Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 08/97/01. The protocol was agreed in August 2009. The assessment report began editorial review in May 2010 and was accepted for publication in December 2010. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Greenhalgh et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
‘Cardiovascular disease’ is an umbrella term that includes coronary heart disease, peripheral arterial disease and cerebrovascular disease. Cardiovascular disease is commonly caused by arteries becoming narrowed through atherosclerosis; it is the main cause of death in the UK, accounting for 35% of deaths each year (almost 198,000). 1 Almost half (48%) of all cardiovascular disease deaths are from coronary heart disease, with stroke making up a further quarter (28%). 1 In addition to being the main cause of death, cardiovascular disease is also the major cause of premature death (< 75 years) in the UK; cardiovascular disease caused 30% of premature death in men and 22% in women in 2006. 1
Occlusive vascular events such as myocardial infarction (MI), ischaemic stroke and transient ischaemic attack (TIA) are classified as subsets of cardiovascular disease. These events are the result of a reduction in blood flow associated with an artery becoming narrow or blocked through atherosclerosis and atherothrombosis. Patients with a history of such events have an increased risk of recurrence when compared with the general population. Peripheral arterial disease is also a subset of cardiovascular disease and is the result of narrowing of the arteries that supply blood to the muscles and other tissues, usually in the lower extremities. Patients with symptomatic peripheral arterial disease (typically intermittent claudication) are at increased risk of experiencing an initial occlusive vascular event. Given the nature of the health problem, some people have what is classified as multivascular disease, i.e. disease in more than one vascular bed, and appear to be at an even greater risk of death, MI or stroke than those with disease in a single bed. 2 Therefore, the primary objective in the treatment of all patients with a history of cardiovascular disease is to prevent the occurrence of new occlusive vascular events.
Aetiology, pathology and prognosis
As noted earlier, the cause of occlusive vascular events is a reduction in blood flow associated with an artery becoming narrow or blocked through atherosclerosis and atherothrombosis. Atherothrombosis involves the formation of a platelet-rich thrombus, frequently at the site of a disrupted atherosclerotic plaque, that leads to local occlusion or distal embolism. Atherosclerotic plaque formation occurs as a result of damage to vascular endothelium. Possible causes of damage include the following: elevated and modified low-density lipoproteins; free radicals caused by cigarette smoking, hypertension and diabetes mellitus; genetic alterations; and, combinations of these and other factors. 3
Epidemiology
The five manifestations of cardiovascular disease considered in this report are MI, ischaemic stroke, TIA, peripheral arterial disease and multivascular disease.
Myocardial infarction
Myocardial infarction (also known as a heart attack) is the interruption of the blood supply to the heart muscle. This is most commonly caused by occlusion of a coronary artery following the rupture of an atherosclerotic plaque. The resulting restriction in blood supply and oxygen starvation can cause damage to, or the death of, the heart muscle. Typical symptoms of MI include sudden chest pain with sweating or nausea, but MIs can also be symptomless. Women may experience different symptoms to men. Based on the results of changes in electrocardiogram (ECG) readings, MIs are classified into two subtypes: non-ST-segment elevated MI (NSTEMI) or ST-segment elevated MI (STEMI). The distinction has implications for future antiplatelet treatment. After a MI, a patient remains at high risk of a further MI or other occlusive vascular event.
Data from 2006 for the UK demonstrate that, across all ages, there were 146,000 cases of MI: 87,000 in men and 59,000 in women. 1 The incidence of MI varies across regions, between men and women, and increases with age. 1 Higher incidence rates are apparent in northern areas of the UK than in southern areas. In the UK, among men and women aged > 35 years, the prevalence is thought to be over 1.4 million. 1 Approximately 30% of people who experience an acute MI die before they reach hospital. 4 Patients who experience a MI and survive are likely to have a further cardiac event. 5
Ischaemic stroke
There are a number of different types of stroke; however, the majority of cases (approximately 70%) are ischaemic, caused through the blockage of an artery in the brain. 6 This leads to damage to, or death of, the brain cells due to lack of oxygen. The symptoms of stroke can include: numbness, weakness or lack of movement on one side of the body, slurred speech, difficulty finding words or understanding speech, problems with vision, confusion and/or severe headache. 7 A stroke happens suddenly and the effects are experienced straight away. 7 Anyone who suddenly has symptoms that might be caused by a stroke should be assessed as soon as possible using a test such as FAST (Face, Arm, Speech Test) and, on arrival at hospital, the ROSIER (Recognition of Stroke in the Emergency Room) test may be used. 7 A stroke may be classified as disabling or non-disabling.
The British Heart Foundation (BHF) reports that approximately 98,000 people experience a first ischaemic stroke every year in the UK, with little difference in rates between men and women but an increased risk with age. 8 Additionally, they estimate from 2006 data that, in the UK, as many as 1.1 million people have experienced a stroke; this is equivalent to a prevalence rate of 1.6% in the population in England and 2% in Wales. 8 The risk of recurrent stroke is greatest in the first 6 months following the initial event, but a patient may remain at greater risk of stroke than the general population for a number of years. 3 As many as 30% of strokes are thought to be recurrent. 9 Patients who have experienced a stroke are also at risk of further occlusive vascular events, including MI. 10,11
Transient ischaemic attack
A TIA is a disorder caused by temporary disturbance of blood supply to an area of the brain that results in a sudden but brief decrease (< 24 hours, usually < 1 hour) in brain functions and causes stroke-like symptoms. If the neurological deficit lasts more than 24 hours it is described as a stroke. Estimates for the UK indicate that between 46,000 and 65,000 people suffer a TIA each year and prevalence of TIA is projected to be 510,000. 8 In contrast with the trend noted in stroke data, there appear to be higher rates of TIA in women, and, as noted for stroke, incidence and prevalence rates increase rapidly with age. 8 Patients experiencing a TIA are at high risk of suffering a subsequent stroke, with 90-day risks of stroke reported to be as high as 10.5%. 12 In patients enrolled in clinical trials after a TIA or non-disabling ischaemic stroke, the annual risk of important vascular events (death from all vascular causes, non-fatal stroke or non-fatal MI) is reported as being between 4% and 11%; the corresponding estimate for population-based studies is 9% per year. 13
Peripheral arterial disease
Peripheral arterial disease is a condition in which the arteries that carry blood to the arms or legs become narrowed or congested, slowing or stopping the flow of blood. Data related to the prevalence of peripheral arterial disease vary. Detailed data from the USA indicate peripheral arterial disease rates of 4.3% in those < 40 years of age and 14.5% in those > 75 years. 14 Other reports estimate rates at 12–14% in the general population and 20% in those > 75 years of age. 15 The scoping document for this review indicated that, for the UK, approximately 20% of people aged from 55 to 75 years of age have evidence of lower extremity peripheral arterial disease and 5% of these appear to have symptoms, the most common of which is intermittent claudication (pain on walking). As the size of the UK population aged ≥ 55 years is approximately 17 million, this equates to a prevalence of around 170,000 with intermittent claudication. 16 It is thought that worldwide, and in the UK, peripheral arterial disease is underdiagnosed and undertreated. 17,18 Patients with peripheral arterial disease may experience significant pain and poor quality of life (QoL). 19 Over 5 years, about 20% of people with intermittent claudication have a non-fatal cardiovascular event (MI or stroke). 15 People with peripheral arterial disease, including those who are asymptomatic, have a high risk of death from MI and ischaemic stroke, their relative risks (RRs) being two to three times that of age- and gender-matched groups. 19 coronary heart disease is the major cause of death in people with peripheral arterial disease of the legs. 20
Although the diagnosis of peripheral arterial disease can generally be made from clinical history and examination, objective evidence of significant peripheral arterial disease can be made by obtaining an ankle–brachial pressure index. This index is the ratio of the ankle to brachial systolic pressure and may be measured using a sphygmomanometer and handheld Doppler device. 19 Obtaining an ankle–brachial pressure index is non-invasive and relatively easy, but is rarely used in clinical practice. 21
Multivascular disease
As noted earlier, there are a number of patients with cardiovascular disease who have disease in more than one vascular bed (otherwise known as multivascular disease patients). The REACH registry (supported by Sanofi–aventis, Bristol–Myers Squibb and the Waksman Foundation) collected data from approximately 67,888 patients who were recruited from 5473 physician practices in 44 countries worldwide. 17,22 Patients in the registry are described as being > 45 years old with least three atherothrombotic risk factors (e.g. treated diabetes mellitus, diabetic nephropathy, ankle–brachial index < 0.9, asymptomatic carotid stenosis of ≥ 70%) or documented cerebrovascular disease, coronary artery disease or peripheral arterial disease. A survey22 of data from the REduction of Atherothrombosis for Continual Health (REACH) registry identified that 15.9% of patients had symptomatic polyvascular disease, defined as coexistent symptomatic (clinically recognised) arterial disease in two or three territories (coronary, cerebral and/or peripheral). A further analysis indicated that rates of cardiovascular death, MI or stroke at 1 year increase substantially with the number of affected vascular beds. 2 This recognition of the importance of multivascular disease, problems with its definition, and its inherent increased risk of further events is explored in Chapter 3 (see Patients with multivascular disease).
Trends in coronary heart disease and stroke
Coronary heart disease causes over 90,000 deaths a year in the UK: approximately one in five deaths in men and one in six deaths in women. There is geographical variation in prevalence, with greater rates in the northern areas of England than in southern areas and intermediate rates in Wales. There are also social inequalities in mortality from coronary heart disease: higher mortality is noted in people from more deprived areas and those working in manual jobs. 1
Death rates from coronary heart disease have been declining since the late 1970s and death rates from stroke have declined in the last 10 years, although these trends appear to be plateauing, particularly in younger people. It is thought that the decline in rates of coronary heart disease is owing to reductions in risk factors (mainly smoking) and better treatment (including secondary prevention). Although mortality appears to be falling, coronary heart disease-related morbidity is rising. 1
Stroke accounts for around 53,000 deaths each year in the UK (approximately 9% of all deaths). According to the BHF8 it is not possible to know how many deaths each year are attributable to each stroke subtype. However, it reports that age-standardised mortality rates from stroke have decreased markedly in the last four decades, with a 90% reduction in ischaemic stroke mortality. 8 There is geographical variation in death rates from stroke in the UK; the highest rates are in Scotland, followed by northern England, Wales and Northern Ireland. The south of England (particularly London) exhibits the lowest stroke mortality rates. Socioeconomic inequalities in stroke mortality are evident; historically, rates have decreased more quickly in adults from higher social classes and mortality increases with deprivation. 8
The majority of people survive an initial stroke, but often have significant morbidity. 7 Stroke causes a greater range of disabilities than any other condition and has a greater disability impact than other chronic diseases. 23 It is thought that more than 900,000 people in England are living with the effects of stroke, with half of these being dependent on other people for help with everyday activities. 7
Impact of health problem
In 2006–7 there were 428,000 inpatient episodes for coronary heart disease in England and over 175,000 for stroke. 1,8 Data from 2006 underline the high cost of coronary heart disease and stroke to the UK health-care system: each cost around £3.2B. A cost per capita of just over £50 for each condition was observed. 1 Hospital care costs for coronary heart disease accounted for 73% of the total cost, whereas for stroke hospital costs accounted for 94%. 1
Production losses from death and illness and from informal care of people with coronary heart disease and cardiovascular disease are a substantial financial burden. 1 Data from 2006 for the UK demonstrate that production losses owing to mortality and morbidity associated with coronary heart disease cost over £3.9B: 65% owing to death and 35% owing to illness in those of working age. Informal care costs were approximately £1.8B. 1 For stroke, 65% of production losses were owing to illness and costs of informal care were £2.9M, reflecting the debilitating impact of stroke on individuals. 1
Current service provision
Management of disease
Secondary prevention of occlusive vascular events is antiplatelet therapy. Current recommendations from the National Institute for Health and Clinical Excellence (NICE) in Technology Appraisal No. 90 (TA90)24 for the secondary prevention of occlusive vascular events in patients with a history of ischaemic stroke or TIA state that modified-release dipyridamole (MRD) in combination with acetylsalicylic acid [ASA (aspirin)] should be used for a period of 2 years from the most recent event. Thereafter, or if MRD is not tolerated, standard care (including long-term, low-dose ASA) should be used. People with a history of occlusive vascular events (except TIA) or peripheral arterial disease who are intolerant to low-dose ASA are advised to use clopidogrel (clopidogrel) alone.
Owing to the evolving nature of treatments, and the different patient groups included in this review, a number of clinical recommendations are relevant. These are described in Table 1.
| Patient population | Guidance | Clinical recommendation | Trial evidence | Trial population | Licensed indication for drug |
|---|---|---|---|---|---|
| MI |
TA90 200524 (MTA) Clopidogrel and modified-release dipyridamole in the prevention of OVEs |
CLOP if ASA intolerant |
CAPRIE26 CLOP vs ASA |
33% MI 34% PAD 33% IS No differentiation between patients with NSTEMI and STEMI |
ASA: for the secondary prevention of thrombotic cerebrovascular or CVD CLOP: prevention of atherosclerotic events in people with a history of MI (from a few days until < 35 days), IS (from 7 days until < 6 months) or established PAD CLOP + ASA: For acute coronary syndromes |
| MI (NSTEMI) |
CG94 201025 (SR) Clopidogrel in the treatment of non-ST-segment elevation acute coronary syndrome |
CLOP + ASA for 12 months after the most recent event. Then standard care (including ASA) or clopidogrel if ASA intolerant |
CURE27 CLOP + ASA vs ASA |
100% | |
| MI (STEMI) |
CG48 200728 (SR) Secondary prevention in primary and secondary care for patients following a myocardial infarction |
CLOP + ASA for 4 weeks after the most recent event. Then standard care (including ASA) or CLOP if ASA intolerant |
COMMIT29 CLOP + ASA vs ASA |
93% STEMI 7% NSTEMI |
CLOP + ASA: for acute coronary syndromes |
| IS |
TA90 200524 (MTA) Clopidogrel and modified-release dipyridamole in the prevention of OVEs |
MRD + ASA for 2 years after the most recent event. Thereafter, or if MRD is not tolerated, standard care (including long-term treatment with low-dose ASA) |
ESPS-230 ASA vs MRD vs MRD + ASA vs placebo |
76% IS 24% TIA |
MRD (with or without ASA): secondary prevention of IS and TIA |
| TIA |
TA90 200524 (MTA) Clopidogrel and modified-release dipyridamole in the prevention of OVEs |
MRD + ASA for 2 years after the most recent event. Thereafter, or if MRD is not tolerated, standard care (including long-term treatment with low-dose ASA) | |||
| PAD |
TA90 200524 (MTA) Clopidogrel and modified-release dipyridamole in the prevention of OVEs |
CLOP if ASA intoleranta |
CAPRIE26 CLOP vs ASA |
33% MI 34% PAD 33% IS |
ASA: for the secondary prevention of thrombotic cerebrovascular or CVD CLOP: prevention of atherosclerotic events in people with a history of MI (from a few days until < 35 days), IS (from 7 days until < 6 months) or established PAD |
| MVD | Not currently included | N/A | N/A | N/A | N/A |
In addition to TA90,24 there are separate (and different) clinical recommendations for the two subtypes of MI: NSTEMI and STEMI. Clopidogrel plus ASA is the recommended treatment for both types, but for a period of 12 months following a NSTEMI25 and 4 weeks in the event of a STEMI. There is currently no guidance for the prevention of occlusive vascular events in patients with multivascular disease.
The purpose of the current review is to update the evidence base that was available to inform NICE’s TA90 guidance. 3,24 Patient groups who are beyond its remit include those who have had, or are at risk of, a stroke associated with atrial fibrillation or who require treatment to prevent occlusive vascular events after coronary revascularisation or carotid artery procedures.
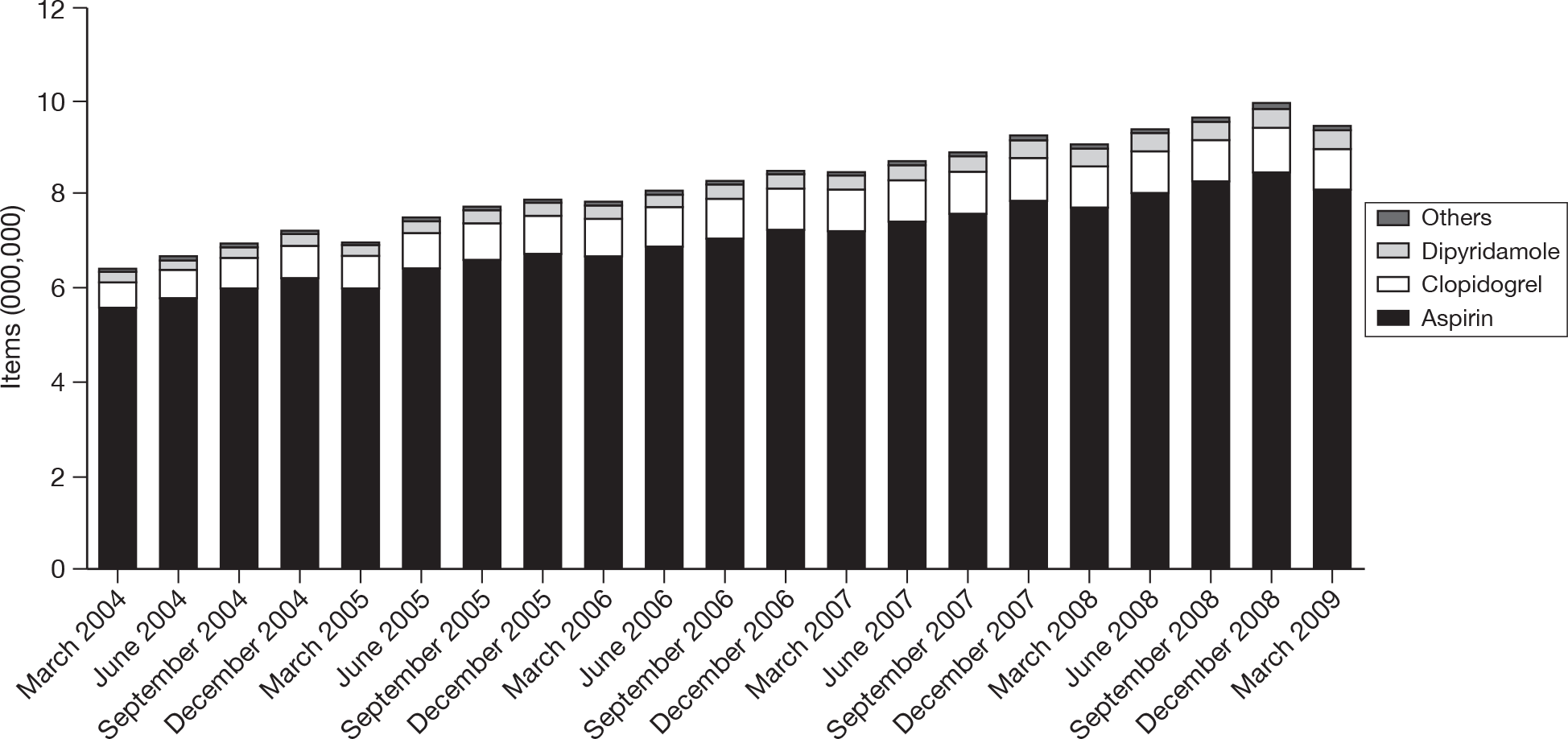
Although explicit data on provision of antiplatelet treatment for patients in the various disease categories are not available, general practitioner (GP) prescribing data for England from 2004 to 200931 indicate a slow and steady increase in prescribing rates over that time period (Figure 1).
FIGURE 1.
Trends in prescribing of antiplatelet drugs in general practice in England. Reproduced with permission from the NHS Business Services Authority (NHSBSA).
Current service cost
The current prices for ASA, MRD and clopidogrel are shown in Table 2. All prices are net and are taken from the British National Formulary (BNF) No. 58. 32 Generic versions of clopidogrel are now licensed; from 1 April 2010, clopidogrel is listed as category M of Part VIII of the Drug Tariff, meaning that pharmacists will be reimbursed at the generic price of £10.90 for 30 tablets of 75 mg clopidogrel. 33,34
| Drug | Price per pack | Price per day (£) |
|---|---|---|
| ASA (75-mg) tablets | £0.94 per 28, £1.07 per 56 | 0.033, 0.019 |
| MRD + ASA dipyridamole (200 mg), ASA (25 mg) | £7.79 per 60 | 0.26 (b.i.d.) |
| MRD (200 mg) | £7.50 per 60 | 0.25 (b.i.d.) |
| CLOP (Plavix) (75 mg) | £36.35 per 30 | 1.21 |
| CLOP (generic) | £10.90 per 30 | 0.36 |
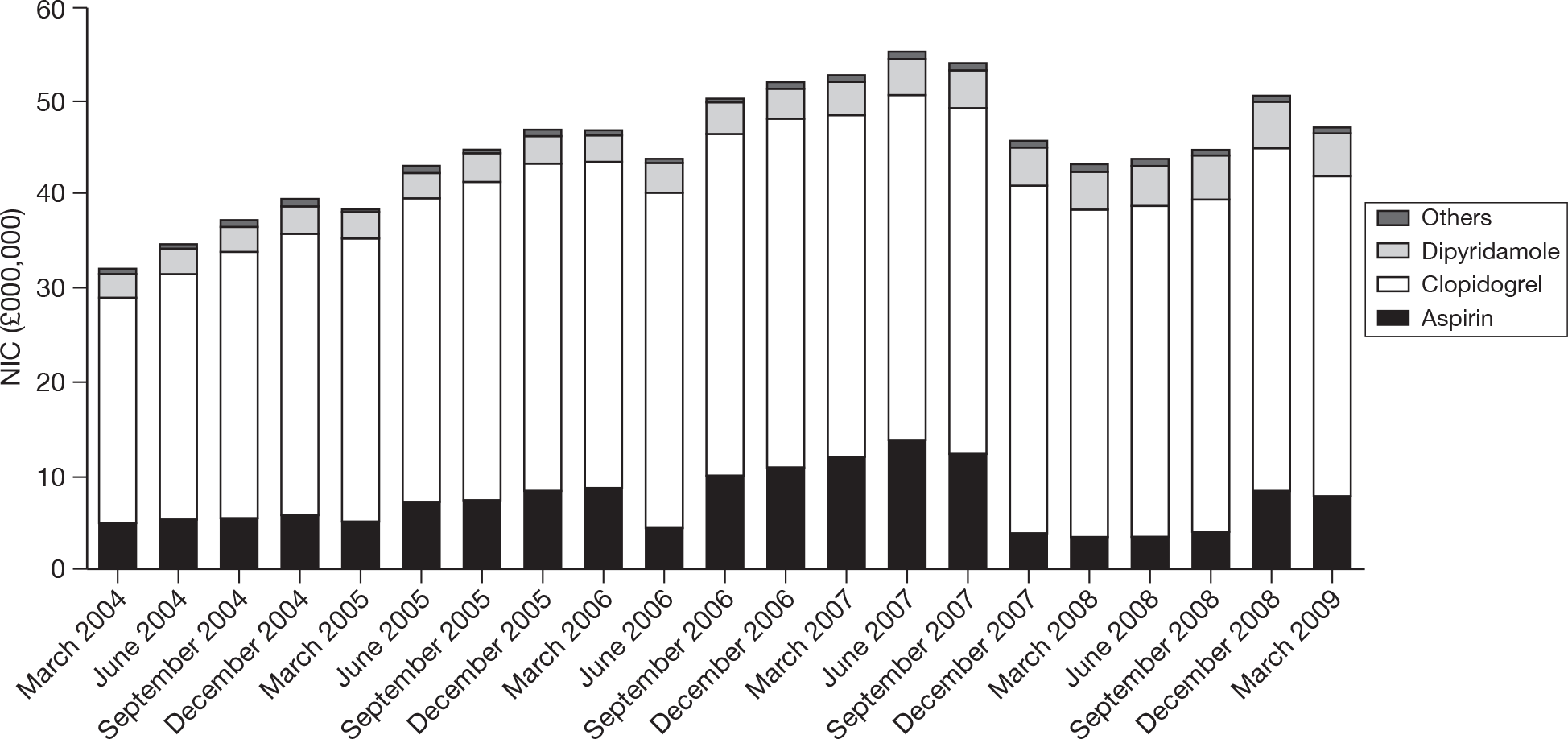
In Figure 2, trends in spending on the various agents prescribed by GPs in England over the period of 2004–9 are shown. 31
FIGURE 2.
Trends in spending on antiplatelet drugs in general practice in England. Reproduced with permission from the NHSBSA. NIC, net ingredient cost.
Variation in services and/or uncertainty about cost
The recent end of patent term for clopidogrel has meant that a number of generic formulations of the drug have been approved by the European Medicines Agency35 and the Medicines and Healthcare products Regulatory Agency (MHRA). 36 At the time of writing, there are at least eight generic products available in the UK, as listed in Table 3. All of those listed are licensed for the prevention of atherothrombotic events in patients suffering from MI (from a few days until < 35 days), ischaemic stroke (from 7 days until < 6 months) or established peripheral arterial disease. It is currently unclear (because of issues relating to patent) whether or not any of these products may also be used in combination with ASA for the treatment of patients with acute coronary syndromes.
Relevant national guidelines including National Service Frameworks
The design of guidelines and National Service Frameworks (NSFs) is based on overall national goals and targets. The government target for England (set in 1999 and 2004) for cardiovascular disease was to reduce the death rate from coronary heart disease, stroke and related diseases in people aged ≤ 75 years by at least two-fifths by 2010, saving up to 200,000 lives in total, with a milestone of a reduction of one-quarter by 2005. 37,38 A further target was to reduce the inequalities gap in death rates from these diseases between the fifth of areas with the worst health and deprivation indicators and the population as a whole in people aged ≤ 75 years by 40% by 2010.
The Welsh Assembly Government39,40 set its target for coronary heart disease as a reduction in mortality rates in 65- to 74-year-olds from 600 per 100,000 in 2002 to 400 per 100,00 in 2012. Its health inequality target is to improve coronary heart disease mortality in all groups and at the same time aim for a more rapid improvement in the most deprived groups. The target for stroke is to reduce mortality in people aged 65–74 years by 20% by 2012.
New GP contracts include points for the number of coronary heart disease and stroke patients who are taking antiplatelet therapy for secondary prevention of occlusive vascular events. 41 The contract does not appear to include patients with peripheral arterial disease. 42
Therefore, the use of antiplatelet agents is the focus of a number of national documents including the National Service Framework43,44 and NICE guidance documents. 25,45 The nature of multivascular disease means that at times these documents apply to overlapping patient populations.
The National Service Framework (NSF) for Coronary Heart Disease: Standards and Quality Requirements (England) states that GPs and primary care teams should identify all patients with established cardiovascular disease and offer them comprehensive advice and appropriate treatment to reduce their risks of coronary heart disease. 43,44
The National Stroke Strategy: Ten Point Plan for Action for England states that in preventing stroke, support for healthier lifestyles should be offered and action to tackle vascular risk taken. 46
As part of the Diabetes, Heart Disease and Stroke (DHDS) prevention project, the UK National Screening Committee commissioned the Handbook of vascular risk assessment, risk reduction and risk management. 47 The handbook is designed to support local health services in meeting the standards for the prevention and early detection of coronary heart disease, set out in the NSF for England. The target population for screening is people aged between 40 and 75 years. The handbook describes the context and outlines evidence for a coordinated vascular disease control programme to identify and reduce risks of cardiovascular disease in the general population; suggest aims, objectives and a delivery strategy framework appropriate for a cardiovascular disease risk management programme; report key messages from the DHDS pilot project; and provide examples of tools, resources and standard operating procedures that can be used by health professionals. 47
Description of technology under assessment
Two antiplatelet agents, used within their licensed indications, are the focus of this review: clopidogrel® (Plavix®, Bristol–Myers Squibb, Sanofi–aventis) and MRD + ASA in a single capsule (Asasantin Retard®, Boehringer Ingelheim) or MRD alone (Persantin Retard®, Boehringer Ingelheim). Clopidogrel produces an immediate and sustained inhibition of ADP-induced platelet aggregation that helps prevent blood clots. 48 Dipyridamole is thought to inhibit adenosine (a potent inhibitor of platelet activation and aggregation) uptake into blood and vascular cells. 3 Summaries of product characteristics for clopidogrel, MRD + ASA and MRD alone are available from the Electronic Medicines Compendium. 49
Clopidogrel
Clopidogrel is licensed in adults for the prevention of atherothrombotic events in patients suffering from MI (from a few days to 35 days), ischaemic stroke (from 7 days to 6 months) or established peripheral arterial disease. Clopidogrel is available as 75 or 300 mg film-coated tablets. The recommended dose is 75 mg as a single daily dose, taken with or without food. As previously noted, generic versions of clopidogrel are now available (see Table 3), although it is currently unclear whether or not any of these generic versions are licensed for prescribing with ASA for the treatment of acute coronary syndromes.
| Name of manufacturer | Licensed name | Active ingredient |
|---|---|---|
| Mylan Pharmaceuticals/Generics UK | Clopidogrel Mylan | Clopidogrel hydrochloride |
| Consilient Health Ltd | Clopidogrel Consilient | |
| Sandoz Ltd | Clopidogrel Sandoz | Clopidogrel besilate |
| Actavis Group PTC EHF | Actavis clopidogrel | |
| Arrow Generics | Arrow clopidogrel | |
| Dr Reddy’s Laboratories (UK) Ltd | Dr Reddy’s clopidogrel | |
| Dexcel Pharma Ltd | Dexcel clopidogrel | |
| Beacon Pharmaceuticals | Beacon clopidogrel (Grepid®) |
Contraindications for clopidogrel include hypersensitivity to the active substance or to any of the excipients, severe liver impairment and active pathological bleeding (such as a peptic ulcer or intracranial haemorrhage). Special warnings for clopidogrel use include (but are not limited to) the following:
-
use with caution in combination with any other anticoagulant or antiplatelet drug or in patients with bleeding diathesis
-
thrombotic thrombocytopenic purpura has been reported very rarely following the use of clopidogrel, sometimes after a short exposure.
Based on literature data, patients with genetically reduced CYP2C19 function have lower systemic exposure to the active metabolite of clopidogrel and diminished antiplatelet responses, and generally exhibit higher cardiovascular event rates after MI than do patients with normal CYP2C19 function. As clopidogrel is metabolised to its active metabolite partly by CYP2C19, use of drugs that inhibit the activity of this enzyme would be expected to result in reduced drug levels of the active metabolite of clopidogrel and a reduction in clinical efficacy. Concomitant use of drugs that inhibit CYP2C19 should be discouraged. Although the evidence of CYP2C19 inhibition varies within the class of proton pump inhibitors (PPIs), clinical studies suggest an interaction between clopidogrel and possibly all members of this class. Therefore, concomitant use of PPIs should be avoided unless absolutely necessary. The Assessment Group is aware that new evidence has led to a new recommendation from the European Medicines Agency50 that only two specific PPIs (omeprazole and esomeprazole) are a problem (see below).
Important subgroups of patients
Clopidogrel is not licensed for secondary prevention of occlusive vascular events in patients who have experienced a TIA, although in UK clinical practice it may be prescribed for these patients if they are unable to tolerate MRD or ASA (Dr Anil Sharma, Aintree Hospitals NHS Trust, 17 March 2010, personal communication).
There is evidence that two PPIs (omeprazole and esomeprazole) reduce the effectiveness of clopidogrel in preventing the recurrence of adverse cardiac events; current advice is that concomitant use of these with clopidogrel should be discouraged. In addition, the concomitant use of other known CYP2C19-inhibiting medicines with clopidogrel is discouraged because these are expected to have a similar effect to omeprazole and esomeprazole. 50
Modified-release dipyridamole
A non-modified-release (often referred to as immediate release) version of dipyridamole is available; however, only the evidence for MRD is considered in this review. MRD is often also referred to as an ‘extended-release dipyridamole’. For clarity, this review will use the term MRD throughout.
Modified-release dipyridamole (alone or with ASA) is licensed for use in adults for the secondary prevention of ischaemic stroke and TIA. It is available in two preparations:
-
Asasantin Retard® (Boehringer Ingelheim) capsules containing both dipyridamole (200 mg) and ASA (25 mg).
-
Persantin Retard® (Boehringer Ingelheim) capsules containing dipyridamole (200 mg).
The recommended dose of MRD is 200 mg twice daily. Capsules should be taken in the morning and again in the evening, preferably with meals.
Contraindications for Asasantin Retard® include hypersensitivity to any component of the product or salicylates, patients with active gastric or duodenal ulcers, and patients in the last trimester of pregnancy. Special warnings and precautions for use include (but are not limited to):
-
Asasantin® should be used with caution in patients with an increased risk of bleeding and such patients should be followed carefully for any signs of bleeding.
-
Caution should be advised in patients receiving concomitant medication that may increase the risk of bleeding.
-
Headache that may occur at the beginning of treatment should not be treated with analgesic doses of ASA.
-
Among other properties, dipyridamole acts as a vasodilator. It should be used with caution in patients with severe coronary artery disease, including unstable angina or recent MI, left ventricular flow obstruction or haemodynamic instability.
-
Owing to the ASA component, all appropriate cautions applicable to ASA should also be observed.
Contraindications for Persantin Retard® are limited to hypersensitivity to any component of the product. The same cautions should be observed as for Asasantin Retard® (with the exception of those related to the ASA content).
Chapter 2 Definition of the decision problem
Decision problem
The remit of this appraisal is to review and update (if necessary) the clinical effectiveness and cost-effectiveness evidence base described in TA90. 24 Table 4 shows the key elements of the decision problem of the appraisal.
| Interventions | Clopidogrel |
| MRD used alone or in combination with ASA | |
| Patient population | For clopidogrel, adults with established PAD or those with a history of MI or IS |
| For MRD, adults with a history of IS or TIA | |
| Comparators | The interventions will be compared with ASA and, where appropriate, with each other |
| Outcomes | Any of the following:
|
| Other considerations |
If the evidence allows, the effectiveness of clopidogrel in people with MVD who are considered to be at high risk of recurrent occlusive vascular events will be considered If the evidence allows, the duration of treatment with the specified interventions will be considered |
The key elements of this appraisal are similar to those that underpin the previous review,3 with the following exceptions: patients with a history of TIA will not be considered in the assessment of the effectiveness of clopidogrel, as clopidogrel is not licensed for this patient group; MI will be divided into STEMI and NSTEMI; and unstable angina has replaced ‘other vascular events’.
Overall aims and objectives of assessment
The purpose of the review is to assess the clinical effectiveness and cost-effectiveness evidence describing the use of clopidogrel and MRD (plus ASA or alone) in the prevention of occlusive vascular events in patients with history of MI, ischaemic stroke or TIA, or established peripheral arterial disease. Evidence relevant to the effectiveness of clopidogrel in patients with multivascular disease will also be considered. This review is an update and focuses on relevant clinical effectiveness and cost-effectiveness evidence that has become available since publication of TA90. 24
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Methods for reviewing clinical effectiveness and cost-effectiveness evidence are described in this chapter.
Search strategies
This review is an update of an existing review. 3 Consequently, the start date for searches of electronic databases is 2003. In addition to searching the two manufacturer’s submissions51,52 for relevant references, the following databases were searched for trials of clopidogrel and MRD:
-
EMBASE (2003–9 week 36)
-
MEDLINE (2003–9 August week 4)
-
Web of Science (2003–9)
-
The Cochrane Library (2003–9, Issue 3).
The results were entered into an Endnote X2 (Thomas Reuters, CA, USA) library and the references were de-duplicated. Full details of the search strategies are presented in Appendix 1.
Inclusion and exclusion criteria
Two reviewers (JG/RD) independently screened all titles and abstracts. Full paper manuscripts of any titles/abstracts that were considered relevant by either reviewer were obtained where possible. The relevance of each study was assessed (JG/JO) according to the criteria set out below. Studies that did not meet the criteria were excluded and their bibliographic details were listed alongside reasons for their exclusion. These are listed in Appendix 5. Any discrepancies were resolved by consensus and, where necessary, a third reviewer was consulted.
Study design
Only randomised controlled trials (RCTs) were included in the assessment of clinical effectiveness. Full economic evaluations were included in the assessment of cost-effectiveness.
The Assessment Group also identified and assessed the quality of existing systematic reviews (SRs) in order to cross-check for the identification of additional studies, as well as to gain an understanding of the issues related to the combining of data in this complex area. A summary and critique of relevant SRs is presented in Appendix 3.
Interventions and comparators
The effectiveness of two antiplatelet agents, used within their licensed indications, was assessed (1) clopidogrel alone and (2) MRD alone or in combination with ASA. Studies that compared clopidogrel alone or MRD (alone or in combination with ASA) with ASA or, where appropriate, with each other were included in the review. Trials in which clopidogrel was used as an adjunct to percutaneous coronary intervention were excluded from the review. Trials in which clopidogrel was combined with ASA were also excluded, as they were not within the remit of the scope. 16
Patient populations
For clopidogrel, patients with a history of MI, ischaemic stroke or established peripheral arterial disease were included. Patients with acute coronary syndromes were not included, and neither were those with atrial fibrillation. For MRD, patients with a history of ischaemic stroke or TIA were included.
Outcomes
Data on any of the following outcomes were included in the assessment of clinical effectiveness: MI, stroke, TIA, death and adverse events including bleeding complications. No data relating to health-related quality of life (HRQoL) or unstable angina were identified. For the assessment of cost-effectiveness, outcomes included incremental cost per life-years gained and incremental cost per quality-adjusted life-year (QALY) gained.
Data extraction strategy
Data relating to both study design and quality were extracted by two reviewers (JO/MB) into an Excel 2007 (Excel Software, Henderson, NV, USA) spreadsheet. The two reviewers cross-checked each other’s extraction and a third independent reviewer (YD) checked for accuracy and was consulted in cases of disagreement. Where multiple publications of the same study were identified, data were extracted and reported as a single study.
Quality assessment strategy
The quality of clinical effectiveness studies was assessed by two reviewers (MB/JO) and checked by a third reviewer (YD) according to criteria based on the NHS Centre for Reviews and Dissemination (CRD) Report 4. 53 The quality of the cost-effectiveness studies was assessed by two reviewers (CMS/AB) according to a checklist updated from that developed by Drummond and Jefferson. 54 All relevant information is tabulated and summarised within the text of the report. Full details and results of the quality assessment strategy for clinical effectiveness and cost-effectiveness studies are reported in Appendix 2.
Methods of data synthesis
Direct evidence
The results of (1) clinical and (2) economic data extraction and quality assessment are summarised in structured tables and as a narrative description. The decision problem of interest to this review was made up of the following comparisons: (1) clopidogrel versus ASA; (2) clopidogrel versus MRD alone; (3) clopidogrel versus MRD + ASA; (4) MRD + ASA versus ASA; and (5) MRD alone versus ASA.
Indirect evidence
Owing to the differences between trials in terms of interventions and comparators, indirect analysis (using a mixed-treatment comparison methodology) was performed on a variety of outcomes. The methods and results of the mixed-treatment comparisons are reported below (see Methods for indirect synthesis).
Additional analysis by the Assessment Group
Using data provided by the manufacturers of clopidogrel, the Assessment Group undertook subgroup analysis and explored the clinical effectiveness of clopidogrel in patients with multivascular disease. The Assessment Group was also able to explore whether or not key outcome events are distributed evenly across the whole period of trial follow-up or if there are particular time points when patients appear to be at a greater risk.
Results
Quantity and quality of research available
A total of 4576 titles and abstracts were screened for inclusion in the review of clinical effectiveness and cost-effectiveness evidence. The process of study selection is shown in Figure 3. 55 The flow chart shows that the two studies identified in our updated searches were added to the two already identified in TA90. 24
FIGURE 3.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart.

Clinical effectiveness (randomised controlled trials)
Four RCTs – CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events),26 ESPS-2 (Second European Stroke Prevention Study),30 ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)56 and PRoFESS (Prevention Regimen For Effectively avoiding Second Strokes)57 – were reported in 28 publications and met the inclusion criteria for this review. These included the two trials26,30 (reported in 20 publications) that were used to inform the previous guidance. 24 The reference provided in the text refers to the primary report and any subsequent publications describing outcomes of the trials are listed by trial in Appendix 4.
The identified trials are summarised in Table 5. We did not include trials in which clopidogrel was combined with ASA, as only clopidogrel alone was specified as an intervention or comparator in the scope issued by NICE. 16 This means that both MATCH (Management of ATherothrombosis with Clopidogrel in High-risk patients)58 and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilisation Management and Avoidance)59 trials are excluded from the review. A full list of publications excluded following the application of the inclusion criteria is presented in Appendix 5.
| Trial | Study design | Patients | Comparators |
|---|---|---|---|
| CAPRIE26 1996 | Double-blind, placebo-controlled trial | 19,185 patients with atherosclerotic vascular diseases manifested as either IS, MI or symptomatic PAD | CLOP (75 mg/day) vs ASA (325 mg/day) |
| ESPS-230 1996 | Double-blind, placebo-controlled trial (2 × 2 factorial) | 6602 patients with prior stroke or TIA | ASA (50 mg/day) vs MRD (400 mg/day) vs ASA (50 mg/day) + MRD (400 mg/day) vs placebo |
| ESPRIT56 2006 | Open-label trial | 2736 patients with prior TIA or strokea | ASA (30–325 mg/day) vs MRD (400 mg/day) + ASA |
| PRoFESS57 (2008) | Double-blind trial | 20,332 patients with prior stroke | MRD (400 mg/day) + ASA (50 mg/day) vs CLOP (75 mg/day) |
In addition, six ongoing trials were identified; these are described in Appendix 6. Limited detail is available relating to these studies and they are not considered in this review. However, it is worthy of note that the majority of the ongoing trials include clopidogrel + ASA as a comparator.
Quality assessment of included randomised controlled trials
All of the included RCTs were of good quality (see Appendix 2). Robust randomisation procedures were used and baseline comparability between treatment groups was achieved. The use of blinding procedures was reported where appropriate and intention-to-treat (ITT) analyses were conducted for each trial. There was no evidence of selective reporting of outcomes in any of the trials.
Trial characteristics
The key characteristics of the included trials are summarised in Table 6. Of the four trials, three were double blind and one was an open-label study (ESPRIT56). The majority of trials were conducted globally, whereas the participating centres in ESPS-230 were located only in Europe. All trials included patients with ischaemic stroke as a qualifying event and two included patients with a qualifying event of TIA. 30,56 Only CAPRIE26 included patients with MI or peripheral arterial disease. The trial sizes ranged from 2763 to 20,332. Mean length of follow-up ranged between 1.91 and 3.5 years. Three trials were industry funded, while ESPRIT56 was funded from a variety of non-industry sources. Two trials (CAPRIE26 and ESPRIT56) utilised a composite as a primary end point, the components of which differed between the trials. In ESPS-2,30 three discrete primary end points were reported, while PRoFESS57 reported on a single primary end point of recurrent stroke. Across the four trials, ASA dosage ranged from 50 mg per day (ESPS-230 and PRoFESS57) to 30–325 mg per day in ESPRIT56 and 325 mg per day in CAPRIE. 26
| Trial name and comparators | Study design | No. of patients (N), location | Qualifying events, no. patients (n) | Follow-up (mean) | Trial support | Outcomes |
|---|---|---|---|---|---|---|
|
CAPRIE26 1996 CLOP (75 mg) vs ASA (325mg) |
Double-blind, placebo-controlled |
N = 19,185 Austria, Australia, Canada, Belgium, France, Finland, Germany, Italy, the Netherlands, New Zealand, Portugal, Spain, Sweden, Switzerland, the UK and the USA |
IS (n = 6431) MI (n = 6302) PAD (n = 6452) |
1.91 years (range 1–3 years) | Sanofi–aventis and Bristol–Myers Squib |
Primary First occurrence of IS, MI or vascular death Secondary First occurrence of IS, MI, amputation or vascular death; vascular death; overall net benefit: any stroke (includes primary intracranial haemorrhage), MI or death from any cause; death from any cause |
|
ESPS-230 1996 ASA (50 mg) vs MRD vs ASA (50 mg) MRD + ASA vs placebo |
Double-blind, placebo-controlled (2 × 2 factorial) |
N = 6602 Austria, Belgium, France, Germany, Ireland, Italy, the Netherlands, Norway, Portugal, Spain, Sweden, Switzerland and the UK |
TIA (n = 1562) IS (n = 5038) |
2 years | Boehringer Ingelheim |
Primary Stroke; all-cause death; stroke and/or all-cause death Secondary TIA; MI; IS events (stroke and/or MI, and/or sudden death of thrombotic origin); other vascular events (pulmonary embolism, deep venous thrombosis, peripheral arterial occlusion, venous retinal thrombosis or combination of these events) |
|
ESPRIT56 2006 ASA (30–325 mg) vs MRD + ASAa (30–325 mg) |
Open-label |
N = 2736 Austria, Belgium, France, Germany, Italy, the Netherlands, Portugal, Spain, Sweden, Switzerland, the UK, Australia, China, Singapore and the USA |
TIA (n = 920) Minor IS (n = 1816) |
3.5 years (SD 2.0) |
Council of Singapore, European Commission; UK Stroke Association; French Ministry of Health Netherlands: Janivo Foundation, AEGON N V; Heart Foundation; Thrombosis Foundation; University Medical Center Utrecht |
Primary First occurrence of death from all vascular causes, non-fatal stroke, non-fatal MI or major bleeding complication Secondary Death from all causes; death from all vascular causes and non-fatal stroke; all major ischaemic events (non-haemorrhagic death from vascular causes, non-fatal IS or non-fatal MI); all vascular events (death from vascular causes, non-fatal stroke or non-fatal MI); major bleeding complications |
|
MRD + ASA (50 mg) vs CLOP (75 mg) |
Double-blind, non-inferiority |
N = 20,332 Argentina, Australia, Austria, Belgium, Brazil, Canada, China, Denmark, Finland, France, Germany, Greece, Hong Kong, India, Ireland, Israel, Italy, Japan, Malaysia, Mexico, the Netherlands, Norway, Portugal, Russian Federation, Singapore, South Africa, Republic of Korea, Spain, Sweden, Taiwan, Thailand, Turkey, Ukraine, the UK and the USA |
Recent IS (n = 20,332) | 2.5 years (range 1.5–4.4 years) | Boehringer Ingelheim. In selected countries also supported by Bayer Schering Pharma and GlaxoSmithKline |
Primary Recurrent stroke of any type Secondary Vascular events; first occurrence of stroke (non-fatal or fatal) or MI (non-fatal or fatal) or vascular death; first occurrence of stroke or major haemorrhagic event; death: IS, haemorrhagic stroke, stroke of uncertain cause, MI, haemorrhage excluding intracranial bleeding, other vascular causes, non-vascular causes; life-threatening or non-life-threatening major haemorrhagic events; other designated vascular events; pulmonary embolism or retinal vascular accidents or deep-vein thrombosis or peripheral arterial occlusion or TIA |
Patient characteristics
The key characteristics of patients in the included trials are summarised in Table 7. The mean age of the patients was similar across trials. The percentage of males appears to be greatest in CAPRIE. 26 The PRoFESS57 trial included the greatest proportion of patients with hypertension and diabetes mellitus. None of the trials characterised the patient population in terms of the number of affected vascular beds, so the number of patients per trial with multivascular disease is unknown. However, the history of vascular events for the whole cohort of patients is reported for each trial; these are described in the right-hand column of Table 7. Compared with the other trials, in ESPS-230 there was a higher percentage of patients with peripheral arterial disease in addition to the qualifying event of ischaemic stroke/TIA. With the exception of CAPRIE,26 the modified Rankin Scale60 was used as a measure of patient disability; this scale is widely used as an outcome measure for stroke in clinical trials. The scale ranges from 0 to 6, where ‘0’ indicates no disability and ‘6’ is death. All patients in ESPRIT56 were rated as between 0 and 3, with 43% having no disability.
| Trial name/comparators | Mean age (SD) | Gender (male) (%) | Modified Rankin Scale status (%) | Other factors (%) | Percentage of patients with history of vascular events |
|---|---|---|---|---|---|
|
CAPRIE26 (CLOP vs ASA) |
62.5 years (11.1) | 72 | NS |
Current smoker: 29.5 Ex-smoker: 49 Hypertension: 51.5 DM: 20 |
MI: 16.5 IS: 9 Intermittent claudication: 4.5 TIA/RIND: 10 |
|
ESPS-230 (ASA vs MRD vs MRD + ASA vs placebo) |
66.7 years | 58 |
0 + 1 + 2 = 69.1 3 = 14.2 4 + 5 = 16.6 |
Current smoker: 24 Hypertension: 60.5 DM: 15.3 |
PAD: 22 |
|
ESPRIT56 (ASA vs MRD + ASA) |
63 years (11) | 66 |
0 = 43 1 = 33 2 = 18 3 = 6 |
Current smoker: 36.5 Hypertension: 59.5 DM: 18.5 |
MI: 7 Intermittent claudication: 5 Stroke: 11.5 |
|
PRoFESS57 (MRD + ASA vs CLOP) |
66.1 years (8.6) | 64 |
0 = 14 1 = 37 2 = 25 3 = 14 4 + 5 = 9 |
Current smoker: 21 Ex-smoker: 36 Never smoker: 42.6 Hypertension: 74 DM: 28 |
MI: 7 TIA: 8.7 PAD: 3 Stroke: 18.25 |
CAPRIE26
The key outcomes of the CAPRIE26 trial are described in Table 8. For the whole trial population, statistically significant outcomes in favour of clopidogrel were noted for the primary outcome (first occurrence of ischaemic stroke, MI or vascular death). The relative risk reduction (RRR) was 8.7% in favour of clopidogrel [95% confidence interval (CI) 0.3% to 16.5%; p = 0.043]. It has been noted,3 elsewhere, that the point estimate favoured clopidogrel, but this benefit appeared to be very small; the boundaries of the CIs raise the possibility that clopidogrel is not more beneficial than ASA. A statistically significant risk reduction (23.8%) in favour of clopidogrel was reported for the subgroup of patients with peripheral arterial disease (95% CI 8.9% to 36.2%; p = 0.0028); however, the trial was not powered to detect differences between patient subgroups and so the finding should be interpreted with caution. No statistically significant differences between clopidogrel and ASA were noted for the subgroup of patients with ischaemic stroke or MI.
| Outcomes | Event rate per year | RRR, % (95% CI) | |
|---|---|---|---|
| CLOP (%) | ASA (%) | ||
| Primary | |||
| First occurrence of IS, MI or vascular death | All patients: 5.32 | All patients: 5.83 | All patients: 8.7 (0.3 to 16.5); p = 0.043 |
| Stroke subgroup: 7.15 | Stroke subgroup: 7.71 | Stroke subgroup: 7.3 (–5.7 to 18.7); p = 0.26 | |
| MI subgroup: 5.03 | MI subgroup: 4.84 | MI subgroup: –3.7 (–22.1 to 12); p = 0.66 | |
| PAD subgroup: 3.71 | PAD subgroup: 4.86 | PAD subgroup: 23.8 (8.9 to 36.2); p = 0.0028 | |
| Secondary | |||
| First occurrence of IS, MI, amputation or vascular death | All patients: 5.56 | All patients: 6.01 | All patients: 7.6 (–0.8 to 15.3); p = 0.076 |
| Vascular death | All patients: 1.90 | All patients: 2.06 | All patients: 7.6 (–6.9 to 20.1); p = 0.29 |
| Overall net benefita | All patients: 6.43 | All patients: 6.90 | All patients: 7.0 (–0.9 to 14.2); p = 0.081 |
| Death from any cause | All patients: 3.05 | All patients: 3.11 | All patients: 2.2 (–9.9 to 12.9); p = 0.71 |
ESPS-230
Table 9 shows the key outcomes of ESPS-2. 3,30 For the first primary outcome of stroke, statistically significant differences in favour of MRD + ASA were observed for two comparisons: MRD + ASA vs ASA [relative risk (RR) 0.76; 95% CI 0.63 to 0.93] and MRD + ASA vs MRD alone (RR 0.75; 95% CI 0.61 to 0.91). No difference was observed for the MRD-versus-ASA comparison. No other primary outcome (all-cause death, stroke and/or all-cause death) showed statistically significant differences between any two treatment arms.
| Outcomes | Total events | RR (95% CI) | ||
|---|---|---|---|---|
| MRD, n (%) | MRD + ASA, n (%) | ASA, n (%) | ||
| Primary | ||||
| MRD + ASA vs ASA | ||||
| Stroke | 157 (9.5) | 206 (12.5) | 0.76 (0.63 to 0.93) | |
| Stroke and/or death | 286 (17.3) | 330 (20.0) | 0.87 (0.75 to 1.00) | |
| All-cause death | 185 (11.2) | 182 (11.0) | 1.02 (0.84 to 1.23) | |
| MRD + ASA v MRD | ||||
| Stroke | 211 (12.8) | 157 (9.5) | 0.75 (0.61 to 0.91) | |
| Stroke and/or death | 321 (19.4) | 286 (17.3) | 0.89 (0.77 to 1.03) | |
| All-cause death | 188 (11.4) | 185 (11.2) | 0.99 (0.81 to 1.19) | |
| MRD vs ASA | ||||
| Stroke | 211 (12.8) | 206 (12.5) | 1.02 (0.85 to 1.22) | |
| Stroke and/or death | 321 (19.4) | 330 (20) | 0.97 (0.85 to 1.11) | |
| All-cause death | 188 (11.4) | 182 (11.37) | 1.03 (0.85 to 1.25) | |
| Secondary | ||||
| MRD + ASA v ASA | ||||
| TIA | 172 (10.4) | 206 (12.5) | 0.83 (0.69 to 1.01) | |
| Stroke/TIA | 18.1 | 22.6 | 0.80 (0.70 to 0.92) | |
| MI | 35 (2.1) | 39 (2.4) | 0.90 (0.57 to 1.41) | |
| Other vascular event | 21 (1.3) | 38 (2.3) | 0.55 (0.33 to 0.94) | |
| Ischaemic eventsa | 206 (12.5) | 307 (16.1) | 0.77 (0.65 to 0.92) | |
| Vascular death | (7.1) | (7.2) | 0.99 (0.77 to 1.27) | |
| Vascular events | (14.9) | (19.0) | 0.78 (0.67 to 0.91) | |
| MRD + ASA v MRD | ||||
| TIA | 215 (13.0) | 172 (10.4) | 0.80 (0.66 to 0.97) | |
| Stroke/TIA | (23.1) | (18.1) | 0.78 (0.69 to 0.90) | |
| MI | 48 (2.9) | 35 (2.1) | 0.73 (0.48 to 1.12) | |
| Other vascular event | 35 (2.1) | 21 (1.3) | 0.60 (0.35 to 1.03) | |
| Ischaemic eventsa | 271 (16.4) | 206 (12.5) | 0.76 (0.64 to 0.90) | |
| Vascular death | (7.6) | (7.1) | 0.94 (0.74 to 1.20) | |
| MRD vs ASA | ||||
| TIA | 215 (3.0) | 206 (12.5) | 1.04 (0.87 to 1.24) | |
| Stroke/TIA | (23.1) | (22.6) | 1.02 (0.90 to 1.16) | |
| MI | 48 (2.9) | 39 (2.4) | 1.23 (0.81 to 1.86) | |
| Other vascular event | 35 (2.1) | 38 (2.3) | 0.92 (0.58 to 1.45) | |
| Ischaemic eventsa | 271 (16.4) | 266 (16.1) | 1.02 (0.87 to 1.19) | |
| Vascular death | (7.6) | (7.2) | 1.06 (0.83 to 1.35) | |
| Vascular events | (19.6) | (19.0) | 1.03 (0.89 to 1.18) | |
Of the secondary outcomes, stroke/TIA, other vascular event, ischaemic events and vascular events, statistically significant differences were recorded in favour of MRD + ASA when compared with ASA (RR 0.80, 95% CI 0.70 to 0.92; RR 0.55, 95% CI 0.33 to 0.94; RR 0.77, 95% CI 0.65 to 0.92; RR 0.78, 95% CI 0.67 to 0.91, respectively).
Of the secondary outcomes of TIA, stroke/TIA, ischaemic events and vascular events, statistically significant differences in favour of MRD + ASA compared with MRD alone were noted (RR 0.80, 95% CI 0.66 to 0.97; RR 0.78, 95% CI 0.69 to 0.90; RR 0.76, 95% CI 0.64 to 0.90; RR 0.76, 95% CI 0.65 to 0.89, respectively).
ESPRIT56
The key outcomes of the ESPRIT56 trial are described in Table 10. For the primary outcome of first occurrence of death from all vascular causes, non-fatal stroke, non-fatal MI or major bleeding complication, the risk of event occurrence was statistically significantly lower in the MRD + ASA arm than in the ASA arm [hazard ratio (HR) 0.80; 95% CI 0.66 to 0.98].
| Outcomes | Total events | HR (95% CI) | |
|---|---|---|---|
| MRD + ASA, n (%) | ASA, n (%) | ||
| Primary | |||
| First occurrence of death from all vascular causes, non-fatal stroke, non-fatal MI or major bleeding complication | 173 (12.69) | 216 (15.20) | 0.80 (0.66 to 0.98) |
| Secondary | |||
| Death from all causes | 93 (6.83) | 107 (7.78) | 0.88 (0.67 to 1.17) |
| Death from all vascular causes | 44 (3.23) | 60 (4.36) | 0.75 (0.51 to 1.10) |
| Death from all vascular causes and non-fatal stroke | 132 (9.69) | 171 (12.42) | 0.78 (0.62 to 0.97) |
| Major bleeding complications | 35 (2.57) | 53 (0.39) | 0.67 (0.44 to 1.03) |
| Non-fatal extracranial | 21 (1.54) | 32 (2.32) | NR |
| Fatal extracranial | 2 (0.15) | 0 | NR |
| Non-fatal intracranial | 9 (0.66) | 17 (12.21) | NR |
| Fatal intracranial | 3 (0.22) | 4 (0.29) | NR |
| Minor bleeding complications | 171 (12.55) | 168 (12.21) | NR |
| All major ischaemic events (non-haemorrhagic death from vascular causes, non-fatal IS or non-fatal MI) | 140 (10.27) | 174 (12.65) | 0.81 (0.65 to 1.01) |
| All vascular events (death from vascular causes, non-fatal stroke or non-fatal MI) | 149 (10.93) | 192 (13.95) | 0.78 (0.63 to 0.97) |
| First IS | 96 (7.0) | 116 (8.43) | 0.84 (0.54 to 1.10) |
| First cardiac event | 43 (3.15) | 60 (4.36) | 0.73 (0.49 to 1.08) |
For the secondary outcome of death from all vascular causes and non-fatal stroke, the rate of event occurrence was also statistically significantly lower in the MRD + ASA arm than in the ASA arm (HR 0.78; 95% CI 0.62 to 0.97). This was also true for the outcome of all vascular events (HR 0.78; 95% CI 0.63 to 0.97).
There were no statistically significant differences reported for any other outcome.
PRoFESS57
The key outcomes from the PRoFESS57 trial are described in Table 11. Although the rate of recurrent stroke of any type was very similar in the MRD + ASA and clopidogrel groups [9% vs 8.8%, HR 1.01 (95% CI 0.92 to 1.11)], the null hypothesis (that MRD + ASA is inferior to clopidogrel) could not be rejected as the predefined non-inferiority margin was –1.075.
| Outcomes | Total events | HR for ASA + MRD (95% CI) | |
|---|---|---|---|
| MRD + ASA (%) | CLOP (%) | ||
| Primary | |||
| Recurrent stroke of any type | 916 (9) | 898 (8.8) | 1.01 (0.92 to 1.11) |
| Secondary/tertiary | |||
| Composite of vascular events (stroke, MI or death from vascular causes) | 1333 (13.1) | 1333 (13.1) | 0.99 (0.92 to 1.07) |
| MI | 178 (1.7) | 197 (1.9) | 0.90 (0.73 to 1.10) |
| Death from vascular causes | 435 (4.3) | 459 (4.5) | 0.94 (0.82 to 1.07) |
| Death from any cause | 739 (7.3) | 756 (7.4) | 0.97 (0.87 to 1.07) |
| New or worsening CHF | 144 (1.4) | 182 (1.8) | 0.78 (0.62 to 0.96) |
| Other vascular event | 533 (5.1) | 517 (5.1) | 1.03 (0.91 to 1.16) |
| First IS | 789 (7.7) | 807 (7.9) | 0.97 (0.88 to 1.07) |
| First recurrence of stroke or major haemorrhagic event | 1194 (11.7) | 1156 (11.4) | 1.03 (0.95 to 1.11) |
| Major haemorrhagic event | 419 (4.1) | 365 (3.6) | 1.15 (1.00 to 1.32) |
| Major haemorrhagic event: life-threatening | 128 (1.3) | 116 (1.1) | |
| Major haemorrhagic event: non-life-threatening | 291 (2.9) | 249 (2.5) | |
| Haemorrhagic event (minor or major) | 535 (5.3) | 494 (4.9) | 1.08 (0.96 to 1.22) |
| Intracranial haemorrhage | 147 (1.4) | 103 (1) | 1.42 (1.11 to 1.83) |
| Intracerebral haemorrhage (haemorrhagic stroke) | 90 (0.9) | 55 (0.5) | |
| Haemorrhagic stroke – fatal | 28 (0.3) | 29 (0.3) | |
| Haemorrhagic stroke – non-fatal | 62 (0.6) | 26 (0.3) | |
| Intraocular haemorrhage | 22 (0.2) | 22 (0.2) | |
| Non-stroke intracranial haemorrhage | 35 (0.3) | 26 (0.3) | |
| Thrombotic thrombocytopenic or neutropenia | 7 (0.1) | 8 (0.1) | 0.89 (0.32 to 2.44) |
For the secondary outcomes, the only statistically significant difference was in favour of MRD + ASA for the outcome of new or worsening congestive heart failure [HR 0.78 (95% CI 0.62 to 0.96)].
Adverse events
Adverse events reported for each trial are described in Table 12. In ESPS-230 and CAPRIE,26 bleeding events in the trials were reported as secondary outcomes rather than as adverse events. The reporting of adverse events differed between trials. In CAPRIE,26 adverse events were recorded as ‘patients ever reporting,’ in ESPS-230 as ‘number of patients reporting at least one adverse event during the study’. In PRoFESS,57 only selected adverse events leading to treatment discontinuation are presented in the published paper. Adverse events other than those related to bleeding were not reported for ESPRIT56 (see Table 10).
| Trial name | Adverse event | CLOP, n (%) | MRD + ASA, n (%) | ASA, n (%) | MRD, n (%) | Placebo, n (%) |
|---|---|---|---|---|---|---|
| aCAPRIE26 | Rashb | 578 (6.02) | 442 (4.61) | |||
| Diarrhoeab | 4 28 (4.46) | 322 (3.36) | ||||
| Indigestion/nausea/vomitingb | 1441 (15.01) | 1686 (17.59) | ||||
| Abnormal liver functionb | 285 (2.97) | 302 (3.15) | ||||
| Any bleeding disorder | 890 (9.27) | 890 (9.28) | ||||
| Intracranial haemorrhage | 34 (0.35) | 47 (0.49) | ||||
| Gastrointestinal haemorrhageb | 191(1.99) | 255 (2.66) | ||||
| Discontinuation due to AEs | (11.94) | (11.92) | ||||
| cESPS-230 | Any AEsb | 1056 (64) | 990 (60) | 1034 (62.57) | 933 (56.58) | |
| GI eventb | 541 (32.80) | 502 (30.44) | 505 (30.53) | 465 (28.20) | ||
| Vomitingb | 133 (8.06) | 93 (5.64) | 119 (7.19) | 109 (6.61) | ||
| Diarrhoeab | 199 (12.06) | 109 (6.6) | 254 (15.36) | 154 (9.33) | ||
| Headacheb | 630 (38.18) | 546 (33.11) | 615 (37.18) | 534 (32.38) | ||
| Bleeding any siteb | 144 (8.73) | 135 (8.19) | 77 (4.66) | 74 (4.49) | ||
| Nausea | 254 (15.39) | 204 (12.37) | 245 (14.81) | 226 (13.71) | ||
| Dyspepsia | 290 (17.58) | 283 (17.69) | 274 (16.57) | 266 (16.13) | ||
| Gastric pain | 274 (16.60) | 242 (14.67) | 240 (14.51) | 219 (13.28) | ||
| Mild bleeding | 84 (5.09) | 82 (5.01) | 53 (3.20) | 52 (3.15) | ||
| Moderate bleeding | 33 (2.0) | 33 (2.0) | 18 (1.09) | 15 (0.91) | ||
| Severe or fatal bleeding | 27 (1.64) | 20 (1.21) | 6 (0.36) | 7 (0.42) | ||
| Dizziness | 486 (29.47) | 481 (29.16) | 498 (30.10) | 509 (30.88) | ||
| Discontinuation due to AEsb | 479 (29) | 366 (22) | 485 (29) | 360 (21) | ||
| dPRoFESS57 | Headache | 87 (0.9) | 593 (5.9) | |||
| Vomiting | 37 (0.4) | 158 (1.6) | ||||
| Nausea | 58 (0.6) | 155 (1.5) | ||||
| Dizziness | 52 (0.5) | 134 (1.3) | ||||
| Atrial fibrillation | 143 (1.2) | 122 (1.4) | ||||
| Diarrhoea | 42 (0.4) | 102 (1.0) | ||||
| Hypotension | 35 (0.3) | 54 (0.5) | ||||
| Thrombotic thrombocytopenia or neutropenia | 8 (0.1) | 7 (0.1) | ||||
| Patients with AEs leading to discontinuationb | 1069 (10.6) | 1650 (16.64) |
In CAPRIE,26 patients in the clopidogrel arm were reported as experiencing significantly higher rates of rash and diarrhoea than in the ASA arm. In the ASA arm, patients reported significantly more incidences of indigestion/nausea/vomiting and abnormal liver function. The number of patients experiencing gastrointestinal haemorrhage was greater in the ASA arm than in the clopidogrel arm, a result reported to be statistically significant. The rates of trial discontinuation because of adverse events were similar in both arms of the trial.
In ESPS-2,30 there was a significant difference between each arm in the occurrence of headaches. These appear to be greater in the arms where MRD was a feature of the treatment regimen. It is recorded in the published paper30 that bleeding episodes were significantly more frequent and more often moderate or severe/fatal in treatment arms that included ASA. Any site bleeding was reported by 8.2% of patients in the ASA arm and by 8.7% in the MRD + ASA arm, but by 4.7% and 4.5% in the MRD alone and placebo groups, respectively. The rates of trial discontinuation because of adverse events differed significantly, with higher rates reported in the two MRD arms than in the ASA or placebo arms.
Of the other reported adverse events in ESPS-2,30 gastrointestinal events, vomiting, diarrhoea and headache were reported as being significantly different between treatment groups, but where the differences lie are unclear. 30
In PRoFESS,57 the rates of trial discontinuation were statistically significantly different between trial arms in favour of clopidogrel. Notably, there was an increased risk of a major haemorrhagic event for MRD + ASA compared with clopidogrel (HR 1.15; 95% CI 1.00 to 1.32) as well as intracranial haemorrhage (HR 1.42; 95% CI 1.11 to 1.83). Headache appears to be reported by many more patients in the MRD + ASA arm – an unsurprising outcome, as MRD acts as a vasodilator.
Assessment Group analysis of time to first event rates
An important consideration in the analysis of trials in this area is the length of patient follow-up. It was noted earlier that the mean length of follow-up for the included trials ranged between 1.91 and 3.5 years (see Table 6). The Assessment Group, using data from CAPRIE,26 assessed the event rates over time for the outcome of ischaemic stroke in the ischaemic stroke-only population of the trial and the outcome of MI in the MI-only population. The assessment indicates that patients appear to be at greatest risk of a recurrent event in the first 6–12 months; thereafter, the risk decreases markedly. Therefore, it is important to explore how event rates change over time.
Methods for indirect synthesis
Justification for indirect analysis
The reported outcomes and their definitions varied significantly across the four trials (Table 13). For instance, in the CAPRIE26 trial, data on first ischaemic stroke are available for the ischaemic stroke population, but other outcomes are available for only the total population (i.e. ischaemic stroke, MI and peripheral arterial disease populations as a single group). The single common qualifying event in the four included trials26,30,56,57 was ischaemic stroke/TIA. Where appropriate, evidence synthesis, using a mixed-treatment comparisons approach, was undertaken using data from the ischaemic stroke/TIA overall populations26,30,56,57 or subpopulation. 26 The Assessment Group notes that the patient populations in the mixed-treatment comparisons are based on those described in the original trial publications and may therefore include patients with multivascular disease.
| All outcomes reported (primary, secondary or tertiary) | CAPRIE26 | ESPS-230 | ESPRIT56 | PRoFESS57 | No. of studies |
|---|---|---|---|---|---|
| First IS event (non-fatal or fatal) | X | X | X | 3 | |
| Stroke (recurrent any type) | X | X | 2 | ||
| MI | X | X | X | 3 | |
| Death from vascular cause | X | X | X | 3 | |
| Death from all causes | X | X | X | 3 | |
| Bleeding complications (major) | X | X | 2 | ||
| Bleeding complications (any) | X | X | X | 3 | |
| First cardiac event (fatal and non-fatal MI, sudden death, cardiac death) | X | 1 | |||
| First event (IS, MI, or death from vascular cause) | X | 1 | |||
| First event [any stroke (includes primary intracranial haemorrhage), MI, fatal bleeding or death from all causes] | X | 1 | |||
| First event (IS, MI, amputation, death from all vascular causes) | X | 1 | |||
| First event (non-fatal stroke, death from all vascular causes) | X | 1 | |||
| First event (non-fatal stroke, non-fatal MI or major bleeding complication, death from all vascular causes) | X | 1 | |||
| First event (non-fatal stroke, non-fatal MI or death from all vascular causes) | X | 1 | |||
| First event (stroke (non-fatal or fatal), MI (non-fatal or fatal) or death from all vascular causes) | X | 1 | |||
| First ischaemic event (stroke and/or MI, and/or sudden death of thrombotic origin) | X | 1 | |||
| First major ischaemic events (non-fatal IS, non-fatal MI or non-haemorrhagic death from vascular causes) | X | 1 | |||
| Other vascular events (pulmonary embolism, retinal vascular accidents, deep-vein thrombosis, peripheral arterial occlusion or TIA) | X | 1 | |||
| Other vascular events (pulmonary embolism, deep-venous thrombosis, peripheral arterial occlusion, venous retinal thrombosis or combination of these events) | X | 1 | |||
| Stroke and/or death from all causes | X | 1 | |||
| TIA | X | 1 |
Indirect comparison of common clinical outcomes (where available in at least two trials) was undertaken to estimate the relative efficacy between interventions in the ischaemic stroke/TIA populations.
Mixed-treatment comparison
The relative treatment effects of clopidogrel, MRD + ASA, MRD alone and ASA ideally would have been derived from a single, direct, head-to-head RCT. However, such a trial does not exist. Instead, we have four trials26,30,56,57 assessing the treatment effects of a subset of the interventions of interest. A mixed-treatment comparison is an alternative approach that is used to estimate relative treatment effects when the objective of the analysis is to compare more than two interventions. A mixed-treatment comparison is an explicit analytical framework and has been presented as an extension of standard meta-analysis by including multiple pairwise comparisons across a range of different interventions. 61 The framework can then be used to derive a relative treatment effect of competing interventions in the absence of direct evidence.
The Assessment Group used a Bayesian approach to mixed-treatment comparison to estimate the relative effectiveness measures for the interventions under comparison, ranking and making probability statements about the most effective intervention in a decision context. A fixed-effects model was chosen for all analyses because random-effect models failed to reach convergence. One possible reason for this failure could be the small number of trials (two to three trials in each analysis) and, hence, overparameterisation.
A non-informative (flat prior) normal distribution was used for the log odds ratio (OR) of each relative comparison; thus, the observed results are completely influenced by the data and not the choice of the priors. We estimated the relative effectiveness for each comparison using Markov chain Monte Carlo for each analysis in Winbugs version 1.4 statistical software (Medical Research Council Biostatistics Unit, Cambridge, UK). 62 Two chains were used to ensure that model convergence was met after 100,000 iterations with a burn-in of 10,000 or more. Formal convergence of the models was assessed using trace plots and the Gelman–Rubin approach. 63 Results are presented with summary statistics for RR and OR along with 95% CIs. Pairwise ORs were estimated and converted to RR using a standard approach. This was implemented in the Winbugs software by applying event rates across included trials from the reference comparator as the baseline probability (prob_baseline). Therefore, the RR = OR/[(1 − prob_baseline) + (prob_baseline × OR)]. The Winbugs codes used in the analysis were adapted from the Multi-parameter Evidence Synthesis Research Group (MPES) and are presented in Appendix 7.
Results of mixed-treatment comparisons for ischaemic stroke/transient ischaemic attack population
All of the results presented in this section are related to ischaemic stroke/TIA populations only.
In this section, for clarity, the data analyses are presented in tables. For ease of reference, significant findings are in bold font within the tables. The networks relevant to each comparison are presented in Appendix 7.
It should be noted that the selection of the outcomes included in the mixed-treatment comparison are driven by the available clinical data. In most analyses, the number of studies is small (two to three trials) and, although a large number of patients were included, the data used from the CAPRIE26 trial were based on a subgroup of patients with ischaemic stroke. The findings of this mixed-treatment comparison analysis should therefore be interpreted with caution.
Stroke
Data on recurrent stroke were available from four trials. 26,30,56,57 However, owing to differences in definition of ‘recurrent stroke’, analysis was performed separately for the ‘first ischaemic stroke’ and ‘any recurrent stroke’. The CAPRIE26 trial did not report data on ‘any recurrent stroke’ and the ESPS-230 trial did not present data on the ‘first ischaemic stroke’.
First ischaemic stroke
Three trials (CAPRIE,26 ESPRIT56 and PRoFESS57) provided direct head-to-head data on the ‘first ischaemic stroke’. Therefore, it was possible to combine these trials through the mixed-treatment comparison approach to calculate the relative efficacy of clopidogrel versus ASA, MRD + ASA versus ASA and MRD + ASA versus clopidogrel.
Table 14 shows head-to-head trial data and the relative estimates calculated using the mixed-treatment comparison analysis. The results show no major differences between the mixed-treatment comparison results and head-to-head estimates from the included trials. Results from the mixed-treatment comparison showed that no single estimated RRs were found to demonstrate a statistically significant difference between any pair of interventions. The observed RR for clopidogrel and MRD + ASA appeared to reflect a lower risk of the ‘first ischaemic stroke’ compared with ASA. A RR of 0.968 was observed for MRD + ASA compared with clopidogrel. However, differences were not significant. There is no evidence to suggest that any intervention is superior to another in terms of prevention of ‘first ischaemic stroke’.
| Trial | ASA | CLOP | MRD + ASA | |
|---|---|---|---|---|
| CAPRIE26 | 226/2370 | 214/2370 | ||
| ESPRIT56 | 116/1376 | – | 96/1363 | |
| PRoFESS57 | 807/10,151 | 789/10,181 | ||
| Comparison | Direct evidence from head-to-head trials | Results from the mixed-treatment comparison analysis | ||
| Trial | RRa (95% CI) | RRa (95% CI) | OR (95% CI) | |
| CLOP vs ASA | CAPRIE26 | 0.947 (0.79 to 1.13) | 0.922 (0.79 to 1.06) | 0.915 (0.77 to 1.07) |
| MRD + ASA vs ASA | ESPRIT56 | 0.835 (0.64 to 1.08) | 0.891 (0.75 to 1.04) | 0.883 (0.74 to 1.04) |
| MRD + ASA vs CLOP | PRoFESS57 | 0.975 (0.88 to 1.07) | 0.968 (0.88 to 1.05) | 0.966 (0.87 to 1.06) |
Any recurrent stroke
Two trials (ESPS-230 and PRoFESS57) provided direct head-to-head data on recurrent stroke outcome. Therefore, it was possible to combine these trials through the mixed-treatment comparison approach to calculate the relative efficacy of MRD + ASA versus ASA, MRD alone versus ASA, MRD + ASA versus clopidogrel, and MRD alone versus MRD + ASA. We were also able to estimate the indirect estimates from the mixed-treatment comparison for clopidogrel versus ASA and MRD versus clopidogrel. Table 15 presents head-to-head trial data and results from the mixed-treatment comparison analysis. No major differences in the mixed-treatment comparison results and head-to-head estimates from the included trials were observed. Results from the mixed-treatment comparison showed that clopidogrel and MRD + ASA were associated with fewer recurrent strokes relative to ASA. An increased risk of recurrent stroke was observed for MRD alone compared with clopidogrel or MRD + ASA. There was no difference between MRD alone compared with ASA or between MRD + ASA and clopidogrel, in terms of reducing recurrent stroke.
| Trial | ASA | CLOP | MRD + ASA | MRD |
|---|---|---|---|---|
| ESPS-230 | 206/1649 | 157/1650 | 211/1654 | |
| PRoFESS57 | 898/10,151 | 916/10,181 | ||
| Comparison | Direct evidence from head-to-head trials | Results from the mixed-treatment comparison analysis | ||
| Trial | RRa (95% CI) | RRa (95% CI) | OR (95% CI) | |
| CLOP vs ASA | None | N/A |
0.752 (0.60 to 0.92) |
0.727 (0.56 to 0.91) |
| MRD + ASA vs ASA | ESPS-230 |
0.762 (0.62 to 0.92) |
0.764 (0.62 to 0.92) |
0.74 (0.59 to 0.91) |
| MRD vs ASA | ESPS-230 |
1.021 (0.85 to 1.22) |
1.025 (0.85 to 1.21) |
1.03 (0.83 to 1.25) |
| MRD + ASA vs CLOP | PRoFESS57 |
1.017 (0.93 to 1.1) |
1.018 (0.93 to 1.11) |
1.02 (0.92 to 1.12) |
| MRD vs CLOP | None | N/A |
1.376 (1.10 to 1.68) |
1.431 (1.11 to 1.80) |
| MRD vs MRD + ASA | ESPS-230 |
1.341 (1.10 to 1.62) |
1.349 (1.10 to 1.61) |
1.403 (1.12 to 1.73) |
Myocardial infarction
Three RCTs (CAPRIE,26 ESPS-230 and PRoFESS57) provided direct head-to-head data on MI outcome. It was possible to combine these trials through the mixed-treatment comparison approach to calculate the relative efficacy of clopidogrel versus ASA, MRD + ASA versus ASA, MRD alone versus ASA, MRD + ASA versus clopidogrel, and MRD alone versus MRD + ASA. We were also able to estimate the indirect estimates for MRD alone versus clopidogrel. Table 16 shows head-to-head trial data and the estimates calculated using the mixed-treatment comparison analysis. No major differences between the mixed-treatment comparison results and head-to-head estimates from the included trials were observed. Results from the mixed-treatment comparison, which are described in Table 16, showed that no single estimated RR was found to demonstrate a statistically significant difference between any pair of interventions in terms of prevention of MI events.
| Trial | ASA | CLOP | MRD + ASA | MRD |
|---|---|---|---|---|
| CAPRIE26 | 20/2370 | 24/2370 | ||
| ESPS-230 | 39/1649 | 35/1650 | 48/1654 | |
| PRoFESS57 | 197/10,151 | 178/10,181 | ||
| Comparison | Direct evidence from head-to-head trials | Results from the mixed-treatment comparison analysis | ||
| Trial | RRa (95% CI) | RRa (95% CI) | OR (95% CI) | |
| CLOP vs ASA | CAPRIE26 | 1.200 (0.66 to 2.16) | 1.094 (0.73 to 1.56) | 1.098 (0.72 to 1.59) |
| MRD + ASA vs ASA | ESPS-230 | 0.897 (0.57 to 1.40) | 0.972 (0.65 to 1.38) | 0.972 (0.65 to 1.39) |
| MRD vs ASA | ESPS-230 | 1.227 (0.80 to 1.86) | 1.291 (0.84 to 1.88) | 1.302 (0.84 to 1.92) |
| MRD + ASA vs CLOP | PRoFESS57 | 0.901 (0.73 to 1.10) | 0.893 (0.73 to 1.07) | 0.892 (0.72 to 1.08) |
| MRD vs CLOP | None | N/A | 1.208 (0.75 to 1.81) | 1.215 (0.75 to 1.85) |
| MRD vs MRD + ASA | ESPS-230 | 1.368 (0.89 to 2.10) | 1.352 (0.88 to 1.98) | 1.365 (0.88 to 2.02) |
Death from vascular causes
Three trials (CAPRIE,26 ESPRIT56 and PRoFESS57) provided direct head-to-head data on vascular death. Therefore, it was possible to combine these trials through the mixed-treatment comparison approach to calculate the relative efficacy of clopidogrel versus ASA, MRD + ASA versus ASA, and MRD + ASA versus clopidogrel. Table 17 shows head-to-head trial data and the estimates calculated using the mixed-treatment comparison analysis. No major differences in the mixed-treatment comparison results and head-to-head estimates from the included trials were noted. Results from the mixed-treatment comparison showed no significant evidence to demonstrate differences in clopidogrel, MRD + ASA and ASA for vascular death outcome. There is no evidence to suggest that any intervention is superior to another in terms of prevention of vascular death.
| Trial | ASA | CLOP | MRD + ASA | |
|---|---|---|---|---|
| CAPRIE26 | 40/2370 | 35/2370 | ||
| ESPRIT56 | 60/1376 | 44/1363 | ||
| PRoFESS57 | 459/10,151 | 435/10,181 | ||
| Comparison | Direct evidence from head-to-head trials | Results from the mixed-treatment comparison analysis | ||
| Trial | RRa (95% CI) | RRa (95% CI) | OR (95% CI) | |
| CLOP vs ASA | CAPRIE26 | 0.875 (0.55 to 1.37) | 0.829 (0.60 to 1.11) | 0.827 (0.59 to 1.12) |
| MRD + ASA vs ASA | ESPRIT56 | 0.750 (0.51 to 1.01) | 0.782 (0.57 to 1.04) | 0.775 (0.56 to 1.04) |
| MRD + ASA vs CLOP | PRoFESS57 | 0.945 (0.83 to 1.07) | 0.942 (0.82 to 1.06) | 0.939 (0.82 to 1.06) |
Death from all causes
Three RCTs (ESPS-2,30 ESPRIT56 and PRoFESS57) provided direct head-to-head data on all-cause death. It was possible to combine these trials through the mixed-treatment comparison approach to calculate the relative efficacy of MRD + ASA versus ASA, MRD alone versus ASA, MRD + ASA versus clopidogrel, and MRD alone versus MRD + ASA. We also estimated the indirect estimates for clopidogrel vs ASA and MRD alone versus clopidogrel, as no head-to-head data were available. Table 18 shows head-to-head trial data and the estimates calculated using the mixed-treatment comparison analysis. No major variation in the mixed-treatment comparison results and head-to-head estimates from the included trials were observed. Results from the mixed-treatment comparison showed that there was no evidence to demonstrate significant differences between clopidogrel, MRD + ASA, MRD and ASA for all-cause death.
| Trial | ASA | CLOP | MRD + ASA | MRD |
|---|---|---|---|---|
| ESPS-230 | 182/1649 | 185/1650 | 188/1654 | |
| ESPRIT56 | 107/1376 | 93/1363 | ||
| PRoFESS57 | 756/10151 | 739/10181 | ||
| Comparison | Direct evidence from head-to-head trials | Results from the mixed-treatment comparison analysis | ||
| Trial | RRa (95% CI) | RRa (95% CI) | OR (95% CI) | |
| CLOP vs ASA | None | N/A | 0.992 (0.82 to 1.18) | 0.992 (0.80 to 1.20) |
| MRD + ASA vs ASA | ESPS-2,30 ESPRIT56 |
ESPS-2:30 1.016 (0.83 to 1.23) ESPRIT:56 0.877 (0.67 to 1.14) |
0.967 (0.82 to 1.12) | 0.964 (0.80 to 1.14) |
| MRD vs ASA | ESPS-230 | 1.030 (0.85 to 1.24) | 1.007 (0.83 to 1.20) | 1.010 (0.81 to 1.23) |
| MRD + ASA vs CLOP | PRoFESS57 | 0.975 (0.88 to 1.07) | 0.976 (0.88 to 1.07) | 0.974 (0.87 to 1.08) |
| MRD vs CLOP | None | N/A | 1.021 (0.81 to 1.25) | 1.024 (0.80 to 1.28) |
| MRD vs MRD + ASA | ESPS-230 | 1.014 (0.83 to 1.22) | 1.044 (0.86 to 1.24) | 1.052 (0.85 to 1.28) |
Bleeding
Data on bleeding were available from three RCTs (ESPS-2,30 ESPRIT56 and PRoFESS57). The CAPRIE26 trial did not present bleeding data for patients in this subpopulation. As there was variation in bleeding reporting across trials, analysis was only possible for ‘any bleeding’ and ‘major bleeding’, as these were the common bleeding definitions used across the trials.
Any bleeding
Three RCTs (ESPS-2,30 ESPRIT56 and PRoFESS57) provided direct head-to-head data on any bleeding. It was possible to combine these trials through the mixed-treatment comparison approach to calculate the relative efficacy of MRD + ASA versus ASA, MRD alone versus ASA, MRD + ASA versus clopidogrel, and MRD alone versus MRD + ASA. We also calculated the indirect estimates for clopidogrel versus ASA and MRD alone versus clopidogrel, as no head-to-head data were available. The category of ‘any bleeding’ includes both minor and major bleeding. Minor events included haematuria, haematemesis, epistaxis, intraocular bleeding, purpura, and gynaecological, internal and intracranial bleeding. Major bleeding included severe or fatal bleeding, life-threatening bleeding, intracranial bleeding, major haemorrhage and major gastrointestinal tract haemorrhage. Table 19 shows head-to-head trial data and the estimates calculated using the mixed-treatment comparison analysis. There were no major differences in the mixed-treatment comparison results and head-to-head estimates from the included trials. Results from the mixed-treatment comparison showed that MRD alone was associated with significantly fewer bleeding events than all comparators; the MRD vs clopidogrel estimates are based on indirect comparisons and are not supported by head-to-head trial data. There was no evidence to suggest any differences between ‘clopidogrel versus ASA’ and ‘MRD + ASA versus ASA’ for any bleeding.
| Trial | ASA | CLOP | MRD + ASA | MRD |
|---|---|---|---|---|
| ESPS-230 | 135/1649 | 144/1650 | 77/1654 | |
| ESPRIT56 | 221/1376 | 206/1363 | ||
| PRoFESS57 | 494/10,151 | 535/10,181 | ||
| Comparison | Direct evidence from head-to-head trials | Results from the mixed-treatment comparison analysis | ||
| Trial | RRa (95% CI) | RRa (95% CI) | OR (95% CI) | |
| CLOP vs ASA | None | N/A | 0.921 (0.75 to 1.10) | 0.916 (0.74 to 1.11) |
| MRD + ASA vs ASA | ESPS-230 | 1.066 (0.85 to 1.33) | ||
| ESPRIT56 | 0.941 (0.79 to 1.12) | 0.991 (0.85 to 1.14) | 0.991 (0.84 to 1.15) | |
| MRD vs ASA | ESPS-230 | 0.569 (0.43 to 0.74) | 0.549 (0.42 to 0.70) | 0.529 (0.39 to 0.68) |
| MRD + ASA vs CLOP | PRoFESS57 | 1.08 (0.95 to 1.21) | 1.082 (0.96 to 1.21) | 1.087 (0.95 to 1.23) |
| MRD vs CLOP | None | N/A | 0.593 (0.44 to 0.78) | 0.582 (0.42 to 0.77) |
| MRD vs MRD + ASA | ESPS-230 | 0.533 (0.40 to 0.69) | 0.557 (0.43 to 0.71) | 0.535 (0.40 to 0.69) |
Major bleeding
Two RCTs (ESPRIT56 and PRoFESS57) provided direct head-to-head data on major bleeding. It was possible to combine these trials through the mixed-treatment comparison approach to calculate the relative efficacy of MRD + ASA versus ASA and MRD + ASA versus clopidogrel. We also estimated the indirect estimates for clopidogrel versus ASA as no head-to-head data were available. The category of ‘major bleeding’ included severe or fatal bleeding, life-threatening bleeding, intracranial bleeding, major haemorrhage and major gastrointestinal tract haemorrhage. Table 20 shows head-to-head trial data and the estimates calculated using the mixed-treatment comparison analysis. There were no major variations in the mixed-treatment comparison results and head-to-head estimates from the included trials. Results from the mixed-treatment comparison showed that clopidogrel was associated with significantly fewer bleeding events than ASA; these estimates are based on indirect comparisons and are not supported by head-to-head trial data. No statistically significant differences among MRD + ASA, clopidogrel and ASA in major bleeding events were observed.
| Trial | ASA | CLOP | MRD + ASA | |
|---|---|---|---|---|
| ESPRIT56 | 53/1376 | 35/1363 | ||
| PRoFESS57 | 365/10,151 | 419/10,181 | ||
| Comparison | Direct evidence from head-to-head trials | Results from the mixed-treatment comparison analysis | ||
| Trial | RRa (95% CI) | RRa (95% CI) | OR (95% CI) | |
| CLOP vs ASA | None | N/A | 0.596 (0.36 to 0.89) | 0.587 (0.35 to 0.89) |
| MRD + ASA vs ASA | ESPRIT56 | 0.667 (0.43 to 1.01) | 0.682 (0.43 to 1.01) | 0.674 (0.42 to 1.00) |
| MRD + ASA vs CLOP | PRoFESS57 | 1.145 (0.99 to 1.31) | 1.147 (0.99 to 1.31) | 1.154 (0.99 to 1.32) |
Results of the mixed-treatment comparison evidence for myocardial infarction and peripheral arterial disease populations
Owing to the lack of available data, we were unable to carry out indirect analyses for the MI and peripheral arterial disease patient populations. Only CAPRIE26 included patients with MI and peripheral arterial disease; data on these individual patients groups were not available from the other included studies. 30,56,57
Summary of the evidence from the mixed-treatment comparison
The mixed-treatment comparison analysis was performed in patients categorised as having an ischaemic stroke/TIA as a qualifying event. The relative effectiveness of clopidogrel, MRD + ASA, MRD alone and ASA was evaluated, based on evidence from four main RCTs26,30,56,57 that reported seven key clinical outcomes. The four trials included in the mixed-treatment comparison analysis were CAPRIE26 (clopidogrel vs ASA), ESPS-230 (ASA vs MRD + ASA vs MRD alone vs placebo), ESPRIT56 (MRD + ASA vs ASA) and PRoFESS57 (MRD + ASA vs clopidogrel). The clinically important outcomes that were included in the mixed-treatment comparison exercise were stroke (‘first ischaemic stroke’ and ‘any recurrent stroke’), MI, vascular death, death from all causes and bleeding (‘any bleeding’ and ‘major bleeding’). The selection of these outcomes was based on the availability of data from two or more of the four RCTs. One study (ESPS-230) contained a placebo arm and was included in the analysis, but placebo results are not presented here. The reference comparator for all the analyses was ASA. The results from the mixed-treatment comparison showed that no single estimated RR was found to demonstrate a statistically important difference between any pair of interventions except for the outcomes of any recurrent stroke, ‘any ‘ and ‘major’ bleeding. The results further showed that MRD alone was statistically significantly associated with increased risk of any recurrent stroke compared with clopidogrel and MRD + ASA. However, it is worth noting that the findings from clopidogrel versus ASA and MRD alone versus clopidogrel were based on the indirect evidence and were not supported by any head-to-head data.
As detailed at the beginning of the section, caveats apply to the findings of our analysis owing to the limited outcomes that were available for selection, the small number of trials and the use of data from subgroups from one trial. 26
The MATCH58 and CHARISMA59 trials were not included in the Assessment Group’s literature review, as these trials included comparators that were not specified in the scope for the appraisal; the combination of clopidogrel + ASA is not licensed in the patient population under evaluation. After much discussion, the Assessment Group decided to also exclude these trials from the indirect comparison exercise undertaken. However, the Assessment Group notes that excluding these trials does not change the ranking of the interventions; their inclusion only strengthens confidence in the results generated. The Assessment Group has checked the methods used by the manufacturer and commends the values from the manufacturer’s indirect comparison.
Patients with multivascular disease
The decision problem matrix (see Table 4) described in the final scope16 issued by NICE specified that, if the evidence allows, the effectiveness of clopidogrel in people with multivascular disease who are considered at high risk of recurrent occlusive vascular events should be considered. The Assessment Group notes that in the literature there is a variety of definitions that characterise this population; this is an issue, as the number of patients included in any multivascular disease analysis will be affected by how the group is defined. The simplest and broadest definition of multivascular disease described in the published literature is ‘patients with disease in more than one vascular bed’. For completeness, the definitions identified by the Assessment Group from the literature are described in Table 21. Owing to the apparent lack of consensus, the Assessment Group has derived a definition of multivascular disease for the purposes of this document that appears to be consistent with the simplest and broadest definition described in the published literature.
| MVD definition source | Definition of MVD |
|---|---|
| Bhatt 200622 (REACH registry) | Polyvascular disease was defined as coexistent symptomatic (clinically recognised) arterial disease in two or three territories (coronary, cerebral and/or peripheral) within each patient |
| CAPRIE26 | No formal definition of MVD was reported (not unusual at time of publication); however, subgroup analysis of 2144 patients with PAD/stroke and previous MI was presented |
| Ringleb 200464 |
Patients with MVD are those with pre-existing symptomatic atherosclerotic disease from the overall CAPRIE26 population defined as having a self-reported history of IS and/or MI before the qualifying event for enrolment into the CAPRIE26 trial (Note: definition does not include PAD or TIA) |
| Sanofi–aventis/Bristol–Myers Squibb submission52 |
Patients with pre-existing symptomatic atherosclerotic disease (IS or MI) in addition to qualifying event (see MS, p. 66) Patients with disease in more than one vascular bed (see MS, p. 2) |
| Assessment Group’s reclassification of populations in CAPRIE26 | Patients with MVD defined as those who had experienced at least two of the following: CAD/MI, IS/TIA or PAD |
Although the original CAPRIE26 publication did not include a formal definition of multivascular disease, the authors did present the results of a subgroup analysis of patients with peripheral arterial disease/stroke and previous MI. The findings support the view that patients with multivascular disease are at a greater risk of recurrent occlusive vascular events than patients with disease in a single vascular bed (Table 22).
| Patient and treatment subgroup | IS, MI or vascular death | RRR (95% CI) | |
|---|---|---|---|
| Events | Rate/year (%) | ||
| PAD/stroke with previous MI (n = 2144) | |||
| CLOP (nyrs 1963) | 164 | 8.35 | 22.7% (4.9 to 37.2) |
| ASA (nyrs 1825) | 196 | 10.74 | |
Post hoc analysis from the CAPRIE trial
One new publication64 using data from the CAPRIE26 trial was identified from the literature review. In this publication, patients with pre-existing symptomatic atherosclerotic disease from the overall CAPRIE26 population were described in a subgroup analysis. As noted in Table 21, this was defined as a self-reported history of ischaemic stroke and/or MI before the qualifying event for enrolment in CAPRIE. 26 The data describing such events had been routinely collected in the case record forms. However, no standard procedures to validate such a pre-existing event were used. 64 The Assessment Group notes that this subgroup of patients does not appear to include patients with peripheral arterial disease or TIA. The key outcomes of the analysis are described in Table 23. Compared with the overall population (n = 19,185), the subgroup of patients with pre-existing symptomatic atherosclerotic disease, which included ischaemic stroke or MI (n = 4496) was found to have elevated event rates for the primary composite end point of ischaemic stroke, MI or vascular death. The results favour clopidogrel over ASA at 1 year and 3 years on both the composite end points.
| Outcomes | Follow-up | Event rate | RRRa (95% CI) | |
|---|---|---|---|---|
| CLOP (%) (n = 2249) | ASA (%) (n = 2247) | |||
| First occurrence of IS, MI or vascular death | 1 year | 8.8 | 10.2 | 14.9 (0.3 to 27.3); p = 0.045 |
| 3 years | 20.4 | 23.8 | ||
| First occurrence of IS, rehospitalisation for ischaemia | 1 year | 16.1 | 18.5 | 12.0 (0.6 to 22.1); p = 0.039 |
| 3 years | 32.7 | 36.6 | ||
The authors64 do not discuss the clinical effectiveness of clopidogrel on individual subpopulations (e.g. ischaemic stroke, MI or peripheral arterial disease) after removal of patients with multivascular disease from the analysis. However, they do comment that the 3-year composite event rate for the subpopulation without any pre-existing atherosclerotic disease is lower than that of the multivascular disease group.
Assessment Group reclassification of patients from CAPRIE
Using the Assessment Group’s definition of multivascular disease (two of the following: coronary artery disease/MI, ischaemic stroke/TIA or peripheral arterial disease) and additional data provided by the manufacturer, the Assessment Group reclassified patients from CAPRIE26 into those with atherosclerotic disease in a single vascular bed (described as ‘MI only’, ‘ischaemic stroke only’ or ‘peripheral arterial disease only’) and those who had disease in more than one vascular bed (e.g. patients who had experienced coronary artery disease/MI and an ischaemic stroke/TIA or who had peripheral arterial disease and experienced a MI). The Assessment Group then compared the risk of two key outcomes (ischaemic stroke and MI) using the original CAPRIE26 patient populations and the Assessment Group’s reclassifications. The results are described below [see Table 24 (ischaemic stroke) and Table 25 (MI)].
| Patient group: qualifying event | Original published, IS rate % (n/N) | Newa IS rate using additional data from manufacturer, % (n/N) | |||||
|---|---|---|---|---|---|---|---|
| CLOP | ASA | RR (95% CI) | Assessment group reclassification | CLOP | ASA | RR (95% CI) | |
| IS | 9.74 (315/3233) | 10.57 (338/3198) | 0.93 (0.80 to 1.07) | IS only | 9.03 (214/2370 | 9.54 (226/2370) | 0.9 (0.79 to 1.13) |
| MI | 1.34 (42/3143) | 1.33 (42/3159) | 1.01 (0.66 to 1.54) | MI only | 0.98 (28/2845) | 1.00 (29/2896) | 0.98 (0.59 to 1.65) |
| PAD |
2.51 (81/3223) |
2.54 (82/3229) | 0.99 (0.73 to 1.34) | PAD only | 2.20 (41/1861) | 1.62 (30/1852) | 1.36 (0.85 to 2.17) |
| MVD | 6.14 (155/2523) | 7.13 (176/2468) | 0.861 (0.70 to 1.06) | ||||
| Patient group: qualifying event | Original published MI rate, % (n/N) | Newa MI rate using additional data from manufacturer, % (n/N) | |||||
|---|---|---|---|---|---|---|---|
| CLOP | ASA | RR (95% CI) | Assessment group reclassification | CLOP | ASA | RR (95% CI) | |
| IS | 1.36 (44/3233) | 1.59 (51/3198) | 0.85 (0.57 to 1.27) | IS only | 1.01 (24/2370) | 0.84 (20/2370) | 1.20 (0.66 to 2.17) |
| MI | 5.19 (163/3143) | 5.51 (174/3159) | 0.93 (0.76 to 1.15) | MI only | 4.53 (129/2845) | 5.18 915/2896) | 0.87 (0.69 to 1.10) |
| PAD | 2.11 (68/3223) | 3.34 (108/3229) | 0.61 (0.42 to 0.83) | PAD only | 1.18 (22/1861) | 1.78 (33/1852) | 0.66 (0.39 to 1.13) |
| MVD | 3.96 (100/2523) | 5.27 (130/2468) | 0.75 (0.58 to 0.97) | ||||
From Table 24 it can be seen that when the patients are reclassified, the risk of a future ischaemic stroke for individual patient groups is different in both treatment arms. The risk for ischaemic stroke-only patients remains stable. The risk for the multivascular disease subgroup is much greater than that of the MI and peripheral arterial disease patients.
From Table 25 it can be seen that when the patients are reclassified, the risk of a future MI for individual patient groups in both treatment arms is different. The risk for MI-only patients remains stable. The risk for the multivascular disease subgroup is greater than that of the ischaemic stroke and peripheral arterial disease patients.
These findings indicate that patients with multivascular disease (as defined by the Assessment Group) constitute an important clinical subgroup. It should be noted that the Assessment Group had access to relevant data from the CAPRIE26 trial only and, therefore, were unable to conduct similar analyses for the other identified trials.
Summary of clinical evidence
For clarity, Table 26 describes the main clinical efficacy findings. The direct evidence from the four included RCTs26,30,56,57 is outlined along with the Assessment Group’s assessment of time to event rates, the indirect evidence from the mixed-treatment comparison and the Assessment Group’s assessment of the evidence for the multivascular disease population. The dearth of new evidence for the MI and peripheral arterial disease populations is notable.
| Trial and population | Outcome | Finding |
|---|---|---|
| Direct evidence | ||
|
CAPRIE:26 MI, IS, PAD |
First occurrence of IS, MI or vascular death | CLOP superior to ASA for overall population |
|
ESPS-2:30 IS/TIA |
Stroke | MRD + ASA superior to MRD alone and superior to ASA |
|
ESPRIT:56 IS/TIA |
First occurrence of death from all vascular causes, non-fatal stroke, non-fatal MI or major bleeding complication | MRD + ASA superior to ASA |
| PRoFESS57 | Recurrent stroke | CLOP and MRD + ASA similar |
| Time to event rates | ||
|
CAPRIE:26 MI and IS |
MI and IS | Recurrent events for patients with disease in a single vascular bed tend to occur within the first 6–12 months |
| Indirect evidence | ||
|
IS/TIA |
Recurrent stroke | CLOP and MRD + ASA superior to ASA |
| Recurrent stroke | MRD alone = increased risk compared with CLOP, MRD + ASA, ASA | |
| Any bleeding | MRD alone = least risk compared with ASA, CLOP, MRD + ASA | |
| Major bleeding | CLOP superior to ASA | |
| MVD subgroup | ||
|
CAPRIE:26 MI, IS, PAD |
IS and MI | Patients with disease in more than one vascular bed are an important clinical subgroup at a greater risk of recurrent OVEs than patients with disease in a single vascular bed |
Discussion of clinical evidence
Direct clinical evidence available
The clinical evidence base supporting the previously published NICE guidance (TA90)24 for the prevention of occlusive vascular events in patients with a prior history of such events and established peripheral arterial disease was constructed from two trials (CAPRIE26 and ESPS-230) relevant to the use of clopidogrel, MRD and ASA. Since publication of this guidance, two more relevant trials have been published (ESPRIT56 and PRoFESS57). The evidence base underpinning this update of TA9024 is therefore focused on four RCTs.
Only CAPRIE26 included patients with MI and peripheral arterial disease; the remaining three trials included just patients with ischaemic stroke/TIA. This means that the clinical evidence base for patients with MI and peripheral arterial disease (except for those with multivascular disease) has not changed since the publication of the TA9024 guidance. Results from CAPRIE26 indicated that clopidogrel was more effective than ASA in preventing a composite of events comprising ischaemic stroke, MI or vascular death; however, the size of the benefit appeared to be small. A subgroup analysis indicated that for the subgroup of patients with peripheral arterial disease, there was a statistically significant benefit of clopidogrel compared with ASA; however, the trial was not powered to detect differences within subgroups and so the chances of a false-negative finding are high. The Assessment Group notes that the CAPRIE26 trial does not distinguish between patients with NSTEMI and STEMI, as the trial was carried out and reported before this distinction was used to differentiate between patient pathways. However, this clearly inhibits the interpretation of the results for these clinically important subgroups of patients.
The manufacturer’s positive response to the Assessment Group’s request for more detailed analyses of the CAPRIE26 trial allowed the Assessment Group to conduct a new post hoc subgroup analysis of patients with multivascular disease (see Summary of the evidence from the mixed-treatment comparison) and explore changes in key event rates for four patient populations (MI, ischaemic stroke, peripheral arterial disease and multivascular disease) instead of the original three (MI, ischaemic stroke and peripheral arterial disease).
For patients with ischaemic stroke/TIA, clinical data from two relevant trials (ESPRIT56 and PRoFESS57) have become available recently in addition to data from ESPS-230 and CAPRIE. 26 Unfortunately, PRoFESS57 yielded inconclusive results, as the trial did not meet the predefined criteria for non-inferiority, but showed similar rates for the primary outcome of recurrent stroke (MRD + ASA vs clopidogrel). Consequently, there is no direct evidence to support the use of clopidogrel instead of MRD + ASA, or vice versa, for the ischaemic stroke/TIA population. ESPS-230 showed that MRD + ASA leads to statistically significant RRRs for the primary outcome of stroke and a range of secondary outcomes compared with ASA and MRD alone. The ESPRIT56 trial also demonstrated statistically significant risk reductions for MRD + ASA versus ASA (first occurrence of death from all vascular causes, non-fatal stroke, non-fatal MI or major bleeding complication; death from all vascular causes and non-fatal stroke; all vascular events). This means that the additional clinical evidence available from the publication of ESPRIT56 supports the original findings of ESPS-230 that MRD + ASA is preferred to ASA across a range of key outcomes.
Key differences between the trials providing direct clinical evidence
All of the trials relevant to the decision problem were considered to be of good quality. However, the trials were disparate in terms of their design, patient populations, interventions and definition/reporting of outcomes (clinical and safety), which means that it is difficult to compare outcomes across the trials or perform evidence synthesis with any confidence using only the summary data reported in the published studies.
-
Design The mean length of follow-up between trials ranged between 1.91 years26 and 3.5 years. 56 The ESPRIT56 trial was the only non-industry-funded trial.
-
Population Patients in ESPRIT56 were randomised within 6 months of a minor ischaemic stroke/TIA, whereas patients in ESPS-230 and PRoFESS57 were randomised within 3 months of ischaemic stroke/TIA and minor ischaemic stroke, respectively. A marked divergence was observed in the disability ratings (as measured by the Rankin Scale65) between the stroke patients in the three trials30,56,57 that exclusively included only ischaemic stroke/TIA patients. To illustrate, in the ESPRIT56 trial the entry criteria limited the study patients to those who had suffered a minor TIA or a minor ischaemic stroke (43% of patients had no stroke symptoms, 53% had minor symptoms), whereas ESPS-230 (17%) and PRoFESS57 (24%) included patients with severe stroke symptoms. The Assessment Group notes that none of the trials identified patients with multivascular disease as being a clinically important subgroup.
-
Interventions There was also disparity in the daily doses of ASA given in the trial: ‘up to 350 mg’,26 30–325 mg56 and 50 mg. 30 In the UK, the current standard dose of ASA is 75 mg per day. However, as there appears to be little variation in the efficacy of doses higher than 75mg, there may be no impact on the main outcomes of the trials, although the bleeding risk may be increased with higher doses. The efficacy of lower doses of ASA (< 75 mg per day) is less well established than the efficacy of higher doses. 9,66
-
Outcomes First, none of the trials had the same primary outcome. Second, two trials utilised a composite event as a primary oucome. 26,57 The use of composite events in clinical trials has been criticised in a number of papers67,68 and guidelines67 for their use have been published. The guidelines67 state that to be meaningful to clinicians, composite events should include components that are similar in importance to patients, occur with similar frequency and are affected to a similar degree by the intervention. When looking at the primary composite event used in CAPRIE,26 ischaemic stroke or MI may not be considered as important to patients as death. In addition, there were many more patients with ischaemic stroke in CAPRIE26 than there were MIs or vascular deaths. The primary composite event described in ESPRIT56 included death from vascular causes, non-fatal stroke, non-fatal MI and non-fatal major bleeding, but these outcomes may not be considered to be similar by patients. Third, it is difficult to summarise the findings related to adverse events, as the classification of these outcomes differed across the trials; this was especially apparent for ‘bleeding events’. However, upon investigation the Assessment Group did not identify any unexpected adverse events associated with any of the drugs – bleeding was associated with ASA and headache was associated with MRD.
Indirect clinical evidence available
As previously discussed, the availability of four good-quality RCTs did not allow the comprehensive comparison of clinical and safety outcomes associated with the relevant interventions across the key populations of interest. In an effort to make best use of all available clinical information, the Assessment Group undertook a mixed-treatment comparison and investigated outcomes, where possible, for the ischaemic stroke/TIA population. The Assessment Group concluded that there were no major differences in the results of the mixed-treatment comparison and the direct estimates from head-to-head trials. However, two of the five newly generated comparisons do yield statistically significant results (1) MRD alone was associated with an increased risk of recurrent stroke when compared with clopidogrel, and (2) clopidogrel was associated with fewer major bleeding events compared with ASA. Owing to the small numbers of trials involved in the mixed-treatment comparison and the forced selection of limited outcomes, caveats apply to the results. In addition, the findings were based on patient populations in which there is no differentiation between patients with vascular disease in a single bed and those with multivascular disease. The results of the indirect analyses, although confirmatory of the direct results, must therefore be interpreted with caution.
Patients with multivascular disease
Recently published data from the REACH52 registry attest to the view that patients with multivascular disease are at an increased risk of future occlusive vascular events when compared with patients with disease in one vascular bed. Based on the post hoc analyses described by the manufacturer in the manufacturer’s submission and the post hoc analyses conducted by the Assessment Group; there is also evidence from CAPRIE26 to support the view that patients with multivascular disease are an important clinical subgroup whose event risk profiles are different from other subgroups of patients. In summary, it appears that patients with multivascular disease have elevated risks for more than one event (ischaemic stroke and MI); this is in contrast with the ischaemic stroke-only and MI-only subgroups, which have been shown to have elevated risks for single events (e.g. ischaemic stroke-only patients have high risks of ischaemic stroke, and MI-only patients have high risks of MI).
Currently, there is no NICE guidance available that identifies a specific treatment for a patient who has multivascular disease, and NICE24 has called for further research in this complex area: ‘Further research is recommended on the effectiveness of clopidogrel in people who are at high risk of recurrent occlusive vascular events … and in people who have recurrent events while taking recommended antiplatelet therapy’.
Evidence from the CAPRIE26 trial allows post hoc exploration of the clinical effectiveness of clopidogrel for patients with multivascular disease and offers a starting point for future discussions regarding appropriate clinical pathways for this subgroup of patients. Existing analyses are based on different definitions of multivascular disease and consensus is required in order to ensure informed and consistent decision-making for patients with multivascular disease.
Commentary on European Medicines Agency approval and guidelines/guidance issued by NICE
The Assessment Group notes that ASA is not licensed for use in patients with peripheral arterial disease, nor is clopidogrel licensed for use in patients with TIA. However, the Assessment Group’s clinical experts are of the opinion that in clinical practice in England and Wales, ASA is routinely prescribed for patients with peripheral arterial disease and sometimes clopidogrel is prescribed for patients with TIA who cannot tolerate MRD or ASA.
The distinction between patients with NSTEMI and STEMI is now important, as recently updated NICE guidelines25 still state that patients diagnosed as NSTEMI who are at moderate to high risk of MI or death should be treated with clopidogrel + ASA for a period of 12 months after the most recent acute event and after 12 months’ treatment should revert to low-dose ASA. At present, there is no NICE guidance for patients diagnosed with STEMI, although NICE clinical guideline 4828 indicates that these patients should receive clopidogrel + ASA for 4 weeks after the most recent event and thereafter revert to standard treatment, usually low-dose ASA. It is not clear how the recommendations in TA9024 fit with the published guidelines, as TA9024 does not differentiate between patients with NSTEMI and STEMI.
Chapter 4 Assessment of cost-effectiveness
Introduction
There are three distinct elements to this section on cost-effectiveness. First, a critical appraisal of the existing economic evidence describing clopidogrel and MRD since the publication of the previous NICE guidance24 (TA90) is presented. Second, a critique of the two economic models submitted by the manufacturers is described. Third, the results of the Assessment Group’s de novo economic evaluation are presented and summarised. It should be noted that a substantive amount of the analysis of cost-effectiveness was based on confidential data provided by the manufacturers. This document has been edited as appropriate so as to maintain confidentiality.
Review of existing cost-effectiveness studies
Full details of the search strategy and the methods for selecting evidence are presented in Chapter 3. Of the 34 potentially relevant studies, 1169–79 met the criteria for inclusion in the cost-effectiveness review; one study69 was also included in the SR that informed the previous guidance. 24 Of the 1169–79 included studies, seven69–75 were published in full, whereas four76–79 were available only in an abstract format. Most of the studies were of reasonable quality; however, more detail and focused critique of the clinical effectiveness evidence used to inform the economic evaluations would have improved the quality of the studies (see Appendix 2).
Characteristics of economic evaluations
Five69,71,72,74,76 of the 1169–79 studies included were described as cost-effectiveness analyses and six70,73,75,77–79 as cost–utility analyses. The cost-effectiveness analyses have used a range of health outcomes including life saved, events avoided, life-years lived, time spent free of stroke recurrence or disability and life expectancy. All of the cost–utility analyses have used QALYs as the main measure of health outcome. As presented in Table 27, seven studies69,71,75–79 compared clopidogrel versus ASA; Karnon et al. 73 compared clopidogrel for the first 2 years followed by ASA indefinitely versus ASA; Chen et al. 72 compared clopidogrel + low-dose ASA versus ASA; Beard et al. 70 compared MRD + ASA versus MRD single agent, low-dose ASA, clopidogrel or no treatment; and Matchar et al. 74 compared placebo versus ASA, ASA + MRD or clopidogrel.
| Study | Source | Type of study | Interventions | Study population | Country | Time period | Industry/author affiliation |
|---|---|---|---|---|---|---|---|
| Annemans 200369 | Full text | CEA | CLOP vs ASA | Patients with MI, IS or PAD; mean age of 62.5 years | Belgium | 2 years | The paper was supported by a grant from Sanofi–Synthelabo and Bristol–Myers Squibb |
| Beard 200470 | Full text | CUA | MRD + ASA vs:
|
Patients who survived an initial acute stroke; mean age of 70 years | UK | 25 years | This project was supported with funding from Boehringer Ingelheim |
| Berger 200871 | Full text | CEA | CLOP vs ASA | Patients with MI, IS or PAD | Germany | 2 years | Supported by Aventis Pharma Deutschland |
| Chen 200972 | Full text | CEA | CLOP + low-dose ASA vs ASA | Patients with established CVD | USA | Follow up of CHARISMA study59 (28 months) | This project has been funded by grants from Sanofi (Paris, France) and Bristol–Myers Squibb (New York, NY, USA) |
| Delea 200376 | Abstract | CEA | CLOP vs ASA | Population with recent IS, MI or diagnosed with PAD; subgroups of 55-, 65- and 75-year-olds | USA | Lifetime of patient | NR |
| Karnon 200573 | Full text | CUA | CLOP for 2 years followed by ASA indefinitely vs ASA | Population with recent IS, MI or PAD aged 60 years | UK | 40 years | This study was supported by Sanofi–Synthelabo and Bristol–Myers Squibb |
| Matchar 200574 | Full text | CEA | Placebo vs:
|
Population with previous IS or TIA aged 70 and with the characteristics of those patients in the Framingham population with first IS | USA | Lifetime of patient | Source of financial support: The Stroke Policy Model80 was developed with support from the Agency for Health Care Research, Quality (1 R03 HS11746–01). The current application was developed while Drs Matchar and Samsa served as consultants to Boehringer Ingelheim |
| Schleinitz 200475 | Full text | CUA | CLOP vs ASA | Population with previous MI or stroke or diagnosed with PAD; mean age 63 years | USA | Lifetime of patient | Dr Schleinitz was supported by an ambulatory care training grant from the Department of Veterans Affairs, a training grant from the Agency for Healthcare Research and Quality (AHRQ), and an NIH BIRCWH grant (HD43447) |
| Palmer 200577 | Abstract | CUA | CLOP vs ASA | Population with previous IS or TIA occurred in the last 90 days (median 15 days) | Belgium, France, Switzerland and the UK | 18 months | NR |
| Stevenson 200878 | Abstract | CUA | CLOP vs ASA | Population with previous MI, who sustain an IS or PAD (high-risk patients) | UK | Lifetime of patient | NR |
| Van Hout 200379 | Abstract | CUA | CLOP vs ASA | Population with previous MI or stroke or diagnosed with PAD | Netherlands | Lifetime of patient | NR |
The study populations in the included studies were made up of patients with a history of cardiovascular disease (MI, ischaemic stroke, TIA or peripheral arterial disease); this matches the populations described in the key clinical trials used to derive efficacy data. Only one study78 explicitly considered patients with multivascular disease. The mean age varied according to the trial source used, ranging from 60 to 70 years. Only four studies70,73,77,78 described a UK population. Most of the studies adopted a lifetime perspective; however, four69,71,72,77 adopted a short-term perspective (e.g. duration of the clinical study follow-up).
Economic models
Only one of the included studies was not based on an economic model; Chen et al. 72 performed an economic evaluation using data from the CHARISMA59 trial without any survival projection beyond 28 months. Matchar et al. 74 used an individual sampling model based on a model previously developed for the secondary prevention of stroke. Berger et al. 71 adapted the model developed by Annemans et al. 69 and Beard et al. 70 based their model on the model developed by Cambers et al. 81 All relevant assumptions and extra information describing the models are summarised in Table 28.
| Study | Type of model | Perspective | Model assumptions | |
|---|---|---|---|---|
| Outcomes | Costs and resource use | |||
| Annemans 200369 |
Markov model Cycle length: 6 months |
Belgian public health payer |
Risk of death from other causes was equal for CLOP and ASA Risk of vascular death was included in the model separately, because it was assumed that over the 2-year study period both drugs affected only vascular death Life expectancy does not decrease further when a patient has more than one additional event Adverse events were only included where a difference between CLOP and ASA was expected, based on pharmacological profiles, and where hospitalisation and intensive resource use would have been required Concomitant medication continued unchanged for the duration of the analysis or until death and, in view of the small difference in concomitant medication profiles for patients receiving ASA or CLOP, an average of the two groups was used for all patients |
DRG derived costs for Belgium were from the year 1997 and were updated to 2002 using an inflation rate of 3% The total cost of patient management was calculated by estimating the total of acute costs and follow-up costs per patient Acute costs covered hospital admission, initial investigations, interventions, re-admission for further interventions and inpatient rehabilitation Follow-up costs comprised outpatient rehabilitation, GP/specialist visits, follow-up examinations, complications, nursing homes and home care |
| Beard 200470 |
Model based on Chambers 199981 model Markov model Cycle length: 90 days |
UK health-care service |
Patients entering the model were assumed to have survived an initial acute stroke event Patients who survived an initial acute episode would be considered suitable for treatment with an antiplatelet therapy Patients had already received rehabilitation treatment for the initial stroke event prior to entering the model, and were being placed on standard long-term care, according to their level of permanent disability/functional status Only adverse events associated with withdrawal from therapy are important to outcomes in the model |
No assumptions made |
| Berger 200871 |
Markov model adapted from Annemans69 Cycle length: 6 months |
German third-party payer | Two scenarios are compared: survival data based on Framingham database and on Saskatchewan databases | German cost data for acute and follow-up treatment of patients with MI, IS or PAD as published by Diener et al.82 were decreased by the included costs for CLOP treatment because of their separate consideration within this Markov model7 |
| Chen 200972 | No model has been developed | US health-care system (payer) | NR | NR |
| Delea 200376 |
Markov model Cycle length: NR |
NR | NR | NR |
| Karnon 200573 |
Markov model Cycle length: 1 year |
UK NHS perspective | The model assumes patients receive lifelong therapy with CLOP or ASA | NR |
| Matchar 200574 | Individual sampling model based on the Duke Stroke Policy Model (DSPM)80 for secondary stroke prevention The model has been run 100 times | Health-care provider |
All patients are assigned an initial Rankin Score of 1 The placebo group was assumed to follow the natural history of 70-year-olds with the characteristics of those patients in the Framingham population with first IS For each antiplatelet group, the cost per month was increased by an estimated cost of antiplatelet medications For each antiplatelet group, the risk of subsequent IS was reduced, using a risk ratio that was estimated from the randomised trials |
NR |
| Schleinitz 200475 |
Markov model Cycle length: 1 month |
Societal perspective |
When more than two events occurred, the Markov state that combined the two events with the lowest utility was used Inclusion of the variable severity of stroke not included in the main trial on which the model is based It is assumed that CLOP did not alter the distribution of severity, based on studies of other antiplatelet therapies As CAPRIE26 results were heterogeneous for the three subgroups, the estimates and 95% CIs for the efficacy of CLOP for each subgroup, rather than the primary study estimate, has been used The efficacy of CLOP in reducing haemorrhagic side effects was varied by a factor of 0.5–2 |
The calculation of chronic care costs after survival of severe stroke or intracranial haemorrhage and other chronic conditions includes 20% of the chronic cost of the other condition to account for overlapping therapy |
| Palmer 200577 |
Markov model Cycle length: NR |
NR | NR | NR |
| Stevenson 200878 |
Markov model Cycle length: NR |
NR | NR | NR |
| Van Hout 200379 |
Markov model Cycle length: NR |
NR | NR | NR |
Cost data and cost sources
All of the studies stated the currency used; five of them also included the currency year, which ranged from 2002 to 2007. Four studies used euros, three used pounds sterling and four used US dollars. The majority of the studies discussed cost items and provided useful definitions of costs. Drugs costs have been taken from a variety of different sources including local cost lists;69 published literature;71 the BNF;70,73 and the WEB of pharmacy wholesale suppliers. 74,75 Costs of acute events, including hospitalisations and acute care, have been taken from the trial-based papers,71,73 Medicare diagnosis-related groups data,74,75 NHS Trust Financial Return data70 and the published literature. 71,78 Only three papers76,77,79 do not state the sources of the cost data used. All papers but one74 have mentioned a discount rate for costs, as Table 29 shows.
| Study | Cost items and cost data sources | Currency and currency year | Discount rate (%) | |
|---|---|---|---|---|
| Annemans 200369 | Ambulatory costs from INAMI tariff list for Belgium; AEs, unit costs from Belgian DRG; cost of CLOP and ASA from ‘Répertoire Commenté des Médicaments’ Public Belgian costing | Euros/2002 | 3 | |
| Beard 200470 | The model considered three specific areas of resource use. Hospitalisation costs from the NHS Trust Financial Returns data; community-based resource costs were based on the Personal Social Services Research Unit Health and Social Care Costs; drugs costs from BNF 2002 prices | £/2002 | 6 | |
| Berger 200871 |
|
Costs from the literature excluding cost of CLOP | Euros/NR | 3 |
| Chen 200972 | Hospitalisations, physician costs, procedures, post acute care and medications. Prices were obtained from price weights derived from comparable populations of US patients | US$/2007 | 3 | |
| Delea 200376 | Antiplatelet therapy; inpatient and outpatient treatment of IS; long-term care for patients with disability; sources NR | US$/NR | 3a | |
| Karnon 200573 |
|
£/2002 | 6 | |
| Matchar 200574 | Cost of events from Medicare claims data; cost of drugs from the WEB of Pharmacy wholesale and Federal Supply Schedule | US$/NR | NR | |
| Schleinitz 200475 |
|
(a) to (d) Medicare diagnostic-related group data and literature and published literature (e) Average US wholesale price for medications and based on prices negotiated by a large volume purchaser |
US$/2002 | 3 |
| Palmer 200577 | NR | NR | Euros/NR | Local guidelines |
| Stevenson 200878 | NR | Literature review | £/NR | 3.5 |
| Van Hout 200379 | NR | NR | Euros/NR | 4 |
Efficacy data and data sources
Only Palmer et al. 77 and Stevenson et al. 78 present data related to efficacy; the rest of the studies only point out that efficacy data are taken from a specific trial. Table 30 describes the information from the main trials used in each of the economic evaluations.
| Study | Efficacy data | Efficacy data sources | Health outcomes | Health outcome data sources | Discount rate (%) |
|---|---|---|---|---|---|
| Annemans 200369 | NR | CAPRIE26 and Saskatchewan database. Inpatient and outpatient management derived from analysis of Belgian and international publications and official Belgian health statistics, and were validated by a group of eight Belgian clinical experts | Cost per LYS; quantity of events; events avoided | CAPRIE trial26 and Saskatchewan database | 3 |
| Beard 200470 | NR | ESPS-2 study30 for all treatments except CLOP where data came from CAPRIE.26 Risks for acute stroke recurrence from years 3 to 5 from the Oxford Community Stroke Project and > 5 years risks assumed to rise with age | Life-years lived; QALYs; time spent free of stroke recurrence or disability; avoided strokes; number of events | Original trials (CAPRIE26 and ESPS-230) and published literature | 1.5 |
| Berger 200871 | NR | CAPRIE trial26 and a Delphi panel to adapt efficacy data to Germany setting | Fatal and non-fatal strokes; LYS | CAPRIE study26 and Delphi panel | 3 |
| Chen 200972 | NR | CHARISMA59 and Saskatchewan database | Lost life expectancy | CHARISMA trial59 and Saskatchewan database | 3 |
| Delea 200376 | NR | CAPRIE study26 | Life expectancy | NR | 3 |
| Karnon 200573 | NR |
UK observational studies CAPRIE trial26 Government Actuary Department (1999–2000) |
QALYs; number of events; LYG | CAPRIE study;26 Harvard utility database; Tengs and Lin;83 Derdeyn and Powers85 Zeckhauser and Shepard;86 Haigh et al.;87 Lee et al.;88 Danese et al.89 | 1.5 |
| Matchar 200574 | NR | Transition functions from Framingham study; CAPRIE study;26 ESPS-2 study30 | QALYs | Duke Stroke Policy Model;80 ‘utilities were estimated from a large survey of patients at risk for major stroke’ (no ref.) | NR |
| Schleinitz 200475 | NR | Based on data from CAPRIE26 and mortality data from life tables. Rate of TTP with CLOP from an observational study | QALYs | Published papers; CAPRIE study26 | 3 |
| Palmer 200577 |
RR increase of CLOP vs ASA: serious vascular events 1.11 RR increase of ASA vs CLOP: major bleedings 1.12 |
‘Cochrane review’ CAPRIE trial26 |
QALYs | NR | ‘Discount rates were applied according to the local guidelines’ |
| Stevenson 200878 |
|
(a) and (b) CAPRIE study26 | QALYs | NR | 3.5 |
| Van Hout 200379 | NR | CAPRIE study26 | QALYs | CAPRIE study26 | 4 |
Health outcome data and data sources
Six of the economic evaluations used QALYs as the main measure of health outcome; other outcomes include life-years saved and life expectancy.
Only Matchar et al. 74 have not discounted health outcomes. In the study by Delea et al. 76 it is not clear if discounting has been applied to both costs and benefits. In the study by Palmer et al. ,77 discounting was used, but the discount rate is not explicitly stated. Health outcome information from the included studies is summarised in Table 30.
Cost-effectiveness ratios
The results of the cost-effectiveness analyses are described in Table 31. In summary, Annemans et al. 69 and Berger et al. 71 conclude that, for the overall population (MI, ischaemic stroke and peripheral arterial disease), clopidogrel is cost-effective compared with ASA, with an incremental cost-effectiveness ratio (ICER) of €13,390 per QALY and €14,380 per life-years saved (scenario 1) or €18,790 per life-years saved (scenario 2). Chen et al. 72 and Delea et al. 76 show an ICER of US$36,343 per life-years saved and a range of US$40,204 to US$49,107 per life-years saved, respectively, concluding that clopidogrel is cost-effective compared with ASA.
| Study | Total costs | Total outcomes | ICERs | Conclusion |
|---|---|---|---|---|
| Annemans 200369 |
|
Events in ASA group: 120.22 Events in CLOP group: 107.2 |
ICER CLOP vs ASA; €13,390/LYG | The findings of this CEA suggest that secondary treatment of MI, IS and PAD patients with CLOP adds approximately 43–114 life-years per 1000 patients compared with ASA (depending on discounting) |
| Beard 200470 |
Primary analysis (per 1000 patients): (a) No treatment: €23,489,812 Secondary analysis (lifetime): (b) No treatment: €37,757,950 |
Primary analysis (per 1000 patients): (a) No treatment: 2357 QALYs (b) Secondary analysis (lifetime): (c) No treatment: 4199 QALYs |
5- and 25-year analysis: |
The current model suggests that, based on a consideration of first recurrence of stroke and the acute treatment impacts of TIAs and non-fatal OVEs, antiplatelet therapy based on MRD + ASA is a cost-effective treatment option over standard ASA. The model is sensitive to the long-term costs of very disabled patients |
| Berger 200871 | Overall, the 2-year costs per 1000 patients under immediately initiated CLOP prophylaxis were calculated to be €1,241,440 |
ASA (events per 1000 patients): CLOP: |
ICER:
|
The presented model shows cost-effectiveness of secondary prevention with CLOP vs ASA in patients with MI, IS or PAD |
| Chen 200972 | Mean cost per patient:
|
Life expectancy without in-trial events (years): Male, age 65 years: 11.63; female, age 65 years: 13.17 Unadjusted lost life expectancy associated with specific in-trial events (years): Male, age 65 years = mild stroke: 6.23; moderate-severe stroke: 8.71; MI: 4.69 Female, age 65 years = mild stroke: 7.53; moderate-severe stroke: 10.34; MI: 5.93 |
|
For the prespecified subgroup of CHARISMA59 patients with established CVD, adding CLOP to ASA for secondary prevention over 28 months of therapy appears to increase life expectancy modestly at a cost commonly considered acceptable within the US health-care system |
| Delea 200376 | NR | NR | ICER ranges from US$40,204 to US$49,107 per LYS | CLOP is cost-effective vs ASA in patients with recent IS, recent MI or PAD |
| Karnon 200573 | Lifetime costs:
|
Total number of events: LYG: QALYs gained: |
ICER:
|
CLOP has been demonstrated to be a cost-effective treatment in patients at risk of secondary OVEs, is clinically superior to ASA and has great potential for reducing the morbidity and mortality caused by these diseases |
| Matchar 200574 | Total cost per patient:
|
Total QALYs per patient:
|
Based on the means for 100 runs of 10,000 patients each.
|
ASA is superior to placebo. Choice between ASA and MRD + ASA is less obvious; but the more the decision-maker is WTP for improved outcomes the more likely it is that MRD + ASA will be preferred. CLOP was seldom judged to be the optimal strategy. But, results were not sufficiently robust to select between MRD + ASA and ASA based on statistical considerations alone |
| Schleinitz 200475 |
CLOP: ASA: |
QALYs (CLOP):
|
PAD: US$25,100/QALY CLOP more effective Stroke: US$31,200/QALY CLOP more effective MI: –US$26,200/QALY ASA more effective |
CLOP provides a large increase in QALYs at a cost that is within traditional societal limits for patients with either PAD or a recent stroke. Current evidence does not support increased efficacy with CLOP vs ASA in patients after MI |
| Palmer 200577 | NR | NR |
20,111€/QALY in Belgium 18,882€/QALY in France 15,620€/QALY in Switzerland 15,713€/QALY in UK |
In the four countries the ICER falls below the acceptable thresholds, showing that CLOP compared with ASA is cost-effective in the studied population |
| Stevenson 200878 | NR | NR | The mean cost per QALY for CLOP compared with ASA was £5443 (95% CI £2332 to dominated) | The model suggests that, in patients with a previous MI event and a subsequent IS or PAD event, CLOP can be considered cost-effective compared with ASA in terms of current UK thresholds |
| Van Hout 200379 | NR | NR | ICER: €17,279/QALY with event-specific risk reductions and €15,776/QALY using constant RRR of 8.7% | CLOP shows as a dominant strategy in patients not eligible for treatment with ASA. The cost-effectiveness is within an acceptable range when compared with ASA, especially in high-risk patients |
Schleinitz et al. ,75 Palmer et al. 77 and van Hout et al. 79 conclude that clopidogrel is cost-effective when compared with ASA (see Table 31), although Schleinitz et al. 75 also conclude that the current evidence does not support increased efficacy of clopidogrel in MI patients. Stevenson et al. 78 estimated that the mean cost per QALY for clopidogrel compared with ASA was £5443 in patients with a previous history of MI who then sustained an ischaemic stroke or a peripheral arterial disease event.
The evaluation by Beard et al. 70 concludes that MRD + ASA is a cost-effective option with an ICER below €5000 per QALY when compared with ASA or MRD alone, and it dominates when compared with clopidogrel or no treatment.
The study by Karnon et al. 73 concludes that the comparison of clopidogrel followed by ASA versus ASA yields an ICER of £21,489 per QALY.
Matchar et al. 74 show that placebo versus ASA and placebo versus MRD + ASA have similarly low ICERs; however, placebo versus clopidogrel yields a high ICER with a low probability of being cost-effective.
The majority of the trials have performed univariate and probabilistic sensitivity analysis. In general, the univariate sensitivity analyses show consistency around the ICER. All univariate sensitivity analyses are summarised in Appendix 8. Beard et al. 70 state that their model is sensitive to the long-term costs of very disabled patients. Matchar et al. 74 conclude that, although the simulations in their model can support the results shown, these are not sufficiently robust.
Summary of evidence and discussion
In general, the results of the literature review of cost-effectiveness evidence show that from a health service perspective, the use of clopidogrel in patients with previous peripheral arterial disease, ischaemic stroke or MI is a cost-effective option compared with ASA in the secondary prevention of occlusive vascular events. However, it is noted that Schleinitz et al. 75 conclude that current evidence does not support increased efficacy of clopidogrel in the MI patient group; this is the only evaluation that includes subgroup analysis to estimate ICERs by patients’ previous event. This is also the only study not funded by a pharmaceutical manufacturer (four papers76–79 did not provide details of industry affiliation).
The combination of MRD + ASA seems to be cost-effective compared with any other treatment (vs ASA, vs clopidogrel, vs no treatment) in patients with previous ischaemic stroke or TIA in the secondary prevention of occlusive vascular events. There is only one evaluation70 that includes this combination (MRD + ASA) and therefore the evidence base is limited.
Although model structures are similar, the length of the cycles differs from one study to another and the assumptions regarding the transition probabilities (e.g. Annemans et al. 69 – life expectancy assumptions) are not always reliable. Data in the models are from a broad variety of sources, which makes it difficult to pool the results and make definitive conclusions.
All evaluations except three71,72,78 were published prior to 2006; this means that more recent trials and papers have not been used to inform the economic evaluations (e.g. clinical data from PRoFESS,57 REACH17 or MATCH58 are not described in the papers). The relevance of this cost-effectiveness review to decision-making is therefore limited as the economic evaluations are not based on the most up-to-date clinical data.
Review of Boehringer Ingelheim submission
See Table 32.
| NICE reference case requirements | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | As per the final scope issued by NICE |
| Comparators | Therapies routinely used in the NHS, including technologies currently regarded as best practice | ASA, CLOP, MRD + ASA and no treatment |
| Perspective on costs | NHS and PSS | As per the final scope issued by NICE |
| Perspective on outcomes | All health effects on individuals | As per the final scope issued by NICE |
| Type of economic evaluation | Cost-effectiveness analysis | Cost-effectiveness analysis |
| Synthesis of evidence on outcomes | Based on a SR | All data are derived from head-to-head trials (mainly PRoFESS57) |
| Measure of health benefits | QALYs | QALYs |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | EQ-5D used to collect data from patients in the PRoFESS57 trial; published literature |
| Source of preference data for valuation of changes in HRQoL | Representative sample of general public | EQ-5D used to collect data from patients in the PRoFESS57 trial; published literature |
| Discount rate | An annual rate of 3.5% on both costs and QALYs | 3.5% per annum for costs and health effects |
| Equity weighting | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | All QALYs estimated by the model have the same weight |
Overview of submitted manufacturer’s submission
A Markov model was designed to assess the cost-effectiveness of MRD + ASA versus ASA alone, clopidogrel and no treatment for the secondary prevention of occlusive vascular events in patients who have experienced:
-
an ischaemic stroke and are tolerant of ASA
-
a TIA and are tolerant of ASA.
The model is based on the model developed by the Technology Appraisal Group to inform the previous guidance. 3
The structure of the manufacturer’s model is shown in Figure 4.
FIGURE 4.
Schematic structure of the Boehringer Ingelheim’s model.
The model estimates costs from the perspective of the UK NHS, and health outcomes in terms of life-years and QALYs in a simulated cohort of 1000 patients initially aged 45–80 years using a time horizon of 2.5–50 years and a cycle length of 6 months.
Costs and benefits have been discounted at a rate of 3.5% per annum.
The model presents five health states:
-
no recurrent stroke
-
recurrent ischaemic stroke
-
haemorrhagic stroke
-
vascular death
-
non-vascular death.
Patients enter into the model in the ‘no recurrent stroke’ health state, from where they may move to any other state or remain in the same state. From the ‘recurrent ischaemic stroke’ state patients may move to ‘haemorrhagic stroke’, ‘vascular death’ or ‘non-vascular death’ or remain in the ‘recurrent ischaemic stroke’ state. In the ‘haemorrhagic stroke’ state, patients will either remain in this state or die. Once patients enter the ‘haemorrhagic stroke’ health state, any additional recurrent haemorrhagic stroke events are not recognised in the model. The manufacturer states that this restriction is introduced to avoid the situation where an additional event (e.g. new ischaemic stroke) leads to a patient’s utility state improving. If multiple events occur in a single cycle, one event is given priority in allocating patients to a health state in the following order of descending priority: death, haemorrhagic stroke, ischaemic stroke. The model also includes two tunnel health states: ‘other haemorrhagic events’ and ‘new or worsening congestive heart failure’.
Summary of clinical effectiveness data
Transition probabilities during the first 4 years are derived from different trials for each of the arms:
-
MRD + ASA and clopidogrel – PRoFESS57 trial
-
ASA alone – combination of ESPRIT56 trial and ESPS-230 trial
-
no treatment – ESPS-230 trial.
Beyond the first 4 years, the transition probabilities are assumed to remain constant at the values of the last monthly cycle of the fourth-year period for the following transitions:
-
new recurrent ischaemic stroke from the ‘no recurrent stroke’ state
-
haemorrhagic stroke from the ‘no recurrent stroke’ state
-
haemorrhagic stroke from the ‘new recurrent ischaemic stroke’ state.
The manufacturer used published data from the Oxfordshire Community Stroke Project90 and the Lothian Stroke Registry91 to estimate the overall death rate among stroke patients compared with the general population. A multiplier of 1.5 was used to generate an overall expected age-related death rate beyond the trial period from the Office for National Statistics death rate data for the general population. The vascular and non-vascular death rates beyond the 4 years of the trial were assumed to sum to this rate.
The manufacturer has assumed that those patients who have experienced a TIA had a rate of previous ischaemic stroke events equal to 80% of those who had experienced a previous ischaemic stroke. This assumption is made on the basis of the previous multiple technology assessment (MTA)3 in which the Assessment Group made the same assumption.
Summary of costs and resource use
Event costs
Separate costs were assigned to the health states of ‘no recurrent stroke’, ‘recurrent ischaemic stroke’ and ‘haemorrhagic stroke’, based on the estimated percentage of patients who were disabled in each health state. Data from the PRoFESS57 trial were used to estimate the percentage of patients in each of these three health states who were disabled and non-disabled, based on the modified Rankin Scale; those who score 0–2 are defined as ‘non-disabled’ and those who score 3–5 are ‘disabled’. The cost data used in the model for disabled and non-disabled stroke patients were taken from the same source used in the original MTA3 updated using an inflation index using data from Personal Social Services Research Unit (PSSRU). 92
Costs are shown in Table 33.
| Health-state event | Costa (£) | Reference | ||
|---|---|---|---|---|
| IS | Institutional cost | Non-disabled (first cycle) | 5930 | bJones et al.3 |
| Non-disabled (subsequent cycle) | 0 | |||
| Disabled (first cycle) | 12,689 | |||
| Disabled (subsequent cycle) | 0 | |||
| Death | 8152 | |||
| Non-institutional cost | Non-disabled (first cycle) | 413 | ||
| Non-disabled (subsequent cycle) | 825 | |||
| Disabled (first cycle) | 1203 | |||
| Disabled (subsequent cycle) | 2406 | |||
| Haemorrhagic stroke | Institutional cost | Non-disabled (first cycle) | 5930 | |
| Non-disabled (subsequent cycle) | 0 | |||
| Disabled (first cycle) | 12,689 | |||
| Disabled (subsequent cycle) | 0 | |||
| Death | 8152 | |||
| Non-institutional cost | Non-disabled (first cycle) | 413 | ||
| Non-disabled (subsequent cycle) | 825 | |||
| Disabled (first cycle) | 1203 | |||
| Disabled (subsequent cycle) | 2406 | |||
Follow-up costs
National reference costs (2006–7) as used in the 2004 Technology Assessment Report3 were used to calculate the hospitalisation costs following congestive heart failure and other haemorrhagic events. The costs used in the model are summarised in Table 34.
Drug costs
Costs of drugs include branded cost for MRD + ASA and clopidogrel and generic costs of ASA. The branded drug costs were taken from MIMS (Monthly Index of Medical Specialities)93 (June 2009) and generic ASA cost from BNF 5794 (March 2009). These costs are shown in Table 35.
Utilities
The utility data for the health states of ‘no recurrent ischaemic stroke’, ‘recurrent ischaemic stroke’ and ‘haemorrhagic stroke’ are taken directly from the PRoFESS57,95 clinical trial, which used the EQ-5D as a measure at 1 year and 4 years. The 1-year data set was used as it contained the largest number of patients.
The manufacturer has used a paper by Miller et al. 96 (based on Galbreath et al. 97 and Smith et al. 98) as the source for the disutility value associated with congestive heart failure using the mean that is calculated when moving from New York Heart Association (NYHA) classification II to NYHA III/IV and NYHA I/II. The disutility value associated with other haemorrhagic events was calculated using utility data presented in Robinson et al. 99 and in Brown et al. 100
Summary of submitted results
The base-case analysis includes second-line treatment with ASA for those patients discontinuing first-line treatment in clopidogrel and MRD + ASA groups. A summary of the results is shown in Table 36 for ischaemic stroke patients and in Table 37 for TIA patients.
| MRD + ASA – long term (first-line); ASA (second-line) | CLOP – long term (first-line); ASA (second-line) | ASA | No treatment | |
|---|---|---|---|---|
| Total costs (£) | 37,430,180 | 39,238,555 | 36,725,769 | 36,678,013 |
| Total QALYs | 8724 | 8739 | 8593 | 8596 |
| ICER (MRD + ASA vs … ) (£) | – | 114,628 | 5377 | 5910 |
| MRD + ASA and ASA – long term (first-line); ASA (second-line) | CLOP – long term (first-line); ASA (second-line) | ASA | No treatment | |
|---|---|---|---|---|
| Total costs (£) | 37,010,692 | 38,871,872 | 36,278,556 | 36,197,693 |
| Total QALYs | 8781 | 8790 | 8660 | 8675 |
| ICER (MRD + ASA vs … ) (£) | – | 199,149 | 6053 | 7684 |
Summary of sensitivity analysis
Deterministic sensitivity analysis
In the scenario sensitivity analysis, statistically significantly different variables were set as central estimates from the PROFESS57 trial for MRD + ASA and clopidogrel arms, i.e. haemorrhagic stroke rates, dropout rates, other haemorrhagic events and congestive heart failure rates; all other transition probabilities were unchanged. Results are shown in Table 38 for ischaemic stroke patients and in Table 39 for TIA patients. For the reference case (ischaemic stroke patients), one-way univariate sensitivity analysis results are also shown in Table 40.
| MRD + ASA – long term (first-line); ASA (second-line) | CLOP – long term (first-line); ASA (second-line) | |
|---|---|---|
| Total costs (£) | 37,430,180 | 39,897,888 |
| Total QALYs | 8724 | 8760 |
| ICER (£) | – | 68,848 |
| MRD + ASA – long term (first-line); ASA (second-line) | CLOP – long term (first-line); ASA (second-line) | |
|---|---|---|
| Total costs (£) | 37,195,638 | 39,634,600 |
| Total QALYs | 8760 | 8799 |
| ICER (£) | – | 62,702 |
| Profile letter | Sensitivity analysis | Source of sensitivity analysis assumption | ICER (£) |
|---|---|---|---|
| Base case | 114,628 | ||
| A | Recurrent IS rate of MRD + ASA used for CLOP | MRD + ASA dominates | |
| B | Haemorrhagic stroke rate of MRD + ASA used for CLOP | MRD + ASA dominates | |
| C | Haemorrhagic stroke rate of MRD + ASA multiplied by factor of 1.12 | Estimated 80th percentile using SD data from PRoFESS57 for IH | 83,105 |
| D | Non-vascular death rate of MRD + ASA used for CLOP | 34,988 | |
| E | Vascular death rate of MRD + ASA used for CLOP | 54,949 | |
| F | Dropout rate of MRD + ASA used for CLOP | 234,647 | |
| G | Dropout rate of MRD + ASA multiplied by a factor of 1.1 | Assumption in the absence of variance data for a categorical variable from PRoFESS57 | 88,872 |
| H | Other haemorrhagic events rate of MRD + ASA used for CLOP | 122,270 | |
| I | CHF rate of MRD + ASA used for CLOP | 113,810 | |
| J | Non-drug costs increased by 50% | Assumption | 88,278 |
| K | Utility of haemorrhagic strokes multiplied by a factor of 0.9 | Estimated 80th percentile using SD data from PROFESS57 for IH | 81,498 |
| L | ESPRIT56 data alone used to estimate ASA vs MRD + ASA (RR) | 95,470 | |
| M | ESPS-230 data alone used to estimate ASA vs MRD + ASA (RR) | 183,875 |
For the scenario sensitivity analysis (ischaemic stroke patients) outlined above, one- and two-way univariate sensitivity analyses were also performed (Table 41).
| Profile letter (see table 8 in MS) | Sensitivity analysis | ICER (£) |
|---|---|---|
| Base case | 68,848 | |
| C | Haemorrhagic stroke rate of MRD + ASA multiplied by factor of 1.12 | 58,696 |
| G | Dropout rate of MRD + ASA multiplied by a factor of 1.1 | 61,142 |
| J | Non-drug costs increased by 50% | 65,838 |
| K | Utility of haemorrhagic strokes multiplied by a factor of 0.9 | 60,397 |
| M | ESPS-230 data alone used to estimate ASA vs MRD + ASA RR | 82,148 |
| CG | 53,242 | |
| CJ | 55,561 | |
| CK | 50,922 | |
| CM | 68,147 | |
| GJ | 58,255 | |
| GK | 54,636 | |
| GM | 70,110 | |
| JK | 57,756 | |
| JM | 78,198 | |
| KM | 70,690 |
A univariate sensitivity analysis was performed to demonstrate the impact on the size of the ICER (MRD + ASA vs ASA) of changing the source (ESPRIT56 or ESPS-230) of the ASA RR data. Using ESPS-230 data, the ICER changes from £5377 per QALY in the base case to £9535 per QALY for ischaemic stroke patients, and using ESPRIT56 data changes the ICER from £6053 per QALY in the base case to £3948 per QALY for TIA patients.
Probabilistic sensitivity analysis
After generating 500 iterations, the results for the probabilistic sensitivity analyses were as follows:
-
ischaemic stroke patients MRD + ASA versus clopidogrel: MRD + ASA has more than 90% probability of being cost-effective at a threshold of £30,000 per QALY.
-
TIA patients MRD + ASA versus clopidogrel: MRD + ASA has more than 90% probability of being cost-effective at a threshold of £30,000 per QALY.
Critique of Boehringer Ingelheim’s economic model by the Assessment Group
The submitted model considers a wide range of treatment alternatives and describes a wide range of resources to populate the model. The model is mainly based on the PRoFESS57 trial, although some data have been taken from ESPS-230 and ESPRIT56 to obtain probability transitions in the ischaemic stroke group. The transition probabilities during the first 4 years for the MRD + ASA and clopidogrel arms are derived from the above-mentioned trials, and beyond that point they have used the same transition probability as used for the last 6-monthly cycle. This is an unreliable basis for long-term projection, as close to the end of the trial patient numbers and the number of events are much reduced. As a consequence, estimated incidence rates are very volatile and should not be relied on to drive the major part of the model calculations.
Death rates amongst patients who have had strokes have been derived from two main papers;90,91 when these papers were checked, the figures quoted in appendix 9 of the manufacturer’s submission do not clearly match with those in the published papers. In relation to the TIA incidence rates, the manufacturer has assumed that patients who experienced TIAs had a rate of ischaemic stroke events equal to 80% of those who had experienced a previous ischaemic stroke; there is no evidence to support this assumption and it has not been tested in the one-way univariate sensitivity analysis.
The design of the model also includes tunnel health states to model adverse events. The tunnel health states are not depicted in the manufacturer’s submission and are poorly addressed in the excel model. The manufacturer’s submission is sometimes hard to follow because of several mistakes in the appendices notation (e.g. manufacturer’s submission, p. 27, section 3.2.1) and within the Excel model (e.g. Overview spreadsheet E35 cell in the excel model says 10 years’ time horizon instead of 50 years). The figure describing the model (manufacturer’s submission, p. 25) has two arrows from ‘no recurrent stroke’ health state to ‘non-vascular death’, which is not consistent with the structure described.
The parameter distributions of costs used in the probabilistic sensitivity analysis are not commonly used distributions and their use is not justified by the manufacturer.
The manufacturer states that ‘MRD + ASA long term first line is cost-effective against clopidogrel … Based on these ICERs at a threshold of £20,000 per QALY, it remains cost-effective until clopidogrel drops by 45% of brand price for ischaemic stroke patients or 51% for TIA patients’ (manufacturer’s submission, pp. 41–2). The Assessment Group notes that the generic price of clopidogrel as listed in the Electronic drug tariff 33 March 2010 is £10.90 (30 × 75-mg tablets); this constitutes a 69% reduction in price [branded Plavix (£36.35) was used in the model] and means that compared with MRD + ASA, clopidogrel is cheaper and more effective for both ischaemic stroke and TIA populations.
Review of the Sanofi–aventis/Bristol–Myers Squibb submission
See Table 42.
| NICE reference case requirements | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by NICE | As per the final scope issued by NICE |
| Comparators | Therapies routinely used in the NHS, including technologies currently regarded as best practice | ASA, CLOP, MRD + ASA, MRD |
| Perspective on costs | NHS and PSS | As per the final scope issued by NICE |
| Perspective on outcomes | All health effects on individuals | As per the final scope issued by NICE |
| Type of economic evaluation | Cost-effectiveness analysis | Cost-effectiveness analysis |
| Synthesis of evidence on outcomes | Based on a systematic review | All data are derived from head to head trials (mainly CAPRIE26) |
| Measure of health benefits | QALYs | QALYs |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Utilities (MI, PAD, stroke) derived from published, population-based studies (TTO or SG); utilities (MVD) based on assumption |
| Source of preference data for valuation of changes in HRQoL | Representative sample of general public | Population-based studies |
| Discount rate | An annual rate of 3.5% on both costs and QALYs | 3.5% per annum for costs and health effects |
| Equity weighting | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | All QALYs estimated by the model have the same weight |
Overview of submitted manufacturer’s submission
A Markov model is designed to assess the cost-effectiveness of clopidogrel, MRD + ASA, ASA and MRD alone for the secondary prevention of occlusive vascular events: MI, ischaemic stroke and vascular death. Cost-effectiveness estimates are calculated for four different patient populations:
-
patients who have previously suffered a MI
-
patients who have previously suffered an ischaemic stroke
-
patients who were diagnosed with peripheral arterial disease
-
patients with multivascular disease which is described as ischaemic disease in more than one vascular bed.
The same model structure is used throughout, but the baseline risks of vascular events differ for each population. The four treatments under consideration are compared against each other only in the ischaemic stroke population; whereas, in the MI, peripheral arterial disease and multivascular disease populations only clopidogrel is compared with ASA.
The model estimates costs from the perspective of the UK NHS and health outcomes in terms of life-years and QALYs. A cohort of 1000 patients with the qualifying diagnosis (MI, stroke peripheral arterial disease or multivascular disease) and aged 65 years progresses through the model over a time horizon of 35 years. The starting age of 65 years was chosen as the average age in the PRoFESS57 trial was 66.1 years, in CAPRIE26 62.5 years and in REACH17 68.6 years. The cycle length is 3 months and only one event can occur in each cycle. The model structure is depicted in Figure 5. Costs and benefits have been discounted at a rate of 3.5% per annum.
FIGURE 5.
Diagram of the Markov model. ACS, acute coronary syndrome; ASP, aspirin; CLOP, clopidogrel; PAD, peripheral arterial disease; TAG, technology assessment guidance.
The model utilises six health states (see Figure 5):
-
Initial state This is the starting condition for all patients, and is considered to be a ‘stable’ state.
-
Death Separately recorded for deaths of non-vascular and vascular origin.
-
History of MI The condition of patients following a non-fatal MI.
-
History of stroke The condition of patients following a non-fatal ischaemic stroke.
-
History of MI and stroke The condition of patients who have suffered both a non-fatal MI and a non-fatal stroke.
-
TA80 state This intermediate state relates to the TA80 guidance,45 which recommends that treatment with clopidogrel+ASA should be continued for up to 12 months (four cycles in the model) after the most recent acute episode of NSTEMI. In the model, after four cycles, patients go back to antiplatelet monotherapy.
All adverse events are included in the cost and QALY calculations, but are not recorded separately as distinct health states or events in the model.
Each patient population (MI, stroke, peripheral arterial disease and multivascular disease) progresses through the model subject to its specific risk profile and parameters depending on previous history. The presence of previous vascular events thus influences the risk of future health states. Patients in the model can either remain stable or experience a MI or a stroke or death (from vascular or non-vascular causes). Deaths within 30 days of a new MI or stroke are defined as vascular deaths and such patients will progress directly to death.
Summary of effectiveness data
The baseline risk of events related to ASA has been taken from the REACH17 registry and from a network meta-analysis of six studies: ESPS-2,30 ESPRIT,56 CAPRIE,26 MATCH,58 CHARISMA59 and PRoFESS. 57 The REACH17 registry recruited a large international cohort of patients (n = 68,236) with either established atherosclerotic arterial disease or at least three risk factors for atherothrombosis, and considered the outcomes of cardiovascular death, non-fatal MI and non-fatal stroke. The event rates were different for year 1 (REACH registry17), year 2 (unpublished – academic in confidence) and year 3 (published online101). The model assumes the 3-year data to be applicable for all subsequent years (years 3–35).
The manufacturer has constructed a matrix to allocate the correct risk of events to patients as they change health states through the model, such that state- and population-specific event rates and probabilities are assigned. This trace matrix is reproduced in Table 43.
| Population no. after new event | Health state | |||||
|---|---|---|---|---|---|---|
| No history | History of NF stroke | History of NF MI | History of stroke and MI | |||
| Population no. at start of model | 1 | Patients with previous stroke | 1 | 1 | 4 | 4 |
| 2 | Patients with previous MI | 2 | 4 | 2 | 4 | |
| 3 | Patients with previous PAD | 3 | 4 | 4 | 4 | |
| 4 | MVD patients | 4 | 4 | 4 | 4 | |
The REACH17 event risks are assumed to be applicable to a population treated with ASA, as 67% of registry patients received ASA monotherapy. Aspirin was chosen to be the treatment of reference to which the three other comparators are modelled. The relative treatment effects of the other three treatments (MRD, MRD + ASA and clopidogrel) versus ASA have been estimated based on direct estimates from clinical trials or indirect estimates from the network meta-analysis of the six studies mentioned above. The network meta-analysis was conducted for the end points – stroke, MI, vascular death, non-vascular death, and major and minor bleeding events.
The base case in the model considers all ASA arms in the network meta-analysis studies to have equal efficacy. Non-vascular death rates have been derived from life tables. The non-vascular mortality rate is estimated by removing deaths owing to the diseases of circulatory system from age-specific deaths from all causes.
The following assumptions were used by the manufacturer in the model:
-
Non-vascular death was assumed to be the difference between ‘all-cause mortality’ and ‘death from vascular causes’.
-
When fatal and non-fatal vascular events were not reported separately then the total of fatal and non-fatal events was used as an approximation for non-fatal events in the dataset.
-
In the absence of any evidence on non-vascular death having a dose–response relationship with ASA (in contrast with the vascular events and adverse events), it was assumed that the risk of non-vascular death was equal for all ASA doses.
-
As the ESPRIT56 trial did not impose a specific ASA dose, but left the decision on dosing to the local investigators, the ASA arm of this trial was assumed to be a weighted average of the low, medium and high ASA dose arms, with weights equal to the proportion of patients observed on the different doses: 46%, 48% and 5%, respectively.
-
The Antithrombotic Trialists’ Collaboration (ATTC) data66 describing the efficacy of ASA versus no treatment reported only on the composite end point of ‘serious vascular events’ but not on the separate components. Therefore, the assumption was made that the relative efficacy of ASA versus no treatment was equal for all these separate end points: MI, stroke and vascular death.
The model presents six different effectiveness analyses derived from the above sources:
-
network meta-analysis with the six studies above and ASA doses pooled (base case)
-
network meta-analysis splitting up the ASA comparator into three separate comparators: low-, medium- and high-dose ASA
-
head-to-head analysis based solely on the PRoFESS57 trial
-
head-to-head analysis based solely on the CAPRIE26 trial
-
head-to-head analysis based on post hoc analysis on multivascular disease patients from CAPRIE26 trial.
To estimate the efficacy of clopidogrel + ASA in the TA8045 state versus ASA, data from the CURE27 trial have been used.
Summary of adverse events data
Baseline risk of adverse events relating to ASA has been derived from three papers: one meta-analysis66 and two RCTs. 26,30
The risk of a major bleeding event is taken from a meta-analysis of RCTs of antiplatelet therapy. 66
The risk of minor bleeding event is derived from the ESPRIT56 trial. The risk of dyspepsia is taken from the ESPS-230 trial comparing ASA with MRD and a combination of MRD + ASA for the secondary prevention of stroke.
Summary of costs and resource use
Event costs
The cost of a non-fatal stroke is a weighted average of the 3-month cost of an acute mild stroke, a moderate stroke and a severe stroke as estimated from a burden-of-illness model using patient-level data. 102
The cost of a non-fatal MI is taken from a regression analysis103 calculating the impact of diabetes-related complications on health-care costs. This paper also estimates the cost of a vascular death as the average of the cost of a fatal MI and a fatal stroke.
The cost of a non-vascular death is based on an assumption from another economic model104 that estimated the cost of dying from unrelated causes to be approximately £250.
The cost of a major bleeding event is an average of all Health Related Groups (HRG) Reference Costs that relate to major bleeding reported in the NICE CG36105 costing report 2006 for atrial fibrillation, which mentions calculations for major and minor bleeding events applicable to atrial fibrillation patients.
The cost of a minor bleeding event is mentioned in the NICE report24 as equal to the cost of a visit to an accident and emergency department, and reported upper and lower limits of £61 and £111, respectively.
The adverse event cost of dyspepsia is taken from a detailed cost analysis106 of the supply and management of upper gastrointestinal and renal toxicity related to low-dose ASA use.
All events costs are summarised in Table 44.
| Event | Cost (£) | Source |
|---|---|---|
| Non-fatal stroke | 6307 | Assumption: these costs are estimated from a range of UK-specific burden-of-illness papers, where necessary costs have been inflated to represent 2007–8 prices |
| Non-fatal MI | 4893 | |
| Vascular death | 2726 | |
| Non-vascular death | 250 | |
| Major bleed | 2805 | |
| Minor bleed | 90 | |
| Dyspepsia | 141 | |
| Three months post stroke | 516 | |
| Three months post MI | 139 |
Follow-up costs
The cost of care 3 months post stroke is estimated using the same weighted severity formula107 used to calculate the costs of non-fatal stroke, and corrects the cost of ongoing care at home and the cost of ongoing care in an institution for the proportion of mild, moderate and severe stroke patients who are discharged to a home or an institution.
The post-MI cost is taken from a regression analysis103 of costs for a cohort of diabetic patients.
Drug costs
All annual costs of the treatment are derived from MIMS93 and are listed in Table 45.
| Treatment | Cost per year (£) |
|---|---|
| ASA (75 mg/day) | 3.50 |
| CLOP (75 mg/day) | 442.26 |
| MRD (2 × 200 mg/day) | 91.25 |
| MRD + ASA (MRD 2 × 200 mg/day + ASA 2 × 25 mg/day) | 94.78 |
Utilities
The utility values for patients with a history of stroke, MI or peripheral arterial disease were estimated from a previously published cost-effectiveness analysis,76 and were derived from published, population-based studies using either the time trade-off or standard gamble techniques. Table 46 provides the utility values used in the model. For the stroke utilities, severity-specific values were given (mild, moderate and severe), and as for costs, these were weighted to reflect the burden of severity in a patient cohort before being aggregated. The utility value for a patient with multivascular disease is not known, so it is assumed to be the minimum of the three other patient population values, which is the utility value for stroke patients (0.61).
| Patients with previous stroke | Patients with previous MI | Patients with previous PAD | MVD patients | |
|---|---|---|---|---|
| Long-term utility values | ||||
| No event | 0.61 | 0.87 | 0.80 | 0.61 |
| After stroke | 0.61 | 0.61 | 0.61 | 0.61 |
| After MI | 0.61 | 0.87 | 0.61 | 0.61 |
| After stroke and MI | 0.61 | 0.61 | 0.61 | 0.61 |
| Short-term decrements after event | ||||
| Stroke | –0.174 | –0.248 | –0.228 | –0.174 |
| MI | –0.058 | –0.082 | –0.076 | –0.058 |
| Major bleed | –0.300 | –0.300 | –0.300 | –0.300 |
| Minor bleed | –0.001 | –0.001 | –0.001 | –0.001 |
| Dyspepsia | –0.184 | –0.184 | –0.184 | –0.184 |
In deriving these utility values, the manufacturer has made several assumptions:
-
Utilities need to be differentiated based on the baseline health state of the patient, acknowledging the fact that stroke patients and peripheral arterial disease patients might be more disabled and have lower QoL than MI patients.
-
The utility value for multivascular disease patients should not be higher than the utility for those patients with disease in one vascular bed.
-
Experiencing a vascular event should decrease QoL temporarily to account for the unpleasantness of the event itself, the time in hospital, recovery time and stress.
-
After experiencing an event patients should not be better off in the long term than before the event (i.e. patients experiencing a MI after stroke could not have their utility increased).
-
Experiencing adverse events (major and minor bleeds) and side effects (dyspepsia) also decreases a patient’s QoL in the short term.
The long-term utility values for each health state reflect the event history of the patient, i.e. a patient with MI who then experiences a stroke is assigned the long-term utility value of a stroke, whereas a patient with MI who experiences another MI is assigned the long-term utility value of a MI (so does not suffer any long-term decrement). A peripheral arterial disease sufferer who then experiences a MI is assigned the long-term utility value of a multivascular disease patient.
Summary of results
Stroke patients
The results of the cost-effectiveness analysis for patients who have a history of stroke show that MRD + ASA (or MRD alone) is the most cost-effective treatment. The manufacturer states that if the NHS is willing to pay £31,200 then clopidogrel could be considered as a second-line treatment followed by ASA. This appears to be consistent with the efficacy results of the main RCTs, where clopidogrel was shown to be superior to ASA in the CAPRIE26 trial, and similar to MRD + ASA in the PRoFESS57 trial (Table 47).
| ASA | CLOP | MRD + ASA | MRD | |
|---|---|---|---|---|
| Total costs (£) | 10,841 | 13,165 | 10,948 | 10,531 |
| Total QALYs | 4.83 | 4.90 | 5.28 | 4.45 |
| Total life-years | 7.60 | 7.75 | 7.96 | 6.78 |
| Incremental net benefit vs ASA (£) | –90 | 13,533 | –10,964 | |
| Incremental net benefit vs CLOP (£) | –13,623 | 10,875 | ||
| ICER vs ASA (£) | 31,204 | 237 | 825 | |
| ICER of CLOP vs comparator (£) | CLOP is dominated | 5850 |
Myocardial infarction patients
Clopidogrel when compared with ASA in the cost-effectiveness model was found to be more effective and more expensive. With an ICER of approximately £21,000 per QALY gained, clopidogrel appears to be a cost-effective treatment for patients with previous history of MI when compared with ASA (Table 48).
| ASA | CLOP | |
|---|---|---|
| Total costs (£) | 6349 | 8992 |
| Total QALYs | 6.70 | 6.83 |
| Total life-years | 7.55 | 7.70 |
| Incremental net benefit (£) | 1194 | |
| ICER (£) | 20,662 |
Peripheral arterial disease patients
Clopidogrel was found to be more expensive and more effective than ASA, with an estimated corresponding ICER of £18,854 (Table 49).
| ASA | CLOP | |
|---|---|---|
| Total costs | £6138 | £8608 |
| Total QALYs | 5.71 | 5.84 |
| Total life-years | 7.06 | 7.22 |
| Incremental net benefit | £1461 | |
| ICER | £18,854 |
Multivascular disease patients
In this population it was found that clopidogrel was cost-effective compared with ASA with an estimated ICER of £15,524 per QALY gained (Table 50).
| ASA | CLOP | |
|---|---|---|
| Total costs (£) | 8678 | 10,483 |
| Total QALYs | 4.68 | 4.80 |
| Total life-years | 6.00 | 6.13 |
| Incremental net benefit (£) | 1683 | |
| ICER (£) | 15,524 |
Summary of sensitivity analysis
The manufacturer has reported a deterministic scenario analysis using the different efficacy analyses included in the model. In the stroke population, clopidogrel is dominated by MRD + ASA in all of the possible efficacy analyses, and with or without treatment effect for non-vascular death. Clopidogrel is shown to be cost-effective when compared with ASA using CAPRIE26 data only in both treatment-effect scenarios for non-vascular death (Table 51).
| Assumption: treatment effect for non-vascular death | With assumption: ICER CLOP vs ASA (£) | Without assumption: ICER CLOP vs ASA (£) |
|---|---|---|
| NMA of ASA doses pooled (base case) | 31,204 | 27,749 |
| NMA of low-, medium- and high-dose ASA | 58,070 | 46,500 |
| CAPRIE26 data only | 28,486 | 24,010 |
The ICERs for the other populations (MI, peripheral arterial disease and multivascular disease) also change slightly with the assumption concerning the treatment effect for non-vascular death in each of the efficacy analyses, resulting in clopidogrel appearing cost-effective with an ICER of < £30,000 per QALY. The best results for clopidogrel are in multivascular disease patients using data from the post hoc CAPRIE26 trial efficacy analysis.
In summary, the cost-effectiveness of treatments for the secondary prevention of occlusive vascular events is sensitive to a range of different scenarios. Removing the treatment effect on non-vascular deaths is found to improve the cost-effectiveness estimates of clopidogrel. Cost-effectiveness is also found to be sensitive to the efficacy estimates: taking account of different ASA doses worsens the cost-effectiveness estimates, whereas using only a head-to-head analysis based on the CAPRIE26 trial improves them. The estimates in the stroke population are least sensitive to a head-to-head analysis using the PRoFESS57 trial.
A probabilistic sensitivity analysis was developed by the manufacturer using a Monte Carlo simulation undertaking 3000 iterations. At a threshold of £30,000 per QALY, the treatment option with the highest probability of being cost-effective in MI, peripheral arterial disease and multivascular disease populations is clopidogrel and in stroke it is MRD + ASA, as Table 52 shows.
| Treatment | Threshold/QALY (£) | Population (%) | |||
|---|---|---|---|---|---|
| Stroke | MI | PAD | MVD | ||
| ASA | 20,000 | 0 | 51 | 48 | 41 |
| CLOP | 20,000 | 0 | 49 | 52 | 59 |
| MRD + ASA | 20,000 | 97 | |||
| MRD | 20,000 | 3 | |||
| ASA | 30,000 | 0 | 40 | 36 | 32 |
| CLOP | 30,000 | 0 | 60 | 64 | 68 |
| MRD + ASA | 30,000 | 97 | |||
| MRD | 30,000 | 3 | |||
In stroke patients, the average incremental net benefit of clopidogrel when compared with ASA is –£6 with an associated 95% CI of –£6320 to £7279.
The probabilistic sensitivity analysis in MI patients reports an incremental net benefit of £1187 (CI –£7692 to £10,260). The cost-effectiveness acceptability curve (CEAC) shows that for a threshold of £30,000 per QALY clopidogrel is cost-effective in 60% of the iterations.
For patients with peripheral arterial disease, the probabilistic sensitivity analysis estimates an average incremental net benefit of clopidogrel versus ASA of £1475 (CI –£6106 to £9476). The CEAC suggests that there is a 64% probability that, at a threshold of £30,000 per QALY, clopidogrel would be considered a cost-effective treatment for the prevention of occlusive vascular events.
For patients with multivascular disease, the average incremental net benefit of clopidogrel versus ASA is £1748 (CI –£5475 to £9179) and the CEAC suggests that there is a 68% probability of clopidogrel being cost-effective at a threshold of £30,000 per QALY.
Critique of Sanofi–aventis/Bristol–Myers Squibb’s economic model
The manufacturer of clopidogrel has presented ‘new’ evidence of the clinical effectiveness and cost-effectiveness of clopidogrel on a set of four re-allocated patient populations (stroke, MI, peripheral arterial disease and multivascular disease); this means that none of the effectiveness results used in their modelling of cost-effectiveness are directly derived from publications from the CAPRIE26 trial. The review group accepts that this new categorisation is more appropriate and results in better-defined and less-heterogeneous patient groups. However, the details that would be required to construct and populate a long-term disease model based on CAPRIE26 are not available beyond the summary statistics presented in the manufacturer’s submission.
The Assessment Group notes that the generic price of clopidogrel as listed in the Electronic drug tariff33 March 2010 is £10.90 (30 × 75-mg tablets); this constitutes a 69% reduction in price [branded Plavix (£36.35) was used in the model]. Using this new price in the model improves the cost-effectiveness of clopidogrel.
The manufacturer’s model is depicted in Figure 5 and includes one health state called ‘TA80 acute coronary syndrome’, which represents treatment after a MI following the TA8045 guidelines in the treatment of patients with NSTEMI. This document refers only to NSTEMI patients, yet the manufacturer’s submission does not differentiate between STEMI and NSTEMI patients so the model does not reflect clearly the recommended treatment of patients following a MI.
The baseline event rates in the ASA arm are taken from the REACH17 registry, whose population is a mixed population of patients with history of MI, stroke or peripheral arterial disease and patients with risk factors of cardiovascular disease. The original scope issued by NICE does not mention risk factors, only history of previous events. Also, these baseline event rates have been applied to patients in the ASA group; however, only 67% of the population of the REACH17 registry have received ASA monotherapy.
The model assumes different transition probabilities every year until year 3. Beyond this point the last-cycle transition probabilities are used for the remainder of the time horizon from years 3 to 35. This is an unreliable basis for long-term projection, as close to the end of the trial patient numbers and the number of events are much reduced. As a consequence, estimated incidence rates are very volatile and should not be relied on to drive the major part of the model calculations.
Calculations used to derive utilities are adequately described in the manufacturer’s submission, but sometimes differences between adverse events utilities are not clearly explained (e.g. decrement utility after major bleed and minor bleed: there is a substantial difference between them which is not discussed). Also, utility values are calculated using an assumption of perfect health for patients before the event 1 (‘utility’ spreadsheet in the model) and this is inappropriate.
In the model, half-cycle correction and discount rate methodologies have been applied incorrectly; this affects the final results of the model and overestimates the number of QALYs generated.
Summary critique of models submitted by the manufacturers
The economic models submitted by the manufacturers are structured in terms of a limited number of disease states that are presumed to be largely homogeneous with respect to health costs and QoL. Moreover, the models do not allow previous health history to be preserved except in the simplest form. There are real dangers that significant interactions between competing risks (e.g. MI vs stroke, vascular death vs non-vascular death) may not be accurately represented in these Markov formulations and that initially minor anomalies can be amplified to large errors when extrapolated over a lifetime. The details that would be required to construct and populate a long-term disease model based on CAPRIE26 and PRoFESS57 are not available beyond the summary statistics presented in the manufacturer’s submissions. Moreover, the revised definitions for assigning patients to the new groups are not completely clear, leading to some concern about how such data should be modelled. To reduce this problem, the Assessment Group requested that a set of analyses should be carried out by the manufacturer to allow a new model to be developed and calibrated for these four patient groups. For this we provided appropriate definitions of each population, and detailed specifications of the three types of analyses required: survival analyses (Kaplan–Meier and Cox regressions), numbers of outcome events and patient exposure to risk, and event fatality (see Appendix 9 for details).
Independent economic assessment
Methods
Approach to modelling occlusive vascular events
Modelling disease-related health and the economic effects of chronic lifetime conditions presents additional and different challenges to those encountered when dealing with conditions of an acute or time-limited nature. In particular, over a lifetime, patients are subject to multiple interacting competing risks of fatal and non-fatal events, and the accumulation of complex and dynamic health histories with a resulting dynamic pattern of prognostic risks. To overcome these challenges the Assessment Group has chosen to develop a new model of occlusive vascular events involving individual patient sampling. Instead of considering patients in aggregated groups with average characteristics, we generate a series of individual patients whose combined characteristics are representative of the specified population. The advantage of this approach is that individual patient histories can be generated according to a number of known competing risks, so that interactions are automatically accounted for.
Obtaining these advantages often involves significant technical costs in terms of complex programming and long processing times, which involve the use of very large numbers of random numbers in order to achieve stable results. To reduce these difficulties the Assessment Group has designed the model structure to operate within a Microsoft Excel workbook with limited additional coding and incorporating several ‘variance reduction’ techniques.
Patient populations
Four mutually exclusive patient populations are modelled using the following definitions:
-
MI only This population is defined as patients suffering a recent acute MI, who may have a prior history of ischaemic heart disease, but have no prior history of ischaemic stroke, TIA or peripheral arterial disease.
-
ischaemic stroke/TIA only This population is defined as patients suffering a recent ischaemic stroke or TIA, who may have a prior history of ischaemic cerebrovascular events, but have no prior history of ischaemic heart disease (including MI) or peripheral arterial disease.
-
Peripheral arterial disease only This population is defined as patients suffering a recent episode of peripheral arterial disease, but who have no prior history of ischaemic stroke or TIA, or ischaemic heart disease (including MI).
-
Multivascular disease This population is defined as patients suffering a recent episode of acute MI, ischaemic stroke or TIA, or peripheral arterial disease, and who have a prior history involving at least one other type of vascular disease.
In order to characterise each of these populations in terms of age and gender, an analysis of data from the Health Survey for England 1996108 has been carried out, using data on self-reported chronic health conditions to identify samples corresponding to the four modelled populations (Table 53). (Note: the Health Survey for England 1996 was commissioned by the Department of Health and carried out by the Joint Surveys Unit of Social and Community Planning Research and the Department of Epidemiology and Public Health at University College London, who bear no responsibility for the analysis or interpretation of its data presented in this report.)
| Gender | IS only | MI only | PAD only | MVD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Proportion | Age (years) | Proportion | Age (years) | Proportion | Age (years) | Proportion | |||||
| Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD | % | |
| Male | 67.75 | 12.95 | 54.9 | 65.01 | 11.96 | 49.9 | 61.75 | 13.96 | 48.6 | 63.92 | 11.33 | 53.1 |
| Female | 67.62 | 12.97 | 45.1 | 70.50 | 9.67 | 50.1 | 65.17 | 15.98 | 51.4 | 70.39 | 11.63 | 46.9 |
Treatment strategies
It is clear from the available evidence56,57 that a significant proportion of patients do not persist with the medication initially prescribed, either because of unacceptable adverse events associated with the drug or for other personal or lifestyle reasons. When discontinuation occurs, it is necessary to prescribe an appropriate alternative treatment if one is available; as a consequence, the effect of treatment on future risks will be modified. It is therefore necessary to assess the effectiveness and cost-effectiveness of preventive medicines within the framework of lifetime treatment strategies. Tables 54 and 55 set out the treatment strategies that may be compared using the economic model for each patient population.
| Intolerance | Strategy stages | |||||
|---|---|---|---|---|---|---|
| None | ASA | MRD | ASA + MRD | Treatment 1 | Treatment 2 | Treatment 3 |
| ✓ | ✓ | ✓ | ✓ | Nothing | Nothing | Nothing |
| ✓ | ✗ | ✓ | ✗ | ASA | ||
| ✓ | ✓ | ✓ | ✓ | CLOP | ||
| ✓ | ✗a | ✗ | ✗ | MRD + ASA | ||
| ✓ | ✗ | ✓ | ✗ | ASA | CLOP | |
| ✓ | ✗ | ✗ | ✗ | MRD + ASA | ||
| ✓ | ✗ | ✓ | ✗ | CLOP | ASA | |
| ✓ | ✗a | ✗ | ✗ | MRD + ASA | ||
| ✓ | ✗ | ✗ | ✗ | MRD + ASA | ASA | |
| ✓ | ✗a | ✗ | ✗ | CLOP | ||
| ✓ | ✗ | ✗ | ✗ | ASA | MRD + ASA | |
| ✓ | ✗ | ✗ | ✗ | MRD + ASA | CLOP | |
| ✓ | ✗ | ✗ | ✗ | CLOP | ASA | MRD + ASA |
| ✓ | ✗ | ✗ | ✗ | MRD + ASA | ASA | |
| ✓ | ✗ | ✗ | ✗ | MRD + ASA | CLOP | |
| ✓ | ✗ | ✗ | ✗ | ASA | CLOP | |
| Intolerant to ASA | Strategy stages | ||
|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | |
| ✓ | Nothing | Nothing | Nothing |
| ✗ | ASA | Nothing | Nothing |
| ✓ | CLOP | Nothing | Nothing |
| ✗ | ASA | CLOP | Nothing |
| ✗ | CLOP | ASA | Nothing |
Model design
The logic flow for generating a full patient history for each sampled patient is shown in Figure 6 for the first two key events. As event times are estimated as continuous variables, it is not possible for a conflict to arise with two events occurring simultaneously. Subsequent events repeat the same pattern. Each patient continues to accumulate additional events until a fatal event is encountered.
FIGURE 6.
Patient sampling model flow chart for a sequence of key events within a single patient history.
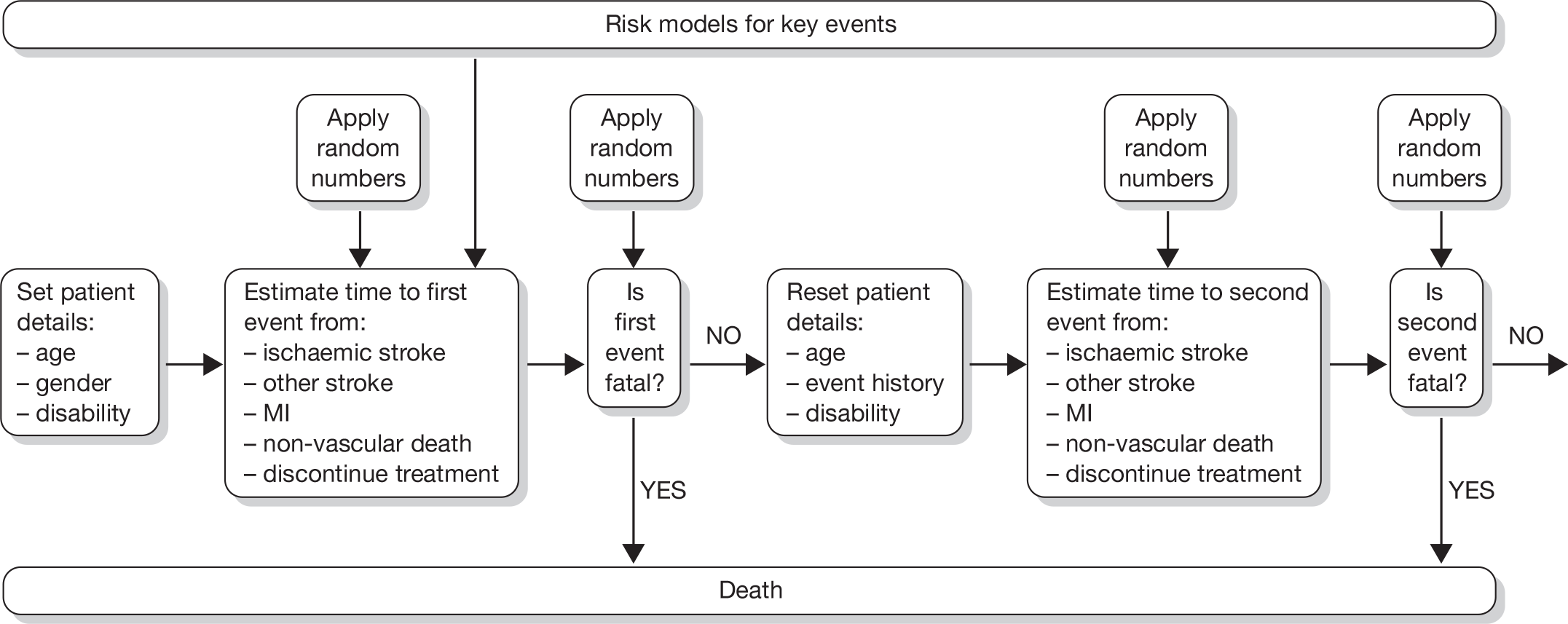
Key events
The following are identified as events that determine the event history of each modelled patient:
-
a new fatal or non-fatal ischaemic stroke event
-
a new fatal or non-fatal non-ischaemic stroke event (haemorrhagic stroke or intracranial haemorrhage)
-
a new fatal or non-fatal MI
-
death from other vascular causes
-
death from non-vascular causes
-
patient discontinues current preventive medication for any reason.
When any of these events occurs, the age, disability status and event history of the patient is updated to the time of the latest event, and the current preventive medication is updated if necessary to the next stage of the defined treatment strategy. The revised patient details are then used to estimate likely event times for the next key patient event until death occurs.
Other events
Additional non-fatal events may also occur to patients and are estimated independently of the main event pathway to ensure their effects on patient experience and health-care resource use are captured by the model. The current model includes several recognised adverse events associated with antiplatelet therapy (major and minor bleeding, gastric problems, etc.) and additionally new/worsened congestive heart failure as a possible event.
Disability
Continuing functional disability resulting from stroke events is known to be a prognostic indicator for high-risk events and greater mortality among affected patients. 91
The model includes a binary measure of functional disability equivalent to scores of ≥ 3 on the modified Rankin Scale. 60
The risk of progression to disabled status following a stroke event was derived from an analysis of PRoFESS results [Boehringer Ingleheim. Clinical Trial Report for PRoFESS (unpublished); 2008] and is used as a risk modifier for subsequent events.
Risk models
Confidential information from the two key clinical trials (CAPRIE26 and PRoFESS57) has been provided to the Assessment Group in order to allow calibration of the model, and, in particular, to facilitate development of risk models incorporating all relevant modifying variables, and avoiding errors arising from incorrect application of competing risks. Full details of the derived parameter values for all model events are provided in Appendix 10.
Event fatality
Data from the CAPRIE26 trial provided by the manufacturer of clopidogrel has allowed separate fatality risk models to be developed for the three primary vascular events. Details of the analysis and parameter values are shown in Appendix 11.
Duration of treatment
Some patients taking continuous preventive medication will eventually discontinue treatment for a variety of reasons. Analysis of clinical trial data from PRoFESS57 and ESPRIT56 (see appendices 5–7 of Boehringer Ingelheim’s manufacturer’s submission) indicates that continuance falls steadily over time, but that a substantial proportion of patients will continue taking the prescribed treatment indefinitely. The most appropriate representation is found to be an exponential survival function, with a minimum ‘floor’ probability of continuing treatment. Survival functions have been estimated for clopidogrel from PRoFESS57 data, for MRD + ASA from PRoFESS57 and ESPRIT,56 and for ASA alone from ESPRIT. 56
A random number is used to place each patient/treatment combination on the appropriate survival curve and to calculate the corresponding time of discontinuation. A facility is included to limit the duration of any treatment to a prespecified maximum duration, after which the patient automatically progresses to the next step in the treatment strategy.
Resource use
Health-care resource use is measured in terms of clinical events and time spent in chronic states, as well as duration of continuing medication as follows:
-
Events:
-
– ischaemic stroke (fatal/non-fatal)
-
– non-ischaemic stroke (fatal/non-fatal)
-
– myocardial infarction (fatal/non-fatal)
-
– other vascular event (fatal)
-
– non-vascular death
-
– adverse events related to medication.
-
-
Chronic states:
-
– prior disabling stroke
-
– no prior disabling stroke
-
– prior MI
-
– history of peripheral arterial disease
-
– history of multivascular disease (disabled/non-disabled).
-
Cost estimates
Unit costs are drawn from a variety of sources, including those used in the two manufacturer’s submissions. 51,52 In all cases the latest costs/prices have been used33,109,110 and, where appropriate, costs have been inflated to 2009 prices using the Hospital and Community Health Services price inflation index reported by the PSSRU. 92
Unit costs for the primary events projected in the model are shown in Table 56, distinguishing between disabling and non-disabling strokes. The model logic uses two parameters for non-fatal stroke and MI events in which an event cost is assigned to a patient at the time of the event (assumed to encompass excess early recovery/rehabilitation costs not covered by long-term service use) and a continuing care cost related to the time following the time of the event until the patient’s status changes.
| Key model event | Patient status | |
|---|---|---|
| Not disabled (Rankin Scale 0–2) | Disabled (Rankin Scale 3–5) | |
| Non-fatal IS | £6409.94 | £13,647.38 |
| Fatal IS | £8767.69 | £8767.69 |
| Non-fatal haemorrhagic stroke/ICH | £6409.94 | £13,647.38 |
| Fatal haemorrhagic stroke/ICH | £8767.69 | £8767.69 |
| Non-fatal MI | £5761.88 | £5761.88 |
| Fatal MI | £2218.39 | £2218.39 |
| Other vascular death | £2225.00 | £2225.00 |
| Other non-vascular death | £2225.00 | £2225.00 |
Costs for stroke events are taken from Youman et al. ,102 uplifted for inflation from 2001. MI costs are more problematic, as the only source cited by either manufacturer [the UK Prospective Diabetes Study (UKPDS) No. 65]103 relates only to patients with type 2 diabetes who are known to incur substantially greater unit costs for all types of health care (in terms of both frequency and intensity of resource use). The main trials (PRoFESS57 and CAPRIE26) only include a minority of patients with diabetes, reflective of the prevalence within the general population of vascular patients, and therefore there is a likelihood that without adjustment these costs will be overestimated. In the UKPDS paper103 two MI costs are estimated: an average for all patients (including 20–26% who received no inpatient care) and a greater average only for those patients admitted to hospital. In recognition of the risk of overestimating MI costs from this source, we selected the lower figure for both fatal and non-fatal MIs and uplifted these unit costs for inflation from 1999.
Estimated unit costs are shown in Table 57. For stroke survivors, the annual costs of ongoing health and social care services are based on the estimates produced by Youman et al. ,102 uplifted for inflation from 2001. For non-disabled stroke survivors the non-institutionalised unit cost was used, and for disabled survivors a weighted average of patients living at home and in institutions was calculated. For non-fatal MI patients, continuing care costs were obtained by combining the inpatient and outpatient costs reported in UKPDS No. 65,103 uplifted for inflation from 1999. Continuing care costs are assumed to be hierarchical on the basis of accumulating patient history; so, a patient suffering a stroke will continue to incur the higher care costs even after surviving a subsequent MI.
| Patient status | Annual continuing care cost (£) |
|---|---|
| No key events | 0.00 |
| Non-fatal MI | 577.60 |
| Non-fatal non-disabling stroke | 1686.04 |
| Non-fatal disabling stroke | 5175.44 |
To estimate the costs of adverse events related to the various treatments we chose to adopt the categories used in the Sanofi–aventis/Bristol–Myers Squibb submission (major/minor bleeding and dyspepsia), but have also incorporated hospital events involving the initiation or worsening of congestive heart failure as used in the Boehringer Ingelheim submission. 51
Table 58 shows the frequency parameters used, as well as the unit costs. Costs have broadly followed the methods used by the manufacturers, but using the latest cost sources and inflating costs to 2009. The overall average annual costs are applied to all patients for the periods when each of the treatments is in use.
| Adverse event | Unit cost (£) | Annual event frequency by treatmenta (%) | ||||
|---|---|---|---|---|---|---|
| ASA | CLOP | MRD | MRD/ASA | None | ||
| Major bleeding event | 2010.35 | 0.54 | 0.41 | 0.13 | 0.46 | 0.00 |
| Minor bleeding event | 111.57 | 0.93 | 0.93 | 0.38 | 0.87 | 0.00 |
| Dyspepsia | 146.61 | 2.33 | 1.99 | 5.85 | 6.19 | 0.00 |
| CHF event/worsening | 1074.92 | 0.63 | 0.75 | 0.63 | 0.63 | 0.63 |
| Combined average cost (£) | 22.08 | 20.10 | 18.42 | 26.18 | 6.80 | |
The estimated NHS cost of each component of antiplatelet therapy is shown in Table 59 for the relevant periods of treatment. Clopidogrel has recently become available to the NHS at a slightly reduced price, although it should be noted that the generic form is not licensed for all indications covered by the branded product.
| Treatment | Dose | Annual cost (£) | 4 weeks’ cost (£) | Single-dose cost (£) | Source |
|---|---|---|---|---|---|
| ASA | 75 mg daily | 6.9888 | 0.5350 | – | BNF 5832 |
| MRD | 200 mg twice daily | 91.3125 | – | – | BNF 58/NHSDT (April 2010)33 |
| MRD + ASA | 200 mg/25 mg twice daily | 94.8433 | – | – | BNF 5832 |
| CLOP (branded) | 300 mg | – | – | 4.8473 | BNF 5832 |
| CLOP (branded) | 75 mg daily | 442.5613 | 33.9267 | – | BNF 5832 |
| CLOP (generic) | 75 mg daily | 132.7075 | 10.1733 | – | NHSDT (April 2010)33 |
Health valuation
Health-utility values are drawn from a variety of sources, including those used in the two manufacturer’s submissions. 51,52
Mean utility values are assigned to each chronic health state and a specific utility decrement effect is applied for each modelled event.
The European Quality of Life-5 Dimensions (EQ-5D) data collected in the PRoFESS57 trial have been used to estimate the utility for ischaemic stroke patients prior to any subsequent key events and to determine the long-term utility decrement applicable to suffering stroke-related disability. In addition, the PRoFESS57 results allowed utility decrements to be applied following the first subsequent non-fatal key event, as well as a single decrement for more than one subsequent key event.
The utility values used in the Sanofi–aventis/Bristol–Myers Squibb model for MI and peripheral arterial disease without a subsequent key event (0.87 and 0.80, respectively, drawn from a study by Schleinitz et al. 75) are adopted here. Although no data can be traced relating to multivascular disease patients, we have assumed that they are likely to begin treatment with a rather worse HRQoL than patients with only a single type of vascular disease and we have adopted a value of 0.75.
The estimate of utility decrement applicable to a congestive heart failure event used in the Boehringer Ingelheim model appears to be well sourced and has been adopted for this model, indicating an event decrement of –0.0163 QALYs. The utility impact of the other events (major/minor bleeding events and dyspepsia) proved more difficult to identify.
The reference given for a minor bleed111 draws upon an earlier paper by O’Brien and Gage,112 which lists the source as ‘assumption’. The suggested decrement (–0.2) is relative to a theoretical ‘perfect health’ state rather that of a patient with established chronic disease and so may be overstated. As this condition is considered to last for only 2 days, the magnitude of this factor in determining cost-effectiveness must be very small and we have adopted a notional decrement of –0.0033 QALYs in the absence of any more reliable source.
The estimate for dyspepsia is drawn directly from Jansen et al. 113 but fails to recognise that each event is estimated to last just 3 weeks rather than the 13 weeks used in the Bristol–Myers Squibb/Sanofi–aventis model. Adjusting for this problem yields an estimated utility decrement per event of –0.0106 QALYs.
The Sanofi–aventis/Bristol–Myers Squibb utility calculations for major bleeding events draw on three patient categories in the paper of Jansen et al. ,113 for gastrointestinal events (outpatient treatment, inpatient treatment and treatment involving surgery) and one for intracranial haemorrhage events. 114 Only one of the figures used from Jansen’s paper113 can be traced and validated from the original sources and the events are taken by Jansen et al. 113 to last for 5 weeks, rather than the 13 weeks implicit in the Sanofi–aventis/Bristol–Myers Squibb model. The paper by Quinn et al. 114 uses a crude approach to estimating the utility decrement of an intracranial haemorrhage event, involving an assumption that utility falls from 1.0 (‘perfect health’) to 0.0 (‘death’) for the whole duration of the event, estimated at 11 weeks. This must be taken as a substantial overestimate. Reworking these calculations suggests a decrement in utility from a major bleeding event of –0.1426 QALYs (compared with the Sanofi–aventis/Bristol–Myers Squibb estimate of –0.3003 QALYs).
In principle, utility decrements should be considered for both long-term state of a patient following a significant event and also associated with the short-term impact of the event in the immediate acute and post-acute periods. Only one study115 has been identified which has attempted in any way to discriminate between these two effects; in table 2 of the paper115 the authors report results of two regression analyses involving parameters that distinguish the effect of events in the last 12 months from those in previous years. Subtracting the estimated long-term value from the short-term value should indicate113 the magnitude of the short-term excess disutility associated with experience of the event itself. However, the results are inconclusive, as this approach appears to indicate a net utility gain from a stroke that is not clinically meaningful. Moreover, the numbers of recorded events are insufficient to generate statistically significant differences between coefficients. As a result it has been concluded that it is not currently possible to assign meaningful disutility estimates to model events in addition to the long-term state-related impact described above, and this element of utility estimation has been omitted.
Discounting
Discount rates of 3.5% for both costs and health outcomes (life-years and QALYs) are used. Discounting is applied annually after the first year.
Time horizon
A lifetime perspective is taken for the model.
Variance reduction
Two specific measures are implemented in the model to limit background random variation and improve efficiency of model performance.
Random assignment of age/gender is not used for individual patients. Instead, 100 points across the standard normal probability distribution are used to define a distinct set of baseline ages for each gender drawn from the specified population providing a fully representative spread of patients by age and gender. This basic set is then reproduced 10 times to yield a total of 2000 individual patients. Finally, results are generated separately for males and females and overall mixed population results are obtained by applying the appropriate gender proportions to yield weighted averages.
The random numbers that govern the occurrence of events are not generated every time that the model is run. Instead, a full set of random numbers is stored and accessed identically for each patient when generating patient histories for different treatment strategies. This ensures that differences apparent in the results obtained are solely because of the difference in treatments and are not arising from the uncontrollable impact of large numbers of ‘in-process’ random fluctuations. The stability of the incremental results obtained can be assessed by comparing results from a number of stored random number sets.
Assessment of uncertainty
Univariate sensitivity analysis is carried out for a full range of model parameters.
Other modelling issues
Three modelling difficulties are apparent from consideration of previous TAs and the related NICE guidance.
Modelling transient ischaemic attack
The Technology Assessment Report3 that led to the development of the current guidance24 on secondary prevention of occlusive vascular events included some consideration of patients suffering from TIA despite the absence of separate trial information for the effectiveness of either treatment for this patient group. A simple assumption was made that TIA patients were at risk of future events at a reduced (80%) rate compared with ischaemic stroke patients. This failed to take into account two published papers presenting results from the Oxfordshire Community Stroke Project, showing the risk of stroke following a first-ever stroke90 or following a TIA. 116
More recently a Canadian population study117 provided similar findings for TIA patients. Table 60 does not suggest that there is strong evidence to make a distinction between TIA patients and those surviving an ischaemic stroke. On this basis it has been assumed that TIA patients may be subsumed within the stroke model population, as long-term risks appear to be similar.
| Population | Stroke risk | |
|---|---|---|
| At 12 months, % (95% CI) | At 5 years, % (95% CI) | |
| Oxford stroke patients | 13.2 (10.0 to 16.4) | 29.5 (19.8 to 39.0) |
| Oxford TIA patients | 11.6 (6.9 to 15.8) | 29.3 (21.3 to 37.3) |
| Alberta TIA patients | 14.5 (12.8 to 16.2) | – |
Technology Appraisal No. 8045 guidance and the myocardial infarction population
On the basis of evidence from the CURE27 trial, NICE guidance document TA8045 recommends that patients surviving a NSTEMI event should receive clopidogrel and low-dose ASA as medication for the prevention of further MI events for a period of 12 months, followed by low-dose ASA alone thereafter. There is no current guidance for surviving STEMI patients beyond the immediate post-MI period.
The only clinical trial evidence submitted for the current appraisal relating to the MI-only patient population is from a subgroup of the CAPRIE26 trial population, which involves a mix of STEMI and NSTEMI patients. No analyses are provided in the CAPRIE26 clinical study report distinguishing between STEMI and NSTEMI patients.
Similar concerns apply to the multivascular disease population, as a proportion of these patients may have MI as the qualifying event. No information is available on the composition of the multivascular disease group in CAPRIE26 by qualifying event so it is difficult to determine how any meaningful subdivisions could be applied.
As reviewing the existing TA80 guidance45 and CG48 guidelines7 is not within the scope of this appraisal, it is necessary to assume that recommendations for post-MI preventive treatment of both NSTEMI and STEMI patients remain valid. However, it would be inappropriate to begin modelling MI-only patients while still subject to these short-term provisions (12 months for NSTEMI and 4 weeks for STEMI patients). We therefore assume that all MI-only patients have survived to the end of the specified period without suffering a further MI, or any other occlusive vascular event (which would require them to be reclassified as multivascular disease patients), prior to embarking on the chosen long-term preventive treatment strategy. This avoids the necessity of identifying MI patients as either STEMI or NSTEMI from the outset.
Technology Appraisal No. 8045 and subsequent myocardial infarction events in all populations
In all four populations defined above there is a risk of future MI events, some of which will be non-fatal. Therefore, the TA80 guidance45 requires that the affected patients (i.e. those suffering an NSTEMI event) should be switched to clopidogrel + ASA for 12 months. For modelling it becomes necessary to estimate the probability of NSTEMI versus STEMI to assign the correct post-event short-term treatment, although none of the available trials provides information on the type of MI suffered. The GRACE (Global Registry of Acute Coronary Events)118 study of acute coronary syndrome patients is used to estimate the proportions of STEMI/NSTEMI in the population as 53.8%/46.2% (MIs excluding unstable angina). To accommodate the effects of TA80 guidance45 in the model, a simplification has been applied, which involves a reduction to the short-term post-MI risk that was estimated from the CAPRIE26 data to reflect the benefits observed in CURE,27 and a corresponding short-term increase in treatment costs for the 12 months post MI, both averaged by the STEMI/NSTEMI proportions in the GRACE118 study.
In addition, the follow-on treatment after 12 months (ASA alone or ‘standard care’) needs to be interpreted in the context of the model treatment strategies. Where an ‘MI-only’ patient suffers subsequent MI events, but no other type of occlusive event, treatment may resume at the stage of the treatment strategy prior to the latest MI(s) requiring short-term follow-up. If an ‘MI-only’ patient suffers a different kind of occlusive event, they attract the higher risks associated with multivascular disease patients for the remainder of their life. In the same way a ‘stroke-only’ or ‘peripheral arterial disease-only’ patient suffering a MI will also be subject to the higher multivascular disease risks once the short-term follow-up care is complete. Equally, an ‘MI-only’ or ‘peripheral arterial disease-only’ patient suffering an ischaemic stroke may receive up to 2 years’ MRD + ASA treatment as required by TA90,24 and, subsequently, resume the long-term care strategy subject to the increased multivascular disease event risks.
Technology Appraisal No. 9024 and subsequent ischaemic stroke events in all populations
The National Institute for Health and Clinical Excellence TA90 guidance24 recommends the use of MRD + ASA for up to 2 years following a non-fatal ischaemic stroke event. The Assessment Group model has been adapted to reflect this feature, which may be rendered active or inactive at the user’s discretion. The adaptation involves introducing a pseudoevent at the end of the TA9024 recommended treatment period, before the patient resumes at his or her prior stage in the assigned treatment strategy. This is an effective mechanism for coping with the added complexity of TA90 guidance. 24
However, it does result in some potential loss of integrity in the matching of random number sequences between comparator model runs (a mechanism used for ‘variance reduction’ in the model); in principle this might introduce some element of bias into the results, but it would occur in only the later stages of a patient’s career when many patients have already died and appears more likely to underestimate incremental differences than to overestimate them. A simple test of this effect is to compare model results with and without this feature activated, as the model results obtained when the TA9024 feature is inactive are not subject to any potential bias. To date the Assessment Group has not detected any evidence of any bias affecting the decision analysis results.
A note of caution is necessary here against attempting to use a comparison of model results with and without the TA9024 feature turned on as a means of reconsidering the validity of TA90 guidance. 24
As currently constructed, the model would not be valid for this purpose and would require important modifications to achieve such an objective. As this is not within the scope of the current appraisal, no effort has been made to pursue this possibility.
Independent economic model results
Results have been generated from the Assessment Group’s model to address two related questions:
-
Which treatment strategy is most cost-effective in avoiding future occlusive vascular events in each of the four specified populations?
-
How does the availability of generic clopidogrel at a lower price than the branded product affect the assessment of cost-effectiveness of clopidogrel containing treatment strategies?
Detailed results are given in this section separately for each of the four populations previously defined and using deterministic analyses. Of particular interest is the stability of cost-effectiveness findings when the size of the sampled patient population is varied. Our initial analyses were based on a sample size of 2000 patients. Further exploratory analyses were then conducted with alternative first-order random number sets and it became evident that there was scope for differing results to be obtained when other random number sets of this size are used to define the sample. Larger sample sizes were found to provide stable results across all populations and scenarios. It was therefore decided to expand the sample size from 2000 to 10,000 simulated patients and re-assess the most cost-effective treatment scenario for each of the four patient groups against the deterministic results reported. For three of the four patient populations (‘MI only’, ‘peripheral arterial disease only’ and multivascular disease) the results of the updated analysis based on a sample size of 10,000 were very similar to those based on the 2000 sample size and did not show any alterations in the optimal treatment strategy. However, for the ‘ischaemic stroke’ population, results for two analyses using generic clopidogrel led to a change in the optimal strategy as reported in the initial Assessment Group submission to NICE. The changes are incorporated into this document.
Ischaemic stroke-only patients
Deterministic analysis
Tables 61 and 62 summarise the main economic results obtained with the Assessment Group model for the ischaemic stroke patient population. Figures 7–10 illustrate these findings in the form of a cost-effectiveness plane plot with cost-effectiveness frontier. The primary analysis (first block in Tables 61 and 62 and Figure 7) reveals that only two strategies lie on the boundary, but neither of these involves initial use of clopidogrel. In all scenarios, the most cost-effective strategy begins with MRD + ASA, followed by ASA and finally clopidogrel.
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | Incremental analysis vs no treatment | Incremental analysis vs ASA | Incremental analysis vs ASA → MRD + ASA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | ||||
| Full | MRD + ASA | None | None | None | 35,202 | 7.056 | |||||||||
| ASA | 32,955 | 7.157 | 0.102 | –2247 | –22,106 a | ||||||||||
| CLOP | 37,910 | 7.620 | 0.564 | 2709 | 4802 | 0.462 | 4956 | 10,716 | –0.060 | 3201 | Dom | ||||
| MRD + ASA | 35,266 | 7.591 | 0.535 | 64 | 120 | 0.434 | 2311 | 5329 | –0.089 | 556 | Dom | ||||
| ASA | CLOP | 35,137 | 7.687 | 0.632 | –64 | –102 | 0.530 | 2183 | 4118 | 0.007 | 427 | 59,033 | |||
| ASA | MRD + ASA | 34,710 | 7.680 | 0.624 | –492 | –787 | 0.523 | 1755 | 3357 a | ||||||
| CLOP | ASA | 37,809 | 7.743 | 0.687 | 2607 | 3796 | 0.585 | 4854 | 8295 | 0.062 | 3099 | 49,665 | |||
| CLOP | MRD + ASA | 38,098 | 7.744 | 0.688 | 2897 | 4211 | 0.586 | 5144 | 8773 | 0.063 | 3388 | 53,370 | |||
| MRD + ASA | ASA | 35,187 | 7.730 | 0.674 | –15 | –22 | 0.573 | 2232 | 3898 | 0.050 | 477 | 9581 | |||
| MRD + ASA | CLOP | 36,321 | 7.734 | 0.678 | 1120 | 1651 | 0.577 | 3367 | 5840 | 0.054 | 1612 | 29,982 | |||
| ASA | CLOP | MRD + ASA | 35,161 | 7.708 | 0.653 | –41 | –62 | 0.551 | 2206 | 4003 | 0.028 | 451 | 15,909 | ||
| ASA | MRD + ASA | CLOP | 34,867 | 7.696 | 0.640 | –335 | –523 | 0.538 | 1912 | 3552 | 0.016 | 157 | 10,098 | ||
| CLOP | ASA | MRD + ASA | 37,811 | 7.769 | 0.713 | 2609 | 3657 | 0.612 | 4856 | 7938 | 0.089 | 3101 | 34,857 | ||
| CLOP | MRD + ASA | ASA | 38,096 | 7.781 | 0.725 | 2895 | 3992 | 0.624 | 5142 | 8246 | 0.101 | 3387 | 33,621 | ||
| MRD + ASA | CLOP | ASA | 36,336 | 7.774 | 0.719 | 1134 | 1579 | 0.617 | 3382 | 5481 | 0.094 | 1626 | 17,267 | ||
| MRD + ASA | ASA | CLOP | 35,356 | 7.759 | 0.703 | 155 | 220 | 0.601 | 2402 | 3994 | 0.079 | 647 | 8222 a | ||
| Not used | None | None | None | 35,267 | 7.126 | ||||||||||
| ASA | 34,594 | 7.644 | 0.518 | –673 | –1299 a | ||||||||||
| CLOP | 38,018 | 7.680 | 0.554 | 2751 | 4966 | 0.036 | 3423 | 95,065 | –0.041 | 3230 | Dom | ||||
| MRD + ASA | None | 35,434 | 7.651 | 0.525 | 167 | 318 | 0.007 | 840 | 116,441 | –0.069 | 646 | Dom | |||
| ASA | CLOP | 35,203 | 7.727 | 0.601 | –64 | –106 | 0.083 | 609 | 7336 | 0.006 | 415 | 64,448 | |||
| ASA | MRD + ASA | 34,788 | 7.721 | 0.594 | –479 | –806 | 0.077 | 194 | 2532 a | ||||||
| CLOP | ASA | 37,981 | 7.804 | 0.678 | 2714 | 4004 | 0.160 | 3386 | 21,189 | 0.083 | 3193 | 38,344 | |||
| CLOP | MRD + ASA | 38,267 | 7.809 | 0.683 | 3000 | 4394 | 0.165 | 3673 | 22,278 | 0.088 | 3479 | 39,394 | |||
| MRD + ASA | ASA | 35,355 | 7.783 | 0.656 | 88 | 134 | 0.138 | 761 | 5494 | 0.062 | 567 | 9159 | |||
| MRD + ASA | CLOP | 36,450 | 7.789 | 0.663 | 1183 | 1785 | 0.145 | 1856 | 12,813 | 0.068 | 1662 | 24,338 | |||
| ASA | CLOP | MRD + ASA | 35,234 | 7.753 | 0.627 | –33 | –52 | 0.109 | 640 | 5869 | 0.033 | 446 | 13,721 | ||
| ASA | MRD + ASA | CLOP | 34,967 | 7.740 | 0.614 | –300 | –489 | 0.096 | 373 | 3878 | 0.020 | 179 | 9150 | ||
| CLOP | ASA | MRD + ASA | 38,015 | 7.832 | 0.705 | 2748 | 3896 | 0.187 | 3421 | 18,249 | 0.111 | 3227 | 29,100 | ||
| CLOP | MRD + ASA | ASA | 38,349 | 7.856 | 0.730 | 3082 | 4222 | 0.212 | 3755 | 17,704 | 0.136 | 3561 | 26,276 | ||
| MRD + ASA | CLOP | ASA | 36,539 | 7.840 | 0.714 | 1272 | 1783 | 0.196 | 1945 | 9933 | 0.119 | 1751 | 14,685 | ||
| MRD + ASA | ASA | CLOP | 35,579 | 7.812 | 0.685 | 312 | 456 | 0.168 | 985 | 5880 | 0.091 | 791 | 8698 a | ||
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | ICER (£) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | ||||
| Generic | MRD + ASA | None | None | None | 35,202 | 7.056 | |||||
| ASA | 32,955 | 7.157 | –22,106 a | ||||||||
| CLOP | 35,534 | 7.620 | 590 | 5578 | Dom | ||||||
| MRD + ASA | 35,266 | 7.591 | 120 | 5329 | Dom | ||||||
| ASA | CLOP | 34,704 | 7.687 | –788 | 3300 | 1116 | |||||
| ASA | MRD + ASA | 34,710 | 7.680 | –787 | 3357 | 623 | |||||
| CLOP | ASA | 35,433 | 7.743 | 337 | 4235 | 20,716 | Dom | ||||
| CLOP | MRD + ASA | 35,722 | 7.744 | 757 | 4720 | 28,305 | Dom | ||||
| MRD + ASA | ASA | 35,187 | 7.730 | –22 | 3898 | 21,432 | 1204 | ||||
| MRD + ASA | CLOP | 35,521 | 7.734 | 471 | 4451 | 31,217 | Dom | ||||
| ASA | CLOP | MRD + ASA | 34,727 | 7.708 | –726 | 3217 a | |||||
| ASA | MRD + ASA | CLOP | 34,737 | 7.696 | –725 | 3312 | Dom | ||||
| CLOP | ASA | MRD + ASA | 35,435 | 7.769 | 327 | 4054 | 11,666 | 20,675 | 19,262 | ||
| CLOP | MRD + ASA | ASA | 35,720 | 7.781 | 715 | 4436 | 13,717 | 22,598 | 28,288 a | ||
| MRD + ASA | CLOP | ASA | 35,535 | 7.774 | 464 | 4183 | 12,269 | 20,200 a | |||
| MRD + ASA | ASA | CLOP | 35,221 | 7.759 | 28 | 3769 | 9820 a | ||||
| Not used | None | None | None | 35,267 | 7.120 | ||||||
| ASA | 34,594 | 7.644 | –1283 a | ||||||||
| CLOP | 35,545 | 7.680 | 496 | 26,406 | Dom | ||||||
| MRD + ASA | 35,434 | 7.651 | 314 | 116,441 | Dom | ||||||
| ASA | CLOP | 34,791 | 7.727 | –783 | 2377 | 1201 | |||||
| ASA | MRD + ASA | 34,788 | 7.721 | –797 | 2532 | 1069 | |||||
| CLOP | ASA | 35,508 | 7.804 | 352 | 5718 | 13,508 | 1249 | ||||
| CLOP | MRD + ASA | 35,795 | 7.809 | 766 | 7282 | 17,424 | Dom | ||||
| MRD + ASA | ASA | 35,355 | 7.783 | 132 | 5494 | 18,127 | 3831 | ||||
| MRD + ASA | CLOP | 35,682 | 7.789 | 620 | 7509 | 24,023 | Dom | ||||
| ASA | CLOP | MRD + ASA | 34,823 | 7.753 | –701 | 2096 a | |||||
| ASA | MRD + ASA | CLOP | 34,823 | 7.740 | –716 | 2377 | |||||
| CLOP | ASA | MRD + ASA | 35,542 | 7.832 | 387 | 5059 | 9185 a | ||||
| CLOP | MRD + ASA | ASA | 35,876 | 7.856 | 827 | 6046 | 10,230 | 13,558 a | |||
| MRD + ASA | CLOP | ASA | 35,771 | 7.840 | 700 | 6010 | 10,934 | 27,336 | |||
| MRD + ASA | ASA | CLOP | 35,426 | 7.812 | 230 | 4967 | 10,328 | 5835 | |||
FIGURE 7.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘ischaemic stroke-only’ patients (using MRD + ASA as per the TA9024 guidance). A = ASA; B = ASA to MRD + ASA; C = MRD + ASA to ASA to clopidogrel; and D = MRD + ASA to clopidogrel to ASA.
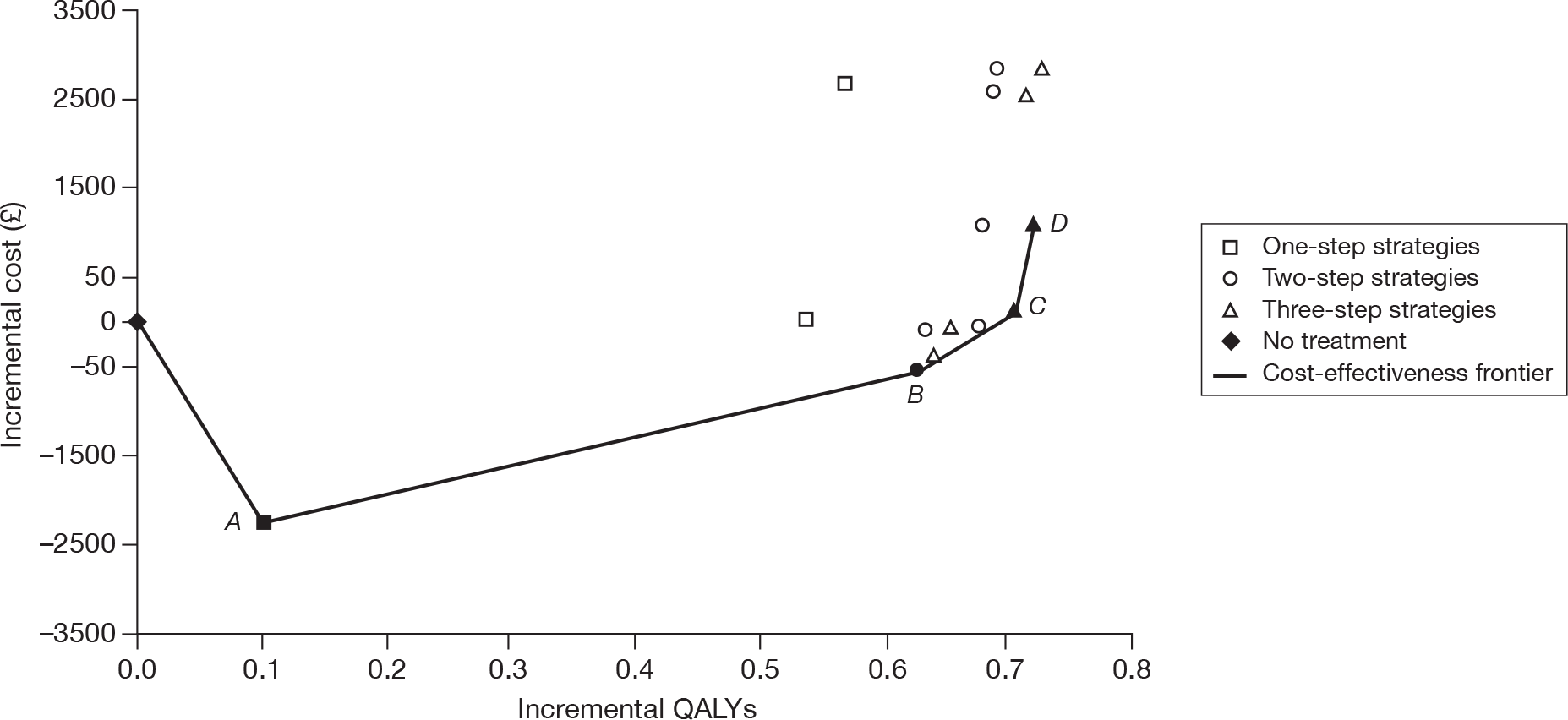
FIGURE 8.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘ischaemic stroke-only’ patients (without applying the TA9024 guidance). A = ASA; B = ASA to MRD + ASA; C = MRD + ASA to ASA to clopidogrel; and D = MRD + ASA to clopidogrel to ASA.
FIGURE 9.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘ischaemic stroke-only’ patients (using MRD + ASA as per the TA9024 guidance and the generic clopidogrel price). A = ASA; B = ASA to clopidogrel to MRD + ASA; C = MRD + ASA to ASA to clopidogrel; D = MRD + ASA to clopidogrel to ASA; and E = clopidogrel to MRD + ASA to ASA.
FIGURE 10.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘ischaemic stroke-only’ patients (without applying the TA9024 guidance and using the generic clopidogrel price). A = ASA; B + ASA to clopidogrel to MRD + ASA; C + clopidogrel to ASA to MRD + ASA; and D = clopidogrel to MRD + ASA to ASA.
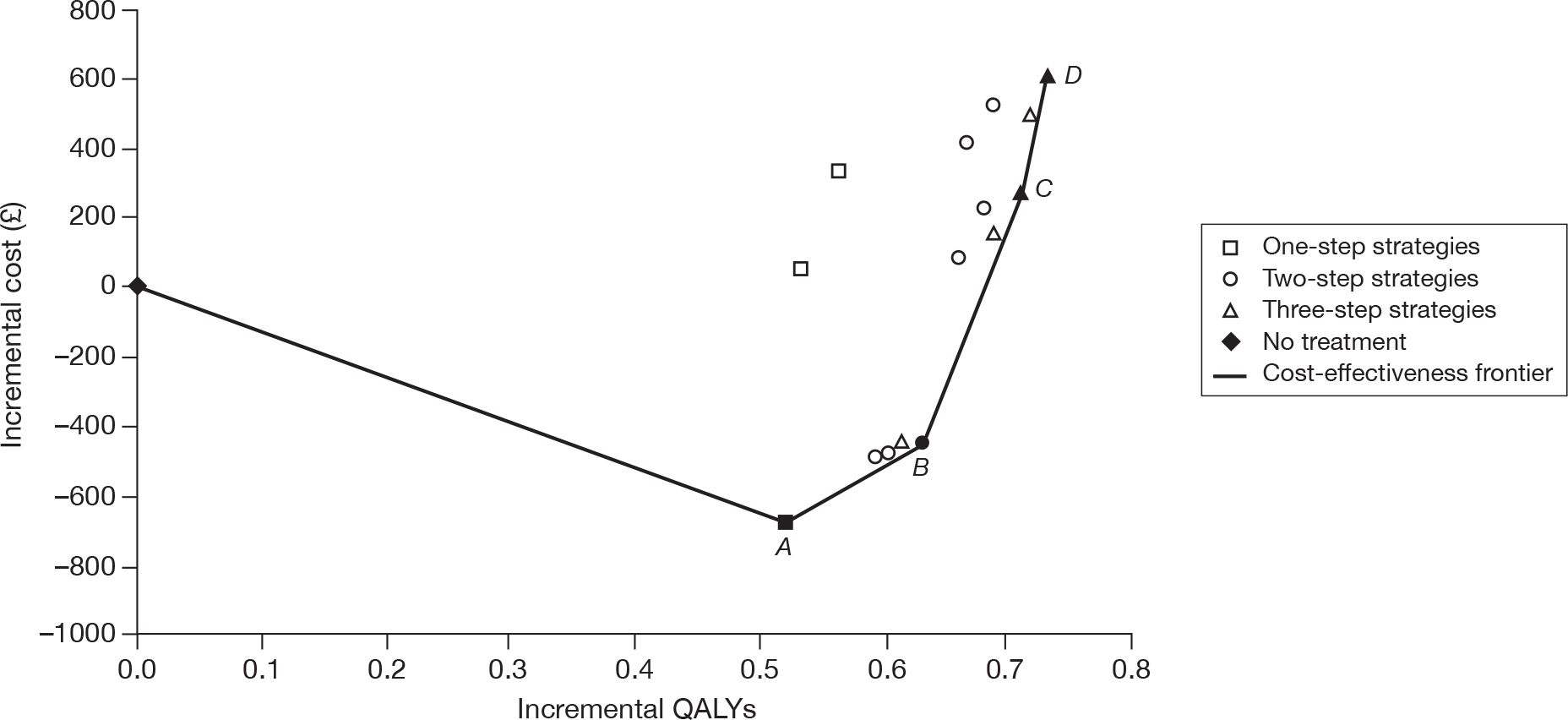
Intolerance to acetylesalicyclic acid and/or modified-release dipyridamole
In patients who are intolerant of ASA, clopidogrel and MRD are the only available long-term therapy options available, and only MRD may be used post ischaemic stroke events as per the TA90 guidance. 24 These are compared with the ‘no-treatment’ scenario in Table 63 and indicate that clopidogrel followed by MRD is the most cost-effective approach to occlusive vascular event prevention, independent of both TA90 guidance24 and the price of clopidogrel.
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | Incremental analysis vs no treatment | Incremental analysis vs MRD | Incremental analysis vs CLOP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | ||||
| ASA intolerant | |||||||||||||||
| Full | MRD | None | None | None | 35,279 | 7.021 | |||||||||
| CLOP | 37,994 | 7.598 | 0.576 | 2715 | 4713 | 0.304 | 1690 | 5556 a | 0.000 | 0 | |||||
| MRD | 36,304 | 7.293 | 0.272 | 1025 | 3770 a | ||||||||||
| CLOP | MRD | 38,413 | 7.653 | 0.632 | 3135 | 4964 | 0.360 | 2110 | 5866 | 0.055 | 419 | 7564 a | |||
| MRD | CLOP | 37,318 | 7.440 | 0.419 | 2039 | 4872 | 0.147 | 1014 | 6915 | –0.158 | –676 | 4292 | |||
| Not used | None | None | None | 35,267 | 7.120 | ||||||||||
| CLOP | 38,018 | 7.680 | 0.560 | 2751 | 4908 | 0.310 | 1580 | 5093 a | |||||||
| MRD | 36,437 | 7.370 | 0.250 | 1170 | 4678 a | ||||||||||
| CLOP | MRD | 38,486 | 7.732 | 0.612 | 3219 | 5257 | 0.362 | 2048 | 5656 | 0.052 | 468 | 9021 a | |||
| MRD | CLOP | 37,457 | 7.512 | 0.392 | 2190 | 5580 | 0.142 | 1020 | 7164 | –0.168 | –561 | 3339 | |||
| Generic | MRD | None | None | None | 35,279 | 7.021 | |||||||||
| CLOP | 35,619 | 7.598 | 0.576 | 341 | 591 a | ||||||||||
| MRD | 36,304 | 7.293 | 0.272 | 1025 | 3770 | –0.304 | 684 | Dom | |||||||
| CLOP | MRD | 36,039 | 7.653 | 0.632 | 760 | 1204 | 0.055 | 419 | 7564 a | ||||||
| MRD | CLOP | 36,525 | 7.440 | 0.419 | 1246 | 2977 | –0.158 | 905 | Dom | ||||||
| Not used | None | None | None | 35,267 | 7.120 | ||||||||||
| CLOP | 35,545 | 7.680 | 0.560 | 278 | 496 a | ||||||||||
| MRD | 36,437 | 7.370 | 0.250 | 1170 | 4678 | –0.310 | 892 | Dom | |||||||
| CLOP | MRD | 36,013 | 7.732 | 0.612 | 746 | 1219 | 0.052 | 468 | 9021 a | ||||||
| MRD | CLOP | 36,688 | 7.512 | 0.392 | 1421 | 3621 | –0.168 | 1143 | Dom | ||||||
For patients who are intolerant of MRD, only clopidogrel and ASA are available for long-term therapy, and TA90 guidance24 is not relevant (Table 64). In this instance the price of clopidogrel is important in determining cost-effectiveness; at the branded price, the preferred strategy is ASA followed by clopidogrel, but for the generic price clopidogrel followed by ASA is more cost-effective. For patients intolerant to both ASA and MRD, only clopidogrel is available for long-term prevention and is seen to be more cost-effective than no preventive therapy.
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | Incremental analysis vs no treatment | Incremental analysis #2 | Incremental analysis #3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | ||||
| MRD intolerant | |||||||||||||||
| Full | Not used | None | None | None | 35,267 | 7.120 | |||||||||
| ASA | 34,594 | 7.644 | 0.524 | –673 | –1283 a | ||||||||||
| CLOP | 38,018 | 7.680 | 0.560 | 2,751 | 4908 | 0.036 | 3423 | 95065 | –0.047 | 2815 | Dom | ||||
| ASA | CLOP | 35,203 | 7.727 | 0.607 | –64 | –105 | 0.083 | 609 | 7336 a | ||||||
| CLOP | ASA | 37,981 | 7.804 | 0.684 | 2714 | 3966 | 0.160 | 3386 | 21,189 | 0.077 | 2778 | 36,155 a | |||
| Generic | Not used | None | None | None | 35,267 | 7.120 | |||||||||
| ASA | 34,594 | 7.644 | 0.524 | –673 | –1283 a | ||||||||||
| CLOP | 35,545 | 7.680 | 0.560 | 278 | 496 | 0.036 | 951 | 26,406 | –0.047 | 754 | Dom | ||||
| ASA | CLOP | 34,791 | 7.727 | 0.607 | –476 | –783 | 0.083 | 197 | 2377 a | ||||||
| CLOP | ASA | 35,508 | 7.804 | 0.684 | 241 | 352 | 0.160 | 914 | 5718 | 0.077 | 717 | 9328 a | |||
| ASA and MRD intolerant | |||||||||||||||
| Full | Not used | None | None | None | 35,267 | 7.120 | |||||||||
| CLOP | 38,018 | 7.680 | 0.560 | 2751 | 4908 a | ||||||||||
| Generic | Not used | None | None | None | 35,267 | 7.120 | |||||||||
| CLOP | 35,545 | 7.680 | 0.560 | 278 | 496 a | ||||||||||
Myocardial infarction-only patients
Deterministic analysis
Table 65 summarises the main economic results obtained with the Assessment Group model for the MI patient population. Figures 11–14 illustrate these findings in the form of a cost-effectiveness plane plot with cost-effectiveness frontier. The primary analysis (first block in Table 65 and Figure 11) reveals that only two strategies lie on the boundary, but both strategies involving initial use of clopidogrel are dominated by those where ASA is the first treatment offered to ‘MI-only’ patients (being both less effective and more expensive), regardless of whether or not TA90 guidance24 is applied, or whether or not the generic price of clopidogrel is used. In all scenarios, the incremental cost-effectiveness of allowing clopidogrel as a subsequent therapy after failure of ASA therapy compared with ASA treatment alone is < £9000 per QALY gained, suggesting that ASA followed by clopidogrel may be the optimal strategy for this patient group.
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | Incremental analysis vs no APT treatment | Incremental analysis vs ASA only strategy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | ||||
| Full | MRD + ASA | None | None | None | 11,781 | 9.305 | – | – | – | – | ||
| ASA | 11,465 | 9.711 | 0.406 | –316 | –778 | – | – | – | ||||
| CLOP | 14,851 | 9.553 | 0.247 | 3070 | 12,406 | –0.158 | 3836 | Dom | ||||
| ASA | CLOP | 11,980 | 9.774 | 0.469 | 199 | 425 | 0.063 | 515 | 8118 | |||
| CLOP | ASA | 14,723 | 9.652 | 0.347 | 2942 | 8480 | –0.059 | 3258 | Dom | |||
| Not used | None | None | None | 11,806 | 9.300 | – | – | – | – | – | – | |
| ASA | 11,493 | 9.716 | 0.416 | –313 | –752 | – | – | – | ||||
| CLOP | 14,891 | 9.554 | 0.255 | 3085 | 12,112 | –0.162 | 3398 | Dom | ||||
| ASA | CLOP | 12,009 | 9.780 | 0.481 | 203 | 422 | 0.064 | 516 | 8044 | |||
| CLOP | ASA | 14,785 | 9.659 | 0.359 | 2978 | 8301 | –0.058 | 3291 | Dom | |||
| Generic | MRD + ASA | None | None | None | 11,781 | 9.305 | ||||||
| ASA | 11,465 | 9.711 | 0.406 | –316 | –778 | 0.000 | 0 | |||||
| CLOP | 12,284 | 9.553 | 0.247 | 503 | 2034 | –0.158 | 819 | Dom | ||||
| ASA | CLOP | 11,552 | 9.774 | 0.469 | –229 | –488 | 0.063 | 86 | 1363 | |||
| CLOP | ASA | 12,156 | 9.652 | 0.347 | 375 | 1,082 | –0.059 | 691 | Dom | |||
| Not used | None | None | None | 11,806 | 9.300 | – | – | – | – | – | – | |
| ASA | 11,493 | 9.716 | 0.416 | –313 | –752 | – | – | – | ||||
| CLOP | 12,306 | 9.554 | 0.255 | 499 | 1961 | –0.162 | 812 | Dom | ||||
| ASA | CLOP | 11,580 | 9.780 | 0.481 | –226 | –471 | 0.064 | 87 | 1352 | |||
| CLOP | ASA | 12,199 | 9.659 | 0.359 | 393 | 1096 | –0.058 | 706 | Dom | |||
FIGURE 11.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘MI-only’ patients (using MRD + ASA as per the TA9024 guidance). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
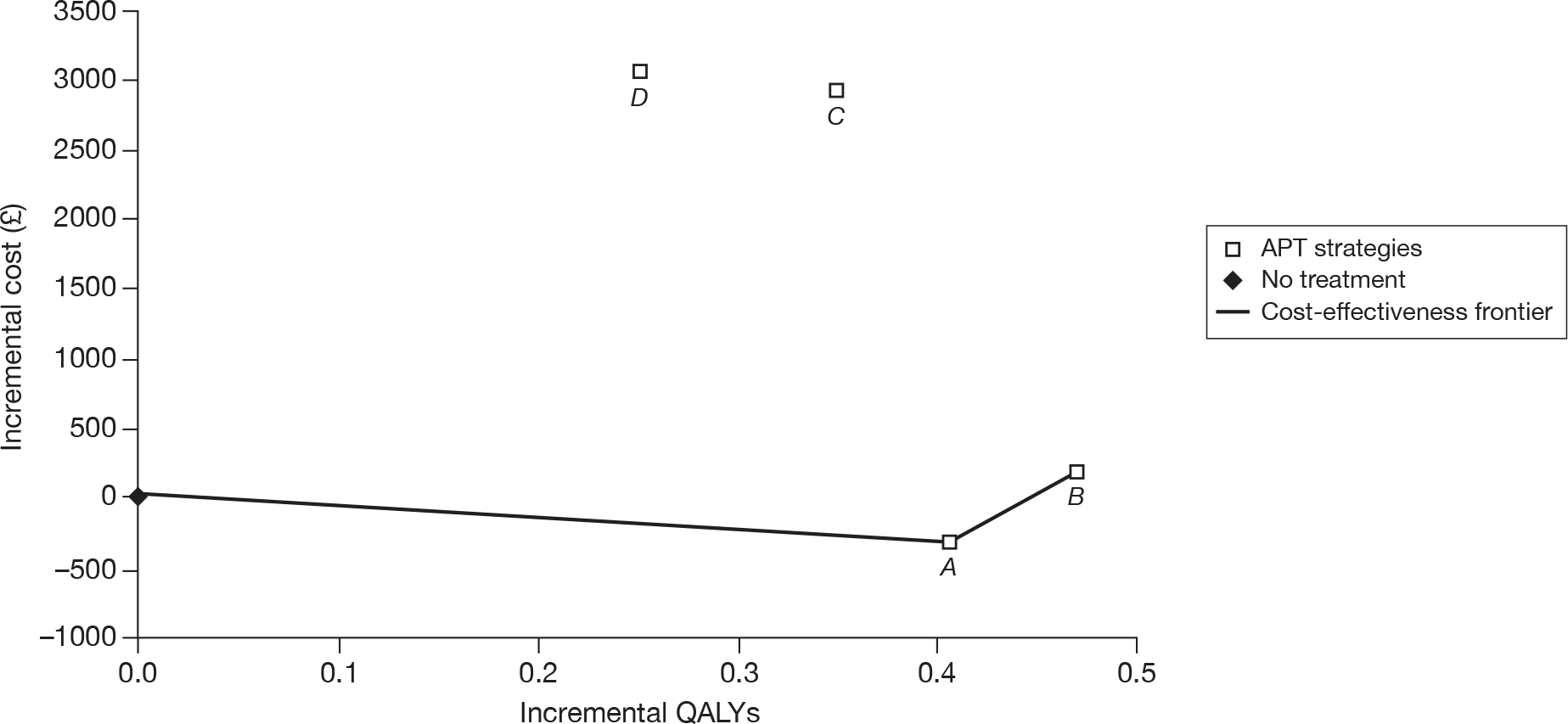
FIGURE 12.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘MI-only’ patients (without applying the TA9024 guidance). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
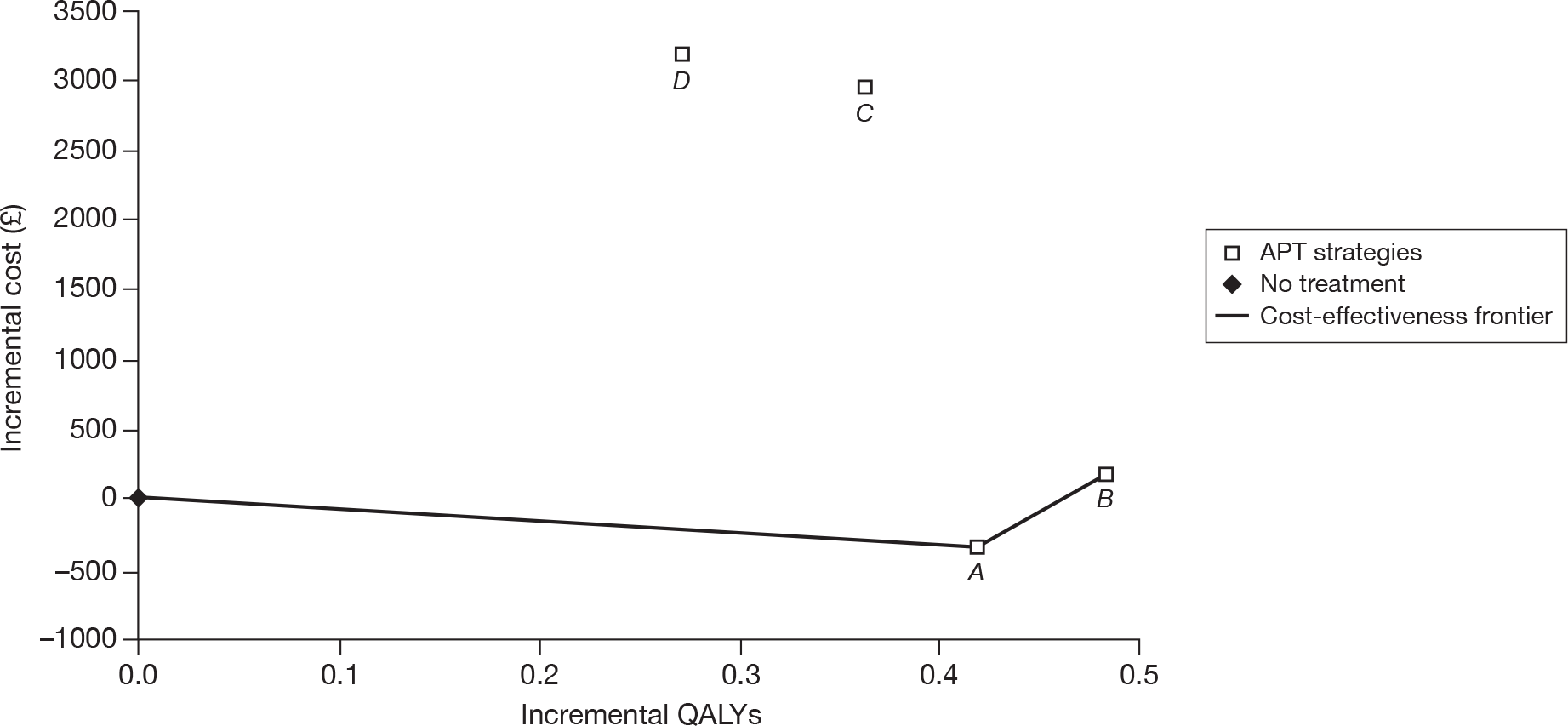
FIGURE 13.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘MI-only’ patients (using MRD + ASA as per the TA9024 guidance and the generic clopidogrel price). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
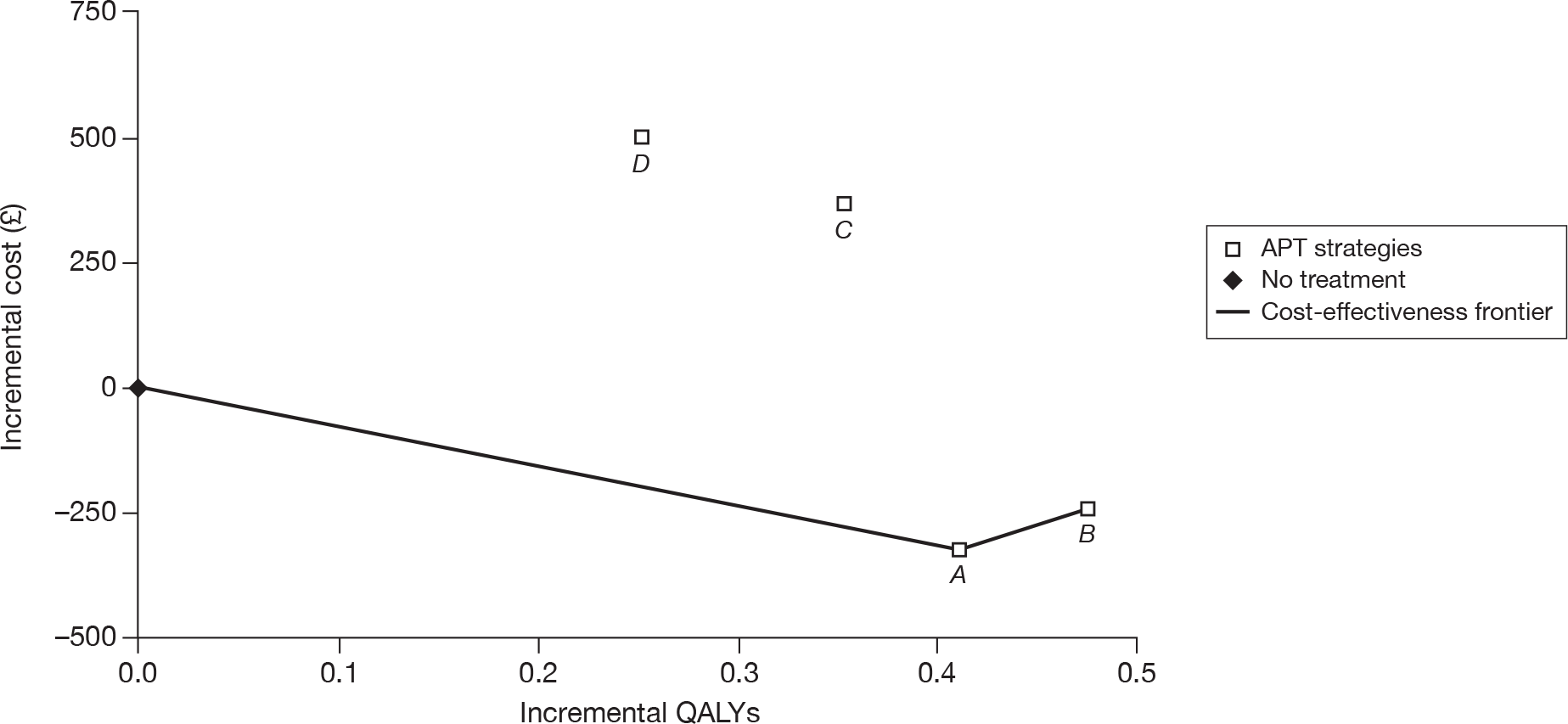
FIGURE 14.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘MI-only’ patients (without applying the TA9024 guidance and using the generic clopidogrel price). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
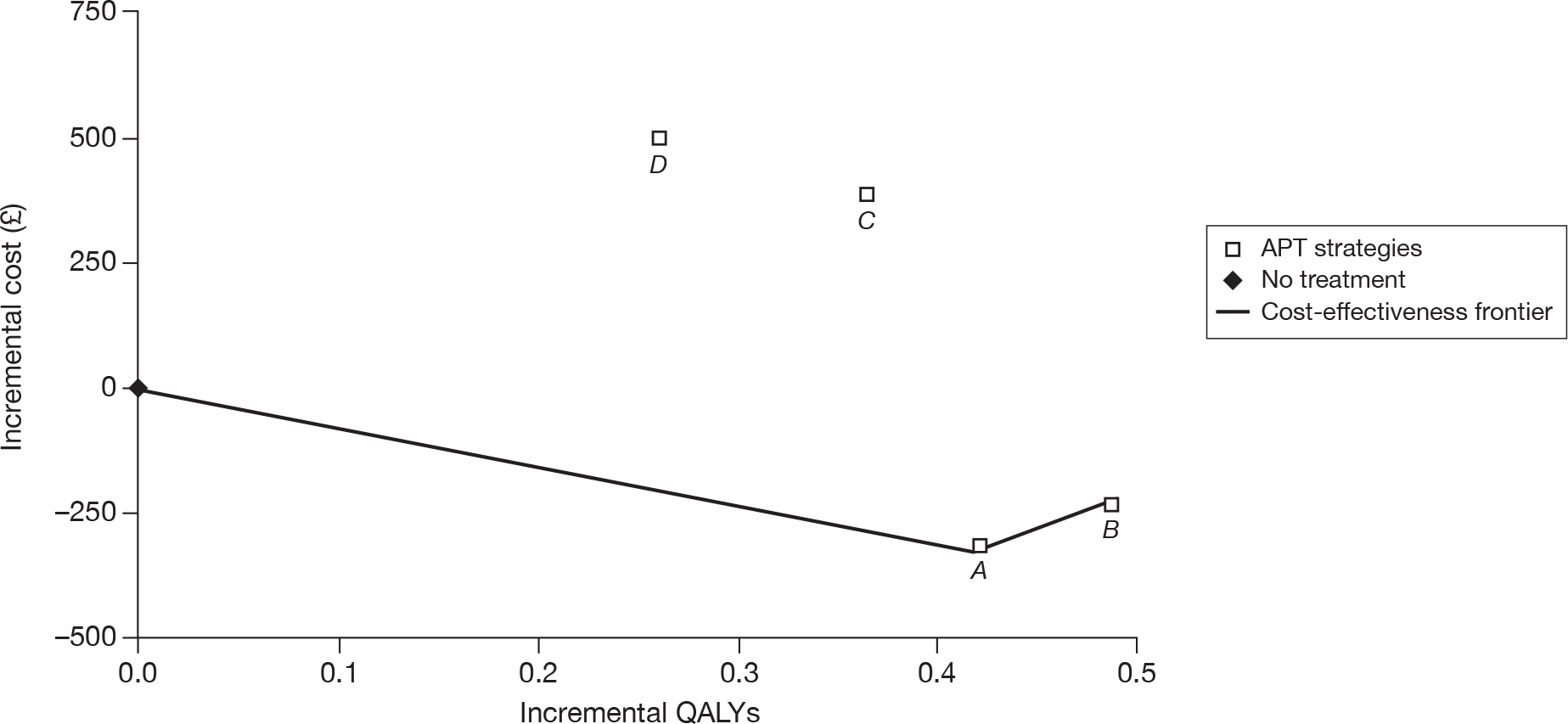
Intolerance to acetylsalicyclic acid
In patients who are intolerant of ASA, clopidogrel is the only available long-term therapy available and, therefore, comparisons have been carried out against the ‘no-treatment’ scenario. The results are given in Table 66 and indicate that clopidogrel is a cost-effective approach to occlusive vascular event prevention independent of both TA90 guidance24 and the price of clopidogrel (ICERs ranging between £1961 and £12,391 per QALY gained).
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | Incremental analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | ||||
| Full | MRD | None | None | None | 11,796 | 9.297 | |||
| CLOP | 14,880 | 9.546 | 0.249 | 3085 | 12,523 | ||||
| Not used | None | 11,806 | 9.300 | ||||||
| CLOP | 14,891 | 9.554 | 0.255 | 3085 | 12,112 | ||||
| Generic | MRD | None | None | None | 11,796 | 9.297 | |||
| CLOP | 12,312 | 9.546 | 0.249 | 516 | 2,073 | ||||
| Not used | None | 11,806 | 9.300 | ||||||
| CLOP | 12,306 | 9.554 | 0.255 | 499 | 1961 | ||||
Peripheral arterial disease-only patients
Deterministic analysis
Table 67 summarises the main economic results obtained with the Assessment Group model for the ‘peripheral arterial disease-only’ patient population. Figures 15–18 illustrate these findings in the form of a cost-effectiveness plane plot with cost-effectiveness frontier. The primary analysis (first block in Table 67 and Figure 15) reveals that three strategies lie on the boundary, but the clopidogrel-only strategy is clearly less cost-effective than all other options. This is true in all peripheral arterial disease scenarios. When the requirement is removed to adhere to the TA90 guidance24 following an ischaemic stroke event, the absolute values of costs and outcomes are modified, but the relativities between strategies remain qualitatively unchanged (see Figures 16 and 18). If the full branded price of clopidogrel is replaced by the NHS generic price, the cost differences between the strategies are markedly reduced, but the broad pattern is unchanged. In all scenarios the ICER for a strategy of clopidogrel followed by ASA when compared with ASA followed by clopidogrel appears to be well within the range considered cost-effective (under £13,000 per QALY gained for branded clopidogrel and under £5000 per QALY for generic clopidogrel), suggesting this as the optimal strategy for this patient group.
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | Incremental analysis vs no APT treatment | Incremental analysis vs ASA only strategy | Incremental analysis vs ASA → CLOP strategy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | ||||
| Full | MRD + ASA | None | None | None | 7283 | 9.567 | – | – | – | – | – | – | – | – | – |
| ASA | 6421 | 9.897 | 0.391 | –862 | –2,612 | – | – | – | – | – | – | ||||
| CLOP | 10,869 | 10.222 | 0.785 | 3586 | 5475 | 0.325 | 4448 | 13,691 | 0.185 | 3864 | 20,891 | ||||
| ASA | CLOP | 7005 | 10.037 | 0.539 | –278 | –591 | 0.140 | 584 | 4176 | – | – | – | |||
| CLOP | ASA | 10,636 | 10.317 | 0.909 | 3353 | 4468 | 0.420 | 4215 | 10,028 | 0.280 | 3631 | 12,948 | |||
| Not used | None | None | None | 7232 | 9.551 | – | – | – | – | – | – | – | – | – | |
| ASA | 6398 | 9.894 | 0.382 | –834 | –2425 | – | – | – | – | – | – | ||||
| CLOP | 10,872 | 10.219 | 0.773 | 3640 | 5443 | 0.325 | 4474 | 13,768 | 0.180 | 3822 | 21,222 | ||||
| ASA | CLOP | 7051 | 10.039 | 0.556 | –181 | –371 | 0.145 | 653 | 4504 | – | – | – | |||
| CLOP | ASA | 10,695 | 10.321 | 0.905 | 3464 | 4498 | 0.426 | 4297 | 10,083 | 0.281 | 3645 | 12,956 | |||
| Generic | MRD + ASA | None | None | None | 7283 | 9.567 | – | – | – | – | – | – | – | – | – |
| ASA | 6421 | 9.897 | 0.391 | –862 | –2612 | – | – | – | – | – | – | ||||
| CLOP | 7840 | 10.222 | 0.785 | 557 | 850 | 0.325 | 1419 | 4368 | 0.185 | 1381 | 7466 | ||||
| ASA | CLOP | 6459 | 10.037 | 0.539 | –824 | –1753 | 0.140 | 38 | 273 | – | – | – | |||
| CLOP | ASA | 7607 | 10.317 | 0.909 | 324 | 432 | 0.420 | 1186 | 2822 | 0.280 | 1148 | 4094 | |||
| Not used | None | None | None | 7232 | 9.551 | – | – | – | – | – | – | – | – | – | |
| ASA | 6398 | 9.894 | 0.382 | –834 | –2425 | – | – | – | – | – | – | ||||
| CLOP | 7819 | 10.219 | 0.773 | 587 | 878 | 0.325 | 1421 | 4373 | 0.180 | 1307 | 7257 | ||||
| ASA | CLOP | 6512 | 10.039 | 0.556 | –720 | –1473 | 0.145 | 114 | 787 | – | – | – | |||
| CLOP | ASA | 7642 | 10.321 | 0.905 | 410 | 533 | 0.426 | 1244 | 2919 | 0.281 | 1130 | 4017 | |||
FIGURE 15.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘peripheral arterial disease-only’ patients (using MRD + ASA as per the TA9024 guidance). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
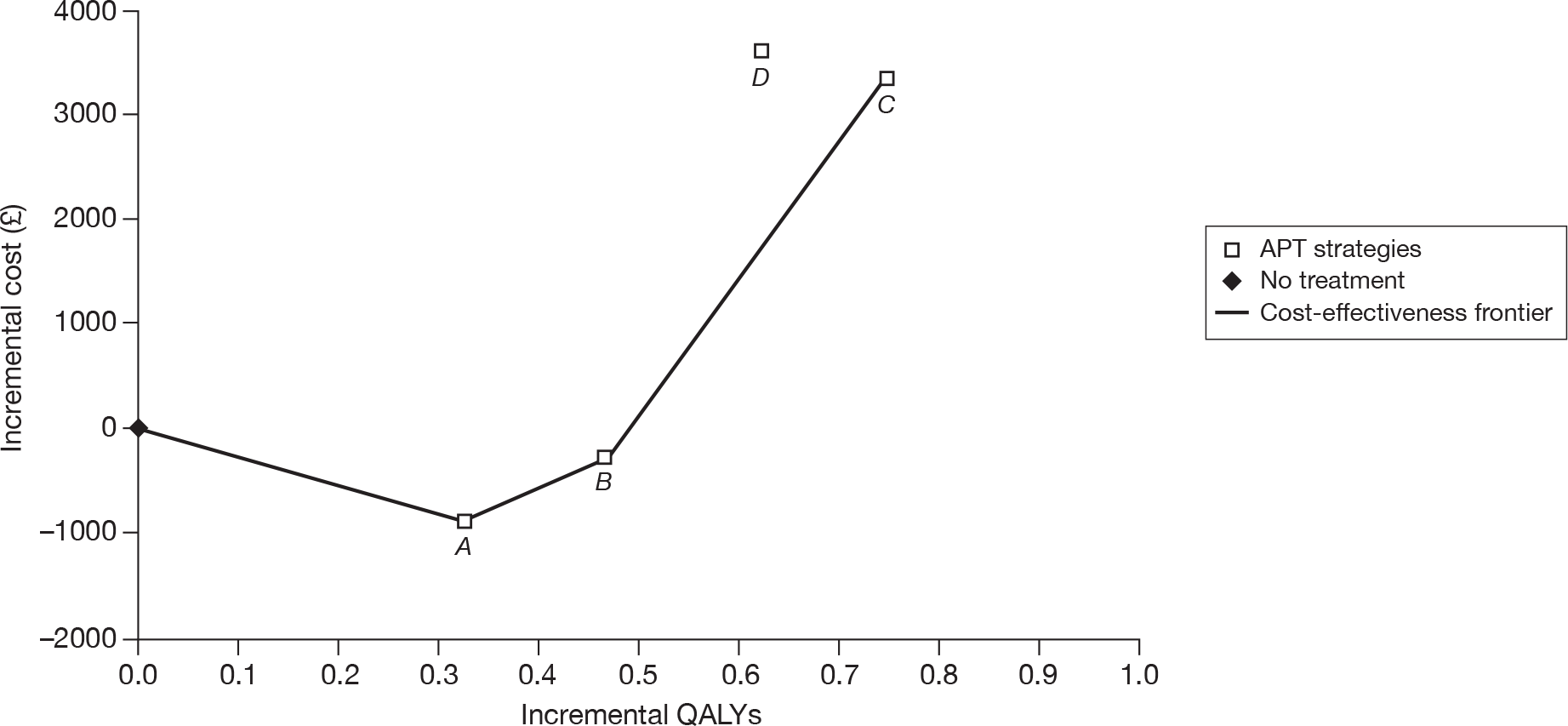
FIGURE 16.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘peripheral arterial disease-only’ patients (without applying the TA9024 guidance). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
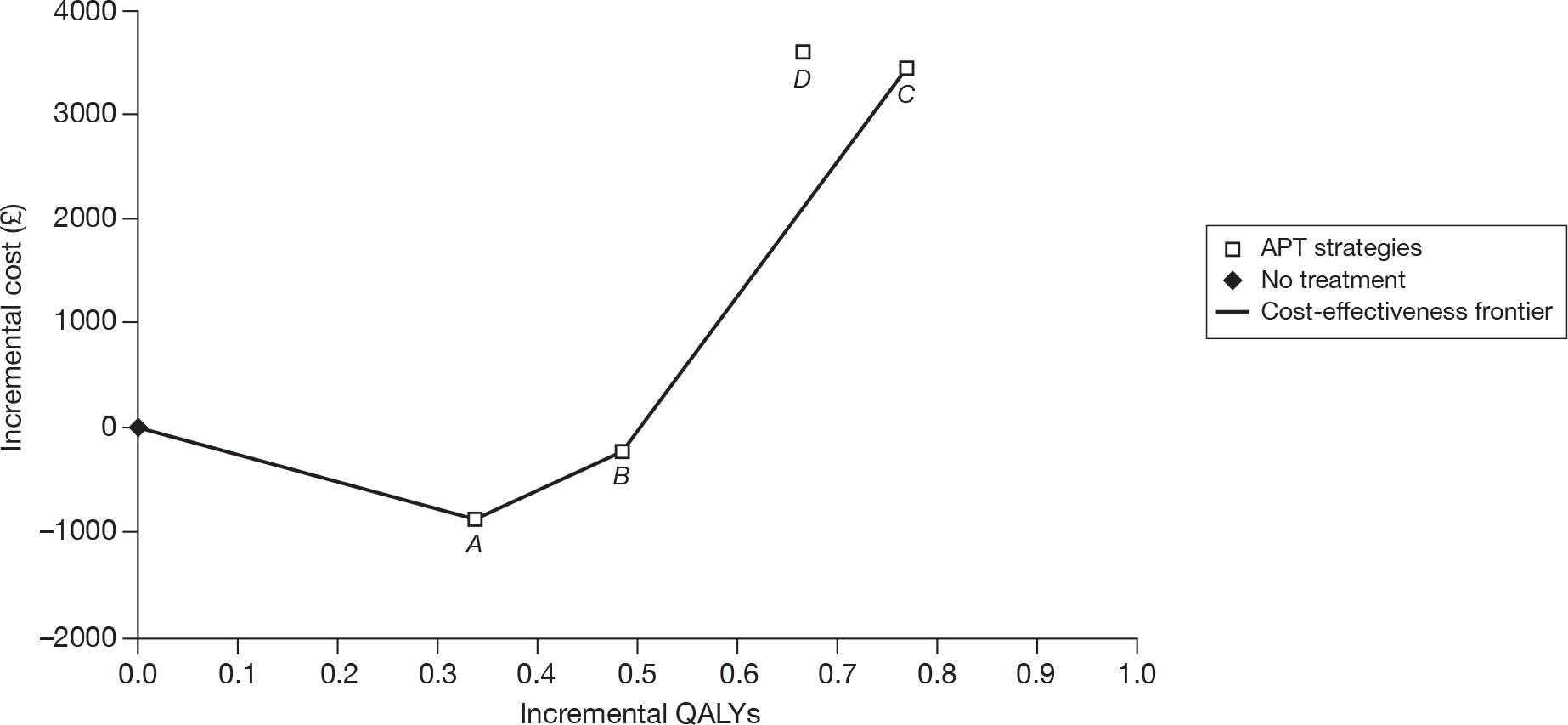
FIGURE 17.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘peripheral arterial disease-only’ patients (using MRD + ASA as per the TA9024 guidance and the generic clopidogrel price). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
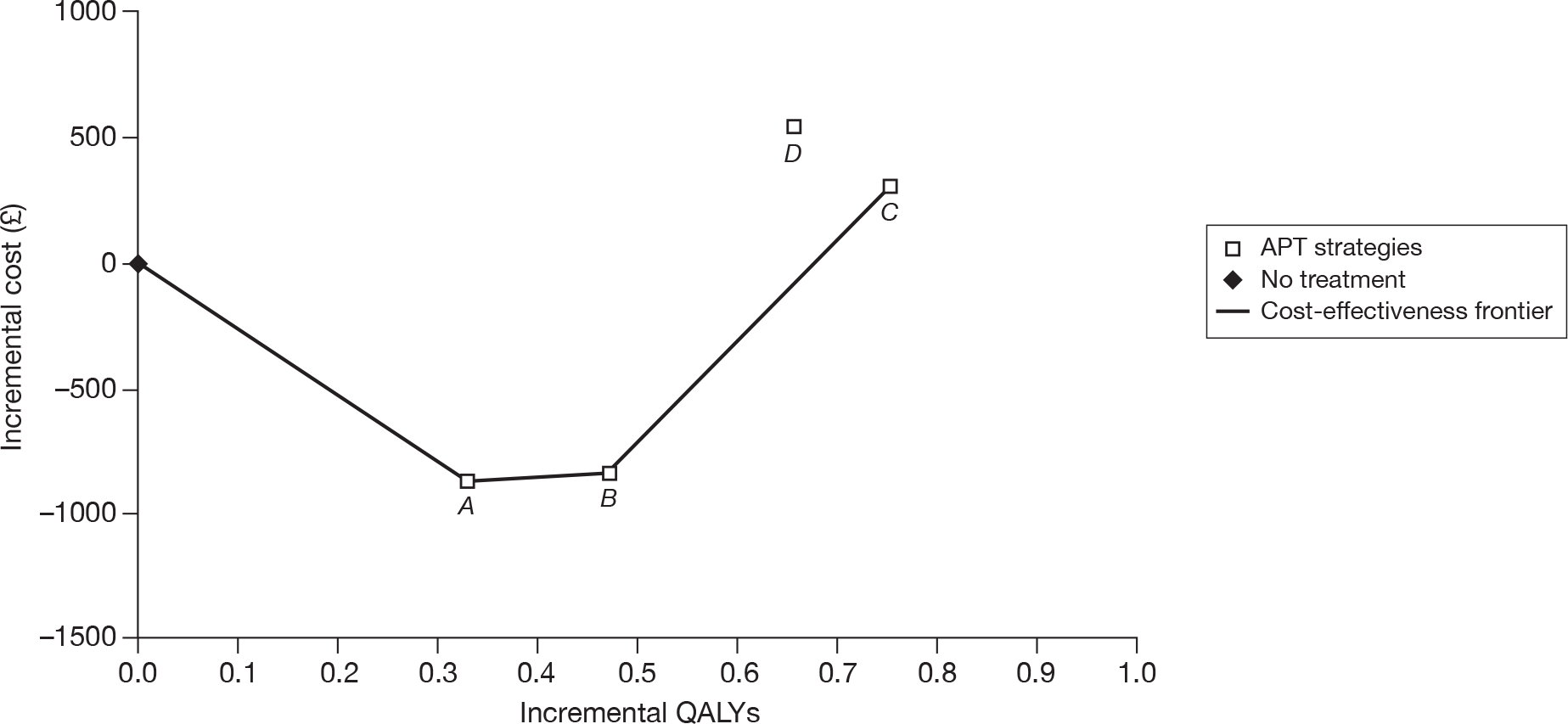
FIGURE 18.
The cost-effectiveness plane and frontier showing available treatment strategies for ‘peripheral arterial disease-only’ patients (without applying the TA9024 guidance and using the generic clopidogrel price). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.

Intolerance to acetylsalicyclic acid
In patients who are intolerant of ASA, clopidogrel is the only available long-term therapy available, and, therefore, comparisons have been carried out against the ‘no-treatment’ scenario. The results are given in Table 68 and indicate that clopidogrel is a cost-effective approach to occlusive vascular event prevention independent of both the TA90 guidance24 and the price of clopidogrel.
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | Incremental analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | ||||
| Full | MRD | None | None | None | 7293 | 9.552 | – | – | – |
| CLOP | 10,901 | 10.210 | 0.659 | 3608 | 5478 | ||||
| Not used | None | None | None | 7232 | 9.551 | – | – | – | |
| CLOP | 10,872 | 10.219 | 0.669 | 3640 | 5443 | ||||
| Generic | MRD | None | None | None | 7293 | 9.552 | – | – | – |
| CLOP | 7671 | 10.210 | 0.659 | 578 | 878 | ||||
| Not used | None | None | None | 7232 | 9.551 | – | – | – | |
| CLOP | 7819 | 10.219 | 0.669 | 587 | 878 | ||||
Patients with multivascular disease
Deterministic analysis
Table 69 summarises the main economic results obtained with the Assessment Group model for the multivascular disease patient population. Figures 19–22 illustrate these findings in the form of a cost-effectiveness plane plot with cost-effectiveness frontier. The primary analysis (first block in Table 69 and Figure 19) reveals that three strategies lie on the boundary, but the clopidogrel-only strategy is clearly less cost-effective than all other options. This is true in all multivascular disease scenarios. When the requirement is removed to adhere to TA90 guidance24 following an ischaemic stroke event, the absolute values of costs and outcomes are modified, but the relativities between strategies remain qualitatively unchanged. If the full branded price of clopidogrel is replaced by the NHS generic price, the cost differences between the strategies are markedly reduced, but the broad pattern is unchanged. In all scenarios, clopidogrel followed by ASA is the most cost-effective strategy.
FIGURE 19.
The cost-effectiveness plane and frontier showing available treatment strategies for multivascular disease patients (using MRD + ASA as per the TA9024 guidance). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
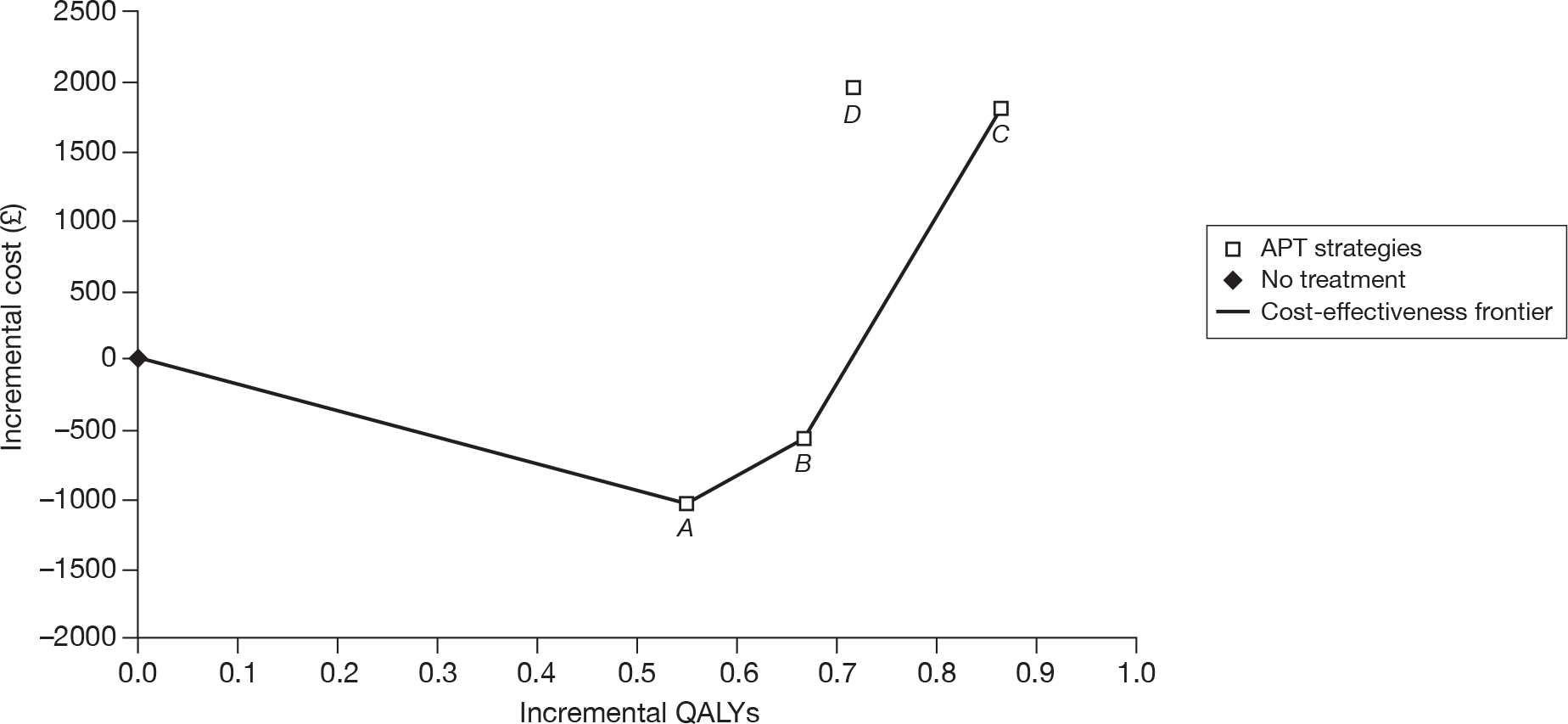
FIGURE 20.
The cost-effectiveness plane and frontier showing available treatment strategies for multivascular disease patients (without applying the TA9024 guidance). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
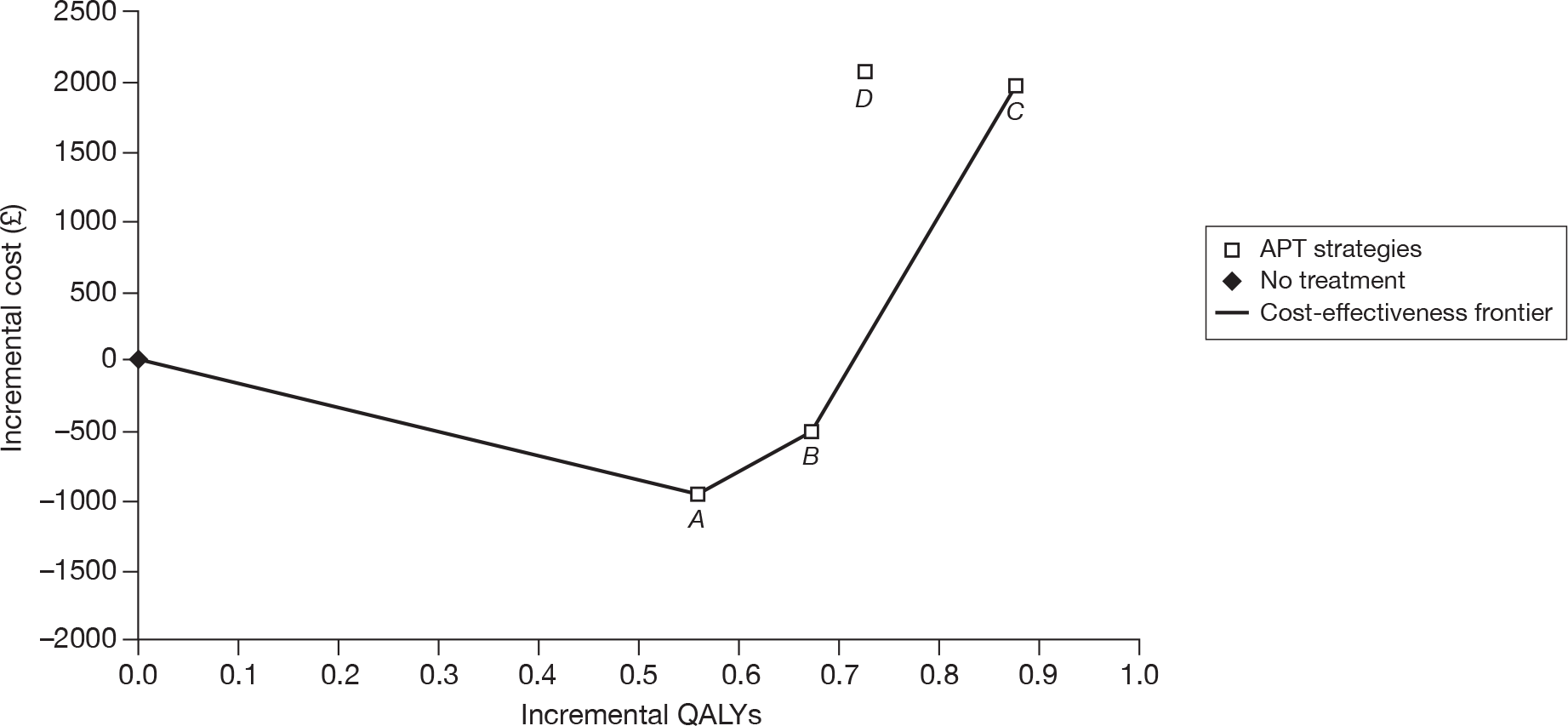
FIGURE 21.
The cost-effectiveness plane and frontier showing available treatment strategies for multivascular disease patients (using MRD + ASA as per the TA9024 guidance and the generic clopidogrel price). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antiplatelet therapy.
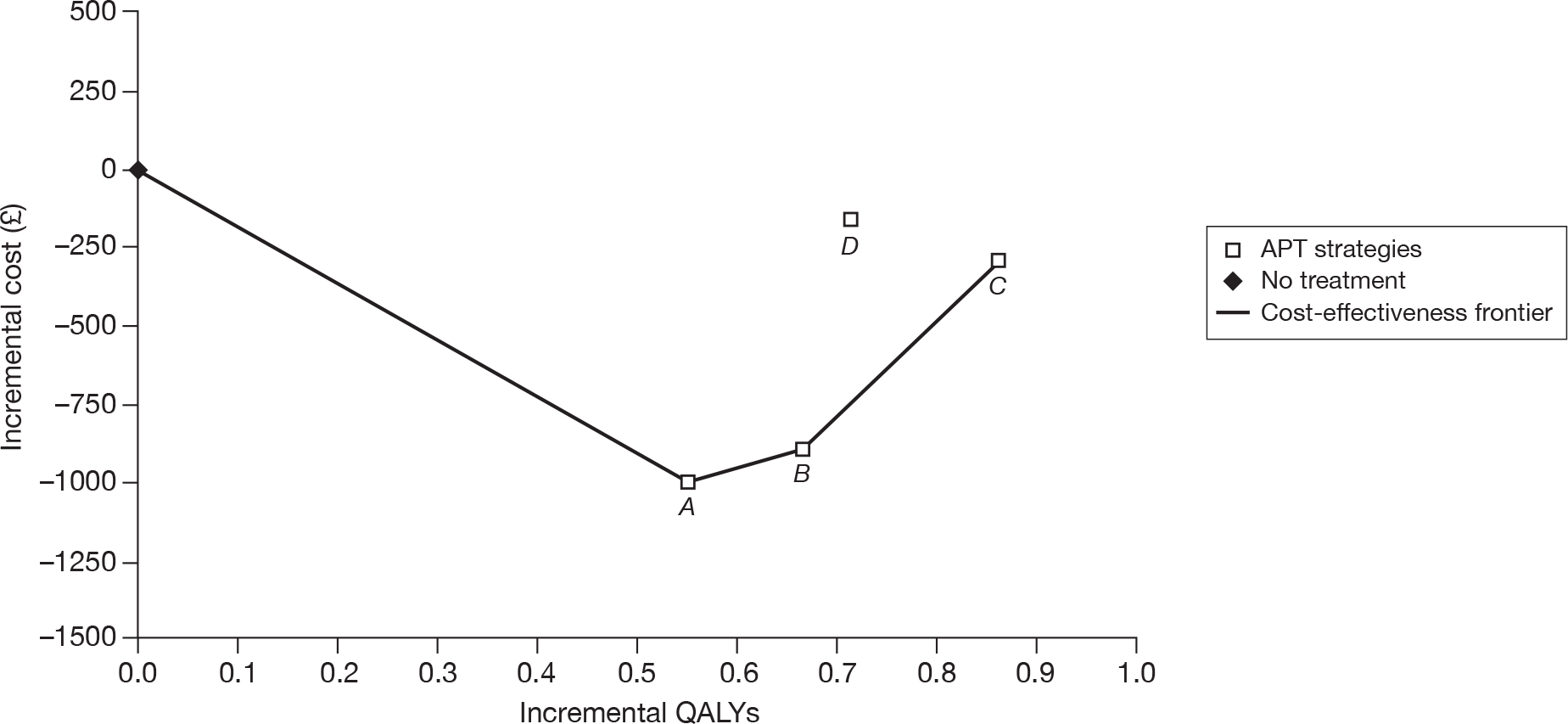
FIGURE 22.
The cost-effectiveness plane and frontier showing available treatment strategies for multivascular disease patients (without applying the TA9024 guidance and using the generic clopidogrel price). A = ASA; B = ASA to clopidogrel; C = clopidogrel to ASA; and D = clopidogrel. APT, antplatelet therapy.
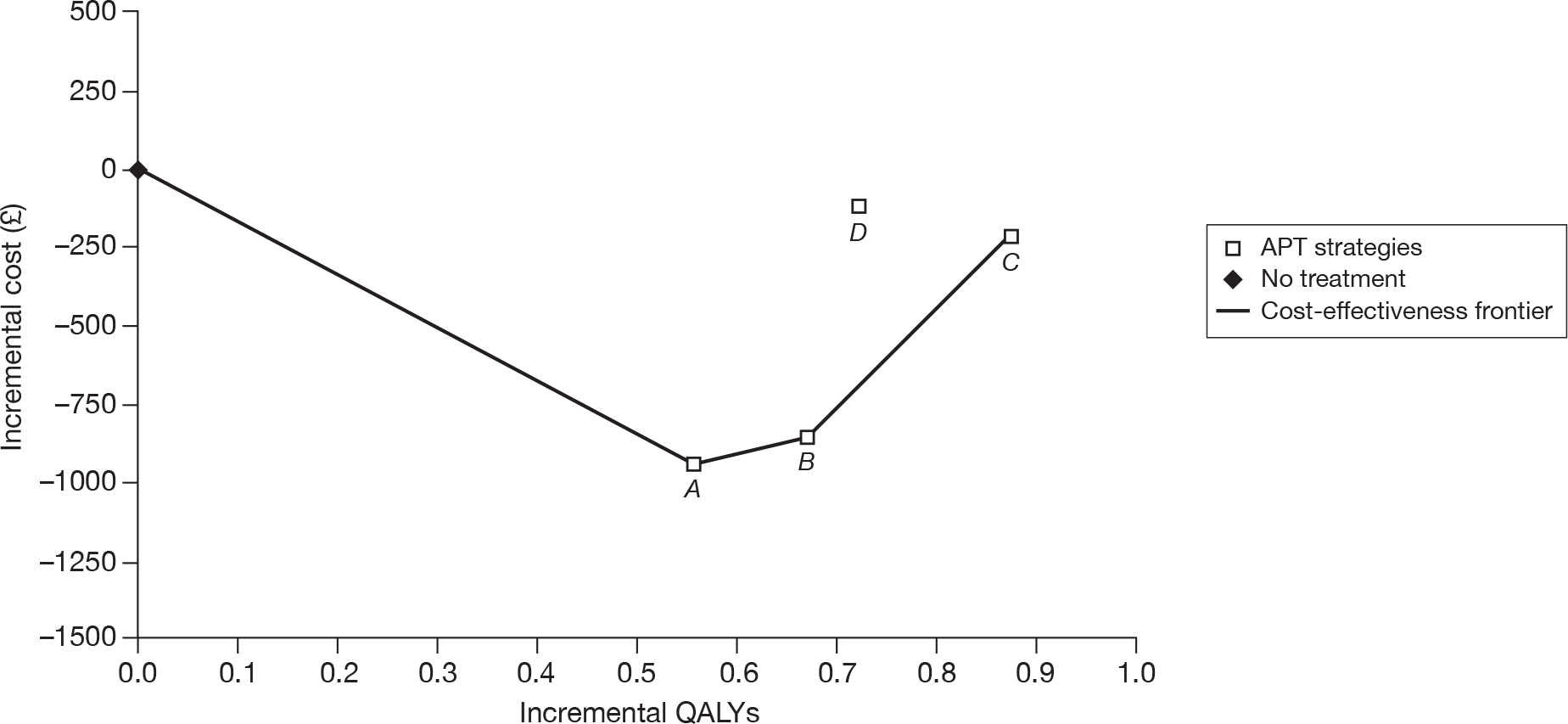
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | Incremental analysis vs no APT treatment | Incremental analysis vs ASA-only strategy | Incremental analysis vs ASA → CLOP strategy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | IQ | IC (£) | ICER (£) | ||||
| Full | MRD + ASA | None | None | None | 20,819 | 5.620 | – | – | – | – | – | – | – | – | – |
| ASA | 19,826 | 6.170 | 0.550 | –993 | –1805 | – | – | – | – | – | – | ||||
| CLOP | 22,809 | 6.333 | 0.713 | 1990 | 2793 | 0.163 | 2983 | 18,331 | 0.047 | 2526 | 53,582 | ||||
| ASA | CLOP | 20,283 | 6.286 | 0.665 | –536 | –805 | 0.116 | 457 | 3952 | – | – | ||||
| CLOP | ASA | 22,671 | 6.481 | 0.860 | 1852 | 2153 | 0.310 | 2844 | 9164 | 0.195 | 2388 | 12,257 | |||
| Not used | None | None | None | 20,733 | 5.625 | – | – | – | – | – | – | – | – | – | |
| ASA | 19,803 | 6.183 | 0.558 | –930 | –1665 | – | – | – | – | – | – | ||||
| CLOP | 22,827 | 6.347 | 0.722 | 2095 | 2,902 | 0.163 | 3,024 | 18,505 | 0.050 | 2579 | 51,729 | ||||
| ASA | CLOP | 20,248 | 6.297 | 0.672 | –484 | –721 | 0.114 | 445 | 3922 | – | – | – | |||
| CLOP | ASA | 22,730 | 6.498 | 0.873 | 1997 | 2287 | 0.315 | 2927 | 9,293 | 0.201 | 2481 | 12,324 | |||
| Generic | MRD + ASA | None | None | None | 20,819 | 5.620 | – | – | – | – | – | – | – | – | – |
| ASA | 19,826 | 6.170 | 0.550 | –993 | –1805 | – | – | – | – | – | |||||
| CLOP | 20,673 | 6.333 | 0.713 | –146 | –205 | 0.163 | 847 | 5203 | 0.047 | 746 | 15,813 | ||||
| ASA | CLOP | 19,927 | 6.286 | 0.665 | –891 | –1339 | 0.116 | 101 | 875 | – | – | – | |||
| CLOP | ASA | 20,534 | 6.481 | 0.860 | –284 | –331 | 0.310 | 708 | 2281 | 0.195 | 607 | 3116 | |||
| Not used | None | None | None | 20,733 | 5.625 | – | – | – | – | – | – | – | – | – | |
| ASA | 19,803 | 6.183 | 0.558 | –930 | –1665 | – | – | – | – | – | – | ||||
| CLOP | 20,625 | 6.347 | 0.722 | –108 | –149 | 0.163 | 822 | 5031 | 0.050 | 727 | 14,574 | ||||
| ASA | CLOP | 19,899 | 6.297 | 0.672 | –834 | –1241 | 0.114 | 96 | 842 | – | – | – | |||
| CLOP | ASA | 20,527 | 6.498 | 0.873 | –205 | –235 | 0.315 | 725 | 2301 | 0.201 | 629 | 3124 | |||
Intolerance to acetylsalicyclic acid
In patients who are intolerant of ASA, clopidogrel is the only long-term therapy available and, therefore, comparisons have been carried out against the ‘no-treatment’ scenario. The results are given in Table 70 and indicate that clopidogrel is a cost-effective approach to occlusive vascular event prevention independent of both the TA90 guidance24 and the price of clopidogrel.
| CLOP price | TA9024 status | Strategy | Costs IQ | Utility IQ | Incremental analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | IQ | IC (£) | ICER (£) | ||||
| Full | MRD | None | None | None | 20,747 | 5.588 | |||
| CLOP | 22,792 | 6.311 | 0.723 | 2046 | 2829 | ||||
| Not used | None | 20,733 | 5.625 | ||||||
| CLOP | 22,827 | 6.347 | 0.722 | 2095 | 2902 | ||||
| Generic | MRD | None | None | None | 20,747 | 5.588 | |||
| CLOP | 21,079 | 6.319 | 0.731 | 332 | 454 | ||||
| Not used | None | 20,733 | 5.625 | ||||||
| CLOP | 20,625 | 6.347 | 0.722 | –108 | –149 | ||||
Univariate sensitivity analysis
The Assessment Group model incorporates 197 parameters involving estimation uncertainty for which their potential influence on the economic results should be examined. Carrying out a comprehensive assessment of each parameter individually was judged to be impractical (because of the model running time involved) and largely uninformative. Instead, the parameters were grouped into 11 sets, which were assessed collectively, taking the maxima of the reasonable value range of all members of a group as a basis for estimating one extreme scenario, and the minima for the other. This is likely to overstate the net effect of the individual factors, as it is very unlikely that all uncertainties within a group will be biased in the same direction. Nonetheless, it was considered to be a helpful approach in identifying which broad categories of parameters that have a greater likelihood of influencing an assessment of cost-effectiveness through parameter uncertainty. In effect, this approach defines an upper limit on the net influence of uncertainty in all the variables within the group.
Wherever possible, the testing intervals have been set to the conventional 95% CI for estimating the parameter value. In the few instances where this information was not available, a general range of ± 10% of the central estimate was adopted. This was used for the duration of effect of the transient component of some event risks (known to have a minimal influence on model results), several events and continuing care costs, and to allow a notional uncertainty to be applied to the assumption, discussed above, that no additional weighting was necessary to the risk of non-vascular mortality in this population.
Ischaemic stroke population
Sensitivity analysis was carried out on the comparison between the strategy recommended above [MRD + ASA followed by ASA followed by clopidogrel (see Ischaemic stroke-only patients)] and the de facto current ‘standard care’ of ASA using the branded price of clopidogrel and without the TA90 guidance24 applied. This scenario exhibits a deterministic ICER of £5880 per QALY gained.
Figure 23 shows the results for each group of parameters. In most cases there is very little variation from the central ICER estimate. There are two exceptions: ‘key event risks’ shows a comparatively larger uncertainty (although still well within the range normally considered acceptable) and the asymmetrical range for ‘antiplatelet cessation risks’ indicates the inherent non-linearity of the model in this feature.
FIGURE 23.
Sensitivity analysis for groups of model parameters for a comparison of the recommended strategy for ‘ischaemic stroke-only’ patients (MRD + ASA → ASA→ clopidogrel) vs ASA alone (branded price of clopidogrel/TA9024 guidance not applied). APT, antiplatelet therapy.
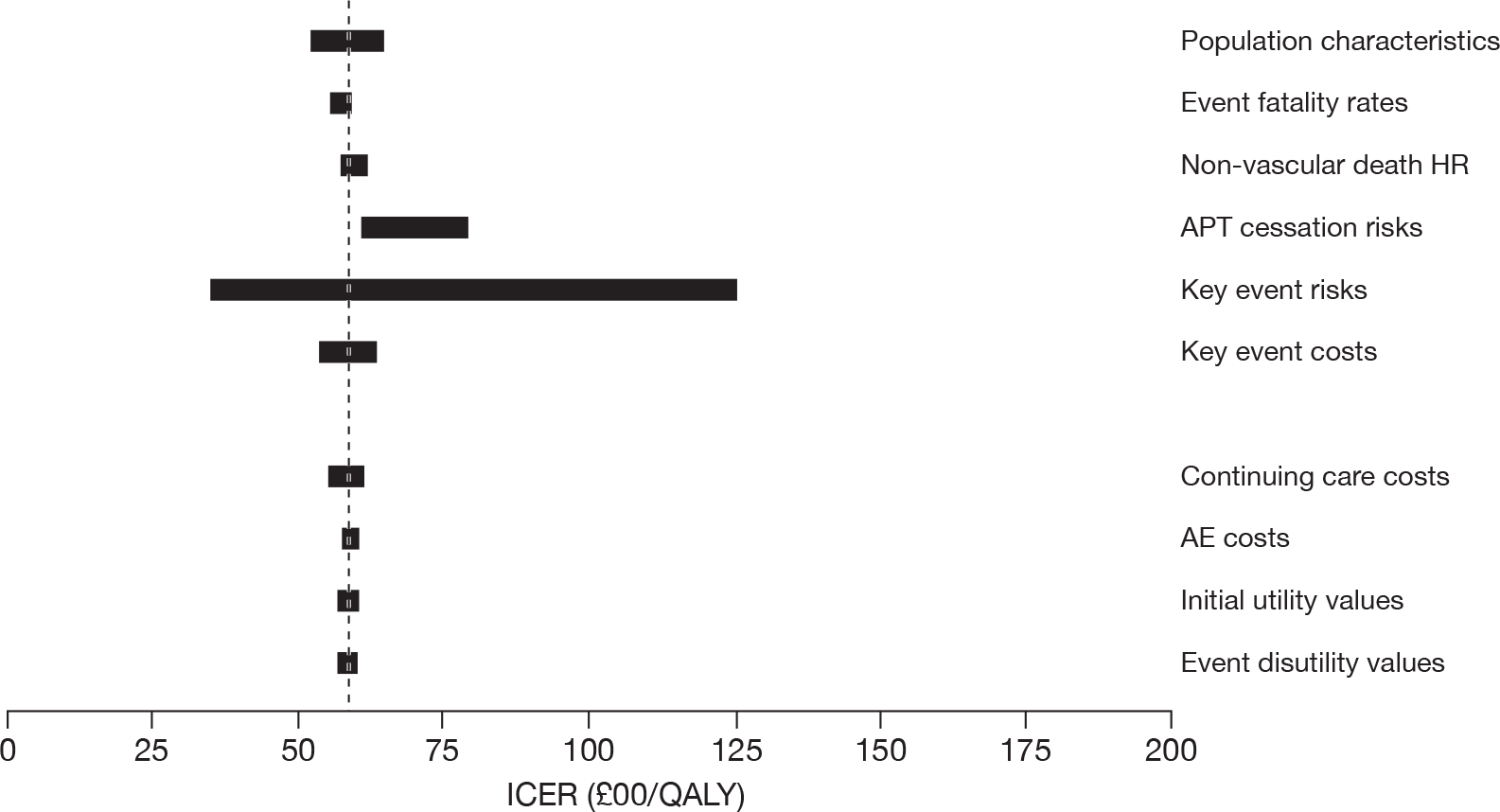
Myocardial infarction-only population
Sensitivity analysis was carried out on the comparison between the strategy recommended above (ASA followed by clopidogrel) and the de facto current ‘standard care’ of ASA using the branded price of clopidogrel and without the TA90 guidance24 applied. This scenario exhibits a deterministic ICER of £8044 per QALY gained.
Figure 24 shows the results for each group of parameters. In most cases there is very little variation from the central ICER estimate. In this case the largest uncertainty is associated with antiplatelet treatment cessation risks, and to a lesser extent to event fatality rates. However, in all cases the ICER remains well below £10,000 per QALY gained.
FIGURE 24.
Sensitivity analysis for groups of model parameters for a comparison of the recommended strategy for ‘MI-only’ patients (ASA → clopidogrel) vs ASA alone (branded price of clopidogrel/TA9024 guidance not applied). APT, antiplatelet therapy.
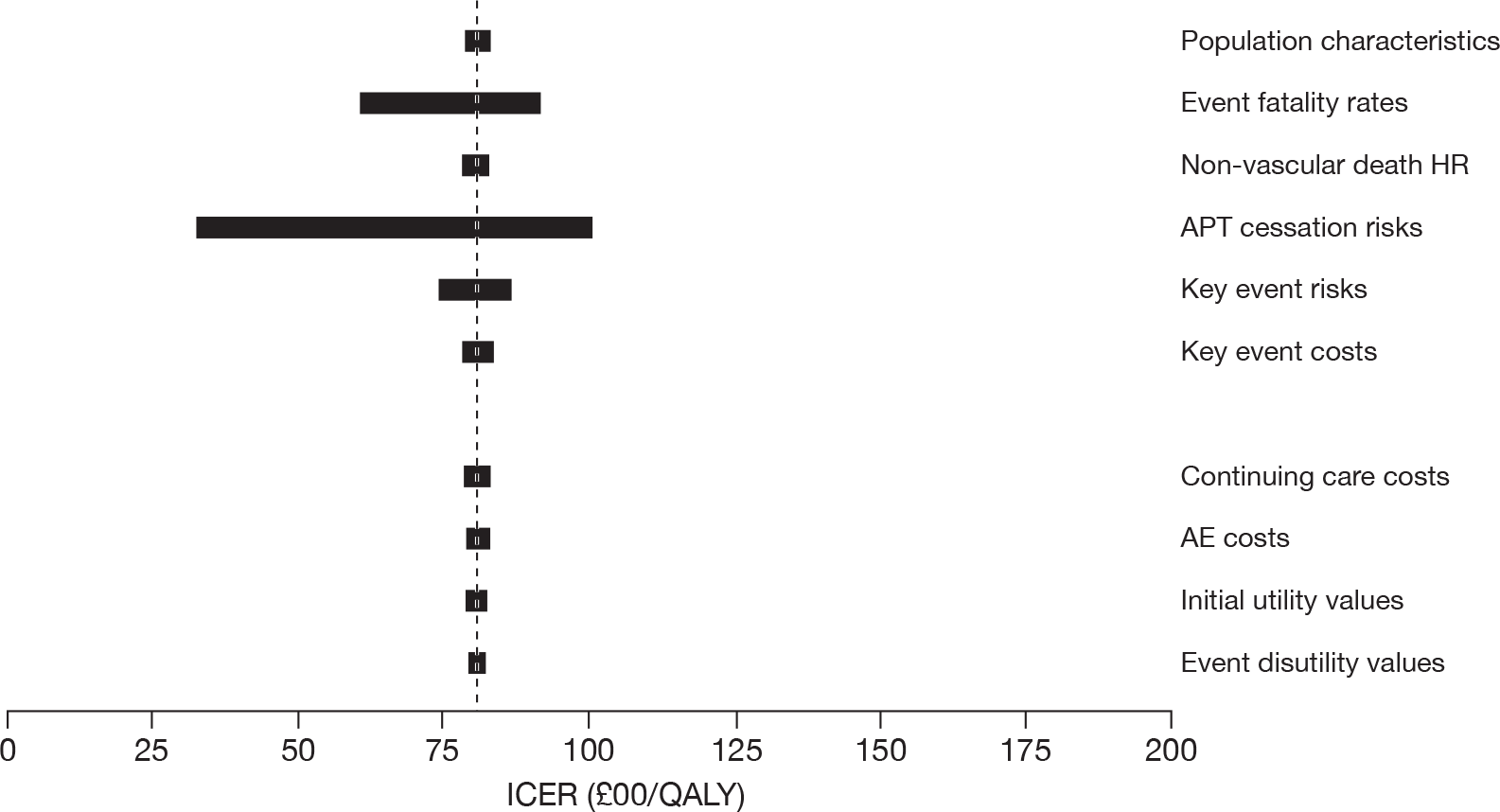
Peripheral arterial disease-only population
Sensitivity analysis was carried out on the comparison between the strategy recommended above (clopidogrel followed by ASA) and the de facto current ‘standard care’ of ASA, using the branded price of clopidogrel and without the TA90 guidance24 applied. This scenario exhibits a deterministic ICER of £10,083 per QALY gained.
Figure 25 shows the results for each group of parameters. In most cases there is very little variation from the central ICER estimate. However, a very large uncertainty range is associated with key event risks. Examination of the underlying parameter values points to a very few instances where there is evidence of a clear advantage for clopidogrel over ASA in this patient group, and where a benefit is indicated the lower confidence limits are closely aligned. As explained above, this effect may in fact be an artefact of the grouping of parameters in this analysis and can be resolved only through full probabilistic sensitivity analysis.
FIGURE 25.
Sensitivity analysis for groups of model parameters for a comparison of the recommended strategy for ‘peripheral arterial disease-only’ patients (ASA → clopidogrel) vs ASA alone (branded price of clopidogrel/TA9024 guidance not applied). APT, antiplatelet therapy.
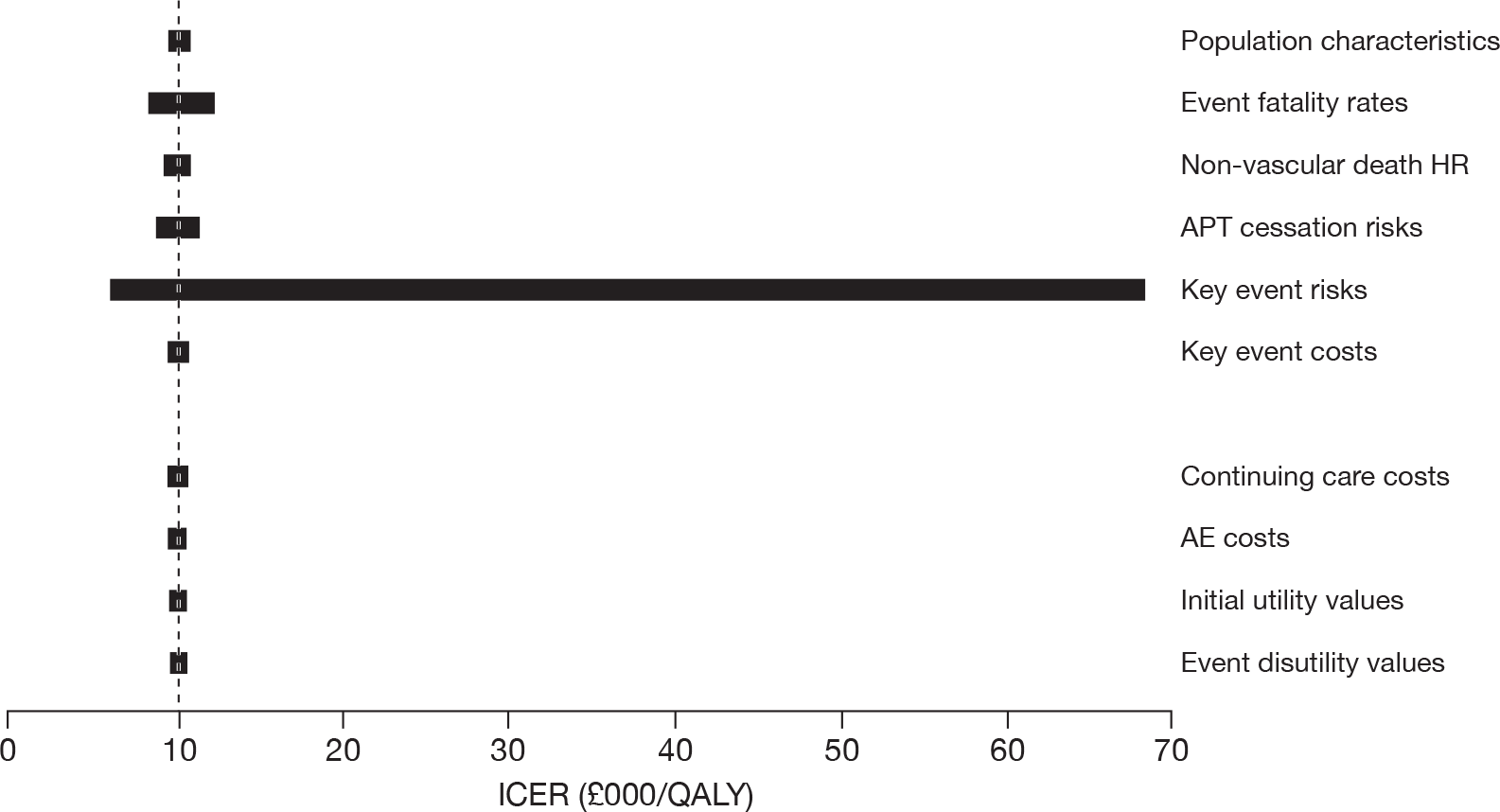
Multivascular disease population
Sensitivity analysis was carried out on the comparison between the strategy recommended above (ASA followed by clopidogrel) and the de facto current ‘standard care’ of ASA using the branded price of clopidogrel and without the TA90 guidance24 applied. This scenario exhibits a deterministic ICER of £9293 per QALY gained.
Figure 26 shows the results for each group of parameters. In most cases there is very little variation from the central ICER estimate. Exceptions are the event fatality rates group and antiplatelet treatment cessation risks. However, in all cases the ICER remains below £11,000 per QALY gained.
FIGURE 26.
Sensitivity analysis for groups of model parameters for a comparison of the recommended strategy for multivascular disease patients (ASA → clopidogrel) vs ASA alone (branded price of clopidogrel/TA9024 guidance not applied). APT, antiplatelet therapy.
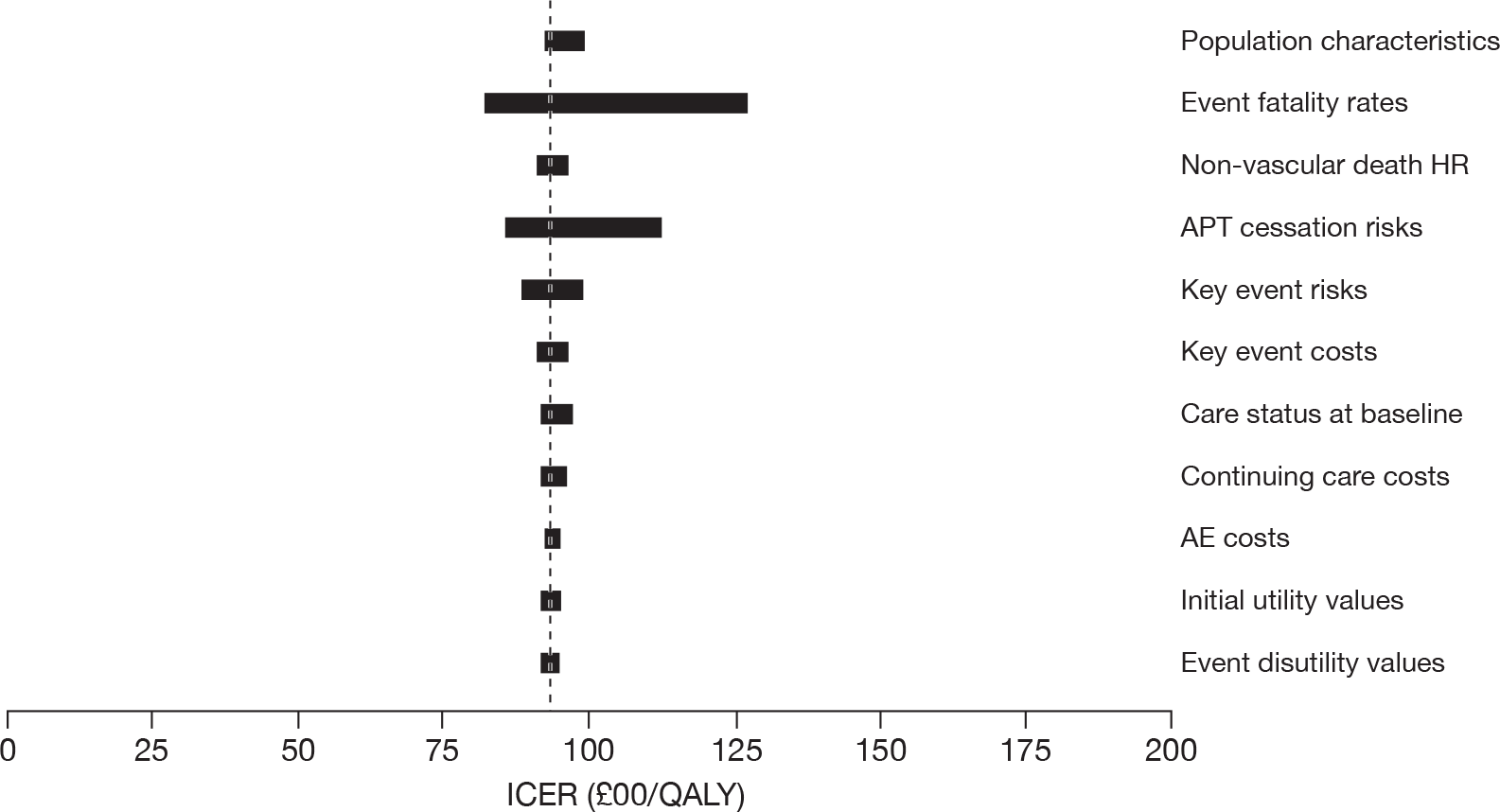
Summary of univariate results
These univariate sensitivity analyses allow the most likely sources of influential uncertainty to be identified. First, there is no indication that cost and utility parameters, population characteristics or non-vascular mortality give rise to significant uncertainty in economic results. Second, three types of parameter are implicated in at least one of the univariate sensitivity analyses as likely to be influential on model results – the risk of events occurring, the fatality of such events and the likelihood that patients will cease taking the prescribed preventive medications. Third, model results for the ‘peripheral arterial disease-only’ population appear to be particularly vulnerable to uncertainty in event risks, which is addressed probabilistically below.
Summary of cost-effective strategies from the Assessment Group’s economic model
The economic results described above are summarised in terms of preferred long-term preventive treatment strategies in Table 71. In only one circumstance (MRD intolerance in the ‘ischaemic stroke-only’ patient) is the pricing of clopidogrel a determining factor in the choice of strategy.
| CLOP price | TA9024 guidance | Patient population | |||
|---|---|---|---|---|---|
| IS only | MI only | PAD only | MVD | ||
| No intolerances | |||||
| Branded | Applied | MRD + ASA → ASA → CLOP | ASA → CLOP | CLOP → ASA | CLOP → ASA |
| Not applied | |||||
| Generic | Applied | CLOP → MRD + ASA → ASA | |||
| Not applied | |||||
| ASA intolerant | |||||
| Branded | Applied | CLOP → MRD | CLOP | CLOP | CLOP |
| Not applied | |||||
| Generic | Applied | ||||
| Not applied | |||||
| MRD intolerant | |||||
| Branded | N/A | ASA → CLOP | N/A | N/A | N/A |
| Generic | CLOP → ASA | ||||
| ASA and MRD intolerant | |||||
| N/A | CLOP | N/A | N/A | N/A | |
Probabilistic sensitivity analyses
Methods
The Assessment Group model was developed as a Microsoft 2007 Excel application, as this facilitated rapid model development and modification within a limited timescale following the availability of the detailed results of trial data (especially from the CAPRIE26 trial) produced by the manufacturer of clopidogrel. Although modest in its design (a maximum of 10 key events for each of 10,000 simulated patients), it became apparent that the limitations of Excel 2007 in the Windows Vista Business Service Pack 2 Build 6002 (Microsoft Corporation, Redmond, WA, USA) operating environment caused serious restrictions in two respects: the speed at which the model could be recalculated and the size of workbook that could be accommodated.
These problems became critical in the context of carrying out probabilistic sensitivity analyses on the Assessment Group’s model results, where it proved necessary to read random number sets from an external data file rather than from within Excel and to severely restrict the number of replications carried out. Commonly, probabilistic sensitivity analysis simulations involve many thousands of replications aimed at achieving stability of results. Instead we used a preprocessing technique previously developed in the context of an earlier MTA project, which uses a much smaller replication random number set designed to ensure full coverage of the distributional space for each parameter together with guaranteed means, variances and correlations with related parameters. By trial and error we determined that a standard set of 100 such replications produced stable and reliable probabilistic sensitivity analysis results, and limited processing times to manageable proportions (typically 45 minutes for each candidate strategy within each population).
In order to limit the amount of probabilistic sensitivity analysis processing required to support decision-making, the Assessment Group restricted attention within each population by not considering any strategy that was subject to dominance or extended dominance within the deterministic analysis, i.e. limiting attention to strategies on the cost-effectiveness frontier.
In all cases but one, consideration was given only to analyses using the full branded price of clopidogrel, on the grounds that previous results had indicated that (with the exception of one subpopulation of ‘ischaemic stroke-only’ patients), a reduced price of clopidogrel does not alter the choice of optimal strategy.
Probabilistic sensitivity analysis findings for optimal strategies
The results of probabilistic sensitivity analysis are compared with the earlier deterministic findings in Table 72. The two sets of ICERs governing the selection of the optimal strategy rather than the ‘next best’ option are very similar in all cases and show no evidence of consistent bias in either direction. Moreover, in all cases the estimated ICERs fall markedly below the ‘standard range’ of cost-effectiveness (£20,000–30,000 per QALY gained).
| Patient population | Treatment strategy | ICER (£/QALY) | WTP threshold | |||||
|---|---|---|---|---|---|---|---|---|
| £20,000/QALY | £30,000/QALY | |||||||
| Optimal | Next best | Deterministic (£) | PSA (£) | Probability of cost-effectiveness (%) | Incremental net benefit (£) | Probability of cost-effectiveness (%) | Incremental net benefit (£) | |
| MI only | ASA → CLOP | ASA | 8118 | 6250 | 100 | 1134 | 100 | 1958 |
| MI only (ASA intolerant) | CLOP | No APT | 12,112 | 12,037 | 98 | 1911 | 100 | 4311 |
| PAD only | CLOP → ASA | ASA → CLOP | 12,956 | 9975 | 98 | 3559 | 100 | 7110 |
| PAD only (ASA intolerant) | CLOP | No APT | 5443 | 4367 | 100 | 12,145 | 100 | 19,914 |
| MVD | CLOP → ASA | ASA → CLOP | 12,324 | 11,121 | 100 | 1790 | 100 | 3806 |
| MVD (ASA intolerant) | CLOP | No APT | 2902 | 2064 | 100 | 12,747 | 100 | 19,853 |
| IS only | MRD + ASA → ASA → CLOP | MRD + ASA → ASA | N/A | 16,833 | 79 | 46 | 89 | 190 |
| IS only (ASA intolerant) | CLOP → MRD | CLOP | 9021 | 6443 | 96 | 2576 | 96 | 4277 |
| IS only (MRD intolerant) | ASA → CLOP | ASA | 7336 | 6185 | 85 | 1347 | 65 | 2322 |
| IS only (MRD intolerant – generic CLOP) | CLOP → ASA | ASA → CLOP | 9328 | 4676 | 85 | 989 | 87 | 1635 |
For three of the four patient populations (MI only, peripheral arterial disease only and multivascular disease) the probability of optimal cost-effectiveness is close to 100% when the willingness to pay exceeds £20,000 per QALY. In the case of the ischaemic stroke-only population, probabilities are somewhat lower, but still well above 50%. It is noticeable that in those smaller patient groups where intolerance to either ASA or MRD leaves only a single antiplatelet treatment option, the incremental net benefit is much greater than when comparing between competing antiplatelet treatment strategies, confirming that using any of the available treatments is preferable to not treating at all.
The CEACs indicating the relative cost-effectiveness performance of each of the eligible treatment strategies for each patient population group across a range of WTP values are illustrated in Figures 27–36.
FIGURE 27.
The cost-effectiveness acceptability curve for the MI-only population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
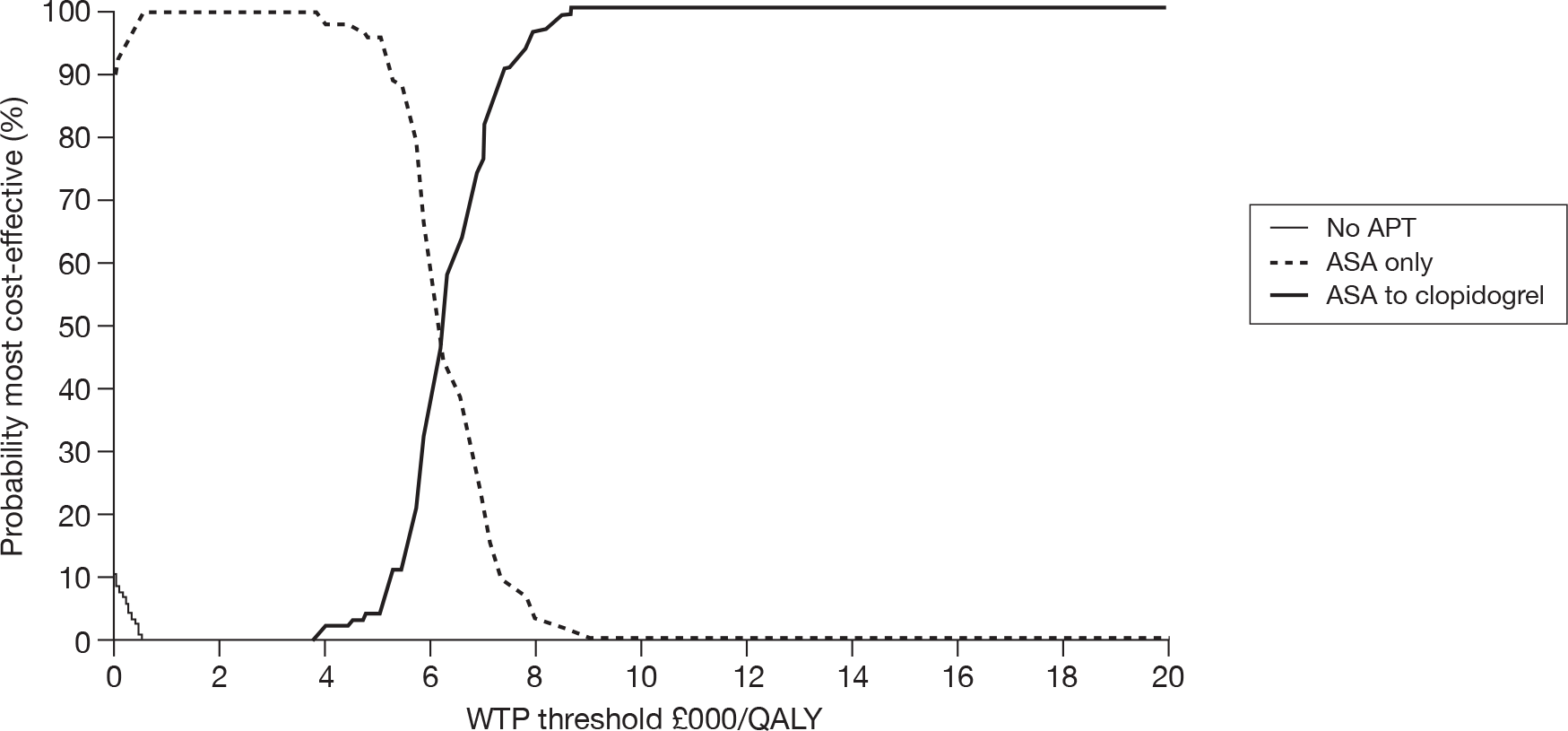
FIGURE 28.
The cost-effectiveness acceptability curve for the MI-only ASA-intolerant population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
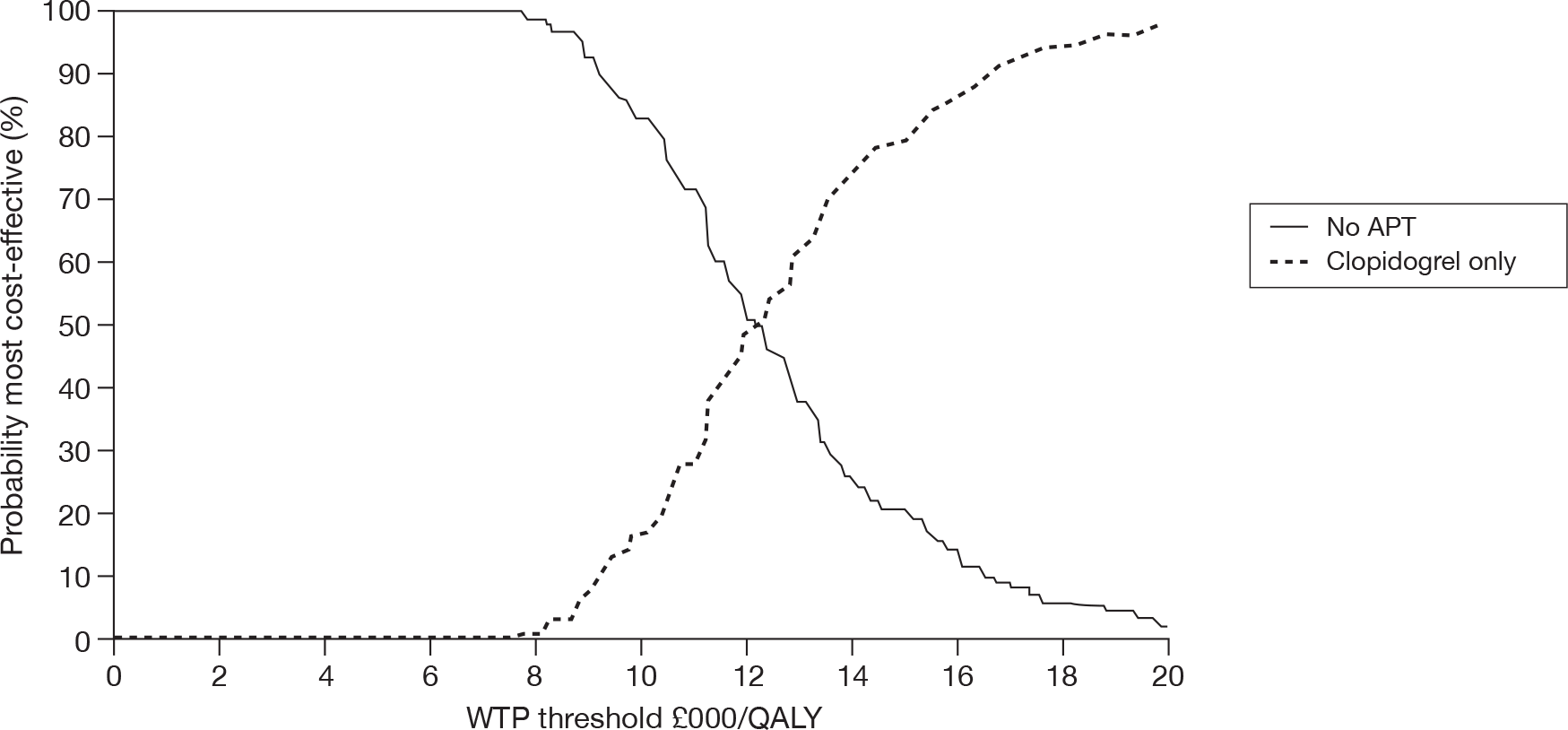
FIGURE 29.
The cost-effectiveness acceptability curve for the peripheral arterial disease-only population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
FIGURE 30.
The cost-effectiveness acceptability curve for the peripheral arterial disease-only ASA-intolerant population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
FIGURE 31.
The cost-effectiveness acceptability curve for the multivascular disease population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
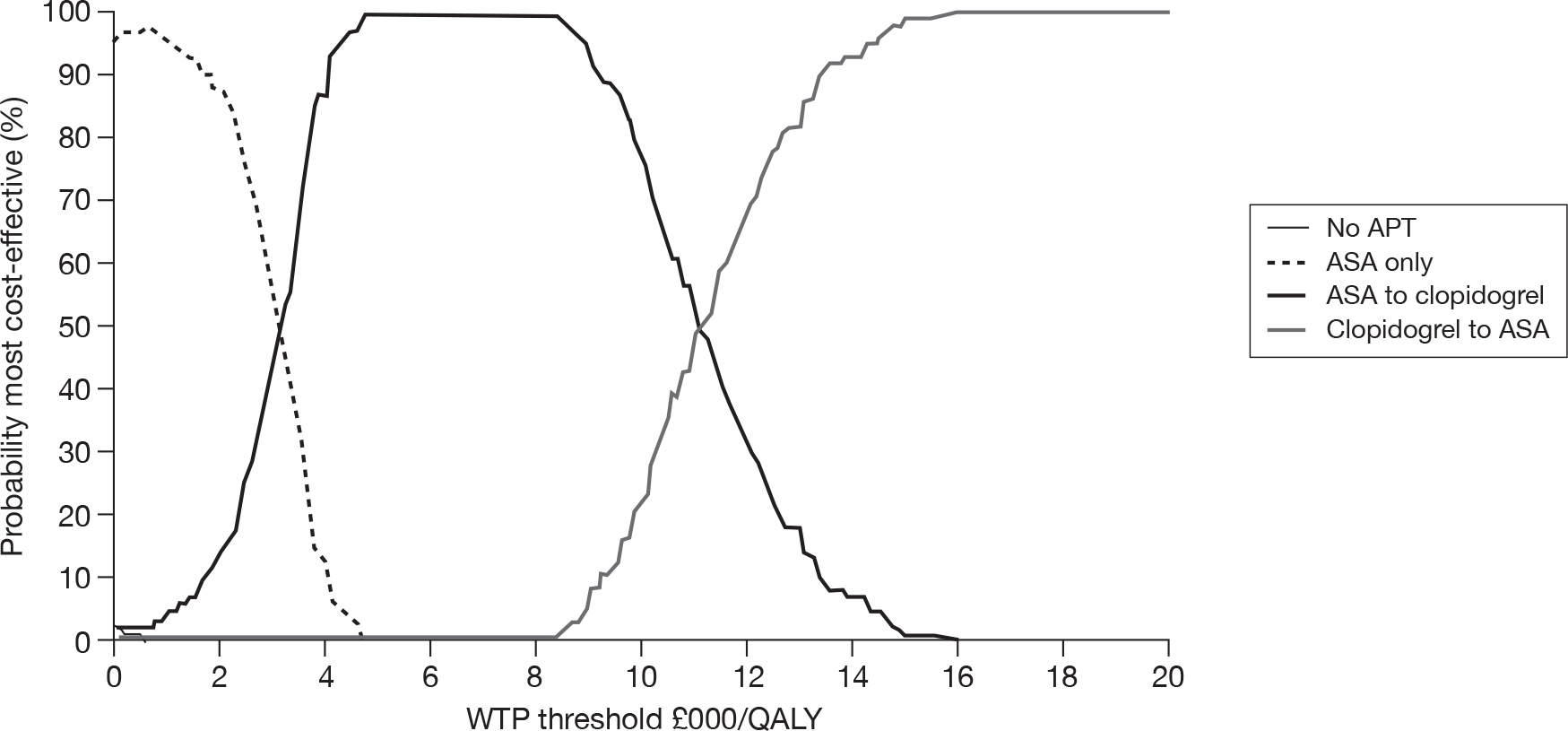
FIGURE 32.
The cost-effectiveness acceptability curve for the multivascular disease ASA-intolerant population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
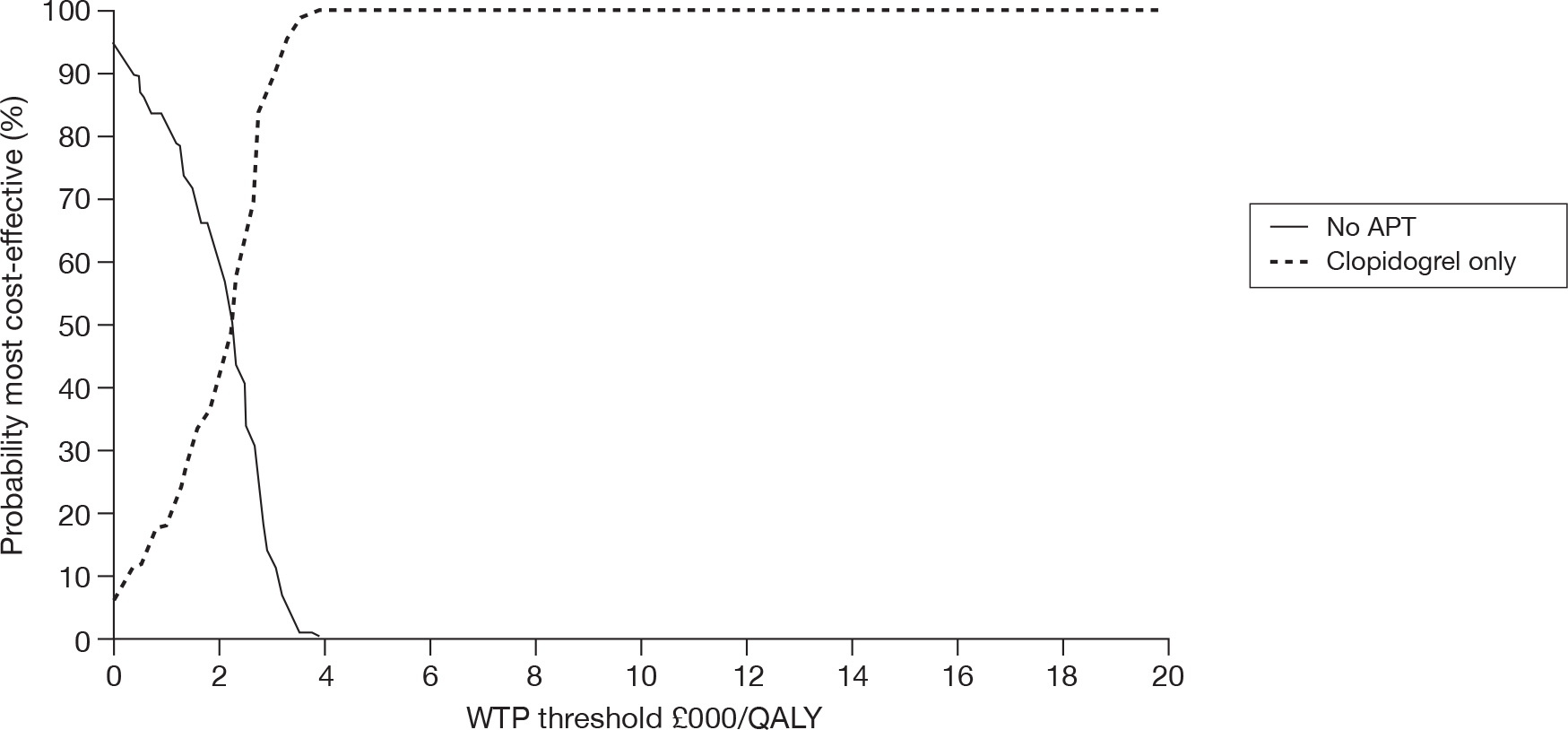
FIGURE 33.
The cost-effectiveness acceptability curve for the ischaemic stroke-only population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
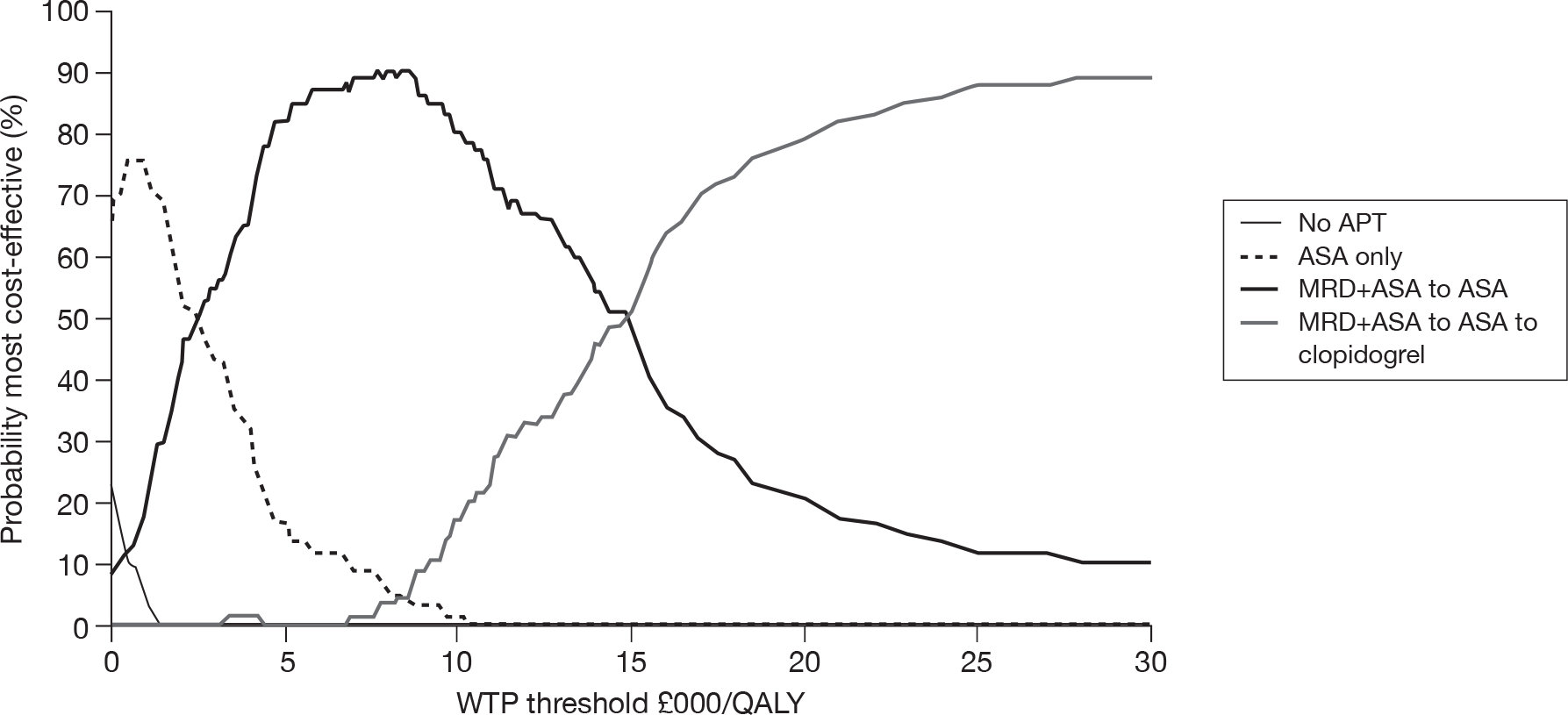
FIGURE 34.
The cost-effectiveness acceptability curve for the ischaemic stroke-only ASA-intolerant population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
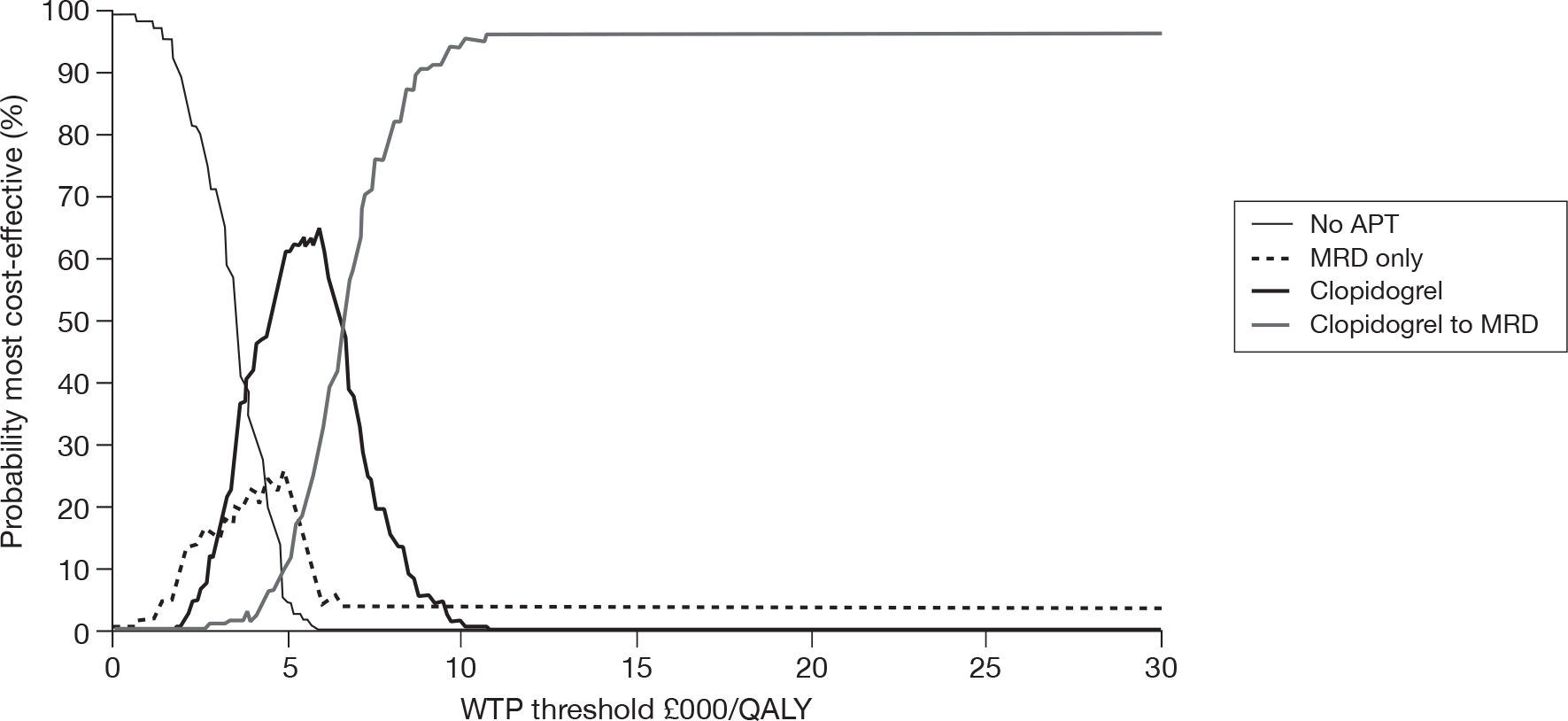
FIGURE 35.
The cost-effectiveness acceptability curve for the ischaemic stroke-only MRD-intolerant population (the branded clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
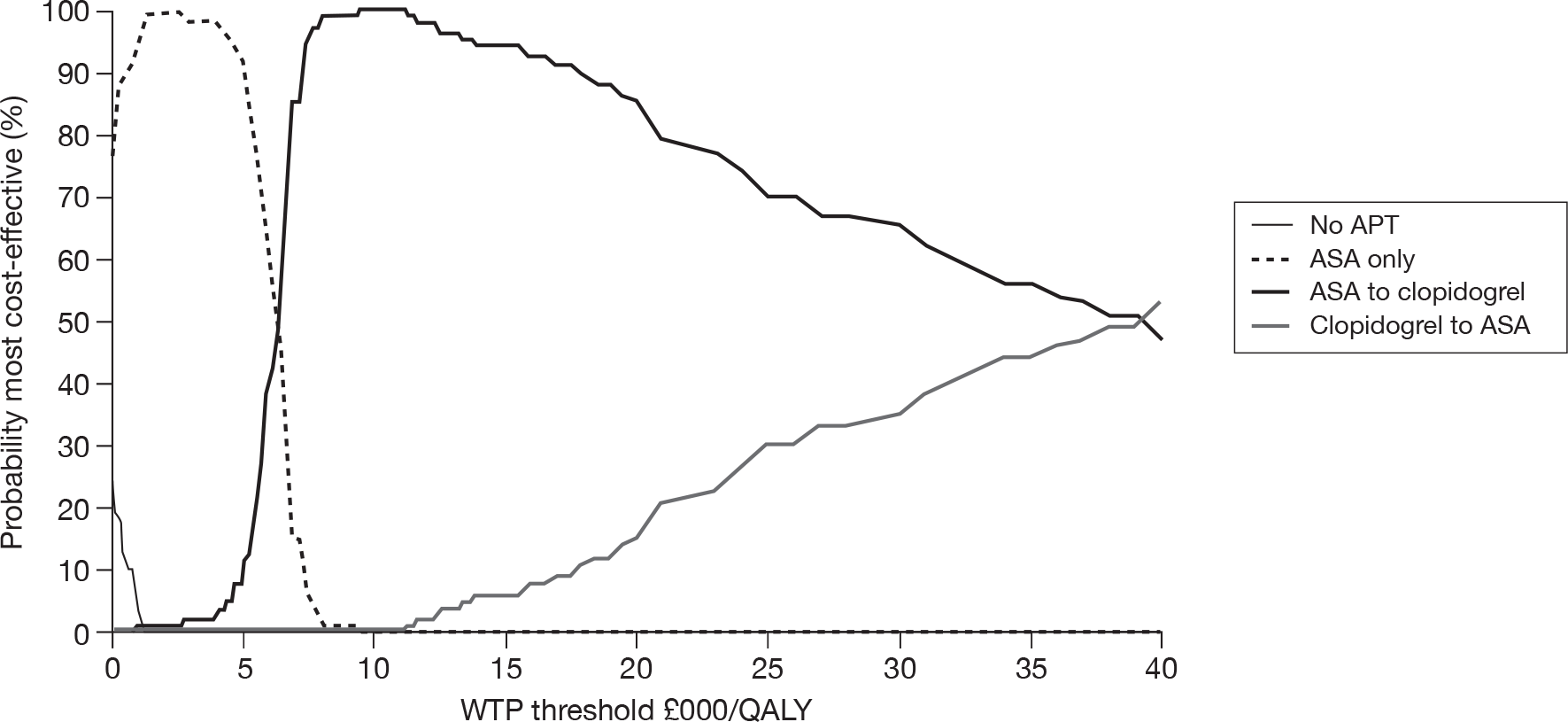
FIGURE 36.
The cost-effectiveness acceptability curve for the ischaemic stroke-only MRD-intolerant population (the generic clopidogrel price and the TA9024 guidance were not applied). APT, antiplatelet therapy.
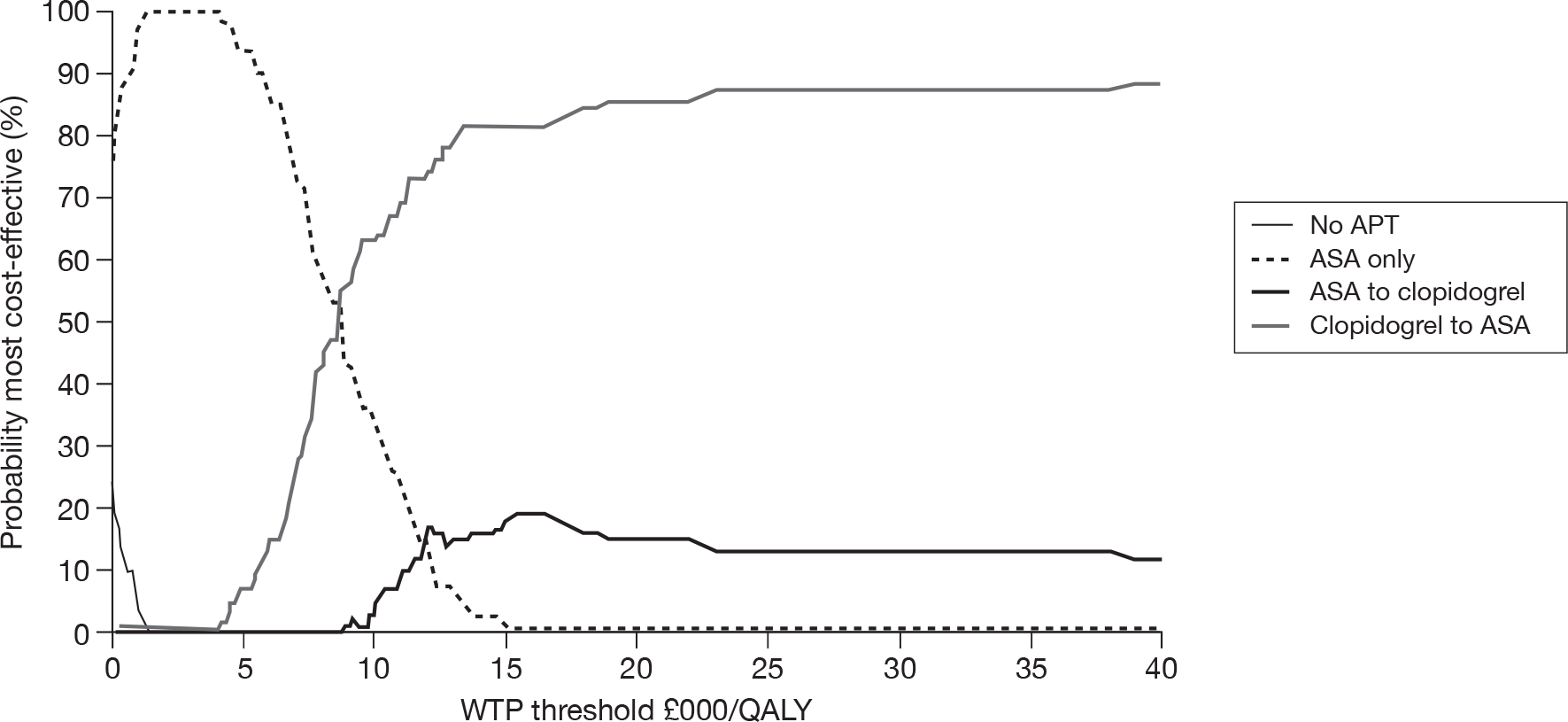
The probabilistic sensitivity analyses undertaken by the Assessment Group are consistent with the results obtained by deterministic use of the Assessment Group’s model. In addition, they have confirmed that the optimal strategies previously described may be considered robust with respect to known parameter uncertainty. In particular, the apparent sensitivity of results in the peripheral arterial disease-only population to uncertainty in event-risk variables is not reflected in greater decision uncertainty when considered in the context of all other model parameters.
Additional probabilistic sensitivity analysis results for the ‘ischaemic stroke-only’ population
Additional results describing the outcome of the probabilistic sensitivity analysis were carried out on the ‘ischaemic stroke-only’ population using the extended sample of 10,000 patients and generic clopidogrel pricing. Owing to the excessive computational time involved, these results relate only to the scenario in which TA90 guidance is not applied in the model.
Probabilistic results showing effect of parameter uncertainty
In Table 73 the calculations identifying scenarios lying on the cost-effectiveness frontier are shown, leading to the final preferred scenario in which clopidogrel is used as the first-line treatment, followed respectively by MRD + ASA and ASA, with a stepwise ICER of just over £10,000 per QALY gained.
| CLOP price | TA9024 status | Strategy | Total costs (£) | Utility QALYs | ICER (£) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | ||||
| Generic | Not used | None | None | None | 34,825 | 7.068 | |||||
| ASA | None | None | 34,289 | 7.588 | –1033 a | ||||||
| ASA | CLOP | MRD + ASA | 34,484 | 7.682 | –557 | 2077 a | |||||
| ASA | MRD + ASA | CLOP | 34,497 | 7.684 | –532 | 2162 | 5227 a | ||||
| CLOP | ASA | MRD + ASA | 35,187 | 7.773 | 513 | 4858 | 7729 | 7803 a | |||
| CLOP | MRD + ASA | ASA | 35,438 | 7.798 | 840 | 5481 | 8240 | 8309 | 10,107 | ||
| MRD + ASA | CLOP | ASA | 35,318 | 7.771 | 701 | 5610 | 9314 | 9436 | Dom | ||
| MRD + ASA | ASA | CLOP | 35,064 | 7.751 | 3500 | 4578 | 8402 | 8526 | Dom | ||
Figure 37 indicates that the MRD + ASA first-line scenarios lie very close to the frontier, but are consistently slightly less effective than the corresponding clopidogrel first-line scenarios.
FIGURE 37.
The cost-effectiveness plane for the ischaemic stroke-only population (the generic clopidogrel price and the TA9024 guidance were not applied).
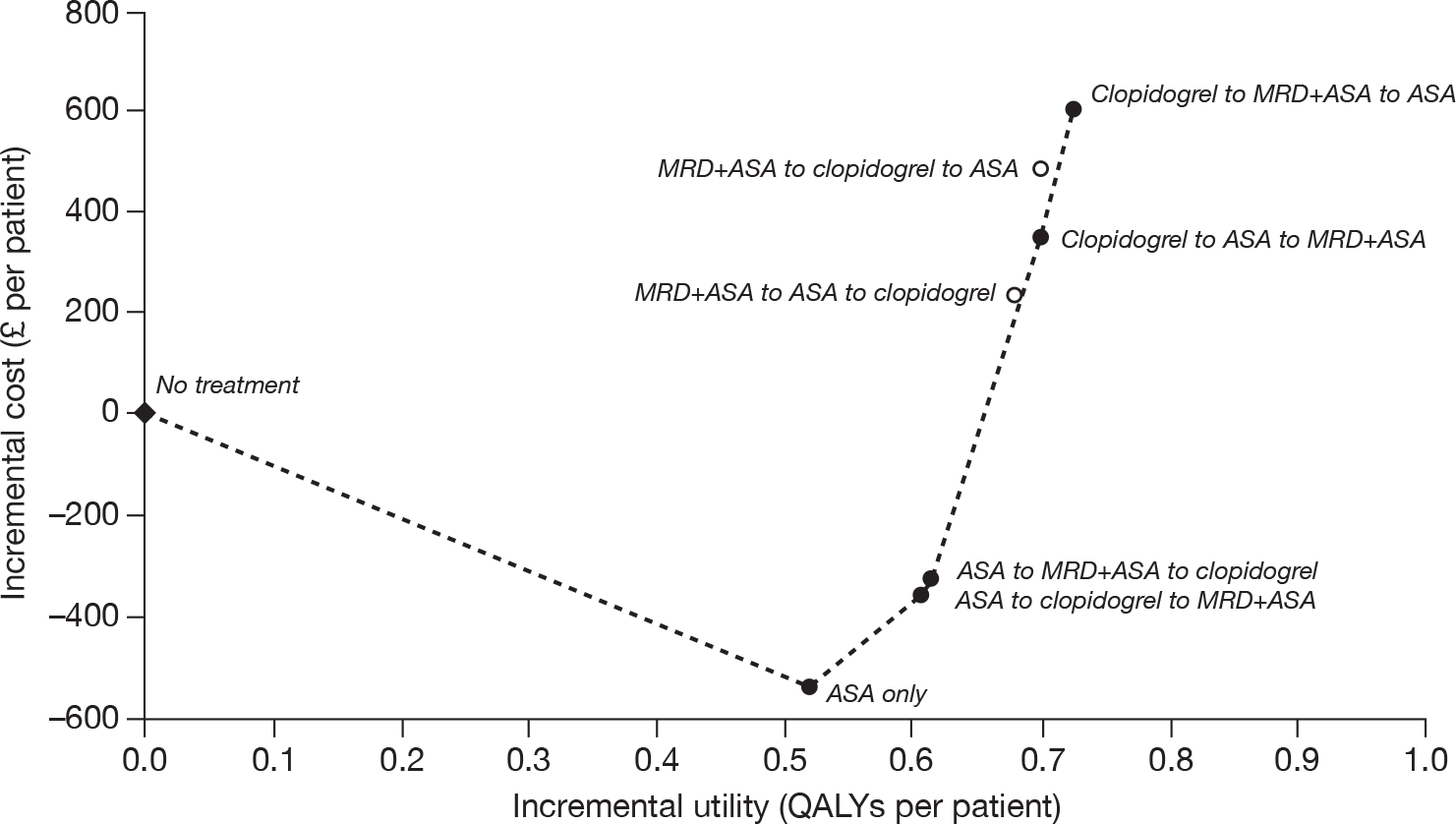
The CEACs (Figure 38) indicate that only three scenarios appear to warrant consideration as ‘most cost-effective’: ‘ASA only’ for WTP threshold of < £2300/QALY, ASA → clopidogrel → MRD + ASA if WTP is < £8300/QALY and clopidogrel → MRD + ASA → ASA for WTP > £8300/QALY. This last scenario demonstrates a probability of being most cost-effective of 68% (WTP = £20,000/QALY) or 73% (WTP = £30,000/QALY). The degree of difference between the probabilistic sensitivity analysis results for the clopidogrel and MRD + ASA scenarios can be gauged from the scatter plot shown in Figure 39. Although there is a consistent indication of greater effectiveness for use of generic clopidogrel as a first-line therapy, the differences in both costs and effectiveness are small.
FIGURE 38.
The cost-effectiveness acceptability curves for the ischaemic stroke-only population (the generic clopidogrel price and the TA9024 guidance were not applied).
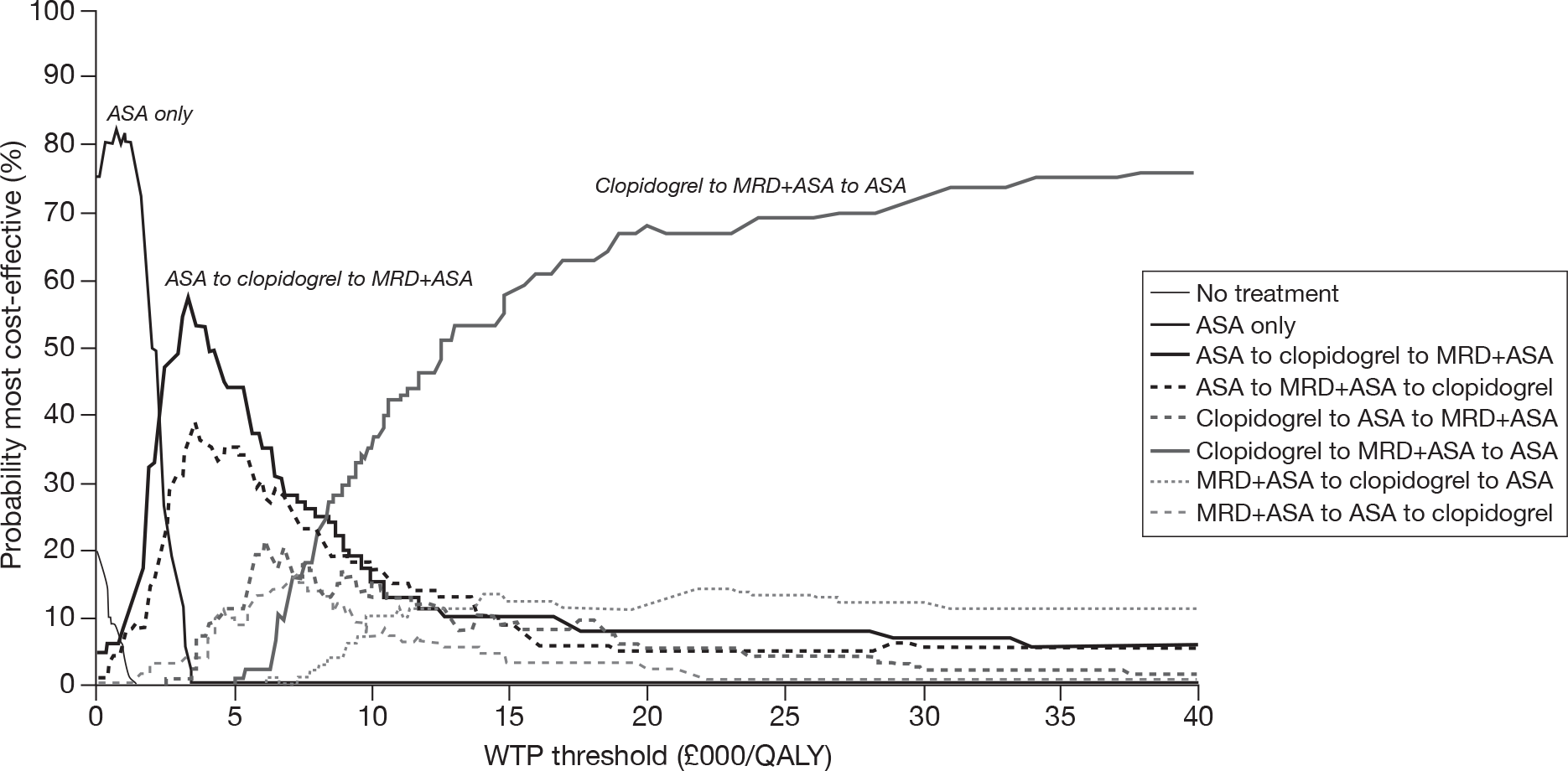
FIGURE 39.
Probabilistic sensitivity analysis scatter plot for the ischaemic stroke-only population (the generic clopidogrel price and the TA9024 guidance were not applied) – comparing two first-line therapies.
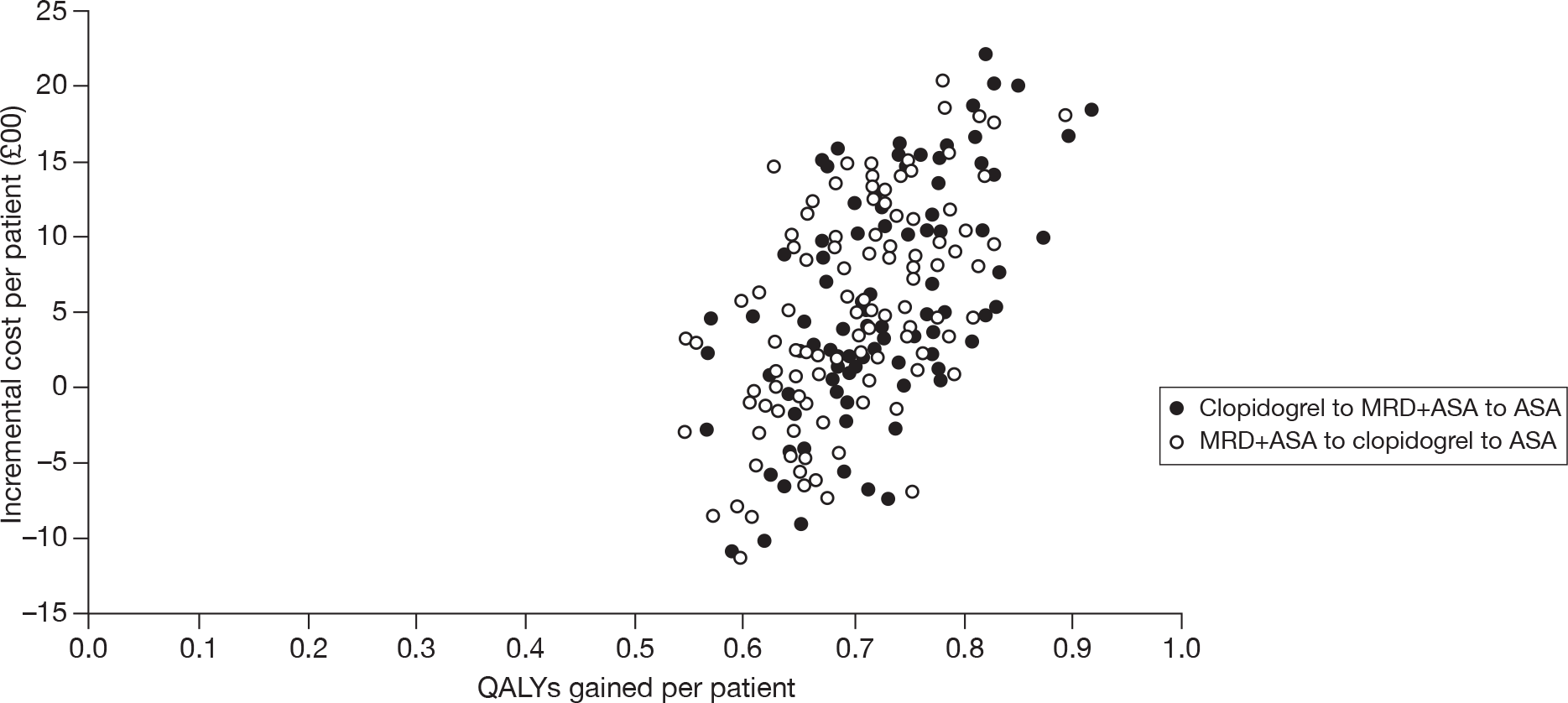
Summary of probabilistic analysis
The probabilistic sensitivity analysis findings confirm deterministic results reported indicating that the use of generic clopidogrel leads to first-line clopidogrel being more cost-effective than first-line MRD + ASA for the ‘ischaemic stroke-only’ population.
Chapter 5 Discussion
Statement of principal findings
The purpose of this report is to assess the clinical effectiveness and cost-effectiveness of (1) clopidogrel and (2) MRD alone or MRD + ASA compared with ASA and, where appropriate, with each other in the prevention of occlusive vascular events in patients with a history of MI or ischaemic stroke/TIA or established peripheral arterial disease. The final scope issued by NICE also called for consideration of the effectiveness of clopidogrel in patients with multivascular disease.
Current NICE guidance in TA9024 recommends that patients with a history of MI or peripheral arterial disease should be treated with ASA (clopidogrel if ASA intolerant); patients with a history of ischaemic stroke/TIA should be treated with MRD + ASA for 2 years (if MRD is not tolerated, standard care, including long-term treatment with low-dose ASA). Patients with multivascular disease are not considered in TA90. 24
Clinical effectiveness: direct evidence
Patients with myocardial infarction and established peripheral arterial disease
Only the CAPRIE26 trial offers evidence of the effectiveness of clopidogrel (vs ASA) in patients with a prior history of MI or established peripheral arterial disease. For the whole population (patients with a prior history of MI or ischaemic stroke or established peripheral arterial disease), the CAPRIE26 trial favoured clopidogrel; statistically significant outcomes were noted for the primary outcome (first occurrence of ischaemic stroke, MI or vascular death). However, the benefit appeared to be small and the boundaries of the CIs raise the possibility that clopidogrel is not more beneficial than ASA across the patient population as a whole. When the results for each of the subgroups were analysed, there was a statistically significant effect only in patients with peripheral arterial disease (favouring clopidogrel).
Patients with multivascular disease
The clinical effectiveness of clopidogrel in patients with multivascular disease is assessed using data from three distinct sources: the original CAPRIE26 publication, a post hoc analysis based on the CAPRIE26 population and the Assessment Group’s reclassification of the original patient groups using additional CAPRIE26 data provided by the manufacturer. The results of all subgroup analyses undertaken suggest that patients with multivascular disease are likely to experience elevated risks of future single and composite events and that treatment with clopidogrel is preferred over ASA.
Patients with ischaemic stroke/transient ischaemic attack
For the ischaemic stroke/TIA population, clinical data are available from four studies: CAPRIE,26 ESPS-2,30 ESPRIT56 and PRoFESS. 57 In the CAPRIE26 trial there were no statistically significant differences in the primary outcome between the treatment groups (MI, ischaemic stroke, peripheral arterial disease) in patients with prior history of ischaemic stroke. In ESPS-230 there was no difference in outcomes when MRD was compared with ASA; there was a statistically significant reduction in incidence of stroke in favour of MRD + ASA compared with ASA and MRD alone. No other primary outcome (all-cause death; stroke and/or all-cause death) showed statistically significant differences between any two treatment arms. In ESPRIT,56 for the primary outcome (first occurrence of death from all vascular causes, non-fatal stroke, non-fatal MI or major bleeding complication), the risk of event occurrence was statistically significantly lower in the MRD + ASA arm than in the ASA arm. In the PRoFESS trial,57 the rate of recurrent stroke of any type (primary outcome) was similar in the MRD + ASA and clopidogrel groups and the null hypothesis (that MRD + ASA is inferior to clopidogrel) could not be rejected. An increased risk of major haemorrhagic event and intracranial haemorrhage was reported for MRD-ASA compared with clopidogrel.
In summary, the clinical evidence appears to suggest that MRD + ASA is preferred to MRD alone and ASA in patients with a prior history of ischaemic stroke/TIA. There is not enough clinical evidence to make an informed decision regarding the use of MRD + ASA vs clopidogrel in patients with a prior history of ischaemic stroke/TIA.
Adverse events
It is difficult to summarise the findings related to adverse events, as the classification of these outcomes differed greatly across the trials; this was especially apparent for ‘bleeding’ events. However, upon investigation, the Assessment Group did not identify any unexpected adverse events associated with any of the drugs, bleeding was associated with ASA and headache was associated with MRD.
Clinical effectiveness: indirect evidence
Ischaemic stroke/transient ischaemic attack-only populations
There were no major differences in the results of the mixed-treatment comparison and the direct estimates from head-to-head trials. However, two of the five newly generated comparisons did yield statistically significant results: MRD alone had an increased risk of recurrent stroke when compared with clopidogrel; clopidogrel had fewer major bleeding events compared with ASA. Owing to the small numbers of trials involved in the mixed-treatment comparison and the forced selection of limited outcomes, caveats apply to the results. Findings were also based on patient populations in which there is no differentiation between patients with vascular disease in a single bed and those with multivascular disease. The results of the indirect analyses, although confirmatory of the direct results, must therefore be interpreted with caution.
Cost-effectiveness evidence
Summary of previously published cost-effectiveness analyses
All of the economic evaluations, except three,71,72,78 were published prior to 2006; this means more recent trials and clinical papers have not been used to inform the economic evaluations. The relevance of this review to decision-making is therefore limited as the economic evaluations are not based on the most up-to-date clinical data. Nonetheless, the results of the literature review of cost-effectiveness evidence show that, from a health service perspective, the use of clopidogrel in patients with previous peripheral arterial disease, ischaemic stroke or MI is a cost-effective option compared with ASA in the secondary prevention of occlusive vascular events. However, it is noted that Schleinitz et al. 75 conclude that the evidence available to them at the time did not support increased efficacy of clopidogrel in the MI patient group; this is the only evaluation that includes subgroup analysis to estimate ICERs by patients’ previous event. The combination of MRD + ASA seems to be cost-effective compared with any other treatment in patients with previous ischaemic stroke/TIA in the secondary prevention of occlusive vascular events. There is only one evaluation that includes this combination (MRD + ASA) and therefore the evidence base is limited.
Summary of industry-submitted economic evaluations
Both manufacturers submitted de novo economic analyses that met the NICE reference case criteria.
Boehringer Ingelheim is the manufacturer of MRD + ASA and the manufacturer’s submission appears to demonstrate that:
-
MRD + ASA (first-line) and ASA (second-line) is cost-effective compared with ASA alone (£5377 per QALY gained) and to no treatment (£5910 per QALY gained) in patients with a history of ischaemic stroke/TIA.
-
MRD + ASA (first-line) and ASA (second-line) compared with clopidogrel yields an ICER of £114,628 per QALY gained (patients with a history of ischaemic stroke) and an ICER of £199,149 (patients with a history of TIA).
The main critique of the Boehringer Ingelheim manufacturer’s submission is focused on the fact that the transition probabilities during the first 4 years for the MRD + ASA and clopidogrel arms are derived from PRoFESS,57 ESPS-230 and ESPRIT56 trials, beyond this point the manufacturers have used the same transition probability as used for the last 6-monthly cycle. This is an unreliable basis for long-term projection, as close to the end of the trial patient numbers and the number of events are much reduced. As a consequence, estimated incidence rates are very volatile and should not be relied on to drive the major part of the model calculations. It is important to note that the manufacturers used Plavix (branded clopidogrel) at a price of £36.35 for 30 tablets (75 mg) in the manufacturer’s submission; the price of clopidogrel is now set at £10.90 for 30 tablets (75 mg). This means that for the ischaemic stroke/TIA populations, clopidogrel is now cheaper and more effective than MRD + ASA.
Sanofi–aventis/Bristol–Myers Squibb are the manufacturers of clopidogrel and the manufacturer’s submission appears to demonstrate that:
-
For patients with a prior history of ischaemic stroke, clopidogrel is dominated by MRD + ASA and that clopidogrel versus MRD yields an ICER of £5850 per QALY gained.
-
For patients with a prior history of MI, clopidogrel versus ASA yields an ICER of £20,662 per QALY gained.
-
For patients with established peripheral arterial disease, clopidogrel versus ASA yields an ICER of £18,845 per QALY gained.
-
For patients with multivascular disease, clopidogrel versus ASA yields an ICER of £15,524 per QALY gained.
The main critique of the Sanofi–aventis/Bristol–Myers Squibb economic model is focused on the approach used to project health outcomes. The model assumes different transition probabilities every year until year 3. Beyond this point, the last cycle transition probabilities are used for the remainder of the time horizon from years 3 to 35. This is an unreliable basis for long-term projection, as patient numbers and the number of events are much reduced close to the end of the trial. As a consequence, estimated incidence rates are very volatile and should not be relied on to drive the major part of the model calculations. It is important to note that using the new generic price of clopidogrel in the economic model improves the cost-effectiveness of clopidogrel.
Summary of the Assessment Group’s cost-effectiveness analysis
Cost-effectiveness results have been generated from the Assessment Group’s economic model to address two related questions:
-
Which treatment strategy is most cost-effective in avoiding future occlusive vascular events in each of the four specified populations?
-
How does the availability of generic clopidogrel at a lower price than the branded product affect the assessment of cost-effectiveness of clopidogrel containing treatment strategies?
-
Patients with ischaemic stroke/TIA:
-
– In all scenarios, the most cost-effective strategy begins with generic clopidogrel followed by MRD + ASA, followed by ASA.
-
– In patients who are intolerant of ASA, compared with no treatment, clopidogrel followed by MRD is the most cost-effective approach, independent of both the TA90 guidance24 and the price of clopidogrel.
-
– In patients who are intolerant of MRD, at the branded price, the preferred strategy is ASA followed by clopidogrel, but for the generic price clopidogrel followed by ASA is more cost-effective.
-
– For patients intolerant to both ASA and MRD, only clopidogrel is available for long-term prevention and it is seen to be more cost-effective than no preventive therapy.
-
-
Patients with MI:
-
– In all scenarios, the incremental cost-effectiveness of allowing clopidogrel as a subsequent therapy after failure of ASA therapy compared with ASA treatment alone is < £9000 per QALY gained suggesting that ASA followed by clopidogrel may be the optimal strategy for this patient group.
-
– In patients who are intolerant of ASA, clopidogrel is a cost-effective approach independent of both the TA90 guidance24 and the price of clopidogrel (ICERs ranging between £1961 and £12,391 per QALY gained).
-
-
Patients with established peripheral arterial disease:
-
– In all scenarios, the ICER for a strategy of clopidogrel followed by ASA when compared with ASA followed by clopidogrel appears to be well within the range considered cost-effective (under £13,000 per QALY gained for branded clopidogrel and under £5000 per QALY for generic clopidogrel), suggesting this as the optimal strategy for this patient group.
-
– In patients who are intolerant to ASA, clopidogrel is a cost-effective approach independent of both the TA90 guidance24 and the price of clopidogrel.
-
-
– Patients with multivascular disease:
-
– In all scenarios, the incremental cost-effectiveness of clopidogrel followed by ASA is the most cost-effective approach, independent of both the TA90 guidance24 and the price of clopidogrel.
-
– In patients who are intolerant to ASA, clopidogrel is a cost-effective approach to occlusive vascular event prevention independent of both the TA90 guidance24 and the price of clopidogrel.
-
Sensitivity analysis
The sensitivity analyses undertaken using the Assessment Group’s de novo model allowed the most likely sources of influential uncertainty to be identified. First, there is no indication that cost and utility parameters, population characteristics or non-vascular mortality give rise to significant uncertainty in economic results. Second, three types of parameter are implicated in at least one of the sensitivity analyses as likely to be influential on model results – the risk of events occurring, the fatality of such events and the likelihood that patients will cease taking the prescribed preventive medications. Third, model results for the ‘peripheral arterial disease-only’ population appear to be particularly vulnerable to uncertainty in event risks, which is addressed probabilistically.
Probabilistic sensitivity analysis
The probabilistic sensitivity analyses undertaken support the findings of the deterministic analyses. In addition, they have confirmed that the optimal strategies previously described may be considered robust with respect to known parameter uncertainty. In particular, the apparent sensitivity of the results in the peripheral arterial disease-only population to uncertainty in event-risk variables is not reflected in greater decision uncertainty when considered in the context of all other model parameters.
Strengths and limitations
The key strengths of the report are threefold. First, the Assessment Group was able to consider the clinical effectiveness and cost-effectiveness of clopidogrel in people with multivascular disease as specified in the final scope issued by NICE. Using information provided by the manufacturer, the Assessment Group reanalysed previously published data from the CAPRIE26 trial and estimated the clinical effectiveness and cost-effectiveness of clopidogrel in this clinically important subgroup of patients. The Assessment Group confirmed the findings of other published clinical papers that patients with multivascular disease are often at high risk of single and composite future clinical events.
Second, the Assessment Group did not simply address the short-term costs and benefits associated with clopidogrel and MRD; the clinical effectiveness and cost-effectiveness of clopidogrel and MRD is considered over time using treatment scenarios. The strength of this approach is that it reflects the real world in which many patients will need to switch between different treatments during their lifetime. Restricting the analysis of costs and benefits of long-term prophylaxis to a few years frequently results in erroneous conclusions.
Finally, the structure of the economic model required to address the questions posed in the final scope issued by NICE necessitated careful planning and execution by the Assessment Group as well as access to further analyses of clinical data from the manufacturers. Working collaboratively, the Assessment Group was able to make the best use of limited evidence and estimate relevant ICERs for individual patient populations using an economic model designed to minimise the scope for multiple cumulative bias inherent in long-term projection of multiple competing risks.
The clinical effectiveness and cost-effectiveness findings of the report are limited by the nature of the clinical evidence available. For the MI, peripheral arterial disease and multivascular disease patient populations, data were available only from the CAPRIE26 trial (clopidogrel vs ASA) and the clinical results favoured clopidogrel. However, use of a single trial to generate clinical evidence for three individual patient populations inevitably attracts criticism. It is also important to note that the CAPRIE26 trial did not distinguish between patients with NSTEMI and STEMI, and this clearly inhibits the interpretation of the trial results for these clinically important subgroups of patients. For the ischaemic stroke/TIA population, relevant evidence was available from four published RCTs to inform the Assessment Group’s assessment of clopidogrel and MRD. However, the studies were all very different in terms of design, patient populations and clinical outcomes, so that even indirect comparisons proved to be fraught with difficulty. The key comparison of interest for patients with ischaemic stroke/TIA was clopidogrel vs MRD + ASA and the results of this trial were inconclusive. This is unfortunate as it is unlikely that a trial of this design will ever be repeated. In summary, the clinical evidence available, particularly for MI, peripheral arterial disease and multivascular disease populations, to answer the key questions set out in the final scope is limited.
Uncertainties
The findings of this report for the MI, peripheral arterial disease and multivascular disease patient populations are reliant on several post hoc subgroup analyses from a single trial; this means that there is inevitable uncertainty associated with the findings of this report. During the Appraisal Committee meeting which led to the publication of TA90,24 the Appraisal Committee ‘… was persuaded that undue reliance on subgroup analysis was inadvisable principally because of insufficient study power. Consequently, it was considered inappropriate to rely on post hoc analyses …’. However, the Assessment Group is of the opinion that reliance on the results of post hoc subgroup analyses from a single trial was unavoidable if the questions set out in the final scope issued by NICE were to be adequately addressed in this report. To illustrate, there are clinical data available from PRoFESS,57 CAPRIE,26 ESPS-230 and ESPRIT56 for the ischaemic stroke/TIA population, but the only clinical data available for patients with prior MI, peripheral arterial disease and multivascular disease are from the CAPRIE26 trial. Patients with MI, peripheral arterial disease and multivascular disease are not considered to constitute a single homogeneous clinical population; this means that use of subgroup analysis to estimate the clinical effectiveness and cost-effectiveness of clopidogrel for these individual subpopulations, although not ideal, is necessary. It is important to note that the size of each of the subgroup populations is considerable (ischaemic stroke 4740, MI 5741, peripheral arterial disease 3713 and multivascular disease 4991) and proved sufficient to demonstrate important differences in risk profiles between these groups.
In the absence of any universally agreed definition, the multivascular disease subgroup analyses were based on a population defined by the Assessment Group. The Assessment Group’s definition appears to be consistent with the simplest and broadest definition described in the published literature; however, it is likely that any differences in definitions of multivascular disease subgroups will lead to differences in patient numbers and RRs.
Additionally, the head-to-head trials and the mixed-treatment comparison results have included subgroups of patients who had disease in more than one vascular bed, as none of the trials distinguished between patients with single and multivascular disease.
Chapter 6 Conclusions
For patients with ischaemic stroke/TIA, MRD + ASA followed by ASA, followed by clopidogrel, appears to be a cost-effective approach to the prevention of future occlusive vascular events.
For patients with MI, ASA followed by clopidogrel appears to be a cost-effective approach to the prevention of future occlusive vascular events.
For patients with established peripheral arterial disease or multivascular disease, clopidogrel followed by ASA appears to be a cost-effective approach to the prevention of future occlusive vascular events.
Suggested research
It is suggested that any future trials in this area should distinguish between patients with single vascular bed and multivascular disease, that definitions of multivascular disease should be prespecified (ideally using a common standard), and that triallists should ensure that trials are sufficiently powered over an extended follow-up period to allow detection of treatment differences between subgroups of patients. To facilitate comparison of primary and secondary outcomes across relevant trials, all outcomes need to be reported consistently and at key time points.
It would be most valuable to have well-audited data on a defined patient group from a long-term clinical registry of all UK patients treated with antiplatelet agents. Such a data source could provide a basis for research and audit to inform future assessments of antiplatelet agents in patients with single vascular bed and multivascular disease over the long term.
Acknowledgements
The authors are pleased to acknowledge Lisa Jones (Centre for Public Health, Liverpool JMU) who provided methodological feedback on the Assessment Group report.
Contributions of authors
Janette Greenhalgh Project lead, review of clinical evidence.
Adrian Bagust Critical appraisal of manufacturers’ economic models, development of de novo model.
Angela Boland Support of review process (clinical and economics).
Carlos Martin Saborido Review of economic literature, description and critique of submitted economic models, and assisted in development of de novo model.
James Oyee Clinical quality assessment, data extraction and statistical advisor.
Michaela Blundell Clinical quality assessment, data extraction and statistical support.
Yenal Dundar Literature searching, quality assessment and data checking.
Rumona Dickson Support of review process.
Chris Proudlove Pharmacological advisor.
Michael Fisher Clinical advisor.
All authors read and commented on draft versions of the Assessment Group report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Allender S, Peto V, Scarborough P, Kaur A, Rayner M. Coronary Heart Disease Statistics 2008. www.heartstats.org/homepage.asp.
- Steg PG, Bhatt DL, Wilson PW, D’Agostino R, Ohman EM, Rother J, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007;297:1197-206.
- Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al. Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation. Health Technol Assess 2004;8.
- National Institute for Health and Clinical Excellence (NICE) . Myocardial Infarction Thrombolysis: Guidance (TA52) 2002. http://guidance.nice.org.uk/TA52/Guidance/pdf/English (accessed 26 January 2010).
- Leonardi-Bee J, Bath PMW, Bousser MG, Davalos A, Diener HC, Guiraud-Chaumeil B, et al. Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke 2005;36:162-8.
- Patient UK. Stroke 2009. www.patient.co.uk/health/Stroke.htm (accessed December 2009).
- National Collaborating Centre for Chronic Conditions . Stroke: National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA) 2008. www.nice.org.uk/nicemedia/pdf/CG68FullGuideline.pdf.
- Scarborough P, Peto V, Bhatnagar P, Kaur A, Leal J, Luengo-Fernandez R, et al. Stroke statistics. Oxford: British Heart Foundation Health Promotion Research Group and Health Economics Research Centre; 2009.
- Verro P, Gorelick PB, Nguyen D. Aspirin plus dipyridamole versus aspirin for prevention of vascular events after stroke or TIA: a meta-analysis. Stroke 2008;39:1358-63.
- National Prescribing Centre . Antiplatelet agents for stroke patients. MeReC Bull 2003;14:5-8.
- Kirshner HS. Therapeutic interventions for prevention of recurrent ischemic stroke. Am J Manag Care 2008;14:212-26.
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006;113:e409-49.
- De Schryver E, Algra A, Van Gijn J. Dipyridamole for preventing stroke and other vascular events in patients with vascular disease. Cochrane Database Syst Rev 2007;4.
- Selvin E, Erlinger T. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004;110:738-43.
- Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag 2007;3:229-34.
- National Institute for Health and Clinical Excellence (NICE) . Final Scope for the Appraisal of Clopidogrel and Modified-Release Dipyridamole for the Prevention of Occlusive Vascular Events (review of TA 90) 2009. http://guidance.nice.org.uk/TA/WaveR/19/FinalScope (accessed July 2009).
- Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Liau C-S, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am Heart J 2006;151:786-10.
- Target PAD. Reducing the risks from leg arterial disease. 2009.
- Scottish Intercollegiate Guidelines Network (SIGN) . Diagnosis and Management of Peripheral Artery Disease: A National Clinical Guideline 2006. www.sign.ac.uk/pdf/sign89.pdf.
- Aronow H, Hiatt WR. The burden of peripheral artery disease and the role of antiplatelet therapy. Postgrad Med J 2009;121:123-35.
- Disabled World . Peripheral Artery Disease (PAD): Facts, Diagnosis and Treatment 2009. www.disabled-world.com/health/cardiovascular/peripheral-artery-disease.php (accessed December 2009).
- Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas J-L, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006;295:180-9.
- Adamson J, Beswick A, Ebrahim S. Stroke and disability. J Stroke Cerebrovasc Dis 2004;13:171-7.
- National Institute for Health and Clinical Excellence (NICE) . Clopidogrel and Modified-Release Dipyridamole for the Prevention of Occlusive Vascular Events (TA90) 2005. http://guidance.nice.org.uk/TA90/Guidance/pdf/English (accessed July 2009).
- National Institute for Health and Clinical Excellence (NICE) . Unstable Angina and NSTEMI: The Early Management of Unstable Angina and Non-ST-Segment-Elevation Myocardial Infarction 2010. http://guidance.nice.org.uk/CG94/Guidance/pdf/English (accessed 25 March 2010).
- The CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;348:1329-39.
- The CURE trial investigators . Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-elevation. N Engl J Med 2001;345:494-502.
- National Institute for Health and Clinical Excellence (NICE) . Secondary Prevention in Primary and Secondary Care for Patients Following a Myocardial Infarction 2007. http://guidance.nice.org.uk/CG48/Guidance/pdf/English.
- Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622-32.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996;143:1-13.
- NHS Business Services Authority . Prescription Services 2009. www.nhsbsa.nhs.uk/PrescriptionServices/Documents/PPDPrescribingAnalysisCharts/NPC_Cardio_Jul_2009.pdf (accessed 2 February 2010).
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary. 2009. http://bnf.org/bnf/index.htm.
- NHS Business Services Authority . Electronic Drug Tariff 2010. www.ppa.org.uk/edt/February_2010/mindex.htm (accessed 3 February 2010).
- NHS Business Services Authority . Category M Prices 2010. www.nhsbsa.nhs.uk/PrescriptionServices/1821.aspx (accessed 11 April 2010).
- European Medicines Agency . Generic Clopidogrel 2010;URL. www.ema.europa.eu/search.php?q=generic+clopidogrel%26spell=1%26site=ema_collection%26client=ema_frontend%26access=p%26ie=UTF-8%26proxystylesheet=ema_frontend%26output=xml_no_dtd.
- Medicines and Healthcare products Regulatory Agency (MHRA) 2009. www.mhra.gov.uk/index.htm.
- Department of Health . Saving Lives: Our Healthier Nation 1999. www.archive.official-documents.co.uk/document/cm43/4386/4386.htm.
- Department of Health (DoH) . National Standards, Local Action: Health and Social Care Standards and Planning Framework 2005 6–2007 8 2004.
- Welsh Assembly Government . Health Status Wales 2004 5: Chief Medical officer’s Report Series 2005. www.p2.wales.nhs.uk/news/3002 (accessed December 2009).
- Welsh Assembly Government . Health Gain Targets: National High-Level Targets and Indicators for Wales 2008. http://wales.gov.uk/topics/health/research/research/gain/;jsessionid=n5WNLBjQd1202WxYDn7H2h2YgY15W0kTWP6mMy6jsMHRWHyJdLTv!–689210129?lang=en (accessed December 2009).
- Quality and Outcomes Framework (QOF) . QOF Database 2009. www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4078659.pdf (accessed 2009).
- Belch J. Peripheral arterial disease: still on the periphery?. Br Med J 2006;332.
- Department of Health (DoH) . National Service Framework for Coronary Heart Disease 2000. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4094275 (accessed December 2009).
- Department of Health (DoH) . National Service Framework for Coronary Heart Disease 2005. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4105281 (accessed December 2009).
- National Institute for Health and Clinical Excellence (NICE) . Clopidogrel in the Treatment of Non-ST-Segment Elevation Acute Coronary Syndrome (TA80) 2004. http://guidance.nice.org.uk/TA80/Guidance/pdf/English (accessed July 2009).
- Department of Health (DoH) . National Stroke Strategy 2007. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081062.
- UK National Screening Centre . The Handbook for Vascular Risk Assessment, Risk Reduction and Risk Management 2008. www.screening.nhs.uk/vascular (accessed 2 February 2010).
- Sanofi-aventis and Bristol-Myers Squibb . Plavix: Summary of Product Characteristics 2009. www.sanofi-aventis.co.uk/products/Plavix_SPC.pdf.
- Electronic Medicines Compendium . Browse Medicines 2009. http://emc.medicines.org.uk/browsedocuments.aspx (accessed December 2009).
- Medicines and Healthcare products Regulatory Agency and Commission on Human Medicines . Drug Safety Update 2010. www.mhra.gov.uk/Publications/Safetyguidance/DrugSafetyUpdate/index.htm.
- Boehringer Ingelheim . Submission to NICE to Support the Review of Health Technology Appraisal 90: Clopidogrel and Modified-Release Dipyridamole and Aspirin for the Prevention of Occlusive Vascular Events 2009.
- Sanofi-aventis and Bristol-Myers Squibb . The Use of Clopidogrel Hydrogen Sulphate (Plavix) in Patients With Atherosclerotic Disease: Manufacturer Submission 2009.
- Centre for Reviews and Dissemination . Systematic Reviews: CRDs Guidance for Undertaking Reviews in Healthcare n.d. www.york.ac.uk/inst/crd/darefaq.htm (accessed December 2009).
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. Br Med J 1996;313:275-83.
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6. www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1000097.
- ESPRIT Study Group . Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006;367:1665-73.
- Sacco RL, Diener H, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008;359:1238-51.
- Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331-7.
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-17.
- Van Swieten J, Koudstaal P, Visser M, Schouten H, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7.
- Multi-parameter Evidence Synthesis Research Group (MPES) . Mixed Treatment Comparisons (MTC) 2009.
- Lunn D, Thomas A, Best N, Spiegelhalter D. Winbugs: a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comp Graph Stats 1998;7:434-55.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004;35:528-32.
- Rankin J. Cerebral vascular accidents in patients over the age of 60: II. Prognosis. Scot Med J 1957;2:200-15.
- Antithrombotic Trialists’ Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patients. BMJ 2002;324:71-86.
- Montori VM, Permanyer-Miralda G, Ferreira-Gonzalez I, Busse JW, Pacheco-Huergo V, Bryant D, et al. Validity of composite end points in clinical trials. BMJ 2005;330:594-6.
- Tomlinson G, Detsky A. Composite end points in randomized trials: there is no free lunch. JAMA 2010;303:267-8.
- Annemans L, Lamotte M, Levy E, Lenne X. Cost-effectiveness analysis of clopidogrel versus aspirin in patients with atherothrombosis based on the CAPRIE trial (Structured abstract). J Med Econ n.d. www.mrw.interscience.wiley.com/cochrane/cleed/articles/NHSEED-22003008271/frame.html.
- Beard SM, Gaffney L, Bamber L, De Platchett J. Economic modelling of antiplatelet therapy in the secondary prevention of stroke. J Med Econ 2004;7:117-34.
- Berger K, Hessel F, Kreuzer J, Smala A, Diener HC. Clopidogrel versus aspirin in patients with atherothrombosis: CAPRIE-based calculation of cost-effectiveness for Germany. Curr Med Res Opin 2008;24:267-74.
- Chen J, Bhatt DL, Dunn ES, Shi CX, Caro JJ, Mahoney EM, et al. Cost-effectiveness of clopidogrel plus aspirin versus aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA trial. Value Health 2009;12:872-9.
- Karnon J, Brennan A, Pandor A, Fowkes G, Lee A, Gray D, et al. Modelling the long term cost effectiveness of clopidogrel for the secondary prevention of occlusive vascular events in the UK. Curr Med Res Opin 2005;21:101-12.
- Matchar DB, Samsa GP, Liu S. Cost-effectiveness of antiplatelet agents in secondary stroke prevention: the limits of certainty. Value Health 2005;8:572-80.
- Schleinitz MD, Weiss JP, Owens DK. Clopidogrel versus aspirin for secondary prophylaxis of vascular events: a cost-effectiveness analysis. Am J Med 2004;116:797-806.
- Delea TE, Edelsberg JS, Richardson E, Singer DE, Oster G. Cost-effectiveness of clopidogrel in patients with ischemic stroke, myocardial infarction, or peripheral arterial disease. Value Health 2003;6:184-5.
- Palmer AJ, Roze S, Hankey G, Hakimi Z, Spiesser J, Carita P, et al. Cost-effectiveness of clopidogrel versus aspirin in the prevention of ischemic events in stroke and TIA patients: a four-European country analysis. Value Health 2005;8.
- Stevenson MD, Rawdin AC, Karnon JD, Brennan A. Clopidogrel is cost-effective compared with aspirin in United Kingdom patients with a myocardial infarction who subsequently sustain an ischaemic stroke or peripheral arterial disease event. Value Health 2008;11:A194-5.
- van Hout BA, Tangelder MJD, Bervoets P, Gabriel S. Cost-effectiveness analysis of antithrombotic treatment with clopidogrel in patients with myocardial infarction, stroke, and peripheral arterial disease in the Netherlands. Value Health 2003;6.
- Matchar DB, Samsa GP, Matthews JR, Ancukiewicz M, Parmigiani G, Hasselblad V, et al. The Stroke Prevention Policy Model: linking evidence and clinical decisions. Ann Intern Med 1997;127:704-11.
- Chambers M, Hutton J, Gladman J. Cost-effectiveness analysis of antiplatelet therapy in the prevention of recurrent stroke in the UK. Aspirin, dipyridamole and aspirin-dipyridamole. Pharmacoeconomics 1999;16:577-93.
- Diener HC, Rupprecht HJ, Willich SN. Cost of atherothrombotic diseases: myocardial infarction (MI), ischaemic stroke (IS) and peripheral arterial occlusive disease (PAD). J Public Health 2005;13:216-24.
- Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics 2003;21:191-200.
- Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al. A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists. Health Technol Assess 2002;6.
- Derdeyn CP, Powers WJ. Cost-effectiveness of screening for asymptomatic carotid atherosclerotic disease. Stroke 1996;27:1944-50.
- Zeckhauser R, Shepard D. Where now for saving lives?. Law Contemp Probl 1976;40:5-45.
- Haigh R, Castleden M, Woods K, Fletcher S, Bowns I, Gibson M, et al. Management of myocardial infarction in the elderly: admission and outcome on a coronary care unit. Health Trends 1991;23:154-7.
- Lee TT, Solomon NA, Heidenreich PA, Oehlert J, Garber AM. Cost-effectiveness of screening for carotid stenosis in asymptomatic persons. Ann Intern Med 1997;126:337-46.
- Danese MD, Powe NR, Sawin CT, Ladenson PW. Screening for mild thyroid failure at the periodic health examination: a decision and cost-effectiveness analysis. JAMA 1996;276:285-92.
- Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke 1994;25:333-7.
- Bruins Slot K. Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ 2008;336.
- Personal Social Services Research Unit (PSSRU) . Unit Costs of Health and Social Care 2007 2007. www.pssru.ac.uk/uc/uc2007contents.htm.
- Medicare Information Management System (MIMS) . The Prescribing Reference for General Practice 2009. www.mims.co.uk/ (accessed June 2009).
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2009. http://bnf.org/bnf/index.htm.
- Diener H-C, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurology 2008;7:875-84.
- Miller G, Randolph S, Forkner E, Smith B, Galbreath AD. Long-term cost-effectiveness of disease management in systolic heart failure. Med Decis Making 2009;29:325-33.
- Galbreath AD, Krasuski RA, Smith B, Stajduhar KC, Kwan MD, Ellis R, et al. Long-term healthcare and cost outcomes of disease management in a large, randomized, community-based population with heart failure. Circulation 2004;110:3518-26.
- Smith B, Forkner E, Zaslow B, Krasuski RA, Stajduhar K, Kwan M, et al. Disease management produces limited quality-of-life improvements in patients with congestive heart failure: evidence from a randomized trial in community-dwelling patients. Am J Manag Care 2005;11:701-13.
- Robinson A, Thomson R, Parkin D, Sudlow M, Eccles M. How patients with atrial fibrillation value different health outcomes: a standard gamble study. J Health Serv Res Policy 2001;6:92-8.
- Brown GC, Sharma S, Brown MM, Kistler J. Utility values and age-related macular degeneration. Arch Ophthalmol 2000;118:47-51.
- Alberts MJ, Bhatt DL, Mas JL, Ohman EM, Hirsch AT, Rother J, et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J 2009;30:2318-26.
- Youman P, Wilson K, Harraf F, Kalra L. The economic burden of stroke in the United Kingdom. Pharmacoeconomics 2003;21:43-50.
- Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med 2003;20:442-50.
- Heeg B, Damen J, Van Hout B. Oral antiplatelet therapy in secondary prevention of cardiovascular events: An assessment from the payer’s perspective. Pharmacoeconomics 2007;25:1063-82.
- National Institute for Health and Clinical Excellence (NICE) . Atrial Fibrillation: The Management of Atrial Fibrillation 2006. www.nice.org.uk/nicemedia/pdf/cg036fullguideline.pdf (accessed July 2009).
- Morant SV, McMahon AD, Cleland JG, Davey PG, MacDonald TM. Cardiovascular prophylaxis with aspirin: costs of supply and management of upper gastrointestinal and renal toxicity. Br J Clin Pharmacol 2004;57:188-98.
- Luengo-Fernandez R, Gray AM, Rothwell PM. Population-based study of determinants of initial secondary care costs of acute stroke in the United Kingdom. Stroke 2006;37:2579-87.
- Department of Health (DoH) . Health Survey for England 1996 2001.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary 2010. http://bnf.org/bnf/index.htm.
- Department of Health (DoH) . NHS Reference Costs 2008–2009 2010. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (accessed March 2010).
- Sullivan P, Arant T, Ellis S, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at risk of stroke in the US. Pharmacoeconomics 2006;24:1021-33.
- O’Brien C, Gage B. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA 2005;293:699-706.
- Jansen JP, Pellissier J, Choy EH, Ostor A, Nash JT, Bacon P, et al. Economic evaluation of etoricoxib versus non-selective NSAIDs in the treatment of ankylosing spondylitis in the UK. Curr Med Res Opin 2007;23:3069-78.
- Quinn R, Naimark D, Oliver M, Bayoumi A. Should hemodialysis patients with atrial fibrillation undergo systemic anticoagulation? A cost-utility analysis. Am J Kidney Dis 2007;50:421-32.
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9.
- Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire community stroke project. Stroke 1990;21:848-53.
- Hill M, Yiannakoulias N, Jeerakathil T, Tu J, Svenson L, Schlopflocher D. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology 2004;62:2015-20.
- Steg P, Goldberg R, Gore J, Fox K, Eagle K, Flather M, et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol 2002;90:358-63.
- Antithrombotic Trialists Collaboration . Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60.
- Berger JS, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA 2009;301:1909-19.
- Halkes PHA, Gray LJ, Bath PMW, Diener HC, Guiraud-Chaumeil B, Yatsu FM, et al. Dipyridamole plus aspirin versus aspirin alone in secondary prevention after TIA or stroke: a meta-analysis by risk. J Neurol Neurosurg Psychiatry 2008;79:1218-23.
- Sudlow C, Mason G, Maurice J, Wedderburn C, Hankey G. Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients. Cochrane Database Syst Rev 2009;4.
Appendix 1 Literature search strategies
EMBASE 2003–9 week 36
-
Clinical trial/
-
Randomized controlled trial/
-
Randomization/
-
Single blind procedure/
-
Double blind procedure/
-
Crossover procedure/
-
Placebo/
-
Randomi?ed controlled trialUS$.tw.
-
Rct.tw.
-
Random allocation.tw.
-
Randomly allocated.tw.
-
Allocated randomly.tw.
-
(allocated adj2 random).tw.
-
Single blindUS$.tw.
-
Double blindUS$.tw.
-
((treble or triple) adj blindUS$).tw.
-
PlaceboUS$.tw.
-
Prospective study/
-
or/1–18
-
Case study/
-
Case report.tw.
-
Abstract report/or letter/
-
or/20–22
-
19 not 23
-
Ticlopidine/
-
Clopidogrel/
-
clopidogrel.ti,ab.
-
plavix.ti,ab.
-
90055–48–4.rn.
-
(asasantin retard or persantin retard).ti,ab.
-
DIPYRIDAMOLE/
-
dipyridamole.ti,ab.
-
58–32–2.rn.
-
or/25–33
-
(myocardUS$infarcUS$or MI).ti.
-
NSTEMI.ti,ab.
-
non ST segment elevation myocardial infarction.ti,ab.
-
stroke.ti.
-
Cerebrovascular Accident/
-
(cerebrovascular accidentUS$or CVA).ti.
-
Transient Ischemic Attack/
-
(isch?emic stroke or transient isch?emic attackUS$).ti,ab.
-
Unstable Angina Pectoris/
-
unstable angina.ti,ab.
-
peripheral arterial disease.ti,ab.
-
(TIA or TIAS).ti.
-
Heart Infarction/
-
or/35–47
-
24 and 34 and 48
-
limit 49 to (human and english language and yr=“2003 – 2009”)
MEDLINE August week 4 2009
-
randomized controlled trial.pt.
-
randomized controlled trials/
-
randomi?ed controlled trialUS$.ti,ab.
-
random allocation/
-
double–blind method/
-
single–blind method/
-
(clinUS$ad2 rialUS$).ti,ab.
-
((singlUS$r doublUS$or treblUS$or triplUS$) adj2 (blindUS$or maskUS$)).ti,ab.
-
placebos/
-
placeboUS$.ti,ab.
-
random.ti,ab.
-
comparative study/
-
exp evaluation studies/
-
follow–up studies/
-
prospective studies/
-
(control or controls or controlled).ti,ab.
-
clinical trials, phase iv/
-
phase iv.ti,ab.
-
phase four.ti,ab.
-
phase 4.ti,ab.
-
post marketUS$surveillance.ti,ab.
-
or/1–21
-
Case report.tw.
-
Letter/
-
Historical article/
-
or/23–25
-
22 not 26
-
Ticlopidine/
-
clopidogrel.ti,ab.
-
plavix.ti,ab.
-
90055–48–4.rn.
-
asasantin retard.ti,ab.
-
persantin retard.ti,ab.
-
dipyridamole.ti,ab.
-
dipyridamole/
-
58–32–2.rn.
-
or/28–36
-
exp MYOCARDIAL INFARCTION/
-
(myocardUS$infarcUS$or MI).ti.
-
NSTEMI.ti,ab.
-
non ST segment elevation myocardial infarction.ti,ab.
-
stroke.ti.
-
CEREBROVASCULAR ACCIDENT/
-
(cerebrovascular accidentUS$or CVA).ti.
-
ISCHEMIC ATTACK, TRANSIENT/
-
sch?emic stroke or transient isch?emic attackUS$).ti,ab.
-
ANGINA, UNSTABLE/
-
unstable angina.ti,ab.
-
peripheral arterial disease.ti,ab.
-
TIA or TIAS).ti.
-
or/38–50
-
52
-
27 and 37 and 51
-
53
-
limit 52 to (english language and humans and yr=“2003 – 2009”)
Web of Science – now with Conference Proceedings
-
2003–2009.
-
Databases searched=SCI-EXPANDED (Science Citation Index Expanded), CPCI-S (Conference Proceedings Citation Index-Science).
-
((Clopidogrel or dipyridamole or plavix or ticlopidine or asasantin or persantin) and (Occlusive vascular event* or ischaemic attack or TIA or ischaemic stroke or myocardial infarction or MI or heart infarction or Peripheral artery disease or cerebrovascular accident* or unstable angina or ST segment elevation)).
-
Results: Document Type=(ARTICLE (1257) OR REVIEW (265) OR PROCEEDINGS PAPER (110) OR MEETING ABSTRACT (93)) AND Languages=(ENGLISH).
-
Total: 1725.
The Cochrane Library
-
2003 – Issue 3, 2009.
-
Databases searched=SCI-EXPANDED (Science Citation Index Expanded), CPCI-S (Conference Proceedings Citation Index- Science).
-
((Clopidogrel or dipyridamole or plavix or ticlopidine or asasantin or persantin) and (Occlusive vascular event* or ischaemic attack or TIA or ischaemic stroke or myocardial infarction or MI or heart infarction or Peripheral artery disease or cerebrovascular accident* or unstable angina or ST segment elevation)) in title, abstract or key words.
-
Cochrane Database of Systematic Reviews (Cochrane Reviews): six Database of Abstracts of Reviews of Effects (Other Reviews): six Cochrane Central Register of Controlled Trials (Clinical Trials): 279 Health Technology Assessment Database (Technology Assessments): sixNHS Economic Evaluation Database (Economic Evaluations): 20.
-
Total number of references identified: 5869 including duplicate references).
-
Total number of references identified: 5109 (excluding duplicate references, removed electronically).
Appendix 2 Quality assessment
Quality assessment of included randomised controlled trials
| Checklist item | CAPRIE26 | ESPS-230 | ESPRIT56 | PRoFESS57 |
|---|---|---|---|---|
| Randomisation | ||||
| Was the randomisation method adequate? | Yes | Yes | Yes | Yes |
| Was the allocation of treatment adequately concealed? | ||||
| Was the number of participants randomised stated? | ||||
| Baseline comparability | ||||
| Were details of baseline comparability presented? | Yes | Yes | Yes | Yes |
| Were the groups similar for prognostic factors? | ||||
| Eligibility criteria and co-interventions | ||||
| Were the eligibility criteria for study entry specified? | Yes | Yes | Yes | Yes |
| Were any co-interventions identified? | ||||
| Blinding | ||||
| Were outcome assessors blinded to treatment allocation? | Yes | Yes | Noa | Yes |
| Were administrators blinded to the treatment allocation? | ||||
| Were patients blinded to the treatment allocation? | ||||
| Was the blinding procedure assessed? | NS | NS | NS | NS |
| Withdrawals | ||||
| Any unexpected imbalances in dropouts between groups? Were they explained or adjusted for? | No/N/A | No/N/A | No/N/A | No/N/A |
| Were ≥ 80% patients included in the final analysis? | Yes | Yes | Yes | Yes |
| Were reasons for withdrawals stated? | ||||
| Was an ITT analysis included? Was this appropriate? Were appropriate methods used to account for missing data? | ||||
| Outcomes | ||||
| Evidence of more outcomes measured than reported? | No | No | No | Noa |
Quality assessment of identified systematic reviews
| Review | Inclusion/exclusion criteria addressed review questions? | Evidence of a substantial effort to search for all relevant research literature? | Validity of included studies adequately assessed? | Sufficient detail of individual studies? | Primary studies summarised appropriately? |
|---|---|---|---|---|---|
| Jones 20043 | Good | Good | Good | Good | Good |
| Leonardi-Bee 20055 | Fair | Fair | |||
| Verro 20089 | Poor | ||||
| De Schryver 200713 | Good | Good | Good | ||
| ATTC 2009119 | |||||
| Berger 2009120 | Fair | ||||
| Halkes 2008121 | Fair | N/A | N/A | ||
| Sudlow 2009122 | Good | Good | Good |
Quality assessment of included cost-effectiveness studies
| Drummond 10-point checklist54 | Annemans 200369 | Beard 200470 | Berger 200871 | Chen 200972 | Karnon 200573 | Matchar 200574 | Schleinitz 200475 | Delea 200376 | Palmer 200577 | Stevenson 200878 | Van Hout 200379 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Well-defined question | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓/✗ | ✓/✗ | ✓/✗ | ||
| Comprehensive description of competing alternatives | ✓ | ✓ | ✓ | ✓ | ✓ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Effectiveness established | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ |
| All important and relevant costs and consequences for each alternative identified | ✓ | ✓ | ✓ | ✓ | ✓ | ✓/✗ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Costs and consequences measured accurately | ✓/✗ | ✓ | ✓ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓/✗ | ✓/✗ | ✓/✗ |
| Costs and consequences valued credibly | ✓ | ✓ | ✓/x | ✓ | ✓/✗ | ✓/✗ | ✓ | ✓ | ✓/✗ | ✓/✗ | ✓/✗ |
| Costs and consequences adjusted for differential timing | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓/✗ | ✓/✗ | ✓/✗ |
| Incremental analysis costs and consequences | ✓ | ✓/✗ | ✓/✗ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓/✗ | ✗ | ✓ |
| Sensitivity analyses to allow for uncertainty in estimates of costs or consequences | ✓ | ✓/✗ | ✓/✗ | ✓ | ✓ | ✓/✗ | ✓ | ✓/✗ | ✓/✗ | ✓/✗ | ✓/✗ |
| Study results/discussion include all issues of concern to users | ✓ | ✓ | ✓ | ✓ | ✓ | ✓/✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
Appendix 3 Description of systematic reviews
Eight relevant SRs were identified via the electronic searches: Jones et al. ,3 Leonardi-Bee et al. ,5 Verro et al. ,9 De Schryver et al. ,13 ATTC,119 Berger et al. ,120 Halkes et al. 121 and Sudlow et al. 122 The majority of these were of good quality; all but two9,121 of the reviews were of generally good quality (i.e. were rated as good on three or more criteria out of five). These generally supported current guidance, but highlighted the variety of patients, the different combinations of drugs and outcomes that have been assessed. No additional trials were identified from the reference lists of the identified SRs for inclusion in the review.
Identifying and assessing the quality of existing reviews allowed the Assessment Group to cross-check for the identification of additional studies, as well as to gain an understanding of the issues related to the combining of data in this complex area. The identified reviews served to demonstrate the heterogeneity of patient populations and interventions as well as the different approaches to data analysis.
The SRs are listed in the table below; most of the included studies assessed immediate-release dipyridamole rather than MRD. One of the identified SRs was the review of Jones et al.,3 which underpins the current NICE TA90 guidance. 24 Three further SRs were updates of those reported by Jones et al. ;3 their conclusions remained unchanged. 13,119,122 These SRs, although meeting the inclusion criteria, included a variety of patient populations. Although included in the Jones et al. review,3 the patient population in De Schryver et al. 13 appears to be different to that described in the scope (those patients with an arterial vascular disease) and is therefore not comparable.
Of the four newly identified SRs (i.e. those that are not updates from Jones et al. 3), three examined dipyridamole (both MRD and the immediate-release version). These reviews had similar patient populations (previous ischaemic stroke or TIA), but Leonardi-Bee et al. 5 compared dipyridamole, with or without ASA, with ASA alone. The other two SRs9,121 only compared dipyridamole + ASA with ASA alone; thus, this was the only comparison that can be considered. The conclusions of all three SRs are generally consistent and favoured the use of dipyridamole + ASA over ASA alone. All three concluded that recurrent stroke was reduced by dipyridamole + ASA, as was the composite of non-fatal stroke, non-fatal MI and vascular death.
Overall, the SRs examine both MRD and the immediate-release version of dipyridamole. De Schryver et al. 13 included three trials that used MRD, Leonardi-Bee et al. 5 included one trial using MRD and six using the immediate-release version. Halkes et al. 121 (an update of Leonardi-Bee et al. 5) included two trials that used MRD; the remainder used the immediate-release version. Verro et al. 9 included two trials that used MRD the other four used the immediate-release formula. In the Jones et al. review,3 all trials and economic reviews that investigated dipyridamole used the modified version.
The SR by Berger et al. 120 investigated the effect of ASA (alone or with dipyridamole) on cardiovascular event rates in patients with peripheral arterial disease. Dipyridamole is not currently licensed in this population. The included patient population was wide and included groups who were post operative. Treatment with ASA alone or with dipyridamole resulted in a non-significant decrease in the primary end point of cardiovascular events, but a statistically significant reduction in non-fatal stroke. This suggests that ASA is of benefit to patients with peripheral arterial disease (in this wider population) for the prevention of stroke, which is consistent with the current guidance. 24
| Review | Title | Patient population | Trials using MRD/immediate-release dipyridamole |
|---|---|---|---|
| Jones 20043 | A rapid and systematic review of the clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of OVEs | MI, IS, PAD, TIA | 1/1 |
| aDe Schryver 200713 | Dipyridamole for preventing stroke and other vascular events in patients with vascular disease | CAD, MI, angina pectoris, retinopathy, nephropathy, PAD, IS, TIA, amaurosis fugax | 3/29 |
| aATTC 2009119 | Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials | MI, IS,TIA | N/A |
| aSudlow 2009122 | Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients | High vascular risk | N/A |
| Leonardi-Bee 20055 | Dipyridamole for preventing recurrent ischaemic stroke and other vascular events | IS, TIA | 1/7 |
| Verro 20089 | Aspirin plus dipyridamole versus aspirin for prevention of vascular events after stroke or TIA: a meta-analysis | IS,TIA | 2/6 |
| Halkes 2008121 | Dipyridamole plus aspirin versus aspirin alone in secondary prevention after TIA or stroke: a meta analysis by risk | IS,TIA | 2/5 |
| Berger 2009120 | Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials | PAD (many following surgical procedures) | Unclear |
Appendix 4 Additional publications associated with each of the main trials
| CAPRIE26 |
|---|
| Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W. Clopidogrel versus aspirin in patients at risk of ischemic events I. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004;35:528–32. |
| Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002;90:625–8. |
| Cannon CP, Investigators C. Effectiveness of clopidogrel versus aspirin in preventing acute myocardial infarction with patients with symptomatic atherothrombosis (CAPRIE trial). Am J Cardiol 2002;90:760–2. |
| Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001;103:363–8. |
| Bhatt DL, Foody J, Hirsch AT, Ringleb P, Hacke W, Topol EJ. Complementary, additive benefit of clopidogrel and lipid-lowering therapy in patients with atherosclerosis. J Am Coll Cardiol 2000;35(Suppl. A):326. |
| Bhatt DL, Hirsch AT, Ringleb P, Hacke W, Topol EJ. Reduction in the need for hospitalisation for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000;140:67–73. |
| Hacke W, Hirsch AT, Topol EJ. The benefit of clopidogrel over aspirin is amplified in high–risk subgroups with a prior history of ischaemic events. Eur Heart J 1999;20(Suppl.). |
| Harker LA, Boissel JP, Pilgrim AJ, Gent M. Comparative safety and tolerability of clopidogrel and aspirin. Results from CAPRIE. Drug Saf 1999;21:325–35. |
| Hacke W. On Behalf of The CAPRIE I. Consistency of the benefit of clopidogrel over aspirin in patients with lacunar and non-lacunar stroke. Cerebrovasc Dis 1998;8:51. |
| Easton JD. Benefit of clopidogrel in patients with evidence of cerebrovascular disease. Neurology 1998;51:S1–2. |
| Morais J. Use of concomitant medications in the CAPRIE trial: clopidogrel is unlikely to be associated with clinically significant drug interactions. Eur Heart J 1998;19:182. |
| Coccheri S. Distribution of symptomatic atherothrombosis and influence of atherosclerotic disease on risk of secondary ischaemic events: Results from CAPRIE. Eur Heart J 1998;19:227. |
| Blecic S. Atherothrombotic events often indicate disseminated atherosclerosis: data from CAPRIE. Cerebrovasc Dis 1998;8:34. |
| Hankey G. The risk of vascular ischaemic events in patients with various clinical manifestations of atherothrombosis: data from CAPRIE. Cerebrovasc Dis 1998;8:30. |
| Rupprecht HJ. Consistency of the benefit of clopidogrel across a range of vascular-related endpoints: results from CAPRIE. Eur Heart J 1998;19(Suppl.):484. |
| Gent M. Benefit of clopidogrel in patients with coronary disease. Circ Res 1997;96:2608. |
| ESPS–230 |
| Ariesen MJ, Algra A, Kappelle LJ. Antiplatelet drugs in the secondary prevention after stroke: Differential efficacy in large versus small vessel disease? A subgroup analysis from ESPS–2. Stroke 2006;37:134–8. |
| Diener HC, Darius H, Bertrand-Hardy JM, Humphreys M. European Stroke Prevention S. Cardiac safety in the European Stroke Prevention Study 2 (ESPS2). Int J Clin Pract 2001;55:162–3. |
| Sivenius J, Cunha L, Diener HC, Forbes C, Laakso M, Lowenthal A. Second European Stroke Prevention Study: antiplatelet therapy is effective regardless of age. ESPS2 Working Group. Acta Neurol Scand 1999;99:54–60. |
| Sivenius J, Cunha L, Diener HC, Forbes C, Laakso M, Lowenthal A. Antiplatelet treatment does not reduce the severity of subsequent stroke. European Stroke Prevention Study 2 Working Group. Neurology 1999;53:825–9. |
| ESPRIT56 |
| Algra A. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurology 2007;6:115–24. |
| Halkes PHA. [Acetylsalicylic acid and dipyridamole offer better secondary protection than acetylsalicylic acid only following transient ischaemic attack or cerebral infarction of arterial origin; the ‘European/Australasian stroke prevention in reversible ischaemia trial’ (ESPRIT)]. Ned Tijdschr Geneeskd 2006;150:1832–8. |
| PRoFESS |
| Diener HC. The PRoFESS trial: Future impact on secondary stroke prevention. Expert Rev Neurother 2007;7:1085–91. |
| Diener HC, Sacco R, Yusuf S. Rationale, design and baseline data of a randomized, double-blind, controlled trial comparing two antithrombotic regimens (a fixed-dose combination of extended-release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: The Prevention Regimen for Effectively Avoiding Second Strokes trial (PRoFESS). Cerebrovasc Dis 2007;23:368–80. |
| Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurring stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol 2008;7:875–84. |
Appendix 5 Excluded publications with rationale
| Published paper | Reason for exclusion | |
|---|---|---|
| 1 | Bezerra DC, Bogousslavsky J. Antiplatelets in stroke prevention: the MATCH trial. Some answers, many questions and countless perspectives. Cerebrovasc Dis 2005;20(Suppl. 2):109–18. | Review |
| 2 | Anand S, Yusuf S, Montague P, Chin SL. The effects of oral anticoagulants in patients with peripheral arterial disease: Rationale, design, and baseline characteristics of the Warfarin and Antiplatelet Vascular Evaluation (WAVE) trial, including a meta-analysis of trials. Am Heart J 2006;151:1–9. | Not relevant intervention |
| 3 | Anand S, Yusuf S, Xie C, Pogue J, Eikelboom J, Budaj A, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med 2007;357:217–27. | Not relevant intervention |
| 4 | Bakhru MR, Bhatt DL. Interpreting the CHARISMA study. What is the role of dual antiplatelet therapy with clopidogrel and aspirin? Cleveland Clinic J Med 2008;75:289–95. | Review |
| 5 | Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA Trial. J Am Coll Cardiol 2007;49:1982–8. | Not relevant intervention |
| 6 | Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. A global view of atherothrombosis: Baseline characteristics in the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance (CHARISMA) trial. Am Heart J 2005;150(3). | Not relevant intervention |
| 7 | Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1744–6. | Not relevant intervention |
| 8 | Bhatt DL, Topol EJ. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high-risk primary prevention: rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J 2004;148:263–8. | Not relevant intervention |
| 9 | Biller J. Antiplatelet therapy in ischemic stroke: variability in clinical trials and its impact on choosing the appropriate therapy. J Neurol Sci 2009;15;284:1–9. | Not RCT or SR |
| 10 | Bjorklund L, Wallander MA, Johansson S, Lesen E. Aspirin in cardiology: benefits and risks. Int J Clin Pract 2009;6:468–77. | Not RCT or SR |
| 11 | Bowry ADK, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol 2008;101:960–6. | Not relevant patient group |
| 12 | Brown J, Lethaby A, Maxwell H, Wawrzyniak AJ, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database Syst Rev 2008;4:CD000535. | Not patient population |
| 13 | Cacoub PP, Bhatt DL, Steg PG, Topol EJ, Creager MA. Patients with peripheral arterial disease in the CHARISMA trial. European Heart J 2009;30:192–201. | Not relevant intervention |
| 14 | Calvet D, Touze E, Mas JL. Adding aspirin to clopidogrel in secondary prevention of ischemic stroke: no significant benefits: results of the MATCH study. Presse Med 2006;35:679–82. | Not relevant intervention |
| 15 | Cassar K, Ford I, Greaves M, Bachoo P, Brittenden J. Randomized clinical trial of the antiplatelet effects of aspirin–clopidogrel combination versus aspirin alone after lower limb angioplasty. Br J Surg 2005;92:159–65. | Not relevant intervention |
| 16 | Chairangsarit P, Sithinamsuwan P, Niyasom S, Udommongkol C, Nidhinandana S, Suwantamee J. Comparison between aspirin combined with dipyridamole versus aspirin alone within 48 hours after ischemic stroke event for prevention of recurrent stroke and improvement of neurological function: a preliminary study. J Med Assoc Thai 2005;88(Suppl. 3):148–54. | Not relevant patient group |
| 17 | Chaturvedi S. Acetylsalicylic acid + extended-release dipyridamole combination therapy for secondary stroke prevention. Clin Ther 2008;30:1196–205. | Review |
| 18 | Culebras A, Borja J, Garcia-Rafanell J. Triflusal versus aspirin for the prevention of stroke. Prog Neurother Neuropsychopharmacol 2008;3:13–33. | Not relevant intervention |
| 19 | de Borst GJ, Hilgevoord AA, de Vries JP, van der Mee M, Moll FL, van de Pavoordt HD, et al. Influence of antiplatelet therapy on cerebral micro-emboli after carotid endarterectomy using postoperative transcranial Doppler monitoring. Eur J Vasc Endovasc Surg 2007;34:135–42. | Not relevant patient group |
| 20 | Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331–7. | Not relevant intervention |
| 21 | Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Management of atherothrombosis with clopidogrel in high-risk patients with recent transient ischaemic attack or ischaemic stroke (MATCH): study design and baseline data. Cerebrovasc Dis 2004;17:253–61. | Not relevant intervention |
| 22 | Diener HC, editor. Management of atherothrombosis with clopidogrel in high-risk patients with recent transient ischaemic attack or ischaemic stroke (MATCH): rationale and study design. Fifth World Stroke Congress, Vancouver, BC, 23–6 June 2004. | Not relevant intervention |
| 23 | Diener HC. Management of atherosclerosis with clopidogrel in high-risk patients with recent transient ischaemic attack or ischemic stroke (MATCH): study results. Stroke 2004. | Not relevant intervention |
| 24 | Donnelly R. Antiplatelet therapy and prevention of ischaemic events: CAPRIE. Br J Diabetes and Vascular Disease. 2005 Br J Diab Vasc Dis 5:203–6. | Not RCT or SR |
| 25 | Eikelboom JW, Hankey GJ, Thom J, Claxton A, Yi Q, Gilmore G, et al. Enhanced antiplatelet effect of clopidogrel in patients whose platelets are least inhibited by aspirin: a randomized crossover trial. J Thrombo Haemost 2005;3:2649–55. | Not relevant intervention |
| 26 | Einhaupl K. ESPRIT study design and outcomes: a critical appraisal. Curr Med Res Opin 2007;23:271–3. | Review |
| 27 | England T, Bath P. Safety and tolerability of clopidogrel when added to aspirin and dipyridamole in high risk patients with recent ischaemic stroke: a randomised controlled trial. Third UK Stroke Forum Conference, Harrogate, 2–4 December 2008. | Not relevant intervention |
| 28 | England TJ, Bath PM. Triple antiplatelets for reducing dependency after ischaemic stroke (TARDIS). Safety and tolerability of clopidogrel when added to aspirin and dipyridamole in high risk patients with recent ischaemic stroke: a randomized controlled trial. International Stroke Conference, San Diego, CA, 17–20 February 2009. | Not relevant intervention |
| 29 | Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004 | Not relevant patient group |
| 30 | Gorelick PB, Richardson D, Kelly M, Ruland S, Hung E, Harris Y, et al. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial. JAMA 2003;289:2947–57. | Not relevant intervention |
| 31 | Greisenegger S, Tentschert S, Weber M, Ferrari J, Lang W, Lalouschek W. Prior therapy with antiplatelet agents is not associated with outcome in patients with acute ischemic stroke/TIA. J Neurol 2006;253:648–52. | Review |
| 32 | Halkes PHA, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Risk indicators for development of headache during dipyridamole treatment after cerebral ischaemia of arterial origin. J Neurol Neurosurg Psychiatry 2009;80:437–9. | Review |
| 33 | Hart RG, Bhatt DL, Hacke W, Fox KA, Hankey GJ, Berger PB, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of stroke in patients with a history of atrial fibrillation: subgroup analysis of the CHARISMA randomized trial. Cerebrovasc Dis 2008;25:344–7. | Not relevant intervention |
| 34 | Hills NK, Johnston SC. Trends in usage of alternative antiplatelet therapy after stroke and transient ischemic attack. Stroke 2008;39:1228–32. | Registry |
| 35 | Hradec J, Spinar J. [CHARISMA. The clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance trial]. Cor et Vasa 2006;5:202–6. | Not relevant intervention |
| 36 | Huang YI, Cheng Y, Wu J, Li YS, Xu E, Hong Z, et al. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol 2008;7:494–9. | Not relevant intervention |
| 37 | Ito E, Takahashi A, Kuzuhara S, Uchiyama S, Nakajima M, Riku S, et al. Ticlopidine alone versus ticlopidine plus aspirin for preventing recurrent stroke. Intern Med 2003;42:793–9. | Not relevant intervention |
| 38 | Karha J, Bhatt DL, Wolski K, Fox KA, Montalescot G, Topol EJ, editors. The use of COX–2 inhibitors and the risk of myocardial infarction in the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance (CHARISMA) trial. 79th Annual Scientific Session of the American Heart Association, Chicago, IL, 12–15 November 2006. | Not RCT |
| 39 | Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007;6:961–9. | Not relevant intervention |
| 40 | Mahmood A, Sintler M, Edwards AT, Smith SRG, Simms MH, Vohra RK. The efficacy of aspirin in patients undergoing infra-inguinal bypass and indentification of high-risk patients. Int Angiol 2003;22:302–7. | Not RCT |
| 41 | Mak KH, Bhatt DL, Shao M, Haffner SM, Hamm CW, Hankey GJ, et al. The influence of body mass index on mortality and bleeding among patients with or at high-risk of atherothrombotic disease. Eur Heart J 2009;30:857–65. | Not relevant intervention |
| 42 | Mak KH, Bhatt DL, Shao M, Hankey GJ, Easton JD, Fox KAA, et al. Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study. Am Heart J 2009;157:658–65. | Not relevant intervention |
| 43 | Markus H. Antiplatelet therapy vs anticoagulation in cervical artery dissection; Rationale and design of the Cervical Artery Dissection in Stroke Study (CADISS). Int J Stroke 2007;2:292–6. | Not a relevant population |
| 44 | Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 2005;111:2233–40. | Not relevant intervention |
| 45 | Matias-Guiu J, Ferro JM, Alvarez-Sabin J, Torres F, Jimenez MD, Lago A, et al. Comparison of triflusal and aspirin for prevention of vascular events in patients after cerebral infarction the TACIP study: a randomized, double-blind, multicenter trial. Stroke 2003;34:840–7. | Not relevant intervention |
| 46 | McKevitt FM, Randall MS, Cleveland TJ, Gaines PA, Tan KT, Venables GS. The benefits of combined anti-platelet treatment in carotid artery stenting. Eur J Vasc Endovasc Surg 2005;29:522–7. | Not relevant intervention |
| 47 | Secondary stroke prevention set to benefit from PRoFESS trial: extended-release dipyridamole plus aspirin (Asasantin Retard) and clopidogrel share very similar benefit–risk ratio in vascular prevention. Cardiovasc J Africa 2008;19:165. | Comment on PRoFESS |
| 48 | Serebruany VL, Malinin AI, Pokov AN, Hanley DF. Randomized single-blind 30-days trial of the antiplatelet profiles after extended-released dipyridamole and low dose aspirin versus clopidogrel with or without aspirin in diabetic patients after TIA. Cerebrovasc Dis 2008; 25 (Suppl. 2):159. | Not relevant intervention |
| 49 | Serebruany VL, Malinin AI, Ziai W, Pokov AN, Bhatt DL, Alberts MJ, et al. Effects of clopidogrel and aspirin in combination versus aspirin alone on platelet activation and major receptor expression in patients after recent ischemic stroke: for the Plavix Use for Treatment of Stroke (PLUTO–Stroke) trial. Stroke 2005;36:2289–92. | Not relevant intervention |
| 50 | Sprigg N, Gray LJ, England T, Willmot MR, Zhao L, Sare GM, et al. A randomised controlled trial of triple antiplatelet therapy (aspirin, clopidogrel and dipyridamole) in the secondary prevention of stroke: safety, tolerability and feasibility. PLoS ONE 2008;3:e2852. | Not relevant intervention |
| 51 | Squizzato A, Keller T, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database Syst Rev 2007;1:CD005158. | Not relevant intervention |
| 52 | Thijs V, Lemmens R, Fieuws S. Network meta-analysis: simultaneous meta-analysis of common antiplatelet regimens after transient ischaemic attack or stroke. Eur Heart J 2008;29:1086–92. | Not relevant intervention |
| 53 | Uchiyama S, Fukuuchi Y, Yamaguchi T. The safety and efficacy of clopidogrel versus ticlopidine in Japanese stroke patients: Combined results of two Phase III, multicenter, randomized clinical trials. J Neurol 2009;256:888–97. | Not relevant intervention |
| 54 | Wang TH, Bhatt DL, Fox KAA, Steinhubl SR, Brennan DM, Hacke W, et al. An analysis of mortality rates with dual-antiplatelet therapy in the primary prevention population of the CHARISMA trial. Eur Heart J 2007;28:2200–7. | Not relevant intervention |
| 55 | Dieker HJ, French JK, Joziasse IC, Brouwer MA, Elliott J, West TM, et al. Antiplatelet therapy and progression of coronary artery disease: a placebo-controlled trial with angiographic and clinical follow-up after myocardial infarction. Am Heart J 2007;153:1–8. | Not relevant intervention |
| 56 | Serebruany VL, Malinin AI, Pokov AN, Hanley DF. Antiplatelet profiles of the fixed-dose combination of extended-release dipyridamole and low-dose aspirin compared with clopidogrel with or without aspirin in patients with type 2 diabetes and a history of transient ischemic attack: A randomized, single-blind, 30-day trial. Clin Ther 2008;30:249–59. | Not relevant outcomes |
Appendix 6 Identified ongoing trials
| Trial name and identification no. | Sponsor | Comparators | Aims of study | Study start date | Estimated primary completion datea | Estimated study completion date |
|---|---|---|---|---|---|---|
|
Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events (CHANCE) NCT00979589 |
Ministry of Science and Technology of the People’s Republic of China |
CLOP + ASA (ASA will be replaced by placebo from day 21) Placebo + ASA |
To assess the effects of a 3-month regimen of CLOP vs a 3–month regimen of aspirin alone on reducing the risk of any stroke when initiated within 24 hours of symptom onset in high-risk patients with TIA or minor stroke | July 2008 | July 2011 | December 2011 |
|
COMbination of clopidogrel and aspirin for Prevention of early REcurrence in acute atherothrombotic Stroke (COMPRESS) NCT00814268 |
Sanofi–aventis |
CLOP + ASA Placebo + ASA |
To compare the efficacy of CLOP + ASA and ASA alone in preventing any recurrent ischaemic lesion | October 2008 | December 2010 | |
|
Platelet-Orientated Inhibition in New Transient ischemic attack (TIA) (POINT) trial NCT00991029 |
University of California, San Francisco, CA |
CLOP + ASA Placebo + ASA |
To evaluate CLOP as a treatment to reduce risk of stroke and MI after TIA in patients also prescribed ASA | October 2009 | June 2016 | |
|
Secondary Prevention of Small Subcortical Strokes trial (SPS3) NCT00059306 |
The University of Texas Health Science Center at San Antonio, TX |
CLOP + ASA Placebo + ASA |
To learn if CLOP + ASA is more effective than ASA alone for prevention of recurrent stroke and cognitive decline | February 2003 | June 2011 | June 2011 |
|
ASpirin non-responsiveness and Clopidogrel Endpoint Trial (ASCET) NCT00222261 |
Ullevaal University Hospital |
CLOP ASA |
To investigate whether or not aspirin non-responders have a higher composite event rate than responders or whether or not CLOP treatment in patients non-responsive to aspirin will reduce their risk of future clinical events | April 2003 | July 2010 | July 2010 |
|
JASAP: Japanese Aggrenox Stroke prevention vs Aspirin Programme NCT00311402 |
Boehringer Ingelheim Pharmaceuticals |
Aggrenox (MRD + ASA) ASA |
To compare the preventive effect of recurrent stroke and safety of Aggrenox vs ASA | April 2006 | March 2009 |
Appendix 7 Example of the mixed-treatment comparison codes for the ‘first ischaemic stroke’ and networks
Mixed-treatment comparison network of RCTs ‘first ischaemic stroke’: ASA/clopidogrel/MRD + ASA. (Solid black lines represent direct head-to-head comparisons and dotted lines represent indirect comparisons.)
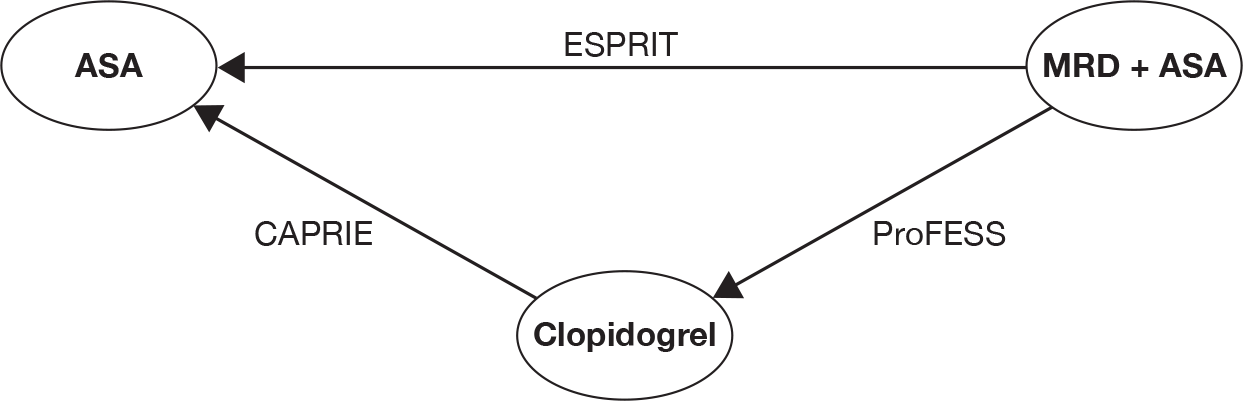
Mixed-treatment comparison network of RCTs ‘recurrent stroke’: ASA/clopidogrel/MRD + ASA. (Solid black lines represent direct head-to-head comparisons and dotted lines represent indirect comparisons.)
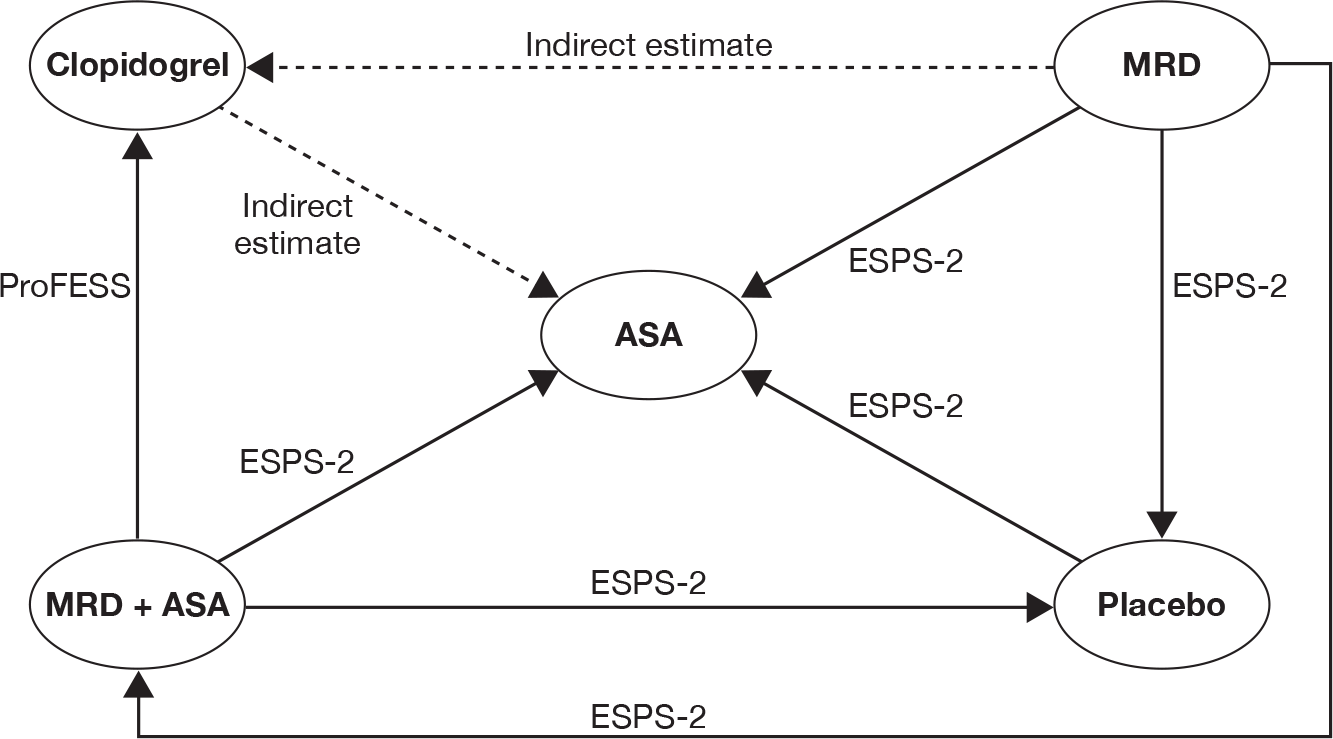
Mixed-treatment comparison network of RCTs ‘MI’: ASA/clopidogrel/MRD + ASA. (Solid black lines represent direct head-to-head comparisons and dotted lines represent indirect comparisons.)
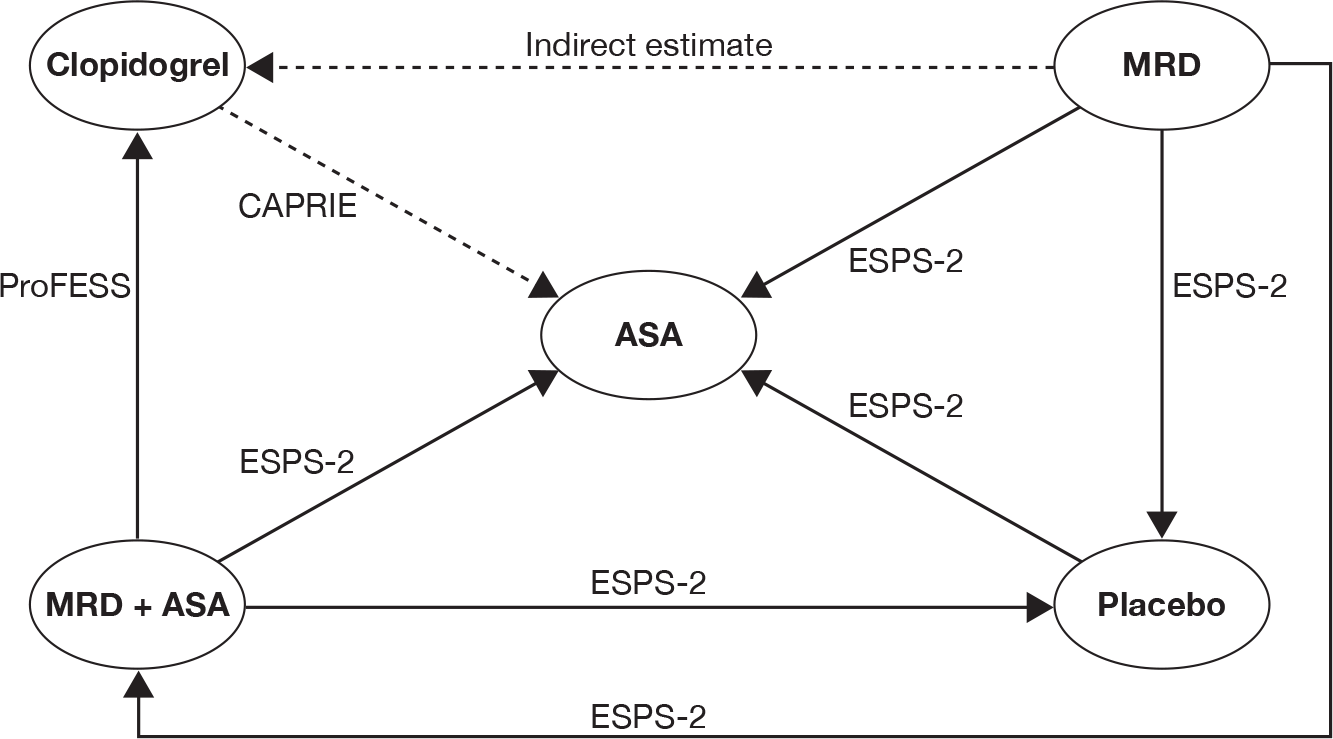
Mixed-treatment comparison network of RCTs ‘death from vascular causes’: ASA/clopidogrel/MRD + ASA. (Solid black lines represent direct head-to-head comparisons and dotted lines represent indirect comparisons.)
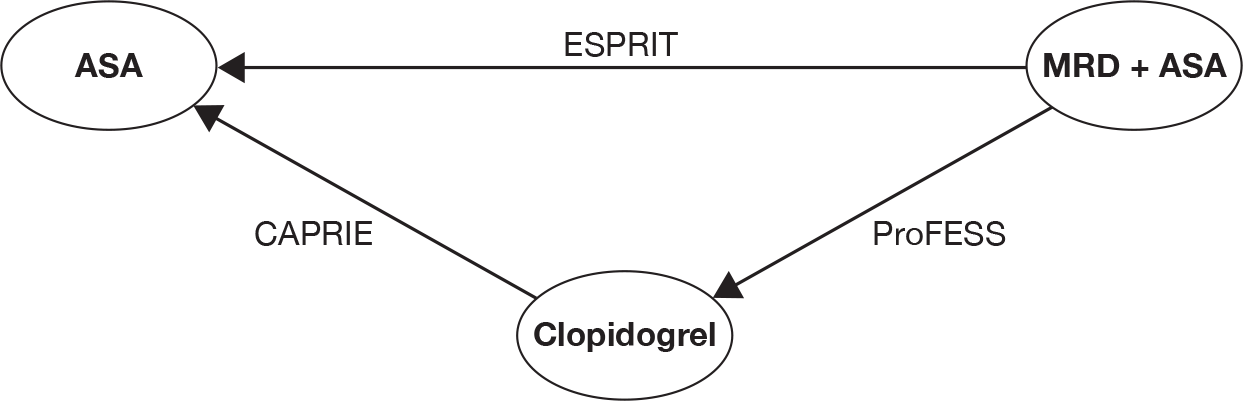
Mixed-treatment comparison network of RCTs ‘all-cause death’: ASA/clopidogrel/MRD + ASA. (Solid black lines represent direct head-to-head comparisons and dotted lines represent indirect comparisons.)
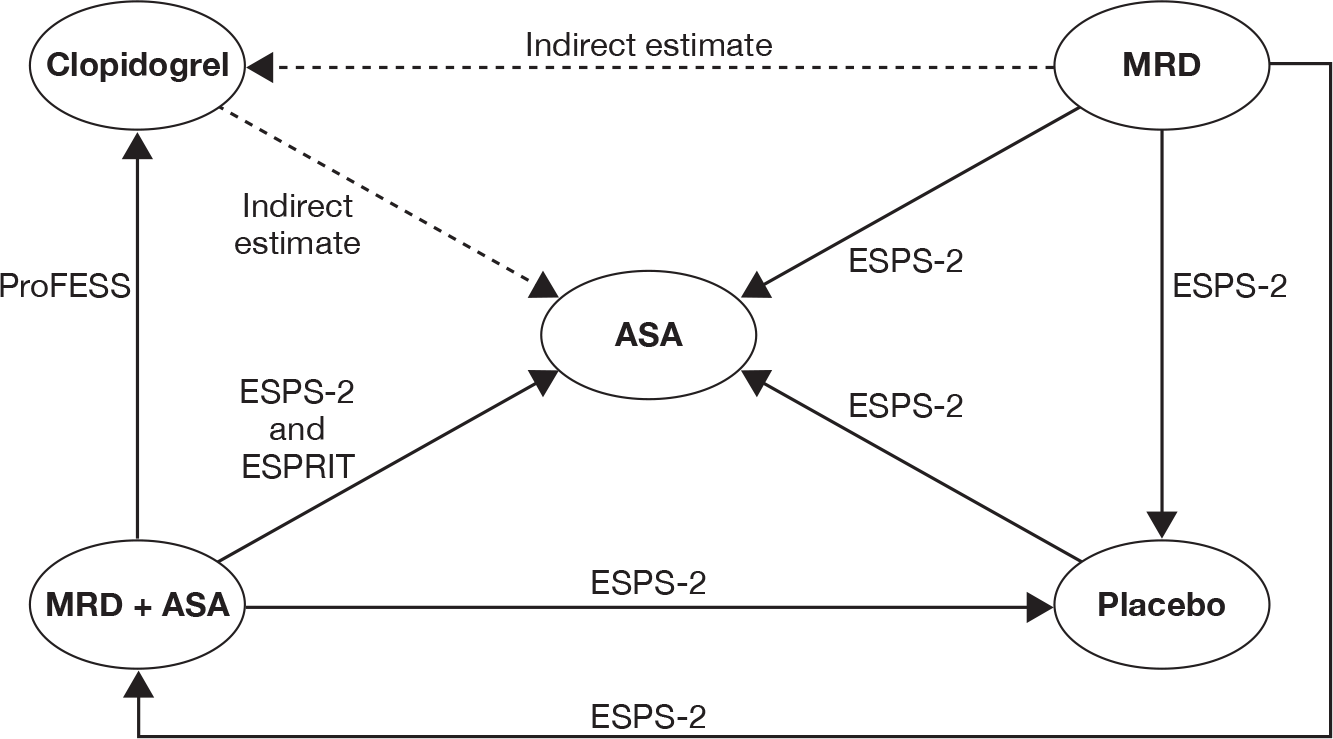
Mixed-treatment comparison network of RCTs ‘any bleeding’: ASA/clopidogrel/MRD + ASA. (Solid black lines represent direct head-to-head comparisons and dotted lines represent indirect comparisons.)
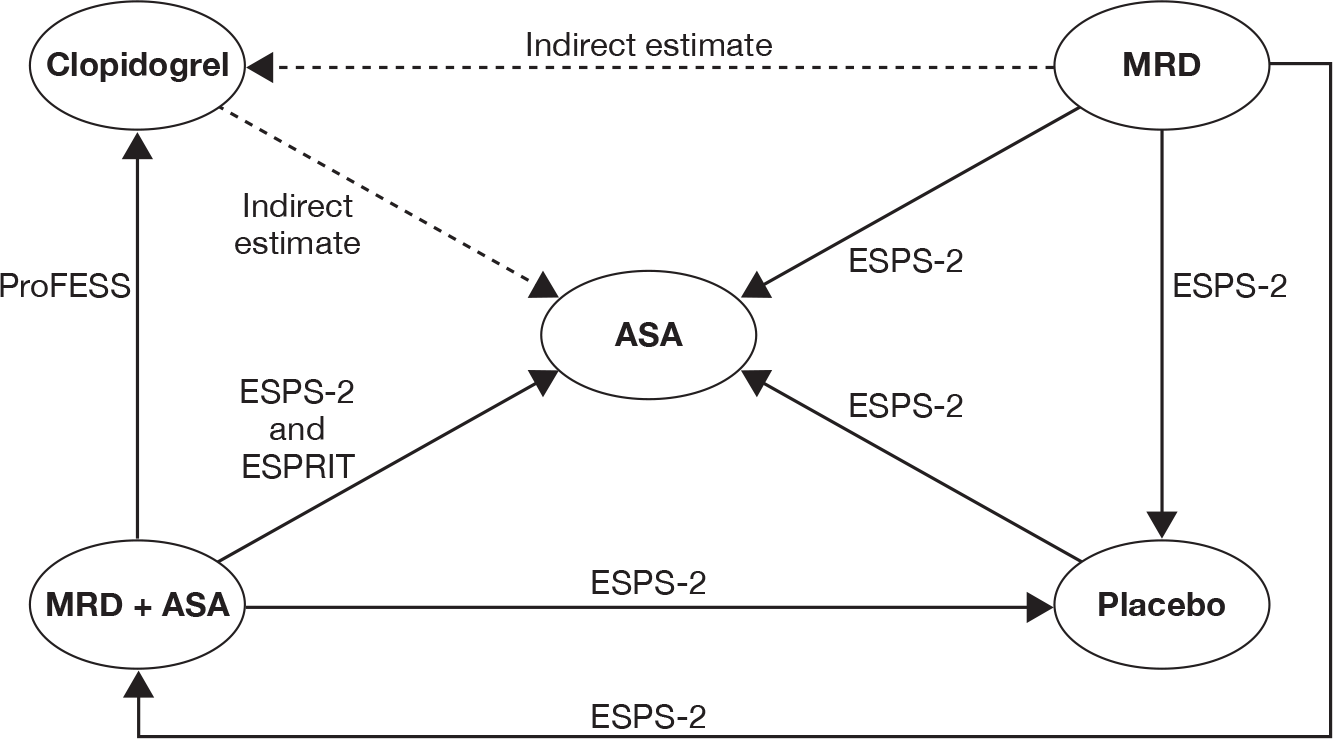
Mixed-treatment comparison network of RCTs ‘death from major bleeding’: ASA/clopidogrel/MRD + ASA. (Solid black lines represent direct head-to-head comparisons and dotted lines represent indirect comparisons.)
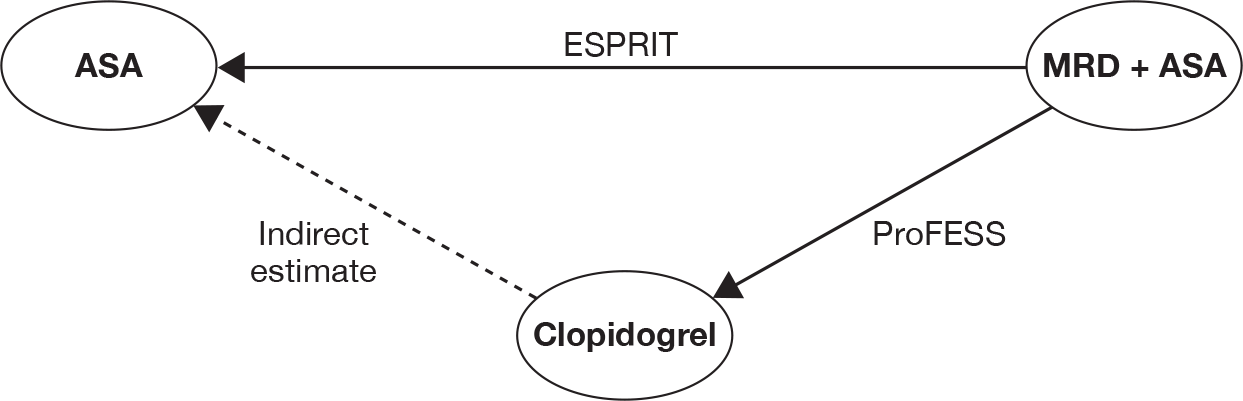
The codes used in the mixed-treatment comparison analysis were adapted from the MPES and are freely available for download from their website (www.bris.ac.uk/cobm/research/mpes).
Appendix 8 Sensitivity analysis table from review of cost-effectiveness literature
| Study | Sensitivity analysis |
|---|---|
| Annemans 200369 |
One-way SA (ICER ranges) Discounting rate 0–6% (€7720–19,640), increase and decrease a 50% costs of AE (ICER: €13,170–13,620), IS (ICER: €12,560–14,220) and life expectancy (ICER: €11,140–20,080) PSA ICER: €14,320 (95% CI €6990 to €26,470). 86% probability of be cost-effective at a threshold of €20,000 |
| Beard 200470 |
Univariate SA (ICER ranges) Cost of acute stroke (ICER: £3155–6959/QALY); costs of OVE (£3475–4908/QALY); cost of TIA (£4012–4374/QALY); cost of long-term care HD stroke (cost saving £–8757/QALY); cost of long-term care N/LD stroke (£639–7446/QALY); cost of rehabilitation (£2952–5647/QALY); cost of ASA (cost saving –£4801/QALY); RRR of ASA + MRD vs placebo (cost saving £–70,407/QALY); background events risks (£1880–5988/QALY); initial disability level (£3347–4869/QALY); disability risk after stroke (£3053–5888/QALY); and utility weights stroke (£4765–5810/QALY) PSA Only with five parameters: 75% chance of being cost-effective at a £35,377 £/QALY threshold |
| Berger 200871 | Univariate SA (ICER ranges) Treatment cost patients: scenario 1: €14,240–14,340/QALY, scenario 2: €18,840–18,740/QALY; AE costs, scenario 1 €14,320–14,430/QALY, scenario 2 €18,710–18,870/QALY; concomitant medication costs, scenario 1 €14,370–14,380/QALY, scenario 2 €18,780–18,800/QALY; CLOP costs, scenario 1 €15,750/QALY, scenario 2 €20,580/QALY; discounting costs and effects, scenario 1 €8350–18,610/QALY, scenario 2 €10,700–24,700/QALY; discounting-only costs 3%, scenario 1 €8150/QALY, scenario 2 €10,440/QALY; discounting-only effects at 3%, scenario 1 €14,740/QALY, scenario 2 €19,260/QALY |
| Chen 200972 |
Univariate SA (ICER ranges) Annual discount rate: US$25,139–44,891/LYG; lost life-years for cardiovascular deaths only US$51,033/LYG; lost life-years for non-fatal events US$31,771–42,453/LYG; CLOP costs average wholesale price US$16,176–56,520/LYG; post acute care costs: US$36,899–35,788/LYG: including indirect costs from lost work productivity US$36,148/LYG; and variation of indirect cost from lost work productivity US$36,051–36,246/LYG PSA The probability of being cost-effective at a threshold of < US$50,000/LYG is 70.6%, and 87.4% at < US$100,000/LYG |
| Delea 200376 | ICER is sensitive to the assumed risk reduction for CLOP |
| Karnon 200573 |
Univariate SA Health-state costs (£21,333–21,819/QALY); initial stroke costs (£24,683/QALY); trial-based compliance (£16,528–24,683/QALY); utilities (£19,232–23,159/QALY); composite outcome RR (£12,835/QALY); RR for MI outcome (£20,026–23,383/QALY), RR for stroke outcome (£15,327–32,894/QALY), RR vascular death (dominated –£7101/QALY); RR for MI, stroke and vascular death (dominated –£5,602/QALY); inclusion of non-vascular death RR (£34,349/QALY); age at start 70 years (£16,222/QALY); age at start 80 years (£16,491/QALY); discount rate 6% for both costs and effects (£32,215/QALY); event rate × 2 (£12,245/QALY); and event rate × 0.5 (£41,486/QALY) Bivariate SA (ICER ranges) Health-state costs and utilities (£23,514/QALY). PSA CLOP is cost-effective at a threshold of £30,000/QALY in approximately 60% of randomly sampled analysis |
| Matchar 200574 |
Univariate SA (ICER) RR for ASA: PBO–ASA US$1681–1700/QALY; PBO–CLOP US$50,762–198,150/QALY; PBO-MRD + ASA US$1769–1769/QALY. Costs based on Pharmacy Benefits Management Strategic Health Care Group. Drug & Pharmaceutical Prices: PBO-ASA US$1562/QALY; PBO–CLOP: dominated; PBO–MRD + ASA US$8321/QALY. Efficacy limited to 24 months: PBO–ASA US$3750/QALY; PBO–CLOP: dominated; PBO–MRD + ASA US$195,950/QALY. Accounting for impact of treatment on MI: PBO–ASA US$1,511/QALY; PBO–CLOP US$46,367/QALY; PBO–MRD + ASA US$1667/QALY. PSA ASA–MRD 65% probability of cost-effectiveness at a threshold of US$30,000/QALY |
| Schleinitz 200475 |
SA Efficacy of CLOP: PAD patients: US$86,400–13,500/QALY per QALY Post stroke patients: US$6300/QALY – CLOP MI patients: more effective and cheaper in the base case to US$42,000/QALY Daily cost of CLOP (US$1.80–7.10): PAD patients: US$14,900/QALY US$–41,800/QALY Stroke patients: dominance of CLOP – US$85,500/QALY PSA CLOP has a 50% probability of being cost-effective at a threshold of US$25,600/QALY for patients with peripheral vascular disease and US$30,300/QALY for those with a recent stroke |
| Palmer 200577 | Paper states: ‘Sensitivity analyses showed that all results were robust under various assumptions’ |
| Stevenson 200878 | PSA The probability of the cost per QALY being below £20,000, a significant threshold for cost-effectiveness in the UK, was 79% |
| Van Hout 200379 | Sensitivity analyses revealed that uncertainties surrounding the outcomes are mainly driven by the expected effectiveness, most notably when defining subgroups. The higher the risk for events, the better the cost-effectiveness ratio. In comparison with no treatment (ASA intolerance or previous failure), CLOP is expected to combine gain in effectiveness (0.158 life-years, 0.210 QALYs) with savings (€332 per patient) |
Appendix 9 Additional data requested from manufacturers to populate the de novo model
Analyses requested by Liverpool Reviews and Implementation Group from the PRoFESS57 trial data
Survival analyses
Kaplan–Meier analysis for each treatment arm, stratified by gender (male/female).
Cox proportional hazards analysis for treatment, using gender, age and Rankin Score at time of prior event as covariates.
| Outcome estimated | Prior event(s) | Censored for |
|---|---|---|
| Time to IS | Randomisation | MI, non-IS, non-vascular death, death from any vascular cause other than IS |
| Time to non-IS | MI, IS, non-vascular death, death from any vascular cause other than non-IS | |
| Time to MI | Any stroke, non-vascular death, death from any non-MI vascular cause | |
| Time to other vascular death | MI, stroke, non-vascular death, death from MI or stroke | |
| Time to non-vascular death | MI, stroke, vascular death | |
| Time to vascular death | Non-vascular death | |
| Time to death | Lost to follow-up or end of trial only | |
| Time to other haemorrhagic event (excluding stroke) | MI, stroke, non-vascular death, death from MI or stroke | |
| Repeat runs 1–8 | Following non-fatal IS as first event | As for runs 1–8 |
| Repeat runs 1–8 | Following non-fatal non-IS as first event | |
| Repeat runs 1–8 | Following non-fatal MI as first event |
For each Kaplan–Meier analysis please provide full survival estimates table [e.g. ‘Product-Limit Survival Estimates’ table from sas version (SAS Institute Inc., Cary, NC, USA) or the ‘Survival’ table from Spss version 15.0 (SPSS Inc., Chicago, IL, USA) and the estimated means table (e.g. ‘Mean Estimate’ table from sas or the ‘Means and Medians for Survival Time’ table from spss)]. Cox analyses should show covariate coefficient estimates with CIs.
Patient outcome events and exposure
For each of the following events for each treatment arm please provide a table showing trial numbers in the format shown:
-
ischaemic strokes
-
non-ischaemic strokes
-
MIs
-
other haemorrhagic events (excluding strokes)
-
congestive heart failure events
-
non-vascular deaths
-
other vascular deaths (excluding strokes and MIs)
-
vascular deaths.
| Time period (months) | Exposure | All events | Fatal events | |||||
|---|---|---|---|---|---|---|---|---|
| Patients at risk in period | Patient-days in period | First trial event for patient | Other events | Total events | First trial event for patient | Other events | Total events | |
| 0–6 | ||||||||
| 7–12 | ||||||||
| 13–18 | ||||||||
| 19–24 | ||||||||
| 25–36 | ||||||||
| 37–42 | ||||||||
| 43–48 | ||||||||
Event fatality
Please complete the following table for each subgroup by treatment arm, showing the proportion of each type of vascular event (occurring at any time) that was fatal, analysed by gender and age at the time of the event.
| Gender | Age range (years) | ISs | Intracerebral haemorrhages | MIs | Other vascular events | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Deaths | Percentage fatal | Events | Deaths | Percentage fatal | Events | Deaths | Percentage fatal | Events | Deaths | Percentage fatal | ||
| Female | < 60 | ||||||||||||
| 60–65 | |||||||||||||
| 66–71 | |||||||||||||
| 72+ | |||||||||||||
| Male | < 60 | ||||||||||||
| 60–65 | |||||||||||||
| 66–71 | |||||||||||||
| 72+ | |||||||||||||
Appendix 10 Model risk parameter values and sources
For patients surviving an ischaemic stroke, four long-term treatment options are available to prevent future occlusive vascular events: low-dose ASA, clopidogrel, MRD and ASA + MRD. For the other three patient groups (MI only, peripheral arterial disease only and multivascular disease) only ASA and clopidogrel are licensed for secondary prevention. In all cases it is also necessary to consider periods when no active long-term drug treatment is being taken to reduce the risk of occlusive vascular event.
NICE Clinical Guidance CG48:28 post myocardial infarction clopidogrel
For patients suffering a new MI, recommendations were made in CG4828 for the short-term use of clopidogrel + ASA to prevent early vascular events (primarily repeat MIs):
-
For patients experiencing a NSTEMI, clopidogrel + ASA is recommended for 12 months.
-
For patients experiencing a STEMI, clopidogrel + ASA is recommended for 4 weeks (30 days).
The CURE27 trial provides the evidence source for the first recommendation. This showed a significant protective effect in relation to repeat MIs, but not for strokes. The absolute risk reduction over 12 months was 1.47% (standard error 0.42%).
The recommendation for STEMI patients derives primarily from the COMMIT29 trial where a modest reduction was seen in the rate of re-infarctions, but not in strokes. During the 30-day follow-up, an absolute risk reduction of 0.33% was reported (standard error 0.14%).
To accommodate the likely impact of these guidelines a weighted-average effect has been estimated of 0.853% (standard error 0.207%), based on the balance of STEMI and NSTEMI patients in the GRACE118 study (54.2% and 45.8%, respectively). This reduction is applied to the transient effect risk parameter values shown below for a second MI event after surviving a non-fatal MI, but not to any other MI risks that are much smaller and where no transient effect was identified.
| Population | Detail | ASA | CLOP | ASA + MRD | MRD | No treatment |
|---|---|---|---|---|---|---|
| IS only | Annual risk (%) | 0.4900 | 0.2610 | 0.4320 | 0.4020 | 0.4020 |
| Standard error (%) | 0.0220 | 0.0170 | 0.0120 | 0.0380 | 0.0380 | |
| Source | CAPRIE26 | CAPRIE26 | PRoFESS57 | CAPRIE26/ATTC66 | ||
| MI only | Annual risk (%) | 0.0956 | 0.0956 | N/A | N/A | 0.0784 |
| Standard error (%) | 0.0003 | 0.0003 | N/A | N/A | 0.0069 | |
| Source | CAPRIE26 | N/A | N/A | CAPRIE26/ATTC66 | ||
| PAD only | Annual risk (%) | 0.0910 | 0.0910 | N/A | N/A | 0.0746 |
| Standard error (%) | 0.0117 | 0.0117 | N/A | N/A | 0.0114 | |
| Source | CAPRIE26 | CAPRIE26 | N/A | N/A | CAPRIE26/ATTC66 | |
| MVD | Annual risk (%) | 0.1960 | 0.1960 | N/A | N/A | 0.1602 |
| Standard error (%) | 0.0120 | 0.0120 | N/A | N/A | 0.0170 | |
| Source | CAPRIE26 | CAPRIE26 | N/A | N/A | CAPRIE26/ATTC66 | |
Risks of first occlusive vascular event
Haemorrhagic stroke as first event
The annual risks of suffering an haemorrhagic stroke are generally very low, but vary significantly between patient types and between different treatment options. Reviewing all of the data available, it appears that this risk is effectively constant over quite long periods of time. Evidence in some cases of a small additional early risk is not confirmed from other sources, and may in part be a consequence of differing qualifying criteria among trials, so that some early acute events (in hospital or in the immediate post-discharge period) are counted within some trials, but excluded in others. In estimating model parameters, such transient effects are ignored and only the longer-term annual event rate is used.
For ASA and clopidogrel treatments, risks are estimated from the CAPRIE26 trial; in the ischaemic stroke-only population, sufficient haemorrhagic stroke events were recorded to allow separate parameter values to be obtained, but for the other groups it was possible to derive only a single risk estimate for the population regardless of the treatment in use.
Haemorrhagic stroke risk for MRD + ASA treatment was estimated from the PRoFESS57 trial (noting that the clopidogrel arm in PRoFESS57 yielded a similar event rate to that in CAPRIE26). The risk appropriate for untreated patients was based on the ASA estimated RR for ‘no treatment’ vs ASA in an ATTC66 analysis of secondary prevention published in 2002: RR 1.22 (95% CI 1.03 to 1.44). Finally, the annual risk of haemorrhagic stroke when using MRD was set at the same level as ‘no treatment’, based on the finding of very similar risks reported from the ESPS-230 trial.
Ischaemic stroke as first event
The risk of suffering a recurrent ischaemic stroke is relatively high for patients in the ‘ischaemic stroke-only’ and multivascular disease populations. In addition to a long-term steady risk level, an important transient increased risk is also present within the trial data, which applies for slightly different periods for each population.
For the ‘ischaemic stroke-only’ population model, parameter values have been estimated from CAPRIE26 for ASA and clopidogrel, and from a comparison of PRoFESS57 and CAPRIE26 for ASA + MRD. The ‘no-treatment’ risk was based on the ATTC66 RR for ASA versus ‘no treatment’ applicable to ischaemic stroke. Finally, the annual risk of ischaemic stroke when using MRD was based on the MRD + ASA estimate adjusted by the RRR (24.7%) compared with MRD reported in the ESPS-230 trial. No consistent differences were observed in any of the trials relating to gender.
In the ‘MI-only’ population, no consistent differences were found in the CAPRIE26 data for the choice of treatment (ASA vs clopidogrel), but long-term risks were much higher for females than for males. Therefore, parameters were estimated for two models (males and females separately), combining patients in the two trial arms.
| Population | Detail | ASA | CLOP | ASA + MRD | MRD | No treatment |
|---|---|---|---|---|---|---|
| IS only | Long-term annual risk (%) | 4.201 | 3.971 | 3.971 | 5.273 | 6.001 |
| Standard error (%) | 0.027 | 0.027 | 0.027 | 0.484 | 0.247 | |
| Transient risk (%) (%) | 1.962 | 1.723 | 1.723 | 2.288 | 2.802 | |
| Standard error (%) | 0.044 | 0.047 | 0.047 | 0.229 | 0.127 | |
| Duration of transient risk (months) | 2.8 | 3.1 | 3.1 | 3.1 | 2.8 | |
| Source | CAPRIE26 | CAPRIE26 | PRoFESS57/CAPRIE26 | ProFESS57/CAPRIE26/ESPS–230 | CAPRIE26/ATTC66 |
| Population | Detail | ASA | CLOP | No treatment |
|---|---|---|---|---|
| MI only (females) | Long-term annual risk (%) | 0.774 | 0.774 | 1.106 |
| Standard error (%) | 0.041 | 0.041 | 0.074 | |
| Transient risk (%) | 0.314 | 0.314 | 0.449 | |
| Standard error (%) | 0.055 | 0.055 | 0.077 | |
| Duration of transient risk (months) | 0.3 | 0.3 | 0.3 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 | |
| MI only (males) | Long-term annual risk (%) | 0.300 | 0.300 | 0.429 |
| Standard error (%) | 0.025 | 0.025 | 0.038 | |
| Transient risk (%) | 0.323 | 0.323 | 0.462 | |
| Standard error (%) | 0.044 | 0.044 | 0.065 | |
| Duration of transient risk (months) | 3.7 | 3.7 | 3.7 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 | |
| PAD only | Long-term annual risk (%) | 1.145 | 1.145 | 1.636 |
| Standard error (%) | 0.012 | 0.012 | 0.067 | |
| Transient risk (%) | –0.099 | –0.099 | –0.141 | |
| Standard error (%) | 0.016 | 0.016 | 0.023 | |
| Duration of transient risk (months) | 0.6 | 0.6 | 0.6 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 | |
| MVD (females) | Long-term annual risk (%) | 4.316 | 3.879 | 6.166 |
| Standard error (%) | 0.070 | 0.086 | 0.272 | |
| Transient risk (%) | 0.413 | 0.265 | 0.591 | |
| Standard error (%) | 0.097 | 0.115 | 0.144 | |
| Duration of transient risk (months) | 0.03 | 0.5 | 0.03 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 | |
| MVD (males) | Long-term annual risk (%) | 3.376 | 2.903 | 4.823 |
| Standard error (%) | 0.030 | 0.029 | 0.192 | |
| Transient risk (%) | 0.808 | 0.627 | 1.154 | |
| Standard error (%) | 0.044 | 0.044 | 0.079 | |
| Duration of transient risk (months) | 1.3 | 1.6 | 1.3 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 |
In the ‘peripheral arterial disease-only’ population, there was no evidence of differences by either gender or treatment, so a single model was calibrated covering all CAPRIE26 trial patients.
In the multivascular disease population, there was equivocal evidence in CAPRIE26 suggesting that females are at greater risk than males, and that ASA may be less effective than clopidogrel at preventing recurrent ischaemic stroke; however, the differences appeared to be quite small. In this case, four separate models were calibrated to ensure that even small differences would be reflected in the economic results.
In all cases, risks for patients not receiving any prophylaxis were estimated by adjusting the ASA rates using the RR from the ATTC66 meta-analysis.
Myocardial infarction as first event
The risk of suffering a MI is relatively high for patients in the ‘MI-only’ and multivascular disease populations. In addition to a long-term steady risk level, an important transient increased risk is also present in some cases within the trial data, which applies for different periods for each population.
For the ‘ischaemic stroke-only’ population model, parameter values have been estimated from CAPRIE26 for ASA and clopidogrel where no difference was observed within the trial. A comparison of PRoFESS57 and CAPRIE26 allowed estimation of the long-term risk when receiving treatment with MRD + ASA. The ‘no-treatment’ risk was based on the ATTC66 RR for ASA vs ‘no treatment’ applicable to MI. Finally, the annual risk of MI when using MRD is assumed to be equal to that of ‘no treatment’ based on comparable event rates reported in the ESPS-230 trial. No consistent differences were observed in any of the trials relating to gender.
| Population | Detail | ASA | CLOP | ASA + MRD | MRD | No treatment |
|---|---|---|---|---|---|---|
| IS only | Long-term annual risk (%) | 0.492 | 0.492 | 0.363 | 0.656 | 0.656 |
| Standard error (%) | 0.006 | 0.006 | 0.006 | 0.019 | 0.019 | |
| Transient risk (%) | N/A | N/A | N/A | N/A | N/A | |
| Standard error | ||||||
| Duration of transient risk (months) | ||||||
| Source | CAPRIE26 | CAPRIE26 | PRoFESS57/CAPRIE26 | CAPRIE26/ESPS–230 | CAPRIE26/ATTC66 |
In the ‘MI-only’ and ‘peripheral arterial disease-only’ populations, separate estimates of risk were obtained from the CAPRIE data for treatment with ASA and clopidogrel. No differences were apparent between male and female patients.
For the multivascular disease population, there was some evidence in the CAPRIE26 data supporting risk differences by both gender and treatment. Four separate models were calibrated to ensure that even small differences would be reflected in the economic results. Transient risks were only evident for ASA treatment.
In all cases, risks for patients not receiving any prophylaxis were estimated by adjusting the ASA rates using the RR from the ATTC66 meta-analysis.
| Population | Detail | ASA | CLOP | No treatment |
|---|---|---|---|---|
| MI only | Long-term annual risk (%) | 2.039 | 1.629 | 2.719 |
| Standard error (%) | 0.019 | 0.019 | 0.076 | |
| Transient risk (%) | 1.477 | 1.589 | 1.969 | |
| Standard error (%) | 0.029 | 0.029 | 0.065 | |
| Duration of transient risk (months) | 2.2 | 2.5 | 2.2 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 | |
| PAD only | Long-term annual risk (%) | 0.964 | 0.953 | 1.285 |
| Standard error (%) | 0.031 | 0.030 | 0.055 | |
| Transient risk (%) | 0.181 | –0.398 | 0.241 | |
| Standard error (%) | 0.043 | 0.045 | 0.058 | |
| Duration of transient risk (months) | 6.6 | 2.6 | 6.6 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 | |
| MVD (females) | Long-term annual risk (%) | 2.386 | 1.497 | 3.182 |
| Standard error (%) | 0.071 | 0.072 | 0.127 | |
| Transient risk (%) | 0.464 | N/A | 0.619 | |
| Standard error (%) | 0.102 | N/A | 0.141 | |
| Duration of transient risk (months) | 0.7 | N/A | 0.7 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 | |
| MVD (males) | Long-term annual risk (%) | 2.794 | 2.486 | 3.726 |
| Standard error (%) | 0.025 | 0.018 | 0.105 | |
| Transient risk (%) | 0.713 | N/A | 0.951 | |
| Standard error (%) | 0.037 | N/A | 0.054 | |
| Duration of transient risk (months) | 1.9 | N/A | 1.9 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ATTC66 |
Other vascular death as first event
The incidence of other vascular death as a first event in the ‘ischaemic stroke-only’ population was estimated directly jointly from the CAPRIE26 trial data for ASA and clopidogrel treatments, where no meaningful differences were observed related to either choice of treatment or to gender. Analysis of the PRoFESS57 trial results similarly show no differences between clopidogrel and MRD + ASA. Occlusive vascular disease was not reported in other trials, but the ESPS-230 report allowed calculation of total deaths excluding fatal strokes and this was considered a reasonable proxy for other vascular death, allowing RR multipliers to be calculated for MRD and ‘no treatment’ compared with ASA + MRD.
| Population | Detail | ASA | CLOP | ASA + MRD | MRD | No treatment |
|---|---|---|---|---|---|---|
| IS only | Long-term annual risk (%) | 1.050 | 1.050 | 1.050 | 1.025 | 1.156 |
| Standard error | 0.026 | 0.026 | 0.026 | 0.100 | 0.094 | |
| Transient risk (%) | –0.457 | –0.457 | –0.457 | –0.446 | –0.503 | |
| Standard error | 0.049 | 0.049 | 0.049 | 0.064 | 0.067 | |
| Duration of transient risk (months) | 6.9 | 6.9 | 6.9 | 6.9 | 6.9 | |
| Source | CAPRIE26 | CAPRIE26 | PRoFESS57/CAPRIE26 | CAPRIE26/ESPS–230 | CAPRIE26/ESPS–230 |
In the ‘MI-only’ population, separate estimates of risk were obtained from the CAPRIE26 data for treatment with ASA and clopidogrel, and for both genders.
In the ‘peripheral arterial disease-only’ population, no differences were observed by gender, so combined estimates were obtained for ASA and clopidogrel after combining results for male and female patients.
For the multivascular disease population, there was clear evidence in the CAPRIE26 data supporting risk differences by gender, but not by treatment. Therefore, two models were calibrated for male and female patients.
In all cases, risks for patients not receiving any prophylaxis were estimated by adjusting the ASA rates using the RR from ESPS-230 trial as described above.
| Population | Detail | ASA | CLOP | No treatment |
|---|---|---|---|---|
| MI only (females) | Long-term annual risk (%) | 0.863 | 1.444 | 0.951 |
| Standard error | 0.137 | 0.234 | 0.167 | |
| Transient risk (%) | 0.709 | 0.658 | 0.780 | |
| Standard error | 0.119 | 0.118 | 0.139 | |
| Duration of transient risk (months) | 0.8 | 1.4 | 0.8 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ESPS–230 | |
| MI only (males) | Long-term annual risk (%) | 0.646 | 1.080 | 0.711 |
| Standard error | 0.019 | 0.039 | 0.060 | |
| Transient risk (%) | 0.530 | 0.492 | 0.583 | |
| Standard error (%) | 0.025 | 0.048 | 0.054 | |
| Duration of transient risk (months) | 0.8 | 1.4 | 0.8 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ESPS–226 | |
| PAD only | Long-term annual risk (%) | 1.499 | 0.583 | 1.650 |
| Standard error (%) | 0.392 | 0.059 | 0.447 | |
| Transient risk (%) | –1.226 | –0.161 | –1.351 | |
| Standard error (%) | 1.561 | 0.111 | 1.751 | |
| Duration of transient risk (months) | 16.6 | 3.4 | 16.6 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ESPS–226 | |
| MVD (females) | Long-term annual risk (%) | 1.427 | 1.427 | 1.571 |
| Standard error (%) | 0.064 | 0.064 | 0.144 | |
| Transient risk (%) | 0.701 | 0.701 | 0.772 | |
| Standard error (%) | 0.109 | 0.109 | 0.137 | |
| Duration of transient risk (months) | 2.3 | 2.3 | 2.3 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ESPS–226 | |
| MVD (males) | Long-term annual risk (%) | 2.653 | 2.653 | 2.922 |
| Standard error (%) | 0.016 | 0.016 | 0.232 | |
| Transient risk (%) | –0.230 | –0.230 | –0.254 | |
| Standard error (%) | 0.027 | 0.027 | 0.035 | |
| Duration of transient risk (months) | 2.4 | 2.4 | 2.4 | |
| Source | CAPRIE26 | CAPRIE26 | CAPRIE26/ESPS–226 |
Risks of subsequent occlusive vascular events
For patients surviving a first occlusive vascular event within the key trials (CAPRIE26 and PRoFESS57), the number of patients suffering a second or third event are very small. In a few cases it is feasible to estimate parameter values relating to specific second events, but in many cases the data are insufficient so it has been necessary to make assumptions based on the available evidence.
Following non-fatal ischaemic stroke as first event: risk of second ischaemic stroke event
A number of patients who survived an ischaemic stroke in the CAPRIE26 trial went on to experience a second ischaemic stroke event. No significant differences in incidence rates were apparent relating to the choice of treatment. However, those belonging to the ‘ischaemic stroke-only’ population experienced a lower level of risk than other patients. The same approach to extending these parameters to cover other treatments used as for ischaemic stroke first events.
| Population | Detail | ASA, CLOP ASA + MRDa | MRD | No treatment |
|---|---|---|---|---|
| IS only | Long-term annual risk (%) | 7.323 | 9.725 | 10.462 |
| Standard error (%) | 0.694 | 1.277 | 1.069 | |
| Transient risk (%) | 7.039 | 9.349 | 10.056 | |
| Standard error (%) | 1.401 | 2.069 | 1.997 | |
| Duration of transient risk (months) | 6.2 | 6.2 | 6.2 | |
| Source | PRoFESS57/CAPRIE26 | ProFESS26/CAPRIE26/ESPS–226 | CAPRIE26/ATTC26 | |
| MI only, PAD only and MVD | Long-term annual risk (%) | 11.627 | N/A | 16.610 |
| Standard error (%) | 0.201 | 0.714 | ||
| Transient risk (%) | 3.335 | 4.764 | ||
| Standard error (%) | 0.224 | 0.365 | ||
| Duration of transient risk (months) | 1.4 | 1.4 | ||
| Source | CAPRIE26 | – | CAPRIE26/ATTC66 |
Following non-fatal ischaemic stroke as first event: risk of myocardial infarction event
Very few ischaemic stroke survivors suffered a subsequent MI in the CAPRIE26 trial. A single overall linear regression hazard model was calibrated for all patient groups, extended additional treatments as before for first MI events.
| Population | Detail | ASA, CLOP | ASA + MRD | MRD, no treatment |
|---|---|---|---|---|
| All patients | Long-term annual risk (%) | 1.212 | 0.892 | 1.616 |
| Standard error (%) | 0.181 | 0.220 | 0.243 | |
| Transient risk (%) | N/A | N/A | N/A | |
| Standard error (%) | N/A | N/A | N/A | |
| Duration of transient risk (months) | N/A | N/A | N/A | |
| Source | CAPRIE26 | PRoFESS/CAPRIE26 | CAPRIE26/ESPS–230/ATTC66 |
Following non-fatal ischaemic stroke as first event: risk of other vascular death event
Very few patients who survived an ischaemic stroke in the CAPRIE26 trial suffered a subsequent other vascular death event. A single projection model was calibrated for all patient groups, extended additional treatments as before for primary other vascular death events.
| Population | Detail | ASA, CLOP, ASA + MRD | MRD | No treatment |
|---|---|---|---|---|
| All patients | Long-term annual risk (%) | 1.853 | 1.809 | 2.041 |
| Standard error (%) | 0.142 | 0.218 | 0.232 | |
| Transient risk (%) | 2.354 | 2.297 | 2.592 | |
| Standard error (%) | 0.211 | 0.300 | 0.310 | |
| Duration of transient risk (months) | 2.0 | 2.0 | 2.0 | |
| Source | CAPRIE26 | PRoFESS57/CAPRIE26 | CAPRIE26/ESPS–230/ATTC66 |
Following non-fatal ischaemic stroke as first event: risk of haemorrhagic stroke event
Insufficient haemorrhagic stroke events occurred among ischaemic stroke survivors to allow any subdivision by patient subgroups or treatments.
Following non-fatal myocardial infarction as first event: risk of myocardial infarction event
No differences in MI risk were detectable by treatment in the CAPRIE26 trial data, but the risk among the multivascular disease population was more than double the risk in the other groups.
| Population | Detail | ASA, CLOP | ASA + MRD | MRD | No treatment |
|---|---|---|---|---|---|
| IS only, MI only and PAD only | Long-term annual risk (%) | 5.787 | 4.261 | 7.716 | 7.716 |
| Standard error (%) | 0.190 | 0.817 | 0.327 | 0.327 | |
| Transient risk (%)a | 3.287 | 3.098 | 4.383 | 4.383 | |
| Standard error (%) | 0.239 | 0.605 | 0.340 | 0.340 | |
| Duration of transient risk (months) | 1.6 | 1.6 | 1.6 | 1.6 | |
| Source | CAPRIE26 | PRoFESS57/CAPRIE26 | CAPRIE26/ESPS–230 | CAPRIE26/ATTC66 | |
| MVD | Long-term annual risk (%) | 12.228 | N/A | N/A | 16.303 |
| Standard error (%) | 0.513 | N/A | N/A | 0.819 | |
| Transient risk (%)a | 8.713 | N/A | N/A | 11.617 | |
| Standard error (%) | 0.462 | N/A | N/A | 0.734 | |
| Duration of transient risk (months) | 0.8 | N/A | N/A | 0.8 | |
| Source | CAPRIE26 | – | – | CAPRIE26/ATTC66 |
Following non-fatal myocardial infarction: risk of ischaemic stroke event
The risk of suffering an ischaemic stroke event following a non-fatal MI was found to be very low and a single projective model was calibrated using all available CAPRIE26 data.
| Population | Detail | ASA, CLOP | ASA + MRD | MRD | No treatment |
|---|---|---|---|---|---|
| All patients | Long-term annual risk (%) | 1.837 | 1.837 | 2.440 | 2.624 |
| Standard error (%) | 0.267 | 0.267 | 0.417 | 0.394 | |
| Transient risk (%) | 1.608 | 1.608 | 2.135 | 2.297 | |
| Standard error (%) | 0.307 | 0.307 | 0.452 | 0.431 | |
| Duration of transient risk (months) | 2.2 | 2.2 | 2.2 | 2.2 | |
| Source | CAPRIE26 | PRoFESS57/CAPRIE26 | CAPRIE26/ESPS–230 | CAPRIE26/ATTC66 |
Following non-fatal myocardial infarction: risk of other vascular death event
Although it was not possible to detect any difference in risk by treatment type in the CAPRIE26 data, it was clear that patients with multivascular disease suffered a threefold risk of other vascular death cause following a non-fatal MI compared with other groups.
| Population | Detail | ASA, CLOP | ASA + MRD | MRD | No treatment |
|---|---|---|---|---|---|
| MI only, IS only and PAD only | Long-term annual risk (%) | 3.110 | 3.110 | 3.035 | 3.425 |
| Standard error (%) | 0.152 | 0.152 | 0.318 | 0.317 | |
| Transient risk (%) | N/A | N/A | N/A | N/A | |
| Standard error (%) | |||||
| Duration of transient risk (months) | |||||
| Source | CAPRIE26 | PRoFESS57/CAPRIE26 | CAPRIE26/ESPS–230 | CAPRIE26/ATTC66 | |
| MVD | Long-term annual risk (%) | 10.850 | N/A | N/A | 11.949 |
| Standard error (%) | 0.304 | 1.000 | |||
| Transient risk (%) | N/A | N/A | |||
| Standard error (%) | |||||
| Duration of transient risk (months) | |||||
| Source | CAPRIE26 | – | – | CAPRIE26/ATTC66 |
Following non-fatal myocardial infarction: risk of a haemorrhagic stroke event
The risk of haemorrhagic stroke following an initial MI event was found to be extremely low.
| Population | Detail | All treatments | No treatment |
|---|---|---|---|
| All patients | Long-term annual risk (%) | 0.190 | 0.156 |
| 95% confidence limits (LCL, UCL) | 0.005% to 0.699% | 0.006% to 0.853% | |
| Transient risk (%) | N/A | N/A | |
| Standard error (%) | N/A | N/A | |
| Duration of transient risk (months) | N/A | N/A | |
| Source | CAPRIE26 | CAPRIE26/ATTC66 |
Following non-fatal haemhorrhagic stroke as a first event
There were too few events of any type recorded in the CAPRIE26 trial to patients surviving an initial haemorrhagic stroke. However, in order to provide parameters for this part of the model, a simple device was employed: the overall event rate was subdivided among the possible four types of event (ischaemic stroke, haemorrhagic stroke, MI and other vascular death) in proportion to their frequency among CAPRIE26 first events, and the figure converted to a single average event rate for each event.
| Population | Event | Detail | All treatments | No treatment |
|---|---|---|---|---|
| All patients | IS | Long-term annual risk (%) | 2.875 | 4.107 |
| Standard error (%) | 0.489 | 0.726 | ||
| Haemorrhagic stroke | Long-term annual risk (%) | 1.944 | 1.594 | |
| Standard error (%) | 0.331 | 0.298 | ||
| MI | Long-term annual risk (%) | 0.182 | 0.243 | |
| Standard error (%) | 0.031 | 0.042 | ||
| Other vascular death | Long-term annual risk (%) | 1.439 | 1.585 | |
| Standard error (%) | 0.245 | 0.311 | ||
| Source | CAPRIE26 | CAPRIE26/ATTC66 |
Risk modifiers
Cox’s proportional hazard regressions were carried out on the CAPRIE26 data to identify the influence of age and stroke-related disability (using the modified Rankin Score) on the key first events in the trial. From these results event modifying factors were derived to allow the risk values described above to be adjusted to the characteristics of individual patients.
| Event | Stroke disability (modified Rankin Score) | Age modifier (per year) | |
|---|---|---|---|
| Not disabled (0–2) | Disabled (3+) | ||
| IS | 1.020 | 0.945 | 1.201 |
| Haemorrhagic stroke | 1.010 | 0.855 | 1.653 |
| MI | 1.041 | 0.981 | 1.064 |
| Other vascular death | 1.043 | 0.774 | 2.283 |
| Non-vascular death | 1.073 | 0.862 | 1.614 |
Appendix 11 Event fatality rates estimated from CAPRIE26 trial data
Ischaemic stroke
There is only evidence to support differences in ischaemic stroke fatality risk arising from patient subgroup and age; gender and type of preventive treatment do not appear to be important predictors. An exponential odds model for risk increasing with age has been calibrated, with separate ORs applied for each patient group (greatest for MI- and peripheral arterial disease-only patients and lowest for ischaemic stroke-only patients). Fatality data from the PRoFESS57 trial are not directly comparable, as the PRoFESS57 population is a combination of ischaemic stroke-only and multivascular disease patients in unknown proportions. In addition, only the clopidogrel arms of the two trials could be included in any data synthesis. Nonetheless, simple rate comparisons did not reveal any marked differences in fatality rates between the two sources.
Fatality odds = 0.00212
-
× exp(0.0520 × age)
-
× population OR
-
× event sequence OR.
Odds ratios for patient subgroups are:
-
ischaemic stroke only, × 0.686
-
MI only, × 1.673
-
peripheral arterial disease only, × 1.691
-
multivascular disease, × 1.175.
Odds ratios for event sequence (MIs or strokes):
-
first, × 0.791
-
second, × 1.931
-
third, × 4.398.
Myocardial infarction
Myocardial infarction fatality is age and gender-specific, but is not influenced by the choice of treatment. Exponential odds models have been calibrated for exponential age relationships – separately for males and females. Important differences are apparent for population subgroups and for interactions between subgroups and gender, so separate age/group OR modifiers are used. As noted above, CAPRIE26 and PRoFESS57 data cannot be compared directly even with the ischaemic stroke population, but visual examination indicates that the PRoFESS57 results are broadly consistent with those obtained from CAPRIE. 26
For females:
-
Fatality odds = 0.00801
-
– × exp(0.0538 × age)
-
– × population OR
-
– × event sequence OR.
-
-
Odds ratios for patient subgroups are:
-
– ischaemic stroke only, × 1.765
-
– MI only, × 0.584
-
– peripheral arterial disease only, × 0.195
-
– multivascular disease, × 1.765.
-
-
Odds ratios for event sequence are:
-
– first, × 0.791
-
– second, × 1.931
-
– third, × 4.398.
-
For males:
-
Fatality odds = 0.00986
-
– × exp(0.0455 × age)
-
– × population OR
-
– × event sequence OR.
-
-
Odds ratios for patient subgroups are:
-
– ischaemic stroke only, × 0.679
-
– MI only, × 0.574
-
– peripheral arterial disease only, × 0.985
-
– multivascular disease, × 1.651.
-
-
Odds ratios for event sequence (MIs or strokes) are:
-
– first, × 0.791
-
– second, × 1.931
-
– third, × 4.398.
-
Non-ischaemic stroke (haemorrhagic stroke)
A small number of non-ischaemic strokes/intracranial haemorrhages were reported in the two trials. When the fatality data from the CAPRIE26 and PRoFESS57 trials were combined, no significant differences attributable to age or patient population were detected, so simple average rates have been estimated for age–treatment combinations:
Glossary
- Acute coronary syndrome
- Acute coronary artery disease including unstable angina and non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI).
- Antiplatelet agent
- Type of anticlotting agent that works by inhibiting blood platelets. Antiplatelet drugs include clopidogrel (CLOP), dipyridamole and aspirin (ASA).
- Cerebrovascular
- Pertaining to the blood vessels of the brain.
- Clopidogrel (CLOP)
- A thienopyridine – an inhibitor of platelet aggregation.
- Coronary arteries
- The arteries that supply the heart muscle with blood.
- Coronary artery disease
- Gradual blockage of the coronary arteries, usually by atherosclerosis.
- Coronary heart disease
- Narrowing or blockage of the coronary arteries of the heart by atheroma; often leads to angina, coronary thrombosis or heart attack, heart failure and/or sudden death.
- Cost-effectiveness
- The consequences of the alternatives are measured in natural units, such as years of life gained. The consequences are not given a monetary value.
- Dipyridamole
- Inhibitor of platelet aggregation, also available in combination with aspirin.
- Dominated
- A technology is dominated if the comparator is less expensive and more effective; a technology dominates if it is cheaper and more effective than the comparator.
- Electrocardiogram (ECG)
- A recording of the electrical signals from the heart.
- Haemorrhagic stroke
- Death of brain cells because of bleeding in the brain.
- Heterogeneity
- Between-study variation. If heterogeneity exists, the pooled effect size in a meta-analysis has no meaning.
- Incremental cost
- The difference in costs between one intervention and an alternative.
- Incremental cost-effectiveness ratio (ICER)
- The difference in costs between one intervention and an alternative, divided by the difference in outcomes.
- Incremental quality-adjusted life-year (QALY)
- The difference in QALYs between one intervention and an alternative.
- Infarction
- Death of tissue following interruption of the blood supply.
- Intention-to-treat (ITT) analysis method
- A method of data analysis in which all patients are analysed in the group to which they were assigned at randomisation, regardless of treatment adherence.
- Intermittent claudication
- The most common peripheral arterial disease symptom, characterised by calf, thigh or buttock pain and weakness brought on by walking. Pain disappears on resting the affected limb.
- Ischaemia
- A low oxygen state, usually due to obstruction of the arterial blood supply or inadequate blood flow leading to hypoxia in the tissue.
- Ischaemic stroke
- Death of brain cells caused by blockage in a cerebral blood vessel.
- Meta-analysis
- A quantitative method for combining the results of many studies into one set of conclusions.
- Myocardial infarction (MI)
- Damage to the heart muscle caused by obstruction of circulation to a region of the heart. Also called a heart attack.
- Non-ST-segment elevation myocardial infarction (NSTEMI)
- A myocardial infarction not associated with elevation of the ST-segment on an electrocardiogram.
- Occlusive vascular event
- An event caused by the blockage of an artery, such as myocardial infarction (MI), unstable angina, ischaemic stroke, transient ischaemic attack (TIA) or peripheral arterial disease.
- Peripheral arterial disease
- A condition in which the arteries that carry blood to the arms or legs become narrowed or clogged, slowing or stopping the flow of blood. Also known as peripheral vascular disease.
- Plaque
- Atheromatous plaque is a swelling on the inner surface of an artery produced by lipid deposition.
- Quality-adjusted life-year (QALY)
- An index of survival that is weighted or adjusted by a patient’s quality of life during the survival period. QALYs are calculated by multiplying the number of life-years by an appropriate utility or preference score.
- Qualifying event
- The event (myocardial infarction, ischaemic stroke, transient ischaemic attack or peripheral arterial disease) for which patients are randomised into a trial.
- Relative risk (RR)
- The proportion of diseased people among those exposed to the relevant risk factor divided by the proportion of diseased people among those not exposed to the risk factor.
- Relative risk reduction (RRR)
- An alternative way of expressing relative risk. It is calculated as RRR = (1 – RR) × 100%. The RRR can be interpreted as the proportion of the baseline ‘risk’ that was eliminated by a given treatment or by avoidance of exposure to a risk factor.
- ST-segment elevation MI (STEMI)
- A myocardial infarction associated with elevation of the ST-segment on the electrocardiogram (ECG).
- Stroke
- The sudden death of brain cells because of a lack of oxygen when blood flow to the brain is impaired by a blockage or rupture of an artery to the brain, causing neurological dysfunction.
- Thrombus
- An aggregation of blood factors, primarily platelets and fibrin with entrapment of cellular elements; frequently causes vascular obstruction at the point of its formation.
- Transient ischaemic attack (TIA)
- A brain disorder caused by temporary disturbance of blood supply to an area of the brain, resulting in a sudden, brief (< 24 hours, usually < 1 hour) decrease in brain function.
- Unstable angina
- Angina pectoris (chest pain) in which the cardiac pain has changed in pattern or occurs at rest.
- Vascular disease
- Any disease of the circulatory system.
List of abbreviations
- ACS
- acute coronary syndrome
- ASA
- acetylsalicylic acid (i.e. aspirin)
- ATTC
- Antithrombotic Trialist’s Collaboration
- BHF
- British Heart Foundation
- BNF
- British National Formulary
- CAD
- coronary artery disease
- CAPRIE
- Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events
- CEAC
- cost-effectiveness acceptability curve
- CHD
- coronary heart disease
- CHF
- congestive heart failure
- CI
- confidence interval
- CLOP
- clopidogrel
- CVD
- cardiovascular disease
- DHDS
- Diabetes, Heart Disease and Stroke prevention project
- ECG
- electrocardiogram
- ESPRIT
- European/Australasian Stroke Prevention in Reversible Ischaemia Trial
- ESPS-2
- Second European Stroke Prevention Study
- GP
- general practitioner
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IS
- ischaemic stroke
- ITT
- intention to treat
- LYG
- life-year gained
- LYS
- life-year saved
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MI
- myocardial infarction
- MIMS
- Monthly Index of Medical Specialities
- MPES
- Multi-parameter Evidence Synthesis Research Group
- MRD
- modified-release dipyridamole
- MS
- manufacturer’s submission
- MTA
- multiple technology assessment
- MTC
- mixed-treatment comparison
- MVD
- multivascular disease
- NICE
- National Institute for Health and Clinical Excellence
- NSF
- National Service Framework
- NSTEMI
- non-ST-segment elevation myocardial infarction
- OR
- odds ratio
- OVE
- occlusive vascular event
- PAD
- peripheral arterial disease
- PPI
- proton pump inhibitor
- PRoFESS
- Prevention Regimen For Effectively avoiding Second Strokes
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RRR
- relative risk reduction
- RR
- relative risk
- SR
- systematic review
- STEMI
- ST-segment elevation myocardial infarction
- TA
- technology appraisal
- TIA
- transient ischaemic attack
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington