the needs and requirements of families; (5) describe their experiences of deciding which families to approach, including their accounts of deciding not to approach eligible families; and, finally, (6) to compare their role in approaching families about trials with other aspects of their role as clinical practitioners or researchers.
Data analysis
Analysis of consultation and interview data will proceed in parallel, but related, courses. One course will involve transcripts from consultations and interviews being analysed for two broad purposes. First, we shall analyse for evidence of the needs and priorities of families with different recruitment responses and experiences, particularly by identifying the cues that they present and the ways that they represent themselves. Second, we shall analyse for evidence about the goals of recruiters with different experiences of recruitment, how they respond to families’ cues and the functions of these responses.
The other main course of the analysis will involve ‘micro’ and ‘macro’ levels. At the ‘micro’ level, transcripts from consultations and interviews will be linked (within families and recruiters) to compare observations of exchanges during consultations with participants’ interpretations of these exchanges. Discrepancies between interpretations and observations will be particularly informative about the ways in which communication may go awry; however, in order to build a complete picture of recruitment processes we will also identify convergences. At a ‘macro’ level we will analyse these discrepancies and convergences for recurrent patterns to illuminate the ways in which exchanges during recruitment serve or thwart families’ needs. The consultation and interview data will therefore be triangulated on the research objectives. Finally, the data sets from each of four trials will be compared to investigate how recruitment processes are associated with particular features of the trials.
Analysis will follow the general principles of the constant comparative method. One member of the research team will lead a process of ‘cycling’ between the developing analysis and new data, and the complete team will develop and test the analysis by periodic discussion. Initially, each transcript will be read several times by the lead analyst for that element, before developing open codes to describe each relevant unit of meaning. Because ‘units of meaning’ can take shape over several (and sometimes widely dispersed) turns, initial open coding will occur at multiple levels, from detailed descriptions of experiences turn by turn, to the general orientation of participants towards clinical research involving children. Through comparison within and across the transcripts, the open codes will gradually be developed into theoretical categories and subcategories to reflect and test the developing analysis. The categories will be organised into a framework to code and index the transcripts using QSR nvivo software (Richards 2002). The framework categories will be continually checked and modified to ensure an adequate ‘fit’ with the data, while also accounting for deviant cases. The categories and the assignment of data to them will be checked by a second member of the project team. A record will be kept of the analysis process, including definitions of the categories and their application.
Analytical approach
As in our previous research (e.g. Dixon-Woodset al. 2001, Younget al. 2003), our analytic approach is informed by writings on quality in qualitative research (Murphyet al. 1998, Seale 1999, Yardley 2000, Spenceret al. 2003). In particular, we will initially focus on participants’ expressed accounts whether in consultations or interviews; however, as the study progresses, it will be necessary to theorise these accounts to identify needs and goals, and examine their consequences for trial recruitment beyond those that the participants make explicit. For example, our analysis will consider how participants’ utterances position them, for example, in relation to childhood illness or to clinical research (Baruch 1981, Hammersley and Atkinson 1995). This is further illustrated by our previous work on communication processes in the care of children with cancer, where parents did not explicitly describe their own executive role but we identified this by interpreting their accounts in the context of their interactions with the practitioners and the researchers (Younget al. 2003). That is, rather than take participants’ accounts only at face value, it is important to understand the interaction work that they are doing through their talk, whether in consultations or in interviews.
Research governance: project steering group
To manage our dual focus on improving recruitment and its conduct, we will form a project steering group to advise on the study and its outputs. The steering group will provide particular guidance in the appropriate prioritisation of the ethical requirement to ensure good conduct in recruitment, and the instrumental objective of improving recruitment rates. It will ensure an appropriate balance is achieved in drawing up strategies to inform recruitment. The committee will have an independent chair and comprise a majority of members outside of the study team. Dr Donal Manning, a consultant paediatrician and Chair of the North West Multicentre Research Ethics Committee (MREC) has agreed to chair the steering group for this study. The other members will comprise two parent representatives, ethics and legal experts, PIs from the four trials and two members of the study team. To preserve participant anonymity, and in line with INVOLVE guidelines, the parent representatives will not be otherwise associated with the study (Hanleyet al. 2004).
Project timetable and milestones
This is a 2-year study. By the end of month 2, the investigators will have liaised with the different RCT sites and prepared and piloted the prompt guides. By end of month 20, we will have completed and transcribed the interviews and, in line with the constant comparative method, the data analysis will be well under way. On average, we expect to recruit two professionals and six to eight parents and children per month during months 3–20, with some concentration of this in the first 12 months of data collection. By the end of month 22, data analysis will be complete. Final writing-up will be in months 23 and 24.
Expertise
The study team is multidisciplinary, including health psychology, academic and clinical paediatrics, medical statistics, and clinical trial design and conduct. Over the last 5 years, BY has authored or co-authored 23 peer-reviewed publications, most of which are directly relevant to psychosocial and communication processes in child health or qualitative methods (e.g. Dixon-Woodset al. 2001; Younget al. 2002a,b, 2003; 2006). Several of these appear in major international journals including, the BMJ, The Lancet and SocialScience & Medicine. BY has also produced numerous other research and scholarship outputs and brings expertise in qualitative methods to the study team. Her recent grant support, all of which has been secured to conduct projects that use qualitative methods, is testament to this. Currently, she is PI on an Economic and Social Research Council (ESRC) study of quality of life in disabled children and a recently awarded multicentre Cancer Research UK (CRUK) study of communication processes in childhood leukaemia. The latter project will employ very similar methods to current study, but involves a different study team, so will add considerable value to this study through cross-fertilisation at methodological and other levels. BY is also a co-investigator on an ERSC study of tumour tissue donation in childhood cancer, which is leading to important new findings on children’s research participation in specialised research intense settings. PRW and RLS have track records of world-class standing in paediatric medicine (RLS), multicentre RCT design, implementation and analysis (PRW and RLS), and collaborations and publications commensurate with this; since 2001 RLS has produced 27 peer-reviewed publications in major international journals, plus numerous Cochrane Reviews, editorial-reviewed papers, and a clutch of highly influential editorials of close relevance to this study in journals such as The Lancet and the BMJ; in the same period, PRW has published more than 30 peer-reviewed papers in high-impact journals, alongside other key outputs including, numerous Cochrane reviews and influential writings of RCT methodology. PRW has provided steering committee support to several international RCTs, while RLS has particular expertise in children’s RCTs, including extensive experience in recruiting children to trials. PRW and RLS have a strong portfolio of research council and other major grants to support clinical and methodological investigations relevant to children’s health, including RCT-related work. Taken together, the value of the current and previous research income secured by PRW and RLS is in excess of £20M. HH has co-authored several papers in high-impact journals, including a report of an RCT, which appeared in the New England Journal of Medicine. She contributes in-depth knowledge and experience of the co-ordination and management of multicentre clinical trials to the study team. Importantly, HH’s pivotal contribution to the study brings no directly allocated or incurred costs owing to the nature of the funding for her post.
Service users
In the context of this study, service users are the children and families who may be approached to participate in trials. Consultation with both of these groups will be assisted by the UKMCRN Consumer Liaison Officer and occur throughout the study, but will particularly focus on the study aims and design before its commencement, and on the appropriateness of the study recommendations towards the end. The inclusion of parent representatives on the steering group will provide further opportunities to consult with parents. Another key interest group are triallists and trial recruiters. Our regular liaison with the trial sites as the study is ongoing, involvement of PIs in the steering group, and consultation with the wider body of triallists through the UKMCRN regarding the recommendations, will ensure representation of this group. Ethics experts are an important group too. The steering group members with ethics expertise will provide representation of the wider ethics community and it is anticipated that these members will direct the study group towards any further consultation activities, as befits the ethical implications of the study.
References
- Bain J. Treatment of acute otis media: are children entered into trials representative?. Br J Gen Pract 2001;51:132-3.
- Barry CA, Bradley CP, Britten N, Stevenson FA, Barber N, Cade A, et al. Patients’ unvoiced agendas in general practice consultations: qualitative study. BMJ 2000;320:1246-50.
- Baruch G. Moral tales: parents’ stories of encounters with the health profession. Soc Health Illn 1981;3:275-96.
- Broome M, Richards DJ. The influence of relationships on children’s and adolescents’ participation in research. Nurs Res 2003;52:191-7.
- Brown JJ, Zacharin MR. Attempted randomized controlled trial of pamidronate versus calcium and calcitriol supplements for management of steroid-induced osteoporosis in children and adolescents. J Paediatr Child Health 2005;41:580-2.
- Burkitt Wright E, Holcombe C, Salmon P. Doctors’ communication of trust, care, and respect in breast cancer: qualitative study. BMJ 2004;328:864-7.
- Byrne PS, Long BEL. Doctors talking to patients. London: HMSO; 1976.
- Cade A, Brownlee KG, Conway SP, . Randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch Dis Child 2000;82:126-30.
- Caldwell P, Butow P, Craig J. Pediatricians’ attitudes towards randomized controlled trials involving children. J Pediatr 2002;141:798-803.
- Caldwell P, Butow P, Craig J. Parents’ attitudes to children’s participation in randomized controlled trials. J Pediatr 2003;142:554-9.
- Caldwell PH, Murphy S, Butow P, Craig JC. Clinical trials in children. Lancet 2004;364:803-11.
- Campbell H, Surry S, Royle E. A review of randomised controlled trials published in Archives of Disease in Childhood from 1982–96. Arch Dis Child 1998;79:192-7.
- Caulton JM, Ward KA, Alsop CW, Dunn G, Adams JE, Mughal MZ. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child 2004;89:131-5.
- Chadwick B. A randomised controlled trials to determine the effectiveness of glass ionomer sealants in pre-school children. London: Department of Health; 2005.
- Cheng K, Smyth RL, Motley J, O’Hea U, Ashby D. Randomized controlled trials in cystic fibrosis (1996–1997) categorized by time, design and intervention. Pediatr Pulmonol 2000;29:1-7.
- Coleman T. Using video-recorded consultations for research in primary care: advantages and limitations. Fam Pract 2000;17:422-7.
- Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Sraube R, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose–response, multicenter study. Pediatrics 1998;101:325-34.
- Dixon-Woods M, Findlay M, Young B, Cox H, Heney D. Parents’ accounts of obtaining a diagnosis of childhood cancer. Lancet 2001;357:670-4.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Improving the design and conduct of randomised trials by embedding them in qualitative research: ProtecT study. BMJ 2002;325:766-9.
- Donovan J. The Quartet Study (Qualitative Research to Improve Recruitment to Randomised Controlled Trials) n.d. URL: www.hsrc.ac.uk/Current_research/research_programmes/quartet.htm (accessed 22 February 2006).
- Gabe J, Olumide G, Bury M. ‘It takes three to tango’: a framework for understanding patient partnership in paediatric clinics. Soc Sci Med 2004;59:1071-9.
- Hammersley M, Atkinson P. Ethnography: principles in practice. London: Routledge; 1995.
- Hanley B, Bradburn J, Barnes M, Evans C, Goodacre H, Kelson M, et al. Involving the public in NHS, public health, and social care research. Eastleigh, UK: INVOLVE; 2004.
- Kodish E, Eder M, Noll R, Ruccione K, Lange B, Angiolillo A, et al. Communication of randomisation in childhood leukemia trials. JAMA 2004;291:470-5.
- Lenney W, Child F. Family genetic studies. Arch Dis Child 2002;87:272-3.
- Little P. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7.
- Makoul G, Arntson P, Schofield T. Health promotion in primary care: physician-patient communication and decision-making about prescription medications. Soc Sci Med 1995;41:1241-54.
- Mason J. Qualitative researching. London: Sage; 2002.
- Mason S, Allmark P. Obtaining informed consent to neonatal randomised controlled trials: interviews with parents and clinicians in the Euricon study. Lancet 2000;356:2045-51.
- Medical Research Council (MRC) . Clinical Trials for Tomorrow 2003.
- Medical Research Council (MRC) . Ethics Guide: Medical Research Involving Children 2004.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Nicklin S, Spencer SA. Recruitment failure in early neonatal research. Arch Dis Child (Fetal and Neonatal Ed) 2004;89.
- Olechnowicz JQ, Eder M, Simon C, Zyzanski S, Kodish E. Assent observed: Children’s involvement in leukemia treatment and research discussions. Pediatrics 2002;109:806-14.
- Postlethwaite RJ, Reynolds JM, Wood AJ, Eminson DM. Recruiting patients to clinical trials: lessons from studies of growth hormone treatment in renal failure. Arch Dis Child 1995;73:30-5.
- Prescott R, Counsell C, Gillespie W, Grant AM, Russell IT, Kiaulka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess 1999;3.
- Punch S. Research with children: the same or different from research with adults?. Childhood 2002;9:321-41.
- Richards L. Using NVivo in qualitative research. Melbourne: QSR International; 2002.
- Rogers M, Todd C. Information exchange in oncology outpatient clinics: source, valence and uncertainty. Psychooncology 2002;11:336-45.
- Roter DL, Hall JA. Doctors Talking with Patients: Improving Communication in Medical Visits. Westport, CT: Auburn House; 1992.
- Royal College of Paediatrics and Child Health (RCPCH) . Guidelines for the ethical conduct of medical research involving children. Arch Dis Child 2000;82:177-82.
- Royal College of Paediatrics and Child Health (RCPCH) . Safer and Better Medicines for Children 2004.
- Salmon P, Young B. Core assumptions and research opportunities in clinical communication. Patient Educ Couns 2005;58:225-34.
- Sammons H, Choonara I. Clinical trials of medication in children, 1996–2002 (Letter). Eur J Clin Pharmacol 2005;61:165-7.
- Seale C. The quality of qualitative research. London: Sage; 1999.
- Sinha SK, Lacaze-Masmonteil T, Valls i Soler A, Wiswell TE, Gadzinowski J, Hajdu J, et al. A multicenter, randomized, controlled trial of lucinactant versus poractant alfa among very premature infants at high risk for respiratory distress syndrome. Pediatrics 2005;115:1030-8.
- Silverman D. The child as a social object: Down’s syndrome children in a paediatric cardiology clinic. Social Health Illn 1981;3:254-74.
- Smyth RL. Research with children. BMJ 2001;322:1377-8.
- Smyth RL, Weindling AM. Research in children: ethical and scientific aspects. Lancet 1999;354:21-4.
- Snowdon C, Garcia J, Elbourne D. Making sense of randomisation: responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Soc Sci Med 1997;45:1337-55.
- Snowdon C, Elbourne D, Garcia J. Zelen randomization: attitudes of parents participating in a neonatal clinical trial. Control Clin Trials 1999;20:149-71.
- Snowdon C, Elbourne D, Garcia J. ‘It was a snap decision’: parental and professional perspectives on the speed of decisions about participation in perinatal randomised controlled trials. Soc Sci Med 2006;62:2279-90.
- Snowdon CE, Elbourne DR, Garcia, J. Perinatal pathology in the context of a clinical trial: attitudes of neonatologists and pathologists. Arch Dis Child (Fetal Neonat Ed) 2004:89-7.
- Snowdon CE, Elbourne DR, Garcia, J. Perinatal pathology in the context of a clinical trial: attitudes of bereaved parents. Arch Dis Child Fetal Neonat Edition 2004;89:F208-11.
- Spencer L, Ritchie J, Lewis J, Dilon L, . Quality in qualitative evaluation. London: Cabinet Office; 2003.
- Steinbrook R. Testing medications in children. N Engl J Med 2002;347:1462-70.
- Sugarman J. Determining the appropriateness of including children in clinical research. JAMA 2004;291:494-6.
- van Stuijvenberg M, Suur M, de Vos S, Tijang GC, Steyerberg EW, Derksen-Lubsen G, et al. Informed consent, parental awareness, and reasons for participating in a randomised controlled study. Arch Dis Child 1998;79:120-5.
- Wendler D, Belsky L, Thompson K, Emanuel EJ. Quantifying the federal minimal risk standard: implications for paediatric research without a prospect of direct benefit. JAMA 2005;294:826-32.
- Yardley L. Dilemmas in qualitative health research. Psych Health 2000;15:215-28.
- Young B, Dixon-Woods M, Findlay M. Parenting in a crisis: conceptualising mothers of children with cancer. Soc Sci Med 2002;55:1835-47.
- Young B, Fitch G, Dixon-Woods M, Lambert PC, Brooke AM. Parents’ accounts of asthma-related symptoms in their children. Arch Dis Child 2002;87:131-4.
- Young B, Dixon-Woods M, Windridge K, Heney D. Managing communication with young people who have a potential life threatening chronic illness: qualitative study of patients and parents. BMJ 2003;326:305-16.
- Young B, Klaber Moffatt J, Jackson D, McNulty A. Decision-making in community-based paediatric physiotherapy: a qualitative study of children, parents and practitioners. Health Soc Care Comm 2006;14:116-24.
- Zimmerman R. Desparately seeking kids for clinical trials. Wall Street Journal 2002.
- Zupancic J, Gillie P, Streiner DL, Watts JL, Schmidt B. Determinants of parental authorization for involvement of newborn infants in clinical trials. Pediatrics 1997;99.
List of abbreviations
- GP
- general practitioner
- HIV
- human immunodeficiency virus
- LRN
- local research network
- MASCOT
- Management of Asthma in School-aged Children On Therapy
- MCRN
- Medicines for Children Research Network
- MENDS
- the use of MElatonin in children with Neurodevelopmental Disorders and impaired Sleep
- MRI
- magnetic resonance imaging
- NIHR
- National Institute of Health Research
- PIL
- participant information leaflet
- POP
- prevention and treatment of steroid-induced osteopenia in children and adolescents with rheumatic disease
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RECRUIT
- Processes in recruitment to randomised controlled trials
- TIPIT
- Thyroxine In Preterm Infants Trial
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr David P Britt, Public contributor
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Public contributor
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington