Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 06/42/01. The contractual start date was in September 2008. The draft report began editorial review in July 2010 and was accepted for publication in October 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
JH-C is director of QResearch, which is a not-for-profit venture between the University of Nottingham and EMIS (commercial supplier of GP clinical systems). RM has received financial support for speaking at meetings sponsored by a number of pharmaceutical companies about the non-pharmacological treatment of depression and bipolar disorder. There are no other competing interests.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Coupland et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Depression is a common and debilitating condition in older people, affecting around 14% of older people living in the community. 1 Depression is largely treated in primary care in the UK, and usually with antidepressant medication, which is one of the most commonly prescribed drug groups in primary care. There were 36 million prescriptions issued in the community for antidepressants in England in 2008, an increase of 6.3% compared with the previous year. 2 For people aged 60 years and over an estimated 14 million antidepressant prescriptions were issued in 2007, an increase of 10.1% compared with the previous year and 79.0% compared with 2000 (figures from data provided by the NHS Information Centre).
Antidepressant medication
The first antidepressant drugs were developed in the 1950s, these were from the drug classes known as monoamine oxidase inhibitors (MAOIs) and tricyclic and related antidepressants (TCAs). Drugs known as selective serotonin reuptake inhibitors (SSRIs) were introduced in the 1980s and other new antidepressant drugs have been introduced since then. Reviews and meta-analyses of trials of these drugs have shown that all classes of antidepressant drug are more effective than placebo in terms of reducing symptoms of depression, particularly for more severe depression, but that the different antidepressant classes have largely similar efficacy. 3–7 A systematic review in older people found that TCAs and SSRIs were equivalent in terms of efficacy but that classical TCAs were associated with a higher discontinuation rate due to side effects. 5 The National Institute for Health and Clinical Excellence (NICE) recommended in 2009 that the choice of an antidepressant should be guided by consideration of side effects and patient preferences, but that normally an SSRI in generic form should be chosen. 3
Although the benefits of antidepressants have been studied in many randomised controlled trials, most such trials are short term, in selected populations and comparatively little is known about their relative safety. Adverse drug events may be more common in the treatment of depression in older people compared with younger groups owing to higher levels of comorbidity, age-related physiological changes and polypharmacy. 8 The under-representation of older people in clinical trials of antidepressants makes it difficult to derive reliable or precise estimates of the incidence of adverse events in this group. 9,10 This problem is further compounded when trial exclusion criteria exclude older people with comorbid conditions. 11
Although some observational studies have examined the effects of antidepressant drugs on single adverse outcomes, few, if any, studies have directly compared adverse event rates across a range of important clinical outcomes. Studies of single outcomes have identified a number of adverse outcomes that may be associated with antidepressants, but an intrinsic problem with these study designs is the difficulty of distinguishing between any effects of antidepressant medication and the effect of depression itself.
Suicide, overdose and poisoning
Antidepressants, particularly TCAs, are an important cause of deaths by overdose and poisoning. 12 Observational studies across all age groups have found associations between antidepressant use and suicide, but have been unable to rule out confounding by indication. 13 There is little evidence to support any difference in terms of class of antidepressant and risk of suicide,14 but studies have tended to look at risks across all ages or among adolescents and young adults. 15
Ischaemic heart disease
An increased risk of ischaemic heart disease was found in one study to be associated with use of the TCA dosulepin (formerly known as dothiepin), but not other TCAs or SSRIs;16 however, other studies have found no evidence of an increased risk of myocardial infarction (MI) among users of antidepressants17 or have suggested that an increased risk of MI may be explained by confounding factors relating to depression itself rather than specific adverse drug effects. 18
Fracture
Findings from case–control19 and case-series studies20 indicate that the risk of hip fracture is elevated with use of TCAs and SSRIs among older people, although the magnitude of the increased risk did not differ between these two classes of antidepressant. 19
Road traffic accidents
Studies that have formally tested the effects of antidepressants on driving performance have found that sedating antidepressants have a similar effect to alcohol,21 but there is little evidence in relation to road traffic accident (RTA) risk.
Other outcomes
Hyponatraemia associated with antidepressant use is rare, but is an adverse event that disproportionately affects older people. 22,23 Gastrointestinal (GI) bleeding has been found to be more common among those taking SSRIs who are aged 80 years or over,24 although there is a lack of consensus as to whether or not the risk of GI bleeding associated with SSRI use is further increased with concurrent use of non-steroidal anti-inflammatory drugs (NSAIDs). 25–27
Other outcomes for which there is some evidence of an association with antidepressant use include all-cause mortality,28,29 sudden cardiac death,30 stroke,31,32 seizures33 and adverse drug reactions (ADRs),34 but results are not consistent and there is a lack of evidence in older people.
Cost-effectiveness
In England, the annual cost of depression to the NHS and Personal Social Services has been estimated to be £1.7B compared with £5.8B in terms of lost employment and absenteeism. 35 More than 4% of hospital admissions have been estimated to be owing to ADRs36,37 and preventable harm from medicines has been estimated to cost the NHS more than £750M per annum. 38 This, coupled with the fact that health-care resources are scarce,39 means that it is important to compare the relative costs associated with different antidepressants and their relative (dis)benefits in terms of adverse events averted.
Need for the current study
The gaps in the research into adverse effects of antidepressant drugs specifically in older people, and the lack of consistent findings, pose problems for policy-makers and clinicians who are prescribing these drugs and making choices as to the most appropriate drug for individual older patients. Primary care databases with their large volumes of high-quality data on representative populations over many years are well suited to the study of unintended effects of medication. In this study we use a large primary care database containing information on prescriptions for antidepressants and a range of potential adverse effects to derive a more integrated picture of the balance of risks for antidepressant drugs in older people who are diagnosed with depression.
Chapter 2 Methods
Aims and objectives
The overall aim of the study was to establish the relative safety and balance of risks for antidepressant drugs in older people, in order to provide a robust evidence base to support decision making for policy-makers and clinicians prescribing these medications to individual patients.
The project had five key objectives:
-
to determine the relative and absolute risks of predefined adverse events in older people diagnosed with depression, comparing classes of antidepressant drugs (TCAs, SSRIs, MAOIs and other antidepressants) and commonly prescribed individual antidepressant drugs with non-use of antidepressant drugs
-
to directly compare the risk of adverse events in patients prescribed SSRIs against TCAs
-
to determine how dose and duration of prescribed antidepressant medication are associated with the risk of an adverse event
-
to describe patterns of antidepressant use in older people diagnosed with depression, in particular the types and doses prescribed, the durations and the proportions switching between different antidepressants (TCAs, SSRIs and other antidepressant drugs)
-
to estimate the costs of antidepressant medication and primary-care visits in older people who are diagnosed with depression, comparing patients by class of antidepressant drug (TCAs, SSRIs and other antidepressants).
Study design summary
The study used a large primary care database (QResearch) to investigate the relative safety and costs of antidepressant drugs in older people.
Two main approaches were used to achieve the study objectives:
-
cohort study analysis
-
nested self-controlled case-series analysis.
The cohort study analysis was used to estimate relative and absolute rates associated with exposures for a number of adverse outcomes, adjusting for potential confounding variables. The self-controlled case-series method40,41 was used to estimate the relative incidence of the adverse outcomes in different risk periods of antidepressant use compared with periods of non-use, using data from only cases with the outcomes. This method is useful for investigating the short-term effects of drug exposures on the risk of acute outcomes, as it eliminates problems of confounding from unmeasured variables, providing that they remain constant throughout the observation period. 41
Setting
The study was undertaken using data from the QResearch primary-care research database (www.qresearch.org). This is a large general practice research database containing the anonymised electronic health-care records of over 12 million patients ever registered with more than 600 general practices throughout England, Wales, Scotland and Northern Ireland. Practices that provide data for QResearch use the Egton Medical Information Systems (EMIS) medical records system. EMIS is the major supplier of primary-care computer systems in the UK and is in use within two-thirds of all UK general practices. The practices that contribute data to QResearch form a representative sample of around 7% of all UK general practices, and there are practices in every strategic health authority and each health board in England, Wales and Scotland. Version 22 of the QResearch database was used for the present study.
The information recorded on the database includes patient demographic data (year of birth, gender, socioeconomic data derived from the UK 2001 census), characteristics (height, weight, smoking status), symptoms, clinical diagnoses, consultations, referrals, prescribed medications and results of investigations.
Detailed analyses have compared QResearch practices with all UK practices and found that practices contributing to QResearch are somewhat larger than UK practices overall but are very similar in other respects. 42 The age–gender structure of the population has been compared with that reported in the 2001 census. There was good correspondence for all of these measures, although the QResearch population is slightly older and has marginally higher prevalence figures for some diagnoses than less recent data. 43
The QResearch database has previously been used to examine the risks and benefits associated with a number of commonly prescribed drugs including statins44,45 and NSAIDs. 46,47
Cohort study design
The target population for the cohort study was all patients in the QResearch database with a recorded diagnosis of depression made between 1 January 1996 and 31 December 2007 and when the patients were aged 65 years and over. We used computer-recorded Read codes to identify a major depressive disorder or unipolar depression, using case definitions similar to those that have been used in previous studies. 15,16 The codes used are listed in Appendix 1.
The cohort was followed up until 31 December 2008. Information was extracted on potential confounding variables at baseline and on all prescriptions for antidepressants during follow-up, along with information on adverse outcomes during follow-up.
Inclusion and exclusion criteria
Patients were eligible for inclusion in the cohort study if:
-
they had a recorded diagnosis of depression in their medical record
-
the diagnosis was made at the age of 65 years or over
-
the diagnosis was recorded between 1 January 1996 to 31 December 2007
-
they were aged no more than 100 years at diagnosis
-
the diagnosis occurred at least 12 months after registration with a study practice and after the installation date of the practice EMIS computer system.
Patients were excluded from the cohort study if any of the following were true:
-
they were temporary residents
-
they had a previous diagnosis of depression in the 12-month period prior to their index-recorded diagnosis of depression
-
they had been prescribed antidepressants in the 12-month period prior to their recorded diagnosis of depression
-
they had a diagnosis of schizophrenia, bipolar disorder or other types of psychoses.
Using these criteria, patients were eligible for inclusion in the study cohort if they did not receive any antidepressant treatment following a diagnosis of depression. They were also eligible for inclusion if they had a previous diagnosis of depression, made before the age of 65 years, as long as it was not in the 12 months before the index diagnosis.
Patients who received prescriptions for antidepressants but did not have a recorded diagnosis of depression were not eligible for inclusion; this was because the prescriptions may have been for indications other than depression (such as insomnia or trigeminal neuralgia), and we wanted to ensure that the cohort was restricted to patients with depression to reduce potential indication bias.
The index date which marked the date of entry into the study cohort was defined as the date of the first recorded diagnosis of depression after the age of 65 years, or the date of the first prescription for an antidepressant after age 65 years in patients if that occurred before the recorded date of depression.
Outcomes
The selected study outcomes were ones for which previous research had indicated some possible associations with use of antidepressants. Information on these outcomes was extracted from the primary-care computer records of patients in the cohort and also the linked death certificates for patients who had died during the study period. Outcomes were included only if they occurred after the date of entry into the study cohort and up to 31 December 2008. Computer-recorded Read codes and ICD-9/ICD-10 codes (International Classification of Diseases, Ninth Revision48/Tenth Revision49), where appropriate, were used to identify patients with each of the outcomes. We used lists of Read codes and ICD-9/ICD-10 codes that had been used in other studies where available, and also searched through lists of Read codes and ICD-9/ICD-10 codes to identify any additional appropriate codes or to define new lists if necessary. Final lists of codes were developed after discussion and agreement between the research team members.
The 13 outcomes that were assessed were:
-
all-cause mortality
-
sudden cardiac death
-
suicide (including open verdicts)
-
attempted suicide/self-harm
-
myocardial infarction
-
stroke/transient ischaemic attack (TIA)
-
falls
-
fractures (upper limb, lower limb, ribs, skull, vertebrae, pelvis)
-
upper GI bleeding
-
epilepsy/seizures
-
RTAs
-
adverse drug reactions (including bullous eruption)
-
hyponatraemia.
An additional prespecified outcome was overdose/poisoning from antidepressants, but the number of patients identified with this outcome was too small for analysis.
We identified suicides as patients either with a code for suicide or an open verdict on their death certificate or patients with a Read code for attempted suicide who died within 30 days. The Read codes used for attempted suicide were based on those used in other studies. 15,50 For RTAs we restricted the Read codes to those that indicated a motor vehicle crash, as in the study by Gibson and colleagues,51 and excluded codes that specified that the patient was a passenger. The date of occurrence of the outcome used in analysis was the first recorded date of the outcome during follow-up.
Exposures
The primary exposure of interest was treatment with antidepressant medication. The QResearch database contains detailed information on prescriptions issued to patients, including the name and formulation, dosage instructions and numbers of tablets issued for each prescription. We extracted details of all prescriptions for antidepressants in patients in our cohort, following their index date (earliest of date of first diagnosis of depression or date of first prescription for an antidepressant after the age of 65 years) and up to 31 December 2008 (or date of death or leaving the practice if this was earlier).
Antidepressant drugs were grouped for analysis according to the major classes of antidepressants as described in section 4.3 (Antidepressant drugs) of the British National Formulary (BNF),52 namely:
-
tricyclic and related antidepressants (TCAs –subsection 4.3.1)
-
monoamine oxidase inhibitors (MAOIs – subsection 4.3.2)
-
selective serotonin reuptake inhibitors (SSRIs – subsection 4.3.3)
-
other antidepressants (subsection 4.3.4).
The drugs in each category were:
-
TCAs – amitriptyline hydrochloride, amoxapine, clomipramine hydrochloride, desipramine, dosulepin hydrochloride, doxepin, imipramine, imipramine hydrochloride, lofepramine, maprotiline hydrochloride, mianserin hydrochloride, nortriptyline, protriptyline hydrochloride, trazodone hydrochloride, trimipramine, viloxazine hydrochloride
-
MAOIs – isocarboxazid, moclobemide, phenelzine, tranylcypromine
-
SSRIs – citalopram hydrobromide, citalopram hydrochloride, escitalopram, fluoxetine hydrochloride, fluvoxamine maleate, paroxetine hydrochloride, sertraline hydrochloride
-
Other antidepressants – duloxetine, flupentixol, l-tryptophan, mirtazapine, nefazodone hydrochloride, reboxetine, tryptophan, venlafaxine hydrochloride.
Some of these drugs have now been withdrawn but were in use at some time during the study period. Some patients received prescriptions for different drugs within a class or drugs from different classes on the same date. These prescriptions were classified as combined prescriptions for some analyses.
We determined the duration of each prescription in days by dividing the number of tablets prescribed by the dosing directions (e.g. number of tablets to be taken per day). In some cases in which the number of tablets prescribed was recorded, but the dosing directions were missing or not sufficiently detailed for this calculation to be made, we used an assumed duration based on the median duration of prescriptions for those prescriptions for which dosing directions were available, taking account of the number of tablets prescribed. On this basis we assumed a duration of 7 days if between 7 and 27 tablets were prescribed, a duration of 28 days if the number of tablets prescribed was between 28 and 99, and a duration of 56 days if the number of tablets prescribed was more than 100. If fewer than seven tablets were prescribed we assumed that the prescription duration in days was equal to the number of tablets prescribed. If the quantity of tablets prescribed was missing we assumed a duration of 28 days.
To calculate the daily dose of each prescription we multiplied the specified dose of each tablet prescribed by the number of tablets to be taken each day. To enable comparison of doses between the antidepressant classes, we converted the dose per day for each prescribed drug to a defined daily dose (DDD), defined as the assumed average maintenance dose per day for a drug used for its main indication in adults. We used the DDD values assigned by the World Health Organization’s Collaborating Centre for Drug Statistics Methodology (www.whocc.no/atc_ddd_index). If patients had two or more prescriptions for the same drug on the same day, we added the doses from these prescriptions.
Confounding variables
We identified potential confounding variables to be included in the cohort study analysis. These were:
-
age at index date (baseline)
-
gender (male, female)
-
year of diagnosis of depression (index date)
-
previous recorded diagnosis of depression before the age of 65 years
-
severity of index diagnosis of depression [categorised as mild, moderate or severe, based on the Read code for the index diagnosis, using codes published by Martinez and colleagues15 and some additional classification by a member of the study team (RM)]
-
deprivation, based on Townsend deprivation score for the patient’s postcode53
-
smoking status (non-smoker, ex-smoker, current smoker)
-
comorbidities at baseline [coronary heart disease (CHD), diabetes, hypertension, stroke/TIA, cancer, dementia, epilepsy/seizures, Parkinson’s disease, hypothyroidism, obsessive–compulsive disorder], identified using appropriate Read codes in the patient’s records
-
use of other drugs at baseline (statins, NSAIDS, antipsychotics, lithium, aspirin, antihypertensive drugs, anticonvulsant drugss, hypnotic/anxiolytic drugs).
In addition, for the analysis of suicide as an outcome, previous attempted suicide at baseline was considered as a confounding variable, and for the analysis of fracture previous falls at baseline was considered as a confounding variable.
Sample size
All eligible patients aged 65 years and over diagnosed with incident depression between 1 January 1996 and 31 December 2007 in the QResearch database were included in the cohort study. A feasibility study showed there are approximately 5.0 million person-years of observation and 18,000 incident cases of depression arising from patients aged 65 years and older between 1996 and 2005 on the database.
Assuming 88% of patients aged 65 years and overdiagnosed with depression are prescribed an antidepressant drug as we found in our feasibility study, and for a rare outcome with an incidence of 5 per 1000 per year (e.g. upper GI event47 or lower limb fracture54), and an average follow-up of 5 years, we anticipated that the study would be able to detect a relative risk of 1.5 with 88% power and a 5% significance level comparing those on antidepressants with those not on antidepressants. For all-cause mortality with a mortality rate of 53 per 1000 per year (Office for National Statistics, 2001 figures for England and Wales; www.statistics.gov.uk) the study would be able to detect a relative risk of 1.15 with 95% power. In direct comparisons between TCAs and SSRIs, assuming that 39% of patients on antidepressants take TCAs and 50% take SSRIs, the study would be able to detect a relative risk of 1.4 with 86% power for rare outcomes and 1.12 with 92% power for all-cause mortality.
Statistical analysis
All analyses were carried out using stata (version 10.1; StataCorp LP, College Station, TX, USA). We calculated incidence rates of diagnosed depression in people aged 65 years and over, using all eligible cases of depression in the study cohort as the numerator and person-years for people aged 65 years and over in the QResearch database as the denominator.
We described baseline characteristics of patients in the study cohort using summary statistics. We described patterns of antidepressant use according to class of antidepressant prescribed, duration of use and dose, and examined which individual drugs were prescribed most frequently. We compared the patients’ baseline characteristics according to the class of antidepressant prescribed. We calculated the proportions of patients who switched between different antidepressant classes at any time within the study period and within the first year of being prescribed an antidepressant.
We calculated the number of treatment episodes for depression during follow-up, where a treatment episode was defined as a period of antidepressant treatment without gaps of more than 90 days between the end of a prescription and the start of the next prescription. A prescription after more than 90 days was counted as the start of a new treatment episode.
We examined variation between practices in patterns of antidepressant prescribing by dividing the total number of prescriptions for each class of antidepressant by the total number of all antidepressant prescriptions in each practice and summarised the variation in these proportions across the practices.
The primary statistical analysis comprised a series of survival analyses to assess the relationship between exposure to antidepressant drugs and the adverse outcomes. We used Cox’s proportion hazards models, with antidepressant exposure treated as a time-varying exposure. The entry date into the analysis was the index date (earliest of first diagnosis of depression or first antidepressant prescription from the age of 65 years or over) and the outcome date was the earliest of either the date of diagnosis of the outcome of interest or the date of death if the outcome was recorded on their death certificate. We used only the first recorded diagnosis of the outcome of interest rather than recurrent events. Patients who did not have the outcome of interest were censored at the earliest of date of death, date of leaving the practice, date of the latest download of data or the study end date. For the analysis of each outcome we excluded patients who had already had the outcome at baseline. The time-varying analysis accounts for patients changing between treatments during follow-up and changing from treatment to no treatment, and the hazard ratio (HR) estimated from this analysis is interpreted as the ratio of the instantaneous rate (i.e. the hazard rate) of the outcome of interest in those on treatment compared with the rate in patients not on treatment at each time point throughout the follow-up period, for those still at risk at each particular time. The model assumes that this ratio has a constant value throughout the follow-up period. For the main analyses, patients were considered to be exposed to a drug if there were no gaps of more than 90 days between the end of one prescription and the start of the next prescription to allow for not having a precise date when the patient finished the prescription. If there were gaps of more than 90 days between the end of one prescription and the start of the next prescription then patients counted as exposed to antidepressant medication for the first 90 days and then unexposed for the remaining period.
The analysis calculated HRs and 95% confidence intervals (CIs) for:
-
current use of each separate class of antidepressants (SSRIs, TCAs and other antidepressants) compared with no current treatment
-
selective serotonin reuptake inhibitors and other antidepressants compared with TCAs
-
antidepressant dose according to antidepressant class, using a time-varying approach based on dose of current prescription. We categorised dose for each class into three groups – (1) ≤ 0.5 DDDs, (2) > 0.5 and ≤ 1.0 DDDs and (3) > 1.0 DDDs – and included dummy variables for these categories in the statistical models. We also carried out tests of trend for each antidepressant class, by fitting a separate model that contained the dose in DDDs for each class as continuous variables
-
duration of use, again using a time-varying approach; this analysis also considered the effects of time since last prescription for an antidepressant drug. We categorised duration of use according to antidepressant class as no use, 1–28 days’ use, 29–84 days’ use, 85+ days’ use, and washout periods of 1–28 days, 29–84 days and 85–182 days since stopping antidepressant medication
-
individual drugs compared with no treatment where numbers were sufficient.
We carried out unadjusted analyses and adjusted for the potential confounding variables described above. For two outcomes, sudden cardiac death and suicide, we used a restricted set of confounding variables as numbers of events were small, namely age, gender, CHD, diabetes, hypertension, and use of statins, aspirin and antihypertensive drugs for sudden cardiac death, and age, gender, severity of depression, previous attempted suicide and use of lithium for suicide, as these were considered likely to be the main confounders for those outcomes. We used a p-value of < 0.01 (two-tailed) to assess statistical significance, but reported 95% CIs for estimation purposes.
In the analyses of antidepressant class we carried out Wald’s significance tests to determine whether there were significant differences between the classes overall, excluding the group with no current treatment. We also did this for the analyses of individual drugs. We performed analyses of interaction between antidepressant class and patient’s characteristics (age, gender), use of other medications and comorbidities using likelihood ratio tests. We carried out an additional complete case analysis where we also adjusted for body mass index (BMI). We checked the assumptions of the Cox proportional hazards model graphically with log minus log plots.
We estimated absolute risks of the adverse events at 1, 2 and 5 years from the baseline date. For each outcome we calculated the absolute risk in patients while they were not taking antidepressant treatment and used this with the adjusted HRs for antidepressant class and individual drugs to calculate adjusted absolute risks in the treated groups using a published formula. 55 From these we determined the number of additional events per 10,000 treated patients compared with those receiving no treatment by subtracting the absolute risk in the group while not on treatment from the adjusted absolute risks in the treated groups, assuming associations to be causal.
Self-controlled case-series study
A limitation of the cohort design approach is that it can be vulnerable to indication bias and residual confounding, whereby relevant confounding variables may be imprecisely recorded or not recorded at all in primary-care records (e.g. diet, physical activity). The self-controlled case-series method has been proposed as a means of addressing this problem. 56,57 It is an internally controlled method whereby analyses are carried out only in patients with the outcome of interest, thereby eliminating the effect of indication bias and unmeasured confounding for variables that do not vary over time. It is of most relevance for acute events occurring within a short period after exposure. The method has previously been used to examine the relationship between antidepressant use and hip fracture20 and MI. 18
Self-controlled case-series study design
The self-controlled case-series analyses included only patients who had the outcomes of interest. Cases with each type of adverse event were identified; these were cases with a diagnosis of the adverse event between 1 January 1996 and 31 December 2008. We used only the first recorded diagnosis of the outcome of interest rather than recurrent events. Patients for whom the outcome was recorded as occurring on the same day as their first antidepressant prescription were distinguished in the analysis. Cases without any antidepressant prescriptions were included in the analyses to improve adjustment for age. 57
We used the extracted information on antidepressant prescriptions during the study period for cases with each outcome to identify periods of exposure to antidepressants and a baseline period. We accounted for multiple periods of exposure in the analysis, defining a period of antidepressant treatment as one without gaps of more than 90 days between the end of a prescription and the start of the next prescription. A prescription after more than 90 days counted as a new treatment episode. We categorised the time periods of exposure as 0 days (day of first prescription in each treatment episode); 1–28 days after the first prescription; 29–84 days and 85+ days (remaining treatment period); and periods after stopping treatment (1–28 days, 29–84 days and 85–182 days after stopping). The day of stopping treatment was taken as the date of the last prescription in the treatment episode plus the duration of the prescription. The 28 days before the first prescription in each treatment episode was considered as a separate category, as occurrence of the outcome of interest in this period could affect the probability of receiving an antidepressant prescription. All time periods outside these specified risk periods contributed to the baseline unexposed time periods. These risk periods were selected as they enable examination of short-term and longer-term effects of antidepressants on the risks of adverse events and are similar to those used in other studies of antidepressants. 18,20 Figure 1 illustrates the time periods used.
FIGURE 1.
Risk periods in the self-controlled case-series design.
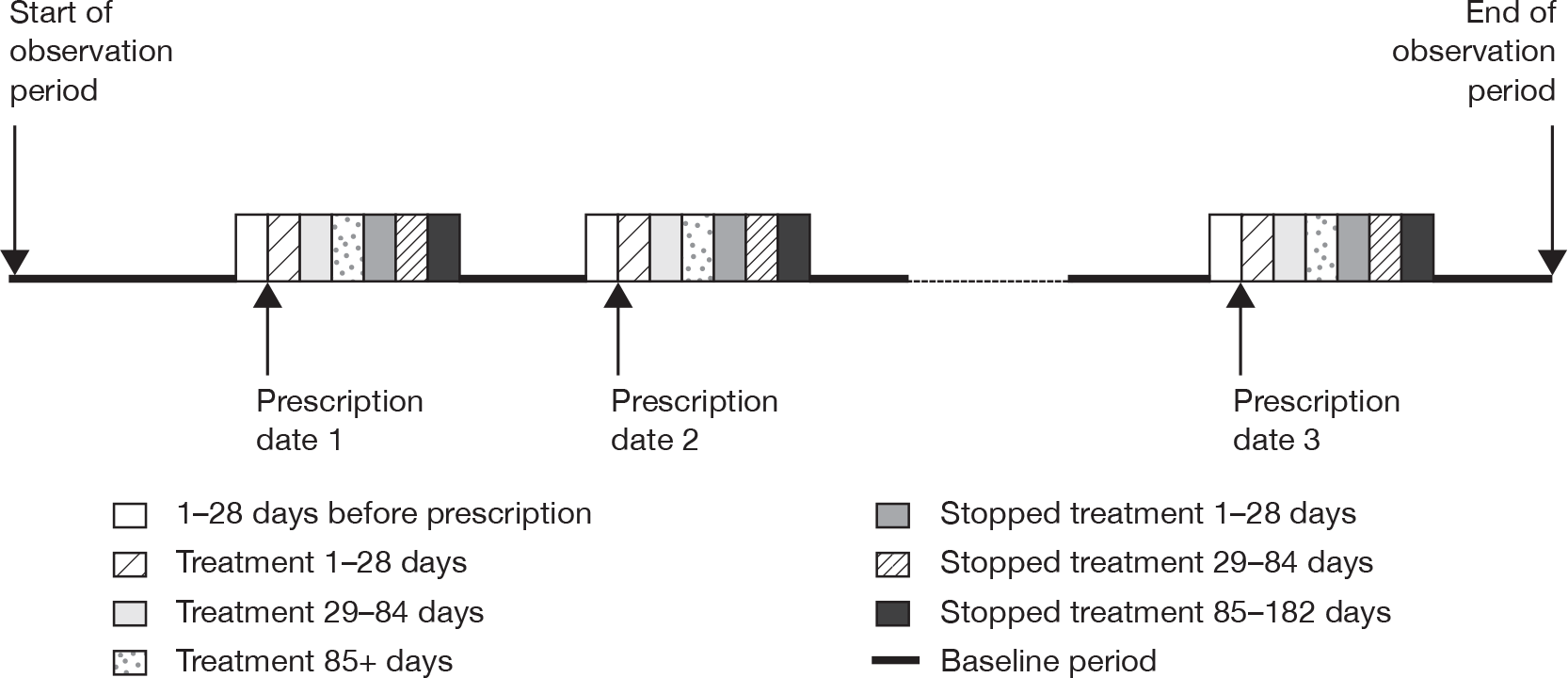
We used conditional Poisson regression to estimate the relative incidence of each of the outcomes of interest for the defined time periods of risk compared with the baseline period. We adjusted for age in the analyses (65–69, 70–74, 75–79, 80–84, 85+ years). We used a p-value of < 0.01 (two-tailed) to assess statistical significance. Where the outcome was a fatal one we used only time from the first prescription in the observation period for analysis, as otherwise the method is invalid.
Sample size for self-controlled case-series study
To detect a rate ratio of 2.0 in a risk period of 1–28 days after the first prescription for an antidepressant with 80% power and 5% significance, with a proportion of 0.015 (28/1825) in the risk period of 28 days compared with an average observation period of 5 years, 1002 exposed cases would be required for each outcome. 58 We anticipated having at least this number for all-cause mortality and falls. To detect a rate ratio of 3.0 in a risk period of 1–28 days then 231 exposed cases would be required. We anticipated having around this number for rarer outcomes such as GI events (incidence rate 5/1000/year).
Cost-effectiveness analysis
Objective
We sought to estimate the costs of health-care resource use in older people who had been diagnosed with depression and compare these for patients who had been prescribed different antidepressant drugs and classes of antidepressant drugs, while controlling for patient characteristics and other factors. Adverse event rates were also estimated enabling us to estimate and compare the cost per adverse event averted for different antidepressants.
Data extraction
The analysis used data from the cohort of patients described above. In addition to data on antidepressant prescriptions, adverse outcomes and patient characteristics, some additional health economic data were extracted. These were the number of practice nurse, community nurse and general practitioner (GP) visits for each patient during the follow-up period from diagnosis with depression up to the earliest of the study end date (31 December 2008), the date of death (if applicable), the date the patient left the study practice (if applicable) or the date of the latest download of data. The use of secondary care is not routinely recorded within the QResearch database, so we were unable to include this information in the analyses.
Analyses
Where technologies have an impact on costs and outcomes over a patient’s lifetime, conducting analysis over a lifetime horizon is appropriate. 59 Within this study, data were available, however, for only up to 13 years, therefore we sought to estimate the costs of antidepressant prescriptions and practice nurse, community nurse and GP visits that would be expected to fall upon the health service within the first 5 years post diagnosis for depression in patients aged ≥ 65 years, and compare these with patients prescribed different antidepressant drugs. In line with the statistical analysis, a 5-year perspective was chosen in the base-case analysis, as the mean length of follow-up was 5.02 years for patients in this study; a large proportion of patients would, thereby, have complete data over this period. We first identified patients for whom at least 5 years of data were available (we therefore excluded those who were diagnosed after 1 January 2004 as the study end date was 31 December 2008). Patients who died before the 5-year follow-up was complete were included in the analysis, but those who moved practices within 5 years (i.e. had incomplete data) were not. Patients who died were included, as resource-use data were available up to the date of death and this constituted the burden upon the NHS over the 5-year period. Patients who moved practices before the 5-year follow-up was complete were not included, as to include only the data up to the point at which they left the GP practice could result in an underestimation of the health-care resources that would be used by such patients over a 5-year period. However, with a view to assess whether those who switched practices had different costs from those who remained with the same practice for the whole 5-year period we compared the 1-year costs (for those patients for whom this was available) for those who were diagnosed on or before 1 January 2004, but switched practices within the 5-year period with the costs for those who were diagnosed within the same period and remained with the same practice for a 5-year period. Costs were estimated in pounds sterling (£) at 2007–8 cost-year levels.
Prescription costs
Unit costs for each drug were estimated using data from the Prescription Cost Analysis database (for the financial year 2007–8),60 which is based on the September 2007 version of the BNF. 52 Within the database, unit costs are estimated at the level of the individual chemical after calculating the weighted average (mean) cost, based on the unit cost and number of prescriptions prescribed for each individual preparation. In order to check that the mean prescription cost for each individual chemical, as calculated within the Prescription Cost Analysis database,60 was applicable to those aged ≥ 65 years, we compared the average dose for each prescription (at the level of the chemical) for patients in our study with that for all prescriptions in the Prescription Cost Analysis database. To do this we extracted the dose for each prescription (including tablets and solutions) for each patient. By taking account of the number of prescriptions at each dose, the weighted average dose was estimated for each type of chemical. The same method was used to estimate the weighted average dose for the Prescription Cost Analysis data. These values were then compared.
To estimate the expected mean cost over a 5-year period post diagnosis for each patient, we assigned the unit cost for each chemical to each prescription, where the unit cost was assumed to be equivalent to the weighted average from the Prescription Cost Analysis database,60 assuming that the mean prescription dosage for each individual chemical, as calculated within the Prescription Cost Analysis database, was applicable to those aged ≥ 65 years, where costs in future years (≥ 1 year post diagnosis) were discounted at 3.5% per annum. 61 Furthermore, to compare costs for patients who were prescribed different antidepressant drugs, we identified patients who were first prescribed each type of antidepressant drug within the first 12 months of diagnosis of depression (of those prescribed an antidepressant within 12 months of diagnosis, 88.3% had a prescription on the date of diagnosis and 93.5% had a prescription within 30 days). Subsequently, for each of the 11 most commonly prescribed antidepressant drugs within our data set, we estimated the mean total prescription cost over the 5-year period post diagnosis. Patients were categorised according to the drug they received first (those initially prescribed more than one antidepressant were excluded from the analyses).
Mean costs were also estimated for the antidepressant classes (TCAs, SSRIs and other antidepressants), based on the weighted average prescription costs for all of the TCA, SSRI and other antidepressant prescriptions. We also identified patients who were not prescribed any antidepressant drugs within the 5-year period. In addition to estimating the mean total prescription cost for each antidepressant drug in question, we estimated the mean total prescription cost for all antidepressant drugs prescribed to each patient in the time period. Again these values were compared for patients who were prescribed the 11 most commonly prescribed antidepressant drugs within our data set and for all TCAs, SSRIs and other antidepressants. Finally, the mean prescription costs (for both the antidepressant drug in question and all antidepressant drugs) associated with each of the 11 antidepressant drugs were ranked from lowest to highest cost.
Costs associated with practice nurse, community nurse and general practitioner visits
We estimated the costs associated with practice nurse, community nurse and GP visits using unit costs extracted from Curtis,62 where all contacts with a practice nurse and GP were assumed to take place at the practice, and all contacts with a community nurse were assumed to be home visits. These visit costs were estimated over the same 5-year period as above, where future costs were discounted and the mean visit cost for each type of health professional was estimated for the 11 most commonly prescribed antidepressant drugs and the antidepressant classes (TCAs, SSRIs, and other antidepressants), as well as for those who received no antidepressant prescriptions within the 5-year period. Additionally, we compared the summation of the total visit cost and the total prescription cost for all antidepressant drugs (referred hereafter as the overall visit plus prescription cost) for the same groups of individuals. The mean overall visit plus prescription costs were ranked from lowest to highest.
Adverse events and their associated costs
Data were extracted on the 13 specific adverse events as detailed above. We used the absolute risks for each of these adverse events for each of the different antidepressant drugs as described previously, along with those for periods of non-use of antidepressants, to estimate the number of additional adverse events per 10,000 patients treated that one would expect to occur in a 5-year period, for each of the different antidepressant drugs compared with no treatment. We used these figures to estimate the mean number of first events that one would estimate each patient to have while receiving a prescription for each of the antidepressants in question. First, we needed to align the time period for the number of events with that for the cost data. This was a period of 5 years, except when a patient died before the end of the time period. Thus, the mean period over which costs were estimated was less than 5 years, if any of the patients prescribed a particular prescription died within the 5-year period. Second, after estimating the mean follow-up time for patients prescribed each type of prescription, we estimated what proportion of the 5-year period this constituted and accordingly estimated the mean expected number of events (per patient) that would be estimated to occur in that period (events in future years were discounted at a rate of 3.5% per annum). These same calculations were performed in order to estimate the number of adverse events (per patient) that would be expected for those prescribed the 11 most commonly prescribed antidepressant drugs, and for the antidepressant classes in the first 5 years post diagnosis. Subsequently, the incremental number of adverse events was estimated by taking the difference between the estimated number of adverse events for patients prescribed each of these antidepressants and the estimated number for patients prescribed no antidepressants. These analyses were preformed for each of the 13 adverse events.
After calculating the mean expected number of events (per person) it was necessary to consider the issue of assigning costs to each of the different types of adverse events. Though we knew whether an event had occurred, the QResearch database does not routinely contain non-primary care resource-use data and, thus, it was difficult to estimate the actual resource use associated with each event. We considered whether we could make an assumption about the mean cost associated with each adverse event, informed by wider literature (where applicable). Though we might, for example, be able to assume the secondary-care costs associated with each fall admission to be equivalent to that for the weighted average of the three fall descriptions contained within the National Schedule of Reference Costs,63 not all falls reported in primary care result in an admission. Alternatively, it could be that secondary-care costs represent an underestimate, as this would exclude additional rehabilitation and community follow-up costs that might occur. Thus, we considered assigning a cost to each of these types of adverse events to be highly speculative. Moreover, to use precise figures might portray an element of robustness that was not appropriate. As a consequence we did not include adverse event costs within our analyses.
Base-case analysis
Of the aforementioned analyses, which included different elements of NHS costs, the mean total prescription cost for all antidepressant drugs was considered to constitute the base case. Visit costs were not included within the base-case analysis as visits would have been made for a number of reasons, many of which might have been unrelated to the particular antidepressant(s) prescribed, the patients’ depression and/or associated comorbidities. That said, within such a large sample it could be argued that non-depression-related costs would be approximately equivalent across different types of antidepressants and that by calculating the incremental cost, between different antidepressants, non-depression-related costs would be excluded.
Before comparing costs for different antidepressant drugs it was necessary to adjust for any patient characteristics and clinical factors that might differ between patients prescribed different antidepressant drugs. Consequently, we used linear regression analyses to estimate the difference between the mean total prescription cost (for all antidepressant drugs) for patients prescribed each of the 11 most commonly prescribed antidepressant drugs, compared with patients not prescribed antidepressant drugs, controlling for the following variables: gender, age at diagnosis, calendar year at diagnosis, depression severity, depression before age 65 years, smoking status, Townsend score, baseline comorbidities (CHD, stroke, diabetes, hypertension, cancer, dementia, epilepsy/seizures, falls, attempted suicide, Parkinson’s disease, hypothyroidism, obsessive–compulsive disorder) and previous use of certain drugs at baseline (statins, NSAIDs, antipsychotics, lithium, aspirin, antihypertensive drugs, anticonvulsant drugs, hypnotic/anxiolytic drugs).
Levels of cost-effectiveness
After adjusting for the aforementioned factors it was possible to estimate the mean incremental total prescription cost (for all antidepressant drugs) for each of the 11 most commonly prescribed antidepressant drugs compared with patients who received no prescriptions. We have described above the calculation of the estimated incremental number of adverse events for each of the 11 most commonly prescribed antidepressant drugs. These cost and event data were used to calculate the incremental cost (mean incremental prescription cost for all antidepressant drugs) per adverse event averted, for different antidepressant drugs, via the efficiency frontier64,65 [the efficiency frontier connects the potentially cost-effective (i.e. non-dominated) options]. The efficiency frontier can be calculated by first identifying the antidepressant drug with the lowest mean prescription cost (for all antidepressant drugs), hereafter referred to as lowest cost. Other antidepressant drugs that are dominated66 by another antidepressant drug (i.e. have a higher mean cost and are estimated to be associated with a greater number of adverse events) can then be excluded, as can antidepressant drugs that are subject to extended dominance66 (i.e. where combinations of other drugs have equivalent or lower mean cost and fewer adverse events). Extended dominance would be apparent if an option was less effective and had a higher incremental cost-effectiveness ratio (ICER) in terms of adverse events than an alternative option. 67 The remaining antidepressant drugs will be located on the efficiency frontier, where one can calculate the incremental cost per averted event (mean incremental cost/expected incremental number of averted events) (ICER) for each antidepressant drug located on the efficiency frontier. Previous studies65,67 provide further details of how ICERs are calculated when evaluating multiple options. These methods were used to calculate the mean incremental cost per averted event (and associated efficiency frontier) for each of the 13 adverse events, comparing the 11 most commonly prescribed antidepressant drugs to each other and the different antidepressant classes (TCAs, SSRIs and other antidepressants).
There is no previously defined threshold (in terms of willingness to pay) against which to compare levels of incremental cost per case averted (in order to assess whether the expected costs would be considered to be worthwhile, i.e. constitute value for money). However, given that the study group patients are aged ≥ 65 years it is unlikely that averting a particular adverse event would, on average, result in a gain of 20 quality-adjusted life-years (QALYs) (after discounting). For example, were one to extend life by 20 years and increase health-related quality of life (HRQoL) by 0.5 years (e.g. assuming HRQoL was initially 0.5, giving a resulting HRQoL of 1.0), then this would equate to a QALY gain of < 20 (after discounting) and it seems unlikely that avoiding an adverse event would (on average) be associated with such a large QALY gain. On that basis, given that NICE has stated that interventions that cost > £30,000 per QALY are unlikely to be deemed cost-effective,59 if the incremental cost per adverse event averted were > £600,000, for a particular option, then, assuming it would not result in a QALY gain of > 20, it would be unlikely to be deemed cost-effective. In the light of this, we assumed that all options which had a cost per adverse event averted of > £600,000 would not form part of the efficiency frontier.
Sensitivity analysis
Sensitivity analysis66 was undertaken in order to estimate the robustness of our results, where the incremental cost of different antidepressant drugs and the incremental cost per adverse event avoided were recalculated using different assumptions. Results were first recalculated using the summation of the total visit cost and the total prescription cost for all antidepressant drugs. One might expect people who have been prescribed different antidepressant drugs to have different consultation rates in primary care, for example consultation rates might be higher for patients prescribed certain antidepressant drugs, as some are more prone to dose changes (this may be more applicable to TCAs than SSRIs) or consultation rates may be higher due to side effects. These results are presented in Appendix 2 and summarised in the main text.
A further sensitivity analysis was to estimate costs and adverse events rates over a 1-year period, enabling data to be used from a greater number of patients, as estimation of 5-year costs resulted in the exclusion of those who were diagnosed post 1 January 2004. Thus, all previously defined costs and adverse events were recalculated for all patients in the study database for a 1-year period (this resulted in the inclusion of all patients, except those who left their practice within 1 year of diagnosis or were initially prescribed more than one antidepressant). The same methods as described previously were used, in which costs and benefits were not discounted as they occurred in the first year of care.
Protocol changes
We specified in the protocol that we would adjust for government office region, BMI and alcohol in addition to other confounders. We did not, however, adjust for these three variables owing to missing data and to avoid having unstable models. We did additionally adjust for study year and adjusted the suicide outcome for attempted suicide at baseline and the fracture outcome for falls at baseline.
We specified in the protocol that in the self-controlled case-series analysis we would use the risk periods 0 days, 1–14 days, 15–28 days and 29–84 days, remaining treatment period and the washout period (a period of 182 days after stopping treatment), and that the 14 days before the first prescription would be considered as a separate category. These categories were changed slightly to those detailed previously after discussion within the study team and prior to statistical analysis to allow more detailed analysis of effects of stopping and increased numbers in some periods.
We specified in the protocol that the cost of adverse events would be estimated using patient-specific resource-use data (identified using the QResearch database). As discussed previously, this was not undertaken, as examination of secondary care resource-use data within the QResearch database revealed that this was not routinely recorded by all GP practices within the database.
We specified in the protocol that a literature search would be performed with a view to identify the quality of life of older people with depression. One of the proposed uses of these data was to aid the comparison of quality of life between patients with depression who had been prescribed different types of antidepressants and those not prescribed antidepressants. Our literature search did not identify any such studies for older people and, hence, this analysis was not undertaken (this issue of disutility associated with different adverse events is discussed further in Chapter 4). In the absence of quality of life data, as outlined in the protocol, we used the ‘incremental cost per adverse event averted’ technique to compare different antidepressants.
Ethical arrangements
The project was independently peer reviewed by the QResearch Scientific Board and has been reported to Trent Research Ethics Committee in accordance with the agreed procedure with the Committee.
Chapter 3 Results
Results of descriptive analyses
Selection of study cohort
A total of 88,701 patients in the QResearch database were diagnosed with depression at age 65 years or over between 1 January 1996 and 31 December 2007. After consecutively excluding 22 patients aged 100 years and over at diagnosis, 3178 patients with schizophrenia, bipolar disorder or other psychoses, 15,690 who had joined the practice in the previous year and 9065 with a diagnosis of depression or a prescription for an antidepressant in the previous year, there were 60,746 eligible patients remaining who formed the study cohort. Figure 2 shows the selection of patients for the study cohort.
FIGURE 2.
Selection of patients for the study cohort.

The 60,746 patients included in the study were from 570 QResearch practices in the UK. These practices included 543 in England, 14 in Wales, 4 in Scotland and 9 in Northern Ireland. The practices in England were spread throughout the regions, with 32 in the North-East, 61 in the North-West, 59 in Yorkshire and the Humber, 85 in the East Midlands, 45 in the West Midlands, 43 in the East of England, 70 in London, 81 in the South-East and 67 in the South-West. The total number of patients registered with eligible practices during the study period was 9,583,082.
Incidence of diagnosed depression
Table 1 shows the incidence rates of diagnosed depression in people aged 65 year and over, by gender and age group. Rates were higher in women than in men, although the difference was less marked with increasing age.
| Age band (years) | Cases of depression | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–69 | 12,532 | 1,049,470 | 119.4 | 117.3 to 121.5 |
| 70–74 | 8278 | 937,795 | 88.3 | 86.4 to 90.2 |
| 75–79 | 8103 | 828,121 | 97.8 | 95.7 to 100.0 |
| 80–84 | 5985 | 623,191 | 96.0 | 93.6 to 98.5 |
| 85–89 | 3808 | 389,302 | 97.8 | 94.8 to 101.0 |
| 90+ | 1810 | 228,490 | 79.2 | 75.6 to 83.0 |
| All ages (65+) | 40,516 | 4,056,369 | 99.9 | 98.9 to 100.9 |
| Men | ||||
| 65–69 | 6027 | 1,015,893 | 59.3 | 57.8 to 60.8 |
| 70–74 | 4496 | 840,855 | 53.5 | 51.9 to 55.1 |
| 75–79 | 4293 | 644,640 | 66.6 | 64.6 to 68.6 |
| 80–84 | 3121 | 399,991 | 78.0 | 75.3 to 80.8 |
| 85–89 | 1713 | 193,199 | 88.7 | 84.6 to 93.0 |
| 90+ | 580 | 81,074 | 71.5 | 65.9 to 77.6 |
| All ages (65+) | 20,230 | 3,175,651 | 63.7 | 62.8 to 64.6 |
Study cohort
Baseline characteristics of the study cohort are shown in Table 2. There were 20,230 (33.3%) men and 40,516 (66.7%) women. There were 31,341 patients who were aged 65–74 years at baseline (51.6%), with 7908 (13.0%) aged 85 years and over. Nearly 20% of patients had a diagnosis of CHD at baseline and 38.9% had hypertension. Substantial proportions were taking prescribed medications at baseline, including antihypertensive drugs (50.0%), aspirin (29.4%), hypnotic/anxiolytic drugs (23.7%) and NSAIDs (57.0%).
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Male | 20,230 | 33.30 |
| Female | 40,516 | 66.70 |
| Age (years) | ||
| 65–74 | 31,341 | 51.59 |
| 75–84 | 21,497 | 35.39 |
| 85+ | 7908 | 13.02 |
| Mean age (SD) | ||
| Overall | 74.98 (7.55) | |
| Male | 74.78 (7.22) | |
| Female | 75.09 (7.71) | |
| Depression severity (index diagnosis) | ||
| Mild | 42,281 | 69.60 |
| Moderate | 15,639 | 25.74 |
| Severe | 2826 | 4.65 |
| Recorded history of depression before age 65 years | ||
| No | 51,803 | 85.28 |
| Yes | 8943 | 14.72 |
| BMI recorded | 43,773 | 72.06 |
| Mean BMI in kg/m2 (SD) | 26.55 (4.70) | |
| Smoking | ||
| Recorded | 57,650 | 94.90 |
| Non smoker | 33,656 | 58.38 |
| Ex smoker | 13,005 | 22.56 |
| Current smoker | 10,989 | 19.06 |
| Comorbidities | ||
| CHD | 11,981 | 19.72 |
| Diabetes | 6169 | 10.16 |
| Hypertension | 23,654 | 38.94 |
| Stroke/TIA | 6448 | 10.61 |
| Any cancer | 5032 | 8.28 |
| Dementia | 1091 | 1.80 |
| Epilepsy/seizures | 953 | 1.57 |
| Parkinson’s disease | 869 | 1.43 |
| Hypothyroidism | 3956 | 6.51 |
| Obsessive–compulsive disorder | 119 | 0.20 |
| Medications at baseline | ||
| Anticonvulsants | 1671 | 2.75 |
| Antihypertensives | 30,363 | 49.98 |
| Antipsychotics | 5332 | 8.78 |
| Aspirin | 17,863 | 29.41 |
| Hypnotics/anxiolytics | 14,391 | 23.69 |
| Lithium | 148 | 0.24 |
| NSAIDs | 34,618 | 56.99 |
| Statins | 10,283 | 16.93 |
Patterns of antidepressant treatment
A total of 1,398,359 prescriptions for antidepressants were received during the study follow-up period. The duration of each prescription could be calculated for 1,244,296 (89.0%) of these, based on the quantity prescribed and dosing directions. The median prescription length was 28 days [interquartile range (IQR) 28 days to 30 days] and more than one-half of these prescriptions were for 28 days’ duration (641,811 prescriptions, 51.6%). For 154,063 prescriptions (11.0% of 1,398,359) there was insufficient information on quantity or dosing directions to enable direct calculation of duration, but values were estimated (as described in Chapter 2) based on the quantity prescribed, which was available for 147,165 of these prescriptions (95.5%), and where this was missing a value of 28 days was assumed.
Details of the first antidepressant drug prescribed and the total number of prescriptions received during follow-up are shown in Table 3. Of the 60,746 patients in the cohort 6708 (11.0%) received no prescriptions for an antidepressant during follow-up and the remaining 54,038 (89.0%) received at least one prescription during follow-up. For nearly half (49.0%) of the patients in the cohort the first antidepressant prescribed was an SSRI, whereas for just over one-third (34.6%) it was a TCA.
| Antidepressant treatment | No. | % of total |
|---|---|---|
| Class of first antidepressant prescribed | ||
| None | 6708 | 11.04 |
| TCA | 21,043 | 34.64 |
| MAOI | 31 | 0.05 |
| SSRI | 29,763 | 49.00 |
| Other | 3060 | 5.04 |
| Combined | 141 | 0.23 |
| Total no. of antidepressant prescriptions per patient in patients with one or more | ||
| Median (IQR) | 12 (3 to 34) | |
| Total antidepressant prescriptions per patient during follow-up | ||
| 0 | 6708 | 11.04 |
| 1 | 6484 | 10.67 |
| 2–3 | 7505 | 12.35 |
| 4–6 | 6340 | 10.44 |
| 7–12 | 7522 | 12.38 |
| 13–24 | 8307 | 13.67 |
| 25–36 | 5233 | 8.61 |
| 37–48 | 3477 | 5.72 |
| 49–60 | 2546 | 4.19 |
| > 60 | 6624 | 10.90 |
| Total antidepressant prescriptions per patient in first year of follow-up | ||
| 0 | 9086 | 14.96 |
| 1 | 12,634 | 20.80 |
| 2–3 | 10,916 | 17.97 |
| 4–6 | 8962 | 14.75 |
| 7–12 | 12,853 | 21.16 |
| 13+ | 6295 | 10.36 |
| Total duration of prescriptions in follow-up (days) | ||
| Median (IQR) | 364 (91 to 1029) | |
| Total duration of prescriptions in first year of follow-up (days) | ||
| Median (IQR) | 140 (56 to 308) | |
| Total duration of antidepressant prescriptions in follow-up | ||
| 1–28 days | 4652 | 8.61 |
| 29–84 days | 7775 | 14.39 |
| 85–182 days | 7219 | 13.36 |
| 182–365 days | 7675 | 14.20 |
| 1–2 years | 8804 | 16.29 |
| 2–3 years | 5255 | 9.72 |
| 3–4 years | 3744 | 6.93 |
| 4–5 years | 2679 | 4.96 |
| 5+ years | 6235 | 11.54 |
| Total episodes of antidepressant treatment during follow-up | ||
| 1 | 25,700 | 47.56 |
| 2 | 14,354 | 26.56 |
| 3 | 7016 | 12.98 |
| 4 | 3470 | 6.42 |
| 5+ | 3498 | 6.47 |
| Duration of antidepressant treatment per treatment episode (days) | ||
| Median (IQR) | 179 (56 to 528) | |
| Duration of antidepressant treatment as percentage of follow-up | ||
| Median (IQR) | 31.3 (8.4 to 74.2) |
The 54,038 patents prescribed antidepressant drugs during follow-up received a median of 12 prescriptions, with a range of 1 to 727. A total of 6484 patients (10.7% of 60,746) had only a single prescription and around one-third (20,697, 34.1%) received three prescriptions or fewer during follow-up. The median total duration of treatment with antidepressants during follow-up was 364 days (IQR 91 days to 1029 days), and during the first year of follow-up it was 140 days (IQR 56 days to 308 days).
Table 3 also shows the number of episodes of antidepressant treatment in patients who received at least one prescription for an antidepressant drug during follow-up, where a new treatment episode was defined as one that occurred after a gap of at least 90 days after the end of the previous prescription. Nearly half of the treated patients (47.6%) had only one treatment episode during follow-up, around one-quarter (26.6%) had two treatment episodes and 25.9% had three or more. The median duration of antidepressant treatment per treatment episode was 179 days and the median duration of treatment as a percentage of total follow-up time was 31.3%.
Table 4 shows the total number of prescriptions received during follow-up for each antidepressant class and also for each specific drug, as well as the numbers of patients with one or more prescriptions for each drug. SSRIs were the most commonly prescribed drug class with more than three-quarters of treated patients being prescribed an SSRI during follow-up and 54.7% of the total antidepressant prescriptions were for this class. The most commonly prescribed SSRI drugs were citalopram hydrobromide (23.0% of all prescriptions) and fluoxetine (14.0%). There were 442,192 prescriptions for TCAs, constituting 31.6% of all antidepressant prescriptions. The most commonly prescribed TCAs were amitriptyline (13.5% of all prescriptions) and dosulepin (10.3%). The group of other antidepressants contributed 13.5% of the total prescriptions. The most commonly prescribed drugs within this group were venlafaxine (6.3% of all prescriptions) and mirtazapine (5.9%). MAOI drugs were the least commonly prescribed class, constituting only 0.16% of the total number of prescriptions issued. The 10 most commonly prescribed antidepressant drugs constituted 93.6% of all prescriptions: these were citalopram hydrobromide, fluoxetine hydrochloride, amitriptyline hydrochloride, dosulepin hydrochloride, paroxetine hydrochloride, venlafaxine hydrochloride, sertraline hydrochloride, mirtazapine, lofepramine and escitalopram. As there were only slightly fewer prescriptions for trazodone hydrochloride, the 11 most commonly prescribed drugs were considered separately in some analyses; these constituted 96.0% of all prescriptions.
| Antidepressant class | Drug name | No. of prescriptions issued | No. of patients who received at least one prescription | ||
|---|---|---|---|---|---|
| n | % | n | %a | ||
| TCA (any) | 442,192 | 31.62 | 29,085 | 53.82 | |
| Amitriptyline hydrochloride | 188,283 | 13.46 | 16,440 | 30.42 | |
| Amoxapine | 4 | 0.00 | 2 | 0.00 | |
| Clomipramine hydrochloride | 6425 | 0.46 | 543 | 1.00 | |
| Desipramine | 2 | 0.00 | 2 | 0.00 | |
| Dosulepin hydrochloride | 144,658 | 10.34 | 10,402 | 19.25 | |
| Doxepin | 6031 | 0.43 | 434 | 0.80 | |
| Imipramine | 24 | 0.00 | 8 | 0.01 | |
| Imipramine hydrochloride | 7218 | 0.52 | 859 | 1.59 | |
| Lofepramine | 43,570 | 3.12 | 5517 | 10.21 | |
| Maprotiline hydrochloride | 321 | 0.02 | 20 | 0.04 | |
| Mianserin hydrochloride | 1840 | 0.13 | 156 | 0.29 | |
| Nortriptyline | 4956 | 0.35 | 565 | 1.05 | |
| Protriptyline hydrochloride | 115 | 0.01 | 15 | 0.03 | |
| Trazodone hydrochloride | 33,675 | 2.41 | 2573 | 4.76 | |
| Trimipramine | 5055 | 0.36 | 314 | 0.58 | |
| Viloxazine hydrochloride | 15 | 0.00 | 4 | 0.01 | |
| MAOI (any) | 2203 | 0.16 | 108 | 0.20 | |
| Isocarboxazid | 390 | 0.03 | 7 | 0.01 | |
| Moclobemide | 665 | 0.05 | 75 | 0.14 | |
| Phenelzine | 376 | 0.03 | 24 | 0.04 | |
| Tranylcypromine | 806 | 0.06 | 14 | 0.03 | |
| SSRI (any) | 764,659 | 54.68 | 42,575 | 78.79 | |
| Citalopram hydrobromide | 321,495 | 22.99 | 22,029 | 40.77 | |
| Citalopram hydrochloride | 1730 | 0.12 | 283 | 0.52 | |
| Escitalopram | 36,014 | 2.58 | 3233 | 5.98 | |
| Fluoxetine hydrochloride | 196,393 | 14.04 | 17,354 | 32.11 | |
| Fluvoxamine maleate | 484 | 0.03 | 68 | 0.13 | |
| Paroxetine hydrochloride | 120,475 | 8.62 | 7519 | 13.91 | |
| Sertraline hydrochloride | 88,068 | 6.30 | 6525 | 12.07 | |
| Other (any) | 189,305 | 13.54 | 10,485 | 19.40 | |
| Duloxetine | 3017 | 0.22 | 327 | 0.61 | |
| Flupentixol | 13,140 | 0.94 | 1698 | 3.14 | |
| l-Tryptophan | 20 | 0.00 | 5 | 0.01 | |
| Mirtazapine | 81,756 | 5.85 | 5258 | 9.73 | |
| Nefazodone hydrochloride | 1529 | 0.11 | 163 | 0.30 | |
| Reboxetine | 1420 | 0.10 | 171 | 0.32 | |
| Tryptophan | 79 | 0.01 | 6 | 0.01 | |
| Venlafaxine hydrochloride | 88,344 | 6.32 | 4686 | 8.67 | |
| Total | 1,398,359 | 100.00 | 54,038 | 100.00 | |
Figure 3 shows the total number of prescriptions during follow-up for the 11 most commonly prescribed antidepressant drugs issued over the study period.
FIGURE 3.
Total number of prescriptions during follow-up for the 11 commonly prescribed antidepressant drugs.
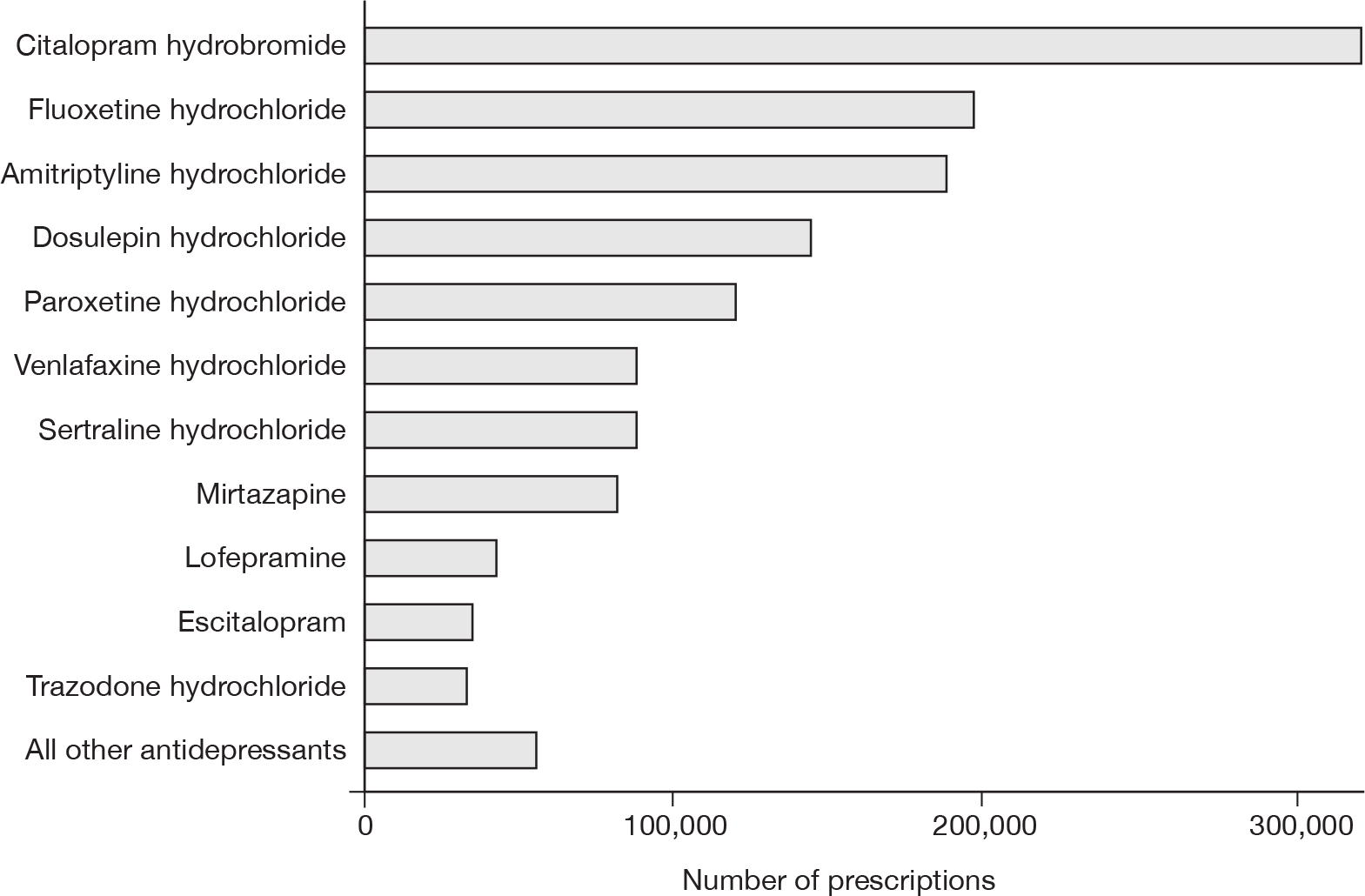
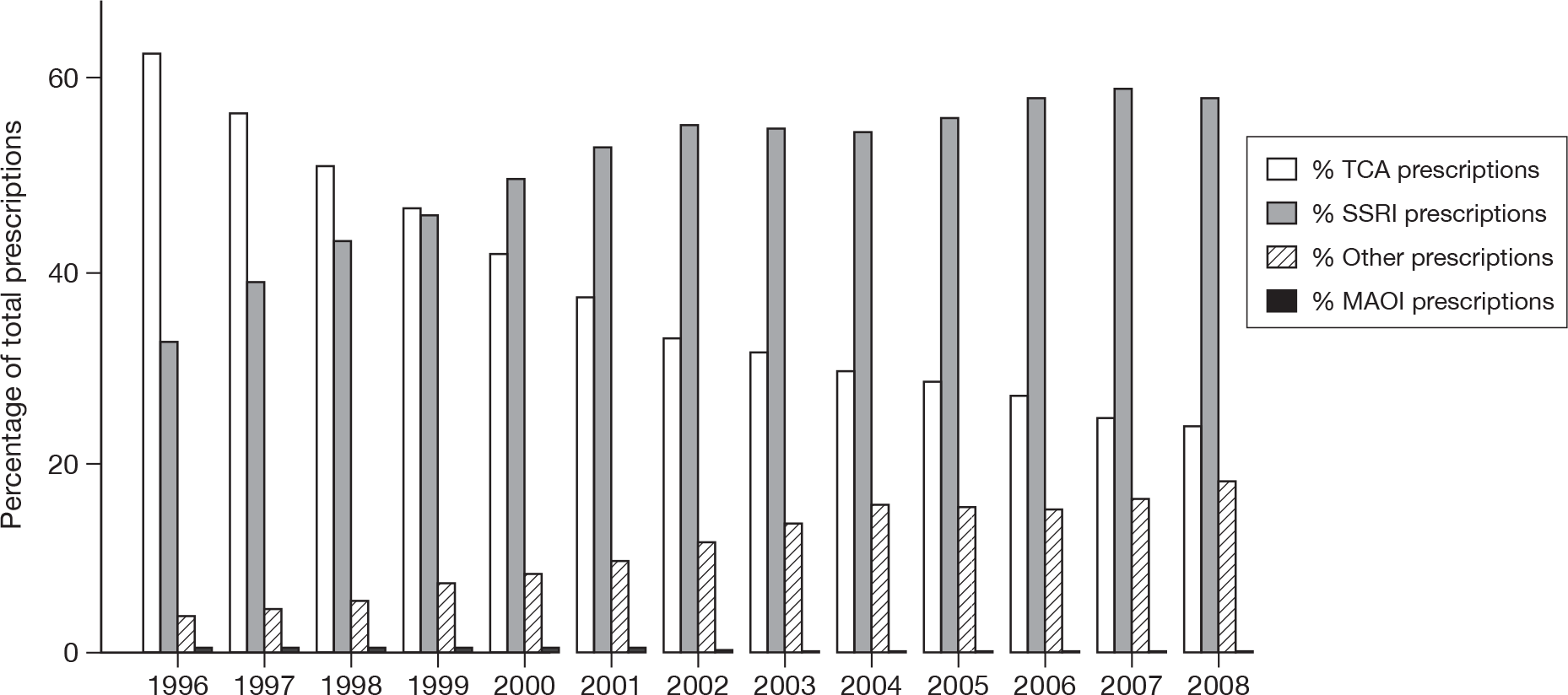
Figure 4 shows the number of prescriptions for each antidepressant class, by year of prescription. There was a steep increase in the proportion of prescriptions which were for an SSRI over time, with a corresponding reduction for TCAs. There was also an increase for the group of other antidepressants over time. In terms of the first antidepressant prescribed, the proportion of patients for whom the first antidepressant prescribed was a TCA fell from 65.7% in 1996 to 18.7% in 2007, whereas the proportion for whom it was an SSRI increased from 29.9% in 1996 to 75.0% in 2007. The proportion of patients in whom the first antidepressant prescribed was in the group of other antidepressants increased from 4.0% in 1996 to 8.0% in 2004 and then decreased to 5.9% in 2007.
For the 11 most commonly prescribed antidepressant drugs, the proportions of total prescriptions that were for the TCAs amitriptyline, dosulepin and lofepramine all decreased over time, while they increased for the SSRIs citalopram hydrobromide, fluoxetine hydrochloride and escitalopram (data not shown). The proportion of prescriptions that was for the SSRI paroxetine hydrochloride decreased from 13.3% in 1996 to 4.0% in 2008, and stayed fairly constant throughout this period for the SSRI sertraline hydrochloride (at around 6.3%) and the TCA trazodone hydrochloride (at around 2.4%). In the group of other antidepressants the proportion prescribed increased for mirtazapine from 1996 to 2008 and increased for venlafaxine hydrochloride from 1.2% in 1996 to 9.1% in 2004, after which it declined to 5.6% in 2008.
FIGURE 4.
Percentages of prescriptions for each antidepressant class by year of prescription.
Antidepressant treatment by baseline characteristics
The baseline characteristics according to the class of antidepressant first prescribed, excluding the combined group, are shown in Table 5. There were significant differences between the groups (excluding MAOIs owing to small numbers) for all baseline characteristics except for obsessive–compulsive disorder and anticonvulsant treatment, although absolute differences were generally small. The most marked differences were that, compared with the treated groups, the untreated group had a higher proportion of men, a higher proportion of patients aged 85 years and over, higher proportions of patients with CHD, diabetes, dementia and epilepsy/seizures at baseline, lower proportions treated with antipsychotics and hypnotics/anxiolytics and a higher proportion treated with statins. Comparing treated groups directly there was a higher proportion of men in the SSRI group than in the other groups and a lower proportion in the TCA group. There were fewer people aged 85 years and over in the TCA and MAOI groups. Patients in the TCA group tended to be less likely to have comorbidities than patients in the SSRI group. For example, 17.4% of patients in the TCA group had CHD compared with 20.6% in the SSRI group; they were also less likely to be treated with antihypertensive drugs, aspirin or statins than patients in the SSRI group.
| Characteristic | First antidepressant class prescribed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No antidepressant | TCA | MAOI | SSRI | Other | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Gender | ||||||||||
| Female | 4201 | 62.63 | 14,929 | 70.95 | 21 | 67.74 | 19,252 | 64.68 | 2028 | 66.27 |
| Male | 2507 | 37.37 | 6114 | 29.05 | 10 | 32.26 | 10,511 | 35.32 | 1032 | 33.73 |
| Age group (years) | ||||||||||
| 65–74 | 2771 | 41.31 | 11,585 | 55.05 | 20 | 64.52 | 15,397 | 51.73 | 1484 | 48.50 |
| 75–84 | 2653 | 39.55 | 7335 | 34.86 | 10 | 32.26 | 10,355 | 34.79 | 1100 | 35.95 |
| 85+ | 1284 | 19.14 | 2123 | 10.09 | 1 | 3.23 | 4011 | 13.48 | 476 | 15.56 |
| Mean age (years) (SD) | ||||||||||
| Overall | 76.74 (8.00) | 74.30 (7.12) | 72.39 (5.74) | 75.02 (7.64) | 75.52 (7.80) | |||||
| Female | 77.19 (8.20) | 74.33 (7.23) | 71.10 (4.91) | 75.16 (7.83) | 75.70 (7.99) | |||||
| Male | 75.98 (7.60) | 74.23 (6.86) | 75.10 (6.64) | 74.77 (7.28) | 75.16 (7.40) | |||||
| Depression severity (index diagnosis) | ||||||||||
| Mild | 4361 | 65.01 | 14,954 | 71.06 | 18 | 58.06 | 20,732 | 69.66 | 2127 | 69.51 |
| Moderate | 2124 | 31.66 | 5105 | 24.26 | 12 | 38.71 | 7630 | 25.64 | 730 | 23.86 |
| Severe | 223 | 3.32 | 984 | 4.68 | 1 | 3.23 | 1401 | 4.71 | 203 | 6.63 |
| Depression before age 65 years | ||||||||||
| Yes | 880 | 13.12 | 3220 | 15.30 | 13 | 41.94 | 4349 | 14.61 | 451 | 14.74 |
| Mean BMI in kg/m2 (SD) | 26.80 (4.85) | 26.62 (4.65) | 25.76 (4.15) | 26.48 (4.70) | 26.21 (4.64) | |||||
| Smoking | ||||||||||
| Non-smoker | 3588 | 59.36 | 11,891 | 59.17 | 14 | 48.28 | 16,332 | 57.41 | 1753 | 60.51 |
| Ex-smoker | 1343 | 22.22 | 4487 | 22.33 | 7 | 24.14 | 6536 | 22.98 | 606 | 20.92 |
| Current smoker | 1,113 | 18.41 | 3720 | 18.51 | 8 | 27.59 | 5580 | 19.61 | 538 | 18.57 |
| Comorbidities | ||||||||||
| CHD | ||||||||||
| Yes | 1581 | 23.57 | 3655 | 17.37 | 4 | 12.90 | 6128 | 20.59 | 595 | 19.44 |
| Diabetes | ||||||||||
| Yes | 1003 | 14.95 | 1857 | 8.82 | 3 | 9.68 | 3023 | 10.16 | 271 | 8.86 |
| Hypertension | ||||||||||
| Yes | 2739 | 40.83 | 7600 | 36.12 | 7 | 22.58 | 12,083 | 40.60 | 1189 | 38.86 |
| Stroke/TIA | ||||||||||
| Yes | 770 | 11.48 | 1788 | 8.50 | 1 | 3.23 | 3535 | 11.88 | 336 | 10.98 |
| Any cancer | ||||||||||
| Yes | 572 | 8.53 | 1545 | 7.34 | 3 | 9.68 | 2666 | 8.96 | 235 | 7.68 |
| Dementia | ||||||||||
| Yes | 215 | 3.21 | 171 | 0.81 | 0 | 0.00 | 629 | 2.11 | 74 | 2.42 |
| Epilepsy/seizures | ||||||||||
| Yes | 144 | 2.15 | 282 | 1.34 | 0 | 0.00 | 468 | 1.57 | 56 | 1.83 |
| Parkinson’s disease | ||||||||||
| Yes | 111 | 1.65 | 216 | 1.03 | 1 | 3.23 | 471 | 1.58 | 66 | 2.16 |
| Hypothyroidism | ||||||||||
| Yes | 495 | 7.38 | 1288 | 6.12 | 1 | 3.23 | 1979 | 6.65 | 186 | 6.08 |
| Obsessive–compulsive disorder | ||||||||||
| Yes | 10 | 0.15 | 45 | 0.21 | 0 | 0.00 | 56 | 0.19 | 8 | 0.26 |
| Medications | ||||||||||
| Anticonvulsants | ||||||||||
| Yes | 165 | 2.46 | 604 | 2.87 | 0 | 0.00 | 790 | 2.65 | 102 | 3.33 |
| Antihypertensives | ||||||||||
| Yes | 3205 | 47.78 | 10,077 | 47.89 | 11 | 35.48 | 15,483 | 52.02 | 1528 | 49.93 |
| Antipsychotic drugs | ||||||||||
| Yes | 410 | 6.11 | 1951 | 9.27 | 3 | 9.68 | 2576 | 8.66 | 382 | 12.48 |
| Aspirin | ||||||||||
| Yes | 2104 | 31.37 | 5365 | 25.50 | 3 | 9.68 | 9497 | 31.91 | 867 | 28.33 |
| Hypnotics/anxiolytics | ||||||||||
| Yes | 866 | 12.91 | 5582 | 26.53 | 10 | 32.26 | 7076 | 23.77 | 813 | 26.57 |
| Lithium | ||||||||||
| Yes | 54 | 0.81 | 38 | 0.18 | 1 | 3.23 | 35 | 0.12 | 19 | 0.62 |
| NSAIDs | ||||||||||
| Yes | 3262 | 48.63 | 12,596 | 59.86 | 14 | 45.16 | 17,065 | 57.34 | 1617 | 52.84 |
| Statins | ||||||||||
| Yes | 1428 | 21.29 | 2538 | 12.06 | 0 | 0.00 | 5784 | 19.43 | 510 | 16.67 |
Antidepressant dose
Table 6 shows the doses prescribed in terms of DDDs by antidepressant class. Dose could not be calculated for 160,170 (11.5%) of the 1,398,359 prescriptions issued during the study period either because dosing directions were not recorded or were unclear or for certain drugs a DDD value was not available. Prescribed doses tended to be lowest for TCAs, with 70.0% of prescriptions being for ≤ 0.5 DDD, compared with 13.8% for SSRIs. Doses prescribed were highest for MAOIs.
| DDD prescribed | Antidepressant class | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCA | MAOI | SSRI | Other | |||||||
| n | % | n | % | n | % | n | % | n | % | |
| ≤ 0.5 | 250,208 | 69.97 | 214 | 12.10 | 96,870 | 13.80 | 27,912 | 19.20 | 375,204 | 31.09 |
| > 0.5/≤ 1 | 75,922 | 21.23 | 601 | 33.99 | 505,093 | 71.95 | 77,286 | 53.16 | 658,902 | 54.60 |
| > 1/≤ 1.5 | 26,139 | 7.31 | 81 | 4.58 | 25,037 | 3.57 | 33,511 | 23.05 | 84,768 | 7.02 |
| > 1.5 | 5308 | 1.48 | 872 | 49.32 | 75,021 | 10.69 | 6664 | 4.58 | 87,865 | 7.28 |
| Total | 357,577 | 1768 | 702,021 | 145,373 | 1,206,739 | |||||
Table 7 summarises doses prescribed for the 11 most commonly prescribed drugs. The median doses were below the DDD values for all of the TCAs, except lofepramine.
| Antidepressant drug | DDDs | Dose prescribed (mg/day) | |||
|---|---|---|---|---|---|
| Median | IQR | Minimum | Maximum | ||
| Amitriptyline hydrochloride (TCA) | 75 | 25 | 20 to 50 | 5 | 225 |
| Dosulepin hydrochloride (TCA) | 150 | 75 | 50 to 75 | 13 | 300 |
| Lofepramine (TCA) | 105 | 140 | 70 to 140 | 35 | 280 |
| Trazodone hydrochloride (TCA) | 300 | 100 | 50 to 150 | 25 | 600 |
| Citalopram hydrobromide (SSRI) | 20 | 20 | 10 to 20 | 5 | 80 |
| Escitalopram (SSRI) | 10 | 10 | 10 to 10 | 3 | 50 |
| Fluoxetine hydrochloride (SSRI) | 20 | 20 | 20 to 20 | 10 | 180 |
| Paroxetine hydrochloride (SSRI) | 20 | 20 | 20 to 20 | 5 | 90 |
| Sertraline hydrochloride (SSRI) | 50 | 50 | 50 to 100 | 25 | 300 |
| Mirtazapine (other) | 30 | 30 | 15 to 30 | 8 | 105 |
| Venlafaxine hydrochloride (other) | 100 | 75 | 75 to 150 | 19 | 450 |
Total number and duration of prescriptions for each antidepressant class
Table 8 shows the number of prescriptions received by patients for each antidepressant class and the median total duration of treatment, both for the whole follow-up period and for the first year of treatment. Of the 29,085 patients who had one or more prescriptions for a TCA during follow-up, one-quarter had only one TCA prescription; this proportion was the same among the 10,485 patients with prescriptions for other antidepressants, whereas 20.2% of the 42,575 patients who had one or more prescriptions for an SSRI had only one SSRI prescription during follow-up. Among the patients with one or more TCA prescriptions, the median duration of use during follow-up was 127 days; it was 117 days for MAOIs, 206 days for SSRIs and 172 days for the group of other antidepressants. In the first year of treatment the median duration of use was lowest for TCAs and highest for MAOIs and the group of other antidepressants.
| Antidepressant class | Other | |||||||
|---|---|---|---|---|---|---|---|---|
| TCA | MAOI | SSRI | ||||||
| n | % | n | % | n | % | n | % | |
| Total no. of prescriptions for each class in follow-up | ||||||||
| 1 | 7338 | 25.23 | 25 | 23.15 | 8597 | 20.19 | 2645 | 25.23 |
| 2–3 | 5980 | 20.56 | 23 | 21.30 | 7215 | 16.95 | 1553 | 14.81 |
| 4–6 | 3782 | 13.00 | 17 | 15.74 | 5221 | 12.26 | 1198 | 11.43 |
| 6–12 | 3148 | 10.82 | 10 | 9.26 | 5060 | 11.88 | 1102 | 10.51 |
| 13–24 | 3517 | 12.09 | 11 | 10.19 | 6686 | 15.70 | 1586 | 15.13 |
| 25–36 | 1677 | 5.77 | 3 | 2.78 | 3414 | 8.02 | 770 | 7.34 |
| 37+ | 3643 | 12.53 | 19 | 17.59 | 6382 | 14.99 | 1631 | 15.56 |
| Total patients | 29,085 | 100.00 | 108 | 100.00 | 42,575 | 100.00 | 10,485 | 100.00 |
| Total duration of prescriptions during follow-up (days) | ||||||||
| Median (IQR) | 127 (46 to 504) | 117 (52 to 532) | 206 (56 to 672) | 172 (45 to 629) | ||||
| No. of prescriptions in the first year of treatment for each classa | ||||||||
| 1 | 7178 | 36.51 | 8 | 25.81 | 7146 | 27.35 | 901 | 33.48 |
| 2–3 | 4628 | 23.54 | 7 | 22.58 | 4699 | 17.99 | 449 | 16.69 |
| 4–6 | 2842 | 14.46 | 5 | 16.13 | 4042 | 15.47 | 367 | 13.64 |
| 6–12 | 3417 | 17.38 | 10 | 32.26 | 6459 | 24.72 | 553 | 20.55 |
| 13+ | 1595 | 8.11 | 1 | 3.23 | 3780 | 14.47 | 421 | 15.64 |
| Total patients | 19,660 | 100.00 | 31 | 100.00 | 26,126 | 100.00 | 2,691 | 100.00 |
| Total duration of prescriptions during the first year of treatment (days)a | ||||||||
| Median (IQR) | 84 (28 to 224) | 224 (56 to 300) | 174 (56 to 343) | 224 (56 to 364) | ||||
Changes between antidepressant classes
Table 9 shows whether patients had prescriptions from only one class, or changed to another class during follow-up, according to the class of the first antidepressant prescribed. For example, in patients whose first antidepressant was a TCA, then 41.9% had prescriptions only for TCAs during follow-up and 58.1% also had prescriptions from other classes of antidepressants. In patients whose first prescription was for an SSRI, then 67.7% had prescriptions only for SSRIs during follow-up, and 32.3% had prescriptions for other antidepressants. Among those patients who changed from a TCA, the majority of patients changed to an SSRI (10,797, 88.3%). Among patients who changed from an SSRI, 6521 (67.8%) changed to a TCA and 3080 (32.0%) changed to a drug from the group of other antidepressants, and among those patientswho changed from the group of other antidepressants, 541 (33.7%) changed to a TCA and 1062 (66.2%) changed to an SSRI.
| Class of first antidepressant drug prescribed | Had prescriptions from another class during follow-up | Total | |||
|---|---|---|---|---|---|
| No change | Changed | ||||
| n | Row % | n | Row % | n | |
| TCA | 8811 | 41.87 | 12,232 | 58.13 | 21,043 |
| MAOI | 9 | 29.03 | 22 | 70.97 | 31 |
| SSRI | 20,148 | 67.69 | 9615 | 32.31 | 29,763 |
| Other | 1456 | 47.58 | 1604 | 52.42 | 3060 |
| Total | 30,424 | 56.45 | 23,473 | 43.55 | 53,897 |
These differences may reflect, in part, differing amounts of follow-up between the classes, as TCAs were more likely to be prescribed as a first antidepressant earlier in the study period. An additional analysis was therefore carried out looking at changes within 1 year restricted to patients who had at least 1 year’s follow-up (Table 10). For patients who were prescribed a TCA as their first antidepressant, 20.1% had prescriptions for other classes of antidepressants within 1 year of their first TCA prescription. Among patients whose first prescription was an SSRI, 15.7% had prescriptions for other classes of antidepressants within 1 year of their first prescription. The highest rate of switching occurred in the group receiving other antidepressants, of whom 30.4% had prescriptions for other antidepressant classes during their first year of treatment. Among those patients who changed from a TCA within 1 year, the majority changed to an SSRI (3446, 87.4%). Among those patients who changed from an SSRI, 2717 (66.3%) changed to a TCA and 1371 (33.5%) changed to a drug from the group of other antidepressants. Among those patients who changed from the group of other antidepressants, 280 (34.5%) changed to a TCA and 532 (65.5%) to an SSRI.
| Class of first antidepressant drug prescribed | Had prescriptions from another class within 1 year | Total | |||
|---|---|---|---|---|---|
| No switch within 1 year | Switch within 1 year | ||||
| n | Row % | n | Row % | n | |
| TCA | 15,653 | 79.87 | 3944 | 20.13 | 19,597 |
| MAOI | 26 | 83.87 | 5 | 16.13 | 31 |
| SSRI | 21,970 | 84.29 | 4096 | 15.71 | 26,066 |
| Other | 1859 | 69.60 | 812 | 30.40 | 2671 |
| Total | 39,508 | 81.69 | 8,857 | 18.31 | 48,365 |
Table 11 distinguishes patients who did not switch class, but had only one prescription in their first year of treatment. Among patients who were prescribed a TCA as their first antidepressant, 27.6% had only one prescription in the year and 20.1% had prescriptions for other classes of antidepressants during the year. This compares with 20.8% and 15.7%, respectively, for SSRIs and 19.7% and 30.4%, respectively, for other antidepressants. Among all patients who switched classes within the first year, 52% switched after only one prescription. The proportions who switched classes within the first year of treatment were similar in male and female patients, and by age group (data not shown).
| Class of first antidepressant drug prescribed | No switch (two or more prescriptions) | No switch (only one prescription) | Switch | Total | |||
|---|---|---|---|---|---|---|---|
| n | Row % | n | Row % | n | Row % | n | |
| TCA | 10,237 | 52.24 | 5416 | 27.64 | 3944 | 20.13 | 19,597 |
| MAOI | 20 | 64.52 | 6 | 19.35 | 5 | 16.13 | 31 |
| SSRI | 16,538 | 63.45 | 5,432 | 20.84 | 4096 | 15.71 | 26,066 |
| Other | 1332 | 49.87 | 527 | 19.73 | 812 | 30.40 | 2671 |
| Total | 28,127 | 58.16 | 11,381 | 23.53 | 8857 | 18.31 | 48,365 |
Table 12 shows whether patients switched or only had one prescription within their first year of treatment by individual drug. The proportion of patients who did not switch, but only had one prescription in the first year of treatment was the highest for amitriptyline hydrochloride (31.3%) and the lowest for mirtazapine (16.8%). The proportion of patients who switched from the first drug they were prescribed within a year was the highest for trazodone hydrochloride (34.7%) and lofepramine (32.9%) and the lowest for citalopram hydrobromide (21.7%).
| First drug prescribed | No switch (two or more prescriptions) | No switch (only one prescription) | Switch | Total | |||
|---|---|---|---|---|---|---|---|
| n | Row % | n | Row % | n | Row % | n | |
| TCAs | |||||||
| Amitriptyline hydrochloride | 3894 | 44.41 | 2740 | 31.25 | 2134 | 24.34 | 8768 |
| Dosulepin hydrochloride | 3329 | 52.24 | 1534 | 24.07 | 1510 | 23.69 | 6373 |
| Lofepramine | 1021 | 40.04 | 691 | 27.10 | 838 | 32.86 | 2550 |
| Trazodone hydrochloride | 319 | 45.70 | 137 | 19.63 | 242 | 34.67 | 698 |
| SSRIs | |||||||
| Citalopram hydrobromide | 5498 | 57.82 | 1944 | 20.45 | 2066 | 21.73 | 9508 |
| Escitalopram | 587 | 52.98 | 237 | 21.39 | 284 | 25.63 | 1108 |
| Fluoxetine hydrochloride | 4827 | 54.23 | 1937 | 21.76 | 2137 | 24.01 | 8901 |
| Paroxetine hydrochloride | 2209 | 55.71 | 770 | 19.42 | 986 | 24.87 | 3965 |
| Sertraline hydrochloride | 1349 | 53.38 | 529 | 20.93 | 649 | 25.68 | 2527 |
| Others | |||||||
| Mirtazapine | 473 | 57.06 | 139 | 16.77 | 217 | 26.18 | 829 |
| Venlafaxine hydrochloride | 528 | 53.77 | 191 | 19.45 | 263 | 26.78 | 982 |
| All other antidepressants | |||||||
| 882 | 40.91 | 532 | 24.68 | 742 | 34.42 | 2156 | |
| Total | 24,916 | 51.52 | 11,381 | 23.53 | 12,068 | 24.95 | 48,365 |
Practice variation in antidepressant prescribing
Table 13 shows variation in practice prescribing by antidepressant class and for the 11 most commonly prescribed drugs across the 570 practices included in the study. The median number of study patients in each practice was 77 (IQR 40 to 131) with a range of 1 to 436. Across practices the median proportion of TCA prescriptions out of all prescriptions for antidepressant drugs was 30.3%, but this ranged from 0% to 100% (IQR 22.6% to 39.4%). The median percentage of SSRI prescriptions was 54.9%, but this also ranged from 0% to 100% (IQR 47.2% to 62.9%). There was considerable variation between practices for all of the individual drugs.
| Percentage of total antidepressant prescriptions | ||||||
|---|---|---|---|---|---|---|
| Median | IQR | Minimum | Maximum | Mean | SD | |
| Antidepressant class | ||||||
| TCAs | 30.29 | 22.60 to 39.41 | 0.00 | 100.00 | 31.21 | 13.48 |
| MAOIs | 0.00 | 0.00 to 0.00 | 0.00 | 13.25 | 0.16 | 0.93 |
| SSRIs | 54.94 | 47.23 to 62.86 | 0.00 | 100.00 | 54.46 | 13.34 |
| Other class | 12.19 | 7.95 to 18.24 | 0.00 | 93.75 | 14.17 | 10.25 |
| Antidepressant drugs | ||||||
| Amitriptyline hydrochloride (TCA) | 11.71 | 7.28 to 17.45 | 0.00 | 72.46 | 13.39 | 9.06 |
| Dosulepin hydrochloride (TCA) | 7.50 | 2.87 to 15.25 | 0.00 | 100.00 | 10.10 | 9.92 |
| Lofepramine (TCA) | 1.87 | 0.48 to 4.07 | 0.00 | 38.83 | 3.09 | 3.89 |
| Trazodone hydrochloride (TCA) | 0.65 | 0.00 to 2.88 | 0.00 | 33.38 | 2.25 | 3.91 |
| Citalopram hydrobromide (SSRI) | 20.87 | 13.15 to 28.72 | 0.00 | 59.74 | 21.59 | 11.51 |
| Escitalopram (SSRI) | 1.09 | 0.05 to 3.55 | 0.00 | 100.00 | 3.13 | 6.85 |
| Fluoxetine hydrochloride (SSRI) | 12.97 | 7.29 to 19.24 | 0.00 | 60.98 | 14.13 | 9.11 |
| Paroxetine hydrochloride (SSRI) | 6.97 | 3.55 to 12.07 | 0.00 | 46.12 | 8.68 | 7.30 |
| Sertraline hydrochloride (SSRI) | 4.64 | 1.43 to 9.91 | 0.00 | 57.25 | 6.76 | 7.27 |
| Mirtazapine (other) | 4.65 | 2.18 to 8.26 | 0.00 | 61.54 | 6.20 | 6.34 |
| Venlafaxine hydrochloride (other) | 5.14 | 1.82 to 8.69 | 0.00 | 87.50 | 6.63 | 7.58 |
| All others | 2.67 | 0.91 to 5.37 | 0.00 | 70.68 | 4.04 | 5.10 |
| All antidepressant prescriptions (n) | 1962 | 945 to 3380 | 12 | 12,207 | 2453 | 1979.27 |
Severity of depression by gender and age band
Table 14 shows the level of severity of the initial diagnosis of depression according to age group and gender. The distribution of the severity of depression was similar in all age bands, and in men and women.
| Depression severity | ||||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| n | Row % | n | Row % | n | Row % | |
| Age band at baseline (years) | ||||||
| 65–74 | 22,338 | 71.27 | 7481 | 23.87 | 1522 | 4.86 |
| 75–84 | 14,609 | 67.96 | 5949 | 27.67 | 939 | 4.37 |
| 85+ | 5334 | 67.45 | 2209 | 27.93 | 365 | 4.62 |
| Gender | ||||||
| Female | 28,414 | 70.13 | 10,288 | 25.39 | 1814 | 4.48 |
| Male | 13,867 | 68.55 | 5351 | 26.45 | 1012 | 5.00 |
Follow-up details
Table 15 gives details of person-years of follow-up for the 60,746 patients in the study cohort. The total number of person-years of follow-up was 305,188, with a mean per patient of 5.0 years [standard deviation (SD) 3.3 years] and a median of 4.6 years (IQR 2.2 years to 7.4 years).
| Age band (years) | Male | Female | Total |
|---|---|---|---|
| 65–74 | 38,182 | 84,317 | 122,498 |
| 75–84 | 40,547 | 92,720 | 133,268 |
| 85+ | 12,341 | 37,082 | 49,422 |
| Total | 91,070 | 214,118 | 305,188 |
Table 16 shows the number of patients who had the outcomes of interest during follow-up and the numbers who had these outcomes at baseline. The most common outcome during follow-up was death (29.4% of the cohort), followed by falls (20.2%), fractures (10.1%) and stroke/TIA (9.9%). A total of 43 people committed suicide. Only four patients had antidepressant poisoning recorded during follow-up, so this outcome was excluded from further analysis.
| Outcome | Had outcome at baseline | Had outcome during follow-upa | ||
|---|---|---|---|---|
| n | % | n | % | |
| Deaths (all causes) | – | 17,834 | 29.36 | |
| Sudden cardiac death | – | 84 | 0.14 | |
| Suicide | – | 43 | 0.07 | |
| Attempted suicide/self-harm | 1107 | 1.82 | 507 | 0.85 |
| MI | 4216 | 6.94 | 2376 | 4.20 |
| Stroke/TIA | 6448 | 10.61 | 5369 | 9.89 |
| Epilepsy/seizures | 953 | 1.57 | 505 | 0.84 |
| Upper GI bleeding | 1251 | 2.06 | 1365 | 2.29 |
| Falls | 4979 | 8.20 | 11,251 | 20.18 |
| Fractures | 7839 | 12.90 | 5330 | 10.07 |
| RTAs | 963 | 1.59 | 423 | 0.71 |
| ADRs | 471 | 0.78 | 833 | 1.38 |
| Hyponatraemia | 341 | 0.56 | 1114 | 1.84 |
| Antidepressant poisoning | 4 | 0.01 | 4 | 0.01 |
Results of time-varying analyses for the study outcomes
Results of analyses for all-cause mortality
Incidence rates for all-cause mortality
All 60,746 patients in the cohort contributed to the analyses of overall mortality. In the follow-up period 17,834 (29.4%) of these patients died, giving a crude mortality rate of 584.4 per 10,000 person-years (95% CI 575.9 to 593.0 person-years). Mortality rates were higher in men than in women and increased steeply with increasing age (Table 17).
| Age band (years) | Deaths | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 1571 | 84,317 | 186.3 | 177.3 to 195.8 |
| 75–84 | 4091 | 92,720 | 441.2 | 427.9 to 455.0 |
| 85+ | 4814 | 37,082 | 1298.2 | 1262.1 to 1335.4 |
| 65+ | 10,476 | 214,118 | 489.3 | 480.0 to 498.7 |
| Men | ||||
| 65–74 | 1551 | 38,182 | 406.2 | 386.5 to 426.9 |
| 75–84 | 3425 | 40,547 | 844.7 | 816.9 to 873.5 |
| 85+ | 2382 | 12,341 | 1930.2 | 1854.3 to 2009.3 |
| 65+ | 7358 | 91,070 | 808.0 | 789.7 to 826.6 |
| Both sexes | ||||
| 65–74 | 3122 | 122,498 | 254.9 | 246.1 to 264.0 |
| 75–84 | 7516 | 133,268 | 564.0 | 551.4 to 576.9 |
| 85+ | 7196 | 49,422 | 1456.0 | 1422.8 to 1490.1 |
| 65+ | 17,834 | 305,188 | 584.4 | 575.9 to 593.0 |
Mortality rates by antidepressant class are shown in Table 18. These exclude patients who had taken MAOIs at any time during follow-up, owing to small numbers. The highest rates occurred in patients having combined prescriptions, then in patients taking the group of other antidepressants.
| Antidepressant class | Deaths | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 8210 | 170,864 | 480.5 | 470.2 to 491.0 |
| TCAs | 2337 | 45,957 | 508.5 | 488.3 to 529.6 |
| SSRIs | 5782 | 70,893 | 815.6 | 794.8 to 836.9 |
| Other antidepressants | 1268 | 14,489 | 875.2 | 828.3 to 924.7 |
| Combination of antidepressants | 216 | 2163 | 998.8 | 874.1 to 1141.3 |
Hazard ratios for all-cause mortality
Table 19 shows the HRs for mortality according to antidepressant class, both unadjusted and adjusted for the potential confounding variables listed in the table footnotes. This shows increased HRs for all classes of antidepressant drugs after adjusting for potential confounding variables. There were significant differences between the classes (p < 0.001). The adjusted HR was highest for combined prescriptions, with an 84% increase in mortality rate compared with no antidepressant use, and then the group of other antidepressants in which there was a 66% increase in mortality rate. In a direct comparison with TCAs, the adjusted HRs were 1.32 (95% CI 1.26 to 1.39) for SSRIs and 1.43 (95% CI 1.33 to 1.54) for the group of other antidepressants.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 0.99 | 0.95 to 1.04 | 0.748 | 1.16 | 1.10 to 1.22 | < 0.001 |
| SSRIs | 1.61 | 1.55 to 1.66 | < 0.001 | 1.54 | 1.48 to 1.59 | < 0.001 |
| Other antidepressants | 1.77 | 1.66 to 1.87 | < 0.001 | 1.66 | 1.56 to 1.77 | < 0.001 |
| Combination of antidepressants | 2.02 | 1.76 to 2.31 | < 0.001 | 1.84 | 1.59 to 2.13 | < 0.001 |
The results of the dose analyses (Table 20) show that the mortality rate was significantly increased for all classes at all dose levels except for lower doses of TCAs (≤ 1.0 DDDs), with evidence of a dose–response relationship for TCAs and SSRIs, but not for the group of other antidepressants.
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 1.05 | 0.98 to 1.12 | 0.149 |
| > 0.5 / ≤ 1.0 DDDs | 1.28 | 1.15 to 1.43 | < 0.001 |
| > 1.0 DDDs | 1.43 | 1.22 to 1.66 | < 0.001 |
| Test for trend | < 0.001 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.48 | 1.38 to 1.60 | < 0.001 |
| > 0.5 / ≤ 1.0 DDDs | 1.46 | 1.40 to 1.52 | < 0.001 |
| > 1.0 DDDs | 1.78 | 1.64 to 1.93 | < 0.001 |
| Test for trend | < 0.001 | ||
| Others | |||
| ≤ 0.5 DDDs | 1.76 | 1.55 to 2.01 | < 0.001 |
| > 0.5 / ≤ 1.0 DDDs | 1.67 | 1.52 to 1.83 | < 0.001 |
| > 1.0 DDDs | 1.77 | 1.55 to 2.04 | < 0.001 |
| Test for trend | 0.696 | ||
Table 21 shows the effects of duration of use and time since stopping an antidepressant on mortality rates. For TCAs the mortality rate was significantly increased in the first 28 days after starting the drug (adjusted HR 1.24, 95% CI 1.06 to 1.45), but was significantly reduced after 85 days of use (adjusted HR 0.60, 95% CI 0.56 to 0.66). The HR was significantly increased in the first 84 days after starting SSRIs, but was significantly reduced after 85 days of use (adjusted HR 0.75, 95% CI 0.71 to 0.80). For the group of other antidepressants the mortality rate was significantly increased in the first 28 days after starting (adjusted HR 2.10, 95% CI 1.73 to 2.56), but was significantly reduced after 85 days of use (adjusted HR 0.81, 95% CI 0.73 to 0.90). The HRs were significantly increased throughout the 182 days after stopping TCAs, SSRIs and the group of other antidepressants, but decreased with time.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.24 | 1.06 to 1.45 | 0.008 |
| 29–84 days | 0.86 | 0.70 to 1.04 | 0.120 |
| 85+ days | 0.60 | 0.56 to 0.66 | < 0.001 |
| Stopped 1–28 days | 6.80 | 6.27 to 7.37 | < 0.001 |
| Stopped 29–84 days | 2.75 | 2.50 to 3.03 | < 0.001 |
| Stopped 85–182 days | 1.29 | 1.15 to 1.45 | < 0.001 |
| SSRIs | |||
| 1–28 days | 1.86 | 1.66 to 2.07 | < 0.001 |
| 29–84 days | 1.41 | 1.26 to 1.57 | < 0.001 |
| 85+ days | 0.75 | 0.71 to 0.80 | < 0.001 |
| Stopped 1–28 days | 11.33 | 10.71 to 11.98 | < 0.001 |
| Stopped 29–84 days | 4.45 | 4.17 to 4.76 | < 0.001 |
| Stopped 85–182 days | 1.87 | 1.72 to 2.03 | < 0.001 |
| Others | |||
| 1–28 days | 2.10 | 1.73 to 2.56 | < 0.001 |
| 29–84 days | 1.15 | 0.90 to 1.48 | 0.259 |
| 85+ days | 0.81 | 0.73 to 0.90 | < 0.001 |
| Stopped 1–28 days | 13.46 | 12.11 to 14.96 | < 0.001 |
| Stopped –84 days | 5.34 | 4.67 to 6.11 | < 0.001 |
| Stopped 85–182 days | 2.07 | 1.72 to 2.50 | < 0.001 |
There were significant interactions for mortality between antidepressant class and age, gender, and use of NSAIDs and antihypertensive drugs at baseline (all p < 0.01). The HRs for all classes of antidepressant drugs were slightly higher for people aged 65–74 years than for those aged 75 and over; the HR for SSRIs was somewhat higher in men than in women, whereas the HR for other antidepressants was slightly lower in men. The HRs for all classes of antidepressant drugs were higher in people taking NSAIDs at baseline and the HR for the class of other antidepressant drugs was slightly higher in people taking antihypertensive drugs at baseline (data not shown).
Table 22 shows the HRs for mortality according to individual antidepressant drugs, both unadjusted and adjusted for the potential confounding variables listed in the table footnotes. There were significant differences between the different drugs (p < 0.001), with significantly increased HRs for all the antidepressant drugs except for dosulepin after adjusting for confounding variables. The highest HRs among these 11 drugs were for trazodone, which was associated with an 82% increased mortality rate compared with no antidepressant use (adjusted HR 1.82, 95% CI 1.59 to 2.08) and mirtazapine (adjusted HR 1.76, 95% CI 1.62 to 1.91).
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 0.94 | 0.88 to 1.00 | 0.065 | 1.10 | 1.03 to 1.18 | 0.008 |
| Dosulepin hydrochloride (TCA) | 0.80 | 0.73 to 0.86 | < 0.001 | 1.03 | 0.95 to 1.13 | 0.469 |
| Lofepramine (TCA) | 1.48 | 1.33 to 1.64 | < 0.001 | 1.51 | 1.35 to 1.69 | < 0.001 |
| Trazodone hydrochloride (TCA) | 2.12 | 1.88 to 2.40 | < 0.001 | 1.82 | 1.59 to 2.08 | < 0.001 |
| Citalopram hydrobromide (SSRI) | 1.76 | 1.68 to 1.84 | < 0.001 | 1.55 | 1.48 to 1.63 | < 0.001 |
| Escitalopram (SSRI) | 1.44 | 1.27 to 1.64 | < 0.001 | 1.45 | 1.27 to 1.66 | < 0.001 |
| Fluoxetine hydrochloride (SSRI) | 1.64 | 1.55 to 1.72 | < 0.001 | 1.66 | 1.57 to 1.76 | < 0.001 |
| Paroxetine hydrochloride (SSRI) | 1.16 | 1.08 to 1.26 | < 0.001 | 1.24 | 1.14 to 1.35 | < 0.001 |
| Sertraline hydrochloride (SSRI) | 1.58 | 1.45 to 1.71 | < 0.001 | 1.47 | 1.35 to 1.61 | < 0.001 |
| Mirtazapine (other) | 2.07 | 1.90 to 2.24 | < 0.001 | 1.76 | 1.62 to 1.91 | < 0.001 |
| Venlafaxine hydrochloride (other) | 1.67 | 1.54 to 1.83 | < 0.001 | 1.66 | 1.51 to 1.82 | < 0.001 |
Absolute risk of death
Table 23 shows the absolute risk of mortality over 1, 2 and 5 years of treatment, using the adjusted HRs presented in Tables 19 and 22, which were significant at p < 0.01, to calculate adjusted absolute risks and numbers of extra deaths per 10,000 treated patients compared with no antidepressant treatment by antidepressant class and individual drug. The results by antidepressant class show that the group of other antidepressants is associated with the highest absolute risks and numbers of extra cases. For individual drugs, trazodone and mirtazapine are associated with the highest number of additional deaths, assuming causality.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 7.04 | 10.85 | 21.66 | |||
| TCAs | 8.12 | 12.48 | 24.68 | 109 | 163 | 302 |
| SSRIs | 10.61 | 16.18 | 31.29 | 357 | 533 | 962 |
| Other antidepressants | 11.43 | 17.39 | 33.37 | 439 | 654 | 1171 |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 7.04 | 10.85 | 21.66 | |||
| Amitriptyline hydrochloride (TCA) | 7.72 | 11.88 | 23.58 | 69 | 103 | 191 |
| Dosulepin hydrochloride (TCA) | 7.26 | 11.18 | 22.28 | NS | NS | NS |
| Lofepramine (TCA) | 10.43 | 15.91 | 30.81 | 339 | 506 | 915 |
| Trazodone hydrochloride (TCA) | 12.44 | 18.87 | 35.88 | 540 | 801 | 1422 |
| Citalopram hydrobromide (SSRI) | 10.69 | 16.29 | 31.48 | 365 | 544 | 982 |
| Escitalopram (SSRI) | 10.06 | 15.37 | 29.86 | 302 | 451 | 819 |
| Fluoxetine hydrochloride (SSRI) | 11.42 | 17.38 | 33.36 | 439 | 653 | 1169 |
| Paroxetine hydrochloride (SSRI) | 8.68 | 13.32 | 26.20 | 164 | 247 | 454 |
| Sertraline hydrochloride (SSRI) | 10.20 | 15.57 | 30.23 | 316 | 472 | 856 |
| Mirtazapine (other) | 12.05 | 18.29 | 34.91 | 501 | 744 | 1324 |
| Venlafaxine hydrochloride (other) | 11.40 | 17.35 | 33.30 | 436 | 649 | 1164 |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 24. For all classes of antidepressants, mortality rates were significantly increased throughout use and during the 182-day period after stopping; however, the self-controlled case-series analysis may produce unreliable results when the outcome under investigation is a fatal one. 57
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 3.99 | 3.43 to 4.65 | < 0.001 |
| 29–84 days | 5.22 | 4.62 to 5.91 | < 0.001 |
| 85+ days | 8.43 | 7.50 to 9.48 | < 0.001 |
| Stopped 1–28 days | 6.01 | 5.29 to 6.82 | < 0.001 |
| Stopped 29–84 days | 3.66 | 3.26 to 4.12 | < 0.001 |
| Stopped 85–182 days | 2.19 | 1.95 to 2.46 | < 0.001 |
| SSRIs | |||
| 1–28 days | 7.87 | 7.10 to 8.72 | < 0.001 |
| 29–84 days | 12.04 | 11.12 to 13.02 | < 0.001 |
| 85+ days | 16.26 | 15.02 to 17.60 | < 0.001 |
| Stopped 1–28 days | 13.29 | 12.20 to 14.47 | < 0.001 |
| Stopped 29–84 days | 6.11 | 5.60 to 6.68 | < 0.001 |
| Stopped 85–182 days | 3.29 | 3.00 to 3.60 | < 0.001 |
| Others | |||
| 1–28 days | 5.99 | 4.79 to 7.51 | < 0.001 |
| 29–84 days | 8.65 | 7.17 to 10.42 | < 0.001 |
| 85+ days | 18.59 | 15.59 to 22.17 | < 0.001 |
| Stopped 1–28 days | 11.75 | 9.63 to 14.34 | < 0.001 |
| Stopped 29–84 days | 5.82 | 4.73 to 7.16 | < 0.001 |
| Stopped 85–182 days | 3.31 | 2.66 to 4.12 | < 0.001 |
Summary of results for all-cause mortality
Mortality rates were significantly increased for all classes of antidepressants compared with no use of antidepressants, with highest rates for the class of other antidepressant drugs. There was some evidence of a dose–response relationship for TCAs and SSRIs, but not for the group of other antidepressants. Among the 11 most commonly prescribed antidepressant drugs, trazodone and mirtazapine were associated with the highest HRs. Mortality rates tended to be highest in the first 28 days of starting an antidepressant, but were reduced after 85 days of use. Rates remained increased during 182 days after stopping antidepressants.
Results of analyses for sudden cardiac death
Incidence rates of sudden cardiac death
All 60,746 patients in the study cohort contributed to the analyses of sudden cardiac death. During the follow-up period, 84 (0.14%) of these patients had a sudden cardiac death, giving a crude incidence rate of 2.8 per 10,000 person-years (95% CI 2.2 to 3.4 per 10,000 person-years). Rates were higher in men than in women and tended to increase with increasing age (Table 25).
| Age band (years) | Sudden cardiac deaths | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 9 | 84,317 | 1.1 | 0.6 to 2.1 |
| 75–84 | 17 | 92,720 | 1.8 | 1.1 to 3.0 |
| 85+ | 18 | 37,082 | 4.9 | 3.1 to 7.7 |
| 65+ | 44 | 214,118 | 2.1 | 1.5 to 2.8 |
| Men | ||||
| 65–74 | 9 | 38,182 | 2.4 | 1.2 to 4.5 |
| 75–84 | 26 | 40,547 | 6.4 | 4.4 to 9.4 |
| 85+ | 5 | 12,341 | 4.1 | 1.7 to 9.7 |
| 65+ | 40 | 91,070 | 4.4 | 3.2 to 6.0 |
| Both sexes | ||||
| 65–74 | 18 | 122,498 | 1.5 | 0.9 to 2.3 |
| 75–84 | 43 | 133,268 | 3.2 | 2.4 to 4.4 |
| 85+ | 23 | 49,422 | 4.7 | 3.1 to 7.0 |
| 65+ | 84 | 305,188 | 2.8 | 2.2 to 3.4 |
Sudden cardiac death rates by antidepressant class are shown in Table 26. These rates are not adjusted for patient characteristics and exclude patients who had taken MAOIs during follow-up. The highest sudden cardiac death rate occurred in patients taking the group of other antidepressants than in patients having combined prescriptions.
| Antidepressant class | Sudden cardiac deaths | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 40 | 170,863 | 2.3 | 1.7 to 3.2 |
| TCAs | 14 | 45,957 | 3.1 | 1.8 to 5.1 |
| SSRIs | 21 | 70,893 | 3.0 | 1.9 to 4.5 |
| Other antidepressants | 8 | 14,489 | 5.5 | 2.8 to 11.0 |
| Combination of antidepressants | 1 | 2163 | 4.6 | 0.7 to 32.8 |
Hazard ratios for sudden cardiac death
Table 27 shows HRs for sudden cardiac death according to antidepressant class. There was an increased HR for the group of other antidepressant drugs (adjusted HR 2.25, 95% CI 1.05 to 4.83), but this was not statistically significant at p < 0.01. There were no significant differences between the classes (p = 0.50); however, numbers were small. In a direct comparison with TCAs, the adjusted HRs were 0.89 (95% CI 0.45 to 1.75) for SSRIs and 1.66 (95% CI 0.69 to 3.97) for the group of other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 1.25 | 0.67 to 2.33 | 0.475 | 1.36 | 0.73 to 2.53 | 0.333 |
| SSRIs | 1.23 | 0.72 to 2.11 | 0.451 | 1.21 | 0.70 to 2.07 | 0.496 |
| Other antidepressants | 2.30 | 1.07 to 4.92 | 0.032 | 2.25 | 1.05 to 4.83 | 0.036 |
| Combination of antidepressants | 1.99 | 0.27 to 14.5 | 0.496 | 1.91 | 0.26 to 13.92 | 0.523 |
Tests of interaction, and analyses of dose, duration and individual drugs were not carried out for sudden cardiac death owing to small patient numbers.
Absolute risk of sudden cardiac death
Table 28 shows the absolute risk of sudden cardiac death by antidepressant class over 1, 2 and 5 years of treatment. There were no excess risks by class which were significant at p < 0.01.
| Antidepressant class | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Not currently on antidepressants | 0.04 | 0.04 | 0.12 | |||
| TCAs | 0.05 | 0.06 | 0.17 | NS | NS | NS |
| SSRIs | 0.04 | 0.05 | 0.15 | NS | NS | NS |
| Other antidepressants | 0.08 | 0.10 | 0.28 | NS | NS | NS |
Self-controlled case-series analyses
The case-series analyses are not presented for sudden cardiac death owing to small patient numbers.
Summary of results for sudden cardiac death
Sudden cardiac death rates were not significantly increased for any class of antidepressant drugs compared with no use of antidepressant drugs. Numbers were too small to examine interactions or effects of dose, duration or individual drugs.
Results of analyses for suicide
Incidence rates of suicide
All 60,746 patients in the study cohort contributed to the analyses of suicide. During follow-up 43 (0.07%) of these patients committed suicide, giving a crude incidence rate of 1.4 per 10,000 person-years (95% CI 1.0 to 1.9 per 10,000 person-years). Rates were higher in men than in women below the age of 85 years, and there was no clear change in rates with increasing age (Table 29).
| Age band (years) | Suicides | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 5 | 84,316 | 0.6 | 0.3 to 1.4 |
| 75–84 | 9 | 92,719 | 1.0 | 0.5 to 1.9 |
| 85+ | 2 | 37,081 | 0.5 | 0.1 to 2.2 |
| 65+ | 16 | 214,117 | 0.8 | 0.5 to 1.2 |
| Men | ||||
| 65–74 | 10 | 38,181 | 2.6 | 1.4 to 4.9 |
| 75–84 | 17 | 40,547 | 4.2 | 2.6 to 6.7 |
| 85+ | 0 | 12,341 | 0.0 | – |
| 65+ | 27 | 91,068 | 3.0 | 2.0 to 4.3 |
| Both sexes | ||||
| 65–74 | 15 | 122,497 | 1.2 | 0.7 to 2.0 |
| 75–84 | 26 | 133,266 | 2.0 | 1.3 to 2.9 |
| 85+ | 2 | 49,422 | 0.4 | 0.1 to 1.6 |
| 65+ | 43 | 305,185 | 1.4 | 1.0 to 1.9 |
Suicide incidence rates by antidepressant class are shown in Table 30. These rates are not adjusted for patient characteristics and exclude patients who had taken MAOIs during follow-up. The highest suicide rates occurred in patients taking the group of other antidepressant drugs than in patients having combined prescriptions.
| Antidepressant class | Suicides | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 7 | 170,863 | 0.4 | 0.2 to 0.9 |
| TCAs | 9 | 45,955 | 2.0 | 1.0 to 3.8 |
| SSRIs | 17 | 70,891 | 2.4 | 1.5 to 3.9 |
| Other antidepressants | 8 | 14,489 | 5.5 | 2.8 to 11.0 |
| Combination of antidepressants | 1 | 2163 | 4.6 | 0.7 to 32.8 |
Hazard ratios for suicide
Table 31 shows the HRs for suicide according to antidepressant class. This shows significantly increased HRs for all classes of antidepressant drugs after adjusting for potential confounding variables. There were no significant differences between the classes (p = 0.16); however, numbers were small. In a direct comparison with TCAs, the adjusted HRs were 1.14 (95% CI 0.51 to 2.57) for SSRIs and 2.64 (95% CI 1.00 to 6.97) for the group of other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 3.91 | 1.43 to 10.69 | 0.008 | 4.27 | 1.56 to 11.70 | 0.005 |
| SSRIs | 4.83 | 1.97 to 11.83 | 0.001 | 4.87 | 1.99 to 11.96 | 0.001 |
| Other antidepressants | 12.97 | 4.69 to 35.89 | < 0.001 | 11.29 | 4.06 to 31.35 | < 0.001 |
| Combination of antidepressants | 13.03 | 1.60 to 106.29 | 0.017 | 12.11 | 1.48 to 98.81 | 0.020 |
Tests of interaction and analyses of dose, duration and individual drugs were not carried out for suicide owing to small patient numbers.
Absolute risk of suicide
Table 32 shows the absolute risk of suicide by antidepressant class over 1, 2 and 5 years of treatment. Absolute risks and numbers of extra cases are greatest for the group of other antidepressant drugs.
| Antidepressant class | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Not currently on antidepressants | 0.01 | 0.02 | 0.03 | |||
| TCAs | 0.04 | 0.08 | 0.13 | 3 | 6 | 10 |
| SSRIs | 0.04 | 0.09 | 0.14 | 3 | 7 | 11 |
| Other antidepressants | 0.09 | 0.20 | 0.33 | 8 | 18 | 30 |
Self-controlled case-series analyses
The case-series analyses are not presented for suicide owing to small patient numbers.
Summary of results for suicide
All classes of antidepressant drugs were associated with significantly increased suicide rates compared with no current use of antidepressant drugs. Numbers were too small to examine interactions or effects of dose, duration or individual drugs.
Results of analyses for attempted suicide/self-harm
Incidence rates of attempted suicide/self-harm
A total of 59,639 patients were included in the analyses of incident attempted suicide/self-harm during follow-up, excluding the 1107 patients who had attempted suicide/self-harm by the baseline date. During the follow-up period, 507 (0.85%) of these patients attempted suicide/self-harm, giving a crude incidence rate of 17.0 per 10,000 person-years (95% CI 15.6 to 18.6 per 10,000 person-years). The rates were higher in men than in women and there was little change in rates with increasing age (Table 33).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 125 | 81,505 | 15.3 | 12.9 to 18.3 |
| 75–84 | 116 | 90,955 | 12.8 | 10.6 to 15.3 |
| 85+ | 50 | 36,568 | 13.7 | 10.4 to 18.0 |
| 65+ | 291 | 209,028 | 13.9 | 12.4 to 15.6 |
| Men | ||||
| 65–74 | 95 | 37,241 | 25.5 | 20.9 to 31.2 |
| 75–84 | 96 | 39,791 | 24.1 | 19.8 to 29.5 |
| 85+ | 25 | 12,120 | 20.6 | 13.9 to 30.5 |
| 65+ | 216 | 89,152 | 24.2 | 21.2 to 27.7 |
| Both sexes | ||||
| 65–74 | 220 | 118,746 | 18.5 | 16.2 to 21.1 |
| 75–84 | 212 | 130,746 | 16.2 | 14.2 to 18.6 |
| 85+ | 75 | 48,688 | 15.4 | 12.3 to 19.3 |
| 65+ | 507 | 298,180 | 17.0 | 15.6 to 18.6 |
Attempted suicide/self-harm incidence rates by antidepressant class are shown in Table 34. These rates exclude patients who had taken MAOIs during follow-up. The highest attempted suicide/self-harm rates occurred in patients having prescriptions for the group of other antidepressant drugs, followed by patients having combined prescriptions.
| Antidepressant class | Events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 150 | 167,507 | 9.0 | 7.6 to 10.5 |
| TCAs | 89 | 44,890 | 19.8 | 16.1 to 24.4 |
| SSRIs | 178 | 69,255 | 25.7 | 22.2 to 29.8 |
| Other antidepressants | 79 | 13,683 | 57.7 | 46.3 to 72.0 |
| Combination of antidepressants | 8 | 2059 | 38.9 | 19.4 to 77.7 |
Hazard ratios for attempted suicide/self-harm
Table 35 shows the HRs for attempted suicide/self-harm according to antidepressant class. This shows increased HRs for all classes of antidepressant drugs, with only small changes after adjusting for potential confounding variables. There were significant differences between the classes (p < 0.001). The HR was highest for the group of other antidepressant drugs, with more than a fivefold increase in attempted suicide/self-harm rate compared with no antidepressant use, and for combined prescriptions, which were associated with a more than fourfold increase in attempted suicide/self-harm rate. In a direct comparison with TCAs, there were adjusted HRs of 1.27 (95% CI 0.97 to 1.66) for SSRIs and 3.04 (95% CI 2.21 to 4.17) for the group of other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 1.67 | 1.27 to 2.18 | < 0.001 | 1.70 | 1.28 to 2.25 | < 0.001 |
| SSRIs | 2.22 | 1.77 to 2.78 | < 0.001 | 2.16 | 1.71 to 2.71 | < 0.001 |
| Other antidepressants | 5.80 | 4.41 to 7.63 | < 0.001 | 5.16 | 3.90 to 6.83 | < 0.001 |
| Combination of antidepressants | 4.60 | 2.25 to 9.37 | < 0.001 | 4.15 | 2.03 to 8.48 | < 0.001 |
The results of the dose analyses are shown in Table 36. This shows that, although the risk of attempted suicide/self-harm tended to increase as dose increased in all classes, the tests for trend were not statistically significant.
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 1.51 | 1.07 to 2.15 | 0.020 |
| > 0.5 / ≤ 1.0 DDDs | 1.76 | 1.01 to 3.06 | 0.046 |
| > 1.0 DDDs | 2.03 | 0.94 to 4.35 | 0.070 |
| Test for trend | 0.282 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 2.19 | 1.41 to 3.41 | 0.001 |
| > 0.5 / ≤ 1.0 DDDs | 1.87 | 1.44 to 2.44 | < 0.001 |
| > 1.0 DDDs | 2.93 | 1.87 to 4.60 | < 0.001 |
| Test for trend | 0.133 | ||
| Others | |||
| ≤ 0.5 DDDs | 4.14 | 2.23 to 7.69 | < 0.001 |
| > 0.5 / ≤ 1.0 DDDs | 5.49 | 3.77 to 8.01 | < 0.001 |
| > 1.0 DDDs | 6.63 | 3.99 to 11.03 | < 0.001 |
| Test for trend | 0.110 | ||
Table 37 shows the effects of duration of use and time since stopping an antidepressant on attempted suicide/self-harm, according to antidepressant class. For TCAs the attempted suicide/self-harm rate was highest in the first 28 days after starting the drug, with no significant increase in risk after 29 days of use. The HR was significantly increased in the first 28 days after stopping TCAs. For SSRIs the attempted suicide/self-harm rate was highest in the first 28 days after starting the drug, but was not significantly increased after 85 days of use. The HR was significantly increased in the first 84 days after stopping SSRIs, but not between 85 and 182 days after stopping. For the group of other antidepressant drugs, the attempted suicide/self-harm rate was significantly increased throughout use and was significantly increased in the first 84 days after stopping, with some indication of an increase between 85 and 182 days after stopping.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 7.11 | 4.66 to 10.83 | < 0.001 |
| 29–84 days | 1.69 | 0.81 to 3.53 | 0.164 |
| 85+ days | 1.10 | 0.72 to 1.68 | 0.659 |
| Stopped 1–28 days | 4.14 | 2.35 to 7.31 | < 0.001 |
| Stopped 29–84 days | 1.31 | 0.63 to 2.72 | 0.464 |
| Stopped 85–182 days | 2.05 | 1.18 to 3.54 | 0.011 |
| SSRIs | |||
| 1–28 days | 12.31 | 8.84 to 17.14 | < 0.001 |
| 29–84 days | 1.96 | 1.11 to 3.46 | 0.020 |
| 85+ days | 0.98 | 0.68 to 1.40 | 0.894 |
| Stopped 1–28 days | 5.91 | 3.76 to 9.30 | < 0.001 |
| Stopped 29–84 days | 3.51 | 2.24 to 5.48 | < 0.001 |
| Stopped 85–182 days | 1.04 | 0.53 to 2.07 | 0.905 |
| Others | |||
| 1–28 days | 17.12 | 10.58 to 27.71 | < 0.001 |
| 29–84 days | 6.63 | 3.34 to 13.16 | < 0.001 |
| 85+ days | 3.99 | 2.69 to 5.92 | < 0.001 |
| Stopped 1–28 days | 17.26 | 9.26 to 32.16 | < 0.001 |
| Stopped 29–84 days | 5.87 | 2.57 to 13.37 | < 0.001 |
| Stopped 85–182 days | 3.36 | 1.24 to 9.13 | 0.017 |
There was a significant interaction between antidepressant class and CHD at baseline (p = 0.006), with higher HRs for attempted suicide in patients without CHD at baseline (adjusted HRs: TCAs 1.99, 95% CI 1.46 to 2.72; SSRIs 2.38, 95% CI 1.82 to 3.10; other antidepressant drugs 6.40, 95% CI 4.72 to 8.69) than in patients with CHD at baseline (adjusted HRs: TCAs 0.98, 95% CI 0.49 to 1.97; SSRIs 1.64, 95% CI 1.01 to 2.69; other antidepressant drugs 1.76, 95% CI 0.73 to 4.27). There were no other significant interactions for attempted suicide/self-harm.
There were significantly (p < 0.01) increased HRs for all individual antidepressant drugs (except for amitriptyline, escitalopram and paroxetine) after adjusting for potential confounding variables (Table 38). There were significant differences in the attempted suicide/self-harm rates between the different antidepressant drugs (p < 0.001), with the highest HRs for mirtazapine, which was associated with a more than sixfold increase in the attempted suicide/self-harm rate compared with no antidepressant use, and trazodone and venlafaxine, which were associated with a more than fourfold increase.
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.03 | 0.67 to 1.60 | 0.879 | 1.07 | 0.69 to 1.67 | 0.761 |
| Dosulepin hydrochloride (TCA) | 1.94 | 1.34 to 2.82 | 0.001 | 1.87 | 1.26 to 2.77 | 0.002 |
| Lofepramine (TCA) | 2.49 | 1.45 to 4.25 | 0.001 | 2.58 | 1.48 to 4.50 | 0.001 |
| Trazodone hydrochloride (TCA) | 4.56 | 2.53 to 8.22 | < 0.001 | 4.70 | 2.60 to 8.49 | < 0.001 |
| Citalopram hydrobromide (SSRI) | 2.88 | 2.20 to 3.77 | < 0.001 | 2.70 | 2.04 to 3.58 | < 0.001 |
| Escitalopram (SSRI) | 2.18 | 1.07 to 4.45 | 0.032 | 2.08 | 1.02 to 4.27 | 0.045 |
| Fluoxetine hydrochloride (SSRI) | 2.06 | 1.49 to 2.86 | < 0.001 | 2.08 | 1.49 to 2.90 | < 0.001 |
| Paroxetine hydrochloride (SSRI) | 1.14 | 0.67 to 1.94 | 0.628 | 1.14 | 0.66 to 1.99 | 0.640 |
| Sertraline hydrochloride (SSRI) | 1.99 | 1.20 to 3.30 | 0.007 | 2.07 | 1.25 to 3.44 | 0.005 |
| Mirtazapine (other) | 7.03 | 4.92 to 10.05 | < 0.001 | 6.11 | 4.24 to 8.80 | < 0.001 |
| Venlafaxine hydrochloride (other) | 5.25 | 3.58 to 7.70 | < 0.001 | 4.60 | 3.11 to 6.80 | < 0.001 |
Absolute risk of attempted suicide/self-harm
Table 39 shows the absolute risks of attempted suicide/self-harm over 1, 2 and 5 years of treatment and the number of extra cases for significant associations at p < 0.01. The results by antidepressant class show that the group of other antidepressant drugs are associated with the highest absolute risks and numbers of extra cases. For individual drugs, mirtazapine, trazodone and venlafaxine are associated with the highest number of additional cases of attempted suicide/self-harm.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 0.25 | 0.36 | 0.55 | |||
| TCAs | 0.43 | 0.62 | 0.93 | 18 | 25 | 38 |
| SSRIs | 0.55 | 0.78 | 1.18 | 29 | 42 | 63 |
| Other antidepressants | 1.30 | 1.86 | 2.81 | 105 | 150 | 226 |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 0.25 | 0.35 | 0.54 | |||
| Amitriptyline hydrochloride (TCA) | 0.27 | 0.39 | 0.59 | NS | NS | NS |
| Dosulepin hydrochloride (TCA) | 0.48 | 0.67 | 1.02 | 23 | 32 | 49 |
| Lofepramine (TCA) | 0.66 | 0.93 | 1.41 | 41 | 58 | 88 |
| Trazodone hydrochloride (TCA) | 1.20 | 1.69 | 2.56 | 95 | 134 | 203 |
| Citalopram hydrobromide (SSRI) | 0.68 | 0.96 | 1.45 | 43 | 60 | 92 |
| Escitalopram (SSRI) | 0.47 | 0.66 | 1.01 | NS | NS | NS |
| Fluoxetine hydrochloride (SSRI) | 0.53 | 0.75 | 1.14 | 28 | 40 | 61 |
| Paroxetine hydrochloride (SSRI) | 0.29 | 0.41 | 0.63 | NS | NS | NS |
| Sertraline hydrochloride (SSRI) | 0.53 | 0.75 | 1.14 | 28 | 40 | 60 |
| Mirtazapine (other) | 1.56 | 2.20 | 3.33 | 131 | 185 | 279 |
| Venlafaxine hydrochloride (other) | 1.17 | 1.65 | 2.50 | 92 | 130 | 197 |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 40. The attempted suicide/self-harm rate was significantly increased during the first 28 days of use for TCAs and the group of other antidepressant drugs, and during the first 84 days of use for SSRIs. The attempted suicide/self-harm rate was significantly increased in the first 28 days and 85–182 days after stopping for TCAs, and in the first 84 days after stopping for SSRIs.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 6.00 | 3.74 to 9.60 | < 0.001 |
| 29–84 days | 2.02 | 0.80 to 5.11 | 0.136 |
| 85+ days | 1.89 | 0.85 to 4.21 | 0.120 |
| Stopped 1–28 days | 2.87 | 1.57 to 5.25 | 0.001 |
| Stopped 29–84 days | 1.47 | 0.75 to 2.89 | 0.257 |
| Stopped 85–182 days | 2.14 | 1.28 to 3.55 | 0.003 |
| SSRIs | |||
| 1–28 days | 12.77 | 9.12 to 17.88 | < 0.001 |
| 29–84 days | 3.47 | 1.93 to 6.25 | < 0.001 |
| 85+ days | 1.05 | 0.59 to 1.88 | 0.872 |
| Stopped 1–28 days | 4.58 | 2.78 to 7.56 | < 0.001 |
| Stopped 29–84 days | 3.17 | 1.90 to 5.29 | < 0.001 |
| Stopped 85–182 days | 1.90 | 1.08 to 3.36 | 0.026 |
| Others | |||
| 1–28 days | 3.20 | 1.68 to 6.06 | < 0.001 |
| 29–84 days | 0.98 | 0.35 to 2.74 | 0.970 |
| 85+ days | 0.38 | 0.16 to 0.86 | 0.020 |
| Stopped 1–28 days | 2.42 | 1.07 to 5.48 | 0.035 |
| Stopped 29–84 days | 1.56 | 0.61 to 4.01 | 0.355 |
| Stopped 85–182 days | 0.39 | 0.09 to 1.69 | 0.211 |
Summary of results for attempted suicide/self-harm
All classes of antidepressant drug were associated with an increased risk of attempted suicide/self-harm risk compared with no current use of antidepressant drugs. The risk varied by antidepressant class, being higher for the group of other antidepressant drugs. The risk tended to increase as dose increased in all classes. There were increased HRs for all 11 most commonly prescribed antidepressant drugs, except for amitriptyline, escitalopram and paroxetine. Mirtazapine, trazodone and venlafaxine were associated with the highest HRs. Attempted suicide/self-harm rates tended to be highest in the first 28 days of starting an antidepressant, and also in the first 28 days after stopping. There were inconsistencies in the patterns of risk between the cohort analyses and case-series analysis, suggesting some indication bias for the group of other antidepressant drugs.
Results of analyses for myocardial infarction outcome
Incidence rates of myocardial infarction
A total of 56,530 patients were included in the analyses of incident MI during follow-up, excluding the 4216 patients who had had a MI by the baseline date. During the follow-up period, 2376 (4.2%) of these patients had an incident MI, giving a crude incidence rate of 84.3 per 10,000 person-years (95% CI 80.9 to 87.7 per 10,000 person-years). The rates were higher in men than women and increased with increasing age (Table 41).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 355 | 80,872 | 43.9 | 39.6 to 48.7 |
| 75–84 | 665 | 87,270 | 76.2 | 70.6 to 82.2 |
| 85+ | 427 | 34,580 | 123.5 | 112.3 to 135.8 |
| 65+ | 1447 | 202,723 | 71.4 | 67.8 to 75.2 |
| Men | ||||
| 65–74 | 309 | 33,561 | 92.1 | 82.4 to 102.9 |
| 75–84 | 435 | 34,964 | 124.4 | 113.3 to 136.7 |
| 85+ | 185 | 10,735 | 172.3 | 149.2 to 199.0 |
| 65+ | 929 | 79,260 | 117.2 | 109.9 to 125.0 |
| Both sexes | ||||
| 65–74 | 664 | 114,434 | 58.0 | 53.8 to 62.6 |
| 75–84 | 1100 | 122,234 | 90.0 | 84.8 to 95.5 |
| 85+ | 612 | 45,316 | 135.1 | 124.8 to 146.2 |
| 65+ | 2376 | 281,983 | 84.3 | 80.9 to 87.7 |
Myocardial infarction incidence rates by antidepressant class are shown in Table 42. These rates exclude patients who had taken MAOIs during follow-up. The highest MI rates occurred in patients taking SSRIs.
| Antidepressant class | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 1264 | 157,723 | 80.1 | 75.8 to 84.7 |
| TCAs | 362 | 43,054 | 84.1 | 75.9 to 93.2 |
| SSRIs | 614 | 64,978 | 94.5 | 87.3 to 102.3 |
| Other antidepressants | 110 | 13,469 | 81.7 | 67.8 to 98.5 |
| Combination of antidepressants | 16 | 1974 | 81.1 | 49.7 to 132.3 |
Table 43 shows the HRs for MI according to antidepressant class. This shows HRs were only significantly increased for SSRIs, which were associated with a 15% increase in MI rate compared with no antidepressant use after adjusting for potential confounding variables. The differences between the classes were not, however, statistically significant (p = 0.72). In a direct comparison with TCAs, there were adjusted HRs of 1.06 (95% CI 0.92 to 1.21) for SSRIs and 0.95 (95% CI 0.76 to 1.19) for the group of other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 1.02 | 0.91 to 1.15 | 0.688 | 1.09 | 0.96 to 1.23 | 0.179 |
| SSRIs | 1.16 | 1.05 to 1.27 | 0.004 | 1.15 | 1.04 to 1.27 | 0.008 |
| Other antidepressant drugs | 1.01 | 0.83 to 1.23 | 0.915 | 1.04 | 0.85 to 1.27 | 0.733 |
| Combination of antidepressants | 1.01 | 0.62 to 1.66 | 0.964 | 1.03 | 0.62 to 1.72 | 0.906 |
The results of the dose analyses are shown in Table 44. The risk of MI tended to increase with dose for TCAs and SSRIs; however, the tests for trend were not statistically significant. The risk was significantly increased only for SSRIs at > 1.0 DDDs.
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 1.05 | 0.91 to 1.23 | 0.495 |
| > 0.5 / ≤ 1.0 DDDs | 1.10 | 0.84 to 1.43 | 0.495 |
| > 1.0 DDDs | 1.30 | 0.90 to 1.88 | 0.166 |
| TCAs: test for trend | 0.323 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.17 | 0.93 to 1.47 | 0.186 |
| > 0.5 / ≤ 1.0 DDDs | 1.12 | 1.00 to 1.26 | 0.048 |
| > 1.0 DDDs | 1.37 | 1.08 to 1.73 | 0.009 |
| SSRIs: test for trend | 0.240 | ||
| ≤ 0.5 DDDs | 0.96 | 0.58 to 1.57 | 0.857 |
| Others | |||
| > 0.5 / ≤ 1.0 DDDs | 1.04 | 0.77 to 1.41 | 0.782 |
| > 1.0 DDDs | 1.00 | 0.63 to 1.60 | 0.986 |
| Others: test for trend | 0.598 | ||
Table 45 shows the effects of duration of use and time since stopping an antidepressant on MI risk. For TCAs and the group of other antidepressant drugs there was there no association during the first 84 days of use, but the MI rate was significantly reduced from 85 days after starting medication. For SSRIs the MI rate was highest in the first 28 days after starting medication, but was significantly reduced from 85 days after starting. There was a significant increase in the first 84 days after stopping TCAs, SSRIs and the group of other antidepressant drugs, but not after 85 days.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 0.96 | 0.65 to 1.42 | 0.839 |
| 29–84 days | 0.81 | 0.51 to 1.26 | 0.345 |
| 85+ days | 0.77 | 0.65 to 0.91 | 0.003 |
| Stopped 1–28 days | 3.74 | 2.95 to 4.76 | < 0.001 |
| Stopped 29–84 days | 1.87 | 1.45 to 2.40 | < 0.001 |
| Stopped 85–182 days | 1.07 | 0.82 to 1.41 | 0.600 |
| SSRIs | |||
| 1–28 days | 1.46 | 1.09 to 1.95 | 0.012 |
| 29–84 days | 1.18 | 0.87 to 1.59 | 0.288 |
| 85+ days | 0.71 | 0.61 to 0.81 | < 0.001 |
| Stopped 1–28 days | 5.97 | 5.01 to 7.12 | < 0.001 |
| Stopped 29–84 days | 1.82 | 1.44 to 2.29 | < 0.001 |
| Stopped 85–182 days | 1.02 | 0.79 to 1.31 | 0.902 |
| Others | |||
| 1–28 days | 1.21 | 0.65 to 2.26 | 0.555 |
| 29–84 days | 0.91 | 0.45 to 1.83 | 0.794 |
| 85+ days | 0.63 | 0.47 to 0.85 | 0.003 |
| Stopped 1–28 days | 4.69 | 3.04 to 7.23 | < 0.001 |
| Stopped 29–84 days | 2.53 | 1.59 to 4.04 | < 0.001 |
| Stopped 85–182 days | 0.83 | 0.41 to 1.66 | 0.596 |
There were no significant interactions for MI.
Table 46 shows the HRs for MI for individual antidepressant drugs. The only significant association was for fluoxetine, with a 31% increased rate compared with no antidepressant use; however, overall there were no significant differences between the drugs (p = 0.65).
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.04 | 0.88 to 1.23 | 0.659 | 1.10 | 0.93 to 1.31 | 0.257 |
| Dosulepin hydrochloride (TCA) | 0.99 | 0.82 to 1.20 | 0.915 | 1.07 | 0.88 to 1.29 | 0.527 |
| Lofepramine (TCA) | 1.15 | 0.85 to 1.57 | 0.369 | 1.18 | 0.86 to 1.61 | 0.300 |
| Trazodone hydrochloride (TCA) | 1.06 | 0.68 to 1.65 | 0.784 | 1.04 | 0.66 to 1.64 | 0.864 |
| Citalopram hydrobromide (SSRI) | 1.11 | 0.96 to 1.28 | 0.164 | 1.10 | 0.95 to 1.28 | 0.198 |
| Escitalopram (SSRI) | 1.10 | 0.75 to 1.61 | 0.628 | 1.31 | 0.89 to 1.93 | 0.166 |
| Fluoxetine hydrochloride (SSRI) | 1.30 | 1.11 to 1.51 | 0.001 | 1.31 | 1.12 to 1.53 | 0.001 |
| Paroxetine hydrochloride (SSRI) | 1.17 | 0.96 to 1.43 | 0.120 | 1.10 | 0.90 to 1.36 | 0.347 |
| Sertraline hydrochloride (SSRI) | 0.95 | 0.73 to 1.25 | 0.730 | 0.89 | 0.67 to 1.19 | 0.427 |
| Mirtazapine (other) | 1.06 | 0.79 to 1.42 | 0.698 | 1.11 | 0.82 to 1.49 | 0.494 |
| Venlafaxine hydrochloride (other) | 1.04 | 0.79 to 1.36 | 0.801 | 1.04 | 0.78 to 1.39 | 0.779 |
Absolute risk of myocardial infarction
Table 47 shows the absolute risk of MI over 1, 2 and 5 years of treatment by antidepressant class and individual drug. The results by class show that the absolute risks and numbers of extra cases are slightly increased for SSRIs.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 1.00 | 1.83 | 4.06 | |||
| TCAs | 1.09 | 1.99 | 4.41 | NS | NS | NS |
| SSRIs | 1.15 | 2.10 | 4.65 | 15 | 27 | 59 |
| Other antidepressants | 1.04 | 1.90 | 4.20 | NS | NS | NS |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 1.00 | 1.83 | 4.06 | |||
| Amitriptyline hydrochloride (TCA) | 1.11 | 2.02 | 4.47 | NS | NS | NS |
| Dosulepin hydrochloride (TCA) | 1.07 | 1.95 | 4.32 | NS | NS | NS |
| Lofepramine (TCA) | 1.18 | 2.16 | 4.77 | NS | NS | NS |
| Trazodone hydrochloride (TCA) | 1.04 | 1.90 | 4.22 | NS | NS | NS |
| Citalopram hydrobromide (SSRI) | 1.11 | 2.02 | 4.46 | NS | NS | NS |
| Escitalopram (SSRI) | 1.32 | 2.40 | 5.29 | NS | NS | NS |
| Fluoxetine hydrochloride (SSRI) | 1.31 | 2.39 | 5.29 | 31 | 56 | 123 |
| Paroxetine hydrochloride (SSRI) | 1.11 | 2.02 | 4.47 | NS | NS | NS |
| Sertraline hydrochloride (SSRI) | 0.89 | 1.63 | 3.62 | NS | NS | NS |
| Mirtazapine (other) | 1.11 | 2.03 | 4.49 | NS | NS | NS |
| Venlafaxine hydrochloride (other) | 1.05 | 1.91 | 4.23 | NS | NS | NS |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 48. For TCAs there were no significant associations during use. The MI rate was significantly increased in the first 28 days after starting an SSRI, but not after 28 days of use. For the group of other antidepressant drugs there was some indication of a reduced risk after 28 days of use. For TCAs, rates were significantly increased in the first 28 days after stopping, and for SSRIs and other antidepressant drugs they were significantly increased in the first 84 days after stopping.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.19 | 0.83 to 1.71 | 0.342 |
| 29–84 days | 1.11 | 0.72 to 1.70 | 0.634 |
| 85+ days | 0.87 | 0.62 to 1.22 | 0.430 |
| Stopped 1–28 days | 2.49 | 1.87 to 3.33 | < 0.001 |
| Stopped 29–84 days | 1.28 | 0.94 to 1.76 | 0.123 |
| Stopped 85–182 days | 1.14 | 0.86 to 1.51 | 0.350 |
| SSRIs | |||
| 1–28 days | 1.44 | 1.10 to 1.88 | 0.008 |
| 29–84 days | 1.01 | 0.73 to 1.39 | 0.955 |
| 85+ days | 1.03 | 0.84 to 1.26 | 0.774 |
| Stopped 1–28 days | 3.49 | 2.80 to 4.34 | < 0.001 |
| Stopped 29–84 days | 1.76 | 1.38 to 2.24 | < 0.001 |
| Stopped 85–182 days | 1.20 | 0.93 to 1.55 | 0.150 |
| Others | |||
| 1–28 days | 1.11 | 0.56 to 2.18 | 0.766 |
| 29–84 days | 0.55 | 0.20 to 1.49 | 0.241 |
| 85+ days | 0.54 | 0.32 to 0.93 | 0.026 |
| Stopped 1–28 days | 3.98 | 2.45 to 6.48 | < 0.001 |
| Stopped 29–84 days | 2.80 | 1.72 to 4.56 | < 0.001 |
| Stopped 85–182 days | 1.14 | 0.60 to 2.17 | 0.679 |
Summary of results for myocardial infarction
Myocardial infarction risk did not differ significantly between the classes of antidepressant drugs, although it was significantly increased for SSRIs compared with no use of antidepressants drugs. Among the most commonly prescribed drugs, only fluoxetine was associated with a significantly increased HR, but overall there were no significant differences between the drugs. MI rates tended to be highest in the first 28 days of starting an SSRI antidepressant and also in the first 28 days after stopping.
Results of analyses for stroke/transient ischaemic attack
Incidence rates of stroke/transient ischaemic attack
A total of 54,298 patients were included in the analyses of stroke/TIA during follow-up, excluding the 6448 patients who had had a stroke/TIA by the baseline date. During the follow-up period, 5369 (9.9%) of these patients had an incident stroke/TIA, giving a crude incidence rate of 202.3 per 10,000 person-years 95% CI (197.0 to 207.8 per 10,000 person-years). Rates were higher in men than in women and increased with increasing age (Table 49).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 820 | 78,380 | 104.6 | 97.7 to 112.0 |
| 75–84 | 1662 | 80,959 | 205.3 | 195.7 to 215.4 |
| 85+ | 1126 | 30,176 | 373.1 | 352.0 to 395.6 |
| 65+ | 3608 | 189,515 | 190.4 | 184.3 to 196.7 |
| Men | ||||
| 65–74 | 511 | 33,473 | 152.7 | 140.0 to 166.5 |
| 75–84 | 881 | 32,763 | 268.9 | 251.7 to 287.3 |
| 85+ | 369 | 9659 | 382.0 | 345.0 to 423.1 |
| 65+ | 1761 | 75,895 | 232.0 | 221.4 to 243.1 |
| Both sexes | ||||
| 65–74 | 1331 | 111,853 | 119.0 | 112.8 to 125.6 |
| 75–84 | 2543 | 113,722 | 223.6 | 215.1 to 232.5 |
| 85+ | 1495 | 39,835 | 375.3 | 356.8 to 394.8 |
| 65+ | 5369 | 265,410 | 202.3 | 197.0 to 207.8 |
Stroke/TIA incidence rates by antidepressant class are shown in Table 50. These exclude patients who had taken MAOIs during follow-up. The highest rates occurred in patients having combined prescriptions than in patients taking the group of other antidepressant drugs.
| Antidepressant class | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 2811 | 149,821 | 187.6 | 180.8 to 194.7 |
| TCAs | 791 | 40,564 | 195.0 | 181.9 to 209.1 |
| SSRIs | 1384 | 60,109 | 230.3 | 218.4 to 242.7 |
| Other antidepressants | 317 | 12,391 | 255.8 | 229.2 to 285.6 |
| Combination of antidepressants | 48 | 1807 | 265.7 | 200.2 to 352.6 |
Hazard ratios for stroke/transient ischaemic attack
Table 51 shows HRs for stroke/TIA according to antidepressant class. This shows that SSRIs and the group of other antidepressant drugs were associated with significantly increased HRs. There were significant differences between the classes (p < 0.001). The HR was highest for combined prescriptions, with a 42% increase in stroke/TIA rate compared with no antidepressant use, and then for the group of other antidepressant drugs in which there was a 37% increase. In a direct comparison with TCAs, the adjusted HRs were 1.15 (95% CI 1.05 to 1.26) for SSRIs and 1.35 (95% CI 1.18 to 1.54) for the group of other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 1.01 | 0.93 to 1.10 | 0.792 | 1.02 | 0.93 to 1.11 | 0.703 |
| SSRIs | 1.19 | 1.12 to 1.27 | < 0.001 | 1.17 | 1.10 to 1.26 | < 0.001 |
| Other antidepressants | 1.35 | 1.21 to 1.52 | < 0.001 | 1.37 | 1.22 to 1.55 | < 0.001 |
| Combination of antidepressants | 1.45 | 1.09 to 1.92 | 0.011 | 1.42 | 1.05 to 1.91 | 0.022 |
The results of the dose analyses are shown in Table 52. This shows little evidence of dose–response relationships.
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 0.98 | 0.88 to 1.09 | 0.713 |
| > 0.5 / ≤ 1.0 DDDs | 1.03 | 0.86 to 1.24 | 0.727 |
| > 1.0 DDDs | 1.30 | 1.01 to 1.68 | 0.041 |
| Test for trend | 0.143 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.20 | 1.03 to 1.39 | 0.017 |
| > 0.5 / ≤ 1.0 DDDs | 1.14 | 1.05 to 1.23 | 0.001 |
| > 1.0 DDDs | 1.37 | 1.17 to 1.62 | < 0.001 |
| Test for trend | 0.158 | ||
| Others | |||
| ≤ 0.5 DDDs | 1.65 | 1.29 to 2.11 | < 0.001 |
| > 0.5 / ≤ 1.0 DDDs | 1.43 | 1.20 to 1.70 | < 0.001 |
| > 1.0 DDDs | 1.37 | 1.04 to 1.80 | 0.025 |
| Test for trend | 0.233 | ||
Table 53 shows the effects of duration of use and time since stopping an antidepressant on stroke/TIA risk. For TCAs there was no association during the first 84 days of use, but the rate of stroke/TIA was significantly reduced from 85 days after starting the drug. For SSRIs the rate was significantly increased in the first 28 days after starting, but was significantly reduced from 85 days after starting. For the group of other antidepressant drugs, the stroke/TIA rate was significantly increased in the first 28 days after starting. The HR was significantly increased in the first 84 days after stopping TCAs, SSRIs and the group of other antidepressant drugs.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.19 | 0.94 to 1.51 | 0.148 |
| 29–84 days | 0.89 | 0.67 to 1.18 | 0.422 |
| 85+ days | 0.78 | 0.70 to 0.88 | < 0.001 |
| Stopped 1–28 days | 3.05 | 2.56 to 3.63 | < 0.001 |
| Stopped 29–84 days | 1.58 | 1.32 to 1.89 | < 0.001 |
| Stopped 85–182 days | 1.09 | 0.91 to 1.30 | 0.344 |
| SSRIs | |||
| 1–28 days | 1.79 | 1.50 to 2.15 | < 0.001 |
| 29–84 days | 1.15 | 0.94 to 1.42 | 0.169 |
| 85+ days | 0.84 | 0.77 to 0.92 | < 0.001 |
| Stopped 1–28 days | 4.05 | 3.52 to 4.66 | < 0.001 |
| Stopped 29–84 days | 2.00 | 1.73 to 2.33 | < 0.001 |
| Stopped 85–182 days | 1.19 | 1.01 to 1.39 | 0.034 |
| Others | |||
| 1–28 days | 1.87 | 1.33 to 2.64 | < 0.001 |
| 29–84 days | 1.49 | 1.03 to 2.15 | 0.035 |
| 85+ days | 0.92 | 0.77 to 1.09 | 0.335 |
| Stopped 1–28 days | 6.29 | 4.87 to 8.12 | < 0.001 |
| Stopped 29–84 days | 2.52 | 1.83 to 3.46 | < 0.001 |
| Stopped 85–182 days | 1.33 | 0.92 to 1.93 | 0.134 |
There were no significant interactions for stroke/TIA between antidepressant class and age, gender, CHD, hypertension or use of aspirin, NSAIDs, antihypertensive drugs or hypnotics/anxiolytics at baseline.
Table 54 shows the HRs for individual antidepressant drugs. There were significantly increased HRs for citalopram, mirtazapine and venlafaxine (at p < 0.01) after adjusting for potential confounding variables. There were significant differences between the drugs (p < 0.001), with the highest HRs associated with venlafaxine, where the stroke/TIA rate was increased by 51% compared with no antidepressant use, and mirtazapine, where there was a 38% increase.
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.00 | 0.89 to 1.12 | 0.980 | 1.01 | 0.90 to 1.13 | 0.901 |
| Dosulepin hydrochloride (TCA) | 0.90 | 0.79 to 1.03 | 0.124 | 0.95 | 0.83 to 1.09 | 0.487 |
| Lofepramine (TCA) | 1.33 | 1.10 to 1.62 | 0.004 | 1.26 | 1.02 to 1.54 | 0.028 |
| Trazodone hydrochloride (TCA) | 1.34 | 1.02 to 1.75 | 0.034 | 1.10 | 0.82 to 1.48 | 0.523 |
| Citalopram hydrobromide (SSRI) | 1.25 | 1.14 to 1.37 | < 0.001 | 1.22 | 1.11 to 1.34 | < 0.001 |
| Escitalopram (SSRI) | 1.07 | 0.83 to 1.40 | 0.598 | 1.21 | 0.93 to 1.59 | 0.152 |
| Fluoxetine hydrochloride (SSRI) | 1.16 | 1.04 to 1.29 | 0.006 | 1.16 | 1.03 to 1.29 | 0.011 |
| Paroxetine hydrochloride (SSRI) | 1.10 | 0.96 to 1.26 | 0.173 | 1.08 | 0.93 to 1.24 | 0.314 |
| Sertraline hydrochloride (SSRI) | 1.29 | 1.10 to 1.52 | 0.001 | 1.22 | 1.03 to 1.44 | 0.021 |
| Mirtazapine (other) | 1.42 | 1.19 to 1.68 | < 0.001 | 1.38 | 1.15 to 1.65 | < 0.001 |
| Venlafaxine hydrochloride (other) | 1.45 | 1.23 to 1.70 | < 0.001 | 1.51 | 1.28 to 1.78 | < 0.001 |
Absolute risk of stroke/transient ischaemic attack
Table 55 shows the absolute risk of stroke/TIA over 1, 2 and 5 years of treatment and number of extra cases for the significant associations at p < 0.01. The results show that the group of other antidepressant drugs is associated with the highest absolute risks and number of extra cases. Of the individual drugs, venlafaxine and mirtazapine are associated with the highest number of additional cases.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 2.23 | 4.04 | 9.09 | |||
| TCAs | 2.26 | 4.10 | 9.23 | NS | NS | NS |
| SSRIs | 2.61 | 4.72 | 10.57 | 38 | 68 | 148 |
| Other antidepressants | 3.04 | 5.49 | 12.24 | 81 | 146 | 316 |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 2.23 | 4.04 | 9.09 | |||
| Amitriptyline hydrochloride (TCA) | 2.24 | 4.07 | 9.15 | NS | NS | NS |
| Dosulepin hydrochloride (TCA) | 2.12 | 3.85 | 8.67 | NS | NS | NS |
| Lofepramine (TCA) | 2.79 | 5.04 | 11.27 | NS | NS | NS |
| Trazodone hydrochloride (TCA) | 2.45 | 4.44 | 9.95 | NS | NS | NS |
| Citalopram hydrobromide (SSRI) | 2.71 | 4.90 | 10.96 | 48 | 86 | 187 |
| Escitalopram (SSRI) | 2.70 | 4.88 | 10.93 | NS | NS | NS |
| Fluoxetine hydrochloride (SSRI) | 2.57 | 4.65 | 10.43 | NS | NS | NS |
| Paroxetine hydrochloride (SSRI) | 2.39 | 4.34 | 9.74 | NS | NS | NS |
| Sertraline hydrochloride (SSRI) | 2.70 | 4.89 | 10.95 | NS | NS | NS |
| Mirtazapine (other) | 3.06 | 5.53 | 12.31 | 83 | 149 | 323 |
| Venlafaxine hydrochloride (other) | 3.34 | 6.03 | 13.40 | 112 | 200 | 431 |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 56. There was a reduction in the stroke/TIA rate after the first 84 days of use of TCAs, but this was not significant at p < 0.01. For SSRIs, the rate was significantly increased in the first 28 days after starting, but was significantly reduced from 85 days after starting treatment. For the group of other antidepressant drugs, there were no significant associations during drug use. For TCAs and SSRIs, rates were significantly increased in the first 84 days after stopping and for the group of other antidepressant drugs rates were significantly increased in the first 28 days after stopping.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.05 | 0.83 to 1.34 | 0.686 |
| 29–84 days | 1.19 | 0.93 to 1.53 | 0.171 |
| 85+ days | 0.79 | 0.65 to 0.96 | 0.020 |
| Stopped 1–28 days | 1.73 | 1.40 to 2.14 | < 0.001 |
| Stopped 29–84 days | 1.35 | 1.11 to 1.64 | 0.002 |
| Stopped 85–182 days | 1.14 | 0.95 to 1.36 | 0.153 |
| SSRIs | |||
| 1–28 days | 1.38 | 1.17 to 1.63 | < 0.001 |
| 29–84 days | 0.98 | 0.80 to 1.18 | 0.806 |
| 85+ days | 0.68 | 0.60 to 0.78 | < 0.001 |
| Stopped 1–28 days | 1.79 | 1.49 to 2.14 | < 0.001 |
| Stopped 29–84 days | 1.42 | 1.20 to 1.68 | < 0.001 |
| Stopped 85–182 days | 1.16 | 1.00 to 1.36 | 0.057 |
| Others | |||
| 1–28 days | 1.37 | 0.93 to 2.03 | 0.114 |
| 29–84 days | 0.82 | 0.50 to 1.36 | 0.450 |
| 85+ days | 0.87 | 0.65 to 1.16 | 0.349 |
| Stopped 1–28 days | 2.36 | 1.62 to 3.43 | < 0.001 |
| Stopped 29–84 days | 1.11 | 0.71 to 1.73 | 0.660 |
| Stopped 85–182 days | 1.24 | 0.86 to 1.80 | 0.250 |
Summary of results for stroke/transient ischaemic attack
Selective serotonin reuptake inhibitors and the group of other antidepressant drugs were associated with a significantly increased stroke/TIA risk compared with no use of antidepressant drugs, but TCAs were not. There was little evidence of a dose–response relationship. Among the most commonly prescribed antidepressant drugs, the highest HRs were associated with venlafaxine and mirtazapine. Rates tended to be highest in the first 28 days of starting an antidepressant and in the first 28 days after stopping. There was an association with a reduced risk of stroke/TIA for TCAs and SSRIs after 85 days of use. There were some differences in the pattern of risks between the cohort and case-series analyses.
Results of analyses for falls
Incidence rates of falls
A total of 55,767 patients were included in the analyses of falls during follow-up, excluding the 4979 patients who had had a fall by the baseline date. During the follow-up period 11,251 (20.2%) of these patients had an incident fall, giving a crude incidence rate of 436.3 per 10,000 person-years (95% CI 428.3 to 444.4 per 10,000 person-years). Rates were higher in women than in men and increased steeply with increasing age (Table 57).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 1777 | 76,536 | 232.2 | 221.6 to 243.2 |
| 75–84 | 3972 | 75,358 | 527.1 | 511.0 to 543.7 |
| 85+ | 2544 | 24,836 | 1024.3 | 985.3 to 1064.9 |
| 65+ | 8293 | 176,730 | 469.3 | 459.3 to 479.5 |
| Men | ||||
| 65–74 | 585 | 36,174 | 161.7 | 149.1 to 175.4 |
| 75–84 | 1502 | 35,613 | 421.8 | 401.0 to 443.6 |
| 85+ | 871 | 9,385 | 928.1 | 868.5 to 991.8 |
| 65+ | 2958 | 81,172 | 364.4 | 351.5 to 377.8 |
| Both sexes | ||||
| 65–74 | 2362 | 112,710 | 209.6 | 201.3 to 218.2 |
| 75–84 | 5474 | 110,971 | 493.3 | 480.4 to 506.5 |
| 85+ | 3415 | 34,221 | 997.9 | 965.0 to 1032.0 |
| 65+ | 11251 | 257,902 | 436.3 | 428.3 to 444.4 |
Falls rates by antidepressant class are shown in Table 58, excluding patients who had taken MAOIs during follow-up. The highest rates occurred in patients having combined prescriptions, followed by patients taking SSRIs.
| Antidepressant class | Events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 5208 | 145,407 | 358.2 | 348.6 to 368.0 |
| TCAs | 1704 | 39,465 | 431.8 | 411.8 to 452.8 |
| SSRIs | 3575 | 58,600 | 610.1 | 590.4 to 630.4 |
| Other antidepressants | 631 | 11,990 | 526.3 | 486.8 to 569.0 |
| Combination of antidepressants | 117 | 1716 | 681.9 | 568.9 to 817.4 |
Hazard ratios for falls
Table 59 shows the HRs for falls according to antidepressant class. This shows significantly increased HRs for all classes of antidepressant drugs, with only small changes after adjusting for potential confounding variables. There were significant differences between the classes (p < 0.001). The HR was highest for combined prescriptions, with a 70% increase in falls rate compared with no antidepressant use, and for SSRIs where there was a 66% increase. In a direct comparison with TCAs, there were adjusted HRs of 1.27 (95% CI 1.20 to 1.35) for SSRIs and 1.07 (95% CI 0.97 to 1.17) for the group of other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 1.20 | 1.14 to 1.27 | < 0.001 | 1.30 | 1.23 to 1.38 | < 0.001 |
| SSRIs | 1.71 | 1.64 to 1.79 | < 0.001 | 1.66 | 1.58 to 1.73 | < 0.001 |
| Other antidepressants | 1.45 | 1.34 to 1.58 | < 0.001 | 1.39 | 1.28 to 1.52 | < 0.001 |
| Combination of antidepressants | 1.80 | 1.50 to 2.16 | < 0.001 | 1.70 | 1.42 to 2.05 | < 0.001 |
Table 60 shows that the fall rate was significantly increased for all classes at all dose levels, with risk tending to increase as dose increased in all classes.
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 1.26 | 1.17 to 1.35 | < 0.001 |
| > 0.5 / ≤ 1.0 DDDs | 1.52 | 1.35 to 1.70 | < 0.001 |
| > 1.0 DDDs | 1.52 | 1.27 to 1.82 | < 0.001 |
| Test for trend | 0.003 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.49 | 1.35 to 1.63 | < 0.001 |
| > 0.5 / ≤ 1.0 DDDs | 1.66 | 1.57 to 1.74 | < 0.001 |
| > 1.0 DDDs | 1.89 | 1.71 to 2.09 | < 0.001 |
| Test for trend | 0.001 | ||
| Others | |||
| ≤ 0.5 DDDs | 1.33 | 1.09 to 1.60 | 0.004 |
| > 0.5 / ≤ 1.0 DDDs | 1.34 | 1.18 to 1.52 | < 0.001 |
| > 1.0 DDDs | 1.82 | 1.53 to 2.15 | < 0.001 |
| Test for trend | 0.040 | ||
Table 61 shows the effects of duration of use and time since stopping an antidepressant on fall risk. For TCAs, SSRIs and the group of other antidepressants, the fall rate was highest in the first 28 days after starting treatment. The HRs were significantly increased in the first 84 days after stopping, but not between 85 and 182 days after stopping across all groups.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.48 | 1.26 to 1.75 | < 0.001 |
| 29–84 days | 1.16 | 0.96 to 1.41 | 0.115 |
| 85+ days | 1.10 | 1.03 to 1.19 | 0.009 |
| Stopped 1–28 days | 3.62 | 3.20 to 4.09 | < 0.001 |
| Stopped 29–84 days | 1.47 | 1.27 to 1.69 | < 0.001 |
| Stopped 85–182 days | 1.08 | 0.94 to 1.24 | 0.266 |
| SSRIs | |||
| 1–28 days | 2.23 | 1.97 to 2.52 | < 0.001 |
| 29–84 days | 2.20 | 1.97 to 2.46 | < 0.001 |
| 85+ days | 1.38 | 1.31 to 1.46 | < 0.001 |
| Stopped 1–28 days | 5.03 | 4.58 to 5.53 | < 0.001 |
| Stopped 29–84 days | 1.47 | 1.29 to 1.66 | < 0.001 |
| Stopped 85–182 days | 1.09 | 0.97 to 1.24 | 0.157 |
| Others | |||
| 1–28 days | 1.86 | 1.45 to 2.38 | < 0.001 |
| 29–84 days | 1.33 | 1.01 to 1.76 | 0.046 |
| 85+ days | 1.12 | 1.00 to 1.25 | 0.051 |
| Stopped 1–28 days | 5.23 | 4.27 to 6.42 | < 0.001 |
| Stopped 29–84 days | 1.71 | 1.29 to 2.26 | < 0.001 |
| Stopped 85–182 days | 0.84 | 0.59 to 1.19 | 0.324 |
There was a significant interaction for falls between antidepressant class and gender (p = 0.002), with slightly higher HRs for men than women across the classes of antidepressant drugs. There were no other significant interactions for falls.
Table 62 shows the HRs for individual antidepressant drugs. This shows that all antidepressant drugs were associated with significantly increased HRs. There were significant differences between the different drugs (p < 0.001). Citalopram, venlafaxine, escitalopram, fluoxetine and sertraline had slightly higher HRs than the other drugs
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.26 | 1.17 to 1.36 | < 0.001 | 1.32 | 1.22 to 1.42 | < 0.001 |
| Dosulepin hydrochloride (TCA) | 1.06 | 0.97 to 1.16 | 0.226 | 1.24 | 1.13 to 1.36 | < 0.001 |
| Lofepramine (TCA) | 1.38 | 1.19 to 1.59 | < 0.001 | 1.34 | 1.15 to 1.55 | < 0.001 |
| Trazodone hydrochloride (TCA) | 1.63 | 1.36 to 1.96 | < 0.001 | 1.55 | 1.29 to 1.87 | < 0.001 |
| Citalopram hydrobromide (SSRI) | 2.00 | 1.89 to 2.11 | < 0.001 | 1.76 | 1.66 to 1.86 | < 0.001 |
| Escitalopram (SSRI) | 1.81 | 1.55 to 2.11 | < 0.001 | 1.66 | 1.42 to 1.94 | < 0.001 |
| Fluoxetine hydrochloride (SSRI) | 1.58 | 1.48 to 1.70 | < 0.001 | 1.64 | 1.52 to 1.76 | < 0.001 |
| Paroxetine hydrochloride (SSRI) | 1.31 | 1.19 to 1.44 | < 0.001 | 1.45 | 1.31 to 1.59 | < 0.001 |
| Sertraline hydrochloride (SSRI) | 1.71 | 1.55 to 1.90 | < 0.001 | 1.63 | 1.46 to 1.81 | < 0.001 |
| Mirtazapine (other) | 1.37 | 1.21 to 1.56 | < 0.001 | 1.19 | 1.05 to 1.36 | 0.009 |
| Venlafaxine hydrochloride (other) | 1.61 | 1.44 to 1.80 | < 0.001 | 1.68 | 1.49 to 1.88 | < 0.001 |
Absolute risk of falls
Table 63 shows the absolute risk of falls over 1, 2 and 5 years of treatment. The results show that SSRIs are associated with the highest absolute risks and number of extra cases. For individual drugs, citalopram, venlafaxine, escitalopram, fluoxetine and sertraline are associated with the highest number of extra cases.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 3.46 | 6.34 | 15.54 | |||
| TCAs | 4.49 | 8.19 | 19.75 | 103 | 184 | 421 |
| SSRIs | 5.67 | 10.28 | 24.38 | 220 | 394 | 884 |
| Other antidepressants | 4.79 | 8.72 | 20.95 | 133 | 238 | 542 |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 3.46 | 6.34 | 15.54 | |||
| Amitriptyline hydrochloride (TCA) | 4.54 | 8.27 | 19.95 | 108 | 193 | 441 |
| Dosulepin hydrochloride (TCA) | 4.28 | 7.80 | 18.89 | 81 | 146 | 335 |
| Lofepramine (TCA) | 4.61 | 8.40 | 20.24 | 115 | 206 | 471 |
| Trazodone hydrochloride (TCA) | 5.32 | 9.67 | 23.05 | 186 | 333 | 751 |
| Citalopram hydrobromide (SSRI) | 6.01 | 10.88 | 25.68 | 255 | 454 | 1015 |
| Escitalopram (SSRI) | 5.70 | 10.33 | 24.50 | 223 | 399 | 896 |
| Fluoxetine hydrochloride (SSRI) | 5.60 | 10.16 | 24.13 | 214 | 382 | 859 |
| Paroxetine hydrochloride (SSRI) | 4.97 | 9.04 | 21.66 | 150 | 270 | 612 |
| Sertraline hydrochloride (SSRI) | 5.57 | 10.11 | 24.01 | 211 | 377 | 847 |
| Mirtazapine (other) | 4.11 | 7.51 | 18.21 | 65 | 116 | 267 |
| Venlafaxine hydrochloride (other) | 5.74 | 10.40 | 24.65 | 227 | 406 | 911 |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 64. The fall rate was significantly increased during all periods of use for all three classes of antidepressants, with SSRIs having the highest rate ratios. For TCAs, rates were significantly increased in the first 84 days after stopping, but for SSRIs rates remained significantly increased during 182 days after stopping. For the group of other antidepressants, there were no significant increases after stopping.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.58 | 1.33 to 1.87 | < 0.001 |
| 29–84 days | 1.75 | 1.46 to 2.11 | < 0.001 |
| 85+ days | 1.60 | 1.39 to 1.85 | < 0.001 |
| Stopped 1–28 days | 1.45 | 1.20 to 1.74 | < 0.001 |
| Stopped 29–84 days | 1.23 | 1.05 to 1.44 | 0.010 |
| Stopped 85–182 days | 1.06 | 0.92 to 1.22 | 0.442 |
| SSRIs | |||
| 1–28 days | 2.65 | 2.37 to 2.97 | < 0.001 |
| 29–84 days | 3.07 | 2.75 to 3.42 | < 0.001 |
| 85+ days | 2.22 | 2.03 to 2.42 | < 0.001 |
| Stopped 1–28 days | 1.88 | 1.62 to 2.18 | < 0.001 |
| Stopped 29–84 days | 1.25 | 1.07 to 1.44 | 0.004 |
| Stopped 85–182 days | 1.24 | 1.09 to 1.40 | 0.001 |
| Others | |||
| 1–28 days | 2.26 | 1.71 to 2.98 | < 0.001 |
| 29–84 days | 1.60 | 1.14 to 2.24 | 0.007 |
| 85+ days | 1.73 | 1.40 to 2.15 | < 0.001 |
| Stopped 1–28 days | 1.47 | 1.00 to 2.16 | 0.051 |
| Stopped 29–84 days | 1.37 | 0.99 to 1.89 | 0.058 |
| Stopped 85–182 days | 0.94 | 0.67 to 1.31 | 0.702 |
Summary of results for falls
All classes of antidepressant drug were associated with significant increases in fall risk, compared with no use of antidepressants. The risk varied by antidepressant class, being higher for SSRIs. The risk tended to increase as dose increased in all classes. All of the most commonly prescribed antidepressant drugs were associated with an increased rate of falls, with citalopram, venlafaxine, escitalopram, fluoxetine and sertraline having slightly higher HRs than the other drugs. Fall rates tended to be highest in the first 28 days after starting an antidepressant and also in the first 28 days after stopping.
Results of analyses for fractures
Incidence rates of fractures
A total of 52,907 patients were included in the analyses of fracture during follow-up, excluding the 7839 patients who had had a fracture by the baseline date. During the follow-up period, 5330 (10.1%) of these patients sustained an incident fracture, giving a crude incidence rate of 210.1 per 10,000 person-years (95% CI 204.5 to 215.8 per 10,000 person-years). Rates were higher in women than in men and increased with increasing age (Table 65).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 1104 | 72,552 | 152.2 | 143.5 to 161.4 |
| 75–84 | 2049 | 73,702 | 278.0 | 266.2 to 290.3 |
| 85+ | 1187 | 26,402 | 449.6 | 424.7 to 475.9 |
| 65+ | 4340 | 172,657 | 251.4 | 244.0 to 259.0 |
| Men | ||||
| 65–74 | 291 | 34,200 | 85.1 | 75.9 to 95.5 |
| 75–84 | 457 | 36,170 | 126.4 | 115.3 to 138.5 |
| 85+ | 242 | 10,700 | 226.2 | 199.4 to 256.5 |
| 65+ | 990 | 81,070 | 122.1 | 114.7 to 130.0 |
| Both sexes | ||||
| 65–74 | 1395 | 106,752 | 130.7 | 124.0 to 137.7 |
| 75–84 | 2506 | 109,872 | 228.1 | 219.3 to 237.2 |
| 85+ | 1429 | 37,102 | 385.2 | 365.7 to 405.7 |
| 65+ | 5330 | 253,726 | 210.1 | 204.5 to 215.8 |
Fracture rates by antidepressant class are shown in Table 66. These exclude patients who had taken MAOIs during follow-up. The highest rates occurred in patients having combined prescriptions.
| Antidepressant class | Events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 2507 | 142,664 | 175.7 | 169.0 to 182.7 |
| TCAs | 809 | 38,575 | 209.7 | 195.8 to 224.7 |
| SSRIs | 1597 | 58,170 | 274.5 | 261.4 to 288.3 |
| Other antidepressants | 341 | 11,883 | 287.0 | 258.1 to 319.1 |
| Combination of antidepressants | 67 | 1737 | 385.7 | 303.6 to 490.1 |
Hazard ratios for fractures
Table 67 shows HRs for fractures according to antidepressant class. This shows significantly increased HRs for all classes of antidepressant drugs, with only small changes after adjusting for potential confounding variables. There were significant differences between the classes (p < 0.001). The HR was highest for combined prescriptions, with more than a doubling of the fracture rate compared with no antidepressant use, and than for the group of other antidepressants, in which there was a 64% increase. In a direct comparison with TCAs, there were adjusted HRs of 1.26 (95% CI 1.15 to 1.37) for SSRIs and 1.31 (95% CI 1.15 to 1.50) for other antidepressants.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 1.23 | 1.13 to 1.33 | < 0.001 | 1.26 | 1.16 to 1.37 | < 0.001 |
| SSRIs | 1.61 | 1.51 to 1.72 | < 0.001 | 1.58 | 1.48 to 1.68 | < 0.001 |
| Other antidepressants | 1.64 | 1.46 to 1.84 | < 0.001 | 1.64 | 1.46 to 1.84 | < 0.001 |
| Combination of antidepressants | 2.11 | 1.65 to 2.69 | < 0.001 | 2.08 | 1.62 to 2.66 | < 0.001 |
The results of the dose analyses (Table 68) show that the risk of fracture was significantly increased for all classes at all dose levels, but there was a significant dose–response relationship only for TCAs.
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 1.16 | 1.04 to 1.28 | 0.006 |
| > 0.5 / ≤ 1.0 DDDs | 1.40 | 1.18 to 1.66 | < 0.001 |
| > 1.0 DDDs | 1.59 | 1.23 to 2.04 | < 0.001 |
| Test for trend | 0.001 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.42 | 1.23 to 1.64 | < 0.001 |
| SSRIs > 0.5 / ≤ 1.0 DDDs | 1.57 | 1.46 to 1.69 | < 0.001 |
| SSRIs > 1.0 DDDs | 1.63 | 1.39 to 1.90 | < 0.001 |
| Test for trend | 0.337 | ||
| Others | |||
| ≤ 0.5 DDDs | 1.44 | 1.10 to 1.89 | 0.008 |
| Others > 0.5 / ≤ 1.0 DDDs | 1.67 | 1.41 to 1.98 | < 0.001 |
| Others > 1.0 DDDs | 2.16 | 1.71 to 2.71 | < 0.001 |
| Test for trend | 0.130 | ||
Table 69 shows the effect of duration and time since stopping an antidepressant on fracture rates. For TCAs the fracture rate was highest in the first 28 days after starting and was not significantly increased after 28 days of use. For SSRIs the rate was significantly increased throughout use. For the group of other antidepressant drugs, the fracture rate was highest in the first 28 days after starting the drug, and was still significantly increased after 85 days of use. The rate was significantly increased in the first 84 days after stopping for TCAs, SSRIs and the group of other antidepressant drugs.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.62 | 1.28 to 2.04 | < 0.001 |
| 29–84 days | 1.19 | 0.91 to 1.56 | 0.210 |
| 85+ days | 1.01 | 0.91 to 1.13 | 0.822 |
| Stopped 1–28 days | 3.68 | 3.09 to 4.38 | < 0.001 |
| Stopped 29–84 days | 1.44 | 1.18 to 1.77 | < 0.001 |
| Stopped 85–182 days | 1.26 | 1.05 to 1.51 | 0.015 |
| SSRIs | |||
| 1–28 days | 1.71 | 1.40 to 2.09 | < 0.001 |
| 29–84 days | 1.76 | 1.47 to 2.11 | < 0.001 |
| 85+ days | 1.29 | 1.19 to 1.40 | < 0.001 |
| Stopped 1–28 days | 5.68 | 4.99 to 6.46 | < 0.001 |
| Stopped 29–84 days | 1.72 | 1.45 to 2.04 | < 0.001 |
| Stopped 85–182 days | 1.06 | 0.89 to 1.27 | 0.520 |
| Others | |||
| 1–28 days | 1.83 | 1.26 to 2.66 | 0.002 |
| 29–84 days | 1.45 | 0.98 to 2.16 | 0.065 |
| 85+ days | 1.37 | 1.18 to 1.59 | < 0.001 |
| Stopped 1–28 days | 6.74 | 5.18 to 8.77 | < 0.001 |
| Stopped 29–84 days | 1.82 | 1.23 to 2.70 | 0.003 |
| Stopped 85–182 days | 1.32 | 0.88 to 1.97 | 0.180 |
There were no significant interactions for fractures.
Table 70 shows the HRs for individual antidepressant drugs. There were significantly increased HRs (at p < 0.01) for all antidepressant drugs, except trazodone and escitalopram, after adjusting for potential confounding variables. There were significant differences between the drugs (p < 0.001), with venlafaxine, citalopram and sertraline having the highest HRs.
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.21 | 1.08 to 1.35 | 0.001 | 1.22 | 1.09 to 1.36 | 0.001 |
| Dosulepin hydrochloride (TCA) | 1.13 | 0.99 to 1.28 | 0.071 | 1.23 | 1.07 to 1.40 | 0.003 |
| Lofepramine (TCA) | 1.53 | 1.26 to 1.87 | < 0.001 | 1.46 | 1.19 to 1.80 | < 0.001 |
| Trazodone hydrochloride (TCA) | 1.01 | 0.72 to 1.40 | 0.965 | 0.97 | 0.70 to 1.35 | 0.848 |
| Citalopram hydrobromide (SSRI) | 1.78 | 1.63 to 1.93 | < 0.001 | 1.62 | 1.48 to 1.77 | < 0.001 |
| Escitalopram (SSRI) | 1.40 | 1.09 to 1.79 | 0.008 | 1.29 | 1.00 to 1.65 | 0.049 |
| Fluoxetine hydrochloride (SSRI) | 1.48 | 1.33 to 1.64 | < 0.001 | 1.58 | 1.42 to 1.75 | < 0.001 |
| Paroxetine hydrochloride (SSRI) | 1.34 | 1.17 to 1.53 | < 0.001 | 1.46 | 1.27 to 1.68 | < 0.001 |
| Sertraline hydrochloride (SSRI) | 1.70 | 1.46 to 1.97 | < 0.001 | 1.60 | 1.37 to 1.87 | < 0.001 |
| Mirtazapine (other) | 1.57 | 1.32 to 1.87 | < 0.001 | 1.46 | 1.23 to 1.74 | < 0.001 |
| Venlafaxine hydrochloride (other) | 1.77 | 1.52 to 2.06 | < 0.001 | 1.87 | 1.60 to 2.19 | < 0.001 |
Absolute risk of fracture
Table 71 shows the absolute risk of fracture over 1, 2 and 5 years of treatment and numbers of extra cases for the significant associations at p < 0.01. The results by class show that the group of other antidepressant drugs is associated with the highest absolute risks and number of extra cases. Among the individual drugs, venlafaxine is associated with the highest number of additional cases.
| Antidepressant class | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Not currently on antidepressants | 1.76 | 3.26 | 8.06 | |||
| TCAs | 2.18 | 4.03 | 9.90 | 42 | 77 | 184 |
| SSRIs | 2.74 | 5.05 | 12.31 | 98 | 179 | 425 |
| Other antidepressants | 2.85 | 5.26 | 12.79 | 109 | 200 | 473 |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 1.76 | 3.26 | 8.06 | |||
| Amitriptyline hydrochloride (TCA) | 2.14 | 3.96 | 9.72 | 38 | 69 | 166 |
| Dosulepin hydrochloride (TCA) | 2.15 | 3.99 | 9.80 | 40 | 73 | 174 |
| Lofepramine (TCA) | 2.56 | 4.73 | 11.56 | 80 | 147 | 350 |
| Trazodone hydrochloride (TCA) | 1.70 | 3.16 | 7.81 | NS | NS | NS |
| Citalopram hydrobromide (SSRI) | 2.83 | 5.23 | 12.71 | 107 | 196 | 465 |
| Escitalopram (SSRI) | 2.26 | 4.18 | 10.25 | NS | NS | NS |
| Fluoxetine hydrochloride (SSRI) | 2.76 | 5.10 | 12.42 | 100 | 184 | 436 |
| Paroxetine hydrochloride (SSRI) | 2.56 | 4.74 | 11.57 | 80 | 147 | 351 |
| Sertraline hydrochloride (SSRI) | 2.80 | 5.17 | 12.58 | 104 | 191 | 452 |
| Mirtazapine (other) | 2.56 | 4.74 | 11.57 | 80 | 147 | 351 |
| Venlafaxine hydrochloride (other) | 3.26 | 6.01 | 14.53 | 150 | 275 | 647 |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 72. For TCAs, the rates were significantly increased during the first 84 days of use, but not after 85 days. Rates were increased throughout all periods of SSRI use. For the group of other antidepressant drugs, rates were significantly increased only from 85 days after starting treatment. Rates decreased with time after stopping TCAs and SSRIs, but remained elevated for other antidepressant drugs.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.64 | 1.31 to 2.05 | < 0.001 |
| 29–84 days | 1.70 | 1.33 to 2.17 | < 0.001 |
| 85+ days | 1.19 | 0.97 to 1.46 | 0.098 |
| Stopped 1–28 days | 1.63 | 1.27 to 2.09 | < 0.001 |
| Stopped 29–84 days | 1.20 | 0.96 to 1.51 | 0.108 |
| Stopped 85–182 days | 1.27 | 1.06 to 1.53 | 0.011 |
| SSRIs | |||
| 1–28 days | 1.52 | 1.25 to 1.83 | < 0.001 |
| 29–84 days | 1.98 | 1.66 to 2.35 | < 0.001 |
| 85+ days | 1.69 | 1.49 to 1.92 | < 0.001 |
| Stopped 1–28 days | 1.71 | 1.39 to 2.12 | < 0.001 |
| Stopped 29–84 days | 1.29 | 1.06 to 1.57 | 0.011 |
| Stopped 85–182 days | 1.02 | 0.85 to 1.24 | 0.800 |
| Others | |||
| 1–28 days | 1.71 | 1.12 to 2.62 | 0.014 |
| 29–84 days | 1.22 | 0.73 to 2.02 | 0.447 |
| 85+ days | 1.63 | 1.22 to 2.17 | 0.001 |
| Stopped 1–28 days | 1.78 | 1.08 to 2.94 | 0.025 |
| Stopped 29–84 days | 1.17 | 0.71 to 1.93 | 0.545 |
| Stopped 85–182 days | 1.87 | 1.32 to 2.65 | < 0.001 |
Summary of results for fractures
All classes of antidepressant drug were associated with a significantly increased fracture risk, compared with no use of antidepressant drugs. The risk varied by antidepressant class, being higher for SSRIs and the group of other antidepressant drugs than for with TCAs; however, there was a significant dose–response relationship only for TCAs. All of the most commonly prescribed antidepressant drugs, except trazodone and escitalopram, were associated with an increased fracture risk, with venlafaxine, citalopram and sertraline having the highest rates. Rates tended to be highest in the first 28 days of starting an antidepressant and also in the first 28 days after stopping.
Results of analyses for upper gastrointestinal bleeding
Incidence rates of upper gastrointestinal bleeding
A total of 59,495 patients were included in the analyses of upper GI bleeding during follow-up, excluding the 1251 patients who had had an upper GI bleed by the baseline date. During the follow-up period, 1365 (2.29%) of these patients had an incident upper GI bleed, giving a crude incidence rate of 46.0 per 10,000 person-years (95% CI 43.6 to 48.5 per 10,000 person-years). Rates were higher in men than in women and increased with increasing age (Table 73).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 186 | 82,928 | 22.4 | 19.4 to 25.9 |
| 75–84 | 368 | 90,554 | 40.6 | 36.7 to 45.0 |
| 85+ | 258 | 35,732 | 72.2 | 63.9 to 81.6 |
| 65+ | 812 | 209,213 | 38.8 | 36.2 to 41.6 |
| Men | ||||
| 65–74 | 158 | 37,113 | 42.6 | 36.4 to 49.8 |
| 75–84 | 258 | 38,745 | 66.6 | 58.9 to 75.2 |
| 85+ | 137 | 11,658 | 117.5 | 99.4 to 138.9 |
| 65+ | 553 | 87,516 | 63.2 | 58.1 to 68.7 |
| Both sexes | ||||
| 65–74 | 344 | 120,041 | 28.7 | 25.8 to 31.9 |
| 75–84 | 626 | 129,298 | 48.4 | 44.8 to 52.4 |
| 85+ | 395 | 47,390 | 83.4 | 75.5 to 92.0 |
| 65+ | 1365 | 296,729 | 46.0 | 43.6 to 48.5 |
Upper GI bleed incidence rates by antidepressant class are shown in Table 74. These exclude patients who had taken MAOIs during follow-up. The highest rate occurred in patients having combined prescriptions.
| Antidepressant class | Events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 671 | 166,182 | 40.4 | 37.4 to 43.6 |
| TCAs | 229 | 44,746 | 51.2 | 45.0 to 58.3 |
| SSRIs | 365 | 68,803 | 53.1 | 47.9 to 58.8 |
| Other antidepressants | 79 | 14,105 | 56.0 | 44.9 to 69.8 |
| Combination of antidepressants | 14 | 2086 | 67.1 | 39.8 to 113.4 |
Hazard ratios for upper gastrointestinal bleed
Table 75 shows HRs for upper GI bleed according to antidepressant class. This shows significantly increased HRs for TCAs, SSRIs and the group of other antidepressant drugs, with only small changes after adjusting for potential confounding variables. There were no significant differences between the classes (p = 0.74). In a direct comparison with TCAs, the adjusted HRs were 0.95 (95% CI 0.80 to 1.12) for SSRIs and 1.06 (95% CI 0.82 to 1.38) for the group of other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 1.21 | 1.04 to 1.41 | 0.014 | 1.29 | 1.10 to 1.51 | 0.002 |
| SSRIs | 1.27 | 1.11 to 1.44 | < 0.001 | 1.22 | 1.07 to 1.40 | 0.004 |
| Other antidepressants | 1.36 | 1.08 to 1.72 | 0.009 | 1.37 | 1.08 to 1.74 | 0.010 |
| Combination of antidepressants | 1.63 | 0.96 to 2.76 | 0.072 | 1.44 | 0.82 to 2.56 | 0.208 |
Table 76 shows that although the risk of upper GI bleed was significantly increased for some dose categories; there were no significant trends with dose.
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 1.23 | 1.01 to 1.49 | 0.042 |
| > 0.5 / ≤ 1.0 DDDs | 1.69 | 1.25 to 2.28 | 0.001 |
| > 1.0 DDDs | 0.45 | 0.19 to 1.09 | 0.077 |
| Test for trend | 0.428 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.45 | 1.10 to 1.91 | 0.007 |
| > 0.5 / ≤ 1.0 DDDs | 1.19 | 1.01 to 1.39 | 0.033 |
| > 1.0 DDDs | 1.21 | 0.87 to 1.70 | 0.263 |
| Test for trend | 0.482 | ||
| Others | |||
| ≤ 0.5 DDDs | 1.01 | 0.54 to 1.90 | 0.964 |
| > 0.5 / ≤ 1.0 DDDs | 1.53 | 1.10 to 2.14 | 0.012 |
| > 1.0 DDDs | 1.56 | 0.95 to 2.57 | 0.078 |
| Test for trend | 0.185 | ||
Table 77 shows the effects of duration of use and time since stopping an antidepressant. For TCAs the upper GI bleed rate was significantly increased in the first 28 days after starting, but not during the remaining period of use. The HR was also significantly increased in the first 84 days after stopping TCAs, but not between 85 and 182 days after stopping. The HR was significantly increased in the first 28 days after stopping SSRIs, but not for the remaining period after stopping. For the group of other antidepressant drugs, the upper GI bleed rate was significantly increased only in the first 28 days after stopping medication, but not in the other time periods.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 1.92 | 1.29 to 2.85 | 0.001 |
| 29–84 days | 1.22 | 0.73 to 2.03 | 0.443 |
| 85+ days | 0.92 | 0.74 to 1.15 | 0.476 |
| stopped 1–28 days | 4.03 | 2.92 to 5.56 | < 0.001 |
| stopped 29–84 days | 2.00 | 1.42 to 2.82 | < 0.001 |
| stopped 85–182 days | 1.26 | 0.87 to 1.81 | 0.218 |
| SSRIs | |||
| 1–28 days | 1.58 | 1.09 to 2.29 | 0.017 |
| 29–84 days | 1.25 | 0.84 to 1.86 | 0.266 |
| 85+ days | 0.97 | 0.82 to 1.16 | 0.760 |
| Stopped 1–28 days | 4.73 | 3.62 to 6.18 | < 0.001 |
| Stopped 29–84 days | 1.49 | 1.05 to 2.12 | 0.026 |
| Stopped 85–182 days | 1.28 | 0.93 to 1.77 | 0.130 |
| Others | |||
| 1–28 days | 1.71 | 0.85 to 3.46 | 0.135 |
| 29–84 days | 0.62 | 0.20 to 1.92 | 0.403 |
| 85+ days | 1.28 | 0.95 to 1.71 | 0.102 |
| Stopped 1–28 days | 4.60 | 2.53 to 8.36 | < 0.001 |
| Stopped 29–84 days | 1.62 | 0.72 to 3.63 | 0.241 |
| Stopped 85–182 days | 1.46 | 0.69 to 3.09 | 0.318 |
There were no significant interactions for upper GI bleed.
Table 78 shows the HRs for upper GI bleed for individual antidepressant drugs. There were significantly (p < 0.01) increased HRs for venlafaxine, amitriptyline and citalopram after adjusting for potential confounding variables; however, there were no significant differences between the different drugs overall (p = 0.44). Although trazodone had the highest HR, it was not significant at p < 0.01.
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.30 | 1.06 to 1.60 | 0.011 | 1.38 | 1.11 to 1.70 | 0.003 |
| Dosulepin hydrochloride (TCA) | 1.08 | 0.84 to 1.39 | 0.527 | 1.21 | 0.93 to 1.56 | 0.152 |
| Lofepramine (TCA) | 1.24 | 0.82 to 1.87 | 0.302 | 1.21 | 0.79 to 1.85 | 0.387 |
| Trazodone hydrochloride (TCA) | 1.90 | 1.20 to 2.99 | 0.006 | 1.79 | 1.12 to 2.87 | 0.015 |
| Citalopram hydrobromide (SSRI) | 1.41 | 1.19 to 1.69 | < 0.001 | 1.34 | 1.12 to 1.61 | 0.001 |
| Escitalopram (SSRI) | 1.13 | 0.68 to 1.89 | 0.631 | 1.07 | 0.62 to 1.86 | 0.811 |
| Fluoxetine hydrochloride (SSRI) | 1.15 | 0.93 to 1.43 | 0.202 | 1.15 | 0.92 to 1.44 | 0.217 |
| Paroxetine hydrochloride (SSRI) | 1.24 | 0.95 to 1.61 | 0.108 | 1.15 | 0.87 to 1.53 | 0.329 |
| Sertraline hydrochloride (SSRI) | 1.08 | 0.77 to 1.53 | 0.660 | 1.04 | 0.73 to 1.49 | 0.825 |
| Mirtazapine (other) | 1.09 | 0.73 to 1.61 | 0.681 | 1.05 | 0.71 to 1.56 | 0.809 |
| Venlafaxine hydrochloride (other) | 1.67 | 1.24 to 2.26 | 0.001 | 1.71 | 1.26 to 2.33 | 0.001 |
Absolute risk of upper gastrointestinal bleed
Table 79 shows the absolute risk of upper GI bleed over 1, 2 and 5 years of treatment and the number of extra cases for the significant associations at p < 0.01. The results show similar absolute risks and the number of extra cases for the three classes of antidepressant drugs. Among the individual drugs, venlafaxine is associated with the highest numbers of additional cases of upper GI bleed compared with no treatment.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 0.42 | 0.78 | 2.02 | |||
| TCAs | 0.54 | 1.00 | 2.60 | 12 | 22 | 58 |
| SSRIs | 0.51 | 0.95 | 2.46 | 9 | 17 | 44 |
| Other antidepressants | 0.57 | 1.06 | 2.76 | 15 | 29 | 74 |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 0.42 | 0.78 | 2.02 | |||
| Amitriptyline hydrochloride (TCA) | 0.58 | 1.07 | 2.77 | 16 | 29 | 75 |
| Dosulepin hydrochloride (TCA) | 0.51 | 0.94 | 2.44 | NS | NS | NS |
| Lofepramine (TCA) | 0.51 | 0.94 | 2.44 | NS | NS | NS |
| Trazodone hydrochloride (TCA) | 0.75 | 1.39 | 3.60 | NS | NS | NS |
| Citalopram hydrobromide (SSRI) | 0.56 | 1.04 | 2.71 | 14 | 27 | 69 |
| Escitalopram (SSRI) | 0.45 | 0.83 | 2.16 | NS | NS | NS |
| Fluoxetine hydrochloride (SSRI) | 0.48 | 0.89 | 2.33 | NS | NS | NS |
| Paroxetine hydrochloride (SSRI) | 0.48 | 0.89 | 2.33 | NS | NS | NS |
| Sertraline hydrochloride (SSRI) | 0.44 | 0.81 | 2.11 | NS | NS | NS |
| Mirtazapine (other) | 0.44 | 0.82 | 2.12 | NS | NS | NS |
| Venlafaxine hydrochloride (other) | 0.72 | 1.33 | 3.44 | 30 | 55 | 142 |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 80. The upper GI bleed rate was significantly increased (p < 0.01) for TCAs during the first 28 days of use and marginally for the remaining period of use (p < 0.05). The rates were significantly increased throughout use for SSRIs. The rates were significantly increased after 85 days of use for the group of other antidepressant drugs. For all three classes, the rates were significantly increased in the first 28 days after stopping then decreased with time, with no significant increase between 85 and 182 days after stopping for TCAs and after 28 days for SSRIs and other antidepressant drugs.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 2.92 | 2.05 to 4.15 | < 0.001 |
| 29–84 days | 1.73 | 1.05 to 2.86 | 0.033 |
| 85+ days | 1.48 | 1.00 to 2.18 | 0.049 |
| Stopped 1–28 days | 2.16 | 1.40 to 3.34 | 0.001 |
| Stopped 29–84 days | 1.90 | 1.31 to 2.76 | 0.001 |
| Stopped 85–182 days | 1.14 | 0.77 to 1.69 | 0.517 |
| SSRIs | |||
| 1–28 days | 1.96 | 1.40 to 2.75 | < 0.001 |
| 29–84 days | 1.81 | 1.27 to 2.60 | 0.001 |
| 85+ days | 1.46 | 1.12 to 1.90 | 0.005 |
| Stopped 1–28 days | 2.61 | 1.86 to 3.67 | < 0.001 |
| Stopped 29–84 days | 1.38 | 0.94 to 2.02 | 0.098 |
| Stopped 85–182 days | 1.33 | 0.95 to 1.85 | 0.095 |
| Others | |||
| 1–28 days | 2.24 | 1.14 to 4.40 | 0.019 |
| 29–84 days | 1.41 | 0.56 to 3.53 | 0.467 |
| 85+ days | 2.21 | 1.26 to 3.90 | 0.006 |
| Stopped 1–28 days | 2.75 | 1.27 to 5.95 | 0.010 |
| Stopped 29–84 days | 1.02 | 0.37 to 2.77 | 0.975 |
| Stopped 85–182 days | 1.46 | 0.71 to 3.02 | 0.304 |
Summary of results for upper gastrointestinal bleeding
All classes of antidepressant drug were associated with a significantly increased risk of upper GI bleeding compared with no use of antidepressant drugs, with no significant differences in risk between the classes. There was no evidence of a dose–response relationship in any class. There were no significant differences between the most commonly prescribed antidepressant drugs. Rates tended to be highest in the first 28 days of starting an antidepressant. Rates were also increased in the first 28 days after stopping for all classes, but were no longer increased after 85 days.
Results of analyses for epilepsy/seizures
Incidence rates of epilepsy/seizures
A total of 59,793 patients were included in the analyses of incident epilepsy/seizures during follow-up, excluding the 953 patients who had recorded diagnoses of epilepsy/seizures by the baseline date. During the follow-up period, 505 (0.84%) of these patients had incident epilepsy/seizures, giving a crude incidence rate of 16.9 per 10,000 person-years (95% CI 15.5 to 18.4 per 10,000 person-years). The rates were higher in men than in women and increased slightly with increasing age (Table 81).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 107 | 82,887 | 12.9 | 10.7 to 15.6 |
| 75–84 | 129 | 91,127 | 14.2 | 11.9 to 16.8 |
| 85+ | 66 | 36,491 | 18.1 | 14.2 to 23.0 |
| 65+ | 302 | 210,505 | 14.4 | 12.8 to 16.1 |
| Men | ||||
| 65–74 | 74 | 37,342 | 19.8 | 15.8 to 24.9 |
| 75–84 | 98 | 39,601 | 24.8 | 20.3 to 30.2 |
| 85+ | 31 | 12,060 | 25.7 | 18.1 to 36.6 |
| 65+ | 203 | 89,004 | 22.8 | 19.9 to 26.2 |
| Both sexes | ||||
| 65–74 | 181 | 120,229 | 15.1 | 13.0 to 17.4 |
| 75–84 | 227 | 130,728 | 17.4 | 15.3 to 19.8 |
| 85+ | 97 | 48,551 | 20.0 | 16.4 to 24.4 |
| 65+ | 505 | 299,508 | 16.9 | 15.5 to 18.4 |
Epilepsy/seizures incidence rates by antidepressant class are shown in Table 82. These rates exclude patients who had taken MAOIs during follow-up. The highest epilepsy/seizure rates occurred in patients having combined prescriptions, followed by patients taking the group of other antidepressant drugs or SSRIs.
| Antidepressant class | Events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 223 | 176,455 | 12.6 | 11.1 to 14.4 |
| TCAs | 58 | 41,623 | 13.9 | 10.8 to 18.0 |
| SSRIs | 177 | 65,074 | 27.2 | 23.5 to 31.5 |
| Other antidepressants | 39 | 13,498 | 28.9 | 21.1 to 39.6 |
| Combination of antidepressants | 8 | 2069 | 38.7 | 19.3 to 77.3 |
Hazard ratios for epilepsy/seizures
Table 83 shows the HRs for epilepsy/seizures according to antidepressant class. This shows significantly increased HRs for SSRIs and the group of other antidepressant drugs after adjustment for potential confounding variables. There were significant differences between the classes (p < 0.001). In a direct comparison with TCAs, there were adjusted HRs of 1.80 (95% CI 1.32 to 2.43) for SSRIs and 2.20 (95% CI 1.46 to 3.30) for the group of other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 0.99 | 0.74 to 1.32 | 0.921 | 1.02 | 0.76 to 1.38 | 0.892 |
| SSRIs | 1.98 | 1.62 to 2.43 | < 0.001 | 1.83 | 1.49 to 2.26 | < 0.001 |
| Other antidepressants | 2.32 | 1.67 to 3.24 | < 0.001 | 2.24 | 1.60 to 3.15 | < 0.001 |
| Combination of antidepressants | 3.13 | 1.54 to 6.35 | 0.002 | 2.61 | 1.23 to 5.55 | 0.013 |
Table 84 shows that the risk of epilepsy/seizures was significantly increased for SSRIs and the group of other antidepressant drugs at dose levels above 0.5 DDDs. There was a significant trend with increasing dose for SSRIs and TCAs, but not for the group of other antidepressant drugs.
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 0.68 | 0.44 to 1.07 | 0.094 |
| > 0.5 / ≤ 1.0 DDDs | 1.20 | 0.65 to 2.21 | 0.560 |
| > 1.0 DDDs | 2.14 | 1.05 to 4.36 | 0.036 |
| Test for trend | 0.010 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.26 | 0.74 to 2.15 | 0.789 |
| > 0.5 / ≤ 1.0 DDDs | 1.83 | 1.43 to 2.35 | < 0.001 |
| > 1.0 DDDs | 3.40 | 2.29 to 5.05 | < 0.001 |
| Test for trend | < 0.001 | ||
| Others | |||
| ≤ 0.5 DDDs | 1.84 | 0.81 to 4.15 | 0.144 |
| > 0.5 / ≤ 1.0 DDDs | 2.46 | 1.53 to 3.94 | < 0.001 |
| > 1.0 DDDs | 3.11 | 1.64 to 5.89 | < 0.001 |
| Test for trend | 0.331 | ||
Table 85 shows the effects of duration of use and time since stopping an antidepressant on epilepsy/seizures risk. The epilepsy/seizures rate was increased in the first 28 days after starting TCAs; however, this was not significant at p < 0.01. The HR was significantly increased in the first 28 days and between 85 and 182 days after stopping TCAs, but not between 29 and 84 days after stopping. For SSRIs the epilepsy/seizures rates were significantly increased after 85 days of use. The HR was highest in the first 28 days after stopping SSRIs. For the group of other antidepressant drugs, the epilepsy/seizures rate was significantly increased in the first 28 days after starting the drug and in the first 84 days after stopping, but not from 85 days after stopping.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 2.31 | 1.16 to 4.59 | 0.017 |
| 29–84 days | 1.20 | 0.52 to 2.77 | 0.670 |
| 85+ days | 0.79 | 0.51 to 1.22 | 0.280 |
| Stopped 1–28 days | 2.99 | 1.60 to 5.58 | 0.001 |
| Stopped 29–84 days | 1.06 | 0.46 to 2.41 | 0.894 |
| Stopped 85–182 days | 2.12 | 1.27 to 3.54 | 0.004 |
| SSRIs | |||
| 1–28 days | 1.40 | 0.68 to 2.89 | 0.366 |
| 29–84 days | 1.75 | 1.01 to 3.04 | 0.046 |
| 85+ days | 1.69 | 1.31 to 2.17 | < 0.001 |
| Stopped 1–28 days | 8.35 | 5.81 to 12.00 | < 0.001 |
| Stopped 29–84 days | 1.59 | 0.88 to 2.88 | 0.126 |
| Stopped 85–182 days | 1.80 | 1.11 to 2.92 | 0.017 |
| Others | |||
| 1–28 days | 4.35 | 1.91 to 9.91 | < 0.001 |
| 29–84 days | 0.59 | 0.08 to 4.21 | 0.596 |
| 85+ days | 1.73 | 1.08 to 2.75 | 0.022 |
| Stopped 1–28 days | 8.79 | 4.11 to 18.81 | < 0.001 |
| Stopped 29–84 days | 5.91 | 2.77 to 12.64 | < 0.001 |
| Stopped 85–182 days | 0.65 | 0.09 to 4.65 | 0.668 |
There were no significant interactions for epilepsy/seizures.
Table 86 shows the HRs for individual antidepressant drugs. This shows significantly (p < 0.01) increased HRs for citalopram, paroxetine, sertraline and venlafaxine. There were significant differences between the drugs (p = 0.003), with the highest HRs for venlafaxine where there was a threefold increase in the epilepsy/seizures rate compared with no current antidepressant use, and sertraline (2.7-fold increase).
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.14 | 0.77 to 1.67 | 0.511 | 1.17 | 0.79 to 1.74 | 0.440 |
| Dosulepin hydrochloride (TCA) | 0.44 | 0.22 to 0.85 | 0.015 | 0.50 | 0.26 to 0.98 | 0.042 |
| Lofepramine (TCA) | 1.23 | 0.61 to 2.50 | 0.566 | 1.37 | 0.67 to 2.79 | 0.388 |
| Trazodone hydrochloride (TCA) | 1.55 | 0.64 to 3.77 | 0.332 | 1.46 | 0.60 to 3.55 | 0.406 |
| Citalopram hydrobromide (SSRI) | 2.03 | 1.55 to 2.66 | < 0.001 | 1.79 | 1.35 to 2.36 | < 0.001 |
| Escitalopram (SSRI) | 1.88 | 0.93 to 3.82 | 0.080 | 1.75 | 0.86 to 3.57 | 0.123 |
| Fluoxetine hydrochloride (SSRI) | 1.58 | 1.14 to 2.21 | 0.007 | 1.49 | 1.06 to 2.09 | 0.022 |
| Paroxetine hydrochloride (SSRI) | 1.96 | 1.34 to 2.87 | < 0.001 | 2.04 | 1.38 to 3.01 | < 0.001 |
| Sertraline hydrochloride (SSRI) | 2.96 | 2.01 to 4.34 | < 0.001 | 2.68 | 1.80 to 3.99 | < 0.001 |
| Mirtazapine (other) | 1.76 | 1.00 to 3.07 | 0.049 | 1.59 | 0.90 to 2.79 | 0.110 |
| Venlafaxine hydrochloride (other) | 3.00 | 1.98 to 4.54 | < 0.001 | 2.99 | 1.95 to 4.57 | < 0.001 |
Absolute risk of epilepsy/seizures
Table 87 shows the absolute risk of epilepsy/seizures over 1, 2 and 5 years of treatment and numbers of extra cases for the significant associations at p < 0.01. The results by class show that the group of other antidepressant drugs is associated with the highest absolute risks and number of extra cases. Among the individual drugs, venlafaxine and sertraline are associated with the highest number of additional cases.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 0.21 | 0.39 | 0.68 | |||
| TCAs | 0.21 | 0.40 | 0.69 | NS | NS | NS |
| SSRIs | 0.38 | 0.71 | 1.24 | 17 | 32 | 56 |
| Other antidepressants | 0.46 | 0.87 | 1.51 | 26 | 48 | 84 |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 0.21 | 0.39 | 0.68 | |||
| Amitriptyline hydrochloride (TCA) | 0.24 | 0.46 | 0.79 | NS | NS | NS |
| Dosulepin hydrochloride (TCA) | 0.10 | 0.19 | 0.34 | NS | NS | NS |
| Lofepramine (TCA) | 0.28 | 0.53 | 0.93 | NS | NS | NS |
| Trazodone hydrochloride (TCA) | 0.30 | 0.57 | 0.99 | NS | NS | NS |
| Citalopram hydrobromide (SSRI) | 0.37 | 0.69 | 1.21 | 16 | 30 | 53 |
| Escitalopram (SSRI) | 0.36 | 0.68 | 1.18 | NS | NS | NS |
| Fluoxetine hydrochloride (SSRI) | 0.31 | 0.58 | 1.01 | NS | NS | NS |
| Paroxetine hydrochloride (SSRI) | 0.42 | 0.79 | 1.38 | 21 | 40 | 70 |
| Sertraline hydrochloride (SSRI) | 0.55 | 1.04 | 1.80 | 34 | 65 | 113 |
| Mirtazapine (other) | 0.33 | 0.62 | 1.07 | NS | NS | NS |
| Venlafaxine hydrochloride (other) | 0.61 | 1.16 | 2.01 | 41 | 77 | 133 |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 88. The epilepsy/seizure rate was significantly increased for TCAs only during the first 28 days of use and only after 28 days of use for SSRIs. The rate ratios for the group of other antidepressant drugs were not significantly increased throughout use, although CIs were wide. For TCAs and SSRIs, rates were significantly increased in the first 28 days after stopping and then decreased with time. For the group of other antidepressant drugs, rates were highest in the 29–84 days after stopping, but this was not significant at p < 0.01.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 2.65 | 1.27 to 5.55 | 0.010 |
| 29–84 days | 1.69 | 0.61 to 4.70 | 0.314 |
| 85+ days | 1.56 | 0.78 to 3.13 | 0.208 |
| Stopped 1–28 days | 5.20 | 2.87 to 9.44 | < 0.001 |
| Stopped 29–84 days | 2.18 | 1.09 to 4.36 | 0.028 |
| Stopped 85–182 days | 1.48 | 0.74 to 2.94 | 0.268 |
| SSRIs | |||
| 1–28 days | 1.69 | 0.93 to 3.08 | 0.083 |
| 29–84 days | 2.99 | 1.86 to 4.81 | < 0.001 |
| 85+ days | 2.49 | 1.73 to 3.59 | < 0.001 |
| Stopped 1–28 days | 3.61 | 2.21 to 5.90 | < 0.001 |
| Stopped 29–84 days | 1.22 | 0.62 to 2.41 | 0.568 |
| Stopped 85–182 days | 1.37 | 0.78 to 2.38 | 0.274 |
| Others | |||
| 1–28 days | 2.77 | 0.97 to 7.87 | 0.057 |
| 29–84 days | 0.90 | 0.12 to 6.69 | 0.921 |
| 85+ days | 2.06 | 0.90 to 4.71 | 0.086 |
| Stopped 1–28 days | 1.08 | 0.15 to 7.91 | 0.938 |
| Stopped 29–84 days | 3.26 | 1.27 to 8.33 | 0.014 |
| Stopped 85–182 days | 1.53 | 0.47 to 5.00 | 0.482 |
Summary of results for epilepsy/seizures
The risk of epilepsy/seizures varied by antidepressant class, and was significantly increased for SSRIs, and the group of other antidepressant drugs compared with no current use of antidepressant drugs, but not for TCAs. The risk tended to increase as dose increased in all classes. Among the most commonly prescribed antidepressant drugs, venlafaxine and sertraline were associated with the highest rates. Epilepsy/seizures rates tended to be highest in the first 28 days of starting an antidepressant.
Results of analyses for road traffic accidents
Incidence rates of road traffic accidents
A total of 59,783 patients were included in the analyses of incident RTAs during follow-up, excluding the 963 patients who had had a RTA recorded by the baseline date. During follow-up, 423 (0.71%) of these patients had a RTA recorded giving a crude incidence rate of 14.2 per 10,000 person-years (95% CI 12.9 to 15.6 per 10,000 person-years). Rates were higher in men than in women and decreased with increasing age (Table 89).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 120 | 82,565 | 14.5 | 12.2 to 17.4 |
| 75–84 | 134 | 91,131 | 14.7 | 12.4 to 17.4 |
| 85+ | 20 | 36,543 | 5.5 | 3.5 to 8.5 |
| 65+ | 274 | 210,239 | 13.0 | 11.6 to 14.7 |
| Men | ||||
| 65–74 | 76 | 37,050 | 20.5 | 16.4 to 25.7 |
| 75–84 | 60 | 39,649 | 15.1 | 11.8 to 19.5 |
| 85+ | 13 | 12,107 | 10.7 | 6.2 to 18.5 |
| 65+ | 149 | 88,806 | 16.8 | 14.3 to 19.7 |
| Both sexes | ||||
| 65–74 | 196 | 119,615 | 16.4 | 14.3 to 18.9 |
| 75–84 | 194 | 130,780 | 14.8 | 12.9 to 17.1 |
| 85+ | 33 | 48,650 | 6.8 | 4.8 to 9.5 |
| 65+ | 423 | 299,045 | 14.2 | 12.9 to 15.6 |
Road traffic accident incidence rates by antidepressant class are shown in Table 90. These rates exclude patients who had taken MAOIs during follow-up. The highest RTA rates occurred in patients having combined prescriptions.
| Antidepressant class | Events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 252 | 167,255 | 15.1 | 13.3 to 17.1 |
| TCAs | 56 | 45,112 | 12.4 | 9.6 to 16.1 |
| SSRIs | 96 | 69,506 | 13.8 | 11.3 to 16.9 |
| Other antidepressants | 15 | 14,261 | 10.5 | 6.3 to 17.5 |
| Combination of antidepressants | 4 | 2099 | 19.1 | 7.2 to 50.8 |
Hazard ratios for road traffic accidents
Table 91 shows the HRs for RTAs according to antidepressant class. There were no significant HRs and no significant differences between the classes (p = 0.62). In a direct comparison with TCAs, the adjusted HRs were 1.03 (95% CI 0.74 to 1.44) for SSRIs and 0.78 (95% CI 0.43 to 1.40) for other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 0.83 | 0.62 to 1.11 | 0.205 | 0.86 | 0.64 to 1.15 | 0.307 |
| SSRIs | 0.92 | 0.72 to 1.16 | 0.473 | 0.89 | 0.70 to 1.13 | 0.328 |
| Other antidepressants | 0.71 | 0.42 to 1.19 | 0.189 | 0.67 | 0.39 to 1.14 | 0.140 |
| Combination of antidepressants | 1.32 | 0.49 to 3.55 | 0.580 | 1.34 | 0.50 to 3.60 | 0.565 |
There was no association between RTAs and either dose of antidepressant or with duration of use, although RTA rates were significantly increased during the first 28 days after stopping TCAs and SSRIs (data not shown).
Table 92 shows the HRs for RTAs for individual antidepressant drugs. This table shows no significant HRs for any of these antidepressant drugs (at p < 0.01) and there were no significant differences between the different drugs overall (p = 0.33).
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 0.85 | 0.56 to 1.29 | 0.453 | 0.84 | 0.55 to 1.27 | 0.412 |
| Dosulepin hydrochloride (TCA) | 0.48 | 0.26 to 0.88 | 0.018 | 0.50 | 0.27 to 0.92 | 0.027 |
| Lofepramine (TCA) | 1.00 | 0.47 to 2.12 | 0.995 | 1.13 | 0.53 to 2.41 | 0.744 |
| Trazodone hydrochloride (TCA) | 1.36 | 0.56 to 3.31 | 0.492 | 1.53 | 0.63 to 3.70 | 0.351 |
| Citalopram hydrobromide (SSRI) | 1.07 | 0.77 to 1.48 | 0.688 | 1.01 | 0.72 to 1.41 | 0.956 |
| Escitalopram (SSRI) | 1.06 | 0.44 to 2.57 | 0.901 | 0.98 | 0.40 to 2.38 | 0.958 |
| Fluoxetine hydrochloride (SSRI) | 0.77 | 0.50 to 1.18 | 0.231 | 0.70 | 0.45 to 1.09 | 0.118 |
| Paroxetine hydrochloride (SSRI) | 0.83 | 0.49 to 1.40 | 0.480 | 0.90 | 0.54 to 1.53 | 0.709 |
| Sertraline hydrochloride (SSRI) | 0.87 | 0.46 to 1.64 | 0.670 | 0.89 | 0.47 to 1.68 | 0.727 |
| Mirtazapine (other) | 0.46 | 0.17 to 1.23 | 0.123 | 0.45 | 0.17 to 1.21 | 0.114 |
| Venlafaxine hydrochloride (other) | 0.80 | 0.40 to 1.62 | 0.532 | 0.72 | 0.34 to 1.53 | 0.391 |
Absolute risk of road traffic accidents
Table 93 shows the absolute risk of having a RTA over 1, 2 and 5 years of treatment. There were no excess risks which were significant at p < 0.01. The results show that the absolute risks are low and similar for all classes and individual drugs.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 0.16 | 0.32 | 0.77 | |||
| TCAs | 0.13 | 0.28 | 0.66 | NS | NS | NS |
| SSRIs | 0.14 | 0.29 | 0.68 | NS | NS | NS |
| Other antidepressants | 0.10 | 0.21 | 0.51 | NS | NS | NS |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 0.16 | 0.32 | 0.77 | |||
| Amitriptyline hydrochloride (TCA) | 0.13 | 0.27 | 0.65 | NS | NS | NS |
| Dosulepin hydrochloride (TCA) | 0.08 | 0.16 | 0.39 | NS | NS | NS |
| Lofepramine (TCA) | 0.18 | 0.37 | 0.88 | NS | NS | NS |
| Trazodone hydrochloride (TCA) | 0.24 | 0.49 | 1.17 | NS | NS | NS |
| Citalopram hydrobromide (SSRI) | 0.16 | 0.33 | 0.78 | NS | NS | NS |
| Escitalopram (SSRI) | 0.15 | 0.32 | 0.75 | NS | NS | NS |
| Fluoxetine hydrochloride (SSRI) | 0.11 | 0.23 | 0.54 | NS | NS | NS |
| Paroxetine hydrochloride (SSRI) | 0.14 | 0.29 | 0.70 | NS | NS | NS |
| Sertraline hydrochloride (SSRI) | 0.14 | 0.29 | 0.69 | NS | NS | NS |
| Mirtazapine (other) | 0.07 | 0.15 | 0.35 | NS | NS | NS |
| Venlafaxine hydrochloride (other) | 0.11 | 0.23 | 0.56 | NS | NS | NS |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 94. There were no significant associations.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 0.18 | 0.02 to 1.26 | 0.083 |
| 29–84 days | 1.49 | 0.64 to 3.48 | 0.355 |
| 85+ days | 1.21 | 0.58 to 2.50 | 0.613 |
| Stopped 1–28 days | 1.74 | 0.84 to 3.57 | 0.133 |
| Stopped 29–84 days | 0.39 | 0.12 to 1.21 | 0.103 |
| Stopped 85–182 days | 1.58 | 0.96 to 2.60 | 0.070 |
| SSRIs | |||
| 1–28 days | 1.05 | 0.51 to 2.15 | 0.896 |
| 29–84 days | 1.27 | 0.64 to 2.52 | 0.494 |
| 85+ days | 0.80 | 0.47 to 1.36 | 0.410 |
| Stopped 1–28 days | 0.73 | 0.27 to 1.98 | 0.537 |
| Stopped 29–84 days | 0.70 | 0.31 to 1.60 | 0.399 |
| Stopped 85–182 days | 1.17 | 0.68 to 1.99 | 0.575 |
| Others | |||
| 1–28 days | 1.13 | 0.15 to 8.35 | 0.902 |
| 29–84 days | 0.00 | – | 0.988 |
| 85+ days | 1.52 | 0.37 to 6.25 | 0.561 |
| Stopped 1–28 days | 1.45 | 0.2 to 10.69 | 0.716 |
| Stopped 29–84 days | 1.70 | 0.41 to 7.18 | 0.467 |
| Stopped 85–182 days | 1.77 | 0.54 to 5.86 | 0.348 |
Summary of results for road traffic accidents
Increased risk of RTAs is not associated with any class of antidepressant drug or with any individual drug.
Results of analyses for adverse drug reactions
Incidence rates of adverse drug reactions
A total of 60,275 patients were included in the analyses of ADRs (including bullous eruptions) during follow-up, excluding the 471 patients who had had an ADR recorded by the baseline date. During the follow-up period, 833 (1.38%) of these patients had an incident ADR giving a crude incidence rate of 27.7 per 10,000 person-years (95% CI 25.9 to 29.7 per 10,000 person-years). Rates were higher in women than in men and there was little change with increasing age (Table 95).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 253 | 83,093 | 30.5 | 26.9 to 34.4 |
| 75–84 | 276 | 90,961 | 30.3 | 27.0 to 34.1 |
| 85+ | 107 | 36,417 | 29.4 | 24.3 to 35.5 |
| 65+ | 636 | 210,472 | 30.2 | 28.0 to 32.7 |
| Men | ||||
| 65–74 | 82 | 37,764 | 21.7 | 17.5 to 27.0 |
| 75–84 | 84 | 39,993 | 21.0 | 17.0 to 26.0 |
| 85+ | 31 | 12,175 | 25.5 | 17.9 to 36.2 |
| 65+ | 197 | 89,932 | 21.9 | 19.1 to 25.2 |
| Both sexes | ||||
| 65–74 | 335 | 120,857 | 27.7 | 24.9 to 30.9 |
| 75–84 | 360 | 130,955 | 27.5 | 24.8 to 30.5 |
| 85+ | 138 | 48,592 | 28.4 | 24.0 to 33.6 |
| 65+ | 833 | 300,404 | 27.7 | 25.9 to 29.7 |
Adverse drug reaction incidence rates by antidepressant class are shown in Table 96. These rates exclude patients who had taken MAOIs during follow-up. The highest ADR rates occurred in patients taking SSRIs, followed by patients taking TCAs.
| Antidepressant class | Events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 417 | 167,913 | 24.8 | 22.6 to 27.3 |
| TCAs | 139 | 45,405 | 30.6 | 25.9 to 36.2 |
| SSRIs | 231 | 69,890 | 33.1 | 29.1 to 37.6 |
| Other antidepressants | 37 | 14,280 | 25.9 | 18.8 to 35.8 |
| Combination of antidepressants | 5 | 2123 | 23.6 | 9.8 to 56.6 |
Hazard ratios for adverse drug reactions
Table 97 shows the HRs for ADRs according to antidepressant class. There were no significant HRs for any class of antidepressant drugs after adjusting for potential confounding variables and no significant difference between the classes (p = 0.60).
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 1.09 | 0.89 to 1.32 | 0.407 | 1.06 | 0.86 to 1.29 | 0.596 |
| SSRIs | 1.20 | 1.02 to 1.42 | 0.030 | 1.16 | 0.98 to 1.37 | 0.087 |
| Other antidepressants | 1.00 | 0.71 to 1.40 | 0.987 | 0.95 | 0.68 to 1.34 | 0.783 |
| Combination of antidepressants | 0.93 | 0.38 to 2.24 | 0.865 | 0.85 | 0.35 to 2.06 | 0.723 |
Table 98 shows that the risk of an ADR was not significantly increased for any class at any dose level at p < 0.01, although there was some indication of an increase (p = 0.02) with high doses of TCAs (> 1.0 DDDs).
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 0.98 | 0.76 to 1.26 | 0.885 |
| > 0.5 / ≤ 1.0 DDDs | 0.77 | 0.46 to 1.27 | 0.300 |
| > 1.0 DDDs | 1.85 | 1.10 to 3.12 | 0.020 |
| Test for trend | 0.158 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.28 | 0.91 to 1.80 | 0.162 |
| > 0.5 / ≤ 1.0 DDDs | 1.11 | 0.91 to 1.35 | 0.291 |
| > 1.0 DDDs | 1.12 | 0.73 to 1.72 | 0.600 |
| Test for trend | 0.660 | ||
| Others | |||
| ≤ 0.5 DDDs | 0.57 | 0.21 to 1.52 | 0.258 |
| > 0.5 / ≤ 1.0 DDDs | 1.05 | 0.65 to 1.71 | 0.835 |
| > 1.0 DDDs | 1.03 | 0.49 to 2.17 | 0.945 |
| Test for trend | 0.134 | ||
Table 99 shows the effects of duration of use and time since stopping an antidepressant on ADR risk. There were significantly increased risks for all classes of antidepressant in the first 28 days after starting and significant decreases in risk for TCAs and SSRIs after 85 days of use. HRs were also significantly increased in the first 28 days after stopping TCAs and SSRIs, but not after 28 days.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 4.13 | 2.99 to 5.70 | < 0.001 |
| 29–84 days | 0.90 | 0.44 to 1.83 | 0.767 |
| 85+ days | 0.45 | 0.31 to 0.65 | < 0.001 |
| Stopped 1–28 days | 3.59 | 2.39 to 5.40 | < 0.001 |
| Stopped 29–84 days | 1.34 | 0.82 to 2.20 | 0.243 |
| Stopped 85–182 days | 1.20 | 0.77 to 1.86 | 0.415 |
| SSRIs | |||
| 1–28 days | 4.86 | 3.68 to 6.42 | < 0.001 |
| 29–84 days | 1.30 | 0.80 to 2.10 | 0.286 |
| 85+ days | 0.68 | 0.53 to 0.86 | 0.002 |
| Stopped 1–28 days | 2.68 | 1.76 to 4.07 | < 0.001 |
| Stopped 29–84 days | 1.15 | 0.71 to 1.86 | 0.565 |
| Stopped 85–182 days | 0.85 | 0.53 to 1.35 | 0.482 |
| Others | |||
| 1–28 days | 3.25 | 1.77 to 5.95 | < 0.001 |
| 29–84 days | 0.61 | 0.15 to 2.46 | 0.489 |
| 85+ days | 0.72 | 0.45 to 1.14 | 0.164 |
| Stopped 1–28 days | 1.23 | 0.31 to 4.96 | 0.768 |
| Stopped 29–84 days | 1.18 | 0.38 to 3.69 | 0.772 |
| Stopped 85–182 days | 0.59 | 0.15 to 2.38 | 0.461 |
There was a significant interaction between antidepressant class and CHD at baseline (p = 0.008), with an indication that HRs for TCAs and SSRIs were somewhat higher in people without CHD at baseline, but for the group of other antidepressant drugs the HR was higher for people with CHD at baseline. There were no other significant interactions.
Table 100 shows the HRs for individual antidepressant drugs. There was some evidence of differences between the different drugs (p = 0.05), with significantly increased HRs for lofepramine and sertraline after adjusting for potential confounding variables.
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.02 | 0.77 to 1.35 | 0.889 | 0.96 | 0.72 to 1.27 | 0.758 |
| Dosulepin hydrochloride (TCA) | 0.89 | 0.64 to 1.24 | 0.484 | 0.88 | 0.63 to 1.24 | 0.482 |
| Lofepramine (TCA) | 2.10 | 1.43 to 3.09 | < 0.001 | 2.11 | 1.42 to 3.13 | < 0.001 |
| Trazodone hydrochloride (TCA) | 1.06 | 0.50 to 2.25 | 0.870 | 1.05 | 0.50 to 2.21 | 0.905 |
| Citalopram hydrobromide (SSRI) | 1.22 | 0.97 to 1.54 | 0.090 | 1.12 | 0.88 to 1.41 | 0.361 |
| Escitalopram (SSRI) | 1.25 | 0.69 to 2.28 | 0.467 | 1.10 | 0.60 to 2.01 | 0.755 |
| Fluoxetine hydrochloride (SSRI) | 1.20 | 0.92 to 1.56 | 0.169 | 1.19 | 0.91 to 1.55 | 0.199 |
| Paroxetine hydrochloride (SSRI) | 0.90 | 0.62 to 1.31 | 0.574 | 0.93 | 0.63 to 1.37 | 0.730 |
| Sertraline hydrochloride (SSRI) | 1.60 | 1.12 to 2.28 | 0.010 | 1.60 | 1.12 to 2.29 | 0.010 |
| Mirtazapine (other) | 1.12 | 0.69 to 1.82 | 0.639 | 1.02 | 0.63 to 1.66 | 0.941 |
| Venlafaxine hydrochloride (other) | 0.88 | 0.53 to 1.47 | 0.626 | 0.88 | 0.52 to 1.47 | 0.619 |
Absolute risk of adverse drug reactions
Table 101 shows the absolute risk of ADR over 1, 2 and 5 years of treatment. The absolute risks are low and similar for all classes and individual drugs, except for lofepramine and sertraline, which are associated with the highest numbers of additional cases.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 0.26 | 0.45 | 1.18 | |||
| TCAs | 0.28 | 0.47 | 1.24 | NS | NS | NS |
| SSRIs | 0.30 | 0.52 | 1.36 | NS | NS | NS |
| Other antidepressants | 0.25 | 0.43 | 1.12 | NS | NS | NS |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 0.26 | 0.45 | 1.18 | |||
| Amitriptyline hydrochloride (TCA) | 0.25 | 0.43 | 1.13 | NS | NS | NS |
| Dosulepin hydrochloride (TCA) | 0.23 | 0.39 | 1.04 | NS | NS | NS |
| Lofepramine (TCA) | 0.55 | 0.94 | 2.47 | 29 | 49 | 129 |
| Trazodone hydrochloride (TCA) | 0.27 | 0.47 | 1.23 | NS | NS | NS |
| Citalopram hydrobromide (SSRI) | 0.29 | 0.50 | 1.31 | NS | NS | NS |
| Escitalopram (SSRI) | 0.29 | 0.49 | 1.30 | NS | NS | NS |
| Fluoxetine hydrochloride (SSRI) | 0.31 | 0.53 | 1.40 | NS | NS | NS |
| Paroxetine hydrochloride (SSRI) | 0.24 | 0.42 | 1.10 | NS | NS | NS |
| Sertraline hydrochloride (SSRI) | 0.42 | 0.71 | 1.88 | 16 | 27 | 70 |
| Mirtazapine (other) | 0.27 | 0.45 | 1.20 | NS | NS | NS |
| Venlafaxine hydrochloride (other) | 0.23 | 0.39 | 1.03 | NS | NS | NS |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 102. ADR rates were significantly increased for all classes of antidepressant in the first 28 days after starting the drugs and remained significantly increased for SSRIs up to 84 days after starting. Rates were significantly increased in the first 28 days after stopping TCAs, but not SSRIs or the group of other drugs.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 7.02 | 5.09 to 9.68 | < 0.001 |
| 29–84 days | 2.19 | 1.15 to 4.19 | 0.018 |
| 85+ days | 0.99 | 0.50 to 1.96 | 0.973 |
| Stopped 1–28 days | 2.14 | 1.29 to 3.54 | 0.003 |
| Stopped 29–84 days | 1.55 | 0.96 to 2.52 | 0.076 |
| Stopped 85–182 days | 0.71 | 0.39 to 1.27 | 0.243 |
| SSRIs | |||
| 1–28 days | 7.39 | 5.62 to 9.72 | < 0.001 |
| 29–84 days | 2.36 | 1.46 to 3.79 | < 0.001 |
| 85+ days | 1.57 | 1.05 to 2.35 | 0.027 |
| Stopped 1–28 days | 1.61 | 0.96 to 2.70 | 0.072 |
| Stopped 29–84 days | 1.29 | 0.78 to 2.12 | 0.316 |
| Stopped 85–182 days | 0.97 | 0.60 to 1.57 | 0.896 |
| Others | |||
| 1–28 days | 4.50 | 2.20 to 9.22 | < 0.001 |
| 29–84 days | 0.68 | 0.09 to 5.00 | 0.709 |
| 85+ days | 0.97 | 0.32 to 2.97 | 0.962 |
| Stopped 1–28 days | 0.92 | 0.22 to 3.87 | 0.911 |
| Stopped 29–84 days | 1.71 | 0.60 to 4.89 | 0.314 |
| Stopped 85–182 days | 0.67 | 0.16 to 2.78 | 0.583 |
Summary of results for adverse drug reactions
Adverse drug reaction rates were not associated with any class of antidepressant drug overall, although rates were increased in the first 28 days of starting an antidepressant. Among the most commonly prescribed antidepressant drugs only lofepramine and sertraline were associated with an increased risk of ADRs.
Results of analyses for hyponatraemia
Incidence rates of hyponatraemia
A total of 60,405 patients were included in the analyses of hyponatraemia during follow-up, excluding the 341 patients who had hyponatraemia recorded by the baseline date. During the follow-up period, 1114 (1.84%) of these patients had incident hyponatraemia, giving a crude incidence rate of 37.0 per 10,000 person-years (95% CI 34.9 to 39.2 per 10,000 person-years). The rates were similar in men and women, and increased with increasing age (Table 103).
| Age band (years) | First events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Women | ||||
| 65–74 | 159 | 83,861 | 19.0 | 16.2 to 22.2 |
| 75–84 | 404 | 91,235 | 44.3 | 40.2 to 48.8 |
| 85+ | 251 | 36,064 | 69.6 | 61.5 to 78.8 |
| 65+ | 814 | 211,160 | 38.6 | 36.0 to 41.3 |
| Men | ||||
| 65–74 | 74 | 37,949 | 19.5 | 15.5 to 24.5 |
| 75–84 | 166 | 40,005 | 41.5 | 35.6 to 48.3 |
| 85+ | 60 | 12,098 | 49.6 | 38.5 to 63.9 |
| 65+ | 300 | 90,052 | 33.3 | 29.8 to 37.3 |
| Both sexes | ||||
| 65–74 | 233 | 121,810 | 19.1 | 16.8 to 21.8 |
| 75–84 | 570 | 131,239 | 43.4 | 40.0 to 47.2 |
| 85+ | 311 | 48,162 | 64.6 | 57.8 to 72.2 |
| 65+ | 1114 | 301,212 | 37.0 | 34.9 to 39.2 |
Hyponatraemia incidence rates by antidepressant class are shown in Table 104. These rates exclude patients who had taken MAOIs during follow-up. The highest rates occurred in patients taking SSRIs, followed by patients taking combined prescriptions or the group of other antidepressant drugs.
| Antidepressant class | Events | Person-years | Rate per 10,000 person-years | 95% CI |
|---|---|---|---|---|
| Not currently on antidepressants | 503 | 168,648 | 29.8 | 27.3 to 32.6 |
| TCAs | 155 | 45,439 | 34.1 | 29.1 to 39.9 |
| SSRIs | 383 | 70,031 | 54.7 | 49.5 to 60.5 |
| Other antidepressants | 62 | 14,151 | 43.8 | 34.2 to 56.2 |
| Combination of antidepressants | 10 | 2132 | 46.9 | 25.2 to 87.2 |
Hazard ratios for hyponatraemia
Table 105 shows the HRs for hyponatraemia according to antidepressant class. There were significant differences between the classes (p = 0.002). The only significant adjusted HR was for SSRIs, which was associated with a 52% increase in hyponatraemia rate compared with no antidepressant use. In a direct comparison with TCAs, the adjusted HRs were 1.44 (95% CI 1.19 to 1.75) for SSRIs and 1.21 (95% 0.90 to 1.64) for other antidepressant drugs.
| Antidepressant class | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| TCAs | 0.99 | 0.82 to 1.18 | 0.875 | 1.05 | 0.87 to 1.27 | 0.580 |
| SSRIs | 1.62 | 1.42 to 1.86 | < 0.001 | 1.52 | 1.33 to 1.75 | < 0.001 |
| Other antidepressants | 1.38 | 1.06 to 1.80 | 0.016 | 1.28 | 0.98 to 1.67 | 0.072 |
| Combination of antidepressants | 1.48 | 0.79 to 2.78 | 0.217 | 1.38 | 0.74 to 2.59 | 0.310 |
Table 106 shows that the risk of hyponatraemia was significantly increased for SSRIs at lower doses, but decreased as the dose of SSRI increased (test for trend, p = 0.014).
| Antidepressant class and dose category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| ≤ 0.5 DDDs | 1.01 | 0.80 to 1.27 | 0.949 |
| > 0.5 / ≤ 1.0 DDDs | 0.86 | 0.54 to 1.37 | 0.530 |
| > 1.0 DDDs | 1.49 | 0.86 to 2.60 | 0.156 |
| Test for trend | 0.442 | ||
| SSRIs | |||
| ≤ 0.5 DDDs | 1.95 | 1.53 to 2.48 | < 0.001 |
| > 0.5 / ≤ 1.0 DDDs | 1.46 | 1.25 to 1.71 | < 0.001 |
| > 1.0 DDDs | 1.07 | 0.71 to 1.61 | 0.754 |
| Test for trend | 0.014 | ||
| Others | |||
| ≤ 0.5 DDDs | 1.27 | 0.71 to 2.25 | 0.420 |
| > 0.5 / ≤ 1.0 DDDs | 1.45 | 1.00 to 2.11 | 0.053 |
| > 1.0 DDDs | 1.06 | 0.53 to 2.13 | 0.870 |
| Test for trend | 0.428 | ||
Table 107 shows the effects of duration of use and time since stopping an antidepressant on hyponatraemia risk. There were significantly increased risks for all classes of antidepressant in the first 28 days after starting, the risk remained increased for SSRIs between 29 and 84 days after starting, and there were significant decreases in risk for TCAs and SSRIs after 85 days of use. There were increased risks for all classes of antidepressant in the first 28 days after stopping, and the risk remained significantly increased for TCAs between 29 and 84 days after stopping.
| Antidepressant class and duration category | Adjusteda | ||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | ||
| TCAs | |||
| 1–28 days | 2.55 | 1.76 to 3.70 | < 0.001 |
| 29–84 days | 0.68 | 0.32 to 1.44 | 0.311 |
| 85+ days | 0.67 | 0.50 to 0.89 | 0.005 |
| Stopped 1–28 days | 4.14 | 2.89 to 5.91 | < 0.001 |
| Stopped 29–84 days | 1.80 | 1.18 to 2.73 | 0.006 |
| Stopped 85–182 days | 0.91 | 0.56 to 1.46 | 0.684 |
| SSRIs | |||
| 1–28 days | 7.72 | 6.19 to 9.63 | < 0.001 |
| 29–84 days | 2.17 | 1.54 to 3.05 | < 0.001 |
| 85+ days | 0.75 | 0.61 to 0.92 | 0.006 |
| Stopped 1–28 days | 4.20 | 3.08 to 5.72 | < 0.001 |
| Stopped 29–84 days | 1.22 | 0.79 to 1.88 | 0.367 |
| Stopped 85–182 days | 1.01 | 0.68 to 1.50 | 0.966 |
| Others | |||
| 1–28 days | 6.33 | 4.21 to 9.53 | < 0.001 |
| 29–84 days | 0.74 | 0.24 to 2.29 | 0.597 |
| 85+ days | 0.62 | 0.39 to 0.97 | 0.035 |
| Stopped 1–28 days | 4.47 | 2.31 to 8.67 | < 0.001 |
| Stopped 29–84 days | 1.01 | 0.32 to 3.15 | 0.983 |
| Stopped 85–182 days | 1.76 | 0.83 to 3.72 | 0.139 |
There were no significant interactions for hyponatraemia.
Table 108 shows the HRs for individual antidepressant drugs. This shows significantly increased HRs (at p < 0.01) for escitalopram, fluoxetine and citalopram after adjusting for potential confounding variables. There were significant differences between the different drugs (p < 0.001). The highest HR was for escitalopram (the hyponatraemia rate was doubled compared with no antidepressant use), then fluoxetine (70% increase) and citalopram (65% increase).
| Antidepressant drug | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Not currently on antidepressants | 1.00 | 1.00 | ||||
| Amitriptyline hydrochloride (TCA) | 1.11 | 0.87 to 1.42 | 0.382 | 1.17 | 0.92 to 1.49 | 0.211 |
| Dosulepin hydrochloride (TCA) | 0.72 | 0.52 to 1.00 | 0.054 | 0.83 | 0.59 to 1.16 | 0.268 |
| Lofepramine (TCA) | 0.92 | 0.55 to 1.53 | 0.739 | 0.93 | 0.55 to 1.59 | 0.791 |
| Trazodone hydrochloride (TCA) | 1.73 | 1.02 to 2.95 | 0.043 | 1.51 | 0.87 to 2.62 | 0.143 |
| Citalopram hydrobromide (SSRI) | 1.96 | 1.64 to 2.33 | < 0.001 | 1.65 | 1.38 to 1.97 | < 0.001 |
| Escitalopram (SSRI) | 2.48 | 1.68 to 3.66 | < 0.001 | 2.08 | 1.40 to 3.09 | < 0.001 |
| Fluoxetine hydrochloride (SSRI) | 1.66 | 1.35 to 2.04 | < 0.001 | 1.70 | 1.38 to 2.09 | < 0.001 |
| Paroxetine hydrochloride (SSRI) | 0.94 | 0.67 to 1.31 | 0.696 | 1.04 | 0.74 to 1.47 | 0.823 |
| Sertraline hydrochloride (SSRI) | 1.06 | 0.72 to 1.57 | 0.761 | 1.00 | 0.68 to 1.49 | 0.986 |
| Mirtazapine (other) | 1.37 | 0.91 to 2.04 | 0.127 | 1.06 | 0.71 to 1.61 | 0.765 |
| Venlafaxine hydrochloride (other) | 1.47 | 1.01 to 2.12 | 0.042 | 1.53 | 1.06 to 2.22 | 0.023 |
Absolute risk of hyponatraemia
Table 109 shows the absolute risk of hyponatraemia over 1, 2 and 5 years of treatment, and numbers of extra cases for the significant associations at p < 0.01. The results by class show that SSRIs are associated with the highest absolute risks and numbers of extra cases. For individual drugs, escitalopram is associated with the highest number of additional cases.
| Antidepressant class/drug | Absolute risk (%) | Extra cases per 10,000 treated | ||||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 5 years | 1 year | 2 years | 5 years | |
| Antidepressant class | ||||||
| Not currently on antidepressants | 0.29 | 0.56 | 1.36 | |||
| TCAs | 0.30 | 0.59 | 1.43 | NS | NS | NS |
| SSRIs | 0.44 | 0.86 | 2.06 | 15 | 29 | 70 |
| Other antidepressants | 0.37 | 0.72 | 1.73 | NS | NS | NS |
| Antidepressant drug | ||||||
| Not currently on antidepressants | 0.29 | 0.56 | 1.36 | |||
| Amitriptyline hydrochloride (TCA) | 0.33 | 0.66 | 1.59 | NS | NS | NS |
| Dosulepin hydrochloride (TCA) | 0.24 | 0.47 | 1.12 | NS | NS | NS |
| Lofepramine (TCA) | 0.27 | 0.52 | 1.27 | NS | NS | NS |
| Trazodone hydrochloride (TCA) | 0.43 | 0.85 | 2.05 | NS | NS | NS |
| Citalopram hydrobromide (SSRI) | 0.47 | 0.93 | 2.23 | 18 | 36 | 87 |
| Escitalopram (SSRI) | 0.59 | 1.17 | 2.80 | 31 | 60 | 144 |
| Fluoxetine hydrochloride (SSRI) | 0.49 | 0.96 | 2.30 | 20 | 39 | 94 |
| Paroxetine hydrochloride (SSRI) | 0.30 | 0.59 | 1.41 | NS | NS | NS |
| Sertraline hydrochloride (SSRI) | 0.29 | 0.57 | 1.36 | NS | NS | NS |
| Mirtazapine (other) | 0.31 | 0.60 | 1.45 | NS | NS | NS |
| Venlafaxine hydrochloride (other) | 0.44 | 0.86 | 2.08 | NS | NS | NS |
Self-controlled case-series analyses
The results of the self-controlled case-series analyses are shown in Table 110. The hyponatraemia rates were significantly increased for TCAs and the group of other antidepressant drugs only in the first 28 days after starting the drugs and in the first 28 days after stopping. The SSRIs rates were significantly increased throughout use and remained significantly increased during the first 84 days after stopping.
| Exposure risk period | IRR | 95% CI | p-value |
|---|---|---|---|
| Baseline period | 1.00 | ||
| TCAs | |||
| 1–28 days | 3.19 | 2.12 to 4.81 | < 0.001 |
| 29–84 days | 1.32 | 0.64 to 2.71 | 0.452 |
| 85+ days | 1.44 | 0.87 to 2.37 | 0.160 |
| Stopped 1–28 days | 2.16 | 1.37 to 3.39 | 0.001 |
| Stopped 29–84 days | 1.28 | 0.78 to 2.08 | 0.329 |
| Stopped 85–182 days | 1.09 | 0.69 to 1.73 | 0.706 |
| SSRIs | |||
| 1–28 days | 13.14 | 10.59 to 16.29 | < 0.001 |
| 29–84 days | 5.97 | 4.31 to 8.28 | < 0.001 |
| 85+ days | 2.09 | 1.48 to 2.95 | < 0.001 |
| Stopped 1–28 days | 3.88 | 2.71 to 5.57 | < 0.001 |
| Stopped 29–84 days | 1.84 | 1.21 to 2.80 | 0.005 |
| Stopped 85–182 days | 1.15 | 0.75 to 1.78 | 0.523 |
| Others | |||
| 1–28 days | 3.63 | 2.03 to 6.51 | < 0.001 |
| 29–84 days | 0.65 | 0.16 to 2.67 | 0.550 |
| 85+ days | 0.78 | 0.38 to 1.59 | 0.494 |
| Stopped 1–28 days | 2.64 | 1.29 to 5.43 | 0.008 |
| Stopped 29–84 days | 0.70 | 0.22 to 2.25 | 0.549 |
| Stopped 85–182 days | 1.28 | 0.58 to 2.81 | 0.544 |
Summary of results for hyponatraemia
Hyponatraemia risk was significantly associated only with use of SSRIs overall; however, there were increased risks for all classes of antidepressant in the first 28 days after starting the drugs. The risk of hyponatraemia tended to decrease as SSRI dose increased. Among the most commonly prescribed antidepressant drugs, there were significantly increased HRs for escitalopram, fluoxetine and citalopram.
Overall summary of results across all outcomes
Table 111 shows the adjusted HRs for all 13 outcomes by antidepressant class. Use of a combination of antidepressant drugs had the highest HRs for many of the outcomes. There were significant differences between the three main classes of antidepressant drugs and their associations with the adverse outcomes for seven of the outcomes. For these outcomes, SSRIs had the highest HRs for falls and hyponatraemia; the group of other antidepressant drugs had the highest HRs for overall mortality, attempted suicide/self-harm, stroke/TIA, fracture and epilepsy/seizures; and TCAs did not have the highest HR for any of the outcomes. The results of complete case analyses where we also adjusted for BMI were very similar. The proportional hazards assumption of the Cox proportional hazards model was reasonable for most outcomes, based on a graphical evaluation, although there was some indication of convergence for stroke/TIA, upper GI bleed and hyponatraemia towards the end of the follow-up period.
| Outcome | HRs (95% CI) | |||
|---|---|---|---|---|
| TCAs | SSRIs | Other antidepressants | Combination of antidepressants | |
| All-cause mortality | 1.16 (1.10 to 1.22) | 1.54 (1.48 to 1.59) | 1.66 (1.56 to 1.77) | 1.84 (1.59 to 2.13) |
| Sudden cardiac death | 1.36 (0.73 to 2.53) | 1.21 (0.70 to 2.07) | 2.25 (1.05 to 4.83) | 1.91 (0.26 to 13.92) |
| Suicide | 4.27 (1.56 to 11.70) | 4.87 (1.99 to 11.96) | 11.29 (4.06 to 31.35) | 12.11 (1.48 to 98.81) |
| Attempted suicide/self-harm | 1.70 (1.28 to 2.25) | 2.16 (1.71 to 2.71) | 5.16 (3.90 to 6.83) | 4.15 (2.03 to 8.48) |
| MI | 1.09 (0.96 to 1.23) | 1.15 (1.04 to 1.27) | 1.04 (0.85 to 1.27) | 1.03 (0.62 to 1.72) |
| Stroke/TIA | 1.02 (0.93 to 1.11) | 1.17 (1.10 to 1.26) | 1.37 (1.22 to 1.55) | 1.42 (1.05 to 1.91) |
| Falls | 1.30 (1.23 to 1.38) | 1.66 (1.58 to 1.73) | 1.39 (1.28 to 1.52) | 1.70 (1.42 to 2.05) |
| Fractures | 1.24 (1.14 to 1.35) | 1.56 (1.46 to 1.67) | 1.63 (1.45 to 1.83) | 2.08 (1.63 to 2.66) |
| Upper GI bleed | 1.29 (1.10 to 1.51) | 1.22 (1.07 to 1.40) | 1.37 (1.08 to 1.74) | 1.44 (0.82 to 2.56) |
| Epilepsy/seizures | 1.02 (0.76 to 1.38) | 1.83 (1.49 to 2.26) | 2.24 (1.60 to 3.15) | 2.61 (1.23 to 5.55) |
| RTAs | 0.86 (0.64 to 1.15) | 0.89 (0.70 to 1.13) | 0.67 (0.39 to 1.14) | 1.34 (0.50 to 3.60) |
| ADRs | 1.06 (0.86 to 1.29) | 1.16 (0.98 to 1.37) | 0.95 (0.68 to 1.34) | 0.85 (0.35 to 2.06) |
| Hyponatraemia | 1.05 (0.87 to 1.27) | 1.52 (1.33 to 1.75) | 1.28 (0.98 to 1.67) | 1.38 (0.74 to 2.59) |
Table 112 shows the adjusted HRs for 11 outcomes according to individual antidepressant drugs. There were significant differences between the drugs for seven outcomes; of these, venlafaxine had the highest HR for three outcomes (stroke/TIA, fracture and epilepsy/seizures) and trazodone had the highest HR for one outcome (all-cause mortality), as did citalopram (falls), escitalopram (hyponatraemia) and mirtazapine (attempted suicide). Amitriptyline, dosulepin, fluoxetine, lofepramine, paroxetine and sertraline did not have the highest HRs for any of these seven outcomes. There was some indication (p = 0.05) of a difference between the drugs for ADRs, with lofepramine having the highest HR for this outcome.
| Outcome | TCAs | SSRIs | Other class | p-valuea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline | Dosulepin | Lofepramine | Trazodone | Citalopram | Escitalopram | Fluoxetine | Paroxetine | Sertraline | Mirtazapine | Venlafaxine | ||
| All-cause mortality | 1.10b | 1.03 | 1.51b | 1.82b | 1.55b | 1.45b | 1.66b | 1.24b | 1.47b | 1.76b | 1.66b | < 0.001 |
| Attempted suicide | 1.07 | 1.87b | 2.58b | 4.70b | 2.70b | 2.08 | 2.08b | 1.14 | 2.07b | 6.11b | 4.60b | < 0.001 |
| MI | 1.10 | 1.07 | 1.18 | 1.04 | 1.10 | 1.31 | 1.31b | 1.10 | 0.89 | 1.11 | 1.04 | 0.650 |
| Stroke/TIA | 1.01 | 0.95 | 1.26 | 1.10 | 1.22b | 1.21 | 1.16 | 1.08 | 1.22 | 1.38b | 1.51b | < 0.001 |
| Falls | 1.32b | 1.24b | 1.34b | 1.55b | 1.76b | 1.66b | 1.64b | 1.45b | 1.63b | 1.19b | 1.68b | < 0.001 |
| Fractures | 1.22b | 1.23b | 1.46b | 0.97 | 1.62b | 1.29 | 1.58b | 1.46b | 1.60b | 1.46b | 1.87b | < 0.001 |
| Upper GI bleed | 1.38b | 1.21 | 1.21 | 1.79 | 1.34b | 1.07 | 1.15 | 1.15 | 1.04 | 1.05 | 1.71b | 0.440 |
| Epilepsy/seizures | 1.17 | 0.50 | 1.37 | 1.46 | 1.79b | 1.75 | 1.49 | 2.04b | 2.68b | 1.59 | 2.99b | 0.003 |
| RTAs | 0.84 | 0.50 | 1.13 | 1.53 | 1.01 | 0.98 | 0.70 | 0.90 | 0.89 | 0.45 | 0.72 | 0.330 |
| ADRs | 0.96 | 0.88 | 2.11b | 1.05 | 1.12 | 1.10 | 1.19 | 0.93 | 1.60b | 1.02 | 0.88 | 0.050 |
| Hyponatraemia | 1.17 | 0.83 | 0.93 | 1.51 | 1.65b | 2.08b | 1.70b | 1.04 | 1.00 | 1.06 | 1.53 | < 0.001 |
Table 113 shows the number of extra cases per 10,000 patients treated over 1 year for the 13 outcomes according to antidepressant class and individual drug. Overall, the number of extra cases were highest for all-cause mortality, falls, fractures and attempted suicide/self-harm, and generally low for the other outcomes.
| Antidepressant class/drug | Extra cases per 10,000 treated in 1 year compared with no antidepressant use | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | Sudden cardiac death | Suicide | Attempted suicide | MI | Stroke/TIA | Falls | Fractures | Upper GI bleed | Epilepsy/seizures | RTAs | ADRs | Hyponatraemia | |
| Antidepressant class | |||||||||||||
| TCAs | 109 | NS | 3 | 18 | NS | NS | 103 | 42 | 12 | NS | NS | NS | NS |
| SSRIs | 357 | NS | 3 | 29 | 15 | 38 | 220 | 98 | 9 | 15 | NS | NS | 15 |
| Other antidepressants | 439 | NS | 8 | 105 | NS | 81 | 133 | 109 | 15 | 18 | NS | NS | NS |
| Antidepressant drug | |||||||||||||
| Amitriptyline hydrochloride | 69 | NS | NS | NS | 108 | 38 | 16 | NS | NS | NS | NS | ||
| Dosulepin hydrochloride | NS | 23 | NS | NS | 81 | 40 | NS | NS | NS | NS | NS | ||
| Lofepramine | 339 | 41 | NS | NS | 115 | 80 | NS | NS | NS | 29 | NS | ||
| Trazodone hydrochloride | 540 | 95 | NS | NS | 186 | NS | NS | NS | NS | NS | NS | ||
| Citalopram hydrobromide | 365 | 43 | NS | 48 | 255 | 107 | 14 | 16 | NS | NS | 18 | ||
| Escitalopram | 302 | 22 | NS | NS | 223 | NS | NS | NS | NS | NS | 31 | ||
| Fluoxetine hydrochloride | 439 | 28 | 31 | NS | 214 | 100 | NS | NS | NS | NS | 20 | ||
| Paroxetine hydrochloride | 164 | NS | NS | NS | 150 | 80 | NS | 21 | NS | NS | NS | ||
| Sertraline hydrochloride | 316 | 28 | NS | 48 | 211 | 104 | NS | 34 | NS | 16 | NS | ||
| Mirtazapine | 501 | 131 | NS | 83 | 65 | 80 | NS | NS | NS | NS | NS | ||
| Venlafaxine hydrochloride | 436 | 92 | NS | 112 | 227 | 150 | 30 | 41 | NS | NS | NS | ||
Results of health economic analyses
Patients
Given the objective of estimating costs for the first 5 years post diagnosis, the exclusion of patients who were diagnosed after 1 January 2004, those who moved practice before 5 years, and those who were initially prescribed more than one type of antidepressant resulted in the identification of 37,268 eligible patients (base-case analysis). When costs were estimated for a 1-year follow-up period data, were available for 58,657 patients (sensitivity analysis).
Levels of resource use
The number of patients (of the 37,268 eligible patients) who were first prescribed each of the 11 most commonly prescribed antidepressant drugs, within 12 months of diagnosis, are shown in Table 114. It can be seen that amitriptyline (TCA) was the most commonly first prescribed antidepressant for the 5-year post-diagnosis period based on patients who were diagnosed before 1 January 2004 and who did not move practice within 5 years, with 6231 of the eligible patients initially receiving a prescription for this drug within 12 months of diagnosis. Citalopram was the most commonly first prescribed antidepressant over the 1-year post-diagnosis period, based on patients who were diagnosed before 1 January 2008 and who did not move practice within 1 year. Figures for the remaining antidepressant drugs are also shown in Table 114, where it should be noted that 4811 of the eligible patients did not receive any antidepressant prescriptions within the 5-year period.
| First antidepressant drug prescribed | No. of patients | No. of prescriptions per user | ||
|---|---|---|---|---|
| Minimum | Maximum | Mean | ||
| Not prescribed antidepressants | 4811 (8599) | – | – | – |
| Amitriptyline hydrochloride (TCA) | 6231 (8647) | 1 (1) | 207 (91) | 10.53 (3.84) |
| Dosulepin hydrochloride (TCA) | 5143 (6461) | 1 (1) | 284 (86) | 11.93 (4.46) |
| Lofepramine (TCA) | 2123 (2675) | 1 (1) | 217 (54) | 7.08 (3.61) |
| Trazodone hydrochloride (TCA) | 490 (734) | 1 (1) | 245 (53) | 12.64 (5.04) |
| Citalopram hydrobromide (SSRI) | 4654 (10,066) | 1 (1) | 263 (100) | 15.49 (5.96) |
| Escitalopram (SSRI) | 320 (1201) | 1 (1) | 137 (50) | 11.13 (5.29) |
| Fluoxetine hydrochloride (SSRI) | 5576 (9489) | 1 (1) | 258 (60) | 11.51 (4.75) |
| Paroxetine hydrochloride (SSRI) | 3474 (4058) | 1 (1) | 242 (55) | 13.21 (4.93) |
| Sertraline hydrochloride (SSRI) | 1760 (2662) | 1 (1) | 245 (53) | 13.43 (5.35) |
| Mirtazapine (other) | 312 (888) | 1 (1) | 253 (64) | 16.32 (6.31) |
| Venlafaxine hydrochloride (other) | 709 (1033) | 1 (1) | 402 (92) | 17.49 (5.97) |
| TCAs | 14973 (19,737) | 1 (1) | 284 (91) | 11.44 (4.35) |
| SSRIs | 15819 (27,544) | 1 (1) | 263 (100) | 14.54 (5.74) |
| Other antidepressants | 1665 (27,77) | 1 (1) | 402 (92) | 13.94 (5.52) |
Patients often received prescriptions for more than one different antidepressant within the first 5 years post diagnosis. Table 115 shows the total number of prescriptions for all antidepressant drugs for patients initially prescribed each of the 11 antidepressant drugs. For example, patients who were first prescribed amitriptyline (TCA) within 12 months of diagnosis received an average of 18.68 antidepressant prescriptions over 5 years (range 1–407). Looking at Table 114, it can be seen that approximately one-half of these prescriptions were for amitriptyline (mean 10.53), which shows that a number of patients who originally started on amitriptyline switched to other antidepressant drugs within the 5-year post-diagnosis period.
| First antidepressant drug prescribed | No. of antidepressant prescriptions | ||
|---|---|---|---|
| Minimum | Maximum | Mean | |
| Not prescribed antidepressants | – | – | – |
| Amitriptyline hydrochloride (TCA) | 1 (1) | 407 (117) | 18.68 (4.93) |
| Dosulepin hydrochloride (TCA) | 1 (1) | 319 (86) | 20.10 (5.53) |
| Lofepramine (TCA) | 1 (1) | 264 (54) | 18.14 (5.30) |
| Trazodone hydrochloride (TCA) | 1 (1) | 245 (66) | 23.27 (7.02) |
| Citalopram hydrobromide (SSRI) | 1 (1) | 417 (100) | 23.32 (7.10) |
| Escitalopram (SSRI) | 1 (1) | 145 (50) | 18.48 (6.51) |
| Fluoxetine hydrochloride (SSRI) | 1 (1) | 389 (81) | 19.33 (5.98) |
| Paroxetine hydrochloride (SSRI) | 1 (1) | 327 (73) | 22.05 (6.12) |
| Sertraline hydrochloride (SSRI) | 1 (1) | 245 (53) | 21.55 (6.49) |
| Mirtazapine (other) | 1 (1) | 253 (67) | 25.78 (7.65) |
| Venlafaxine hydrochloride (other) | 1 (1) | 402 (92) | 26.42 (7.20) |
| TCAs | 1 (1) | 407 (117) | 19.43 (5.29) |
| SSRIs | 1 (1) | 417 (100) | 21.33 (6.48) |
| Other antidepressants | 1 (1) | 402 (92) | 23.90 (6.94) |
The number of visits to practice nurses, community nurses and GPs are shown in Tables 116–118. For example, those initially prescribed amitriptyline (TCA) visited the practice nurse on average 13.4 times over the 5-year post-diagnosis period, compared with 5.2 visits to community nurses and 50.9 to GPs. On average, those not prescribed any antidepressant drugs had the fewest GP and practice nurse visits, although this was not always the case for community nurse visits.
| First antidepressant drug prescribed | Number of practice nurse visits | ||
|---|---|---|---|
| Minimum | Maximum | Mean | |
| Not prescribed antidepressants | 0 (0) | 177 (115) | 9.40 (2.67) |
| Amitriptyline hydrochloride (TCA) | 0 (0) | 419 (93) | 13.44 (2.81) |
| Dosulepin hydrochloride (TCA) | 0 (0) | 249 (89) | 10.97 (2.02) |
| Lofepramine (TCA) | 0 (0) | 348 (102) | 10.28 (1.99) |
| Trazodone hydrochloride (TCA) | 0 (0) | 154 (61) | 10.14 (2.78) |
| Citalopram hydrobromide (SSRI) | 0 (0) | 547 (176) | 12.68 (3.06) |
| Escitalopram (SSRI) | 0 (0) | 118 (64) | 12.37 (2.95) |
| Fluoxetine hydrochloride (SSRI) | 0 (0) | 271 (76) | 11.30 (2.70) |
| Paroxetine hydrochloride (SSRI) | 0 (0) | 227 (73) | 10.79 (1.98) |
| Sertraline hydrochloride (SSRI) | 0 (0) | 145 (139) | 10.39 (2.43) |
| Mirtazapine (other) | 0 (0) | 74 (51) | 9.82 (2.81) |
| Venlafaxine hydrochloride (other) | 0 (0) | 193 (51) | 11.47 (2.32) |
| TCAs | 0 (0) | 419 (102) | 11.86 (2.40) |
| SSRIs | 0 (0) | 547 (1.76) | 11.51 (2.71) |
| Other antidepressants | 0 (0) | 193 (52) | 11.10 (2.48) |
| First antidepressant drug prescribed | No. of community nurse visits | ||
|---|---|---|---|
| Minimum | Maximum | Mean | |
| Not prescribed antidepressants | 0 (0) | 624 (287) | 4.24 (1.14) |
| Amitriptyline hydrochloride (TCA) | 0 (0) | 578 (172) | 5.16 (1.10) |
| Dosulepin hydrochloride (TCA) | 0 (0) | 327 (161) | 3.97 (0.68) |
| Lofepramine (TCA) | 0 (0) | 219 (73) | 3.31 (0.69) |
| Trazodone hydrochloride (TCA) | 0 (0) | 222 (57) | 4.92 (1.38) |
| Citalopram hydrobromide (SSRI) | 0 (0) | 565 (227) | 5.51 (1.48) |
| Escitalopram (SSRI) | 0 (0) | 273 (72) | 5.53 (1.17) |
| Fluoxetine hydrochloride (SSRI) | 0 (0) | 393 (168) | 4.56 (1.15) |
| Paroxetine hydrochloride (SSRI) | 0 (0) | 305 (101) | 3.36 (0.66) |
| Sertraline hydrochloride (SSRI) | 0 (0) | 204 (138) | 4.11 (1.02) |
| Mirtazapine (other) | 0 (0) | 129 (256) | 4.81 (2.11) |
| Venlafaxine hydrochloride (other) | 0 (0) | 161 (308) | 5.33 (1.59) |
| TCAs | 0 (0) | 578 (172) | 4.37 (0.88) |
| SSRIs | 0 (0) | 565 (227) | 4.54 (1.19) |
| Other antidepressants | 0 (0) | 257 (308) | 4.28 (1.42) |
| First antidepressant drug prescribed | No. of GP visits | ||
|---|---|---|---|
| Minimum | Maximum | Mean | |
| Not prescribed antidepressants | 0 (0) | 294 (115) | 33.96 (9.30) |
| Amitriptyline hydrochloride (TCA) | 0 (0) | 429 (110) | 50.92 (13.42) |
| Dosulepin hydrochloride (TCA) | 0 (0) | 625 (97) | 42.46 (9.37) |
| Lofepramine (TCA) | 0 (0) | 283 (105) | 42.71 (10.08) |
| Trazodone hydrochloride (TCA) | 0 (0) | 291 (96) | 48.35 (11.87) |
| Citalopram hydrobromide (SSRI) | 0 (0) | 328 (97) | 49.22 (9.37) |
| Escitalopram (SSRI) | 1 (0) | 230 (90) | 54.60 (14.58) |
| Fluoxetine hydrochloride (SSRI) | 0 (0) | 528 (136) | 44.19 (11.96) |
| Paroxetine hydrochloride (SSRI) | 0 (0) | 266 (105) | 44.27 (9.78) |
| Sertraline hydrochloride (SSRI) | 0 (0) | 413 (84) | 45.08 (11.23) |
| Mirtazapine (other) | 0 (0) | 222 (118) | 50.91 (14.43) |
| Venlafaxine hydrochloride (other) | 0 (0) | 277 (88) | 48.78 (11.82) |
| TCAs | 0 (0) | 625 (144) | 45.86 (10.64) |
| SSRIs | 0 (0) | 528 (136) | 45.23 (12.22) |
| Other antidepressants | 0 (0) | 277 (118) | 46.73 (12.18) |
Dosage
The estimated weighted-average dose for each type of chemical, across all prescriptions, in both our study data set and for the Prescription Cost Analysis database60 are shown in Table 119. It can be seen that patients within our study cohort (all of whom were aged ≥ 65 years) received, on average, a slightly lower dose than the mean from the Prescription Cost Analysis database. However, for simplicity, the mean unit costs were based on the weighted average (at the individual chemical level) within the Prescription Cost Analysis database. 60 Further justification for this was provided by the fact that the cost/mg does not vary systematically according to dosage52 (the cost/mg reduces with dose for some drugs, whereas it increases for others).
| Mean prescription dosage (mg) in study database | Mean prescription dosage (mg) in the Prescription Cost Analysis database60 | |
|---|---|---|
| Amitriptyline hydrochloride (TCA) | 20.77 | 21.89 |
| Dosulepin hydrochloride (TCA) | 45.37 | 50.20 |
| Lofepramine (TCA) | 70.00 | 70.00 |
| Trazodone hydrochloride (TCA) | 75.15 | 87.66 |
| Citalopram hydrobromide (SSRI) | 17.85 | 19.92 |
| Escitalopram (SSRI) | 10.66 | 12.45 |
| Fluoxetine hydrochloride (SSRI) | 20.02 | 20.56 |
| Paroxetine hydrochloride (SSRI) | 21.02 | 21.58 |
| Sertraline hydrochloride (SSRI) | 61.80 | 69.42 |
| Mirtazapine (other) | 27.79 | 28.90 |
| Venlafaxine hydrochloride (other) | 79.38 | 95.46 |
Unit costs
The unit cost (per prescription), at the level of the individual chemical, for each of the different antidepressant drugs was extracted from the Prescription Cost Analysis database. 60 Figures for the 11 most commonly prescribed antidepressant drugs are shown in Table 120, where it can be seen that the prescription costs vary between £1.64 [amitriptyline (TCA)] and £39.29 [lofepramine (TCA)] per prescription. In terms of visit costs, Curtis62 estimated the unit cost for a visit to a practice nurse to be £11.00 compared with £36.00 for a GP consultation and £26.00 for a home visit by a community nurse (costs were estimated at 2007–8 financial year levels). These unit costs include salary costs, employers’ costs (national insurance and superannuation), qualifications, overheads and travel (if applicable). 62 Owing to the fact that these costs are estimated to the nearest pound (and not pence), when estimating costs over a 1-year period, pence values are not reported as these are equivalent to zero (discounting means that this is not the case over the 5-year period).
| Unit cost per prescription (£) | |
|---|---|
| Amitriptyline hydrochloride (TCA) | 1.64 |
| Dosulepin hydrochloride (TCA) | 2.90 |
| Lofepramine (TCA) | 39.29 |
| Trazodone hydrochloride (TCA) | 11.67 |
| Citalopram hydrobromide (SSRI) | 1.73 |
| Escitalopram (SSRI) | 19.79 |
| Fluoxetine hydrochloride (SSRI) | 2.43 |
| Paroxetine hydrochloride (SSRI) | 6.89 |
| Sertraline hydrochloride (SSRI) | 2.74 |
| Mirtazapine (other) | 12.15 |
| Venlafaxine hydrochloride (other) | 34.79 |
Prescription costs
Mean total prescription costs over the 5-year study period, for the 11 most commonly prescribed antidepressant drugs, were estimated using the prescription numbers and unit costs (as reported in Tables 114 and 120, respectively) and are shown in Table 121. Amitriptyline (TCA) had the lowest mean cost over the 5-year period (£16.44) and venlafaxine (other) had the highest mean cost (£578.05), and there was wide variation across different patients who were initially prescribed the same drug. Figures for the 1-year post-diagnosis period are also shown in Table 121, where it can be seen that the rankings, in terms of lowest (1) to highest (11) cost, are broadly similar to those over a 5-year period.
| Antidepressant drug | Minimum (£) | Maximum (£) | Mean (£) | Ranked costb |
|---|---|---|---|---|
| Not prescribed antidepressants | – | – | – | |
| Amitriptyline hydrochloride (TCA) | 1.64 (1.64) | 319.26 (149.41) | 16.44 (6.31) | 1 (1) |
| Dosulepin hydrochloride (TCA) | 2.90 (2.90) | 747.48 (249.40) | 32.98 (12.94) | 4 (4) |
| Lofepramine (TCA) | 39.29 (39.29) | 7941.47 (2121.85) | 268.01 (141.81) | 10 (9) |
| Trazodone hydrochloride (TCA) | 11.67 (11.67) | 2662.67 (618.77) | 141.08 (58.87) | 7 (7) |
| Citalopram hydrobromide (SSRI) | 1.73 (1.73) | 424.49 (173.31) | 25.55 (10.32) | 2 (2) |
| Escitalopram (SSRI) | 19.79 (19.79) | 2520.27 (989.71) | 211.04 (184.81) | 9 (10) |
| Fluoxetine hydrochloride (SSRI) | 2.43 (2.43) | 585.50 (145.73) | 26.72 (11.53) | 3 (3) |
| Paroxetine hydrochloride (SSRI) | 6.89 (6.89) | 1510.94 (378.93) | 85.45 (33.96) | 6 (6) |
| Sertraline hydrochloride (SSRI) | 2.74 (2.74) | 634.45 (145.44) | 35.18 (14.69) | 5 (5) |
| Mirtazapine (other) | 12.15 (12.15) | 2869.04 (777.78) | 188.34 (76.71) | 8 (8) |
| Venlafaxine hydrochloride (other) | 34.79 (34.79) | 12,827.79 (320.42) | 578.05 (207.85) | 11 (11) |
| TCAs | 1.64 (1.64) | 7,941.47 (2121.85) | 74.08 (33.66) | |
| SSRIs | 1.73 (1.73) | 2520.27 (989.71) | 49.02 (20.89) | |
| Other antidepressants | 3.08 (3.08) | 12,827.79 (3200.42) | 331.82 (119.59) |
When the mean total prescription costs for all antidepressant drugs were estimated over the 5- and 1-year post-diagnosis period, it can be seen (Table 122) that the rank ordering of lowest (1) to highest (11) cost was broadly similar across the 11 most commonly prescribed antidepressant drugs. When these figures were collated across different classes of antidepressant drugs (TCAs, SSRIs and other antidepressant drugs), the mean cost was estimated to be lowest for SSRIs and highest for other antidepressant drugs.
| Antidepressant drug | Minimum (£) | Maximum (£) | Mean (£) | Ranked cost |
|---|---|---|---|---|
| Not prescribed antidepressants | – | – | – | |
| Amitriptyline hydrochloride (TCA) | 1.64 (1.64) | 7800.59 (1,599.62) | 40.13 (15.34) | 1 (1) |
| Dosulepin hydrochloride (TCA) | 2.90 (2.90) | 7703.10 (2,719.20) | 60.79 (22.49) | 2 (2) |
| Lofepramine (TCA) | 39.29 (39.29) | 8271.83 (2,121.85) | 308.08 (153.09) | 10 (10) |
| Trazodone hydrochloride (TCA) | 11.67 (11.67) | 2662.67 (1,415.31) | 196.10 (80.48) | 7 (7) |
| Citalopram hydrobromide (SSRI) | 1.73 (1.73) | 10,406.06 (1,599.62) | 69.11 (24.29) | 3 (3) |
| Escitalopram (SSRI) | 19.79 (19.79) | 3101.71 (989.71) | 249.58 (116.98) | 8 (9) |
| Fluoxetine hydrochloride (SSRI) | 2.43 (2.43) | 4644.71 (1,867.28) | 69.34 (25.92) | 4 (4) |
| Paroxetine hydrochloride (SSRI) | 6.89 (6.89) | 10,448.22 (1,302.64) | 135.07 (48.04) | 6 (6) |
| Sertraline hydrochloride (SSRI) | 2.74 (2.74) | 2441.36 (1,466.55) | 75.48 (30.14) | 5 (5) |
| Mirtazapine (other) | 12.15 (12.15) | 2869.04 (1,589.73) | 251.44 (94.25) | 9 (8) |
| Venlafaxine hydrochloride (other) | 34.79 (34.79) | 12,827.79 (3,200.42) | 602.81 (216.35) | 11 (11) |
| TCAs | 1.64 (1.64) | 8271.83 (2,719.20) | 95.98 (40.75) | |
| SSRIs | 1.73 (1.73) | 10,448.22 (1,867.28) | 88.22 (33.08) | |
| Other antidepressants | 3.08 (3.08) | 12,827.79 (3,200.42) | 359.26 (128.84) |
We assessed whether those patients who changed practices (within the 5-year period) had different costs to those who remained with the same practice for the whole 5-year period. Of those who were otherwise eligible for the base-case analysis, but who changed practices, 5334 had a cost over 1 year, the mean value of which was estimated to be £40.22. Conversely, the 37,268 who were included in the base-case analysis had a mean cost of £35.87 over 1 year.
The total costs associated with practice nurse, community nurse and GP visits are reported in Tables 123–125, respectively. It can be seen that total costs for both practice nurse and community nurse visits are broadly comparable, although there is variation across antidepressant drugs. Conversely, GP visit costs are substantially higher, varying between a mean of £1145.35 over 5 years for those who received no antidepressant prescriptions and £1707.58 for those prescribed escitalopram (SSRI).
| Antidepressant drug | Total costs associated with practice nurse visits | ||
|---|---|---|---|
| Minimum (£) | Maximum (£) | Mean (£) | |
| Not prescribed antidepressants | 0 (0) | 1841.81 (1265) | 96.49 (29.24) |
| Amitriptyline hydrochloride (TCA) | 0 (0) | 4236.66 (1023) | 137.60 (30.96) |
| Dosulepin hydrochloride (TCA) | 0 (0) | 2523.37 (979) | 112.25 (22.21) |
| Lofepramine (TCA) | 0 (0) | 3607.07 (1122) | 105.05 (21.94) |
| Trazodone hydrochloride (TCA) | 0 (0) | 1545.66 (671) | 103.86 (30.53) |
| Citalopram hydrobromide (SSRI) | 0 (0) | 5716.46 (1936) | 130.35 (33.61) |
| Escitalopram (SSRI) | 0 (0) | 1191.42 (704) | 127.41 (32.46) |
| Fluoxetine hydrochloride (SSRI) | 0 (0) | 2790.60 (836) | 115.97 (29.67) |
| Paroxetine hydrochloride (SSRI) | 0 (0) | 2371.77 (803) | 110.46 (21.75) |
| Sertraline hydrochloride (SSRI) | 0 (0) | 1,488.86 (1529) | 106.51 (26.73) |
| Mirtazapine (other) | 0 (0) | 729.48 (561) | 101.16 (30.92) |
| Venlafaxine hydrochloride (other) | 0 (0) | 2005.76 (572) | 117.97 (25.47) |
| TCAs | 0 (0) | 4236.66 (1122) | 121.29 (26.35) |
| SSRIs | 0 (0) | 5716.46 (1936) | 118.14 (29.77) |
| Other antidepressants | 0 (0) | 2005.76 (572) | 113.99 (27.25) |
| Antidepressant drug | Total costs associated with community nurse visits | ||
|---|---|---|---|
| Minimum (£) | Maximum (£) | Mean (£) | |
| Not prescribed antidepressants | 0 (0) | 14,605.77 (7462) | 102.65 (29.53) |
| Amitriptyline hydrochloride (TCA) | 0 (0) | 14,075.80 (4472) | 124.64 (28.51) |
| Dosulepin hydrochloride (TCA) | 0 (0) | 7684.30 (4186) | 95.24 (17.67) |
| Lofepramine (TCA) | 0 (0) | 5208.10 (1898) | 79.61 (17.91) |
| Trazodone hydrochloride (TCA) | 0 (0) | 5479.25 (1482) | 119.14 (35.99) |
| Citalopram hydrobromide (SSRI) | 0 (0) | 13,810.28 (5902) | 133.23 (38.42) |
| Escitalopram (SSRI) | 0 (0) | 6649.84 (1872) | 134.40 (30.55) |
| Fluoxetine hydrochloride (SSRI) | 0 (0) | 9577.72 (4368) | 110.20 (29.79) |
| Paroxetine hydrochloride (SSRI) | 0 (0) | 7559.93 (2626) | 81.09 (17.11) |
| Sertraline hydrochloride (SSRI) | 0 (0) | 4950.92 (3588) | 99.79 (26.48) |
| Mirtazapine (other) | 0 (0) | 3260.28 (6656) | 117.20 (54.90) |
| Venlafaxine hydrochloride (other) | 0 (0) | 4053.24 (8008) | 129.61 (29.53) |
| TCAs | 0 (0) | 14,075.80 (4472) | 105.32 (22.80) |
| SSRIs | 0 (0) | 13,810.28 (5902) | 109.92 (30.87) |
| Other antidepressants | 0 (0) | 6070.45 (8008) | 103.78 (36.99) |
| Antidepressant drug | Total costs associated with GP visits | ||
|---|---|---|---|
| Minimum (£) | Maximum (£) | Mean (£) | |
| Not prescribed antidepressants | 0 (0) | 9800.19 (4140) | 1145.35 (334.70) |
| Amitriptyline hydrochloride (TCA) | 0 (0) | 14,749.99 (5184) | 1685.53 (422.24) |
| Dosulepin hydrochloride (TCA) | 0 (0) | 20,631.76 (3492) | 1364.22 (337.32) |
| Lofepramine (TCA) | 0 (0) | 9543.30 (3780) | 1355.88 (362.77) |
| Trazodone hydrochloride (TCA) | 0 (0) | 9794.84 (3456) | 1512.14 (427.39) |
| Citalopram hydrobromide (SSRI) | 0 (0) | 10,801.56 (3960) | 1593.10 (483.06) |
| Escitalopram (SSRI) | 32.47 (0) | 7691.73 (3240) | 1707.58 (524.98) |
| Fluoxetine hydrochloride (SSRI) | 0 (0) | 17,702.75 (4896) | 1440.51 (430.38) |
| Paroxetine hydrochloride (SSRI) | 0 (0) | 8956.14 (3780) | 1444.79 (352.05) |
| Sertraline hydrochloride (SSRI) | 0 (0) | 7858.29 (3024) | 1439.86 (404.34) |
| Mirtazapine (other) | 0 (0) | 6691.40 (3024) | 1553.25 (519.49) |
| Venlafaxine hydrochloride (other) | 0 (0) | 9320.32 (3168) | 1548.64 (425.48) |
| TCAs | 0 (0) | 20,631.76 (5184) | 1514.67 (382.99) |
| SSRIs | 0 (0) | 17,702.75 (4896) | 1491.30 (439.74) |
| Other antidepressants | 0 (0) | 9320.32 (4248) | 1458.20 (438.30) |
When the costs associated with practice nurse, community nurse and GP visits were summed, the total visit cost over 5 years varied between an average of £1344.49 for those who received no antidepressant drugs and £1969.39 for those prescribed escitalopram (SSRI) (Table 126). There was also wide variation across different patients who were initially prescribed the same drug.
| Antidepressant drug | Total visit costs (practice nurse, community nurse and GP) | ||
|---|---|---|---|
| Minimum (£) | Maximum (£) | Mean (£) | |
| Not prescribed antidepressants | 0 (0) | 21,993.03 (7697) | 1344.49 (393.58) |
| Amitriptyline hydrochloride (TCA) | 0 (0) | 21,765.24 (5564) | 1947.76 (481.71) |
| Dosulepin hydrochloride (TCA) | 0 (0) | 20,702.63 (4797) | 1571.60 (377.20) |
| Lofepramine (TCA) | 0 (0) | 10,912.18 (4642) | 1540.55 (402.63) |
| Trazodone hydrochloride (TCA) | 0 (0) | 12,518.13 (3630) | 1735.14 (493.91) |
| Citalopram hydrobromide (SSRI) | 0 (0) | 17,606.22 (6417) | 1856.68 (555.09) |
| Escitalopram (SSRI) | 32.47 (0) | 9220.75 (3240) | 1969.39 (587.99) |
| Fluoxetine hydrochloride (SSRI) | 0 (0) | 20,695.92 (6153) | 1666.68 (489.84) |
| Paroxetine hydrochloride (SSRI) | 0 (0) | 10,446.79 (4563) | 1636.34 (390.92) |
| Sertraline hydrochloride (SSRI) | 0 (0) | 9686.08 (4769) | 1646.15 (457.55) |
| Mirtazapine (other) | 0 (0) | 8715.47 (7229) | 1771.60 (605.30) |
| Venlafaxine hydrochloride (other) | 0 (0) | 12,882.21 (8091) | 1796.22 (492.23) |
| TCAs | 0 (0) | 21,765.24 (5564) | 1741.29 (432.15) |
| SSRIs | 0 (0) | 20,695.92 (6417) | 1719.36 (500.38) |
| Other antidepressants | 0 (0) | 13,610.63 (8091) | 1675.97 (502.54) |
When the total prescription costs for all antidepressant drugs were added to the total visit costs, in order to estimate the overall visit plus prescription cost, of the 11 most commonly prescribed antidepressant drugs, dosulepin (TCA) was estimated to have the lowest (1) mean cost (£1632.39) and venlafaxine (other) the highest (11; £2399.04) (Table 127). The mean cost for the different classes of antidepressant drugs ranged between £1807.58 for SSRIs and £2035.23 for other antidepressant drugs.
| Antidepressant drug | Total visits plus prescription costs | Ranked cost | ||
|---|---|---|---|---|
| Minimum (£) | Maximum (£) | Mean (£) | ||
| Not prescribed antidepressants | 0.00 (0.00) | 21,993.03 (7697.00) | 1344.49 (393.58) | |
| Amitriptyline hydrochloride (TCA) | 1.64 (1.64) | 21,771.80 (5565.64) | 1987.90 (497.05) | 8 (4) |
| Dosulepin hydrochloride (TCA) | 2.90 (2.90) | 20,731.63 (4802.33) | 1632.39 (399.68) | 1 (1) |
| Lofepramine (TCA) | 39.29 (39.29) | 11,030.06 (5108.97) | 1848.63 (555.72) | 5 (6) |
| Trazodone hydrochloride (TCA) | 11.67 (11.67) | 12,589.96 (3641.67) | 1931.24 (574.38) | 7 (7) |
| Citalopram hydrobromide (SSRI) | 1.73 (1.73) | 17,607.95 (6439.53) | 1925.80 (579.38) | 6 (8) |
| Escitalopram (SSRI) | 87.17 (19.79) | 9240.55 (3420.30) | 2218.98 (704.97) | 10 (10) |
| Fluoxetine hydrochloride (SSRI) | 2.43 (2.43) | 20,925.47 (6174.86) | 1736.03 (515.76) | 3 (5) |
| Paroxetine hydrochloride (SSRI) | 6.89 (6.89) | 13,211.86 (4718.43) | 1771.42 (438.95) | 4 (2) |
| Sertraline hydrochloride (SSRI) | 2.74 (2.74) | 9702.55 (4887.00) | 1721.64 (487.69) | 2 (3) |
| Mirtazapine (other) | 84.15 (12.15) | 9501.16 (7800.19) | 2023.04 (699.56) | 9 (9) |
| Venlafaxine hydrochloride (other) | 34.79 (34.79) | 15,934.83 (9726.00) | 2399.04 (708.58) | 11 (11) |
| TCAs | 1.64 (1.64) | 21,771.80 (5565.64) | 1837.26 (472.89) | |
| SSRIs | 1.73 (1.73) | 20,925.47 (6439.53) | 1807.58 (533.45) | |
| Other antidepressants | 3.08 (3.08) | 15,934.83 (9726.00) | 2035.23 (631.38) | |
The costs presented do not control for potential differences between patients prescribed different antidepressant drugs. Thus, we sought to control for the patient characteristics, baseline comorbidities and use of certain drugs at baseline as detailed in Chapter 2. Complete data were available for each of these variables with the exception of smoking status (2097 had missing data) and Townsend score (1090 had missing data), so 2069 of the 37,268 eligible patients were excluded from subsequent analyses. All subsequently presented 5-year costs are thereby based on 35,217 patients (base case) and, for similar reasons, 1-year costs are based on 55,907 patients (sensitivity analysis).
The incremental cost estimates from the regression analysis, i.e. the mean incremental total prescription cost for all antidepressant drugs compared with prescription of no antidepressant drugs over the 5-year study period, after controlling for other factors, are presented in Table 128. All cost differences were significant (p < 0.001) when the 11 most commonly prescribed antidepressant drugs were compared with no antidepressant prescriptions, with the adjusted R2-value ranging between 0.021 [paroxetine (SSRI)] and 0.070 [venlafaxine (other)]. The mean incremental cost ranged between £46.36 for amitriptyline (TCA) and £611.14 for venlafaxine (other), and the ranking [from lowest (1) to highest cost] was identical to that when other factors were not controlled for (see Table 122). The mean incremental cost for the different classes of antidepressant drugs ranged from £90.30 for SSRIs to £364.95 for the group of other antidepressant drugs. Incremental costs for the 1-year post-diagnosis period are also shown in Table 128.
| Antidepressant drug | Mean incremental cost (£) | Ranked cost |
|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 (15.34) | 1 (1) |
| Citalopram hydrobromide (SSRI) | 66.89 (24.29) | 3 (3) |
| Dosulepin hydrochloride (TCA) | 66.48 (22.49) | 2 (2) |
| Escitalopram (SSRI) | 232.45 (116.98) | 8 (9) |
| Fluoxetine hydrochloride (SSRI) | 72.96 (25.92) | 4 (4) |
| Lofepramine (TCA) | 314.32 (153.09) | 10 (10) |
| Mirtazapine (other) | 241.40 (94.25) | 9 (8) |
| Paroxetine hydrochloride (SSRI) | 142.83 (48.04) | 6 (6) |
| Sertraline hydrochloride (SSRI) | 77.36 (30.14) | 5 (5) |
| Trazodone hydrochloride (TCA) | 193.49 (80.48) | 7 (7) |
| Venlafaxine hydrochloride (other) | 611.14 (216.35) | 11 (11) |
| TCAs | 100.62 (40.75) | |
| SSRIs | 90.30 (33.08) | |
| Other antidepressants | 364.95 (128.84) |
Sensitivity analysis
When the overall visit plus prescription costs were adjusted for differences between patients prescribed different antidepressant drugs, all cost differences were significant (p < 0.001) when the 11 most commonly prescribed antidepressant drugs were compared with those patients who were prescribed no antidepressant prescriptions. Also, the adjusted R2-value ranged between 0.122 [escitalopram (SSRI)] and 0.125 [venlafaxine (other)], and venlafaxine (other) was again found to have the highest mean incremental cost. However, fluoxetine (SSRI) was now found to have the lowest mean incremental cost (£217.30) and the incremental costs were higher, after the inclusion of visit costs, than when only prescription costs were assessed (Table 129).
| Antidepressant drug | Mean incremental cost (£) | Ranked cost |
|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 (141.50) | 8 (6) |
| Citalopram hydrobromide (SSRI) | 280.95 (123.03) | 4 (4) |
| Dosulepin hydrochloride (TCA) | 248.61 (95.26) | 3 (1) |
| Escitalopram (SSRI) | 345.79 (198.39) | 6 (7) |
| Fluoxetine hydrochloride (SSRI) | 217.30 (116.15) | 1 (3) |
| Lofepramine (TCA) | 536.83 (260.59) | 9 (10) |
| Mirtazapine (other) | 330.97 (212.18) | 5 (9) |
| Paroxetine hydrochloride (SSRI) | 370.12 (140.95) | 7 (5) |
| Sertraline hydrochloride (SSRI) | 235.88 (109.16) | 2 (2) |
| Trazodone hydrochloride (TCA) | 541.30 (209.75) | 10 (8) |
| Venlafaxine hydrochloride (other) | 851.55 (309.09) | 11 (11) |
| TCAs | 425.51 (146.06) | |
| SSRIs | 272.70 (125.55) | |
| Other antidepressants | 526.56 (226.11) |
Levels of cost-effectiveness
We now estimate the incremental number of adverse events for each of the 11 most commonly prescribed antidepressant drugs, and for the antidepressant classes, compared with no antidepressant drugs. Additionally, we also estimate the incremental cost per averted event (ICER) for those antidepressant drugs that are located on the efficiency frontier. These analyses are presented for each of the 13 adverse events in turn (the results of the sensitivity analysis are presented in Appendix 2). Finally, summary ICER results for both the base-case and sensitivity analyses, over the 5- and 1-year periods, are presented.
Mortality
The absolute risk of mortality when patients were not prescribed antidepressant drugs was estimated to be 21.66% over 5 years (see Table 23). However, of the 4811 patients who were prescribed no antidepressant drugs, 1691 (35.1%) died within 5 years of being diagnosed with depression, which meant that the mean follow-up time over which costs were estimated was 3.95 years. Consequently, when account was taken of this, and deaths in future years were discounted, the expected mortality rate for those who were prescribed no antidepressant drugs, was estimated to be to 0.1626 per patient over 5 years. Similar methods were used to estimate the expected mortality rate and the incremental number of deaths for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over 5 years, and for each of the different classes of antidepressant drugs. These values are presented in Table 130.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of deaths | Difference in incremental mean cost (£) | Difference in incremental no. of deaths | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0401 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0291 | 20.12 | 0.011 | 1828.79 |
| Lofepramine (TCA) | 314.32 | 0.0888 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.1146 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0836 | |||
| Escitalopram (SSRI) | 232.45 | 0.0600 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.1010 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0551 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0776 | |||
| Mirtazapine (other) | 241.40 | 0.1000 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0989 | |||
| TCAs | 100.62 | 0.0474 | 10.32 | 0.0391 | 263.96 |
| SSRIs | 90.30 | 0.0865 | LC | LC | |
| Other antidepressants | 364.95 | 0.1039 |
The values for the incremental number of deaths were subsequently combined with the previously estimated incremental costs [the base-case incremental total prescription costs (for all antidepressant drugs) reported in Table 128] in order to estimate the efficiency frontier. Amitriptyline (TCA) had the lowest mean prescription cost (of all antidepressant drugs) (hereafter referred to as lowest cost) and was also estimated to be associated with fewer deaths than lofepramine (TCA), trazodone (TCA), citalopram (SSRI), escitalopram (SSRI), fluoxetine (SSRI), paroxetine (SSRI), sertraline (SSRI) mirtazapine (other) and venlafaxine (other). Consequently, amitriptyline was deemed to dominate these nine antidepressant drugs. The remaining antidepressant [dosulepin (TCA)] made up the efficiency frontier. Amitriptyline had the lowest cost; dosulepin had a mean incremental cost of £20.12 compared with amitriptyline, but was associated with 0.0110 fewer deaths. This equated to an incremental cost per averted death (mean incremental cost/incremental number of deaths) (ICER) of £1829 per averted death. With regard to the different classes of antidepressant drugs (see Table 130), SSRIs had the lowest cost and dominated other antidepressant drugs. The mean cost was on average higher for those patients prescribed a TCA than for those prescribed an SSRI, but TCAs were estimated to be associated with fewer deaths, giving an incremental cost per averted death of £264 for TCAs, compared with SSRIs.
Sudden cardiac death
The estimated incremental number of sudden cardiac deaths for each of the 11 most commonly prescribed antidepressant drugs over the 5-year follow-up period are presented in Table 131. A negative incremental number of sudden cardiac deaths, as, for example for trazodone (TCA), can be explained by the fact that the estimated absolute risk of a sudden cardiac death is lower for these antidepressants than for no antidepressant drugs. Of the 11 most commonly prescribed antidepressant drugs amitriptyline (TCA) had the lowest cost. After excluding dominated options, citalopram (SSRI) was estimated to have an incremental cost per averted sudden cardiac death of £32,791 compared with amitriptyline; fluoxetine (SSRI) was estimated to have an incremental cost per averted sudden cardiac death of £56,882 compared with citalopram; and trazodone (TCA) was estimated to have an incremental cost per averted sudden cardiac death of £138,536 compared with fluoxetine. In terms of class, SSRIs had the lowest cost and dominated both TCAs and other antidepressant drugs.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of sudden cardiac deaths | Difference in incremental mean cost (£) | Difference in incremental no. of sudden cardiac deaths | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0007 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0011 | |||
| Lofepramine (TCA) | 314.32 | 0.0001 | |||
| Trazodone hydrochloride (TCA) | 193.49 | −0.0009 | 120.53 | 0.0009 | 138,535.53 |
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0000 | 20.53 | 0.0006 | 32,790.81 |
| Escitalopram (SSRI) | 232.45 | −0.0009 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | −0.0001 | 6.07 | 0.0001 | 56,882.32 |
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0008 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0001 | |||
| Mirtazapine (other) | 241.40 | 0.0015 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0017 | |||
| TCAs | 100.62 | 0.0005 | |||
| SSRIs | 90.30 | 0.0001 | LC | Dominates | |
| Other antidepressants | 364.95 | 0.0014 |
Suicide
The estimated incremental number of suicides that would be expected for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 132. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, paroxetine (SSRI) was estimated to have an incremental cost per averted suicide of £228,598 compared with amitriptyline. When looking at the different classes, SSRIs had the lowest cost and TCAs were estimated to have an incremental cost per averted suicide of £48,339 compared with SSRIs.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of suicides | Difference in incremental mean cost (£) | Difference in incremental no. of suicides | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0002 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0013 | |||
| Lofepramine (TCA) | 314.32 | 0.0027 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0027 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0014 | |||
| Escitalopram (SSRI) | 232.45 | −0.0002 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0022 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | −0.0002 | 96.47 | 0.0004 | 228,598.47 |
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0006 | |||
| Mirtazapine (other) | 241.40 | 0.0036 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0025 | |||
| TCAs | 100.62 | 0.0010 | 10.32 | 0.0002 | 48,339.10 |
| SSRIs | 90.30 | 0.0012 | LC | LC | |
| Other antidepressants | 364.95 | 0.0028 |
Attempted suicide/self-harm
The estimated incremental number of attempted suicides for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 133. Amitriptyline (TCA) had the lowest absolute risk, as well as the lowest incremental number of attempted suicides and lowest cost (compared with those not prescribed antidepressant drugs). Consequently, amitriptyline was deemed to dominate all of the other 10 most commonly prescribed antidepressant drugs, when estimating the cost per attempted suicide averted. When looking at the different classes, SSRIs had the lowest cost and TCAs were estimated to be associated with an incremental cost per averted attempted suicide of £7597 compared with SSRIs.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of attempted suicides | Difference in incremental mean cost (£) | Difference in incremental no. of attempted suicides | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0010 | LC | D | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0048 | |||
| Lofepramine (TCA) | 314.32 | 0.0075 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0158 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0073 | |||
| Escitalopram (SSRI) | 232.45 | 0.0035 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0050 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0012 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0050 | |||
| Mirtazapine (other) | 241.40 | 0.0210 | |||
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0010 | LC | D | |
| TCAs | 100.62 | 0.0039 | 10.32 | 0.0014 | 7596.58 |
| SSRIs | 90.30 | 0.0053 | LC | LC | |
| Other antidepressants | 364.95 | 0.0184 |
Myocardial infarction
The estimated incremental number of MIs for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 134. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, sertraline (SSRI) was estimated to have an incremental cost per averted MI of £3227 compared with amitriptyline. When looking at the different classes of antidepressant drugs, SSRIs had the lowest cost and other antidepressant drugs were estimated to be associated with an incremental cost per averted MI of £79,799.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of MIs | Difference in incremental mean cost (£) | Difference in incremental no. of MIs | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0079 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0067 | |||
| Lofepramine (TCA) | 314.32 | 0.0085 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0021 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0044 | |||
| Escitalopram (SSRI) | 232.45 | 0.0090 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0113 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0067 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | −0.0017 | 31.01 | 0.01 | 3226.98 |
| Mirtazapine (other) | 241.40 | 0.0033 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0027 | |||
| TCAs | 100.62 | 0.0070 | |||
| SSRIs | 90.30 | 0.0065 | LC | LC | |
| Other antidepressants | 364.95 | 0.0031 | 274.65 | 0.0034 | 79,799.09 |
Stroke/transient ischaemic attack
The estimated incremental number of strokes/TIAs for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 135. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, dosulepin (TCA) was estimated to have an incremental cost per averted stroke/TIA of £4961 compared with amitriptyline. When looking at the different classes of antidepressant drugs, SSRIs had the lowest cost and TCAs were estimated to be associated with an incremental cost per averted stroke/TIA of £1833.
| Antidepressant drug | Incremental mean cost (£) | Incremental no/ of strokes/TIAs | Difference in incremental mean cost (£) | Difference in incremental no. of strokes/TIAs | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0105 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0064 | 20.12 | 0.0041 | 4961.16 |
| Lofepramine (TCA) | 314.32 | 0.0238 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0087 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0175 | |||
| Escitalopram (SSRI) | 232.45 | 0.0132 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0142 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0128 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0188 | |||
| Mirtazapine (other) | 241.40 | 0.0244 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0370 | |||
| TCAs | 100.62 | 0.0103 | 10.32 | 0.0056 | 1832.83 |
| SSRIs | 90.30 | 0.0160 | LC | LC | |
| Other antidepressants | 364.95 | 0.0296 |
Falls
The estimated incremental number of falls for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are shown in Table 136. Amitriptyline (TCA) had the lowest cost, yet other antidepressant drugs were associated with fewer expected falls. After excluding dominated options, dosulepin (TCA) had an incremental cost per averted fall of £2234 compared with amitriptyline (TCA) and mirtazapine (other) had an incremental cost per averted fall of £6868 compared with dosulepin (TCA). When looking at the different classes of antidepressant drugs, SSRIs had the lowest cost and TCAs were estimated to be associated with an incremental cost per averted fall of £396.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of falls | Difference in incremental mean cost (£) | Difference in incremental no. of falls | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0549 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0459 | 20.12 | 0.009 | 2234.36 |
| Lofepramine (TCA) | 314.32 | 0.0486 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0614 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0842 | |||
| Escitalopram (SSRI) | 232.45 | 0.0660 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0741 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0634 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0742 | |||
| Mirtazapine (other) | 241.40 | 0.0204 | 174.92 | 0.0255 | 6868.42 |
| Venlafaxine hydrochloride (other) | 611.14 | 0.0769 | |||
| TCAs | 100.62 | 0.0514 | 10.32 | 0.0261 | 395.62 |
| SSRIs | 90.30 | 0.0775 | LC | LC | |
| Other antidepressants | 364.95 | 0.0507 |
Fractures
The estimated incremental number of fractures for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 137. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, trazodone (TCA) was estimated to have an incremental cost per averted fracture of £6342 compared with amitriptyline. When looking at the different classes, SSRIs had the lowest cost and TCAs were estimated to be associated with an incremental cost per averted fracture of £750.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of fractures | Difference in incremental mean cost (£) | Difference in incremental no. of fractures | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0231 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0238 | |||
| Lofepramine (TCA) | 314.32 | 0.0338 | |||
| Trazodone hydrochloride (TCA) | 193.49 | −0.0001 | 147.14 | 0.0232 | 6342.21 |
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0389 | |||
| Escitalopram (SSRI) | 232.45 | 0.0159 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0376 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0356 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0395 | |||
| Mirtazapine (other) | 241.40 | 0.0265 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0536 | |||
| TCAs | 100.62 | 0.0237 | 10.32 | 0.0138 | 750.28 |
| SSRIs | 90.30 | 0.0375 | LC | LC | |
| Other antidepressants | 364.95 | 0.0417 |
Upper gastrointestinal bleed
The estimated incremental number of upper GI bleeds for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 138. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, the estimated incremental costs per averted upper GI bleed were £23 for dosulepin (TCA) compared with amitriptyline, £2527 for fluoxetine (SSRI) compared with dosulepin, £2655 for sertraline (SSRI) compared with fluoxetine and £215,955 for mirtazapine (other) compared with sertraline. Escitalopram (SSRI) was estimated to be subject to extended dominance (it was estimated to be associated with more upper GI bleeds than the other antidepressant drugs, but had a higher ICER, i.e. combinations of sertraline (SSRI) and mirtazapine (other) would be estimated to be associated with a lower cost and fewer upper GI bleeds. When looking at the different classes, SSRIs had the lowest cost and were estimated to dominate both TCAs and other antidepressant drugs.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of upper GI bleeds | Difference in incremental mean cost (£) | Difference in incremental no. of upper GI bleeds | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0086 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0058 | 20.12 | 0.8800 | 22.89 |
| Lofepramine (TCA) | 314.32 | 0.0047 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0126 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0060 | |||
| Escitalopram (SSRI) | 232.45 | 0.0009 | ED | ||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0032 | 6.48 | 0.0026 | 2527.25 |
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0041 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0015 | 4.4 | 0.0000 | 2655.44 |
| Mirtazapine (other) | 241.40 | 0.0008 | 164.04 | 0.0000 | 215,954.79 |
| Venlafaxine hydrochloride (other) | 611.14 | 0.0118 | |||
| TCAs | 100.62 | 0.0070 | |||
| SSRIs | 90.30 | 0.0044 | LC | D | |
| Other antidepressants | 364.95 | 0.0069 |
Epilepsy/seizures
The estimated incremental number of epilepsy/seizure cases for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 139. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, dosulepin (TCA) was estimated to have an incremental cost per averted epilepsy/seizure of £5159 compared with amitriptyline. When looking at the different classes, SSRIs had the lowest cost and TCAs had an incremental cost per averted epilepsy/seizure case of £2594 compared with SSRIs.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of epilepsy/seizure cases | Difference in incremental mean cost (£) | Difference in incremental no. of epilepsy/seizure cases | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0017 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0022 | 20.12 | 0.0039 | 5158.97 |
| Lofepramine (TCA) | 314.32 | 0.0025 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0026 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0044 | |||
| Escitalopram (SSRI) | 232.45 | 0.0039 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0029 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0064 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0094 | |||
| Mirtazapine (other) | 241.4 | 0.0031 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0109 | |||
| TCAs | 100.62 | 0.0008 | 10.32 | 0.0040 | 2593.95 |
| SSRIs | 90.30 | 0.0048 | LC | LC | |
| Other antidepressants | 364.95 | 0.0070 |
Road traffic accidents
The estimated incremental number of RTAs for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 140. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, dosulepin (TCA) was estimated to have an incremental cost per averted RTA of £9009 compared with amitriptyline, and mirtazapine (other) was estimated to have an incremental cost per averted RTA of £240,044 compared with dosulepin. When looking at the different classes, SSRIs had the lowest cost and TCAs were estimated to be associated with an incremental cost per averted RTA of £204,943.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of RTAs | Difference in incremental mean cost (£) | Difference in incremental no. of RTAs | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | −0.0002 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | −0.0024 | 20.12 | 0.0022 | 9008.60 |
| Lofepramine (TCA) | 314.32 | 0.0013 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0033 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0003 | |||
| Escitalopram (SSRI) | 232.45 | −0.0002 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | −0.0015 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0000 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | −0.0003 | |||
| Mirtazapine (other) | 241.40 | −0.0032 | 174.92 | 0.0007 | 240,043.92 |
| Venlafaxine hydrochloride (other) | 611.14 | −0.0014 | |||
| TCAs | 100.62 | −0.0002 | |||
| SSRIs | 90.30 | −0.0003 | LC | LC | |
| Other antidepressants | 364.95 | −0.0017 | 274.65 | 0.0013 | 204,943.04 |
Adverse drug reactions
The estimated incremental number of ADRs for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 141. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, dosulepin (TCA) had a mean incremental cost of £28,209 per averted ADR and venlafaxine (other) had an incremental cost of £637,960 per averted ADR. Those prescribed SSRIs had the lowest cost. The incremental cost per averted ADR was £39,280 for TCAs compared with SSRIs and £164,896 for the group of other antidepressant drugs compared with TCAs.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of ADRs | Difference in incremental mean cost (£) | Difference in incremental no. of ADRs | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0008 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0001 | 20.12 | 0.0007 | 28,209.46 |
| Lofepramine (TCA) | 314.32 | 0.0113 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0007 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0014 | |||
| Escitalopram (SSRI) | 232.45 | 0.0008 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.0022 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0003 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0061 | |||
| Mirtazapine (other) | 241.40 | 0.0002 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0007 | 544.66 | 0.0009 | 637,960.00 |
| TCAs | 100.62 | 0.0017 | 10.32 | 0.0003 | 39,280.15 |
| SSRIs | 90.30 | 0.0020 | LC | LC | |
| Other antidepressants | 364.95 | 0.0001 | 264.33 | 0.0016 | 164,896.44 |
Hyponatraemia
The estimated incremental number of hyponatraemia cases for each of the 11 most commonly prescribed antidepressant drugs (after discounting) over the 5-year follow-up period are presented in Table 142. Amitriptyline (TCA) had the lowest cost and, after excluding dominated options, dosulepin (TCA) was estimated to have an incremental cost per averted hyponatraemia case of £5087 compared with amitriptyline. When looking at the different classes, SSRIs had the lowest cost and TCAs were estimated to be associated with an incremental cost per averted hyponatraemia case of £2433.
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of hyponatraemia cases | Difference in incremental mean cost (£) | Difference in incremental no. of hyponatraemia cases | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 46.36 | 0.0034 | LC | LC | |
| Dosulepin hydrochloride (TCA) | 66.48 | 0.0005 | 20.12 | 0.004 | 5087.28 |
| Lofepramine (TCA) | 314.32 | 0.0001 | |||
| Trazodone hydrochloride (TCA) | 193.49 | 0.0056 | |||
| Citalopram hydrobromide (SSRI) | 66.89 | 0.0072 | |||
| Escitalopram (SSRI) | 232.45 | 0.0107 | |||
| Fluoxetine hydrochloride (SSRI) | 72.96 | 0.008 | |||
| Paroxetine hydrochloride (SSRI) | 142.83 | 0.0015 | |||
| Sertraline hydrochloride (SSRI) | 77.36 | 0.0006 | |||
| Mirtazapine (other) | 241.4 | 0.0007 | |||
| Venlafaxine hydrochloride (other) | 611.14 | 0.0061 | |||
| TCAs | 100.62 | 0.0020 | 10.32 | 0.0042 | 2433.15 |
| SSRIs | 90.30 | 0.0062 | LC | LC | |
| Other antidepressants | 364.95 | 0.0036 |
Summary
The ICER values for each of the adverse events for those antidepressant drugs located on the efficiency frontier are presented in Table 143 (base-case analysis: prescription costs for all antidepressant drugs) and Table 144 [sensitivity analysis: overall costs (total visit costs and total prescription costs)] for the 5-year post-diagnosis period. None of the 11 most commonly prescribed antidepressant drugs was estimated to be consistently the most cost-effective across the different types of adverse events; this was the case for both prescription costs and overall costs, and for both 1- and 5-year time periods. Moreover, as it is unclear what one would be willing to pay to avert an adverse event, one cannot determine the most cost-effective antidepressant for averting different adverse events (with the exception of where dominance occurs). That said, when focusing on prescription costs over the 5-year perspective, patients prescribed amitriptyline (TCA) had the lowest mean cost and this drug also had the lowest predicted number of attempted suicides (i.e. here it dominated other options). Dosulepin (TCA) was the other drug that was located, more often than not, on the efficiency frontier (this was the case for ADRs, epilepsy/seizures, falls, hyponatraemia, mortality, RTAs, stroke/TIA and upper GI bleeds) and, therefore, could potentially be cost-effective if one were willing to pay the specific values to avoid the different adverse events. Additionally, escitalopram (SSRI) and lofepramine (TCA) were always dominated by at least one other option, for each of the 13 adverse events. Conversely, when looking at overall costs (total visit cost and the total prescription cost) (see Appendix 2 for results) over the 5-year perspective, those prescribed fluoxetine (SSRI) had the lowest mean cost. However, fluoxetine (SSRI) never dominated any of the other options, and dosulepin (TCA) was again located, more often than not, on the efficiency frontier (this was the case for ADRs, epilepsy/seizures, falls, fractures, hyponatraemia, mortality, RTAs and stroke/TIA). Moreover, escitalopram (SSRI), citalopram (SSRI) and lofepramine (TCA) were always dominated by at least one other option, for each of the 13 adverse events. Additionally, it should be noted that these 5-year perspective results are in line with those for the 1-year period (sensitivity analysis: see Tables 145 and 146, respectively), although dosulepin (TCA) was located on the efficiency frontier on a greater number of occasions for overall costs for the 1-year perspective as it was estimated to have the lowest cost. Finally, given that there is variation in levels of cost-effectiveness within classes, it is difficult to conclude that a particular class of drugs are more cost-effective than another in terms of these adverse events.
| Mortality | Sudden cardiac death | Suicide | Attempted suicide | MI | Stroke/TIA | Falls | Fractures | Upper GI bleed | Epilepsy/seizures | RTA | ADR | Hyponatraemia | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | LC | LC | LC | D | LC | LC | LC | LC | LC | LC | LC | LC | LC |
| Dosulepin hydrochloride (TCA) | 1829 | 4961 | 2234 | 23 | 5159 | 9009 | 28,209 | 5087 | |||||
| Lofepramine (TCA) | |||||||||||||
| Trazodone hydrochloride (TCA) | 138,536 | 6342 | |||||||||||
| Citalopram hydrobromide (SSRI) | 32,791 | ||||||||||||
| Escitalopram (SSRI) | ED | ||||||||||||
| Fluoxetine hydrochloride (SSRI) | 56,882 | 2527 | |||||||||||
| Paroxetine hydrochloride (SSRI) | 228,598 | ||||||||||||
| Sertraline hydrochloride (SSRI) | 3227 | 2655 | |||||||||||
| Mirtazapine (other) | 6868 | 215,955 | 240,044 | ||||||||||
| Venlafaxine hydrochloride (other) | 637,960 | ||||||||||||
| TCAs | 264 | 48,339 | 7597 | 1833 | 396 | 750 | 2594 | 39,280 | 2433 | ||||
| SSRIs | LC | D | LC | LC | LC | LC | LC | LC | D | LC | LC | LC | LC |
| Other antidepressants | 79,799 | 204,943 | 164,896 |
| Mortality | Sudden cardiac death | Suicide | Attempted suicide | MI | Stroke/TIA | Falls | Fractures | Upper GI bleed | Epilepsy/seizures | RTA | ADR | Hyponatraemia | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 882,758 | ED | |||||||||||
| Dosulepin hydrochloride (TCA) | 435 | ED | ED | 3993 | 1109 | 2260 | 6210 | 32,854 | 15,090 | 10,914 | |||
| Lofepramine (TCA) | |||||||||||||
| Trazodone hydrochloride (TCA) | 372,401 | 12,240 | |||||||||||
| Citalopram hydrobromide (SSRI) | |||||||||||||
| Escitalopram (SSRI) | ED | ||||||||||||
| Fluoxetine hydrochloride (SSRI) | LC | LC | LC | LC | LC | LC | LC | LC | LC | LC | LC | LC | LC |
| Paroxetine hydrochloride (SSRI) | 158,763 | 39,865 | |||||||||||
| Sertraline hydrochloride (SSRI) | ED | 12,123 | 1428 | 961 | 2532 | ||||||||
| Mirtazapine (other) | 3234 | 125,188 | 113,029 | ||||||||||
| Venlafaxine hydrochloride (other) | 706,227 | ||||||||||||
| TCAs | 3908 | 715,767 | 112,484 | 27,139 | 5858 | 11,109 | 38,409 | ED | 36,028 | ||||
| SSRIs | LC | D | LC | LC | LC | LC | LC | LC | D | LC | LC | LC | LC |
| Other antidepressants | 73,758 | 189,427 | 136,063 |
| Mortality | Sudden cardiac death | Suicide | Attempted suicide | MI | Stroke/TIA | Falls | Fracture | Upper GI bleed | Epilepsy/seizures | RTA | ADR | Hyponatraemia | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | LC | LC | LC | D | LC | LC | LC | LC | LC | LC | LC | LC | LC |
| Dosulepin hydrochloride (TCA) | 1300 | ED | 4968 | 2,297 | 7 | 4499 | 11,851 | 32,723 | 6345 | ||||
| Lofepramine (TCA) | |||||||||||||
| Trazodone hydrochloride (TCA) | 171,388 | 13,466 | |||||||||||
| Citalopram hydrobromide (SSRI) | 37,843 | ||||||||||||
| Escitalopram (SSRI) | |||||||||||||
| Fluoxetine hydrochloride (SSRI) | 86,674 | ED | |||||||||||
| Paroxetine hydrochloride (SSRI) | 246,675 | ||||||||||||
| Sertraline hydrochloride (SSRI) | 5858 | 9522 | |||||||||||
| Mirtazapine (other) | 20,653 | 1,423,810 | 637,171 | ||||||||||
| Venlafaxine hydrochloride (other) | 2,840,352 | ||||||||||||
| TCAs | 355 | 88,010 | 8207 | 24,566 | 2834 | 760 | 1609 | 4997 | ED | 41,939 | 6386 | ||
| SSRIs | LC | D | LC | LC | LC | LC | LC | LC | D | LC | LC | LC | LC |
| Other antidepressants | 121,278 | 292,702 | 278,602 |
| Mortality | Sudden cardiac death | Suicide | Attempted suicide | MI | Stroke/TIA | Falls | Fracture | Upper GI bleed | Epilepsy/seizures | RTA | ADR | Hyponatraemia | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 23,339 | ED | |||||||||||
| Dosulepin hydrochloride (TCA) | D | LC | LC | LC | LC | D | LC | LC | LC | D | LC | LC | D |
| Lofepramine (TCA) | |||||||||||||
| Trazodone hydrochloride (TCA) | 30,8907 | 23,968 | |||||||||||
| Citalopram hydrobromide (SSRI) | |||||||||||||
| Escitalopram (SSRI) | |||||||||||||
| Fluoxetine hydrochloride (SSRI) | 58,384 | ||||||||||||
| Paroxetine hydrochloride (SSRI) | 112,872 | ED | |||||||||||
| Sertraline hydrochloride (SSRI) | 69,588 | 7166 | 17,581 | ||||||||||
| Mirtazapine (other) | 34,230 | 2,327,655 | 1,056,032 | ||||||||||
| Venlafaxine hydrochloride (other) | 3,148,619 | ||||||||||||
| TCAs | 945 | 234,451 | 21,863 | 65,441 | 7548 | 2025 | 4286 | 13,312 | ED | 111,721 | 17,012 | ||
| SSRIs | LC | D | LC | LC | LC | LC | LC | LC | D | LC | LC | LC | LC |
| Other antidepressants | 112,264 | 312,544 | 257,897 |
Chapter 4 Discussion
Summary of the main findings
Findings by antidepressant class
All classes of antidepressant drugs were associated with significantly increased rates of all-cause mortality, suicide, attempted suicide/self-harm, falls, fracture and upper GI bleeds compared with periods of no use of antidepressant drugs in this cohort of older people who had been diagnosed with depression. There were significant differences between the three main classes of antidepressant drugs and their associations with 7 of the 13 adverse outcomes examined; all-cause mortality, attempted suicide/self-harm, stroke/TIA, falls, fracture, epilepsy/seizures and hyponatraemia. For these outcomes, SSRIs was associated with the highest rates for falls and hyponatraemia, and the group of other antidepressant drugs was associated with the highest rates for overall mortality, attempted suicide/self-harm, stroke/TIA, fracture and epilepsy/seizures. TCAs did not have the highest rates for any of these outcomes.
The rates of sudden cardiac death, suicide, MI, upper GI bleeds, RTAs and ADRs were not significantly different between the different antidepressant classes.
Patients who had been prescribed combined antidepressant drugs from different classes or different drugs within a class had the highest overall rates for several of the adverse outcomes: all-cause mortality, suicide, stroke/TIA, falls, fracture, upper GI bleed, epilepsy/seizures and RTAs.
Findings for individual antidepressant drugs
There were significant differences between the associations of the most commonly prescribed individual drugs and seven of the adverse outcomes: all-cause mortality, attempted suicide/self-harm, stroke/TIA, falls, fracture, epilepsy/seizures and hyponatraemia. Patients who had been prescribed trazodone had the highest rate of all-cause mortality and one of the highest rates of attempted suicide/self-harm. Mirtazapine was associated with the highest rate of attempted suicide/self-harm and one of the highest rates for all-cause mortality and stroke/TIA. Patients prescribed venlafaxine had higher rates of stroke/TIA, fracture and epilepsy/seizures than patients prescribed the other drugs, and one of the highest rates for all-cause mortality, attempted suicide/self-harm and falls. Citalopram was associated with the highest rate of falls, but rates were similar for all of the SSRIs. There were significantly increased risks of hyponatraemia associated with three SSRIs (citalopram, escitalopram and fluoxetine), but not paroxetine or sertraline. Amitriptyline and dosulepin were associated with the lowest rates for several of these outcomes.
There were no significant differences between individual drugs for MI, upper GI bleeds and RTAs. There was some evidence of a difference between individual drugs for ADRs, with lofepramine and sertraline being associated with the highest rates. The number of cases of sudden cardiac death and suicide were too small to enable comparisons of individual drugs.
Findings according to dose and duration of use
There was considerable variation in the prescribed doses between the antidepressant classes, and between individual drugs. TCAs tended to have the lowest prescribed doses, with nearly 70% of prescriptions being ≤ 0.5 of a DDD, compared with 14% for SSRIs and 19% for the class of other antidepressant drugs.
There was evidence of a dose–response relationship with mortality rates for TCAs and SSRIs, but not for the group of other antidepressant drugs. The rate of falls tended to increase as dose increased in all classes, whereas the fracture rate increased significantly as dose increased for TCAs, but less markedly for SSRIs and other antidepressant drugs. The rates of epilepsy/seizures tended to increase as dose increased in all classes, although the trend was not significant for the group of other antidepressant drugs. Hyponatraemia was significantly associated only with use of SSRIs; however, the rate was highest for low doses of SSRIs and decreased as SSRI dose increased. There were no significant dose–response relationships for any class for ADRs, although there was some indication of an increased rate associated with high doses of TCAs.
Although TCAs had the lowest prescribed doses, when comparisons were made within separate categories of dose (≤ 0.5 DDDs, 0.5–1.0 DDDs and > 1.0 DDDs) TCAs tended to be associated with lower adjusted HRs for all-cause mortality, attempted suicide/self-harm, stroke/TIA and epilepsy/seizures within each dose category. There is also some evidence suggesting that low-dose TCAs are similar to higher-dose TCAs in terms of reducing symptoms of depression. 3,68
Rates of most outcomes were highest in the first 28 days after starting an antidepressant, and also in the first 28 days after stopping. For all-cause mortality, MI, stroke/TIA, ADRs and hyponatraemia, there was some evidence that rates were reduced after 85 days of use.
For most outcomes risks were no longer increased from 85 days after stopping antidepressant treatment; however, they remained increased for overall mortality and epilepsy/seizures.
The high rates in the first 28 days after stopping may reflect a direct effect of withdrawal from the antidepressant drug, but are more likely, given the similar pattern for many outcomes, to reflect patients stopping the drugs because of an onset of symptoms or after being admitted to hospital or a residential home following an adverse event that may be recorded at a later date. In addition, these findings are hard to interpret as we cannot tell the precise date when patients stopped taking antidepressant medication, as they may not have taken all of the tablets in their last prescription.
Findings on patterns of antidepressant prescribing
Selective serotonin reuptake inhibitors were the most commonly prescribed drug class in the cohort; more than three-quarters of treated patients were prescribed an SSRI during follow-up, compared with 54% for TCAs and 19% for the other class of antidepressant drugs. Very few patients were prescribed a MAOI (0.2%). There was a steep increase in the proportion of prescriptions that were for SSRIs over the study period, with a corresponding reduction for TCAs. There was also an increase for the group of other antidepressant drugs and a slight reduction in MAOI prescribing. These trends, are likely to reflect the availability of new SSRIs and other antidepressant drugs, and concerns that TCAs have more side effects and are more toxic in overdose than SSRIs, as well as recommendations in guidelines. MAOIs have never been recommended for older people because of possible interaction effects with other medicines that older people, in particular, are likely to take and with certain foods.
Patients who had been prescribed SSRIs were slightly less likely than patients prescribed TCAs or other antidepressant drugs to either stop after a single prescription or switch to another drug class in the year following their first prescription: 37% for SSRIs, 48% for TCAs, 50% for the group of other antidepressant drugs. Among the individual antidepressant drugs, the proportions of patients who either stopped after a single prescription or switched to another drug in the year following their first prescription were lowest for citalopram (42%) and mirtazapine (43%), and highest for lofepramine (60%), amitriptyline (56%) and trazodone (54%).
Findings from analyses of costs
It was difficult to conclude that one particular class was more cost-effective than another in terms of adverse events avoided. Although SSRIs were estimated to have the lowest mean cost, they often had higher estimated adverse event rates than other classes (sudden cardiac death and upper GI bleed were the exceptions). Conversely, TCAs were often estimated to be associated with lower adverse event rates (this was the case for attempted suicide, epilepsy/seizures, falls, fractures, MI, stroke/TIA and suicide), but higher costs. However, as it is unclear what one would be willing to pay to avert the different types of adverse events it is difficult to assess whether the provision of TCAs would constitute value for money. The group of other antidepressant drugs was dominated, however, by either SSRIs or TCAs for 10 of the 13 adverse events, as they had higher mean cost and adverse event rates (the exceptions were ADRs, hyponatraemia and RTAs).
In terms of individual drugs, amitriptyline was estimated to have the lowest prescription costs (for all antidepressant drugs), although when practice/community nurse and GP visits (which far outweigh prescription costs) were included fluoxetine had the lowest cost. Venlafaxine was estimated to have the highest mean cost from both these perspectives. Dominance occurred only with regard to attempted suicide, for which amitriptyline was estimated to have both the lowest prescription cost and adverse event rate. In all other cases, the most cost-effective drug in terms of adverse events avoided was estimated to be dependent on what one would be willing to pay to avert an adverse event, which is an unknown factor. That said, in the base-case analysis, as it appeared on the efficiency frontier, dosulepin was estimated to be potentially cost-effective for 8 of the 13 types of adverse events. Finally, it should be noted that the conclusions drawn from the results of the sensitivity analyses, where costs were conducted over a 1-year period and additionally included visit costs, were similar to those discussed above.
Strengths of the study
This study has a number of strengths. It is a large study, comprising over 60,000 patients over the age of 65 years who had been diagnosed with depression, followed up for up to 13 years, with a mean length of follow-up of 5.0 years. This study size enabled us to detect associations with relatively rare adverse events, which would not be possible with clinical trials of antidepressant drugs which are smaller and have shorter follow-up periods and so are generally underpowered to detect effects on adverse events unless they are very common.
The study had broad inclusion criteria and so the findings are generalisable to the population of older people diagnosed with depression in primary care. This, again, is in contrast with clinical trials, which generally have strict inclusion and exclusion criteria, tending to lead to the exclusion of many older people who have comorbidities or are taking medication for other conditions. In addition, we included all eligible patients, as individual consent to participate was not required, which reduces selection bias and increases external validity compared with clinical trials or many cohort and case–control studies, and for which patients need to consent to participate, which may lead to a highly selected group participating in the study. As it has been shown that practices within the QResearch database are generally representative of those within England and Wales,42 this will also increase the generalisability of results.
The data on the database are recorded prospectively, so all information on prescriptions for antidepressant drugs and potential confounding variables was recorded before occurrence of any adverse event. This means that recall bias will not have occurred in this study, which can be a problem in case–control studies collecting information after the occurrence of adverse events. We were able to adjust our analyses for a number of potential confounding variables, including comorbidities and use of other medications.
We had details of all prescriptions for antidepressant drugs issued in primary care throughout the follow-up period, so were able to carry out detailed analyses investigating effects of individual drugs, dose and duration. This contrasts with many cohort or case–control studies in which information on antidepressant use is self-reported or is collected only at the start of the study.
The detailed information about the number of GP and nurse visits enabled us to assess whether patients prescribed certain antidepressant drugs were more likely to visit these health-care professionals, for example to renew prescriptions or to have their symptoms monitored. The collation of such detailed information may not have been possible with other study designs or would be susceptible to recall bias.
Limitations of the study
The main concerns from observational studies such as this one are indication and ascertainment bias. Indication bias occurs when patients are prescribed medication for a condition that is itself associated with the outcome of interest. This means that apparent associations with a medication may be in fact owing to the condition for which it was prescribed rather than the medication itself. To reduce this bias we restricted our study cohort to patients with a recorded GP diagnosis of depression, as depression itself is associated with many adverse outcomes. 69–72 There are still likely to be systematic differences between those who are treated and those who are not. The latter are more likely to have less severe and chronic depression, and to express a preference for psychological treatment, and they may have poorer physical health, such that they are considered too frail for antidepressant medication. We adjusted the analyses for many of factors that could differ between groups and which are risk factors for the adverse outcomes, including age, gender, severity of depression, a number of comorbidities and use of other medications. Generally, the adjustment did not have a large effect on the results (this was the case for both adverse events and costs), as there were no big differences in these factors according to whether or not antidepressant medication was prescribed. However, although we adjusted for severity of the initial diagnosis of depression, we were able to use only a crude measure, as we did not have a detailed depression severity score. We cannot therefore exclude the possible effect of residual confounding on our results.
Another concern in direct comparisons of drugs is channelling bias, whereby different antidepressant drugs might be prescribed according to various patient characteristics. An example of this would be preferentially prescribing SSRIs rather than TCAs to frail patients who were at greater risk of falling. 20 Again, adjusting for a range of potential confounding factors would be expected to reduce the effect of this bias, for example in the analysis of fracture we adjusted for falls at baseline as well as for a large number of other confounders. This bias would be less likely to apply to comparisons between individual drugs within a class than to comparisons between classes, and we have found differences between individual drugs within classes for some of the outcomes.
Residual confounding may remain in the findings, as certain potential confounding variables may not be recorded on the database or may not be recorded in sufficient detail to completely remove their confounding effect. For these reasons we also carried out a self-controlled case-series analysis, which can largely remove the problems of residual confounding and selection and indication bias. 57 This is a within-patients comparison, which implicitly removes the effects of all patient characteristics that vary between patients, irrespective of whether or in how much detail they have been recorded on the database, assuming that they do not vary over time within the observation period. 57 This means that factors such as patient frailty or level of physical activity, which may not be recorded on the database, are implicitly accounted for, and, as the analysis compares outcome rates across different periods of exposure in treated patients rather than comparing treated with untreated patients, the issue of indication bias arising from the cohort analyses discussed above is reduced. The results from the case-series analyses were generally in accordance with the findings from the cohort analyses, although there were some differences for attempted suicide/self-harm and stroke/TIA, suggesting possible indication bias. However, case-series analyses are less valid when the adverse event is a fatal one, so we do not consider the findings of high increase in mortality rates from the case-series analysis to be reliable.
The main remaining bias is due to changes in severity of depression over time. The presence and severity of depression can vary considerably over time, particularly after starting antidepressant treatment, and our analysis was not able to accommodate this. This is a source of bias in both the cohort analyses and the case-series analysis, which implicitly adjusts only for confounders which do not change over time. As antidepressant prescribing and the presence and severity of depression change over time and will be highly correlated, it is difficult to separate their effects in these analyses. This could explain why increases were generally most marked in the first 28 days after a prescription, when the depression is likely to be more severe, and could also explain the reductions in rates for some outcomes after 85 days of use, when the depression may be resolving. This will have less impact on direct comparisons between classes or individual antidepressant drugs than on comparisons with non-use of antidepressant drugs. So, for example, where analyses show similar increases for all classes of antidepressant use compared with periods of non-use we cannot be sure that these are not due to the effects of depression itself, but where there are differences between classes or individual drugs these are more likely to be direct effects of the drugs.
The outcome measures we used were not specifically validated in this study, although some have been validated in other UK primary care databases and we would expect similar levels of validity in QResearch. We included information from death certificates to identify additional patients with the outcomes, and this will have reduced misclassification. However, some outcomes may be more likely to be recorded by a GP if a patient is known to be taking antidepressant drugs which could increase the HRs – this could be the case for ADRs, for example. In addition, patients taking antidepressant drugs visit their GPs and practice nurses more frequently, and this could lead to additional tests for certain outcomes (hyponatraemia) and an increased likelihood of reporting more minor events, such as some ADRs or some falls. This is an ascertainment bias, and could affect comparisons with the group not currently taking antidepressant drugs; however, the numbers of visits to GPs and practice nurses were similar for each class of antidepressant drugs, so the direct comparisons between classes should be less affected by this source of bias. We restricted our analyses to first events for each outcome, as it is difficult to distinguish whether subsequent recorded events are new events or reviews of previous events; this also reduces confounding effects due to previous events, but does mean we were unable to assess the effects of antidepressant medication in people with previous events.
The data on prescriptions for antidepressant drugs are likely to be reliably recorded; however, prescriptions in secondary care may not be included. As the majority of people with depression are treated in primary care, this should not have much of an effect on our results. Furthermore, we do not know whether prescriptions dispensed were actually taken, and there is research showing that adherence with antidepressant medication is low in patients with depression;73,74 for example, one study in older people found that nearly one-third of patients were not fully adherent with their medication. 73 This would tend to reduce the HRs comparing drug use with non-use, but if the adherence with medication varies by drug class or individual drugs then this may distort direct comparisons between drugs.
There were some missing data in our study, for example smoking status was missing for 5% of the cohort. We did not adjust for BMI, which was missing for 28% of the cohort in our main analyses; however, there were only small differences when we did adjust for BMI in a complete case analysis.
In our analyses we treated antidepressant use as a time-varying exposure, as this relates the rate of events to the antidepressant currently being used, rather than basing results on the first antidepressant prescribed, for example. This is particularly important given the large amount of switching between antidepressant drugs during the follow-up period; however, we did not directly account for changes in dose or previous switches between antidepressant drugs in the models owing to the complex patterns of antidepressant use over time.
We have presented absolute as well as relative rates for the outcomes studied; however, we did not account for death as a competing risk in our analyses so the estimates of absolute rates will tend to overestimate the true values.
With regard to the health economic analysis, one limitation was that we were unable to estimate costs from the NHS and Personal Social Services viewpoint as, among other things, levels of resource use in secondary care are not routinely recorded in the QResearch database. The collation of secondary-care costs would have enabled us to estimate and include the costs associated with adverse events. Their inclusion would have been likely to mean that antidepressant drugs that had a low adverse event rate would have had relatively lower overall costs, which may have led to improvements in the cost-effectiveness of such antidepressant drugs in terms of adverse events.
Estimation of the cost per adverse event averted is in line with previous cost-effectiveness studies;75 however, a weakness of this technique is that, as it is unclear what one would be willing to pay to avert the different types of adverse events, it is difficult to make recommendations with regard to cost-effectiveness. Thus, in the absence of dominance we have been able to identify only those antidepressants that are potentially cost-effective, as they appear on the efficiency frontier. This limitation could potentially be overcome by seeking to estimate the loss in utility associated with each of the different types of adverse events, as discussed in the subsequent section on the implications for further research.
Finally, it should be reiterated that the above analyses focus on adverse events. This is justified on the basis that different antidepressant classes have largely similar efficacy. 3–7 A further limitation of this study, however, is that efficacy data for the different antidepressant drugs are not available in the QResearch database.
Interpretation of the study findings in light of previous research
All-cause mortality
There is a complex picture in relation to antidepressants and mortality in the literature with differential effects according to age, gender, underlying physical morbidity, response of depression to antidepressant treatment and class of antidepressant.
A cohort study from Finland found that current use of all antidepressant drugs and each class of antidepressant (SSRIs, TCAs and other antidepressant drugs) was associated with a reduced mortality rate compared with no current use or one antidepressant prescription only. 76 Another cohort study in Finland of subjects hospitalised because of a suicide attempt also found reduced mortality during use of all antidepressant drugs;77 however, in this study only 5% of the sample was aged 65 years or older and the analysis did not control for physical comorbidity, although it did control for the number of previous suicide attempts.
In a cohort study from Sweden, antidepressant treatment in patients over 65 years of age was associated with increased all-cause mortality and mortality from cardiovascular disease. 28 The analysis controlled for baseline comorbidity but not for gender, current or past depression, suicidal ideation or self-harm. A cohort of union members found that antidepressant drugs were not associated with an increase in all-cause mortality after adjustment for confounding variables;78 however, this study contained few people aged over 65 years.
A more complex picture emerges in a French prospective study of non-institutionalised patients aged over 65 years. 29 After adjustment for confounders there was a difference in the effects of antidepressant drugs according to gender and severity of depression. In men, antidepressant drugs were associated with increased mortality, especially in those with severe depression. In women, use of antidepressant drugs was not associated with increased mortality and the only increase was in women with severe depression who were not taking antidepressant drugs. There was no increase in mortality in men or women who were on antidepressant drugs but not currently depressed. 29 Given that both mild and especially severe depression increased mortality, there is a possibility that the increase in mortality in men taking antidepressant drugs is due to indication bias, whereby antidepressant drugs were prescribed for depression, which is the cause of increased mortality rather than the treatment itself.
This observation has support from prospective studies. In one study, severe depression in the first 2 weeks of hospitalisation for an acute coronary syndrome and failure to improve from depression after treatment with the SSRI antidepressant sertraline or placebo was associated with a twofold increase in mortality rate in the following 7 years. 79 Persistent depression was associated with poor adherence to antidepressant drugs. The sample included a substantial proportion of older people and the effects were not age or gender dependent. Both major and minor depression at the time of acute MI reduced survival in the ENRICHD (Enhancing Recovery in Coronary Heart Disease) study. 80 In women with suspected CHD, an analysis that controlled for cardiovascular risk factors and severity of depression and anxiety symptoms showed that a combination of antidepressant drugs and sedatives was associated with increased all-cause mortality compared with antidepressant drugs alone, sedatives alone or neither antidepressant drugs nor sedatives. 81 All of these data point to the severity of depression and its response to treatment or the ability to tolerate antidepressant drugs to be predictive of mortality rather than antidepressant drugs themselves.
Many of these studies were carried out in people at high risk of cardiovascular disease and in the elderly. In a primary-care population that contained mostly people of middle age, there were no significant differences between mortality rates for six antidepressant drugs after adjustment for age and gender. 82
In summary, the literature shows that the severity of depression and its previous course, gender, response to antidepressant treatment and the ability to tolerate and adhere to antidepressant treatment seem to be more likely to be associated with mortality rather than the effects of antidepressant treatment alone. In our study we found that increased rates of mortality were particularly associated with the group of other antidepressant drugs and SSRIs during the first 28 days of use of antidepressant drugs, with a reduced risk after 85 days of use. This pattern could reflect the effects of an improvement in severity of depression after starting treatment, and a subsequent reduction in mortality rates.
Sudden cardiac death
Few studies have looked specifically at the relationship between sudden cardiac death and antidepressant use. One study using data from the Nurses’ Health Study30 found an increased risk of sudden cardiac death among those with depression, and, more specifically, there was more than a threefold increase among those treated with antidepressant drugs. The risk did not differ by class of antidepressant and was independent of a proxy measure for severity of clinical depression. In another study an elevated risk of sudden cardiac death was observed for TCA doses of more than 100 mg (amitriptyline equivalents) compared with non-users of antidepressant drugs but not with lower doses of TCAs or SSRIs. 83 A nested case–control study in another UK primary-care data set found that venlafaxine was not associated with an increased risk of sudden cardiac death when compared with fluoxetine, dosulepin or citalopram. 84
Other associations indirectly provide some evidence of an association between antidepressant use and sudden cardiac death. In a study of survival among patients with heart failure,85 antidepressant drugs were associated with increased mortality rates after adjustment for depression severity. However, the study was not large enough to look at mortality specific to antidepressant class. Compared with other antidepressant drugs, SSRI use was found to be associated with increased mortality among patients with coronary artery bypass grafting. 86 No studies have been restricted to older patients.
In our study the number of sudden cardiac deaths was small and we are unable to draw firm conclusions on any associations with antidepressant treatment.
Suicide
Twenty per cent of fatal self-poisonings (whether there was suicide intent or not) involve antidepressant drugs,87,88 although there are lower rates of detectable antidepressant drugs in the over-85-year age group, possibly reflecting lower rates of antidepressant prescribing at this age. Among suicides involving people with a recent physician-recorded diagnosis of depression, around one-third had detectable antidepressant drugs at the time of death. 89 Sedatives, rather than antidepressant drugs, are found more often in older suicide victims. 90 A study of suicides in older people found that the commonest methods of suicide were hanging in men and drug overdose in women. 91
There is some evidence that tricyclic antidepressant drugs, with the exception of lofepramine, may result in a higher relative mortality from overdose of that antidepressant than MAOIs, other antidepressant drugs and particularly SSRI antidepressant drugs. 92–98 A national study relating primary-care prescription data to mortality data in England, Scotland and Wales93 found that amoxapine, dosulepin, amitriptyline, trimipramine and nortriptyline were particularly associated with high mortality from overdose, whereas fluoxetine, lofepramine, paroxetine, mianserin and fluvoxamine were associated with a lower mortality from overdose.
Arguably the more important question is whether antidepressant drugs in general and specific classes of antidepressant drugs or individual antidepressant drugs are associated with changes in suicide rates. A number of ecological studies have shown that the suicide rate has decreased as prescribing rates for SSRI antidepressant drugs have increased, but they do not fully account for the decline that started before SSRI antidepressant drugs were introduced. 99–105 There are mixed results concerning whether use of tricyclic antidepressant drugs has changed suicide rate with claims both that they have increased102 and decreased rates. 106–108 Most studies claim that the largest effects are in the elderly, but absolute risk reductions in suicide with antidepressant drugs in the elderly are low,109 perhaps contributing to only 10% of the decline in elderly suicides because of underprescribing of effective doses of antidepressant drugs. 110 However, a study in England found no clear relationship between antidepressant drugs or class of antidepressant and suicide. 111 Furthermore, most ecological studies do not adequately correct for risk factors for suicide, such as gender, age, alcohol use, previous suicide attempts, previous depression, divorce and unemployment, and, when they do, the relationship between antidepressant use and suicide may disappear. 112
Meta-analyses of RCTs of antidepressant drugs in adults have tended to show no effect of antidepressant drugs overall or class of antidepressant drugs on suicide rates. 113–115 Record linkage studies also tend to show no effect of antidepressant drugs overall, SSRIs or tricyclic antidepressant drugs on suicide. 116–117 However, a national cohort study in Finland found that SSRIs were associated with slightly decreased suicide rates, whereas TCAs and other antidepressant drugs had no effect. 77 Among specific antidepressant agents, fluoxetine was associated with decreased suicide rates and venlafaxine with increased suicide rates. In a case–control study with an analysis confined to the over-65-year age group, there was an increased risk of suicide, and particularly suicide by violent means, in the first month of SSRI antidepressant treatment compared with other antidepressant drugs. 118 After the first month, use of SSRIs was not associated with an increased risk of suicide. These results were not confirmed in a study of suicidal thoughts in the elderly, which declined in the first 2 weeks of treatment with antidepressant drugs. 119 A cohort study of elderly patients dispensed SSRIs found that the risk of suicide was not higher during periods of SSRI use than when antidepressant drugs were not being used. 120 Other studies have shown the emergence of new suicidal ideation and worsening of existing suicidal thoughts in 8–23% of patients in the first month of treatment, which usually subside in subsequent months with SSRIs and TCAs, particularly in men and retired people. 121,122
In summary, there is little evidence from the literature that use of SSRI antidepressant drugs increases suicide rates in the elderly, but some evidence that they may reduce rates, although numbers are small in absolute terms and some studies are not adequately controlled for confounding variables. The evidence for the effects of TCAs is less clear, and there is an absence of evidence concerning other antidepressant drugs and specific antidepressant agents. There is a possible risk of increased suicide in the first month of antidepressant treatment, particularly for SSRI antidepressant drugs, but this subsides in subsequent months.
We found that suicide rates were significantly associated with all classes of antidepressant drugs compared with non-use of antidepressant drugs, with highest rates associated with the group of other antidepressant drugs. The number of suicides in our study was small, so we were unable to carry out detailed analyses of individual drugs, dose or duration.
Attempted suicide/self-harm
There has been growing concern about the potential of antidepressant drugs to increase suicidality, especially in children, adolescents and young adults,123 but there is also concern about these effects in the elderly, who have the highest suicide rates. 119 Often suicidality is considered from the perspectives of suicidal ideation and suicidal behaviour, such as self-harm and mortality, but these are not necessarily on a continuum of severity of suicidality because suicides are more common in males and the elderly and self-harm is more common in females and younger adults. 77 Therefore, antidepressant drugs may have effects on suicidal ideation and self-harm that do not necessarily translate into an increased risk of suicide, so a complex picture emerges in relation to antidepressant drugs and suicidality. 117 On the other hand, self-harm is an important clinical outcome in its own right as it often results in hospital admission. 124
The most comprehensive meta-analysis of suicidal behaviour or ideation using individual patient data from randomised controlled trials registered with the US Food and Drug Administration (FDA) found that age has an important mediating effect in relation to antidepressant drugs and suicidal behaviour and ideation. 125 In the group aged 65 years and over, antidepressant drugs reduced suicidal behaviour and ideation, especially in patients with a diagnosis of a major depressive episode. The biggest reductions in suicidal behaviour and ideation were in the group aged 75 years and over. There were no significant differences between drugs in adults overall. Typically, these trials exclude patients who are actively suicidal, which reduces their generalisability.
An earlier meta-analysis of study using FDA-registered RCTs comparing SSRIs and other antidepressant drugs with placebo or a comparator group of older antidepressant drugs (amitriptyline, imipramine or trazodone) found no significant differences in attempted suicide rates between the groups. 113 There was no analysis by age group.
An analysis of data from RCTs submitted to the UK Medicines and Healthcare products Regulatory Agency (MHRA) showed weak evidence of an increase in self-harm with SSRI antidepressant drugs versus placebo;126 however, this increase was not reflected in suicidal ideation or suicides. There was no analysis by age group. Meta-analyses of RCT data of the single agents fluoxetine,127,128 sertraline129 and duloxetine130 showed no change in self-harm compared with placebo or tricyclic antidepressant drugs. No analyses were performed by age and the studies involved few elderly participants.
In a nationwide cohort study in Finland of patients hospitalised for attempted suicide, all classes of antidepressant were associated with an increased rate of future attempted suicide when compared with no antidepressant use. 77 However, these increases were not reflected in increases in suicides or mortality. The sample included a relatively small number of patients over the age of 65 years. A sample of psychiatric outpatients with depressive episodes found a 50% decrease in self-harm over 6 months with antidepressant treatment. 131 In a case–control study of attempted suicide,132 antidepressant use was associated with a reduced risk, whereas discontinuation, initiation and titration of dose up or down were associated with an increased risk of attempted suicide that did not diminish for 8 weeks. There was no analysis by age or gender.
In another large study of antidepressant drugs mostly prescribed by primary-care physicians, the risk of a suicide attempt was highest in the month before starting an antidepressant and progressively declined in the next 6 months. 133 The pattern was similar in those aged over 50 years and in younger age groups. In contrast with some other studies, in this study the risk of suicide attempts in the first month after treatment was higher in patients on TCAs and trazodone than in those on SSRIs and other antidepressant drugs.
In a case–control study using the UK General Practice Research Database (GPRD), patients with depression taking the TCAs amitriptyline or dosulepine or SSRIs fluoxetine or paroxetine showed an increase in suicidal ideation and behaviour in the first 3 months after starting the antidepressant compared with patients who were not prescribed antidepressant drugs. 14 These findings were more marked in the first month of treatment, and on days 1–9 in particular. There were no differences in the results between the four antidepressant drugs. However, there were relatively few patients aged over 60 years and none was older than 69 years. The results were similar in a nested case–control study using a GP database in New Zealand, where use of SSRI antidepressant drugs was associated with an increase in self-harm but not suicide once age, gender and the presence of depression and suicidal ideation was controlled. 134
In another study also using the UK GPRD, but with a much larger sample of patients over the age of 60 years, rates of self-harm were not increased with use of SSRIs or other antidepressant drugs compared with TCAs. 15 There were no differences between specific antidepressant agents, but the prescription of more than one antidepressant was associated with increased rates of self-harm. Another case–control study confined to patients who received psychiatric in-patient care for depression in the USA did not find an association between antidepressant drugs and self-harm in adults under 65 years,123 although there were trends for more suicide attempts with venlafaxine and mirtazapine. No patients in this study were over the age of 64 years.
A problem with naturalistic studies is that antidepressant treatment may be inadequate, in terms of dosage and duration, to measure the antisuicidal effects of antidepressant drugs, particularly in patients who are at known high risk of self-harm. 135 These concerns may be less with the elderly, for whom a previous history of self-harm is not so closely associated with further self-harm. 135,136 Furthermore, most studies do not adequately control for risk factors such as previous suicide attempts, previous depression, medical and psychiatric comorbidity other than depression, alcohol use disorders and marital status. 137
Around 20–30% of non-fatal self-poisoning episodes presenting to general hospitals involve antidepressant drugs. 138 In a study of patients with antidepressant overdose presenting to one Edinburgh hospital, the likelihood of self-harm was decreased by sertaline and amitriptyline, and increased by mirtazapine, venlafaxine and trazodone. 124 There was no analysis by age, so it is unclear how the findings relate to the elderly.
In conclusion, there are relatively few data on antidepressant drugs and self-harm in people over 65 years. The data suggest that in people over 65 years with depressive episodes antidepressant drugs tend to be associated with either reductions or no change in rates of self-harm, with no clear evidence of differences between classes of antidepressant drugs or specific antidepressant agents. Our findings of increased rates of attempted suicide for all classes of antidepressant drugs, which were most marked in the 28 days after an antidepressant prescription, suggest an effect of depression itself rather than a direct causal effect; however, the findings of particularly increased rates for mirtazapine, venlafaxine and trazodone compared with other antidepressant drugs are in accordance, to some extent, with the study by Bateman and colleagues124 and warrant further investigation.
Myocardial infarction
A case–control study of patients with MI, aged between 40 and 75 years, found SSRI use (but not TCA or atypical antidepressant use) to be protective against MI. 139 A slight protective effect of current use of SSRIs on acute MI was also found in a study using GPRD data,140 but not for other antidepressant drugs. However, recent past SSRI use was associated with a slightly raised risk. Among a large cohort of people who were hospitalised in Finland for a suicide attempt, SSRIs were associated with a lower rate of cardiovascular deaths. 77
In a Danish case–control study17 there was a protective effect of all classes of antidepressant on MI, but only when restricted to those with a history of cardiovascular disease. In a cohort study of 136,293 post menopausal women, antidepressant use was not associated with incident CHD (defined as fatal plus non-fatal MI or death due to definite or possible CHD). 32 This was one of the few studies to have adjusted for severity of depression.
Another study using GPRD data found antidepressant drugs to be associated with an elevated risk of MI, but this was not specific to any antidepressant class. 18 The authors argued that this association is most likely to be explained by the nature of depression itself (indication bias) and health utilisation rather than by the antidepressant drugs themselves. However, a large cohort study carried out in North America found a twofold increased risk of MI in those prescribed TCAs, with no elevated risk among those treated with SSRIs. 78 One study confined to older people found that SSRI users were at increased risk of MI compared with non-users after adjusting for depression. 141 A case–control study found no association between risk of ischaemic heart disease and use of SSRIs or the TCAs amitriptyline and lofepramine after adjustment for confounders, but dosulepin (formerly known as dothiepin) was associated with an increased risk which increased with the number of prescriptions. 16
Overall, there are no clear findings in the literature for MI risk and antidepressant use in older people. While some studies have provided evidence of increased risk of MI with antidepressant use,17,18,78,141 others have found that antidepressant drugs confer a protective effect,77,139,140 with others finding no association. 32 Increased risk has been observed as being restricted to TCAs,78 SSRIs141 or across classes17,18 or restricted to particular drugs. 16 Evidence of a protective effect is largely limited to SSRIs. 139,140
Our study found no clear evidence of a difference in myocardial risk between antidepressant classes, although there was some indication of an increased risk associated with SSRIs, mostly confined to fluoxetine and occurring during the first 28 days of use.
Stroke
There have been a number of trials of antidepressant treatment for post-stroke depression, but few have looked at stroke outcomes. 142 In a cohort of post menopausal women, after adjusting for severity of depression, use of SSRIs and the group of other antidepressant drugs were associated with an increased risk of stroke, with other antidepressant drugs having the highest HR. 32 SSRIs were a risk factor for haemorrhagic stroke in particular. In a case–control study of patients with depression, the risk of stroke was increased for all antidepressant classes compared with no antidepressant use. 31
In a Finnish study of subjects who were hospitalised for suicide attempts, there was reduced mortality among those treated with SSRIs, due to a decrease in cerebrovascular-related deaths. 77 A Danish case–control study143 found no association between antidepressant use and intracerebral haemorrhage or ischaemic stroke, although there was some evidence of an increased risk of intracerebral haemorrhage among those taking both SSRIs and NSAIDs.
A comparison of antidepressant drugs in terms of their degree of serotonin reuptake inhibition found no difference in the risk of haemorrhagic stroke across groups (including non-users). 144 Data from the Framingham Heart Study indicated that depressive symptoms were predictive of stroke/TIA among only those aged < 65 years, but revealed no evidence to support an association between antidepressant use and stroke. 145 In a matched case–control study there was no evidence of an increased risk of haemorrhagic stroke among SSRI users. 146 An analysis of GPRD data found no evidence of an increased risk of intracranial haemorrhage among users of antidepressant drugs,147 although the level of antidepressant use was not high enough to exclude the possibility of anything other than fairly large effects.
Findings from previous studies are therefore inconsistent, but some suggest a possible increase in risk among users of antidepressant drugs. Our study findings showed a significantly increased risk of stroke/TIA associated with SSRIs and the group of other antidepressant drugs.
Falls
There is a large literature on the risk of falls in relation to antidepressant drugs among older people,148,149 with some studies suggesting that the risk for TCAs is similar to that for SSRIs,150,151 while others suggest that SSRIs have the highest risk. 152 Few studies have examined effects of individual drugs. Sedation, insomnia and impaired sleep, nocturia, impaired postural reflexes and increased reaction times, postural hypotension, and cardiac rhythm and movement disorders have all been proposed as contributing factors to falls in patients who are taking antidepressant drugs; however, it is difficult to distinguish whether these are due to antidepressant treatment or effects of depression itself.
A review of 78 studies148 found that although there are extensive data for TCAs and SSRIs, there are few data for other antidepressant drugs. The effects of TCAs and SSRIs on the risk of falls were found to be generally similar across studies. There were insufficient data to exonerate any individual antidepressant or class of antidepressant drugs as a potential cause of falls. The authors reported that the magnitude of the increased risk of falling with an antidepressant is about the same as the excess risk found in patients with untreated depression.
A large meta-analysis of RCTs and observational studies of falls in patients older than 60 years found that antidepressant drugs had the strongest association with falls risk out of a number of different types of medication reviewed. 149 This analysis did not distinguish by class or individual drug.
A cross-sectional survey of patients aged 60 years and over152 found that use of antidepressant drugs – SSRIs in particular – was strongly associated with the risk of falls, regardless of the presence of depressive symptoms. Another cross-sectional survey of patients aged 65 and over153 found that SSRIs were significantly associated with the risk of falls but that other antidepressant drugs were not; however, only a small number of patients were taking other antidepressant drugs.
A cohort study of patients aged 60 years and over154 found that SSRIs were associated with over a twofold increase in risk of falls and injurious falls, but there was no significant increase in risk for non-SSRI antidepressant drugs. A cohort study of nursing home residents151 found that patients taking TCAs had a twofold increased rate of falling compared with non-antidepressant users, and SSRIs were associated with an 80% increase; however, trazodone was not associated with an increased falls rate. The study found dose–response effects for TCAs and SSRIs, and a persistent effect throughout treatment.
Our finding of an increased rate of falls for all antidepressant drugs, being slightly higher for SSRIs, is in general accordance with other studies, as are our findings of a dose–response effect and a persistent effect during treatment.
Fracture
A case–control study and case-series analysis20 found that both SSRIs and TCAs were associated with an increased risk of hip fracture, which was most marked in the first 15 days of treatment. The case-series analysis showed lower effects than the case–control study, suggesting that the case–control study was subject to some indication bias. The increased risk with SSRIs and TCAs remained throughout the treatment period, but decreased more steeply for TCAs.
A case–control study of hip fracture in people over 65 years old19 found more than a twofold increased risk for SSRIs and for secondary amine TCAs (nortriptyline, protriptyline and desipramine), and a 50% increase for tertiary amine TCAs (amitriptyline, clomipramine, doxepin, imipramine and trimipramine). Risks were higher for current use than for former use, and for new current users than for continuous current users, in all three drug classes.
A cohort study of patients aged 55 years and over155 found that the risk of non-vertebral fractures increased by over twofold among patients taking SSRIs compared with past antidepressant users, and there was a 50% increase for TCAs users, which was not statistically significant. The association increased with prolonged use for SSRIs but decreased for TCAs. Another cohort study of patients aged 50 years and over156 found that daily SSRI use was associated with a twofold increased rate of low-trauma fractures, but there was no statistically significant effect for TCAs (HR 1.2, 95% CI 0.7 to 2.2). This study also found an increased risk of falling among SSRI users, and a reduced bone mineral density. A case–control study that examined individual drugs in the group of other antidepressant drugs as well as TCAs and SSRIs found dose–response relationships for some TCAs and SSRIs but not for other drugs. 157
The increased risk of fracture associated with antidepressant use may be due to an increased risk of falls, but there is also evidence of reduced bone mineral density in SSRIs users. For example, a study of 5995 men aged 65 years and older158 found that bone mineral density was lower among those reporting current SSRI use, but not among users of other antidepressant drugs. A cohort study of older women found that use of SSRIs but not of TCAs was associated with an increased rate of bone loss at the hip. 159
Overall findings from the literature suggest that SSRIs and TCAs are associated with increased fracture rates, with possibly somewhat higher rates for SSRI use than for TCAs. There is little evidence for other antidepressant drugs. Our findings of a more marked and prolonged increase in risk for SSRIs, compared with TCAs, are in general agreement with this. Our finding of an increased risk associated with the group of other antidepressant drugs warrants confirmation in other studies.
Upper gastrointestinal bleeds
In a systematic review comparing SSRIs with TCAs in the treatment of depression in older people there was some suggestion that GI problems were more common in those patients who were treated with classical TCAs, although the number of adverse outcomes was too low to be conclusive. 160 In the absence of trials of sufficient size to identify differences in rare adverse outcomes, studies examining associations between antidepressant use and GI bleeding have been largely confined to cohort studies24–26 and case–control studies. 27,161–163 Whereas some studies have found an increased risk of GI bleeds to be associated with antidepressant use,24,27,161 others have found no evidence for an effect. 25,163 There is conflicting evidence as to whether25,26,162 or not27 that risk is increased in the presence of NSAID use.
Much of the evidence is not specific to older age groups; however, in a retrospective cohort of Canadians aged 65 years and over, the risk of GI bleeding increased with higher levels of inhibition of serotonin reuptake,24 and was greatest in the oldest age groups and those with previous upper GI bleeding. Among adults admitted to hospital, a modest increased risk of GI bleeding was found with antidepressant use, but this was restricted to the group of other antidepressant drugs rather than TCAs or SSRIs. 161 A case–control study found that SSRIs overall were associated with an increased risk of upper GI tract bleeding, and also found a particularly increased risk for venlafaxine. 162
In a study of medication data, combined use of SSRIs and NSAIDs strongly increased the risk of GI adverse effects. 25 A study of hospitalisation data in Denmark reported similar findings of an increased risk of upper GI bleeding with use of SSRIs, which was increased by concurrent use of NSAIDs or low-dose aspirin. 26 There is some evidence that this risk is attenuated with the use of acid-suppressing agents. 162 In contrast, a case–control study of incident cases of upper GI bleeds found no evidence of an increased risk of GI bleeding for SSRI use and no evidence of an interaction with NSAIDs. 163 A case-control analysis of GPRD data estimated that individually SSRIs and NSAIDs doubled the risk of GI bleeding but that this risk was not substantially increased when these drugs were prescribed together. 27 The relationship between antidepressant drugs and GI bleeding did not differ between those above and below 80 years.
In our study we found similar increased risks for all classes of antidepressant drugs. Our finding of a particularly increased risk associated with venlafaxine is in agreement with the findings of de Abajo and colleagues. 162 Unlike some other studies, we did not find a significant interaction with use of NSAIDs or aspirin.
Epilepsy/seizures
Antidepressant drugs can result in seizures as an ADR and as a complication of an overdose. 164 Both are more likely in people with a history of epilepsy and with other disorders of the brain that might be associated with seizures, such as stroke. Depressive episodes are more likely in those with epilepsy and with other disorders of the brain than in the general population, so antidepressant drugs are used in more than 10% of people with these conditions. 165 Polypharmacy of drugs with the potential to induce seizures, including SSRIs and TCAs, increased two- or threefold in women and men with epilepsy, respectively, from age 34 to 85 years. 165 There are also claims that depression itself can increase the risk of seizures in people over the age of 65 years independently of taking antidepressant drugs. 166
Interrogation of the World Health Organization (WHO) Program for International Drug Monitoring database of ADRs shows a 12-fold variation in the reporting of seizures as a proportion of ADRs. 167 In general, tetracyclic drugs have higher rates of seizures than tricyclic drugs and these, in turn, have higher rates of seizures than SSRI antidepressant drugs. 33,168
Other literature also points to different potentials for specific drugs to induce seizures within classes of antidepressant. Desipramine and dosulepin may be more proconvulsant than other tricyclic antidepressant drugs. 169,170 However, there are exceptions, with the growing realisation that escitalopram and citalopram may have a proconvulsive effect compared with other SSRI antidepressant drugs. 171 A review of consecutive overdose patients admitted to one Edinburgh hospital also suggested that citalopram and venlafaxine are proconvulsants. 172 Paradoxically, doxepin (TCA) and fluoxetine (SSRI) may have anticonvulsant effects,173,174 although there are no randomised controlled trials supporting these claims. However, nothing in the literature points to differential effects of age on the capacity for an antidepressant to produce a seizure unless there is polypharmacy or a greater risk due to underlying medical problems.
Our analyses found that the risk of epilepsy/seizures varied by antidepressant class and was increased for SSRIs and the group of other antidepressant drugs compared with no use of antidepressant drugs, but not for TCAs. Of individual drugs, venlafaxine had the highest rates. There is limited support for these findings in the literature.
Road traffic accidents
Studies that have tested the effects of antidepressant drugs on driving performance have found that sedating antidepressant drugs have a similar effect to alcohol. 21
A study that examined risk factors for vehicle crashes specifically in older people175 found that the use of antidepressant drugs increased the risk of a crash in men but not in women, with an approximate doubling in risk. Another study in older people found that current use of TCAs increased the risk of a motor vehicle crash in men and women, and that the risk increased with dose and was substantial for high doses. 176 A case–control study also found an increased risk in older drivers taking TCAs. 177
In studies across all ages, a Norwegian study found similar increased risks for sedating (including TCAs and mirtazapine) and non-sedating antidepressant drugs (including SSRIs and venlafaxine). 178 In a self-controlled case-series study, Gibson and colleagues51 found that use of SSRIs for more than 4 weeks was associated with an increased risk of a motor vehicle crash, but shorter-term use was not, nor was the use of TCAs. Other studies have found no associations with antidepressant use. 179,180
Many of these studies have been unable to distinguish between effects of antidepressant use and direct effects of depression itself on risk of a crash, or account for possible changes in driving patterns that may occur in people with depression. Our findings of no association with RTAs for antidepressant medication in a study restricted to people diagnosed with depression suggests that at least part of the increased risk in other studies may be due to depression itself rather than its treatment.
Adverse drug reactions
A Swedish death registry study reported that antidepressant drugs account for approximately 7% of all fatal ADRs. 181 Antidepressant drugs also have the highest Adverse Drug Reaction Hospitalization index based on a 7-year study of 454,520 events reported to a national drug poisoning data system related to commonly implicated therapeutic agents,34 although this is across all age groups rather than the elderly.
The German drug safety programme in psychiatry reported an assessment of severe or new ADRs in patients treated with antidepressant drugs. 182 The overall incidence of severe ADRs was 1.4% for exposed patients. Rates were higher for TCAs and lower for MAOIs and SSRIs. In particular, TCAs were associated with known risks, such as toxic delirium, grand mal seizures, and hepatic, urological, allergic and cardiovascular reactions. In SSRI-treated patients, psychic and neurological ADRs were most common, followed by GI, dermatological and endocrinological/electrolyte reactions, with agitation, hyponatraemia, increased liver enzymes, nausea, and the serotonin syndrome as the main unwanted symptoms. Venlafaxine was associated with adverse central nervous systems and somatic symptoms, such as severe agitation, diarrhoea, increased liver enzymes, hypertension and hyponatraemia. Mirtazapine was mostly connected with increased liver enzymes, cutaneous oedema and collapse.
A large multinational case–control study, conducted in Europe between 1997 and 2001, evaluated the risk of medications to induce severe cutaneous adverse reactions. 183 An association was found for sertraline (odds ratio 11, 95% CI 2.7 to 46) based on six cases and five controls. No association was found for fluoxetine or other SSRIs, although the authors mentioned the need to monitor fluoxetine closely.
We found little evidence of any difference between drugs for ADRs, although there was some indication of an increased risk for TCAs at high doses and for lofepramine and sertraline.
Hyponatraemia
Reviews of hyponatraemia184,185 have concluded that SSRIs cause hyponatraemia more frequently than other antidepressant drugs. The incidence of hyponatraemia caused by SSRIs was reported to vary widely from 0.5% to 32%, usually occurring within the first few weeks of treatment and returning to normal within 2 weeks after drug withdrawal. Older age was an important risk factor for the development of hyponatraemia associated with SSRIs. However, a review of the risks and benefits of newer antidepressant drugs concluded that there is a lack of data on hyponatraemia. 186
A case–control study23 found that SSRIs were associated with a threefold increased risk of hyponatraemia compared with non-use, and that hyponatraemia was more common in older patients. Another study by the same authors found that serotonergic antidepressant drugs (SSRIs and venlafaxine) were associated with the development of hyponatraemia, with the highest risk occurring in the first 2 weeks. 187
A study to determine risk factors associated with hyponatraemia during treatment with antidepressant drugs using the WHO database for spontaneous reporting of ADRs22 found that the risk for hyponatraemia during treatment with antidepressant drugs was highest in women, in the elderly, during the summer, and during the first weeks of treatment.
A study of elderly patients in a psychogeriatric inpatient unit188 reported that the odds of hyponatraemia were increased in patients taking either an SSRI or venlafaxine compared with patients not taking these drugs, with venlafaxine having the larger risk.
There is fairly consistent evidence from these studies of an increased risk of hyponatraemia with SSRI use, and the findings of our study are in accordance with this, although we found increased risks only for the SSRIs citalopram, escitalopram and fluoxetine, but not paroxetine or sertraline. We also found some evidence of an increased risk associated with venlafaxine. As in other studies, the highest risks occurred during the first few weeks of starting the antidepressant and were no longer increased a few weeks after stopping treatment.
Analyses of costs
A draft National Clinical Practice Guideline189 undertook a literature review to identify economic evaluations, comparing different antidepressant drugs, and identified nine studies. The report concluded that the pharmacoeconomic data were piecemeal as no study had compared all relevant antidepressant drugs in a single evaluation and went on to develop a cost–utility model to compare 10 antidepressant drugs. 189 It is difficult to make comparisons with any of these nine previous studies as they sought to identify the most cost-effective antidepressant, where successful treatment/QALY gain was the measure of effect, whereas the measures of effect within our analyses were the different adverse events (estimated number averted with different drugs). In terms of costs, the National Clinical Practice Guideline estimated that of the drugs assessed citalopram/fluoxetine had the lowest unit cost and venlafaxine the highest, which is in line with our estimates;189 however, it did point out that venlafaxine has recently been released in generic form, and that escitalopram will be shortly. This means that the price for these drugs will fall in the future, which may have implications for estimates of cost-effectiveness.
Chapter 5 Conclusions
Implications for health care
The finding that SSRIs and drugs in the group of other antidepressant drugs were not associated with a reduced risk of any of the adverse outcomes compared with TCAs and may even be associated with an increased risk for certain outcomes implies that a careful evaluation of benefits and adverse outcomes is needed when prescribing antidepressant drugs to older people, which should include consideration of TCAs and tailoring of drugs to individual patients.
In this study, mirtazapine, venlafaxine and trazodone were associated with higher rates than the other antidepressant drugs for a number of outcomes, including all-cause mortality and attempted suicide/self-harm. Venlafaxine was also associated with the highest rates of stroke/TIA, fracture and epilepsy/seizures. These risks should be considered when prescribing these drugs.
There was evidence from the current study that use of a combination of antidepressant drugs was associated with an increased risk for many of the adverse events studied. Although this may reflect increased severity of depression and lack of response to monotherapy, it is a matter of concern, and use of a higher dose of a single antidepressant should be considered as an alternative to combined treatment where appropriate.
This study found that rates of most outcomes were highest in the first 28 days after starting an antidepressant, which would support careful monitoring during the first weeks after prescribing antidepressant drugs in older people.
The evidence suggests that all classes of antidepressant drugs are associated with an increased risk of falls and fracture in older people. These risks should be considered when prescribing these drugs.
There is fairly consistent evidence of an increased risk of hyponatraemia associated with SSRI use; we found increased risks associated with citalopram, escitalopram and fluoxetine but not with paroxetine or sertraline. We also found some evidence of an increased risk with venlafaxine. These risks should be considered when prescribing these drugs.
Implications for further research
There are few randomised trials of antidepressant drugs in older people, particularly in a primary-care setting, with sufficient size and length of follow-up to assess adverse outcomes as well as benefits. Thus, there is a need for a long-term randomised trial of antidepressant drugs in older people with depression in primary care comparing benefits and risks of more common adverse events between an SSRI and a low-dose TCA.
As all observational studies are susceptible to indication biases and residual confounding, and as it is particularly difficult in observational studies of antidepressant drugs to separate the effects of treatment from the effects of depression itself and changes in severity of depression, there is a need for meta-analyses of randomised controlled trials of antidepressant drugs in relation to adverse events in older people to be carried out to confirm these findings.
Some of our findings are unexpected, and there is limited information in the literature on some of these adverse events in older people, particularly for individual drugs. Research is needed to confirm our findings using other data sources of older people in a community setting.
Further studies are needed to develop algorithms to individualise the risks associated with antidepressant use so that patients at highest risk of these adverse events can be monitored closely.
A number of adverse events have been examined within this study, but it is unclear which of the different adverse events it is most important to avert, what the overall loss is expected to be for the different types of antidepressant drugs, and what one would be willing to pay to avert an adverse event. Further research might be conducted with a view to estimate the loss in utility (disutility) associated with each of the different types of adverse events. This would enable calculation of expected QALY loss associated with the different types of antidepressant drugs for each adverse event. When combined with cost information this would enable one to estimate the incremental cost per loss in QALY averted, i.e. level of cost-effectiveness associated with different antidepressant drugs.
Conclusions
There are associations between use of antidepressant drugs and a number of adverse events in people with depression aged 65 years and older. These associations vary by antidepressant class and between individual drugs. There is no evidence that SSRIs or drugs in the group of other antidepressant drugs are associated with a reduced risk of any of the adverse outcomes compared with TCAs, and they may even be associated with an increased risk for certain outcomes. The risks of prescribing different antidepressant drugs need to be weighed against the potential benefits of these drugs. Limitations of this study include possible indication bias, and residual confounding.
Acknowledgements
We thank the practices using EMIS who provide data to QResearch and their patients, and David Stables (Medical Director, EMIS) for his expertise in establishing, developing and supporting the database.
We thank colleagues and patient representatives for their comments on our research findings.
We also wish to thank the National Institute for Health Research Health Technology Assessment programme for providing the funding for this project.
Contribution of authors
Carol Coupland (Associate Professor of Medical Statistics) was the chief investigator of the study and was involved in the conception and design of the study, carrying out statistical analysis, interpretation of data, reviewing the literature, and drafting and revising the report.
Paula Dhiman (Research Statistician) was involved in the design of the study, data checking, statistical analysis, interpreting data and reviewing the literature.
Garry Barton (Senior Lecturer in Health Economics) conducted the health-economic analyses, and was involved in interpretation of data, reviewing the literature and drafting the report.
Richard Morriss (Professor of Psychiatry and Community Mental Health) was involved in the conception and design of the study, interpretation of data, reviewing the literature, and drafting the report.
Antony Arthur (Senior Lecturer in Elder Care) was involved in the conception and design of the study, interpretation of data, reviewing the literature, and drafting the report.
Tracey Sach (Senior Lecturer in Health Economics) was involved in the conception and design of the study, health-economic analyses, interpretation of data, and drafting the report.
Julia Hippisley-Cox (Professor of Clinical Epidemiology and General Practice) was involved in the conception and design of the study, extraction of the data, interpretation of data, reviewing the literature and drafting the report.
Publication
Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ 2011;343:d4551. DOI: 10.1136/bmj.d4551.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry 1999;174:307-11.
- The NHS Information Centre Prescribing Support Unit (PSU) . Prescriptions Dispensed in the Community. Statistics for 1998 to 2008 2009.
- National Institute for Health and Clinical Excellence (NICE) . Depression: The Treatment and Management of Depression in Adults (update) 2009.
- Arroll B, Elley CR, Fishman T, Goodyear-Smith FA, Kenealy T, Blashki G, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database Syst Rev 2009.
- Mottram PG, Wilson K, Strobl JJ. Antidepressants for depressed elderly. Cochrane Database Syst Rev 2006;1. 10.1002/14651858.CD003491.pub2.
- Wilson K, Mottram PG, Sivananthan A, Nightingale A. Antidepressants versus placebo for the depressed elderly. Cochrane Database Syst Rev 2009;1. 10.1002/14651858.CD000561.
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 2010;303:47-53.
- Cadieux RJ. Antidepressant drug interactions in the elderly. Understanding the P450 system is half the battle in reducing risks. Postgrad Med 1999;106:231-2.
- Giron MS, Fastbom J, Winblad B. Clinical trials of potential antidepressants: to what extent are the elderly represented: a review. Int J Geriatr Psychiatry 2005;20:201-17.
- Pollock BG. Adverse reactions of antidepressants in elderly patients. J Clin Psychiatry 1999;60:4-8.
- Parikh C. Antidepressants in the elderly: challenges for study design and their interpretation. Br J Clin Pharmacol 2000;49:539-47.
- Shah R, Uren Z, Baker A, Majeed A. Deaths from antidepressants in England and Wales 1993–1997: analysis of a new national database. Psychol Med 2001;31:1203-10.
- Neutel CI, Patten SB. Risk of suicide attempts after benzodiazepine and/or antidepressant use. Ann Epidemiol 1997;7:568-74.
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA 2004;292:338-43.
- Martinez C, Rietbrock S, Wise L, Ashby D, Chick J, Moseley J, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case–control study. BMJ 2005;330.
- Hippisley-Cox J, Pringle M, Hammersley V, Crown N, Wynn A, Meal A, et al. Antidepressants as risk factor for ischaemic heart disease: case–control study in primary care. BMJ 2001;323:666-9.
- Monster TBM, Johnsen SP, Olsen ML, McLaughlin JK, Sorensen HT. Antidepressants and risk of first-time hospitalization for myocardial infarction: a population-based case–control study. Am J Med 2004;117:732-7.
- Tata LJ, West J, Smith C, Farrington P, Card T, Smeeth L, et al. General population based study of the impact of tricyclic and selective serotonin reuptake inhibitor antidepressants on the risk of acute myocardial infarction. Heart 2005;91:465-71.
- Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N. Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 1998;351:1303-7.
- Hubbard R, Farrington P, Smith C, Smeeth L, Tattersfield A. Exposure to tricyclic and selective serotonin reuptake inhibitor antidepressants and the risk of hip fracture. Am J Epidemiol 2003;158:77-84.
- Ramaekers JG. Antidepressants and driver impairment: empirical evidence from a standard on-the-road test. J Clin Psychiatry 2003;64:20-9.
- Spigset O, Hedenmalm K. Hyponatremia in relation to treatment with antidepressants: a survey of reports in the World Health Organization data base for spontaneous reporting of adverse drug reactions. Pharmacotherapy 1997;17:348-52.
- Movig KLL, Leufkens HGM, Lenderink AW, van den Akker VGA, Hodiamont PPG, Goldschmidt HMJ, et al. Association between antidepressant drug use and hyponatraemia: a case–control study. Br J Clin Pharmacol 2002;53:363-9.
- van Walraven C, Mamdani MM, Wells PS, Williams JI. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ 2001;323:655-8.
- de Jong JC, van den Berg PB, Tobi H, de Jong-van den Berg LT. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol 2003;55:591-5.
- Dalton SO, Johansen C, Mellemkjaer L, Norgard B, Sorensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med 2003;163:59-64.
- Tata LJ, Fortun PJ, Hubbard RB, Smeeth L, Hawkey CJ, Smith CJ, et al. Does concurrent prescription of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs substantially increase the risk of upper gastrointestinal bleeding?. Aliment Pharmacol Ther 2005;22:175-81.
- Bingefors K, Isacson D, vonKnorring L, Smedby B, Wicknertz K. Antidepressant-treated patients in ambulatory care mortality during a nine-year period after first treatment. Br J Psychiatry 1996;169:647-54.
- Ryan J, Carriere I, Ritchie K, Stewart R, Toulemonde G, Dartigues J-F, et al. Late-life depression and mortality: influence of gender and antidepressant use. Br J Psychiatry 2008;192:12-8.
- Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, et al. Depression and risk of sudden cardiac death and coronary heart disease in women. Results from the Nurses’ Health Study. J Am Coll Cardiol 2009;53:950-8.
- Chen Y, Guo JJ, Li H, Wulsin L, Patel NC. Risk of cerebrovascular events associated with antidepressant use in patients with depression: a population-based, nested case–control study. Ann Pharmacother 2008;42:177-84.
- Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, Robinson JG, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative Study. Arch Intern Med 2009;169:2128-39.
- Rosenstein DL, Nelson JC, Jacobs SC. Seizures associated with antidepressants: a review. J Clin Psychiatry 1993;54:289-99.
- Vassilev ZP, Chu AF, Ruck B, Adams EH, Marcus SM. Evaluation of adverse drug reactions reported to a poison control center between 2000 and 2007. Am J Health Syst Pharm 2009;66:481-7.
- McCrone P, Dhanasiri S, Patel A. Paying the price: the cost of mental health care in England to 2026. London: King’s Fund; 2008.
- Pirmohamed M, James S, Meakin A, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004;329:15-9.
- Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007;63:136-47.
- The fourth report from the Patient Safety Observatory . Safety in Doses: Medication Safety Incidents in the NHS 2007.
- Sach T, Barton GR, Jenkinson C, Doherty M, Avery A, Muir KR. Comparing cost-utility estimates: Does the choice of E D or S D matter?. Med Care 2009;47:889-94.
- Farrington CP. Relative incidence estimation from case series for vaccine safety evaluation. Biometrics 1995;51:228-35.
- Whitaker H. The self controlled case series method. BMJ 2008;337.
- Hippisley-Cox J, Vinogradova Y, Coupland C, Pringle M. Comparison of key practice characteristics between general practices in England and Wales and general practices in the QResearch database. Report to the Health and Social Care Information Centre. Nottingham: University of Nottingham; 2005.
- Hippisley-Cox J, Pringle M. Prevalence, care and outcomes for patients with diet controlled diabetes in general practice: cross-sectional survey. Lancet 2004;364:423-5.
- Hippisley-Cox J, Coupland C. Effect of combinations of drugs on all-cause mortality in patients with ischaemic heart disease: nested case control analysis. BMJ 2005;330:1059-63.
- Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340.
- Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients on Cox 2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case–control analysis. BMJ 2005;366:1366-74.
- Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case–control analysis. BMJ 2005;331:1310-16.
- World Health Organization (WHO) . Manual of the International Statistical Classification of Diseases, Injuries and Causes of Death 1997;1.
- World Health Organization (WHO) . World Health Organization:International Statistical Classification of Disease and Related Health Problems. Tenth Revision (ICD-10) 1992.
- Rubino A, Roskell N, Tennis P, Mines D, Weich S, Andrews E. Risk of suicide during treatment with venlafaxine, citalopram, fluoxetine, and dothiepin: retrospective cohort study. BMJ 2007;334.
- Gibson JE, Hubbard RB, Smith CJP, Tata LJ, Britton JR, Fogarty AW. Use of self-controlled analytical techniques to assess the association between use of prescription medications and the risk of motor vehicle crashes. Am J Epidemiol 2009;169:761-8.
- British Medical Association and the Royal Pharmaceutical Society of Great Britain . British National Formulary 2007.
- Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the north. London: Croom Helm; 1988.
- Kaye JA, Jick H. Epidemiology of lower limb fractures in general practice in the United Kingdom. Inj Prev 2004;10:368-74.
- Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492-5.
- Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. Am J Epidemiol 1996;143:1165-73.
- Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006;25:1768-97.
- Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case series studies. Stat Med 2006;25:2618-31.
- National Institute of Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2008.
- Health and Social Care Information Centre Prescribing Support Unit (PSU) . Prescription Cost Analysis: England 2008 2009. www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescriptions/prescription-cost-analysis-2008.
- HM Treasury . The Green Book: Appraisal and Evaluation in Central Government 2003.
- Curtis L. Unit costs of health and social care. The University of Kent: Personal Social Services Research Unit; 2008.
- Department of Health . NHS Reference Costs 2006–07 2008.
- Briggs AH, Sculpher MJ, Claxton K. Decision modelling for health economic evaluation. New York: Oxford University Press; 2006.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effective decisions: the role of the cost-effectiveness acceptability curve (CEAC), cost-effectiveness acceptability frontier (CEAF) and expected value of perfect information (EVPI). Value Health 2008;11:886-97.
- Drummond MF, Sculpher MJ, Torrance GW, O Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. New York, NY: Oxford University Press; 2005.
- UK BEAM Trial Team . United Kingdom back pain exercise and manipulation (UK BEAM) randomised trial: cost effectiveness of physical treatments for back pain in primary care. BMJ 2004;329.
- Furukawa TA, McGuire H, Barbui C. Low dosage tricyclic antidepressants for depression. Cochrane Database Syst Rev 2003;3. 10.1002/14651858.CD0031972003.
- Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res 2002;53:849-57.
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006;27:2763-74.
- Hippisley-Cox J, Fielding K, Pringle M. Depression as a risk factor for ischaemic heart disease in men: population based case–control study. BMJ 1998;316:1714-19.
- Empana JP, Jouven X, Lemaitre RN, Sotoodehnia N, Rea T, Raghunathan TE, et al. Clinical depression and risk of out-of-hospital cardiac arrest. Arch Intern Med 2006;166:195-200.
- Maidment R, Livingston G, Katona C. Just keep taking the tablets: adherence to antidepressant treatment in older people in primary care. Int J Geriatr Psychiatry 2002;17:752-7.
- Olfson M, Marcus SC, Tedeschi M, Wan GJ. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry 2006;163:101-8.
- Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al. A comparison of the cost-effectiveness of five strategies for the prevention of non-steroidal anti-inflammatory drug induced gastrointestinal toxicity: a systematic review with economic modelling. Health Technol Assess 2006;10.
- Haukka J, Arffman M, Partonen T, Sihvo S, Elovainio M, Tiihonen J, et al. Antidepressant use and mortality in Finland: a register-linkage study from a nationwide cohort. Eur J Clin Pharmacol 2009;65:715-20.
- Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Tanskanen A, Haukka J. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry 2006;63:1358-67.
- Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med 2000;108:2-8.
- Glassman AH, O Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002;288:701-9.
- Carney RM, Freedland KE, Steinmeyer B, Blumenthal JA, Berkman LF, Watkins LL, et al. Depression and five year survival following acute myocardial infarction: a prospective study. Affect Disord 2008;109:133-8.
- Krantz DS, Whittaker KS, Francis JL, Rutledge T, Johnson BD, Barrow G, et al. Psychotropic medication use and risk of adverse cardiovascular events in women with suspected coronary artery disease: outcomes from the Women’s Ischemia Syndrome Evaluation (WISE) study. Heart 2009;95:1901-6.
- Mackay FR, Dunn NR, Martin RM, Pearce GL, Freemantle SN, Mann RD. Newer antidepressants: a comparison of tolerability in general practice. Br J Gen Pract 1999;49:892-6.
- Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther 2004;75:234-41.
- Martinez C, Assimes TL, Mines D, Dell Aniello S, Suissa S. Use of venlafaxine compared with other antidepressants and the risk of sudden cardiac death or near death: a nested case–control study. BMJ 2010;340.
- Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O Connor CM, Adams KF, et al. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern 2007;167:367-73.
- Xiong GL, Jiang W, Clare R, Shaw LK, Smith PK, Mahaffey KW, et al. Prognosis of patients taking selective serotonin reuptake inhibitors before coronary artery bypass grafting. Am J Cardiol 2006;98:42-7.
- Office for National Statistics (ONS) . Deaths related to drug poisoning in England and Wales, 2002–06. Health Stat Q 2007;36:66-72.
- Abrams RC, Leon AC, Tardiff K, Marzuk PM, Li CS, Galea S. Antidepressant use in elderly suicide victims in New York City: an analysis of 255 cases. J Clin Psychiatry 2009;70:312-17.
- Isacsson G, Bergman U, Rich CL. Antidepressants, depression and suicide: an analysis of the San Diego study. J Affect Disord 1994;32:277-86.
- Jonasson B, Jonasson U, Saldeen T. Among fatal poisonings dextropropoxyphene predominates in younger people, antidepressants in the middle aged and sedatives in the elderly. J Forensic Sci 2000;45:7-10.
- Harwood DMJ, Hawton K, Hope T, Jacoby R. Suicide in older people: mode of death, demographic factors, and medical contact before death. Int J Geriatr Psychiatry 2000;15:736-43.
- Cassidy S, Henry J. Fatal toxicity of antidepressant drugs in overdose. Br Med J 1987;295:1021-4.
- Henry JA, Alexander CA, Sener EK. Relative mortality from overdose of antidepressants. BMJ 1995;310:221-4.
- Montgomery SA, Baldwin D, Green M. Why do amitriptyline and dothiepin appear to be so dangerous in overdose?. Acta Psychiatr Scand 1989;80:47-53.
- Buckley NA, McManus PR. Fatal toxicity of serotoninergic and other antidepressant drugs: analysis of United Kingdom mortality data. BMJ 2002;325:1332-3.
- Bauer M, Monz BU, Montejo AL, Quail D, Dantchev N, Dernyttenaere K, et al. Prescribing patterns of antidepressants in Europe: results from the factors influencing depression endpoints research (FINDER) study. Eur Psychiatry 2008;23:66-73.
- Flanagan RJ. Fatal toxicity of drugs used in psychiatry. Human Psychopharmacol Clin Exp 2008;23:43-51.
- Tournier M, Grolleau A, Cougnard A, Verdoux H, Molimard M. The prognostic impact of psychotropic drugs in intentional drug overdose. Pharmacopsychiatry 2009;42:51-6.
- Carlsten A, Waern M, Ekedahl A, Ranstam J. Antidepressant medication and suicide in Sweden. Pharmacoepidemiol Drug Saf 2001;10:525-30.
- Grunebaum MF, Ellis SP, Li SH, Oquendo MA, Mann JJ. Antidepressants and suicide risk in the United States, 1985–1999. J Clin Psychiatry 2004;65:1456-62.
- Morgan OW, Griffiths C, Majeed A. Association between mortality from suicide in England and antidepressant prescribing: an ecological study. BMC Public Health 2004;4.
- Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry 2005;62:165-72.
- Reseland S, Bray I, Gunnell D. Relationship between antidepressant sales and secular trends in suicide rates in the Nordic countries. Br J Psychiatry 2006;188:354-8.
- Nakagawa A, Grunebaum MF, Ellis SP, Oquendo MA, Kashima H, Gibbons RD, et al. Association of suicide and antidepressant prescription rates in Japan, 1999–2003. J Clin Psychiatry 2007;68:908-16.
- Ludwig J, Marcotte DE, Norberg K. Anti-depressants and suicide. J Health Econ 2009;28:659-76.
- Hall WD, Mant A, Mitchell PB, Rendle VA, Hickie IB, McManus P. Association between antidepressant prescribing and suicide in Australia, 1991–2000: trend analysis. BMJ 2003;326.
- Søndergård L, Kvist K, Lopez AG, Andersen PK, Kessing LV. Temporal changes in suicide rates for persons treated and not treated with antidepressants in Denmark during 1995–1999. Acta Psychiatr Scand 2006;114:168-76.
- Nettelbladt P, Mattisson C, Bogren M, Holmqvist M. Suicide rates in the Lundby cohort before and after the introduction of tricyclic antidepressant drugs. Arch Suicide Res 2007;11:57-6.
- Hall WD, Lucke J. How have the selective serotonin reuptake inhibitor antidepressants affected suicide mortality?. Aust N Z J Psychiatry 2006;40:941-50.
- Erlangsen A, Canudas-Romo V, Conwell Y. Increased use of antidepressants and decreasing suicide rates: a population-based study using Danish register data. J Epidemiol Community Health 2008;62:448-54.
- Morgan O, Griffiths C, Majeed A. Antidepressant prescribing and changes in antidepressant poisoning mortality and suicide in England, 1993–2004. J Public Health 2008;30:60-8.
- Kalmar S, Szanto K, Rihmer Z, Mazumdar S, Harrison K, Mann JJ. Antidepressant prescription and suicide rates: effect of age and gender. Suicide Life Threat Behav 2008;38:363-74.
- Khan A, Warner HA, Brown WA. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the Food and Drug Administration database. Arch Gen Psychiatry 2000;57:311-17.
- Khan A, Khan S, Kolts R, Brown WA. Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports. Am J Psychiatry 2003;160:790-2.
- Hammad TA, Laughren TP, Racoosin JA. Suicide rates in short-term randomized controlled trials of newer antidepressants. J Clin Psychopharmacol 2006;26:203-7.
- Simon GE, VonKorff M. Suicide mortality among patients treated for depression in an insured population. Am J Epidemiol 1998;147:155-60.
- Simon GE. How can we know whether antidepressants increase suicide risk?. Am J Psychiatry 2006;163:1861-3.
- Juurlink DN, Mamdani MM, Kopp A, Redelmeier DA. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry 2006;163:813-21.
- Szanto K, Mulsant BH, Houck P, Dew MA, Reynolds CF III. Occurrence and course of suicidality during short-term treatment of late-life depression. Arch Gen Psychiatry 2003;60:610-17.
- Rahme E, Dasgupta K, Turecki G, Nedjar H, Galbaud du Fort G. Risks of suicide and poisoning among elderly patients prescribed selective serotonin reuptake inhibitors: a retrospective cohort study. J Clin Psychiatry 2008;69:349-57.
- Perlis RH, Beasley CM, Wines JD, Tamura RN, Cusin C, Shear D, et al. Treatment-associated suicidal ideation and adverse effects in an open, multicenter trial of fluoxetine for major depressive episodes. Psychother Psychosom 2007;76:40-6.
- Perroud N, Uher R, Marusic A, Rietschel M, Mors O, Henigsberg N, et al. Suicidal ideation during treatment of depression with escitalopram and nortriptyline in genome-based therapeutic drugs for depression (GENDEP): a clinical trial. BMC Med 2009;7.
- Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: a case–control study. Arch Gen Psychiatry 2006;63:865-72.
- Bateman DN, Chick J, Good AM, Kelly CA, Masterton G. Are selective serotonin re-uptake inhibitors associated with an increased risk of self-harm by antidepressant overdose?. Eur J 2004;60:221-4.
- Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, et al. Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. BMJ 2009;339.
- Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ 2005;330.
- Beasley CM, Dornseif BE, Bosomworth JC, Sayler ME, Rampey AH, Heiligenstein JH, et al. Fluoxetine and suicide: a meta-analysis of controlled trials of treatment for depression. BMJ 1991;303:685-92.
- Tauscher-Wisniewski S, Disch D, Plewes J, Ball S, Beasley CM. Evaluating suicide-related adverse events in clinical trials of fluoxetine treatment in adults for indications other than major depressive disorder. Psychol Med 2007;37:1585-93.
- Vanderburg DG, Batzar E, Fogel I, Kremer CM. A pooled analysis of suicidality in double-blind, placebo-controlled studies of sertraline in adults. J Clin Psychiatry 2009;70:674-83.
- Acharya N, Rosen AS, Polzer JP, D Souza DN, Perahia DG, Cavazzoni PA, et al. Duloxetine: meta-analyses of suicidal behaviors and ideation in clinical trials for major depressive disorder. J Clin Psychopharmacol 2006;26:587-94.
- Mulder RT, Joyce PR, Frampton CMA, Luty SE. Antidepressant treatment is associated with a reduction in suicidal ideation and suicide attempts. Acta Psychiatr Scand 2008;118:116-22.
- Valuck RJ, Orton HD, Libby AM. Antidepressant discontinuation and risk of suicide attempt: a retrospective, nested case–control study. J Clin Psychiatry 2009;70:1069-77.
- Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry 2006;163:41-7.
- Didham RC, McConnell DW, Blair HJ, Reith DM. Suicide and self-harm following prescription of SSRIs and other antidepressants: confounding by indication. Br J Clin Pharmacol 2005;60:519-25.
- Oquendo MA, Kamali M, Ellis SP, Grunebaum MF, Malone KM, Brodsky BS, et al. Adequacy of antidepressant treatment after discharge and the occurrence of suicidal acts in major depression: a prospective study. Am J Psychiatry 2002;159:1746-51.
- Taylor DM, Cameron PA, Eddey D. Recurrent overdose: patient characteristics, habits, and outcomes. J Accid Emerg Med 1998;15:257-61.
- Bernal M, Haro JM, Bernert S, Brugha T, de Graaf R, Bruffaerts R, et al. Risk factors for suicidality in Europe: results from the ESEMED study. J Affect Disord 2007;101:27-34.
- Hawton K, Bergen H, Casey D, Simkin S, Palmer B, Cooper J, et al. Self-harm in England: a tale of three cities. Multicentre study of self-harm. Soc Psychiatry Psychiatr Epidemiol 2007;42:513-21.
- Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation 2003;108:32-6.
- Schlienger RG, Fischer LM, Jick H, Meier CR. Current use of selective serotonin reuptake inhibitors and risk of acute myocardial infarction. Drug Safety 2004;27:1157-65.
- Blanchette CM, Simoni-Wastila L, Zuckerman IH, Stuart B. A secondary analysis of a duration response association between selective serotonin reuptake inhibitor use and the risk of acute myocardial infarction in the aging population. Ann Epidemiol 2008;18:316-21.
- Starkstein SE, Mizrahi R, Power BD. Antidepressant therapy in post-stroke depression. Expert Opin Pharmacother 2008;9:1291-8.
- Bak S, Tsiropoulos I, Kjaersgaard JO, Andersen M, Mellerup E, Hallas J, et al. Selective serotonin reuptake inhibitors and the risk of stroke: a population-based case-control study. Stroke 2002;33:1465-73.
- Chen Y, Guo JJ, Patel NC. Hemorrhagic stroke associated with antidepressant use in patients with depression: does degree of serotonin reuptake inhibition matter?. Pharmacoepidemiology Drug Saf 2009;18:196-202.
- Salaycik KJ, Kelly-Hayes M, Beiser A, Nguyen AH, Brady SM, Kase CS, et al. Depressive symptoms and risk of stroke the Framingham Study. Stroke 2007;38:16-21.
- Kharofa J, Sekar P, Haverbusch M, Moomaw C, Flaherty M, Kissela B, et al. Selective serotonin reuptake inhibitors and risk of hemorrhagic stroke. Stroke 2007;38:3049-51.
- de Abajo FJ, Jick H, Derby L, Jick S, Schmitz S. Intracranial haemorrhage and use of selective serotonin reuptake inhibitors. Br J Clin Pharmacol 2000;50:43-7.
- Darowski A, Chambers SACF, Chambers DJ. Antidepressants and falls in the elderly. Drugs Aging 2009;26:381-94.
- Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med 2009;169:1952-60.
- Ensrud KE, Blackwell TL, Mangione CM, Bowman PJ, Whooley MA, Bauer DC, et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc 2002;50:1629-37.
- Thapa PB, Gideon P, Cost TW, Milam AB, Ray WA. Antidepressants and the risk of falls among nursing home residents. N Engl J Med 1998;339:875-82.
- Kerse N, Flicker L, Pfaff JJ, Draper B, Lautenschlager NT, Sim M, et al. Falls, depression and antidepressants in later life: a large primary care appraisal. PLoS ONE 2008;3.
- Kallin K, Gustafson Y, Sandman P-O, Karlsson S. Drugs and falls in older people in geriatric care settings. Aging Clin Exp Res 2004;16:270-6.
- Arfken CL, Wilson JG, Aronson SM. Retrospective review of selective serotonin reuptake inhibitors and falling in older nursing home residents. Int Psychogeriatr 2001;13:85-91.
- Ziere G, Dieleman JP, van der Cammen TJM, Hofman A, Pols HAP, Stricker BHC. Selective serotonin reuptake inhibiting antidepressants are associated with an increased risk of nonvertebral fractures. J Clin Psychopharmacol 2008;28:411-17.
- Richards JB, Papaioannou A, Adachi JD, Joseph L, Whitson HE, Prior JC, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med 2007;167:188-94.
- Vestergaard P, Rejnmark L, Mosekilde L. Selective serotonin reuptake inhibitors and other antidepressants and risk of fracture. Calcif Tissue Int 2008;82:92-101.
- Haney EM, Chan BK, Diem SJ, Ensrud KE, Cauley JA, Barrett-Connor E, et al. Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med 2007;167:1246-51.
- Diem SJ, Blackwell TL, Stone KL, Yaffe K, Cauley JA, Whooley MA, et al. Depressive symptoms and rates of bone loss at the hip in older women. J Am Geriatr Soc 2007;55:824-31.
- Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database Syst Rev 2006.
- Barbui C, Andretta M, De Vitis G, Rossi E, D Arienzo F, Mezzalira L, et al. Antidepressant drug prescription and risk of abnormal bleeding: a case–control study. J Clin Psychopharmacol 2009;29:33-8.
- de Abajo FJ, Garcia-Rodriguez LA. Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents. Arch Gen Psychiatry 2008;65:795-803.
- Vidal X, Ibanez L, Vendrell L, Conforti A, Laporte JR. Risk of upper gastrointestinal bleeding and the degree of serotonin reuptake inhibition by antidepressants: a case–control study. Drug Saf 2008;31:159-68.
- Montgomery SA. Antidepressants and seizures: emphasis on newer agents and clinical implications. Int J Clin Pract 2005;59:1435-40.
- Gidal BE, French JA, Grossman P, Le Teuff G. Assessment of potential drug interactions in patients with epilepsy: impact of age and sex. Neurology 2009;72:419-25.
- Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol 2000;47:246-9.
- Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure 2010;19:69-73.
- Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R. Effects of psychotropic drugs on seizure threshold. Drug Saf 2002;25:91-110.
- Wedin GP, Oderda GM, Klein-Schwartz W, Gorman RL. Relative toxicity of cyclic antidepressants. Ann Emerg Med 1986;15:797-804.
- Buckley NA, Dawson AH, White IM, Henry DA. Greater toxicity in overdose of dothiepin than of other tricyclic antidepressants. Lancet 1994;343:159-62.
- Grundemar L, Wohlfart B, Lagerstedt C, Bengtsson F, Eklundh G. Symptoms and signs of severe citalopram overdose. Lancet 1997;349.
- Kelly CA, Dhaun N, Laing WJ, Strachan FE, Good AM, Bateman DN. Comparative toxicity of citalopram and the newer antidepressants after overdose. Clin Toxicol 2004;42:67-71.
- Ojemann LM, Friel PN, Trejo WJ, Dudley DL. Effect of doxepin on seizure frequency in depressed epileptic patients. Neurology 1983;33.
- Favale E, Rubino V, Mainardi P, Lunardi G, Albano C. Anticonvulsant effect of fluoxetine in humans. Neurology 1995;45:1926-7.
- Hu PS, Trumble DA, Foley DJ, Eberhard JW, Wallace RB. Crash risks of older drivers: a panel data analysis. Accid Anal Prev 1998;30:569-81.
- Ray WA, Fought RL, Decker MD. Psychoactive drugs and the risk of injurious motor vehicle crashes in elderly drivers. Am J Epidemiol 1992;136:873-83.
- Leveille S, Buchner D, Koepsell T, McCloskey L, Wolf M, Wagner E. Psychoactive medications and injurious motor vehicle collisions involving older drivers. Epidemiology 1994;5:591-8.
- Bramness JG, Skurtveit S, Neutel CI, Morland J, Engeland A. Minor increase in risk of road traffic accidents after prescriptions of antidepressants: a study of population registry data in Norway. J Clin Psychiatry 2008;69:1099-103.
- Movig KLL, Mathijssen MPM, Nagel PHA, van Egmond T, de Gier JJ, Leufkens HGM, et al. Psychoactive substance use and the risk of motor vehicle accidents. Accid Anal Prev 2004;36:631-6.
- Barbone F, McMahon AD, Davey PG, Morris AD, Reid IC, McDevitt DG, et al. Association of road-traffic accidents with benzodiazepine use. Lancet 1998;352:1331-6.
- Wester K, Jonsson AK, Spigset O, Druid H, Hagg S. Incidence of fatal adverse drug reactions: a population based study. Br J Clin Pharmacol 2008;65:573-9.
- Degner D, Grohmann R, Kropp S, Ruther E, Bender S, Engel RR, et al. Severe adverse drug reactions of antidepressants: results of the German multicenter drug surveillance program AMSP. Pharmacopsychiatry 2004;37:39-45.
- Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128:35-44.
- Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis 2008;52:144-53.
- Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother 2006;40:1618-22.
- Gartlehner G, Gaynes BN, Hansen RA, Thieda P, DeVeaugh-Geiss A, Krebs EE, et al. Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians. Ann Intern Med 2008;149:734-50.
- Movig KLL, Leufkens HGM, Lenderink AW, Egberts ACG. Serotonergic antidepressants associated with an increased risk for hyponatraemia in the elderly. Eur J Clin Pharmacol 2002;58:143-8.
- Kirby D, Harrigan S, Ames D. Hyponatraemia in elderly psychiatric patients treated with selective serotonin reuptake inhibitors and venlafaxine: a retrospective controlled study in an inpatient unit. Int J Geriatr Psychiatry 2002;17:231-7.
- National Collaborating Centre for Mental Health . Depression: The Treatment and Management of Depression in Adults 2009.
Appendix 1 Read codes used for depression and severity
Read codes used for identification of patients diagnosed with depression and their severity classification. The severity classification uses codes published by Martinez and colleagues15 and some additional classification by a member of the study team (RM).
| Read code | Read code description | Severity |
|---|---|---|
| 1465 | H/O – depression | Mild |
| 1B17 | Depressed | Mild |
| 1B17–1 | C/O – feeling depressed | Mild |
| E1121 | Single major depressive episode, mild | Mild |
| E1126 | Single major depressive episode, in full remission | Mild |
| E1131 | Recurrent major depressive episodes, mild | Mild |
| E1136 | Recurrent major depressive episodes, in full remission | Mild |
| E118 | Seasonal affective disorder | Mild |
| E2003 | Anxiety with depression | Mild |
| E204 | Neurotic depression reactive type | Mild |
| E2112 | Depressive personality disorder | Mild |
| E290 | Brief depressive reaction | Mild |
| E2B0 | Postviral depression | Mild |
| Eu320 | [X]Mild depressive episode | Mild |
| Eu320–99 | Mild depression | Mild |
| Eu32–1 | [X]Single episode of depressive reaction | Mild |
| Eu32–2 | [X]Single episode of psychogenic depression | Mild |
| Eu324 | [X]Mild depression | Mild |
| Eu32y | [X]Other depressive episodes | Mild |
| Eu32y-2 | [X]Single episode of masked depression NOS | Mild |
| Eu32z-1 | [X]Depression NOS | Mild |
| Eu32z-2 | [X]Depressive disorder NOS | Mild |
| Eu32z-4 | [X]Reactive depression NOS | Mild |
| Eu330 | [X]Recurrent depressive disorder, current episode mild | Mild |
| Eu33–1 | [X]Recurrent episodes of depressive reaction | Mild |
| Eu33–2 | [X]Recurrent episodes of psychogenic depression | Mild |
| Eu33–3 | [X]Recurrent episodes of reactive depression | Mild |
| Eu33–4 | [X]Seasonal depressive disorder | Mild |
| Eu33–5 | [X]SAD – seasonal affective disorder | Mild |
| Eu341 | [X]Dysthymia | Mild |
| Eu341–1 | [X]Depressive neurosis | Mild |
| Eu3y1–1 | [X]Recurrent brief depressive episodes | Mild |
| Eu412–1 | [X]Mild anxiety depression | Mild |
| R007z-3 | [D]Postoperative depression | Mild |
| 2257 | O/E – depressed | Moderate |
| E002 | Senile dementia with depressive or paranoid features | Moderate |
| E0021 | Senile dementia with depression | Moderate |
| E002z | Senile dementia with depressive or paranoid features NOS | Moderate |
| E112 | Single major depressive episode | Moderate |
| E1122 | Single major depressive episode, moderate | Moderate |
| E112–2 | Endogenous depression first episode | Moderate |
| E1123 | Single major depressive episode, severe, without psychosis | Moderate |
| E112–3 | Endogenous depression first episode | Moderate |
| E1125 | Single major depressive episode, partial or unspec remission | Moderate |
| E112z | Single major depressive episode NOS | Moderate |
| E1132 | Recurrent major depressive episodes, moderate | Moderate |
| E1135 | Recurrent major depressive episodes, partial/unspec remission | Moderate |
| E1137 | Recurrent depression | Moderate |
| E115–1 | Manic–depressive – now depressed | Moderate |
| E11y | Other and unspecified manic–depressive psychoses | Moderate |
| E11y2 | Atypical depressive disorder | Moderate |
| E11z2 | Masked depression | Moderate |
| E291 | Prolonged depressive reaction | Moderate |
| E2B | Depressive disorder NEC | Moderate |
| E2B1 | Chronic depression | Moderate |
| Eu32 | [X]Depressive episode | Moderate |
| Eu321 | [X]Moderate depressive episode | Moderate |
| Eu321–99 | Moderate depression | Moderate |
| Eu322–3 | [X]Single episode vital depression without psychotic symptoms | Moderate |
| Eu32y-1 | [X]Atypical depression | Moderate |
| Eu32z | [X]Depressive episode, unspecified | Moderate |
| Eu32z-3 | [X]Prolonged single episode of reactive depression | Moderate |
| Eu33 | [X]Recurrent depressive disorder | Moderate |
| Eu331 | [X]Recurrent depressive disorder, current episode moderate | Moderate |
| Eu332–1 | [X]Endogenous depression without psychotic symptoms | Moderate |
| Eu332–2 | [X]Major depression, recurrent without psychotic symptoms | Moderate |
| Eu332–3 | [X]Manic–depressive psychosis, depressed, no psychotic symptoms | Moderate |
| Eu332–4 | [X]Vital depression, recurrent without psychotic symptoms | Moderate |
| Eu334 | [X]Recurrent depressive disorder, currently in remission | Moderate |
| Eu33y | [X]Other recurrent depressive disorders | Moderate |
| Eu33z | [X]Recurrent depressive disorder, unspecified | Moderate |
| Eu33z-1 | [X]Monopolar depression NOS | Moderate |
| Eu341–4 | [X]Persistent anxiety depression | Moderate |
| Eu3y0–1 | [X]Mixed affective episode | Moderate |
| Eu412 | [X]Mixed anxiety and depressive disorder | Moderate |
| ZV111–1 | [V]Personal history of manic–depressive psychosis | Moderate |
| E0013 | Presenile dementia with depression | Severe |
| E11–2 | Depressive psychoses | Severe |
| E1120 | Single major depressive episode, unspecified | Severe |
| E112–1 | Agitated depression | Severe |
| E1124 | Single major depressive episode, severe, with psychosis | Severe |
| E112–4 | Endogenous depression | Severe |
| E113 | Recurrent major depressive episode | Severe |
| E1130 | Recurrent major depressive episodes, unspecified | Severe |
| E113–1 | Endogenous depression – recurrent | Severe |
| E1133 | Recurrent major depressive episodes, severe, no psychosis | Severe |
| E1134 | Recurrent major depressive episodes, severe, with psychosis | Severe |
| E113z | Recurrent major depressive episode NOS | Severe |
| E11y0 | Unspecified manic–depressive psychoses | Severe |
| E130 | Reactive depressive psychosis | Severe |
| E130–1 | Psychotic reactive depression | Severe |
| E135 | Agitated depression | Severe |
| Eu322 | [X]Severe depressive episode without psychotic symptoms | Severe |
| Eu322–1 | [X]Single episode agitated depression without psychotic symptoms | Severe |
| Eu322–2 | [X]Single episode major depression without psychotic symptoms | Severe |
| Eu322–99 | Severe depression | Severe |
| Eu323 | [X]Severe depressive episode with psychotic symptoms | Severe |
| Eu323–1 | [X]Single episode of major depression and psychotic symptoms | Severe |
| Eu323–2 | [X]Single episode of psychogenic depressive psychosis | Severe |
| Eu323–3 | [X]Single episode of psychotic depression | Severe |
| Eu323–4 | [X]Single episode of reactive depressive psychosis | Severe |
| Eu332 | [X]Recurrent depressive disorder, current episode severe without psychotic symptoms | Severe |
| Eu333 | [X]Recurrent depressive disorder, current episode severe with psychotic symptoms | Severe |
| Eu333–1 | [X]Endogenous depression with psychotic symptoms | Severe |
| Eu333–2 | [X]Manic-depressive psychosis, depressed type + psychotic symptoms | Severe |
| Eu333–3 | [X]Recurrent severe episodes/major depression + psychotic symptom | Severe |
| Eu333–5 | [X]Recurrent severe episodes of psychotic depression | Severe |
| Eu333–6 | [X]Recurrent severe episodes/reactive depressive psychosis | Severe |
| ZV111–2 | [V]Personal history of manic–depressive psychosis | Severe |
Appendix 2 Cost-effectiveness analysis – sensitivity analysis
Levels of cost-effectiveness
Mortality
Sensitivity analysis was performed with regard to the incremental cost, in order to assess whether results were robust to the inclusion of visit costs. The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of deaths figures (see Table 130). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, dosulepin (TCA) had an incremental cost per averted death of £435 compared with fluoxetine (Table 147). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and TCAs were estimated to have an incremental cost per averted death of £3909 compared with SSRIs (see Table 147).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of deaths | Difference in incremental mean cost (£) | Difference in incremental no. of deaths | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0000 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0291 | 31.30 | 0.0719 | 435.13 |
| Lofepramine (TCA) | 536.83 | 0.0888 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.1146 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0836 | |||
| Escitalopram (SSRI) | 345.79 | 0.0600 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.1010 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0551 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0776 | ED | ||
| Mirtazapine (other) | 330.97 | 0.1000 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0989 | |||
| TCAs | 425.51 | 0.0474 | 152.81 | 0.0391 | 3908.54 |
| SSRIs | 272.70 | 0.0865 | LC | LC | |
| Other antidepressants | 526.56 | 0.1039 |
Sudden cardiac death
The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of sudden cardiac death figures (see Table 131). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, trazodone (TCA) had an incremental cost per averted sudden cardiac death of £372,401 compared with fluoxetine (Table 148). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and dominated both TCAs and other antidepressants (see Table 148).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of sudden cardiac deaths | Difference in incremental mean cost (£) | Difference in incremental no. of sudden cardiac deaths | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0007 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0011 | |||
| Lofepramine (TCA) | 536.83 | 0.0001 | |||
| Trazodone hydrochloride (TCA) | 541.30 | −0.0009 | 324.00 | 0.0009 | 372,400.56 |
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0000 | |||
| Escitalopram (SSRI) | 345.79 | −0.0009 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | −0.0001 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0008 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0001 | |||
| Mirtazapine (other) | 330.97 | 0.0015 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0017 | |||
| TCAs | 425.51 | 0.0005 | |||
| SSRIs | 272.70 | 0.0001 | LC | D | |
| Other antidepressants | 526.56 | 0.0014 |
Suicide
The incremental costs, in terms of overall visit plus prescription cost for (all antidepressant drugs) (reported in Table 129) were combined with the previously reported incremental number of suicide figures. Fluoxetine (SSRI) had the lowest mean cost (see Table 132) and, after excluding dominated options, sertraline (SSRI) had an incremental cost per averted suicide of £12,123 compared with fluoxetine, and paroxetine (SSRI) had an incremental cost per averted suicide of £158,763 compared with sertraline (Table 149). With regard to the different classes of antidepressant drugs SSRIs had the lowest cost and TCAs had an incremental cost per averted suicide of £715,767 compared with SSRIs (see Table 149).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of suicides | Difference in incremental mean cost (£) | Difference in incremental no. of suicides | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0002 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0013 | |||
| Lofepramine (TCA) | 536.83 | 0.0027 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0027 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0014 | |||
| Escitalopram (SSRI) | 345.79 | −0.0002 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0022 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | −0.0002 | 134.24 | 0.0008 | 158,763.22 |
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0006 | 18.58 | 0.0015 | 12,123.51 |
| Mirtazapine (other) | 330.97 | 0.0036 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0025 | |||
| TCAs | 425.51 | 0.0010 | 152.81 | 0.0002 | 715,766.57 |
| SSRIs | 272.70 | 0.0012 | LC | LC | |
| Other antidepressants | 526.56 | 0.0028 |
Attempted suicide/self-harm
The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of attempted suicide figures (see Table 133). Fluoxetine (SSRI) had the lowest mean cost, and, after excluding dominated options, paroxetine had an incremental cost per averted attempted suicide of £39,865 compared with fluoxetine (Table 150). Amitriptyline was estimated to have an incremental cost per averted attempted suicide of £882,758 compared with paroxetine (see Table 150). With regard to the different classes of antidepressant drugs, other antidepressants were dominated by SSRIs and TCAs were estimated to have an incremental cost per averted attempted suicide of £112,484 compared with SSRIs (see Table 150).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of attempted suicides | Difference in incremental mean cost (£) | Difference in incremental no. of attempted suicides | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0010 | 128.63 | 0.0001 | 882,758.26 |
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0048 | ED | ||
| Lofepramine (TCA) | 536.83 | 0.0075 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0158 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0073 | |||
| Escitalopram (SSRI) | 345.79 | 0.0035 | ED | ||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0050 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0012 | 152.82 | 0.0038 | 39,865.25 |
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0050 | |||
| Mirtazapine (other) | 330.97 | 0.0210 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0156 | |||
| TCAs | 425.51 | 0.0039 | 152.81 | 0.0014 | 112,484.12 |
| SSRIs | 272.70 | 0.0053 | LC | LC | |
| Other antidepressants | 526.56 | 0.0184 |
Myocardial infarction
The incremental costs, in terms of overall visit plus prescription cost for (all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of MI figures (see Table 134). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, sertraline (SSRI) had an incremental cost per averted MI of £1428 compared with fluoxetine (Table 151). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and other antidepressants were estimated to have an incremental cost per averted MI of £73,358 compared with SSRIs (see Table 151).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of MIs | Difference in incremental mean cost (£) | Difference in incremental no. of MIs | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0079 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0067 | ED | ||
| Lofepramine (TCA) | 536.83 | 0.0085 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0021 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0044 | |||
| Escitalopram (SSRI) | 345.79 | 0.0090 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0113 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0067 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | −0.0017 | 18.58 | 0.013 | 1428.02 |
| Mirtazapine (other) | 330.97 | 0.0033 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0027 | |||
| TCAs | 425.51 | 0.0070 | |||
| SSRIs | 272.70 | 0.0065 | LC | LC | |
| Other antidepressants | 526.56 | 0.0031 | 253.85 | 0.0034 | 73,757.69 |
Stroke/transient ischaemic attack
The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of stroke/TIA figures (see Table 135). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, dosulepin (TCA) had an incremental cost per averted stroke/TIA of £3993 compared with fluoxetine (Table 152). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and other antidepressants were estimated to have an incremental cost per averted stroke/TIA of £27,139 compared with SSRIs (see Table 152).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of strokes/TIAs | Difference in incremental mean cost (£) | Difference in incremental no. of strokes/TIAs | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0105 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0064 | 31.30 | 0.0078 | 3992.93 |
| Lofepramine (TCA) | 536.83 | 0.0238 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0087 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0175 | |||
| Escitalopram (SSRI) | 345.79 | 0.0132 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0142 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0128 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0188 | |||
| Mirtazapine (other) | 330.97 | 0.0244 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0370 | |||
| TCAs | 425.51 | 0.0103 | 152.81 | 0.0056 | 27,139.05 |
| SSRIs | 272.70 | 0.0160 | LC | LC | |
| Other antidepressants | 526.56 | 0.0296 |
Falls
The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129) were combined with the previously reported incremental number of falls figures (see Table 136). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, dosulepin (TCA) had an incremental cost per averted fall of £1109 compared with fluoxetine and mirtazapine (other) had an incremental cost per averted fall of £3234 compared with dosulepin (Table 153). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and TCAs were estimated to have an incremental cost per averted fall of £5858 compared with SSRIs (see Table 153).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of falls | Difference in incremental mean cost (£) | Difference in incremental no. of falls | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0549 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0459 | 31.30 | 0.0282 | 1109.34 |
| Lofepramine (TCA) | 536.83 | 0.0486 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0614 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0842 | |||
| Escitalopram (SSRI) | 345.79 | 0.0660 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0741 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0634 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0742 | |||
| Mirtazapine (other) | 330.97 | 0.0204 | 82.36 | 0.0255 | 3234.11 |
| Venlafaxine hydrochloride (other) | 851.55 | 0.0769 | |||
| TCAs | 425.51 | 0.0514 | 152.81 | 0.0261 | 5858.06 |
| SSRIs | 272.70 | 0.0775 | LC | LC | |
| Other antidepressants | 526.56 | 0.0507 |
Fractures
The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of fractures figures (see Table 137). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, dosulepin (TCA) had an incremental cost per averted fracture of £2260 compared with fluoxetine and trazodone (TCA) had an incremental cost per averted fall of £12,240 compared with dosulepin (Table 154). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and TCAs were estimated to have an incremental cost per averted fracture of £11,109 compared with SSRIs (see Table 154).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of fractures | Difference in incremental mean cost (£) | Difference in incremental no. of fractures | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0231 | ED | ||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0238 | 31.30 | 0.0139 | 2260.05 |
| Lofepramine (TCA) | 536.83 | 0.0338 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0001 | 292.70 | 0.0239 | 12,240.01 |
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0389 | |||
| Escitalopram (SSRI) | 345.79 | 0.0159 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0376 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0356 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0395 | |||
| Mirtazapine (other) | 330.97 | 0.0265 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0536 | |||
| TCAs | 425.51 | 0.0237 | 152.81 | 0.0138 | 11,109.49 |
| SSRIs | 272.70 | 0.0375 | LC | LC | |
| Other antidepressants | 526.56 | 0.0417 |
Upper gastrointestinal bleed
The incremental costs, in terms of overall visit plus prescription cost for (all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of upper GI bleed figures (see Table 138). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, sertraline (SSRI) had an incremental cost per averted upper GI bleed of £961 compared with fluoxetine, and mirtazapine (other) had an incremental cost per averted upper GI bleed of £125,188 compared with sertraline (Table 155). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and dominated both TCAs and other antidepressants (see Table 155).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of upper GI bleeds | Difference in incremental mean cost (£) | Difference in incremental no. of upper GI bleeds | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0086 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0058 | |||
| Lofepramine (TCA) | 536.83 | 0.0047 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0126 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0060 | |||
| Escitalopram (SSRI) | 345.79 | 0.0009 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0032 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0041 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0015 | 18.58 | 0.0193 | 961.12 |
| Mirtazapine (other) | 330.97 | 0.0008 | 95.09 | 0.0008 | 125,188.46 |
| Venlafaxine hydrochloride (other) | 851.55 | 0.0118 | |||
| TCAs | 425.51 | 0.0070 | |||
| SSRIs | 272.70 | 0.0044 | LC | D | |
| Other antidepressants | 526.56 | 0.0069 |
Epilepsy/seizures
The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of epilepsy/seizure figures (see Table 139). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, dosulepin (TCA) had an incremental cost per averted epilepsy/seizure of £6211 compared with fluoxetine (Table 156). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and TCAs were estimated to have an incremental cost per averted epilepsy/seizure of £38,409 compared with SSRIs (see Table 156).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of epilepsy/seizure cases | Difference in incremental mean cost (£) | Difference in incremental no. of epilepsy/seizure cases | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0017 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | −0.0022 | 31.30 | 0.0050 | 6210.77 |
| Lofepramine (TCA) | 536.83 | 0.0025 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0026 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0044 | |||
| Escitalopram (SSRI) | 345.79 | 0.0039 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0029 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0064 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0094 | |||
| Mirtazapine (other) | 330.97 | 0.0031 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0109 | |||
| TCAs | 425.51 | 0.0008 | 152.81 | 0.0040 | 38,409.14 |
| SSRIs | 272.70 | 0.0048 | LC | LC | |
| Other antidepressants | 526.56 | 0.0070 |
Road traffic accident
The incremental costs, in terms of overall visit plus prescription cost for (all antidepressant drugs) (reported in Table 129) were combined with the previously reported incremental number of RTA figures (see Table 140). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, dosulepin (TCA) had an incremental cost per averted RTA of £32,854 compared with fluoxetine (Table 157) and mirtazapine (other) had an incremental cost per averted RTA of £113,029 compared with dosulepin. With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and other antidepressants were estimated to have an incremental cost per averted RTA of £189,427 compared with SSRIs (see Table 157).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of RTAs | Difference in incremental mean cost (£) | Difference in incremental no. of RTAs | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | −0.0002 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | −0.0024 | 31.30 | 0.0010 | 32,854.05 |
| Lofepramine (TCA) | 536.83 | 0.0013 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0033 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0003 | |||
| Escitalopram (SSRI) | 345.79 | −0.0002 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | −0.0015 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0000 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | −0.0003 | |||
| Mirtazapine (other) | 330.97 | −0.0032 | 82.36 | 0.0007 | 113,028.70 |
| Venlafaxine hydrochloride (other) | 851.55 | −0.0014 | |||
| TCAs | 425.51 | −0.0002 | |||
| SSRIs | 272.70 | −0.0003 | LC | LC | |
| Other antidepressants | 526.56 | −0.0017 | 253.85 | 0.0013 | 189,427.28 |
Adverse drug reactions
The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of ADR figures (see Table 141). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, dosulepin had an incremental cost per averted ADR of £15,090 compared with fluoxetine (Table 158). Venlafaxine was estimated to have an incremental cost per averted ADR of £706,227 compared with dosulepin. With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and other antidepressants were estimated to have an incremental cost per averted ADR of £136,063 compared with SSRIs (see Table 158).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of ADRs | Difference in incremental mean cost (£) | Difference in incremental no. of ADRs | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0008 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | 0.0001 | 31.30 | 0.0021 | 15,090.47 |
| Lofepramine (TCA) | 536.83 | 0.0113 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0007 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0014 | |||
| Escitalopram (SSRI) | 345.79 | 0.0008 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0022 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0003 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0061 | |||
| Mirtazapine (other) | 330.97 | 0.0002 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0007 | 602.94 | 0.0009 | 706,227.34 |
| TCAs | 425.51 | 0.0017 | ED | ||
| SSRIs | 272.70 | 0.0020 | LC | LC | |
| Other antidepressants | 526.56 | 0.0001 | 253.85 | 0.0019 | 136,062.86 |
Hyponatraemia
The incremental costs, in terms of overall visit plus prescription costs (for all antidepressant drugs) (reported in Table 129), were combined with the previously reported incremental number of hyponatraemia figures (see Table 142). Fluoxetine (SSRI) had the lowest mean cost and, after excluding dominated options, sertraline (SSRI) had an incremental cost per averted case of hyponatraemia of £2532 compared with fluoxetine, and dosulepin (TCA) had an incremental cost per averted hyponatraemia case of £10,914 compared with sertraline (SSRI) (Table 159). With regard to the different classes of antidepressant drugs, SSRIs had the lowest cost and TCAs were estimated to have an incremental cost per averted hyponatraemia case of £36,028 compared with SSRIs (see Table 159).
| Antidepressant drug | Incremental mean cost (£) | Incremental no. of hyponatraemia cases | Difference in incremental mean cost (£) | Difference in incremental no. of hyponatraemia cases | ICER (£) |
|---|---|---|---|---|---|
| Amitriptyline hydrochloride (TCA) | 498.75 | 0.0034 | |||
| Dosulepin hydrochloride (TCA) | 248.61 | −0.0005 | 12.73 | 0.0012 | 10,914.37 |
| Lofepramine (TCA) | 536.83 | 0.0001 | |||
| Trazodone hydrochloride (TCA) | 541.30 | 0.0056 | |||
| Citalopram hydrobromide (SSRI) | 280.95 | 0.0072 | |||
| Escitalopram (SSRI) | 345.79 | 0.0107 | |||
| Fluoxetine hydrochloride (SSRI) | 217.30 | 0.0080 | LC | LC | |
| Paroxetine hydrochloride (SSRI) | 370.12 | 0.0015 | |||
| Sertraline hydrochloride (SSRI) | 235.88 | 0.0006 | 18.58 | 0.0073 | 2532.67 |
| Mirtazapine (other) | 330.97 | 0.0007 | |||
| Venlafaxine hydrochloride (other) | 851.55 | 0.0061 | |||
| TCAs | 425.51 | 0.0020 | 152.81 | 0.0042 | 36,028.14 |
| SSRIs | 272.70 | 0.0062 | LC | LC | |
| Other antidepressants | 526.56 | 0.0036 |
Appendix 3 Final protocol
Protocol
A study of the safety and harms of antidepressant drugs for older people: an analysis using a large primary care database.
Investigators
Carol Coupland, Division of Primary Care, University of Nottingham
Julia Hippisley-Cox, Division of Primary Care, University of Nottingham
Antony Arthur, School of Nursing, University of Nottingham
Garry Barton, School of Medicine, Health Policy and Practice, University of East Anglia
Tracey Sach, School of Medicine, Health Policy and Practice, University of East Anglia
Richard Morriss, Division of Psychiatry, University of Nottingham
Paula Dhiman, Division of Primary Care, University of Nottingham
Funding
-
NCCHTA (ref: 06/42/01).
Protocol details
-
Version 1.7. Date: 2 April 2009.
Investigators
Carol Coupland
Senior Lecturer in Medical Statistics
Division of Primary Care
University of Nottingham
Nottingham NG7 2RD
Julia Hippisley-Cox
Professor of Clinical Epidemiology and General Practice
Division of Primary Care
University of Nottingham
Nottingham NG7 2RD
Antony Arthur
Senior Lecturer in Elder Care
School of Nursing
University of Nottingham
Queen’s Medical Centre
Nottingham NG7 2HA
Garry Barton
Senior Lecturer in Health Economics
Health Economics Group (HEG)
School of Medicine, Health Policy and Practice
University of East Anglia
Norwich NR4 7TJ
Tracey Sach
Senior Lecturer in Health Economics
School of Chemical Sciences and Pharmacy
University of East Anglia
Norwich NR4 7TJ
Richard Morriss
Professor of Psychiatry & Community Mental Health
Division of Psychiatry
University of Nottingham
Queen’s Medical Centre
Nottingham NG7 2UH
Paula Dhiman
Research Statistician
Division of Primary Care
University of Nottingham
Nottingham NG7 2RD
Detailed project description
Project title
Safety and harms of antidepressant drugs for older people: an analysis using a large primary care database.
HTA project number: 06/42/01.
Summary
Depression is a common and debilitating condition in older people. Adverse drug events may be more common in the treatment of depression in older people compared with younger age groups owing to higher levels of comorbidity, age-related physiological changes and polypharmacy. The under-representation of older people in clinical trials of antidepressants makes it difficult to make reliable or precise estimates of the incidence of adverse events. This problem is further compounded when trial exclusion criteria exclude older people with comorbid conditions.
The overall aim of this study is to establish the relative safety and balance of risks for individual antidepressant drugs in older people. The study is a cohort study of people aged 65 years and over who have been diagnosed with a major depressive disorder or with unipolar depression identified from a large primary care database (QResearch). Prescribing data for these patients will be used to ascertain their use of antidepressant drugs following diagnosis of depression including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs) and other antidepressants. Prospectively recorded data on these patients will be used to ascertain harms and adverse events that occurred in these patients over a minimum of 12 months’ follow-up after their diagnosis. Primary outcomes will include the following events: all-cause mortality, suicide, sudden cardiac death, overdose/poisoning, attempted suicide, myocardial infarction (MI), stroke, seizures, gastointestinal (GI) bleeding, falls and fractures, road traffic accidents (RTAs), adverse drug reactions (ADRs) and hyponatraemia. The analysis will examine the associations between exposure to the different classes of antidepressant and risk of the adverse events. Annual costs of antidepressant medication and costs of adverse events will be calculated and compared. A further analysis will use the self-controlled case-series approach to reduce effects of residual confounding and indication biases.
Background
Depression is a common and debilitating condition in older people. A pooled estimate of prevalence of depression from community-based studies of older people is 13.5%. 1 Across all ages, 29 million prescriptions for antidepressant drugs were issued in 2004. 2 Adverse drug events may be more common in the treatment of depression in older people compared with younger age groups owing to higher levels of comorbidity, age-related physiological changes and polypharmacy. 3 The under-representation of older people in clinical trials of antidepressants makes it difficult to make reliable or precise estimates of the incidence of adverse events. 4,5 This problem is further compounded when trial exclusion criteria exclude older people with comorbid conditions. 6 Even though older people with depression are more likely to be treated since the introduction of the newer generation of antidepressants,7,8 under treatment of depression among older people is a global problem. 9 Evidence from a systematic review suggests that TCAs and SSRIs are equivalent in terms of efficacy, but classical TCAs are associated with a higher discontinuation rate owing to the side effect profile. 10
Antidepressants, and particularly TCAs are an important cause of deaths by overdose and poisoning. 11 There appears to be some evidence from a meta-analysis of placebo-controlled trials that SSRIs are associated with a small increase in risk of fatal and non-fatal suicide attempts. 12 Lack of sufficient trial data meant that it was not possible to see whether this finding held within those aged 60 years and over. Observational studies across all age groups have found associations between antidepressant use and suicide, but have been unable to rule out confounding by indication. 13 There is little evidence to support any difference in terms of class of antidepressant and risk of suicide,14 but studies have tended to look at risks across all ages or among adolescents and young adults. 15
There may be an increased risk of subsequent ischaemic heart disease associated with dosulepin (formerly known as dothiepin) use, but not other TCAs or SSRIs. 16 Some studies have found no evidence of an increased risk of MI among users of antidepressants17 or have suggested that an increased risk of MI may be explained by confounding factors relating to depression itself rather than specific adverse drug effects. 18
Findings from both case-control,19 and case-series analysis studies,20 indicate that risk of hip fracture is elevated with the use of TCAs and SSRIs among older people, although the magnitude of the increased risk did not differ between the two classes of antidepressant. 19 The likely mechanism underlying this increased risk appearing to be changes in orthostatic blood pressure,21 rather than altered bone mineral density. 22
Older people who use lithium may be at increased risk of being involved in an injurious motor vehicle accident. 23 In studies that have formally tested the effects of antidepressants on driving performance, sedating antidepressants have a similar effect to alcohol. 24
Hyponatraemia associated with antidepressant use is rare, but it is an adverse event that disproportionately affects older people. 25,26 Similarly, GI bleeding is more common among those taking SSRIs who are aged 80 years or over,27 although there is a lack of consensus as to whether the risk of GI bleeding associated with SSRI use is further increased with concurrent use of non-steroidal anti-inflammatory drugs28,29 or not. 30
The gaps in the research into adverse effects for these drugs specifically in older people and the lack of consistent findings pose problems for clinicians prescribing these drugs and making choices as to the most appropriate drug for individual older patients. In this study we will use a large primary care database containing information on virtually all prescriptions for antidepressants and a range of potential adverse effects to derive a unified picture of the balance of risks for antidepressant drugs in older people with depression.
Specific aims and objectives
The overall aim of this study is to establish the relative safety and balance of risks for individual antidepressant drugs in older people, in order to provide a robust evidence base to support decision making for clinicians prescribing these medications to individual patients.
The project has five key objectives, which are:
-
to determine the relative and absolute risks of predefined adverse events in older people diagnosed with depression. Comparisons will be made between classes of antidepressant drugs: TCAs; SSRIs; monoamine oxidase inhibitors (MAOIs); other antidepressants; and non-use of antidepressant drugs
-
to directly compare the risk of adverse events in patients prescribed SSRIs compared with TCAs in older people diagnosed with depression
-
to determine how the dose and duration of prescribed antidepressant medication is associated with the risk of an adverse event
-
to describe patterns of antidepressant use in older people diagnosed with depression, in particular the types and doses prescribed, the durations and the proportions switched between different antidepressants (TCAs, SSRIs and other antidepressant drugs)
-
to determine the annual costs of antidepressant medication, the costs of the adverse events, and the costs of health-care resource use in older people diagnosed with depression, comparing patients by type of antidepressant drugs (TCAs, SSRIs, MAOIs and other antidepressants).
Study design
Design
The planned investigation will use a large primary care database (QResearch) to investigate the relative safety and costs of antidepressant drugs in older people.
Two main approaches will be used to achieve the study objectives:
-
a cohort study
-
a self-controlled case-series study.
The cohort study is a well-established powerful method for determining absolute and relative risks associated with exposures. The self-controlled case-series method is a newer approach that estimates relative incidence of an outcome in high-risk compared with low-risk periods of time, based only on data from cases. It is useful for investigating the short-term effect of drug exposures on the risk of acute outcomes, as it eliminates problems of confounding from unmeasured variables, such as severity of disease. Both of these studies will derive data from a large primary-care research database (QResearch).
Cohort study
Our target population for the cohort study will be all patients aged 65 years and over with a recorded diagnosis of depression (major depressive disorder or unipolar depression) between 1 January 1996 and 31 December 2007. Patients with previous diagnoses of depression before the age of 65 years will be included. We will use Read codes to identify a major depressive disorder or unipolar depression, using case definitions that have been used in previous studies. The cohort will be followed up until 1 January 2009. Information on all prescriptions for antidepressants will be extracted, along with information on potential confounding variables and adverse events during follow-up.
Self-controlled case-series study
The self-controlled case-series study only uses the patients in the cohort who have the outcomes of interest. Cases with each type of adverse event will be identified; these will be cases with a diagnosis of the adverse event between 1 January 1996 to 31 December 2006, who had a previous diagnosis of depression between 1 January 1996 to 31 December 2005. Information on prescriptions for antidepressants in these cases will be extracted and the analysis will compare rates of the adverse events in periods following a first prescription for an antidepressant compared with a baseline period.
Setting: QResearch database
We will undertake the study using data from the QResearch primary-care research database (www.qresearch.org). This validated database is the largest general practice research database in the UK and it contains the anonymised electronic health-care records of over 10 million patients ever registered with 525 general practices throughout England, Wales, Scotland and Northern Ireland. Consent to provide data for QResearch was sought from all UK practices using the Egton Medical Information Systems (EMIS) medical records system. EMIS is the major supplier of primary-care computer systems in the UK and is in use in two-thirds of all UK general practices. The consenting practices form a representative sample of 6–7% of all UK general practices, and there are practices in every strategic health authority and each health board in England, Wales and Scotland.
The information recorded on the QResearch database includes patient demographic data (year of birth, gender, socioeconomic data derived from the UK 2001 census), characteristics (height, weight, smoking status), symptoms, clinical diagnoses, consultations, referrals, prescribed medications and results of investigations. The latest version of the QResearch database, which is updated quarterly, will be used for the analysis.
Detailed analyses have compared QResearch practices with all UK practices and found that practices contributing to QResearch are somewhat larger than UK practices overall, but are very similar in other respects. 31 The database has been validated by comparing birth rates, death rates, consultation rates, prevalence and mortality rates with other data sources including the General Household Survey and the General Practice Research Database (http://secure.qresearch.org/SiteSections/DataValidation/DataValidationMain.aspx). The age–gender structure of the population has been compared with that reported in the 2001 census. There was good correspondence for all of these measures, although the QResearch population is slightly older and has marginally higher prevalence figures for some diagnoses compared with less recent data,32 but they are almost identical to current prevalence data from the new General Medical Services Contract for General Practitioners.
Compared with other primary care databases QResearch is the largest [currently 525 practices compared with around 200–400 [depending on selection criteria) in the General Practice Research Database (GPRD) and 100 in The Health Improvement Network (THIN)33], and it has information on deprivation derived from postcode data, which is not currently available in the other databases. The database is completely independent from commercial organisations and QResearch receives no funding from pharmaceutical companies. The database contains only anonymised data, which are encrypted and kept in secure conditions. One of the coapplicants (Professor Julia Hippisley-Cox) is the chief custodian of the database and QResearch has been used to examine the risks and benefits associated with a number of commonly prescribed drugs including statins34,35 and non-steroidal anti-inflammatory drugs (NSAIDs). 36 The applicants have also published studies examining antidepressants as risk factors for ischaemic heart disease. 16
Outcome measures
The outcomes to be assessed will be extracted from the routine primary-care computer records of patients in the cohort. Outcomes will only be included if they occurred after the initial diagnosis of depression and up until 31 December 2008. The relevant computer recorded Read codes and ICD-9/ICD-10 codes, where appropriate, will be used to identify patients with the outcomes.
The outcomes that will be assessed will include:
-
all-cause mortality
-
suicide (including open verdicts)
-
attempted suicide/self-harm
-
sudden cardiac death
-
overdose/poisoning from antidepressants
-
myocardial infarction
-
stroke/TIA
-
epilepsy/seizures
-
upper GI bleeding
-
falls
-
fractures (upper limb, lower limb, ribs, skull, vertebrae and pelvis)
-
road traffic accidents
-
adverse drug reactions (including bullous eruption)
-
hyponatraemia.
QResearch is undertaking a national audit on care for patients with osteoporosis and falls in primary care. As part of this project, funded by the Information Centre, we will be examining the clinical coding of falls and fractures in some detail, and will be able to utilise the definitions for the proposed project. We have consulted professional groupings regarding the diagnostic codes (Read codes) which are likely to be used in clinical practice. Other outcomes are likely to be well recorded, although sudden cardiac deaths may be difficult to identify.
Exposures
Our exposure of interest is antidepressant medication. We will extract details of all prescriptions for antidepressants in patients in our cohort, following their diagnosis of depression; this will include the date of prescription, the type of drug, the dose and the duration.
The antidepressant drugs will be grouped for analysis according to the major classes as described in the British National Formulary (BNF), namely tricyclic and related antidepressants (TCAs: section 4.3.1), selective serotonin reuptake inhibitors (SSRIs: section 4.3.3), monoamine oxidase inhibitors (MAOIs: section 4.3.2) and other antidepressants (section 4.3.4). Effects of individual antidepressant drugs will also be assessed where numbers are sufficient. The number of prescriptions, duration and dose of the antidepressant drugs will be examined in the analyses.
We will determine the proportions of patients who switch between antidepressants, including switches between classes of drugs and between different drugs within a class. We will examine the proportions for patients who discontinue a drug before the recommended time by examining the proportions who have only one prescription, have two to three and have four to six prescriptions for a particular drug.
Confounding variables
Data will be extracted on the following variables, and these will be considered as confounding variables in the analysis of the cohort study:
-
age, gender, year of diagnosis of depression, previous recorded diagnosis of depression before age 65 years, severity of index diagnosis of depression, deprivation, smoking status, comorbidities (ischaemic heart disease, diabetes, hypertension, stroke/TIA, cancer, dementia, epilepsy/seizures, Parkinson’s disease, hypothyroidism, obsessive–compulsive disorder), and use of other drugs (including statins, NSAIDS, antipsychotics, lithium, aspirin, antihypertensive drugs, anticonvulsants, hypnotics/anxiolytics).
In addition for the analysis of suicide as an outcome, previous attempted suicide at baseline was considered as a confounding variable, and, for the analysis of fracture, previous falls at baseline was considered as a confounding variable.
Inclusion/exclusion criteria
Patients will be eligible for inclusion in the cohort study if:
-
they have a recorded diagnosis of depression (a major depressive disorder or unipolar depression including depression mixed with anxiety)
-
they are aged between 65 and 100 years
-
the diagnosis of depression was made at the age of 65 years or over (but does not need to be their first recorded diagnosis of depression)
-
the diagnosis was recorded between 1 January 1996 and 31 December 2007
-
the diagnosis occurred at least 12 months after registration with a study practice and after the date of the installation of the practice EMIS computer system.
Patients will be excluded from the cohort study if:
-
they are temporary residents
-
they have a previous diagnosis of depression in the 12-month period prior to their index recorded diagnosis of depression
-
they have been prescribed antidepressants in the 12-month period prior to their recorded diagnosis of depression
-
they have a diagnosis of schizophrenia or bipolar disorder
-
they have a diagnosis of other types of psychoses.
Quality of life
Quality of life is not recorded in primary-care consultations, so we are unable to examine this outcome in our database; however, as part of our review of the literature we will search for literature on quality of life and antidepressant medication in older people with depression to see if some estimations can be made about the likely effectiveness and cost-effectiveness of different antidepressants in older people. However, a basic literature search undertaken to support the development of this proposal found very few cost–utility studies37 comparing different types of antidepressants in a population aged 18 years or over and therefore, there may well be limited published economic evidence specific to a more elderly population.
Strengths and limitations of study design
The strengths of using a cohort study design for this project are that it will include a large and representative number of older people with depression, it can calculate absolute as well as relative risks, it can take account of exposures changing over time and it is able to adjust for a number of potential confounding variables. The recording of prescriptions in primary-care records is high, and the exposures under consideration are available only on prescription. The outcomes we have included are likely to be well recorded, and we will compare their rates in this study against other published data where possible.
The limitations of the cohort design approach are that it can be vulnerable to indication bias and residual confounding whereby relevant confounding variables may be imprecisely recorded or not recorded at all in primary-care records (for example diet, physical activity). Indication bias can cause difficulties in the interpretation of results on effects of drugs in observational studies; in this instance whether or not an antidepressant is prescribed or the type of antidepressant prescribed may be related to important prognostic factors for the outcome in question, such as the severity of depression or the attitude of the patient towards taking medication. These characteristics could influence the outcome but are unlikely to be recorded well in a patient’s medical records. The self-controlled case-series method has been proposed as a means of addressing this problem. 38,39 This is an internally controlled method whereby analyses are carried out only in patients with the outcome of interest, thereby eliminating the effect of indication bias and unmeasured confounding variables that do not vary over time. This method has previously been used to examine the relationship between antidepressants and hip fracture,20 and is of most relevance for acute events occurring within a short period after exposure. A limitation of the case-series design is that it requires that probability of exposure is not affected by occurrence of an outcome event, which is a particular problem for fatal outcomes, but this can be resolved by using only time from first prescription in the observation period for analysis.
Sample size
Cohort study
All eligible patients aged 65 years and over diagnosed with incident depression between 1 January 1996 and 31 December 2007 in the QResearch database will be included in the cohort study. A feasibility study shows there are approximately 5.0 million years of observation and 18,000 incident cases of depression arising from patients aged 65 and older between 1996 and 2005 on the database.
Assuming 88% of patients aged 65 years and over, diagnosed with depression, are prescribed an antidepressant drug as we found in our feasibility study, and for a rare outcome with an incidence of 5 per 1000 per year (e.g. upper GI event40 or lower limb fracture41), and an average follow-up of 5 years, we will be able to detect a relative risk of 1.5 with 88% power and a 5% significance level comparing those on antidepressants with those not on antidepressants. For all-cause mortality with a mortality rate of 47 per 1000 per year (Office for National Statistics for 2001) we will be able to detect a relative risk of 1.15 with 95% power. In comparisons between TCAs and SSRIs, assuming 39% of patients on antidepressants take TCAs and 50% take SSRIs we will be able to detect a relative risk of 1.4 with 86% power for rare outcomes and 1.12 with 92% power for all-cause mortality.
Self-controlled case-series study
The exposed cases contribute to the statistical power in the case-series method, under certain conditions the power of the analysis can be similar to that of the cohort study from which the cases are derived. To detect a rate ratio of 2.0 in a risk period of 1–14 days after the first prescription for an antidepressant with 80% power and 5% significance, then with a proportion of 0.0077 in the risk period of 14 days compared with an average observation period of 5 years (3/1825) then 1435 exposed cases would be required for each outcome. 42 We would anticipate having at least this number for all-cause mortality and falls/fracture. To detect a rate ratio of 3.0 in a risk period of 1–14 days then 448 exposed cases would be required. We would anticipate having around this number for rare outcomes such as GI events (incidence rate 5/1000/year).
Statistical analysis
Descriptive statistics
Descriptive statistics will be derived primarily using the data from the cohort study.
-
We will calculate incidence rates of diagnosed depression in people aged 65 years and over, and examine these rates by gender, age group (65–74, 75–84, 85+ years) and study year. We will compare these rates with other published rates of depression.
-
In patients with a diagnosis of depression in the study cohort we will describe patterns of antidepressant use according to type of antidepressant prescribed, duration of use and dose, and will examine these patterns by gender, age group, use of other medications, comorbidities and study year. We will also examine variations between practices in patterns of antidepressant prescribing.
-
We will calculate the proportions of people switched between different antidepressants (TCAs, SSRIs and other antidepressant drugs) by gender, age group and study year, and examine duration of use before switching.
-
Discontinuation rates for each drug will also be determined by examining the proportion of those with at least one prescription for a drug who only have one prescription, have two to three and have four to six prescriptions.
-
We will describe the severity of depression (classified as mild, moderate or severe) in the study cohort, overall and by age group and gender. We will describe patterns of antidepressant use according to severity of depression.
Analysis of cohort study
The analysis of the cohort study will determine absolute and relative risks of adverse events in older people with depression according to type of antidepressant prescribed, and will also examine risks according to dose and duration of treatment.
Incidence rates of the adverse events will be calculated in the study cohort. The statistical analysis will comprise a series of survival analyses to assess the relationship between exposure to antidepressant drugs and a number of potential adverse effects. These analyses will be restricted to the cohort of older people with a diagnosis of depression. The exposure variables will be use of antidepressant drugs, including SSRIs, TCAs and other antidepressants. The number of prescriptions, duration and dose of the antidepressant drugs will be examined in the analyses. The date of entry into the survival analyses will be the date of diagnosis of depression (their earliest date at the age of 65 years or over or the date of the first prescription for an antidepressant after the age of 65 years in patients if that occurred before the recorded date of depression), and the right censor date will be the earliest of the following: date of diagnosis of the outcome of interest, date of death, date of leaving the practice, date of the latest download of data or the study end date.
Cox’s proportion hazards models will be used with antidepressant exposure treated as a time-varying exposure. The analysis will calculate HRs and 95% CIs comparing:
-
The risk of each adverse effect in patients on any type of antidepressant compared with patients with no antidepressant treatment.
-
Each separate class of antidepressants (SSRIs, TCAs and other antidepressants) compared with no treatment.
-
The risk of each adverse effect for each class of antidepressant will be directly compared with each other class (in particular SSRIs will be directly compared with TCAs).
-
Analyses will also calculate HRs according to duration of use and prescribed dose of antidepressant. Where numbers are sufficient, subcategories and individual antidepressants within each class will be examined.
-
Analyses will calculate HRs according to time since stopping antidepressant medication.
-
Analyses of interaction will be carried out to examine the extent to which patient’s characteristics (age, gender), use of other medications and comorbidities modify the relationship between antidepressant use and adverse outcomes.
Adjustment will be made for potential confounders including:
-
age, gender, year of diagnosis of depression, previous recorded diagnosis of depression before age 65 years, severity of index diagnosis of depression, deprivation, smoking status, comorbidities (ischaemic heart disease, diabetes, hypertension, stroke/TIA, cancer, dementia, epilepsy/seizures, Parkinson’s disease, hypothyroidism, obsessive–compulsive disorder) and use of other drugs (including statins, NSAIDS, antipsychotics, lithium, aspirin, antihypertensive drugs, anticonvulsants, hypnotics/anxiolytics).
The analysis will also compare these patient characteristics according to the type of antidepressant prescribed. The assumptions of the Cox proportional hazards model will be checked. Absolute risks of the adverse events will also be estimated and presented.
Self-controlled case-series analysis
We will perform self-controlled case-series analyses using data only on patients who have had an adverse event. We will carry out a separate analysis for each type of outcome. The case-series method will enable us to determine the relative incidence of the outcomes of interest for high-risk versus low-risk time periods relative to commencing use of antidepressants in individuals who have the outcome of interest.
In the case-series analyses we will include cases who have the outcomes of interest as in the cohort study. We will use only the first recorded diagnosis of the outcome of interest rather than recurrent events. Patients who have the outcome of interest occurring on the same day as their first prescription for antidepressants will be distinguished in the analysis. Cases without any prescriptions for antidepressants will be included in the analyses to improve adjustment for age.
We will use conditional Poisson regression to estimate the relative incidence of the outcomes of interest for defined time periods of risk after the first prescription for antidepressants in a treatment episode. We will account for multiple periods of exposure in the analysis, defining a period of antidepressant treatment as one without gaps of more than 90 days between the end of a prescription and the start of the next prescription. A prescription after more than 90 days will count as a new treatment episode. The time periods for assessing potential short term effects of antidepressants will be defined as follows as shown in the figure below: 0 days (day of first prescription in each treatment episode); 1–28 days after the first prescription; 29–84 days and 85+ days (remaining treatment period); and periods after stopping treatment (1–28 days, 29–84 days and 85 to 182 days after stopping). The 28 days before the first prescription will be considered as a separate category, as occurrence of the outcome of interest in this period could affect the probability of an antidepressant prescription. All other time periods outside these specified risk periods will contribute to the baseline person time, i.e. the unexposed periods. These periods will enable us to examine short- and longer-term effects of antidepressants on the risks of adverse events and are similar to those used in other studies of antidepressants. 18,20 Where the outcome is a fatal one we will only use time from the first prescription in the observation period for analysis, as otherwise the method is invalid. We will adjust for age in the analyses and also take account of repeated prescriptions over time.
Figure showing risk periods in case-series design:
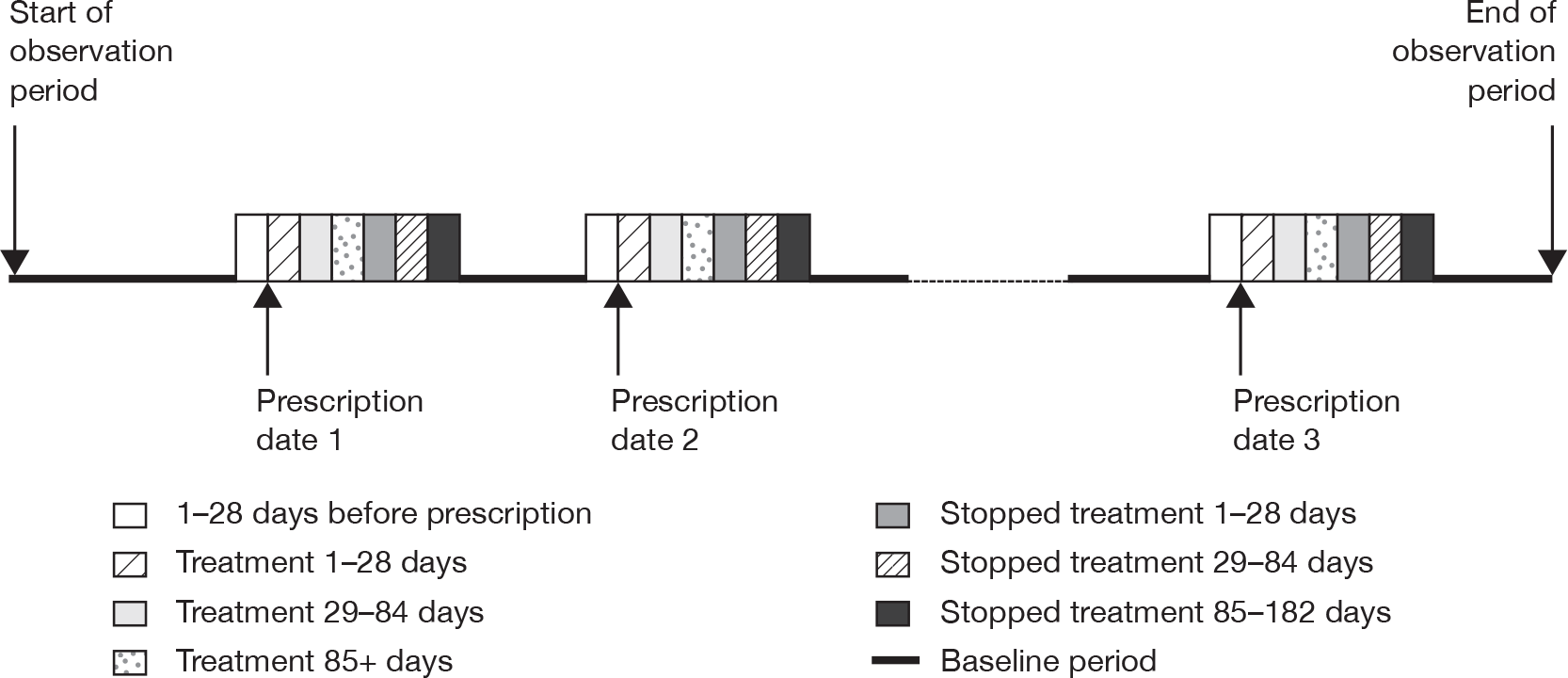
Measurement and analysis of costs
The cost analysis will be undertaken from an NHS health-care perspective with the aim of detecting whether there are any significant differences in health-care costs for patients on different types of antidepressants. Patient-specific resource use data (identified using the QResearch database), including data on antidepressant medication and primary-care consultations will be captured. The unit costs of these resources will be estimated using published data for a common price year from, for example the British National Formulary (BNF) and Curtis and Netten. 43 This will enable the overall aggregate cost per patient to be calculated over 1 year and 5 years following diagnosis of depression. In turn, the incremental mean cost associated with each type of antidepressant (SSRIs, TCAs, MAOIs and others) will be estimated, controlling for patient characteristics (age, gender), and other factors (comorbidities, whether they switched treatment, and severity of depression, for instance), which may be associated with differences in mean costs between the antidepressant groups that are not a result of the antidepressant they are taking. Sensitivity analyses will be undertaken to assess the robustness of results. Accepted methods will be used. 44 The cost of adverse events cannot be estimated using patient-specific resource use data as secondary care resource use data within the QResearch database is not routinely recorded by all GP practices within the database.
We will measure and analyse costs based on the cohort analysis to ensure representative costs are estimated for the population as a whole rather than just for those who experience the outcome of interest as would be the case using the self-controlled case-series study. We will estimate and compare, for instance, the incremental cost per adverse event avoided across the different antidepressants and those not taking antidepressants.
However, we recognise that cost-effectiveness ratios, such as cost per adverse event avoided, may capture only intermediate as opposed to final outcomes. In order to capture final outcomes it is usual to estimate quality-adjusted life-years (QALYs). As stated on p. 10, health-related quality of life, is not recorded in primary care databases, and whilst we shall search for literature on quality of life of older people with depression and for different antidepressants, initial searches do not reveal a vast literature on this and our ability to extrapolate from published studies therefore, is likely to be limited. However, we will explore this possibility in order to try and estimate the incremental cost per QALY between different types of antidepressants and those not on antidepressants but diagnosed with depression. Our primary focus, however, will relate to adverse events that may be associated with antidepressant use in older people, and we will be able to determine the cost per adverse event avoided based on data recorded in the database.
Research governance
All projects using QResearch are independently reviewed by the QResearch scientific committee and reported both to Trent Research Ethics Committee (REC) and also the national QResearch advisory board.
R&D governance of QResearch projects undertaken by the applicants is undertaken by Nottinghamshire County Teaching Primary Care Trust.
Ethical arrangements
The project will be independently peer reviewed by the QResearch scientific board and has been reported to Trent REC in accordance with the agreed procedure with the Committee.
Funding
This study is funded by the NIHR Coordinating Centre for Health Technology Assessment.
Project timetable and milestones
Duration
-
15 months.
Project timetable
| Months 1–3 | Finalise protocol |
| Specify detailed data definitions for data extraction | |
| Commence data extraction | |
| Months 4–7 | Refresh literature review |
| Complete data extraction | |
| Carry out data validation and manipulation | |
| Produce full statistical analysis plan | |
| Month 8 | Undertake descriptive statistical analyses |
| Months 9–10 | Analyse cohort study data |
| Months 11–12 | Analyse case-series study data |
| Undertake analyses of cost data | |
| Months 13–15 | Prepare reports and papers for publication |
Service users
As part of this project we will arrange meetings with the ‘Consumer Involvement in Research’ group at the Nottingham Primary Care Research Partnership. Members of this group have undertaken introductory-level research training. The Partnership also has strong links with a local mental health service users’ group. At these meetings the consumers will consider with us the implications of the study findings, and help us to identify means for dissemination of our findings to service users. We will not include consumers in those meetings that are focused on the technical and statistical aspects of the study.
Flow diagram
- Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry 1999;174:307-11.
- Department of Health (DoH) . Prescriptions Dispensed in the Community: Statistics for 1994–2004 2005.
- Cadieux RJ. Antidepressant drug interactions in the elderly. Understanding the P450 system is half the battle in reducing risks. Postgrad n.d.:231-2.
- Giron MS, Fastbom J, Winblad B. Clinical trials of potential antidepressants: to what extent are the elderly represented: a review. Int J Geriatr Psychiatry 2005;20:201-17.
- Pollock BG. Adverse reactions of antidepressants in elderly patients. J Clin Psychiatry 1999;60:4-8.
- Parikh C. Antidepressants in the elderly: challenges for study design and their interpretation. Br J Clin Pharmacol 2000;49:539-47.
- Blazer DG, Hybels CF, Fillenbaum GG, Pieper CF. Predictors of antidepressant use among older adults: have they changed over time?. Am J Psychiatry 2005;162:705-10.
- Arthur A, Matthews R, Jagger C, Lindesay J. Factors associated with antidepressant treatment in residential care: changes between 1990 and 1997. Int J Geriatr Psychiatry 2002;17:54-60.
- Rojas-Fernandez C, Thomas VS, Carver D, Tonks R. Suboptimal use of antidepressants in the elderly: a population-based study in Nova Scotia. Clin Ther 1999;21:1937-50.
- Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database Syst Rev 2006;1.
- Shah R, Uren Z, Baker A, Majeed A. Deaths from antidepressants in England and Wales 1993–1997: analysis of a new national database. Psychol Med 2001;31:1203-10.
- Fergusson D, Doucette S, Glass KC, Shapiro S, Healy D, Hebert P. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ 2005;330.
- Neutel CI, Patten SB. Risk of suicide attempts after benzodiazepine and/or antidepressant use. Ann Epidemiol 1997;7:568-74.
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA 2004;292:338-43.
- Martinez C, Rietbrock S, Wise L, Ashby D, Chick J, Moseley J. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ 2005;330.
- Hippisley-Cox J, Pringle M, Hammersley V, Crown N, Wynn A, Meal A. Antidepressants as risk factor for ischaemic heart disease: case-control study in primary care. BMJ 2001;323:666-9.
- Monster TBM, Johnsen SP, Olsen ML, McLaughlin JK, Sorensen HT. Antidepressants and risk of first-time hospitalization for myocardial infarction: a population-based case-control study. Am J Med 2004;117:732-7.
- Tata LJ, West J, Smith C, Farrington P, Card T, Smeeth L. General population based study of the impact of tricyclic and selective serotonin reuptake inhibitor antidepressants on the risk of acute myocardial infarction. Heart 2005;91:465-71.
- Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N. Use of selective serotonin-reuptake inhibitors of tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 1998;351:1303-7.
- Hubbard R, Farrington P, Smith C, Smeeth L, Tattersfield A. Exposure to tricyclic and selective serotonin reuptake inhibitor antidepressants and the risk of hip fracture. Am J Epidemiol 2003;158:77-84.
- Stage KBB. Danish University Antidepressant G . Orthostatic side effects of clomipramine and moclobemide during treatment for depression. Nordic J Psychiatry 2005;59:298-301.
- Kinjo M, Setoguchi S, Schneeweiss S, Solomon DH. Bone mineral density in subjects using central nervous system-active medications. Am J Med 2005;118.
- Etminan M, Hemmelgarn B, Delaney JAC, Suissa S. Use of lithium and the risk of injurious motor vehicle crash in elderly adults: case-control study nested within a cohort. BMJ 2004;328:558-9.
- Ramaekers JG. Antidepressants and driver impairment: empirical evidence from a standard on-the-road test. J Clin Psychiatry 2003;64:20-9.
- Spigset O, Hedenmalm K. Hyponatremia in relation to treatment with antidepressants: a survey of reports in the World Health Organization data base for spontaneous reporting of adverse drug reactions. Pharmacotherapy 1997;17:348-52.
- Movig KLL, Leufkens HGM, Lenderink AW, van den Akker VGA, Hodiamont PPG, Goldschmidt HMJ. Association between antidepressant drug use and hyponatraemia: a case-control study. Br J Clin Pharmacol 2002;53:363-9.
- van Walraven C, Mamdani MM, Wells PS, Williams JI. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ 2001;323:655-8.
- de Jong JCF, van den Berg PB, Tobi H, de Jong-van den Berg LTW. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol 2003;55:591-5.
- Dalton SO, Johansen C, Mellemkjaer L, Norgard B, Sorensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Int Med 2003;163:59-64.
- Tata LJ, Fortun PJ, Hubbard RB, Smeeth L, Hawkey CJ, Smith CJ. Does concurrent prescription of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs substantially increase the risk of upper gastrointestinal bleeding?. Aliment Pharmacol Ther 2005;22:175-81.
- Hippisley-Cox J, Vinogradova Y, Coupland C, Pringle M. Comparison of key practice characteristics between general practices in England and Wales and general practices in the QResearch database. Nottingham: University of Nottingham; 2005.
- Hippisley-Cox J, Pringle M. Prevalence, Care and Outcomes for patients with diet controlled diabetes in general practice: cross-sectional survey. Lancet 2004;364:423-5.
- Gnani S, Majeed A. A user’s guide to data collected in primary care in England. Cambridge: Eastern Region Public Health Observatory, Cambridge; 2006.
- Hippisley-Cox J, Coupland C. Effect of combinations of drugs on all cause mortality in patients with ischaemic heart disease: nested case control analysis. BMJ 2005;330:1059-63.
- Hippisley-Cox J, Coupland C. Statins and all cause mortality in ischaemic heart disease: nested case control analysis. Heart 2006;92:752-58.
- Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients on Cox 2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case control analysis. BMJ 2005;366:1366-74.
- Barrett B, Byford S, Knapp M. Evidence of cost-effective treatments for depression: a systematic review. J Affect Disord 2005;84:1-13.
- Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. Am J Epidemiol 1996;143:1165-73.
- Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006;25:1768-97.
- Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase inhibitors or conventional non-steroidal anti-inflammatory drugs: population-based nested case-control analysis. BMJ 2005;331:1310-16.
- Kaye JA, Jick H. Epidemiology of lower limb fractures in general practice in the United Kingdom. Inj Prev 2004;10:368-74.
- Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case-series studies. Stat Med 2006;25:2618-31.
- Curtis L, Netten A. Unit costs of health and social care, PSSRU, 2006. London: Pharmaceutical Press; 2006.
- Drummond MF, Sculpher MJ, Torrance GW, O Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
Appendix 4 Original protocol
Protocol
A study of the safety and harms of antidepressant drugs for older people: an analysis using a large primary care database.
Investigators
Carol Coupland, Division of Primary Care, University of Nottingham.
Julia Hippisley-Cox, Division of Primary Care, University of Nottingham.
Antony Arthur, School of Nursing, University of Nottingham.
Tracey Sach, School of Chemical Sciences and Pharmacy, University of East Anglia.
Richard Morriss, Division of Psychiatry, University of Nottingham.
Funding
-
NCCHTA.
Protocol details
-
Version 1.1.
Investigators
Carol Coupland
Senior Lecturer in Medical Statistics
Division of Primary Care
University of Nottingham
Nottingham NG7 2RD
Julia Hippisley-Cox
Professor of Clinical Epidemiology and General Practice
Division of Primary Care
University of Nottingham
Nottingham NG7 2RD
Antony Arthur
Senior Lecturer in Elder Care
School of Nursing
University of Nottingham
Queen’s Medical Centre
Nottingham NG7 2HA
Tracey Sach
Senior Lecturer in Health Economics
School of Chemical Sciences and Pharmacy
University of East Anglia
Norwich NR4 7TJ
Richard Morriss
Professor of Psychiatry & Community Mental Health
Division of Psychiatry
University of Nottingham
Queen’s Medical Centre
Nottingham NG7 2UH
Detailed project description
Project title
Safety and harms of antidepressant drugs for older people: an analysis using a large primary care database.
HTA project number: 06/42/01.
Summary
Depression is a common and debilitating condition in older people. Adverse drug events may be more common in the treatment of depression in older people compared with younger age groups owing to higher levels of comorbidity, age-related physiological changes and polypharmacy. The under-representation of older people in clinical trials of antidepressants makes it difficult to make reliable or precise estimates of the incidence of adverse events. This problem is further compounded when trial exclusion criteria exclude older people with comorbid conditions.
The overall aim of this study is to establish the relative safety and balance of risks for individual antidepressant drugs in older people. The study is a cohort study of people aged 65 years and over who have been diagnosed with a major depressive disorder or with unipolar depression identified from a large primary care database (QResearch). Prescribing data for these patients will be used to ascertain their use of antidepressant drugs following diagnosis of depression, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs) and other antidepressants. Prospectively recorded data on these patients will be used to ascertain harms and adverse events that occurred in these patients over a minimum of 12 months’ follow-up after their diagnosis. Primary outcomes will include the following events: all-cause mortality, suicide, sudden cardiac death, overdose/poisoning, attempted suicide, myocardial infarction (MI), stroke, seizures, gastrointestinal (GI) bleeding, falls and fractures, road traffic accidents (RTAs), adverse drug reactions (ADRs) and hyponatraemia. The analysis will examine the associations between exposure to the different classes of antidepressant and risk of the adverse events. Annual costs of antidepressant medication and costs of adverse events will be calculated and compared. A further analysis will use the self-controlled case-series approach to reduce effects of residual confounding and indication biases.
Background
Depression is a common and debilitating condition in older people. A pooled estimate of prevalence of depression from community-based studies of older people is 13.5%. 1 Across all ages 29 million prescriptions for antidepressant drugs were issued in 2004. 2 Adverse drug events may be more common in the treatment of depression in older people compared with younger age groups owing to higher levels of comorbidity, age-related physiological changes, and polypharmacy. 3 The under-representation of older people in clinical trials of antidepressants makes it difficult to make reliable or precise estimates of the incidence of adverse events. 4,5 This problem is further compounded when trial exclusion criteria exclude older people with comorbid conditions. 6 Even though older people with depression are more likely to be treated since the introduction of the newer generation of antidepressants,7,8 under treatment of depression among older people is a global problem. 9 Evidence from a systematic review suggests that TCAs and SSRIs are equivalent in terms of efficacy but classical TCAs are associated with a higher discontinuation rate due to the side effect profile. 10
Antidepressants, and particularly TCAs are an important cause of deaths by overdose and poisoning. 11 There appears to be some evidence from a meta-analysis of placebo-controlled trials that SSRIs are associated with a small increase in risk of fatal and non-fatal suicide attempts. 12 Lack of sufficient trial data meant it was not possible to see whether this finding held within those aged 60 years and over. Observational studies across all age groups have found associations between antidepressant use and suicide but have been unable to rule out confounding by indication. 13 There is little evidence to support any difference in terms of class of antidepressant and risk of suicide,14 but studies have tended to look at risks across all ages or among adolescents and young adults. 15
There appears to be an increased risk of subsequent ischaemic heart disease associated with dosulepin (formerly known as dothiepin) use but not other TCAs or SSRIs. 16 Some studies have found no evidence of an increased risk of MI among users of antidepressants,17 or have suggested that an increased risk of MI may be explained by confounding factors relating to depression itself rather than specific adverse drug effects. 18
Findings from both case-control,19 and case-series analysis studies,20 indicate that risk of hip fracture is elevated with the use of TCAs and SSRIs among older people although the magnitude of the increased risk did not differ between the two classes of antidepressant. 19 The likely mechanism underlying this increased risk appearing to be changes in orthostatic blood pressure,21 rather than altered bone mineral density. 22
Older people who use lithium may be at increased risk of being involved in an injurious motor vehicle accident. 23 In studies that have formally tested the effects of antidepressants on driving performance, sedating antidepressants have a similar effect to alcohol. 24
Hyponatraemia associated with antidepressant use is rare but is an adverse event that disproportionately affects older people. 25,26 Similarly, GI bleeding is more common among those taking SSRIs who are aged 80 years or over,27 although there is a lack of consensus as to whether the risk of GI bleeding associated with SSRI use is further increased with concurrent use of non-steroidal anti-inflammatory drugs,28,29 or not. 30
The gaps in the research into adverse effects for these drugs specifically in older people and the lack of consistent findings pose problems for clinicians prescribing these drugs and making choices as to the most appropriate drug for individual older patients. In this study we will use a large primary care database containing information on virtually all prescriptions for antidepressants and a range of potential adverse effects to derive a unified picture of the balance of risks for antidepressant drugs in older people with depression.
Specific aims and objectives
The overall aim of this study is to establish the relative safety and balance of risks for individual antidepressant drugs in older people, in order to provide a robust evidence base to support decision making for clinicians prescribing these medications to individual patients.
The project has five key objectives which are:
-
to determine the relative and absolute risks of predefined adverse events in older people diagnosed with depression. Comparisons will be made between classes of antidepressant drugs (tricyclic and related antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs) and other antidepressants) and non-use of antidepressant drugs
-
to directly compare the risk of adverse events in patients prescribed SSRIs compared with TCAs in older people diagnosed with depression
-
to determine how the dose and duration of prescribed antidepressant medication is associated with the risk of an adverse event
-
to describe patterns of antidepressant use in older people diagnosed with depression, in particular the types and doses prescribed, the durations and the proportions switched between different antidepressants (TCAs, SSRIs and other antidepressant drugs)
-
to determine the annual costs of antidepressant medication, the costs of the adverse events, and the costs of health-care resource use in older people diagnosed with depression, comparing patients by type of antidepressant drugs (TCAs, SSRIs, MAOIs, and other antidepressants).
Study design
Design
The planned investigation will use a large primary care database (QResearch) to investigate the relative safety and costs of antidepressant drugs in older people.
Two main approaches will be used to achieve the study objectives:
-
a cohort study
-
a self-controlled case-series study.
The cohort study is a well-established powerful method for determining absolute and relative risks associated with exposures. The self-controlled case-series method is a newer approach that estimates relative incidence of an outcome in high-risk compared with low-risk periods of time based only on data from cases. It is useful for investigating the short term effect of drug exposures on the risk of acute outcomes, since it eliminates problems of confounding from unmeasured variables, such as severity of disease. Both of these studies will derive data from a large primary-care research database (QResearch).
Cohort study
Our target population for the cohort study will be all patients aged 65 years and over with a first recorded diagnosis of depression (major depressive disorder or unipolar depression) between 1 January 1996 and 31 December 2005. We will use Read codes to identify a major depressive disorder or unipolar depression, using case definitions that have been used in previous studies. The cohort will be followed up until 31 December 2006. Information on all prescriptions for antidepressants will be extracted, along with information on potential confounding variables and adverse events during follow-up.
Self-controlled case-series study
The self-controlled case-series study only uses the patients in the cohort who have the outcomes of interest. Cases with each type of adverse event will be identified; these will be cases with a diagnosis of the adverse event between 1 January 1996 and 31 December 2006, who had a previous diagnosis of depression between 1 January 1996 and 31 December 2005. Information on prescriptions for antidepressants in these cases will be extracted and the analysis will compare rates of the adverse events in periods following a first prescription for an antidepressant compared with a baseline period.
Setting: QResearch database
We will undertake the study using data from the QResearch primary-care research database (www.qresearch.org). This validated database is the largest general practice research database in the UK and it contains the anonymised electronic health-care records of over 10 million patients ever registered with 525 general practices throughout England, Wales, Scotland and Northern Ireland. Consent to provide data for QResearch was sought from all UK practices using the Egton Medical Information Systems (EMIS) medical records system. EMIS is the major supplier of primary-care computer systems in the UK and is in use in two-thirds of all UK general practices. The consenting practices form a representative sample of 6–7% of all UK general practices, and there are practices in every strategic health authority and each health board in England, Wales and Scotland.
The information recorded on the QResearch database includes patient demographic data (year of birth, gender, socio-economic data derived from the UK 2001 census), characteristics (height, weight, smoking status), symptoms, clinical diagnoses, consultations, referrals, prescribed medications and results of investigations. The latest version of the QResearch database, which is updated quarterly, will be used for the analysis.
Detailed analyses have compared QResearch practices with all UK practices and found that practices contributing to QResearch are somewhat larger than UK practices overall but are very similar in other respects. 31 The database has been validated by comparing birth rates, death rates, consultation rates, prevalence and mortality rates with other data sources including the General Household Survey and the General Practice Research Database http://secure.qresearch.org/SiteSections/DataValidation/DataValidationMain.aspx. The age–gender structure of the population has been compared with that reported in the 2001 census. There was good correspondence for all of these measures, although the QResearch population is slightly older and has marginally higher prevalence figures for some diagnoses compared with less recent data. 32 but they are almost identical to current prevalence data from the new General Medical Services (GMS) contract for General Practitioners.
Compared with other primary care databases QResearch is the largest (currently 525 practices compared with around 200–400 (depending on selection criteria) in GPRD and 100 in The Health Improvement Network (THIN33), and it has information on deprivation derived from postcode data which is not currently available in the other databases. The database is completely independent from commercial organisations and QResearch receives no funding from pharmaceutical companies. The database contains only anonymised data, which are encrypted and kept in secure conditions. One of the co-applicants (Professor Julia Hippisley-Cox) is the chief custodian of the database and QResearch has been used to examine the risks and benefits associated with a number of commonly prescribed drugs including statins34,35 and non-steroidal anti-inflammatory drugs (NSAIDs). 36 The applicants have also published studies examining antidepressants as risk factors for ischaemic heart disease. 16
Outcome measures
The outcomes to be assessed will be extracted from the routine primary-care computer records of patients in the cohort. Outcomes will only be included if they occurred after the initial diagnosis of depression. The relevant computer recorded Read codes will be used to identify patients with the outcomes.
The outcomes that will be assessed will include:
-
all-cause mortality
-
suicide
-
sudden cardiac death
-
overdose/poisoning
-
attempted suicide
-
myocardial infarction
-
stroke
-
seizures
-
gastrointestinal bleeding
-
falls and fractures
-
road traffic accidents
-
adverse drug reactions.
We will examine hyponatraemia in a subset of practices with electronic links for pathology results.
QResearch is undertaking a national audit on care for patients with osteoporosis and falls in primary care. As part of this project, funded by the Information Centre, we will be examining the clinical coding of falls and fractures in some detail and will be able to utilise the definitions for the proposed project. We have consulted professional groupings regarding the diagnostic codes (Read codes) which are likely to be used in clinical practice. Other outcomes are likely to be well recorded, although sudden cardiac deaths may be difficult to identify.
Exposures
Our exposure of interest is antidepressant medication. We will extract details of all prescriptions for antidepressants in patients in our cohort, following their diagnosis of depression; this will include the date of prescription, the type of drug, the dose and the duration. The antidepressant drugs will be grouped for analysis according to the major classes as described in the British National Formulary (BNF), namely: tricyclic and related antidepressants (TCAs: section 4.3.1), selective serotonin reuptake inhibitors (SSRIs: section 4.3.3), monoamine oxidase inhibitors (MAOIs: section 4.3.2) and other antidepressants (section 4.3.4). Effects of individual antidepressant drugs will also be assessed where numbers are sufficient. The number of prescriptions, duration and dose of the antidepressant drugs will be examined in the analyses.
We will determine the proportions of patients who switch between antidepressants, including switches between classes of drugs and between different drugs within a class. We will examine the proportions for patients who discontinue a drug before the recommended time by examining the proportions who have only one prescription, have two to three and four to six prescriptions for a particular drug.
Confounding variables
Data will be extracted on the following variables, and these will be considered as confounding variables in the analysis of the cohort study:
-
age, gender, deprivation, government office region, comorbidities (e.g. ischaemic heart disease, diabetes, hypertension, cancer, dementia, epilepsy, Parkinson’s), body mass index (BMI), smoking and use of other drugs (including statins, NSAIDS, anti-psychotics, aspirin, antihypertensive drugs, anticonvulsants).
Inclusion/exclusion criteria
Patients will be eligible for inclusion in the cohort study if:
-
they have a recorded diagnosis of depression (a major depressive disorder or unipolar depression)
-
the diagnosis was made at the age of 65 years or over
-
the diagnosis was recorded between 1 January 1996 to 31 December 2005
-
the diagnosis occurred at least 12 months after registration with a study practice and after the date of the installation of the practice EMIS computer system.
Patients will be excluded from the cohort study if:
-
they are temporary residents
-
they have a previous recorded diagnosis of depression
-
they have been prescribed antidepressants more than 1 month prior to their recorded diagnosis of depression.
Quality of life
Quality of life is not recorded in primary-care consultations so we are unable to examine this outcome in our database, however, as part of our review of the literature we will search for literature on quality of life and antidepressant medication in older people with depression to see if some estimations can be made about the likely effectiveness and cost-effectiveness of different antidepressants in older people. However, a basic literature search undertaken to support the development of this proposal found very few cost–utility studies37 comparing different types of antidepressants in a population aged 18 years or over and therefore, there may well be limited published economic evidence specific to a more elderly population.
Strengths and limitations of study design
The strengths of using a cohort study design for this project are that it will include a large and representative number of older people with depression, it can calculate absolute as well as relative risks, it can take account of exposures changing over time and it is able to adjust for a number of potential confounding variables. The recording of prescriptions in primary-care records is high, and the exposures under consideration are available only on prescription. The outcomes we have included are likely to be well recorded, and we will compare their rates in this study against other published data where possible.
The limitations of the cohort design approach are that it can be vulnerable to indication bias and residual confounding whereby relevant confounding variables may be imprecisely recorded or not recorded at all in primary-care records (for example diet, physical activity). Indication bias can cause difficulties in the interpretation of results on effects of drugs in observational studies; in this instance whether or not an antidepressant is prescribed or the type of antidepressant prescribed may be related to important prognostic factors for the outcome in question such as the severity of depression or the attitude of the patient towards taking medication. These characteristics could influence the outcome but are unlikely to be recorded well in a patient’s medical records. The self-controlled case-series method has been proposed as a means of addressing this problem. 38,39 This is an internally controlled method whereby analyses are carried out only in patients with the outcome of interest, thereby eliminating the effect of indication bias and unmeasured confounding variables that do not vary over time. This method has previously been used to examine the relationship between antidepressants and hip fracture,20 and is of most relevance for acute events occurring within a short period after exposure. A limitation of the case-series design is that it requires that probability of exposure is not affected by occurrence of an outcome event, which is a particular problem for fatal outcomes, but this can be resolved by using only time from first prescription in the observation period for analysis.
Sample size
Cohort study
All eligible patients aged 65 years and over diagnosed with incident depression between 1 January 1996 to 31 December 2005 in the QResearch database will be included in the cohort study. A feasibility study shows there are approximately 5.0 million years of observation and 18,000 incident cases of depression arising from patients aged 65 years and older between 1996 and 2005 on the database.
Assuming 88% of patients aged 65 years and over diagnosed with depression are prescribed an antidepressant drug as we found in our feasibility study, and for a rare outcome with an incidence of 5 per 1000 per year (e.g. upper GI event40 or lower limb fracture,41 and an average follow-up of 5 years, we will be able to detect a relative risk of 1.5 with 88% power and a 5% significance level comparing those on antidepressants with those not on antidepressants. For all-cause mortality with a mortality rate of 47 per 1000 per year (Office for National Statistics for 2001) we will be able to detect a relative risk of 1.15 with 95% power. In comparisons between TCAs and SSRIs, assuming 39% of patients on antidepressants take TCAs and 50% take SSRIs we will be able to detect a relative risk of 1.4 with 86% power for rare outcomes and 1.12 with 92% power for all-cause mortality.
Self-controlled case-series study
The exposed cases contribute to the statistical power in the case-series method, under certain conditions the power of the analysis can be similar to that of the cohort study from which the cases are derived. To detect a rate ratio of 2.0 in a risk period of 1–14 days after the first prescription for an antidepressant with 80% power and 5% significance, then with a proportion of 0.0077 in the risk period of 14 days compared with an average observation period of 5 years (3/1825) then 1435 exposed cases would be required for each outcome. 42 We would anticipate having at least this number for all-cause mortality and falls/fracture. To detect a rate ratio of 3.0 in a risk period of 1–14 days then 448 exposed cases would be required. We would anticipate having around this number for rare outcomes such as GI events (incidence rate 5/1000/year).
Statistical analysis
Descriptive statistics
Descriptive statistics will be derived primarily using the data from the cohort study.
-
We will calculate incidence rates of diagnosed depression in people aged 65 years and over, and examine these rates by gender, age group (65–74, 75–84, 85+ years) and study year. We will compare these rates with other published rates of depression.
-
In patients with a diagnosis of depression in the study cohort we will describe patterns of antidepressant use according to type of antidepressant prescribed, duration of use and dose, and will examine these patterns by gender, age group, use of other medications, comorbidities and study year. We will also examine variations between practices in patterns of antidepressant prescribing.
-
We will calculate the proportions of people switched between different antidepressants (TCAs, SSRIs and other antidepressant drugs) by gender, age group and study year and examine duration of use before switching.
-
Discontinuation rates for each drug will also be determined by examining the proportion of those with at least one prescription for a drug who only have one prescription, have 2–3 and have 4–6 prescriptions.
Analysis of cohort study
The analysis of the cohort study will determine absolute and relative risks of adverse events in older people with depression according to type of antidepressant prescribed, and will also examine risks according to dose and duration of treatment.
Incidence rates of the adverse events will be calculated in the study cohort. The statistical analysis will comprise a series of survival analyses to assess the relationship between exposure to antidepressant drugs and a number of potential adverse effects. These analyses will be restricted to the cohort of older people with a diagnosis of depression. The exposure variables will be use of antidepressant drugs including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs) and other antidepressants. The number of prescriptions, duration and dose of the antidepressant drugs will be examined in the analyses. The date of entry into the survival analyses will be the date of diagnosis of depression, and the right censor date will be the earliest of the following: date of diagnosis of the outcome of interest, date of death, date of leaving the practice, date of the latest download of data or the study end date.
Cox’s proportion hazards models will be used with antidepressant exposure treated as a time-varying exposure. The analysis will calculate HRs and 95% confidence intervals (CIs) comparing:
-
The risk of each adverse effect in patients on any type of antidepressant compared with patients with no antidepressant treatment.
-
Each separate class of antidepressants (SSRIs, TCAs and other antidepressants) compared with no treatment.
-
The risk of each adverse effect for each class of antidepressant will be directly compared with each other class (in particular SSRIs will be directly compared with TCAs).
-
Analyses will also calculate HRs according to duration of use and prescribed dose of antidepressant; where numbers are sufficient individual antidepressants within each class will be examined.
-
Analyses of interaction will be carried out to examine the extent to which patient’s characteristics (age, gender), use of other medications and comorbidities modify the relationship between antidepressant use and adverse outcomes.
Adjustment will be made for potential confounders including:
-
age, gender, deprivation, government office region, comorbidities (e.g. ischaemic heart disease, diabetes, hypertension, cancer, dementia, epilepsy, Parkinson’s), BMI, smoking and use of other drugs (including statins, NSAIDS, anti-psychotics, aspirin, antihypertensive drugs, anticonvulsants). The analysis will also compare these patient characteristics according to the type of antidepressant prescribed. The assumptions of the Cox proportional hazards model will be checked. Absolute risks of the adverse events will also be estimated and presented.
Self-controlled case-series analysis
We will perform self-controlled case-series analyses using data only on patients who have had an adverse event. We will carry out a separate analysis for each type of outcome. The case-series method will enable us to determine the relative incidence of the outcomes of interest for high risk versus low risk time periods relative to commencing use of antidepressants in individuals who have the outcome of interest.
In the case-series analyses we will include cases who have the outcomes of interest as in the cohort study. We will use only the first recorded diagnosis of the outcome of interest rather than recurrent events. Patients who have the outcome of interest occurring on the same day as their first prescription for antidepressants will be distinguished in the analysis. Cases without any prescriptions for antidepressants will be included in the analyses to improve adjustment for age.
We will use conditional Poisson regression to estimate the relative incidence of the outcomes of interest for defined time periods of risk after the first prescription for antidepressants. The time periods for assessing potential short term effects of antidepressants will be defined as follows as shown in the figure below: 0 days (outcome occurs on same day as first prescription); 1–14 days after the first prescription; 15–28 days and 29–84 days; remaining treatment period; washout period (a period of 182 days after stopping treatment). The 14 days before the first prescription will be considered as a separate category, as occurrence of the outcome of interest in this period could affect the probability of an antidepressant prescription. All other time periods outside these specified risk periods will contribute to the baseline person time, i.e. the unexposed periods. These periods will enable us to examine short-term and longer-term effects of antidepressants on the risks of adverse events and are similar to those used in other studies of antidepressants. 18,20 Where the outcome is a fatal one we will only use time from the first prescription in the observation period for analysis, as otherwise the method is invalid. We will adjust for age in the analyses and also take account of repeated prescriptions over time.
Figure showing risk periods in case-series design:
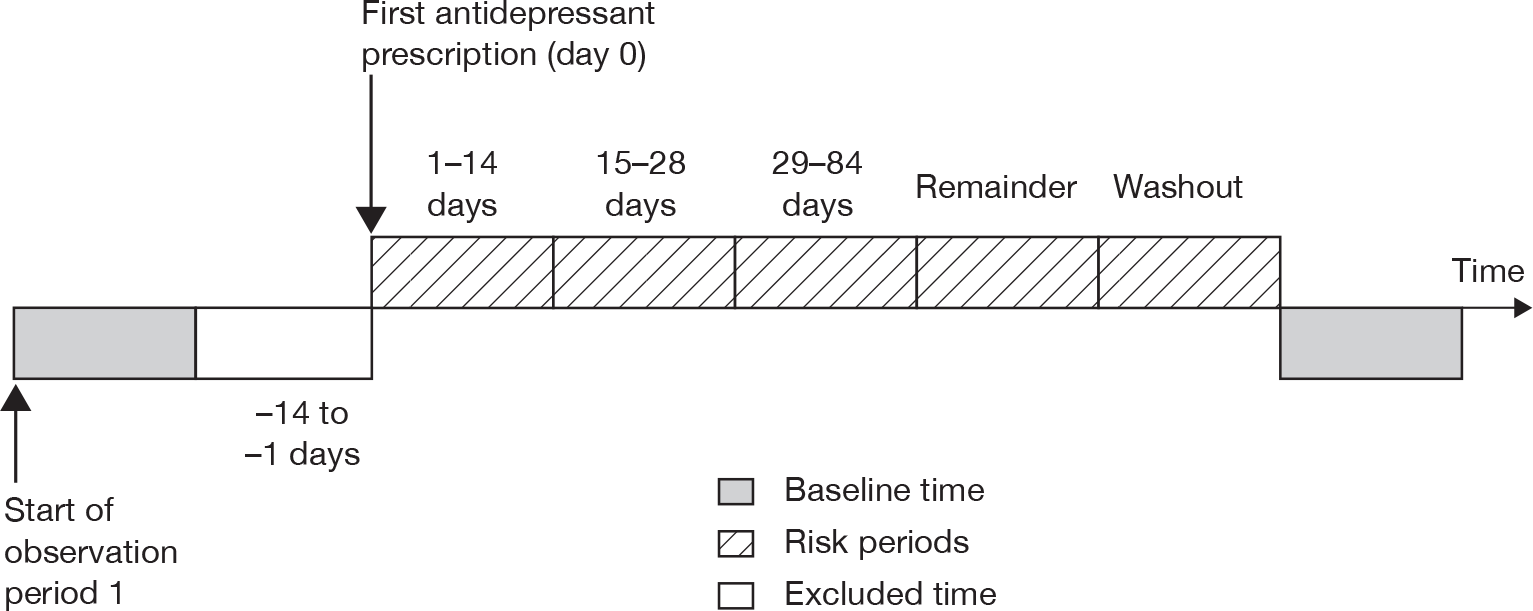
Measurement and analysis of costs
The cost analysis will be undertaken from an NHS health-care perspective with the aim of detecting whether there are any significant differences in health-care costs for patients on different types of antidepressants. Patient-specific resource use data (identified using the QResearch database), including those specific to depression such as antidepressant medication and resource use as a result of an adverse reaction, will be captured alongside wider health service utilisation in case different medications for depression are associated with distinct impacts on the patients wider health needs. The unit costs of these resources will be estimated using published data for a common price year from, for example, the British National Formulary (BNF) and Curtis and Netten. 43 This will enable the overall aggregate cost per patient to be calculated over 1 year following diagnosis of depression. In turn, the incremental mean cost associated with each type of antidepressant (SSRIs, TCAs, MAOIs, and others) will be estimated, controlling for patient characteristics (age, gender), and other factors (comorbidities, whether they switched treatment, and severity of depression, for instance), which may be associated with differences in mean costs between the antidepressant groups that are not a result of the antidepressant they are taking. Sensitivity analyses will be undertaken to assess the robustness of results. Accepted methods will be used. 44
We will measure and analyse costs based on the cohort analysis to ensure representative costs are estimated for the population as a whole rather than just for those who experience the outcome of interest as would be the case using the self-controlled case-series study. We will estimate and compare, for instance, the incremental cost per adverse event avoided across the different antidepressants and those not taking antidepressants. We will explore the relationship between cost and explanatory factors such as severity, comorbidities, use of other medications and patients’ characteristics. In addition, the mean cost of an adverse event, will be estimated using only the sample in the self-controlled case-series study. We will also explore the relationship between time of adverse event relative to time since first antidepressant prescription to see if this has a relationship with the scale of the adverse event cost.
However, we recognise that cost-effectiveness ratios, such as cost per adverse event avoided, may capture only intermediate as opposed to final outcomes. In order to capture final outcomes it is usual to estimate quality-adjusted life years (QALYs). As stated on p. 10, health-related quality of life, is not recorded in primary care databases, and whilst we shall search for literature on quality of life of older people with depression and for different antidepressants, initial searches do not reveal a vast literature on this and our ability to extrapolate from published studies therefore, is likely to be limited. However, we will explore this possibility in order to try and estimate the incremental cost per QALY between different types of antidepressants and those not on antidepressants but diagnosed with depression. Our primary focus however will relate to adverse events that may be associated with antidepressant use in older people, and we will be able to determine the cost per adverse event avoided based on data recorded in the database.
Research governance
All projects using QResearch are independently reviewed by the QResearch scientific committee and reported both to Trent Research Ethics Committee (REC) and also the national QResearch advisory board.
R&D governance of QResearch projects undertaken by the applicants is undertaken by Nottinghamshire County Teaching Primary Care Trust.
Ethical arrangements
The project will be independently peer reviewed by the QResearch Scientific board and has been reported to Trent Research Ethics Committee in accordance with the agreed procedure with the Committee.
Funding
This study is funded by the NCCHTA.
Project timetable and milestones
Proposed start date
-
1 April 2008.
Duration
-
15 months.
Project timetable
| Months 1–3 | Finalise protocol |
| Specify detailed data definitions for data extraction | |
| Commence data extraction | |
| Months 4–7 | Refresh literature review |
| Complete data extraction | |
| Carry out data validation and manipulation | |
| Produce full statistical analysis plan | |
| Month 8 | Undertake descriptive statistical analyses |
| Months 9–10 | Analyse cohort study data |
| Months 11–12 | Analyse case-series study data |
| Undertake analyses of cost data | |
| Months 13–15 | Prepare reports and papers for publication |
Service users
As part of this project we will arrange meetings with the ‘Consumer Involvement in Research’ group at the Nottingham Primary Care Research Partnership. Members of this group have undertaken introductory-level research training. The Partnership also has strong links with a local mental health service users’ group. At these meetings the consumers will consider with us the implications of the study findings, and help us to identify means for dissemination of our findings to service users. We will not include consumers in those meetings that are focused on the technical and statistical aspects of the study.
Flow diagram
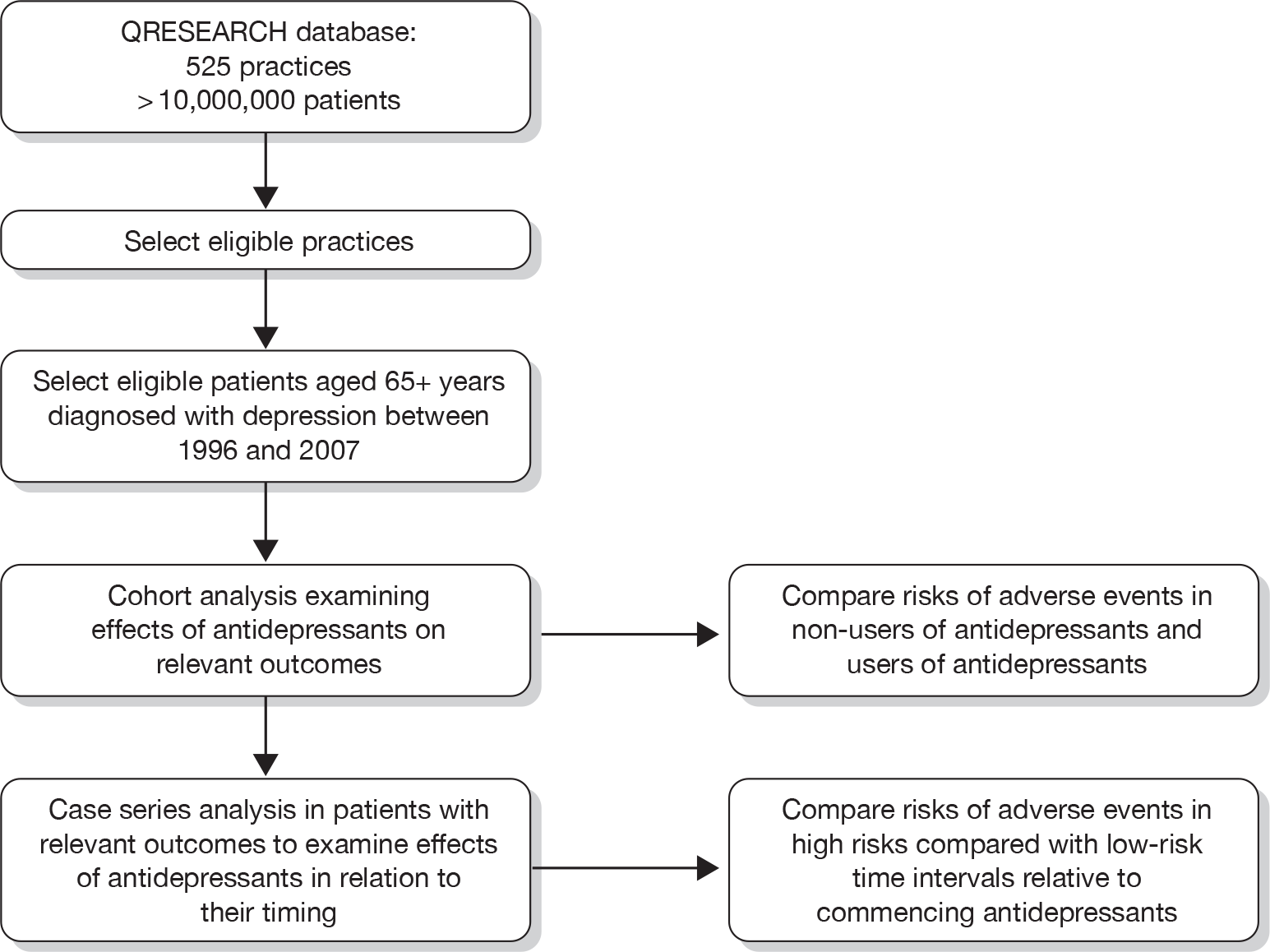
- Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry 1999;174:307-11.
- Department of Health (DoH) . Prescriptions Dispensed in the Community: Statistics for 1994–2004 2005.
- Cadieux RJ. Antidepressant drug interactions in the elderly. Understanding the P450 system is half the battle in reducing risks. Postgrad n.d.:231-2.
- Giron MS, Fastbom J, Winblad B. Clinical trials of potential antidepressants: to what extent are the elderly represented: a review. Int J Geriatr Psychiatry 2005;20:201-17.
- Pollock BG. Adverse reactions of antidepressants in elderly patients. J Clin Psychiatry 1999;60:4-8.
- Parikh C. Antidepressants in the elderly: challenges for study design and their interpretation. Br J Clin Pharmacol 2000;49:539-47.
- Blazer DG, Hybels CF, Fillenbaum GG, Pieper CF. Predictors of antidepressant use among older adults: have they changed over time?. Am J Psychiatry 2005;162:705-10.
- Arthur A, Matthews R, Jagger C, Lindesay J. Factors associated with antidepressant treatment in residential care: changes between 1990 and 1997. Int J Geriatr Psychiatry 2002;17:54-60.
- Rojas-Fernandez C, Thomas VS, Carver D, Tonks R. Suboptimal use of antidepressants in the elderly: a population-based study in Nova Scotia. Clin Ther 1999;21:1937-50.
- Mottram P, Wilson K, Strobl J. Antidepressants for depressed elderly. Cochrane Database Syst Rev 2006;1.
- Shah R, Uren Z, Baker A, Majeed A. Deaths from antidepressants in England and Wales 1993–1997: analysis of a new national database. Psychol Med 2001;31:1203-10.
- Fergusson D, Doucette S, Glass KC, Shapiro S, Healy D, Hebert P. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ 2005;330.
- Neutel CI, Patten SB. Risk of suicide attempts after benzodiazepine and/or antidepressant use. Ann Epidemiol 1997;7:568-74.
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA 2004;292:338-43.
- Martinez C, Rietbrock S, Wise L, Ashby D, Chick J, Moseley J. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ 2005;330.
- Hippisley-Cox J, Pringle M, Hammersley V, Crown N, Wynn A, Meal A. Antidepressants as risk factor for ischaemic heart disease: case-control study in primary care. BMJ 2001;323:666-9.
- Monster TBM, Johnsen SP, Olsen ML, McLaughlin JK, Sorensen HT. Antidepressants and risk of first-time hospitalization for myocardial infarction: a population-based case-control study. Am J Med 2004;117:732-7.
- Tata LJ, West J, Smith C, Farrington P, Card T, Smeeth L. General population based study of the impact of tricyclic and selective serotonin reuptake inhibitor antidepressants on the risk of acute myocardial infarction. Heart 2005;91:465-71.
- Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N. Use of selective serotonin-reuptake inhibitors of tricyclic antidepressants and risk of hip fractures in elderly people. Lancet 1998;351:1303-7.
- Hubbard R, Farrington P, Smith C, Smeeth L, Tattersfield A. Exposure to tricyclic and selective serotonin reuptake inhibitor antidepressants and the risk of hip fracture. Am J Epidemiol 2003;158:77-84.
- Stage KBB. Danish University Antidepressant G . Orthostatic side effects of clomipramine and moclobemide during treatment for depression. Nordic J Psychiatry 2005;59:298-301.
- Kinjo M, Setoguchi S, Schneeweiss S, Solomon DH. Bone mineral density in subjects using central nervous system-active medications. Am J Med 2005;118.
- Etminan M, Hemmelgarn B, Delaney JAC, Suissa S. Use of lithium and the risk of injurious motor vehicle crash in elderly adults: case-control study nested within a cohort. BMJ 2004;328:558-9.
- Ramaekers JG. Antidepressants and driver impairment: empirical evidence from a standard on-the-road test. J Clin Psychiatry 2003;64:20-9.
- Spigset O, Hedenmalm K. Hyponatremia in relation to treatment with antidepressants: a survey of reports in the World Health Organization data base for spontaneous reporting of adverse drug reactions. Pharmacotherapy 1997;17:348-52.
- Movig KLL, Leufkens HGM, Lenderink AW, van den Akker VGA, Hodiamont PPG, Goldschmidt HMJ. Association between antidepressant drug use and hyponatraemia: a case-control study. Br J Clin Pharmacol 2002;53:363-9.
- van Walraven C, Mamdani MM, Wells PS, Williams JI. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ 2001;323:655-8.
- de Jong JCF, van den Berg PB, Tobi H, de Jong-van den Berg LTW. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol 2003;55:591-5.
- Dalton SO, Johansen C, Mellemkjaer L, Norgard B, Sorensen HT, Olsen JH. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Int Med 2003;163:59-64.
- Tata LJ, Fortun PJ, Hubbard RB, Smeeth L, Hawkey CJ, Smith CJ. Does concurrent prescription of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs substantially increase the risk of upper gastrointestinal bleeding?. Aliment Pharmacol Ther 2005;22:175-81.
- Hippisley-Cox J, Vinogradova Y, Coupland C, Pringle M. Comparison of key practice characteristics between general practices in England and Wales and general practices in the QResearch database. Nottingham: University of Nottingham; 2005.
- Hippisley-Cox J, Pringle M. Prevalence, Care and Outcomes for patients with diet controlled diabetes in general practice: cross-sectional survey. Lancet 2004;364:423-5.
- Gnani S, Majeed A. A user’s guide to data collected in primary care in England. Cambridge: Eastern Region Public Health Observatory, Cambridge; 2006.
- Hippisley-Cox J, Coupland C. Effect of combinations of drugs on all cause mortality in patients with ischaemic heart disease: nested case control analysis. BMJ 2005;330:1059-63.
- Hippisley-Cox J, Coupland C. Statins and all cause mortality in ischaemic heart disease: nested case control analysis. Heart 2006;92:752-58.
- Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients on Cox 2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case control analysis. BMJ 2005;366:1366-74.
- Barrett B, Byford S, Knapp M. Evidence of cost-effective treatments for depression: a systematic review. J Affect Disord 2005;84:1-13.
- Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. Am J Epidemiol 1996;143:1165-73.
- Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006;25:1768-97.
- Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase inhibitors or conventional non-steroidal anti-inflammatory drugs: population-based nested case-control analysis. BMJ 2005;331:1310-16.
- Kaye JA, Jick H. Epidemiology of lower limb fractures in general practice in the United Kingdom. Inj Prev 2004;10:368-74.
- Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case-series studies. Stat Med 2006;25:2618-31.
- Curtis L, Netten A. Unit costs of health and social care, PSSRU, 2006. London: Pharmaceutical Press; 2006.
- Drummond MF, Sculpher MJ, Torrance GW, O Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
List of abbreviations
- ADR
- adverse drug reaction
- BMI
- body mass index
- BNF
- British National Formulary
- CHD
- coronary heart disease
- CI
- confidence interval
- DDD
- defined daily dose
- EMIS
- Egton Medical Information Systems
- GI
- gastrointestinal
- GP
- general practitioner
- GPRD
- General Practice Research Database
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICD-9
- International Classification of Diseases, Ninth Revision
- ICD-10
- International Classification of Disease, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- MAOI
- monoamine oxidase inhibitor
- MI
- myocardial infarction
- NICE
- National Institute for Health and Clinical Excellence
- NSAID
- non-steroidal anti-inflammatory drug
- QALY
- quality-adjusted life-year
- RTA
- road traffic accident
- SD
- standard deviation
- SSRI
- selective serotonin reuptake inhibitor
- TCA
- tricyclic and related antidepressant
- TIA
- transient ischaemic attack
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington