Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/34/02. The contractual start date was in December 2009. The draft report began editorial review in May 2010 and was accepted for publication in September 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Auguste et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Breast cancer
One in thirteen women in the UK will develop breast cancer in their lifetime. Breast cancer is a serious life-threatening disease. In women, breast cancer accounts for one-third of all cases of cancer and, in the UK, from 2004 to 2006, the incidence rate of new breast cancer was 122 per 10,000 women. 1 Most women fortunately present with early-stage breast cancer and the UK NHS screening programme currently offers screening at 50 years of age. After diagnosis, initial treatment often involves surgery with curative intent and subsequent combinations of hormone therapy, chemotherapy and radiation therapy. 2 Treatment options have developed significantly over the past decade and, with early diagnosis, rates of 5-year survival are currently > 80% and have increased steadily over the past 10–20 years. 1 However, a number of women will develop metastatic disease and die of their breast cancer. Recurrence of breast cancer may be an increasing problem as survival rates increase.
Follow-up and treatment in the setting of recurrence
In most breast cancer units, after treatment for the initial disease, patients are routinely followed up for at least 5 years3 with clinical examination and mammography specifically looking for treatable local recurrence and symptoms to suggest metastatic disease. Investigational screening for metastatic disease is not performed routinely. If symptoms suggest relapse with metastatic disease, further investigations may be conducted where there is suspicion of disease. Rates of breast cancer recurrence have been shown to be around 20%4,5 and recurrence may be local (in the breast), regional (lymph nodes in the ipsilateral axilla) or distant metastases (in tissues such as bone, liver, lungs and brain). Of patients with breast cancer recurrence, one study showed that 27% had bone metastases, 27% had local recurrence, 16% had lung metastases and 13% had liver metastases. 4
If metastatic disease is established, patients are generally not curable and treatment is aimed at palliating symptoms and improving survival if possible. Useful and sometimes lengthy clinical responses can be obtained by using hormone therapy in hormone-responsive disease and with chemotherapy, particularly with the newer agents. Taxane-based chemotherapy is considered likely to increase overall survival, time to progression and overall response in the second-line setting,2 and trastuzumab (Herceptin, Roche) is showing promising results in HER-2 (human epidermal growth factor receptor-2)-positive disease. Radiotherapy, combined with appropriate analgesia, may be effective in reducing persistent localised bone pain. 2 Therapy with selective aromatase inhibitors in postmenopausal women has been shown to prolong survival in the second-line setting, although no difference was shown in time to progression. 2
Existing diagnostic strategies
Women with a past history of breast cancer may present with symptoms that may be innocent or indicate disease. There are a range of strategies that may be involved in patient diagnosis, and the choice of tests used depends on the presenting symptoms. Conventional work-up often includes conventional radiography, computed tomography (CT), ultrasound, bone scintigraphy and measurement of serum tumour markers, and, in a limited number of settings, magnetic resonance imaging (MRI) may be available for use.
Conventional radiography is widely available and may routinely be used for cases of suspected breast cancer recurrence. Radiographs may be particularly useful for the diagnosis of metastases in the lung and bones (chest or individual bone radiography), but this technique is limited in other applications where there may be a smaller difference in the amount of radiation taken up by the tumour than by surrounding tissues. 6 CT scanning is a technology that uses X-rays, but is different to the conventional method as it uses multiple X-ray projections from different angles to produce three-dimensional images. 5 CT scans can be used to detect cancer in a range of tissue types (lung, bone, soft tissue, etc.), but settings must be selected for examination of the appropriate tissue density. 5,7 It is possible to conduct whole-body CT examinations, and CT images may also be enhanced with the administration of oral or intravenous contrast agents. 5 Ultrasound uses information from reflected high-frequency sound waves to produce images of tissues. 4 More recently, contrast agents have been developed for use with ultrasound that enhance images. 4 Ultrasonography in oncology is commonly used in the detection of distant metastases and can be used to detect metastases in the liver and kidneys, but the technique relies, to a considerable extent, on the expertise of the operator. 6 Bone scintigraphy uses radionuclides of technetium-99m-labelled disphosphonates previously injected into patients. 6 The degree of uptake into bone depends largely on the extent of blood flow and the rate of new bone formation, and sites of high radio-emission may represent the presence of a tumour. In oncology, bone scintigraphy may be used for the identification of bone metastases. 8
Several biochemical compounds in the serum/plasma may act as indicators of the presence, risk or prognosis of cancer. 9 In patients with a history of breast cancer, elevated tumour marker levels may represent cases of tumour relapse. 9 It has been identified that increasing levels of these markers are associated with disease recurrence and may indicate the need for further investigation. MRI uses magnetic fields and radiowave pulses to cause signals to be produced from tissues, and these signals are subsequently converted to an image. MRI may be used for the detection of local breast cancer recurrence10 or, with imaging of the whole body, for the additional detection of bone metastases11 and other distant metastases. 12 However, access to MRI is limited6 and it is not routinely used for diagnosing suspected breast cancer recurrence.
In the setting of diagnosis of breast cancer recurrence, conventional work-up is likely to comprise a combination of these technologies (in most cases, conventional work-up will not include MRI). Patients may undergo a variety of tests depending on their presenting symptoms and on the basis of the results of other imaging tests.
Positron emission tomography and positron emission tomography/computed tomography
Positron emission tomography (PET) and, more recently, positron emission tomography/computed tomography (PET/CT) are new technologies which have been increasingly shown to have application in the detection and management of cancer since the introduction of whole-body PET and PET/CT in the late 1990s. These technologies involve oral administration of a radioactive isotope and detection of photons produced in the process of radioactive decay and interaction with surrounding tissues. 11,13 In oncology, the most commonly used radionuclide is 18F-fluorodeoxyglucose (FDG), which is taken up into cells in the same way as glucose. FDG accumulates in tumour tissue owing to increased glucose requirements and therefore increased glucose uptake. Also, in most tissues, FDG accumulates following uptake and phosphorylation, as, unlike glucose, it cannot enter the normal glycolytic pathway. 13 FDG is administered intravenously to patients and, following an interval of time (usually 60–90 minutes) to allow approximately 1 hour uptake time, PET scans are conducted. The whole body may be imaged during a single session,14 and these technologies may be used to detect both local and metastatic tumours.
Positron emission tomography/computed tomography combines information obtained from PET with data from CT scanning. 15 As these technologies provide different types of data (PET gives metabolic and CT anatomical data), their combination provides greater diagnostic information. CT data may also be used for attenuation correction of PET images. 15 In PET scanning, attenuation, the loss of emitted photons through scatter and absorption, can be the biggest source of inaccuracy. 11 An attenuation map of CT can be used to estimate attenuation factors for PET, and this correction applied to increase the accuracy of the images produced. 11,13
Current guidelines
Guidelines have been developed by the National Institute for Health and Clinical Excellence (NICE) for the treatment of early/locally advanced3 and advanced16 breast cancer. In the early breast cancer guidance, key recommendations for the follow-up of breast cancer patients include provision of designated health-care professionals, dates for review of any adjuvant therapy, surveillance with mammography and contact points for specialist care. 3 The guidance suggests that future research should involve determination of the appropriate length of follow-up and suitable methods for detecting disease recurrence. 3
The advanced breast cancer guidelines give advice on the treatment of advanced/metastatic disease that is generally amenable only to palliative care without curative intent. These guidelines do not specifically relate to the diagnosis of breast cancer recurrence. However, some guidance given may be applicable. The NICE guidelines state that ‘Positron emission tomography fused with computed tomography (PET-CT) should only be used to make a new diagnosis of metastases for patients with breast cancer whose imaging is suspicious but not diagnostic of metastatic disease’. 16 In other words, PET/CT would be used only for diagnosis in cases in which conventional imaging techniques fail to properly diagnose the presence or absence of metastases. Although these guidelines relate to the use of PET/CT, all of the evidence reviewed to inform them are studies or systematic reviews of PET and not PET/CT. Additionally, these guidelines refer only to the diagnosis of metastatic, and not local, recurrence, and the evidence base relating to the application of PET and PET/CT for the detection of recurrent breast cancer is not clear. However, there are no detailed recommendations for the types of investigations to be used for diagnosis of disease in context of recurrence. The guidance suggests that future research should involve determination of the appropriate length of follow-up and suitable methods for detecting disease recurrence. 3
Imaging procedures such as radiography, CT, ultrasound and bone scintigraphy are currently conventionally used in many hospitals for the diagnosis of recurrent breast cancer, whereas MRI, PET and PET/CT are not routinely used in this context (Dr Theodoros Arvanitis, University of Birmingham, personal communication). In 2005, the Royal College of Radiologists published a strategy document detailing the provision of PET/CT instruments across the UK. 14 At that time, there were 11 fixed scanning PET/CT units installed in the UK, predominantly for clinical use. Recommendations included a hub and satellite system, where central hub staff and resources would be used to maintain PET/CT scanners in more satellite settings. 14 Initially, provision was to be made for one PET/CT system per 1.5 million of the population,14 equating to approximately 40 machines for the current UK population.
Recent guidelines from the UK PET/CT Advisory Board have recommended the use of PET/CT for patients with suspected cancer recurrence who are negative for other imaging procedures and in whom detection would alter therapy and benefit outcome. 15 However, this recommendation is not specific to breast cancer and the evidence base relating to the Advisory Board’s application for use for detection of recurrent breast cancer is not clear.
Evidence on accuracy and effectiveness of positron emission tomography and positron emission tomography/computed tomography
This report has been carried out in close collaboration with another which has reviewed the evidence on the accuracy and effectiveness of PET and PET/CT in the diagnosis of suspected distant recurrence in breast cancer. 17
This report employed a systematic review and meta-analysis. A search for primary studies in MEDLINE (Ovid) and EMBASE (Ovid) was conducted with no language restrictions to May 2009. Studies of PET or PET/CT in patients with a history of breast cancer and suspicion of recurrence were selected for inclusion. Studies were excluded if investigations were conducted for screening or staging of primary breast cancer, if a non-standard PET or PET/CT technology was used, if there was an inadequate or undefined reference standard or if raw data for calculation of diagnostic accuracy were not available. Both comparative and non-comparative studies were included.
Data extraction and quality assessment were conducted independently by two reviewers, with any disagreements resolved by consensus. Direct and indirect comparisons were made between PET and PET/CT and between these technologies and methods of conventional imaging, and meta-analysis was performed using a bivariate random effects model. Analysis was conducted separately on patient- and lesion-based data. Subgroup analysis was conducted to investigate variation in the accuracy of PET in certain populations or contexts, and sensitivity analysis was conducted to examine the reliability of the primary outcome measures.
The key findings were that 28 studies18–45 were included and, of these, 2618–36,38,39,41–45 investigated the diagnostic accuracy of PET. Twenty-five18–36,38,39,41–44 presented patient-based data and seven18,24,29–31,34,45 presented lesion-based data for PET. Six21,22,27,37,40,42 studies investigated the accuracy of PET/CT, five21,22,27,37,42 presenting patient-based data and one40 presenting lesion-based data. Sixteen18,20,21,23,24,26–28,35,37,38,40,42–45 studies conducted direct comparisons and, of these, 1218,21,23,26–28,35,37,38,40,42–45 compared the accuracy of PET or PET/CT with conventional diagnostic tests, and four20,24,26,40 compared PET or PET/CT with an MRI technology. Quality varied between studies; the major quality issue identified was the time delay between conventional tests and PET or PET/CT in comparative studies. The PET or PET/CT technology used was similar across the studies.
The results of the systematic review were:
-
For patient-based data, in studies in which direct comparisons were made, compared with conventional imaging tests (n = 10), PET had a significantly higher sensitivity [89%, 95% confidence interval (CI) 83% to 93%, vs 79%, 95% CI 72% to 85%; relative sensitivity 1.12, 95% CI 1.04 to 1.21, p = 0.005] and a significantly higher specificity (93%, 95% CI 83% to 97%, vs 83%, 95% CI 67% to 92%; relative specificity 1.12, 95% CI 1.01 to 1.24, p = 0.036). Test performance did not appear to vary according to the type of conventional imaging test that was compared with PET (p = 0.500). Indirect comparisons, in which all conventional imaging test18,21,23,27,28,35,37,38,42–44 (n = 11) and PET18–34,36,38–44 (n = 25) studies were included, gave the same findings. For lesion-based data, no significant differences in sensitivity or specificity between PET and conventional imaging testing were observed for studies making direct comparisons18,23,45 (n = 3) or for indirect comparisons for all PET18,23,29–31,34,45 (n = 7) and conventional imaging test18,23,45 (n = 3) studies. In the sensitivity analysis of patient data, for studies in which the time period between PET and comparator tests was clearly < 1 month18,21,27,35,38,42 (n = 6), differences between PET and conventional imaging test tended to be smaller, and the difference in sensitivity became non-significant.
-
For patient-based data, in all studies in which direct comparisons were made21,27,37,42 (n = 4), the conventional imaging test used was CT. In these studies, compared with CT, PET/CT had significantly higher sensitivity (95%, 95% CI 88% to 98%, vs 80%, 95% CI 65% to 90%; relative sensitivity 1.19, 95% CI 1.03 to 1.37, p = 0.015), but the increase in specificity was not significant (89%, 95% CI 69% to 97%, vs 77%, 95% CI 50% to 92%; relative specificity 1.15, 95% CI 0.95 to 1.41, p = 0.157). Indirect comparisons, in which all conventional imaging test18,21,23,27,28,35,37,38,42–44 (n = 11) and PET/CT21,22,27,37,42 (n = 5) studies were included, gave the same findings. No lesion-based data compared PET/CT with conventional imaging testing. In the sensitivity analysis of patient data, for studies in which the time period between PET/CT and comparator tests was clearly < 1 month21,27,42 (n = 3), differences between PET/CT and CT became non-significant, largely owing to the small sample size.
-
For patient-based data, three studies compared PET with different types of MRI technology. 20,24,26 In each of these studies, there were no significant differences in the sensitivity or specificity of PET compared with MRI. One study compared PET/CT and MRI on a lesion basis and there were no significant differences in sensitivity or specificity for PET/CT compared with MRI. 40
-
For patient-based data, in the analysis of studies directly comparing PET/CT and PET (n = 4),21,22,27,42 PET/CT had a significantly higher sensitivity (96%, 95% CI 90% to 98%, vs 85%, 95% CI 77% to 91%; relative sensitivity 1.11, 95% CI 1.03 to 1.18, p = 0.006), but the increase in specificity was not significant (89%, 95% CI 74% to 96%, vs 82%, 95% CI 64% to 92%; relative specificity 1.08, 95% CI 0.94 to 1.20, p = 0.267) compared with PET. The same pattern of results was observed for the indirect comparison of all PET/CT21,22,27,37,42 (n = 5) and PET18–36,38,39,41–44 (n = 25) studies. In the lesion-based analysis, indirect comparison of PET/CT37,40 (n = 2) and PET18,23,29–31,34,45 (n = 7) showed no significant differences in sensitivity or specificity between PET/CT and PET.
-
For overall diagnostic accuracy, on a patient basis, PET/CT21,22,27,37,42 (n = 5) and PET18–36,38,39,41–44 (n = 25) had sensitivities of 96% (95% CI 89% to 99%) and 91% (95% CI 86% to 94%) and specificities of 89% (95% CI 75% to 95%) and 86% (95% CI 79% to 91%), respectively. On a lesion basis, PET/CT37,40 (n = 2) and PET18,23,29–31,34,45 (n = 7) had sensitivities of 96% (95% CI 80% to 99%) and 89% (95% CI 78% to 95%) and specificities of 83% (95% CI 61% to 94%) and 91% (95% CI 83% to 96%), respectively. There was considerable heterogeneity in the spread of the results for PET.
-
Changes in patient management in study participants ranged from 11% to 74% (median 27%). These changes included initiation and avoidance of medical treatment such as hormone therapy and chemotherapy. In the three42,43,46 studies in which only changes in management directly due to PET or PET/CT were considered (patients were not correctly diagnosed by conventional imaging techniques), estimates ranged from 11% to 25%.
-
In subgroup analysis, the accuracy of PET did not appear to be related to the location of disease or to whether PET was conducted with or without knowledge of previous clinical history and imaging studies. Characteristics of patient populations varied in many respects and it was not possible to draw definite conclusions about patient characteristics that may affect test accuracy.
The report concluded that:
-
For detection of breast cancer recurrence, in addition to conventional imaging techniques, PET may generally offer improved diagnostic accuracy compared with current standard practice. Uncertainty remains around its incremental value compared with specific conventional diagnostic tests, and around its use as a replacement, rather than an add-on, to existing imaging technologies.
-
PET/CT appears to show a clear advantage over CT for the diagnosis of breast cancer recurrence. Although PET/CT may give an advantage over other conventional imaging tests, its incremental value over other tests has yet to be assessed in studies directly. Concurrent use with, rather than as replacement of, other conventional tests may be appropriate.
-
PET/CT appears to show a clear advantage over PET and, if found to be more cost-effective, it may be preferable to PET for use in this context.
-
PET and PET/CT appear to have some effect on patient management, but there is currently no evidence of the effect of their use on patient outcome.
Chapter 2 Methods and results of review of published economic studies
Objective
A systematic review was conducted to determine the existence of published literature on the economic evaluation of PET and PET/CT to detect breast cancer recurrence. The purpose of this review was to search available literature on the suitability of existing cost-effectiveness models and model design, and to identify information that could be used to populate the model. The aim was also to identify useful information relating to the cost of tests and treatments commonly used to diagnose and treat breast cancer recurrence.
Methods
Using systematic reviews and meta-analyses of clinical studies, particularly randomised controlled trials (RCTs), is a well-established research method, but the approach for reviewing economic evaluations and costing studies is necessarily different and more qualitative, primarily because of the heterogeneity that exists in economic studies, which means that data synthesis and meta-analyses are rarely possible. This review was carried out using established methods for systematically reviewing economic evaluation and costing studies. 47
Identifying studies
Search strategy
The original databases searched include MEDLINE (Ovid) (1950 to week 5 May 2009), EMBASE (Ovid) (1980 to 2009 week 22) and the NHS Economic Evaluation Database (NHS EED) using the search strategies detailed in Appendix 1.1. An updated search was conducted for each database from May 2009 to week 4 April 2010 using the same search strategies detailed in Appendix 1.2.
Studies were independently reviewed on the basis of their title, abstract and Medical Subject Heading (MeSH) by independent researchers: MEDLINE and EMBASE (CH, MP and PA) and NHS EED (CH and PA). The screening process used by PA follows similar methods used by Roberts et al. 47 to identify economic evaluation and costing studies. Briefly, a three-stage process is adopted for the review of cost and economic studies. In stage 1 each study is categorised on the basis of its title and abstract, where available, according to five classification criteria. For example, studies are classified into group A if they are suspected of being a full economic evaluation on PET/CT recurrence (such studies would have terms such as cost-effectiveness or economic evaluation as well as the clinically relevant terms in the title or abstract). Studies are classified into group B if they are cost studies (costs would be mentioned somewhere in the title or abstract as well as the clinically relevant terms). Any studies that were categorised into the relevant classification for these review groups A or B would proceed to stage 2. In stage 2, all potentially relevant studies would be read in full and, if confirmed in their classification, proceed to stage 3 for a quality review. For example, a confirmed economic evaluation would be coded A1, a study that was initially considered to be cost study, but discovered to be an economic evaluation on full review would be coded as B1, etc.
Inclusion criteria
To be included in the review, published literature should have studied a population of women who had completed a course of treatment for primary breast cancer, and those who had undergone PET or PET/CT with recurrent breast cancer were also included.
Exclusion criteria
Studies that conducted research on primary breast cancer or staging of breast cancer and studies that monitored the response to primary breast cancer treatment were excluded.
Results
Database searches resulted in 1540 MEDLINE, 1353 EMBASE and 3 NHS EED citations. No relevant studies to the economic evaluation were identified that related to breast cancer recurrence. Two published economic evaluation studies48,49 were identified, but were based on primary and axilliary staging of breast cancer. These two studies were reviewed completely to determine the usefulness of the information to the current study. However, no information was taken from these studies; a synopsis of the studies can be found in Appendix 1.2. Wider ad hoc searches led to additional papers being retrieved that would not have been picked up by the database search. 50–52 From the NICE guidelines,52 we extracted data that related to treatment costs and quality-adjusted life-year (QALY) data as explained in the subsequent chapter. Cost data for PET and PET/CT imaging tests were taken from Jacklin et al. 51 and Heinrich et al. ,50 respectively. Some authors were contacted by e-mail to explore any further cost information that may have been useful for this study. However, no cost data were utilised from this source.
Chapter 3 Model-based economic evaluation
Developing the model structure
To assess the cost-effectiveness of the various diagnostic procedures, a decision tree was developed in treeage software (TreeAge Software Inc., Williamstown, MA, USA). The model begins with patients receiving one of four diagnostic procedure packages: (1) conventional work-up (this refers to a diagnostic package comprising ultrasound, radiography, CT, bone scintigraphy and measurement of serum tumour markers) (2) PET; (3) PET and CT, referred to as PET/CT; and (4) PET/CT as an adjunct to conventional work-up.
Many assumptions are required in order to develop a workable model structure and which would allow the model-based analysis to be carried out:
-
For the branches of the decision tree that include the diagnostic package of conventional work-up, it is assumed that all patients will have symptoms that require all the elements of conventional work-up. But it is possible for patients to have initiating symptoms that do not require full work-up, and symptoms may be more specific, such as bone pain, which would be more appropriately investigated with only a bone scan.
-
The assumption is also made that patients are being investigated only for distant recurrence. The possibility of concurrent local recurrence that could be detected exists, but this is likely to be established with other imaging techniques and it is unlikely that PET/CT would be introduced to specifically improve the accuracy of the diagnosis of local recurrence.
-
It is also assumed that recurrence occurs on one occasion only; however, in reality, one possible recurrence is likely to increase the risk of further possible recurrence episodes. In addition, it is assumed in the model that a confirmatory biopsy test will be used when a positive result from imaging with or without PET/CT occurs. This limits the effect of false-positives. However, in practice, such biopsy may not occur, partly because of concerns about the accuracy of biopsy itself, particularly where the suspected cancerous areas are small.
-
The model population has an assumed starting age of 50 years because it is above this age that women are most at risk (the UK NHS screening programme for early-stage breast cancer starts at 50 years of age), and, therefore, the expected survival for breast cancer recurrence has been calculated based on women starting at this age.
Figure 1 depicts the general structure of the decision tree.
FIGURE 1.
Model structure.
Data required for the model
Prevalence data
We populated the decision model with the prevalence estimated in the meta-analysis of the systematic review carried out by Pennant et al. 17 We estimated an average of the whole-body prevalence values reported in the study for use in the model. 17 However, the data reported in the review were noted to be variable and, whereas in the base case we use an estimated prevalence of 0.69, the range in the literature was from 0.19 to 0.93 (Table 1). We explored the effect of the wide range on prevalence in the sensitivity analysis. The distribution associated with the 95% CI is explored in the probabilistic sensitivity analysis.
| Screening strategy | Sensitivity (95% CI) | Specificity (95% CI) | Source |
|---|---|---|---|
| PET | 0.91 (0.87 to 0.93) | 0.86 (0.79 to 0.91) | Pennant et al.17 |
| PET/CT | 0.95 (0.89 to 0.97) | 0.89 (0.76 to 0.96) | Pennant et al.17 |
| Conventional work-up | 0.80 (0.72 to 0.87) | 0.76 (0.59 to 0.88) | Derived from data presented in Pennant et al.17 |
| PET/CT as an adjunct to conventional work-upa | 0.99a (0.97 to 1.00) | 0.68a (0.45 to 0.89) | Derived from data presented in Pennant et al.17 |
| Base-case prevalence (95% CI) |
0.69 (0.66 to 0.72) (range 0.19–0.93) |
Derived average of whole body prevalence reported by Pennant et al.17 | |
Test accuracy data
Test accuracy data used in the model-based economic evaluation are based on the values estimated by the authors of the clinical systematic review. 17 The most reliable results from the systematic review are the relative sensitivity and specificity of the test, but these could not be accommodated in the model, which requires absolute sensitivity and specificity. The choice of which accuracy estimates are used as parameters is therefore likely to have an impact on the cost-effectiveness estimates.
The sensitivity and specificity of PET (see Table 1, row 1) of 91% and 86%, respectively, are estimated by the review authors from an indirect comparison between PET and conventional work-up (referred to as conventional imaging in their report) and presented in Table 8 (row 2) of that report. 17 The relevant tables from Pennant et al. 17 are presented in Appendix 2.2.
The sensitivity and specificity of PET/CT (see Table 1, row 2) of 95% and 89%, respectively, are also estimated by indirect comparison between PET/CT and conventional work-up and are presented in Table 11 (row 2) of Pennant et al. 17
The sensitivity and specificity of conventional work-up (see Table 1, row 3) of 80% and 76%, respectively, have been derived by the current authors for use in the economic model from data presented by Pennant et al. ,17 and have not been estimated by the review authors. The sensitivity of conventional work-up is based on an average of 81% and 78%. Eighty-one per cent is the sensitivity of conventional work-up [referred to as conventional imaging test (CIT)] estimated as an indirect comparison of PET versus conventional work-up in Table 8 of the report by Pennant et al. 17 Seventy-eight per cent is the sensitivity of CIT estimated as an indirect comparison of PET/CT versus conventional work-up, presented in Table 11 of the report by Pennant et al. 17 The conventional work-up in these two tables (i.e. Tables 8 and 11 of Pennant et al. 17) is based on slightly different techniques, which is why an average of the two is used. The specificity estimate of 76% is an average of 73% and 79% from the same tables (8 and 11 respectively), for the reasons given above.
The sensitivity and specificity of PET/CT as an adjunct to conventional work-up (see Table 1, row 4) have also been derived by the current authors. We assumed that the overall result would be positive if either test is positive on its own. As a result of this rule, the sensitivity increases as a result of combining the two tests – as all individual patients who test positive on either (but not necessarily both) test are considered a potential positive case. This very interpretation means that there will be more false-positives, and so the specificity of the combined test is necessarily lower than the specificity of either test individually. In the base case, we assumed that testing errors were statistically independent between the two tests. Then the specificity of the combined test is simply the product of the two individual test specificities: in the base case, this becomes 0.89 × 0.76 = 0.68, while for the sensitivity (sens) we have the formula
which can be rearranged to give
where CW refers to conventional work-up. In the base case, this gives a combined sensitivity of (0.95 + 0.80) – (0.95 × 0.80) = 0.99. For probabilistic sensitivity analysis, we preserved the relationship between these characteristics by sampling the test characteristics for PET/CT and conventional work-up separately, and then calculating the test characteristics for the combined test using the methods just described. The methods we have used have been used elsewhere53–55 and are referred to by some authors as the ‘either positive rule’. 55
In the sensitivity analysis, we considered the situation in which the sensitivity of the test increases, as per the base case, but we assumed that these additional positive cases were ‘true-positives’ and so we maintained the specificity at the level estimated for conventional work-up alone – for which the value is 0.76.
The data presented in Table 1 require a number of cautionary explanations. First, the number of indirect comparisons described above is necessary to estimate accuracy data, because the systematic review of accuracy studies found no studies that had made the relevant direct comparison. 17 For instance, an indirect comparison was used to estimate the accuracy data for conventional work-up (see Table 1, row 3) because there are no primary studies comparing PET/CT with conventional work-up.
Second, when PET and PET/CT are introduced into clinical practice, it would be a fair assumption, that their introduction is in the role of an adjunct to conventional management (referred to as conventional work-up in the current study) in clinical practice. Therefore, to many familiar with this sort of technology, the data in rows 1 and 2 of Table 1 would be assumed to represent the diagnostic tests of both PET and PET/CT as an adjunct to the conventional work-up. However, it was very apparent to Pennant et al. ,19 who had scrutinised the papers in their review, that the tests reported in the papers had been introduced not as an adjunct, but as a replacement. It was apparently clear in these studies that previous test results were not consulted or considered in the interpretation of the PET and PET/CT results. Hence, we present these data (see Table 1, rows 1–3) as representing the accuracy of the individual test alone, and we derived the data presented in Table 1, row 4, in which the accuracy of PET/CT is combined with conventional work-up to represent the strategy of PET/CT as an adjunct to conventional work-up.
It was also noted by Pennant et al. 19 that PET as a strategy was never really introduced into clinical practice, it was soon subsumed by PET/CT, and so for this reason we carried out a sensitivity analysis in which PET as a single strategy was removed from the analysis.
Costs and resource data
The costs of resources utilised were those that were directly incurred by the NHS. Resources included were the costs of the index, confirmatory tests and blood tests, and treatment costs. Costs that were not considered were those incurred during the primary diagnosis and the treatment of breast cancer. Costs for long-term and end-of-life care were also not included. As stated earlier, recurrence is assumed to occur once only. Diagnostic procedure costs were taken from published sources. Cost estimates for PET were taken from Jacklin et al. ,51 and were adjusted to 2008 prices by the use of the Hospital and Community Health Services combined pay and price inflation index. 56 Estimated costs for conventional work-up included costs of ultrasound, bone scintigraphy, radiography, CT and measurement of serum tumour markers. These cost data were taken from the NHS national schedule for reference costs 2002–3,57 2007–858 and published sources. 59 As a result of a paucity of cost-effectiveness studies comparing PET/CT as an adjunct to conventional work-up, we assumed an additive procedural cost of PET/CT as an adjunct to conventional work-up as shown in Table 2. All costs were adjusted to 2007–8 prices and not discounted as the time period of the model was 1 year.
| Unit costs (£) | Description | Source | |
|---|---|---|---|
| Index test | |||
| PET | 1006 | Full description could be found in Jacklin et al.51 | Jacklin et al.51 cost inflated to 2008 prices |
| PET/CT | 1236 | Full description of PET/CT procedure could be found in Heinrich et al.50 | Heinrich et al.50 cost inflated to 2008 prices |
| Conventional work-up | 485 | ||
| Ultrasound | 53 | Ultrasound scan < 20 minutes | NHS reference costs 2008,58 RA23Z |
| Bone scintigraphy | 164 | Procedure that required diagnostic level radiation, basic gamma camera, standard technologist time of up to 1 hour and low-to-medium isotope costs | NHS reference costs 2008,58 Nuclear Medicine – Category 2, RA36Z |
| Radiography (chest) | 95 | J35 op ultrasound scan | NHS reference costs 2003,57 inflated to 2008 prices |
| CT (chest, brain and abdomen) | 135 | CT scan, three areas with contrast | NHS reference costs 2008,58 RA13Z |
| Measurement of serum tumour markers | 38 | Carcinoembryonic antigen blood test | Guadagni et al.59 inflated to 2008 pricesa |
| PET/CT together with conventional work-up | 1721 | PET/CT together with routine conventional work-up | Heinrich et al.,50 NHS reference costs 2003,57 NHS reference costs 200858 and Guadagni et al.59 |
| Reference standard test | |||
| Biopsy | 141 | Core-needle biopsy (J30 op) in HRG | NHS reference costs 2003,57 inflated to 2008 prices |
| Treatment | |||
| Docetaxel, capecitabine and no chemotherapy | 18,643 | Full description in NICE guidelines52 | cNICE guidelines52 |
The treatment cost presented in Table 2 is based on an estimate of the most cost-effective treatment strategy estimated in a study reported by NICE which presents estimates of costs for treatment received by patients with confirmatory diagnosis of breast cancer recurrence. 52 The authors of the NICE report conducted an economic evaluation to determine the most cost-effective treatment strategy for breast cancer using chemotherapy. The treatment cost used in the current model is based on the treatment cost for the most cost-effective strategy (strategy 14) in their results. 52 The assumptions regarding population, treatment, survival and cost estimation upon which their analysis is based are presented in Appendix 2.1. The authors concluded that docetaxel (monotherapy) used as a first-line treatment followed by capecitabine (monotherapy) as a second-line treatment then ‘no chemotherapy’ (supportive and palliative care) as a final treatment was the most cost-effective strategy at the £20,000 threshold. The treatment cost for this strategy was £18,118 in 2007 prices. This estimate was updated to £18,643 in 2008 prices using the Hospital and Community Health Services combined pay and price inflation index. 56
Outcomes
We used three different effectiveness/outcome measures in the model.
Outcome in terms of quality-adjusted life-years
Two sets of QALY values were estimated for use in the model: first, the QALY values for the true-positives and second the QALY values for the false-positives and true-negatives.
True-positives: The utility weights for patients who had been treated with the most cost-effective strategy (as reported above based on the report by NICE52) were originally obtained from oncology nurses by the use of the standard gamble technique. 60 These utility weights were then used to calculate the QALYs expected for patients who had been treated and for the most cost-effective strategy (strategy 14);52 the total expected QALYs were 0.8737.
False-positives and true-negatives: The utility weight for those patients who were not treated, i.e. those who were breast cancer recurrence free, was derived from the Kind et al. 61 study, and the estimate is 0.848. The authors used the EuroQol European Quality of Life-5 Dimensions questionnaire to value differences in the health-related quality of life between age groups in a UK setting. 61 This figure was used to calculate the QALYs from the lifetime tables. In the base-case analysis we assumed that patients who are false-positive (patients who had a positive index test, but were breast cancer recurrent free) and go on to have a confirmatory biopsy will experience no disutility and will have QALYs of 13.8724. 62 The assumption here is that the biopsy is 100% accurate and, because it is a relatively minor procedure, we have assumed that it induces no QALY decrement. If the biopsy is not 100% accurate, there will be a finite number of people who think they have recurrence when they have not. However, we have no data on this potential event and we did not address it in the sensitivity analysis. An important aspect of these data is the longer life expectancy associated with non-recurrence than with recurrence. The survival of those who do not experience recurrence is based on general population life tables. 62 In contrast, recurrent breast cancer has a median life expectancy of 16.747 months, even if patients are treated optimally. 52
Outcome in terms of recurrent cancer appropriately diagnosed and treated
For recurrent cancer appropriately diagnosed, only the difference between true-positive and false-negative matters. We have given these values 1 and zero respectively. For convenience we have given a value of 1 for false-positive and true-negatives, and therefore reserved the value of zero for important error.
Outcome in terms of diagnostic error avoided
In relation to the outcome of diagnostic error avoided, no effectiveness data were required. The true-positive and true-negative cases were given the value of one. False-positives and false-negatives were given the value of zero.
Analysis
The decision tree model was constructed and programmed to select the base-case model input parameters. The model estimated the mean cost associated with each diagnostic procedure and assumed that patients entering the model would be aged 50–75 years.
The results of the cost-effectiveness analysis are presented in terms of the incremental cost-effectiveness ratios (ICERs). It was appropriate to use this method of presentation as it gives the benefit associated with an additional cost per QALY. The base-case analysis is based on a number of outcomes. The primary outcome is cost per QALY, but secondary outcome measures of case of recurrent cancer appropriately diagnosed and treated and cost per diagnostic error avoided are also considered.
A deterministic analysis was carried out for the base-case results for the primary and secondary outcome measures.
In addition, a number of sensitivity analyses were required, and these have been highlighted where appropriate during the description of the data. The sensitivity analyses that we carried out are summarised below:
-
Threshold-focused sensitivity analyses were carried out, deterministically, to establish the critical values for the cost and accuracy of PET/CT that could change deterministic results in terms of the ICERs, in such a way that might affect the decisions of policy-makers.
-
A deterministic sensitivity analysis was carried out on the extreme values in the range of prevalence (0.19–0.93) found in the systematic review by Pennant et al. 17
-
In response to the ‘either positive rule’ used in the base case for the derivation of the sensitivity and specificity for the strategy of PET/CT as an adjunct to conventional work-up, we carried out a deterministic analysis using the derived sensitivity which was used in the base case, but instead of using the derived specificity value used in the base case of 0.68, which was necessarily low to reflect increased number of false-positives, we used the specificity for conventional work-up alone (value 0.76) to explore the assumption that the additional cases detected were in fact true-positive (0.76 is the lower of the two specificities for the tests being combined).
-
It was clear to the authors of the accuracy review17 that PET alone was never introduced into clinical practice, so we carry out sensitivity analysis which removes this diagnostic strategy from the base case.
Probabilistic sensitivity analysis was undertaken to determine the uncertainty of the model input parameters of prevalence, sensitivity and specificity, treatment costs and expected QALYs. We carried out probabilistic sensitivity analysis based on an outcome of cost per QALY only. In probabilistic sensitivity analysis, each model parameter is assigned a distribution reflecting the amount and pattern of its variation, and cost-effectiveness results are calculated by simultaneously selecting random values from each distribution. The process is repeated 10,000 times in a Monte Carlo simulation of the model to give an indication of how variation in the model parameters leads to variation in the ICERs for a given test combination. The appropriate distribution for the data on test accuracy (sensitivity and specificity) and prevalence is a beta distribution. The beta and normal distributions that were used in the probabilistic sensitivity analysis are presented in Appendix 2.3.
For the cost and effectiveness of treatment, only point estimates were available from the report. 52 For the purpose of probabilistic sensitivity analysis, we placed independent normal distributions around each figure, taking the standard deviation to be 0.1 times the mean. This assumption ensures that both cost and QALY outcomes will remain positive. Given the arbitrary nature of this assumption, we also carried out probabilistic sensitivity analysis in which these values were fixed at the point estimates.
Chapter 4 Results of the cost-effectiveness modelling
Results in terms of cost per quality-adjusted life-year
The base-case deterministic results of the strategies based on the outcomes of QALYs, diagnostic error avoided and the case of recurrent cancer appropriately diagnosed and treated are presented in Tables 3–5. In Table 3, the results are presented in terms of cost per QALY. Conventional work-up with a mean cost of £10,864 was the least costly diagnostic strategy with corresponding QALYs of 4.7826 (see Table 3b). The conventional work-up diagnostic strategy provided the lowest number of QALYs. PET diagnostic strategy had a mean cost of £12,807 and was £1942 more costly than conventional work-up. The total effectiveness of PET was 4.8490 QALYs, an incremental QALY gained of 0.0663 compared with conventional work-up. The estimated ICER for this diagnostic strategy was £29,300 per QALY. This indicates that for every additional QALY from PET there is an incremental cost of £29,300. PET/CT costs £747 more than PET alone, but has an incremental QALY gained of 0.0241 compared with PET alone. The ICER for PET/CT compared with PET is approximately £31,000 per QALY. Finally, the diagnostic strategy of PET/CT combined with conventional work-up has a mean cost of £14,566 and costs approximately £1012 more than PET/CT and has a total effectiveness of 4.8972 QALYs, which is an incremental QALY gain of 0.0241 compared with PET/CT. The ICER for PET/CT as an adjunct to conventional work-up compared with PET/CT is £42,000 per QALY.
| Strategy | Mean cost per strategy | Difference in costs | Effectiveness (QALY) | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| Conventional work-up | £10,864 | – | 4.7826 | – | – |
| PET | £12,807 | £1942 | 4.8490 | 0.0663 | £29,300 |
| PET/CT | £13,554 | £747 | 4.8732 | 0.0241 | £31,000 |
| PET/CT together with conventional work-up | £14,566 | £1013 | 4.8972 | 0.0241 | £42,100 |
| Outcome | Probability | QALY | Probability and QALY |
|---|---|---|---|
| True-positive | 0.552 | 0.8737 | 0.4823 |
| False-negative | 0.138 | 0 | 0 |
| False-positive | 0.0744 | 13.8724 | 1.0321 |
| True-negative | 0.2356 | 13.8724 | 3.2683 |
| Effectiveness for conventional work-up | – | – | 4.7827 |
| Strategy | Mean cost per strategy | Difference in costs | Effectiveness | Incremental case of recurrent cancer appropriately diagnosed and treated | ICER |
|---|---|---|---|---|---|
| Conventional work-up | £534 | – | 0.8620 | – | – |
| PET | £1101 | £527 | 0.9379 | 0.0759 | £6900 |
| PET/CT | £1333 | £233 | 0.9655 | 0.0276 | £8400 |
| PET/CT together with conventional work-up | £1831 | £498 | 0.9931 | 0.0276 | £18,100 |
| Strategy | Mean costs | Difference in costs | Effectiveness | Incremental diagnostic error avoided | ICER |
|---|---|---|---|---|---|
| Conventional work-up | £573 | – | 0.7876 | – | – |
| PET | £1101 | £527 | 0.8945 | 0.1069 | £4900 |
| PET/CT | £1333 | £233 | 0.9314 | 0.0369 | £6300 |
| PET/CT as an adjunct to conventional work-upa | £1831 | £498 | 0.8928 | –0.0386 | Dominated |
Results based on an outcome of cost per case of recurrent cancer appropriately diagnosed and treated
Table 4 presents the results based on an outcome of case of recurrent cancer appropriately diagnosed and treated. The results show that, based on this outcome, PET is both more costly and more effective than conventional work-up, with an ICER of £6900. PET/CT is more costly and more effective than PET alone, with an ICER of £8400. The final diagnostic strategy of PET/CT combined with conventional work-up is more costly and more effective than PET/CT, with an estimated ICER of £18,100.
The ICERs presented indicate the additional cost required to accurately diagnose and treat one case of recurrent cancer. For the final diagnostic strategy of PET/CT as an adjunct to conventional work-up the sharp increase in the ICER compared with the preceding strategy of PET/CT is a result of the tests of PET/CT and conventional work-up combining to produce more false-positives.
Results based on an outcome of cost per diagnostic error avoided
Table 5 presents the results of the analysis based on an outcome of cost per diagnostic error avoided. The results for PET alone and PET/CT show that PET alone is both more costly and more accurate than conventional work-up and that PET/CT is correspondingly more costly, but also more accurate than PET alone. As the components of the test increase, so do the cost and the accuracy of the test. However, PET/CT as an adjunct to conventional work-up is the exception, as this combined package of available tests has a high cost, but is less accurate than both PET/CT and PET alone in terms of errors avoided because it detected more false-positives. Thus, PET/CT as an adjunct to conventional work-up is more costly than both PET alone and PET/CT, but also less effective than either strategy in terms of reducing errors, and so this combined strategy of PET/CT as an adjunct to conventional work-up is dominated by the preceding two diagnostic strategies presented in the table.
Deterministic sensitivity analysis results
Threshold analysis to find a value for cost and positron emission tomography/computed tomography test accuracy at which positron emission tomography/computed tomography might be considered cost-effective
Finding the threshold sensitivity level
In the model, the sensitivity of the test was increased from the base-case value of 95%, in order to find a value for which PET/CT became a cost-effective diagnostic strategy – all other parameters including specificity were held at their base-case values. When the sensitivity of PET/CT was increased to a value of 97%, the ICER for PET/CT versus PET fell slightly to £27,800 per QALY, reflecting the improved accuracy for this test. This result also shows dominance over PET alone as, with improved sensitivity, the strategy has the most favourable ICER and shows extended dominance over the ICER for PET versus conventional work-up. The ICER for PET/CT as an adjunct to conventional work-up increased to approximately £55,000 per QALY, reflecting the increase in the number of false-positives.
Finding the threshold specificity level
In the model, the specificity of the test was increased from the base-case value of 89% to find a value for which PET/CT became a cost-effective diagnostic strategy – all other parameters including sensitivity were held at their base-case values. The specificity of PET/CT was increased to a value of 100%, and the ICER for PET/CT versus PET was estimated at £30,800 per QALY. The conclusion that specificity does not affect cost-effectiveness is dependent on the structural assumption about use of biopsy. Without biopsy confirmation, the effect of specificity is likely to be much greater because the impact of false-positive results is magnified. Clearly, this shows that there is clear sensitivity of the results to costs of the test.
Finding the threshold cost value
The cost of PET/CT was reduced from the base-case value of £1236 to £1210, a reduction of just £26, which had the effect of reducing the ICER for PET/CT versus PET in the deterministic analysis to just under the £30,000 per QALY threshold to an estimated ICER of £29,900 per QALY.
Changing the prevalence to use the extreme values of the range identified in the literature review
Two analyses, the first using the upper and the second using the lower limit from the range (0.93–0.19) of prevalence estimates identified by the clinical literature review, were used in the model to see the impact on the ICERs. 17 Reducing the prevalence increased the ICERs and, thus, reduced cost-effectiveness, while increasing the prevalence had the effect of improving the ICERs, but only slightly under the base-case values.
Changing the specificity of positron emission tomography/computed tomography as an adjunct to conventional work-up to 0.76
The effect of changing the specificity when PET/CT is used as an adjunct to conventional work-up, from the base case of 0.68 (calculated according to the ‘either positive rule’) to 0.76, the value representing the lowest for either of the two individual tests – represented by the specificity of conventional work-up. The change makes no difference to the results and, as before (sensitivity analysis 1b, Table 6), this may be related to the assumption about biopsy.
| Strategy | ICER of diagnostic strategies compared with conventional work-up (cost per QALY) | ||
|---|---|---|---|
| PET vs conventional work-up | PET/CT vs PET | PET/CT as an adjunct to conventional work-up vs PET/CT | |
| Base case | £29,300 | £31,000 | £42,100 |
| Sensitivity analysis | |||
| 1a. Increasing the sensitivity of PET/CT to 97% – all other parameters as base case (cost per QALY) | £29,300 | £27,800 | £55,600 |
| 1b. Increasing the specificity of PET/CT to 100% all other parameters as base case (cost per QALY) | £29,300 | £30,800 | £42,000 |
| 1c. Reducing the cost of the PET/CT strategy from the base-case value of £1236 to £1210 | £29,300 | £29,900 | £43,100 |
| 2a. Reducing prevalence to lowest value in range (0.19) | £49,400 | £55,600 | £98,200 |
| 2b. Increasing prevalence to highest value in range (0.93) | £27,300 | £28,600 | £36,500 |
| 3. Using specificity from just one (conventional work-up) of the two combined tests PET/CT as an adjunct to conventional work-up | £29,300 | £31,000 | £41,800 |
| 4. Omitting PET as a strategy | £29,700 | £41,900 | |
Omitting positron emission tomography from the analysis
Removing PET from the analysis provides a direct comparison between PET/CT and conventional work-up. Exploring the impact on this is important because, in practice, it appears that the use of PET has not been implemented at all. The removal of PET from the analysis produces an ICER for PET/CT versus conventional work-up of £29,700 per QALY. Compared with the base-case results, this ICER represents a weighted average of the ‘ICER for PET/CT versus PET’ and the ‘ICER for PET versus conventional work-up’ as expected.
Given the increasingly clear irrelevance of PET in the reviews of the clinical literature,17 the strategy of PET/CT versus PET should be interpreted with some scepticism as to its meaning, as PET alone is not really used in practice. The ICER for the comparison of PET/CT versus conventional work-up is calculated by taking the weighted average of the ICER of PET/CT versus PET and the ICER of PET versus conventional work-up. Thus, the results for such a comparison would be only slightly different from that presented in the middle column of results for Table 6 (PET/CT vs PET), and can be estimated intuitively. As a result, the analysis for PET/CT versus conventional work-up has not been repeated for all the items explored in the sensitivity analysis.
Results of probabilistic sensitivity analysis for the base-case cost per quality-adjusted life-year outcome
Figure 2 presents the four-way cost-effectiveness acceptability curve (CEAC) with and without the distributions around the treatment costs and outcomes. There is very little difference between the two sets of curves. Whereas these results suggest a clear preference for conventional work-up at thresholds up to £20,000/QALY, and for PET/CT combined with conventional work-up at much higher thresholds, they show considerable uncertainty at thresholds around £30,000/QALY. It has been shown that CEACs can be misleading when correlated strategies are compared. 63 The four-way CEAC is not informative between the options at thresholds around £30,000/QALY. We have also produced bilateral CEACs for the comparisons specified in the protocol (see Appendix 3).
FIGURE 2.
Cost-effectiveness acceptability curves for all strategies compared together. (a) Distributions around treatment costs and outcomes; and (b) point estimates for treatment costs and outcomes.
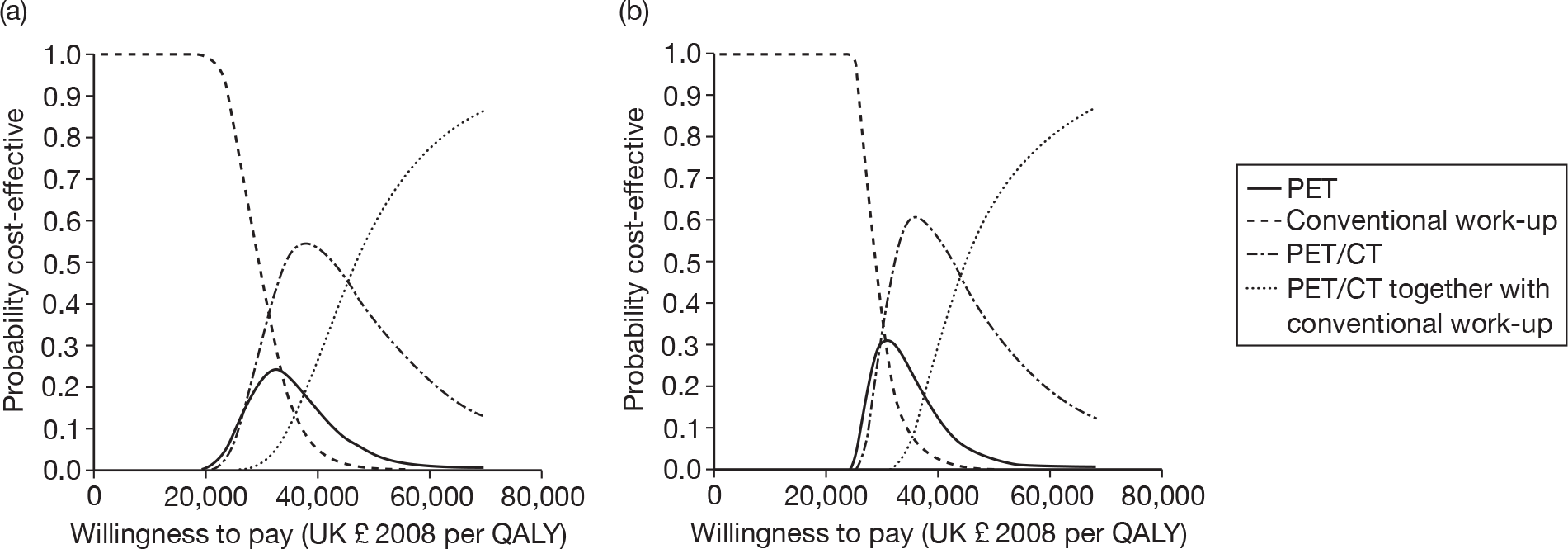
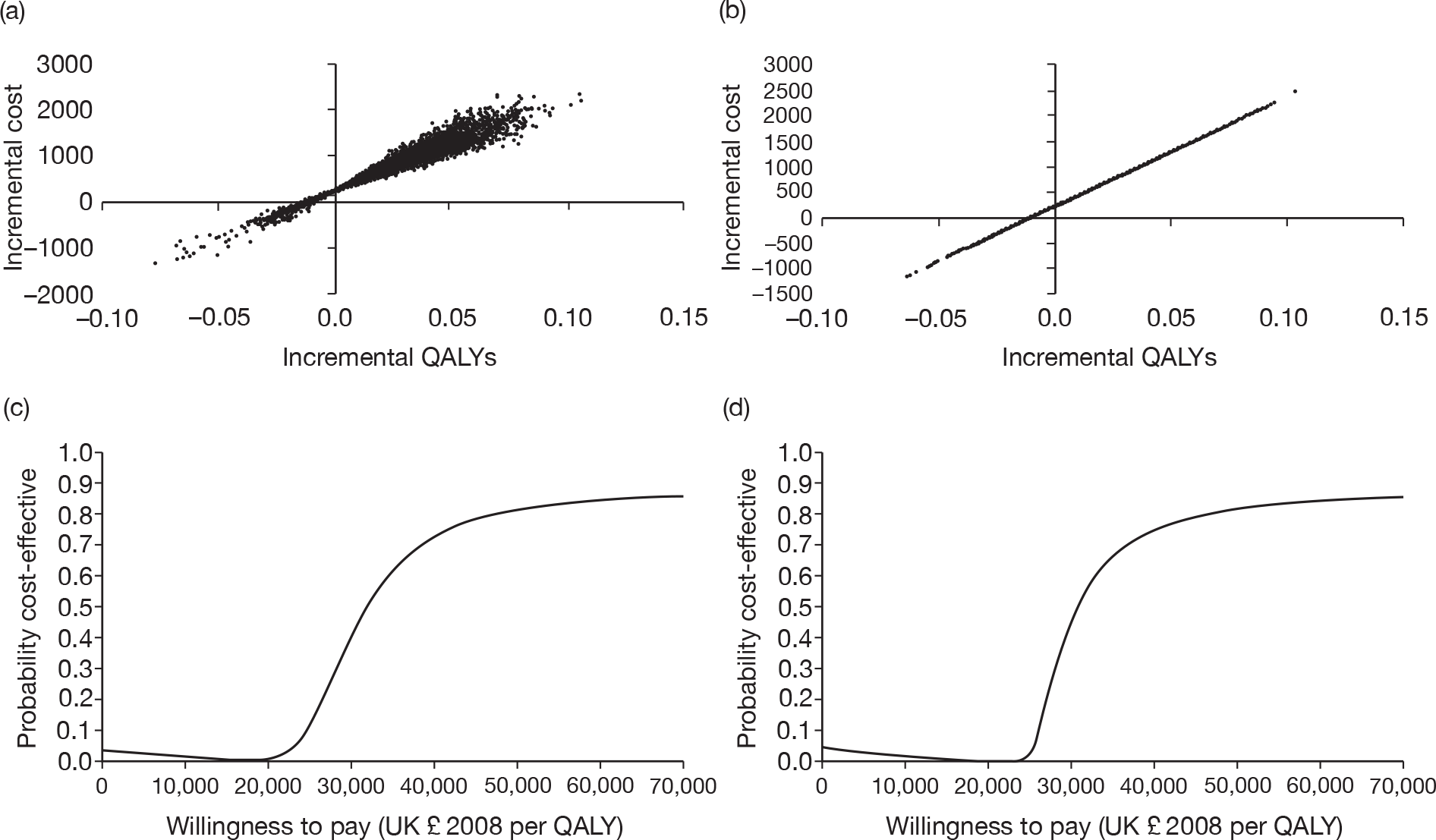
Figure 3 shows the results for PET/CT as a replacement for conventional work-up. Figure 3a shows the scatter plot, including the distributions around treatment outcomes, while Figure 3b shows the scatter plot when only the point estimates for treatment outcomes are included in the analysis. The unusual shape of the scatter plot in Figure 3b requires comment. We explored this further by looking at the effect of the distribution of each parameter separately. The QALY outcomes are completely insensitive to variations in the specificities of the two tests. This makes perfect sense in modelling terms, as we have assumed no loss in QALYs from a false-positive result. Varying the specificity of either strategy makes a small difference to the cost of that strategy, which is why the scatter plot in Figure 3b is not quite confined to a straight line, although the difference is not clearly visible. Varying the prevalence makes a visible difference to costs and QALYs, but the greater part of the uncertainty is due to the test sensitivities. In effect, almost all of the uncertainty shown on Figure 3b is due to the relative sensitivity of the two tests: as the model outcomes are linearly related to the relative sensitivity, the scatter plot is close to a straight line.
FIGURE 3.
Probabilistic sensitivity analysis results for PET/CT as a replacement for conventional work-up. (a) Scatter plot using distributions around treatment costs and outcomes; (b) scatter plot using point estimates for treatment costs and outcomes; (c) CEAC using distributions around treatment costs and outcomes; and (d) CEAC using point estimates for treatment costs and outcomes.
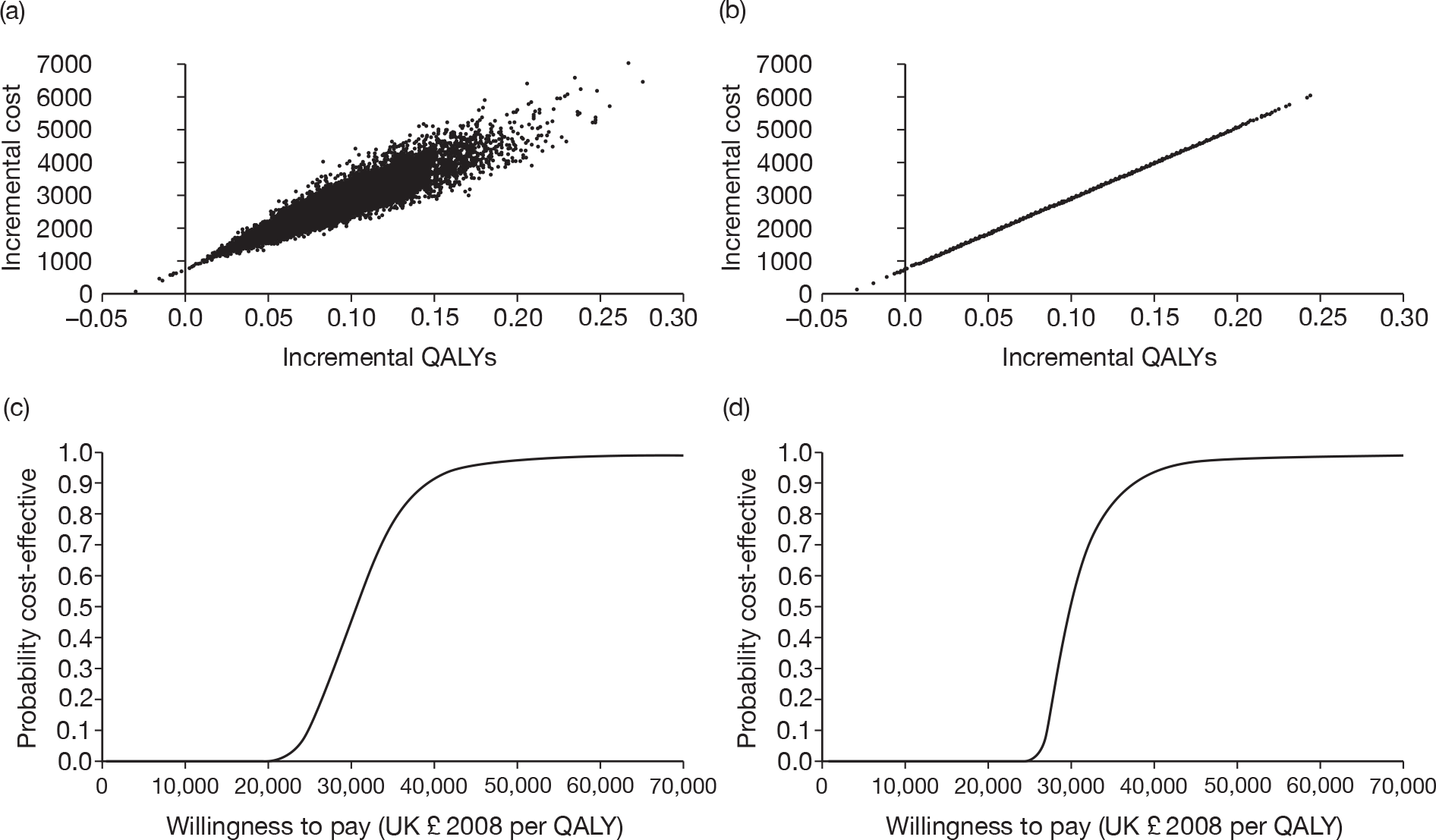
Figures 3c and 3d show the CEACs for this comparison, with and without the distribution around treatment costs and outcomes. There is very little difference between the CEACs, and each suggests that PET/CT is highly likely to be considered cost-effective at willingness-to-pay thresholds > £40,000.
Figure 4 shows the results for PET/CT as an adjunct to conventional work-up. The results are similar to those shown in Figure 3.
FIGURE 4.
Probabilistic sensitivity analysis results for PET/CT as an adjunct to conventional work-up. (a) Scatter plot using distributions around treatment costs and outcomes; (b) scatter plot using point estimates for treatment costs and outcomes; (c) CEAC using distributions around treatment costs and outcomes; and (d) CEAC using point estimates for treatment costs and outcomes.
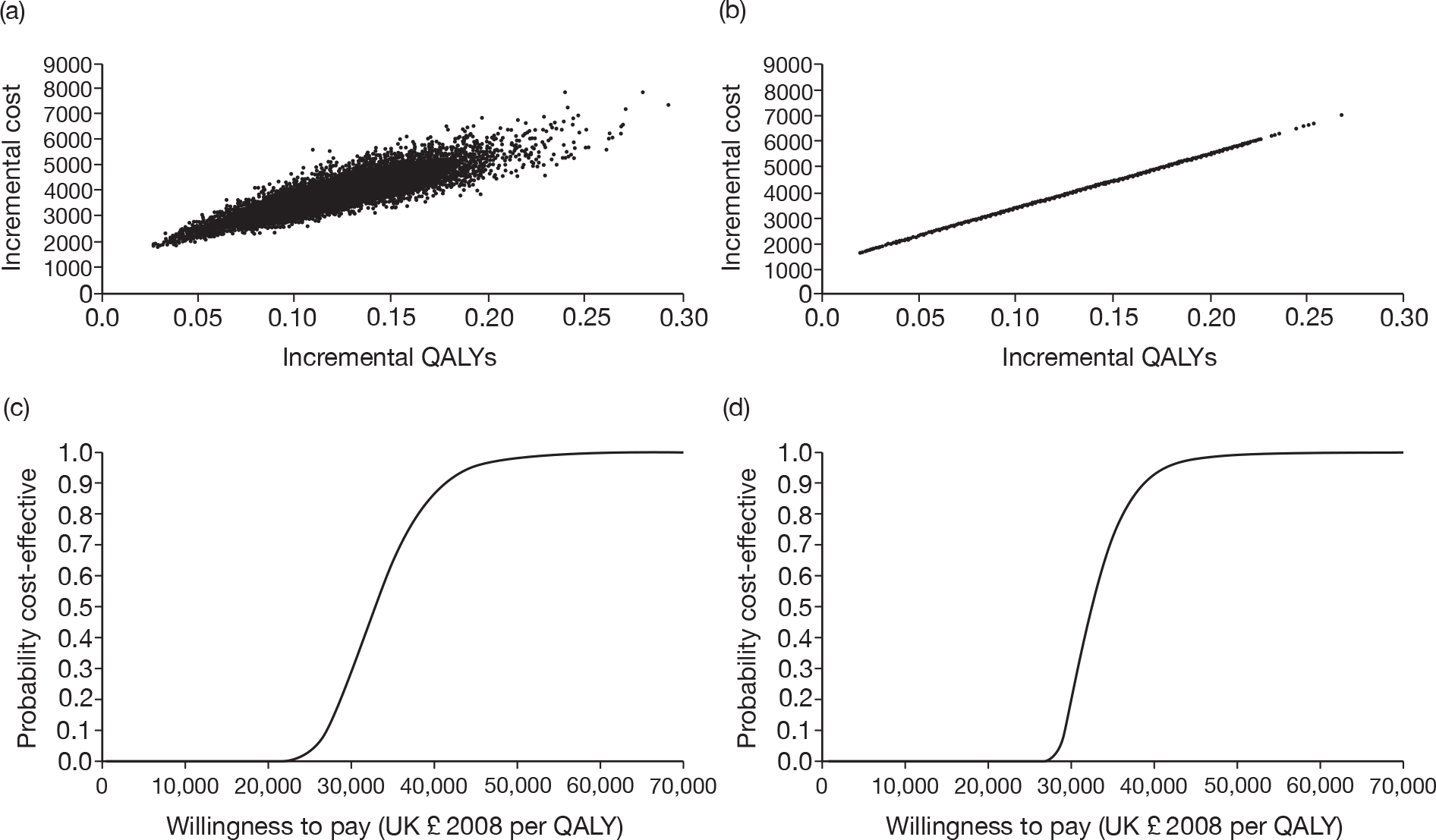
Figure 5 shows the results for PET/CT versus PET alone. Again, the scatter plot based on point estimates is very close to a straight line. In this case, both scatter plots show a substantial part of the distribution in the south-west quadrant, where PET/CT appears to be cost-saving, but less effective, compared with PET. This is reflected in the CEACs, which start above zero, but reduce towards zero at around £20,000/QALY, before assuming the more usual increasing shape. Note that the modelled probability that PET/CT is cost-effective does not reach 1 in either curve. It should be noted that a CEAC is not the same as a cumulative probability curve when the distribution goes beyond the north-east quadrant. 64
FIGURE 5.
Probabilistic sensitivity analysis results for PET/CT compared with PET. (a) Scatter plot using distributions around treatment costs and outcomes; (b) scatter plot using point estimates for treatment costs and outcomes; (c) CEAC using distributions around treatment costs and outcomes; and (d) CEAC using point estimates for treatment costs and outcomes.
Chapter 5 Discussion
Principal findings
The results of the base-case deterministic analysis for the outcome of cost per QALY based on the current model suggest that the strategy of conventional work-up is both the least costly and the least effective strategy. The ICER for the strategy of PET compared with conventional work-up was estimated at £29,300 per QALY; the ICER for PET/CT compared with PET was £31,000 per QALY; and the ICER for PET/CT as an adjunct to conventional work-up versus PET/CT was £42,000 per QALY. The ICER for PET/CT compared with conventional work-up was £29,700 per QALY. Clearly for each additional diagnostic test that is added to PET, the more expensive the package becomes, but also the more effective it becomes in terms of QALYs gained.
The probabilistic sensitivity analysis suggests that at a willingness-to-pay threshold of £20,000 per QALY, which is the threshold below which technologies are accepted and considered cost-effective by NICE, conventional work-up is the preferred option given the current model. Neither of the PET nor PET/CT strategies appear cost-effective at this threshold given the current model. At the upper end of the accepted threshold, at the £30,000 per QALY level, there is considerable uncertainty regarding what would be considered the preferred strategy. Based on the current model-based analysis, PET/CT appears to be the most cost-effective strategy only at thresholds > £40,000 per QALY, which exceeds the threshold currently considered acceptable by NICE.
The deterministic threshold analysis showed that relatively small increases in the sensitivity of PET/CT which were well within the margin of observed results of the review by Pennant et al. ,17 and relatively small reductions in the cost of PET/CT, can change the ICERs estimated in the deterministic base-case analysis to within the acceptable range of £20,000–30,000 per QALY.
Other changes in the one-way deterministic sensitivity have been explored, but, with the exception of changes to sensitivity of the test and cost, the impact of the deterministic sensitivity analysis on the results was very modest. Furthermore, the deterministic analysis results do not take into account the uncertainty highlighted by the base-case probabilistic sensitivity analysis which highlighted great uncertainty at the £30,000 per QALY level for all comparisons made.
Strengths and limitations of the study
The strengths of this study are that the analysis is based on test accuracy data from a systematic review of the accuracy literature for PET/CT and alternative strategies, which represents the best available data. 17 The report by Pennant et al. 17 represented the work of a multidisciplinary team which included both oncologists and radiologists, and the authors used their expertise to design the protocol for the current model-based economic analysis (see Appendix 3).There are some major limitations in the analysis that must be considered in the interpretation of the results. Serious concerns are based on the availability of suitable data identified in the clinical literature review by Pennant et al. 17 For example, we have assumed that all patients undergo conventional work-up, whereas, in reality, all patients would not receive all tests that constitute conventional work-up, but perhaps one specific test such as a bone scan if they complain of bone pain. This assumption serves to make conventional work-up more expensive than it would be in reality; hence, disadvantaging the conventional work-up strategy. However, the conclusions of our analysis remain unaffected by the assumption as conventional work-up was shown to be the most cost-effective strategy despite being disadvantaged. Furthermore, in the model it is assumed that confirmatory biopsy testing will be used when a positive result from any imaging diagnostic strategy is obtained. This assumption has the effect of reducing the false-positive cases and the impact these cases would have on follow-up treatment, and their associated cost. Pennant et al. 17 were clear that some of the data (presented in Table 1) have been attributed to tests such as PET/CT in the context of PET/CT being an adjunct to conventional work-up, whereas they realised that what the studies were reporting on should be perceived not as an adjunct but as a replacement. Thus, the data have been interpreted by us as representing replacement, which meant that we had to derive the data for PET/CT as an adjunct to conventional work-up from the available data. It must also be emphasised that many of the accuracy data were not available from studies that had used direct comparison, so that indirect comparisons had to be used instead. It also became apparent that PET alone was never absorbed into clinical practice, and this must be considered in the interpretation of the results. Other limitations include the fact that the model structure was compromised in the pathways for which data were scarce, and many assumptions were required in the development of the model structure as a result of paucity of data: for instance it was assumed that women who were diagnosed as false-negative for recurrent breast cancer died immediately. In reality, symptoms may have prompted at least some of these women to seek further medical advice and treatment, but the lack of robust data meant that this was not accounted for in the model. The model-based analysis is also restricted to women aged ≥ 50 years, based on available data. But breast cancer in women aged < 50 years can often be more aggressive and thus this population may have a higher rate of recurrence. Cost data for tests were available in very few published studies, and only unit costs for relevant resource use were available. Typically, cost data were supported only by one source and this is also true for the data available for quality of life associated with recurrent breast cancer. The deterministic sensitivity analysis showed that very small changes in the cost of PET/CT were sufficient to change results to fall within the acceptable threshold set by NICE. Accurate cost data for PET/CT are essential to reduce the uncertainty associated with such changes. Limited availability of data also means that any correlations that may exist between the sensitivity and specificity data, for the range of diagnostic tests, have been ignored.
Given its brief, the current study was presented with some major challenges regarding the extent of objectives defined at the outset and the availability of both clinical and economic data required to populate the model. Both the resources and the time frame available for the current study were severely limited, and there are many issues which can be resolved only by further primary research.
Comparison with existing studies
No studies were identified that had considered the relative cost-effectiveness of available technologies for recurrent breast cancer, and therefore appropriate comparisons with other existing studies are not possible. At the time of writing, the authors are aware of an ongoing National Institute for Health Research-funded study modelling the cost-effectiveness of PET and PET/CT for primary breast cancer. It is possible that some data or results from that study could reduce the uncertainty for some aspects of the current study.
Chapter 6 Conclusion
Meaning of the study and implications for health care
Based on the current model and given the limitations that have been highlighted in terms of limited availability of data, resources and time restrictions, the results of the current analysis suggest that use of PET/CT in the diagnosis of recurrent breast cancer, in the use of every woman suspected of having a recurrence, is unlikely to be cost-effective given the current willingness-to-pay thresholds that are accepted in the UK by decision-making bodies such as NICE. Our modelling suggests that conventional work-up could be the most cost-effective diagnostic strategy given current data. The results are affected by uncertainty at many levels, and so a better expression of our current understanding is that the cost-effectiveness of PET/CT for detecting distantly recurrent breast cancer is largely unknown. There is certainly insufficient evidence to firmly exclude PET/CT as a cost-effective diagnostic option for every woman suspected of having a recurrence. It also needs to be considered that examining the value of PET/CT for detecting metastases in suspected recurrence in isolation from the same role in primary breast cancer is not necessarily appropriate. The wider context of the use of PET/CT in the investigation of all breast cancer needs to be taken into account. Our results on distant recurrence contrast with the cost-effectiveness estimates of PET in the initial investigation of suspected breast cancer, which highlights the importance of not viewing these results in isolation.
Unanswered questions and future research
The evidence on the cost-effectiveness of PET/CT in recurrent breast cancer seems finely poised, so further research would thus seem to be the priority. The key question remains: what is the cost-effectiveness of PET/CT for the diagnosis of breast cancer recurrence? Here we acknowledge that PET alone is no longer a relevant technology to be appraised. This could be addressed by a model or primary economic evaluation; both approaches remain relevant. The model we presented could be enhanced and developed further, but considerably more resources and time would be required than have been afforded to the current analysis. No primary economic evaluations have been conducted as of May 2010. There are also aspects of model structure and approach that would merit further development too, particularly examining the impact of alternative assumptions about the patient pathway.
It will also be important to ascertain whether the relative cost-effectiveness of the diagnostic tests associated with PET/CT would improve if more reliable data on accuracy of the tests, resource use costs or the outcome in QALYs were available, or whether PET/CT is just not yet accurate enough in this application to compete with conventional work-up on cost-effectiveness grounds. Future studies need to secure robust cost data that can be verified from more than one source for the diagnostic tests involved in PET/CT. Reliable and verifiable data on quality of life associated with this clinical condition is also crucial.
It is possible that data on both quality of life and costs are available in literature that have a wider focus than PET and PET/CT for recurrent breast cancer, but searching for this literature was beyond the scope of the current study.
Acknowledgements
The authors wish to acknowledge Mary Pennant, Anne Fry Smith and Lazaros Andronis.
Contributions of authors
Peter Auguste (Research Associate, Health Economics) conducted the review of economic evaluations and the analysis of the economic model.
Pelham Barton (Senior Lecturer in Mathematical Modelling) supervised the development of the model and all aspects of the model-based economic evaluation, and contributed to the write-up of the results.
Chris Hyde (Professor of Health Technology Assessment) was coapplicant on the grant and co-author of the protocol for the study. He coreviewed papers for the systematic review and provided specific guidance on the clinical aspects of PET/CT and the results of the clinical review that were relevant for the model-based economic analysis, and contributed to the writing of the report.
Tracy Roberts (Professor of Health Economics) supervised the review and model-based economic evaluation, and wrote the first draft the report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Office for National Statistics . Breast Cancer Incidence, Mortality and Survival 2009.
- The BMJ Publishing Group . Clinical Evidence 2006.
- National Institute for Health and Clinical Excellence . CG80 Early and Locally Advanced Breast Cancer: Full Guideline 2009. http://guidance.nice.org.uk/CG80/Guidance/pdf/English (accessed January 2010).
- Barentsz J, Takahashi S, Oyen W, Mus R, De MP, Reznek R, et al. Commonly used imaging techniques for diagnosis and staging. J Clin Oncol 2006;24:3234-44.
- Schnall M, Rosen M. Primer on imaging technologies for cancer. J Clin Oncol 2006;24:3225-33.
- Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ. Radionuclide bone imaging: an illustrative review. Radiographics 2003;23:341-58.
- Hayes DF. Tumor markers for breast cancer. Ann Oncol 1993;4:807-19.
- Belli P, Costantini M, Romani M, Marano P, Pastore G. Magnetic resonance imaging in breast cancer recurrence. Breast Cancer Res Treat 2002;73:223-35.
- Engelhard K, Hollenbach HP, Wohlfart K, von IE, Fellner FA. Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur Radiol 2004;14:99-105.
- Walker R, Kessar P, Blanchard R, Dimasi M, Harper K, DeCarvalho V, et al. Turbo STIR magnetic resonance imaging as a whole-body screening tool for metastases in patients with breast carcinoma: preliminary clinical experience. J Magn Reson Imaging 2000;11:343-50.
- Rohren EM, Turkington TG, Coleman RE, Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology 2004;231:305-32.
- Kostakoglu L, Agress H, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics 2003;23:315-40.
- von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology 2006;238:405-22.
- The Royal College of Radiologists . PET-CT in the UK: A Strategy for Development and Integration of a Leading Edge Technology Within Routein Clinical Practice 2005.
- UK PETCT. Advisory Board Oncology FDG PETCT Scan Referral Criteria 2009. http://bnmsonline.co.uk/dmdocuments/referral_indications_pet-ct_board_2009.pdf (accessed June 2009).
- Isasi CR, Moadel RM, Blaufox MD. A meta-analysis of FDG-PET for the evaluation of breast cancer recurrence and metastases. Breast Cancer Res Treat 2005;90:105-12.
- Pennant M, Takwoingi Y, Pennant L, Davenport C, Fry-Smith A, Eisinga A, et al. A systematic review of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess 2010;14.
- Abe K, Sasaki M, Kuwabara Y, Koga H, Baba S, Hayashi K, et al. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med 2005;19:573-9.
- Aide N, Huchet V, Switsers O, Heutte N, Delozier T, Hardouin A, et al. Influence of CA 15-3 blood level and doubling time on diagnostic performances of 18F–FDG PET in breast cancer patients with occult recurrence. Nucl Med Commun 2007;28:267-72.
- Bender H, Kirst J, Palmedo H, Schomburg A, Wagner U, Ruhlmann J, et al. Value of 18-fluoro-deoxyglucose positron emission tomography in the staging of recurrent breast carcinoma. Anticancer Res 1997;17:1687-92.
- Dirisamer A, Halpern BS, Flory D, Wolf F, Beheshti M, Mayerhoefer ME, et al. Integrated contrast-enhanced diagnostic whole-body PET/CT as a first-line restaging modality in patients with suspected metastatic recurrence of breast cancer. Eur J Radiol 2010;73:294-9.
- Fueger BJ, Weber WA, Quon A, Crawford TL, len-Auerbach MS, Halpern BS, et al. Performance of 2-deoxy-2-[F-18]fluoro-d-glucose positron emission tomography and integrated PET/CT in restaged breast cancer patients. Mol Imaging Biol 2005;7:369-76.
- Gallowitsch HJ, Kresnik E, Gasser J, Kumnig G, Igerc I, Mikosch P, et al. F-18 fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the follow-up of patients with breast carcinoma: a comparison to conventional imaging. Investigative Radiology 2003;38:250-6.
- Goerres GW, Michel SCA, Fehr MK, Kaim AH, Steinert HC, Seifert B, et al. Follow-up of women with breast cancer: Comparison between MRI and FDG PET. Eur Radiol 2003;13:1635-44.
- Guillemard S, Eberle Pouzeratte MC, Lamy P-J, Romieu G, Rossi M, Artus JC. 18FDG PET/CT and CA 15-3 in the early diagnosis of recurrent breast cancer [French]. Med Nucl 2006;30:209-16.
- Hathaway PB, Mankoff DA, Maravilla KR, ustin-Seymour MM, Ellis GK, Gralow JR, et al. Value of combined FDG PET and MR imaging in the evaluation of suspected recurrent local-regional breast cancer: preliminary experience. Radiology 1999;210:807-14.
- Haug AR, Schmidt GP, Klingenstein A, Heinemann V, Stieber P, Priebe M, et al. F-18-fluoro-2-deoxyglucose positron emission tomography/computed tomography in the follow-up of breast cancer with elevated levels of tumor markers. J Comput Assist Tomogr 2007;31:629-34.
- Hubner KF, Smith GT, Thie JA, Bell JL, Nelson HS, Hanna WT. The potential of F-18-FDG PET in breast cancer. Detection of primary lesions, axillary lymph node metastases, or distant metastases. Clin Positron Imaging 2000;3:197-205.
- Kamel EM, Wyss MT, Fehr MK, von Schulthess GK, Goerres GW. [18F]-fluorodeoxyglucose positron emission tomography in patients with suspected recurrence of breast cancer. J Cancer Res Clin Oncol 2003;129:147-53.
- Kim T-S, Moon WK, Lee D-S, Chung J-K, Lee MC, Youn Y-K, et al. Fluorodeoxyglucose positron emission tomography for detection of recurrent or metastatic breast cancer. World J Surg 2001;25:829-34.
- Lin W-Y, Tsai S-C, Cheng K-Y, Yen R-F, Kao C-H. Fluorine-18 FDG-PET in detecting local recurrence and distant metastases in breast cancer Taiwanese experiences. Cancer Invest 2002;20:725-9.
- Liu CS, Shen YY, Lin CC, Yen RF, Kao CH, Liu CS, et al. Clinical impact of [(18)F]FDG-PET in patients with suspected recurrent breast cancer based on asymptomatically elevated tumor marker serum levels: a preliminary report. Jap J Clin Oncol 2002;32:244-7.
- Lonneux M, Borbath I, Berliere M, Kirkove C, Pauwels S. The place of whole-body PET FDG for the diagnosis of distant recurrence of breast cancer. Clin Positron Imaging 2000;3:45-9.
- Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK, et al. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med 1998;39:431-5.
- Ohta M, Tokuda Y, Suzuki Y, Kubota M, Makuuchi H, Tajima T, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Commun 2001;22:875-9.
- Pecking AP, Corone-Mechelany C, Alberini JL, Gutman F, Sarandi F, Bertrand-Kermorgant F, et al. Positron Emission Tomography (PET) using 18FDG and occult diseases in cancerology: The experiment of the Rene Huguenin Center [French]. Immuno-Anal Biol Special 2004;19:269-73.
- Radan L, Ben-Haim S, Bar-Shalom R, Guralnik L, Israel O, Radan L, et al. The role of FDG-PET/CT in suspected recurrence of breast cancer. Cancer 2006;107:2545-51.
- Raileanu I, Grahek D, Montravers F, Kerrou K, Aide N, Younsi N, et al. Comparison of [18F]-fluorodeoxyglucose positron emission tomography and technetium bisphosphonate bone scintigraphy to detect bone metastases in patients with breast cancer [French]. Med Nucl 2004;28:297-303.
- Santiago JF, Gonen M, Yeung H, Macapinlac H, Larson S, Santiago JFY, et al. A retrospective analysis of the impact of 18F-FDG PET scans on clinical management of 133 breast cancer patients. Q J Nucl Med Mol Imaging 2006;50:61-7.
- Schmidt GP, Baur-Melnyk A, Haug A, Heinemann V, Bauerfeind I, Reiser MF, et al. Comprehensive imaging of tumor recurrence in breast cancer patients using whole-body MRI at 1.5 and 3 T compared to FDG-PET-CT. Eur J Radiol 2008;65:47-58.
- Suarez M, Perez-Castejon MJ, Jimenez A, Domper M, Ruiz G, Montz R, et al. Early diagnosis of recurrent breast cancer with FDG-PET in patients with progressive elevation of serum tumor markers. Q J Nucl Med 2002;46:113-21.
- Veit-Haibach P, Antoch G, Beyer T, Stergar H, Schleucher R, Hauth EA, et al. FDG-PET/CT in restaging of patients with recurrent breast cancer: possible impact on staging and therapy. Br J Radiol 2007;80:508-15.
- Vranjesevic D, Filmont JE, Meta J, Silverman DH, Phelps ME, Rao J, et al. Whole-body (18)F-FDG PET and conventional imaging for predicting outcome in previously treated breast cancer patients. J Nucl Med 2002;43:325-9.
- Wolfort RM, Li BDL, Johnson LW, Turnage RH, Lilien D, Ampil F, et al. The role of whole-body fluorine-18-FDG positron emission tomography in the detection of recurrence in symptomatic patients with stages II and III breast cancer. World J Surg 2006;30:1422-7.
- Yang SN, Liang JA, Lin FJ, Kao CH, Lin CC, Lee CC, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol 2002;128:325-8.
- Eubank WB, Mankoff D, Bhattacharya M, Gralow J, Linden H, Ellis G, et al. Impact of FDG PET on defining the extent of disease and on the treatment of patients with recurrent or metastatic breast cancer. AJR Am J Roentgenol 2004;183:479-86.
- Roberts T, Henderson J, Mugford M, Bricker L, Neilson J, Garcia J. Antenatal ultrasound screening for fetal abnormalities: a systematic review of studies of cost and cost effectiveness. BJOG 2002;109:44-56.
- Miles KA. An approach to demonstrating cost-effectiveness of diagnostic imaging modalities in Australia illustrated by positron emission tomography. Australas Radiol 2001;45:9-18.
- Sloka J, Hollet D, Mathews M. Cost-effectiveness of positron emission tomography in breast cancer. Mol Imaging Biol 2005;7:351-60.
- Heinrich SM, Goerres GWM, Schafer MM, Sagmeister MM, Bauerfeind PM, Pestalozzi BCM, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005;242:235-43.
- Jacklin PB, Barrington SF, Roxburgh JC, Jackson G, Sariklis D, West PA, et al. Cost-effectiveness of preoperative positron emission tomography in ischemic heart disease. Ann Thorac Surg 2002;73:1403-9.
- National Institute for Health and Clinical Excelence . CG81 Advanced Breast Cancer: Diagnosis and Treatment 2009.
- Hayen A, Macaskill P, Irwig L, Bossuyt P. Appropriate statistical methods are required to assess diagnostic tests for replacement, add-on, and triage. J Clin Epidemiol 2010;63:883-91.
- Lin SCC. Some results on combinations of two binary screening tests. J Biopharm Stat 1999;9:81-8.
- Tang M-L. On simultaneous assessment of sensitivity and specificity when combining two diagnostic tests. Stat Med 2004;23:3593-605.
- Curtis L. Unit Costs of Health Care and Social Care 2009 2009.
- Department of Health . Reference Costs 2002–03 Collection: Costing and Activity Guidance and Requirements 2004. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4070195.
- Department of Health . Collection: Costing and Activity Guidance and Requirements 2004. www.dh.gov/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945.
- Guadagni F, Ferroni P, Carlini S, Mariotti S, Spila A, Aloe S, et al. A re-evaluation of Carcinoembryonic Antigen (CEA) as a serum marker for breast cancer: a prospective longitudinal study. Clin Can Res 2001;7:2357-62.
- Cooper NJ, Abrams KR, Sutton AJ, Turner D, Lambert PC. A Bayesian approach to Markov modelling in cost-effectiveness analyses: application to taxane use in advanced breast cancer. J Roy Stat Soc Stat Soc 2003;166:389-405.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41.
- Government Actuary’s Department . Life Tables 2009.
- Sadatsafavi M, Najafzadeh M, Marra C. Technical note: acceptability curves could be misleading when correlated strategies are compared. Med Decis Making 2008;28:306-7.
- Fenwick E, Bernie JO, Andrew B. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15.
Appendix 1 Review of published economic evaluation studies
Appendix 1.1: Original database search strategy
Database: EMBASE 1980 to week 22 2009
-
exp emission tomography/ (64,606)
-
exp whole body imaging/or whole body ct/or whole body pet/ (3384)
-
(positron adj2 emission adj2 tomograph$).tw. (21,306)
-
(emission adj2 comput$adj2 tomograph$).tw. (9371)
-
(radionuclide adj2 cat scan$).tw. (3)
-
(radionuclide adj2 ct scan$).tw. (22)
-
(radionuclide-comput$adj2 tomograph$).tw. (12)
-
(scintigraph$adj2 comput$adj2 tomograph$).tw. (332)
-
(tomograph$adj2 emission adj2 comput$).tw. (9601)
-
(pet or petct).tw. (29,731)
-
or/1-10 (75,284)
-
exp breast cancer/ (154,137)
-
exp breast tumor/ (161,246)
-
(breast$adj5 (cancer$or carcinoma$or adenocarcinoma$or carcinogen$or sarcoma$or malignan$or tumo?r$or neoplas$)).tw. (128,069)
-
or/12-14 (177,409)
-
11 and 15 (2224)
-
(recur$or relaps$or metasta$or restag$or re-stag$).mp. (529,809)
-
16 and 17 (1096)
-
from 18 keep 1-1000 (1000)
Database: Ovid MEDLINE(R) 1950 to week 5 May 2009
-
exp tomography, emission-computed/ (53,082)
-
(emission adj2 comput$adj2 tomograph$).tw. (9756)
-
(tomograph$adj2 emission adj2 comput$).tw. (9987)
-
(radionuclide-comput$adj2 tomograph$).tw. (19)
-
(radionuclide adj2 cat scan$).tw. (4)
-
(radionuclide adj2 ct scan$).tw. (29)
-
(scintigraph$adj2 comput$adj2 tomograph$).tw. (373)
-
(positron adj2 emission adj2 tomograph$).tw. (21,497)
-
(pet or petct).tw. (30,376)
-
or/1-9 (66,222)
-
exp breast neoplasms/ (162,938)
-
(breast$adj5 (cancer$or carcinoma$or adenocarcinoma$or carcinogen$or sarcoma$or malignan$or tumo?r$or neoplas$)).tw. (149,549)
-
or/11-12 (191,686)
-
10 and 13 (1433)
-
from 14 keep 1-1000 (1000)
Appendix 1.2: Updated search strategy
Database: EMBASE May 2009 to week 4 April 2010
-
exp emission tomography/ (72,363)
-
exp whole body imaging/or whole body ct/or whole body pet/ (3920)
-
(positron adj2 emission adj2 tomograph$).tw. (23,570)
-
(emission adj2 comput$adj2 tomograph$).tw. (10,282)
-
(radionuclide adj2 cat scan$).tw. (3)
-
(radionuclide adj2 ct scan$).tw. (22)
-
(radionuclide-comput$adj2 tomograph$).tw. (12)
-
(scintigraph$adj2 comput$adj2 tomograph$).tw. (348)
-
(tomograph$adj2 emission adj2 comput$).tw. (10,623)
-
(pet or petct).tw. (33,611)
-
or/1-10 (84,228)
-
exp breast cancer/ (167,175)
-
exp breast tumor/ (175,984)
-
(breast$adj5 (cancer$or carcinoma$or adenocarcinoma$or carcinogen$or sarcoma$or malignan$or tumo?r$or neoplas$)).tw. (139,514)
-
or/12-14 (193,763)
-
11 and 15 (2633)
-
(recur$or relaps$or metasta$or restag$or re-stag$).mp. (575,047)
-
16 and 17 (1321)
-
limit 18 to yr=“2009 -Current” (257)
-
from 19 keep 1-257 (257)
Database: Ovid MEDLINE(R) May 2009 to week 4 April 2010
-
exp tomography, emission-computed/ (57,549)
-
(positron adj2 emission adj2 tomograph$).tw. (23,435)
-
(emission adj2 comput$adj2 tomograph$).tw. (10,528)
-
(radionuclide adj2 cat scan$).tw. (4)
-
(radionuclide adj2 ct scan$).tw. (29)
-
(radionuclide-comput$adj2 tomograph$).tw. (19)
-
(scintigraph$adj2 comput$adj2 tomograph$).tw. (387)
-
(tomograph$adj2 emission adj2 comput$).tw. (10,852)
-
(pet or petct).tw. (33,726)
-
or/1-9 (71,942)
-
exp breast neoplasms/ (172,296)
-
(breast$adj5 (cancer$or carcinoma$or adenocarcinoma$or carcinogen$or sarcoma$or malignan$or tumo?r$or neoplas$)).tw. (159,884)
-
or/11-12 (203,535)
-
10 and 13 (1622)
-
(recur$or relaps$or metasta$or restag$or re-stag$).mp. (671,924)
-
14 and 15 (832)
-
limit 16 to yr=“2009 -Current” (107)
-
from 17 keep 1-107 (107)
Appendix 1.3: Published economic evaluation studies reviewed
Two published papers which were independently identified by the reviewers (CH and PA), but were rejected because they were based on primary and axillary staging of breast cancer. The studies identified were by Miles48 and Sloka et al. 49 The quality of the study by Sloka et al. 49 was better than that for Miles. 20 Both studies used decision tree analysis to determine the cost-effectiveness of diagnostic imaging techniques, one in primary breast cancer and other the axillary staging of breast cancer.
Miles48 used a previously existing and published model and applied this to the Australian context by using new prevalence and cost data to determine the cost-effectiveness of diagnostic imaging techniques to detect breast cancer. The study population was not clearly stated. The results from the economic evaluation stated that the cost saving per patient examined by PET is AUS $550 presented in 1997 prices for axillary staging of breast cancer. Miles48 further used sensitivity analysis for the prevalence of the disease and the specificity of PET. He concluded in the Australian context that the use of PET was cost-effective.
Comments: This paper48 passed the majority of the quality assessment criteria. However, given that the model was constructed and results presented for the staging of breast cancer, this paper was not relevant in developing a decision tree model for breast cancer recurrence, and thus not deemed relevant to the current study.
Sloka et al. ’s49 objective was to establish the cost-effectiveness of PET in the management of patients. Two groups of patients were considered, all patients receiving axillary lymph node dissection, but one group undergoing a PET scan and the other no PET. The study population was 1000 women aged ≥ 55 years; they justified this study population as a representative average age of patients presenting with breast cancer in Canada. The results from the cost-effectiveness analysis suggested that cost savings of CAN$695 per person could arise from the use of PET scanning with an increase in life expectancy of 7.4 days. Based on the results presented, Sloka et al. 49 concluded that the use of a PET management strategy with axillary lymph node dissection of primary breast cancer would be economically beneficial in Canada, even under varying conditions.
Comments: The results and conclusion presented were based on a population of women being diagnosed with primary breast cancer. Therefore this paper does not provide any assistance in modelling breast cancer recurrence.
Appendix 2 Model-based assumptions
Appendix 2.1: assumptions from NICE (2009) guidelines
Here we present the assumptions regarding the study population, treatment and survival used by NICE in its economic evaluation. The most cost-effective strategy was used as the basis of the treatment cost presented in Table 2.
| Assumptions |
|---|
| Study population |
| Women with metastatic breast cancer who had previously been treated with anthracycline (treatment may have been used as adjuvant treatment) |
| Patients in whom the disease is hormone responsive will receive alternative/additional treatment |
| Treatment |
| Patients will receive first-line therapy; there is the possibility of the patient dying from a toxic death. The assumption is that a toxic death can only occur after first-line therapy |
| There is a time lag of one month between discontinuing first-line therapy and commencing second-line therapy. In the absence of toxicity the patient will continue first-line therapy. At this point it is assumed that response can be assessed, to the patient faces a probability of responding to therapy, or having stable disease or not |
| Capecitabine used as first-line treatment or part of a combination of first-line treatment will not be used in second-line treatment |
| The ‘no chemotherapy’ treatment would result in no progression-free survival and 5 months’ survival with progressive disease |
| Survival |
| Overall survival was assumed to be time to progression of first-line treatment, time to progression of second-line treatment, time to progression of third-line treatment and progression to death (5 months) |
| Survival and time to progression followed exponential distributions |
| Authors assumed the utility with progressive disease, 0.45 |
| Utility associated with stable disease, 0.65 |
| Utility associated with progressive disease, 0.45 |
| Cost estimation |
| For treatment which required a combination of two drugs, it was assumed that there will be one administration cost |
| At the beginning of each cycle, it was assumed that the patient will have an oncologist consultation |
We used strategy 14, the most cost-effective strategy, which leads to an expected mean cost of £18,118, which we inflated to 2008 prices.
Appendix 2.2: accuracy data for conventional work-up, positron emission tomography and positron emission tomography/computed tomography
| Comparison | PET sensitivity, % (95% CI) | CIT sensitivity, % (95% CI) | Relative sensitivity (95% CI) | PET specificity, % (95% CI) | CIT specificity, % (95% CI) | Relative specificity (95% CI) |
|---|---|---|---|---|---|---|
| Direct PET vs CIT | 89 (83 to 93), n = 10 | 79 (72 to 85), n = 10 | 1.12 (1.04 to 1.21), p = 0.005 | 93 (83 to 97) | 83 (67 to 92) | 1.12 (1.01 to 1.24), p = 0.036 |
| Indirect PET vs CIT | 91 (87 to 93), n = 25 | 81 (73 to 87), n = 11 | 1.12 (1.04 to 1.21), p = 0.005 | 86 (79 to 91) | 73 (59 to 83) | 1.18 (1.03 to 1.36), p = 0.017 |
| Comparison | PET/CT sensitivity, % (95% CI) | CIT sensitivity, % (95% CI) | Relative sensitivity (95% CI) | PET/CT specificity, % (95% CI) | CIT specificity, % (95% CI) | Relative specificity (95% CI) |
|---|---|---|---|---|---|---|
| Direct PET/CT vs CT | 95 (88 to 98), n = 4 | 80 (65 to 90), n = 4 | 1.19 (1.03 to 1.37), p = 0.015 | 89 (69 to 97) | 77 (50 to 92) | 1.15 (0.95 to 1.41), p = 0.157 |
| Indirect PET/CT vs CIT | 95 (89 to 97), n = 5 | 78 (72 to 84), n = 11 | 1.21 (1.11 to 1.31), p < 0.0001 | 89 (76 to 96) | 79 (65 to 88) | 1.13 (0.99 to 1.29), p = 0.063 |
Appendix 2.3: beta and normal distributions for accuracy, prevalence, treatment and expected quality-adjusted life-years
Beta distributions
| Strategy | Base-case sensitivities | Range (95% CI) | Probability distribution | Source |
|---|---|---|---|---|
| Conventional work-up | 0.80 | 0.72 to 0.87 | Beta (85.6, 21.4) | Derived from data presented in Pennant et al.17 |
| PET | 0.91 | 0.87 to 0.93 | Beta (282.1, 27.9) | Derived from data presented in Pennant et al.17 |
| PET/CT | 0.95 | 0.89 to 0.97 | Beta (71.25, 3.75) | Derived from data presented in Pennant et al.17 |
| PET together with conventional work-up | 0.99 | 0.97 to 1.00 | Beta (168.3, 1.7) | Derived from data presented in Pennant et al.17 |
| Strategy | Base-case specificities | Range (95% CI) | Probability distribution | Source |
|---|---|---|---|---|
| Conventional work-up | 0.76 | 0.59 to 0.88 | Beta (20.52,6.48) | Derived from data presented in Pennant et al.17 |
| PET | 0.86 | 0.79 to 0.91 | Beta (103.2, 16.8) | Derived from data presented in Pennant et al.17 |
| PET/CT | 0.89 | 0.76 to 0.96 | Beta (27.145, 3.355) | Derived from data presented in Pennant et al.17 |
| PET together with conventional work-up | 0.68 | 0.45 to 0.89 | Beta (12.24, 5.76) | Derived from data presented in Pennant et al.17 |
| Base-case prevalence | Range (95% CI) | Probability distribution | Source | |
|---|---|---|---|---|
| Prevalence | 0.69 | 0.66 to 0.72 | Beta (655.5, 294.5) | Derived from data presented in Pennant et al.17 |
Normal distributions
| Cost (£) | Probability distribution | Standard deviation | Source | |
|---|---|---|---|---|
| Treatment (docetaxel, capecitabine and no chemotherapy | 18, 643 | Normal | 1864.3 | aNICE23 |
| QALY | Probability distribution | Standard deviation | Source | |
|---|---|---|---|---|
| Total QALYs expected for treatment | 0.8737 | Normal | 0.08737 | NICE24 |
Appendix 2.4: detailed results of the sensitivity analysis
-
1(a) Increasing the sensitivity of PET/CT to 97% – all other parameters as base case (cost per QALY).
-
1(b) Increasing the specificity of PET/CT to 100% – all other parameters as base case (cost per QALY).
-
1(c) Reducing the cost of the PET/CT strategy from the base-case value of £1236 to £1210.
-
2(a) Reducing the prevalence to the lowest value in range (0.19).
-
2(b) Increasing the prevalence to the highest value in the range (0.93).
-
3 Using specificity from just one (conventional work-up) of the two combined tests for PET/CT as an adjunct to conventional work-up.
-
4 Omitting PET alone as a strategy.
Appendix 3 Approved protocol for the study
Positron emission tomography and positron emission tomography/computed tomography in breast cancer recurrence: draft protocol for economic evaluation
Produced by the West Midlands Health Technology Assessment Collaboration and the Department of Public Health, Epidemiology & Biostatistics, University of Birmingham, Birmingham
Authors: Mary Pennant1, Lazaros Andronis2, Anne Fry-Smith1, Daniel Rea4, Theo Arvanitis5 and Chris Hyde1
1Department of Public Health, Epidemiology & Biostatistics, University of Birmingham
2Department of Health Economics, University of Birmingham
3UK Cochrane Centre
4Cancer Sciences Clinical Trials Unit, University of Birmingham
5Electronic & Electrical Engineering, University of Birmingham
Correspondence to: Chris Hyde, Department of Public Health, Epidemiology & Biostatistics, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
Fax.: 0121 414 7878
Date Completed: June 2009
This report was commissioned by the NHS National Institute for Health Research HTA Programme.
HTA no. 08/34.
Background
Breast cancer is a serious life-threatening disease. Treatment options have developed significantly over the past decade and have affected survival. Inevitably, recurrence of breast cancer has increased and its diagnosis is important, as early appropriate treatment also has small but clear associated advantage for survival.
Breast cancer recurrence may be local (in the breast), regional (lymph nodes, collar bone, etc., same side of body as original cancer) or distant (in other body tissues such as bone, liver, lungs and brain), and is associated with symptoms such as weight loss, abdominal pain, respiratory symptoms, bone pain and neurological signs. Women with a past history of breast cancer may present with symptoms that may be innocent or the first indication of recurrence. The nature of these symptoms will dictate the nature of the investigation, but tests will often include bone scans, chest X-rays, CT scans and ultrasound.
| Lead/senior reviewer | Hyde, Christopher, Dr | Senior Lecturer/Director of WMHTAC |
| Reviewer | Pennant, Mary, Dr |
Systematic Reviewer m.pennant@bham.ac.uk |
| Administrator | Farren, Janet, Mrs |
Project Administrator J.A.Farren@bham.ac.uk |
| Health economist | Andronis, Lazaros, Mr |
Research Fellow L.Andronis@bham.ac.uk |
| Information specialist | Fry-Smith, Anne, Ms |
Information Specialist A.S.Fry-Smith@bham.ac.uk |
| Clinical advisor | Rea, Dan, Dr |
Senior Lecturer and Honorary Consultant in Medical Oncology D.W.Rea@bham.ac.uk |
| Technical advisor | Arvanitis, Theodoros, Dr |
Senior Lecturer t.arvanitis@bham.ac.uk |
Positron emission spectroscopy and, more recently, PET/CT are new tools that may be used to diagnose breast cancer recurrence. These technologies trace radioactive isotopes in the body. Isotopes of glucose, most commonly fluorodeoxyglucose, are used for the detection of tumours as glucose is taken up and retained in tumour tissue, making it visible in PET images.
As well as exploring whether PET/CT is more effective than conventional diagnostic strategies and PET alone, there is a need to investigate and establish its relative cost-effectiveness in the diagnosis of breast cancer recurrence.
Objectives
This study aims to explore the cost-effectiveness of PET/CT in the diagnosis and treatment of breast cancer recurrence by modelling the relative costs and benefits accruing from the use of PET/CT as replacement to PET, as well as a replacement for, or in addition to, conventional work-up involving conventional X-rays, CT, ultrasound, bone scintigraphy and measurement of serum tumour markers.
Population
As in the main report,1 the population to be studied are patients with a history of breast cancer who have previously been treated for primary disease and are under investigation for the diagnosis of recurrence or secondary metastases (distinct from the primary disease).
Index tests and reference standard
The index test under assessment is PET/CT. It may be used in addition to standard tests, e.g. in combination with clinical examination/bone scanning, etc., and also instead of standard tests. Studies in which whole-body PET/CT is conducted as well as studies using only breast imaging may be considered. The reference standard used to define the true disease status of patients may be histological diagnosis (operation/biopsy) or clinical follow-up/autopsy findings.
Comparators
The study will set out to compare the costs and benefits associated with the use of PET/CT:
-
as a replacement for PET
-
as a replacement for conventional work-up consisting of ultrasound, bone scintigraphy, X-rays, CT and measurement or serum tumour markers
-
as a supplement to conventional work-up.
It is noted that, although PET scanners have ceased to be produced and can be found only in a limited number of hospitals, it is possible to carry out PET testing in isolation by using PET/CT scanners. The uncertainty on whether a comparison between PET and PET/CT is policy relevant will be explored further and this will be taken into account in possible changes in the study comparators.
Methods
Review of existing cost-effectiveness studies and input for economic modelling
A systematic review will be conducted to identify existing economic evaluation studies. The review will be carried out in a way similar to the review of effectiveness studies undertaken for the main report; however, this will differ in the following:
-
Searches will be appropriately modified for the purpose of identifying cost-effectiveness studies.
-
Criteria for inclusion with respect to study design will target cost-effectiveness, cost–consequences, cost–utility, cost–analysis and cost–minimisation studies.
-
The quality of the retrieved economic studies will be assessed against the commonly used checklist proposed by Drummond et al. 2 A preliminary search was conducted, and revealed limited evidence on the cost-effectiveness of PET/CT against other commonly used diagnostic tests for the purpose of diagnosing recurrent breast cancer. Given this, it is expected that a de novo economic model will need to be built. Thus, apart from identifying existing cost-effectiveness studies, the purpose of the review is to extract useful information to be used in the de novo model.
Model structure
The study will involve constructing a decision analytic model through which we will estimate the cost-effectiveness of the assessed interventions. This will take the form of a decision tree. Each of its branches will represent a diagnostic test under assessment and it will be populated with data on diagnostic accuracy, costs and benefits obtained from the effectiveness report, and available literature. If data on health-related quality of life exist, the study results will be expressed in terms of cost per QALY gained, which is the principle measure of outcome used by NICE. 3 If this is not plausible, results will be expressed as cost per unit of relevant health outcome, e.g. cost per diagnostic error avoided or cost per death due to recurrent cancer prevented.
Uncertainty in the cost-effectiveness results will be explored through deterministic sensitivity analysis, in which input values for one or more parameters will be varied across a plausible range, probabilistic sensitivity analysis, in which values for uncertain parameters are drawn from distributions assigned to those parameters and threshold analysis, which will allow identifying the values of an uncertain parameter (i.e. sensitivity of PET/CT) above and below which an intervention becomes cost effective. 4 The study will adopt the perspective of the NHS.
Data required
The study will make use of data obtained from the main report, existing literature on the topic and published national sources. In specific, data on the diagnostic accuracy of the assessed technologies will be obtained from the systematic review of effectiveness, while cost data will be acquired from sources including the NHS national schedule for reference costs5 and the Unit Costs of Health and Social Care 2008 report,6 supplemented by data extracted from cost analyses and cost-effectiveness studies identified in the literature. Data input for the model will include the following:
-
effectiveness-related information:
-
– accuracy of PET, PET/CT and conventional imaging technologies to diagnose BC recurrence
-
– prevalence of breast cancer (BC) recurrence in different locations of the body
-
– age at which BC recurrence might typically be diagnosed
-
-
cost-related information
-
cost of staff time, equipment and other facilities associated with use of PET and PET/CT
-
cost of staff time, equipment and other associated costs for CITs
-
costs of any additional tests required for further staging after the diagnosis of BC recurrence
-
cost of treatments for patients diagnosed with BC recurrence (surgery/chemotherapy/radiotherapy, etc.):
-
– quality of life
-
-
quality of life in patients under suspicion, cleared and diagnosed with BC recurrence
-
quality of life of patients undergoing treatments (necessary or unnecessary).
References
- Pennant M, Takwoingi Y, Pennant L, Davenport C, Fry-Smith A, Eisinga A, et al. A systematic review of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess 2010;14.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- NICE . Guide to the Methods of Technology Appraisal 2008. URL: www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf.
- Andronis L, Barton P, Bryan S. Sensitivity analysis in economic evaluation. An audit of NICE current practise and a review of its value and use in NICE decision making. Health Technol Assess 2009;13.
- Department of Health . NHS Reference Costs 2007–2008 n.d. URL: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945.
- Curtis L. Unit Costs of Health and Social Care 2008. Kent: Personal Social Services Research Unit, University of Kent; 2008.
List of abbreviations
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CIT
- conventional imaging test
- CT
- computed tomography
- FDG
- 18F-fluorodeoxyglucose
- ICER
- incremental cost-effectiveness ratio
- MRI
- magnetic resonance imaging
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Clinical Excellence
- PET
- positron emission tomography
- PET/CT
- positron emission tomography/computed tomography
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr David P Britt, Public contributor
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Public contributor
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington